User login
AHA: COPD Doubles Sudden Cardiac Death Risk in Hypertensives
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
AT THE AHA SCIENTIFIC SESSIONS
Home apnea monitors—when to discontinue use
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
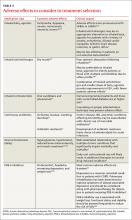
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
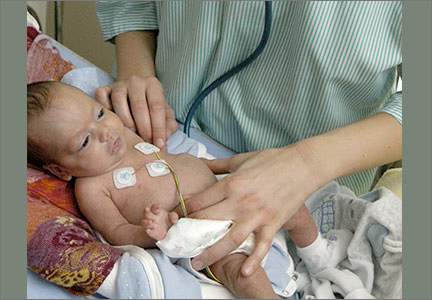
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
AHA: Spirometry Identifies Mortality Risk in Asymptomatic Adults
ORLANDO – Unselected people from the general population without clinically apparent lung disease but with low lung function had significantly increased mortality during follow-up that was independent of cardiac function, in results from more than 13,000 middle-aged Germans.
“Subtle, subclinical pulmonary impairment is a risk indicator for increased mortality independent of cardiac performance,” Dr. Christina Baum said at the American Heart Association scientific sessions.
The researchers used spirometry to measure each subject’s forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). The results showed that “spirometry is a good screening tool that is not very expensive,” making spirometry an effective risk assessment tool for use in the general adult population, said Dr. Baum of the department of general and interventional cardiology at the University Heart Center in Hamburg, Germany.
She and her associates used data collected in the Gutenberg Health Study, which enrolled more than 15,000 German women and men aged 35-74 years during 2007-2012. The investigators excluded people with a history of pulmonary disease, resulting in a study cohort of 13,191, who averaged 55 years old, with 51% men.
At enrollment into the study, all people underwent screening spirometry and echocardiography. Their average baseline FEV1 was 2.9 L and their average FVC was 3.7 L, and 4% had heart failure based on assessments of left ventricular size and function by echocardiography. The first 5,000 enrollees also had measurements taken of their serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I through use of a high-sensitivity assay. The researchers used data from patients followed for a median of 5.5 years.
During follow-up, people in the lowest tertile for FEV1 and those in the lowest tertile for FVC had higher rates of all-cause mortality, compared with those in the highest tertile for each of these two parameters.
In a multivariate analysis that adjusted for age, sex, body mass index, smoking status, hypertension, dyslipidemia, heart failure status, serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I, and other parameters, people with lower FEV1 and FVC readings had significantly worse survival, Dr. Baum said. Every 1–standard deviation increase in FEV1 was linked with a statistically significant, 38% reduced mortality rate; furthermore, a similar significant inverse association existed between FVC and mortality, she reported.
ORLANDO – Unselected people from the general population without clinically apparent lung disease but with low lung function had significantly increased mortality during follow-up that was independent of cardiac function, in results from more than 13,000 middle-aged Germans.
“Subtle, subclinical pulmonary impairment is a risk indicator for increased mortality independent of cardiac performance,” Dr. Christina Baum said at the American Heart Association scientific sessions.
The researchers used spirometry to measure each subject’s forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). The results showed that “spirometry is a good screening tool that is not very expensive,” making spirometry an effective risk assessment tool for use in the general adult population, said Dr. Baum of the department of general and interventional cardiology at the University Heart Center in Hamburg, Germany.
She and her associates used data collected in the Gutenberg Health Study, which enrolled more than 15,000 German women and men aged 35-74 years during 2007-2012. The investigators excluded people with a history of pulmonary disease, resulting in a study cohort of 13,191, who averaged 55 years old, with 51% men.
At enrollment into the study, all people underwent screening spirometry and echocardiography. Their average baseline FEV1 was 2.9 L and their average FVC was 3.7 L, and 4% had heart failure based on assessments of left ventricular size and function by echocardiography. The first 5,000 enrollees also had measurements taken of their serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I through use of a high-sensitivity assay. The researchers used data from patients followed for a median of 5.5 years.
During follow-up, people in the lowest tertile for FEV1 and those in the lowest tertile for FVC had higher rates of all-cause mortality, compared with those in the highest tertile for each of these two parameters.
In a multivariate analysis that adjusted for age, sex, body mass index, smoking status, hypertension, dyslipidemia, heart failure status, serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I, and other parameters, people with lower FEV1 and FVC readings had significantly worse survival, Dr. Baum said. Every 1–standard deviation increase in FEV1 was linked with a statistically significant, 38% reduced mortality rate; furthermore, a similar significant inverse association existed between FVC and mortality, she reported.
ORLANDO – Unselected people from the general population without clinically apparent lung disease but with low lung function had significantly increased mortality during follow-up that was independent of cardiac function, in results from more than 13,000 middle-aged Germans.
“Subtle, subclinical pulmonary impairment is a risk indicator for increased mortality independent of cardiac performance,” Dr. Christina Baum said at the American Heart Association scientific sessions.
The researchers used spirometry to measure each subject’s forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). The results showed that “spirometry is a good screening tool that is not very expensive,” making spirometry an effective risk assessment tool for use in the general adult population, said Dr. Baum of the department of general and interventional cardiology at the University Heart Center in Hamburg, Germany.
She and her associates used data collected in the Gutenberg Health Study, which enrolled more than 15,000 German women and men aged 35-74 years during 2007-2012. The investigators excluded people with a history of pulmonary disease, resulting in a study cohort of 13,191, who averaged 55 years old, with 51% men.
At enrollment into the study, all people underwent screening spirometry and echocardiography. Their average baseline FEV1 was 2.9 L and their average FVC was 3.7 L, and 4% had heart failure based on assessments of left ventricular size and function by echocardiography. The first 5,000 enrollees also had measurements taken of their serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I through use of a high-sensitivity assay. The researchers used data from patients followed for a median of 5.5 years.
During follow-up, people in the lowest tertile for FEV1 and those in the lowest tertile for FVC had higher rates of all-cause mortality, compared with those in the highest tertile for each of these two parameters.
In a multivariate analysis that adjusted for age, sex, body mass index, smoking status, hypertension, dyslipidemia, heart failure status, serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I, and other parameters, people with lower FEV1 and FVC readings had significantly worse survival, Dr. Baum said. Every 1–standard deviation increase in FEV1 was linked with a statistically significant, 38% reduced mortality rate; furthermore, a similar significant inverse association existed between FVC and mortality, she reported.
AT THE AHA SCIENTIFIC SESSIONS
AHA: Asthma History Boosts Heart Disease Risk in Postmenopausal Women
ORLANDO – A history of asthma was independently associated with a 24% increase in the risk of new-onset coronary heart disease among postmenopausal women in an analysis from the Women’s Health Initiative.
The study cohort included 90,168 women aged 50-79 years who were free of cardiovascular disease at enrollment in the Women’s Health Initiative (WHI), of whom 6,921 reported a history of physician-diagnosed asthma at baseline. During follow-up in the prospective study, the incidence of a CHD event was 8.6% in subjects with a history of asthma and 6.97% in the no-asthma group, Dr. Fady Y. Marmoush reported at the American Heart Association scientific sessions.
Moreover, the incidence of a first cardiovascular event was 11.6% in the asthma group, compared with 9.7% in the no-asthma controls, added Dr. Marmoush of Memorial Hospital of Rhode Island, Pawtucket.
