User login
Coronary Artery Calcium Linked to Cancer, Kidney Disease, COPD
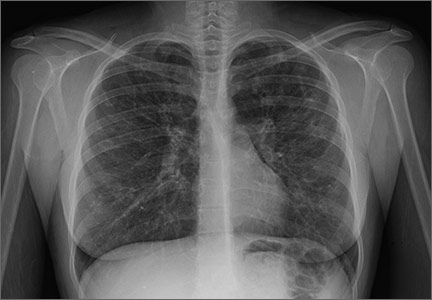
Patients whose coronary artery calcium scores exceeded 400 were significantly more likely to develop cancer, chronic obstructive pulmonary disease, chronic kidney disease, and hip fractures, compared with adults with undetectable CAC, in an analysis of the Multi-Ethnic Study of Atherosclerosis reported March 9 in JACC Cardiovascular Imaging.
The study is the first to examine the relationship between CAC and significant noncardiovascular diseases, said Dr. Catherine Handy of the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore. Patients with CAC scores of zero represent a unique group of “healthy agers,” she and her associates said. Conversely, 20% of initial non-CVD events occurred in the 10% of patients with CAC scores over 400, and 70% of events occurred in patients with scores greater than zero, they reported.
While CAC is an established indicator of vascular aging, CVD risk, and all-cause mortality, its relationship with non-CVD is unclear. To elucidate the issue, the researchers analyzed data from the prospective, observational Multi-Ethnic Study of Atherosclerosis, which included 6,814 adults aged 45-84 years from six U.S. cities. Patients had no CVD and were not receiving cancer treatment.
Over a median follow-up period of 10.2 years, and after controlling for demographic factors and predictors of CVD, patients with CAC scores exceeding 400 were significantly more likely to develop cancer (hazard ratio, 1.53), chronic kidney disease (HR, 1.70), pneumonia (HR, 1.97), chronic obstructive pulmonary disease (HR, 2.71) and hip fracture (HR, 4.29), compared with patients without detectable CAC. Patients with CAC scores of zero were at significantly lower risk of these diagnoses, compared with patients with scores greater than zero (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.02).
Doubling of CAC was a modest but significant predictor of cancer, chronic kidney disease, pneumonia, and chronic obstructive pulmonary disease in the subgroup of adults aged 65 years and older. However, CAC was not associated with dementia or deep vein thrombosis or pulmonary embolism.
Sparse diagnoses of hip fractures and DVT/PE meant that the study might be underpowered to clearly link CAC with risk of these events, said the researchers. There also might not have been enough follow-up time to uncover risk in participants with the lowest CAC scores, they said. “At this time, our data are not powered for stratifying results based on gender or race,” they added.
The National Institutes of Health funded the study. The researchers had no conflicts of interest.
The current report from the Multi-Ethnic Study of Atherosclerosis further expands the evidence base supporting the concept of coronary artery calcium as a marker of global health by examining its prognostic power across a diversity of noncardiovascular conditions.
Regardless of the directionality or magnitude of the connections between cardiovascular disease and non-CVD conditions, the extent to which coronary artery calcium–guided patient adherence to risk factor modification and lifestyle recommendations [affected] non-CVD conditions remains an additional link that should be explored further.
A synthesis of evidence, including the study by Handy et al., now supports the predictive ability of coronary artery calcium to estimate cardiac, cerebrovascular, and noncardiovascular conditions. We likely should come full circle in our discussion and acknowledge the far reaching implications of its predictive ability. Perhaps our index response that CAC should be fully integrated into all adult wellness and screening evaluations was on target after all!
Although CAC has not been without its critics and is not supported as a reimbursable procedure, its expansive evidence warrants a more thoughtful discussion within the CVD community that this powerful procedure provides valuable information to guide health care decision making.
Dr. Mosaab Awad, Dr. Parham Eshtehardi, and Leslee J. Shaw, Ph.D., of Emory University Clinical Cardiovascular Research Institute, Emory University, Atlanta, made these comments in an editorial (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.021). They had no disclosures.
The current report from the Multi-Ethnic Study of Atherosclerosis further expands the evidence base supporting the concept of coronary artery calcium as a marker of global health by examining its prognostic power across a diversity of noncardiovascular conditions.
Regardless of the directionality or magnitude of the connections between cardiovascular disease and non-CVD conditions, the extent to which coronary artery calcium–guided patient adherence to risk factor modification and lifestyle recommendations [affected] non-CVD conditions remains an additional link that should be explored further.
A synthesis of evidence, including the study by Handy et al., now supports the predictive ability of coronary artery calcium to estimate cardiac, cerebrovascular, and noncardiovascular conditions. We likely should come full circle in our discussion and acknowledge the far reaching implications of its predictive ability. Perhaps our index response that CAC should be fully integrated into all adult wellness and screening evaluations was on target after all!
Although CAC has not been without its critics and is not supported as a reimbursable procedure, its expansive evidence warrants a more thoughtful discussion within the CVD community that this powerful procedure provides valuable information to guide health care decision making.
Dr. Mosaab Awad, Dr. Parham Eshtehardi, and Leslee J. Shaw, Ph.D., of Emory University Clinical Cardiovascular Research Institute, Emory University, Atlanta, made these comments in an editorial (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.021). They had no disclosures.
The current report from the Multi-Ethnic Study of Atherosclerosis further expands the evidence base supporting the concept of coronary artery calcium as a marker of global health by examining its prognostic power across a diversity of noncardiovascular conditions.
Regardless of the directionality or magnitude of the connections between cardiovascular disease and non-CVD conditions, the extent to which coronary artery calcium–guided patient adherence to risk factor modification and lifestyle recommendations [affected] non-CVD conditions remains an additional link that should be explored further.
A synthesis of evidence, including the study by Handy et al., now supports the predictive ability of coronary artery calcium to estimate cardiac, cerebrovascular, and noncardiovascular conditions. We likely should come full circle in our discussion and acknowledge the far reaching implications of its predictive ability. Perhaps our index response that CAC should be fully integrated into all adult wellness and screening evaluations was on target after all!
Although CAC has not been without its critics and is not supported as a reimbursable procedure, its expansive evidence warrants a more thoughtful discussion within the CVD community that this powerful procedure provides valuable information to guide health care decision making.
Dr. Mosaab Awad, Dr. Parham Eshtehardi, and Leslee J. Shaw, Ph.D., of Emory University Clinical Cardiovascular Research Institute, Emory University, Atlanta, made these comments in an editorial (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.021). They had no disclosures.
Patients whose coronary artery calcium scores exceeded 400 were significantly more likely to develop cancer, chronic obstructive pulmonary disease, chronic kidney disease, and hip fractures, compared with adults with undetectable CAC, in an analysis of the Multi-Ethnic Study of Atherosclerosis reported March 9 in JACC Cardiovascular Imaging.
The study is the first to examine the relationship between CAC and significant noncardiovascular diseases, said Dr. Catherine Handy of the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore. Patients with CAC scores of zero represent a unique group of “healthy agers,” she and her associates said. Conversely, 20% of initial non-CVD events occurred in the 10% of patients with CAC scores over 400, and 70% of events occurred in patients with scores greater than zero, they reported.
While CAC is an established indicator of vascular aging, CVD risk, and all-cause mortality, its relationship with non-CVD is unclear. To elucidate the issue, the researchers analyzed data from the prospective, observational Multi-Ethnic Study of Atherosclerosis, which included 6,814 adults aged 45-84 years from six U.S. cities. Patients had no CVD and were not receiving cancer treatment.
Over a median follow-up period of 10.2 years, and after controlling for demographic factors and predictors of CVD, patients with CAC scores exceeding 400 were significantly more likely to develop cancer (hazard ratio, 1.53), chronic kidney disease (HR, 1.70), pneumonia (HR, 1.97), chronic obstructive pulmonary disease (HR, 2.71) and hip fracture (HR, 4.29), compared with patients without detectable CAC. Patients with CAC scores of zero were at significantly lower risk of these diagnoses, compared with patients with scores greater than zero (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.02).
Doubling of CAC was a modest but significant predictor of cancer, chronic kidney disease, pneumonia, and chronic obstructive pulmonary disease in the subgroup of adults aged 65 years and older. However, CAC was not associated with dementia or deep vein thrombosis or pulmonary embolism.
Sparse diagnoses of hip fractures and DVT/PE meant that the study might be underpowered to clearly link CAC with risk of these events, said the researchers. There also might not have been enough follow-up time to uncover risk in participants with the lowest CAC scores, they said. “At this time, our data are not powered for stratifying results based on gender or race,” they added.
The National Institutes of Health funded the study. The researchers had no conflicts of interest.
Patients whose coronary artery calcium scores exceeded 400 were significantly more likely to develop cancer, chronic obstructive pulmonary disease, chronic kidney disease, and hip fractures, compared with adults with undetectable CAC, in an analysis of the Multi-Ethnic Study of Atherosclerosis reported March 9 in JACC Cardiovascular Imaging.
The study is the first to examine the relationship between CAC and significant noncardiovascular diseases, said Dr. Catherine Handy of the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore. Patients with CAC scores of zero represent a unique group of “healthy agers,” she and her associates said. Conversely, 20% of initial non-CVD events occurred in the 10% of patients with CAC scores over 400, and 70% of events occurred in patients with scores greater than zero, they reported.
While CAC is an established indicator of vascular aging, CVD risk, and all-cause mortality, its relationship with non-CVD is unclear. To elucidate the issue, the researchers analyzed data from the prospective, observational Multi-Ethnic Study of Atherosclerosis, which included 6,814 adults aged 45-84 years from six U.S. cities. Patients had no CVD and were not receiving cancer treatment.
Over a median follow-up period of 10.2 years, and after controlling for demographic factors and predictors of CVD, patients with CAC scores exceeding 400 were significantly more likely to develop cancer (hazard ratio, 1.53), chronic kidney disease (HR, 1.70), pneumonia (HR, 1.97), chronic obstructive pulmonary disease (HR, 2.71) and hip fracture (HR, 4.29), compared with patients without detectable CAC. Patients with CAC scores of zero were at significantly lower risk of these diagnoses, compared with patients with scores greater than zero (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.02).
Doubling of CAC was a modest but significant predictor of cancer, chronic kidney disease, pneumonia, and chronic obstructive pulmonary disease in the subgroup of adults aged 65 years and older. However, CAC was not associated with dementia or deep vein thrombosis or pulmonary embolism.
Sparse diagnoses of hip fractures and DVT/PE meant that the study might be underpowered to clearly link CAC with risk of these events, said the researchers. There also might not have been enough follow-up time to uncover risk in participants with the lowest CAC scores, they said. “At this time, our data are not powered for stratifying results based on gender or race,” they added.
The National Institutes of Health funded the study. The researchers had no conflicts of interest.
FROM JACC CARDIOVASCULAR IMAGING
AAAAI: Albuterol Dry Powder Inhaler Offers Simplified Approach for Young Kids
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
AT 2016 AAAAI ANNUAL MEETING
AAAAI: Albuterol Dry Powder Inhaler Offers Simplified Approach for Young Kids
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
AT 2016 AAAAI ANNUAL MEETING
Helping patients with cystic fibrosis live longer
› Prescribe inhaled dornase alpha and inhaled tobramycin for maintenance pulmonary treatment of moderate to severe cystic fibrosis (CF). A
› Give aggressive nutritional supplementation to maintain a patient’s body mass index and blood sugar control and to attain maximal forced expiratory volume in one second (FEV1). B
› Consider prescribing cystic fibrosis transmembrane conductance regulator modulators, which have demonstrated a 5% to 10% improvement in FEV1 for CF patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The focus of treatment. CF is not limited to the classic picture of lung and pancreas destruction with subsequent loss of function. The underlying pathology can occur in body epithelial tissues from the intestinal lining to sweat glands. These tissues contain cystic fibrosis transmembrane conductance regulator (CFTR), a protein that allows for the transport of chloride across epithelial cell membranes.2 In individuals homozygous for mutated CFTR genes, chloride transport can be impaired. In addition to regulating chloride transport, CFTR is part of a larger, complex interaction of ion transport proteins such as the epithelial sodium channel (ENaC) and others that regulate bicarbonate secretion.2 Decreased chloride ion transport in mutant CFTR negatively affects the ion transport complex; the result is a higher-than-normal viscosity of secreted body fluids.
Reason for hope. It is this impairment of chloride ion transport that leads to the classic phenotypic features of CF (eg, pulmonary function decline, pancreatic insufficiency, malnutrition, chronic respiratory infection), and is the target of both established and emerging therapies3—both of which I will review here.
When to consider a CF diagnosis
Cystic fibrosis remains a clinical diagnosis when evidence of at least one phenotypic feature of the disease (TABLE 1) exists in the presence of laboratory evidence of a CFTR abnormality.4 Confirmation of CFTR dysfunction is demonstrated by an abnormality on sweat testing or identification of a CF-causing mutation in each copy of CFTR (ie, one on each chromosome).5 All 50 states now have neonatal laboratory screening programs;4 despite this, 30% of cases in 2012 were still diagnosed in those older than 1 year of age, with 3% to 5% diagnosed after age 18.1
A sweat chloride reading in the abnormal range (>60 mmol/L) is present in 90% of patients diagnosed with CF in adulthood; this test remains the gold standard in the diagnosis of CF and the initial test of choice in suspected cases.4 Newborn screening programs identify those at risk by detecting persistent hypertrypsinogenemia and referring those with positive results for definitive testing with sweat chloride evaluation. Keep CF in mind when evaluating adolescents and adults who have chronic sinusitis, chronic/recurrent pulmonary infections, chronic/recurrent pancreatitis, or infertility from absence of the vas deferens.4 When features of the CF phenotype are present, especially if there is a known positive family history of CF or CF carrier status, order sweat chloride testing.
Traditional therapies
Both maintenance and acute therapies are directed throughout the body at decreasing fluid viscosity, clearing fluid with a high viscosity, or treating the tissue destruction that results from highly-viscous fluid.3 The traditional classic picture of CF is one of lung and pancreas destruction with subsequent loss of function. However, CF is, in reality, a full-body disease.
Respiratory system: Lungs
CFTR dysfunction in the lungs results in thick pulmonary secretions as the aqueous surface layer (ASL) lining the alveolar epithelium becomes dehydrated and creates a prime environment for the development of chronic infection. What ensues is a recurrent cycle of chronic infection, inflammation, and tissue destruction with loss of lung volume and function. Current therapies interrupt this cycle at multiple points.6
Airway clearance is one of the hallmarks of CF therapy, using both chemical and mechanical treatments. Daily, most patients will use either a therapy vest that administers sheering forces to the chest cavity or an airflow device that creates positive expiratory pressure and laminar flow to aid in expectorating pulmonary secretions.7 Because exercise has yielded comparable results to mechanical or airflow clearance devices, it is recommended that all CF patients who are not otherwise prohibited engage in regular, vigorous exercise in accordance with standard recommendations for the general public.7
Mechanical therapies are often preceded by airway dilation with short- and long-acting bronchodilators and inhaled steroids that open airways for optimal airway clearance.4 Thick secretions can be treated directly and enzymatically with nebulized dornase alpha,4,8 which is also best administered before mechanical clearance therapy. Finally, viscosity of airway secretions can be decreased by improving the hydration of the ASL with nebulized 7% hypertonic saline.4,8
Infection suppression. Thickened pulmonary secretions create a fertile environment for the development of chronic infection. By the time most CF patients reach adulthood, many are colonized with mucoid producing strains of Pseudomonas aeruginosa.4,8-10 Many may also have chronic infection with Staphylococcus aureus, some strains of which may be methicillin-resistant. Quarterly culture and sensitivity results can be essential in directing acute antibiotic therapy, both in the hospital and ambulatory settings. In addition, in the case of Pseudomonas, inhaled antibiotics suppress chronic infection, improve lung function, decrease pulmonary secretions, and reduce inflammation.
Formulations are available for tobramycin and aztreonam, both of which are administered every other month to reduce toxicities and to deter antibiotic resistance. Some patients may use a single agent or may alternate agents every month. When acute antibiotic therapy is necessary for a pulmonary exacerbation, the inhaled agent is generally withheld. If outpatient treatment is warranted, the only available oral antibiotics with anti-pseudomonal activity are ciprofloxacin and levofloxacin.4,8-10S aureus can be treated with trimethoprim/sulfamethoxazole or doxycycline.4,8-10
Inflammation reduction is addressed with high-dose ibuprofen twice daily, azithromycin daily or 3 times weekly, or both. Children up to age 18 benefit from ibuprofen, which also improves forced expiratory volume in one second (FEV1) to a greater extent than azithromycin.8 Adults, however, face the risk of gastrointestinal bleeding and renal dysfunction with ibuprofen, which must be weighed against its potential anti-inflammatory benefit. Both populations, however, benefit from chronic azithromycin, whose mechanism of action in this setting is believed to be more anti-inflammatory than bacterial suppression, since it has no direct bactericidal effect on the primary colonizing microbe, P aeruginosa.4,11
Gastrointestinal system: Pancreas
Cystic fibrosis was first comprehensively described in 1938 and was named for the diseased appearance of the pancreas.12 As happens in the lungs, thick pancreatic duct secretions create a cycle of tissue destruction, inflammation, and dysfunction.2 CF patients lack adequate secretion of pancreatic enzymes and bicarbonate into the small bowel, which progressively leads to pancreatic dysfunction in most patients.
