User login
Singulair-induced anaphylaxis?
When L.O., an African American boy, was 13 months old, he was taken to the emergency room by his mother for an episode of diffuse expiratory wheezing. The family had a history of asthma. L.O.’s wheezing was effectively treated with albuterol, which was prescribed for use at home. At 17 months, L.O. was diagnosed with eczema and allergy to eggs.
When the boy was 3 years old, his mother brought him to St. Dominic’s Health Clinic in Jamaica, NY, for a well-child visit. She reported that L.O. had experienced only 2 asthma attacks in the past year. We diagnosed mild intermittent asthma and advised the mother to continue using albuterol as needed. The patient returned to the clinic at age 4, with redness and swelling of both eyes typical of allergic conjunctivitis. Four months later L.O. returned with rhinorrhea, which, in conjunction with asthma, eczema, and allergic conjunctivitis, led us to diagnose atopic syndrome. This time, we prescribed 4 mg Singulair (montelukast sodium), to be taken once daily.
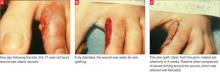
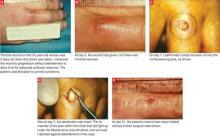
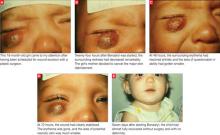
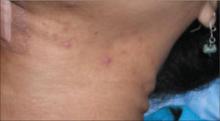
Immediately after taking a single Singulair tablet in the afternoon, L.O. developed pruritus. That evening he awoke from his sleep screaming; he had prominent lip, facial, and pedal edema. He also had trouble breathing and had red, blotchy hives over his entire back. His mother was unable to administer epinephrine (EpiPen), which had been prescribed for L.O.’s egg allergy. She called 911 and L.O. was taken to an emergency room. He had tachycardia and a low-grade fever. Epinephrine and diphenhydramine (Benadryl) began to lower his temperature and gradually lessened his edema and urticaria. Upon L.O.’s discharge, his mother was cautioned not to give him any more Singulair.
How common is L.O.’s experience? In a review of the literature, we found just 4 mentions of an anaphylactic response to Singulair treatment. We describe these reports here and discuss the implications.
A drug with few reported side effects
Singulair is a leukotriene receptor antagonist commonly prescribed for the prevention and treatment of asthma and for the treatment of allergic rhinitis. It is an orally active compound that binds with high affinity to the CysLT type-1 receptor, a leukotriene receptor found in a variety of human airway cells, including smooth muscle cells, macrophages, and eosinophils.1 At this receptor, Singulair inhibits the physiologic action of LTD4, a leukotriene released by various inflammatory cells that normally initiates the symptoms of asthma.
Singulair has been shown to dramatically increase forced expiratory volume, decrease usage of inhaled beta-agonists, and improve other asthma-related outcomes in both adults and children. In clinical studies, Singulair has proven safe, with few reported side effects. Some benign adverse events have been associated with this drug when compared with placebo, but causality between these events and Singulair is uncertain. Anaphylaxis was not reported in any of the premarketing clinical studies of Singulair.
4 other accounts of anaphylaxis
Singulair’s package insert mentions anaphylaxis as an adverse reaction reported after the US Food and Drug Administration approved the drug in 1998.1 Merck & Co., producer and distributor of Singulair, did not provide any specific information on reports of anaphylaxis for this review.
The Drug Safety Research Unit, an independent body associated with the University of Portsmouth in England, mentioned just one instance of anaphylaxis in a study of adverse reactions to montelukast among a cohort of more than 15,000 patients.2
A presentation given at a Healthcare Information Management Systems Society conference also briefly mentioned the case of an 8-year-old boy who experienced an anaphylactic reaction to Singulair.3
The only published description of a possible case of anaphylaxis in response to Singulair appeared in a report published by Lareb, the Dutch national pharmacovigilance system.4 A 4-year-old boy suffered facial edema, rash, coughing, and fatigue 2 days after starting montelukast 5 mg daily for asthma. The patient’s age and symptoms were strikingly similar to those of L.O.
Anaphylaxis: Always a possibility
Clearly, anaphylaxis as an adverse reaction to Singulair is rare, with only a handful of cases being reported worldwide. Nevertheless, anaphylaxis is life threatening, and we should be alert to its possibility when prescribing Singulair, especially for patients with a history of atopy.
Correspondence
Adriel Gerard, State University of New York at Buffalo School of Medicine, 99 Gold Street, Apt 1L, Brooklyn, NY 11201; [email protected]
1. Singulair (montelukast sodium) [prescribing information]. Whitehouse Station, NJ: Merck & Co., Inc; 2008.
2. Biswas P, Wilton L, Pearce G, et al. Pharmacosurveillance and safety of the leukotriene receptor antagonist (LTRA), montelukast. Clin Exp All Rev. 2001;3:300-304.
3. Millikan E. XML drug information modeling: linking evidence-based medicine with the bedside. In Proceedings of Health Information Management Systems Society. February 13-17, 2005. Available at: www.himss.org/content/files/2005proceedings/sessions/edu031.pdf. Accessed February 2, 2009.
4. An overview of reports on montelukast. Available at: www.lareb.nl/documents/kwb_2002_3_monte.pdf. Accessed February 2, 2009.
When L.O., an African American boy, was 13 months old, he was taken to the emergency room by his mother for an episode of diffuse expiratory wheezing. The family had a history of asthma. L.O.’s wheezing was effectively treated with albuterol, which was prescribed for use at home. At 17 months, L.O. was diagnosed with eczema and allergy to eggs.
When the boy was 3 years old, his mother brought him to St. Dominic’s Health Clinic in Jamaica, NY, for a well-child visit. She reported that L.O. had experienced only 2 asthma attacks in the past year. We diagnosed mild intermittent asthma and advised the mother to continue using albuterol as needed. The patient returned to the clinic at age 4, with redness and swelling of both eyes typical of allergic conjunctivitis. Four months later L.O. returned with rhinorrhea, which, in conjunction with asthma, eczema, and allergic conjunctivitis, led us to diagnose atopic syndrome. This time, we prescribed 4 mg Singulair (montelukast sodium), to be taken once daily.
Immediately after taking a single Singulair tablet in the afternoon, L.O. developed pruritus. That evening he awoke from his sleep screaming; he had prominent lip, facial, and pedal edema. He also had trouble breathing and had red, blotchy hives over his entire back. His mother was unable to administer epinephrine (EpiPen), which had been prescribed for L.O.’s egg allergy. She called 911 and L.O. was taken to an emergency room. He had tachycardia and a low-grade fever. Epinephrine and diphenhydramine (Benadryl) began to lower his temperature and gradually lessened his edema and urticaria. Upon L.O.’s discharge, his mother was cautioned not to give him any more Singulair.
How common is L.O.’s experience? In a review of the literature, we found just 4 mentions of an anaphylactic response to Singulair treatment. We describe these reports here and discuss the implications.
A drug with few reported side effects
Singulair is a leukotriene receptor antagonist commonly prescribed for the prevention and treatment of asthma and for the treatment of allergic rhinitis. It is an orally active compound that binds with high affinity to the CysLT type-1 receptor, a leukotriene receptor found in a variety of human airway cells, including smooth muscle cells, macrophages, and eosinophils.1 At this receptor, Singulair inhibits the physiologic action of LTD4, a leukotriene released by various inflammatory cells that normally initiates the symptoms of asthma.
Singulair has been shown to dramatically increase forced expiratory volume, decrease usage of inhaled beta-agonists, and improve other asthma-related outcomes in both adults and children. In clinical studies, Singulair has proven safe, with few reported side effects. Some benign adverse events have been associated with this drug when compared with placebo, but causality between these events and Singulair is uncertain. Anaphylaxis was not reported in any of the premarketing clinical studies of Singulair.
4 other accounts of anaphylaxis
Singulair’s package insert mentions anaphylaxis as an adverse reaction reported after the US Food and Drug Administration approved the drug in 1998.1 Merck & Co., producer and distributor of Singulair, did not provide any specific information on reports of anaphylaxis for this review.
The Drug Safety Research Unit, an independent body associated with the University of Portsmouth in England, mentioned just one instance of anaphylaxis in a study of adverse reactions to montelukast among a cohort of more than 15,000 patients.2
A presentation given at a Healthcare Information Management Systems Society conference also briefly mentioned the case of an 8-year-old boy who experienced an anaphylactic reaction to Singulair.3
The only published description of a possible case of anaphylaxis in response to Singulair appeared in a report published by Lareb, the Dutch national pharmacovigilance system.4 A 4-year-old boy suffered facial edema, rash, coughing, and fatigue 2 days after starting montelukast 5 mg daily for asthma. The patient’s age and symptoms were strikingly similar to those of L.O.
Anaphylaxis: Always a possibility
Clearly, anaphylaxis as an adverse reaction to Singulair is rare, with only a handful of cases being reported worldwide. Nevertheless, anaphylaxis is life threatening, and we should be alert to its possibility when prescribing Singulair, especially for patients with a history of atopy.
Correspondence
Adriel Gerard, State University of New York at Buffalo School of Medicine, 99 Gold Street, Apt 1L, Brooklyn, NY 11201; [email protected]
When L.O., an African American boy, was 13 months old, he was taken to the emergency room by his mother for an episode of diffuse expiratory wheezing. The family had a history of asthma. L.O.’s wheezing was effectively treated with albuterol, which was prescribed for use at home. At 17 months, L.O. was diagnosed with eczema and allergy to eggs.
When the boy was 3 years old, his mother brought him to St. Dominic’s Health Clinic in Jamaica, NY, for a well-child visit. She reported that L.O. had experienced only 2 asthma attacks in the past year. We diagnosed mild intermittent asthma and advised the mother to continue using albuterol as needed. The patient returned to the clinic at age 4, with redness and swelling of both eyes typical of allergic conjunctivitis. Four months later L.O. returned with rhinorrhea, which, in conjunction with asthma, eczema, and allergic conjunctivitis, led us to diagnose atopic syndrome. This time, we prescribed 4 mg Singulair (montelukast sodium), to be taken once daily.
Immediately after taking a single Singulair tablet in the afternoon, L.O. developed pruritus. That evening he awoke from his sleep screaming; he had prominent lip, facial, and pedal edema. He also had trouble breathing and had red, blotchy hives over his entire back. His mother was unable to administer epinephrine (EpiPen), which had been prescribed for L.O.’s egg allergy. She called 911 and L.O. was taken to an emergency room. He had tachycardia and a low-grade fever. Epinephrine and diphenhydramine (Benadryl) began to lower his temperature and gradually lessened his edema and urticaria. Upon L.O.’s discharge, his mother was cautioned not to give him any more Singulair.
How common is L.O.’s experience? In a review of the literature, we found just 4 mentions of an anaphylactic response to Singulair treatment. We describe these reports here and discuss the implications.
A drug with few reported side effects
Singulair is a leukotriene receptor antagonist commonly prescribed for the prevention and treatment of asthma and for the treatment of allergic rhinitis. It is an orally active compound that binds with high affinity to the CysLT type-1 receptor, a leukotriene receptor found in a variety of human airway cells, including smooth muscle cells, macrophages, and eosinophils.1 At this receptor, Singulair inhibits the physiologic action of LTD4, a leukotriene released by various inflammatory cells that normally initiates the symptoms of asthma.
Singulair has been shown to dramatically increase forced expiratory volume, decrease usage of inhaled beta-agonists, and improve other asthma-related outcomes in both adults and children. In clinical studies, Singulair has proven safe, with few reported side effects. Some benign adverse events have been associated with this drug when compared with placebo, but causality between these events and Singulair is uncertain. Anaphylaxis was not reported in any of the premarketing clinical studies of Singulair.
4 other accounts of anaphylaxis
Singulair’s package insert mentions anaphylaxis as an adverse reaction reported after the US Food and Drug Administration approved the drug in 1998.1 Merck & Co., producer and distributor of Singulair, did not provide any specific information on reports of anaphylaxis for this review.
The Drug Safety Research Unit, an independent body associated with the University of Portsmouth in England, mentioned just one instance of anaphylaxis in a study of adverse reactions to montelukast among a cohort of more than 15,000 patients.2
A presentation given at a Healthcare Information Management Systems Society conference also briefly mentioned the case of an 8-year-old boy who experienced an anaphylactic reaction to Singulair.3
The only published description of a possible case of anaphylaxis in response to Singulair appeared in a report published by Lareb, the Dutch national pharmacovigilance system.4 A 4-year-old boy suffered facial edema, rash, coughing, and fatigue 2 days after starting montelukast 5 mg daily for asthma. The patient’s age and symptoms were strikingly similar to those of L.O.
Anaphylaxis: Always a possibility
Clearly, anaphylaxis as an adverse reaction to Singulair is rare, with only a handful of cases being reported worldwide. Nevertheless, anaphylaxis is life threatening, and we should be alert to its possibility when prescribing Singulair, especially for patients with a history of atopy.
Correspondence
Adriel Gerard, State University of New York at Buffalo School of Medicine, 99 Gold Street, Apt 1L, Brooklyn, NY 11201; [email protected]
1. Singulair (montelukast sodium) [prescribing information]. Whitehouse Station, NJ: Merck & Co., Inc; 2008.
2. Biswas P, Wilton L, Pearce G, et al. Pharmacosurveillance and safety of the leukotriene receptor antagonist (LTRA), montelukast. Clin Exp All Rev. 2001;3:300-304.
3. Millikan E. XML drug information modeling: linking evidence-based medicine with the bedside. In Proceedings of Health Information Management Systems Society. February 13-17, 2005. Available at: www.himss.org/content/files/2005proceedings/sessions/edu031.pdf. Accessed February 2, 2009.
4. An overview of reports on montelukast. Available at: www.lareb.nl/documents/kwb_2002_3_monte.pdf. Accessed February 2, 2009.
1. Singulair (montelukast sodium) [prescribing information]. Whitehouse Station, NJ: Merck & Co., Inc; 2008.
2. Biswas P, Wilton L, Pearce G, et al. Pharmacosurveillance and safety of the leukotriene receptor antagonist (LTRA), montelukast. Clin Exp All Rev. 2001;3:300-304.
3. Millikan E. XML drug information modeling: linking evidence-based medicine with the bedside. In Proceedings of Health Information Management Systems Society. February 13-17, 2005. Available at: www.himss.org/content/files/2005proceedings/sessions/edu031.pdf. Accessed February 2, 2009.
4. An overview of reports on montelukast. Available at: www.lareb.nl/documents/kwb_2002_3_monte.pdf. Accessed February 2, 2009.
Tips for talking to teens about STDs
- Annual physical exams and sports physicals are ideal opportunities to raise issues of sex and sexually transmitted infections (STIs) (B).
- Meet with young patients alone when asking about sexual habits, activity, preferences, or other sensitive matters (C).
- Evidence supports the use of expedited partner therapy (EPT) in treating gonorrhea and Chlamydia (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
As you conclude a routine annual physical exam of a 14-year-old girl, you ask her if she has any questions. With some hesitation, she tells you about a “friend” of hers who recently had sex with her boyfriend. She has heard many rumors from her friends, television, and chat lines. She doesn’t think her friend got pregnant, but is worried that she might have caught something. She tells you that her friend is also uncertain about whether—and how—to tell her parents.
How would you respond? Would you be comfortable helping your patient, and her parents, discuss this delicate matter in a meaningful way?
Though most clinicians are adept at diagnosing and treating sexually transmitted infections, far fewer feel skilled enough to counsel teens and parents about them. STIs (the new and politically appropriate terminology for STDs) are commonly encountered in family medicine; knowing how to discuss the many associated issues with teens and parents is paramount—especially when you consider the statistics.
According to the Centers for Disease Control and Prevention’s Youth Risk Behavior Surveillance System (YRBSS):1
- 47.8% of 9th to 12th graders have had sexual intercourse.
- 7.1% of children have had intercourse before age 13.
- 14.9% of high school students have had sexual intercourse with 4 or more people.
- 38.5% of sexually active 9th to 12th graders did not use condoms during their last sexual encounter.
Opening Pandora’s box—carefully
As family physicians, we must be willing not only to discuss sexual issues if asked, but to raise them with patients and parents, when appropriate. The CDC offers training for health professionals in obtaining a sexual history and counseling and educating patients (http://depts.washington.edu/nnptc). Unfortunately, little information exists on how to tailor education to the values and beliefs of an individual family or to the broader cultural mores of the society in which the young person lives.2 Nonetheless, there are many things you can do to improve the conversations you have.
For starters, ask open-ended questions and use understandable and normalizing language.3 Educate patients regarding the various STIs and methods of prevention—ie, proper condom use, abstinence, limiting the number of sexual partners, changes in sexual practices, and vaccination.4-6 Ask patients about their use of and motivation to use condoms and contraceptive methods, as well as their sexual orientation, number of sexual partners, if they have ever exchanged sex for money or drugs, and prior history of STIs.7 To facilitate communication, familiarize yourself with sexual slang terms.
Focus on dispelling myths such as the misconception that contraceptive pills protect against STIs ( TABLE W1 , available online at www.jfponline.com). Educate parents, too, about STIs, how to counsel their children, and prevention strategies, including available vaccinations.
TABLE W1
Dispelling common myths about STIs
| MYTH | FACT |
|---|---|
| Oral contraceptives protect against STIs | Oral contraceptives do not protect against STIs. Neither do vaginal rings or IuDs. use condoms to minimize the risk of infection transmission. |
| Condoms are 100% effective | Effectiveness depends on proper technique and a condom must be used every time to be effective. |
| Nonvaginal sex (oral, anal, sex toys) doesn’t carry risk | Oral and anal sex can spread STIs, as can sharing sex toys. |
| Risk is only present if there is ejaculation | Sexual contact even in the absence of ejaculation (coitus interruptus) is potentially risky, especially if skin or mucosal integrity is damaged. any exposure to body fluids is a risk. |
| All STIs are treatable | Many STIs, such as hepatitis, HIV, herpes, and syphilis cannot be permanently cured. |
| HIV, human immunodeficiency virus; IUDs, intrauterine devices; STIs, sexually transmitted infections. | |
TABLE W2
Additional recommended reading
|
When should I raise these issues?
As depicted in the opening vignette, an adolescent may raise a myriad of issues concerning sexual activity, which makes your preparedness all the more important.
Most of the time, however, you will need to broach the subject. Sports physicals and annual well-child/adolescent exams are ideal opportunities to bring up issues regarding sex. In fact, it is usually easier to address this topic—and information is better received—when children are in their late preteen years and seen during nonacute visits.
Other opportunities to address these issues are during an STI screening visit, upon discovery of an STI, or during evaluations for amenorrhea or dysmenorrhea (when concerns about pregnancy and sex would naturally arise).
How should I start the discussion?
It is difficult to address sensitive issues in a 15-minute visit. Consider scheduling an extended appointment so that critical messages are not rushed. Another option is to introduce the topic to the parent or patient, and then schedule a separate counseling visit to discuss things in detail. To create a relaxed atmosphere, conduct the initial interview (with patient alone or with parent attending) in your office rather than in an examination room.
Put patients at ease. Many young people feel shame and embarrassment when talking about their sexual activity or lack of sexual activity. They may also be self conscious about asking “stupid” questions. They may have difficulty making eye contact with you during the discussion, or may display such nervous behaviors as nail biting or foot shaking. However, they may also have a false sense of confidence about their activities. It could take time to get adolescents to open up. Victims of sexual assault may need much longer periods of time to get comfortable discussing sexual behavior.
To encourage rapport, put your pen away, sit down, make eye contact, and speak in a neutral, nonjudgmental tone ( TABLE 1 ). Talk with, and not at, the patient (with parents, too, if present) as you assess baseline knowledge and readiness to change behavior.8 After posing a question, listen without interrupting. Lean forward while listening, to let the patient know he or she has your complete attention. Once the patient or parent finishes stating their concerns, ask additional open-ended questions—eg, “Is there anything else you’d like to discuss?”
Promote risk reduction. Applaud any positive risk reduction the patient has already adopted, and offer other specific and achievable risk-reduction tactics. Suggest options rather than giving directives.8 Also explore any possible barriers to further risk reduction, such as mental health concerns, addiction, abuse, lack of education, or lack of social support.
Risk-reduction efforts include condom use, reducing or limiting the number of partners, enhancing partner communication, self-testing and partner-testing for STIs, and avoiding recreational drug use.8 Recommend that the patient ask his or her partner(s) about present and past sexual practices and prior partners.
Role playing, training in the use of condoms, or sessions with peer counselors can often help patients learn how to communicate with partners and take responsibility for their own behavior.
TABLE 1
Talking to teens about sex and their health: A conversation starter
| DR: So, Bryan, I’m wondering if we could talk a little bit about sex and your health. Bryan: Fine, I guess. DR: First, I want to mention that sexual experimentation in young men is normal. But a whole range of dangerous illnesses/infections can come from risky sexual behaviors, and that’s what I’d like to focus on today. So, before I start I’m curious if you have any questions about things you’ve heard from your friends about sex or sexually transmitted diseases? Bryan: Nope. DR: OK. I’m guessing this is pretty uncomfortable for you. Is there anything that might make this discussion a little easier? Bryan: For you to do all of the talking. DR: Got it. I might ask some simple yes or no questions just to make sure that you understand what I’m saying as we go along, but that will be it. I may also use some photos so you can have a clear idea about the way some of these things actually look so you can come to me if you notice anything unusual. At least you’ll have an idea about what might be happening and can come talk to me about it. Bryan: Yeah, sure. DR: So, the thing I want to make really clear is that I am going to start with the most basic information, show you what a condom is, and even give you one to try out privately at home. You don’t have to use it, but it makes sense for you to become familiar with it now, so that if there is ever a time when you might need it, you’ll know how. I’m not going to make any assumptions about what you know or don’t know, I’m going to give you some broad guidelines, and you can take whatever information you think is helpful. You with me so far? Bryan: Yes. DR: Your family may have certain rules about who you hang out with or what you do, and I want to make sure you know that I don’t want to contradict your parents. I just want to make sure that you have accurate information, so you can make decisions that will keep you safe and healthy. |
Provide information on STI consequences. Several surveys and studies4,6,9 have shown that most patients would like additional information about symptoms, treatment, and consequences of various STIs. The research suggests that you should tell patients which STIs are curable, merely treatable, or life threatening.9 Include information on asymptomatic conditions, carrier states, and false-negative results that can occur when testing for human immunodeficiency virus (HIV) and herpes virus.6
Set aside your biases and assumptions. Avoid using labels and making gender-role assumptions. Pay attention to the patient’s (and parent’s) emotions, mannerisms, omissions, and comprehension.10 Note nonverbal cues, and keep in mind that your own attitude and subtle nonverbal communication will be detected, and may determine how patients respond. (Cultural competence training is often invaluable in establishing rapport and avoiding an unintended connotation.)
Evaluation, testing, and treatment are indicated for partners of a patient who has Chlamydia, gonorrhea, granuloma inguinale, or lymphogranuloma venereum, and who had unprotected intercourse with the patient within 60 days preceding the index patient’s symptom onset. In the case of chancroid, partner evaluation is warranted even for asymptomatic individuals if sexual activity occurred within 10 days of the index patient’s onset of symptoms. Similarly, partners of patients diagnosed with syphilis, HIV, or herpes should seek immediate medical evaluation.
Partners can be notified by the provider, the patient, or both—with the patient notifying partners, and the provider following up. Card referral is another option, in which the patient is given appointment cards to hand out to partners.3,17
Despite these efforts, partners often fail to come to the office for an evaluation. An alternative is expedited partner therapy (EPT), with patient-delivered partner therapy (PDPT) the most common means of implementation.17 For PDPT, the provider gives the index patient a prescription or drug samples, and offers precautions and instructions for treating the partners without prior clinical assessment.3,17,18
Evidence supports the use of EPT in treating gonorrhea and Chlamydia.17 Evidence also suggests that EPT decreases recurrence and incidence rates for gonorrhea and Chlamydia.17 Evidence is insufficient, however, to advocate EPT for syphilis, trichomoniasis, or gonorrhea/Chlamydia in men who have sex with men.17 In addition, EPT has led to beneficial behavioral changes, such as decreased unprotected intercourse with untreated partners and reduction of high-risk sexual behaviors.17
However, EPT comes with its share of obstacles. Noting a partner’s information and treatment plan in the index patient’s chart may violate HIPAA regulations. Also, adverse drug reactions are possible in a person whom the clinician has not interviewed or examined. There is no way to know if medications are taken as prescribed; thus, the potential exists for drug resistance or inappropriate use of the unused portions. Another concern is the legal implication of providing treatment to a person with whom there is no doctor-patient relationship.
Know the law, as far as it is knowable
Treating individuals without establishing a patient-provider relationship is illegal in many states; in other states it is not, but may be assumed to be illegal by physicians and pharmacists practicing in these states.17,18 Some states have no formal legal guidelines, and even those that do may not publicize them clearly or may have conflicting guidelines from different medical, legal, and public health societies.
Despite these legal complications, the Centers for Disease Control and Prevention advocates EPT in situations where there is clear medical benefit.3,17 The list below provides some general guidance on where individual states stand on EPT,2,3,18 although you should contact your local state medical board or Department of Health and Human Services for specific guidelines.
- EPT is permitted in the following states: California, Colorado, Maryland, Minnesota, Mississippi, Nevada, New Mexico, Pennsylvania, Tennessee, Utah, Washington, and Wyoming.
- EPT is potentially allowable in the following locations: Alabama, Alaska, Connecticut, Delaware, District of Columbia, Georgia, Hawaii, Idaho, Indiana, Iowa, Kansas, Maine, Massachusetts, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Oregon, Puerto Rico, Rhode Island, South Dakota, Texas, Virginia, and Wisconsin.
- EPT is probably prohibited in the following states: Arizona, Arkansas, Florida, Illinois, Kentucky, Louisiana, Michigan, North Dakota, Ohio, Oklahoma, South Carolina, Vermont, and West Virginia.
Speak with patients alone, or with parents? It is not unusual for parents or teens who have not had much experience discussing physical anatomy to become embarrassed or anxious when the topic of sexuality comes up. Counseling teens alone or with their parents depends on the nature of the conversation.
Consider introducing the general topic of sexual health—physical anatomy, physiology, risks of STIs, myths—with the teen and parent together.
Meet with patients alone when questions relate directly to their sexual habits, activity, or preferences. You may want to start with an open invitation designed to engage the adolescent in a dialogue: “At your age, I was very confused and misinformed. I am here to answer any of your questions. I also want to remind you that everything we discuss stays here. I know you have some information about this, but I am here to help you understand what is true and what is not.”
Legality. With a few exceptions, adolescents in the United States can legally consent to the confidential diagnosis and treatment of STIs, and medical care for STIs can be provided without parental consent or knowledge. In addition, in the majority of states, adolescents can consent to HIV counseling and testing.3
Plan for follow-up visits. Rather than overwhelm a teen at the initial visit, answer pressing questions and provide an overview. Have the teen make a follow-up appointment for 1 to 2 weeks after the initial visit. Provide print or online resources and phone numbers to call for additional information, and say you’ll be happy to address any questions or concerns at the next visit. (For reimbursement, these sessions can be coded as time-based counseling visits. When done in conjunction with other problem visits, your counseling can be coded with modifiers for prolonged services.11 )
How important is it to raise this topic?
Adolescents and teens are prone to receiving and accepting misinformation from a variety of sources, such as peers, the general media, and the Internet. Not providing them with sound, accurate information about risks puts their health in jeopardy.
Most parents want to discuss this issue with their children, but they are often afraid or ill prepared to do so. Based on the literature2-4,6 and our conversations with therapists, it seems most parents don’t object to physicians giving their children sound information as much as they object to being left out of the process. One technique to encourage parental cooperation is to offer choices about the way information will be delivered.
How should I advise parents?
Parents who really want to talk with their teens about sexual behaviors will likely find it easier to ask questions of their child in the doctor’s office, and to accept in that setting the answers they receive. Parents and physicians can collaborate ( TABLE 2 ) to create a 3-way conversation with the child in which any topic can be discussed safely and objectively. Such discourse in your office will hopefully translate to the home, as well.
In addition to answering parents’ questions and giving them medical information and counseling suggestions, offer them handouts and a list of online resources. Many groups have used MySpace to deliver counseling and support group information to children and teens.12-15 These include Congress (health promotion, safety); Rape, Abuse, & Incest National Network; National Suicide Prevention Hotline (20,000 hits/month on MySpace); and gay/lesbian counseling forums. We suggest parents get texting capabilities on their phones and set up MySpace accounts to augment communication with their teens.
TABLE 2
Gaining parental buy-in before the teen talk: A conversation starter
| DR: Mr. Jones, I wanted to take this opportunity to discuss an important matter with you. As you know, Eva is now a teenager. There are a lot of issues that teenagers are faced with, have questions about, and are misinformed about. One of these is sex. First, I would like to know your thoughts on the matter and what kind of discussions—if any—you have had with your daughter. Mr. Jones: She is only 13, and she is not having sex. I want to keep it that way. I don’t want to talk about it and thus encourage her to have sex. DR: I can appreciate what you’re saying. I want you to know that I respect your views and how you would like to raise your child. I have some concerns because teens nowadays have many questions and get a lot of misinformation from their peers and the media. Mr. Jones: What do you want to do? DR: Well, I would suggest we ask her about what she knows about sex, what she has heard, and what questions she may have. I would like to do that with both of us in the room. Then, I would like to go over some of the terminology along with issues such as STDs and pregnancy/complications. Mr. Jones: As long as I’m there and as long as you are not encouraging it. I don’t want her to think it’s OK to have sex at 13. But I’m also scared about what she sees on TV and at school and what she thinks she should be doing. DR: I understand. Before the 3 of us talk, I would like to speak to her privately and see if there are any specific issues she may want addressed. I want to ensure that she feels comfortable enough to ask anything. I also want to assure you that if there is anything of concern, I will address it and let you know. What do you think? Mr. Jones: That’s fine. DR: One more thing: If you would like any information regarding how to continue this discussion at home, or even medical information for your own reference, I would be happy to provide that for you. Mr. Jones: Yes, that would be great. |
Remain vigilant
Actively screen patients (from preteen years onward) for STIs.4-7,10 Screening may range from questions that assess risk to formal STI testing. According to The National Health and Social Life Survey, 71% of individuals with STIs were diagnosed and treated in a private clinic or emergency room.16 Only 29% of sexually active individuals sought care at an STI clinic. Thus, most patients present to the primary care office.4-6 It is crucial that we become as familiar with the guidelines for reporting and partner testing and treatment as with knowing how to treat index patients for STIs.
Reporting specifics. Syphilis, gonorrhea, Chlamydia, chancroid, HIV infection, and AIDS are reportable in every state.17 Hospital and commercial laboratories report all abnormal findings of reportable diseases; however, it is ultimately the physician’s responsibility to report the STI to the local health department. Each state has a complete list of reportable conditions and the time frame for reporting, accessible through its Department of Health Services. Once reported, information becomes confidential, and public health officials may contact you to verify the diagnosis before initiating a follow-up protocol.
Consider notifying patients directly regarding negative and positive results so you can answer any questions they may have and discuss treatment for themselves—and their partners. (See “Getting treatment to partners: The expedited partner therapy option”.) Directly notifying patients offers another opportunity to further educate teens regarding prevention strategies and to dispel any misconceptions they may have.
Correspondence
Anush S. Pillai, DO, FAAFP, 424 Hahlo, Houston, TX 77020; [email protected]
1. Eaton DK, Kann L, Kinchen S, et al. and the CDC. Youth risk behavior surveillance—United States, 2007. Morb Mortal Wkly Rep. 2008;57(SS-4):1-136.
2. Diller J. Cultural Diversity: A Primer for the Human Services. Belmont, Calif: Wadsworth Publishing Company; 1999:8-26.
3. Centers for Disease Control and Prevention. Sexually transmitted diseases: treatment guidelines, 2006. Morb Mortal Wkly Rep. 2006;55(RR-11):1-100.
4. Rietmeijer CA. Risk reduction counseling for prevention of sexually transmitted infections: how it works and how to make it work. Sex Transm Inf. 2007;83:2-9.
5. Ma R. Special report: one-to-one counseling can reduce STI risk. The Practitioner. April 26;2007:81.-
6. The clinical approach to the patient with possible STD. In: Marrazzo J, Ocbamichael N, Meegan A, Stamm WE. The Practitioner’s Handbook for the Management of STDs. 4th ed. Seattle, Wash: University of Washington; 2007:1-7.
7. American Medical Association (AMA). Guidelines for Adolescent Preventative Services (GAPS). [Recommendations Monograph]. Chicago, Ill: AMA; 1997.
8. Creegan L, ed. Behavioral Counseling for STD/HIV Risk Reduction. National Network of STD/HIV Prevention Training Centers; August 2007. Available at: http://www.stdhivtraining.org/resource.php?id=19&ret=clinical_resources. Accessed December 31, 2008.
9. CDC. STD Health Communication, Executive Summary HPV. 2004. Available at: http://www.cdc.gov/std/HealthComm/ExecSumHPVGenPub2004.pdf. Accessed December 31, 2008.
10. Bauer HM, ed. The Clinical Approach to the STD Patient. National Network of STD/HIV Prevention Training Centers; August 2007. Available at: http://www.stdhivtraining.org/resource.php?id=21&ret=clinical_resources. Accessed December 31, 2008.
11. Sophocles A. Time is of the essence: coding on the basis of time for physician services. Fam Pract Manag. 2003;10:27-31.
12. The House Committee on Energy and Commerce. Making the Internet safe for kids: the role of ISP’s and social networking sites, Day 2. Hearing Webcast. Available at: http://archives.energy-commerce.house.gov/reparchives/108/Hearings/06282006hearing1955/hearing.htm. Accessed January 7, 2009.
13. National Suicide Prevention Lifeline home page. Available at: www.suicidepreventionlifeline.org. Accessed December 31, 2008.
14. Brown NL. Internet-based hotline to counsel abused young people. Healthline.com Web site. Available at: www.healthline.com/blogs/teen_health/2008/04/internet-based-hotline-to-counsel.html. Accessed December 31, 2008.
15. Rape, Abuse & Incest National Network home page. Available at: www.rainn.org. Accessed December 31, 2008.
16. Brackbill R, Sternberg M, Fishbein M. Where do people go for treatment of sexually transmitted diseases? Fam Plann Perspect. 1999;3:10-15.
17. CDC. Expedited Partner Therapy in the Management of Sexually Transmitted Diseases. Atlanta, Ga: US Department of Health and Human Services; 2006.
18. Hodge JG, Jr, Pulver A, Hogben M, et al. Expedited partner therapy for sexually transmitted diseases: assessing the legal environment. Am J Public Health. 2008;98:238-243.
- Annual physical exams and sports physicals are ideal opportunities to raise issues of sex and sexually transmitted infections (STIs) (B).
- Meet with young patients alone when asking about sexual habits, activity, preferences, or other sensitive matters (C).
- Evidence supports the use of expedited partner therapy (EPT) in treating gonorrhea and Chlamydia (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
As you conclude a routine annual physical exam of a 14-year-old girl, you ask her if she has any questions. With some hesitation, she tells you about a “friend” of hers who recently had sex with her boyfriend. She has heard many rumors from her friends, television, and chat lines. She doesn’t think her friend got pregnant, but is worried that she might have caught something. She tells you that her friend is also uncertain about whether—and how—to tell her parents.
How would you respond? Would you be comfortable helping your patient, and her parents, discuss this delicate matter in a meaningful way?
Though most clinicians are adept at diagnosing and treating sexually transmitted infections, far fewer feel skilled enough to counsel teens and parents about them. STIs (the new and politically appropriate terminology for STDs) are commonly encountered in family medicine; knowing how to discuss the many associated issues with teens and parents is paramount—especially when you consider the statistics.
According to the Centers for Disease Control and Prevention’s Youth Risk Behavior Surveillance System (YRBSS):1
- 47.8% of 9th to 12th graders have had sexual intercourse.
- 7.1% of children have had intercourse before age 13.
- 14.9% of high school students have had sexual intercourse with 4 or more people.
- 38.5% of sexually active 9th to 12th graders did not use condoms during their last sexual encounter.
Opening Pandora’s box—carefully
As family physicians, we must be willing not only to discuss sexual issues if asked, but to raise them with patients and parents, when appropriate. The CDC offers training for health professionals in obtaining a sexual history and counseling and educating patients (http://depts.washington.edu/nnptc). Unfortunately, little information exists on how to tailor education to the values and beliefs of an individual family or to the broader cultural mores of the society in which the young person lives.2 Nonetheless, there are many things you can do to improve the conversations you have.
For starters, ask open-ended questions and use understandable and normalizing language.3 Educate patients regarding the various STIs and methods of prevention—ie, proper condom use, abstinence, limiting the number of sexual partners, changes in sexual practices, and vaccination.4-6 Ask patients about their use of and motivation to use condoms and contraceptive methods, as well as their sexual orientation, number of sexual partners, if they have ever exchanged sex for money or drugs, and prior history of STIs.7 To facilitate communication, familiarize yourself with sexual slang terms.
Focus on dispelling myths such as the misconception that contraceptive pills protect against STIs ( TABLE W1 , available online at www.jfponline.com). Educate parents, too, about STIs, how to counsel their children, and prevention strategies, including available vaccinations.
TABLE W1
Dispelling common myths about STIs
| MYTH | FACT |
|---|---|
| Oral contraceptives protect against STIs | Oral contraceptives do not protect against STIs. Neither do vaginal rings or IuDs. use condoms to minimize the risk of infection transmission. |
| Condoms are 100% effective | Effectiveness depends on proper technique and a condom must be used every time to be effective. |
| Nonvaginal sex (oral, anal, sex toys) doesn’t carry risk | Oral and anal sex can spread STIs, as can sharing sex toys. |
| Risk is only present if there is ejaculation | Sexual contact even in the absence of ejaculation (coitus interruptus) is potentially risky, especially if skin or mucosal integrity is damaged. any exposure to body fluids is a risk. |
| All STIs are treatable | Many STIs, such as hepatitis, HIV, herpes, and syphilis cannot be permanently cured. |
| HIV, human immunodeficiency virus; IUDs, intrauterine devices; STIs, sexually transmitted infections. | |
TABLE W2
Additional recommended reading
|
When should I raise these issues?
As depicted in the opening vignette, an adolescent may raise a myriad of issues concerning sexual activity, which makes your preparedness all the more important.
Most of the time, however, you will need to broach the subject. Sports physicals and annual well-child/adolescent exams are ideal opportunities to bring up issues regarding sex. In fact, it is usually easier to address this topic—and information is better received—when children are in their late preteen years and seen during nonacute visits.
Other opportunities to address these issues are during an STI screening visit, upon discovery of an STI, or during evaluations for amenorrhea or dysmenorrhea (when concerns about pregnancy and sex would naturally arise).
How should I start the discussion?
It is difficult to address sensitive issues in a 15-minute visit. Consider scheduling an extended appointment so that critical messages are not rushed. Another option is to introduce the topic to the parent or patient, and then schedule a separate counseling visit to discuss things in detail. To create a relaxed atmosphere, conduct the initial interview (with patient alone or with parent attending) in your office rather than in an examination room.
Put patients at ease. Many young people feel shame and embarrassment when talking about their sexual activity or lack of sexual activity. They may also be self conscious about asking “stupid” questions. They may have difficulty making eye contact with you during the discussion, or may display such nervous behaviors as nail biting or foot shaking. However, they may also have a false sense of confidence about their activities. It could take time to get adolescents to open up. Victims of sexual assault may need much longer periods of time to get comfortable discussing sexual behavior.
To encourage rapport, put your pen away, sit down, make eye contact, and speak in a neutral, nonjudgmental tone ( TABLE 1 ). Talk with, and not at, the patient (with parents, too, if present) as you assess baseline knowledge and readiness to change behavior.8 After posing a question, listen without interrupting. Lean forward while listening, to let the patient know he or she has your complete attention. Once the patient or parent finishes stating their concerns, ask additional open-ended questions—eg, “Is there anything else you’d like to discuss?”
Promote risk reduction. Applaud any positive risk reduction the patient has already adopted, and offer other specific and achievable risk-reduction tactics. Suggest options rather than giving directives.8 Also explore any possible barriers to further risk reduction, such as mental health concerns, addiction, abuse, lack of education, or lack of social support.
Risk-reduction efforts include condom use, reducing or limiting the number of partners, enhancing partner communication, self-testing and partner-testing for STIs, and avoiding recreational drug use.8 Recommend that the patient ask his or her partner(s) about present and past sexual practices and prior partners.
Role playing, training in the use of condoms, or sessions with peer counselors can often help patients learn how to communicate with partners and take responsibility for their own behavior.
TABLE 1
Talking to teens about sex and their health: A conversation starter
| DR: So, Bryan, I’m wondering if we could talk a little bit about sex and your health. Bryan: Fine, I guess. DR: First, I want to mention that sexual experimentation in young men is normal. But a whole range of dangerous illnesses/infections can come from risky sexual behaviors, and that’s what I’d like to focus on today. So, before I start I’m curious if you have any questions about things you’ve heard from your friends about sex or sexually transmitted diseases? Bryan: Nope. DR: OK. I’m guessing this is pretty uncomfortable for you. Is there anything that might make this discussion a little easier? Bryan: For you to do all of the talking. DR: Got it. I might ask some simple yes or no questions just to make sure that you understand what I’m saying as we go along, but that will be it. I may also use some photos so you can have a clear idea about the way some of these things actually look so you can come to me if you notice anything unusual. At least you’ll have an idea about what might be happening and can come talk to me about it. Bryan: Yeah, sure. DR: So, the thing I want to make really clear is that I am going to start with the most basic information, show you what a condom is, and even give you one to try out privately at home. You don’t have to use it, but it makes sense for you to become familiar with it now, so that if there is ever a time when you might need it, you’ll know how. I’m not going to make any assumptions about what you know or don’t know, I’m going to give you some broad guidelines, and you can take whatever information you think is helpful. You with me so far? Bryan: Yes. DR: Your family may have certain rules about who you hang out with or what you do, and I want to make sure you know that I don’t want to contradict your parents. I just want to make sure that you have accurate information, so you can make decisions that will keep you safe and healthy. |
Provide information on STI consequences. Several surveys and studies4,6,9 have shown that most patients would like additional information about symptoms, treatment, and consequences of various STIs. The research suggests that you should tell patients which STIs are curable, merely treatable, or life threatening.9 Include information on asymptomatic conditions, carrier states, and false-negative results that can occur when testing for human immunodeficiency virus (HIV) and herpes virus.6
Set aside your biases and assumptions. Avoid using labels and making gender-role assumptions. Pay attention to the patient’s (and parent’s) emotions, mannerisms, omissions, and comprehension.10 Note nonverbal cues, and keep in mind that your own attitude and subtle nonverbal communication will be detected, and may determine how patients respond. (Cultural competence training is often invaluable in establishing rapport and avoiding an unintended connotation.)
Evaluation, testing, and treatment are indicated for partners of a patient who has Chlamydia, gonorrhea, granuloma inguinale, or lymphogranuloma venereum, and who had unprotected intercourse with the patient within 60 days preceding the index patient’s symptom onset. In the case of chancroid, partner evaluation is warranted even for asymptomatic individuals if sexual activity occurred within 10 days of the index patient’s onset of symptoms. Similarly, partners of patients diagnosed with syphilis, HIV, or herpes should seek immediate medical evaluation.
Partners can be notified by the provider, the patient, or both—with the patient notifying partners, and the provider following up. Card referral is another option, in which the patient is given appointment cards to hand out to partners.3,17
Despite these efforts, partners often fail to come to the office for an evaluation. An alternative is expedited partner therapy (EPT), with patient-delivered partner therapy (PDPT) the most common means of implementation.17 For PDPT, the provider gives the index patient a prescription or drug samples, and offers precautions and instructions for treating the partners without prior clinical assessment.3,17,18
Evidence supports the use of EPT in treating gonorrhea and Chlamydia.17 Evidence also suggests that EPT decreases recurrence and incidence rates for gonorrhea and Chlamydia.17 Evidence is insufficient, however, to advocate EPT for syphilis, trichomoniasis, or gonorrhea/Chlamydia in men who have sex with men.17 In addition, EPT has led to beneficial behavioral changes, such as decreased unprotected intercourse with untreated partners and reduction of high-risk sexual behaviors.17
However, EPT comes with its share of obstacles. Noting a partner’s information and treatment plan in the index patient’s chart may violate HIPAA regulations. Also, adverse drug reactions are possible in a person whom the clinician has not interviewed or examined. There is no way to know if medications are taken as prescribed; thus, the potential exists for drug resistance or inappropriate use of the unused portions. Another concern is the legal implication of providing treatment to a person with whom there is no doctor-patient relationship.
Know the law, as far as it is knowable
Treating individuals without establishing a patient-provider relationship is illegal in many states; in other states it is not, but may be assumed to be illegal by physicians and pharmacists practicing in these states.17,18 Some states have no formal legal guidelines, and even those that do may not publicize them clearly or may have conflicting guidelines from different medical, legal, and public health societies.
Despite these legal complications, the Centers for Disease Control and Prevention advocates EPT in situations where there is clear medical benefit.3,17 The list below provides some general guidance on where individual states stand on EPT,2,3,18 although you should contact your local state medical board or Department of Health and Human Services for specific guidelines.
- EPT is permitted in the following states: California, Colorado, Maryland, Minnesota, Mississippi, Nevada, New Mexico, Pennsylvania, Tennessee, Utah, Washington, and Wyoming.
- EPT is potentially allowable in the following locations: Alabama, Alaska, Connecticut, Delaware, District of Columbia, Georgia, Hawaii, Idaho, Indiana, Iowa, Kansas, Maine, Massachusetts, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Oregon, Puerto Rico, Rhode Island, South Dakota, Texas, Virginia, and Wisconsin.
- EPT is probably prohibited in the following states: Arizona, Arkansas, Florida, Illinois, Kentucky, Louisiana, Michigan, North Dakota, Ohio, Oklahoma, South Carolina, Vermont, and West Virginia.
Speak with patients alone, or with parents? It is not unusual for parents or teens who have not had much experience discussing physical anatomy to become embarrassed or anxious when the topic of sexuality comes up. Counseling teens alone or with their parents depends on the nature of the conversation.
Consider introducing the general topic of sexual health—physical anatomy, physiology, risks of STIs, myths—with the teen and parent together.
Meet with patients alone when questions relate directly to their sexual habits, activity, or preferences. You may want to start with an open invitation designed to engage the adolescent in a dialogue: “At your age, I was very confused and misinformed. I am here to answer any of your questions. I also want to remind you that everything we discuss stays here. I know you have some information about this, but I am here to help you understand what is true and what is not.”
Legality. With a few exceptions, adolescents in the United States can legally consent to the confidential diagnosis and treatment of STIs, and medical care for STIs can be provided without parental consent or knowledge. In addition, in the majority of states, adolescents can consent to HIV counseling and testing.3
Plan for follow-up visits. Rather than overwhelm a teen at the initial visit, answer pressing questions and provide an overview. Have the teen make a follow-up appointment for 1 to 2 weeks after the initial visit. Provide print or online resources and phone numbers to call for additional information, and say you’ll be happy to address any questions or concerns at the next visit. (For reimbursement, these sessions can be coded as time-based counseling visits. When done in conjunction with other problem visits, your counseling can be coded with modifiers for prolonged services.11 )
How important is it to raise this topic?
Adolescents and teens are prone to receiving and accepting misinformation from a variety of sources, such as peers, the general media, and the Internet. Not providing them with sound, accurate information about risks puts their health in jeopardy.
Most parents want to discuss this issue with their children, but they are often afraid or ill prepared to do so. Based on the literature2-4,6 and our conversations with therapists, it seems most parents don’t object to physicians giving their children sound information as much as they object to being left out of the process. One technique to encourage parental cooperation is to offer choices about the way information will be delivered.
How should I advise parents?
Parents who really want to talk with their teens about sexual behaviors will likely find it easier to ask questions of their child in the doctor’s office, and to accept in that setting the answers they receive. Parents and physicians can collaborate ( TABLE 2 ) to create a 3-way conversation with the child in which any topic can be discussed safely and objectively. Such discourse in your office will hopefully translate to the home, as well.
In addition to answering parents’ questions and giving them medical information and counseling suggestions, offer them handouts and a list of online resources. Many groups have used MySpace to deliver counseling and support group information to children and teens.12-15 These include Congress (health promotion, safety); Rape, Abuse, & Incest National Network; National Suicide Prevention Hotline (20,000 hits/month on MySpace); and gay/lesbian counseling forums. We suggest parents get texting capabilities on their phones and set up MySpace accounts to augment communication with their teens.
TABLE 2
Gaining parental buy-in before the teen talk: A conversation starter
| DR: Mr. Jones, I wanted to take this opportunity to discuss an important matter with you. As you know, Eva is now a teenager. There are a lot of issues that teenagers are faced with, have questions about, and are misinformed about. One of these is sex. First, I would like to know your thoughts on the matter and what kind of discussions—if any—you have had with your daughter. Mr. Jones: She is only 13, and she is not having sex. I want to keep it that way. I don’t want to talk about it and thus encourage her to have sex. DR: I can appreciate what you’re saying. I want you to know that I respect your views and how you would like to raise your child. I have some concerns because teens nowadays have many questions and get a lot of misinformation from their peers and the media. Mr. Jones: What do you want to do? DR: Well, I would suggest we ask her about what she knows about sex, what she has heard, and what questions she may have. I would like to do that with both of us in the room. Then, I would like to go over some of the terminology along with issues such as STDs and pregnancy/complications. Mr. Jones: As long as I’m there and as long as you are not encouraging it. I don’t want her to think it’s OK to have sex at 13. But I’m also scared about what she sees on TV and at school and what she thinks she should be doing. DR: I understand. Before the 3 of us talk, I would like to speak to her privately and see if there are any specific issues she may want addressed. I want to ensure that she feels comfortable enough to ask anything. I also want to assure you that if there is anything of concern, I will address it and let you know. What do you think? Mr. Jones: That’s fine. DR: One more thing: If you would like any information regarding how to continue this discussion at home, or even medical information for your own reference, I would be happy to provide that for you. Mr. Jones: Yes, that would be great. |
Remain vigilant
Actively screen patients (from preteen years onward) for STIs.4-7,10 Screening may range from questions that assess risk to formal STI testing. According to The National Health and Social Life Survey, 71% of individuals with STIs were diagnosed and treated in a private clinic or emergency room.16 Only 29% of sexually active individuals sought care at an STI clinic. Thus, most patients present to the primary care office.4-6 It is crucial that we become as familiar with the guidelines for reporting and partner testing and treatment as with knowing how to treat index patients for STIs.
Reporting specifics. Syphilis, gonorrhea, Chlamydia, chancroid, HIV infection, and AIDS are reportable in every state.17 Hospital and commercial laboratories report all abnormal findings of reportable diseases; however, it is ultimately the physician’s responsibility to report the STI to the local health department. Each state has a complete list of reportable conditions and the time frame for reporting, accessible through its Department of Health Services. Once reported, information becomes confidential, and public health officials may contact you to verify the diagnosis before initiating a follow-up protocol.
Consider notifying patients directly regarding negative and positive results so you can answer any questions they may have and discuss treatment for themselves—and their partners. (See “Getting treatment to partners: The expedited partner therapy option”.) Directly notifying patients offers another opportunity to further educate teens regarding prevention strategies and to dispel any misconceptions they may have.
Correspondence
Anush S. Pillai, DO, FAAFP, 424 Hahlo, Houston, TX 77020; [email protected]
- Annual physical exams and sports physicals are ideal opportunities to raise issues of sex and sexually transmitted infections (STIs) (B).
- Meet with young patients alone when asking about sexual habits, activity, preferences, or other sensitive matters (C).
- Evidence supports the use of expedited partner therapy (EPT) in treating gonorrhea and Chlamydia (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
As you conclude a routine annual physical exam of a 14-year-old girl, you ask her if she has any questions. With some hesitation, she tells you about a “friend” of hers who recently had sex with her boyfriend. She has heard many rumors from her friends, television, and chat lines. She doesn’t think her friend got pregnant, but is worried that she might have caught something. She tells you that her friend is also uncertain about whether—and how—to tell her parents.
How would you respond? Would you be comfortable helping your patient, and her parents, discuss this delicate matter in a meaningful way?
Though most clinicians are adept at diagnosing and treating sexually transmitted infections, far fewer feel skilled enough to counsel teens and parents about them. STIs (the new and politically appropriate terminology for STDs) are commonly encountered in family medicine; knowing how to discuss the many associated issues with teens and parents is paramount—especially when you consider the statistics.
According to the Centers for Disease Control and Prevention’s Youth Risk Behavior Surveillance System (YRBSS):1
- 47.8% of 9th to 12th graders have had sexual intercourse.
- 7.1% of children have had intercourse before age 13.
- 14.9% of high school students have had sexual intercourse with 4 or more people.
- 38.5% of sexually active 9th to 12th graders did not use condoms during their last sexual encounter.
Opening Pandora’s box—carefully
As family physicians, we must be willing not only to discuss sexual issues if asked, but to raise them with patients and parents, when appropriate. The CDC offers training for health professionals in obtaining a sexual history and counseling and educating patients (http://depts.washington.edu/nnptc). Unfortunately, little information exists on how to tailor education to the values and beliefs of an individual family or to the broader cultural mores of the society in which the young person lives.2 Nonetheless, there are many things you can do to improve the conversations you have.
For starters, ask open-ended questions and use understandable and normalizing language.3 Educate patients regarding the various STIs and methods of prevention—ie, proper condom use, abstinence, limiting the number of sexual partners, changes in sexual practices, and vaccination.4-6 Ask patients about their use of and motivation to use condoms and contraceptive methods, as well as their sexual orientation, number of sexual partners, if they have ever exchanged sex for money or drugs, and prior history of STIs.7 To facilitate communication, familiarize yourself with sexual slang terms.
Focus on dispelling myths such as the misconception that contraceptive pills protect against STIs ( TABLE W1 , available online at www.jfponline.com). Educate parents, too, about STIs, how to counsel their children, and prevention strategies, including available vaccinations.
TABLE W1
Dispelling common myths about STIs
| MYTH | FACT |
|---|---|
| Oral contraceptives protect against STIs | Oral contraceptives do not protect against STIs. Neither do vaginal rings or IuDs. use condoms to minimize the risk of infection transmission. |
| Condoms are 100% effective | Effectiveness depends on proper technique and a condom must be used every time to be effective. |
| Nonvaginal sex (oral, anal, sex toys) doesn’t carry risk | Oral and anal sex can spread STIs, as can sharing sex toys. |
| Risk is only present if there is ejaculation | Sexual contact even in the absence of ejaculation (coitus interruptus) is potentially risky, especially if skin or mucosal integrity is damaged. any exposure to body fluids is a risk. |
| All STIs are treatable | Many STIs, such as hepatitis, HIV, herpes, and syphilis cannot be permanently cured. |
| HIV, human immunodeficiency virus; IUDs, intrauterine devices; STIs, sexually transmitted infections. | |
TABLE W2
Additional recommended reading
|
When should I raise these issues?
As depicted in the opening vignette, an adolescent may raise a myriad of issues concerning sexual activity, which makes your preparedness all the more important.
Most of the time, however, you will need to broach the subject. Sports physicals and annual well-child/adolescent exams are ideal opportunities to bring up issues regarding sex. In fact, it is usually easier to address this topic—and information is better received—when children are in their late preteen years and seen during nonacute visits.
Other opportunities to address these issues are during an STI screening visit, upon discovery of an STI, or during evaluations for amenorrhea or dysmenorrhea (when concerns about pregnancy and sex would naturally arise).
How should I start the discussion?
It is difficult to address sensitive issues in a 15-minute visit. Consider scheduling an extended appointment so that critical messages are not rushed. Another option is to introduce the topic to the parent or patient, and then schedule a separate counseling visit to discuss things in detail. To create a relaxed atmosphere, conduct the initial interview (with patient alone or with parent attending) in your office rather than in an examination room.
Put patients at ease. Many young people feel shame and embarrassment when talking about their sexual activity or lack of sexual activity. They may also be self conscious about asking “stupid” questions. They may have difficulty making eye contact with you during the discussion, or may display such nervous behaviors as nail biting or foot shaking. However, they may also have a false sense of confidence about their activities. It could take time to get adolescents to open up. Victims of sexual assault may need much longer periods of time to get comfortable discussing sexual behavior.
To encourage rapport, put your pen away, sit down, make eye contact, and speak in a neutral, nonjudgmental tone ( TABLE 1 ). Talk with, and not at, the patient (with parents, too, if present) as you assess baseline knowledge and readiness to change behavior.8 After posing a question, listen without interrupting. Lean forward while listening, to let the patient know he or she has your complete attention. Once the patient or parent finishes stating their concerns, ask additional open-ended questions—eg, “Is there anything else you’d like to discuss?”
Promote risk reduction. Applaud any positive risk reduction the patient has already adopted, and offer other specific and achievable risk-reduction tactics. Suggest options rather than giving directives.8 Also explore any possible barriers to further risk reduction, such as mental health concerns, addiction, abuse, lack of education, or lack of social support.
Risk-reduction efforts include condom use, reducing or limiting the number of partners, enhancing partner communication, self-testing and partner-testing for STIs, and avoiding recreational drug use.8 Recommend that the patient ask his or her partner(s) about present and past sexual practices and prior partners.
Role playing, training in the use of condoms, or sessions with peer counselors can often help patients learn how to communicate with partners and take responsibility for their own behavior.
TABLE 1
Talking to teens about sex and their health: A conversation starter
| DR: So, Bryan, I’m wondering if we could talk a little bit about sex and your health. Bryan: Fine, I guess. DR: First, I want to mention that sexual experimentation in young men is normal. But a whole range of dangerous illnesses/infections can come from risky sexual behaviors, and that’s what I’d like to focus on today. So, before I start I’m curious if you have any questions about things you’ve heard from your friends about sex or sexually transmitted diseases? Bryan: Nope. DR: OK. I’m guessing this is pretty uncomfortable for you. Is there anything that might make this discussion a little easier? Bryan: For you to do all of the talking. DR: Got it. I might ask some simple yes or no questions just to make sure that you understand what I’m saying as we go along, but that will be it. I may also use some photos so you can have a clear idea about the way some of these things actually look so you can come to me if you notice anything unusual. At least you’ll have an idea about what might be happening and can come talk to me about it. Bryan: Yeah, sure. DR: So, the thing I want to make really clear is that I am going to start with the most basic information, show you what a condom is, and even give you one to try out privately at home. You don’t have to use it, but it makes sense for you to become familiar with it now, so that if there is ever a time when you might need it, you’ll know how. I’m not going to make any assumptions about what you know or don’t know, I’m going to give you some broad guidelines, and you can take whatever information you think is helpful. You with me so far? Bryan: Yes. DR: Your family may have certain rules about who you hang out with or what you do, and I want to make sure you know that I don’t want to contradict your parents. I just want to make sure that you have accurate information, so you can make decisions that will keep you safe and healthy. |
Provide information on STI consequences. Several surveys and studies4,6,9 have shown that most patients would like additional information about symptoms, treatment, and consequences of various STIs. The research suggests that you should tell patients which STIs are curable, merely treatable, or life threatening.9 Include information on asymptomatic conditions, carrier states, and false-negative results that can occur when testing for human immunodeficiency virus (HIV) and herpes virus.6
Set aside your biases and assumptions. Avoid using labels and making gender-role assumptions. Pay attention to the patient’s (and parent’s) emotions, mannerisms, omissions, and comprehension.10 Note nonverbal cues, and keep in mind that your own attitude and subtle nonverbal communication will be detected, and may determine how patients respond. (Cultural competence training is often invaluable in establishing rapport and avoiding an unintended connotation.)
Evaluation, testing, and treatment are indicated for partners of a patient who has Chlamydia, gonorrhea, granuloma inguinale, or lymphogranuloma venereum, and who had unprotected intercourse with the patient within 60 days preceding the index patient’s symptom onset. In the case of chancroid, partner evaluation is warranted even for asymptomatic individuals if sexual activity occurred within 10 days of the index patient’s onset of symptoms. Similarly, partners of patients diagnosed with syphilis, HIV, or herpes should seek immediate medical evaluation.
Partners can be notified by the provider, the patient, or both—with the patient notifying partners, and the provider following up. Card referral is another option, in which the patient is given appointment cards to hand out to partners.3,17
Despite these efforts, partners often fail to come to the office for an evaluation. An alternative is expedited partner therapy (EPT), with patient-delivered partner therapy (PDPT) the most common means of implementation.17 For PDPT, the provider gives the index patient a prescription or drug samples, and offers precautions and instructions for treating the partners without prior clinical assessment.3,17,18
Evidence supports the use of EPT in treating gonorrhea and Chlamydia.17 Evidence also suggests that EPT decreases recurrence and incidence rates for gonorrhea and Chlamydia.17 Evidence is insufficient, however, to advocate EPT for syphilis, trichomoniasis, or gonorrhea/Chlamydia in men who have sex with men.17 In addition, EPT has led to beneficial behavioral changes, such as decreased unprotected intercourse with untreated partners and reduction of high-risk sexual behaviors.17
However, EPT comes with its share of obstacles. Noting a partner’s information and treatment plan in the index patient’s chart may violate HIPAA regulations. Also, adverse drug reactions are possible in a person whom the clinician has not interviewed or examined. There is no way to know if medications are taken as prescribed; thus, the potential exists for drug resistance or inappropriate use of the unused portions. Another concern is the legal implication of providing treatment to a person with whom there is no doctor-patient relationship.
Know the law, as far as it is knowable
Treating individuals without establishing a patient-provider relationship is illegal in many states; in other states it is not, but may be assumed to be illegal by physicians and pharmacists practicing in these states.17,18 Some states have no formal legal guidelines, and even those that do may not publicize them clearly or may have conflicting guidelines from different medical, legal, and public health societies.
Despite these legal complications, the Centers for Disease Control and Prevention advocates EPT in situations where there is clear medical benefit.3,17 The list below provides some general guidance on where individual states stand on EPT,2,3,18 although you should contact your local state medical board or Department of Health and Human Services for specific guidelines.
- EPT is permitted in the following states: California, Colorado, Maryland, Minnesota, Mississippi, Nevada, New Mexico, Pennsylvania, Tennessee, Utah, Washington, and Wyoming.
- EPT is potentially allowable in the following locations: Alabama, Alaska, Connecticut, Delaware, District of Columbia, Georgia, Hawaii, Idaho, Indiana, Iowa, Kansas, Maine, Massachusetts, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Oregon, Puerto Rico, Rhode Island, South Dakota, Texas, Virginia, and Wisconsin.
- EPT is probably prohibited in the following states: Arizona, Arkansas, Florida, Illinois, Kentucky, Louisiana, Michigan, North Dakota, Ohio, Oklahoma, South Carolina, Vermont, and West Virginia.
Speak with patients alone, or with parents? It is not unusual for parents or teens who have not had much experience discussing physical anatomy to become embarrassed or anxious when the topic of sexuality comes up. Counseling teens alone or with their parents depends on the nature of the conversation.
Consider introducing the general topic of sexual health—physical anatomy, physiology, risks of STIs, myths—with the teen and parent together.
Meet with patients alone when questions relate directly to their sexual habits, activity, or preferences. You may want to start with an open invitation designed to engage the adolescent in a dialogue: “At your age, I was very confused and misinformed. I am here to answer any of your questions. I also want to remind you that everything we discuss stays here. I know you have some information about this, but I am here to help you understand what is true and what is not.”
Legality. With a few exceptions, adolescents in the United States can legally consent to the confidential diagnosis and treatment of STIs, and medical care for STIs can be provided without parental consent or knowledge. In addition, in the majority of states, adolescents can consent to HIV counseling and testing.3
Plan for follow-up visits. Rather than overwhelm a teen at the initial visit, answer pressing questions and provide an overview. Have the teen make a follow-up appointment for 1 to 2 weeks after the initial visit. Provide print or online resources and phone numbers to call for additional information, and say you’ll be happy to address any questions or concerns at the next visit. (For reimbursement, these sessions can be coded as time-based counseling visits. When done in conjunction with other problem visits, your counseling can be coded with modifiers for prolonged services.11 )
How important is it to raise this topic?
Adolescents and teens are prone to receiving and accepting misinformation from a variety of sources, such as peers, the general media, and the Internet. Not providing them with sound, accurate information about risks puts their health in jeopardy.
Most parents want to discuss this issue with their children, but they are often afraid or ill prepared to do so. Based on the literature2-4,6 and our conversations with therapists, it seems most parents don’t object to physicians giving their children sound information as much as they object to being left out of the process. One technique to encourage parental cooperation is to offer choices about the way information will be delivered.
How should I advise parents?
Parents who really want to talk with their teens about sexual behaviors will likely find it easier to ask questions of their child in the doctor’s office, and to accept in that setting the answers they receive. Parents and physicians can collaborate ( TABLE 2 ) to create a 3-way conversation with the child in which any topic can be discussed safely and objectively. Such discourse in your office will hopefully translate to the home, as well.
In addition to answering parents’ questions and giving them medical information and counseling suggestions, offer them handouts and a list of online resources. Many groups have used MySpace to deliver counseling and support group information to children and teens.12-15 These include Congress (health promotion, safety); Rape, Abuse, & Incest National Network; National Suicide Prevention Hotline (20,000 hits/month on MySpace); and gay/lesbian counseling forums. We suggest parents get texting capabilities on their phones and set up MySpace accounts to augment communication with their teens.
TABLE 2
Gaining parental buy-in before the teen talk: A conversation starter
| DR: Mr. Jones, I wanted to take this opportunity to discuss an important matter with you. As you know, Eva is now a teenager. There are a lot of issues that teenagers are faced with, have questions about, and are misinformed about. One of these is sex. First, I would like to know your thoughts on the matter and what kind of discussions—if any—you have had with your daughter. Mr. Jones: She is only 13, and she is not having sex. I want to keep it that way. I don’t want to talk about it and thus encourage her to have sex. DR: I can appreciate what you’re saying. I want you to know that I respect your views and how you would like to raise your child. I have some concerns because teens nowadays have many questions and get a lot of misinformation from their peers and the media. Mr. Jones: What do you want to do? DR: Well, I would suggest we ask her about what she knows about sex, what she has heard, and what questions she may have. I would like to do that with both of us in the room. Then, I would like to go over some of the terminology along with issues such as STDs and pregnancy/complications. Mr. Jones: As long as I’m there and as long as you are not encouraging it. I don’t want her to think it’s OK to have sex at 13. But I’m also scared about what she sees on TV and at school and what she thinks she should be doing. DR: I understand. Before the 3 of us talk, I would like to speak to her privately and see if there are any specific issues she may want addressed. I want to ensure that she feels comfortable enough to ask anything. I also want to assure you that if there is anything of concern, I will address it and let you know. What do you think? Mr. Jones: That’s fine. DR: One more thing: If you would like any information regarding how to continue this discussion at home, or even medical information for your own reference, I would be happy to provide that for you. Mr. Jones: Yes, that would be great. |
Remain vigilant
Actively screen patients (from preteen years onward) for STIs.4-7,10 Screening may range from questions that assess risk to formal STI testing. According to The National Health and Social Life Survey, 71% of individuals with STIs were diagnosed and treated in a private clinic or emergency room.16 Only 29% of sexually active individuals sought care at an STI clinic. Thus, most patients present to the primary care office.4-6 It is crucial that we become as familiar with the guidelines for reporting and partner testing and treatment as with knowing how to treat index patients for STIs.
