User login
Black Salve and Bloodroot Extract in Dermatologic Conditions
Black salve is composed of various ingredients, many of which are inert; however, some black salves contain escharotics, the 2 most common are zinc chloride and bloodroot (Sanguinaria canadensis) extract. In high doses, such as those contained in most black salve products, these corrosive agents can indiscriminately damage both healthy and diseased tissue.1 Nevertheless, many black salve products currently are advertised as safe and natural methods for curing skin cancer2-4 or treating a variety of other skin conditions (eg, moles, warts, skin tags, boils, abscesses, bee stings, other minor wounds)1,5 and even nondermatologic conditions such as a sore throat.6 Despite the information and testimonials that are widely available on the Internet, black salve use has not been validated by rigorous studies. Black salve is not regulated by the US Food and Drug Administration, resulting in poor quality control and inconsistent user instructions. We report the case of application of black salve to a biopsy site of a compound nevus with moderate atypia that resulted in the formation of a dermatitis plaque with subsequent scarring and basal layer pigmentation.
Case Report
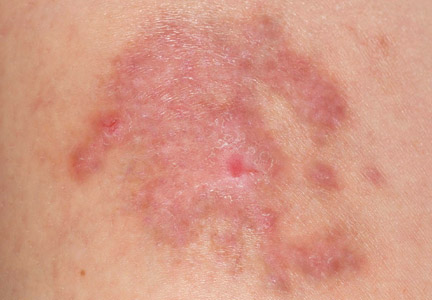
A 35-year-old woman with a family history of melanoma presented for follow-up of a compound nevus with moderate atypia on the right anterior thigh that had been biopsied 6 months prior. Complete excision of the lesion was recommended at the initial presentation but was not performed due to scheduling conflicts. The patient reported applying black salve to the biopsy site and also to the left thigh 3 months later. There was no reaction on the left thigh after one 24-hour application of black salve, but an area around the biopsy site on the right thigh became thickened and irritated with superficial erosion of the skin following 2 applications of black salve, each of 24 hours’ duration. Physical examination revealed a granulomatous plaque at the biopsy site that was approximately 5 cm in diameter (Figure 1A). One year later the lesion had completely healed (Figure 1B) and a biopsy revealed scarring with basal layer pigmentation (Figure 2).
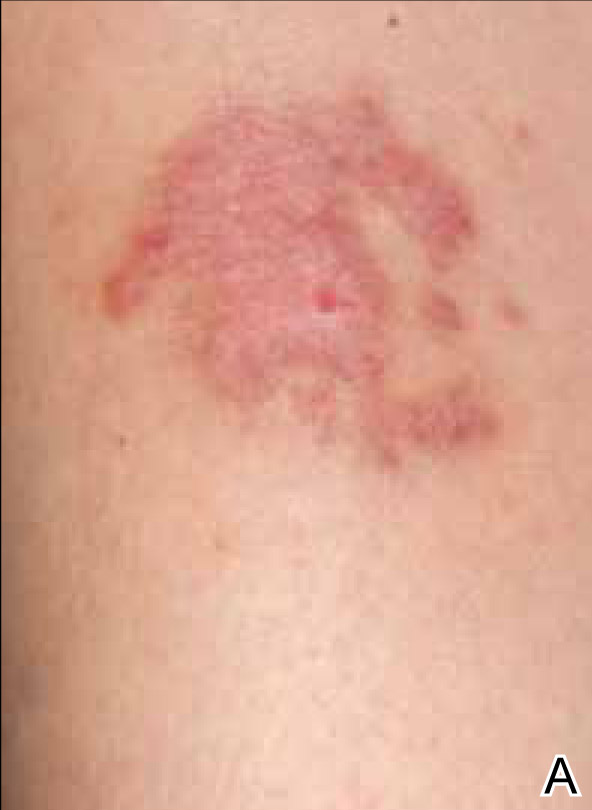 | 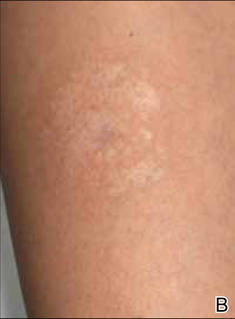 | 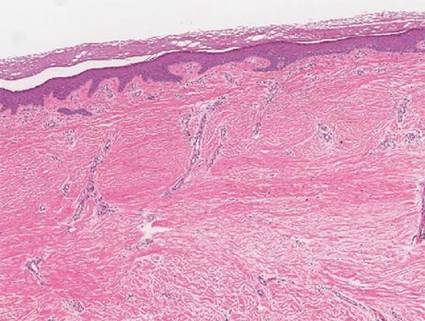 | |||
| Figure 1. A 5-cm granulomatous reaction surrounding a biopsy site on the right anterior thigh 3 months after application of black salve (A). One year later, the lesion had completely healed (B). | Figure 2. A biopsy one year following application of black salve demonstrated scarring with basal layer pigmentation (H&E, original magnification ×4). | ||||
Comment
A Web search using the term black salve yields a large number of products labeled as skin cancer salves, many showing glowing reviews and some being sold by major US retailers. The ingredients in black salves often vary in the innocuous substances they contain, but most products include the escharotics zinc chloride and bloodroot extract, which is derived from the plant S canadensis.1,3 For example, the ingredients of one popular black salve product include zinc chloride, chaparral (active ingredient is nordihydroguaiaretic acid), graviola leaf extract, oleander leaf extract, bloodroot extract, and glycerine,7 while another product includes bloodroot extract, zinc chloride, chaparral, cayenne pepper, red clover, birch bark, dimethyl sulfoxide, and burdock root.4
Bloodroot extract’s antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory effects derive from its benzylisoquinoline alkaloids including sanguinarine, allocryptopine, berberine, coptisine, protopine, and stylopine.3,8 Bloodroot extract possesses some degree of tumoricidal potency, with one study finding that it selectively targets cancer cells.9 However, this differential response is seen only at low doses and not at the high concentrations contained in most black salve products.1 According to fluorometric assays, sanguinarine is not selective for tumor cells and therefore damages healthy tissue in addition to the unwanted lesions.6,10,11 The US Food and Drug Administration includes black salve products on its list of fake cancer cures that consumers should avoid.12 Reports of extensive damage from black salve use include skin ulceration2,10 and complete loss of a naris1 and nasal ala.5 Our case suggests the possible association between black salve use and an irritant reaction and erosion of the skin.
Furthermore, reliance on black salve alone in the treatment of skin cancer poses the threat of recurrence or metastasis of cancer because there is no way to know if the salve completely removed the cancer without a biopsy. Self-treatment can delay more effective therapy and may require further treatments.
Black salve should be subject to standarddrug regulations and its use discouraged by dermatologists due to the associated harmful effects and the availability of safer treatments. To better treat and inform their patients, dermatologists should be aware that patients may be attracted to alternative treatments such as black salves.
1. Eastman KL, McFarland LV, Raugi GJ. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289.
2. Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:e154-e155.
3. Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80.
4. McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
5. Payne CE. ‘Black Salve’ and melanomas [published online ahead of print August 11, 2010]. J Plast Reconstr Aesthet Surg. 2011;64:422.
6. Cienki JJ, Zaret L. An Internet misadventure: bloodroot salve toxicity. J Altern Complement Med. 2010;16:1125-1127.
7. Cansema and escharotics. Alpha Omega Labs Web site. http://www.altcancer.com/faqcan.htm. Accessed May 6, 2015.
8. Vlachojannis C, Magora F, Chrubasik S. Rise and fall of oral health products with Canadian bloodroot extract. Phytother Res. 2012;26:1423-1426.
9. Ahmad N, Gupta S, Husain MM, et al. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6:1524-1528.
10. Saltzberg F, Barron G, Fenske N. Deforming self-treatment with herbal “black salve.” Dermatol Surg. 2009;35:1152-1154.
11. Debiton E, Madelmont JC, Legault J, et al. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol. 2003;51:474-482.
12. 187 fake cancer “cures” consumers should avoid. US Food and Drug Administration Web site. http://www.fda.gov/Drugs/GuidanceCompliance RegulatoryInformation/EnforcementActivitiesbyFDA/ucm171057.htm. Updated July 9, 2009. Accessed May 6, 2015.
Black salve is composed of various ingredients, many of which are inert; however, some black salves contain escharotics, the 2 most common are zinc chloride and bloodroot (Sanguinaria canadensis) extract. In high doses, such as those contained in most black salve products, these corrosive agents can indiscriminately damage both healthy and diseased tissue.1 Nevertheless, many black salve products currently are advertised as safe and natural methods for curing skin cancer2-4 or treating a variety of other skin conditions (eg, moles, warts, skin tags, boils, abscesses, bee stings, other minor wounds)1,5 and even nondermatologic conditions such as a sore throat.6 Despite the information and testimonials that are widely available on the Internet, black salve use has not been validated by rigorous studies. Black salve is not regulated by the US Food and Drug Administration, resulting in poor quality control and inconsistent user instructions. We report the case of application of black salve to a biopsy site of a compound nevus with moderate atypia that resulted in the formation of a dermatitis plaque with subsequent scarring and basal layer pigmentation.
Case Report
A 35-year-old woman with a family history of melanoma presented for follow-up of a compound nevus with moderate atypia on the right anterior thigh that had been biopsied 6 months prior. Complete excision of the lesion was recommended at the initial presentation but was not performed due to scheduling conflicts. The patient reported applying black salve to the biopsy site and also to the left thigh 3 months later. There was no reaction on the left thigh after one 24-hour application of black salve, but an area around the biopsy site on the right thigh became thickened and irritated with superficial erosion of the skin following 2 applications of black salve, each of 24 hours’ duration. Physical examination revealed a granulomatous plaque at the biopsy site that was approximately 5 cm in diameter (Figure 1A). One year later the lesion had completely healed (Figure 1B) and a biopsy revealed scarring with basal layer pigmentation (Figure 2).
 |  |  | |||
| Figure 1. A 5-cm granulomatous reaction surrounding a biopsy site on the right anterior thigh 3 months after application of black salve (A). One year later, the lesion had completely healed (B). | Figure 2. A biopsy one year following application of black salve demonstrated scarring with basal layer pigmentation (H&E, original magnification ×4). | ||||
Comment
A Web search using the term black salve yields a large number of products labeled as skin cancer salves, many showing glowing reviews and some being sold by major US retailers. The ingredients in black salves often vary in the innocuous substances they contain, but most products include the escharotics zinc chloride and bloodroot extract, which is derived from the plant S canadensis.1,3 For example, the ingredients of one popular black salve product include zinc chloride, chaparral (active ingredient is nordihydroguaiaretic acid), graviola leaf extract, oleander leaf extract, bloodroot extract, and glycerine,7 while another product includes bloodroot extract, zinc chloride, chaparral, cayenne pepper, red clover, birch bark, dimethyl sulfoxide, and burdock root.4
Bloodroot extract’s antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory effects derive from its benzylisoquinoline alkaloids including sanguinarine, allocryptopine, berberine, coptisine, protopine, and stylopine.3,8 Bloodroot extract possesses some degree of tumoricidal potency, with one study finding that it selectively targets cancer cells.9 However, this differential response is seen only at low doses and not at the high concentrations contained in most black salve products.1 According to fluorometric assays, sanguinarine is not selective for tumor cells and therefore damages healthy tissue in addition to the unwanted lesions.6,10,11 The US Food and Drug Administration includes black salve products on its list of fake cancer cures that consumers should avoid.12 Reports of extensive damage from black salve use include skin ulceration2,10 and complete loss of a naris1 and nasal ala.5 Our case suggests the possible association between black salve use and an irritant reaction and erosion of the skin.
Furthermore, reliance on black salve alone in the treatment of skin cancer poses the threat of recurrence or metastasis of cancer because there is no way to know if the salve completely removed the cancer without a biopsy. Self-treatment can delay more effective therapy and may require further treatments.
Black salve should be subject to standarddrug regulations and its use discouraged by dermatologists due to the associated harmful effects and the availability of safer treatments. To better treat and inform their patients, dermatologists should be aware that patients may be attracted to alternative treatments such as black salves.
Black salve is composed of various ingredients, many of which are inert; however, some black salves contain escharotics, the 2 most common are zinc chloride and bloodroot (Sanguinaria canadensis) extract. In high doses, such as those contained in most black salve products, these corrosive agents can indiscriminately damage both healthy and diseased tissue.1 Nevertheless, many black salve products currently are advertised as safe and natural methods for curing skin cancer2-4 or treating a variety of other skin conditions (eg, moles, warts, skin tags, boils, abscesses, bee stings, other minor wounds)1,5 and even nondermatologic conditions such as a sore throat.6 Despite the information and testimonials that are widely available on the Internet, black salve use has not been validated by rigorous studies. Black salve is not regulated by the US Food and Drug Administration, resulting in poor quality control and inconsistent user instructions. We report the case of application of black salve to a biopsy site of a compound nevus with moderate atypia that resulted in the formation of a dermatitis plaque with subsequent scarring and basal layer pigmentation.
Case Report
A 35-year-old woman with a family history of melanoma presented for follow-up of a compound nevus with moderate atypia on the right anterior thigh that had been biopsied 6 months prior. Complete excision of the lesion was recommended at the initial presentation but was not performed due to scheduling conflicts. The patient reported applying black salve to the biopsy site and also to the left thigh 3 months later. There was no reaction on the left thigh after one 24-hour application of black salve, but an area around the biopsy site on the right thigh became thickened and irritated with superficial erosion of the skin following 2 applications of black salve, each of 24 hours’ duration. Physical examination revealed a granulomatous plaque at the biopsy site that was approximately 5 cm in diameter (Figure 1A). One year later the lesion had completely healed (Figure 1B) and a biopsy revealed scarring with basal layer pigmentation (Figure 2).
 |  |  | |||
| Figure 1. A 5-cm granulomatous reaction surrounding a biopsy site on the right anterior thigh 3 months after application of black salve (A). One year later, the lesion had completely healed (B). | Figure 2. A biopsy one year following application of black salve demonstrated scarring with basal layer pigmentation (H&E, original magnification ×4). | ||||
Comment
A Web search using the term black salve yields a large number of products labeled as skin cancer salves, many showing glowing reviews and some being sold by major US retailers. The ingredients in black salves often vary in the innocuous substances they contain, but most products include the escharotics zinc chloride and bloodroot extract, which is derived from the plant S canadensis.1,3 For example, the ingredients of one popular black salve product include zinc chloride, chaparral (active ingredient is nordihydroguaiaretic acid), graviola leaf extract, oleander leaf extract, bloodroot extract, and glycerine,7 while another product includes bloodroot extract, zinc chloride, chaparral, cayenne pepper, red clover, birch bark, dimethyl sulfoxide, and burdock root.4
Bloodroot extract’s antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory effects derive from its benzylisoquinoline alkaloids including sanguinarine, allocryptopine, berberine, coptisine, protopine, and stylopine.3,8 Bloodroot extract possesses some degree of tumoricidal potency, with one study finding that it selectively targets cancer cells.9 However, this differential response is seen only at low doses and not at the high concentrations contained in most black salve products.1 According to fluorometric assays, sanguinarine is not selective for tumor cells and therefore damages healthy tissue in addition to the unwanted lesions.6,10,11 The US Food and Drug Administration includes black salve products on its list of fake cancer cures that consumers should avoid.12 Reports of extensive damage from black salve use include skin ulceration2,10 and complete loss of a naris1 and nasal ala.5 Our case suggests the possible association between black salve use and an irritant reaction and erosion of the skin.
Furthermore, reliance on black salve alone in the treatment of skin cancer poses the threat of recurrence or metastasis of cancer because there is no way to know if the salve completely removed the cancer without a biopsy. Self-treatment can delay more effective therapy and may require further treatments.
Black salve should be subject to standarddrug regulations and its use discouraged by dermatologists due to the associated harmful effects and the availability of safer treatments. To better treat and inform their patients, dermatologists should be aware that patients may be attracted to alternative treatments such as black salves.
1. Eastman KL, McFarland LV, Raugi GJ. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289.
2. Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:e154-e155.
3. Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80.
4. McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
5. Payne CE. ‘Black Salve’ and melanomas [published online ahead of print August 11, 2010]. J Plast Reconstr Aesthet Surg. 2011;64:422.
6. Cienki JJ, Zaret L. An Internet misadventure: bloodroot salve toxicity. J Altern Complement Med. 2010;16:1125-1127.
7. Cansema and escharotics. Alpha Omega Labs Web site. http://www.altcancer.com/faqcan.htm. Accessed May 6, 2015.
8. Vlachojannis C, Magora F, Chrubasik S. Rise and fall of oral health products with Canadian bloodroot extract. Phytother Res. 2012;26:1423-1426.
9. Ahmad N, Gupta S, Husain MM, et al. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6:1524-1528.
10. Saltzberg F, Barron G, Fenske N. Deforming self-treatment with herbal “black salve.” Dermatol Surg. 2009;35:1152-1154.
11. Debiton E, Madelmont JC, Legault J, et al. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol. 2003;51:474-482.
12. 187 fake cancer “cures” consumers should avoid. US Food and Drug Administration Web site. http://www.fda.gov/Drugs/GuidanceCompliance RegulatoryInformation/EnforcementActivitiesbyFDA/ucm171057.htm. Updated July 9, 2009. Accessed May 6, 2015.
1. Eastman KL, McFarland LV, Raugi GJ. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289.
2. Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:e154-e155.
3. Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80.
4. McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
5. Payne CE. ‘Black Salve’ and melanomas [published online ahead of print August 11, 2010]. J Plast Reconstr Aesthet Surg. 2011;64:422.
6. Cienki JJ, Zaret L. An Internet misadventure: bloodroot salve toxicity. J Altern Complement Med. 2010;16:1125-1127.
7. Cansema and escharotics. Alpha Omega Labs Web site. http://www.altcancer.com/faqcan.htm. Accessed May 6, 2015.
8. Vlachojannis C, Magora F, Chrubasik S. Rise and fall of oral health products with Canadian bloodroot extract. Phytother Res. 2012;26:1423-1426.
9. Ahmad N, Gupta S, Husain MM, et al. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6:1524-1528.
10. Saltzberg F, Barron G, Fenske N. Deforming self-treatment with herbal “black salve.” Dermatol Surg. 2009;35:1152-1154.
11. Debiton E, Madelmont JC, Legault J, et al. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol. 2003;51:474-482.
12. 187 fake cancer “cures” consumers should avoid. US Food and Drug Administration Web site. http://www.fda.gov/Drugs/GuidanceCompliance RegulatoryInformation/EnforcementActivitiesbyFDA/ucm171057.htm. Updated July 9, 2009. Accessed May 6, 2015.
Practice Points
- Clinicians should be aware that black salve containing bloodroot extract is a popular alternative treatment used to cure a variety of skin ailments.
- Black salve containing bloodroot extract is not selective for tumor cells. Various case reports have shown that black salve can result in extensive tissue damage and recurrence or metastasis of skin cancer.
- Damage to healthy tissue can occur with as few as 2 applications of black salve.
Diagnosis and Management of Complex Pelvic Floor Disorders in Women
From Beaumont Health System, Royal Oak, MI.
Abstract
- Objective: To review the evaluation and management of complex pelvic floor disorders in elderly women.
- Methods: Literature review and presentation of a clinical case.
- Results: Pelvic floor disorders are a common problem in elderly women. Pelvic organ prolapse and voiding complaints often coexist and several treatment options are available. A step-wise approach should be used in which management of the most bothersome symptoms occurs first. Conservative, medication, and surgical options should be discussed with each patient depending on treatment goals and health status. Some effects do overlap; however, treatment of one condition may not preclude treatment of other symptoms.
- Conclusion: In women with complex pelvic floor disorders, addressing the most bothersome symptom first will increase patient satisfaction. Patients should be counseled about the potential need for multiple treatments for optimal results.
The female pelvic floor consists of a complex relationship of muscles, connective tissue and fascia, ligaments, and neurovascular support. These structures are responsible for support of the pelvic organs (uterus, bladder, rectum, and vagina), maintain continence, and assist in normal bowel function. Pelvic floor disorders occur when there is a compromise in these structures, resulting in prolapse, urinary incontinence, bowel complaints, or pain. Often several symptoms coexist with overlapping pathophysiology. Examinations and studies should aim to correctly diagnose the disorders and guide treatments toward the most bothersome symptoms.
Pelvic organ prolapse occurs when there is a weakening of the pelvic floor connective tissue, muscles, and nerves, allowing a bulge or protrusion of the vaginal walls and their associated pelvic organs. Between 3% to 50% of women in the United States have some degree of pelvic organ prolapse depending on whether the definition is based on symptoms or anatomic evaluation [1–3]. Risk factors include vaginal delivery, obesity, Caucasian race, and prior prolapse surgery. Despite the non–life-threatening nature of pelvic organ prolapse, the associated social and physical restrictions can significantly impact quality of life [4]. The cost of prolapse surgery has been estimated to be over $1.4 billion per year [3].
The sensation of a vaginal bulge is the only symptom consistently related to pelvic organ prolapse, with patients typically reporting symptoms once the prolapse extends beyond the hymenal ring [5]. The diagnosis of pelvic organ prolapse is made based on symptoms and confirmed by physical exam.
Patients with pelvic organ prolapse may experience obstructive voiding symptoms, such as hesitancy, straining, or incomplete bladder emptying. In some cases, patients may have to manually reduce the bulge to be able to void, a practice known as “splinting.” Overactive bladder (OAB), a syndrome of urinary urgency, frequency, and nocturia with or without urgency incontinence, can also occur. In patients with lower urinary tract complaints, repair of a vaginal bulge, especially a cystocele, can be associated with improved voiding symptoms [6]. Additionally, prolapse treatment can unmask de novo stress urinary incontinence (SUI), leaking with cough, sneeze or other activity that increases abdominal pressure. Urinary tract infections, pelvic pain, dyspareunia and defecatory problems can also be present.
When evaluating a woman with pelvic organ prolapse and voiding complaints, the clinician should strive to illicit which symptoms bother the patient most. A patient with primarily OAB symptoms and minimal prolapse may be treated with physical therapy or medications addressing the OAB rather than reconstructive surgery. In contrast, the patient with OAB symptoms and bothersome prolapse must be counseled on possible need for additional treatment of voiding complaints following surgical repair. This may include management of persistent OAB symptoms or SUI occurring following prolapse repair. Defecatory problems may be independent of a small rectocele present on exam, especially if long-term constipation is present. Choice of treatment depends on the severity of symptoms, the degree of prolapse, and the patient’s health status and activity level.
Case Study
Initial Presentation
A 68-year-old woman with a 15-month history of urinary urgency, frequency, incontinence and vaginal pressure presents to a urologist.
History and Physical Examination
The patient’s symptoms began shortly after the death of her husband. She initially saw her internist who prescribed antibiotics for a suspected urinary tract infection (UTI) based on office urinalysis. The symptoms did not resolve so another course of antibiotics was tried, again without relief. At her 3rd visit, a urine culture was done which was negative and she was referred to a urologist.
The patient reports 3 UTIs in the last 6 months. Following antibiotic treatment, the burning improves but she still complains of urgency and frequency. She also wears 2 to 3 pads per day for leakage that occurs with coughing and also when she feels an urge but cannot make it to the bathroom. She wakes 1 to 3 times at night to void. She feels that she empties her bladder well but often has to strain to void and sometimes feels a “bulge” in her vagina. All of these symptoms increase after being on her feet all day while she works as a grocery store cashier.
Physical exam demonstrates mild suprapubic tenderness and mild atrophic vaginitis. The anterior vaginal wall protrudes to the hymen with straining and her vaginal apex is supported 5 cm above the hymenal ring. With reduction of the cystocele there was urine leakage with cough. The cervix is surgically absent and her posterior vaginal wall is without bulge on valsalva. Her catheterized post-void residual (PVR) was 105 mL. Urine dipstick analysis was negative for infection or blood.
What is the initial evaluation of a woman with pelvic organ prolapse and voiding complaints?
The initial evaluation of a woman with pelvic organ prolapse and voiding complaints consists of a detailed history and physical examination. The nature, duration, and severity of symptoms should be assessed. Complaints of vaginal pressure or bulge are important, as well as exacerbating instances (standing, straining, defecation). Local irritation or vaginal spotting is common if prolapse is beyond the hymen. Splinting or reduction of a bulge to void or defecate are important elements of the history. Sexual history should never be overlooked, including both sexual status (active or not) as well as goals for future sexual activity. Voiding symptoms such as dysuria, frequency, urgency, nocturia and incontinence should be discussed. A 3-day voiding diary that captures number of voids per day, voided volumes, and fluid intake can be obtained. If incontinence is present, the clinician should determine what causes the incontinence. Incontinence that is associated with urgency or no warning (urge incontinence) should be treated differently than incontinence associated with activity (SUI). Mixed urinary incontinence is the presence of both stress and urgency incontinence.
Past medical history should include common medical comorbidities such as diabetes, hypertension and cardiovascular disease. Obstetric history is important due to the increased risk for pelvic floor disorders in women with multiple pregnancies and vaginal deliveries [2]. Prior hysterectomy, colon resection, or other pelvic surgeries may also contribute to symptoms. Smokers have a greater risk of genitourinary malignancy and high caffeine consumption is implicated in urgency-frequency syndromes. Exercise, sleep, and work may also be affected.
Pelvic examination should evaluate for vaginal atrophy or other vaginal mucosal abnormalities such as tears, ulcerations, lichen sclerosis, or erythema. To evaluate for prolapse, using one-half of a Graves or Pederson speculum, examine the 3 compartments of the vagina: anterior, posterior and apical. To view the anterior wall, the speculum is placed posteriorly to retract the posterior wall downward. Next it is rotated anteriorly to retract the anterior wall up and examine the posterior compartment. The uterus or the apex is evaluated with 2 halves of the speculum, one pushing anteriorly and the other posteriorly. At each point in the evaluation, the patient is told to strain or valsalva. The pelvic organ prolapse quantification system (POP-Q) is a systematic description of site-specific measurements of a woman’s pelvic support [7]. Using this classification system, a standardized and reproducible method of documenting the severity of the prolapse is done based on 6 points of the vaginal wall in relation to the hymen (2 on the anterior wall, 2 in the superior vagina, and 2 on the posterior vaginal wall). A corresponding prolapse stage can then be assigned to the patient based on POP-Q measurements. If unable to reproduce the patient’s symptoms, or exam findings do not correlate with the history, a standing exam can be helpful. Close evaluation of the urethra is also important. In severe prolapse the urethra may become kinked and mask a potential underlying problem (occult SUI). Patients should be asked to valsalva or cough with prolapse reduction and a full bladder to evaluate for this. Lastly, the pelvic floor muscles should be palpated to assess for pain or pelvic floor atrophy, hypertonicity, tenderness, or spasms.
If the patient complains of urgency, frequency, and/or dysuria, urine cultures should be performed to exclude infection even if the urinalysis is negative. Antibiotics should be given based on culture results. A postvoid ultrasound or catheterization is used to evaluate for incomplete bladder emptying. Patients with microscopic or gross hematuria should undergo further testing with radiologic and cystoscopic evaluation as indicated, especially with a history of smoking. Women should be questioned regarding their menstrual history and if postmenopausal, about any vaginal bleeding. A pelvic ultrasound should be considered if the patient has a history of endometriosis, gynecological cancers, uterine fibroids, or ovarian cysts or if considering uterine preserving surgery or colpocleisis. Urodynamics are often indicated in complex patients with prolapse and lower urinary tract complaints or prior pelvic surgery.
Diagnosis
The patient was diagnosed with mixed urinary incontinence and a grade 2 cystocele. Treatment options were discussed and she was most interested in conservative management options.
What is first-line treatment for the complaints of urgency, frequency, and incontinence?
In an older patient with complaints of urgency, frequency, and incontinence, dietary and behavioral modifications as well as pelvic floor physical therapy are considered first-line minimally invasive treatments.
Dietary irritants such as coffee, tea, soda, and other caffeinated beverages can contribute to worsening of symptoms [8]. A randomized study measuring the effects of caffeine noted a significant reduction in urgency and frequency of voids and in symptom scores with reduction of caffeine use [9]. Some elderly patients are reluctant to change their lifestyle, but even small changes can significantly improve their urgency symptoms.
Timed voiding is an effective method for bladder retraining, which can be critical for managing symptoms both alone and as an adjunct to other interventions. Studies of behavioral therapy show significant improvement in urgency, frequency, and incontinence episodes. In a study by Wyman and Fanti, patients participating in bladder training and Kegel exercises noted a 57% decrease in incontinence episodes and 54% decrease in urine loss without medications [10]. Burgio et al compared behavioral therapy to anticholinergic medication administration. After 4 sessions over 8 weeks they reported 81% reduction in incontinence episodes compared to 69% in the drug group and 39% in the placebo group [11].
Elderly patients may take several medications, some of which can affect urine volume and timing of urine production. Diuretics given later in the day can increase nighttime urine production and worsen nocturia. Similarly, lower extremity edema can increase nocturnal urine volumes when the patient reclines. Compressive stockings and leg elevation 2-3 hours prior to bedtime will help evenly distribute fluids and decrease reabsorption when supine at night.
Pelvic Floor Physical Therapy
Pelvic floor physical therapy (PFPT) can be an effective treatment for OAB, SUI, and pelvic organ prolapse. PFPT is used as an urge suppression strategy for OAB by teaching patients how to contract their pelvic muscles to occlude the urethra and prevent leakage during a detrusor contraction. Strategies to help suppress urge and manage stress situations can reduce incontinence episodes up to 60% to 80% [12]. Behavioral programs can include bladder diaries, scheduled voiding, delayed voiding, double voiding, fluid management, and caffeine reduction. When combined with PFPT they can be very effective in the management of OAB symptoms and incontinence. The BE-DRI study showed that combined behavioral training and drug therapy yielded better outcomes over time in OAB symptoms, patient distress and treatment satisfaction than drug therapy alone [13]. PFPT is considered a first-line treatment for OAB and is a noninvasive and effective treatment for these symptoms [14].
Pelvic floor programs for SUI aim to teach pelvic floor muscle contraction to help prevent stress leakage and use a variety of methods including biofeedback and personalized training programs. A recent Cochrane review included 18 studies of PFPT for incontinence. They concluded that there was high quality evidence that PFPT was associated with cure and moderate evidence for improvement in SUI [15]. In a study comparing surgery versus PFPT at 1 year, subjective improvement in the surgery group was 91% compared to 64% in the PFPT group. While PFPT was not as effective as surgery, over 50% had improvement. PFPT remains an effective noninvasive option that should be considered, particularly in an older patient [16].
PFPT has also been studied as a treatment option for pelvic organ prolapse. In a randomized controlled trial (RCT) that compared PFPT to controls over time, more women in the PFPT group improved 1 POP-Q stage compared to the control group. They also had significantly improved pelvic floor symptom bother [17]. In the POPPY study examining PFPT versus a control condition, researchers were not able to show statistically significant improvement in prolapse stages but did show improvement in secondary outcomes, including symptom bother and the feeling of “bulge.” Fewer women sought further treatment for prolapse after undergoing PFPT [18]. PFPT can be effective in managing prolapse symptoms and may help improve prolapse stage.
Pessary
Pessaries are commonly used for management of pelvic organ prolapse in patients who choose nonoperative management. In a large study of pessary use in the Medicare population, it was noted that of 34,782 women diagnosed with prolapse between 1999 and 2000, 11.6% were treated with a pessary. Complications noted during the 9 years of follow-up included 3% with vesicovaginal or rectovaginal fistulas and 5% with a device-associated complication [19]. Use increased with age, with 24% of women over 85 being managed with a pessary. In a review examining quality of life, improvement in bulge, irritative symptoms, and sexual satisfaction occurred with pessary use. In the medium-term, prolapse-related bother symptoms, quality of life, and overall perception of body image improved with the use of a pessary [20]. For SUI, rings with a knob or an incontinence dish can provide support to the urethra and help to pinch it closed with coughing, sneezing, and laughing, preventing leakage. In an RCT comparing women who received behavioral therapy, an incontinence pessary, or both, at 3 months 33% of those assigned to pessary reported improved incontinence symptoms compared to 49% with behavioral therapy, and 63% were satisfied with pessary treatment compared to 75% with behavioral therapy [21,22]. Differences did not persist to 12 months with over one third of all women improved and even more satisfied. A pessary can be safely used in the elderly population but does require office management and regular follow-up to prevent complications.
Initital Treatment
The patient was treated with 3 months of PFPT with biofeedback and pelvic floor muscle strengthening. In addition, she was able to decrease her caffeine use from 4 cups of coffee per day to 1 cup in the morning. At her 3-month follow-up visit, she noticed significant improvement in her voiding symptoms, and her voiding diary showed improved voided volumes and decreased frequency and nocturia. However, she was becoming more active in her community, going to aerobics and dance classes. She was more bothered by the “bulge” feeling in her vagina. She was not interested in a pessary but wanted to hear about surgical options for prolapse treatment.
What is operative management of pelvic organ prolapse?
The goals for surgical pelvic organ prolapse repair are to resolve symptoms, restore normal or near-normal anatomy, preserve sexual, urinary and bowel function, and minimize patient morbidity. The extent of prolapse, patient risk factors for recurrence, patient preference, and overall medical condition all influence the method for surgical repair. Surgeon familiarity and experience is also important when selecting the appropriate repair. Recent concerns regarding the use of synthetic mesh material has become a factor in counseling patients since the 2011 US Food and Drug Administration safety communication on transvaginal mesh [23].
Vaginal Approaches
Numerous techniques for pelvic organ prolapse repair have been described, though most repairs can broadly be divided into vaginal and abdominal procedures. Vaginal surgery is consistently associated with shorter operative times, less postoperative pain, and a shorter length of stay than abdominal approaches. All prolapsing compartments can be addressed vaginally using a patient’s own tissue, often called a “native tissue repair.” The vaginal apex is suspended from either the uterosacral ligament (USL) condensations or to the sacrospinous ligaments (SSL). Sutures are placed through these structures and tied to hold the vaginal vault in place, often at the time of concomitant enterocele, cystocele, or rectocele repairs. A recent randomized trial comparing USL and SSL repair showed composite functional and symptomatic success was 60% at 2 years and did not differ by technique [24]. While overall success may appear low, symptomatic vaginal bulge was present in only 17% to 19% of women at 2 years and only 5% underwent re-treatment with surgery or pessary during follow-up. Similar outcomes have been demonstrated for isolated cystocele repairs plicating the pubo-cervical fascia (anterior colporrhaphy). Cited failure rates have been as high as 70% [25], though this depends on the definition of success. When symptoms of bulge and/or prolapse beyond the hymen are used, success rates are closer to 89% at 2 years [26]. In one study comparing mesh-augmented cystocele repair with native tissue anterior colporrhaphy, 49% of women had a successful composite outcome at 2 years of grade 0 or 1 prolapse and no symptoms of bulge without the use of mesh graft [27]. Despite lower anatomic success rates, anterior colporrhaphy consistently relieves symptoms of bulge with low retreatment rates.
The high failure rates of native tissue vaginal repairs, especially in women with high-grade or recurrent prolapse, led to an interest in graft-augmented repairs. Furthermore, anatomic studies showed that up to 88% of cystoceles were associated with a lateral defect, or tearing of the pubocervical fascia from the pelvic sidewall (arcus tendineous fascia pelvis) [28]. Plicating the already weak fascia centrally would not repair an underlying lateral defect resulting in treatment failure. Replacing this weak fascia with a graft and anchoring it laterally and proximally should result in better anatomic and functional outcomes. These patches can be made from autologous tissue (rectus fascia), donor allograft material (fascia lata), xenografts (porcine dermis, bovine pericardium), or synthetic mesh. Initial studies using cadaveric dermis grafts for recurrent stage II or stage III/IV pelvic organ prolapse resulted in 50% failure at 4 years, but symptomatic failure was only 11% [29]. Further publications utilizing cadaveric tissue patches showed lower rates of cystocele recurrences of 0 to 17% between 20 and 56 months of follow-up [30].
As interest in patch repairs became popular, the use of synthetic mesh was applied to tension-free mid-urethral tapes for SUI. Studies were also showing rapid cadaveric and xenograft graft metabolism, graft extrusion, and early failure in some women [31]. This led to the use of larger pieces of synthetic mesh for prolapse repair, as it had been for abdominal wall and inguinal hernia repairs. Ultimately large-pore, light-weight polypropylene mesh was seen as the most favorable material and large randomized studies were performed to compare outcomes. Theoretically, a synthetic material would provide a replacement for the weakened and torn pubocervical fascia and not be subjected to enzymatic degradation. Altman et al published a widely cited randomized trial comparing native tissue vs. synthetic mesh showing that improvement in the composite primary outcome (no prolapse on the basis of both objective and subjective assessments) was more common in the mesh group (61% vs. 35%) at 1 year. Mesh placement was associated with longer operative times, higher blood loss, and 3.2% of women underwent secondary procedures for vaginal mesh exposure [27]. While there is still debate on the routine use of transvaginal mesh placement, current recommendations generally limit its use for recurrent or high grade pelvic organ prolapse (> Stage III), and possibly those at higher risks for recurrence. The American Urological Association has supported the FDA recommendation that patients undergo a thorough consent process and that surgeons are properly trained in pelvic reconstruction and mesh placement techniques. Furthermore, surgeons placing transvaginal mesh should be equipped to diagnose and treat any complications that may arise subsequent to its use.
Abdominal Approaches
Pelvic organ prolapse can also be approached through an abdominal technique. The classic description for vaginal vault prolapse repair is the abdominal sacrocolpopexy. This involves fixating the vaginal apex to the anterior longitudinal ligament at the sacral promontory. Hysterectomy is performed at the same setting if still in situ. A strip of lightweight polypropylene mesh is sutured to the anterior and posterior vaginal walls after dissecting the bladder and rectum off, then suspended in a tension-free manner to the sacrum. Large trials with long-term follow-up show durability of this repair. Seven-year follow-up of a large NIH-sponsored trial comparing sacrocolpopexy with and without urethropexy found 31/181 (17%) with anatomic prolapse beyond the hymen [32]. Of these women one-third had involvement of the vaginal apex, though 50% of women were asymptomatic. Overall, 95% of women had no retreatment for pelvic organ prolapse. A surprising finding was a 10.5% mesh exposure rate with a mean follow-up of 6.1 years. Previously, abdominally placed mesh was thought to be much safer than transvaginal mesh, but exposure rates are roughly similar in newer studies at high-volume, fellowship-trained centers [33]. The largest advance in abdominal prolapse surgery has come with the adoption of laparoscopic and robotic-assisted technology. Minimally invasive approaches to abdominal surgery have resulted in less blood loss and shorter length of stay, though longer operative times [34]. Short- and medium-term outcomes have been compared to the open techniques in smaller single-center series. At least 1 randomized trial comparing laparoscopic to robotic sacrocolpopexy showed similar complications and perioperative outcomes, though the robotic technique was more costly [35].
Stress Urinary Incontinence Procedures
When SUI is identified preoperatively, treatment should be considered at the time of prolapse repair [32,36]. The gold standard for treatment of SUI with urethral hypermobility has been placement of a synthetic mid-urethral sling. There are several types of slings available, mainly categorized as retropubic, transobturator, or single-incision “mini-slings.” In a multicenter study by the Urinary Incontinence Treatment Network (UITN), patient satisfaction after retropubic and transobturator sling placement was studied 12 months after surgery. Both groups had a high satisfaction rate (from 85% to 90%) for urine leakage, urgency, and frequency [37]. There was no significant difference in outcomes between the 2 approaches. Several other studies and systematic reviews have also shown excellent long-term results with sling treatment. In the recently published 5-year follow-up of the Trial of Mid-Urethral Slings (TOMUS), researchers demonstrated an 80% to 85% patient satisfaction rate with a 10% adverse event rate. Of these adverse events, only 6 were classified as serious requiring surgical, radiologic, or endoscopic intervention [38].
If the patient has SUI but no urethral hypermobility, consider intrinsic sphincter deficiency as the etiology of her incontinence. In that case, injectable therapy with urethral bulking agents is an effective treatment. Some commonly used injectables include carbon beads (Durasphere), calcium hydroxylapatite (Coaptite), bovine collagen (Contigen), and silicon particles (Macroplastique). In a Cochrane review of injectable therapy, they compared urethral injection to conservative treatment with physical therapy and noted an improvement with injection at 3 months. Surgical treatment was overall more effective; however, 50% of the women that received a collagen injection were satisfied at 12 months after the procedure. They also note lower morbidity for this procedure compared to surgery [39].
Treatment in This Patient
The patient underwent successful robotic sacrocolpopexy with mesh and a transobturator sling. There were no complications during the procedure and she reports no bulge or SUI symptoms. She has not been straining to void and has been emptying her bladder well since the Foley catheter was removed the day after surgery. However, she continues to complain of bothersome urgency, frequency, and urge incontinence. She is wearing 1 to 3 pads daily for leakage. At her 6-week postoperative visit, the exam showed excellent vaginal support, no SUI, low PVR, and her urine culture was negative.
What are the clinical implications of these findings?
At this point it is reasonable to continue treatment of OAB. The patient may continue to see improvement as she gets further out from surgery but especially in a patient that had preoperative OAB symptoms, treatment is indicated and may consist of reminding her of behavioral modifications, returning to pelvic floor physical therapy, or starting her on a medication.
What medications are used to treat OAB?
Anticholinergic Drugs
Anticholinergics are second-line therapy for OAB; these medications prevent the binding of acetylcholine to the M3 muscarinic receptor in the detrusor muscle and inhibit uncontrolled bladder contraction. There are numerous medications and delivery methods (pills, patches, gels) but efficacy is similar among the different drugs and all are limited by side effects such as dry mouth, constipation, and central nervous system side effects. Mirabegron, approved by the FDA in June 2012 and released in October 2012, is an agonist of the β3-adrenoceptor receptor in the detrusor muscle promoting bladder storage. A phase III trial found that mirabegron significantly decreased incontinence episodes and micturition frequency compared to placebo [40]. Dry mouth, common with anticholinergics, was 3 times less likely compared to tolterodine [41]. The most common side effects (headache, urinary tract infection, hypertension, and nasopharyngitis) were similar between treatment and placebo groups.
Long-term compliance, side effects, and decreased efficacy limit the benefits of medication therapy [42]. In one survey, 25% of patients taking OAB medications discontinued them within 12 months with 89% reporting unmet treatment expectations and/or tolerability [43].
6 Months Later
The patient continues to complain of persistent OAB symptoms despite anticholinergic and beta-3 agonist therapy. She reported significant constipation and dry mouth with an anticholinergic and symptoms did not improve with mirebegron. Despite having OAB symptoms prior to her prolapse repair, it is important to evaluate for any other cause of her persistent symptoms. Her surgical repair remains intact and urodynamics and cystoscopy were performed showing no evidence of bladder outlet obstruction and no mesh or suture material in the bladder. There was no leakage with valsalva, though she had some early sensation of fullness (sensory urge). With a negative evaluation, refractory OAB is diagnosed and the patient is a candidate for third-line OAB treatment.
What are third-line OAB treatments?
OnabotulinumtoxinA
OnabotulinumtoxinA (Botox) was approved in 2013 for patients intolerant or unresponsive to behavioral therapy and oral medications. OnabotulinumtoxinA is a chemical neuromodulator that cleaves the SNARE protein SNAP-25, inhibits the fusion of the cytoplasmic vesical to the nerve terminal, and prevents the release of acetylcholine. This causes detrusor muscle relaxation and may also inhibit sensory afferent pathways [44].
Nitti et al compared Botox 100 U to placebo in 557 patients that were refractory to anticholinergics [45]. Botox decreased the frequency of daily urinary incontinence episodes vs placebo (–2.65 vs –0.87, P < 0.001) and 22.9% vs 6.5% of patients became completely continent. A 5.4% rate of urinary retention occurred and UTI was the most common side effect (16%) in those receiving active drug. A dose of 100 U is recommended to limit side effects while maintaining efficacy [46].
Comparision of a daily anticholinergic (solifenacin) versus Botox 100 U for 6 months was done in a randomized double-blind, double-placebo-controlled trial [47]. Patients underwent saline injection or took an oral placebo in the anticholinergic and Botox groups, respectively. Complete resolution of urinary symptoms occurred in 13% of the medication group and 27% of the Botox group (P = 0.003). Dry mouth was more common in the medication group (46% vs. 31%) and the Botox group had a higher rate of catheter use and urinary tract infections (5% vs. 0%; 33% vs. 13%). Quality of life measures have also been shown to improve significantly following Botox injection [45,48].
When considering whether Botox is appropriate for a particular patient, physicians must determine whether the patient is willing and able to perform clean intermittent catheterization. Contraindications include active UTI, urinary retention, unwilling or unable to do clean intermittent catheterization, and known hypersenstivitiy to botulinum toxin type A. Although the definition of urinary retention and the PVR at which clean intermittent catheterization should be initiated varies, one study found a 94% rate of urinary retention with a preoperative PVR > 100 mL [49].
Botox can be administered in the clinic with or without local anesthetic but general anesthetic may be used in patients who might be poorly tolerant of the procedure. Using flexible or rigid cystoscopy, the bladder is filled to 100 to 200 mL. An injection needle is used to inject 0.5 cc aliquots of reconstituted onabotulinumtoxinA in 20 areas spaced 1 cm apart. Periprocedure antibiotics are recommended by the manufacturer but actual usage varies [50]. Patients should understand that the effects of Botox may take up to 4 weeks and an appointment should be scheduled within 2 weeks to evaluate PVR and any other adverse reactions. Repeat injections are needed between 3 to 9 months as symptoms return; however, efficacy is maintained with subsequent treatments [51].
Neuromodulation
Additional third-line treatment options include sacral or posterior tibial nerve neuromodulation. Sacral neuromodulation has been FDA approved for treatment of urgency, frequency and urgency incontinence since 1997. Also known as InterStim (Medtronic, Minneapolis, MN), this involves placement of a tined electrode adjacent to the S3 nerve root and is thought to result in modulation of the afferent nerve signals from the bladder to the spinal cord and the pontine micturition center.
