User login
This adjunct medication can speed CAP recovery
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Hot flashes and night sweats • amenorrhea • positive home pregnancy test • Dx?
THE CASE
A 25-year-old G2P2 woman came to our family practice clinic because she had multiple positive home pregnancy test results despite having undergone a sterilization procedure 4 years earlier. She said that 9 months ago, she had begun to experience hot flashes and night sweats that were getting progressively worse. Her menstrual cycles had been regular until 6 months earlier, when her bleeding became very light and irregular (2- to 6-week cycles with only one day of menstruation). Then 3 months ago, she stopped menstruating.
She’d had 2 uncomplicated pregnancies with normal vaginal deliveries 3 and 4 years ago, and had undergone a transcervical sterilization procedure after delivering her second child. Her medical history included hypothyroidism diagnosed at age 15, moderate persistent asthma, and seasonal allergies. She was taking levothyroxine 250 mcg/d, inhaled fluticasone/salmeterol, albuterol, and intranasal mometasone.
Transvaginal ultrasound failed to identify an intrauterine or ectopic pregnancy, and the patient’s ovaries were not visualized (uterine anatomy was normal with an endometrial stripe of 5.7 mm). The result of a serum human chorionic gonadotropin (hCG) test was 6.73 mIU/ mL. (In a nonpregnant, premenopausal woman, hCG is typically undetectable.) Subsequent serial hCG measurements remained low (6.72-7.09 mIU/mL), but persistent. Given these low hCG levels, it was imperative to rule out an intrauterine or ectopic pregnancy. A urine hCG was negative.
THE DIAGNOSIS
Because of our patient’s vasomotor symptoms, we ordered additional laboratory studies, which revealed an elevated follicle-stimulating hormone (FSH) level (66.08 mIU/mL and 42.2 mIU/mL taken one year apart; normal, 1.98-9.58 mIU/mL in a premenopausal female), an elevated luteinizing hormone (LH) level (46.1 mIU/mL; normal, 2.58-15.5 mIU/mL in a premenopausal female), a low thyroid-stimulating hormone (TSH) level (0.445 mIU/mL; normal, 0.465-4.65 mIU/mL), and a normal prolactin level (12.5 mIU/mL). Based on these results, we diagnosed primary ovarian insufficiency (POI).
DISCUSSION
POI, formerly known as premature ovarian failure, is defined as 4 to 6 months of amenorrhea or oligomenorrhea in a woman younger than 40 with an elevated FSH on 2 occasions, at least 4 weeks apart.1-3
The etiology of POI is broad. It can be caused by a failure of the pituitary gland or hypothalamus to secrete regulating hormones to stimulate the ovaries. Possible genetic causes include Turner’s syndrome, fragile X permutation, and other autosomal disorders that cause follicle dysfunction or destruction.1 Infections such as mumps, varicella, and tuberculosis are known to affect ovary function, as well.1,4 In addition, women who are exposed to chemotherapy or radiation are at higher risk for developing POI.1
Because POI and autoimmune disorders tend to occur together, consider screening any patient with POI for disorders such as hypothyroidism and Addison’s disease. A serum analysis to evaluate for autoantibodies against steroid-producing cells may be a potential marker for POI in patients with an autoimmune disease that affects the adrenal glands or thyroid. However, patients with isolated Addison’s disease, autoimmune hypothyroidism, or diabetes mellitus in the absence of POI do not appear to have steroid-specific antibodies.2 In our patient’s case, her hypothyroidism may have placed her at higher risk of having a second organ system adversely affected by her immune system.
What causes a false-positive pregnancy test? This case is unique because our patient reported multiple positive home pregnancy test results and had persistently low serum hCG levels. While she had symptoms that suggested menopause (hot flashes, oligomenorrhea that progressed to amenorrhea), she believed these symptoms were related to pregnancy. In addition to pregnancy, an elevated serum hCG measurement can be due to various malignancies, molar pregnancy, pituitary production of hCG, elevated LH, cross-reactivity with multiple animal exposures (due to the production of human anti-animal antibodies that react with testing), and recent mononucleosis infection.5
Other potential causes for false-positive urine pregnancy test results include tuboovarian abscess,6 adenomyosis,7 and cancers that produce hCG, such as colon, pancreatic, lung, liver, and urothelial bladder carcinoma.8,9 Urine with significant proteinuria can also cause a positive pregnancy test result.10
Our patient likely had a false-positive hCG due to elevated LH, secondary to POI, that demonstrated cross-reactivity on the hCG assay. The similarity in the chemical structure of the beta subunits of hCG and LH have been reported as false-positive tests in the absence of pregnancy.5
Because home pregnancy tests are designed to detect pregnancy as early as possible, they typically feature a high sensitivity by detecting very low levels of hCG, which leads to more frequent false-positive results. It is possible that different assay methods could account for the discrepancy between our patient’s positive home pregnancy tests and our negative laboratory urine pregnancy test.
Our patient and her husband were both counseled regarding her POI diagnosis. We conducted further studies to establish a possible etiology. She was found to have a normal karyotype of 46, XX, which ruled out Turner’s syndrome. Testing for permutations of the FMR1 gene was negative for fragile X syndrome, and antibody testing for thyroid and adrenal glands was negative for autoimmune disease.
Hormone therapy and supplemental calcium and vitamin D are recommended for women with POI to help prevent bone loss and other negative effects of low estrogen.11 We did not take this tack with our patient, however, because she decided she wanted to pursue a tubal ligation reversal in order to get pregnant. So instead, we decreased her dose of levothyroxine to 150 mcg (since her TSH was low) and we referred her to the Reproductive Endocrinology Department.
THE TAKEAWAY
Although many cases of POI have no discernible etiology, it is important to rule out malignancies, failure of pituitary production, genetic causes, infections, and other possible causes. Hormone therapy and prophylactic doses of calcium and vitamin D are recommended for patients diagnosed with POI.
1. Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68:499-509.
2. Betterle C, Rossi A, Dalla Pria S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf). 1993;39:35-43.
3. Fox H. The pathology of premature ovarian failure. J Pathol. 1992;167:357-363.
4. Panay N, Kalu E. Management of premature ovarian failure. Best Practice & Research Clinical Obstetrics and Gynaecology. 2009;23;129-140.
5. Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol. 2002;187:217-224.
6. Levsky ME, Handler JA, Suarez RD, et al. False-positive urine beta-HCG in a woman with a tubo-ovarian abscess. J Emerg Med. 2001;21:407-409.
7. Er TK, Chiang CH, Cheng BH, et al. False-positive urine pregnancy test in a woman with adenomysosis. Am J Emerg Med. 2009;27:1019.e5-7.
8. Rajabi B, Khoury J, Brewer C, et al. Urothelial bladder carcinoma with choriocarcinomatous differentiation presenting with a false-positive pregnancy test. Am J Med Sci. 2013;346:256-258.
9. Marcillac I, Troalen F, Bidart JM, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901-3907.
10. Kountz DS, Kolander SA, Rozovsky A. False positive urinary pregnancy test in the nephrotic syndrome. N Engl J Med. 1989;321:1416.
11. National Institute of Health, National Institute of Child Health and Human Development. What are the treatments for POI? National Institute of Child Health and Human Development Web site. Available at: https://www.nichd.nih.gov/health/topics/poi/conditioninfo/Pages/treatments.aspx. Accessed August 5, 2015.
THE CASE
A 25-year-old G2P2 woman came to our family practice clinic because she had multiple positive home pregnancy test results despite having undergone a sterilization procedure 4 years earlier. She said that 9 months ago, she had begun to experience hot flashes and night sweats that were getting progressively worse. Her menstrual cycles had been regular until 6 months earlier, when her bleeding became very light and irregular (2- to 6-week cycles with only one day of menstruation). Then 3 months ago, she stopped menstruating.
She’d had 2 uncomplicated pregnancies with normal vaginal deliveries 3 and 4 years ago, and had undergone a transcervical sterilization procedure after delivering her second child. Her medical history included hypothyroidism diagnosed at age 15, moderate persistent asthma, and seasonal allergies. She was taking levothyroxine 250 mcg/d, inhaled fluticasone/salmeterol, albuterol, and intranasal mometasone.
Transvaginal ultrasound failed to identify an intrauterine or ectopic pregnancy, and the patient’s ovaries were not visualized (uterine anatomy was normal with an endometrial stripe of 5.7 mm). The result of a serum human chorionic gonadotropin (hCG) test was 6.73 mIU/ mL. (In a nonpregnant, premenopausal woman, hCG is typically undetectable.) Subsequent serial hCG measurements remained low (6.72-7.09 mIU/mL), but persistent. Given these low hCG levels, it was imperative to rule out an intrauterine or ectopic pregnancy. A urine hCG was negative.
THE DIAGNOSIS
Because of our patient’s vasomotor symptoms, we ordered additional laboratory studies, which revealed an elevated follicle-stimulating hormone (FSH) level (66.08 mIU/mL and 42.2 mIU/mL taken one year apart; normal, 1.98-9.58 mIU/mL in a premenopausal female), an elevated luteinizing hormone (LH) level (46.1 mIU/mL; normal, 2.58-15.5 mIU/mL in a premenopausal female), a low thyroid-stimulating hormone (TSH) level (0.445 mIU/mL; normal, 0.465-4.65 mIU/mL), and a normal prolactin level (12.5 mIU/mL). Based on these results, we diagnosed primary ovarian insufficiency (POI).
DISCUSSION
POI, formerly known as premature ovarian failure, is defined as 4 to 6 months of amenorrhea or oligomenorrhea in a woman younger than 40 with an elevated FSH on 2 occasions, at least 4 weeks apart.1-3
The etiology of POI is broad. It can be caused by a failure of the pituitary gland or hypothalamus to secrete regulating hormones to stimulate the ovaries. Possible genetic causes include Turner’s syndrome, fragile X permutation, and other autosomal disorders that cause follicle dysfunction or destruction.1 Infections such as mumps, varicella, and tuberculosis are known to affect ovary function, as well.1,4 In addition, women who are exposed to chemotherapy or radiation are at higher risk for developing POI.1
Because POI and autoimmune disorders tend to occur together, consider screening any patient with POI for disorders such as hypothyroidism and Addison’s disease. A serum analysis to evaluate for autoantibodies against steroid-producing cells may be a potential marker for POI in patients with an autoimmune disease that affects the adrenal glands or thyroid. However, patients with isolated Addison’s disease, autoimmune hypothyroidism, or diabetes mellitus in the absence of POI do not appear to have steroid-specific antibodies.2 In our patient’s case, her hypothyroidism may have placed her at higher risk of having a second organ system adversely affected by her immune system.