Reporting specifics. Syphilis, gonorrhea, Chlamydia, chancroid, HIV infection, and AIDS are reportable in every state.17 Hospital and commercial laboratories report all abnormal findings of reportable diseases; however, it is ultimately the physician’s responsibility to report the STI to the local health department. Each state has a complete list of reportable conditions and the time frame for reporting, accessible through its Department of Health Services. Once reported, information becomes confidential, and public health officials may contact you to verify the diagnosis before initiating a follow-up protocol.
Consider notifying patients directly regarding negative and positive results so you can answer any questions they may have and discuss treatment for themselves—and their partners. (See “Getting treatment to partners: The expedited partner therapy option”.) Directly notifying patients offers another opportunity to further educate teens regarding prevention strategies and to dispel any misconceptions they may have.
Correspondence
Anush S. Pillai, DO, FAAFP, 424 Hahlo, Houston, TX 77020; [email protected]
1. Eaton DK, Kann L, Kinchen S, et al. and the CDC. Youth risk behavior surveillance—United States, 2007. Morb Mortal Wkly Rep. 2008;57(SS-4):1-136.
2. Diller J. Cultural Diversity: A Primer for the Human Services. Belmont, Calif: Wadsworth Publishing Company; 1999:8-26.
3. Centers for Disease Control and Prevention. Sexually transmitted diseases: treatment guidelines, 2006. Morb Mortal Wkly Rep. 2006;55(RR-11):1-100.
4. Rietmeijer CA. Risk reduction counseling for prevention of sexually transmitted infections: how it works and how to make it work. Sex Transm Inf. 2007;83:2-9.
5. Ma R. Special report: one-to-one counseling can reduce STI risk. The Practitioner. April 26;2007:81.-
6. The clinical approach to the patient with possible STD. In: Marrazzo J, Ocbamichael N, Meegan A, Stamm WE. The Practitioner’s Handbook for the Management of STDs. 4th ed. Seattle, Wash: University of Washington; 2007:1-7.
7. American Medical Association (AMA). Guidelines for Adolescent Preventative Services (GAPS). [Recommendations Monograph]. Chicago, Ill: AMA; 1997.
8. Creegan L, ed. Behavioral Counseling for STD/HIV Risk Reduction. National Network of STD/HIV Prevention Training Centers; August 2007. Available at: http://www.stdhivtraining.org/resource.php?id=19&ret=clinical_resources. Accessed December 31, 2008.
9. CDC. STD Health Communication, Executive Summary HPV. 2004. Available at: http://www.cdc.gov/std/HealthComm/ExecSumHPVGenPub2004.pdf. Accessed December 31, 2008.
10. Bauer HM, ed. The Clinical Approach to the STD Patient. National Network of STD/HIV Prevention Training Centers; August 2007. Available at: http://www.stdhivtraining.org/resource.php?id=21&ret=clinical_resources. Accessed December 31, 2008.
11. Sophocles A. Time is of the essence: coding on the basis of time for physician services. Fam Pract Manag. 2003;10:27-31.
12. The House Committee on Energy and Commerce. Making the Internet safe for kids: the role of ISP’s and social networking sites, Day 2. Hearing Webcast. Available at: http://archives.energy-commerce.house.gov/reparchives/108/Hearings/06282006hearing1955/hearing.htm. Accessed January 7, 2009.
13. National Suicide Prevention Lifeline home page. Available at: www.suicidepreventionlifeline.org. Accessed December 31, 2008.
14. Brown NL. Internet-based hotline to counsel abused young people. Healthline.com Web site. Available at: www.healthline.com/blogs/teen_health/2008/04/internet-based-hotline-to-counsel.html. Accessed December 31, 2008.
15. Rape, Abuse & Incest National Network home page. Available at: www.rainn.org. Accessed December 31, 2008.
16. Brackbill R, Sternberg M, Fishbein M. Where do people go for treatment of sexually transmitted diseases? Fam Plann Perspect. 1999;3:10-15.
17. CDC. Expedited Partner Therapy in the Management of Sexually Transmitted Diseases. Atlanta, Ga: US Department of Health and Human Services; 2006.
18. Hodge JG, Jr, Pulver A, Hogben M, et al. Expedited partner therapy for sexually transmitted diseases: assessing the legal environment. Am J Public Health. 2008;98:238-243.
1. Eaton DK, Kann L, Kinchen S, et al. and the CDC. Youth risk behavior surveillance—United States, 2007. Morb Mortal Wkly Rep. 2008;57(SS-4):1-136.
2. Diller J. Cultural Diversity: A Primer for the Human Services. Belmont, Calif: Wadsworth Publishing Company; 1999:8-26.
3. Centers for Disease Control and Prevention. Sexually transmitted diseases: treatment guidelines, 2006. Morb Mortal Wkly Rep. 2006;55(RR-11):1-100.
4. Rietmeijer CA. Risk reduction counseling for prevention of sexually transmitted infections: how it works and how to make it work. Sex Transm Inf. 2007;83:2-9.
5. Ma R. Special report: one-to-one counseling can reduce STI risk. The Practitioner. April 26;2007:81.-
6. The clinical approach to the patient with possible STD. In: Marrazzo J, Ocbamichael N, Meegan A, Stamm WE. The Practitioner’s Handbook for the Management of STDs. 4th ed. Seattle, Wash: University of Washington; 2007:1-7.
7. American Medical Association (AMA). Guidelines for Adolescent Preventative Services (GAPS). [Recommendations Monograph]. Chicago, Ill: AMA; 1997.
8. Creegan L, ed. Behavioral Counseling for STD/HIV Risk Reduction. National Network of STD/HIV Prevention Training Centers; August 2007. Available at: http://www.stdhivtraining.org/resource.php?id=19&ret=clinical_resources. Accessed December 31, 2008.
9. CDC. STD Health Communication, Executive Summary HPV. 2004. Available at: http://www.cdc.gov/std/HealthComm/ExecSumHPVGenPub2004.pdf. Accessed December 31, 2008.
10. Bauer HM, ed. The Clinical Approach to the STD Patient. National Network of STD/HIV Prevention Training Centers; August 2007. Available at: http://www.stdhivtraining.org/resource.php?id=21&ret=clinical_resources. Accessed December 31, 2008.
11. Sophocles A. Time is of the essence: coding on the basis of time for physician services. Fam Pract Manag. 2003;10:27-31.
12. The House Committee on Energy and Commerce. Making the Internet safe for kids: the role of ISP’s and social networking sites, Day 2. Hearing Webcast. Available at: http://archives.energy-commerce.house.gov/reparchives/108/Hearings/06282006hearing1955/hearing.htm. Accessed January 7, 2009.
13. National Suicide Prevention Lifeline home page. Available at: www.suicidepreventionlifeline.org. Accessed December 31, 2008.
14. Brown NL. Internet-based hotline to counsel abused young people. Healthline.com Web site. Available at: www.healthline.com/blogs/teen_health/2008/04/internet-based-hotline-to-counsel.html. Accessed December 31, 2008.
15. Rape, Abuse & Incest National Network home page. Available at: www.rainn.org. Accessed December 31, 2008.
16. Brackbill R, Sternberg M, Fishbein M. Where do people go for treatment of sexually transmitted diseases? Fam Plann Perspect. 1999;3:10-15.
17. CDC. Expedited Partner Therapy in the Management of Sexually Transmitted Diseases. Atlanta, Ga: US Department of Health and Human Services; 2006.
18. Hodge JG, Jr, Pulver A, Hogben M, et al. Expedited partner therapy for sexually transmitted diseases: assessing the legal environment. Am J Public Health. 2008;98:238-243.
Brown recluse spider bite? Consider this uniquely conservative treatment
- Be concerned about brown recluse envenomation when a patient reports intensifying localized pain disproportionate to physical findings after a “bite” (C).
- Prescribe an oral antihistamine alone to control symptoms, even with a necrotic wound, and mark the patient’s progress over 24 hours (C).
- If the patient improves dramatically, continue the antihistamine; with little or no improvement, consider giving an antibiotic with the antihistamine (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
What is the best way to treat the bite of a brown recluse spider? Having treated more than 185 bite wounds on 150 patients over the past 30 years, I have found that an oral antihistamine works best and makes surgery unnecessary.
I did not arrive at this treatment protocol immediately but, rather, developed it in 4 phases, which I describe in this article. Not only does this conservative approach consistently heal confirmed brown recluse bite wounds, but should a bite be mistakenly attributed to the brown recluse (or one of its relatives in the Loxosceles genus of spider), there is no harm to the patient, nor any big expense.
Is a brown recluse to blame?
Due to limited experience among the wider medical community in identifying spider envenomation,1-4 bite recognition and selection of appropriate therapy can be difficult.
Early findings can be confusing. Brown recluse bites typically feel like a pin prick. A vasoconstrictive halo may surround the bite, but this sign is inconsistent. Usually there is nothing to see immediately and pain from the bite goes away, so most patients dismiss the incident.5 Soon, however, pain from the envenomation begins and, importantly, becomes disproportionate to physical findings—the patient shows you where it hurts, but there is nothing visible to support a diagnosis or suggest a course of action.
Progression of the wound usually tells more than a captured specimen. If you are fortunate enough to examine an intact spider caught by the patient, a “fiddle” mark on the spider’s back confirms it is a brown recluse (FIGURE 1). However, most specimens brought in for examination are so misshapen from the patient’s retribution as to make identification futile. I focus instead on the resultant wound.
Spider’s venom causes the wound. Spiders lack a mechanical digestive system; their so-called venom actually is a set of enzymes that liquefy a prey’s tissues.6 In the 48 hours after a bite, these digestive enzymes cause a progressive necrosis of fat under the skin. Eventually the overlying skin also turns necrotic. The venom does not penetrate underlying fascia planes, as many infectious processes do.
Necrosis can, rarely, cause disseminated intravascular coagulation and death. A more likely scenario, though still relatively uncommon, is that the necrotic sequence leads to an indolent wound that heals only with difficulty. Most patients recover without medical care and do not even seek it.7-9
Wounds can be categorized, as outlined by Auer et al,10 into groups 1 through 4, from least severe to most severe. However, this classification scheme has made no difference in my management decisions.
FIGURE 1
The distinctive “fiddle” mark
How my treatment protocol evolved
My protocol for treating necrotic bite wounds of the brown recluse, and similar wounds from other insects, progressed over 30 years through 4 phases of treatment concepts.
Phase 1: Surgical excision
From 1979 to 1980, I treated necrotic wounds with surgical excision and secondary closure or skin grafting.1 Many patients complained of severe itching around these wounds, which I treated empirically with the antihistamine Benadryl. All patients experienced symptom relief and had a reasonable cosmetic result (FIGURE 2).
In writing up the case results for a presentation to the Society of Air Force Clinical Surgeons, I conducted background research and found a postulate suggesting that antigen recognition was an important factor in wound progression and that it took approximately 7 days to achieve. That postulate drove the second phase of discovery.1
FIGURE 2
Bite wound treatment: Debridement and closure or skin grafting
From 1980 to 1981, I focused on the postulate, which also suggested that the antigen–antibody reaction following envenomation could make reactions to subsequent (“second set”) bites less severe.1 This made sense, though good clinical data were lacking.
I decided to simply observe patients’ wounds for 7 to 10 days before performing debridement, advising patients to use an antihistamine to control symptoms during the waiting period. I took photographs to document clinical progression of wounds. In reviewing these photographs, I noted that the necrotic wounds stabilized immediately on starting an antihistamine—specifically, Benadryl, 5 mg/kg or 50 mg qid. After 7 to 10 days, I debrided the wounds, using fluorescein and a Woods lamp to guide the procedure. I found that tissue of doubtful viability survived if debridement was conservative and the patient had used an antihistamine (FIGURE 3).
FIGURE 3
Bite wound treatment: Observation for 7 to 10 days
In reviewing phase 2 results in preparation for a presentation to the Southwestern Surgical Congress, I realized that debridement was rarely indicated. So, from 1981 to 1982, I relied less on surgery and more on conservative therapy. Patients got better simply with antihistamine therapy. Redness diminished very quickly once patients took the antihistamine, and pockets of purulent material drained on their own.
This approach also allowed antigen recognition to occur and seemed to prove the postulate of a less severe second bite. I saw 4 patients with proven “second set” spider bites (intact specimens were available) and all had an immediate inflammatory response and no subsequent necrosis. An 18-month-old patient with a bite below her right eye healed with Benadryl therapy alone (FIGURE 4).
FIGURE 4
Bite wound treatment: Antihistamine alone
Phase 4: Exclusive antihistamine therapy
Since 1982, I have treated more than 100 patients (myself included) with necrotic bite wounds from spiders and other insects. None have required operative debridement, and all have achieved much more acceptable cosmetic results using Benadryl therapy than with surgical therapy.
My encounter with a brown recluse. The bite I received was rather typical. I felt a sharp sting when outside working on the lawn. I looked down, saw nothing, and assumed I had run into a pin or small stick that was now not visible. For 3 days I noticed nothing unusual. On day 4, after showering I noticed a large amount of purulent material on the towel after drying my anterior left leg. On inspecting the area, I saw an area of necrosis approximately 6 × 6 cm with a central area oozing a purulent material. This appeared just like the necrotic insect bites I had treated with antihistamines, so I started myself on that regimen. The redness cleared up in 2 days and the wound healed completely in 10 days without antibiotics. A 3 × 3 mm circular scar remained; I lost none of the surrounding marginal skin.
Further evidence of less severe second bites. Another physician—my coauthor on the presentation to the Southwestern Surgical Congress—was bitten at age 12 by an unidentified insect, resulting in a large necrotic wound on his thigh. The lesion, reportedly 10 to 12 cm in diameter, healed with topical treatment only. After our joint report in 1982, a brown recluse spider bit him on the abdomen while he was working in the yard. He captured the spider and verified its genus and species. Guessing that the bite he received as a 12 year old was also from a brown recluse, he decided to wait and see if he had a “second set” reaction to the bite. He had an immediate inflammatory reaction, no skin necrosis, and the entire area returned to normal in 3 days. His experience became the fifth “second set” spider bite reaction in our series.
Another dramatic experience. One 22-year-old woman’s experience is worth a longer discussion. She visited her physician because 4 small areas of necrosis on her lower abdomen and chest had lasted several days and were getting worse. He assumed these were small abscesses and treated her with antibiotics. The lesions slowly enlarged to become 6 to 8 cm in diameter, and the physician hospitalized her for intravenous (IV) antibiotic therapy.
When the patient worsened after 2 days of IV antibiotics and incision and drainage, I was called to examine her for possible surgical debridement. I thought the lesions were necrotic insect bites and recommended starting Benadryl, 50 mg qid. She improved remarkably over the next 24 hours, her wounds decreasing in size by 50%. She was discharged from the hospital and, against my advice, the Benadryl was stopped. Within 48 hours the area of inflammation around the wounds had doubled in size. She was readmitted and given Benadryl. Again she improved remarkably in 24 hours. The purulent discharge was sent for culture, which grew an organism resistant to all of her previous antibiotics. She healed uneventfully in 10 days on antihistamines, and without antibiotics. This is typical in my experience. Antibiotics have seldom proved useful, and culture results are generally confusing.
Broader support for my observations
On January 27, 2006, on eMedicine (link is no longer active), dermatologist Adam S. Stibich, MD, published a physiology review of the brown recluse bite. He acknowledged that neutrophils accumulate in the wound at 24 to 72 hours, consistent with the antigen–antibody response postulated in my clinical review. Neutrophils are products of the histamine cascade that most likely brings about tissue necrosis.
Stibich’s plea for conservative management was well founded. He noted that only 10% of envenomation episodes result in large open wounds. The physical findings he described were in keeping with my observation that a subcutaneous necrotic process precedes surface changes.
His treatment of choice was dapsone. He pointed out that other clinicians, too, had reported success with dapsone, and with steroids, antibiotics, hyperbaric oxygen, electric shock therapy, and surgical therapy.3,4,11,12 Stibich commented extensively on these treatment modalities, except electrical shock. However, while the laboratory results achieved were good, clinical outcomes were mixed. Of these treatments, I have experience only with surgery, and I have found that it does not improve the healing process.
Antihistamine and observation: The ideal Tx
The ideal treatment for necrotic wounds from envenomation would account for an underlying antigen–antibody process, shorten the natural history of the illness, result in the least deformity, minimize cost, and allow for errors in diagnosis without harming patients.
The brown recluse spider is found primarily in the Mississippi River Valley and its tributaries. Its genus members can be found in Arizona, Texas, and South America. They are nocturnal creatures, typically living in woodpiles and secluded dark areas. Usually they are 8 to 9 mm in diameter, but they can reach several centimeters.
Unfortunately, the spider travels well hidden in clothing, so even if you don’t practice medicine in Arizona, Texas, or South America, you could still find yourself treating a patient with a brown recluse spider bite. I saw these wounds in Germany and Spain, on patients who had just arrived from the United States or whose family members had been there. Hite and others have also described this experience.5,12-14
Many people seek care in emergency departments for nasty looking spontaneous abscesses, some of which are in fact spider bites. Increasingly, though, lesions in this setting are colonized with methicillin-resistant Staphylococcus aureus (MRSA). One recent study showed that among patients who visited EDs because of skin infections, more than 58% were infected with MRSA.15 The difficulty is in determining whether MRSA has caused the nasty wound, is a secondary colonization of a spider bite, or if the wound is actually an uninfected spider bite.
Given the simplicity and low cost of antihistamine treatment, I recommend it for wound care in these instances. If the clinical response is good within 24 hours, you are probably dealing with an uninfected spider bite. If the response is slow or the wound worsens after 24 hours, consider adding an antibiotic or performing surgical debridement.
Based on my observations since 1982, I am convinced these necrotic wounds involve an antigen–antibody reaction, are histamine driven, and are adequately treated with oral antihistamines. I now treat presumed necrotic spider bite with antihistamines only. Laboratory or serum testing to confirm a diagnosis has not been useful. It takes too long, costs too much, and does not contribute to management decisions.
If the wound improves dramatically in 24 hours, which is the norm, I continue the antihistamines for 7 to 10 days. If the wound does not improve, I suspect a bacterial component and add an antibiotic. Not once in the last 26 years have I had to resort to surgery.
Correspondence
Paul K. Carlton, Jr, MD, FACS, The Texas A&M University Health Science Center, Office of Homeland Security, 301 Tarrow, 7th Floor, John Connally Building, College Station, TX 77840; [email protected]
1. Hershey FB, Aulenbacher CE. Surgical treatment of brown spider bites. Ann Surg. 1969;170:300-308.
2. Arnold RE. Brown recluse spider bites: five cases with a review of the literature. JACEP. 1976;5:262-264.
3. DeLozier JB, Reaves L, King LE, Jr, et al. Brown recluse spider bites of the upper extremity. South Med J. 1988;81:181-184.
4. Wright SW, Wrenn KD, Murray L, et al. Clinical presentation and outcome of brown recluse spider bite. Ann Emerg Med. 1997;30:28-32.
5. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13:415-423.
6. Spiders: Digestive system. Available at: http://www.greensmiths.com/spiders.htm. Accessed November 20, 2007.
7. Nance WE. Hemolytic anemia of necrotic arachnidism. Am J Med. 1981;31:801-807.
8. Russell FE, Waldron WG, Madon MB. Bites by the brown spiders Loxosceles unicolor and Loxosceles arizonica in California and Arizona. Toxicon. 1969;17:109-117.
9. Atkins JA, Wingo CW, Sodeman WA, et al. Necrotic arachnidism. Am J Trop Med Hyg. 1958;7:165-184.
10. Auer A, Hershey FB. Proceedings: surgery for necrotic bites of the brown spider. Arch Surg. 1974;108:612-618.
11. Rees RS, Shack RB, Withers EH, et al. Management of the brown recluse spider bite. Plast Reconstr Surg. 1981;68:768-773.
12. Swanson DL, Vetter RS. Bites of brown recluse spiders and suspected necrotic arachnidism. N Engl J Med. 2005;352:700-707.
13. Barnes JK. Brown recluse and Mediterranean recluse spiders. University of Arkansas Arthropod Museum Notes. Revised May 2003; #11. Available at: http://entomology.uark.edu/museum/browrec.html. Accessed January 16, 2009.
14. Hite JL, Gladney WJ, Lancaster JL, et al. Biology of the brown recluse spider. Arkansas Experiment Station Bulletin. 1966; #711.
15. Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
- Be concerned about brown recluse envenomation when a patient reports intensifying localized pain disproportionate to physical findings after a “bite” (C).
- Prescribe an oral antihistamine alone to control symptoms, even with a necrotic wound, and mark the patient’s progress over 24 hours (C).
- If the patient improves dramatically, continue the antihistamine; with little or no improvement, consider giving an antibiotic with the antihistamine (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
What is the best way to treat the bite of a brown recluse spider? Having treated more than 185 bite wounds on 150 patients over the past 30 years, I have found that an oral antihistamine works best and makes surgery unnecessary.
I did not arrive at this treatment protocol immediately but, rather, developed it in 4 phases, which I describe in this article. Not only does this conservative approach consistently heal confirmed brown recluse bite wounds, but should a bite be mistakenly attributed to the brown recluse (or one of its relatives in the Loxosceles genus of spider), there is no harm to the patient, nor any big expense.
Is a brown recluse to blame?
Due to limited experience among the wider medical community in identifying spider envenomation,1-4 bite recognition and selection of appropriate therapy can be difficult.
Early findings can be confusing. Brown recluse bites typically feel like a pin prick. A vasoconstrictive halo may surround the bite, but this sign is inconsistent. Usually there is nothing to see immediately and pain from the bite goes away, so most patients dismiss the incident.5 Soon, however, pain from the envenomation begins and, importantly, becomes disproportionate to physical findings—the patient shows you where it hurts, but there is nothing visible to support a diagnosis or suggest a course of action.
Progression of the wound usually tells more than a captured specimen. If you are fortunate enough to examine an intact spider caught by the patient, a “fiddle” mark on the spider’s back confirms it is a brown recluse (FIGURE 1). However, most specimens brought in for examination are so misshapen from the patient’s retribution as to make identification futile. I focus instead on the resultant wound.
Spider’s venom causes the wound. Spiders lack a mechanical digestive system; their so-called venom actually is a set of enzymes that liquefy a prey’s tissues.6 In the 48 hours after a bite, these digestive enzymes cause a progressive necrosis of fat under the skin. Eventually the overlying skin also turns necrotic. The venom does not penetrate underlying fascia planes, as many infectious processes do.
Necrosis can, rarely, cause disseminated intravascular coagulation and death. A more likely scenario, though still relatively uncommon, is that the necrotic sequence leads to an indolent wound that heals only with difficulty. Most patients recover without medical care and do not even seek it.7-9
Wounds can be categorized, as outlined by Auer et al,10 into groups 1 through 4, from least severe to most severe. However, this classification scheme has made no difference in my management decisions.
FIGURE 1
The distinctive “fiddle” mark
How my treatment protocol evolved
My protocol for treating necrotic bite wounds of the brown recluse, and similar wounds from other insects, progressed over 30 years through 4 phases of treatment concepts.
Phase 1: Surgical excision
From 1979 to 1980, I treated necrotic wounds with surgical excision and secondary closure or skin grafting.1 Many patients complained of severe itching around these wounds, which I treated empirically with the antihistamine Benadryl. All patients experienced symptom relief and had a reasonable cosmetic result (FIGURE 2).
In writing up the case results for a presentation to the Society of Air Force Clinical Surgeons, I conducted background research and found a postulate suggesting that antigen recognition was an important factor in wound progression and that it took approximately 7 days to achieve. That postulate drove the second phase of discovery.1
FIGURE 2
Bite wound treatment: Debridement and closure or skin grafting
From 1980 to 1981, I focused on the postulate, which also suggested that the antigen–antibody reaction following envenomation could make reactions to subsequent (“second set”) bites less severe.1 This made sense, though good clinical data were lacking.
I decided to simply observe patients’ wounds for 7 to 10 days before performing debridement, advising patients to use an antihistamine to control symptoms during the waiting period. I took photographs to document clinical progression of wounds. In reviewing these photographs, I noted that the necrotic wounds stabilized immediately on starting an antihistamine—specifically, Benadryl, 5 mg/kg or 50 mg qid. After 7 to 10 days, I debrided the wounds, using fluorescein and a Woods lamp to guide the procedure. I found that tissue of doubtful viability survived if debridement was conservative and the patient had used an antihistamine (FIGURE 3).
FIGURE 3
Bite wound treatment: Observation for 7 to 10 days
In reviewing phase 2 results in preparation for a presentation to the Southwestern Surgical Congress, I realized that debridement was rarely indicated. So, from 1981 to 1982, I relied less on surgery and more on conservative therapy. Patients got better simply with antihistamine therapy. Redness diminished very quickly once patients took the antihistamine, and pockets of purulent material drained on their own.
This approach also allowed antigen recognition to occur and seemed to prove the postulate of a less severe second bite. I saw 4 patients with proven “second set” spider bites (intact specimens were available) and all had an immediate inflammatory response and no subsequent necrosis. An 18-month-old patient with a bite below her right eye healed with Benadryl therapy alone (FIGURE 4).
FIGURE 4
Bite wound treatment: Antihistamine alone
Phase 4: Exclusive antihistamine therapy
Since 1982, I have treated more than 100 patients (myself included) with necrotic bite wounds from spiders and other insects. None have required operative debridement, and all have achieved much more acceptable cosmetic results using Benadryl therapy than with surgical therapy.
My encounter with a brown recluse. The bite I received was rather typical. I felt a sharp sting when outside working on the lawn. I looked down, saw nothing, and assumed I had run into a pin or small stick that was now not visible. For 3 days I noticed nothing unusual. On day 4, after showering I noticed a large amount of purulent material on the towel after drying my anterior left leg. On inspecting the area, I saw an area of necrosis approximately 6 × 6 cm with a central area oozing a purulent material. This appeared just like the necrotic insect bites I had treated with antihistamines, so I started myself on that regimen. The redness cleared up in 2 days and the wound healed completely in 10 days without antibiotics. A 3 × 3 mm circular scar remained; I lost none of the surrounding marginal skin.
Further evidence of less severe second bites. Another physician—my coauthor on the presentation to the Southwestern Surgical Congress—was bitten at age 12 by an unidentified insect, resulting in a large necrotic wound on his thigh. The lesion, reportedly 10 to 12 cm in diameter, healed with topical treatment only. After our joint report in 1982, a brown recluse spider bit him on the abdomen while he was working in the yard. He captured the spider and verified its genus and species. Guessing that the bite he received as a 12 year old was also from a brown recluse, he decided to wait and see if he had a “second set” reaction to the bite. He had an immediate inflammatory reaction, no skin necrosis, and the entire area returned to normal in 3 days. His experience became the fifth “second set” spider bite reaction in our series.
Another dramatic experience. One 22-year-old woman’s experience is worth a longer discussion. She visited her physician because 4 small areas of necrosis on her lower abdomen and chest had lasted several days and were getting worse. He assumed these were small abscesses and treated her with antibiotics. The lesions slowly enlarged to become 6 to 8 cm in diameter, and the physician hospitalized her for intravenous (IV) antibiotic therapy.
When the patient worsened after 2 days of IV antibiotics and incision and drainage, I was called to examine her for possible surgical debridement. I thought the lesions were necrotic insect bites and recommended starting Benadryl, 50 mg qid. She improved remarkably over the next 24 hours, her wounds decreasing in size by 50%. She was discharged from the hospital and, against my advice, the Benadryl was stopped. Within 48 hours the area of inflammation around the wounds had doubled in size. She was readmitted and given Benadryl. Again she improved remarkably in 24 hours. The purulent discharge was sent for culture, which grew an organism resistant to all of her previous antibiotics. She healed uneventfully in 10 days on antihistamines, and without antibiotics. This is typical in my experience. Antibiotics have seldom proved useful, and culture results are generally confusing.
Broader support for my observations
On January 27, 2006, on eMedicine (link is no longer active), dermatologist Adam S. Stibich, MD, published a physiology review of the brown recluse bite. He acknowledged that neutrophils accumulate in the wound at 24 to 72 hours, consistent with the antigen–antibody response postulated in my clinical review. Neutrophils are products of the histamine cascade that most likely brings about tissue necrosis.
Stibich’s plea for conservative management was well founded. He noted that only 10% of envenomation episodes result in large open wounds. The physical findings he described were in keeping with my observation that a subcutaneous necrotic process precedes surface changes.
His treatment of choice was dapsone. He pointed out that other clinicians, too, had reported success with dapsone, and with steroids, antibiotics, hyperbaric oxygen, electric shock therapy, and surgical therapy.3,4,11,12 Stibich commented extensively on these treatment modalities, except electrical shock. However, while the laboratory results achieved were good, clinical outcomes were mixed. Of these treatments, I have experience only with surgery, and I have found that it does not improve the healing process.
Antihistamine and observation: The ideal Tx
The ideal treatment for necrotic wounds from envenomation would account for an underlying antigen–antibody process, shorten the natural history of the illness, result in the least deformity, minimize cost, and allow for errors in diagnosis without harming patients.
The brown recluse spider is found primarily in the Mississippi River Valley and its tributaries. Its genus members can be found in Arizona, Texas, and South America. They are nocturnal creatures, typically living in woodpiles and secluded dark areas. Usually they are 8 to 9 mm in diameter, but they can reach several centimeters.
Unfortunately, the spider travels well hidden in clothing, so even if you don’t practice medicine in Arizona, Texas, or South America, you could still find yourself treating a patient with a brown recluse spider bite. I saw these wounds in Germany and Spain, on patients who had just arrived from the United States or whose family members had been there. Hite and others have also described this experience.5,12-14
Many people seek care in emergency departments for nasty looking spontaneous abscesses, some of which are in fact spider bites. Increasingly, though, lesions in this setting are colonized with methicillin-resistant Staphylococcus aureus (MRSA). One recent study showed that among patients who visited EDs because of skin infections, more than 58% were infected with MRSA.15 The difficulty is in determining whether MRSA has caused the nasty wound, is a secondary colonization of a spider bite, or if the wound is actually an uninfected spider bite.
Given the simplicity and low cost of antihistamine treatment, I recommend it for wound care in these instances. If the clinical response is good within 24 hours, you are probably dealing with an uninfected spider bite. If the response is slow or the wound worsens after 24 hours, consider adding an antibiotic or performing surgical debridement.
Based on my observations since 1982, I am convinced these necrotic wounds involve an antigen–antibody reaction, are histamine driven, and are adequately treated with oral antihistamines. I now treat presumed necrotic spider bite with antihistamines only. Laboratory or serum testing to confirm a diagnosis has not been useful. It takes too long, costs too much, and does not contribute to management decisions.
If the wound improves dramatically in 24 hours, which is the norm, I continue the antihistamines for 7 to 10 days. If the wound does not improve, I suspect a bacterial component and add an antibiotic. Not once in the last 26 years have I had to resort to surgery.
Correspondence
Paul K. Carlton, Jr, MD, FACS, The Texas A&M University Health Science Center, Office of Homeland Security, 301 Tarrow, 7th Floor, John Connally Building, College Station, TX 77840; [email protected]
- Be concerned about brown recluse envenomation when a patient reports intensifying localized pain disproportionate to physical findings after a “bite” (C).
- Prescribe an oral antihistamine alone to control symptoms, even with a necrotic wound, and mark the patient’s progress over 24 hours (C).
- If the patient improves dramatically, continue the antihistamine; with little or no improvement, consider giving an antibiotic with the antihistamine (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
What is the best way to treat the bite of a brown recluse spider? Having treated more than 185 bite wounds on 150 patients over the past 30 years, I have found that an oral antihistamine works best and makes surgery unnecessary.
I did not arrive at this treatment protocol immediately but, rather, developed it in 4 phases, which I describe in this article. Not only does this conservative approach consistently heal confirmed brown recluse bite wounds, but should a bite be mistakenly attributed to the brown recluse (or one of its relatives in the Loxosceles genus of spider), there is no harm to the patient, nor any big expense.
Is a brown recluse to blame?
Due to limited experience among the wider medical community in identifying spider envenomation,1-4 bite recognition and selection of appropriate therapy can be difficult.
Early findings can be confusing. Brown recluse bites typically feel like a pin prick. A vasoconstrictive halo may surround the bite, but this sign is inconsistent. Usually there is nothing to see immediately and pain from the bite goes away, so most patients dismiss the incident.5 Soon, however, pain from the envenomation begins and, importantly, becomes disproportionate to physical findings—the patient shows you where it hurts, but there is nothing visible to support a diagnosis or suggest a course of action.
Progression of the wound usually tells more than a captured specimen. If you are fortunate enough to examine an intact spider caught by the patient, a “fiddle” mark on the spider’s back confirms it is a brown recluse (FIGURE 1). However, most specimens brought in for examination are so misshapen from the patient’s retribution as to make identification futile. I focus instead on the resultant wound.
Spider’s venom causes the wound. Spiders lack a mechanical digestive system; their so-called venom actually is a set of enzymes that liquefy a prey’s tissues.6 In the 48 hours after a bite, these digestive enzymes cause a progressive necrosis of fat under the skin. Eventually the overlying skin also turns necrotic. The venom does not penetrate underlying fascia planes, as many infectious processes do.
Necrosis can, rarely, cause disseminated intravascular coagulation and death. A more likely scenario, though still relatively uncommon, is that the necrotic sequence leads to an indolent wound that heals only with difficulty. Most patients recover without medical care and do not even seek it.7-9
Wounds can be categorized, as outlined by Auer et al,10 into groups 1 through 4, from least severe to most severe. However, this classification scheme has made no difference in my management decisions.
FIGURE 1
The distinctive “fiddle” mark
How my treatment protocol evolved
My protocol for treating necrotic bite wounds of the brown recluse, and similar wounds from other insects, progressed over 30 years through 4 phases of treatment concepts.
Phase 1: Surgical excision
From 1979 to 1980, I treated necrotic wounds with surgical excision and secondary closure or skin grafting.1 Many patients complained of severe itching around these wounds, which I treated empirically with the antihistamine Benadryl. All patients experienced symptom relief and had a reasonable cosmetic result (FIGURE 2).
In writing up the case results for a presentation to the Society of Air Force Clinical Surgeons, I conducted background research and found a postulate suggesting that antigen recognition was an important factor in wound progression and that it took approximately 7 days to achieve. That postulate drove the second phase of discovery.1
FIGURE 2
Bite wound treatment: Debridement and closure or skin grafting
From 1980 to 1981, I focused on the postulate, which also suggested that the antigen–antibody reaction following envenomation could make reactions to subsequent (“second set”) bites less severe.1 This made sense, though good clinical data were lacking.
I decided to simply observe patients’ wounds for 7 to 10 days before performing debridement, advising patients to use an antihistamine to control symptoms during the waiting period. I took photographs to document clinical progression of wounds. In reviewing these photographs, I noted that the necrotic wounds stabilized immediately on starting an antihistamine—specifically, Benadryl, 5 mg/kg or 50 mg qid. After 7 to 10 days, I debrided the wounds, using fluorescein and a Woods lamp to guide the procedure. I found that tissue of doubtful viability survived if debridement was conservative and the patient had used an antihistamine (FIGURE 3).
FIGURE 3
Bite wound treatment: Observation for 7 to 10 days
In reviewing phase 2 results in preparation for a presentation to the Southwestern Surgical Congress, I realized that debridement was rarely indicated. So, from 1981 to 1982, I relied less on surgery and more on conservative therapy. Patients got better simply with antihistamine therapy. Redness diminished very quickly once patients took the antihistamine, and pockets of purulent material drained on their own.
This approach also allowed antigen recognition to occur and seemed to prove the postulate of a less severe second bite. I saw 4 patients with proven “second set” spider bites (intact specimens were available) and all had an immediate inflammatory response and no subsequent necrosis. An 18-month-old patient with a bite below her right eye healed with Benadryl therapy alone (FIGURE 4).
FIGURE 4
Bite wound treatment: Antihistamine alone
Phase 4: Exclusive antihistamine therapy
Since 1982, I have treated more than 100 patients (myself included) with necrotic bite wounds from spiders and other insects. None have required operative debridement, and all have achieved much more acceptable cosmetic results using Benadryl therapy than with surgical therapy.
My encounter with a brown recluse. The bite I received was rather typical. I felt a sharp sting when outside working on the lawn. I looked down, saw nothing, and assumed I had run into a pin or small stick that was now not visible. For 3 days I noticed nothing unusual. On day 4, after showering I noticed a large amount of purulent material on the towel after drying my anterior left leg. On inspecting the area, I saw an area of necrosis approximately 6 × 6 cm with a central area oozing a purulent material. This appeared just like the necrotic insect bites I had treated with antihistamines, so I started myself on that regimen. The redness cleared up in 2 days and the wound healed completely in 10 days without antibiotics. A 3 × 3 mm circular scar remained; I lost none of the surrounding marginal skin.
Further evidence of less severe second bites. Another physician—my coauthor on the presentation to the Southwestern Surgical Congress—was bitten at age 12 by an unidentified insect, resulting in a large necrotic wound on his thigh. The lesion, reportedly 10 to 12 cm in diameter, healed with topical treatment only. After our joint report in 1982, a brown recluse spider bit him on the abdomen while he was working in the yard. He captured the spider and verified its genus and species. Guessing that the bite he received as a 12 year old was also from a brown recluse, he decided to wait and see if he had a “second set” reaction to the bite. He had an immediate inflammatory reaction, no skin necrosis, and the entire area returned to normal in 3 days. His experience became the fifth “second set” spider bite reaction in our series.
Another dramatic experience. One 22-year-old woman’s experience is worth a longer discussion. She visited her physician because 4 small areas of necrosis on her lower abdomen and chest had lasted several days and were getting worse. He assumed these were small abscesses and treated her with antibiotics. The lesions slowly enlarged to become 6 to 8 cm in diameter, and the physician hospitalized her for intravenous (IV) antibiotic therapy.
When the patient worsened after 2 days of IV antibiotics and incision and drainage, I was called to examine her for possible surgical debridement. I thought the lesions were necrotic insect bites and recommended starting Benadryl, 50 mg qid. She improved remarkably over the next 24 hours, her wounds decreasing in size by 50%. She was discharged from the hospital and, against my advice, the Benadryl was stopped. Within 48 hours the area of inflammation around the wounds had doubled in size. She was readmitted and given Benadryl. Again she improved remarkably in 24 hours. The purulent discharge was sent for culture, which grew an organism resistant to all of her previous antibiotics. She healed uneventfully in 10 days on antihistamines, and without antibiotics. This is typical in my experience. Antibiotics have seldom proved useful, and culture results are generally confusing.
Broader support for my observations
On January 27, 2006, on eMedicine (link is no longer active), dermatologist Adam S. Stibich, MD, published a physiology review of the brown recluse bite. He acknowledged that neutrophils accumulate in the wound at 24 to 72 hours, consistent with the antigen–antibody response postulated in my clinical review. Neutrophils are products of the histamine cascade that most likely brings about tissue necrosis.
Stibich’s plea for conservative management was well founded. He noted that only 10% of envenomation episodes result in large open wounds. The physical findings he described were in keeping with my observation that a subcutaneous necrotic process precedes surface changes.
His treatment of choice was dapsone. He pointed out that other clinicians, too, had reported success with dapsone, and with steroids, antibiotics, hyperbaric oxygen, electric shock therapy, and surgical therapy.3,4,11,12 Stibich commented extensively on these treatment modalities, except electrical shock. However, while the laboratory results achieved were good, clinical outcomes were mixed. Of these treatments, I have experience only with surgery, and I have found that it does not improve the healing process.
Antihistamine and observation: The ideal Tx
The ideal treatment for necrotic wounds from envenomation would account for an underlying antigen–antibody process, shorten the natural history of the illness, result in the least deformity, minimize cost, and allow for errors in diagnosis without harming patients.
The brown recluse spider is found primarily in the Mississippi River Valley and its tributaries. Its genus members can be found in Arizona, Texas, and South America. They are nocturnal creatures, typically living in woodpiles and secluded dark areas. Usually they are 8 to 9 mm in diameter, but they can reach several centimeters.
Unfortunately, the spider travels well hidden in clothing, so even if you don’t practice medicine in Arizona, Texas, or South America, you could still find yourself treating a patient with a brown recluse spider bite. I saw these wounds in Germany and Spain, on patients who had just arrived from the United States or whose family members had been there. Hite and others have also described this experience.5,12-14
Many people seek care in emergency departments for nasty looking spontaneous abscesses, some of which are in fact spider bites. Increasingly, though, lesions in this setting are colonized with methicillin-resistant Staphylococcus aureus (MRSA). One recent study showed that among patients who visited EDs because of skin infections, more than 58% were infected with MRSA.15 The difficulty is in determining whether MRSA has caused the nasty wound, is a secondary colonization of a spider bite, or if the wound is actually an uninfected spider bite.
Given the simplicity and low cost of antihistamine treatment, I recommend it for wound care in these instances. If the clinical response is good within 24 hours, you are probably dealing with an uninfected spider bite. If the response is slow or the wound worsens after 24 hours, consider adding an antibiotic or performing surgical debridement.
Based on my observations since 1982, I am convinced these necrotic wounds involve an antigen–antibody reaction, are histamine driven, and are adequately treated with oral antihistamines. I now treat presumed necrotic spider bite with antihistamines only. Laboratory or serum testing to confirm a diagnosis has not been useful. It takes too long, costs too much, and does not contribute to management decisions.
If the wound improves dramatically in 24 hours, which is the norm, I continue the antihistamines for 7 to 10 days. If the wound does not improve, I suspect a bacterial component and add an antibiotic. Not once in the last 26 years have I had to resort to surgery.
Correspondence
Paul K. Carlton, Jr, MD, FACS, The Texas A&M University Health Science Center, Office of Homeland Security, 301 Tarrow, 7th Floor, John Connally Building, College Station, TX 77840; [email protected]
1. Hershey FB, Aulenbacher CE. Surgical treatment of brown spider bites. Ann Surg. 1969;170:300-308.
2. Arnold RE. Brown recluse spider bites: five cases with a review of the literature. JACEP. 1976;5:262-264.
3. DeLozier JB, Reaves L, King LE, Jr, et al. Brown recluse spider bites of the upper extremity. South Med J. 1988;81:181-184.
4. Wright SW, Wrenn KD, Murray L, et al. Clinical presentation and outcome of brown recluse spider bite. Ann Emerg Med. 1997;30:28-32.
5. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13:415-423.
6. Spiders: Digestive system. Available at: http://www.greensmiths.com/spiders.htm. Accessed November 20, 2007.
7. Nance WE. Hemolytic anemia of necrotic arachnidism. Am J Med. 1981;31:801-807.
8. Russell FE, Waldron WG, Madon MB. Bites by the brown spiders Loxosceles unicolor and Loxosceles arizonica in California and Arizona. Toxicon. 1969;17:109-117.
9. Atkins JA, Wingo CW, Sodeman WA, et al. Necrotic arachnidism. Am J Trop Med Hyg. 1958;7:165-184.
10. Auer A, Hershey FB. Proceedings: surgery for necrotic bites of the brown spider. Arch Surg. 1974;108:612-618.
11. Rees RS, Shack RB, Withers EH, et al. Management of the brown recluse spider bite. Plast Reconstr Surg. 1981;68:768-773.
12. Swanson DL, Vetter RS. Bites of brown recluse spiders and suspected necrotic arachnidism. N Engl J Med. 2005;352:700-707.
13. Barnes JK. Brown recluse and Mediterranean recluse spiders. University of Arkansas Arthropod Museum Notes. Revised May 2003; #11. Available at: http://entomology.uark.edu/museum/browrec.html. Accessed January 16, 2009.
14. Hite JL, Gladney WJ, Lancaster JL, et al. Biology of the brown recluse spider. Arkansas Experiment Station Bulletin. 1966; #711.
15. Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
1. Hershey FB, Aulenbacher CE. Surgical treatment of brown spider bites. Ann Surg. 1969;170:300-308.
2. Arnold RE. Brown recluse spider bites: five cases with a review of the literature. JACEP. 1976;5:262-264.
3. DeLozier JB, Reaves L, King LE, Jr, et al. Brown recluse spider bites of the upper extremity. South Med J. 1988;81:181-184.
4. Wright SW, Wrenn KD, Murray L, et al. Clinical presentation and outcome of brown recluse spider bite. Ann Emerg Med. 1997;30:28-32.
5. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13:415-423.
6. Spiders: Digestive system. Available at: http://www.greensmiths.com/spiders.htm. Accessed November 20, 2007.
7. Nance WE. Hemolytic anemia of necrotic arachnidism. Am J Med. 1981;31:801-807.
8. Russell FE, Waldron WG, Madon MB. Bites by the brown spiders Loxosceles unicolor and Loxosceles arizonica in California and Arizona. Toxicon. 1969;17:109-117.
9. Atkins JA, Wingo CW, Sodeman WA, et al. Necrotic arachnidism. Am J Trop Med Hyg. 1958;7:165-184.
10. Auer A, Hershey FB. Proceedings: surgery for necrotic bites of the brown spider. Arch Surg. 1974;108:612-618.
11. Rees RS, Shack RB, Withers EH, et al. Management of the brown recluse spider bite. Plast Reconstr Surg. 1981;68:768-773.
12. Swanson DL, Vetter RS. Bites of brown recluse spiders and suspected necrotic arachnidism. N Engl J Med. 2005;352:700-707.
13. Barnes JK. Brown recluse and Mediterranean recluse spiders. University of Arkansas Arthropod Museum Notes. Revised May 2003; #11. Available at: http://entomology.uark.edu/museum/browrec.html. Accessed January 16, 2009.
14. Hite JL, Gladney WJ, Lancaster JL, et al. Biology of the brown recluse spider. Arkansas Experiment Station Bulletin. 1966; #711.
15. Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
An aid for spotting basal cell carcinoma
Basal cell carcinoma (BCC) is the most common skin malignancy, but it can still be a tricky and difficult diagnosis even in the most experienced hands. Many BCCs exhibit only some of the defining clinical characteristics, and even when these findings are present, they can be hard to detect without excellent background and oblique lighting and handheld magnification that is 5-power or greater.
Most clinicians are familiar with classic BCC characteristics of pearliness, rolled edges, telangiectasia, bleeding, and crusting. However, the telangiectasias may be so fine that they are difficult or impossible to see, even with excellent lighting and magnification. FIGURE 1A illustrates a histologically verified nodular BCC with telangiectasias that are not readily visible, even with camera macro magnification.
Some BCC papules or plaques lack the classic rolled edges, even when viewed with oblique lighting and magnification. Some lesions may be more scaly than pearly, and others do not bleed and crust until later in their clinical course. With such variation in clinical presentation, all possible clues to BCC are welcome.
Dark dots: Connecting them to BCC
One underappreciated sign that may help with the diagnosis of BCC is the “dark dot sign.”1,2 Dermoscopists are familiar with patterns of pigmentation associated with BCCs, and introductory dermoscopy texts contain pictures of these patterns (blue/blue-gray blotches, maple leaf structures, and spoke-wheel structures).3,4 What is less well known is that this pigmentation may be visible on routine skin examination as “dark dots”—blue, gray, black, or a combination thereof, such as blue-gray. When visible clinically, these dark dots may bolster one’s confidence in the diagnosis—or, in my experience, occasionally even be the best visible hint for a tentative diagnosis of BCC.
Case in point. During a routine skin examination of a patient’s back, I identified an asymptomatic lesion the patient was unaware of, exhibiting no bleeding or crusting. It drew attention because it was different from surrounding lesions (FIGURE 2A), which has been called the “ugly duckling sign.”5 On closer examination, I noted the lesion had a pearly sheen, telangiectasias, and a plethora of dark dots (FIGURE 2B)—the poster child for the “dark dot sign.” Unlike this profound example, most lesions that have dark dots have just one or a few visible dots.
Now review FIGURE 1A again carefully. Above the lesion’s central crater there is an arcuate hemorrhagic crust, and, between the 12 and 1 o’clock positions, a small dark dot, which helps corroborate the clinical impression of BCC. FIGURE 1B is a dermoscopic image of the same lesion. It shows the dark dot well and illustrates the ease with which telangiectasias are seen on dermoscopy, lending a high degree of certainty to the diagnosis of BCC.
Dermoscopy (FIGURE 2C) for the lesion on the patient’s back in FIGURE 2B, however, adds nothing (beyond magnification) because telangiectasias and dark dots were visible clinically.
CASE #1
FIGURE 1A
Basal cell carcinoma. Even with photography, which is often clearer than magnified clinical inspection, telangiectasias are not well seen in this basal cell carcinoma. A dark dot, however, is visible between the 12 and 1 o’clock positions.
FIGURE 1B
A dermoscopic view of the BCC in FIGURE 1A clearly shows telangiectasias and a dark dot.
CASE #2
FIGURE 2A
Ugly duckling sign. The circled lesion on this patient’s back wasn’t crusty or bleeding, but it drew attention because it was different from surrounding lesions. This has been called the “ugly duckling sign.”
FIGURE 2B
Dark dots. A closer look at the lesion on the patient’s back revealed multiple dark dots and telangiectasias, virtually diagnostic of basal cell carcinoma.
FIGURE 2C
A dermoscopic view of the lesion in FIGURE 2B adds little to what was well visualized clinically.
Caution: Context is critical
Because dots or blotches of pigment may occur in squamous cell carcinoma, pigmented actinic keratoses, seborrheic keratoses, and hypomelanotic or “amelanotic” melanoma, it is not possible clinically to confirm a diagnosis of BCC solely on the basis of dark dots. (In fact, even in the absence of pigment, any “red and white” lesion could be an amelanotic melanoma.) However, when added to other findings, such as a firm, pearly papule, the finding increases the likelihood of BCC.
How to proceed to biopsy
Lesions such as the ones I’ve shown should never be treated without preserving a specimen for pathology examination (eg, directly frozen). That said, there is no one right biopsy technique. The choice will depend on your clinical suspicion and perhaps other factors in your patient’s circumstance.
If you believe the lesion could be a cutaneous melanoma, the ideal option is full excision (to reduce sampling error pathologically), with a narrow margin (to preserve lymphatics, should sentinel node biopsy be considered later) and full thickness (to subcutaneous fat for prognostic staging; remember the dermis on the back is very thick). If you are confident the lesion is BCC, an initial shave biopsy preserves the option for treatment via curettage should the histology reveal superficial or nodular BCC. Pathology findings that indicate infiltrative, morpheaform, or micronodular BCC require excision; curettage is not adequate treatment for these lesions.
Treating the 2 patients
The patient in FIGURE 1A had no symptoms and no history of skin cancer. Because he was skeptical of the diagnosis, I performed a shave biopsy for histologic verification. The pathology report confirmed nodular BCC. We discussed options, and the patient elected excision.
In the case of the second lesion (FIGURE 2B), the diagnosis of BCC was fairly certain. Because of the patient’s advanced age, declining health, and difficulty arranging transportation, we decided to perform a primary excision at the outset. Had histology shown BCC with micronodular architecture or infitrative features, a shave biopsy for diagnosis, plus curettage, would not have been ideal treatment. Histology showed a nodular BCC.
Acknowledgements
The author thanks the St. Vincent Mercy Medical Center (Toledo) staff for its expert assistance.
Correspondence
Gary N. Fox, MD, Defiance Clinic, 1400 E 2nd Street, Defiance, OH 43512; [email protected]
1. Goldberg LH, Friedman RH, Silapunt S. Pigmented speckling as a sign of basal cell carcinoma. Dermatol Surg. 2004;30:1553-1555.
2. Bates B. A black dot appears to flag early basal cell carcinoma. Family Practice News. 2006;36(11):38.-Available at: http://www.familypracticenews.com/article/PIIS0300707306733047/fulltext. Accessed December 4, 2008.
3. Johr R, Soyer HP, Argenziano G, Hofmann-Wellenhof R, Scalvenzi M. Dermoscopy: The Essentials. New York: Mosby; 2004:107-117, 157.
4. Polsky D. Non-melanocytic lesions: pigmented basal cell carcinoma [Chapter 6a]. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:55-59.
5. Grob JJ, Bonerandi JJ. The ‘ugly duckling’ sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998;134:103-104.
Basal cell carcinoma (BCC) is the most common skin malignancy, but it can still be a tricky and difficult diagnosis even in the most experienced hands. Many BCCs exhibit only some of the defining clinical characteristics, and even when these findings are present, they can be hard to detect without excellent background and oblique lighting and handheld magnification that is 5-power or greater.
Most clinicians are familiar with classic BCC characteristics of pearliness, rolled edges, telangiectasia, bleeding, and crusting. However, the telangiectasias may be so fine that they are difficult or impossible to see, even with excellent lighting and magnification. FIGURE 1A illustrates a histologically verified nodular BCC with telangiectasias that are not readily visible, even with camera macro magnification.
Some BCC papules or plaques lack the classic rolled edges, even when viewed with oblique lighting and magnification. Some lesions may be more scaly than pearly, and others do not bleed and crust until later in their clinical course. With such variation in clinical presentation, all possible clues to BCC are welcome.
Dark dots: Connecting them to BCC
One underappreciated sign that may help with the diagnosis of BCC is the “dark dot sign.”1,2 Dermoscopists are familiar with patterns of pigmentation associated with BCCs, and introductory dermoscopy texts contain pictures of these patterns (blue/blue-gray blotches, maple leaf structures, and spoke-wheel structures).3,4 What is less well known is that this pigmentation may be visible on routine skin examination as “dark dots”—blue, gray, black, or a combination thereof, such as blue-gray. When visible clinically, these dark dots may bolster one’s confidence in the diagnosis—or, in my experience, occasionally even be the best visible hint for a tentative diagnosis of BCC.
Case in point. During a routine skin examination of a patient’s back, I identified an asymptomatic lesion the patient was unaware of, exhibiting no bleeding or crusting. It drew attention because it was different from surrounding lesions (FIGURE 2A), which has been called the “ugly duckling sign.”5 On closer examination, I noted the lesion had a pearly sheen, telangiectasias, and a plethora of dark dots (FIGURE 2B)—the poster child for the “dark dot sign.” Unlike this profound example, most lesions that have dark dots have just one or a few visible dots.
Now review FIGURE 1A again carefully. Above the lesion’s central crater there is an arcuate hemorrhagic crust, and, between the 12 and 1 o’clock positions, a small dark dot, which helps corroborate the clinical impression of BCC. FIGURE 1B is a dermoscopic image of the same lesion. It shows the dark dot well and illustrates the ease with which telangiectasias are seen on dermoscopy, lending a high degree of certainty to the diagnosis of BCC.
Dermoscopy (FIGURE 2C) for the lesion on the patient’s back in FIGURE 2B, however, adds nothing (beyond magnification) because telangiectasias and dark dots were visible clinically.
CASE #1
FIGURE 1A
Basal cell carcinoma. Even with photography, which is often clearer than magnified clinical inspection, telangiectasias are not well seen in this basal cell carcinoma. A dark dot, however, is visible between the 12 and 1 o’clock positions.
FIGURE 1B
A dermoscopic view of the BCC in FIGURE 1A clearly shows telangiectasias and a dark dot.
CASE #2
FIGURE 2A
Ugly duckling sign. The circled lesion on this patient’s back wasn’t crusty or bleeding, but it drew attention because it was different from surrounding lesions. This has been called the “ugly duckling sign.”
FIGURE 2B
Dark dots. A closer look at the lesion on the patient’s back revealed multiple dark dots and telangiectasias, virtually diagnostic of basal cell carcinoma.
FIGURE 2C
A dermoscopic view of the lesion in FIGURE 2B adds little to what was well visualized clinically.
Caution: Context is critical
Because dots or blotches of pigment may occur in squamous cell carcinoma, pigmented actinic keratoses, seborrheic keratoses, and hypomelanotic or “amelanotic” melanoma, it is not possible clinically to confirm a diagnosis of BCC solely on the basis of dark dots. (In fact, even in the absence of pigment, any “red and white” lesion could be an amelanotic melanoma.) However, when added to other findings, such as a firm, pearly papule, the finding increases the likelihood of BCC.
How to proceed to biopsy
Lesions such as the ones I’ve shown should never be treated without preserving a specimen for pathology examination (eg, directly frozen). That said, there is no one right biopsy technique. The choice will depend on your clinical suspicion and perhaps other factors in your patient’s circumstance.
If you believe the lesion could be a cutaneous melanoma, the ideal option is full excision (to reduce sampling error pathologically), with a narrow margin (to preserve lymphatics, should sentinel node biopsy be considered later) and full thickness (to subcutaneous fat for prognostic staging; remember the dermis on the back is very thick). If you are confident the lesion is BCC, an initial shave biopsy preserves the option for treatment via curettage should the histology reveal superficial or nodular BCC. Pathology findings that indicate infiltrative, morpheaform, or micronodular BCC require excision; curettage is not adequate treatment for these lesions.
Treating the 2 patients
The patient in FIGURE 1A had no symptoms and no history of skin cancer. Because he was skeptical of the diagnosis, I performed a shave biopsy for histologic verification. The pathology report confirmed nodular BCC. We discussed options, and the patient elected excision.
In the case of the second lesion (FIGURE 2B), the diagnosis of BCC was fairly certain. Because of the patient’s advanced age, declining health, and difficulty arranging transportation, we decided to perform a primary excision at the outset. Had histology shown BCC with micronodular architecture or infitrative features, a shave biopsy for diagnosis, plus curettage, would not have been ideal treatment. Histology showed a nodular BCC.
Acknowledgements
The author thanks the St. Vincent Mercy Medical Center (Toledo) staff for its expert assistance.
Correspondence
Gary N. Fox, MD, Defiance Clinic, 1400 E 2nd Street, Defiance, OH 43512; [email protected]
Basal cell carcinoma (BCC) is the most common skin malignancy, but it can still be a tricky and difficult diagnosis even in the most experienced hands. Many BCCs exhibit only some of the defining clinical characteristics, and even when these findings are present, they can be hard to detect without excellent background and oblique lighting and handheld magnification that is 5-power or greater.
Most clinicians are familiar with classic BCC characteristics of pearliness, rolled edges, telangiectasia, bleeding, and crusting. However, the telangiectasias may be so fine that they are difficult or impossible to see, even with excellent lighting and magnification. FIGURE 1A illustrates a histologically verified nodular BCC with telangiectasias that are not readily visible, even with camera macro magnification.
Some BCC papules or plaques lack the classic rolled edges, even when viewed with oblique lighting and magnification. Some lesions may be more scaly than pearly, and others do not bleed and crust until later in their clinical course. With such variation in clinical presentation, all possible clues to BCC are welcome.
Dark dots: Connecting them to BCC
One underappreciated sign that may help with the diagnosis of BCC is the “dark dot sign.”1,2 Dermoscopists are familiar with patterns of pigmentation associated with BCCs, and introductory dermoscopy texts contain pictures of these patterns (blue/blue-gray blotches, maple leaf structures, and spoke-wheel structures).3,4 What is less well known is that this pigmentation may be visible on routine skin examination as “dark dots”—blue, gray, black, or a combination thereof, such as blue-gray. When visible clinically, these dark dots may bolster one’s confidence in the diagnosis—or, in my experience, occasionally even be the best visible hint for a tentative diagnosis of BCC.
Case in point. During a routine skin examination of a patient’s back, I identified an asymptomatic lesion the patient was unaware of, exhibiting no bleeding or crusting. It drew attention because it was different from surrounding lesions (FIGURE 2A), which has been called the “ugly duckling sign.”5 On closer examination, I noted the lesion had a pearly sheen, telangiectasias, and a plethora of dark dots (FIGURE 2B)—the poster child for the “dark dot sign.” Unlike this profound example, most lesions that have dark dots have just one or a few visible dots.
Now review FIGURE 1A again carefully. Above the lesion’s central crater there is an arcuate hemorrhagic crust, and, between the 12 and 1 o’clock positions, a small dark dot, which helps corroborate the clinical impression of BCC. FIGURE 1B is a dermoscopic image of the same lesion. It shows the dark dot well and illustrates the ease with which telangiectasias are seen on dermoscopy, lending a high degree of certainty to the diagnosis of BCC.
Dermoscopy (FIGURE 2C) for the lesion on the patient’s back in FIGURE 2B, however, adds nothing (beyond magnification) because telangiectasias and dark dots were visible clinically.
CASE #1
FIGURE 1A
Basal cell carcinoma. Even with photography, which is often clearer than magnified clinical inspection, telangiectasias are not well seen in this basal cell carcinoma. A dark dot, however, is visible between the 12 and 1 o’clock positions.
FIGURE 1B
A dermoscopic view of the BCC in FIGURE 1A clearly shows telangiectasias and a dark dot.
CASE #2
FIGURE 2A
Ugly duckling sign. The circled lesion on this patient’s back wasn’t crusty or bleeding, but it drew attention because it was different from surrounding lesions. This has been called the “ugly duckling sign.”
FIGURE 2B
Dark dots. A closer look at the lesion on the patient’s back revealed multiple dark dots and telangiectasias, virtually diagnostic of basal cell carcinoma.
FIGURE 2C
A dermoscopic view of the lesion in FIGURE 2B adds little to what was well visualized clinically.
Caution: Context is critical
Because dots or blotches of pigment may occur in squamous cell carcinoma, pigmented actinic keratoses, seborrheic keratoses, and hypomelanotic or “amelanotic” melanoma, it is not possible clinically to confirm a diagnosis of BCC solely on the basis of dark dots. (In fact, even in the absence of pigment, any “red and white” lesion could be an amelanotic melanoma.) However, when added to other findings, such as a firm, pearly papule, the finding increases the likelihood of BCC.
How to proceed to biopsy
Lesions such as the ones I’ve shown should never be treated without preserving a specimen for pathology examination (eg, directly frozen). That said, there is no one right biopsy technique. The choice will depend on your clinical suspicion and perhaps other factors in your patient’s circumstance.
If you believe the lesion could be a cutaneous melanoma, the ideal option is full excision (to reduce sampling error pathologically), with a narrow margin (to preserve lymphatics, should sentinel node biopsy be considered later) and full thickness (to subcutaneous fat for prognostic staging; remember the dermis on the back is very thick). If you are confident the lesion is BCC, an initial shave biopsy preserves the option for treatment via curettage should the histology reveal superficial or nodular BCC. Pathology findings that indicate infiltrative, morpheaform, or micronodular BCC require excision; curettage is not adequate treatment for these lesions.
Treating the 2 patients
The patient in FIGURE 1A had no symptoms and no history of skin cancer. Because he was skeptical of the diagnosis, I performed a shave biopsy for histologic verification. The pathology report confirmed nodular BCC. We discussed options, and the patient elected excision.
In the case of the second lesion (FIGURE 2B), the diagnosis of BCC was fairly certain. Because of the patient’s advanced age, declining health, and difficulty arranging transportation, we decided to perform a primary excision at the outset. Had histology shown BCC with micronodular architecture or infitrative features, a shave biopsy for diagnosis, plus curettage, would not have been ideal treatment. Histology showed a nodular BCC.
Acknowledgements
The author thanks the St. Vincent Mercy Medical Center (Toledo) staff for its expert assistance.
Correspondence
Gary N. Fox, MD, Defiance Clinic, 1400 E 2nd Street, Defiance, OH 43512; [email protected]
1. Goldberg LH, Friedman RH, Silapunt S. Pigmented speckling as a sign of basal cell carcinoma. Dermatol Surg. 2004;30:1553-1555.
2. Bates B. A black dot appears to flag early basal cell carcinoma. Family Practice News. 2006;36(11):38.-Available at: http://www.familypracticenews.com/article/PIIS0300707306733047/fulltext. Accessed December 4, 2008.
3. Johr R, Soyer HP, Argenziano G, Hofmann-Wellenhof R, Scalvenzi M. Dermoscopy: The Essentials. New York: Mosby; 2004:107-117, 157.
4. Polsky D. Non-melanocytic lesions: pigmented basal cell carcinoma [Chapter 6a]. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:55-59.
5. Grob JJ, Bonerandi JJ. The ‘ugly duckling’ sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998;134:103-104.
1. Goldberg LH, Friedman RH, Silapunt S. Pigmented speckling as a sign of basal cell carcinoma. Dermatol Surg. 2004;30:1553-1555.
2. Bates B. A black dot appears to flag early basal cell carcinoma. Family Practice News. 2006;36(11):38.-Available at: http://www.familypracticenews.com/article/PIIS0300707306733047/fulltext. Accessed December 4, 2008.
3. Johr R, Soyer HP, Argenziano G, Hofmann-Wellenhof R, Scalvenzi M. Dermoscopy: The Essentials. New York: Mosby; 2004:107-117, 157.
4. Polsky D. Non-melanocytic lesions: pigmented basal cell carcinoma [Chapter 6a]. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:55-59.
5. Grob JJ, Bonerandi JJ. The ‘ugly duckling’ sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998;134:103-104.
Atrial fibrillation: Ways to refine your care
- Pursue a rate-control strategy for most patients with atrial fibrillation (AF); rhythm control may be preferable for younger (<65 years) symptomatic patients (A).
- Use a risk stratification scheme to guide decisions regarding anticoagulation therapy; adjusted-dose warfarin is extremely effective at preventing strokes in patients with AF (A).
- Hemodynamically unstable patients require urgent cardioversion, so you should not delay the procedure in order to provide anticoagulation therapy (C).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Atrial fibrillation (AF), the most common arrhythmia seen in clinical practice, affects an estimated 2.2 million American adults.1 The condition is associated with a 1.5- to 1.9-fold mortality risk independent of other risk factors2 and about a 4- to 5-fold increase in the risk of strokes.3 Achieving rate control; restoring or maintaining sinus rhythm, when it’s feasible; and preventing stroke are the primary goals in treating patients with AF. Yet many physicians are not always sure about the best ways to achieve them.
Failure to provide adequate anticoagulation therapy—despite clear evidence that anticoagulation significantly reduces the risk of thromboembolic complications—may be the most common misstep physicians make in treating AF.4 But anticoagulation is not the only trouble spot. Choosing between a rate-control and rhythm-control strategy also has its share of challenges, as does deciding which drugs are best for which patients.
AF is an age-related condition, with the prevalence increasing from 0.5% among individuals <60 years old to 9% of those >80.5 An aging population will make your ability to manage AF even more critical in the years ahead. The text and tables that follow will help you refine your care. But first, a quick review.
