User login
Epithelial Ovarian Cancer: Evaluation, Staging, Surgery, and Stage I and II Disease Management
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Ovarian cancer is the second most common gynecologic cancer among women in the United States. It is also the fifth leading cause of cancer mortality in women and the leading cause of death among women with gynecologic malignancies. The American Cancer Society statistics released in 2015 estimate that 21,290 new cases of ovarian cancer will occur during the year, with approximately 14,180 deaths. Globally, there were 238,719 new cases of ovarian cancer diagnosed in 2012, representing 3.6% of all cancers in women, and nearly 151,905 deaths. The highest incidence of ovarian cancer occurs in northern, central, and eastern Europe, followed by western Europe and North America, with the lowest incidence in parts of Africa and Asia. The majority of women presenting with ovarian cancer will present at an advanced stage, and the 5-year survival in this group is less than 30%.
To read the full article in PDF:
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Ovarian cancer is the second most common gynecologic cancer among women in the United States. It is also the fifth leading cause of cancer mortality in women and the leading cause of death among women with gynecologic malignancies. The American Cancer Society statistics released in 2015 estimate that 21,290 new cases of ovarian cancer will occur during the year, with approximately 14,180 deaths. Globally, there were 238,719 new cases of ovarian cancer diagnosed in 2012, representing 3.6% of all cancers in women, and nearly 151,905 deaths. The highest incidence of ovarian cancer occurs in northern, central, and eastern Europe, followed by western Europe and North America, with the lowest incidence in parts of Africa and Asia. The majority of women presenting with ovarian cancer will present at an advanced stage, and the 5-year survival in this group is less than 30%.
To read the full article in PDF:
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Ovarian cancer is the second most common gynecologic cancer among women in the United States. It is also the fifth leading cause of cancer mortality in women and the leading cause of death among women with gynecologic malignancies. The American Cancer Society statistics released in 2015 estimate that 21,290 new cases of ovarian cancer will occur during the year, with approximately 14,180 deaths. Globally, there were 238,719 new cases of ovarian cancer diagnosed in 2012, representing 3.6% of all cancers in women, and nearly 151,905 deaths. The highest incidence of ovarian cancer occurs in northern, central, and eastern Europe, followed by western Europe and North America, with the lowest incidence in parts of Africa and Asia. The majority of women presenting with ovarian cancer will present at an advanced stage, and the 5-year survival in this group is less than 30%.
To read the full article in PDF:
A teen with seizures, amnesia, and troubled family dynamics
CASE Seizures, amnesia
Ms. A, age 13, who has a history of seizures, presents to the emergency department (ED) with sudden onset of memory loss. Her family reports that she had been spending a normal evening at home with family and friends. After going to the bathroom, Ms. A became acutely confused and extremely upset, had slurred speech, and did not recognize anyone in the room except her mother.
Initial neurologic examination in the ED reports that Ms. A does not remember recent or remote past events. Her family denies any recent stressors.
Vital signs are within normal range. She has mild muscle soreness and gait instability, which is attributed to a presumed postictal phase. Her medication regimen includes: levetiracetam, 500 mg, 3 times a day; valproic acid, 1,000 mg/d; and oxcarbazepine, 2,400 mg/d, for seizure management.
Complete blood count and comprehensive metabolic panel are within normal limits. Pregnancy test is negative. Urine toxicology report is negative. Serum valproic acid level is 71 μg/mL; oxcarbazepine level, <2 μg/mL; ammonia level, 71 μg/dL (reference range, 15 to 45 μg/dL). Other than the aforementioned deficits, she is neurologically intact. The team thinks that her symptoms are part of a postictal phase of an unwitnessed seizure.
Ms. A is admitted to the inpatient medical unit for further work up. Along with the memory loss and seizures, she reports visual hallucinations.
What could be causing Ms. A’s amnesia?
a) a seizure disorder
b) malingering
c) posttraumatic stress disorder
d) traumatic brain injury
HISTORY Repeat ED visits
Ms. A’s mother reports that 3 years ago her daughter was treated for tics with quetiapine and aripiprazole, prescribed by a primary care physician. She received a short course of counseling 6 years ago after her sister was sexually abused by her grandfather. Approximately 6 months ago, Ms. A engaged in self-injurious behavior by cutting herself, and she briefly received counseling. There is no history of suicide attempts, psychiatric hospitalization, or a psychiatric diagnosis.
Medical and surgical history include viral meningitis at age 6 months. Medical records show a visit to the ED for abdominal pain after a classmate punched her in the abdomen, which resolved with supportive care. She was given a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections 6 years ago.
Ms. A developed multiple recurrent methicillin-resistant Staphylococcus aureus abscesses a year ago, which lasted for 4 months; it was noted that she was self-inoculating by scratching eczema. She had a possible syncopal episode 5 months ago, but the medical work-up was normal. The pediatric neurology service diagnosed and treated seizures 4 months ago.
Levetiracetam was prescribed after a possible syncopal episode followed by a tonic-clonic seizure. Because she was still having seizure-like episodes with a single antiepileptic drug (AED), oxcarbazepine, then valproic acid were added. Whether her seizures were generalized or partial was inconclusive. The seizures were followed by a postictal phase lasting 3 minutes to 1 hour. Her last generalized tonic-clonic seizure was 1 month before admission.
Ms. A had 3 MRI studies of the brain over the past 3 years, which showed consistent and unchanged multifocal punctate white matter lesions. The findings represented gliosis from an old perivascular inflammation, trauma, or ischemic damage. There is no history of traumatic brain injury.
Her perinatal history is unremarkable, with normal vaginal delivery at 36 weeks (pre-term birth). All developmental milestones were on target.
Ms. A lives at home with her mother, 6-year-old brother, and stepfather. Her parents are divorced, but her biological father has been involved in her upbringing. She is in seventh grade, but is home schooled after she withdrew from school because of multiple seizure episodes. Ms. A denied bullying at school although she had been punched by a peer. It was unclear if it was a single incident or bullying continued and she was hesitant to disclose it.
The authors’ observations
We focus on the amnesia because it has an acute onset and it seems this is the first time Ms. A presented with this symptom. There is no need to wait for neurology consultation, even though organic causes of amnesia need to be ruled out. Our plan is to develop rapport with Ms. A, and then administer a mental status examination focusing on memory assessment. We understand that, because Ms. A’s chief concern is amnesia, she might not be able to provide many details. We start the initial interview with the family in the patient’s room to understand family dynamics, and then interview Ms. A alone.
EVALUATION Memory problems
On initial psychiatric interview, Ms. A can recognize some of her family members. She is seen in clean attire, with short hair, lying in the bed with good eye contact and a calm demeanor. She seems to be difficult to engage because of her reserved nature.
Ms. A displays some psychomotor retardation. She reports her mood as tired, and her affect is flat and mood incongruent. She is alert and oriented to person only; not to place, time, or situation. She can do a simple spelling task, perform 5-minute recall of 3 words, complete serial 3 subtractions, repeat phrases, read aloud, focus on a coin task, and name simple objects. She does not compare similar objects or answer simple historical or factual questions.
Ms. A replies “I don’t know” to most historical questions, such as her birthday, favorite color, and family members; she does not answer when asked how many legs a dog has, who is the current or past president, what month the Fourth of July is in, or when Christmas is. She can complete some memory tasks on the Mini-Mental State Examination, but does not attempt many others. Ms. A says she is upset about her memory deficit, but her affect was flat. Her mood and her affect were incongruent. She describes a vision of a “girl with black holes [for eyes]” in the corner of her hospital room telling her not to believe anyone and that the interviewers are lying to her. Also, she reports that “the girl” tells her to hurt herself and others, but she is not going to act on the commands because she knows it is not the right thing to do. When we ask Ms. A about a history of substance abuse, she says she has never heard of drugs or alcohol.
Overall, she displays multiple apparent deficits in declarative memory, both episodic and semantic. Regarding non-declarative or procedural memory, she can dress herself, use the bathroom independently, order meals off the menu, and feed herself, among other routine tasks, without difficulty.
According to Ms. A’s mother, Ms. A has shown a decline in overall functioning and personality changes during the past 5 months. She started to cut herself superficially on her forearms 6 months ago and also tried to change her appearance with a new hairstyle when school started. She displayed noticeably intense and disturbing writings, artwork, and conversations with others over 3 to 4 months.
She started experiencing seizures, with 3 to 4 seizures a day; however, she could attend sleepovers seizure-free. She had prolonged periods of seizures lasting up to an hour, much longer than would be expected clinically. She also had requested to go to the cemetery for unclear reasons (because the spirit wanted her to visit), and was observed mumbling under her breath.
Six years ago, Ms. A’s 6-year-old sister tried to suffocate her infant brother. Child protective services was involved and the sister was hospitalized in a psychiatric facility, where she was given a diagnosis of bipolar disorder; she was then transferred to foster care, and later placed in residential treatment. Her mother relinquished her parental rights and gave custody of Ms. A’s sister to the state.
Ms. A’s mother has a history of depression, but her younger brother is healthy. There is no history of autism, attention problems, tics, substance abuse, brain tumor, or intellectual disabilities in the family.
Which diagnosis does Ms. A’s presentation and history suggest?
a) dissociative amnesia
b) factitious disorder imposed on self
c) conversion disorder (neurological symptom disorder)
d) psychosis not otherwise specified
e) malingering
The authors’ observations
The history of unwitnessed seizures, sudden onset of visual hallucinations, and transient amnesia points to a possible postictal cause. Selective amnesia brings up the question of whether psychological components are driving the symptoms.
Her psychotic symptoms appear to be mediated by anxiety and possibly related to the trauma of losing her only sister when her mother relinquished custody to the state; the circumstances might have aroused feelings of insecurity or fear of abandonment and raised questions about her mother’s love toward her. Her sister’s abuse by a family member might have created reticence to trust others. These background experiences could be intensely conflicting at this age when the second separation individuation process commences, especially in an emotionally immature adolescent.
OUTCOME Medication change
The neurology team recommends discontinuing levetiracetam because the visual hallucinations, mood disturbance, and personality change could be adverse effects of the drug. Because of generalized uncontrolled body movements with staring episodes and unresponsiveness, an EEG is ordered to rule out ongoing seizures.
Ms. A recognizes the psychosomatic medicine team members when they interview her again. The team employs consistent reassurance and a non-confrontational approach. She spends 3 days in the medical unit during which she reports that the frequency of visual and auditory hallucinations decreases and her memory symptoms resolve. Her 24-hour EEG is negative for seizure activity, and the 24-hour video EEG does not show any signs of epileptogenic foci. Ms. A’s family declines inpatient psychiatric hospitalization.
Because of gradual improvement in Ms. A’s symptoms and no imminent safety concerns, she is discharged home with valproic acid, 1,000 mg/d, and oxcarbazepine, 1,200 mg/d, and follow-up appointments with her primary care physician, a neurologist, and a psychiatrist.
The authors’ observations
Dissociative amnesia
Generalized dissociative amnesia is difficult to differentiate from factitious disorder or malingering. According to DSM-5, there is loss of episodic memory in dissociative amnesia, in which the person is unable to recall the stressful event after trauma (Table 1).1 Although there have been case reports of dissociative amnesia with loss of semantic and procedural memory, episodic memory is the last to return.2 In Ms. A’s case, there was no immediate basis to explain amnesia onset, although she had experienced the trauma of losing her sister. She had episodic and mostly semantic memory loss.
Although organic causes can precipitate amnesia,3 Ms. A’s EEG and MRI results did not reflect that. Patients with a dissociative disorder often report some physical, sexual, or emotional abuse.4 Although Ms. A did not report any abuse, it cannot be completely ruled out because of her sister’s history of abuse.
Suicidality or self-injurious behavior is common among adults with dissociative amnesia, although it is not well studied in children.4,5 Generally, the constellation of primary dissociative symptoms that patients develop are forgetfulness, fragmentation, and emotional numbing. Ms. A presented with some of these features; did she, in fact, have dissociative amnesia?
Factitious amnesia
Factious amnesia (Table 2)6 is a symptom of factious disorder in which amnesia appears with the motivation to assume a sick role.3 Ms. A’s amnesia garnered significant attention from her mother and other family members; this may have been related to insecurity in her family relationships because her sister was given up to the state. She also could be afraid of entering adolescence and leaving her sister behind. Did she want more time to bond with her mother? Did she experience emotional benefit from being cared for by medical professionals?7 Her affect during interviews was blunted and her attitude was nonchalant, and her multiple visits to the hospital since childhood for abdominal pain, abscesses (it isn’t clear whether the abscesses were related to self-injury and scratching), tics, seizures, and, recently, amnesia and hallucinations indicated some desire to occupy a sick role. Furthermore, the severity of her symptoms seemed to be increasing over time, from somatic to neurologic (seizure-like episodes) to significant and less frequent psychiatric symptoms (amnesia and hallucinations). One could speculate that her symptoms were escalating because she was not receiving the attention she needed.
Malingered amnesia
Although malingering is not a psychiatric diagnosis, it can be a focus of clinical attention. It is challenging to identify malingered cognitive impairments.8 Children often have difficulty malingering symptoms because they have limited understanding of the illness they are trying to simulate.9 Many malingerers do not want to participate in their medical work up and might exhibit a hostile attitude toward examiners (Table 26). Clinicians could rely on family to provide information regarding history and inconsistencies in clinical deficits.9 The clinical interview, mental status examination, and collateral information are crucial for identifying malingering.
Most of Ms. A’s seizure-like episodes happened in specific contexts, such as in school, but not at friends’ houses, raising the question of whether she is aware of her episodes. Ms. A’s grades are consistently good; because she is being home schooled, there is no secondary gain from not going to school. There is no other reason to speculate that she was malingering.
The inconsistency of Ms. A’s symptoms and her compliance with assessment and treatment did not reflect malingering. Interestingly, Ms. A’s amnesia was retrograde in nature. There have been more studies on malingered anterograde amnesia8 than on retrograde amnesia, making her presentation even more unusual.
Amnesia presenting as conversion disorder
Amnesia as a symptom of conversion disorder is referred as psychogenic amnesia; the memory loss mostly is isolated retrograde amnesia.10 Ms. A likely had unconsciously produced symptoms of non-epileptic seizures, followed by auditory and visual hallucinations not related to her seizures, and then later developed selective transient amnesia. Conversion disorder seemed to be the diagnosis most consistent with her indifference (“la belle indifference”) and the significant attention she gained from the acute memory loss (Table 3).1 It seemed that she developed multiple symptoms in progression leading toward a conversion disorder diagnosis. The question arises whether Ms. A’s presentation is a gradually increasing cry for help or reflects depressive or anxiety symptoms, which often are comorbid with conversion disorder.
FOLLOW-UP Suicide attempt
Ms. A has frequent visits to the ED with symptoms of syncope and seizures and undergoes medical work-up and multiple EEGs. A prolonged 5-day video EEG is performed to assess seizure episodes after AEDs were withdrawn, but no seizure activity is elicited. She also has an ED visit for recurrent tic emergence.
The last visit in the ED is for a suicide attempt with overdose of an unknown quantity of unspecified pills. Ms. A talks to a social worker, who reports that Ms. A needed answers to such questions as why her grandfather abused her sister? Could she have stopped them and made a difference for the family?
The authors’ observations
Conversion disorder arises from unconscious psychological conflicts, needs, or responses to trauma. Ms. A’s consistent conflict about her sister and grandfather’s relationship was evident from occasions when she tried to confide in hospital staff. During an ED visit, she reported her sister’s abuse to a staff member. Another time, while recovering from sedation, she spontaneously spoke about her sister’s abuse. When asked again, she said she did not remember saying it.
Freud said that patients develop conversion disorder to avoid unacceptable conflicting thoughts and feelings.10 It appeared that Ms. A was struggling with these questions because she brought them up again when she visited the ED after the suicide attempt.
Dissociative symptoms arise from unstable parenting and disciplining styles with variable family dynamics. Patients show extreme detachment and emotional unresponsiveness akin to attachment disorder.11 Ms. A had inconsistent parenting because both her stepfather and biological father were involved with her care. Her mother had relinquished her parental rights to her sister, which indicated some attachment issues.
Ms. A’s idea that her mother was indifferent stemmed from her uncaring approach toward her sister and not able to understand her emotionally. Her amnesia could be thought of as “I don’t know you because I don’t remember that I am related to you.” The traumas of infancy (referred to as hidden traumas) that were a result of parent-child mismatch of needs and availability at times of distress might not be obvious to the examiner.11
Although Ms. A’s infancy was reported to be unremarkable, there always is a question, especially in a consultation-liaison setting, of whether conversion disorder might be masking an attachment problem. Perhaps with long-term psychotherapy, an attachment issue would be revealed.
Excluding an organic cause or a neurologic disorder is important when diagnosing conversion disorder10; Ms. A’s negative neurologic tests favored a diagnosis of amnesia due to conversion disorder. It appears that, although Ms. A presented with “transient amnesia,” she had underlying psychiatric symptoms, likely depression or anxiety. We were concerned about possible psychiatric comorbidity and recommended inpatient hospitalization to clarify the diagnosis and provide intensive therapy, but her family declined. She may have received outpatient services, but that was not documented.
Bottom Line
Psychogenic amnesia can be a form of conversion disorder or a symptom of
malingering; can occur in dissociative disorder; and can be factitious in nature.
Regardless of the cause, the condition requires continuous close follow up. Although organic causes of amnesia should be ruled out, mental health care can help address comorbid psychiatric symptoms and might change the course of the illness.
Related Resources
• Byatt N, Toor R. Young, pregnant, ataxic—and jilted. Current Psychiatry. 2015;14(1):44-49.
• Leipsic J. A teen who is wasting away. Current Psychiatry. 2013;12(6):40-45.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Levetiracetam • Keppra Valproic acid • Depakote
Oxcarbazepine • Trileptal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry. 2001;35(5):589-560.
3. Ehrlich S, Pfeiffer E, Salbach H, et al. Factitious disorder in children and adolescents: a retrospective study. Psychosomatics. 2008;45(5):392-398.
4. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
5. Kisiel CL, Lyons JS. Dissociation as a mediator of psychopathology among sexually abused children and adolescents. Am J Psychiatry. 2001;158(7):1034-1039.
6. Worley CB, Feldman MD, Hamilton JC. The case of factitious disorder versus malingering. http://www.psychiatrictimes. com/munchausen-syndrome/case-factitious-disorder-versus-malingering. Published October 30, 2009. Accessed January 27, 2015.
7. Hagglund LA. Challenges in the treatment of factitious disorder: a case study. Arch Psychiatr Nurs. 2009;23(1):58-64.
8. Jenkins KG, Kapur N, Kopelman MD. Retrograde amnesia and malingering. Curr Opin Neurol. 2009;22(6):601-605.
9. Walker JS. Malingering in children: fibs and faking. Child Adolesc Psychiatr Clin N Am. 2011;20(3):547-556.
10. Levenson JL. Psychiatric issues in neurology, part 4: amnestic syndromes and conversion disorder. Primary Psychiatry. http://primarypsychiatry.com/psychiatric-issues-in-neurology-part-4-amnestic-syndromes-and-conversion-disorder. Published March 1, 2008. Accessed February 3, 2015.
11. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
CASE Seizures, amnesia
Ms. A, age 13, who has a history of seizures, presents to the emergency department (ED) with sudden onset of memory loss. Her family reports that she had been spending a normal evening at home with family and friends. After going to the bathroom, Ms. A became acutely confused and extremely upset, had slurred speech, and did not recognize anyone in the room except her mother.
Initial neurologic examination in the ED reports that Ms. A does not remember recent or remote past events. Her family denies any recent stressors.
Vital signs are within normal range. She has mild muscle soreness and gait instability, which is attributed to a presumed postictal phase. Her medication regimen includes: levetiracetam, 500 mg, 3 times a day; valproic acid, 1,000 mg/d; and oxcarbazepine, 2,400 mg/d, for seizure management.
Complete blood count and comprehensive metabolic panel are within normal limits. Pregnancy test is negative. Urine toxicology report is negative. Serum valproic acid level is 71 μg/mL; oxcarbazepine level, <2 μg/mL; ammonia level, 71 μg/dL (reference range, 15 to 45 μg/dL). Other than the aforementioned deficits, she is neurologically intact. The team thinks that her symptoms are part of a postictal phase of an unwitnessed seizure.
Ms. A is admitted to the inpatient medical unit for further work up. Along with the memory loss and seizures, she reports visual hallucinations.
What could be causing Ms. A’s amnesia?
a) a seizure disorder
b) malingering
c) posttraumatic stress disorder
d) traumatic brain injury
HISTORY Repeat ED visits
Ms. A’s mother reports that 3 years ago her daughter was treated for tics with quetiapine and aripiprazole, prescribed by a primary care physician. She received a short course of counseling 6 years ago after her sister was sexually abused by her grandfather. Approximately 6 months ago, Ms. A engaged in self-injurious behavior by cutting herself, and she briefly received counseling. There is no history of suicide attempts, psychiatric hospitalization, or a psychiatric diagnosis.
Medical and surgical history include viral meningitis at age 6 months. Medical records show a visit to the ED for abdominal pain after a classmate punched her in the abdomen, which resolved with supportive care. She was given a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections 6 years ago.
Ms. A developed multiple recurrent methicillin-resistant Staphylococcus aureus abscesses a year ago, which lasted for 4 months; it was noted that she was self-inoculating by scratching eczema. She had a possible syncopal episode 5 months ago, but the medical work-up was normal. The pediatric neurology service diagnosed and treated seizures 4 months ago.
Levetiracetam was prescribed after a possible syncopal episode followed by a tonic-clonic seizure. Because she was still having seizure-like episodes with a single antiepileptic drug (AED), oxcarbazepine, then valproic acid were added. Whether her seizures were generalized or partial was inconclusive. The seizures were followed by a postictal phase lasting 3 minutes to 1 hour. Her last generalized tonic-clonic seizure was 1 month before admission.
Ms. A had 3 MRI studies of the brain over the past 3 years, which showed consistent and unchanged multifocal punctate white matter lesions. The findings represented gliosis from an old perivascular inflammation, trauma, or ischemic damage. There is no history of traumatic brain injury.
Her perinatal history is unremarkable, with normal vaginal delivery at 36 weeks (pre-term birth). All developmental milestones were on target.
Ms. A lives at home with her mother, 6-year-old brother, and stepfather. Her parents are divorced, but her biological father has been involved in her upbringing. She is in seventh grade, but is home schooled after she withdrew from school because of multiple seizure episodes. Ms. A denied bullying at school although she had been punched by a peer. It was unclear if it was a single incident or bullying continued and she was hesitant to disclose it.
The authors’ observations
We focus on the amnesia because it has an acute onset and it seems this is the first time Ms. A presented with this symptom. There is no need to wait for neurology consultation, even though organic causes of amnesia need to be ruled out. Our plan is to develop rapport with Ms. A, and then administer a mental status examination focusing on memory assessment. We understand that, because Ms. A’s chief concern is amnesia, she might not be able to provide many details. We start the initial interview with the family in the patient’s room to understand family dynamics, and then interview Ms. A alone.
EVALUATION Memory problems
On initial psychiatric interview, Ms. A can recognize some of her family members. She is seen in clean attire, with short hair, lying in the bed with good eye contact and a calm demeanor. She seems to be difficult to engage because of her reserved nature.
Ms. A displays some psychomotor retardation. She reports her mood as tired, and her affect is flat and mood incongruent. She is alert and oriented to person only; not to place, time, or situation. She can do a simple spelling task, perform 5-minute recall of 3 words, complete serial 3 subtractions, repeat phrases, read aloud, focus on a coin task, and name simple objects. She does not compare similar objects or answer simple historical or factual questions.
Ms. A replies “I don’t know” to most historical questions, such as her birthday, favorite color, and family members; she does not answer when asked how many legs a dog has, who is the current or past president, what month the Fourth of July is in, or when Christmas is. She can complete some memory tasks on the Mini-Mental State Examination, but does not attempt many others. Ms. A says she is upset about her memory deficit, but her affect was flat. Her mood and her affect were incongruent. She describes a vision of a “girl with black holes [for eyes]” in the corner of her hospital room telling her not to believe anyone and that the interviewers are lying to her. Also, she reports that “the girl” tells her to hurt herself and others, but she is not going to act on the commands because she knows it is not the right thing to do. When we ask Ms. A about a history of substance abuse, she says she has never heard of drugs or alcohol.
Overall, she displays multiple apparent deficits in declarative memory, both episodic and semantic. Regarding non-declarative or procedural memory, she can dress herself, use the bathroom independently, order meals off the menu, and feed herself, among other routine tasks, without difficulty.
According to Ms. A’s mother, Ms. A has shown a decline in overall functioning and personality changes during the past 5 months. She started to cut herself superficially on her forearms 6 months ago and also tried to change her appearance with a new hairstyle when school started. She displayed noticeably intense and disturbing writings, artwork, and conversations with others over 3 to 4 months.
She started experiencing seizures, with 3 to 4 seizures a day; however, she could attend sleepovers seizure-free. She had prolonged periods of seizures lasting up to an hour, much longer than would be expected clinically. She also had requested to go to the cemetery for unclear reasons (because the spirit wanted her to visit), and was observed mumbling under her breath.
Six years ago, Ms. A’s 6-year-old sister tried to suffocate her infant brother. Child protective services was involved and the sister was hospitalized in a psychiatric facility, where she was given a diagnosis of bipolar disorder; she was then transferred to foster care, and later placed in residential treatment. Her mother relinquished her parental rights and gave custody of Ms. A’s sister to the state.
Ms. A’s mother has a history of depression, but her younger brother is healthy. There is no history of autism, attention problems, tics, substance abuse, brain tumor, or intellectual disabilities in the family.
Which diagnosis does Ms. A’s presentation and history suggest?
a) dissociative amnesia
b) factitious disorder imposed on self
c) conversion disorder (neurological symptom disorder)
d) psychosis not otherwise specified
e) malingering
The authors’ observations
The history of unwitnessed seizures, sudden onset of visual hallucinations, and transient amnesia points to a possible postictal cause. Selective amnesia brings up the question of whether psychological components are driving the symptoms.
Her psychotic symptoms appear to be mediated by anxiety and possibly related to the trauma of losing her only sister when her mother relinquished custody to the state; the circumstances might have aroused feelings of insecurity or fear of abandonment and raised questions about her mother’s love toward her. Her sister’s abuse by a family member might have created reticence to trust others. These background experiences could be intensely conflicting at this age when the second separation individuation process commences, especially in an emotionally immature adolescent.
OUTCOME Medication change
The neurology team recommends discontinuing levetiracetam because the visual hallucinations, mood disturbance, and personality change could be adverse effects of the drug. Because of generalized uncontrolled body movements with staring episodes and unresponsiveness, an EEG is ordered to rule out ongoing seizures.
Ms. A recognizes the psychosomatic medicine team members when they interview her again. The team employs consistent reassurance and a non-confrontational approach. She spends 3 days in the medical unit during which she reports that the frequency of visual and auditory hallucinations decreases and her memory symptoms resolve. Her 24-hour EEG is negative for seizure activity, and the 24-hour video EEG does not show any signs of epileptogenic foci. Ms. A’s family declines inpatient psychiatric hospitalization.
Because of gradual improvement in Ms. A’s symptoms and no imminent safety concerns, she is discharged home with valproic acid, 1,000 mg/d, and oxcarbazepine, 1,200 mg/d, and follow-up appointments with her primary care physician, a neurologist, and a psychiatrist.
The authors’ observations
Dissociative amnesia
Generalized dissociative amnesia is difficult to differentiate from factitious disorder or malingering. According to DSM-5, there is loss of episodic memory in dissociative amnesia, in which the person is unable to recall the stressful event after trauma (Table 1).1 Although there have been case reports of dissociative amnesia with loss of semantic and procedural memory, episodic memory is the last to return.2 In Ms. A’s case, there was no immediate basis to explain amnesia onset, although she had experienced the trauma of losing her sister. She had episodic and mostly semantic memory loss.
Although organic causes can precipitate amnesia,3 Ms. A’s EEG and MRI results did not reflect that. Patients with a dissociative disorder often report some physical, sexual, or emotional abuse.4 Although Ms. A did not report any abuse, it cannot be completely ruled out because of her sister’s history of abuse.
Suicidality or self-injurious behavior is common among adults with dissociative amnesia, although it is not well studied in children.4,5 Generally, the constellation of primary dissociative symptoms that patients develop are forgetfulness, fragmentation, and emotional numbing. Ms. A presented with some of these features; did she, in fact, have dissociative amnesia?
Factitious amnesia
Factious amnesia (Table 2)6 is a symptom of factious disorder in which amnesia appears with the motivation to assume a sick role.3 Ms. A’s amnesia garnered significant attention from her mother and other family members; this may have been related to insecurity in her family relationships because her sister was given up to the state. She also could be afraid of entering adolescence and leaving her sister behind. Did she want more time to bond with her mother? Did she experience emotional benefit from being cared for by medical professionals?7 Her affect during interviews was blunted and her attitude was nonchalant, and her multiple visits to the hospital since childhood for abdominal pain, abscesses (it isn’t clear whether the abscesses were related to self-injury and scratching), tics, seizures, and, recently, amnesia and hallucinations indicated some desire to occupy a sick role. Furthermore, the severity of her symptoms seemed to be increasing over time, from somatic to neurologic (seizure-like episodes) to significant and less frequent psychiatric symptoms (amnesia and hallucinations). One could speculate that her symptoms were escalating because she was not receiving the attention she needed.
Malingered amnesia
Although malingering is not a psychiatric diagnosis, it can be a focus of clinical attention. It is challenging to identify malingered cognitive impairments.8 Children often have difficulty malingering symptoms because they have limited understanding of the illness they are trying to simulate.9 Many malingerers do not want to participate in their medical work up and might exhibit a hostile attitude toward examiners (Table 26). Clinicians could rely on family to provide information regarding history and inconsistencies in clinical deficits.9 The clinical interview, mental status examination, and collateral information are crucial for identifying malingering.
Most of Ms. A’s seizure-like episodes happened in specific contexts, such as in school, but not at friends’ houses, raising the question of whether she is aware of her episodes. Ms. A’s grades are consistently good; because she is being home schooled, there is no secondary gain from not going to school. There is no other reason to speculate that she was malingering.
The inconsistency of Ms. A’s symptoms and her compliance with assessment and treatment did not reflect malingering. Interestingly, Ms. A’s amnesia was retrograde in nature. There have been more studies on malingered anterograde amnesia8 than on retrograde amnesia, making her presentation even more unusual.
Amnesia presenting as conversion disorder
Amnesia as a symptom of conversion disorder is referred as psychogenic amnesia; the memory loss mostly is isolated retrograde amnesia.10 Ms. A likely had unconsciously produced symptoms of non-epileptic seizures, followed by auditory and visual hallucinations not related to her seizures, and then later developed selective transient amnesia. Conversion disorder seemed to be the diagnosis most consistent with her indifference (“la belle indifference”) and the significant attention she gained from the acute memory loss (Table 3).1 It seemed that she developed multiple symptoms in progression leading toward a conversion disorder diagnosis. The question arises whether Ms. A’s presentation is a gradually increasing cry for help or reflects depressive or anxiety symptoms, which often are comorbid with conversion disorder.
FOLLOW-UP Suicide attempt
Ms. A has frequent visits to the ED with symptoms of syncope and seizures and undergoes medical work-up and multiple EEGs. A prolonged 5-day video EEG is performed to assess seizure episodes after AEDs were withdrawn, but no seizure activity is elicited. She also has an ED visit for recurrent tic emergence.
The last visit in the ED is for a suicide attempt with overdose of an unknown quantity of unspecified pills. Ms. A talks to a social worker, who reports that Ms. A needed answers to such questions as why her grandfather abused her sister? Could she have stopped them and made a difference for the family?
The authors’ observations
Conversion disorder arises from unconscious psychological conflicts, needs, or responses to trauma. Ms. A’s consistent conflict about her sister and grandfather’s relationship was evident from occasions when she tried to confide in hospital staff. During an ED visit, she reported her sister’s abuse to a staff member. Another time, while recovering from sedation, she spontaneously spoke about her sister’s abuse. When asked again, she said she did not remember saying it.
Freud said that patients develop conversion disorder to avoid unacceptable conflicting thoughts and feelings.10 It appeared that Ms. A was struggling with these questions because she brought them up again when she visited the ED after the suicide attempt.
Dissociative symptoms arise from unstable parenting and disciplining styles with variable family dynamics. Patients show extreme detachment and emotional unresponsiveness akin to attachment disorder.11 Ms. A had inconsistent parenting because both her stepfather and biological father were involved with her care. Her mother had relinquished her parental rights to her sister, which indicated some attachment issues.
Ms. A’s idea that her mother was indifferent stemmed from her uncaring approach toward her sister and not able to understand her emotionally. Her amnesia could be thought of as “I don’t know you because I don’t remember that I am related to you.” The traumas of infancy (referred to as hidden traumas) that were a result of parent-child mismatch of needs and availability at times of distress might not be obvious to the examiner.11
Although Ms. A’s infancy was reported to be unremarkable, there always is a question, especially in a consultation-liaison setting, of whether conversion disorder might be masking an attachment problem. Perhaps with long-term psychotherapy, an attachment issue would be revealed.
Excluding an organic cause or a neurologic disorder is important when diagnosing conversion disorder10; Ms. A’s negative neurologic tests favored a diagnosis of amnesia due to conversion disorder. It appears that, although Ms. A presented with “transient amnesia,” she had underlying psychiatric symptoms, likely depression or anxiety. We were concerned about possible psychiatric comorbidity and recommended inpatient hospitalization to clarify the diagnosis and provide intensive therapy, but her family declined. She may have received outpatient services, but that was not documented.
Bottom Line
Psychogenic amnesia can be a form of conversion disorder or a symptom of
malingering; can occur in dissociative disorder; and can be factitious in nature.
Regardless of the cause, the condition requires continuous close follow up. Although organic causes of amnesia should be ruled out, mental health care can help address comorbid psychiatric symptoms and might change the course of the illness.
