User login
What to assess before stepping up asthma therapy
Depression drops COPD medication adherence
Patients with chronic obstructive pulmonary disease (COPD) who also suffer from depression are less likely to take their COPD maintenance medications, according to a review of Medicare claims by researchers at the University of Maryland, Baltimore. Researchers found that patients with newly diagnosed depression were about 7% less likely to have good adherence to their medications. For more on this research, see the article in CHEST Physician, available at http://www.mdedge.com/chestphysician/article/115659/depression/depression-drops-copd-medication-adherence.
Patients with chronic obstructive pulmonary disease (COPD) who also suffer from depression are less likely to take their COPD maintenance medications, according to a review of Medicare claims by researchers at the University of Maryland, Baltimore. Researchers found that patients with newly diagnosed depression were about 7% less likely to have good adherence to their medications. For more on this research, see the article in CHEST Physician, available at http://www.mdedge.com/chestphysician/article/115659/depression/depression-drops-copd-medication-adherence.
Patients with chronic obstructive pulmonary disease (COPD) who also suffer from depression are less likely to take their COPD maintenance medications, according to a review of Medicare claims by researchers at the University of Maryland, Baltimore. Researchers found that patients with newly diagnosed depression were about 7% less likely to have good adherence to their medications. For more on this research, see the article in CHEST Physician, available at http://www.mdedge.com/chestphysician/article/115659/depression/depression-drops-copd-medication-adherence.
Pulmonary Function Tests
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
COPD: Diagnostic and Treatment Update for the Primary Care Setting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
COPD patient characteristics predict response to maintenance drug
Azithromycin maintenance therapy may be best reserved for patients with mild to moderate chronic obstructive pulmonary disease (COPD) and few symptoms, according to an analysis from the COLUMBUS randomized controlled trial. The study, reported on in Family Practice News, also revealed that patients with a high serum eosinophil level… http://www.familypracticenews.com/specialty-focus/pulmonary-sleep-medicine/single-article-page/copd-patient-characteristics-predict-response-to-maintenance-drug/f29efaba9a4874ed9b754fb87b77b663.html.
Azithromycin maintenance therapy may be best reserved for patients with mild to moderate chronic obstructive pulmonary disease (COPD) and few symptoms, according to an analysis from the COLUMBUS randomized controlled trial. The study, reported on in Family Practice News, also revealed that patients with a high serum eosinophil level… http://www.familypracticenews.com/specialty-focus/pulmonary-sleep-medicine/single-article-page/copd-patient-characteristics-predict-response-to-maintenance-drug/f29efaba9a4874ed9b754fb87b77b663.html.
Azithromycin maintenance therapy may be best reserved for patients with mild to moderate chronic obstructive pulmonary disease (COPD) and few symptoms, according to an analysis from the COLUMBUS randomized controlled trial. The study, reported on in Family Practice News, also revealed that patients with a high serum eosinophil level… http://www.familypracticenews.com/specialty-focus/pulmonary-sleep-medicine/single-article-page/copd-patient-characteristics-predict-response-to-maintenance-drug/f29efaba9a4874ed9b754fb87b77b663.html.
Elevated HDL levels predict reduced lung function
LONDON – Having an elevated level of high-density lipoprotein cholesterol (HDL-C) is associated with an increased rate of lung function decline over time, according to results from a cohort analysis of more than 30,000 adults presented at the annual congress of the European Respiratory Society.
For forced expiratory volume in 1 second (FEV1), “there was a highly statistically significant inverse association for HDL-C for both cross-sectional and longitudinal measures of lung function,” reported Elizabeth C. Oelsner, MD, Columbia University Medical Center, New York. Those in the top quartile for HDL-C, on average, had a 9-mL greater decline in FEV1, compared with patients in the lowest quartile (P less than .001). To put this in perspective, Dr. Oelsner said this decline is comparable “to a 10-year increment in pack-years of smoking.”
The study, which pooled six population-based cohorts in the United States, included 31,843 adults for whom there were baseline HDL-C levels and at least two longitudinally collected spirometry readings. According to Dr. Oelsner, quality control criteria were rigorously applied. For example, spirometry measures were obtained according to contemporary standards issued by the American Thoracic Society (ATS).
The average age of the study patients was 57 years, and 45% were classified as never smokers. The mean FEV1 decline over a median follow-up of 5 years was 37 mL per year (range of 22-49 mL/year across the six cohorts). Approximately 15% of individuals had airflow limitation at baseline. There were more than 300,000 total person-years of observation in the pooled data.
In a fully adjusted cross-sectional analysis, each 1 mmol/L increase (38.67 mg/dL) in HDL-C was associated with a 9-mL lower FEV1, according to Dr. Oelsner. He said the list of adjusted variables included age, gender, pack-years of smoking, weight, and height.
Results were consistent across age groups, presence or absence of smoking history, body mass index, and the presence or absence of airflow limitations at baseline, according to Dr. Oelsner.
HDL-C’s inverse correlation with lung function has been shown in other studies, such as the MESA Lung Study, another population-based analysis, according to Dr. Oelsner. In that study, a 0.4% increase in emphysema on CT lung scans was observed for every 10 mg/dL increase in HDL-C (Am J Respir Crit Care Med. 2010;181:A2878).
In this study, “being in the highest quartile for HDL at baseline was associated with an odds ratio of 1.2 for incident airflow limitation relative to being in the lowest [quartile],” Dr. Oelsner said.
The risk of a decline in airway function from an elevated HDL-C, if confirmed, should be considered in the context of the well-known protective effect exerted by HDL against cardiovascular events, according to Dr. Oelsner. However, she added, these data suggest that “having an excessively high HDL-C may incur risk just as an excessively low HDL may incur risk.” She noted, “there may be a limitation to the good of the good cholesterol.”
When asked after these data were presented whether she would prefer to have a low or high HDL-C, Dr. Oelsner responded, “Everything in moderation.” She also suggested that studies of treatments designed to raise HDL-C to reduce cardiovascular risk should take lung function into consideration. She warned that adverse effects on lung function are a potential “off-target risk” from such therapies.
LONDON – Having an elevated level of high-density lipoprotein cholesterol (HDL-C) is associated with an increased rate of lung function decline over time, according to results from a cohort analysis of more than 30,000 adults presented at the annual congress of the European Respiratory Society.
For forced expiratory volume in 1 second (FEV1), “there was a highly statistically significant inverse association for HDL-C for both cross-sectional and longitudinal measures of lung function,” reported Elizabeth C. Oelsner, MD, Columbia University Medical Center, New York. Those in the top quartile for HDL-C, on average, had a 9-mL greater decline in FEV1, compared with patients in the lowest quartile (P less than .001). To put this in perspective, Dr. Oelsner said this decline is comparable “to a 10-year increment in pack-years of smoking.”
The study, which pooled six population-based cohorts in the United States, included 31,843 adults for whom there were baseline HDL-C levels and at least two longitudinally collected spirometry readings. According to Dr. Oelsner, quality control criteria were rigorously applied. For example, spirometry measures were obtained according to contemporary standards issued by the American Thoracic Society (ATS).
The average age of the study patients was 57 years, and 45% were classified as never smokers. The mean FEV1 decline over a median follow-up of 5 years was 37 mL per year (range of 22-49 mL/year across the six cohorts). Approximately 15% of individuals had airflow limitation at baseline. There were more than 300,000 total person-years of observation in the pooled data.
In a fully adjusted cross-sectional analysis, each 1 mmol/L increase (38.67 mg/dL) in HDL-C was associated with a 9-mL lower FEV1, according to Dr. Oelsner. He said the list of adjusted variables included age, gender, pack-years of smoking, weight, and height.
