User login
Sulfur Spring Dermatitis
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
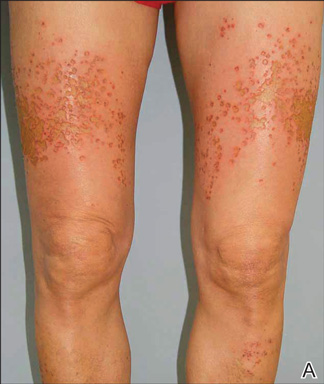
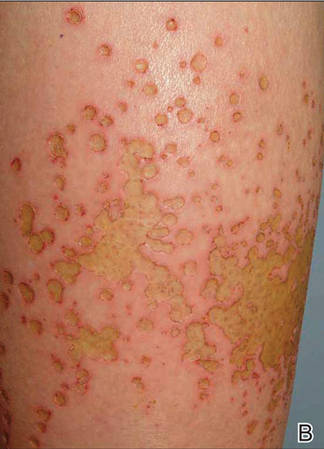
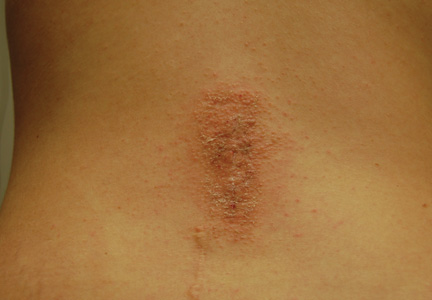
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
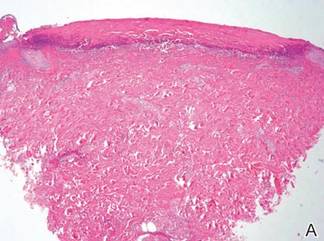
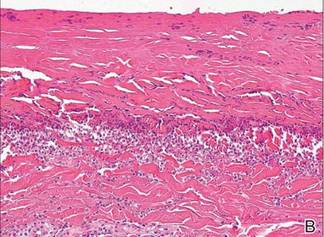
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
Practice Points
- The clinical findings of sulfur spring dermatitis are similar to those of a superficial second-degree burn.
- Careful evaluation of the patient’s clinical history and recognition of characteristic findings are important for correct diagnosis.
- Patients with preexisting skin disorders who engage in thermal sulfur baths should be aware of the potential adverse effect of sulfur spring dermatitis.
Stage III Non–Small Cell Lung Cancer
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Each year approximately 228,000 Americans will be diagnosed with lung cancer, and 159,000 will die of this disease. An estimated 85% of lung cancer cases are non–small cell lung cancer (NSCLC), more than 50% of NSCLC is comprised of adenocarcinoma, the median age at diagnosis is 71 years, and 25% of patients with this diagnosis present with stage III disease. In 2010 the seventh edition of the American Joint Committee on Cancer (AJCC) TNM staging system for lung cancer was released, and several changes were made which affect the patient population designated as having stage III disease.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Each year approximately 228,000 Americans will be diagnosed with lung cancer, and 159,000 will die of this disease. An estimated 85% of lung cancer cases are non–small cell lung cancer (NSCLC), more than 50% of NSCLC is comprised of adenocarcinoma, the median age at diagnosis is 71 years, and 25% of patients with this diagnosis present with stage III disease. In 2010 the seventh edition of the American Joint Committee on Cancer (AJCC) TNM staging system for lung cancer was released, and several changes were made which affect the patient population designated as having stage III disease.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Each year approximately 228,000 Americans will be diagnosed with lung cancer, and 159,000 will die of this disease. An estimated 85% of lung cancer cases are non–small cell lung cancer (NSCLC), more than 50% of NSCLC is comprised of adenocarcinoma, the median age at diagnosis is 71 years, and 25% of patients with this diagnosis present with stage III disease. In 2010 the seventh edition of the American Joint Committee on Cancer (AJCC) TNM staging system for lung cancer was released, and several changes were made which affect the patient population designated as having stage III disease.
To read the full article in PDF:
Acting strange after trying to ‘get numb’
CASE Numb and confused
Mr. L, age 17, is admitted to the hospital after ingesting 24 diphenhydramine 25-mg tablets in 3 hours as a possible suicide attempt. His parents witnessed him behaving strangely and brought him to the hospital. They state that their son was visibly agitated and acting inappropriately. He was seen talking to birds, trees, and the walls of the house.
Mr. L says he is upset because he broke up with his girlfriend a week earlier after she asked if they could “take a break.” He says that he took the diphenhydramine because he wanted to “get numb” to deal with the emotional stress caused by the break-up.
After the break-up, Mr. L experienced middle-to-late insomnia and was unable to get more than 3 or 4 hours of sleep a night. He reports significant fatigue, depressed mood, anhedonia, impaired concentration, and psychomotor retardation. He denies homicidal ideation or auditory and visual hallucinations.
As an aside, Mr. L reports that, for the past year, he had difficulties with gender identity, sometimes thinking that he might be better off if he had been born a girl and that he felt uncomfortable in a male body.
Which treatment option would you choose for Mr. L’s substance abuse?
a) refer him to a 12-step program
b) begin supportive measures
c) administer activated charcoal
d) prescribe a benzodiazepine to control agitation
The authors’ observations
As youths gain increasing access to medical and pharmaceutical knowledge through the Internet and other sources, it appears that adolescent drug abuse has, in part, shifted toward more easily attainable over-the-counter (OTC) medications. Diphenhydramine, a first-generation antihistamine, can be abused for its effects on the CNS, such as disturbed coordination, irritability, paresthesia, blurred vision, and depression. Effects of diphenhydramine are increased by the presence of alcohol, monoamine oxidase inhibitors, diazepam, hypnotics, sedatives, tranquilizers, and other CNS depressants. In 2011, diphenhydramine abuse was involved in 19,012 emergency room visits, of which 9,301 were for drug-related suicide attempts.1
Diphenhydramine is an inverse agonist of the histamine H1 receptor.2 It is a member of the ethanolamine subclass of antihistaminergic agents.3 By reversing the effects of histamine on capillaries, diphenhydramine can reduce the intensity of allergic symptoms. Diphenhydramine also crosses the blood–brain barrier and antagonizes H1 receptors centrally.
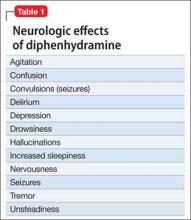
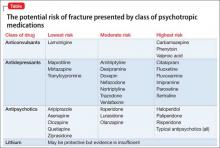
Used as a common sleep aid and allergy medication, the drug works primarily as an H1 receptor partial agonist, but also is a strong competitive antagonist at muscarinic acetylcholine receptors.4 It is abused for its sedative effects and its capacity to cause delirium and hallucinations.5 Diphenhydramine can have a stimulatory effect in children and young adults, instead of the sedating properties seen in adults.6 Such misuse is concerning because diphenhydramine overdose can lead to delirium, confusion, and hallucinations, tachycardia, seizures, mydriasis, xerostomia, urinary retention, ileus, anhidrosis, and hyperthermia. In severe cases it has been associated with cardiac arrhythmias, rhabdomyolysis, status epilepticus, and death.4,6 Neurologic symptoms of diphenhydramine overdose are listed in Table 1.
HISTORY Polysubstance abuse
Mr. L has a 2-year history of major depressive disorder and a history of Cannabis abuse with physiological dependence; Robitussin (base active ingredient, guaifenesin) and hydrocodone abuse with physiological dependence; 3,4-methylenedioxymethamphetamine (MDMA) abuse; and diphenhydramine abuse. He also has a history of gender dysphoria, although he reports that these feelings have become less severe over the past year.
Mr. L attends bi-weekly appointments with an outpatient psychiatrist and reportedly adheres to his medication regimen: fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime. He denies previous suicidal ideation, suicide attempts, homicidal ideation, or homicidal attempts. He reports no history of physical, sexual, or emotional abuse. He gets good grades in school and has no outstanding academic problems.
Mr. L began using Cannabis at age 14; his last use was 3 weeks before admission. He is guarded about his use of Robitussin, hydrocodone, and MDMA. However, Mr. L reports that he has researched diphenhydramine on the internet and believes that he can safely take up to 1,200 mg without overdosing. He reports normally taking 450 mg of diphenhydramine daily. Mr. L reports difficulty urinating after using diphenhydramine but no other physical complaints.
Mr. L lives with his father and stepmother and has a history of one psychiatric hospitalization at a different facility 2 months ago, followed by outpatient therapy. He obtained his Graduate Equivalency Diploma (GED) and plans to attend college.
At age 5, Mr. L emigrated from Turkey to the United States with his parents. His mother returned to Turkey when he was age 6 and has had no contact with her son since. Whenever Mr. L visits Turkey with his father, the patient refuses to see her, as per collaterals. He gets along well with his stepmother, who is his maternal aunt. Mr. L has been bullied at school and reportedly has few friends.
On mental status examination, Mr. L has an appropriate appearance and appears to be his stated age. He shows good eye contact and is cooperative. Muscle tone and gait are within normal limits. He has no abnormal movements. Speech, thought processes, and associations are normal. He denies auditory hallucinations, visual hallucinations, suicidal ideation (although he presented with a probable suicide attempt), or homicidal ideation. No delusions are elicited.
Mr. L shows poor judgment about his drug use and situation. He demonstrates limited insight, because he says his only goal is to get out of the hospital. He is alert, awake, and oriented to person, place, and time. He shows no memory or knowledge impairment. He appears euthymic with an inappropriate and constricted affect. On neurologic exam, he had mild tremors in his hands. The authors’ observationsTreatment for diphenhydramine overdose should begin quickly to prevent life-threatening effects and reduce the risk for mortality. The toxin can be removed from the patient’s GI tract with activated charcoal or gastric lavage if the patient presents within 1 hour of ingesting the substance. Administering IV fluids will prevent dehydration. Cardiac functioning is monitored and benzodiazepines could be administered to manage seizures.
Key elements of a toxicologic physical examination include:
• eyes: pupillary size, symmetry, and response to light (vertical or horizontal nystagmus)
• oropharynx: moist or dry mucous membranes, presence or absence of the gag reflex, distinctive odors
• abdomen: presence or absence and quality of bowel sounds
• skin: warm and dry, warm and sweaty, or cool
• neurologic: level of consciousness and mental status, presence of tremors, seizures, or other movement disorders, presence or absence and quality of deep tendon reflexes.7
If a child or adolescent patient cannot communicate how much of a drug he (she) has ingested, questions to ask parents or other informants include:
• Was the medication purchased recently, and if so was the bottle or box full before the patient took the pills?
• If the medication was not new, how many pills were in the bottle before the patient got to it?
• If the medication was prescribed, how many pills were originally prescribed, when was the medication prescribed, and how many pills were already taken prior to the patient getting to the bottle?
• How many pills were left in the bottle?
• How many pills were seen around the area where the patient was found?
• How many pills were found in the patient’s mouth?7
Recommendations
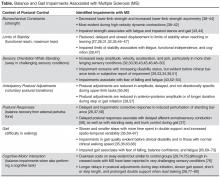
It is well known that OTC medication abuse is a growing medical problem (Table 2). Antihistamines, including diphenhydramine, are readily available to minors and adults. Because of the powerful sedating effects of antihistamines, many adolescent health practitioners give them to patients who have insomnia as a sleep aid.8 As in our case, antihistamines are used recreationally for their hallucinogenic effects, at dosages of 300 to 700 mg.9 Severe symptoms of toxicity, such as delirium and psychosis, seizures, and coma, occur at dosages ≥1,000 mg.9
With growing abuse of these medications, we aim to encourage detailed history taking about abuse of OTC drugs, especially diphenhydramine in adolescent patients.
Outcome Improvement, discharge
Mr. L is given a dual diagnosis of diphenhydramine-induced psychotic disorder with
hallucinations and diphenhydramine-induced depressive disorder, both with onset during intoxication. He also is given a provisional diagnosis of psychotic disorder not otherwise specified and major depressive disorder. Last, he is given a diagnosis of Cannabis dependence with physiological dependence, MDMA abuse, hydrocodone abuse, and Robitussin abuse.
Mr. L is maintained on fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime and 0.5 mg in the morning. He receives milieu, individual, group, recreational, and medical therapy while in the hospital. Symptoms abate and he is discharged with a plan to follow up with outpatient providers.
Bottom Line
Abuse of over-the-counter (OTC) drugs, such as diphenhydramine, among youths is a growing problem. Remember to question adolescents who appear intoxicated or to have overdosed not only about abuse of alcohol and illicit substances but also of common—and easily and legally accessible—OTC drugs.
Related Resources
• Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18(2):184-188.
• Thomas A, Nallur DG, Jones N, et al. Diphenhydramine abuse and detoxification: a brief review and case report. J Psychopharmacol. 2009;23(1):101-105.
Drug Brand Names
Diazepam • Valium Hydrocodone • Vicodin
Diphenhydramine • Benadryl Risperidone • Risperdal
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa. gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on September 29, 2014.
2. Yamashiro K, Kiryu J, Tsujikawa A, et al. Suppressive effects of histamine H1 receptor antagonist diphenhydramine on the leukocyte infiltration during endotoxin-induced uveitis. Exp Eye Res. 2001;73(1):69-80.
3. Skidgel RA, Kaplan AP, Erdos EG. Histamine, bradykinin, and their antagonists. In: Brunton L, Chabner B, Knollman B, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw Hill; 2011: 911-935.
4. Vearrier D, Curtis JA. Case files of the medical toxicology fellowship at Drexel University. Rhabdomyolysis and compartment syndrome following acute diphenhydramine overdose. J Med Toxicol. 2011;7(3):213-219.
5. Ho M, Tsai K, Liu C. Diphenhydramine overdose related delirium: a case report. Journal of Emergency and Critical Care Medicine. 2006;17(2):77-79.
6. Krenzelok EP, Anderson GM, Mirick M. Massive diphenhydramine overdose resulting in death. Ann Emerg Med. 1982;11(4):212-213.
7. Inaba AS. Toxicologic teasers: Testing your knowledge of clinical toxicology. Hawaii Med J. 1998;57(4):471-473.
8. Kaplan SL. Busner J. The use of prn and stat medication in three child psychiatric inpatient settings. Psychopharmacol Bull. 1997;33(1):161-164.
9. Radovanovic D, Meier PJ, Guirguis M, et al. Dose-dependent toxicity of diphenhydramine overdose. Hum Exp Toxicol. 2000;19(9):489-495.
CASE Numb and confused
Mr. L, age 17, is admitted to the hospital after ingesting 24 diphenhydramine 25-mg tablets in 3 hours as a possible suicide attempt. His parents witnessed him behaving strangely and brought him to the hospital. They state that their son was visibly agitated and acting inappropriately. He was seen talking to birds, trees, and the walls of the house.
Mr. L says he is upset because he broke up with his girlfriend a week earlier after she asked if they could “take a break.” He says that he took the diphenhydramine because he wanted to “get numb” to deal with the emotional stress caused by the break-up.
After the break-up, Mr. L experienced middle-to-late insomnia and was unable to get more than 3 or 4 hours of sleep a night. He reports significant fatigue, depressed mood, anhedonia, impaired concentration, and psychomotor retardation. He denies homicidal ideation or auditory and visual hallucinations.
As an aside, Mr. L reports that, for the past year, he had difficulties with gender identity, sometimes thinking that he might be better off if he had been born a girl and that he felt uncomfortable in a male body.
Which treatment option would you choose for Mr. L’s substance abuse?
a) refer him to a 12-step program
b) begin supportive measures
c) administer activated charcoal
d) prescribe a benzodiazepine to control agitation
The authors’ observations
As youths gain increasing access to medical and pharmaceutical knowledge through the Internet and other sources, it appears that adolescent drug abuse has, in part, shifted toward more easily attainable over-the-counter (OTC) medications. Diphenhydramine, a first-generation antihistamine, can be abused for its effects on the CNS, such as disturbed coordination, irritability, paresthesia, blurred vision, and depression. Effects of diphenhydramine are increased by the presence of alcohol, monoamine oxidase inhibitors, diazepam, hypnotics, sedatives, tranquilizers, and other CNS depressants. In 2011, diphenhydramine abuse was involved in 19,012 emergency room visits, of which 9,301 were for drug-related suicide attempts.1
Diphenhydramine is an inverse agonist of the histamine H1 receptor.2 It is a member of the ethanolamine subclass of antihistaminergic agents.3 By reversing the effects of histamine on capillaries, diphenhydramine can reduce the intensity of allergic symptoms. Diphenhydramine also crosses the blood–brain barrier and antagonizes H1 receptors centrally.
Used as a common sleep aid and allergy medication, the drug works primarily as an H1 receptor partial agonist, but also is a strong competitive antagonist at muscarinic acetylcholine receptors.4 It is abused for its sedative effects and its capacity to cause delirium and hallucinations.5 Diphenhydramine can have a stimulatory effect in children and young adults, instead of the sedating properties seen in adults.6 Such misuse is concerning because diphenhydramine overdose can lead to delirium, confusion, and hallucinations, tachycardia, seizures, mydriasis, xerostomia, urinary retention, ileus, anhidrosis, and hyperthermia. In severe cases it has been associated with cardiac arrhythmias, rhabdomyolysis, status epilepticus, and death.4,6 Neurologic symptoms of diphenhydramine overdose are listed in Table 1.
HISTORY Polysubstance abuse
Mr. L has a 2-year history of major depressive disorder and a history of Cannabis abuse with physiological dependence; Robitussin (base active ingredient, guaifenesin) and hydrocodone abuse with physiological dependence; 3,4-methylenedioxymethamphetamine (MDMA) abuse; and diphenhydramine abuse. He also has a history of gender dysphoria, although he reports that these feelings have become less severe over the past year.
Mr. L attends bi-weekly appointments with an outpatient psychiatrist and reportedly adheres to his medication regimen: fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime. He denies previous suicidal ideation, suicide attempts, homicidal ideation, or homicidal attempts. He reports no history of physical, sexual, or emotional abuse. He gets good grades in school and has no outstanding academic problems.
Mr. L began using Cannabis at age 14; his last use was 3 weeks before admission. He is guarded about his use of Robitussin, hydrocodone, and MDMA. However, Mr. L reports that he has researched diphenhydramine on the internet and believes that he can safely take up to 1,200 mg without overdosing. He reports normally taking 450 mg of diphenhydramine daily. Mr. L reports difficulty urinating after using diphenhydramine but no other physical complaints.
Mr. L lives with his father and stepmother and has a history of one psychiatric hospitalization at a different facility 2 months ago, followed by outpatient therapy. He obtained his Graduate Equivalency Diploma (GED) and plans to attend college.
At age 5, Mr. L emigrated from Turkey to the United States with his parents. His mother returned to Turkey when he was age 6 and has had no contact with her son since. Whenever Mr. L visits Turkey with his father, the patient refuses to see her, as per collaterals. He gets along well with his stepmother, who is his maternal aunt. Mr. L has been bullied at school and reportedly has few friends.
On mental status examination, Mr. L has an appropriate appearance and appears to be his stated age. He shows good eye contact and is cooperative. Muscle tone and gait are within normal limits. He has no abnormal movements. Speech, thought processes, and associations are normal. He denies auditory hallucinations, visual hallucinations, suicidal ideation (although he presented with a probable suicide attempt), or homicidal ideation. No delusions are elicited.
Mr. L shows poor judgment about his drug use and situation. He demonstrates limited insight, because he says his only goal is to get out of the hospital. He is alert, awake, and oriented to person, place, and time. He shows no memory or knowledge impairment. He appears euthymic with an inappropriate and constricted affect. On neurologic exam, he had mild tremors in his hands. The authors’ observationsTreatment for diphenhydramine overdose should begin quickly to prevent life-threatening effects and reduce the risk for mortality. The toxin can be removed from the patient’s GI tract with activated charcoal or gastric lavage if the patient presents within 1 hour of ingesting the substance. Administering IV fluids will prevent dehydration. Cardiac functioning is monitored and benzodiazepines could be administered to manage seizures.
Key elements of a toxicologic physical examination include:
• eyes: pupillary size, symmetry, and response to light (vertical or horizontal nystagmus)
• oropharynx: moist or dry mucous membranes, presence or absence of the gag reflex, distinctive odors
• abdomen: presence or absence and quality of bowel sounds
• skin: warm and dry, warm and sweaty, or cool
• neurologic: level of consciousness and mental status, presence of tremors, seizures, or other movement disorders, presence or absence and quality of deep tendon reflexes.7
If a child or adolescent patient cannot communicate how much of a drug he (she) has ingested, questions to ask parents or other informants include:
• Was the medication purchased recently, and if so was the bottle or box full before the patient took the pills?
• If the medication was not new, how many pills were in the bottle before the patient got to it?
• If the medication was prescribed, how many pills were originally prescribed, when was the medication prescribed, and how many pills were already taken prior to the patient getting to the bottle?
• How many pills were left in the bottle?
• How many pills were seen around the area where the patient was found?
• How many pills were found in the patient’s mouth?7
Recommendations
It is well known that OTC medication abuse is a growing medical problem (Table 2). Antihistamines, including diphenhydramine, are readily available to minors and adults. Because of the powerful sedating effects of antihistamines, many adolescent health practitioners give them to patients who have insomnia as a sleep aid.8 As in our case, antihistamines are used recreationally for their hallucinogenic effects, at dosages of 300 to 700 mg.9 Severe symptoms of toxicity, such as delirium and psychosis, seizures, and coma, occur at dosages ≥1,000 mg.9
With growing abuse of these medications, we aim to encourage detailed history taking about abuse of OTC drugs, especially diphenhydramine in adolescent patients.
Outcome Improvement, discharge
Mr. L is given a dual diagnosis of diphenhydramine-induced psychotic disorder with
hallucinations and diphenhydramine-induced depressive disorder, both with onset during intoxication. He also is given a provisional diagnosis of psychotic disorder not otherwise specified and major depressive disorder. Last, he is given a diagnosis of Cannabis dependence with physiological dependence, MDMA abuse, hydrocodone abuse, and Robitussin abuse.
Mr. L is maintained on fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime and 0.5 mg in the morning. He receives milieu, individual, group, recreational, and medical therapy while in the hospital. Symptoms abate and he is discharged with a plan to follow up with outpatient providers.
Bottom Line
Abuse of over-the-counter (OTC) drugs, such as diphenhydramine, among youths is a growing problem. Remember to question adolescents who appear intoxicated or to have overdosed not only about abuse of alcohol and illicit substances but also of common—and easily and legally accessible—OTC drugs.
Related Resources
• Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18(2):184-188.
• Thomas A, Nallur DG, Jones N, et al. Diphenhydramine abuse and detoxification: a brief review and case report. J Psychopharmacol. 2009;23(1):101-105.
Drug Brand Names
Diazepam • Valium Hydrocodone • Vicodin
Diphenhydramine • Benadryl Risperidone • Risperdal
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Numb and confused
Mr. L, age 17, is admitted to the hospital after ingesting 24 diphenhydramine 25-mg tablets in 3 hours as a possible suicide attempt. His parents witnessed him behaving strangely and brought him to the hospital. They state that their son was visibly agitated and acting inappropriately. He was seen talking to birds, trees, and the walls of the house.
Mr. L says he is upset because he broke up with his girlfriend a week earlier after she asked if they could “take a break.” He says that he took the diphenhydramine because he wanted to “get numb” to deal with the emotional stress caused by the break-up.
After the break-up, Mr. L experienced middle-to-late insomnia and was unable to get more than 3 or 4 hours of sleep a night. He reports significant fatigue, depressed mood, anhedonia, impaired concentration, and psychomotor retardation. He denies homicidal ideation or auditory and visual hallucinations.
As an aside, Mr. L reports that, for the past year, he had difficulties with gender identity, sometimes thinking that he might be better off if he had been born a girl and that he felt uncomfortable in a male body.
Which treatment option would you choose for Mr. L’s substance abuse?
a) refer him to a 12-step program
b) begin supportive measures
c) administer activated charcoal
d) prescribe a benzodiazepine to control agitation
The authors’ observations
As youths gain increasing access to medical and pharmaceutical knowledge through the Internet and other sources, it appears that adolescent drug abuse has, in part, shifted toward more easily attainable over-the-counter (OTC) medications. Diphenhydramine, a first-generation antihistamine, can be abused for its effects on the CNS, such as disturbed coordination, irritability, paresthesia, blurred vision, and depression. Effects of diphenhydramine are increased by the presence of alcohol, monoamine oxidase inhibitors, diazepam, hypnotics, sedatives, tranquilizers, and other CNS depressants. In 2011, diphenhydramine abuse was involved in 19,012 emergency room visits, of which 9,301 were for drug-related suicide attempts.1
Diphenhydramine is an inverse agonist of the histamine H1 receptor.2 It is a member of the ethanolamine subclass of antihistaminergic agents.3 By reversing the effects of histamine on capillaries, diphenhydramine can reduce the intensity of allergic symptoms. Diphenhydramine also crosses the blood–brain barrier and antagonizes H1 receptors centrally.
Used as a common sleep aid and allergy medication, the drug works primarily as an H1 receptor partial agonist, but also is a strong competitive antagonist at muscarinic acetylcholine receptors.4 It is abused for its sedative effects and its capacity to cause delirium and hallucinations.5 Diphenhydramine can have a stimulatory effect in children and young adults, instead of the sedating properties seen in adults.6 Such misuse is concerning because diphenhydramine overdose can lead to delirium, confusion, and hallucinations, tachycardia, seizures, mydriasis, xerostomia, urinary retention, ileus, anhidrosis, and hyperthermia. In severe cases it has been associated with cardiac arrhythmias, rhabdomyolysis, status epilepticus, and death.4,6 Neurologic symptoms of diphenhydramine overdose are listed in Table 1.
HISTORY Polysubstance abuse
Mr. L has a 2-year history of major depressive disorder and a history of Cannabis abuse with physiological dependence; Robitussin (base active ingredient, guaifenesin) and hydrocodone abuse with physiological dependence; 3,4-methylenedioxymethamphetamine (MDMA) abuse; and diphenhydramine abuse. He also has a history of gender dysphoria, although he reports that these feelings have become less severe over the past year.
Mr. L attends bi-weekly appointments with an outpatient psychiatrist and reportedly adheres to his medication regimen: fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime. He denies previous suicidal ideation, suicide attempts, homicidal ideation, or homicidal attempts. He reports no history of physical, sexual, or emotional abuse. He gets good grades in school and has no outstanding academic problems.
Mr. L began using Cannabis at age 14; his last use was 3 weeks before admission. He is guarded about his use of Robitussin, hydrocodone, and MDMA. However, Mr. L reports that he has researched diphenhydramine on the internet and believes that he can safely take up to 1,200 mg without overdosing. He reports normally taking 450 mg of diphenhydramine daily. Mr. L reports difficulty urinating after using diphenhydramine but no other physical complaints.
Mr. L lives with his father and stepmother and has a history of one psychiatric hospitalization at a different facility 2 months ago, followed by outpatient therapy. He obtained his Graduate Equivalency Diploma (GED) and plans to attend college.
At age 5, Mr. L emigrated from Turkey to the United States with his parents. His mother returned to Turkey when he was age 6 and has had no contact with her son since. Whenever Mr. L visits Turkey with his father, the patient refuses to see her, as per collaterals. He gets along well with his stepmother, who is his maternal aunt. Mr. L has been bullied at school and reportedly has few friends.
On mental status examination, Mr. L has an appropriate appearance and appears to be his stated age. He shows good eye contact and is cooperative. Muscle tone and gait are within normal limits. He has no abnormal movements. Speech, thought processes, and associations are normal. He denies auditory hallucinations, visual hallucinations, suicidal ideation (although he presented with a probable suicide attempt), or homicidal ideation. No delusions are elicited.
Mr. L shows poor judgment about his drug use and situation. He demonstrates limited insight, because he says his only goal is to get out of the hospital. He is alert, awake, and oriented to person, place, and time. He shows no memory or knowledge impairment. He appears euthymic with an inappropriate and constricted affect. On neurologic exam, he had mild tremors in his hands. The authors’ observationsTreatment for diphenhydramine overdose should begin quickly to prevent life-threatening effects and reduce the risk for mortality. The toxin can be removed from the patient’s GI tract with activated charcoal or gastric lavage if the patient presents within 1 hour of ingesting the substance. Administering IV fluids will prevent dehydration. Cardiac functioning is monitored and benzodiazepines could be administered to manage seizures.
Key elements of a toxicologic physical examination include:
• eyes: pupillary size, symmetry, and response to light (vertical or horizontal nystagmus)
• oropharynx: moist or dry mucous membranes, presence or absence of the gag reflex, distinctive odors
• abdomen: presence or absence and quality of bowel sounds
• skin: warm and dry, warm and sweaty, or cool
• neurologic: level of consciousness and mental status, presence of tremors, seizures, or other movement disorders, presence or absence and quality of deep tendon reflexes.7
If a child or adolescent patient cannot communicate how much of a drug he (she) has ingested, questions to ask parents or other informants include:
• Was the medication purchased recently, and if so was the bottle or box full before the patient took the pills?
• If the medication was not new, how many pills were in the bottle before the patient got to it?
• If the medication was prescribed, how many pills were originally prescribed, when was the medication prescribed, and how many pills were already taken prior to the patient getting to the bottle?
• How many pills were left in the bottle?
• How many pills were seen around the area where the patient was found?
• How many pills were found in the patient’s mouth?7
Recommendations
It is well known that OTC medication abuse is a growing medical problem (Table 2). Antihistamines, including diphenhydramine, are readily available to minors and adults. Because of the powerful sedating effects of antihistamines, many adolescent health practitioners give them to patients who have insomnia as a sleep aid.8 As in our case, antihistamines are used recreationally for their hallucinogenic effects, at dosages of 300 to 700 mg.9 Severe symptoms of toxicity, such as delirium and psychosis, seizures, and coma, occur at dosages ≥1,000 mg.9
With growing abuse of these medications, we aim to encourage detailed history taking about abuse of OTC drugs, especially diphenhydramine in adolescent patients.
Outcome Improvement, discharge
Mr. L is given a dual diagnosis of diphenhydramine-induced psychotic disorder with
hallucinations and diphenhydramine-induced depressive disorder, both with onset during intoxication. He also is given a provisional diagnosis of psychotic disorder not otherwise specified and major depressive disorder. Last, he is given a diagnosis of Cannabis dependence with physiological dependence, MDMA abuse, hydrocodone abuse, and Robitussin abuse.
Mr. L is maintained on fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime and 0.5 mg in the morning. He receives milieu, individual, group, recreational, and medical therapy while in the hospital. Symptoms abate and he is discharged with a plan to follow up with outpatient providers.
Bottom Line
Abuse of over-the-counter (OTC) drugs, such as diphenhydramine, among youths is a growing problem. Remember to question adolescents who appear intoxicated or to have overdosed not only about abuse of alcohol and illicit substances but also of common—and easily and legally accessible—OTC drugs.
Related Resources
• Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18(2):184-188.
• Thomas A, Nallur DG, Jones N, et al. Diphenhydramine abuse and detoxification: a brief review and case report. J Psychopharmacol. 2009;23(1):101-105.
Drug Brand Names
Diazepam • Valium Hydrocodone • Vicodin
Diphenhydramine • Benadryl Risperidone • Risperdal
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa. gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on September 29, 2014.
2. Yamashiro K, Kiryu J, Tsujikawa A, et al. Suppressive effects of histamine H1 receptor antagonist diphenhydramine on the leukocyte infiltration during endotoxin-induced uveitis. Exp Eye Res. 2001;73(1):69-80.
3. Skidgel RA, Kaplan AP, Erdos EG. Histamine, bradykinin, and their antagonists. In: Brunton L, Chabner B, Knollman B, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw Hill; 2011: 911-935.
4. Vearrier D, Curtis JA. Case files of the medical toxicology fellowship at Drexel University. Rhabdomyolysis and compartment syndrome following acute diphenhydramine overdose. J Med Toxicol. 2011;7(3):213-219.
5. Ho M, Tsai K, Liu C. Diphenhydramine overdose related delirium: a case report. Journal of Emergency and Critical Care Medicine. 2006;17(2):77-79.
6. Krenzelok EP, Anderson GM, Mirick M. Massive diphenhydramine overdose resulting in death. Ann Emerg Med. 1982;11(4):212-213.
7. Inaba AS. Toxicologic teasers: Testing your knowledge of clinical toxicology. Hawaii Med J. 1998;57(4):471-473.
8. Kaplan SL. Busner J. The use of prn and stat medication in three child psychiatric inpatient settings. Psychopharmacol Bull. 1997;33(1):161-164.
9. Radovanovic D, Meier PJ, Guirguis M, et al. Dose-dependent toxicity of diphenhydramine overdose. Hum Exp Toxicol. 2000;19(9):489-495.
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa. gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on September 29, 2014.
2. Yamashiro K, Kiryu J, Tsujikawa A, et al. Suppressive effects of histamine H1 receptor antagonist diphenhydramine on the leukocyte infiltration during endotoxin-induced uveitis. Exp Eye Res. 2001;73(1):69-80.
3. Skidgel RA, Kaplan AP, Erdos EG. Histamine, bradykinin, and their antagonists. In: Brunton L, Chabner B, Knollman B, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw Hill; 2011: 911-935.
4. Vearrier D, Curtis JA. Case files of the medical toxicology fellowship at Drexel University. Rhabdomyolysis and compartment syndrome following acute diphenhydramine overdose. J Med Toxicol. 2011;7(3):213-219.
5. Ho M, Tsai K, Liu C. Diphenhydramine overdose related delirium: a case report. Journal of Emergency and Critical Care Medicine. 2006;17(2):77-79.
6. Krenzelok EP, Anderson GM, Mirick M. Massive diphenhydramine overdose resulting in death. Ann Emerg Med. 1982;11(4):212-213.
7. Inaba AS. Toxicologic teasers: Testing your knowledge of clinical toxicology. Hawaii Med J. 1998;57(4):471-473.
8. Kaplan SL. Busner J. The use of prn and stat medication in three child psychiatric inpatient settings. Psychopharmacol Bull. 1997;33(1):161-164.
9. Radovanovic D, Meier PJ, Guirguis M, et al. Dose-dependent toxicity of diphenhydramine overdose. Hum Exp Toxicol. 2000;19(9):489-495.
Allergic Contact Dermatitis to 2-Octyl Cyanoacrylate
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
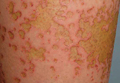
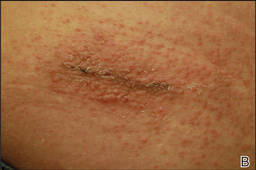
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
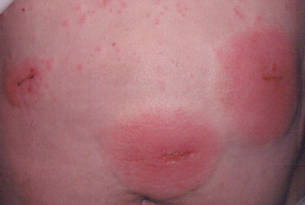
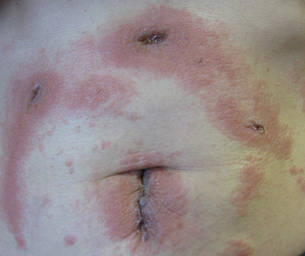
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.
|
| Figure 2. Acute eczematous plaques at wound closures. |
|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
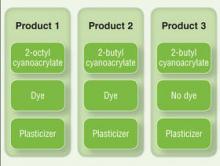
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.

|
| Figure 2. Acute eczematous plaques at wound closures. |

|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.

|
| Figure 2. Acute eczematous plaques at wound closures. |

|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Practice Points
- It is important for physicians to recognize that skin adhesives are a potential source of allergic contact dermatitis (ACD) in a postsurgical setting.
- There are 3 primary components of skin adhesives that are potential contactants, including a cyanoacrylate, a plasticizer, and a dye.
- Treatment of ACD to skin adhesives is straightforward, including removal of any remaining adhesive and applying topical steroids.
Metastatic Brain Tumors
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Systemic cancer can affect the central nervous system in several different ways, including direct tumor metastasis and indirect remote effects. Intracranial metastasis can involve the skull, dura, and leptomeninges (arachnoid and pia mater), as well as the brain parenchyma. Of these, parenchymal brain metastases are the most common and have been found in as many as 24% of cancer patients in autopsy studies. It has been reported that metastatic brain tumors outnumber primary brain tumors 10 to 1.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Systemic cancer can affect the central nervous system in several different ways, including direct tumor metastasis and indirect remote effects. Intracranial metastasis can involve the skull, dura, and leptomeninges (arachnoid and pia mater), as well as the brain parenchyma. Of these, parenchymal brain metastases are the most common and have been found in as many as 24% of cancer patients in autopsy studies. It has been reported that metastatic brain tumors outnumber primary brain tumors 10 to 1.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Systemic cancer can affect the central nervous system in several different ways, including direct tumor metastasis and indirect remote effects. Intracranial metastasis can involve the skull, dura, and leptomeninges (arachnoid and pia mater), as well as the brain parenchyma. Of these, parenchymal brain metastases are the most common and have been found in as many as 24% of cancer patients in autopsy studies. It has been reported that metastatic brain tumors outnumber primary brain tumors 10 to 1.
To read the full article in PDF:
Defecation Disorders: Diagnosis and Treatment
From the Digestive Health Center, Medical College of Georgia, Georgia Regents University, Augusta, GA
Defecation is a coordinated process that involves generation of sufficient propulsive forces in the abdomen and rectum together with relaxation of the puborectalis and external anal sphincter. Likewise, continence involves conscious retention of bowel contents until stool or gas can be voluntarily eliminated in an appropriate fashion. A failure of these processes leads to altered bowel function and disorders of defecation that are commonly encountered in clinical practice. They include a diverse group of maladies that result in altered defecation. Among them are functional disorders, such as dyssynergic defecation, and mechanical/structural disorders, such as rectocele, solitary rectal ulcer syndrome (SRUS), excessive perineal descent, and rectal prolapse. This article discusses 3 cases that illustrate the clinical features and management approaches to dyssynergic defecation, SRUS, and fecal incontinence.
Case Study 1
Presentation and History
A 26-year-old white woman with a 10-year history of constipation presents to a gastroenterologist after referral from her primary care physician. She reports spontaneous bowel movements once every 2 weeks, and often she has to induce stools by using enemas or suppositories. Stooling became progressively more difficult for her during her teenage years, with infrequent bowel movements and hard stools (type 1–2 on Bristol stool scale). She also reports having to strain excessively during bowel movements, and on average she spends 30 minutes in the bathroom. She denies experiencing any perianal pain or bleeding or using manual maneuvers to defecate, but she often feels a sense of incomplete evacuation. She also describes intermittent abdominal pain and bloating.
She has tried several over-the-counter laxatives, includ-ing milk of magnesia, senna, and magnesium citrate. Most recently, she tried lubiprostone and polyethylene glycol without improvement. Her past medical history is significant for endometriosis, exploratory laparotomy, and 1 vaginal delivery. There is no family history of colorectal cancer or inflammatory bowel disease. She works as a truck driver and does not use alcohol, illicit drugs, or tobacco. There is no history of physical or sexual abuse. Her current medications include lubiprostone 24 µg twice daily, polyethylene glycol 17 g twice daily, and a birth control pill.
Physical Examination
On physical examination, the patient appears healthy without any distress. Her body mass index is 26 kg/m2, and vital signs are normal. General examination is normal. Abdomen is flat, and bowel sounds are normal. Mild tenderness is noted in both lower quadrants. Rectal examination reveals normal anal skin folds. Digital exam-ination reveals a normal resting tone with pellet-like stool that is heme-negative. When asked to attempt defecation, she shows poor perineal descent and paradoxical contraction of the anal sphincter.
Laboratory Evaluation
Laboratory testing reveals normal levels of thyrotropin and thyroxine, no anemia on complete blood count, and normal levels of calcium, glucose, and electrolytes.
What are the possible causes for this patient’s altered bowel habits?
What is the approach to physical examination in patients with constipation?
Causes of Constipation
Constipation is a common digestive disorder, affecting up to 20% of the world’s population [1]. Primary or idiopathic constipation consists of 3 common overlapping subtypes: slow-transit constipation, dyssynergic defecation, and constipation-predominant irritable bowel syndrome. Slow-transit constipation involves the slow movement of stool through the colon. This is usually seen on a colonic transit study or with wireless motility capsule study. Dyssynergia in general is caused by functional outlet obstruction with or without normal colonic transit. Patients with dyssynergia often complain of incomplete evacuation, excessive straining, bloating, and blockage [2]. Often patients with dyssynergia resort to manual disimpaction/vaginal splinting and/or abdominal pressure to facilitate bowel movements. Secondary constipation may result from metabolic disorders (eg, hypercalcemia and hypokalemia, disorders associated with renal failure, hypothyroidism, and diabetes) as well as medications, including narcotics, anticholinergics, and antidepressants.
Rectal Examination
Physical examination in patients with constipation should include a detailed rectal examination. The perianal skin should be inspected closely for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with resting and squeeze anal tone. A study by Rao et al[3] showed that rectal examination could identify 76% of patients with dyssynergia. The sensitivity and positive predictive value for diagnosing dyssynergia with digital rectal examination was 81% and 99%, respectively, making it a good screening test for dyssynergia [3].
When is colonoscopy indicated in the workup of constipation?
What imaging studies may be useful?
Colonoscopy
Colonoscopic evaluation is only indicated in patients with alarming features such as rectal bleeding, weight loss, unex-plained abdominal pain, palpable mass in the abdomen or rectum, persistent and unexplained anal/rectal pain, or anemia, as well as in patients over age 50 years [4].
Colonic Transit Study
Two imaging studies can be useful in the evaluation of a patient with constipation: colonic transit study and defeco-graphy. A colonic transit study provides useful information regarding the rate at which stool travels through the colon. This test is performed by administering one capsule (Sitzmarks, Konsyl Pharmaceuticals, Fort Worth, TX) containing radiopaque markers. A plain radiograph of the abdomen is obtained on day 6 (120 hr after ingestion of capsule). A transit study is considered abnormal if more than 20% of markers (> 5) are present on a plain radiograph of the abdomen. Approximately two-thirds of patients with dyssynergia have an abnormal colonic transit study, with retention of markers either in the rectosigmoid region or throughout the colon [5]. Wireless motility capsule is a newer test that is comprised of ingesting a capsule and wearing a recorder for up to 5 days. This test measures regional transit (ie, gastric emptying, colonic transit time, and whole gut transit time), is standardized and validated, and avoids use of radiation [6].
Defecography
Defecography is conducted by instilling a barium paste in the rectum and monitoring evacuation of the barium radiologically. It can reveal poor activation of the levator ani muscles, prolonged retention of the barium, inability to expel the barium, absence of a striping wave, rectal mucosal intussusception, rectocele, abnormal perineal descent, or rectal prolapse [5]. Although abnormalities are frequently found on defecography, they may not translate into clinical dysfunction. In one study, 77% of women with complaints of defecation disorders had abnormalities on defecography, but there was no relationship between the abnormalities and the patients’ symptoms [7]. Hence, defecography is not recommended unless there is clinical suspicion of prolapse or excessive descent. Endoanal and dynamic pelvic magnetic resonance imaging (MRI) can evaluate global pelvic floor anatomy in dynamic function [8]. Dynamic MRI in the seated position provides the most physiologic approach.
What testing is needed to make a diagnosis of dyssynergic defecation?
Both an abnormal balloon expulsion test and an abnormal pattern of defecation on anal rectal manometry are required to diagnose dyssynergic defecation [9]. Anorectal manometry provides information regarding rectal and anal pressures at rest and during maneuvers of simulated defecation as well as information on rectal sensation, rectoanal reflexes, and compliance [2,10]. There are 4 patterns of dyssynergia found on anorectal manometry: type 1, normal push effort with paradoxical contraction of the anal sphincter; type 2, poor push effort with paradoxical contraction of the anal sphincter; type 3, normal push effort with incomplete or absent relaxation of the anal sphincter; and type 4, poor push with incomplete anal relaxation. The balloon expulsion test should be included in the work-up of dyssynergia.
Normal subjects can expel a 50-mL water-filled balloon in less than 1 minute. Although normal patients can show a dyssynergic pattern in the left lateral decubitus position, when seated on a commode and with a sensation of stooling most exhibit a normal pattern of defecation [9].
Diagnosis
What treatment options are available for dyssynergia?
The treatment of patients with dyssynergic defecation consists of standard therapies for constipation, including diet, laxatives, and timed toileting. Medical therapy includes laxatives, polyethylene glycol, and lubiprostone.
Case Study 2
Initial Presentation and History
A 39-year-old woman presents with a 5-year history of intermittent bright red blood with stooling. Most often, she notices blood on the toilet paper or when wiping and rarely in the commode. She reports having experienced difficulty with bowel movements since her teens. She does not have a daily urge but strains up to 30 minutes to pass stool that is hard in consistency (type 1–2 on the Bristol stool scale). Over the past year, she has started using fingers to remove stool.
The patient reports bloating and abdominal discomfort that is improved with stooling. Her weight has been stable. Current medications include polyethylene glycol 17 g twice daily, sodium docusate 100 mg twice daily, iron sulfate 325 mg 3 times daily, and a birth control pill. Her past medical history is significant for iron deficiency anemia. Family history is notable for her mother and sister with similar “bowel troubles,” but no family history of inflammatory bowel disease or colorectal cancer. She is a salesperson and has been married for 7 years. She does not use tobacco or alcohol. As a child, she was sexually abused. She did not receive any formal counseling for the abuse. Review of systems is negative.
Physical Examination
General and neurologic examinations are normal. The abdomen is mildly distended, bowel sounds are normal, there is mild tenderness, and stool is palpable in the left lower quadrant. Rectal examination reveals normal anal skin with no fissures, intact anocutaneous reflex, and hard stool in the rectal vault that is guaiac-positive. The resting anal sphincter tone is elevated, and when asked to attempt defecation, there is excessive perineal descent and rectal mucosal intussusception with paradoxical anal contraction.
Laboratory Evaluation and Endoscopy
What is SRUS and how is it diagnosed?
Evaluation and Diagnosis
SRUS is characterized by single or multiple ulcerations of the rectal mucosa along with distinct pathologic changes [17]. The term solitary rectal ulcer is a misnomer because many patients have more than 1 lesion, and it is not always an ulcer. Patients with SRUS present with several symptoms, but the most common is passage of blood or mucus, and up to 26% may be asymptomatic [18]. The pathophysiology of this condition is poorly understood. Multiple mechanisms have been implicated, including occult or overt rectal prolapse, dyssynergia, rectal mucosal intussusception, rectal hypersensitivity with a persistent feeling of a need to defecate, and reduced mucosal blood flow [19].
The diagnosis of SRUS is based on the patient’s clinical history combined with endoscopy and histopathology findings. Endoscopically, the lesions may vary in appearance. Shallow ulcerations on hyperemic surrounding mucosa located on the anterior wall is the most common finding [17]. Lesions vary in size, although most are 1 to 1.5 cm in diameter [17] and rarely involve more than half the circumference of the rectal wall. Polypoid lesions occur in approximately 25% of patients with SRUS, and multiple lesions occur in 30% [17].
Obtaining specimens for histology is an important step in the evaluation of SRUS. The differential diagnosis includes Crohn’s disease, ulcerative colitis, ischemic colitis, and malignancy. The typical histologic findings include fibromuscular hyperplasia with smooth muscle infiltration of the lamina propria, thickening of the muscularis mucosa, regenerative changes, and distortion of the crypt architecture [17].
Are physiologic or imaging studies helpful in the diagnosis of SRUS?
Two complementary physiologic tests for SRUS are anorectal manometry and defecography. Anorectal manometry often shows evidence of dyssynergia and rectal hypersensitivity in patients with SRUS [20,21]. Hyper-sensitivity may produce a sensation of incomplete evacuation, which in turn results in excessive straining. Defecography may reveal rectal mucosal intussusception or overt rectal prolapse. The patient in this case had evidence of rectal hypersensitivity on anorectal manometry along with excessive perineal descent on defecography.
What are treatment options for SRUS?
Treatment of SRUS is not standardized. The options include topical medical therapy, biofeedback, and surgery. Uncontrolled studies have suggested that 5-aminosalicylic acid enema [22], sucralfate enema [23], steroid enema [24], and fibrin glue [25] may improve symptoms. Patients who fail topical therapy and have evidence of dyssynergia on anorectal manometry should receive biofeedback therapy. A case-control study of biofeedback involving 11 patients with refractory SRUS and 15 healthy controls showed improvement in anorectal function, including dyssynergia [21]. At follow-up endoscopy, 36% had complete mucosal healing and more than 50% showed partial healing. In a study involving 16 patients with SRUS and 26 healthy controls, Jarrett et al [26] showed that 75% of patients who underwent biofeedback therapy had improved and 31% had ulcer resolution. Surgical therapy should be considered in rare patients who are refractory to medical therapy. The Delorme procedure is commonly performed with a success rate of 42% to 100% [27].