As malabsorption of nutrients advances, patients suffer varying degrees of malnutrition and vitamin deficiency, especially of the fat-soluble vitamins A, D, E, and K. Over 85% of CF patients have deficient pancreatic function, requiring pancreatic enzyme supplementation with all food intake and daily vitamin supplementation.2
Ensuring adequate nutrition. Most CF patients experience a chronic mismatch of dietary intake against caloric expenditure and benefit from aggressive nutritional management featuring a high-calorie diet with supplementation in the form of nutrition shakes or bars.2 There is a well-documented linear relationship between BMI and FEV1. Lung function declines in CF when a man’s body mass index (BMI) falls below 23 kg/m2 and a woman’s BMI drops below 22 kg/m2.2 For this reason, the goal for caloric intake can be as high as 200% of the customary recommended daily allowance.2
Watch for CF-related diabetes. Since the pancreas is also the major source of endogenous insulin, nearly half of adults with CF will develop cystic fibrosis-related diabetes (CFRD) as pancreatic deficiency progresses.13 Similar to the relationship between BMI and FEV1, there is a relationship between glucose intolerance and FEV1. For this reason, annual diabetes screening is recommended for all CF patients ages 10 years and older.13 Because glycated hemoglobin (HbA1c) may not accurately reflect low levels of glucose intolerance, screen for CFRD with a 2-hour 75-g oral glucose tolerance test.13 Early insulin therapy can help maintain BMI and lower average blood sugar in support of FEV1. Once CFRD is diagnosed, the goals and recommendations for control are largely the same as those recommended by the American Diabetes Association for other forms of diabetes.13
Cystic Fibrosis Resources
Cystic Fibrosis Foundation
www.cff.org
Consensus report on cystic fibrosis management
Yankaskas JR, Marchall BC, Sufian B, et al. Cystic fibrosis adult care. Chest. 2004;125:1S-39S.
Consensus report on cystic fibrosis diagnostic guidelines
Farrell PM, Rosenstein BJ, White RB. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation Consensus Report. J Pediatr. 2008;153:S4-S14.
Gastrointestinal system: Alimentary canal
CF is often mistakenly believed to be primarily a pulmonary disease since 85% of the mortality is due to lung dysfunction,7 but intermittent abdominal pain is a common experience for most patients, and disorders can range from gastroesophageal reflux disease (GERD) to small bowel bacterial overgrowth (SBBO) to constipation. Up to 85% of adult patients experience symptoms of reflux, with as many as 40% of cases occurring silently.2 Proton pump inhibitors are a first-line treatment, but they can also contribute to intestinal bacterial overgrowth and pulmonary infections.
In SBBO, gram-negative colonic bacteria colonize the small bowel and can contribute to abdominal pain and malabsorption, weight loss, and malnutrition. Treatment requires antibiotics with activity against gram-negative organisms, or non-absorbable agents such as rifamyxin, sometimes on a chronic, recurrent, or rotating basis.2
Chronic constipation is also quite common among CF patients and many require daily administration of poly-ethylene-glycol. Before newborn screening programs were introduced, infants would on occasion present with complete distal intestinal obstruction. Adults are not immune to obstructive complications and may require hospitalization for bowel cleansing.
Gastrointestinal system: Liver
Liver disease is relatively common in CF, with up to 24% of adults experiencing hepatomegaly or persistently elevated liver function tests (LFT).4 Progressive biliary fibrosis and cirrhosis are encountered more often as the median survival age has increased. There is evidence that ursodeoxycholic acid (UDCA) can be a useful adjunct in the treatment of cholestasis, but it is not clear if it alters mortality or progression to cirrhosis. Only CF patients with elevated LFTs should be started on UDCA.4
Other areas of concern: Sinuses, serum sodium levels
Chronic, symptomatic sinus disease in CF patients—chiefly polyposis—is common and may require repeat surgery, although most patients with extensive nasal polyps find symptom relief with daily sinus rinses. Intranasal steroids and intranasal antibiotics are also often employed, and many CF patients need to be in regular contact with an otolaryngologist.14 For symptoms of allergic rhinitis, recommend OTC antihistamines in standard dosages.
Exercise is recommended for all CF patients, as noted earlier, and as life expectancy increases, many are engaging in more strenuous and longer duration activities.15 Due to high sweat sodium loss, CF patients are at risk for hyponatremia, especially when exercising on days with high temperatures and humidity. CF patients need to replace sodium losses in these conditions and when exercising for extended periods.
There are no evidence-based guidelines for sodium replacement. The Cystic Fibrosis Foundation (CFF) recommends that patients increase salt in the diet when under conditions likely to result in increased sodium loss, such as exercise. It has been thought that CF patients can easily dehydrate due to an impaired thirst mechanism and, when exercising, should consume fluids beyond the need to quench thirst.16,17 More recent evidence suggests, however, that the thirst mechanism in those with CF remains normally intact and that overconsumption of fluids beyond the level of thirst may predispose the individual to exercise-associated hyponatremia as serum sodium is diluted.15
New therapies
Small-molecule CFTR-modulating compounds are a promising development in the treatment of CF. The first such available medication was ivacaftor in 2012. Because these molecules are mutation specific, ivacaftor was available at first only for patients with at least one copy of the G551D mutation,18 which means about 5% of patients with CF.3
Ivacaftor increases the likelihood that the CFTR chloride channel will open and patients will exhibit a reduction in sweat chloride levels. In the first reported clinical trial of ivacaftor involving patients with the G551D mutation, FEV1 improvements of 10% occurred by the second week of therapy and persisted for 48 weeks.18 The drug has now been approved by the US Food and Drug Administration (FDA) for patients 12 years of age and older with at least one of the following mutations: R117H, G551D, G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P, or G1349D.19
A medication combining ivacaftor with lumacaftor is also now available for patients with a copy of F508del on both chromosomes. F508del is the most common CFTR mutation, with one copy present in almost 87% of people with CF in the United States.1 Since 47% of CF patients have 2 copies of F508del,1 about half of those with CF in the United States are now eligible for small-molecule therapy. Lumacaftor acts by facilitating transport of a misfolded CFTR to the cell membrane where ivacaftor then increases the probability of an open chloride channel. This combination medication has improved lung function by about 5%.
The ivacaftor/lumacaftor combination was approved by the FDA in July 2015. Both ivacaftor and the ivacaftor/lumacaftor combination were deemed by the FDA to demonstrate statistically significant and sustained FEV1 improvements over placebo.
The CFF was instrumental in providing financial support for the development of both ivacaftor and the ivacaftor/lumacaftor combination and continues to provide significant research advancement. According to the CFF (www.cff.org), medications currently in the development pipeline include compounds that provide CFTR modulation, surface airway liquid restoration, anti-inflammation, inhaled anti-infection, and pancreatic enzyme function. For more on CFF, see "The traditional CF care model.”4,20
The traditional CF care model
The Cystic Fibrosis Foundation (CFF) has been a driving force behind the increased life expectancy CF patients have seen over the last 3 decades. Its contributions include the development of medication through the CFF Therapeutics Development Network (TDN) and disease management through a network of CF Care Centers throughout the United States. The CFF recommends a minimum of quarterly visits to a CF Care Center, and the primary care physician can play a critical role alongside the multidisciplinary CF team.20
At every CF Care Center encounter, the entire team (nurse, physician, dietician, social worker, psychologist) interacts with each patient and their families to maximize overall medical care. Respiratory cultures are generally obtained at each visit. Dual-energy x-ray absorptiometry is performed biannually. Lab work (complete blood count, comprehensive metabolic panel, glycated hemoglobin, vitamins A, D, E, and K, 2-hour glucose tolerance test), and chest x-ray are obtained at least annually (TABLE 2).4
Since CF generally involves both restrictive and obstructive lung components, complete spirometry evaluation is performed annually in the pulmonary function lab, with static lung volumes in addition to airflow measurement. Office spirometry to measure airflow alone is performed at each visit. FEV1 is tracked both as an indicator of disease progression and as a measure of current pulmonary status.
The CFF recommends that each patient receive full genetic testing and encourages patient participation in the CFF Registry, where mutation data are documented among other disease parameters to ensure that patients receive mutation specific therapies as they become available.4 The vaccine schedule recommended for CF patients is the same as for the general population.
CORRESPONDENCE
Douglas Lewis, MD, 1121 S. Clifton, Wichita, KS 67218; [email protected].
1. Cystic Fibrosis Foundation. Patient registry 2012 annual data report. Cystic Fibrosis Foundation Web site. Available at: http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf. Accessed August 14, 2014.
2. Haller W, Ledder O, Lewindon PJ, et al. Cystic fibrosis: An update for clinicians. Part 1: Nutrition and gastrointestinal complications. J Gastroenterol Hepatol. 2014;29:1344-1355.
3. Hoffman LR, Ramsey BW. Cystic fibrosis therapeutics: the road ahead. Chest. 2013;143:207-213.
4. Yankaskas JR, Marshall BC, Sufian B, et al. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S-39S.
5. Farrell PM, Rosenstein BJ, White TB, et al; Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4-S14.
6. Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631-1636.
7. Flume PA, Robinson KA, O’Sullivan BP, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54:522-537.
8. Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957-969.
9. Döring G, Flume P, Heijerman H, et al; Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461-479.
10. Flume PA, Mogayzel PJ Jr, Robinson KA, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802-808.
11. Southern KW, Barker PM. Azithromycin for cystic fibrosis. Eur Respir J. 2004;24:834-838.
12. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344-399.
13. Moran A, Brunzell C, Cohen RC, et al; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697-2708.
14. Kerem E, Conway S, Elborn S, et al; Consensus Committee. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2005;4:7-26.
15. Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin J Sport Med. 2015;25:303-320.
16. Brown MB, McCarty NA, Millard-Stafford M. High-sweat Na+ in cystic fibrosis and healthy individuals does not diminish thirst during exercise in the heat. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1177-R1185.
17. Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exerc Sport Sci Rev. 2011;39:155-160.
18. Ramsey BW, Davies J, McElvaney NG, et al; VX08-770-102 Study Group. ACFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663-1672.
19. Pettit RS, Fellner C. CFTR Modulators for the Treatment of Cystic Fibrosis. P T. 2014;39:500-511.
20. Lewis D. Role of the family physician in the management of cystic fibrosis. Am Fam Physician. 2015;91:822-824.
› Prescribe inhaled dornase alpha and inhaled tobramycin for maintenance pulmonary treatment of moderate to severe cystic fibrosis (CF). A
› Give aggressive nutritional supplementation to maintain a patient’s body mass index and blood sugar control and to attain maximal forced expiratory volume in one second (FEV1). B
› Consider prescribing cystic fibrosis transmembrane conductance regulator modulators, which have demonstrated a 5% to 10% improvement in FEV1 for CF patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The focus of treatment. CF is not limited to the classic picture of lung and pancreas destruction with subsequent loss of function. The underlying pathology can occur in body epithelial tissues from the intestinal lining to sweat glands. These tissues contain cystic fibrosis transmembrane conductance regulator (CFTR), a protein that allows for the transport of chloride across epithelial cell membranes.2 In individuals homozygous for mutated CFTR genes, chloride transport can be impaired. In addition to regulating chloride transport, CFTR is part of a larger, complex interaction of ion transport proteins such as the epithelial sodium channel (ENaC) and others that regulate bicarbonate secretion.2 Decreased chloride ion transport in mutant CFTR negatively affects the ion transport complex; the result is a higher-than-normal viscosity of secreted body fluids.
Reason for hope. It is this impairment of chloride ion transport that leads to the classic phenotypic features of CF (eg, pulmonary function decline, pancreatic insufficiency, malnutrition, chronic respiratory infection), and is the target of both established and emerging therapies3—both of which I will review here.
When to consider a CF diagnosis
Cystic fibrosis remains a clinical diagnosis when evidence of at least one phenotypic feature of the disease (TABLE 1) exists in the presence of laboratory evidence of a CFTR abnormality.4 Confirmation of CFTR dysfunction is demonstrated by an abnormality on sweat testing or identification of a CF-causing mutation in each copy of CFTR (ie, one on each chromosome).5 All 50 states now have neonatal laboratory screening programs;4 despite this, 30% of cases in 2012 were still diagnosed in those older than 1 year of age, with 3% to 5% diagnosed after age 18.1
A sweat chloride reading in the abnormal range (>60 mmol/L) is present in 90% of patients diagnosed with CF in adulthood; this test remains the gold standard in the diagnosis of CF and the initial test of choice in suspected cases.4 Newborn screening programs identify those at risk by detecting persistent hypertrypsinogenemia and referring those with positive results for definitive testing with sweat chloride evaluation. Keep CF in mind when evaluating adolescents and adults who have chronic sinusitis, chronic/recurrent pulmonary infections, chronic/recurrent pancreatitis, or infertility from absence of the vas deferens.4 When features of the CF phenotype are present, especially if there is a known positive family history of CF or CF carrier status, order sweat chloride testing.
Traditional therapies
Both maintenance and acute therapies are directed throughout the body at decreasing fluid viscosity, clearing fluid with a high viscosity, or treating the tissue destruction that results from highly-viscous fluid.3 The traditional classic picture of CF is one of lung and pancreas destruction with subsequent loss of function. However, CF is, in reality, a full-body disease.
Respiratory system: Lungs
CFTR dysfunction in the lungs results in thick pulmonary secretions as the aqueous surface layer (ASL) lining the alveolar epithelium becomes dehydrated and creates a prime environment for the development of chronic infection. What ensues is a recurrent cycle of chronic infection, inflammation, and tissue destruction with loss of lung volume and function. Current therapies interrupt this cycle at multiple points.6
Airway clearance is one of the hallmarks of CF therapy, using both chemical and mechanical treatments. Daily, most patients will use either a therapy vest that administers sheering forces to the chest cavity or an airflow device that creates positive expiratory pressure and laminar flow to aid in expectorating pulmonary secretions.7 Because exercise has yielded comparable results to mechanical or airflow clearance devices, it is recommended that all CF patients who are not otherwise prohibited engage in regular, vigorous exercise in accordance with standard recommendations for the general public.7
Mechanical therapies are often preceded by airway dilation with short- and long-acting bronchodilators and inhaled steroids that open airways for optimal airway clearance.4 Thick secretions can be treated directly and enzymatically with nebulized dornase alpha,4,8 which is also best administered before mechanical clearance therapy. Finally, viscosity of airway secretions can be decreased by improving the hydration of the ASL with nebulized 7% hypertonic saline.4,8
Infection suppression. Thickened pulmonary secretions create a fertile environment for the development of chronic infection. By the time most CF patients reach adulthood, many are colonized with mucoid producing strains of Pseudomonas aeruginosa.4,8-10 Many may also have chronic infection with Staphylococcus aureus, some strains of which may be methicillin-resistant. Quarterly culture and sensitivity results can be essential in directing acute antibiotic therapy, both in the hospital and ambulatory settings. In addition, in the case of Pseudomonas, inhaled antibiotics suppress chronic infection, improve lung function, decrease pulmonary secretions, and reduce inflammation.
Formulations are available for tobramycin and aztreonam, both of which are administered every other month to reduce toxicities and to deter antibiotic resistance. Some patients may use a single agent or may alternate agents every month. When acute antibiotic therapy is necessary for a pulmonary exacerbation, the inhaled agent is generally withheld. If outpatient treatment is warranted, the only available oral antibiotics with anti-pseudomonal activity are ciprofloxacin and levofloxacin.4,8-10S aureus can be treated with trimethoprim/sulfamethoxazole or doxycycline.4,8-10
Inflammation reduction is addressed with high-dose ibuprofen twice daily, azithromycin daily or 3 times weekly, or both. Children up to age 18 benefit from ibuprofen, which also improves forced expiratory volume in one second (FEV1) to a greater extent than azithromycin.8 Adults, however, face the risk of gastrointestinal bleeding and renal dysfunction with ibuprofen, which must be weighed against its potential anti-inflammatory benefit. Both populations, however, benefit from chronic azithromycin, whose mechanism of action in this setting is believed to be more anti-inflammatory than bacterial suppression, since it has no direct bactericidal effect on the primary colonizing microbe, P aeruginosa.4,11
Gastrointestinal system: Pancreas
Cystic fibrosis was first comprehensively described in 1938 and was named for the diseased appearance of the pancreas.12 As happens in the lungs, thick pancreatic duct secretions create a cycle of tissue destruction, inflammation, and dysfunction.2 CF patients lack adequate secretion of pancreatic enzymes and bicarbonate into the small bowel, which progressively leads to pancreatic dysfunction in most patients.