Related Resources
• Byatt N, Toor R. Young, pregnant, ataxic—and jilted. Current Psychiatry. 2015;14(1):44-49.
• Leipsic J. A teen who is wasting away. Current Psychiatry. 2013;12(6):40-45.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Levetiracetam • Keppra Valproic acid • Depakote
Oxcarbazepine • Trileptal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Seizures, amnesia
Ms. A, age 13, who has a history of seizures, presents to the emergency department (ED) with sudden onset of memory loss. Her family reports that she had been spending a normal evening at home with family and friends. After going to the bathroom, Ms. A became acutely confused and extremely upset, had slurred speech, and did not recognize anyone in the room except her mother.
Initial neurologic examination in the ED reports that Ms. A does not remember recent or remote past events. Her family denies any recent stressors.
Vital signs are within normal range. She has mild muscle soreness and gait instability, which is attributed to a presumed postictal phase. Her medication regimen includes: levetiracetam, 500 mg, 3 times a day; valproic acid, 1,000 mg/d; and oxcarbazepine, 2,400 mg/d, for seizure management.
Complete blood count and comprehensive metabolic panel are within normal limits. Pregnancy test is negative. Urine toxicology report is negative. Serum valproic acid level is 71 μg/mL; oxcarbazepine level, <2 μg/mL; ammonia level, 71 μg/dL (reference range, 15 to 45 μg/dL). Other than the aforementioned deficits, she is neurologically intact. The team thinks that her symptoms are part of a postictal phase of an unwitnessed seizure.
Ms. A is admitted to the inpatient medical unit for further work up. Along with the memory loss and seizures, she reports visual hallucinations.
What could be causing Ms. A’s amnesia?
a) a seizure disorder
b) malingering
c) posttraumatic stress disorder
d) traumatic brain injury
HISTORY Repeat ED visits
Ms. A’s mother reports that 3 years ago her daughter was treated for tics with quetiapine and aripiprazole, prescribed by a primary care physician. She received a short course of counseling 6 years ago after her sister was sexually abused by her grandfather. Approximately 6 months ago, Ms. A engaged in self-injurious behavior by cutting herself, and she briefly received counseling. There is no history of suicide attempts, psychiatric hospitalization, or a psychiatric diagnosis.
Medical and surgical history include viral meningitis at age 6 months. Medical records show a visit to the ED for abdominal pain after a classmate punched her in the abdomen, which resolved with supportive care. She was given a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections 6 years ago.
Ms. A developed multiple recurrent methicillin-resistant Staphylococcus aureus abscesses a year ago, which lasted for 4 months; it was noted that she was self-inoculating by scratching eczema. She had a possible syncopal episode 5 months ago, but the medical work-up was normal. The pediatric neurology service diagnosed and treated seizures 4 months ago.
Levetiracetam was prescribed after a possible syncopal episode followed by a tonic-clonic seizure. Because she was still having seizure-like episodes with a single antiepileptic drug (AED), oxcarbazepine, then valproic acid were added. Whether her seizures were generalized or partial was inconclusive. The seizures were followed by a postictal phase lasting 3 minutes to 1 hour. Her last generalized tonic-clonic seizure was 1 month before admission.
Ms. A had 3 MRI studies of the brain over the past 3 years, which showed consistent and unchanged multifocal punctate white matter lesions. The findings represented gliosis from an old perivascular inflammation, trauma, or ischemic damage. There is no history of traumatic brain injury.
Her perinatal history is unremarkable, with normal vaginal delivery at 36 weeks (pre-term birth). All developmental milestones were on target.
Ms. A lives at home with her mother, 6-year-old brother, and stepfather. Her parents are divorced, but her biological father has been involved in her upbringing. She is in seventh grade, but is home schooled after she withdrew from school because of multiple seizure episodes. Ms. A denied bullying at school although she had been punched by a peer. It was unclear if it was a single incident or bullying continued and she was hesitant to disclose it.
The authors’ observations
We focus on the amnesia because it has an acute onset and it seems this is the first time Ms. A presented with this symptom. There is no need to wait for neurology consultation, even though organic causes of amnesia need to be ruled out. Our plan is to develop rapport with Ms. A, and then administer a mental status examination focusing on memory assessment. We understand that, because Ms. A’s chief concern is amnesia, she might not be able to provide many details. We start the initial interview with the family in the patient’s room to understand family dynamics, and then interview Ms. A alone.
EVALUATION Memory problems
On initial psychiatric interview, Ms. A can recognize some of her family members. She is seen in clean attire, with short hair, lying in the bed with good eye contact and a calm demeanor. She seems to be difficult to engage because of her reserved nature.
Ms. A displays some psychomotor retardation. She reports her mood as tired, and her affect is flat and mood incongruent. She is alert and oriented to person only; not to place, time, or situation. She can do a simple spelling task, perform 5-minute recall of 3 words, complete serial 3 subtractions, repeat phrases, read aloud, focus on a coin task, and name simple objects. She does not compare similar objects or answer simple historical or factual questions.
Ms. A replies “I don’t know” to most historical questions, such as her birthday, favorite color, and family members; she does not answer when asked how many legs a dog has, who is the current or past president, what month the Fourth of July is in, or when Christmas is. She can complete some memory tasks on the Mini-Mental State Examination, but does not attempt many others. Ms. A says she is upset about her memory deficit, but her affect was flat. Her mood and her affect were incongruent. She describes a vision of a “girl with black holes [for eyes]” in the corner of her hospital room telling her not to believe anyone and that the interviewers are lying to her. Also, she reports that “the girl” tells her to hurt herself and others, but she is not going to act on the commands because she knows it is not the right thing to do. When we ask Ms. A about a history of substance abuse, she says she has never heard of drugs or alcohol.
Overall, she displays multiple apparent deficits in declarative memory, both episodic and semantic. Regarding non-declarative or procedural memory, she can dress herself, use the bathroom independently, order meals off the menu, and feed herself, among other routine tasks, without difficulty.
According to Ms. A’s mother, Ms. A has shown a decline in overall functioning and personality changes during the past 5 months. She started to cut herself superficially on her forearms 6 months ago and also tried to change her appearance with a new hairstyle when school started. She displayed noticeably intense and disturbing writings, artwork, and conversations with others over 3 to 4 months.
She started experiencing seizures, with 3 to 4 seizures a day; however, she could attend sleepovers seizure-free. She had prolonged periods of seizures lasting up to an hour, much longer than would be expected clinically. She also had requested to go to the cemetery for unclear reasons (because the spirit wanted her to visit), and was observed mumbling under her breath.
Six years ago, Ms. A’s 6-year-old sister tried to suffocate her infant brother. Child protective services was involved and the sister was hospitalized in a psychiatric facility, where she was given a diagnosis of bipolar disorder; she was then transferred to foster care, and later placed in residential treatment. Her mother relinquished her parental rights and gave custody of Ms. A’s sister to the state.
Ms. A’s mother has a history of depression, but her younger brother is healthy. There is no history of autism, attention problems, tics, substance abuse, brain tumor, or intellectual disabilities in the family.
Which diagnosis does Ms. A’s presentation and history suggest?
a) dissociative amnesia
b) factitious disorder imposed on self
c) conversion disorder (neurological symptom disorder)
d) psychosis not otherwise specified
e) malingering
The authors’ observations
The history of unwitnessed seizures, sudden onset of visual hallucinations, and transient amnesia points to a possible postictal cause. Selective amnesia brings up the question of whether psychological components are driving the symptoms.
Her psychotic symptoms appear to be mediated by anxiety and possibly related to the trauma of losing her only sister when her mother relinquished custody to the state; the circumstances might have aroused feelings of insecurity or fear of abandonment and raised questions about her mother’s love toward her. Her sister’s abuse by a family member might have created reticence to trust others. These background experiences could be intensely conflicting at this age when the second separation individuation process commences, especially in an emotionally immature adolescent.
OUTCOME Medication change
The neurology team recommends discontinuing levetiracetam because the visual hallucinations, mood disturbance, and personality change could be adverse effects of the drug. Because of generalized uncontrolled body movements with staring episodes and unresponsiveness, an EEG is ordered to rule out ongoing seizures.
Ms. A recognizes the psychosomatic medicine team members when they interview her again. The team employs consistent reassurance and a non-confrontational approach. She spends 3 days in the medical unit during which she reports that the frequency of visual and auditory hallucinations decreases and her memory symptoms resolve. Her 24-hour EEG is negative for seizure activity, and the 24-hour video EEG does not show any signs of epileptogenic foci. Ms. A’s family declines inpatient psychiatric hospitalization.
Because of gradual improvement in Ms. A’s symptoms and no imminent safety concerns, she is discharged home with valproic acid, 1,000 mg/d, and oxcarbazepine, 1,200 mg/d, and follow-up appointments with her primary care physician, a neurologist, and a psychiatrist.
The authors’ observations
Dissociative amnesia
Generalized dissociative amnesia is difficult to differentiate from factitious disorder or malingering. According to DSM-5, there is loss of episodic memory in dissociative amnesia, in which the person is unable to recall the stressful event after trauma (Table 1).1 Although there have been case reports of dissociative amnesia with loss of semantic and procedural memory, episodic memory is the last to return.2 In Ms. A’s case, there was no immediate basis to explain amnesia onset, although she had experienced the trauma of losing her sister. She had episodic and mostly semantic memory loss.
Although organic causes can precipitate amnesia,3 Ms. A’s EEG and MRI results did not reflect that. Patients with a dissociative disorder often report some physical, sexual, or emotional abuse.4 Although Ms. A did not report any abuse, it cannot be completely ruled out because of her sister’s history of abuse.
Suicidality or self-injurious behavior is common among adults with dissociative amnesia, although it is not well studied in children.4,5 Generally, the constellation of primary dissociative symptoms that patients develop are forgetfulness, fragmentation, and emotional numbing. Ms. A presented with some of these features; did she, in fact, have dissociative amnesia?
Factitious amnesia
Factious amnesia (Table 2)6 is a symptom of factious disorder in which amnesia appears with the motivation to assume a sick role.3 Ms. A’s amnesia garnered significant attention from her mother and other family members; this may have been related to insecurity in her family relationships because her sister was given up to the state. She also could be afraid of entering adolescence and leaving her sister behind. Did she want more time to bond with her mother? Did she experience emotional benefit from being cared for by medical professionals?7 Her affect during interviews was blunted and her attitude was nonchalant, and her multiple visits to the hospital since childhood for abdominal pain, abscesses (it isn’t clear whether the abscesses were related to self-injury and scratching), tics, seizures, and, recently, amnesia and hallucinations indicated some desire to occupy a sick role. Furthermore, the severity of her symptoms seemed to be increasing over time, from somatic to neurologic (seizure-like episodes) to significant and less frequent psychiatric symptoms (amnesia and hallucinations). One could speculate that her symptoms were escalating because she was not receiving the attention she needed.
Malingered amnesia
Although malingering is not a psychiatric diagnosis, it can be a focus of clinical attention. It is challenging to identify malingered cognitive impairments.8 Children often have difficulty malingering symptoms because they have limited understanding of the illness they are trying to simulate.9 Many malingerers do not want to participate in their medical work up and might exhibit a hostile attitude toward examiners (Table 26). Clinicians could rely on family to provide information regarding history and inconsistencies in clinical deficits.9 The clinical interview, mental status examination, and collateral information are crucial for identifying malingering.
Most of Ms. A’s seizure-like episodes happened in specific contexts, such as in school, but not at friends’ houses, raising the question of whether she is aware of her episodes. Ms. A’s grades are consistently good; because she is being home schooled, there is no secondary gain from not going to school. There is no other reason to speculate that she was malingering.
The inconsistency of Ms. A’s symptoms and her compliance with assessment and treatment did not reflect malingering. Interestingly, Ms. A’s amnesia was retrograde in nature. There have been more studies on malingered anterograde amnesia8 than on retrograde amnesia, making her presentation even more unusual.
Amnesia presenting as conversion disorder
Amnesia as a symptom of conversion disorder is referred as psychogenic amnesia; the memory loss mostly is isolated retrograde amnesia.10 Ms. A likely had unconsciously produced symptoms of non-epileptic seizures, followed by auditory and visual hallucinations not related to her seizures, and then later developed selective transient amnesia. Conversion disorder seemed to be the diagnosis most consistent with her indifference (“la belle indifference”) and the significant attention she gained from the acute memory loss (Table 3).1 It seemed that she developed multiple symptoms in progression leading toward a conversion disorder diagnosis. The question arises whether Ms. A’s presentation is a gradually increasing cry for help or reflects depressive or anxiety symptoms, which often are comorbid with conversion disorder.
FOLLOW-UP Suicide attempt
Ms. A has frequent visits to the ED with symptoms of syncope and seizures and undergoes medical work-up and multiple EEGs. A prolonged 5-day video EEG is performed to assess seizure episodes after AEDs were withdrawn, but no seizure activity is elicited. She also has an ED visit for recurrent tic emergence.
The last visit in the ED is for a suicide attempt with overdose of an unknown quantity of unspecified pills. Ms. A talks to a social worker, who reports that Ms. A needed answers to such questions as why her grandfather abused her sister? Could she have stopped them and made a difference for the family?
The authors’ observations
Conversion disorder arises from unconscious psychological conflicts, needs, or responses to trauma. Ms. A’s consistent conflict about her sister and grandfather’s relationship was evident from occasions when she tried to confide in hospital staff. During an ED visit, she reported her sister’s abuse to a staff member. Another time, while recovering from sedation, she spontaneously spoke about her sister’s abuse. When asked again, she said she did not remember saying it.
Freud said that patients develop conversion disorder to avoid unacceptable conflicting thoughts and feelings.10 It appeared that Ms. A was struggling with these questions because she brought them up again when she visited the ED after the suicide attempt.
Dissociative symptoms arise from unstable parenting and disciplining styles with variable family dynamics. Patients show extreme detachment and emotional unresponsiveness akin to attachment disorder.11 Ms. A had inconsistent parenting because both her stepfather and biological father were involved with her care. Her mother had relinquished her parental rights to her sister, which indicated some attachment issues.
Ms. A’s idea that her mother was indifferent stemmed from her uncaring approach toward her sister and not able to understand her emotionally. Her amnesia could be thought of as “I don’t know you because I don’t remember that I am related to you.” The traumas of infancy (referred to as hidden traumas) that were a result of parent-child mismatch of needs and availability at times of distress might not be obvious to the examiner.11
Although Ms. A’s infancy was reported to be unremarkable, there always is a question, especially in a consultation-liaison setting, of whether conversion disorder might be masking an attachment problem. Perhaps with long-term psychotherapy, an attachment issue would be revealed.
Excluding an organic cause or a neurologic disorder is important when diagnosing conversion disorder10; Ms. A’s negative neurologic tests favored a diagnosis of amnesia due to conversion disorder. It appears that, although Ms. A presented with “transient amnesia,” she had underlying psychiatric symptoms, likely depression or anxiety. We were concerned about possible psychiatric comorbidity and recommended inpatient hospitalization to clarify the diagnosis and provide intensive therapy, but her family declined. She may have received outpatient services, but that was not documented.
Bottom Line
Psychogenic amnesia can be a form of conversion disorder or a symptom of
malingering; can occur in dissociative disorder; and can be factitious in nature.
Regardless of the cause, the condition requires continuous close follow up. Although organic causes of amnesia should be ruled out, mental health care can help address comorbid psychiatric symptoms and might change the course of the illness.
Related Resources
• Byatt N, Toor R. Young, pregnant, ataxic—and jilted. Current Psychiatry. 2015;14(1):44-49.
• Leipsic J. A teen who is wasting away. Current Psychiatry. 2013;12(6):40-45.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Levetiracetam • Keppra Valproic acid • Depakote
Oxcarbazepine • Trileptal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry. 2001;35(5):589-560.
3. Ehrlich S, Pfeiffer E, Salbach H, et al. Factitious disorder in children and adolescents: a retrospective study. Psychosomatics. 2008;45(5):392-398.
4. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
5. Kisiel CL, Lyons JS. Dissociation as a mediator of psychopathology among sexually abused children and adolescents. Am J Psychiatry. 2001;158(7):1034-1039.
6. Worley CB, Feldman MD, Hamilton JC. The case of factitious disorder versus malingering. http://www.psychiatrictimes. com/munchausen-syndrome/case-factitious-disorder-versus-malingering. Published October 30, 2009. Accessed January 27, 2015.
7. Hagglund LA. Challenges in the treatment of factitious disorder: a case study. Arch Psychiatr Nurs. 2009;23(1):58-64.
8. Jenkins KG, Kapur N, Kopelman MD. Retrograde amnesia and malingering. Curr Opin Neurol. 2009;22(6):601-605.
9. Walker JS. Malingering in children: fibs and faking. Child Adolesc Psychiatr Clin N Am. 2011;20(3):547-556.
10. Levenson JL. Psychiatric issues in neurology, part 4: amnestic syndromes and conversion disorder. Primary Psychiatry. http://primarypsychiatry.com/psychiatric-issues-in-neurology-part-4-amnestic-syndromes-and-conversion-disorder. Published March 1, 2008. Accessed February 3, 2015.
11. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry. 2001;35(5):589-560.
3. Ehrlich S, Pfeiffer E, Salbach H, et al. Factitious disorder in children and adolescents: a retrospective study. Psychosomatics. 2008;45(5):392-398.
4. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
5. Kisiel CL, Lyons JS. Dissociation as a mediator of psychopathology among sexually abused children and adolescents. Am J Psychiatry. 2001;158(7):1034-1039.
6. Worley CB, Feldman MD, Hamilton JC. The case of factitious disorder versus malingering. http://www.psychiatrictimes. com/munchausen-syndrome/case-factitious-disorder-versus-malingering. Published October 30, 2009. Accessed January 27, 2015.
7. Hagglund LA. Challenges in the treatment of factitious disorder: a case study. Arch Psychiatr Nurs. 2009;23(1):58-64.
8. Jenkins KG, Kapur N, Kopelman MD. Retrograde amnesia and malingering. Curr Opin Neurol. 2009;22(6):601-605.
9. Walker JS. Malingering in children: fibs and faking. Child Adolesc Psychiatr Clin N Am. 2011;20(3):547-556.
10. Levenson JL. Psychiatric issues in neurology, part 4: amnestic syndromes and conversion disorder. Primary Psychiatry. http://primarypsychiatry.com/psychiatric-issues-in-neurology-part-4-amnestic-syndromes-and-conversion-disorder. Published March 1, 2008. Accessed February 3, 2015.
11. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
Abnormal Uterine Bleeding in Reproductive-Aged Women
From the University of Wisconsin School of Medicine and Public Health, Madison, WI.
Abstract
- Objective: To describe the contributing etiologies, common presentations, diagnosis, evaluation, and management of abnormal uterine bleeding (AUB).
- Methods: Review of the literature in the context of 3 cases.
- Results: AUB is one of the most common reasons that reproductive-aged women seek health care. The causes are varied, depending in large part on the age and life stage of the woman. Diagnosis requires a systematic approach that is driven by a thorough health history and review of presenting symptoms. Determining whether the bleeding is ovulatory or anovulatory is a central part of the evaluation. A methodical history, physical examination, and laboratory evaluation may enable the physician to rule out causes such as pregnancy and pregnancy-related disorders, medications, iatrogenic causes, systemic conditions, and obvious genital tract pathology.
- Conclusion: Clinicians must be knowledgeable about AUB and partner with women to develop appropriate, individualized treatment plans.
Abnormal vaginal bleeding is a common complaint in primary care. The prevalence of some type of abnormal bleeding is up to 30% among women of reproductive age [1].Over 18% of all gynecology outpatient visits in the United States are for menorrhagia alone [2].A retrospective analysis of medical expenditures data compared 1.4 million women with abnormal uterine bleeding to over 50 million women without abnormal bleeding. This study found that women with abnormal bleeding were more likely to be younger, Caucasian, and obese and had poorer physical and mental health quality of life scores [3].
The estimated direct and indirect costs of abnormal bleeding are $1 billion and $12 billion annually, respectively [4]. Indirect costs of abnormal bleeding include time off from work and cost of products to protect clothing from bleeding (eg, tampons and pads). Abnormal bleeding is also a common reason for women to be referred to gynecologists and is an indication for up to 25% of all gynecologic surgeries [5].
History Taking
Taking a menstrual history is an important step in determining whether the current bleeding pattern is normal or abnormal. Regularity of menstrual bleeding is clarified by asking about the frequency of the menses and their duration. Other important questions include age at menarche, presence of premenstrual syndrome symptoms, breast tenderness, cervical mucus changes, and amount of bleeding. An ovulatory cycle will usually include premenstrual symptoms whereas an anovulatory cycle will be random in its symptomatology. Women’s estimates of the amount of menstrual bleeding are notoriously inaccurate. Traditionally, more than 80 cc of menstrual blood loss per cycle is considered menorrhagia. However, women and their health care providers do not measure menstrual blood volume outside of study settings, and one study found that only half of women who presented with menorrhagia actually had more than 80 cc of blood loss [6]. There is movement toward use of more patient-centered measures to diagnose men-orrhagia, such as bleeding interfering with a woman’s daily activities, needing to wake up at night to change tampons or pads, or inability to exercise during menses. Anemia in the setting of menorrhagia by history is a less subjective way to diagnose menorrhagia.
Nomenclature and Differential Diagnosis
Differential diagnosis will vary based on symptomatology as well as age. Pregnancy is a possible cause of any type of abnormal bleeding in any woman of reproductive age (ie, after menarche and before menopause). Many systemic illnesses and medications can affect menstrual bleeding and should be included in a broad differential diagnosis of a presenting woman.
Case 1—Heavy Menstrual Bleeding
Initial Presentation
A 42-year-old woman presents reporting increasingly heavy, somewhat painful periods over the last 6 to 8 months. She experienced menarche at age 12 and has had regular, moderately heavy periods throughout her adult life. She denies any inter-menstrual bleeding.
What additional history should be obtained?
Heavy menstrual bleeding refers to abnormally heavy bleeding that occurs in an ovulatory, cyclical pattern. Women with anovulatory cycles can also have heavy bleeding as well, and distinguishing ovulatory vs anovulatory cycles is often the first step in the evaluation.
The initial evaluation of a woman presenting with heavy menstrual bleeding includes a detailed history and physical examination. The first goal of the history is to establish the severity of bleeding, including any symptoms of hemodynamically significant anemia such as dizziness or exertional dyspnea. Next, the clinician should determine whether the bleeding pattern is ovulatory or anovulatory. Ovulatory heavy menstrual bleeding is most often caused by structural lesions (leiomyomas, endometriosis, adenomyosis, cervical polyps, and endometrial polyps) or a coagulopathy (von Willebrand disease, anticoagulant use, etc). Less commonly, ovulatory heavy menstrual bleeding may be due to systemic illness (including thyroid disease, renal disease, and liver disease) or endometrial hyperplasia or carcinoma.
Once an ovulatory pattern is confirmed, a history of dysmenorrhea, pelvic pain, lower urinary tract symptoms, constipation, dyspareunia, or infertility should be elicited.
Further history taking should seek to identify any symptoms suggestive of thyroid, kidney, or liver disease,
What are key elements of the physical examination?
The physical examination should include visual inspection and palpation of the thyroid gland as well as an abdominal exam to evaluate for hepatosplenomegaly or lower abdominal tenderness or masses. Signs of anemia such as pallor should also be noted. The gynecologic exam should include visual inspection of the external genitalia, a bimanual exam, and a speculum exam. Cervical and endometrial polyps may be visible as masses at the cervical os or extending into the vaginal canal. An enlarged mobile uterus with irregular contours is consistent with leiomyomas [8].Endometriosis may manifest as tenderness, thickening, or nodularity of the uterine corpus, the vaginal canal, the uterosacral ligaments, or the adnexa. Endometriosis may also cause an asymmetric, fixed position of the uterus, the cervix, or the adnexa [9].Adenomyosis may cause diffuse moderate uterine enlargement with or without tenderness [10].Endometrial carcinoma may also cause uterine enlargement and/or immobility.
What laboratory testing should be performed?
What additional testing would be useful in narrowing the differential diagnosis?
If the physical examination and initial laboratory testing is nondiagnostic, the decision to initiate a trial of symptom management or proceed with further testing (imaging and/or tissue sampling) is based on risk of endometrial cancer, severity of symptoms, and patient preference. In many women, body habitus makes a confirmatory pelvic examination difficult, which may lower the threshold for obtaining a pelvic ultrasound.
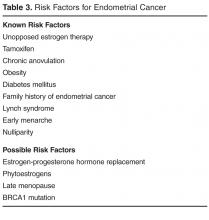
Women with risk factors for endometrial cancer should undergo office-based endometrial biopsy as the first step in evaluation of heavy menstrual bleeding [7].Risk factors include older age (45 years and older), obesity (BMI > 30), diabetes mellitus, nulliparity, and history of chronic anovulation (eg, polycystic ovary syndrome). Pelvic ultrasound is the first step in the evaluation of women with an abnormal physical exam suggesting a structural lesion [7].If the physical exam is abnormal and the pelvic ultrasound is nondiagnostic, a hysteroscopy or saline-infusion sonohysterogram should be performed, as these tests are more sensitive for the detection of intracavitary lesions and submucosal fibroids [13].Most endometrial polyps will appear as a thickened or irregular endometrium on pelvic ultrasound, but be clearly delineated on sonohysterogram. Women who have a negative initial evaluation but then go on to have persistent bleeding despite a trial of therapy also require further evaluation.
Case Continued
The patient reports that her periods are regular, with a cycle length of 30 to 31 days. She usually notes some bloating and breast tenderness in the days leading up to onset of menses. She experiences lower abdominal cramping during days 1–3 of her period. This has worsened somewhat over the last year, and sometimes radiates to her low back. Her reproductive history is significant for 3 uncomplicated vaginal deliveries and 1 first trimester spontaneous abortion. She did not experience postpartum hemorrhage, and has no history of significant oropharyngeal bleeding or unexplained bruising. Her BMI is 23.3. Her physical exam is unremarkable, including a normal thyroid, abdominal, bimanual and speculum exam. Laboratory evaluation demonstrates a low-normal hemoglobin, hematocrit, and MCV. The TSH is normal and a urine pregnancy test is negative. She had a normal pap smear and HPV assay 2 years ago.
What is the most likely diagnosis?
What treatment is recommended?
Oral tranexamic acid is an anti-fibrinolytic that was recently approved by the FDA for treatment of menorrhagia or heavy menstrual bleeding. It has been used for many years to prevent bleeding during surgery and to treat bleeding disorders. It has been used for over 30 years to treat menorrhagia in Europe. It has a different mechanism of action than NSAIDs and hormonal contraceptives, and is therefore an appropriate alternative for women who cannot tolerate other medication options [16,17].Tranexamic acid is contraindicated in women with an elevated risk of thromboembolic disease.
For women who have insufficient response to medical management or for women who present with more severe symptoms, anemia, or prominent bulk-related symptoms due to fibroids, gynecologic referral should be made for consideration of surgical intervention. The preferred interventional approach to the treatment of uterine fibroid tumors depends upon the type of fibroid (eg submucosal, intramural, subserosal), the number of fibroids, desire for future childbearing, risk for surgical complications, and patient preference. Effective options include myomectomy, uterine artery embolization, endometrial ablation, and hysterectomy [18].
By contrast, good evidence supports the use of medication as first-line therapy for heavy menstrual bleeding when it occurs in the setting of endometriosis. Estrogen-progestin oral contraceptive pills, oral progestins, and depot medroxyprogesterone have all been demonstrated to be effective in decreasing pain [19,20].The levonorgestrel-releasing intrauterine system is also effective in decreasing pain due to endometriosis [21].
Women who do not respond to first-line therapy should be referred to a gynecologist for consideration of other treatment options. Effective second-line treatment options include oral danazol, intramuscular GnRH agonists, and surgical approaches such as laparoscopic ablation and/or excision of endometriosis implants [22].
A similar range of treatment options appears to be effective in the management of heavy menstrual bleeding due to adenomyosis. First-line therapies include oral NSAIDs, oral tranexamic acid, estrogen-progestin oral contraceptive pills, and the levonorgestrel-releasing intrauterine system [23,24].Women with an inadequate response to first-line treatment should be referred to a gynecologist for consideration definitive treatment with hysterectomy versus uterine artery embolization or a trial of a GnRH agonist [24].
For some women with heavy menstrual bleeding, no specific underlying cause is identified. Current evidence suggests that such patients may have disorders of local endometrial hemostasis leading to increased blood loss during otherwise normal menstrual cycles [25].The levonorgestrel-releasing intrauterine system may be the most effective medical therapy for heavy menstrual bleeding in the absence of a specific target lesion [26].For women wishing to avoid hormonal treatment, scheduled oral NSAIDs or oral tranexamic acid are inexpensive and effective options for reducing blood loss [27–29].Other medical treatment options include estrogen-progestin contraceptive pills, cyclic oral progestin, and depot medroxy-progesterone.
For patients who experience treatment failure with pharmaceutical therapy or who desire definitive treatment, both endometrial ablation and hysterectomy have been shown to be effective and associated with high rates of patient satisfaction [30].
Follow-up
The patient reports that she would like to avoid invasive testing if possible. Given her relatively low risk for endometrial cancer, she elects a trial of scheduled NSAIDs. Unfortunately, after a couple of cycles she reports that her heavy bleeding has not been well-controlled. A pelvic ultrasound demonstrates an anterior submucosal fibroid measuring 2.4 cm and a posterior intramural fibroid measuring 1.5 cm. She agrees to insertion of a levonorgestrel IUD and calls 6 months later to report a significant decrease in her bleeding.
Case 2—Anovulation
Initial Presentation
A 27-year-old female presents for pregnancy testing. She is 2 weeks late for her period. She and her husband are attempting pregnancy and she seems disappointed that the pregnancy test is negative. She is having trouble tracking her periods. Her cycles range from 24 to 45 days apart and often she skips cycles altogether. Her flow is scant at times but some months are heavy with soaking tampons/pads.
What are diagnostic considerations in evaluating this bleeding pattern?
Menstrual history can help differentiate between of ovulatory and anovulatory abnormal bleeding. Typically, anovulatory bleeding is marked by irregular or infrequent periods. Flow can be scant to excessive. Women experiencing anovulatory cycles may fail to notice common ovulation symptoms (thin watery cervical mucus) or pre-menstrual symptoms (breast tenderness) [31].
The International Federation of Gynecology and Obstetrics (FIGO) designates AUD-O as “abnormal uterine bleeding due to ovulatory dysfunction” or “anovulatory abnormal uterine bleeding” [7,31].In general, if women are having menses at regular cycles their bleeding is likely to be ovulatory.
Differential Diagnosis
Anovulatory bleeding may be physiologic. After menarche, the hypothalamic-pituitary-ovarian axis is immature. This may result in anovulatory cycles for 2 to 3 years. Women entering perimenopausal transition may also experience intermittent anovulation and subsequent abnormal uterine bleeding. Other physiologic examples include lactation and pregnancy [31].
Physical Examination
A thorough history will help to narrow the differential diagnosis. The physical exam can evaluate for other findings that indicate endocrine dysfunction such as low body weight, hirsutism, balding, acne, high blood pressure, obesity (especially centripetal fat distribution). Acanthosis nigricans is a sign of insulin resistance which is part of the pathophysiology of PCOS. The gynecologic exam is often unremarkable in AUB-O although a bimanual exam can reveal adnexal enlargement indicative of cystic ovaries. Of note, clitoromegally is not common in PCOS. This finding would increase the likelihood of other causes of hyperandrogenism [32].
What is the pathophysiologic basis for this patient’s bleeding pattern?
Pathophysiology of Anovulatory Bleeding
Anovulatory bleeding presumes that there is a normal anatomic and genetic makeup. For example, a woman without ovaries will be, by definition, anovulatory. Using current terminology anovulatory bleeding implies a disruption in the hypothalamic-pituitary-ovarian axis and is therefore primarily an endocrine disorder [31,33].
At the level of the ovary and uterus, anovulation results in prolonged estrogen effect on the endometrium. After ovulation, the corpus luteum produces progesterone which stops endometrial thickening and stabilizes the endometrium. Without ovulation, estrogen continues endometrial stimulation and excess proliferation of endometrial lining. The endometrium becomes unstable, undifferentiated, and sheds unpredictably. The blood vessels become larger, more tortuous and have increased fragility. The result is light or heavy menstrual bleeding, decreased frequency of periods but overall unpredictable menstrual bleeding [33].
Effects of Chronic Anovulation
Irregular cycles can be more than a mere inconvenience. Women who have anovulatory cycles associated with heavy menstrual bleeding are at risk for anemia. Anovulation that is a result of hyperandrogen state or other endocrine disorder has other health ramifications. Infertility and its treatment are common sequelae. Finally, over time, unopposed estrogen in anovulation increases the risk of endometrial hyperplasia, or cancer [7,34].
Case Continued
The patient reports menarche at age 12. Her periods were irregular for the first 1–2 years but became more regular after that. She has been taking combination oral contraceptives since the age of 20 to prevent pregnancy. She stopped this 1 year ago and she and her husband began actively trying to conceive 6 months ago. Her family history is notable for diabetes and hypertension in her father. Her mother had heavy periods leading up to menopause and had a hysterectomy with no malignancy at the age of 47. She has a BMI of 33; blood pressure is mildly elevated at 134/84 mm Hg. She has oily skin and acne along her chin and neck. She has mild hirsutism of her face. Otherwise her skin is normal appearing. She has an elevated waist circumference of 35 inches. The remainder of her exam is normal.
What is the likely diagnosis?
Women with PCOS are at increased risk for metabolic syndrome, nonalcoholic fatty liver disease, type 2 diabetes and cardiovascular disease, endometrial cancer, and infertility. Women with PCOS who become pregnant have increased risk of pregnancy complications such as hypertensive disorders and gestational diabetes.
What tests are indicated in this patient?