The case patient underwent biofeedback therapy, and after 5 sessions had complete healing of the lesion and resolution of rectal bleeding and bowel symptoms.
Case Study 3
Initial Presentation and History
A 75-year-old woman is referred to a gastroenterologist with complaints of incomplete stool evacuation and intermittent fecal seepage. She passes stools daily but sits on the toilet for 15 to 20 minutes, and after straining will pass only a small amount of stool. She describes stools as type 4 on the Bristol scale with no blood or mucus. One to 2 hours after a bowel movement, she experiences some wetness in the perineal region and upon checking often notices that a tablespoon full of stool material has leaked out. Sometimes, she will pass another large stool. She denies any leakage of stool while sleeping. Occasionally, she has urgency and leaks stool before reaching the toilet. In the past, she has used digital maneuvers to facilitate stooling. This problem has interfered with shopping, socializing, and taking vacations.
Her past medical history is significant for narcolepsy, hypertension, tubal ligation, appendectomy, and inguinal hernia repair. Obstetric history is significant for 6 vaginal deliveries, 1 requiring episiotomy but no forceps use. Her current medications include estradiol vaginal cream, hydrochlorothiazide, pilocarpine, and amitriptyline 10 mg 3 times daily. She also reports stress urinary incontinence, particularly with sneezing and coughing.
Physical Examination
Physical examination reveals a well-nourished woman with normal vital signs and a normal general examination. Abdominal examination is normal. A rectal examination shows no fissures, but the anocutaneous reflex is absent on the right side. Resting and squeeze sphincter tones are normal, with good perineal descent and normal anal relaxation.
Laboratory Evaluation
What are the mechanisms involved in fecal incontinence?
What are the 3 clinical subtypes of fecal incontinence?
Mechanisms and Subtypes
Fecal incontinence is often an unvoiced problem that causes significant social stigma. Approximately 2% of the US population suffers from fecal incontinence [28], with a higher prevalence among women and elderly persons. Several mechanisms are involved in the pathogenesis of fecal incontinence. A common cause is injury to the external or internal anal sphincter, puborectalis muscle, or pudendal nerves, often after obstetric trauma. Hence, a detailed obstetric history including number of vaginal deliveries, use of forceps, tears, and episiotomy is important. Sphincter disruption, most commonly after surgery for hemorrhoid or anal fissure, can result in incontinence. Likewise, reduced rectal compliance causes urgency and fecal incontinence. Impaired rectal sensation results in the accumulation of stool and overflow. Patients rarely have a single cause, with 80% having more than one factor that leads to incontinence [29].
Clinically, fecal incontinence can be classified into 3 categories. Urge incontinence is characterized by the inability to control stool discharge despite active attempts to retain contents. These patients often have disruption or injury to the external anal sphincter. Fecal seepage is the involuntary discharge of less than 2 tablespoons of stool matter without awareness. Seepage can result from impaired rectal evacuation and dyssynergia. Often patients with seepage complain of incomplete evacuation. Passive incontinence refers to the involuntary discharge of stool contents without awareness. These patients often have underlying neuropathy and sphincter weakness [30,31].
What is the approach to evaluation and diagnosis?
Evaluation and Diagnosis
Physical examination of patients with fecal incontinence should include a detailed rectal examination, similar to the exam performed in patients who present with constipation. It should include perineal inspection for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with the resting and squeeze sphincter tone and sphincter relaxation. Further investigations should focus on determining the underlying mechanism in order to facilitate treatment.
Endoscopic investigation should be performed to exclude mucosal disease or malignancy. Anorectal manometry provides objective information regarding resting and squeeze anal sphincter tone, rectal compliance, rectal sensitivity, and rectoanal reflexes [29]. Some experts believe that anorectal manometry is not needed for diagnosis and emphasize the importance of rectal examination and history [32]. Proponents of anorectal manometry point out the importance of physiologic data that can be gained and how it may direct therapy. For example, anorectal manometry and sensory testing may reveal weak anal sphincters and impaired rectal sensation. The latter cannot be identified by clinical evaluation alone. These 2 pathophysiologic findings could enable the biofeedback therapist to focus on improving both anal sphincter tone and rectal sensation [33]. Defecography may reveal anterior rectocele, mucosal intussusception, or rectal prolapse. Anal ultrasound provides information on the structural integrity of the external and internal anal sphincters [34]. Ultrasound is widely available and is relatively inexpensive. Endoanal MRI may provide better information regarding the integrity of the external anal sphincter [35].
What are the treatment options?
The goal of treatment is to restore continence and quality of life. General considerations include stool bulking agents such as fiber supplements. Antidiarrheal agents, such as loperamide and diphenoxylate/atropine, are useful as they can decrease stool volume and increase and prolong sphincter pressure and colonic transit time [36,37]. Patients with diarrhea and functional incontinence may benefit from treatment with cholestyramine [38]. Biofeedback therapy improves sphincter tone and rectal sensation [39]. The number of biofeedback sessions is titrated to the patient’s needs, but often 6 sessions are required [40]. Generally, a 70% success rate has been described. Table 4 summarizes recent evidence supporting the use of biofeedback in the treatment of fecal incontinence [41–46].
Surgery for incontinence should be reserved for patients who have failed aggressive conservative management and biofeedback therapy. Overlapping sphincteroplasty is the most common surgery performed for fecal incontinence, with a success rate between 35% and 70% [47,48]. Creation of a neosphincter via dynamic graciloplasty or artificial sphincter has been tried in patients with an irreversibly damaged anal sphincter, but the success rate is low and the complication rate is high [49].
Sacral nerve stimulation (SNS) involves inserting electrodes in the lower back and connecting them to a pulse generator that produces pulses of electricity that innervate the nerves controlling the anal sphincters. Two double-blind crossover studies have reported a beneficial effect of SNS in fecal incontinence [50,51]. In 19 patients who preferred the periods when the stimulator was turned on, the median number of fecal incontinence episodes per week decreased from 1.7 to 0.7, and in the 5 patients who preferred the off period, the median number of fecal incontinence episodes per week increased from 1.7 to 3.7. SNS is now approved by FDA and insurance payers. Recently, hyaluronic acid/dextranomer injection (Solesta, Salix Pharmaceuticals, Raleigh, NC) has also been approved by FDA and has been shown to improve incontinence. A randomized controlled trial showed a 52% response rate to hyaluronic acid/dextranomer compared to a 31% response with placebo [52].
Conclusion
The 3 cases presented illustrate the complexities of several common anorectal disorders. A definitive diagnosis can be established in patients with defecation disorders through systematic evaluations and physiologic and imaging studies. Diagnosis in turn can pave the way for appropriate medical, behavioral, or surgical treatment. If facilities for appropriate testing are unavailable, it is important to refer these patients to appropriate specialists instead of embarking on empirical therapies which may prove futile. Treatment is often possible, and in a majority of patients their symptoms can be ameliorated.
Corresponding author: Satish S.C. Rao, MD, PhD, Section of Gastroenterology and Hepatology, Medical College of Georgia, Georgia Regents University, BB R2540, 1120 15th St., Augusta, GA 30912.
Funding/support: Portions of this work were supported by National Institutes of Health grant RO1 DK 57100-05.
1. Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004;99:750–9.
2. Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterol Motil 2004;16:580–96.
3. Rao SS, Tantiphlachiva K, Rao PS, et al. How useful is digital rectal examination in the assessment of patients with dyssynergia? Am J Gastroenterol 2007;S268:419.
4. Pepin C, Ladabuam U. The yield of lower endoscopy in patients with constipation: survey of a university hospital, public county hospital, and a Veterans Administration medical center. Gastrointest Endosc 2002;56:325–32.
5. Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am 2003;32:659–83.
6. Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil; 2011; 23: 8-23.
7. Savoye-Collet C, Savoye G, Koning E, et al. Defecography in symptomatic older women living at home. Age Ageing 2003;32:347–50.
8. Bolog N, Weishaupt D. Dynamic MR imaging of outlet obstruction. Rom J Gastroenterol 2005;14:293–302.
9. Rao SS, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol 2006;101:2790–6.
10. Karlbom U, Lundin E, Graf W, Pahlman L. Anorectal physiology in relation to clinical subgroups of patients with severe constipation. Colorectal Dis 2004;6:343–9.
11. Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130:657–64.
12. Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007;5:331–8.
13. Chiarioni G, Saladini L, Whitehead W. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129:86–97.
14. Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to alternate treatments for patients with pelvic floor dyssynergia-type defecation [abstract]. Am J Gastroenterol 2005;100:S335.
15. Hallan RI, Williams NS, Melling J, et al. Treatment of anismus in intractable constipation with botulinum A toxin. Lancet 1988;2:714–7.
16. Boccasanta P, Venturi M, Stuto A, et al. Stapled transanal rectal resection for outlet obstruction: a prospective, multicenter trial. Dis Colon Rectum 2004;47:1285–97.
17. Sharara AI, Azar C, Amr SS, et al. Solitary rectal ulcer syndrome: endoscopic spectrum and review of the literature. Gastrointest Endosc 2005;62:755–62.
18. Tjandra JJ, Fazio VW, Church JM, et al. Clinical conundrum of solitary rectal ulcer. Dis Colon Rectum 1992;35:227–34.
19. Felt-Bersma RJ, Cuesta A. Rectal prolapse, rectal intussusception, rectocele, and solitary rectal ulcer syndrome. Gastroenterol Clin North Am 2001;30:199–222.
20. Vaizey CJ, van den Bogaerde JB, Emmanuel AV, et al. Solitary rectal ulcer syndrome. Br J Surg 1998;85:1617–23.
21. Rao SS, Ozturk R, De Ocampo S, Stessman M. Pathophysiology and role of biofeedback therapy in solitary rectal ulcer syndrome. Am J Gastroenterol 2006;101:613–8.
22. Kumar M, Puri AS, Srivastava R, Yachha SK. Solitary rectal ulcer syndrome in a child treated with local sulfasalazine. Indian Pediatr 1994;31:1553–5.
23. Zargar SA, Khuroo MS, Mahajan R. Sucralfate retention enemas in solitary rectal ulcer. Dis Colon Rectum 1991;34:455–7.
24. Bishop PR, Nowicki MJ. Nonsurgical therapy for solitary rectal ulcer syndrome. Curr Treat Options Gastroenterol 2002;5:215–23.
25. Ederle A, Bulighin G, Orlandin PG, Pilati S. Endoscopic application of human fibrin sealant in the treatment of solitary rectal ulcer syndrome [letter]. Endoscopy 1992;24:736–7.
26. Jarrett ME, Emmanuel AV, Vaizey CJ, Kamm MA. Behavioural therapy (biofeedback) for solitary rectal ulcer syndrome improves symptoms and mucosal blood flow. Gut 2004;53:368–70.
27. Tweedie DJ, Varma JS. Long-term outcome of laparoscopic mesh rectopexy for solitary rectal ulcer syndrome. Colorectal Dis 2005;7:151–5.
28. Rao SS. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastrenterol 2004;99:1585–604.
29. Rao SS, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol 1997;92:469–75.
30. Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 2004;126(1 Suppl 1):S14–22.
31. Deutekom M, Dobben AC, Terra MP, et al. Clinical presentation of fecal incontinence and anorectal function: what is the relationship? Am J Gastroenterol 2007;102:351–61.
32. Wald A. Con: anorectal manometry and imaging are not necessary in patients with fecal incontinence. Am J Gastroenterol 2006;101:2681–3.
33. Bharucha AE. Pro: anorectal testing is useful in fecal incontinence. Am J Gastroenterol 2006;101:2679–81.
34. Tuteja AK, Rao SS. Review article: recent trends in diagnosis and treatment of faecal incontinence. Aliment Pharmacol Ther 2004;19:829–40.
35. Terra MP, Beets-Tan RG, van der Hulst VP, et al. MRI in evaluating atrophy of the external anal sphincter in patients with fecal incontinence. AJR Am J Roentgenol 2006;187:991–9.
36. Sun WM, Read NW, Verlinden M. Effects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinence. Scan J Gastroenterol 1997;32:34–8.
37. Harford WV, Krejs GJ, Santa Ana CA, Fordtran JS. Acute effect of diphenoxylate with atropine (Lomotil) in patients with chronic diarrhea and fecal incontinence. Gastroenterol 1980;78:440–3.
38. Remes-Troche JM, Ozturk R, Philips C, et al. Cholesteryramine—a useful adjunct for the treatment of patients with fecal incontinence. Int J Colorectal Dis 2008;23:189–99.
39. Rao SS. The technical aspects of biofeedback therapy for defecation disorders. Gastroenterologist 1998;6:96–103.
40. Rao SS, Welcher KD, Happel J. Can biofeedback therapy improve anorectal function in fecal incontinence? Am J Gastroenterol 1966;91:2360–6.
41. Byrne CM, Solomon MJ, Young JM, et al. Biofeedback for fecal incontinence: short-term outcomes of 513 consecutive patients and predictors of successful treatment. Dis Colon Rectum 2007;50:417–27.
42. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenteroloy 2003;125:1320–9.
43. Heymen S, Whitehead W. EMG biofeedback vs. Kegel exercise for the treatment of fecal incontinence. Gastroenterology 2007;132:A–83.
44. Pager CK, Solomon MJ, Rex J, Roberts RA. Long-term outcomes of pelvic floor exercise and biofeedback treatment for patients with fecal incontinence. Dis Colon Rectum 2002;45:997–1003.
45. Ozturk R, Niazi S, Stessman M, Rao SS. Long-term and objective changes of anorectal function after biofeedback therapy for faecal incontinence. Aliment Pharmacol Ther 2004;20:667–74.
46. Byrne CM, Solomon MJ, Rex J, et al. Telephone vs. face-to-face biofeedback for fecal incontinence: comparison of two techniques in 239 patients. Dis Colon Rectum 2005;48:2281–8.
47. Zorcolo L, Covotta L, Bartolo DC. Outcome of anterior sphincter repair for obstetric injury: comparison of early and late results. Dis Colon Rectum 2005;48:524–31.
48. Madoff RD. Surgical treatment options for fecal incontinence. Gastroenterology 2004;126(1 Suppl 1)S48–54.
49. Thornton MJ, Kennedy ML, Lubowski DZ, King DW. Long-term follow-up of dynamic graciloplasty for faecal incontinence. Colorectal Dis 2004;6:470–6.
50. Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg 2005;242:662–9.
51. Vaizey CJ, Kamm MA, Nicholls RJ. Double-blind crossover study of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 2000;43:298–302.
52. Graf W, Mellgren A, Matzel KE, et al. . Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet;2011;377:997–1003.
From the Digestive Health Center, Medical College of Georgia, Georgia Regents University, Augusta, GA
Defecation is a coordinated process that involves generation of sufficient propulsive forces in the abdomen and rectum together with relaxation of the puborectalis and external anal sphincter. Likewise, continence involves conscious retention of bowel contents until stool or gas can be voluntarily eliminated in an appropriate fashion. A failure of these processes leads to altered bowel function and disorders of defecation that are commonly encountered in clinical practice. They include a diverse group of maladies that result in altered defecation. Among them are functional disorders, such as dyssynergic defecation, and mechanical/structural disorders, such as rectocele, solitary rectal ulcer syndrome (SRUS), excessive perineal descent, and rectal prolapse. This article discusses 3 cases that illustrate the clinical features and management approaches to dyssynergic defecation, SRUS, and fecal incontinence.
Case Study 1
Presentation and History
A 26-year-old white woman with a 10-year history of constipation presents to a gastroenterologist after referral from her primary care physician. She reports spontaneous bowel movements once every 2 weeks, and often she has to induce stools by using enemas or suppositories. Stooling became progressively more difficult for her during her teenage years, with infrequent bowel movements and hard stools (type 1–2 on Bristol stool scale). She also reports having to strain excessively during bowel movements, and on average she spends 30 minutes in the bathroom. She denies experiencing any perianal pain or bleeding or using manual maneuvers to defecate, but she often feels a sense of incomplete evacuation. She also describes intermittent abdominal pain and bloating.
She has tried several over-the-counter laxatives, includ-ing milk of magnesia, senna, and magnesium citrate. Most recently, she tried lubiprostone and polyethylene glycol without improvement. Her past medical history is significant for endometriosis, exploratory laparotomy, and 1 vaginal delivery. There is no family history of colorectal cancer or inflammatory bowel disease. She works as a truck driver and does not use alcohol, illicit drugs, or tobacco. There is no history of physical or sexual abuse. Her current medications include lubiprostone 24 µg twice daily, polyethylene glycol 17 g twice daily, and a birth control pill.
Physical Examination
On physical examination, the patient appears healthy without any distress. Her body mass index is 26 kg/m2, and vital signs are normal. General examination is normal. Abdomen is flat, and bowel sounds are normal. Mild tenderness is noted in both lower quadrants. Rectal examination reveals normal anal skin folds. Digital exam-ination reveals a normal resting tone with pellet-like stool that is heme-negative. When asked to attempt defecation, she shows poor perineal descent and paradoxical contraction of the anal sphincter.
Laboratory Evaluation
Laboratory testing reveals normal levels of thyrotropin and thyroxine, no anemia on complete blood count, and normal levels of calcium, glucose, and electrolytes.
What are the possible causes for this patient’s altered bowel habits?
What is the approach to physical examination in patients with constipation?
Causes of Constipation
Constipation is a common digestive disorder, affecting up to 20% of the world’s population [1]. Primary or idiopathic constipation consists of 3 common overlapping subtypes: slow-transit constipation, dyssynergic defecation, and constipation-predominant irritable bowel syndrome. Slow-transit constipation involves the slow movement of stool through the colon. This is usually seen on a colonic transit study or with wireless motility capsule study. Dyssynergia in general is caused by functional outlet obstruction with or without normal colonic transit. Patients with dyssynergia often complain of incomplete evacuation, excessive straining, bloating, and blockage [2]. Often patients with dyssynergia resort to manual disimpaction/vaginal splinting and/or abdominal pressure to facilitate bowel movements. Secondary constipation may result from metabolic disorders (eg, hypercalcemia and hypokalemia, disorders associated with renal failure, hypothyroidism, and diabetes) as well as medications, including narcotics, anticholinergics, and antidepressants.
Rectal Examination
Physical examination in patients with constipation should include a detailed rectal examination. The perianal skin should be inspected closely for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with resting and squeeze anal tone. A study by Rao et al[3] showed that rectal examination could identify 76% of patients with dyssynergia. The sensitivity and positive predictive value for diagnosing dyssynergia with digital rectal examination was 81% and 99%, respectively, making it a good screening test for dyssynergia [3].
When is colonoscopy indicated in the workup of constipation?
What imaging studies may be useful?
Colonoscopy
Colonoscopic evaluation is only indicated in patients with alarming features such as rectal bleeding, weight loss, unex-plained abdominal pain, palpable mass in the abdomen or rectum, persistent and unexplained anal/rectal pain, or anemia, as well as in patients over age 50 years [4].
Colonic Transit Study
Two imaging studies can be useful in the evaluation of a patient with constipation: colonic transit study and defeco-graphy. A colonic transit study provides useful information regarding the rate at which stool travels through the colon. This test is performed by administering one capsule (Sitzmarks, Konsyl Pharmaceuticals, Fort Worth, TX) containing radiopaque markers. A plain radiograph of the abdomen is obtained on day 6 (120 hr after ingestion of capsule). A transit study is considered abnormal if more than 20% of markers (> 5) are present on a plain radiograph of the abdomen. Approximately two-thirds of patients with dyssynergia have an abnormal colonic transit study, with retention of markers either in the rectosigmoid region or throughout the colon [5]. Wireless motility capsule is a newer test that is comprised of ingesting a capsule and wearing a recorder for up to 5 days. This test measures regional transit (ie, gastric emptying, colonic transit time, and whole gut transit time), is standardized and validated, and avoids use of radiation [6].
Defecography
Defecography is conducted by instilling a barium paste in the rectum and monitoring evacuation of the barium radiologically. It can reveal poor activation of the levator ani muscles, prolonged retention of the barium, inability to expel the barium, absence of a striping wave, rectal mucosal intussusception, rectocele, abnormal perineal descent, or rectal prolapse [5]. Although abnormalities are frequently found on defecography, they may not translate into clinical dysfunction. In one study, 77% of women with complaints of defecation disorders had abnormalities on defecography, but there was no relationship between the abnormalities and the patients’ symptoms [7]. Hence, defecography is not recommended unless there is clinical suspicion of prolapse or excessive descent. Endoanal and dynamic pelvic magnetic resonance imaging (MRI) can evaluate global pelvic floor anatomy in dynamic function [8]. Dynamic MRI in the seated position provides the most physiologic approach.
What testing is needed to make a diagnosis of dyssynergic defecation?
Both an abnormal balloon expulsion test and an abnormal pattern of defecation on anal rectal manometry are required to diagnose dyssynergic defecation [9]. Anorectal manometry provides information regarding rectal and anal pressures at rest and during maneuvers of simulated defecation as well as information on rectal sensation, rectoanal reflexes, and compliance [2,10]. There are 4 patterns of dyssynergia found on anorectal manometry: type 1, normal push effort with paradoxical contraction of the anal sphincter; type 2, poor push effort with paradoxical contraction of the anal sphincter; type 3, normal push effort with incomplete or absent relaxation of the anal sphincter; and type 4, poor push with incomplete anal relaxation. The balloon expulsion test should be included in the work-up of dyssynergia.
Normal subjects can expel a 50-mL water-filled balloon in less than 1 minute. Although normal patients can show a dyssynergic pattern in the left lateral decubitus position, when seated on a commode and with a sensation of stooling most exhibit a normal pattern of defecation [9].
Diagnosis
What treatment options are available for dyssynergia?
The treatment of patients with dyssynergic defecation consists of standard therapies for constipation, including diet, laxatives, and timed toileting. Medical therapy includes laxatives, polyethylene glycol, and lubiprostone.
Case Study 2
Initial Presentation and History
A 39-year-old woman presents with a 5-year history of intermittent bright red blood with stooling. Most often, she notices blood on the toilet paper or when wiping and rarely in the commode. She reports having experienced difficulty with bowel movements since her teens. She does not have a daily urge but strains up to 30 minutes to pass stool that is hard in consistency (type 1–2 on the Bristol stool scale). Over the past year, she has started using fingers to remove stool.
The patient reports bloating and abdominal discomfort that is improved with stooling. Her weight has been stable. Current medications include polyethylene glycol 17 g twice daily, sodium docusate 100 mg twice daily, iron sulfate 325 mg 3 times daily, and a birth control pill. Her past medical history is significant for iron deficiency anemia. Family history is notable for her mother and sister with similar “bowel troubles,” but no family history of inflammatory bowel disease or colorectal cancer. She is a salesperson and has been married for 7 years. She does not use tobacco or alcohol. As a child, she was sexually abused. She did not receive any formal counseling for the abuse. Review of systems is negative.
Physical Examination
General and neurologic examinations are normal. The abdomen is mildly distended, bowel sounds are normal, there is mild tenderness, and stool is palpable in the left lower quadrant. Rectal examination reveals normal anal skin with no fissures, intact anocutaneous reflex, and hard stool in the rectal vault that is guaiac-positive. The resting anal sphincter tone is elevated, and when asked to attempt defecation, there is excessive perineal descent and rectal mucosal intussusception with paradoxical anal contraction.
Laboratory Evaluation and Endoscopy
What is SRUS and how is it diagnosed?
Evaluation and Diagnosis
SRUS is characterized by single or multiple ulcerations of the rectal mucosa along with distinct pathologic changes [17]. The term solitary rectal ulcer is a misnomer because many patients have more than 1 lesion, and it is not always an ulcer. Patients with SRUS present with several symptoms, but the most common is passage of blood or mucus, and up to 26% may be asymptomatic [18]. The pathophysiology of this condition is poorly understood. Multiple mechanisms have been implicated, including occult or overt rectal prolapse, dyssynergia, rectal mucosal intussusception, rectal hypersensitivity with a persistent feeling of a need to defecate, and reduced mucosal blood flow [19].
The diagnosis of SRUS is based on the patient’s clinical history combined with endoscopy and histopathology findings. Endoscopically, the lesions may vary in appearance. Shallow ulcerations on hyperemic surrounding mucosa located on the anterior wall is the most common finding [17]. Lesions vary in size, although most are 1 to 1.5 cm in diameter [17] and rarely involve more than half the circumference of the rectal wall. Polypoid lesions occur in approximately 25% of patients with SRUS, and multiple lesions occur in 30% [17].
Obtaining specimens for histology is an important step in the evaluation of SRUS. The differential diagnosis includes Crohn’s disease, ulcerative colitis, ischemic colitis, and malignancy. The typical histologic findings include fibromuscular hyperplasia with smooth muscle infiltration of the lamina propria, thickening of the muscularis mucosa, regenerative changes, and distortion of the crypt architecture [17].
Are physiologic or imaging studies helpful in the diagnosis of SRUS?
Two complementary physiologic tests for SRUS are anorectal manometry and defecography. Anorectal manometry often shows evidence of dyssynergia and rectal hypersensitivity in patients with SRUS [20,21]. Hyper-sensitivity may produce a sensation of incomplete evacuation, which in turn results in excessive straining. Defecography may reveal rectal mucosal intussusception or overt rectal prolapse. The patient in this case had evidence of rectal hypersensitivity on anorectal manometry along with excessive perineal descent on defecography.
What are treatment options for SRUS?
Treatment of SRUS is not standardized. The options include topical medical therapy, biofeedback, and surgery. Uncontrolled studies have suggested that 5-aminosalicylic acid enema [22], sucralfate enema [23], steroid enema [24], and fibrin glue [25] may improve symptoms. Patients who fail topical therapy and have evidence of dyssynergia on anorectal manometry should receive biofeedback therapy. A case-control study of biofeedback involving 11 patients with refractory SRUS and 15 healthy controls showed improvement in anorectal function, including dyssynergia [21]. At follow-up endoscopy, 36% had complete mucosal healing and more than 50% showed partial healing. In a study involving 16 patients with SRUS and 26 healthy controls, Jarrett et al [26] showed that 75% of patients who underwent biofeedback therapy had improved and 31% had ulcer resolution. Surgical therapy should be considered in rare patients who are refractory to medical therapy. The Delorme procedure is commonly performed with a success rate of 42% to 100% [27].
The case patient underwent biofeedback therapy, and after 5 sessions had complete healing of the lesion and resolution of rectal bleeding and bowel symptoms.
Case Study 3
Initial Presentation and History
A 75-year-old woman is referred to a gastroenterologist with complaints of incomplete stool evacuation and intermittent fecal seepage. She passes stools daily but sits on the toilet for 15 to 20 minutes, and after straining will pass only a small amount of stool. She describes stools as type 4 on the Bristol scale with no blood or mucus. One to 2 hours after a bowel movement, she experiences some wetness in the perineal region and upon checking often notices that a tablespoon full of stool material has leaked out. Sometimes, she will pass another large stool. She denies any leakage of stool while sleeping. Occasionally, she has urgency and leaks stool before reaching the toilet. In the past, she has used digital maneuvers to facilitate stooling. This problem has interfered with shopping, socializing, and taking vacations.
Her past medical history is significant for narcolepsy, hypertension, tubal ligation, appendectomy, and inguinal hernia repair. Obstetric history is significant for 6 vaginal deliveries, 1 requiring episiotomy but no forceps use. Her current medications include estradiol vaginal cream, hydrochlorothiazide, pilocarpine, and amitriptyline 10 mg 3 times daily. She also reports stress urinary incontinence, particularly with sneezing and coughing.
Physical Examination
Physical examination reveals a well-nourished woman with normal vital signs and a normal general examination. Abdominal examination is normal. A rectal examination shows no fissures, but the anocutaneous reflex is absent on the right side. Resting and squeeze sphincter tones are normal, with good perineal descent and normal anal relaxation.
Laboratory Evaluation
What are the mechanisms involved in fecal incontinence?
What are the 3 clinical subtypes of fecal incontinence?
Mechanisms and Subtypes
Fecal incontinence is often an unvoiced problem that causes significant social stigma. Approximately 2% of the US population suffers from fecal incontinence [28], with a higher prevalence among women and elderly persons. Several mechanisms are involved in the pathogenesis of fecal incontinence. A common cause is injury to the external or internal anal sphincter, puborectalis muscle, or pudendal nerves, often after obstetric trauma. Hence, a detailed obstetric history including number of vaginal deliveries, use of forceps, tears, and episiotomy is important. Sphincter disruption, most commonly after surgery for hemorrhoid or anal fissure, can result in incontinence. Likewise, reduced rectal compliance causes urgency and fecal incontinence. Impaired rectal sensation results in the accumulation of stool and overflow. Patients rarely have a single cause, with 80% having more than one factor that leads to incontinence [29].
Clinically, fecal incontinence can be classified into 3 categories. Urge incontinence is characterized by the inability to control stool discharge despite active attempts to retain contents. These patients often have disruption or injury to the external anal sphincter. Fecal seepage is the involuntary discharge of less than 2 tablespoons of stool matter without awareness. Seepage can result from impaired rectal evacuation and dyssynergia. Often patients with seepage complain of incomplete evacuation. Passive incontinence refers to the involuntary discharge of stool contents without awareness. These patients often have underlying neuropathy and sphincter weakness [30,31].
What is the approach to evaluation and diagnosis?
Evaluation and Diagnosis
Physical examination of patients with fecal incontinence should include a detailed rectal examination, similar to the exam performed in patients who present with constipation. It should include perineal inspection for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with the resting and squeeze sphincter tone and sphincter relaxation. Further investigations should focus on determining the underlying mechanism in order to facilitate treatment.
Endoscopic investigation should be performed to exclude mucosal disease or malignancy. Anorectal manometry provides objective information regarding resting and squeeze anal sphincter tone, rectal compliance, rectal sensitivity, and rectoanal reflexes [29]. Some experts believe that anorectal manometry is not needed for diagnosis and emphasize the importance of rectal examination and history [32]. Proponents of anorectal manometry point out the importance of physiologic data that can be gained and how it may direct therapy. For example, anorectal manometry and sensory testing may reveal weak anal sphincters and impaired rectal sensation. The latter cannot be identified by clinical evaluation alone. These 2 pathophysiologic findings could enable the biofeedback therapist to focus on improving both anal sphincter tone and rectal sensation [33]. Defecography may reveal anterior rectocele, mucosal intussusception, or rectal prolapse. Anal ultrasound provides information on the structural integrity of the external and internal anal sphincters [34]. Ultrasound is widely available and is relatively inexpensive. Endoanal MRI may provide better information regarding the integrity of the external anal sphincter [35].
What are the treatment options?
The goal of treatment is to restore continence and quality of life. General considerations include stool bulking agents such as fiber supplements. Antidiarrheal agents, such as loperamide and diphenoxylate/atropine, are useful as they can decrease stool volume and increase and prolong sphincter pressure and colonic transit time [36,37]. Patients with diarrhea and functional incontinence may benefit from treatment with cholestyramine [38]. Biofeedback therapy improves sphincter tone and rectal sensation [39]. The number of biofeedback sessions is titrated to the patient’s needs, but often 6 sessions are required [40]. Generally, a 70% success rate has been described. Table 4 summarizes recent evidence supporting the use of biofeedback in the treatment of fecal incontinence [41–46].
Surgery for incontinence should be reserved for patients who have failed aggressive conservative management and biofeedback therapy. Overlapping sphincteroplasty is the most common surgery performed for fecal incontinence, with a success rate between 35% and 70% [47,48]. Creation of a neosphincter via dynamic graciloplasty or artificial sphincter has been tried in patients with an irreversibly damaged anal sphincter, but the success rate is low and the complication rate is high [49].
Sacral nerve stimulation (SNS) involves inserting electrodes in the lower back and connecting them to a pulse generator that produces pulses of electricity that innervate the nerves controlling the anal sphincters. Two double-blind crossover studies have reported a beneficial effect of SNS in fecal incontinence [50,51]. In 19 patients who preferred the periods when the stimulator was turned on, the median number of fecal incontinence episodes per week decreased from 1.7 to 0.7, and in the 5 patients who preferred the off period, the median number of fecal incontinence episodes per week increased from 1.7 to 3.7. SNS is now approved by FDA and insurance payers. Recently, hyaluronic acid/dextranomer injection (Solesta, Salix Pharmaceuticals, Raleigh, NC) has also been approved by FDA and has been shown to improve incontinence. A randomized controlled trial showed a 52% response rate to hyaluronic acid/dextranomer compared to a 31% response with placebo [52].
Conclusion
The 3 cases presented illustrate the complexities of several common anorectal disorders. A definitive diagnosis can be established in patients with defecation disorders through systematic evaluations and physiologic and imaging studies. Diagnosis in turn can pave the way for appropriate medical, behavioral, or surgical treatment. If facilities for appropriate testing are unavailable, it is important to refer these patients to appropriate specialists instead of embarking on empirical therapies which may prove futile. Treatment is often possible, and in a majority of patients their symptoms can be ameliorated.
Corresponding author: Satish S.C. Rao, MD, PhD, Section of Gastroenterology and Hepatology, Medical College of Georgia, Georgia Regents University, BB R2540, 1120 15th St., Augusta, GA 30912.
Funding/support: Portions of this work were supported by National Institutes of Health grant RO1 DK 57100-05.
From the Digestive Health Center, Medical College of Georgia, Georgia Regents University, Augusta, GA
Defecation is a coordinated process that involves generation of sufficient propulsive forces in the abdomen and rectum together with relaxation of the puborectalis and external anal sphincter. Likewise, continence involves conscious retention of bowel contents until stool or gas can be voluntarily eliminated in an appropriate fashion. A failure of these processes leads to altered bowel function and disorders of defecation that are commonly encountered in clinical practice. They include a diverse group of maladies that result in altered defecation. Among them are functional disorders, such as dyssynergic defecation, and mechanical/structural disorders, such as rectocele, solitary rectal ulcer syndrome (SRUS), excessive perineal descent, and rectal prolapse. This article discusses 3 cases that illustrate the clinical features and management approaches to dyssynergic defecation, SRUS, and fecal incontinence.
Case Study 1
Presentation and History
A 26-year-old white woman with a 10-year history of constipation presents to a gastroenterologist after referral from her primary care physician. She reports spontaneous bowel movements once every 2 weeks, and often she has to induce stools by using enemas or suppositories. Stooling became progressively more difficult for her during her teenage years, with infrequent bowel movements and hard stools (type 1–2 on Bristol stool scale). She also reports having to strain excessively during bowel movements, and on average she spends 30 minutes in the bathroom. She denies experiencing any perianal pain or bleeding or using manual maneuvers to defecate, but she often feels a sense of incomplete evacuation. She also describes intermittent abdominal pain and bloating.
She has tried several over-the-counter laxatives, includ-ing milk of magnesia, senna, and magnesium citrate. Most recently, she tried lubiprostone and polyethylene glycol without improvement. Her past medical history is significant for endometriosis, exploratory laparotomy, and 1 vaginal delivery. There is no family history of colorectal cancer or inflammatory bowel disease. She works as a truck driver and does not use alcohol, illicit drugs, or tobacco. There is no history of physical or sexual abuse. Her current medications include lubiprostone 24 µg twice daily, polyethylene glycol 17 g twice daily, and a birth control pill.
Physical Examination
On physical examination, the patient appears healthy without any distress. Her body mass index is 26 kg/m2, and vital signs are normal. General examination is normal. Abdomen is flat, and bowel sounds are normal. Mild tenderness is noted in both lower quadrants. Rectal examination reveals normal anal skin folds. Digital exam-ination reveals a normal resting tone with pellet-like stool that is heme-negative. When asked to attempt defecation, she shows poor perineal descent and paradoxical contraction of the anal sphincter.
Laboratory Evaluation
Laboratory testing reveals normal levels of thyrotropin and thyroxine, no anemia on complete blood count, and normal levels of calcium, glucose, and electrolytes.
What are the possible causes for this patient’s altered bowel habits?
What is the approach to physical examination in patients with constipation?
Causes of Constipation
Constipation is a common digestive disorder, affecting up to 20% of the world’s population [1]. Primary or idiopathic constipation consists of 3 common overlapping subtypes: slow-transit constipation, dyssynergic defecation, and constipation-predominant irritable bowel syndrome. Slow-transit constipation involves the slow movement of stool through the colon. This is usually seen on a colonic transit study or with wireless motility capsule study. Dyssynergia in general is caused by functional outlet obstruction with or without normal colonic transit. Patients with dyssynergia often complain of incomplete evacuation, excessive straining, bloating, and blockage [2]. Often patients with dyssynergia resort to manual disimpaction/vaginal splinting and/or abdominal pressure to facilitate bowel movements. Secondary constipation may result from metabolic disorders (eg, hypercalcemia and hypokalemia, disorders associated with renal failure, hypothyroidism, and diabetes) as well as medications, including narcotics, anticholinergics, and antidepressants.
Rectal Examination
Physical examination in patients with constipation should include a detailed rectal examination. The perianal skin should be inspected closely for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with resting and squeeze anal tone. A study by Rao et al[3] showed that rectal examination could identify 76% of patients with dyssynergia. The sensitivity and positive predictive value for diagnosing dyssynergia with digital rectal examination was 81% and 99%, respectively, making it a good screening test for dyssynergia [3].
When is colonoscopy indicated in the workup of constipation?
What imaging studies may be useful?
Colonoscopy
Colonoscopic evaluation is only indicated in patients with alarming features such as rectal bleeding, weight loss, unex-plained abdominal pain, palpable mass in the abdomen or rectum, persistent and unexplained anal/rectal pain, or anemia, as well as in patients over age 50 years [4].
Colonic Transit Study
Two imaging studies can be useful in the evaluation of a patient with constipation: colonic transit study and defeco-graphy. A colonic transit study provides useful information regarding the rate at which stool travels through the colon. This test is performed by administering one capsule (Sitzmarks, Konsyl Pharmaceuticals, Fort Worth, TX) containing radiopaque markers. A plain radiograph of the abdomen is obtained on day 6 (120 hr after ingestion of capsule). A transit study is considered abnormal if more than 20% of markers (> 5) are present on a plain radiograph of the abdomen. Approximately two-thirds of patients with dyssynergia have an abnormal colonic transit study, with retention of markers either in the rectosigmoid region or throughout the colon [5]. Wireless motility capsule is a newer test that is comprised of ingesting a capsule and wearing a recorder for up to 5 days. This test measures regional transit (ie, gastric emptying, colonic transit time, and whole gut transit time), is standardized and validated, and avoids use of radiation [6].
Defecography
Defecography is conducted by instilling a barium paste in the rectum and monitoring evacuation of the barium radiologically. It can reveal poor activation of the levator ani muscles, prolonged retention of the barium, inability to expel the barium, absence of a striping wave, rectal mucosal intussusception, rectocele, abnormal perineal descent, or rectal prolapse [5]. Although abnormalities are frequently found on defecography, they may not translate into clinical dysfunction. In one study, 77% of women with complaints of defecation disorders had abnormalities on defecography, but there was no relationship between the abnormalities and the patients’ symptoms [7]. Hence, defecography is not recommended unless there is clinical suspicion of prolapse or excessive descent. Endoanal and dynamic pelvic magnetic resonance imaging (MRI) can evaluate global pelvic floor anatomy in dynamic function [8]. Dynamic MRI in the seated position provides the most physiologic approach.
What testing is needed to make a diagnosis of dyssynergic defecation?
Both an abnormal balloon expulsion test and an abnormal pattern of defecation on anal rectal manometry are required to diagnose dyssynergic defecation [9]. Anorectal manometry provides information regarding rectal and anal pressures at rest and during maneuvers of simulated defecation as well as information on rectal sensation, rectoanal reflexes, and compliance [2,10]. There are 4 patterns of dyssynergia found on anorectal manometry: type 1, normal push effort with paradoxical contraction of the anal sphincter; type 2, poor push effort with paradoxical contraction of the anal sphincter; type 3, normal push effort with incomplete or absent relaxation of the anal sphincter; and type 4, poor push with incomplete anal relaxation. The balloon expulsion test should be included in the work-up of dyssynergia.
Normal subjects can expel a 50-mL water-filled balloon in less than 1 minute. Although normal patients can show a dyssynergic pattern in the left lateral decubitus position, when seated on a commode and with a sensation of stooling most exhibit a normal pattern of defecation [9].
Diagnosis
What treatment options are available for dyssynergia?
The treatment of patients with dyssynergic defecation consists of standard therapies for constipation, including diet, laxatives, and timed toileting. Medical therapy includes laxatives, polyethylene glycol, and lubiprostone.
Case Study 2
Initial Presentation and History
A 39-year-old woman presents with a 5-year history of intermittent bright red blood with stooling. Most often, she notices blood on the toilet paper or when wiping and rarely in the commode. She reports having experienced difficulty with bowel movements since her teens. She does not have a daily urge but strains up to 30 minutes to pass stool that is hard in consistency (type 1–2 on the Bristol stool scale). Over the past year, she has started using fingers to remove stool.
The patient reports bloating and abdominal discomfort that is improved with stooling. Her weight has been stable. Current medications include polyethylene glycol 17 g twice daily, sodium docusate 100 mg twice daily, iron sulfate 325 mg 3 times daily, and a birth control pill. Her past medical history is significant for iron deficiency anemia. Family history is notable for her mother and sister with similar “bowel troubles,” but no family history of inflammatory bowel disease or colorectal cancer. She is a salesperson and has been married for 7 years. She does not use tobacco or alcohol. As a child, she was sexually abused. She did not receive any formal counseling for the abuse. Review of systems is negative.
Physical Examination
General and neurologic examinations are normal. The abdomen is mildly distended, bowel sounds are normal, there is mild tenderness, and stool is palpable in the left lower quadrant. Rectal examination reveals normal anal skin with no fissures, intact anocutaneous reflex, and hard stool in the rectal vault that is guaiac-positive. The resting anal sphincter tone is elevated, and when asked to attempt defecation, there is excessive perineal descent and rectal mucosal intussusception with paradoxical anal contraction.
Laboratory Evaluation and Endoscopy
What is SRUS and how is it diagnosed?
Evaluation and Diagnosis
SRUS is characterized by single or multiple ulcerations of the rectal mucosa along with distinct pathologic changes [17]. The term solitary rectal ulcer is a misnomer because many patients have more than 1 lesion, and it is not always an ulcer. Patients with SRUS present with several symptoms, but the most common is passage of blood or mucus, and up to 26% may be asymptomatic [18]. The pathophysiology of this condition is poorly understood. Multiple mechanisms have been implicated, including occult or overt rectal prolapse, dyssynergia, rectal mucosal intussusception, rectal hypersensitivity with a persistent feeling of a need to defecate, and reduced mucosal blood flow [19].
The diagnosis of SRUS is based on the patient’s clinical history combined with endoscopy and histopathology findings. Endoscopically, the lesions may vary in appearance. Shallow ulcerations on hyperemic surrounding mucosa located on the anterior wall is the most common finding [17]. Lesions vary in size, although most are 1 to 1.5 cm in diameter [17] and rarely involve more than half the circumference of the rectal wall. Polypoid lesions occur in approximately 25% of patients with SRUS, and multiple lesions occur in 30% [17].
Obtaining specimens for histology is an important step in the evaluation of SRUS. The differential diagnosis includes Crohn’s disease, ulcerative colitis, ischemic colitis, and malignancy. The typical histologic findings include fibromuscular hyperplasia with smooth muscle infiltration of the lamina propria, thickening of the muscularis mucosa, regenerative changes, and distortion of the crypt architecture [17].
Are physiologic or imaging studies helpful in the diagnosis of SRUS?
Two complementary physiologic tests for SRUS are anorectal manometry and defecography. Anorectal manometry often shows evidence of dyssynergia and rectal hypersensitivity in patients with SRUS [20,21]. Hyper-sensitivity may produce a sensation of incomplete evacuation, which in turn results in excessive straining. Defecography may reveal rectal mucosal intussusception or overt rectal prolapse. The patient in this case had evidence of rectal hypersensitivity on anorectal manometry along with excessive perineal descent on defecography.
What are treatment options for SRUS?
Treatment of SRUS is not standardized. The options include topical medical therapy, biofeedback, and surgery. Uncontrolled studies have suggested that 5-aminosalicylic acid enema [22], sucralfate enema [23], steroid enema [24], and fibrin glue [25] may improve symptoms. Patients who fail topical therapy and have evidence of dyssynergia on anorectal manometry should receive biofeedback therapy. A case-control study of biofeedback involving 11 patients with refractory SRUS and 15 healthy controls showed improvement in anorectal function, including dyssynergia [21]. At follow-up endoscopy, 36% had complete mucosal healing and more than 50% showed partial healing. In a study involving 16 patients with SRUS and 26 healthy controls, Jarrett et al [26] showed that 75% of patients who underwent biofeedback therapy had improved and 31% had ulcer resolution. Surgical therapy should be considered in rare patients who are refractory to medical therapy. The Delorme procedure is commonly performed with a success rate of 42% to 100% [27].
The case patient underwent biofeedback therapy, and after 5 sessions had complete healing of the lesion and resolution of rectal bleeding and bowel symptoms.
Case Study 3
Initial Presentation and History
A 75-year-old woman is referred to a gastroenterologist with complaints of incomplete stool evacuation and intermittent fecal seepage. She passes stools daily but sits on the toilet for 15 to 20 minutes, and after straining will pass only a small amount of stool. She describes stools as type 4 on the Bristol scale with no blood or mucus. One to 2 hours after a bowel movement, she experiences some wetness in the perineal region and upon checking often notices that a tablespoon full of stool material has leaked out. Sometimes, she will pass another large stool. She denies any leakage of stool while sleeping. Occasionally, she has urgency and leaks stool before reaching the toilet. In the past, she has used digital maneuvers to facilitate stooling. This problem has interfered with shopping, socializing, and taking vacations.
Her past medical history is significant for narcolepsy, hypertension, tubal ligation, appendectomy, and inguinal hernia repair. Obstetric history is significant for 6 vaginal deliveries, 1 requiring episiotomy but no forceps use. Her current medications include estradiol vaginal cream, hydrochlorothiazide, pilocarpine, and amitriptyline 10 mg 3 times daily. She also reports stress urinary incontinence, particularly with sneezing and coughing.
Physical Examination
Physical examination reveals a well-nourished woman with normal vital signs and a normal general examination. Abdominal examination is normal. A rectal examination shows no fissures, but the anocutaneous reflex is absent on the right side. Resting and squeeze sphincter tones are normal, with good perineal descent and normal anal relaxation.
Laboratory Evaluation
What are the mechanisms involved in fecal incontinence?
What are the 3 clinical subtypes of fecal incontinence?
Mechanisms and Subtypes
Fecal incontinence is often an unvoiced problem that causes significant social stigma. Approximately 2% of the US population suffers from fecal incontinence [28], with a higher prevalence among women and elderly persons. Several mechanisms are involved in the pathogenesis of fecal incontinence. A common cause is injury to the external or internal anal sphincter, puborectalis muscle, or pudendal nerves, often after obstetric trauma. Hence, a detailed obstetric history including number of vaginal deliveries, use of forceps, tears, and episiotomy is important. Sphincter disruption, most commonly after surgery for hemorrhoid or anal fissure, can result in incontinence. Likewise, reduced rectal compliance causes urgency and fecal incontinence. Impaired rectal sensation results in the accumulation of stool and overflow. Patients rarely have a single cause, with 80% having more than one factor that leads to incontinence [29].
Clinically, fecal incontinence can be classified into 3 categories. Urge incontinence is characterized by the inability to control stool discharge despite active attempts to retain contents. These patients often have disruption or injury to the external anal sphincter. Fecal seepage is the involuntary discharge of less than 2 tablespoons of stool matter without awareness. Seepage can result from impaired rectal evacuation and dyssynergia. Often patients with seepage complain of incomplete evacuation. Passive incontinence refers to the involuntary discharge of stool contents without awareness. These patients often have underlying neuropathy and sphincter weakness [30,31].
What is the approach to evaluation and diagnosis?
Evaluation and Diagnosis
Physical examination of patients with fecal incontinence should include a detailed rectal examination, similar to the exam performed in patients who present with constipation. It should include perineal inspection for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with the resting and squeeze sphincter tone and sphincter relaxation. Further investigations should focus on determining the underlying mechanism in order to facilitate treatment.