Results were consistent across age groups, presence or absence of smoking history, body mass index, and the presence or absence of airflow limitations at baseline, according to Dr. Oelsner.
HDL-C’s inverse correlation with lung function has been shown in other studies, such as the MESA Lung Study, another population-based analysis, according to Dr. Oelsner. In that study, a 0.4% increase in emphysema on CT lung scans was observed for every 10 mg/dL increase in HDL-C (Am J Respir Crit Care Med. 2010;181:A2878).
In this study, “being in the highest quartile for HDL at baseline was associated with an odds ratio of 1.2 for incident airflow limitation relative to being in the lowest [quartile],” Dr. Oelsner said.
The risk of a decline in airway function from an elevated HDL-C, if confirmed, should be considered in the context of the well-known protective effect exerted by HDL against cardiovascular events, according to Dr. Oelsner. However, she added, these data suggest that “having an excessively high HDL-C may incur risk just as an excessively low HDL may incur risk.” She noted, “there may be a limitation to the good of the good cholesterol.”
When asked after these data were presented whether she would prefer to have a low or high HDL-C, Dr. Oelsner responded, “Everything in moderation.” She also suggested that studies of treatments designed to raise HDL-C to reduce cardiovascular risk should take lung function into consideration. She warned that adverse effects on lung function are a potential “off-target risk” from such therapies.
LONDON – Having an elevated level of high-density lipoprotein cholesterol (HDL-C) is associated with an increased rate of lung function decline over time, according to results from a cohort analysis of more than 30,000 adults presented at the annual congress of the European Respiratory Society.
For forced expiratory volume in 1 second (FEV1), “there was a highly statistically significant inverse association for HDL-C for both cross-sectional and longitudinal measures of lung function,” reported Elizabeth C. Oelsner, MD, Columbia University Medical Center, New York. Those in the top quartile for HDL-C, on average, had a 9-mL greater decline in FEV1, compared with patients in the lowest quartile (P less than .001). To put this in perspective, Dr. Oelsner said this decline is comparable “to a 10-year increment in pack-years of smoking.”
The study, which pooled six population-based cohorts in the United States, included 31,843 adults for whom there were baseline HDL-C levels and at least two longitudinally collected spirometry readings. According to Dr. Oelsner, quality control criteria were rigorously applied. For example, spirometry measures were obtained according to contemporary standards issued by the American Thoracic Society (ATS).
The average age of the study patients was 57 years, and 45% were classified as never smokers. The mean FEV1 decline over a median follow-up of 5 years was 37 mL per year (range of 22-49 mL/year across the six cohorts). Approximately 15% of individuals had airflow limitation at baseline. There were more than 300,000 total person-years of observation in the pooled data.
In a fully adjusted cross-sectional analysis, each 1 mmol/L increase (38.67 mg/dL) in HDL-C was associated with a 9-mL lower FEV1, according to Dr. Oelsner. He said the list of adjusted variables included age, gender, pack-years of smoking, weight, and height.
Results were consistent across age groups, presence or absence of smoking history, body mass index, and the presence or absence of airflow limitations at baseline, according to Dr. Oelsner.
HDL-C’s inverse correlation with lung function has been shown in other studies, such as the MESA Lung Study, another population-based analysis, according to Dr. Oelsner. In that study, a 0.4% increase in emphysema on CT lung scans was observed for every 10 mg/dL increase in HDL-C (Am J Respir Crit Care Med. 2010;181:A2878).
In this study, “being in the highest quartile for HDL at baseline was associated with an odds ratio of 1.2 for incident airflow limitation relative to being in the lowest [quartile],” Dr. Oelsner said.
The risk of a decline in airway function from an elevated HDL-C, if confirmed, should be considered in the context of the well-known protective effect exerted by HDL against cardiovascular events, according to Dr. Oelsner. However, she added, these data suggest that “having an excessively high HDL-C may incur risk just as an excessively low HDL may incur risk.” She noted, “there may be a limitation to the good of the good cholesterol.”
When asked after these data were presented whether she would prefer to have a low or high HDL-C, Dr. Oelsner responded, “Everything in moderation.” She also suggested that studies of treatments designed to raise HDL-C to reduce cardiovascular risk should take lung function into consideration. She warned that adverse effects on lung function are a potential “off-target risk” from such therapies.
AT THE ERS CONGRESS 2016
Key clinical point: In an evaluation of greater than 30,000 patients in six study cohorts, higher high-density lipoprotein cholesterol (HDL-C) was associated with accelerated lung function decline.
Major finding: Those in the top quartile for HDL-C, on average, had a 9-mL greater decline in forced expiratory volume in 1 second, compared with patients in the lowest quartile (P less than .001).
Data source: Observational cohort study.
Disclosures: Dr. Oelsner reported no relevant financial relationships.
Did Somebody Say “Precepting”?
But First, a Word About Vaping …
As advocates for tobacco control, my colleagues and I took great interest in Randy D. Danielsen’s editorial, “Vaping: Are Its ‘Benefits’ a Lot of Hot Air?” (Clinician Reviews. 2016;26[6]:15-16). Our practice offers evidence-based cessation treatment for individuals with nicotine addiction through counseling, pharmacotherapy, and the use of nicotine replacement products.
At our center, we often interact with clients who have had multiple quit attempts. Many of our clients state that they have been unsuccessful using an e-cigarette as a smoking cessation strategy. More often than not, they report smoking a cigarette “here and there” along with “vaping,” until they eventually relapse to their usual smoking pattern. Some report that they smoke even more than before they tried to quit. We have concerns about how vaping may renormalize the behaviors associated with smoking. Our clients say that when they vape, it reminds them of the “social” aspects of smoking— “being part of a group” and participating in an activity that keeps their hands busy.
Recent literature suggests that curiosity is the primary reason adolescents engage in e-cigarette use. While the newly implemented FDA regulations on e-cigarettes may keep these products out of the hands of some adolescents by prohibiting sales to those younger than 18, there is much more to consider. Along with exposure to nicotine, these devices offer a variety of kid-friendly flavorings that make these products attractive to middle and high school youth. Flavorings will not be regulated at this point in time.
According to researchers, this is a major concern. Findings from studies report that when inhaled, certain flavors are more harmful than others. For example, very high—even toxic—levels of benzaldehyde are inhaled by the user when cherry-flavored e-liquid is heated at high temperatures. The chemical diacetyl, a respiratory irritant known to be associated with bronchiolitis obliterans (popcorn lung), is produced by the aerosol vapors from buttered popcorn and certain fruit-flavored e-cigarette liquids.
As public health advocates, we must provide research to the FDA about the health hazards of the flavoring added to e-cigarettes and continue to fight for this regulation. We must support evidence-based tobacco control interventions, such as hard-hitting media campaigns and tobacco excise taxes, and promote access to cessation treatment, smoke-free policies, and statewide funding. Elimination of tobacco products will reduce the public health burden of tobacco-related illness.
Andrea Spatarella, DNP, RN, FNP-BC, Christine Fardellone, DNP, RN, Raisa Abramova, FNP-BC, RN
Great Neck, NY
Continue for Precepting & E-Quality of Care >>
Precepting & E-Quality of Care
As a woman of the baby-boomer generation, I was raised in an era when feminism was a focus for many. There was a great deal being written and discussed to encourage women to attain equal pay for equal work. Because nursing was (and still is) a profession dominated by women, this was a frequent topic in the classroom. We were repeatedly told, “Don’t give away your knowledge for free” and “You deserve to be paid what you’re worth, don’t discount yourself.”
I find it very telling that the same female-dominated academic programs that encouraged me to seek proper payment are now taking advantage of my free labor. I am somewhat offended by this attitude and consider it a step backward. Each time NPs are guilted or browbeaten into teaching without proper compensation, the profession is devalued. To continue to participate is to enable a problematic, if not broken, system.