Since the FDA approved sacral neuromodulation, long-term results for this therapy have been positive. A multicenter study with a 5-year follow-up showed a statistically significant reduction in daily leakage episodes, number of daily voids, and increase in voided volume, with a 5-year success rate of 68% for urgency incontinence and 56% for urgency/frequency [52]. Al-Zahrani et al followed 96 patients (35% with urgency incontinence) for a mean of 50.7 months and approximately 85% of the incontinent patients remained improved [53]. Conversely, Groen et al observed a gradual decrease in success rate from 1 month to 5 years in 60 women with urge incontinence, with only 15% completely continent at 5 years [54].
Sacral neuromodulation is typically performed in 2 stages. The first stage is electrode placement and trial period. A percutaneous nerve evaluation is a temporary electrode placement in the office or a permanent lead placement can be performed in the operating room. Correct placement stimulating the S3 nerve root is confirmed by motor and/or sensory testing. If there is an appropriate response, the electrode lead is connected to a temporary external pulse generator and is worn by the patient for a 2–14 day test period. If more than a 50% improvement in symptoms occur, a permanent lead and battery is placed in the operating room. If there is inadequate symptom response, the lead is removed. There are several recognized limitations of the office percutaneous nerve evaluation compared to operating room lead placement, including false-negative responses, possibly due to lead migration [55], incorrect lead placement, or an inadequate test period [56]. However, it is relatively noninvasive and potentially avoids 2 operating room procedures. Regardless of the choice of the initial test period, sacral neuromodulation offers a minimally invasive, long-term treatment option for refractory OAB.
Percutaneous tibial nerve stimulation (PTNS) is an office procedure that stimulates the posterior tibial nerve. This nerve contains L4–S3 fibers that originate from the same spinal segments that innervate the bladder and pelvic floor. In comparision to sacral neuromodulation, percutaneous tibial nerve stimulation is less invasive, less expensive and there is no permanent implant required [57].
Percutaneous tibial nerve stimulation is performed by a physician, nurse, or other advanced practice provider. Patients sit with knees abducted and the leg externally rotated. A 34-gauge needle is inserted 3 cm into the skin 3 fingerbreadths above the medial malleolus. The Urgent PC Neuromodulation System (Uroplasty, Minnetonka, MN) is attached and the amplitude of the stimulation is increased until the large toe curls or the toes fan. Each session lasts 30 minutes and 12 weekly treatments provides the best improvement in patient symptoms [58, 59]. There is a strong carry-over effect and patients generally need re-treatments every 4-6 weeks for 30 minutes.
Percutaneous tibial nerve stimulation has been compared favorably to both anticholinergic and sham treatments. The Overactive Bladder Innovative Therapy Trial (OrBIT) randomized 100 patients to PTNS or tolterodine for 12 weeks. The global response assessment demonstrated a statistically significant subjective improvement or cure over baseline in OAB symptoms in 79.5% of the PTNS group vs. 54.8% of the tolterodine group (P = 0.01)[60]. The SUmiT trial compared the efficacy of PTNS to sham for 12 weeks of therapy [59]. In this multicenter study, subjects were assessed at 13 weeks using the global response assessment for overall bladder symptoms. 55% of PTNS subjects achieved moderately or marked improvement in bladder symptoms compared to 20.9% of sham subjects (P < 0.001). Voiding diary parameters also improved compared to sham. In an earlier sham controlled trial, 12 patients (71%) in the treatment arm compared to none of the 15 placebo patients, demonstrated more than 50% improvement in diary and quality of life scores [61].
To evaluate long-term efficacy and safety, 36-month results of 29 positive responders of the initial SUmiT trial was reported [59]. In addition, a maintenance regimen was developed so patients received PTNS at tapering intervals over a 3-month period followed by a personalized treatment plan to sustain subjective improvement in their symptoms. With an average of 1 treatment a month, symptom severity scores and health related quality of life scores were statistically significant for improvement at each tested time-point. Yoong et al followed patients for 2 years following initial treatment with PTNS and confirmed a durable improvement in nocturia, frequency, urgency incontinence and symptom scores with a longer median length between treatments of 64 days [62].
PTNS is office-based, has few side effects, and avoids an implantable device. In addition, continuous stimulation is not necessary and a decreased treatment frequency is needed over time. Limitations include the time commitment that is required for both the initial treatment phase and the maintenance phase. Logistical concerns of weekly and monthly office visits or arranging for transportation can limit treatment.
Additional Treatment
The patient received injection of 100 U of Botox in the office. At her 2-week follow up appointment, her PVR was 90 mL and she was already seeing improvement in her incontinence episodes. At 6 weeks she was wearing 1 pad per day but using it mainly for protection. She still notices urgency, particularly if she drinks more than 1 cup of coffee in the morning, but overall she reports significant improvement in her symptoms. She has no complaints of a vaginal bulge and on exam has a grade 1 distal rectocele and no SUI with a full bladder. The physician discussed need for continued yearly examinations and repeat injections due to the duration of action of Botox.
Conclusion
This case demonstrates the complex step-wise management strategy of a patient with pelvic organ prolapse and voiding dysfunction. Interventions directed at patient bother and recognition of the various modalities and timing of treatment are essential to provide the greatest chance of positive treatment outcomes and patient satisfaction.
Corresponding author: Jaimie M Bartley, DO, 3601 W. 13 Mile Rd., Royal Oak, MI 48073.
Financial disclosures: None.
1. Nygaard I, Barber MD, Burgio KL, et al; Pelvic Floor Disorders Network. Prevalence of symptomatic pelvic floor disorders in US women. JAMA 2008;300:1311–6.
2. Hendrix SL, Clark A, Nygaard I, et al. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol 2002;186:1160–6.
3. Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J 2013;24:1783–90.
4. Zhang C, Hai T, Yu L, et al. Association between occupational stress and risk of overactive bladder and other lower urinary tract symptoms: a cross-sectional study of female nurses in China. Neurourol Urodyn 2013;32:254–60.
5. Swift SE, Tate SB, Nicholas J. Correlation of symptoms with degree of pelvic organ support in a general population of women: what is pelvic organ prolapse? Am J Obstet Gynecol 2003;189:372–7.
6. Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J 2013;24:1843–52.
7. Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996;175:10–7.
8. Lohsiriwat S, Hirunsai M, Chaiyaprasithi B. Effect of caffeine on bladder function in patients with overactive bladder symptoms. Urol Ann 2011;3:14–8.
9. Wells MJ, Jamieson K, Markham TC, et al. The effect of caffeinated versus decaffeinated drinks on overactive bladder: a double-blind, randomized, crossover study. J Wound Ostomy Continence Nurs 2014;41:371–8.
10. Fantl JA, Wyman JF, McClish DK, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA 1991;265:609–13.
11. Burgio KL, Locher JL, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA 1998;280:1995–2000.
12. Burgio KL. Update on behavioral and physical therapies for incontinence and overactive bladder: the role of pelvic floor muscle training. Curr Urol Rep 2013;14:457–64.
13. Burgio KL, Kraus SR, Menefee S, et al; Urinary Incontinence Treatment Network. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med 2008;149:161–9.
14. Gormley EA, Lightner DJ, Faraday M, Vasavada SP. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment. J Urol 2015;193:1572–80.
15. Dumoulin C, Hay-Smith J, Habée-Séguin GM, Mercier J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: A short version Cochrane systematic review with meta-analysis. Neurourol Urodyn 2015;34:300–8.
16. Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. N Engl J Med 2013;369:1124–33.
17. Braekken IH, Majida M, Engh ME, Bø K. Can pelvic floor muscle training reverse pelvic organ prolapse and reduce prolapse symptoms? An assessor-blinded, randomized, controlled trial. Am J Obstet Gynecol 2010;203:170.e1–7.
18. Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet 2014;383:796–806.
19. Alperin M, Khan A, Dubina E, et al. Patterns of pessary care and outcomes for medicare beneficiaries with pelvic organ prolapse. Female Pelvic Med Reconstr Surg 2013;19:142-7.
20. Lamers BH, Broekman BM, Milani AL. Pessary treatment for pelvic organ prolapse and health-related quality of life: a review. Int Urogynecol J 2011;22:637–44.
21. Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol 2010;115:609–17.
22. Wood LN, Anger JT. Urinary incontinence in women. BMJ 2014;349:g4531.
23. FDA Safety Communication. Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse. Available at http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm.
24. Barber MD, Brubaker L, Burgio KL, Meikle SF; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA 2014;311:1023–34.
25. Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol 2001;185:1299–304.
26. Chmielewski L, Walters MD, Weber AM, Barber MD. Reanalysis of a randomized trial of 3 techniques of anterior colporrhaphy using clinically relevan tdefinitions of success. Am J Obstet Gynecol 2011;205:69.e1–8.
27. Altman D, Väyrynen T, Engh ME, et al; Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364:1826–36. Erratum in: N Engl J Med 2013;368:394.
28. Delancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol 2002;187:93–8.
29. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol 2003;189:1612–8.
30. Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ prolapse procedures. J Urol 2004;171:1581–4.
31. Handel LN, Frenkl TL, Kim YH. Results of cystocele repair: a comparison of traditional anterior colporrhaphy, polypropylene mesh and porcine dermis. J Urol 2007;178:153–6.
32. Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA 2013;309:2016–24.
33. Sirls LT, McLennan GP, Killinger KA, et al. Exploring predictors of mesh exposure after vaginal prolapse repair. Female Pelvic Med Reconstr Surg 2013;19:206–9.
34. Hsiao KC, Latchamsetty K, Govier FE, et al. Comparison of laparoscopic and abdominal sacrocolpopexy for the treatment of vaginal vault prolapse. J Endourol 2007;21:926–30.
35. Anger JT, Mueller ER, Tarnay C, et al. Robotic compared with laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol 2014;123:5–12.
36. Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med 2012;366:2358–67.
37. Wai CY, Curto TM, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Patient satisfaction after midurethral sling surgery for stress urinary incontinence. Obstet Gynecol 2013;121:1009–16.
38. Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. J Urol 2015;193:203–10.
39. Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev 2012;2:CD003881.
40. Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur Urol 2013;63:296–305.
41. Nitti VW, Auerbach S, Martin N, et al. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol 2013;189:1388–95.
42. Dmochowski RR, Newman DK. Impact of overactive bladder on women in the United States: results of a national survey. Curr Med Res Opin 2007;23:65–76.
43. Benner JS, Nichol MB, Rovner ES, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 2010;105:1276–82.
44. Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol 2006;49:644–50.
45. Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol 2013;189:2186–93.
46. Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol 2010;184:2416–22.
47. Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial. Contemp Clin Trials 2012;33:184–96.
48. Chapple C, Sievert KD, MacDiarmid S, et al. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double-blind, placebo-controlled trial. Eur Urol 2013;64:249–56.
49. Osborn DJ, Kaufman MR, Mock S, et al. Urinary retention rates after intravesical onabotulinumtoxinA injection for idiopathic overactive bladder in clinical practice and predictors of this outcome. Neurourol Urodyn 2014 Jun 29.
50. Rovner E. Chapter 6: Practical aspects of administration of onabotulinumtoxinA. Neurourol Urodyn 2014;33 Suppl 3:S32–7.
51. Duthie JB, Vincent M, Herbison GP, et al. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database Syst Rev 2011;(12):CD005493.
52. van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 2007;178:2029–34.
53. Al-zahrani AA, Elzayat EA, Gajewski JB. Long-term outcome and surgical interventions after sacral neuromodulation implant for lower urinary tract symptoms: 14-year experience at 1 center. J Urol 2011;185:981–6.
54. Groen J, Blok BF, Bosch JL. Sacral neuromodulation as treatment for refractory idiopathic urge urinary incontinence: 5-year results of a longitudinal study in 60 women. J Urol 2011;186:954–9.
55. Carey M, Fynes M, Murray C, Maher C. Sacral nerve root stimulation for lower urinary tract dysfunction: overcoming the problem of lead migration. BJU Int 2001;87:15–8.
56. Everaert K, Kerckhaert W, Caluwaerts H, et al. A prospective randomized trial comparing the 1-stage with the 2-stage implantation of a pulse generator in patients with pelvic floor dysfunction selected for sacral nerve stimulation. Eur Urol 2004;45:649–54.
57. Staskin DR, Peters KM, MacDiarmid S, et al. Percutaneous tibial nerve stimulation: a clinically and cost effective addition to the overactive bladder algorithm of care. Curr Urol Rep 2012;13:327–34.
58. Peters KM, Carrico DJ, Wooldridge LS, et al. Percutaneous tibial nerve stimulation for the long-term treatment of overactive bladder: 3-year results of the STEP study. J Urol 2013;189:2194–201.
59. Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 2010;183:1438–43.
60. Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 2009;182:1055–61.
61. Finazzi-Agrò E, Petta F, Sciobica F, et al. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, double-blind, placebo controlled trial. J Urol 2010;184:2001–6.
62. Yoong W, Shah P, Dadswell R, Green L. Sustained effectiveness of percutaneous tibial nerve stimulation for overactive bladder syndrome: 2-year follow-up of positive responders. Int Urogynecol J 2013;24:795–9.
From Beaumont Health System, Royal Oak, MI.
Abstract
- Objective: To review the evaluation and management of complex pelvic floor disorders in elderly women.
- Methods: Literature review and presentation of a clinical case.
- Results: Pelvic floor disorders are a common problem in elderly women. Pelvic organ prolapse and voiding complaints often coexist and several treatment options are available. A step-wise approach should be used in which management of the most bothersome symptoms occurs first. Conservative, medication, and surgical options should be discussed with each patient depending on treatment goals and health status. Some effects do overlap; however, treatment of one condition may not preclude treatment of other symptoms.
- Conclusion: In women with complex pelvic floor disorders, addressing the most bothersome symptom first will increase patient satisfaction. Patients should be counseled about the potential need for multiple treatments for optimal results.
The female pelvic floor consists of a complex relationship of muscles, connective tissue and fascia, ligaments, and neurovascular support. These structures are responsible for support of the pelvic organs (uterus, bladder, rectum, and vagina), maintain continence, and assist in normal bowel function. Pelvic floor disorders occur when there is a compromise in these structures, resulting in prolapse, urinary incontinence, bowel complaints, or pain. Often several symptoms coexist with overlapping pathophysiology. Examinations and studies should aim to correctly diagnose the disorders and guide treatments toward the most bothersome symptoms.
Pelvic organ prolapse occurs when there is a weakening of the pelvic floor connective tissue, muscles, and nerves, allowing a bulge or protrusion of the vaginal walls and their associated pelvic organs. Between 3% to 50% of women in the United States have some degree of pelvic organ prolapse depending on whether the definition is based on symptoms or anatomic evaluation [1–3]. Risk factors include vaginal delivery, obesity, Caucasian race, and prior prolapse surgery. Despite the non–life-threatening nature of pelvic organ prolapse, the associated social and physical restrictions can significantly impact quality of life [4]. The cost of prolapse surgery has been estimated to be over $1.4 billion per year [3].
The sensation of a vaginal bulge is the only symptom consistently related to pelvic organ prolapse, with patients typically reporting symptoms once the prolapse extends beyond the hymenal ring [5]. The diagnosis of pelvic organ prolapse is made based on symptoms and confirmed by physical exam.
Patients with pelvic organ prolapse may experience obstructive voiding symptoms, such as hesitancy, straining, or incomplete bladder emptying. In some cases, patients may have to manually reduce the bulge to be able to void, a practice known as “splinting.” Overactive bladder (OAB), a syndrome of urinary urgency, frequency, and nocturia with or without urgency incontinence, can also occur. In patients with lower urinary tract complaints, repair of a vaginal bulge, especially a cystocele, can be associated with improved voiding symptoms [6]. Additionally, prolapse treatment can unmask de novo stress urinary incontinence (SUI), leaking with cough, sneeze or other activity that increases abdominal pressure. Urinary tract infections, pelvic pain, dyspareunia and defecatory problems can also be present.
When evaluating a woman with pelvic organ prolapse and voiding complaints, the clinician should strive to illicit which symptoms bother the patient most. A patient with primarily OAB symptoms and minimal prolapse may be treated with physical therapy or medications addressing the OAB rather than reconstructive surgery. In contrast, the patient with OAB symptoms and bothersome prolapse must be counseled on possible need for additional treatment of voiding complaints following surgical repair. This may include management of persistent OAB symptoms or SUI occurring following prolapse repair. Defecatory problems may be independent of a small rectocele present on exam, especially if long-term constipation is present. Choice of treatment depends on the severity of symptoms, the degree of prolapse, and the patient’s health status and activity level.
Case Study
Initial Presentation
A 68-year-old woman with a 15-month history of urinary urgency, frequency, incontinence and vaginal pressure presents to a urologist.
History and Physical Examination
The patient’s symptoms began shortly after the death of her husband. She initially saw her internist who prescribed antibiotics for a suspected urinary tract infection (UTI) based on office urinalysis. The symptoms did not resolve so another course of antibiotics was tried, again without relief. At her 3rd visit, a urine culture was done which was negative and she was referred to a urologist.
The patient reports 3 UTIs in the last 6 months. Following antibiotic treatment, the burning improves but she still complains of urgency and frequency. She also wears 2 to 3 pads per day for leakage that occurs with coughing and also when she feels an urge but cannot make it to the bathroom. She wakes 1 to 3 times at night to void. She feels that she empties her bladder well but often has to strain to void and sometimes feels a “bulge” in her vagina. All of these symptoms increase after being on her feet all day while she works as a grocery store cashier.
Physical exam demonstrates mild suprapubic tenderness and mild atrophic vaginitis. The anterior vaginal wall protrudes to the hymen with straining and her vaginal apex is supported 5 cm above the hymenal ring. With reduction of the cystocele there was urine leakage with cough. The cervix is surgically absent and her posterior vaginal wall is without bulge on valsalva. Her catheterized post-void residual (PVR) was 105 mL. Urine dipstick analysis was negative for infection or blood.
What is the initial evaluation of a woman with pelvic organ prolapse and voiding complaints?
The initial evaluation of a woman with pelvic organ prolapse and voiding complaints consists of a detailed history and physical examination. The nature, duration, and severity of symptoms should be assessed. Complaints of vaginal pressure or bulge are important, as well as exacerbating instances (standing, straining, defecation). Local irritation or vaginal spotting is common if prolapse is beyond the hymen. Splinting or reduction of a bulge to void or defecate are important elements of the history. Sexual history should never be overlooked, including both sexual status (active or not) as well as goals for future sexual activity. Voiding symptoms such as dysuria, frequency, urgency, nocturia and incontinence should be discussed. A 3-day voiding diary that captures number of voids per day, voided volumes, and fluid intake can be obtained. If incontinence is present, the clinician should determine what causes the incontinence. Incontinence that is associated with urgency or no warning (urge incontinence) should be treated differently than incontinence associated with activity (SUI). Mixed urinary incontinence is the presence of both stress and urgency incontinence.
Past medical history should include common medical comorbidities such as diabetes, hypertension and cardiovascular disease. Obstetric history is important due to the increased risk for pelvic floor disorders in women with multiple pregnancies and vaginal deliveries [2]. Prior hysterectomy, colon resection, or other pelvic surgeries may also contribute to symptoms. Smokers have a greater risk of genitourinary malignancy and high caffeine consumption is implicated in urgency-frequency syndromes. Exercise, sleep, and work may also be affected.
Pelvic examination should evaluate for vaginal atrophy or other vaginal mucosal abnormalities such as tears, ulcerations, lichen sclerosis, or erythema. To evaluate for prolapse, using one-half of a Graves or Pederson speculum, examine the 3 compartments of the vagina: anterior, posterior and apical. To view the anterior wall, the speculum is placed posteriorly to retract the posterior wall downward. Next it is rotated anteriorly to retract the anterior wall up and examine the posterior compartment. The uterus or the apex is evaluated with 2 halves of the speculum, one pushing anteriorly and the other posteriorly. At each point in the evaluation, the patient is told to strain or valsalva. The pelvic organ prolapse quantification system (POP-Q) is a systematic description of site-specific measurements of a woman’s pelvic support [7]. Using this classification system, a standardized and reproducible method of documenting the severity of the prolapse is done based on 6 points of the vaginal wall in relation to the hymen (2 on the anterior wall, 2 in the superior vagina, and 2 on the posterior vaginal wall). A corresponding prolapse stage can then be assigned to the patient based on POP-Q measurements. If unable to reproduce the patient’s symptoms, or exam findings do not correlate with the history, a standing exam can be helpful. Close evaluation of the urethra is also important. In severe prolapse the urethra may become kinked and mask a potential underlying problem (occult SUI). Patients should be asked to valsalva or cough with prolapse reduction and a full bladder to evaluate for this. Lastly, the pelvic floor muscles should be palpated to assess for pain or pelvic floor atrophy, hypertonicity, tenderness, or spasms.
If the patient complains of urgency, frequency, and/or dysuria, urine cultures should be performed to exclude infection even if the urinalysis is negative. Antibiotics should be given based on culture results. A postvoid ultrasound or catheterization is used to evaluate for incomplete bladder emptying. Patients with microscopic or gross hematuria should undergo further testing with radiologic and cystoscopic evaluation as indicated, especially with a history of smoking. Women should be questioned regarding their menstrual history and if postmenopausal, about any vaginal bleeding. A pelvic ultrasound should be considered if the patient has a history of endometriosis, gynecological cancers, uterine fibroids, or ovarian cysts or if considering uterine preserving surgery or colpocleisis. Urodynamics are often indicated in complex patients with prolapse and lower urinary tract complaints or prior pelvic surgery.
Diagnosis
The patient was diagnosed with mixed urinary incontinence and a grade 2 cystocele. Treatment options were discussed and she was most interested in conservative management options.
What is first-line treatment for the complaints of urgency, frequency, and incontinence?
In an older patient with complaints of urgency, frequency, and incontinence, dietary and behavioral modifications as well as pelvic floor physical therapy are considered first-line minimally invasive treatments.
Dietary irritants such as coffee, tea, soda, and other caffeinated beverages can contribute to worsening of symptoms [8]. A randomized study measuring the effects of caffeine noted a significant reduction in urgency and frequency of voids and in symptom scores with reduction of caffeine use [9]. Some elderly patients are reluctant to change their lifestyle, but even small changes can significantly improve their urgency symptoms.
Timed voiding is an effective method for bladder retraining, which can be critical for managing symptoms both alone and as an adjunct to other interventions. Studies of behavioral therapy show significant improvement in urgency, frequency, and incontinence episodes. In a study by Wyman and Fanti, patients participating in bladder training and Kegel exercises noted a 57% decrease in incontinence episodes and 54% decrease in urine loss without medications [10]. Burgio et al compared behavioral therapy to anticholinergic medication administration. After 4 sessions over 8 weeks they reported 81% reduction in incontinence episodes compared to 69% in the drug group and 39% in the placebo group [11].
Elderly patients may take several medications, some of which can affect urine volume and timing of urine production. Diuretics given later in the day can increase nighttime urine production and worsen nocturia. Similarly, lower extremity edema can increase nocturnal urine volumes when the patient reclines. Compressive stockings and leg elevation 2-3 hours prior to bedtime will help evenly distribute fluids and decrease reabsorption when supine at night.
Pelvic Floor Physical Therapy
Pelvic floor physical therapy (PFPT) can be an effective treatment for OAB, SUI, and pelvic organ prolapse. PFPT is used as an urge suppression strategy for OAB by teaching patients how to contract their pelvic muscles to occlude the urethra and prevent leakage during a detrusor contraction. Strategies to help suppress urge and manage stress situations can reduce incontinence episodes up to 60% to 80% [12]. Behavioral programs can include bladder diaries, scheduled voiding, delayed voiding, double voiding, fluid management, and caffeine reduction. When combined with PFPT they can be very effective in the management of OAB symptoms and incontinence. The BE-DRI study showed that combined behavioral training and drug therapy yielded better outcomes over time in OAB symptoms, patient distress and treatment satisfaction than drug therapy alone [13]. PFPT is considered a first-line treatment for OAB and is a noninvasive and effective treatment for these symptoms [14].
Pelvic floor programs for SUI aim to teach pelvic floor muscle contraction to help prevent stress leakage and use a variety of methods including biofeedback and personalized training programs. A recent Cochrane review included 18 studies of PFPT for incontinence. They concluded that there was high quality evidence that PFPT was associated with cure and moderate evidence for improvement in SUI [15]. In a study comparing surgery versus PFPT at 1 year, subjective improvement in the surgery group was 91% compared to 64% in the PFPT group. While PFPT was not as effective as surgery, over 50% had improvement. PFPT remains an effective noninvasive option that should be considered, particularly in an older patient [16].
PFPT has also been studied as a treatment option for pelvic organ prolapse. In a randomized controlled trial (RCT) that compared PFPT to controls over time, more women in the PFPT group improved 1 POP-Q stage compared to the control group. They also had significantly improved pelvic floor symptom bother [17]. In the POPPY study examining PFPT versus a control condition, researchers were not able to show statistically significant improvement in prolapse stages but did show improvement in secondary outcomes, including symptom bother and the feeling of “bulge.” Fewer women sought further treatment for prolapse after undergoing PFPT [18]. PFPT can be effective in managing prolapse symptoms and may help improve prolapse stage.
Pessary
Pessaries are commonly used for management of pelvic organ prolapse in patients who choose nonoperative management. In a large study of pessary use in the Medicare population, it was noted that of 34,782 women diagnosed with prolapse between 1999 and 2000, 11.6% were treated with a pessary. Complications noted during the 9 years of follow-up included 3% with vesicovaginal or rectovaginal fistulas and 5% with a device-associated complication [19]. Use increased with age, with 24% of women over 85 being managed with a pessary. In a review examining quality of life, improvement in bulge, irritative symptoms, and sexual satisfaction occurred with pessary use. In the medium-term, prolapse-related bother symptoms, quality of life, and overall perception of body image improved with the use of a pessary [20]. For SUI, rings with a knob or an incontinence dish can provide support to the urethra and help to pinch it closed with coughing, sneezing, and laughing, preventing leakage. In an RCT comparing women who received behavioral therapy, an incontinence pessary, or both, at 3 months 33% of those assigned to pessary reported improved incontinence symptoms compared to 49% with behavioral therapy, and 63% were satisfied with pessary treatment compared to 75% with behavioral therapy [21,22]. Differences did not persist to 12 months with over one third of all women improved and even more satisfied. A pessary can be safely used in the elderly population but does require office management and regular follow-up to prevent complications.
Initital Treatment
The patient was treated with 3 months of PFPT with biofeedback and pelvic floor muscle strengthening. In addition, she was able to decrease her caffeine use from 4 cups of coffee per day to 1 cup in the morning. At her 3-month follow-up visit, she noticed significant improvement in her voiding symptoms, and her voiding diary showed improved voided volumes and decreased frequency and nocturia. However, she was becoming more active in her community, going to aerobics and dance classes. She was more bothered by the “bulge” feeling in her vagina. She was not interested in a pessary but wanted to hear about surgical options for prolapse treatment.
What is operative management of pelvic organ prolapse?
The goals for surgical pelvic organ prolapse repair are to resolve symptoms, restore normal or near-normal anatomy, preserve sexual, urinary and bowel function, and minimize patient morbidity. The extent of prolapse, patient risk factors for recurrence, patient preference, and overall medical condition all influence the method for surgical repair. Surgeon familiarity and experience is also important when selecting the appropriate repair. Recent concerns regarding the use of synthetic mesh material has become a factor in counseling patients since the 2011 US Food and Drug Administration safety communication on transvaginal mesh [23].
Vaginal Approaches
Numerous techniques for pelvic organ prolapse repair have been described, though most repairs can broadly be divided into vaginal and abdominal procedures. Vaginal surgery is consistently associated with shorter operative times, less postoperative pain, and a shorter length of stay than abdominal approaches. All prolapsing compartments can be addressed vaginally using a patient’s own tissue, often called a “native tissue repair.” The vaginal apex is suspended from either the uterosacral ligament (USL) condensations or to the sacrospinous ligaments (SSL). Sutures are placed through these structures and tied to hold the vaginal vault in place, often at the time of concomitant enterocele, cystocele, or rectocele repairs. A recent randomized trial comparing USL and SSL repair showed composite functional and symptomatic success was 60% at 2 years and did not differ by technique [24]. While overall success may appear low, symptomatic vaginal bulge was present in only 17% to 19% of women at 2 years and only 5% underwent re-treatment with surgery or pessary during follow-up. Similar outcomes have been demonstrated for isolated cystocele repairs plicating the pubo-cervical fascia (anterior colporrhaphy). Cited failure rates have been as high as 70% [25], though this depends on the definition of success. When symptoms of bulge and/or prolapse beyond the hymen are used, success rates are closer to 89% at 2 years [26]. In one study comparing mesh-augmented cystocele repair with native tissue anterior colporrhaphy, 49% of women had a successful composite outcome at 2 years of grade 0 or 1 prolapse and no symptoms of bulge without the use of mesh graft [27]. Despite lower anatomic success rates, anterior colporrhaphy consistently relieves symptoms of bulge with low retreatment rates.
The high failure rates of native tissue vaginal repairs, especially in women with high-grade or recurrent prolapse, led to an interest in graft-augmented repairs. Furthermore, anatomic studies showed that up to 88% of cystoceles were associated with a lateral defect, or tearing of the pubocervical fascia from the pelvic sidewall (arcus tendineous fascia pelvis) [28]. Plicating the already weak fascia centrally would not repair an underlying lateral defect resulting in treatment failure. Replacing this weak fascia with a graft and anchoring it laterally and proximally should result in better anatomic and functional outcomes. These patches can be made from autologous tissue (rectus fascia), donor allograft material (fascia lata), xenografts (porcine dermis, bovine pericardium), or synthetic mesh. Initial studies using cadaveric dermis grafts for recurrent stage II or stage III/IV pelvic organ prolapse resulted in 50% failure at 4 years, but symptomatic failure was only 11% [29]. Further publications utilizing cadaveric tissue patches showed lower rates of cystocele recurrences of 0 to 17% between 20 and 56 months of follow-up [30].
As interest in patch repairs became popular, the use of synthetic mesh was applied to tension-free mid-urethral tapes for SUI. Studies were also showing rapid cadaveric and xenograft graft metabolism, graft extrusion, and early failure in some women [31]. This led to the use of larger pieces of synthetic mesh for prolapse repair, as it had been for abdominal wall and inguinal hernia repairs. Ultimately large-pore, light-weight polypropylene mesh was seen as the most favorable material and large randomized studies were performed to compare outcomes. Theoretically, a synthetic material would provide a replacement for the weakened and torn pubocervical fascia and not be subjected to enzymatic degradation. Altman et al published a widely cited randomized trial comparing native tissue vs. synthetic mesh showing that improvement in the composite primary outcome (no prolapse on the basis of both objective and subjective assessments) was more common in the mesh group (61% vs. 35%) at 1 year. Mesh placement was associated with longer operative times, higher blood loss, and 3.2% of women underwent secondary procedures for vaginal mesh exposure [27]. While there is still debate on the routine use of transvaginal mesh placement, current recommendations generally limit its use for recurrent or high grade pelvic organ prolapse (> Stage III), and possibly those at higher risks for recurrence. The American Urological Association has supported the FDA recommendation that patients undergo a thorough consent process and that surgeons are properly trained in pelvic reconstruction and mesh placement techniques. Furthermore, surgeons placing transvaginal mesh should be equipped to diagnose and treat any complications that may arise subsequent to its use.
Abdominal Approaches
Pelvic organ prolapse can also be approached through an abdominal technique. The classic description for vaginal vault prolapse repair is the abdominal sacrocolpopexy. This involves fixating the vaginal apex to the anterior longitudinal ligament at the sacral promontory. Hysterectomy is performed at the same setting if still in situ. A strip of lightweight polypropylene mesh is sutured to the anterior and posterior vaginal walls after dissecting the bladder and rectum off, then suspended in a tension-free manner to the sacrum. Large trials with long-term follow-up show durability of this repair. Seven-year follow-up of a large NIH-sponsored trial comparing sacrocolpopexy with and without urethropexy found 31/181 (17%) with anatomic prolapse beyond the hymen [32]. Of these women one-third had involvement of the vaginal apex, though 50% of women were asymptomatic. Overall, 95% of women had no retreatment for pelvic organ prolapse. A surprising finding was a 10.5% mesh exposure rate with a mean follow-up of 6.1 years. Previously, abdominally placed mesh was thought to be much safer than transvaginal mesh, but exposure rates are roughly similar in newer studies at high-volume, fellowship-trained centers [33]. The largest advance in abdominal prolapse surgery has come with the adoption of laparoscopic and robotic-assisted technology. Minimally invasive approaches to abdominal surgery have resulted in less blood loss and shorter length of stay, though longer operative times [34]. Short- and medium-term outcomes have been compared to the open techniques in smaller single-center series. At least 1 randomized trial comparing laparoscopic to robotic sacrocolpopexy showed similar complications and perioperative outcomes, though the robotic technique was more costly [35].
Stress Urinary Incontinence Procedures
When SUI is identified preoperatively, treatment should be considered at the time of prolapse repair [32,36]. The gold standard for treatment of SUI with urethral hypermobility has been placement of a synthetic mid-urethral sling. There are several types of slings available, mainly categorized as retropubic, transobturator, or single-incision “mini-slings.” In a multicenter study by the Urinary Incontinence Treatment Network (UITN), patient satisfaction after retropubic and transobturator sling placement was studied 12 months after surgery. Both groups had a high satisfaction rate (from 85% to 90%) for urine leakage, urgency, and frequency [37]. There was no significant difference in outcomes between the 2 approaches. Several other studies and systematic reviews have also shown excellent long-term results with sling treatment. In the recently published 5-year follow-up of the Trial of Mid-Urethral Slings (TOMUS), researchers demonstrated an 80% to 85% patient satisfaction rate with a 10% adverse event rate. Of these adverse events, only 6 were classified as serious requiring surgical, radiologic, or endoscopic intervention [38].
If the patient has SUI but no urethral hypermobility, consider intrinsic sphincter deficiency as the etiology of her incontinence. In that case, injectable therapy with urethral bulking agents is an effective treatment. Some commonly used injectables include carbon beads (Durasphere), calcium hydroxylapatite (Coaptite), bovine collagen (Contigen), and silicon particles (Macroplastique). In a Cochrane review of injectable therapy, they compared urethral injection to conservative treatment with physical therapy and noted an improvement with injection at 3 months. Surgical treatment was overall more effective; however, 50% of the women that received a collagen injection were satisfied at 12 months after the procedure. They also note lower morbidity for this procedure compared to surgery [39].
Treatment in This Patient
The patient underwent successful robotic sacrocolpopexy with mesh and a transobturator sling. There were no complications during the procedure and she reports no bulge or SUI symptoms. She has not been straining to void and has been emptying her bladder well since the Foley catheter was removed the day after surgery. However, she continues to complain of bothersome urgency, frequency, and urge incontinence. She is wearing 1 to 3 pads daily for leakage. At her 6-week postoperative visit, the exam showed excellent vaginal support, no SUI, low PVR, and her urine culture was negative.
What are the clinical implications of these findings?
At this point it is reasonable to continue treatment of OAB. The patient may continue to see improvement as she gets further out from surgery but especially in a patient that had preoperative OAB symptoms, treatment is indicated and may consist of reminding her of behavioral modifications, returning to pelvic floor physical therapy, or starting her on a medication.
What medications are used to treat OAB?
Anticholinergic Drugs
Anticholinergics are second-line therapy for OAB; these medications prevent the binding of acetylcholine to the M3 muscarinic receptor in the detrusor muscle and inhibit uncontrolled bladder contraction. There are numerous medications and delivery methods (pills, patches, gels) but efficacy is similar among the different drugs and all are limited by side effects such as dry mouth, constipation, and central nervous system side effects. Mirabegron, approved by the FDA in June 2012 and released in October 2012, is an agonist of the β3-adrenoceptor receptor in the detrusor muscle promoting bladder storage. A phase III trial found that mirabegron significantly decreased incontinence episodes and micturition frequency compared to placebo [40]. Dry mouth, common with anticholinergics, was 3 times less likely compared to tolterodine [41]. The most common side effects (headache, urinary tract infection, hypertension, and nasopharyngitis) were similar between treatment and placebo groups.
Long-term compliance, side effects, and decreased efficacy limit the benefits of medication therapy [42]. In one survey, 25% of patients taking OAB medications discontinued them within 12 months with 89% reporting unmet treatment expectations and/or tolerability [43].
6 Months Later
The patient continues to complain of persistent OAB symptoms despite anticholinergic and beta-3 agonist therapy. She reported significant constipation and dry mouth with an anticholinergic and symptoms did not improve with mirebegron. Despite having OAB symptoms prior to her prolapse repair, it is important to evaluate for any other cause of her persistent symptoms. Her surgical repair remains intact and urodynamics and cystoscopy were performed showing no evidence of bladder outlet obstruction and no mesh or suture material in the bladder. There was no leakage with valsalva, though she had some early sensation of fullness (sensory urge). With a negative evaluation, refractory OAB is diagnosed and the patient is a candidate for third-line OAB treatment.
What are third-line OAB treatments?
OnabotulinumtoxinA
OnabotulinumtoxinA (Botox) was approved in 2013 for patients intolerant or unresponsive to behavioral therapy and oral medications. OnabotulinumtoxinA is a chemical neuromodulator that cleaves the SNARE protein SNAP-25, inhibits the fusion of the cytoplasmic vesical to the nerve terminal, and prevents the release of acetylcholine. This causes detrusor muscle relaxation and may also inhibit sensory afferent pathways [44].
Nitti et al compared Botox 100 U to placebo in 557 patients that were refractory to anticholinergics [45]. Botox decreased the frequency of daily urinary incontinence episodes vs placebo (–2.65 vs –0.87, P < 0.001) and 22.9% vs 6.5% of patients became completely continent. A 5.4% rate of urinary retention occurred and UTI was the most common side effect (16%) in those receiving active drug. A dose of 100 U is recommended to limit side effects while maintaining efficacy [46].
Comparision of a daily anticholinergic (solifenacin) versus Botox 100 U for 6 months was done in a randomized double-blind, double-placebo-controlled trial [47]. Patients underwent saline injection or took an oral placebo in the anticholinergic and Botox groups, respectively. Complete resolution of urinary symptoms occurred in 13% of the medication group and 27% of the Botox group (P = 0.003). Dry mouth was more common in the medication group (46% vs. 31%) and the Botox group had a higher rate of catheter use and urinary tract infections (5% vs. 0%; 33% vs. 13%). Quality of life measures have also been shown to improve significantly following Botox injection [45,48].
When considering whether Botox is appropriate for a particular patient, physicians must determine whether the patient is willing and able to perform clean intermittent catheterization. Contraindications include active UTI, urinary retention, unwilling or unable to do clean intermittent catheterization, and known hypersenstivitiy to botulinum toxin type A. Although the definition of urinary retention and the PVR at which clean intermittent catheterization should be initiated varies, one study found a 94% rate of urinary retention with a preoperative PVR > 100 mL [49].
Botox can be administered in the clinic with or without local anesthetic but general anesthetic may be used in patients who might be poorly tolerant of the procedure. Using flexible or rigid cystoscopy, the bladder is filled to 100 to 200 mL. An injection needle is used to inject 0.5 cc aliquots of reconstituted onabotulinumtoxinA in 20 areas spaced 1 cm apart. Periprocedure antibiotics are recommended by the manufacturer but actual usage varies [50]. Patients should understand that the effects of Botox may take up to 4 weeks and an appointment should be scheduled within 2 weeks to evaluate PVR and any other adverse reactions. Repeat injections are needed between 3 to 9 months as symptoms return; however, efficacy is maintained with subsequent treatments [51].
Neuromodulation
Additional third-line treatment options include sacral or posterior tibial nerve neuromodulation. Sacral neuromodulation has been FDA approved for treatment of urgency, frequency and urgency incontinence since 1997. Also known as InterStim (Medtronic, Minneapolis, MN), this involves placement of a tined electrode adjacent to the S3 nerve root and is thought to result in modulation of the afferent nerve signals from the bladder to the spinal cord and the pontine micturition center.
Since the FDA approved sacral neuromodulation, long-term results for this therapy have been positive. A multicenter study with a 5-year follow-up showed a statistically significant reduction in daily leakage episodes, number of daily voids, and increase in voided volume, with a 5-year success rate of 68% for urgency incontinence and 56% for urgency/frequency [52]. Al-Zahrani et al followed 96 patients (35% with urgency incontinence) for a mean of 50.7 months and approximately 85% of the incontinent patients remained improved [53]. Conversely, Groen et al observed a gradual decrease in success rate from 1 month to 5 years in 60 women with urge incontinence, with only 15% completely continent at 5 years [54].
Sacral neuromodulation is typically performed in 2 stages. The first stage is electrode placement and trial period. A percutaneous nerve evaluation is a temporary electrode placement in the office or a permanent lead placement can be performed in the operating room. Correct placement stimulating the S3 nerve root is confirmed by motor and/or sensory testing. If there is an appropriate response, the electrode lead is connected to a temporary external pulse generator and is worn by the patient for a 2–14 day test period. If more than a 50% improvement in symptoms occur, a permanent lead and battery is placed in the operating room. If there is inadequate symptom response, the lead is removed. There are several recognized limitations of the office percutaneous nerve evaluation compared to operating room lead placement, including false-negative responses, possibly due to lead migration [55], incorrect lead placement, or an inadequate test period [56]. However, it is relatively noninvasive and potentially avoids 2 operating room procedures. Regardless of the choice of the initial test period, sacral neuromodulation offers a minimally invasive, long-term treatment option for refractory OAB.
Percutaneous tibial nerve stimulation (PTNS) is an office procedure that stimulates the posterior tibial nerve. This nerve contains L4–S3 fibers that originate from the same spinal segments that innervate the bladder and pelvic floor. In comparision to sacral neuromodulation, percutaneous tibial nerve stimulation is less invasive, less expensive and there is no permanent implant required [57].
Percutaneous tibial nerve stimulation is performed by a physician, nurse, or other advanced practice provider. Patients sit with knees abducted and the leg externally rotated. A 34-gauge needle is inserted 3 cm into the skin 3 fingerbreadths above the medial malleolus. The Urgent PC Neuromodulation System (Uroplasty, Minnetonka, MN) is attached and the amplitude of the stimulation is increased until the large toe curls or the toes fan. Each session lasts 30 minutes and 12 weekly treatments provides the best improvement in patient symptoms [58, 59]. There is a strong carry-over effect and patients generally need re-treatments every 4-6 weeks for 30 minutes.
Percutaneous tibial nerve stimulation has been compared favorably to both anticholinergic and sham treatments. The Overactive Bladder Innovative Therapy Trial (OrBIT) randomized 100 patients to PTNS or tolterodine for 12 weeks. The global response assessment demonstrated a statistically significant subjective improvement or cure over baseline in OAB symptoms in 79.5% of the PTNS group vs. 54.8% of the tolterodine group (P = 0.01)[60]. The SUmiT trial compared the efficacy of PTNS to sham for 12 weeks of therapy [59]. In this multicenter study, subjects were assessed at 13 weeks using the global response assessment for overall bladder symptoms. 55% of PTNS subjects achieved moderately or marked improvement in bladder symptoms compared to 20.9% of sham subjects (P < 0.001). Voiding diary parameters also improved compared to sham. In an earlier sham controlled trial, 12 patients (71%) in the treatment arm compared to none of the 15 placebo patients, demonstrated more than 50% improvement in diary and quality of life scores [61].
To evaluate long-term efficacy and safety, 36-month results of 29 positive responders of the initial SUmiT trial was reported [59]. In addition, a maintenance regimen was developed so patients received PTNS at tapering intervals over a 3-month period followed by a personalized treatment plan to sustain subjective improvement in their symptoms. With an average of 1 treatment a month, symptom severity scores and health related quality of life scores were statistically significant for improvement at each tested time-point. Yoong et al followed patients for 2 years following initial treatment with PTNS and confirmed a durable improvement in nocturia, frequency, urgency incontinence and symptom scores with a longer median length between treatments of 64 days [62].
PTNS is office-based, has few side effects, and avoids an implantable device. In addition, continuous stimulation is not necessary and a decreased treatment frequency is needed over time. Limitations include the time commitment that is required for both the initial treatment phase and the maintenance phase. Logistical concerns of weekly and monthly office visits or arranging for transportation can limit treatment.
Additional Treatment
The patient received injection of 100 U of Botox in the office. At her 2-week follow up appointment, her PVR was 90 mL and she was already seeing improvement in her incontinence episodes. At 6 weeks she was wearing 1 pad per day but using it mainly for protection. She still notices urgency, particularly if she drinks more than 1 cup of coffee in the morning, but overall she reports significant improvement in her symptoms. She has no complaints of a vaginal bulge and on exam has a grade 1 distal rectocele and no SUI with a full bladder. The physician discussed need for continued yearly examinations and repeat injections due to the duration of action of Botox.
Conclusion
This case demonstrates the complex step-wise management strategy of a patient with pelvic organ prolapse and voiding dysfunction. Interventions directed at patient bother and recognition of the various modalities and timing of treatment are essential to provide the greatest chance of positive treatment outcomes and patient satisfaction.
Corresponding author: Jaimie M Bartley, DO, 3601 W. 13 Mile Rd., Royal Oak, MI 48073.
Financial disclosures: None.
From Beaumont Health System, Royal Oak, MI.
Abstract
- Objective: To review the evaluation and management of complex pelvic floor disorders in elderly women.
- Methods: Literature review and presentation of a clinical case.
- Results: Pelvic floor disorders are a common problem in elderly women. Pelvic organ prolapse and voiding complaints often coexist and several treatment options are available. A step-wise approach should be used in which management of the most bothersome symptoms occurs first. Conservative, medication, and surgical options should be discussed with each patient depending on treatment goals and health status. Some effects do overlap; however, treatment of one condition may not preclude treatment of other symptoms.
- Conclusion: In women with complex pelvic floor disorders, addressing the most bothersome symptom first will increase patient satisfaction. Patients should be counseled about the potential need for multiple treatments for optimal results.