As malabsorption of nutrients advances, patients suffer varying degrees of malnutrition and vitamin deficiency, especially of the fat-soluble vitamins A, D, E, and K. Over 85% of CF patients have deficient pancreatic function, requiring pancreatic enzyme supplementation with all food intake and daily vitamin supplementation.2
Ensuring adequate nutrition. Most CF patients experience a chronic mismatch of dietary intake against caloric expenditure and benefit from aggressive nutritional management featuring a high-calorie diet with supplementation in the form of nutrition shakes or bars.2 There is a well-documented linear relationship between BMI and FEV1. Lung function declines in CF when a man’s body mass index (BMI) falls below 23 kg/m2 and a woman’s BMI drops below 22 kg/m2.2 For this reason, the goal for caloric intake can be as high as 200% of the customary recommended daily allowance.2
Watch for CF-related diabetes. Since the pancreas is also the major source of endogenous insulin, nearly half of adults with CF will develop cystic fibrosis-related diabetes (CFRD) as pancreatic deficiency progresses.13 Similar to the relationship between BMI and FEV1, there is a relationship between glucose intolerance and FEV1. For this reason, annual diabetes screening is recommended for all CF patients ages 10 years and older.13 Because glycated hemoglobin (HbA1c) may not accurately reflect low levels of glucose intolerance, screen for CFRD with a 2-hour 75-g oral glucose tolerance test.13 Early insulin therapy can help maintain BMI and lower average blood sugar in support of FEV1. Once CFRD is diagnosed, the goals and recommendations for control are largely the same as those recommended by the American Diabetes Association for other forms of diabetes.13
Cystic Fibrosis Resources
Cystic Fibrosis Foundation
www.cff.org
Consensus report on cystic fibrosis management
Yankaskas JR, Marchall BC, Sufian B, et al. Cystic fibrosis adult care. Chest. 2004;125:1S-39S.
Consensus report on cystic fibrosis diagnostic guidelines
Farrell PM, Rosenstein BJ, White RB. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation Consensus Report. J Pediatr. 2008;153:S4-S14.
Gastrointestinal system: Alimentary canal
CF is often mistakenly believed to be primarily a pulmonary disease since 85% of the mortality is due to lung dysfunction,7 but intermittent abdominal pain is a common experience for most patients, and disorders can range from gastroesophageal reflux disease (GERD) to small bowel bacterial overgrowth (SBBO) to constipation. Up to 85% of adult patients experience symptoms of reflux, with as many as 40% of cases occurring silently.2 Proton pump inhibitors are a first-line treatment, but they can also contribute to intestinal bacterial overgrowth and pulmonary infections.
In SBBO, gram-negative colonic bacteria colonize the small bowel and can contribute to abdominal pain and malabsorption, weight loss, and malnutrition. Treatment requires antibiotics with activity against gram-negative organisms, or non-absorbable agents such as rifamyxin, sometimes on a chronic, recurrent, or rotating basis.2
Chronic constipation is also quite common among CF patients and many require daily administration of poly-ethylene-glycol. Before newborn screening programs were introduced, infants would on occasion present with complete distal intestinal obstruction. Adults are not immune to obstructive complications and may require hospitalization for bowel cleansing.
Gastrointestinal system: Liver
Liver disease is relatively common in CF, with up to 24% of adults experiencing hepatomegaly or persistently elevated liver function tests (LFT).4 Progressive biliary fibrosis and cirrhosis are encountered more often as the median survival age has increased. There is evidence that ursodeoxycholic acid (UDCA) can be a useful adjunct in the treatment of cholestasis, but it is not clear if it alters mortality or progression to cirrhosis. Only CF patients with elevated LFTs should be started on UDCA.4
Other areas of concern: Sinuses, serum sodium levels
Chronic, symptomatic sinus disease in CF patients—chiefly polyposis—is common and may require repeat surgery, although most patients with extensive nasal polyps find symptom relief with daily sinus rinses. Intranasal steroids and intranasal antibiotics are also often employed, and many CF patients need to be in regular contact with an otolaryngologist.14 For symptoms of allergic rhinitis, recommend OTC antihistamines in standard dosages.
Exercise is recommended for all CF patients, as noted earlier, and as life expectancy increases, many are engaging in more strenuous and longer duration activities.15 Due to high sweat sodium loss, CF patients are at risk for hyponatremia, especially when exercising on days with high temperatures and humidity. CF patients need to replace sodium losses in these conditions and when exercising for extended periods.
There are no evidence-based guidelines for sodium replacement. The Cystic Fibrosis Foundation (CFF) recommends that patients increase salt in the diet when under conditions likely to result in increased sodium loss, such as exercise. It has been thought that CF patients can easily dehydrate due to an impaired thirst mechanism and, when exercising, should consume fluids beyond the need to quench thirst.16,17 More recent evidence suggests, however, that the thirst mechanism in those with CF remains normally intact and that overconsumption of fluids beyond the level of thirst may predispose the individual to exercise-associated hyponatremia as serum sodium is diluted.15
New therapies
Small-molecule CFTR-modulating compounds are a promising development in the treatment of CF. The first such available medication was ivacaftor in 2012. Because these molecules are mutation specific, ivacaftor was available at first only for patients with at least one copy of the G551D mutation,18 which means about 5% of patients with CF.3
Ivacaftor increases the likelihood that the CFTR chloride channel will open and patients will exhibit a reduction in sweat chloride levels. In the first reported clinical trial of ivacaftor involving patients with the G551D mutation, FEV1 improvements of 10% occurred by the second week of therapy and persisted for 48 weeks.18 The drug has now been approved by the US Food and Drug Administration (FDA) for patients 12 years of age and older with at least one of the following mutations: R117H, G551D, G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P, or G1349D.19
A medication combining ivacaftor with lumacaftor is also now available for patients with a copy of F508del on both chromosomes. F508del is the most common CFTR mutation, with one copy present in almost 87% of people with CF in the United States.1 Since 47% of CF patients have 2 copies of F508del,1 about half of those with CF in the United States are now eligible for small-molecule therapy. Lumacaftor acts by facilitating transport of a misfolded CFTR to the cell membrane where ivacaftor then increases the probability of an open chloride channel. This combination medication has improved lung function by about 5%.
The ivacaftor/lumacaftor combination was approved by the FDA in July 2015. Both ivacaftor and the ivacaftor/lumacaftor combination were deemed by the FDA to demonstrate statistically significant and sustained FEV1 improvements over placebo.
The CFF was instrumental in providing financial support for the development of both ivacaftor and the ivacaftor/lumacaftor combination and continues to provide significant research advancement. According to the CFF (www.cff.org), medications currently in the development pipeline include compounds that provide CFTR modulation, surface airway liquid restoration, anti-inflammation, inhaled anti-infection, and pancreatic enzyme function. For more on CFF, see "The traditional CF care model.”4,20
The traditional CF care model
The Cystic Fibrosis Foundation (CFF) has been a driving force behind the increased life expectancy CF patients have seen over the last 3 decades. Its contributions include the development of medication through the CFF Therapeutics Development Network (TDN) and disease management through a network of CF Care Centers throughout the United States. The CFF recommends a minimum of quarterly visits to a CF Care Center, and the primary care physician can play a critical role alongside the multidisciplinary CF team.20
At every CF Care Center encounter, the entire team (nurse, physician, dietician, social worker, psychologist) interacts with each patient and their families to maximize overall medical care. Respiratory cultures are generally obtained at each visit. Dual-energy x-ray absorptiometry is performed biannually. Lab work (complete blood count, comprehensive metabolic panel, glycated hemoglobin, vitamins A, D, E, and K, 2-hour glucose tolerance test), and chest x-ray are obtained at least annually (TABLE 2).4
Since CF generally involves both restrictive and obstructive lung components, complete spirometry evaluation is performed annually in the pulmonary function lab, with static lung volumes in addition to airflow measurement. Office spirometry to measure airflow alone is performed at each visit. FEV1 is tracked both as an indicator of disease progression and as a measure of current pulmonary status.
The CFF recommends that each patient receive full genetic testing and encourages patient participation in the CFF Registry, where mutation data are documented among other disease parameters to ensure that patients receive mutation specific therapies as they become available.4 The vaccine schedule recommended for CF patients is the same as for the general population.
CORRESPONDENCE
Douglas Lewis, MD, 1121 S. Clifton, Wichita, KS 67218; [email protected].
› Prescribe inhaled dornase alpha and inhaled tobramycin for maintenance pulmonary treatment of moderate to severe cystic fibrosis (CF). A
› Give aggressive nutritional supplementation to maintain a patient’s body mass index and blood sugar control and to attain maximal forced expiratory volume in one second (FEV1). B
› Consider prescribing cystic fibrosis transmembrane conductance regulator modulators, which have demonstrated a 5% to 10% improvement in FEV1 for CF patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The focus of treatment. CF is not limited to the classic picture of lung and pancreas destruction with subsequent loss of function. The underlying pathology can occur in body epithelial tissues from the intestinal lining to sweat glands. These tissues contain cystic fibrosis transmembrane conductance regulator (CFTR), a protein that allows for the transport of chloride across epithelial cell membranes.2 In individuals homozygous for mutated CFTR genes, chloride transport can be impaired. In addition to regulating chloride transport, CFTR is part of a larger, complex interaction of ion transport proteins such as the epithelial sodium channel (ENaC) and others that regulate bicarbonate secretion.2 Decreased chloride ion transport in mutant CFTR negatively affects the ion transport complex; the result is a higher-than-normal viscosity of secreted body fluids.
Reason for hope. It is this impairment of chloride ion transport that leads to the classic phenotypic features of CF (eg, pulmonary function decline, pancreatic insufficiency, malnutrition, chronic respiratory infection), and is the target of both established and emerging therapies3—both of which I will review here.
When to consider a CF diagnosis
Cystic fibrosis remains a clinical diagnosis when evidence of at least one phenotypic feature of the disease (TABLE 1) exists in the presence of laboratory evidence of a CFTR abnormality.4 Confirmation of CFTR dysfunction is demonstrated by an abnormality on sweat testing or identification of a CF-causing mutation in each copy of CFTR (ie, one on each chromosome).5 All 50 states now have neonatal laboratory screening programs;4 despite this, 30% of cases in 2012 were still diagnosed in those older than 1 year of age, with 3% to 5% diagnosed after age 18.1
A sweat chloride reading in the abnormal range (>60 mmol/L) is present in 90% of patients diagnosed with CF in adulthood; this test remains the gold standard in the diagnosis of CF and the initial test of choice in suspected cases.4 Newborn screening programs identify those at risk by detecting persistent hypertrypsinogenemia and referring those with positive results for definitive testing with sweat chloride evaluation. Keep CF in mind when evaluating adolescents and adults who have chronic sinusitis, chronic/recurrent pulmonary infections, chronic/recurrent pancreatitis, or infertility from absence of the vas deferens.4 When features of the CF phenotype are present, especially if there is a known positive family history of CF or CF carrier status, order sweat chloride testing.
Traditional therapies
Both maintenance and acute therapies are directed throughout the body at decreasing fluid viscosity, clearing fluid with a high viscosity, or treating the tissue destruction that results from highly-viscous fluid.3 The traditional classic picture of CF is one of lung and pancreas destruction with subsequent loss of function. However, CF is, in reality, a full-body disease.
Respiratory system: Lungs
CFTR dysfunction in the lungs results in thick pulmonary secretions as the aqueous surface layer (ASL) lining the alveolar epithelium becomes dehydrated and creates a prime environment for the development of chronic infection. What ensues is a recurrent cycle of chronic infection, inflammation, and tissue destruction with loss of lung volume and function. Current therapies interrupt this cycle at multiple points.6
Airway clearance is one of the hallmarks of CF therapy, using both chemical and mechanical treatments. Daily, most patients will use either a therapy vest that administers sheering forces to the chest cavity or an airflow device that creates positive expiratory pressure and laminar flow to aid in expectorating pulmonary secretions.7 Because exercise has yielded comparable results to mechanical or airflow clearance devices, it is recommended that all CF patients who are not otherwise prohibited engage in regular, vigorous exercise in accordance with standard recommendations for the general public.7
Mechanical therapies are often preceded by airway dilation with short- and long-acting bronchodilators and inhaled steroids that open airways for optimal airway clearance.4 Thick secretions can be treated directly and enzymatically with nebulized dornase alpha,4,8 which is also best administered before mechanical clearance therapy. Finally, viscosity of airway secretions can be decreased by improving the hydration of the ASL with nebulized 7% hypertonic saline.4,8
Infection suppression. Thickened pulmonary secretions create a fertile environment for the development of chronic infection. By the time most CF patients reach adulthood, many are colonized with mucoid producing strains of Pseudomonas aeruginosa.4,8-10 Many may also have chronic infection with Staphylococcus aureus, some strains of which may be methicillin-resistant. Quarterly culture and sensitivity results can be essential in directing acute antibiotic therapy, both in the hospital and ambulatory settings. In addition, in the case of Pseudomonas, inhaled antibiotics suppress chronic infection, improve lung function, decrease pulmonary secretions, and reduce inflammation.
Formulations are available for tobramycin and aztreonam, both of which are administered every other month to reduce toxicities and to deter antibiotic resistance. Some patients may use a single agent or may alternate agents every month. When acute antibiotic therapy is necessary for a pulmonary exacerbation, the inhaled agent is generally withheld. If outpatient treatment is warranted, the only available oral antibiotics with anti-pseudomonal activity are ciprofloxacin and levofloxacin.4,8-10S aureus can be treated with trimethoprim/sulfamethoxazole or doxycycline.4,8-10
Inflammation reduction is addressed with high-dose ibuprofen twice daily, azithromycin daily or 3 times weekly, or both. Children up to age 18 benefit from ibuprofen, which also improves forced expiratory volume in one second (FEV1) to a greater extent than azithromycin.8 Adults, however, face the risk of gastrointestinal bleeding and renal dysfunction with ibuprofen, which must be weighed against its potential anti-inflammatory benefit. Both populations, however, benefit from chronic azithromycin, whose mechanism of action in this setting is believed to be more anti-inflammatory than bacterial suppression, since it has no direct bactericidal effect on the primary colonizing microbe, P aeruginosa.4,11
Gastrointestinal system: Pancreas
Cystic fibrosis was first comprehensively described in 1938 and was named for the diseased appearance of the pancreas.12 As happens in the lungs, thick pancreatic duct secretions create a cycle of tissue destruction, inflammation, and dysfunction.2 CF patients lack adequate secretion of pancreatic enzymes and bicarbonate into the small bowel, which progressively leads to pancreatic dysfunction in most patients.
As malabsorption of nutrients advances, patients suffer varying degrees of malnutrition and vitamin deficiency, especially of the fat-soluble vitamins A, D, E, and K. Over 85% of CF patients have deficient pancreatic function, requiring pancreatic enzyme supplementation with all food intake and daily vitamin supplementation.2
Ensuring adequate nutrition. Most CF patients experience a chronic mismatch of dietary intake against caloric expenditure and benefit from aggressive nutritional management featuring a high-calorie diet with supplementation in the form of nutrition shakes or bars.2 There is a well-documented linear relationship between BMI and FEV1. Lung function declines in CF when a man’s body mass index (BMI) falls below 23 kg/m2 and a woman’s BMI drops below 22 kg/m2.2 For this reason, the goal for caloric intake can be as high as 200% of the customary recommended daily allowance.2
Watch for CF-related diabetes. Since the pancreas is also the major source of endogenous insulin, nearly half of adults with CF will develop cystic fibrosis-related diabetes (CFRD) as pancreatic deficiency progresses.13 Similar to the relationship between BMI and FEV1, there is a relationship between glucose intolerance and FEV1. For this reason, annual diabetes screening is recommended for all CF patients ages 10 years and older.13 Because glycated hemoglobin (HbA1c) may not accurately reflect low levels of glucose intolerance, screen for CFRD with a 2-hour 75-g oral glucose tolerance test.13 Early insulin therapy can help maintain BMI and lower average blood sugar in support of FEV1. Once CFRD is diagnosed, the goals and recommendations for control are largely the same as those recommended by the American Diabetes Association for other forms of diabetes.13
Cystic Fibrosis Resources
Cystic Fibrosis Foundation
www.cff.org
Consensus report on cystic fibrosis management
Yankaskas JR, Marchall BC, Sufian B, et al. Cystic fibrosis adult care. Chest. 2004;125:1S-39S.