The asthma group had an absolute 1%-2% greater baseline prevalence of hypertension, diabetes, and family history of CHD. Those with asthma also were more likely to be obese. On the other hand, they were less likely to have ever smoked.
In a multivariate analysis adjusted for these and other potential confounders, including age, dyslipidemia, and waist-hip ratio, the women with a history of asthma had a 24% greater risk of CHD during prospective follow-up in the WHI, as well as a 21% increased rate of cardiovascular events, including stroke, compared with the no-asthma group.
Thus, a history of asthma could be a useful consideration – a tie breaker of sorts – in older women whose calculated 10-year atherosclerotic cardiovascular disease risk based on the standard risk factors places them on the borderline as candidates for statin therapy. The most likely mechanism for the observed association between asthma history and increased risk of cardiovascular disease is the chronic inflammatory state that’s a hallmark of asthma accelerating the atherosclerotic process, which also is inflammatory, she said.
The WHI is funded by the National Heart, Lung, and Blood Institute. Dr. Marmoush reported having no financial conflicts.
ORLANDO – A history of asthma was independently associated with a 24% increase in the risk of new-onset coronary heart disease among postmenopausal women in an analysis from the Women’s Health Initiative.
The study cohort included 90,168 women aged 50-79 years who were free of cardiovascular disease at enrollment in the Women’s Health Initiative (WHI), of whom 6,921 reported a history of physician-diagnosed asthma at baseline. During follow-up in the prospective study, the incidence of a CHD event was 8.6% in subjects with a history of asthma and 6.97% in the no-asthma group, Dr. Fady Y. Marmoush reported at the American Heart Association scientific sessions.
Moreover, the incidence of a first cardiovascular event was 11.6% in the asthma group, compared with 9.7% in the no-asthma controls, added Dr. Marmoush of Memorial Hospital of Rhode Island, Pawtucket.
The asthma group had an absolute 1%-2% greater baseline prevalence of hypertension, diabetes, and family history of CHD. Those with asthma also were more likely to be obese. On the other hand, they were less likely to have ever smoked.
In a multivariate analysis adjusted for these and other potential confounders, including age, dyslipidemia, and waist-hip ratio, the women with a history of asthma had a 24% greater risk of CHD during prospective follow-up in the WHI, as well as a 21% increased rate of cardiovascular events, including stroke, compared with the no-asthma group.
Thus, a history of asthma could be a useful consideration – a tie breaker of sorts – in older women whose calculated 10-year atherosclerotic cardiovascular disease risk based on the standard risk factors places them on the borderline as candidates for statin therapy. The most likely mechanism for the observed association between asthma history and increased risk of cardiovascular disease is the chronic inflammatory state that’s a hallmark of asthma accelerating the atherosclerotic process, which also is inflammatory, she said.
The WHI is funded by the National Heart, Lung, and Blood Institute. Dr. Marmoush reported having no financial conflicts.
ORLANDO – A history of asthma was independently associated with a 24% increase in the risk of new-onset coronary heart disease among postmenopausal women in an analysis from the Women’s Health Initiative.
The study cohort included 90,168 women aged 50-79 years who were free of cardiovascular disease at enrollment in the Women’s Health Initiative (WHI), of whom 6,921 reported a history of physician-diagnosed asthma at baseline. During follow-up in the prospective study, the incidence of a CHD event was 8.6% in subjects with a history of asthma and 6.97% in the no-asthma group, Dr. Fady Y. Marmoush reported at the American Heart Association scientific sessions.
Moreover, the incidence of a first cardiovascular event was 11.6% in the asthma group, compared with 9.7% in the no-asthma controls, added Dr. Marmoush of Memorial Hospital of Rhode Island, Pawtucket.
The asthma group had an absolute 1%-2% greater baseline prevalence of hypertension, diabetes, and family history of CHD. Those with asthma also were more likely to be obese. On the other hand, they were less likely to have ever smoked.
In a multivariate analysis adjusted for these and other potential confounders, including age, dyslipidemia, and waist-hip ratio, the women with a history of asthma had a 24% greater risk of CHD during prospective follow-up in the WHI, as well as a 21% increased rate of cardiovascular events, including stroke, compared with the no-asthma group.
Thus, a history of asthma could be a useful consideration – a tie breaker of sorts – in older women whose calculated 10-year atherosclerotic cardiovascular disease risk based on the standard risk factors places them on the borderline as candidates for statin therapy. The most likely mechanism for the observed association between asthma history and increased risk of cardiovascular disease is the chronic inflammatory state that’s a hallmark of asthma accelerating the atherosclerotic process, which also is inflammatory, she said.
The WHI is funded by the National Heart, Lung, and Blood Institute. Dr. Marmoush reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Prophylactic Antibiotics Don’t Prevent Poststroke Pneumonia
Prophylactic antibiotics don’t prevent poststroke pneumonia or reduce mortality, even in patients who have stroke-induced dysphagia and are at high risk of aspiration, according to a report published in the Lancet.
In a prospective open-label cluster-randomized clinical trial, researchers randomly assigned 37 stroke units in the United Kingdom to give new patients either prophylactic antibiotics for 7 days plus standard stroke care (564 patients) or standard stroke care alone (524 patients). All study participants were considered “unsafe to swallow” because they had impaired consciousness, they failed a bedside swallow test, or they had a nasogastric tube, said Lalit Kalra, Ph.D., of the department of basic and clinical neurosciences and the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, and his associates.
Each hospital was allowed to choose which prophylactic antibiotics to use according to their local guidelines, as well as which dosage and route of administration. The primary outcome was the incidence of post-stroke pneumonia within 2 weeks of hospitalization, which was assessed by two separate methods: a statistician masked to treatment assignment diagnosed pneumonia according to a criteria-based hierarchical algorithm, and a local treating physician diagnosed pneumonia according to clinical findings.
According to the algorithm, poststroke pneumonia developed in 13% of patients given prophylactic antibiotics and 10% of the control group, for an OR of 1.21. According to the clinical findings, poststroke pneumonia developed in 16% of the intervention group and 15% of the control group, for an OR of 1.01. By either definition, prophylactic antibiotics failed to reduce the incidence of poststroke pneumonia, the investigators said (Lancet 2015;386:1835-44).
In addition, all-cause mortality at 14 days (10%) and at 90 days (39%) was not significantly different between the two study groups. And there was no significant difference in the percentage of patients with good functional outcomes. Prophylactic antibiotics were associated with longer hospital stays than standard treatment.
On the positive side, prophylactic antibiotics did reduce the number of nonpneumonia infections, especially urosepsis.
Adverse effects, including cases of Clostridium difficile-positive diarrhea and MRSA colonization, were rare and occurred in equal numbers across the two study groups.
The findings indicate that routine use of antibiotics to prevent poststroke pneumonia “cannot be recommended and should be used judiciously ... in patients after stroke who are managed on stroke units, even if they are at high risk of aspiration,” Dr. Kalra and associates said.
The most likely explanation for this study’s negative findings is that prophylactic antibiotics “do not add to existing preventive measures such as positioning, regular suction, swallowing techniques, modified diets, and early initiation of antibiotics” if patients are suspected of developing pneumonia. It also is possible that poststroke pneumonia is not just a straightforward infection but a complex respiratory syndrome stemming from multiple bacterial, chemical, and immunologic causes that might not respond to antibiotics alone, they added.