What causes a false-positive pregnancy test? This case is unique because our patient reported multiple positive home pregnancy test results and had persistently low serum hCG levels. While she had symptoms that suggested menopause (hot flashes, oligomenorrhea that progressed to amenorrhea), she believed these symptoms were related to pregnancy. In addition to pregnancy, an elevated serum hCG measurement can be due to various malignancies, molar pregnancy, pituitary production of hCG, elevated LH, cross-reactivity with multiple animal exposures (due to the production of human anti-animal antibodies that react with testing), and recent mononucleosis infection.5
Other potential causes for false-positive urine pregnancy test results include tuboovarian abscess,6 adenomyosis,7 and cancers that produce hCG, such as colon, pancreatic, lung, liver, and urothelial bladder carcinoma.8,9 Urine with significant proteinuria can also cause a positive pregnancy test result.10
Our patient likely had a false-positive hCG due to elevated LH, secondary to POI, that demonstrated cross-reactivity on the hCG assay. The similarity in the chemical structure of the beta subunits of hCG and LH have been reported as false-positive tests in the absence of pregnancy.5
Because home pregnancy tests are designed to detect pregnancy as early as possible, they typically feature a high sensitivity by detecting very low levels of hCG, which leads to more frequent false-positive results. It is possible that different assay methods could account for the discrepancy between our patient’s positive home pregnancy tests and our negative laboratory urine pregnancy test.
Our patient and her husband were both counseled regarding her POI diagnosis. We conducted further studies to establish a possible etiology. She was found to have a normal karyotype of 46, XX, which ruled out Turner’s syndrome. Testing for permutations of the FMR1 gene was negative for fragile X syndrome, and antibody testing for thyroid and adrenal glands was negative for autoimmune disease.
Hormone therapy and supplemental calcium and vitamin D are recommended for women with POI to help prevent bone loss and other negative effects of low estrogen.11 We did not take this tack with our patient, however, because she decided she wanted to pursue a tubal ligation reversal in order to get pregnant. So instead, we decreased her dose of levothyroxine to 150 mcg (since her TSH was low) and we referred her to the Reproductive Endocrinology Department.
THE TAKEAWAY
Although many cases of POI have no discernible etiology, it is important to rule out malignancies, failure of pituitary production, genetic causes, infections, and other possible causes. Hormone therapy and prophylactic doses of calcium and vitamin D are recommended for patients diagnosed with POI.
THE CASE
A 25-year-old G2P2 woman came to our family practice clinic because she had multiple positive home pregnancy test results despite having undergone a sterilization procedure 4 years earlier. She said that 9 months ago, she had begun to experience hot flashes and night sweats that were getting progressively worse. Her menstrual cycles had been regular until 6 months earlier, when her bleeding became very light and irregular (2- to 6-week cycles with only one day of menstruation). Then 3 months ago, she stopped menstruating.
She’d had 2 uncomplicated pregnancies with normal vaginal deliveries 3 and 4 years ago, and had undergone a transcervical sterilization procedure after delivering her second child. Her medical history included hypothyroidism diagnosed at age 15, moderate persistent asthma, and seasonal allergies. She was taking levothyroxine 250 mcg/d, inhaled fluticasone/salmeterol, albuterol, and intranasal mometasone.
Transvaginal ultrasound failed to identify an intrauterine or ectopic pregnancy, and the patient’s ovaries were not visualized (uterine anatomy was normal with an endometrial stripe of 5.7 mm). The result of a serum human chorionic gonadotropin (hCG) test was 6.73 mIU/ mL. (In a nonpregnant, premenopausal woman, hCG is typically undetectable.) Subsequent serial hCG measurements remained low (6.72-7.09 mIU/mL), but persistent. Given these low hCG levels, it was imperative to rule out an intrauterine or ectopic pregnancy. A urine hCG was negative.
THE DIAGNOSIS
Because of our patient’s vasomotor symptoms, we ordered additional laboratory studies, which revealed an elevated follicle-stimulating hormone (FSH) level (66.08 mIU/mL and 42.2 mIU/mL taken one year apart; normal, 1.98-9.58 mIU/mL in a premenopausal female), an elevated luteinizing hormone (LH) level (46.1 mIU/mL; normal, 2.58-15.5 mIU/mL in a premenopausal female), a low thyroid-stimulating hormone (TSH) level (0.445 mIU/mL; normal, 0.465-4.65 mIU/mL), and a normal prolactin level (12.5 mIU/mL). Based on these results, we diagnosed primary ovarian insufficiency (POI).
DISCUSSION
POI, formerly known as premature ovarian failure, is defined as 4 to 6 months of amenorrhea or oligomenorrhea in a woman younger than 40 with an elevated FSH on 2 occasions, at least 4 weeks apart.1-3
The etiology of POI is broad. It can be caused by a failure of the pituitary gland or hypothalamus to secrete regulating hormones to stimulate the ovaries. Possible genetic causes include Turner’s syndrome, fragile X permutation, and other autosomal disorders that cause follicle dysfunction or destruction.1 Infections such as mumps, varicella, and tuberculosis are known to affect ovary function, as well.1,4 In addition, women who are exposed to chemotherapy or radiation are at higher risk for developing POI.1
Because POI and autoimmune disorders tend to occur together, consider screening any patient with POI for disorders such as hypothyroidism and Addison’s disease. A serum analysis to evaluate for autoantibodies against steroid-producing cells may be a potential marker for POI in patients with an autoimmune disease that affects the adrenal glands or thyroid. However, patients with isolated Addison’s disease, autoimmune hypothyroidism, or diabetes mellitus in the absence of POI do not appear to have steroid-specific antibodies.2 In our patient’s case, her hypothyroidism may have placed her at higher risk of having a second organ system adversely affected by her immune system.
What causes a false-positive pregnancy test? This case is unique because our patient reported multiple positive home pregnancy test results and had persistently low serum hCG levels. While she had symptoms that suggested menopause (hot flashes, oligomenorrhea that progressed to amenorrhea), she believed these symptoms were related to pregnancy. In addition to pregnancy, an elevated serum hCG measurement can be due to various malignancies, molar pregnancy, pituitary production of hCG, elevated LH, cross-reactivity with multiple animal exposures (due to the production of human anti-animal antibodies that react with testing), and recent mononucleosis infection.5
Other potential causes for false-positive urine pregnancy test results include tuboovarian abscess,6 adenomyosis,7 and cancers that produce hCG, such as colon, pancreatic, lung, liver, and urothelial bladder carcinoma.8,9 Urine with significant proteinuria can also cause a positive pregnancy test result.10
Our patient likely had a false-positive hCG due to elevated LH, secondary to POI, that demonstrated cross-reactivity on the hCG assay. The similarity in the chemical structure of the beta subunits of hCG and LH have been reported as false-positive tests in the absence of pregnancy.5
Because home pregnancy tests are designed to detect pregnancy as early as possible, they typically feature a high sensitivity by detecting very low levels of hCG, which leads to more frequent false-positive results. It is possible that different assay methods could account for the discrepancy between our patient’s positive home pregnancy tests and our negative laboratory urine pregnancy test.
Our patient and her husband were both counseled regarding her POI diagnosis. We conducted further studies to establish a possible etiology. She was found to have a normal karyotype of 46, XX, which ruled out Turner’s syndrome. Testing for permutations of the FMR1 gene was negative for fragile X syndrome, and antibody testing for thyroid and adrenal glands was negative for autoimmune disease.
Hormone therapy and supplemental calcium and vitamin D are recommended for women with POI to help prevent bone loss and other negative effects of low estrogen.11 We did not take this tack with our patient, however, because she decided she wanted to pursue a tubal ligation reversal in order to get pregnant. So instead, we decreased her dose of levothyroxine to 150 mcg (since her TSH was low) and we referred her to the Reproductive Endocrinology Department.
THE TAKEAWAY
Although many cases of POI have no discernible etiology, it is important to rule out malignancies, failure of pituitary production, genetic causes, infections, and other possible causes. Hormone therapy and prophylactic doses of calcium and vitamin D are recommended for patients diagnosed with POI.
1. Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68:499-509.
2. Betterle C, Rossi A, Dalla Pria S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf). 1993;39:35-43.
3. Fox H. The pathology of premature ovarian failure. J Pathol. 1992;167:357-363.
4. Panay N, Kalu E. Management of premature ovarian failure. Best Practice & Research Clinical Obstetrics and Gynaecology. 2009;23;129-140.
5. Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol. 2002;187:217-224.
6. Levsky ME, Handler JA, Suarez RD, et al. False-positive urine beta-HCG in a woman with a tubo-ovarian abscess. J Emerg Med. 2001;21:407-409.
7. Er TK, Chiang CH, Cheng BH, et al. False-positive urine pregnancy test in a woman with adenomysosis. Am J Emerg Med. 2009;27:1019.e5-7.
8. Rajabi B, Khoury J, Brewer C, et al. Urothelial bladder carcinoma with choriocarcinomatous differentiation presenting with a false-positive pregnancy test. Am J Med Sci. 2013;346:256-258.
9. Marcillac I, Troalen F, Bidart JM, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901-3907.
10. Kountz DS, Kolander SA, Rozovsky A. False positive urinary pregnancy test in the nephrotic syndrome. N Engl J Med. 1989;321:1416.
11. National Institute of Health, National Institute of Child Health and Human Development. What are the treatments for POI? National Institute of Child Health and Human Development Web site. Available at: https://www.nichd.nih.gov/health/topics/poi/conditioninfo/Pages/treatments.aspx. Accessed August 5, 2015.
1. Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68:499-509.
2. Betterle C, Rossi A, Dalla Pria S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf). 1993;39:35-43.
3. Fox H. The pathology of premature ovarian failure. J Pathol. 1992;167:357-363.
4. Panay N, Kalu E. Management of premature ovarian failure. Best Practice & Research Clinical Obstetrics and Gynaecology. 2009;23;129-140.
5. Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol. 2002;187:217-224.