Appropriate laboratory testing is often determined based on findings in the history and physical as well as the patient’s age. Anovulation in the first 18 months to 3 years after menarche is common and testing for pregnancy, infection, and anemia are often sufficient. Menorrhagia in adolescents warrants testing for bleeding disorders as well [7].Within 3 years of menarche, menstrual cycles should become more regular. Persistent anovulatory cycles increase the likelihood of pathologic causes and warrant additional evaluation. Pregnancy testing, thyroid stimulation hormone and prolactin levels are recommended first line evaluation [7,32,34].If PCOS is suspected an ultrasound can be performed but as noted above, polycystic ovaries are not required to make the diagnosis after adolescence.
Additional testing includes testosterone levels to look for androgen secreting tumors. Late onset congenital adrenal hyperplasia is an uncommon cause of hyperandrogenism but is more common in women of Ashkenazi Jewish descent and those with a family history [34].Morning hydroxyprogesterone can be performed to evaluate for this. If women exhibit abrupt change in menstrual pattern and other signs of cortisol excess (hypertension, abdominal striae) 24-hour urine cortisol can detect Cushing’s syndrome [34].
In patients with PCOS, additional testing to evaluate for medical comorbidities is recommended. This includes screening for diabetes, dyslipidemia, and liver dysfunction.
Case Continued
The patient’s prolactin and TSH are normal. Tests for diabetes are normal. Her LDL is elevated to 162, triglycerides are 200, and her HDL is 38. The physician informs her that she meets criteria for PCOS and also that she has obesity and metabolic syndrome.
What factors should be considered when making treatment recommendations for this patient?
Treatment for anovulation is guided by the goals of therapy. Since anovulation is an endocrine abnormality, medical treatment is first line [31].If secondary causes are diagnosed, these should be treated first. Other goals of treatment can include reducing amount and irregularity of menstruation, provide contraception, increasing ovulation in women with desired fertility, and reducing androgenic sequelae such as acne and hirsutism.
When treating the irregular or heavy bleeding associated with anovulation, first-line treatment is exogenous hormone. This can be in the form of combined estrogen/progesterone formulations (pill, patch, and ring). Medroxyprogesterone (medroxyprogesterone acetate 5–10 mg daily) taken 10 to 14 days per month is another option. Standard consideration for medical eligibility in prescribing these agents should be considered (see U.S. medical eligibility criteria for contraceptive use available at www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm). Combined formulations offer contraception, while cyclic progesterone does not. Both offer cyclic withdrawal bleeding. A Cochrane review did not find any RCTs comparing one to the other and therefore either are reasonable options [36].The levonorgestrel IUD is effective at treating AUB as well [31].Women may still experience intermittent vaginal bleeding or amenorrhea so it is less likely to result in cyclic withdrawal bleeding.
All of the above treatments provide the additional benefit of thinning the endometrium and preventing unopposed estrogen effect. This provides further protection the endometrial hyperplasia with chronic anovulatory cycles and unopposed estrogen [31].
In women with PCOS and associated metabolic conditions, first-line treatment is weight loss and other lifestyle interventions to improve or prevent other sequelae of the condition. Weight loss has been shown to reduce circulating androgen levels and increase ovulation. It has been shown to reduce glucose and lipid levels and hirsutism. Pregnancy rates increase as well. Weight loss achieved through medications and gastric bypass has similar effects. There is no special diet that has been shown to be more effective than another [32]. As little as a 5% weight reduction from baseline can improve PCOS symptoms [34,35].
Metformin is also commonly added to lifestyle modifications in women with PCOS to reduce risks for developing diabetes. There is little high quality evidence of added benefit above lifestyle modifications [34].Statin therapy can be considered in women with hyperlipidemia and PCOS [32].
For women with PCOS who desire to conceive, treatment should target increased ovulation. Pre-conception counseling and lifestyle modifications are again first line [32].Ovulation induction interventions carry increased risk of multiple gestation. For ovulation induction, clomiphene citrate is first line therapy. Metformin is commonly used as noted above to improve comorbidities associated with PCOS and can increase ovulation compared to placebo [37].However, RCTs do not support its use as first-line treatment of infertility treatment in PCOS. Clomiphene is 3 times more effective than metformin alone [32].
Medications can improve but often do not resolve hirsutism in women with PCOS. Combined hormonal contraceptives are commonly used off-label and no one type of pill has been shown to be superior. Anti-androgens are also off-label but empirically used. They can also improve lipid and other metabolic variables. They are all teratogenic and therefore should not be used in women who desire conception, and be used with effective contraceptives. Spironolactone is an androgen receptor antagonist. It takes months for effect. Some women will have improved menstrual frequency with this medication as well. Often adjunctive therapy such as eflornithine facial cream or laser therapy or a combination is needed to further treat hirsutism [32,35,38].
Follow-up
After discussion, the patient decides to adopt therapeutic lifestyle changes. She desires to get pregnant and does not opt for hormonal contraceptives at this time. She sees a nutritionist and begins calorie restriction and exercise. Three months later she has lost 20 pounds and feels “healthier.” Her lipid panel shows LDL of 125 and HDL of 43. Her triglycerides are now 160. Her blood pressure in the office is 118/78 mm Hg. She has lost “inches” around her middle. She has had more regular periods as well. She is still not pregnant so the physician asks her to begin tracking ovulation with cervical mucus evaluation and basal body temperature prior to considering further infertility evaluations. Three months after that she misses a period but is pleased to report a positive home pregnancy test.
Case 3—Breakthough Bleeding On Combined Hormonal Contraceptives
A 28-year-old G0P0 in a monogamous relationship presents to her physician. She has been on oral contraceptive pills for 8 years. For the last 3 years she has been taking the pills on an extended cycle schedule. She normally takes an active pill daily for 3 cycles of pills (9 weeks), and then takes a 7-day pill-free week when she gets a menstrual period. This had been working fine until the last 6 months. She has noticed breakthrough spotting up to 2 weeks at a time during the 2nd and 3rd pack of pills.
What is the approach to evaluation and treatment in this patient?
Bleeding in Women on Combined Hormonal Contraception
Many women are now using combined hormonal contraceptives on different schedules. Extended-cycle contraception has been shown to be as effective as the traditional 21/7 schedule of active pills/pill-free week. The FDA has approved several packaged extended-cycle contraceptives. Extended-cycle contraception decreases overall number of bleeding days and improves many menstrual-related symptoms [39].Breakthrough bleeding is the most common side effect of extended cycle contraception. It is classified as AUB-I (abnormal uterine bleeding—iatrogenic). It is most common in the first few months of use, and decreases as use continues. Up to 86% of women will have unscheduled bleeding during the first 3 months of use of extended cycle contraception, but this bleedingdecreases as use continues [40].
There is no consensus as to the underlying mechanism causing this abnormal bleeding. Most clinicians believe that it is related to the balance of estrogen/progestin in each combined hormonal contraceptive. Each woman reacts differently to this combination, making it difficult to predict who will have abnormal bleeding. In women who are beginning an extended-cycle regimen, reassurance is sufficient. Most abnormal bleeding will normalize within the first 2 to 3 months. Missed pills and smoking are consistently related to breakthrough bleeding in women who take combined oral contraceptive pills [41].In women who have previously had stable bleeding patterns and who present with new breakthrough bleeding, evaluation for secondary causes of bleeding may be considered (ie, urine hCG, TSH, STI cultures, evaluation for cervical cancer screening). A pelvic examination may help determine a possible secondary cause of bleeding, but is not necessary.
Treatment of unscheduled bleeding in women on extended-cycle contraception includes shortening the hormone-free interval and adding medications for prevention/treatment of bleeding episodes. The 7-day hormone-free interval in the context of low-dose hormonal contraception may be too long. One study demonstrated that a 7-day hormone-free interval was associated with a lack of pituitary-ovarian suppression, follicular development, and possible ovulation [42].A systematic review found that shortened hormone-free intervals decreased the amount of unscheduled bleeding [39].A small RCT (65 women) of continuous contraceptive ring users found that the group that removed the ring for 4 days during an episode of unscheduled bleeding, and then reinserted it had overall reduction in unscheduled bleeding [43].Some clinicians will also recommend trying a different pill formlation or a different schedule. There is no evidence to support this recommendation, but it can be helpful in some women.
Low-dose doxycycline (40 mg daily) for prevention of unscheduled bleeding shows promise [44].This low- dose doxycycline is also helpful to prevent more unscheduled bleeding in extended-cycle oral contraceptive users [44].However, an RCT found that traditional-dose doxycline (100 mg BID) taken for 5 days at the onset of a bleeding episode, did not decrease the amount or length of unscheduled bleeding [40].Neither estrogen dose [45]nor progestin dose [45]affected bleeding patterns. There is some suggestion based on a small study that women on pills with norethindrone may have less unscheduled bleeding than those who are on pills with levonorgestrel, but more research needs to be done before clinicians change practice [46].A Cochrane review looked at one small study that suggested third-generation progestins had more favorable bleeding profiles than second-generation progestins [47].
Follow-up
The physician investigates for secondary causes of the bleeding. The patient’s urine hCG, TSH, and prolactin levels are all normal. No fibroids or polyps are seen on ultrasound. The physician and patient discuss treatment options, including a low-dose doxycycline pill to help minimize bleeding, trying a different pill formulation, or use of naproxen during the bleeding episodes, but the patient does not want to take 2 pills every day. After further discussion, the patient decides she would like to change to the contraceptive ring with the plan of removing the ring for 4 days at the onset of any unscheduled bleeding. In a phone call 6 months later, the patient states that her unscheduled bleeding has been controlled.
Corresponding author: Sarina Schrager, MD, MS, Dept. of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Ct., Madison, WI 53715, [email protected].
1. Singh S, Best C, Dunn S, et al; Society of Obstetricians and Gynaecologists of Canada. Abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can 2013 May;35:473-9.
2. Nicholson WR, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Ob Gyn 2001;184:523-30.
3. Matteson KA, Raker CA, Clark MA, Frick KD. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Wom Health 2013;22:959-65.
4. Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183-94.
5. Goodman A. Abnormal genital tract bleeding. Clin Cornerstone 2000;3:25-35.
6. Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol 1999;82:73-6.
7. ACOG Practice Bulletin No. 128. Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol 2012;120:197-206.
8. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007; 87:725-736.
9. Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008;115:1382-91.
10. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril 2012;98(3):572-9.
11. Krassas GE, Pontikides N, Kaltsas T, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol 1999;50:655-9.
12. Knol HM, Mulder AB, Bogchelman DH, et al. The prevalence of underlying bleeding disorders in patients with heavy menstrual bleeding with and without gynecologic abnormalities. Am J Obstet Gynecol 2013;209:202.e1-7.
13. Kelekci S, Kaya E, Alan M, et al. Comparison of transvaginal sonography, saline infusion sonography, and office hysteroscopy in reproductive-aged women with or without abnormal uterine bleeding. Fertil Steril 2005;84:682–6.
14. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010;82:41-55.
15. Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BWJ. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Systematic Rev 2013, Issue 2.
16. Phillip CS. Antifibrinolytics in women with menorrhagia. Thrombosis Research 2011;127(Sup 3):S113-S115.
17. Hrometz SL. Oral modified release tranexamic acid for heavy menstrual bleeding. Ann Pharmacother 2012;46:1047-53.
18. NICE clinical guidelines. Heavy menstrual bleeding. London: National Institute for Health and Care Excellence.
19. Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD001019.
20. Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev. 2012 Mar 14;3:CD002122.
21. Management of endometriosis. Practice Bulletin No. 114. American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;116:223-36.
22. B. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2014, Issue 3.
23. Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006 Aug;20:603-16.
24. Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009;79:189-93.
25. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet 2011;113:3–13.
26. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37.
27. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database System Rev 2013;1: CD000400.
28. Naoulou BB, MC Ming C Tsai. Efficacy of tranexamic acid in the treatment of idiopathic and non-functional heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand 2012;91:529-37.
29. Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:865-75.
30. Fergusson RJ, Lethaby A, Shepperd, S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding Cochrane Database System Rev 2013;11:CD000329.
31. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin 136. Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol 2013;122:176-85.
32. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. Polycystic ovary syndrome. Obstet Gynecol 2009;114 :936-49.
33. Livinstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Human Reproduction Update 2002;8:60-67.
34. Setji TL, Brown AJ. Polycystic ovary syndrome: diagnosis and treatment. Am J Med 2007;120: 128-132.
35. Cahill D. PCOS. Clinical Evidence. BMJ Publishing Group. 2009;01:1-45.
36. Hickey M, Higam JM, Fraser I. Progestogens with our without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst Reviews 2012;9:CD001895.
37. Nothinagle M, Scott-Taylor J. Does metformin improve clinical features of polycystic ovary syndrome? Cochrane for clinicians: putting evidence into practice. Am Fam Physician 2003;68:2163-4.
38. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Systematic Rev 2009;(4): CD002249.
39. Godfrey EM, Whiteman MK, Curtis KM. Treatment of unscheduled bleeding in women using extended- or continuous-use combined hormonal contraception: a systematic review. Contraception 2013;87:567-75.
40. Kaneshiro B, Edelman A, Carlson N, et al. Treatment of unscheduled bleeding in continuous oral contraceptive users with doxycycline: a randomized controlled trial. Obstet &Gynecol 2010;115 :1141-9.
41. Grossman MP, Nakajima SP. Menstrual cycle bleeding patterns in smokers. Contraception 2006;73:562-5.
42. Schlaff WD, Lynch Am, Hughes HD, et al. Manipulation of the pill-free interval in oral contraceptive pill users: the effects on follicular suppression. Am J Obstet Gynecol 2004;190:943-51.
43. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol 2008;112:563-71.
44. Kaneshiro B, Edelman A, Carlson NE, et al. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception 2012;85:351-8.
45. Kaneshiro B, Edelman A, Carlson NE, et al. Unscheduled bleeding with continuous oral contraceptive pills: a comparison of progestin dose. Contraception 2012;86:22-
46. Edelman AB, Koontz SL, Nichols MD, Jensen JT. Continuous oral contraceptives: are bleeding patterns dependent on the hormones given? Obstet Gynecol 2006;107:657-65.
47. Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev 2011; (5):CD0004681.
From the University of Wisconsin School of Medicine and Public Health, Madison, WI.
Abstract
- Objective: To describe the contributing etiologies, common presentations, diagnosis, evaluation, and management of abnormal uterine bleeding (AUB).
- Methods: Review of the literature in the context of 3 cases.
- Results: AUB is one of the most common reasons that reproductive-aged women seek health care. The causes are varied, depending in large part on the age and life stage of the woman. Diagnosis requires a systematic approach that is driven by a thorough health history and review of presenting symptoms. Determining whether the bleeding is ovulatory or anovulatory is a central part of the evaluation. A methodical history, physical examination, and laboratory evaluation may enable the physician to rule out causes such as pregnancy and pregnancy-related disorders, medications, iatrogenic causes, systemic conditions, and obvious genital tract pathology.
- Conclusion: Clinicians must be knowledgeable about AUB and partner with women to develop appropriate, individualized treatment plans.
Abnormal vaginal bleeding is a common complaint in primary care. The prevalence of some type of abnormal bleeding is up to 30% among women of reproductive age [1].Over 18% of all gynecology outpatient visits in the United States are for menorrhagia alone [2].A retrospective analysis of medical expenditures data compared 1.4 million women with abnormal uterine bleeding to over 50 million women without abnormal bleeding. This study found that women with abnormal bleeding were more likely to be younger, Caucasian, and obese and had poorer physical and mental health quality of life scores [3].
The estimated direct and indirect costs of abnormal bleeding are $1 billion and $12 billion annually, respectively [4]. Indirect costs of abnormal bleeding include time off from work and cost of products to protect clothing from bleeding (eg, tampons and pads). Abnormal bleeding is also a common reason for women to be referred to gynecologists and is an indication for up to 25% of all gynecologic surgeries [5].
History Taking
Taking a menstrual history is an important step in determining whether the current bleeding pattern is normal or abnormal. Regularity of menstrual bleeding is clarified by asking about the frequency of the menses and their duration. Other important questions include age at menarche, presence of premenstrual syndrome symptoms, breast tenderness, cervical mucus changes, and amount of bleeding. An ovulatory cycle will usually include premenstrual symptoms whereas an anovulatory cycle will be random in its symptomatology. Women’s estimates of the amount of menstrual bleeding are notoriously inaccurate. Traditionally, more than 80 cc of menstrual blood loss per cycle is considered menorrhagia. However, women and their health care providers do not measure menstrual blood volume outside of study settings, and one study found that only half of women who presented with menorrhagia actually had more than 80 cc of blood loss [6]. There is movement toward use of more patient-centered measures to diagnose men-orrhagia, such as bleeding interfering with a woman’s daily activities, needing to wake up at night to change tampons or pads, or inability to exercise during menses. Anemia in the setting of menorrhagia by history is a less subjective way to diagnose menorrhagia.
Nomenclature and Differential Diagnosis
Differential diagnosis will vary based on symptomatology as well as age. Pregnancy is a possible cause of any type of abnormal bleeding in any woman of reproductive age (ie, after menarche and before menopause). Many systemic illnesses and medications can affect menstrual bleeding and should be included in a broad differential diagnosis of a presenting woman.
Case 1—Heavy Menstrual Bleeding
Initial Presentation
A 42-year-old woman presents reporting increasingly heavy, somewhat painful periods over the last 6 to 8 months. She experienced menarche at age 12 and has had regular, moderately heavy periods throughout her adult life. She denies any inter-menstrual bleeding.
What additional history should be obtained?
Heavy menstrual bleeding refers to abnormally heavy bleeding that occurs in an ovulatory, cyclical pattern. Women with anovulatory cycles can also have heavy bleeding as well, and distinguishing ovulatory vs anovulatory cycles is often the first step in the evaluation.
The initial evaluation of a woman presenting with heavy menstrual bleeding includes a detailed history and physical examination. The first goal of the history is to establish the severity of bleeding, including any symptoms of hemodynamically significant anemia such as dizziness or exertional dyspnea. Next, the clinician should determine whether the bleeding pattern is ovulatory or anovulatory. Ovulatory heavy menstrual bleeding is most often caused by structural lesions (leiomyomas, endometriosis, adenomyosis, cervical polyps, and endometrial polyps) or a coagulopathy (von Willebrand disease, anticoagulant use, etc). Less commonly, ovulatory heavy menstrual bleeding may be due to systemic illness (including thyroid disease, renal disease, and liver disease) or endometrial hyperplasia or carcinoma.
Once an ovulatory pattern is confirmed, a history of dysmenorrhea, pelvic pain, lower urinary tract symptoms, constipation, dyspareunia, or infertility should be elicited.
Further history taking should seek to identify any symptoms suggestive of thyroid, kidney, or liver disease,
What are key elements of the physical examination?
The physical examination should include visual inspection and palpation of the thyroid gland as well as an abdominal exam to evaluate for hepatosplenomegaly or lower abdominal tenderness or masses. Signs of anemia such as pallor should also be noted. The gynecologic exam should include visual inspection of the external genitalia, a bimanual exam, and a speculum exam. Cervical and endometrial polyps may be visible as masses at the cervical os or extending into the vaginal canal. An enlarged mobile uterus with irregular contours is consistent with leiomyomas [8].Endometriosis may manifest as tenderness, thickening, or nodularity of the uterine corpus, the vaginal canal, the uterosacral ligaments, or the adnexa. Endometriosis may also cause an asymmetric, fixed position of the uterus, the cervix, or the adnexa [9].Adenomyosis may cause diffuse moderate uterine enlargement with or without tenderness [10].Endometrial carcinoma may also cause uterine enlargement and/or immobility.
What laboratory testing should be performed?
What additional testing would be useful in narrowing the differential diagnosis?
If the physical examination and initial laboratory testing is nondiagnostic, the decision to initiate a trial of symptom management or proceed with further testing (imaging and/or tissue sampling) is based on risk of endometrial cancer, severity of symptoms, and patient preference. In many women, body habitus makes a confirmatory pelvic examination difficult, which may lower the threshold for obtaining a pelvic ultrasound.
Women with risk factors for endometrial cancer should undergo office-based endometrial biopsy as the first step in evaluation of heavy menstrual bleeding [7].Risk factors include older age (45 years and older), obesity (BMI > 30), diabetes mellitus, nulliparity, and history of chronic anovulation (eg, polycystic ovary syndrome). Pelvic ultrasound is the first step in the evaluation of women with an abnormal physical exam suggesting a structural lesion [7].If the physical exam is abnormal and the pelvic ultrasound is nondiagnostic, a hysteroscopy or saline-infusion sonohysterogram should be performed, as these tests are more sensitive for the detection of intracavitary lesions and submucosal fibroids [13].Most endometrial polyps will appear as a thickened or irregular endometrium on pelvic ultrasound, but be clearly delineated on sonohysterogram. Women who have a negative initial evaluation but then go on to have persistent bleeding despite a trial of therapy also require further evaluation.
Case Continued
The patient reports that her periods are regular, with a cycle length of 30 to 31 days. She usually notes some bloating and breast tenderness in the days leading up to onset of menses. She experiences lower abdominal cramping during days 1–3 of her period. This has worsened somewhat over the last year, and sometimes radiates to her low back. Her reproductive history is significant for 3 uncomplicated vaginal deliveries and 1 first trimester spontaneous abortion. She did not experience postpartum hemorrhage, and has no history of significant oropharyngeal bleeding or unexplained bruising. Her BMI is 23.3. Her physical exam is unremarkable, including a normal thyroid, abdominal, bimanual and speculum exam. Laboratory evaluation demonstrates a low-normal hemoglobin, hematocrit, and MCV. The TSH is normal and a urine pregnancy test is negative. She had a normal pap smear and HPV assay 2 years ago.
What is the most likely diagnosis?
What treatment is recommended?
Oral tranexamic acid is an anti-fibrinolytic that was recently approved by the FDA for treatment of menorrhagia or heavy menstrual bleeding. It has been used for many years to prevent bleeding during surgery and to treat bleeding disorders. It has been used for over 30 years to treat menorrhagia in Europe. It has a different mechanism of action than NSAIDs and hormonal contraceptives, and is therefore an appropriate alternative for women who cannot tolerate other medication options [16,17].Tranexamic acid is contraindicated in women with an elevated risk of thromboembolic disease.
For women who have insufficient response to medical management or for women who present with more severe symptoms, anemia, or prominent bulk-related symptoms due to fibroids, gynecologic referral should be made for consideration of surgical intervention. The preferred interventional approach to the treatment of uterine fibroid tumors depends upon the type of fibroid (eg submucosal, intramural, subserosal), the number of fibroids, desire for future childbearing, risk for surgical complications, and patient preference. Effective options include myomectomy, uterine artery embolization, endometrial ablation, and hysterectomy [18].
By contrast, good evidence supports the use of medication as first-line therapy for heavy menstrual bleeding when it occurs in the setting of endometriosis. Estrogen-progestin oral contraceptive pills, oral progestins, and depot medroxyprogesterone have all been demonstrated to be effective in decreasing pain [19,20].The levonorgestrel-releasing intrauterine system is also effective in decreasing pain due to endometriosis [21].
Women who do not respond to first-line therapy should be referred to a gynecologist for consideration of other treatment options. Effective second-line treatment options include oral danazol, intramuscular GnRH agonists, and surgical approaches such as laparoscopic ablation and/or excision of endometriosis implants [22].
A similar range of treatment options appears to be effective in the management of heavy menstrual bleeding due to adenomyosis. First-line therapies include oral NSAIDs, oral tranexamic acid, estrogen-progestin oral contraceptive pills, and the levonorgestrel-releasing intrauterine system [23,24].Women with an inadequate response to first-line treatment should be referred to a gynecologist for consideration definitive treatment with hysterectomy versus uterine artery embolization or a trial of a GnRH agonist [24].
For some women with heavy menstrual bleeding, no specific underlying cause is identified. Current evidence suggests that such patients may have disorders of local endometrial hemostasis leading to increased blood loss during otherwise normal menstrual cycles [25].The levonorgestrel-releasing intrauterine system may be the most effective medical therapy for heavy menstrual bleeding in the absence of a specific target lesion [26].For women wishing to avoid hormonal treatment, scheduled oral NSAIDs or oral tranexamic acid are inexpensive and effective options for reducing blood loss [27–29].Other medical treatment options include estrogen-progestin contraceptive pills, cyclic oral progestin, and depot medroxy-progesterone.
For patients who experience treatment failure with pharmaceutical therapy or who desire definitive treatment, both endometrial ablation and hysterectomy have been shown to be effective and associated with high rates of patient satisfaction [30].
Follow-up
The patient reports that she would like to avoid invasive testing if possible. Given her relatively low risk for endometrial cancer, she elects a trial of scheduled NSAIDs. Unfortunately, after a couple of cycles she reports that her heavy bleeding has not been well-controlled. A pelvic ultrasound demonstrates an anterior submucosal fibroid measuring 2.4 cm and a posterior intramural fibroid measuring 1.5 cm. She agrees to insertion of a levonorgestrel IUD and calls 6 months later to report a significant decrease in her bleeding.
Case 2—Anovulation
Initial Presentation
A 27-year-old female presents for pregnancy testing. She is 2 weeks late for her period. She and her husband are attempting pregnancy and she seems disappointed that the pregnancy test is negative. She is having trouble tracking her periods. Her cycles range from 24 to 45 days apart and often she skips cycles altogether. Her flow is scant at times but some months are heavy with soaking tampons/pads.
What are diagnostic considerations in evaluating this bleeding pattern?
Menstrual history can help differentiate between of ovulatory and anovulatory abnormal bleeding. Typically, anovulatory bleeding is marked by irregular or infrequent periods. Flow can be scant to excessive. Women experiencing anovulatory cycles may fail to notice common ovulation symptoms (thin watery cervical mucus) or pre-menstrual symptoms (breast tenderness) [31].
The International Federation of Gynecology and Obstetrics (FIGO) designates AUD-O as “abnormal uterine bleeding due to ovulatory dysfunction” or “anovulatory abnormal uterine bleeding” [7,31].In general, if women are having menses at regular cycles their bleeding is likely to be ovulatory.
Differential Diagnosis
Anovulatory bleeding may be physiologic. After menarche, the hypothalamic-pituitary-ovarian axis is immature. This may result in anovulatory cycles for 2 to 3 years. Women entering perimenopausal transition may also experience intermittent anovulation and subsequent abnormal uterine bleeding. Other physiologic examples include lactation and pregnancy [31].
Physical Examination
A thorough history will help to narrow the differential diagnosis. The physical exam can evaluate for other findings that indicate endocrine dysfunction such as low body weight, hirsutism, balding, acne, high blood pressure, obesity (especially centripetal fat distribution). Acanthosis nigricans is a sign of insulin resistance which is part of the pathophysiology of PCOS. The gynecologic exam is often unremarkable in AUB-O although a bimanual exam can reveal adnexal enlargement indicative of cystic ovaries. Of note, clitoromegally is not common in PCOS. This finding would increase the likelihood of other causes of hyperandrogenism [32].
What is the pathophysiologic basis for this patient’s bleeding pattern?
Pathophysiology of Anovulatory Bleeding
Anovulatory bleeding presumes that there is a normal anatomic and genetic makeup. For example, a woman without ovaries will be, by definition, anovulatory. Using current terminology anovulatory bleeding implies a disruption in the hypothalamic-pituitary-ovarian axis and is therefore primarily an endocrine disorder [31,33].
At the level of the ovary and uterus, anovulation results in prolonged estrogen effect on the endometrium. After ovulation, the corpus luteum produces progesterone which stops endometrial thickening and stabilizes the endometrium. Without ovulation, estrogen continues endometrial stimulation and excess proliferation of endometrial lining. The endometrium becomes unstable, undifferentiated, and sheds unpredictably. The blood vessels become larger, more tortuous and have increased fragility. The result is light or heavy menstrual bleeding, decreased frequency of periods but overall unpredictable menstrual bleeding [33].
Effects of Chronic Anovulation
Irregular cycles can be more than a mere inconvenience. Women who have anovulatory cycles associated with heavy menstrual bleeding are at risk for anemia. Anovulation that is a result of hyperandrogen state or other endocrine disorder has other health ramifications. Infertility and its treatment are common sequelae. Finally, over time, unopposed estrogen in anovulation increases the risk of endometrial hyperplasia, or cancer [7,34].
Case Continued
The patient reports menarche at age 12. Her periods were irregular for the first 1–2 years but became more regular after that. She has been taking combination oral contraceptives since the age of 20 to prevent pregnancy. She stopped this 1 year ago and she and her husband began actively trying to conceive 6 months ago. Her family history is notable for diabetes and hypertension in her father. Her mother had heavy periods leading up to menopause and had a hysterectomy with no malignancy at the age of 47. She has a BMI of 33; blood pressure is mildly elevated at 134/84 mm Hg. She has oily skin and acne along her chin and neck. She has mild hirsutism of her face. Otherwise her skin is normal appearing. She has an elevated waist circumference of 35 inches. The remainder of her exam is normal.
What is the likely diagnosis?
Women with PCOS are at increased risk for metabolic syndrome, nonalcoholic fatty liver disease, type 2 diabetes and cardiovascular disease, endometrial cancer, and infertility. Women with PCOS who become pregnant have increased risk of pregnancy complications such as hypertensive disorders and gestational diabetes.
What tests are indicated in this patient?
Appropriate laboratory testing is often determined based on findings in the history and physical as well as the patient’s age. Anovulation in the first 18 months to 3 years after menarche is common and testing for pregnancy, infection, and anemia are often sufficient. Menorrhagia in adolescents warrants testing for bleeding disorders as well [7].Within 3 years of menarche, menstrual cycles should become more regular. Persistent anovulatory cycles increase the likelihood of pathologic causes and warrant additional evaluation. Pregnancy testing, thyroid stimulation hormone and prolactin levels are recommended first line evaluation [7,32,34].If PCOS is suspected an ultrasound can be performed but as noted above, polycystic ovaries are not required to make the diagnosis after adolescence.
Additional testing includes testosterone levels to look for androgen secreting tumors. Late onset congenital adrenal hyperplasia is an uncommon cause of hyperandrogenism but is more common in women of Ashkenazi Jewish descent and those with a family history [34].Morning hydroxyprogesterone can be performed to evaluate for this. If women exhibit abrupt change in menstrual pattern and other signs of cortisol excess (hypertension, abdominal striae) 24-hour urine cortisol can detect Cushing’s syndrome [34].
In patients with PCOS, additional testing to evaluate for medical comorbidities is recommended. This includes screening for diabetes, dyslipidemia, and liver dysfunction.
Case Continued
The patient’s prolactin and TSH are normal. Tests for diabetes are normal. Her LDL is elevated to 162, triglycerides are 200, and her HDL is 38. The physician informs her that she meets criteria for PCOS and also that she has obesity and metabolic syndrome.
What factors should be considered when making treatment recommendations for this patient?
Treatment for anovulation is guided by the goals of therapy. Since anovulation is an endocrine abnormality, medical treatment is first line [31].If secondary causes are diagnosed, these should be treated first. Other goals of treatment can include reducing amount and irregularity of menstruation, provide contraception, increasing ovulation in women with desired fertility, and reducing androgenic sequelae such as acne and hirsutism.
When treating the irregular or heavy bleeding associated with anovulation, first-line treatment is exogenous hormone. This can be in the form of combined estrogen/progesterone formulations (pill, patch, and ring). Medroxyprogesterone (medroxyprogesterone acetate 5–10 mg daily) taken 10 to 14 days per month is another option. Standard consideration for medical eligibility in prescribing these agents should be considered (see U.S. medical eligibility criteria for contraceptive use available at www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm). Combined formulations offer contraception, while cyclic progesterone does not. Both offer cyclic withdrawal bleeding. A Cochrane review did not find any RCTs comparing one to the other and therefore either are reasonable options [36].The levonorgestrel IUD is effective at treating AUB as well [31].Women may still experience intermittent vaginal bleeding or amenorrhea so it is less likely to result in cyclic withdrawal bleeding.
All of the above treatments provide the additional benefit of thinning the endometrium and preventing unopposed estrogen effect. This provides further protection the endometrial hyperplasia with chronic anovulatory cycles and unopposed estrogen [31].
In women with PCOS and associated metabolic conditions, first-line treatment is weight loss and other lifestyle interventions to improve or prevent other sequelae of the condition. Weight loss has been shown to reduce circulating androgen levels and increase ovulation. It has been shown to reduce glucose and lipid levels and hirsutism. Pregnancy rates increase as well. Weight loss achieved through medications and gastric bypass has similar effects. There is no special diet that has been shown to be more effective than another [32]. As little as a 5% weight reduction from baseline can improve PCOS symptoms [34,35].
Metformin is also commonly added to lifestyle modifications in women with PCOS to reduce risks for developing diabetes. There is little high quality evidence of added benefit above lifestyle modifications [34].Statin therapy can be considered in women with hyperlipidemia and PCOS [32].
For women with PCOS who desire to conceive, treatment should target increased ovulation. Pre-conception counseling and lifestyle modifications are again first line [32].Ovulation induction interventions carry increased risk of multiple gestation. For ovulation induction, clomiphene citrate is first line therapy. Metformin is commonly used as noted above to improve comorbidities associated with PCOS and can increase ovulation compared to placebo [37].However, RCTs do not support its use as first-line treatment of infertility treatment in PCOS. Clomiphene is 3 times more effective than metformin alone [32].
Medications can improve but often do not resolve hirsutism in women with PCOS. Combined hormonal contraceptives are commonly used off-label and no one type of pill has been shown to be superior. Anti-androgens are also off-label but empirically used. They can also improve lipid and other metabolic variables. They are all teratogenic and therefore should not be used in women who desire conception, and be used with effective contraceptives. Spironolactone is an androgen receptor antagonist. It takes months for effect. Some women will have improved menstrual frequency with this medication as well. Often adjunctive therapy such as eflornithine facial cream or laser therapy or a combination is needed to further treat hirsutism [32,35,38].