This study was funded by the U.K. National Institute for Health Research. Dr. Kalra and associates reported having no relevant financial disclosures.
Prophylactic antibiotics don’t prevent poststroke pneumonia or reduce mortality, even in patients who have stroke-induced dysphagia and are at high risk of aspiration, according to a report published in the Lancet.
In a prospective open-label cluster-randomized clinical trial, researchers randomly assigned 37 stroke units in the United Kingdom to give new patients either prophylactic antibiotics for 7 days plus standard stroke care (564 patients) or standard stroke care alone (524 patients). All study participants were considered “unsafe to swallow” because they had impaired consciousness, they failed a bedside swallow test, or they had a nasogastric tube, said Lalit Kalra, Ph.D., of the department of basic and clinical neurosciences and the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, and his associates.
Each hospital was allowed to choose which prophylactic antibiotics to use according to their local guidelines, as well as which dosage and route of administration. The primary outcome was the incidence of post-stroke pneumonia within 2 weeks of hospitalization, which was assessed by two separate methods: a statistician masked to treatment assignment diagnosed pneumonia according to a criteria-based hierarchical algorithm, and a local treating physician diagnosed pneumonia according to clinical findings.
According to the algorithm, poststroke pneumonia developed in 13% of patients given prophylactic antibiotics and 10% of the control group, for an OR of 1.21. According to the clinical findings, poststroke pneumonia developed in 16% of the intervention group and 15% of the control group, for an OR of 1.01. By either definition, prophylactic antibiotics failed to reduce the incidence of poststroke pneumonia, the investigators said (Lancet 2015;386:1835-44).
In addition, all-cause mortality at 14 days (10%) and at 90 days (39%) was not significantly different between the two study groups. And there was no significant difference in the percentage of patients with good functional outcomes. Prophylactic antibiotics were associated with longer hospital stays than standard treatment.
On the positive side, prophylactic antibiotics did reduce the number of nonpneumonia infections, especially urosepsis.
Adverse effects, including cases of Clostridium difficile-positive diarrhea and MRSA colonization, were rare and occurred in equal numbers across the two study groups.
The findings indicate that routine use of antibiotics to prevent poststroke pneumonia “cannot be recommended and should be used judiciously ... in patients after stroke who are managed on stroke units, even if they are at high risk of aspiration,” Dr. Kalra and associates said.
The most likely explanation for this study’s negative findings is that prophylactic antibiotics “do not add to existing preventive measures such as positioning, regular suction, swallowing techniques, modified diets, and early initiation of antibiotics” if patients are suspected of developing pneumonia. It also is possible that poststroke pneumonia is not just a straightforward infection but a complex respiratory syndrome stemming from multiple bacterial, chemical, and immunologic causes that might not respond to antibiotics alone, they added.
This study was funded by the U.K. National Institute for Health Research. Dr. Kalra and associates reported having no relevant financial disclosures.
Prophylactic antibiotics don’t prevent poststroke pneumonia or reduce mortality, even in patients who have stroke-induced dysphagia and are at high risk of aspiration, according to a report published in the Lancet.
In a prospective open-label cluster-randomized clinical trial, researchers randomly assigned 37 stroke units in the United Kingdom to give new patients either prophylactic antibiotics for 7 days plus standard stroke care (564 patients) or standard stroke care alone (524 patients). All study participants were considered “unsafe to swallow” because they had impaired consciousness, they failed a bedside swallow test, or they had a nasogastric tube, said Lalit Kalra, Ph.D., of the department of basic and clinical neurosciences and the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, and his associates.
Each hospital was allowed to choose which prophylactic antibiotics to use according to their local guidelines, as well as which dosage and route of administration. The primary outcome was the incidence of post-stroke pneumonia within 2 weeks of hospitalization, which was assessed by two separate methods: a statistician masked to treatment assignment diagnosed pneumonia according to a criteria-based hierarchical algorithm, and a local treating physician diagnosed pneumonia according to clinical findings.
According to the algorithm, poststroke pneumonia developed in 13% of patients given prophylactic antibiotics and 10% of the control group, for an OR of 1.21. According to the clinical findings, poststroke pneumonia developed in 16% of the intervention group and 15% of the control group, for an OR of 1.01. By either definition, prophylactic antibiotics failed to reduce the incidence of poststroke pneumonia, the investigators said (Lancet 2015;386:1835-44).
In addition, all-cause mortality at 14 days (10%) and at 90 days (39%) was not significantly different between the two study groups. And there was no significant difference in the percentage of patients with good functional outcomes. Prophylactic antibiotics were associated with longer hospital stays than standard treatment.
On the positive side, prophylactic antibiotics did reduce the number of nonpneumonia infections, especially urosepsis.
Adverse effects, including cases of Clostridium difficile-positive diarrhea and MRSA colonization, were rare and occurred in equal numbers across the two study groups.
The findings indicate that routine use of antibiotics to prevent poststroke pneumonia “cannot be recommended and should be used judiciously ... in patients after stroke who are managed on stroke units, even if they are at high risk of aspiration,” Dr. Kalra and associates said.
The most likely explanation for this study’s negative findings is that prophylactic antibiotics “do not add to existing preventive measures such as positioning, regular suction, swallowing techniques, modified diets, and early initiation of antibiotics” if patients are suspected of developing pneumonia. It also is possible that poststroke pneumonia is not just a straightforward infection but a complex respiratory syndrome stemming from multiple bacterial, chemical, and immunologic causes that might not respond to antibiotics alone, they added.
This study was funded by the U.K. National Institute for Health Research. Dr. Kalra and associates reported having no relevant financial disclosures.
FROM THE LANCET
AHA Releases First-ever Pediatric Pulmonary Hypertension Guideline
The American Heart Association and the American Thoracic Society jointly released the first-ever clinical practice guideline for assessing and managing pulmonary hypertension (PH) in the pediatric population, which was published online Nov. 3 in Circulation.
The two organizations developed this guideline because the causes and treatments of PH in neonates, infants, and children are often different from those in adults. The literature for adult PH is “robust,” and there are several treatment guidelines available, whereas pediatric PH has not been well studied, “and little is understood about the natural history, fundamental mechanisms, and treatment of childhood PH,” said Dr. Steven H. Abman, cochair of the guideline committee and a pediatric pulmonologist at the University of Colorado and Children’s Hospital, both in Denver.
“It’s important to note that, although these guidelines provide a foundation for taking care of children with pulmonary hypertension, we still have a huge need for more specific data and research to further improve outcomes,” he said in a statement accompanying the guideline.
This guideline was developed by a working group of 27 clinicians and researchers with expertise in pediatric pulmonology, pediatric and adult cardiology, pediatric intensivism, neonatology, and translational science. They reviewed more than 600 articles in the literature, but given the paucity of high-quality data regarding pediatric PH, the guideline relies heavily on expert opinion and primarily describes “generally acceptable approaches” to diagnosis and management; more specific and detailed recommendations await the findings of future research, Dr. Abman and his associates said (Circulation. 2015 Oct 26. doi:10.1161/CIR.0000000000000329).