6. Levsky ME, Handler JA, Suarez RD, et al. False-positive urine beta-HCG in a woman with a tubo-ovarian abscess. J Emerg Med. 2001;21:407-409.
7. Er TK, Chiang CH, Cheng BH, et al. False-positive urine pregnancy test in a woman with adenomysosis. Am J Emerg Med. 2009;27:1019.e5-7.
8. Rajabi B, Khoury J, Brewer C, et al. Urothelial bladder carcinoma with choriocarcinomatous differentiation presenting with a false-positive pregnancy test. Am J Med Sci. 2013;346:256-258.
9. Marcillac I, Troalen F, Bidart JM, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901-3907.
10. Kountz DS, Kolander SA, Rozovsky A. False positive urinary pregnancy test in the nephrotic syndrome. N Engl J Med. 1989;321:1416.
11. National Institute of Health, National Institute of Child Health and Human Development. What are the treatments for POI? National Institute of Child Health and Human Development Web site. Available at: https://www.nichd.nih.gov/health/topics/poi/conditioninfo/Pages/treatments.aspx. Accessed August 5, 2015.
Tuberculosis testing: Which patients, which test?
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
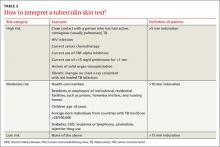
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
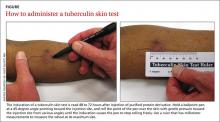
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
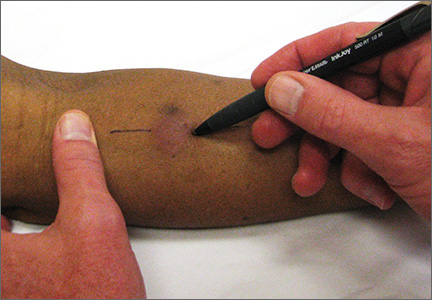
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.
Severe Anaphylaxis in Children Predicts Biphasic Reactions
Children who present with a severe anaphylactic reaction are two to three times more likely to develop a second delayed, or late-phase, reaction, Dr. Waleed Alqurashi and his colleagues reported.
Their retrospective study found that of 484 children who came to the emergency department for anaphylaxis, 15% developed a biphasic reaction. Most of these (69%) were respiratory and/or cardiovascular; half required epinephrine (Ann. Allerg. Asth. Immunol. 2015 [doi.org/10.1016/j.anai.2015.05.013]).
Dr. Alqurashi of the pediatric department at the University of Ottawa and his associates identified five independent predictors of a biphasic reaction: age 6-9 years (odds ratio, 3.6); a delay in presentation of more than 90 minutes after the onset of the initial reaction (OR, 2.58); wide pulse pressure at triage (OR, 2.92); requiring more than one epinephrine dose for initial reaction (OR, 2.7); and needing inhaled beta-agonists at the ED (OR, 2.39).
Children with these characteristics should stay for observation, rather than being discharged when their initial reactions are stabilized, the authors wrote.
“This period should be individualized and based primarily on the degree of severity of the initial anaphylactic reaction. Given the relatively high sensitivity of the present predictors, children with mild anaphylaxis who do not match any of these predictors could be considered for early disposition from the ED (less than 6 hours timed from the onset of the anaphylactic reaction) as long as an appropriate counseling has been provided,” the investigators said. Nonetheless, Dr. Alqurashi and his associates think large-scale prospective pediatric studies are necessary to validate these predictors.
Dr Alqurashi and his associates have no financial disclosures.
Children who present with a severe anaphylactic reaction are two to three times more likely to develop a second delayed, or late-phase, reaction, Dr. Waleed Alqurashi and his colleagues reported.
Their retrospective study found that of 484 children who came to the emergency department for anaphylaxis, 15% developed a biphasic reaction. Most of these (69%) were respiratory and/or cardiovascular; half required epinephrine (Ann. Allerg. Asth. Immunol. 2015 [doi.org/10.1016/j.anai.2015.05.013]).
Dr. Alqurashi of the pediatric department at the University of Ottawa and his associates identified five independent predictors of a biphasic reaction: age 6-9 years (odds ratio, 3.6); a delay in presentation of more than 90 minutes after the onset of the initial reaction (OR, 2.58); wide pulse pressure at triage (OR, 2.92); requiring more than one epinephrine dose for initial reaction (OR, 2.7); and needing inhaled beta-agonists at the ED (OR, 2.39).
Children with these characteristics should stay for observation, rather than being discharged when their initial reactions are stabilized, the authors wrote.
“This period should be individualized and based primarily on the degree of severity of the initial anaphylactic reaction. Given the relatively high sensitivity of the present predictors, children with mild anaphylaxis who do not match any of these predictors could be considered for early disposition from the ED (less than 6 hours timed from the onset of the anaphylactic reaction) as long as an appropriate counseling has been provided,” the investigators said. Nonetheless, Dr. Alqurashi and his associates think large-scale prospective pediatric studies are necessary to validate these predictors.
Dr Alqurashi and his associates have no financial disclosures.
Children who present with a severe anaphylactic reaction are two to three times more likely to develop a second delayed, or late-phase, reaction, Dr. Waleed Alqurashi and his colleagues reported.
Their retrospective study found that of 484 children who came to the emergency department for anaphylaxis, 15% developed a biphasic reaction. Most of these (69%) were respiratory and/or cardiovascular; half required epinephrine (Ann. Allerg. Asth. Immunol. 2015 [doi.org/10.1016/j.anai.2015.05.013]).
Dr. Alqurashi of the pediatric department at the University of Ottawa and his associates identified five independent predictors of a biphasic reaction: age 6-9 years (odds ratio, 3.6); a delay in presentation of more than 90 minutes after the onset of the initial reaction (OR, 2.58); wide pulse pressure at triage (OR, 2.92); requiring more than one epinephrine dose for initial reaction (OR, 2.7); and needing inhaled beta-agonists at the ED (OR, 2.39).
Children with these characteristics should stay for observation, rather than being discharged when their initial reactions are stabilized, the authors wrote.
“This period should be individualized and based primarily on the degree of severity of the initial anaphylactic reaction. Given the relatively high sensitivity of the present predictors, children with mild anaphylaxis who do not match any of these predictors could be considered for early disposition from the ED (less than 6 hours timed from the onset of the anaphylactic reaction) as long as an appropriate counseling has been provided,” the investigators said. Nonetheless, Dr. Alqurashi and his associates think large-scale prospective pediatric studies are necessary to validate these predictors.
Dr Alqurashi and his associates have no financial disclosures.
FROM THE ANNALS OF ALLERGY, ASTHMA, AND IMMUNOLOGY
July 2015: Click for Credit
Here are 7 articles in the July issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. BSR: Multiple Benefits Seen With Intensive Psoriatic Arthritis Therapy
Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis (PsA) until they achieve a set of minimal disease activity (MDA) criteria (see Table), an expert said at the British Society for Rheumatology annual conference.
To take the posttest, go to: http://bit.ly/1KaikxW
2. Subclinical Hyperthyroidism Linked to Higher Fracture Risk
Individuals with subclinical hyperthyroidism are at increased risk for hip and other fractures, according to the authors of a meta-analysis. The researchers examined data from 70,298 individuals—4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism—enrolled in 13 prospective cohort studies.
To take the posttest, go to: http://bit.ly/1H13j0t
3. Newer Oral Contraceptives Pose Higher VTE Risk
The risk for venous thromboembolism (VTE) is generally greater for women using oral contraceptives with newer types of progestogen hormones than for those taking older, second-generation birth control pills, study results showed.
To take the posttest, go to: http://bit.ly/1AKQert
4. Statins, Fibrates Lower Stroke Risk in Elderly
Both statin and fibrate therapies taken to improve lipid profiles decreased risk for stroke by 30% in a community-dwelling population of elderly people, according to a prospective European study published online in the British Medical Journal.
To take the posttest, go to: http://bit.ly/1FuyYCb
5. Cystic Fibrosis–related Diabetes Requires Different Approach
Cystic fibrosis–related diabetes (CFRD) is a unique disease that requires a different mindset on the part of the treating clinician.
To take the posttest, go to: http://bit.ly/1BKGZCm
6. CVD Risk Persists for 40 Years in Hodgkin Survivors
People who survive Hodgkin lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease (CVD) for at least 40 years—the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
To take the posttest, go to: http://bit.ly/1M5ymYG
7. Asymptomatic Carotid Stenosis and Central Sleep Apnea Linked
More than two-thirds of patients with asymptomatic carotid stenosis are likely to have sleep apnea, according to an observational study. The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea: 42% had obstructive sleep apnea (OSA) and 27%, central sleep apnea (CSA).
To take the posttest, go to: http://bit.ly/1SWGPmb
Here are 7 articles in the July issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. BSR: Multiple Benefits Seen With Intensive Psoriatic Arthritis Therapy
Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis (PsA) until they achieve a set of minimal disease activity (MDA) criteria (see Table), an expert said at the British Society for Rheumatology annual conference.
To take the posttest, go to: http://bit.ly/1KaikxW
2. Subclinical Hyperthyroidism Linked to Higher Fracture Risk
Individuals with subclinical hyperthyroidism are at increased risk for hip and other fractures, according to the authors of a meta-analysis. The researchers examined data from 70,298 individuals—4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism—enrolled in 13 prospective cohort studies.
To take the posttest, go to: http://bit.ly/1H13j0t
3. Newer Oral Contraceptives Pose Higher VTE Risk
The risk for venous thromboembolism (VTE) is generally greater for women using oral contraceptives with newer types of progestogen hormones than for those taking older, second-generation birth control pills, study results showed.
To take the posttest, go to: http://bit.ly/1AKQert
4. Statins, Fibrates Lower Stroke Risk in Elderly
Both statin and fibrate therapies taken to improve lipid profiles decreased risk for stroke by 30% in a community-dwelling population of elderly people, according to a prospective European study published online in the British Medical Journal.
To take the posttest, go to: http://bit.ly/1FuyYCb
5. Cystic Fibrosis–related Diabetes Requires Different Approach
Cystic fibrosis–related diabetes (CFRD) is a unique disease that requires a different mindset on the part of the treating clinician.