Endoscopic investigation should be performed to exclude mucosal disease or malignancy. Anorectal manometry provides objective information regarding resting and squeeze anal sphincter tone, rectal compliance, rectal sensitivity, and rectoanal reflexes [29]. Some experts believe that anorectal manometry is not needed for diagnosis and emphasize the importance of rectal examination and history [32]. Proponents of anorectal manometry point out the importance of physiologic data that can be gained and how it may direct therapy. For example, anorectal manometry and sensory testing may reveal weak anal sphincters and impaired rectal sensation. The latter cannot be identified by clinical evaluation alone. These 2 pathophysiologic findings could enable the biofeedback therapist to focus on improving both anal sphincter tone and rectal sensation [33]. Defecography may reveal anterior rectocele, mucosal intussusception, or rectal prolapse. Anal ultrasound provides information on the structural integrity of the external and internal anal sphincters [34]. Ultrasound is widely available and is relatively inexpensive. Endoanal MRI may provide better information regarding the integrity of the external anal sphincter [35].
What are the treatment options?
The goal of treatment is to restore continence and quality of life. General considerations include stool bulking agents such as fiber supplements. Antidiarrheal agents, such as loperamide and diphenoxylate/atropine, are useful as they can decrease stool volume and increase and prolong sphincter pressure and colonic transit time [36,37]. Patients with diarrhea and functional incontinence may benefit from treatment with cholestyramine [38]. Biofeedback therapy improves sphincter tone and rectal sensation [39]. The number of biofeedback sessions is titrated to the patient’s needs, but often 6 sessions are required [40]. Generally, a 70% success rate has been described. Table 4 summarizes recent evidence supporting the use of biofeedback in the treatment of fecal incontinence [41–46].
Surgery for incontinence should be reserved for patients who have failed aggressive conservative management and biofeedback therapy. Overlapping sphincteroplasty is the most common surgery performed for fecal incontinence, with a success rate between 35% and 70% [47,48]. Creation of a neosphincter via dynamic graciloplasty or artificial sphincter has been tried in patients with an irreversibly damaged anal sphincter, but the success rate is low and the complication rate is high [49].
Sacral nerve stimulation (SNS) involves inserting electrodes in the lower back and connecting them to a pulse generator that produces pulses of electricity that innervate the nerves controlling the anal sphincters. Two double-blind crossover studies have reported a beneficial effect of SNS in fecal incontinence [50,51]. In 19 patients who preferred the periods when the stimulator was turned on, the median number of fecal incontinence episodes per week decreased from 1.7 to 0.7, and in the 5 patients who preferred the off period, the median number of fecal incontinence episodes per week increased from 1.7 to 3.7. SNS is now approved by FDA and insurance payers. Recently, hyaluronic acid/dextranomer injection (Solesta, Salix Pharmaceuticals, Raleigh, NC) has also been approved by FDA and has been shown to improve incontinence. A randomized controlled trial showed a 52% response rate to hyaluronic acid/dextranomer compared to a 31% response with placebo [52].
Conclusion
The 3 cases presented illustrate the complexities of several common anorectal disorders. A definitive diagnosis can be established in patients with defecation disorders through systematic evaluations and physiologic and imaging studies. Diagnosis in turn can pave the way for appropriate medical, behavioral, or surgical treatment. If facilities for appropriate testing are unavailable, it is important to refer these patients to appropriate specialists instead of embarking on empirical therapies which may prove futile. Treatment is often possible, and in a majority of patients their symptoms can be ameliorated.
Corresponding author: Satish S.C. Rao, MD, PhD, Section of Gastroenterology and Hepatology, Medical College of Georgia, Georgia Regents University, BB R2540, 1120 15th St., Augusta, GA 30912.
Funding/support: Portions of this work were supported by National Institutes of Health grant RO1 DK 57100-05.
1. Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004;99:750–9.
2. Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterol Motil 2004;16:580–96.
3. Rao SS, Tantiphlachiva K, Rao PS, et al. How useful is digital rectal examination in the assessment of patients with dyssynergia? Am J Gastroenterol 2007;S268:419.
4. Pepin C, Ladabuam U. The yield of lower endoscopy in patients with constipation: survey of a university hospital, public county hospital, and a Veterans Administration medical center. Gastrointest Endosc 2002;56:325–32.
5. Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am 2003;32:659–83.
6. Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil; 2011; 23: 8-23.
7. Savoye-Collet C, Savoye G, Koning E, et al. Defecography in symptomatic older women living at home. Age Ageing 2003;32:347–50.
8. Bolog N, Weishaupt D. Dynamic MR imaging of outlet obstruction. Rom J Gastroenterol 2005;14:293–302.
9. Rao SS, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol 2006;101:2790–6.
10. Karlbom U, Lundin E, Graf W, Pahlman L. Anorectal physiology in relation to clinical subgroups of patients with severe constipation. Colorectal Dis 2004;6:343–9.
11. Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130:657–64.
12. Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007;5:331–8.
13. Chiarioni G, Saladini L, Whitehead W. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129:86–97.
14. Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to alternate treatments for patients with pelvic floor dyssynergia-type defecation [abstract]. Am J Gastroenterol 2005;100:S335.
15. Hallan RI, Williams NS, Melling J, et al. Treatment of anismus in intractable constipation with botulinum A toxin. Lancet 1988;2:714–7.
16. Boccasanta P, Venturi M, Stuto A, et al. Stapled transanal rectal resection for outlet obstruction: a prospective, multicenter trial. Dis Colon Rectum 2004;47:1285–97.
17. Sharara AI, Azar C, Amr SS, et al. Solitary rectal ulcer syndrome: endoscopic spectrum and review of the literature. Gastrointest Endosc 2005;62:755–62.
18. Tjandra JJ, Fazio VW, Church JM, et al. Clinical conundrum of solitary rectal ulcer. Dis Colon Rectum 1992;35:227–34.
19. Felt-Bersma RJ, Cuesta A. Rectal prolapse, rectal intussusception, rectocele, and solitary rectal ulcer syndrome. Gastroenterol Clin North Am 2001;30:199–222.
20. Vaizey CJ, van den Bogaerde JB, Emmanuel AV, et al. Solitary rectal ulcer syndrome. Br J Surg 1998;85:1617–23.
21. Rao SS, Ozturk R, De Ocampo S, Stessman M. Pathophysiology and role of biofeedback therapy in solitary rectal ulcer syndrome. Am J Gastroenterol 2006;101:613–8.
22. Kumar M, Puri AS, Srivastava R, Yachha SK. Solitary rectal ulcer syndrome in a child treated with local sulfasalazine. Indian Pediatr 1994;31:1553–5.
23. Zargar SA, Khuroo MS, Mahajan R. Sucralfate retention enemas in solitary rectal ulcer. Dis Colon Rectum 1991;34:455–7.
24. Bishop PR, Nowicki MJ. Nonsurgical therapy for solitary rectal ulcer syndrome. Curr Treat Options Gastroenterol 2002;5:215–23.
25. Ederle A, Bulighin G, Orlandin PG, Pilati S. Endoscopic application of human fibrin sealant in the treatment of solitary rectal ulcer syndrome [letter]. Endoscopy 1992;24:736–7.
26. Jarrett ME, Emmanuel AV, Vaizey CJ, Kamm MA. Behavioural therapy (biofeedback) for solitary rectal ulcer syndrome improves symptoms and mucosal blood flow. Gut 2004;53:368–70.
27. Tweedie DJ, Varma JS. Long-term outcome of laparoscopic mesh rectopexy for solitary rectal ulcer syndrome. Colorectal Dis 2005;7:151–5.
28. Rao SS. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastrenterol 2004;99:1585–604.
29. Rao SS, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol 1997;92:469–75.
30. Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 2004;126(1 Suppl 1):S14–22.
31. Deutekom M, Dobben AC, Terra MP, et al. Clinical presentation of fecal incontinence and anorectal function: what is the relationship? Am J Gastroenterol 2007;102:351–61.
32. Wald A. Con: anorectal manometry and imaging are not necessary in patients with fecal incontinence. Am J Gastroenterol 2006;101:2681–3.
33. Bharucha AE. Pro: anorectal testing is useful in fecal incontinence. Am J Gastroenterol 2006;101:2679–81.
34. Tuteja AK, Rao SS. Review article: recent trends in diagnosis and treatment of faecal incontinence. Aliment Pharmacol Ther 2004;19:829–40.
35. Terra MP, Beets-Tan RG, van der Hulst VP, et al. MRI in evaluating atrophy of the external anal sphincter in patients with fecal incontinence. AJR Am J Roentgenol 2006;187:991–9.
36. Sun WM, Read NW, Verlinden M. Effects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinence. Scan J Gastroenterol 1997;32:34–8.
37. Harford WV, Krejs GJ, Santa Ana CA, Fordtran JS. Acute effect of diphenoxylate with atropine (Lomotil) in patients with chronic diarrhea and fecal incontinence. Gastroenterol 1980;78:440–3.
38. Remes-Troche JM, Ozturk R, Philips C, et al. Cholesteryramine—a useful adjunct for the treatment of patients with fecal incontinence. Int J Colorectal Dis 2008;23:189–99.
39. Rao SS. The technical aspects of biofeedback therapy for defecation disorders. Gastroenterologist 1998;6:96–103.
40. Rao SS, Welcher KD, Happel J. Can biofeedback therapy improve anorectal function in fecal incontinence? Am J Gastroenterol 1966;91:2360–6.
41. Byrne CM, Solomon MJ, Young JM, et al. Biofeedback for fecal incontinence: short-term outcomes of 513 consecutive patients and predictors of successful treatment. Dis Colon Rectum 2007;50:417–27.
42. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenteroloy 2003;125:1320–9.
43. Heymen S, Whitehead W. EMG biofeedback vs. Kegel exercise for the treatment of fecal incontinence. Gastroenterology 2007;132:A–83.
44. Pager CK, Solomon MJ, Rex J, Roberts RA. Long-term outcomes of pelvic floor exercise and biofeedback treatment for patients with fecal incontinence. Dis Colon Rectum 2002;45:997–1003.
45. Ozturk R, Niazi S, Stessman M, Rao SS. Long-term and objective changes of anorectal function after biofeedback therapy for faecal incontinence. Aliment Pharmacol Ther 2004;20:667–74.
46. Byrne CM, Solomon MJ, Rex J, et al. Telephone vs. face-to-face biofeedback for fecal incontinence: comparison of two techniques in 239 patients. Dis Colon Rectum 2005;48:2281–8.
47. Zorcolo L, Covotta L, Bartolo DC. Outcome of anterior sphincter repair for obstetric injury: comparison of early and late results. Dis Colon Rectum 2005;48:524–31.
48. Madoff RD. Surgical treatment options for fecal incontinence. Gastroenterology 2004;126(1 Suppl 1)S48–54.
49. Thornton MJ, Kennedy ML, Lubowski DZ, King DW. Long-term follow-up of dynamic graciloplasty for faecal incontinence. Colorectal Dis 2004;6:470–6.
50. Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg 2005;242:662–9.
51. Vaizey CJ, Kamm MA, Nicholls RJ. Double-blind crossover study of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 2000;43:298–302.
52. Graf W, Mellgren A, Matzel KE, et al. . Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet;2011;377:997–1003.
1. Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004;99:750–9.
2. Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterol Motil 2004;16:580–96.
3. Rao SS, Tantiphlachiva K, Rao PS, et al. How useful is digital rectal examination in the assessment of patients with dyssynergia? Am J Gastroenterol 2007;S268:419.
4. Pepin C, Ladabuam U. The yield of lower endoscopy in patients with constipation: survey of a university hospital, public county hospital, and a Veterans Administration medical center. Gastrointest Endosc 2002;56:325–32.
5. Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am 2003;32:659–83.
6. Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil; 2011; 23: 8-23.
7. Savoye-Collet C, Savoye G, Koning E, et al. Defecography in symptomatic older women living at home. Age Ageing 2003;32:347–50.
8. Bolog N, Weishaupt D. Dynamic MR imaging of outlet obstruction. Rom J Gastroenterol 2005;14:293–302.
9. Rao SS, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol 2006;101:2790–6.
10. Karlbom U, Lundin E, Graf W, Pahlman L. Anorectal physiology in relation to clinical subgroups of patients with severe constipation. Colorectal Dis 2004;6:343–9.
11. Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130:657–64.
12. Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007;5:331–8.
13. Chiarioni G, Saladini L, Whitehead W. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129:86–97.
14. Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to alternate treatments for patients with pelvic floor dyssynergia-type defecation [abstract]. Am J Gastroenterol 2005;100:S335.
15. Hallan RI, Williams NS, Melling J, et al. Treatment of anismus in intractable constipation with botulinum A toxin. Lancet 1988;2:714–7.
16. Boccasanta P, Venturi M, Stuto A, et al. Stapled transanal rectal resection for outlet obstruction: a prospective, multicenter trial. Dis Colon Rectum 2004;47:1285–97.
17. Sharara AI, Azar C, Amr SS, et al. Solitary rectal ulcer syndrome: endoscopic spectrum and review of the literature. Gastrointest Endosc 2005;62:755–62.
18. Tjandra JJ, Fazio VW, Church JM, et al. Clinical conundrum of solitary rectal ulcer. Dis Colon Rectum 1992;35:227–34.
19. Felt-Bersma RJ, Cuesta A. Rectal prolapse, rectal intussusception, rectocele, and solitary rectal ulcer syndrome. Gastroenterol Clin North Am 2001;30:199–222.
20. Vaizey CJ, van den Bogaerde JB, Emmanuel AV, et al. Solitary rectal ulcer syndrome. Br J Surg 1998;85:1617–23.
21. Rao SS, Ozturk R, De Ocampo S, Stessman M. Pathophysiology and role of biofeedback therapy in solitary rectal ulcer syndrome. Am J Gastroenterol 2006;101:613–8.
22. Kumar M, Puri AS, Srivastava R, Yachha SK. Solitary rectal ulcer syndrome in a child treated with local sulfasalazine. Indian Pediatr 1994;31:1553–5.
23. Zargar SA, Khuroo MS, Mahajan R. Sucralfate retention enemas in solitary rectal ulcer. Dis Colon Rectum 1991;34:455–7.
24. Bishop PR, Nowicki MJ. Nonsurgical therapy for solitary rectal ulcer syndrome. Curr Treat Options Gastroenterol 2002;5:215–23.
25. Ederle A, Bulighin G, Orlandin PG, Pilati S. Endoscopic application of human fibrin sealant in the treatment of solitary rectal ulcer syndrome [letter]. Endoscopy 1992;24:736–7.
26. Jarrett ME, Emmanuel AV, Vaizey CJ, Kamm MA. Behavioural therapy (biofeedback) for solitary rectal ulcer syndrome improves symptoms and mucosal blood flow. Gut 2004;53:368–70.
27. Tweedie DJ, Varma JS. Long-term outcome of laparoscopic mesh rectopexy for solitary rectal ulcer syndrome. Colorectal Dis 2005;7:151–5.
28. Rao SS. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastrenterol 2004;99:1585–604.
29. Rao SS, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol 1997;92:469–75.
30. Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 2004;126(1 Suppl 1):S14–22.
31. Deutekom M, Dobben AC, Terra MP, et al. Clinical presentation of fecal incontinence and anorectal function: what is the relationship? Am J Gastroenterol 2007;102:351–61.
32. Wald A. Con: anorectal manometry and imaging are not necessary in patients with fecal incontinence. Am J Gastroenterol 2006;101:2681–3.
33. Bharucha AE. Pro: anorectal testing is useful in fecal incontinence. Am J Gastroenterol 2006;101:2679–81.
34. Tuteja AK, Rao SS. Review article: recent trends in diagnosis and treatment of faecal incontinence. Aliment Pharmacol Ther 2004;19:829–40.
35. Terra MP, Beets-Tan RG, van der Hulst VP, et al. MRI in evaluating atrophy of the external anal sphincter in patients with fecal incontinence. AJR Am J Roentgenol 2006;187:991–9.
36. Sun WM, Read NW, Verlinden M. Effects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinence. Scan J Gastroenterol 1997;32:34–8.
37. Harford WV, Krejs GJ, Santa Ana CA, Fordtran JS. Acute effect of diphenoxylate with atropine (Lomotil) in patients with chronic diarrhea and fecal incontinence. Gastroenterol 1980;78:440–3.
38. Remes-Troche JM, Ozturk R, Philips C, et al. Cholesteryramine—a useful adjunct for the treatment of patients with fecal incontinence. Int J Colorectal Dis 2008;23:189–99.
39. Rao SS. The technical aspects of biofeedback therapy for defecation disorders. Gastroenterologist 1998;6:96–103.
40. Rao SS, Welcher KD, Happel J. Can biofeedback therapy improve anorectal function in fecal incontinence? Am J Gastroenterol 1966;91:2360–6.
41. Byrne CM, Solomon MJ, Young JM, et al. Biofeedback for fecal incontinence: short-term outcomes of 513 consecutive patients and predictors of successful treatment. Dis Colon Rectum 2007;50:417–27.
42. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenteroloy 2003;125:1320–9.
43. Heymen S, Whitehead W. EMG biofeedback vs. Kegel exercise for the treatment of fecal incontinence. Gastroenterology 2007;132:A–83.
44. Pager CK, Solomon MJ, Rex J, Roberts RA. Long-term outcomes of pelvic floor exercise and biofeedback treatment for patients with fecal incontinence. Dis Colon Rectum 2002;45:997–1003.
45. Ozturk R, Niazi S, Stessman M, Rao SS. Long-term and objective changes of anorectal function after biofeedback therapy for faecal incontinence. Aliment Pharmacol Ther 2004;20:667–74.
46. Byrne CM, Solomon MJ, Rex J, et al. Telephone vs. face-to-face biofeedback for fecal incontinence: comparison of two techniques in 239 patients. Dis Colon Rectum 2005;48:2281–8.
47. Zorcolo L, Covotta L, Bartolo DC. Outcome of anterior sphincter repair for obstetric injury: comparison of early and late results. Dis Colon Rectum 2005;48:524–31.
48. Madoff RD. Surgical treatment options for fecal incontinence. Gastroenterology 2004;126(1 Suppl 1)S48–54.
49. Thornton MJ, Kennedy ML, Lubowski DZ, King DW. Long-term follow-up of dynamic graciloplasty for faecal incontinence. Colorectal Dis 2004;6:470–6.
50. Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg 2005;242:662–9.
51. Vaizey CJ, Kamm MA, Nicholls RJ. Double-blind crossover study of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 2000;43:298–302.
52. Graf W, Mellgren A, Matzel KE, et al. . Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet;2011;377:997–1003.
Depressed, suicidal, and brittle in her bones
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
Understanding and Treating Balance Impairment in Multiple Sclerosis
From the Department of Rehabilitation and Movement Science, University of Vermont, Burlington, VT.
Abstract
- Objective: To provide insight into the mechanisms and treatment options associated with balance impairments in individuals with multiple sclerosis (MS).
- Methods: Systematic reviews, randomized controlled trials, and noncontrolled studies were examined to collect current data regarding treatment options aimed at improving balance in MS.
- Results: Balance deficits are common in individuals with MS and result from a diverse set of constraints across multiple systems of postural control. Poor balance often leads to increased fall risk, reduced physical activity, added comorbidities, and decreased quality of life. A variety of exercise options are available for individuals with MS who experience balance and mobility problems. Physical interventions include targeted therapies, such as vestibular rehabilitation and weighted torso training, as well as more general exercise and balance training prescriptions.
- Conclusion: The evidence, albeit preliminary, suggests that therapeutic intervention aimed at ameliorating balance deficits associated with MS be multimodal. Exercise prescriptions should include sensory and motor strategy training, strength development, as well as functional gait activities. Further evidence-based research is needed to improve the management of balance deficits in those with MS and to identify the impact of improved balance on activity participation and quality of life.
Multiple sclerosis (MS) is one of the most common nontraumatic neurologic causes of disability among young adults. With greater awareness and improved diagnostics, more people are being diagnosed with the disease today than in the past. Prevalence estimates in the United States range from 90 to 135 per 100,000 individuals [1], with approximately 400,000 people currently diagnosed [2,3].
MS is a chronic inflammatory disease of the central nervous system typically characterized by increasing muscle weakness, spasticity, fatigue, pain, depression, visual and sensory disturbances, and cognitive difficulties. The clinical course of MS is highly variable and often unpredictable with increasing disability and physical decline spanning a 30- to 40-year period post diagnosis [4]. During this time, advancing symptoms can lead to a number of comorbidities and negatively impact daily functioning, mobility, and community participation [5–7]. From a public health standpoint, the early and disabling impact of symptoms and prolonged physical decline create a significant economic burden. The projected national heath care costs of MS are greater than $7 billion annually [8], with the average total annual cost per patient estimated at over $47,000 [9]. Of this annual cost, indirect costs associated with lost productivity represent the single highest component cost [9,10].
Of the wide range of disease-related challenges, mobility difficulties are most significant. Over 90% of people with MS report mobility difficulties [11], and maintaining mobility is consistently ranked as one of the highest priorities for this group, independent of disease duration or disability level [10,12]. Several studies have demonstrated that loss of balance and mobility contributes to substantial patient burden [13] and lower perceived quality of life [10]. Moreover, poor balance and increased fall risk have been associated with reduced physical activity and other health-related behaviors [14,15].
Because balance and mobility limitations are so prevalent and impacting, targeted treatments aimed at maintaining ambulation and function are critical goals in the management of MS. It is important for physicians and rehabilitation professionals to understand and recognize the underlying sensorimotor mechanisms related to postural instability and initiate appropriate evidenced-based treatments that can improve balance, reduce fall risk, and enhance quality of life for individuals with MS. This review seeks to analyze the evidence on the physical interventions aimed at ameliorating balance and mobility impairments associated with MS in the context of a case example.
Case Study
Initital Presentation and History
Ms. D is a 41-year-old woman with relapse-remitting MS. She was diagnosed 6 years ago after experiencing initial symptoms of optic neuritis and some numbness in her right hand. Since then, she has developed greater weakness in both her legs and reports that her MS significantly impacts her ability to walk, both in terms of distance and the effort needed to ambulate.
Ms. D is independently ambulatory without the use of any assistive device. She reports that her balance is worse when walking on uneven surfaces, moving about in dimly lit environments, turning, or when walking in crowded spaces. Ms. D also shares that she has difficulty standing on one leg while pulling on socks. She states that she must concentrate and focus on her balance when in these challenging situations and that she has to consistently look where she is stepping.
Ms. D does not have any spasticity in muscles of the lower extremities, but on occasion does experience some numbness and tingling in her left foot. She experiences moderate fatigue that requires her to pace herself throughout her daily activities. She reports that her fatigue impacts her ability to concentrate or pay attention for long periods of time and impacts her motivation to engage in social activities. She states that she sleeps restlessly and is consequently tired when she wakes in the morning. Although she is sedentary, she has no history of cardiopulmonary issues or orthopedic problems.
Physical Examination
Ms. D is 5’7” and weighs 175 pounds, with a BMI of 27.4. She presents with observable gait and balance impairment. On physical examination, she exhibits reduced bilateral strength of knee flexors and extensors as well as hip adductors, although the weakness is more evident on the left. On neurologic exam, she exhibits moderate disability in both sensory and cerebellar functioning (resulting in an Expanded Disability Status Scale score of 3.5) [16].
What is postural control?
What balance impairments are associated with MS?
Postural Equilibrium and Balance
For all individuals, postural orientation and equilibrium underlie the effective performance of life’s daily tasks. Postural orientation refers to the alignment of body segments to a reference (such as gravity, the support surface, or an object in the visual field), while postural equilibrium—often equated with balance—refers to maintaining or re-acquiring the body’s center of gravity (CoG) within the base of support (BoS) [17,18]. This paper will focus on postural equilibrium with MS across multiple contexts of balance tasks.
Horak [18] described contexts of balance tasks that affect the mechanisms of maintaining postural equilibrium. Some of these contextual variables include
- Biomechanical constraints (eg, strength)
- Limits of stability (functional reach, maximum lean)
- Anticipatory postural adjustments (voluntary postural transitions)
- Automatic postural responses (balance recovery from external perturbations)
- Sensory orientation (ability to reweight sensory information [somatosensory, visual, vestibular] depending on context
- Dynamic control during gait
- Cognitive-motor interaction (balance impairments when also performing a cognitive task)
Emotion represents another contextual variable of interest, because mood and fear can significantly modify postural control [19–23]. Knowing the contextual factors that modify balance control provides insight into underlying neuropathology associated with impairments of these postural control variables [24,25] as well as insight into what should be included during the examination of patients with MS based on patient descriptions of their symptoms and functional challenges.
Balance Assessment
Balance assessment indicates that Ms. D cannot abduct and hold either leg to her side for any noticeable length of time, cannot reach forward adequately without lifting her heels off the ground or falling forward, and cannot stand on one leg for more than 10 seconds without losing balance. She also needs to take multiple steps to recover balance with any slight perturbation and is unable to maintain stability while standing on foam with her eyes closed. She shows significant imbalance when rising from a chair, walking forward, and turning to come back to sit.
For Ms. D, the clinical balance exam suggests pervasive impairment of hip strength, limits of stability, anticipatory postural adjustments, postural responses, sensory integration, and gait. Furthermore, her reported need to focus vision on her gait is in accordance with compensation for existing sensory impairments. Lastly, fatigue and attention demand likely enhance the presentation of balance impairment.
What are the consequences of balance impairments associated with MS?
Balance impairments present considerable health problems for adults with MS. Greater than 50% of individuals with MS report falling in any 6-month period [81–85], with the incidence of recurrent falls reported to be as high as 9 falls per year [86]. In addition, fall-related injuries, including fractures, are more common with MS, although this increased risk is considerably greater for women with MS than men [86–90].
Common risk factors for falling in people with MS include variable or deteriorating MS status [90–96], problems with balance or mobility [88,92–94,96–99], use of walking aids [88,93,97], lower balance confidence [86,98], reduced executive functioning [99] and greater fatigue [85]. Increased postural sway [52,99,100], slower walking speed [99], greater gait asymmetry and variability [92,101], slower choice stepping reaction time [99], impaired forward limits of stability [92,99], impaired visually dependent sway [92,99], and leg weakness [88,92] have also been found predictive of future falls in MS. A link has also emerged between cognitive impairment and fall risk [86,95,99,102].
Fear of falling and fall-induced injuries are also the most common causes of restricted activity and disability for individuals with MS [14]. Research has shown that future physical activity associates with fear of falling, and fear of falling subsequently associated with lower-limb strength asymmetry and decreased limits of stability rather than past experience of falling [103]. Similarly, the perceived benefits of physical activity and an individual’s self-efficacy to engage in physical activity predict reported levels of physical activity independent of disability level for individuals with MS [104]. Thus, psychological perception represents an important, and potentially modifiable, correlate of physical activity.
Moreover, individuals with MS experience a high risk of cardiovascular disease and other chronic health conditions associated with deconditioning, as unfavorable blood lipid levels, poor glucose profiles, and obesity have been observed in this population [105]. Comorbid conditions, secondary conditions, and health behaviors are increasingly recognized to be important factors influencing a range of outcomes in MS [107].
Further History
Consistent with the consequences of balance and mobility impairment, Ms. D reports that she loses her balance and nearly falls at least 1 time per week while engaged in daily activities. She also shares that she fell 2 months ago while walking outside and across the lawn to get the mail. Her confidence is low for many daily tasks such as climbing stairs, picking up objects from the floor, reaching when on tiptoes, or walking on ramps or on slippery surfaces. While Ms. D is independent in all activities of daily living, she currently does not work due to her fatigue and poor balance. She indicates that she is not very physically active and feels somewhat isolated and depressed because her balance and mobility challenges keep her from going out with friends and socializing.
What exercise approaches are available to ameliorate the balance deficits associated with MS?
There are a variety of therapeutic approaches for the treatment of poor balance in MS. While pharmacologic treatment typically encompasses disease-modifying therapies, specific medications can also help in the management of symptoms (ie, fatigue, spasticity, gait variability) that can negatively impact balance and mobility. Other rehabilitative strategies for balance impairment include gait training, assistive devices for mobility, and environmental modifications for fall prevention. Although all of these avenues offer viable treatment options for improving balance, exercise is increasingly appreciated as an important adjunct to the rehabilitation management of MS [107], especially in terms of improving balance deficits, optimizing daily functioning, and increasing participation across various life contexts.
The diversity of exercise options available for individuals with MS who experience balance and mobility problems is expanding. Moreover, mounting evidence suggests that exercise is well tolerated by participants with the disease[108–110] and that individuals with MS can exercise sufficiently to improve their fitness, function, and quality of life [109,110]. Given the inherent variability of MS and the heterogeneity of symptoms and disease course across individuals, however, no one exercise prescription is optimal for all those diagnosed. Instead, treatment goals must be individualized and functionally based [107] with ongoing evaluation and modification of treatment plans due to disease progression, symptom fluctuations, and functional decline [107,111]. Regardless of specific approach, the aim of any exercise intervention is to reduce activity limitations, encourage participation, and facilitate independence and life satisfaction in those with the disease [112].
Resistance Training
There have been several structured reviews of exercise research in MS [108,110,113,114]. The existing evidence supports resistance exercise as compared with no exercise for improving general balance [115] or performing tasks such as a chair transfer [116] or sit-to-stand [117]. Two randomized controlled trials (RCTs) also revealed significant increases in functional reach (ie, limits of stability) as a result of progressive resistance exercise [118,119]. Resistance exercise has not, however, facilitated greater benefit over traditional rehabilitation in other postural control contexts such as those involving postural transitions, sensory integration, or postural sway [120–122].
The effects of resistance training on mobility have also been inconsistent. While several studies showed no significant improvement in functional mobility [118,122,123], a positive improvement was observed in other research [119,124,125]. Likewise, stair climbing was shown to improve in 2 noncontrolled studies [125,126] and one RCT [117] but not in another [127].
In a recent RCT to evaluate the comparative effectiveness of different methods of resistance training, Hayes et al [123] determined that the addition of high-intensity, eccentric resistance training offered no additional benefit over standard concentric resistance exercise in improving static standing balance and stair climbing. In addition, compared with no exercise or a home-based program to improve strength and balance, progressive resistance cycling showed significantly greater effect on functional reach and timed up-and-go in individuals with moderate MS [128]. Nonetheless, evidence for the efficacy of home-based training remains equivocal given issues of motivation, adherence, and training intensity [115,118,128].
Taken together, the systematic reviews to date conclude that there is insufficient evidence for the effects of resistance exercise on balance in MS, thus making solid evidenced-based conclusions difficult [108,110,113,129]. Moreover, it is difficult to ascertain a definitive and most efficacious exercise prescription for improving balance in MS given the inconsistency in protocols and findings across studies. There is some support, albeit preliminary, for progressive resistance training as a modality to improve balance, especially those functional tasks demanding greater strength [113]. Nonetheless, resistance training may contribute to improved posture and gait given it directly addresses one context of postural control, but it may not be fully effective due to lack of training to modify central neural control of posture in other contexts.
Aerobic Exercise
Many of the studies examining aerobic exercise in MS more often target walking capacity, exercise tolerance, fatigue, and quality of life than balance [130]. The limited research that has focused on aerobic exercise for balance improvement has shown equal benefit to that achieved from resistance exercise in those contexts involving limits of stability and dynamic balance while stepping or walking [119]. This finding was reasonable given that the aerobic exercise included step-up and treadmill walking. Still, it has been recommended that, for most people with MS, aerobic exercise also incorporate a degree of balance training [109].
Combined Exercise
The more recent exercise research involving people with MS often combines some aspect of aerobic, strengthening, and/or balance exercise. While only a few RCTs have examined the effects of combined training in this population, preliminary evidence suggests it is well tolerated and may have some benefit for improving function [110]. While one study found no differences in static balance after a combined strength and aerobic training program [131], review of the exercise protocol revealed that the training regime had only incorporated 2 standing exercises. Other studies more intentionally combining strength and balance exercise have demonstrated benefits in balance confidence [132], standing static balance or postural sway [132–134], step climbing [133], and functional mobility [135]. Combining aerobic exercise and strengthening has also been effective in reducing falls in those with MS [85].
Balance-Specific Exercise
Only one balance-specific RCT has been published to date. In this study, outcomes from balance training involving both motor and sensory strategies were compared to training of only motor strategies and to standard therapy [136]. Both the balance training groups significantly reduced the number of falls post intervention as compared to the conventional treatment group. There were no observed differences in self-reported balance confidence across the groups, although both the balance training groups significantly improved in static and dynamic standing balance over that achieved by the standard treatment group. The fact that only the group engaged in sensory training differed significantly on dynamic gait highlights the importance of sensory integration for dynamic balance and gait.
Video Game–Assisted Exercises
Novel rehabilitative approaches have taken advantage of advances in virtual reality and visual feedback training to improve balance and mobility deficits in people with MS. Exercise using the general physical activity games on the Nintendo Wii Fit provided short-term improvement in standing balance, strength, gait and physical activity in people with MS [137]. This general exercise offered no significant gains in self-efficacy, fatigue impact or quality of life, and physical activity levels returned to baseline levels 14 weeks after exercising. Subsequent review has, however, highlighted concerns that current commercially available video options for general exercise may not be sufficiently adaptive for people with moderate disability, leading to intimidation and low adherence [138].
Beyond general physical activity, the Wii Balance Board System has also been used to specifically target balance and mobility deficits in MS. Although one study found no significant benefit from Wii Fit balance exercise in balance performance and walking ability [139], other studies have shown positive effects in standing sway, static balance, dynamic stepping, walking speed, and MS impact [140–142].
The evidence, albeit preliminary, thus suggests that the Wii Fit may offer a feasible adjunct to traditional rehabilitation approaches, especially because the exercise can be done at home without the need for continuous support from a practitioner and because the technology aids in overcoming access barriers often associated with community-based physical activity programs [138]. Nonetheless, research shows that Wii Balance Board System training is more specific for static standing balance than for dynamic balance or mobility, the technology is not positively viewed by those with more advanced symptoms, and there exists a risk of adverse affects and training-related injuries associated with home-based use of the Wii [137,140].
Vestibular Rehabilitation Exercise
Vestibular rehabilitation is a specialized treatment approach that strengthens the vestibular sensory system by retraining the brain to recognize and process signals from the vestibular system and coordinate these with visual and proprioceptive inputs. To date, there has only been one RCT investigating the effects of vestibular rehabilitation on balance in adults with MS [143]. In this study, the outcomes of a standard vestibular rehabilitation program to those of an exercise regime as well as to no intervention were compared. The vestibular rehabilitation program consisted of static and dynamic tasks performed with changing bases of support, on various surfaces, with eyes open or closed, and different head movements. The 6-week vestibular rehabilitation program resulted in both statistically significant and clinically relevant change in standing balance under various sensory conditions compared with either of the other two groups, although no significant difference was found in walking capacity across groups.
Weighted Torso Training
Balance-based torso weighting (BBTW) involves strategically placing small weights on the trunk of an individual to decrease balance deviations observed during quiet stance, perturbed standing, walking, and transitioning [144]. While the specific mechanism underlying the therapeutic effect of rehabilitative weighting has been debated [145], various suggestions include joint compression to encourage co-contraction, enhanced conscious awareness of body segments, and biomechanical changes via shifting of the center of mass [146].
The one RCT examining the effectiveness of BBTW in people with MS found immediate and significant effects of BBTW on postural control and upright mobility [146]. The research confirmed preliminary investigations of BBTW in MS [144,147], demonstrating that BBTW can improve walking speed as well as functional tasks involving standing, walking, turning, and sitting down.
Whole Body Vibration
Whole body vibration (WBV) has been employed across a variety of neurological populations as a means of improving muscle tone, sensation, strength, stability, and functional performance. In WBV, multidimensional vibrations are transferred to an individual performing static or dynamic movements on an oscillating platform. The vibrations are believed to facilitate both neuroendocrine responses as well as motor unit recruitment [148–150].
Results have been inconsistent regarding the effectiveness of WBV as a way of improving postural control and functional mobility in individuals with MS. A few studies have shown significant positive effects of WBV lasting from 1 to 4 weeks on functional mobility [151–153], strength [151,153,154], walking speed [152,155], and standing balance [152]. Walking endurance has also been affected by vibration training designed to improve muscular endurance [156]. Although there have been noted benefits of WBV, these benefits were not significantly more advantageous than those offered by a vibration program in conjunction with lower-limb stretching and strengthening exercises [157] or in addition to a traditional rehabilitation program [154].
There has also been some evidence to show that prolonged WBV does not improve postural stability or functional mobility in individuals with MS after training [155,156,158]. Likewise, there is contradictory evidence supporting the use of WBV in improving walking speed [157], functional reaching [152,153] or overall quality of life [152].
While WBV does not appear to have a detrimental effect on symptoms of MS, there is insufficient evidence regarding its beneficial effects on balance, gait, muscle strength and quality of life compared to other interventions. Future research is necessary to examine various protocols in terms of vibratory parameters and length of intervention before specific prescriptions can be offered [159].
Aquatics
Although aquatic exercise has often been recommended for individuals with MS, much of the research employing this therapeutic modality has focused on outcomes of pain, fatigue, cardiorespiratory fitness, gait, and quality of life [160–164]. Research focused on aquatic exercise for improved balance is limited. Nonetheless, significant improvements in standing balance and functional mobility have been shown for individuals with MS following aquatic exercise [165,166]. Similar results on standing balance and functional mobility have also been shown from Ai Chi, a program in which Tai Chi is combined with other techniques and performed standing in shoulder-depth water using a combination of deep breathing and slow, broad movements of the arms, legs, and torso [167]. These methods of intervention, however, still lack evidence from rigorous designs involving control groups and randomization.
Yoga
Yoga has also been explored as a means to improve physical and mental health outcomes in MS. While an initial study showed no significant changes in one-leg stance from an Iyengar yoga program [168], more recent research found Ananda yoga practice effective in improving standing balance [169]. Likewise, other research has shown that static and dynamic standing balance improved after yoga practice, although not significantly better than that from treadmill exercise training [170].
Kickboxing
There has been only one study to date, albeit not an RCT, that has examined kickboxing as a training modality to improve balance in MS. Although kickboxing was found to be a feasible exercise activity, not all participants demonstrated improved balance and mobility outcomes [171]. As such, further investigation of this novel treatment approach is warranted.
Hippotherapy
Hippotherapy has also been employed as a means of balance training because the multidimensional and random nature of the horse’s movement requires the rider to process increased sensory information and make the necessary anticipatory and reactive adjustments for postural control. While one study reported no improvement in postural sway after hippotherapy [172], other research has shown some benefit in balance and gait after riding [173,174]. Although preliminary, findings from 2 of the studies reveal that hippotherapy may be most beneficial for those with primary progressive MS compared to other subtypes of MS [175]. While hippotherapy may have a positive effect on balance in individuals with MS, the data is limited and lacks rigorous examination through randomized controlled study of large samples in order to allow for its advocacy as a primary rehabilitation modality at this time.
What exercise prescription is indicated for Ms. D?
Because Ms. D’s balance deficits have begun to limit her daily functioning and increase her risk of falling, a formal and targeted balance intervention is warranted. Research confirms that exercise would be well tolerated by Ms. D and supports the feasibility of her engaging in various exercise modalities. Although a number of exercise inter-vention studies involving people with MS have been described in the literature, their clinical utility and results in improving balance and mobility are varied. Nonetheless, there is preliminary evidence suggesting that exercise training may have positive effects on balance and functional mobility and could offer Ms. D benefit. This is especially true given that much of the exercise research included individuals with mild or minimal disability and at same stage of disease progression as Ms. D.
Since Ms. D’s balance problems stem from a range of postural impairments across multiple contexts of balance control, her treatment approach must incorporate exercises that include and integrate these underlying control systems. A targeted and multimodal balance exercise program, rather than general physical activity, may be most efficacious toward this end.
Intervention Prescription
Ms. D has poor ability to utilize somatosensory and vestibular inputs in order to dynamically weight the influence of multiple sensory modalities for the control of standing sway under varying sensory conditions. This visual dependence contributes to her poor balance and increases her fall risk when visual inputs are absent (ie, walking in dimly lit rooms) or when optic flow is incongruent or when visual distractions are present (ie, walking in dynamic contexts such as crowded spaces). Ms. D would benefit from exercises requiring greater use of proprioceptive and vestibular inputs, thereby facilitating improved sensory integration. Exercises performed with eyes closed as well as those completed on mats, foam, or other compliant surfaces would be beneficial. She might also benefit from specific vestibular rehabilitation exercises as this approach has resulted in improved sensory integration [143]. Given that Ms. D must regularly concentrate and focus on her balance and consistently look where she is stepping, her balance exercise program should also address her central processing and attentional deficits by including dual-task training [26].
Ms. D also noted that her MS significantly impacts her ability to walk both in terms of effort and distance and adversely affects her participation in social events. Supplemental to her balance exercise program, aerobic exercise, particularly treadmill walking, may offer some benefit both in terms of her endurance as well as gait. While some of the more targeted modalities such as hippotherapy, yoga, and kickboxing have not been extensively studied, they do offer promise and could be used as adjuncts in order to facilitate Ms. D’s motivation and adherence through more diverse programming. Lastly, and although requiring further study, cognitive-behavioral interventions and patient education may be warranted to help Ms. D overcome her fear of falling, low exercise self-efficacy, and any negative beliefs regarding the potential benefits of exercise.
What additional research is needed?
Although valuable insight has been gained from studies of balance and gait impairment with MS, many contexts remain understudied, particularly with regard to understanding both the neuroanatomical and neurophysiologic pathologies that underlie the behavioral impairments of balance and gait in MS. Further, the value of applying this knowledge of balance impairment to clinical diagnostics and prognostics requires further study in order to develop the most cost- and time-effective exams and evidence-based treatment approaches.
Based on the research to date, it remains difficult to draw definitive evidenced-based conclusions regarding what specific exercise mode or training dose would be most beneficial for Ms. D and others with MS. Moreover, while there exists some evidence of efficacious balance outcomes from exercise training, many of the studies involved individuals with mild MS. Only a few studies to date have included those with more advanced disability, thus making prescription generalizations to those more moderately affected by MS tenuous. Irrespective of specific approach, all modalities of balance-oriented interventions require larger controlled studies, inclusion of those with advancing disability status, long-term follow-up, an evaluation of optimal dose or duration, and outcomes on the neural mechanisms of effect.
Summary
Challenges to balance and mobility present serious consequences for those with MS, as falls and fear of falling lead to poor health outcomes and low quality of life. Given that postural impairments result from a diverse set of deficits in different underlying control systems, therapeutic intervention should be multimodal. Exercise prescription should address all affected contexts of postural control, including sensory and motor strategy training during postural transitions as well as induced postural perturbations, strength development, and gait activity. Evidence from clinical trials suggests that targeted balance oriented exercise in people with MS has the potential to improve balance and functional mobility, although more rigorous study on the topic is needed.
Corresponding author: Susan L. Kasser, PhD, Dept. of Rehabilitation and Movement Science, Univ. of Vermont, 306 Rowell Bldg, 106 Carrigan Dr, Burlington, VT 05405, [email protected]
Financial disclosures: None.
1. Hirtz D, Thurman DJ, Gwinn-Hardy K, et al. How common are the “common” neurologic disorders? Neurology 2007;68:326–37.
2. Keegan BM, Noseworthy JH. Multiple sclerosis. Annu Rev Med 2002;53:285–302.
3. National Multiple Sclerosis Society: Who gets MS? Accessed 5 Mar 2014 at http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/who-gets-ms/index.aspx.
4. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000;343:1430–8.
5. Overs S, Hughes C, Haselkorn J, Turner A. Modifiable comorbidities and disability in multiple sclerosis. Curr Neurol Neurosci Rep 2012;12:610–7.
6. Motl R. Physical activity and irreversible disability in multiple sclerosis. Exerc Sport Sci Rev 2010;38:186–91.
7. Naci H, Fleurence R, Birt J, Duhig A. The impact of increasing neurological disability of multiple sclerosis on health utilities: a systematic review of the literature. J Med Econ 2010;13:78–89.
8. Bainbridge JL. Economics of multiple sclerosis. Adv Stud Pharm 2007;4:330–3.
9. Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross sectional study in the United States. Neurology 2006;66:1696–702.
10. Zwibel H. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv Ther 2009;26:1043–57.
11. Hemmett L, Holmes J, Barnes M, Russell N. What drives quality of life in multiple sclerosis? QJM 2004;97:671–6.
12. Heesen C, Bohm J, Reich C, et al. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler 2008;14:988–91.
13. Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin 2010; 26:109–19.
14. Peterson EW, Cho CC, Finlayson ML. Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult Scler 2007;13:1168–75.
15. Matsuda PN, Shumway-Cook A, Ciol MA, et al. Understanding falls in multiple sclerosis: association of mobility status, concerns about falling, and accumulated impairments. Phys Ther 2012;92:407–15.
16. Kurtzke JF: Rating neurologic impairment in multiple sclerosis: an expended disability status scale (EDSS). Neurology 1983,33:1444–52.
17. Pollock AS, Durward BR, Rowe PJ, Paul JP. What is balance? Clin Rehabil 2000;14:402–6.
18. Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 2006;35 Suppl 2: ii7–ii11.
19. Carpenter MG, Frank JS, Silcher CP, Peysar GW. The influence of postural threat on the control of upright stance. Exp Brain Res 2001;138:210–8.
20. Adkin Al, Frank JS, Carpenter MG, Petsar GW. Fear of falling modifies anticipatory postural control. Exp Brain Res 2002;143:160–70.
21. Bolmont B, Gangloff P, Vouriot A, Perrin P. Mood states and anxiety influence abilities to maintain balance control in healthy human subjects. Neurosci Lett 2002;329:96–100.
22. Carpenter MG, Frank JS, Adkin AL, et al. Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol 2004;92:3255–65.
23. Kitaoka K, Ito R, Araki H, et al. Effect of mood state on anticipatory postural adjustments. Neurosci Lett 2004;370:65–8.
24. Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm 2007; 114:1339–48.
25. Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Movement Disord 2013;28:1483–91.
26. Cameron MH, Lord S. Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep 2010;10:407–12.
27. Jacobs JV, Kasser SL. Balance impairment in people with multiple sclerosis: preliminary evidence for the Balance Evaluation Systems Test. Gait Posture 2012;36:414–8.
28. Jacobs JV, Kasser SL. Effects of dual tasking on the postural performance of people with and without multiple sclerosis: a pilot study. J Neurol 2012;259:1166–76.
29. Boes MK, Sosnoff JJ, Socie MJ, et al. Postural control in multiple sclerosis: effect of disability status and dual task. J Neurol Sci 2012;315:44–8.
30. Wajda A, Achiron A, Dvir Z. Motor impairments at presentation of clinically isolated syndrome suggestive of multiple sclerosis: characterization of different disease subtypes. NeuroRehab 2012;31:147–55.
31. Karst GM, Venema DM, Roehrs TG, Tyler AE. Center of pressure measures during standing tasks in minimally impaired persons with multiple sclerosis. J Neurol Phys Ther 2005;29:170–80.
32. Soyuer F, Mirza M, Erkorkmaz U. Balance performance in three forms of multiple sclerosis. Neurol Res 2006;28:555–62.
33. Findling O, Sellner J, Meler N, et al. Trunk sway in mildly disables multiple sclerosis patients with and without balance impairment. Exp Brain Res 2011;213:363–70.
34. Corporaal SH, Gensicke H, Kuhle J, et al. Balance control in multiple sclerosis: correlations of trunk sway during stance and gait tests with disease severity. Gait Posture 2013;37:55–60.
35. Martin CL, Phillips BA, Kilpatrick TJ, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler 2006;12:620–8.
36. Spain RI, St. George RJ, Salarian A, et al. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture 2012;35:573–8.
37. Huisinga JM, St George RJ, Spain R, et al. Postural response latencies are related to balance control during standing and walking in patients with multiple sclerosis. Arch Phys Med Rehab 2014;
38. Lambert CP, Archer RL, Evans WJ. Muscle strength and fatigue during isokinetic exercise in individuals with multiple sclerosis. Med Sci Sports Exerc 2001;33:1613–9.
39. Carroll CC, Gallagher PM, Seidle ME, Trappe SW. Skeletal muscle characteristics of people with multiple sclerosis. Arch Phys Med Rehab 2005;86:224–9.
40. Ng AV, Miller RG, Gelinas D, Kent-Braun JA. Functional relationships of central and peripheral muscle alterations in multiple sclerosis. Muscle Nerve 2004;29:843–52.
41. Garner DJ, Widrick JJ. Cross-bridge mechanisms of muscle weakness in multiple sclerosis. Muscle Nerve 2003;27:456–64.
42. Ponichtera JA. Concentric and eccentric isokinetic lower extremity strength in multiple sclerosis and able-bodied. J Orthop Sports Phys Ther 2006;16:114–22.