The female pelvic floor consists of a complex relationship of muscles, connective tissue and fascia, ligaments, and neurovascular support. These structures are responsible for support of the pelvic organs (uterus, bladder, rectum, and vagina), maintain continence, and assist in normal bowel function. Pelvic floor disorders occur when there is a compromise in these structures, resulting in prolapse, urinary incontinence, bowel complaints, or pain. Often several symptoms coexist with overlapping pathophysiology. Examinations and studies should aim to correctly diagnose the disorders and guide treatments toward the most bothersome symptoms.
Pelvic organ prolapse occurs when there is a weakening of the pelvic floor connective tissue, muscles, and nerves, allowing a bulge or protrusion of the vaginal walls and their associated pelvic organs. Between 3% to 50% of women in the United States have some degree of pelvic organ prolapse depending on whether the definition is based on symptoms or anatomic evaluation [1–3]. Risk factors include vaginal delivery, obesity, Caucasian race, and prior prolapse surgery. Despite the non–life-threatening nature of pelvic organ prolapse, the associated social and physical restrictions can significantly impact quality of life [4]. The cost of prolapse surgery has been estimated to be over $1.4 billion per year [3].
The sensation of a vaginal bulge is the only symptom consistently related to pelvic organ prolapse, with patients typically reporting symptoms once the prolapse extends beyond the hymenal ring [5]. The diagnosis of pelvic organ prolapse is made based on symptoms and confirmed by physical exam.
Patients with pelvic organ prolapse may experience obstructive voiding symptoms, such as hesitancy, straining, or incomplete bladder emptying. In some cases, patients may have to manually reduce the bulge to be able to void, a practice known as “splinting.” Overactive bladder (OAB), a syndrome of urinary urgency, frequency, and nocturia with or without urgency incontinence, can also occur. In patients with lower urinary tract complaints, repair of a vaginal bulge, especially a cystocele, can be associated with improved voiding symptoms [6]. Additionally, prolapse treatment can unmask de novo stress urinary incontinence (SUI), leaking with cough, sneeze or other activity that increases abdominal pressure. Urinary tract infections, pelvic pain, dyspareunia and defecatory problems can also be present.
When evaluating a woman with pelvic organ prolapse and voiding complaints, the clinician should strive to illicit which symptoms bother the patient most. A patient with primarily OAB symptoms and minimal prolapse may be treated with physical therapy or medications addressing the OAB rather than reconstructive surgery. In contrast, the patient with OAB symptoms and bothersome prolapse must be counseled on possible need for additional treatment of voiding complaints following surgical repair. This may include management of persistent OAB symptoms or SUI occurring following prolapse repair. Defecatory problems may be independent of a small rectocele present on exam, especially if long-term constipation is present. Choice of treatment depends on the severity of symptoms, the degree of prolapse, and the patient’s health status and activity level.
Case Study
Initial Presentation
A 68-year-old woman with a 15-month history of urinary urgency, frequency, incontinence and vaginal pressure presents to a urologist.
History and Physical Examination
The patient’s symptoms began shortly after the death of her husband. She initially saw her internist who prescribed antibiotics for a suspected urinary tract infection (UTI) based on office urinalysis. The symptoms did not resolve so another course of antibiotics was tried, again without relief. At her 3rd visit, a urine culture was done which was negative and she was referred to a urologist.
The patient reports 3 UTIs in the last 6 months. Following antibiotic treatment, the burning improves but she still complains of urgency and frequency. She also wears 2 to 3 pads per day for leakage that occurs with coughing and also when she feels an urge but cannot make it to the bathroom. She wakes 1 to 3 times at night to void. She feels that she empties her bladder well but often has to strain to void and sometimes feels a “bulge” in her vagina. All of these symptoms increase after being on her feet all day while she works as a grocery store cashier.
Physical exam demonstrates mild suprapubic tenderness and mild atrophic vaginitis. The anterior vaginal wall protrudes to the hymen with straining and her vaginal apex is supported 5 cm above the hymenal ring. With reduction of the cystocele there was urine leakage with cough. The cervix is surgically absent and her posterior vaginal wall is without bulge on valsalva. Her catheterized post-void residual (PVR) was 105 mL. Urine dipstick analysis was negative for infection or blood.
What is the initial evaluation of a woman with pelvic organ prolapse and voiding complaints?
The initial evaluation of a woman with pelvic organ prolapse and voiding complaints consists of a detailed history and physical examination. The nature, duration, and severity of symptoms should be assessed. Complaints of vaginal pressure or bulge are important, as well as exacerbating instances (standing, straining, defecation). Local irritation or vaginal spotting is common if prolapse is beyond the hymen. Splinting or reduction of a bulge to void or defecate are important elements of the history. Sexual history should never be overlooked, including both sexual status (active or not) as well as goals for future sexual activity. Voiding symptoms such as dysuria, frequency, urgency, nocturia and incontinence should be discussed. A 3-day voiding diary that captures number of voids per day, voided volumes, and fluid intake can be obtained. If incontinence is present, the clinician should determine what causes the incontinence. Incontinence that is associated with urgency or no warning (urge incontinence) should be treated differently than incontinence associated with activity (SUI). Mixed urinary incontinence is the presence of both stress and urgency incontinence.
Past medical history should include common medical comorbidities such as diabetes, hypertension and cardiovascular disease. Obstetric history is important due to the increased risk for pelvic floor disorders in women with multiple pregnancies and vaginal deliveries [2]. Prior hysterectomy, colon resection, or other pelvic surgeries may also contribute to symptoms. Smokers have a greater risk of genitourinary malignancy and high caffeine consumption is implicated in urgency-frequency syndromes. Exercise, sleep, and work may also be affected.
Pelvic examination should evaluate for vaginal atrophy or other vaginal mucosal abnormalities such as tears, ulcerations, lichen sclerosis, or erythema. To evaluate for prolapse, using one-half of a Graves or Pederson speculum, examine the 3 compartments of the vagina: anterior, posterior and apical. To view the anterior wall, the speculum is placed posteriorly to retract the posterior wall downward. Next it is rotated anteriorly to retract the anterior wall up and examine the posterior compartment. The uterus or the apex is evaluated with 2 halves of the speculum, one pushing anteriorly and the other posteriorly. At each point in the evaluation, the patient is told to strain or valsalva. The pelvic organ prolapse quantification system (POP-Q) is a systematic description of site-specific measurements of a woman’s pelvic support [7]. Using this classification system, a standardized and reproducible method of documenting the severity of the prolapse is done based on 6 points of the vaginal wall in relation to the hymen (2 on the anterior wall, 2 in the superior vagina, and 2 on the posterior vaginal wall). A corresponding prolapse stage can then be assigned to the patient based on POP-Q measurements. If unable to reproduce the patient’s symptoms, or exam findings do not correlate with the history, a standing exam can be helpful. Close evaluation of the urethra is also important. In severe prolapse the urethra may become kinked and mask a potential underlying problem (occult SUI). Patients should be asked to valsalva or cough with prolapse reduction and a full bladder to evaluate for this. Lastly, the pelvic floor muscles should be palpated to assess for pain or pelvic floor atrophy, hypertonicity, tenderness, or spasms.
If the patient complains of urgency, frequency, and/or dysuria, urine cultures should be performed to exclude infection even if the urinalysis is negative. Antibiotics should be given based on culture results. A postvoid ultrasound or catheterization is used to evaluate for incomplete bladder emptying. Patients with microscopic or gross hematuria should undergo further testing with radiologic and cystoscopic evaluation as indicated, especially with a history of smoking. Women should be questioned regarding their menstrual history and if postmenopausal, about any vaginal bleeding. A pelvic ultrasound should be considered if the patient has a history of endometriosis, gynecological cancers, uterine fibroids, or ovarian cysts or if considering uterine preserving surgery or colpocleisis. Urodynamics are often indicated in complex patients with prolapse and lower urinary tract complaints or prior pelvic surgery.
Diagnosis
The patient was diagnosed with mixed urinary incontinence and a grade 2 cystocele. Treatment options were discussed and she was most interested in conservative management options.
What is first-line treatment for the complaints of urgency, frequency, and incontinence?
In an older patient with complaints of urgency, frequency, and incontinence, dietary and behavioral modifications as well as pelvic floor physical therapy are considered first-line minimally invasive treatments.
Dietary irritants such as coffee, tea, soda, and other caffeinated beverages can contribute to worsening of symptoms [8]. A randomized study measuring the effects of caffeine noted a significant reduction in urgency and frequency of voids and in symptom scores with reduction of caffeine use [9]. Some elderly patients are reluctant to change their lifestyle, but even small changes can significantly improve their urgency symptoms.
Timed voiding is an effective method for bladder retraining, which can be critical for managing symptoms both alone and as an adjunct to other interventions. Studies of behavioral therapy show significant improvement in urgency, frequency, and incontinence episodes. In a study by Wyman and Fanti, patients participating in bladder training and Kegel exercises noted a 57% decrease in incontinence episodes and 54% decrease in urine loss without medications [10]. Burgio et al compared behavioral therapy to anticholinergic medication administration. After 4 sessions over 8 weeks they reported 81% reduction in incontinence episodes compared to 69% in the drug group and 39% in the placebo group [11].
Elderly patients may take several medications, some of which can affect urine volume and timing of urine production. Diuretics given later in the day can increase nighttime urine production and worsen nocturia. Similarly, lower extremity edema can increase nocturnal urine volumes when the patient reclines. Compressive stockings and leg elevation 2-3 hours prior to bedtime will help evenly distribute fluids and decrease reabsorption when supine at night.
Pelvic Floor Physical Therapy
Pelvic floor physical therapy (PFPT) can be an effective treatment for OAB, SUI, and pelvic organ prolapse. PFPT is used as an urge suppression strategy for OAB by teaching patients how to contract their pelvic muscles to occlude the urethra and prevent leakage during a detrusor contraction. Strategies to help suppress urge and manage stress situations can reduce incontinence episodes up to 60% to 80% [12]. Behavioral programs can include bladder diaries, scheduled voiding, delayed voiding, double voiding, fluid management, and caffeine reduction. When combined with PFPT they can be very effective in the management of OAB symptoms and incontinence. The BE-DRI study showed that combined behavioral training and drug therapy yielded better outcomes over time in OAB symptoms, patient distress and treatment satisfaction than drug therapy alone [13]. PFPT is considered a first-line treatment for OAB and is a noninvasive and effective treatment for these symptoms [14].
Pelvic floor programs for SUI aim to teach pelvic floor muscle contraction to help prevent stress leakage and use a variety of methods including biofeedback and personalized training programs. A recent Cochrane review included 18 studies of PFPT for incontinence. They concluded that there was high quality evidence that PFPT was associated with cure and moderate evidence for improvement in SUI [15]. In a study comparing surgery versus PFPT at 1 year, subjective improvement in the surgery group was 91% compared to 64% in the PFPT group. While PFPT was not as effective as surgery, over 50% had improvement. PFPT remains an effective noninvasive option that should be considered, particularly in an older patient [16].
PFPT has also been studied as a treatment option for pelvic organ prolapse. In a randomized controlled trial (RCT) that compared PFPT to controls over time, more women in the PFPT group improved 1 POP-Q stage compared to the control group. They also had significantly improved pelvic floor symptom bother [17]. In the POPPY study examining PFPT versus a control condition, researchers were not able to show statistically significant improvement in prolapse stages but did show improvement in secondary outcomes, including symptom bother and the feeling of “bulge.” Fewer women sought further treatment for prolapse after undergoing PFPT [18]. PFPT can be effective in managing prolapse symptoms and may help improve prolapse stage.
Pessary
Pessaries are commonly used for management of pelvic organ prolapse in patients who choose nonoperative management. In a large study of pessary use in the Medicare population, it was noted that of 34,782 women diagnosed with prolapse between 1999 and 2000, 11.6% were treated with a pessary. Complications noted during the 9 years of follow-up included 3% with vesicovaginal or rectovaginal fistulas and 5% with a device-associated complication [19]. Use increased with age, with 24% of women over 85 being managed with a pessary. In a review examining quality of life, improvement in bulge, irritative symptoms, and sexual satisfaction occurred with pessary use. In the medium-term, prolapse-related bother symptoms, quality of life, and overall perception of body image improved with the use of a pessary [20]. For SUI, rings with a knob or an incontinence dish can provide support to the urethra and help to pinch it closed with coughing, sneezing, and laughing, preventing leakage. In an RCT comparing women who received behavioral therapy, an incontinence pessary, or both, at 3 months 33% of those assigned to pessary reported improved incontinence symptoms compared to 49% with behavioral therapy, and 63% were satisfied with pessary treatment compared to 75% with behavioral therapy [21,22]. Differences did not persist to 12 months with over one third of all women improved and even more satisfied. A pessary can be safely used in the elderly population but does require office management and regular follow-up to prevent complications.
Initital Treatment
The patient was treated with 3 months of PFPT with biofeedback and pelvic floor muscle strengthening. In addition, she was able to decrease her caffeine use from 4 cups of coffee per day to 1 cup in the morning. At her 3-month follow-up visit, she noticed significant improvement in her voiding symptoms, and her voiding diary showed improved voided volumes and decreased frequency and nocturia. However, she was becoming more active in her community, going to aerobics and dance classes. She was more bothered by the “bulge” feeling in her vagina. She was not interested in a pessary but wanted to hear about surgical options for prolapse treatment.
What is operative management of pelvic organ prolapse?
The goals for surgical pelvic organ prolapse repair are to resolve symptoms, restore normal or near-normal anatomy, preserve sexual, urinary and bowel function, and minimize patient morbidity. The extent of prolapse, patient risk factors for recurrence, patient preference, and overall medical condition all influence the method for surgical repair. Surgeon familiarity and experience is also important when selecting the appropriate repair. Recent concerns regarding the use of synthetic mesh material has become a factor in counseling patients since the 2011 US Food and Drug Administration safety communication on transvaginal mesh [23].
Vaginal Approaches
Numerous techniques for pelvic organ prolapse repair have been described, though most repairs can broadly be divided into vaginal and abdominal procedures. Vaginal surgery is consistently associated with shorter operative times, less postoperative pain, and a shorter length of stay than abdominal approaches. All prolapsing compartments can be addressed vaginally using a patient’s own tissue, often called a “native tissue repair.” The vaginal apex is suspended from either the uterosacral ligament (USL) condensations or to the sacrospinous ligaments (SSL). Sutures are placed through these structures and tied to hold the vaginal vault in place, often at the time of concomitant enterocele, cystocele, or rectocele repairs. A recent randomized trial comparing USL and SSL repair showed composite functional and symptomatic success was 60% at 2 years and did not differ by technique [24]. While overall success may appear low, symptomatic vaginal bulge was present in only 17% to 19% of women at 2 years and only 5% underwent re-treatment with surgery or pessary during follow-up. Similar outcomes have been demonstrated for isolated cystocele repairs plicating the pubo-cervical fascia (anterior colporrhaphy). Cited failure rates have been as high as 70% [25], though this depends on the definition of success. When symptoms of bulge and/or prolapse beyond the hymen are used, success rates are closer to 89% at 2 years [26]. In one study comparing mesh-augmented cystocele repair with native tissue anterior colporrhaphy, 49% of women had a successful composite outcome at 2 years of grade 0 or 1 prolapse and no symptoms of bulge without the use of mesh graft [27]. Despite lower anatomic success rates, anterior colporrhaphy consistently relieves symptoms of bulge with low retreatment rates.
The high failure rates of native tissue vaginal repairs, especially in women with high-grade or recurrent prolapse, led to an interest in graft-augmented repairs. Furthermore, anatomic studies showed that up to 88% of cystoceles were associated with a lateral defect, or tearing of the pubocervical fascia from the pelvic sidewall (arcus tendineous fascia pelvis) [28]. Plicating the already weak fascia centrally would not repair an underlying lateral defect resulting in treatment failure. Replacing this weak fascia with a graft and anchoring it laterally and proximally should result in better anatomic and functional outcomes. These patches can be made from autologous tissue (rectus fascia), donor allograft material (fascia lata), xenografts (porcine dermis, bovine pericardium), or synthetic mesh. Initial studies using cadaveric dermis grafts for recurrent stage II or stage III/IV pelvic organ prolapse resulted in 50% failure at 4 years, but symptomatic failure was only 11% [29]. Further publications utilizing cadaveric tissue patches showed lower rates of cystocele recurrences of 0 to 17% between 20 and 56 months of follow-up [30].
As interest in patch repairs became popular, the use of synthetic mesh was applied to tension-free mid-urethral tapes for SUI. Studies were also showing rapid cadaveric and xenograft graft metabolism, graft extrusion, and early failure in some women [31]. This led to the use of larger pieces of synthetic mesh for prolapse repair, as it had been for abdominal wall and inguinal hernia repairs. Ultimately large-pore, light-weight polypropylene mesh was seen as the most favorable material and large randomized studies were performed to compare outcomes. Theoretically, a synthetic material would provide a replacement for the weakened and torn pubocervical fascia and not be subjected to enzymatic degradation. Altman et al published a widely cited randomized trial comparing native tissue vs. synthetic mesh showing that improvement in the composite primary outcome (no prolapse on the basis of both objective and subjective assessments) was more common in the mesh group (61% vs. 35%) at 1 year. Mesh placement was associated with longer operative times, higher blood loss, and 3.2% of women underwent secondary procedures for vaginal mesh exposure [27]. While there is still debate on the routine use of transvaginal mesh placement, current recommendations generally limit its use for recurrent or high grade pelvic organ prolapse (> Stage III), and possibly those at higher risks for recurrence. The American Urological Association has supported the FDA recommendation that patients undergo a thorough consent process and that surgeons are properly trained in pelvic reconstruction and mesh placement techniques. Furthermore, surgeons placing transvaginal mesh should be equipped to diagnose and treat any complications that may arise subsequent to its use.
Abdominal Approaches
Pelvic organ prolapse can also be approached through an abdominal technique. The classic description for vaginal vault prolapse repair is the abdominal sacrocolpopexy. This involves fixating the vaginal apex to the anterior longitudinal ligament at the sacral promontory. Hysterectomy is performed at the same setting if still in situ. A strip of lightweight polypropylene mesh is sutured to the anterior and posterior vaginal walls after dissecting the bladder and rectum off, then suspended in a tension-free manner to the sacrum. Large trials with long-term follow-up show durability of this repair. Seven-year follow-up of a large NIH-sponsored trial comparing sacrocolpopexy with and without urethropexy found 31/181 (17%) with anatomic prolapse beyond the hymen [32]. Of these women one-third had involvement of the vaginal apex, though 50% of women were asymptomatic. Overall, 95% of women had no retreatment for pelvic organ prolapse. A surprising finding was a 10.5% mesh exposure rate with a mean follow-up of 6.1 years. Previously, abdominally placed mesh was thought to be much safer than transvaginal mesh, but exposure rates are roughly similar in newer studies at high-volume, fellowship-trained centers [33]. The largest advance in abdominal prolapse surgery has come with the adoption of laparoscopic and robotic-assisted technology. Minimally invasive approaches to abdominal surgery have resulted in less blood loss and shorter length of stay, though longer operative times [34]. Short- and medium-term outcomes have been compared to the open techniques in smaller single-center series. At least 1 randomized trial comparing laparoscopic to robotic sacrocolpopexy showed similar complications and perioperative outcomes, though the robotic technique was more costly [35].
Stress Urinary Incontinence Procedures
When SUI is identified preoperatively, treatment should be considered at the time of prolapse repair [32,36]. The gold standard for treatment of SUI with urethral hypermobility has been placement of a synthetic mid-urethral sling. There are several types of slings available, mainly categorized as retropubic, transobturator, or single-incision “mini-slings.” In a multicenter study by the Urinary Incontinence Treatment Network (UITN), patient satisfaction after retropubic and transobturator sling placement was studied 12 months after surgery. Both groups had a high satisfaction rate (from 85% to 90%) for urine leakage, urgency, and frequency [37]. There was no significant difference in outcomes between the 2 approaches. Several other studies and systematic reviews have also shown excellent long-term results with sling treatment. In the recently published 5-year follow-up of the Trial of Mid-Urethral Slings (TOMUS), researchers demonstrated an 80% to 85% patient satisfaction rate with a 10% adverse event rate. Of these adverse events, only 6 were classified as serious requiring surgical, radiologic, or endoscopic intervention [38].
If the patient has SUI but no urethral hypermobility, consider intrinsic sphincter deficiency as the etiology of her incontinence. In that case, injectable therapy with urethral bulking agents is an effective treatment. Some commonly used injectables include carbon beads (Durasphere), calcium hydroxylapatite (Coaptite), bovine collagen (Contigen), and silicon particles (Macroplastique). In a Cochrane review of injectable therapy, they compared urethral injection to conservative treatment with physical therapy and noted an improvement with injection at 3 months. Surgical treatment was overall more effective; however, 50% of the women that received a collagen injection were satisfied at 12 months after the procedure. They also note lower morbidity for this procedure compared to surgery [39].
Treatment in This Patient
The patient underwent successful robotic sacrocolpopexy with mesh and a transobturator sling. There were no complications during the procedure and she reports no bulge or SUI symptoms. She has not been straining to void and has been emptying her bladder well since the Foley catheter was removed the day after surgery. However, she continues to complain of bothersome urgency, frequency, and urge incontinence. She is wearing 1 to 3 pads daily for leakage. At her 6-week postoperative visit, the exam showed excellent vaginal support, no SUI, low PVR, and her urine culture was negative.
What are the clinical implications of these findings?
At this point it is reasonable to continue treatment of OAB. The patient may continue to see improvement as she gets further out from surgery but especially in a patient that had preoperative OAB symptoms, treatment is indicated and may consist of reminding her of behavioral modifications, returning to pelvic floor physical therapy, or starting her on a medication.
What medications are used to treat OAB?
Anticholinergic Drugs
Anticholinergics are second-line therapy for OAB; these medications prevent the binding of acetylcholine to the M3 muscarinic receptor in the detrusor muscle and inhibit uncontrolled bladder contraction. There are numerous medications and delivery methods (pills, patches, gels) but efficacy is similar among the different drugs and all are limited by side effects such as dry mouth, constipation, and central nervous system side effects. Mirabegron, approved by the FDA in June 2012 and released in October 2012, is an agonist of the β3-adrenoceptor receptor in the detrusor muscle promoting bladder storage. A phase III trial found that mirabegron significantly decreased incontinence episodes and micturition frequency compared to placebo [40]. Dry mouth, common with anticholinergics, was 3 times less likely compared to tolterodine [41]. The most common side effects (headache, urinary tract infection, hypertension, and nasopharyngitis) were similar between treatment and placebo groups.
Long-term compliance, side effects, and decreased efficacy limit the benefits of medication therapy [42]. In one survey, 25% of patients taking OAB medications discontinued them within 12 months with 89% reporting unmet treatment expectations and/or tolerability [43].
6 Months Later
The patient continues to complain of persistent OAB symptoms despite anticholinergic and beta-3 agonist therapy. She reported significant constipation and dry mouth with an anticholinergic and symptoms did not improve with mirebegron. Despite having OAB symptoms prior to her prolapse repair, it is important to evaluate for any other cause of her persistent symptoms. Her surgical repair remains intact and urodynamics and cystoscopy were performed showing no evidence of bladder outlet obstruction and no mesh or suture material in the bladder. There was no leakage with valsalva, though she had some early sensation of fullness (sensory urge). With a negative evaluation, refractory OAB is diagnosed and the patient is a candidate for third-line OAB treatment.
What are third-line OAB treatments?
OnabotulinumtoxinA
OnabotulinumtoxinA (Botox) was approved in 2013 for patients intolerant or unresponsive to behavioral therapy and oral medications. OnabotulinumtoxinA is a chemical neuromodulator that cleaves the SNARE protein SNAP-25, inhibits the fusion of the cytoplasmic vesical to the nerve terminal, and prevents the release of acetylcholine. This causes detrusor muscle relaxation and may also inhibit sensory afferent pathways [44].
Nitti et al compared Botox 100 U to placebo in 557 patients that were refractory to anticholinergics [45]. Botox decreased the frequency of daily urinary incontinence episodes vs placebo (–2.65 vs –0.87, P < 0.001) and 22.9% vs 6.5% of patients became completely continent. A 5.4% rate of urinary retention occurred and UTI was the most common side effect (16%) in those receiving active drug. A dose of 100 U is recommended to limit side effects while maintaining efficacy [46].
Comparision of a daily anticholinergic (solifenacin) versus Botox 100 U for 6 months was done in a randomized double-blind, double-placebo-controlled trial [47]. Patients underwent saline injection or took an oral placebo in the anticholinergic and Botox groups, respectively. Complete resolution of urinary symptoms occurred in 13% of the medication group and 27% of the Botox group (P = 0.003). Dry mouth was more common in the medication group (46% vs. 31%) and the Botox group had a higher rate of catheter use and urinary tract infections (5% vs. 0%; 33% vs. 13%). Quality of life measures have also been shown to improve significantly following Botox injection [45,48].
When considering whether Botox is appropriate for a particular patient, physicians must determine whether the patient is willing and able to perform clean intermittent catheterization. Contraindications include active UTI, urinary retention, unwilling or unable to do clean intermittent catheterization, and known hypersenstivitiy to botulinum toxin type A. Although the definition of urinary retention and the PVR at which clean intermittent catheterization should be initiated varies, one study found a 94% rate of urinary retention with a preoperative PVR > 100 mL [49].
Botox can be administered in the clinic with or without local anesthetic but general anesthetic may be used in patients who might be poorly tolerant of the procedure. Using flexible or rigid cystoscopy, the bladder is filled to 100 to 200 mL. An injection needle is used to inject 0.5 cc aliquots of reconstituted onabotulinumtoxinA in 20 areas spaced 1 cm apart. Periprocedure antibiotics are recommended by the manufacturer but actual usage varies [50]. Patients should understand that the effects of Botox may take up to 4 weeks and an appointment should be scheduled within 2 weeks to evaluate PVR and any other adverse reactions. Repeat injections are needed between 3 to 9 months as symptoms return; however, efficacy is maintained with subsequent treatments [51].
Neuromodulation
Additional third-line treatment options include sacral or posterior tibial nerve neuromodulation. Sacral neuromodulation has been FDA approved for treatment of urgency, frequency and urgency incontinence since 1997. Also known as InterStim (Medtronic, Minneapolis, MN), this involves placement of a tined electrode adjacent to the S3 nerve root and is thought to result in modulation of the afferent nerve signals from the bladder to the spinal cord and the pontine micturition center.
Since the FDA approved sacral neuromodulation, long-term results for this therapy have been positive. A multicenter study with a 5-year follow-up showed a statistically significant reduction in daily leakage episodes, number of daily voids, and increase in voided volume, with a 5-year success rate of 68% for urgency incontinence and 56% for urgency/frequency [52]. Al-Zahrani et al followed 96 patients (35% with urgency incontinence) for a mean of 50.7 months and approximately 85% of the incontinent patients remained improved [53]. Conversely, Groen et al observed a gradual decrease in success rate from 1 month to 5 years in 60 women with urge incontinence, with only 15% completely continent at 5 years [54].
Sacral neuromodulation is typically performed in 2 stages. The first stage is electrode placement and trial period. A percutaneous nerve evaluation is a temporary electrode placement in the office or a permanent lead placement can be performed in the operating room. Correct placement stimulating the S3 nerve root is confirmed by motor and/or sensory testing. If there is an appropriate response, the electrode lead is connected to a temporary external pulse generator and is worn by the patient for a 2–14 day test period. If more than a 50% improvement in symptoms occur, a permanent lead and battery is placed in the operating room. If there is inadequate symptom response, the lead is removed. There are several recognized limitations of the office percutaneous nerve evaluation compared to operating room lead placement, including false-negative responses, possibly due to lead migration [55], incorrect lead placement, or an inadequate test period [56]. However, it is relatively noninvasive and potentially avoids 2 operating room procedures. Regardless of the choice of the initial test period, sacral neuromodulation offers a minimally invasive, long-term treatment option for refractory OAB.
Percutaneous tibial nerve stimulation (PTNS) is an office procedure that stimulates the posterior tibial nerve. This nerve contains L4–S3 fibers that originate from the same spinal segments that innervate the bladder and pelvic floor. In comparision to sacral neuromodulation, percutaneous tibial nerve stimulation is less invasive, less expensive and there is no permanent implant required [57].
Percutaneous tibial nerve stimulation is performed by a physician, nurse, or other advanced practice provider. Patients sit with knees abducted and the leg externally rotated. A 34-gauge needle is inserted 3 cm into the skin 3 fingerbreadths above the medial malleolus. The Urgent PC Neuromodulation System (Uroplasty, Minnetonka, MN) is attached and the amplitude of the stimulation is increased until the large toe curls or the toes fan. Each session lasts 30 minutes and 12 weekly treatments provides the best improvement in patient symptoms [58, 59]. There is a strong carry-over effect and patients generally need re-treatments every 4-6 weeks for 30 minutes.
Percutaneous tibial nerve stimulation has been compared favorably to both anticholinergic and sham treatments. The Overactive Bladder Innovative Therapy Trial (OrBIT) randomized 100 patients to PTNS or tolterodine for 12 weeks. The global response assessment demonstrated a statistically significant subjective improvement or cure over baseline in OAB symptoms in 79.5% of the PTNS group vs. 54.8% of the tolterodine group (P = 0.01)[60]. The SUmiT trial compared the efficacy of PTNS to sham for 12 weeks of therapy [59]. In this multicenter study, subjects were assessed at 13 weeks using the global response assessment for overall bladder symptoms. 55% of PTNS subjects achieved moderately or marked improvement in bladder symptoms compared to 20.9% of sham subjects (P < 0.001). Voiding diary parameters also improved compared to sham. In an earlier sham controlled trial, 12 patients (71%) in the treatment arm compared to none of the 15 placebo patients, demonstrated more than 50% improvement in diary and quality of life scores [61].
To evaluate long-term efficacy and safety, 36-month results of 29 positive responders of the initial SUmiT trial was reported [59]. In addition, a maintenance regimen was developed so patients received PTNS at tapering intervals over a 3-month period followed by a personalized treatment plan to sustain subjective improvement in their symptoms. With an average of 1 treatment a month, symptom severity scores and health related quality of life scores were statistically significant for improvement at each tested time-point. Yoong et al followed patients for 2 years following initial treatment with PTNS and confirmed a durable improvement in nocturia, frequency, urgency incontinence and symptom scores with a longer median length between treatments of 64 days [62].
PTNS is office-based, has few side effects, and avoids an implantable device. In addition, continuous stimulation is not necessary and a decreased treatment frequency is needed over time. Limitations include the time commitment that is required for both the initial treatment phase and the maintenance phase. Logistical concerns of weekly and monthly office visits or arranging for transportation can limit treatment.
Additional Treatment
The patient received injection of 100 U of Botox in the office. At her 2-week follow up appointment, her PVR was 90 mL and she was already seeing improvement in her incontinence episodes. At 6 weeks she was wearing 1 pad per day but using it mainly for protection. She still notices urgency, particularly if she drinks more than 1 cup of coffee in the morning, but overall she reports significant improvement in her symptoms. She has no complaints of a vaginal bulge and on exam has a grade 1 distal rectocele and no SUI with a full bladder. The physician discussed need for continued yearly examinations and repeat injections due to the duration of action of Botox.
Conclusion
This case demonstrates the complex step-wise management strategy of a patient with pelvic organ prolapse and voiding dysfunction. Interventions directed at patient bother and recognition of the various modalities and timing of treatment are essential to provide the greatest chance of positive treatment outcomes and patient satisfaction.
Corresponding author: Jaimie M Bartley, DO, 3601 W. 13 Mile Rd., Royal Oak, MI 48073.
Financial disclosures: None.
1. Nygaard I, Barber MD, Burgio KL, et al; Pelvic Floor Disorders Network. Prevalence of symptomatic pelvic floor disorders in US women. JAMA 2008;300:1311–6.
2. Hendrix SL, Clark A, Nygaard I, et al. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol 2002;186:1160–6.
3. Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J 2013;24:1783–90.
4. Zhang C, Hai T, Yu L, et al. Association between occupational stress and risk of overactive bladder and other lower urinary tract symptoms: a cross-sectional study of female nurses in China. Neurourol Urodyn 2013;32:254–60.
5. Swift SE, Tate SB, Nicholas J. Correlation of symptoms with degree of pelvic organ support in a general population of women: what is pelvic organ prolapse? Am J Obstet Gynecol 2003;189:372–7.
6. Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J 2013;24:1843–52.
7. Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996;175:10–7.
8. Lohsiriwat S, Hirunsai M, Chaiyaprasithi B. Effect of caffeine on bladder function in patients with overactive bladder symptoms. Urol Ann 2011;3:14–8.
9. Wells MJ, Jamieson K, Markham TC, et al. The effect of caffeinated versus decaffeinated drinks on overactive bladder: a double-blind, randomized, crossover study. J Wound Ostomy Continence Nurs 2014;41:371–8.
10. Fantl JA, Wyman JF, McClish DK, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA 1991;265:609–13.
11. Burgio KL, Locher JL, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA 1998;280:1995–2000.
12. Burgio KL. Update on behavioral and physical therapies for incontinence and overactive bladder: the role of pelvic floor muscle training. Curr Urol Rep 2013;14:457–64.
13. Burgio KL, Kraus SR, Menefee S, et al; Urinary Incontinence Treatment Network. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med 2008;149:161–9.
14. Gormley EA, Lightner DJ, Faraday M, Vasavada SP. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment. J Urol 2015;193:1572–80.
15. Dumoulin C, Hay-Smith J, Habée-Séguin GM, Mercier J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: A short version Cochrane systematic review with meta-analysis. Neurourol Urodyn 2015;34:300–8.
16. Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. N Engl J Med 2013;369:1124–33.
17. Braekken IH, Majida M, Engh ME, Bø K. Can pelvic floor muscle training reverse pelvic organ prolapse and reduce prolapse symptoms? An assessor-blinded, randomized, controlled trial. Am J Obstet Gynecol 2010;203:170.e1–7.
18. Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet 2014;383:796–806.
19. Alperin M, Khan A, Dubina E, et al. Patterns of pessary care and outcomes for medicare beneficiaries with pelvic organ prolapse. Female Pelvic Med Reconstr Surg 2013;19:142-7.
20. Lamers BH, Broekman BM, Milani AL. Pessary treatment for pelvic organ prolapse and health-related quality of life: a review. Int Urogynecol J 2011;22:637–44.
21. Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol 2010;115:609–17.
22. Wood LN, Anger JT. Urinary incontinence in women. BMJ 2014;349:g4531.
23. FDA Safety Communication. Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse. Available at http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm.
24. Barber MD, Brubaker L, Burgio KL, Meikle SF; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA 2014;311:1023–34.
25. Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol 2001;185:1299–304.
26. Chmielewski L, Walters MD, Weber AM, Barber MD. Reanalysis of a randomized trial of 3 techniques of anterior colporrhaphy using clinically relevan tdefinitions of success. Am J Obstet Gynecol 2011;205:69.e1–8.
27. Altman D, Väyrynen T, Engh ME, et al; Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364:1826–36. Erratum in: N Engl J Med 2013;368:394.
28. Delancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol 2002;187:93–8.
29. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol 2003;189:1612–8.
30. Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ prolapse procedures. J Urol 2004;171:1581–4.
31. Handel LN, Frenkl TL, Kim YH. Results of cystocele repair: a comparison of traditional anterior colporrhaphy, polypropylene mesh and porcine dermis. J Urol 2007;178:153–6.
32. Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA 2013;309:2016–24.
33. Sirls LT, McLennan GP, Killinger KA, et al. Exploring predictors of mesh exposure after vaginal prolapse repair. Female Pelvic Med Reconstr Surg 2013;19:206–9.
34. Hsiao KC, Latchamsetty K, Govier FE, et al. Comparison of laparoscopic and abdominal sacrocolpopexy for the treatment of vaginal vault prolapse. J Endourol 2007;21:926–30.
35. Anger JT, Mueller ER, Tarnay C, et al. Robotic compared with laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol 2014;123:5–12.
36. Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med 2012;366:2358–67.
37. Wai CY, Curto TM, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Patient satisfaction after midurethral sling surgery for stress urinary incontinence. Obstet Gynecol 2013;121:1009–16.
38. Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. J Urol 2015;193:203–10.
39. Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev 2012;2:CD003881.
40. Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur Urol 2013;63:296–305.
41. Nitti VW, Auerbach S, Martin N, et al. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol 2013;189:1388–95.
42. Dmochowski RR, Newman DK. Impact of overactive bladder on women in the United States: results of a national survey. Curr Med Res Opin 2007;23:65–76.
43. Benner JS, Nichol MB, Rovner ES, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 2010;105:1276–82.
44. Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol 2006;49:644–50.
45. Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol 2013;189:2186–93.
46. Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol 2010;184:2416–22.
47. Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial. Contemp Clin Trials 2012;33:184–96.
48. Chapple C, Sievert KD, MacDiarmid S, et al. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double-blind, placebo-controlled trial. Eur Urol 2013;64:249–56.
49. Osborn DJ, Kaufman MR, Mock S, et al. Urinary retention rates after intravesical onabotulinumtoxinA injection for idiopathic overactive bladder in clinical practice and predictors of this outcome. Neurourol Urodyn 2014 Jun 29.
50. Rovner E. Chapter 6: Practical aspects of administration of onabotulinumtoxinA. Neurourol Urodyn 2014;33 Suppl 3:S32–7.
51. Duthie JB, Vincent M, Herbison GP, et al. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database Syst Rev 2011;(12):CD005493.
52. van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 2007;178:2029–34.
53. Al-zahrani AA, Elzayat EA, Gajewski JB. Long-term outcome and surgical interventions after sacral neuromodulation implant for lower urinary tract symptoms: 14-year experience at 1 center. J Urol 2011;185:981–6.
54. Groen J, Blok BF, Bosch JL. Sacral neuromodulation as treatment for refractory idiopathic urge urinary incontinence: 5-year results of a longitudinal study in 60 women. J Urol 2011;186:954–9.
55. Carey M, Fynes M, Murray C, Maher C. Sacral nerve root stimulation for lower urinary tract dysfunction: overcoming the problem of lead migration. BJU Int 2001;87:15–8.
56. Everaert K, Kerckhaert W, Caluwaerts H, et al. A prospective randomized trial comparing the 1-stage with the 2-stage implantation of a pulse generator in patients with pelvic floor dysfunction selected for sacral nerve stimulation. Eur Urol 2004;45:649–54.
57. Staskin DR, Peters KM, MacDiarmid S, et al. Percutaneous tibial nerve stimulation: a clinically and cost effective addition to the overactive bladder algorithm of care. Curr Urol Rep 2012;13:327–34.
58. Peters KM, Carrico DJ, Wooldridge LS, et al. Percutaneous tibial nerve stimulation for the long-term treatment of overactive bladder: 3-year results of the STEP study. J Urol 2013;189:2194–201.
59. Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 2010;183:1438–43.
60. Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 2009;182:1055–61.
61. Finazzi-Agrò E, Petta F, Sciobica F, et al. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, double-blind, placebo controlled trial. J Urol 2010;184:2001–6.
62. Yoong W, Shah P, Dadswell R, Green L. Sustained effectiveness of percutaneous tibial nerve stimulation for overactive bladder syndrome: 2-year follow-up of positive responders. Int Urogynecol J 2013;24:795–9.
1. Nygaard I, Barber MD, Burgio KL, et al; Pelvic Floor Disorders Network. Prevalence of symptomatic pelvic floor disorders in US women. JAMA 2008;300:1311–6.
2. Hendrix SL, Clark A, Nygaard I, et al. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol 2002;186:1160–6.
3. Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J 2013;24:1783–90.
4. Zhang C, Hai T, Yu L, et al. Association between occupational stress and risk of overactive bladder and other lower urinary tract symptoms: a cross-sectional study of female nurses in China. Neurourol Urodyn 2013;32:254–60.
5. Swift SE, Tate SB, Nicholas J. Correlation of symptoms with degree of pelvic organ support in a general population of women: what is pelvic organ prolapse? Am J Obstet Gynecol 2003;189:372–7.
6. Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J 2013;24:1843–52.
7. Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996;175:10–7.
8. Lohsiriwat S, Hirunsai M, Chaiyaprasithi B. Effect of caffeine on bladder function in patients with overactive bladder symptoms. Urol Ann 2011;3:14–8.
9. Wells MJ, Jamieson K, Markham TC, et al. The effect of caffeinated versus decaffeinated drinks on overactive bladder: a double-blind, randomized, crossover study. J Wound Ostomy Continence Nurs 2014;41:371–8.
10. Fantl JA, Wyman JF, McClish DK, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA 1991;265:609–13.
11. Burgio KL, Locher JL, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA 1998;280:1995–2000.
12. Burgio KL. Update on behavioral and physical therapies for incontinence and overactive bladder: the role of pelvic floor muscle training. Curr Urol Rep 2013;14:457–64.
13. Burgio KL, Kraus SR, Menefee S, et al; Urinary Incontinence Treatment Network. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med 2008;149:161–9.
14. Gormley EA, Lightner DJ, Faraday M, Vasavada SP. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment. J Urol 2015;193:1572–80.
15. Dumoulin C, Hay-Smith J, Habée-Séguin GM, Mercier J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: A short version Cochrane systematic review with meta-analysis. Neurourol Urodyn 2015;34:300–8.
16. Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. N Engl J Med 2013;369:1124–33.
17. Braekken IH, Majida M, Engh ME, Bø K. Can pelvic floor muscle training reverse pelvic organ prolapse and reduce prolapse symptoms? An assessor-blinded, randomized, controlled trial. Am J Obstet Gynecol 2010;203:170.e1–7.
18. Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet 2014;383:796–806.
19. Alperin M, Khan A, Dubina E, et al. Patterns of pessary care and outcomes for medicare beneficiaries with pelvic organ prolapse. Female Pelvic Med Reconstr Surg 2013;19:142-7.
20. Lamers BH, Broekman BM, Milani AL. Pessary treatment for pelvic organ prolapse and health-related quality of life: a review. Int Urogynecol J 2011;22:637–44.
21. Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol 2010;115:609–17.
22. Wood LN, Anger JT. Urinary incontinence in women. BMJ 2014;349:g4531.
23. FDA Safety Communication. Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse. Available at http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm.
24. Barber MD, Brubaker L, Burgio KL, Meikle SF; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA 2014;311:1023–34.
25. Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol 2001;185:1299–304.
26. Chmielewski L, Walters MD, Weber AM, Barber MD. Reanalysis of a randomized trial of 3 techniques of anterior colporrhaphy using clinically relevan tdefinitions of success. Am J Obstet Gynecol 2011;205:69.e1–8.
27. Altman D, Väyrynen T, Engh ME, et al; Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364:1826–36. Erratum in: N Engl J Med 2013;368:394.
28. Delancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol 2002;187:93–8.
29. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol 2003;189:1612–8.
30. Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ prolapse procedures. J Urol 2004;171:1581–4.
31. Handel LN, Frenkl TL, Kim YH. Results of cystocele repair: a comparison of traditional anterior colporrhaphy, polypropylene mesh and porcine dermis. J Urol 2007;178:153–6.
32. Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA 2013;309:2016–24.
33. Sirls LT, McLennan GP, Killinger KA, et al. Exploring predictors of mesh exposure after vaginal prolapse repair. Female Pelvic Med Reconstr Surg 2013;19:206–9.
34. Hsiao KC, Latchamsetty K, Govier FE, et al. Comparison of laparoscopic and abdominal sacrocolpopexy for the treatment of vaginal vault prolapse. J Endourol 2007;21:926–30.
35. Anger JT, Mueller ER, Tarnay C, et al. Robotic compared with laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol 2014;123:5–12.
36. Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med 2012;366:2358–67.
37. Wai CY, Curto TM, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Patient satisfaction after midurethral sling surgery for stress urinary incontinence. Obstet Gynecol 2013;121:1009–16.
38. Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. J Urol 2015;193:203–10.
39. Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev 2012;2:CD003881.
40. Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur Urol 2013;63:296–305.
41. Nitti VW, Auerbach S, Martin N, et al. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol 2013;189:1388–95.
42. Dmochowski RR, Newman DK. Impact of overactive bladder on women in the United States: results of a national survey. Curr Med Res Opin 2007;23:65–76.
43. Benner JS, Nichol MB, Rovner ES, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 2010;105:1276–82.
44. Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol 2006;49:644–50.
45. Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol 2013;189:2186–93.
46. Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol 2010;184:2416–22.
47. Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial. Contemp Clin Trials 2012;33:184–96.
48. Chapple C, Sievert KD, MacDiarmid S, et al. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double-blind, placebo-controlled trial. Eur Urol 2013;64:249–56.
49. Osborn DJ, Kaufman MR, Mock S, et al. Urinary retention rates after intravesical onabotulinumtoxinA injection for idiopathic overactive bladder in clinical practice and predictors of this outcome. Neurourol Urodyn 2014 Jun 29.
50. Rovner E. Chapter 6: Practical aspects of administration of onabotulinumtoxinA. Neurourol Urodyn 2014;33 Suppl 3:S32–7.
51. Duthie JB, Vincent M, Herbison GP, et al. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database Syst Rev 2011;(12):CD005493.
52. van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 2007;178:2029–34.
53. Al-zahrani AA, Elzayat EA, Gajewski JB. Long-term outcome and surgical interventions after sacral neuromodulation implant for lower urinary tract symptoms: 14-year experience at 1 center. J Urol 2011;185:981–6.
54. Groen J, Blok BF, Bosch JL. Sacral neuromodulation as treatment for refractory idiopathic urge urinary incontinence: 5-year results of a longitudinal study in 60 women. J Urol 2011;186:954–9.
55. Carey M, Fynes M, Murray C, Maher C. Sacral nerve root stimulation for lower urinary tract dysfunction: overcoming the problem of lead migration. BJU Int 2001;87:15–8.