Classification, causes, and clinical features
AF is classified primarily on the basis of duration:
Paroxysmal AF is the term for brief episodes (lasting <24 hours) and episodes that last up to 7 days but terminate spontaneously. Cardioversion is not needed for this self-limiting condition.
Persistent AF lasts longer than 7 days, and often requires electrical or pharmacological cardioversion.
Permanent AF is used to describe instances in which cardioversion has failed (or has not been attempted) and the arrhythmia is continuous.
These categories are not mutually exclusive—a patient may primarily have paroxysmal AF, with an occasional episode of persistent AF. The term recurrent AF is used when 2 or more episodes of paroxysmal or persistent AF occur.
CHADS2 is a validated risk stratification scheme that offers help in making decisions about anticoagulation therapy. Each of the letters in this acronym represents a risk factor, and carries a certain number of points:
Congestive heart failure (1 point)
Hypertension (1 point)
Age >75 years (1 point)
Diabetes (1 point)
Stroke (2 points)
Patients with a score of ≥3 are at high risk and need to be treated with warfarin; those with a score of 0 are at low risk and can be managed with aspirin. For patients with a score of 1 or 2, the choice of warfarin or aspirin should be based on clinician assessment and patient preference.
Source: Gage BF, et al. JAMA. 2001.24
AF typically linked to heart conditions—but not always
Chronic cardiac conditions commonly associated with AF include ischemic heart disease, congestive heart failure (CHF), hypertension, and rheumatic mitral valve disease. Recurrent AF may also be associated with atrial flutter, Wolff-Parkinson-White (WPW) syndrome, or atrioventricular (AV) nodal reentrant tachycardia. It is essential to recognize the presence of such conditions, because treatment of the primary arrhythmia may reduce or eliminate the incidence of recurrent AF. 6
There are also noncardiac causes of AF—eg, excessive alcohol intake (“holiday heart syndrome”), pulmonary embolism and other pulmonary diseases, and hyperthyroidism and other metabolic disorders. Lone AF, a term used when the patient is younger than 60 years of age and has neither clinical nor echocardiographic evidence of cardiopulmonary disease, is a diagnosis of exclusion. About 30% to 45% of cases of paroxysmal AF and 20% to 25% of persistent AF are considered to be lone AF.1
EKG, x-ray, and echo: The role of each
Although some patients are asymptomatic, AF patients typically present with palpitations, dyspnea, fatigue, chest pain, or dizziness. A stroke may also be the first indication that a patient has AF.
A normal pulse rules out AF,7 and an irregular pulse should be an indication for an electrocardiogram (EKG). In most cases, a diagnosis can be made from the results of a 12-lead EKG. However, when diagnosis is uncertain or symptoms are paroxysmal, a Holter monitor or event recorder may be required.
Thyroid, renal, and hepatic function tests, serum electrolytes, and hemograms may help to rule out reversible causes of AF. Chest x-ray is valuable in diagnosing CHF, as well as lung pathology. Recent guidelines recommend that all patients who present with AF undergo echocardiography to evaluate for valvular heart disease, left and right atrial size, left ventricular size and function, left ventricular hypertrophy, and pericardial disease.1 Transesophageal echocardiogram (TEE) should be used to detect intracardiac clots in patients who have had an embolic event or when AF has lasted for more than 48 hours and cardioversion is being considered.
Rate vs rhythm control: What the research reveals
For hemodynamically unstable patients who present with AF and a rapid rate associated with cardiogenic shock, pulmonary edema, acute myocardial infarction, or unstable angina, urgent direct-current cardioversion is indicated. In less urgent cases, treatment is not so clear cut. Spontaneous conversion to sinus rhythm occurs in up to 60% of patients within 24 hours, and in about 80% of patients within 48 hours.8
Intuitively, restoring normal sinus rhythm seems superior to rate control, but several randomized trials9-12 and one meta-analysis13 found no support for that belief when researchers looked at mortality, thromboembolic events, and major hemorrhage.
One of the largest studies was the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM), which involved more than 4000 patients with paroxysmal and persistent AF who were randomized to either rate control or rhythm control.9 The research revealed a nonsignificant trend toward an increased death rate with the rhythm-control strategy—a 5-year mortality rate of 24% vs 21% for patients in the rate-control group. A trend toward higher risk of ischemic stroke, particularly associated with the lack of anticoagulation therapy, was also found in the rhythm-control group. That finding emphasizes the need for indefinite anticoagulation, independent of the use of a rate-control or rhythm-control approach.
A retrospective subanalysis of the AFFIRM trial that evaluated patients on the basis of a number of independent treatment variables found that sinus rhythm, in and of itself, was actually associated with a lower risk of death. But the antiarrhythmic agents that are often needed to achieve sinus rhythm are not associated with higher rates of survival. According to the researchers, this finding suggests that the drugs’ beneficial antiarrhythmic effects are offset by their adverse effects.14
Age is another confounding factor. Most of the AFFIRM subjects were relatively older, with a mean age of 69.7 years. In another study, rhythm control was found to be beneficial in young patients (mean age of 38.6 years) with AF and rheumatic valvular heart disease, in terms of morbidity and mortality.15
With no single treatment strategy emerging as the best approach, guidelines offer help in determining whether to pursue a rate-control or rhythm-control strategy for a particular patient. The recommendations of the British National Institute for Health and Clinical Excellence (NICE) guideline for AF,16 developed on the basis of a systematic literature review as well as expert consensus, are summarized here.
When should you opt for rate control?
The NICE guideline recommends rate control as the initial choice for patients who have persistent AF and:
- are >65 years of age
- have coronary artery disease
- do not have CHF
- are not candidates for cardioversion
- have contraindications to antiarrhythmic drugs.16
The American College of Cardiology (ACC), American Heart Association (AHA), and European Society of Cardiology (ESC) guidelines recommend maintaining a ventricular rate during AF of 60 to 80 beats per minute at rest and 90 to 115 beats per minute during exercise.1
Which drug for which patient?
Beta-blockers and nondihydropyridine calcium channel blockers (verapamil and diltiazem) and digoxin slow conduction through the AV node. Compared with placebo, beta-blockers and calcium channel blockers are effective for controlling the ventricular rate in patients with AF, both at rest and during exercise.17 In the AFFIRM trial, rate control was achieved in 70% of patients treated with beta-blockers vs 54% of patients taking calcium channel blockers.9
That said, the type of drug you use to achieve rate control should be an individual decision based on characteristics of your particular patient. In general, beta-blockers are preferable for patients with myocardial infarction or ischemia, and for any patient in a high adrenergic state, whereas calcium channel blockers should be used for patients with severe asthma or chronic obstructive pulmonary disease. Consider using digoxin for patients with CHF or hypotension, because both beta-blockers and calcium channel blockers can precipitate hemodynamic deterioration in these patients.
Digoxin has a relatively slow onset of action, however, and is less effective than beta-blockers or calcium channel blockers for rate control. What’s more, digoxin is ineffective for slowing the heart rate during exercise or in hyperadrenergic states. Thus, combination therapy will be needed to achieve adequate rate control in many cases.
Agents that predominantly block AV conduction, such as beta-blockers, calcium channel blockers, and digoxin, are contraindicated in patients with WPW syndrome and wide-complex ventricular response related to the preexcitation syndrome. That’s because these drugs can trigger an antegrade conduction along the accessory pathway.18 In this subset of patients, use a Class 1 antiarrhythmic such as flecainide or procainamide, or amiodarone for rate control1 (TABLE 1).
TABLE 1
Rate-control agents: A review of the options
| DRUG | LOADING DOSE (ONSET) | MAINTENANCE DOSE | MAJOR ADVERSE EFFECTS |
|---|---|---|---|
| Amiodarone* | IV: 150 mg over 10 min (days) | Acute care: 0.5-1 mg/min IV Outpatient: 200 mg/d oral | Hypotension, HB, bradycardia; pulmonary toxicity; skin discoloration, thyroid dysfunction; corneal deposits, optic neuropathy; warfarin interaction |
| Digoxin† | IV: 0.25 mg/2h, up to 1.5 mg (≥60 min) | Acute care: 0.125-0.375 mg/d IV or oral Outpatient: 0.125-0.375 mg/d oral | Digitalis toxicity, HB, bradycardia |
| Diltiazem | IV: 0.25 mg/kg over 2 min (2-7 min) | Acute care: 5-15 mg/h IV Outpatient: 120-360 mg/d oral in divided doses (slow release available) | Hypotension, HB, HF |
| Esmolol‡ | IV: 500 mcg/kg over 1 min (5 min) | Acute care: 60-200 mcg/kg per min IV | Hypotension, HB, HF, bradycardia; asthma |
| Metoprolol‡ | IV: 2.5-5 mg bolus over 2 min; up to 3 doses (5 min) | Outpatient: 25-100 mg BID oral | Hypotension, HB, HF, bradycardia; asthma |
| Propranolol‡ | IV: 0.15 mg/kg (5 min) | Outpatient: 80-240 mg/d oral in divided doses | Hypotension, HB, HF, bradycardia; asthma |
| Verapamil | IV: 0.075-0.15 mg/kg over 2 min (3-5 min) | Outpatient: 120-360 mg/d oral in divided doses (slow release available) | Hypotension, HB, HF; digoxin interaction |
| HB, heart block; HF, heart failure; IV, intravenous. | |||
| * Recommended for patients with accessory pathway and those with heart failure without accessory pathway; often useful when other measures are unsuccessful or contraindicated. | |||
| †For patients with heart failure without accessory pathway. | |||
| ‡The beta-blockers listed here are representative; other similar agents can also be used to achieve rate control. | |||
| Adapted from: Fuster V, et al. Circulation. 2006.1 | |||
When should you consider cardioversion?
The NICE guidelines recommend rhythm control as the initial choice for patients who:
- are symptomatic
- are <65 years old
- are presenting for the first time with lone AF or AF secondary to a condition that has been treated or corrected
- have CHF.16
While the guidelines recommend restoring sinus rhythm in patients with heart failure, a recent study suggests that rhythm control is no more effective for reducing the rate of death from cardiovascular causes compared to a rate-control strategy in this patient population.19 As with other aspects of AF management for which there is no definitive approach, individualized factors—including patient preference—should be your guide.
Electrical vs pharmacological cardioversion. Sinus rhythm can be established with electrical or pharmacological cardioversion. Electrical cardioversion, in which an external defibrillator delivers an electric shock that’s synchronized with the QRS complex, is usually well tolerated; embolization, pulmonary edema, and other arrhythmias are infrequent complications. Cardioversion with biphasic waveform defibrillation typically uses less energy and may have greater efficacy than monophasic waveforms.
The success rate of electrical cardioversion is higher than that of pharmacological cardioversion.8 But the use of electrical cardioversion is limited by the need for general anesthesia or conscious sedation for pain control. Pharmacological cardioversion is more effective for patients who have had AF for <48 hours; after that, the conversion rate drops considerably, and electrical cardioversion is often needed to restore sinus rhythm in a patient whose AF has lasted more than 7 days. A variety of antiarrhythmic drugs (TABLE 2), including propafenone, flecainide, ibutilide, and amiodarone, can be used to restore sinus rhythm. But because of the proarrhythmic potential of most of these agents, patients should be monitored in the hospital while drug therapy is initiated. After sinus rhythm is restored, maintenance therapy may be required.
Whether cardioversion is achieved by electrical or pharmacological means, it is associated with an increased risk of thromboembolism, especially in patients whose AF has persisted for >48 hours. Adequate anticoagulation with warfarin (international normalized rate of 2-3) should be achieved 3 weeks prior to cardioversion and continued for 4 weeks thereafter. Alternatively, excluding atrial thrombus by TEE paves the way for early cardioversion, using IV heparin or low-molecular-weight heparin for anticoagulation.
TABLE 2
Pharmacological cardioversion: Typical drugs and doses
| DRUG | ROUTE OF ADMINISTRATION | TYPICAL DOSAGE | POTENTIAL ADVERSE EFFECTS |
|---|---|---|---|
| Amiodarone | Oral | Inpatient: 1.2-1.8 g/d in divided dose to 10 g total, then 200-400 mg/d or 30 mg/kg as single dose | Hypotension, bradycardia, QT prolongation, torsades de pointes (rare); GI upset, constipation; phlebitis (IV) |
| IV | 5-7 mg/kg over 30-60 min, then 1.2-1.8 g/d continuous | ||
| Dofetilide | Oral | 125-500 mcg BID* | QT prolongation, torsades de pointes |
| Flecainide | Oral | 200-300 mg | Hypotension, atrial flutter with high ventricular rate |
| IV | 1.5-3 mg/kg over 10-20 min | ||
| Ibutilide | IV | 1 mg/10 min; repeat 1 mg PRN | QT prolongation, torsades de pointes |
| Propafenone | Oral | 600 mg | Hypotension, atrial flutter with high ventricular rate |
| IV | 1.5-2 mg/kg over 10-20 min | ||
| AF, atrial fibrillation; BID, twice a day; GI, gastrointestinal; IV, intravenous; PRN, as needed. | |||
| *Dosage adjusted based on renal function, body size, and age. | |||
| Adapted from: Fuster V, et al. Circulation. 2006.1 | |||
Maintaining sinus rhythm: Choosing the right drug
Without chronic antiarrhythmic therapy, only about 30% of patients with AF will remain in normal sinus rhythm after a year.20 Of the drugs that can be used to maintain sinus rhythm—amiodarone, disopyramide, flecainide, propafenone, and sotalol—amiodarone is the most effective. In the Canadian Trial of Atrial Fibrillation,21 403 patients treated with amiodarone, sotalol, or propafenone were followed for 16 months. The recurrence rate for the amiodarone group was 35%, compared with 63% for those being treated with sotalol or propafenone.
Adverse effects to consider
Amiodarone is less proarrhythmic than the other antiarrhythmic agents, but it is associated with serious noncardiac toxicities, including pulmonary, thyroid, neurologic, hepatic, optic, and dermatologic adverse effects. In addition, amiodarone can increase plasma levels of several drugs, including digoxin and warfarin, and periodic monitoring of the doses of these medications is essential. Adding enalapril, an angiotensin-converting enzyme inhibitor, or irbesartan, an angiotensin receptor blocker, can enhance the efficacy of amiodarone in maintaining normal sinus rhythm after cardioversion.
Thus, the choice of medication to maintain sinus rhythm should be individualized, based on the patient’s underlying cardiac condition and the safety profile of the antiarrhythmics being considered. (TABLE 3). The ACC/AHA/ESC guidelines recommend class 1C agents flecainide and propafenone as first-line therapy for maintaining sinus rhythm in patients with structurally normal hearts.1 But because of their proarrhythmic and negative ionotropic effects, class 1C agents should not be given to patients who have heart failure or ischemia. Amiodarone and dofetilide are the preferred agents for maintaining sinus rhythm in patients with heart failure and severe left ventricular hypertrophy, and dofetilide, amiodarone, and sotalol are best suited for patients with ischemic heart disease.
Pill-in-the-pocket. For selected patients with paroxysmal AF and a structurally normal heart, a “pill-in-the-pocket” strategy is an option—provided it has been tried in the hospital and proven to be safe. A patient using this strategy would self-administer a single dose of a class 1C antiarrhythmic agent—eg, 600 mg propafenone or 300 mg flecainide—at the onset of an acute episode of AF. Concomitant administration of a beta-blocker or calcium channel blocker is recommended to prevent development of atrial flutter with rapid AV conduction.
TABLE 3
Maintaining sinus rhythm in patients with AF
| DRUG | DAILY DOSE | INDICATION | POTENTIAL ADVERSE EFFECTS | COMMENTS |
|---|---|---|---|---|
| Amiodarone | 100-400 mg | Hypertension with LVH, impaired LV function, HF, ischemic heart disease | Photosensitivity, pulmonary toxicity, polyneuropathy, GI upset, bradycardia, torsades de pointes (rare), hepatic toxicity, thyroid dysfunction, eye complications | Use with care in patients with asthma or bradycardia. |
| Disopyramide | 400-750 mg | Asthma, thyroid disease | Torsades de pointes, HF, glaucoma, urinary retention, dry mouth | |
| Dofetilide | 500-1000 mcg | Cardiomyopathy, ischemic heart disease, significant LV dysfunction | QT prolongation, torsades de pointes | In inpatient setting, adjust dose for renal function and QT-interval response. Avoid in patients with renal failure. |
| Flecainide | 200-300 mg | First-line therapy for patients with a structurally normal heart | VT, HF, conversion to atrial flutter with rapid conduction through AV node | May be used in patients with asthma and thyroid disease. |
| Propafenone | 450-900 mg | First-line therapy for patients with a structurally normal heart | VT, HF, conversion to atrial flutter with rapid conduction through AV node | Use with care in patients with asthma or bradycardia. |
| Sotalol | 160-320 mg | Ischemic heart disease, thyroid disease | Torsades de pointes, HF, bradycardia, exacerbation of chronic obstructive or bronchospastic lung disease | In inpatient setting, adjust dose for renal function and QT-interval response. Avoid in patients with renal failure. |
| AF, atrial fibrillation; AV, atrioventricular; GI, gastrointestinal; HF, heart failure; LV, left ventricular; LVH, left ventricular hypertrophy; VT, ventricular tachycardia. | ||||
| Adapted from: Fuster V, et al. Circulation. 2006.1 | ||||
Using anticoagulation as prophylaxis
Judicious use of antithrombotic prophylaxis can significantly reduce the incidence of strokes associated with AF, regardless of whether you pursue a rate-control or rhythm-control strategy. Despite clear evidence of the efficacy of warfarin and aspirin in this patient population, anticoagulation remains underused in clinical practice.
If AF recurs or the patient develops chronic AF, the AFFIRM trial suggests the need for long-term anticoagulation for patients with thromboembolic risk factors.9
Adjusted-dose warfarin gets best results. A meta-analysis of 29 randomized trials from 1996 to 2007 involving 28,044 patients (mean age, 71 years; mean follow-up, 1.5 years) assessed the benefits of antithrombotic therapy for patients with AF.22 Compared with the controls, adjusted-dose warfarin (6 trials, 2900 participants) and antiplatelet agents (8 trials, 4876 participants) reduced stroke by 64% (95% confidence interval [CI], 49%-74%) and 22% (95% CI, 6%-35%), respectively.
Adjusted-dose warfarin was substantially more effective than antiplatelet therapy (12 trials, 12,963 participants; relative risk reduction, 39% [95% CI, 22%-52%]). The absolute risk reduction (ARR) with adjusted-dose warfarin in all strokes was 2.7% per year (number needed to treat [NNT] for 1 year to prevent 1 stroke was 37) for primary prevention and 8.4% per year (NNT, 12) for secondary prevention. Aspirin showed an ARR of 0.8% per year (NNT, 125) for primary prevention trials and 2.5% per year (NNT, 40) for secondary prevention trials. The absolute increase in major extracranial hemorrhage was small (≤0.3% per year).22
A recent Cochrane review of 8 randomized trials with a total of 9598 patients concluded that adjusted-dose warfarin reduces stroke and other major vascular events in patients with nonvalvular AF by about one third, compared with antiplatelet therapy alone.23
Warfarin or aspirin? Tools to help you decide
The risk of stroke varies considerably among patients with AF, depending on age and history of thromboembolic events, among other risk factors. What’s more, anticoagulation therapy carries an inherent risk of increased bleeding, making its use a complicated decision. A validated stroke risk stratification scheme like the CHADS2 can help.24 (See “Warfarin or aspirin? An anticoagulation risk tool”.)
The ACC/AHA/ESC guidelines recommend an alternate means of determining when anticoagulation is needed. The recommended risk stratification scheme divides risk factors for stroke into 3 categories:
- weak/less validated (female gender, age 65-74 years, coronary artery disease, thyrotoxicosis)
- moderate (≥75 years of age, hypertension, heart failure, LV ejection fraction ≤35%, diabetes mellitus)
- high (previous stroke, TIA, or embolism; mitral stenosis, prosthetic heart valve).
The guidelines recommend warfarin therapy for any patient with any high-risk factor or 2 or more moderate-risk factors; aspirin therapy for patients with no moderate- or high-risk factors; and aspirin or warfarin for patients with 1 moderate-risk factor.1
When conventional therapy fails
For patients who do not respond to conventional therapy, other options, including radiofrequency catheter ablation and pacemakers, may be effective in controlling symptoms and improving quality of life. In a recent RCT of 70 patients 18 to 75 years of age who experienced monthly symptomatic episodes of AF, the recurrence rate at the end of the 12-month follow-up was 13% after pulmonary vein isolation with radiofrequency ablation compared with 63% after treatment with antiarrhythmic drugs (P<.001). The rate of hospitalization was also significantly lower in the radiofrequency ablation group: 9% compared with 54% in the antiarrhythmia group (P<.001).25 Another option to consider for patients who require cardiac surgery for other reasons is left atrial appendage (LAA) occlusion or ligation at the time of surgery. This may prevent cardiac embolization, because the vast majority of thrombi in nonvalvular AF involve the LAA.
Correspondence
Shobha Rao, MD, University of Texas Southwestern Family Medicine Residency Program, 6263 Harry Hines Blvd., Clinical 1 Building, Dallas, TX 75390; [email protected].
1. Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257-354.
2. Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946-952.
3. Wolf PA, Abbot RD, Kannel WB. Atrial fibrillation as independent risk factor for stroke: the Framingham Study. Stroke. 1991;22;983-988.
4. Singer DE, Albers GW, Dalen JE, et al. Anti-thrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):s429S-s456.
5. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrilllation: population-based estimates. Am J Cardiol. 1998;82(8A):2N-9N.
6. Prystowsky EN. Tachycardia-induced tachycardia: a mechanism of initiation of atrial fibrillation. In: DiMarco JP, Prystowsky EN, eds. Atrial Arrhythmias: State of the Art. Armonk, NY: Futura Publishing; 1995:123-149.
7. Cooke G, Doust J, Sanders S. Is pulse palpation helpful in detecting atrial fibrillation? A systematic review. J Fam Pract. 2006;55:130-134.
8. Nattel S, Opie LH. Controversies in atrial fibrillation. Lancet. 2006;367:262-272.
9. Wyse DG, Waldo AL, DiMarco JP, et al. Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
10. Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789-1794.
11. Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690-1696.
12. Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
13. de Denus S, Sanoski CA, Carlsson J, et al. Rate vs rhythm control in patients with atrial fibrillation: a meta-analysis. Arch Intern Med. 2005;165:258-262.
14. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509-1513.
15. Vora A, Karnad D, Goyal V, et al. Control of rate versus rhythm in rheumatic atrial fibrillation: a randomized study. Indian Heart J. 2004;56:110-116.
16. National Institute for Health and Clinical Excellence. National Collaborating Centre for Chronic Conditions. Atrial Fibrillation: National Clinical Guideline for Management in Primary and Secondary Care. London: Royal College of Physicians, 2006.
17. McNamara RL, Bass EB, Miller MR, et al. Management of new onset atrial fibrillation. Evid Rep Technol Assess (Summ). 2000;May(12):1-7.
18. Prystowsky EN. Atrial fibrillation. In: Topol EJ, ed. Textbook of Cardiovascular Medicine. Philadelphia: Lippincott Williams & Wilkins;1998:1661.
19. Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667-2677.
20. Coplen SE, Antman E, Berlin JA, et al. Efficacy and safety of quinidine therapy for maintenance of sinus rhythm after cardioversion. A meta-analysis of randomized control trials. Circulation. 1990;82:1106-1116.
21. Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913-920.
22. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867.
23. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.-
24. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864-2870.
25. Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634-2640.
- Pursue a rate-control strategy for most patients with atrial fibrillation (AF); rhythm control may be preferable for younger (<65 years) symptomatic patients (A).
- Use a risk stratification scheme to guide decisions regarding anticoagulation therapy; adjusted-dose warfarin is extremely effective at preventing strokes in patients with AF (A).
- Hemodynamically unstable patients require urgent cardioversion, so you should not delay the procedure in order to provide anticoagulation therapy (C).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Atrial fibrillation (AF), the most common arrhythmia seen in clinical practice, affects an estimated 2.2 million American adults.1 The condition is associated with a 1.5- to 1.9-fold mortality risk independent of other risk factors2 and about a 4- to 5-fold increase in the risk of strokes.3 Achieving rate control; restoring or maintaining sinus rhythm, when it’s feasible; and preventing stroke are the primary goals in treating patients with AF. Yet many physicians are not always sure about the best ways to achieve them.
Failure to provide adequate anticoagulation therapy—despite clear evidence that anticoagulation significantly reduces the risk of thromboembolic complications—may be the most common misstep physicians make in treating AF.4 But anticoagulation is not the only trouble spot. Choosing between a rate-control and rhythm-control strategy also has its share of challenges, as does deciding which drugs are best for which patients.
AF is an age-related condition, with the prevalence increasing from 0.5% among individuals <60 years old to 9% of those >80.5 An aging population will make your ability to manage AF even more critical in the years ahead. The text and tables that follow will help you refine your care. But first, a quick review.
Classification, causes, and clinical features
AF is classified primarily on the basis of duration:
Paroxysmal AF is the term for brief episodes (lasting <24 hours) and episodes that last up to 7 days but terminate spontaneously. Cardioversion is not needed for this self-limiting condition.
Persistent AF lasts longer than 7 days, and often requires electrical or pharmacological cardioversion.
Permanent AF is used to describe instances in which cardioversion has failed (or has not been attempted) and the arrhythmia is continuous.
These categories are not mutually exclusive—a patient may primarily have paroxysmal AF, with an occasional episode of persistent AF. The term recurrent AF is used when 2 or more episodes of paroxysmal or persistent AF occur.
CHADS2 is a validated risk stratification scheme that offers help in making decisions about anticoagulation therapy. Each of the letters in this acronym represents a risk factor, and carries a certain number of points:
Congestive heart failure (1 point)
Hypertension (1 point)
Age >75 years (1 point)
Diabetes (1 point)
Stroke (2 points)
Patients with a score of ≥3 are at high risk and need to be treated with warfarin; those with a score of 0 are at low risk and can be managed with aspirin. For patients with a score of 1 or 2, the choice of warfarin or aspirin should be based on clinician assessment and patient preference.
Source: Gage BF, et al. JAMA. 2001.24
AF typically linked to heart conditions—but not always
Chronic cardiac conditions commonly associated with AF include ischemic heart disease, congestive heart failure (CHF), hypertension, and rheumatic mitral valve disease. Recurrent AF may also be associated with atrial flutter, Wolff-Parkinson-White (WPW) syndrome, or atrioventricular (AV) nodal reentrant tachycardia. It is essential to recognize the presence of such conditions, because treatment of the primary arrhythmia may reduce or eliminate the incidence of recurrent AF. 6
There are also noncardiac causes of AF—eg, excessive alcohol intake (“holiday heart syndrome”), pulmonary embolism and other pulmonary diseases, and hyperthyroidism and other metabolic disorders. Lone AF, a term used when the patient is younger than 60 years of age and has neither clinical nor echocardiographic evidence of cardiopulmonary disease, is a diagnosis of exclusion. About 30% to 45% of cases of paroxysmal AF and 20% to 25% of persistent AF are considered to be lone AF.1
EKG, x-ray, and echo: The role of each
Although some patients are asymptomatic, AF patients typically present with palpitations, dyspnea, fatigue, chest pain, or dizziness. A stroke may also be the first indication that a patient has AF.
A normal pulse rules out AF,7 and an irregular pulse should be an indication for an electrocardiogram (EKG). In most cases, a diagnosis can be made from the results of a 12-lead EKG. However, when diagnosis is uncertain or symptoms are paroxysmal, a Holter monitor or event recorder may be required.
Thyroid, renal, and hepatic function tests, serum electrolytes, and hemograms may help to rule out reversible causes of AF. Chest x-ray is valuable in diagnosing CHF, as well as lung pathology. Recent guidelines recommend that all patients who present with AF undergo echocardiography to evaluate for valvular heart disease, left and right atrial size, left ventricular size and function, left ventricular hypertrophy, and pericardial disease.1 Transesophageal echocardiogram (TEE) should be used to detect intracardiac clots in patients who have had an embolic event or when AF has lasted for more than 48 hours and cardioversion is being considered.
Rate vs rhythm control: What the research reveals
For hemodynamically unstable patients who present with AF and a rapid rate associated with cardiogenic shock, pulmonary edema, acute myocardial infarction, or unstable angina, urgent direct-current cardioversion is indicated. In less urgent cases, treatment is not so clear cut. Spontaneous conversion to sinus rhythm occurs in up to 60% of patients within 24 hours, and in about 80% of patients within 48 hours.8
Intuitively, restoring normal sinus rhythm seems superior to rate control, but several randomized trials9-12 and one meta-analysis13 found no support for that belief when researchers looked at mortality, thromboembolic events, and major hemorrhage.
One of the largest studies was the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM), which involved more than 4000 patients with paroxysmal and persistent AF who were randomized to either rate control or rhythm control.9 The research revealed a nonsignificant trend toward an increased death rate with the rhythm-control strategy—a 5-year mortality rate of 24% vs 21% for patients in the rate-control group. A trend toward higher risk of ischemic stroke, particularly associated with the lack of anticoagulation therapy, was also found in the rhythm-control group. That finding emphasizes the need for indefinite anticoagulation, independent of the use of a rate-control or rhythm-control approach.
A retrospective subanalysis of the AFFIRM trial that evaluated patients on the basis of a number of independent treatment variables found that sinus rhythm, in and of itself, was actually associated with a lower risk of death. But the antiarrhythmic agents that are often needed to achieve sinus rhythm are not associated with higher rates of survival. According to the researchers, this finding suggests that the drugs’ beneficial antiarrhythmic effects are offset by their adverse effects.14
Age is another confounding factor. Most of the AFFIRM subjects were relatively older, with a mean age of 69.7 years. In another study, rhythm control was found to be beneficial in young patients (mean age of 38.6 years) with AF and rheumatic valvular heart disease, in terms of morbidity and mortality.15
With no single treatment strategy emerging as the best approach, guidelines offer help in determining whether to pursue a rate-control or rhythm-control strategy for a particular patient. The recommendations of the British National Institute for Health and Clinical Excellence (NICE) guideline for AF,16 developed on the basis of a systematic literature review as well as expert consensus, are summarized here.
When should you opt for rate control?
The NICE guideline recommends rate control as the initial choice for patients who have persistent AF and:
- are >65 years of age
- have coronary artery disease
- do not have CHF
- are not candidates for cardioversion
- have contraindications to antiarrhythmic drugs.16
The American College of Cardiology (ACC), American Heart Association (AHA), and European Society of Cardiology (ESC) guidelines recommend maintaining a ventricular rate during AF of 60 to 80 beats per minute at rest and 90 to 115 beats per minute during exercise.1
Which drug for which patient?
Beta-blockers and nondihydropyridine calcium channel blockers (verapamil and diltiazem) and digoxin slow conduction through the AV node. Compared with placebo, beta-blockers and calcium channel blockers are effective for controlling the ventricular rate in patients with AF, both at rest and during exercise.17 In the AFFIRM trial, rate control was achieved in 70% of patients treated with beta-blockers vs 54% of patients taking calcium channel blockers.9
That said, the type of drug you use to achieve rate control should be an individual decision based on characteristics of your particular patient. In general, beta-blockers are preferable for patients with myocardial infarction or ischemia, and for any patient in a high adrenergic state, whereas calcium channel blockers should be used for patients with severe asthma or chronic obstructive pulmonary disease. Consider using digoxin for patients with CHF or hypotension, because both beta-blockers and calcium channel blockers can precipitate hemodynamic deterioration in these patients.
Digoxin has a relatively slow onset of action, however, and is less effective than beta-blockers or calcium channel blockers for rate control. What’s more, digoxin is ineffective for slowing the heart rate during exercise or in hyperadrenergic states. Thus, combination therapy will be needed to achieve adequate rate control in many cases.
Agents that predominantly block AV conduction, such as beta-blockers, calcium channel blockers, and digoxin, are contraindicated in patients with WPW syndrome and wide-complex ventricular response related to the preexcitation syndrome. That’s because these drugs can trigger an antegrade conduction along the accessory pathway.18 In this subset of patients, use a Class 1 antiarrhythmic such as flecainide or procainamide, or amiodarone for rate control1 (TABLE 1).
TABLE 1
Rate-control agents: A review of the options
| DRUG | LOADING DOSE (ONSET) | MAINTENANCE DOSE | MAJOR ADVERSE EFFECTS |
|---|---|---|---|
| Amiodarone* | IV: 150 mg over 10 min (days) | Acute care: 0.5-1 mg/min IV Outpatient: 200 mg/d oral | Hypotension, HB, bradycardia; pulmonary toxicity; skin discoloration, thyroid dysfunction; corneal deposits, optic neuropathy; warfarin interaction |
| Digoxin† | IV: 0.25 mg/2h, up to 1.5 mg (≥60 min) | Acute care: 0.125-0.375 mg/d IV or oral Outpatient: 0.125-0.375 mg/d oral | Digitalis toxicity, HB, bradycardia |
| Diltiazem | IV: 0.25 mg/kg over 2 min (2-7 min) | Acute care: 5-15 mg/h IV Outpatient: 120-360 mg/d oral in divided doses (slow release available) | Hypotension, HB, HF |
| Esmolol‡ | IV: 500 mcg/kg over 1 min (5 min) | Acute care: 60-200 mcg/kg per min IV | Hypotension, HB, HF, bradycardia; asthma |
| Metoprolol‡ | IV: 2.5-5 mg bolus over 2 min; up to 3 doses (5 min) | Outpatient: 25-100 mg BID oral | Hypotension, HB, HF, bradycardia; asthma |
| Propranolol‡ | IV: 0.15 mg/kg (5 min) | Outpatient: 80-240 mg/d oral in divided doses | Hypotension, HB, HF, bradycardia; asthma |
| Verapamil | IV: 0.075-0.15 mg/kg over 2 min (3-5 min) | Outpatient: 120-360 mg/d oral in divided doses (slow release available) | Hypotension, HB, HF; digoxin interaction |
| HB, heart block; HF, heart failure; IV, intravenous. | |||
| * Recommended for patients with accessory pathway and those with heart failure without accessory pathway; often useful when other measures are unsuccessful or contraindicated. | |||
| †For patients with heart failure without accessory pathway. | |||
| ‡The beta-blockers listed here are representative; other similar agents can also be used to achieve rate control. | |||
| Adapted from: Fuster V, et al. Circulation. 2006.1 | |||
When should you consider cardioversion?
The NICE guidelines recommend rhythm control as the initial choice for patients who:
- are symptomatic
- are <65 years old
- are presenting for the first time with lone AF or AF secondary to a condition that has been treated or corrected
- have CHF.16
While the guidelines recommend restoring sinus rhythm in patients with heart failure, a recent study suggests that rhythm control is no more effective for reducing the rate of death from cardiovascular causes compared to a rate-control strategy in this patient population.19 As with other aspects of AF management for which there is no definitive approach, individualized factors—including patient preference—should be your guide.
Electrical vs pharmacological cardioversion. Sinus rhythm can be established with electrical or pharmacological cardioversion. Electrical cardioversion, in which an external defibrillator delivers an electric shock that’s synchronized with the QRS complex, is usually well tolerated; embolization, pulmonary edema, and other arrhythmias are infrequent complications. Cardioversion with biphasic waveform defibrillation typically uses less energy and may have greater efficacy than monophasic waveforms.
The success rate of electrical cardioversion is higher than that of pharmacological cardioversion.8 But the use of electrical cardioversion is limited by the need for general anesthesia or conscious sedation for pain control. Pharmacological cardioversion is more effective for patients who have had AF for <48 hours; after that, the conversion rate drops considerably, and electrical cardioversion is often needed to restore sinus rhythm in a patient whose AF has lasted more than 7 days. A variety of antiarrhythmic drugs (TABLE 2), including propafenone, flecainide, ibutilide, and amiodarone, can be used to restore sinus rhythm. But because of the proarrhythmic potential of most of these agents, patients should be monitored in the hospital while drug therapy is initiated. After sinus rhythm is restored, maintenance therapy may be required.
Whether cardioversion is achieved by electrical or pharmacological means, it is associated with an increased risk of thromboembolism, especially in patients whose AF has persisted for >48 hours. Adequate anticoagulation with warfarin (international normalized rate of 2-3) should be achieved 3 weeks prior to cardioversion and continued for 4 weeks thereafter. Alternatively, excluding atrial thrombus by TEE paves the way for early cardioversion, using IV heparin or low-molecular-weight heparin for anticoagulation.
TABLE 2
Pharmacological cardioversion: Typical drugs and doses
| DRUG | ROUTE OF ADMINISTRATION | TYPICAL DOSAGE | POTENTIAL ADVERSE EFFECTS |
|---|---|---|---|
| Amiodarone | Oral | Inpatient: 1.2-1.8 g/d in divided dose to 10 g total, then 200-400 mg/d or 30 mg/kg as single dose | Hypotension, bradycardia, QT prolongation, torsades de pointes (rare); GI upset, constipation; phlebitis (IV) |
| IV | 5-7 mg/kg over 30-60 min, then 1.2-1.8 g/d continuous | ||
| Dofetilide | Oral | 125-500 mcg BID* | QT prolongation, torsades de pointes |
| Flecainide | Oral | 200-300 mg | Hypotension, atrial flutter with high ventricular rate |
| IV | 1.5-3 mg/kg over 10-20 min | ||
| Ibutilide | IV | 1 mg/10 min; repeat 1 mg PRN | QT prolongation, torsades de pointes |
| Propafenone | Oral | 600 mg | Hypotension, atrial flutter with high ventricular rate |
| IV | 1.5-2 mg/kg over 10-20 min | ||
| AF, atrial fibrillation; BID, twice a day; GI, gastrointestinal; IV, intravenous; PRN, as needed. | |||
| *Dosage adjusted based on renal function, body size, and age. | |||
| Adapted from: Fuster V, et al. Circulation. 2006.1 | |||
Maintaining sinus rhythm: Choosing the right drug
Without chronic antiarrhythmic therapy, only about 30% of patients with AF will remain in normal sinus rhythm after a year.20 Of the drugs that can be used to maintain sinus rhythm—amiodarone, disopyramide, flecainide, propafenone, and sotalol—amiodarone is the most effective. In the Canadian Trial of Atrial Fibrillation,21 403 patients treated with amiodarone, sotalol, or propafenone were followed for 16 months. The recurrence rate for the amiodarone group was 35%, compared with 63% for those being treated with sotalol or propafenone.
Adverse effects to consider
Amiodarone is less proarrhythmic than the other antiarrhythmic agents, but it is associated with serious noncardiac toxicities, including pulmonary, thyroid, neurologic, hepatic, optic, and dermatologic adverse effects. In addition, amiodarone can increase plasma levels of several drugs, including digoxin and warfarin, and periodic monitoring of the doses of these medications is essential. Adding enalapril, an angiotensin-converting enzyme inhibitor, or irbesartan, an angiotensin receptor blocker, can enhance the efficacy of amiodarone in maintaining normal sinus rhythm after cardioversion.
Thus, the choice of medication to maintain sinus rhythm should be individualized, based on the patient’s underlying cardiac condition and the safety profile of the antiarrhythmics being considered. (TABLE 3). The ACC/AHA/ESC guidelines recommend class 1C agents flecainide and propafenone as first-line therapy for maintaining sinus rhythm in patients with structurally normal hearts.1 But because of their proarrhythmic and negative ionotropic effects, class 1C agents should not be given to patients who have heart failure or ischemia. Amiodarone and dofetilide are the preferred agents for maintaining sinus rhythm in patients with heart failure and severe left ventricular hypertrophy, and dofetilide, amiodarone, and sotalol are best suited for patients with ischemic heart disease.
Pill-in-the-pocket. For selected patients with paroxysmal AF and a structurally normal heart, a “pill-in-the-pocket” strategy is an option—provided it has been tried in the hospital and proven to be safe. A patient using this strategy would self-administer a single dose of a class 1C antiarrhythmic agent—eg, 600 mg propafenone or 300 mg flecainide—at the onset of an acute episode of AF. Concomitant administration of a beta-blocker or calcium channel blocker is recommended to prevent development of atrial flutter with rapid AV conduction.
TABLE 3
Maintaining sinus rhythm in patients with AF
| DRUG | DAILY DOSE | INDICATION | POTENTIAL ADVERSE EFFECTS | COMMENTS |
|---|---|---|---|---|
| Amiodarone | 100-400 mg | Hypertension with LVH, impaired LV function, HF, ischemic heart disease | Photosensitivity, pulmonary toxicity, polyneuropathy, GI upset, bradycardia, torsades de pointes (rare), hepatic toxicity, thyroid dysfunction, eye complications | Use with care in patients with asthma or bradycardia. |
| Disopyramide | 400-750 mg | Asthma, thyroid disease | Torsades de pointes, HF, glaucoma, urinary retention, dry mouth | |
| Dofetilide | 500-1000 mcg | Cardiomyopathy, ischemic heart disease, significant LV dysfunction | QT prolongation, torsades de pointes | In inpatient setting, adjust dose for renal function and QT-interval response. Avoid in patients with renal failure. |
| Flecainide | 200-300 mg | First-line therapy for patients with a structurally normal heart | VT, HF, conversion to atrial flutter with rapid conduction through AV node | May be used in patients with asthma and thyroid disease. |
| Propafenone | 450-900 mg | First-line therapy for patients with a structurally normal heart | VT, HF, conversion to atrial flutter with rapid conduction through AV node | Use with care in patients with asthma or bradycardia. |
| Sotalol | 160-320 mg | Ischemic heart disease, thyroid disease | Torsades de pointes, HF, bradycardia, exacerbation of chronic obstructive or bronchospastic lung disease | In inpatient setting, adjust dose for renal function and QT-interval response. Avoid in patients with renal failure. |
| AF, atrial fibrillation; AV, atrioventricular; GI, gastrointestinal; HF, heart failure; LV, left ventricular; LVH, left ventricular hypertrophy; VT, ventricular tachycardia. | ||||
| Adapted from: Fuster V, et al. Circulation. 2006.1 | ||||
Using anticoagulation as prophylaxis
Judicious use of antithrombotic prophylaxis can significantly reduce the incidence of strokes associated with AF, regardless of whether you pursue a rate-control or rhythm-control strategy. Despite clear evidence of the efficacy of warfarin and aspirin in this patient population, anticoagulation remains underused in clinical practice.
If AF recurs or the patient develops chronic AF, the AFFIRM trial suggests the need for long-term anticoagulation for patients with thromboembolic risk factors.9
Adjusted-dose warfarin gets best results. A meta-analysis of 29 randomized trials from 1996 to 2007 involving 28,044 patients (mean age, 71 years; mean follow-up, 1.5 years) assessed the benefits of antithrombotic therapy for patients with AF.22 Compared with the controls, adjusted-dose warfarin (6 trials, 2900 participants) and antiplatelet agents (8 trials, 4876 participants) reduced stroke by 64% (95% confidence interval [CI], 49%-74%) and 22% (95% CI, 6%-35%), respectively.
Adjusted-dose warfarin was substantially more effective than antiplatelet therapy (12 trials, 12,963 participants; relative risk reduction, 39% [95% CI, 22%-52%]). The absolute risk reduction (ARR) with adjusted-dose warfarin in all strokes was 2.7% per year (number needed to treat [NNT] for 1 year to prevent 1 stroke was 37) for primary prevention and 8.4% per year (NNT, 12) for secondary prevention. Aspirin showed an ARR of 0.8% per year (NNT, 125) for primary prevention trials and 2.5% per year (NNT, 40) for secondary prevention trials. The absolute increase in major extracranial hemorrhage was small (≤0.3% per year).22
A recent Cochrane review of 8 randomized trials with a total of 9598 patients concluded that adjusted-dose warfarin reduces stroke and other major vascular events in patients with nonvalvular AF by about one third, compared with antiplatelet therapy alone.23
Warfarin or aspirin? Tools to help you decide
The risk of stroke varies considerably among patients with AF, depending on age and history of thromboembolic events, among other risk factors. What’s more, anticoagulation therapy carries an inherent risk of increased bleeding, making its use a complicated decision. A validated stroke risk stratification scheme like the CHADS2 can help.24 (See “Warfarin or aspirin? An anticoagulation risk tool”.)
The ACC/AHA/ESC guidelines recommend an alternate means of determining when anticoagulation is needed. The recommended risk stratification scheme divides risk factors for stroke into 3 categories:
- weak/less validated (female gender, age 65-74 years, coronary artery disease, thyrotoxicosis)
- moderate (≥75 years of age, hypertension, heart failure, LV ejection fraction ≤35%, diabetes mellitus)
- high (previous stroke, TIA, or embolism; mitral stenosis, prosthetic heart valve).
The guidelines recommend warfarin therapy for any patient with any high-risk factor or 2 or more moderate-risk factors; aspirin therapy for patients with no moderate- or high-risk factors; and aspirin or warfarin for patients with 1 moderate-risk factor.1
When conventional therapy fails
For patients who do not respond to conventional therapy, other options, including radiofrequency catheter ablation and pacemakers, may be effective in controlling symptoms and improving quality of life. In a recent RCT of 70 patients 18 to 75 years of age who experienced monthly symptomatic episodes of AF, the recurrence rate at the end of the 12-month follow-up was 13% after pulmonary vein isolation with radiofrequency ablation compared with 63% after treatment with antiarrhythmic drugs (P<.001). The rate of hospitalization was also significantly lower in the radiofrequency ablation group: 9% compared with 54% in the antiarrhythmia group (P<.001).25 Another option to consider for patients who require cardiac surgery for other reasons is left atrial appendage (LAA) occlusion or ligation at the time of surgery. This may prevent cardiac embolization, because the vast majority of thrombi in nonvalvular AF involve the LAA.
Correspondence
Shobha Rao, MD, University of Texas Southwestern Family Medicine Residency Program, 6263 Harry Hines Blvd., Clinical 1 Building, Dallas, TX 75390; [email protected].
- Pursue a rate-control strategy for most patients with atrial fibrillation (AF); rhythm control may be preferable for younger (<65 years) symptomatic patients (A).
- Use a risk stratification scheme to guide decisions regarding anticoagulation therapy; adjusted-dose warfarin is extremely effective at preventing strokes in patients with AF (A).
- Hemodynamically unstable patients require urgent cardioversion, so you should not delay the procedure in order to provide anticoagulation therapy (C).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Atrial fibrillation (AF), the most common arrhythmia seen in clinical practice, affects an estimated 2.2 million American adults.1 The condition is associated with a 1.5- to 1.9-fold mortality risk independent of other risk factors2 and about a 4- to 5-fold increase in the risk of strokes.3 Achieving rate control; restoring or maintaining sinus rhythm, when it’s feasible; and preventing stroke are the primary goals in treating patients with AF. Yet many physicians are not always sure about the best ways to achieve them.
Failure to provide adequate anticoagulation therapy—despite clear evidence that anticoagulation significantly reduces the risk of thromboembolic complications—may be the most common misstep physicians make in treating AF.4 But anticoagulation is not the only trouble spot. Choosing between a rate-control and rhythm-control strategy also has its share of challenges, as does deciding which drugs are best for which patients.
AF is an age-related condition, with the prevalence increasing from 0.5% among individuals <60 years old to 9% of those >80.5 An aging population will make your ability to manage AF even more critical in the years ahead. The text and tables that follow will help you refine your care. But first, a quick review.
Classification, causes, and clinical features
AF is classified primarily on the basis of duration:
Paroxysmal AF is the term for brief episodes (lasting <24 hours) and episodes that last up to 7 days but terminate spontaneously. Cardioversion is not needed for this self-limiting condition.
Persistent AF lasts longer than 7 days, and often requires electrical or pharmacological cardioversion.
Permanent AF is used to describe instances in which cardioversion has failed (or has not been attempted) and the arrhythmia is continuous.
These categories are not mutually exclusive—a patient may primarily have paroxysmal AF, with an occasional episode of persistent AF. The term recurrent AF is used when 2 or more episodes of paroxysmal or persistent AF occur.
CHADS2 is a validated risk stratification scheme that offers help in making decisions about anticoagulation therapy. Each of the letters in this acronym represents a risk factor, and carries a certain number of points:
Congestive heart failure (1 point)
Hypertension (1 point)
Age >75 years (1 point)
Diabetes (1 point)
Stroke (2 points)
Patients with a score of ≥3 are at high risk and need to be treated with warfarin; those with a score of 0 are at low risk and can be managed with aspirin. For patients with a score of 1 or 2, the choice of warfarin or aspirin should be based on clinician assessment and patient preference.
Source: Gage BF, et al. JAMA. 2001.24
AF typically linked to heart conditions—but not always
Chronic cardiac conditions commonly associated with AF include ischemic heart disease, congestive heart failure (CHF), hypertension, and rheumatic mitral valve disease. Recurrent AF may also be associated with atrial flutter, Wolff-Parkinson-White (WPW) syndrome, or atrioventricular (AV) nodal reentrant tachycardia. It is essential to recognize the presence of such conditions, because treatment of the primary arrhythmia may reduce or eliminate the incidence of recurrent AF. 6
There are also noncardiac causes of AF—eg, excessive alcohol intake (“holiday heart syndrome”), pulmonary embolism and other pulmonary diseases, and hyperthyroidism and other metabolic disorders. Lone AF, a term used when the patient is younger than 60 years of age and has neither clinical nor echocardiographic evidence of cardiopulmonary disease, is a diagnosis of exclusion. About 30% to 45% of cases of paroxysmal AF and 20% to 25% of persistent AF are considered to be lone AF.1
EKG, x-ray, and echo: The role of each
Although some patients are asymptomatic, AF patients typically present with palpitations, dyspnea, fatigue, chest pain, or dizziness. A stroke may also be the first indication that a patient has AF.
A normal pulse rules out AF,7 and an irregular pulse should be an indication for an electrocardiogram (EKG). In most cases, a diagnosis can be made from the results of a 12-lead EKG. However, when diagnosis is uncertain or symptoms are paroxysmal, a Holter monitor or event recorder may be required.
Thyroid, renal, and hepatic function tests, serum electrolytes, and hemograms may help to rule out reversible causes of AF. Chest x-ray is valuable in diagnosing CHF, as well as lung pathology. Recent guidelines recommend that all patients who present with AF undergo echocardiography to evaluate for valvular heart disease, left and right atrial size, left ventricular size and function, left ventricular hypertrophy, and pericardial disease.1 Transesophageal echocardiogram (TEE) should be used to detect intracardiac clots in patients who have had an embolic event or when AF has lasted for more than 48 hours and cardioversion is being considered.
Rate vs rhythm control: What the research reveals
For hemodynamically unstable patients who present with AF and a rapid rate associated with cardiogenic shock, pulmonary edema, acute myocardial infarction, or unstable angina, urgent direct-current cardioversion is indicated. In less urgent cases, treatment is not so clear cut. Spontaneous conversion to sinus rhythm occurs in up to 60% of patients within 24 hours, and in about 80% of patients within 48 hours.8
Intuitively, restoring normal sinus rhythm seems superior to rate control, but several randomized trials9-12 and one meta-analysis13 found no support for that belief when researchers looked at mortality, thromboembolic events, and major hemorrhage.
One of the largest studies was the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM), which involved more than 4000 patients with paroxysmal and persistent AF who were randomized to either rate control or rhythm control.9 The research revealed a nonsignificant trend toward an increased death rate with the rhythm-control strategy—a 5-year mortality rate of 24% vs 21% for patients in the rate-control group. A trend toward higher risk of ischemic stroke, particularly associated with the lack of anticoagulation therapy, was also found in the rhythm-control group. That finding emphasizes the need for indefinite anticoagulation, independent of the use of a rate-control or rhythm-control approach.
A retrospective subanalysis of the AFFIRM trial that evaluated patients on the basis of a number of independent treatment variables found that sinus rhythm, in and of itself, was actually associated with a lower risk of death. But the antiarrhythmic agents that are often needed to achieve sinus rhythm are not associated with higher rates of survival. According to the researchers, this finding suggests that the drugs’ beneficial antiarrhythmic effects are offset by their adverse effects.14
Age is another confounding factor. Most of the AFFIRM subjects were relatively older, with a mean age of 69.7 years. In another study, rhythm control was found to be beneficial in young patients (mean age of 38.6 years) with AF and rheumatic valvular heart disease, in terms of morbidity and mortality.15
With no single treatment strategy emerging as the best approach, guidelines offer help in determining whether to pursue a rate-control or rhythm-control strategy for a particular patient. The recommendations of the British National Institute for Health and Clinical Excellence (NICE) guideline for AF,16 developed on the basis of a systematic literature review as well as expert consensus, are summarized here.
When should you opt for rate control?
The NICE guideline recommends rate control as the initial choice for patients who have persistent AF and:
- are >65 years of age
- have coronary artery disease
- do not have CHF
- are not candidates for cardioversion
- have contraindications to antiarrhythmic drugs.16
The American College of Cardiology (ACC), American Heart Association (AHA), and European Society of Cardiology (ESC) guidelines recommend maintaining a ventricular rate during AF of 60 to 80 beats per minute at rest and 90 to 115 beats per minute during exercise.1
Which drug for which patient?
Beta-blockers and nondihydropyridine calcium channel blockers (verapamil and diltiazem) and digoxin slow conduction through the AV node. Compared with placebo, beta-blockers and calcium channel blockers are effective for controlling the ventricular rate in patients with AF, both at rest and during exercise.17 In the AFFIRM trial, rate control was achieved in 70% of patients treated with beta-blockers vs 54% of patients taking calcium channel blockers.9
That said, the type of drug you use to achieve rate control should be an individual decision based on characteristics of your particular patient. In general, beta-blockers are preferable for patients with myocardial infarction or ischemia, and for any patient in a high adrenergic state, whereas calcium channel blockers should be used for patients with severe asthma or chronic obstructive pulmonary disease. Consider using digoxin for patients with CHF or hypotension, because both beta-blockers and calcium channel blockers can precipitate hemodynamic deterioration in these patients.
Digoxin has a relatively slow onset of action, however, and is less effective than beta-blockers or calcium channel blockers for rate control. What’s more, digoxin is ineffective for slowing the heart rate during exercise or in hyperadrenergic states. Thus, combination therapy will be needed to achieve adequate rate control in many cases.
Agents that predominantly block AV conduction, such as beta-blockers, calcium channel blockers, and digoxin, are contraindicated in patients with WPW syndrome and wide-complex ventricular response related to the preexcitation syndrome. That’s because these drugs can trigger an antegrade conduction along the accessory pathway.18 In this subset of patients, use a Class 1 antiarrhythmic such as flecainide or procainamide, or amiodarone for rate control1 (TABLE 1).
TABLE 1
Rate-control agents: A review of the options
| DRUG | LOADING DOSE (ONSET) | MAINTENANCE DOSE | MAJOR ADVERSE EFFECTS |
|---|---|---|---|
| Amiodarone* | IV: 150 mg over 10 min (days) | Acute care: 0.5-1 mg/min IV Outpatient: 200 mg/d oral | Hypotension, HB, bradycardia; pulmonary toxicity; skin discoloration, thyroid dysfunction; corneal deposits, optic neuropathy; warfarin interaction |
| Digoxin† | IV: 0.25 mg/2h, up to 1.5 mg (≥60 min) | Acute care: 0.125-0.375 mg/d IV or oral Outpatient: 0.125-0.375 mg/d oral | Digitalis toxicity, HB, bradycardia |
| Diltiazem | IV: 0.25 mg/kg over 2 min (2-7 min) | Acute care: 5-15 mg/h IV Outpatient: 120-360 mg/d oral in divided doses (slow release available) | Hypotension, HB, HF |
| Esmolol‡ | IV: 500 mcg/kg over 1 min (5 min) | Acute care: 60-200 mcg/kg per min IV | Hypotension, HB, HF, bradycardia; asthma |
| Metoprolol‡ | IV: 2.5-5 mg bolus over 2 min; up to 3 doses (5 min) | Outpatient: 25-100 mg BID oral | Hypotension, HB, HF, bradycardia; asthma |
| Propranolol‡ | IV: 0.15 mg/kg (5 min) | Outpatient: 80-240 mg/d oral in divided doses | Hypotension, HB, HF, bradycardia; asthma |
| Verapamil | IV: 0.075-0.15 mg/kg over 2 min (3-5 min) | Outpatient: 120-360 mg/d oral in divided doses (slow release available) | Hypotension, HB, HF; digoxin interaction |
| HB, heart block; HF, heart failure; IV, intravenous. | |||
| * Recommended for patients with accessory pathway and those with heart failure without accessory pathway; often useful when other measures are unsuccessful or contraindicated. | |||
| †For patients with heart failure without accessory pathway. | |||
| ‡The beta-blockers listed here are representative; other similar agents can also be used to achieve rate control. | |||
| Adapted from: Fuster V, et al. Circulation. 2006.1 | |||
When should you consider cardioversion?
The NICE guidelines recommend rhythm control as the initial choice for patients who:
- are symptomatic
- are <65 years old
- are presenting for the first time with lone AF or AF secondary to a condition that has been treated or corrected
- have CHF.16
While the guidelines recommend restoring sinus rhythm in patients with heart failure, a recent study suggests that rhythm control is no more effective for reducing the rate of death from cardiovascular causes compared to a rate-control strategy in this patient population.19 As with other aspects of AF management for which there is no definitive approach, individualized factors—including patient preference—should be your guide.
Electrical vs pharmacological cardioversion. Sinus rhythm can be established with electrical or pharmacological cardioversion. Electrical cardioversion, in which an external defibrillator delivers an electric shock that’s synchronized with the QRS complex, is usually well tolerated; embolization, pulmonary edema, and other arrhythmias are infrequent complications. Cardioversion with biphasic waveform defibrillation typically uses less energy and may have greater efficacy than monophasic waveforms.
The success rate of electrical cardioversion is higher than that of pharmacological cardioversion.8 But the use of electrical cardioversion is limited by the need for general anesthesia or conscious sedation for pain control. Pharmacological cardioversion is more effective for patients who have had AF for <48 hours; after that, the conversion rate drops considerably, and electrical cardioversion is often needed to restore sinus rhythm in a patient whose AF has lasted more than 7 days. A variety of antiarrhythmic drugs (TABLE 2), including propafenone, flecainide, ibutilide, and amiodarone, can be used to restore sinus rhythm. But because of the proarrhythmic potential of most of these agents, patients should be monitored in the hospital while drug therapy is initiated. After sinus rhythm is restored, maintenance therapy may be required.
Whether cardioversion is achieved by electrical or pharmacological means, it is associated with an increased risk of thromboembolism, especially in patients whose AF has persisted for >48 hours. Adequate anticoagulation with warfarin (international normalized rate of 2-3) should be achieved 3 weeks prior to cardioversion and continued for 4 weeks thereafter. Alternatively, excluding atrial thrombus by TEE paves the way for early cardioversion, using IV heparin or low-molecular-weight heparin for anticoagulation.
TABLE 2
Pharmacological cardioversion: Typical drugs and doses
| DRUG | ROUTE OF ADMINISTRATION | TYPICAL DOSAGE | POTENTIAL ADVERSE EFFECTS |
|---|---|---|---|
| Amiodarone | Oral | Inpatient: 1.2-1.8 g/d in divided dose to 10 g total, then 200-400 mg/d or 30 mg/kg as single dose | Hypotension, bradycardia, QT prolongation, torsades de pointes (rare); GI upset, constipation; phlebitis (IV) |
| IV | 5-7 mg/kg over 30-60 min, then 1.2-1.8 g/d continuous | ||
| Dofetilide | Oral | 125-500 mcg BID* | QT prolongation, torsades de pointes |
| Flecainide | Oral | 200-300 mg | Hypotension, atrial flutter with high ventricular rate |
| IV | 1.5-3 mg/kg over 10-20 min | ||
| Ibutilide | IV | 1 mg/10 min; repeat 1 mg PRN | QT prolongation, torsades de pointes |
| Propafenone | Oral | 600 mg | Hypotension, atrial flutter with high ventricular rate |
| IV | 1.5-2 mg/kg over 10-20 min | ||
| AF, atrial fibrillation; BID, twice a day; GI, gastrointestinal; IV, intravenous; PRN, as needed. | |||
| *Dosage adjusted based on renal function, body size, and age. | |||
| Adapted from: Fuster V, et al. Circulation. 2006.1 | |||
Maintaining sinus rhythm: Choosing the right drug
Without chronic antiarrhythmic therapy, only about 30% of patients with AF will remain in normal sinus rhythm after a year.20 Of the drugs that can be used to maintain sinus rhythm—amiodarone, disopyramide, flecainide, propafenone, and sotalol—amiodarone is the most effective. In the Canadian Trial of Atrial Fibrillation,21 403 patients treated with amiodarone, sotalol, or propafenone were followed for 16 months. The recurrence rate for the amiodarone group was 35%, compared with 63% for those being treated with sotalol or propafenone.
Adverse effects to consider
Amiodarone is less proarrhythmic than the other antiarrhythmic agents, but it is associated with serious noncardiac toxicities, including pulmonary, thyroid, neurologic, hepatic, optic, and dermatologic adverse effects. In addition, amiodarone can increase plasma levels of several drugs, including digoxin and warfarin, and periodic monitoring of the doses of these medications is essential. Adding enalapril, an angiotensin-converting enzyme inhibitor, or irbesartan, an angiotensin receptor blocker, can enhance the efficacy of amiodarone in maintaining normal sinus rhythm after cardioversion.
Thus, the choice of medication to maintain sinus rhythm should be individualized, based on the patient’s underlying cardiac condition and the safety profile of the antiarrhythmics being considered. (TABLE 3). The ACC/AHA/ESC guidelines recommend class 1C agents flecainide and propafenone as first-line therapy for maintaining sinus rhythm in patients with structurally normal hearts.1 But because of their proarrhythmic and negative ionotropic effects, class 1C agents should not be given to patients who have heart failure or ischemia. Amiodarone and dofetilide are the preferred agents for maintaining sinus rhythm in patients with heart failure and severe left ventricular hypertrophy, and dofetilide, amiodarone, and sotalol are best suited for patients with ischemic heart disease.
Pill-in-the-pocket. For selected patients with paroxysmal AF and a structurally normal heart, a “pill-in-the-pocket” strategy is an option—provided it has been tried in the hospital and proven to be safe. A patient using this strategy would self-administer a single dose of a class 1C antiarrhythmic agent—eg, 600 mg propafenone or 300 mg flecainide—at the onset of an acute episode of AF. Concomitant administration of a beta-blocker or calcium channel blocker is recommended to prevent development of atrial flutter with rapid AV conduction.
TABLE 3
Maintaining sinus rhythm in patients with AF
| DRUG | DAILY DOSE | INDICATION | POTENTIAL ADVERSE EFFECTS | COMMENTS |
|---|---|---|---|---|
| Amiodarone | 100-400 mg | Hypertension with LVH, impaired LV function, HF, ischemic heart disease | Photosensitivity, pulmonary toxicity, polyneuropathy, GI upset, bradycardia, torsades de pointes (rare), hepatic toxicity, thyroid dysfunction, eye complications | Use with care in patients with asthma or bradycardia. |
| Disopyramide | 400-750 mg | Asthma, thyroid disease | Torsades de pointes, HF, glaucoma, urinary retention, dry mouth | |
| Dofetilide | 500-1000 mcg | Cardiomyopathy, ischemic heart disease, significant LV dysfunction | QT prolongation, torsades de pointes | In inpatient setting, adjust dose for renal function and QT-interval response. Avoid in patients with renal failure. |
| Flecainide | 200-300 mg | First-line therapy for patients with a structurally normal heart | VT, HF, conversion to atrial flutter with rapid conduction through AV node | May be used in patients with asthma and thyroid disease. |
| Propafenone | 450-900 mg | First-line therapy for patients with a structurally normal heart | VT, HF, conversion to atrial flutter with rapid conduction through AV node | Use with care in patients with asthma or bradycardia. |
| Sotalol | 160-320 mg | Ischemic heart disease, thyroid disease | Torsades de pointes, HF, bradycardia, exacerbation of chronic obstructive or bronchospastic lung disease | In inpatient setting, adjust dose for renal function and QT-interval response. Avoid in patients with renal failure. |
| AF, atrial fibrillation; AV, atrioventricular; GI, gastrointestinal; HF, heart failure; LV, left ventricular; LVH, left ventricular hypertrophy; VT, ventricular tachycardia. | ||||
| Adapted from: Fuster V, et al. Circulation. 2006.1 | ||||
Using anticoagulation as prophylaxis
Judicious use of antithrombotic prophylaxis can significantly reduce the incidence of strokes associated with AF, regardless of whether you pursue a rate-control or rhythm-control strategy. Despite clear evidence of the efficacy of warfarin and aspirin in this patient population, anticoagulation remains underused in clinical practice.
If AF recurs or the patient develops chronic AF, the AFFIRM trial suggests the need for long-term anticoagulation for patients with thromboembolic risk factors.9
Adjusted-dose warfarin gets best results. A meta-analysis of 29 randomized trials from 1996 to 2007 involving 28,044 patients (mean age, 71 years; mean follow-up, 1.5 years) assessed the benefits of antithrombotic therapy for patients with AF.22 Compared with the controls, adjusted-dose warfarin (6 trials, 2900 participants) and antiplatelet agents (8 trials, 4876 participants) reduced stroke by 64% (95% confidence interval [CI], 49%-74%) and 22% (95% CI, 6%-35%), respectively.
Adjusted-dose warfarin was substantially more effective than antiplatelet therapy (12 trials, 12,963 participants; relative risk reduction, 39% [95% CI, 22%-52%]). The absolute risk reduction (ARR) with adjusted-dose warfarin in all strokes was 2.7% per year (number needed to treat [NNT] for 1 year to prevent 1 stroke was 37) for primary prevention and 8.4% per year (NNT, 12) for secondary prevention. Aspirin showed an ARR of 0.8% per year (NNT, 125) for primary prevention trials and 2.5% per year (NNT, 40) for secondary prevention trials. The absolute increase in major extracranial hemorrhage was small (≤0.3% per year).22
A recent Cochrane review of 8 randomized trials with a total of 9598 patients concluded that adjusted-dose warfarin reduces stroke and other major vascular events in patients with nonvalvular AF by about one third, compared with antiplatelet therapy alone.23
Warfarin or aspirin? Tools to help you decide
The risk of stroke varies considerably among patients with AF, depending on age and history of thromboembolic events, among other risk factors. What’s more, anticoagulation therapy carries an inherent risk of increased bleeding, making its use a complicated decision. A validated stroke risk stratification scheme like the CHADS2 can help.24 (See “Warfarin or aspirin? An anticoagulation risk tool”.)
The ACC/AHA/ESC guidelines recommend an alternate means of determining when anticoagulation is needed. The recommended risk stratification scheme divides risk factors for stroke into 3 categories:
- weak/less validated (female gender, age 65-74 years, coronary artery disease, thyrotoxicosis)
- moderate (≥75 years of age, hypertension, heart failure, LV ejection fraction ≤35%, diabetes mellitus)
- high (previous stroke, TIA, or embolism; mitral stenosis, prosthetic heart valve).