NP education is in need of major reform. The precepting issue is the weak link in becoming a qualified professional who is able to meet the demands and responsibilities that academics and politicos are pushing harder and harder for. Our physician and PA colleagues can rightly argue that their clinical education is superior to ours—and I cannot fault our colleagues for expressing concern about quality of care. If nursing really wants an equal place at the table, this weakness must be improved, or the naysayers will have plenty of evidence that they were correct in the years to come.
Rebecca Shively, MSN, RN, FNP-BC
San Marcos, TX
Continue for NP Schools & Their Rigid Rules >>
NP Schools & Their Rigid Rules
I have been a preceptor for at least a dozen NP students and have yet to be offered compensation. Preceptors take the place of a paid instructor, giving away free advice and experiences. I don’t mind doing this, but at times it can be a struggle. Some students, for example, have never done a pelvic exam. Letting an inexperienced NP student practice a pelvic exam on a patient who made an appointment to see an experienced provider is unjust and unfair to the patient—I won’t do it. These schools need to provide practice sessions on paid patients so their students can learn these skills.
I have my beef with the institutes of higher learning, not the students. It feels like a one-way street. You fill out the forms they require in order to precept, which takes up valuable work time. You equip their students with the skills they need to practice safely and correctly, and then try to fill out their evaluation sheets on things that students are not licensed to do.
Schools present their contracts and won’t adapt them to match what your employer wants. We are doing them a service, yet they dictate how we do it. My practice no longer takes students from certain schools, simply because we do not agree with their contracts. These poor students are thrown out without a life raft to find their preceptors! Aren’t their schools getting paid to do something?
Carol Glascock, WHNP-BC
Columbia, MO
Continue for Teaching & Precepting: Two Sides of the Same Coin >>
Teaching & Precepting: Two Sides of the Same Coin
I am a 64-year-old NP who has been precepting in Montana for the past four years. The students I precept are responsible for finding their own preceptors, just as I was 20+ years ago. However, preceptors are hard to find here, as the population is widely scattered; this places an emotional burden on students. They cannot be picky in choosing where they go. Thus, students may not be familiar with the preceptor’s practice or ability to teach.
The students I precept are in doctorate programs. My experience has shown that these students have very little understanding of practical application and instead have an overabundance of theoretical knowledge that does not always apply to seeing and treating patients. I believe that this, and the suggested “lack of preparedness,” is the fault of the program—not of the student.
Regardless of program faults, students are looking to learn from our experience. Teaching is part of being a preceptor; if you do not want to teach, being a preceptor is not for you. If you want to share your experience and knowledge with those following you (mindful that they may treat you in the future), precepting is an enjoyable experience. But—a good practitioner does not always make a good teacher.
Before becoming a preceptor, you must consider your time constraints, as well as your staff’s. You also must consider how your patients will react to seeing a student in your place.
Preceptors need to have a relationship with the student’s university apart from signing a paper saying they, the NP, will be the student’s preceptor. The university needs to be more proactive, as medical schools are, when finding preceptors willing to take students.
Compensation is another consideration that is rarely mentioned or discussed. Compensation would eliminate some of the negative reactions and might get more preceptors to sign on.
Harold W. Bruce, MSN, FNP-BC
Butte, MT
Continue for Collision of Causes for Precepting Hurdles >>
Collision of Causes for Precepting Hurdles
I am a family NP practicing in a large internal medicine practice owned by a university-based health care system. I precept NP students because I feel an obligation to my profession. However, the stress and additional workload that precepting places on me will probably lead me to stop sooner than I would like.
The inability to locate enough quality preceptors is a multifaceted issue. Too many students in too many programs, as mentioned in the editorial, is one contributing problem. I have been told by nursing professors that universities profit from their NP programs. They have an incentive to admit a large quantity of students and push them through. We could learn from our MD colleagues, who recognize the value of limiting student numbers.
The rise in NP students has led to a high number of poorly prepared students who enter their programs with no experience as RNs. Preceptors should not teach the basics, and professors should not expect preceptors to do so. Likewise, professors should not expect employers to fill in the gaps for new NPs they hire.
Many NP students have no “real-life” clinical experience to supplement their knowledge and skills. A strong foundation that combines nursing and medical knowledge, clinical experiences, basic assessment skills, and an understanding of human nature and human responses is crucial to being a successful NP. The latter is only developed through experience with patients. Students cannot develop these skills when their professors push them to immediately enroll in NP or DNP programs upon graduation from their BSN or basic non-NP MSN programs.
Our programs would do well to provide all the didactic classroom hours prior to the start of clinical rotations. Thus, the limited clinical hours can be used to hone clinical skills, instead of the current practice of students learning basics while also trying to incorporate knowledge with practice. It is a disservice to our NP students not to have completed classroom learning before starting their limited clinical rotations.
Preceptor overload and “burnout” occurs when very busy NPs are expected to fit precepting into their usual clinical sessions. There are strict mandates that dictate the number of residents a physician can precept. Those rules also allot physicians time reserved just for precepting. Why are NPs expected to precept during their already overworked day? Why haven’t our Boards of Nursing and nursing educators demanded this?
Precepting puts us behind during our clinical sessions. In some cases, it can impact our relative value units or patient numbers and salaries. We are teaching on our own time, with no incentives or monetary gain, yet we are expected to devote time and resources to our students.
Most of us do not receive merit-based financial rewards for the extra work. When did it become wrong to expect to be paid for our work? No other profession has this sense of guilt or self-recrimination when asking to be paid for services.
Preceptor training is another issue. Unlike physicians, we are not acculturated in the “see one, do one, teach one” manner. In nursing, we are trained that we must be taught, observed, and tested before being allowed to do anything new. We have a need to be taught everything, including how to precept. That being said, precepting is both an art and a science that involves grasping the basic tenets of learning and mentoring. These are skills that should be taught through observation or in classes so that we can pass on our knowledge. If our NP programs were longer and more step-by-step—in terms of first acquiring knowledge, then incorporating clinical skills with practice—we might learn the skills of teaching and mentoring without feeling we need additional “education” in precepting.
I have been in nursing for more than 40 years and love my profession. There are challenges ahead of us that we can only meet if we are brave enough to look clearly at the way we teach younger nurses, create improved ways of teaching those who will replace us, and actually recognize the value and efforts of those we ask to precept the next generation.
Theresa Dippolito, MSN, NP-C, CRNP, APN, CCM
Levittown, PA
Continue for Raising the Bar >>
Raising the Bar
I no longer want to be involved in precepting. I, too, find the students to be poorly prepared, and I was flabbergasted when I read a recent post on Facebook—a student offered to pay her preceptor to sign off on her clinicals!
I graduated from an FNP program in 1998 and also felt unprepared at first. My class thought like nurses, in that we expected things to be presented to us. Very few of us were aware that we should prepare ourselves, and the program I went through did nothing to inform us of this. It was a rude awakening.
NP programs should have improved since then, but they certainly have not. I have precepted multiple students who did not know how to do a proper physical exam, despite having passed their related courses. I have also precepted students who thought they knew everything and felt I should let them practice solo. Sadly, the majority were simultaneously in both groups.
There is still the stigma that we should remain within a nursing philosophy when we practice, when the reality is that we practice side by side with the doctors. We need to think critically, as they do, and have our programs teach such thinking via competent instructors.
My suggestions include a competency exam for NP instructors so that we can assure a higher, more standardized level of teaching. There should also be a prep course for potential NP students on how to think, including an explanation that it will be their responsibility to go after knowledge as well. Finally, we need to stray from the nursing philosophy-type teaching in NP programs and instead focus on stronger clinical knowledge and competence.