In the pediatric population, PH is defined as a resting mean pulmonary artery pressure greater than 25 mm Hg after the first few months of life and is usually related to cardiac, lung, or systemic diseases. Idiopathic PH, a pulmonary vasculopathy, is a diagnosis of exclusion after diseases of the left side of the heart, lung parenchyma, heart valves, thromboembolism, and other miscellaneous causes have been ruled out.
The guideline emphasizes that children thought to have PH should be evaluated and treated at comprehensive, multidisciplinary clinics at specialized pediatric centers. “When children are diagnosed, parents often feel helpless. However, it’s important that parents seek doctors and centers that see these children on a regular basis and can offer them access to new molecular diagnostics, new drug therapies, and new devices, as well as surgeries that have recently been developed,” Dr. Stephen L. Archer, cochair of the guideline committee and head of medicine at Queen’s University, Kingston, Ont., said in the statement.
“These children suffer with health issues throughout their lives or die prematurely, particularly if they’re not properly diagnosed and managed. But with the proper diagnosis and treatment at a specialized center for PH, the prognosis for many of these children is excellent,” he noted.
Properly classifying the type of PH is a key first step in determining treatment. The guideline addresses numerous methods for diagnosing and monitoring PH, including imaging studies, echocardiograms, cardiac catheterization, brain natriuretic peptide and other laboratory testing, 6-minute walk distance (at appropriate ages), sleep studies, and genetic testing. It specifically deals with persistent PH of the newborn and PH arising from congenital diaphragmatic hernia; bronchopulmonary dysplasia or other lung diseases; heart disease such as atrial-septal defect or patent ductus arteriosus; and systemic diseases such as hemolytic hemoglobinopathies and hepatic, renal, or metabolic illness; as well as idiopathic PH and PH related to high-altitude pulmonary edema.
Regarding ongoing outpatient care, the guideline recommends that children with PH receive influenza and pneumococcal vaccinations and prophylaxis for respiratory syncytial virus (if they are eligible), as well as antibiotic prophylaxis to prevent subacute bacterial endocarditis in those who are cyanotic or have indwelling central lines. Growth must be monitored rigorously, and infections and respiratory illnesses must be recognized and treated promptly. Any surgeries require careful preoperative planning and should be performed at hospitals with expertise in PH.
The guideline includes an extensive section on pharmacotherapy for childhood PH, including the use of digitalis, diuretics, long-term anticoagulation, oxygen therapy, calcium channel blockers, phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, intravenous and subcutaneous prostacyclin therapy, and the transition from parenteral to oral or inhaled treatment.
In addition, the guideline addresses exercise and sports participation, travel restrictions, and contraceptive counseling for adolescent patients. Finally, “given the impact of childhood PH on the entire family, [patients], siblings, and caregivers should be assessed for psychosocial stress and be readily provided support and referral as needed,” the guideline recommends.
A copy of the guideline is available at http://my.americanheart.org/statements.
The pediatric pulmonary, pediatric cardiology, and neonatal and pediatric intensivists all have greatly anticipated directions for the care of pediatric pulmonary hypertension. The guidelines have excellent care maps for the diagnosis and evaluation of the various etiologies of pulmonary hypertension.
The new guidelines also should help also with insurance authorizations for the expensive medications for pulmonary hypertension! Dr. Robyn J. Barst, a renowned leader in pediatric pulmonary hypertension, who passed away in 2013, would be so proud of this AHA guideline!
Dr. Susan L. Millard is director of research, pediatric pulmonary & sleep medicine at Helen DeVos Children’s Hospital in Grand Rapids, MI.
The pediatric pulmonary, pediatric cardiology, and neonatal and pediatric intensivists all have greatly anticipated directions for the care of pediatric pulmonary hypertension. The guidelines have excellent care maps for the diagnosis and evaluation of the various etiologies of pulmonary hypertension.
The new guidelines also should help also with insurance authorizations for the expensive medications for pulmonary hypertension! Dr. Robyn J. Barst, a renowned leader in pediatric pulmonary hypertension, who passed away in 2013, would be so proud of this AHA guideline!
Dr. Susan L. Millard is director of research, pediatric pulmonary & sleep medicine at Helen DeVos Children’s Hospital in Grand Rapids, MI.
The pediatric pulmonary, pediatric cardiology, and neonatal and pediatric intensivists all have greatly anticipated directions for the care of pediatric pulmonary hypertension. The guidelines have excellent care maps for the diagnosis and evaluation of the various etiologies of pulmonary hypertension.
The new guidelines also should help also with insurance authorizations for the expensive medications for pulmonary hypertension! Dr. Robyn J. Barst, a renowned leader in pediatric pulmonary hypertension, who passed away in 2013, would be so proud of this AHA guideline!
Dr. Susan L. Millard is director of research, pediatric pulmonary & sleep medicine at Helen DeVos Children’s Hospital in Grand Rapids, MI.
The American Heart Association and the American Thoracic Society jointly released the first-ever clinical practice guideline for assessing and managing pulmonary hypertension (PH) in the pediatric population, which was published online Nov. 3 in Circulation.
The two organizations developed this guideline because the causes and treatments of PH in neonates, infants, and children are often different from those in adults. The literature for adult PH is “robust,” and there are several treatment guidelines available, whereas pediatric PH has not been well studied, “and little is understood about the natural history, fundamental mechanisms, and treatment of childhood PH,” said Dr. Steven H. Abman, cochair of the guideline committee and a pediatric pulmonologist at the University of Colorado and Children’s Hospital, both in Denver.
“It’s important to note that, although these guidelines provide a foundation for taking care of children with pulmonary hypertension, we still have a huge need for more specific data and research to further improve outcomes,” he said in a statement accompanying the guideline.
This guideline was developed by a working group of 27 clinicians and researchers with expertise in pediatric pulmonology, pediatric and adult cardiology, pediatric intensivism, neonatology, and translational science. They reviewed more than 600 articles in the literature, but given the paucity of high-quality data regarding pediatric PH, the guideline relies heavily on expert opinion and primarily describes “generally acceptable approaches” to diagnosis and management; more specific and detailed recommendations await the findings of future research, Dr. Abman and his associates said (Circulation. 2015 Oct 26. doi:10.1161/CIR.0000000000000329).
In the pediatric population, PH is defined as a resting mean pulmonary artery pressure greater than 25 mm Hg after the first few months of life and is usually related to cardiac, lung, or systemic diseases. Idiopathic PH, a pulmonary vasculopathy, is a diagnosis of exclusion after diseases of the left side of the heart, lung parenchyma, heart valves, thromboembolism, and other miscellaneous causes have been ruled out.
The guideline emphasizes that children thought to have PH should be evaluated and treated at comprehensive, multidisciplinary clinics at specialized pediatric centers. “When children are diagnosed, parents often feel helpless. However, it’s important that parents seek doctors and centers that see these children on a regular basis and can offer them access to new molecular diagnostics, new drug therapies, and new devices, as well as surgeries that have recently been developed,” Dr. Stephen L. Archer, cochair of the guideline committee and head of medicine at Queen’s University, Kingston, Ont., said in the statement.
“These children suffer with health issues throughout their lives or die prematurely, particularly if they’re not properly diagnosed and managed. But with the proper diagnosis and treatment at a specialized center for PH, the prognosis for many of these children is excellent,” he noted.