Consensus report on cystic fibrosis diagnostic guidelines
Farrell PM, Rosenstein BJ, White RB. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation Consensus Report. J Pediatr. 2008;153:S4-S14.
Gastrointestinal system: Alimentary canal
CF is often mistakenly believed to be primarily a pulmonary disease since 85% of the mortality is due to lung dysfunction,7 but intermittent abdominal pain is a common experience for most patients, and disorders can range from gastroesophageal reflux disease (GERD) to small bowel bacterial overgrowth (SBBO) to constipation. Up to 85% of adult patients experience symptoms of reflux, with as many as 40% of cases occurring silently.2 Proton pump inhibitors are a first-line treatment, but they can also contribute to intestinal bacterial overgrowth and pulmonary infections.
In SBBO, gram-negative colonic bacteria colonize the small bowel and can contribute to abdominal pain and malabsorption, weight loss, and malnutrition. Treatment requires antibiotics with activity against gram-negative organisms, or non-absorbable agents such as rifamyxin, sometimes on a chronic, recurrent, or rotating basis.2
Chronic constipation is also quite common among CF patients and many require daily administration of poly-ethylene-glycol. Before newborn screening programs were introduced, infants would on occasion present with complete distal intestinal obstruction. Adults are not immune to obstructive complications and may require hospitalization for bowel cleansing.
Gastrointestinal system: Liver
Liver disease is relatively common in CF, with up to 24% of adults experiencing hepatomegaly or persistently elevated liver function tests (LFT).4 Progressive biliary fibrosis and cirrhosis are encountered more often as the median survival age has increased. There is evidence that ursodeoxycholic acid (UDCA) can be a useful adjunct in the treatment of cholestasis, but it is not clear if it alters mortality or progression to cirrhosis. Only CF patients with elevated LFTs should be started on UDCA.4
Other areas of concern: Sinuses, serum sodium levels
Chronic, symptomatic sinus disease in CF patients—chiefly polyposis—is common and may require repeat surgery, although most patients with extensive nasal polyps find symptom relief with daily sinus rinses. Intranasal steroids and intranasal antibiotics are also often employed, and many CF patients need to be in regular contact with an otolaryngologist.14 For symptoms of allergic rhinitis, recommend OTC antihistamines in standard dosages.
Exercise is recommended for all CF patients, as noted earlier, and as life expectancy increases, many are engaging in more strenuous and longer duration activities.15 Due to high sweat sodium loss, CF patients are at risk for hyponatremia, especially when exercising on days with high temperatures and humidity. CF patients need to replace sodium losses in these conditions and when exercising for extended periods.
There are no evidence-based guidelines for sodium replacement. The Cystic Fibrosis Foundation (CFF) recommends that patients increase salt in the diet when under conditions likely to result in increased sodium loss, such as exercise. It has been thought that CF patients can easily dehydrate due to an impaired thirst mechanism and, when exercising, should consume fluids beyond the need to quench thirst.16,17 More recent evidence suggests, however, that the thirst mechanism in those with CF remains normally intact and that overconsumption of fluids beyond the level of thirst may predispose the individual to exercise-associated hyponatremia as serum sodium is diluted.15
New therapies
Small-molecule CFTR-modulating compounds are a promising development in the treatment of CF. The first such available medication was ivacaftor in 2012. Because these molecules are mutation specific, ivacaftor was available at first only for patients with at least one copy of the G551D mutation,18 which means about 5% of patients with CF.3
Ivacaftor increases the likelihood that the CFTR chloride channel will open and patients will exhibit a reduction in sweat chloride levels. In the first reported clinical trial of ivacaftor involving patients with the G551D mutation, FEV1 improvements of 10% occurred by the second week of therapy and persisted for 48 weeks.18 The drug has now been approved by the US Food and Drug Administration (FDA) for patients 12 years of age and older with at least one of the following mutations: R117H, G551D, G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P, or G1349D.19
A medication combining ivacaftor with lumacaftor is also now available for patients with a copy of F508del on both chromosomes. F508del is the most common CFTR mutation, with one copy present in almost 87% of people with CF in the United States.1 Since 47% of CF patients have 2 copies of F508del,1 about half of those with CF in the United States are now eligible for small-molecule therapy. Lumacaftor acts by facilitating transport of a misfolded CFTR to the cell membrane where ivacaftor then increases the probability of an open chloride channel. This combination medication has improved lung function by about 5%.
The ivacaftor/lumacaftor combination was approved by the FDA in July 2015. Both ivacaftor and the ivacaftor/lumacaftor combination were deemed by the FDA to demonstrate statistically significant and sustained FEV1 improvements over placebo.
The CFF was instrumental in providing financial support for the development of both ivacaftor and the ivacaftor/lumacaftor combination and continues to provide significant research advancement. According to the CFF (www.cff.org), medications currently in the development pipeline include compounds that provide CFTR modulation, surface airway liquid restoration, anti-inflammation, inhaled anti-infection, and pancreatic enzyme function. For more on CFF, see "The traditional CF care model.”4,20
The traditional CF care model
The Cystic Fibrosis Foundation (CFF) has been a driving force behind the increased life expectancy CF patients have seen over the last 3 decades. Its contributions include the development of medication through the CFF Therapeutics Development Network (TDN) and disease management through a network of CF Care Centers throughout the United States. The CFF recommends a minimum of quarterly visits to a CF Care Center, and the primary care physician can play a critical role alongside the multidisciplinary CF team.20
At every CF Care Center encounter, the entire team (nurse, physician, dietician, social worker, psychologist) interacts with each patient and their families to maximize overall medical care. Respiratory cultures are generally obtained at each visit. Dual-energy x-ray absorptiometry is performed biannually. Lab work (complete blood count, comprehensive metabolic panel, glycated hemoglobin, vitamins A, D, E, and K, 2-hour glucose tolerance test), and chest x-ray are obtained at least annually (TABLE 2).4
Since CF generally involves both restrictive and obstructive lung components, complete spirometry evaluation is performed annually in the pulmonary function lab, with static lung volumes in addition to airflow measurement. Office spirometry to measure airflow alone is performed at each visit. FEV1 is tracked both as an indicator of disease progression and as a measure of current pulmonary status.
The CFF recommends that each patient receive full genetic testing and encourages patient participation in the CFF Registry, where mutation data are documented among other disease parameters to ensure that patients receive mutation specific therapies as they become available.4 The vaccine schedule recommended for CF patients is the same as for the general population.
CORRESPONDENCE
Douglas Lewis, MD, 1121 S. Clifton, Wichita, KS 67218; [email protected].
1. Cystic Fibrosis Foundation. Patient registry 2012 annual data report. Cystic Fibrosis Foundation Web site. Available at: http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf. Accessed August 14, 2014.
2. Haller W, Ledder O, Lewindon PJ, et al. Cystic fibrosis: An update for clinicians. Part 1: Nutrition and gastrointestinal complications. J Gastroenterol Hepatol. 2014;29:1344-1355.
3. Hoffman LR, Ramsey BW. Cystic fibrosis therapeutics: the road ahead. Chest. 2013;143:207-213.
4. Yankaskas JR, Marshall BC, Sufian B, et al. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S-39S.
5. Farrell PM, Rosenstein BJ, White TB, et al; Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4-S14.
6. Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631-1636.
7. Flume PA, Robinson KA, O’Sullivan BP, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54:522-537.
8. Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957-969.
9. Döring G, Flume P, Heijerman H, et al; Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461-479.
10. Flume PA, Mogayzel PJ Jr, Robinson KA, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802-808.
11. Southern KW, Barker PM. Azithromycin for cystic fibrosis. Eur Respir J. 2004;24:834-838.
12. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344-399.
13. Moran A, Brunzell C, Cohen RC, et al; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697-2708.
14. Kerem E, Conway S, Elborn S, et al; Consensus Committee. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2005;4:7-26.
15. Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin J Sport Med. 2015;25:303-320.
16. Brown MB, McCarty NA, Millard-Stafford M. High-sweat Na+ in cystic fibrosis and healthy individuals does not diminish thirst during exercise in the heat. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1177-R1185.
17. Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exerc Sport Sci Rev. 2011;39:155-160.
18. Ramsey BW, Davies J, McElvaney NG, et al; VX08-770-102 Study Group. ACFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663-1672.
19. Pettit RS, Fellner C. CFTR Modulators for the Treatment of Cystic Fibrosis. P T. 2014;39:500-511.
20. Lewis D. Role of the family physician in the management of cystic fibrosis. Am Fam Physician. 2015;91:822-824.
1. Cystic Fibrosis Foundation. Patient registry 2012 annual data report. Cystic Fibrosis Foundation Web site. Available at: http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf. Accessed August 14, 2014.
2. Haller W, Ledder O, Lewindon PJ, et al. Cystic fibrosis: An update for clinicians. Part 1: Nutrition and gastrointestinal complications. J Gastroenterol Hepatol. 2014;29:1344-1355.
3. Hoffman LR, Ramsey BW. Cystic fibrosis therapeutics: the road ahead. Chest. 2013;143:207-213.
4. Yankaskas JR, Marshall BC, Sufian B, et al. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S-39S.
5. Farrell PM, Rosenstein BJ, White TB, et al; Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4-S14.
6. Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631-1636.
7. Flume PA, Robinson KA, O’Sullivan BP, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54:522-537.
8. Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957-969.
9. Döring G, Flume P, Heijerman H, et al; Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461-479.
10. Flume PA, Mogayzel PJ Jr, Robinson KA, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802-808.
11. Southern KW, Barker PM. Azithromycin for cystic fibrosis. Eur Respir J. 2004;24:834-838.
12. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344-399.
13. Moran A, Brunzell C, Cohen RC, et al; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697-2708.
14. Kerem E, Conway S, Elborn S, et al; Consensus Committee. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2005;4:7-26.
15. Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin J Sport Med. 2015;25:303-320.
16. Brown MB, McCarty NA, Millard-Stafford M. High-sweat Na+ in cystic fibrosis and healthy individuals does not diminish thirst during exercise in the heat. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1177-R1185.
17. Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exerc Sport Sci Rev. 2011;39:155-160.
18. Ramsey BW, Davies J, McElvaney NG, et al; VX08-770-102 Study Group. ACFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663-1672.
19. Pettit RS, Fellner C. CFTR Modulators for the Treatment of Cystic Fibrosis. P T. 2014;39:500-511.
20. Lewis D. Role of the family physician in the management of cystic fibrosis. Am Fam Physician. 2015;91:822-824.
COPD Exacerbation Amps Up Stroke Risk
People with chronic obstructive pulmonary disease have an approximately 20% increased risk of stroke, and the risk is highest during the time after an acute exacerbation of COPD, data from a large epidemiologic study indicate.
The study also indicated that cigarette smoking was a strong risk factor for stroke and that hypertension management is important in COPD patients given the elevated risk for hemorrhagic strokes observed, according to Dr. Marileen L. P. Portegies of Erasmus MC University Medical Center, Rotterdam, the Netherlands, and her colleagues.
In 13,115 participants from the Rotterdam study, people with COPD had a 6.7-fold increase in the risk of stroke within the first 7 weeks of a severe exacerbation (hazard ratio, 6.66; 95% confidence interval, 2.42-18.20).
The study (Am J Respir Crit Care Med. 2016; 193:251-8) found that 1,250 of the participants had a stroke (701 were ischemic and 107 hemorrhagic) over 126,347 person-years of follow-up.
After researchers adjusted for age and sex, COPD was significantly associated with all stroke (HR, 1.20; 95% CI, 1.00-1.43), ischemic stroke (HR, 1.27; 95% CI, 1.02-1.59), and hemorrhagic stroke (HR, 1.70; 95% CI, 1.01-2.84).
Smoking was the strongest explanatory factor for the association between COPD and stroke, the researchers said. Adjustments for cardiovascular risk factors gave similar effect sizes, whereas adjustments for smoking attenuated the effect sizes: for all stroke, (HR, 1.09; 95% CI, 0.91-1.31); for ischemic stroke, (HR, 1.13; 95% CI, 0.91-1.42) ; and for hemorrhagic stroke, (HR 1.53; 95% CI, 0.91-2.59) .
“Our study reveals the importance of smoking as a shared risk factor and implicates that clinicians should be aware of the higher risk of both stroke subtypes in subjects with COPD, especially after severe exacerbations,” they concluded.
Writing in an accompanying editorial Dr. Janice M. Leung and Dr. Don D. Sin from St. Paul’s Hospital University of British Columbia, Vancouver, said there was no doubt that cardiovascular disease, stroke, and COPD all shared smoking as a common risk factor.
But smoking alone failed to account for the particularly critical period of a COPD exacerbation during which the risk for strokes and myocardial infarctions exponentially increase.
“Surely, this acute period must offer clues as to why such a strong link exists among the lung, heart, and brain in COPD,” the researchers wrote.
COPD exacerbations may represent a time of intense oxidative stress and systemic inflammation that drive endothelial dysfunction, vascular reactivity, and even atherosclerotic plaque rupture.
“Pulmonologists must work closer with cardiologists and neurologists to enable optimal vascular care for patients with COPD, because it is clear that the lungs do not stand alone and that COPD, although a lung disease, is a major risk factor for stroke, especially during exacerbations,” they concluded.
People with chronic obstructive pulmonary disease have an approximately 20% increased risk of stroke, and the risk is highest during the time after an acute exacerbation of COPD, data from a large epidemiologic study indicate.
The study also indicated that cigarette smoking was a strong risk factor for stroke and that hypertension management is important in COPD patients given the elevated risk for hemorrhagic strokes observed, according to Dr. Marileen L. P. Portegies of Erasmus MC University Medical Center, Rotterdam, the Netherlands, and her colleagues.
In 13,115 participants from the Rotterdam study, people with COPD had a 6.7-fold increase in the risk of stroke within the first 7 weeks of a severe exacerbation (hazard ratio, 6.66; 95% confidence interval, 2.42-18.20).
The study (Am J Respir Crit Care Med. 2016; 193:251-8) found that 1,250 of the participants had a stroke (701 were ischemic and 107 hemorrhagic) over 126,347 person-years of follow-up.
After researchers adjusted for age and sex, COPD was significantly associated with all stroke (HR, 1.20; 95% CI, 1.00-1.43), ischemic stroke (HR, 1.27; 95% CI, 1.02-1.59), and hemorrhagic stroke (HR, 1.70; 95% CI, 1.01-2.84).
Smoking was the strongest explanatory factor for the association between COPD and stroke, the researchers said. Adjustments for cardiovascular risk factors gave similar effect sizes, whereas adjustments for smoking attenuated the effect sizes: for all stroke, (HR, 1.09; 95% CI, 0.91-1.31); for ischemic stroke, (HR, 1.13; 95% CI, 0.91-1.42) ; and for hemorrhagic stroke, (HR 1.53; 95% CI, 0.91-2.59) .
“Our study reveals the importance of smoking as a shared risk factor and implicates that clinicians should be aware of the higher risk of both stroke subtypes in subjects with COPD, especially after severe exacerbations,” they concluded.
Writing in an accompanying editorial Dr. Janice M. Leung and Dr. Don D. Sin from St. Paul’s Hospital University of British Columbia, Vancouver, said there was no doubt that cardiovascular disease, stroke, and COPD all shared smoking as a common risk factor.
But smoking alone failed to account for the particularly critical period of a COPD exacerbation during which the risk for strokes and myocardial infarctions exponentially increase.
“Surely, this acute period must offer clues as to why such a strong link exists among the lung, heart, and brain in COPD,” the researchers wrote.
COPD exacerbations may represent a time of intense oxidative stress and systemic inflammation that drive endothelial dysfunction, vascular reactivity, and even atherosclerotic plaque rupture.
“Pulmonologists must work closer with cardiologists and neurologists to enable optimal vascular care for patients with COPD, because it is clear that the lungs do not stand alone and that COPD, although a lung disease, is a major risk factor for stroke, especially during exacerbations,” they concluded.
People with chronic obstructive pulmonary disease have an approximately 20% increased risk of stroke, and the risk is highest during the time after an acute exacerbation of COPD, data from a large epidemiologic study indicate.
The study also indicated that cigarette smoking was a strong risk factor for stroke and that hypertension management is important in COPD patients given the elevated risk for hemorrhagic strokes observed, according to Dr. Marileen L. P. Portegies of Erasmus MC University Medical Center, Rotterdam, the Netherlands, and her colleagues.