Nikki Knight, MSN, FNP-C
San Francisco, CA
But First, a Word About Vaping …
As advocates for tobacco control, my colleagues and I took great interest in Randy D. Danielsen’s editorial, “Vaping: Are Its ‘Benefits’ a Lot of Hot Air?” (Clinician Reviews. 2016;26[6]:15-16). Our practice offers evidence-based cessation treatment for individuals with nicotine addiction through counseling, pharmacotherapy, and the use of nicotine replacement products.
At our center, we often interact with clients who have had multiple quit attempts. Many of our clients state that they have been unsuccessful using an e-cigarette as a smoking cessation strategy. More often than not, they report smoking a cigarette “here and there” along with “vaping,” until they eventually relapse to their usual smoking pattern. Some report that they smoke even more than before they tried to quit. We have concerns about how vaping may renormalize the behaviors associated with smoking. Our clients say that when they vape, it reminds them of the “social” aspects of smoking— “being part of a group” and participating in an activity that keeps their hands busy.
Recent literature suggests that curiosity is the primary reason adolescents engage in e-cigarette use. While the newly implemented FDA regulations on e-cigarettes may keep these products out of the hands of some adolescents by prohibiting sales to those younger than 18, there is much more to consider. Along with exposure to nicotine, these devices offer a variety of kid-friendly flavorings that make these products attractive to middle and high school youth. Flavorings will not be regulated at this point in time.
According to researchers, this is a major concern. Findings from studies report that when inhaled, certain flavors are more harmful than others. For example, very high—even toxic—levels of benzaldehyde are inhaled by the user when cherry-flavored e-liquid is heated at high temperatures. The chemical diacetyl, a respiratory irritant known to be associated with bronchiolitis obliterans (popcorn lung), is produced by the aerosol vapors from buttered popcorn and certain fruit-flavored e-cigarette liquids.
As public health advocates, we must provide research to the FDA about the health hazards of the flavoring added to e-cigarettes and continue to fight for this regulation. We must support evidence-based tobacco control interventions, such as hard-hitting media campaigns and tobacco excise taxes, and promote access to cessation treatment, smoke-free policies, and statewide funding. Elimination of tobacco products will reduce the public health burden of tobacco-related illness.
Andrea Spatarella, DNP, RN, FNP-BC, Christine Fardellone, DNP, RN, Raisa Abramova, FNP-BC, RN
Great Neck, NY
Continue for Precepting & E-Quality of Care >>
Precepting & E-Quality of Care
As a woman of the baby-boomer generation, I was raised in an era when feminism was a focus for many. There was a great deal being written and discussed to encourage women to attain equal pay for equal work. Because nursing was (and still is) a profession dominated by women, this was a frequent topic in the classroom. We were repeatedly told, “Don’t give away your knowledge for free” and “You deserve to be paid what you’re worth, don’t discount yourself.”
I find it very telling that the same female-dominated academic programs that encouraged me to seek proper payment are now taking advantage of my free labor. I am somewhat offended by this attitude and consider it a step backward. Each time NPs are guilted or browbeaten into teaching without proper compensation, the profession is devalued. To continue to participate is to enable a problematic, if not broken, system.
NP education is in need of major reform. The precepting issue is the weak link in becoming a qualified professional who is able to meet the demands and responsibilities that academics and politicos are pushing harder and harder for. Our physician and PA colleagues can rightly argue that their clinical education is superior to ours—and I cannot fault our colleagues for expressing concern about quality of care. If nursing really wants an equal place at the table, this weakness must be improved, or the naysayers will have plenty of evidence that they were correct in the years to come.
Rebecca Shively, MSN, RN, FNP-BC
San Marcos, TX
Continue for NP Schools & Their Rigid Rules >>
NP Schools & Their Rigid Rules
I have been a preceptor for at least a dozen NP students and have yet to be offered compensation. Preceptors take the place of a paid instructor, giving away free advice and experiences. I don’t mind doing this, but at times it can be a struggle. Some students, for example, have never done a pelvic exam. Letting an inexperienced NP student practice a pelvic exam on a patient who made an appointment to see an experienced provider is unjust and unfair to the patient—I won’t do it. These schools need to provide practice sessions on paid patients so their students can learn these skills.
I have my beef with the institutes of higher learning, not the students. It feels like a one-way street. You fill out the forms they require in order to precept, which takes up valuable work time. You equip their students with the skills they need to practice safely and correctly, and then try to fill out their evaluation sheets on things that students are not licensed to do.
Schools present their contracts and won’t adapt them to match what your employer wants. We are doing them a service, yet they dictate how we do it. My practice no longer takes students from certain schools, simply because we do not agree with their contracts. These poor students are thrown out without a life raft to find their preceptors! Aren’t their schools getting paid to do something?
Carol Glascock, WHNP-BC
Columbia, MO
Continue for Teaching & Precepting: Two Sides of the Same Coin >>
Teaching & Precepting: Two Sides of the Same Coin
I am a 64-year-old NP who has been precepting in Montana for the past four years. The students I precept are responsible for finding their own preceptors, just as I was 20+ years ago. However, preceptors are hard to find here, as the population is widely scattered; this places an emotional burden on students. They cannot be picky in choosing where they go. Thus, students may not be familiar with the preceptor’s practice or ability to teach.
The students I precept are in doctorate programs. My experience has shown that these students have very little understanding of practical application and instead have an overabundance of theoretical knowledge that does not always apply to seeing and treating patients. I believe that this, and the suggested “lack of preparedness,” is the fault of the program—not of the student.
Regardless of program faults, students are looking to learn from our experience. Teaching is part of being a preceptor; if you do not want to teach, being a preceptor is not for you. If you want to share your experience and knowledge with those following you (mindful that they may treat you in the future), precepting is an enjoyable experience. But—a good practitioner does not always make a good teacher.
Before becoming a preceptor, you must consider your time constraints, as well as your staff’s. You also must consider how your patients will react to seeing a student in your place.
Preceptors need to have a relationship with the student’s university apart from signing a paper saying they, the NP, will be the student’s preceptor. The university needs to be more proactive, as medical schools are, when finding preceptors willing to take students.
Compensation is another consideration that is rarely mentioned or discussed. Compensation would eliminate some of the negative reactions and might get more preceptors to sign on.
Harold W. Bruce, MSN, FNP-BC
Butte, MT
Continue for Collision of Causes for Precepting Hurdles >>
Collision of Causes for Precepting Hurdles
I am a family NP practicing in a large internal medicine practice owned by a university-based health care system. I precept NP students because I feel an obligation to my profession. However, the stress and additional workload that precepting places on me will probably lead me to stop sooner than I would like.
The inability to locate enough quality preceptors is a multifaceted issue. Too many students in too many programs, as mentioned in the editorial, is one contributing problem. I have been told by nursing professors that universities profit from their NP programs. They have an incentive to admit a large quantity of students and push them through. We could learn from our MD colleagues, who recognize the value of limiting student numbers.
The rise in NP students has led to a high number of poorly prepared students who enter their programs with no experience as RNs. Preceptors should not teach the basics, and professors should not expect preceptors to do so. Likewise, professors should not expect employers to fill in the gaps for new NPs they hire.
Many NP students have no “real-life” clinical experience to supplement their knowledge and skills. A strong foundation that combines nursing and medical knowledge, clinical experiences, basic assessment skills, and an understanding of human nature and human responses is crucial to being a successful NP. The latter is only developed through experience with patients. Students cannot develop these skills when their professors push them to immediately enroll in NP or DNP programs upon graduation from their BSN or basic non-NP MSN programs.