To take the posttest, go to: http://bit.ly/1BKGZCm
6. CVD Risk Persists for 40 Years in Hodgkin Survivors
People who survive Hodgkin lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease (CVD) for at least 40 years—the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
To take the posttest, go to: http://bit.ly/1M5ymYG
7. Asymptomatic Carotid Stenosis and Central Sleep Apnea Linked
More than two-thirds of patients with asymptomatic carotid stenosis are likely to have sleep apnea, according to an observational study. The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea: 42% had obstructive sleep apnea (OSA) and 27%, central sleep apnea (CSA).
To take the posttest, go to: http://bit.ly/1SWGPmb
Here are 7 articles in the July issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. BSR: Multiple Benefits Seen With Intensive Psoriatic Arthritis Therapy
Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis (PsA) until they achieve a set of minimal disease activity (MDA) criteria (see Table), an expert said at the British Society for Rheumatology annual conference.
To take the posttest, go to: http://bit.ly/1KaikxW
2. Subclinical Hyperthyroidism Linked to Higher Fracture Risk
Individuals with subclinical hyperthyroidism are at increased risk for hip and other fractures, according to the authors of a meta-analysis. The researchers examined data from 70,298 individuals—4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism—enrolled in 13 prospective cohort studies.
To take the posttest, go to: http://bit.ly/1H13j0t
3. Newer Oral Contraceptives Pose Higher VTE Risk
The risk for venous thromboembolism (VTE) is generally greater for women using oral contraceptives with newer types of progestogen hormones than for those taking older, second-generation birth control pills, study results showed.
To take the posttest, go to: http://bit.ly/1AKQert
4. Statins, Fibrates Lower Stroke Risk in Elderly
Both statin and fibrate therapies taken to improve lipid profiles decreased risk for stroke by 30% in a community-dwelling population of elderly people, according to a prospective European study published online in the British Medical Journal.
To take the posttest, go to: http://bit.ly/1FuyYCb
5. Cystic Fibrosis–related Diabetes Requires Different Approach
Cystic fibrosis–related diabetes (CFRD) is a unique disease that requires a different mindset on the part of the treating clinician.
To take the posttest, go to: http://bit.ly/1BKGZCm
6. CVD Risk Persists for 40 Years in Hodgkin Survivors
People who survive Hodgkin lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease (CVD) for at least 40 years—the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
To take the posttest, go to: http://bit.ly/1M5ymYG
7. Asymptomatic Carotid Stenosis and Central Sleep Apnea Linked
More than two-thirds of patients with asymptomatic carotid stenosis are likely to have sleep apnea, according to an observational study. The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea: 42% had obstructive sleep apnea (OSA) and 27%, central sleep apnea (CSA).
To take the posttest, go to: http://bit.ly/1SWGPmb
Mind the Gap
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications was brought to the ED with a two-week history of intermittent fever and poor oral intake. His current medications included sodium bromide (185 mg bid, orally) for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were sodium, 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
WHAT DOES THE ANION GAP REPRESENT?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo and is accomplished by the presence of unmeasured anions (UA; eg, lactate and phosphate) and unmeasured cations (UC; eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus unmeasured anions must equal the sum of the measured plus unmeasured cations.
WHAT CAUSES A LOW OR NEGATIVE ANION GAP?
While most health care providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased concentration of unmeasured anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than that of the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of serum chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride.
However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
Continue for causes of the falsely elevated chloride >>
WHAT CAUSES THE FALSELY ELEVATED CHLORIDE?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride.
Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration; rather, it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
HOW ARE PATIENTS EXPOSED TO BROMIDE SALTS?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurologic and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide, as well as the brominated vegetable oils found in some soft drinks.5
Continue for how bromism is treated >>
HOW IS BROMISM TREATED?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10 to 12 days), but in patients with normal renal function, it is possible to reduce this half-life to approximately three days with hydration and diuresis with sodium chloride.3
Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
CASE CONCLUSION
The patient was admitted for observation and treated with IV sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide (185 mg bid, orally) since his seizures were refractory to other anticonvulsant medications.
REFERENCES
1. Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
2. Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
3. Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, et al. A negative anion gap as a clue to diagnose bromide intoxication. Nephron. 1995;69(3):311-313.
4. Yamamoto K, Kobayashi H, Kobayashi T, Murakami S. False hyperchloremia in bromism. J Anesth. 1991;5(1):88-91.
5. Ng YY, Lin WL, Chen TW. Spurious hyperchloremia and decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications was brought to the ED with a two-week history of intermittent fever and poor oral intake. His current medications included sodium bromide (185 mg bid, orally) for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were sodium, 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
WHAT DOES THE ANION GAP REPRESENT?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo and is accomplished by the presence of unmeasured anions (UA; eg, lactate and phosphate) and unmeasured cations (UC; eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus unmeasured anions must equal the sum of the measured plus unmeasured cations.
WHAT CAUSES A LOW OR NEGATIVE ANION GAP?
While most health care providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased concentration of unmeasured anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than that of the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of serum chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride.
However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
Continue for causes of the falsely elevated chloride >>
WHAT CAUSES THE FALSELY ELEVATED CHLORIDE?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride.
Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration; rather, it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
HOW ARE PATIENTS EXPOSED TO BROMIDE SALTS?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurologic and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide, as well as the brominated vegetable oils found in some soft drinks.5
Continue for how bromism is treated >>
HOW IS BROMISM TREATED?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10 to 12 days), but in patients with normal renal function, it is possible to reduce this half-life to approximately three days with hydration and diuresis with sodium chloride.3
Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
CASE CONCLUSION
The patient was admitted for observation and treated with IV sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide (185 mg bid, orally) since his seizures were refractory to other anticonvulsant medications.
REFERENCES
1. Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
2. Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
3. Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, et al. A negative anion gap as a clue to diagnose bromide intoxication. Nephron. 1995;69(3):311-313.
4. Yamamoto K, Kobayashi H, Kobayashi T, Murakami S. False hyperchloremia in bromism. J Anesth. 1991;5(1):88-91.
5. Ng YY, Lin WL, Chen TW. Spurious hyperchloremia and decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications was brought to the ED with a two-week history of intermittent fever and poor oral intake. His current medications included sodium bromide (185 mg bid, orally) for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were sodium, 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
WHAT DOES THE ANION GAP REPRESENT?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo and is accomplished by the presence of unmeasured anions (UA; eg, lactate and phosphate) and unmeasured cations (UC; eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus unmeasured anions must equal the sum of the measured plus unmeasured cations.
WHAT CAUSES A LOW OR NEGATIVE ANION GAP?
While most health care providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased concentration of unmeasured anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than that of the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of serum chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride.
However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
Continue for causes of the falsely elevated chloride >>
WHAT CAUSES THE FALSELY ELEVATED CHLORIDE?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride.
Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration; rather, it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
HOW ARE PATIENTS EXPOSED TO BROMIDE SALTS?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurologic and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide, as well as the brominated vegetable oils found in some soft drinks.5
Continue for how bromism is treated >>
HOW IS BROMISM TREATED?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10 to 12 days), but in patients with normal renal function, it is possible to reduce this half-life to approximately three days with hydration and diuresis with sodium chloride.3
Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
CASE CONCLUSION
The patient was admitted for observation and treated with IV sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide (185 mg bid, orally) since his seizures were refractory to other anticonvulsant medications.
REFERENCES
1. Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
2. Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
3. Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, et al. A negative anion gap as a clue to diagnose bromide intoxication. Nephron. 1995;69(3):311-313.
4. Yamamoto K, Kobayashi H, Kobayashi T, Murakami S. False hyperchloremia in bromism. J Anesth. 1991;5(1):88-91.
5. Ng YY, Lin WL, Chen TW. Spurious hyperchloremia and decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
Broad spectrum–antibiotic Use Shifted Following National Guideline Publication
Use of broad-spectrum antibiotics to treat pediatric pneumonia dropped considerably following the publication of national guidelines that recommended narrow-spectrum antibiotics, according to a recent study.
The guidelines released by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) in August 2011 “emphasized the use of a single, narrow-spectrum antibiotic (i.e., penicillin/ampicillin) for vaccinated children hospitalized with uncomplicated community-acquired pneumonia,” based on evidence that Streptococcus pneumoniae most commonly caused the illness and that incidence of penicillin-resistant pneumococcal disease had dropped after the vaccine’s introduction.
“Overall, use of third-generation cephalosporins declined significantly after release of the guidelines, whereas penicillin/ampicillin use increased,” reported Dr. Derek Williams of Monroe Carell Jr. Children’s Hospital and Vanderbilt University in Nashville, Tenn., and his associates. “We noted consistent trends across study sites, although changes were most apparent in institutions that conducted active hospital-based educational efforts to disseminate the PIDS/IDSA guidelines,” they wrote (Pediatrics 2015 June 22 [doi:10.1542/peds.2014-3047]).
The researchers analyzed the records of all 2,121 children hospitalized with community-acquired pneumonia between January 2010 and June 2012 at three hospitals in Tennessee and Utah. In the year before the new guidelines, all three hospitals most commonly used the broad-spectrum antibiotics, with prescription rates ranging from 43% to 61% for third-generation cephalosporins, compared with rates of 1%-9% for penicillin and ampicillin prescriptions. Overall, 52.8% of children received third-generation cephalosporins to treat their pneumonia and 2.7% received penicillin or ampicillin before the guidelines.
Nine months after the guidelines had been published, the use of third-generation cephalosporins dropped 12.4 percentage points and the use of penicillin and ampicillin increased 11.3 percentage points, Dr. Williams and his associates said.
The largest shift in prescribing patterns occurred at the two hospitals that held pediatric departmental educational conferences within 4 months of the new guidelines. The third hospital, which did not formally distribute information abut the guidelines, saw the smallest reduction in broad spectrum–antibiotic use.
Although all three hospitals reported having antimicrobial stewardship programs, none had a community-acquired pneumonia practice guideline during the study period and none of the programs specifically focused on community-acquired pneumonia or restrictions on third-generation cephalosporins, aminopenicillins, or macrolides.
The research was supported by the National Institute of Allergy and Infectious Diseases, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention. The authors reported no relevant financial disclosures.
Use of broad-spectrum antibiotics to treat pediatric pneumonia dropped considerably following the publication of national guidelines that recommended narrow-spectrum antibiotics, according to a recent study.