43. Chung LH, Remelius JG, Van Emmerik RE, Kent-Braun JA. Leg power asymmetry and postural control in women with multiple sclerosis. Med Sci Sports Exerc 2008;40:1717–24.
44. Yahia A, Ghroubi S, Mhiri C, Elleuch MH. Relationship between muscle strength, gait and postural parameters in multiple sclerosis. Ann Phys Rehab Med 2011;54:144–55.
45. Frzovic D, Morris ME, Vowels L. Clinical tests of standing balance: performance of persons with multiple sclerosis. Arch Phys Med Rehab 2000;81:215–21.
46. van Emmerik REA, Remelius JG, Johnson MB, et al. Postural control in women with multiple sclerosis: effects of task, vision and symptomatic fatigue. Gait Posture 2010; 32:608–14.
47. Kanekar N, Aruin AS. Clinical and instrumented outcomes measures in balance control of individuals with multiple sclerosis. Mult Scler Int 2013;
48. Huisinga JM, Yentes JM, Filipi ML, Stergiou N. Postural control strategy during standing is altered in patients with multiple sclerosis. Neurosci Lett 2012;524:124–8.
49. Cattaneo D, Jonsdottir J. Sensory impairments in quiet standing in subjects with multiple sclerosis. Mult Scler 2009;15:59–67.
50. Kanekar N, Lee YJ, Aruin AS. Frequency analysis approach to study balance control in individuals with multiple sclerosis. J Neurosci Meth 2014:222:91–6.
51. Cao H, Peyrodie L, Boudet S, et al. Expanded disability status scale (EDSS) estimation in multiple sclerosis from posturographic data. Gait Posture 2013;37:242–5.
52. Kalron A, Achiron A. Postural control, falls and fear of falling in people with multiple sclerosis without mobility aids. J Neurol Sci 2013;335:186–90.
53. Hebert JR, Corboy JR. The association between multiple sclerosis-related fatigue and balance as a function of central sensory integration. Gait Posture 2013;38:37–42.
54. Jackson K, Bigelow KE. Measures of balance performance are affected by a rested versus fatigued testing condition in people with multiple sclerosis. Phys Med Rehabil 2013;5:949–56.
55. Krishnan V, Kanekar N, Aruin AS. Anticipatory postural adjustments in individuals with multiple sclerosis. Neurosci Lett 2012;506:256–60.
56. Krishnan V, Kanekar N, Aruin AS. Feedforard postural control in individuals with multiple sclerosis during load release. Gait Posture 2012;36:225–30.
57. Remelius JG, Hamill J, Kent-Braun J, Van Emmerik R. Gait initiation in multiple sclerosis. Motor Control 2008;12:93–106.
58. Cameron MH, Horak FB, Herndon RR, Bourdette D. Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosens Mot Res 2008,25:113–22.
59. Crenshaw SJ, Royer TD, Richards JG, Hudson DJ. Gait variability in people with multiple sclerosis. Mult Scler 2006;12:613–9.
60. Sosnoff JJ, Sandroff BM, Motl RW. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait Posture 2012;36:154–6.
61. Givon U, Zeilig G, Achiron A. Gait analysis in multiple sclerosis: characterization of temporal–spatial parameters using GAITRite functional ambulation system. Gait Posture 2009;29:138–42.
62. Sosnoff JJ, Weikert M, Dlugonski D, et al. Quantifying gait impairment in multiple sclerosis using GAITRite technology. Gait Posture 2011;34:145–7.
63. Benedetti MG, Piperno R, Simoncini L, et al. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult Scler 1999;5:363–8.
64. Kelleher KJ, Spence W, Solomonidis S, Apatsidis D. The characterisation of gait patterns of people with multiple sclerosis. Disabil Rehabil 2010;32:1242–50.
65. Sacco R, Bussman R, Oesch P, et al. Assessment of gait parameters and fatigue in MS patients during inpatient rehabilitation: a pilot trial. J Neurol 2011;258:889–94.
66. Gianfrancesco MA, Triche EW, Fawcett JA, et al. Speed- and cane-related alterations in gait parameters in individuals with multiple sclerosis. Gait Posture 2011;33:140–2.
67. Morris ME, Cantwell C, Vowels L, Dodd K. Changes in gait and fatigue from morning to afternoon in people with multiple sclerosis.J Neurol Neurosurg Psychiatry 2002;72:361–5.
68. Nogueira LAC, Teixeira L, Sabino P, et al. Gait characteristics of multiple sclerosis patients in the absence of clinical disability. Disabil Rehabil 2013;35:1472–8.
69. Nilsagard Y, Carling A, Forsberg A. Activities-specific balance confidence in people with multiple sclerosis. Mult Scler Int 2012;2012:613925.
70. Kalron A, Achiron A. Relationship between fear of falling to spatiotemporal gait parameters measured by an instrumented treadmill in people with multiple sclerosis. Gait Posture 2014;39:739–44.
71. Huisinga JM, Filipi ML, Schmid KK, Stergiou N. Is there a relationship between fatigue questionnaires and gait mechanics in persons with multiple sclerosis? Arch Phys Med Rehabil 2011;92:1594–601.
72. Motl RW, Sandroff BM, Suh Y, Sosnoff JJ. Energy cost of walking and its association with gait parameters, daily activity, and fatigue in persons with mild multiple sclerosis. Neurorehabil Neural Repair 2012;26:1015–21.
73. Burschka JM, Keune PM, Menge U, et al. An exploration of impaired walking dynamics and fatigue in multiple sclerosis. BMC Neurol 2012;12:161.
74. Kalron A, Dvir Z, Achiron A. Effect of a cognitive task on postural control in patients with a clinically isolated syndrome suggestive of multiple sclerosis. Eur J Phys Rehabil Med 2011;47:579–86.
75. Negahban H, Sanjari M, Mofateh R, Parnianpou M. Nonlinear dynamical structure of sway path during standing in patients with multiple sclerosis and in healthy controls is affected by changes in sensory input and cognitive load. Neurosci Lett 2013;553:126–31.
76. Negahban H, Mofateh R, Arastoo AA, et al. The effect of cognitive loading on balance control in patients with multiple sclerosis. Gait Posture 2011;34:479–84.
77. Sosnoff JJ, Boes MK, Sandroff BM, et al. Walking and thinking in persons with multiple sclerosis who vary in disability. Arch Phys Med Rehabil 2011;92:2028–33.
78. Hamilton F, Rochester L, Paul L, et al. Walking and talking: an investigation of cognitive-motor dual tasking in multiple sclerosis. Mult Scler 2010;15:1215–27.
79. Kalron A, Dvir Z, Achiron A. Walking while talking - difficulties incurred during the initial stages of multiple sclerosis disease process. Gait Posture 2010;32:332–5.
80. Wajda DA, Motl RW, Sosnoff JJ. Dual task cost of walking is related to fall risk in persons with multiple sclerosis. J Neuro Sci 2013;335:160–3.
81. Cameron MH, Asano M, Bourdette D, Finlayson ML. People with multiple sclerosis use many fall prevention strategies but still fall frequently. Arch Phys Med Rehabil 2013;94;1562–6.
82. Cameron MH, Thielman E, Mazumder R, Bourdette D. Predicting falls in people with multiple sclerosis: fall history is as accurate as more complex measures. Mult Scler Int 2013;2013:496325
83. Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil 2006;87:1274–9.
84. Gunn H, Creanor S, Haas B, et al. Risk factors for falls in multiple sclerosis: an observational study. Mult Scler J 2013;19:1913–22.
85. Coote S, Hogan N. Franklin S. Falls in people with multiple sclerosis who use a walking aid: prevalence, factors, and effect of strength and balance interventions. Arch Phys Med Rehabil 2013;94:616–21.
86. Peterson EW, Cho CC, von Koch L, Finlayson ML. Injurious falls among middle aged and older adults with multiple sclerosis. Arch Phys Med Rehabil 2008;89:1031–7.
87. Cameron MH, Poel AJ, Haselkorn JK, et al. Falls requiring medical attention among veterans with multiple sclerosis: a cohort study. J Rehab Res Dev 2011;48:13–20.
88. Matsuda PN, Shumway-Cook A, Bamer AM, et al. Falls in multiple sclerosis. Phys Med Rehabil 2011;3:624–32.
89. Tremlet H, Lucas R. The risks for falls and fractures in multiple sclerosis. Neurology 2012;78:1902–3.
90. Zikan V. Bone health in patients with multiple sclerosis. J Osteoporos 2011;2011:596294.
91. Nilsagard Y, Lundholm C, Denison E, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis: a longitudinal study. Clin Rehabil 2009;23:259–69.
92. Kasser SL, Jacobs JV, Foley JT, et al. A prospective evaluation of balance, gait, and strength to predict falling in women with multiple sclerosis. Arch Phys Med Rehabil 2011;92:1840–6.
93. Gunn H, Newell P, Haas B, et al. Identification of risk factors for falls in multiple sclerosis: a systematic review and meta-analysis. Phys Ther 2013;93:504–13.
94. Sosnoff JJ, Socie MJ, Boes MK, et al. Mobility, balance and falls in persons with multiple sclerosis. PLoS ONE 2011;6(11):e28021.
95. D’Orio VL, Foley FW, Armentano F, et al. Cognitive and motor functioning in patients with multiple sclerosis: neuropsychological predictors of walking speed and falls. J Neurol Sci 2012;316:42–6.
96. Prosperini L, Kouleridou A, Petsas N, et al. The relationship between infratentorial lesions, balance deficit, and accidental falls in multiple sclerosis. J Neuro Sci 2011; 304:55–60.
97. Cattaneo D, De Nuzzo C, Fascia T, et al. Risks of falls in subjects with multiple sclerosis. Arch Phys Med Rehabil 2002;83:864–7.
98. Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil 2006;87:1274–9.
99. Hoang PD, Cameron MH, Gandevia SC, Lord SR. Neuropsychologcial, balance, and mobility risk factors for falls in people with multiple sclerosis: a prospective cohort study. Arch Phys Med Rehabil 2014;95:480–6.
100. Prosperini L, Fortuna D, Gianni C, et al. The diagnostic accuracy of static posturography in predicting accidental falls in people with multiple sclerosis. Neurorehabi Neural Repair 2013;27:45–52.
101. Socie MJ, Sandroff BM, Pula JH, et al. Footfall placement variability and falls in multiple sclerosis. Ann Biomed Eng 2013;41:1740–7.
102. Sosnoff JJ, Balantrapu S, Pilutti L, et al. Cognitive processing speed is related to fall frequency in older adults with multiple sclerosis. Arch Phys Med Rehabil 2013;94:1567–72.
103. Kasser SL, Jacobs JV, Littenberg B, et al. Exploring physical activity in women with multiple sclerosis: associations with fear of falling and underlying impairments. Am J Phys Med Rehabil 2014; Jan 6. [Epub ahead of print].
104. Kasser SL, Kosma M. Health beliefs and physical activity behavior in adults with multiple sclerosis. Disabil Health J 2012;5:261–8.
105. Slawta JN, Wilcox AR, McCubbin JA, et al. Health behaviors, body composition, and coronary heart disease risk in women with multiple sclerosis. Arch Phys Med Rehabil 2003;84:
1823–30.
106. Marrie RA, Hanwell H. General health issues in multiple sclerosis: comorbidities, secondary conditions, and health behaviors. Continuum (Minneap Minn). 2013;19:1046–57.
107. Beer S, Khan F, Kesselring J. Rehabilitation interventions in multiple sclerosis. J Neurol 2012;259:1994–2008.
108. Rietberg MB, Brooks D, Uitdehaag BMJ, Kwakkel G. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev 2004;(3):CD003980.
109. Brown TR, Kraft GH. Exercise and rehabilitation for individuals with multiple sclerosis. Phys Med Rehabil Clin N Am 2005;16:513–55.
110. Dalgas U, Stenager E, Ingemann-Hansen T. Multiple sclerosis and physical exercise: recommendations for the application of resistance-, endurance- and combined training. Mult Scler 2008;14:35–53.
111. Khan F, Turner-Stokes L, Ng L, et al. Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database Syst Rev 2007;2:CD006036.
112. Kesslering J, Beer S. Symptomatic therapy and neurorehabilitation in multiple sclerosis. Lancet Neurol 2005;4:643–52.
113. Kjolhede T, Vissing K, Dalgas U. Multiple sclerosis and preogressive resistance training: a systematic review. Mult Scler 2012;18:1215–28.
114. Paltamaa J, Sjogren T, Peurala, SH, Heinonen A. Effects of physiotherapy interventions on balance in multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. J Rehabil Med 2012;44:811–23.
115. Wiles CM, Newcombe RG, Fuller KJ, et al. Controlled randomized crossover trial of the effects of physiotherapy on mobility in chronic multiple sclerosis. J Neurol Neurosurg Psychiatry 2001;70:174–9.
116. Harvey L, Davies Smith A, Jones R. The effect of weighted leg raises on quadriceps strength, EMG parameters and functional activities in people with multiple sclerosis. Physiother 1999;85:154–61.
117. Dalgas U, Stenager E, Jakobsen J, et al. Resistance training improves muscle strength and functional capacity in multiple sclerosis. Neurology 2009;73:1478–84.
118. Broekmans T, Roelants M, Feys P, et al. Effects of long-term resistance training and simultaneous electro-stimulation on muscle strength and functional mobility in multiple sclerosis. Mult Scler 2010;17:468–77.
119. Sabapathy NM, Minihan CL, Turner GT, Broadley SA. Comparing endurance- and resitance-exercise training in people with multiple sclerosis: a randomized pilot study. Clin Rehabil 2011;25:14–24.
120. Lord SE, Wade DT, Halligan PW. A comparison of two physiotherapy treatment approaches to improve walking in multiple sclerosis: a pilot randomized controlled study. Clin Rehabil 1998;12:477–86.
121. Plow MA, Mathiowetz V, Lowe DA. Comparing individualized rehabilitation to a group wellness intervention for persons with multiple sclerosis. Am J Health Prom 2009;24:23–6.
122. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil 2004;85:290–7.
123. Hayes HA, Gappmaier E, LaStayo PC. Effects of high-intensity resistance training on strength, mobility, balance, and fatigue in individuals with multiple sclerosis: a randomized controlled trial. J Neurol Phys Ther 2011;35:2–10.
124. de Souza-Teixeira F, Costilla S, Ayan C, et al. Effects of resistance training in multiple sclerosis. Int J Sports Med 2009;30:245–50.
125. Kraft G, Alquist A, Lateur B. Effects of resistive exercise on function in multiple sclerosis (MS). Arch Phys Med Rehabil 1996;77:984.
126. White LJ, McCoy SC, Castellano V, et al. Resistance training improves strength and functional capacity in persons with multiple sclerosis. Mult Scler 2004;10:668–74.
127. Taylor NF, Dodd KJ, Prasad D, Denisenko S. Progressive resistance exercise for people with multiple sclerosis. Disabil Rehabil 2006;28:1119–26.
128. Cakit BD, Nacir B, Genc¸ H, et al. Cycling progressive resistance training for people with multiple sclerosis: a randomized controlled study. Am J Phys Med Rehabil 2010;89:446–57.
129. Motl RW, Pilutti, LA. The benefits of exercise training in multiple sclerosis. Nat Rev Neurol 2012;8:487–97.
130. Asano M, Arafah A, Moriello C, Mayo NE. What does a structured review of the effectiveness of exercise interventions for persons with multiple sclerosis tell us about the challenges of designing trials? Mult Scler 2009;15:412–21.
131. Romberg A, Virtanen A, Ruutiainen J, et al. Effects of a 6-month exercise program on patients with multiple sclerosis. Neurology 2004;63:2034–8.
132. Learmonth YC, Paul L, Miller L, et al. The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil 2011;26:579–93.
133. Tarakci E, Yeldan I, Huseyinsinoglu B, et al. Group exercise training for balance, functional status, spasticity, fatigue and quality of life in multiple sclerosis: a randomized controlled trial. Clin Rehabil 2013;27:813–22.
134. Huisinga JM, Filipi ML, Stergiou N. Supervised resistance training results in changes in postural control in patients with multiple sclerosis. Motor Control 2012;16:50–63.
135. Negahban H, Rezaie S, Goharpey S. Massage therapy and exercise therapy in patients with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil 2013;27:1126–36.
136. Cattaneo D, Jonsdottir J, Zocchi M, Regola A. Effects of balance exercises on people with multiple sclerosis: a pilot study. Clin Rehabil 2007;21:771–81.
137. Plow M, Finlayson M. Potential benefits of Nintendo Wii Fit among people with multiple sclerosis: a longitudinal pilot study. Int J MS Care 2011;13:21–30.
138. Plow M, Finlayson M. A qualitative study exploring the usability of Nintendo Wii Fit among persons with multiple sclerosis. Occup Ther Int 2014;21:21–32.
139. Nilsgard YE, Forsberg AS, von Koch L. Balance exercise for persons with multiple sclerosis using Wii games: a randomized, controlled multi-centre study. Mult Scler J 2012;19:209–16.
140. Prosperini L, Fortuna D, Gianni C, et al. Home-based balance training using the Wii Balance Board: a randomized, crossover pilot study in multiple sclerosis. Neurorehabil Neural Repair 2013;27:516–25.
141. Bruchetta G, Spallarossa P, Lopes de Carvalho ML, Battaglia MA. The effect of Nintendo Wii on balance in people with multiple sclerosis: a pilot randomized control study. Mult Scler J 2013;19:1219–21.
142. Guidi I, Giovannelli T, Paci M. Effects of Wii exercise on balance in people with multiple sclerosis. Mult Scler 2013;19:965.
143. Hebert JR, Corboy JR, Manago MM, Schenkman M. Effects of vestibular rehabilitation on multiple sclerosis–related fatigue and upright postural control: a randomized controlled trial. Phys Ther 2011;91:1166–83.
144. Gibson-Horn C. Balance-based torso-weighting in a patient with ataxia and multiple sclerosis: a case report. J Neurol Phys Ther 2008;32:139-146.
145. Crittendon A, O’Neill D, Widener GL, Allen DD. Standing data disproves biomechanical mechanism for balance-based torso-weighting. Arch Phys Med Rehabil 2014;95:43–9.
146. Widener GL, Allen DD, Gibson-Horn C. Balance-based torso-weighting may enhance balance in persons with multiple sclerosis: preliminary evidence. Arch Phys Med Rehabil 2009;90:602–9.
147. Widener GL, Allen DD, Gibson C. Randomized clinical trial of balance-based torso weighting for improving upright mobility in people with multiple sclerosis. Neurorehabil Neural Repair 2009;23:784–91.
148. Abercromby AF, Amonette WE, Layne CS, et al. Vibration exposure and biodynamic responses during whole-body vibration training. Med Sci Sports Exerc 2007;39:1794–800.
149. Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Phys 2010;108:877–904.
150. Prisby RD, Lafage-Proust MH, Malaval L, et al. Effects of whole body vibration on the skeleton and other organ systems in man and animal models: what we know and what we need to know. Age Res Rev 2008;7:319–29.
151. Wunderer K, Schabrun SM, Chipchase LS. Effects of whole body vibration on strength and functional mobility in multiple sclerosis. Physiother Theory Practice 2010;26:374–84.
152. Mason RR, Cochrane DJ, Denny GJ, et al. Is 8 weeks of side-alternating whole-body vibration a safe and acceptable modality to improve functional performance in multiple sclerosis? Dis Rehabil 2012;34:647–54.
153. Schuhfried O, Mittermaier C, Jovanovic T, et al. Effects of whole-body vibration in patients with multiple sclerosis: a pilot study. Clin Rehabil 2005;19:834–42.
154. Claerbout M, Gebara B, Ilsbroukx S, et al. Effects of 3 weeks’ whole body vibration training on muscle strength and functional mobility in hospitalized persons with multiple sclerosis. Mult Scler J 2012;18:498–505.
155. Eftekhari E, Mostahfezian M, Etemadifar M, Zafari A. Resistance training and vibration improve musle strength and functional capacity in female patients with multiple sclerosis. Asian J Sports Med 2012;3:279–84.
156. Hilgers C, Mundermann A, Riehle H, Dettmers C. Effects of whole-body vibration training on physical function inpatients with multiple sclerosis. Neurorehabil 2013;32:655–63.
157. Schyns F, Paul L, Finlay K, et al. Vibration therapy in multiple sclerosis: a pilot study exploring its effects on tone, muscle force, sensation and functional performance. Clin Rehabil 2009;23:771–81.
158. Broekmans T, Roelants M, Alders G, et al. Exploring the effects of a 20-week whole-body vibration training program on leg muscle performance and function in persons with multiple sclerosis. J Rehabil Med 2010;42:866–72.
159. Santos-Fihlo SD, Cameron MH, Bernardo-Filho M. Benefits of whole-body vibration with an oscillating platform for people with multiple sclerosis: a systematic review. Mult Scler Int 2012;2012:274728.
160. Castro-Sanchez AM, Mataran-Penarrocha GA, Lara-Palomo I, et al. Hydrotherapy for the treatment of pain in people with multiple sclerosis: a randomized controlled trial. eCAM. 2012;473963.
161. Kargarfard M, Etemadifar M, Baker P, et al. Effects of aquatic exercise training on fatigue and health-related quality of life in patients with multiple sclerosis. Arch Phys Med Rehabil 2012;93:1701–8.
162. Pariser G, Madras D, Weiss E. Outcomes of an aquatic exercise program including aerobic capacity, lactate threshold, and fatigue in two individuals with multiple sclerosis. J Neurol Phys Ther 2006;30:82–90.
163. Rafeeyan Z, Azarbarzin M, Moosa FM, Hasanzadeh A. Effect of aquatic exercise on the multiple sclerosis patients’ quality of life. Iranian J Nurs Midwifery Res 2010;15:43–7.
164. Gehlsen G, Beekman K, Assmann N, et al. Carter A. Gait characteristics in multiple sclerosis: progressive changes and effects of exercise on parameters. Arch Phys Med Rehabil 1986;67:536–9.
165. Salem Y, Scott AH, Karpatkin H, et al. Community-based group aquatic programme for individuals with multiple sclerosis: a pilot study. Dis Rehabil 2011;33:720–8.
166. Marandi SM, Nejad VS, Shanazari Z, Zolaktaf V. A comparison of 12 weeks of pilates and aquatic training on the dynamic balance of women with multiple sclerosis. Int J Preventive Med 2013;4(Suppl 1):S110-7.
167. Bayraktar D, Guclu-Gunduz A, Yazici G, et al. Effects of Ai-Chi on balance, functional mobility, sytrength and fatigue in patients with multiple sclerosis: a pilot study. Neurorehabil 2013;33:431–7.
168. Oken BS, Kishiyama S, Zajdel D, et al. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology 2004;62:2058–64.
169. Salgado BC, Jones M, Ilgun S, et al. Effects of a 4-month Ananda yoga program on physical and mental health outcomes for persons with multiple sclerosis. Int J Yoga Ther 2013;23:27–38.
170. Ahmadi A, Arastoo AA, Nikbakht Met al. Comparison of the effect of 8 weeks aerobic and yoga training on ambulatory function, fatigue and mood status in MS patients. Iranian Red Crescent Med J 2013;15:449–54.
171. Jackson K, Edginton-Bigelow K, Bowsheir C, et al. Feasibility and effects of a group kickboxing program for individuals with multiple sclerosis: a pilot report. J Bodywork Movement Ther 2012;16:7–13.
172. Mackay-Lyons M, Conway C, Roberts W. Effects of therapeutic riding on patients with multiple sclerosis: a preliminary trial. Physiother Can 1988;40:104–9.
173. Hammer A, Nilsagard Y, Forsberg A, et al. Evaluation of therapeutic riding (Sweden)/hippotherapy (United States): a single-subject experimental design study replicated in eleven patients with multiple sclerosis. Physiother Theory Prac 2005;21:51–77.
174. Silkwood-Sherer D, Warmbier H. Effects of hippotherapy on postural stability in persons with multiple sclerosis: a pilot study. J Neurol Phys Ther 2007;31:77–84.
175. Bronson C, Brewerton K, Ong J, et al. Does hippotherapy improve balance in persons with multiple sclerosis: a systematic review. Eur J Phys Rehabil Med 2010;46:347–53.
From the Department of Rehabilitation and Movement Science, University of Vermont, Burlington, VT.
Abstract
- Objective: To provide insight into the mechanisms and treatment options associated with balance impairments in individuals with multiple sclerosis (MS).
- Methods: Systematic reviews, randomized controlled trials, and noncontrolled studies were examined to collect current data regarding treatment options aimed at improving balance in MS.
- Results: Balance deficits are common in individuals with MS and result from a diverse set of constraints across multiple systems of postural control. Poor balance often leads to increased fall risk, reduced physical activity, added comorbidities, and decreased quality of life. A variety of exercise options are available for individuals with MS who experience balance and mobility problems. Physical interventions include targeted therapies, such as vestibular rehabilitation and weighted torso training, as well as more general exercise and balance training prescriptions.
- Conclusion: The evidence, albeit preliminary, suggests that therapeutic intervention aimed at ameliorating balance deficits associated with MS be multimodal. Exercise prescriptions should include sensory and motor strategy training, strength development, as well as functional gait activities. Further evidence-based research is needed to improve the management of balance deficits in those with MS and to identify the impact of improved balance on activity participation and quality of life.
Multiple sclerosis (MS) is one of the most common nontraumatic neurologic causes of disability among young adults. With greater awareness and improved diagnostics, more people are being diagnosed with the disease today than in the past. Prevalence estimates in the United States range from 90 to 135 per 100,000 individuals [1], with approximately 400,000 people currently diagnosed [2,3].
MS is a chronic inflammatory disease of the central nervous system typically characterized by increasing muscle weakness, spasticity, fatigue, pain, depression, visual and sensory disturbances, and cognitive difficulties. The clinical course of MS is highly variable and often unpredictable with increasing disability and physical decline spanning a 30- to 40-year period post diagnosis [4]. During this time, advancing symptoms can lead to a number of comorbidities and negatively impact daily functioning, mobility, and community participation [5–7]. From a public health standpoint, the early and disabling impact of symptoms and prolonged physical decline create a significant economic burden. The projected national heath care costs of MS are greater than $7 billion annually [8], with the average total annual cost per patient estimated at over $47,000 [9]. Of this annual cost, indirect costs associated with lost productivity represent the single highest component cost [9,10].
Of the wide range of disease-related challenges, mobility difficulties are most significant. Over 90% of people with MS report mobility difficulties [11], and maintaining mobility is consistently ranked as one of the highest priorities for this group, independent of disease duration or disability level [10,12]. Several studies have demonstrated that loss of balance and mobility contributes to substantial patient burden [13] and lower perceived quality of life [10]. Moreover, poor balance and increased fall risk have been associated with reduced physical activity and other health-related behaviors [14,15].
Because balance and mobility limitations are so prevalent and impacting, targeted treatments aimed at maintaining ambulation and function are critical goals in the management of MS. It is important for physicians and rehabilitation professionals to understand and recognize the underlying sensorimotor mechanisms related to postural instability and initiate appropriate evidenced-based treatments that can improve balance, reduce fall risk, and enhance quality of life for individuals with MS. This review seeks to analyze the evidence on the physical interventions aimed at ameliorating balance and mobility impairments associated with MS in the context of a case example.
Case Study
Initital Presentation and History
Ms. D is a 41-year-old woman with relapse-remitting MS. She was diagnosed 6 years ago after experiencing initial symptoms of optic neuritis and some numbness in her right hand. Since then, she has developed greater weakness in both her legs and reports that her MS significantly impacts her ability to walk, both in terms of distance and the effort needed to ambulate.
Ms. D is independently ambulatory without the use of any assistive device. She reports that her balance is worse when walking on uneven surfaces, moving about in dimly lit environments, turning, or when walking in crowded spaces. Ms. D also shares that she has difficulty standing on one leg while pulling on socks. She states that she must concentrate and focus on her balance when in these challenging situations and that she has to consistently look where she is stepping.
Ms. D does not have any spasticity in muscles of the lower extremities, but on occasion does experience some numbness and tingling in her left foot. She experiences moderate fatigue that requires her to pace herself throughout her daily activities. She reports that her fatigue impacts her ability to concentrate or pay attention for long periods of time and impacts her motivation to engage in social activities. She states that she sleeps restlessly and is consequently tired when she wakes in the morning. Although she is sedentary, she has no history of cardiopulmonary issues or orthopedic problems.
Physical Examination
Ms. D is 5’7” and weighs 175 pounds, with a BMI of 27.4. She presents with observable gait and balance impairment. On physical examination, she exhibits reduced bilateral strength of knee flexors and extensors as well as hip adductors, although the weakness is more evident on the left. On neurologic exam, she exhibits moderate disability in both sensory and cerebellar functioning (resulting in an Expanded Disability Status Scale score of 3.5) [16].
What is postural control?
What balance impairments are associated with MS?
Postural Equilibrium and Balance
For all individuals, postural orientation and equilibrium underlie the effective performance of life’s daily tasks. Postural orientation refers to the alignment of body segments to a reference (such as gravity, the support surface, or an object in the visual field), while postural equilibrium—often equated with balance—refers to maintaining or re-acquiring the body’s center of gravity (CoG) within the base of support (BoS) [17,18]. This paper will focus on postural equilibrium with MS across multiple contexts of balance tasks.
Horak [18] described contexts of balance tasks that affect the mechanisms of maintaining postural equilibrium. Some of these contextual variables include
- Biomechanical constraints (eg, strength)
- Limits of stability (functional reach, maximum lean)
- Anticipatory postural adjustments (voluntary postural transitions)
- Automatic postural responses (balance recovery from external perturbations)
- Sensory orientation (ability to reweight sensory information [somatosensory, visual, vestibular] depending on context
- Dynamic control during gait
- Cognitive-motor interaction (balance impairments when also performing a cognitive task)
Emotion represents another contextual variable of interest, because mood and fear can significantly modify postural control [19–23]. Knowing the contextual factors that modify balance control provides insight into underlying neuropathology associated with impairments of these postural control variables [24,25] as well as insight into what should be included during the examination of patients with MS based on patient descriptions of their symptoms and functional challenges.
Balance Assessment
Balance assessment indicates that Ms. D cannot abduct and hold either leg to her side for any noticeable length of time, cannot reach forward adequately without lifting her heels off the ground or falling forward, and cannot stand on one leg for more than 10 seconds without losing balance. She also needs to take multiple steps to recover balance with any slight perturbation and is unable to maintain stability while standing on foam with her eyes closed. She shows significant imbalance when rising from a chair, walking forward, and turning to come back to sit.
For Ms. D, the clinical balance exam suggests pervasive impairment of hip strength, limits of stability, anticipatory postural adjustments, postural responses, sensory integration, and gait. Furthermore, her reported need to focus vision on her gait is in accordance with compensation for existing sensory impairments. Lastly, fatigue and attention demand likely enhance the presentation of balance impairment.
What are the consequences of balance impairments associated with MS?
Balance impairments present considerable health problems for adults with MS. Greater than 50% of individuals with MS report falling in any 6-month period [81–85], with the incidence of recurrent falls reported to be as high as 9 falls per year [86]. In addition, fall-related injuries, including fractures, are more common with MS, although this increased risk is considerably greater for women with MS than men [86–90].
Common risk factors for falling in people with MS include variable or deteriorating MS status [90–96], problems with balance or mobility [88,92–94,96–99], use of walking aids [88,93,97], lower balance confidence [86,98], reduced executive functioning [99] and greater fatigue [85]. Increased postural sway [52,99,100], slower walking speed [99], greater gait asymmetry and variability [92,101], slower choice stepping reaction time [99], impaired forward limits of stability [92,99], impaired visually dependent sway [92,99], and leg weakness [88,92] have also been found predictive of future falls in MS. A link has also emerged between cognitive impairment and fall risk [86,95,99,102].
Fear of falling and fall-induced injuries are also the most common causes of restricted activity and disability for individuals with MS [14]. Research has shown that future physical activity associates with fear of falling, and fear of falling subsequently associated with lower-limb strength asymmetry and decreased limits of stability rather than past experience of falling [103]. Similarly, the perceived benefits of physical activity and an individual’s self-efficacy to engage in physical activity predict reported levels of physical activity independent of disability level for individuals with MS [104]. Thus, psychological perception represents an important, and potentially modifiable, correlate of physical activity.
Moreover, individuals with MS experience a high risk of cardiovascular disease and other chronic health conditions associated with deconditioning, as unfavorable blood lipid levels, poor glucose profiles, and obesity have been observed in this population [105]. Comorbid conditions, secondary conditions, and health behaviors are increasingly recognized to be important factors influencing a range of outcomes in MS [107].
Further History
Consistent with the consequences of balance and mobility impairment, Ms. D reports that she loses her balance and nearly falls at least 1 time per week while engaged in daily activities. She also shares that she fell 2 months ago while walking outside and across the lawn to get the mail. Her confidence is low for many daily tasks such as climbing stairs, picking up objects from the floor, reaching when on tiptoes, or walking on ramps or on slippery surfaces. While Ms. D is independent in all activities of daily living, she currently does not work due to her fatigue and poor balance. She indicates that she is not very physically active and feels somewhat isolated and depressed because her balance and mobility challenges keep her from going out with friends and socializing.
What exercise approaches are available to ameliorate the balance deficits associated with MS?
There are a variety of therapeutic approaches for the treatment of poor balance in MS. While pharmacologic treatment typically encompasses disease-modifying therapies, specific medications can also help in the management of symptoms (ie, fatigue, spasticity, gait variability) that can negatively impact balance and mobility. Other rehabilitative strategies for balance impairment include gait training, assistive devices for mobility, and environmental modifications for fall prevention. Although all of these avenues offer viable treatment options for improving balance, exercise is increasingly appreciated as an important adjunct to the rehabilitation management of MS [107], especially in terms of improving balance deficits, optimizing daily functioning, and increasing participation across various life contexts.
The diversity of exercise options available for individuals with MS who experience balance and mobility problems is expanding. Moreover, mounting evidence suggests that exercise is well tolerated by participants with the disease[108–110] and that individuals with MS can exercise sufficiently to improve their fitness, function, and quality of life [109,110]. Given the inherent variability of MS and the heterogeneity of symptoms and disease course across individuals, however, no one exercise prescription is optimal for all those diagnosed. Instead, treatment goals must be individualized and functionally based [107] with ongoing evaluation and modification of treatment plans due to disease progression, symptom fluctuations, and functional decline [107,111]. Regardless of specific approach, the aim of any exercise intervention is to reduce activity limitations, encourage participation, and facilitate independence and life satisfaction in those with the disease [112].
Resistance Training
There have been several structured reviews of exercise research in MS [108,110,113,114]. The existing evidence supports resistance exercise as compared with no exercise for improving general balance [115] or performing tasks such as a chair transfer [116] or sit-to-stand [117]. Two randomized controlled trials (RCTs) also revealed significant increases in functional reach (ie, limits of stability) as a result of progressive resistance exercise [118,119]. Resistance exercise has not, however, facilitated greater benefit over traditional rehabilitation in other postural control contexts such as those involving postural transitions, sensory integration, or postural sway [120–122].
The effects of resistance training on mobility have also been inconsistent. While several studies showed no significant improvement in functional mobility [118,122,123], a positive improvement was observed in other research [119,124,125]. Likewise, stair climbing was shown to improve in 2 noncontrolled studies [125,126] and one RCT [117] but not in another [127].
In a recent RCT to evaluate the comparative effectiveness of different methods of resistance training, Hayes et al [123] determined that the addition of high-intensity, eccentric resistance training offered no additional benefit over standard concentric resistance exercise in improving static standing balance and stair climbing. In addition, compared with no exercise or a home-based program to improve strength and balance, progressive resistance cycling showed significantly greater effect on functional reach and timed up-and-go in individuals with moderate MS [128]. Nonetheless, evidence for the efficacy of home-based training remains equivocal given issues of motivation, adherence, and training intensity [115,118,128].
Taken together, the systematic reviews to date conclude that there is insufficient evidence for the effects of resistance exercise on balance in MS, thus making solid evidenced-based conclusions difficult [108,110,113,129]. Moreover, it is difficult to ascertain a definitive and most efficacious exercise prescription for improving balance in MS given the inconsistency in protocols and findings across studies. There is some support, albeit preliminary, for progressive resistance training as a modality to improve balance, especially those functional tasks demanding greater strength [113]. Nonetheless, resistance training may contribute to improved posture and gait given it directly addresses one context of postural control, but it may not be fully effective due to lack of training to modify central neural control of posture in other contexts.
Aerobic Exercise
Many of the studies examining aerobic exercise in MS more often target walking capacity, exercise tolerance, fatigue, and quality of life than balance [130]. The limited research that has focused on aerobic exercise for balance improvement has shown equal benefit to that achieved from resistance exercise in those contexts involving limits of stability and dynamic balance while stepping or walking [119]. This finding was reasonable given that the aerobic exercise included step-up and treadmill walking. Still, it has been recommended that, for most people with MS, aerobic exercise also incorporate a degree of balance training [109].
Combined Exercise
The more recent exercise research involving people with MS often combines some aspect of aerobic, strengthening, and/or balance exercise. While only a few RCTs have examined the effects of combined training in this population, preliminary evidence suggests it is well tolerated and may have some benefit for improving function [110]. While one study found no differences in static balance after a combined strength and aerobic training program [131], review of the exercise protocol revealed that the training regime had only incorporated 2 standing exercises. Other studies more intentionally combining strength and balance exercise have demonstrated benefits in balance confidence [132], standing static balance or postural sway [132–134], step climbing [133], and functional mobility [135]. Combining aerobic exercise and strengthening has also been effective in reducing falls in those with MS [85].
Balance-Specific Exercise
Only one balance-specific RCT has been published to date. In this study, outcomes from balance training involving both motor and sensory strategies were compared to training of only motor strategies and to standard therapy [136]. Both the balance training groups significantly reduced the number of falls post intervention as compared to the conventional treatment group. There were no observed differences in self-reported balance confidence across the groups, although both the balance training groups significantly improved in static and dynamic standing balance over that achieved by the standard treatment group. The fact that only the group engaged in sensory training differed significantly on dynamic gait highlights the importance of sensory integration for dynamic balance and gait.
Video Game–Assisted Exercises
Novel rehabilitative approaches have taken advantage of advances in virtual reality and visual feedback training to improve balance and mobility deficits in people with MS. Exercise using the general physical activity games on the Nintendo Wii Fit provided short-term improvement in standing balance, strength, gait and physical activity in people with MS [137]. This general exercise offered no significant gains in self-efficacy, fatigue impact or quality of life, and physical activity levels returned to baseline levels 14 weeks after exercising. Subsequent review has, however, highlighted concerns that current commercially available video options for general exercise may not be sufficiently adaptive for people with moderate disability, leading to intimidation and low adherence [138].
Beyond general physical activity, the Wii Balance Board System has also been used to specifically target balance and mobility deficits in MS. Although one study found no significant benefit from Wii Fit balance exercise in balance performance and walking ability [139], other studies have shown positive effects in standing sway, static balance, dynamic stepping, walking speed, and MS impact [140–142].
The evidence, albeit preliminary, thus suggests that the Wii Fit may offer a feasible adjunct to traditional rehabilitation approaches, especially because the exercise can be done at home without the need for continuous support from a practitioner and because the technology aids in overcoming access barriers often associated with community-based physical activity programs [138]. Nonetheless, research shows that Wii Balance Board System training is more specific for static standing balance than for dynamic balance or mobility, the technology is not positively viewed by those with more advanced symptoms, and there exists a risk of adverse affects and training-related injuries associated with home-based use of the Wii [137,140].
Vestibular Rehabilitation Exercise
Vestibular rehabilitation is a specialized treatment approach that strengthens the vestibular sensory system by retraining the brain to recognize and process signals from the vestibular system and coordinate these with visual and proprioceptive inputs. To date, there has only been one RCT investigating the effects of vestibular rehabilitation on balance in adults with MS [143]. In this study, the outcomes of a standard vestibular rehabilitation program to those of an exercise regime as well as to no intervention were compared. The vestibular rehabilitation program consisted of static and dynamic tasks performed with changing bases of support, on various surfaces, with eyes open or closed, and different head movements. The 6-week vestibular rehabilitation program resulted in both statistically significant and clinically relevant change in standing balance under various sensory conditions compared with either of the other two groups, although no significant difference was found in walking capacity across groups.
Weighted Torso Training
Balance-based torso weighting (BBTW) involves strategically placing small weights on the trunk of an individual to decrease balance deviations observed during quiet stance, perturbed standing, walking, and transitioning [144]. While the specific mechanism underlying the therapeutic effect of rehabilitative weighting has been debated [145], various suggestions include joint compression to encourage co-contraction, enhanced conscious awareness of body segments, and biomechanical changes via shifting of the center of mass [146].
The one RCT examining the effectiveness of BBTW in people with MS found immediate and significant effects of BBTW on postural control and upright mobility [146]. The research confirmed preliminary investigations of BBTW in MS [144,147], demonstrating that BBTW can improve walking speed as well as functional tasks involving standing, walking, turning, and sitting down.
Whole Body Vibration
Whole body vibration (WBV) has been employed across a variety of neurological populations as a means of improving muscle tone, sensation, strength, stability, and functional performance. In WBV, multidimensional vibrations are transferred to an individual performing static or dynamic movements on an oscillating platform. The vibrations are believed to facilitate both neuroendocrine responses as well as motor unit recruitment [148–150].
Results have been inconsistent regarding the effectiveness of WBV as a way of improving postural control and functional mobility in individuals with MS. A few studies have shown significant positive effects of WBV lasting from 1 to 4 weeks on functional mobility [151–153], strength [151,153,154], walking speed [152,155], and standing balance [152]. Walking endurance has also been affected by vibration training designed to improve muscular endurance [156]. Although there have been noted benefits of WBV, these benefits were not significantly more advantageous than those offered by a vibration program in conjunction with lower-limb stretching and strengthening exercises [157] or in addition to a traditional rehabilitation program [154].
There has also been some evidence to show that prolonged WBV does not improve postural stability or functional mobility in individuals with MS after training [155,156,158]. Likewise, there is contradictory evidence supporting the use of WBV in improving walking speed [157], functional reaching [152,153] or overall quality of life [152].
While WBV does not appear to have a detrimental effect on symptoms of MS, there is insufficient evidence regarding its beneficial effects on balance, gait, muscle strength and quality of life compared to other interventions. Future research is necessary to examine various protocols in terms of vibratory parameters and length of intervention before specific prescriptions can be offered [159].
Aquatics
Although aquatic exercise has often been recommended for individuals with MS, much of the research employing this therapeutic modality has focused on outcomes of pain, fatigue, cardiorespiratory fitness, gait, and quality of life [160–164]. Research focused on aquatic exercise for improved balance is limited. Nonetheless, significant improvements in standing balance and functional mobility have been shown for individuals with MS following aquatic exercise [165,166]. Similar results on standing balance and functional mobility have also been shown from Ai Chi, a program in which Tai Chi is combined with other techniques and performed standing in shoulder-depth water using a combination of deep breathing and slow, broad movements of the arms, legs, and torso [167]. These methods of intervention, however, still lack evidence from rigorous designs involving control groups and randomization.
Yoga
Yoga has also been explored as a means to improve physical and mental health outcomes in MS. While an initial study showed no significant changes in one-leg stance from an Iyengar yoga program [168], more recent research found Ananda yoga practice effective in improving standing balance [169]. Likewise, other research has shown that static and dynamic standing balance improved after yoga practice, although not significantly better than that from treadmill exercise training [170].
Kickboxing
There has been only one study to date, albeit not an RCT, that has examined kickboxing as a training modality to improve balance in MS. Although kickboxing was found to be a feasible exercise activity, not all participants demonstrated improved balance and mobility outcomes [171]. As such, further investigation of this novel treatment approach is warranted.
Hippotherapy
Hippotherapy has also been employed as a means of balance training because the multidimensional and random nature of the horse’s movement requires the rider to process increased sensory information and make the necessary anticipatory and reactive adjustments for postural control. While one study reported no improvement in postural sway after hippotherapy [172], other research has shown some benefit in balance and gait after riding [173,174]. Although preliminary, findings from 2 of the studies reveal that hippotherapy may be most beneficial for those with primary progressive MS compared to other subtypes of MS [175]. While hippotherapy may have a positive effect on balance in individuals with MS, the data is limited and lacks rigorous examination through randomized controlled study of large samples in order to allow for its advocacy as a primary rehabilitation modality at this time.
What exercise prescription is indicated for Ms. D?
Because Ms. D’s balance deficits have begun to limit her daily functioning and increase her risk of falling, a formal and targeted balance intervention is warranted. Research confirms that exercise would be well tolerated by Ms. D and supports the feasibility of her engaging in various exercise modalities. Although a number of exercise inter-vention studies involving people with MS have been described in the literature, their clinical utility and results in improving balance and mobility are varied. Nonetheless, there is preliminary evidence suggesting that exercise training may have positive effects on balance and functional mobility and could offer Ms. D benefit. This is especially true given that much of the exercise research included individuals with mild or minimal disability and at same stage of disease progression as Ms. D.
Since Ms. D’s balance problems stem from a range of postural impairments across multiple contexts of balance control, her treatment approach must incorporate exercises that include and integrate these underlying control systems. A targeted and multimodal balance exercise program, rather than general physical activity, may be most efficacious toward this end.
Intervention Prescription
Ms. D has poor ability to utilize somatosensory and vestibular inputs in order to dynamically weight the influence of multiple sensory modalities for the control of standing sway under varying sensory conditions. This visual dependence contributes to her poor balance and increases her fall risk when visual inputs are absent (ie, walking in dimly lit rooms) or when optic flow is incongruent or when visual distractions are present (ie, walking in dynamic contexts such as crowded spaces). Ms. D would benefit from exercises requiring greater use of proprioceptive and vestibular inputs, thereby facilitating improved sensory integration. Exercises performed with eyes closed as well as those completed on mats, foam, or other compliant surfaces would be beneficial. She might also benefit from specific vestibular rehabilitation exercises as this approach has resulted in improved sensory integration [143]. Given that Ms. D must regularly concentrate and focus on her balance and consistently look where she is stepping, her balance exercise program should also address her central processing and attentional deficits by including dual-task training [26].
Ms. D also noted that her MS significantly impacts her ability to walk both in terms of effort and distance and adversely affects her participation in social events. Supplemental to her balance exercise program, aerobic exercise, particularly treadmill walking, may offer some benefit both in terms of her endurance as well as gait. While some of the more targeted modalities such as hippotherapy, yoga, and kickboxing have not been extensively studied, they do offer promise and could be used as adjuncts in order to facilitate Ms. D’s motivation and adherence through more diverse programming. Lastly, and although requiring further study, cognitive-behavioral interventions and patient education may be warranted to help Ms. D overcome her fear of falling, low exercise self-efficacy, and any negative beliefs regarding the potential benefits of exercise.
What additional research is needed?
Although valuable insight has been gained from studies of balance and gait impairment with MS, many contexts remain understudied, particularly with regard to understanding both the neuroanatomical and neurophysiologic pathologies that underlie the behavioral impairments of balance and gait in MS. Further, the value of applying this knowledge of balance impairment to clinical diagnostics and prognostics requires further study in order to develop the most cost- and time-effective exams and evidence-based treatment approaches.
Based on the research to date, it remains difficult to draw definitive evidenced-based conclusions regarding what specific exercise mode or training dose would be most beneficial for Ms. D and others with MS. Moreover, while there exists some evidence of efficacious balance outcomes from exercise training, many of the studies involved individuals with mild MS. Only a few studies to date have included those with more advanced disability, thus making prescription generalizations to those more moderately affected by MS tenuous. Irrespective of specific approach, all modalities of balance-oriented interventions require larger controlled studies, inclusion of those with advancing disability status, long-term follow-up, an evaluation of optimal dose or duration, and outcomes on the neural mechanisms of effect.
Summary
Challenges to balance and mobility present serious consequences for those with MS, as falls and fear of falling lead to poor health outcomes and low quality of life. Given that postural impairments result from a diverse set of deficits in different underlying control systems, therapeutic intervention should be multimodal. Exercise prescription should address all affected contexts of postural control, including sensory and motor strategy training during postural transitions as well as induced postural perturbations, strength development, and gait activity. Evidence from clinical trials suggests that targeted balance oriented exercise in people with MS has the potential to improve balance and functional mobility, although more rigorous study on the topic is needed.
Corresponding author: Susan L. Kasser, PhD, Dept. of Rehabilitation and Movement Science, Univ. of Vermont, 306 Rowell Bldg, 106 Carrigan Dr, Burlington, VT 05405, [email protected]
Financial disclosures: None.