56. Everaert K, Kerckhaert W, Caluwaerts H, et al. A prospective randomized trial comparing the 1-stage with the 2-stage implantation of a pulse generator in patients with pelvic floor dysfunction selected for sacral nerve stimulation. Eur Urol 2004;45:649–54.
57. Staskin DR, Peters KM, MacDiarmid S, et al. Percutaneous tibial nerve stimulation: a clinically and cost effective addition to the overactive bladder algorithm of care. Curr Urol Rep 2012;13:327–34.
58. Peters KM, Carrico DJ, Wooldridge LS, et al. Percutaneous tibial nerve stimulation for the long-term treatment of overactive bladder: 3-year results of the STEP study. J Urol 2013;189:2194–201.
59. Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 2010;183:1438–43.
60. Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 2009;182:1055–61.
61. Finazzi-Agrò E, Petta F, Sciobica F, et al. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, double-blind, placebo controlled trial. J Urol 2010;184:2001–6.
62. Yoong W, Shah P, Dadswell R, Green L. Sustained effectiveness of percutaneous tibial nerve stimulation for overactive bladder syndrome: 2-year follow-up of positive responders. Int Urogynecol J 2013;24:795–9.
A physician who feels hopeless and worthless and complains of pain
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
Grapefruit juice and psychotropics: How to avoid potential interactions
Ms. H, age 42, was given a diagnosis of bipolar disorder 10 years ago and has been taking carbamazepine, 1,200 mg/d, and olanzapine, 10 mg/d, for the past 2 years. She has not experienced a mood episode while on this regimen, and her carbamazepine level was 9.2 μg/mL 6 months ago. The only adverse effect she experienced was weight gain of approximately 10 lb. Ms. H takes a calcium supplement, but no other medications.
Ms. H reports to her psychiatrist that, for the past few days, she has been feeling nauseated, fatigued, and dizzy, but has continued taking her medications as prescribed. Her carbamazepine level is found to be 13.1 μg/mL. Ms. H states she has not started any new medications or supplements; her serum creatinine and liver function test results are within normal limits.
Upon further questioning, Ms. H says that an upper respiratory infection has been “going around her office,” so she increased her vitamin C intake by drinking 2 glasses of grapefruit juice a day (she doesn’t like orange juice). She has heard grapefruit juice can cause problems with some drugs so she is careful not to drink it at the same time she takes her medications. Her psychiatrist recognizes there may be a drug interaction involved, and recommends Ms. H hold her carbamazepine for 1 day and not consume any more grapefruit juice. A few days later, she reports feeling much better during a follow-up call and she makes an appointment to have her carbamazepine level rechecked in a we
Although grapefruit products are high in vitamins and low in calories, they can be associated with potentially serious drug interactions. The interaction between grapefruit juice and the calcium channel blocker felodipine was discovered inadvertently >20 years ago; since that time, possible interactions with >85 medications have been identified.1 Interactions with grapefruit products are complicated because, although most result in increased drug exposure, reduced exposure of the medication also can occur. Additionally, the degree and clinical significance of the interaction varies among individuals and from one drug to another.
Mechanism of action
Most interactions with grapefruit products are thought to result from the inhibition of intestinal cytochrome P450 3A4 (CYP3A4). CYP3A4 is involved in the metabolism of numerous drugs, and is the most abundant cytochrome P450 enzyme in the liver and epithelial cells lining the intestine.2 Although hepatic CYP3A4 is thought to be minimally affected by grapefruit, inhibition of intestinal CYP3A4 can result in an overall increase in bioavailability of medications that are substrates and raise the risk of potential toxicity.3 Grapefruit contains various chemicals collectively known as furanocoumarins, which are largely responsible for inhibition of intestinal CYP3A4.4 Additionally, Seville oranges and the pomelo (a large, sweet grapefruit-like citrus fruit) also contain furanocoumarins and could have a similar effect, warranting caution with certain medications.5
Inhibition of CYP3A4 by furanocoumarins cannot be reversed, and new enzymes must be synthesized to return to the previous level of function.6 Therefore, drug interactions resulting from CYP3A4 inhibition can last for as long as 72 hours after ingesting grapefruit products.7 Separating consumption of grapefruit products and medication administration will not help manage this interaction.
Grapefruit products also could affect drug disposition through effects on various drug transporters. Decreased systemic exposure to certain medications could occur through grapefruit’s inhibition of organic anion-transporting polypeptides (OATPs). OATPs form a family of drug uptake transporters found in the intestine, liver, kidney, and brain.8 For drugs that are substrates of OATPs, grapefruit’s inhibition of this transporter can result in decreased absorption and a resulting decrease in efficacy. Flavanoids in grapefruit, such as naringin, inhibit OATPs, which is competitive in nature.9 Unlike the irreversible inhibition of CYP3A4 by furanocoumarins, flavanoids effects on OATPs have been shown to decrease within 4 hours.10
No psychotropic medications have been identified as being susceptible to this interaction, but for those medications affected—including fexofenadine and levothyroxine—separating consumption of grapefruit and medication administration by 4 hours could avoid this interaction.11 Additional data indicate that orange juice and apple juice could have similar effects on OATPs.12
Perhaps the most well-known drug transporter, P-glycoprotein is part of the multidrug-resistant subfamily of transporters. It is located throughout the body, including in the intestine, kidneys, liver, and blood-brain barrier. P-glycoprotein acts as an export pump to decrease the cellular concentration of many different drug substrates, and many agents can alter P-glycoprotein’s expression or function.
Small changes in P-glycoprotein’s activity can result in substantial changes in the disposition of substrates, which can include certain antineoplastics and antiretrovirals. Most reports have found grapefruit juice inhibits P-glycoprotein-mediated efflux; however, there also are reports of transporter activation.6 Additionally, P-glycoprotein and CYP3A4 share many substrates, so it can be difficult to isolate the contribution of P-glycoprotein to grapefruit−drug interactions.13 The effect of grapefruit on P-glycoprotein activity has been difficult to fully elucidate; more studies are needed.
Grapefruit consumption and its effect
Drug interactions can occur by consuming commercially produced grapefruit juice and juice from concentrate, as well as freshly squeezed juice and grapefruit segments.14 CYP3A4-inhibiting furanocoumarins also have been isolated in grapefruit peel; it is not known, however, whether items made from peel (marmalade, candied peel) contain concentrations high enough to pose a risk of a drug interaction.14 Contributing to the unpredictability of grapefruit-drug interactions, the amount or concentration of furanocoumarins can vary among grapefruit products and brands.15 This variability can be influenced by the variety or maturity of the fruit and the fruit’s exposure to environmental stress.4
The frequency of consuming a grapefruit product can influence the degree of a drug interaction. In general, consuming one 8-oz glass of grapefruit juice or the segments from a whole grapefruit is enough to alter a susceptible drug’s pharmacokinetics.14 Regular grapefruit product consumption, however, can result in an overall greater effect.16,17
Lilja et al16 conducted a randomized, 4-phase, crossover study to look at the effect of grapefruit juice dose on kinetics of triazolam. Grapefruit juice was found to increase the mean area under the concentration-time curve (AUC) of triazolam compared with water, but no difference was found between single glasses of normal-strength and double-strength grapefruit juice. However, repeated consumption of double-strength grapefruit juice (200 mL, 3 times/d for 3 days) increased triazolam’s mean AUC by 143%, compared with an increase of 49% with just a single 200-mL glass of double-strength juice.16 Recurrent consumption of grapefruit juice (8 oz, 3 times/d for 6 days) also was found to increase the kinetics of the antihypertensive felodipine more than a single glass of grapefruit juice.17
Clinical consequences of an interaction between a drug and grapefruit can be difficult to predict. Drug concentration changes caused by a grapefruit interaction could vary based on interindividual differences. The amount and activity of intestinal CYP3A4 can vary from person to person, and can be influenced by genetic polymorphisms in addition to race, age, and environmental variables.18 Interindividual sensitivity to a change in a drug’s concentration also will differ, and patient-specific factors, such as concomitant drugs or diseases, could influence the likelihood of harm.
Interactions with grapefruit products are not necessarily a “class effect,” and specific drugs within a therapeutic category can be affected (although others might not). Several drug-specific characteristics can help gauge the risk of a clinically relevant interaction with grapefruit, including:
• metabolism through CYP3A4
• low bioavailability
• oral administration
• a narrow therapeutic index.1
For drugs with low bioavailability because of first-pass metabolism, grapefruit’s inhibition of intestinal CYP3A4 can result in a greater relative increase in plasma concentrations compared with a drug with high bioavailability.19
For example, an increase in bioavailability from 5% to 10% will result in a much larger increase in AUC and overall clinical exposure compared with an increase from 85% to 90% even though both represent an absolute increase of 5%. Although a drug does not have to have low oral bioavailability for an interaction to occur, lower bioavailability means that a drug has a higher likelihood of causing a significant interaction because of altered pharmacokinetics. Of note, injectable medications will not interact with grapefruit because metabolism through intestinal CYP3A4 is bypassed and grapefruit does not significantly inhibit hepatic CYP3A4.
Although grapefruit products could alter the pharmacokinetics of susceptible drugs, those changes might not be associated with adverse effects. Therefore, a factor to consider in evaluating a potential interaction with grapefruit is the drug’s therapeutic index and its risk of serious adverse effects. Drugs with a narrow therapeutic index are of particular concern because a significant increase in therapeutic or adverse effects could result from a relatively small increase in the drug’s concentration.7
Which medications are affected?
Among medications identified as interacting with grapefruit, some cardiovascular agents and several of the HMG-CoA reductase inhibitors (statins) have garnered the most attention. However, grapefruit also can affect the metabolism of several psychotropic medications through inhibition of intestinal CYP3A4 (Table).16,20-35 Prescribing information for some drugs warns against consuming grapefruit while using the medication. Among CNS agents, buspirone, carbamazepine, lurasidone, pimozide, triazolam, and oral midazolam all have such warnings in their product labeling.
Buspirone currently is not recommended with “large quantities of grapefruit juice.”20 A randomized, 2-phase crossover study looking at the effects of grapefruit juice on buspirone’s pharmacokinetics found that double-strength grapefruit juice (200 mL, administered 3 times/d for 3 days) resulted in a 9.2-fold increase in mean AUC and a 4.3-fold increase in mean Cmax after a single 10-mg buspirone dose.22 Highlighting the wide interindividual variability seen with drug-grapefruit interactions, the increase found in buspirone’s AUC ranged from 3-fold to 20-fold among study participants.22
Carbamazepine product labeling lists grapefruit juice as a CYP3A4 inhibitor that is expected to or has been found to increase plasma levels of the drug.20 Carbamazepine’s bioavailability is influenced by intestinal CYP3A4 activity; in a randomized, 2-phase crossover study of 10 patients with epilepsy, grapefruit juice was found to increase AUC of carbamazepine by 41% and Cmax by 40%.23,36
Lurasidone and pimozide, although not specifically studied, have product labels that recommend avoiding grapefruit juice because it could inhibit metabolism of these agents by CYP3A4.20 Of particular concern is the potential for elevated levels of pimozide to increase the risk of adverse cardiovascular effects including QT interval prolongation.19
Midazolam. Although grapefruit juice does not affect the disposition of IV midazolam, pretreatment with grapefruit juice was found to increase the AUC and Cmax of oral midazolam by 52% and 56%, respectively.30
Other considerations in drug-grapefruit interactions
Cautionary statements about a possible interaction with grapefruit juice for many other psychotropics can be found in commonly used drug information references or online sources. If you are concerned about a possible interaction and avoiding grapefruit products is not feasible, consider a different medication in the same class.
However, you also should consider the level of evidence supporting any purported interaction. Several psychotropic agents do have studies or case reports supporting an interaction with grapefruit, but cautionary statements could be based on theoretical concerns because of a medication’s bioavailability, metabolic pathway, and concern for increased adverse events related to higher drug concentrations. Adding to the confusion, cautionary statements can be found about medications, such as clozapine, that have not been shown to have an interaction with grapefruit juice when studied.
With many of the drugs that have a reported or theoretical interaction with grapefruit, data are inconsistent as to whether the resulting interaction will be clinically relevant. A number of variables relating to the individual patient, grapefruit product, or particular drug can play a role in the significance of an interaction. Additionally, effects on drug disposition can last for a few days after consuming a grapefruit product.
Keep alert to situations of increased risk
Recall that the case patient, Ms. H, presented with an elevated carbamazepine level and suffered resulting adverse effects because of an interaction between the drug and grapefruit juice. Although Ms. H was careful to separate intake of grapefruit juice from carbamazepine administration, grapefruit’s inhibition of intestinal CYP3A4 still was present, leading to the interaction.
It is important for health care professionals to recognize this potential risk and to advise patients regarding possible interactions between medications and grapefruit products.
Related Resources
• U.S. Food and Drug Administration. Grapefruit juice and medicine may not mix. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm292276.htm.
• Hanley MJ, Cancalon P, Widmer WW, et al. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol. 2011;7(3):267-286.
• Andrade C. Fruit juice, organic anion transporting polypeptides, and drug interactions in psychiatry. J Clin Psychiatry. 2014;75(11):e1323-e1325.
Drug Brand Names
Alprazolam • Xanax Lurasidone • Latuda
Buspirone • BuSpar Midazolam • Versed
Carbamazepine • Tegretol Methadone • Dolophine
Clomipramine • Anafranil Nefazodone • Serzone
Clozapine • Clozaril Olanzapine • Zyprexa
Diazepam • Valium Pimozide • Orap
Felodipine • Plendil Quetiapine • Seroquel
Fexofenadine • Allegra Sertraline • Zoloft
Fluoxetine • Prozac Trazodone • Desyrel
Fluvoxamine • Luvox Triazolam • Halcion
Haloperidol • Haldol Ziprasidone • Geodon
Levothyroxine • Levoxyl, Synthroid
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bailey DG, Dresser G, Arnold JM. Grapefruit-medication interactions: forbidden fruit or avoidable consequences? CMAJ. 2013;185(4):309-316.
2. Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352(21): 2211-2221.
3. Saito M, Hirata-Koizumi M, Matsumoto M, et al. Undesirable effects of citrus juice on the pharmacokinetics of drugs: focus on recent studies. Drug Saf. 2005;28(8):677- 694.
4. Cancalon PF, Barros SM, Haun C, et al. Effect of maturity, processing, and storage on the furanocoumarin composition of grapefruit and grapefruit juice. J Food Sci. 2011;76(4):C543-C548.
5. Pirmohamed M. Drug-grapefruit juice interactions: two mechanisms are clear but individual responses vary. BMJ. 2013;346:f1. doi: 10.1136/bmj.f1.
6. Dahan A, Altman H. Food-drug interaction: grapefruit juice augments drug bioavailability–mechanism, extent and relevance. Eur J Clin Nutr. 2004;58:1-9.
7. Stump AL, Mayo T, Blum A. Management of grapefruit-drug interactions. Am Fam Physician. 2006;74(4):605-608.
8. Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest. 2003;33(suppl 2):1-5.
9. Bailey DG, Dressker GK, Leak BF, et al. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81(4):495-502.
10. Glaeser H, Bailey DG, Dresser GK, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81(3):362-370.
11. Bailey DG. Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010;70(5): 645-655.
12. Dresser GK, Bailey DG, Leake BF, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11-20.
13. Seden K, Dickinson L, Khoo S, et al. Grapefruit-drug interactions. Drugs. 2010;70(18):2373-2407.
14. Bailey DG, Dresser GK, Kreeft JH, et al. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther. 2000;68(5):468-477.
15. De Castro WV, Mertens-Talcott S, Rubner A, et al. Variation of flavonoids and furanocoumarins in grapefruit juices: a potential source of variability in grapefruit juice-drug interaction studies. J Agric Food Chem. 2006;54(1):249-255.
16. Lilja JJ, Kivistö KT, Backman JT, et al. Effect of grapefruit juice on grapefruit juice-triazolam interaction: repeated consumption prolongs triazolam half-life. Eur J Clin Pharmacol. 2000;56(5):411-415.
17. Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99(10):2545-2553.
18. Lin JH, Lu AY. Interindividual variability in inhibition and induction of cytochrome P450 enzymes. Annu Rev Pharmacol Toxicol. 2001;41:535-567.
19. Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000; 38(1):41-57.
20. U.S. Food and Drug Administration. Drugs@FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed July 14, 2014.
21. Yasui, N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;15(2):185-190.
22. Lilja JJ, Kivistö KT, Backman JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64(6):655-660.
23. Garg SK, Kumar N, Bhargava VK, et al. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin Pharmacol Ther. 1998;64(3):286-288.
24. Oesterheld J, Kallepalli BR. Grapefruit juice and clomipramine: shifting metabolic ratios. J Clin Psychopharm. 1997;17(1):62-63.
25. Lane HY, Jann MW, Chang YC, et al. Repeated ingestion of grapefruit juice does not alter clozapine’s steady-state plasma levels, effectiveness, and tolerability. J Clin Psychiatry. 2001;62(10):812-817.
26. Ozdemir M, Aktan Y, Boydag BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59.
27. DeSilva KE, Le Flore DB, Marston BJ, et al. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS. 2001;15(10):1281-1285.
28. Hori H, Yoshimura R, Ueda N, et al. Grapefruit juice-fluvoxamine interaction—is it risky or not? J Clin Psychopharmacol. 2003;23(4):422-424.
29. Yasui N, Kondo T, Suzuki A, et al. Lack of significant pharmacokinetic interaction between haloperidol and grapefruit juice. Int Clin Psychopharmacol. 1999;142(2):113-118.
30. Kupferschmidt HH, Ha HR, Ziegler WH, et al. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58(1):20-28.
31. Benmebarek M, Cevaud C, Gex-Fabry M, et al. Effects of grapefruit juice on the pharmacokinetics of the enantiomers of methadone. Clin Pharmacol Ther. 2004;76(1):55-63.
32. DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40(7):509-522.
33. Ueda N, Yoshimura R, Umene-Nakano W, et al. Grapefruit juice alters plasma sertraline levels after single ingestion of sertraline in healthy volunteers. World J Biol Psychiatry. 2009;10(4 pt 3):832-835.
34. Lee AJ, Chan WK, Harralson AF, et al. The effects of grapefruit juice on sertraline metabolism: an in vitro and in vivo study. Clin Ther. 1999;21(11):1890-1899.
35. Sugimoto K, Araki N, Ohmori M, et al. Interaction between grapefruit juice and hypnotic drugs: comparison of triazolam and quazepam. Eur J Clin Pharmacol. 2006;62(3):209-215.
36. Fagiolino P, Vazquez M, Olano I, et al. Systemic and presystemic conversion of carbamazepine to carbamazepine- 10-11-epoxide during long term treatment. Journal of Epilepsy and Clinical Neurophysiology. 2006;12(1):13-16.
Ms. H, age 42, was given a diagnosis of bipolar disorder 10 years ago and has been taking carbamazepine, 1,200 mg/d, and olanzapine, 10 mg/d, for the past 2 years. She has not experienced a mood episode while on this regimen, and her carbamazepine level was 9.2 μg/mL 6 months ago. The only adverse effect she experienced was weight gain of approximately 10 lb. Ms. H takes a calcium supplement, but no other medications.
Ms. H reports to her psychiatrist that, for the past few days, she has been feeling nauseated, fatigued, and dizzy, but has continued taking her medications as prescribed. Her carbamazepine level is found to be 13.1 μg/mL. Ms. H states she has not started any new medications or supplements; her serum creatinine and liver function test results are within normal limits.
Upon further questioning, Ms. H says that an upper respiratory infection has been “going around her office,” so she increased her vitamin C intake by drinking 2 glasses of grapefruit juice a day (she doesn’t like orange juice). She has heard grapefruit juice can cause problems with some drugs so she is careful not to drink it at the same time she takes her medications. Her psychiatrist recognizes there may be a drug interaction involved, and recommends Ms. H hold her carbamazepine for 1 day and not consume any more grapefruit juice. A few days later, she reports feeling much better during a follow-up call and she makes an appointment to have her carbamazepine level rechecked in a we
Although grapefruit products are high in vitamins and low in calories, they can be associated with potentially serious drug interactions. The interaction between grapefruit juice and the calcium channel blocker felodipine was discovered inadvertently >20 years ago; since that time, possible interactions with >85 medications have been identified.1 Interactions with grapefruit products are complicated because, although most result in increased drug exposure, reduced exposure of the medication also can occur. Additionally, the degree and clinical significance of the interaction varies among individuals and from one drug to another.
Mechanism of action
Most interactions with grapefruit products are thought to result from the inhibition of intestinal cytochrome P450 3A4 (CYP3A4). CYP3A4 is involved in the metabolism of numerous drugs, and is the most abundant cytochrome P450 enzyme in the liver and epithelial cells lining the intestine.2 Although hepatic CYP3A4 is thought to be minimally affected by grapefruit, inhibition of intestinal CYP3A4 can result in an overall increase in bioavailability of medications that are substrates and raise the risk of potential toxicity.3 Grapefruit contains various chemicals collectively known as furanocoumarins, which are largely responsible for inhibition of intestinal CYP3A4.4 Additionally, Seville oranges and the pomelo (a large, sweet grapefruit-like citrus fruit) also contain furanocoumarins and could have a similar effect, warranting caution with certain medications.5
Inhibition of CYP3A4 by furanocoumarins cannot be reversed, and new enzymes must be synthesized to return to the previous level of function.6 Therefore, drug interactions resulting from CYP3A4 inhibition can last for as long as 72 hours after ingesting grapefruit products.7 Separating consumption of grapefruit products and medication administration will not help manage this interaction.
Grapefruit products also could affect drug disposition through effects on various drug transporters. Decreased systemic exposure to certain medications could occur through grapefruit’s inhibition of organic anion-transporting polypeptides (OATPs). OATPs form a family of drug uptake transporters found in the intestine, liver, kidney, and brain.8 For drugs that are substrates of OATPs, grapefruit’s inhibition of this transporter can result in decreased absorption and a resulting decrease in efficacy. Flavanoids in grapefruit, such as naringin, inhibit OATPs, which is competitive in nature.9 Unlike the irreversible inhibition of CYP3A4 by furanocoumarins, flavanoids effects on OATPs have been shown to decrease within 4 hours.10
No psychotropic medications have been identified as being susceptible to this interaction, but for those medications affected—including fexofenadine and levothyroxine—separating consumption of grapefruit and medication administration by 4 hours could avoid this interaction.11 Additional data indicate that orange juice and apple juice could have similar effects on OATPs.12
Perhaps the most well-known drug transporter, P-glycoprotein is part of the multidrug-resistant subfamily of transporters. It is located throughout the body, including in the intestine, kidneys, liver, and blood-brain barrier. P-glycoprotein acts as an export pump to decrease the cellular concentration of many different drug substrates, and many agents can alter P-glycoprotein’s expression or function.
Small changes in P-glycoprotein’s activity can result in substantial changes in the disposition of substrates, which can include certain antineoplastics and antiretrovirals. Most reports have found grapefruit juice inhibits P-glycoprotein-mediated efflux; however, there also are reports of transporter activation.6 Additionally, P-glycoprotein and CYP3A4 share many substrates, so it can be difficult to isolate the contribution of P-glycoprotein to grapefruit−drug interactions.13 The effect of grapefruit on P-glycoprotein activity has been difficult to fully elucidate; more studies are needed.
Grapefruit consumption and its effect
Drug interactions can occur by consuming commercially produced grapefruit juice and juice from concentrate, as well as freshly squeezed juice and grapefruit segments.14 CYP3A4-inhibiting furanocoumarins also have been isolated in grapefruit peel; it is not known, however, whether items made from peel (marmalade, candied peel) contain concentrations high enough to pose a risk of a drug interaction.14 Contributing to the unpredictability of grapefruit-drug interactions, the amount or concentration of furanocoumarins can vary among grapefruit products and brands.15 This variability can be influenced by the variety or maturity of the fruit and the fruit’s exposure to environmental stress.4
The frequency of consuming a grapefruit product can influence the degree of a drug interaction. In general, consuming one 8-oz glass of grapefruit juice or the segments from a whole grapefruit is enough to alter a susceptible drug’s pharmacokinetics.14 Regular grapefruit product consumption, however, can result in an overall greater effect.16,17
Lilja et al16 conducted a randomized, 4-phase, crossover study to look at the effect of grapefruit juice dose on kinetics of triazolam. Grapefruit juice was found to increase the mean area under the concentration-time curve (AUC) of triazolam compared with water, but no difference was found between single glasses of normal-strength and double-strength grapefruit juice. However, repeated consumption of double-strength grapefruit juice (200 mL, 3 times/d for 3 days) increased triazolam’s mean AUC by 143%, compared with an increase of 49% with just a single 200-mL glass of double-strength juice.16 Recurrent consumption of grapefruit juice (8 oz, 3 times/d for 6 days) also was found to increase the kinetics of the antihypertensive felodipine more than a single glass of grapefruit juice.17
Clinical consequences of an interaction between a drug and grapefruit can be difficult to predict. Drug concentration changes caused by a grapefruit interaction could vary based on interindividual differences. The amount and activity of intestinal CYP3A4 can vary from person to person, and can be influenced by genetic polymorphisms in addition to race, age, and environmental variables.18 Interindividual sensitivity to a change in a drug’s concentration also will differ, and patient-specific factors, such as concomitant drugs or diseases, could influence the likelihood of harm.
Interactions with grapefruit products are not necessarily a “class effect,” and specific drugs within a therapeutic category can be affected (although others might not). Several drug-specific characteristics can help gauge the risk of a clinically relevant interaction with grapefruit, including:
• metabolism through CYP3A4
• low bioavailability
• oral administration
• a narrow therapeutic index.1
For drugs with low bioavailability because of first-pass metabolism, grapefruit’s inhibition of intestinal CYP3A4 can result in a greater relative increase in plasma concentrations compared with a drug with high bioavailability.19
For example, an increase in bioavailability from 5% to 10% will result in a much larger increase in AUC and overall clinical exposure compared with an increase from 85% to 90% even though both represent an absolute increase of 5%. Although a drug does not have to have low oral bioavailability for an interaction to occur, lower bioavailability means that a drug has a higher likelihood of causing a significant interaction because of altered pharmacokinetics. Of note, injectable medications will not interact with grapefruit because metabolism through intestinal CYP3A4 is bypassed and grapefruit does not significantly inhibit hepatic CYP3A4.
Although grapefruit products could alter the pharmacokinetics of susceptible drugs, those changes might not be associated with adverse effects. Therefore, a factor to consider in evaluating a potential interaction with grapefruit is the drug’s therapeutic index and its risk of serious adverse effects. Drugs with a narrow therapeutic index are of particular concern because a significant increase in therapeutic or adverse effects could result from a relatively small increase in the drug’s concentration.7
Which medications are affected?
Among medications identified as interacting with grapefruit, some cardiovascular agents and several of the HMG-CoA reductase inhibitors (statins) have garnered the most attention. However, grapefruit also can affect the metabolism of several psychotropic medications through inhibition of intestinal CYP3A4 (Table).16,20-35 Prescribing information for some drugs warns against consuming grapefruit while using the medication. Among CNS agents, buspirone, carbamazepine, lurasidone, pimozide, triazolam, and oral midazolam all have such warnings in their product labeling.
Buspirone currently is not recommended with “large quantities of grapefruit juice.”20 A randomized, 2-phase crossover study looking at the effects of grapefruit juice on buspirone’s pharmacokinetics found that double-strength grapefruit juice (200 mL, administered 3 times/d for 3 days) resulted in a 9.2-fold increase in mean AUC and a 4.3-fold increase in mean Cmax after a single 10-mg buspirone dose.22 Highlighting the wide interindividual variability seen with drug-grapefruit interactions, the increase found in buspirone’s AUC ranged from 3-fold to 20-fold among study participants.22
Carbamazepine product labeling lists grapefruit juice as a CYP3A4 inhibitor that is expected to or has been found to increase plasma levels of the drug.20 Carbamazepine’s bioavailability is influenced by intestinal CYP3A4 activity; in a randomized, 2-phase crossover study of 10 patients with epilepsy, grapefruit juice was found to increase AUC of carbamazepine by 41% and Cmax by 40%.23,36
Lurasidone and pimozide, although not specifically studied, have product labels that recommend avoiding grapefruit juice because it could inhibit metabolism of these agents by CYP3A4.20 Of particular concern is the potential for elevated levels of pimozide to increase the risk of adverse cardiovascular effects including QT interval prolongation.19
Midazolam. Although grapefruit juice does not affect the disposition of IV midazolam, pretreatment with grapefruit juice was found to increase the AUC and Cmax of oral midazolam by 52% and 56%, respectively.30
Other considerations in drug-grapefruit interactions
Cautionary statements about a possible interaction with grapefruit juice for many other psychotropics can be found in commonly used drug information references or online sources. If you are concerned about a possible interaction and avoiding grapefruit products is not feasible, consider a different medication in the same class.
However, you also should consider the level of evidence supporting any purported interaction. Several psychotropic agents do have studies or case reports supporting an interaction with grapefruit, but cautionary statements could be based on theoretical concerns because of a medication’s bioavailability, metabolic pathway, and concern for increased adverse events related to higher drug concentrations. Adding to the confusion, cautionary statements can be found about medications, such as clozapine, that have not been shown to have an interaction with grapefruit juice when studied.
With many of the drugs that have a reported or theoretical interaction with grapefruit, data are inconsistent as to whether the resulting interaction will be clinically relevant. A number of variables relating to the individual patient, grapefruit product, or particular drug can play a role in the significance of an interaction. Additionally, effects on drug disposition can last for a few days after consuming a grapefruit product.
Keep alert to situations of increased risk
Recall that the case patient, Ms. H, presented with an elevated carbamazepine level and suffered resulting adverse effects because of an interaction between the drug and grapefruit juice. Although Ms. H was careful to separate intake of grapefruit juice from carbamazepine administration, grapefruit’s inhibition of intestinal CYP3A4 still was present, leading to the interaction.
It is important for health care professionals to recognize this potential risk and to advise patients regarding possible interactions between medications and grapefruit products.
Related Resources
• U.S. Food and Drug Administration. Grapefruit juice and medicine may not mix. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm292276.htm.
• Hanley MJ, Cancalon P, Widmer WW, et al. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol. 2011;7(3):267-286.
• Andrade C. Fruit juice, organic anion transporting polypeptides, and drug interactions in psychiatry. J Clin Psychiatry. 2014;75(11):e1323-e1325.
Drug Brand Names
Alprazolam • Xanax Lurasidone • Latuda
Buspirone • BuSpar Midazolam • Versed
Carbamazepine • Tegretol Methadone • Dolophine
Clomipramine • Anafranil Nefazodone • Serzone
Clozapine • Clozaril Olanzapine • Zyprexa
Diazepam • Valium Pimozide • Orap
Felodipine • Plendil Quetiapine • Seroquel
Fexofenadine • Allegra Sertraline • Zoloft
Fluoxetine • Prozac Trazodone • Desyrel
Fluvoxamine • Luvox Triazolam • Halcion
Haloperidol • Haldol Ziprasidone • Geodon
Levothyroxine • Levoxyl, Synthroid
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. H, age 42, was given a diagnosis of bipolar disorder 10 years ago and has been taking carbamazepine, 1,200 mg/d, and olanzapine, 10 mg/d, for the past 2 years. She has not experienced a mood episode while on this regimen, and her carbamazepine level was 9.2 μg/mL 6 months ago. The only adverse effect she experienced was weight gain of approximately 10 lb. Ms. H takes a calcium supplement, but no other medications.
Ms. H reports to her psychiatrist that, for the past few days, she has been feeling nauseated, fatigued, and dizzy, but has continued taking her medications as prescribed. Her carbamazepine level is found to be 13.1 μg/mL. Ms. H states she has not started any new medications or supplements; her serum creatinine and liver function test results are within normal limits.
Upon further questioning, Ms. H says that an upper respiratory infection has been “going around her office,” so she increased her vitamin C intake by drinking 2 glasses of grapefruit juice a day (she doesn’t like orange juice). She has heard grapefruit juice can cause problems with some drugs so she is careful not to drink it at the same time she takes her medications. Her psychiatrist recognizes there may be a drug interaction involved, and recommends Ms. H hold her carbamazepine for 1 day and not consume any more grapefruit juice. A few days later, she reports feeling much better during a follow-up call and she makes an appointment to have her carbamazepine level rechecked in a we
Although grapefruit products are high in vitamins and low in calories, they can be associated with potentially serious drug interactions. The interaction between grapefruit juice and the calcium channel blocker felodipine was discovered inadvertently >20 years ago; since that time, possible interactions with >85 medications have been identified.1 Interactions with grapefruit products are complicated because, although most result in increased drug exposure, reduced exposure of the medication also can occur. Additionally, the degree and clinical significance of the interaction varies among individuals and from one drug to another.
Mechanism of action
Most interactions with grapefruit products are thought to result from the inhibition of intestinal cytochrome P450 3A4 (CYP3A4). CYP3A4 is involved in the metabolism of numerous drugs, and is the most abundant cytochrome P450 enzyme in the liver and epithelial cells lining the intestine.2 Although hepatic CYP3A4 is thought to be minimally affected by grapefruit, inhibition of intestinal CYP3A4 can result in an overall increase in bioavailability of medications that are substrates and raise the risk of potential toxicity.3 Grapefruit contains various chemicals collectively known as furanocoumarins, which are largely responsible for inhibition of intestinal CYP3A4.4 Additionally, Seville oranges and the pomelo (a large, sweet grapefruit-like citrus fruit) also contain furanocoumarins and could have a similar effect, warranting caution with certain medications.5
Inhibition of CYP3A4 by furanocoumarins cannot be reversed, and new enzymes must be synthesized to return to the previous level of function.6 Therefore, drug interactions resulting from CYP3A4 inhibition can last for as long as 72 hours after ingesting grapefruit products.7 Separating consumption of grapefruit products and medication administration will not help manage this interaction.
Grapefruit products also could affect drug disposition through effects on various drug transporters. Decreased systemic exposure to certain medications could occur through grapefruit’s inhibition of organic anion-transporting polypeptides (OATPs). OATPs form a family of drug uptake transporters found in the intestine, liver, kidney, and brain.8 For drugs that are substrates of OATPs, grapefruit’s inhibition of this transporter can result in decreased absorption and a resulting decrease in efficacy. Flavanoids in grapefruit, such as naringin, inhibit OATPs, which is competitive in nature.9 Unlike the irreversible inhibition of CYP3A4 by furanocoumarins, flavanoids effects on OATPs have been shown to decrease within 4 hours.10
No psychotropic medications have been identified as being susceptible to this interaction, but for those medications affected—including fexofenadine and levothyroxine—separating consumption of grapefruit and medication administration by 4 hours could avoid this interaction.11 Additional data indicate that orange juice and apple juice could have similar effects on OATPs.12
Perhaps the most well-known drug transporter, P-glycoprotein is part of the multidrug-resistant subfamily of transporters. It is located throughout the body, including in the intestine, kidneys, liver, and blood-brain barrier. P-glycoprotein acts as an export pump to decrease the cellular concentration of many different drug substrates, and many agents can alter P-glycoprotein’s expression or function.
Small changes in P-glycoprotein’s activity can result in substantial changes in the disposition of substrates, which can include certain antineoplastics and antiretrovirals. Most reports have found grapefruit juice inhibits P-glycoprotein-mediated efflux; however, there also are reports of transporter activation.6 Additionally, P-glycoprotein and CYP3A4 share many substrates, so it can be difficult to isolate the contribution of P-glycoprotein to grapefruit−drug interactions.13 The effect of grapefruit on P-glycoprotein activity has been difficult to fully elucidate; more studies are needed.
Grapefruit consumption and its effect
Drug interactions can occur by consuming commercially produced grapefruit juice and juice from concentrate, as well as freshly squeezed juice and grapefruit segments.14 CYP3A4-inhibiting furanocoumarins also have been isolated in grapefruit peel; it is not known, however, whether items made from peel (marmalade, candied peel) contain concentrations high enough to pose a risk of a drug interaction.14 Contributing to the unpredictability of grapefruit-drug interactions, the amount or concentration of furanocoumarins can vary among grapefruit products and brands.15 This variability can be influenced by the variety or maturity of the fruit and the fruit’s exposure to environmental stress.4
The frequency of consuming a grapefruit product can influence the degree of a drug interaction. In general, consuming one 8-oz glass of grapefruit juice or the segments from a whole grapefruit is enough to alter a susceptible drug’s pharmacokinetics.14 Regular grapefruit product consumption, however, can result in an overall greater effect.16,17
Lilja et al16 conducted a randomized, 4-phase, crossover study to look at the effect of grapefruit juice dose on kinetics of triazolam. Grapefruit juice was found to increase the mean area under the concentration-time curve (AUC) of triazolam compared with water, but no difference was found between single glasses of normal-strength and double-strength grapefruit juice. However, repeated consumption of double-strength grapefruit juice (200 mL, 3 times/d for 3 days) increased triazolam’s mean AUC by 143%, compared with an increase of 49% with just a single 200-mL glass of double-strength juice.16 Recurrent consumption of grapefruit juice (8 oz, 3 times/d for 6 days) also was found to increase the kinetics of the antihypertensive felodipine more than a single glass of grapefruit juice.17
Clinical consequences of an interaction between a drug and grapefruit can be difficult to predict. Drug concentration changes caused by a grapefruit interaction could vary based on interindividual differences. The amount and activity of intestinal CYP3A4 can vary from person to person, and can be influenced by genetic polymorphisms in addition to race, age, and environmental variables.18 Interindividual sensitivity to a change in a drug’s concentration also will differ, and patient-specific factors, such as concomitant drugs or diseases, could influence the likelihood of harm.
Interactions with grapefruit products are not necessarily a “class effect,” and specific drugs within a therapeutic category can be affected (although others might not). Several drug-specific characteristics can help gauge the risk of a clinically relevant interaction with grapefruit, including:
• metabolism through CYP3A4
• low bioavailability
• oral administration
• a narrow therapeutic index.1
For drugs with low bioavailability because of first-pass metabolism, grapefruit’s inhibition of intestinal CYP3A4 can result in a greater relative increase in plasma concentrations compared with a drug with high bioavailability.19
For example, an increase in bioavailability from 5% to 10% will result in a much larger increase in AUC and overall clinical exposure compared with an increase from 85% to 90% even though both represent an absolute increase of 5%. Although a drug does not have to have low oral bioavailability for an interaction to occur, lower bioavailability means that a drug has a higher likelihood of causing a significant interaction because of altered pharmacokinetics. Of note, injectable medications will not interact with grapefruit because metabolism through intestinal CYP3A4 is bypassed and grapefruit does not significantly inhibit hepatic CYP3A4.
Although grapefruit products could alter the pharmacokinetics of susceptible drugs, those changes might not be associated with adverse effects. Therefore, a factor to consider in evaluating a potential interaction with grapefruit is the drug’s therapeutic index and its risk of serious adverse effects. Drugs with a narrow therapeutic index are of particular concern because a significant increase in therapeutic or adverse effects could result from a relatively small increase in the drug’s concentration.7
Which medications are affected?
Among medications identified as interacting with grapefruit, some cardiovascular agents and several of the HMG-CoA reductase inhibitors (statins) have garnered the most attention. However, grapefruit also can affect the metabolism of several psychotropic medications through inhibition of intestinal CYP3A4 (Table).16,20-35 Prescribing information for some drugs warns against consuming grapefruit while using the medication. Among CNS agents, buspirone, carbamazepine, lurasidone, pimozide, triazolam, and oral midazolam all have such warnings in their product labeling.
Buspirone currently is not recommended with “large quantities of grapefruit juice.”20 A randomized, 2-phase crossover study looking at the effects of grapefruit juice on buspirone’s pharmacokinetics found that double-strength grapefruit juice (200 mL, administered 3 times/d for 3 days) resulted in a 9.2-fold increase in mean AUC and a 4.3-fold increase in mean Cmax after a single 10-mg buspirone dose.22 Highlighting the wide interindividual variability seen with drug-grapefruit interactions, the increase found in buspirone’s AUC ranged from 3-fold to 20-fold among study participants.22
Carbamazepine product labeling lists grapefruit juice as a CYP3A4 inhibitor that is expected to or has been found to increase plasma levels of the drug.20 Carbamazepine’s bioavailability is influenced by intestinal CYP3A4 activity; in a randomized, 2-phase crossover study of 10 patients with epilepsy, grapefruit juice was found to increase AUC of carbamazepine by 41% and Cmax by 40%.23,36
Lurasidone and pimozide, although not specifically studied, have product labels that recommend avoiding grapefruit juice because it could inhibit metabolism of these agents by CYP3A4.20 Of particular concern is the potential for elevated levels of pimozide to increase the risk of adverse cardiovascular effects including QT interval prolongation.19
Midazolam. Although grapefruit juice does not affect the disposition of IV midazolam, pretreatment with grapefruit juice was found to increase the AUC and Cmax of oral midazolam by 52% and 56%, respectively.30
Other considerations in drug-grapefruit interactions
Cautionary statements about a possible interaction with grapefruit juice for many other psychotropics can be found in commonly used drug information references or online sources. If you are concerned about a possible interaction and avoiding grapefruit products is not feasible, consider a different medication in the same class.
However, you also should consider the level of evidence supporting any purported interaction. Several psychotropic agents do have studies or case reports supporting an interaction with grapefruit, but cautionary statements could be based on theoretical concerns because of a medication’s bioavailability, metabolic pathway, and concern for increased adverse events related to higher drug concentrations. Adding to the confusion, cautionary statements can be found about medications, such as clozapine, that have not been shown to have an interaction with grapefruit juice when studied.
With many of the drugs that have a reported or theoretical interaction with grapefruit, data are inconsistent as to whether the resulting interaction will be clinically relevant. A number of variables relating to the individual patient, grapefruit product, or particular drug can play a role in the significance of an interaction. Additionally, effects on drug disposition can last for a few days after consuming a grapefruit product.
Keep alert to situations of increased risk
Recall that the case patient, Ms. H, presented with an elevated carbamazepine level and suffered resulting adverse effects because of an interaction between the drug and grapefruit juice. Although Ms. H was careful to separate intake of grapefruit juice from carbamazepine administration, grapefruit’s inhibition of intestinal CYP3A4 still was present, leading to the interaction.
It is important for health care professionals to recognize this potential risk and to advise patients regarding possible interactions between medications and grapefruit products.
Related Resources
• U.S. Food and Drug Administration. Grapefruit juice and medicine may not mix. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm292276.htm.
• Hanley MJ, Cancalon P, Widmer WW, et al. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol. 2011;7(3):267-286.
• Andrade C. Fruit juice, organic anion transporting polypeptides, and drug interactions in psychiatry. J Clin Psychiatry. 2014;75(11):e1323-e1325.
Drug Brand Names
Alprazolam • Xanax Lurasidone • Latuda
Buspirone • BuSpar Midazolam • Versed
Carbamazepine • Tegretol Methadone • Dolophine
Clomipramine • Anafranil Nefazodone • Serzone
Clozapine • Clozaril Olanzapine • Zyprexa
Diazepam • Valium Pimozide • Orap
Felodipine • Plendil Quetiapine • Seroquel
Fexofenadine • Allegra Sertraline • Zoloft
Fluoxetine • Prozac Trazodone • Desyrel
Fluvoxamine • Luvox Triazolam • Halcion
Haloperidol • Haldol Ziprasidone • Geodon
Levothyroxine • Levoxyl, Synthroid
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bailey DG, Dresser G, Arnold JM. Grapefruit-medication interactions: forbidden fruit or avoidable consequences? CMAJ. 2013;185(4):309-316.
2. Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352(21): 2211-2221.
3. Saito M, Hirata-Koizumi M, Matsumoto M, et al. Undesirable effects of citrus juice on the pharmacokinetics of drugs: focus on recent studies. Drug Saf. 2005;28(8):677- 694.
4. Cancalon PF, Barros SM, Haun C, et al. Effect of maturity, processing, and storage on the furanocoumarin composition of grapefruit and grapefruit juice. J Food Sci. 2011;76(4):C543-C548.
5. Pirmohamed M. Drug-grapefruit juice interactions: two mechanisms are clear but individual responses vary. BMJ. 2013;346:f1. doi: 10.1136/bmj.f1.
6. Dahan A, Altman H. Food-drug interaction: grapefruit juice augments drug bioavailability–mechanism, extent and relevance. Eur J Clin Nutr. 2004;58:1-9.
7. Stump AL, Mayo T, Blum A. Management of grapefruit-drug interactions. Am Fam Physician. 2006;74(4):605-608.
8. Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest. 2003;33(suppl 2):1-5.
9. Bailey DG, Dressker GK, Leak BF, et al. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81(4):495-502.
10. Glaeser H, Bailey DG, Dresser GK, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81(3):362-370.
11. Bailey DG. Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010;70(5): 645-655.
12. Dresser GK, Bailey DG, Leake BF, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11-20.
13. Seden K, Dickinson L, Khoo S, et al. Grapefruit-drug interactions. Drugs. 2010;70(18):2373-2407.
14. Bailey DG, Dresser GK, Kreeft JH, et al. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther. 2000;68(5):468-477.
15. De Castro WV, Mertens-Talcott S, Rubner A, et al. Variation of flavonoids and furanocoumarins in grapefruit juices: a potential source of variability in grapefruit juice-drug interaction studies. J Agric Food Chem. 2006;54(1):249-255.
16. Lilja JJ, Kivistö KT, Backman JT, et al. Effect of grapefruit juice on grapefruit juice-triazolam interaction: repeated consumption prolongs triazolam half-life. Eur J Clin Pharmacol. 2000;56(5):411-415.
17. Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99(10):2545-2553.
18. Lin JH, Lu AY. Interindividual variability in inhibition and induction of cytochrome P450 enzymes. Annu Rev Pharmacol Toxicol. 2001;41:535-567.
19. Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000; 38(1):41-57.
20. U.S. Food and Drug Administration. Drugs@FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed July 14, 2014.
21. Yasui, N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;15(2):185-190.
22. Lilja JJ, Kivistö KT, Backman JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64(6):655-660.
23. Garg SK, Kumar N, Bhargava VK, et al. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin Pharmacol Ther. 1998;64(3):286-288.