The guidelines released by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) in August 2011 “emphasized the use of a single, narrow-spectrum antibiotic (i.e., penicillin/ampicillin) for vaccinated children hospitalized with uncomplicated community-acquired pneumonia,” based on evidence that Streptococcus pneumoniae most commonly caused the illness and that incidence of penicillin-resistant pneumococcal disease had dropped after the vaccine’s introduction.
“Overall, use of third-generation cephalosporins declined significantly after release of the guidelines, whereas penicillin/ampicillin use increased,” reported Dr. Derek Williams of Monroe Carell Jr. Children’s Hospital and Vanderbilt University in Nashville, Tenn., and his associates. “We noted consistent trends across study sites, although changes were most apparent in institutions that conducted active hospital-based educational efforts to disseminate the PIDS/IDSA guidelines,” they wrote (Pediatrics 2015 June 22 [doi:10.1542/peds.2014-3047]).
The researchers analyzed the records of all 2,121 children hospitalized with community-acquired pneumonia between January 2010 and June 2012 at three hospitals in Tennessee and Utah. In the year before the new guidelines, all three hospitals most commonly used the broad-spectrum antibiotics, with prescription rates ranging from 43% to 61% for third-generation cephalosporins, compared with rates of 1%-9% for penicillin and ampicillin prescriptions. Overall, 52.8% of children received third-generation cephalosporins to treat their pneumonia and 2.7% received penicillin or ampicillin before the guidelines.
Nine months after the guidelines had been published, the use of third-generation cephalosporins dropped 12.4 percentage points and the use of penicillin and ampicillin increased 11.3 percentage points, Dr. Williams and his associates said.
The largest shift in prescribing patterns occurred at the two hospitals that held pediatric departmental educational conferences within 4 months of the new guidelines. The third hospital, which did not formally distribute information abut the guidelines, saw the smallest reduction in broad spectrum–antibiotic use.
Although all three hospitals reported having antimicrobial stewardship programs, none had a community-acquired pneumonia practice guideline during the study period and none of the programs specifically focused on community-acquired pneumonia or restrictions on third-generation cephalosporins, aminopenicillins, or macrolides.
The research was supported by the National Institute of Allergy and Infectious Diseases, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention. The authors reported no relevant financial disclosures.
Use of broad-spectrum antibiotics to treat pediatric pneumonia dropped considerably following the publication of national guidelines that recommended narrow-spectrum antibiotics, according to a recent study.
The guidelines released by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) in August 2011 “emphasized the use of a single, narrow-spectrum antibiotic (i.e., penicillin/ampicillin) for vaccinated children hospitalized with uncomplicated community-acquired pneumonia,” based on evidence that Streptococcus pneumoniae most commonly caused the illness and that incidence of penicillin-resistant pneumococcal disease had dropped after the vaccine’s introduction.
“Overall, use of third-generation cephalosporins declined significantly after release of the guidelines, whereas penicillin/ampicillin use increased,” reported Dr. Derek Williams of Monroe Carell Jr. Children’s Hospital and Vanderbilt University in Nashville, Tenn., and his associates. “We noted consistent trends across study sites, although changes were most apparent in institutions that conducted active hospital-based educational efforts to disseminate the PIDS/IDSA guidelines,” they wrote (Pediatrics 2015 June 22 [doi:10.1542/peds.2014-3047]).
The researchers analyzed the records of all 2,121 children hospitalized with community-acquired pneumonia between January 2010 and June 2012 at three hospitals in Tennessee and Utah. In the year before the new guidelines, all three hospitals most commonly used the broad-spectrum antibiotics, with prescription rates ranging from 43% to 61% for third-generation cephalosporins, compared with rates of 1%-9% for penicillin and ampicillin prescriptions. Overall, 52.8% of children received third-generation cephalosporins to treat their pneumonia and 2.7% received penicillin or ampicillin before the guidelines.
Nine months after the guidelines had been published, the use of third-generation cephalosporins dropped 12.4 percentage points and the use of penicillin and ampicillin increased 11.3 percentage points, Dr. Williams and his associates said.
The largest shift in prescribing patterns occurred at the two hospitals that held pediatric departmental educational conferences within 4 months of the new guidelines. The third hospital, which did not formally distribute information abut the guidelines, saw the smallest reduction in broad spectrum–antibiotic use.
Although all three hospitals reported having antimicrobial stewardship programs, none had a community-acquired pneumonia practice guideline during the study period and none of the programs specifically focused on community-acquired pneumonia or restrictions on third-generation cephalosporins, aminopenicillins, or macrolides.
The research was supported by the National Institute of Allergy and Infectious Diseases, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention. The authors reported no relevant financial disclosures.
FROM PEDIATRICS
FDA Panel Gives Nod to Mepolizumab for Severe Asthma in Adults
GAITHERSBURG, MD. – The biologic agent mepolizumab was unanimously recommended for approval as a treatment for severe asthma in adults at a June 4 meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
All 14 members of the advisory panel agreed that the efficacy data provide substantial evidence of a clinically meaningful benefit of mepolizumab for the treatment of severe asthma in adults; 13 of the 14 members agreed that the data adequately demonstrated safety in adults. However, only four panel members recommended approval for adolescents aged 12-17 years, with the majority of panel members citing concerns that the low number of adolescents studied to date did not allow safety to be adequately evaluated in a younger population, especially for a medication that would be taken for many years – perhaps for a lifetime.
If approved by the FDA, the biologic agent would be available as a once-monthly treatment that is injected subcutaneously by a health care professional. Mepolizumab would be marketed by Glaxo Smith Kline under the trade name Nucala. Mepolizumab is a first-in-class humanized monoclonal antibody that targets interleukin-5, a glycoprotein cytokine that mediates production of eosinophils. Elevation of eosinophils in blood and tissue is associated with an increase in cytokines and other inflammatory molecules that can trigger or exacerbate airway inflammation in asthma. One other monoclonal antibody, the anti-IgE biologic omulizimab (Xolair), has been approved to treat asthma. Glaxo Smith Kline brought mepolizumab to the FDA for use as an add-on therapy for the small subset of asthma patients whose disease remains uncontrolled despite the optimal use of inhaled corticosteroids and additional therapies such as leukotriene inhibitors or theophylline. This population experiences more frequent asthma exacerbations, has more emergency department visits and hospitalizations, and uses higher doses of oral corticosteroids. Approximately 60% of those with severe asthma have marked eosinophilia.Panel members uniformly cited the efficacy data for adults with severe asthma; several panelists also remarked on the importance of developing more steroid-sparing alternatives for this population.
The panel endorsed neither efficacy nor safety for those aged 12-17 years, with 9 of the 14 panelists voting not to endorse efficacy and 13 members voting not to endorse safety findings. Mepolizumab’s efficacy, many panelists said, was not clearly established from the data presented, which drew from small numbers of adolescents enrolled in the studies.
Dr. David Au, acting director of Health Services Research and Development at Seattle’s VA Puget Sound Health Care system, observed that “adolescents are not small adults – their lungs continue to mature over time.” Many panelists, however, also called for ongoing study, noting the significant unmet need for steroid alternatives in the adolescent population.
Several panelists advocated postmarketing surveillance for long-term use, with particular attention to those with parasitic disease, to monitoring any sign of malignancy, and to tracking opportunistic diseases such as herpes zoster.
An early clinical trial of mepolizumab, conducted in 1999, failed to show benefit for an undifferentiated population of patients with moderate to severe asthma. However, independent research later identified marked eosinophilia as a factor associated with more frequent asthma exacerbations and a series of clinical trials begun in 2011 targeted patients with severe asthma and eosinophilic inflammation. A global program was initiated, with 12% of patients overall coming from the United States.
Pivotal phase 2b/3, double blind, placebo-controlled clinical trials included a dose-ranging study tracking asthma exacerbations enrolling 616 patients for 52 weeks.
The recommended dose of 100 mg subcutaneously every 4 weeks, as well as a 75-mg IV dose, was used for an additional 576 patients for 32 weeks, with the primary outcome measure being the number of asthma exacerbations. A final 24-week study of 135 patients with severe asthma measured the reduction in oral corticosteroid use, compared with placebo, as well as the number of asthma exacerbations. In all of the studies, patients’ asthma treatment was optimized according to standard of care before adding mepolizumab.
In each study and in pooled data, mepolizumab approximately halved the number of asthma exacerbations for study participants when compared with those using placebo. A 24-week corticosteroid-sparing study showed significant reduction in oral corticosteroid use, without loss of asthma control, for the mepolizumab group. Prespecified subgroup analyses were hampered because of low participation numbers for African Americans and adolescents, and because confidence intervals for these subgroups often ranged over 1, limiting interpretation of benefit results for these groups. The overall safety profile was good, with adverse event rates similar in the treatment and placebo arms. Headache and injection-site reactions were the most commonly reported adverse events but were similar between treatment and placebo arms. No episodes of anaphylaxis were reported, and neutralizing antibodies developed in one patient total across all studies. Ongoing open-label studies continue.The FDA’s independent biostatistical analysis showed clear evidence of efficacy for mepolizumab, with demonstrated consistent, statistically significant decreases of about one exacerbation per year, according to the agency.In its analysis, the agency observed a positive association between higher eosinophil count and mepolizumab treatment effect, meaning that those with higher eosinophil counts saw a greater benefit from mepolizumab, as measured by a reduction in exacerbations.
The number of deaths was balanced across treatment arms, though a larger number of respiratory-related deaths than expected was seen overall. This higher number of deaths may reflect the severity of asthma in the study population. The respiratory-related serious adverse events, according to the FDA, favor treatment over placebo.
No treatment-related cardiovascular risks were identified.
The FDA usually follows the recommendations of its advisory panels. The FDA panelists reported no relevant conflicts of interest.
GAITHERSBURG, MD. – The biologic agent mepolizumab was unanimously recommended for approval as a treatment for severe asthma in adults at a June 4 meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
All 14 members of the advisory panel agreed that the efficacy data provide substantial evidence of a clinically meaningful benefit of mepolizumab for the treatment of severe asthma in adults; 13 of the 14 members agreed that the data adequately demonstrated safety in adults. However, only four panel members recommended approval for adolescents aged 12-17 years, with the majority of panel members citing concerns that the low number of adolescents studied to date did not allow safety to be adequately evaluated in a younger population, especially for a medication that would be taken for many years – perhaps for a lifetime.