From the Department of Rehabilitation and Movement Science, University of Vermont, Burlington, VT.
Abstract
- Objective: To provide insight into the mechanisms and treatment options associated with balance impairments in individuals with multiple sclerosis (MS).
- Methods: Systematic reviews, randomized controlled trials, and noncontrolled studies were examined to collect current data regarding treatment options aimed at improving balance in MS.
- Results: Balance deficits are common in individuals with MS and result from a diverse set of constraints across multiple systems of postural control. Poor balance often leads to increased fall risk, reduced physical activity, added comorbidities, and decreased quality of life. A variety of exercise options are available for individuals with MS who experience balance and mobility problems. Physical interventions include targeted therapies, such as vestibular rehabilitation and weighted torso training, as well as more general exercise and balance training prescriptions.
- Conclusion: The evidence, albeit preliminary, suggests that therapeutic intervention aimed at ameliorating balance deficits associated with MS be multimodal. Exercise prescriptions should include sensory and motor strategy training, strength development, as well as functional gait activities. Further evidence-based research is needed to improve the management of balance deficits in those with MS and to identify the impact of improved balance on activity participation and quality of life.
Multiple sclerosis (MS) is one of the most common nontraumatic neurologic causes of disability among young adults. With greater awareness and improved diagnostics, more people are being diagnosed with the disease today than in the past. Prevalence estimates in the United States range from 90 to 135 per 100,000 individuals [1], with approximately 400,000 people currently diagnosed [2,3].
MS is a chronic inflammatory disease of the central nervous system typically characterized by increasing muscle weakness, spasticity, fatigue, pain, depression, visual and sensory disturbances, and cognitive difficulties. The clinical course of MS is highly variable and often unpredictable with increasing disability and physical decline spanning a 30- to 40-year period post diagnosis [4]. During this time, advancing symptoms can lead to a number of comorbidities and negatively impact daily functioning, mobility, and community participation [5–7]. From a public health standpoint, the early and disabling impact of symptoms and prolonged physical decline create a significant economic burden. The projected national heath care costs of MS are greater than $7 billion annually [8], with the average total annual cost per patient estimated at over $47,000 [9]. Of this annual cost, indirect costs associated with lost productivity represent the single highest component cost [9,10].
Of the wide range of disease-related challenges, mobility difficulties are most significant. Over 90% of people with MS report mobility difficulties [11], and maintaining mobility is consistently ranked as one of the highest priorities for this group, independent of disease duration or disability level [10,12]. Several studies have demonstrated that loss of balance and mobility contributes to substantial patient burden [13] and lower perceived quality of life [10]. Moreover, poor balance and increased fall risk have been associated with reduced physical activity and other health-related behaviors [14,15].
Because balance and mobility limitations are so prevalent and impacting, targeted treatments aimed at maintaining ambulation and function are critical goals in the management of MS. It is important for physicians and rehabilitation professionals to understand and recognize the underlying sensorimotor mechanisms related to postural instability and initiate appropriate evidenced-based treatments that can improve balance, reduce fall risk, and enhance quality of life for individuals with MS. This review seeks to analyze the evidence on the physical interventions aimed at ameliorating balance and mobility impairments associated with MS in the context of a case example.
Case Study
Initital Presentation and History
Ms. D is a 41-year-old woman with relapse-remitting MS. She was diagnosed 6 years ago after experiencing initial symptoms of optic neuritis and some numbness in her right hand. Since then, she has developed greater weakness in both her legs and reports that her MS significantly impacts her ability to walk, both in terms of distance and the effort needed to ambulate.
Ms. D is independently ambulatory without the use of any assistive device. She reports that her balance is worse when walking on uneven surfaces, moving about in dimly lit environments, turning, or when walking in crowded spaces. Ms. D also shares that she has difficulty standing on one leg while pulling on socks. She states that she must concentrate and focus on her balance when in these challenging situations and that she has to consistently look where she is stepping.
Ms. D does not have any spasticity in muscles of the lower extremities, but on occasion does experience some numbness and tingling in her left foot. She experiences moderate fatigue that requires her to pace herself throughout her daily activities. She reports that her fatigue impacts her ability to concentrate or pay attention for long periods of time and impacts her motivation to engage in social activities. She states that she sleeps restlessly and is consequently tired when she wakes in the morning. Although she is sedentary, she has no history of cardiopulmonary issues or orthopedic problems.
Physical Examination
Ms. D is 5’7” and weighs 175 pounds, with a BMI of 27.4. She presents with observable gait and balance impairment. On physical examination, she exhibits reduced bilateral strength of knee flexors and extensors as well as hip adductors, although the weakness is more evident on the left. On neurologic exam, she exhibits moderate disability in both sensory and cerebellar functioning (resulting in an Expanded Disability Status Scale score of 3.5) [16].
What is postural control?
What balance impairments are associated with MS?
Postural Equilibrium and Balance
For all individuals, postural orientation and equilibrium underlie the effective performance of life’s daily tasks. Postural orientation refers to the alignment of body segments to a reference (such as gravity, the support surface, or an object in the visual field), while postural equilibrium—often equated with balance—refers to maintaining or re-acquiring the body’s center of gravity (CoG) within the base of support (BoS) [17,18]. This paper will focus on postural equilibrium with MS across multiple contexts of balance tasks.
Horak [18] described contexts of balance tasks that affect the mechanisms of maintaining postural equilibrium. Some of these contextual variables include
- Biomechanical constraints (eg, strength)
- Limits of stability (functional reach, maximum lean)
- Anticipatory postural adjustments (voluntary postural transitions)
- Automatic postural responses (balance recovery from external perturbations)
- Sensory orientation (ability to reweight sensory information [somatosensory, visual, vestibular] depending on context
- Dynamic control during gait
- Cognitive-motor interaction (balance impairments when also performing a cognitive task)
Emotion represents another contextual variable of interest, because mood and fear can significantly modify postural control [19–23]. Knowing the contextual factors that modify balance control provides insight into underlying neuropathology associated with impairments of these postural control variables [24,25] as well as insight into what should be included during the examination of patients with MS based on patient descriptions of their symptoms and functional challenges.
Balance Assessment
Balance assessment indicates that Ms. D cannot abduct and hold either leg to her side for any noticeable length of time, cannot reach forward adequately without lifting her heels off the ground or falling forward, and cannot stand on one leg for more than 10 seconds without losing balance. She also needs to take multiple steps to recover balance with any slight perturbation and is unable to maintain stability while standing on foam with her eyes closed. She shows significant imbalance when rising from a chair, walking forward, and turning to come back to sit.
For Ms. D, the clinical balance exam suggests pervasive impairment of hip strength, limits of stability, anticipatory postural adjustments, postural responses, sensory integration, and gait. Furthermore, her reported need to focus vision on her gait is in accordance with compensation for existing sensory impairments. Lastly, fatigue and attention demand likely enhance the presentation of balance impairment.
What are the consequences of balance impairments associated with MS?
Balance impairments present considerable health problems for adults with MS. Greater than 50% of individuals with MS report falling in any 6-month period [81–85], with the incidence of recurrent falls reported to be as high as 9 falls per year [86]. In addition, fall-related injuries, including fractures, are more common with MS, although this increased risk is considerably greater for women with MS than men [86–90].
Common risk factors for falling in people with MS include variable or deteriorating MS status [90–96], problems with balance or mobility [88,92–94,96–99], use of walking aids [88,93,97], lower balance confidence [86,98], reduced executive functioning [99] and greater fatigue [85]. Increased postural sway [52,99,100], slower walking speed [99], greater gait asymmetry and variability [92,101], slower choice stepping reaction time [99], impaired forward limits of stability [92,99], impaired visually dependent sway [92,99], and leg weakness [88,92] have also been found predictive of future falls in MS. A link has also emerged between cognitive impairment and fall risk [86,95,99,102].
Fear of falling and fall-induced injuries are also the most common causes of restricted activity and disability for individuals with MS [14]. Research has shown that future physical activity associates with fear of falling, and fear of falling subsequently associated with lower-limb strength asymmetry and decreased limits of stability rather than past experience of falling [103]. Similarly, the perceived benefits of physical activity and an individual’s self-efficacy to engage in physical activity predict reported levels of physical activity independent of disability level for individuals with MS [104]. Thus, psychological perception represents an important, and potentially modifiable, correlate of physical activity.
Moreover, individuals with MS experience a high risk of cardiovascular disease and other chronic health conditions associated with deconditioning, as unfavorable blood lipid levels, poor glucose profiles, and obesity have been observed in this population [105]. Comorbid conditions, secondary conditions, and health behaviors are increasingly recognized to be important factors influencing a range of outcomes in MS [107].
Further History
Consistent with the consequences of balance and mobility impairment, Ms. D reports that she loses her balance and nearly falls at least 1 time per week while engaged in daily activities. She also shares that she fell 2 months ago while walking outside and across the lawn to get the mail. Her confidence is low for many daily tasks such as climbing stairs, picking up objects from the floor, reaching when on tiptoes, or walking on ramps or on slippery surfaces. While Ms. D is independent in all activities of daily living, she currently does not work due to her fatigue and poor balance. She indicates that she is not very physically active and feels somewhat isolated and depressed because her balance and mobility challenges keep her from going out with friends and socializing.
What exercise approaches are available to ameliorate the balance deficits associated with MS?
There are a variety of therapeutic approaches for the treatment of poor balance in MS. While pharmacologic treatment typically encompasses disease-modifying therapies, specific medications can also help in the management of symptoms (ie, fatigue, spasticity, gait variability) that can negatively impact balance and mobility. Other rehabilitative strategies for balance impairment include gait training, assistive devices for mobility, and environmental modifications for fall prevention. Although all of these avenues offer viable treatment options for improving balance, exercise is increasingly appreciated as an important adjunct to the rehabilitation management of MS [107], especially in terms of improving balance deficits, optimizing daily functioning, and increasing participation across various life contexts.
The diversity of exercise options available for individuals with MS who experience balance and mobility problems is expanding. Moreover, mounting evidence suggests that exercise is well tolerated by participants with the disease[108–110] and that individuals with MS can exercise sufficiently to improve their fitness, function, and quality of life [109,110]. Given the inherent variability of MS and the heterogeneity of symptoms and disease course across individuals, however, no one exercise prescription is optimal for all those diagnosed. Instead, treatment goals must be individualized and functionally based [107] with ongoing evaluation and modification of treatment plans due to disease progression, symptom fluctuations, and functional decline [107,111]. Regardless of specific approach, the aim of any exercise intervention is to reduce activity limitations, encourage participation, and facilitate independence and life satisfaction in those with the disease [112].
Resistance Training
There have been several structured reviews of exercise research in MS [108,110,113,114]. The existing evidence supports resistance exercise as compared with no exercise for improving general balance [115] or performing tasks such as a chair transfer [116] or sit-to-stand [117]. Two randomized controlled trials (RCTs) also revealed significant increases in functional reach (ie, limits of stability) as a result of progressive resistance exercise [118,119]. Resistance exercise has not, however, facilitated greater benefit over traditional rehabilitation in other postural control contexts such as those involving postural transitions, sensory integration, or postural sway [120–122].
The effects of resistance training on mobility have also been inconsistent. While several studies showed no significant improvement in functional mobility [118,122,123], a positive improvement was observed in other research [119,124,125]. Likewise, stair climbing was shown to improve in 2 noncontrolled studies [125,126] and one RCT [117] but not in another [127].
In a recent RCT to evaluate the comparative effectiveness of different methods of resistance training, Hayes et al [123] determined that the addition of high-intensity, eccentric resistance training offered no additional benefit over standard concentric resistance exercise in improving static standing balance and stair climbing. In addition, compared with no exercise or a home-based program to improve strength and balance, progressive resistance cycling showed significantly greater effect on functional reach and timed up-and-go in individuals with moderate MS [128]. Nonetheless, evidence for the efficacy of home-based training remains equivocal given issues of motivation, adherence, and training intensity [115,118,128].
Taken together, the systematic reviews to date conclude that there is insufficient evidence for the effects of resistance exercise on balance in MS, thus making solid evidenced-based conclusions difficult [108,110,113,129]. Moreover, it is difficult to ascertain a definitive and most efficacious exercise prescription for improving balance in MS given the inconsistency in protocols and findings across studies. There is some support, albeit preliminary, for progressive resistance training as a modality to improve balance, especially those functional tasks demanding greater strength [113]. Nonetheless, resistance training may contribute to improved posture and gait given it directly addresses one context of postural control, but it may not be fully effective due to lack of training to modify central neural control of posture in other contexts.
Aerobic Exercise
Many of the studies examining aerobic exercise in MS more often target walking capacity, exercise tolerance, fatigue, and quality of life than balance [130]. The limited research that has focused on aerobic exercise for balance improvement has shown equal benefit to that achieved from resistance exercise in those contexts involving limits of stability and dynamic balance while stepping or walking [119]. This finding was reasonable given that the aerobic exercise included step-up and treadmill walking. Still, it has been recommended that, for most people with MS, aerobic exercise also incorporate a degree of balance training [109].
Combined Exercise
The more recent exercise research involving people with MS often combines some aspect of aerobic, strengthening, and/or balance exercise. While only a few RCTs have examined the effects of combined training in this population, preliminary evidence suggests it is well tolerated and may have some benefit for improving function [110]. While one study found no differences in static balance after a combined strength and aerobic training program [131], review of the exercise protocol revealed that the training regime had only incorporated 2 standing exercises. Other studies more intentionally combining strength and balance exercise have demonstrated benefits in balance confidence [132], standing static balance or postural sway [132–134], step climbing [133], and functional mobility [135]. Combining aerobic exercise and strengthening has also been effective in reducing falls in those with MS [85].
Balance-Specific Exercise
Only one balance-specific RCT has been published to date. In this study, outcomes from balance training involving both motor and sensory strategies were compared to training of only motor strategies and to standard therapy [136]. Both the balance training groups significantly reduced the number of falls post intervention as compared to the conventional treatment group. There were no observed differences in self-reported balance confidence across the groups, although both the balance training groups significantly improved in static and dynamic standing balance over that achieved by the standard treatment group. The fact that only the group engaged in sensory training differed significantly on dynamic gait highlights the importance of sensory integration for dynamic balance and gait.
Video Game–Assisted Exercises
Novel rehabilitative approaches have taken advantage of advances in virtual reality and visual feedback training to improve balance and mobility deficits in people with MS. Exercise using the general physical activity games on the Nintendo Wii Fit provided short-term improvement in standing balance, strength, gait and physical activity in people with MS [137]. This general exercise offered no significant gains in self-efficacy, fatigue impact or quality of life, and physical activity levels returned to baseline levels 14 weeks after exercising. Subsequent review has, however, highlighted concerns that current commercially available video options for general exercise may not be sufficiently adaptive for people with moderate disability, leading to intimidation and low adherence [138].
Beyond general physical activity, the Wii Balance Board System has also been used to specifically target balance and mobility deficits in MS. Although one study found no significant benefit from Wii Fit balance exercise in balance performance and walking ability [139], other studies have shown positive effects in standing sway, static balance, dynamic stepping, walking speed, and MS impact [140–142].
The evidence, albeit preliminary, thus suggests that the Wii Fit may offer a feasible adjunct to traditional rehabilitation approaches, especially because the exercise can be done at home without the need for continuous support from a practitioner and because the technology aids in overcoming access barriers often associated with community-based physical activity programs [138]. Nonetheless, research shows that Wii Balance Board System training is more specific for static standing balance than for dynamic balance or mobility, the technology is not positively viewed by those with more advanced symptoms, and there exists a risk of adverse affects and training-related injuries associated with home-based use of the Wii [137,140].
Vestibular Rehabilitation Exercise
Vestibular rehabilitation is a specialized treatment approach that strengthens the vestibular sensory system by retraining the brain to recognize and process signals from the vestibular system and coordinate these with visual and proprioceptive inputs. To date, there has only been one RCT investigating the effects of vestibular rehabilitation on balance in adults with MS [143]. In this study, the outcomes of a standard vestibular rehabilitation program to those of an exercise regime as well as to no intervention were compared. The vestibular rehabilitation program consisted of static and dynamic tasks performed with changing bases of support, on various surfaces, with eyes open or closed, and different head movements. The 6-week vestibular rehabilitation program resulted in both statistically significant and clinically relevant change in standing balance under various sensory conditions compared with either of the other two groups, although no significant difference was found in walking capacity across groups.
Weighted Torso Training
Balance-based torso weighting (BBTW) involves strategically placing small weights on the trunk of an individual to decrease balance deviations observed during quiet stance, perturbed standing, walking, and transitioning [144]. While the specific mechanism underlying the therapeutic effect of rehabilitative weighting has been debated [145], various suggestions include joint compression to encourage co-contraction, enhanced conscious awareness of body segments, and biomechanical changes via shifting of the center of mass [146].
The one RCT examining the effectiveness of BBTW in people with MS found immediate and significant effects of BBTW on postural control and upright mobility [146]. The research confirmed preliminary investigations of BBTW in MS [144,147], demonstrating that BBTW can improve walking speed as well as functional tasks involving standing, walking, turning, and sitting down.
Whole Body Vibration
Whole body vibration (WBV) has been employed across a variety of neurological populations as a means of improving muscle tone, sensation, strength, stability, and functional performance. In WBV, multidimensional vibrations are transferred to an individual performing static or dynamic movements on an oscillating platform. The vibrations are believed to facilitate both neuroendocrine responses as well as motor unit recruitment [148–150].
Results have been inconsistent regarding the effectiveness of WBV as a way of improving postural control and functional mobility in individuals with MS. A few studies have shown significant positive effects of WBV lasting from 1 to 4 weeks on functional mobility [151–153], strength [151,153,154], walking speed [152,155], and standing balance [152]. Walking endurance has also been affected by vibration training designed to improve muscular endurance [156]. Although there have been noted benefits of WBV, these benefits were not significantly more advantageous than those offered by a vibration program in conjunction with lower-limb stretching and strengthening exercises [157] or in addition to a traditional rehabilitation program [154].
There has also been some evidence to show that prolonged WBV does not improve postural stability or functional mobility in individuals with MS after training [155,156,158]. Likewise, there is contradictory evidence supporting the use of WBV in improving walking speed [157], functional reaching [152,153] or overall quality of life [152].
While WBV does not appear to have a detrimental effect on symptoms of MS, there is insufficient evidence regarding its beneficial effects on balance, gait, muscle strength and quality of life compared to other interventions. Future research is necessary to examine various protocols in terms of vibratory parameters and length of intervention before specific prescriptions can be offered [159].
Aquatics
Although aquatic exercise has often been recommended for individuals with MS, much of the research employing this therapeutic modality has focused on outcomes of pain, fatigue, cardiorespiratory fitness, gait, and quality of life [160–164]. Research focused on aquatic exercise for improved balance is limited. Nonetheless, significant improvements in standing balance and functional mobility have been shown for individuals with MS following aquatic exercise [165,166]. Similar results on standing balance and functional mobility have also been shown from Ai Chi, a program in which Tai Chi is combined with other techniques and performed standing in shoulder-depth water using a combination of deep breathing and slow, broad movements of the arms, legs, and torso [167]. These methods of intervention, however, still lack evidence from rigorous designs involving control groups and randomization.
Yoga
Yoga has also been explored as a means to improve physical and mental health outcomes in MS. While an initial study showed no significant changes in one-leg stance from an Iyengar yoga program [168], more recent research found Ananda yoga practice effective in improving standing balance [169]. Likewise, other research has shown that static and dynamic standing balance improved after yoga practice, although not significantly better than that from treadmill exercise training [170].
Kickboxing
There has been only one study to date, albeit not an RCT, that has examined kickboxing as a training modality to improve balance in MS. Although kickboxing was found to be a feasible exercise activity, not all participants demonstrated improved balance and mobility outcomes [171]. As such, further investigation of this novel treatment approach is warranted.
Hippotherapy
Hippotherapy has also been employed as a means of balance training because the multidimensional and random nature of the horse’s movement requires the rider to process increased sensory information and make the necessary anticipatory and reactive adjustments for postural control. While one study reported no improvement in postural sway after hippotherapy [172], other research has shown some benefit in balance and gait after riding [173,174]. Although preliminary, findings from 2 of the studies reveal that hippotherapy may be most beneficial for those with primary progressive MS compared to other subtypes of MS [175]. While hippotherapy may have a positive effect on balance in individuals with MS, the data is limited and lacks rigorous examination through randomized controlled study of large samples in order to allow for its advocacy as a primary rehabilitation modality at this time.
What exercise prescription is indicated for Ms. D?
Because Ms. D’s balance deficits have begun to limit her daily functioning and increase her risk of falling, a formal and targeted balance intervention is warranted. Research confirms that exercise would be well tolerated by Ms. D and supports the feasibility of her engaging in various exercise modalities. Although a number of exercise inter-vention studies involving people with MS have been described in the literature, their clinical utility and results in improving balance and mobility are varied. Nonetheless, there is preliminary evidence suggesting that exercise training may have positive effects on balance and functional mobility and could offer Ms. D benefit. This is especially true given that much of the exercise research included individuals with mild or minimal disability and at same stage of disease progression as Ms. D.
Since Ms. D’s balance problems stem from a range of postural impairments across multiple contexts of balance control, her treatment approach must incorporate exercises that include and integrate these underlying control systems. A targeted and multimodal balance exercise program, rather than general physical activity, may be most efficacious toward this end.
Intervention Prescription
Ms. D has poor ability to utilize somatosensory and vestibular inputs in order to dynamically weight the influence of multiple sensory modalities for the control of standing sway under varying sensory conditions. This visual dependence contributes to her poor balance and increases her fall risk when visual inputs are absent (ie, walking in dimly lit rooms) or when optic flow is incongruent or when visual distractions are present (ie, walking in dynamic contexts such as crowded spaces). Ms. D would benefit from exercises requiring greater use of proprioceptive and vestibular inputs, thereby facilitating improved sensory integration. Exercises performed with eyes closed as well as those completed on mats, foam, or other compliant surfaces would be beneficial. She might also benefit from specific vestibular rehabilitation exercises as this approach has resulted in improved sensory integration [143]. Given that Ms. D must regularly concentrate and focus on her balance and consistently look where she is stepping, her balance exercise program should also address her central processing and attentional deficits by including dual-task training [26].
Ms. D also noted that her MS significantly impacts her ability to walk both in terms of effort and distance and adversely affects her participation in social events. Supplemental to her balance exercise program, aerobic exercise, particularly treadmill walking, may offer some benefit both in terms of her endurance as well as gait. While some of the more targeted modalities such as hippotherapy, yoga, and kickboxing have not been extensively studied, they do offer promise and could be used as adjuncts in order to facilitate Ms. D’s motivation and adherence through more diverse programming. Lastly, and although requiring further study, cognitive-behavioral interventions and patient education may be warranted to help Ms. D overcome her fear of falling, low exercise self-efficacy, and any negative beliefs regarding the potential benefits of exercise.
What additional research is needed?
Although valuable insight has been gained from studies of balance and gait impairment with MS, many contexts remain understudied, particularly with regard to understanding both the neuroanatomical and neurophysiologic pathologies that underlie the behavioral impairments of balance and gait in MS. Further, the value of applying this knowledge of balance impairment to clinical diagnostics and prognostics requires further study in order to develop the most cost- and time-effective exams and evidence-based treatment approaches.
Based on the research to date, it remains difficult to draw definitive evidenced-based conclusions regarding what specific exercise mode or training dose would be most beneficial for Ms. D and others with MS. Moreover, while there exists some evidence of efficacious balance outcomes from exercise training, many of the studies involved individuals with mild MS. Only a few studies to date have included those with more advanced disability, thus making prescription generalizations to those more moderately affected by MS tenuous. Irrespective of specific approach, all modalities of balance-oriented interventions require larger controlled studies, inclusion of those with advancing disability status, long-term follow-up, an evaluation of optimal dose or duration, and outcomes on the neural mechanisms of effect.
Summary
Challenges to balance and mobility present serious consequences for those with MS, as falls and fear of falling lead to poor health outcomes and low quality of life. Given that postural impairments result from a diverse set of deficits in different underlying control systems, therapeutic intervention should be multimodal. Exercise prescription should address all affected contexts of postural control, including sensory and motor strategy training during postural transitions as well as induced postural perturbations, strength development, and gait activity. Evidence from clinical trials suggests that targeted balance oriented exercise in people with MS has the potential to improve balance and functional mobility, although more rigorous study on the topic is needed.
Corresponding author: Susan L. Kasser, PhD, Dept. of Rehabilitation and Movement Science, Univ. of Vermont, 306 Rowell Bldg, 106 Carrigan Dr, Burlington, VT 05405, [email protected]
Financial disclosures: None.
1. Hirtz D, Thurman DJ, Gwinn-Hardy K, et al. How common are the “common” neurologic disorders? Neurology 2007;68:326–37.
2. Keegan BM, Noseworthy JH. Multiple sclerosis. Annu Rev Med 2002;53:285–302.
3. National Multiple Sclerosis Society: Who gets MS? Accessed 5 Mar 2014 at http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/who-gets-ms/index.aspx.
4. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000;343:1430–8.
5. Overs S, Hughes C, Haselkorn J, Turner A. Modifiable comorbidities and disability in multiple sclerosis. Curr Neurol Neurosci Rep 2012;12:610–7.
6. Motl R. Physical activity and irreversible disability in multiple sclerosis. Exerc Sport Sci Rev 2010;38:186–91.
7. Naci H, Fleurence R, Birt J, Duhig A. The impact of increasing neurological disability of multiple sclerosis on health utilities: a systematic review of the literature. J Med Econ 2010;13:78–89.
8. Bainbridge JL. Economics of multiple sclerosis. Adv Stud Pharm 2007;4:330–3.
9. Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross sectional study in the United States. Neurology 2006;66:1696–702.
10. Zwibel H. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv Ther 2009;26:1043–57.
11. Hemmett L, Holmes J, Barnes M, Russell N. What drives quality of life in multiple sclerosis? QJM 2004;97:671–6.
12. Heesen C, Bohm J, Reich C, et al. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler 2008;14:988–91.
13. Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin 2010; 26:109–19.
14. Peterson EW, Cho CC, Finlayson ML. Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult Scler 2007;13:1168–75.
15. Matsuda PN, Shumway-Cook A, Ciol MA, et al. Understanding falls in multiple sclerosis: association of mobility status, concerns about falling, and accumulated impairments. Phys Ther 2012;92:407–15.
16. Kurtzke JF: Rating neurologic impairment in multiple sclerosis: an expended disability status scale (EDSS). Neurology 1983,33:1444–52.
17. Pollock AS, Durward BR, Rowe PJ, Paul JP. What is balance? Clin Rehabil 2000;14:402–6.
18. Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 2006;35 Suppl 2: ii7–ii11.
19. Carpenter MG, Frank JS, Silcher CP, Peysar GW. The influence of postural threat on the control of upright stance. Exp Brain Res 2001;138:210–8.
20. Adkin Al, Frank JS, Carpenter MG, Petsar GW. Fear of falling modifies anticipatory postural control. Exp Brain Res 2002;143:160–70.
21. Bolmont B, Gangloff P, Vouriot A, Perrin P. Mood states and anxiety influence abilities to maintain balance control in healthy human subjects. Neurosci Lett 2002;329:96–100.
22. Carpenter MG, Frank JS, Adkin AL, et al. Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol 2004;92:3255–65.
23. Kitaoka K, Ito R, Araki H, et al. Effect of mood state on anticipatory postural adjustments. Neurosci Lett 2004;370:65–8.
24. Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm 2007; 114:1339–48.
25. Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Movement Disord 2013;28:1483–91.
26. Cameron MH, Lord S. Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep 2010;10:407–12.
27. Jacobs JV, Kasser SL. Balance impairment in people with multiple sclerosis: preliminary evidence for the Balance Evaluation Systems Test. Gait Posture 2012;36:414–8.
28. Jacobs JV, Kasser SL. Effects of dual tasking on the postural performance of people with and without multiple sclerosis: a pilot study. J Neurol 2012;259:1166–76.
29. Boes MK, Sosnoff JJ, Socie MJ, et al. Postural control in multiple sclerosis: effect of disability status and dual task. J Neurol Sci 2012;315:44–8.
30. Wajda A, Achiron A, Dvir Z. Motor impairments at presentation of clinically isolated syndrome suggestive of multiple sclerosis: characterization of different disease subtypes. NeuroRehab 2012;31:147–55.
31. Karst GM, Venema DM, Roehrs TG, Tyler AE. Center of pressure measures during standing tasks in minimally impaired persons with multiple sclerosis. J Neurol Phys Ther 2005;29:170–80.
32. Soyuer F, Mirza M, Erkorkmaz U. Balance performance in three forms of multiple sclerosis. Neurol Res 2006;28:555–62.
33. Findling O, Sellner J, Meler N, et al. Trunk sway in mildly disables multiple sclerosis patients with and without balance impairment. Exp Brain Res 2011;213:363–70.
34. Corporaal SH, Gensicke H, Kuhle J, et al. Balance control in multiple sclerosis: correlations of trunk sway during stance and gait tests with disease severity. Gait Posture 2013;37:55–60.
35. Martin CL, Phillips BA, Kilpatrick TJ, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler 2006;12:620–8.
36. Spain RI, St. George RJ, Salarian A, et al. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture 2012;35:573–8.
37. Huisinga JM, St George RJ, Spain R, et al. Postural response latencies are related to balance control during standing and walking in patients with multiple sclerosis. Arch Phys Med Rehab 2014;
38. Lambert CP, Archer RL, Evans WJ. Muscle strength and fatigue during isokinetic exercise in individuals with multiple sclerosis. Med Sci Sports Exerc 2001;33:1613–9.
39. Carroll CC, Gallagher PM, Seidle ME, Trappe SW. Skeletal muscle characteristics of people with multiple sclerosis. Arch Phys Med Rehab 2005;86:224–9.
40. Ng AV, Miller RG, Gelinas D, Kent-Braun JA. Functional relationships of central and peripheral muscle alterations in multiple sclerosis. Muscle Nerve 2004;29:843–52.
41. Garner DJ, Widrick JJ. Cross-bridge mechanisms of muscle weakness in multiple sclerosis. Muscle Nerve 2003;27:456–64.
42. Ponichtera JA. Concentric and eccentric isokinetic lower extremity strength in multiple sclerosis and able-bodied. J Orthop Sports Phys Ther 2006;16:114–22.
43. Chung LH, Remelius JG, Van Emmerik RE, Kent-Braun JA. Leg power asymmetry and postural control in women with multiple sclerosis. Med Sci Sports Exerc 2008;40:1717–24.
44. Yahia A, Ghroubi S, Mhiri C, Elleuch MH. Relationship between muscle strength, gait and postural parameters in multiple sclerosis. Ann Phys Rehab Med 2011;54:144–55.
45. Frzovic D, Morris ME, Vowels L. Clinical tests of standing balance: performance of persons with multiple sclerosis. Arch Phys Med Rehab 2000;81:215–21.
46. van Emmerik REA, Remelius JG, Johnson MB, et al. Postural control in women with multiple sclerosis: effects of task, vision and symptomatic fatigue. Gait Posture 2010; 32:608–14.
47. Kanekar N, Aruin AS. Clinical and instrumented outcomes measures in balance control of individuals with multiple sclerosis. Mult Scler Int 2013;
48. Huisinga JM, Yentes JM, Filipi ML, Stergiou N. Postural control strategy during standing is altered in patients with multiple sclerosis. Neurosci Lett 2012;524:124–8.
49. Cattaneo D, Jonsdottir J. Sensory impairments in quiet standing in subjects with multiple sclerosis. Mult Scler 2009;15:59–67.
50. Kanekar N, Lee YJ, Aruin AS. Frequency analysis approach to study balance control in individuals with multiple sclerosis. J Neurosci Meth 2014:222:91–6.
51. Cao H, Peyrodie L, Boudet S, et al. Expanded disability status scale (EDSS) estimation in multiple sclerosis from posturographic data. Gait Posture 2013;37:242–5.
52. Kalron A, Achiron A. Postural control, falls and fear of falling in people with multiple sclerosis without mobility aids. J Neurol Sci 2013;335:186–90.
53. Hebert JR, Corboy JR. The association between multiple sclerosis-related fatigue and balance as a function of central sensory integration. Gait Posture 2013;38:37–42.
54. Jackson K, Bigelow KE. Measures of balance performance are affected by a rested versus fatigued testing condition in people with multiple sclerosis. Phys Med Rehabil 2013;5:949–56.
55. Krishnan V, Kanekar N, Aruin AS. Anticipatory postural adjustments in individuals with multiple sclerosis. Neurosci Lett 2012;506:256–60.
56. Krishnan V, Kanekar N, Aruin AS. Feedforard postural control in individuals with multiple sclerosis during load release. Gait Posture 2012;36:225–30.
57. Remelius JG, Hamill J, Kent-Braun J, Van Emmerik R. Gait initiation in multiple sclerosis. Motor Control 2008;12:93–106.
58. Cameron MH, Horak FB, Herndon RR, Bourdette D. Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosens Mot Res 2008,25:113–22.
59. Crenshaw SJ, Royer TD, Richards JG, Hudson DJ. Gait variability in people with multiple sclerosis. Mult Scler 2006;12:613–9.
60. Sosnoff JJ, Sandroff BM, Motl RW. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait Posture 2012;36:154–6.
61. Givon U, Zeilig G, Achiron A. Gait analysis in multiple sclerosis: characterization of temporal–spatial parameters using GAITRite functional ambulation system. Gait Posture 2009;29:138–42.
62. Sosnoff JJ, Weikert M, Dlugonski D, et al. Quantifying gait impairment in multiple sclerosis using GAITRite technology. Gait Posture 2011;34:145–7.
63. Benedetti MG, Piperno R, Simoncini L, et al. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult Scler 1999;5:363–8.
64. Kelleher KJ, Spence W, Solomonidis S, Apatsidis D. The characterisation of gait patterns of people with multiple sclerosis. Disabil Rehabil 2010;32:1242–50.
65. Sacco R, Bussman R, Oesch P, et al. Assessment of gait parameters and fatigue in MS patients during inpatient rehabilitation: a pilot trial. J Neurol 2011;258:889–94.
66. Gianfrancesco MA, Triche EW, Fawcett JA, et al. Speed- and cane-related alterations in gait parameters in individuals with multiple sclerosis. Gait Posture 2011;33:140–2.
67. Morris ME, Cantwell C, Vowels L, Dodd K. Changes in gait and fatigue from morning to afternoon in people with multiple sclerosis.J Neurol Neurosurg Psychiatry 2002;72:361–5.
68. Nogueira LAC, Teixeira L, Sabino P, et al. Gait characteristics of multiple sclerosis patients in the absence of clinical disability. Disabil Rehabil 2013;35:1472–8.
69. Nilsagard Y, Carling A, Forsberg A. Activities-specific balance confidence in people with multiple sclerosis. Mult Scler Int 2012;2012:613925.
70. Kalron A, Achiron A. Relationship between fear of falling to spatiotemporal gait parameters measured by an instrumented treadmill in people with multiple sclerosis. Gait Posture 2014;39:739–44.
71. Huisinga JM, Filipi ML, Schmid KK, Stergiou N. Is there a relationship between fatigue questionnaires and gait mechanics in persons with multiple sclerosis? Arch Phys Med Rehabil 2011;92:1594–601.
72. Motl RW, Sandroff BM, Suh Y, Sosnoff JJ. Energy cost of walking and its association with gait parameters, daily activity, and fatigue in persons with mild multiple sclerosis. Neurorehabil Neural Repair 2012;26:1015–21.
73. Burschka JM, Keune PM, Menge U, et al. An exploration of impaired walking dynamics and fatigue in multiple sclerosis. BMC Neurol 2012;12:161.
74. Kalron A, Dvir Z, Achiron A. Effect of a cognitive task on postural control in patients with a clinically isolated syndrome suggestive of multiple sclerosis. Eur J Phys Rehabil Med 2011;47:579–86.
75. Negahban H, Sanjari M, Mofateh R, Parnianpou M. Nonlinear dynamical structure of sway path during standing in patients with multiple sclerosis and in healthy controls is affected by changes in sensory input and cognitive load. Neurosci Lett 2013;553:126–31.
76. Negahban H, Mofateh R, Arastoo AA, et al. The effect of cognitive loading on balance control in patients with multiple sclerosis. Gait Posture 2011;34:479–84.
77. Sosnoff JJ, Boes MK, Sandroff BM, et al. Walking and thinking in persons with multiple sclerosis who vary in disability. Arch Phys Med Rehabil 2011;92:2028–33.
78. Hamilton F, Rochester L, Paul L, et al. Walking and talking: an investigation of cognitive-motor dual tasking in multiple sclerosis. Mult Scler 2010;15:1215–27.
79. Kalron A, Dvir Z, Achiron A. Walking while talking - difficulties incurred during the initial stages of multiple sclerosis disease process. Gait Posture 2010;32:332–5.
80. Wajda DA, Motl RW, Sosnoff JJ. Dual task cost of walking is related to fall risk in persons with multiple sclerosis. J Neuro Sci 2013;335:160–3.
81. Cameron MH, Asano M, Bourdette D, Finlayson ML. People with multiple sclerosis use many fall prevention strategies but still fall frequently. Arch Phys Med Rehabil 2013;94;1562–6.
82. Cameron MH, Thielman E, Mazumder R, Bourdette D. Predicting falls in people with multiple sclerosis: fall history is as accurate as more complex measures. Mult Scler Int 2013;2013:496325
83. Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil 2006;87:1274–9.
84. Gunn H, Creanor S, Haas B, et al. Risk factors for falls in multiple sclerosis: an observational study. Mult Scler J 2013;19:1913–22.
85. Coote S, Hogan N. Franklin S. Falls in people with multiple sclerosis who use a walking aid: prevalence, factors, and effect of strength and balance interventions. Arch Phys Med Rehabil 2013;94:616–21.
86. Peterson EW, Cho CC, von Koch L, Finlayson ML. Injurious falls among middle aged and older adults with multiple sclerosis. Arch Phys Med Rehabil 2008;89:1031–7.
87. Cameron MH, Poel AJ, Haselkorn JK, et al. Falls requiring medical attention among veterans with multiple sclerosis: a cohort study. J Rehab Res Dev 2011;48:13–20.
88. Matsuda PN, Shumway-Cook A, Bamer AM, et al. Falls in multiple sclerosis. Phys Med Rehabil 2011;3:624–32.
89. Tremlet H, Lucas R. The risks for falls and fractures in multiple sclerosis. Neurology 2012;78:1902–3.
90. Zikan V. Bone health in patients with multiple sclerosis. J Osteoporos 2011;2011:596294.
91. Nilsagard Y, Lundholm C, Denison E, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis: a longitudinal study. Clin Rehabil 2009;23:259–69.
92. Kasser SL, Jacobs JV, Foley JT, et al. A prospective evaluation of balance, gait, and strength to predict falling in women with multiple sclerosis. Arch Phys Med Rehabil 2011;92:1840–6.
93. Gunn H, Newell P, Haas B, et al. Identification of risk factors for falls in multiple sclerosis: a systematic review and meta-analysis. Phys Ther 2013;93:504–13.
94. Sosnoff JJ, Socie MJ, Boes MK, et al. Mobility, balance and falls in persons with multiple sclerosis. PLoS ONE 2011;6(11):e28021.
95. D’Orio VL, Foley FW, Armentano F, et al. Cognitive and motor functioning in patients with multiple sclerosis: neuropsychological predictors of walking speed and falls. J Neurol Sci 2012;316:42–6.
96. Prosperini L, Kouleridou A, Petsas N, et al. The relationship between infratentorial lesions, balance deficit, and accidental falls in multiple sclerosis. J Neuro Sci 2011; 304:55–60.
97. Cattaneo D, De Nuzzo C, Fascia T, et al. Risks of falls in subjects with multiple sclerosis. Arch Phys Med Rehabil 2002;83:864–7.
98. Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil 2006;87:1274–9.
99. Hoang PD, Cameron MH, Gandevia SC, Lord SR. Neuropsychologcial, balance, and mobility risk factors for falls in people with multiple sclerosis: a prospective cohort study. Arch Phys Med Rehabil 2014;95:480–6.
100. Prosperini L, Fortuna D, Gianni C, et al. The diagnostic accuracy of static posturography in predicting accidental falls in people with multiple sclerosis. Neurorehabi Neural Repair 2013;27:45–52.
101. Socie MJ, Sandroff BM, Pula JH, et al. Footfall placement variability and falls in multiple sclerosis. Ann Biomed Eng 2013;41:1740–7.
102. Sosnoff JJ, Balantrapu S, Pilutti L, et al. Cognitive processing speed is related to fall frequency in older adults with multiple sclerosis. Arch Phys Med Rehabil 2013;94:1567–72.
103. Kasser SL, Jacobs JV, Littenberg B, et al. Exploring physical activity in women with multiple sclerosis: associations with fear of falling and underlying impairments. Am J Phys Med Rehabil 2014; Jan 6. [Epub ahead of print].
104. Kasser SL, Kosma M. Health beliefs and physical activity behavior in adults with multiple sclerosis. Disabil Health J 2012;5:261–8.
105. Slawta JN, Wilcox AR, McCubbin JA, et al. Health behaviors, body composition, and coronary heart disease risk in women with multiple sclerosis. Arch Phys Med Rehabil 2003;84:
1823–30.
106. Marrie RA, Hanwell H. General health issues in multiple sclerosis: comorbidities, secondary conditions, and health behaviors. Continuum (Minneap Minn). 2013;19:1046–57.
107. Beer S, Khan F, Kesselring J. Rehabilitation interventions in multiple sclerosis. J Neurol 2012;259:1994–2008.
108. Rietberg MB, Brooks D, Uitdehaag BMJ, Kwakkel G. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev 2004;(3):CD003980.
109. Brown TR, Kraft GH. Exercise and rehabilitation for individuals with multiple sclerosis. Phys Med Rehabil Clin N Am 2005;16:513–55.
110. Dalgas U, Stenager E, Ingemann-Hansen T. Multiple sclerosis and physical exercise: recommendations for the application of resistance-, endurance- and combined training. Mult Scler 2008;14:35–53.
111. Khan F, Turner-Stokes L, Ng L, et al. Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database Syst Rev 2007;2:CD006036.
112. Kesslering J, Beer S. Symptomatic therapy and neurorehabilitation in multiple sclerosis. Lancet Neurol 2005;4:643–52.
113. Kjolhede T, Vissing K, Dalgas U. Multiple sclerosis and preogressive resistance training: a systematic review. Mult Scler 2012;18:1215–28.
114. Paltamaa J, Sjogren T, Peurala, SH, Heinonen A. Effects of physiotherapy interventions on balance in multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. J Rehabil Med 2012;44:811–23.
115. Wiles CM, Newcombe RG, Fuller KJ, et al. Controlled randomized crossover trial of the effects of physiotherapy on mobility in chronic multiple sclerosis. J Neurol Neurosurg Psychiatry 2001;70:174–9.
116. Harvey L, Davies Smith A, Jones R. The effect of weighted leg raises on quadriceps strength, EMG parameters and functional activities in people with multiple sclerosis. Physiother 1999;85:154–61.
117. Dalgas U, Stenager E, Jakobsen J, et al. Resistance training improves muscle strength and functional capacity in multiple sclerosis. Neurology 2009;73:1478–84.
118. Broekmans T, Roelants M, Feys P, et al. Effects of long-term resistance training and simultaneous electro-stimulation on muscle strength and functional mobility in multiple sclerosis. Mult Scler 2010;17:468–77.
119. Sabapathy NM, Minihan CL, Turner GT, Broadley SA. Comparing endurance- and resitance-exercise training in people with multiple sclerosis: a randomized pilot study. Clin Rehabil 2011;25:14–24.
120. Lord SE, Wade DT, Halligan PW. A comparison of two physiotherapy treatment approaches to improve walking in multiple sclerosis: a pilot randomized controlled study. Clin Rehabil 1998;12:477–86.
121. Plow MA, Mathiowetz V, Lowe DA. Comparing individualized rehabilitation to a group wellness intervention for persons with multiple sclerosis. Am J Health Prom 2009;24:23–6.
122. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil 2004;85:290–7.
123. Hayes HA, Gappmaier E, LaStayo PC. Effects of high-intensity resistance training on strength, mobility, balance, and fatigue in individuals with multiple sclerosis: a randomized controlled trial. J Neurol Phys Ther 2011;35:2–10.
124. de Souza-Teixeira F, Costilla S, Ayan C, et al. Effects of resistance training in multiple sclerosis. Int J Sports Med 2009;30:245–50.
125. Kraft G, Alquist A, Lateur B. Effects of resistive exercise on function in multiple sclerosis (MS). Arch Phys Med Rehabil 1996;77:984.
126. White LJ, McCoy SC, Castellano V, et al. Resistance training improves strength and functional capacity in persons with multiple sclerosis. Mult Scler 2004;10:668–74.
127. Taylor NF, Dodd KJ, Prasad D, Denisenko S. Progressive resistance exercise for people with multiple sclerosis. Disabil Rehabil 2006;28:1119–26.
128. Cakit BD, Nacir B, Genc¸ H, et al. Cycling progressive resistance training for people with multiple sclerosis: a randomized controlled study. Am J Phys Med Rehabil 2010;89:446–57.
129. Motl RW, Pilutti, LA. The benefits of exercise training in multiple sclerosis. Nat Rev Neurol 2012;8:487–97.
130. Asano M, Arafah A, Moriello C, Mayo NE. What does a structured review of the effectiveness of exercise interventions for persons with multiple sclerosis tell us about the challenges of designing trials? Mult Scler 2009;15:412–21.
131. Romberg A, Virtanen A, Ruutiainen J, et al. Effects of a 6-month exercise program on patients with multiple sclerosis. Neurology 2004;63:2034–8.
132. Learmonth YC, Paul L, Miller L, et al. The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil 2011;26:579–93.
133. Tarakci E, Yeldan I, Huseyinsinoglu B, et al. Group exercise training for balance, functional status, spasticity, fatigue and quality of life in multiple sclerosis: a randomized controlled trial. Clin Rehabil 2013;27:813–22.
134. Huisinga JM, Filipi ML, Stergiou N. Supervised resistance training results in changes in postural control in patients with multiple sclerosis. Motor Control 2012;16:50–63.
135. Negahban H, Rezaie S, Goharpey S. Massage therapy and exercise therapy in patients with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil 2013;27:1126–36.
136. Cattaneo D, Jonsdottir J, Zocchi M, Regola A. Effects of balance exercises on people with multiple sclerosis: a pilot study. Clin Rehabil 2007;21:771–81.
137. Plow M, Finlayson M. Potential benefits of Nintendo Wii Fit among people with multiple sclerosis: a longitudinal pilot study. Int J MS Care 2011;13:21–30.
138. Plow M, Finlayson M. A qualitative study exploring the usability of Nintendo Wii Fit among persons with multiple sclerosis. Occup Ther Int 2014;21:21–32.
139. Nilsgard YE, Forsberg AS, von Koch L. Balance exercise for persons with multiple sclerosis using Wii games: a randomized, controlled multi-centre study. Mult Scler J 2012;19:209–16.
140. Prosperini L, Fortuna D, Gianni C, et al. Home-based balance training using the Wii Balance Board: a randomized, crossover pilot study in multiple sclerosis. Neurorehabil Neural Repair 2013;27:516–25.
141. Bruchetta G, Spallarossa P, Lopes de Carvalho ML, Battaglia MA. The effect of Nintendo Wii on balance in people with multiple sclerosis: a pilot randomized control study. Mult Scler J 2013;19:1219–21.
142. Guidi I, Giovannelli T, Paci M. Effects of Wii exercise on balance in people with multiple sclerosis. Mult Scler 2013;19:965.
143. Hebert JR, Corboy JR, Manago MM, Schenkman M. Effects of vestibular rehabilitation on multiple sclerosis–related fatigue and upright postural control: a randomized controlled trial. Phys Ther 2011;91:1166–83.
144. Gibson-Horn C. Balance-based torso-weighting in a patient with ataxia and multiple sclerosis: a case report. J Neurol Phys Ther 2008;32:139-146.
145. Crittendon A, O’Neill D, Widener GL, Allen DD. Standing data disproves biomechanical mechanism for balance-based torso-weighting. Arch Phys Med Rehabil 2014;95:43–9.
146. Widener GL, Allen DD, Gibson-Horn C. Balance-based torso-weighting may enhance balance in persons with multiple sclerosis: preliminary evidence. Arch Phys Med Rehabil 2009;90:602–9.
147. Widener GL, Allen DD, Gibson C. Randomized clinical trial of balance-based torso weighting for improving upright mobility in people with multiple sclerosis. Neurorehabil Neural Repair 2009;23:784–91.