Follow-up
After discussion, the patient decides to adopt therapeutic lifestyle changes. She desires to get pregnant and does not opt for hormonal contraceptives at this time. She sees a nutritionist and begins calorie restriction and exercise. Three months later she has lost 20 pounds and feels “healthier.” Her lipid panel shows LDL of 125 and HDL of 43. Her triglycerides are now 160. Her blood pressure in the office is 118/78 mm Hg. She has lost “inches” around her middle. She has had more regular periods as well. She is still not pregnant so the physician asks her to begin tracking ovulation with cervical mucus evaluation and basal body temperature prior to considering further infertility evaluations. Three months after that she misses a period but is pleased to report a positive home pregnancy test.
Case 3—Breakthough Bleeding On Combined Hormonal Contraceptives
A 28-year-old G0P0 in a monogamous relationship presents to her physician. She has been on oral contraceptive pills for 8 years. For the last 3 years she has been taking the pills on an extended cycle schedule. She normally takes an active pill daily for 3 cycles of pills (9 weeks), and then takes a 7-day pill-free week when she gets a menstrual period. This had been working fine until the last 6 months. She has noticed breakthrough spotting up to 2 weeks at a time during the 2nd and 3rd pack of pills.
What is the approach to evaluation and treatment in this patient?
Bleeding in Women on Combined Hormonal Contraception
Many women are now using combined hormonal contraceptives on different schedules. Extended-cycle contraception has been shown to be as effective as the traditional 21/7 schedule of active pills/pill-free week. The FDA has approved several packaged extended-cycle contraceptives. Extended-cycle contraception decreases overall number of bleeding days and improves many menstrual-related symptoms [39].Breakthrough bleeding is the most common side effect of extended cycle contraception. It is classified as AUB-I (abnormal uterine bleeding—iatrogenic). It is most common in the first few months of use, and decreases as use continues. Up to 86% of women will have unscheduled bleeding during the first 3 months of use of extended cycle contraception, but this bleedingdecreases as use continues [40].
There is no consensus as to the underlying mechanism causing this abnormal bleeding. Most clinicians believe that it is related to the balance of estrogen/progestin in each combined hormonal contraceptive. Each woman reacts differently to this combination, making it difficult to predict who will have abnormal bleeding. In women who are beginning an extended-cycle regimen, reassurance is sufficient. Most abnormal bleeding will normalize within the first 2 to 3 months. Missed pills and smoking are consistently related to breakthrough bleeding in women who take combined oral contraceptive pills [41].In women who have previously had stable bleeding patterns and who present with new breakthrough bleeding, evaluation for secondary causes of bleeding may be considered (ie, urine hCG, TSH, STI cultures, evaluation for cervical cancer screening). A pelvic examination may help determine a possible secondary cause of bleeding, but is not necessary.
Treatment of unscheduled bleeding in women on extended-cycle contraception includes shortening the hormone-free interval and adding medications for prevention/treatment of bleeding episodes. The 7-day hormone-free interval in the context of low-dose hormonal contraception may be too long. One study demonstrated that a 7-day hormone-free interval was associated with a lack of pituitary-ovarian suppression, follicular development, and possible ovulation [42].A systematic review found that shortened hormone-free intervals decreased the amount of unscheduled bleeding [39].A small RCT (65 women) of continuous contraceptive ring users found that the group that removed the ring for 4 days during an episode of unscheduled bleeding, and then reinserted it had overall reduction in unscheduled bleeding [43].Some clinicians will also recommend trying a different pill formlation or a different schedule. There is no evidence to support this recommendation, but it can be helpful in some women.
Low-dose doxycycline (40 mg daily) for prevention of unscheduled bleeding shows promise [44].This low- dose doxycycline is also helpful to prevent more unscheduled bleeding in extended-cycle oral contraceptive users [44].However, an RCT found that traditional-dose doxycline (100 mg BID) taken for 5 days at the onset of a bleeding episode, did not decrease the amount or length of unscheduled bleeding [40].Neither estrogen dose [45]nor progestin dose [45]affected bleeding patterns. There is some suggestion based on a small study that women on pills with norethindrone may have less unscheduled bleeding than those who are on pills with levonorgestrel, but more research needs to be done before clinicians change practice [46].A Cochrane review looked at one small study that suggested third-generation progestins had more favorable bleeding profiles than second-generation progestins [47].
Follow-up
The physician investigates for secondary causes of the bleeding. The patient’s urine hCG, TSH, and prolactin levels are all normal. No fibroids or polyps are seen on ultrasound. The physician and patient discuss treatment options, including a low-dose doxycycline pill to help minimize bleeding, trying a different pill formulation, or use of naproxen during the bleeding episodes, but the patient does not want to take 2 pills every day. After further discussion, the patient decides she would like to change to the contraceptive ring with the plan of removing the ring for 4 days at the onset of any unscheduled bleeding. In a phone call 6 months later, the patient states that her unscheduled bleeding has been controlled.
Corresponding author: Sarina Schrager, MD, MS, Dept. of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Ct., Madison, WI 53715, [email protected].
From the University of Wisconsin School of Medicine and Public Health, Madison, WI.
Abstract
- Objective: To describe the contributing etiologies, common presentations, diagnosis, evaluation, and management of abnormal uterine bleeding (AUB).
- Methods: Review of the literature in the context of 3 cases.
- Results: AUB is one of the most common reasons that reproductive-aged women seek health care. The causes are varied, depending in large part on the age and life stage of the woman. Diagnosis requires a systematic approach that is driven by a thorough health history and review of presenting symptoms. Determining whether the bleeding is ovulatory or anovulatory is a central part of the evaluation. A methodical history, physical examination, and laboratory evaluation may enable the physician to rule out causes such as pregnancy and pregnancy-related disorders, medications, iatrogenic causes, systemic conditions, and obvious genital tract pathology.
- Conclusion: Clinicians must be knowledgeable about AUB and partner with women to develop appropriate, individualized treatment plans.
Abnormal vaginal bleeding is a common complaint in primary care. The prevalence of some type of abnormal bleeding is up to 30% among women of reproductive age [1].Over 18% of all gynecology outpatient visits in the United States are for menorrhagia alone [2].A retrospective analysis of medical expenditures data compared 1.4 million women with abnormal uterine bleeding to over 50 million women without abnormal bleeding. This study found that women with abnormal bleeding were more likely to be younger, Caucasian, and obese and had poorer physical and mental health quality of life scores [3].
The estimated direct and indirect costs of abnormal bleeding are $1 billion and $12 billion annually, respectively [4]. Indirect costs of abnormal bleeding include time off from work and cost of products to protect clothing from bleeding (eg, tampons and pads). Abnormal bleeding is also a common reason for women to be referred to gynecologists and is an indication for up to 25% of all gynecologic surgeries [5].
History Taking
Taking a menstrual history is an important step in determining whether the current bleeding pattern is normal or abnormal. Regularity of menstrual bleeding is clarified by asking about the frequency of the menses and their duration. Other important questions include age at menarche, presence of premenstrual syndrome symptoms, breast tenderness, cervical mucus changes, and amount of bleeding. An ovulatory cycle will usually include premenstrual symptoms whereas an anovulatory cycle will be random in its symptomatology. Women’s estimates of the amount of menstrual bleeding are notoriously inaccurate. Traditionally, more than 80 cc of menstrual blood loss per cycle is considered menorrhagia. However, women and their health care providers do not measure menstrual blood volume outside of study settings, and one study found that only half of women who presented with menorrhagia actually had more than 80 cc of blood loss [6]. There is movement toward use of more patient-centered measures to diagnose men-orrhagia, such as bleeding interfering with a woman’s daily activities, needing to wake up at night to change tampons or pads, or inability to exercise during menses. Anemia in the setting of menorrhagia by history is a less subjective way to diagnose menorrhagia.
Nomenclature and Differential Diagnosis
Differential diagnosis will vary based on symptomatology as well as age. Pregnancy is a possible cause of any type of abnormal bleeding in any woman of reproductive age (ie, after menarche and before menopause). Many systemic illnesses and medications can affect menstrual bleeding and should be included in a broad differential diagnosis of a presenting woman.
Case 1—Heavy Menstrual Bleeding
Initial Presentation
A 42-year-old woman presents reporting increasingly heavy, somewhat painful periods over the last 6 to 8 months. She experienced menarche at age 12 and has had regular, moderately heavy periods throughout her adult life. She denies any inter-menstrual bleeding.
What additional history should be obtained?
Heavy menstrual bleeding refers to abnormally heavy bleeding that occurs in an ovulatory, cyclical pattern. Women with anovulatory cycles can also have heavy bleeding as well, and distinguishing ovulatory vs anovulatory cycles is often the first step in the evaluation.
The initial evaluation of a woman presenting with heavy menstrual bleeding includes a detailed history and physical examination. The first goal of the history is to establish the severity of bleeding, including any symptoms of hemodynamically significant anemia such as dizziness or exertional dyspnea. Next, the clinician should determine whether the bleeding pattern is ovulatory or anovulatory. Ovulatory heavy menstrual bleeding is most often caused by structural lesions (leiomyomas, endometriosis, adenomyosis, cervical polyps, and endometrial polyps) or a coagulopathy (von Willebrand disease, anticoagulant use, etc). Less commonly, ovulatory heavy menstrual bleeding may be due to systemic illness (including thyroid disease, renal disease, and liver disease) or endometrial hyperplasia or carcinoma.
Once an ovulatory pattern is confirmed, a history of dysmenorrhea, pelvic pain, lower urinary tract symptoms, constipation, dyspareunia, or infertility should be elicited.
Further history taking should seek to identify any symptoms suggestive of thyroid, kidney, or liver disease,
What are key elements of the physical examination?
The physical examination should include visual inspection and palpation of the thyroid gland as well as an abdominal exam to evaluate for hepatosplenomegaly or lower abdominal tenderness or masses. Signs of anemia such as pallor should also be noted. The gynecologic exam should include visual inspection of the external genitalia, a bimanual exam, and a speculum exam. Cervical and endometrial polyps may be visible as masses at the cervical os or extending into the vaginal canal. An enlarged mobile uterus with irregular contours is consistent with leiomyomas [8].Endometriosis may manifest as tenderness, thickening, or nodularity of the uterine corpus, the vaginal canal, the uterosacral ligaments, or the adnexa. Endometriosis may also cause an asymmetric, fixed position of the uterus, the cervix, or the adnexa [9].Adenomyosis may cause diffuse moderate uterine enlargement with or without tenderness [10].Endometrial carcinoma may also cause uterine enlargement and/or immobility.
What laboratory testing should be performed?
What additional testing would be useful in narrowing the differential diagnosis?
If the physical examination and initial laboratory testing is nondiagnostic, the decision to initiate a trial of symptom management or proceed with further testing (imaging and/or tissue sampling) is based on risk of endometrial cancer, severity of symptoms, and patient preference. In many women, body habitus makes a confirmatory pelvic examination difficult, which may lower the threshold for obtaining a pelvic ultrasound.
Women with risk factors for endometrial cancer should undergo office-based endometrial biopsy as the first step in evaluation of heavy menstrual bleeding [7].Risk factors include older age (45 years and older), obesity (BMI > 30), diabetes mellitus, nulliparity, and history of chronic anovulation (eg, polycystic ovary syndrome). Pelvic ultrasound is the first step in the evaluation of women with an abnormal physical exam suggesting a structural lesion [7].If the physical exam is abnormal and the pelvic ultrasound is nondiagnostic, a hysteroscopy or saline-infusion sonohysterogram should be performed, as these tests are more sensitive for the detection of intracavitary lesions and submucosal fibroids [13].Most endometrial polyps will appear as a thickened or irregular endometrium on pelvic ultrasound, but be clearly delineated on sonohysterogram. Women who have a negative initial evaluation but then go on to have persistent bleeding despite a trial of therapy also require further evaluation.
Case Continued
The patient reports that her periods are regular, with a cycle length of 30 to 31 days. She usually notes some bloating and breast tenderness in the days leading up to onset of menses. She experiences lower abdominal cramping during days 1–3 of her period. This has worsened somewhat over the last year, and sometimes radiates to her low back. Her reproductive history is significant for 3 uncomplicated vaginal deliveries and 1 first trimester spontaneous abortion. She did not experience postpartum hemorrhage, and has no history of significant oropharyngeal bleeding or unexplained bruising. Her BMI is 23.3. Her physical exam is unremarkable, including a normal thyroid, abdominal, bimanual and speculum exam. Laboratory evaluation demonstrates a low-normal hemoglobin, hematocrit, and MCV. The TSH is normal and a urine pregnancy test is negative. She had a normal pap smear and HPV assay 2 years ago.
What is the most likely diagnosis?
What treatment is recommended?
Oral tranexamic acid is an anti-fibrinolytic that was recently approved by the FDA for treatment of menorrhagia or heavy menstrual bleeding. It has been used for many years to prevent bleeding during surgery and to treat bleeding disorders. It has been used for over 30 years to treat menorrhagia in Europe. It has a different mechanism of action than NSAIDs and hormonal contraceptives, and is therefore an appropriate alternative for women who cannot tolerate other medication options [16,17].Tranexamic acid is contraindicated in women with an elevated risk of thromboembolic disease.
For women who have insufficient response to medical management or for women who present with more severe symptoms, anemia, or prominent bulk-related symptoms due to fibroids, gynecologic referral should be made for consideration of surgical intervention. The preferred interventional approach to the treatment of uterine fibroid tumors depends upon the type of fibroid (eg submucosal, intramural, subserosal), the number of fibroids, desire for future childbearing, risk for surgical complications, and patient preference. Effective options include myomectomy, uterine artery embolization, endometrial ablation, and hysterectomy [18].
By contrast, good evidence supports the use of medication as first-line therapy for heavy menstrual bleeding when it occurs in the setting of endometriosis. Estrogen-progestin oral contraceptive pills, oral progestins, and depot medroxyprogesterone have all been demonstrated to be effective in decreasing pain [19,20].The levonorgestrel-releasing intrauterine system is also effective in decreasing pain due to endometriosis [21].
Women who do not respond to first-line therapy should be referred to a gynecologist for consideration of other treatment options. Effective second-line treatment options include oral danazol, intramuscular GnRH agonists, and surgical approaches such as laparoscopic ablation and/or excision of endometriosis implants [22].
A similar range of treatment options appears to be effective in the management of heavy menstrual bleeding due to adenomyosis. First-line therapies include oral NSAIDs, oral tranexamic acid, estrogen-progestin oral contraceptive pills, and the levonorgestrel-releasing intrauterine system [23,24].Women with an inadequate response to first-line treatment should be referred to a gynecologist for consideration definitive treatment with hysterectomy versus uterine artery embolization or a trial of a GnRH agonist [24].
For some women with heavy menstrual bleeding, no specific underlying cause is identified. Current evidence suggests that such patients may have disorders of local endometrial hemostasis leading to increased blood loss during otherwise normal menstrual cycles [25].The levonorgestrel-releasing intrauterine system may be the most effective medical therapy for heavy menstrual bleeding in the absence of a specific target lesion [26].For women wishing to avoid hormonal treatment, scheduled oral NSAIDs or oral tranexamic acid are inexpensive and effective options for reducing blood loss [27–29].Other medical treatment options include estrogen-progestin contraceptive pills, cyclic oral progestin, and depot medroxy-progesterone.
For patients who experience treatment failure with pharmaceutical therapy or who desire definitive treatment, both endometrial ablation and hysterectomy have been shown to be effective and associated with high rates of patient satisfaction [30].
Follow-up
The patient reports that she would like to avoid invasive testing if possible. Given her relatively low risk for endometrial cancer, she elects a trial of scheduled NSAIDs. Unfortunately, after a couple of cycles she reports that her heavy bleeding has not been well-controlled. A pelvic ultrasound demonstrates an anterior submucosal fibroid measuring 2.4 cm and a posterior intramural fibroid measuring 1.5 cm. She agrees to insertion of a levonorgestrel IUD and calls 6 months later to report a significant decrease in her bleeding.
Case 2—Anovulation
Initial Presentation
A 27-year-old female presents for pregnancy testing. She is 2 weeks late for her period. She and her husband are attempting pregnancy and she seems disappointed that the pregnancy test is negative. She is having trouble tracking her periods. Her cycles range from 24 to 45 days apart and often she skips cycles altogether. Her flow is scant at times but some months are heavy with soaking tampons/pads.
What are diagnostic considerations in evaluating this bleeding pattern?
Menstrual history can help differentiate between of ovulatory and anovulatory abnormal bleeding. Typically, anovulatory bleeding is marked by irregular or infrequent periods. Flow can be scant to excessive. Women experiencing anovulatory cycles may fail to notice common ovulation symptoms (thin watery cervical mucus) or pre-menstrual symptoms (breast tenderness) [31].
The International Federation of Gynecology and Obstetrics (FIGO) designates AUD-O as “abnormal uterine bleeding due to ovulatory dysfunction” or “anovulatory abnormal uterine bleeding” [7,31].In general, if women are having menses at regular cycles their bleeding is likely to be ovulatory.
Differential Diagnosis
Anovulatory bleeding may be physiologic. After menarche, the hypothalamic-pituitary-ovarian axis is immature. This may result in anovulatory cycles for 2 to 3 years. Women entering perimenopausal transition may also experience intermittent anovulation and subsequent abnormal uterine bleeding. Other physiologic examples include lactation and pregnancy [31].
Physical Examination
A thorough history will help to narrow the differential diagnosis. The physical exam can evaluate for other findings that indicate endocrine dysfunction such as low body weight, hirsutism, balding, acne, high blood pressure, obesity (especially centripetal fat distribution). Acanthosis nigricans is a sign of insulin resistance which is part of the pathophysiology of PCOS. The gynecologic exam is often unremarkable in AUB-O although a bimanual exam can reveal adnexal enlargement indicative of cystic ovaries. Of note, clitoromegally is not common in PCOS. This finding would increase the likelihood of other causes of hyperandrogenism [32].
What is the pathophysiologic basis for this patient’s bleeding pattern?
Pathophysiology of Anovulatory Bleeding
Anovulatory bleeding presumes that there is a normal anatomic and genetic makeup. For example, a woman without ovaries will be, by definition, anovulatory. Using current terminology anovulatory bleeding implies a disruption in the hypothalamic-pituitary-ovarian axis and is therefore primarily an endocrine disorder [31,33].
At the level of the ovary and uterus, anovulation results in prolonged estrogen effect on the endometrium. After ovulation, the corpus luteum produces progesterone which stops endometrial thickening and stabilizes the endometrium. Without ovulation, estrogen continues endometrial stimulation and excess proliferation of endometrial lining. The endometrium becomes unstable, undifferentiated, and sheds unpredictably. The blood vessels become larger, more tortuous and have increased fragility. The result is light or heavy menstrual bleeding, decreased frequency of periods but overall unpredictable menstrual bleeding [33].
Effects of Chronic Anovulation
Irregular cycles can be more than a mere inconvenience. Women who have anovulatory cycles associated with heavy menstrual bleeding are at risk for anemia. Anovulation that is a result of hyperandrogen state or other endocrine disorder has other health ramifications. Infertility and its treatment are common sequelae. Finally, over time, unopposed estrogen in anovulation increases the risk of endometrial hyperplasia, or cancer [7,34].
Case Continued
The patient reports menarche at age 12. Her periods were irregular for the first 1–2 years but became more regular after that. She has been taking combination oral contraceptives since the age of 20 to prevent pregnancy. She stopped this 1 year ago and she and her husband began actively trying to conceive 6 months ago. Her family history is notable for diabetes and hypertension in her father. Her mother had heavy periods leading up to menopause and had a hysterectomy with no malignancy at the age of 47. She has a BMI of 33; blood pressure is mildly elevated at 134/84 mm Hg. She has oily skin and acne along her chin and neck. She has mild hirsutism of her face. Otherwise her skin is normal appearing. She has an elevated waist circumference of 35 inches. The remainder of her exam is normal.
What is the likely diagnosis?
Women with PCOS are at increased risk for metabolic syndrome, nonalcoholic fatty liver disease, type 2 diabetes and cardiovascular disease, endometrial cancer, and infertility. Women with PCOS who become pregnant have increased risk of pregnancy complications such as hypertensive disorders and gestational diabetes.
What tests are indicated in this patient?
Appropriate laboratory testing is often determined based on findings in the history and physical as well as the patient’s age. Anovulation in the first 18 months to 3 years after menarche is common and testing for pregnancy, infection, and anemia are often sufficient. Menorrhagia in adolescents warrants testing for bleeding disorders as well [7].Within 3 years of menarche, menstrual cycles should become more regular. Persistent anovulatory cycles increase the likelihood of pathologic causes and warrant additional evaluation. Pregnancy testing, thyroid stimulation hormone and prolactin levels are recommended first line evaluation [7,32,34].If PCOS is suspected an ultrasound can be performed but as noted above, polycystic ovaries are not required to make the diagnosis after adolescence.
Additional testing includes testosterone levels to look for androgen secreting tumors. Late onset congenital adrenal hyperplasia is an uncommon cause of hyperandrogenism but is more common in women of Ashkenazi Jewish descent and those with a family history [34].Morning hydroxyprogesterone can be performed to evaluate for this. If women exhibit abrupt change in menstrual pattern and other signs of cortisol excess (hypertension, abdominal striae) 24-hour urine cortisol can detect Cushing’s syndrome [34].
In patients with PCOS, additional testing to evaluate for medical comorbidities is recommended. This includes screening for diabetes, dyslipidemia, and liver dysfunction.
Case Continued
The patient’s prolactin and TSH are normal. Tests for diabetes are normal. Her LDL is elevated to 162, triglycerides are 200, and her HDL is 38. The physician informs her that she meets criteria for PCOS and also that she has obesity and metabolic syndrome.
What factors should be considered when making treatment recommendations for this patient?
Treatment for anovulation is guided by the goals of therapy. Since anovulation is an endocrine abnormality, medical treatment is first line [31].If secondary causes are diagnosed, these should be treated first. Other goals of treatment can include reducing amount and irregularity of menstruation, provide contraception, increasing ovulation in women with desired fertility, and reducing androgenic sequelae such as acne and hirsutism.
When treating the irregular or heavy bleeding associated with anovulation, first-line treatment is exogenous hormone. This can be in the form of combined estrogen/progesterone formulations (pill, patch, and ring). Medroxyprogesterone (medroxyprogesterone acetate 5–10 mg daily) taken 10 to 14 days per month is another option. Standard consideration for medical eligibility in prescribing these agents should be considered (see U.S. medical eligibility criteria for contraceptive use available at www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm). Combined formulations offer contraception, while cyclic progesterone does not. Both offer cyclic withdrawal bleeding. A Cochrane review did not find any RCTs comparing one to the other and therefore either are reasonable options [36].The levonorgestrel IUD is effective at treating AUB as well [31].Women may still experience intermittent vaginal bleeding or amenorrhea so it is less likely to result in cyclic withdrawal bleeding.
All of the above treatments provide the additional benefit of thinning the endometrium and preventing unopposed estrogen effect. This provides further protection the endometrial hyperplasia with chronic anovulatory cycles and unopposed estrogen [31].
In women with PCOS and associated metabolic conditions, first-line treatment is weight loss and other lifestyle interventions to improve or prevent other sequelae of the condition. Weight loss has been shown to reduce circulating androgen levels and increase ovulation. It has been shown to reduce glucose and lipid levels and hirsutism. Pregnancy rates increase as well. Weight loss achieved through medications and gastric bypass has similar effects. There is no special diet that has been shown to be more effective than another [32]. As little as a 5% weight reduction from baseline can improve PCOS symptoms [34,35].
Metformin is also commonly added to lifestyle modifications in women with PCOS to reduce risks for developing diabetes. There is little high quality evidence of added benefit above lifestyle modifications [34].Statin therapy can be considered in women with hyperlipidemia and PCOS [32].
For women with PCOS who desire to conceive, treatment should target increased ovulation. Pre-conception counseling and lifestyle modifications are again first line [32].Ovulation induction interventions carry increased risk of multiple gestation. For ovulation induction, clomiphene citrate is first line therapy. Metformin is commonly used as noted above to improve comorbidities associated with PCOS and can increase ovulation compared to placebo [37].However, RCTs do not support its use as first-line treatment of infertility treatment in PCOS. Clomiphene is 3 times more effective than metformin alone [32].
Medications can improve but often do not resolve hirsutism in women with PCOS. Combined hormonal contraceptives are commonly used off-label and no one type of pill has been shown to be superior. Anti-androgens are also off-label but empirically used. They can also improve lipid and other metabolic variables. They are all teratogenic and therefore should not be used in women who desire conception, and be used with effective contraceptives. Spironolactone is an androgen receptor antagonist. It takes months for effect. Some women will have improved menstrual frequency with this medication as well. Often adjunctive therapy such as eflornithine facial cream or laser therapy or a combination is needed to further treat hirsutism [32,35,38].
Follow-up
After discussion, the patient decides to adopt therapeutic lifestyle changes. She desires to get pregnant and does not opt for hormonal contraceptives at this time. She sees a nutritionist and begins calorie restriction and exercise. Three months later she has lost 20 pounds and feels “healthier.” Her lipid panel shows LDL of 125 and HDL of 43. Her triglycerides are now 160. Her blood pressure in the office is 118/78 mm Hg. She has lost “inches” around her middle. She has had more regular periods as well. She is still not pregnant so the physician asks her to begin tracking ovulation with cervical mucus evaluation and basal body temperature prior to considering further infertility evaluations. Three months after that she misses a period but is pleased to report a positive home pregnancy test.
Case 3—Breakthough Bleeding On Combined Hormonal Contraceptives
A 28-year-old G0P0 in a monogamous relationship presents to her physician. She has been on oral contraceptive pills for 8 years. For the last 3 years she has been taking the pills on an extended cycle schedule. She normally takes an active pill daily for 3 cycles of pills (9 weeks), and then takes a 7-day pill-free week when she gets a menstrual period. This had been working fine until the last 6 months. She has noticed breakthrough spotting up to 2 weeks at a time during the 2nd and 3rd pack of pills.
What is the approach to evaluation and treatment in this patient?
Bleeding in Women on Combined Hormonal Contraception
Many women are now using combined hormonal contraceptives on different schedules. Extended-cycle contraception has been shown to be as effective as the traditional 21/7 schedule of active pills/pill-free week. The FDA has approved several packaged extended-cycle contraceptives. Extended-cycle contraception decreases overall number of bleeding days and improves many menstrual-related symptoms [39].Breakthrough bleeding is the most common side effect of extended cycle contraception. It is classified as AUB-I (abnormal uterine bleeding—iatrogenic). It is most common in the first few months of use, and decreases as use continues. Up to 86% of women will have unscheduled bleeding during the first 3 months of use of extended cycle contraception, but this bleedingdecreases as use continues [40].
There is no consensus as to the underlying mechanism causing this abnormal bleeding. Most clinicians believe that it is related to the balance of estrogen/progestin in each combined hormonal contraceptive. Each woman reacts differently to this combination, making it difficult to predict who will have abnormal bleeding. In women who are beginning an extended-cycle regimen, reassurance is sufficient. Most abnormal bleeding will normalize within the first 2 to 3 months. Missed pills and smoking are consistently related to breakthrough bleeding in women who take combined oral contraceptive pills [41].In women who have previously had stable bleeding patterns and who present with new breakthrough bleeding, evaluation for secondary causes of bleeding may be considered (ie, urine hCG, TSH, STI cultures, evaluation for cervical cancer screening). A pelvic examination may help determine a possible secondary cause of bleeding, but is not necessary.
Treatment of unscheduled bleeding in women on extended-cycle contraception includes shortening the hormone-free interval and adding medications for prevention/treatment of bleeding episodes. The 7-day hormone-free interval in the context of low-dose hormonal contraception may be too long. One study demonstrated that a 7-day hormone-free interval was associated with a lack of pituitary-ovarian suppression, follicular development, and possible ovulation [42].A systematic review found that shortened hormone-free intervals decreased the amount of unscheduled bleeding [39].A small RCT (65 women) of continuous contraceptive ring users found that the group that removed the ring for 4 days during an episode of unscheduled bleeding, and then reinserted it had overall reduction in unscheduled bleeding [43].Some clinicians will also recommend trying a different pill formlation or a different schedule. There is no evidence to support this recommendation, but it can be helpful in some women.
Low-dose doxycycline (40 mg daily) for prevention of unscheduled bleeding shows promise [44].This low- dose doxycycline is also helpful to prevent more unscheduled bleeding in extended-cycle oral contraceptive users [44].However, an RCT found that traditional-dose doxycline (100 mg BID) taken for 5 days at the onset of a bleeding episode, did not decrease the amount or length of unscheduled bleeding [40].Neither estrogen dose [45]nor progestin dose [45]affected bleeding patterns. There is some suggestion based on a small study that women on pills with norethindrone may have less unscheduled bleeding than those who are on pills with levonorgestrel, but more research needs to be done before clinicians change practice [46].A Cochrane review looked at one small study that suggested third-generation progestins had more favorable bleeding profiles than second-generation progestins [47].
Follow-up
The physician investigates for secondary causes of the bleeding. The patient’s urine hCG, TSH, and prolactin levels are all normal. No fibroids or polyps are seen on ultrasound. The physician and patient discuss treatment options, including a low-dose doxycycline pill to help minimize bleeding, trying a different pill formulation, or use of naproxen during the bleeding episodes, but the patient does not want to take 2 pills every day. After further discussion, the patient decides she would like to change to the contraceptive ring with the plan of removing the ring for 4 days at the onset of any unscheduled bleeding. In a phone call 6 months later, the patient states that her unscheduled bleeding has been controlled.
Corresponding author: Sarina Schrager, MD, MS, Dept. of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Ct., Madison, WI 53715, [email protected].
1. Singh S, Best C, Dunn S, et al; Society of Obstetricians and Gynaecologists of Canada. Abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can 2013 May;35:473-9.
2. Nicholson WR, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Ob Gyn 2001;184:523-30.
3. Matteson KA, Raker CA, Clark MA, Frick KD. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Wom Health 2013;22:959-65.
4. Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183-94.
5. Goodman A. Abnormal genital tract bleeding. Clin Cornerstone 2000;3:25-35.
6. Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol 1999;82:73-6.
7. ACOG Practice Bulletin No. 128. Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol 2012;120:197-206.
8. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007; 87:725-736.
9. Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008;115:1382-91.
10. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril 2012;98(3):572-9.
11. Krassas GE, Pontikides N, Kaltsas T, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol 1999;50:655-9.
12. Knol HM, Mulder AB, Bogchelman DH, et al. The prevalence of underlying bleeding disorders in patients with heavy menstrual bleeding with and without gynecologic abnormalities. Am J Obstet Gynecol 2013;209:202.e1-7.
13. Kelekci S, Kaya E, Alan M, et al. Comparison of transvaginal sonography, saline infusion sonography, and office hysteroscopy in reproductive-aged women with or without abnormal uterine bleeding. Fertil Steril 2005;84:682–6.
14. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010;82:41-55.
15. Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BWJ. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Systematic Rev 2013, Issue 2.
16. Phillip CS. Antifibrinolytics in women with menorrhagia. Thrombosis Research 2011;127(Sup 3):S113-S115.
17. Hrometz SL. Oral modified release tranexamic acid for heavy menstrual bleeding. Ann Pharmacother 2012;46:1047-53.
18. NICE clinical guidelines. Heavy menstrual bleeding. London: National Institute for Health and Care Excellence.
19. Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD001019.
20. Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev. 2012 Mar 14;3:CD002122.
21. Management of endometriosis. Practice Bulletin No. 114. American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;116:223-36.
22. B. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2014, Issue 3.
23. Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006 Aug;20:603-16.
24. Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009;79:189-93.
25. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet 2011;113:3–13.
26. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37.
27. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database System Rev 2013;1: CD000400.
28. Naoulou BB, MC Ming C Tsai. Efficacy of tranexamic acid in the treatment of idiopathic and non-functional heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand 2012;91:529-37.
29. Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:865-75.
30. Fergusson RJ, Lethaby A, Shepperd, S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding Cochrane Database System Rev 2013;11:CD000329.
31. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin 136. Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol 2013;122:176-85.
32. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. Polycystic ovary syndrome. Obstet Gynecol 2009;114 :936-49.
33. Livinstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Human Reproduction Update 2002;8:60-67.
34. Setji TL, Brown AJ. Polycystic ovary syndrome: diagnosis and treatment. Am J Med 2007;120: 128-132.
35. Cahill D. PCOS. Clinical Evidence. BMJ Publishing Group. 2009;01:1-45.
36. Hickey M, Higam JM, Fraser I. Progestogens with our without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst Reviews 2012;9:CD001895.
37. Nothinagle M, Scott-Taylor J. Does metformin improve clinical features of polycystic ovary syndrome? Cochrane for clinicians: putting evidence into practice. Am Fam Physician 2003;68:2163-4.
38. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Systematic Rev 2009;(4): CD002249.
39. Godfrey EM, Whiteman MK, Curtis KM. Treatment of unscheduled bleeding in women using extended- or continuous-use combined hormonal contraception: a systematic review. Contraception 2013;87:567-75.
40. Kaneshiro B, Edelman A, Carlson N, et al. Treatment of unscheduled bleeding in continuous oral contraceptive users with doxycycline: a randomized controlled trial. Obstet &Gynecol 2010;115 :1141-9.
41. Grossman MP, Nakajima SP. Menstrual cycle bleeding patterns in smokers. Contraception 2006;73:562-5.
42. Schlaff WD, Lynch Am, Hughes HD, et al. Manipulation of the pill-free interval in oral contraceptive pill users: the effects on follicular suppression. Am J Obstet Gynecol 2004;190:943-51.
43. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol 2008;112:563-71.
44. Kaneshiro B, Edelman A, Carlson NE, et al. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception 2012;85:351-8.