In 13,115 participants from the Rotterdam study, people with COPD had a 6.7-fold increase in the risk of stroke within the first 7 weeks of a severe exacerbation (hazard ratio, 6.66; 95% confidence interval, 2.42-18.20).
The study (Am J Respir Crit Care Med. 2016; 193:251-8) found that 1,250 of the participants had a stroke (701 were ischemic and 107 hemorrhagic) over 126,347 person-years of follow-up.
After researchers adjusted for age and sex, COPD was significantly associated with all stroke (HR, 1.20; 95% CI, 1.00-1.43), ischemic stroke (HR, 1.27; 95% CI, 1.02-1.59), and hemorrhagic stroke (HR, 1.70; 95% CI, 1.01-2.84).
Smoking was the strongest explanatory factor for the association between COPD and stroke, the researchers said. Adjustments for cardiovascular risk factors gave similar effect sizes, whereas adjustments for smoking attenuated the effect sizes: for all stroke, (HR, 1.09; 95% CI, 0.91-1.31); for ischemic stroke, (HR, 1.13; 95% CI, 0.91-1.42) ; and for hemorrhagic stroke, (HR 1.53; 95% CI, 0.91-2.59) .
“Our study reveals the importance of smoking as a shared risk factor and implicates that clinicians should be aware of the higher risk of both stroke subtypes in subjects with COPD, especially after severe exacerbations,” they concluded.
Writing in an accompanying editorial Dr. Janice M. Leung and Dr. Don D. Sin from St. Paul’s Hospital University of British Columbia, Vancouver, said there was no doubt that cardiovascular disease, stroke, and COPD all shared smoking as a common risk factor.
But smoking alone failed to account for the particularly critical period of a COPD exacerbation during which the risk for strokes and myocardial infarctions exponentially increase.
“Surely, this acute period must offer clues as to why such a strong link exists among the lung, heart, and brain in COPD,” the researchers wrote.
COPD exacerbations may represent a time of intense oxidative stress and systemic inflammation that drive endothelial dysfunction, vascular reactivity, and even atherosclerotic plaque rupture.
“Pulmonologists must work closer with cardiologists and neurologists to enable optimal vascular care for patients with COPD, because it is clear that the lungs do not stand alone and that COPD, although a lung disease, is a major risk factor for stroke, especially during exacerbations,” they concluded.
FROM AJRCCM
Recent Active Asthma Raises AAA Rupture Risk
Patients aged 50 and older with recent active asthma are at elevated risk of abdominal aortic aneurysm and aneurysm rupture, according to new research.
A common inflammatory pathway between asthma and AAA, first observed nearly a decade ago in mice, is thought to be responsible.
The new findings, published online Feb. 11 in Arteriosclerosis, Thrombosis, and Vascular Biology (Arterioscler Thromb Vasc Biol. 2016. doi: 10.1161/ATVBAHA.115.306497) support the association in humans.
In a news release accompanying the findings, lead study author Guo-Ping Shi, D.Sc., of Brigham and Women’s Hospital and Harvard Medical School, Boston, said that the findings had clear clinical implications for older patients with a recent asthma diagnosis. Such patients, particularly older men, “should be checked for signs” of abdominal aortic aneurysm, Dr. Shi said.
For their research, Dr. Shi, along with colleagues at Zhengzhou (China) University, used data from a large Danish population-based cohort of nearly 16,000 patients (81% men) with AAA between 1996 and 2012, of which about 4,500 patients had rupture. They also looked at data from a comparison cohort of patients with and without AAA from a slightly larger population-based vascular screening trial of men in Denmark.
The investigators showed that hospital diagnosis of asthma within the previous year (n = 514) was associated with significantly higher risk of hospital admission with AAA rupture (n = 146) both before and after adjustment for AAA comorbidities (adjusted odds ratio 1.51-2.06). Higher risk of rupture also was seen for patients filling prescriptions for bronchodilators within the previous 3 months (aOR = 1.10-1.31), and for patients prescribed anti-asthma drugs (aOR OR = 1.09-1.48), which were seen as indicative of an outpatient asthma diagnosis.
“A hospital diagnosis of asthma or a recently filled prescription of an anti-asthmatic drug is associated with an increased risk of admission with rAAA compared with admission with intact AAA, both before and after adjusting for AAA comorbidities and relevant medications,” the researchers wrote in their analysis.
Moreover, “an asthma diagnosis or the use of bronchodilators or other anti-asthmatic drug prescriptions closer to the date of admission with AAA correlated directly with a higher risk of aortic rupture. The results remained robust after adjusting for a wide range of relevant possible confounders,” the researchers wrote.
In the cohort of patients from the vascular screening study, which included age-matched controls without AAA, asthma (measured by recent anti-asthmatic medication use) was seen associated with a significantly elevated risk of AAA before (OR = 1.45) and after adjustment for smoking (OR = 1.45) or other risk factors (OR = 1.46). This does not refer to rupture but just AAA.
Dr. Shi and colleagues noted that the AAA cohort lacked sufficient information on cigarette smoking, a known risk factor for AAA and AAA rupture, to preclude the possibility of confounding; however, the second all-male cohort did have extensive data on smoking, “and the risk of AAA among patients with asthma remained 45% higher than that of patients with nonasthma” even after adjustment.
The researchers hypothesized that an inflammatory response characterized by elevated immunoglobulin E may be the link between AAA pathogenesis and asthma, and that other allergic inflammatory diseases, including atopic dermatitis, allergic rhinitis, and some ocular allergic diseases, could potentially carry risks for AAA formation and rupture. Dr. Shi and colleagues previously investigated the IgE and aneurysm link in animal studies.
“The results have implications for the development of much needed advances in the prevention, screening criteria, and treatment of AAA, common conditions for which we currently lack sufficiently effective approaches,” the investigators wrote.
The Chinese, Danish, and U.S. governments sponsored the study. The investigators disclosed no conflicts of interest.
Patients aged 50 and older with recent active asthma are at elevated risk of abdominal aortic aneurysm and aneurysm rupture, according to new research.
A common inflammatory pathway between asthma and AAA, first observed nearly a decade ago in mice, is thought to be responsible.
The new findings, published online Feb. 11 in Arteriosclerosis, Thrombosis, and Vascular Biology (Arterioscler Thromb Vasc Biol. 2016. doi: 10.1161/ATVBAHA.115.306497) support the association in humans.
In a news release accompanying the findings, lead study author Guo-Ping Shi, D.Sc., of Brigham and Women’s Hospital and Harvard Medical School, Boston, said that the findings had clear clinical implications for older patients with a recent asthma diagnosis. Such patients, particularly older men, “should be checked for signs” of abdominal aortic aneurysm, Dr. Shi said.
For their research, Dr. Shi, along with colleagues at Zhengzhou (China) University, used data from a large Danish population-based cohort of nearly 16,000 patients (81% men) with AAA between 1996 and 2012, of which about 4,500 patients had rupture. They also looked at data from a comparison cohort of patients with and without AAA from a slightly larger population-based vascular screening trial of men in Denmark.
The investigators showed that hospital diagnosis of asthma within the previous year (n = 514) was associated with significantly higher risk of hospital admission with AAA rupture (n = 146) both before and after adjustment for AAA comorbidities (adjusted odds ratio 1.51-2.06). Higher risk of rupture also was seen for patients filling prescriptions for bronchodilators within the previous 3 months (aOR = 1.10-1.31), and for patients prescribed anti-asthma drugs (aOR OR = 1.09-1.48), which were seen as indicative of an outpatient asthma diagnosis.
“A hospital diagnosis of asthma or a recently filled prescription of an anti-asthmatic drug is associated with an increased risk of admission with rAAA compared with admission with intact AAA, both before and after adjusting for AAA comorbidities and relevant medications,” the researchers wrote in their analysis.
Moreover, “an asthma diagnosis or the use of bronchodilators or other anti-asthmatic drug prescriptions closer to the date of admission with AAA correlated directly with a higher risk of aortic rupture. The results remained robust after adjusting for a wide range of relevant possible confounders,” the researchers wrote.
In the cohort of patients from the vascular screening study, which included age-matched controls without AAA, asthma (measured by recent anti-asthmatic medication use) was seen associated with a significantly elevated risk of AAA before (OR = 1.45) and after adjustment for smoking (OR = 1.45) or other risk factors (OR = 1.46). This does not refer to rupture but just AAA.
Dr. Shi and colleagues noted that the AAA cohort lacked sufficient information on cigarette smoking, a known risk factor for AAA and AAA rupture, to preclude the possibility of confounding; however, the second all-male cohort did have extensive data on smoking, “and the risk of AAA among patients with asthma remained 45% higher than that of patients with nonasthma” even after adjustment.
The researchers hypothesized that an inflammatory response characterized by elevated immunoglobulin E may be the link between AAA pathogenesis and asthma, and that other allergic inflammatory diseases, including atopic dermatitis, allergic rhinitis, and some ocular allergic diseases, could potentially carry risks for AAA formation and rupture. Dr. Shi and colleagues previously investigated the IgE and aneurysm link in animal studies.
“The results have implications for the development of much needed advances in the prevention, screening criteria, and treatment of AAA, common conditions for which we currently lack sufficiently effective approaches,” the investigators wrote.
The Chinese, Danish, and U.S. governments sponsored the study. The investigators disclosed no conflicts of interest.
Patients aged 50 and older with recent active asthma are at elevated risk of abdominal aortic aneurysm and aneurysm rupture, according to new research.
A common inflammatory pathway between asthma and AAA, first observed nearly a decade ago in mice, is thought to be responsible.
The new findings, published online Feb. 11 in Arteriosclerosis, Thrombosis, and Vascular Biology (Arterioscler Thromb Vasc Biol. 2016. doi: 10.1161/ATVBAHA.115.306497) support the association in humans.
In a news release accompanying the findings, lead study author Guo-Ping Shi, D.Sc., of Brigham and Women’s Hospital and Harvard Medical School, Boston, said that the findings had clear clinical implications for older patients with a recent asthma diagnosis. Such patients, particularly older men, “should be checked for signs” of abdominal aortic aneurysm, Dr. Shi said.
For their research, Dr. Shi, along with colleagues at Zhengzhou (China) University, used data from a large Danish population-based cohort of nearly 16,000 patients (81% men) with AAA between 1996 and 2012, of which about 4,500 patients had rupture. They also looked at data from a comparison cohort of patients with and without AAA from a slightly larger population-based vascular screening trial of men in Denmark.
The investigators showed that hospital diagnosis of asthma within the previous year (n = 514) was associated with significantly higher risk of hospital admission with AAA rupture (n = 146) both before and after adjustment for AAA comorbidities (adjusted odds ratio 1.51-2.06). Higher risk of rupture also was seen for patients filling prescriptions for bronchodilators within the previous 3 months (aOR = 1.10-1.31), and for patients prescribed anti-asthma drugs (aOR OR = 1.09-1.48), which were seen as indicative of an outpatient asthma diagnosis.
“A hospital diagnosis of asthma or a recently filled prescription of an anti-asthmatic drug is associated with an increased risk of admission with rAAA compared with admission with intact AAA, both before and after adjusting for AAA comorbidities and relevant medications,” the researchers wrote in their analysis.
Moreover, “an asthma diagnosis or the use of bronchodilators or other anti-asthmatic drug prescriptions closer to the date of admission with AAA correlated directly with a higher risk of aortic rupture. The results remained robust after adjusting for a wide range of relevant possible confounders,” the researchers wrote.
In the cohort of patients from the vascular screening study, which included age-matched controls without AAA, asthma (measured by recent anti-asthmatic medication use) was seen associated with a significantly elevated risk of AAA before (OR = 1.45) and after adjustment for smoking (OR = 1.45) or other risk factors (OR = 1.46). This does not refer to rupture but just AAA.
Dr. Shi and colleagues noted that the AAA cohort lacked sufficient information on cigarette smoking, a known risk factor for AAA and AAA rupture, to preclude the possibility of confounding; however, the second all-male cohort did have extensive data on smoking, “and the risk of AAA among patients with asthma remained 45% higher than that of patients with nonasthma” even after adjustment.
The researchers hypothesized that an inflammatory response characterized by elevated immunoglobulin E may be the link between AAA pathogenesis and asthma, and that other allergic inflammatory diseases, including atopic dermatitis, allergic rhinitis, and some ocular allergic diseases, could potentially carry risks for AAA formation and rupture. Dr. Shi and colleagues previously investigated the IgE and aneurysm link in animal studies.
“The results have implications for the development of much needed advances in the prevention, screening criteria, and treatment of AAA, common conditions for which we currently lack sufficiently effective approaches,” the investigators wrote.
The Chinese, Danish, and U.S. governments sponsored the study. The investigators disclosed no conflicts of interest.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Early-wheezing Patterns Prefigure Adolescent Respiratory Outcomes and Asthma
Wheezing patterns in early childhood can predict pulmonary function and the development of asthma in adolescence in a high-risk population, a study finds.
The study validates four clinically distinct early-life wheezing phenotypes identified by the landmark Tucson Children’s Respiratory Study in a high-risk population:
• Never.
• Transient early – wheezing before age 3 years but not at age 6 years.
• Late onset – wheezing at age 6 years but not before age 3 years.
• Persistent – wheezing before age 3 years and at age 6 years.
Previously, these phenotypes were shown to be associated with respiratory outcomes in adolescence but not with diagnosed asthma or in genetically predisposed children. The present study extends the associations with these early phenotypes to a high-risk adolescent population, said Meghan B. Azad, Ph.D., of the University of Manitoba, Winnipeg, and her associates.
The study findings were based on findings in 459 children previously enrolled in the Canadian Asthma Primary Prevention Study cohort; this was a prenatally randomized prevention trial in children at high genetic risk for asthma. The distribution of early-wheeze phenotypes was 51% never, 28% transient early, 9% late onset, and 13% persistent (JAMA Pediatrics. 2016 Feb 8. doi: 10.1001/jamapediatrics.2015.4127).
Across all four phenotypes, the authors found a strong gradient of decreasing lung function and increasing asthma risk by age 15 years. Asthma, assessed at 15 years in 320 adolescents, was associated with early-wheeze phenotypes: the prevalence of asthma was 5% among never, 19% among transient early, 27% among late onset, and 42% among persistent phenotypes.
At age 15, early-wheezing phenotypes were not associated with atopic dermatitis or allergic rhinitis. Atopy before 2 years of age was associated with persistent wheeze, which was in turn associated with a 12-fold increased risk of diagnosed asthma by age 15.
“Our results are consistent with other cohorts” and the study “extends these findings through adolescence in a high-risk cohort and demonstrates that asthma-associated deficits are already present at a young age. Collectively, these data show that early wheezing patterns provide clinically meaningful information and suggest that strategies to reduce early-life wheezing and atopic sensitization could have long-term health benefits,” Dr. Azad and her colleagues concluded.
Proven strategies to prevent wheezing include avoiding dust, pets, and tobacco smoke; encouragement of breastfeeding; and delayed introduction of solid foods.
The research was supported by the Canadian Institutes of Health Research. The investigators reported no relevant financial disclosures.
Wheezing patterns in early childhood can predict pulmonary function and the development of asthma in adolescence in a high-risk population, a study finds.
The study validates four clinically distinct early-life wheezing phenotypes identified by the landmark Tucson Children’s Respiratory Study in a high-risk population:
• Never.
• Transient early – wheezing before age 3 years but not at age 6 years.
• Late onset – wheezing at age 6 years but not before age 3 years.
• Persistent – wheezing before age 3 years and at age 6 years.
Previously, these phenotypes were shown to be associated with respiratory outcomes in adolescence but not with diagnosed asthma or in genetically predisposed children. The present study extends the associations with these early phenotypes to a high-risk adolescent population, said Meghan B. Azad, Ph.D., of the University of Manitoba, Winnipeg, and her associates.
The study findings were based on findings in 459 children previously enrolled in the Canadian Asthma Primary Prevention Study cohort; this was a prenatally randomized prevention trial in children at high genetic risk for asthma. The distribution of early-wheeze phenotypes was 51% never, 28% transient early, 9% late onset, and 13% persistent (JAMA Pediatrics. 2016 Feb 8. doi: 10.1001/jamapediatrics.2015.4127).
Across all four phenotypes, the authors found a strong gradient of decreasing lung function and increasing asthma risk by age 15 years. Asthma, assessed at 15 years in 320 adolescents, was associated with early-wheeze phenotypes: the prevalence of asthma was 5% among never, 19% among transient early, 27% among late onset, and 42% among persistent phenotypes.