148. Abercromby AF, Amonette WE, Layne CS, et al. Vibration exposure and biodynamic responses during whole-body vibration training. Med Sci Sports Exerc 2007;39:1794–800.
149. Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Phys 2010;108:877–904.
150. Prisby RD, Lafage-Proust MH, Malaval L, et al. Effects of whole body vibration on the skeleton and other organ systems in man and animal models: what we know and what we need to know. Age Res Rev 2008;7:319–29.
151. Wunderer K, Schabrun SM, Chipchase LS. Effects of whole body vibration on strength and functional mobility in multiple sclerosis. Physiother Theory Practice 2010;26:374–84.
152. Mason RR, Cochrane DJ, Denny GJ, et al. Is 8 weeks of side-alternating whole-body vibration a safe and acceptable modality to improve functional performance in multiple sclerosis? Dis Rehabil 2012;34:647–54.
153. Schuhfried O, Mittermaier C, Jovanovic T, et al. Effects of whole-body vibration in patients with multiple sclerosis: a pilot study. Clin Rehabil 2005;19:834–42.
154. Claerbout M, Gebara B, Ilsbroukx S, et al. Effects of 3 weeks’ whole body vibration training on muscle strength and functional mobility in hospitalized persons with multiple sclerosis. Mult Scler J 2012;18:498–505.
155. Eftekhari E, Mostahfezian M, Etemadifar M, Zafari A. Resistance training and vibration improve musle strength and functional capacity in female patients with multiple sclerosis. Asian J Sports Med 2012;3:279–84.
156. Hilgers C, Mundermann A, Riehle H, Dettmers C. Effects of whole-body vibration training on physical function inpatients with multiple sclerosis. Neurorehabil 2013;32:655–63.
157. Schyns F, Paul L, Finlay K, et al. Vibration therapy in multiple sclerosis: a pilot study exploring its effects on tone, muscle force, sensation and functional performance. Clin Rehabil 2009;23:771–81.
158. Broekmans T, Roelants M, Alders G, et al. Exploring the effects of a 20-week whole-body vibration training program on leg muscle performance and function in persons with multiple sclerosis. J Rehabil Med 2010;42:866–72.
159. Santos-Fihlo SD, Cameron MH, Bernardo-Filho M. Benefits of whole-body vibration with an oscillating platform for people with multiple sclerosis: a systematic review. Mult Scler Int 2012;2012:274728.
160. Castro-Sanchez AM, Mataran-Penarrocha GA, Lara-Palomo I, et al. Hydrotherapy for the treatment of pain in people with multiple sclerosis: a randomized controlled trial. eCAM. 2012;473963.
161. Kargarfard M, Etemadifar M, Baker P, et al. Effects of aquatic exercise training on fatigue and health-related quality of life in patients with multiple sclerosis. Arch Phys Med Rehabil 2012;93:1701–8.
162. Pariser G, Madras D, Weiss E. Outcomes of an aquatic exercise program including aerobic capacity, lactate threshold, and fatigue in two individuals with multiple sclerosis. J Neurol Phys Ther 2006;30:82–90.
163. Rafeeyan Z, Azarbarzin M, Moosa FM, Hasanzadeh A. Effect of aquatic exercise on the multiple sclerosis patients’ quality of life. Iranian J Nurs Midwifery Res 2010;15:43–7.
164. Gehlsen G, Beekman K, Assmann N, et al. Carter A. Gait characteristics in multiple sclerosis: progressive changes and effects of exercise on parameters. Arch Phys Med Rehabil 1986;67:536–9.
165. Salem Y, Scott AH, Karpatkin H, et al. Community-based group aquatic programme for individuals with multiple sclerosis: a pilot study. Dis Rehabil 2011;33:720–8.
166. Marandi SM, Nejad VS, Shanazari Z, Zolaktaf V. A comparison of 12 weeks of pilates and aquatic training on the dynamic balance of women with multiple sclerosis. Int J Preventive Med 2013;4(Suppl 1):S110-7.
167. Bayraktar D, Guclu-Gunduz A, Yazici G, et al. Effects of Ai-Chi on balance, functional mobility, sytrength and fatigue in patients with multiple sclerosis: a pilot study. Neurorehabil 2013;33:431–7.
168. Oken BS, Kishiyama S, Zajdel D, et al. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology 2004;62:2058–64.
169. Salgado BC, Jones M, Ilgun S, et al. Effects of a 4-month Ananda yoga program on physical and mental health outcomes for persons with multiple sclerosis. Int J Yoga Ther 2013;23:27–38.
170. Ahmadi A, Arastoo AA, Nikbakht Met al. Comparison of the effect of 8 weeks aerobic and yoga training on ambulatory function, fatigue and mood status in MS patients. Iranian Red Crescent Med J 2013;15:449–54.
171. Jackson K, Edginton-Bigelow K, Bowsheir C, et al. Feasibility and effects of a group kickboxing program for individuals with multiple sclerosis: a pilot report. J Bodywork Movement Ther 2012;16:7–13.
172. Mackay-Lyons M, Conway C, Roberts W. Effects of therapeutic riding on patients with multiple sclerosis: a preliminary trial. Physiother Can 1988;40:104–9.
173. Hammer A, Nilsagard Y, Forsberg A, et al. Evaluation of therapeutic riding (Sweden)/hippotherapy (United States): a single-subject experimental design study replicated in eleven patients with multiple sclerosis. Physiother Theory Prac 2005;21:51–77.
174. Silkwood-Sherer D, Warmbier H. Effects of hippotherapy on postural stability in persons with multiple sclerosis: a pilot study. J Neurol Phys Ther 2007;31:77–84.
175. Bronson C, Brewerton K, Ong J, et al. Does hippotherapy improve balance in persons with multiple sclerosis: a systematic review. Eur J Phys Rehabil Med 2010;46:347–53.
1. Hirtz D, Thurman DJ, Gwinn-Hardy K, et al. How common are the “common” neurologic disorders? Neurology 2007;68:326–37.
2. Keegan BM, Noseworthy JH. Multiple sclerosis. Annu Rev Med 2002;53:285–302.
3. National Multiple Sclerosis Society: Who gets MS? Accessed 5 Mar 2014 at http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/who-gets-ms/index.aspx.
4. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000;343:1430–8.
5. Overs S, Hughes C, Haselkorn J, Turner A. Modifiable comorbidities and disability in multiple sclerosis. Curr Neurol Neurosci Rep 2012;12:610–7.
6. Motl R. Physical activity and irreversible disability in multiple sclerosis. Exerc Sport Sci Rev 2010;38:186–91.
7. Naci H, Fleurence R, Birt J, Duhig A. The impact of increasing neurological disability of multiple sclerosis on health utilities: a systematic review of the literature. J Med Econ 2010;13:78–89.
8. Bainbridge JL. Economics of multiple sclerosis. Adv Stud Pharm 2007;4:330–3.
9. Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross sectional study in the United States. Neurology 2006;66:1696–702.
10. Zwibel H. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv Ther 2009;26:1043–57.
11. Hemmett L, Holmes J, Barnes M, Russell N. What drives quality of life in multiple sclerosis? QJM 2004;97:671–6.
12. Heesen C, Bohm J, Reich C, et al. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler 2008;14:988–91.
13. Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin 2010; 26:109–19.
14. Peterson EW, Cho CC, Finlayson ML. Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult Scler 2007;13:1168–75.
15. Matsuda PN, Shumway-Cook A, Ciol MA, et al. Understanding falls in multiple sclerosis: association of mobility status, concerns about falling, and accumulated impairments. Phys Ther 2012;92:407–15.
16. Kurtzke JF: Rating neurologic impairment in multiple sclerosis: an expended disability status scale (EDSS). Neurology 1983,33:1444–52.
17. Pollock AS, Durward BR, Rowe PJ, Paul JP. What is balance? Clin Rehabil 2000;14:402–6.
18. Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 2006;35 Suppl 2: ii7–ii11.
19. Carpenter MG, Frank JS, Silcher CP, Peysar GW. The influence of postural threat on the control of upright stance. Exp Brain Res 2001;138:210–8.
20. Adkin Al, Frank JS, Carpenter MG, Petsar GW. Fear of falling modifies anticipatory postural control. Exp Brain Res 2002;143:160–70.
21. Bolmont B, Gangloff P, Vouriot A, Perrin P. Mood states and anxiety influence abilities to maintain balance control in healthy human subjects. Neurosci Lett 2002;329:96–100.
22. Carpenter MG, Frank JS, Adkin AL, et al. Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol 2004;92:3255–65.
23. Kitaoka K, Ito R, Araki H, et al. Effect of mood state on anticipatory postural adjustments. Neurosci Lett 2004;370:65–8.
24. Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm 2007; 114:1339–48.
25. Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Movement Disord 2013;28:1483–91.
26. Cameron MH, Lord S. Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep 2010;10:407–12.
27. Jacobs JV, Kasser SL. Balance impairment in people with multiple sclerosis: preliminary evidence for the Balance Evaluation Systems Test. Gait Posture 2012;36:414–8.
28. Jacobs JV, Kasser SL. Effects of dual tasking on the postural performance of people with and without multiple sclerosis: a pilot study. J Neurol 2012;259:1166–76.
29. Boes MK, Sosnoff JJ, Socie MJ, et al. Postural control in multiple sclerosis: effect of disability status and dual task. J Neurol Sci 2012;315:44–8.
30. Wajda A, Achiron A, Dvir Z. Motor impairments at presentation of clinically isolated syndrome suggestive of multiple sclerosis: characterization of different disease subtypes. NeuroRehab 2012;31:147–55.
31. Karst GM, Venema DM, Roehrs TG, Tyler AE. Center of pressure measures during standing tasks in minimally impaired persons with multiple sclerosis. J Neurol Phys Ther 2005;29:170–80.
32. Soyuer F, Mirza M, Erkorkmaz U. Balance performance in three forms of multiple sclerosis. Neurol Res 2006;28:555–62.
33. Findling O, Sellner J, Meler N, et al. Trunk sway in mildly disables multiple sclerosis patients with and without balance impairment. Exp Brain Res 2011;213:363–70.
34. Corporaal SH, Gensicke H, Kuhle J, et al. Balance control in multiple sclerosis: correlations of trunk sway during stance and gait tests with disease severity. Gait Posture 2013;37:55–60.
35. Martin CL, Phillips BA, Kilpatrick TJ, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler 2006;12:620–8.
36. Spain RI, St. George RJ, Salarian A, et al. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture 2012;35:573–8.
37. Huisinga JM, St George RJ, Spain R, et al. Postural response latencies are related to balance control during standing and walking in patients with multiple sclerosis. Arch Phys Med Rehab 2014;
38. Lambert CP, Archer RL, Evans WJ. Muscle strength and fatigue during isokinetic exercise in individuals with multiple sclerosis. Med Sci Sports Exerc 2001;33:1613–9.
39. Carroll CC, Gallagher PM, Seidle ME, Trappe SW. Skeletal muscle characteristics of people with multiple sclerosis. Arch Phys Med Rehab 2005;86:224–9.
40. Ng AV, Miller RG, Gelinas D, Kent-Braun JA. Functional relationships of central and peripheral muscle alterations in multiple sclerosis. Muscle Nerve 2004;29:843–52.
41. Garner DJ, Widrick JJ. Cross-bridge mechanisms of muscle weakness in multiple sclerosis. Muscle Nerve 2003;27:456–64.
42. Ponichtera JA. Concentric and eccentric isokinetic lower extremity strength in multiple sclerosis and able-bodied. J Orthop Sports Phys Ther 2006;16:114–22.
43. Chung LH, Remelius JG, Van Emmerik RE, Kent-Braun JA. Leg power asymmetry and postural control in women with multiple sclerosis. Med Sci Sports Exerc 2008;40:1717–24.
44. Yahia A, Ghroubi S, Mhiri C, Elleuch MH. Relationship between muscle strength, gait and postural parameters in multiple sclerosis. Ann Phys Rehab Med 2011;54:144–55.
45. Frzovic D, Morris ME, Vowels L. Clinical tests of standing balance: performance of persons with multiple sclerosis. Arch Phys Med Rehab 2000;81:215–21.
46. van Emmerik REA, Remelius JG, Johnson MB, et al. Postural control in women with multiple sclerosis: effects of task, vision and symptomatic fatigue. Gait Posture 2010; 32:608–14.
47. Kanekar N, Aruin AS. Clinical and instrumented outcomes measures in balance control of individuals with multiple sclerosis. Mult Scler Int 2013;
48. Huisinga JM, Yentes JM, Filipi ML, Stergiou N. Postural control strategy during standing is altered in patients with multiple sclerosis. Neurosci Lett 2012;524:124–8.
49. Cattaneo D, Jonsdottir J. Sensory impairments in quiet standing in subjects with multiple sclerosis. Mult Scler 2009;15:59–67.
50. Kanekar N, Lee YJ, Aruin AS. Frequency analysis approach to study balance control in individuals with multiple sclerosis. J Neurosci Meth 2014:222:91–6.
51. Cao H, Peyrodie L, Boudet S, et al. Expanded disability status scale (EDSS) estimation in multiple sclerosis from posturographic data. Gait Posture 2013;37:242–5.
52. Kalron A, Achiron A. Postural control, falls and fear of falling in people with multiple sclerosis without mobility aids. J Neurol Sci 2013;335:186–90.
53. Hebert JR, Corboy JR. The association between multiple sclerosis-related fatigue and balance as a function of central sensory integration. Gait Posture 2013;38:37–42.
54. Jackson K, Bigelow KE. Measures of balance performance are affected by a rested versus fatigued testing condition in people with multiple sclerosis. Phys Med Rehabil 2013;5:949–56.
55. Krishnan V, Kanekar N, Aruin AS. Anticipatory postural adjustments in individuals with multiple sclerosis. Neurosci Lett 2012;506:256–60.
56. Krishnan V, Kanekar N, Aruin AS. Feedforard postural control in individuals with multiple sclerosis during load release. Gait Posture 2012;36:225–30.
57. Remelius JG, Hamill J, Kent-Braun J, Van Emmerik R. Gait initiation in multiple sclerosis. Motor Control 2008;12:93–106.
58. Cameron MH, Horak FB, Herndon RR, Bourdette D. Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosens Mot Res 2008,25:113–22.
59. Crenshaw SJ, Royer TD, Richards JG, Hudson DJ. Gait variability in people with multiple sclerosis. Mult Scler 2006;12:613–9.
60. Sosnoff JJ, Sandroff BM, Motl RW. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait Posture 2012;36:154–6.
61. Givon U, Zeilig G, Achiron A. Gait analysis in multiple sclerosis: characterization of temporal–spatial parameters using GAITRite functional ambulation system. Gait Posture 2009;29:138–42.
62. Sosnoff JJ, Weikert M, Dlugonski D, et al. Quantifying gait impairment in multiple sclerosis using GAITRite technology. Gait Posture 2011;34:145–7.
63. Benedetti MG, Piperno R, Simoncini L, et al. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult Scler 1999;5:363–8.
64. Kelleher KJ, Spence W, Solomonidis S, Apatsidis D. The characterisation of gait patterns of people with multiple sclerosis. Disabil Rehabil 2010;32:1242–50.
65. Sacco R, Bussman R, Oesch P, et al. Assessment of gait parameters and fatigue in MS patients during inpatient rehabilitation: a pilot trial. J Neurol 2011;258:889–94.
66. Gianfrancesco MA, Triche EW, Fawcett JA, et al. Speed- and cane-related alterations in gait parameters in individuals with multiple sclerosis. Gait Posture 2011;33:140–2.
67. Morris ME, Cantwell C, Vowels L, Dodd K. Changes in gait and fatigue from morning to afternoon in people with multiple sclerosis.J Neurol Neurosurg Psychiatry 2002;72:361–5.
68. Nogueira LAC, Teixeira L, Sabino P, et al. Gait characteristics of multiple sclerosis patients in the absence of clinical disability. Disabil Rehabil 2013;35:1472–8.
69. Nilsagard Y, Carling A, Forsberg A. Activities-specific balance confidence in people with multiple sclerosis. Mult Scler Int 2012;2012:613925.
70. Kalron A, Achiron A. Relationship between fear of falling to spatiotemporal gait parameters measured by an instrumented treadmill in people with multiple sclerosis. Gait Posture 2014;39:739–44.
71. Huisinga JM, Filipi ML, Schmid KK, Stergiou N. Is there a relationship between fatigue questionnaires and gait mechanics in persons with multiple sclerosis? Arch Phys Med Rehabil 2011;92:1594–601.
72. Motl RW, Sandroff BM, Suh Y, Sosnoff JJ. Energy cost of walking and its association with gait parameters, daily activity, and fatigue in persons with mild multiple sclerosis. Neurorehabil Neural Repair 2012;26:1015–21.
73. Burschka JM, Keune PM, Menge U, et al. An exploration of impaired walking dynamics and fatigue in multiple sclerosis. BMC Neurol 2012;12:161.
74. Kalron A, Dvir Z, Achiron A. Effect of a cognitive task on postural control in patients with a clinically isolated syndrome suggestive of multiple sclerosis. Eur J Phys Rehabil Med 2011;47:579–86.
75. Negahban H, Sanjari M, Mofateh R, Parnianpou M. Nonlinear dynamical structure of sway path during standing in patients with multiple sclerosis and in healthy controls is affected by changes in sensory input and cognitive load. Neurosci Lett 2013;553:126–31.
76. Negahban H, Mofateh R, Arastoo AA, et al. The effect of cognitive loading on balance control in patients with multiple sclerosis. Gait Posture 2011;34:479–84.
77. Sosnoff JJ, Boes MK, Sandroff BM, et al. Walking and thinking in persons with multiple sclerosis who vary in disability. Arch Phys Med Rehabil 2011;92:2028–33.
78. Hamilton F, Rochester L, Paul L, et al. Walking and talking: an investigation of cognitive-motor dual tasking in multiple sclerosis. Mult Scler 2010;15:1215–27.
79. Kalron A, Dvir Z, Achiron A. Walking while talking - difficulties incurred during the initial stages of multiple sclerosis disease process. Gait Posture 2010;32:332–5.
80. Wajda DA, Motl RW, Sosnoff JJ. Dual task cost of walking is related to fall risk in persons with multiple sclerosis. J Neuro Sci 2013;335:160–3.
81. Cameron MH, Asano M, Bourdette D, Finlayson ML. People with multiple sclerosis use many fall prevention strategies but still fall frequently. Arch Phys Med Rehabil 2013;94;1562–6.
82. Cameron MH, Thielman E, Mazumder R, Bourdette D. Predicting falls in people with multiple sclerosis: fall history is as accurate as more complex measures. Mult Scler Int 2013;2013:496325
83. Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil 2006;87:1274–9.
84. Gunn H, Creanor S, Haas B, et al. Risk factors for falls in multiple sclerosis: an observational study. Mult Scler J 2013;19:1913–22.
85. Coote S, Hogan N. Franklin S. Falls in people with multiple sclerosis who use a walking aid: prevalence, factors, and effect of strength and balance interventions. Arch Phys Med Rehabil 2013;94:616–21.
86. Peterson EW, Cho CC, von Koch L, Finlayson ML. Injurious falls among middle aged and older adults with multiple sclerosis. Arch Phys Med Rehabil 2008;89:1031–7.
87. Cameron MH, Poel AJ, Haselkorn JK, et al. Falls requiring medical attention among veterans with multiple sclerosis: a cohort study. J Rehab Res Dev 2011;48:13–20.
88. Matsuda PN, Shumway-Cook A, Bamer AM, et al. Falls in multiple sclerosis. Phys Med Rehabil 2011;3:624–32.
89. Tremlet H, Lucas R. The risks for falls and fractures in multiple sclerosis. Neurology 2012;78:1902–3.
90. Zikan V. Bone health in patients with multiple sclerosis. J Osteoporos 2011;2011:596294.
91. Nilsagard Y, Lundholm C, Denison E, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis: a longitudinal study. Clin Rehabil 2009;23:259–69.
92. Kasser SL, Jacobs JV, Foley JT, et al. A prospective evaluation of balance, gait, and strength to predict falling in women with multiple sclerosis. Arch Phys Med Rehabil 2011;92:1840–6.
93. Gunn H, Newell P, Haas B, et al. Identification of risk factors for falls in multiple sclerosis: a systematic review and meta-analysis. Phys Ther 2013;93:504–13.
94. Sosnoff JJ, Socie MJ, Boes MK, et al. Mobility, balance and falls in persons with multiple sclerosis. PLoS ONE 2011;6(11):e28021.
95. D’Orio VL, Foley FW, Armentano F, et al. Cognitive and motor functioning in patients with multiple sclerosis: neuropsychological predictors of walking speed and falls. J Neurol Sci 2012;316:42–6.
96. Prosperini L, Kouleridou A, Petsas N, et al. The relationship between infratentorial lesions, balance deficit, and accidental falls in multiple sclerosis. J Neuro Sci 2011; 304:55–60.
97. Cattaneo D, De Nuzzo C, Fascia T, et al. Risks of falls in subjects with multiple sclerosis. Arch Phys Med Rehabil 2002;83:864–7.
98. Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil 2006;87:1274–9.
99. Hoang PD, Cameron MH, Gandevia SC, Lord SR. Neuropsychologcial, balance, and mobility risk factors for falls in people with multiple sclerosis: a prospective cohort study. Arch Phys Med Rehabil 2014;95:480–6.
100. Prosperini L, Fortuna D, Gianni C, et al. The diagnostic accuracy of static posturography in predicting accidental falls in people with multiple sclerosis. Neurorehabi Neural Repair 2013;27:45–52.
101. Socie MJ, Sandroff BM, Pula JH, et al. Footfall placement variability and falls in multiple sclerosis. Ann Biomed Eng 2013;41:1740–7.
102. Sosnoff JJ, Balantrapu S, Pilutti L, et al. Cognitive processing speed is related to fall frequency in older adults with multiple sclerosis. Arch Phys Med Rehabil 2013;94:1567–72.
103. Kasser SL, Jacobs JV, Littenberg B, et al. Exploring physical activity in women with multiple sclerosis: associations with fear of falling and underlying impairments. Am J Phys Med Rehabil 2014; Jan 6. [Epub ahead of print].
104. Kasser SL, Kosma M. Health beliefs and physical activity behavior in adults with multiple sclerosis. Disabil Health J 2012;5:261–8.
105. Slawta JN, Wilcox AR, McCubbin JA, et al. Health behaviors, body composition, and coronary heart disease risk in women with multiple sclerosis. Arch Phys Med Rehabil 2003;84:
1823–30.
106. Marrie RA, Hanwell H. General health issues in multiple sclerosis: comorbidities, secondary conditions, and health behaviors. Continuum (Minneap Minn). 2013;19:1046–57.
107. Beer S, Khan F, Kesselring J. Rehabilitation interventions in multiple sclerosis. J Neurol 2012;259:1994–2008.
108. Rietberg MB, Brooks D, Uitdehaag BMJ, Kwakkel G. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev 2004;(3):CD003980.
109. Brown TR, Kraft GH. Exercise and rehabilitation for individuals with multiple sclerosis. Phys Med Rehabil Clin N Am 2005;16:513–55.
110. Dalgas U, Stenager E, Ingemann-Hansen T. Multiple sclerosis and physical exercise: recommendations for the application of resistance-, endurance- and combined training. Mult Scler 2008;14:35–53.
111. Khan F, Turner-Stokes L, Ng L, et al. Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database Syst Rev 2007;2:CD006036.
112. Kesslering J, Beer S. Symptomatic therapy and neurorehabilitation in multiple sclerosis. Lancet Neurol 2005;4:643–52.
113. Kjolhede T, Vissing K, Dalgas U. Multiple sclerosis and preogressive resistance training: a systematic review. Mult Scler 2012;18:1215–28.
114. Paltamaa J, Sjogren T, Peurala, SH, Heinonen A. Effects of physiotherapy interventions on balance in multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. J Rehabil Med 2012;44:811–23.
115. Wiles CM, Newcombe RG, Fuller KJ, et al. Controlled randomized crossover trial of the effects of physiotherapy on mobility in chronic multiple sclerosis. J Neurol Neurosurg Psychiatry 2001;70:174–9.
116. Harvey L, Davies Smith A, Jones R. The effect of weighted leg raises on quadriceps strength, EMG parameters and functional activities in people with multiple sclerosis. Physiother 1999;85:154–61.
117. Dalgas U, Stenager E, Jakobsen J, et al. Resistance training improves muscle strength and functional capacity in multiple sclerosis. Neurology 2009;73:1478–84.
118. Broekmans T, Roelants M, Feys P, et al. Effects of long-term resistance training and simultaneous electro-stimulation on muscle strength and functional mobility in multiple sclerosis. Mult Scler 2010;17:468–77.
119. Sabapathy NM, Minihan CL, Turner GT, Broadley SA. Comparing endurance- and resitance-exercise training in people with multiple sclerosis: a randomized pilot study. Clin Rehabil 2011;25:14–24.
120. Lord SE, Wade DT, Halligan PW. A comparison of two physiotherapy treatment approaches to improve walking in multiple sclerosis: a pilot randomized controlled study. Clin Rehabil 1998;12:477–86.
121. Plow MA, Mathiowetz V, Lowe DA. Comparing individualized rehabilitation to a group wellness intervention for persons with multiple sclerosis. Am J Health Prom 2009;24:23–6.
122. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil 2004;85:290–7.
123. Hayes HA, Gappmaier E, LaStayo PC. Effects of high-intensity resistance training on strength, mobility, balance, and fatigue in individuals with multiple sclerosis: a randomized controlled trial. J Neurol Phys Ther 2011;35:2–10.
124. de Souza-Teixeira F, Costilla S, Ayan C, et al. Effects of resistance training in multiple sclerosis. Int J Sports Med 2009;30:245–50.
125. Kraft G, Alquist A, Lateur B. Effects of resistive exercise on function in multiple sclerosis (MS). Arch Phys Med Rehabil 1996;77:984.
126. White LJ, McCoy SC, Castellano V, et al. Resistance training improves strength and functional capacity in persons with multiple sclerosis. Mult Scler 2004;10:668–74.
127. Taylor NF, Dodd KJ, Prasad D, Denisenko S. Progressive resistance exercise for people with multiple sclerosis. Disabil Rehabil 2006;28:1119–26.
128. Cakit BD, Nacir B, Genc¸ H, et al. Cycling progressive resistance training for people with multiple sclerosis: a randomized controlled study. Am J Phys Med Rehabil 2010;89:446–57.
129. Motl RW, Pilutti, LA. The benefits of exercise training in multiple sclerosis. Nat Rev Neurol 2012;8:487–97.
130. Asano M, Arafah A, Moriello C, Mayo NE. What does a structured review of the effectiveness of exercise interventions for persons with multiple sclerosis tell us about the challenges of designing trials? Mult Scler 2009;15:412–21.
131. Romberg A, Virtanen A, Ruutiainen J, et al. Effects of a 6-month exercise program on patients with multiple sclerosis. Neurology 2004;63:2034–8.
132. Learmonth YC, Paul L, Miller L, et al. The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil 2011;26:579–93.
133. Tarakci E, Yeldan I, Huseyinsinoglu B, et al. Group exercise training for balance, functional status, spasticity, fatigue and quality of life in multiple sclerosis: a randomized controlled trial. Clin Rehabil 2013;27:813–22.
134. Huisinga JM, Filipi ML, Stergiou N. Supervised resistance training results in changes in postural control in patients with multiple sclerosis. Motor Control 2012;16:50–63.
135. Negahban H, Rezaie S, Goharpey S. Massage therapy and exercise therapy in patients with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil 2013;27:1126–36.
136. Cattaneo D, Jonsdottir J, Zocchi M, Regola A. Effects of balance exercises on people with multiple sclerosis: a pilot study. Clin Rehabil 2007;21:771–81.
137. Plow M, Finlayson M. Potential benefits of Nintendo Wii Fit among people with multiple sclerosis: a longitudinal pilot study. Int J MS Care 2011;13:21–30.
138. Plow M, Finlayson M. A qualitative study exploring the usability of Nintendo Wii Fit among persons with multiple sclerosis. Occup Ther Int 2014;21:21–32.
139. Nilsgard YE, Forsberg AS, von Koch L. Balance exercise for persons with multiple sclerosis using Wii games: a randomized, controlled multi-centre study. Mult Scler J 2012;19:209–16.
140. Prosperini L, Fortuna D, Gianni C, et al. Home-based balance training using the Wii Balance Board: a randomized, crossover pilot study in multiple sclerosis. Neurorehabil Neural Repair 2013;27:516–25.
141. Bruchetta G, Spallarossa P, Lopes de Carvalho ML, Battaglia MA. The effect of Nintendo Wii on balance in people with multiple sclerosis: a pilot randomized control study. Mult Scler J 2013;19:1219–21.
142. Guidi I, Giovannelli T, Paci M. Effects of Wii exercise on balance in people with multiple sclerosis. Mult Scler 2013;19:965.
143. Hebert JR, Corboy JR, Manago MM, Schenkman M. Effects of vestibular rehabilitation on multiple sclerosis–related fatigue and upright postural control: a randomized controlled trial. Phys Ther 2011;91:1166–83.
144. Gibson-Horn C. Balance-based torso-weighting in a patient with ataxia and multiple sclerosis: a case report. J Neurol Phys Ther 2008;32:139-146.
145. Crittendon A, O’Neill D, Widener GL, Allen DD. Standing data disproves biomechanical mechanism for balance-based torso-weighting. Arch Phys Med Rehabil 2014;95:43–9.
146. Widener GL, Allen DD, Gibson-Horn C. Balance-based torso-weighting may enhance balance in persons with multiple sclerosis: preliminary evidence. Arch Phys Med Rehabil 2009;90:602–9.
147. Widener GL, Allen DD, Gibson C. Randomized clinical trial of balance-based torso weighting for improving upright mobility in people with multiple sclerosis. Neurorehabil Neural Repair 2009;23:784–91.
148. Abercromby AF, Amonette WE, Layne CS, et al. Vibration exposure and biodynamic responses during whole-body vibration training. Med Sci Sports Exerc 2007;39:1794–800.
149. Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Phys 2010;108:877–904.
150. Prisby RD, Lafage-Proust MH, Malaval L, et al. Effects of whole body vibration on the skeleton and other organ systems in man and animal models: what we know and what we need to know. Age Res Rev 2008;7:319–29.
151. Wunderer K, Schabrun SM, Chipchase LS. Effects of whole body vibration on strength and functional mobility in multiple sclerosis. Physiother Theory Practice 2010;26:374–84.
152. Mason RR, Cochrane DJ, Denny GJ, et al. Is 8 weeks of side-alternating whole-body vibration a safe and acceptable modality to improve functional performance in multiple sclerosis? Dis Rehabil 2012;34:647–54.
153. Schuhfried O, Mittermaier C, Jovanovic T, et al. Effects of whole-body vibration in patients with multiple sclerosis: a pilot study. Clin Rehabil 2005;19:834–42.
154. Claerbout M, Gebara B, Ilsbroukx S, et al. Effects of 3 weeks’ whole body vibration training on muscle strength and functional mobility in hospitalized persons with multiple sclerosis. Mult Scler J 2012;18:498–505.
155. Eftekhari E, Mostahfezian M, Etemadifar M, Zafari A. Resistance training and vibration improve musle strength and functional capacity in female patients with multiple sclerosis. Asian J Sports Med 2012;3:279–84.
156. Hilgers C, Mundermann A, Riehle H, Dettmers C. Effects of whole-body vibration training on physical function inpatients with multiple sclerosis. Neurorehabil 2013;32:655–63.
157. Schyns F, Paul L, Finlay K, et al. Vibration therapy in multiple sclerosis: a pilot study exploring its effects on tone, muscle force, sensation and functional performance. Clin Rehabil 2009;23:771–81.
158. Broekmans T, Roelants M, Alders G, et al. Exploring the effects of a 20-week whole-body vibration training program on leg muscle performance and function in persons with multiple sclerosis. J Rehabil Med 2010;42:866–72.
159. Santos-Fihlo SD, Cameron MH, Bernardo-Filho M. Benefits of whole-body vibration with an oscillating platform for people with multiple sclerosis: a systematic review. Mult Scler Int 2012;2012:274728.
160. Castro-Sanchez AM, Mataran-Penarrocha GA, Lara-Palomo I, et al. Hydrotherapy for the treatment of pain in people with multiple sclerosis: a randomized controlled trial. eCAM. 2012;473963.
161. Kargarfard M, Etemadifar M, Baker P, et al. Effects of aquatic exercise training on fatigue and health-related quality of life in patients with multiple sclerosis. Arch Phys Med Rehabil 2012;93:1701–8.
162. Pariser G, Madras D, Weiss E. Outcomes of an aquatic exercise program including aerobic capacity, lactate threshold, and fatigue in two individuals with multiple sclerosis. J Neurol Phys Ther 2006;30:82–90.
163. Rafeeyan Z, Azarbarzin M, Moosa FM, Hasanzadeh A. Effect of aquatic exercise on the multiple sclerosis patients’ quality of life. Iranian J Nurs Midwifery Res 2010;15:43–7.
164. Gehlsen G, Beekman K, Assmann N, et al. Carter A. Gait characteristics in multiple sclerosis: progressive changes and effects of exercise on parameters. Arch Phys Med Rehabil 1986;67:536–9.
165. Salem Y, Scott AH, Karpatkin H, et al. Community-based group aquatic programme for individuals with multiple sclerosis: a pilot study. Dis Rehabil 2011;33:720–8.
166. Marandi SM, Nejad VS, Shanazari Z, Zolaktaf V. A comparison of 12 weeks of pilates and aquatic training on the dynamic balance of women with multiple sclerosis. Int J Preventive Med 2013;4(Suppl 1):S110-7.
167. Bayraktar D, Guclu-Gunduz A, Yazici G, et al. Effects of Ai-Chi on balance, functional mobility, sytrength and fatigue in patients with multiple sclerosis: a pilot study. Neurorehabil 2013;33:431–7.
168. Oken BS, Kishiyama S, Zajdel D, et al. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology 2004;62:2058–64.
169. Salgado BC, Jones M, Ilgun S, et al. Effects of a 4-month Ananda yoga program on physical and mental health outcomes for persons with multiple sclerosis. Int J Yoga Ther 2013;23:27–38.
170. Ahmadi A, Arastoo AA, Nikbakht Met al. Comparison of the effect of 8 weeks aerobic and yoga training on ambulatory function, fatigue and mood status in MS patients. Iranian Red Crescent Med J 2013;15:449–54.
171. Jackson K, Edginton-Bigelow K, Bowsheir C, et al. Feasibility and effects of a group kickboxing program for individuals with multiple sclerosis: a pilot report. J Bodywork Movement Ther 2012;16:7–13.
172. Mackay-Lyons M, Conway C, Roberts W. Effects of therapeutic riding on patients with multiple sclerosis: a preliminary trial. Physiother Can 1988;40:104–9.
173. Hammer A, Nilsagard Y, Forsberg A, et al. Evaluation of therapeutic riding (Sweden)/hippotherapy (United States): a single-subject experimental design study replicated in eleven patients with multiple sclerosis. Physiother Theory Prac 2005;21:51–77.
174. Silkwood-Sherer D, Warmbier H. Effects of hippotherapy on postural stability in persons with multiple sclerosis: a pilot study. J Neurol Phys Ther 2007;31:77–84.
175. Bronson C, Brewerton K, Ong J, et al. Does hippotherapy improve balance in persons with multiple sclerosis: a systematic review. Eur J Phys Rehabil Med 2010;46:347–53.
Noninvasive Bladder Cancer: Diagnosis and Management
From the William Beaumont Hospital, Royal Oak, MI.
Abstract
- Objective: To review the diagnosis and management of noninvasive bladder cancer.
- Methods: Literature review.
- Results: Nonmuscle invasive bladder cancer is a common malignancy that affects more men than women. It is estimated that smoking accounts for half of all cases. Direct visualization of the bladder mucosa remains the standard in diagnosing bladder malignancy. The natural history of superficial bladder cancer is characterized by disease recurrence and disease progression. First-line treatment of patients with noninvasive bladder cancer is transurethral resection of bladder tumor. Adjuvant treatment with intravesical chemotherapy and immunotherapy has become an important component of therapy.
- Conclusion: The results of ongoing studies are eagerly anticipated and will improve our understanding of the disease.
Nonmuscle invasive bladder cancer is a common malignancy and the second most common urologic malignancy after prostate cancer. It accounts for approximately 73,500 new cancer diagnoses yearly in the United States [1]. An estimated 14,880 persons die each year as a result of the disease. Despite improvements in diagnosis and management of noninvasive bladder tumors, the risk of both recurrence and progression remains significant. In this article, we review the etiology, diagnosis, and management of noninvasive bladder cancer.
Epidemiology And Risk Factors
Bladder cancer affects men more commonly than women, with an approximate 3 to 4:1 ratio [1,2].The incidence in men over the past 8 years has been stable, and the incidence in women has decreased by 0.3% over the same time period. Bladder cancer affects Caucasians twice as often as African Americans, and affects Hispanics and Asians even less frequently than African Americans [2]. More than 90% of patients diagnosed with bladder cancer will be older than 55 years of age.
Histologically, urothelial (transitional cell) carcinoma accounts for over 90% of all diagnosed bladder cancers [3].Other subtypes in order of prevalence include squamous cell carcinoma, adenocarcinoma, and small cell carcinomas. Of those diagnosed with urothelial carcinoma, nonmuscle invasive (superficial) bladder cancer (NMIBC) accounts for almost 75% of cases [2]. Muscle invasion is seen in 20% of newly diagnosed cases, and metastatic disease is seen approximately 5% of the time.
It is estimated that smoking accounts for half of all cases of bladder cancer, with smokers having a 2- to 6-fold greater risk of bladder cancer as compared with nonsmokers [4–6]. At 25 years after smoking cessation, the risk of bladder cancer continues to decrease but is still higher than that of nonsmokers [7]. Continued smoking despite the diagnosis of urothelial carcinoma increases the risk of recurrence 2.2-fold [8].
Environmental exposures also have been linked to the development of urothelial carcinoma, particularly exposure to aromatic amines [9]. Occupations associated with an increased risk of bladder cancer include tire/rubber workers, leather workers, textile workers, hairdressers, painters, dry cleaners, and chemical workers.
Exposure to certain medications has been associated with an increased risk of bladder cancer, including the analgesic phenacetin, which has since been taken off the market [10]. Additionally, patients treated with the chemotherapeutic agent cyclophosphamide have a higher risk of bladder cancer, with a dose-response relationship between cyclophosphamide and the risk for bladder cancer [11,12]. The increased risk of bladder cancer and risk of hemorrhagic cystitis associated with cyclophosphamide therapy is secondary to exposure to the urinary metabolite acrolein. Concomitant administration of sodium 2-mercaptoethanesulfonate (MESNA) provides regional detoxification of acrolein in the urinary tract [13].
Urothelial carcinoma does not have a strong inherited disease association. It is felt, however, that there are 2 separate molecular pathways that may lead to the development of bladder cancer [14]. Mutation of the p53 gene has been shown to be associated with carcinoma in situ and invasive disease, whereas mutation of FGFR3 is seen more frequently with Ta disease [15]. Accumulation of p53 in cell nuclei is an independent predictor of tumor recurrence and overall poor prognosis [16]. The identification of molecular markers of tumor progression is an active field of research in bladder cancer [17].
Case Patient 1
Initial Presentation and Evaluation
A 63-year-old man with a 60 pack-year history of smoking presents to a urologist with a urinalysis from his primary care physician showing 20 to 50 red blood cells per high-power field (RBCs/HPF). He denies any urgency, frequency, or recent urinary tract infections. A urine culture from his primary care doctor is negative.
What are the common presenting features of bladder cancer?
Hematuria is the most common presenting feature of bladder cancer. It is present as the initial symptom in up to 90% of patients with urothelial carcinoma [18]. Other symptoms include irritative voiding symptoms such as urgency, frequency, and dysuria. Irritative voiding symptoms tend to occur more commonly with carcinoma in situ [19].
What are the next steps in the workup of this patient?
Initial Evaluation
American Urological Association (AUA) guidelines for the evaluation and management of asymptomatic microhematuria were updated in 2011 [20]. They recommend that every patient who presents with microscopic hematuria (> 3 RBCs/HPF) undergo a thorough history and physical exam, including rectal exam and bimanual evaluation in females to assess for any masses or pelvic fixation. Once benign sources of hematuria (eg, infection, menstruation, vigorous exercise, medical renal disease, viral illness, trauma, or recent urological procedures) have been ruled out, further testing will include a renal function panel, upper tract imaging, as well as cystoscopy in high-risk patients and those older than age 35 years. Urine cytology may be utilized in high-risk patients, but it is no longer generally recommended for routine workup.
Imaging
The imaging modality of choice during the hematuria workup is the computed tomography urogram (CTU), a multiphasic CT scan that images the urinary tract before and after contrast administration and includes excretory stage imaging [21]. Sadow et al found that CTU had a negative predictive value (NPV) of 95% for the detection of bladder cancer, while cystoscopy had an NPV of 99% [22]. In addition to radiographic evaluation of the urinary system, CT offers useful staging information regarding metastatic disease. In patients with renal failure or other contraindications to CTU, magnetic resonance urography (MRU) has become an acceptable alternative for hematuria evaluation. MRU allows for improved characterization of tissue and does not utilize ionizing radiation. During MRU, the high T2 signal intensity of urine is utilized to provide contrast in the images in static phase MRU and after gadolinium administration for excretory-phase MRU [21]. The bladder is typically best evaluated in T1-weighted images a few minutes after gadolinium administration, before the contrast reaches the bladder; it may also be evaluated during the late excretory phase when signal enhancement from gadolinium is greatest. The effectiveness of MRU in collecting system evaluation is still evolving, and therefore, in appropriately selected patients who would benefit from further collecting system evaluation, MRU should be utilized in conjunction with retrograde pyelograms [20]. Though previously considered the gold standard in imaging, intravenous pyelography is no longer a recommended imaging modality for hematuria evaluation.
Urine Cytology and Urine Markers
Urine markers and urine cytology are a debated topic in the workup and follow-up of bladder cancer. Urine cytology evaluates sloughed cells for malignant features [23]. Due to the lack of cohesion of carcinoma in situ cells and high-grade lesions, these cells are more likely to slough than are low-grade lesions [24]. The range of sensitivity of urine cytology reported in the literature varies widely. Studies report that the sensitivity of urine cytology in high-grade tumors approaches 95%, and in carcinoma in situ is up to 100% when 3 consecutive specimens are obtained [25]. However, Yafi et al recently reported that the sensitivity of urine cytology in high-grade tumors is 51% and in low-grade tumors is only 10% [26]. It is recommended that urine cytology be evaluated as part of a hematuria work-up in high-risk patients.
Aside from cytology, more than a dozen urine marker tests for bladder cancer detection and surveillance have been developed [27]. Current urine markers tests include protein-based assays such as the nuclear matrix protein 22 (NMP22) assay (NMP22 Test Kit; Alere, Waltham, MA) and bladder tumor antigen assays (BTA stat and BTA-TRAK; Polymedco, Cortlandt, NY) as well as cellular marker tests such as UroVysion FISH (Abbott Molecular, Abbott Park, IL) and ImmunoCyt (Scimedx, Denville, NJ) [27–31]. NMP22 is a nuclear matrix protein that is elevated in bladder cancer patients, and BTA stat/TRAK (qualitative/quantitative) detects complement factor H. Much controversy surrounds the utilization of these markers for screening and monitoring of bladder cancer, and currently they are not routinely recommended for these purposes nor are they recommended for follow-up in patients with bowel interposition [32].
Cystoscopy
Ultimately, direct visualization of the bladder mucosa remains a gold standard in diagnosing bladder malignancy. Office-based cystoscopy allows for rapid assessment and also allows biopsy to be performed for suspicious lesions. It can be performed easily with local anesthetic.
The use of fluorescence and narrow-band cystoscopy has been evaluated in recent years. The premise of fluorescence cystoscopy is that there is preferential accumulation of porphyrin in neoplastic cells. Therefore, intravesically instilled photoactive heme precursors such as 5-aminolevulinic acid (5-ALA) or hexaminolevulinate (HAL) have increased uptake within these neoplastic cells and subsequent enhancement. Preliminary studies have shown that approximately one quarter to one third more cases of small papillary tumors and carcinoma in situ are identified using fluorescence cystoscopy as compared with standard white light cystoscopy [33–36]. In one prospective study, the use of fluorescence cystoscopy resulted in a 16% decrease in the recurrence rate [37]. Denzinger et al found that 8-year recurrence-free survival in those who underwent fluorescence transurethral resection (TUR) was 71% as compared with 45% in conventional TUR patients [36]. Caution is required, however, because false-positives may occur in patients with inflammatory lesions.
Narrow-band cystoscopy works by filtering white light into bandwidths of 415 and 540 nm, wavelengths absorbed by hemoglobin. This allows for added contrast between vascular structures and normal urothelium [38]. Narrow-band imaging has an advantage over fluorescence cystoscopy in that no preoperative intravesical instillations are required. Detection rates of NMIBC were as high as 94.7% with narrow-band imaging, as compared to 79.2% with white light cystoscopy [39]. In the case of recurrent low-grade papillary lesions, resection with narrow-band imaging reduces recurrence rates by approximately 30% when patients are followed for 3 years [40]. While both fluorescence cystoscopy and narrow-band imaging appear to be promising technology, higher false-positive rates are seen with both as compared to white light cystoscopy [3,41]. Neither modality is a recommended treatment option [42].
Case 1 Continued
On office-based cystoscopy, a 2.5-cm papillary lesion is noted on the left lateral wall of the bladder. There are no other suspicious lesions within the bladder. A CTU is obtained, which reveals no hydronephrosis or lymphadenopathy and correlates with the cystoscopic examination of a bladder lesion on the left lateral wall.
What are the next steps in management?
Transurethral Resection
Transurethral resection of bladder tumor (TURBT) is paramount in the treatment and diagnosis of bladder tumors. TURBT allows for complete resection of the tumor and also allows for histologic diagnosis, staging, and grading. The bladder wall consists of 3 principle layers: the mucosa, submucosa, and muscularis. An important factor in identifying the stage of disease is determining the depth of invasion as well as the size and mobility of masses. Adequate resection, with inclusion of muscle in the TURBT specimen, allows for proper staging of urothelial carcinoma. When pathology reveals high-grade Ta or T1 disease or does not contain muscle, re-resection is recommended [42]. In a study involving 150 patients with bladder tumors, when re-resection was undertaken within 2 to 6 weeks, 29% of NMIBC lesions were upstaged, and treatment options were changed based on re-resection results in one third of patients [43].
TURBT is a relatively safe procedure that can be performed in an outpatient setting. The most common complications of TURBT are urinary tract infection and hematuria [44]. Other complications include the risk of bladder perforation with deep resection. In the event of bladder perforation, it is important to determine the location and depth of the perforation to decide on appropriate treatment. Many small extraperitoneal perforations may be managed with simple Foley drainage, whereas large perforations may require open or laparoscopic repair [45–46]. The incidence of extravesical recurrence of NMIBC after bladder perforation varies in the literature from 0% to 6% [47]. Numerous studies report open bladder repair following any intraperitoneal perforation, but laparoscopic repair is becoming more common [48,49].In any case of intraperitoneal rupture, the recommendation is for close follow-up for the rare event of recurrence.
While performing TURBT, one must be cognizant of the obturator nerve reflex. The obturator nerve runs in close proximity to the inferolateral wall of the bladder. Stimulation from the electrocautery current will cause external rotation and adduction of the thigh in a sudden jerking movement, thus increasing the risk of bladder perforation [50]. Bipolar technology has been found to be a safe alternative to conventional monopolar electrocautery for resection of bladder tumors, with decreased length of catheterization and fewer bladder perforations documented [51]. While bipolar technology may decrease stimulation of the obturator reflex, it is important to note that it still may occur, resulting in bladder perforation [52.53].
Staging, Grading, and Risk Stratification
In 2004 the World Health Organization revised the classification of urothelial malignancies to include tumors designated as either high- or low-grade as well as carcinoma in situ [55]. The differentiation of low- and high-grade is based on the degree of nuclear anaplasia and architectural abnormalities. Those with high-grade tumors as well as increased depth of invasion have an increased risk of recurrence and progression of disease compared to low-grade tumors [56].