45. Kaneshiro B, Edelman A, Carlson NE, et al. Unscheduled bleeding with continuous oral contraceptive pills: a comparison of progestin dose. Contraception 2012;86:22-
46. Edelman AB, Koontz SL, Nichols MD, Jensen JT. Continuous oral contraceptives: are bleeding patterns dependent on the hormones given? Obstet Gynecol 2006;107:657-65.
47. Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev 2011; (5):CD0004681.
1. Singh S, Best C, Dunn S, et al; Society of Obstetricians and Gynaecologists of Canada. Abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can 2013 May;35:473-9.
2. Nicholson WR, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Ob Gyn 2001;184:523-30.
3. Matteson KA, Raker CA, Clark MA, Frick KD. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Wom Health 2013;22:959-65.
4. Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183-94.
5. Goodman A. Abnormal genital tract bleeding. Clin Cornerstone 2000;3:25-35.
6. Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol 1999;82:73-6.
7. ACOG Practice Bulletin No. 128. Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol 2012;120:197-206.
8. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007; 87:725-736.
9. Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008;115:1382-91.
10. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril 2012;98(3):572-9.
11. Krassas GE, Pontikides N, Kaltsas T, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol 1999;50:655-9.
12. Knol HM, Mulder AB, Bogchelman DH, et al. The prevalence of underlying bleeding disorders in patients with heavy menstrual bleeding with and without gynecologic abnormalities. Am J Obstet Gynecol 2013;209:202.e1-7.
13. Kelekci S, Kaya E, Alan M, et al. Comparison of transvaginal sonography, saline infusion sonography, and office hysteroscopy in reproductive-aged women with or without abnormal uterine bleeding. Fertil Steril 2005;84:682–6.
14. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010;82:41-55.
15. Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BWJ. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Systematic Rev 2013, Issue 2.
16. Phillip CS. Antifibrinolytics in women with menorrhagia. Thrombosis Research 2011;127(Sup 3):S113-S115.
17. Hrometz SL. Oral modified release tranexamic acid for heavy menstrual bleeding. Ann Pharmacother 2012;46:1047-53.
18. NICE clinical guidelines. Heavy menstrual bleeding. London: National Institute for Health and Care Excellence.
19. Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD001019.
20. Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev. 2012 Mar 14;3:CD002122.
21. Management of endometriosis. Practice Bulletin No. 114. American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;116:223-36.
22. B. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2014, Issue 3.
23. Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006 Aug;20:603-16.
24. Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009;79:189-93.
25. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet 2011;113:3–13.
26. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37.
27. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database System Rev 2013;1: CD000400.
28. Naoulou BB, MC Ming C Tsai. Efficacy of tranexamic acid in the treatment of idiopathic and non-functional heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand 2012;91:529-37.
29. Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:865-75.
30. Fergusson RJ, Lethaby A, Shepperd, S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding Cochrane Database System Rev 2013;11:CD000329.
31. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin 136. Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol 2013;122:176-85.
32. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. Polycystic ovary syndrome. Obstet Gynecol 2009;114 :936-49.
33. Livinstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Human Reproduction Update 2002;8:60-67.
34. Setji TL, Brown AJ. Polycystic ovary syndrome: diagnosis and treatment. Am J Med 2007;120: 128-132.
35. Cahill D. PCOS. Clinical Evidence. BMJ Publishing Group. 2009;01:1-45.
36. Hickey M, Higam JM, Fraser I. Progestogens with our without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst Reviews 2012;9:CD001895.
37. Nothinagle M, Scott-Taylor J. Does metformin improve clinical features of polycystic ovary syndrome? Cochrane for clinicians: putting evidence into practice. Am Fam Physician 2003;68:2163-4.
38. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Systematic Rev 2009;(4): CD002249.
39. Godfrey EM, Whiteman MK, Curtis KM. Treatment of unscheduled bleeding in women using extended- or continuous-use combined hormonal contraception: a systematic review. Contraception 2013;87:567-75.
40. Kaneshiro B, Edelman A, Carlson N, et al. Treatment of unscheduled bleeding in continuous oral contraceptive users with doxycycline: a randomized controlled trial. Obstet &Gynecol 2010;115 :1141-9.
41. Grossman MP, Nakajima SP. Menstrual cycle bleeding patterns in smokers. Contraception 2006;73:562-5.
42. Schlaff WD, Lynch Am, Hughes HD, et al. Manipulation of the pill-free interval in oral contraceptive pill users: the effects on follicular suppression. Am J Obstet Gynecol 2004;190:943-51.
43. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol 2008;112:563-71.
44. Kaneshiro B, Edelman A, Carlson NE, et al. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception 2012;85:351-8.
45. Kaneshiro B, Edelman A, Carlson NE, et al. Unscheduled bleeding with continuous oral contraceptive pills: a comparison of progestin dose. Contraception 2012;86:22-
46. Edelman AB, Koontz SL, Nichols MD, Jensen JT. Continuous oral contraceptives: are bleeding patterns dependent on the hormones given? Obstet Gynecol 2006;107:657-65.
47. Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev 2011; (5):CD0004681.
Delusional and aggressive, while playing the lottery
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
Do glutamatergic drugs have a role in treating depression?
Mrs. S, age 46, has been struggling to manage depression for 7 years. She completed adequate trials of several selective serotonin reuptake inhibitors and bupropion. Currently, she is taking duloxetine, 60 mg/d, and aripiprazole, 5 mg/d.
At her most recent clinic visit, Mrs. S reports that she is doing “OK,” but that she still feels sad and disengaged most days of the week. She wants to know more about ketamine for treating depression after reading about it on the Internet and hearing it mentioned in a support group she attends. She asks if you think it would work for her, and gives you with a copy of an article about its use in patients with treatment-resistant depression. Mrs. S has no other health conditions and takes a daily vitamin D and calcium supplement.
The monoamine hypothesis of depression postulates that symptoms originate from underactivity of monoamines, such as serotonin, norepinephrine, and dopamine, in the brain. This hypothesis was formulated in the 1960s after researchers observed that monoamine oxidase inhibitors and tricyclic antidepressants relieved depressive symptoms; both were known to increase monoamine concentrations in the synaptic cleft.1
Regrettably, these medications do not adequately relieve depressive symptoms for many people. In fact, symptom remission occurs in only one-third of treated patients.2 This low remission rate reflects a lack of understanding of the pathophysiology of depression, and the need for drugs with unique mechanisms of action.
One of the newest drug targets shown to be relevant in psychiatric illness is the
glutamatergic system. Glutamate is the predominant excitatory neurotransmitter in the CNS, and it is responsible for many key functions, including synaptic plasticity, learning, memory, and locomotion.3 Normally, the glutamatergic system tightly regulates the amount of glutamate in the neuronal synapse via receptors on presynaptic and postsynaptic neurons, as well as on glial cells (Figure). When this equilibrium is disrupted in stressful situations, such as ischemia, trauma, or seizures, excess glutamate is released into the synapse. The resulting glutamatergic hyperactivity can lead to neurotoxicity and cell death when neuronal receptors are activated for an extended period.
A key component of the glutamatergic system that is responsible for removing excess glutamate from the synapse is membrane-bound transporters, which are similar to serotonin and norepinephrine transporters. These excitatory amino acid transporters (EAATs) are important because glutamate metabolism does not occur within the synapse and EAATS are responsible for removing most of the glutamate from the synapse into glial cells.3
The network of receptors within the synapse that are activated by glutamate is extensive and complex. There are at least 11 glutamate-responsive receptors: 3 are ionotropic action channels, and the remaining 8 are metabotropic G protein-coupled receptors. Previous studies have shown regional changes in glutamate receptors, as well as elevated levels of glutamate, in the brains of patients with major depressive disorder (MDD).4
Ketamine. The ionotropic receptor N-methyl-d-aspartate (NMDA) is one of the most studied glutamate receptors. Pharmacologically, ketamine is a noncompetitive NMDA receptor antagonist that also activates the amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, which is another subtype of ionotropic glutamate receptors. In open-label clinical trials, ketamine has demonstrated rapid antidepressant action in patients with treatment-resistant MDD.4,5
Recently, Murrough et al6 performed the first randomized, psychoactive controlled trial using a single IV infusion of ketamine dosed below anesthesia ranges (0.5 mg/kg), or midazolam (0.045 mg/kg), in patients with treatment-resistant depression who had been antidepressant-free for at least 4 weeks. They found that 24 hours after medication administration, the likelihood of response to ketamine was significantly higher than the response to midazolam (OR: 2.18; 95% CI: 1.21 to 4.14), with a response rate of 64% in the ketamine group and 28% in the midazolam group.6
Psychotropic side effects, such as hallucinations, are a major concern with ketamine tolerability and abuse potential. This is largely because of ketamine’s antagonism of the NMDA receptor, which is a property shared with other abused drugs such as phencyclidine (PCP) and dextromethorphan. In the Murrough et al6 study, there were no reported cases of paranoia or hallucinations, but dissociative symptoms were relatively common (17%).
Although the results in this trial appear encouraging, there are several limitations to using ketamine to treat MDD, especially in an ambulatory setting. Concerns include ketamine’s IV administration, potential for abuse, long-term efficacy, and side-effect profile—particularly psychotic symptoms and hemodynamic changes. An ideal compound would have the rapid efficacy of ketamine, but with a safer side-effect profile, easier administration, and less potential for abuse.
Riluzole also acts on the glutamatergic system, but has not shown antidepressant efficacy as consistently as ketamine. Riluzole is FDA-approved for treating amyotrophic lateral sclerosis.5 Pharmacologically, riluzole is a glutamatergic modulator that increases glutamate reuptake into glial cells, decreases glutamate release, and increases AMPA trafficking. In open-label studies riluzole has shown efficacy in reducing depressive symptoms.4,5 However, when compared with placebo as a means of sustaining treatment response after a 1-time dose of ketamine, riluzole showed was no significant improvement in time to depressive relapse.7
Acamprosate, often used for treating alcohol abuse, is another a drug with glutamatergic activity that has been studied for possible use as an antidepressant.5
A review by Lapidus et al5 has a more extensive listing of current medications and investigational compounds that modulate glutamate transmission, and are of interest for their possible antidepressant activity. Given the relatively new “glutamatergic hypothesis” of depression, it is exciting that so many current and novel glutamatergic drug therapies are being evaluated.
Future of ketamine treatment
Glutamate has been shown to play an important part in the pathophysiology of depression. The rapid antidepressant efficacy of ketamine provides evidence that future medications with glutamate-modulating activity could be useful for patients who struggle to achieve symptom relief using available antidepressants. Several limitations exist regarding ketamine use, and more work in this important therapeutic area needs to be done. This last point is important to remember when speaking with patients such as Mrs. S. Although it is understandable for her to be excited about novel treatment options such as ketamine, stress to her that treating depression with ketamine at this time is strictly investigational, and that the drug needs to be thoroughly evaluated for safety and efficacy before it can be prescribed for this indication.
CASE CONTINUED
Mrs. S realizes that ketamine may not be the best next step for her, and she agrees to explore other approaches to treat her residual depressive symptoms.
Related Resources
• Machado-Vieira R, Ibrahim L, Henter ID, et al. Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol Biochem Behav. 2012;100(4):678-687.
• Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72(10):1313-1333.
Drug Brand Names
Acamprosate • Campral Duloxetine • Cymbalta
Aripiprazole • Abilify Ketamine • Ketalar
Bupropion • Wellbutrin, Zyban Riluzole • Rilutek
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. Niciu MJ, Ionescu DF, Richards EM, et al. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm. 2014;121(8):907-924.
2. Gaynes BN, Dusetzina SB, Ellis AR, et al. Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32(1):114-119.
3. Curry SC, Mills KC, Ruha A, et al. Neurotransmitters and neuromodulators. In: Nelson LS, Lewin NA, Howland MA, et al, eds. Goldfrank’s toxicologic emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:189-220.
4. Zarate C Jr, Machado-Vieira R, Henter I, et al. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18(5):293-303.
5. Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr Dis Treat. 2013;9:1101-1112.
6. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
7. Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37(6):1526-1533.
Mrs. S, age 46, has been struggling to manage depression for 7 years. She completed adequate trials of several selective serotonin reuptake inhibitors and bupropion. Currently, she is taking duloxetine, 60 mg/d, and aripiprazole, 5 mg/d.
At her most recent clinic visit, Mrs. S reports that she is doing “OK,” but that she still feels sad and disengaged most days of the week. She wants to know more about ketamine for treating depression after reading about it on the Internet and hearing it mentioned in a support group she attends. She asks if you think it would work for her, and gives you with a copy of an article about its use in patients with treatment-resistant depression. Mrs. S has no other health conditions and takes a daily vitamin D and calcium supplement.
The monoamine hypothesis of depression postulates that symptoms originate from underactivity of monoamines, such as serotonin, norepinephrine, and dopamine, in the brain. This hypothesis was formulated in the 1960s after researchers observed that monoamine oxidase inhibitors and tricyclic antidepressants relieved depressive symptoms; both were known to increase monoamine concentrations in the synaptic cleft.1
Regrettably, these medications do not adequately relieve depressive symptoms for many people. In fact, symptom remission occurs in only one-third of treated patients.2 This low remission rate reflects a lack of understanding of the pathophysiology of depression, and the need for drugs with unique mechanisms of action.
One of the newest drug targets shown to be relevant in psychiatric illness is the
glutamatergic system. Glutamate is the predominant excitatory neurotransmitter in the CNS, and it is responsible for many key functions, including synaptic plasticity, learning, memory, and locomotion.3 Normally, the glutamatergic system tightly regulates the amount of glutamate in the neuronal synapse via receptors on presynaptic and postsynaptic neurons, as well as on glial cells (Figure). When this equilibrium is disrupted in stressful situations, such as ischemia, trauma, or seizures, excess glutamate is released into the synapse. The resulting glutamatergic hyperactivity can lead to neurotoxicity and cell death when neuronal receptors are activated for an extended period.
A key component of the glutamatergic system that is responsible for removing excess glutamate from the synapse is membrane-bound transporters, which are similar to serotonin and norepinephrine transporters. These excitatory amino acid transporters (EAATs) are important because glutamate metabolism does not occur within the synapse and EAATS are responsible for removing most of the glutamate from the synapse into glial cells.3
The network of receptors within the synapse that are activated by glutamate is extensive and complex. There are at least 11 glutamate-responsive receptors: 3 are ionotropic action channels, and the remaining 8 are metabotropic G protein-coupled receptors. Previous studies have shown regional changes in glutamate receptors, as well as elevated levels of glutamate, in the brains of patients with major depressive disorder (MDD).4
Ketamine. The ionotropic receptor N-methyl-d-aspartate (NMDA) is one of the most studied glutamate receptors. Pharmacologically, ketamine is a noncompetitive NMDA receptor antagonist that also activates the amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, which is another subtype of ionotropic glutamate receptors. In open-label clinical trials, ketamine has demonstrated rapid antidepressant action in patients with treatment-resistant MDD.4,5
Recently, Murrough et al6 performed the first randomized, psychoactive controlled trial using a single IV infusion of ketamine dosed below anesthesia ranges (0.5 mg/kg), or midazolam (0.045 mg/kg), in patients with treatment-resistant depression who had been antidepressant-free for at least 4 weeks. They found that 24 hours after medication administration, the likelihood of response to ketamine was significantly higher than the response to midazolam (OR: 2.18; 95% CI: 1.21 to 4.14), with a response rate of 64% in the ketamine group and 28% in the midazolam group.6
Psychotropic side effects, such as hallucinations, are a major concern with ketamine tolerability and abuse potential. This is largely because of ketamine’s antagonism of the NMDA receptor, which is a property shared with other abused drugs such as phencyclidine (PCP) and dextromethorphan. In the Murrough et al6 study, there were no reported cases of paranoia or hallucinations, but dissociative symptoms were relatively common (17%).
Although the results in this trial appear encouraging, there are several limitations to using ketamine to treat MDD, especially in an ambulatory setting. Concerns include ketamine’s IV administration, potential for abuse, long-term efficacy, and side-effect profile—particularly psychotic symptoms and hemodynamic changes. An ideal compound would have the rapid efficacy of ketamine, but with a safer side-effect profile, easier administration, and less potential for abuse.
Riluzole also acts on the glutamatergic system, but has not shown antidepressant efficacy as consistently as ketamine. Riluzole is FDA-approved for treating amyotrophic lateral sclerosis.5 Pharmacologically, riluzole is a glutamatergic modulator that increases glutamate reuptake into glial cells, decreases glutamate release, and increases AMPA trafficking. In open-label studies riluzole has shown efficacy in reducing depressive symptoms.4,5 However, when compared with placebo as a means of sustaining treatment response after a 1-time dose of ketamine, riluzole showed was no significant improvement in time to depressive relapse.7
Acamprosate, often used for treating alcohol abuse, is another a drug with glutamatergic activity that has been studied for possible use as an antidepressant.5
A review by Lapidus et al5 has a more extensive listing of current medications and investigational compounds that modulate glutamate transmission, and are of interest for their possible antidepressant activity. Given the relatively new “glutamatergic hypothesis” of depression, it is exciting that so many current and novel glutamatergic drug therapies are being evaluated.
Future of ketamine treatment
Glutamate has been shown to play an important part in the pathophysiology of depression. The rapid antidepressant efficacy of ketamine provides evidence that future medications with glutamate-modulating activity could be useful for patients who struggle to achieve symptom relief using available antidepressants. Several limitations exist regarding ketamine use, and more work in this important therapeutic area needs to be done. This last point is important to remember when speaking with patients such as Mrs. S. Although it is understandable for her to be excited about novel treatment options such as ketamine, stress to her that treating depression with ketamine at this time is strictly investigational, and that the drug needs to be thoroughly evaluated for safety and efficacy before it can be prescribed for this indication.
CASE CONTINUED
Mrs. S realizes that ketamine may not be the best next step for her, and she agrees to explore other approaches to treat her residual depressive symptoms.
Related Resources
• Machado-Vieira R, Ibrahim L, Henter ID, et al. Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol Biochem Behav. 2012;100(4):678-687.
• Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72(10):1313-1333.
Drug Brand Names
Acamprosate • Campral Duloxetine • Cymbalta
Aripiprazole • Abilify Ketamine • Ketalar
Bupropion • Wellbutrin, Zyban Riluzole • Rilutek
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Mrs. S, age 46, has been struggling to manage depression for 7 years. She completed adequate trials of several selective serotonin reuptake inhibitors and bupropion. Currently, she is taking duloxetine, 60 mg/d, and aripiprazole, 5 mg/d.
At her most recent clinic visit, Mrs. S reports that she is doing “OK,” but that she still feels sad and disengaged most days of the week. She wants to know more about ketamine for treating depression after reading about it on the Internet and hearing it mentioned in a support group she attends. She asks if you think it would work for her, and gives you with a copy of an article about its use in patients with treatment-resistant depression. Mrs. S has no other health conditions and takes a daily vitamin D and calcium supplement.
The monoamine hypothesis of depression postulates that symptoms originate from underactivity of monoamines, such as serotonin, norepinephrine, and dopamine, in the brain. This hypothesis was formulated in the 1960s after researchers observed that monoamine oxidase inhibitors and tricyclic antidepressants relieved depressive symptoms; both were known to increase monoamine concentrations in the synaptic cleft.1
Regrettably, these medications do not adequately relieve depressive symptoms for many people. In fact, symptom remission occurs in only one-third of treated patients.2 This low remission rate reflects a lack of understanding of the pathophysiology of depression, and the need for drugs with unique mechanisms of action.
One of the newest drug targets shown to be relevant in psychiatric illness is the
glutamatergic system. Glutamate is the predominant excitatory neurotransmitter in the CNS, and it is responsible for many key functions, including synaptic plasticity, learning, memory, and locomotion.3 Normally, the glutamatergic system tightly regulates the amount of glutamate in the neuronal synapse via receptors on presynaptic and postsynaptic neurons, as well as on glial cells (Figure). When this equilibrium is disrupted in stressful situations, such as ischemia, trauma, or seizures, excess glutamate is released into the synapse. The resulting glutamatergic hyperactivity can lead to neurotoxicity and cell death when neuronal receptors are activated for an extended period.
A key component of the glutamatergic system that is responsible for removing excess glutamate from the synapse is membrane-bound transporters, which are similar to serotonin and norepinephrine transporters. These excitatory amino acid transporters (EAATs) are important because glutamate metabolism does not occur within the synapse and EAATS are responsible for removing most of the glutamate from the synapse into glial cells.3
The network of receptors within the synapse that are activated by glutamate is extensive and complex. There are at least 11 glutamate-responsive receptors: 3 are ionotropic action channels, and the remaining 8 are metabotropic G protein-coupled receptors. Previous studies have shown regional changes in glutamate receptors, as well as elevated levels of glutamate, in the brains of patients with major depressive disorder (MDD).4
Ketamine. The ionotropic receptor N-methyl-d-aspartate (NMDA) is one of the most studied glutamate receptors. Pharmacologically, ketamine is a noncompetitive NMDA receptor antagonist that also activates the amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, which is another subtype of ionotropic glutamate receptors. In open-label clinical trials, ketamine has demonstrated rapid antidepressant action in patients with treatment-resistant MDD.4,5
Recently, Murrough et al6 performed the first randomized, psychoactive controlled trial using a single IV infusion of ketamine dosed below anesthesia ranges (0.5 mg/kg), or midazolam (0.045 mg/kg), in patients with treatment-resistant depression who had been antidepressant-free for at least 4 weeks. They found that 24 hours after medication administration, the likelihood of response to ketamine was significantly higher than the response to midazolam (OR: 2.18; 95% CI: 1.21 to 4.14), with a response rate of 64% in the ketamine group and 28% in the midazolam group.6
Psychotropic side effects, such as hallucinations, are a major concern with ketamine tolerability and abuse potential. This is largely because of ketamine’s antagonism of the NMDA receptor, which is a property shared with other abused drugs such as phencyclidine (PCP) and dextromethorphan. In the Murrough et al6 study, there were no reported cases of paranoia or hallucinations, but dissociative symptoms were relatively common (17%).
Although the results in this trial appear encouraging, there are several limitations to using ketamine to treat MDD, especially in an ambulatory setting. Concerns include ketamine’s IV administration, potential for abuse, long-term efficacy, and side-effect profile—particularly psychotic symptoms and hemodynamic changes. An ideal compound would have the rapid efficacy of ketamine, but with a safer side-effect profile, easier administration, and less potential for abuse.
Riluzole also acts on the glutamatergic system, but has not shown antidepressant efficacy as consistently as ketamine. Riluzole is FDA-approved for treating amyotrophic lateral sclerosis.5 Pharmacologically, riluzole is a glutamatergic modulator that increases glutamate reuptake into glial cells, decreases glutamate release, and increases AMPA trafficking. In open-label studies riluzole has shown efficacy in reducing depressive symptoms.4,5 However, when compared with placebo as a means of sustaining treatment response after a 1-time dose of ketamine, riluzole showed was no significant improvement in time to depressive relapse.7
Acamprosate, often used for treating alcohol abuse, is another a drug with glutamatergic activity that has been studied for possible use as an antidepressant.5
A review by Lapidus et al5 has a more extensive listing of current medications and investigational compounds that modulate glutamate transmission, and are of interest for their possible antidepressant activity. Given the relatively new “glutamatergic hypothesis” of depression, it is exciting that so many current and novel glutamatergic drug therapies are being evaluated.
Future of ketamine treatment
Glutamate has been shown to play an important part in the pathophysiology of depression. The rapid antidepressant efficacy of ketamine provides evidence that future medications with glutamate-modulating activity could be useful for patients who struggle to achieve symptom relief using available antidepressants. Several limitations exist regarding ketamine use, and more work in this important therapeutic area needs to be done. This last point is important to remember when speaking with patients such as Mrs. S. Although it is understandable for her to be excited about novel treatment options such as ketamine, stress to her that treating depression with ketamine at this time is strictly investigational, and that the drug needs to be thoroughly evaluated for safety and efficacy before it can be prescribed for this indication.
CASE CONTINUED
Mrs. S realizes that ketamine may not be the best next step for her, and she agrees to explore other approaches to treat her residual depressive symptoms.
Related Resources
• Machado-Vieira R, Ibrahim L, Henter ID, et al. Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol Biochem Behav. 2012;100(4):678-687.
• Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72(10):1313-1333.
Drug Brand Names
Acamprosate • Campral Duloxetine • Cymbalta
Aripiprazole • Abilify Ketamine • Ketalar
Bupropion • Wellbutrin, Zyban Riluzole • Rilutek
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. Niciu MJ, Ionescu DF, Richards EM, et al. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm. 2014;121(8):907-924.
2. Gaynes BN, Dusetzina SB, Ellis AR, et al. Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32(1):114-119.
3. Curry SC, Mills KC, Ruha A, et al. Neurotransmitters and neuromodulators. In: Nelson LS, Lewin NA, Howland MA, et al, eds. Goldfrank’s toxicologic emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:189-220.
4. Zarate C Jr, Machado-Vieira R, Henter I, et al. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18(5):293-303.
5. Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr Dis Treat. 2013;9:1101-1112.
6. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
7. Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37(6):1526-1533.
1. Niciu MJ, Ionescu DF, Richards EM, et al. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm. 2014;121(8):907-924.
2. Gaynes BN, Dusetzina SB, Ellis AR, et al. Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32(1):114-119.
3. Curry SC, Mills KC, Ruha A, et al. Neurotransmitters and neuromodulators. In: Nelson LS, Lewin NA, Howland MA, et al, eds. Goldfrank’s toxicologic emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:189-220.
4. Zarate C Jr, Machado-Vieira R, Henter I, et al. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18(5):293-303.
5. Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr Dis Treat. 2013;9:1101-1112.
6. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
7. Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37(6):1526-1533.
Management of Plasma Cell Disorders
The plasma cell disorders are a spectrum of conditions that include asymptomatic precursor conditions—monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM)—as well as symptomatic multiple myeloma (MM) and solitary plasmacytoma. Other plasma cell disorders include immunoglobulin light chain amyloidosis and POEMS syndrome, which are characterized by a unique set of end-organ manifestations. There are other related plasma cell and B-cell proliferations, such as light chain deposition disease and cryoglobulinemia, that are beyond the scope of this review but are relevant to the hematologist/oncologist and have been reviewed in detail elsewhere.
To read the full article in PDF:
The plasma cell disorders are a spectrum of conditions that include asymptomatic precursor conditions—monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM)—as well as symptomatic multiple myeloma (MM) and solitary plasmacytoma. Other plasma cell disorders include immunoglobulin light chain amyloidosis and POEMS syndrome, which are characterized by a unique set of end-organ manifestations. There are other related plasma cell and B-cell proliferations, such as light chain deposition disease and cryoglobulinemia, that are beyond the scope of this review but are relevant to the hematologist/oncologist and have been reviewed in detail elsewhere.
To read the full article in PDF:
The plasma cell disorders are a spectrum of conditions that include asymptomatic precursor conditions—monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM)—as well as symptomatic multiple myeloma (MM) and solitary plasmacytoma. Other plasma cell disorders include immunoglobulin light chain amyloidosis and POEMS syndrome, which are characterized by a unique set of end-organ manifestations. There are other related plasma cell and B-cell proliferations, such as light chain deposition disease and cryoglobulinemia, that are beyond the scope of this review but are relevant to the hematologist/oncologist and have been reviewed in detail elsewhere.
To read the full article in PDF:
Young, pregnant, ataxic—and jilted
CASE Difficulty walking
Ms. M, age 15, is a pregnant, Spanish-speaking Guatemalan woman who is brought to obstetrics triage in a large academic medical center at 35 weeks gestational age. She complains of dizziness, tinnitus, left orbital headache, and difficulty walking.
The neurology service finds profound truncal ataxia, astasia-abasia, and buckling of the knees; a normal brain and spine MRI are not consistent with a neurologic etiology. Otolaryngology service evaluates Ms. M to rule out a cholesteatoma and suggests a head CT and endoscopy, which are normal.
Ms. M’s symptoms resolve after 3 days, although the gait disturbances persist. When no clear cause is found for her difficulty walking, the psychiatry service is consulted to evaluate whether an underlying psychiatric disorder is contributing to symptoms.
What could be causing Ms. M’s symptoms?
a) malingering
b) factitious disorder
c) undiagnosed neurologic disorder
d) conversion disorder
The authors’ observations
Women are vulnerable to a variety of psychiatric illnesses during pregnancy1 that have deleterious effects on mother, baby, and family.2-6 Although there is a burgeoning literature on affective and anxiety disorders occurring in pregnancy, there is a dearth of information about somatoform disorders.
HISTORY Abandonment
Ms. M reports that, although her boyfriend deserted her after learning about the unexpected pregnancy, she will welcome the baby and looks forward to motherhood. She seems unaware of the responsibilities of being a mother.
Ms. M acknowledges a history of depression and self-harm a few years earlier, yet says she feels better now and thinks that psychiatric care is unnecessary. Because she does not endorse a history of trauma or symptoms suggesting an affective, anxiety, or psychotic illness, the psychiatrist does not recommend treatment with psychotropic medication.
At age 5, Ms. M’s parents sent her to the United States with her aunt, hoping that she would have a better life than she would have had in Guatemala. Her aunt reports that Ms. M initially had difficulty adjusting to life in the United States without her parents, yet she has made substantial strides over the years and is now quite accustomed to the country. Her aunt describes Ms. M as an independent high school student who earns good grades.
During the interview, the psychiatrist observes that Ms. M exhibits childlike mannerisms, including sleeping with stuffed toys and coloring in Disney books with crayons. She also is indifferent to her gait difficulty, pregnancy, and psychosocial stressors. Her affect is inconsistent with the content of her speech and she is alexithymic.
Ms. M’s aunt reports that her niece is becoming more dependent on her, which is not consistent with her baseline. Her aunt also notes that several years earlier, Ms. M’s nephew was diagnosed with a cholesteatoma after he presented with similar symptoms.
The combination of (1) Ms. M’s clinical presentation, which was causing her significant impairment in her social functioning, (2) the incompatibility of symptoms with any recognized neurologic and medical disease, and (3) prior family experience with cholesteatoma leads the consulting psychiatrist to suspect conversion disorder. Ms. M’s alexithymia, indifference to her symptoms, and recent abandonment by the baby’s father also support a conversion disorder diagnosis.
From a psychodynamic perspective, the ataxia appears to be her way of protecting herself from the abandonment she is experiencing by being left again to “stand alone” by her boyfriend as she had been when her parents sent her to the United States. Her regressive behavior could be her way of securing her aunt’s love and support.
The authors’ observations
This is the first case of psychogenic gait disturbance during pregnancy described in the literature. Authors have reported on pseudotoxemia,7 hyperemesis gravidarum,8 and pesudocyesis,9 yet there is a paucity of information on psychogenic gait disturbance during pregnancy. Ms. M’s case elucidates many of the clinical quandaries that occur when managing psychiatric illness—and, more specifically, conversion disorder— during pregnancy. Many women are hesitant to seek psychiatric treatment during pregnancy because of shame, stigma, and fear of loss of personal or parental rights10,11; it is not surprising that emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms.
Likely diagnosis
Conversion disorder is the presence of neurologic symptoms in the absence of a neurologic diagnosis that fully explains those symptoms. Conversion disorder, previously known as hysteria, is called functional neurologic symptom disorder in DSM-5 (Box).12 Symptoms are not feigned; instead, they represent “conversion” of emotional distress into neurologic symptoms.13,14 Although misdiagnosing conversion disorder in patients with true neurologic disease is uncommon, clinicians often are uncomfortable making the diagnosis until all medical causes have been ruled out.14 It is not always possible to find a psychological explanation for conversion disorder, but a history of childhood abuse, particularly sexual abuse, could play a role.14
Because of the variety of presentations, clinicians in all specialties should be familiar with somatoform disorders; this is especially important in obstetrics and gynecology because women are more likely than men to develop these disorders.15 It is important to consider that Ms. M is a teenager and somatoform disorders can present differently in adults. The diagnostic process should include a diligent somatic workup and a personal and social history to identify the patient’s developmental tasks, stressors, and coping style.15
How would you treat Ms. M?
a) destigmatize psychiatric illness and provide psychoeducation regarding treatment benefits
b) identify and treat any comorbid psychiatric disorders
c) maintain a proactive and multidisciplinary approach that includes assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy
d) all of the above
TREATMENT Close follow-up
The psychiatrist recommends continued close psychiatric follow-up as well as multidisciplinary involvement, including physical therapy, neurology, and obstetrics.
Ms. M initially is resistant to psychiatric follow-up because she says that “people on the street” told her that, if she saw a psychiatrist, her baby would be taken away. After the psychiatrist explains that it is unlikely her baby would be taken away, Ms. M immediately appears relieved, smiles, and readily agrees to outpatient psychotherapy.
Over the next 24 hours, she continues to work with a physical therapist and her gait significantly improves. She is discharged home 2 days later with a walking aid (Zimmer frame) for assistance.
Four days later, however, Ms. M is readmitted with worsening ataxia. Her aunt reports that, at home, Ms. M’s regressed behaviors are worsening; she is sleeping in bed with her and had several episodes of enuresis at home.
Ms. M continues to deny psychiatric symptoms or anxiety about the delivery. Although she shows some improvement when working with physical therapists, they note that Ms. M is still unable to ambulate or stand on her own. The psychiatrist is increasingly concerned about her regressed behavior and continued ataxia.
A family meeting is held and the psychiatrist and social worker educate Ms. M and her aunt about conversion disorder, including how some emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms and how that may apply to Ms. M. During the meeting, the team also destigmatizes psychiatric illness and treatment and provides psychoeducation regarding its benefits. The psychiatrist and social worker also provide a psychodynamic interpretation that her ataxia could be a way of protecting herself against the abandonment she is experiencing by being left to “stand alone” by her boyfriend— as she had been when her parents sent her to the United States, and that her behavior could be her way of securing her aunt’s love and support.Ms. M and her aunt both readily agree with this interpretation. The aunt notes that her niece is more anxious about motherhood than she acknowledges and is concerned that Ms. M expects her to be the primary caregiver for the baby. Those present note that Ms. M is becoming increasingly dependent on her aunt, and that it is important for her to retain her independence, especially once she becomes a mother.