At age 15, early-wheezing phenotypes were not associated with atopic dermatitis or allergic rhinitis. Atopy before 2 years of age was associated with persistent wheeze, which was in turn associated with a 12-fold increased risk of diagnosed asthma by age 15.
“Our results are consistent with other cohorts” and the study “extends these findings through adolescence in a high-risk cohort and demonstrates that asthma-associated deficits are already present at a young age. Collectively, these data show that early wheezing patterns provide clinically meaningful information and suggest that strategies to reduce early-life wheezing and atopic sensitization could have long-term health benefits,” Dr. Azad and her colleagues concluded.
Proven strategies to prevent wheezing include avoiding dust, pets, and tobacco smoke; encouragement of breastfeeding; and delayed introduction of solid foods.
The research was supported by the Canadian Institutes of Health Research. The investigators reported no relevant financial disclosures.
Wheezing patterns in early childhood can predict pulmonary function and the development of asthma in adolescence in a high-risk population, a study finds.
The study validates four clinically distinct early-life wheezing phenotypes identified by the landmark Tucson Children’s Respiratory Study in a high-risk population:
• Never.
• Transient early – wheezing before age 3 years but not at age 6 years.
• Late onset – wheezing at age 6 years but not before age 3 years.
• Persistent – wheezing before age 3 years and at age 6 years.
Previously, these phenotypes were shown to be associated with respiratory outcomes in adolescence but not with diagnosed asthma or in genetically predisposed children. The present study extends the associations with these early phenotypes to a high-risk adolescent population, said Meghan B. Azad, Ph.D., of the University of Manitoba, Winnipeg, and her associates.
The study findings were based on findings in 459 children previously enrolled in the Canadian Asthma Primary Prevention Study cohort; this was a prenatally randomized prevention trial in children at high genetic risk for asthma. The distribution of early-wheeze phenotypes was 51% never, 28% transient early, 9% late onset, and 13% persistent (JAMA Pediatrics. 2016 Feb 8. doi: 10.1001/jamapediatrics.2015.4127).
Across all four phenotypes, the authors found a strong gradient of decreasing lung function and increasing asthma risk by age 15 years. Asthma, assessed at 15 years in 320 adolescents, was associated with early-wheeze phenotypes: the prevalence of asthma was 5% among never, 19% among transient early, 27% among late onset, and 42% among persistent phenotypes.
At age 15, early-wheezing phenotypes were not associated with atopic dermatitis or allergic rhinitis. Atopy before 2 years of age was associated with persistent wheeze, which was in turn associated with a 12-fold increased risk of diagnosed asthma by age 15.
“Our results are consistent with other cohorts” and the study “extends these findings through adolescence in a high-risk cohort and demonstrates that asthma-associated deficits are already present at a young age. Collectively, these data show that early wheezing patterns provide clinically meaningful information and suggest that strategies to reduce early-life wheezing and atopic sensitization could have long-term health benefits,” Dr. Azad and her colleagues concluded.
Proven strategies to prevent wheezing include avoiding dust, pets, and tobacco smoke; encouragement of breastfeeding; and delayed introduction of solid foods.
The research was supported by the Canadian Institutes of Health Research. The investigators reported no relevant financial disclosures.
FROM JAMA PEDIATRICS
Smoking Cessation What Should You Recommend?
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
Updates in Pediatrics
TALKING OUT CHILDHOOD OBESITY
Resnicow K, McMaster F, Bocian A, et al. Motivational interviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics. 2015;135(4):649-657.
Counseling parents of overweight children using motivational interviewing from both health care providers and registered dietitians can significantly improve BMI, according to a study of 42 practices in the Pediatric Research in Office Settings Network of the American Academy of Pediatrics.
Researchers randomly assigned parents of overweight children, ages 2 through 8, to one of three groups: (1) usual care, (2) four provider-delivered motivational interviewing sessions over two years, or (3) four provider-delivered motivational interviewing sessions plus six sessions with a registered dietitian over two years. At study end, BMI percentile and change in BMI for the different groups were as follows:
COMMENTARY
The results of this study are exciting. Motivational interviewing is a technique in which the practitioner asks questions of a patient and allows the patient to discover his/her own conclusions about the topic. By so doing, the patient is more engaged in the discussion and is less resistant to input. This technique, with excellent evidence of effectiveness in the area of drug and alcohol abuse, has been shown to facilitate effective behavioral change in many areas and is recommended by the American Heart Association for behavioral change in adults.1,2 This is an exciting paper demonstrating evidence-based efficacy in addressing childhood obesity—a critical health issue—and is worth trying in the office.
1. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
2. Spring B, Ockene JK, Gidding SS, et al; American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, and Council on Cardiovascular and Stroke Nursing. Better population health through behavior change in adults: a call to action. Circulation. 2013;128(19):2169-2176. doi: 10.1161/01.cir.0000435173.25936.e1.
Continue for long-acting reversible contraception among teens >>
LONG-ACTING REVERSIBLE CONTRACEPTION AMONG TEENS
Romero L, Pazol K, Warner L, et al. Vital signs: trends in use of long-acting reversible contraception among teens aged 15-19 years seeking contraceptive services – United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2015;64(13):363-369.
Efforts to improve teen access to long-acting reversible contraception (LARC) have increased use of these methods, according to a CDC review of services provided at Title X National Family Planning Program centers. The report found
• LARC rates among teen patients increased from 0.4% in 2005 to 7.2% in 2013.
• In 2013, 2.8% of those seeking contraception used IUDs and 4.3% used implants.
• Among Title X patients, 7.6% of 18- and 19-year-olds used LARC, compared with 6.5% of 15- to 17-year-olds.
• Rates of LARC were lowest in Mississippi (0.7%) and highest in Colorado (25.8%).
COMMENTARY
LARCs, which include IUD and implantable hormonal contraceptive devices, require no effort for adherence on the part of the user; once in place, they are effective without further action. Current CDC guidelines on contraceptive use clearly recommend LARC for teenagers based on the efficacy and safety.1 LARCs are favored for teenagers because poor compliance has yielded suboptimal effectiveness of oral contraceptives and condoms in teenagers, who often forget to take their birth control pills or don’t use condoms when they should. Many clinicians have been slow to recommend LARCs in teenagers based on safety concerns related to adverse experience with IUDs 20 to 30 years ago. According to CDC guidelines, IUDs and implantable contraceptive devices now have robust safety data, and this article shows that they are being increasingly made available to teenagers who need them.
1. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. US Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62(RR-05):1-60.
Continue to testing for celiac in pediatric rheumatology patients >>
TESTING FOR CELIAC IN PEDIATRIC RHEUMATOLOGY PATIENTS
Sherman Y, Karanicolas R, DiMarco B, et al. Unrecognized celiac disease in children presenting for rheumatology evaluation. Pediatrics. 2015; [Epub ahead of print].
Children presenting for rheumatology evaluation should be screened for celiac disease, according to a review of 2,125 pediatric patients who were screened for celiac as part of the standard initial serologic evaluation.
Researchers identified 36 new cases of celiac disease (2.0% prevalence rate). The most common presenting complaints among these patients were myalgia, arthralgia, and rash. Less frequent complaints included gastrointestinal complaints of abdominal pain, nausea, and diarrhea.
After initiating a gluten-free diet, all of the patients with celiac disease reported improvement or complete resolution of musculoskeletal symptoms.
COMMENTARY
Celiac disease has a prevalence of 0.5% to 1% in the US and can present with a range of symptoms that include diarrhea, abdominal discomfort, and weight loss. In addition to these typical symptoms, celiac disease can cause a wide range of systemic symptoms, including osteopenia, abnormal liver function tests, anemia, neurologic symptoms, and general malaise and fatigue.1 This study suggests that celiac testing be considered in children presenting with rheumatologic symptoms, including myalgia, arthralgia, and rash.
1. Presutti RJ, Cangemi JR, Cassidy HD, et al. Celiac disease. Am Fam Physician. 2007;76(12):1795-1802, 1809-1810.
Continue for educating parents about antibiotic use >>
EDUCATING PARENTS ABOUT ANTIBIOTIC USE
Vaz LE, Kleinman KP, Lakoma MD, et al. Prevalence of parental misconceptions about antibiotic use. Pediatrics. 2015;136:221-231.
Misperceptions about antibiotic use persist and continue to be more prevalent among parents of Medicaid-insured children, according to a study of 1,500 Massachusetts parents.
Investigators examined antibiotic-related knowledge and attitudes among both Medicaid-insured and private-insured parents and found
• Fewer Medicaid parents answered questions correctly, except for one regarding bronchitis.
• Medicaid patients were more likely to request unnecessary antibiotics.
• More parents in 2013 understood that green nasal discharge did not require antibiotics than in 2000.
• Medicaid-insured parents were younger, less likely to be white, and had less education than those commercially insured.
COMMENTARY
Decreasing the unnecessary use of antibiotics for viral infections is an important component of decreasing the development of antibiotic-resistant organisms. An important driver of clinician use of antibiotics is patients’ expectations for antibiotics. This study shows that much work remains to be done in changing patient expectations, which is not surprising to any practicing clinician. This study also suggests that the expectation for antibiotics is greater among those individuals with Medicaid insurance, which suggests that an opportunity exists for Medicaid insurance plans to do targeted patient education on this issue—which should improve patient outcomes, decrease cost from use of unneeded antibiotics, and decrease the development of antibiotic-resistant organisms.
Continue for newborn pulmonary hypertension and maternal antidepressant use >>
NEWBORN PULMONARY HYPERTENSION AND MATERNAL ANTIDEPRESSANT USE
Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313(21):2142-2151.
Taking antidepressants during late pregnancy may increase the risk for persistent pulmonary hypertension of the newborn (PPHN), according to a nested cohort study of more than 3.7 million pregnant women in the 2000-2010 Medicaid Analytic eXtract.
Investigators compared offspring of mothers who used selective serotonin reuptake inhibitors (SSRIs) or non-SSRI monotherapy in the last 90 days of pregnancy to those who did not and found 3.4% of women filled at least one prescription for antidepressants late in pregnancy, primarily SSRIs.
Rates and odds ratios (ORs) of PPHN stratified by use and type of antidepressant were as follows:
The study authors note the absolute risk is small, and the increased risk is more modest than previous studies found.
COMMENTARY
Depression affects more than 12% of pregnancies and has important consequences, including increased risk for suicide, preterm birth, poor fetal growth, and impaired fetal and infant development.1 PPHN is a serious condition that can require intubation and can be fatal in 10% to 20% of cases. This study adds to the conflicting data suggesting that SSRI use in pregnancy can lead to an increase in this rare condition. The decision to use an antidepressant during pregnancy is a difficult one, because depression has serious consequences but so, potentially, does treatment. The treatment of depression during pregnancy requires thoughtful, informed discussion between patient and provider.
1. Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med. 2011;365(17):1605-1611. doi:10.1056/NEJMcp1102730.
Continue for new infant vaccine treats 6 diseases >>
NEW INFANT VACCINE TREATS 6 DISEASES
Marshall GS, Adams GL, Leonardi ML, et al. Immunogenicity, safety, and tolerability of a hexavalent vaccine in infants. Pediatrics. 2015:136(2):323-332.
The safety and immunogenicity of DTaP5-IPV-Hib-HepB fully liquid investigational hexavalent vaccine are comparable with the analogous licensed component vaccines and provide a new combination vaccine option aligned with the recommended US infant immunization schedule, according to a phase III study of 1,465 participants. Overall, 981 healthy infants were vaccinated in group 1 with the hexavalent vaccine and 484 in group 2 with the analogous licensed component vaccines.
The study found
• Immune responses in group 1 to all antigens contained in the vaccine one month after dose 3 were essentially noninferior to those in group 2.
• Adverse event rates after any dose were similar in both groups.
COMMENTARY
Vaccine administration has led to many diseases, including Haemophilus influenzae type b, polio, and measles, becoming quite rare. As we have recently seen with the resurgence of measles, continued vigilance and high immunization rates are important in ensuring that these diseases remain rare. The development of a hexavalent vaccine is another step in making immunization easier for clinicians to administer and for patients to accept.
Continue for treating infants with bronchiolitis >>
TREATING INFANTS WITH BRONCHIOLITIS
Silver AH, Esteban-Cruciani N, Azzarone G, et al. 3% hypertonic saline versus normal saline in inpatient bronchiolitis: a randomized controlled trial. Pediatrics. 2015;136:1036-1043.
Infants hospitalized with bronchiolitis saw no difference in length of stay or seven-day readmission rates when treated with nebulized 3% hypertonic saline (HS) compared with nebulized normal saline (NS), according to a randomized, controlled study of 227 infants who were younger than 12 months when admitted. Patients received either 4 mL nebulized 3% HS (113 infants) or 4 mL 0.9% NS (114 infants) every four hours from enrollment until hospital discharge. Researchers found
• Median length of stay of HS and NS groups was 2.1 days vs 2.1 days, respectively.
• Seven-day readmission rates for HS and NS groups were 4.3% vs 3.1%, respectively.
• Clinical worsening events were similar between groups.
COMMENTARY
Bronchiolitis, the most common lower respiratory tract infection in infants, is usually due to a viral infection, most often respiratory syncytial virus, and can cause disease that ranges in severity from mild to life-threatening. Infants with bronchiolitis typically present with rhinitis, tachypnea, wheezing, and cough, and occasionally crackles and use of accessory muscles. While many medications are used, supportive care and monitoring are the mainstays of therapy. Maintaining pulse above 90% is important, using supplemental oxygen when needed to achieve this. Alpha-adrenergic and beta-adrenergic bronchodilators are often used; though the evidence suggests that they are not usually helpful, they can be tried and continued if they appear to help. Systemic steroids are often used, but they too lack evidence of efficacy.1 This study shows that hypertonic saline can now join the list of interventions used with evidence of a lack of efficacy.
1. Diagnosis and management of bronchiolitis. Subcommittee on diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. doi:10.1542/peds.2006-2223.
Continue for azithromycin and preschool children >>
AZITHROMYCIN AND PRESCHOOL CHILDREN: CAN ANTIBIOTICS LESSEN ONSET OF SEVERE LRTIs?
Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034-2044.
The use of azithromycin early during an apparent respiratory tract illness (RTI) reduced the likelihood of severe lower RTI (LRTI) among young children with a history of recurrent severe LRTI, compared with placebo, according to a study of 607 children ages 12 to 71 months. Participants were randomized in a 1:1 ratio to receive either azithromycin (12 mg/kg/d for 5 d) or matching placebo at the start of an RTI. Researchers found
• A total of 937 treated RTIs were experienced by 443 children, including 92 severe LRTIs (azithromycin group, 35; placebo group, 57).
• Azithromycin significantly reduced the risk for progression to severe LRTI relative to placebo (HR, 0.64).
• Induction of azithromycin-resistant organisms and adverse events were infrequent.
COMMENTARY
Recurrent episodes of severe wheezing with RTI are an important and common occurrence, affecting up to 15% to 20% of children prior to age 6.1 LRTI was defined in this study as RTI that required the use of additional rescue medication. The current approach to RTI is to try to minimize the use of antibiotics unless an infection is clearly bacterial in origin, and to treat severe LRTI when it occurs. The results of this trial suggest that in children at high risk for severe LRTI, identified by their episodes of recurrent wheezing, early treatment of RTI with azithromycin may decrease the development of severe LRTI by more than 35%—an important result. It is important to recognize that this study does not suggest treating all RTIs with antibiotics, but rather that the use of a macrolide antibiotic may be considered, perhaps, in the select group of children similar to those studied, with a history of recurrent wheezing with previous RTIs.
1. Ly NP, Gold DR, Weiss ST, Celedón JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132-e1138.
TALKING OUT CHILDHOOD OBESITY
Resnicow K, McMaster F, Bocian A, et al. Motivational interviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics. 2015;135(4):649-657.
Counseling parents of overweight children using motivational interviewing from both health care providers and registered dietitians can significantly improve BMI, according to a study of 42 practices in the Pediatric Research in Office Settings Network of the American Academy of Pediatrics.
Researchers randomly assigned parents of overweight children, ages 2 through 8, to one of three groups: (1) usual care, (2) four provider-delivered motivational interviewing sessions over two years, or (3) four provider-delivered motivational interviewing sessions plus six sessions with a registered dietitian over two years. At study end, BMI percentile and change in BMI for the different groups were as follows:
COMMENTARY
The results of this study are exciting. Motivational interviewing is a technique in which the practitioner asks questions of a patient and allows the patient to discover his/her own conclusions about the topic. By so doing, the patient is more engaged in the discussion and is less resistant to input. This technique, with excellent evidence of effectiveness in the area of drug and alcohol abuse, has been shown to facilitate effective behavioral change in many areas and is recommended by the American Heart Association for behavioral change in adults.1,2 This is an exciting paper demonstrating evidence-based efficacy in addressing childhood obesity—a critical health issue—and is worth trying in the office.
1. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
2. Spring B, Ockene JK, Gidding SS, et al; American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, and Council on Cardiovascular and Stroke Nursing. Better population health through behavior change in adults: a call to action. Circulation. 2013;128(19):2169-2176. doi: 10.1161/01.cir.0000435173.25936.e1.
Continue for long-acting reversible contraception among teens >>
LONG-ACTING REVERSIBLE CONTRACEPTION AMONG TEENS
Romero L, Pazol K, Warner L, et al. Vital signs: trends in use of long-acting reversible contraception among teens aged 15-19 years seeking contraceptive services – United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2015;64(13):363-369.
Efforts to improve teen access to long-acting reversible contraception (LARC) have increased use of these methods, according to a CDC review of services provided at Title X National Family Planning Program centers. The report found
• LARC rates among teen patients increased from 0.4% in 2005 to 7.2% in 2013.
• In 2013, 2.8% of those seeking contraception used IUDs and 4.3% used implants.
• Among Title X patients, 7.6% of 18- and 19-year-olds used LARC, compared with 6.5% of 15- to 17-year-olds.
• Rates of LARC were lowest in Mississippi (0.7%) and highest in Colorado (25.8%).
COMMENTARY
LARCs, which include IUD and implantable hormonal contraceptive devices, require no effort for adherence on the part of the user; once in place, they are effective without further action. Current CDC guidelines on contraceptive use clearly recommend LARC for teenagers based on the efficacy and safety.1 LARCs are favored for teenagers because poor compliance has yielded suboptimal effectiveness of oral contraceptives and condoms in teenagers, who often forget to take their birth control pills or don’t use condoms when they should. Many clinicians have been slow to recommend LARCs in teenagers based on safety concerns related to adverse experience with IUDs 20 to 30 years ago. According to CDC guidelines, IUDs and implantable contraceptive devices now have robust safety data, and this article shows that they are being increasingly made available to teenagers who need them.
1. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. US Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62(RR-05):1-60.
Continue to testing for celiac in pediatric rheumatology patients >>
TESTING FOR CELIAC IN PEDIATRIC RHEUMATOLOGY PATIENTS
Sherman Y, Karanicolas R, DiMarco B, et al. Unrecognized celiac disease in children presenting for rheumatology evaluation. Pediatrics. 2015; [Epub ahead of print].
Children presenting for rheumatology evaluation should be screened for celiac disease, according to a review of 2,125 pediatric patients who were screened for celiac as part of the standard initial serologic evaluation.
Researchers identified 36 new cases of celiac disease (2.0% prevalence rate). The most common presenting complaints among these patients were myalgia, arthralgia, and rash. Less frequent complaints included gastrointestinal complaints of abdominal pain, nausea, and diarrhea.
After initiating a gluten-free diet, all of the patients with celiac disease reported improvement or complete resolution of musculoskeletal symptoms.
COMMENTARY
Celiac disease has a prevalence of 0.5% to 1% in the US and can present with a range of symptoms that include diarrhea, abdominal discomfort, and weight loss. In addition to these typical symptoms, celiac disease can cause a wide range of systemic symptoms, including osteopenia, abnormal liver function tests, anemia, neurologic symptoms, and general malaise and fatigue.1 This study suggests that celiac testing be considered in children presenting with rheumatologic symptoms, including myalgia, arthralgia, and rash.
1. Presutti RJ, Cangemi JR, Cassidy HD, et al. Celiac disease. Am Fam Physician. 2007;76(12):1795-1802, 1809-1810.
Continue for educating parents about antibiotic use >>
EDUCATING PARENTS ABOUT ANTIBIOTIC USE
Vaz LE, Kleinman KP, Lakoma MD, et al. Prevalence of parental misconceptions about antibiotic use. Pediatrics. 2015;136:221-231.
Misperceptions about antibiotic use persist and continue to be more prevalent among parents of Medicaid-insured children, according to a study of 1,500 Massachusetts parents.
Investigators examined antibiotic-related knowledge and attitudes among both Medicaid-insured and private-insured parents and found
• Fewer Medicaid parents answered questions correctly, except for one regarding bronchitis.
• Medicaid patients were more likely to request unnecessary antibiotics.
• More parents in 2013 understood that green nasal discharge did not require antibiotics than in 2000.
• Medicaid-insured parents were younger, less likely to be white, and had less education than those commercially insured.
COMMENTARY
Decreasing the unnecessary use of antibiotics for viral infections is an important component of decreasing the development of antibiotic-resistant organisms. An important driver of clinician use of antibiotics is patients’ expectations for antibiotics. This study shows that much work remains to be done in changing patient expectations, which is not surprising to any practicing clinician. This study also suggests that the expectation for antibiotics is greater among those individuals with Medicaid insurance, which suggests that an opportunity exists for Medicaid insurance plans to do targeted patient education on this issue—which should improve patient outcomes, decrease cost from use of unneeded antibiotics, and decrease the development of antibiotic-resistant organisms.
Continue for newborn pulmonary hypertension and maternal antidepressant use >>
NEWBORN PULMONARY HYPERTENSION AND MATERNAL ANTIDEPRESSANT USE
Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313(21):2142-2151.
Taking antidepressants during late pregnancy may increase the risk for persistent pulmonary hypertension of the newborn (PPHN), according to a nested cohort study of more than 3.7 million pregnant women in the 2000-2010 Medicaid Analytic eXtract.
Investigators compared offspring of mothers who used selective serotonin reuptake inhibitors (SSRIs) or non-SSRI monotherapy in the last 90 days of pregnancy to those who did not and found 3.4% of women filled at least one prescription for antidepressants late in pregnancy, primarily SSRIs.
Rates and odds ratios (ORs) of PPHN stratified by use and type of antidepressant were as follows:
The study authors note the absolute risk is small, and the increased risk is more modest than previous studies found.
COMMENTARY
Depression affects more than 12% of pregnancies and has important consequences, including increased risk for suicide, preterm birth, poor fetal growth, and impaired fetal and infant development.1 PPHN is a serious condition that can require intubation and can be fatal in 10% to 20% of cases. This study adds to the conflicting data suggesting that SSRI use in pregnancy can lead to an increase in this rare condition. The decision to use an antidepressant during pregnancy is a difficult one, because depression has serious consequences but so, potentially, does treatment. The treatment of depression during pregnancy requires thoughtful, informed discussion between patient and provider.
1. Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med. 2011;365(17):1605-1611. doi:10.1056/NEJMcp1102730.
Continue for new infant vaccine treats 6 diseases >>
NEW INFANT VACCINE TREATS 6 DISEASES
Marshall GS, Adams GL, Leonardi ML, et al. Immunogenicity, safety, and tolerability of a hexavalent vaccine in infants. Pediatrics. 2015:136(2):323-332.
The safety and immunogenicity of DTaP5-IPV-Hib-HepB fully liquid investigational hexavalent vaccine are comparable with the analogous licensed component vaccines and provide a new combination vaccine option aligned with the recommended US infant immunization schedule, according to a phase III study of 1,465 participants. Overall, 981 healthy infants were vaccinated in group 1 with the hexavalent vaccine and 484 in group 2 with the analogous licensed component vaccines.
The study found
• Immune responses in group 1 to all antigens contained in the vaccine one month after dose 3 were essentially noninferior to those in group 2.
• Adverse event rates after any dose were similar in both groups.
COMMENTARY
Vaccine administration has led to many diseases, including Haemophilus influenzae type b, polio, and measles, becoming quite rare. As we have recently seen with the resurgence of measles, continued vigilance and high immunization rates are important in ensuring that these diseases remain rare. The development of a hexavalent vaccine is another step in making immunization easier for clinicians to administer and for patients to accept.
Continue for treating infants with bronchiolitis >>
TREATING INFANTS WITH BRONCHIOLITIS
Silver AH, Esteban-Cruciani N, Azzarone G, et al. 3% hypertonic saline versus normal saline in inpatient bronchiolitis: a randomized controlled trial. Pediatrics. 2015;136:1036-1043.
Infants hospitalized with bronchiolitis saw no difference in length of stay or seven-day readmission rates when treated with nebulized 3% hypertonic saline (HS) compared with nebulized normal saline (NS), according to a randomized, controlled study of 227 infants who were younger than 12 months when admitted. Patients received either 4 mL nebulized 3% HS (113 infants) or 4 mL 0.9% NS (114 infants) every four hours from enrollment until hospital discharge. Researchers found
• Median length of stay of HS and NS groups was 2.1 days vs 2.1 days, respectively.
• Seven-day readmission rates for HS and NS groups were 4.3% vs 3.1%, respectively.
• Clinical worsening events were similar between groups.
COMMENTARY
Bronchiolitis, the most common lower respiratory tract infection in infants, is usually due to a viral infection, most often respiratory syncytial virus, and can cause disease that ranges in severity from mild to life-threatening. Infants with bronchiolitis typically present with rhinitis, tachypnea, wheezing, and cough, and occasionally crackles and use of accessory muscles. While many medications are used, supportive care and monitoring are the mainstays of therapy. Maintaining pulse above 90% is important, using supplemental oxygen when needed to achieve this. Alpha-adrenergic and beta-adrenergic bronchodilators are often used; though the evidence suggests that they are not usually helpful, they can be tried and continued if they appear to help. Systemic steroids are often used, but they too lack evidence of efficacy.1 This study shows that hypertonic saline can now join the list of interventions used with evidence of a lack of efficacy.
1. Diagnosis and management of bronchiolitis. Subcommittee on diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. doi:10.1542/peds.2006-2223.
Continue for azithromycin and preschool children >>
AZITHROMYCIN AND PRESCHOOL CHILDREN: CAN ANTIBIOTICS LESSEN ONSET OF SEVERE LRTIs?
Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034-2044.
The use of azithromycin early during an apparent respiratory tract illness (RTI) reduced the likelihood of severe lower RTI (LRTI) among young children with a history of recurrent severe LRTI, compared with placebo, according to a study of 607 children ages 12 to 71 months. Participants were randomized in a 1:1 ratio to receive either azithromycin (12 mg/kg/d for 5 d) or matching placebo at the start of an RTI. Researchers found
• A total of 937 treated RTIs were experienced by 443 children, including 92 severe LRTIs (azithromycin group, 35; placebo group, 57).
• Azithromycin significantly reduced the risk for progression to severe LRTI relative to placebo (HR, 0.64).
• Induction of azithromycin-resistant organisms and adverse events were infrequent.
COMMENTARY
Recurrent episodes of severe wheezing with RTI are an important and common occurrence, affecting up to 15% to 20% of children prior to age 6.1 LRTI was defined in this study as RTI that required the use of additional rescue medication. The current approach to RTI is to try to minimize the use of antibiotics unless an infection is clearly bacterial in origin, and to treat severe LRTI when it occurs. The results of this trial suggest that in children at high risk for severe LRTI, identified by their episodes of recurrent wheezing, early treatment of RTI with azithromycin may decrease the development of severe LRTI by more than 35%—an important result. It is important to recognize that this study does not suggest treating all RTIs with antibiotics, but rather that the use of a macrolide antibiotic may be considered, perhaps, in the select group of children similar to those studied, with a history of recurrent wheezing with previous RTIs.
1. Ly NP, Gold DR, Weiss ST, Celedón JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132-e1138.
TALKING OUT CHILDHOOD OBESITY
Resnicow K, McMaster F, Bocian A, et al. Motivational interviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics. 2015;135(4):649-657.
Counseling parents of overweight children using motivational interviewing from both health care providers and registered dietitians can significantly improve BMI, according to a study of 42 practices in the Pediatric Research in Office Settings Network of the American Academy of Pediatrics.
Researchers randomly assigned parents of overweight children, ages 2 through 8, to one of three groups: (1) usual care, (2) four provider-delivered motivational interviewing sessions over two years, or (3) four provider-delivered motivational interviewing sessions plus six sessions with a registered dietitian over two years. At study end, BMI percentile and change in BMI for the different groups were as follows:
COMMENTARY
The results of this study are exciting. Motivational interviewing is a technique in which the practitioner asks questions of a patient and allows the patient to discover his/her own conclusions about the topic. By so doing, the patient is more engaged in the discussion and is less resistant to input. This technique, with excellent evidence of effectiveness in the area of drug and alcohol abuse, has been shown to facilitate effective behavioral change in many areas and is recommended by the American Heart Association for behavioral change in adults.1,2 This is an exciting paper demonstrating evidence-based efficacy in addressing childhood obesity—a critical health issue—and is worth trying in the office.
1. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
2. Spring B, Ockene JK, Gidding SS, et al; American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, and Council on Cardiovascular and Stroke Nursing. Better population health through behavior change in adults: a call to action. Circulation. 2013;128(19):2169-2176. doi: 10.1161/01.cir.0000435173.25936.e1.
Continue for long-acting reversible contraception among teens >>
LONG-ACTING REVERSIBLE CONTRACEPTION AMONG TEENS
Romero L, Pazol K, Warner L, et al. Vital signs: trends in use of long-acting reversible contraception among teens aged 15-19 years seeking contraceptive services – United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2015;64(13):363-369.
Efforts to improve teen access to long-acting reversible contraception (LARC) have increased use of these methods, according to a CDC review of services provided at Title X National Family Planning Program centers. The report found
• LARC rates among teen patients increased from 0.4% in 2005 to 7.2% in 2013.
• In 2013, 2.8% of those seeking contraception used IUDs and 4.3% used implants.
• Among Title X patients, 7.6% of 18- and 19-year-olds used LARC, compared with 6.5% of 15- to 17-year-olds.
• Rates of LARC were lowest in Mississippi (0.7%) and highest in Colorado (25.8%).
COMMENTARY
LARCs, which include IUD and implantable hormonal contraceptive devices, require no effort for adherence on the part of the user; once in place, they are effective without further action. Current CDC guidelines on contraceptive use clearly recommend LARC for teenagers based on the efficacy and safety.1 LARCs are favored for teenagers because poor compliance has yielded suboptimal effectiveness of oral contraceptives and condoms in teenagers, who often forget to take their birth control pills or don’t use condoms when they should. Many clinicians have been slow to recommend LARCs in teenagers based on safety concerns related to adverse experience with IUDs 20 to 30 years ago. According to CDC guidelines, IUDs and implantable contraceptive devices now have robust safety data, and this article shows that they are being increasingly made available to teenagers who need them.
1. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. US Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62(RR-05):1-60.
Continue to testing for celiac in pediatric rheumatology patients >>
TESTING FOR CELIAC IN PEDIATRIC RHEUMATOLOGY PATIENTS
Sherman Y, Karanicolas R, DiMarco B, et al. Unrecognized celiac disease in children presenting for rheumatology evaluation. Pediatrics. 2015; [Epub ahead of print].
Children presenting for rheumatology evaluation should be screened for celiac disease, according to a review of 2,125 pediatric patients who were screened for celiac as part of the standard initial serologic evaluation.
Researchers identified 36 new cases of celiac disease (2.0% prevalence rate). The most common presenting complaints among these patients were myalgia, arthralgia, and rash. Less frequent complaints included gastrointestinal complaints of abdominal pain, nausea, and diarrhea.
After initiating a gluten-free diet, all of the patients with celiac disease reported improvement or complete resolution of musculoskeletal symptoms.
COMMENTARY
Celiac disease has a prevalence of 0.5% to 1% in the US and can present with a range of symptoms that include diarrhea, abdominal discomfort, and weight loss. In addition to these typical symptoms, celiac disease can cause a wide range of systemic symptoms, including osteopenia, abnormal liver function tests, anemia, neurologic symptoms, and general malaise and fatigue.1 This study suggests that celiac testing be considered in children presenting with rheumatologic symptoms, including myalgia, arthralgia, and rash.
1. Presutti RJ, Cangemi JR, Cassidy HD, et al. Celiac disease. Am Fam Physician. 2007;76(12):1795-1802, 1809-1810.
Continue for educating parents about antibiotic use >>
EDUCATING PARENTS ABOUT ANTIBIOTIC USE
Vaz LE, Kleinman KP, Lakoma MD, et al. Prevalence of parental misconceptions about antibiotic use. Pediatrics. 2015;136:221-231.
Misperceptions about antibiotic use persist and continue to be more prevalent among parents of Medicaid-insured children, according to a study of 1,500 Massachusetts parents.
Investigators examined antibiotic-related knowledge and attitudes among both Medicaid-insured and private-insured parents and found
• Fewer Medicaid parents answered questions correctly, except for one regarding bronchitis.
• Medicaid patients were more likely to request unnecessary antibiotics.