If approved by the FDA, the biologic agent would be available as a once-monthly treatment that is injected subcutaneously by a health care professional. Mepolizumab would be marketed by Glaxo Smith Kline under the trade name Nucala. Mepolizumab is a first-in-class humanized monoclonal antibody that targets interleukin-5, a glycoprotein cytokine that mediates production of eosinophils. Elevation of eosinophils in blood and tissue is associated with an increase in cytokines and other inflammatory molecules that can trigger or exacerbate airway inflammation in asthma. One other monoclonal antibody, the anti-IgE biologic omulizimab (Xolair), has been approved to treat asthma. Glaxo Smith Kline brought mepolizumab to the FDA for use as an add-on therapy for the small subset of asthma patients whose disease remains uncontrolled despite the optimal use of inhaled corticosteroids and additional therapies such as leukotriene inhibitors or theophylline. This population experiences more frequent asthma exacerbations, has more emergency department visits and hospitalizations, and uses higher doses of oral corticosteroids. Approximately 60% of those with severe asthma have marked eosinophilia.Panel members uniformly cited the efficacy data for adults with severe asthma; several panelists also remarked on the importance of developing more steroid-sparing alternatives for this population.
The panel endorsed neither efficacy nor safety for those aged 12-17 years, with 9 of the 14 panelists voting not to endorse efficacy and 13 members voting not to endorse safety findings. Mepolizumab’s efficacy, many panelists said, was not clearly established from the data presented, which drew from small numbers of adolescents enrolled in the studies.
Dr. David Au, acting director of Health Services Research and Development at Seattle’s VA Puget Sound Health Care system, observed that “adolescents are not small adults – their lungs continue to mature over time.” Many panelists, however, also called for ongoing study, noting the significant unmet need for steroid alternatives in the adolescent population.
Several panelists advocated postmarketing surveillance for long-term use, with particular attention to those with parasitic disease, to monitoring any sign of malignancy, and to tracking opportunistic diseases such as herpes zoster.
An early clinical trial of mepolizumab, conducted in 1999, failed to show benefit for an undifferentiated population of patients with moderate to severe asthma. However, independent research later identified marked eosinophilia as a factor associated with more frequent asthma exacerbations and a series of clinical trials begun in 2011 targeted patients with severe asthma and eosinophilic inflammation. A global program was initiated, with 12% of patients overall coming from the United States.
Pivotal phase 2b/3, double blind, placebo-controlled clinical trials included a dose-ranging study tracking asthma exacerbations enrolling 616 patients for 52 weeks.
The recommended dose of 100 mg subcutaneously every 4 weeks, as well as a 75-mg IV dose, was used for an additional 576 patients for 32 weeks, with the primary outcome measure being the number of asthma exacerbations. A final 24-week study of 135 patients with severe asthma measured the reduction in oral corticosteroid use, compared with placebo, as well as the number of asthma exacerbations. In all of the studies, patients’ asthma treatment was optimized according to standard of care before adding mepolizumab.
In each study and in pooled data, mepolizumab approximately halved the number of asthma exacerbations for study participants when compared with those using placebo. A 24-week corticosteroid-sparing study showed significant reduction in oral corticosteroid use, without loss of asthma control, for the mepolizumab group. Prespecified subgroup analyses were hampered because of low participation numbers for African Americans and adolescents, and because confidence intervals for these subgroups often ranged over 1, limiting interpretation of benefit results for these groups. The overall safety profile was good, with adverse event rates similar in the treatment and placebo arms. Headache and injection-site reactions were the most commonly reported adverse events but were similar between treatment and placebo arms. No episodes of anaphylaxis were reported, and neutralizing antibodies developed in one patient total across all studies. Ongoing open-label studies continue.The FDA’s independent biostatistical analysis showed clear evidence of efficacy for mepolizumab, with demonstrated consistent, statistically significant decreases of about one exacerbation per year, according to the agency.In its analysis, the agency observed a positive association between higher eosinophil count and mepolizumab treatment effect, meaning that those with higher eosinophil counts saw a greater benefit from mepolizumab, as measured by a reduction in exacerbations.
The number of deaths was balanced across treatment arms, though a larger number of respiratory-related deaths than expected was seen overall. This higher number of deaths may reflect the severity of asthma in the study population. The respiratory-related serious adverse events, according to the FDA, favor treatment over placebo.
No treatment-related cardiovascular risks were identified.
The FDA usually follows the recommendations of its advisory panels. The FDA panelists reported no relevant conflicts of interest.
GAITHERSBURG, MD. – The biologic agent mepolizumab was unanimously recommended for approval as a treatment for severe asthma in adults at a June 4 meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
All 14 members of the advisory panel agreed that the efficacy data provide substantial evidence of a clinically meaningful benefit of mepolizumab for the treatment of severe asthma in adults; 13 of the 14 members agreed that the data adequately demonstrated safety in adults. However, only four panel members recommended approval for adolescents aged 12-17 years, with the majority of panel members citing concerns that the low number of adolescents studied to date did not allow safety to be adequately evaluated in a younger population, especially for a medication that would be taken for many years – perhaps for a lifetime.
If approved by the FDA, the biologic agent would be available as a once-monthly treatment that is injected subcutaneously by a health care professional. Mepolizumab would be marketed by Glaxo Smith Kline under the trade name Nucala. Mepolizumab is a first-in-class humanized monoclonal antibody that targets interleukin-5, a glycoprotein cytokine that mediates production of eosinophils. Elevation of eosinophils in blood and tissue is associated with an increase in cytokines and other inflammatory molecules that can trigger or exacerbate airway inflammation in asthma. One other monoclonal antibody, the anti-IgE biologic omulizimab (Xolair), has been approved to treat asthma. Glaxo Smith Kline brought mepolizumab to the FDA for use as an add-on therapy for the small subset of asthma patients whose disease remains uncontrolled despite the optimal use of inhaled corticosteroids and additional therapies such as leukotriene inhibitors or theophylline. This population experiences more frequent asthma exacerbations, has more emergency department visits and hospitalizations, and uses higher doses of oral corticosteroids. Approximately 60% of those with severe asthma have marked eosinophilia.Panel members uniformly cited the efficacy data for adults with severe asthma; several panelists also remarked on the importance of developing more steroid-sparing alternatives for this population.
The panel endorsed neither efficacy nor safety for those aged 12-17 years, with 9 of the 14 panelists voting not to endorse efficacy and 13 members voting not to endorse safety findings. Mepolizumab’s efficacy, many panelists said, was not clearly established from the data presented, which drew from small numbers of adolescents enrolled in the studies.
Dr. David Au, acting director of Health Services Research and Development at Seattle’s VA Puget Sound Health Care system, observed that “adolescents are not small adults – their lungs continue to mature over time.” Many panelists, however, also called for ongoing study, noting the significant unmet need for steroid alternatives in the adolescent population.
Several panelists advocated postmarketing surveillance for long-term use, with particular attention to those with parasitic disease, to monitoring any sign of malignancy, and to tracking opportunistic diseases such as herpes zoster.
An early clinical trial of mepolizumab, conducted in 1999, failed to show benefit for an undifferentiated population of patients with moderate to severe asthma. However, independent research later identified marked eosinophilia as a factor associated with more frequent asthma exacerbations and a series of clinical trials begun in 2011 targeted patients with severe asthma and eosinophilic inflammation. A global program was initiated, with 12% of patients overall coming from the United States.
Pivotal phase 2b/3, double blind, placebo-controlled clinical trials included a dose-ranging study tracking asthma exacerbations enrolling 616 patients for 52 weeks.
The recommended dose of 100 mg subcutaneously every 4 weeks, as well as a 75-mg IV dose, was used for an additional 576 patients for 32 weeks, with the primary outcome measure being the number of asthma exacerbations. A final 24-week study of 135 patients with severe asthma measured the reduction in oral corticosteroid use, compared with placebo, as well as the number of asthma exacerbations. In all of the studies, patients’ asthma treatment was optimized according to standard of care before adding mepolizumab.
In each study and in pooled data, mepolizumab approximately halved the number of asthma exacerbations for study participants when compared with those using placebo. A 24-week corticosteroid-sparing study showed significant reduction in oral corticosteroid use, without loss of asthma control, for the mepolizumab group. Prespecified subgroup analyses were hampered because of low participation numbers for African Americans and adolescents, and because confidence intervals for these subgroups often ranged over 1, limiting interpretation of benefit results for these groups. The overall safety profile was good, with adverse event rates similar in the treatment and placebo arms. Headache and injection-site reactions were the most commonly reported adverse events but were similar between treatment and placebo arms. No episodes of anaphylaxis were reported, and neutralizing antibodies developed in one patient total across all studies. Ongoing open-label studies continue.The FDA’s independent biostatistical analysis showed clear evidence of efficacy for mepolizumab, with demonstrated consistent, statistically significant decreases of about one exacerbation per year, according to the agency.In its analysis, the agency observed a positive association between higher eosinophil count and mepolizumab treatment effect, meaning that those with higher eosinophil counts saw a greater benefit from mepolizumab, as measured by a reduction in exacerbations.
The number of deaths was balanced across treatment arms, though a larger number of respiratory-related deaths than expected was seen overall. This higher number of deaths may reflect the severity of asthma in the study population. The respiratory-related serious adverse events, according to the FDA, favor treatment over placebo.
No treatment-related cardiovascular risks were identified.
The FDA usually follows the recommendations of its advisory panels. The FDA panelists reported no relevant conflicts of interest.
AT AN FDA ADVISORY COMMITTEE MEETING
Traditional Diagnostic Criteria Miss OSA in Coronary Artery Disease Patients
DENVER – Obstructive sleep apnea was common among patients in the multinational Sleep and Stent Study who underwent successful percutaneous coronary intervention, but most of those affected had no daytime sleepiness, and only about half had positive findings on the Berlin Questionnaire.
Further, findings at up to 4 years of follow-up show that coronary artery disease patients with vs. without obstructive sleep apnea (OSA) had nearly twice the risk of adverse events.
The findings suggest that OSA has important clinical implications for coronary artery disease (CAD), and that validated tools for identifying OSA risk in the general population may not be useful in those with cardiovascular disease, Sofia Furlan, Ph.D. said in a press briefing at an international conference of the American Thoracic Society.