Properly classifying the type of PH is a key first step in determining treatment. The guideline addresses numerous methods for diagnosing and monitoring PH, including imaging studies, echocardiograms, cardiac catheterization, brain natriuretic peptide and other laboratory testing, 6-minute walk distance (at appropriate ages), sleep studies, and genetic testing. It specifically deals with persistent PH of the newborn and PH arising from congenital diaphragmatic hernia; bronchopulmonary dysplasia or other lung diseases; heart disease such as atrial-septal defect or patent ductus arteriosus; and systemic diseases such as hemolytic hemoglobinopathies and hepatic, renal, or metabolic illness; as well as idiopathic PH and PH related to high-altitude pulmonary edema.
Regarding ongoing outpatient care, the guideline recommends that children with PH receive influenza and pneumococcal vaccinations and prophylaxis for respiratory syncytial virus (if they are eligible), as well as antibiotic prophylaxis to prevent subacute bacterial endocarditis in those who are cyanotic or have indwelling central lines. Growth must be monitored rigorously, and infections and respiratory illnesses must be recognized and treated promptly. Any surgeries require careful preoperative planning and should be performed at hospitals with expertise in PH.
The guideline includes an extensive section on pharmacotherapy for childhood PH, including the use of digitalis, diuretics, long-term anticoagulation, oxygen therapy, calcium channel blockers, phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, intravenous and subcutaneous prostacyclin therapy, and the transition from parenteral to oral or inhaled treatment.
In addition, the guideline addresses exercise and sports participation, travel restrictions, and contraceptive counseling for adolescent patients. Finally, “given the impact of childhood PH on the entire family, [patients], siblings, and caregivers should be assessed for psychosocial stress and be readily provided support and referral as needed,” the guideline recommends.
A copy of the guideline is available at http://my.americanheart.org/statements.
The American Heart Association and the American Thoracic Society jointly released the first-ever clinical practice guideline for assessing and managing pulmonary hypertension (PH) in the pediatric population, which was published online Nov. 3 in Circulation.
The two organizations developed this guideline because the causes and treatments of PH in neonates, infants, and children are often different from those in adults. The literature for adult PH is “robust,” and there are several treatment guidelines available, whereas pediatric PH has not been well studied, “and little is understood about the natural history, fundamental mechanisms, and treatment of childhood PH,” said Dr. Steven H. Abman, cochair of the guideline committee and a pediatric pulmonologist at the University of Colorado and Children’s Hospital, both in Denver.
“It’s important to note that, although these guidelines provide a foundation for taking care of children with pulmonary hypertension, we still have a huge need for more specific data and research to further improve outcomes,” he said in a statement accompanying the guideline.
This guideline was developed by a working group of 27 clinicians and researchers with expertise in pediatric pulmonology, pediatric and adult cardiology, pediatric intensivism, neonatology, and translational science. They reviewed more than 600 articles in the literature, but given the paucity of high-quality data regarding pediatric PH, the guideline relies heavily on expert opinion and primarily describes “generally acceptable approaches” to diagnosis and management; more specific and detailed recommendations await the findings of future research, Dr. Abman and his associates said (Circulation. 2015 Oct 26. doi:10.1161/CIR.0000000000000329).
In the pediatric population, PH is defined as a resting mean pulmonary artery pressure greater than 25 mm Hg after the first few months of life and is usually related to cardiac, lung, or systemic diseases. Idiopathic PH, a pulmonary vasculopathy, is a diagnosis of exclusion after diseases of the left side of the heart, lung parenchyma, heart valves, thromboembolism, and other miscellaneous causes have been ruled out.
The guideline emphasizes that children thought to have PH should be evaluated and treated at comprehensive, multidisciplinary clinics at specialized pediatric centers. “When children are diagnosed, parents often feel helpless. However, it’s important that parents seek doctors and centers that see these children on a regular basis and can offer them access to new molecular diagnostics, new drug therapies, and new devices, as well as surgeries that have recently been developed,” Dr. Stephen L. Archer, cochair of the guideline committee and head of medicine at Queen’s University, Kingston, Ont., said in the statement.
“These children suffer with health issues throughout their lives or die prematurely, particularly if they’re not properly diagnosed and managed. But with the proper diagnosis and treatment at a specialized center for PH, the prognosis for many of these children is excellent,” he noted.
Properly classifying the type of PH is a key first step in determining treatment. The guideline addresses numerous methods for diagnosing and monitoring PH, including imaging studies, echocardiograms, cardiac catheterization, brain natriuretic peptide and other laboratory testing, 6-minute walk distance (at appropriate ages), sleep studies, and genetic testing. It specifically deals with persistent PH of the newborn and PH arising from congenital diaphragmatic hernia; bronchopulmonary dysplasia or other lung diseases; heart disease such as atrial-septal defect or patent ductus arteriosus; and systemic diseases such as hemolytic hemoglobinopathies and hepatic, renal, or metabolic illness; as well as idiopathic PH and PH related to high-altitude pulmonary edema.
Regarding ongoing outpatient care, the guideline recommends that children with PH receive influenza and pneumococcal vaccinations and prophylaxis for respiratory syncytial virus (if they are eligible), as well as antibiotic prophylaxis to prevent subacute bacterial endocarditis in those who are cyanotic or have indwelling central lines. Growth must be monitored rigorously, and infections and respiratory illnesses must be recognized and treated promptly. Any surgeries require careful preoperative planning and should be performed at hospitals with expertise in PH.
The guideline includes an extensive section on pharmacotherapy for childhood PH, including the use of digitalis, diuretics, long-term anticoagulation, oxygen therapy, calcium channel blockers, phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, intravenous and subcutaneous prostacyclin therapy, and the transition from parenteral to oral or inhaled treatment.
In addition, the guideline addresses exercise and sports participation, travel restrictions, and contraceptive counseling for adolescent patients. Finally, “given the impact of childhood PH on the entire family, [patients], siblings, and caregivers should be assessed for psychosocial stress and be readily provided support and referral as needed,” the guideline recommends.
A copy of the guideline is available at http://my.americanheart.org/statements.
FROM CIRCULATION
This Adjunct Medication Can Speed CAP Recovery
PRACTICE CHANGER
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild-to-moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
STRENGTH OF RECOMMENDATION
A: Based on a single good-quality randomized controlled trial (RCT) and meta-analysis.1
ILLUSTRATIVE CASE
A 75-year-old woman with hypertension and diabetes presents to the emergency department with shortness of breath, cough, and fever that she’s had for four days. On examination, her temperature is 38.2°C; heart rate, 110 beats/min; respiratory rate, 28 breaths/min; and O2 saturation, 91%. Rhonchi are heard in her right lower lung field; chest x-ray reveals infiltrate in her right lower lobe. The patient is admitted and started on IV antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than 1 million hospitalizations annually in the United States and is the eighth leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures (eg, IV fluids and antipyretics). Because the disease process involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified six small RCTs that evaluated the impact of corticosteroids on CAP recovery.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk for bias.
Subsequently, a 2012 meta-analysis of nine RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of eight moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY
Prednisone hastens clinical stabilization, cuts hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP who were admitted to one of seven tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible if they were 18 or older, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had a contraindication to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for seven days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on day 30.