The guidelines recommend warfarin therapy for any patient with any high-risk factor or 2 or more moderate-risk factors; aspirin therapy for patients with no moderate- or high-risk factors; and aspirin or warfarin for patients with 1 moderate-risk factor.1
When conventional therapy fails
For patients who do not respond to conventional therapy, other options, including radiofrequency catheter ablation and pacemakers, may be effective in controlling symptoms and improving quality of life. In a recent RCT of 70 patients 18 to 75 years of age who experienced monthly symptomatic episodes of AF, the recurrence rate at the end of the 12-month follow-up was 13% after pulmonary vein isolation with radiofrequency ablation compared with 63% after treatment with antiarrhythmic drugs (P<.001). The rate of hospitalization was also significantly lower in the radiofrequency ablation group: 9% compared with 54% in the antiarrhythmia group (P<.001).25 Another option to consider for patients who require cardiac surgery for other reasons is left atrial appendage (LAA) occlusion or ligation at the time of surgery. This may prevent cardiac embolization, because the vast majority of thrombi in nonvalvular AF involve the LAA.
Correspondence
Shobha Rao, MD, University of Texas Southwestern Family Medicine Residency Program, 6263 Harry Hines Blvd., Clinical 1 Building, Dallas, TX 75390; [email protected].
1. Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257-354.
2. Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946-952.
3. Wolf PA, Abbot RD, Kannel WB. Atrial fibrillation as independent risk factor for stroke: the Framingham Study. Stroke. 1991;22;983-988.
4. Singer DE, Albers GW, Dalen JE, et al. Anti-thrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):s429S-s456.
5. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrilllation: population-based estimates. Am J Cardiol. 1998;82(8A):2N-9N.
6. Prystowsky EN. Tachycardia-induced tachycardia: a mechanism of initiation of atrial fibrillation. In: DiMarco JP, Prystowsky EN, eds. Atrial Arrhythmias: State of the Art. Armonk, NY: Futura Publishing; 1995:123-149.
7. Cooke G, Doust J, Sanders S. Is pulse palpation helpful in detecting atrial fibrillation? A systematic review. J Fam Pract. 2006;55:130-134.
8. Nattel S, Opie LH. Controversies in atrial fibrillation. Lancet. 2006;367:262-272.
9. Wyse DG, Waldo AL, DiMarco JP, et al. Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
10. Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789-1794.
11. Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690-1696.
12. Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
13. de Denus S, Sanoski CA, Carlsson J, et al. Rate vs rhythm control in patients with atrial fibrillation: a meta-analysis. Arch Intern Med. 2005;165:258-262.
14. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509-1513.
15. Vora A, Karnad D, Goyal V, et al. Control of rate versus rhythm in rheumatic atrial fibrillation: a randomized study. Indian Heart J. 2004;56:110-116.
16. National Institute for Health and Clinical Excellence. National Collaborating Centre for Chronic Conditions. Atrial Fibrillation: National Clinical Guideline for Management in Primary and Secondary Care. London: Royal College of Physicians, 2006.
17. McNamara RL, Bass EB, Miller MR, et al. Management of new onset atrial fibrillation. Evid Rep Technol Assess (Summ). 2000;May(12):1-7.
18. Prystowsky EN. Atrial fibrillation. In: Topol EJ, ed. Textbook of Cardiovascular Medicine. Philadelphia: Lippincott Williams & Wilkins;1998:1661.
19. Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667-2677.
20. Coplen SE, Antman E, Berlin JA, et al. Efficacy and safety of quinidine therapy for maintenance of sinus rhythm after cardioversion. A meta-analysis of randomized control trials. Circulation. 1990;82:1106-1116.
21. Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913-920.
22. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867.
23. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.-
24. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864-2870.
25. Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634-2640.
1. Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257-354.
2. Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946-952.
3. Wolf PA, Abbot RD, Kannel WB. Atrial fibrillation as independent risk factor for stroke: the Framingham Study. Stroke. 1991;22;983-988.
4. Singer DE, Albers GW, Dalen JE, et al. Anti-thrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):s429S-s456.
5. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrilllation: population-based estimates. Am J Cardiol. 1998;82(8A):2N-9N.
6. Prystowsky EN. Tachycardia-induced tachycardia: a mechanism of initiation of atrial fibrillation. In: DiMarco JP, Prystowsky EN, eds. Atrial Arrhythmias: State of the Art. Armonk, NY: Futura Publishing; 1995:123-149.
7. Cooke G, Doust J, Sanders S. Is pulse palpation helpful in detecting atrial fibrillation? A systematic review. J Fam Pract. 2006;55:130-134.
8. Nattel S, Opie LH. Controversies in atrial fibrillation. Lancet. 2006;367:262-272.
9. Wyse DG, Waldo AL, DiMarco JP, et al. Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
10. Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789-1794.
11. Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690-1696.
12. Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
13. de Denus S, Sanoski CA, Carlsson J, et al. Rate vs rhythm control in patients with atrial fibrillation: a meta-analysis. Arch Intern Med. 2005;165:258-262.
14. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509-1513.
15. Vora A, Karnad D, Goyal V, et al. Control of rate versus rhythm in rheumatic atrial fibrillation: a randomized study. Indian Heart J. 2004;56:110-116.
16. National Institute for Health and Clinical Excellence. National Collaborating Centre for Chronic Conditions. Atrial Fibrillation: National Clinical Guideline for Management in Primary and Secondary Care. London: Royal College of Physicians, 2006.
17. McNamara RL, Bass EB, Miller MR, et al. Management of new onset atrial fibrillation. Evid Rep Technol Assess (Summ). 2000;May(12):1-7.
18. Prystowsky EN. Atrial fibrillation. In: Topol EJ, ed. Textbook of Cardiovascular Medicine. Philadelphia: Lippincott Williams & Wilkins;1998:1661.
19. Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667-2677.
20. Coplen SE, Antman E, Berlin JA, et al. Efficacy and safety of quinidine therapy for maintenance of sinus rhythm after cardioversion. A meta-analysis of randomized control trials. Circulation. 1990;82:1106-1116.
21. Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913-920.
22. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867.
23. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.-
24. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864-2870.
25. Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634-2640.
Performance-enhancing drugs snare nonathletes, too
- Multiple adverse effects, including serious cardiovascular effects, have prompted bans on the sale of anabolic androgenic steroids (AAS) and their use in competition (A).
- Most users of AAS and other performance-enhancing drugs are nonathletes or recreational body builders who begin using these substances in their teen years. Ask about steroid or supplement use during yearly physicals (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
JC, a 23-year-old man, is in your office for evaluation of high blood pressure, after failing a commercial driver’s license exam the previous week. He has been your patient for the past 10 years, and his previous annual physicals have been unremarkable. He is 5’10’’ tall, weighs 209 pounds, and has a muscular build. His blood pressure today is 160/90 and his heart rate is 62 and regular. The rest of his physical exam is normal.
He is a nonsmoker, rarely uses alcohol, and denies illicit drug use. He exercises regularly, has been taking some protein shakes and what he refers to as a “natural” supplement. His lab work shows some elevation in his aspartate aminotransferase (AST) and alanine aminotransferase (ALT), with a negative hepatitis panel. The rest of his metabolic panel is within normal limits.
JC was on the track team in high school, and since graduation has continued to work out and stay fit. You ask him if he takes steroids, and he tells you he was warned about the risks of anabolic androgenic steroids (AAS) in high school. He sticks to a “natural” supplement, which he buys online or through friends at the gym. Still, you know that elevated liver enzymes and hypertension can be associated with AAS use and that dietary supplements don’t have to meet the same standards the Food and Drug Administration (FDA) imposes on drugs. (See “What’s in that supplement? Labels don’t always help” on page 18.) You warn him that supplements aren’t always safe, and ask him to bring in his supplement bottle so you can go over the label and, possibly, have the contents tested.
Pursuit of that “edge” extends beyond Olympians
Even before the start of the modern Olympic games, athletes have used ergogenic aids—substances used to enhance performance, energy, or work capacity—to give themselves a “competitive edge.”1,2 Athletes still use these substances today, and they have been joined by nonathletes—some of whom simply want to look good.
A 2004 Internet study of AAS users reported that the majority are recreational bodybuilders or nonathletes. Twenty-five percent of participants in this survey reported starting using steroids during their teenage years.3
An ongoing study of high school students and young adults indicates an AAS use prevalence rate of 1.1% to 2.3% in boys and 0.4% to 0.6% in girls. Approximately 40% of survey participants noted that obtaining steroids was relatively easy.4
The Centers for Disease Control and Prevention (CDC) reports that 4.4% to 5.7% of boys (grades 9 through 12) have used illegal steroids and that 1.9% to 3.8% of girls have.5
Few AAS users tell their physicians of their steroid use. Part of the reason, of course, is that illegal substance use is stigmatized and can lead to prosecution. Another reason, though, is that these patients think physicians don’t know much about these substances.3 Still other patients, like JC, don’t tell because they may not even be aware that some substances billed as “natural” conceal potential dangers.
For help in spotting patients who are using these agents, see “Red flags for performance-enhancing drug use” on page 20.
Performance-enhancing drugs go by many names
Refining your care of patients who are taking performance-enhancing drugs requires that you know the various names these drugs go by, the reason your patients may be taking them, and the adverse effects associated with them. This review, and the TABLE, will help.
Table
Performance-enhancing agents: What to watch for
| DRUG/SUPPLEMENT | ERGOGENIC USE | ADVERSE EFFECTS | COMMENTS |
|---|---|---|---|
| Anabolic androgenic steroids (AAS) |
| Acne, gynecomastia,* testicular atrophy,* virilization in females,* premature physeal closure, elevated liver enzymes, increased aggression, hypertension, CAD, sudden death |
|
| Tetrahydrogestrinone (THG) | Data on ergogenic use are insufficient | Hepatotoxicity; side effect profile probably similar to AAS |
|
| Androstenedione (Andro) | Increase testosterone levels in order to build muscle | Increased estradiol levels, feminization, priapism; side effect profile probably similar to AAS |
|
| Dehydroepiandrosterone (DHEA) | Increase testosterone levels for anabolic effects | Increased estrogen and estradiol levels, virilization, increased risk of endometrial cancer in females |
|
| Human growth hormone (HGH) | Increase protein synthesis and muscle mass without unwanted androgenic effects, decrease body fat | Insulin resistance, premature physeal closure, acromegaly, hypertension, cardiomegaly |
|
| Ephedrine | Weight loss, increase energy, increase concentration | Anxiety, panic attacks, hypertension, tachycardia, MI, stroke | Banned by the FDA because of cardiovascular and stroke risk |
| Caffeine | Increase alertness and energy, weight loss, improve endurance | Agitation; potential for withdrawal symptoms; hypertension, arrhythmia, and stroke when used with ephedrine or other stimulants | Urinary threshold in NCAA and Olympic competition |
| Erythropoietin (EPO) | Increase oxygen-carrying capacity of blood in endurance athletes | Pulmonary embolism, MI, stroke, development of anti-EPO antibodies | Banned in all sports competition |
| Creatine | Increase production of ATP in skeletal muscle during anaerobic exercise | Muscle cramps, weight gain, minor gastrointestinal upset |
|
| Sildenafil | Vasodilation, increase oxygenation and exercise capacity | Headache, flushing, dyspepsia, blurring of vision | No action yet to ban in athletic competition |
| ATP, adenosine triphosphate; CAD, coronary artery disease; FDA, Food and Drug Administration; MI, myocardial infarction; NCAA, National Collegiate Athletic Association. | |||
| * These adverse effects may be irreversible. | |||
Anabolic androgenic steroids: Often paired with energy drinks
Teenagers may refer to AAS as “pumpers,” “gym candy,” or “juice.” Trade names for AAS are Dianabol, Anadrol, Deca Durabolin, Parabolin, and Winstrol. AAS are often used with nutritional supplements like creatine, multivitamins, and energy drinks, in the belief that these regimens will make the user stronger, more muscular, and a better athlete.6,7
AAS are synthetic analogues of testosterone and come in oral, injectable, and transdermal forms.8,9 At supraphysiologic doses, testosterone has been found to increase lean body (fat-free) mass and muscle strength in humans.10 The anabolic effects are more pronounced when AAS are used at higher doses over longer periods of time, especially when combined with a strength training program.9,10 AAS have also been found to stimulate the production of growth hormone and insulin-like growth factor and to counteract the catabolic effects of cortisol.11
The use and possession of AAS without a doctor’s prescription is illegal in the United States. A majority of AAS users buy their medications through Internet suppliers, with some of the drugs being manufactured overseas or in illicit labs.3 Substandard quality control in manufacture poses an increased health risk to consumers.
Adverse effects include injection site pain, acne, baldness, gynecomastia, testicular atrophy, sexual dysfunction, and psychological disturbances (also known as “roid rage”).8,9,11-13 Increases in liver enzymes with the oral forms of AAS have also been noted.8 In the prepubertal athlete, premature physeal closure may occur, resulting in permanent short stature.14 Women who take AAS may have virilization effects, menstrual irregularities, and early menopause.11
The cardiovascular risks of AAS use are substantial. High-dose and long-term AAS use has been linked to cardiomyopathy and sudden death.15-20 Some data suggest the development of accelerated atherosclerosis with AAS use, leading to hypertension, coronary artery disease (CAD), and acute myocardial infarction.15,16,18-21 An unfavorable lipid panel has also been noted, with an increase in LDL and decreased HDL.18-21
Under the provisions of the 1994 Dietary Supplement Health and Education Act (DSHEA), supplement manufacturers, not the Food and Drug Administration (FDA), are responsible for guaranteeing the safety of their products.52 Components of the various supplements available are not uniform, and do not need to be submitted to the FDA for analysis. A study analyzing several nutritional supplements revealed the presence of anabolic androgenic steroids (AAS) (14.8% of 634 products) not mentioned in the labeling.53
Using supplements can result in positive drug tests for banned substances and unwanted side effects. It is important to ask about supplement use during annual checkups and sports physicals—especially if the patient has unexplained high blood pressure or other somatic complaints.
Tetrahydrogestrinone: A “designer” steroid
Tetrahydrogestrinone (THG) was initially developed to avoid detection by testing protocols current at the time.22-24 This drug has garnered significant media attention in the past few years because of scandals involving professional and Olympic athletes. THG is chemically related to 2 other banned steroids, trenbolone and gestrinone.22,24 It is used similarly to AAS to increase muscle bulk and enhance performance. It is more hepatotoxic than AAS, with highly potent androgenic and progestin properties in in vitro bioassay studies.22,25
Marketing of this agent is banned in the United States. There are no long-term studies of its effectiveness or side effect profile.
Androstenedione: Initially an anti-aging drug
Androstenedione, aka Andromax and Androstat 100, is a precursor of testosterone. This substance is produced in the adrenal glands and gonads.2 Initially marketed as a dietary supplement and anti-aging drug, it was banned by the FDA in 2004 because of its potent anabolic and androgenic effects.26 Ergogenic use includes promoting muscle building and strength and fat reduction.2 Studies on healthy young men found no improvement in skeletal muscle adaptation to resistance training with androstenedione supplementation for 8 to 12 weeks.27,28 Studies of its effect on increasing blood testosterone levels are conflicting.27,29 Several studies noted an increase in estradiol levels after oral androstenedione supplementation.9,11,27-29
Endocrine pathways with this drug are similar to AAS, and the side effect profile is similar as well, although not as pronounced. Larger, long-term studies are needed to fill out this drug’s profile and document its effects on the athletes who use it.
- Rapid increase in muscle bulk and loss of body fat
- Unexplained high blood pressure, cardiomyopathy, or arrhythmia in a previously healthy adolescent or young adult
- Signs and symptoms of feminization in males or virilization in females
- Increased aggression, violent behavior, or insomnia
- Abnormal lab work, including increases in liver enzymes or hematocrit
- Polypharmacy or increased use of medications and dietary supplements.
Dehydroepiandrosterone: Marketed as a “wonder drug”
Dehydroepiandrosterone (DHEA), marketed under the names Prastera, Fidelin, and Fluasterone, is another precursor of testosterone. It is produced in the adrenal cortex and has weak androgenic properties.2 DHEA is a dietary supplement marketed as a “wonder drug” and, like androstenedione, is advertised to promote muscle-building and fat-burning. It is also said to have anti-aging properties.11,14 DHEA has been used by athletes in the belief that it will increase testosterone levels and muscle bulk.30
In studies done in healthy men, however, even large doses of DHEA (1600 mg/d) did not result in an increase in testosterone levels. An increase in estradiol levels was noted in elderly men. Women who supplement with DHEA were found to have increased levels of testosterone and virilization effects, even at small doses (25-50 mg/d).31 Because of the risk of these side effects and the lack of long-term studies, DHEA supplementation is not recommended for use by adolescents or women.30 There is no convincing evidence to support claims of the anabolic and anti-aging effects of DHEA.
Human growth hormone: Side effects include hypertension
Human growth hormone (HGH) is an endogenous pituitary hormone with anabolic functions that increases muscle mass without the androgenic side effects. It is used medically for patients with decreased endogenous levels of GH or dwarfism. As an ergogenic aid, it has been found to increase levels of insulin-like growth factors, and the combination leads to increased protein synthesis and muscle mass.32
Side effects of HGH include insulin resistance, GH-induced myopathy, and acromegaly-like effects.11 There have been reports of hypertension, cardiomegaly, ventricular hypertrophy, and abnormal lipids with excessive use.19,33 Premature physeal closure may occur in the adolescent HGH user.8 It’s unclear whether HGH actually enhances sports performance, because the evidence is insufficient.34
Ephedrine: Used by hockey players
Ephedrine is a stimulant derived from the herb ma huang. It goes by many names, among them Ma Huang, Bolt-ephedrine, Asia Black 25, Hot Body Ephedra, and Thin Quick. Its chemical structure is related to amphetamine. Among college athletes, ephedrine and amphetamine use is more common in power sports, those requiring increased concentration (eg, rifle shooting, fencing), ice hockey, and field sports.35 Users feel less fatigue, experience bursts of energy, and lose weight.8,36
Users may experience irritability, anxiety, insomnia, and tremors, especially if stimulants are used in conjunction with high doses of caffeine.35,37 Ephedrine stimulates the release of norepinephrine, which produces increases in blood pressure, peripheral vascular resistance, and heart rate. These norepinephrine effects are the proposed mechanism for reported cases of myocardial infarction, cerebral artery vasoconstriction, and stroke associated with ephedrine use.13
Marketing of dietary supplements that contain ephedrine has been banned by the FDA because of the stimulant’s potential for increasing cardiovascular and stroke risks.38
Caffeine: May give sprinters a leg up
Caffeine—which is found in everything from coffee to energy tablets and energy drinks—increases a person’s energy level. In endurance sports, it also increases time to exhaustion.32 Studies in endurance-trained cyclists have shown that caffeine intake reduced leg pain, increased maximal leg force, and lengthened time to fatigue.39,40 A recent study in Australia also showed that caffeine may improve intermittent-sprint performance in competitive male athletes.41
Serious cardiovascular risks and even death have been documented when caffeine has been used with other stimulants, such as ephedrine or amphetamines. The combination of high doses of caffeine and ephedrine has a potential for life-threatening arrhythmia, hypertension, and stroke.42 Other psychomotor side effects include anxiety, irritability, tremor, and the potential for withdrawal symptoms.42,43 Because of caffeine’s stimulant nature, the International Olympic Committee and the National Collegiate Athletic Association have set urinary thresholds for its use in competition.
Erythropoietin: Promotes endurance
Erythropoietin (EPO) is a hormone produced in the kidneys that stimulates production of red blood cells (erythropoiesis). Marketed under the brand names Epogen and Procrit, EPO has legitimate medical uses. As an ergogenic substance, EPO is used to promote endurance by increasing the oxygen-carrying capacity of the blood with the increased red blood cell mass. In endurance athletes, the benefits of recombinant erythropoietin (rEpo) may last several weeks.23 There is also a practice called “blood doping,” which is a transfusion prior to competition, to produce the same effect.
Adverse effects of EPO use are attributed to increased blood viscosity and thrombotic potential. Pulmonary embolism, stroke, myocardial infarction, and sudden death can occur.19 Cases of death due to severe bradycardia, usually occurring during the night, have also been reported.23 Development of anti-EPO antibodies may also occur, causing paradoxical anemia.23 Athletes found to be using rEPO are banned from competition by sports-governing organizations.
Creatine: Popular among body builders
Creatine is a popular supplement used by athletes and recreational bodybuilders to provide energy to skeletal muscles in short-duration, maximal exercise.44 It is an endogenous substance found mainly in skeletal muscle and is synthesized by the liver from the amino acids glycine, arginine, and methionine.11,44 It is also found in meat.
Creatine monohydrate supplements have been found to increase creatine stores in muscles.45 In the phosphorylated form, creatine serves as a substrate for adenosine triphosphate resynthesis during intense anaerobic exercise.11,44-46 Numerous studies support its ergogenic effect on short-term, intermittent maximal activities such as bodybuilding, swimming, and jumping. Similar benefits have not been proven for endurance aerobic activities, such as long-distance cycling or running.46,47
This supplement is sold in many forms under such names as Rejuvinix, Cell Tech Hardcore, Muscle Marketing, Femme Advantage, and NOZ. Although not recommended for those under age 18, creatine is actually used by approximately 5.6% of high school athletes, with the highest levels of use (44%) occurring in the 11th and 12th grades.48 Reported side effects of creatine include muscle cramps, weight gain, and some minor gastrointestinal upset. Long-term studies on creatine supplementation are still needed.
Viagra (that’s right, Viagra)
Viagra (sildenafil) is the latest entry in the list of drugs competitive athletes may be using to try to improve sports performance. The World Anti-Doping Agency is financing a study investigating whether sildenafil can create an unfair competitive advantage by dilating blood vessels and increasing oxygen-carrying capacity.49 Studies of the impact of sildenafil on exercise capacity of climbers at the Mt. Everest base camp and on exercise performance during acute hypoxia have been published.50,51 Sildenafil was found to improve athletic capacity in both. To date, no action has been taken to ban the substance in athletic competition.
Are your patients using these agents? Ask them
Family physicians need to be alert to the red flags that may indicate steroid use and gently explore the full list of medications, over-the-counter products, and dietary supplements patients may be using. Take advantage of annual checkups and sports physicals to ask about use of performance-enhancing substances, educate patients on the risks involved, and emphasize good nutrition and sensible exercise routines as healthy ways to build a strong, attractive physique.
www.usdoj.gov/dea/pubs/abuse/10-steroids.htm
Education was certainly in order for your patient, JC, described at the beginning of this article. He thought the dietary supplement he used was natural and therefore harmless. Not so. It contained potentially dangerous substances, so you advised him to stop using it. Nutritional counseling and a vigorous exercise routine have allowed JC to maintain his fitness ideal. His blood pressure and liver enzymes returned to normal levels, and he passed his commercial driver’s license exam.
Correspondence
Marifel Mitzi F. Fernandez, MD, 600 Woodside Drive, Cornell, WI 54732; [email protected]
1. De Rose E. Doping in athletes – an update. Clin Sports Med. 2008;27:107-130.
2. Di Luigi L. Supplements and the endocrine system in athletes. Clin Sports Med. 2008;27:131-151.
3. Parkinson A, Evans N. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc. 2006;38:644-651.
4. National Institute on Drug Abuse Monitoring the future. National results on adolescent drug use. Overview of key findings. National Institutes of Health. 2007. Available at: http://monitoringthefuture.org/pubs/monographs/overview2007.pdf. Accessed November 22, 2008.
5. Centers for Disease Control and Prevention. Youth risk behavior surveillance-United States, 2007. MMWR. 2008;57 (SS-4). Available at http://www.cdc.gov/HealthyYouth/yrbs/pdf/yrbss07_mmwr.pdf. Accessed November 22, 2008.
6. Hoffman J, Faigenbaum A, Ratamess NA, et al. Nutritional supplementation and anabolic steroid use in adolescents. Med Sci Sports Exerc. 2008;40:15-24.
7. Faigenbaum A, Zaichkowsky L, Gardner DE, et al. Anabolic steroid use by male and female middle school students. Pediatrics. 1998;101:E6.-
8. Calfee R, Fadale P. Popular ergogenic drugs and supplements in young athletes. Pediatrics. 2006;117:e577-589.
9. Tokish J, Kocher M, Hawkins R. Ergogenic aids: a review of basic science, performance, side effects, and status in sports. Am J Sports Med. 2004;32:1543-1553.
10. Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1-7.
11. McDevitt E. Ergogenic drugs in sports. DeLee & Drez’s Orthopaedic Sports Medicine. Philadelphia: Elsevier Science; 2003:471-483.
12. Kibble M, Ross M. Adverse effects of anabolic steroids in athletes. Clin Pharmpoe. 1987;6:686-692.
13. Kutscher EC, Lund BC, Perry PJ. Anabolic steroids: a review for the clinician. Sports Med. 2002;32:285-296.
14. Blue JG, Lombardo JA. Steroids and steroid-like compounds. Clin Sports Med. 1999;18:667-689.
15. Rockhold R. Cardiovascular toxicity of anabolic steroids. Ann Rev Pharmacol Toxicol. 1993;33:497-520.
16. Parssinen M, Kujale U, Vartainen E, et al. Increased premature mortality of competitive power lifters suspected to have used anabolic agents. Int J Sports Med. 2000;21:225-227.
17. Nieminen MS, Ramo MP, Viitasalo M, et al. Serious cardiovascular side effects of large doses of anabolic steroids in weight lifters. Eur Heart J. 1996;17:1576-1583.
18. Parssinen M, Seppala T. Steroid use and long-term health risks in former athletes. Sports Med. 2002;32:83-94.
19. Dhar R, Stout W, Link MS, et al. Cardiovascular toxicities of performance-enhancing substances in sports. Mayo Clin Proc. 2005;80:1307-1315.
20. Melchert RB, Welder AA. Cardiovascular effects of androgenic anabolic steroids. Med Sci Sports Exerc. 1995;27:1252-1262.
21. Sullivan M, Martinez C, Gennis P, et al. The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis. 1998;41:1-15.
22. Malvey T, Armsey T. Tetrahydrogestrinone: the discovery of a designer steroid. Curr Sports Med Rep. 2005;4:227-230.
23. Noakes TD. Tainted glory – doping and athletic performance. N Engl J Med. 2004;351:847-849.
24. Fourcroy J. Designer steroids: past, present and future. Curr Opin Endocrinol Diabetes Obes. 2006;13:306-309.
25. Death A, McGrath K, Kazlauskas R, et al. Tetrahydrogestrinone is a potent androgen and progestin. J Clin Endocrinol Metabol. 2004;89:2498-2500.
26. Center for Food Safety and Applied Nutrition. Food and Drug Administration. Questions and Answers. Androstenedione. March 11, 2004. Available at: http://www.cfsan.fda.gov/~dms/androqa.html. Accessed November 22, 2008.
27. King DS, Sharp RL, Vukovich MD, et al. Effect of oral androstenedione on serum testosterone & adaptations to resistance training in young men. A randomized control trial. JAMA. 1999;281:2020-2028.
28. Broeder C, Quindry J, Brittingham K, et al. The Andro project. Physiological & hormonal influences of androstenedione supplement in men 35 to 65 years old participating in a high-intensity resistance training program. Arch Intern Med. 2000;160:3093-3104.
29. Leder B, Longcope C, Catlin DH, et al. Oral androstenedione administration and serum testosterone concentrations in young men. JAMA. 2000;283:779-782.
30. Yesalis C, Bahrke M. Anabolic-androgenic steroids and related substances. Curr Sports Med Rep. 2002;1:246-252.
31. Arlt W, Justl HG, Callies F, et al. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab. 1998;83:1928-1934.
32. Boyce EG. Use and effectiveness of performance-enhancing substances. J Pharm Pract. 2003;16:22-36.
33. Meyers DE, Cuneo RC. Controversies regarding the effects of growth hormone on the heart. Mayo Clin Proc. 2003;78:1521-1526.
34. Dean H. Does exogenous growth hormone improve performance? Clin J Sports Med. 2002;12:250-253.
35. McDuff DR, Baron D. Substance use in athletics: a sports psychiatry perspective. Clin Sports Med. 2005;24:885-897.
36. Keisler BD, Hosey RG. Ergogenic aids: an update on ephedra. Curr Sports Med Rep. 2005;4:231-235.
37. Sinclair CJ, Geiger JD. Caffeine use in sports. A pharmacologic review. J Sports Med Phys Fitness. 2000;40:71-79.
38. US Food and Drug Administration. Questions and answers about FDA’s actions on dietary supplements containing ephedrine alkaloids. February 6, 2004. Available at: http://www.fda.gov/oc/initiatives/ephedra/february2004/qa_020604.html. Accessed November 23, 2008.
39. Motl R, O’Connor P, Tubandi L, et al. Effect of caffeine on leg muscle pain during cycling exercise among females. Med Sci Sports Exerc. 2006;38:598-604.
40. Del Coso J, Estevez E, Mora-Rodriguez R. Caffeine effects on short-term performance during prolonged exercise in the heat. Med Sci Sports Exerc. 2008;40:744-751.
41. Schneiker KN, Bishop D, Dawson B, et al. Effects of caffeine on prolonged intermittent-sprint ability in team sport athletes. Med Sci Sports Exerc. 2006;38:578-585.
42. Keisler BD, Armsey TD. Caffeine as an ergogenic aid. Curr Sports Med Rep. 2006;5:215-219.
43. Rogers N, Dinges D. Caffeine: implications for alertness in athletes. Clin Sport Med. 2005;24:e1-e13.
44. Kraemer W, Volek J. Creatine supplementation. its role in human performance. Clin Sports Med. 1999;18:651-666.
45. Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci. 1992;83:367-374.
46. Demant TW, Rhodes EC. Effects of creatine supplementation on exercise performance. Sports Med. 1999;28:49-60.
47. Balsom PD, Harridge S, Soderlund K, et al. Creatine supplementation per se does not enhance endurance exercise performance. Acta Physiol Scand. 1993;149:521-523.
48. Metzl J, Small E, Levine SR, et al. Creatine use among young athletes. Pediatrics. 2001;108:421-425.
49. Longman J. New suspect in sports doping is, no joke, Viagra. The New York Times. November 23, 2008. Available at: http://www.nytimes.com/2008/11/23/sports/23viagra.html?scp=2&sq=Viagra&st=cse. Accessed November 23, 2008
50. Ghofrani HA, Reichenberger F, Kohstall MG, et al. Sildenafil increased exercise capacity during hypoxia at low altitudes and at Mt. Everest base camp: a randomized, double-blind, placebo-controlled crossover trial. Ann Intern Med. 2004;141:169-177.
51. Hsu AR, Barnholt KE, Grundman NK, et al. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J Appl Physiol. 2006;100:2031.-
52. Kurtzweil P. An FDA guide to dietary supplements. FDA Consumer. Sept-Oct, 1998. Available at: http://www.fda.gov/fdac/features/1998/598_guid.html. Accessed November 23, 2008.
53. Geyer H, Parr M, Mareck U, et al. Analysis of nonhormonal nutritional supplements for anabolic-androgenic steroids—results of an international study. Int J Sports Med. 2004;25:124-129.
Anticoagulation therapy:
ACC/AHA/ESC risk assessment …
Weak or less validated Moderate risk High risk
Female ≥75 years Previous stroke;TIA;or embolism
Age 65-74 y Hypertension Mitral stenosis
Coronary artery disease Heart failure Prosthetic heart valve
Thyrotoxicosis LV ejection fraction ≤35%
Diabetes mellitus
…and therapy recommendations
Marifel Mitzi F. Fernandez; anabolic androgenic steroids; elevated liver enzymes; ephedrine
- Multiple adverse effects, including serious cardiovascular effects, have prompted bans on the sale of anabolic androgenic steroids (AAS) and their use in competition (A).
- Most users of AAS and other performance-enhancing drugs are nonathletes or recreational body builders who begin using these substances in their teen years. Ask about steroid or supplement use during yearly physicals (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
JC, a 23-year-old man, is in your office for evaluation of high blood pressure, after failing a commercial driver’s license exam the previous week. He has been your patient for the past 10 years, and his previous annual physicals have been unremarkable. He is 5’10’’ tall, weighs 209 pounds, and has a muscular build. His blood pressure today is 160/90 and his heart rate is 62 and regular. The rest of his physical exam is normal.
He is a nonsmoker, rarely uses alcohol, and denies illicit drug use. He exercises regularly, has been taking some protein shakes and what he refers to as a “natural” supplement. His lab work shows some elevation in his aspartate aminotransferase (AST) and alanine aminotransferase (ALT), with a negative hepatitis panel. The rest of his metabolic panel is within normal limits.
JC was on the track team in high school, and since graduation has continued to work out and stay fit. You ask him if he takes steroids, and he tells you he was warned about the risks of anabolic androgenic steroids (AAS) in high school. He sticks to a “natural” supplement, which he buys online or through friends at the gym. Still, you know that elevated liver enzymes and hypertension can be associated with AAS use and that dietary supplements don’t have to meet the same standards the Food and Drug Administration (FDA) imposes on drugs. (See “What’s in that supplement? Labels don’t always help” on page 18.) You warn him that supplements aren’t always safe, and ask him to bring in his supplement bottle so you can go over the label and, possibly, have the contents tested.
Pursuit of that “edge” extends beyond Olympians
Even before the start of the modern Olympic games, athletes have used ergogenic aids—substances used to enhance performance, energy, or work capacity—to give themselves a “competitive edge.”1,2 Athletes still use these substances today, and they have been joined by nonathletes—some of whom simply want to look good.
A 2004 Internet study of AAS users reported that the majority are recreational bodybuilders or nonathletes. Twenty-five percent of participants in this survey reported starting using steroids during their teenage years.3
An ongoing study of high school students and young adults indicates an AAS use prevalence rate of 1.1% to 2.3% in boys and 0.4% to 0.6% in girls. Approximately 40% of survey participants noted that obtaining steroids was relatively easy.4
The Centers for Disease Control and Prevention (CDC) reports that 4.4% to 5.7% of boys (grades 9 through 12) have used illegal steroids and that 1.9% to 3.8% of girls have.5
Few AAS users tell their physicians of their steroid use. Part of the reason, of course, is that illegal substance use is stigmatized and can lead to prosecution. Another reason, though, is that these patients think physicians don’t know much about these substances.3 Still other patients, like JC, don’t tell because they may not even be aware that some substances billed as “natural” conceal potential dangers.
For help in spotting patients who are using these agents, see “Red flags for performance-enhancing drug use” on page 20.
Performance-enhancing drugs go by many names
Refining your care of patients who are taking performance-enhancing drugs requires that you know the various names these drugs go by, the reason your patients may be taking them, and the adverse effects associated with them. This review, and the TABLE, will help.
Table
Performance-enhancing agents: What to watch for
| DRUG/SUPPLEMENT | ERGOGENIC USE | ADVERSE EFFECTS | COMMENTS |
|---|---|---|---|
| Anabolic androgenic steroids (AAS) |
| Acne, gynecomastia,* testicular atrophy,* virilization in females,* premature physeal closure, elevated liver enzymes, increased aggression, hypertension, CAD, sudden death |
|
| Tetrahydrogestrinone (THG) | Data on ergogenic use are insufficient | Hepatotoxicity; side effect profile probably similar to AAS |
|
| Androstenedione (Andro) | Increase testosterone levels in order to build muscle | Increased estradiol levels, feminization, priapism; side effect profile probably similar to AAS |
|
| Dehydroepiandrosterone (DHEA) | Increase testosterone levels for anabolic effects | Increased estrogen and estradiol levels, virilization, increased risk of endometrial cancer in females |
|
| Human growth hormone (HGH) | Increase protein synthesis and muscle mass without unwanted androgenic effects, decrease body fat | Insulin resistance, premature physeal closure, acromegaly, hypertension, cardiomegaly |
|
| Ephedrine | Weight loss, increase energy, increase concentration | Anxiety, panic attacks, hypertension, tachycardia, MI, stroke | Banned by the FDA because of cardiovascular and stroke risk |
| Caffeine | Increase alertness and energy, weight loss, improve endurance | Agitation; potential for withdrawal symptoms; hypertension, arrhythmia, and stroke when used with ephedrine or other stimulants | Urinary threshold in NCAA and Olympic competition |
| Erythropoietin (EPO) | Increase oxygen-carrying capacity of blood in endurance athletes | Pulmonary embolism, MI, stroke, development of anti-EPO antibodies | Banned in all sports competition |
| Creatine | Increase production of ATP in skeletal muscle during anaerobic exercise | Muscle cramps, weight gain, minor gastrointestinal upset |
|
| Sildenafil | Vasodilation, increase oxygenation and exercise capacity | Headache, flushing, dyspepsia, blurring of vision | No action yet to ban in athletic competition |
| ATP, adenosine triphosphate; CAD, coronary artery disease; FDA, Food and Drug Administration; MI, myocardial infarction; NCAA, National Collegiate Athletic Association. | |||
| * These adverse effects may be irreversible. | |||
Anabolic androgenic steroids: Often paired with energy drinks
Teenagers may refer to AAS as “pumpers,” “gym candy,” or “juice.” Trade names for AAS are Dianabol, Anadrol, Deca Durabolin, Parabolin, and Winstrol. AAS are often used with nutritional supplements like creatine, multivitamins, and energy drinks, in the belief that these regimens will make the user stronger, more muscular, and a better athlete.6,7
AAS are synthetic analogues of testosterone and come in oral, injectable, and transdermal forms.8,9 At supraphysiologic doses, testosterone has been found to increase lean body (fat-free) mass and muscle strength in humans.10 The anabolic effects are more pronounced when AAS are used at higher doses over longer periods of time, especially when combined with a strength training program.9,10 AAS have also been found to stimulate the production of growth hormone and insulin-like growth factor and to counteract the catabolic effects of cortisol.11
The use and possession of AAS without a doctor’s prescription is illegal in the United States. A majority of AAS users buy their medications through Internet suppliers, with some of the drugs being manufactured overseas or in illicit labs.3 Substandard quality control in manufacture poses an increased health risk to consumers.
Adverse effects include injection site pain, acne, baldness, gynecomastia, testicular atrophy, sexual dysfunction, and psychological disturbances (also known as “roid rage”).8,9,11-13 Increases in liver enzymes with the oral forms of AAS have also been noted.8 In the prepubertal athlete, premature physeal closure may occur, resulting in permanent short stature.14 Women who take AAS may have virilization effects, menstrual irregularities, and early menopause.11
The cardiovascular risks of AAS use are substantial. High-dose and long-term AAS use has been linked to cardiomyopathy and sudden death.15-20 Some data suggest the development of accelerated atherosclerosis with AAS use, leading to hypertension, coronary artery disease (CAD), and acute myocardial infarction.15,16,18-21 An unfavorable lipid panel has also been noted, with an increase in LDL and decreased HDL.18-21
Under the provisions of the 1994 Dietary Supplement Health and Education Act (DSHEA), supplement manufacturers, not the Food and Drug Administration (FDA), are responsible for guaranteeing the safety of their products.52 Components of the various supplements available are not uniform, and do not need to be submitted to the FDA for analysis. A study analyzing several nutritional supplements revealed the presence of anabolic androgenic steroids (AAS) (14.8% of 634 products) not mentioned in the labeling.53
Using supplements can result in positive drug tests for banned substances and unwanted side effects. It is important to ask about supplement use during annual checkups and sports physicals—especially if the patient has unexplained high blood pressure or other somatic complaints.
Tetrahydrogestrinone: A “designer” steroid
Tetrahydrogestrinone (THG) was initially developed to avoid detection by testing protocols current at the time.22-24 This drug has garnered significant media attention in the past few years because of scandals involving professional and Olympic athletes. THG is chemically related to 2 other banned steroids, trenbolone and gestrinone.22,24 It is used similarly to AAS to increase muscle bulk and enhance performance. It is more hepatotoxic than AAS, with highly potent androgenic and progestin properties in in vitro bioassay studies.22,25
Marketing of this agent is banned in the United States. There are no long-term studies of its effectiveness or side effect profile.
Androstenedione: Initially an anti-aging drug
Androstenedione, aka Andromax and Androstat 100, is a precursor of testosterone. This substance is produced in the adrenal glands and gonads.2 Initially marketed as a dietary supplement and anti-aging drug, it was banned by the FDA in 2004 because of its potent anabolic and androgenic effects.26 Ergogenic use includes promoting muscle building and strength and fat reduction.2 Studies on healthy young men found no improvement in skeletal muscle adaptation to resistance training with androstenedione supplementation for 8 to 12 weeks.27,28 Studies of its effect on increasing blood testosterone levels are conflicting.27,29 Several studies noted an increase in estradiol levels after oral androstenedione supplementation.9,11,27-29
Endocrine pathways with this drug are similar to AAS, and the side effect profile is similar as well, although not as pronounced. Larger, long-term studies are needed to fill out this drug’s profile and document its effects on the athletes who use it.
- Rapid increase in muscle bulk and loss of body fat
- Unexplained high blood pressure, cardiomyopathy, or arrhythmia in a previously healthy adolescent or young adult
- Signs and symptoms of feminization in males or virilization in females
- Increased aggression, violent behavior, or insomnia
- Abnormal lab work, including increases in liver enzymes or hematocrit
- Polypharmacy or increased use of medications and dietary supplements.
Dehydroepiandrosterone: Marketed as a “wonder drug”
Dehydroepiandrosterone (DHEA), marketed under the names Prastera, Fidelin, and Fluasterone, is another precursor of testosterone. It is produced in the adrenal cortex and has weak androgenic properties.2 DHEA is a dietary supplement marketed as a “wonder drug” and, like androstenedione, is advertised to promote muscle-building and fat-burning. It is also said to have anti-aging properties.11,14 DHEA has been used by athletes in the belief that it will increase testosterone levels and muscle bulk.30
In studies done in healthy men, however, even large doses of DHEA (1600 mg/d) did not result in an increase in testosterone levels. An increase in estradiol levels was noted in elderly men. Women who supplement with DHEA were found to have increased levels of testosterone and virilization effects, even at small doses (25-50 mg/d).31 Because of the risk of these side effects and the lack of long-term studies, DHEA supplementation is not recommended for use by adolescents or women.30 There is no convincing evidence to support claims of the anabolic and anti-aging effects of DHEA.
Human growth hormone: Side effects include hypertension
Human growth hormone (HGH) is an endogenous pituitary hormone with anabolic functions that increases muscle mass without the androgenic side effects. It is used medically for patients with decreased endogenous levels of GH or dwarfism. As an ergogenic aid, it has been found to increase levels of insulin-like growth factors, and the combination leads to increased protein synthesis and muscle mass.32
Side effects of HGH include insulin resistance, GH-induced myopathy, and acromegaly-like effects.11 There have been reports of hypertension, cardiomegaly, ventricular hypertrophy, and abnormal lipids with excessive use.19,33 Premature physeal closure may occur in the adolescent HGH user.8 It’s unclear whether HGH actually enhances sports performance, because the evidence is insufficient.34
Ephedrine: Used by hockey players
Ephedrine is a stimulant derived from the herb ma huang. It goes by many names, among them Ma Huang, Bolt-ephedrine, Asia Black 25, Hot Body Ephedra, and Thin Quick. Its chemical structure is related to amphetamine. Among college athletes, ephedrine and amphetamine use is more common in power sports, those requiring increased concentration (eg, rifle shooting, fencing), ice hockey, and field sports.35 Users feel less fatigue, experience bursts of energy, and lose weight.8,36
Users may experience irritability, anxiety, insomnia, and tremors, especially if stimulants are used in conjunction with high doses of caffeine.35,37 Ephedrine stimulates the release of norepinephrine, which produces increases in blood pressure, peripheral vascular resistance, and heart rate. These norepinephrine effects are the proposed mechanism for reported cases of myocardial infarction, cerebral artery vasoconstriction, and stroke associated with ephedrine use.13
Marketing of dietary supplements that contain ephedrine has been banned by the FDA because of the stimulant’s potential for increasing cardiovascular and stroke risks.38
Caffeine: May give sprinters a leg up
Caffeine—which is found in everything from coffee to energy tablets and energy drinks—increases a person’s energy level. In endurance sports, it also increases time to exhaustion.32 Studies in endurance-trained cyclists have shown that caffeine intake reduced leg pain, increased maximal leg force, and lengthened time to fatigue.39,40 A recent study in Australia also showed that caffeine may improve intermittent-sprint performance in competitive male athletes.41
Serious cardiovascular risks and even death have been documented when caffeine has been used with other stimulants, such as ephedrine or amphetamines. The combination of high doses of caffeine and ephedrine has a potential for life-threatening arrhythmia, hypertension, and stroke.42 Other psychomotor side effects include anxiety, irritability, tremor, and the potential for withdrawal symptoms.42,43 Because of caffeine’s stimulant nature, the International Olympic Committee and the National Collegiate Athletic Association have set urinary thresholds for its use in competition.
Erythropoietin: Promotes endurance
Erythropoietin (EPO) is a hormone produced in the kidneys that stimulates production of red blood cells (erythropoiesis). Marketed under the brand names Epogen and Procrit, EPO has legitimate medical uses. As an ergogenic substance, EPO is used to promote endurance by increasing the oxygen-carrying capacity of the blood with the increased red blood cell mass. In endurance athletes, the benefits of recombinant erythropoietin (rEpo) may last several weeks.23 There is also a practice called “blood doping,” which is a transfusion prior to competition, to produce the same effect.
Adverse effects of EPO use are attributed to increased blood viscosity and thrombotic potential. Pulmonary embolism, stroke, myocardial infarction, and sudden death can occur.19 Cases of death due to severe bradycardia, usually occurring during the night, have also been reported.23 Development of anti-EPO antibodies may also occur, causing paradoxical anemia.23 Athletes found to be using rEPO are banned from competition by sports-governing organizations.
Creatine: Popular among body builders
Creatine is a popular supplement used by athletes and recreational bodybuilders to provide energy to skeletal muscles in short-duration, maximal exercise.44 It is an endogenous substance found mainly in skeletal muscle and is synthesized by the liver from the amino acids glycine, arginine, and methionine.11,44 It is also found in meat.
Creatine monohydrate supplements have been found to increase creatine stores in muscles.45 In the phosphorylated form, creatine serves as a substrate for adenosine triphosphate resynthesis during intense anaerobic exercise.11,44-46 Numerous studies support its ergogenic effect on short-term, intermittent maximal activities such as bodybuilding, swimming, and jumping. Similar benefits have not been proven for endurance aerobic activities, such as long-distance cycling or running.46,47
This supplement is sold in many forms under such names as Rejuvinix, Cell Tech Hardcore, Muscle Marketing, Femme Advantage, and NOZ. Although not recommended for those under age 18, creatine is actually used by approximately 5.6% of high school athletes, with the highest levels of use (44%) occurring in the 11th and 12th grades.48 Reported side effects of creatine include muscle cramps, weight gain, and some minor gastrointestinal upset. Long-term studies on creatine supplementation are still needed.
Viagra (that’s right, Viagra)
Viagra (sildenafil) is the latest entry in the list of drugs competitive athletes may be using to try to improve sports performance. The World Anti-Doping Agency is financing a study investigating whether sildenafil can create an unfair competitive advantage by dilating blood vessels and increasing oxygen-carrying capacity.49 Studies of the impact of sildenafil on exercise capacity of climbers at the Mt. Everest base camp and on exercise performance during acute hypoxia have been published.50,51 Sildenafil was found to improve athletic capacity in both. To date, no action has been taken to ban the substance in athletic competition.
Are your patients using these agents? Ask them
Family physicians need to be alert to the red flags that may indicate steroid use and gently explore the full list of medications, over-the-counter products, and dietary supplements patients may be using. Take advantage of annual checkups and sports physicals to ask about use of performance-enhancing substances, educate patients on the risks involved, and emphasize good nutrition and sensible exercise routines as healthy ways to build a strong, attractive physique.
www.usdoj.gov/dea/pubs/abuse/10-steroids.htm
Education was certainly in order for your patient, JC, described at the beginning of this article. He thought the dietary supplement he used was natural and therefore harmless. Not so. It contained potentially dangerous substances, so you advised him to stop using it. Nutritional counseling and a vigorous exercise routine have allowed JC to maintain his fitness ideal. His blood pressure and liver enzymes returned to normal levels, and he passed his commercial driver’s license exam.
Correspondence
Marifel Mitzi F. Fernandez, MD, 600 Woodside Drive, Cornell, WI 54732; [email protected]
- Multiple adverse effects, including serious cardiovascular effects, have prompted bans on the sale of anabolic androgenic steroids (AAS) and their use in competition (A).
- Most users of AAS and other performance-enhancing drugs are nonathletes or recreational body builders who begin using these substances in their teen years. Ask about steroid or supplement use during yearly physicals (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
JC, a 23-year-old man, is in your office for evaluation of high blood pressure, after failing a commercial driver’s license exam the previous week. He has been your patient for the past 10 years, and his previous annual physicals have been unremarkable. He is 5’10’’ tall, weighs 209 pounds, and has a muscular build. His blood pressure today is 160/90 and his heart rate is 62 and regular. The rest of his physical exam is normal.
He is a nonsmoker, rarely uses alcohol, and denies illicit drug use. He exercises regularly, has been taking some protein shakes and what he refers to as a “natural” supplement. His lab work shows some elevation in his aspartate aminotransferase (AST) and alanine aminotransferase (ALT), with a negative hepatitis panel. The rest of his metabolic panel is within normal limits.
JC was on the track team in high school, and since graduation has continued to work out and stay fit. You ask him if he takes steroids, and he tells you he was warned about the risks of anabolic androgenic steroids (AAS) in high school. He sticks to a “natural” supplement, which he buys online or through friends at the gym. Still, you know that elevated liver enzymes and hypertension can be associated with AAS use and that dietary supplements don’t have to meet the same standards the Food and Drug Administration (FDA) imposes on drugs. (See “What’s in that supplement? Labels don’t always help” on page 18.) You warn him that supplements aren’t always safe, and ask him to bring in his supplement bottle so you can go over the label and, possibly, have the contents tested.
Pursuit of that “edge” extends beyond Olympians
Even before the start of the modern Olympic games, athletes have used ergogenic aids—substances used to enhance performance, energy, or work capacity—to give themselves a “competitive edge.”1,2 Athletes still use these substances today, and they have been joined by nonathletes—some of whom simply want to look good.
A 2004 Internet study of AAS users reported that the majority are recreational bodybuilders or nonathletes. Twenty-five percent of participants in this survey reported starting using steroids during their teenage years.3
An ongoing study of high school students and young adults indicates an AAS use prevalence rate of 1.1% to 2.3% in boys and 0.4% to 0.6% in girls. Approximately 40% of survey participants noted that obtaining steroids was relatively easy.4
The Centers for Disease Control and Prevention (CDC) reports that 4.4% to 5.7% of boys (grades 9 through 12) have used illegal steroids and that 1.9% to 3.8% of girls have.5
Few AAS users tell their physicians of their steroid use. Part of the reason, of course, is that illegal substance use is stigmatized and can lead to prosecution. Another reason, though, is that these patients think physicians don’t know much about these substances.3 Still other patients, like JC, don’t tell because they may not even be aware that some substances billed as “natural” conceal potential dangers.
For help in spotting patients who are using these agents, see “Red flags for performance-enhancing drug use” on page 20.
Performance-enhancing drugs go by many names
Refining your care of patients who are taking performance-enhancing drugs requires that you know the various names these drugs go by, the reason your patients may be taking them, and the adverse effects associated with them. This review, and the TABLE, will help.
Table
Performance-enhancing agents: What to watch for
| DRUG/SUPPLEMENT | ERGOGENIC USE | ADVERSE EFFECTS | COMMENTS |
|---|---|---|---|
| Anabolic androgenic steroids (AAS) |
| Acne, gynecomastia,* testicular atrophy,* virilization in females,* premature physeal closure, elevated liver enzymes, increased aggression, hypertension, CAD, sudden death |
|
| Tetrahydrogestrinone (THG) | Data on ergogenic use are insufficient | Hepatotoxicity; side effect profile probably similar to AAS |
|
| Androstenedione (Andro) | Increase testosterone levels in order to build muscle | Increased estradiol levels, feminization, priapism; side effect profile probably similar to AAS |
|
| Dehydroepiandrosterone (DHEA) | Increase testosterone levels for anabolic effects | Increased estrogen and estradiol levels, virilization, increased risk of endometrial cancer in females |
|
| Human growth hormone (HGH) | Increase protein synthesis and muscle mass without unwanted androgenic effects, decrease body fat | Insulin resistance, premature physeal closure, acromegaly, hypertension, cardiomegaly |
|
| Ephedrine | Weight loss, increase energy, increase concentration | Anxiety, panic attacks, hypertension, tachycardia, MI, stroke | Banned by the FDA because of cardiovascular and stroke risk |
| Caffeine | Increase alertness and energy, weight loss, improve endurance | Agitation; potential for withdrawal symptoms; hypertension, arrhythmia, and stroke when used with ephedrine or other stimulants | Urinary threshold in NCAA and Olympic competition |
| Erythropoietin (EPO) | Increase oxygen-carrying capacity of blood in endurance athletes | Pulmonary embolism, MI, stroke, development of anti-EPO antibodies | Banned in all sports competition |
| Creatine | Increase production of ATP in skeletal muscle during anaerobic exercise | Muscle cramps, weight gain, minor gastrointestinal upset |
|
| Sildenafil | Vasodilation, increase oxygenation and exercise capacity | Headache, flushing, dyspepsia, blurring of vision | No action yet to ban in athletic competition |
| ATP, adenosine triphosphate; CAD, coronary artery disease; FDA, Food and Drug Administration; MI, myocardial infarction; NCAA, National Collegiate Athletic Association. | |||
| * These adverse effects may be irreversible. | |||
Anabolic androgenic steroids: Often paired with energy drinks
Teenagers may refer to AAS as “pumpers,” “gym candy,” or “juice.” Trade names for AAS are Dianabol, Anadrol, Deca Durabolin, Parabolin, and Winstrol. AAS are often used with nutritional supplements like creatine, multivitamins, and energy drinks, in the belief that these regimens will make the user stronger, more muscular, and a better athlete.6,7
AAS are synthetic analogues of testosterone and come in oral, injectable, and transdermal forms.8,9 At supraphysiologic doses, testosterone has been found to increase lean body (fat-free) mass and muscle strength in humans.10 The anabolic effects are more pronounced when AAS are used at higher doses over longer periods of time, especially when combined with a strength training program.9,10 AAS have also been found to stimulate the production of growth hormone and insulin-like growth factor and to counteract the catabolic effects of cortisol.11
The use and possession of AAS without a doctor’s prescription is illegal in the United States. A majority of AAS users buy their medications through Internet suppliers, with some of the drugs being manufactured overseas or in illicit labs.3 Substandard quality control in manufacture poses an increased health risk to consumers.
Adverse effects include injection site pain, acne, baldness, gynecomastia, testicular atrophy, sexual dysfunction, and psychological disturbances (also known as “roid rage”).8,9,11-13 Increases in liver enzymes with the oral forms of AAS have also been noted.8 In the prepubertal athlete, premature physeal closure may occur, resulting in permanent short stature.14 Women who take AAS may have virilization effects, menstrual irregularities, and early menopause.11
The cardiovascular risks of AAS use are substantial. High-dose and long-term AAS use has been linked to cardiomyopathy and sudden death.15-20 Some data suggest the development of accelerated atherosclerosis with AAS use, leading to hypertension, coronary artery disease (CAD), and acute myocardial infarction.15,16,18-21 An unfavorable lipid panel has also been noted, with an increase in LDL and decreased HDL.18-21
Under the provisions of the 1994 Dietary Supplement Health and Education Act (DSHEA), supplement manufacturers, not the Food and Drug Administration (FDA), are responsible for guaranteeing the safety of their products.52 Components of the various supplements available are not uniform, and do not need to be submitted to the FDA for analysis. A study analyzing several nutritional supplements revealed the presence of anabolic androgenic steroids (AAS) (14.8% of 634 products) not mentioned in the labeling.53
Using supplements can result in positive drug tests for banned substances and unwanted side effects. It is important to ask about supplement use during annual checkups and sports physicals—especially if the patient has unexplained high blood pressure or other somatic complaints.
Tetrahydrogestrinone: A “designer” steroid
Tetrahydrogestrinone (THG) was initially developed to avoid detection by testing protocols current at the time.22-24 This drug has garnered significant media attention in the past few years because of scandals involving professional and Olympic athletes. THG is chemically related to 2 other banned steroids, trenbolone and gestrinone.22,24 It is used similarly to AAS to increase muscle bulk and enhance performance. It is more hepatotoxic than AAS, with highly potent androgenic and progestin properties in in vitro bioassay studies.22,25
Marketing of this agent is banned in the United States. There are no long-term studies of its effectiveness or side effect profile.
Androstenedione: Initially an anti-aging drug
Androstenedione, aka Andromax and Androstat 100, is a precursor of testosterone. This substance is produced in the adrenal glands and gonads.2 Initially marketed as a dietary supplement and anti-aging drug, it was banned by the FDA in 2004 because of its potent anabolic and androgenic effects.26 Ergogenic use includes promoting muscle building and strength and fat reduction.2 Studies on healthy young men found no improvement in skeletal muscle adaptation to resistance training with androstenedione supplementation for 8 to 12 weeks.27,28 Studies of its effect on increasing blood testosterone levels are conflicting.27,29 Several studies noted an increase in estradiol levels after oral androstenedione supplementation.9,11,27-29
Endocrine pathways with this drug are similar to AAS, and the side effect profile is similar as well, although not as pronounced. Larger, long-term studies are needed to fill out this drug’s profile and document its effects on the athletes who use it.
- Rapid increase in muscle bulk and loss of body fat
- Unexplained high blood pressure, cardiomyopathy, or arrhythmia in a previously healthy adolescent or young adult
- Signs and symptoms of feminization in males or virilization in females
- Increased aggression, violent behavior, or insomnia
- Abnormal lab work, including increases in liver enzymes or hematocrit
- Polypharmacy or increased use of medications and dietary supplements.
Dehydroepiandrosterone: Marketed as a “wonder drug”
Dehydroepiandrosterone (DHEA), marketed under the names Prastera, Fidelin, and Fluasterone, is another precursor of testosterone. It is produced in the adrenal cortex and has weak androgenic properties.2 DHEA is a dietary supplement marketed as a “wonder drug” and, like androstenedione, is advertised to promote muscle-building and fat-burning. It is also said to have anti-aging properties.11,14 DHEA has been used by athletes in the belief that it will increase testosterone levels and muscle bulk.30
In studies done in healthy men, however, even large doses of DHEA (1600 mg/d) did not result in an increase in testosterone levels. An increase in estradiol levels was noted in elderly men. Women who supplement with DHEA were found to have increased levels of testosterone and virilization effects, even at small doses (25-50 mg/d).31 Because of the risk of these side effects and the lack of long-term studies, DHEA supplementation is not recommended for use by adolescents or women.30 There is no convincing evidence to support claims of the anabolic and anti-aging effects of DHEA.
Human growth hormone: Side effects include hypertension
Human growth hormone (HGH) is an endogenous pituitary hormone with anabolic functions that increases muscle mass without the androgenic side effects. It is used medically for patients with decreased endogenous levels of GH or dwarfism. As an ergogenic aid, it has been found to increase levels of insulin-like growth factors, and the combination leads to increased protein synthesis and muscle mass.32
Side effects of HGH include insulin resistance, GH-induced myopathy, and acromegaly-like effects.11 There have been reports of hypertension, cardiomegaly, ventricular hypertrophy, and abnormal lipids with excessive use.19,33 Premature physeal closure may occur in the adolescent HGH user.8 It’s unclear whether HGH actually enhances sports performance, because the evidence is insufficient.34
Ephedrine: Used by hockey players
Ephedrine is a stimulant derived from the herb ma huang. It goes by many names, among them Ma Huang, Bolt-ephedrine, Asia Black 25, Hot Body Ephedra, and Thin Quick. Its chemical structure is related to amphetamine. Among college athletes, ephedrine and amphetamine use is more common in power sports, those requiring increased concentration (eg, rifle shooting, fencing), ice hockey, and field sports.35 Users feel less fatigue, experience bursts of energy, and lose weight.8,36
Users may experience irritability, anxiety, insomnia, and tremors, especially if stimulants are used in conjunction with high doses of caffeine.35,37 Ephedrine stimulates the release of norepinephrine, which produces increases in blood pressure, peripheral vascular resistance, and heart rate. These norepinephrine effects are the proposed mechanism for reported cases of myocardial infarction, cerebral artery vasoconstriction, and stroke associated with ephedrine use.13
Marketing of dietary supplements that contain ephedrine has been banned by the FDA because of the stimulant’s potential for increasing cardiovascular and stroke risks.38
Caffeine: May give sprinters a leg up
Caffeine—which is found in everything from coffee to energy tablets and energy drinks—increases a person’s energy level. In endurance sports, it also increases time to exhaustion.32 Studies in endurance-trained cyclists have shown that caffeine intake reduced leg pain, increased maximal leg force, and lengthened time to fatigue.39,40 A recent study in Australia also showed that caffeine may improve intermittent-sprint performance in competitive male athletes.41
Serious cardiovascular risks and even death have been documented when caffeine has been used with other stimulants, such as ephedrine or amphetamines. The combination of high doses of caffeine and ephedrine has a potential for life-threatening arrhythmia, hypertension, and stroke.42 Other psychomotor side effects include anxiety, irritability, tremor, and the potential for withdrawal symptoms.42,43 Because of caffeine’s stimulant nature, the International Olympic Committee and the National Collegiate Athletic Association have set urinary thresholds for its use in competition.
Erythropoietin: Promotes endurance
Erythropoietin (EPO) is a hormone produced in the kidneys that stimulates production of red blood cells (erythropoiesis). Marketed under the brand names Epogen and Procrit, EPO has legitimate medical uses. As an ergogenic substance, EPO is used to promote endurance by increasing the oxygen-carrying capacity of the blood with the increased red blood cell mass. In endurance athletes, the benefits of recombinant erythropoietin (rEpo) may last several weeks.23 There is also a practice called “blood doping,” which is a transfusion prior to competition, to produce the same effect.
Adverse effects of EPO use are attributed to increased blood viscosity and thrombotic potential. Pulmonary embolism, stroke, myocardial infarction, and sudden death can occur.19 Cases of death due to severe bradycardia, usually occurring during the night, have also been reported.23 Development of anti-EPO antibodies may also occur, causing paradoxical anemia.23 Athletes found to be using rEPO are banned from competition by sports-governing organizations.
Creatine: Popular among body builders
Creatine is a popular supplement used by athletes and recreational bodybuilders to provide energy to skeletal muscles in short-duration, maximal exercise.44 It is an endogenous substance found mainly in skeletal muscle and is synthesized by the liver from the amino acids glycine, arginine, and methionine.11,44 It is also found in meat.
Creatine monohydrate supplements have been found to increase creatine stores in muscles.45 In the phosphorylated form, creatine serves as a substrate for adenosine triphosphate resynthesis during intense anaerobic exercise.11,44-46 Numerous studies support its ergogenic effect on short-term, intermittent maximal activities such as bodybuilding, swimming, and jumping. Similar benefits have not been proven for endurance aerobic activities, such as long-distance cycling or running.46,47
This supplement is sold in many forms under such names as Rejuvinix, Cell Tech Hardcore, Muscle Marketing, Femme Advantage, and NOZ. Although not recommended for those under age 18, creatine is actually used by approximately 5.6% of high school athletes, with the highest levels of use (44%) occurring in the 11th and 12th grades.48 Reported side effects of creatine include muscle cramps, weight gain, and some minor gastrointestinal upset. Long-term studies on creatine supplementation are still needed.
Viagra (that’s right, Viagra)
Viagra (sildenafil) is the latest entry in the list of drugs competitive athletes may be using to try to improve sports performance. The World Anti-Doping Agency is financing a study investigating whether sildenafil can create an unfair competitive advantage by dilating blood vessels and increasing oxygen-carrying capacity.49 Studies of the impact of sildenafil on exercise capacity of climbers at the Mt. Everest base camp and on exercise performance during acute hypoxia have been published.50,51 Sildenafil was found to improve athletic capacity in both. To date, no action has been taken to ban the substance in athletic competition.
Are your patients using these agents? Ask them
Family physicians need to be alert to the red flags that may indicate steroid use and gently explore the full list of medications, over-the-counter products, and dietary supplements patients may be using. Take advantage of annual checkups and sports physicals to ask about use of performance-enhancing substances, educate patients on the risks involved, and emphasize good nutrition and sensible exercise routines as healthy ways to build a strong, attractive physique.
www.usdoj.gov/dea/pubs/abuse/10-steroids.htm
Education was certainly in order for your patient, JC, described at the beginning of this article. He thought the dietary supplement he used was natural and therefore harmless. Not so. It contained potentially dangerous substances, so you advised him to stop using it. Nutritional counseling and a vigorous exercise routine have allowed JC to maintain his fitness ideal. His blood pressure and liver enzymes returned to normal levels, and he passed his commercial driver’s license exam.
Correspondence
Marifel Mitzi F. Fernandez, MD, 600 Woodside Drive, Cornell, WI 54732; [email protected]
1. De Rose E. Doping in athletes – an update. Clin Sports Med. 2008;27:107-130.
2. Di Luigi L. Supplements and the endocrine system in athletes. Clin Sports Med. 2008;27:131-151.
3. Parkinson A, Evans N. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc. 2006;38:644-651.
4. National Institute on Drug Abuse Monitoring the future. National results on adolescent drug use. Overview of key findings. National Institutes of Health. 2007. Available at: http://monitoringthefuture.org/pubs/monographs/overview2007.pdf. Accessed November 22, 2008.
5. Centers for Disease Control and Prevention. Youth risk behavior surveillance-United States, 2007. MMWR. 2008;57 (SS-4). Available at http://www.cdc.gov/HealthyYouth/yrbs/pdf/yrbss07_mmwr.pdf. Accessed November 22, 2008.
6. Hoffman J, Faigenbaum A, Ratamess NA, et al. Nutritional supplementation and anabolic steroid use in adolescents. Med Sci Sports Exerc. 2008;40:15-24.
7. Faigenbaum A, Zaichkowsky L, Gardner DE, et al. Anabolic steroid use by male and female middle school students. Pediatrics. 1998;101:E6.-
8. Calfee R, Fadale P. Popular ergogenic drugs and supplements in young athletes. Pediatrics. 2006;117:e577-589.
9. Tokish J, Kocher M, Hawkins R. Ergogenic aids: a review of basic science, performance, side effects, and status in sports. Am J Sports Med. 2004;32:1543-1553.
10. Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1-7.
11. McDevitt E. Ergogenic drugs in sports. DeLee & Drez’s Orthopaedic Sports Medicine. Philadelphia: Elsevier Science; 2003:471-483.