Our programs would do well to provide all the didactic classroom hours prior to the start of clinical rotations. Thus, the limited clinical hours can be used to hone clinical skills, instead of the current practice of students learning basics while also trying to incorporate knowledge with practice. It is a disservice to our NP students not to have completed classroom learning before starting their limited clinical rotations.
Preceptor overload and “burnout” occurs when very busy NPs are expected to fit precepting into their usual clinical sessions. There are strict mandates that dictate the number of residents a physician can precept. Those rules also allot physicians time reserved just for precepting. Why are NPs expected to precept during their already overworked day? Why haven’t our Boards of Nursing and nursing educators demanded this?
Precepting puts us behind during our clinical sessions. In some cases, it can impact our relative value units or patient numbers and salaries. We are teaching on our own time, with no incentives or monetary gain, yet we are expected to devote time and resources to our students.
Most of us do not receive merit-based financial rewards for the extra work. When did it become wrong to expect to be paid for our work? No other profession has this sense of guilt or self-recrimination when asking to be paid for services.
Preceptor training is another issue. Unlike physicians, we are not acculturated in the “see one, do one, teach one” manner. In nursing, we are trained that we must be taught, observed, and tested before being allowed to do anything new. We have a need to be taught everything, including how to precept. That being said, precepting is both an art and a science that involves grasping the basic tenets of learning and mentoring. These are skills that should be taught through observation or in classes so that we can pass on our knowledge. If our NP programs were longer and more step-by-step—in terms of first acquiring knowledge, then incorporating clinical skills with practice—we might learn the skills of teaching and mentoring without feeling we need additional “education” in precepting.
I have been in nursing for more than 40 years and love my profession. There are challenges ahead of us that we can only meet if we are brave enough to look clearly at the way we teach younger nurses, create improved ways of teaching those who will replace us, and actually recognize the value and efforts of those we ask to precept the next generation.
Theresa Dippolito, MSN, NP-C, CRNP, APN, CCM
Levittown, PA
Continue for Raising the Bar >>
Raising the Bar
I no longer want to be involved in precepting. I, too, find the students to be poorly prepared, and I was flabbergasted when I read a recent post on Facebook—a student offered to pay her preceptor to sign off on her clinicals!
I graduated from an FNP program in 1998 and also felt unprepared at first. My class thought like nurses, in that we expected things to be presented to us. Very few of us were aware that we should prepare ourselves, and the program I went through did nothing to inform us of this. It was a rude awakening.
NP programs should have improved since then, but they certainly have not. I have precepted multiple students who did not know how to do a proper physical exam, despite having passed their related courses. I have also precepted students who thought they knew everything and felt I should let them practice solo. Sadly, the majority were simultaneously in both groups.
There is still the stigma that we should remain within a nursing philosophy when we practice, when the reality is that we practice side by side with the doctors. We need to think critically, as they do, and have our programs teach such thinking via competent instructors.
My suggestions include a competency exam for NP instructors so that we can assure a higher, more standardized level of teaching. There should also be a prep course for potential NP students on how to think, including an explanation that it will be their responsibility to go after knowledge as well. Finally, we need to stray from the nursing philosophy-type teaching in NP programs and instead focus on stronger clinical knowledge and competence.
Nikki Knight, MSN, FNP-C
San Francisco, CA
But First, a Word About Vaping …
As advocates for tobacco control, my colleagues and I took great interest in Randy D. Danielsen’s editorial, “Vaping: Are Its ‘Benefits’ a Lot of Hot Air?” (Clinician Reviews. 2016;26[6]:15-16). Our practice offers evidence-based cessation treatment for individuals with nicotine addiction through counseling, pharmacotherapy, and the use of nicotine replacement products.
At our center, we often interact with clients who have had multiple quit attempts. Many of our clients state that they have been unsuccessful using an e-cigarette as a smoking cessation strategy. More often than not, they report smoking a cigarette “here and there” along with “vaping,” until they eventually relapse to their usual smoking pattern. Some report that they smoke even more than before they tried to quit. We have concerns about how vaping may renormalize the behaviors associated with smoking. Our clients say that when they vape, it reminds them of the “social” aspects of smoking— “being part of a group” and participating in an activity that keeps their hands busy.
Recent literature suggests that curiosity is the primary reason adolescents engage in e-cigarette use. While the newly implemented FDA regulations on e-cigarettes may keep these products out of the hands of some adolescents by prohibiting sales to those younger than 18, there is much more to consider. Along with exposure to nicotine, these devices offer a variety of kid-friendly flavorings that make these products attractive to middle and high school youth. Flavorings will not be regulated at this point in time.
According to researchers, this is a major concern. Findings from studies report that when inhaled, certain flavors are more harmful than others. For example, very high—even toxic—levels of benzaldehyde are inhaled by the user when cherry-flavored e-liquid is heated at high temperatures. The chemical diacetyl, a respiratory irritant known to be associated with bronchiolitis obliterans (popcorn lung), is produced by the aerosol vapors from buttered popcorn and certain fruit-flavored e-cigarette liquids.
As public health advocates, we must provide research to the FDA about the health hazards of the flavoring added to e-cigarettes and continue to fight for this regulation. We must support evidence-based tobacco control interventions, such as hard-hitting media campaigns and tobacco excise taxes, and promote access to cessation treatment, smoke-free policies, and statewide funding. Elimination of tobacco products will reduce the public health burden of tobacco-related illness.
Andrea Spatarella, DNP, RN, FNP-BC, Christine Fardellone, DNP, RN, Raisa Abramova, FNP-BC, RN
Great Neck, NY
Continue for Precepting & E-Quality of Care >>
Precepting & E-Quality of Care
As a woman of the baby-boomer generation, I was raised in an era when feminism was a focus for many. There was a great deal being written and discussed to encourage women to attain equal pay for equal work. Because nursing was (and still is) a profession dominated by women, this was a frequent topic in the classroom. We were repeatedly told, “Don’t give away your knowledge for free” and “You deserve to be paid what you’re worth, don’t discount yourself.”
I find it very telling that the same female-dominated academic programs that encouraged me to seek proper payment are now taking advantage of my free labor. I am somewhat offended by this attitude and consider it a step backward. Each time NPs are guilted or browbeaten into teaching without proper compensation, the profession is devalued. To continue to participate is to enable a problematic, if not broken, system.
NP education is in need of major reform. The precepting issue is the weak link in becoming a qualified professional who is able to meet the demands and responsibilities that academics and politicos are pushing harder and harder for. Our physician and PA colleagues can rightly argue that their clinical education is superior to ours—and I cannot fault our colleagues for expressing concern about quality of care. If nursing really wants an equal place at the table, this weakness must be improved, or the naysayers will have plenty of evidence that they were correct in the years to come.
Rebecca Shively, MSN, RN, FNP-BC
San Marcos, TX
Continue for NP Schools & Their Rigid Rules >>
NP Schools & Their Rigid Rules
I have been a preceptor for at least a dozen NP students and have yet to be offered compensation. Preceptors take the place of a paid instructor, giving away free advice and experiences. I don’t mind doing this, but at times it can be a struggle. Some students, for example, have never done a pelvic exam. Letting an inexperienced NP student practice a pelvic exam on a patient who made an appointment to see an experienced provider is unjust and unfair to the patient—I won’t do it. These schools need to provide practice sessions on paid patients so their students can learn these skills.
I have my beef with the institutes of higher learning, not the students. It feels like a one-way street. You fill out the forms they require in order to precept, which takes up valuable work time. You equip their students with the skills they need to practice safely and correctly, and then try to fill out their evaluation sheets on things that students are not licensed to do.