The primary outcome was length of time to clinical stability (eg, at least 24 hours of stable vital signs). This composite endpoint required all of the following: temperature ≤ 37.8°C; heart rate ≤ 100 beats/min; spontaneous respiratory rate ≤ 24 breaths/min; systolic blood pressure ≥ 90 mm Hg (≥ 100 mm Hg for patients diagnosed with hypertension) without vasopressor support; mental status back to baseline; ability to take food by mouth; and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR], 2.5 to 3.4) compared to the placebo group at 4.4 days (IQR, 4 - 5; hazard ratio [HR], 1.33). Median time to hospital discharge was also shorter for the prednisone group (6 d vs 7 d; HR, 1.19), as was duration of IV antibiotic treatment (4 d vs 5 d; difference, –0.89 d).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio, 1.96).
WHAT’S NEW
This large, good-quality study reinforces previous evidence
This is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay. It also used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS
It’s unclear if steroids benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, although this was a large, well-designed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP.
Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the US.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION
Risk for adverse events
Treatment with prednisone increases risk for corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these effects appear to resolve quickly after treatment and do not impact the overall time to clinical stability.
REFERENCES
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. CDC. FastStats: Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed September 29, 2015.
3. CDC/National Center for Health Statistics. Top 10 leading causes of death: United States, 1999–2013. http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 29, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:519-528.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(10):648-650.
PRACTICE CHANGER
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild-to-moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
STRENGTH OF RECOMMENDATION
A: Based on a single good-quality randomized controlled trial (RCT) and meta-analysis.1
ILLUSTRATIVE CASE
A 75-year-old woman with hypertension and diabetes presents to the emergency department with shortness of breath, cough, and fever that she’s had for four days. On examination, her temperature is 38.2°C; heart rate, 110 beats/min; respiratory rate, 28 breaths/min; and O2 saturation, 91%. Rhonchi are heard in her right lower lung field; chest x-ray reveals infiltrate in her right lower lobe. The patient is admitted and started on IV antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than 1 million hospitalizations annually in the United States and is the eighth leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures (eg, IV fluids and antipyretics). Because the disease process involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified six small RCTs that evaluated the impact of corticosteroids on CAP recovery.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk for bias.
Subsequently, a 2012 meta-analysis of nine RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of eight moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY
Prednisone hastens clinical stabilization, cuts hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP who were admitted to one of seven tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible if they were 18 or older, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had a contraindication to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for seven days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on day 30.
The primary outcome was length of time to clinical stability (eg, at least 24 hours of stable vital signs). This composite endpoint required all of the following: temperature ≤ 37.8°C; heart rate ≤ 100 beats/min; spontaneous respiratory rate ≤ 24 breaths/min; systolic blood pressure ≥ 90 mm Hg (≥ 100 mm Hg for patients diagnosed with hypertension) without vasopressor support; mental status back to baseline; ability to take food by mouth; and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR], 2.5 to 3.4) compared to the placebo group at 4.4 days (IQR, 4 - 5; hazard ratio [HR], 1.33). Median time to hospital discharge was also shorter for the prednisone group (6 d vs 7 d; HR, 1.19), as was duration of IV antibiotic treatment (4 d vs 5 d; difference, –0.89 d).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio, 1.96).
WHAT’S NEW
This large, good-quality study reinforces previous evidence
This is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay. It also used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS
It’s unclear if steroids benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, although this was a large, well-designed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP.
Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the US.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION
Risk for adverse events
Treatment with prednisone increases risk for corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these effects appear to resolve quickly after treatment and do not impact the overall time to clinical stability.
REFERENCES
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. CDC. FastStats: Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed September 29, 2015.
3. CDC/National Center for Health Statistics. Top 10 leading causes of death: United States, 1999–2013. http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 29, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:519-528.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(10):648-650.
PRACTICE CHANGER
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild-to-moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
STRENGTH OF RECOMMENDATION
A: Based on a single good-quality randomized controlled trial (RCT) and meta-analysis.1
ILLUSTRATIVE CASE
A 75-year-old woman with hypertension and diabetes presents to the emergency department with shortness of breath, cough, and fever that she’s had for four days. On examination, her temperature is 38.2°C; heart rate, 110 beats/min; respiratory rate, 28 breaths/min; and O2 saturation, 91%. Rhonchi are heard in her right lower lung field; chest x-ray reveals infiltrate in her right lower lobe. The patient is admitted and started on IV antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than 1 million hospitalizations annually in the United States and is the eighth leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures (eg, IV fluids and antipyretics). Because the disease process involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified six small RCTs that evaluated the impact of corticosteroids on CAP recovery.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk for bias.
Subsequently, a 2012 meta-analysis of nine RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of eight moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY
Prednisone hastens clinical stabilization, cuts hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP who were admitted to one of seven tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible if they were 18 or older, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had a contraindication to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for seven days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on day 30.
The primary outcome was length of time to clinical stability (eg, at least 24 hours of stable vital signs). This composite endpoint required all of the following: temperature ≤ 37.8°C; heart rate ≤ 100 beats/min; spontaneous respiratory rate ≤ 24 breaths/min; systolic blood pressure ≥ 90 mm Hg (≥ 100 mm Hg for patients diagnosed with hypertension) without vasopressor support; mental status back to baseline; ability to take food by mouth; and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR], 2.5 to 3.4) compared to the placebo group at 4.4 days (IQR, 4 - 5; hazard ratio [HR], 1.33). Median time to hospital discharge was also shorter for the prednisone group (6 d vs 7 d; HR, 1.19), as was duration of IV antibiotic treatment (4 d vs 5 d; difference, –0.89 d).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio, 1.96).
WHAT’S NEW
This large, good-quality study reinforces previous evidence
This is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay. It also used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS
It’s unclear if steroids benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, although this was a large, well-designed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP.
Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the US.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION
Risk for adverse events
Treatment with prednisone increases risk for corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these effects appear to resolve quickly after treatment and do not impact the overall time to clinical stability.
REFERENCES
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. CDC. FastStats: Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed September 29, 2015.
3. CDC/National Center for Health Statistics. Top 10 leading causes of death: United States, 1999–2013. http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 29, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:519-528.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(10):648-650.
Triglycerides and Cardiovascular Risk
Joyce Ross explains the role of triglycerides in cardiovascular health and how to manage high levels.
This video provides three takeaways in less than three minutes from the presentation at the 2015 MEDS conference, entitled “Triglycerides on the Rise: Confronting Residual Cardiometabolic Risk,” co-presented with Gregory S. Pokrywka, MD, CACP, FNLA, NCMP, jointly provided by Postgraduate Institute for Medicine and Medtelligence, and supported by an educational grant from Amarin Pharma Inc.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Joyce Ross explains the role of triglycerides in cardiovascular health and how to manage high levels.
This video provides three takeaways in less than three minutes from the presentation at the 2015 MEDS conference, entitled “Triglycerides on the Rise: Confronting Residual Cardiometabolic Risk,” co-presented with Gregory S. Pokrywka, MD, CACP, FNLA, NCMP, jointly provided by Postgraduate Institute for Medicine and Medtelligence, and supported by an educational grant from Amarin Pharma Inc.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Joyce Ross explains the role of triglycerides in cardiovascular health and how to manage high levels.