Ms. M immediately begins to display more affect; she smiles and reports feeling relieved. Similar to the previous admission, her gait significantly improves over the next 2 days and she is discharged home with a walking aid.
The authors’ observations
A broad differential diagnosis and early multidisciplinary involvement might facilitate earlier diagnosis and treatment.16 Assessment of psychosocial stressors in the patient’s personal and family life, including circumstances around the pregnancy and the meaning of motherhood, as well as investigation of what the patient may gain from the sick role, are paramount. In Ms. M’s case, cultural background, separation from her parents at a young age, and recent abandonment by her boyfriend have contributed to her inability to “stand alone,” which manifested as ataxia. Young age, regressed behavior, and her minimization of stressors also point to her difficulty acknowledging and coping with psychosocial stressors.
Successful delivery of the diagnosis is key to treatment success. After building a therapeutic alliance, a multidisciplinary discussion should take place that allows the patient to understand the diagnosis and treatment plan.17,18 The patient and family should be reassured that the fetus is healthy and all organic causes of symptoms have been investigated.17 Although management of conversion disorder during pregnancy is similar to that in non-pregnant women, several additional avenues of investigation should be considered:
• Explore the psychodynamic basis of the disorder and the role of the pregnancy and motherhood.
• Identify any comorbid psychiatric disorders, particularly those specific to pregnancy or the postpartum period.
• Because of the shame and stigma associated with seeking psychiatric treatment during pregnancy,10,11 it is imperative to destigmatize treatment and provide psychoeducation regarding its benefits.
A treatment plan can then be developed that involves psychotherapy, psychoeducation, stress management, and, when appropriate, pharmacotherapy.17
Providing psychoeducation about postpartum depression and other perinatal psychiatric illness could be beneficial. Physical therapy often is culturally acceptable and can help re-establish healthy patterns of motor function.19 Ms. M’s gait showed some improvement with physical therapy as part of the multidisciplinary approach, which also should include a thorough medical workup. Appropriate psychiatric treatment can help patients give up the sick role and return to their previous level of functioning.17
Maintain close communication with the outpatient perinatal care team as they monitor the patient’s parenting capacity. The outpatient perinatal care team also should engage pregnant or postpartum women in prioritizing their emotional well-being and encourage outpatient mental health treatment. Despite a dearth of data on the regressive symptoms and prognosis for future pregnancies, it is important to monitor maternal capacity and discuss the possibility of symptom recurrence.
OUTCOME Healthy baby
Three days later, Ms. M returns in labor with improved gait yet still using a walking aid. She has a normal vaginal delivery of a healthy baby boy at 37 weeks’ gestational age.
After the birth, Ms. M reports feeling well and enjoying motherhood, and denies psychiatric symptoms. She is ambulating without assistance within hours of delivery. This spontaneous resolution of symptoms could have been because of the psychodynamically oriented multidisciplinary approach to her care, which may have helped her realize that she did not have to “stand alone” as she embarked on motherhood.
Before being discharged home, Ms. M and her aunt meet with the inpatient obstetric social worker to assess Ms. M’s ability to care for the baby and discuss the importance of continued emotional support. The social worker does not contact the Department of Children and Families because Ms. M is walking independently and not endorsing or exhibiting regressive behaviors. Ms. M also reports that she will ask her aunt to take care of the baby should ataxia recur. Her aunt reassures the social workers that she will encourage Ms. M to attend outpatient psychotherapy and will contact the social worker if she becomes concerned about Ms. M’s or the baby’s well-being.
During her postpartum obstetric visit, Ms. M is walking independently and does not exhibit or endorse neurologic symptoms. The social worker provides psychoeducation about the importance of outpatient psychotherapy and schedules an initial appointment; Ms. M does not attend outpatient psychotherapy after discharge.
Bottom Line
Consider conversion disorder in obstetric patients who present with ataxia without a neurologic cause. Management involves a proactive and multidisciplinary approach that includes a thorough medical workup and assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy. Early identification and delivery of the diagnosis, destigmatization, and provision of appropriate psychiatric treatment can facilitate treatment success.
Disclosures
Dr. Byatt has received grant funding/support for this project from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Toor reports no financial relationships with any company whose products are mentioned in this article or manufacturers of competing products.
1. Vesga-Lopez O, Blanco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
2. Britton HL, Gronwaldt V, Britton JR. Maternal postpartum behaviors and mother-infant relationship during the first year of life. J Pediatr. 2001;138(6):905-909.
3. Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
4. Paulson JF, Keefe HA, Leiferman JA. Early parental depression and child language development. J Child Psychol Psychiatry. 2009;50(3):254-262.
5. Zuckerman B, Amaro H, Bauchner H, et al. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160(5 pt 1):1107-1111.
6. Forman DR, O’Hara MW, Stuart S, et al. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Dev Psychopathol. 2007;19(2):585-602.
7. Brady WJ Jr, Huff JS. Pseudotoxemia: new onset psychogenic seizure in third trimester pregnancy. J Emerg Med. 1997;15(6):815-820.
8. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis. 1990; 178(10):655-659.
9. Paulman PM, Sadat A. Pseudocyesis. J Fam Pract. 1990;30(5):575-576.
10. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment p: a qualitative systematic review. Birth. 2006;33(4):323-331.
11. Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstetr Gynaecol. 2012;33(4):143-161.
12. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
13. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183(8):915-920.
14. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
15. Bitzer J. Somatization disorders in obstetrics and gynecology. Arch Womens Mental health, 2003;6(2):99-107.
16. Smith HE, Rynning RE, Okafor C, et al. Evaluation of neurologic deficit without apparent cause: the importance of a multidisciplinary approach. J Spinal Cord Med. 2007;30(5):509-517.
17. Hinson VK, Haren WB. Psychogenic movement disorders. Lancet Neurol. 2006;5(8):695-700.
18. Oyama O, Paltoo C, Greengold J. Somatoform disorders. Am Fam Physician. 2007;76(9):1333-1338.
19. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31(1):30-39.
CASE Difficulty walking
Ms. M, age 15, is a pregnant, Spanish-speaking Guatemalan woman who is brought to obstetrics triage in a large academic medical center at 35 weeks gestational age. She complains of dizziness, tinnitus, left orbital headache, and difficulty walking.
The neurology service finds profound truncal ataxia, astasia-abasia, and buckling of the knees; a normal brain and spine MRI are not consistent with a neurologic etiology. Otolaryngology service evaluates Ms. M to rule out a cholesteatoma and suggests a head CT and endoscopy, which are normal.
Ms. M’s symptoms resolve after 3 days, although the gait disturbances persist. When no clear cause is found for her difficulty walking, the psychiatry service is consulted to evaluate whether an underlying psychiatric disorder is contributing to symptoms.
What could be causing Ms. M’s symptoms?
a) malingering
b) factitious disorder
c) undiagnosed neurologic disorder
d) conversion disorder
The authors’ observations
Women are vulnerable to a variety of psychiatric illnesses during pregnancy1 that have deleterious effects on mother, baby, and family.2-6 Although there is a burgeoning literature on affective and anxiety disorders occurring in pregnancy, there is a dearth of information about somatoform disorders.
HISTORY Abandonment
Ms. M reports that, although her boyfriend deserted her after learning about the unexpected pregnancy, she will welcome the baby and looks forward to motherhood. She seems unaware of the responsibilities of being a mother.
Ms. M acknowledges a history of depression and self-harm a few years earlier, yet says she feels better now and thinks that psychiatric care is unnecessary. Because she does not endorse a history of trauma or symptoms suggesting an affective, anxiety, or psychotic illness, the psychiatrist does not recommend treatment with psychotropic medication.
At age 5, Ms. M’s parents sent her to the United States with her aunt, hoping that she would have a better life than she would have had in Guatemala. Her aunt reports that Ms. M initially had difficulty adjusting to life in the United States without her parents, yet she has made substantial strides over the years and is now quite accustomed to the country. Her aunt describes Ms. M as an independent high school student who earns good grades.
During the interview, the psychiatrist observes that Ms. M exhibits childlike mannerisms, including sleeping with stuffed toys and coloring in Disney books with crayons. She also is indifferent to her gait difficulty, pregnancy, and psychosocial stressors. Her affect is inconsistent with the content of her speech and she is alexithymic.
Ms. M’s aunt reports that her niece is becoming more dependent on her, which is not consistent with her baseline. Her aunt also notes that several years earlier, Ms. M’s nephew was diagnosed with a cholesteatoma after he presented with similar symptoms.
The combination of (1) Ms. M’s clinical presentation, which was causing her significant impairment in her social functioning, (2) the incompatibility of symptoms with any recognized neurologic and medical disease, and (3) prior family experience with cholesteatoma leads the consulting psychiatrist to suspect conversion disorder. Ms. M’s alexithymia, indifference to her symptoms, and recent abandonment by the baby’s father also support a conversion disorder diagnosis.
From a psychodynamic perspective, the ataxia appears to be her way of protecting herself from the abandonment she is experiencing by being left again to “stand alone” by her boyfriend as she had been when her parents sent her to the United States. Her regressive behavior could be her way of securing her aunt’s love and support.
The authors’ observations
This is the first case of psychogenic gait disturbance during pregnancy described in the literature. Authors have reported on pseudotoxemia,7 hyperemesis gravidarum,8 and pesudocyesis,9 yet there is a paucity of information on psychogenic gait disturbance during pregnancy. Ms. M’s case elucidates many of the clinical quandaries that occur when managing psychiatric illness—and, more specifically, conversion disorder— during pregnancy. Many women are hesitant to seek psychiatric treatment during pregnancy because of shame, stigma, and fear of loss of personal or parental rights10,11; it is not surprising that emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms.
Likely diagnosis
Conversion disorder is the presence of neurologic symptoms in the absence of a neurologic diagnosis that fully explains those symptoms. Conversion disorder, previously known as hysteria, is called functional neurologic symptom disorder in DSM-5 (Box).12 Symptoms are not feigned; instead, they represent “conversion” of emotional distress into neurologic symptoms.13,14 Although misdiagnosing conversion disorder in patients with true neurologic disease is uncommon, clinicians often are uncomfortable making the diagnosis until all medical causes have been ruled out.14 It is not always possible to find a psychological explanation for conversion disorder, but a history of childhood abuse, particularly sexual abuse, could play a role.14
Because of the variety of presentations, clinicians in all specialties should be familiar with somatoform disorders; this is especially important in obstetrics and gynecology because women are more likely than men to develop these disorders.15 It is important to consider that Ms. M is a teenager and somatoform disorders can present differently in adults. The diagnostic process should include a diligent somatic workup and a personal and social history to identify the patient’s developmental tasks, stressors, and coping style.15
How would you treat Ms. M?
a) destigmatize psychiatric illness and provide psychoeducation regarding treatment benefits
b) identify and treat any comorbid psychiatric disorders
c) maintain a proactive and multidisciplinary approach that includes assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy
d) all of the above
TREATMENT Close follow-up
The psychiatrist recommends continued close psychiatric follow-up as well as multidisciplinary involvement, including physical therapy, neurology, and obstetrics.
Ms. M initially is resistant to psychiatric follow-up because she says that “people on the street” told her that, if she saw a psychiatrist, her baby would be taken away. After the psychiatrist explains that it is unlikely her baby would be taken away, Ms. M immediately appears relieved, smiles, and readily agrees to outpatient psychotherapy.
Over the next 24 hours, she continues to work with a physical therapist and her gait significantly improves. She is discharged home 2 days later with a walking aid (Zimmer frame) for assistance.
Four days later, however, Ms. M is readmitted with worsening ataxia. Her aunt reports that, at home, Ms. M’s regressed behaviors are worsening; she is sleeping in bed with her and had several episodes of enuresis at home.
Ms. M continues to deny psychiatric symptoms or anxiety about the delivery. Although she shows some improvement when working with physical therapists, they note that Ms. M is still unable to ambulate or stand on her own. The psychiatrist is increasingly concerned about her regressed behavior and continued ataxia.
A family meeting is held and the psychiatrist and social worker educate Ms. M and her aunt about conversion disorder, including how some emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms and how that may apply to Ms. M. During the meeting, the team also destigmatizes psychiatric illness and treatment and provides psychoeducation regarding its benefits. The psychiatrist and social worker also provide a psychodynamic interpretation that her ataxia could be a way of protecting herself against the abandonment she is experiencing by being left to “stand alone” by her boyfriend— as she had been when her parents sent her to the United States, and that her behavior could be her way of securing her aunt’s love and support.Ms. M and her aunt both readily agree with this interpretation. The aunt notes that her niece is more anxious about motherhood than she acknowledges and is concerned that Ms. M expects her to be the primary caregiver for the baby. Those present note that Ms. M is becoming increasingly dependent on her aunt, and that it is important for her to retain her independence, especially once she becomes a mother.
Ms. M immediately begins to display more affect; she smiles and reports feeling relieved. Similar to the previous admission, her gait significantly improves over the next 2 days and she is discharged home with a walking aid.
The authors’ observations
A broad differential diagnosis and early multidisciplinary involvement might facilitate earlier diagnosis and treatment.16 Assessment of psychosocial stressors in the patient’s personal and family life, including circumstances around the pregnancy and the meaning of motherhood, as well as investigation of what the patient may gain from the sick role, are paramount. In Ms. M’s case, cultural background, separation from her parents at a young age, and recent abandonment by her boyfriend have contributed to her inability to “stand alone,” which manifested as ataxia. Young age, regressed behavior, and her minimization of stressors also point to her difficulty acknowledging and coping with psychosocial stressors.
Successful delivery of the diagnosis is key to treatment success. After building a therapeutic alliance, a multidisciplinary discussion should take place that allows the patient to understand the diagnosis and treatment plan.17,18 The patient and family should be reassured that the fetus is healthy and all organic causes of symptoms have been investigated.17 Although management of conversion disorder during pregnancy is similar to that in non-pregnant women, several additional avenues of investigation should be considered:
• Explore the psychodynamic basis of the disorder and the role of the pregnancy and motherhood.
• Identify any comorbid psychiatric disorders, particularly those specific to pregnancy or the postpartum period.
• Because of the shame and stigma associated with seeking psychiatric treatment during pregnancy,10,11 it is imperative to destigmatize treatment and provide psychoeducation regarding its benefits.
A treatment plan can then be developed that involves psychotherapy, psychoeducation, stress management, and, when appropriate, pharmacotherapy.17
Providing psychoeducation about postpartum depression and other perinatal psychiatric illness could be beneficial. Physical therapy often is culturally acceptable and can help re-establish healthy patterns of motor function.19 Ms. M’s gait showed some improvement with physical therapy as part of the multidisciplinary approach, which also should include a thorough medical workup. Appropriate psychiatric treatment can help patients give up the sick role and return to their previous level of functioning.17
Maintain close communication with the outpatient perinatal care team as they monitor the patient’s parenting capacity. The outpatient perinatal care team also should engage pregnant or postpartum women in prioritizing their emotional well-being and encourage outpatient mental health treatment. Despite a dearth of data on the regressive symptoms and prognosis for future pregnancies, it is important to monitor maternal capacity and discuss the possibility of symptom recurrence.
OUTCOME Healthy baby
Three days later, Ms. M returns in labor with improved gait yet still using a walking aid. She has a normal vaginal delivery of a healthy baby boy at 37 weeks’ gestational age.
After the birth, Ms. M reports feeling well and enjoying motherhood, and denies psychiatric symptoms. She is ambulating without assistance within hours of delivery. This spontaneous resolution of symptoms could have been because of the psychodynamically oriented multidisciplinary approach to her care, which may have helped her realize that she did not have to “stand alone” as she embarked on motherhood.
Before being discharged home, Ms. M and her aunt meet with the inpatient obstetric social worker to assess Ms. M’s ability to care for the baby and discuss the importance of continued emotional support. The social worker does not contact the Department of Children and Families because Ms. M is walking independently and not endorsing or exhibiting regressive behaviors. Ms. M also reports that she will ask her aunt to take care of the baby should ataxia recur. Her aunt reassures the social workers that she will encourage Ms. M to attend outpatient psychotherapy and will contact the social worker if she becomes concerned about Ms. M’s or the baby’s well-being.
During her postpartum obstetric visit, Ms. M is walking independently and does not exhibit or endorse neurologic symptoms. The social worker provides psychoeducation about the importance of outpatient psychotherapy and schedules an initial appointment; Ms. M does not attend outpatient psychotherapy after discharge.
Bottom Line
Consider conversion disorder in obstetric patients who present with ataxia without a neurologic cause. Management involves a proactive and multidisciplinary approach that includes a thorough medical workup and assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy. Early identification and delivery of the diagnosis, destigmatization, and provision of appropriate psychiatric treatment can facilitate treatment success.
Disclosures
Dr. Byatt has received grant funding/support for this project from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Toor reports no financial relationships with any company whose products are mentioned in this article or manufacturers of competing products.
CASE Difficulty walking
Ms. M, age 15, is a pregnant, Spanish-speaking Guatemalan woman who is brought to obstetrics triage in a large academic medical center at 35 weeks gestational age. She complains of dizziness, tinnitus, left orbital headache, and difficulty walking.
The neurology service finds profound truncal ataxia, astasia-abasia, and buckling of the knees; a normal brain and spine MRI are not consistent with a neurologic etiology. Otolaryngology service evaluates Ms. M to rule out a cholesteatoma and suggests a head CT and endoscopy, which are normal.
Ms. M’s symptoms resolve after 3 days, although the gait disturbances persist. When no clear cause is found for her difficulty walking, the psychiatry service is consulted to evaluate whether an underlying psychiatric disorder is contributing to symptoms.
What could be causing Ms. M’s symptoms?
a) malingering
b) factitious disorder
c) undiagnosed neurologic disorder
d) conversion disorder
The authors’ observations
Women are vulnerable to a variety of psychiatric illnesses during pregnancy1 that have deleterious effects on mother, baby, and family.2-6 Although there is a burgeoning literature on affective and anxiety disorders occurring in pregnancy, there is a dearth of information about somatoform disorders.
HISTORY Abandonment
Ms. M reports that, although her boyfriend deserted her after learning about the unexpected pregnancy, she will welcome the baby and looks forward to motherhood. She seems unaware of the responsibilities of being a mother.
Ms. M acknowledges a history of depression and self-harm a few years earlier, yet says she feels better now and thinks that psychiatric care is unnecessary. Because she does not endorse a history of trauma or symptoms suggesting an affective, anxiety, or psychotic illness, the psychiatrist does not recommend treatment with psychotropic medication.
At age 5, Ms. M’s parents sent her to the United States with her aunt, hoping that she would have a better life than she would have had in Guatemala. Her aunt reports that Ms. M initially had difficulty adjusting to life in the United States without her parents, yet she has made substantial strides over the years and is now quite accustomed to the country. Her aunt describes Ms. M as an independent high school student who earns good grades.
During the interview, the psychiatrist observes that Ms. M exhibits childlike mannerisms, including sleeping with stuffed toys and coloring in Disney books with crayons. She also is indifferent to her gait difficulty, pregnancy, and psychosocial stressors. Her affect is inconsistent with the content of her speech and she is alexithymic.
Ms. M’s aunt reports that her niece is becoming more dependent on her, which is not consistent with her baseline. Her aunt also notes that several years earlier, Ms. M’s nephew was diagnosed with a cholesteatoma after he presented with similar symptoms.
The combination of (1) Ms. M’s clinical presentation, which was causing her significant impairment in her social functioning, (2) the incompatibility of symptoms with any recognized neurologic and medical disease, and (3) prior family experience with cholesteatoma leads the consulting psychiatrist to suspect conversion disorder. Ms. M’s alexithymia, indifference to her symptoms, and recent abandonment by the baby’s father also support a conversion disorder diagnosis.
From a psychodynamic perspective, the ataxia appears to be her way of protecting herself from the abandonment she is experiencing by being left again to “stand alone” by her boyfriend as she had been when her parents sent her to the United States. Her regressive behavior could be her way of securing her aunt’s love and support.
The authors’ observations
This is the first case of psychogenic gait disturbance during pregnancy described in the literature. Authors have reported on pseudotoxemia,7 hyperemesis gravidarum,8 and pesudocyesis,9 yet there is a paucity of information on psychogenic gait disturbance during pregnancy. Ms. M’s case elucidates many of the clinical quandaries that occur when managing psychiatric illness—and, more specifically, conversion disorder— during pregnancy. Many women are hesitant to seek psychiatric treatment during pregnancy because of shame, stigma, and fear of loss of personal or parental rights10,11; it is not surprising that emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms.
Likely diagnosis
Conversion disorder is the presence of neurologic symptoms in the absence of a neurologic diagnosis that fully explains those symptoms. Conversion disorder, previously known as hysteria, is called functional neurologic symptom disorder in DSM-5 (Box).12 Symptoms are not feigned; instead, they represent “conversion” of emotional distress into neurologic symptoms.13,14 Although misdiagnosing conversion disorder in patients with true neurologic disease is uncommon, clinicians often are uncomfortable making the diagnosis until all medical causes have been ruled out.14 It is not always possible to find a psychological explanation for conversion disorder, but a history of childhood abuse, particularly sexual abuse, could play a role.14
Because of the variety of presentations, clinicians in all specialties should be familiar with somatoform disorders; this is especially important in obstetrics and gynecology because women are more likely than men to develop these disorders.15 It is important to consider that Ms. M is a teenager and somatoform disorders can present differently in adults. The diagnostic process should include a diligent somatic workup and a personal and social history to identify the patient’s developmental tasks, stressors, and coping style.15
How would you treat Ms. M?
a) destigmatize psychiatric illness and provide psychoeducation regarding treatment benefits
b) identify and treat any comorbid psychiatric disorders
c) maintain a proactive and multidisciplinary approach that includes assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy
d) all of the above
TREATMENT Close follow-up
The psychiatrist recommends continued close psychiatric follow-up as well as multidisciplinary involvement, including physical therapy, neurology, and obstetrics.
Ms. M initially is resistant to psychiatric follow-up because she says that “people on the street” told her that, if she saw a psychiatrist, her baby would be taken away. After the psychiatrist explains that it is unlikely her baby would be taken away, Ms. M immediately appears relieved, smiles, and readily agrees to outpatient psychotherapy.
Over the next 24 hours, she continues to work with a physical therapist and her gait significantly improves. She is discharged home 2 days later with a walking aid (Zimmer frame) for assistance.
Four days later, however, Ms. M is readmitted with worsening ataxia. Her aunt reports that, at home, Ms. M’s regressed behaviors are worsening; she is sleeping in bed with her and had several episodes of enuresis at home.
Ms. M continues to deny psychiatric symptoms or anxiety about the delivery. Although she shows some improvement when working with physical therapists, they note that Ms. M is still unable to ambulate or stand on her own. The psychiatrist is increasingly concerned about her regressed behavior and continued ataxia.
A family meeting is held and the psychiatrist and social worker educate Ms. M and her aunt about conversion disorder, including how some emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms and how that may apply to Ms. M. During the meeting, the team also destigmatizes psychiatric illness and treatment and provides psychoeducation regarding its benefits. The psychiatrist and social worker also provide a psychodynamic interpretation that her ataxia could be a way of protecting herself against the abandonment she is experiencing by being left to “stand alone” by her boyfriend— as she had been when her parents sent her to the United States, and that her behavior could be her way of securing her aunt’s love and support.Ms. M and her aunt both readily agree with this interpretation. The aunt notes that her niece is more anxious about motherhood than she acknowledges and is concerned that Ms. M expects her to be the primary caregiver for the baby. Those present note that Ms. M is becoming increasingly dependent on her aunt, and that it is important for her to retain her independence, especially once she becomes a mother.
Ms. M immediately begins to display more affect; she smiles and reports feeling relieved. Similar to the previous admission, her gait significantly improves over the next 2 days and she is discharged home with a walking aid.
The authors’ observations
A broad differential diagnosis and early multidisciplinary involvement might facilitate earlier diagnosis and treatment.16 Assessment of psychosocial stressors in the patient’s personal and family life, including circumstances around the pregnancy and the meaning of motherhood, as well as investigation of what the patient may gain from the sick role, are paramount. In Ms. M’s case, cultural background, separation from her parents at a young age, and recent abandonment by her boyfriend have contributed to her inability to “stand alone,” which manifested as ataxia. Young age, regressed behavior, and her minimization of stressors also point to her difficulty acknowledging and coping with psychosocial stressors.
Successful delivery of the diagnosis is key to treatment success. After building a therapeutic alliance, a multidisciplinary discussion should take place that allows the patient to understand the diagnosis and treatment plan.17,18 The patient and family should be reassured that the fetus is healthy and all organic causes of symptoms have been investigated.17 Although management of conversion disorder during pregnancy is similar to that in non-pregnant women, several additional avenues of investigation should be considered:
• Explore the psychodynamic basis of the disorder and the role of the pregnancy and motherhood.
• Identify any comorbid psychiatric disorders, particularly those specific to pregnancy or the postpartum period.
• Because of the shame and stigma associated with seeking psychiatric treatment during pregnancy,10,11 it is imperative to destigmatize treatment and provide psychoeducation regarding its benefits.
A treatment plan can then be developed that involves psychotherapy, psychoeducation, stress management, and, when appropriate, pharmacotherapy.17
Providing psychoeducation about postpartum depression and other perinatal psychiatric illness could be beneficial. Physical therapy often is culturally acceptable and can help re-establish healthy patterns of motor function.19 Ms. M’s gait showed some improvement with physical therapy as part of the multidisciplinary approach, which also should include a thorough medical workup. Appropriate psychiatric treatment can help patients give up the sick role and return to their previous level of functioning.17
Maintain close communication with the outpatient perinatal care team as they monitor the patient’s parenting capacity. The outpatient perinatal care team also should engage pregnant or postpartum women in prioritizing their emotional well-being and encourage outpatient mental health treatment. Despite a dearth of data on the regressive symptoms and prognosis for future pregnancies, it is important to monitor maternal capacity and discuss the possibility of symptom recurrence.
OUTCOME Healthy baby
Three days later, Ms. M returns in labor with improved gait yet still using a walking aid. She has a normal vaginal delivery of a healthy baby boy at 37 weeks’ gestational age.
After the birth, Ms. M reports feeling well and enjoying motherhood, and denies psychiatric symptoms. She is ambulating without assistance within hours of delivery. This spontaneous resolution of symptoms could have been because of the psychodynamically oriented multidisciplinary approach to her care, which may have helped her realize that she did not have to “stand alone” as she embarked on motherhood.
Before being discharged home, Ms. M and her aunt meet with the inpatient obstetric social worker to assess Ms. M’s ability to care for the baby and discuss the importance of continued emotional support. The social worker does not contact the Department of Children and Families because Ms. M is walking independently and not endorsing or exhibiting regressive behaviors. Ms. M also reports that she will ask her aunt to take care of the baby should ataxia recur. Her aunt reassures the social workers that she will encourage Ms. M to attend outpatient psychotherapy and will contact the social worker if she becomes concerned about Ms. M’s or the baby’s well-being.
During her postpartum obstetric visit, Ms. M is walking independently and does not exhibit or endorse neurologic symptoms. The social worker provides psychoeducation about the importance of outpatient psychotherapy and schedules an initial appointment; Ms. M does not attend outpatient psychotherapy after discharge.
Bottom Line
Consider conversion disorder in obstetric patients who present with ataxia without a neurologic cause. Management involves a proactive and multidisciplinary approach that includes a thorough medical workup and assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy. Early identification and delivery of the diagnosis, destigmatization, and provision of appropriate psychiatric treatment can facilitate treatment success.
Disclosures
Dr. Byatt has received grant funding/support for this project from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Toor reports no financial relationships with any company whose products are mentioned in this article or manufacturers of competing products.
1. Vesga-Lopez O, Blanco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
2. Britton HL, Gronwaldt V, Britton JR. Maternal postpartum behaviors and mother-infant relationship during the first year of life. J Pediatr. 2001;138(6):905-909.
3. Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
4. Paulson JF, Keefe HA, Leiferman JA. Early parental depression and child language development. J Child Psychol Psychiatry. 2009;50(3):254-262.
5. Zuckerman B, Amaro H, Bauchner H, et al. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160(5 pt 1):1107-1111.
6. Forman DR, O’Hara MW, Stuart S, et al. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Dev Psychopathol. 2007;19(2):585-602.
7. Brady WJ Jr, Huff JS. Pseudotoxemia: new onset psychogenic seizure in third trimester pregnancy. J Emerg Med. 1997;15(6):815-820.
8. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis. 1990; 178(10):655-659.
9. Paulman PM, Sadat A. Pseudocyesis. J Fam Pract. 1990;30(5):575-576.
10. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment p: a qualitative systematic review. Birth. 2006;33(4):323-331.
11. Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstetr Gynaecol. 2012;33(4):143-161.
12. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
13. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183(8):915-920.
14. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
15. Bitzer J. Somatization disorders in obstetrics and gynecology. Arch Womens Mental health, 2003;6(2):99-107.
16. Smith HE, Rynning RE, Okafor C, et al. Evaluation of neurologic deficit without apparent cause: the importance of a multidisciplinary approach. J Spinal Cord Med. 2007;30(5):509-517.
17. Hinson VK, Haren WB. Psychogenic movement disorders. Lancet Neurol. 2006;5(8):695-700.
18. Oyama O, Paltoo C, Greengold J. Somatoform disorders. Am Fam Physician. 2007;76(9):1333-1338.
19. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31(1):30-39.
1. Vesga-Lopez O, Blanco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
2. Britton HL, Gronwaldt V, Britton JR. Maternal postpartum behaviors and mother-infant relationship during the first year of life. J Pediatr. 2001;138(6):905-909.
3. Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
4. Paulson JF, Keefe HA, Leiferman JA. Early parental depression and child language development. J Child Psychol Psychiatry. 2009;50(3):254-262.
5. Zuckerman B, Amaro H, Bauchner H, et al. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160(5 pt 1):1107-1111.
6. Forman DR, O’Hara MW, Stuart S, et al. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Dev Psychopathol. 2007;19(2):585-602.
7. Brady WJ Jr, Huff JS. Pseudotoxemia: new onset psychogenic seizure in third trimester pregnancy. J Emerg Med. 1997;15(6):815-820.
8. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis. 1990; 178(10):655-659.
9. Paulman PM, Sadat A. Pseudocyesis. J Fam Pract. 1990;30(5):575-576.
10. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment p: a qualitative systematic review. Birth. 2006;33(4):323-331.
11. Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstetr Gynaecol. 2012;33(4):143-161.
12. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
13. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183(8):915-920.
14. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
15. Bitzer J. Somatization disorders in obstetrics and gynecology. Arch Womens Mental health, 2003;6(2):99-107.
16. Smith HE, Rynning RE, Okafor C, et al. Evaluation of neurologic deficit without apparent cause: the importance of a multidisciplinary approach. J Spinal Cord Med. 2007;30(5):509-517.
17. Hinson VK, Haren WB. Psychogenic movement disorders. Lancet Neurol. 2006;5(8):695-700.
18. Oyama O, Paltoo C, Greengold J. Somatoform disorders. Am Fam Physician. 2007;76(9):1333-1338.
19. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31(1):30-39.
Metastatic Prostate Cancer: A Case Study
Prostate cancer remains the second leading cause of death in men in the United States as of 2012. It is estimated that prostate cancer affected more than 241,000 new men in 2012, with 15% of these patients presenting with advanced disease. As one would expect, compared to localized prostate cancer, metastatic disease remains the more challenging type to treat. In 1941 Huggins and Hodges demonstrated the dependence of prostatic tissues on androgens and from this work hormonal therapy was developed as the primary treatment for metastatic prostate cancer. Since then, significant progress has been made in the treatment of metastatic prostate cancer, including advances in androgen deprivation therapy and in the treatment of castrationresistant prostate cancer (CRPC), with many advances yet to come. CPRC has been an exciting topic for recent research and advancement, as our understanding of how prostate cancer utilizes very low levels of androgen has evolved considerably.
To read the full article in PDF:
Prostate cancer remains the second leading cause of death in men in the United States as of 2012. It is estimated that prostate cancer affected more than 241,000 new men in 2012, with 15% of these patients presenting with advanced disease. As one would expect, compared to localized prostate cancer, metastatic disease remains the more challenging type to treat. In 1941 Huggins and Hodges demonstrated the dependence of prostatic tissues on androgens and from this work hormonal therapy was developed as the primary treatment for metastatic prostate cancer. Since then, significant progress has been made in the treatment of metastatic prostate cancer, including advances in androgen deprivation therapy and in the treatment of castrationresistant prostate cancer (CRPC), with many advances yet to come. CPRC has been an exciting topic for recent research and advancement, as our understanding of how prostate cancer utilizes very low levels of androgen has evolved considerably.
To read the full article in PDF:
Prostate cancer remains the second leading cause of death in men in the United States as of 2012. It is estimated that prostate cancer affected more than 241,000 new men in 2012, with 15% of these patients presenting with advanced disease. As one would expect, compared to localized prostate cancer, metastatic disease remains the more challenging type to treat. In 1941 Huggins and Hodges demonstrated the dependence of prostatic tissues on androgens and from this work hormonal therapy was developed as the primary treatment for metastatic prostate cancer. Since then, significant progress has been made in the treatment of metastatic prostate cancer, including advances in androgen deprivation therapy and in the treatment of castrationresistant prostate cancer (CRPC), with many advances yet to come. CPRC has been an exciting topic for recent research and advancement, as our understanding of how prostate cancer utilizes very low levels of androgen has evolved considerably.
To read the full article in PDF:
He’s been making new ‘friends’
CASE Seeing friends
Mr. B, age 91, presents to the emergency room (ER) for hip pain. As he is being evaluated, he asks a nurse to tell the “other people” around her to leave so that he can have privacy. As clarification, Mr. B reports visual hallucinations, which prompts the ER physician to request a psychiatry consult.