• More parents in 2013 understood that green nasal discharge did not require antibiotics than in 2000.
• Medicaid-insured parents were younger, less likely to be white, and had less education than those commercially insured.
COMMENTARY
Decreasing the unnecessary use of antibiotics for viral infections is an important component of decreasing the development of antibiotic-resistant organisms. An important driver of clinician use of antibiotics is patients’ expectations for antibiotics. This study shows that much work remains to be done in changing patient expectations, which is not surprising to any practicing clinician. This study also suggests that the expectation for antibiotics is greater among those individuals with Medicaid insurance, which suggests that an opportunity exists for Medicaid insurance plans to do targeted patient education on this issue—which should improve patient outcomes, decrease cost from use of unneeded antibiotics, and decrease the development of antibiotic-resistant organisms.
Continue for newborn pulmonary hypertension and maternal antidepressant use >>
NEWBORN PULMONARY HYPERTENSION AND MATERNAL ANTIDEPRESSANT USE
Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313(21):2142-2151.
Taking antidepressants during late pregnancy may increase the risk for persistent pulmonary hypertension of the newborn (PPHN), according to a nested cohort study of more than 3.7 million pregnant women in the 2000-2010 Medicaid Analytic eXtract.
Investigators compared offspring of mothers who used selective serotonin reuptake inhibitors (SSRIs) or non-SSRI monotherapy in the last 90 days of pregnancy to those who did not and found 3.4% of women filled at least one prescription for antidepressants late in pregnancy, primarily SSRIs.
Rates and odds ratios (ORs) of PPHN stratified by use and type of antidepressant were as follows:
The study authors note the absolute risk is small, and the increased risk is more modest than previous studies found.
COMMENTARY
Depression affects more than 12% of pregnancies and has important consequences, including increased risk for suicide, preterm birth, poor fetal growth, and impaired fetal and infant development.1 PPHN is a serious condition that can require intubation and can be fatal in 10% to 20% of cases. This study adds to the conflicting data suggesting that SSRI use in pregnancy can lead to an increase in this rare condition. The decision to use an antidepressant during pregnancy is a difficult one, because depression has serious consequences but so, potentially, does treatment. The treatment of depression during pregnancy requires thoughtful, informed discussion between patient and provider.
1. Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med. 2011;365(17):1605-1611. doi:10.1056/NEJMcp1102730.
Continue for new infant vaccine treats 6 diseases >>
NEW INFANT VACCINE TREATS 6 DISEASES
Marshall GS, Adams GL, Leonardi ML, et al. Immunogenicity, safety, and tolerability of a hexavalent vaccine in infants. Pediatrics. 2015:136(2):323-332.
The safety and immunogenicity of DTaP5-IPV-Hib-HepB fully liquid investigational hexavalent vaccine are comparable with the analogous licensed component vaccines and provide a new combination vaccine option aligned with the recommended US infant immunization schedule, according to a phase III study of 1,465 participants. Overall, 981 healthy infants were vaccinated in group 1 with the hexavalent vaccine and 484 in group 2 with the analogous licensed component vaccines.
The study found
• Immune responses in group 1 to all antigens contained in the vaccine one month after dose 3 were essentially noninferior to those in group 2.
• Adverse event rates after any dose were similar in both groups.
COMMENTARY
Vaccine administration has led to many diseases, including Haemophilus influenzae type b, polio, and measles, becoming quite rare. As we have recently seen with the resurgence of measles, continued vigilance and high immunization rates are important in ensuring that these diseases remain rare. The development of a hexavalent vaccine is another step in making immunization easier for clinicians to administer and for patients to accept.
Continue for treating infants with bronchiolitis >>
TREATING INFANTS WITH BRONCHIOLITIS
Silver AH, Esteban-Cruciani N, Azzarone G, et al. 3% hypertonic saline versus normal saline in inpatient bronchiolitis: a randomized controlled trial. Pediatrics. 2015;136:1036-1043.
Infants hospitalized with bronchiolitis saw no difference in length of stay or seven-day readmission rates when treated with nebulized 3% hypertonic saline (HS) compared with nebulized normal saline (NS), according to a randomized, controlled study of 227 infants who were younger than 12 months when admitted. Patients received either 4 mL nebulized 3% HS (113 infants) or 4 mL 0.9% NS (114 infants) every four hours from enrollment until hospital discharge. Researchers found
• Median length of stay of HS and NS groups was 2.1 days vs 2.1 days, respectively.
• Seven-day readmission rates for HS and NS groups were 4.3% vs 3.1%, respectively.
• Clinical worsening events were similar between groups.
COMMENTARY
Bronchiolitis, the most common lower respiratory tract infection in infants, is usually due to a viral infection, most often respiratory syncytial virus, and can cause disease that ranges in severity from mild to life-threatening. Infants with bronchiolitis typically present with rhinitis, tachypnea, wheezing, and cough, and occasionally crackles and use of accessory muscles. While many medications are used, supportive care and monitoring are the mainstays of therapy. Maintaining pulse above 90% is important, using supplemental oxygen when needed to achieve this. Alpha-adrenergic and beta-adrenergic bronchodilators are often used; though the evidence suggests that they are not usually helpful, they can be tried and continued if they appear to help. Systemic steroids are often used, but they too lack evidence of efficacy.1 This study shows that hypertonic saline can now join the list of interventions used with evidence of a lack of efficacy.
1. Diagnosis and management of bronchiolitis. Subcommittee on diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. doi:10.1542/peds.2006-2223.
Continue for azithromycin and preschool children >>
AZITHROMYCIN AND PRESCHOOL CHILDREN: CAN ANTIBIOTICS LESSEN ONSET OF SEVERE LRTIs?
Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034-2044.
The use of azithromycin early during an apparent respiratory tract illness (RTI) reduced the likelihood of severe lower RTI (LRTI) among young children with a history of recurrent severe LRTI, compared with placebo, according to a study of 607 children ages 12 to 71 months. Participants were randomized in a 1:1 ratio to receive either azithromycin (12 mg/kg/d for 5 d) or matching placebo at the start of an RTI. Researchers found
• A total of 937 treated RTIs were experienced by 443 children, including 92 severe LRTIs (azithromycin group, 35; placebo group, 57).
• Azithromycin significantly reduced the risk for progression to severe LRTI relative to placebo (HR, 0.64).
• Induction of azithromycin-resistant organisms and adverse events were infrequent.
COMMENTARY
Recurrent episodes of severe wheezing with RTI are an important and common occurrence, affecting up to 15% to 20% of children prior to age 6.1 LRTI was defined in this study as RTI that required the use of additional rescue medication. The current approach to RTI is to try to minimize the use of antibiotics unless an infection is clearly bacterial in origin, and to treat severe LRTI when it occurs. The results of this trial suggest that in children at high risk for severe LRTI, identified by their episodes of recurrent wheezing, early treatment of RTI with azithromycin may decrease the development of severe LRTI by more than 35%—an important result. It is important to recognize that this study does not suggest treating all RTIs with antibiotics, but rather that the use of a macrolide antibiotic may be considered, perhaps, in the select group of children similar to those studied, with a history of recurrent wheezing with previous RTIs.
1. Ly NP, Gold DR, Weiss ST, Celedón JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132-e1138.
Yoga Performs Like Pulmonary Rehab for COPD Patients
MONTREAL – A structured regimen of regular yoga exercises was as effective as a standard pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease for improving lung function, exercise tolerance, dyspnea severity, and quality of life in a single-center, randomized comparison of the two strategies with 60 patients.
In addition, chronic obstructive pulmonary disease (COPD) patients had a higher level of acceptance of yoga and were more comfortable doing it, compared with standard pulmonary rehabilitation, and it is a cost-effective approach given the minimal equipment required, Dr. Randeep Guleria said at the annual meeting of the American College of Chest Physicians.
“Patients with difficulty walking, osteoarthritis, knee problems, or unable to do exercises like cycling or treadmill found yoga to be much more acceptable,” said Dr. Guleria in an interview. Acceptance of yoga was also higher than standard rehabilitation among patients with more severe COPD, said Dr. Guleria, professor and head of the department of pulmonary medicine and sleep disorders at the All India Institute of Medical Sciences in New Delhi.
“I think that yoga could be a very valuable adjunct” to pulmonary rehabilitation in COPD patients, commented Dr. Roger S. Goldstein, director of the divisional program in respiratory rehabilitation at the University of Toronto. Dr. Goldstein speculated that even better than comparing yoga against conventional pulmonary rehabilitation would be a study that compared a combined yoga plus rehabilitation program against standard rehabilitation alone. Yoga is “a tremendous opportunity,” he said.
The 12-week study enrolled 60 patients who averaged 56 years old who had been diagnosed with COPD for an average of 8 years. Just under a third of the patients had moderate COPD, 42% had severe COPD, and 28% had very severe COPD.
Dr. Guleria and his associates randomized 30 patients into a yoga program that included 4 weeks of biweekly 1-hour sessions that instructed patients in a series of specially designed yoga exercises. That was followed by 8 weeks during which patients were mostly left to perform their learned exercises on their own, but with a supervised session once every 2 weeks. The other 30 patients participated in a standard pulmonary rehabilitation program for 12 weeks.
The researchers measured several parameters at baseline and after 12 weeks, including two measures of dyspnea severity, 6-minute walk distance, a quality of life assessment, and two serum markers of inflammation, C-reactive protein and interleukin 6.
Both interventions resulted in modest but statistically significant improvements, such as increases in 6-minute walk distance and a reduced modified Borg scale assessment. The Borg scale score fell from an average of 1.5 at baseline to 1.0 after 12 weeks in the yoga patients, and from an average 3.0 at baseline to 0.5 after 12 weeks in the rehabilitation patients.
A score that measured total quality of life improved by an average of 32% in the yoga patients and by an average of 21% in the rehabilitation patients, changes that approached statistical significance in both subgroups.
Comparing the assessment measures after 12 weeks in both arms of the study showed no statistically significant between-group differences, Dr. Guleria reported.
The researchers commissioned a specially designed yoga program from a professional yoga instructor who had been briefed about COPD. The yoga exercises included physical postures, breathing technique, and meditation and relaxation. The pulmonary rehabilitation program included patient education, upper and lower limb exercises, and breathing exercises.
Dr. Guleria said he believed the opportunity exists to further modify the yoga program to better optimize its potential to benefit COPD patients.
Dr. Guleria had no relevant disclosures. Dr. Goldstein had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
MONTREAL – A structured regimen of regular yoga exercises was as effective as a standard pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease for improving lung function, exercise tolerance, dyspnea severity, and quality of life in a single-center, randomized comparison of the two strategies with 60 patients.
In addition, chronic obstructive pulmonary disease (COPD) patients had a higher level of acceptance of yoga and were more comfortable doing it, compared with standard pulmonary rehabilitation, and it is a cost-effective approach given the minimal equipment required, Dr. Randeep Guleria said at the annual meeting of the American College of Chest Physicians.
“Patients with difficulty walking, osteoarthritis, knee problems, or unable to do exercises like cycling or treadmill found yoga to be much more acceptable,” said Dr. Guleria in an interview. Acceptance of yoga was also higher than standard rehabilitation among patients with more severe COPD, said Dr. Guleria, professor and head of the department of pulmonary medicine and sleep disorders at the All India Institute of Medical Sciences in New Delhi.
“I think that yoga could be a very valuable adjunct” to pulmonary rehabilitation in COPD patients, commented Dr. Roger S. Goldstein, director of the divisional program in respiratory rehabilitation at the University of Toronto. Dr. Goldstein speculated that even better than comparing yoga against conventional pulmonary rehabilitation would be a study that compared a combined yoga plus rehabilitation program against standard rehabilitation alone. Yoga is “a tremendous opportunity,” he said.
The 12-week study enrolled 60 patients who averaged 56 years old who had been diagnosed with COPD for an average of 8 years. Just under a third of the patients had moderate COPD, 42% had severe COPD, and 28% had very severe COPD.
Dr. Guleria and his associates randomized 30 patients into a yoga program that included 4 weeks of biweekly 1-hour sessions that instructed patients in a series of specially designed yoga exercises. That was followed by 8 weeks during which patients were mostly left to perform their learned exercises on their own, but with a supervised session once every 2 weeks. The other 30 patients participated in a standard pulmonary rehabilitation program for 12 weeks.
The researchers measured several parameters at baseline and after 12 weeks, including two measures of dyspnea severity, 6-minute walk distance, a quality of life assessment, and two serum markers of inflammation, C-reactive protein and interleukin 6.
Both interventions resulted in modest but statistically significant improvements, such as increases in 6-minute walk distance and a reduced modified Borg scale assessment. The Borg scale score fell from an average of 1.5 at baseline to 1.0 after 12 weeks in the yoga patients, and from an average 3.0 at baseline to 0.5 after 12 weeks in the rehabilitation patients.
A score that measured total quality of life improved by an average of 32% in the yoga patients and by an average of 21% in the rehabilitation patients, changes that approached statistical significance in both subgroups.
Comparing the assessment measures after 12 weeks in both arms of the study showed no statistically significant between-group differences, Dr. Guleria reported.
The researchers commissioned a specially designed yoga program from a professional yoga instructor who had been briefed about COPD. The yoga exercises included physical postures, breathing technique, and meditation and relaxation. The pulmonary rehabilitation program included patient education, upper and lower limb exercises, and breathing exercises.
Dr. Guleria said he believed the opportunity exists to further modify the yoga program to better optimize its potential to benefit COPD patients.
Dr. Guleria had no relevant disclosures. Dr. Goldstein had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
MONTREAL – A structured regimen of regular yoga exercises was as effective as a standard pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease for improving lung function, exercise tolerance, dyspnea severity, and quality of life in a single-center, randomized comparison of the two strategies with 60 patients.
In addition, chronic obstructive pulmonary disease (COPD) patients had a higher level of acceptance of yoga and were more comfortable doing it, compared with standard pulmonary rehabilitation, and it is a cost-effective approach given the minimal equipment required, Dr. Randeep Guleria said at the annual meeting of the American College of Chest Physicians.
“Patients with difficulty walking, osteoarthritis, knee problems, or unable to do exercises like cycling or treadmill found yoga to be much more acceptable,” said Dr. Guleria in an interview. Acceptance of yoga was also higher than standard rehabilitation among patients with more severe COPD, said Dr. Guleria, professor and head of the department of pulmonary medicine and sleep disorders at the All India Institute of Medical Sciences in New Delhi.
“I think that yoga could be a very valuable adjunct” to pulmonary rehabilitation in COPD patients, commented Dr. Roger S. Goldstein, director of the divisional program in respiratory rehabilitation at the University of Toronto. Dr. Goldstein speculated that even better than comparing yoga against conventional pulmonary rehabilitation would be a study that compared a combined yoga plus rehabilitation program against standard rehabilitation alone. Yoga is “a tremendous opportunity,” he said.
The 12-week study enrolled 60 patients who averaged 56 years old who had been diagnosed with COPD for an average of 8 years. Just under a third of the patients had moderate COPD, 42% had severe COPD, and 28% had very severe COPD.
Dr. Guleria and his associates randomized 30 patients into a yoga program that included 4 weeks of biweekly 1-hour sessions that instructed patients in a series of specially designed yoga exercises. That was followed by 8 weeks during which patients were mostly left to perform their learned exercises on their own, but with a supervised session once every 2 weeks. The other 30 patients participated in a standard pulmonary rehabilitation program for 12 weeks.
The researchers measured several parameters at baseline and after 12 weeks, including two measures of dyspnea severity, 6-minute walk distance, a quality of life assessment, and two serum markers of inflammation, C-reactive protein and interleukin 6.
Both interventions resulted in modest but statistically significant improvements, such as increases in 6-minute walk distance and a reduced modified Borg scale assessment. The Borg scale score fell from an average of 1.5 at baseline to 1.0 after 12 weeks in the yoga patients, and from an average 3.0 at baseline to 0.5 after 12 weeks in the rehabilitation patients.
A score that measured total quality of life improved by an average of 32% in the yoga patients and by an average of 21% in the rehabilitation patients, changes that approached statistical significance in both subgroups.
Comparing the assessment measures after 12 weeks in both arms of the study showed no statistically significant between-group differences, Dr. Guleria reported.
The researchers commissioned a specially designed yoga program from a professional yoga instructor who had been briefed about COPD. The yoga exercises included physical postures, breathing technique, and meditation and relaxation. The pulmonary rehabilitation program included patient education, upper and lower limb exercises, and breathing exercises.
Dr. Guleria said he believed the opportunity exists to further modify the yoga program to better optimize its potential to benefit COPD patients.
Dr. Guleria had no relevant disclosures. Dr. Goldstein had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
AT CHEST 2015