This video provides three takeaways in less than three minutes from the presentation at the 2015 MEDS conference, entitled “Triglycerides on the Rise: Confronting Residual Cardiometabolic Risk,” co-presented with Gregory S. Pokrywka, MD, CACP, FNLA, NCMP, jointly provided by Postgraduate Institute for Medicine and Medtelligence, and supported by an educational grant from Amarin Pharma Inc.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Antibiotic Prescribing Patterns for Pediatric CAP Vary Widely
SAN DIEGO – Antibiotic prescribing patterns for pediatric community-acquired pneumonia vary substantially across both children’s hospitals and facilities that are not children’s hospitals, a large analysis found.
Specifically, children’s hospitals are far more likely to prescribe in accordance with national guidelines than are other hospitals.
“Moving forward, I think there’s a need for further study to understand these differences, so we can begin to narrow this gap between children’s and non–children’s hospitals,” lead study author Dr. Alison Tribble said at an annual scientific meeting on infectious diseases. “Across the board, we need to continue efforts to improve guideline adherence for all children hospitalized with community-acquired pneumonia.”
In 2012, community-acquired pneumonia (CAP) accounted for 120,000 known pneumonia admissions among children in the United States and about 7% of all pediatric hospitalizations, said Dr. Tribble, a pediatric infectious disease specialist at C.S. Mott Children’s Hospital and the University of Michigan Medical Center, both in Ann Arbor. “We also know that pneumonia accounts for more days of antibiotic therapy than any other indication for admission to U.S. children’s hospitals,” she said.
In 2011, the Infectious Diseases Society of America and Pediatric Infectious Diseases Society released guidelines for pediatric CAP, which recommend a first-line therapy with penicillin, ampicillin, or amoxicillin for most children who are immunized and healthy. “Only in situations where there’s a significant concern for an atypical organism should we be adding coverage for that – even in older children,” Dr. Tribble said. Following the release of the guidelines, she continued, multiple studies have shown that the use of first-line therapy is increasing in children’s hospitals. “However, a substantial proportion of children with pneumonia are admitted to non–children’s hospitals,” she said. “Prior to release of the guidelines, one study showed that use of first-line therapy for pediatric CAP was low in non–children’s hospitals (J Pediatr. 2014 165[3]:585-91), but postguideline CAP therapy in non–children’s hospitals has not yet been evaluated.”
For the current study, Dr. Tribble and her associates set out to evaluate antibiotic prescribing patterns for pediatric CAP in non–children’s hospitals and to compare prescribing patterns between children’s and non–children’s hospitals. They conducted a retrospective cross-sectional study of children aged 1-17 years admitted for CAP in 2013 to 323 hospitals, captured via the Pediatric Health Information System (PHIS) and Premier Perspective databases. PHIS is an administrative database that includes billing data, diagnosis codes, and procedure codes for about 44 freestanding children’s hospitals nationwide, while Premier Perspective encompasses data from 522 hospitals nationwide. The researchers used a validated ICD-9 code-based algorithm to identify patients with CAP and excluded those with complicated pneumonia or complex chronic conditions, those who received intensive care, and those with methicillin-resistant Staphylococcus aureus infection or colonization.
Children’s hospitals were defined as those with pediatric admissions accounting for more than 75% of all admissions. “This was after excluding newborns and admission for childbirth, because many community hospitals will have a birthing center or a NICU, but otherwise would not be considered a children’s hospital,” Dr. Tribble explained. Any other hospital was considered a non–children’s hospital.
Three different outcomes for antibiotic use were examined: those who ever received penicillin, amoxicillin, or ampicillin (guideline therapy); those who ever received a macrolide, fluoroquinolone, or tetracycline (atypical therapy); and those who received anything other than penicillin, amoxicillin, or ampicillin (nonguideline therapy). The standardized probability of exposure to select antibiotics was compared between children’s and non–children’s hospitals, adjusted for age, sex, and insurance provider.
In all, 323 hospitals contributed 15,495 CAP cases. Of the 323 hospitals, 49 were identified as children’s hospitals (44 from the PHIS database and 5 from the Premier database). Dr. Tribble reported results from 9,224 subjects admitted to children’s hospitals and 6,271 subjects admitted to non–children’s hospitals. The demographics between the two groups were similar: The patients’ mean age was 3 years, and 66% were younger than age 5 years.
After adjustment of data, patients admitted to children’s hospitals were found to be more likely to receive guideline therapy, compared with those admitted to non–children’s hospitals (46% vs. 15%, respectively), were less likely to received atypical therapy (36% vs. 51%), and were less likely to receive nonguideline therapy (78% vs. 94%; P less than .001 for all comparisons).
Dr. Tribble acknowledged certain limitations of the study, including the potential for misclassification of children’s hospitals in the Premier database, “although most likely I think we would have failed to identify a children’s hospital, and this would have biased us toward the null and made our difference less significant,” she said. “We are developing an absolute volume classification so we can look at this in another way.” Another limitation is that the study design did not account for the potential of combination therapy, “and you can’t account for change in therapy during hospitalization. Lastly, we compared data across different databases and across different hospital types.”
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The study was supported by a training grant from the National Institute of Child Health and Human Development. The researchers reported having no relevant financial disclosures.
SAN DIEGO – Antibiotic prescribing patterns for pediatric community-acquired pneumonia vary substantially across both children’s hospitals and facilities that are not children’s hospitals, a large analysis found.
Specifically, children’s hospitals are far more likely to prescribe in accordance with national guidelines than are other hospitals.
“Moving forward, I think there’s a need for further study to understand these differences, so we can begin to narrow this gap between children’s and non–children’s hospitals,” lead study author Dr. Alison Tribble said at an annual scientific meeting on infectious diseases. “Across the board, we need to continue efforts to improve guideline adherence for all children hospitalized with community-acquired pneumonia.”
In 2012, community-acquired pneumonia (CAP) accounted for 120,000 known pneumonia admissions among children in the United States and about 7% of all pediatric hospitalizations, said Dr. Tribble, a pediatric infectious disease specialist at C.S. Mott Children’s Hospital and the University of Michigan Medical Center, both in Ann Arbor. “We also know that pneumonia accounts for more days of antibiotic therapy than any other indication for admission to U.S. children’s hospitals,” she said.
In 2011, the Infectious Diseases Society of America and Pediatric Infectious Diseases Society released guidelines for pediatric CAP, which recommend a first-line therapy with penicillin, ampicillin, or amoxicillin for most children who are immunized and healthy. “Only in situations where there’s a significant concern for an atypical organism should we be adding coverage for that – even in older children,” Dr. Tribble said. Following the release of the guidelines, she continued, multiple studies have shown that the use of first-line therapy is increasing in children’s hospitals. “However, a substantial proportion of children with pneumonia are admitted to non–children’s hospitals,” she said. “Prior to release of the guidelines, one study showed that use of first-line therapy for pediatric CAP was low in non–children’s hospitals (J Pediatr. 2014 165[3]:585-91), but postguideline CAP therapy in non–children’s hospitals has not yet been evaluated.”