24. Oesterheld J, Kallepalli BR. Grapefruit juice and clomipramine: shifting metabolic ratios. J Clin Psychopharm. 1997;17(1):62-63.
25. Lane HY, Jann MW, Chang YC, et al. Repeated ingestion of grapefruit juice does not alter clozapine’s steady-state plasma levels, effectiveness, and tolerability. J Clin Psychiatry. 2001;62(10):812-817.
26. Ozdemir M, Aktan Y, Boydag BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59.
27. DeSilva KE, Le Flore DB, Marston BJ, et al. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS. 2001;15(10):1281-1285.
28. Hori H, Yoshimura R, Ueda N, et al. Grapefruit juice-fluvoxamine interaction—is it risky or not? J Clin Psychopharmacol. 2003;23(4):422-424.
29. Yasui N, Kondo T, Suzuki A, et al. Lack of significant pharmacokinetic interaction between haloperidol and grapefruit juice. Int Clin Psychopharmacol. 1999;142(2):113-118.
30. Kupferschmidt HH, Ha HR, Ziegler WH, et al. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58(1):20-28.
31. Benmebarek M, Cevaud C, Gex-Fabry M, et al. Effects of grapefruit juice on the pharmacokinetics of the enantiomers of methadone. Clin Pharmacol Ther. 2004;76(1):55-63.
32. DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40(7):509-522.
33. Ueda N, Yoshimura R, Umene-Nakano W, et al. Grapefruit juice alters plasma sertraline levels after single ingestion of sertraline in healthy volunteers. World J Biol Psychiatry. 2009;10(4 pt 3):832-835.
34. Lee AJ, Chan WK, Harralson AF, et al. The effects of grapefruit juice on sertraline metabolism: an in vitro and in vivo study. Clin Ther. 1999;21(11):1890-1899.
35. Sugimoto K, Araki N, Ohmori M, et al. Interaction between grapefruit juice and hypnotic drugs: comparison of triazolam and quazepam. Eur J Clin Pharmacol. 2006;62(3):209-215.
36. Fagiolino P, Vazquez M, Olano I, et al. Systemic and presystemic conversion of carbamazepine to carbamazepine- 10-11-epoxide during long term treatment. Journal of Epilepsy and Clinical Neurophysiology. 2006;12(1):13-16.
1. Bailey DG, Dresser G, Arnold JM. Grapefruit-medication interactions: forbidden fruit or avoidable consequences? CMAJ. 2013;185(4):309-316.
2. Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352(21): 2211-2221.
3. Saito M, Hirata-Koizumi M, Matsumoto M, et al. Undesirable effects of citrus juice on the pharmacokinetics of drugs: focus on recent studies. Drug Saf. 2005;28(8):677- 694.
4. Cancalon PF, Barros SM, Haun C, et al. Effect of maturity, processing, and storage on the furanocoumarin composition of grapefruit and grapefruit juice. J Food Sci. 2011;76(4):C543-C548.
5. Pirmohamed M. Drug-grapefruit juice interactions: two mechanisms are clear but individual responses vary. BMJ. 2013;346:f1. doi: 10.1136/bmj.f1.
6. Dahan A, Altman H. Food-drug interaction: grapefruit juice augments drug bioavailability–mechanism, extent and relevance. Eur J Clin Nutr. 2004;58:1-9.
7. Stump AL, Mayo T, Blum A. Management of grapefruit-drug interactions. Am Fam Physician. 2006;74(4):605-608.
8. Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest. 2003;33(suppl 2):1-5.
9. Bailey DG, Dressker GK, Leak BF, et al. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81(4):495-502.
10. Glaeser H, Bailey DG, Dresser GK, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81(3):362-370.
11. Bailey DG. Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010;70(5): 645-655.
12. Dresser GK, Bailey DG, Leake BF, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11-20.
13. Seden K, Dickinson L, Khoo S, et al. Grapefruit-drug interactions. Drugs. 2010;70(18):2373-2407.
14. Bailey DG, Dresser GK, Kreeft JH, et al. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther. 2000;68(5):468-477.
15. De Castro WV, Mertens-Talcott S, Rubner A, et al. Variation of flavonoids and furanocoumarins in grapefruit juices: a potential source of variability in grapefruit juice-drug interaction studies. J Agric Food Chem. 2006;54(1):249-255.
16. Lilja JJ, Kivistö KT, Backman JT, et al. Effect of grapefruit juice on grapefruit juice-triazolam interaction: repeated consumption prolongs triazolam half-life. Eur J Clin Pharmacol. 2000;56(5):411-415.
17. Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99(10):2545-2553.
18. Lin JH, Lu AY. Interindividual variability in inhibition and induction of cytochrome P450 enzymes. Annu Rev Pharmacol Toxicol. 2001;41:535-567.
19. Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000; 38(1):41-57.
20. U.S. Food and Drug Administration. Drugs@FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed July 14, 2014.
21. Yasui, N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;15(2):185-190.
22. Lilja JJ, Kivistö KT, Backman JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64(6):655-660.
23. Garg SK, Kumar N, Bhargava VK, et al. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin Pharmacol Ther. 1998;64(3):286-288.
24. Oesterheld J, Kallepalli BR. Grapefruit juice and clomipramine: shifting metabolic ratios. J Clin Psychopharm. 1997;17(1):62-63.
25. Lane HY, Jann MW, Chang YC, et al. Repeated ingestion of grapefruit juice does not alter clozapine’s steady-state plasma levels, effectiveness, and tolerability. J Clin Psychiatry. 2001;62(10):812-817.
26. Ozdemir M, Aktan Y, Boydag BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59.
27. DeSilva KE, Le Flore DB, Marston BJ, et al. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS. 2001;15(10):1281-1285.
28. Hori H, Yoshimura R, Ueda N, et al. Grapefruit juice-fluvoxamine interaction—is it risky or not? J Clin Psychopharmacol. 2003;23(4):422-424.
29. Yasui N, Kondo T, Suzuki A, et al. Lack of significant pharmacokinetic interaction between haloperidol and grapefruit juice. Int Clin Psychopharmacol. 1999;142(2):113-118.
30. Kupferschmidt HH, Ha HR, Ziegler WH, et al. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58(1):20-28.
31. Benmebarek M, Cevaud C, Gex-Fabry M, et al. Effects of grapefruit juice on the pharmacokinetics of the enantiomers of methadone. Clin Pharmacol Ther. 2004;76(1):55-63.
32. DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40(7):509-522.
33. Ueda N, Yoshimura R, Umene-Nakano W, et al. Grapefruit juice alters plasma sertraline levels after single ingestion of sertraline in healthy volunteers. World J Biol Psychiatry. 2009;10(4 pt 3):832-835.
34. Lee AJ, Chan WK, Harralson AF, et al. The effects of grapefruit juice on sertraline metabolism: an in vitro and in vivo study. Clin Ther. 1999;21(11):1890-1899.
35. Sugimoto K, Araki N, Ohmori M, et al. Interaction between grapefruit juice and hypnotic drugs: comparison of triazolam and quazepam. Eur J Clin Pharmacol. 2006;62(3):209-215.
36. Fagiolino P, Vazquez M, Olano I, et al. Systemic and presystemic conversion of carbamazepine to carbamazepine- 10-11-epoxide during long term treatment. Journal of Epilepsy and Clinical Neurophysiology. 2006;12(1):13-16.
Epithelial Ovarian Cancer: Management of Advanced Disease
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Epithelial ovarian cancer is the fifth leading cause of cancer death among women in the United States. Most women with ovarian cancer present at an advanced stage (International Federation of Gynecology and Obstetrics stage III), for which the standard treatment remains cytoreductive surgery followed by platinum- and taxane-based combination chemotherapy. Although this treatment frequently is curative for patients with early-stage disease, more than 60% of women with advanced disease will develop recurrent disease with progressively shorter disease-free intervals. However, there are many clinical trials in progress that are aimed at refining current therapy and evaluating different approaches to postoperative therapy, with the goal of improving prognosis and quality of life.
To read the full article in PDF:
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Epithelial ovarian cancer is the fifth leading cause of cancer death among women in the United States. Most women with ovarian cancer present at an advanced stage (International Federation of Gynecology and Obstetrics stage III), for which the standard treatment remains cytoreductive surgery followed by platinum- and taxane-based combination chemotherapy. Although this treatment frequently is curative for patients with early-stage disease, more than 60% of women with advanced disease will develop recurrent disease with progressively shorter disease-free intervals. However, there are many clinical trials in progress that are aimed at refining current therapy and evaluating different approaches to postoperative therapy, with the goal of improving prognosis and quality of life.
To read the full article in PDF:
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Epithelial ovarian cancer is the fifth leading cause of cancer death among women in the United States. Most women with ovarian cancer present at an advanced stage (International Federation of Gynecology and Obstetrics stage III), for which the standard treatment remains cytoreductive surgery followed by platinum- and taxane-based combination chemotherapy. Although this treatment frequently is curative for patients with early-stage disease, more than 60% of women with advanced disease will develop recurrent disease with progressively shorter disease-free intervals. However, there are many clinical trials in progress that are aimed at refining current therapy and evaluating different approaches to postoperative therapy, with the goal of improving prognosis and quality of life.
To read the full article in PDF:
Sober today, but lethargic and confused
CASE Confused and weak
Mr. W, age 26, is brought to the emergency department (ED) by his parents for intermittent confusion, weakness, and increasing lethargy over the past 4 days. He is jaundiced with mild abdominal pain, nausea, and vomiting.
Mr. W has a history of alcohol use disorder, drinking as much as 1 L of vodka a day. Six months ago, he was hospitalized for alcoholic hepatitis and severe hyponatremia.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse 89 beats per minute; blood pressure, 117/50 mm Hg; respirations, 15 breaths per minute; and temperature, 98.5ºF. Physical examination is notable for scleral icterus, jaundice, tender hepatomegaly, and asterixis.
Mr. W is not taking any medications. He reports that his most recent drink was the day before; however, his current alcohol intake is unknown.
Laboratory tests reveal altered hepatic function, including elevated aspartate aminotransferase (251 U/L), alanine aminotransferase (56 U/L), alkaline phosphatase (179 U/L), total bilirubin (15.4 mg/dL), and ammonia (143 U/L), impaired coagulation (international normalized ratio 2.39), and decreased albumin (2.7 g/dL). Other metabolic disturbances include: sodium, 104 mEq/L; chloride, <60 mEq/L; potassium, 2.2 mEq/L; and CO2, 44.5 mEq/L.
What is your differential diagnosis for Mr. W’s altered mental status?
a) hepatic encephalopathy
b) Wernicke’s encephalopathy
c) hyponatremia
d) drug intoxication
e) head trauma
The authors’ observations
Hyponatremia is defined as a serum sodium concentration <136 mEq/L. Mr. W is considered to have severe hyponatremia because his serum sodium concentration is <125 mEq/L. Although commonly caused by an inability to suppress antidiuretic hormone, hyponatremia has several possible causes (Figure 1).1 Symptoms are nonspecific and are more visible when there is a large or rapid decrease in the serum sodium concentration. Most patients with a serum sodium concentration >125 mEq/L are asymptomatic. Mr. W, who had a serum sodium of 104 mEq/L, presented with several symptoms, including confusion, lethargy, nausea, vomiting, and weakness. Headache, muscle spasms, depressed reflexes, restlessness, and disorientation also might be observed.2
Because of Mr. W’s impaired hepatic function, elevated ammonia, and asterixis, hepatic encephalopathy could be contributing to his altered mental status. Suspect Wernicke’s encephalopathy in a patient with neurologic symptoms and a history of chronic alcohol abuse. In its classic form, Wernicke’s encephalopathy has acute onset, characterized by the triad of ataxia, global confusion, and ocular abnormalities. However, this triad is not consistently or frequently encountered.3
Which tests would you order next?
a) blood ethanol level
b) urine drug screen
c) serum osmolality
d) CT of the head
EVALUATION Sober, yet sick
To rule out intoxication as the cause of Mr. W’s altered mental status, blood ethanol level and urine drug screens are obtained and found to be negative. CT of the head is negative for acute intracranial pathology.
Mr. W is admitted to the medical intensive care unit (MICU) for severe hyponatremia and altered mental status. Serum osmolality is 220 mOsm/kg (normal range 281 to 304 mOsm/kg). To further classify his hypotonic hyponatremia, volume status is assessed, and Mr. W is determined to be euvolemic. Thyroid-stimulating hormone and cortisol are within normal limits, eliminating hypothyroidism and adrenal insufficiency as causes of his euvolemic hypotonic hyponatremia. Mr. W is treated for hyponatremia likely secondary to syndrome of inappropriate antidiuretic hormone (SIADH). SIADH is a diagnosis of exclusion that first requires ruling out hypothyroidism and glucocorticoid insufficiency (Figure 1).1
The authors’ observations
Because hypokalemia is an independent predictive factor for development of hyponatremia, it is necessary to evaluate the potassium level in all hyponatremic patients. Mr. W’s potassium level was 2.2 mEq/L on admission. Serum sodium concentration is related to total exchangeable sodium, total body water, and total exchangeable potassium. Potassium depletion causes a shift of sodium into cells with a comparable exit of potassium from cells into extracellular fluid. The reverse process occurs during potassium repletion, leading to an increase in serum sodium concentration and making hypokalemia a risk factor for developing osmotic demyelination syndrome (ODS).4
Treating hyponatremia
Hyponatremia treatment depends on its severity, presence or absence of symptoms, and whether the hyponatremia is acute (<24 hours) or chronic (>48 hours).5
Because of Mr. W’s extremely low serum sodium concentration, predisposition to hyponatremia secondary to alcoholism, and history of severe hyponatremia, it is likely he is chronically hyponatremic.
In patients with chronic hyponatremia, neurological sequelae are associated with the need for a more rapid rate of correction of serum sodium. For most patients with chronic hyponatremia, a correction rate of ≤10 to 12 mEq/L during the first 24 hours and <18 mEq/L over 48 hours is recommended to avoid ODS.6
Evidence suggests, however, that this 1-day limit might be too high for some patients. Alcoholism, hypokalemia, malnutrition, and liver disease are present in a high percentage of patients who develop
ODS after correcting hyponatremia (Table 1).6 Therefore, for patients such as Mr. W who are at high risk of ODS, experts recommend a goal of 4 to 6 mEq/L/d with a correction rate of ≤8 mEq/L in any 24-hour period (Table 2).6
TREATMENT Sodium normalizes
Mr. W receives 1 L of normal saline in the ED before admission to the MICU. Once in the MICU, despite likely chronic hyponatremia, he receives hypertonic (3%) saline, followed by normal saline. Initially, he responds when the serum sodium concentration improves. Because of his likely SIADH, Mr. W is fluid-restricted for 4 days. Serum sodium returns to normal over 7 hospital days (Figure 2). To address the profound hypokalemia, Mr. S receives 30 mEq of potassium chloride in the ED, and potassium is repeated daily throughout his stay in the MICU.
Mr. W remains lethargic, with intermittent periods of confusion throughout the hospital stay. His altered mental status is attributed to hepatic encephalopathy secondary to alcoholic hepatitis. The Maddrey discriminant function is a calculation that stratifies patients with alcoholic hepatitis for risk of mortality and the use of steroids. Because Mr. W shows a Maddrey discriminant function ≥32, he receives methylprednisolone, followed by pentoxifylline, and liver function tests trend down. He also receives lactulose throughout hospitalization.
By discharge on hospital day 9, Mr. W’s serum sodium is 138 mEq/L; serum potassium, 4.1 mEq/L. Total bilirubin and prothrombin remain elevated. Mr. W is discharged on lactulose, thiamine, folic acid, and a 1-month course of pentoxifylline, 400 mg, 3 times a day.
READMISSION Unsteady gait, nausea
Three days after discharge, Mr. W returns to the ED after experiencing a 20-second episode of total body rigidity. He has an unsteady gait and worsening nausea and vomiting.
When Mr. W arrives in the ED, he confirms he is taking his discharge medications as prescribed. His parents report that he has consumed alcohol and Cannabis since discharge and has been taking his sibling’s prescription medications, including quetiapine.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse, 118 beats per minute; blood pressure, 128/73 mm Hg; respirations, 16 breaths per minute; and temperature, 98.5ºF. Physical examination, again, is notable for scleral icterus, jaundice, and asterixis. No focal neurologic deficits are noted.
Consistent with Mr. W’s previous admission, laboratory values reveal altered hepatic function and impaired coagulation. The serum sodium level remains within normal limits at 136 mEq/L. However, again, metabolic disturbances include decreased chloride (97 mEq/L), potassium (2.9 mEq/L), and CO2 (18.2 mEq/L). CT on readmission is unchanged from the earlier hospitalization.
What is your differential diagnosis for Mr. W’s total body rigidity?
a) seizure
b) ODS
c) drug intoxication
d) neuroleptic malignant syndrome
EVALUATION Shaking and weakness
Once admitted to the hospital, Mr. W reports an episode of right upper-extremity “shaking,” followed by weakness. He remembers the entire event and denies tongue biting or incontinence. He is evaluated for possible seizure, given his multiple risk factors, including drug and alcohol use, ingestion of quetiapine, and history of hyponatremia. Routine EEG is negative but prolactin level is elevated.
Mr. W’s mental status continues to wax and wane, prompting a neurology consult and MRI for further evaluation. MRI of the brain without contrast reveals restricted diffusion in the pons centrally, with extension bilaterally to the midbrain and thalami—findings consistent with central pontine myelinolysis. A neurology consultation reveals quadriparesis, paraparesis, dysarthria, and diplopia on examination, all symptoms associated with central pontine myelinolysis.
The authors’ observations
ODS, including central and extrapontine myelinolysis, is a demyelinating condition that occurs because of severe osmotic stress, most commonly secondary to the overly rapid correction of hyponatremia in patients with conditions leading to nutritional or electrolyte stress.7 Mr. W is considered at high risk of developing ODS because he fulfills the 5 criteria listed in Table 1.
Several psychiatric illnesses and neuropsychiatric medications could lead to hyponatremia. Many studies8-10 have documented hyponatremia and resulting ODS in patients with alcoholism, schizophrenia, anorexia, primary psychogenic polydipsia, and MDMA (3,4-methylenedioxymethamphetamine) abuse. Hyponatremia is a side effect of several neuropsychiatric medications, including serotonin reuptake inhibitors, lithium, tricyclic antidepressants, opioids, carbamazepine, oxcarbazepine, and antipsychotic polypharmacy. Other commonly used medications associated with hyponatremia include salt-losing diuretics, nonsteroidal anti-inflammatory drugs, and acetaminophen.7
Disease severity varies from asymptomatic to coma or death. Symptoms, although some could reverse completely, typically are a combination of neuropsychiatric (ie, emotional lability, disinhibition, and other bizarre behaviors) and neurologic. Neurologic symptoms include confusion, impaired cognition, dysarthria, dysphagia, gait instability, weakness or paralysis, and generalized seizures. Severely affected patients could experience “locked-in syndrome,” in which they are awake but unable to move or communicate. Also consistent with Mr. W’s case, ODS often presents initially with delirium, seizures, or encephalopathy, followed by a lucid interval before symptoms develop.7
Diagnosis is based on the appearance of demyelinating brain lesions on CT or MRI. MRI is more sensitive than CT; however, even an MRI scan can appear normal for as long as 4 weeks after symptoms appear.7 Therefore, an initial negative radiologic study in a high-risk patient who develops neurologic symptoms does not exclude ODS. Earlier detection is possible with diffusion-weighted MRI, which is most sensitive and can detect lesions within 24 hours of developing symptoms.11 The severity of the lesion does not correlate with severity of symptoms.
Studies reveal a considerable range in prognosis of patients with clinically symptomatic ODS. A study of 44 patients with central pontine myelinolysis, of which 42 had chronic alcoholism, reported that 34% had no significant functional deficits at follow-up, 34% had minor neurologic deficits, and 31% became dependent on personal help. Outcome did not depend on the extent or severity of neurologic symptoms or the severity of concomitant systemic complications.12
Because of its poor prognosis, prevention of ODS is important. Because ODS commonly is caused by overly rapid correction of hyponatremia, it is necessary to adhere to guidelines for treating chronic hyponatremia (Table 2). If overcorrection occurs, therapeutic re-lowering of serum sodium can be considered, but has not been validated in controlled trials. Based mainly on case reports that suggest benefit from early re-lowering serum sodium in patients with ODS symptoms, experts recommend the following:
• administer desmopressin, 2 to 4 μg, every 8 hours parenterally
• replace water orally or as 5% dextrose in water intravenously (3 mL/kg/hr)
• check serum sodium hourly until serum is reduced to goal.6
Bottom Line
Hyponatremia is the most common electrolyte disorder encountered in practice. Osmotic demyelination syndrome often is preventable, with considerable morbidity and mortality. Psychiatrists should be aware of this condition because it could be an adverse effect of many psychiatric medications and there are some psychiatric illnesses in which hyponatremia is a potential risk. In hyponatremic patients with persistent nonspecific neurologic or neuropsychiatric symptoms and negative CT imaging, additional imaging, such as MRI, is warranted.
Related Resources
- Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
- Vaidya C, Ho W, Freda BJ. Management of hyponatremia: providing treatment and avoiding harm. Cleve Clin J Med. 2010;77(10):715-726.
Drug Brand Names
Carbamazepine • Tegretol
Oxcarbazepine • Trileptal
Desmopressin • Stimate, DDAVP
Lithium • Eskalith, Lithobid
Pentoxifylline • Trental, Pentoxil
Methylprednisolone • Medrol
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Elhassen EA, Schrier RW. Disorders of sodium and water balance. In: McKean SC, Ross JJ, Dressler DD, et al, eds. Principles and practice of hospital medicine. New York, NY: McGraw-Hill; 2012:2084-2093.
2. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
3. Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312(16):1035-1039.
4. Edelman IS, Leibman J, O’Meara MP, et al. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236-1256.
5. Reynolds RM, Seckl JR. Hyponatraemia for the clinical endocrinologist. Clin Endocrinol (Oxf). 2005;63(4):366-374.
6. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 suppl 1):S1-S42.
7. Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci. 2011;23(4):369-374.
8. Goldman MB. The assessment and treatment of water imbalance in patients with psychosis. Clin Schizophr Related Psychoses. 2010;4(2):115-123.
9. Patel AS, Matthews L, Bruce-Jones W. Central pontine myelinolysis as a complication of refeeding syndrome in a patient with anorexia nervosa. J Neuropsychiatry Clin Neurosci. 2008;20(3):371-373.
10. Bhuvaneswar CG, Baldessarini RJ, Harsh VL, et al. Adverse endocrine and metabolic effects of psychotropic drugs: selective clinical review. CNS Drugs. 2009;23(12):1003-1021.
11. Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25(2):210-213.
12. Menger H, Jörg J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J Neurol. 1999;246(8):700-705.
sodium concentration, Wernicke’s
encephalopathy, osmotic demyelination syndrome, electrolyte disorder
CASE Confused and weak
Mr. W, age 26, is brought to the emergency department (ED) by his parents for intermittent confusion, weakness, and increasing lethargy over the past 4 days. He is jaundiced with mild abdominal pain, nausea, and vomiting.
Mr. W has a history of alcohol use disorder, drinking as much as 1 L of vodka a day. Six months ago, he was hospitalized for alcoholic hepatitis and severe hyponatremia.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse 89 beats per minute; blood pressure, 117/50 mm Hg; respirations, 15 breaths per minute; and temperature, 98.5ºF. Physical examination is notable for scleral icterus, jaundice, tender hepatomegaly, and asterixis.
Mr. W is not taking any medications. He reports that his most recent drink was the day before; however, his current alcohol intake is unknown.
Laboratory tests reveal altered hepatic function, including elevated aspartate aminotransferase (251 U/L), alanine aminotransferase (56 U/L), alkaline phosphatase (179 U/L), total bilirubin (15.4 mg/dL), and ammonia (143 U/L), impaired coagulation (international normalized ratio 2.39), and decreased albumin (2.7 g/dL). Other metabolic disturbances include: sodium, 104 mEq/L; chloride, <60 mEq/L; potassium, 2.2 mEq/L; and CO2, 44.5 mEq/L.
What is your differential diagnosis for Mr. W’s altered mental status?
a) hepatic encephalopathy
b) Wernicke’s encephalopathy
c) hyponatremia
d) drug intoxication
e) head trauma
The authors’ observations
Hyponatremia is defined as a serum sodium concentration <136 mEq/L. Mr. W is considered to have severe hyponatremia because his serum sodium concentration is <125 mEq/L. Although commonly caused by an inability to suppress antidiuretic hormone, hyponatremia has several possible causes (Figure 1).1 Symptoms are nonspecific and are more visible when there is a large or rapid decrease in the serum sodium concentration. Most patients with a serum sodium concentration >125 mEq/L are asymptomatic. Mr. W, who had a serum sodium of 104 mEq/L, presented with several symptoms, including confusion, lethargy, nausea, vomiting, and weakness. Headache, muscle spasms, depressed reflexes, restlessness, and disorientation also might be observed.2
Because of Mr. W’s impaired hepatic function, elevated ammonia, and asterixis, hepatic encephalopathy could be contributing to his altered mental status. Suspect Wernicke’s encephalopathy in a patient with neurologic symptoms and a history of chronic alcohol abuse. In its classic form, Wernicke’s encephalopathy has acute onset, characterized by the triad of ataxia, global confusion, and ocular abnormalities. However, this triad is not consistently or frequently encountered.3
Which tests would you order next?
a) blood ethanol level
b) urine drug screen
c) serum osmolality
d) CT of the head
EVALUATION Sober, yet sick
To rule out intoxication as the cause of Mr. W’s altered mental status, blood ethanol level and urine drug screens are obtained and found to be negative. CT of the head is negative for acute intracranial pathology.
Mr. W is admitted to the medical intensive care unit (MICU) for severe hyponatremia and altered mental status. Serum osmolality is 220 mOsm/kg (normal range 281 to 304 mOsm/kg). To further classify his hypotonic hyponatremia, volume status is assessed, and Mr. W is determined to be euvolemic. Thyroid-stimulating hormone and cortisol are within normal limits, eliminating hypothyroidism and adrenal insufficiency as causes of his euvolemic hypotonic hyponatremia. Mr. W is treated for hyponatremia likely secondary to syndrome of inappropriate antidiuretic hormone (SIADH). SIADH is a diagnosis of exclusion that first requires ruling out hypothyroidism and glucocorticoid insufficiency (Figure 1).1
The authors’ observations
Because hypokalemia is an independent predictive factor for development of hyponatremia, it is necessary to evaluate the potassium level in all hyponatremic patients. Mr. W’s potassium level was 2.2 mEq/L on admission. Serum sodium concentration is related to total exchangeable sodium, total body water, and total exchangeable potassium. Potassium depletion causes a shift of sodium into cells with a comparable exit of potassium from cells into extracellular fluid. The reverse process occurs during potassium repletion, leading to an increase in serum sodium concentration and making hypokalemia a risk factor for developing osmotic demyelination syndrome (ODS).4
Treating hyponatremia
Hyponatremia treatment depends on its severity, presence or absence of symptoms, and whether the hyponatremia is acute (<24 hours) or chronic (>48 hours).5
Because of Mr. W’s extremely low serum sodium concentration, predisposition to hyponatremia secondary to alcoholism, and history of severe hyponatremia, it is likely he is chronically hyponatremic.
In patients with chronic hyponatremia, neurological sequelae are associated with the need for a more rapid rate of correction of serum sodium. For most patients with chronic hyponatremia, a correction rate of ≤10 to 12 mEq/L during the first 24 hours and <18 mEq/L over 48 hours is recommended to avoid ODS.6
Evidence suggests, however, that this 1-day limit might be too high for some patients. Alcoholism, hypokalemia, malnutrition, and liver disease are present in a high percentage of patients who develop
ODS after correcting hyponatremia (Table 1).6 Therefore, for patients such as Mr. W who are at high risk of ODS, experts recommend a goal of 4 to 6 mEq/L/d with a correction rate of ≤8 mEq/L in any 24-hour period (Table 2).6
TREATMENT Sodium normalizes
Mr. W receives 1 L of normal saline in the ED before admission to the MICU. Once in the MICU, despite likely chronic hyponatremia, he receives hypertonic (3%) saline, followed by normal saline. Initially, he responds when the serum sodium concentration improves. Because of his likely SIADH, Mr. W is fluid-restricted for 4 days. Serum sodium returns to normal over 7 hospital days (Figure 2). To address the profound hypokalemia, Mr. S receives 30 mEq of potassium chloride in the ED, and potassium is repeated daily throughout his stay in the MICU.
Mr. W remains lethargic, with intermittent periods of confusion throughout the hospital stay. His altered mental status is attributed to hepatic encephalopathy secondary to alcoholic hepatitis. The Maddrey discriminant function is a calculation that stratifies patients with alcoholic hepatitis for risk of mortality and the use of steroids. Because Mr. W shows a Maddrey discriminant function ≥32, he receives methylprednisolone, followed by pentoxifylline, and liver function tests trend down. He also receives lactulose throughout hospitalization.
By discharge on hospital day 9, Mr. W’s serum sodium is 138 mEq/L; serum potassium, 4.1 mEq/L. Total bilirubin and prothrombin remain elevated. Mr. W is discharged on lactulose, thiamine, folic acid, and a 1-month course of pentoxifylline, 400 mg, 3 times a day.
READMISSION Unsteady gait, nausea
Three days after discharge, Mr. W returns to the ED after experiencing a 20-second episode of total body rigidity. He has an unsteady gait and worsening nausea and vomiting.
When Mr. W arrives in the ED, he confirms he is taking his discharge medications as prescribed. His parents report that he has consumed alcohol and Cannabis since discharge and has been taking his sibling’s prescription medications, including quetiapine.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse, 118 beats per minute; blood pressure, 128/73 mm Hg; respirations, 16 breaths per minute; and temperature, 98.5ºF. Physical examination, again, is notable for scleral icterus, jaundice, and asterixis. No focal neurologic deficits are noted.
Consistent with Mr. W’s previous admission, laboratory values reveal altered hepatic function and impaired coagulation. The serum sodium level remains within normal limits at 136 mEq/L. However, again, metabolic disturbances include decreased chloride (97 mEq/L), potassium (2.9 mEq/L), and CO2 (18.2 mEq/L). CT on readmission is unchanged from the earlier hospitalization.
What is your differential diagnosis for Mr. W’s total body rigidity?
a) seizure
b) ODS
c) drug intoxication
d) neuroleptic malignant syndrome
EVALUATION Shaking and weakness
Once admitted to the hospital, Mr. W reports an episode of right upper-extremity “shaking,” followed by weakness. He remembers the entire event and denies tongue biting or incontinence. He is evaluated for possible seizure, given his multiple risk factors, including drug and alcohol use, ingestion of quetiapine, and history of hyponatremia. Routine EEG is negative but prolactin level is elevated.
Mr. W’s mental status continues to wax and wane, prompting a neurology consult and MRI for further evaluation. MRI of the brain without contrast reveals restricted diffusion in the pons centrally, with extension bilaterally to the midbrain and thalami—findings consistent with central pontine myelinolysis. A neurology consultation reveals quadriparesis, paraparesis, dysarthria, and diplopia on examination, all symptoms associated with central pontine myelinolysis.
The authors’ observations
ODS, including central and extrapontine myelinolysis, is a demyelinating condition that occurs because of severe osmotic stress, most commonly secondary to the overly rapid correction of hyponatremia in patients with conditions leading to nutritional or electrolyte stress.7 Mr. W is considered at high risk of developing ODS because he fulfills the 5 criteria listed in Table 1.
Several psychiatric illnesses and neuropsychiatric medications could lead to hyponatremia. Many studies8-10 have documented hyponatremia and resulting ODS in patients with alcoholism, schizophrenia, anorexia, primary psychogenic polydipsia, and MDMA (3,4-methylenedioxymethamphetamine) abuse. Hyponatremia is a side effect of several neuropsychiatric medications, including serotonin reuptake inhibitors, lithium, tricyclic antidepressants, opioids, carbamazepine, oxcarbazepine, and antipsychotic polypharmacy. Other commonly used medications associated with hyponatremia include salt-losing diuretics, nonsteroidal anti-inflammatory drugs, and acetaminophen.7
Disease severity varies from asymptomatic to coma or death. Symptoms, although some could reverse completely, typically are a combination of neuropsychiatric (ie, emotional lability, disinhibition, and other bizarre behaviors) and neurologic. Neurologic symptoms include confusion, impaired cognition, dysarthria, dysphagia, gait instability, weakness or paralysis, and generalized seizures. Severely affected patients could experience “locked-in syndrome,” in which they are awake but unable to move or communicate. Also consistent with Mr. W’s case, ODS often presents initially with delirium, seizures, or encephalopathy, followed by a lucid interval before symptoms develop.7
Diagnosis is based on the appearance of demyelinating brain lesions on CT or MRI. MRI is more sensitive than CT; however, even an MRI scan can appear normal for as long as 4 weeks after symptoms appear.7 Therefore, an initial negative radiologic study in a high-risk patient who develops neurologic symptoms does not exclude ODS. Earlier detection is possible with diffusion-weighted MRI, which is most sensitive and can detect lesions within 24 hours of developing symptoms.11 The severity of the lesion does not correlate with severity of symptoms.
Studies reveal a considerable range in prognosis of patients with clinically symptomatic ODS. A study of 44 patients with central pontine myelinolysis, of which 42 had chronic alcoholism, reported that 34% had no significant functional deficits at follow-up, 34% had minor neurologic deficits, and 31% became dependent on personal help. Outcome did not depend on the extent or severity of neurologic symptoms or the severity of concomitant systemic complications.12
Because of its poor prognosis, prevention of ODS is important. Because ODS commonly is caused by overly rapid correction of hyponatremia, it is necessary to adhere to guidelines for treating chronic hyponatremia (Table 2). If overcorrection occurs, therapeutic re-lowering of serum sodium can be considered, but has not been validated in controlled trials. Based mainly on case reports that suggest benefit from early re-lowering serum sodium in patients with ODS symptoms, experts recommend the following:
• administer desmopressin, 2 to 4 μg, every 8 hours parenterally
• replace water orally or as 5% dextrose in water intravenously (3 mL/kg/hr)
• check serum sodium hourly until serum is reduced to goal.6
Bottom Line
Hyponatremia is the most common electrolyte disorder encountered in practice. Osmotic demyelination syndrome often is preventable, with considerable morbidity and mortality. Psychiatrists should be aware of this condition because it could be an adverse effect of many psychiatric medications and there are some psychiatric illnesses in which hyponatremia is a potential risk. In hyponatremic patients with persistent nonspecific neurologic or neuropsychiatric symptoms and negative CT imaging, additional imaging, such as MRI, is warranted.
Related Resources
- Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
- Vaidya C, Ho W, Freda BJ. Management of hyponatremia: providing treatment and avoiding harm. Cleve Clin J Med. 2010;77(10):715-726.
Drug Brand Names
Carbamazepine • Tegretol
Oxcarbazepine • Trileptal
Desmopressin • Stimate, DDAVP
Lithium • Eskalith, Lithobid
Pentoxifylline • Trental, Pentoxil
Methylprednisolone • Medrol
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Confused and weak
Mr. W, age 26, is brought to the emergency department (ED) by his parents for intermittent confusion, weakness, and increasing lethargy over the past 4 days. He is jaundiced with mild abdominal pain, nausea, and vomiting.
Mr. W has a history of alcohol use disorder, drinking as much as 1 L of vodka a day. Six months ago, he was hospitalized for alcoholic hepatitis and severe hyponatremia.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse 89 beats per minute; blood pressure, 117/50 mm Hg; respirations, 15 breaths per minute; and temperature, 98.5ºF. Physical examination is notable for scleral icterus, jaundice, tender hepatomegaly, and asterixis.
Mr. W is not taking any medications. He reports that his most recent drink was the day before; however, his current alcohol intake is unknown.
Laboratory tests reveal altered hepatic function, including elevated aspartate aminotransferase (251 U/L), alanine aminotransferase (56 U/L), alkaline phosphatase (179 U/L), total bilirubin (15.4 mg/dL), and ammonia (143 U/L), impaired coagulation (international normalized ratio 2.39), and decreased albumin (2.7 g/dL). Other metabolic disturbances include: sodium, 104 mEq/L; chloride, <60 mEq/L; potassium, 2.2 mEq/L; and CO2, 44.5 mEq/L.
What is your differential diagnosis for Mr. W’s altered mental status?
a) hepatic encephalopathy
b) Wernicke’s encephalopathy
c) hyponatremia
d) drug intoxication
e) head trauma
The authors’ observations
Hyponatremia is defined as a serum sodium concentration <136 mEq/L. Mr. W is considered to have severe hyponatremia because his serum sodium concentration is <125 mEq/L. Although commonly caused by an inability to suppress antidiuretic hormone, hyponatremia has several possible causes (Figure 1).1 Symptoms are nonspecific and are more visible when there is a large or rapid decrease in the serum sodium concentration. Most patients with a serum sodium concentration >125 mEq/L are asymptomatic. Mr. W, who had a serum sodium of 104 mEq/L, presented with several symptoms, including confusion, lethargy, nausea, vomiting, and weakness. Headache, muscle spasms, depressed reflexes, restlessness, and disorientation also might be observed.2
Because of Mr. W’s impaired hepatic function, elevated ammonia, and asterixis, hepatic encephalopathy could be contributing to his altered mental status. Suspect Wernicke’s encephalopathy in a patient with neurologic symptoms and a history of chronic alcohol abuse. In its classic form, Wernicke’s encephalopathy has acute onset, characterized by the triad of ataxia, global confusion, and ocular abnormalities. However, this triad is not consistently or frequently encountered.3
Which tests would you order next?
a) blood ethanol level
b) urine drug screen
c) serum osmolality
d) CT of the head
EVALUATION Sober, yet sick
To rule out intoxication as the cause of Mr. W’s altered mental status, blood ethanol level and urine drug screens are obtained and found to be negative. CT of the head is negative for acute intracranial pathology.
Mr. W is admitted to the medical intensive care unit (MICU) for severe hyponatremia and altered mental status. Serum osmolality is 220 mOsm/kg (normal range 281 to 304 mOsm/kg). To further classify his hypotonic hyponatremia, volume status is assessed, and Mr. W is determined to be euvolemic. Thyroid-stimulating hormone and cortisol are within normal limits, eliminating hypothyroidism and adrenal insufficiency as causes of his euvolemic hypotonic hyponatremia. Mr. W is treated for hyponatremia likely secondary to syndrome of inappropriate antidiuretic hormone (SIADH). SIADH is a diagnosis of exclusion that first requires ruling out hypothyroidism and glucocorticoid insufficiency (Figure 1).1
The authors’ observations
Because hypokalemia is an independent predictive factor for development of hyponatremia, it is necessary to evaluate the potassium level in all hyponatremic patients. Mr. W’s potassium level was 2.2 mEq/L on admission. Serum sodium concentration is related to total exchangeable sodium, total body water, and total exchangeable potassium. Potassium depletion causes a shift of sodium into cells with a comparable exit of potassium from cells into extracellular fluid. The reverse process occurs during potassium repletion, leading to an increase in serum sodium concentration and making hypokalemia a risk factor for developing osmotic demyelination syndrome (ODS).4
Treating hyponatremia
Hyponatremia treatment depends on its severity, presence or absence of symptoms, and whether the hyponatremia is acute (<24 hours) or chronic (>48 hours).5
Because of Mr. W’s extremely low serum sodium concentration, predisposition to hyponatremia secondary to alcoholism, and history of severe hyponatremia, it is likely he is chronically hyponatremic.
In patients with chronic hyponatremia, neurological sequelae are associated with the need for a more rapid rate of correction of serum sodium. For most patients with chronic hyponatremia, a correction rate of ≤10 to 12 mEq/L during the first 24 hours and <18 mEq/L over 48 hours is recommended to avoid ODS.6
Evidence suggests, however, that this 1-day limit might be too high for some patients. Alcoholism, hypokalemia, malnutrition, and liver disease are present in a high percentage of patients who develop
ODS after correcting hyponatremia (Table 1).6 Therefore, for patients such as Mr. W who are at high risk of ODS, experts recommend a goal of 4 to 6 mEq/L/d with a correction rate of ≤8 mEq/L in any 24-hour period (Table 2).6
TREATMENT Sodium normalizes
Mr. W receives 1 L of normal saline in the ED before admission to the MICU. Once in the MICU, despite likely chronic hyponatremia, he receives hypertonic (3%) saline, followed by normal saline. Initially, he responds when the serum sodium concentration improves. Because of his likely SIADH, Mr. W is fluid-restricted for 4 days. Serum sodium returns to normal over 7 hospital days (Figure 2). To address the profound hypokalemia, Mr. S receives 30 mEq of potassium chloride in the ED, and potassium is repeated daily throughout his stay in the MICU.
Mr. W remains lethargic, with intermittent periods of confusion throughout the hospital stay. His altered mental status is attributed to hepatic encephalopathy secondary to alcoholic hepatitis. The Maddrey discriminant function is a calculation that stratifies patients with alcoholic hepatitis for risk of mortality and the use of steroids. Because Mr. W shows a Maddrey discriminant function ≥32, he receives methylprednisolone, followed by pentoxifylline, and liver function tests trend down. He also receives lactulose throughout hospitalization.
By discharge on hospital day 9, Mr. W’s serum sodium is 138 mEq/L; serum potassium, 4.1 mEq/L. Total bilirubin and prothrombin remain elevated. Mr. W is discharged on lactulose, thiamine, folic acid, and a 1-month course of pentoxifylline, 400 mg, 3 times a day.
READMISSION Unsteady gait, nausea
Three days after discharge, Mr. W returns to the ED after experiencing a 20-second episode of total body rigidity. He has an unsteady gait and worsening nausea and vomiting.
When Mr. W arrives in the ED, he confirms he is taking his discharge medications as prescribed. His parents report that he has consumed alcohol and Cannabis since discharge and has been taking his sibling’s prescription medications, including quetiapine.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse, 118 beats per minute; blood pressure, 128/73 mm Hg; respirations, 16 breaths per minute; and temperature, 98.5ºF. Physical examination, again, is notable for scleral icterus, jaundice, and asterixis. No focal neurologic deficits are noted.
Consistent with Mr. W’s previous admission, laboratory values reveal altered hepatic function and impaired coagulation. The serum sodium level remains within normal limits at 136 mEq/L. However, again, metabolic disturbances include decreased chloride (97 mEq/L), potassium (2.9 mEq/L), and CO2 (18.2 mEq/L). CT on readmission is unchanged from the earlier hospitalization.
What is your differential diagnosis for Mr. W’s total body rigidity?
a) seizure
b) ODS
c) drug intoxication
d) neuroleptic malignant syndrome
EVALUATION Shaking and weakness
Once admitted to the hospital, Mr. W reports an episode of right upper-extremity “shaking,” followed by weakness. He remembers the entire event and denies tongue biting or incontinence. He is evaluated for possible seizure, given his multiple risk factors, including drug and alcohol use, ingestion of quetiapine, and history of hyponatremia. Routine EEG is negative but prolactin level is elevated.
Mr. W’s mental status continues to wax and wane, prompting a neurology consult and MRI for further evaluation. MRI of the brain without contrast reveals restricted diffusion in the pons centrally, with extension bilaterally to the midbrain and thalami—findings consistent with central pontine myelinolysis. A neurology consultation reveals quadriparesis, paraparesis, dysarthria, and diplopia on examination, all symptoms associated with central pontine myelinolysis.
The authors’ observations
ODS, including central and extrapontine myelinolysis, is a demyelinating condition that occurs because of severe osmotic stress, most commonly secondary to the overly rapid correction of hyponatremia in patients with conditions leading to nutritional or electrolyte stress.7 Mr. W is considered at high risk of developing ODS because he fulfills the 5 criteria listed in Table 1.
Several psychiatric illnesses and neuropsychiatric medications could lead to hyponatremia. Many studies8-10 have documented hyponatremia and resulting ODS in patients with alcoholism, schizophrenia, anorexia, primary psychogenic polydipsia, and MDMA (3,4-methylenedioxymethamphetamine) abuse. Hyponatremia is a side effect of several neuropsychiatric medications, including serotonin reuptake inhibitors, lithium, tricyclic antidepressants, opioids, carbamazepine, oxcarbazepine, and antipsychotic polypharmacy. Other commonly used medications associated with hyponatremia include salt-losing diuretics, nonsteroidal anti-inflammatory drugs, and acetaminophen.7
Disease severity varies from asymptomatic to coma or death. Symptoms, although some could reverse completely, typically are a combination of neuropsychiatric (ie, emotional lability, disinhibition, and other bizarre behaviors) and neurologic. Neurologic symptoms include confusion, impaired cognition, dysarthria, dysphagia, gait instability, weakness or paralysis, and generalized seizures. Severely affected patients could experience “locked-in syndrome,” in which they are awake but unable to move or communicate. Also consistent with Mr. W’s case, ODS often presents initially with delirium, seizures, or encephalopathy, followed by a lucid interval before symptoms develop.7
Diagnosis is based on the appearance of demyelinating brain lesions on CT or MRI. MRI is more sensitive than CT; however, even an MRI scan can appear normal for as long as 4 weeks after symptoms appear.7 Therefore, an initial negative radiologic study in a high-risk patient who develops neurologic symptoms does not exclude ODS. Earlier detection is possible with diffusion-weighted MRI, which is most sensitive and can detect lesions within 24 hours of developing symptoms.11 The severity of the lesion does not correlate with severity of symptoms.