Of 1,305 patients included in the ongoing observational study, 45% had OSA, including 21.8% who had severe OSA defined by an apnea-hypopnea index of 30 or more events/hour. Excessive daytime sleepiness, defined as an Epworth Sleepiness Scale score of greater than 10, was identified in 24.5% of the OSA patients, and a Berlin Questionnaire score indicative of high risk for OSA was found in 54.3% of patients with OSA, said Dr. Furlan of the University of Sao Paulo, Brazil.
The adverse event rate was 11% in CAD patients with OSA, compared with 6.5% in those without OSA – a significant difference thus far in the study, Dr. Furlan noted.
Patients in the Sleep and Stent Study, which was designed to look at the relationships between OSA and cardiovascular outcomes in adults treated with percutaneous coronary intervention, were aged 18-80 years and were enrolled from eight centers in five countries: Singapore, China and Hong Kong, India, Myanmar, and Brazil.
Indications for percutaneous coronary intervention included ST segment elevation myocardial infarction in 33% of subjects, non-ST segment elevation myocardial infarction in 20% of subjects, unstable angina in 16% of subjects, and stable angina in 31% of subjects. All patients underwent an overnight sleep study using a level-3 portable diagnostic device prior to hospital discharge, and the tracings were analyzed by a blinded sleep physician.
The prevalence of OSA was comparable across study sites, Dr. Furlan said.
The findings reinforce the known association between OSA and cardiovascular disease, lead researcher Dr. Luciano Drager, also of the University of Sao Paulo, said in a press statement.
“Earlier studies have shown strong relationships between sleep apnea and a number of cardiovascular conditions, including high blood pressure, arrhythmia, stroke, and heart failure. Our study supports this strong association between OSA and heart disease and also suggests that the methods used to screen for OSA in patients with cardiovascular disease need to be improved,” Dr. Drager said.
Given the apparent association between sleep apnea and adverse outcomes in patients with CAD, and the fact that sleep apnea is underdiagnosed, it may be time to consider monitoring for sleep apnea in CAD patients, Dr. Furlan added.
Non–sleep related predictors of OSA in patients with cardiovascular disease also should be explored, she said.
Press briefing moderator Dr. Mihaela Teodorescu of the University of Wisconsin, Madison, noted that other studies also have suggested that coexistent OSA manifests differently in different disease states.
“This is a very good example. Whereas in the general population sleepiness is one of the major presenting symptoms, in this particularly vulnerable population with heart attacks and severe coronary artery disease, these people are not sleepy, ” she said, adding that “current screening questionnaires, which are relatively widely used in clinical populations, may not do the job.”
In fact, if traditional criteria for diagnosis are used, a large proportion of patients with OSA will be missed, she said.
“To me as a clinician, this is the major take-home message: Don’t rely on symptoms, because we will miss a large proportion of patients simply based on that.”
The investigators reported having no disclosures. The Sleep and Stent Study is sponsored by Boston Scientific Corporation.
DENVER – Obstructive sleep apnea was common among patients in the multinational Sleep and Stent Study who underwent successful percutaneous coronary intervention, but most of those affected had no daytime sleepiness, and only about half had positive findings on the Berlin Questionnaire.
Further, findings at up to 4 years of follow-up show that coronary artery disease patients with vs. without obstructive sleep apnea (OSA) had nearly twice the risk of adverse events.
The findings suggest that OSA has important clinical implications for coronary artery disease (CAD), and that validated tools for identifying OSA risk in the general population may not be useful in those with cardiovascular disease, Sofia Furlan, Ph.D. said in a press briefing at an international conference of the American Thoracic Society.
Of 1,305 patients included in the ongoing observational study, 45% had OSA, including 21.8% who had severe OSA defined by an apnea-hypopnea index of 30 or more events/hour. Excessive daytime sleepiness, defined as an Epworth Sleepiness Scale score of greater than 10, was identified in 24.5% of the OSA patients, and a Berlin Questionnaire score indicative of high risk for OSA was found in 54.3% of patients with OSA, said Dr. Furlan of the University of Sao Paulo, Brazil.
The adverse event rate was 11% in CAD patients with OSA, compared with 6.5% in those without OSA – a significant difference thus far in the study, Dr. Furlan noted.
Patients in the Sleep and Stent Study, which was designed to look at the relationships between OSA and cardiovascular outcomes in adults treated with percutaneous coronary intervention, were aged 18-80 years and were enrolled from eight centers in five countries: Singapore, China and Hong Kong, India, Myanmar, and Brazil.
Indications for percutaneous coronary intervention included ST segment elevation myocardial infarction in 33% of subjects, non-ST segment elevation myocardial infarction in 20% of subjects, unstable angina in 16% of subjects, and stable angina in 31% of subjects. All patients underwent an overnight sleep study using a level-3 portable diagnostic device prior to hospital discharge, and the tracings were analyzed by a blinded sleep physician.
The prevalence of OSA was comparable across study sites, Dr. Furlan said.
The findings reinforce the known association between OSA and cardiovascular disease, lead researcher Dr. Luciano Drager, also of the University of Sao Paulo, said in a press statement.
“Earlier studies have shown strong relationships between sleep apnea and a number of cardiovascular conditions, including high blood pressure, arrhythmia, stroke, and heart failure. Our study supports this strong association between OSA and heart disease and also suggests that the methods used to screen for OSA in patients with cardiovascular disease need to be improved,” Dr. Drager said.
Given the apparent association between sleep apnea and adverse outcomes in patients with CAD, and the fact that sleep apnea is underdiagnosed, it may be time to consider monitoring for sleep apnea in CAD patients, Dr. Furlan added.
Non–sleep related predictors of OSA in patients with cardiovascular disease also should be explored, she said.
Press briefing moderator Dr. Mihaela Teodorescu of the University of Wisconsin, Madison, noted that other studies also have suggested that coexistent OSA manifests differently in different disease states.
“This is a very good example. Whereas in the general population sleepiness is one of the major presenting symptoms, in this particularly vulnerable population with heart attacks and severe coronary artery disease, these people are not sleepy, ” she said, adding that “current screening questionnaires, which are relatively widely used in clinical populations, may not do the job.”
In fact, if traditional criteria for diagnosis are used, a large proportion of patients with OSA will be missed, she said.
“To me as a clinician, this is the major take-home message: Don’t rely on symptoms, because we will miss a large proportion of patients simply based on that.”
The investigators reported having no disclosures. The Sleep and Stent Study is sponsored by Boston Scientific Corporation.
DENVER – Obstructive sleep apnea was common among patients in the multinational Sleep and Stent Study who underwent successful percutaneous coronary intervention, but most of those affected had no daytime sleepiness, and only about half had positive findings on the Berlin Questionnaire.
Further, findings at up to 4 years of follow-up show that coronary artery disease patients with vs. without obstructive sleep apnea (OSA) had nearly twice the risk of adverse events.
The findings suggest that OSA has important clinical implications for coronary artery disease (CAD), and that validated tools for identifying OSA risk in the general population may not be useful in those with cardiovascular disease, Sofia Furlan, Ph.D. said in a press briefing at an international conference of the American Thoracic Society.
Of 1,305 patients included in the ongoing observational study, 45% had OSA, including 21.8% who had severe OSA defined by an apnea-hypopnea index of 30 or more events/hour. Excessive daytime sleepiness, defined as an Epworth Sleepiness Scale score of greater than 10, was identified in 24.5% of the OSA patients, and a Berlin Questionnaire score indicative of high risk for OSA was found in 54.3% of patients with OSA, said Dr. Furlan of the University of Sao Paulo, Brazil.
The adverse event rate was 11% in CAD patients with OSA, compared with 6.5% in those without OSA – a significant difference thus far in the study, Dr. Furlan noted.
Patients in the Sleep and Stent Study, which was designed to look at the relationships between OSA and cardiovascular outcomes in adults treated with percutaneous coronary intervention, were aged 18-80 years and were enrolled from eight centers in five countries: Singapore, China and Hong Kong, India, Myanmar, and Brazil.
Indications for percutaneous coronary intervention included ST segment elevation myocardial infarction in 33% of subjects, non-ST segment elevation myocardial infarction in 20% of subjects, unstable angina in 16% of subjects, and stable angina in 31% of subjects. All patients underwent an overnight sleep study using a level-3 portable diagnostic device prior to hospital discharge, and the tracings were analyzed by a blinded sleep physician.
The prevalence of OSA was comparable across study sites, Dr. Furlan said.
The findings reinforce the known association between OSA and cardiovascular disease, lead researcher Dr. Luciano Drager, also of the University of Sao Paulo, said in a press statement.
“Earlier studies have shown strong relationships between sleep apnea and a number of cardiovascular conditions, including high blood pressure, arrhythmia, stroke, and heart failure. Our study supports this strong association between OSA and heart disease and also suggests that the methods used to screen for OSA in patients with cardiovascular disease need to be improved,” Dr. Drager said.
Given the apparent association between sleep apnea and adverse outcomes in patients with CAD, and the fact that sleep apnea is underdiagnosed, it may be time to consider monitoring for sleep apnea in CAD patients, Dr. Furlan added.
Non–sleep related predictors of OSA in patients with cardiovascular disease also should be explored, she said.
Press briefing moderator Dr. Mihaela Teodorescu of the University of Wisconsin, Madison, noted that other studies also have suggested that coexistent OSA manifests differently in different disease states.
“This is a very good example. Whereas in the general population sleepiness is one of the major presenting symptoms, in this particularly vulnerable population with heart attacks and severe coronary artery disease, these people are not sleepy, ” she said, adding that “current screening questionnaires, which are relatively widely used in clinical populations, may not do the job.”
In fact, if traditional criteria for diagnosis are used, a large proportion of patients with OSA will be missed, she said.
“To me as a clinician, this is the major take-home message: Don’t rely on symptoms, because we will miss a large proportion of patients simply based on that.”
The investigators reported having no disclosures. The Sleep and Stent Study is sponsored by Boston Scientific Corporation.
AT ATS 2015
June 2015: Click for Credit
Here are 6 articles in the June issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. ACCP and CTS Issue Joint Guideline on COPD Exacerbations
To take the posttest, go to: http://bit.ly/1EIadmv
VITALS
Key clinical point: The American College of Chest Physicians and the Canadian Thoracic Society have issued a guideline for prevention of acute exacerbations of COPD.
Major finding: COPD exacerbations are acute, trajectory changing, and often deadly manifestations of a chronic disease.
Data source: A comprehensive literature review on prevention of acute COPD exacerbations and a compilation of 33 recommendations and suggestions for clinicians in clinical practice.