Schools present their contracts and won’t adapt them to match what your employer wants. We are doing them a service, yet they dictate how we do it. My practice no longer takes students from certain schools, simply because we do not agree with their contracts. These poor students are thrown out without a life raft to find their preceptors! Aren’t their schools getting paid to do something?
Carol Glascock, WHNP-BC
Columbia, MO
Continue for Teaching & Precepting: Two Sides of the Same Coin >>
Teaching & Precepting: Two Sides of the Same Coin
I am a 64-year-old NP who has been precepting in Montana for the past four years. The students I precept are responsible for finding their own preceptors, just as I was 20+ years ago. However, preceptors are hard to find here, as the population is widely scattered; this places an emotional burden on students. They cannot be picky in choosing where they go. Thus, students may not be familiar with the preceptor’s practice or ability to teach.
The students I precept are in doctorate programs. My experience has shown that these students have very little understanding of practical application and instead have an overabundance of theoretical knowledge that does not always apply to seeing and treating patients. I believe that this, and the suggested “lack of preparedness,” is the fault of the program—not of the student.
Regardless of program faults, students are looking to learn from our experience. Teaching is part of being a preceptor; if you do not want to teach, being a preceptor is not for you. If you want to share your experience and knowledge with those following you (mindful that they may treat you in the future), precepting is an enjoyable experience. But—a good practitioner does not always make a good teacher.
Before becoming a preceptor, you must consider your time constraints, as well as your staff’s. You also must consider how your patients will react to seeing a student in your place.
Preceptors need to have a relationship with the student’s university apart from signing a paper saying they, the NP, will be the student’s preceptor. The university needs to be more proactive, as medical schools are, when finding preceptors willing to take students.
Compensation is another consideration that is rarely mentioned or discussed. Compensation would eliminate some of the negative reactions and might get more preceptors to sign on.
Harold W. Bruce, MSN, FNP-BC
Butte, MT
Continue for Collision of Causes for Precepting Hurdles >>
Collision of Causes for Precepting Hurdles
I am a family NP practicing in a large internal medicine practice owned by a university-based health care system. I precept NP students because I feel an obligation to my profession. However, the stress and additional workload that precepting places on me will probably lead me to stop sooner than I would like.
The inability to locate enough quality preceptors is a multifaceted issue. Too many students in too many programs, as mentioned in the editorial, is one contributing problem. I have been told by nursing professors that universities profit from their NP programs. They have an incentive to admit a large quantity of students and push them through. We could learn from our MD colleagues, who recognize the value of limiting student numbers.
The rise in NP students has led to a high number of poorly prepared students who enter their programs with no experience as RNs. Preceptors should not teach the basics, and professors should not expect preceptors to do so. Likewise, professors should not expect employers to fill in the gaps for new NPs they hire.
Many NP students have no “real-life” clinical experience to supplement their knowledge and skills. A strong foundation that combines nursing and medical knowledge, clinical experiences, basic assessment skills, and an understanding of human nature and human responses is crucial to being a successful NP. The latter is only developed through experience with patients. Students cannot develop these skills when their professors push them to immediately enroll in NP or DNP programs upon graduation from their BSN or basic non-NP MSN programs.
Our programs would do well to provide all the didactic classroom hours prior to the start of clinical rotations. Thus, the limited clinical hours can be used to hone clinical skills, instead of the current practice of students learning basics while also trying to incorporate knowledge with practice. It is a disservice to our NP students not to have completed classroom learning before starting their limited clinical rotations.
Preceptor overload and “burnout” occurs when very busy NPs are expected to fit precepting into their usual clinical sessions. There are strict mandates that dictate the number of residents a physician can precept. Those rules also allot physicians time reserved just for precepting. Why are NPs expected to precept during their already overworked day? Why haven’t our Boards of Nursing and nursing educators demanded this?
Precepting puts us behind during our clinical sessions. In some cases, it can impact our relative value units or patient numbers and salaries. We are teaching on our own time, with no incentives or monetary gain, yet we are expected to devote time and resources to our students.
Most of us do not receive merit-based financial rewards for the extra work. When did it become wrong to expect to be paid for our work? No other profession has this sense of guilt or self-recrimination when asking to be paid for services.
Preceptor training is another issue. Unlike physicians, we are not acculturated in the “see one, do one, teach one” manner. In nursing, we are trained that we must be taught, observed, and tested before being allowed to do anything new. We have a need to be taught everything, including how to precept. That being said, precepting is both an art and a science that involves grasping the basic tenets of learning and mentoring. These are skills that should be taught through observation or in classes so that we can pass on our knowledge. If our NP programs were longer and more step-by-step—in terms of first acquiring knowledge, then incorporating clinical skills with practice—we might learn the skills of teaching and mentoring without feeling we need additional “education” in precepting.
I have been in nursing for more than 40 years and love my profession. There are challenges ahead of us that we can only meet if we are brave enough to look clearly at the way we teach younger nurses, create improved ways of teaching those who will replace us, and actually recognize the value and efforts of those we ask to precept the next generation.
Theresa Dippolito, MSN, NP-C, CRNP, APN, CCM
Levittown, PA
Continue for Raising the Bar >>
Raising the Bar
I no longer want to be involved in precepting. I, too, find the students to be poorly prepared, and I was flabbergasted when I read a recent post on Facebook—a student offered to pay her preceptor to sign off on her clinicals!
I graduated from an FNP program in 1998 and also felt unprepared at first. My class thought like nurses, in that we expected things to be presented to us. Very few of us were aware that we should prepare ourselves, and the program I went through did nothing to inform us of this. It was a rude awakening.
NP programs should have improved since then, but they certainly have not. I have precepted multiple students who did not know how to do a proper physical exam, despite having passed their related courses. I have also precepted students who thought they knew everything and felt I should let them practice solo. Sadly, the majority were simultaneously in both groups.
There is still the stigma that we should remain within a nursing philosophy when we practice, when the reality is that we practice side by side with the doctors. We need to think critically, as they do, and have our programs teach such thinking via competent instructors.
My suggestions include a competency exam for NP instructors so that we can assure a higher, more standardized level of teaching. There should also be a prep course for potential NP students on how to think, including an explanation that it will be their responsibility to go after knowledge as well. Finally, we need to stray from the nursing philosophy-type teaching in NP programs and instead focus on stronger clinical knowledge and competence.
Nikki Knight, MSN, FNP-C
San Francisco, CA
Shortness of breath: Looking beyond the usual suspects
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.
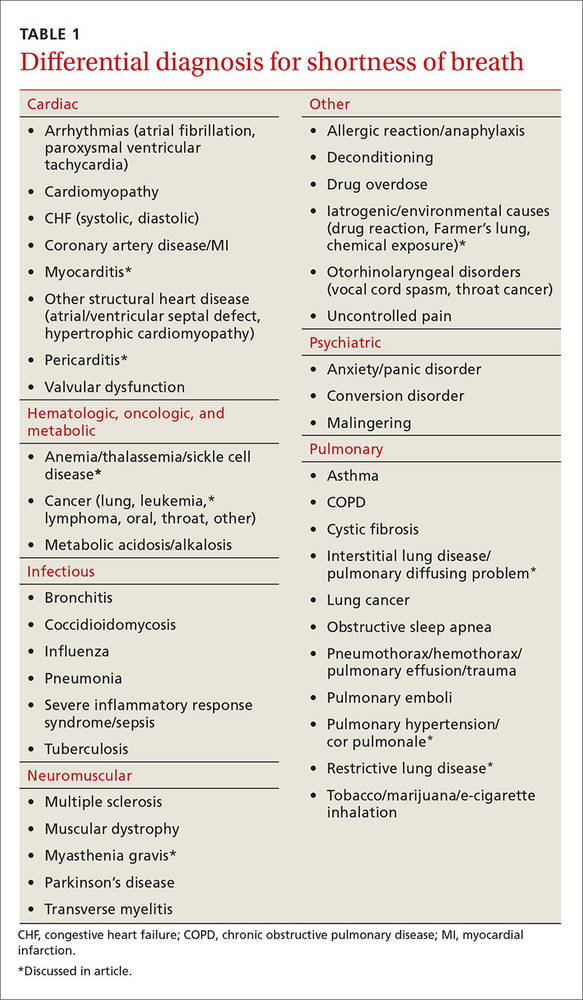
Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.

Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.

Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
Does oseltamivir shorten flu symptom duration?
Yes. Treatment of influenza virus infection with oral oseltamivir reduces time to alleviation of symptoms in adults and children by approximately one day compared with placebo. It reduces symptom duration even when initiated more than 2 days after symptom onset (strength of recommendation: A, systematic review of randomized controlled trials [RCTs], meta-analysis of observation trials, RCT).
Evidence summary
A 2014 systematic review included 8 RCTs in adults (3954 patients) and one RCT in children (669 patients) with influenza and compared time to alleviation of symptoms with oseltamivir and placebo.1 Symptoms were defined as local (nasal discharge, dry cough, sore throat) and systemic (fever, myalgia, headache, fatigue). Methodology for diagnosis varied by trial.
Oral oseltamivir (75, 150, or 300 mg for 5 to 10 days) reduced time to first alleviation of symptoms by 17 hours (95% confidence interval [CI], 8.4-25 hours) compared with placebo for adults and 29 hours (95% CI, 12-47 hours) for the otherwise healthy children.
The systematic review also included 2 RCTs involving 660 children with chronic asthma who received treatment with oseltamivir. Researchers found no reduction in time to symptom alleviation with the oseltamivir.
Treatment with oseltamivir increased the risk of nausea (number needed to harm [NNH]=28) and vomiting (NNH=22) in adults and the risk of vomiting (NNH=19) in children. Sources of bias included industry sponsorship of all trials, differing placebo components, inadequate recruitment, and use of other medication.
Shorter fever duration?
A 2012 meta-analysis of 6 observational studies (5842 patients) compared the effect of oral oseltamivir with no treatment on duration of signs and symptoms (definition not given) in patients with influenza (method of diagnosis not stated).2 Oseltamivir reduced fever duration by 33 hours (95% CI, 21-45 hours) compared with no treatment.
The authors describe the evidence as being of very low quality because of study heterogeneity, lack of control for confounding variables, selection bias, and study sources (many unpublished industry studies).
There’s benefit even with late Tx
A 2014 double-blind RCT, not included in the previously described reviews, of 130 adults and 1070 children with a positive rapid influenza test examined the effect of oseltamivir and placebo on symptom duration.3 Research assistants visited participants at home each day until patients were asymptomatic for 7 consecutive days.
Treatment with oseltamivir reduced symptom duration by a median of one day compared with no treatment (hazard ratio=0.87; 95% CI, 0.79-0.95). This benefit was observed regardless of whether treatment was initiated fewer or more than 48 hours after symptom onset. One notable limitation was failure to control for paracetamol (acetaminophen) usage, a possible confounder for duration of symptoms, such as fever.
1. Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;(4):CD008965.
2. Hsu J, Santesso N, Mustafa R, et al. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med. 2012;156:512-524.
3. Fry AM, Goswami D, Nahar K, et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014;14:109-118.
Yes. Treatment of influenza virus infection with oral oseltamivir reduces time to alleviation of symptoms in adults and children by approximately one day compared with placebo. It reduces symptom duration even when initiated more than 2 days after symptom onset (strength of recommendation: A, systematic review of randomized controlled trials [RCTs], meta-analysis of observation trials, RCT).
Evidence summary
A 2014 systematic review included 8 RCTs in adults (3954 patients) and one RCT in children (669 patients) with influenza and compared time to alleviation of symptoms with oseltamivir and placebo.1 Symptoms were defined as local (nasal discharge, dry cough, sore throat) and systemic (fever, myalgia, headache, fatigue). Methodology for diagnosis varied by trial.
Oral oseltamivir (75, 150, or 300 mg for 5 to 10 days) reduced time to first alleviation of symptoms by 17 hours (95% confidence interval [CI], 8.4-25 hours) compared with placebo for adults and 29 hours (95% CI, 12-47 hours) for the otherwise healthy children.
The systematic review also included 2 RCTs involving 660 children with chronic asthma who received treatment with oseltamivir. Researchers found no reduction in time to symptom alleviation with the oseltamivir.
Treatment with oseltamivir increased the risk of nausea (number needed to harm [NNH]=28) and vomiting (NNH=22) in adults and the risk of vomiting (NNH=19) in children. Sources of bias included industry sponsorship of all trials, differing placebo components, inadequate recruitment, and use of other medication.
Shorter fever duration?
A 2012 meta-analysis of 6 observational studies (5842 patients) compared the effect of oral oseltamivir with no treatment on duration of signs and symptoms (definition not given) in patients with influenza (method of diagnosis not stated).2 Oseltamivir reduced fever duration by 33 hours (95% CI, 21-45 hours) compared with no treatment.
The authors describe the evidence as being of very low quality because of study heterogeneity, lack of control for confounding variables, selection bias, and study sources (many unpublished industry studies).
There’s benefit even with late Tx
A 2014 double-blind RCT, not included in the previously described reviews, of 130 adults and 1070 children with a positive rapid influenza test examined the effect of oseltamivir and placebo on symptom duration.3 Research assistants visited participants at home each day until patients were asymptomatic for 7 consecutive days.
Treatment with oseltamivir reduced symptom duration by a median of one day compared with no treatment (hazard ratio=0.87; 95% CI, 0.79-0.95). This benefit was observed regardless of whether treatment was initiated fewer or more than 48 hours after symptom onset. One notable limitation was failure to control for paracetamol (acetaminophen) usage, a possible confounder for duration of symptoms, such as fever.
Yes. Treatment of influenza virus infection with oral oseltamivir reduces time to alleviation of symptoms in adults and children by approximately one day compared with placebo. It reduces symptom duration even when initiated more than 2 days after symptom onset (strength of recommendation: A, systematic review of randomized controlled trials [RCTs], meta-analysis of observation trials, RCT).
Evidence summary
A 2014 systematic review included 8 RCTs in adults (3954 patients) and one RCT in children (669 patients) with influenza and compared time to alleviation of symptoms with oseltamivir and placebo.1 Symptoms were defined as local (nasal discharge, dry cough, sore throat) and systemic (fever, myalgia, headache, fatigue). Methodology for diagnosis varied by trial.
Oral oseltamivir (75, 150, or 300 mg for 5 to 10 days) reduced time to first alleviation of symptoms by 17 hours (95% confidence interval [CI], 8.4-25 hours) compared with placebo for adults and 29 hours (95% CI, 12-47 hours) for the otherwise healthy children.
The systematic review also included 2 RCTs involving 660 children with chronic asthma who received treatment with oseltamivir. Researchers found no reduction in time to symptom alleviation with the oseltamivir.
Treatment with oseltamivir increased the risk of nausea (number needed to harm [NNH]=28) and vomiting (NNH=22) in adults and the risk of vomiting (NNH=19) in children. Sources of bias included industry sponsorship of all trials, differing placebo components, inadequate recruitment, and use of other medication.
Shorter fever duration?
A 2012 meta-analysis of 6 observational studies (5842 patients) compared the effect of oral oseltamivir with no treatment on duration of signs and symptoms (definition not given) in patients with influenza (method of diagnosis not stated).2 Oseltamivir reduced fever duration by 33 hours (95% CI, 21-45 hours) compared with no treatment.