Studies reveal a considerable range in prognosis of patients with clinically symptomatic ODS. A study of 44 patients with central pontine myelinolysis, of which 42 had chronic alcoholism, reported that 34% had no significant functional deficits at follow-up, 34% had minor neurologic deficits, and 31% became dependent on personal help. Outcome did not depend on the extent or severity of neurologic symptoms or the severity of concomitant systemic complications.12
Because of its poor prognosis, prevention of ODS is important. Because ODS commonly is caused by overly rapid correction of hyponatremia, it is necessary to adhere to guidelines for treating chronic hyponatremia (Table 2). If overcorrection occurs, therapeutic re-lowering of serum sodium can be considered, but has not been validated in controlled trials. Based mainly on case reports that suggest benefit from early re-lowering serum sodium in patients with ODS symptoms, experts recommend the following:
• administer desmopressin, 2 to 4 μg, every 8 hours parenterally
• replace water orally or as 5% dextrose in water intravenously (3 mL/kg/hr)
• check serum sodium hourly until serum is reduced to goal.6
Bottom Line
Hyponatremia is the most common electrolyte disorder encountered in practice. Osmotic demyelination syndrome often is preventable, with considerable morbidity and mortality. Psychiatrists should be aware of this condition because it could be an adverse effect of many psychiatric medications and there are some psychiatric illnesses in which hyponatremia is a potential risk. In hyponatremic patients with persistent nonspecific neurologic or neuropsychiatric symptoms and negative CT imaging, additional imaging, such as MRI, is warranted.
Related Resources
- Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
- Vaidya C, Ho W, Freda BJ. Management of hyponatremia: providing treatment and avoiding harm. Cleve Clin J Med. 2010;77(10):715-726.
Drug Brand Names
Carbamazepine • Tegretol
Oxcarbazepine • Trileptal
Desmopressin • Stimate, DDAVP
Lithium • Eskalith, Lithobid
Pentoxifylline • Trental, Pentoxil
Methylprednisolone • Medrol
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Elhassen EA, Schrier RW. Disorders of sodium and water balance. In: McKean SC, Ross JJ, Dressler DD, et al, eds. Principles and practice of hospital medicine. New York, NY: McGraw-Hill; 2012:2084-2093.
2. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
3. Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312(16):1035-1039.
4. Edelman IS, Leibman J, O’Meara MP, et al. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236-1256.
5. Reynolds RM, Seckl JR. Hyponatraemia for the clinical endocrinologist. Clin Endocrinol (Oxf). 2005;63(4):366-374.
6. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 suppl 1):S1-S42.
7. Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci. 2011;23(4):369-374.
8. Goldman MB. The assessment and treatment of water imbalance in patients with psychosis. Clin Schizophr Related Psychoses. 2010;4(2):115-123.
9. Patel AS, Matthews L, Bruce-Jones W. Central pontine myelinolysis as a complication of refeeding syndrome in a patient with anorexia nervosa. J Neuropsychiatry Clin Neurosci. 2008;20(3):371-373.
10. Bhuvaneswar CG, Baldessarini RJ, Harsh VL, et al. Adverse endocrine and metabolic effects of psychotropic drugs: selective clinical review. CNS Drugs. 2009;23(12):1003-1021.
11. Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25(2):210-213.
12. Menger H, Jörg J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J Neurol. 1999;246(8):700-705.
1. Elhassen EA, Schrier RW. Disorders of sodium and water balance. In: McKean SC, Ross JJ, Dressler DD, et al, eds. Principles and practice of hospital medicine. New York, NY: McGraw-Hill; 2012:2084-2093.
2. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
3. Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312(16):1035-1039.
4. Edelman IS, Leibman J, O’Meara MP, et al. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236-1256.
5. Reynolds RM, Seckl JR. Hyponatraemia for the clinical endocrinologist. Clin Endocrinol (Oxf). 2005;63(4):366-374.
6. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 suppl 1):S1-S42.
7. Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci. 2011;23(4):369-374.
8. Goldman MB. The assessment and treatment of water imbalance in patients with psychosis. Clin Schizophr Related Psychoses. 2010;4(2):115-123.
9. Patel AS, Matthews L, Bruce-Jones W. Central pontine myelinolysis as a complication of refeeding syndrome in a patient with anorexia nervosa. J Neuropsychiatry Clin Neurosci. 2008;20(3):371-373.
10. Bhuvaneswar CG, Baldessarini RJ, Harsh VL, et al. Adverse endocrine and metabolic effects of psychotropic drugs: selective clinical review. CNS Drugs. 2009;23(12):1003-1021.
11. Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25(2):210-213.
12. Menger H, Jörg J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J Neurol. 1999;246(8):700-705.
sodium concentration, Wernicke’s
encephalopathy, osmotic demyelination syndrome, electrolyte disorder
sodium concentration, Wernicke’s
encephalopathy, osmotic demyelination syndrome, electrolyte disorder
Management of Acute Decompensated Heart Failure in Hospitalized Patients
From Ohio Health, Riverside Methodist Hospital, Columbus, OH.
Abstract
- Objective: To review the current in-hospital management of patients with acute decompensated heart failure (ADHF).
- Methods: Review of the literature.
- Results: Heart failure is a leading cause of hospitalization in the elderly, and morbidity, mortality, and hospital readmission rates for ADHF remain high. The patient’s hemodynamic status along with the use of prognostic models for short-term mortality may facilitate patient triage and encourage the use of evidence-based therapy, especially in high-risk patients. Initial treatment should target the relief of congestive symptoms, and intravenous loop diuretics are the mainstay of therapy. The preferred IV vasoactive medication has yet to be determined in a large prospective randomized trial. Positive inotropic agents should be reserved for patients with signs of low cardiac output and tissue hypoperfusion; however, the risk/benefit equation should be evaluated judiciously with each treatment option before initiating therapy. For patients with refractory hemodynamic collapse, ventricular assist devices can allow stabilization until recovery or decision regarding transplantation versus destination therapy.
- Conclusion: Patients with ADHF are at increased risk for readmission to the hospital as well as at increased risk for death. Risk factors need to be identified and referral to a heart disease management program should be considered for those patients deemed at increased risk for rehospitalization.
Heart failure is a major public health problem in the United States and the leading cause of hospitalization in patients 65 years of age and older [1]. Patients hospitalized with acute decompensated heart failure (ADHF) have a readmission rate as high as 50% within 6 months and 25% within 30 days [2]. It is estimated that $32 billion is spent on heart failure care each year, the majority of which is directly related to inpatient care. Projections show that by 2030 the total cost of heart failure will increase to $70 billion per year [1]. Despite the growing burden, advances in treatment have been limited [2,3] and management continues to be a challenge. In this article, we review the current in-hospital management of patients with ADHF.
Case Study
Initial Presentation
A 64-year-old woman with a nonischemic dilated cardiomyopathy presents to the emergency department (ED) with a 4-day history of progressive dyspnea on exertion. She can not ambulate more than 50 feet without having to stop due to dyspnea and reports increased lower extremity edema. She is found to have a heart rate of 105 bpm, a respiratory rate of 30 breaths/min, and a blood pressure of 90/51 mm Hg. Physical examination is remarkable for distended neck vein, S3 gallop, end expiratory wheezing in the bases, and lower extremity edema. Blood tests, including a B-type natriuretic peptide level, are pending. Electrocardiogram and chest radiograph are ordered. The physician suspects that the patient has ADHF and admits her for further management.
What are aspects of initial management in the ED?
Most patients that present for evaluation and management of ADHF are first evaluated in the ED. Initial management includes an assessment of oxygenation, hemodynamic status, and adequacy of tissue perfusion, as well as for possibility of an acute coronary syndrome. A complete history, physical examination, chest radiography, 12-lead electrocardiogram, cardiac troponin T or I, electrolytes, and complete blood count should be obtained to allow rapid diagnosis and triage followed by prompt, aggressive treatment in the ED or observation unit. This should alleviate the patient’s symptoms sooner, and it is intuitive that this would lessen morbidity and length of hospital stay [4].
How are patients with ADHF classified?
What risk assessment tools are available?
B-type natriuretic peptide (BNP) and N-terminal fragment proBNP (NT-proBNP) were recently validated as diagnostic aids for the differentiation of etiologies of dypnea in patients in the ED with possible symptoms of ADHF. Use of these biomarkers can help reduce diagnostic uncertainty and associated mismanagement of patients presenting with nonspecific symptoms of dysp-nea [4,5,7]. Low or normal levels (BNP < 100 pg/ml or NT-proBNP < 500 pg/ml) have a high negative predictive value for excluding heart failure.
Elevated BNP or NT-proBNP levels may also yield prognostic information, identifying patients at increased risk of mortality or rehospitalization when value does not fall after aggressive heart failure management [8,9].In a recent study by Fonarow et al, the levels of BNP on hospital admission correlated directly with the risk of in-hospital mortality in patients admitted with ADHF independent of left ventricular ejection fraction. When the levels of BNP were below 430 pg/ml, the in-hospital mortality was 1.9%, and when the levels were above 1730 pg/ml, the mortality went up to 6% (P < 0.001) [8]. Additionally, elevated pre-discharge BNP levels (BNP > 350 ng/l; P < 0.001) in patients with ADHF seem to identify those at increased risk of death or readmission after in-patient management [9]. Elevated cardiac troponin T or I in hospitalized patients with ADHF also are associated with increased mortality, including in those without acute coronary syndrome or underlying coronary artery disease [10,11].
Case Continued
Upon further evaluation by a cardiologist, the patient is cool and clammy with elevated neck veins and prominent S3 confirmed. She continues to report severe shortness of breath after 1 dose of intravenous (IV) furosemide in the ED. Repeat vital signs shows a blood pressure of 83/49 mm Hg and respiratory rate of 33. Her electrocardiogram shows sinus tachycardia. The cardiologist determines that the patient’s clinical profile is “cold and wet” and admits the patient to the cardiac care unit (CCU) with a diagnosis of ADHF.
Initial blood tests show a BNP level of 1830 pg/ml, troponin I is 0.63 and stable after 2 measurements, serum creatinine is 1.6 mg/dL, BUN is 44 mg/dL, and serum sodium is 132 mg/dL. The GWTG-HF risk score for in-hospital mortality was calculated based on admission data and the probability of death was estimated at > 5% to 10% [12]. Prompt aggressive medical therapy was instituted in the CCU consisting of furosemide infusion to reduce congestion and IV dobutamine to improve systemic perfusion. Enoxoparin 40 mg subcutaneously once daily was initiated for venous thromboembolism prophylaxis.
What are important aspects of therapy for ADHF?
Several days to weeks prior to the appearance of signs and symptoms of volume overload, patients may develop hemodynamic congestion, defined as an elevation of ventricular filling pressure/pulmonary capillary wedge pressure independent of clinical evidence of fluid overload [17]. Elevated filling pressure is the culprit in the development of most of the signs and symptoms of ADHF and is the target for treatment.
Although use of vasoactive medications such as nitroglycerin or nitroprusside are not routinely recommended for use in all ADHF patients admitted to the hospital, retrospective analysis of the ADHEREdatabase suggests that there is a significant reduction of mortality, hospital length of stay, admission to intensive care unit, invasive procedures, and prolonged hospitalizations when IV diuretics, vasodilators (nitroglycerin, nitroprusside, nesiritide,) and/or positive inotropes (milrinone, dobutamine) are initiated in the ED within 6 hours of an ADHF presentation [18,19].However, whether prompt ED intervention impacts intermediate- to long-term outcomes is unknown [4].
Hospitalized patients with ADHF are at increased risk of venous thromboembolism mainly due to reduced cardiac output, increased systemic venous pressure, and reduced activity levels. Therefore, it is recommended that during the hospitalization ADHF patients receive prophylaxis against venous thromboembolism with low-dose unfractionated heparin or low-molecular-weight heparin if there is no contraindication [5].Individual therapeutic choices for ADHF are reviewed in detail below.
What treatments are used to relieve congestion?
Diuresis
In patients admitted to the hospital with ADHF, initial effective diuresis is vital to lowering cardiac filling pressures and relieving symptoms of congestion. Intravenous loop diuretics represent the first line of treatment and have long been the mainstay of therapy for decompensated heart failure with preserved or reduced ejection fraction, reducing fluid overload, and relieving symptoms.
Despite its long track record, the dose administration of IV diuretics is more of an art than a science. Medication dosage sufficient to produce a rate of diuresis that will optimize volume status and relieve signs and symptoms of congestion without causing kidney injury or hypotension is recommended [5].Due to the relatively short half-life of loop diuretics and concerns about tubular sodium reabsorption in the kidneys, continuous IV diuretic infusion has been suggested to enhance diuresis and avoid sodium and fluid rebound [5,20,21]. However, continuous loop diuretic infusion has not proven superior to intermittent IV bolus dosing in clinical studies. Recent data from the Diuretic Optimization Strategies Evaluation (DOSE) trial comparing bolus versus continuous infusion diuretic strategy in patients with ADHF showed no difference in global symptom relief, diuresis, or any of the clinical secondary endpoints including composite of death, re-hospitalization, or ED visits with either IV bolus versus continuous infusion or low versus high doses of furosemide [22]. Concern has also been previously raised about adverse outcomes utilizing high doses of loop diuretics in the treatment of ADHF [20,23,24]. However, the DOSE trial also evaluated the safety of 2 strategies for furosemide dosing in patients with ADHF. The study randomized ADHF patients with a prior diagnosis of chronic heart failure to 4 different treatment groups, either a high dose (2.5x their daily chronic oral furosemide dose) or low dose (1x their daily chronic oral furosemide dose), which was given either twice daily via IV bolus or via continuous infusion. The study showed no difference in change in renal function from baseline to 72 hours with either IV bolus versus continuous infusion or low versus high doses of furosemide [22].
Ultrafiltration
For patients with marked fluid overload who are unresponsive to diuretic therapy, peripheral ultrafiltration may be considered. Initial data demonstrated that early ultrafiltration effectively and safely reduced congestion in patients with ADHF with diuretic resistance and renal insufficiency. Length of stay was reduced, with 60% of discharges in 3 days or less and 1 readmission at 30 days. Neurohormonal activation, indicated by reduction in BNP level, was reduced without worsening glomerular filtration rate, hypotension or electrolyte abnormalities [28]. The UNLOAD trial confirmed these results and extended their findings to show that patients undergoing peripheral ultrafiltration had greater weight and net fluid loss at 48 hours and reduced rate of rehospitalization at 90 days when compared with IV diuretic therapy alone in ADHF patients. Interestingly, there was no difference in the dyspnea score at 48 hours and there was a trend toward worsening of renal function in the ultrafiltration group. The study was not powered to document a survival benefit [29]. However, the more recent Cardiorenal Rescue Study in ADHF (CARRESS-HF) trial involving patients with ADHF and worsening renal function showed that there was no difference in weight loss between patients randomized to ultrafiltration or a strategy of stepped pharmacologic therapy. Additionally, ultrafiltration was associated with a significant increase in creatinine at 96 hours and a higher rate of adverse events related to the procedure, driven by complications from intravenous catheter insertion. There was no difference between the 2 groups in death or rehospitalization for heart failure [30]. At present, ultrafiltration may be a reasonable option if all diuretic strategies are unsuccessful in relieving congestion [5].
Vasopressin-Receptor Antagonists
The vasopressin-receptor antagonists represent a relatively new class of medications that target the vasopressin receptors V1a and V2. Activation of the vasopressin V2 receptors by arginine vasopressin in heart failure causes inappropriate free water retention contributing to the symptoms of congestion and hyponatremia [31]. Currently, the only 2 vasopressin-receptor antagonists available for clinical use are conivaptan (V1a /V2 receptor antagonist) and tolvaptan (V2 receptor antagonist). The effectiveness of tolvaptan was tested in a randomized study (EVEREST) in patients hospitalized with ADHF [32,33]. At 1 year there was no difference seen in the primary endpoints of all-cause mortality, death from cardiovascular causes, or first hospitalization for heart failure [32,33]. However, hyponatremia, when present, was improved in the tolvaptan group. Conivaptan has a similar hemodynamic profile compared to tolvaptan, but without improving signs and symptoms in hospitalized patients with ADHF [34]. Currently, vasopressin antagonists are recommended in the management of ADHF by professional guidelines as only a class IIb indication in hospitalized patients with volume overload and severe hyponatremia [5].
Case Continued
After 24 hours of medical therapy in the CCU, the patient is no longer clammy and cool but continues to have shortness of breath, and peripheral edema is not improving. She continues to have elevated JVP and S3. Her blood pressure is now 120/79 mm Hg and her heart rate is 110. A Swan-Ganz catheter placed this morning showed a cardiac index of 1.8 L/minute/m2 (reference range, 2.5–4.0 L/min/m2); pulmonary capillary wedge pressure is 28 mm Hg (reference range, 6–12 mm Hg) and systemic vascular resistance is 1932 dyne/second/cm5 (reference range, 800–1200 dynes/sec/cm5). The physician decides to add nitroprusside to lower her filling pressure and systemic vascular resistance.
What is the role of vasoactive medications in treatment?
Vasodilators
Nitroglycerin is a venodilating medication with preload reduction properties at low doses and an arterial dilator at high doses [35]. Preload reduction improves left ventricular filling pressures and pulmonary congestion without increasing the oxygen demand in the heart in patients with ADHF. This leads to an improvement of symptoms, including dyspnea, in as early as 5 minutes [36]. For a highly symptomatic patient, nitroglycerin given sublingually can be useful in an acute situation because it is typically immediately available while preparations are made for administration of IV medications. Limitations of nitroglycerin include rapid tachyphylaxis within several hours of continuous exposure at high doses, resistance to the hemodynamic effects of nitroglycerin in up to 20% of patients, and hypotension, which may occur before significant preload reduction effect can be obtained [37]. When symptomatic hypotension becomes a problem, the highest hemodynamically tolerable dose should be given. Another agent with a potent vasodilator effect used in the treatment of heart failure is sodium nitroprusside (SNP). As opposed to nitroglycerin, this drug has an equally potent preload- and afterload-reducing effect [35]. Afterload reduction through its arteriodilator effect has the benefit of increasing cardiac output and decreasing myocardial oxygen demand with improvement of pulmonary congestion [36]. SNP is used in less than 1% of patients hospitalized with heart failure [38], probably due to the potential for causing marked hypotension, its need for invasive hemodynamic monitoring, and the rare risk for thiocyanate toxicity with high doses and/or longer infusions, especially in patients with reduced hepatic perfusion and renal function, as in the case of low-output heart failure [35]. However, data demonstrating safety and efficacy of SNP infusion in patients with ADHF are limited [39].A single-center, retrospective case-control study suggested that the administration of SNP in carefully selected patients with advanced low-output ADHF was safe and may be associated with favorable long-term clinical outcomes [39]. SNP can be attractive in severely congested patients with hypertension or severe mitral regurgitation complicating left ventricular failure, but prospective trials are needed to clarify the safety and efficacy in this patient population.
Nesiritide is a human recombinant form of BNP that has a direct effect on the vascular endothelium by increasing the bioavailability of nitric oxide through stimulation of cyclic guanosine monophosphate. Its primary mechanism of action is to reduce left ventricular filling pressures by a systemic and pulmonary vasodilator effect. It also promotes diuresis and natriuresis [40].The initial efficacy of nesiritide was demonstrated in the VMAC (Vasodilation in the Management of Acute Congestive Heart Failure) study, a randomized trial of IV nesiritide versus IV nitroglycerin or placebo in decompensated heart failure patients. A significant reduction in pulmonary capillary wedge pressure was demonstrated within 15 minutes in the nesiritide group and maintained at 3 hours compared to either nitroglycerin or placebo, with a similar improvement in dyspnea extending out to 24 hours [41].
The large ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) randomized ADHF patients to nesiritide or placebo and tested the hypothesis that nesiritide would be superior to placebo in improving acute dyspnea, all-cause mortality, and heart failure readmission in patients presenting with ADHF [42]. Nesiritide-treated patients showed only a modest early improvement in self-assessed dyspnea and no difference in the composite endpoint of death or rehospitalization at 30 days in patients admitted with ADHF. Reassuringly, there was no increase in renal failure compared to placebo; however, the incidence of symptomatic hypotension was higher with nesiritide [42]. Although nesiritide remains in the armentarium of vasoactive medications for ADHF, less expensive vasodilators such as nitroglycerin or nitroprusside may be preferred by many clinicians.
Overall, vasodilators represent a good treatment option for patients presenting with ADHF characterized by low cardiac output, high filling pressures, and elevated systemic vascular resistance. There is no clear evidence, however, to suggest that IV vasodilators improve survival in hospitalized patients with ADHF; thus, its use should be restricted to the relief of dyspnea in patients with stable blood pressure [5].
Inotropic Therapy
The most commonly used positive inotropic agents in the management of patients with ADHF in the United States are dobutamine (beta-1, beta-2, and alpha adrenoreceptor agonist) and milrinone (phosphodiesterase-III inhibitor) [38]. Inotropes increase cardiac output by increasing myocardial contractility, reduce left and right ventricular filling pressures, and improve hemodynamic parameters. Despite these hemodynamic effects, inotropic agents have not demonstrated a survival benefit in patients with ADHF. A major limitation regarding these agents is that they increase the risk of cardiac arrhythmias by increasing intracellular calcium in cardiac myocytes. In fact, retrospective analyses suggest that most inotropic agents are associated with an increased risk of death [38,43].
Milrinone inhibits type III isoform of the enzyme phosphodiasterase leading to an increase in intracellular cyclic AMP to exert its positive inotropic effect on the myocardium. Milrinone also exerts systemic and pulmonary vasodilator effects in the circulation decreasing right atrial, pulmonary capillary wedge, and mean arterial pressure. In the OPTIME-CHF trial, patients with chronic heart failure admitted to the hospital with ADHF were randomized to short term infusion of milrinone vs. placebo plus standard therapy. Milrinone resulted in more hypotension, atrial fibrillation and ventricular arrhythmias without any benefit on mortality or re-hospitalization [44].A retrospective analysis from the ADHEREregistry showed that in-hospital mortality was twofold higher with the use of dobutamine or milrinone in patients with ADHF when compared to treatment with vasodilators [38].
Dobutamine is a beta-1, beta-2, and alpha adrenoreceptor agonist that works by increasing myocardial contractility leading to an increase in cardiac output as its primary cardiovascular effect. Currently, routine use of IV positive inotropic agents in the absence of imminent cardiogenic shock or low output ADHF with systemic hypoperfusion is generally not recommended due to concerns of adverse effects [5]. The ACCF/AHA guidelines recommend the use of positive inotropic agents to relieve symptoms, improve systemic perfusion and preserve end-organ function in patients with severe left ventricular systolic failure and low output syndrome with evidence of end-organ dysfunction (such as hypotension, altered mentation, cool extremities, low urine output and serum markers indicative of renal and/or hepatic dysfunction) with or without congestion [5].
Continuous outpatient therapy with inotropes may be a viable option in patients with stage D (end stage) heart failure who are deemed unlikely to survive hospital discharge [45].This is also supported by the ACCF/AHA practice guidelines where IV inotropic support may be considered for the previous reasons only after all alternative therapies to achieve stability have failed (Class IIB indication) [5].
Is there a role for morphine?
For decades morphine has been considered an essential component in the armamentarium for the treatment of ADHF. Its preload-reducing effect, anti-anxiety properties, and breathlessness suppression has made morphine a popular medication in the treatment of ADHF. Despite its common use, there is a lack of prospective randomized trials demonstrating the safety and benefit of this drug. In a retrospective analysis from the ADHERE database, IV morphine used for ADHF was associated with higher rates of adverse events, including increase use of mechanical ventilation, prolonged hospitalization, increased intensive care unit admissions, and higher mortality, bringing into question its safety profile [46]. Until a randomized trial is completed demonstrating safety and benefit, caution is advised regarding the use of morphine in ADHF.
Case Continued
Over the next 72 hours the patient’s symptoms improved. She no longer has dyspnea at rest, she has had a proper urine-output response to therapy, her serum creatinine has returned to normal, and her vital signs have remained stable. The IV vasodilator was discontinued, dobutamine was weaned off, and the patient was transitioned to guideline-directed medical therapy with an angiotensin-converting enzyme (ACE) inhibitor while continuing IV furosemide. Hospitalized patients who are hemodynamically stable should be transitioned to guideline-directed medical therapy with an oral ACE inhibitor unless the patient has a contraindication, such as marked azotemia or hyperkalemia. Low-dose carvedilol was initiated after optimization of volume status was confirmed. In the absence of shock and after optimization of volume status, every effort should be made to initiate low-dose beta blockers prior to hospital discharge.
When is mechanical circulatory support indicated in ADHF patients?
Mechanical circulatory support has emerged as a reasonable option in selected patients with acute and reversible cardiogenic shock (ie, acute coronary syndrome or an acute mechanical problem such as a torn papillary muscle or ventricular septal defect) [5]. Recently, the utility of intraaortic balloon pump (IABP) in the setting of cardiogenic shock resulting from acute coronary syndrome was called into question with the negative results from the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial [47]. The study compared IABP with best available medical therapy alone among patients with acute myocardial infarction complicated by cardiogenic shock for who early revascularization was planned. Use of IABP did not reduce 30-day mortality compared with medical therapy in this patient population [47]. Whether IABP has a significant role in mechanical complications, such as acute ventricular septal rupture or papillary muscle rupture, is unknown due to the paucity of data in the management of patients with such complications. Therefore, when patients present with severe acute cardiogenic shock refractory to medical therapy, mechanical circulatory support with either ventricular assist devices (VAD) or extracorporeal membrane oxygenation (ECMO) is the preferred means to reverse terminal circulatory collapse. VADs are effective in the short-term as a “bridge-to-recovery” or as a “bridge-to-decision” when recovery, transplant candidacy, or neurologic status are still uncertain [48,49]. There are several options currently available for mechanical circulatory support, including surgically implanted VADs or the percutaneously implanted VADs, such as the Impella 2.5, 3.5 and 5.0 (Abiomed, Danvers, MA) and the TandemHeart pump (Cardiac Assist, Pittsburgh, PA).The ideal device and optimal duration of temporary support are yet to be defined. A detailed description of the function and clinical effects of mechanical support devices is beyond the scope of this article, although thorough reviews are available [48,49].
What elements of care may help optimize the discharge process?
Transition of care in hospitalized patients with ADHF to outpatient care is a critical and vulnerable period for patients given the complexity of the discharge planning for heart failure. A multidisciplinary heart failure disease management program is recommended in both the inpatient and outpatient setting to address the barriers to successful transition of care [5]. Physicians and physician extenders, nurses, pharmacists, and social workers can work together to identify risk factors for readmission and bridge the gap between the inpatient and outpatient setting.
Patients at high risk for hospital readmission should be referred to a heart failure disease management program [5,37]. Patients at high risk for hospital readmission include patients with renal insufficiency, low output state, diabetes mellitus, chronic lung disease, persistent NYHA functional class III, IV symptoms, frequent hospitalizations, multiple comorbidities, history of depression, cognitive impairment, or recurrent problems with noncompliance. There is strong evidence that a heart failure disease management program will reduce rehospitalization rates and costs while improving functional status and quality of life of the patient [37].In addition, a heart failure disease management clinic often can see the patient shortly after discharge, which may allow earlier discharge of the patient and shorter length of stay. Proven therapies such as ACE inhibitors, angiotensin-receptor blockers, beta blockers, and aldosterone antagonists can be titrated frequently in this setting.
It is strongly recommended that comprehensive written discharge instructions be provided at the end of hospitalization with special emphasis on diet, discharge medications, activity level, follow-up appointment, daily weight monitoring, and instructions for recurrence of symptoms [5].
Case Conclusion
The patient tolerated well the initiation of guideline-directed medical therapy and is continued on the ACE inhibitor and beta-blocker medications. After 4 days IV furosemide is discontinued and transitioned to oral furosemide. Precipitant causes of heart failure were addressed throughout hospitalization. It was determined that the patient had been taking high doses of nonsteroidal anti-inflammatory drugs due to knee pain. She was educated on this and other potential precipitant factors. Heart failure education was reinforced, including self-care, emergency plans, and need for medication and diet adherence. She is scheduled an early follow-up visit within 2 weeks of hospital discharge in the multidisciplinary heart failure disease management clinic.
Summary
ADHF is a major public health problem commonly encountered and often initially managed in the ED. Initial history and physical examination are important to estimate the degree of congestion and peripheral perfusion. The patient’s hemodynamic status along with the use prognostic models for short-term mortality may facilitate patient triage and encourage the use of evidence-based therapy, especially in high-risk patients. Initial treatment should target the relief of congestive symptoms and intravenous loop diuretics are the mainstay of therapy. The preferred IV vasoactive medication has yet to be determined in a large prospective randomized trial. Positive inotropic agents should be reserved for patients with signs of low cardiac output and tissue hypoperfusion, however, the risk/benefit equation should be evaluated judiciously with each treatment option before initiating therapy. For patients with refractory hemodynamic collapse, ventricular assist devices can allow stabilization until recovery or decision regarding transplantation versus destination therapy. Patients with ADHF are at increased risk for readmission to the hospital as well as increased risk for death. Risk factors need to be identified and referral to a heart disease management program should be considered for those patients deemed at increased risk for rehospitalization.
Corresponding author: Carlos E. Sanchez, MD, 3705 Olentanfy River Rd., Columbus, OH 43214, [email protected].
Financial disclosures: None.
Author contributions: conception and design, CES; analysis and interpretation of data, CES; drafting of article, CES; critical revision of the article, CES, DRR; collection and assembly of data, CES.
1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 2013;127: e6–245.
2. Fonarow GC. ADHERE Scientific Advisory Committee. The ADHF National Registry (ADHERE): opportunities to improve care of patients hospitalized with ADHF. Rev Cardiovasc Med 2003;4(suppl 7):S21-S30.
3. Philbin EF, Dec GW, Enkins PL, et al. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol 2001;87:1367–71.
4. Weintraub NL, Collins SP, Pang PS, et al.; on behalf of the American Heart Association Council on Clinical Cardiology and Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation 2010;122:1975–96.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-239.
6. Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003;41:1797–1804.
7. Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161–7.
8. Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in ADHF. J Am Coll Cardiol 2007;49:1943–50.
9. Logeart D, Thabut G, Jourdian P, et al. Pre-discharge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol 2004;43:635–41.
10. Peacock WFIV, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117–26.
11. Ilva T, Lassus J, Siirila-Waris K, et al. Clinical significance of cardiac troponins I and T in acute heart failure. Eur J Heart Fail 2008;10:772–9.
12. Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for inhospital mortality in patients with heart failure from the American Heart Association Get With The Guidelines program. Circ Cardiovasc Qual Outcomes 2010;3:25–32.
13. Fonarow GC, Adams KF, Abraham WT, et al, for the ADHERE Scientific Advisory Committee, Study Groups and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572–80.
14. Felker GM, Leimberger JD, Califf RM, et al. Risk stratification after hospitalization for decompensated heart failure. J Card Fail 2004;10:460–6.
15. Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 2003;290:2581–7.
16. Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure. J Am Coll Cardiol 2008; 52:347–56.
17. Gheorghiade M, Shin DD, Thomas TO, et al. Congestion is an important diagnostic and therapeutic target in heart failure. Rev Cardiovasc Med 2006;7(suppl l):S12-S24.
18. Peacock WF, Emerman C, Costanzo MR, et al. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail 2009;15:256–64.
19. Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE analysis. J Am Coll Cardiol 2008;52:534–40.
20. Salvador DRK, Rey NR, Ramos GC, et al. Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst Rev 2005(3):CD003178.
21. Pivac N, Rumboldt Z, Sardelic S, et al. Diuretic effects of furosemide infusion versus bolus injection in congestive heart failure. Int J Clin Pharmacol Res 1998;18:121–8.
22. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with ADHF. N Engl J Med 2011;364:797–805.
23. Cotter G, Metzkor E, Kaluski E, et al. Randomized trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary edema. Lancet 1998;351:389–93.
24. Butler J, Forman DE, Abraham WT, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J 2004;147:331–8.
25. Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in ADHF (DAD-HF) Trial. J Card Fail 2010;16:922–30.
26. Cotter G, Weissgarten J, Metzkor E, et al. Increased toxicity of high-dose furosemide versus low-dose dopamine in the treatment of refractory congestive heart failure. Clin Pharmacol Ther 1997;62:187–93.
27. Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43.
28. Costanzo MR, Saltzberg M, O’Sullivan J, et al. Early ultrafiltration in patients with decompensated heart failure and diuretic resistance. J Am Coll Cardiol 2005;46:2047–51.
29. Costanzo MR, Guglin ME, Saltzberg MT, et al; UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for ADHF. J Am Coll Cardiol 2007;49:675–83.
30. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296-304.
31. Shrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999;341:577–85.
32. Konstam MA, Gheorghiade M, Burnett Jr JC, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297:1319–31.
33. Gheorghiade M, Konstam MA, Burnett Jr JC, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007;297:1332–43.
34. Goldsmith SR, Elkayam U, Haught WH, et al. Efficacy and safety of the vasopressin V1A/V2-receptor antagonist conivaptan in ADHF: a dose-ranging pilot study. J Card Fail 2008;14:641–-7.
35. Shin DD, Brandimarte F, DeLuca L, et al. Review of current and investigational pharmacologic agents for acute heart failure syndromes. Am J Cardiol 2007;99:4A–23A.
36. Mattu A, Martinez JP, Kelly BS. Modern management of cardiogenic pulmonary edema. Emerg Med Clin North Am 2005;23:1105–25.
37. Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail 2010;16:e475-e539
38. Abraham WT, Adams KF, Fonarow GC, et al. ADHERE Scientific Advisory Committee and Investigators; ADHERE Study Group. In-hospital mortality in patients with ADHF requiring intravenous vasoactive medications: an analysis from the ADHF national registry (ADHERE). J Am Coll Cardiol 2005;46:57–64.
39. Mullens W, Abrahams Z, Francis GS, et al. Sodium nitroprusside for advanced low-output heart failure. J Am Coll Cardiol 2008;52:200–7.
40. Bhalla V, Willis S, Maisel AS. B-type natriuretic peptide: the level and the drug-partners in the diagnosis and management of congestive heart failure. Congest Heart Fail 2004;10(1 suppl 1):3–27.
41. Publication Committee for the VMAC investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA 2002;287:1531–40.
42. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with ADHF. N Engl J Med 2011;365:32–43.
43. Elkayam U, Tasissa G, Binanay C, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J 2007;153:98–104.
44. Cuffe MS, Califf RM, Adams KF Jr, et al. Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized control trial. JAMA 2002;287:1541–7.
45. Hershberger RE, Nauman D, Walker TL, et al. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail 2003;9:180–7.
46. Peacock WF, Hollander JE, Diercks DB, et al. Morphine and outcomes in ADHF: an ADHERE analysis. Emerg Med J 2008;25:205–9.
47. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96.
48. Abu-Omar Y, Tsui S. Mechanical circulatory support for AMI and cardiogenic shock. J Card Surg 2010;25:434–41.
49. Ziemba EA, John R. Mechanical circulatory support for bridge to decision: which device and when to decide. J Card Surg 2010;25:425–33.
50. Sanchez CE, Richards DR. Contemporary in-hospital management strategies for ADHF. Cardiol Rev 2011;19:122–9.
From Ohio Health, Riverside Methodist Hospital, Columbus, OH.
Abstract
- Objective: To review the current in-hospital management of patients with acute decompensated heart failure (ADHF).
- Methods: Review of the literature.
- Results: Heart failure is a leading cause of hospitalization in the elderly, and morbidity, mortality, and hospital readmission rates for ADHF remain high. The patient’s hemodynamic status along with the use of prognostic models for short-term mortality may facilitate patient triage and encourage the use of evidence-based therapy, especially in high-risk patients. Initial treatment should target the relief of congestive symptoms, and intravenous loop diuretics are the mainstay of therapy. The preferred IV vasoactive medication has yet to be determined in a large prospective randomized trial. Positive inotropic agents should be reserved for patients with signs of low cardiac output and tissue hypoperfusion; however, the risk/benefit equation should be evaluated judiciously with each treatment option before initiating therapy. For patients with refractory hemodynamic collapse, ventricular assist devices can allow stabilization until recovery or decision regarding transplantation versus destination therapy.
- Conclusion: Patients with ADHF are at increased risk for readmission to the hospital as well as at increased risk for death. Risk factors need to be identified and referral to a heart disease management program should be considered for those patients deemed at increased risk for rehospitalization.
Heart failure is a major public health problem in the United States and the leading cause of hospitalization in patients 65 years of age and older [1]. Patients hospitalized with acute decompensated heart failure (ADHF) have a readmission rate as high as 50% within 6 months and 25% within 30 days [2]. It is estimated that $32 billion is spent on heart failure care each year, the majority of which is directly related to inpatient care. Projections show that by 2030 the total cost of heart failure will increase to $70 billion per year [1]. Despite the growing burden, advances in treatment have been limited [2,3] and management continues to be a challenge. In this article, we review the current in-hospital management of patients with ADHF.
Case Study
Initial Presentation
A 64-year-old woman with a nonischemic dilated cardiomyopathy presents to the emergency department (ED) with a 4-day history of progressive dyspnea on exertion. She can not ambulate more than 50 feet without having to stop due to dyspnea and reports increased lower extremity edema. She is found to have a heart rate of 105 bpm, a respiratory rate of 30 breaths/min, and a blood pressure of 90/51 mm Hg. Physical examination is remarkable for distended neck vein, S3 gallop, end expiratory wheezing in the bases, and lower extremity edema. Blood tests, including a B-type natriuretic peptide level, are pending. Electrocardiogram and chest radiograph are ordered. The physician suspects that the patient has ADHF and admits her for further management.
What are aspects of initial management in the ED?
Most patients that present for evaluation and management of ADHF are first evaluated in the ED. Initial management includes an assessment of oxygenation, hemodynamic status, and adequacy of tissue perfusion, as well as for possibility of an acute coronary syndrome. A complete history, physical examination, chest radiography, 12-lead electrocardiogram, cardiac troponin T or I, electrolytes, and complete blood count should be obtained to allow rapid diagnosis and triage followed by prompt, aggressive treatment in the ED or observation unit. This should alleviate the patient’s symptoms sooner, and it is intuitive that this would lessen morbidity and length of hospital stay [4].
How are patients with ADHF classified?
What risk assessment tools are available?
B-type natriuretic peptide (BNP) and N-terminal fragment proBNP (NT-proBNP) were recently validated as diagnostic aids for the differentiation of etiologies of dypnea in patients in the ED with possible symptoms of ADHF. Use of these biomarkers can help reduce diagnostic uncertainty and associated mismanagement of patients presenting with nonspecific symptoms of dysp-nea [4,5,7]. Low or normal levels (BNP < 100 pg/ml or NT-proBNP < 500 pg/ml) have a high negative predictive value for excluding heart failure.
Elevated BNP or NT-proBNP levels may also yield prognostic information, identifying patients at increased risk of mortality or rehospitalization when value does not fall after aggressive heart failure management [8,9].In a recent study by Fonarow et al, the levels of BNP on hospital admission correlated directly with the risk of in-hospital mortality in patients admitted with ADHF independent of left ventricular ejection fraction. When the levels of BNP were below 430 pg/ml, the in-hospital mortality was 1.9%, and when the levels were above 1730 pg/ml, the mortality went up to 6% (P < 0.001) [8]. Additionally, elevated pre-discharge BNP levels (BNP > 350 ng/l; P < 0.001) in patients with ADHF seem to identify those at increased risk of death or readmission after in-patient management [9]. Elevated cardiac troponin T or I in hospitalized patients with ADHF also are associated with increased mortality, including in those without acute coronary syndrome or underlying coronary artery disease [10,11].
Case Continued
Upon further evaluation by a cardiologist, the patient is cool and clammy with elevated neck veins and prominent S3 confirmed. She continues to report severe shortness of breath after 1 dose of intravenous (IV) furosemide in the ED. Repeat vital signs shows a blood pressure of 83/49 mm Hg and respiratory rate of 33. Her electrocardiogram shows sinus tachycardia. The cardiologist determines that the patient’s clinical profile is “cold and wet” and admits the patient to the cardiac care unit (CCU) with a diagnosis of ADHF.
Initial blood tests show a BNP level of 1830 pg/ml, troponin I is 0.63 and stable after 2 measurements, serum creatinine is 1.6 mg/dL, BUN is 44 mg/dL, and serum sodium is 132 mg/dL. The GWTG-HF risk score for in-hospital mortality was calculated based on admission data and the probability of death was estimated at > 5% to 10% [12]. Prompt aggressive medical therapy was instituted in the CCU consisting of furosemide infusion to reduce congestion and IV dobutamine to improve systemic perfusion. Enoxoparin 40 mg subcutaneously once daily was initiated for venous thromboembolism prophylaxis.
What are important aspects of therapy for ADHF?
Several days to weeks prior to the appearance of signs and symptoms of volume overload, patients may develop hemodynamic congestion, defined as an elevation of ventricular filling pressure/pulmonary capillary wedge pressure independent of clinical evidence of fluid overload [17]. Elevated filling pressure is the culprit in the development of most of the signs and symptoms of ADHF and is the target for treatment.
Although use of vasoactive medications such as nitroglycerin or nitroprusside are not routinely recommended for use in all ADHF patients admitted to the hospital, retrospective analysis of the ADHEREdatabase suggests that there is a significant reduction of mortality, hospital length of stay, admission to intensive care unit, invasive procedures, and prolonged hospitalizations when IV diuretics, vasodilators (nitroglycerin, nitroprusside, nesiritide,) and/or positive inotropes (milrinone, dobutamine) are initiated in the ED within 6 hours of an ADHF presentation [18,19].However, whether prompt ED intervention impacts intermediate- to long-term outcomes is unknown [4].
Hospitalized patients with ADHF are at increased risk of venous thromboembolism mainly due to reduced cardiac output, increased systemic venous pressure, and reduced activity levels. Therefore, it is recommended that during the hospitalization ADHF patients receive prophylaxis against venous thromboembolism with low-dose unfractionated heparin or low-molecular-weight heparin if there is no contraindication [5].Individual therapeutic choices for ADHF are reviewed in detail below.
What treatments are used to relieve congestion?
Diuresis
In patients admitted to the hospital with ADHF, initial effective diuresis is vital to lowering cardiac filling pressures and relieving symptoms of congestion. Intravenous loop diuretics represent the first line of treatment and have long been the mainstay of therapy for decompensated heart failure with preserved or reduced ejection fraction, reducing fluid overload, and relieving symptoms.
Despite its long track record, the dose administration of IV diuretics is more of an art than a science. Medication dosage sufficient to produce a rate of diuresis that will optimize volume status and relieve signs and symptoms of congestion without causing kidney injury or hypotension is recommended [5].Due to the relatively short half-life of loop diuretics and concerns about tubular sodium reabsorption in the kidneys, continuous IV diuretic infusion has been suggested to enhance diuresis and avoid sodium and fluid rebound [5,20,21]. However, continuous loop diuretic infusion has not proven superior to intermittent IV bolus dosing in clinical studies. Recent data from the Diuretic Optimization Strategies Evaluation (DOSE) trial comparing bolus versus continuous infusion diuretic strategy in patients with ADHF showed no difference in global symptom relief, diuresis, or any of the clinical secondary endpoints including composite of death, re-hospitalization, or ED visits with either IV bolus versus continuous infusion or low versus high doses of furosemide [22]. Concern has also been previously raised about adverse outcomes utilizing high doses of loop diuretics in the treatment of ADHF [20,23,24]. However, the DOSE trial also evaluated the safety of 2 strategies for furosemide dosing in patients with ADHF. The study randomized ADHF patients with a prior diagnosis of chronic heart failure to 4 different treatment groups, either a high dose (2.5x their daily chronic oral furosemide dose) or low dose (1x their daily chronic oral furosemide dose), which was given either twice daily via IV bolus or via continuous infusion. The study showed no difference in change in renal function from baseline to 72 hours with either IV bolus versus continuous infusion or low versus high doses of furosemide [22].
Ultrafiltration
For patients with marked fluid overload who are unresponsive to diuretic therapy, peripheral ultrafiltration may be considered. Initial data demonstrated that early ultrafiltration effectively and safely reduced congestion in patients with ADHF with diuretic resistance and renal insufficiency. Length of stay was reduced, with 60% of discharges in 3 days or less and 1 readmission at 30 days. Neurohormonal activation, indicated by reduction in BNP level, was reduced without worsening glomerular filtration rate, hypotension or electrolyte abnormalities [28]. The UNLOAD trial confirmed these results and extended their findings to show that patients undergoing peripheral ultrafiltration had greater weight and net fluid loss at 48 hours and reduced rate of rehospitalization at 90 days when compared with IV diuretic therapy alone in ADHF patients. Interestingly, there was no difference in the dyspnea score at 48 hours and there was a trend toward worsening of renal function in the ultrafiltration group. The study was not powered to document a survival benefit [29]. However, the more recent Cardiorenal Rescue Study in ADHF (CARRESS-HF) trial involving patients with ADHF and worsening renal function showed that there was no difference in weight loss between patients randomized to ultrafiltration or a strategy of stepped pharmacologic therapy. Additionally, ultrafiltration was associated with a significant increase in creatinine at 96 hours and a higher rate of adverse events related to the procedure, driven by complications from intravenous catheter insertion. There was no difference between the 2 groups in death or rehospitalization for heart failure [30]. At present, ultrafiltration may be a reasonable option if all diuretic strategies are unsuccessful in relieving congestion [5].
Vasopressin-Receptor Antagonists
The vasopressin-receptor antagonists represent a relatively new class of medications that target the vasopressin receptors V1a and V2. Activation of the vasopressin V2 receptors by arginine vasopressin in heart failure causes inappropriate free water retention contributing to the symptoms of congestion and hyponatremia [31]. Currently, the only 2 vasopressin-receptor antagonists available for clinical use are conivaptan (V1a /V2 receptor antagonist) and tolvaptan (V2 receptor antagonist). The effectiveness of tolvaptan was tested in a randomized study (EVEREST) in patients hospitalized with ADHF [32,33]. At 1 year there was no difference seen in the primary endpoints of all-cause mortality, death from cardiovascular causes, or first hospitalization for heart failure [32,33]. However, hyponatremia, when present, was improved in the tolvaptan group. Conivaptan has a similar hemodynamic profile compared to tolvaptan, but without improving signs and symptoms in hospitalized patients with ADHF [34]. Currently, vasopressin antagonists are recommended in the management of ADHF by professional guidelines as only a class IIb indication in hospitalized patients with volume overload and severe hyponatremia [5].