For the current study, Dr. Tribble and her associates set out to evaluate antibiotic prescribing patterns for pediatric CAP in non–children’s hospitals and to compare prescribing patterns between children’s and non–children’s hospitals. They conducted a retrospective cross-sectional study of children aged 1-17 years admitted for CAP in 2013 to 323 hospitals, captured via the Pediatric Health Information System (PHIS) and Premier Perspective databases. PHIS is an administrative database that includes billing data, diagnosis codes, and procedure codes for about 44 freestanding children’s hospitals nationwide, while Premier Perspective encompasses data from 522 hospitals nationwide. The researchers used a validated ICD-9 code-based algorithm to identify patients with CAP and excluded those with complicated pneumonia or complex chronic conditions, those who received intensive care, and those with methicillin-resistant Staphylococcus aureus infection or colonization.
Children’s hospitals were defined as those with pediatric admissions accounting for more than 75% of all admissions. “This was after excluding newborns and admission for childbirth, because many community hospitals will have a birthing center or a NICU, but otherwise would not be considered a children’s hospital,” Dr. Tribble explained. Any other hospital was considered a non–children’s hospital.
Three different outcomes for antibiotic use were examined: those who ever received penicillin, amoxicillin, or ampicillin (guideline therapy); those who ever received a macrolide, fluoroquinolone, or tetracycline (atypical therapy); and those who received anything other than penicillin, amoxicillin, or ampicillin (nonguideline therapy). The standardized probability of exposure to select antibiotics was compared between children’s and non–children’s hospitals, adjusted for age, sex, and insurance provider.
In all, 323 hospitals contributed 15,495 CAP cases. Of the 323 hospitals, 49 were identified as children’s hospitals (44 from the PHIS database and 5 from the Premier database). Dr. Tribble reported results from 9,224 subjects admitted to children’s hospitals and 6,271 subjects admitted to non–children’s hospitals. The demographics between the two groups were similar: The patients’ mean age was 3 years, and 66% were younger than age 5 years.
After adjustment of data, patients admitted to children’s hospitals were found to be more likely to receive guideline therapy, compared with those admitted to non–children’s hospitals (46% vs. 15%, respectively), were less likely to received atypical therapy (36% vs. 51%), and were less likely to receive nonguideline therapy (78% vs. 94%; P less than .001 for all comparisons).
Dr. Tribble acknowledged certain limitations of the study, including the potential for misclassification of children’s hospitals in the Premier database, “although most likely I think we would have failed to identify a children’s hospital, and this would have biased us toward the null and made our difference less significant,” she said. “We are developing an absolute volume classification so we can look at this in another way.” Another limitation is that the study design did not account for the potential of combination therapy, “and you can’t account for change in therapy during hospitalization. Lastly, we compared data across different databases and across different hospital types.”
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The study was supported by a training grant from the National Institute of Child Health and Human Development. The researchers reported having no relevant financial disclosures.
SAN DIEGO – Antibiotic prescribing patterns for pediatric community-acquired pneumonia vary substantially across both children’s hospitals and facilities that are not children’s hospitals, a large analysis found.
Specifically, children’s hospitals are far more likely to prescribe in accordance with national guidelines than are other hospitals.
“Moving forward, I think there’s a need for further study to understand these differences, so we can begin to narrow this gap between children’s and non–children’s hospitals,” lead study author Dr. Alison Tribble said at an annual scientific meeting on infectious diseases. “Across the board, we need to continue efforts to improve guideline adherence for all children hospitalized with community-acquired pneumonia.”
In 2012, community-acquired pneumonia (CAP) accounted for 120,000 known pneumonia admissions among children in the United States and about 7% of all pediatric hospitalizations, said Dr. Tribble, a pediatric infectious disease specialist at C.S. Mott Children’s Hospital and the University of Michigan Medical Center, both in Ann Arbor. “We also know that pneumonia accounts for more days of antibiotic therapy than any other indication for admission to U.S. children’s hospitals,” she said.
In 2011, the Infectious Diseases Society of America and Pediatric Infectious Diseases Society released guidelines for pediatric CAP, which recommend a first-line therapy with penicillin, ampicillin, or amoxicillin for most children who are immunized and healthy. “Only in situations where there’s a significant concern for an atypical organism should we be adding coverage for that – even in older children,” Dr. Tribble said. Following the release of the guidelines, she continued, multiple studies have shown that the use of first-line therapy is increasing in children’s hospitals. “However, a substantial proportion of children with pneumonia are admitted to non–children’s hospitals,” she said. “Prior to release of the guidelines, one study showed that use of first-line therapy for pediatric CAP was low in non–children’s hospitals (J Pediatr. 2014 165[3]:585-91), but postguideline CAP therapy in non–children’s hospitals has not yet been evaluated.”
For the current study, Dr. Tribble and her associates set out to evaluate antibiotic prescribing patterns for pediatric CAP in non–children’s hospitals and to compare prescribing patterns between children’s and non–children’s hospitals. They conducted a retrospective cross-sectional study of children aged 1-17 years admitted for CAP in 2013 to 323 hospitals, captured via the Pediatric Health Information System (PHIS) and Premier Perspective databases. PHIS is an administrative database that includes billing data, diagnosis codes, and procedure codes for about 44 freestanding children’s hospitals nationwide, while Premier Perspective encompasses data from 522 hospitals nationwide. The researchers used a validated ICD-9 code-based algorithm to identify patients with CAP and excluded those with complicated pneumonia or complex chronic conditions, those who received intensive care, and those with methicillin-resistant Staphylococcus aureus infection or colonization.
Children’s hospitals were defined as those with pediatric admissions accounting for more than 75% of all admissions. “This was after excluding newborns and admission for childbirth, because many community hospitals will have a birthing center or a NICU, but otherwise would not be considered a children’s hospital,” Dr. Tribble explained. Any other hospital was considered a non–children’s hospital.
Three different outcomes for antibiotic use were examined: those who ever received penicillin, amoxicillin, or ampicillin (guideline therapy); those who ever received a macrolide, fluoroquinolone, or tetracycline (atypical therapy); and those who received anything other than penicillin, amoxicillin, or ampicillin (nonguideline therapy). The standardized probability of exposure to select antibiotics was compared between children’s and non–children’s hospitals, adjusted for age, sex, and insurance provider.
In all, 323 hospitals contributed 15,495 CAP cases. Of the 323 hospitals, 49 were identified as children’s hospitals (44 from the PHIS database and 5 from the Premier database). Dr. Tribble reported results from 9,224 subjects admitted to children’s hospitals and 6,271 subjects admitted to non–children’s hospitals. The demographics between the two groups were similar: The patients’ mean age was 3 years, and 66% were younger than age 5 years.
After adjustment of data, patients admitted to children’s hospitals were found to be more likely to receive guideline therapy, compared with those admitted to non–children’s hospitals (46% vs. 15%, respectively), were less likely to received atypical therapy (36% vs. 51%), and were less likely to receive nonguideline therapy (78% vs. 94%; P less than .001 for all comparisons).
Dr. Tribble acknowledged certain limitations of the study, including the potential for misclassification of children’s hospitals in the Premier database, “although most likely I think we would have failed to identify a children’s hospital, and this would have biased us toward the null and made our difference less significant,” she said. “We are developing an absolute volume classification so we can look at this in another way.” Another limitation is that the study design did not account for the potential of combination therapy, “and you can’t account for change in therapy during hospitalization. Lastly, we compared data across different databases and across different hospital types.”
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The study was supported by a training grant from the National Institute of Child Health and Human Development. The researchers reported having no relevant financial disclosures.
AT IDWEEK 2015
COPD: Optimizing treatment
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.