12. Kibble M, Ross M. Adverse effects of anabolic steroids in athletes. Clin Pharmpoe. 1987;6:686-692.
13. Kutscher EC, Lund BC, Perry PJ. Anabolic steroids: a review for the clinician. Sports Med. 2002;32:285-296.
14. Blue JG, Lombardo JA. Steroids and steroid-like compounds. Clin Sports Med. 1999;18:667-689.
15. Rockhold R. Cardiovascular toxicity of anabolic steroids. Ann Rev Pharmacol Toxicol. 1993;33:497-520.
16. Parssinen M, Kujale U, Vartainen E, et al. Increased premature mortality of competitive power lifters suspected to have used anabolic agents. Int J Sports Med. 2000;21:225-227.
17. Nieminen MS, Ramo MP, Viitasalo M, et al. Serious cardiovascular side effects of large doses of anabolic steroids in weight lifters. Eur Heart J. 1996;17:1576-1583.
18. Parssinen M, Seppala T. Steroid use and long-term health risks in former athletes. Sports Med. 2002;32:83-94.
19. Dhar R, Stout W, Link MS, et al. Cardiovascular toxicities of performance-enhancing substances in sports. Mayo Clin Proc. 2005;80:1307-1315.
20. Melchert RB, Welder AA. Cardiovascular effects of androgenic anabolic steroids. Med Sci Sports Exerc. 1995;27:1252-1262.
21. Sullivan M, Martinez C, Gennis P, et al. The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis. 1998;41:1-15.
22. Malvey T, Armsey T. Tetrahydrogestrinone: the discovery of a designer steroid. Curr Sports Med Rep. 2005;4:227-230.
23. Noakes TD. Tainted glory – doping and athletic performance. N Engl J Med. 2004;351:847-849.
24. Fourcroy J. Designer steroids: past, present and future. Curr Opin Endocrinol Diabetes Obes. 2006;13:306-309.
25. Death A, McGrath K, Kazlauskas R, et al. Tetrahydrogestrinone is a potent androgen and progestin. J Clin Endocrinol Metabol. 2004;89:2498-2500.
26. Center for Food Safety and Applied Nutrition. Food and Drug Administration. Questions and Answers. Androstenedione. March 11, 2004. Available at: http://www.cfsan.fda.gov/~dms/androqa.html. Accessed November 22, 2008.
27. King DS, Sharp RL, Vukovich MD, et al. Effect of oral androstenedione on serum testosterone & adaptations to resistance training in young men. A randomized control trial. JAMA. 1999;281:2020-2028.
28. Broeder C, Quindry J, Brittingham K, et al. The Andro project. Physiological & hormonal influences of androstenedione supplement in men 35 to 65 years old participating in a high-intensity resistance training program. Arch Intern Med. 2000;160:3093-3104.
29. Leder B, Longcope C, Catlin DH, et al. Oral androstenedione administration and serum testosterone concentrations in young men. JAMA. 2000;283:779-782.
30. Yesalis C, Bahrke M. Anabolic-androgenic steroids and related substances. Curr Sports Med Rep. 2002;1:246-252.
31. Arlt W, Justl HG, Callies F, et al. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab. 1998;83:1928-1934.
32. Boyce EG. Use and effectiveness of performance-enhancing substances. J Pharm Pract. 2003;16:22-36.
33. Meyers DE, Cuneo RC. Controversies regarding the effects of growth hormone on the heart. Mayo Clin Proc. 2003;78:1521-1526.
34. Dean H. Does exogenous growth hormone improve performance? Clin J Sports Med. 2002;12:250-253.
35. McDuff DR, Baron D. Substance use in athletics: a sports psychiatry perspective. Clin Sports Med. 2005;24:885-897.
36. Keisler BD, Hosey RG. Ergogenic aids: an update on ephedra. Curr Sports Med Rep. 2005;4:231-235.
37. Sinclair CJ, Geiger JD. Caffeine use in sports. A pharmacologic review. J Sports Med Phys Fitness. 2000;40:71-79.
38. US Food and Drug Administration. Questions and answers about FDA’s actions on dietary supplements containing ephedrine alkaloids. February 6, 2004. Available at: http://www.fda.gov/oc/initiatives/ephedra/february2004/qa_020604.html. Accessed November 23, 2008.
39. Motl R, O’Connor P, Tubandi L, et al. Effect of caffeine on leg muscle pain during cycling exercise among females. Med Sci Sports Exerc. 2006;38:598-604.
40. Del Coso J, Estevez E, Mora-Rodriguez R. Caffeine effects on short-term performance during prolonged exercise in the heat. Med Sci Sports Exerc. 2008;40:744-751.
41. Schneiker KN, Bishop D, Dawson B, et al. Effects of caffeine on prolonged intermittent-sprint ability in team sport athletes. Med Sci Sports Exerc. 2006;38:578-585.
42. Keisler BD, Armsey TD. Caffeine as an ergogenic aid. Curr Sports Med Rep. 2006;5:215-219.
43. Rogers N, Dinges D. Caffeine: implications for alertness in athletes. Clin Sport Med. 2005;24:e1-e13.
44. Kraemer W, Volek J. Creatine supplementation. its role in human performance. Clin Sports Med. 1999;18:651-666.
45. Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci. 1992;83:367-374.
46. Demant TW, Rhodes EC. Effects of creatine supplementation on exercise performance. Sports Med. 1999;28:49-60.
47. Balsom PD, Harridge S, Soderlund K, et al. Creatine supplementation per se does not enhance endurance exercise performance. Acta Physiol Scand. 1993;149:521-523.
48. Metzl J, Small E, Levine SR, et al. Creatine use among young athletes. Pediatrics. 2001;108:421-425.
49. Longman J. New suspect in sports doping is, no joke, Viagra. The New York Times. November 23, 2008. Available at: http://www.nytimes.com/2008/11/23/sports/23viagra.html?scp=2&sq=Viagra&st=cse. Accessed November 23, 2008
50. Ghofrani HA, Reichenberger F, Kohstall MG, et al. Sildenafil increased exercise capacity during hypoxia at low altitudes and at Mt. Everest base camp: a randomized, double-blind, placebo-controlled crossover trial. Ann Intern Med. 2004;141:169-177.
51. Hsu AR, Barnholt KE, Grundman NK, et al. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J Appl Physiol. 2006;100:2031.-
52. Kurtzweil P. An FDA guide to dietary supplements. FDA Consumer. Sept-Oct, 1998. Available at: http://www.fda.gov/fdac/features/1998/598_guid.html. Accessed November 23, 2008.
53. Geyer H, Parr M, Mareck U, et al. Analysis of nonhormonal nutritional supplements for anabolic-androgenic steroids—results of an international study. Int J Sports Med. 2004;25:124-129.
1. De Rose E. Doping in athletes – an update. Clin Sports Med. 2008;27:107-130.
2. Di Luigi L. Supplements and the endocrine system in athletes. Clin Sports Med. 2008;27:131-151.
3. Parkinson A, Evans N. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc. 2006;38:644-651.
4. National Institute on Drug Abuse Monitoring the future. National results on adolescent drug use. Overview of key findings. National Institutes of Health. 2007. Available at: http://monitoringthefuture.org/pubs/monographs/overview2007.pdf. Accessed November 22, 2008.
5. Centers for Disease Control and Prevention. Youth risk behavior surveillance-United States, 2007. MMWR. 2008;57 (SS-4). Available at http://www.cdc.gov/HealthyYouth/yrbs/pdf/yrbss07_mmwr.pdf. Accessed November 22, 2008.
6. Hoffman J, Faigenbaum A, Ratamess NA, et al. Nutritional supplementation and anabolic steroid use in adolescents. Med Sci Sports Exerc. 2008;40:15-24.
7. Faigenbaum A, Zaichkowsky L, Gardner DE, et al. Anabolic steroid use by male and female middle school students. Pediatrics. 1998;101:E6.-
8. Calfee R, Fadale P. Popular ergogenic drugs and supplements in young athletes. Pediatrics. 2006;117:e577-589.
9. Tokish J, Kocher M, Hawkins R. Ergogenic aids: a review of basic science, performance, side effects, and status in sports. Am J Sports Med. 2004;32:1543-1553.
10. Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1-7.
11. McDevitt E. Ergogenic drugs in sports. DeLee & Drez’s Orthopaedic Sports Medicine. Philadelphia: Elsevier Science; 2003:471-483.
12. Kibble M, Ross M. Adverse effects of anabolic steroids in athletes. Clin Pharmpoe. 1987;6:686-692.
13. Kutscher EC, Lund BC, Perry PJ. Anabolic steroids: a review for the clinician. Sports Med. 2002;32:285-296.
14. Blue JG, Lombardo JA. Steroids and steroid-like compounds. Clin Sports Med. 1999;18:667-689.
15. Rockhold R. Cardiovascular toxicity of anabolic steroids. Ann Rev Pharmacol Toxicol. 1993;33:497-520.
16. Parssinen M, Kujale U, Vartainen E, et al. Increased premature mortality of competitive power lifters suspected to have used anabolic agents. Int J Sports Med. 2000;21:225-227.
17. Nieminen MS, Ramo MP, Viitasalo M, et al. Serious cardiovascular side effects of large doses of anabolic steroids in weight lifters. Eur Heart J. 1996;17:1576-1583.
18. Parssinen M, Seppala T. Steroid use and long-term health risks in former athletes. Sports Med. 2002;32:83-94.
19. Dhar R, Stout W, Link MS, et al. Cardiovascular toxicities of performance-enhancing substances in sports. Mayo Clin Proc. 2005;80:1307-1315.
20. Melchert RB, Welder AA. Cardiovascular effects of androgenic anabolic steroids. Med Sci Sports Exerc. 1995;27:1252-1262.
21. Sullivan M, Martinez C, Gennis P, et al. The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis. 1998;41:1-15.
22. Malvey T, Armsey T. Tetrahydrogestrinone: the discovery of a designer steroid. Curr Sports Med Rep. 2005;4:227-230.
23. Noakes TD. Tainted glory – doping and athletic performance. N Engl J Med. 2004;351:847-849.
24. Fourcroy J. Designer steroids: past, present and future. Curr Opin Endocrinol Diabetes Obes. 2006;13:306-309.
25. Death A, McGrath K, Kazlauskas R, et al. Tetrahydrogestrinone is a potent androgen and progestin. J Clin Endocrinol Metabol. 2004;89:2498-2500.
26. Center for Food Safety and Applied Nutrition. Food and Drug Administration. Questions and Answers. Androstenedione. March 11, 2004. Available at: http://www.cfsan.fda.gov/~dms/androqa.html. Accessed November 22, 2008.
27. King DS, Sharp RL, Vukovich MD, et al. Effect of oral androstenedione on serum testosterone & adaptations to resistance training in young men. A randomized control trial. JAMA. 1999;281:2020-2028.
28. Broeder C, Quindry J, Brittingham K, et al. The Andro project. Physiological & hormonal influences of androstenedione supplement in men 35 to 65 years old participating in a high-intensity resistance training program. Arch Intern Med. 2000;160:3093-3104.
29. Leder B, Longcope C, Catlin DH, et al. Oral androstenedione administration and serum testosterone concentrations in young men. JAMA. 2000;283:779-782.
30. Yesalis C, Bahrke M. Anabolic-androgenic steroids and related substances. Curr Sports Med Rep. 2002;1:246-252.
31. Arlt W, Justl HG, Callies F, et al. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab. 1998;83:1928-1934.
32. Boyce EG. Use and effectiveness of performance-enhancing substances. J Pharm Pract. 2003;16:22-36.
33. Meyers DE, Cuneo RC. Controversies regarding the effects of growth hormone on the heart. Mayo Clin Proc. 2003;78:1521-1526.
34. Dean H. Does exogenous growth hormone improve performance? Clin J Sports Med. 2002;12:250-253.
35. McDuff DR, Baron D. Substance use in athletics: a sports psychiatry perspective. Clin Sports Med. 2005;24:885-897.
36. Keisler BD, Hosey RG. Ergogenic aids: an update on ephedra. Curr Sports Med Rep. 2005;4:231-235.
37. Sinclair CJ, Geiger JD. Caffeine use in sports. A pharmacologic review. J Sports Med Phys Fitness. 2000;40:71-79.
38. US Food and Drug Administration. Questions and answers about FDA’s actions on dietary supplements containing ephedrine alkaloids. February 6, 2004. Available at: http://www.fda.gov/oc/initiatives/ephedra/february2004/qa_020604.html. Accessed November 23, 2008.
39. Motl R, O’Connor P, Tubandi L, et al. Effect of caffeine on leg muscle pain during cycling exercise among females. Med Sci Sports Exerc. 2006;38:598-604.
40. Del Coso J, Estevez E, Mora-Rodriguez R. Caffeine effects on short-term performance during prolonged exercise in the heat. Med Sci Sports Exerc. 2008;40:744-751.
41. Schneiker KN, Bishop D, Dawson B, et al. Effects of caffeine on prolonged intermittent-sprint ability in team sport athletes. Med Sci Sports Exerc. 2006;38:578-585.
42. Keisler BD, Armsey TD. Caffeine as an ergogenic aid. Curr Sports Med Rep. 2006;5:215-219.
43. Rogers N, Dinges D. Caffeine: implications for alertness in athletes. Clin Sport Med. 2005;24:e1-e13.
44. Kraemer W, Volek J. Creatine supplementation. its role in human performance. Clin Sports Med. 1999;18:651-666.
45. Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci. 1992;83:367-374.
46. Demant TW, Rhodes EC. Effects of creatine supplementation on exercise performance. Sports Med. 1999;28:49-60.
47. Balsom PD, Harridge S, Soderlund K, et al. Creatine supplementation per se does not enhance endurance exercise performance. Acta Physiol Scand. 1993;149:521-523.
48. Metzl J, Small E, Levine SR, et al. Creatine use among young athletes. Pediatrics. 2001;108:421-425.
49. Longman J. New suspect in sports doping is, no joke, Viagra. The New York Times. November 23, 2008. Available at: http://www.nytimes.com/2008/11/23/sports/23viagra.html?scp=2&sq=Viagra&st=cse. Accessed November 23, 2008
50. Ghofrani HA, Reichenberger F, Kohstall MG, et al. Sildenafil increased exercise capacity during hypoxia at low altitudes and at Mt. Everest base camp: a randomized, double-blind, placebo-controlled crossover trial. Ann Intern Med. 2004;141:169-177.
51. Hsu AR, Barnholt KE, Grundman NK, et al. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J Appl Physiol. 2006;100:2031.-
52. Kurtzweil P. An FDA guide to dietary supplements. FDA Consumer. Sept-Oct, 1998. Available at: http://www.fda.gov/fdac/features/1998/598_guid.html. Accessed November 23, 2008.
53. Geyer H, Parr M, Mareck U, et al. Analysis of nonhormonal nutritional supplements for anabolic-androgenic steroids—results of an international study. Int J Sports Med. 2004;25:124-129.
Anticoagulation therapy:
ACC/AHA/ESC risk assessment …
Weak or less validated Moderate risk High risk
Female ≥75 years Previous stroke;TIA;or embolism
Age 65-74 y Hypertension Mitral stenosis
Coronary artery disease Heart failure Prosthetic heart valve
Thyrotoxicosis LV ejection fraction ≤35%
Diabetes mellitus
…and therapy recommendations
Marifel Mitzi F. Fernandez; anabolic androgenic steroids; elevated liver enzymes; ephedrine
Anticoagulation therapy:
ACC/AHA/ESC risk assessment …
Weak or less validated Moderate risk High risk
Female ≥75 years Previous stroke;TIA;or embolism
Age 65-74 y Hypertension Mitral stenosis
Coronary artery disease Heart failure Prosthetic heart valve
Thyrotoxicosis LV ejection fraction ≤35%
Diabetes mellitus
…and therapy recommendations
Marifel Mitzi F. Fernandez; anabolic androgenic steroids; elevated liver enzymes; ephedrine
How to prevent a delayed Alzheimer’s diagnosis
- Avoid cognitive screening solely on the basis of age (SOR A).
- Screen vulnerable elderly patients at their initial visit and annually thereafter (SOR A).
- Ensure that all patients who undergo cognitive screening are tested for depression (SOR A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented
Janet M, a 69-year-old woman with a history of hypertension, comes for a visit because she thinks she has Alzheimer’s disease (AD). She recently had an episode of acute confusion while shopping at the mall; when she returned to her car, she couldn’t remember how to get home. The episode cleared within minutes and hasn’t recurred.
Jack S, an 84-year-old man, seeks medical care for pain in his right shoulder. He injured the rotator cuff several years ago, but it’s been fine since he completed physical therapy—until he tripped and fell while walking outside about a week ago. His daughter is concerned about his “forgetfulness” and increasing inability to remember certain words, but the patient thinks this is a natural consequence of age.
Fran B, a 72-year-old, presents with complaints of memory problems that began about 6 months ago. She’s worried about her son and has had increasing difficulty concentrating, sleeping, and keeping track of her things.
If these were your patients, whom would you screen for dementia? Would you decide whether to screen based on your “gut,” or a defined set of criteria? Would you have several screening tools on hand, and know enough about them to determine which one might be best suited for a particular patient?
If you make decisions about screening based on your gut or aren’t sure which tools are best for which patients, you’re far from alone. Cognitive impairment, particularly in the early stages, can be difficult and time-consuming to detect, and community physicians fail to diagnose mild-to-moderate dementia more than 50% of the time.1-5
Family members and caregivers often overlook declines in cognitive function, as well. In a study of 741 caregivers of patients with AD, an average of 4 months went by between the time symptoms were first noticed and the patient was seen by a physician—and 22% of the caregivers waited more than a year.6
Cognitive decline can be slowed with early Dx
Early diagnosis of AD or any dementia is important for a number of reasons. In some cases, cognitive impairment may be related to medical conditions—head trauma, Parkinson’s disease, human immunodeficiency virus, thyroid disorder, among others—that can be modified or reversed with treatment.7 There is evidence, too, that medical, behavioral, and social interventions can delay the cognitive and functional decline associated with AD, thereby helping to prolong the time the patient can remain at home. Early diagnosis also facilitates legal and financial family planning, and makes it possible to take appropriate safety measures.8-13
AD affects approximately 5 million US residents.14 With an aging population, that number is expected to surge in the decades ahead. To help you provide optimal care for your geriatric patients, this review will detail when to screen, which tools to use, and how best to care for the 3 elderly patients in the opener.
When and whether to screen
Despite the benefits of early detection, population-based screening based on age alone is not recommended. Guidelines recommend focused screening of patients in high-risk groups and on a case-by-case basis.15
US Preventive Services Task Force (USPSTF) guidelines recommend that physicians evaluate older patients for dementia whenever there is a suggestion of cognitive impairment, based on clinical observation or concern expressed by the individuals themselves or by family members, caretakers, or friends.15
However, cognitive screening generally provides better results in populations at higher risk of dementia.16,17 With that in mind, the Assessing Care of Vulnerable Elders (ACOVE), a collaborative project to develop a set of quality indicators for care of the elderly, recently recommended cognitive and functional screening of all “vulnerable elderly.”18,19
Who are the vulnerable elderly?
ACOVE defines the vulnerable elderly as individuals who are 65 years of age and older who live in the community and are at high risk of death or functional decline over the next 2 years. You can use a screening tool to identify members of this high-risk group. Or you can simply ask a few questions and classify any noninstitutionalized older person who reports being in poor health and/or acknowledges difficulty with activities of daily living—eg, money or medication management, dressing, grooming, or preparing simple meals—as a vulnerable elderly patient.
ACOVE recommends screening all vulnerable elderly patients who are new to a primary care practice or inpatient service, and following up with an annual evaluation to detect any changes in memory and function.18,19 Based on the recommendations of ACOVE and the USPSTF, all 3 patients described earlier would be candidates for screening.
Choosing the best screening tool
There is no serum or radiographic test available for the diagnosis of AD, and a thorough clinical evaluation can be extremely time-consuming. In the primary care setting, focused evaluations are both useful and cost effective.
There are a number of valid and reliable cognitive screening tools, including the Mini-Mental State Examination (MMSE), the Mini-Cog, the Montreal Cognitive Assessment (MoCA), the AD 8 Dementia Screening Interview, and the 7-Minute Neurocognitive Screen. In evaluating these and other screening tools, consider the sensitivity and specificity of each. Consider, too, features that may make one test better suited than another for a particular patient or specific circumstance (TABLE).
TABLE
Cognitive screening tools: How they compare
| TOOL (TIME TO ADMINISTER) | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| MMSE (5-10 min) | Tracks/quantifies changes; easy to administer; widely accepted; available in >50 languages | Not specific to AD or sensitive to mild dementia; influenced by age, education, and language skills |
| Mini-Cog (3 min) | Brief, easy to administer and score; unaffected by education, language skill; high sensitivity; easily paired with FAQ | |
| MoCA (10 min) | High sensitivity for MCI and mild AD; available in >20 languages; especially useful for patients with memory complaints but normal MMSE score | More time-consuming than other screening tools |
| AD 8 (3 min) | Administered in person or by phone; identifies earliest stages of dementia | Designed for informant, who may not be available, but can be given to patient |
| 7-Minute Neurocognitive Screen | Highly sensitive to early stages of AD | |
| AD, Alzheimer’s disease; FAQ, Functional Activities Questionnaire; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment. | ||
The Mini-Mental State Exam is the gold standard
Considered the gold standard in dementia assessment, the MMSE is the most widely used cognitive screening tool in the United States.20 The MMSE samples 6 cognitive areas—orientation, registration, attention and calculation, recall, language, and constructional skill.21 It has a high degree of validity in detecting dementia, and is particularly useful in tracking and quantifying changes over time. Well-known features include the serial 7s, in which patients are asked to count backwards from 100 by 7s, and the 3-stage command: Take a paper in your right hand, fold it in half, and put it on the floor.
Besides being relatively short (testing takes 5-10 minutes), the MMSE is easy to administer. It has been translated into more than 50 languages, and can therefore be used in many cultural settings.
Interpreting the MMSE. A total score of ≤23 out of a maximum of 30 points is suggestive of dementia. However, patient performance on the MMSE is influenced by a number of factors unrelated to cognitive function, such as age, educational level, deficits in language skills, and motor or visual impairment.22 Thus, the cut point may be adjusted depending on the patient or population being screened, and the positive predictive value and sensitivity and specificity for dementia vary accordingly. In a population-based sample of >18,000 individuals with a cut point of 24, the sensitivity was 87% and the specificity was 82%.23
The usefulness of the MMSE is also limited because the test is not specific to AD and is not sensitive to mild dementia. When the test is used to screen highly intelligent, well-educated patients, it may fail to detect any decline in memory or cognitive function.
The Mini-Cog is a quick and easy alternative
The Mini-Cog screen consists of 2 simple components that evaluate executive function: a clock-drawing test and word recall.24 The patient is given paper and pencil and asked to draw a clock with hands set on a specified time—5:10, say. First, however, the screener recites 3 common but unrelated words; the patient is asked to recite the words before drawing the clock and to recall them afterwards. The entire test takes about 3 minutes.
Interpreting the Mini-Cog. Besides being quick and easy to administer, the Mini-Cog is easy to score:
- Patients who do not recall any of the words are classified as demented.
- Patients who recall all of the words are classified as nondemented
- Patients who recall 1 or 2 words are classified based on their clock drawing: They’re considered nondemented if the clock is normal and demented if it is not.
When clock-drawing is part of the Mini-Cog screen, the results are simply considered normal or abnormal. For a clock to be considered normal, all the numbers must be in the correct sequence and the hands correctly positioned to show the designated time.24 (The clock-drawing can also be used as an independent screen. For more about that, see “Clock-drawing: What to look for”.)
The Mini-Cog has been found to be highly accurate. And, unlike the MMSE, the Mini-Cog is not skewed by education level or language skill. One study based on interviews with “informants”—the family, friends, or caregivers of patients being tested for dementia—examined the Mini-Cog’s ability to differentiate between 129 demented and 120 nondemented older adults who were culturally, linguistically, and educationally heterogeneous. The Mini-Cog classified 96% of participants correctly and had a sensitivity of 99%.24
Another retrospective analysis of a random sample of more than 1100 elderly people compared the Mini-Cog to the MMSE. In this case, the Mini-Cog and MMSE (with a cut point of 25) demonstrated similar sensitivity (76% vs 79%) and specificity (89% vs 88%) for identifying individuals with dementia.25
Jack S, whose daughter worried about his “forgetfulness,” was given the Mini-Cog. He drew a clock with the big hand incorrectly placed, and recalled only 2 words. Because his daughter was with him, the physician asked her to complete a Functional Activities Questionnaire (FAQ), a test designed for family members, caregivers, and other informants. The findings indicated that Jack had dementia, and the physician gave the family a referral to a specialist in elder care.
The Mini-Cog–FAQ. A recent study paired the Mini-Cog with the FAQ, which asks the informant to rate the patient’s ability to perform a variety of activities—eg, paying bills, shopping, engaging in hobbies, and preparing a meal. When used alone, the FAQ has a high sensitivity and specificity, but patient testing is still necessary. Used together, the Mini-Cog–FAQ allowed researchers to classify patients as cognitively normal, demented, or mildly cognitively impaired with 83% accuracy.26
Montreal Cognitive Assessment detects mild impairment
The MoCA is a 10-minute screening tool designed to help physicians detect mild cognitive impairment, which is considered to be predictive of dementia.27 This tool is especially useful for individuals who present with memory complaints but achieve a normal score (26-30) on the MMSE, as the MoCA is a better measure of executive function. It tests visuospatial skills, for example, by asking patients to draw lines connecting letters and numbers in a numerical/alphabetical sequence. It also requires abstract reasoning, by asking patients to explain the similarity between, say, a banana and an orange or a train and a bicycle.
Indicators point to ischemic disease. Janet M, the patient who had an episode of acute confusion at the mall, was an ideal candidate for the MoCA. But her physician was more familiar with the MMSE and screened her with that tool first. She received a perfect score on the MMSE, but continued to worry that the episode at the mall was the beginning of dementia, so her physician followed up with the MoCA. Only after receiving a 28 of a possible 30 on the MoCA (≥26 is considered normal) was Janet reassured that she was not suffering from dementia. Based on evidence of poorly controlled blood pressure (167/89 mm Hg), the physician determined that the brief episode was more consistent with a transient ischemic attack. The patient was referred for brain imaging to be evaluated for ischemic disease.
Like the MMSE, the MoCA has been widely translated. It is available online in more than 20 languages.
Interpreting the MoCA. In a validation study of patients from a community clinic and an academic center, the test was administered to 94 patients with mild cognitive impairment, 93 patients with mild AD (MMSE score ≥17), and 90 healthy elderly adults.27 At a cut point of 26, the MMSE demonstrated a sensitivity of 18% in detecting mild impairment; among patients with mild AD, its sensitivity was 78%. In contrast, the MoCA detected 90% of cases of mild impairment and had 100% sensitivity for mild AD. Specificity was excellent for both the MMSE and the MoCA (100% and 87%, respectively). Thus, physicians can reassure patients who achieve high scores on the MoCA that there is no indication of cognitive impairment, and schedule follow-up testing in a year. Those whose MoCA scores indicate some impairment can be referred to memory clinics or consultants for a more thorough work-up.
The AD 8 Dementia Screening Interview detects early changes
This brief screening tool—a simple 8-question interview—takes about 3 minutes to administer, and accurately identifies patients in the earliest stages of AD or another dementing disorder.28 The questions examine the domains of memory, orientation, judgment, and function.
The test, which is designed primarily for an informant but is sometimes given to patients themselves, can be administered in a variety of ways: by reading the questions to the respondent, either in person or by telephone, or on a clipboard for self-administration. The AD 8 simply asks whether specific changes have been noted, without attributing causality. The respondent answers Yes, No, or Don’t Know. The final score is a sum of the number of Yes answers. A score of ≥2 is suggestive of cognitive impairment. The AD 8 has a sensitivity of 84% and specificity of 80%.28
7-Minute Neurocognitive Screen considers normal aging
This neurocognitive screen is highly sensitive and specific (mean, 92% and 96%, respectively) for the early stages of AD and can distinguish between cognitive changes due to normal aging and those due to dementia.29 The screen consists of 4 brief tests: orientation for date and time, a 5-word recall test to assess memory function, a clock-drawing test to evaluate visuospatial skills, and a semantic verbal fluency or output task. Examples of verbal fluency measures include category generation (eg, names of animals), letter (eg, number of words beginning with a particular letter), and first names.
Interpreting the 7-minute screen. This instrument was initially validated on 60 patients diagnosed as having probable AD and 60 community-dwelling volunteers of comparable age, sex distribution, and education. The neurocognitive screen has reasonable interrater and test-retest reliability and can be administered in a short time by an individual with little clinical judgment and minimal training. Unlike the MMSE, its outcome is not affected by age or education.29
When clock drawing is used as an independent screen, it is generally graded 0 to 5, based on the shape of the circle, the distribution and inclusion of all the numbers, and the length and placement of the hands. A score of ≤3 is suggestive of dementia. All the patients here were asked to show the time as 10 minutes past 5.
The numbers on this clock are not proportionally distributed, and only 1 hand is correctly placed.
Total score: 2
The numbers are outside the circle, and there are 2 number 10s on this clock, but both hands are correctly placed.
Total score: 3
A reasonable circle with both hands correctly placed, but the numbers are disproportionately distributed.
Total score: 4
Could it be depression?
Patients with suspected dementia should also be screened for depression, because this psychiatric disorder can impersonate dementia, or exist concurrently. Depression occurs in 25% of dementia patients; it is an independent risk factor for institutionalization, and should be detected and treated.30 Subclinical depression in older women is a risk factor for cognitive decline, as well.31 Although patient presentation is not a substitute for a screening tool, the following can be helpful in distinguishing dementia and depression:
Memory. A patient suffering from depression alone is more likely to complain of memory problems than a patient with cognitive dysfunction.
Psychomotor. A depressed patient is also more likely to exhibit psychomotor retardation. Function is rarely, or only mildly, impaired in the early stages of dementia, and the movements and reactions of a patient with mild cognitive impairment often appear normal.
Concentration. Depression interferes with the ability to concentrate, and the patient may exhibit an obvious disturbance. Patients with dementia often appear to have a normal ability to concentrate, but demonstrate greater intellectual strain in attempting to answer test questions.
A 5-item geriatric screen for depression
One useful screen for depression is the 5-item version of the Geriatric Depression Scale, which has been validated in older adults.32,33 It asks the following questions:
Are you basically satisfied with your life? Do you often get bored? Do you often feel helpless? Do you prefer to stay at home rather than going out and doing new things? Do you feel pretty worthless the way you are now? This 5-item test is scored by awarding 1 point if the answer to the first question is No, and 1 additional point for each Yes answer to the remaining questions. A score of 2 or more is considered to be diagnostic for depression.
Did you suspect depression?
As you may have suspected, Fran B—the last of our 3 patients—was suffering from depression rather than dementia. Fran had a score of 4, and was referred to a psychotherapist.
Mini-Mental State Examination
http://www.nia.nih.gov/NR/rdonlyres/1C0BFA48-8280-422B-97E6-195BBFD84BA2/0/mmse.PDF
Montreal Cognitive Assessment
www.mocatest.org
AD 8 Dementia Screening Interview
http://alzheimer.wustl.edu/About_Us/PDFs/AD8form2005.pdf
7-Minute Neurocognitive Screen
http://memorydoc.org/7minutescreen
Keep these tools on hand
The assessment tools discussed in this review can help you identify mild cognitive impairment or early dementia, rule out depression, and—in many cases—reassure patients (or concerned family members) that their experiences are consistent with normal aging. All are easy to incorporate into a busy primary care practice without undue concern about the time they will take to administer.
Correspondence
Diana R. Kerwin, MD, Division of Geriatrics and Gerontology, Medical College of Wisconsin, 9200 West Wisconsin Avenue, Milwaukee, WI 53226; [email protected]
1. O’Connor DW, Pollitt PA, Hyde JB, et al. Do general practitioners miss dementia in elderly patients? BMJ. 1988;297:1107-1110.
2. Lagaay AM, van der Meij JC, Hijmans W. Validation of medical history taking as part of a population based survey in subjects aged 85 and over. BMJ. 1992;304:1091-1092.
3. Cooper B, Bickel H, Schaufele M. Early development and progression of dementing illness in the elderly: a general-practice based study. Psychol Med. 1996;26:411-419.
4. Olafsdottir M, Skoog I, Marcusson J. Detection of dementia in primary care: the Linkoping study. Dement Geriatr Cogn Disord. 2000;11:223-229.
5. Valcour VG, Masaki KH, Curb JD, et al. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964-2968.
6. Wilkinson D, Stave C, Keohane D, et al. The role of general practitioners in the diagnosis and treatment of Alzheimer’s disease: a multinational survey. J Int Med Res. 2004;32:149-159.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
8. Ritchie CW, Ames D, Clayton T, et al. Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am J Geriatr Psych. 2004;12:358-369.
9. Winblad B, Engedal K, Soinenen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489-495.
10. Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136-145.
11. Doody RS, Geldmacher DS, Gordon B, et al. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58:427-433.
12. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia. Arch Intern Med. 2006;166:2182-2188.
13. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease. A randomized controlled trial. JAMA. 1996;276:1725-1731.
14. 2008 Alzheimer’s disease facts and figures. Available at: http://www.alz.org/alzheimers_disease_publications_reports.asp. Accessed March 19, 2008.
15. US Preventive Services Task Force. Screening for dementia: recommendations and rationale. June 2003. Available at: http://www.ahcpr.gov/clinic/3rduspstf/dementia/dementrr.htm. Accessed March 18, 2008.
16. AGS Clinical Practice Committee. Guidelines abstracted from the American Academy of Neurology’s Dementia Guidelines for early detection, diagnosis, and management of dementia. J Am Geriatr Soc. 2003;51:869-873.
17. Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: Mild Cognitive Impairment(an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133-1142.
18. Wenger NS, Roth CP, Shekelle P. ACOVE Investigators. Introduction to the Assessing Care of Vulnerable Elders-3 Quality Indicator Measurement Set. J Am Geriatr Soc. 2007;55(suppl 2):s247-s251.
19. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):s293-s301.
20. Adelman AM. Initial evaluation of the patient with suspected dementia. Am Fam Physician. 2005;71:1745-1750.
21. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
22. Boustani M, Peterson B, Hanson L, et al. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138:927-937.
23. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level. JAMA. 1993;18:2386-2391.
24. Borson S, Scanlan J, Brush M, et al. The Mini-Cog: a cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021-1027.
25. Borson S, Scanlan JM, Chen P, et al. The mini-cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451-1454.
26. Steenland NK, Auman CM, Patel PM, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis. 2008;15:419-427.
27. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
28. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559-564.
29. Solomon PR, Hirschoff A, Kelly B, et al. A 7 minute neurocognitive screening battery highly sensitive to Alzheimer’s disease. Arch Neurol. 1998;55:349-355.
30. Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer’s disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction. The DIADS. Arch Gen Psychol. 2003;60:737-746.
31. Artero S, Ancelin ML, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979-984.
32. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51:694-698.
33. Sheikh JL, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173.
- Avoid cognitive screening solely on the basis of age (SOR A).
- Screen vulnerable elderly patients at their initial visit and annually thereafter (SOR A).
- Ensure that all patients who undergo cognitive screening are tested for depression (SOR A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented
Janet M, a 69-year-old woman with a history of hypertension, comes for a visit because she thinks she has Alzheimer’s disease (AD). She recently had an episode of acute confusion while shopping at the mall; when she returned to her car, she couldn’t remember how to get home. The episode cleared within minutes and hasn’t recurred.
Jack S, an 84-year-old man, seeks medical care for pain in his right shoulder. He injured the rotator cuff several years ago, but it’s been fine since he completed physical therapy—until he tripped and fell while walking outside about a week ago. His daughter is concerned about his “forgetfulness” and increasing inability to remember certain words, but the patient thinks this is a natural consequence of age.
Fran B, a 72-year-old, presents with complaints of memory problems that began about 6 months ago. She’s worried about her son and has had increasing difficulty concentrating, sleeping, and keeping track of her things.
If these were your patients, whom would you screen for dementia? Would you decide whether to screen based on your “gut,” or a defined set of criteria? Would you have several screening tools on hand, and know enough about them to determine which one might be best suited for a particular patient?
If you make decisions about screening based on your gut or aren’t sure which tools are best for which patients, you’re far from alone. Cognitive impairment, particularly in the early stages, can be difficult and time-consuming to detect, and community physicians fail to diagnose mild-to-moderate dementia more than 50% of the time.1-5
Family members and caregivers often overlook declines in cognitive function, as well. In a study of 741 caregivers of patients with AD, an average of 4 months went by between the time symptoms were first noticed and the patient was seen by a physician—and 22% of the caregivers waited more than a year.6
Cognitive decline can be slowed with early Dx
Early diagnosis of AD or any dementia is important for a number of reasons. In some cases, cognitive impairment may be related to medical conditions—head trauma, Parkinson’s disease, human immunodeficiency virus, thyroid disorder, among others—that can be modified or reversed with treatment.7 There is evidence, too, that medical, behavioral, and social interventions can delay the cognitive and functional decline associated with AD, thereby helping to prolong the time the patient can remain at home. Early diagnosis also facilitates legal and financial family planning, and makes it possible to take appropriate safety measures.8-13
AD affects approximately 5 million US residents.14 With an aging population, that number is expected to surge in the decades ahead. To help you provide optimal care for your geriatric patients, this review will detail when to screen, which tools to use, and how best to care for the 3 elderly patients in the opener.
When and whether to screen
Despite the benefits of early detection, population-based screening based on age alone is not recommended. Guidelines recommend focused screening of patients in high-risk groups and on a case-by-case basis.15
US Preventive Services Task Force (USPSTF) guidelines recommend that physicians evaluate older patients for dementia whenever there is a suggestion of cognitive impairment, based on clinical observation or concern expressed by the individuals themselves or by family members, caretakers, or friends.15
However, cognitive screening generally provides better results in populations at higher risk of dementia.16,17 With that in mind, the Assessing Care of Vulnerable Elders (ACOVE), a collaborative project to develop a set of quality indicators for care of the elderly, recently recommended cognitive and functional screening of all “vulnerable elderly.”18,19
Who are the vulnerable elderly?
ACOVE defines the vulnerable elderly as individuals who are 65 years of age and older who live in the community and are at high risk of death or functional decline over the next 2 years. You can use a screening tool to identify members of this high-risk group. Or you can simply ask a few questions and classify any noninstitutionalized older person who reports being in poor health and/or acknowledges difficulty with activities of daily living—eg, money or medication management, dressing, grooming, or preparing simple meals—as a vulnerable elderly patient.
ACOVE recommends screening all vulnerable elderly patients who are new to a primary care practice or inpatient service, and following up with an annual evaluation to detect any changes in memory and function.18,19 Based on the recommendations of ACOVE and the USPSTF, all 3 patients described earlier would be candidates for screening.
Choosing the best screening tool
There is no serum or radiographic test available for the diagnosis of AD, and a thorough clinical evaluation can be extremely time-consuming. In the primary care setting, focused evaluations are both useful and cost effective.
There are a number of valid and reliable cognitive screening tools, including the Mini-Mental State Examination (MMSE), the Mini-Cog, the Montreal Cognitive Assessment (MoCA), the AD 8 Dementia Screening Interview, and the 7-Minute Neurocognitive Screen. In evaluating these and other screening tools, consider the sensitivity and specificity of each. Consider, too, features that may make one test better suited than another for a particular patient or specific circumstance (TABLE).
TABLE
Cognitive screening tools: How they compare
| TOOL (TIME TO ADMINISTER) | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| MMSE (5-10 min) | Tracks/quantifies changes; easy to administer; widely accepted; available in >50 languages | Not specific to AD or sensitive to mild dementia; influenced by age, education, and language skills |
| Mini-Cog (3 min) | Brief, easy to administer and score; unaffected by education, language skill; high sensitivity; easily paired with FAQ | |
| MoCA (10 min) | High sensitivity for MCI and mild AD; available in >20 languages; especially useful for patients with memory complaints but normal MMSE score | More time-consuming than other screening tools |
| AD 8 (3 min) | Administered in person or by phone; identifies earliest stages of dementia | Designed for informant, who may not be available, but can be given to patient |
| 7-Minute Neurocognitive Screen | Highly sensitive to early stages of AD | |
| AD, Alzheimer’s disease; FAQ, Functional Activities Questionnaire; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment. | ||
The Mini-Mental State Exam is the gold standard
Considered the gold standard in dementia assessment, the MMSE is the most widely used cognitive screening tool in the United States.20 The MMSE samples 6 cognitive areas—orientation, registration, attention and calculation, recall, language, and constructional skill.21 It has a high degree of validity in detecting dementia, and is particularly useful in tracking and quantifying changes over time. Well-known features include the serial 7s, in which patients are asked to count backwards from 100 by 7s, and the 3-stage command: Take a paper in your right hand, fold it in half, and put it on the floor.
Besides being relatively short (testing takes 5-10 minutes), the MMSE is easy to administer. It has been translated into more than 50 languages, and can therefore be used in many cultural settings.
Interpreting the MMSE. A total score of ≤23 out of a maximum of 30 points is suggestive of dementia. However, patient performance on the MMSE is influenced by a number of factors unrelated to cognitive function, such as age, educational level, deficits in language skills, and motor or visual impairment.22 Thus, the cut point may be adjusted depending on the patient or population being screened, and the positive predictive value and sensitivity and specificity for dementia vary accordingly. In a population-based sample of >18,000 individuals with a cut point of 24, the sensitivity was 87% and the specificity was 82%.23
The usefulness of the MMSE is also limited because the test is not specific to AD and is not sensitive to mild dementia. When the test is used to screen highly intelligent, well-educated patients, it may fail to detect any decline in memory or cognitive function.
The Mini-Cog is a quick and easy alternative
The Mini-Cog screen consists of 2 simple components that evaluate executive function: a clock-drawing test and word recall.24 The patient is given paper and pencil and asked to draw a clock with hands set on a specified time—5:10, say. First, however, the screener recites 3 common but unrelated words; the patient is asked to recite the words before drawing the clock and to recall them afterwards. The entire test takes about 3 minutes.
Interpreting the Mini-Cog. Besides being quick and easy to administer, the Mini-Cog is easy to score:
- Patients who do not recall any of the words are classified as demented.
- Patients who recall all of the words are classified as nondemented
- Patients who recall 1 or 2 words are classified based on their clock drawing: They’re considered nondemented if the clock is normal and demented if it is not.
When clock-drawing is part of the Mini-Cog screen, the results are simply considered normal or abnormal. For a clock to be considered normal, all the numbers must be in the correct sequence and the hands correctly positioned to show the designated time.24 (The clock-drawing can also be used as an independent screen. For more about that, see “Clock-drawing: What to look for”.)
The Mini-Cog has been found to be highly accurate. And, unlike the MMSE, the Mini-Cog is not skewed by education level or language skill. One study based on interviews with “informants”—the family, friends, or caregivers of patients being tested for dementia—examined the Mini-Cog’s ability to differentiate between 129 demented and 120 nondemented older adults who were culturally, linguistically, and educationally heterogeneous. The Mini-Cog classified 96% of participants correctly and had a sensitivity of 99%.24
Another retrospective analysis of a random sample of more than 1100 elderly people compared the Mini-Cog to the MMSE. In this case, the Mini-Cog and MMSE (with a cut point of 25) demonstrated similar sensitivity (76% vs 79%) and specificity (89% vs 88%) for identifying individuals with dementia.25
Jack S, whose daughter worried about his “forgetfulness,” was given the Mini-Cog. He drew a clock with the big hand incorrectly placed, and recalled only 2 words. Because his daughter was with him, the physician asked her to complete a Functional Activities Questionnaire (FAQ), a test designed for family members, caregivers, and other informants. The findings indicated that Jack had dementia, and the physician gave the family a referral to a specialist in elder care.
The Mini-Cog–FAQ. A recent study paired the Mini-Cog with the FAQ, which asks the informant to rate the patient’s ability to perform a variety of activities—eg, paying bills, shopping, engaging in hobbies, and preparing a meal. When used alone, the FAQ has a high sensitivity and specificity, but patient testing is still necessary. Used together, the Mini-Cog–FAQ allowed researchers to classify patients as cognitively normal, demented, or mildly cognitively impaired with 83% accuracy.26
Montreal Cognitive Assessment detects mild impairment
The MoCA is a 10-minute screening tool designed to help physicians detect mild cognitive impairment, which is considered to be predictive of dementia.27 This tool is especially useful for individuals who present with memory complaints but achieve a normal score (26-30) on the MMSE, as the MoCA is a better measure of executive function. It tests visuospatial skills, for example, by asking patients to draw lines connecting letters and numbers in a numerical/alphabetical sequence. It also requires abstract reasoning, by asking patients to explain the similarity between, say, a banana and an orange or a train and a bicycle.
Indicators point to ischemic disease. Janet M, the patient who had an episode of acute confusion at the mall, was an ideal candidate for the MoCA. But her physician was more familiar with the MMSE and screened her with that tool first. She received a perfect score on the MMSE, but continued to worry that the episode at the mall was the beginning of dementia, so her physician followed up with the MoCA. Only after receiving a 28 of a possible 30 on the MoCA (≥26 is considered normal) was Janet reassured that she was not suffering from dementia. Based on evidence of poorly controlled blood pressure (167/89 mm Hg), the physician determined that the brief episode was more consistent with a transient ischemic attack. The patient was referred for brain imaging to be evaluated for ischemic disease.
Like the MMSE, the MoCA has been widely translated. It is available online in more than 20 languages.
Interpreting the MoCA. In a validation study of patients from a community clinic and an academic center, the test was administered to 94 patients with mild cognitive impairment, 93 patients with mild AD (MMSE score ≥17), and 90 healthy elderly adults.27 At a cut point of 26, the MMSE demonstrated a sensitivity of 18% in detecting mild impairment; among patients with mild AD, its sensitivity was 78%. In contrast, the MoCA detected 90% of cases of mild impairment and had 100% sensitivity for mild AD. Specificity was excellent for both the MMSE and the MoCA (100% and 87%, respectively). Thus, physicians can reassure patients who achieve high scores on the MoCA that there is no indication of cognitive impairment, and schedule follow-up testing in a year. Those whose MoCA scores indicate some impairment can be referred to memory clinics or consultants for a more thorough work-up.
The AD 8 Dementia Screening Interview detects early changes
This brief screening tool—a simple 8-question interview—takes about 3 minutes to administer, and accurately identifies patients in the earliest stages of AD or another dementing disorder.28 The questions examine the domains of memory, orientation, judgment, and function.
The test, which is designed primarily for an informant but is sometimes given to patients themselves, can be administered in a variety of ways: by reading the questions to the respondent, either in person or by telephone, or on a clipboard for self-administration. The AD 8 simply asks whether specific changes have been noted, without attributing causality. The respondent answers Yes, No, or Don’t Know. The final score is a sum of the number of Yes answers. A score of ≥2 is suggestive of cognitive impairment. The AD 8 has a sensitivity of 84% and specificity of 80%.28
7-Minute Neurocognitive Screen considers normal aging
This neurocognitive screen is highly sensitive and specific (mean, 92% and 96%, respectively) for the early stages of AD and can distinguish between cognitive changes due to normal aging and those due to dementia.29 The screen consists of 4 brief tests: orientation for date and time, a 5-word recall test to assess memory function, a clock-drawing test to evaluate visuospatial skills, and a semantic verbal fluency or output task. Examples of verbal fluency measures include category generation (eg, names of animals), letter (eg, number of words beginning with a particular letter), and first names.
Interpreting the 7-minute screen. This instrument was initially validated on 60 patients diagnosed as having probable AD and 60 community-dwelling volunteers of comparable age, sex distribution, and education. The neurocognitive screen has reasonable interrater and test-retest reliability and can be administered in a short time by an individual with little clinical judgment and minimal training. Unlike the MMSE, its outcome is not affected by age or education.29
When clock drawing is used as an independent screen, it is generally graded 0 to 5, based on the shape of the circle, the distribution and inclusion of all the numbers, and the length and placement of the hands. A score of ≤3 is suggestive of dementia. All the patients here were asked to show the time as 10 minutes past 5.
The numbers on this clock are not proportionally distributed, and only 1 hand is correctly placed.
Total score: 2
The numbers are outside the circle, and there are 2 number 10s on this clock, but both hands are correctly placed.
Total score: 3
A reasonable circle with both hands correctly placed, but the numbers are disproportionately distributed.
Total score: 4
Could it be depression?
Patients with suspected dementia should also be screened for depression, because this psychiatric disorder can impersonate dementia, or exist concurrently. Depression occurs in 25% of dementia patients; it is an independent risk factor for institutionalization, and should be detected and treated.30 Subclinical depression in older women is a risk factor for cognitive decline, as well.31 Although patient presentation is not a substitute for a screening tool, the following can be helpful in distinguishing dementia and depression:
Memory. A patient suffering from depression alone is more likely to complain of memory problems than a patient with cognitive dysfunction.
Psychomotor. A depressed patient is also more likely to exhibit psychomotor retardation. Function is rarely, or only mildly, impaired in the early stages of dementia, and the movements and reactions of a patient with mild cognitive impairment often appear normal.
Concentration. Depression interferes with the ability to concentrate, and the patient may exhibit an obvious disturbance. Patients with dementia often appear to have a normal ability to concentrate, but demonstrate greater intellectual strain in attempting to answer test questions.
A 5-item geriatric screen for depression
One useful screen for depression is the 5-item version of the Geriatric Depression Scale, which has been validated in older adults.32,33 It asks the following questions:
Are you basically satisfied with your life? Do you often get bored? Do you often feel helpless? Do you prefer to stay at home rather than going out and doing new things? Do you feel pretty worthless the way you are now? This 5-item test is scored by awarding 1 point if the answer to the first question is No, and 1 additional point for each Yes answer to the remaining questions. A score of 2 or more is considered to be diagnostic for depression.
Did you suspect depression?
As you may have suspected, Fran B—the last of our 3 patients—was suffering from depression rather than dementia. Fran had a score of 4, and was referred to a psychotherapist.
Mini-Mental State Examination
http://www.nia.nih.gov/NR/rdonlyres/1C0BFA48-8280-422B-97E6-195BBFD84BA2/0/mmse.PDF
Montreal Cognitive Assessment
www.mocatest.org
AD 8 Dementia Screening Interview
http://alzheimer.wustl.edu/About_Us/PDFs/AD8form2005.pdf
7-Minute Neurocognitive Screen
http://memorydoc.org/7minutescreen
Keep these tools on hand
The assessment tools discussed in this review can help you identify mild cognitive impairment or early dementia, rule out depression, and—in many cases—reassure patients (or concerned family members) that their experiences are consistent with normal aging. All are easy to incorporate into a busy primary care practice without undue concern about the time they will take to administer.
Correspondence
Diana R. Kerwin, MD, Division of Geriatrics and Gerontology, Medical College of Wisconsin, 9200 West Wisconsin Avenue, Milwaukee, WI 53226; [email protected]
- Avoid cognitive screening solely on the basis of age (SOR A).
- Screen vulnerable elderly patients at their initial visit and annually thereafter (SOR A).
- Ensure that all patients who undergo cognitive screening are tested for depression (SOR A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented
Janet M, a 69-year-old woman with a history of hypertension, comes for a visit because she thinks she has Alzheimer’s disease (AD). She recently had an episode of acute confusion while shopping at the mall; when she returned to her car, she couldn’t remember how to get home. The episode cleared within minutes and hasn’t recurred.
Jack S, an 84-year-old man, seeks medical care for pain in his right shoulder. He injured the rotator cuff several years ago, but it’s been fine since he completed physical therapy—until he tripped and fell while walking outside about a week ago. His daughter is concerned about his “forgetfulness” and increasing inability to remember certain words, but the patient thinks this is a natural consequence of age.
Fran B, a 72-year-old, presents with complaints of memory problems that began about 6 months ago. She’s worried about her son and has had increasing difficulty concentrating, sleeping, and keeping track of her things.
If these were your patients, whom would you screen for dementia? Would you decide whether to screen based on your “gut,” or a defined set of criteria? Would you have several screening tools on hand, and know enough about them to determine which one might be best suited for a particular patient?
If you make decisions about screening based on your gut or aren’t sure which tools are best for which patients, you’re far from alone. Cognitive impairment, particularly in the early stages, can be difficult and time-consuming to detect, and community physicians fail to diagnose mild-to-moderate dementia more than 50% of the time.1-5
Family members and caregivers often overlook declines in cognitive function, as well. In a study of 741 caregivers of patients with AD, an average of 4 months went by between the time symptoms were first noticed and the patient was seen by a physician—and 22% of the caregivers waited more than a year.6
Cognitive decline can be slowed with early Dx
Early diagnosis of AD or any dementia is important for a number of reasons. In some cases, cognitive impairment may be related to medical conditions—head trauma, Parkinson’s disease, human immunodeficiency virus, thyroid disorder, among others—that can be modified or reversed with treatment.7 There is evidence, too, that medical, behavioral, and social interventions can delay the cognitive and functional decline associated with AD, thereby helping to prolong the time the patient can remain at home. Early diagnosis also facilitates legal and financial family planning, and makes it possible to take appropriate safety measures.8-13
AD affects approximately 5 million US residents.14 With an aging population, that number is expected to surge in the decades ahead. To help you provide optimal care for your geriatric patients, this review will detail when to screen, which tools to use, and how best to care for the 3 elderly patients in the opener.
When and whether to screen
Despite the benefits of early detection, population-based screening based on age alone is not recommended. Guidelines recommend focused screening of patients in high-risk groups and on a case-by-case basis.15
US Preventive Services Task Force (USPSTF) guidelines recommend that physicians evaluate older patients for dementia whenever there is a suggestion of cognitive impairment, based on clinical observation or concern expressed by the individuals themselves or by family members, caretakers, or friends.15
However, cognitive screening generally provides better results in populations at higher risk of dementia.16,17 With that in mind, the Assessing Care of Vulnerable Elders (ACOVE), a collaborative project to develop a set of quality indicators for care of the elderly, recently recommended cognitive and functional screening of all “vulnerable elderly.”18,19
Who are the vulnerable elderly?
ACOVE defines the vulnerable elderly as individuals who are 65 years of age and older who live in the community and are at high risk of death or functional decline over the next 2 years. You can use a screening tool to identify members of this high-risk group. Or you can simply ask a few questions and classify any noninstitutionalized older person who reports being in poor health and/or acknowledges difficulty with activities of daily living—eg, money or medication management, dressing, grooming, or preparing simple meals—as a vulnerable elderly patient.
ACOVE recommends screening all vulnerable elderly patients who are new to a primary care practice or inpatient service, and following up with an annual evaluation to detect any changes in memory and function.18,19 Based on the recommendations of ACOVE and the USPSTF, all 3 patients described earlier would be candidates for screening.
Choosing the best screening tool
There is no serum or radiographic test available for the diagnosis of AD, and a thorough clinical evaluation can be extremely time-consuming. In the primary care setting, focused evaluations are both useful and cost effective.
There are a number of valid and reliable cognitive screening tools, including the Mini-Mental State Examination (MMSE), the Mini-Cog, the Montreal Cognitive Assessment (MoCA), the AD 8 Dementia Screening Interview, and the 7-Minute Neurocognitive Screen. In evaluating these and other screening tools, consider the sensitivity and specificity of each. Consider, too, features that may make one test better suited than another for a particular patient or specific circumstance (TABLE).
TABLE
Cognitive screening tools: How they compare
| TOOL (TIME TO ADMINISTER) | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| MMSE (5-10 min) | Tracks/quantifies changes; easy to administer; widely accepted; available in >50 languages | Not specific to AD or sensitive to mild dementia; influenced by age, education, and language skills |
| Mini-Cog (3 min) | Brief, easy to administer and score; unaffected by education, language skill; high sensitivity; easily paired with FAQ | |
| MoCA (10 min) | High sensitivity for MCI and mild AD; available in >20 languages; especially useful for patients with memory complaints but normal MMSE score | More time-consuming than other screening tools |
| AD 8 (3 min) | Administered in person or by phone; identifies earliest stages of dementia | Designed for informant, who may not be available, but can be given to patient |
| 7-Minute Neurocognitive Screen | Highly sensitive to early stages of AD | |
| AD, Alzheimer’s disease; FAQ, Functional Activities Questionnaire; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment. | ||
The Mini-Mental State Exam is the gold standard
Considered the gold standard in dementia assessment, the MMSE is the most widely used cognitive screening tool in the United States.20 The MMSE samples 6 cognitive areas—orientation, registration, attention and calculation, recall, language, and constructional skill.21 It has a high degree of validity in detecting dementia, and is particularly useful in tracking and quantifying changes over time. Well-known features include the serial 7s, in which patients are asked to count backwards from 100 by 7s, and the 3-stage command: Take a paper in your right hand, fold it in half, and put it on the floor.
Besides being relatively short (testing takes 5-10 minutes), the MMSE is easy to administer. It has been translated into more than 50 languages, and can therefore be used in many cultural settings.
Interpreting the MMSE. A total score of ≤23 out of a maximum of 30 points is suggestive of dementia. However, patient performance on the MMSE is influenced by a number of factors unrelated to cognitive function, such as age, educational level, deficits in language skills, and motor or visual impairment.22 Thus, the cut point may be adjusted depending on the patient or population being screened, and the positive predictive value and sensitivity and specificity for dementia vary accordingly. In a population-based sample of >18,000 individuals with a cut point of 24, the sensitivity was 87% and the specificity was 82%.23
The usefulness of the MMSE is also limited because the test is not specific to AD and is not sensitive to mild dementia. When the test is used to screen highly intelligent, well-educated patients, it may fail to detect any decline in memory or cognitive function.
The Mini-Cog is a quick and easy alternative
The Mini-Cog screen consists of 2 simple components that evaluate executive function: a clock-drawing test and word recall.24 The patient is given paper and pencil and asked to draw a clock with hands set on a specified time—5:10, say. First, however, the screener recites 3 common but unrelated words; the patient is asked to recite the words before drawing the clock and to recall them afterwards. The entire test takes about 3 minutes.
Interpreting the Mini-Cog. Besides being quick and easy to administer, the Mini-Cog is easy to score:
- Patients who do not recall any of the words are classified as demented.
- Patients who recall all of the words are classified as nondemented
- Patients who recall 1 or 2 words are classified based on their clock drawing: They’re considered nondemented if the clock is normal and demented if it is not.
When clock-drawing is part of the Mini-Cog screen, the results are simply considered normal or abnormal. For a clock to be considered normal, all the numbers must be in the correct sequence and the hands correctly positioned to show the designated time.24 (The clock-drawing can also be used as an independent screen. For more about that, see “Clock-drawing: What to look for”.)
The Mini-Cog has been found to be highly accurate. And, unlike the MMSE, the Mini-Cog is not skewed by education level or language skill. One study based on interviews with “informants”—the family, friends, or caregivers of patients being tested for dementia—examined the Mini-Cog’s ability to differentiate between 129 demented and 120 nondemented older adults who were culturally, linguistically, and educationally heterogeneous. The Mini-Cog classified 96% of participants correctly and had a sensitivity of 99%.24
Another retrospective analysis of a random sample of more than 1100 elderly people compared the Mini-Cog to the MMSE. In this case, the Mini-Cog and MMSE (with a cut point of 25) demonstrated similar sensitivity (76% vs 79%) and specificity (89% vs 88%) for identifying individuals with dementia.25
Jack S, whose daughter worried about his “forgetfulness,” was given the Mini-Cog. He drew a clock with the big hand incorrectly placed, and recalled only 2 words. Because his daughter was with him, the physician asked her to complete a Functional Activities Questionnaire (FAQ), a test designed for family members, caregivers, and other informants. The findings indicated that Jack had dementia, and the physician gave the family a referral to a specialist in elder care.
The Mini-Cog–FAQ. A recent study paired the Mini-Cog with the FAQ, which asks the informant to rate the patient’s ability to perform a variety of activities—eg, paying bills, shopping, engaging in hobbies, and preparing a meal. When used alone, the FAQ has a high sensitivity and specificity, but patient testing is still necessary. Used together, the Mini-Cog–FAQ allowed researchers to classify patients as cognitively normal, demented, or mildly cognitively impaired with 83% accuracy.26
Montreal Cognitive Assessment detects mild impairment
The MoCA is a 10-minute screening tool designed to help physicians detect mild cognitive impairment, which is considered to be predictive of dementia.27 This tool is especially useful for individuals who present with memory complaints but achieve a normal score (26-30) on the MMSE, as the MoCA is a better measure of executive function. It tests visuospatial skills, for example, by asking patients to draw lines connecting letters and numbers in a numerical/alphabetical sequence. It also requires abstract reasoning, by asking patients to explain the similarity between, say, a banana and an orange or a train and a bicycle.
Indicators point to ischemic disease. Janet M, the patient who had an episode of acute confusion at the mall, was an ideal candidate for the MoCA. But her physician was more familiar with the MMSE and screened her with that tool first. She received a perfect score on the MMSE, but continued to worry that the episode at the mall was the beginning of dementia, so her physician followed up with the MoCA. Only after receiving a 28 of a possible 30 on the MoCA (≥26 is considered normal) was Janet reassured that she was not suffering from dementia. Based on evidence of poorly controlled blood pressure (167/89 mm Hg), the physician determined that the brief episode was more consistent with a transient ischemic attack. The patient was referred for brain imaging to be evaluated for ischemic disease.
Like the MMSE, the MoCA has been widely translated. It is available online in more than 20 languages.
Interpreting the MoCA. In a validation study of patients from a community clinic and an academic center, the test was administered to 94 patients with mild cognitive impairment, 93 patients with mild AD (MMSE score ≥17), and 90 healthy elderly adults.27 At a cut point of 26, the MMSE demonstrated a sensitivity of 18% in detecting mild impairment; among patients with mild AD, its sensitivity was 78%. In contrast, the MoCA detected 90% of cases of mild impairment and had 100% sensitivity for mild AD. Specificity was excellent for both the MMSE and the MoCA (100% and 87%, respectively). Thus, physicians can reassure patients who achieve high scores on the MoCA that there is no indication of cognitive impairment, and schedule follow-up testing in a year. Those whose MoCA scores indicate some impairment can be referred to memory clinics or consultants for a more thorough work-up.
The AD 8 Dementia Screening Interview detects early changes
This brief screening tool—a simple 8-question interview—takes about 3 minutes to administer, and accurately identifies patients in the earliest stages of AD or another dementing disorder.28 The questions examine the domains of memory, orientation, judgment, and function.
The test, which is designed primarily for an informant but is sometimes given to patients themselves, can be administered in a variety of ways: by reading the questions to the respondent, either in person or by telephone, or on a clipboard for self-administration. The AD 8 simply asks whether specific changes have been noted, without attributing causality. The respondent answers Yes, No, or Don’t Know. The final score is a sum of the number of Yes answers. A score of ≥2 is suggestive of cognitive impairment. The AD 8 has a sensitivity of 84% and specificity of 80%.28
7-Minute Neurocognitive Screen considers normal aging
This neurocognitive screen is highly sensitive and specific (mean, 92% and 96%, respectively) for the early stages of AD and can distinguish between cognitive changes due to normal aging and those due to dementia.29 The screen consists of 4 brief tests: orientation for date and time, a 5-word recall test to assess memory function, a clock-drawing test to evaluate visuospatial skills, and a semantic verbal fluency or output task. Examples of verbal fluency measures include category generation (eg, names of animals), letter (eg, number of words beginning with a particular letter), and first names.
Interpreting the 7-minute screen. This instrument was initially validated on 60 patients diagnosed as having probable AD and 60 community-dwelling volunteers of comparable age, sex distribution, and education. The neurocognitive screen has reasonable interrater and test-retest reliability and can be administered in a short time by an individual with little clinical judgment and minimal training. Unlike the MMSE, its outcome is not affected by age or education.29
When clock drawing is used as an independent screen, it is generally graded 0 to 5, based on the shape of the circle, the distribution and inclusion of all the numbers, and the length and placement of the hands. A score of ≤3 is suggestive of dementia. All the patients here were asked to show the time as 10 minutes past 5.
The numbers on this clock are not proportionally distributed, and only 1 hand is correctly placed.
Total score: 2
The numbers are outside the circle, and there are 2 number 10s on this clock, but both hands are correctly placed.
Total score: 3
A reasonable circle with both hands correctly placed, but the numbers are disproportionately distributed.
Total score: 4
Could it be depression?
Patients with suspected dementia should also be screened for depression, because this psychiatric disorder can impersonate dementia, or exist concurrently. Depression occurs in 25% of dementia patients; it is an independent risk factor for institutionalization, and should be detected and treated.30 Subclinical depression in older women is a risk factor for cognitive decline, as well.31 Although patient presentation is not a substitute for a screening tool, the following can be helpful in distinguishing dementia and depression:
Memory. A patient suffering from depression alone is more likely to complain of memory problems than a patient with cognitive dysfunction.
Psychomotor. A depressed patient is also more likely to exhibit psychomotor retardation. Function is rarely, or only mildly, impaired in the early stages of dementia, and the movements and reactions of a patient with mild cognitive impairment often appear normal.
Concentration. Depression interferes with the ability to concentrate, and the patient may exhibit an obvious disturbance. Patients with dementia often appear to have a normal ability to concentrate, but demonstrate greater intellectual strain in attempting to answer test questions.
A 5-item geriatric screen for depression
One useful screen for depression is the 5-item version of the Geriatric Depression Scale, which has been validated in older adults.32,33 It asks the following questions:
Are you basically satisfied with your life? Do you often get bored? Do you often feel helpless? Do you prefer to stay at home rather than going out and doing new things? Do you feel pretty worthless the way you are now? This 5-item test is scored by awarding 1 point if the answer to the first question is No, and 1 additional point for each Yes answer to the remaining questions. A score of 2 or more is considered to be diagnostic for depression.
Did you suspect depression?
As you may have suspected, Fran B—the last of our 3 patients—was suffering from depression rather than dementia. Fran had a score of 4, and was referred to a psychotherapist.
Mini-Mental State Examination
http://www.nia.nih.gov/NR/rdonlyres/1C0BFA48-8280-422B-97E6-195BBFD84BA2/0/mmse.PDF
Montreal Cognitive Assessment
www.mocatest.org
AD 8 Dementia Screening Interview
http://alzheimer.wustl.edu/About_Us/PDFs/AD8form2005.pdf
7-Minute Neurocognitive Screen
http://memorydoc.org/7minutescreen
Keep these tools on hand
The assessment tools discussed in this review can help you identify mild cognitive impairment or early dementia, rule out depression, and—in many cases—reassure patients (or concerned family members) that their experiences are consistent with normal aging. All are easy to incorporate into a busy primary care practice without undue concern about the time they will take to administer.
Correspondence
Diana R. Kerwin, MD, Division of Geriatrics and Gerontology, Medical College of Wisconsin, 9200 West Wisconsin Avenue, Milwaukee, WI 53226; [email protected]
1. O’Connor DW, Pollitt PA, Hyde JB, et al. Do general practitioners miss dementia in elderly patients? BMJ. 1988;297:1107-1110.