The authors describe the evidence as being of very low quality because of study heterogeneity, lack of control for confounding variables, selection bias, and study sources (many unpublished industry studies).
There’s benefit even with late Tx
A 2014 double-blind RCT, not included in the previously described reviews, of 130 adults and 1070 children with a positive rapid influenza test examined the effect of oseltamivir and placebo on symptom duration.3 Research assistants visited participants at home each day until patients were asymptomatic for 7 consecutive days.
Treatment with oseltamivir reduced symptom duration by a median of one day compared with no treatment (hazard ratio=0.87; 95% CI, 0.79-0.95). This benefit was observed regardless of whether treatment was initiated fewer or more than 48 hours after symptom onset. One notable limitation was failure to control for paracetamol (acetaminophen) usage, a possible confounder for duration of symptoms, such as fever.
1. Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;(4):CD008965.
2. Hsu J, Santesso N, Mustafa R, et al. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med. 2012;156:512-524.
3. Fry AM, Goswami D, Nahar K, et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014;14:109-118.
1. Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;(4):CD008965.
2. Hsu J, Santesso N, Mustafa R, et al. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med. 2012;156:512-524.
3. Fry AM, Goswami D, Nahar K, et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014;14:109-118.
Evidence-based answers from the Family Physicians Inquiries Network
Practical “pearls” to help improve your care
Although we reserve the term “PURL” for our popular feature, Priority Updates from the Research Literature, I’m proud to comment on the collection of articles in this issue of JFP, each of which contains important “pearls” of information for family physicians and other primary care clinicians.
Managing sport-related concussion. Revelations about serious head injuries in the National Football League have catalyzed important research regarding the management of sports-related head injuries, and the evidence for diagnosis and treatment is evolving. The article in this issue by Dr. Sprouse and colleagues provides some of the latest information regarding brain changes after concussion straight from the American Academy of Neurology’s 2016 Sports Concussion Conference held in Chicago in July, as well as valuable return-to-play recommendations.
Family medicine ultrasound. Because of advances in technology and reductions in the cost of portable machines, ultrasound use is rapidly moving into family medicine offices. Drs. Steinmetz and Oleskevich provide a no-nonsense review of the current uses of ultrasound in family medicine, leading me to wonder whether ultrasound might become the stethoscope of the future.
Shortness of breath. Although the diagnosis of shortness of breath is straightforward in many cases, misdiagnosis is not uncommon. Recently, I cared for a new patient who was diagnosed with asthma 15 years ago. Because of fine rales on exam, I suspected the patient’s diagnosis was incorrect. Indeed, he had pulmonary fibrosis, not asthma, and he is doing fine now without his asthma inhalers. Dr. Taggart outlines a thoughtful approach to the evaluation of shortness of breath, one that alerts you to when to suspect something beyond the usual culprits.
Cervical cancer screening. The days of yearly Pap smears for all women are over. Combined screening with cytology and human papillomavirus testing is now recommended at 5-year intervals for women 30 to 65 years of age who are at low risk for cervical cancer. In addition, Dr. Hofmeister reviews recent randomized trials that suggest HPV screening alone may be sufficient for low-risk women.
On-demand HIV prophylaxis. Our PURL for the month discusses an effective prevention strategy—other than condoms—that can be used as needed by people at high risk for human immunodeficiency virus.
We hope you enjoy this PURL—and the other “pearls”—this month. As diagnosis and treatments evolve, JFP will continue to bring you the information you need to provide the best possible care for your patients.
Although we reserve the term “PURL” for our popular feature, Priority Updates from the Research Literature, I’m proud to comment on the collection of articles in this issue of JFP, each of which contains important “pearls” of information for family physicians and other primary care clinicians.
Managing sport-related concussion. Revelations about serious head injuries in the National Football League have catalyzed important research regarding the management of sports-related head injuries, and the evidence for diagnosis and treatment is evolving. The article in this issue by Dr. Sprouse and colleagues provides some of the latest information regarding brain changes after concussion straight from the American Academy of Neurology’s 2016 Sports Concussion Conference held in Chicago in July, as well as valuable return-to-play recommendations.
Family medicine ultrasound. Because of advances in technology and reductions in the cost of portable machines, ultrasound use is rapidly moving into family medicine offices. Drs. Steinmetz and Oleskevich provide a no-nonsense review of the current uses of ultrasound in family medicine, leading me to wonder whether ultrasound might become the stethoscope of the future.
Shortness of breath. Although the diagnosis of shortness of breath is straightforward in many cases, misdiagnosis is not uncommon. Recently, I cared for a new patient who was diagnosed with asthma 15 years ago. Because of fine rales on exam, I suspected the patient’s diagnosis was incorrect. Indeed, he had pulmonary fibrosis, not asthma, and he is doing fine now without his asthma inhalers. Dr. Taggart outlines a thoughtful approach to the evaluation of shortness of breath, one that alerts you to when to suspect something beyond the usual culprits.
Cervical cancer screening. The days of yearly Pap smears for all women are over. Combined screening with cytology and human papillomavirus testing is now recommended at 5-year intervals for women 30 to 65 years of age who are at low risk for cervical cancer. In addition, Dr. Hofmeister reviews recent randomized trials that suggest HPV screening alone may be sufficient for low-risk women.
On-demand HIV prophylaxis. Our PURL for the month discusses an effective prevention strategy—other than condoms—that can be used as needed by people at high risk for human immunodeficiency virus.
We hope you enjoy this PURL—and the other “pearls”—this month. As diagnosis and treatments evolve, JFP will continue to bring you the information you need to provide the best possible care for your patients.
Although we reserve the term “PURL” for our popular feature, Priority Updates from the Research Literature, I’m proud to comment on the collection of articles in this issue of JFP, each of which contains important “pearls” of information for family physicians and other primary care clinicians.
Managing sport-related concussion. Revelations about serious head injuries in the National Football League have catalyzed important research regarding the management of sports-related head injuries, and the evidence for diagnosis and treatment is evolving. The article in this issue by Dr. Sprouse and colleagues provides some of the latest information regarding brain changes after concussion straight from the American Academy of Neurology’s 2016 Sports Concussion Conference held in Chicago in July, as well as valuable return-to-play recommendations.
Family medicine ultrasound. Because of advances in technology and reductions in the cost of portable machines, ultrasound use is rapidly moving into family medicine offices. Drs. Steinmetz and Oleskevich provide a no-nonsense review of the current uses of ultrasound in family medicine, leading me to wonder whether ultrasound might become the stethoscope of the future.
Shortness of breath. Although the diagnosis of shortness of breath is straightforward in many cases, misdiagnosis is not uncommon. Recently, I cared for a new patient who was diagnosed with asthma 15 years ago. Because of fine rales on exam, I suspected the patient’s diagnosis was incorrect. Indeed, he had pulmonary fibrosis, not asthma, and he is doing fine now without his asthma inhalers. Dr. Taggart outlines a thoughtful approach to the evaluation of shortness of breath, one that alerts you to when to suspect something beyond the usual culprits.
Cervical cancer screening. The days of yearly Pap smears for all women are over. Combined screening with cytology and human papillomavirus testing is now recommended at 5-year intervals for women 30 to 65 years of age who are at low risk for cervical cancer. In addition, Dr. Hofmeister reviews recent randomized trials that suggest HPV screening alone may be sufficient for low-risk women.
On-demand HIV prophylaxis. Our PURL for the month discusses an effective prevention strategy—other than condoms—that can be used as needed by people at high risk for human immunodeficiency virus.
We hope you enjoy this PURL—and the other “pearls”—this month. As diagnosis and treatments evolve, JFP will continue to bring you the information you need to provide the best possible care for your patients.