When determining treatment and surveillance options for NMIBC patients, not only are the stage and grade determining factors, but future risk of recurrence and progression dictates
Intravesical Chemotherapy/Immunotherapy
Intravesical therapy is the use of chemotherapeutic or immunotherapeutic substances instilled within the bladder. It is indicated for the treatment of NMIBC but is not the recommended treatment for T2 or greater lesions. The goals of intravesical therapy are to reduce recurrence and progression of resected disease and eradicate carcinoma in situ as well as incompletely resected papillary tumor [42].
Intravesical chemotherapeutic agents include mitomycin C, thiotepa, doxorubicin, valrubicin, epirubicin, and gemcitabine [42]. Mitomycin C is an alkylating agent that acts by inhibiting DNA synthesis. Because of mitomycin C’s relatively high molecular weight, systemic absorption is minimal, although there is a small risk of myelosuppression. Thiotepa is an alkylating agent that cross-links nucleic acids. Doxorubicin, epirubicin, and valrubicin are intercalating agents that inhibit DNA synthesis. Gemcitabine is a deoxycytidine analog that also inhibits DNA synthesis.
Immunotherapy utilizes bacillus Calmette-Guérin (BCG), a live, attenuated strain of Mycobacterium bovis. Though the mechanism of action of BCG is not fully understood, it is known that instillation of BCG stimulates a large immune response [57]. BCG is taken up by antigen-presenting cells as well as urothelial cells and bladder cancer cells, initiating the immune response. Cytokine release in response to BCG is thought to be mediated by macrophages and activated lymphocytes as well as urothelial cells directly [58]. Recent studies have found that interleukin-17 plays an important role in neutrophil recruitment and the generation of the Th1- cell response, which mediates the antitumor effect [59,60]. The innate immune response is also felt to be important in the antitumor effect of BCG, with studies suggesting that BCG is ineffective in the absence of natural killer cell activity and that neutrophils and macrophages are important in the immune response [58,61,62].
Administration of BCG is typically held for at least 2 weeks following TURBT to minimize the risk of sepsis and adverse events. BCG also should not be used in patients who have had traumatic catheterization, recent gross hematuria, or urinary tract infection, in immunocompromised hosts, or in patients with active autoimmune disease, known allergy, or history of BCG sepsis. Adverse events associated with BCG use include sepsis, prostatitis, epididymitis, cystitis, and flu-like symptoms [63].
Interferon alpha-2b is a cytokine that helps modulate the immune response. In cases of refractory bladder cancer that have failed BCG treatment, modulation with interferon alfa-2b therapy has been investigated. In vitro studies show that administration of interferon alfa-2b enhanced the ability of BCG to induce interferon-gamma production, upregulated tumor necrosis factor-α and interleukin-12, and down-regulated interleukin-10, thus favoring the upregulation of the Th1 immune-mediated response [64]. Used in conjunction with BCG in patients who have failed BCG therapy, interferon alfa-2b has been shown to have a 2-year recurrence-free survival rate of up to 45% [65].
Immediately following TURBT, it is recommended that patients with low-risk disease undergo single-dose intravesical chemotherapy [66]. When performed within 24 hours (and ideally 6 hours) of resection, intravesical chemotherapy has been shown to decrease the odds of bladder cancer recurrence by up to 40% in low-risk disease [67].The mechanism of action of single-dose intravesical chemotherapy instilled immediately after resection is not definitively known, but it is hypothesized that it destroys any remaining microscopic disease and prevents reimplantation of any freely circulating cells [67]. Single-dose mitomycin C, however, does not decrease the rate of progression in incompletely resected tumors [68]. Administration of intravesical chemotherapeutic agents should be avoided when there is bladder perforation [69].
There is some debate regarding the best approach to treating intermediate-risk bladder cancer. In guidelines released by the International Bladder Cancer Group, a group of experts who evaluated and set forth guidelines based on current recommendations from the NCCN, AUA, European Association of Urology, and the First International Consultation on Bladder Tumors, initiation of BCG therapy with maintenance or intravesical chemotherapy for up to 1 year of adjuvant treatment is recommended following the diagnosis of intermediate-risk bladder cancer [66]. Induction treatments are single intravesical instillations administered weekly for 6 weeks and begun 2 to 4 weeks after resection. Maintenance courses consist of once weekly instillations for 3 weeks undertaken at 3 months, 6 months, and then every 6 months for up to a total of 3 years of treatment [70].
For the management of high-risk disease, most guidelines concur that the optimal treatment is BCG with maintenance, although the recommended length of maintenance varies from 1 to 3 years [66]. The EORTC-GU recently reported the results of a randomized study in which high-risk Ta and T1 lesions were treated with BCG maintenance; they found that a full-dose, 3-year maintenance course of BCG decreased recurrences without increasing toxicity [71].
Although both intravesical chemotherapy and immunotherapy are recommended treatments for NMIBC, there is a preference in the published guidelines toward the use of BCG over intravesical chemotherapy. In multiple meta-analyses, BCG, and especially BCG with maintenance, has been shown to have improved disease-free recurrence when compared with intravesical chemotherapy [72,73]. Malmström et al showed a 32% reduction in the recurrence rate in BCG-treated patients compared with those treated with mitomycin C [74]. Similarly, high-risk patients treated with gemcitabine therapy had a higher recurrence rate and more rapid time to recurrence as compared with those treated with BCG therapy; in intermediate-risk patients, the rate of recurrence was not statistically significant [75].
Cystectomy
In certain high-risk patients, it is also appropriate to offer cystectomy as initial therapy. Though much more invasive than other treatment options, it does offer a chance for cure in a select group of patients with high likelihood of progression of disease. Risk factors associated with progression and consideration for immediate or early cystectomy include large tumor size (> 3 cm), inability to completely resect tumor, difficult resection site, multifocal/ diffuse disease, presence of carcinoma in situ, prostatic urethral involvement, female sex, suspected understaging secondary to lymphovascular invasion, or unfavorable histology [76–81]. While tumor upstaging has been noted in up to one-quarter of high-risk immediate cystectomy patients, it is important to note that multiple retrospective reviews have not found a cancer-specific survival (CSS) benefit to immediate cystectomy versus conservative treatment [82–85]. Hautmann et al examined immediate cystectomy versus deferred cystectomy until after recurrence in high-risk patients and demonstrated a clear 10-year CSS benefit of 79% versus 65% [86]. Because the number of patients who have undergone immediate cystectomy is still relatively small and predictors of aggressive disease are still evolving, immediate cystectomy is still considered a viable treatment option in the appropriately selected patient.
Case Patient 2
A 72-year-old woman with a history of T1 bladder cancer presents for routine follow-up. She has completed a course of BCG with maintenance for her initial lesion. On follow-up cystoscopy, she is found to have multiple velvety red patches throughout the bladder and a 1-cm sessile lesion.
What is the follow-up for bladder cancer?
Bladder cancer causes what is known as a field defect. As urine bathes the urothelium, theoretically, so do the carcinogens within the urine, exposing cells throughout the bladder. Bladder cancer therefore does not just recur at the initial site of the tumor, but can occur anywhere in the bladder. For example, Heney et al found that initial tumors were only occasionally located at the dome (5% of the time), whereas new tumor occurrences were found at the dome in 29% of patients [87].
Though there is no consensus in the literature as to the ideal timing of cystoscopic follow-up, NCCN guidelines recommend cystoscopy every 3 months with increasing intervals as indicated for low-risk lesions [88]. For all other lesions, they recommend cystoscopy and cytology every 3 to 6 months with increasing intervals as indicated, upper tract imaging every 1 to 2 years for high-grade tumors, and the optional use of urine markers for follow-up. The AUA varies slightly in recommending cystoscopy and cytology for all patients every 3 months for 2 years, followed by every 6 months for 2 to 3 years, and then annually. They recommend imaging of the upper tracts but do not specify timing, and current recommendations do not support the use of urine markers [89].
How are recurrences/treatment failures managed?
When recurrence or treatment failure is identified, it is important to consider the initial lesion and treatment as well as stage and grade of any follow-up lesions. Low-risk disease may be treated with re-resection and BCG or mitomycin C with or without maintenance [42]. With treatment failure of intermediate disease, resection followed by a change in the modality of intravesical treatment is an option. When recurrences occur in intermediate-risk disease, one might change modalities or reinstitute a second induction therapy course after resection [66].
High-risk NMIBC provides a challenging dilemma in management. In a systematic literature review of 19 published trials, van den Bosch and Witjes [90] reported a 21% progression to muscle-invasive disease in high-risk NMIBC patients. Management of recurrences in this population in an effort to decrease progression and increase CSS is a highly debated topic, with no clear answer currently available. In the case of high-risk disease that has recurred, treatment options include a second induction course of BCG, cystectomy, or alternative intravesical chemotherapeutic options. Those patients who underwent early cystectomy for high-risk recurrence after BCG therapy had an overall greater survival compared to those who delayed cystectomy over 2 years [91]. In their study evaluating early versus delayed cystectomy, Jäger et al [92] found that as the number of TURBTs performed before cystectomy for high-risk disease went from 1 to 2–4 to greater than 4, the 10-year CSS decreased from 84% to 77% to 45%. Additionally, they found that when cystectomy was performed 1 year after initial TURBT, the 10-year CSS decreased from 79% to 61%.
In patients who have failed BCG treatment and are not surgical candidates or do not desire surgical intervention, intravesical valrubicin is emerging as a treatment alternative. It is currently the only therapy that is approved by the U.S. Food and Drug Administration for treatment of BCG-refractory carcinoma in situ in nonsurgical candidates. Dinney et al examined the efficacy and safety of valrubicin in BCG-refractory carcinoma in situ and found an 18% complete response rate over the 6-month follow-up period, which correlated with the previously reported response rates in phase II/III trials [93]. Other therapies being investigated for BCG failure include thermochemotherapy, photodynamic therapy, as well as combination intravesical chemotherapies [94].
Conclusion
Though much research is under way on the surveillance, diagnosis, and treatment of NMIBC, time-tested modalities remain the mainstay of management. Ongoing studies will improve our understanding of the disease as new information regarding novel ways of delivering intravesical therapeutics, surveillance modalities, and optimal treatment and follow-up strategies becomes available.
Corresponding author: Frank N. Burks, MD, 31157 Woodward Ave., Royal Oak, MI 48073, [email protected].
Financial disclosures: None.
1. American Cancer Society. Cancer facts & figures 2012. Accessed 2 May 2013 at www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012.
2. National Cancer Institute. SEER Stat Fact Sheets: Bladder. Accessed 14 December 2012 at seer.cancer.gov/statfacts/html/urinb.html.
3. Lynch CF, Davila JA, Platz CE. Cancer of the urinary bladder. In: Ries LAG, Young JL, Keel GE, et al, editors. SEER survival monograph: cancer survival among adults: US SEER program 1988-2001, patient and tumor characteristics. NIH Pub. No. 07-6215. Bethesda (MD): National Cancer Institute; 2007:181–92.
4. Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer 2000;86:289–94.
5. Castelao JE, Yuan JM, Skipper PL, et al. Gender and smoking-related bladder cancer risk. J Natl Cancer Inst 2001;93:538–45.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. World Health Organization International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, volume 83. Tobacco smoke and involuntary smoking. Lyon, France: World Health Organization; 2004. Accessed 2 May 2013 at http://monographs.iarc.fr/ENG/Monographs/vol83/index.php.
8. Chen CH, Shun CT, Huang KH, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer, BJU Int 2007;100:281–6.
9. Murta-Nascimento C, Schmitz-Dräger BJ, Zeegers MP, et al. Epidemiology of urinary bladder cancer: from tumor development to patient’s death. World J Urology 2007;25:285–95.
10. Piper JM, Tonascia J, Matanoski GM. Heavy phenacetin use and bladder cancer in women aged 20 to 49 years. N Engl J Med 1985;313:292–5.
11. Knight A, Askling J, Granath F, et al. Urinary bladder cancer in Wegener’s granulomatosis: risks and relation to cyclophosphamide. Ann Rheum Dis 2004;63:1307–11.
12. Fairchild WV, Spence CR, Solomon HD, Gangai MP. The incidence of bladder cancer after cyclophosphamide therapy. J Urology 1979; 122:163.
13. Brock N. The development of mesna for the inhibition of urotoxic side effects of cyclophosphamide, ifosfamide, and other oxazaphosphorine cytostatics. Recent Results Cancer Res 1980;74:270–8.
14. Spruck CH, Ohneseit PF, Gonzalez-Zulueta M, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res 1994;54:784–8.
15. Bakkar AA, Wallerand H, Radvanyi F, et al. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer Res 2003;63:8108–12.
16. Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med 1994;331:1259–64.
17. Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol 2006;24:5552–64.
18. National Cancer Institute. Bladder and other urothelial cancers screening (PDQ). January 23, 2012. Accessed 14 December 2012 at www.cancer.gov/cancertopics/pdq/screening/bladder/HealthProfessional.
19. Farrow GM, Utz DC, Rife CC, Greene LF. Clinical observations on sixty-nine cases of in situ carcinoma of the urinary bladder. Cancer Res 1977;37:2794–8.
20. Davis R, Jones J, Barocas DA, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol 2012;188(6 Suppl):2473–81.
21. Silverman SG, Leyendecker JR, Amis ES Jr. What is the current role of CT urography and MR urography in the evaluation of the urinary tract? Radiology 2009;250:309–23.
22. Sadow CA, Silverman SG, O’Leary MP, Signorovitch JE. Bladder cancer detection with CT urography in an academic medical center. Radiology 2008;249:195–202.
23. Murphy WM, Soloway MS, Jukkola AF, et al. Urinary cytology and bladder cancer. The cellular features of transitional cell neoplasms. Cancer 1984;53:1555–65.
24. Halachmi S, Linn JF, Amiel GE, et al. Urine cytology, tumour markers and bladder cancer. Br J Urol 1998;82:647–54.
25. Koss LG, Deitch D, Ramanathan R, Sherman AB. Diagnostic value of cytology of voided urine. Acta Cytol 1985;29:810–6.
26. Yafi FA, Brimo F, Auger M, et al. Is the performance of urinary cytology as high as reported historically? A contemporary analysis in the detection and surveillance of bladder cancer. Urol Oncol 11 Feb 2013. [Epub ahead of print]
27. van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol 2005;47:736–48.
28. Vrooman OPJ, Witjes JA. Urinary markers in bladder cancer. Eur Urol 2008;53:909–16.
29. Toma MI, Friedrich MG, Hautmann SH, et al. Comparison of the ImmunoCyt test and urinary cytology with other urine tests in the detection and surveillance of bladder cancer. World J Urol 2004;22:145–9.
30. Jones JS. DNA–based molecular cytology for bladder cancer surveillance. Urology 2006;67(3 Suppl 1):35–45.
31. Glas AS, Roos D, Deutekom M, et al. Tumor markers in the diagnosis of primary bladder cancer. A systematic review. J Urol 2003;169:1975–82.
32. Sharma S, Zippe CD, Pandrangi L, et al. Exclusion criteria enhance the specificity and positive predictive value of NMP22 and BTA stat. J Urol 1999;162:53–7.
33. Fradet Y, Grossman HB, Gomella L, et al. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. J Urol 2007;178:68–73.
34. Grossman HB, Gomella L, Fradet Y, et al. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J Urol 2007;178:62–7.
35. Schmidbauer J, Witjes F, Schmeller N, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol 2004;171:135–8.
36. Denzinger S, Burger M, Walter B, et al. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology 2007;69:675–9.
37. Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol 2010;184:
1907–14.
38. Cauberg EC, Mamoulakis C, de la Rosette JJ, de Reijke TM. Narrow band imaging-assisted transurethral resection for non-muscle invasive bladder cancer significantly reduces residual tumour rate. World J Urol 2011;29:503–9.
39. Cauberg EC, Kloen S, Visser M, et al. Narrow band imaging cystoscopy improves the detection of non–muscle-invasive bladder cancer. Urology 2010;76:658–63.
40. Herr HW, Donat SM. Reduced bladder tumour recurrence rate associated with narrow-band imaging surveillance cystoscopy. Br J Urol Intl 211;107:396–8.
41. Zaak D, Karl A, Knüchel R, et al. Diagnosis of urothelial carcinoma of the bladder using fluorescence endoscopy. Br J Urol Intl 2005;96:217–22.
42. Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007;178:2314–30.
43. Herr HW. The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol 1999;162:74–6.
44. Hollenbeck BK, Miller DC, Taub D, et al. Risk factors for adverse outcomes after transurethral resection of bladder tumors. Cancer 2006;106:1527–35.
45. Nieder AM, Meinbach DS, Kim SS, Soloway MS. Transurethral bladder tumor resection: intraoperative and postoperative complications in a residency setting. J Urol 2005;174:2307–9.
46. Traxer O, Pasqui F, Gattegno B, Pearle MS. Technique and complications of transurethral surgery for bladder tumours. Br J Urol Intl 2004;94:492–6.
47. Mydlo JH, Weinstein R, Shah S, et al. Long-term consequences from bladder perforation and/or violation in the presence of transitional cell carcinoma: results of a small series and a review of the literature. J Urol 1999;161:1128–32.
48. Frachet O, Cordier G, Henry N, et al. Bladder perforation during transurethral resection of bladder tumour: a review. Prog Urol 2007;17:1310–2.
49. Golan S, Baniel J, Lask D, et al. Transurethral resection of bladder tumour complicated by perforation requiring open surgical repair - clinical characteristics and oncological outcomes. Br J Urol Intl 2011; 107:1065–8.
50. Kihl B, Nilson AE, Pettersson S. Thigh adductor contraction during transurethral resection of bladder tumours: evaluation of inactive electrode placement and obturator nerve topography. Scand J Urol Nephrol 1981;15:121–5.
51. Del Rosso A, Pace G, Masciovecchio S, et al. Plasmakinetic bipolar versus monopolar transurethral resection of non-muscle invasive bladder cancer: a single center randomized controlled trial. Intl J Urol 2013;20:399–403.
52. Puppo P, Bertolotto F, Introini C, et al. Bipolar transurethral resection in saline (TURis): outcome and complication rates after the first 1000 cases. J Endourol 2009;23:1145–9.
53. Kitamura T, Mori Y, Ohno N, et al. Case of bladder perforation due to the obturator nerve reflex during transurethral resection (TUR) of bladder tumor using the TUR in saline (Turis) system under spinal anesthesia [in Japanese]. Masui 2010;59:386–9.
54. American Joint Committee on Cancer.: Urinary bladder. In: Edge SB, Byrd DR, Compton CC, et al, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010:497–505.
55. Elbe J, Sauter G, Epstein J, Sesterhenn I. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary and male genital organs. Lyon, France: IARC Press;2004.
56. Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, et al. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol 2000;164:680–4.
57. Böhle A, Brandau S. Immune mechanisms in bacillus Calmette-Guérin immunotherapy for superficial bladder cancer. J Urol 2003;170964–9.
58. Kawai K, Miyazaki J, Joraku A, et al. Bacillus Calmette-Guérin (BCG) immunotherapy for bladder cancer: current understanding and perspectives on engineered BCG vaccine. Cancer Sci 2013;104:22–7.
59. Takeuchi A, Dejima T, Yamada H, et al. IL-17 production by γδ T cells is important for the antitumor effect of Mycobacterium bovis bacillus Calmette-Guérin treatment against bladder cancer. Eur J Immunol 2011;41:246–51.
60. Gopal R, Lin Y, Obermajer N, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol 2012;42:364–73.
61. Suttmann H, Jacobsen M, Reiss K, et al. Mechanisms of bacillus Calmette-Guerin mediated natural killer cell activation. J Urol 2004;172:1490–5.
62. Luo Y, Knudson MJ. Mycobacterium bovis bacillus Calmette-Guérin-induced macrophage cytotoxicity against bladder cancer cells. Clin Dev Immunol 2010;2010:357591.
63. Rischmann P, Desgrandchamps F, Malavaud B, Chopin DK. BCG intravesical instillations: recommendations for side-effects management. Eur Urol 2000;37(Suppl 1):33–6.
64. Luo Y, Chen X, Downs TM, et al. IFN-α 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette-Guérin immunotherapy. J Immnuol 1999;162:2399–2405.
65. Joudi FN, Smith BJ, O’Donnell MA. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guérin plus interferon α-2B for reducing recurrence of superficial bladder cancer. Urol Oncol 2006;24:344–8.
66. Brausi M, Witjes JA, Lamm D, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol 2011;186:2158–67.
67. Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol 2004;171:2186–90.
68. Divrik RT, Yildirim U, Zorlu F, Ozen H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J Urol 2006;175:1641–4.
69. Oddens JR, Van der Meijden AP, Sylvester R. One immediate postoperative instillation of chemotherapy in low risk Ta, T1 bladder cancer patients. Is it always safe? Eur Urol 2004;46:336–8.
70. Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163:1124–9.
71. Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 2013;63:462–72.
72. Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol 2003;169:90–5.
73. Sylvester RJ, van der Meijden AP, Witjes JA, Kurth J. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol 2005;174:86–91.
74. Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol 2009;56:247–56.
75. Jones G, Cleves A, Wilt TJ, et al. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database Syst Rev 2012;CD009294.
76. Kurth H, Denis L, Bouffioux C, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer 1995;31A:1840–6.
77. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:465–66.
78. Rodríguez Faba O, Palou J. Predictive factors for recurrence progression and cancer specific survival in high-risk bladder cancer. Curr Opin Urol 2012;22:415–20.
79. Streeper NM, Simons CM, Konety BR, et al. The significance of lymphovascular invasion in transurethral resection of bladder tumour and cystectomy specimens on the survival of patients with urothelial bladder cancer. Br J Urol Intl 2009;103:475–9.
80. Witjes JA. Prognosis of T1G3 bladder cancer: how well can we predict progression? Eur Urol 2012; 62:126–7.
81. Khochikar M. Early vs delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: delay of cystectomy reduces cancer-specific survival. Br J Urol Intl 2011;108(Pt 2):E288–9.
82. De Berardinis E, Busetto GM, Antonini G, et al. T1G3 high-risk NMIBC (non-muscle invasive bladder cancer): conservative treatment versus immediate cystectomy. Intl Urol Nephrol 2011;43:1047–57.
83. Badalato GM, Gaya JM, Hruby G, et al. Immediate radical cystectomy vs conservative management for high grade cT1 bladder cancer: is there a survival difference? Br J Urol Intl 2012;110:1471–7.
84. Sternberg IA, Keren Paz GE, Chen LY, et al. Role of immediate radical cystectomy in the treatment of patients with residual T1 bladder cancer on restaging transurethral resection. BJU Intl 2012;112:54–9.
85. Canter D, Egleston B, Wong YN, et al. Use of radical cystectomy as initial therapy for the treatment of high-grade T1 urothelial carcinoma of the bladder: A SEER database analysis. Urol Oncol 2013;31:866–70.
86. Hautmann RE, Volkmer BG, Gust K. Quantification of the survival benefit of early versus deferred cystectomy in high-risk non-muscle invasive bladder cancer (T1 G3). World J Urol 2009;27:347–51.
87. Heney NM, Nocks BN, Daly JJ, et al. Ta and T1 Bladder cancer: location, recurrence and progression. Br J Urol 2008;54:152–7.
88. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN Guidelines): Bladder cancer. Jenkintown (PA): NCCN; 2012.
89. Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1 and Tis): a 2007 update. J Urol 2007;178:2314–30.
90. van den Bosch S, Witjes JA. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol 2011;60:493–500.
91. Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol 2001;166:1296–9.
92. Jäger W, Thomas C, Haag S, et al. Early vs delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: delay of cystectomy reduces cancer-specific survival. BJU Int; 2011;108(Pt 2):E284–8.
93. Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol Oncol 2012 May 9. [Epub ahead of print]
94. Yates DR, Rouprêt M. Contemporary management of patients with high-risk non-muscle-invasive bladder cancer who fail intravesical BCG therapy. World J Urol 2011;29:415–22.
From the William Beaumont Hospital, Royal Oak, MI.
Abstract
- Objective: To review the diagnosis and management of noninvasive bladder cancer.
- Methods: Literature review.
- Results: Nonmuscle invasive bladder cancer is a common malignancy that affects more men than women. It is estimated that smoking accounts for half of all cases. Direct visualization of the bladder mucosa remains the standard in diagnosing bladder malignancy. The natural history of superficial bladder cancer is characterized by disease recurrence and disease progression. First-line treatment of patients with noninvasive bladder cancer is transurethral resection of bladder tumor. Adjuvant treatment with intravesical chemotherapy and immunotherapy has become an important component of therapy.
- Conclusion: The results of ongoing studies are eagerly anticipated and will improve our understanding of the disease.
Nonmuscle invasive bladder cancer is a common malignancy and the second most common urologic malignancy after prostate cancer. It accounts for approximately 73,500 new cancer diagnoses yearly in the United States [1]. An estimated 14,880 persons die each year as a result of the disease. Despite improvements in diagnosis and management of noninvasive bladder tumors, the risk of both recurrence and progression remains significant. In this article, we review the etiology, diagnosis, and management of noninvasive bladder cancer.
Epidemiology And Risk Factors
Bladder cancer affects men more commonly than women, with an approximate 3 to 4:1 ratio [1,2].The incidence in men over the past 8 years has been stable, and the incidence in women has decreased by 0.3% over the same time period. Bladder cancer affects Caucasians twice as often as African Americans, and affects Hispanics and Asians even less frequently than African Americans [2]. More than 90% of patients diagnosed with bladder cancer will be older than 55 years of age.
Histologically, urothelial (transitional cell) carcinoma accounts for over 90% of all diagnosed bladder cancers [3].Other subtypes in order of prevalence include squamous cell carcinoma, adenocarcinoma, and small cell carcinomas. Of those diagnosed with urothelial carcinoma, nonmuscle invasive (superficial) bladder cancer (NMIBC) accounts for almost 75% of cases [2]. Muscle invasion is seen in 20% of newly diagnosed cases, and metastatic disease is seen approximately 5% of the time.
It is estimated that smoking accounts for half of all cases of bladder cancer, with smokers having a 2- to 6-fold greater risk of bladder cancer as compared with nonsmokers [4–6]. At 25 years after smoking cessation, the risk of bladder cancer continues to decrease but is still higher than that of nonsmokers [7]. Continued smoking despite the diagnosis of urothelial carcinoma increases the risk of recurrence 2.2-fold [8].
Environmental exposures also have been linked to the development of urothelial carcinoma, particularly exposure to aromatic amines [9]. Occupations associated with an increased risk of bladder cancer include tire/rubber workers, leather workers, textile workers, hairdressers, painters, dry cleaners, and chemical workers.
Exposure to certain medications has been associated with an increased risk of bladder cancer, including the analgesic phenacetin, which has since been taken off the market [10]. Additionally, patients treated with the chemotherapeutic agent cyclophosphamide have a higher risk of bladder cancer, with a dose-response relationship between cyclophosphamide and the risk for bladder cancer [11,12]. The increased risk of bladder cancer and risk of hemorrhagic cystitis associated with cyclophosphamide therapy is secondary to exposure to the urinary metabolite acrolein. Concomitant administration of sodium 2-mercaptoethanesulfonate (MESNA) provides regional detoxification of acrolein in the urinary tract [13].
Urothelial carcinoma does not have a strong inherited disease association. It is felt, however, that there are 2 separate molecular pathways that may lead to the development of bladder cancer [14]. Mutation of the p53 gene has been shown to be associated with carcinoma in situ and invasive disease, whereas mutation of FGFR3 is seen more frequently with Ta disease [15]. Accumulation of p53 in cell nuclei is an independent predictor of tumor recurrence and overall poor prognosis [16]. The identification of molecular markers of tumor progression is an active field of research in bladder cancer [17].
Case Patient 1
Initial Presentation and Evaluation
A 63-year-old man with a 60 pack-year history of smoking presents to a urologist with a urinalysis from his primary care physician showing 20 to 50 red blood cells per high-power field (RBCs/HPF). He denies any urgency, frequency, or recent urinary tract infections. A urine culture from his primary care doctor is negative.
What are the common presenting features of bladder cancer?
Hematuria is the most common presenting feature of bladder cancer. It is present as the initial symptom in up to 90% of patients with urothelial carcinoma [18]. Other symptoms include irritative voiding symptoms such as urgency, frequency, and dysuria. Irritative voiding symptoms tend to occur more commonly with carcinoma in situ [19].
What are the next steps in the workup of this patient?
Initial Evaluation
American Urological Association (AUA) guidelines for the evaluation and management of asymptomatic microhematuria were updated in 2011 [20]. They recommend that every patient who presents with microscopic hematuria (> 3 RBCs/HPF) undergo a thorough history and physical exam, including rectal exam and bimanual evaluation in females to assess for any masses or pelvic fixation. Once benign sources of hematuria (eg, infection, menstruation, vigorous exercise, medical renal disease, viral illness, trauma, or recent urological procedures) have been ruled out, further testing will include a renal function panel, upper tract imaging, as well as cystoscopy in high-risk patients and those older than age 35 years. Urine cytology may be utilized in high-risk patients, but it is no longer generally recommended for routine workup.
Imaging
The imaging modality of choice during the hematuria workup is the computed tomography urogram (CTU), a multiphasic CT scan that images the urinary tract before and after contrast administration and includes excretory stage imaging [21]. Sadow et al found that CTU had a negative predictive value (NPV) of 95% for the detection of bladder cancer, while cystoscopy had an NPV of 99% [22]. In addition to radiographic evaluation of the urinary system, CT offers useful staging information regarding metastatic disease. In patients with renal failure or other contraindications to CTU, magnetic resonance urography (MRU) has become an acceptable alternative for hematuria evaluation. MRU allows for improved characterization of tissue and does not utilize ionizing radiation. During MRU, the high T2 signal intensity of urine is utilized to provide contrast in the images in static phase MRU and after gadolinium administration for excretory-phase MRU [21]. The bladder is typically best evaluated in T1-weighted images a few minutes after gadolinium administration, before the contrast reaches the bladder; it may also be evaluated during the late excretory phase when signal enhancement from gadolinium is greatest. The effectiveness of MRU in collecting system evaluation is still evolving, and therefore, in appropriately selected patients who would benefit from further collecting system evaluation, MRU should be utilized in conjunction with retrograde pyelograms [20]. Though previously considered the gold standard in imaging, intravenous pyelography is no longer a recommended imaging modality for hematuria evaluation.
Urine Cytology and Urine Markers
Urine markers and urine cytology are a debated topic in the workup and follow-up of bladder cancer. Urine cytology evaluates sloughed cells for malignant features [23]. Due to the lack of cohesion of carcinoma in situ cells and high-grade lesions, these cells are more likely to slough than are low-grade lesions [24]. The range of sensitivity of urine cytology reported in the literature varies widely. Studies report that the sensitivity of urine cytology in high-grade tumors approaches 95%, and in carcinoma in situ is up to 100% when 3 consecutive specimens are obtained [25]. However, Yafi et al recently reported that the sensitivity of urine cytology in high-grade tumors is 51% and in low-grade tumors is only 10% [26]. It is recommended that urine cytology be evaluated as part of a hematuria work-up in high-risk patients.
Aside from cytology, more than a dozen urine marker tests for bladder cancer detection and surveillance have been developed [27]. Current urine markers tests include protein-based assays such as the nuclear matrix protein 22 (NMP22) assay (NMP22 Test Kit; Alere, Waltham, MA) and bladder tumor antigen assays (BTA stat and BTA-TRAK; Polymedco, Cortlandt, NY) as well as cellular marker tests such as UroVysion FISH (Abbott Molecular, Abbott Park, IL) and ImmunoCyt (Scimedx, Denville, NJ) [27–31]. NMP22 is a nuclear matrix protein that is elevated in bladder cancer patients, and BTA stat/TRAK (qualitative/quantitative) detects complement factor H. Much controversy surrounds the utilization of these markers for screening and monitoring of bladder cancer, and currently they are not routinely recommended for these purposes nor are they recommended for follow-up in patients with bowel interposition [32].
Cystoscopy
Ultimately, direct visualization of the bladder mucosa remains a gold standard in diagnosing bladder malignancy. Office-based cystoscopy allows for rapid assessment and also allows biopsy to be performed for suspicious lesions. It can be performed easily with local anesthetic.
The use of fluorescence and narrow-band cystoscopy has been evaluated in recent years. The premise of fluorescence cystoscopy is that there is preferential accumulation of porphyrin in neoplastic cells. Therefore, intravesically instilled photoactive heme precursors such as 5-aminolevulinic acid (5-ALA) or hexaminolevulinate (HAL) have increased uptake within these neoplastic cells and subsequent enhancement. Preliminary studies have shown that approximately one quarter to one third more cases of small papillary tumors and carcinoma in situ are identified using fluorescence cystoscopy as compared with standard white light cystoscopy [33–36]. In one prospective study, the use of fluorescence cystoscopy resulted in a 16% decrease in the recurrence rate [37]. Denzinger et al found that 8-year recurrence-free survival in those who underwent fluorescence transurethral resection (TUR) was 71% as compared with 45% in conventional TUR patients [36]. Caution is required, however, because false-positives may occur in patients with inflammatory lesions.
Narrow-band cystoscopy works by filtering white light into bandwidths of 415 and 540 nm, wavelengths absorbed by hemoglobin. This allows for added contrast between vascular structures and normal urothelium [38]. Narrow-band imaging has an advantage over fluorescence cystoscopy in that no preoperative intravesical instillations are required. Detection rates of NMIBC were as high as 94.7% with narrow-band imaging, as compared to 79.2% with white light cystoscopy [39]. In the case of recurrent low-grade papillary lesions, resection with narrow-band imaging reduces recurrence rates by approximately 30% when patients are followed for 3 years [40]. While both fluorescence cystoscopy and narrow-band imaging appear to be promising technology, higher false-positive rates are seen with both as compared to white light cystoscopy [3,41]. Neither modality is a recommended treatment option [42].
Case 1 Continued
On office-based cystoscopy, a 2.5-cm papillary lesion is noted on the left lateral wall of the bladder. There are no other suspicious lesions within the bladder. A CTU is obtained, which reveals no hydronephrosis or lymphadenopathy and correlates with the cystoscopic examination of a bladder lesion on the left lateral wall.
What are the next steps in management?
Transurethral Resection
Transurethral resection of bladder tumor (TURBT) is paramount in the treatment and diagnosis of bladder tumors. TURBT allows for complete resection of the tumor and also allows for histologic diagnosis, staging, and grading. The bladder wall consists of 3 principle layers: the mucosa, submucosa, and muscularis. An important factor in identifying the stage of disease is determining the depth of invasion as well as the size and mobility of masses. Adequate resection, with inclusion of muscle in the TURBT specimen, allows for proper staging of urothelial carcinoma. When pathology reveals high-grade Ta or T1 disease or does not contain muscle, re-resection is recommended [42]. In a study involving 150 patients with bladder tumors, when re-resection was undertaken within 2 to 6 weeks, 29% of NMIBC lesions were upstaged, and treatment options were changed based on re-resection results in one third of patients [43].
TURBT is a relatively safe procedure that can be performed in an outpatient setting. The most common complications of TURBT are urinary tract infection and hematuria [44]. Other complications include the risk of bladder perforation with deep resection. In the event of bladder perforation, it is important to determine the location and depth of the perforation to decide on appropriate treatment. Many small extraperitoneal perforations may be managed with simple Foley drainage, whereas large perforations may require open or laparoscopic repair [45–46]. The incidence of extravesical recurrence of NMIBC after bladder perforation varies in the literature from 0% to 6% [47]. Numerous studies report open bladder repair following any intraperitoneal perforation, but laparoscopic repair is becoming more common [48,49].In any case of intraperitoneal rupture, the recommendation is for close follow-up for the rare event of recurrence.
While performing TURBT, one must be cognizant of the obturator nerve reflex. The obturator nerve runs in close proximity to the inferolateral wall of the bladder. Stimulation from the electrocautery current will cause external rotation and adduction of the thigh in a sudden jerking movement, thus increasing the risk of bladder perforation [50]. Bipolar technology has been found to be a safe alternative to conventional monopolar electrocautery for resection of bladder tumors, with decreased length of catheterization and fewer bladder perforations documented [51]. While bipolar technology may decrease stimulation of the obturator reflex, it is important to note that it still may occur, resulting in bladder perforation [52.53].
Staging, Grading, and Risk Stratification
In 2004 the World Health Organization revised the classification of urothelial malignancies to include tumors designated as either high- or low-grade as well as carcinoma in situ [55]. The differentiation of low- and high-grade is based on the degree of nuclear anaplasia and architectural abnormalities. Those with high-grade tumors as well as increased depth of invasion have an increased risk of recurrence and progression of disease compared to low-grade tumors [56].
When determining treatment and surveillance options for NMIBC patients, not only are the stage and grade determining factors, but future risk of recurrence and progression dictates
Intravesical Chemotherapy/Immunotherapy
Intravesical therapy is the use of chemotherapeutic or immunotherapeutic substances instilled within the bladder. It is indicated for the treatment of NMIBC but is not the recommended treatment for T2 or greater lesions. The goals of intravesical therapy are to reduce recurrence and progression of resected disease and eradicate carcinoma in situ as well as incompletely resected papillary tumor [42].
Intravesical chemotherapeutic agents include mitomycin C, thiotepa, doxorubicin, valrubicin, epirubicin, and gemcitabine [42]. Mitomycin C is an alkylating agent that acts by inhibiting DNA synthesis. Because of mitomycin C’s relatively high molecular weight, systemic absorption is minimal, although there is a small risk of myelosuppression. Thiotepa is an alkylating agent that cross-links nucleic acids. Doxorubicin, epirubicin, and valrubicin are intercalating agents that inhibit DNA synthesis. Gemcitabine is a deoxycytidine analog that also inhibits DNA synthesis.
Immunotherapy utilizes bacillus Calmette-Guérin (BCG), a live, attenuated strain of Mycobacterium bovis. Though the mechanism of action of BCG is not fully understood, it is known that instillation of BCG stimulates a large immune response [57]. BCG is taken up by antigen-presenting cells as well as urothelial cells and bladder cancer cells, initiating the immune response. Cytokine release in response to BCG is thought to be mediated by macrophages and activated lymphocytes as well as urothelial cells directly [58]. Recent studies have found that interleukin-17 plays an important role in neutrophil recruitment and the generation of the Th1- cell response, which mediates the antitumor effect [59,60]. The innate immune response is also felt to be important in the antitumor effect of BCG, with studies suggesting that BCG is ineffective in the absence of natural killer cell activity and that neutrophils and macrophages are important in the immune response [58,61,62].
Administration of BCG is typically held for at least 2 weeks following TURBT to minimize the risk of sepsis and adverse events. BCG also should not be used in patients who have had traumatic catheterization, recent gross hematuria, or urinary tract infection, in immunocompromised hosts, or in patients with active autoimmune disease, known allergy, or history of BCG sepsis. Adverse events associated with BCG use include sepsis, prostatitis, epididymitis, cystitis, and flu-like symptoms [63].
Interferon alpha-2b is a cytokine that helps modulate the immune response. In cases of refractory bladder cancer that have failed BCG treatment, modulation with interferon alfa-2b therapy has been investigated. In vitro studies show that administration of interferon alfa-2b enhanced the ability of BCG to induce interferon-gamma production, upregulated tumor necrosis factor-α and interleukin-12, and down-regulated interleukin-10, thus favoring the upregulation of the Th1 immune-mediated response [64]. Used in conjunction with BCG in patients who have failed BCG therapy, interferon alfa-2b has been shown to have a 2-year recurrence-free survival rate of up to 45% [65].
Immediately following TURBT, it is recommended that patients with low-risk disease undergo single-dose intravesical chemotherapy [66]. When performed within 24 hours (and ideally 6 hours) of resection, intravesical chemotherapy has been shown to decrease the odds of bladder cancer recurrence by up to 40% in low-risk disease [67].The mechanism of action of single-dose intravesical chemotherapy instilled immediately after resection is not definitively known, but it is hypothesized that it destroys any remaining microscopic disease and prevents reimplantation of any freely circulating cells [67]. Single-dose mitomycin C, however, does not decrease the rate of progression in incompletely resected tumors [68]. Administration of intravesical chemotherapeutic agents should be avoided when there is bladder perforation [69].
There is some debate regarding the best approach to treating intermediate-risk bladder cancer. In guidelines released by the International Bladder Cancer Group, a group of experts who evaluated and set forth guidelines based on current recommendations from the NCCN, AUA, European Association of Urology, and the First International Consultation on Bladder Tumors, initiation of BCG therapy with maintenance or intravesical chemotherapy for up to 1 year of adjuvant treatment is recommended following the diagnosis of intermediate-risk bladder cancer [66]. Induction treatments are single intravesical instillations administered weekly for 6 weeks and begun 2 to 4 weeks after resection. Maintenance courses consist of once weekly instillations for 3 weeks undertaken at 3 months, 6 months, and then every 6 months for up to a total of 3 years of treatment [70].
For the management of high-risk disease, most guidelines concur that the optimal treatment is BCG with maintenance, although the recommended length of maintenance varies from 1 to 3 years [66]. The EORTC-GU recently reported the results of a randomized study in which high-risk Ta and T1 lesions were treated with BCG maintenance; they found that a full-dose, 3-year maintenance course of BCG decreased recurrences without increasing toxicity [71].
Although both intravesical chemotherapy and immunotherapy are recommended treatments for NMIBC, there is a preference in the published guidelines toward the use of BCG over intravesical chemotherapy. In multiple meta-analyses, BCG, and especially BCG with maintenance, has been shown to have improved disease-free recurrence when compared with intravesical chemotherapy [72,73]. Malmström et al showed a 32% reduction in the recurrence rate in BCG-treated patients compared with those treated with mitomycin C [74]. Similarly, high-risk patients treated with gemcitabine therapy had a higher recurrence rate and more rapid time to recurrence as compared with those treated with BCG therapy; in intermediate-risk patients, the rate of recurrence was not statistically significant [75].
Cystectomy
In certain high-risk patients, it is also appropriate to offer cystectomy as initial therapy. Though much more invasive than other treatment options, it does offer a chance for cure in a select group of patients with high likelihood of progression of disease. Risk factors associated with progression and consideration for immediate or early cystectomy include large tumor size (> 3 cm), inability to completely resect tumor, difficult resection site, multifocal/ diffuse disease, presence of carcinoma in situ, prostatic urethral involvement, female sex, suspected understaging secondary to lymphovascular invasion, or unfavorable histology [76–81]. While tumor upstaging has been noted in up to one-quarter of high-risk immediate cystectomy patients, it is important to note that multiple retrospective reviews have not found a cancer-specific survival (CSS) benefit to immediate cystectomy versus conservative treatment [82–85]. Hautmann et al examined immediate cystectomy versus deferred cystectomy until after recurrence in high-risk patients and demonstrated a clear 10-year CSS benefit of 79% versus 65% [86]. Because the number of patients who have undergone immediate cystectomy is still relatively small and predictors of aggressive disease are still evolving, immediate cystectomy is still considered a viable treatment option in the appropriately selected patient.
Case Patient 2
A 72-year-old woman with a history of T1 bladder cancer presents for routine follow-up. She has completed a course of BCG with maintenance for her initial lesion. On follow-up cystoscopy, she is found to have multiple velvety red patches throughout the bladder and a 1-cm sessile lesion.
What is the follow-up for bladder cancer?
Bladder cancer causes what is known as a field defect. As urine bathes the urothelium, theoretically, so do the carcinogens within the urine, exposing cells throughout the bladder. Bladder cancer therefore does not just recur at the initial site of the tumor, but can occur anywhere in the bladder. For example, Heney et al found that initial tumors were only occasionally located at the dome (5% of the time), whereas new tumor occurrences were found at the dome in 29% of patients [87].
Though there is no consensus in the literature as to the ideal timing of cystoscopic follow-up, NCCN guidelines recommend cystoscopy every 3 months with increasing intervals as indicated for low-risk lesions [88]. For all other lesions, they recommend cystoscopy and cytology every 3 to 6 months with increasing intervals as indicated, upper tract imaging every 1 to 2 years for high-grade tumors, and the optional use of urine markers for follow-up. The AUA varies slightly in recommending cystoscopy and cytology for all patients every 3 months for 2 years, followed by every 6 months for 2 to 3 years, and then annually. They recommend imaging of the upper tracts but do not specify timing, and current recommendations do not support the use of urine markers [89].
How are recurrences/treatment failures managed?
When recurrence or treatment failure is identified, it is important to consider the initial lesion and treatment as well as stage and grade of any follow-up lesions. Low-risk disease may be treated with re-resection and BCG or mitomycin C with or without maintenance [42]. With treatment failure of intermediate disease, resection followed by a change in the modality of intravesical treatment is an option. When recurrences occur in intermediate-risk disease, one might change modalities or reinstitute a second induction therapy course after resection [66].
High-risk NMIBC provides a challenging dilemma in management. In a systematic literature review of 19 published trials, van den Bosch and Witjes [90] reported a 21% progression to muscle-invasive disease in high-risk NMIBC patients. Management of recurrences in this population in an effort to decrease progression and increase CSS is a highly debated topic, with no clear answer currently available. In the case of high-risk disease that has recurred, treatment options include a second induction course of BCG, cystectomy, or alternative intravesical chemotherapeutic options. Those patients who underwent early cystectomy for high-risk recurrence after BCG therapy had an overall greater survival compared to those who delayed cystectomy over 2 years [91]. In their study evaluating early versus delayed cystectomy, Jäger et al [92] found that as the number of TURBTs performed before cystectomy for high-risk disease went from 1 to 2–4 to greater than 4, the 10-year CSS decreased from 84% to 77% to 45%. Additionally, they found that when cystectomy was performed 1 year after initial TURBT, the 10-year CSS decreased from 79% to 61%.
In patients who have failed BCG treatment and are not surgical candidates or do not desire surgical intervention, intravesical valrubicin is emerging as a treatment alternative. It is currently the only therapy that is approved by the U.S. Food and Drug Administration for treatment of BCG-refractory carcinoma in situ in nonsurgical candidates. Dinney et al examined the efficacy and safety of valrubicin in BCG-refractory carcinoma in situ and found an 18% complete response rate over the 6-month follow-up period, which correlated with the previously reported response rates in phase II/III trials [93]. Other therapies being investigated for BCG failure include thermochemotherapy, photodynamic therapy, as well as combination intravesical chemotherapies [94].
Conclusion
Though much research is under way on the surveillance, diagnosis, and treatment of NMIBC, time-tested modalities remain the mainstay of management. Ongoing studies will improve our understanding of the disease as new information regarding novel ways of delivering intravesical therapeutics, surveillance modalities, and optimal treatment and follow-up strategies becomes available.
Corresponding author: Frank N. Burks, MD, 31157 Woodward Ave., Royal Oak, MI 48073, [email protected].
Financial disclosures: None.
From the William Beaumont Hospital, Royal Oak, MI.
Abstract
- Objective: To review the diagnosis and management of noninvasive bladder cancer.
- Methods: Literature review.
- Results: Nonmuscle invasive bladder cancer is a common malignancy that affects more men than women. It is estimated that smoking accounts for half of all cases. Direct visualization of the bladder mucosa remains the standard in diagnosing bladder malignancy. The natural history of superficial bladder cancer is characterized by disease recurrence and disease progression. First-line treatment of patients with noninvasive bladder cancer is transurethral resection of bladder tumor. Adjuvant treatment with intravesical chemotherapy and immunotherapy has become an important component of therapy.
- Conclusion: The results of ongoing studies are eagerly anticipated and will improve our understanding of the disease.
Nonmuscle invasive bladder cancer is a common malignancy and the second most common urologic malignancy after prostate cancer. It accounts for approximately 73,500 new cancer diagnoses yearly in the United States [1]. An estimated 14,880 persons die each year as a result of the disease. Despite improvements in diagnosis and management of noninvasive bladder tumors, the risk of both recurrence and progression remains significant. In this article, we review the etiology, diagnosis, and management of noninvasive bladder cancer.
Epidemiology And Risk Factors
Bladder cancer affects men more commonly than women, with an approximate 3 to 4:1 ratio [1,2].The incidence in men over the past 8 years has been stable, and the incidence in women has decreased by 0.3% over the same time period. Bladder cancer affects Caucasians twice as often as African Americans, and affects Hispanics and Asians even less frequently than African Americans [2]. More than 90% of patients diagnosed with bladder cancer will be older than 55 years of age.
Histologically, urothelial (transitional cell) carcinoma accounts for over 90% of all diagnosed bladder cancers [3].Other subtypes in order of prevalence include squamous cell carcinoma, adenocarcinoma, and small cell carcinomas. Of those diagnosed with urothelial carcinoma, nonmuscle invasive (superficial) bladder cancer (NMIBC) accounts for almost 75% of cases [2]. Muscle invasion is seen in 20% of newly diagnosed cases, and metastatic disease is seen approximately 5% of the time.