2. Lagaay AM, van der Meij JC, Hijmans W. Validation of medical history taking as part of a population based survey in subjects aged 85 and over. BMJ. 1992;304:1091-1092.
3. Cooper B, Bickel H, Schaufele M. Early development and progression of dementing illness in the elderly: a general-practice based study. Psychol Med. 1996;26:411-419.
4. Olafsdottir M, Skoog I, Marcusson J. Detection of dementia in primary care: the Linkoping study. Dement Geriatr Cogn Disord. 2000;11:223-229.
5. Valcour VG, Masaki KH, Curb JD, et al. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964-2968.
6. Wilkinson D, Stave C, Keohane D, et al. The role of general practitioners in the diagnosis and treatment of Alzheimer’s disease: a multinational survey. J Int Med Res. 2004;32:149-159.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
8. Ritchie CW, Ames D, Clayton T, et al. Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am J Geriatr Psych. 2004;12:358-369.
9. Winblad B, Engedal K, Soinenen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489-495.
10. Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136-145.
11. Doody RS, Geldmacher DS, Gordon B, et al. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58:427-433.
12. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia. Arch Intern Med. 2006;166:2182-2188.
13. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease. A randomized controlled trial. JAMA. 1996;276:1725-1731.
14. 2008 Alzheimer’s disease facts and figures. Available at: http://www.alz.org/alzheimers_disease_publications_reports.asp. Accessed March 19, 2008.
15. US Preventive Services Task Force. Screening for dementia: recommendations and rationale. June 2003. Available at: http://www.ahcpr.gov/clinic/3rduspstf/dementia/dementrr.htm. Accessed March 18, 2008.
16. AGS Clinical Practice Committee. Guidelines abstracted from the American Academy of Neurology’s Dementia Guidelines for early detection, diagnosis, and management of dementia. J Am Geriatr Soc. 2003;51:869-873.
17. Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: Mild Cognitive Impairment(an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133-1142.
18. Wenger NS, Roth CP, Shekelle P. ACOVE Investigators. Introduction to the Assessing Care of Vulnerable Elders-3 Quality Indicator Measurement Set. J Am Geriatr Soc. 2007;55(suppl 2):s247-s251.
19. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):s293-s301.
20. Adelman AM. Initial evaluation of the patient with suspected dementia. Am Fam Physician. 2005;71:1745-1750.
21. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
22. Boustani M, Peterson B, Hanson L, et al. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138:927-937.
23. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level. JAMA. 1993;18:2386-2391.
24. Borson S, Scanlan J, Brush M, et al. The Mini-Cog: a cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021-1027.
25. Borson S, Scanlan JM, Chen P, et al. The mini-cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451-1454.
26. Steenland NK, Auman CM, Patel PM, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis. 2008;15:419-427.
27. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
28. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559-564.
29. Solomon PR, Hirschoff A, Kelly B, et al. A 7 minute neurocognitive screening battery highly sensitive to Alzheimer’s disease. Arch Neurol. 1998;55:349-355.
30. Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer’s disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction. The DIADS. Arch Gen Psychol. 2003;60:737-746.
31. Artero S, Ancelin ML, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979-984.
32. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51:694-698.
33. Sheikh JL, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173.
1. O’Connor DW, Pollitt PA, Hyde JB, et al. Do general practitioners miss dementia in elderly patients? BMJ. 1988;297:1107-1110.
2. Lagaay AM, van der Meij JC, Hijmans W. Validation of medical history taking as part of a population based survey in subjects aged 85 and over. BMJ. 1992;304:1091-1092.
3. Cooper B, Bickel H, Schaufele M. Early development and progression of dementing illness in the elderly: a general-practice based study. Psychol Med. 1996;26:411-419.
4. Olafsdottir M, Skoog I, Marcusson J. Detection of dementia in primary care: the Linkoping study. Dement Geriatr Cogn Disord. 2000;11:223-229.
5. Valcour VG, Masaki KH, Curb JD, et al. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964-2968.
6. Wilkinson D, Stave C, Keohane D, et al. The role of general practitioners in the diagnosis and treatment of Alzheimer’s disease: a multinational survey. J Int Med Res. 2004;32:149-159.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
8. Ritchie CW, Ames D, Clayton T, et al. Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am J Geriatr Psych. 2004;12:358-369.
9. Winblad B, Engedal K, Soinenen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489-495.
10. Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136-145.
11. Doody RS, Geldmacher DS, Gordon B, et al. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58:427-433.
12. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia. Arch Intern Med. 2006;166:2182-2188.
13. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease. A randomized controlled trial. JAMA. 1996;276:1725-1731.
14. 2008 Alzheimer’s disease facts and figures. Available at: http://www.alz.org/alzheimers_disease_publications_reports.asp. Accessed March 19, 2008.
15. US Preventive Services Task Force. Screening for dementia: recommendations and rationale. June 2003. Available at: http://www.ahcpr.gov/clinic/3rduspstf/dementia/dementrr.htm. Accessed March 18, 2008.
16. AGS Clinical Practice Committee. Guidelines abstracted from the American Academy of Neurology’s Dementia Guidelines for early detection, diagnosis, and management of dementia. J Am Geriatr Soc. 2003;51:869-873.
17. Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: Mild Cognitive Impairment(an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133-1142.
18. Wenger NS, Roth CP, Shekelle P. ACOVE Investigators. Introduction to the Assessing Care of Vulnerable Elders-3 Quality Indicator Measurement Set. J Am Geriatr Soc. 2007;55(suppl 2):s247-s251.
19. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):s293-s301.
20. Adelman AM. Initial evaluation of the patient with suspected dementia. Am Fam Physician. 2005;71:1745-1750.
21. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
22. Boustani M, Peterson B, Hanson L, et al. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138:927-937.
23. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level. JAMA. 1993;18:2386-2391.
24. Borson S, Scanlan J, Brush M, et al. The Mini-Cog: a cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021-1027.
25. Borson S, Scanlan JM, Chen P, et al. The mini-cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451-1454.
26. Steenland NK, Auman CM, Patel PM, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis. 2008;15:419-427.
27. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
28. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559-564.
29. Solomon PR, Hirschoff A, Kelly B, et al. A 7 minute neurocognitive screening battery highly sensitive to Alzheimer’s disease. Arch Neurol. 1998;55:349-355.
30. Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer’s disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction. The DIADS. Arch Gen Psychol. 2003;60:737-746.
31. Artero S, Ancelin ML, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979-984.
32. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51:694-698.
33. Sheikh JL, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173.
YOU HAVE A NEW JOB: Monitor the lipid profile
Dr. Dayspring serves on the advisory board for LipoScience. Dr. Helmbold reports no financial relationships relevant to this article.
Add another item to your ever-growing list of responsibilities: monitoring your patients’ risk of atherosclerosis.
This task used to be the purview of internists and cardiologists but, because gynecologists are increasingly serving as a primary care provider, you need to learn to recognize and diagnose the many clinical expressions of atherosclerosis in your aging patients.
A crucial part of that knowledge is a thorough understanding of each and every lipid concentration parameter reported within the standard lipid profile. This article reviews those parameters, explains how to interpret them individually and in combination, and introduces a new paradigm: the analysis of lipoprotein particle concentrations as a more precise way to determine risk.
If used in its entirety, the lipid profile provides a significant amount of information about the presence or absence of pathologic lipoprotein concentrations. Far too many clinicians focus solely on low-density lipoprotein cholesterol (LDL-C) and ignore the rest of the profile. Failure to consider the other variables is one reason why atherosclerotic disease is underdiagnosed and undertreated in the United States in many patients—especially women.1
1. Look at the triglyceride (TG) level. If it is >500 mg/dL, treatment is indicated, and TG reduction takes precedence over all other lipid concentrations. If TG is <500 mg/dL, go to Step 2.
2. Look at the low-density lipoprotein cholesterol (LDL-C) level. If it is >190 mg/dL, drug therapy is indicated regardless of other findings. At lower levels, the need for therapy is based on the patient’s overall risk of cardiovascular disease (CVD). Therapeutic lifestyle recommendations are always indicated.
3. Look at high-density lipoprotein cholesterol (HDL-C). Increased risk is present if it is <50 mg/dL, the threshold for women. Do not assume that high HDL-C always means low CVD risk.
4. Calculate the total cholesterol (TC)/HDL-C ratio (a surrogate of apoB/apoA-I ratio). Increased risk is present if it is >4.0.
5. Calculate the non-HDL-C level (TC minus HDL-C). If it is >130 mg/dL (or >100 mg/dL in very-high-risk women), therapy is warranted. Newer data reveal that this calculation is always equal to, or better than, LDL-C at predicting CVD risk. Non-HDL-C is less valuable if TG is >500 mg/dL.
6. Calculate the TG/HDL-C ratio to estimate the size of LDL. If the ratio is >3.8, the likelihood of small LDL is 80%. (Small LDL usually has very high LDL-P.)
Why lipoproteins are important
There is only one absolute in atherosclerosis: Sterols—predominantly cholesterol—enter the artery wall, where they are oxidized, internalized by macrophages, and transformed into foam cells, the histologic hallmark of atherosclerosis. With the accumulation of foam cells, fatty streaks develop and, ultimately, so does complex plaque.
Lipids associated with cardiovascular disease (CVD) include:
- cholesterol
- noncholesterol sterols such as sitosterol, campesterol, and others of mostly plant or shellfish origin
- triacylglycerol, or triglycerides (TG)
- phospholipids.
Because lipids are insoluble in aqueous solutions such as plasma, they must be “trafficked” within protein-enwrapped particles called lipoproteins. The surface proteins that provide structure and solubility to lipoproteins are called apolipoproteins. A key concept is that, with their surface apolipoproteins and cholesterol core, certain lipoproteins are potential agents of atherogenesis in that they transport sterols into the artery wall.2
Estimation of the risk of CVD involves careful analysis of all standard lipid concentrations and their various ratios, and prediction of the potential presence of atherogenic lipoproteins. Successful prevention or treatment of atherosclerosis entails limiting the presence of atherogenic lipoproteins.
A new paradigm is on its way
The atherogenicity of lipoprotein particles is determined by particle concentration as well as other variables, including particle size, lipid composition, and distinct surface apolipoproteins.
Lipoproteins smaller than 70 nm in diameter are driven into the arterial intima primarily by concentration gradients, regardless of lipid composition or particle size.3 A recent Consensus Statement from the American Diabetes Association and the American College of Cardiology observed that quantitative analysis of these potentially atherogenic lipoproteins is one of the best lipid/lipoprotein-related determinants of CVD risk.4 Lipoprotein particle concentrations have emerged not only as superb predictors of risk, but also as goals of therapy.5-7
Because of cost, third-party reimbursement, varying test availability, and lack of interpretive knowledge, few clinicians routinely order lipoprotein quantification. Historically, CVD risk and goals of therapy have been based on lipid concentrations (the amount of lipids trafficked within lipoprotein cores) reported in the lipid profile. Guidelines from the National Cholesterol Education Program, Adult Treatment Panel III (NCEP ATP-III)8,9 and the American Heart Association (AHA) CVD Prevention in Women10,11 use lipid concentrations such as total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), and TG as estimates or surrogates of lipoprotein concentrations ( TABLE 1 ).
The day is rapidly approaching, however, when lipoprotein concentrations may replace the lipid profile in clinical practice. It is critical that clinicians develop a solid understanding of lipoprotein physiology and pathology.7,12 It also is crucial that we be as skilled as possible in accurately predicting lipoprotein pathology using all of the lipid concentration parameters present in the lipid panel.
TABLE 1
Desirable lipid values for women
| Lipid | Level (mg/dL) |
|---|---|
| Total cholesterol | <200 |
| Low-density lipoprotein (LDL) cholesterol | <100 |
| High-density lipoprotein (HDL) cholesterol | ≥50 |
| Triglycerides | <150 |
| Non-HDL-cholesterol | <130 |
| FOR VERY HIGH-RISK PATIENTS | |
| LDL-C | <70 |
| Non-HDL-C | <100 |
| Source: American Heart Association | |
How lipoproteins are analyzed
Lipoproteins can be separated into their components using any of several methodologies, including ultracentrifugation, electrophoresis, apolipoprotein content analysis, and nuclear magnetic resonance (NMR) spectroscopy. Of these, only the last two provide information on particle concentrations.13,14
Apolipoprotein content analysis reveals two major categories of particles:
- alpha-lipoproteins, or HDL, which contain two to four molecules of apolipoprotein A-I (apoA-I)
- beta-lipoproteins, a collective group of chylomicrons, very-low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), and LDL, each containing a single molecule of apolipoprotein B (apoB). Because of very different half-lives (chylomicrons, 1 hour; VLDL, 2–6 hours; IDL, 1–2 hours; LDL, 2–3 days), the great majority (90% to 95%) of apoB-containing particles are LDL. Although apoB measurement yields quantification of all beta-lipoproteins, it is primarily a surrogate of LDL particle (LDL-P) concentration.15
Individual particle concentrations, determined by NMR spectroscopy, are reported as VLDL-P, IDL-P, LDL-P, and HDL-P (see the “Glossary”).14
Several epidemiologic studies that enrolled both genders found the best predictors of risk to be:
- elevated levels of apoB or LDL-P and reduced levels of apoA-I or HDL-P
- a high apoB/apoA-I ratio or LDL-P/HDL-P ratio.6,13,14
After adjustment for lipoprotein concentration data (apoB or LDL-P), other lipoprotein characteristics such as particle lipid content, size, or composition, for the most part, had no statistically significant relationship with the risk of cardiovascular disease.16,17
Lipids and lipoproteins: A glossary
| Variable | What is it? |
|---|---|
| Triglycerides (TG) | The triacylglycerol concentration within all of the TG-trafficking lipoproteins in 100 mL or 1 dL of plasma |
| Total cholesterol (TC) | Cholesterol content of all lipoproteins in 1 dL of plasma |
| Low-density lipoprotein (LDL) cholesterol | Cholesterol content of all intermediate-density lipoprotein (IDL) and LDL particles in 1 dL of plasma |
| High-density lipoprotein (HDL) cholesterol | Cholesterol content of all HDL particles in 1 dL of plasma |
| Very-low-density lipoprotein (VLDL) cholesterol | Cholesterol content of all VLDL particles in 1 dL of plasma |
| Remnant-C | Cholesterol content of all remnants in 1 dL of plasma |
| Lipoprotein (a) [Lp(a)] cholesterol | Cholesterol content of LDL particles that have apo(a) attached |
| Lp(a) concentration | Concentration of apo(a) in 1 dL of plasma |
| Non-HDL cholesterol | Cholesterol within all apoB particles in 1 dL of plasma |
| LDL-P | Number of LDL particles in 1 L of plasma (expressed in nmol/L). This represents LDL particles of all sizes |
| Small LDL-P | Number of small and intermediate LDL particles in 1 L of plasma (nmol/L) |
| HDL-P | Number of HDL particles in 1 L of plasma (μmol/L). HDL-P is also reported as large, intermediate, and small HDL-P (μmol/L) |
| VLDL-P | Number of VLDL particles in 1 L of plasma (nmol/L) |
| IDL-P | Number of IDL particles in 1 L of plasma (nmol/L) |
| LDL size | Diameter of the predominant LDL species:
|
Using lipid measurements to estimate lipoproteins
Total cholesterol represents the cholesterol content within all lipoproteins in 1 dL of plasma. Because beta-lipoproteins are considerably larger than alpha-lipoproteins, approximately 75% of total cholesterol is carried in the apoB-containing particles, making TC an apoB surrogate.
VLDL-C, an often ignored variable, is not measured but calculated using the Friedewald formula, dividing TG by five. This calculation assumes—often erroneously as TG levels rise—that TG consists only of VLDL particles and that VLDL composition contains five times more TG than cholesterol molecules.
A desirable TG level is <150 mg/dL, so normal VLDL-C is 150/5 or <30 mg/dL.
LDL-C is also an apoB surrogate
Although VLDL-C is a weak apoB surrogate,15 data from the Framingham Heart Study showed it to be a good predictor of VLDL remnant particles.18 However, because the vast majority of beta-lipoproteins are LDL, LDL-C (especially if elevated) is a better apoB surrogate than VLDL-C and is the primary CVD risk factor and goal of therapy in every current guideline.
LDL-C is usually a calculated value using the formula:
LDL-C = TC – (HDL-C + VLDL-C)
Upon special order, laboratories can directly measure LDL-C. This option is most useful when TG levels are high, rendering the Friedewald formula less accurate ( TABLE 2 ).19 For population cut points and desirable goals of therapy for lipid and lipoprotein concentrations, see the FIGURE .
TABLE 2
How lipid concentrations are determined
| TC = apoA-I-C + apoB-C |
| TC = HDL-C + LDL-C + VLDL-C + IDL-C + Chylomicron-C + Lp(a)-C + Remnant-C |
| In a fasting patient under normal circumstances, there are no chylomicrons and remnants (smaller chylomicrons or VLDL particles) and very few, if any, IDL particles. These are postprandial lipoproteins. Most patients do not have Lp(a) pathology. Therefore, the lipid concentration formula simplifies: |
| TC = HDL-C + LDL-C + VLDL-C |
| VLDL-C is estimated by TG/5 (assumes that all TG is in VLDL and that VLDL TG:cholesterol composition is 5:1). Therefore: |
| TC = HDL-C + LDL-C + TG/5 |
| LDL-C = TC – (HDL-C + TG/5) |
| Non-HDL-C = TC – HDL-C |
| In actuality, the calculated or directly measured LDL-C values in the standard lipid panel represent LDL-C + IDL-C + Lp(a)-C. However, because labs do not usually separate IDL and Lp(a) particles from LDL (without significant added expense), only total LDL-C is reported. |
FIGURE Population percentile cut points and goals for LDL-C, LDL-P, ApoB, and non-HDL-C
HDL-C, apoA-I are inversely related to cardiovascular risk
The epidemiologic data strongly indicate that both HDL-C and apoA-I are strongly and inversely related to CVD risk.6 HDL particles are a heterogenous collection of:
- unlipidated apoA-I
- very small pre-beta HDL
- more mature, lipidated HDL3 and HDL2 species (HDL3 smaller than HDL2).
NMR nomenclature identifies the smaller HDL species as H1 and H2 and the larger HDL species as H4 and H5.14 The smaller HDL species also contain apoA-II.
Although HDL can acquire cholesterol from any cell, including arterial-wall foam cells, the majority of HDL lipidation occurs in the liver or proximal small intestine, after which it is trafficked to steroidogenic tissue, adipocytes, or back to the liver. Normally, HDL carries little TG.20 The only lipid concentration that can serve as a surrogate of apoA-I or HDL-P is HDL-C, where the assumption is that higher HDL-C indicates higher apoA-I, and vice versa.
In reality, the correlation between apoA-I and HDL-C varies because each HDL particle can have from two to four apoA-I molecules, and the volume of cholesterol within the particle is a function of particle size and its TG content. For the most part, total HDL-C is indicative of the cholesterol carried in the larger, mature HDL2 (H4, H5) particles; patients with low HDL-C typically lack these mature, lipidated HDL particles.
Because HDL rapidly and repeatedly lipidates and then delipidates, there is no relationship between the HDL-C level and the complex dynamic process termed reverse cholesterol transport process. Neither HDL-C, nor apoA-I, nor HDL-P, nor HDL size is consistently related to HDL particle functionality—i.e., the ability of HDL to lipidate or delipidate, appropriately traffic cholesterol, or perform numerous other nonlipid antiatherogenic functions.20,21
Two premenopausal women undergo assessment of their basic lipid panel, with these results:
| LIPID | PATIENT 1 | PATIENT 2 |
|---|---|---|
| Total cholesterol (TC) | 180 | 180 |
| LDL-C | 100 | 100 |
| HDL-C | 60 | 40 |
| VLDL-C | 20 | 40 |
| Triglycerides (TG) | 100 | 200 |
| Non-HDL-C | 120 | 160 |
| TC/HDL-C ratio | 3.0 | 4.5 |
| TG/HDL-C ratio | 1.6 | 5.0 |
| LDL-C, low-density lipoprotein cholesterol | ||
| HDL-C, high-density lipoprotein cholesterol | ||
| VLDL-C, very-low-density lipoprotein cholesterol | ||
Both patients have the same desirable TC and LDL-C values. However, further analysis reveals an abnormal TC/HDL-C ratio and an abnormal non-HDL-C level in patient 2. This finding indicates a higher risk of CVD.
In addition, the TG/HDL-C ratio of 5.0 in patient 2 is highly suggestive of small-LDL phenotype B. That designation means that this patient will have 40% to 70% more LDL particles to traffic her LDL-C than patient 1, who appears to have LDL of normal size.27 The elevated VLDL-C of patient 2 indicates the presence of VLDL remnants, which predict risk above that conveyed by LDL-C.7
The typical clinician, looking only at TC or LDL-C, would miss the increased risk (high apoB) in patient 2. Obvious clues to her lipoprotein pathology are the elevated TG and reduced HDL-C (TG-HDL axis disorder). Beyond elevated TG and reduced HDL-C, patient 2 is also likely to have increased waist size, subtle hypertension, and possibly impaired fasting glucose—three additional parameters of metabolic syndrome.7,10,25
Focus on lipoprotein particle concentrations
To most accurately predict lipid-related CVD risk, you must determine which patients have elevated numbers of atherogenic lipoproteins using actual particle concentrations. In most practices, lipoprotein particle numbers must be estimated by scrutinizing all of the lipid concentrations and ratios (not simply LDL-C).
TC and, especially, LDL-C are apoB and LDL-P surrogates, but the best lipid concentration estimate of apoB is the calculated non-HDL-C value. By subtracting HDL-C from TC, it is possible to identify the cholesterol not in the HDL particles but in all of the potentially atherogenic apoB particles. In essence, non-HDL-C is VLDL-C plus LDL-C. This equation yields a better apoB or LDL-P proxy, compared with LDL-C alone.18 If a patient has reached her LDL-C goal but still has a high non-HDL-C level, we can assume that there are still too many apoB particles and that they are contributing to residual risk.
Because LDL is the predominant apoB species, non-HDL-C is the best lipid concentration predictor of LDL-P.15 Because neither TC nor HDL-C assays require a patient to fast, non-HDL-C is accurate in nonfasting patients, making it a very practical way to screen for CVD risk.8 In the Women’s Health Study, which involved mostly healthy women, non-HDL-C predicted the risk of coronary heart disease as well as apoB did, but not as well as LDL-P.22,23 In independent, separately published analyses from the Framingham Off-spring Study, LDL-P was a better predictor of risk than LDL-C and apoB.15,24
NCEP ATP-III guidelines introduced non-HDL-C as a secondary goal of therapy in patients with TG >200 mg/dL. Subsequent data indicate that non-HDL-C is always a better predictor of risk than LDL-C is, regardless of TG levels.18
The AHA Women’s Guideline was the first to set a desired non-HDL-C level (130 mg/dL) independent of the TG value.10 Because a normal VLDL-C concentration is 30 mg/dL, the non-HDL-C goal is 30 mg/dL above the desired LDL-C goal. For example, if the desired LDL-C value is 100 mg/dL, the non-HDL-C goal is 130 mg/dL. If the desired LDL-C goal is 70 mg/dL—as it is in a patient at very high risk—the non-HDL-C goal would be 100 mg/dL ( FIGURE ).9,11
Insulin resistance diminishes accuracy of lipid profile
The ability to predict lipoprotein particle concentrations using the lipid profile becomes far less accurate in situations associated with insulin resistance and metabolic syndrome in patients who have TG-HDL axis disorders. In women, these disorders are typified by an elevation of TG >150 mg/dL and a decrease in HDL-C <50 mg/dL, with borderline or normal LDL-C levels.25
As TG begins to rise above 120 mg/dL, hepatic secretion of TG-rich VLDL particles increases. As VLDL-TG is hydrolyzed by lipoprotein lipase in muscle and fat cells, in a process termed lipolysis, VLDL shrinks and transforms into IDL. Ultimately, unless it is cleared by hepatic LDL receptors, the IDL undergoes additional lipolysis by hepatic lipase and transforms into LDL particles. Because of their longer half-life, these LDL particles accumulate, further elevating apoB and LDL-P.
In the presence of TG-rich VLDL and chylomicrons, additional pathologic particle remodeling occurs. By way of a lipid transfer protein called cholesteryl ester transfer protein (CETP), some of the TG molecules present in TG-rich lipoproteins are exchanged for cholesteryl esters in LDL and HDL. This lipid transfer creates LDL and HDL that are TG-rich and cholesterol-poor, enabling additional TG lipolysis by hepatic lipase to create smaller LDL and HDL. The latter is so small that it can pass through renal glomeruli and be excreted, leading to reductions of HDL-P, apoA-I, and HDL-C.
Also created in this process are smaller, atherogenic, cholesterol-rich VLDL and chylomicron remnants, diagnosable by an elevated VLDL-C. Patients who have this pathology typically have elevated TG, reduced HDL-C, variable LDL-C, and an increased TG/HDL-C ratio (>3.8), which are indicative of too many small LDL particles (high apoB, LDL-P) and reduced number of HDL particles (high apoB/A-I ratio).26,27
Such a scenario, typical of TG-HDL axis disorders, explains much of the risk associated with rising TG levels and is very common in premenopausal women who have insulin-resistant states such as type 2 diabetes or polycystic ovary syndrome and in menopausal women who have insulin resistance and coronary artery disease.1
LDL-C and LDL-P do not always correlate
Because the volume of a lipoprotein is a function of its radius cubed (V = 4/3πr3),14 a patient who has small LDL will require up to 40% to 70% more LDL particles to traffic a given amount of LDL-C. In such a patient, there is often little correlation between LDL-C and LDL-P or apoB values. Regardless of the LDL-C, the apoB, LDL-P, or non-HDL-C is often elevated.28 This risk, which cannot be predicted by looking only at LDL-C, is the main reason guidelines advocate the use of non-HDL-C or the TC/HDL-C ratio.8,11 (See the case studies.)
In summary, a large part of the risk of CVD seen in patients who have low HDL-C derives from the associated increase in the number of apoB particles, mostly composed of small LDL, as well as an increase in remnant particles.15,21,28 This crucial point explains why treatment of low HDL-C states should always first target apoB or LDL-P (LDL-C and non-HDL-C), rather than apoA-I or HDL-C ( TABLES 3 and 4 ).8,9
TABLE 3
Lipid markers of small low-density lipoproteins
| High-density lipoprotein cholesterol (HDL-C) <50 mg/dL |
| Triglyceride (TG) >130–150 mg/dL |
| Total cholesterol/HDL-C ratio >4.0 with normal low-density lipoprotein cholesterol (LDL-C) |
| TG/HDL-C ratio >3.8 in women |
| Unremarkable LDL-C but elevated non-HDL-C |
TABLE 4
Lipid markers of remnant lipoproteins
| Triglyceride (TG) >150–200 mg/dL |
| Very-low-density lipoprotein cholesterol >30 mg/dL |
| Unremarkable low-density lipoprotein cholesterol with elevated non-high-density lipoprotein cholesterol (HDL-C) |
| Low HDL-C in insulin-resistant patients |
| Elevated total cholesterol/HDL-C ratio and TG >150 mg/dL |
A few words of advice
The driving forces of atherogenesis are increased numbers of apoB-containing lipoproteins and impaired endothelial integrity. ApoB and LDL-P are the available lab assays that most accurately quantify atherogenic particle number.
The lipid-concentration surrogates that you should be using to better predict apoB and CVD risk are:
- TC (unless HDL-C is very high)
- LDL-C
- Non-HDL-C
- TC/HDL-C ratio
- TG/HDL-C ratio.
Because LDL is by far the most numerous of the apoB particles present in plasma, it is the primary agent of atherogenesis. However, apoB and LDL-P do not correlate with LDL-C when LDL particles are small, are TG-rich and cholesterol-poor, or simply cholesterol-poor (seen in some patients who have low LDL-C levels).7,15
Both NCEP ATP-III and AHA Women’s Guidelines use the TC/HDL ratio as a powerful risk predictor. However, as a goal of therapy, these guidelines recommend normalizing LDL-C and then non-HDL-C.8,11 In reality, normalization of non-HDL-C takes care of LDL-C as well. For example, say a patient has LDL-C <100 mg/dL, but non-HDL-C >130 mg/dL or TC/HDL-C ratio >4. These readings indicate residual risk and suggest that an elevated number of apoB particles is present. Therapy to normalize non-HDL-C or, better yet, apoB/LDL-P, is warranted. The clue that residual risk is present even when LDL-C is normal is the reduction of HDL-C and elevation of TG and non-HDL-C.
1. Lloyd-Jones DM, O’Donnell CJ, D’Agostino RB, et al. Applicability of cholesterol-lowering primary prevention trials to a general population. The Framingham Heart Study. Arch Intern Med. 2001;161:949-954.
2. Biggerstaff KD, Wooten JS. Understanding lipoproteins as transporters of cholesterol and other lipids. Adv Physiol Educ. 2004;28:105-106.
3. Nordestgaard BG, Wooten R, Lewis B. Selective retention of VLDL, IDL and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. 1995;15:534-542.
4. Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk. Consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811-822.
5. Barter PJ, Ballantyne CM, Carmena R, et al. ApoB versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259:247-258.
6. Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026-2033.
7. Mudd JO, Borlaug BA, Johnson PV, et al. Beyond low-density lipoprotein cholesterol: defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J Am Coll Cardiol. 2007;50:1735-1741.
8. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
9. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227-239.
10. Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672-693.
11. Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481.-
12. Sniderman AD. Apolipoprotein B versus non-high-density lipoprotein cholesterol. And the winner is… Circulation. 2005;112:3366-3367.
13. Sniderman AD, Marcovina SM. Apolipoprotein A-I and B. Clin Lab Med. 2006;26:733-750.
14. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847-870.
15. Cromwell WC, Otvos JD, Keyes MJ, et al. LDL particle number and risk of future cardiovascular disease in the Framingham Off spring Study—implications for LDL management. J Clin Lipidol. 2007;1:583-592.
16. El Harchaoui K, van der Steeg WA, Stroes ES, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547-553.
17. Mora S, Szklo M, Otvos JD, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2007;192:211-217.
18. Liu J, Sempos CT, Donahue RP, et al. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their predictive risk values in coronary heart disease. Am J Cardiol. 2006;98:1363-1368.
19. National Cholesterol Education Program. Recommendations on lipoprotein measurement from the Working Group on Lipoprotein Measurement. National Institutes of Health. National Heart, Lung, and Blood Institute. NIH Publication No. 95-3044. Bethesda, Md: September 1995.
20. Dayspring T. High density lipoproteins: emerging knowledge. J Cardiometabol Syndr. 2007;2:59-62.
21. Cromwell WC. High-density lipoprotein associations with coronary heart disease: does measurement of cholesterol content give the best result? J Clin Lipidol. 2007;1:57-64.
22. Ridker PM, Rifai N, Cook NR, et al. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326.-
23. Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930-1937.
24. Ingelsson E, Schaefer EJ, Contois JH, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776-785.
25. Szapary PO, Rader DJ. The triglyceride-high-density lipoprotein axis: an important target of therapy. Am Heart J. 2004;148:211-221.
26. Davidson MH, Yannicelli D. New concepts in dyslipidemia in the metabolic syndrome and diabetes. Metab Syndr Relat Disord. 2006;4:299-314.
27. Hanak V, Munoz J, Teague J, Stanley A, Jr, Bittner V. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94:219-222.
28. Kathiresan S, Otvos JD, Sullivan LM, et al. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20-29.
Dr. Dayspring serves on the advisory board for LipoScience. Dr. Helmbold reports no financial relationships relevant to this article.
Add another item to your ever-growing list of responsibilities: monitoring your patients’ risk of atherosclerosis.
This task used to be the purview of internists and cardiologists but, because gynecologists are increasingly serving as a primary care provider, you need to learn to recognize and diagnose the many clinical expressions of atherosclerosis in your aging patients.
A crucial part of that knowledge is a thorough understanding of each and every lipid concentration parameter reported within the standard lipid profile. This article reviews those parameters, explains how to interpret them individually and in combination, and introduces a new paradigm: the analysis of lipoprotein particle concentrations as a more precise way to determine risk.
If used in its entirety, the lipid profile provides a significant amount of information about the presence or absence of pathologic lipoprotein concentrations. Far too many clinicians focus solely on low-density lipoprotein cholesterol (LDL-C) and ignore the rest of the profile. Failure to consider the other variables is one reason why atherosclerotic disease is underdiagnosed and undertreated in the United States in many patients—especially women.1
1. Look at the triglyceride (TG) level. If it is >500 mg/dL, treatment is indicated, and TG reduction takes precedence over all other lipid concentrations. If TG is <500 mg/dL, go to Step 2.
2. Look at the low-density lipoprotein cholesterol (LDL-C) level. If it is >190 mg/dL, drug therapy is indicated regardless of other findings. At lower levels, the need for therapy is based on the patient’s overall risk of cardiovascular disease (CVD). Therapeutic lifestyle recommendations are always indicated.
3. Look at high-density lipoprotein cholesterol (HDL-C). Increased risk is present if it is <50 mg/dL, the threshold for women. Do not assume that high HDL-C always means low CVD risk.
4. Calculate the total cholesterol (TC)/HDL-C ratio (a surrogate of apoB/apoA-I ratio). Increased risk is present if it is >4.0.
5. Calculate the non-HDL-C level (TC minus HDL-C). If it is >130 mg/dL (or >100 mg/dL in very-high-risk women), therapy is warranted. Newer data reveal that this calculation is always equal to, or better than, LDL-C at predicting CVD risk. Non-HDL-C is less valuable if TG is >500 mg/dL.
6. Calculate the TG/HDL-C ratio to estimate the size of LDL. If the ratio is >3.8, the likelihood of small LDL is 80%. (Small LDL usually has very high LDL-P.)
Why lipoproteins are important
There is only one absolute in atherosclerosis: Sterols—predominantly cholesterol—enter the artery wall, where they are oxidized, internalized by macrophages, and transformed into foam cells, the histologic hallmark of atherosclerosis. With the accumulation of foam cells, fatty streaks develop and, ultimately, so does complex plaque.
Lipids associated with cardiovascular disease (CVD) include:
- cholesterol
- noncholesterol sterols such as sitosterol, campesterol, and others of mostly plant or shellfish origin
- triacylglycerol, or triglycerides (TG)
- phospholipids.
Because lipids are insoluble in aqueous solutions such as plasma, they must be “trafficked” within protein-enwrapped particles called lipoproteins. The surface proteins that provide structure and solubility to lipoproteins are called apolipoproteins. A key concept is that, with their surface apolipoproteins and cholesterol core, certain lipoproteins are potential agents of atherogenesis in that they transport sterols into the artery wall.2
Estimation of the risk of CVD involves careful analysis of all standard lipid concentrations and their various ratios, and prediction of the potential presence of atherogenic lipoproteins. Successful prevention or treatment of atherosclerosis entails limiting the presence of atherogenic lipoproteins.
A new paradigm is on its way
The atherogenicity of lipoprotein particles is determined by particle concentration as well as other variables, including particle size, lipid composition, and distinct surface apolipoproteins.
Lipoproteins smaller than 70 nm in diameter are driven into the arterial intima primarily by concentration gradients, regardless of lipid composition or particle size.3 A recent Consensus Statement from the American Diabetes Association and the American College of Cardiology observed that quantitative analysis of these potentially atherogenic lipoproteins is one of the best lipid/lipoprotein-related determinants of CVD risk.4 Lipoprotein particle concentrations have emerged not only as superb predictors of risk, but also as goals of therapy.5-7
Because of cost, third-party reimbursement, varying test availability, and lack of interpretive knowledge, few clinicians routinely order lipoprotein quantification. Historically, CVD risk and goals of therapy have been based on lipid concentrations (the amount of lipids trafficked within lipoprotein cores) reported in the lipid profile. Guidelines from the National Cholesterol Education Program, Adult Treatment Panel III (NCEP ATP-III)8,9 and the American Heart Association (AHA) CVD Prevention in Women10,11 use lipid concentrations such as total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), and TG as estimates or surrogates of lipoprotein concentrations ( TABLE 1 ).
The day is rapidly approaching, however, when lipoprotein concentrations may replace the lipid profile in clinical practice. It is critical that clinicians develop a solid understanding of lipoprotein physiology and pathology.7,12 It also is crucial that we be as skilled as possible in accurately predicting lipoprotein pathology using all of the lipid concentration parameters present in the lipid panel.
TABLE 1
Desirable lipid values for women
| Lipid | Level (mg/dL) |
|---|---|
| Total cholesterol | <200 |
| Low-density lipoprotein (LDL) cholesterol | <100 |
| High-density lipoprotein (HDL) cholesterol | ≥50 |
| Triglycerides | <150 |
| Non-HDL-cholesterol | <130 |
| FOR VERY HIGH-RISK PATIENTS | |
| LDL-C | <70 |
| Non-HDL-C | <100 |
| Source: American Heart Association | |
How lipoproteins are analyzed
Lipoproteins can be separated into their components using any of several methodologies, including ultracentrifugation, electrophoresis, apolipoprotein content analysis, and nuclear magnetic resonance (NMR) spectroscopy. Of these, only the last two provide information on particle concentrations.13,14
Apolipoprotein content analysis reveals two major categories of particles:
- alpha-lipoproteins, or HDL, which contain two to four molecules of apolipoprotein A-I (apoA-I)
- beta-lipoproteins, a collective group of chylomicrons, very-low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), and LDL, each containing a single molecule of apolipoprotein B (apoB). Because of very different half-lives (chylomicrons, 1 hour; VLDL, 2–6 hours; IDL, 1–2 hours; LDL, 2–3 days), the great majority (90% to 95%) of apoB-containing particles are LDL. Although apoB measurement yields quantification of all beta-lipoproteins, it is primarily a surrogate of LDL particle (LDL-P) concentration.15
Individual particle concentrations, determined by NMR spectroscopy, are reported as VLDL-P, IDL-P, LDL-P, and HDL-P (see the “Glossary”).14
Several epidemiologic studies that enrolled both genders found the best predictors of risk to be:
- elevated levels of apoB or LDL-P and reduced levels of apoA-I or HDL-P
- a high apoB/apoA-I ratio or LDL-P/HDL-P ratio.6,13,14
After adjustment for lipoprotein concentration data (apoB or LDL-P), other lipoprotein characteristics such as particle lipid content, size, or composition, for the most part, had no statistically significant relationship with the risk of cardiovascular disease.16,17
Lipids and lipoproteins: A glossary
| Variable | What is it? |
|---|---|
| Triglycerides (TG) | The triacylglycerol concentration within all of the TG-trafficking lipoproteins in 100 mL or 1 dL of plasma |
| Total cholesterol (TC) | Cholesterol content of all lipoproteins in 1 dL of plasma |
| Low-density lipoprotein (LDL) cholesterol | Cholesterol content of all intermediate-density lipoprotein (IDL) and LDL particles in 1 dL of plasma |
| High-density lipoprotein (HDL) cholesterol | Cholesterol content of all HDL particles in 1 dL of plasma |
| Very-low-density lipoprotein (VLDL) cholesterol | Cholesterol content of all VLDL particles in 1 dL of plasma |
| Remnant-C | Cholesterol content of all remnants in 1 dL of plasma |
| Lipoprotein (a) [Lp(a)] cholesterol | Cholesterol content of LDL particles that have apo(a) attached |
| Lp(a) concentration | Concentration of apo(a) in 1 dL of plasma |
| Non-HDL cholesterol | Cholesterol within all apoB particles in 1 dL of plasma |
| LDL-P | Number of LDL particles in 1 L of plasma (expressed in nmol/L). This represents LDL particles of all sizes |
| Small LDL-P | Number of small and intermediate LDL particles in 1 L of plasma (nmol/L) |
| HDL-P | Number of HDL particles in 1 L of plasma (μmol/L). HDL-P is also reported as large, intermediate, and small HDL-P (μmol/L) |
| VLDL-P | Number of VLDL particles in 1 L of plasma (nmol/L) |
| IDL-P | Number of IDL particles in 1 L of plasma (nmol/L) |
| LDL size | Diameter of the predominant LDL species:
|
Using lipid measurements to estimate lipoproteins
Total cholesterol represents the cholesterol content within all lipoproteins in 1 dL of plasma. Because beta-lipoproteins are considerably larger than alpha-lipoproteins, approximately 75% of total cholesterol is carried in the apoB-containing particles, making TC an apoB surrogate.
VLDL-C, an often ignored variable, is not measured but calculated using the Friedewald formula, dividing TG by five. This calculation assumes—often erroneously as TG levels rise—that TG consists only of VLDL particles and that VLDL composition contains five times more TG than cholesterol molecules.
A desirable TG level is <150 mg/dL, so normal VLDL-C is 150/5 or <30 mg/dL.
LDL-C is also an apoB surrogate
Although VLDL-C is a weak apoB surrogate,15 data from the Framingham Heart Study showed it to be a good predictor of VLDL remnant particles.18 However, because the vast majority of beta-lipoproteins are LDL, LDL-C (especially if elevated) is a better apoB surrogate than VLDL-C and is the primary CVD risk factor and goal of therapy in every current guideline.
LDL-C is usually a calculated value using the formula:
LDL-C = TC – (HDL-C + VLDL-C)
Upon special order, laboratories can directly measure LDL-C. This option is most useful when TG levels are high, rendering the Friedewald formula less accurate ( TABLE 2 ).19 For population cut points and desirable goals of therapy for lipid and lipoprotein concentrations, see the FIGURE .
TABLE 2
How lipid concentrations are determined
| TC = apoA-I-C + apoB-C |
| TC = HDL-C + LDL-C + VLDL-C + IDL-C + Chylomicron-C + Lp(a)-C + Remnant-C |
| In a fasting patient under normal circumstances, there are no chylomicrons and remnants (smaller chylomicrons or VLDL particles) and very few, if any, IDL particles. These are postprandial lipoproteins. Most patients do not have Lp(a) pathology. Therefore, the lipid concentration formula simplifies: |
| TC = HDL-C + LDL-C + VLDL-C |
| VLDL-C is estimated by TG/5 (assumes that all TG is in VLDL and that VLDL TG:cholesterol composition is 5:1). Therefore: |
| TC = HDL-C + LDL-C + TG/5 |
| LDL-C = TC – (HDL-C + TG/5) |
| Non-HDL-C = TC – HDL-C |
| In actuality, the calculated or directly measured LDL-C values in the standard lipid panel represent LDL-C + IDL-C + Lp(a)-C. However, because labs do not usually separate IDL and Lp(a) particles from LDL (without significant added expense), only total LDL-C is reported. |
FIGURE Population percentile cut points and goals for LDL-C, LDL-P, ApoB, and non-HDL-C
HDL-C, apoA-I are inversely related to cardiovascular risk
The epidemiologic data strongly indicate that both HDL-C and apoA-I are strongly and inversely related to CVD risk.6 HDL particles are a heterogenous collection of:
- unlipidated apoA-I
- very small pre-beta HDL
- more mature, lipidated HDL3 and HDL2 species (HDL3 smaller than HDL2).
NMR nomenclature identifies the smaller HDL species as H1 and H2 and the larger HDL species as H4 and H5.14 The smaller HDL species also contain apoA-II.
Although HDL can acquire cholesterol from any cell, including arterial-wall foam cells, the majority of HDL lipidation occurs in the liver or proximal small intestine, after which it is trafficked to steroidogenic tissue, adipocytes, or back to the liver. Normally, HDL carries little TG.20 The only lipid concentration that can serve as a surrogate of apoA-I or HDL-P is HDL-C, where the assumption is that higher HDL-C indicates higher apoA-I, and vice versa.
In reality, the correlation between apoA-I and HDL-C varies because each HDL particle can have from two to four apoA-I molecules, and the volume of cholesterol within the particle is a function of particle size and its TG content. For the most part, total HDL-C is indicative of the cholesterol carried in the larger, mature HDL2 (H4, H5) particles; patients with low HDL-C typically lack these mature, lipidated HDL particles.
Because HDL rapidly and repeatedly lipidates and then delipidates, there is no relationship between the HDL-C level and the complex dynamic process termed reverse cholesterol transport process. Neither HDL-C, nor apoA-I, nor HDL-P, nor HDL size is consistently related to HDL particle functionality—i.e., the ability of HDL to lipidate or delipidate, appropriately traffic cholesterol, or perform numerous other nonlipid antiatherogenic functions.20,21
Two premenopausal women undergo assessment of their basic lipid panel, with these results:
| LIPID | PATIENT 1 | PATIENT 2 |
|---|---|---|
| Total cholesterol (TC) | 180 | 180 |
| LDL-C | 100 | 100 |
| HDL-C | 60 | 40 |
| VLDL-C | 20 | 40 |
| Triglycerides (TG) | 100 | 200 |
| Non-HDL-C | 120 | 160 |
| TC/HDL-C ratio | 3.0 | 4.5 |
| TG/HDL-C ratio | 1.6 | 5.0 |
| LDL-C, low-density lipoprotein cholesterol | ||
| HDL-C, high-density lipoprotein cholesterol | ||
| VLDL-C, very-low-density lipoprotein cholesterol | ||
Both patients have the same desirable TC and LDL-C values. However, further analysis reveals an abnormal TC/HDL-C ratio and an abnormal non-HDL-C level in patient 2. This finding indicates a higher risk of CVD.
In addition, the TG/HDL-C ratio of 5.0 in patient 2 is highly suggestive of small-LDL phenotype B. That designation means that this patient will have 40% to 70% more LDL particles to traffic her LDL-C than patient 1, who appears to have LDL of normal size.27 The elevated VLDL-C of patient 2 indicates the presence of VLDL remnants, which predict risk above that conveyed by LDL-C.7
The typical clinician, looking only at TC or LDL-C, would miss the increased risk (high apoB) in patient 2. Obvious clues to her lipoprotein pathology are the elevated TG and reduced HDL-C (TG-HDL axis disorder). Beyond elevated TG and reduced HDL-C, patient 2 is also likely to have increased waist size, subtle hypertension, and possibly impaired fasting glucose—three additional parameters of metabolic syndrome.7,10,25
Focus on lipoprotein particle concentrations
To most accurately predict lipid-related CVD risk, you must determine which patients have elevated numbers of atherogenic lipoproteins using actual particle concentrations. In most practices, lipoprotein particle numbers must be estimated by scrutinizing all of the lipid concentrations and ratios (not simply LDL-C).
TC and, especially, LDL-C are apoB and LDL-P surrogates, but the best lipid concentration estimate of apoB is the calculated non-HDL-C value. By subtracting HDL-C from TC, it is possible to identify the cholesterol not in the HDL particles but in all of the potentially atherogenic apoB particles. In essence, non-HDL-C is VLDL-C plus LDL-C. This equation yields a better apoB or LDL-P proxy, compared with LDL-C alone.18 If a patient has reached her LDL-C goal but still has a high non-HDL-C level, we can assume that there are still too many apoB particles and that they are contributing to residual risk.
Because LDL is the predominant apoB species, non-HDL-C is the best lipid concentration predictor of LDL-P.15 Because neither TC nor HDL-C assays require a patient to fast, non-HDL-C is accurate in nonfasting patients, making it a very practical way to screen for CVD risk.8 In the Women’s Health Study, which involved mostly healthy women, non-HDL-C predicted the risk of coronary heart disease as well as apoB did, but not as well as LDL-P.22,23 In independent, separately published analyses from the Framingham Off-spring Study, LDL-P was a better predictor of risk than LDL-C and apoB.15,24
NCEP ATP-III guidelines introduced non-HDL-C as a secondary goal of therapy in patients with TG >200 mg/dL. Subsequent data indicate that non-HDL-C is always a better predictor of risk than LDL-C is, regardless of TG levels.18
The AHA Women’s Guideline was the first to set a desired non-HDL-C level (130 mg/dL) independent of the TG value.10 Because a normal VLDL-C concentration is 30 mg/dL, the non-HDL-C goal is 30 mg/dL above the desired LDL-C goal. For example, if the desired LDL-C value is 100 mg/dL, the non-HDL-C goal is 130 mg/dL. If the desired LDL-C goal is 70 mg/dL—as it is in a patient at very high risk—the non-HDL-C goal would be 100 mg/dL ( FIGURE ).9,11
Insulin resistance diminishes accuracy of lipid profile
The ability to predict lipoprotein particle concentrations using the lipid profile becomes far less accurate in situations associated with insulin resistance and metabolic syndrome in patients who have TG-HDL axis disorders. In women, these disorders are typified by an elevation of TG >150 mg/dL and a decrease in HDL-C <50 mg/dL, with borderline or normal LDL-C levels.25
As TG begins to rise above 120 mg/dL, hepatic secretion of TG-rich VLDL particles increases. As VLDL-TG is hydrolyzed by lipoprotein lipase in muscle and fat cells, in a process termed lipolysis, VLDL shrinks and transforms into IDL. Ultimately, unless it is cleared by hepatic LDL receptors, the IDL undergoes additional lipolysis by hepatic lipase and transforms into LDL particles. Because of their longer half-life, these LDL particles accumulate, further elevating apoB and LDL-P.
In the presence of TG-rich VLDL and chylomicrons, additional pathologic particle remodeling occurs. By way of a lipid transfer protein called cholesteryl ester transfer protein (CETP), some of the TG molecules present in TG-rich lipoproteins are exchanged for cholesteryl esters in LDL and HDL. This lipid transfer creates LDL and HDL that are TG-rich and cholesterol-poor, enabling additional TG lipolysis by hepatic lipase to create smaller LDL and HDL. The latter is so small that it can pass through renal glomeruli and be excreted, leading to reductions of HDL-P, apoA-I, and HDL-C.
Also created in this process are smaller, atherogenic, cholesterol-rich VLDL and chylomicron remnants, diagnosable by an elevated VLDL-C. Patients who have this pathology typically have elevated TG, reduced HDL-C, variable LDL-C, and an increased TG/HDL-C ratio (>3.8), which are indicative of too many small LDL particles (high apoB, LDL-P) and reduced number of HDL particles (high apoB/A-I ratio).26,27
Such a scenario, typical of TG-HDL axis disorders, explains much of the risk associated with rising TG levels and is very common in premenopausal women who have insulin-resistant states such as type 2 diabetes or polycystic ovary syndrome and in menopausal women who have insulin resistance and coronary artery disease.1
LDL-C and LDL-P do not always correlate
Because the volume of a lipoprotein is a function of its radius cubed (V = 4/3πr3),14 a patient who has small LDL will require up to 40% to 70% more LDL particles to traffic a given amount of LDL-C. In such a patient, there is often little correlation between LDL-C and LDL-P or apoB values. Regardless of the LDL-C, the apoB, LDL-P, or non-HDL-C is often elevated.28 This risk, which cannot be predicted by looking only at LDL-C, is the main reason guidelines advocate the use of non-HDL-C or the TC/HDL-C ratio.8,11 (See the case studies.)
In summary, a large part of the risk of CVD seen in patients who have low HDL-C derives from the associated increase in the number of apoB particles, mostly composed of small LDL, as well as an increase in remnant particles.15,21,28 This crucial point explains why treatment of low HDL-C states should always first target apoB or LDL-P (LDL-C and non-HDL-C), rather than apoA-I or HDL-C ( TABLES 3 and 4 ).8,9
TABLE 3
Lipid markers of small low-density lipoproteins
| High-density lipoprotein cholesterol (HDL-C) <50 mg/dL |
| Triglyceride (TG) >130–150 mg/dL |
| Total cholesterol/HDL-C ratio >4.0 with normal low-density lipoprotein cholesterol (LDL-C) |
| TG/HDL-C ratio >3.8 in women |
| Unremarkable LDL-C but elevated non-HDL-C |
TABLE 4
Lipid markers of remnant lipoproteins
| Triglyceride (TG) >150–200 mg/dL |
| Very-low-density lipoprotein cholesterol >30 mg/dL |
| Unremarkable low-density lipoprotein cholesterol with elevated non-high-density lipoprotein cholesterol (HDL-C) |
| Low HDL-C in insulin-resistant patients |
| Elevated total cholesterol/HDL-C ratio and TG >150 mg/dL |
A few words of advice
The driving forces of atherogenesis are increased numbers of apoB-containing lipoproteins and impaired endothelial integrity. ApoB and LDL-P are the available lab assays that most accurately quantify atherogenic particle number.
The lipid-concentration surrogates that you should be using to better predict apoB and CVD risk are:
- TC (unless HDL-C is very high)
- LDL-C
- Non-HDL-C
- TC/HDL-C ratio
- TG/HDL-C ratio.
Because LDL is by far the most numerous of the apoB particles present in plasma, it is the primary agent of atherogenesis. However, apoB and LDL-P do not correlate with LDL-C when LDL particles are small, are TG-rich and cholesterol-poor, or simply cholesterol-poor (seen in some patients who have low LDL-C levels).7,15
Both NCEP ATP-III and AHA Women’s Guidelines use the TC/HDL ratio as a powerful risk predictor. However, as a goal of therapy, these guidelines recommend normalizing LDL-C and then non-HDL-C.8,11 In reality, normalization of non-HDL-C takes care of LDL-C as well. For example, say a patient has LDL-C <100 mg/dL, but non-HDL-C >130 mg/dL or TC/HDL-C ratio >4. These readings indicate residual risk and suggest that an elevated number of apoB particles is present. Therapy to normalize non-HDL-C or, better yet, apoB/LDL-P, is warranted. The clue that residual risk is present even when LDL-C is normal is the reduction of HDL-C and elevation of TG and non-HDL-C.
Dr. Dayspring serves on the advisory board for LipoScience. Dr. Helmbold reports no financial relationships relevant to this article.
Add another item to your ever-growing list of responsibilities: monitoring your patients’ risk of atherosclerosis.
This task used to be the purview of internists and cardiologists but, because gynecologists are increasingly serving as a primary care provider, you need to learn to recognize and diagnose the many clinical expressions of atherosclerosis in your aging patients.
A crucial part of that knowledge is a thorough understanding of each and every lipid concentration parameter reported within the standard lipid profile. This article reviews those parameters, explains how to interpret them individually and in combination, and introduces a new paradigm: the analysis of lipoprotein particle concentrations as a more precise way to determine risk.
If used in its entirety, the lipid profile provides a significant amount of information about the presence or absence of pathologic lipoprotein concentrations. Far too many clinicians focus solely on low-density lipoprotein cholesterol (LDL-C) and ignore the rest of the profile. Failure to consider the other variables is one reason why atherosclerotic disease is underdiagnosed and undertreated in the United States in many patients—especially women.1
1. Look at the triglyceride (TG) level. If it is >500 mg/dL, treatment is indicated, and TG reduction takes precedence over all other lipid concentrations. If TG is <500 mg/dL, go to Step 2.
2. Look at the low-density lipoprotein cholesterol (LDL-C) level. If it is >190 mg/dL, drug therapy is indicated regardless of other findings. At lower levels, the need for therapy is based on the patient’s overall risk of cardiovascular disease (CVD). Therapeutic lifestyle recommendations are always indicated.
3. Look at high-density lipoprotein cholesterol (HDL-C). Increased risk is present if it is <50 mg/dL, the threshold for women. Do not assume that high HDL-C always means low CVD risk.
4. Calculate the total cholesterol (TC)/HDL-C ratio (a surrogate of apoB/apoA-I ratio). Increased risk is present if it is >4.0.
5. Calculate the non-HDL-C level (TC minus HDL-C). If it is >130 mg/dL (or >100 mg/dL in very-high-risk women), therapy is warranted. Newer data reveal that this calculation is always equal to, or better than, LDL-C at predicting CVD risk. Non-HDL-C is less valuable if TG is >500 mg/dL.
6. Calculate the TG/HDL-C ratio to estimate the size of LDL. If the ratio is >3.8, the likelihood of small LDL is 80%. (Small LDL usually has very high LDL-P.)
Why lipoproteins are important
There is only one absolute in atherosclerosis: Sterols—predominantly cholesterol—enter the artery wall, where they are oxidized, internalized by macrophages, and transformed into foam cells, the histologic hallmark of atherosclerosis. With the accumulation of foam cells, fatty streaks develop and, ultimately, so does complex plaque.
Lipids associated with cardiovascular disease (CVD) include:
- cholesterol
- noncholesterol sterols such as sitosterol, campesterol, and others of mostly plant or shellfish origin
- triacylglycerol, or triglycerides (TG)
- phospholipids.
Because lipids are insoluble in aqueous solutions such as plasma, they must be “trafficked” within protein-enwrapped particles called lipoproteins. The surface proteins that provide structure and solubility to lipoproteins are called apolipoproteins. A key concept is that, with their surface apolipoproteins and cholesterol core, certain lipoproteins are potential agents of atherogenesis in that they transport sterols into the artery wall.2
Estimation of the risk of CVD involves careful analysis of all standard lipid concentrations and their various ratios, and prediction of the potential presence of atherogenic lipoproteins. Successful prevention or treatment of atherosclerosis entails limiting the presence of atherogenic lipoproteins.
A new paradigm is on its way
The atherogenicity of lipoprotein particles is determined by particle concentration as well as other variables, including particle size, lipid composition, and distinct surface apolipoproteins.
Lipoproteins smaller than 70 nm in diameter are driven into the arterial intima primarily by concentration gradients, regardless of lipid composition or particle size.3 A recent Consensus Statement from the American Diabetes Association and the American College of Cardiology observed that quantitative analysis of these potentially atherogenic lipoproteins is one of the best lipid/lipoprotein-related determinants of CVD risk.4 Lipoprotein particle concentrations have emerged not only as superb predictors of risk, but also as goals of therapy.5-7
Because of cost, third-party reimbursement, varying test availability, and lack of interpretive knowledge, few clinicians routinely order lipoprotein quantification. Historically, CVD risk and goals of therapy have been based on lipid concentrations (the amount of lipids trafficked within lipoprotein cores) reported in the lipid profile. Guidelines from the National Cholesterol Education Program, Adult Treatment Panel III (NCEP ATP-III)8,9 and the American Heart Association (AHA) CVD Prevention in Women10,11 use lipid concentrations such as total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), and TG as estimates or surrogates of lipoprotein concentrations ( TABLE 1 ).
The day is rapidly approaching, however, when lipoprotein concentrations may replace the lipid profile in clinical practice. It is critical that clinicians develop a solid understanding of lipoprotein physiology and pathology.7,12 It also is crucial that we be as skilled as possible in accurately predicting lipoprotein pathology using all of the lipid concentration parameters present in the lipid panel.
TABLE 1
Desirable lipid values for women
| Lipid | Level (mg/dL) |
|---|---|
| Total cholesterol | <200 |
| Low-density lipoprotein (LDL) cholesterol | <100 |
| High-density lipoprotein (HDL) cholesterol | ≥50 |
| Triglycerides | <150 |
| Non-HDL-cholesterol | <130 |
| FOR VERY HIGH-RISK PATIENTS | |
| LDL-C | <70 |
| Non-HDL-C | <100 |
| Source: American Heart Association | |
How lipoproteins are analyzed
Lipoproteins can be separated into their components using any of several methodologies, including ultracentrifugation, electrophoresis, apolipoprotein content analysis, and nuclear magnetic resonance (NMR) spectroscopy. Of these, only the last two provide information on particle concentrations.13,14
Apolipoprotein content analysis reveals two major categories of particles:
- alpha-lipoproteins, or HDL, which contain two to four molecules of apolipoprotein A-I (apoA-I)
- beta-lipoproteins, a collective group of chylomicrons, very-low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), and LDL, each containing a single molecule of apolipoprotein B (apoB). Because of very different half-lives (chylomicrons, 1 hour; VLDL, 2–6 hours; IDL, 1–2 hours; LDL, 2–3 days), the great majority (90% to 95%) of apoB-containing particles are LDL. Although apoB measurement yields quantification of all beta-lipoproteins, it is primarily a surrogate of LDL particle (LDL-P) concentration.15
Individual particle concentrations, determined by NMR spectroscopy, are reported as VLDL-P, IDL-P, LDL-P, and HDL-P (see the “Glossary”).14
Several epidemiologic studies that enrolled both genders found the best predictors of risk to be:
- elevated levels of apoB or LDL-P and reduced levels of apoA-I or HDL-P
- a high apoB/apoA-I ratio or LDL-P/HDL-P ratio.6,13,14
After adjustment for lipoprotein concentration data (apoB or LDL-P), other lipoprotein characteristics such as particle lipid content, size, or composition, for the most part, had no statistically significant relationship with the risk of cardiovascular disease.16,17
Lipids and lipoproteins: A glossary
| Variable | What is it? |
|---|---|
| Triglycerides (TG) | The triacylglycerol concentration within all of the TG-trafficking lipoproteins in 100 mL or 1 dL of plasma |
| Total cholesterol (TC) | Cholesterol content of all lipoproteins in 1 dL of plasma |
| Low-density lipoprotein (LDL) cholesterol | Cholesterol content of all intermediate-density lipoprotein (IDL) and LDL particles in 1 dL of plasma |
| High-density lipoprotein (HDL) cholesterol | Cholesterol content of all HDL particles in 1 dL of plasma |
| Very-low-density lipoprotein (VLDL) cholesterol | Cholesterol content of all VLDL particles in 1 dL of plasma |
| Remnant-C | Cholesterol content of all remnants in 1 dL of plasma |
| Lipoprotein (a) [Lp(a)] cholesterol | Cholesterol content of LDL particles that have apo(a) attached |
| Lp(a) concentration | Concentration of apo(a) in 1 dL of plasma |
| Non-HDL cholesterol | Cholesterol within all apoB particles in 1 dL of plasma |
| LDL-P | Number of LDL particles in 1 L of plasma (expressed in nmol/L). This represents LDL particles of all sizes |
| Small LDL-P | Number of small and intermediate LDL particles in 1 L of plasma (nmol/L) |
| HDL-P | Number of HDL particles in 1 L of plasma (μmol/L). HDL-P is also reported as large, intermediate, and small HDL-P (μmol/L) |
| VLDL-P | Number of VLDL particles in 1 L of plasma (nmol/L) |
| IDL-P | Number of IDL particles in 1 L of plasma (nmol/L) |
| LDL size | Diameter of the predominant LDL species:
|
Using lipid measurements to estimate lipoproteins
Total cholesterol represents the cholesterol content within all lipoproteins in 1 dL of plasma. Because beta-lipoproteins are considerably larger than alpha-lipoproteins, approximately 75% of total cholesterol is carried in the apoB-containing particles, making TC an apoB surrogate.
VLDL-C, an often ignored variable, is not measured but calculated using the Friedewald formula, dividing TG by five. This calculation assumes—often erroneously as TG levels rise—that TG consists only of VLDL particles and that VLDL composition contains five times more TG than cholesterol molecules.
A desirable TG level is <150 mg/dL, so normal VLDL-C is 150/5 or <30 mg/dL.
LDL-C is also an apoB surrogate
Although VLDL-C is a weak apoB surrogate,15 data from the Framingham Heart Study showed it to be a good predictor of VLDL remnant particles.18 However, because the vast majority of beta-lipoproteins are LDL, LDL-C (especially if elevated) is a better apoB surrogate than VLDL-C and is the primary CVD risk factor and goal of therapy in every current guideline.
LDL-C is usually a calculated value using the formula:
LDL-C = TC – (HDL-C + VLDL-C)
Upon special order, laboratories can directly measure LDL-C. This option is most useful when TG levels are high, rendering the Friedewald formula less accurate ( TABLE 2 ).19 For population cut points and desirable goals of therapy for lipid and lipoprotein concentrations, see the FIGURE .
TABLE 2
How lipid concentrations are determined
| TC = apoA-I-C + apoB-C |
| TC = HDL-C + LDL-C + VLDL-C + IDL-C + Chylomicron-C + Lp(a)-C + Remnant-C |
| In a fasting patient under normal circumstances, there are no chylomicrons and remnants (smaller chylomicrons or VLDL particles) and very few, if any, IDL particles. These are postprandial lipoproteins. Most patients do not have Lp(a) pathology. Therefore, the lipid concentration formula simplifies: |
| TC = HDL-C + LDL-C + VLDL-C |
| VLDL-C is estimated by TG/5 (assumes that all TG is in VLDL and that VLDL TG:cholesterol composition is 5:1). Therefore: |
| TC = HDL-C + LDL-C + TG/5 |
| LDL-C = TC – (HDL-C + TG/5) |
| Non-HDL-C = TC – HDL-C |
| In actuality, the calculated or directly measured LDL-C values in the standard lipid panel represent LDL-C + IDL-C + Lp(a)-C. However, because labs do not usually separate IDL and Lp(a) particles from LDL (without significant added expense), only total LDL-C is reported. |
FIGURE Population percentile cut points and goals for LDL-C, LDL-P, ApoB, and non-HDL-C
HDL-C, apoA-I are inversely related to cardiovascular risk
The epidemiologic data strongly indicate that both HDL-C and apoA-I are strongly and inversely related to CVD risk.6 HDL particles are a heterogenous collection of:
- unlipidated apoA-I
- very small pre-beta HDL
- more mature, lipidated HDL3 and HDL2 species (HDL3 smaller than HDL2).
NMR nomenclature identifies the smaller HDL species as H1 and H2 and the larger HDL species as H4 and H5.14 The smaller HDL species also contain apoA-II.
Although HDL can acquire cholesterol from any cell, including arterial-wall foam cells, the majority of HDL lipidation occurs in the liver or proximal small intestine, after which it is trafficked to steroidogenic tissue, adipocytes, or back to the liver. Normally, HDL carries little TG.20 The only lipid concentration that can serve as a surrogate of apoA-I or HDL-P is HDL-C, where the assumption is that higher HDL-C indicates higher apoA-I, and vice versa.
In reality, the correlation between apoA-I and HDL-C varies because each HDL particle can have from two to four apoA-I molecules, and the volume of cholesterol within the particle is a function of particle size and its TG content. For the most part, total HDL-C is indicative of the cholesterol carried in the larger, mature HDL2 (H4, H5) particles; patients with low HDL-C typically lack these mature, lipidated HDL particles.
Because HDL rapidly and repeatedly lipidates and then delipidates, there is no relationship between the HDL-C level and the complex dynamic process termed reverse cholesterol transport process. Neither HDL-C, nor apoA-I, nor HDL-P, nor HDL size is consistently related to HDL particle functionality—i.e., the ability of HDL to lipidate or delipidate, appropriately traffic cholesterol, or perform numerous other nonlipid antiatherogenic functions.20,21
Two premenopausal women undergo assessment of their basic lipid panel, with these results:
| LIPID | PATIENT 1 | PATIENT 2 |
|---|---|---|
| Total cholesterol (TC) | 180 | 180 |
| LDL-C | 100 | 100 |
| HDL-C | 60 | 40 |
| VLDL-C | 20 | 40 |
| Triglycerides (TG) | 100 | 200 |
| Non-HDL-C | 120 | 160 |
| TC/HDL-C ratio | 3.0 | 4.5 |
| TG/HDL-C ratio | 1.6 | 5.0 |
| LDL-C, low-density lipoprotein cholesterol | ||
| HDL-C, high-density lipoprotein cholesterol | ||
| VLDL-C, very-low-density lipoprotein cholesterol | ||
Both patients have the same desirable TC and LDL-C values. However, further analysis reveals an abnormal TC/HDL-C ratio and an abnormal non-HDL-C level in patient 2. This finding indicates a higher risk of CVD.