Case Continued
After 24 hours of medical therapy in the CCU, the patient is no longer clammy and cool but continues to have shortness of breath, and peripheral edema is not improving. She continues to have elevated JVP and S3. Her blood pressure is now 120/79 mm Hg and her heart rate is 110. A Swan-Ganz catheter placed this morning showed a cardiac index of 1.8 L/minute/m2 (reference range, 2.5–4.0 L/min/m2); pulmonary capillary wedge pressure is 28 mm Hg (reference range, 6–12 mm Hg) and systemic vascular resistance is 1932 dyne/second/cm5 (reference range, 800–1200 dynes/sec/cm5). The physician decides to add nitroprusside to lower her filling pressure and systemic vascular resistance.
What is the role of vasoactive medications in treatment?
Vasodilators
Nitroglycerin is a venodilating medication with preload reduction properties at low doses and an arterial dilator at high doses [35]. Preload reduction improves left ventricular filling pressures and pulmonary congestion without increasing the oxygen demand in the heart in patients with ADHF. This leads to an improvement of symptoms, including dyspnea, in as early as 5 minutes [36]. For a highly symptomatic patient, nitroglycerin given sublingually can be useful in an acute situation because it is typically immediately available while preparations are made for administration of IV medications. Limitations of nitroglycerin include rapid tachyphylaxis within several hours of continuous exposure at high doses, resistance to the hemodynamic effects of nitroglycerin in up to 20% of patients, and hypotension, which may occur before significant preload reduction effect can be obtained [37]. When symptomatic hypotension becomes a problem, the highest hemodynamically tolerable dose should be given. Another agent with a potent vasodilator effect used in the treatment of heart failure is sodium nitroprusside (SNP). As opposed to nitroglycerin, this drug has an equally potent preload- and afterload-reducing effect [35]. Afterload reduction through its arteriodilator effect has the benefit of increasing cardiac output and decreasing myocardial oxygen demand with improvement of pulmonary congestion [36]. SNP is used in less than 1% of patients hospitalized with heart failure [38], probably due to the potential for causing marked hypotension, its need for invasive hemodynamic monitoring, and the rare risk for thiocyanate toxicity with high doses and/or longer infusions, especially in patients with reduced hepatic perfusion and renal function, as in the case of low-output heart failure [35]. However, data demonstrating safety and efficacy of SNP infusion in patients with ADHF are limited [39].A single-center, retrospective case-control study suggested that the administration of SNP in carefully selected patients with advanced low-output ADHF was safe and may be associated with favorable long-term clinical outcomes [39]. SNP can be attractive in severely congested patients with hypertension or severe mitral regurgitation complicating left ventricular failure, but prospective trials are needed to clarify the safety and efficacy in this patient population.
Nesiritide is a human recombinant form of BNP that has a direct effect on the vascular endothelium by increasing the bioavailability of nitric oxide through stimulation of cyclic guanosine monophosphate. Its primary mechanism of action is to reduce left ventricular filling pressures by a systemic and pulmonary vasodilator effect. It also promotes diuresis and natriuresis [40].The initial efficacy of nesiritide was demonstrated in the VMAC (Vasodilation in the Management of Acute Congestive Heart Failure) study, a randomized trial of IV nesiritide versus IV nitroglycerin or placebo in decompensated heart failure patients. A significant reduction in pulmonary capillary wedge pressure was demonstrated within 15 minutes in the nesiritide group and maintained at 3 hours compared to either nitroglycerin or placebo, with a similar improvement in dyspnea extending out to 24 hours [41].
The large ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) randomized ADHF patients to nesiritide or placebo and tested the hypothesis that nesiritide would be superior to placebo in improving acute dyspnea, all-cause mortality, and heart failure readmission in patients presenting with ADHF [42]. Nesiritide-treated patients showed only a modest early improvement in self-assessed dyspnea and no difference in the composite endpoint of death or rehospitalization at 30 days in patients admitted with ADHF. Reassuringly, there was no increase in renal failure compared to placebo; however, the incidence of symptomatic hypotension was higher with nesiritide [42]. Although nesiritide remains in the armentarium of vasoactive medications for ADHF, less expensive vasodilators such as nitroglycerin or nitroprusside may be preferred by many clinicians.
Overall, vasodilators represent a good treatment option for patients presenting with ADHF characterized by low cardiac output, high filling pressures, and elevated systemic vascular resistance. There is no clear evidence, however, to suggest that IV vasodilators improve survival in hospitalized patients with ADHF; thus, its use should be restricted to the relief of dyspnea in patients with stable blood pressure [5].
Inotropic Therapy
The most commonly used positive inotropic agents in the management of patients with ADHF in the United States are dobutamine (beta-1, beta-2, and alpha adrenoreceptor agonist) and milrinone (phosphodiesterase-III inhibitor) [38]. Inotropes increase cardiac output by increasing myocardial contractility, reduce left and right ventricular filling pressures, and improve hemodynamic parameters. Despite these hemodynamic effects, inotropic agents have not demonstrated a survival benefit in patients with ADHF. A major limitation regarding these agents is that they increase the risk of cardiac arrhythmias by increasing intracellular calcium in cardiac myocytes. In fact, retrospective analyses suggest that most inotropic agents are associated with an increased risk of death [38,43].
Milrinone inhibits type III isoform of the enzyme phosphodiasterase leading to an increase in intracellular cyclic AMP to exert its positive inotropic effect on the myocardium. Milrinone also exerts systemic and pulmonary vasodilator effects in the circulation decreasing right atrial, pulmonary capillary wedge, and mean arterial pressure. In the OPTIME-CHF trial, patients with chronic heart failure admitted to the hospital with ADHF were randomized to short term infusion of milrinone vs. placebo plus standard therapy. Milrinone resulted in more hypotension, atrial fibrillation and ventricular arrhythmias without any benefit on mortality or re-hospitalization [44].A retrospective analysis from the ADHEREregistry showed that in-hospital mortality was twofold higher with the use of dobutamine or milrinone in patients with ADHF when compared to treatment with vasodilators [38].
Dobutamine is a beta-1, beta-2, and alpha adrenoreceptor agonist that works by increasing myocardial contractility leading to an increase in cardiac output as its primary cardiovascular effect. Currently, routine use of IV positive inotropic agents in the absence of imminent cardiogenic shock or low output ADHF with systemic hypoperfusion is generally not recommended due to concerns of adverse effects [5]. The ACCF/AHA guidelines recommend the use of positive inotropic agents to relieve symptoms, improve systemic perfusion and preserve end-organ function in patients with severe left ventricular systolic failure and low output syndrome with evidence of end-organ dysfunction (such as hypotension, altered mentation, cool extremities, low urine output and serum markers indicative of renal and/or hepatic dysfunction) with or without congestion [5].
Continuous outpatient therapy with inotropes may be a viable option in patients with stage D (end stage) heart failure who are deemed unlikely to survive hospital discharge [45].This is also supported by the ACCF/AHA practice guidelines where IV inotropic support may be considered for the previous reasons only after all alternative therapies to achieve stability have failed (Class IIB indication) [5].
Is there a role for morphine?
For decades morphine has been considered an essential component in the armamentarium for the treatment of ADHF. Its preload-reducing effect, anti-anxiety properties, and breathlessness suppression has made morphine a popular medication in the treatment of ADHF. Despite its common use, there is a lack of prospective randomized trials demonstrating the safety and benefit of this drug. In a retrospective analysis from the ADHERE database, IV morphine used for ADHF was associated with higher rates of adverse events, including increase use of mechanical ventilation, prolonged hospitalization, increased intensive care unit admissions, and higher mortality, bringing into question its safety profile [46]. Until a randomized trial is completed demonstrating safety and benefit, caution is advised regarding the use of morphine in ADHF.
Case Continued
Over the next 72 hours the patient’s symptoms improved. She no longer has dyspnea at rest, she has had a proper urine-output response to therapy, her serum creatinine has returned to normal, and her vital signs have remained stable. The IV vasodilator was discontinued, dobutamine was weaned off, and the patient was transitioned to guideline-directed medical therapy with an angiotensin-converting enzyme (ACE) inhibitor while continuing IV furosemide. Hospitalized patients who are hemodynamically stable should be transitioned to guideline-directed medical therapy with an oral ACE inhibitor unless the patient has a contraindication, such as marked azotemia or hyperkalemia. Low-dose carvedilol was initiated after optimization of volume status was confirmed. In the absence of shock and after optimization of volume status, every effort should be made to initiate low-dose beta blockers prior to hospital discharge.
When is mechanical circulatory support indicated in ADHF patients?
Mechanical circulatory support has emerged as a reasonable option in selected patients with acute and reversible cardiogenic shock (ie, acute coronary syndrome or an acute mechanical problem such as a torn papillary muscle or ventricular septal defect) [5]. Recently, the utility of intraaortic balloon pump (IABP) in the setting of cardiogenic shock resulting from acute coronary syndrome was called into question with the negative results from the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial [47]. The study compared IABP with best available medical therapy alone among patients with acute myocardial infarction complicated by cardiogenic shock for who early revascularization was planned. Use of IABP did not reduce 30-day mortality compared with medical therapy in this patient population [47]. Whether IABP has a significant role in mechanical complications, such as acute ventricular septal rupture or papillary muscle rupture, is unknown due to the paucity of data in the management of patients with such complications. Therefore, when patients present with severe acute cardiogenic shock refractory to medical therapy, mechanical circulatory support with either ventricular assist devices (VAD) or extracorporeal membrane oxygenation (ECMO) is the preferred means to reverse terminal circulatory collapse. VADs are effective in the short-term as a “bridge-to-recovery” or as a “bridge-to-decision” when recovery, transplant candidacy, or neurologic status are still uncertain [48,49]. There are several options currently available for mechanical circulatory support, including surgically implanted VADs or the percutaneously implanted VADs, such as the Impella 2.5, 3.5 and 5.0 (Abiomed, Danvers, MA) and the TandemHeart pump (Cardiac Assist, Pittsburgh, PA).The ideal device and optimal duration of temporary support are yet to be defined. A detailed description of the function and clinical effects of mechanical support devices is beyond the scope of this article, although thorough reviews are available [48,49].
What elements of care may help optimize the discharge process?
Transition of care in hospitalized patients with ADHF to outpatient care is a critical and vulnerable period for patients given the complexity of the discharge planning for heart failure. A multidisciplinary heart failure disease management program is recommended in both the inpatient and outpatient setting to address the barriers to successful transition of care [5]. Physicians and physician extenders, nurses, pharmacists, and social workers can work together to identify risk factors for readmission and bridge the gap between the inpatient and outpatient setting.
Patients at high risk for hospital readmission should be referred to a heart failure disease management program [5,37]. Patients at high risk for hospital readmission include patients with renal insufficiency, low output state, diabetes mellitus, chronic lung disease, persistent NYHA functional class III, IV symptoms, frequent hospitalizations, multiple comorbidities, history of depression, cognitive impairment, or recurrent problems with noncompliance. There is strong evidence that a heart failure disease management program will reduce rehospitalization rates and costs while improving functional status and quality of life of the patient [37].In addition, a heart failure disease management clinic often can see the patient shortly after discharge, which may allow earlier discharge of the patient and shorter length of stay. Proven therapies such as ACE inhibitors, angiotensin-receptor blockers, beta blockers, and aldosterone antagonists can be titrated frequently in this setting.
It is strongly recommended that comprehensive written discharge instructions be provided at the end of hospitalization with special emphasis on diet, discharge medications, activity level, follow-up appointment, daily weight monitoring, and instructions for recurrence of symptoms [5].
Case Conclusion
The patient tolerated well the initiation of guideline-directed medical therapy and is continued on the ACE inhibitor and beta-blocker medications. After 4 days IV furosemide is discontinued and transitioned to oral furosemide. Precipitant causes of heart failure were addressed throughout hospitalization. It was determined that the patient had been taking high doses of nonsteroidal anti-inflammatory drugs due to knee pain. She was educated on this and other potential precipitant factors. Heart failure education was reinforced, including self-care, emergency plans, and need for medication and diet adherence. She is scheduled an early follow-up visit within 2 weeks of hospital discharge in the multidisciplinary heart failure disease management clinic.
Summary
ADHF is a major public health problem commonly encountered and often initially managed in the ED. Initial history and physical examination are important to estimate the degree of congestion and peripheral perfusion. The patient’s hemodynamic status along with the use prognostic models for short-term mortality may facilitate patient triage and encourage the use of evidence-based therapy, especially in high-risk patients. Initial treatment should target the relief of congestive symptoms and intravenous loop diuretics are the mainstay of therapy. The preferred IV vasoactive medication has yet to be determined in a large prospective randomized trial. Positive inotropic agents should be reserved for patients with signs of low cardiac output and tissue hypoperfusion, however, the risk/benefit equation should be evaluated judiciously with each treatment option before initiating therapy. For patients with refractory hemodynamic collapse, ventricular assist devices can allow stabilization until recovery or decision regarding transplantation versus destination therapy. Patients with ADHF are at increased risk for readmission to the hospital as well as increased risk for death. Risk factors need to be identified and referral to a heart disease management program should be considered for those patients deemed at increased risk for rehospitalization.
Corresponding author: Carlos E. Sanchez, MD, 3705 Olentanfy River Rd., Columbus, OH 43214, [email protected].
Financial disclosures: None.
Author contributions: conception and design, CES; analysis and interpretation of data, CES; drafting of article, CES; critical revision of the article, CES, DRR; collection and assembly of data, CES.
From Ohio Health, Riverside Methodist Hospital, Columbus, OH.
Abstract
- Objective: To review the current in-hospital management of patients with acute decompensated heart failure (ADHF).
- Methods: Review of the literature.
- Results: Heart failure is a leading cause of hospitalization in the elderly, and morbidity, mortality, and hospital readmission rates for ADHF remain high. The patient’s hemodynamic status along with the use of prognostic models for short-term mortality may facilitate patient triage and encourage the use of evidence-based therapy, especially in high-risk patients. Initial treatment should target the relief of congestive symptoms, and intravenous loop diuretics are the mainstay of therapy. The preferred IV vasoactive medication has yet to be determined in a large prospective randomized trial. Positive inotropic agents should be reserved for patients with signs of low cardiac output and tissue hypoperfusion; however, the risk/benefit equation should be evaluated judiciously with each treatment option before initiating therapy. For patients with refractory hemodynamic collapse, ventricular assist devices can allow stabilization until recovery or decision regarding transplantation versus destination therapy.
- Conclusion: Patients with ADHF are at increased risk for readmission to the hospital as well as at increased risk for death. Risk factors need to be identified and referral to a heart disease management program should be considered for those patients deemed at increased risk for rehospitalization.
Heart failure is a major public health problem in the United States and the leading cause of hospitalization in patients 65 years of age and older [1]. Patients hospitalized with acute decompensated heart failure (ADHF) have a readmission rate as high as 50% within 6 months and 25% within 30 days [2]. It is estimated that $32 billion is spent on heart failure care each year, the majority of which is directly related to inpatient care. Projections show that by 2030 the total cost of heart failure will increase to $70 billion per year [1]. Despite the growing burden, advances in treatment have been limited [2,3] and management continues to be a challenge. In this article, we review the current in-hospital management of patients with ADHF.
Case Study
Initial Presentation
A 64-year-old woman with a nonischemic dilated cardiomyopathy presents to the emergency department (ED) with a 4-day history of progressive dyspnea on exertion. She can not ambulate more than 50 feet without having to stop due to dyspnea and reports increased lower extremity edema. She is found to have a heart rate of 105 bpm, a respiratory rate of 30 breaths/min, and a blood pressure of 90/51 mm Hg. Physical examination is remarkable for distended neck vein, S3 gallop, end expiratory wheezing in the bases, and lower extremity edema. Blood tests, including a B-type natriuretic peptide level, are pending. Electrocardiogram and chest radiograph are ordered. The physician suspects that the patient has ADHF and admits her for further management.
What are aspects of initial management in the ED?
Most patients that present for evaluation and management of ADHF are first evaluated in the ED. Initial management includes an assessment of oxygenation, hemodynamic status, and adequacy of tissue perfusion, as well as for possibility of an acute coronary syndrome. A complete history, physical examination, chest radiography, 12-lead electrocardiogram, cardiac troponin T or I, electrolytes, and complete blood count should be obtained to allow rapid diagnosis and triage followed by prompt, aggressive treatment in the ED or observation unit. This should alleviate the patient’s symptoms sooner, and it is intuitive that this would lessen morbidity and length of hospital stay [4].
How are patients with ADHF classified?
What risk assessment tools are available?
B-type natriuretic peptide (BNP) and N-terminal fragment proBNP (NT-proBNP) were recently validated as diagnostic aids for the differentiation of etiologies of dypnea in patients in the ED with possible symptoms of ADHF. Use of these biomarkers can help reduce diagnostic uncertainty and associated mismanagement of patients presenting with nonspecific symptoms of dysp-nea [4,5,7]. Low or normal levels (BNP < 100 pg/ml or NT-proBNP < 500 pg/ml) have a high negative predictive value for excluding heart failure.
Elevated BNP or NT-proBNP levels may also yield prognostic information, identifying patients at increased risk of mortality or rehospitalization when value does not fall after aggressive heart failure management [8,9].In a recent study by Fonarow et al, the levels of BNP on hospital admission correlated directly with the risk of in-hospital mortality in patients admitted with ADHF independent of left ventricular ejection fraction. When the levels of BNP were below 430 pg/ml, the in-hospital mortality was 1.9%, and when the levels were above 1730 pg/ml, the mortality went up to 6% (P < 0.001) [8]. Additionally, elevated pre-discharge BNP levels (BNP > 350 ng/l; P < 0.001) in patients with ADHF seem to identify those at increased risk of death or readmission after in-patient management [9]. Elevated cardiac troponin T or I in hospitalized patients with ADHF also are associated with increased mortality, including in those without acute coronary syndrome or underlying coronary artery disease [10,11].
Case Continued
Upon further evaluation by a cardiologist, the patient is cool and clammy with elevated neck veins and prominent S3 confirmed. She continues to report severe shortness of breath after 1 dose of intravenous (IV) furosemide in the ED. Repeat vital signs shows a blood pressure of 83/49 mm Hg and respiratory rate of 33. Her electrocardiogram shows sinus tachycardia. The cardiologist determines that the patient’s clinical profile is “cold and wet” and admits the patient to the cardiac care unit (CCU) with a diagnosis of ADHF.
Initial blood tests show a BNP level of 1830 pg/ml, troponin I is 0.63 and stable after 2 measurements, serum creatinine is 1.6 mg/dL, BUN is 44 mg/dL, and serum sodium is 132 mg/dL. The GWTG-HF risk score for in-hospital mortality was calculated based on admission data and the probability of death was estimated at > 5% to 10% [12]. Prompt aggressive medical therapy was instituted in the CCU consisting of furosemide infusion to reduce congestion and IV dobutamine to improve systemic perfusion. Enoxoparin 40 mg subcutaneously once daily was initiated for venous thromboembolism prophylaxis.
What are important aspects of therapy for ADHF?
Several days to weeks prior to the appearance of signs and symptoms of volume overload, patients may develop hemodynamic congestion, defined as an elevation of ventricular filling pressure/pulmonary capillary wedge pressure independent of clinical evidence of fluid overload [17]. Elevated filling pressure is the culprit in the development of most of the signs and symptoms of ADHF and is the target for treatment.
Although use of vasoactive medications such as nitroglycerin or nitroprusside are not routinely recommended for use in all ADHF patients admitted to the hospital, retrospective analysis of the ADHEREdatabase suggests that there is a significant reduction of mortality, hospital length of stay, admission to intensive care unit, invasive procedures, and prolonged hospitalizations when IV diuretics, vasodilators (nitroglycerin, nitroprusside, nesiritide,) and/or positive inotropes (milrinone, dobutamine) are initiated in the ED within 6 hours of an ADHF presentation [18,19].However, whether prompt ED intervention impacts intermediate- to long-term outcomes is unknown [4].
Hospitalized patients with ADHF are at increased risk of venous thromboembolism mainly due to reduced cardiac output, increased systemic venous pressure, and reduced activity levels. Therefore, it is recommended that during the hospitalization ADHF patients receive prophylaxis against venous thromboembolism with low-dose unfractionated heparin or low-molecular-weight heparin if there is no contraindication [5].Individual therapeutic choices for ADHF are reviewed in detail below.
What treatments are used to relieve congestion?
Diuresis
In patients admitted to the hospital with ADHF, initial effective diuresis is vital to lowering cardiac filling pressures and relieving symptoms of congestion. Intravenous loop diuretics represent the first line of treatment and have long been the mainstay of therapy for decompensated heart failure with preserved or reduced ejection fraction, reducing fluid overload, and relieving symptoms.
Despite its long track record, the dose administration of IV diuretics is more of an art than a science. Medication dosage sufficient to produce a rate of diuresis that will optimize volume status and relieve signs and symptoms of congestion without causing kidney injury or hypotension is recommended [5].Due to the relatively short half-life of loop diuretics and concerns about tubular sodium reabsorption in the kidneys, continuous IV diuretic infusion has been suggested to enhance diuresis and avoid sodium and fluid rebound [5,20,21]. However, continuous loop diuretic infusion has not proven superior to intermittent IV bolus dosing in clinical studies. Recent data from the Diuretic Optimization Strategies Evaluation (DOSE) trial comparing bolus versus continuous infusion diuretic strategy in patients with ADHF showed no difference in global symptom relief, diuresis, or any of the clinical secondary endpoints including composite of death, re-hospitalization, or ED visits with either IV bolus versus continuous infusion or low versus high doses of furosemide [22]. Concern has also been previously raised about adverse outcomes utilizing high doses of loop diuretics in the treatment of ADHF [20,23,24]. However, the DOSE trial also evaluated the safety of 2 strategies for furosemide dosing in patients with ADHF. The study randomized ADHF patients with a prior diagnosis of chronic heart failure to 4 different treatment groups, either a high dose (2.5x their daily chronic oral furosemide dose) or low dose (1x their daily chronic oral furosemide dose), which was given either twice daily via IV bolus or via continuous infusion. The study showed no difference in change in renal function from baseline to 72 hours with either IV bolus versus continuous infusion or low versus high doses of furosemide [22].
Ultrafiltration
For patients with marked fluid overload who are unresponsive to diuretic therapy, peripheral ultrafiltration may be considered. Initial data demonstrated that early ultrafiltration effectively and safely reduced congestion in patients with ADHF with diuretic resistance and renal insufficiency. Length of stay was reduced, with 60% of discharges in 3 days or less and 1 readmission at 30 days. Neurohormonal activation, indicated by reduction in BNP level, was reduced without worsening glomerular filtration rate, hypotension or electrolyte abnormalities [28]. The UNLOAD trial confirmed these results and extended their findings to show that patients undergoing peripheral ultrafiltration had greater weight and net fluid loss at 48 hours and reduced rate of rehospitalization at 90 days when compared with IV diuretic therapy alone in ADHF patients. Interestingly, there was no difference in the dyspnea score at 48 hours and there was a trend toward worsening of renal function in the ultrafiltration group. The study was not powered to document a survival benefit [29]. However, the more recent Cardiorenal Rescue Study in ADHF (CARRESS-HF) trial involving patients with ADHF and worsening renal function showed that there was no difference in weight loss between patients randomized to ultrafiltration or a strategy of stepped pharmacologic therapy. Additionally, ultrafiltration was associated with a significant increase in creatinine at 96 hours and a higher rate of adverse events related to the procedure, driven by complications from intravenous catheter insertion. There was no difference between the 2 groups in death or rehospitalization for heart failure [30]. At present, ultrafiltration may be a reasonable option if all diuretic strategies are unsuccessful in relieving congestion [5].
Vasopressin-Receptor Antagonists
The vasopressin-receptor antagonists represent a relatively new class of medications that target the vasopressin receptors V1a and V2. Activation of the vasopressin V2 receptors by arginine vasopressin in heart failure causes inappropriate free water retention contributing to the symptoms of congestion and hyponatremia [31]. Currently, the only 2 vasopressin-receptor antagonists available for clinical use are conivaptan (V1a /V2 receptor antagonist) and tolvaptan (V2 receptor antagonist). The effectiveness of tolvaptan was tested in a randomized study (EVEREST) in patients hospitalized with ADHF [32,33]. At 1 year there was no difference seen in the primary endpoints of all-cause mortality, death from cardiovascular causes, or first hospitalization for heart failure [32,33]. However, hyponatremia, when present, was improved in the tolvaptan group. Conivaptan has a similar hemodynamic profile compared to tolvaptan, but without improving signs and symptoms in hospitalized patients with ADHF [34]. Currently, vasopressin antagonists are recommended in the management of ADHF by professional guidelines as only a class IIb indication in hospitalized patients with volume overload and severe hyponatremia [5].
Case Continued
After 24 hours of medical therapy in the CCU, the patient is no longer clammy and cool but continues to have shortness of breath, and peripheral edema is not improving. She continues to have elevated JVP and S3. Her blood pressure is now 120/79 mm Hg and her heart rate is 110. A Swan-Ganz catheter placed this morning showed a cardiac index of 1.8 L/minute/m2 (reference range, 2.5–4.0 L/min/m2); pulmonary capillary wedge pressure is 28 mm Hg (reference range, 6–12 mm Hg) and systemic vascular resistance is 1932 dyne/second/cm5 (reference range, 800–1200 dynes/sec/cm5). The physician decides to add nitroprusside to lower her filling pressure and systemic vascular resistance.
What is the role of vasoactive medications in treatment?
Vasodilators
Nitroglycerin is a venodilating medication with preload reduction properties at low doses and an arterial dilator at high doses [35]. Preload reduction improves left ventricular filling pressures and pulmonary congestion without increasing the oxygen demand in the heart in patients with ADHF. This leads to an improvement of symptoms, including dyspnea, in as early as 5 minutes [36]. For a highly symptomatic patient, nitroglycerin given sublingually can be useful in an acute situation because it is typically immediately available while preparations are made for administration of IV medications. Limitations of nitroglycerin include rapid tachyphylaxis within several hours of continuous exposure at high doses, resistance to the hemodynamic effects of nitroglycerin in up to 20% of patients, and hypotension, which may occur before significant preload reduction effect can be obtained [37]. When symptomatic hypotension becomes a problem, the highest hemodynamically tolerable dose should be given. Another agent with a potent vasodilator effect used in the treatment of heart failure is sodium nitroprusside (SNP). As opposed to nitroglycerin, this drug has an equally potent preload- and afterload-reducing effect [35]. Afterload reduction through its arteriodilator effect has the benefit of increasing cardiac output and decreasing myocardial oxygen demand with improvement of pulmonary congestion [36]. SNP is used in less than 1% of patients hospitalized with heart failure [38], probably due to the potential for causing marked hypotension, its need for invasive hemodynamic monitoring, and the rare risk for thiocyanate toxicity with high doses and/or longer infusions, especially in patients with reduced hepatic perfusion and renal function, as in the case of low-output heart failure [35]. However, data demonstrating safety and efficacy of SNP infusion in patients with ADHF are limited [39].A single-center, retrospective case-control study suggested that the administration of SNP in carefully selected patients with advanced low-output ADHF was safe and may be associated with favorable long-term clinical outcomes [39]. SNP can be attractive in severely congested patients with hypertension or severe mitral regurgitation complicating left ventricular failure, but prospective trials are needed to clarify the safety and efficacy in this patient population.
Nesiritide is a human recombinant form of BNP that has a direct effect on the vascular endothelium by increasing the bioavailability of nitric oxide through stimulation of cyclic guanosine monophosphate. Its primary mechanism of action is to reduce left ventricular filling pressures by a systemic and pulmonary vasodilator effect. It also promotes diuresis and natriuresis [40].The initial efficacy of nesiritide was demonstrated in the VMAC (Vasodilation in the Management of Acute Congestive Heart Failure) study, a randomized trial of IV nesiritide versus IV nitroglycerin or placebo in decompensated heart failure patients. A significant reduction in pulmonary capillary wedge pressure was demonstrated within 15 minutes in the nesiritide group and maintained at 3 hours compared to either nitroglycerin or placebo, with a similar improvement in dyspnea extending out to 24 hours [41].
The large ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) randomized ADHF patients to nesiritide or placebo and tested the hypothesis that nesiritide would be superior to placebo in improving acute dyspnea, all-cause mortality, and heart failure readmission in patients presenting with ADHF [42]. Nesiritide-treated patients showed only a modest early improvement in self-assessed dyspnea and no difference in the composite endpoint of death or rehospitalization at 30 days in patients admitted with ADHF. Reassuringly, there was no increase in renal failure compared to placebo; however, the incidence of symptomatic hypotension was higher with nesiritide [42]. Although nesiritide remains in the armentarium of vasoactive medications for ADHF, less expensive vasodilators such as nitroglycerin or nitroprusside may be preferred by many clinicians.
Overall, vasodilators represent a good treatment option for patients presenting with ADHF characterized by low cardiac output, high filling pressures, and elevated systemic vascular resistance. There is no clear evidence, however, to suggest that IV vasodilators improve survival in hospitalized patients with ADHF; thus, its use should be restricted to the relief of dyspnea in patients with stable blood pressure [5].
Inotropic Therapy
The most commonly used positive inotropic agents in the management of patients with ADHF in the United States are dobutamine (beta-1, beta-2, and alpha adrenoreceptor agonist) and milrinone (phosphodiesterase-III inhibitor) [38]. Inotropes increase cardiac output by increasing myocardial contractility, reduce left and right ventricular filling pressures, and improve hemodynamic parameters. Despite these hemodynamic effects, inotropic agents have not demonstrated a survival benefit in patients with ADHF. A major limitation regarding these agents is that they increase the risk of cardiac arrhythmias by increasing intracellular calcium in cardiac myocytes. In fact, retrospective analyses suggest that most inotropic agents are associated with an increased risk of death [38,43].
Milrinone inhibits type III isoform of the enzyme phosphodiasterase leading to an increase in intracellular cyclic AMP to exert its positive inotropic effect on the myocardium. Milrinone also exerts systemic and pulmonary vasodilator effects in the circulation decreasing right atrial, pulmonary capillary wedge, and mean arterial pressure. In the OPTIME-CHF trial, patients with chronic heart failure admitted to the hospital with ADHF were randomized to short term infusion of milrinone vs. placebo plus standard therapy. Milrinone resulted in more hypotension, atrial fibrillation and ventricular arrhythmias without any benefit on mortality or re-hospitalization [44].A retrospective analysis from the ADHEREregistry showed that in-hospital mortality was twofold higher with the use of dobutamine or milrinone in patients with ADHF when compared to treatment with vasodilators [38].
Dobutamine is a beta-1, beta-2, and alpha adrenoreceptor agonist that works by increasing myocardial contractility leading to an increase in cardiac output as its primary cardiovascular effect. Currently, routine use of IV positive inotropic agents in the absence of imminent cardiogenic shock or low output ADHF with systemic hypoperfusion is generally not recommended due to concerns of adverse effects [5]. The ACCF/AHA guidelines recommend the use of positive inotropic agents to relieve symptoms, improve systemic perfusion and preserve end-organ function in patients with severe left ventricular systolic failure and low output syndrome with evidence of end-organ dysfunction (such as hypotension, altered mentation, cool extremities, low urine output and serum markers indicative of renal and/or hepatic dysfunction) with or without congestion [5].
Continuous outpatient therapy with inotropes may be a viable option in patients with stage D (end stage) heart failure who are deemed unlikely to survive hospital discharge [45].This is also supported by the ACCF/AHA practice guidelines where IV inotropic support may be considered for the previous reasons only after all alternative therapies to achieve stability have failed (Class IIB indication) [5].
Is there a role for morphine?
For decades morphine has been considered an essential component in the armamentarium for the treatment of ADHF. Its preload-reducing effect, anti-anxiety properties, and breathlessness suppression has made morphine a popular medication in the treatment of ADHF. Despite its common use, there is a lack of prospective randomized trials demonstrating the safety and benefit of this drug. In a retrospective analysis from the ADHERE database, IV morphine used for ADHF was associated with higher rates of adverse events, including increase use of mechanical ventilation, prolonged hospitalization, increased intensive care unit admissions, and higher mortality, bringing into question its safety profile [46]. Until a randomized trial is completed demonstrating safety and benefit, caution is advised regarding the use of morphine in ADHF.
Case Continued
Over the next 72 hours the patient’s symptoms improved. She no longer has dyspnea at rest, she has had a proper urine-output response to therapy, her serum creatinine has returned to normal, and her vital signs have remained stable. The IV vasodilator was discontinued, dobutamine was weaned off, and the patient was transitioned to guideline-directed medical therapy with an angiotensin-converting enzyme (ACE) inhibitor while continuing IV furosemide. Hospitalized patients who are hemodynamically stable should be transitioned to guideline-directed medical therapy with an oral ACE inhibitor unless the patient has a contraindication, such as marked azotemia or hyperkalemia. Low-dose carvedilol was initiated after optimization of volume status was confirmed. In the absence of shock and after optimization of volume status, every effort should be made to initiate low-dose beta blockers prior to hospital discharge.
When is mechanical circulatory support indicated in ADHF patients?
Mechanical circulatory support has emerged as a reasonable option in selected patients with acute and reversible cardiogenic shock (ie, acute coronary syndrome or an acute mechanical problem such as a torn papillary muscle or ventricular septal defect) [5]. Recently, the utility of intraaortic balloon pump (IABP) in the setting of cardiogenic shock resulting from acute coronary syndrome was called into question with the negative results from the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial [47]. The study compared IABP with best available medical therapy alone among patients with acute myocardial infarction complicated by cardiogenic shock for who early revascularization was planned. Use of IABP did not reduce 30-day mortality compared with medical therapy in this patient population [47]. Whether IABP has a significant role in mechanical complications, such as acute ventricular septal rupture or papillary muscle rupture, is unknown due to the paucity of data in the management of patients with such complications. Therefore, when patients present with severe acute cardiogenic shock refractory to medical therapy, mechanical circulatory support with either ventricular assist devices (VAD) or extracorporeal membrane oxygenation (ECMO) is the preferred means to reverse terminal circulatory collapse. VADs are effective in the short-term as a “bridge-to-recovery” or as a “bridge-to-decision” when recovery, transplant candidacy, or neurologic status are still uncertain [48,49]. There are several options currently available for mechanical circulatory support, including surgically implanted VADs or the percutaneously implanted VADs, such as the Impella 2.5, 3.5 and 5.0 (Abiomed, Danvers, MA) and the TandemHeart pump (Cardiac Assist, Pittsburgh, PA).The ideal device and optimal duration of temporary support are yet to be defined. A detailed description of the function and clinical effects of mechanical support devices is beyond the scope of this article, although thorough reviews are available [48,49].
What elements of care may help optimize the discharge process?
Transition of care in hospitalized patients with ADHF to outpatient care is a critical and vulnerable period for patients given the complexity of the discharge planning for heart failure. A multidisciplinary heart failure disease management program is recommended in both the inpatient and outpatient setting to address the barriers to successful transition of care [5]. Physicians and physician extenders, nurses, pharmacists, and social workers can work together to identify risk factors for readmission and bridge the gap between the inpatient and outpatient setting.
Patients at high risk for hospital readmission should be referred to a heart failure disease management program [5,37]. Patients at high risk for hospital readmission include patients with renal insufficiency, low output state, diabetes mellitus, chronic lung disease, persistent NYHA functional class III, IV symptoms, frequent hospitalizations, multiple comorbidities, history of depression, cognitive impairment, or recurrent problems with noncompliance. There is strong evidence that a heart failure disease management program will reduce rehospitalization rates and costs while improving functional status and quality of life of the patient [37].In addition, a heart failure disease management clinic often can see the patient shortly after discharge, which may allow earlier discharge of the patient and shorter length of stay. Proven therapies such as ACE inhibitors, angiotensin-receptor blockers, beta blockers, and aldosterone antagonists can be titrated frequently in this setting.
It is strongly recommended that comprehensive written discharge instructions be provided at the end of hospitalization with special emphasis on diet, discharge medications, activity level, follow-up appointment, daily weight monitoring, and instructions for recurrence of symptoms [5].
Case Conclusion
The patient tolerated well the initiation of guideline-directed medical therapy and is continued on the ACE inhibitor and beta-blocker medications. After 4 days IV furosemide is discontinued and transitioned to oral furosemide. Precipitant causes of heart failure were addressed throughout hospitalization. It was determined that the patient had been taking high doses of nonsteroidal anti-inflammatory drugs due to knee pain. She was educated on this and other potential precipitant factors. Heart failure education was reinforced, including self-care, emergency plans, and need for medication and diet adherence. She is scheduled an early follow-up visit within 2 weeks of hospital discharge in the multidisciplinary heart failure disease management clinic.
Summary
ADHF is a major public health problem commonly encountered and often initially managed in the ED. Initial history and physical examination are important to estimate the degree of congestion and peripheral perfusion. The patient’s hemodynamic status along with the use prognostic models for short-term mortality may facilitate patient triage and encourage the use of evidence-based therapy, especially in high-risk patients. Initial treatment should target the relief of congestive symptoms and intravenous loop diuretics are the mainstay of therapy. The preferred IV vasoactive medication has yet to be determined in a large prospective randomized trial. Positive inotropic agents should be reserved for patients with signs of low cardiac output and tissue hypoperfusion, however, the risk/benefit equation should be evaluated judiciously with each treatment option before initiating therapy. For patients with refractory hemodynamic collapse, ventricular assist devices can allow stabilization until recovery or decision regarding transplantation versus destination therapy. Patients with ADHF are at increased risk for readmission to the hospital as well as increased risk for death. Risk factors need to be identified and referral to a heart disease management program should be considered for those patients deemed at increased risk for rehospitalization.
Corresponding author: Carlos E. Sanchez, MD, 3705 Olentanfy River Rd., Columbus, OH 43214, [email protected].
Financial disclosures: None.
Author contributions: conception and design, CES; analysis and interpretation of data, CES; drafting of article, CES; critical revision of the article, CES, DRR; collection and assembly of data, CES.
1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 2013;127: e6–245.
2. Fonarow GC. ADHERE Scientific Advisory Committee. The ADHF National Registry (ADHERE): opportunities to improve care of patients hospitalized with ADHF. Rev Cardiovasc Med 2003;4(suppl 7):S21-S30.
3. Philbin EF, Dec GW, Enkins PL, et al. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol 2001;87:1367–71.
4. Weintraub NL, Collins SP, Pang PS, et al.; on behalf of the American Heart Association Council on Clinical Cardiology and Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation 2010;122:1975–96.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-239.
6. Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003;41:1797–1804.
7. Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161–7.
8. Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in ADHF. J Am Coll Cardiol 2007;49:1943–50.
9. Logeart D, Thabut G, Jourdian P, et al. Pre-discharge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol 2004;43:635–41.
10. Peacock WFIV, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117–26.
11. Ilva T, Lassus J, Siirila-Waris K, et al. Clinical significance of cardiac troponins I and T in acute heart failure. Eur J Heart Fail 2008;10:772–9.
12. Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for inhospital mortality in patients with heart failure from the American Heart Association Get With The Guidelines program. Circ Cardiovasc Qual Outcomes 2010;3:25–32.
13. Fonarow GC, Adams KF, Abraham WT, et al, for the ADHERE Scientific Advisory Committee, Study Groups and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572–80.
14. Felker GM, Leimberger JD, Califf RM, et al. Risk stratification after hospitalization for decompensated heart failure. J Card Fail 2004;10:460–6.
15. Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 2003;290:2581–7.
16. Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure. J Am Coll Cardiol 2008; 52:347–56.
17. Gheorghiade M, Shin DD, Thomas TO, et al. Congestion is an important diagnostic and therapeutic target in heart failure. Rev Cardiovasc Med 2006;7(suppl l):S12-S24.
18. Peacock WF, Emerman C, Costanzo MR, et al. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail 2009;15:256–64.
19. Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE analysis. J Am Coll Cardiol 2008;52:534–40.
20. Salvador DRK, Rey NR, Ramos GC, et al. Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst Rev 2005(3):CD003178.
21. Pivac N, Rumboldt Z, Sardelic S, et al. Diuretic effects of furosemide infusion versus bolus injection in congestive heart failure. Int J Clin Pharmacol Res 1998;18:121–8.
22. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with ADHF. N Engl J Med 2011;364:797–805.
23. Cotter G, Metzkor E, Kaluski E, et al. Randomized trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary edema. Lancet 1998;351:389–93.
24. Butler J, Forman DE, Abraham WT, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J 2004;147:331–8.
25. Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in ADHF (DAD-HF) Trial. J Card Fail 2010;16:922–30.
26. Cotter G, Weissgarten J, Metzkor E, et al. Increased toxicity of high-dose furosemide versus low-dose dopamine in the treatment of refractory congestive heart failure. Clin Pharmacol Ther 1997;62:187–93.
27. Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43.
28. Costanzo MR, Saltzberg M, O’Sullivan J, et al. Early ultrafiltration in patients with decompensated heart failure and diuretic resistance. J Am Coll Cardiol 2005;46:2047–51.
29. Costanzo MR, Guglin ME, Saltzberg MT, et al; UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for ADHF. J Am Coll Cardiol 2007;49:675–83.
30. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296-304.
31. Shrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999;341:577–85.
32. Konstam MA, Gheorghiade M, Burnett Jr JC, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297:1319–31.
33. Gheorghiade M, Konstam MA, Burnett Jr JC, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007;297:1332–43.
34. Goldsmith SR, Elkayam U, Haught WH, et al. Efficacy and safety of the vasopressin V1A/V2-receptor antagonist conivaptan in ADHF: a dose-ranging pilot study. J Card Fail 2008;14:641–-7.
35. Shin DD, Brandimarte F, DeLuca L, et al. Review of current and investigational pharmacologic agents for acute heart failure syndromes. Am J Cardiol 2007;99:4A–23A.
36. Mattu A, Martinez JP, Kelly BS. Modern management of cardiogenic pulmonary edema. Emerg Med Clin North Am 2005;23:1105–25.
37. Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail 2010;16:e475-e539
38. Abraham WT, Adams KF, Fonarow GC, et al. ADHERE Scientific Advisory Committee and Investigators; ADHERE Study Group. In-hospital mortality in patients with ADHF requiring intravenous vasoactive medications: an analysis from the ADHF national registry (ADHERE). J Am Coll Cardiol 2005;46:57–64.
39. Mullens W, Abrahams Z, Francis GS, et al. Sodium nitroprusside for advanced low-output heart failure. J Am Coll Cardiol 2008;52:200–7.
40. Bhalla V, Willis S, Maisel AS. B-type natriuretic peptide: the level and the drug-partners in the diagnosis and management of congestive heart failure. Congest Heart Fail 2004;10(1 suppl 1):3–27.
41. Publication Committee for the VMAC investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA 2002;287:1531–40.
42. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with ADHF. N Engl J Med 2011;365:32–43.
43. Elkayam U, Tasissa G, Binanay C, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J 2007;153:98–104.
44. Cuffe MS, Califf RM, Adams KF Jr, et al. Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized control trial. JAMA 2002;287:1541–7.
45. Hershberger RE, Nauman D, Walker TL, et al. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail 2003;9:180–7.
46. Peacock WF, Hollander JE, Diercks DB, et al. Morphine and outcomes in ADHF: an ADHERE analysis. Emerg Med J 2008;25:205–9.
47. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96.
48. Abu-Omar Y, Tsui S. Mechanical circulatory support for AMI and cardiogenic shock. J Card Surg 2010;25:434–41.
49. Ziemba EA, John R. Mechanical circulatory support for bridge to decision: which device and when to decide. J Card Surg 2010;25:425–33.
50. Sanchez CE, Richards DR. Contemporary in-hospital management strategies for ADHF. Cardiol Rev 2011;19:122–9.
1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 2013;127: e6–245.
2. Fonarow GC. ADHERE Scientific Advisory Committee. The ADHF National Registry (ADHERE): opportunities to improve care of patients hospitalized with ADHF. Rev Cardiovasc Med 2003;4(suppl 7):S21-S30.
3. Philbin EF, Dec GW, Enkins PL, et al. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol 2001;87:1367–71.
4. Weintraub NL, Collins SP, Pang PS, et al.; on behalf of the American Heart Association Council on Clinical Cardiology and Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation 2010;122:1975–96.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-239.
6. Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003;41:1797–1804.
7. Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161–7.
8. Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in ADHF. J Am Coll Cardiol 2007;49:1943–50.
9. Logeart D, Thabut G, Jourdian P, et al. Pre-discharge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol 2004;43:635–41.
10. Peacock WFIV, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117–26.
11. Ilva T, Lassus J, Siirila-Waris K, et al. Clinical significance of cardiac troponins I and T in acute heart failure. Eur J Heart Fail 2008;10:772–9.
12. Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for inhospital mortality in patients with heart failure from the American Heart Association Get With The Guidelines program. Circ Cardiovasc Qual Outcomes 2010;3:25–32.
13. Fonarow GC, Adams KF, Abraham WT, et al, for the ADHERE Scientific Advisory Committee, Study Groups and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572–80.
14. Felker GM, Leimberger JD, Califf RM, et al. Risk stratification after hospitalization for decompensated heart failure. J Card Fail 2004;10:460–6.
15. Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 2003;290:2581–7.
16. Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure. J Am Coll Cardiol 2008; 52:347–56.
17. Gheorghiade M, Shin DD, Thomas TO, et al. Congestion is an important diagnostic and therapeutic target in heart failure. Rev Cardiovasc Med 2006;7(suppl l):S12-S24.
18. Peacock WF, Emerman C, Costanzo MR, et al. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail 2009;15:256–64.
19. Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE analysis. J Am Coll Cardiol 2008;52:534–40.
20. Salvador DRK, Rey NR, Ramos GC, et al. Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst Rev 2005(3):CD003178.
21. Pivac N, Rumboldt Z, Sardelic S, et al. Diuretic effects of furosemide infusion versus bolus injection in congestive heart failure. Int J Clin Pharmacol Res 1998;18:121–8.
22. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with ADHF. N Engl J Med 2011;364:797–805.