It is estimated that smoking accounts for half of all cases of bladder cancer, with smokers having a 2- to 6-fold greater risk of bladder cancer as compared with nonsmokers [4–6]. At 25 years after smoking cessation, the risk of bladder cancer continues to decrease but is still higher than that of nonsmokers [7]. Continued smoking despite the diagnosis of urothelial carcinoma increases the risk of recurrence 2.2-fold [8].
Environmental exposures also have been linked to the development of urothelial carcinoma, particularly exposure to aromatic amines [9]. Occupations associated with an increased risk of bladder cancer include tire/rubber workers, leather workers, textile workers, hairdressers, painters, dry cleaners, and chemical workers.
Exposure to certain medications has been associated with an increased risk of bladder cancer, including the analgesic phenacetin, which has since been taken off the market [10]. Additionally, patients treated with the chemotherapeutic agent cyclophosphamide have a higher risk of bladder cancer, with a dose-response relationship between cyclophosphamide and the risk for bladder cancer [11,12]. The increased risk of bladder cancer and risk of hemorrhagic cystitis associated with cyclophosphamide therapy is secondary to exposure to the urinary metabolite acrolein. Concomitant administration of sodium 2-mercaptoethanesulfonate (MESNA) provides regional detoxification of acrolein in the urinary tract [13].
Urothelial carcinoma does not have a strong inherited disease association. It is felt, however, that there are 2 separate molecular pathways that may lead to the development of bladder cancer [14]. Mutation of the p53 gene has been shown to be associated with carcinoma in situ and invasive disease, whereas mutation of FGFR3 is seen more frequently with Ta disease [15]. Accumulation of p53 in cell nuclei is an independent predictor of tumor recurrence and overall poor prognosis [16]. The identification of molecular markers of tumor progression is an active field of research in bladder cancer [17].
Case Patient 1
Initial Presentation and Evaluation
A 63-year-old man with a 60 pack-year history of smoking presents to a urologist with a urinalysis from his primary care physician showing 20 to 50 red blood cells per high-power field (RBCs/HPF). He denies any urgency, frequency, or recent urinary tract infections. A urine culture from his primary care doctor is negative.
What are the common presenting features of bladder cancer?
Hematuria is the most common presenting feature of bladder cancer. It is present as the initial symptom in up to 90% of patients with urothelial carcinoma [18]. Other symptoms include irritative voiding symptoms such as urgency, frequency, and dysuria. Irritative voiding symptoms tend to occur more commonly with carcinoma in situ [19].
What are the next steps in the workup of this patient?
Initial Evaluation
American Urological Association (AUA) guidelines for the evaluation and management of asymptomatic microhematuria were updated in 2011 [20]. They recommend that every patient who presents with microscopic hematuria (> 3 RBCs/HPF) undergo a thorough history and physical exam, including rectal exam and bimanual evaluation in females to assess for any masses or pelvic fixation. Once benign sources of hematuria (eg, infection, menstruation, vigorous exercise, medical renal disease, viral illness, trauma, or recent urological procedures) have been ruled out, further testing will include a renal function panel, upper tract imaging, as well as cystoscopy in high-risk patients and those older than age 35 years. Urine cytology may be utilized in high-risk patients, but it is no longer generally recommended for routine workup.
Imaging
The imaging modality of choice during the hematuria workup is the computed tomography urogram (CTU), a multiphasic CT scan that images the urinary tract before and after contrast administration and includes excretory stage imaging [21]. Sadow et al found that CTU had a negative predictive value (NPV) of 95% for the detection of bladder cancer, while cystoscopy had an NPV of 99% [22]. In addition to radiographic evaluation of the urinary system, CT offers useful staging information regarding metastatic disease. In patients with renal failure or other contraindications to CTU, magnetic resonance urography (MRU) has become an acceptable alternative for hematuria evaluation. MRU allows for improved characterization of tissue and does not utilize ionizing radiation. During MRU, the high T2 signal intensity of urine is utilized to provide contrast in the images in static phase MRU and after gadolinium administration for excretory-phase MRU [21]. The bladder is typically best evaluated in T1-weighted images a few minutes after gadolinium administration, before the contrast reaches the bladder; it may also be evaluated during the late excretory phase when signal enhancement from gadolinium is greatest. The effectiveness of MRU in collecting system evaluation is still evolving, and therefore, in appropriately selected patients who would benefit from further collecting system evaluation, MRU should be utilized in conjunction with retrograde pyelograms [20]. Though previously considered the gold standard in imaging, intravenous pyelography is no longer a recommended imaging modality for hematuria evaluation.
Urine Cytology and Urine Markers
Urine markers and urine cytology are a debated topic in the workup and follow-up of bladder cancer. Urine cytology evaluates sloughed cells for malignant features [23]. Due to the lack of cohesion of carcinoma in situ cells and high-grade lesions, these cells are more likely to slough than are low-grade lesions [24]. The range of sensitivity of urine cytology reported in the literature varies widely. Studies report that the sensitivity of urine cytology in high-grade tumors approaches 95%, and in carcinoma in situ is up to 100% when 3 consecutive specimens are obtained [25]. However, Yafi et al recently reported that the sensitivity of urine cytology in high-grade tumors is 51% and in low-grade tumors is only 10% [26]. It is recommended that urine cytology be evaluated as part of a hematuria work-up in high-risk patients.
Aside from cytology, more than a dozen urine marker tests for bladder cancer detection and surveillance have been developed [27]. Current urine markers tests include protein-based assays such as the nuclear matrix protein 22 (NMP22) assay (NMP22 Test Kit; Alere, Waltham, MA) and bladder tumor antigen assays (BTA stat and BTA-TRAK; Polymedco, Cortlandt, NY) as well as cellular marker tests such as UroVysion FISH (Abbott Molecular, Abbott Park, IL) and ImmunoCyt (Scimedx, Denville, NJ) [27–31]. NMP22 is a nuclear matrix protein that is elevated in bladder cancer patients, and BTA stat/TRAK (qualitative/quantitative) detects complement factor H. Much controversy surrounds the utilization of these markers for screening and monitoring of bladder cancer, and currently they are not routinely recommended for these purposes nor are they recommended for follow-up in patients with bowel interposition [32].
Cystoscopy
Ultimately, direct visualization of the bladder mucosa remains a gold standard in diagnosing bladder malignancy. Office-based cystoscopy allows for rapid assessment and also allows biopsy to be performed for suspicious lesions. It can be performed easily with local anesthetic.
The use of fluorescence and narrow-band cystoscopy has been evaluated in recent years. The premise of fluorescence cystoscopy is that there is preferential accumulation of porphyrin in neoplastic cells. Therefore, intravesically instilled photoactive heme precursors such as 5-aminolevulinic acid (5-ALA) or hexaminolevulinate (HAL) have increased uptake within these neoplastic cells and subsequent enhancement. Preliminary studies have shown that approximately one quarter to one third more cases of small papillary tumors and carcinoma in situ are identified using fluorescence cystoscopy as compared with standard white light cystoscopy [33–36]. In one prospective study, the use of fluorescence cystoscopy resulted in a 16% decrease in the recurrence rate [37]. Denzinger et al found that 8-year recurrence-free survival in those who underwent fluorescence transurethral resection (TUR) was 71% as compared with 45% in conventional TUR patients [36]. Caution is required, however, because false-positives may occur in patients with inflammatory lesions.
Narrow-band cystoscopy works by filtering white light into bandwidths of 415 and 540 nm, wavelengths absorbed by hemoglobin. This allows for added contrast between vascular structures and normal urothelium [38]. Narrow-band imaging has an advantage over fluorescence cystoscopy in that no preoperative intravesical instillations are required. Detection rates of NMIBC were as high as 94.7% with narrow-band imaging, as compared to 79.2% with white light cystoscopy [39]. In the case of recurrent low-grade papillary lesions, resection with narrow-band imaging reduces recurrence rates by approximately 30% when patients are followed for 3 years [40]. While both fluorescence cystoscopy and narrow-band imaging appear to be promising technology, higher false-positive rates are seen with both as compared to white light cystoscopy [3,41]. Neither modality is a recommended treatment option [42].
Case 1 Continued
On office-based cystoscopy, a 2.5-cm papillary lesion is noted on the left lateral wall of the bladder. There are no other suspicious lesions within the bladder. A CTU is obtained, which reveals no hydronephrosis or lymphadenopathy and correlates with the cystoscopic examination of a bladder lesion on the left lateral wall.
What are the next steps in management?
Transurethral Resection
Transurethral resection of bladder tumor (TURBT) is paramount in the treatment and diagnosis of bladder tumors. TURBT allows for complete resection of the tumor and also allows for histologic diagnosis, staging, and grading. The bladder wall consists of 3 principle layers: the mucosa, submucosa, and muscularis. An important factor in identifying the stage of disease is determining the depth of invasion as well as the size and mobility of masses. Adequate resection, with inclusion of muscle in the TURBT specimen, allows for proper staging of urothelial carcinoma. When pathology reveals high-grade Ta or T1 disease or does not contain muscle, re-resection is recommended [42]. In a study involving 150 patients with bladder tumors, when re-resection was undertaken within 2 to 6 weeks, 29% of NMIBC lesions were upstaged, and treatment options were changed based on re-resection results in one third of patients [43].
TURBT is a relatively safe procedure that can be performed in an outpatient setting. The most common complications of TURBT are urinary tract infection and hematuria [44]. Other complications include the risk of bladder perforation with deep resection. In the event of bladder perforation, it is important to determine the location and depth of the perforation to decide on appropriate treatment. Many small extraperitoneal perforations may be managed with simple Foley drainage, whereas large perforations may require open or laparoscopic repair [45–46]. The incidence of extravesical recurrence of NMIBC after bladder perforation varies in the literature from 0% to 6% [47]. Numerous studies report open bladder repair following any intraperitoneal perforation, but laparoscopic repair is becoming more common [48,49].In any case of intraperitoneal rupture, the recommendation is for close follow-up for the rare event of recurrence.
While performing TURBT, one must be cognizant of the obturator nerve reflex. The obturator nerve runs in close proximity to the inferolateral wall of the bladder. Stimulation from the electrocautery current will cause external rotation and adduction of the thigh in a sudden jerking movement, thus increasing the risk of bladder perforation [50]. Bipolar technology has been found to be a safe alternative to conventional monopolar electrocautery for resection of bladder tumors, with decreased length of catheterization and fewer bladder perforations documented [51]. While bipolar technology may decrease stimulation of the obturator reflex, it is important to note that it still may occur, resulting in bladder perforation [52.53].
Staging, Grading, and Risk Stratification
In 2004 the World Health Organization revised the classification of urothelial malignancies to include tumors designated as either high- or low-grade as well as carcinoma in situ [55]. The differentiation of low- and high-grade is based on the degree of nuclear anaplasia and architectural abnormalities. Those with high-grade tumors as well as increased depth of invasion have an increased risk of recurrence and progression of disease compared to low-grade tumors [56].
When determining treatment and surveillance options for NMIBC patients, not only are the stage and grade determining factors, but future risk of recurrence and progression dictates
Intravesical Chemotherapy/Immunotherapy
Intravesical therapy is the use of chemotherapeutic or immunotherapeutic substances instilled within the bladder. It is indicated for the treatment of NMIBC but is not the recommended treatment for T2 or greater lesions. The goals of intravesical therapy are to reduce recurrence and progression of resected disease and eradicate carcinoma in situ as well as incompletely resected papillary tumor [42].
Intravesical chemotherapeutic agents include mitomycin C, thiotepa, doxorubicin, valrubicin, epirubicin, and gemcitabine [42]. Mitomycin C is an alkylating agent that acts by inhibiting DNA synthesis. Because of mitomycin C’s relatively high molecular weight, systemic absorption is minimal, although there is a small risk of myelosuppression. Thiotepa is an alkylating agent that cross-links nucleic acids. Doxorubicin, epirubicin, and valrubicin are intercalating agents that inhibit DNA synthesis. Gemcitabine is a deoxycytidine analog that also inhibits DNA synthesis.
Immunotherapy utilizes bacillus Calmette-Guérin (BCG), a live, attenuated strain of Mycobacterium bovis. Though the mechanism of action of BCG is not fully understood, it is known that instillation of BCG stimulates a large immune response [57]. BCG is taken up by antigen-presenting cells as well as urothelial cells and bladder cancer cells, initiating the immune response. Cytokine release in response to BCG is thought to be mediated by macrophages and activated lymphocytes as well as urothelial cells directly [58]. Recent studies have found that interleukin-17 plays an important role in neutrophil recruitment and the generation of the Th1- cell response, which mediates the antitumor effect [59,60]. The innate immune response is also felt to be important in the antitumor effect of BCG, with studies suggesting that BCG is ineffective in the absence of natural killer cell activity and that neutrophils and macrophages are important in the immune response [58,61,62].
Administration of BCG is typically held for at least 2 weeks following TURBT to minimize the risk of sepsis and adverse events. BCG also should not be used in patients who have had traumatic catheterization, recent gross hematuria, or urinary tract infection, in immunocompromised hosts, or in patients with active autoimmune disease, known allergy, or history of BCG sepsis. Adverse events associated with BCG use include sepsis, prostatitis, epididymitis, cystitis, and flu-like symptoms [63].
Interferon alpha-2b is a cytokine that helps modulate the immune response. In cases of refractory bladder cancer that have failed BCG treatment, modulation with interferon alfa-2b therapy has been investigated. In vitro studies show that administration of interferon alfa-2b enhanced the ability of BCG to induce interferon-gamma production, upregulated tumor necrosis factor-α and interleukin-12, and down-regulated interleukin-10, thus favoring the upregulation of the Th1 immune-mediated response [64]. Used in conjunction with BCG in patients who have failed BCG therapy, interferon alfa-2b has been shown to have a 2-year recurrence-free survival rate of up to 45% [65].
Immediately following TURBT, it is recommended that patients with low-risk disease undergo single-dose intravesical chemotherapy [66]. When performed within 24 hours (and ideally 6 hours) of resection, intravesical chemotherapy has been shown to decrease the odds of bladder cancer recurrence by up to 40% in low-risk disease [67].The mechanism of action of single-dose intravesical chemotherapy instilled immediately after resection is not definitively known, but it is hypothesized that it destroys any remaining microscopic disease and prevents reimplantation of any freely circulating cells [67]. Single-dose mitomycin C, however, does not decrease the rate of progression in incompletely resected tumors [68]. Administration of intravesical chemotherapeutic agents should be avoided when there is bladder perforation [69].
There is some debate regarding the best approach to treating intermediate-risk bladder cancer. In guidelines released by the International Bladder Cancer Group, a group of experts who evaluated and set forth guidelines based on current recommendations from the NCCN, AUA, European Association of Urology, and the First International Consultation on Bladder Tumors, initiation of BCG therapy with maintenance or intravesical chemotherapy for up to 1 year of adjuvant treatment is recommended following the diagnosis of intermediate-risk bladder cancer [66]. Induction treatments are single intravesical instillations administered weekly for 6 weeks and begun 2 to 4 weeks after resection. Maintenance courses consist of once weekly instillations for 3 weeks undertaken at 3 months, 6 months, and then every 6 months for up to a total of 3 years of treatment [70].
For the management of high-risk disease, most guidelines concur that the optimal treatment is BCG with maintenance, although the recommended length of maintenance varies from 1 to 3 years [66]. The EORTC-GU recently reported the results of a randomized study in which high-risk Ta and T1 lesions were treated with BCG maintenance; they found that a full-dose, 3-year maintenance course of BCG decreased recurrences without increasing toxicity [71].
Although both intravesical chemotherapy and immunotherapy are recommended treatments for NMIBC, there is a preference in the published guidelines toward the use of BCG over intravesical chemotherapy. In multiple meta-analyses, BCG, and especially BCG with maintenance, has been shown to have improved disease-free recurrence when compared with intravesical chemotherapy [72,73]. Malmström et al showed a 32% reduction in the recurrence rate in BCG-treated patients compared with those treated with mitomycin C [74]. Similarly, high-risk patients treated with gemcitabine therapy had a higher recurrence rate and more rapid time to recurrence as compared with those treated with BCG therapy; in intermediate-risk patients, the rate of recurrence was not statistically significant [75].
Cystectomy
In certain high-risk patients, it is also appropriate to offer cystectomy as initial therapy. Though much more invasive than other treatment options, it does offer a chance for cure in a select group of patients with high likelihood of progression of disease. Risk factors associated with progression and consideration for immediate or early cystectomy include large tumor size (> 3 cm), inability to completely resect tumor, difficult resection site, multifocal/ diffuse disease, presence of carcinoma in situ, prostatic urethral involvement, female sex, suspected understaging secondary to lymphovascular invasion, or unfavorable histology [76–81]. While tumor upstaging has been noted in up to one-quarter of high-risk immediate cystectomy patients, it is important to note that multiple retrospective reviews have not found a cancer-specific survival (CSS) benefit to immediate cystectomy versus conservative treatment [82–85]. Hautmann et al examined immediate cystectomy versus deferred cystectomy until after recurrence in high-risk patients and demonstrated a clear 10-year CSS benefit of 79% versus 65% [86]. Because the number of patients who have undergone immediate cystectomy is still relatively small and predictors of aggressive disease are still evolving, immediate cystectomy is still considered a viable treatment option in the appropriately selected patient.
Case Patient 2
A 72-year-old woman with a history of T1 bladder cancer presents for routine follow-up. She has completed a course of BCG with maintenance for her initial lesion. On follow-up cystoscopy, she is found to have multiple velvety red patches throughout the bladder and a 1-cm sessile lesion.
What is the follow-up for bladder cancer?
Bladder cancer causes what is known as a field defect. As urine bathes the urothelium, theoretically, so do the carcinogens within the urine, exposing cells throughout the bladder. Bladder cancer therefore does not just recur at the initial site of the tumor, but can occur anywhere in the bladder. For example, Heney et al found that initial tumors were only occasionally located at the dome (5% of the time), whereas new tumor occurrences were found at the dome in 29% of patients [87].
Though there is no consensus in the literature as to the ideal timing of cystoscopic follow-up, NCCN guidelines recommend cystoscopy every 3 months with increasing intervals as indicated for low-risk lesions [88]. For all other lesions, they recommend cystoscopy and cytology every 3 to 6 months with increasing intervals as indicated, upper tract imaging every 1 to 2 years for high-grade tumors, and the optional use of urine markers for follow-up. The AUA varies slightly in recommending cystoscopy and cytology for all patients every 3 months for 2 years, followed by every 6 months for 2 to 3 years, and then annually. They recommend imaging of the upper tracts but do not specify timing, and current recommendations do not support the use of urine markers [89].
How are recurrences/treatment failures managed?
When recurrence or treatment failure is identified, it is important to consider the initial lesion and treatment as well as stage and grade of any follow-up lesions. Low-risk disease may be treated with re-resection and BCG or mitomycin C with or without maintenance [42]. With treatment failure of intermediate disease, resection followed by a change in the modality of intravesical treatment is an option. When recurrences occur in intermediate-risk disease, one might change modalities or reinstitute a second induction therapy course after resection [66].
High-risk NMIBC provides a challenging dilemma in management. In a systematic literature review of 19 published trials, van den Bosch and Witjes [90] reported a 21% progression to muscle-invasive disease in high-risk NMIBC patients. Management of recurrences in this population in an effort to decrease progression and increase CSS is a highly debated topic, with no clear answer currently available. In the case of high-risk disease that has recurred, treatment options include a second induction course of BCG, cystectomy, or alternative intravesical chemotherapeutic options. Those patients who underwent early cystectomy for high-risk recurrence after BCG therapy had an overall greater survival compared to those who delayed cystectomy over 2 years [91]. In their study evaluating early versus delayed cystectomy, Jäger et al [92] found that as the number of TURBTs performed before cystectomy for high-risk disease went from 1 to 2–4 to greater than 4, the 10-year CSS decreased from 84% to 77% to 45%. Additionally, they found that when cystectomy was performed 1 year after initial TURBT, the 10-year CSS decreased from 79% to 61%.
In patients who have failed BCG treatment and are not surgical candidates or do not desire surgical intervention, intravesical valrubicin is emerging as a treatment alternative. It is currently the only therapy that is approved by the U.S. Food and Drug Administration for treatment of BCG-refractory carcinoma in situ in nonsurgical candidates. Dinney et al examined the efficacy and safety of valrubicin in BCG-refractory carcinoma in situ and found an 18% complete response rate over the 6-month follow-up period, which correlated with the previously reported response rates in phase II/III trials [93]. Other therapies being investigated for BCG failure include thermochemotherapy, photodynamic therapy, as well as combination intravesical chemotherapies [94].
Conclusion
Though much research is under way on the surveillance, diagnosis, and treatment of NMIBC, time-tested modalities remain the mainstay of management. Ongoing studies will improve our understanding of the disease as new information regarding novel ways of delivering intravesical therapeutics, surveillance modalities, and optimal treatment and follow-up strategies becomes available.
Corresponding author: Frank N. Burks, MD, 31157 Woodward Ave., Royal Oak, MI 48073, [email protected].
Financial disclosures: None.
1. American Cancer Society. Cancer facts & figures 2012. Accessed 2 May 2013 at www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012.
2. National Cancer Institute. SEER Stat Fact Sheets: Bladder. Accessed 14 December 2012 at seer.cancer.gov/statfacts/html/urinb.html.
3. Lynch CF, Davila JA, Platz CE. Cancer of the urinary bladder. In: Ries LAG, Young JL, Keel GE, et al, editors. SEER survival monograph: cancer survival among adults: US SEER program 1988-2001, patient and tumor characteristics. NIH Pub. No. 07-6215. Bethesda (MD): National Cancer Institute; 2007:181–92.
4. Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer 2000;86:289–94.
5. Castelao JE, Yuan JM, Skipper PL, et al. Gender and smoking-related bladder cancer risk. J Natl Cancer Inst 2001;93:538–45.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. World Health Organization International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, volume 83. Tobacco smoke and involuntary smoking. Lyon, France: World Health Organization; 2004. Accessed 2 May 2013 at http://monographs.iarc.fr/ENG/Monographs/vol83/index.php.
8. Chen CH, Shun CT, Huang KH, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer, BJU Int 2007;100:281–6.
9. Murta-Nascimento C, Schmitz-Dräger BJ, Zeegers MP, et al. Epidemiology of urinary bladder cancer: from tumor development to patient’s death. World J Urology 2007;25:285–95.
10. Piper JM, Tonascia J, Matanoski GM. Heavy phenacetin use and bladder cancer in women aged 20 to 49 years. N Engl J Med 1985;313:292–5.
11. Knight A, Askling J, Granath F, et al. Urinary bladder cancer in Wegener’s granulomatosis: risks and relation to cyclophosphamide. Ann Rheum Dis 2004;63:1307–11.
12. Fairchild WV, Spence CR, Solomon HD, Gangai MP. The incidence of bladder cancer after cyclophosphamide therapy. J Urology 1979; 122:163.
13. Brock N. The development of mesna for the inhibition of urotoxic side effects of cyclophosphamide, ifosfamide, and other oxazaphosphorine cytostatics. Recent Results Cancer Res 1980;74:270–8.
14. Spruck CH, Ohneseit PF, Gonzalez-Zulueta M, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res 1994;54:784–8.
15. Bakkar AA, Wallerand H, Radvanyi F, et al. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer Res 2003;63:8108–12.
16. Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med 1994;331:1259–64.
17. Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol 2006;24:5552–64.
18. National Cancer Institute. Bladder and other urothelial cancers screening (PDQ). January 23, 2012. Accessed 14 December 2012 at www.cancer.gov/cancertopics/pdq/screening/bladder/HealthProfessional.
19. Farrow GM, Utz DC, Rife CC, Greene LF. Clinical observations on sixty-nine cases of in situ carcinoma of the urinary bladder. Cancer Res 1977;37:2794–8.
20. Davis R, Jones J, Barocas DA, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol 2012;188(6 Suppl):2473–81.
21. Silverman SG, Leyendecker JR, Amis ES Jr. What is the current role of CT urography and MR urography in the evaluation of the urinary tract? Radiology 2009;250:309–23.
22. Sadow CA, Silverman SG, O’Leary MP, Signorovitch JE. Bladder cancer detection with CT urography in an academic medical center. Radiology 2008;249:195–202.
23. Murphy WM, Soloway MS, Jukkola AF, et al. Urinary cytology and bladder cancer. The cellular features of transitional cell neoplasms. Cancer 1984;53:1555–65.
24. Halachmi S, Linn JF, Amiel GE, et al. Urine cytology, tumour markers and bladder cancer. Br J Urol 1998;82:647–54.
25. Koss LG, Deitch D, Ramanathan R, Sherman AB. Diagnostic value of cytology of voided urine. Acta Cytol 1985;29:810–6.
26. Yafi FA, Brimo F, Auger M, et al. Is the performance of urinary cytology as high as reported historically? A contemporary analysis in the detection and surveillance of bladder cancer. Urol Oncol 11 Feb 2013. [Epub ahead of print]
27. van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol 2005;47:736–48.
28. Vrooman OPJ, Witjes JA. Urinary markers in bladder cancer. Eur Urol 2008;53:909–16.
29. Toma MI, Friedrich MG, Hautmann SH, et al. Comparison of the ImmunoCyt test and urinary cytology with other urine tests in the detection and surveillance of bladder cancer. World J Urol 2004;22:145–9.
30. Jones JS. DNA–based molecular cytology for bladder cancer surveillance. Urology 2006;67(3 Suppl 1):35–45.
31. Glas AS, Roos D, Deutekom M, et al. Tumor markers in the diagnosis of primary bladder cancer. A systematic review. J Urol 2003;169:1975–82.
32. Sharma S, Zippe CD, Pandrangi L, et al. Exclusion criteria enhance the specificity and positive predictive value of NMP22 and BTA stat. J Urol 1999;162:53–7.
33. Fradet Y, Grossman HB, Gomella L, et al. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. J Urol 2007;178:68–73.
34. Grossman HB, Gomella L, Fradet Y, et al. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J Urol 2007;178:62–7.
35. Schmidbauer J, Witjes F, Schmeller N, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol 2004;171:135–8.
36. Denzinger S, Burger M, Walter B, et al. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology 2007;69:675–9.
37. Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol 2010;184:
1907–14.
38. Cauberg EC, Mamoulakis C, de la Rosette JJ, de Reijke TM. Narrow band imaging-assisted transurethral resection for non-muscle invasive bladder cancer significantly reduces residual tumour rate. World J Urol 2011;29:503–9.
39. Cauberg EC, Kloen S, Visser M, et al. Narrow band imaging cystoscopy improves the detection of non–muscle-invasive bladder cancer. Urology 2010;76:658–63.
40. Herr HW, Donat SM. Reduced bladder tumour recurrence rate associated with narrow-band imaging surveillance cystoscopy. Br J Urol Intl 211;107:396–8.
41. Zaak D, Karl A, Knüchel R, et al. Diagnosis of urothelial carcinoma of the bladder using fluorescence endoscopy. Br J Urol Intl 2005;96:217–22.
42. Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007;178:2314–30.
43. Herr HW. The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol 1999;162:74–6.
44. Hollenbeck BK, Miller DC, Taub D, et al. Risk factors for adverse outcomes after transurethral resection of bladder tumors. Cancer 2006;106:1527–35.
45. Nieder AM, Meinbach DS, Kim SS, Soloway MS. Transurethral bladder tumor resection: intraoperative and postoperative complications in a residency setting. J Urol 2005;174:2307–9.
46. Traxer O, Pasqui F, Gattegno B, Pearle MS. Technique and complications of transurethral surgery for bladder tumours. Br J Urol Intl 2004;94:492–6.
47. Mydlo JH, Weinstein R, Shah S, et al. Long-term consequences from bladder perforation and/or violation in the presence of transitional cell carcinoma: results of a small series and a review of the literature. J Urol 1999;161:1128–32.
48. Frachet O, Cordier G, Henry N, et al. Bladder perforation during transurethral resection of bladder tumour: a review. Prog Urol 2007;17:1310–2.
49. Golan S, Baniel J, Lask D, et al. Transurethral resection of bladder tumour complicated by perforation requiring open surgical repair - clinical characteristics and oncological outcomes. Br J Urol Intl 2011; 107:1065–8.
50. Kihl B, Nilson AE, Pettersson S. Thigh adductor contraction during transurethral resection of bladder tumours: evaluation of inactive electrode placement and obturator nerve topography. Scand J Urol Nephrol 1981;15:121–5.
51. Del Rosso A, Pace G, Masciovecchio S, et al. Plasmakinetic bipolar versus monopolar transurethral resection of non-muscle invasive bladder cancer: a single center randomized controlled trial. Intl J Urol 2013;20:399–403.
52. Puppo P, Bertolotto F, Introini C, et al. Bipolar transurethral resection in saline (TURis): outcome and complication rates after the first 1000 cases. J Endourol 2009;23:1145–9.
53. Kitamura T, Mori Y, Ohno N, et al. Case of bladder perforation due to the obturator nerve reflex during transurethral resection (TUR) of bladder tumor using the TUR in saline (Turis) system under spinal anesthesia [in Japanese]. Masui 2010;59:386–9.
54. American Joint Committee on Cancer.: Urinary bladder. In: Edge SB, Byrd DR, Compton CC, et al, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010:497–505.
55. Elbe J, Sauter G, Epstein J, Sesterhenn I. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary and male genital organs. Lyon, France: IARC Press;2004.
56. Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, et al. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol 2000;164:680–4.
57. Böhle A, Brandau S. Immune mechanisms in bacillus Calmette-Guérin immunotherapy for superficial bladder cancer. J Urol 2003;170964–9.
58. Kawai K, Miyazaki J, Joraku A, et al. Bacillus Calmette-Guérin (BCG) immunotherapy for bladder cancer: current understanding and perspectives on engineered BCG vaccine. Cancer Sci 2013;104:22–7.
59. Takeuchi A, Dejima T, Yamada H, et al. IL-17 production by γδ T cells is important for the antitumor effect of Mycobacterium bovis bacillus Calmette-Guérin treatment against bladder cancer. Eur J Immunol 2011;41:246–51.
60. Gopal R, Lin Y, Obermajer N, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol 2012;42:364–73.
61. Suttmann H, Jacobsen M, Reiss K, et al. Mechanisms of bacillus Calmette-Guerin mediated natural killer cell activation. J Urol 2004;172:1490–5.
62. Luo Y, Knudson MJ. Mycobacterium bovis bacillus Calmette-Guérin-induced macrophage cytotoxicity against bladder cancer cells. Clin Dev Immunol 2010;2010:357591.
63. Rischmann P, Desgrandchamps F, Malavaud B, Chopin DK. BCG intravesical instillations: recommendations for side-effects management. Eur Urol 2000;37(Suppl 1):33–6.
64. Luo Y, Chen X, Downs TM, et al. IFN-α 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette-Guérin immunotherapy. J Immnuol 1999;162:2399–2405.
65. Joudi FN, Smith BJ, O’Donnell MA. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guérin plus interferon α-2B for reducing recurrence of superficial bladder cancer. Urol Oncol 2006;24:344–8.
66. Brausi M, Witjes JA, Lamm D, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol 2011;186:2158–67.
67. Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol 2004;171:2186–90.
68. Divrik RT, Yildirim U, Zorlu F, Ozen H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J Urol 2006;175:1641–4.
69. Oddens JR, Van der Meijden AP, Sylvester R. One immediate postoperative instillation of chemotherapy in low risk Ta, T1 bladder cancer patients. Is it always safe? Eur Urol 2004;46:336–8.
70. Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163:1124–9.
71. Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 2013;63:462–72.
72. Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol 2003;169:90–5.
73. Sylvester RJ, van der Meijden AP, Witjes JA, Kurth J. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol 2005;174:86–91.
74. Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol 2009;56:247–56.
75. Jones G, Cleves A, Wilt TJ, et al. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database Syst Rev 2012;CD009294.
76. Kurth H, Denis L, Bouffioux C, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer 1995;31A:1840–6.
77. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:465–66.
78. Rodríguez Faba O, Palou J. Predictive factors for recurrence progression and cancer specific survival in high-risk bladder cancer. Curr Opin Urol 2012;22:415–20.
79. Streeper NM, Simons CM, Konety BR, et al. The significance of lymphovascular invasion in transurethral resection of bladder tumour and cystectomy specimens on the survival of patients with urothelial bladder cancer. Br J Urol Intl 2009;103:475–9.
80. Witjes JA. Prognosis of T1G3 bladder cancer: how well can we predict progression? Eur Urol 2012; 62:126–7.
81. Khochikar M. Early vs delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: delay of cystectomy reduces cancer-specific survival. Br J Urol Intl 2011;108(Pt 2):E288–9.
82. De Berardinis E, Busetto GM, Antonini G, et al. T1G3 high-risk NMIBC (non-muscle invasive bladder cancer): conservative treatment versus immediate cystectomy. Intl Urol Nephrol 2011;43:1047–57.
83. Badalato GM, Gaya JM, Hruby G, et al. Immediate radical cystectomy vs conservative management for high grade cT1 bladder cancer: is there a survival difference? Br J Urol Intl 2012;110:1471–7.
84. Sternberg IA, Keren Paz GE, Chen LY, et al. Role of immediate radical cystectomy in the treatment of patients with residual T1 bladder cancer on restaging transurethral resection. BJU Intl 2012;112:54–9.
85. Canter D, Egleston B, Wong YN, et al. Use of radical cystectomy as initial therapy for the treatment of high-grade T1 urothelial carcinoma of the bladder: A SEER database analysis. Urol Oncol 2013;31:866–70.
86. Hautmann RE, Volkmer BG, Gust K. Quantification of the survival benefit of early versus deferred cystectomy in high-risk non-muscle invasive bladder cancer (T1 G3). World J Urol 2009;27:347–51.
87. Heney NM, Nocks BN, Daly JJ, et al. Ta and T1 Bladder cancer: location, recurrence and progression. Br J Urol 2008;54:152–7.
88. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN Guidelines): Bladder cancer. Jenkintown (PA): NCCN; 2012.
89. Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1 and Tis): a 2007 update. J Urol 2007;178:2314–30.
90. van den Bosch S, Witjes JA. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol 2011;60:493–500.
91. Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol 2001;166:1296–9.
92. Jäger W, Thomas C, Haag S, et al. Early vs delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: delay of cystectomy reduces cancer-specific survival. BJU Int; 2011;108(Pt 2):E284–8.
93. Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol Oncol 2012 May 9. [Epub ahead of print]
94. Yates DR, Rouprêt M. Contemporary management of patients with high-risk non-muscle-invasive bladder cancer who fail intravesical BCG therapy. World J Urol 2011;29:415–22.
1. American Cancer Society. Cancer facts & figures 2012. Accessed 2 May 2013 at www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012.
2. National Cancer Institute. SEER Stat Fact Sheets: Bladder. Accessed 14 December 2012 at seer.cancer.gov/statfacts/html/urinb.html.
3. Lynch CF, Davila JA, Platz CE. Cancer of the urinary bladder. In: Ries LAG, Young JL, Keel GE, et al, editors. SEER survival monograph: cancer survival among adults: US SEER program 1988-2001, patient and tumor characteristics. NIH Pub. No. 07-6215. Bethesda (MD): National Cancer Institute; 2007:181–92.
4. Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer 2000;86:289–94.
5. Castelao JE, Yuan JM, Skipper PL, et al. Gender and smoking-related bladder cancer risk. J Natl Cancer Inst 2001;93:538–45.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. World Health Organization International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, volume 83. Tobacco smoke and involuntary smoking. Lyon, France: World Health Organization; 2004. Accessed 2 May 2013 at http://monographs.iarc.fr/ENG/Monographs/vol83/index.php.
8. Chen CH, Shun CT, Huang KH, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer, BJU Int 2007;100:281–6.
9. Murta-Nascimento C, Schmitz-Dräger BJ, Zeegers MP, et al. Epidemiology of urinary bladder cancer: from tumor development to patient’s death. World J Urology 2007;25:285–95.
10. Piper JM, Tonascia J, Matanoski GM. Heavy phenacetin use and bladder cancer in women aged 20 to 49 years. N Engl J Med 1985;313:292–5.
11. Knight A, Askling J, Granath F, et al. Urinary bladder cancer in Wegener’s granulomatosis: risks and relation to cyclophosphamide. Ann Rheum Dis 2004;63:1307–11.
12. Fairchild WV, Spence CR, Solomon HD, Gangai MP. The incidence of bladder cancer after cyclophosphamide therapy. J Urology 1979; 122:163.
13. Brock N. The development of mesna for the inhibition of urotoxic side effects of cyclophosphamide, ifosfamide, and other oxazaphosphorine cytostatics. Recent Results Cancer Res 1980;74:270–8.
14. Spruck CH, Ohneseit PF, Gonzalez-Zulueta M, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res 1994;54:784–8.
15. Bakkar AA, Wallerand H, Radvanyi F, et al. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer Res 2003;63:8108–12.
16. Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med 1994;331:1259–64.
17. Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol 2006;24:5552–64.
18. National Cancer Institute. Bladder and other urothelial cancers screening (PDQ). January 23, 2012. Accessed 14 December 2012 at www.cancer.gov/cancertopics/pdq/screening/bladder/HealthProfessional.
19. Farrow GM, Utz DC, Rife CC, Greene LF. Clinical observations on sixty-nine cases of in situ carcinoma of the urinary bladder. Cancer Res 1977;37:2794–8.
20. Davis R, Jones J, Barocas DA, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol 2012;188(6 Suppl):2473–81.
21. Silverman SG, Leyendecker JR, Amis ES Jr. What is the current role of CT urography and MR urography in the evaluation of the urinary tract? Radiology 2009;250:309–23.
22. Sadow CA, Silverman SG, O’Leary MP, Signorovitch JE. Bladder cancer detection with CT urography in an academic medical center. Radiology 2008;249:195–202.
23. Murphy WM, Soloway MS, Jukkola AF, et al. Urinary cytology and bladder cancer. The cellular features of transitional cell neoplasms. Cancer 1984;53:1555–65.
24. Halachmi S, Linn JF, Amiel GE, et al. Urine cytology, tumour markers and bladder cancer. Br J Urol 1998;82:647–54.
25. Koss LG, Deitch D, Ramanathan R, Sherman AB. Diagnostic value of cytology of voided urine. Acta Cytol 1985;29:810–6.
26. Yafi FA, Brimo F, Auger M, et al. Is the performance of urinary cytology as high as reported historically? A contemporary analysis in the detection and surveillance of bladder cancer. Urol Oncol 11 Feb 2013. [Epub ahead of print]
27. van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol 2005;47:736–48.
28. Vrooman OPJ, Witjes JA. Urinary markers in bladder cancer. Eur Urol 2008;53:909–16.
29. Toma MI, Friedrich MG, Hautmann SH, et al. Comparison of the ImmunoCyt test and urinary cytology with other urine tests in the detection and surveillance of bladder cancer. World J Urol 2004;22:145–9.
30. Jones JS. DNA–based molecular cytology for bladder cancer surveillance. Urology 2006;67(3 Suppl 1):35–45.
31. Glas AS, Roos D, Deutekom M, et al. Tumor markers in the diagnosis of primary bladder cancer. A systematic review. J Urol 2003;169:1975–82.
32. Sharma S, Zippe CD, Pandrangi L, et al. Exclusion criteria enhance the specificity and positive predictive value of NMP22 and BTA stat. J Urol 1999;162:53–7.
33. Fradet Y, Grossman HB, Gomella L, et al. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. J Urol 2007;178:68–73.
34. Grossman HB, Gomella L, Fradet Y, et al. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J Urol 2007;178:62–7.
35. Schmidbauer J, Witjes F, Schmeller N, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol 2004;171:135–8.
36. Denzinger S, Burger M, Walter B, et al. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology 2007;69:675–9.
37. Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol 2010;184:
1907–14.
38. Cauberg EC, Mamoulakis C, de la Rosette JJ, de Reijke TM. Narrow band imaging-assisted transurethral resection for non-muscle invasive bladder cancer significantly reduces residual tumour rate. World J Urol 2011;29:503–9.
39. Cauberg EC, Kloen S, Visser M, et al. Narrow band imaging cystoscopy improves the detection of non–muscle-invasive bladder cancer. Urology 2010;76:658–63.
40. Herr HW, Donat SM. Reduced bladder tumour recurrence rate associated with narrow-band imaging surveillance cystoscopy. Br J Urol Intl 211;107:396–8.
41. Zaak D, Karl A, Knüchel R, et al. Diagnosis of urothelial carcinoma of the bladder using fluorescence endoscopy. Br J Urol Intl 2005;96:217–22.
42. Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007;178:2314–30.
43. Herr HW. The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol 1999;162:74–6.
44. Hollenbeck BK, Miller DC, Taub D, et al. Risk factors for adverse outcomes after transurethral resection of bladder tumors. Cancer 2006;106:1527–35.
45. Nieder AM, Meinbach DS, Kim SS, Soloway MS. Transurethral bladder tumor resection: intraoperative and postoperative complications in a residency setting. J Urol 2005;174:2307–9.
46. Traxer O, Pasqui F, Gattegno B, Pearle MS. Technique and complications of transurethral surgery for bladder tumours. Br J Urol Intl 2004;94:492–6.
47. Mydlo JH, Weinstein R, Shah S, et al. Long-term consequences from bladder perforation and/or violation in the presence of transitional cell carcinoma: results of a small series and a review of the literature. J Urol 1999;161:1128–32.
48. Frachet O, Cordier G, Henry N, et al. Bladder perforation during transurethral resection of bladder tumour: a review. Prog Urol 2007;17:1310–2.
49. Golan S, Baniel J, Lask D, et al. Transurethral resection of bladder tumour complicated by perforation requiring open surgical repair - clinical characteristics and oncological outcomes. Br J Urol Intl 2011; 107:1065–8.
50. Kihl B, Nilson AE, Pettersson S. Thigh adductor contraction during transurethral resection of bladder tumours: evaluation of inactive electrode placement and obturator nerve topography. Scand J Urol Nephrol 1981;15:121–5.
51. Del Rosso A, Pace G, Masciovecchio S, et al. Plasmakinetic bipolar versus monopolar transurethral resection of non-muscle invasive bladder cancer: a single center randomized controlled trial. Intl J Urol 2013;20:399–403.
52. Puppo P, Bertolotto F, Introini C, et al. Bipolar transurethral resection in saline (TURis): outcome and complication rates after the first 1000 cases. J Endourol 2009;23:1145–9.
53. Kitamura T, Mori Y, Ohno N, et al. Case of bladder perforation due to the obturator nerve reflex during transurethral resection (TUR) of bladder tumor using the TUR in saline (Turis) system under spinal anesthesia [in Japanese]. Masui 2010;59:386–9.
54. American Joint Committee on Cancer.: Urinary bladder. In: Edge SB, Byrd DR, Compton CC, et al, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010:497–505.
55. Elbe J, Sauter G, Epstein J, Sesterhenn I. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary and male genital organs. Lyon, France: IARC Press;2004.
56. Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, et al. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol 2000;164:680–4.
57. Böhle A, Brandau S. Immune mechanisms in bacillus Calmette-Guérin immunotherapy for superficial bladder cancer. J Urol 2003;170964–9.
58. Kawai K, Miyazaki J, Joraku A, et al. Bacillus Calmette-Guérin (BCG) immunotherapy for bladder cancer: current understanding and perspectives on engineered BCG vaccine. Cancer Sci 2013;104:22–7.
59. Takeuchi A, Dejima T, Yamada H, et al. IL-17 production by γδ T cells is important for the antitumor effect of Mycobacterium bovis bacillus Calmette-Guérin treatment against bladder cancer. Eur J Immunol 2011;41:246–51.
60. Gopal R, Lin Y, Obermajer N, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol 2012;42:364–73.
61. Suttmann H, Jacobsen M, Reiss K, et al. Mechanisms of bacillus Calmette-Guerin mediated natural killer cell activation. J Urol 2004;172:1490–5.
62. Luo Y, Knudson MJ. Mycobacterium bovis bacillus Calmette-Guérin-induced macrophage cytotoxicity against bladder cancer cells. Clin Dev Immunol 2010;2010:357591.
63. Rischmann P, Desgrandchamps F, Malavaud B, Chopin DK. BCG intravesical instillations: recommendations for side-effects management. Eur Urol 2000;37(Suppl 1):33–6.
64. Luo Y, Chen X, Downs TM, et al. IFN-α 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette-Guérin immunotherapy. J Immnuol 1999;162:2399–2405.
65. Joudi FN, Smith BJ, O’Donnell MA. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guérin plus interferon α-2B for reducing recurrence of superficial bladder cancer. Urol Oncol 2006;24:344–8.
66. Brausi M, Witjes JA, Lamm D, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol 2011;186:2158–67.
67. Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol 2004;171:2186–90.
68. Divrik RT, Yildirim U, Zorlu F, Ozen H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J Urol 2006;175:1641–4.
69. Oddens JR, Van der Meijden AP, Sylvester R. One immediate postoperative instillation of chemotherapy in low risk Ta, T1 bladder cancer patients. Is it always safe? Eur Urol 2004;46:336–8.
70. Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163:1124–9.
71. Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 2013;63:462–72.
72. Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol 2003;169:90–5.
73. Sylvester RJ, van der Meijden AP, Witjes JA, Kurth J. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol 2005;174:86–91.
74. Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol 2009;56:247–56.
75. Jones G, Cleves A, Wilt TJ, et al. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database Syst Rev 2012;CD009294.
76. Kurth H, Denis L, Bouffioux C, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer 1995;31A:1840–6.
77. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:465–66.
78. Rodríguez Faba O, Palou J. Predictive factors for recurrence progression and cancer specific survival in high-risk bladder cancer. Curr Opin Urol 2012;22:415–20.
79. Streeper NM, Simons CM, Konety BR, et al. The significance of lymphovascular invasion in transurethral resection of bladder tumour and cystectomy specimens on the survival of patients with urothelial bladder cancer. Br J Urol Intl 2009;103:475–9.
80. Witjes JA. Prognosis of T1G3 bladder cancer: how well can we predict progression? Eur Urol 2012; 62:126–7.
81. Khochikar M. Early vs delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: delay of cystectomy reduces cancer-specific survival. Br J Urol Intl 2011;108(Pt 2):E288–9.
82. De Berardinis E, Busetto GM, Antonini G, et al. T1G3 high-risk NMIBC (non-muscle invasive bladder cancer): conservative treatment versus immediate cystectomy. Intl Urol Nephrol 2011;43:1047–57.
83. Badalato GM, Gaya JM, Hruby G, et al. Immediate radical cystectomy vs conservative management for high grade cT1 bladder cancer: is there a survival difference? Br J Urol Intl 2012;110:1471–7.
84. Sternberg IA, Keren Paz GE, Chen LY, et al. Role of immediate radical cystectomy in the treatment of patients with residual T1 bladder cancer on restaging transurethral resection. BJU Intl 2012;112:54–9.
85. Canter D, Egleston B, Wong YN, et al. Use of radical cystectomy as initial therapy for the treatment of high-grade T1 urothelial carcinoma of the bladder: A SEER database analysis. Urol Oncol 2013;31:866–70.
86. Hautmann RE, Volkmer BG, Gust K. Quantification of the survival benefit of early versus deferred cystectomy in high-risk non-muscle invasive bladder cancer (T1 G3). World J Urol 2009;27:347–51.
87. Heney NM, Nocks BN, Daly JJ, et al. Ta and T1 Bladder cancer: location, recurrence and progression. Br J Urol 2008;54:152–7.
88. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN Guidelines): Bladder cancer. Jenkintown (PA): NCCN; 2012.
89. Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1 and Tis): a 2007 update. J Urol 2007;178:2314–30.
90. van den Bosch S, Witjes JA. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol 2011;60:493–500.
91. Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol 2001;166:1296–9.
92. Jäger W, Thomas C, Haag S, et al. Early vs delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: delay of cystectomy reduces cancer-specific survival. BJU Int; 2011;108(Pt 2):E284–8.
93. Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol Oncol 2012 May 9. [Epub ahead of print]
94. Yates DR, Rouprêt M. Contemporary management of patients with high-risk non-muscle-invasive bladder cancer who fail intravesical BCG therapy. World J Urol 2011;29:415–22.
Allergic Contact Dermatitis From Ketoconazole
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.

|
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |
|
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.

|
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |

|
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.

|
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |

|
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
- Contact allergy to topical ketoconazole is rare and its cross-reactivity with other imidazole antifungals is unpredictable.
- Patch testing to personal products often is important for detecting rare allergies.