In addition, the TG/HDL-C ratio of 5.0 in patient 2 is highly suggestive of small-LDL phenotype B. That designation means that this patient will have 40% to 70% more LDL particles to traffic her LDL-C than patient 1, who appears to have LDL of normal size.27 The elevated VLDL-C of patient 2 indicates the presence of VLDL remnants, which predict risk above that conveyed by LDL-C.7
The typical clinician, looking only at TC or LDL-C, would miss the increased risk (high apoB) in patient 2. Obvious clues to her lipoprotein pathology are the elevated TG and reduced HDL-C (TG-HDL axis disorder). Beyond elevated TG and reduced HDL-C, patient 2 is also likely to have increased waist size, subtle hypertension, and possibly impaired fasting glucose—three additional parameters of metabolic syndrome.7,10,25
Focus on lipoprotein particle concentrations
To most accurately predict lipid-related CVD risk, you must determine which patients have elevated numbers of atherogenic lipoproteins using actual particle concentrations. In most practices, lipoprotein particle numbers must be estimated by scrutinizing all of the lipid concentrations and ratios (not simply LDL-C).
TC and, especially, LDL-C are apoB and LDL-P surrogates, but the best lipid concentration estimate of apoB is the calculated non-HDL-C value. By subtracting HDL-C from TC, it is possible to identify the cholesterol not in the HDL particles but in all of the potentially atherogenic apoB particles. In essence, non-HDL-C is VLDL-C plus LDL-C. This equation yields a better apoB or LDL-P proxy, compared with LDL-C alone.18 If a patient has reached her LDL-C goal but still has a high non-HDL-C level, we can assume that there are still too many apoB particles and that they are contributing to residual risk.
Because LDL is the predominant apoB species, non-HDL-C is the best lipid concentration predictor of LDL-P.15 Because neither TC nor HDL-C assays require a patient to fast, non-HDL-C is accurate in nonfasting patients, making it a very practical way to screen for CVD risk.8 In the Women’s Health Study, which involved mostly healthy women, non-HDL-C predicted the risk of coronary heart disease as well as apoB did, but not as well as LDL-P.22,23 In independent, separately published analyses from the Framingham Off-spring Study, LDL-P was a better predictor of risk than LDL-C and apoB.15,24
NCEP ATP-III guidelines introduced non-HDL-C as a secondary goal of therapy in patients with TG >200 mg/dL. Subsequent data indicate that non-HDL-C is always a better predictor of risk than LDL-C is, regardless of TG levels.18
The AHA Women’s Guideline was the first to set a desired non-HDL-C level (130 mg/dL) independent of the TG value.10 Because a normal VLDL-C concentration is 30 mg/dL, the non-HDL-C goal is 30 mg/dL above the desired LDL-C goal. For example, if the desired LDL-C value is 100 mg/dL, the non-HDL-C goal is 130 mg/dL. If the desired LDL-C goal is 70 mg/dL—as it is in a patient at very high risk—the non-HDL-C goal would be 100 mg/dL ( FIGURE ).9,11
Insulin resistance diminishes accuracy of lipid profile
The ability to predict lipoprotein particle concentrations using the lipid profile becomes far less accurate in situations associated with insulin resistance and metabolic syndrome in patients who have TG-HDL axis disorders. In women, these disorders are typified by an elevation of TG >150 mg/dL and a decrease in HDL-C <50 mg/dL, with borderline or normal LDL-C levels.25
As TG begins to rise above 120 mg/dL, hepatic secretion of TG-rich VLDL particles increases. As VLDL-TG is hydrolyzed by lipoprotein lipase in muscle and fat cells, in a process termed lipolysis, VLDL shrinks and transforms into IDL. Ultimately, unless it is cleared by hepatic LDL receptors, the IDL undergoes additional lipolysis by hepatic lipase and transforms into LDL particles. Because of their longer half-life, these LDL particles accumulate, further elevating apoB and LDL-P.
In the presence of TG-rich VLDL and chylomicrons, additional pathologic particle remodeling occurs. By way of a lipid transfer protein called cholesteryl ester transfer protein (CETP), some of the TG molecules present in TG-rich lipoproteins are exchanged for cholesteryl esters in LDL and HDL. This lipid transfer creates LDL and HDL that are TG-rich and cholesterol-poor, enabling additional TG lipolysis by hepatic lipase to create smaller LDL and HDL. The latter is so small that it can pass through renal glomeruli and be excreted, leading to reductions of HDL-P, apoA-I, and HDL-C.
Also created in this process are smaller, atherogenic, cholesterol-rich VLDL and chylomicron remnants, diagnosable by an elevated VLDL-C. Patients who have this pathology typically have elevated TG, reduced HDL-C, variable LDL-C, and an increased TG/HDL-C ratio (>3.8), which are indicative of too many small LDL particles (high apoB, LDL-P) and reduced number of HDL particles (high apoB/A-I ratio).26,27
Such a scenario, typical of TG-HDL axis disorders, explains much of the risk associated with rising TG levels and is very common in premenopausal women who have insulin-resistant states such as type 2 diabetes or polycystic ovary syndrome and in menopausal women who have insulin resistance and coronary artery disease.1
LDL-C and LDL-P do not always correlate
Because the volume of a lipoprotein is a function of its radius cubed (V = 4/3πr3),14 a patient who has small LDL will require up to 40% to 70% more LDL particles to traffic a given amount of LDL-C. In such a patient, there is often little correlation between LDL-C and LDL-P or apoB values. Regardless of the LDL-C, the apoB, LDL-P, or non-HDL-C is often elevated.28 This risk, which cannot be predicted by looking only at LDL-C, is the main reason guidelines advocate the use of non-HDL-C or the TC/HDL-C ratio.8,11 (See the case studies.)
In summary, a large part of the risk of CVD seen in patients who have low HDL-C derives from the associated increase in the number of apoB particles, mostly composed of small LDL, as well as an increase in remnant particles.15,21,28 This crucial point explains why treatment of low HDL-C states should always first target apoB or LDL-P (LDL-C and non-HDL-C), rather than apoA-I or HDL-C ( TABLES 3 and 4 ).8,9
TABLE 3
Lipid markers of small low-density lipoproteins
| High-density lipoprotein cholesterol (HDL-C) <50 mg/dL |
| Triglyceride (TG) >130–150 mg/dL |
| Total cholesterol/HDL-C ratio >4.0 with normal low-density lipoprotein cholesterol (LDL-C) |
| TG/HDL-C ratio >3.8 in women |
| Unremarkable LDL-C but elevated non-HDL-C |
TABLE 4
Lipid markers of remnant lipoproteins
| Triglyceride (TG) >150–200 mg/dL |
| Very-low-density lipoprotein cholesterol >30 mg/dL |
| Unremarkable low-density lipoprotein cholesterol with elevated non-high-density lipoprotein cholesterol (HDL-C) |
| Low HDL-C in insulin-resistant patients |
| Elevated total cholesterol/HDL-C ratio and TG >150 mg/dL |
A few words of advice
The driving forces of atherogenesis are increased numbers of apoB-containing lipoproteins and impaired endothelial integrity. ApoB and LDL-P are the available lab assays that most accurately quantify atherogenic particle number.
The lipid-concentration surrogates that you should be using to better predict apoB and CVD risk are:
- TC (unless HDL-C is very high)
- LDL-C
- Non-HDL-C
- TC/HDL-C ratio
- TG/HDL-C ratio.
Because LDL is by far the most numerous of the apoB particles present in plasma, it is the primary agent of atherogenesis. However, apoB and LDL-P do not correlate with LDL-C when LDL particles are small, are TG-rich and cholesterol-poor, or simply cholesterol-poor (seen in some patients who have low LDL-C levels).7,15
Both NCEP ATP-III and AHA Women’s Guidelines use the TC/HDL ratio as a powerful risk predictor. However, as a goal of therapy, these guidelines recommend normalizing LDL-C and then non-HDL-C.8,11 In reality, normalization of non-HDL-C takes care of LDL-C as well. For example, say a patient has LDL-C <100 mg/dL, but non-HDL-C >130 mg/dL or TC/HDL-C ratio >4. These readings indicate residual risk and suggest that an elevated number of apoB particles is present. Therapy to normalize non-HDL-C or, better yet, apoB/LDL-P, is warranted. The clue that residual risk is present even when LDL-C is normal is the reduction of HDL-C and elevation of TG and non-HDL-C.
1. Lloyd-Jones DM, O’Donnell CJ, D’Agostino RB, et al. Applicability of cholesterol-lowering primary prevention trials to a general population. The Framingham Heart Study. Arch Intern Med. 2001;161:949-954.
2. Biggerstaff KD, Wooten JS. Understanding lipoproteins as transporters of cholesterol and other lipids. Adv Physiol Educ. 2004;28:105-106.
3. Nordestgaard BG, Wooten R, Lewis B. Selective retention of VLDL, IDL and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. 1995;15:534-542.
4. Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk. Consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811-822.
5. Barter PJ, Ballantyne CM, Carmena R, et al. ApoB versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259:247-258.
6. Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026-2033.
7. Mudd JO, Borlaug BA, Johnson PV, et al. Beyond low-density lipoprotein cholesterol: defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J Am Coll Cardiol. 2007;50:1735-1741.
8. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
9. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227-239.
10. Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672-693.
11. Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481.-
12. Sniderman AD. Apolipoprotein B versus non-high-density lipoprotein cholesterol. And the winner is… Circulation. 2005;112:3366-3367.
13. Sniderman AD, Marcovina SM. Apolipoprotein A-I and B. Clin Lab Med. 2006;26:733-750.
14. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847-870.
15. Cromwell WC, Otvos JD, Keyes MJ, et al. LDL particle number and risk of future cardiovascular disease in the Framingham Off spring Study—implications for LDL management. J Clin Lipidol. 2007;1:583-592.
16. El Harchaoui K, van der Steeg WA, Stroes ES, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547-553.
17. Mora S, Szklo M, Otvos JD, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2007;192:211-217.
18. Liu J, Sempos CT, Donahue RP, et al. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their predictive risk values in coronary heart disease. Am J Cardiol. 2006;98:1363-1368.
19. National Cholesterol Education Program. Recommendations on lipoprotein measurement from the Working Group on Lipoprotein Measurement. National Institutes of Health. National Heart, Lung, and Blood Institute. NIH Publication No. 95-3044. Bethesda, Md: September 1995.
20. Dayspring T. High density lipoproteins: emerging knowledge. J Cardiometabol Syndr. 2007;2:59-62.
21. Cromwell WC. High-density lipoprotein associations with coronary heart disease: does measurement of cholesterol content give the best result? J Clin Lipidol. 2007;1:57-64.
22. Ridker PM, Rifai N, Cook NR, et al. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326.-
23. Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930-1937.
24. Ingelsson E, Schaefer EJ, Contois JH, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776-785.
25. Szapary PO, Rader DJ. The triglyceride-high-density lipoprotein axis: an important target of therapy. Am Heart J. 2004;148:211-221.
26. Davidson MH, Yannicelli D. New concepts in dyslipidemia in the metabolic syndrome and diabetes. Metab Syndr Relat Disord. 2006;4:299-314.
27. Hanak V, Munoz J, Teague J, Stanley A, Jr, Bittner V. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94:219-222.
28. Kathiresan S, Otvos JD, Sullivan LM, et al. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20-29.
1. Lloyd-Jones DM, O’Donnell CJ, D’Agostino RB, et al. Applicability of cholesterol-lowering primary prevention trials to a general population. The Framingham Heart Study. Arch Intern Med. 2001;161:949-954.
2. Biggerstaff KD, Wooten JS. Understanding lipoproteins as transporters of cholesterol and other lipids. Adv Physiol Educ. 2004;28:105-106.
3. Nordestgaard BG, Wooten R, Lewis B. Selective retention of VLDL, IDL and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. 1995;15:534-542.
4. Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk. Consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811-822.
5. Barter PJ, Ballantyne CM, Carmena R, et al. ApoB versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259:247-258.
6. Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026-2033.
7. Mudd JO, Borlaug BA, Johnson PV, et al. Beyond low-density lipoprotein cholesterol: defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J Am Coll Cardiol. 2007;50:1735-1741.
8. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
9. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227-239.
10. Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672-693.
11. Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481.-
12. Sniderman AD. Apolipoprotein B versus non-high-density lipoprotein cholesterol. And the winner is… Circulation. 2005;112:3366-3367.
13. Sniderman AD, Marcovina SM. Apolipoprotein A-I and B. Clin Lab Med. 2006;26:733-750.
14. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847-870.
15. Cromwell WC, Otvos JD, Keyes MJ, et al. LDL particle number and risk of future cardiovascular disease in the Framingham Off spring Study—implications for LDL management. J Clin Lipidol. 2007;1:583-592.
16. El Harchaoui K, van der Steeg WA, Stroes ES, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547-553.
17. Mora S, Szklo M, Otvos JD, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2007;192:211-217.
18. Liu J, Sempos CT, Donahue RP, et al. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their predictive risk values in coronary heart disease. Am J Cardiol. 2006;98:1363-1368.
19. National Cholesterol Education Program. Recommendations on lipoprotein measurement from the Working Group on Lipoprotein Measurement. National Institutes of Health. National Heart, Lung, and Blood Institute. NIH Publication No. 95-3044. Bethesda, Md: September 1995.
20. Dayspring T. High density lipoproteins: emerging knowledge. J Cardiometabol Syndr. 2007;2:59-62.
21. Cromwell WC. High-density lipoprotein associations with coronary heart disease: does measurement of cholesterol content give the best result? J Clin Lipidol. 2007;1:57-64.
22. Ridker PM, Rifai N, Cook NR, et al. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326.-
23. Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930-1937.
24. Ingelsson E, Schaefer EJ, Contois JH, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776-785.
25. Szapary PO, Rader DJ. The triglyceride-high-density lipoprotein axis: an important target of therapy. Am Heart J. 2004;148:211-221.
26. Davidson MH, Yannicelli D. New concepts in dyslipidemia in the metabolic syndrome and diabetes. Metab Syndr Relat Disord. 2006;4:299-314.
27. Hanak V, Munoz J, Teague J, Stanley A, Jr, Bittner V. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94:219-222.
28. Kathiresan S, Otvos JD, Sullivan LM, et al. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20-29.
A curious case study
- Skin folds on the neck
- Decreasing visual acuity
- Skin biopsy showing irregular aggregates of clumped, thickened, elastic fibers in the papillary and reticular dermis
A 21-year-old woman came to the British American Hospital in Lima, Peru, complaining of skin folds that had been growing on her neck for 3 years, as well as decreasing visual acuity over the last several months. She said that the skin folds were neither itchy nor painful.
She had a history of heartburn that worsened after eating, but that was being treated successfully with ranitidine. She reported no fever, chills, sweats, weight loss, alopecia, photosensitivity, malar rash, or any other skin rashes. Her history was otherwise unremarkable. She was taking no medications other than ranitidine, and had no known drug allergies. She didn’t smoke and drank alcohol only occasionally. Our patient worked as a salesperson at a music store. Interestingly, she had a sister with the same skin lesions and ophthalmologic complaint.
We found no lymphadenopathy. On her antecubital fossae and neck, numerous small, yellow papules had coalesced to form plaques with a cobblestone appearance (FIGURE 1). Her skin was redundant in the neck and axillae. We noted no other skin folds. She had a prominent horizontal crease of the chin (positive mental crease sign).
There were angioid streaks on funduscopic examination (FIGURE 2). Her lungs were clear to auscultation and percussion. Her cardiovascular exam was normal; there were no carotid bruits, and peripheral pulses were normal. The abdomen was soft and nontender; no hepatosplenomegaly or masses were present. We found no peripheral edema or cyanosis. A neurological exam was unremarkable.
Blood work revealed a hemoglobin level of 11.8 g/dL. The rest of the complete blood count was within normal limits. Electrolytes, liver profile, lipid profile, and urinalysis were within normal limits. A chest film showed no acute process. Skin biopsy of the neck folds showed irregular aggregates of clumped, thickened, elasticfibers in the papillary and reticular dermis (FIGURE 3).
FIGURE 1
Skin folds that had been growing for 3 years
In addition to the skin folds on her neck, the patient had several small, yellow papules that had coalesced to form plaques with a cobblestone appearance.
FIGURE 2
Cause of the patient’s declining vision
A funduscopic exam revealed angioid streaks (arrows).
FIGURE 3
Skin biopsy results
Biopsy of the patient’s skin folds showed irregular aggregates of clumped, thickened, elastic fibers in the papillary and reticular dermis. No epidermal changes were appreciated (hematoxylin and eosin stain).
What is your diagnosis?
Diagnosis: Pseudoxanthoma elasticum
Pseudoxanthoma elasticum is an autosomally inherited disorder associated with the accumulation of mineralized and fragmented elastic fibers in the skin, Bruch’s membrane in the retina, and vessel walls.1,2 The patient’s dermatologic and ocular findings were highly suggestive of this disorder, particularly given the absence of findings indicative of other diseases.
The average age of patients diagnosed with pseudoxanthoma elasticum skin abnormalities is 22 years.3 Risk of cardiovascular disease may correlate with the presence of angioid streaks of the fundus—irregular, reddish-brown, or gray lines radiating from the optic disc.4 Patients may have severe ophthalmologic or cardiac disease with little or no skin involvement, or vice versa.5-8 Onset, progression, and severity of the disease vary considerably among patients.8,9
With timely diagnosis, many complications of pseudoxanthoma elasticum can be prevented or treated. In particular, prognosis improves with early recognition of extracutaneous organ involvement. Screen first-degree family members for any cutaneous or ophthalmologic markers of pseudoxanthoma elasticum, and offer genetic counseling to family members of affected patients.
Estimated prevalence ranges from 1:70,000 to 1:100,000 live births,10 which may vary in specific areas. In 2000, the patient support group PXE International suggested that there is a prevalence of 1:25,000 in New England.11 The female-to-male ratio is thought to be almost 2:1, but there is no satisfactory explanation for this. All races are affected.
Clues to look for in the 3 systems affected
Dermatologic manifestations. The most common features of pseudoxanthoma elasticum are ivory to yellowish, raised papules varying in size from 1 to 3 mm. The papules may have a linear or reticular arrangement and may coalesce into plaques.12 In many patients the skin becomes wrinkled and redundant, hanging in folds, as was the case with our patient. The lesions occur first on the lateral part of the neck and the flexural areas.
Isolated periumbilical skin lesions are called periumbilical perforating pseudoxanthoma elasticum, but there is no known relation with hereditary pseudoxanthoma elasticum.13 The mental crease sign is considered a sign of pseudoxanthoma elasticum.14 The scalp, palms, and soles are unaffected. Lesions on the mucous membranes are common, especially on the inner aspect of the lower lip.
Importantly, an absence of skin lesions does not exclude the diagnosis of pseudoxanthoma elasticum.4
Ocular changes. Angioid streaks of the fundus are the most common ocular findings and are associated with pseudoxanthoma elasticum 85% of the time.2 They result from a rupture of the elastic tissue in the Bruch’s membrane of the retina. Angioid streaks may become less marked with time or disappear in conjunction with generalized atrophy of the retinal pigmentum epithelium.15 The visual prognosis for patients with angioid streaks is often poor.
Other retinal changes include macular degeneration, altered pigmentation (eg, peau d’orange appearance), subretinal neovascularization, and chorioretinal scarring. Ocular hemorrhage can occur even with minimal trauma. Hu et al described one fundus feature that seems to be typical of pseudoxanthoma elasticum: comet-like tails.1
Cardiovascular involvement. Elastic tissue degeneration and calcification of arterial media within medium-sized arteries results in diminished peripheral pulses, intermittent claudication, coronary insufficiency, premature calcification of peripheral arteries, intracranial aneurysms, cerebral ischemia, and hypertension.16 Twenty-five percent of patients acquire renovascular hypertension secondary to renal blood flow obstruction from calcium deposition in the renal arteries.
Premature cardiovascular disease may begin as early as 4 years of age and may lead to angina or sudden death.2 Consider pseudoxanthoma elasticum in young patients with coronary artery disease and no cardiovascular risk factors.17
Calcification of elastic fibers in the thin-walled arteries directly under the gastric mucosa can cause gastrointestinal bleeding.18 Melena and hematemesis are reported in up to 15% of cases.19 Subarachnoid, nasal, renal, bladder, and joint bleeding are much less common.
Skin biopsy is key
The classic histologic picture with pseudoxanthoma elasticum skin lesions is fragmentation and clumping of elastic tissue evident on Verhoeff-van Gieson stain, and calcification on von Kossa stain.7 Patients who have angioid streaks on funduscopic examination but no visible skin lesions have received a diagnosis of pseudoxanthoma elasticum based on the biopsy results of scars or flexural skin of the neck or axillae.20 Hausser described a specific ultrastructural aberrant pattern in 3 siblings without any clinical symptoms: elastin of elastic fibers regularly contained small foci of calcification resembling those in perilesional skin of the mother and other pseudoxanthoma elasticum patients.21
Another reason for biopsy. Clinically visible pseudoxanthoma elasticum-like skin lesions are not pathognomonic for this disease, because they also occur in late-onset focal dermal elastosis,22 beta-thalassemia,23 adult patients with deforming osteitis (Paget’s disease) or osteoectasia,24 farmers exposed to saltpeter fertilizers,25 pseudoxanthoma elasticum-like papillary dermal elastolysis,26 and patients who have had penicillamine therapy.
Management
Watch out for complications
Management of pseudoxanthoma elasticum focuses on preventing, screening for, and monitoring complications. One study of adolescents with pseudoxanthoma elasticum demonstrated a positive correlation between a high intake of dietary calcium and cardiovascular manifestations—but not skin lesions. At least one authority on the subject recommends that daily ingestion of calcium be restricted to 500 to 600 mg.27
Redundant skin folds can be treated with surgical excision.28 A case report describes the temporary treatment of chin folds with injectable collagen.29 Laser photocoagulation can prevent retinal hemorrhage, but recurrence is relatively common.30
How our patient’s case evolved
Once the patient’s presumptive diagnosis of pseudoxanthoma elasticum was confirmed by biopsy, we established the same diagnosis in her 18-year-old sister. The parents did not exhibit any clinical findings of pseudoxanthoma elasticum. We did refer the father to ophthalmology and to dermatology for skin biopsies of scars, but he never returned for follow-up.
To rule out cardiovascular involvement, we referred the patient to our cardiology colleagues. No abnormality was found on physical exam, and the EKG and echocardiography results were within normal limits. Results of myocardial perfusion testing were also within normal limits, and the patient achieved 92% of the maximum predicted heart rate.
Since the patient was not a smoker, we simply reinforced the importance of not smoking. We advised her to follow a diet low in fat and calcium, and to avoid platelet inhibitors and contact sports to prevent gastrointestinal bleeding and retinal hemorrhage, respectively. We also recommended that she have periodic funduscopic evaluations, as well as cardiovascular evaluations to monitor blood pressure and lipids.
Acknowledgments
The authors thank Rafael Doig, MD (Nuclear Medicine) and Jose Barriga, MD (Department of Ophthalmology) of British American Hospital, Lima, Peru, for their assistance in the preparation of this article.
Correspondence
Gustavo Vasquez, MD, Division of Infectious Diseases, Department of Medicine, Thomas Jefferson University, 834 Walnut Street, Suite 650, Philadelphia, PA 19107; [email protected]
1. Hu X, Plomp A, van Soest S, et al. Pseudoxanthoma elasticum: A clinical, histopathological, and molecular update. Survey Ophthalmol. 2003;48:424-438.
2. Abdelmalek NF, Gerber TL, Menter A. Cardiocutaneous syndromes and associations. J Am Acad Dermatol. 2002;46:161-183.
3. Jacobi H, Schreiber G. Pseudoxanthoma elasticum. Skin changes as a marker of systemic illness. Der Hautarzt. 1997;48:191-194.
4. Sherer DW, Sapadin AN, Lebwohl MG. Pseudoxanthoma elasticum: an update. Dermatology. 1999;199:3-7.
5. De Paepe A, Viljoen D, Matton M, et al. Pseudoxanthoma elasticum: similar autosomal recessive subtype in Belgian and Afrikaner families. Am J Med Genet. 1991;38:16-20.
6. Kevorkian JP, Masquet C, Kural-Menasche S, et al. New report of severe coronary artery disease in an eighteen-year-old girl with pseudoxanthoma elasticum. Case report and review of the literature. Angiology. 1997;48:735-741.
7. Lebwohl M, Halperin J, Phelps RG. Brief report: occult pseudoxanthoma elasticum in patients with premature cardiovascular disease. N Engl J Med. 1993;329:1237-1239.
8. Sherer DW, Bercovitch L, Lebwohl M. Pseudoxanthoma elasticum: significance of limited phenotypic expression in parents of affected offspring. J Am Acad Dermatol. 2001;44:534-537.
9. Uitto J, Boyd CD, Lebwohl MG, et al. International Centennial Meeting on Pseudoxanthoma Elasticum: progress in PXE research. J Invest Dermatol. 1998;110:840-842.
10. Struk B, Neldner KH, Rao VS, et al. Mapping of both autosomal recessive and dominant variants of pseudoxanthoma elasticum to chromosome 16p13.1. Hum Mol Genet. 1997;6:1823-1828.
11. Chassaing N, Martin L, Calvas P. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet. 2005;42:881-892.
12. Schneider T, Appel H-P, Kuhlwein A, et al. Groenblad-Strandberg-Syndrome. Z Hautkr. 1984;59:1290-1300.
13. Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
14. Lebwohl M, Lebwohl E, Bercovitch L. Prominent mental (chin) crease: a new sign of pseudoxanthoma elasticum. J Am Acad Dermatol. 2003;48:620-622.
15. Shilling JS, Blach RK. Prognosis and therapy of angioid streaks. Trans Ophthalmol Soc UK. 1975;95:301-306.
16. Schievink WI, Michels VV, Piepgras DG. Neurovascular manifestations of heritable connective tissue disorders: a review. Stroke. 1994;25:889-903.
17. Kiec-Wilk B, Surdacki A, Dembinska-Kiec A. Acute myocardial infarction and a new ABCC6 mutation in a 16-year-old boy with pseudoxanthoma elasticum. Int J Cardiol. 2007;116:261-262.
18. Lever WF, Elder DE, Elenitsas R. Congenital diseases (Genodermatoses). In: Lever WF, Schaumburg-Lever G, eds. Histopathology of the Skin. 8th ed. Philadelphia, Pa: Lippincott-Raven; 1997:117-151.
19. Fah L. Pseudoxanthoma elasticum—a visual diagnosis. Schweiz Med Wochenschr. 1991;121:660-663.
20. Lebwohl M, Phelps RG, Yannuzzi L, et al. Diagnosis of pseudoxanthoma elasticum by scar biopsy in patients without characteristic skin lesions. N Engl J Med. 1987;317:347-350.
21. Hausser I. Early preclinical diagnosis of dominant pseudoxanthoma elasticum by specific ultrastructural changes of dermal elastic and collagen tissue in a family at risk. Hum Genet. 1991;87:693-700.
22. Limas C. Late onset focal dermal elastosis: a distinct clinicopathologic entity? Am J Dermatopathol. 1999;21:381-383.
23. Baccarani-Contri M, Bacchelli B, Boraldi F, et al. Characterization of pseudoxanthoma elasticum-like lesions in the skin of patients with beta-thalassemia. J Am Acad Dermatol. 2001;44:33-39.
24. Rook A, Wilkinson D, Ebling F, et al. PXE. In: Rook A, ed. Textbook of Dermatology. 4th ed. Oxford, England: Blackwell Scientific; 1986:1841-1844.
25. Christensen OB. An exogenous variety of pseudoxanthoma elasticum in old farmers. Acta Derm Venereol. 1978;58:319-321.
26. Rongioletti F, Rebora A. Fibroelastolytic patterns of intrinsic skin aging: pseudoxanthoma-elasticum-like papillary dermal elastolysis and white fibrous papulosis of the neck. Dermatology. 1995;191:19-24.
27. Neldner KH. Pseudoxanthoma elasticum. Int J Dermatol. 2007;27:98-100.
28. Ng AB, O’Sullivan ST, Sharpe DT. Plastic surgery and pseudoxanthoma elasticum. Br J Plast Surg. 1999;52:594-596.
29. Galadari H, Lebwohl M. Pseudoxanthoma elasticum: Temporary treatment of chin folds and lines with injectable collagen. J Am Acad Dermatol. 2003;49(suppl 5):S265-S266.
30. Uitto J, Ringpfeil F, Pulkkinen L. Pseudoxanthoma elasticum. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 1st ed. London, England: Mosby; 2003:1524-1526.
- Skin folds on the neck
- Decreasing visual acuity
- Skin biopsy showing irregular aggregates of clumped, thickened, elastic fibers in the papillary and reticular dermis
A 21-year-old woman came to the British American Hospital in Lima, Peru, complaining of skin folds that had been growing on her neck for 3 years, as well as decreasing visual acuity over the last several months. She said that the skin folds were neither itchy nor painful.
She had a history of heartburn that worsened after eating, but that was being treated successfully with ranitidine. She reported no fever, chills, sweats, weight loss, alopecia, photosensitivity, malar rash, or any other skin rashes. Her history was otherwise unremarkable. She was taking no medications other than ranitidine, and had no known drug allergies. She didn’t smoke and drank alcohol only occasionally. Our patient worked as a salesperson at a music store. Interestingly, she had a sister with the same skin lesions and ophthalmologic complaint.
We found no lymphadenopathy. On her antecubital fossae and neck, numerous small, yellow papules had coalesced to form plaques with a cobblestone appearance (FIGURE 1). Her skin was redundant in the neck and axillae. We noted no other skin folds. She had a prominent horizontal crease of the chin (positive mental crease sign).
There were angioid streaks on funduscopic examination (FIGURE 2). Her lungs were clear to auscultation and percussion. Her cardiovascular exam was normal; there were no carotid bruits, and peripheral pulses were normal. The abdomen was soft and nontender; no hepatosplenomegaly or masses were present. We found no peripheral edema or cyanosis. A neurological exam was unremarkable.
Blood work revealed a hemoglobin level of 11.8 g/dL. The rest of the complete blood count was within normal limits. Electrolytes, liver profile, lipid profile, and urinalysis were within normal limits. A chest film showed no acute process. Skin biopsy of the neck folds showed irregular aggregates of clumped, thickened, elasticfibers in the papillary and reticular dermis (FIGURE 3).
FIGURE 1
Skin folds that had been growing for 3 years
In addition to the skin folds on her neck, the patient had several small, yellow papules that had coalesced to form plaques with a cobblestone appearance.
FIGURE 2
Cause of the patient’s declining vision
A funduscopic exam revealed angioid streaks (arrows).
FIGURE 3
Skin biopsy results
Biopsy of the patient’s skin folds showed irregular aggregates of clumped, thickened, elastic fibers in the papillary and reticular dermis. No epidermal changes were appreciated (hematoxylin and eosin stain).
What is your diagnosis?
Diagnosis: Pseudoxanthoma elasticum
Pseudoxanthoma elasticum is an autosomally inherited disorder associated with the accumulation of mineralized and fragmented elastic fibers in the skin, Bruch’s membrane in the retina, and vessel walls.1,2 The patient’s dermatologic and ocular findings were highly suggestive of this disorder, particularly given the absence of findings indicative of other diseases.
The average age of patients diagnosed with pseudoxanthoma elasticum skin abnormalities is 22 years.3 Risk of cardiovascular disease may correlate with the presence of angioid streaks of the fundus—irregular, reddish-brown, or gray lines radiating from the optic disc.4 Patients may have severe ophthalmologic or cardiac disease with little or no skin involvement, or vice versa.5-8 Onset, progression, and severity of the disease vary considerably among patients.8,9
With timely diagnosis, many complications of pseudoxanthoma elasticum can be prevented or treated. In particular, prognosis improves with early recognition of extracutaneous organ involvement. Screen first-degree family members for any cutaneous or ophthalmologic markers of pseudoxanthoma elasticum, and offer genetic counseling to family members of affected patients.
Estimated prevalence ranges from 1:70,000 to 1:100,000 live births,10 which may vary in specific areas. In 2000, the patient support group PXE International suggested that there is a prevalence of 1:25,000 in New England.11 The female-to-male ratio is thought to be almost 2:1, but there is no satisfactory explanation for this. All races are affected.
Clues to look for in the 3 systems affected
Dermatologic manifestations. The most common features of pseudoxanthoma elasticum are ivory to yellowish, raised papules varying in size from 1 to 3 mm. The papules may have a linear or reticular arrangement and may coalesce into plaques.12 In many patients the skin becomes wrinkled and redundant, hanging in folds, as was the case with our patient. The lesions occur first on the lateral part of the neck and the flexural areas.
Isolated periumbilical skin lesions are called periumbilical perforating pseudoxanthoma elasticum, but there is no known relation with hereditary pseudoxanthoma elasticum.13 The mental crease sign is considered a sign of pseudoxanthoma elasticum.14 The scalp, palms, and soles are unaffected. Lesions on the mucous membranes are common, especially on the inner aspect of the lower lip.
Importantly, an absence of skin lesions does not exclude the diagnosis of pseudoxanthoma elasticum.4
Ocular changes. Angioid streaks of the fundus are the most common ocular findings and are associated with pseudoxanthoma elasticum 85% of the time.2 They result from a rupture of the elastic tissue in the Bruch’s membrane of the retina. Angioid streaks may become less marked with time or disappear in conjunction with generalized atrophy of the retinal pigmentum epithelium.15 The visual prognosis for patients with angioid streaks is often poor.
Other retinal changes include macular degeneration, altered pigmentation (eg, peau d’orange appearance), subretinal neovascularization, and chorioretinal scarring. Ocular hemorrhage can occur even with minimal trauma. Hu et al described one fundus feature that seems to be typical of pseudoxanthoma elasticum: comet-like tails.1
Cardiovascular involvement. Elastic tissue degeneration and calcification of arterial media within medium-sized arteries results in diminished peripheral pulses, intermittent claudication, coronary insufficiency, premature calcification of peripheral arteries, intracranial aneurysms, cerebral ischemia, and hypertension.16 Twenty-five percent of patients acquire renovascular hypertension secondary to renal blood flow obstruction from calcium deposition in the renal arteries.
Premature cardiovascular disease may begin as early as 4 years of age and may lead to angina or sudden death.2 Consider pseudoxanthoma elasticum in young patients with coronary artery disease and no cardiovascular risk factors.17
Calcification of elastic fibers in the thin-walled arteries directly under the gastric mucosa can cause gastrointestinal bleeding.18 Melena and hematemesis are reported in up to 15% of cases.19 Subarachnoid, nasal, renal, bladder, and joint bleeding are much less common.
Skin biopsy is key
The classic histologic picture with pseudoxanthoma elasticum skin lesions is fragmentation and clumping of elastic tissue evident on Verhoeff-van Gieson stain, and calcification on von Kossa stain.7 Patients who have angioid streaks on funduscopic examination but no visible skin lesions have received a diagnosis of pseudoxanthoma elasticum based on the biopsy results of scars or flexural skin of the neck or axillae.20 Hausser described a specific ultrastructural aberrant pattern in 3 siblings without any clinical symptoms: elastin of elastic fibers regularly contained small foci of calcification resembling those in perilesional skin of the mother and other pseudoxanthoma elasticum patients.21
Another reason for biopsy. Clinically visible pseudoxanthoma elasticum-like skin lesions are not pathognomonic for this disease, because they also occur in late-onset focal dermal elastosis,22 beta-thalassemia,23 adult patients with deforming osteitis (Paget’s disease) or osteoectasia,24 farmers exposed to saltpeter fertilizers,25 pseudoxanthoma elasticum-like papillary dermal elastolysis,26 and patients who have had penicillamine therapy.
Management
Watch out for complications
Management of pseudoxanthoma elasticum focuses on preventing, screening for, and monitoring complications. One study of adolescents with pseudoxanthoma elasticum demonstrated a positive correlation between a high intake of dietary calcium and cardiovascular manifestations—but not skin lesions. At least one authority on the subject recommends that daily ingestion of calcium be restricted to 500 to 600 mg.27
Redundant skin folds can be treated with surgical excision.28 A case report describes the temporary treatment of chin folds with injectable collagen.29 Laser photocoagulation can prevent retinal hemorrhage, but recurrence is relatively common.30
How our patient’s case evolved
Once the patient’s presumptive diagnosis of pseudoxanthoma elasticum was confirmed by biopsy, we established the same diagnosis in her 18-year-old sister. The parents did not exhibit any clinical findings of pseudoxanthoma elasticum. We did refer the father to ophthalmology and to dermatology for skin biopsies of scars, but he never returned for follow-up.
To rule out cardiovascular involvement, we referred the patient to our cardiology colleagues. No abnormality was found on physical exam, and the EKG and echocardiography results were within normal limits. Results of myocardial perfusion testing were also within normal limits, and the patient achieved 92% of the maximum predicted heart rate.
Since the patient was not a smoker, we simply reinforced the importance of not smoking. We advised her to follow a diet low in fat and calcium, and to avoid platelet inhibitors and contact sports to prevent gastrointestinal bleeding and retinal hemorrhage, respectively. We also recommended that she have periodic funduscopic evaluations, as well as cardiovascular evaluations to monitor blood pressure and lipids.
Acknowledgments
The authors thank Rafael Doig, MD (Nuclear Medicine) and Jose Barriga, MD (Department of Ophthalmology) of British American Hospital, Lima, Peru, for their assistance in the preparation of this article.
Correspondence
Gustavo Vasquez, MD, Division of Infectious Diseases, Department of Medicine, Thomas Jefferson University, 834 Walnut Street, Suite 650, Philadelphia, PA 19107; [email protected]
- Skin folds on the neck
- Decreasing visual acuity
- Skin biopsy showing irregular aggregates of clumped, thickened, elastic fibers in the papillary and reticular dermis
A 21-year-old woman came to the British American Hospital in Lima, Peru, complaining of skin folds that had been growing on her neck for 3 years, as well as decreasing visual acuity over the last several months. She said that the skin folds were neither itchy nor painful.
She had a history of heartburn that worsened after eating, but that was being treated successfully with ranitidine. She reported no fever, chills, sweats, weight loss, alopecia, photosensitivity, malar rash, or any other skin rashes. Her history was otherwise unremarkable. She was taking no medications other than ranitidine, and had no known drug allergies. She didn’t smoke and drank alcohol only occasionally. Our patient worked as a salesperson at a music store. Interestingly, she had a sister with the same skin lesions and ophthalmologic complaint.
We found no lymphadenopathy. On her antecubital fossae and neck, numerous small, yellow papules had coalesced to form plaques with a cobblestone appearance (FIGURE 1). Her skin was redundant in the neck and axillae. We noted no other skin folds. She had a prominent horizontal crease of the chin (positive mental crease sign).
There were angioid streaks on funduscopic examination (FIGURE 2). Her lungs were clear to auscultation and percussion. Her cardiovascular exam was normal; there were no carotid bruits, and peripheral pulses were normal. The abdomen was soft and nontender; no hepatosplenomegaly or masses were present. We found no peripheral edema or cyanosis. A neurological exam was unremarkable.
Blood work revealed a hemoglobin level of 11.8 g/dL. The rest of the complete blood count was within normal limits. Electrolytes, liver profile, lipid profile, and urinalysis were within normal limits. A chest film showed no acute process. Skin biopsy of the neck folds showed irregular aggregates of clumped, thickened, elasticfibers in the papillary and reticular dermis (FIGURE 3).
FIGURE 1
Skin folds that had been growing for 3 years
In addition to the skin folds on her neck, the patient had several small, yellow papules that had coalesced to form plaques with a cobblestone appearance.
FIGURE 2
Cause of the patient’s declining vision
A funduscopic exam revealed angioid streaks (arrows).
FIGURE 3
Skin biopsy results
Biopsy of the patient’s skin folds showed irregular aggregates of clumped, thickened, elastic fibers in the papillary and reticular dermis. No epidermal changes were appreciated (hematoxylin and eosin stain).
What is your diagnosis?
Diagnosis: Pseudoxanthoma elasticum
Pseudoxanthoma elasticum is an autosomally inherited disorder associated with the accumulation of mineralized and fragmented elastic fibers in the skin, Bruch’s membrane in the retina, and vessel walls.1,2 The patient’s dermatologic and ocular findings were highly suggestive of this disorder, particularly given the absence of findings indicative of other diseases.
The average age of patients diagnosed with pseudoxanthoma elasticum skin abnormalities is 22 years.3 Risk of cardiovascular disease may correlate with the presence of angioid streaks of the fundus—irregular, reddish-brown, or gray lines radiating from the optic disc.4 Patients may have severe ophthalmologic or cardiac disease with little or no skin involvement, or vice versa.5-8 Onset, progression, and severity of the disease vary considerably among patients.8,9
With timely diagnosis, many complications of pseudoxanthoma elasticum can be prevented or treated. In particular, prognosis improves with early recognition of extracutaneous organ involvement. Screen first-degree family members for any cutaneous or ophthalmologic markers of pseudoxanthoma elasticum, and offer genetic counseling to family members of affected patients.
Estimated prevalence ranges from 1:70,000 to 1:100,000 live births,10 which may vary in specific areas. In 2000, the patient support group PXE International suggested that there is a prevalence of 1:25,000 in New England.11 The female-to-male ratio is thought to be almost 2:1, but there is no satisfactory explanation for this. All races are affected.
Clues to look for in the 3 systems affected
Dermatologic manifestations. The most common features of pseudoxanthoma elasticum are ivory to yellowish, raised papules varying in size from 1 to 3 mm. The papules may have a linear or reticular arrangement and may coalesce into plaques.12 In many patients the skin becomes wrinkled and redundant, hanging in folds, as was the case with our patient. The lesions occur first on the lateral part of the neck and the flexural areas.
Isolated periumbilical skin lesions are called periumbilical perforating pseudoxanthoma elasticum, but there is no known relation with hereditary pseudoxanthoma elasticum.13 The mental crease sign is considered a sign of pseudoxanthoma elasticum.14 The scalp, palms, and soles are unaffected. Lesions on the mucous membranes are common, especially on the inner aspect of the lower lip.
Importantly, an absence of skin lesions does not exclude the diagnosis of pseudoxanthoma elasticum.4
Ocular changes. Angioid streaks of the fundus are the most common ocular findings and are associated with pseudoxanthoma elasticum 85% of the time.2 They result from a rupture of the elastic tissue in the Bruch’s membrane of the retina. Angioid streaks may become less marked with time or disappear in conjunction with generalized atrophy of the retinal pigmentum epithelium.15 The visual prognosis for patients with angioid streaks is often poor.
Other retinal changes include macular degeneration, altered pigmentation (eg, peau d’orange appearance), subretinal neovascularization, and chorioretinal scarring. Ocular hemorrhage can occur even with minimal trauma. Hu et al described one fundus feature that seems to be typical of pseudoxanthoma elasticum: comet-like tails.1
Cardiovascular involvement. Elastic tissue degeneration and calcification of arterial media within medium-sized arteries results in diminished peripheral pulses, intermittent claudication, coronary insufficiency, premature calcification of peripheral arteries, intracranial aneurysms, cerebral ischemia, and hypertension.16 Twenty-five percent of patients acquire renovascular hypertension secondary to renal blood flow obstruction from calcium deposition in the renal arteries.
Premature cardiovascular disease may begin as early as 4 years of age and may lead to angina or sudden death.2 Consider pseudoxanthoma elasticum in young patients with coronary artery disease and no cardiovascular risk factors.17
Calcification of elastic fibers in the thin-walled arteries directly under the gastric mucosa can cause gastrointestinal bleeding.18 Melena and hematemesis are reported in up to 15% of cases.19 Subarachnoid, nasal, renal, bladder, and joint bleeding are much less common.
Skin biopsy is key
The classic histologic picture with pseudoxanthoma elasticum skin lesions is fragmentation and clumping of elastic tissue evident on Verhoeff-van Gieson stain, and calcification on von Kossa stain.7 Patients who have angioid streaks on funduscopic examination but no visible skin lesions have received a diagnosis of pseudoxanthoma elasticum based on the biopsy results of scars or flexural skin of the neck or axillae.20 Hausser described a specific ultrastructural aberrant pattern in 3 siblings without any clinical symptoms: elastin of elastic fibers regularly contained small foci of calcification resembling those in perilesional skin of the mother and other pseudoxanthoma elasticum patients.21
Another reason for biopsy. Clinically visible pseudoxanthoma elasticum-like skin lesions are not pathognomonic for this disease, because they also occur in late-onset focal dermal elastosis,22 beta-thalassemia,23 adult patients with deforming osteitis (Paget’s disease) or osteoectasia,24 farmers exposed to saltpeter fertilizers,25 pseudoxanthoma elasticum-like papillary dermal elastolysis,26 and patients who have had penicillamine therapy.
Management
Watch out for complications
Management of pseudoxanthoma elasticum focuses on preventing, screening for, and monitoring complications. One study of adolescents with pseudoxanthoma elasticum demonstrated a positive correlation between a high intake of dietary calcium and cardiovascular manifestations—but not skin lesions. At least one authority on the subject recommends that daily ingestion of calcium be restricted to 500 to 600 mg.27
Redundant skin folds can be treated with surgical excision.28 A case report describes the temporary treatment of chin folds with injectable collagen.29 Laser photocoagulation can prevent retinal hemorrhage, but recurrence is relatively common.30
How our patient’s case evolved
Once the patient’s presumptive diagnosis of pseudoxanthoma elasticum was confirmed by biopsy, we established the same diagnosis in her 18-year-old sister. The parents did not exhibit any clinical findings of pseudoxanthoma elasticum. We did refer the father to ophthalmology and to dermatology for skin biopsies of scars, but he never returned for follow-up.
To rule out cardiovascular involvement, we referred the patient to our cardiology colleagues. No abnormality was found on physical exam, and the EKG and echocardiography results were within normal limits. Results of myocardial perfusion testing were also within normal limits, and the patient achieved 92% of the maximum predicted heart rate.
Since the patient was not a smoker, we simply reinforced the importance of not smoking. We advised her to follow a diet low in fat and calcium, and to avoid platelet inhibitors and contact sports to prevent gastrointestinal bleeding and retinal hemorrhage, respectively. We also recommended that she have periodic funduscopic evaluations, as well as cardiovascular evaluations to monitor blood pressure and lipids.
Acknowledgments
The authors thank Rafael Doig, MD (Nuclear Medicine) and Jose Barriga, MD (Department of Ophthalmology) of British American Hospital, Lima, Peru, for their assistance in the preparation of this article.
Correspondence
Gustavo Vasquez, MD, Division of Infectious Diseases, Department of Medicine, Thomas Jefferson University, 834 Walnut Street, Suite 650, Philadelphia, PA 19107; [email protected]
1. Hu X, Plomp A, van Soest S, et al. Pseudoxanthoma elasticum: A clinical, histopathological, and molecular update. Survey Ophthalmol. 2003;48:424-438.
2. Abdelmalek NF, Gerber TL, Menter A. Cardiocutaneous syndromes and associations. J Am Acad Dermatol. 2002;46:161-183.
3. Jacobi H, Schreiber G. Pseudoxanthoma elasticum. Skin changes as a marker of systemic illness. Der Hautarzt. 1997;48:191-194.
4. Sherer DW, Sapadin AN, Lebwohl MG. Pseudoxanthoma elasticum: an update. Dermatology. 1999;199:3-7.
5. De Paepe A, Viljoen D, Matton M, et al. Pseudoxanthoma elasticum: similar autosomal recessive subtype in Belgian and Afrikaner families. Am J Med Genet. 1991;38:16-20.
6. Kevorkian JP, Masquet C, Kural-Menasche S, et al. New report of severe coronary artery disease in an eighteen-year-old girl with pseudoxanthoma elasticum. Case report and review of the literature. Angiology. 1997;48:735-741.
7. Lebwohl M, Halperin J, Phelps RG. Brief report: occult pseudoxanthoma elasticum in patients with premature cardiovascular disease. N Engl J Med. 1993;329:1237-1239.
8. Sherer DW, Bercovitch L, Lebwohl M. Pseudoxanthoma elasticum: significance of limited phenotypic expression in parents of affected offspring. J Am Acad Dermatol. 2001;44:534-537.
9. Uitto J, Boyd CD, Lebwohl MG, et al. International Centennial Meeting on Pseudoxanthoma Elasticum: progress in PXE research. J Invest Dermatol. 1998;110:840-842.
10. Struk B, Neldner KH, Rao VS, et al. Mapping of both autosomal recessive and dominant variants of pseudoxanthoma elasticum to chromosome 16p13.1. Hum Mol Genet. 1997;6:1823-1828.
11. Chassaing N, Martin L, Calvas P. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet. 2005;42:881-892.
12. Schneider T, Appel H-P, Kuhlwein A, et al. Groenblad-Strandberg-Syndrome. Z Hautkr. 1984;59:1290-1300.
13. Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
14. Lebwohl M, Lebwohl E, Bercovitch L. Prominent mental (chin) crease: a new sign of pseudoxanthoma elasticum. J Am Acad Dermatol. 2003;48:620-622.
15. Shilling JS, Blach RK. Prognosis and therapy of angioid streaks. Trans Ophthalmol Soc UK. 1975;95:301-306.
16. Schievink WI, Michels VV, Piepgras DG. Neurovascular manifestations of heritable connective tissue disorders: a review. Stroke. 1994;25:889-903.
17. Kiec-Wilk B, Surdacki A, Dembinska-Kiec A. Acute myocardial infarction and a new ABCC6 mutation in a 16-year-old boy with pseudoxanthoma elasticum. Int J Cardiol. 2007;116:261-262.
18. Lever WF, Elder DE, Elenitsas R. Congenital diseases (Genodermatoses). In: Lever WF, Schaumburg-Lever G, eds. Histopathology of the Skin. 8th ed. Philadelphia, Pa: Lippincott-Raven; 1997:117-151.
19. Fah L. Pseudoxanthoma elasticum—a visual diagnosis. Schweiz Med Wochenschr. 1991;121:660-663.
20. Lebwohl M, Phelps RG, Yannuzzi L, et al. Diagnosis of pseudoxanthoma elasticum by scar biopsy in patients without characteristic skin lesions. N Engl J Med. 1987;317:347-350.
21. Hausser I. Early preclinical diagnosis of dominant pseudoxanthoma elasticum by specific ultrastructural changes of dermal elastic and collagen tissue in a family at risk. Hum Genet. 1991;87:693-700.
22. Limas C. Late onset focal dermal elastosis: a distinct clinicopathologic entity? Am J Dermatopathol. 1999;21:381-383.
23. Baccarani-Contri M, Bacchelli B, Boraldi F, et al. Characterization of pseudoxanthoma elasticum-like lesions in the skin of patients with beta-thalassemia. J Am Acad Dermatol. 2001;44:33-39.
24. Rook A, Wilkinson D, Ebling F, et al. PXE. In: Rook A, ed. Textbook of Dermatology. 4th ed. Oxford, England: Blackwell Scientific; 1986:1841-1844.
25. Christensen OB. An exogenous variety of pseudoxanthoma elasticum in old farmers. Acta Derm Venereol. 1978;58:319-321.
26. Rongioletti F, Rebora A. Fibroelastolytic patterns of intrinsic skin aging: pseudoxanthoma-elasticum-like papillary dermal elastolysis and white fibrous papulosis of the neck. Dermatology. 1995;191:19-24.
27. Neldner KH. Pseudoxanthoma elasticum. Int J Dermatol. 2007;27:98-100.
28. Ng AB, O’Sullivan ST, Sharpe DT. Plastic surgery and pseudoxanthoma elasticum. Br J Plast Surg. 1999;52:594-596.
29. Galadari H, Lebwohl M. Pseudoxanthoma elasticum: Temporary treatment of chin folds and lines with injectable collagen. J Am Acad Dermatol. 2003;49(suppl 5):S265-S266.
30. Uitto J, Ringpfeil F, Pulkkinen L. Pseudoxanthoma elasticum. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 1st ed. London, England: Mosby; 2003:1524-1526.
1. Hu X, Plomp A, van Soest S, et al. Pseudoxanthoma elasticum: A clinical, histopathological, and molecular update. Survey Ophthalmol. 2003;48:424-438.
2. Abdelmalek NF, Gerber TL, Menter A. Cardiocutaneous syndromes and associations. J Am Acad Dermatol. 2002;46:161-183.
3. Jacobi H, Schreiber G. Pseudoxanthoma elasticum. Skin changes as a marker of systemic illness. Der Hautarzt. 1997;48:191-194.
4. Sherer DW, Sapadin AN, Lebwohl MG. Pseudoxanthoma elasticum: an update. Dermatology. 1999;199:3-7.
5. De Paepe A, Viljoen D, Matton M, et al. Pseudoxanthoma elasticum: similar autosomal recessive subtype in Belgian and Afrikaner families. Am J Med Genet. 1991;38:16-20.
6. Kevorkian JP, Masquet C, Kural-Menasche S, et al. New report of severe coronary artery disease in an eighteen-year-old girl with pseudoxanthoma elasticum. Case report and review of the literature. Angiology. 1997;48:735-741.
7. Lebwohl M, Halperin J, Phelps RG. Brief report: occult pseudoxanthoma elasticum in patients with premature cardiovascular disease. N Engl J Med. 1993;329:1237-1239.
8. Sherer DW, Bercovitch L, Lebwohl M. Pseudoxanthoma elasticum: significance of limited phenotypic expression in parents of affected offspring. J Am Acad Dermatol. 2001;44:534-537.
9. Uitto J, Boyd CD, Lebwohl MG, et al. International Centennial Meeting on Pseudoxanthoma Elasticum: progress in PXE research. J Invest Dermatol. 1998;110:840-842.
10. Struk B, Neldner KH, Rao VS, et al. Mapping of both autosomal recessive and dominant variants of pseudoxanthoma elasticum to chromosome 16p13.1. Hum Mol Genet. 1997;6:1823-1828.
11. Chassaing N, Martin L, Calvas P. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet. 2005;42:881-892.
12. Schneider T, Appel H-P, Kuhlwein A, et al. Groenblad-Strandberg-Syndrome. Z Hautkr. 1984;59:1290-1300.
13. Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
14. Lebwohl M, Lebwohl E, Bercovitch L. Prominent mental (chin) crease: a new sign of pseudoxanthoma elasticum. J Am Acad Dermatol. 2003;48:620-622.
15. Shilling JS, Blach RK. Prognosis and therapy of angioid streaks. Trans Ophthalmol Soc UK. 1975;95:301-306.
16. Schievink WI, Michels VV, Piepgras DG. Neurovascular manifestations of heritable connective tissue disorders: a review. Stroke. 1994;25:889-903.
17. Kiec-Wilk B, Surdacki A, Dembinska-Kiec A. Acute myocardial infarction and a new ABCC6 mutation in a 16-year-old boy with pseudoxanthoma elasticum. Int J Cardiol. 2007;116:261-262.
18. Lever WF, Elder DE, Elenitsas R. Congenital diseases (Genodermatoses). In: Lever WF, Schaumburg-Lever G, eds. Histopathology of the Skin. 8th ed. Philadelphia, Pa: Lippincott-Raven; 1997:117-151.
19. Fah L. Pseudoxanthoma elasticum—a visual diagnosis. Schweiz Med Wochenschr. 1991;121:660-663.
20. Lebwohl M, Phelps RG, Yannuzzi L, et al. Diagnosis of pseudoxanthoma elasticum by scar biopsy in patients without characteristic skin lesions. N Engl J Med. 1987;317:347-350.
21. Hausser I. Early preclinical diagnosis of dominant pseudoxanthoma elasticum by specific ultrastructural changes of dermal elastic and collagen tissue in a family at risk. Hum Genet. 1991;87:693-700.
22. Limas C. Late onset focal dermal elastosis: a distinct clinicopathologic entity? Am J Dermatopathol. 1999;21:381-383.
23. Baccarani-Contri M, Bacchelli B, Boraldi F, et al. Characterization of pseudoxanthoma elasticum-like lesions in the skin of patients with beta-thalassemia. J Am Acad Dermatol. 2001;44:33-39.
24. Rook A, Wilkinson D, Ebling F, et al. PXE. In: Rook A, ed. Textbook of Dermatology. 4th ed. Oxford, England: Blackwell Scientific; 1986:1841-1844.
25. Christensen OB. An exogenous variety of pseudoxanthoma elasticum in old farmers. Acta Derm Venereol. 1978;58:319-321.
26. Rongioletti F, Rebora A. Fibroelastolytic patterns of intrinsic skin aging: pseudoxanthoma-elasticum-like papillary dermal elastolysis and white fibrous papulosis of the neck. Dermatology. 1995;191:19-24.
27. Neldner KH. Pseudoxanthoma elasticum. Int J Dermatol. 2007;27:98-100.
28. Ng AB, O’Sullivan ST, Sharpe DT. Plastic surgery and pseudoxanthoma elasticum. Br J Plast Surg. 1999;52:594-596.
29. Galadari H, Lebwohl M. Pseudoxanthoma elasticum: Temporary treatment of chin folds and lines with injectable collagen. J Am Acad Dermatol. 2003;49(suppl 5):S265-S266.
30. Uitto J, Ringpfeil F, Pulkkinen L. Pseudoxanthoma elasticum. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 1st ed. London, England: Mosby; 2003:1524-1526.
The latest contraceptive options: What you must know
- Consider an oral contraceptive for women who would prefer less frequent menstrual periods (A).
- An intrauterine device may be appropriate for women with prior pelvic inflammatory disease, ectopic pregnancy, or an abnormal Papanicolaou (Pap) smear result, and for many adolescents (A).
- There are no medical contraindications to progestin-only emergency contraception (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 35-year-old woman with a family history of breast cancer (mother diagnosed with breast cancer at age 55) requests your help in choosing an appropriate method of contraception. She is a nonsmoker, has a body mass index of 25, and dislikes taking pills. Which options would you recommend to her? Are there any that you would rule out?
Helping your patient make the best choice requires that you be as up to date as possible. In this review, we discuss select new options in a clinically relevant manner. Specifically, we explore the newest oral contraceptives (OCs), including extended-cycle, continuous, and shortened hormone-free interval formulations. In addition, we review the latest data and updated recommendations for the contraceptive patch and ring, intrauterine devices (IUDs), implants, and emergency contraception (TABLE). We conclude by describing appropriate choices for the patient described above. (See “So what do you recommend?” on page 803.)
Oral contraceptives
Since OCs became available in the 1960s, the standard regimen has been 21 active pills followed by 7 placebo pills, simulating the average unassisted monthly menstrual cycle in which “menstrual” or withdrawal bleeding occurs. Clinicians have successfully lengthened intermenstrual intervals with OCs, without incurring additional risk, to control symptoms of endometriosis, premenstrual syndrome, and menstrual-withdrawal headaches, or to satisfy many patients’ preference for fewer menses per year.1,2
Any monophasic active OC can be used without a placebo interval to delay menses for extended periods. Until recently, such usage was off-label. Based on extensive safety and efficacy studies, however, the US Food and Drug Administration (FDA) has now approved several formulations for extended-cycle and continuous-cycle use.
Extended-cycle OCs: Fewer menses per year
Two FDA-approved extended-cycle OCs are available: Seasonale and Seasonique.3,4 Both products enable 4 scheduled menstrual intervals per year, as opposed to about 13 with 28-day cycles. Each regimen uses 84 consecutive pills of levonorgestrel 0.15 mg and ethinyl estradiol (EE) 0.03 mg, followed by 7 placebo pills (Seasonale) or 7 pills of EE 0.01 mg (Seasonique).
Other potential advantages. With Seasonique, the average length of menses is 3 days, which is shorter than the average unassisted menstrual period. Seasonique’s 7 additional low-dose estrogen pills may help decrease estrogen withdrawal symptoms, such as headaches in women with menstrual migraine and vasomotor instability in perimenopausal women. Though this effect has also been reported with other OCs containing low-dose estrogen during the traditional placebo week, specific supportive evidence is not yet available for these formulations.5,6
Disadvantages to address. With Seasonale and Seasonique, unscheduled spotting or bleeding has been reported—especially during initial use—at rates considerably higher than those associated with comparable traditional OCs.3,4 Effective counseling will help ensure patient compliance and satisfaction.
During the first cycle (days 1-91), about 65% of women taking either formulation reported ≥7 days of spotting, and 29% to 35% reported ≥20 days of spotting. By the 4th cycle (days 273-364), 39% to 42% of patients reported ≥7 days of spotting and 11% to 15% reported ≥20 days of spotting. For patients taking comparable progestin and EE doses in traditional monthly regimens, 38% reported ≥7 days of spotting and 6% reported ≥20 days of spotting during the first cycle. Thirty-nine percent and 4%, respectively, reported spotting during the fourth cycle.3,4
Continuous OC: Consistent hormonal milieu
Lybrel, the only FDA-approved OC for continuous use, contains levonorgestrel 90 mcg and EE 20 mcg; pills are taken daily throughout the year.7 Progestin and estrogen doses are lower than those found in many monthly OCs and in all extended-cycle formulations. A phase 3 trial of 2134 women reported the safety and efficacy of Lybrel to be comparable to cyclic OCs.8 Again, unscheduled bleeding and spotting rates were relatively high but decreased at pack 3 from 47% and 26%, respectively, to 21% and 20%, respectively, at pack 13. Predictably, amenorrhea rates increased from 27% to 59% between pack 3 and pack 13.
Shortened hormone-free interval OCs: Less breakthrough ovulation
The shortened hormone-free interval OC is an alternative for patients who want regular, but shorter, menstrual intervals. With 24 active and 4 placebo pills in each cycle, this regimen suppresses the pituitary/ovarian axis to a greater extent than traditional 21/7-day regimens and thus lowers the rate of breakthrough ovulation.9
Loestrin 24 Fe contains norethindrone 1 mg and EE 20 mcg; placebo pills contain 75 mg of ferrous fumarate.
Yaz contains the newer progestin drospirenone 3 mg and EE 20 mcg. Drospirenone, an analog of the antihypertensive spironolactone, was introduced in a 21/7 formulation, Yasmin, and proved to have beneficial effects on mood, water retention, and acne.10 Yaz, which contains a lower dose of EE than Yasmin, provides 3 additional days of antimineralocorticoid and antiandrogenic activity, and is indicated for the treatment of premenstrual dysphoric disorder, a more severe form of premenstrual syndrome. Drospirenone-containing OCs are contraindicated for patients with renal, adrenal, or hepatic impairment because of the progestin’s metabolism via these routes.
An established OC with a twist: Chewable pills
For patients unable to swallow OCs, a chewable formulation, Femcon Fe, is available.11 It is hormonally identical to Ovcon 35, a well-established OC containing norethindrone acetate 0.4 mg and EE 35 mcg. The 7 placebo pills contain ferrous fumarate 75 mg. The spearmint-flavored chewable pill (which can also be swallowed) must be taken with 8 ounces of water.
Noncontraceptive benefits of OCs—there are many
An extensive body of evidence supports the noncontraceptive health benefits of OCs. These include a decreased risk of:
- endometrial and ovarian cancer
- bone loss
- benign breast disease
- pelvic inflammatory disease
- ectopic pregnancy
- rheumatoid arthritis.
Women with symptoms of androgen excess, premenstrual mood disorders, or endometriosis pain have long benefited from treatment with OCs.10,12-14 Healthy perimenopausal women are excellent candidates for OCs to regulate menses and treat symptoms of estrogen deficiency. OCs with added estrogen during the menstrual interval or shortened hormone-free interval may be more effective in moderating the perimenopausal transition. However, specific evidence about these effects is not yet available for the newest OC formulations.
Risks of OCs have been reduced, but some remain
Many of the well-known risks and side effects of OCs have been minimized over the years as total doses of estrogen have decreased and less androgenic progestins have been incorporated into OC formulations. Nonetheless, OCs are contraindicated for women who have a history of venous thromboembolism (VTE) or coronary artery disease (or are at risk for these complications), are over the age of 35 and smoke, are pregnant or newly postpartum, or are immobilized after surgery.
OCs remain relatively contraindicated for women with a history of migraines and focal auras, due to the increased risk for ischemic stroke.15 Breast and other estrogen-dependent cancers as well as liver disease preclude the use of OCs.16 Additional studies using the newer formulations of OCs are needed to definitively determine their long-term risk compared with traditional monthly formulations.
Contraceptive patch: Improving compliance
The contraceptive patch Evra is applied weekly and releases norelgestromin 150 mcg and EE 20 mcg each day, providing an OC alternative that is less dependent on compliance. However, in November 2005, the FDA modified product labeling to inform providers and the public that, based on pharmacokinetic studies, patients using the patch were exposed to hormone levels about 60% higher than with OCs of similar dosage.17 Another FDA labeling change made in 2008 states that “it is not known whether there are changes in the risk of serious adverse events based on the differences in pharmacokinetic profiles of EE in women using [the patch] as compared with women using oral contraceptives containing 35 mcg of EE.”
What is the real risk of VTE? One case-control study reported that the rates of VTE events in patch users and OC users were 52 per 100,000 woman-years and 42 per 100,000 woman-years, respectively.18 Another large case-control study showed the odds ratio (OR) of VTE to be 2.4 (95% confidence interval [CI], 1.1-5.5) with the patch compared with OCs; data were corrected for high-risk factors.19 However, the absolute risks for the patch and OCs were 40 and 18 per 100,000 woman-years, respectively—both lower than the risk of VTE associated with pregnancy.
The risk of myocardial infarction. The OR for myocardial infarction among patch users in the same population was 1.8 (95% CI, 0.5-6.8), and there was no statistically significant increase in the rate of cerebrovascular accidents.19 Thus, it is reasonable to use the patch with caution in patients without cardiac risk factors and to limit total hormone dosage by not using the patch in an extended-cycle manner. Of note, the patch is reported to have decreased efficacy in patients weighing over 90 kg (198 lb).20
Vaginal ring: Fewer drug interactions
NuvaRing, the ethylene vinyl vaginal ring, releases etonorgestrel (ENG) 120 mcg and EE 15 mcg each day (a lower estrogen dose than is contained in OCs or the patch).21 The device is 5.4 cm in diameter and 4 mm thick. Patients insert the ring intravaginally, remove it 3 weeks later for menses, and insert a new ring 1 week later.
Continuous use regimen. A randomized controlled trial evaluating the frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring reported a reduction in bleeding, flow, and pelvic pain, and a high continuation rate. Most patients considered the bleeding profile with the continuous vaginal ring acceptable compared with the baseline 21/7 use.22 Each ring contains up to a 28-day supply of hormones.