Disclosures: The American College of Chest Physicians, the Canadian Thoracic Society, and the American Thoracic Society supported the project. Dr Criner reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
2. GI Symptoms Common in Parkinson Disease and Related Disorders
To take the posttest, go to: http://bit.ly/1AZSaXA
VITALS
Key clinical point: GI symptoms were linked with specific motor and nonmotor features of Parkinson disease and related disorders.
Major finding: Constipation was the most common symptom among all three parkinsonian disorders.
Data source: Multicenter, cross-sectional survey of 473 patients with Parkinson disease, atypical parkinsonism, or vascular parkinsonism.
Disclosures: The Collaborative Clinical Research Fund of Boramae Medical Center partially funded the work. The authors declared no relevant conflicts of interest.
3. Aerosolized Measles Vaccine Inferior to Subcutaneous
To take the posttest, go to: http://bit.ly/1RKJizC
VITALS
Key clinical point: An aerosolized measles vaccine was immunogenic but inferior to the subcutaneous vaccine at inducing seropositivity among babies residing in rural India.
Major finding: The primary endpoint—seropositivity for antibodies against measles at 91 days after vaccination—was 85.4% for aerosolized vaccine and 94.6% for subcutaneous.
Data source: An open-label, randomized noninferiority trial comparing aerosolized vs subcutaneous measles vaccination in 2,004 infants ages 9 to 11.9 months in villages in India.
Disclosures: This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge, and Aerogen provided the delivery devices free of charge. Dr Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
4. Unrecognized Diabetes Common in Acute MI
To take the posttest, go to: http://bit.ly/1IB9sC8
VITALS
Key clinical point: Many patients presenting with acute MI had unrecognized diabetes, which, in most cases, remained undiagnosed, untreated, and unrecorded.
Major finding: Of 2,854 (10%) patients enrolled in an MI registry, 287 had A1C levels of 6.5% or higher on routine laboratory testing during hospitalization for acute MI, but treating physicians recognized only 101 of these cases of diabetes (35%).
Data source: A retrospective cohort study involving 2,854 adults presenting with acute MI to 24 US medical centers in a 3.5-year period.
Disclosures: This study was sponsored by the National Heart, Lung, and Blood Institute and supported by a research grant from Genentech. Dr Arnold reported receiving honoraria from Novartis; her associates reported ties to numerous industry sources.
5. Methotrexate and Biologics Linked to Higher Zoster Risk in Psoriasis
To take the posttest, go to: http://bit.ly/1AZScyF
VITALS
Key clinical point: The combination of methotrexate and biologics for the treatment of psoriasis may increase risk for herpes zoster.
Major finding: Combination therapy with both biologic medications and methotrexate was associated with a significant 66% increase in the incidence of herpes zoster over more than 11 years of follow-up.
Data source: Analysis of medical records for 95,941 patients with psoriasis.
Disclosures: One author reported consultancies and research grants from a range of pharmaceutical companies. There were no other disclosures.
6. ACP: Avoid ECG, MPI Cardiac Screening in Low-risk Patients
To take the posttest, go to: http://bit.ly/1e3NLha
Here are 6 articles in the June issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. ACCP and CTS Issue Joint Guideline on COPD Exacerbations
To take the posttest, go to: http://bit.ly/1EIadmv
VITALS
Key clinical point: The American College of Chest Physicians and the Canadian Thoracic Society have issued a guideline for prevention of acute exacerbations of COPD.
Major finding: COPD exacerbations are acute, trajectory changing, and often deadly manifestations of a chronic disease.
Data source: A comprehensive literature review on prevention of acute COPD exacerbations and a compilation of 33 recommendations and suggestions for clinicians in clinical practice.
Disclosures: The American College of Chest Physicians, the Canadian Thoracic Society, and the American Thoracic Society supported the project. Dr Criner reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
2. GI Symptoms Common in Parkinson Disease and Related Disorders
To take the posttest, go to: http://bit.ly/1AZSaXA
VITALS
Key clinical point: GI symptoms were linked with specific motor and nonmotor features of Parkinson disease and related disorders.
Major finding: Constipation was the most common symptom among all three parkinsonian disorders.
Data source: Multicenter, cross-sectional survey of 473 patients with Parkinson disease, atypical parkinsonism, or vascular parkinsonism.
Disclosures: The Collaborative Clinical Research Fund of Boramae Medical Center partially funded the work. The authors declared no relevant conflicts of interest.
3. Aerosolized Measles Vaccine Inferior to Subcutaneous
To take the posttest, go to: http://bit.ly/1RKJizC
VITALS
Key clinical point: An aerosolized measles vaccine was immunogenic but inferior to the subcutaneous vaccine at inducing seropositivity among babies residing in rural India.
Major finding: The primary endpoint—seropositivity for antibodies against measles at 91 days after vaccination—was 85.4% for aerosolized vaccine and 94.6% for subcutaneous.
Data source: An open-label, randomized noninferiority trial comparing aerosolized vs subcutaneous measles vaccination in 2,004 infants ages 9 to 11.9 months in villages in India.
Disclosures: This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge, and Aerogen provided the delivery devices free of charge. Dr Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
4. Unrecognized Diabetes Common in Acute MI
To take the posttest, go to: http://bit.ly/1IB9sC8
VITALS
Key clinical point: Many patients presenting with acute MI had unrecognized diabetes, which, in most cases, remained undiagnosed, untreated, and unrecorded.
Major finding: Of 2,854 (10%) patients enrolled in an MI registry, 287 had A1C levels of 6.5% or higher on routine laboratory testing during hospitalization for acute MI, but treating physicians recognized only 101 of these cases of diabetes (35%).
Data source: A retrospective cohort study involving 2,854 adults presenting with acute MI to 24 US medical centers in a 3.5-year period.
Disclosures: This study was sponsored by the National Heart, Lung, and Blood Institute and supported by a research grant from Genentech. Dr Arnold reported receiving honoraria from Novartis; her associates reported ties to numerous industry sources.
5. Methotrexate and Biologics Linked to Higher Zoster Risk in Psoriasis
To take the posttest, go to: http://bit.ly/1AZScyF
VITALS
Key clinical point: The combination of methotrexate and biologics for the treatment of psoriasis may increase risk for herpes zoster.
Major finding: Combination therapy with both biologic medications and methotrexate was associated with a significant 66% increase in the incidence of herpes zoster over more than 11 years of follow-up.
Data source: Analysis of medical records for 95,941 patients with psoriasis.
Disclosures: One author reported consultancies and research grants from a range of pharmaceutical companies. There were no other disclosures.
6. ACP: Avoid ECG, MPI Cardiac Screening in Low-risk Patients
To take the posttest, go to: http://bit.ly/1e3NLha
Here are 6 articles in the June issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. ACCP and CTS Issue Joint Guideline on COPD Exacerbations
To take the posttest, go to: http://bit.ly/1EIadmv
VITALS
Key clinical point: The American College of Chest Physicians and the Canadian Thoracic Society have issued a guideline for prevention of acute exacerbations of COPD.
Major finding: COPD exacerbations are acute, trajectory changing, and often deadly manifestations of a chronic disease.
Data source: A comprehensive literature review on prevention of acute COPD exacerbations and a compilation of 33 recommendations and suggestions for clinicians in clinical practice.
Disclosures: The American College of Chest Physicians, the Canadian Thoracic Society, and the American Thoracic Society supported the project. Dr Criner reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
2. GI Symptoms Common in Parkinson Disease and Related Disorders
To take the posttest, go to: http://bit.ly/1AZSaXA
VITALS
Key clinical point: GI symptoms were linked with specific motor and nonmotor features of Parkinson disease and related disorders.
Major finding: Constipation was the most common symptom among all three parkinsonian disorders.
Data source: Multicenter, cross-sectional survey of 473 patients with Parkinson disease, atypical parkinsonism, or vascular parkinsonism.
Disclosures: The Collaborative Clinical Research Fund of Boramae Medical Center partially funded the work. The authors declared no relevant conflicts of interest.
3. Aerosolized Measles Vaccine Inferior to Subcutaneous
To take the posttest, go to: http://bit.ly/1RKJizC
VITALS
Key clinical point: An aerosolized measles vaccine was immunogenic but inferior to the subcutaneous vaccine at inducing seropositivity among babies residing in rural India.
Major finding: The primary endpoint—seropositivity for antibodies against measles at 91 days after vaccination—was 85.4% for aerosolized vaccine and 94.6% for subcutaneous.
Data source: An open-label, randomized noninferiority trial comparing aerosolized vs subcutaneous measles vaccination in 2,004 infants ages 9 to 11.9 months in villages in India.
Disclosures: This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge, and Aerogen provided the delivery devices free of charge. Dr Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
4. Unrecognized Diabetes Common in Acute MI
To take the posttest, go to: http://bit.ly/1IB9sC8
VITALS
Key clinical point: Many patients presenting with acute MI had unrecognized diabetes, which, in most cases, remained undiagnosed, untreated, and unrecorded.
Major finding: Of 2,854 (10%) patients enrolled in an MI registry, 287 had A1C levels of 6.5% or higher on routine laboratory testing during hospitalization for acute MI, but treating physicians recognized only 101 of these cases of diabetes (35%).
Data source: A retrospective cohort study involving 2,854 adults presenting with acute MI to 24 US medical centers in a 3.5-year period.
Disclosures: This study was sponsored by the National Heart, Lung, and Blood Institute and supported by a research grant from Genentech. Dr Arnold reported receiving honoraria from Novartis; her associates reported ties to numerous industry sources.
5. Methotrexate and Biologics Linked to Higher Zoster Risk in Psoriasis
To take the posttest, go to: http://bit.ly/1AZScyF
VITALS
Key clinical point: The combination of methotrexate and biologics for the treatment of psoriasis may increase risk for herpes zoster.
Major finding: Combination therapy with both biologic medications and methotrexate was associated with a significant 66% increase in the incidence of herpes zoster over more than 11 years of follow-up.
Data source: Analysis of medical records for 95,941 patients with psoriasis.
Disclosures: One author reported consultancies and research grants from a range of pharmaceutical companies. There were no other disclosures.
6. ACP: Avoid ECG, MPI Cardiac Screening in Low-risk Patients
To take the posttest, go to: http://bit.ly/1e3NLha