Mr. B is alert and oriented to time, place, and person when he is evaluated by the on-call psychiatry resident. He reports that he has been seeing several unusual things for the last 4 to 5 months. Asked to elaborate, Mr. B admits seeing colorful and vivid images of people around him. These people come and go as they like; rarely, they talk to him. He describes the conversations as “a constant chatter” in the background and adds that it is difficult to understand what they are talking about.
Mr. B states that he has been “seeing” a couple of people on a regular basis, and they are “sort of like my friends.” He endorses that these people often sing songs or dance for him. He states that, sometimes, these “friends” bring 3 or 4 friends and, although he could not make out their faces clearly, “they all are around me.” He describes the people he sees as “nice people” and does not report being scared or frightened by them.
Mr. B does not report paranoia, and denies command-type hallucinations. He and his family report no unusual changes in behavior in recent months. The medical history is remarkable for atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, age-related macular degeneration, and glaucoma.
Mr. B denies having any ongoing mood or anxiety symptoms. He states that he knows these people are “probably not real,” and they do not bother him and just keep him company.
What could be causing Mr. B’s hallucinations?
a) a stroke
b) late-onset schizophrenia
c) dementia
d) Charles Bonnet syndrome
The authors’ observations
Visual hallucinations among geriatric pa-tients are a common and confusing presentation. In addition to several medical causes for this presentation (Table 1), consider Charles Bonnet syndrome in patients with visual loss, presenting as visual hallucinations with intact insight and absence of a mental illness. Other conditions to consider in the differential diagnosis include Parkinson’s disease, dementia with Lewy bodies, schizophrenia, seizures, migraine, and stroke, including lesions of the thalamus or brain stem.
Charles Bonnet syndrome was first described by Swiss philosopher Charles Bonnet in the 18th century. He reported vivid visual hallucinations in his visually impaired grandfather (bilateral cataracts).1
It is important to recognize this syndrome because patients can present across different specialties, including psychiatry, ophthalmology, neurology, geriatric medicine, and family medicine.2 As life expectancy increases, this condition might be seen more often. It is prudent to identify, intervene, and refer as appropriate, in addition to educating patients and caregivers about the nature and course of the condition.
EVALUATION Not psychotic
Mr. B reports good sleep and appetite. He denies using alcohol or illicit drugs. He states he slipped in the bathroom the day before coming to the ER, but denies other recent falls or injuries. Other than hip pain, he has no other physical complaints. His medication regimen includes aspirin, lisinopril, lovastatin, and metoprolol.
The ER team diagnoses a hip fracture. Mr. B is transferred to the orthopedic service; the psychiatry consult team continues to follow him. Mental status examination is unremarkable other than the visual hallucinations. His speech is clear, non-pressured, with goal-directed thought processing. Mini-Mental State Examination score is 23/30 with Mr. B having difficulty with object drawing and 3-object recall. Brief cognitive examination in the ER is unremarkable.
The orthopedic team decides on conservative management of the hip fracture. There is no evidence of infection. Mr. B is afebrile with clear sensorium; complete blood cell count and normal liver function tests are normal; urinalysis and urine drug screen are negative; and chest radiography is unremarkable. CT and MRI of the head are unremarkable.
After 1 week in the hospital, Mr. B continues to experience vivid visual imagery. No signs of active infection are found. An ophthalmologist is consulted, who confirms Mr. B’s earlier diagnosis of glaucoma and age-related macular degeneration but does not recommend further treatment. Visual field test by confrontation is normal, with normal visual reflexes.
The authors’ observations
The reported prevalence of Charles Bonnet syndrome among visually impaired people varies from study to study—from as low as 0.4% to as high as 63%.3-6 The reason for such variation can be attributed to several variables:
• underdiagnosis
• misdiagnosis
• underreporting by patients because of the benign nature of the hallucinations
• patients’ reluctance to report visual hallucinations because of fear of being labeled “mentally ill.”7,8
Symptoms
There are no specific diagnostic criteria for Charles Bonnet syndrome (Table 2). However, the following are generally accepted for diagnosis9:
• grossly intact cognition, although mild cognitive impairment may be present in some cases10
• underlying visual disorder, usually acquired, such as glaucoma, age-related macular degeneration, diabetic retinopathy, central retinal artery occlusion, and optic neuritis3,4,11
• no hallucinations or perceptive difficulties in other sensory modalities
• generally intact insight
• absence of delusions
• absence of other neurologic, psychiatric, toxic, or metabolic conditions; medical causes of delirium must be ruled out.
Hallucinations might not be disturbing to the patient. Hallucinations could be simple (light flashes, lines, or geometric shapes) or complex (faces, figures, or scenes),12 and perceived as in color or in black and white. Hallucinations mostly are pleasant and rarely have any emotional impact or meaning. Although hallucinations are almost exclusively visual, they can be accompanied by noise or auditory hallucinations.13,14
Other characteristics of Charles Bonnet syndrome include:
• typical age of onset is approximately 72 years (range, 70 to 92 years)
• no sex distinction has been identified
• episodes can last from a few seconds to few hours; the syndrome may last a few days or a few years5
• it is not uncommon for episodes to occur in clusters, followed by symptom-free intervals and recurrences
• symptoms tend to fade away as patients progress to complete loss of sight.15
The course of Charles Bonnet syndrome is uncertain and unpredictable and the episodic nature can be frustrating for both patient and clinician. The syndrome could be misdiagnosed as a psychiatric condition.
Pathophysiology
The precise mechanism behind simple or complex vivid hallucinations in persons with Charles Bonnet syndrome is unclear. Several theories have been proposed.
Release theory proposes a loss of input to the primary visual areas, which decreases cortical inhibition and further causes disinhibition of visual association areas, thereby “releasing” visual hallucinations.16 Research suggests that this might be an attempt by surviving neurons to recover vision. Loss of input somehow causes surviving neurons to adapt by increased sensitivity to residual visual stimuli.
Deafferentation theory. This relatively new theory proposes deafferentation of the visual sensory pathway, which, in turn, causes disinhibition of neurons in the visual cortical regions, thereby causing them to fire spontaneously. This could cause a sensation analogous to phantom limb pain, which would be called “phantom vision presence of brain activity in the absence of an actual visual input.” Further, biochemical and molecular changes have been proposed to explain the deafferentation theory.17
Neurobiological evidence. Limited data are available for a neurobiological basis to visual hallucinations in Charles Bonnet syndrome. A few studies have used functional MRI and single-photon emission CT and reported possible association of visual hallucinations to specific visual areas.18,19
Risk factors
Social or physical isolation, loneliness, low extraversion, and shyness are risk factors for Charles Bonnet syndrome in visually impaired people.20 Sensory deprivation and low level of arousal favor the occurrence of hallucinations.5 Rate of vision loss—not the nature of pathology or severity of visual impairment—has been suggested to increase the risk of developing Charles Bonnet syndrome.21
What are the treatment options for Charles Bonnet syndrome?
a) begin an antipsychotic
b) do nothing; there is no cure
c) educate the patient about the nature of the hallucinations
d) refer the patient to an ophthalmologist for evaluation of vision loss
Treatment
There are several modalities to manage visual hallucinations in a patient with Charles Bonnet syndrome (Table 3). After ruling out medical and other psychiatric causes of visual hallucinations, treatment might not be indicated if the patient is not disturbed by the hallucinations. In most cases, reassurance and educating the patient and family about the benign nature of the visual hallucinations is all that is needed.
For patients who are disturbed by these visions or for whom there is a treatable cause, treatment could include cataract removal, medical therapy to reduce intraocular pressure in glaucoma, treatment of diabetic retinopathy, or laser photocoagulation. These treatments are associated with a reduction in hallucinations.22
In some cases, hallucinations disappear as visual acuity deteriorates. Psychotropics have been used to treat Charles Bonnet syndrome, including:
• antipsychotics, including haloperidol, risperidone, and olanzapine
• anticonvulsants, including valproic acid, gabapentin, and carbamazepine
• antidepressants, including mirtazapine and venlafaxine.23-30
Some experts recommend a conservative approach, which might be justified because some cases of Charles Bonnet syndrome are episodic and remit spontaneously.31 Again, however, consider pharmacotherapy if a patient is disturbed by hallucinations or if hallucinations impair overall functioning.
TREATMENT Education
After discussion with Mr. B and his family, he is started on risperidone, 1 mg at bedtime, and the psychiatric team provides information about the nature of Charles Bonnet syndrome. Mr. B reportedly takes this medication for a few days and then stops because he does not want the visual hallucinations to go away.
The psychiatry team sees Mr. B before discharge. He and his family are educated about the benign nature of the syndrome, the need for continued family support, and the fact that hallucinations will have minimal or no implications for his life.
The authors’ observations
It is important to remember that a visual description of hallucinations in Charles Bonnet syndrome can be quite vivid, and that the patient might not identify his hallucinations as such or consider them as a problem. Be careful not to dismiss the patient’s complaints as a primary psychiatric condition. It also is important to be mindful of the patient’s concerns with a psychiatric diagnosis; detailed discussion with the patient is helpful in most cases. A more comprehensive and empathetic approach to care could go a long way to sustain quality of life for these patients.
Bottom Line
Charles Bonnet syndrome is characterized by visual hallucinations in patients with visual impairment who have intact insight and an absence of mental illness. Taking a thorough history can help rule out medical and psychiatric causes of visual hallucinations. Educate patients and family about the nature of the hallucinations. In some cases, a psychotropic may be indicated.
Related Resources
• Nguyen ND, Osterweil D, Hoffman J. Charles Bonnet syndrome: treating nonpsychiatric hallucinations. Consult Pharm. 2013;28(3):184-188.
• Lapid MI, Burton MC, Chang MT, et al. Clinical phenomenology and mortality in Charles Bonnet syndrome. J Geriatr Psychiatry Neurol. 2013;26(1):3-9.
Drug Brand Names
Carbamazepine • Tegretol Mirtazapine • Remeron
Gabapentin • Neurontin Olanzapine • Zyprexa
Haloperidol • Haldol Risperidone • Risperdal
Lisinopril • Prinivil, Zestril Valproic acid • Depakene
Lovastatin • Mevacor Venlafaxine • Effexor
Metoprolol • Lopressor
Acknowledgement
The authors acknowledge Barry Liskow, MD, Vice Chair of Psychiatry, Kansas University Medical Center, Kansas City, Kansas, for providing both insight into the topic and useful feedback on the manuscript.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bonnet C. Essai analytique sur les facultes de l’ame. Copenhagen, Denmark: Chez le Ferres CI. & Ant. Philibert; 1760:426-429.
2. Plummer C, Kleinitz A, Vroomen P, et al. Of Roman chariots and goats in overcoats: the syndrome of Charles Bonnet. J Clin Neurosci. 2007;14(8):709-714.
3. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
4. Tan CS, Lim VS, Ho DY, et al. Charles Bonnet syndrome in Asian patients in a tertiary ophthalmic centre. Br J Ophthalmol. 2004;88(10):1325-1329.
5. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Visual hallucinations in psychologically normal people: Charles Bonnet’s syndrome. Lancet. 1996;347(9004):794-797.
6. Menon GJ. Complex visual hallucinations in the visually impaired: a structured history-taking approach. Arch Ophthalmol. 2005;123(3):349-355.
7. Hart CT. Formed visual hallucinations: a symptom of cranial arteritis. Br Med J. 1967;3(5566):643-644.
8. Norton-Wilson L, Munir M. Visual perceptual disorders resembling the Charles Bonnet syndrome. A study of 434 consecutive patients referred to a psychogeriatric unit. Fam Pract. 1987;4(1):27-35.
9. Eperjesi F, Akbarali N. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
10. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients from an ophthalmology clinic and medical clinic population. J Nerv Ment Dis. 1994;182(5):273-276.
11. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(pt 10):1819-1840.
12. Kester EM. Charles Bonnet syndrome: case presentation and literature review. Optometry. 2009;80(7):360-366.
13. Hori H, Terao T, Nakamura JL. Charles Bonnet syndrome with auditory hallucinations: a diagnostic dilemma. Psychopathology. 2001;34(3):164-166.
14. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003;48(1):58-72.
15. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis. 1997;185(3):195-200.
16. Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973;188(2):139-150.
17. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
18. Ffytche DH, Howard RJ, Brammer MJ, et al. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1(8):738-742.
19. Adachi N, Watanabe T, Matsuda H, et al. Hyperperfusion in the lateral temporal cortex, the striatum and the thalamus during complex visual hallucinations: single photon emission computed tomography findings in patients with Charles Bonnet syndrome. Psychiatry Clin Neurosci. 2000;54(2):157-162.
20. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry. 1999;40(4):315-319.
21. Shiraishi Y, Terao T, Ibi K, et al. Charles Bonnet syndrome and visual acuity—the involvement of dynamic or acute sensory deprivation. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):362-364.
22. Tueth MJ, Cheong JA, Samander J. The Charles Bonnet syndrome: a type of organic visual hallucinosis. J Geriatr Psychiatry Neurol. 1995;8(1):1-3.
23. Nguyen H, Le C, Nguyen H. Charles Bonnet syndrome in an elderly patient concurrent with acute cerebellar infarction treated successfully with haloperidol. J Am Geriatr Soc. 2011;59(4):761-762.
24. Campbell JJ, Ngo G. Risperidone treatment of complex hallucinations in a patient with posterior cortical atrophy. J Neuropsychiatry Clin Neurosci. 2008;20(3):378-379.
25. Colletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
26. Jang JW, Youn YC, Seok JW, et al. Hypermetabolism in the left thalamus and right inferior temporal area on positron emission tomography-statistical parametric mapping (PET-SPM) in a patient with Charles Bonnet syndrome resolving after treatment with valproic acid. J Clin Neurosci. 2011;18(8):1130-1132.
27. Paulig M, Mentrup H. Charles Bonnet’s syndrome; Complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
28. Terao T. Effect of carbamazepine and clonazepam combination on Charles Bonnet syndrome: a case report. Hum Psychopharmacol. 1998;13(6):451-453.
29. Siddiqui Z, Ramaswmay S, Petty F. Mirtazapine for Charles Bonnet syndrome. Can J Psychiatry. 2004;49(11):787-788.
30. Lang UE, Stogowski D, Schulze D, et al. Charles Bonnet Syndrome: successful treatment of visual hallucinations due to vision loss with selective serotonin reuptake inhibitors. J Psychopharmacol. 2007;21(5):553-555.
31. Hartney KE, Catalano G, Catalano MC. Charles Bonnet syndrome: are medications necessary? J Psychiatr Pract. 2011;17(2):137-141.
CASE Seeing friends
Mr. B, age 91, presents to the emergency room (ER) for hip pain. As he is being evaluated, he asks a nurse to tell the “other people” around her to leave so that he can have privacy. As clarification, Mr. B reports visual hallucinations, which prompts the ER physician to request a psychiatry consult.
Mr. B is alert and oriented to time, place, and person when he is evaluated by the on-call psychiatry resident. He reports that he has been seeing several unusual things for the last 4 to 5 months. Asked to elaborate, Mr. B admits seeing colorful and vivid images of people around him. These people come and go as they like; rarely, they talk to him. He describes the conversations as “a constant chatter” in the background and adds that it is difficult to understand what they are talking about.
Mr. B states that he has been “seeing” a couple of people on a regular basis, and they are “sort of like my friends.” He endorses that these people often sing songs or dance for him. He states that, sometimes, these “friends” bring 3 or 4 friends and, although he could not make out their faces clearly, “they all are around me.” He describes the people he sees as “nice people” and does not report being scared or frightened by them.
Mr. B does not report paranoia, and denies command-type hallucinations. He and his family report no unusual changes in behavior in recent months. The medical history is remarkable for atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, age-related macular degeneration, and glaucoma.
Mr. B denies having any ongoing mood or anxiety symptoms. He states that he knows these people are “probably not real,” and they do not bother him and just keep him company.
What could be causing Mr. B’s hallucinations?
a) a stroke
b) late-onset schizophrenia
c) dementia
d) Charles Bonnet syndrome
The authors’ observations
Visual hallucinations among geriatric pa-tients are a common and confusing presentation. In addition to several medical causes for this presentation (Table 1), consider Charles Bonnet syndrome in patients with visual loss, presenting as visual hallucinations with intact insight and absence of a mental illness. Other conditions to consider in the differential diagnosis include Parkinson’s disease, dementia with Lewy bodies, schizophrenia, seizures, migraine, and stroke, including lesions of the thalamus or brain stem.
Charles Bonnet syndrome was first described by Swiss philosopher Charles Bonnet in the 18th century. He reported vivid visual hallucinations in his visually impaired grandfather (bilateral cataracts).1
It is important to recognize this syndrome because patients can present across different specialties, including psychiatry, ophthalmology, neurology, geriatric medicine, and family medicine.2 As life expectancy increases, this condition might be seen more often. It is prudent to identify, intervene, and refer as appropriate, in addition to educating patients and caregivers about the nature and course of the condition.
EVALUATION Not psychotic
Mr. B reports good sleep and appetite. He denies using alcohol or illicit drugs. He states he slipped in the bathroom the day before coming to the ER, but denies other recent falls or injuries. Other than hip pain, he has no other physical complaints. His medication regimen includes aspirin, lisinopril, lovastatin, and metoprolol.
The ER team diagnoses a hip fracture. Mr. B is transferred to the orthopedic service; the psychiatry consult team continues to follow him. Mental status examination is unremarkable other than the visual hallucinations. His speech is clear, non-pressured, with goal-directed thought processing. Mini-Mental State Examination score is 23/30 with Mr. B having difficulty with object drawing and 3-object recall. Brief cognitive examination in the ER is unremarkable.
The orthopedic team decides on conservative management of the hip fracture. There is no evidence of infection. Mr. B is afebrile with clear sensorium; complete blood cell count and normal liver function tests are normal; urinalysis and urine drug screen are negative; and chest radiography is unremarkable. CT and MRI of the head are unremarkable.
After 1 week in the hospital, Mr. B continues to experience vivid visual imagery. No signs of active infection are found. An ophthalmologist is consulted, who confirms Mr. B’s earlier diagnosis of glaucoma and age-related macular degeneration but does not recommend further treatment. Visual field test by confrontation is normal, with normal visual reflexes.
The authors’ observations
The reported prevalence of Charles Bonnet syndrome among visually impaired people varies from study to study—from as low as 0.4% to as high as 63%.3-6 The reason for such variation can be attributed to several variables:
• underdiagnosis
• misdiagnosis
• underreporting by patients because of the benign nature of the hallucinations
• patients’ reluctance to report visual hallucinations because of fear of being labeled “mentally ill.”7,8
Symptoms
There are no specific diagnostic criteria for Charles Bonnet syndrome (Table 2). However, the following are generally accepted for diagnosis9:
• grossly intact cognition, although mild cognitive impairment may be present in some cases10
• underlying visual disorder, usually acquired, such as glaucoma, age-related macular degeneration, diabetic retinopathy, central retinal artery occlusion, and optic neuritis3,4,11
• no hallucinations or perceptive difficulties in other sensory modalities
• generally intact insight
• absence of delusions
• absence of other neurologic, psychiatric, toxic, or metabolic conditions; medical causes of delirium must be ruled out.
Hallucinations might not be disturbing to the patient. Hallucinations could be simple (light flashes, lines, or geometric shapes) or complex (faces, figures, or scenes),12 and perceived as in color or in black and white. Hallucinations mostly are pleasant and rarely have any emotional impact or meaning. Although hallucinations are almost exclusively visual, they can be accompanied by noise or auditory hallucinations.13,14
Other characteristics of Charles Bonnet syndrome include:
• typical age of onset is approximately 72 years (range, 70 to 92 years)
• no sex distinction has been identified
• episodes can last from a few seconds to few hours; the syndrome may last a few days or a few years5
• it is not uncommon for episodes to occur in clusters, followed by symptom-free intervals and recurrences
• symptoms tend to fade away as patients progress to complete loss of sight.15
The course of Charles Bonnet syndrome is uncertain and unpredictable and the episodic nature can be frustrating for both patient and clinician. The syndrome could be misdiagnosed as a psychiatric condition.
Pathophysiology
The precise mechanism behind simple or complex vivid hallucinations in persons with Charles Bonnet syndrome is unclear. Several theories have been proposed.
Release theory proposes a loss of input to the primary visual areas, which decreases cortical inhibition and further causes disinhibition of visual association areas, thereby “releasing” visual hallucinations.16 Research suggests that this might be an attempt by surviving neurons to recover vision. Loss of input somehow causes surviving neurons to adapt by increased sensitivity to residual visual stimuli.
Deafferentation theory. This relatively new theory proposes deafferentation of the visual sensory pathway, which, in turn, causes disinhibition of neurons in the visual cortical regions, thereby causing them to fire spontaneously. This could cause a sensation analogous to phantom limb pain, which would be called “phantom vision presence of brain activity in the absence of an actual visual input.” Further, biochemical and molecular changes have been proposed to explain the deafferentation theory.17
Neurobiological evidence. Limited data are available for a neurobiological basis to visual hallucinations in Charles Bonnet syndrome. A few studies have used functional MRI and single-photon emission CT and reported possible association of visual hallucinations to specific visual areas.18,19
Risk factors
Social or physical isolation, loneliness, low extraversion, and shyness are risk factors for Charles Bonnet syndrome in visually impaired people.20 Sensory deprivation and low level of arousal favor the occurrence of hallucinations.5 Rate of vision loss—not the nature of pathology or severity of visual impairment—has been suggested to increase the risk of developing Charles Bonnet syndrome.21
What are the treatment options for Charles Bonnet syndrome?
a) begin an antipsychotic
b) do nothing; there is no cure
c) educate the patient about the nature of the hallucinations
d) refer the patient to an ophthalmologist for evaluation of vision loss
Treatment
There are several modalities to manage visual hallucinations in a patient with Charles Bonnet syndrome (Table 3). After ruling out medical and other psychiatric causes of visual hallucinations, treatment might not be indicated if the patient is not disturbed by the hallucinations. In most cases, reassurance and educating the patient and family about the benign nature of the visual hallucinations is all that is needed.
For patients who are disturbed by these visions or for whom there is a treatable cause, treatment could include cataract removal, medical therapy to reduce intraocular pressure in glaucoma, treatment of diabetic retinopathy, or laser photocoagulation. These treatments are associated with a reduction in hallucinations.22
In some cases, hallucinations disappear as visual acuity deteriorates. Psychotropics have been used to treat Charles Bonnet syndrome, including:
• antipsychotics, including haloperidol, risperidone, and olanzapine
• anticonvulsants, including valproic acid, gabapentin, and carbamazepine
• antidepressants, including mirtazapine and venlafaxine.23-30
Some experts recommend a conservative approach, which might be justified because some cases of Charles Bonnet syndrome are episodic and remit spontaneously.31 Again, however, consider pharmacotherapy if a patient is disturbed by hallucinations or if hallucinations impair overall functioning.
TREATMENT Education
After discussion with Mr. B and his family, he is started on risperidone, 1 mg at bedtime, and the psychiatric team provides information about the nature of Charles Bonnet syndrome. Mr. B reportedly takes this medication for a few days and then stops because he does not want the visual hallucinations to go away.
The psychiatry team sees Mr. B before discharge. He and his family are educated about the benign nature of the syndrome, the need for continued family support, and the fact that hallucinations will have minimal or no implications for his life.
The authors’ observations
It is important to remember that a visual description of hallucinations in Charles Bonnet syndrome can be quite vivid, and that the patient might not identify his hallucinations as such or consider them as a problem. Be careful not to dismiss the patient’s complaints as a primary psychiatric condition. It also is important to be mindful of the patient’s concerns with a psychiatric diagnosis; detailed discussion with the patient is helpful in most cases. A more comprehensive and empathetic approach to care could go a long way to sustain quality of life for these patients.
Bottom Line
Charles Bonnet syndrome is characterized by visual hallucinations in patients with visual impairment who have intact insight and an absence of mental illness. Taking a thorough history can help rule out medical and psychiatric causes of visual hallucinations. Educate patients and family about the nature of the hallucinations. In some cases, a psychotropic may be indicated.
Related Resources
• Nguyen ND, Osterweil D, Hoffman J. Charles Bonnet syndrome: treating nonpsychiatric hallucinations. Consult Pharm. 2013;28(3):184-188.
• Lapid MI, Burton MC, Chang MT, et al. Clinical phenomenology and mortality in Charles Bonnet syndrome. J Geriatr Psychiatry Neurol. 2013;26(1):3-9.
Drug Brand Names
Carbamazepine • Tegretol Mirtazapine • Remeron
Gabapentin • Neurontin Olanzapine • Zyprexa
Haloperidol • Haldol Risperidone • Risperdal
Lisinopril • Prinivil, Zestril Valproic acid • Depakene
Lovastatin • Mevacor Venlafaxine • Effexor
Metoprolol • Lopressor
Acknowledgement
The authors acknowledge Barry Liskow, MD, Vice Chair of Psychiatry, Kansas University Medical Center, Kansas City, Kansas, for providing both insight into the topic and useful feedback on the manuscript.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Seeing friends
Mr. B, age 91, presents to the emergency room (ER) for hip pain. As he is being evaluated, he asks a nurse to tell the “other people” around her to leave so that he can have privacy. As clarification, Mr. B reports visual hallucinations, which prompts the ER physician to request a psychiatry consult.
Mr. B is alert and oriented to time, place, and person when he is evaluated by the on-call psychiatry resident. He reports that he has been seeing several unusual things for the last 4 to 5 months. Asked to elaborate, Mr. B admits seeing colorful and vivid images of people around him. These people come and go as they like; rarely, they talk to him. He describes the conversations as “a constant chatter” in the background and adds that it is difficult to understand what they are talking about.
Mr. B states that he has been “seeing” a couple of people on a regular basis, and they are “sort of like my friends.” He endorses that these people often sing songs or dance for him. He states that, sometimes, these “friends” bring 3 or 4 friends and, although he could not make out their faces clearly, “they all are around me.” He describes the people he sees as “nice people” and does not report being scared or frightened by them.
Mr. B does not report paranoia, and denies command-type hallucinations. He and his family report no unusual changes in behavior in recent months. The medical history is remarkable for atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, age-related macular degeneration, and glaucoma.
Mr. B denies having any ongoing mood or anxiety symptoms. He states that he knows these people are “probably not real,” and they do not bother him and just keep him company.
What could be causing Mr. B’s hallucinations?
a) a stroke
b) late-onset schizophrenia
c) dementia
d) Charles Bonnet syndrome
The authors’ observations
Visual hallucinations among geriatric pa-tients are a common and confusing presentation. In addition to several medical causes for this presentation (Table 1), consider Charles Bonnet syndrome in patients with visual loss, presenting as visual hallucinations with intact insight and absence of a mental illness. Other conditions to consider in the differential diagnosis include Parkinson’s disease, dementia with Lewy bodies, schizophrenia, seizures, migraine, and stroke, including lesions of the thalamus or brain stem.
Charles Bonnet syndrome was first described by Swiss philosopher Charles Bonnet in the 18th century. He reported vivid visual hallucinations in his visually impaired grandfather (bilateral cataracts).1
It is important to recognize this syndrome because patients can present across different specialties, including psychiatry, ophthalmology, neurology, geriatric medicine, and family medicine.2 As life expectancy increases, this condition might be seen more often. It is prudent to identify, intervene, and refer as appropriate, in addition to educating patients and caregivers about the nature and course of the condition.
EVALUATION Not psychotic
Mr. B reports good sleep and appetite. He denies using alcohol or illicit drugs. He states he slipped in the bathroom the day before coming to the ER, but denies other recent falls or injuries. Other than hip pain, he has no other physical complaints. His medication regimen includes aspirin, lisinopril, lovastatin, and metoprolol.
The ER team diagnoses a hip fracture. Mr. B is transferred to the orthopedic service; the psychiatry consult team continues to follow him. Mental status examination is unremarkable other than the visual hallucinations. His speech is clear, non-pressured, with goal-directed thought processing. Mini-Mental State Examination score is 23/30 with Mr. B having difficulty with object drawing and 3-object recall. Brief cognitive examination in the ER is unremarkable.
The orthopedic team decides on conservative management of the hip fracture. There is no evidence of infection. Mr. B is afebrile with clear sensorium; complete blood cell count and normal liver function tests are normal; urinalysis and urine drug screen are negative; and chest radiography is unremarkable. CT and MRI of the head are unremarkable.
After 1 week in the hospital, Mr. B continues to experience vivid visual imagery. No signs of active infection are found. An ophthalmologist is consulted, who confirms Mr. B’s earlier diagnosis of glaucoma and age-related macular degeneration but does not recommend further treatment. Visual field test by confrontation is normal, with normal visual reflexes.
The authors’ observations
The reported prevalence of Charles Bonnet syndrome among visually impaired people varies from study to study—from as low as 0.4% to as high as 63%.3-6 The reason for such variation can be attributed to several variables:
• underdiagnosis
• misdiagnosis
• underreporting by patients because of the benign nature of the hallucinations
• patients’ reluctance to report visual hallucinations because of fear of being labeled “mentally ill.”7,8
Symptoms
There are no specific diagnostic criteria for Charles Bonnet syndrome (Table 2). However, the following are generally accepted for diagnosis9:
• grossly intact cognition, although mild cognitive impairment may be present in some cases10
• underlying visual disorder, usually acquired, such as glaucoma, age-related macular degeneration, diabetic retinopathy, central retinal artery occlusion, and optic neuritis3,4,11
• no hallucinations or perceptive difficulties in other sensory modalities
• generally intact insight
• absence of delusions
• absence of other neurologic, psychiatric, toxic, or metabolic conditions; medical causes of delirium must be ruled out.
Hallucinations might not be disturbing to the patient. Hallucinations could be simple (light flashes, lines, or geometric shapes) or complex (faces, figures, or scenes),12 and perceived as in color or in black and white. Hallucinations mostly are pleasant and rarely have any emotional impact or meaning. Although hallucinations are almost exclusively visual, they can be accompanied by noise or auditory hallucinations.13,14
Other characteristics of Charles Bonnet syndrome include:
• typical age of onset is approximately 72 years (range, 70 to 92 years)
• no sex distinction has been identified
• episodes can last from a few seconds to few hours; the syndrome may last a few days or a few years5
• it is not uncommon for episodes to occur in clusters, followed by symptom-free intervals and recurrences
• symptoms tend to fade away as patients progress to complete loss of sight.15
The course of Charles Bonnet syndrome is uncertain and unpredictable and the episodic nature can be frustrating for both patient and clinician. The syndrome could be misdiagnosed as a psychiatric condition.
Pathophysiology
The precise mechanism behind simple or complex vivid hallucinations in persons with Charles Bonnet syndrome is unclear. Several theories have been proposed.
Release theory proposes a loss of input to the primary visual areas, which decreases cortical inhibition and further causes disinhibition of visual association areas, thereby “releasing” visual hallucinations.16 Research suggests that this might be an attempt by surviving neurons to recover vision. Loss of input somehow causes surviving neurons to adapt by increased sensitivity to residual visual stimuli.
Deafferentation theory. This relatively new theory proposes deafferentation of the visual sensory pathway, which, in turn, causes disinhibition of neurons in the visual cortical regions, thereby causing them to fire spontaneously. This could cause a sensation analogous to phantom limb pain, which would be called “phantom vision presence of brain activity in the absence of an actual visual input.” Further, biochemical and molecular changes have been proposed to explain the deafferentation theory.17
Neurobiological evidence. Limited data are available for a neurobiological basis to visual hallucinations in Charles Bonnet syndrome. A few studies have used functional MRI and single-photon emission CT and reported possible association of visual hallucinations to specific visual areas.18,19
Risk factors
Social or physical isolation, loneliness, low extraversion, and shyness are risk factors for Charles Bonnet syndrome in visually impaired people.20 Sensory deprivation and low level of arousal favor the occurrence of hallucinations.5 Rate of vision loss—not the nature of pathology or severity of visual impairment—has been suggested to increase the risk of developing Charles Bonnet syndrome.21
What are the treatment options for Charles Bonnet syndrome?
a) begin an antipsychotic
b) do nothing; there is no cure
c) educate the patient about the nature of the hallucinations
d) refer the patient to an ophthalmologist for evaluation of vision loss
Treatment
There are several modalities to manage visual hallucinations in a patient with Charles Bonnet syndrome (Table 3). After ruling out medical and other psychiatric causes of visual hallucinations, treatment might not be indicated if the patient is not disturbed by the hallucinations. In most cases, reassurance and educating the patient and family about the benign nature of the visual hallucinations is all that is needed.
For patients who are disturbed by these visions or for whom there is a treatable cause, treatment could include cataract removal, medical therapy to reduce intraocular pressure in glaucoma, treatment of diabetic retinopathy, or laser photocoagulation. These treatments are associated with a reduction in hallucinations.22
In some cases, hallucinations disappear as visual acuity deteriorates. Psychotropics have been used to treat Charles Bonnet syndrome, including:
• antipsychotics, including haloperidol, risperidone, and olanzapine
• anticonvulsants, including valproic acid, gabapentin, and carbamazepine
• antidepressants, including mirtazapine and venlafaxine.23-30
Some experts recommend a conservative approach, which might be justified because some cases of Charles Bonnet syndrome are episodic and remit spontaneously.31 Again, however, consider pharmacotherapy if a patient is disturbed by hallucinations or if hallucinations impair overall functioning.
TREATMENT Education
After discussion with Mr. B and his family, he is started on risperidone, 1 mg at bedtime, and the psychiatric team provides information about the nature of Charles Bonnet syndrome. Mr. B reportedly takes this medication for a few days and then stops because he does not want the visual hallucinations to go away.
The psychiatry team sees Mr. B before discharge. He and his family are educated about the benign nature of the syndrome, the need for continued family support, and the fact that hallucinations will have minimal or no implications for his life.
The authors’ observations
It is important to remember that a visual description of hallucinations in Charles Bonnet syndrome can be quite vivid, and that the patient might not identify his hallucinations as such or consider them as a problem. Be careful not to dismiss the patient’s complaints as a primary psychiatric condition. It also is important to be mindful of the patient’s concerns with a psychiatric diagnosis; detailed discussion with the patient is helpful in most cases. A more comprehensive and empathetic approach to care could go a long way to sustain quality of life for these patients.