LNG-IUS (Mirena)
Fewer interactions. Transvaginal absorption of hormones with the vaginal ring avoids a first pass through the liver, thus decreasing many medication interactions. Irregular bleeding experienced with OCs or the patch may be effectively reduced with the steadily released, rapidly acting hormones in the ring.23
Intrauterine contraception
Intrauterine contraception is increasingly accepted by women who want long-term and effective contraception without having to comply with a particular regimen.
Copper IUD: Many contraindications are lifted
The nonhormonal IUD ParaGard T 380A is indicated for contraception for up to 10 years in women who are 16 years of age and older.24 This IUD’s active ingredient is the spermicidal copper wire wound around the short arms of the device. A recent meta-analysis reported an association between the use of a copper IUD and a decrease in the risk of endometrial cancer (OR=0.39; 95% CI, 0.29-0.51), though the mechanism for this association is unclear.25
In late 2005, the FDA broadened the use of copper IUDs to include women who are nulliparous; have a history of pelvic inflammatory disease (PID), sexually transmitted disease, or ectopic pregnancy; are in nonmonogamous relationships; or have a history of premenopausal breast cancer. This method also may be used by women with asymptomatic human immunodeficiency virus infection, Actinomyces infection, abnormal Papanicolaou (Pap) test results, or vaginitis. Furthermore, data support its use in adolescents who are at particularly high risk for unintended pregnancy.26
The copper IUD remains contra-indicated for patients with acute PID or current high-risk behavior for sexually transmitted infections, as well as for those who have mucopurulent cervicitis or have had postpartum endometritis within the past 3 months.24 Wilson’s disease is also a contraindication.
Insertion tip. Misoprostol may be used to soften the nulliparous cervix for insertion.27
Levonorgestrel intrauterine system: An alternative to the copper IUD
The levonorgestrel intrauterine system (LNG IUS), sold under the brand name Mirena, is gaining tremendous popularity in the United States. Multiple mechanisms of action, including endometrial thinning, cervical mucus thickening, inhibition of sperm function, and intermittent ovulation suppression are responsible for the >99% efficacy of this 5-year contraceptive.
Irregular menses can be expected initially, and 20% of patients reported amenorrhea at 1 year of use. In the unlikely event that a woman becomes pregnant while using Mirena, evaluate for ectopic pregnancy, which occurs in about half of pregnancies in women using this system.
Noncontraceptive benefits. The system’s primary noncontraceptive benefit is the dramatic reduction of menstrual blood loss, reported to be up to 90%.28 This contraceptive has been used as a cost-effective alternative to hysterectomy and endometrial ablation.29,30 Mirena imparts a protective effect against PID, likely secondary to progestin-mediated cervical mucus thickening.31
Subdermal implant (Implanon)
It appears safe and expeditious to provide both counseling and intrauterine contraception insertion in one visit, provided pregnancy is excluded.32 Confirm normalcy of cervical cytology and screen for sexually transmitted disease, if indicated. Prophylactic antibiotics are unnecessary, as the risk of PID within 20 days of insertion is only 9.7 per 1000 woman- years.33 After 20 days, the risk declines to 1.4 per 1000 woman-years, the same as that of the general population.34
Subdermal implant: Easily reversible
In July 2006, the FDA approved Implanon, a subdermal contraceptive implant.35 It has been available worldwide since 1998. The 40 × 2 mm single-rod implant containing etonogestrel (ENG) 68 mg diffuses the hormone at a rate of 60 mcg/d immediately after insertion and then steadily at 30 mcg/d for up to 3 years.
Its primary mechanism of action is ovulation suppression, with no ovulation detected for 30 months in a study group of more than 17,000 women.35 Increased cervical mucus viscosity also contributes to its effectiveness.35 In a large clinical trial, no pregnancies were reported in more than 6100 cycles.36 However, this trial excluded women weighing more than 130% of their ideal body weight, so no data are available to support the effectiveness of Implanon in obese women.36
Benefits and risks. This implant does not cause a hypoestrogenic state and ovulation suppression is rapidly reversible, with ENG levels undetectable within 10 days of implant removal.35 Furthermore, this method has no reported deleterious effects on bone mineral density or lactation.37,38 When counseling women about the implant, emphasize its propensity to result in “irregular and unpredictable” bleeding. An average of 7 bleeding and 10 spotting days within a 90-day period has been reported. Most women had fewer bleeding/spotting days than they would without contraception, but unscheduled bleeding was the leading reason for method discontinuation (11%), followed by weight gain, emotional lability, acne, headache, and depression (each ≈1%-2%).35
Implant insertion. The device is inserted in the sulcus between the biceps and triceps muscles of the nondominant arm. It is crucial to place the implant subdermally, tenting the skin during insertion to prevent deep insertion. High-frequency ultrasound can be used to detect nonpalpable implants. The FDA has mandated 3 hours of training for clinicians before they can obtain the device.
Depot medroxyprogesterone: Tried and true alternative
The depot medroxyprogesterone acetate (DMPA) injection has been a mainstay of contraception for decades. Available under the brand name Depo-Provera, it’s an option for women in whom estrogen-containing contraceptives are contraindicated. Its convenience, reduced risk of anemia, and postpartum benefits are all well known, and we have thus limited our discussion of DMPA to the summary in the TABLE.
TABLE
How do these contraceptives compare?
| METHOD | ROUTE OF ADMINISTRATION | FREQUENCY OF ADMINISTRATION | FAILURES/YR WITH TYPICAL USE 33,35,51 | EXPECTED MENSTRUAL PATTERN | ADVERSE EFFECTS/CONTRAINDICATIONS |
|---|---|---|---|---|---|
| Cyclic OCs | Oral | Daily | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Extended-cycle OCs (Seasonale, Seasonique) | Oral | Daily | 8% | Menses 4/yr, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Continuous OCs (Lybrel) | Oral | Daily | 8% | No scheduled menses, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Shortened hormone-free interval OCs (Loestrin 24 Fe, Yaz) | Oral | Daily | 8% | Shorter monthly menses | Hormonal adverse effects, unscheduled bleeding |
| Transdermal patch (Evra) | Patch applied to skin | New patch applied weekly for 3 wk; off for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects, increased risk of VTE higher than OCs but lower than pregnancy; MI risk higher than comparable OCs, but use is reasonable if no cardiac risk factors |
| Vaginal ring (NuvaRing) | Ring inserted in vagina by patient | Ring inserted for 3 wk, removed for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Copper IUD (ParaGard T 380A) | IUD inserted & removed by clinician | Every 10 yr | 0. 8% | Heavier menses, may have BTB | Menorrhagia. Contraindications: Acute PID or high risk for STI; postpartum endometritis within 3 mo; mucopurulent cervicitis; Wilson’s disease |
| Levonorgestrel IUS (LNG IUS, Mirena) | IUS inserted & removed by clinician | Every 5 yr | < 0.1% | Lighter, shorter menses or amenorrhea | Minimal hormonal adverse effects. Contraindications: Acute PID, history of or high risk for PID; postpartum endometritis within 3 mo; mucopurulent cervicitis |
| Subdermal implant (Implanon) | Inserted subdermally & removed by clinician | Every 3 yr | 0.3% | Irregular, unpredictable bleeding | Unscheduled bleeding, mood symptoms, headache, weight gain, acne |
| Depot medroxyprogesterone acetate (Depo-Provera) | IM injection | Every 3 mo | 3% | Irregular bleeding, amenorrhea | Unscheduled bleeding, reversible bone loss |
| BTB, breakthrough bleeding; IM, intramuscular; IUD, intrauterine device; IUS, intrauterine system; MI, myocardial infarction; OC, oral contraceptive; PID, pelvic inflammatory disease; STI, sexually transmitted infection; VTE, venous thromboembolism. | |||||
Emergency contraception: 2 pills, 12 hours apart
Plan B contains 2 tablets of levonorgestrel 0.75 mg, to be taken 12 hours apart as soon as possible after unprotected intercourse. However, taking both doses together is as effective as taking them separately, and doing so may improve compliance.39
How it works. Emergency contraception (EC) works by inhibiting or delaying the surge of luteinizing hormone and follicular rupture before ovulation. It does not affect implantation or corpus luteum function, and it poses no risk to an established pregnancy or embryo. EC is ineffective when administered after ovulation.40
In a World Health Organization (WHO) multicenter randomized trial, EC prevented 79% to 84% of pregnancies if taken 1 to 3 days after intercourse, and 60% to 63% of pregnancies if taken 4 to 5 days after intercourse.41 Plan B is available without a prescription for women ages 18 and older. It is important to screen for pregnancy before prescribing Plan B for younger patients.
Adverse effects include nausea and vomiting, occurring in 23% and 5% of patients, respectively. Intermenstrual bleeding occurs in 8% of patients taking progestin-only EC. Menses are expected within 21 days of EC administration, and the second cycle after EC should be of normal length.42 The authors of a WHO report concluded that “there are no medical conditions wherein risks outweigh benefits of EC.”43
Combination estrogen/progestin for EC is no more effective than progestin-only EC and results in higher rates of adverse effects, especially nausea and vomiting.44
IUD can also be used in an emergency
Insertion of a copper IUD provides EC as well as ongoing contraception. It is hormone free and can be used effectively for EC up to 5 days after sexual intercourse, then continued for primary contraception for up to 10 years.45 Its estimated failure rate was less than 0.1% in more than 8400 postcoital insertions.46 The IUD works by impairing fertilization and implantation and by altering sperm motility and integrity. With a copper IUD, additional primary contraception is unnecessary. The LNG IUS (Mirena) has not been studied as an alternative EC.
Some worry that EC’s availability will encourage unprotected sex
Some authorities have wondered if increased access to EC might paradoxically lead to more pregnancies by encouraging unprotected sex. Researchers are exploring this issue. One study reported that unfettered access to free EC resulted in an increase in EC use, and another study reported that patients with unrestricted EC access had inadequately protected sex more often than those in the control group.47,48
A systematic review of 23 articles studying the effect of increased access to EC confirmed an increase in EC use,49 but no statistically significant differences in pregnancy or abortion rates.
For patients like the 35-year-old woman discussed earlier, who do not like taking pills, there are many contraception options to choose from, including the patch, vaginal ring, chewable OC, IUD, depot medroxyprogesterone injection, and subdermal implant.
Hormonal contraception would not be an issue for this patient—even though she has a family history of breast cancer. Using hormonal contraception does not increase the risk of breast cancer for individuals with a family history of breast cancer in a first-or second-degree relative.50
Correspondence
Petra M. Casey, MD, Department of Obstetrics and Gynecology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected].
1. Andrist LC, Arias RD, Nucatola D, et al. Women’s and providers’ attitudes toward menstrual suppression with extended use of oral contraceptives. Contraception. 2004;70:359-363.
2. Glasier AF, Smith KB, van der Spuy ZM, et al. Amenorrhea associated with contraception—an international study on acceptability. Contraception. 2003;67:1-8.
3. Seasonale [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2008.
4. Seasonique [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
5. Shortened pill-free interval delivered by new 20 mcg pill, Organon’s Mircette, schedule for US debut this summer Contraception Technol Update. 1998;19:85-87.
6. Casper RF, Dodin S, Reid RL. For the Study Investigators. The effect of 20 mcg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
7. Lybrel [package insert] Philadelphia, Pa: Wyeth Pharmaceuticals, Inc; 2007.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Willis SA, Kuehl TJ, Spiekerman AM, et al. Greater inhibition of the pituitary: ovarian axis in oral contraceptive regimens with a shortened hormone-free interval. Contraception. 2006;74:100-103.
10. Parsey KS, Pong A. An open-label, multicenter study to evaluate Yasmin, a low-dose combination oral contraceptive containing drospirenone, a new progestogen. Contraception. 2000;61:105-111.
11. Femcon Fe [package insert]. Rockaway, NJ: Warner Chilcott (US), Inc; 2007.
12. Burkman R. The evolution of oral contraceptives: 40 years of continuous improvement. Clinician. 2002;20:3-30.
13. Vercellini P, Frontino G, De Giorgi O, et al. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril. 2003;80:560-563.
14. Guido M, Romualdi D, Giuliani M. Drospirenone for the treatment of hirsute women with PCOS; a clinical endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004;89:2817-2823.
15. Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. Br Med J. 1995;310:830-833.
16. Steinauer J, Autry AM. Extended cycle combined hormonal contraception. Obstet Gynecol Clin North Am. 2007;34:43-55, viii.
17. Ortho Evra [prescribing information]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001.
18. Jick SS, Kaye JA, Russmann S, et al. Risk of non-fatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg/l of ethinyl estradiol. Contraception. 2006;73:223-228.
19. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
20. American College of Obstetricians and Gynecologists. ACOG practice bulletin. Use of hormonal contraceptives in women with coexisting medical conditions. No. 73, June 2006. Obstet Gynecol. 2006;107:1453-1472.
21. Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril. 2001;75:865-870.
22. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol. 2008;112:563-571.
23. Bjarnadottir RI, Tuppurainen M, Killick SR. Comparison of cycle control with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl estradiol. Am J Obstet Gynecol. 2002;186:389-395.
24. ParaGard T 380A [prescribing information]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
25. Beining RM, Dennis LK, Smith EM, et al. Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann Epidem. 2008;18:492-499.
26. ACOG Committee Opinion. Intrauterine Device and Adolescents No. 392. Obstet Gynecol. 2007;110:1493-1495.
27. McNaught J. Adolescents and IUCDs—Not a contraindication. J Ped Adolesc Gyn. 2006;19:303-305.
28. Lethaby AE, Cooke I, Rees M. Progesterone/progestogen releasing intrauterine systems versus either placebo or any other medication for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.-
29. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273-277.
30. Römer T. Prospective comparison study of levonorgestrel IUD versus Roller-Ball endometrial ablation in the management of refractory recurrent hypermenorrhea. Eur J Obstet Gynecol Reprod Biol. 2000;90:27-29.
31. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’ comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77:261-264.
32. Ball CE. News and controversies in contraception. AudioDigest Obstet Gynecol Clin Updates. 2007;54:18.-
33. Grimes DA. Intrauterine device and upper genital tract infection. Lancet. 2000;356:1013-1019.
34. Farley TM, Rosenberg MJ, Rowe PJ, et al. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785-788.
35. Implanon [package insert]. Roseland, NJ: Organon USA Inc; 2007.
36. Funk S, Miller MM, Mishell DR, Jr, et al. The Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
37. Beerthuizen R, van Beek A, Massai R, et al. Bone mineral density during long-term use of the progestagen contraceptive implant Implanon compared to a non-hormonal method of contraception. Hum Reprod. 2000;15:118-122.
38. Reinprayoon D, Taneepanichskul S, Bunyavejchevin S, et al. Effects of the etonogestrel-releasing contraceptive implant (Implanon) on parameters of breastfeeding compared to those of an intrauterine device. Contraception. 2000;62:239-246.
39. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
40. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 69: emergency contraception. Obstet Gynecol. 2005;106:1443-1452.
41. Von Hertzen H, Piaggio G, Ding J. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. WHO Research Group on Post-Ovulatory Methods of Fertility Regulation. Lancet. 2002;360:1803-1810.
42. Raymond EG, Goldberg A, Trussell J, et al. Bleeding patterns after use of levonorgestrel emergency contraceptive pills. Contraception. 2006;73:376-381.
43. World Health Organization. Medical eligibility criteria for contraceptive use. 3rd ed. 2004. Available at: www.who.int/reproductive-health/publication/med/mec.pdf. Accessed February 7, 2008.
44. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
45. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 59. Intrauterine device. Obstet Gynecol. 2005;105:223-232.
46. LaValleur J. Emergency contraception. Obstet Gynecol Clin North Am. 2000;27:817-839, vii.
47. Raymond EG, Stewart F, Weaver M, et al. Impact of increased access to emergency contraceptive pills: a randomized controlled trial. Obstet Gynecol. 2006;108:1098-1106.
48. Raymond EG, Weaver MA. Effect of an emergency contraceptive pill intervention on pregnancy risk behavior. Contraception. 2008;77:333-336.
49. Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol. 2007;109:181-188.
50. Casey PM, Cerhan JR, Pruthi S. Oral contraceptive use and risk of breast cancer Mayo Clinic Proc. 2008;83:86-89.
51. Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart FH, et al, eds. Contraceptive Technology, 18th ed. New York, NY: Ardent Media, Inc; 2004:773-845.
- Consider an oral contraceptive for women who would prefer less frequent menstrual periods (A).
- An intrauterine device may be appropriate for women with prior pelvic inflammatory disease, ectopic pregnancy, or an abnormal Papanicolaou (Pap) smear result, and for many adolescents (A).
- There are no medical contraindications to progestin-only emergency contraception (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 35-year-old woman with a family history of breast cancer (mother diagnosed with breast cancer at age 55) requests your help in choosing an appropriate method of contraception. She is a nonsmoker, has a body mass index of 25, and dislikes taking pills. Which options would you recommend to her? Are there any that you would rule out?
Helping your patient make the best choice requires that you be as up to date as possible. In this review, we discuss select new options in a clinically relevant manner. Specifically, we explore the newest oral contraceptives (OCs), including extended-cycle, continuous, and shortened hormone-free interval formulations. In addition, we review the latest data and updated recommendations for the contraceptive patch and ring, intrauterine devices (IUDs), implants, and emergency contraception (TABLE). We conclude by describing appropriate choices for the patient described above. (See “So what do you recommend?” on page 803.)
Oral contraceptives
Since OCs became available in the 1960s, the standard regimen has been 21 active pills followed by 7 placebo pills, simulating the average unassisted monthly menstrual cycle in which “menstrual” or withdrawal bleeding occurs. Clinicians have successfully lengthened intermenstrual intervals with OCs, without incurring additional risk, to control symptoms of endometriosis, premenstrual syndrome, and menstrual-withdrawal headaches, or to satisfy many patients’ preference for fewer menses per year.1,2
Any monophasic active OC can be used without a placebo interval to delay menses for extended periods. Until recently, such usage was off-label. Based on extensive safety and efficacy studies, however, the US Food and Drug Administration (FDA) has now approved several formulations for extended-cycle and continuous-cycle use.
Extended-cycle OCs: Fewer menses per year
Two FDA-approved extended-cycle OCs are available: Seasonale and Seasonique.3,4 Both products enable 4 scheduled menstrual intervals per year, as opposed to about 13 with 28-day cycles. Each regimen uses 84 consecutive pills of levonorgestrel 0.15 mg and ethinyl estradiol (EE) 0.03 mg, followed by 7 placebo pills (Seasonale) or 7 pills of EE 0.01 mg (Seasonique).
Other potential advantages. With Seasonique, the average length of menses is 3 days, which is shorter than the average unassisted menstrual period. Seasonique’s 7 additional low-dose estrogen pills may help decrease estrogen withdrawal symptoms, such as headaches in women with menstrual migraine and vasomotor instability in perimenopausal women. Though this effect has also been reported with other OCs containing low-dose estrogen during the traditional placebo week, specific supportive evidence is not yet available for these formulations.5,6
Disadvantages to address. With Seasonale and Seasonique, unscheduled spotting or bleeding has been reported—especially during initial use—at rates considerably higher than those associated with comparable traditional OCs.3,4 Effective counseling will help ensure patient compliance and satisfaction.
During the first cycle (days 1-91), about 65% of women taking either formulation reported ≥7 days of spotting, and 29% to 35% reported ≥20 days of spotting. By the 4th cycle (days 273-364), 39% to 42% of patients reported ≥7 days of spotting and 11% to 15% reported ≥20 days of spotting. For patients taking comparable progestin and EE doses in traditional monthly regimens, 38% reported ≥7 days of spotting and 6% reported ≥20 days of spotting during the first cycle. Thirty-nine percent and 4%, respectively, reported spotting during the fourth cycle.3,4
Continuous OC: Consistent hormonal milieu
Lybrel, the only FDA-approved OC for continuous use, contains levonorgestrel 90 mcg and EE 20 mcg; pills are taken daily throughout the year.7 Progestin and estrogen doses are lower than those found in many monthly OCs and in all extended-cycle formulations. A phase 3 trial of 2134 women reported the safety and efficacy of Lybrel to be comparable to cyclic OCs.8 Again, unscheduled bleeding and spotting rates were relatively high but decreased at pack 3 from 47% and 26%, respectively, to 21% and 20%, respectively, at pack 13. Predictably, amenorrhea rates increased from 27% to 59% between pack 3 and pack 13.
Shortened hormone-free interval OCs: Less breakthrough ovulation
The shortened hormone-free interval OC is an alternative for patients who want regular, but shorter, menstrual intervals. With 24 active and 4 placebo pills in each cycle, this regimen suppresses the pituitary/ovarian axis to a greater extent than traditional 21/7-day regimens and thus lowers the rate of breakthrough ovulation.9
Loestrin 24 Fe contains norethindrone 1 mg and EE 20 mcg; placebo pills contain 75 mg of ferrous fumarate.
Yaz contains the newer progestin drospirenone 3 mg and EE 20 mcg. Drospirenone, an analog of the antihypertensive spironolactone, was introduced in a 21/7 formulation, Yasmin, and proved to have beneficial effects on mood, water retention, and acne.10 Yaz, which contains a lower dose of EE than Yasmin, provides 3 additional days of antimineralocorticoid and antiandrogenic activity, and is indicated for the treatment of premenstrual dysphoric disorder, a more severe form of premenstrual syndrome. Drospirenone-containing OCs are contraindicated for patients with renal, adrenal, or hepatic impairment because of the progestin’s metabolism via these routes.
An established OC with a twist: Chewable pills
For patients unable to swallow OCs, a chewable formulation, Femcon Fe, is available.11 It is hormonally identical to Ovcon 35, a well-established OC containing norethindrone acetate 0.4 mg and EE 35 mcg. The 7 placebo pills contain ferrous fumarate 75 mg. The spearmint-flavored chewable pill (which can also be swallowed) must be taken with 8 ounces of water.
Noncontraceptive benefits of OCs—there are many
An extensive body of evidence supports the noncontraceptive health benefits of OCs. These include a decreased risk of:
- endometrial and ovarian cancer
- bone loss
- benign breast disease
- pelvic inflammatory disease
- ectopic pregnancy
- rheumatoid arthritis.
Women with symptoms of androgen excess, premenstrual mood disorders, or endometriosis pain have long benefited from treatment with OCs.10,12-14 Healthy perimenopausal women are excellent candidates for OCs to regulate menses and treat symptoms of estrogen deficiency. OCs with added estrogen during the menstrual interval or shortened hormone-free interval may be more effective in moderating the perimenopausal transition. However, specific evidence about these effects is not yet available for the newest OC formulations.
Risks of OCs have been reduced, but some remain
Many of the well-known risks and side effects of OCs have been minimized over the years as total doses of estrogen have decreased and less androgenic progestins have been incorporated into OC formulations. Nonetheless, OCs are contraindicated for women who have a history of venous thromboembolism (VTE) or coronary artery disease (or are at risk for these complications), are over the age of 35 and smoke, are pregnant or newly postpartum, or are immobilized after surgery.
OCs remain relatively contraindicated for women with a history of migraines and focal auras, due to the increased risk for ischemic stroke.15 Breast and other estrogen-dependent cancers as well as liver disease preclude the use of OCs.16 Additional studies using the newer formulations of OCs are needed to definitively determine their long-term risk compared with traditional monthly formulations.
Contraceptive patch: Improving compliance
The contraceptive patch Evra is applied weekly and releases norelgestromin 150 mcg and EE 20 mcg each day, providing an OC alternative that is less dependent on compliance. However, in November 2005, the FDA modified product labeling to inform providers and the public that, based on pharmacokinetic studies, patients using the patch were exposed to hormone levels about 60% higher than with OCs of similar dosage.17 Another FDA labeling change made in 2008 states that “it is not known whether there are changes in the risk of serious adverse events based on the differences in pharmacokinetic profiles of EE in women using [the patch] as compared with women using oral contraceptives containing 35 mcg of EE.”
What is the real risk of VTE? One case-control study reported that the rates of VTE events in patch users and OC users were 52 per 100,000 woman-years and 42 per 100,000 woman-years, respectively.18 Another large case-control study showed the odds ratio (OR) of VTE to be 2.4 (95% confidence interval [CI], 1.1-5.5) with the patch compared with OCs; data were corrected for high-risk factors.19 However, the absolute risks for the patch and OCs were 40 and 18 per 100,000 woman-years, respectively—both lower than the risk of VTE associated with pregnancy.
The risk of myocardial infarction. The OR for myocardial infarction among patch users in the same population was 1.8 (95% CI, 0.5-6.8), and there was no statistically significant increase in the rate of cerebrovascular accidents.19 Thus, it is reasonable to use the patch with caution in patients without cardiac risk factors and to limit total hormone dosage by not using the patch in an extended-cycle manner. Of note, the patch is reported to have decreased efficacy in patients weighing over 90 kg (198 lb).20
Vaginal ring: Fewer drug interactions
NuvaRing, the ethylene vinyl vaginal ring, releases etonorgestrel (ENG) 120 mcg and EE 15 mcg each day (a lower estrogen dose than is contained in OCs or the patch).21 The device is 5.4 cm in diameter and 4 mm thick. Patients insert the ring intravaginally, remove it 3 weeks later for menses, and insert a new ring 1 week later.
Continuous use regimen. A randomized controlled trial evaluating the frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring reported a reduction in bleeding, flow, and pelvic pain, and a high continuation rate. Most patients considered the bleeding profile with the continuous vaginal ring acceptable compared with the baseline 21/7 use.22 Each ring contains up to a 28-day supply of hormones.
LNG-IUS (Mirena)
Fewer interactions. Transvaginal absorption of hormones with the vaginal ring avoids a first pass through the liver, thus decreasing many medication interactions. Irregular bleeding experienced with OCs or the patch may be effectively reduced with the steadily released, rapidly acting hormones in the ring.23
Intrauterine contraception
Intrauterine contraception is increasingly accepted by women who want long-term and effective contraception without having to comply with a particular regimen.
Copper IUD: Many contraindications are lifted
The nonhormonal IUD ParaGard T 380A is indicated for contraception for up to 10 years in women who are 16 years of age and older.24 This IUD’s active ingredient is the spermicidal copper wire wound around the short arms of the device. A recent meta-analysis reported an association between the use of a copper IUD and a decrease in the risk of endometrial cancer (OR=0.39; 95% CI, 0.29-0.51), though the mechanism for this association is unclear.25
In late 2005, the FDA broadened the use of copper IUDs to include women who are nulliparous; have a history of pelvic inflammatory disease (PID), sexually transmitted disease, or ectopic pregnancy; are in nonmonogamous relationships; or have a history of premenopausal breast cancer. This method also may be used by women with asymptomatic human immunodeficiency virus infection, Actinomyces infection, abnormal Papanicolaou (Pap) test results, or vaginitis. Furthermore, data support its use in adolescents who are at particularly high risk for unintended pregnancy.26
The copper IUD remains contra-indicated for patients with acute PID or current high-risk behavior for sexually transmitted infections, as well as for those who have mucopurulent cervicitis or have had postpartum endometritis within the past 3 months.24 Wilson’s disease is also a contraindication.
Insertion tip. Misoprostol may be used to soften the nulliparous cervix for insertion.27
Levonorgestrel intrauterine system: An alternative to the copper IUD
The levonorgestrel intrauterine system (LNG IUS), sold under the brand name Mirena, is gaining tremendous popularity in the United States. Multiple mechanisms of action, including endometrial thinning, cervical mucus thickening, inhibition of sperm function, and intermittent ovulation suppression are responsible for the >99% efficacy of this 5-year contraceptive.
Irregular menses can be expected initially, and 20% of patients reported amenorrhea at 1 year of use. In the unlikely event that a woman becomes pregnant while using Mirena, evaluate for ectopic pregnancy, which occurs in about half of pregnancies in women using this system.
Noncontraceptive benefits. The system’s primary noncontraceptive benefit is the dramatic reduction of menstrual blood loss, reported to be up to 90%.28 This contraceptive has been used as a cost-effective alternative to hysterectomy and endometrial ablation.29,30 Mirena imparts a protective effect against PID, likely secondary to progestin-mediated cervical mucus thickening.31
Subdermal implant (Implanon)
It appears safe and expeditious to provide both counseling and intrauterine contraception insertion in one visit, provided pregnancy is excluded.32 Confirm normalcy of cervical cytology and screen for sexually transmitted disease, if indicated. Prophylactic antibiotics are unnecessary, as the risk of PID within 20 days of insertion is only 9.7 per 1000 woman- years.33 After 20 days, the risk declines to 1.4 per 1000 woman-years, the same as that of the general population.34
Subdermal implant: Easily reversible
In July 2006, the FDA approved Implanon, a subdermal contraceptive implant.35 It has been available worldwide since 1998. The 40 × 2 mm single-rod implant containing etonogestrel (ENG) 68 mg diffuses the hormone at a rate of 60 mcg/d immediately after insertion and then steadily at 30 mcg/d for up to 3 years.
Its primary mechanism of action is ovulation suppression, with no ovulation detected for 30 months in a study group of more than 17,000 women.35 Increased cervical mucus viscosity also contributes to its effectiveness.35 In a large clinical trial, no pregnancies were reported in more than 6100 cycles.36 However, this trial excluded women weighing more than 130% of their ideal body weight, so no data are available to support the effectiveness of Implanon in obese women.36
Benefits and risks. This implant does not cause a hypoestrogenic state and ovulation suppression is rapidly reversible, with ENG levels undetectable within 10 days of implant removal.35 Furthermore, this method has no reported deleterious effects on bone mineral density or lactation.37,38 When counseling women about the implant, emphasize its propensity to result in “irregular and unpredictable” bleeding. An average of 7 bleeding and 10 spotting days within a 90-day period has been reported. Most women had fewer bleeding/spotting days than they would without contraception, but unscheduled bleeding was the leading reason for method discontinuation (11%), followed by weight gain, emotional lability, acne, headache, and depression (each ≈1%-2%).35
Implant insertion. The device is inserted in the sulcus between the biceps and triceps muscles of the nondominant arm. It is crucial to place the implant subdermally, tenting the skin during insertion to prevent deep insertion. High-frequency ultrasound can be used to detect nonpalpable implants. The FDA has mandated 3 hours of training for clinicians before they can obtain the device.
Depot medroxyprogesterone: Tried and true alternative
The depot medroxyprogesterone acetate (DMPA) injection has been a mainstay of contraception for decades. Available under the brand name Depo-Provera, it’s an option for women in whom estrogen-containing contraceptives are contraindicated. Its convenience, reduced risk of anemia, and postpartum benefits are all well known, and we have thus limited our discussion of DMPA to the summary in the TABLE.
TABLE
How do these contraceptives compare?
| METHOD | ROUTE OF ADMINISTRATION | FREQUENCY OF ADMINISTRATION | FAILURES/YR WITH TYPICAL USE 33,35,51 | EXPECTED MENSTRUAL PATTERN | ADVERSE EFFECTS/CONTRAINDICATIONS |
|---|---|---|---|---|---|
| Cyclic OCs | Oral | Daily | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Extended-cycle OCs (Seasonale, Seasonique) | Oral | Daily | 8% | Menses 4/yr, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Continuous OCs (Lybrel) | Oral | Daily | 8% | No scheduled menses, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Shortened hormone-free interval OCs (Loestrin 24 Fe, Yaz) | Oral | Daily | 8% | Shorter monthly menses | Hormonal adverse effects, unscheduled bleeding |
| Transdermal patch (Evra) | Patch applied to skin | New patch applied weekly for 3 wk; off for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects, increased risk of VTE higher than OCs but lower than pregnancy; MI risk higher than comparable OCs, but use is reasonable if no cardiac risk factors |
| Vaginal ring (NuvaRing) | Ring inserted in vagina by patient | Ring inserted for 3 wk, removed for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Copper IUD (ParaGard T 380A) | IUD inserted & removed by clinician | Every 10 yr | 0. 8% | Heavier menses, may have BTB | Menorrhagia. Contraindications: Acute PID or high risk for STI; postpartum endometritis within 3 mo; mucopurulent cervicitis; Wilson’s disease |
| Levonorgestrel IUS (LNG IUS, Mirena) | IUS inserted & removed by clinician | Every 5 yr | < 0.1% | Lighter, shorter menses or amenorrhea | Minimal hormonal adverse effects. Contraindications: Acute PID, history of or high risk for PID; postpartum endometritis within 3 mo; mucopurulent cervicitis |
| Subdermal implant (Implanon) | Inserted subdermally & removed by clinician | Every 3 yr | 0.3% | Irregular, unpredictable bleeding | Unscheduled bleeding, mood symptoms, headache, weight gain, acne |
| Depot medroxyprogesterone acetate (Depo-Provera) | IM injection | Every 3 mo | 3% | Irregular bleeding, amenorrhea | Unscheduled bleeding, reversible bone loss |
| BTB, breakthrough bleeding; IM, intramuscular; IUD, intrauterine device; IUS, intrauterine system; MI, myocardial infarction; OC, oral contraceptive; PID, pelvic inflammatory disease; STI, sexually transmitted infection; VTE, venous thromboembolism. | |||||
Emergency contraception: 2 pills, 12 hours apart
Plan B contains 2 tablets of levonorgestrel 0.75 mg, to be taken 12 hours apart as soon as possible after unprotected intercourse. However, taking both doses together is as effective as taking them separately, and doing so may improve compliance.39
How it works. Emergency contraception (EC) works by inhibiting or delaying the surge of luteinizing hormone and follicular rupture before ovulation. It does not affect implantation or corpus luteum function, and it poses no risk to an established pregnancy or embryo. EC is ineffective when administered after ovulation.40
In a World Health Organization (WHO) multicenter randomized trial, EC prevented 79% to 84% of pregnancies if taken 1 to 3 days after intercourse, and 60% to 63% of pregnancies if taken 4 to 5 days after intercourse.41 Plan B is available without a prescription for women ages 18 and older. It is important to screen for pregnancy before prescribing Plan B for younger patients.
Adverse effects include nausea and vomiting, occurring in 23% and 5% of patients, respectively. Intermenstrual bleeding occurs in 8% of patients taking progestin-only EC. Menses are expected within 21 days of EC administration, and the second cycle after EC should be of normal length.42 The authors of a WHO report concluded that “there are no medical conditions wherein risks outweigh benefits of EC.”43
Combination estrogen/progestin for EC is no more effective than progestin-only EC and results in higher rates of adverse effects, especially nausea and vomiting.44
IUD can also be used in an emergency
Insertion of a copper IUD provides EC as well as ongoing contraception. It is hormone free and can be used effectively for EC up to 5 days after sexual intercourse, then continued for primary contraception for up to 10 years.45 Its estimated failure rate was less than 0.1% in more than 8400 postcoital insertions.46 The IUD works by impairing fertilization and implantation and by altering sperm motility and integrity. With a copper IUD, additional primary contraception is unnecessary. The LNG IUS (Mirena) has not been studied as an alternative EC.
Some worry that EC’s availability will encourage unprotected sex
Some authorities have wondered if increased access to EC might paradoxically lead to more pregnancies by encouraging unprotected sex. Researchers are exploring this issue. One study reported that unfettered access to free EC resulted in an increase in EC use, and another study reported that patients with unrestricted EC access had inadequately protected sex more often than those in the control group.47,48
A systematic review of 23 articles studying the effect of increased access to EC confirmed an increase in EC use,49 but no statistically significant differences in pregnancy or abortion rates.
For patients like the 35-year-old woman discussed earlier, who do not like taking pills, there are many contraception options to choose from, including the patch, vaginal ring, chewable OC, IUD, depot medroxyprogesterone injection, and subdermal implant.
Hormonal contraception would not be an issue for this patient—even though she has a family history of breast cancer. Using hormonal contraception does not increase the risk of breast cancer for individuals with a family history of breast cancer in a first-or second-degree relative.50
Correspondence
Petra M. Casey, MD, Department of Obstetrics and Gynecology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected].
- Consider an oral contraceptive for women who would prefer less frequent menstrual periods (A).
- An intrauterine device may be appropriate for women with prior pelvic inflammatory disease, ectopic pregnancy, or an abnormal Papanicolaou (Pap) smear result, and for many adolescents (A).
- There are no medical contraindications to progestin-only emergency contraception (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 35-year-old woman with a family history of breast cancer (mother diagnosed with breast cancer at age 55) requests your help in choosing an appropriate method of contraception. She is a nonsmoker, has a body mass index of 25, and dislikes taking pills. Which options would you recommend to her? Are there any that you would rule out?
Helping your patient make the best choice requires that you be as up to date as possible. In this review, we discuss select new options in a clinically relevant manner. Specifically, we explore the newest oral contraceptives (OCs), including extended-cycle, continuous, and shortened hormone-free interval formulations. In addition, we review the latest data and updated recommendations for the contraceptive patch and ring, intrauterine devices (IUDs), implants, and emergency contraception (TABLE). We conclude by describing appropriate choices for the patient described above. (See “So what do you recommend?” on page 803.)
Oral contraceptives
Since OCs became available in the 1960s, the standard regimen has been 21 active pills followed by 7 placebo pills, simulating the average unassisted monthly menstrual cycle in which “menstrual” or withdrawal bleeding occurs. Clinicians have successfully lengthened intermenstrual intervals with OCs, without incurring additional risk, to control symptoms of endometriosis, premenstrual syndrome, and menstrual-withdrawal headaches, or to satisfy many patients’ preference for fewer menses per year.1,2
Any monophasic active OC can be used without a placebo interval to delay menses for extended periods. Until recently, such usage was off-label. Based on extensive safety and efficacy studies, however, the US Food and Drug Administration (FDA) has now approved several formulations for extended-cycle and continuous-cycle use.
Extended-cycle OCs: Fewer menses per year
Two FDA-approved extended-cycle OCs are available: Seasonale and Seasonique.3,4 Both products enable 4 scheduled menstrual intervals per year, as opposed to about 13 with 28-day cycles. Each regimen uses 84 consecutive pills of levonorgestrel 0.15 mg and ethinyl estradiol (EE) 0.03 mg, followed by 7 placebo pills (Seasonale) or 7 pills of EE 0.01 mg (Seasonique).
Other potential advantages. With Seasonique, the average length of menses is 3 days, which is shorter than the average unassisted menstrual period. Seasonique’s 7 additional low-dose estrogen pills may help decrease estrogen withdrawal symptoms, such as headaches in women with menstrual migraine and vasomotor instability in perimenopausal women. Though this effect has also been reported with other OCs containing low-dose estrogen during the traditional placebo week, specific supportive evidence is not yet available for these formulations.5,6
Disadvantages to address. With Seasonale and Seasonique, unscheduled spotting or bleeding has been reported—especially during initial use—at rates considerably higher than those associated with comparable traditional OCs.3,4 Effective counseling will help ensure patient compliance and satisfaction.
During the first cycle (days 1-91), about 65% of women taking either formulation reported ≥7 days of spotting, and 29% to 35% reported ≥20 days of spotting. By the 4th cycle (days 273-364), 39% to 42% of patients reported ≥7 days of spotting and 11% to 15% reported ≥20 days of spotting. For patients taking comparable progestin and EE doses in traditional monthly regimens, 38% reported ≥7 days of spotting and 6% reported ≥20 days of spotting during the first cycle. Thirty-nine percent and 4%, respectively, reported spotting during the fourth cycle.3,4
Continuous OC: Consistent hormonal milieu
Lybrel, the only FDA-approved OC for continuous use, contains levonorgestrel 90 mcg and EE 20 mcg; pills are taken daily throughout the year.7 Progestin and estrogen doses are lower than those found in many monthly OCs and in all extended-cycle formulations. A phase 3 trial of 2134 women reported the safety and efficacy of Lybrel to be comparable to cyclic OCs.8 Again, unscheduled bleeding and spotting rates were relatively high but decreased at pack 3 from 47% and 26%, respectively, to 21% and 20%, respectively, at pack 13. Predictably, amenorrhea rates increased from 27% to 59% between pack 3 and pack 13.
Shortened hormone-free interval OCs: Less breakthrough ovulation
The shortened hormone-free interval OC is an alternative for patients who want regular, but shorter, menstrual intervals. With 24 active and 4 placebo pills in each cycle, this regimen suppresses the pituitary/ovarian axis to a greater extent than traditional 21/7-day regimens and thus lowers the rate of breakthrough ovulation.9
Loestrin 24 Fe contains norethindrone 1 mg and EE 20 mcg; placebo pills contain 75 mg of ferrous fumarate.
Yaz contains the newer progestin drospirenone 3 mg and EE 20 mcg. Drospirenone, an analog of the antihypertensive spironolactone, was introduced in a 21/7 formulation, Yasmin, and proved to have beneficial effects on mood, water retention, and acne.10 Yaz, which contains a lower dose of EE than Yasmin, provides 3 additional days of antimineralocorticoid and antiandrogenic activity, and is indicated for the treatment of premenstrual dysphoric disorder, a more severe form of premenstrual syndrome. Drospirenone-containing OCs are contraindicated for patients with renal, adrenal, or hepatic impairment because of the progestin’s metabolism via these routes.
An established OC with a twist: Chewable pills
For patients unable to swallow OCs, a chewable formulation, Femcon Fe, is available.11 It is hormonally identical to Ovcon 35, a well-established OC containing norethindrone acetate 0.4 mg and EE 35 mcg. The 7 placebo pills contain ferrous fumarate 75 mg. The spearmint-flavored chewable pill (which can also be swallowed) must be taken with 8 ounces of water.
Noncontraceptive benefits of OCs—there are many
An extensive body of evidence supports the noncontraceptive health benefits of OCs. These include a decreased risk of:
- endometrial and ovarian cancer
- bone loss
- benign breast disease
- pelvic inflammatory disease
- ectopic pregnancy
- rheumatoid arthritis.
Women with symptoms of androgen excess, premenstrual mood disorders, or endometriosis pain have long benefited from treatment with OCs.10,12-14 Healthy perimenopausal women are excellent candidates for OCs to regulate menses and treat symptoms of estrogen deficiency. OCs with added estrogen during the menstrual interval or shortened hormone-free interval may be more effective in moderating the perimenopausal transition. However, specific evidence about these effects is not yet available for the newest OC formulations.
Risks of OCs have been reduced, but some remain
Many of the well-known risks and side effects of OCs have been minimized over the years as total doses of estrogen have decreased and less androgenic progestins have been incorporated into OC formulations. Nonetheless, OCs are contraindicated for women who have a history of venous thromboembolism (VTE) or coronary artery disease (or are at risk for these complications), are over the age of 35 and smoke, are pregnant or newly postpartum, or are immobilized after surgery.
OCs remain relatively contraindicated for women with a history of migraines and focal auras, due to the increased risk for ischemic stroke.15 Breast and other estrogen-dependent cancers as well as liver disease preclude the use of OCs.16 Additional studies using the newer formulations of OCs are needed to definitively determine their long-term risk compared with traditional monthly formulations.
Contraceptive patch: Improving compliance
The contraceptive patch Evra is applied weekly and releases norelgestromin 150 mcg and EE 20 mcg each day, providing an OC alternative that is less dependent on compliance. However, in November 2005, the FDA modified product labeling to inform providers and the public that, based on pharmacokinetic studies, patients using the patch were exposed to hormone levels about 60% higher than with OCs of similar dosage.17 Another FDA labeling change made in 2008 states that “it is not known whether there are changes in the risk of serious adverse events based on the differences in pharmacokinetic profiles of EE in women using [the patch] as compared with women using oral contraceptives containing 35 mcg of EE.”
What is the real risk of VTE? One case-control study reported that the rates of VTE events in patch users and OC users were 52 per 100,000 woman-years and 42 per 100,000 woman-years, respectively.18 Another large case-control study showed the odds ratio (OR) of VTE to be 2.4 (95% confidence interval [CI], 1.1-5.5) with the patch compared with OCs; data were corrected for high-risk factors.19 However, the absolute risks for the patch and OCs were 40 and 18 per 100,000 woman-years, respectively—both lower than the risk of VTE associated with pregnancy.
The risk of myocardial infarction. The OR for myocardial infarction among patch users in the same population was 1.8 (95% CI, 0.5-6.8), and there was no statistically significant increase in the rate of cerebrovascular accidents.19 Thus, it is reasonable to use the patch with caution in patients without cardiac risk factors and to limit total hormone dosage by not using the patch in an extended-cycle manner. Of note, the patch is reported to have decreased efficacy in patients weighing over 90 kg (198 lb).20
Vaginal ring: Fewer drug interactions
NuvaRing, the ethylene vinyl vaginal ring, releases etonorgestrel (ENG) 120 mcg and EE 15 mcg each day (a lower estrogen dose than is contained in OCs or the patch).21 The device is 5.4 cm in diameter and 4 mm thick. Patients insert the ring intravaginally, remove it 3 weeks later for menses, and insert a new ring 1 week later.
Continuous use regimen. A randomized controlled trial evaluating the frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring reported a reduction in bleeding, flow, and pelvic pain, and a high continuation rate. Most patients considered the bleeding profile with the continuous vaginal ring acceptable compared with the baseline 21/7 use.22 Each ring contains up to a 28-day supply of hormones.
LNG-IUS (Mirena)
Fewer interactions. Transvaginal absorption of hormones with the vaginal ring avoids a first pass through the liver, thus decreasing many medication interactions. Irregular bleeding experienced with OCs or the patch may be effectively reduced with the steadily released, rapidly acting hormones in the ring.23
Intrauterine contraception
Intrauterine contraception is increasingly accepted by women who want long-term and effective contraception without having to comply with a particular regimen.
Copper IUD: Many contraindications are lifted
The nonhormonal IUD ParaGard T 380A is indicated for contraception for up to 10 years in women who are 16 years of age and older.24 This IUD’s active ingredient is the spermicidal copper wire wound around the short arms of the device. A recent meta-analysis reported an association between the use of a copper IUD and a decrease in the risk of endometrial cancer (OR=0.39; 95% CI, 0.29-0.51), though the mechanism for this association is unclear.25
In late 2005, the FDA broadened the use of copper IUDs to include women who are nulliparous; have a history of pelvic inflammatory disease (PID), sexually transmitted disease, or ectopic pregnancy; are in nonmonogamous relationships; or have a history of premenopausal breast cancer. This method also may be used by women with asymptomatic human immunodeficiency virus infection, Actinomyces infection, abnormal Papanicolaou (Pap) test results, or vaginitis. Furthermore, data support its use in adolescents who are at particularly high risk for unintended pregnancy.26
The copper IUD remains contra-indicated for patients with acute PID or current high-risk behavior for sexually transmitted infections, as well as for those who have mucopurulent cervicitis or have had postpartum endometritis within the past 3 months.24 Wilson’s disease is also a contraindication.
Insertion tip. Misoprostol may be used to soften the nulliparous cervix for insertion.27
Levonorgestrel intrauterine system: An alternative to the copper IUD
The levonorgestrel intrauterine system (LNG IUS), sold under the brand name Mirena, is gaining tremendous popularity in the United States. Multiple mechanisms of action, including endometrial thinning, cervical mucus thickening, inhibition of sperm function, and intermittent ovulation suppression are responsible for the >99% efficacy of this 5-year contraceptive.
Irregular menses can be expected initially, and 20% of patients reported amenorrhea at 1 year of use. In the unlikely event that a woman becomes pregnant while using Mirena, evaluate for ectopic pregnancy, which occurs in about half of pregnancies in women using this system.
Noncontraceptive benefits. The system’s primary noncontraceptive benefit is the dramatic reduction of menstrual blood loss, reported to be up to 90%.28 This contraceptive has been used as a cost-effective alternative to hysterectomy and endometrial ablation.29,30 Mirena imparts a protective effect against PID, likely secondary to progestin-mediated cervical mucus thickening.31
Subdermal implant (Implanon)
It appears safe and expeditious to provide both counseling and intrauterine contraception insertion in one visit, provided pregnancy is excluded.32 Confirm normalcy of cervical cytology and screen for sexually transmitted disease, if indicated. Prophylactic antibiotics are unnecessary, as the risk of PID within 20 days of insertion is only 9.7 per 1000 woman- years.33 After 20 days, the risk declines to 1.4 per 1000 woman-years, the same as that of the general population.34
Subdermal implant: Easily reversible
In July 2006, the FDA approved Implanon, a subdermal contraceptive implant.35 It has been available worldwide since 1998. The 40 × 2 mm single-rod implant containing etonogestrel (ENG) 68 mg diffuses the hormone at a rate of 60 mcg/d immediately after insertion and then steadily at 30 mcg/d for up to 3 years.
Its primary mechanism of action is ovulation suppression, with no ovulation detected for 30 months in a study group of more than 17,000 women.35 Increased cervical mucus viscosity also contributes to its effectiveness.35 In a large clinical trial, no pregnancies were reported in more than 6100 cycles.36 However, this trial excluded women weighing more than 130% of their ideal body weight, so no data are available to support the effectiveness of Implanon in obese women.36
Benefits and risks. This implant does not cause a hypoestrogenic state and ovulation suppression is rapidly reversible, with ENG levels undetectable within 10 days of implant removal.35 Furthermore, this method has no reported deleterious effects on bone mineral density or lactation.37,38 When counseling women about the implant, emphasize its propensity to result in “irregular and unpredictable” bleeding. An average of 7 bleeding and 10 spotting days within a 90-day period has been reported. Most women had fewer bleeding/spotting days than they would without contraception, but unscheduled bleeding was the leading reason for method discontinuation (11%), followed by weight gain, emotional lability, acne, headache, and depression (each ≈1%-2%).35
Implant insertion. The device is inserted in the sulcus between the biceps and triceps muscles of the nondominant arm. It is crucial to place the implant subdermally, tenting the skin during insertion to prevent deep insertion. High-frequency ultrasound can be used to detect nonpalpable implants. The FDA has mandated 3 hours of training for clinicians before they can obtain the device.
Depot medroxyprogesterone: Tried and true alternative
The depot medroxyprogesterone acetate (DMPA) injection has been a mainstay of contraception for decades. Available under the brand name Depo-Provera, it’s an option for women in whom estrogen-containing contraceptives are contraindicated. Its convenience, reduced risk of anemia, and postpartum benefits are all well known, and we have thus limited our discussion of DMPA to the summary in the TABLE.
TABLE
How do these contraceptives compare?
| METHOD | ROUTE OF ADMINISTRATION | FREQUENCY OF ADMINISTRATION | FAILURES/YR WITH TYPICAL USE 33,35,51 | EXPECTED MENSTRUAL PATTERN | ADVERSE EFFECTS/CONTRAINDICATIONS |
|---|---|---|---|---|---|
| Cyclic OCs | Oral | Daily | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Extended-cycle OCs (Seasonale, Seasonique) | Oral | Daily | 8% | Menses 4/yr, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Continuous OCs (Lybrel) | Oral | Daily | 8% | No scheduled menses, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Shortened hormone-free interval OCs (Loestrin 24 Fe, Yaz) | Oral | Daily | 8% | Shorter monthly menses | Hormonal adverse effects, unscheduled bleeding |
| Transdermal patch (Evra) | Patch applied to skin | New patch applied weekly for 3 wk; off for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects, increased risk of VTE higher than OCs but lower than pregnancy; MI risk higher than comparable OCs, but use is reasonable if no cardiac risk factors |
| Vaginal ring (NuvaRing) | Ring inserted in vagina by patient | Ring inserted for 3 wk, removed for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Copper IUD (ParaGard T 380A) | IUD inserted & removed by clinician | Every 10 yr | 0. 8% | Heavier menses, may have BTB | Menorrhagia. Contraindications: Acute PID or high risk for STI; postpartum endometritis within 3 mo; mucopurulent cervicitis; Wilson’s disease |
| Levonorgestrel IUS (LNG IUS, Mirena) | IUS inserted & removed by clinician | Every 5 yr | < 0.1% | Lighter, shorter menses or amenorrhea | Minimal hormonal adverse effects. Contraindications: Acute PID, history of or high risk for PID; postpartum endometritis within 3 mo; mucopurulent cervicitis |
| Subdermal implant (Implanon) | Inserted subdermally & removed by clinician | Every 3 yr | 0.3% | Irregular, unpredictable bleeding | Unscheduled bleeding, mood symptoms, headache, weight gain, acne |
| Depot medroxyprogesterone acetate (Depo-Provera) | IM injection | Every 3 mo | 3% | Irregular bleeding, amenorrhea | Unscheduled bleeding, reversible bone loss |
| BTB, breakthrough bleeding; IM, intramuscular; IUD, intrauterine device; IUS, intrauterine system; MI, myocardial infarction; OC, oral contraceptive; PID, pelvic inflammatory disease; STI, sexually transmitted infection; VTE, venous thromboembolism. | |||||
Emergency contraception: 2 pills, 12 hours apart
Plan B contains 2 tablets of levonorgestrel 0.75 mg, to be taken 12 hours apart as soon as possible after unprotected intercourse. However, taking both doses together is as effective as taking them separately, and doing so may improve compliance.39
How it works. Emergency contraception (EC) works by inhibiting or delaying the surge of luteinizing hormone and follicular rupture before ovulation. It does not affect implantation or corpus luteum function, and it poses no risk to an established pregnancy or embryo. EC is ineffective when administered after ovulation.40
In a World Health Organization (WHO) multicenter randomized trial, EC prevented 79% to 84% of pregnancies if taken 1 to 3 days after intercourse, and 60% to 63% of pregnancies if taken 4 to 5 days after intercourse.41 Plan B is available without a prescription for women ages 18 and older. It is important to screen for pregnancy before prescribing Plan B for younger patients.
Adverse effects include nausea and vomiting, occurring in 23% and 5% of patients, respectively. Intermenstrual bleeding occurs in 8% of patients taking progestin-only EC. Menses are expected within 21 days of EC administration, and the second cycle after EC should be of normal length.42 The authors of a WHO report concluded that “there are no medical conditions wherein risks outweigh benefits of EC.”43
Combination estrogen/progestin for EC is no more effective than progestin-only EC and results in higher rates of adverse effects, especially nausea and vomiting.44
IUD can also be used in an emergency
Insertion of a copper IUD provides EC as well as ongoing contraception. It is hormone free and can be used effectively for EC up to 5 days after sexual intercourse, then continued for primary contraception for up to 10 years.45 Its estimated failure rate was less than 0.1% in more than 8400 postcoital insertions.46 The IUD works by impairing fertilization and implantation and by altering sperm motility and integrity. With a copper IUD, additional primary contraception is unnecessary. The LNG IUS (Mirena) has not been studied as an alternative EC.
Some worry that EC’s availability will encourage unprotected sex
Some authorities have wondered if increased access to EC might paradoxically lead to more pregnancies by encouraging unprotected sex. Researchers are exploring this issue. One study reported that unfettered access to free EC resulted in an increase in EC use, and another study reported that patients with unrestricted EC access had inadequately protected sex more often than those in the control group.47,48
A systematic review of 23 articles studying the effect of increased access to EC confirmed an increase in EC use,49 but no statistically significant differences in pregnancy or abortion rates.
For patients like the 35-year-old woman discussed earlier, who do not like taking pills, there are many contraception options to choose from, including the patch, vaginal ring, chewable OC, IUD, depot medroxyprogesterone injection, and subdermal implant.
Hormonal contraception would not be an issue for this patient—even though she has a family history of breast cancer. Using hormonal contraception does not increase the risk of breast cancer for individuals with a family history of breast cancer in a first-or second-degree relative.50
Correspondence
Petra M. Casey, MD, Department of Obstetrics and Gynecology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected].
1. Andrist LC, Arias RD, Nucatola D, et al. Women’s and providers’ attitudes toward menstrual suppression with extended use of oral contraceptives. Contraception. 2004;70:359-363.
2. Glasier AF, Smith KB, van der Spuy ZM, et al. Amenorrhea associated with contraception—an international study on acceptability. Contraception. 2003;67:1-8.
3. Seasonale [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2008.
4. Seasonique [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
5. Shortened pill-free interval delivered by new 20 mcg pill, Organon’s Mircette, schedule for US debut this summer Contraception Technol Update. 1998;19:85-87.
6. Casper RF, Dodin S, Reid RL. For the Study Investigators. The effect of 20 mcg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
7. Lybrel [package insert] Philadelphia, Pa: Wyeth Pharmaceuticals, Inc; 2007.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Willis SA, Kuehl TJ, Spiekerman AM, et al. Greater inhibition of the pituitary: ovarian axis in oral contraceptive regimens with a shortened hormone-free interval. Contraception. 2006;74:100-103.
10. Parsey KS, Pong A. An open-label, multicenter study to evaluate Yasmin, a low-dose combination oral contraceptive containing drospirenone, a new progestogen. Contraception. 2000;61:105-111.
11. Femcon Fe [package insert]. Rockaway, NJ: Warner Chilcott (US), Inc; 2007.
12. Burkman R. The evolution of oral contraceptives: 40 years of continuous improvement. Clinician. 2002;20:3-30.
13. Vercellini P, Frontino G, De Giorgi O, et al. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril. 2003;80:560-563.
14. Guido M, Romualdi D, Giuliani M. Drospirenone for the treatment of hirsute women with PCOS; a clinical endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004;89:2817-2823.
15. Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. Br Med J. 1995;310:830-833.
16. Steinauer J, Autry AM. Extended cycle combined hormonal contraception. Obstet Gynecol Clin North Am. 2007;34:43-55, viii.
17. Ortho Evra [prescribing information]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001.
18. Jick SS, Kaye JA, Russmann S, et al. Risk of non-fatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg/l of ethinyl estradiol. Contraception. 2006;73:223-228.
19. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
20. American College of Obstetricians and Gynecologists. ACOG practice bulletin. Use of hormonal contraceptives in women with coexisting medical conditions. No. 73, June 2006. Obstet Gynecol. 2006;107:1453-1472.
21. Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril. 2001;75:865-870.
22. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol. 2008;112:563-571.
23. Bjarnadottir RI, Tuppurainen M, Killick SR. Comparison of cycle control with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl estradiol. Am J Obstet Gynecol. 2002;186:389-395.
24. ParaGard T 380A [prescribing information]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
25. Beining RM, Dennis LK, Smith EM, et al. Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann Epidem. 2008;18:492-499.
26. ACOG Committee Opinion. Intrauterine Device and Adolescents No. 392. Obstet Gynecol. 2007;110:1493-1495.
27. McNaught J. Adolescents and IUCDs—Not a contraindication. J Ped Adolesc Gyn. 2006;19:303-305.
28. Lethaby AE, Cooke I, Rees M. Progesterone/progestogen releasing intrauterine systems versus either placebo or any other medication for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.-
29. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273-277.
30. Römer T. Prospective comparison study of levonorgestrel IUD versus Roller-Ball endometrial ablation in the management of refractory recurrent hypermenorrhea. Eur J Obstet Gynecol Reprod Biol. 2000;90:27-29.
31. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’ comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77:261-264.
32. Ball CE. News and controversies in contraception. AudioDigest Obstet Gynecol Clin Updates. 2007;54:18.-
33. Grimes DA. Intrauterine device and upper genital tract infection. Lancet. 2000;356:1013-1019.
34. Farley TM, Rosenberg MJ, Rowe PJ, et al. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785-788.
35. Implanon [package insert]. Roseland, NJ: Organon USA Inc; 2007.
36. Funk S, Miller MM, Mishell DR, Jr, et al. The Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
37. Beerthuizen R, van Beek A, Massai R, et al. Bone mineral density during long-term use of the progestagen contraceptive implant Implanon compared to a non-hormonal method of contraception. Hum Reprod. 2000;15:118-122.
38. Reinprayoon D, Taneepanichskul S, Bunyavejchevin S, et al. Effects of the etonogestrel-releasing contraceptive implant (Implanon) on parameters of breastfeeding compared to those of an intrauterine device. Contraception. 2000;62:239-246.
39. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
40. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 69: emergency contraception. Obstet Gynecol. 2005;106:1443-1452.
41. Von Hertzen H, Piaggio G, Ding J. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. WHO Research Group on Post-Ovulatory Methods of Fertility Regulation. Lancet. 2002;360:1803-1810.
42. Raymond EG, Goldberg A, Trussell J, et al. Bleeding patterns after use of levonorgestrel emergency contraceptive pills. Contraception. 2006;73:376-381.
43. World Health Organization. Medical eligibility criteria for contraceptive use. 3rd ed. 2004. Available at: www.who.int/reproductive-health/publication/med/mec.pdf. Accessed February 7, 2008.
44. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
45. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 59. Intrauterine device. Obstet Gynecol. 2005;105:223-232.
46. LaValleur J. Emergency contraception. Obstet Gynecol Clin North Am. 2000;27:817-839, vii.
47. Raymond EG, Stewart F, Weaver M, et al. Impact of increased access to emergency contraceptive pills: a randomized controlled trial. Obstet Gynecol. 2006;108:1098-1106.
48. Raymond EG, Weaver MA. Effect of an emergency contraceptive pill intervention on pregnancy risk behavior. Contraception. 2008;77:333-336.
49. Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol. 2007;109:181-188.
50. Casey PM, Cerhan JR, Pruthi S. Oral contraceptive use and risk of breast cancer Mayo Clinic Proc. 2008;83:86-89.
51. Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart FH, et al, eds. Contraceptive Technology, 18th ed. New York, NY: Ardent Media, Inc; 2004:773-845.
1. Andrist LC, Arias RD, Nucatola D, et al. Women’s and providers’ attitudes toward menstrual suppression with extended use of oral contraceptives. Contraception. 2004;70:359-363.
2. Glasier AF, Smith KB, van der Spuy ZM, et al. Amenorrhea associated with contraception—an international study on acceptability. Contraception. 2003;67:1-8.
3. Seasonale [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2008.
4. Seasonique [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
5. Shortened pill-free interval delivered by new 20 mcg pill, Organon’s Mircette, schedule for US debut this summer Contraception Technol Update. 1998;19:85-87.
6. Casper RF, Dodin S, Reid RL. For the Study Investigators. The effect of 20 mcg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
7. Lybrel [package insert] Philadelphia, Pa: Wyeth Pharmaceuticals, Inc; 2007.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Willis SA, Kuehl TJ, Spiekerman AM, et al. Greater inhibition of the pituitary: ovarian axis in oral contraceptive regimens with a shortened hormone-free interval. Contraception. 2006;74:100-103.
10. Parsey KS, Pong A. An open-label, multicenter study to evaluate Yasmin, a low-dose combination oral contraceptive containing drospirenone, a new progestogen. Contraception. 2000;61:105-111.
11. Femcon Fe [package insert]. Rockaway, NJ: Warner Chilcott (US), Inc; 2007.
12. Burkman R. The evolution of oral contraceptives: 40 years of continuous improvement. Clinician. 2002;20:3-30.
13. Vercellini P, Frontino G, De Giorgi O, et al. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril. 2003;80:560-563.
14. Guido M, Romualdi D, Giuliani M. Drospirenone for the treatment of hirsute women with PCOS; a clinical endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004;89:2817-2823.
15. Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. Br Med J. 1995;310:830-833.
16. Steinauer J, Autry AM. Extended cycle combined hormonal contraception. Obstet Gynecol Clin North Am. 2007;34:43-55, viii.
17. Ortho Evra [prescribing information]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001.
18. Jick SS, Kaye JA, Russmann S, et al. Risk of non-fatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg/l of ethinyl estradiol. Contraception. 2006;73:223-228.
19. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
20. American College of Obstetricians and Gynecologists. ACOG practice bulletin. Use of hormonal contraceptives in women with coexisting medical conditions. No. 73, June 2006. Obstet Gynecol. 2006;107:1453-1472.
21. Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril. 2001;75:865-870.
22. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol. 2008;112:563-571.
23. Bjarnadottir RI, Tuppurainen M, Killick SR. Comparison of cycle control with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl estradiol. Am J Obstet Gynecol. 2002;186:389-395.
24. ParaGard T 380A [prescribing information]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
25. Beining RM, Dennis LK, Smith EM, et al. Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann Epidem. 2008;18:492-499.
26. ACOG Committee Opinion. Intrauterine Device and Adolescents No. 392. Obstet Gynecol. 2007;110:1493-1495.
27. McNaught J. Adolescents and IUCDs—Not a contraindication. J Ped Adolesc Gyn. 2006;19:303-305.
28. Lethaby AE, Cooke I, Rees M. Progesterone/progestogen releasing intrauterine systems versus either placebo or any other medication for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.-
29. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273-277.
30. Römer T. Prospective comparison study of levonorgestrel IUD versus Roller-Ball endometrial ablation in the management of refractory recurrent hypermenorrhea. Eur J Obstet Gynecol Reprod Biol. 2000;90:27-29.
31. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’ comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77:261-264.
32. Ball CE. News and controversies in contraception. AudioDigest Obstet Gynecol Clin Updates. 2007;54:18.-
33. Grimes DA. Intrauterine device and upper genital tract infection. Lancet. 2000;356:1013-1019.
34. Farley TM, Rosenberg MJ, Rowe PJ, et al. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785-788.
35. Implanon [package insert]. Roseland, NJ: Organon USA Inc; 2007.
36. Funk S, Miller MM, Mishell DR, Jr, et al. The Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
37. Beerthuizen R, van Beek A, Massai R, et al. Bone mineral density during long-term use of the progestagen contraceptive implant Implanon compared to a non-hormonal method of contraception. Hum Reprod. 2000;15:118-122.
38. Reinprayoon D, Taneepanichskul S, Bunyavejchevin S, et al. Effects of the etonogestrel-releasing contraceptive implant (Implanon) on parameters of breastfeeding compared to those of an intrauterine device. Contraception. 2000;62:239-246.
39. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
40. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 69: emergency contraception. Obstet Gynecol. 2005;106:1443-1452.
41. Von Hertzen H, Piaggio G, Ding J. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. WHO Research Group on Post-Ovulatory Methods of Fertility Regulation. Lancet. 2002;360:1803-1810.
42. Raymond EG, Goldberg A, Trussell J, et al. Bleeding patterns after use of levonorgestrel emergency contraceptive pills. Contraception. 2006;73:376-381.
43. World Health Organization. Medical eligibility criteria for contraceptive use. 3rd ed. 2004. Available at: www.who.int/reproductive-health/publication/med/mec.pdf. Accessed February 7, 2008.
44. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
45. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 59. Intrauterine device. Obstet Gynecol. 2005;105:223-232.
46. LaValleur J. Emergency contraception. Obstet Gynecol Clin North Am. 2000;27:817-839, vii.
47. Raymond EG, Stewart F, Weaver M, et al. Impact of increased access to emergency contraceptive pills: a randomized controlled trial. Obstet Gynecol. 2006;108:1098-1106.
48. Raymond EG, Weaver MA. Effect of an emergency contraceptive pill intervention on pregnancy risk behavior. Contraception. 2008;77:333-336.
49. Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol. 2007;109:181-188.
50. Casey PM, Cerhan JR, Pruthi S. Oral contraceptive use and risk of breast cancer Mayo Clinic Proc. 2008;83:86-89.
51. Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart FH, et al, eds. Contraceptive Technology, 18th ed. New York, NY: Ardent Media, Inc; 2004:773-845.