Bottom Line
Charles Bonnet syndrome is characterized by visual hallucinations in patients with visual impairment who have intact insight and an absence of mental illness. Taking a thorough history can help rule out medical and psychiatric causes of visual hallucinations. Educate patients and family about the nature of the hallucinations. In some cases, a psychotropic may be indicated.
Related Resources
• Nguyen ND, Osterweil D, Hoffman J. Charles Bonnet syndrome: treating nonpsychiatric hallucinations. Consult Pharm. 2013;28(3):184-188.
• Lapid MI, Burton MC, Chang MT, et al. Clinical phenomenology and mortality in Charles Bonnet syndrome. J Geriatr Psychiatry Neurol. 2013;26(1):3-9.
Drug Brand Names
Carbamazepine • Tegretol Mirtazapine • Remeron
Gabapentin • Neurontin Olanzapine • Zyprexa
Haloperidol • Haldol Risperidone • Risperdal
Lisinopril • Prinivil, Zestril Valproic acid • Depakene
Lovastatin • Mevacor Venlafaxine • Effexor
Metoprolol • Lopressor
Acknowledgement
The authors acknowledge Barry Liskow, MD, Vice Chair of Psychiatry, Kansas University Medical Center, Kansas City, Kansas, for providing both insight into the topic and useful feedback on the manuscript.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bonnet C. Essai analytique sur les facultes de l’ame. Copenhagen, Denmark: Chez le Ferres CI. & Ant. Philibert; 1760:426-429.
2. Plummer C, Kleinitz A, Vroomen P, et al. Of Roman chariots and goats in overcoats: the syndrome of Charles Bonnet. J Clin Neurosci. 2007;14(8):709-714.
3. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
4. Tan CS, Lim VS, Ho DY, et al. Charles Bonnet syndrome in Asian patients in a tertiary ophthalmic centre. Br J Ophthalmol. 2004;88(10):1325-1329.
5. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Visual hallucinations in psychologically normal people: Charles Bonnet’s syndrome. Lancet. 1996;347(9004):794-797.
6. Menon GJ. Complex visual hallucinations in the visually impaired: a structured history-taking approach. Arch Ophthalmol. 2005;123(3):349-355.
7. Hart CT. Formed visual hallucinations: a symptom of cranial arteritis. Br Med J. 1967;3(5566):643-644.
8. Norton-Wilson L, Munir M. Visual perceptual disorders resembling the Charles Bonnet syndrome. A study of 434 consecutive patients referred to a psychogeriatric unit. Fam Pract. 1987;4(1):27-35.
9. Eperjesi F, Akbarali N. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
10. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients from an ophthalmology clinic and medical clinic population. J Nerv Ment Dis. 1994;182(5):273-276.
11. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(pt 10):1819-1840.
12. Kester EM. Charles Bonnet syndrome: case presentation and literature review. Optometry. 2009;80(7):360-366.
13. Hori H, Terao T, Nakamura JL. Charles Bonnet syndrome with auditory hallucinations: a diagnostic dilemma. Psychopathology. 2001;34(3):164-166.
14. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003;48(1):58-72.
15. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis. 1997;185(3):195-200.
16. Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973;188(2):139-150.
17. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
18. Ffytche DH, Howard RJ, Brammer MJ, et al. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1(8):738-742.
19. Adachi N, Watanabe T, Matsuda H, et al. Hyperperfusion in the lateral temporal cortex, the striatum and the thalamus during complex visual hallucinations: single photon emission computed tomography findings in patients with Charles Bonnet syndrome. Psychiatry Clin Neurosci. 2000;54(2):157-162.
20. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry. 1999;40(4):315-319.
21. Shiraishi Y, Terao T, Ibi K, et al. Charles Bonnet syndrome and visual acuity—the involvement of dynamic or acute sensory deprivation. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):362-364.
22. Tueth MJ, Cheong JA, Samander J. The Charles Bonnet syndrome: a type of organic visual hallucinosis. J Geriatr Psychiatry Neurol. 1995;8(1):1-3.
23. Nguyen H, Le C, Nguyen H. Charles Bonnet syndrome in an elderly patient concurrent with acute cerebellar infarction treated successfully with haloperidol. J Am Geriatr Soc. 2011;59(4):761-762.
24. Campbell JJ, Ngo G. Risperidone treatment of complex hallucinations in a patient with posterior cortical atrophy. J Neuropsychiatry Clin Neurosci. 2008;20(3):378-379.
25. Colletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
26. Jang JW, Youn YC, Seok JW, et al. Hypermetabolism in the left thalamus and right inferior temporal area on positron emission tomography-statistical parametric mapping (PET-SPM) in a patient with Charles Bonnet syndrome resolving after treatment with valproic acid. J Clin Neurosci. 2011;18(8):1130-1132.
27. Paulig M, Mentrup H. Charles Bonnet’s syndrome; Complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
28. Terao T. Effect of carbamazepine and clonazepam combination on Charles Bonnet syndrome: a case report. Hum Psychopharmacol. 1998;13(6):451-453.
29. Siddiqui Z, Ramaswmay S, Petty F. Mirtazapine for Charles Bonnet syndrome. Can J Psychiatry. 2004;49(11):787-788.
30. Lang UE, Stogowski D, Schulze D, et al. Charles Bonnet Syndrome: successful treatment of visual hallucinations due to vision loss with selective serotonin reuptake inhibitors. J Psychopharmacol. 2007;21(5):553-555.
31. Hartney KE, Catalano G, Catalano MC. Charles Bonnet syndrome: are medications necessary? J Psychiatr Pract. 2011;17(2):137-141.
1. Bonnet C. Essai analytique sur les facultes de l’ame. Copenhagen, Denmark: Chez le Ferres CI. & Ant. Philibert; 1760:426-429.
2. Plummer C, Kleinitz A, Vroomen P, et al. Of Roman chariots and goats in overcoats: the syndrome of Charles Bonnet. J Clin Neurosci. 2007;14(8):709-714.
3. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
4. Tan CS, Lim VS, Ho DY, et al. Charles Bonnet syndrome in Asian patients in a tertiary ophthalmic centre. Br J Ophthalmol. 2004;88(10):1325-1329.
5. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Visual hallucinations in psychologically normal people: Charles Bonnet’s syndrome. Lancet. 1996;347(9004):794-797.
6. Menon GJ. Complex visual hallucinations in the visually impaired: a structured history-taking approach. Arch Ophthalmol. 2005;123(3):349-355.
7. Hart CT. Formed visual hallucinations: a symptom of cranial arteritis. Br Med J. 1967;3(5566):643-644.
8. Norton-Wilson L, Munir M. Visual perceptual disorders resembling the Charles Bonnet syndrome. A study of 434 consecutive patients referred to a psychogeriatric unit. Fam Pract. 1987;4(1):27-35.
9. Eperjesi F, Akbarali N. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
10. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients from an ophthalmology clinic and medical clinic population. J Nerv Ment Dis. 1994;182(5):273-276.
11. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(pt 10):1819-1840.
12. Kester EM. Charles Bonnet syndrome: case presentation and literature review. Optometry. 2009;80(7):360-366.
13. Hori H, Terao T, Nakamura JL. Charles Bonnet syndrome with auditory hallucinations: a diagnostic dilemma. Psychopathology. 2001;34(3):164-166.
14. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003;48(1):58-72.
15. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis. 1997;185(3):195-200.
16. Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973;188(2):139-150.
17. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
18. Ffytche DH, Howard RJ, Brammer MJ, et al. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1(8):738-742.
19. Adachi N, Watanabe T, Matsuda H, et al. Hyperperfusion in the lateral temporal cortex, the striatum and the thalamus during complex visual hallucinations: single photon emission computed tomography findings in patients with Charles Bonnet syndrome. Psychiatry Clin Neurosci. 2000;54(2):157-162.
20. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry. 1999;40(4):315-319.
21. Shiraishi Y, Terao T, Ibi K, et al. Charles Bonnet syndrome and visual acuity—the involvement of dynamic or acute sensory deprivation. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):362-364.
22. Tueth MJ, Cheong JA, Samander J. The Charles Bonnet syndrome: a type of organic visual hallucinosis. J Geriatr Psychiatry Neurol. 1995;8(1):1-3.
23. Nguyen H, Le C, Nguyen H. Charles Bonnet syndrome in an elderly patient concurrent with acute cerebellar infarction treated successfully with haloperidol. J Am Geriatr Soc. 2011;59(4):761-762.
24. Campbell JJ, Ngo G. Risperidone treatment of complex hallucinations in a patient with posterior cortical atrophy. J Neuropsychiatry Clin Neurosci. 2008;20(3):378-379.
25. Colletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
26. Jang JW, Youn YC, Seok JW, et al. Hypermetabolism in the left thalamus and right inferior temporal area on positron emission tomography-statistical parametric mapping (PET-SPM) in a patient with Charles Bonnet syndrome resolving after treatment with valproic acid. J Clin Neurosci. 2011;18(8):1130-1132.
27. Paulig M, Mentrup H. Charles Bonnet’s syndrome; Complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
28. Terao T. Effect of carbamazepine and clonazepam combination on Charles Bonnet syndrome: a case report. Hum Psychopharmacol. 1998;13(6):451-453.
29. Siddiqui Z, Ramaswmay S, Petty F. Mirtazapine for Charles Bonnet syndrome. Can J Psychiatry. 2004;49(11):787-788.
30. Lang UE, Stogowski D, Schulze D, et al. Charles Bonnet Syndrome: successful treatment of visual hallucinations due to vision loss with selective serotonin reuptake inhibitors. J Psychopharmacol. 2007;21(5):553-555.
31. Hartney KE, Catalano G, Catalano MC. Charles Bonnet syndrome: are medications necessary? J Psychiatr Pract. 2011;17(2):137-141.
How to modify psychotropic therapy for patients who have liver dysfunction
Police bring Ms. R, age 35, to the psychiatric ER after they find her asleep in a park. She is awake but drowsy, and states that she has a history of bipolar disorder. She claims that she had been stable on valproic acid (VPA), 1,500 mg/d, bupropion XL, 300 mg/d, quetiapine, 400 mg/d, and trazodone, 100 mg/d, until 2 weeks ago, when her best friend died and she stopped taking her medications all together. The previous evening, feeling “alone, hopeless, and sad,” she attempted suicide by ingesting a handful of VPA and clonazepam, obtained from a friend, and 2 liters of vodka. She complains of nausea, vomiting, and abdominal pain. Elevated laboratory chemistries included aspartate aminotransferase (AST), 220 U/L; alanine aminotransferase (ALT), 182 U/L; alkaline phosphatase (AP), 75 U/L; γ-glutamyltransferase (GGT), 104 U/L; total bilirubin, 1.4 mg/dL; and an elevated VPA serum concentration of 152 μg/mL.
Drug-induced hepatotoxicity accounts for approximately 50% of acute liver failure cases, and almost 10% of liver transplants in some facilities.1 The incidence of drug-induced hepatotoxicity is between 0.001% and 0.1% in patients on standard medication doses.2 Drug-induced hepatotoxicity is characterized by:
• abnormalities in laboratory parameters (hepatocellular, cholestatic, or mixed)
• mechanisms of toxicity (direct, immune-mediated, idiosyncratic, mitochondrial toxicity)
• liver biopsy histology (steatosis, sinusoidal obstruction syndrome).3
Liver function test results of hepatocellular injury are characterized by ALT elevation and minimal AP elevation, whereas cholestatic injury manifests as high AP. Table 13 categorizes psychotropics based on type of liver injury and how each injury manifest in liver function tests. Delayed idiosyncratic reactions occur after taking the drug, whereas direct toxicities are dose-dependent and more predictable. By definition, a clinically significant hepatotoxicity is associated with an ALT >3 times the upper limit of normal.3
VPA-induced liver injury occurs in approximately 1 in 37,000 persons taking the drug.4 Patients at an increased risk of VPA-induced liver injury include:
• children
• patients with mitochondrial enzyme deficiencies
• Reye’s syndrome
• Friedreich’s ataxia
• polypharmacy patients
• patients with a sibling who has experienced VPA toxicity.4
Benign enzyme elevations occur in approximately 20% of patients taking VPA.5 In Ms. R’s case, concomitant VPA, clonazepam, and alcohol may have led to elevations in ALT, AST, and GGT. Her nausea, vomiting, and abdominal pain are consistent with hepatic dysfunction.
Carnitine is effective in increasing survival of patients with VPA-induced hepatotoxicity.4 Because Ms. R is symptomatic, discontinuing VPA and administering IV L-carnitine is warranted.5 L-carnitine can be initiated at 100 mg/kg as an IV bolus, followed by 50 mg/kg as an IV infusion every 8 hours, with a maximum dosage of 3,000 mg.6 Patients may require several days of therapy based on symptoms. L-carnitine should be continued until a patient shows clinical improvement, such as decreases in ALT and AST.
Ms. R experienced a VPA-induced hepatotoxic reaction. However, continuous monitoring is appropriate for all patients who are prescribed any potentially hepatotoxic psychotropic, especially after hepatic injuries resolve. This includes mood stabilizers, antipsychotics, benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors, especially when given concomitantly with other hepatotoxic agents.
Table 2 lists dosing recommendations for commonly used psychotropics in patients with hepatic impairment. Among mood stabilizers, carbamazepine and VPA are associated with the highest incidence of hepatotoxicity.2 A follow-up study of more than 1,000,000 VPA prescriptions found 29 cases of fatal hepatotoxicity in a 7-year period.7 Although there are case reports of hepatotoxicity with oxcarbazepine, it may have a better liver safety profile than carbamazepine.2 Hepatotoxicity with lamotrigine is rare, although fatal cases have been reported.5
When initiating an antipsychotic, a temporary, benign increase in liver enzymes can be expected, but typically discontinuation is unnecessary.2 Phenothiazines in particular can cause increases in liver enzymes in 20% of patients.2 Hepatotoxicity with benzodiazepines is infrequent, with a few cases of cholestatic injury reported with diazepam, chlordiazepoxide, and flurazepam.2
SSRIs are relatively safe; incidents of hepatic injury are rare. Among SSRIs, paroxetine is most frequently associated with hepatotoxicity. Abnormal liver function tests have been observed with fluoxetine (0.5% of long-term recipients) and other SSRIs.1,2,4
Among antidepressants with dual serotonergic action, nefazodone carries a black-box warning for hepatotoxicity and is used rarely, whereas trazodone is not regarded as hepatotoxic.2 Antidepressants with dual norepinephrine and serotonin reuptake inhibitor properties carry a higher risk of liver injury, especially duloxetine. Hepatocellular, cholestatic, and mixed types of hepatotoxicity are associated with duloxetine-induced hepatotoxicity.2
Monitoring recommendations
Before prescribing potentially hepatotoxic medications, order baseline liver function tests. During therapy, periodic liver function monitoring is recommended. Elevated transaminase concentrations (>3 × the upper limit of normal), bilirubin (>2 × the upper limit of normal), and prolonged prothrombin times are indicators of hepatic injury.2 Caution should be taken to prevent polypharmacy with multiple hepatotoxic medications and over-the-counter use of hepatotoxic drugs and supplements.
When choosing a psychotropic, take into account patient-specific factors, such as underlying liver disease and alcohol consumption. Patients on potentially hepatotoxic medications should be counseled to recognize and report symptoms of liver dysfunction, including nausea, vomiting, jaundice, and lower-extremity edema.2 If liver injury occurs, modify therapy with the potential offending agent and check liver function periodically.
Related Resourcesa
• Bleibel W, Kim S, D’Silva K, et al. Drug-induced liver injury: review article. Dig Dis Sci. 2007;52(10):2463-2471.
• U.S. National Library of Medicine. LiverTox. National Institute of Health. www.livertox.nih.gov.
Drug Brand Names
Amitriptyline • Elavil Lurasidone • Latuda
Molindone • Moban Molindone • Moban
Aripiprazole • Abilify Nefazodone • Serzone
Asenapine • Saphris Nortriptyline • Pamelor
Bupropion XL • Wellbutrin XL Olanzapine • Zyprexa
Citalopram • Celexa Oxcarbazepine • Trileptal
Carbamazepine • Tegretol Paroxetine • Paxil
Chlordiazepoxide • Librium Perphenazine • Trilafon
Chlorpromazine • Thorazine Phenobarbital • Luminal
Clonazepam • Klonopin Phenytoin • Dilantin
Clozapine • Clozaril Quetiapine • Seroquel
Desvenlafaxine • Pristiq Risperidone • Risperdal
Diazepam • Valium Sertraline • Zoloft
Duloxetine • Cymbalta Thiothixene • Navane
Escitalopram • Lexapro Trazodone • Desyrel
Fluoxetine • Prozac Trifluoperazine • Stelazine
Fluphenazine • Prolixin Topiramate • Topamax
Flurazepam • Dalmane Valproic acid • Depakote
Haloperidol • Haldol Venlafaxine • Effexor
Iloperidone • Fanapt Ziprasidone • Geodon
Lamotrigine • Lamictal
Levocarnitine • L-carnitine
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pugh AJ, Barve AJ, Falkner K, et al. Drug-induced hepatotoxicity or drug-induced liver injury. Clin Liver Dis. 2009;13(2):277-294.
2. Sedky K, Nazir R, Joshi A, et al. Which psychotropic medications induce hepatotoxicity? Gen Hosp Psychiatry. 2012;34(1):53-61.
3. Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. 2007;25(10):1135-1151.
4. Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169-183.
5. Murray KF, Hadzic N, Wirth S, et al. Drug-related hepatotoxicity and acute liver failure. J Pediatr Gastroenterol Nutr. 2008;47(4):395-405.
6. Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010;44(7-8):1287-1293.
7. Bryant AE 3rd, Dreifuss FE. Valproic acid hepatic fatalities. III. U.S. experience since 1986. Neurology. 1996;46(2):465-469.
Police bring Ms. R, age 35, to the psychiatric ER after they find her asleep in a park. She is awake but drowsy, and states that she has a history of bipolar disorder. She claims that she had been stable on valproic acid (VPA), 1,500 mg/d, bupropion XL, 300 mg/d, quetiapine, 400 mg/d, and trazodone, 100 mg/d, until 2 weeks ago, when her best friend died and she stopped taking her medications all together. The previous evening, feeling “alone, hopeless, and sad,” she attempted suicide by ingesting a handful of VPA and clonazepam, obtained from a friend, and 2 liters of vodka. She complains of nausea, vomiting, and abdominal pain. Elevated laboratory chemistries included aspartate aminotransferase (AST), 220 U/L; alanine aminotransferase (ALT), 182 U/L; alkaline phosphatase (AP), 75 U/L; γ-glutamyltransferase (GGT), 104 U/L; total bilirubin, 1.4 mg/dL; and an elevated VPA serum concentration of 152 μg/mL.
Drug-induced hepatotoxicity accounts for approximately 50% of acute liver failure cases, and almost 10% of liver transplants in some facilities.1 The incidence of drug-induced hepatotoxicity is between 0.001% and 0.1% in patients on standard medication doses.2 Drug-induced hepatotoxicity is characterized by:
• abnormalities in laboratory parameters (hepatocellular, cholestatic, or mixed)
• mechanisms of toxicity (direct, immune-mediated, idiosyncratic, mitochondrial toxicity)
• liver biopsy histology (steatosis, sinusoidal obstruction syndrome).3
Liver function test results of hepatocellular injury are characterized by ALT elevation and minimal AP elevation, whereas cholestatic injury manifests as high AP. Table 13 categorizes psychotropics based on type of liver injury and how each injury manifest in liver function tests. Delayed idiosyncratic reactions occur after taking the drug, whereas direct toxicities are dose-dependent and more predictable. By definition, a clinically significant hepatotoxicity is associated with an ALT >3 times the upper limit of normal.3
VPA-induced liver injury occurs in approximately 1 in 37,000 persons taking the drug.4 Patients at an increased risk of VPA-induced liver injury include:
• children
• patients with mitochondrial enzyme deficiencies
• Reye’s syndrome
• Friedreich’s ataxia
• polypharmacy patients
• patients with a sibling who has experienced VPA toxicity.4
Benign enzyme elevations occur in approximately 20% of patients taking VPA.5 In Ms. R’s case, concomitant VPA, clonazepam, and alcohol may have led to elevations in ALT, AST, and GGT. Her nausea, vomiting, and abdominal pain are consistent with hepatic dysfunction.
Carnitine is effective in increasing survival of patients with VPA-induced hepatotoxicity.4 Because Ms. R is symptomatic, discontinuing VPA and administering IV L-carnitine is warranted.5 L-carnitine can be initiated at 100 mg/kg as an IV bolus, followed by 50 mg/kg as an IV infusion every 8 hours, with a maximum dosage of 3,000 mg.6 Patients may require several days of therapy based on symptoms. L-carnitine should be continued until a patient shows clinical improvement, such as decreases in ALT and AST.
Ms. R experienced a VPA-induced hepatotoxic reaction. However, continuous monitoring is appropriate for all patients who are prescribed any potentially hepatotoxic psychotropic, especially after hepatic injuries resolve. This includes mood stabilizers, antipsychotics, benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors, especially when given concomitantly with other hepatotoxic agents.
Table 2 lists dosing recommendations for commonly used psychotropics in patients with hepatic impairment. Among mood stabilizers, carbamazepine and VPA are associated with the highest incidence of hepatotoxicity.2 A follow-up study of more than 1,000,000 VPA prescriptions found 29 cases of fatal hepatotoxicity in a 7-year period.7 Although there are case reports of hepatotoxicity with oxcarbazepine, it may have a better liver safety profile than carbamazepine.2 Hepatotoxicity with lamotrigine is rare, although fatal cases have been reported.5
When initiating an antipsychotic, a temporary, benign increase in liver enzymes can be expected, but typically discontinuation is unnecessary.2 Phenothiazines in particular can cause increases in liver enzymes in 20% of patients.2 Hepatotoxicity with benzodiazepines is infrequent, with a few cases of cholestatic injury reported with diazepam, chlordiazepoxide, and flurazepam.2
SSRIs are relatively safe; incidents of hepatic injury are rare. Among SSRIs, paroxetine is most frequently associated with hepatotoxicity. Abnormal liver function tests have been observed with fluoxetine (0.5% of long-term recipients) and other SSRIs.1,2,4
Among antidepressants with dual serotonergic action, nefazodone carries a black-box warning for hepatotoxicity and is used rarely, whereas trazodone is not regarded as hepatotoxic.2 Antidepressants with dual norepinephrine and serotonin reuptake inhibitor properties carry a higher risk of liver injury, especially duloxetine. Hepatocellular, cholestatic, and mixed types of hepatotoxicity are associated with duloxetine-induced hepatotoxicity.2
Monitoring recommendations
Before prescribing potentially hepatotoxic medications, order baseline liver function tests. During therapy, periodic liver function monitoring is recommended. Elevated transaminase concentrations (>3 × the upper limit of normal), bilirubin (>2 × the upper limit of normal), and prolonged prothrombin times are indicators of hepatic injury.2 Caution should be taken to prevent polypharmacy with multiple hepatotoxic medications and over-the-counter use of hepatotoxic drugs and supplements.
When choosing a psychotropic, take into account patient-specific factors, such as underlying liver disease and alcohol consumption. Patients on potentially hepatotoxic medications should be counseled to recognize and report symptoms of liver dysfunction, including nausea, vomiting, jaundice, and lower-extremity edema.2 If liver injury occurs, modify therapy with the potential offending agent and check liver function periodically.
Related Resourcesa
• Bleibel W, Kim S, D’Silva K, et al. Drug-induced liver injury: review article. Dig Dis Sci. 2007;52(10):2463-2471.
• U.S. National Library of Medicine. LiverTox. National Institute of Health. www.livertox.nih.gov.
Drug Brand Names
Amitriptyline • Elavil Lurasidone • Latuda
Molindone • Moban Molindone • Moban
Aripiprazole • Abilify Nefazodone • Serzone
Asenapine • Saphris Nortriptyline • Pamelor
Bupropion XL • Wellbutrin XL Olanzapine • Zyprexa
Citalopram • Celexa Oxcarbazepine • Trileptal
Carbamazepine • Tegretol Paroxetine • Paxil
Chlordiazepoxide • Librium Perphenazine • Trilafon
Chlorpromazine • Thorazine Phenobarbital • Luminal
Clonazepam • Klonopin Phenytoin • Dilantin
Clozapine • Clozaril Quetiapine • Seroquel
Desvenlafaxine • Pristiq Risperidone • Risperdal
Diazepam • Valium Sertraline • Zoloft
Duloxetine • Cymbalta Thiothixene • Navane
Escitalopram • Lexapro Trazodone • Desyrel
Fluoxetine • Prozac Trifluoperazine • Stelazine
Fluphenazine • Prolixin Topiramate • Topamax
Flurazepam • Dalmane Valproic acid • Depakote
Haloperidol • Haldol Venlafaxine • Effexor
Iloperidone • Fanapt Ziprasidone • Geodon
Lamotrigine • Lamictal
Levocarnitine • L-carnitine
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Police bring Ms. R, age 35, to the psychiatric ER after they find her asleep in a park. She is awake but drowsy, and states that she has a history of bipolar disorder. She claims that she had been stable on valproic acid (VPA), 1,500 mg/d, bupropion XL, 300 mg/d, quetiapine, 400 mg/d, and trazodone, 100 mg/d, until 2 weeks ago, when her best friend died and she stopped taking her medications all together. The previous evening, feeling “alone, hopeless, and sad,” she attempted suicide by ingesting a handful of VPA and clonazepam, obtained from a friend, and 2 liters of vodka. She complains of nausea, vomiting, and abdominal pain. Elevated laboratory chemistries included aspartate aminotransferase (AST), 220 U/L; alanine aminotransferase (ALT), 182 U/L; alkaline phosphatase (AP), 75 U/L; γ-glutamyltransferase (GGT), 104 U/L; total bilirubin, 1.4 mg/dL; and an elevated VPA serum concentration of 152 μg/mL.
Drug-induced hepatotoxicity accounts for approximately 50% of acute liver failure cases, and almost 10% of liver transplants in some facilities.1 The incidence of drug-induced hepatotoxicity is between 0.001% and 0.1% in patients on standard medication doses.2 Drug-induced hepatotoxicity is characterized by:
• abnormalities in laboratory parameters (hepatocellular, cholestatic, or mixed)
• mechanisms of toxicity (direct, immune-mediated, idiosyncratic, mitochondrial toxicity)
• liver biopsy histology (steatosis, sinusoidal obstruction syndrome).3
Liver function test results of hepatocellular injury are characterized by ALT elevation and minimal AP elevation, whereas cholestatic injury manifests as high AP. Table 13 categorizes psychotropics based on type of liver injury and how each injury manifest in liver function tests. Delayed idiosyncratic reactions occur after taking the drug, whereas direct toxicities are dose-dependent and more predictable. By definition, a clinically significant hepatotoxicity is associated with an ALT >3 times the upper limit of normal.3
VPA-induced liver injury occurs in approximately 1 in 37,000 persons taking the drug.4 Patients at an increased risk of VPA-induced liver injury include:
• children
• patients with mitochondrial enzyme deficiencies
• Reye’s syndrome
• Friedreich’s ataxia
• polypharmacy patients
• patients with a sibling who has experienced VPA toxicity.4
Benign enzyme elevations occur in approximately 20% of patients taking VPA.5 In Ms. R’s case, concomitant VPA, clonazepam, and alcohol may have led to elevations in ALT, AST, and GGT. Her nausea, vomiting, and abdominal pain are consistent with hepatic dysfunction.
Carnitine is effective in increasing survival of patients with VPA-induced hepatotoxicity.4 Because Ms. R is symptomatic, discontinuing VPA and administering IV L-carnitine is warranted.5 L-carnitine can be initiated at 100 mg/kg as an IV bolus, followed by 50 mg/kg as an IV infusion every 8 hours, with a maximum dosage of 3,000 mg.6 Patients may require several days of therapy based on symptoms. L-carnitine should be continued until a patient shows clinical improvement, such as decreases in ALT and AST.
Ms. R experienced a VPA-induced hepatotoxic reaction. However, continuous monitoring is appropriate for all patients who are prescribed any potentially hepatotoxic psychotropic, especially after hepatic injuries resolve. This includes mood stabilizers, antipsychotics, benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors, especially when given concomitantly with other hepatotoxic agents.
Table 2 lists dosing recommendations for commonly used psychotropics in patients with hepatic impairment. Among mood stabilizers, carbamazepine and VPA are associated with the highest incidence of hepatotoxicity.2 A follow-up study of more than 1,000,000 VPA prescriptions found 29 cases of fatal hepatotoxicity in a 7-year period.7 Although there are case reports of hepatotoxicity with oxcarbazepine, it may have a better liver safety profile than carbamazepine.2 Hepatotoxicity with lamotrigine is rare, although fatal cases have been reported.5
When initiating an antipsychotic, a temporary, benign increase in liver enzymes can be expected, but typically discontinuation is unnecessary.2 Phenothiazines in particular can cause increases in liver enzymes in 20% of patients.2 Hepatotoxicity with benzodiazepines is infrequent, with a few cases of cholestatic injury reported with diazepam, chlordiazepoxide, and flurazepam.2
SSRIs are relatively safe; incidents of hepatic injury are rare. Among SSRIs, paroxetine is most frequently associated with hepatotoxicity. Abnormal liver function tests have been observed with fluoxetine (0.5% of long-term recipients) and other SSRIs.1,2,4
Among antidepressants with dual serotonergic action, nefazodone carries a black-box warning for hepatotoxicity and is used rarely, whereas trazodone is not regarded as hepatotoxic.2 Antidepressants with dual norepinephrine and serotonin reuptake inhibitor properties carry a higher risk of liver injury, especially duloxetine. Hepatocellular, cholestatic, and mixed types of hepatotoxicity are associated with duloxetine-induced hepatotoxicity.2
Monitoring recommendations
Before prescribing potentially hepatotoxic medications, order baseline liver function tests. During therapy, periodic liver function monitoring is recommended. Elevated transaminase concentrations (>3 × the upper limit of normal), bilirubin (>2 × the upper limit of normal), and prolonged prothrombin times are indicators of hepatic injury.2 Caution should be taken to prevent polypharmacy with multiple hepatotoxic medications and over-the-counter use of hepatotoxic drugs and supplements.
When choosing a psychotropic, take into account patient-specific factors, such as underlying liver disease and alcohol consumption. Patients on potentially hepatotoxic medications should be counseled to recognize and report symptoms of liver dysfunction, including nausea, vomiting, jaundice, and lower-extremity edema.2 If liver injury occurs, modify therapy with the potential offending agent and check liver function periodically.
Related Resourcesa
• Bleibel W, Kim S, D’Silva K, et al. Drug-induced liver injury: review article. Dig Dis Sci. 2007;52(10):2463-2471.
• U.S. National Library of Medicine. LiverTox. National Institute of Health. www.livertox.nih.gov.
Drug Brand Names
Amitriptyline • Elavil Lurasidone • Latuda
Molindone • Moban Molindone • Moban
Aripiprazole • Abilify Nefazodone • Serzone
Asenapine • Saphris Nortriptyline • Pamelor
Bupropion XL • Wellbutrin XL Olanzapine • Zyprexa
Citalopram • Celexa Oxcarbazepine • Trileptal
Carbamazepine • Tegretol Paroxetine • Paxil
Chlordiazepoxide • Librium Perphenazine • Trilafon
Chlorpromazine • Thorazine Phenobarbital • Luminal
Clonazepam • Klonopin Phenytoin • Dilantin
Clozapine • Clozaril Quetiapine • Seroquel
Desvenlafaxine • Pristiq Risperidone • Risperdal
Diazepam • Valium Sertraline • Zoloft
Duloxetine • Cymbalta Thiothixene • Navane
Escitalopram • Lexapro Trazodone • Desyrel
Fluoxetine • Prozac Trifluoperazine • Stelazine
Fluphenazine • Prolixin Topiramate • Topamax
Flurazepam • Dalmane Valproic acid • Depakote
Haloperidol • Haldol Venlafaxine • Effexor
Iloperidone • Fanapt Ziprasidone • Geodon
Lamotrigine • Lamictal
Levocarnitine • L-carnitine
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pugh AJ, Barve AJ, Falkner K, et al. Drug-induced hepatotoxicity or drug-induced liver injury. Clin Liver Dis. 2009;13(2):277-294.
2. Sedky K, Nazir R, Joshi A, et al. Which psychotropic medications induce hepatotoxicity? Gen Hosp Psychiatry. 2012;34(1):53-61.
3. Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. 2007;25(10):1135-1151.
4. Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169-183.
5. Murray KF, Hadzic N, Wirth S, et al. Drug-related hepatotoxicity and acute liver failure. J Pediatr Gastroenterol Nutr. 2008;47(4):395-405.
6. Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010;44(7-8):1287-1293.
7. Bryant AE 3rd, Dreifuss FE. Valproic acid hepatic fatalities. III. U.S. experience since 1986. Neurology. 1996;46(2):465-469.
1. Pugh AJ, Barve AJ, Falkner K, et al. Drug-induced hepatotoxicity or drug-induced liver injury. Clin Liver Dis. 2009;13(2):277-294.
2. Sedky K, Nazir R, Joshi A, et al. Which psychotropic medications induce hepatotoxicity? Gen Hosp Psychiatry. 2012;34(1):53-61.
3. Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. 2007;25(10):1135-1151.
4. Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169-183.
5. Murray KF, Hadzic N, Wirth S, et al. Drug-related hepatotoxicity and acute liver failure. J Pediatr Gastroenterol Nutr. 2008;47(4):395-405.
6. Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010;44(7-8):1287-1293.
7. Bryant AE 3rd, Dreifuss FE. Valproic acid hepatic fatalities. III. U.S. experience since 1986. Neurology. 1996;46(2):465-469.