23. Cotter G, Metzkor E, Kaluski E, et al. Randomized trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary edema. Lancet 1998;351:389–93.
24. Butler J, Forman DE, Abraham WT, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J 2004;147:331–8.
25. Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in ADHF (DAD-HF) Trial. J Card Fail 2010;16:922–30.
26. Cotter G, Weissgarten J, Metzkor E, et al. Increased toxicity of high-dose furosemide versus low-dose dopamine in the treatment of refractory congestive heart failure. Clin Pharmacol Ther 1997;62:187–93.
27. Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43.
28. Costanzo MR, Saltzberg M, O’Sullivan J, et al. Early ultrafiltration in patients with decompensated heart failure and diuretic resistance. J Am Coll Cardiol 2005;46:2047–51.
29. Costanzo MR, Guglin ME, Saltzberg MT, et al; UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for ADHF. J Am Coll Cardiol 2007;49:675–83.
30. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296-304.
31. Shrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999;341:577–85.
32. Konstam MA, Gheorghiade M, Burnett Jr JC, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297:1319–31.
33. Gheorghiade M, Konstam MA, Burnett Jr JC, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007;297:1332–43.
34. Goldsmith SR, Elkayam U, Haught WH, et al. Efficacy and safety of the vasopressin V1A/V2-receptor antagonist conivaptan in ADHF: a dose-ranging pilot study. J Card Fail 2008;14:641–-7.
35. Shin DD, Brandimarte F, DeLuca L, et al. Review of current and investigational pharmacologic agents for acute heart failure syndromes. Am J Cardiol 2007;99:4A–23A.
36. Mattu A, Martinez JP, Kelly BS. Modern management of cardiogenic pulmonary edema. Emerg Med Clin North Am 2005;23:1105–25.
37. Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail 2010;16:e475-e539
38. Abraham WT, Adams KF, Fonarow GC, et al. ADHERE Scientific Advisory Committee and Investigators; ADHERE Study Group. In-hospital mortality in patients with ADHF requiring intravenous vasoactive medications: an analysis from the ADHF national registry (ADHERE). J Am Coll Cardiol 2005;46:57–64.
39. Mullens W, Abrahams Z, Francis GS, et al. Sodium nitroprusside for advanced low-output heart failure. J Am Coll Cardiol 2008;52:200–7.
40. Bhalla V, Willis S, Maisel AS. B-type natriuretic peptide: the level and the drug-partners in the diagnosis and management of congestive heart failure. Congest Heart Fail 2004;10(1 suppl 1):3–27.
41. Publication Committee for the VMAC investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA 2002;287:1531–40.
42. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with ADHF. N Engl J Med 2011;365:32–43.
43. Elkayam U, Tasissa G, Binanay C, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J 2007;153:98–104.
44. Cuffe MS, Califf RM, Adams KF Jr, et al. Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized control trial. JAMA 2002;287:1541–7.
45. Hershberger RE, Nauman D, Walker TL, et al. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail 2003;9:180–7.
46. Peacock WF, Hollander JE, Diercks DB, et al. Morphine and outcomes in ADHF: an ADHERE analysis. Emerg Med J 2008;25:205–9.
47. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96.
48. Abu-Omar Y, Tsui S. Mechanical circulatory support for AMI and cardiogenic shock. J Card Surg 2010;25:434–41.
49. Ziemba EA, John R. Mechanical circulatory support for bridge to decision: which device and when to decide. J Card Surg 2010;25:425–33.
50. Sanchez CE, Richards DR. Contemporary in-hospital management strategies for ADHF. Cardiol Rev 2011;19:122–9.
Depressed and sick with ‘nothing to live for’
CASE ‘I’ve had enough’
The psychiatry consultation team is asked to evaluate Mr. M, age 76, for a passive death wish and depression 2 months after he was admitted to the hospital after a traumatic fall.
Mr. M has several chronic medical conditions, including hypertension, type 2 diabetes mellitus, and coronary artery disease. Within 2 weeks of his admission, he developed Proteus mirabilis pneumonia and persistent respiratory failure requiring tracheostomy. Records indicate that Mr. M has told family and his treatment team, “I’m tired, just let me go.” He then developed antibiotic-induced Clostridium difficile colitis and acute renal failure requiring temporary renal replacement therapy (RRT).
Mr. M’s clinical status improves, allowing his transfer to a transitional unit, where he continues to state, “I have had enough. I’m done.” He asks for the tracheostomy tube to be removed and RRT discontinued. He is treated again for persistent C. difficile colitis and, within 2 weeks, develops hypotension, hypoxia, emesis, and abdominal distension, requiring transfer to the ICU for management of ileus.
He is stabilized with vasopressors and artificial nutritional support by nasogastric tube. Renal function improves, RRT is discontinued, and he is transferred to the general medical floor.
After a few days on the general medical floor, Mr. M develops a urinary tract infection and develops antibiotic-induced acute renal failure requiring re-initiation of RRT. A percutaneous endoscopic gastrostomy (PEG) tube is placed for nutrition when he shows little improvement with swallowing exercises. Two days after placing the PEG tube, he develops respiratory failure secondary to a left-sided pneumothorax and is transferred to the ICU for the third time, where he undergoes repeated bronchoscopies and requires pressure support ventilation.
One week later, Mr. M is weaned off the ventilator and transferred to the general medical floor with aggressive respiratory therapy, tube feeding, and RRT. Mr. M’s chart indicates that he expresses an ongoing desire to withdraw RRT, the tracheostomy, and feeding tube.
Which of the following would you consider when assessing Mr. M’s decision-making capacity (DMC)?
a) his ability to understand information relevant to treatment decision-making
b) his ability to appreciate the significance of his diagnoses and treatment options and consequences in the context of his own life circumstances
c) his ability to communicate a preference
d) his ability to reason through the relevant information to weigh the potential costs and benefits of treatment options
e) all of the above
HISTORY Guilt and regret
Mr. M reports a 30-year history of depression that has responded poorly to a variety of medications, outpatient psychotherapy, and electroconvulsive therapy. Before admission, he says, he was adherent to citalopram, 20 mg/d, and buspirone, 30 mg/d. Citalopram is continued throughout his hospitalization, although buspirone was discontinued for unknown reasons during admission.
Mr. M is undergoing hemodialysis during his initial encounter with the psychiatry team. He struggles to communicate clearly because of the tracheostomy but is alert, oriented to person and location, answers questions appropriately, maintains good eye contact, and does not demonstrate any psychomotor abnormalities. He describes his disposition as “tired,” and is on the verge of tears during the interview.
Mr. M denies physical discomfort and states, “I have just had enough. I do not want all of this done.” He clarifies that he is not suicidal and denies a history of suicidal or self-injurious behaviors.
Mr. M describes having low mood, anhedonia, and insomnia to varying degrees throughout his adult life. He also reports feeling guilt and regret about earlier experiences, but does not elaborate. He denies symptoms of panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder, mania, or hypomania. He reports an episode of visual hallucinations during an earlier hospitalization, likely a symptom of delirium, but denies any recent visual disturbances.
Mr. M’s thought process is linear and logical, with intact abstract reasoning and no evidence of delusions. Attention and concentration are intact for most of the interview but diminish as he becomes fatigued. Mr. M can describe past treatments in detail and recounts the events leading to this hospitalization.
The authors’ observations
Literature on assessment of DMC recently has centered on the 4-ability model, proposed by Grisso and Appelbaum.1 With this approach, impairment to any of the 4 processes of understanding, appreciation, ability to express a choice, and ability to use reasoning to weigh treatment options could interfere with capacity to make decisions. Few studies have clarified the mechanism and degree to which depression may impair these 4 elements, making capacity assessments in a depressed patient challenging.
Preliminary evidence suggests that depression severity, not the presence of depression, determines the degree to which DMC is impaired, if at all. In several studies, depressed patients did not demonstrate more impaired DMC compared with non-depressed patients based on standardized assessments.2-4 In depressed patients who lack DMC, case reports5-7 and cross-sectional studies8 indicate that appreciation—one’s ability to comprehend the personal relevance of illness and potential consequences of treatments in the context of one’s life—is most often impaired. Other studies suggest that the ability to reason through decision-specific information and weigh the risks and benefits of treatment options is commonly impaired in depressed patients.9,10
Even when a depressed patient demonstrates the 4 elements of DMC, providers might be concerned that the patient’s preferences are skewed by the negative emotions associated with depression.11-13 In such a case, the patient’s expressed wishes might not be consistent with views and priorities that were expressed during an earlier, euthymic period.
Rather than focusing on whether cognitive elements of DMC are impaired, some experts advocate for assessing how depression might lead to “unbalanced” decision-making that is impaired by a patient’s tendency to undervalue positive outcomes and overvalue negative ones.14 Some depressed patients will decide to forego additional medical interventions because they do not see the potential benefits of treatment, view events through a negative lens, and lack hope for the future; however, studies indicate this is not typically the case.15-17
In a study of >2,500 patients age >65 with chronic medical conditions, Garrett et al15 found that those who were depressed communicated a desire for more treatment compared with non-depressed patients. Another study of patients’ wishes for life-sustaining treatment among those who had mild or moderate depression found that most patients did not express a greater desire for life-sustaining medical interventions after their depressive episode remitted. An increased desire for life-sustaining medical interventions occurred only among the most severely depressed patients.16 Similarly, Lee and Ganzini17 found that treatment preferences among patients with mild or moderate depression and serious physical illness were unchanged after the mood disorder was treated.
These findings demonstrate that a clinician charged with assessing DMC must evaluate the severity of a patient’s depression and carefully consider how mood is influencing his (her) perspective and cognitive abilities. It is important to observe how the depressed patient perceives feelings of sadness or hopelessness in the context of decision-making, and how he (she) integrates these feelings when assigning relative value to potential outcomes and alternative treatment options. Because the intensity of depression could vary over time, assessment of the depressed patient’s decision-making abilities must be viewed as a dynamic process.
Clinical application
Recent studies indicate that, although the in-hospital mortality rate for critically ill patients who develop acute renal failure is high, it is variable, ranging from 28% to 90%.18 In one study, patients who required more interventions over the course of a hospital stay (eg, mechanical ventilation, vasopressors) had an in-hospital mortality rate closer to 60% after initiating RRT.19 In a similar trial,20,21 mean survival for critically ill patients with acute renal failure was 32 days from initiation of dialysis; only 27% of these patients were alive 6 months later.21
Given his complicated hospital course, the medical team estimates that Mr. M has a reasonable chance of surviving to discharge, although his longer-term prognosis is poor.
EVALUATION Conflicting preferences
Mr. M expresses reasonable understanding of the medical indications for temporary RRT, respiratory therapy, and enteral tube feedings, and the consequences of withdrawing these interventions. He understands that the primary team recommended ongoing but temporary use of life-sustaining interventions, anticipating that he would recover from his acute medical conditions. Mr. M clearly articulates that he wants to terminate RRT knowing that this would cause a buildup of urea and other toxins, to resume eating by mouth despite the risk of aspiration, and to be allowed to die “naturally.”
Mr. M declines to speak with a clergy member, explaining that he preferred direct contact with God and had reconciled himself to the “consequences” of his actions. He reports having “nothing left to live for” and “nothing left to do.” He says that he is “tired of being a burden” to his wife and son, regrets the way he treated them in the past, and believes they would be better off without him.
Although Mr. M’s abilities to understand, reason, and express a preference are intact, the psychiatry team is concerned that depression could be influencing his perspective, thereby compromising his appreciation for the personal relevance of his request to withdraw life-sustaining treatments. The psychiatrist shares this concern with Mr. M, who voices an understanding that undertreated depression could lead him to make irreversible decisions about his medical treatment that he might not make if he were not depressed; nevertheless, he continues to state that he is “ready” to die. With his permission, the team seeks additional information from Mr. M’s family.
Mr. M’s wife recalls a conversation with her husband 5 years ago in which he said that, were he to become seriously ill, “he would want everything done.” However, she also reports that Mr. M has been expressing a passive death wish “for years,” as he was struggling with chronic medical conditions that led to recurrent hospital admissions.
“He has always been a negative person,” she adds, and confirms that he has been depressed for most of their marriage.
The conflict between Mr. M’s earlier expressed preference for full care and his current wish to withdraw life-sustaining therapies and experience a “natural death” raises significant concern that depression could explain this change in perspective. When asked about this discrepancy, Mr. M admits that he “wanted everything done” in the past, when he was younger and healthier, but his preferences changed as his chronic medical problems progressed.
OUTCOME Better mood, discharge
We encourage Mr. M to continue discussing his treatment preferences with his family, while meeting with the palliative care team to address medical conditions that could be exacerbating depression and to clarify his goals of care. The medical team and Mr. M report feeling relieved when a palliative care consult is suggested, although his wife and son ask that it be delayed until Mr. M is more medically stable. The treatment team acknowledges the competing risks of proceeding too hastily with Mr. M’s request to withdraw life-sustaining treatments because of depression, and of delaying his decision, which could prolong suffering and violate his right to refuse medical treatment.
Mr. M agrees to increase citalopram to 40 mg/d to target depressive symptoms. We monitor Mr. M for treatment response and side effects, to provide ongoing support, to facilitate communication with the medical team, and to evaluate the influence of depression on treatment preferences and decision-making.
As Mr. M is stabilized over the next 3 weeks, he begins to reply, “I’m alive,” when asked about passive death wish. His renal function improves and RRT is discontinued. Mr. M reports a slight improvement in his mood and is discharged to a skilled nursing facility, with plans for closing his tracheostomy.
The authors’ observations
Capacity assessments can be challenging in depressed patients, often because of the uncertain role of features such as hopelessness, anhedonia, and passive death wish in the decision-making process. Depressed patients do not automatically lack DMC, and existing studies suggest that decisions regarding life-saving interventions typically are stable across time. The 4-ability model for capacity assessment is a useful starting point, but additional considerations are warranted in depressed patients with chronic illness (Figure). There is no evidence to date to guide these assessments in chronically depressed or dysthymic patients; therefore additional safeguards may be needed (Table).
In Mr. M’s case, the team’s decision to optimize depression treatment while continuing unwanted life-sustaining therapies led to improved mood and a positive health outcome. In some cases, patients do not respond quickly, if at all, to depression treatment. Also, what constitutes a reasonable attempt to treat depression, or an appropriate delay in decision-making related to life-sustaining therapies, is debatable.
When positive outcomes are not achieved or ethical dilemmas arise, health care providers could experience high moral distress.21 In Mr. M’s case, the consultation team felt moral distress because of the delayed involvement of palliative care, especially because this decision was driven by the family rather than the patient.
Related Resources
• Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
• American Academy of Hospice and Palliative Medicine. www. aahpm.org.
Drug Brand Names
Buspirone • Buspar Citalopram • Celexa
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen BJ, McGarvey El, Pinkerton RC, et al. Willingness and competence of depressed and schizophrenic inpatients to consent to research. J Am Acad Psychiatry Law. 2004;32(2):134-143.
3. Lapid MI, Rummans TA, Poole KL, et al. Decisional capacity of severely depressed patients requiring electroconvulsive therapy. J ECT. 2003;19(2):67-72.
4. Appelbaum PS, Grisso T, Frank E, et al. Competence of depressed patients for consent to research. Am J Psychiatry. 1999;156(9):1380-1384.
5. Leeman CP. Depression and the right to die. Gen Hosp Psychiatry. 1999;21(2):112-115.
6. Young EW, Corby JC, Johnson R. Does depression invalidate competence? Consultants’ ethical, psychiatric, and legal considerations. Camb Q Healthc Ethics. 1993;2(4):505-515.
7. Halpern J. When concretized emotion-belief complexes derail decision-making capacity. Bioethics. 2012;26(2):108-116.
8. Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19(2):149-174.
9. Bean G, Nishisato S, Rector NA, et al. The assessment of competence to make a treatment decision: an empirical approach. Can J Psychiatry. 1996;41(2):85-92.
10. Vollmann J, Bauer A, Danker-Hopfe H, et al. Competence of mentally ill patients: a comparative empirical study. Psychol Med. 2003;33(8):1463-1471.
11. Sullivan MD, Youngner SJ. Depression, competence, and the right to refuse lifesaving medical-treatment. Am J Psychiatry. 1994;151(7):971-978.
12. Meynen G. Depression, possibilities, and competence: a phenomenological perspective. Theor Med Bioeth. 2011;32(3):181-193.
13. Elliott C. Caring about risks. Are severely depressed patients competent to consent to research? Arch Gen Psychiatry. 1997;54(2):113-116.
14. Bursztajn HJ, Harding HP Jr, Gutheil TG, et al. Beyond cognition: the role of disordered affective states in impairing competence to consent to treatment. Bull Am Acad Psychiatry Law. 1991;19(4):383-388.
15. Garrett JM, Harris RP, Norburn JK, et al. Life-sustaining treatments during terminal illness: who wants what? J Gen Intern Med. 1993;8(7):361-368.
16. Ganzini L, Lee MA, Heintz RT, et al. The effect of depression treatment on elderly patients’ p for life-sustaining medical therapy. Am J Psychiatry. 1994;151(11):1631-1636.
17. Lee M, Ganzini L. The effect of recovery from depression on p for life-sustaining therapy in older patients. J Gerontol. 1994;49(1):M15-M21.
18. Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2003;30(9):2051-2058.
19. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
20. The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and p for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
21. Kälvemark S, Höglund AT, Hansson MG, et al. Living the conflicts-ethical dilemmas and moral distress in the health care system. Soc Sci Med. 2004;58(6):1075-1084.
CASE ‘I’ve had enough’
The psychiatry consultation team is asked to evaluate Mr. M, age 76, for a passive death wish and depression 2 months after he was admitted to the hospital after a traumatic fall.
Mr. M has several chronic medical conditions, including hypertension, type 2 diabetes mellitus, and coronary artery disease. Within 2 weeks of his admission, he developed Proteus mirabilis pneumonia and persistent respiratory failure requiring tracheostomy. Records indicate that Mr. M has told family and his treatment team, “I’m tired, just let me go.” He then developed antibiotic-induced Clostridium difficile colitis and acute renal failure requiring temporary renal replacement therapy (RRT).
Mr. M’s clinical status improves, allowing his transfer to a transitional unit, where he continues to state, “I have had enough. I’m done.” He asks for the tracheostomy tube to be removed and RRT discontinued. He is treated again for persistent C. difficile colitis and, within 2 weeks, develops hypotension, hypoxia, emesis, and abdominal distension, requiring transfer to the ICU for management of ileus.
He is stabilized with vasopressors and artificial nutritional support by nasogastric tube. Renal function improves, RRT is discontinued, and he is transferred to the general medical floor.
After a few days on the general medical floor, Mr. M develops a urinary tract infection and develops antibiotic-induced acute renal failure requiring re-initiation of RRT. A percutaneous endoscopic gastrostomy (PEG) tube is placed for nutrition when he shows little improvement with swallowing exercises. Two days after placing the PEG tube, he develops respiratory failure secondary to a left-sided pneumothorax and is transferred to the ICU for the third time, where he undergoes repeated bronchoscopies and requires pressure support ventilation.
One week later, Mr. M is weaned off the ventilator and transferred to the general medical floor with aggressive respiratory therapy, tube feeding, and RRT. Mr. M’s chart indicates that he expresses an ongoing desire to withdraw RRT, the tracheostomy, and feeding tube.
Which of the following would you consider when assessing Mr. M’s decision-making capacity (DMC)?
a) his ability to understand information relevant to treatment decision-making
b) his ability to appreciate the significance of his diagnoses and treatment options and consequences in the context of his own life circumstances
c) his ability to communicate a preference
d) his ability to reason through the relevant information to weigh the potential costs and benefits of treatment options
e) all of the above
HISTORY Guilt and regret
Mr. M reports a 30-year history of depression that has responded poorly to a variety of medications, outpatient psychotherapy, and electroconvulsive therapy. Before admission, he says, he was adherent to citalopram, 20 mg/d, and buspirone, 30 mg/d. Citalopram is continued throughout his hospitalization, although buspirone was discontinued for unknown reasons during admission.
Mr. M is undergoing hemodialysis during his initial encounter with the psychiatry team. He struggles to communicate clearly because of the tracheostomy but is alert, oriented to person and location, answers questions appropriately, maintains good eye contact, and does not demonstrate any psychomotor abnormalities. He describes his disposition as “tired,” and is on the verge of tears during the interview.
Mr. M denies physical discomfort and states, “I have just had enough. I do not want all of this done.” He clarifies that he is not suicidal and denies a history of suicidal or self-injurious behaviors.
Mr. M describes having low mood, anhedonia, and insomnia to varying degrees throughout his adult life. He also reports feeling guilt and regret about earlier experiences, but does not elaborate. He denies symptoms of panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder, mania, or hypomania. He reports an episode of visual hallucinations during an earlier hospitalization, likely a symptom of delirium, but denies any recent visual disturbances.
Mr. M’s thought process is linear and logical, with intact abstract reasoning and no evidence of delusions. Attention and concentration are intact for most of the interview but diminish as he becomes fatigued. Mr. M can describe past treatments in detail and recounts the events leading to this hospitalization.
The authors’ observations
Literature on assessment of DMC recently has centered on the 4-ability model, proposed by Grisso and Appelbaum.1 With this approach, impairment to any of the 4 processes of understanding, appreciation, ability to express a choice, and ability to use reasoning to weigh treatment options could interfere with capacity to make decisions. Few studies have clarified the mechanism and degree to which depression may impair these 4 elements, making capacity assessments in a depressed patient challenging.
Preliminary evidence suggests that depression severity, not the presence of depression, determines the degree to which DMC is impaired, if at all. In several studies, depressed patients did not demonstrate more impaired DMC compared with non-depressed patients based on standardized assessments.2-4 In depressed patients who lack DMC, case reports5-7 and cross-sectional studies8 indicate that appreciation—one’s ability to comprehend the personal relevance of illness and potential consequences of treatments in the context of one’s life—is most often impaired. Other studies suggest that the ability to reason through decision-specific information and weigh the risks and benefits of treatment options is commonly impaired in depressed patients.9,10
Even when a depressed patient demonstrates the 4 elements of DMC, providers might be concerned that the patient’s preferences are skewed by the negative emotions associated with depression.11-13 In such a case, the patient’s expressed wishes might not be consistent with views and priorities that were expressed during an earlier, euthymic period.
Rather than focusing on whether cognitive elements of DMC are impaired, some experts advocate for assessing how depression might lead to “unbalanced” decision-making that is impaired by a patient’s tendency to undervalue positive outcomes and overvalue negative ones.14 Some depressed patients will decide to forego additional medical interventions because they do not see the potential benefits of treatment, view events through a negative lens, and lack hope for the future; however, studies indicate this is not typically the case.15-17
In a study of >2,500 patients age >65 with chronic medical conditions, Garrett et al15 found that those who were depressed communicated a desire for more treatment compared with non-depressed patients. Another study of patients’ wishes for life-sustaining treatment among those who had mild or moderate depression found that most patients did not express a greater desire for life-sustaining medical interventions after their depressive episode remitted. An increased desire for life-sustaining medical interventions occurred only among the most severely depressed patients.16 Similarly, Lee and Ganzini17 found that treatment preferences among patients with mild or moderate depression and serious physical illness were unchanged after the mood disorder was treated.
These findings demonstrate that a clinician charged with assessing DMC must evaluate the severity of a patient’s depression and carefully consider how mood is influencing his (her) perspective and cognitive abilities. It is important to observe how the depressed patient perceives feelings of sadness or hopelessness in the context of decision-making, and how he (she) integrates these feelings when assigning relative value to potential outcomes and alternative treatment options. Because the intensity of depression could vary over time, assessment of the depressed patient’s decision-making abilities must be viewed as a dynamic process.
Clinical application
Recent studies indicate that, although the in-hospital mortality rate for critically ill patients who develop acute renal failure is high, it is variable, ranging from 28% to 90%.18 In one study, patients who required more interventions over the course of a hospital stay (eg, mechanical ventilation, vasopressors) had an in-hospital mortality rate closer to 60% after initiating RRT.19 In a similar trial,20,21 mean survival for critically ill patients with acute renal failure was 32 days from initiation of dialysis; only 27% of these patients were alive 6 months later.21
Given his complicated hospital course, the medical team estimates that Mr. M has a reasonable chance of surviving to discharge, although his longer-term prognosis is poor.
EVALUATION Conflicting preferences
Mr. M expresses reasonable understanding of the medical indications for temporary RRT, respiratory therapy, and enteral tube feedings, and the consequences of withdrawing these interventions. He understands that the primary team recommended ongoing but temporary use of life-sustaining interventions, anticipating that he would recover from his acute medical conditions. Mr. M clearly articulates that he wants to terminate RRT knowing that this would cause a buildup of urea and other toxins, to resume eating by mouth despite the risk of aspiration, and to be allowed to die “naturally.”
Mr. M declines to speak with a clergy member, explaining that he preferred direct contact with God and had reconciled himself to the “consequences” of his actions. He reports having “nothing left to live for” and “nothing left to do.” He says that he is “tired of being a burden” to his wife and son, regrets the way he treated them in the past, and believes they would be better off without him.
Although Mr. M’s abilities to understand, reason, and express a preference are intact, the psychiatry team is concerned that depression could be influencing his perspective, thereby compromising his appreciation for the personal relevance of his request to withdraw life-sustaining treatments. The psychiatrist shares this concern with Mr. M, who voices an understanding that undertreated depression could lead him to make irreversible decisions about his medical treatment that he might not make if he were not depressed; nevertheless, he continues to state that he is “ready” to die. With his permission, the team seeks additional information from Mr. M’s family.
Mr. M’s wife recalls a conversation with her husband 5 years ago in which he said that, were he to become seriously ill, “he would want everything done.” However, she also reports that Mr. M has been expressing a passive death wish “for years,” as he was struggling with chronic medical conditions that led to recurrent hospital admissions.
“He has always been a negative person,” she adds, and confirms that he has been depressed for most of their marriage.
The conflict between Mr. M’s earlier expressed preference for full care and his current wish to withdraw life-sustaining therapies and experience a “natural death” raises significant concern that depression could explain this change in perspective. When asked about this discrepancy, Mr. M admits that he “wanted everything done” in the past, when he was younger and healthier, but his preferences changed as his chronic medical problems progressed.
OUTCOME Better mood, discharge
We encourage Mr. M to continue discussing his treatment preferences with his family, while meeting with the palliative care team to address medical conditions that could be exacerbating depression and to clarify his goals of care. The medical team and Mr. M report feeling relieved when a palliative care consult is suggested, although his wife and son ask that it be delayed until Mr. M is more medically stable. The treatment team acknowledges the competing risks of proceeding too hastily with Mr. M’s request to withdraw life-sustaining treatments because of depression, and of delaying his decision, which could prolong suffering and violate his right to refuse medical treatment.
Mr. M agrees to increase citalopram to 40 mg/d to target depressive symptoms. We monitor Mr. M for treatment response and side effects, to provide ongoing support, to facilitate communication with the medical team, and to evaluate the influence of depression on treatment preferences and decision-making.
As Mr. M is stabilized over the next 3 weeks, he begins to reply, “I’m alive,” when asked about passive death wish. His renal function improves and RRT is discontinued. Mr. M reports a slight improvement in his mood and is discharged to a skilled nursing facility, with plans for closing his tracheostomy.
The authors’ observations
Capacity assessments can be challenging in depressed patients, often because of the uncertain role of features such as hopelessness, anhedonia, and passive death wish in the decision-making process. Depressed patients do not automatically lack DMC, and existing studies suggest that decisions regarding life-saving interventions typically are stable across time. The 4-ability model for capacity assessment is a useful starting point, but additional considerations are warranted in depressed patients with chronic illness (Figure). There is no evidence to date to guide these assessments in chronically depressed or dysthymic patients; therefore additional safeguards may be needed (Table).
In Mr. M’s case, the team’s decision to optimize depression treatment while continuing unwanted life-sustaining therapies led to improved mood and a positive health outcome. In some cases, patients do not respond quickly, if at all, to depression treatment. Also, what constitutes a reasonable attempt to treat depression, or an appropriate delay in decision-making related to life-sustaining therapies, is debatable.
When positive outcomes are not achieved or ethical dilemmas arise, health care providers could experience high moral distress.21 In Mr. M’s case, the consultation team felt moral distress because of the delayed involvement of palliative care, especially because this decision was driven by the family rather than the patient.
Related Resources
• Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
• American Academy of Hospice and Palliative Medicine. www. aahpm.org.
Drug Brand Names
Buspirone • Buspar Citalopram • Celexa
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE ‘I’ve had enough’
The psychiatry consultation team is asked to evaluate Mr. M, age 76, for a passive death wish and depression 2 months after he was admitted to the hospital after a traumatic fall.
Mr. M has several chronic medical conditions, including hypertension, type 2 diabetes mellitus, and coronary artery disease. Within 2 weeks of his admission, he developed Proteus mirabilis pneumonia and persistent respiratory failure requiring tracheostomy. Records indicate that Mr. M has told family and his treatment team, “I’m tired, just let me go.” He then developed antibiotic-induced Clostridium difficile colitis and acute renal failure requiring temporary renal replacement therapy (RRT).
Mr. M’s clinical status improves, allowing his transfer to a transitional unit, where he continues to state, “I have had enough. I’m done.” He asks for the tracheostomy tube to be removed and RRT discontinued. He is treated again for persistent C. difficile colitis and, within 2 weeks, develops hypotension, hypoxia, emesis, and abdominal distension, requiring transfer to the ICU for management of ileus.
He is stabilized with vasopressors and artificial nutritional support by nasogastric tube. Renal function improves, RRT is discontinued, and he is transferred to the general medical floor.
After a few days on the general medical floor, Mr. M develops a urinary tract infection and develops antibiotic-induced acute renal failure requiring re-initiation of RRT. A percutaneous endoscopic gastrostomy (PEG) tube is placed for nutrition when he shows little improvement with swallowing exercises. Two days after placing the PEG tube, he develops respiratory failure secondary to a left-sided pneumothorax and is transferred to the ICU for the third time, where he undergoes repeated bronchoscopies and requires pressure support ventilation.
One week later, Mr. M is weaned off the ventilator and transferred to the general medical floor with aggressive respiratory therapy, tube feeding, and RRT. Mr. M’s chart indicates that he expresses an ongoing desire to withdraw RRT, the tracheostomy, and feeding tube.
Which of the following would you consider when assessing Mr. M’s decision-making capacity (DMC)?
a) his ability to understand information relevant to treatment decision-making
b) his ability to appreciate the significance of his diagnoses and treatment options and consequences in the context of his own life circumstances
c) his ability to communicate a preference
d) his ability to reason through the relevant information to weigh the potential costs and benefits of treatment options
e) all of the above
HISTORY Guilt and regret
Mr. M reports a 30-year history of depression that has responded poorly to a variety of medications, outpatient psychotherapy, and electroconvulsive therapy. Before admission, he says, he was adherent to citalopram, 20 mg/d, and buspirone, 30 mg/d. Citalopram is continued throughout his hospitalization, although buspirone was discontinued for unknown reasons during admission.
Mr. M is undergoing hemodialysis during his initial encounter with the psychiatry team. He struggles to communicate clearly because of the tracheostomy but is alert, oriented to person and location, answers questions appropriately, maintains good eye contact, and does not demonstrate any psychomotor abnormalities. He describes his disposition as “tired,” and is on the verge of tears during the interview.
Mr. M denies physical discomfort and states, “I have just had enough. I do not want all of this done.” He clarifies that he is not suicidal and denies a history of suicidal or self-injurious behaviors.
Mr. M describes having low mood, anhedonia, and insomnia to varying degrees throughout his adult life. He also reports feeling guilt and regret about earlier experiences, but does not elaborate. He denies symptoms of panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder, mania, or hypomania. He reports an episode of visual hallucinations during an earlier hospitalization, likely a symptom of delirium, but denies any recent visual disturbances.
Mr. M’s thought process is linear and logical, with intact abstract reasoning and no evidence of delusions. Attention and concentration are intact for most of the interview but diminish as he becomes fatigued. Mr. M can describe past treatments in detail and recounts the events leading to this hospitalization.
The authors’ observations
Literature on assessment of DMC recently has centered on the 4-ability model, proposed by Grisso and Appelbaum.1 With this approach, impairment to any of the 4 processes of understanding, appreciation, ability to express a choice, and ability to use reasoning to weigh treatment options could interfere with capacity to make decisions. Few studies have clarified the mechanism and degree to which depression may impair these 4 elements, making capacity assessments in a depressed patient challenging.
Preliminary evidence suggests that depression severity, not the presence of depression, determines the degree to which DMC is impaired, if at all. In several studies, depressed patients did not demonstrate more impaired DMC compared with non-depressed patients based on standardized assessments.2-4 In depressed patients who lack DMC, case reports5-7 and cross-sectional studies8 indicate that appreciation—one’s ability to comprehend the personal relevance of illness and potential consequences of treatments in the context of one’s life—is most often impaired. Other studies suggest that the ability to reason through decision-specific information and weigh the risks and benefits of treatment options is commonly impaired in depressed patients.9,10
Even when a depressed patient demonstrates the 4 elements of DMC, providers might be concerned that the patient’s preferences are skewed by the negative emotions associated with depression.11-13 In such a case, the patient’s expressed wishes might not be consistent with views and priorities that were expressed during an earlier, euthymic period.
Rather than focusing on whether cognitive elements of DMC are impaired, some experts advocate for assessing how depression might lead to “unbalanced” decision-making that is impaired by a patient’s tendency to undervalue positive outcomes and overvalue negative ones.14 Some depressed patients will decide to forego additional medical interventions because they do not see the potential benefits of treatment, view events through a negative lens, and lack hope for the future; however, studies indicate this is not typically the case.15-17
In a study of >2,500 patients age >65 with chronic medical conditions, Garrett et al15 found that those who were depressed communicated a desire for more treatment compared with non-depressed patients. Another study of patients’ wishes for life-sustaining treatment among those who had mild or moderate depression found that most patients did not express a greater desire for life-sustaining medical interventions after their depressive episode remitted. An increased desire for life-sustaining medical interventions occurred only among the most severely depressed patients.16 Similarly, Lee and Ganzini17 found that treatment preferences among patients with mild or moderate depression and serious physical illness were unchanged after the mood disorder was treated.
These findings demonstrate that a clinician charged with assessing DMC must evaluate the severity of a patient’s depression and carefully consider how mood is influencing his (her) perspective and cognitive abilities. It is important to observe how the depressed patient perceives feelings of sadness or hopelessness in the context of decision-making, and how he (she) integrates these feelings when assigning relative value to potential outcomes and alternative treatment options. Because the intensity of depression could vary over time, assessment of the depressed patient’s decision-making abilities must be viewed as a dynamic process.
Clinical application
Recent studies indicate that, although the in-hospital mortality rate for critically ill patients who develop acute renal failure is high, it is variable, ranging from 28% to 90%.18 In one study, patients who required more interventions over the course of a hospital stay (eg, mechanical ventilation, vasopressors) had an in-hospital mortality rate closer to 60% after initiating RRT.19 In a similar trial,20,21 mean survival for critically ill patients with acute renal failure was 32 days from initiation of dialysis; only 27% of these patients were alive 6 months later.21
Given his complicated hospital course, the medical team estimates that Mr. M has a reasonable chance of surviving to discharge, although his longer-term prognosis is poor.
EVALUATION Conflicting preferences
Mr. M expresses reasonable understanding of the medical indications for temporary RRT, respiratory therapy, and enteral tube feedings, and the consequences of withdrawing these interventions. He understands that the primary team recommended ongoing but temporary use of life-sustaining interventions, anticipating that he would recover from his acute medical conditions. Mr. M clearly articulates that he wants to terminate RRT knowing that this would cause a buildup of urea and other toxins, to resume eating by mouth despite the risk of aspiration, and to be allowed to die “naturally.”
Mr. M declines to speak with a clergy member, explaining that he preferred direct contact with God and had reconciled himself to the “consequences” of his actions. He reports having “nothing left to live for” and “nothing left to do.” He says that he is “tired of being a burden” to his wife and son, regrets the way he treated them in the past, and believes they would be better off without him.
Although Mr. M’s abilities to understand, reason, and express a preference are intact, the psychiatry team is concerned that depression could be influencing his perspective, thereby compromising his appreciation for the personal relevance of his request to withdraw life-sustaining treatments. The psychiatrist shares this concern with Mr. M, who voices an understanding that undertreated depression could lead him to make irreversible decisions about his medical treatment that he might not make if he were not depressed; nevertheless, he continues to state that he is “ready” to die. With his permission, the team seeks additional information from Mr. M’s family.
Mr. M’s wife recalls a conversation with her husband 5 years ago in which he said that, were he to become seriously ill, “he would want everything done.” However, she also reports that Mr. M has been expressing a passive death wish “for years,” as he was struggling with chronic medical conditions that led to recurrent hospital admissions.
“He has always been a negative person,” she adds, and confirms that he has been depressed for most of their marriage.
The conflict between Mr. M’s earlier expressed preference for full care and his current wish to withdraw life-sustaining therapies and experience a “natural death” raises significant concern that depression could explain this change in perspective. When asked about this discrepancy, Mr. M admits that he “wanted everything done” in the past, when he was younger and healthier, but his preferences changed as his chronic medical problems progressed.
OUTCOME Better mood, discharge
We encourage Mr. M to continue discussing his treatment preferences with his family, while meeting with the palliative care team to address medical conditions that could be exacerbating depression and to clarify his goals of care. The medical team and Mr. M report feeling relieved when a palliative care consult is suggested, although his wife and son ask that it be delayed until Mr. M is more medically stable. The treatment team acknowledges the competing risks of proceeding too hastily with Mr. M’s request to withdraw life-sustaining treatments because of depression, and of delaying his decision, which could prolong suffering and violate his right to refuse medical treatment.
Mr. M agrees to increase citalopram to 40 mg/d to target depressive symptoms. We monitor Mr. M for treatment response and side effects, to provide ongoing support, to facilitate communication with the medical team, and to evaluate the influence of depression on treatment preferences and decision-making.
As Mr. M is stabilized over the next 3 weeks, he begins to reply, “I’m alive,” when asked about passive death wish. His renal function improves and RRT is discontinued. Mr. M reports a slight improvement in his mood and is discharged to a skilled nursing facility, with plans for closing his tracheostomy.
The authors’ observations
Capacity assessments can be challenging in depressed patients, often because of the uncertain role of features such as hopelessness, anhedonia, and passive death wish in the decision-making process. Depressed patients do not automatically lack DMC, and existing studies suggest that decisions regarding life-saving interventions typically are stable across time. The 4-ability model for capacity assessment is a useful starting point, but additional considerations are warranted in depressed patients with chronic illness (Figure). There is no evidence to date to guide these assessments in chronically depressed or dysthymic patients; therefore additional safeguards may be needed (Table).
In Mr. M’s case, the team’s decision to optimize depression treatment while continuing unwanted life-sustaining therapies led to improved mood and a positive health outcome. In some cases, patients do not respond quickly, if at all, to depression treatment. Also, what constitutes a reasonable attempt to treat depression, or an appropriate delay in decision-making related to life-sustaining therapies, is debatable.
When positive outcomes are not achieved or ethical dilemmas arise, health care providers could experience high moral distress.21 In Mr. M’s case, the consultation team felt moral distress because of the delayed involvement of palliative care, especially because this decision was driven by the family rather than the patient.
Related Resources
• Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
• American Academy of Hospice and Palliative Medicine. www. aahpm.org.
Drug Brand Names
Buspirone • Buspar Citalopram • Celexa
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen BJ, McGarvey El, Pinkerton RC, et al. Willingness and competence of depressed and schizophrenic inpatients to consent to research. J Am Acad Psychiatry Law. 2004;32(2):134-143.
3. Lapid MI, Rummans TA, Poole KL, et al. Decisional capacity of severely depressed patients requiring electroconvulsive therapy. J ECT. 2003;19(2):67-72.
4. Appelbaum PS, Grisso T, Frank E, et al. Competence of depressed patients for consent to research. Am J Psychiatry. 1999;156(9):1380-1384.
5. Leeman CP. Depression and the right to die. Gen Hosp Psychiatry. 1999;21(2):112-115.
6. Young EW, Corby JC, Johnson R. Does depression invalidate competence? Consultants’ ethical, psychiatric, and legal considerations. Camb Q Healthc Ethics. 1993;2(4):505-515.
7. Halpern J. When concretized emotion-belief complexes derail decision-making capacity. Bioethics. 2012;26(2):108-116.
8. Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19(2):149-174.
9. Bean G, Nishisato S, Rector NA, et al. The assessment of competence to make a treatment decision: an empirical approach. Can J Psychiatry. 1996;41(2):85-92.
10. Vollmann J, Bauer A, Danker-Hopfe H, et al. Competence of mentally ill patients: a comparative empirical study. Psychol Med. 2003;33(8):1463-1471.
11. Sullivan MD, Youngner SJ. Depression, competence, and the right to refuse lifesaving medical-treatment. Am J Psychiatry. 1994;151(7):971-978.
12. Meynen G. Depression, possibilities, and competence: a phenomenological perspective. Theor Med Bioeth. 2011;32(3):181-193.
13. Elliott C. Caring about risks. Are severely depressed patients competent to consent to research? Arch Gen Psychiatry. 1997;54(2):113-116.
14. Bursztajn HJ, Harding HP Jr, Gutheil TG, et al. Beyond cognition: the role of disordered affective states in impairing competence to consent to treatment. Bull Am Acad Psychiatry Law. 1991;19(4):383-388.
15. Garrett JM, Harris RP, Norburn JK, et al. Life-sustaining treatments during terminal illness: who wants what? J Gen Intern Med. 1993;8(7):361-368.
16. Ganzini L, Lee MA, Heintz RT, et al. The effect of depression treatment on elderly patients’ p for life-sustaining medical therapy. Am J Psychiatry. 1994;151(11):1631-1636.
17. Lee M, Ganzini L. The effect of recovery from depression on p for life-sustaining therapy in older patients. J Gerontol. 1994;49(1):M15-M21.
18. Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2003;30(9):2051-2058.
19. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
20. The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and p for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
21. Kälvemark S, Höglund AT, Hansson MG, et al. Living the conflicts-ethical dilemmas and moral distress in the health care system. Soc Sci Med. 2004;58(6):1075-1084.
1. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen BJ, McGarvey El, Pinkerton RC, et al. Willingness and competence of depressed and schizophrenic inpatients to consent to research. J Am Acad Psychiatry Law. 2004;32(2):134-143.
3. Lapid MI, Rummans TA, Poole KL, et al. Decisional capacity of severely depressed patients requiring electroconvulsive therapy. J ECT. 2003;19(2):67-72.
4. Appelbaum PS, Grisso T, Frank E, et al. Competence of depressed patients for consent to research. Am J Psychiatry. 1999;156(9):1380-1384.
5. Leeman CP. Depression and the right to die. Gen Hosp Psychiatry. 1999;21(2):112-115.
6. Young EW, Corby JC, Johnson R. Does depression invalidate competence? Consultants’ ethical, psychiatric, and legal considerations. Camb Q Healthc Ethics. 1993;2(4):505-515.
7. Halpern J. When concretized emotion-belief complexes derail decision-making capacity. Bioethics. 2012;26(2):108-116.
8. Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19(2):149-174.
9. Bean G, Nishisato S, Rector NA, et al. The assessment of competence to make a treatment decision: an empirical approach. Can J Psychiatry. 1996;41(2):85-92.
10. Vollmann J, Bauer A, Danker-Hopfe H, et al. Competence of mentally ill patients: a comparative empirical study. Psychol Med. 2003;33(8):1463-1471.
11. Sullivan MD, Youngner SJ. Depression, competence, and the right to refuse lifesaving medical-treatment. Am J Psychiatry. 1994;151(7):971-978.
12. Meynen G. Depression, possibilities, and competence: a phenomenological perspective. Theor Med Bioeth. 2011;32(3):181-193.
13. Elliott C. Caring about risks. Are severely depressed patients competent to consent to research? Arch Gen Psychiatry. 1997;54(2):113-116.
14. Bursztajn HJ, Harding HP Jr, Gutheil TG, et al. Beyond cognition: the role of disordered affective states in impairing competence to consent to treatment. Bull Am Acad Psychiatry Law. 1991;19(4):383-388.
15. Garrett JM, Harris RP, Norburn JK, et al. Life-sustaining treatments during terminal illness: who wants what? J Gen Intern Med. 1993;8(7):361-368.
16. Ganzini L, Lee MA, Heintz RT, et al. The effect of depression treatment on elderly patients’ p for life-sustaining medical therapy. Am J Psychiatry. 1994;151(11):1631-1636.
17. Lee M, Ganzini L. The effect of recovery from depression on p for life-sustaining therapy in older patients. J Gerontol. 1994;49(1):M15-M21.
18. Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2003;30(9):2051-2058.
19. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
20. The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and p for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
21. Kälvemark S, Höglund AT, Hansson MG, et al. Living the conflicts-ethical dilemmas and moral distress in the health care system. Soc Sci Med. 2004;58(6):1075-1084.
What to tell your bipolar disorder patient who wants to breast-feed
Ms. K, age 35, soon will deliver her second child. She has a 12-year history of bipolar disorder, which was well controlled with lithium, 1,200 mg/d. During her first pregnancy 3 years ago, Ms. K stopped taking lithium because she was concerned about the risk of Ebstein’s anomaly. She experienced a bipolar relapse after her healthy baby was born, and developed postpartum psychosis that was treated by restarting lithium, 1,200 mg/d, and adding olanzapine, 10 mg/d.
Ms. K has continued these medications throughout her current pregnancy. She wants to breast-feed her infant and is concerned about the effects that psychotropics might have on her newborn.
Breast-feeding and medications
The benefits of breast-feeding for mother and infant are well-known. Despite this, some women with bipolar disorder are advised not to breast-feed or, worse, to discontinue their medications in order to breast-feed. Decisions about breast-feeding while taking medications should be based on evidence of benefits and risks to the infant, along with a discussion of the risks of untreated illness, which is high postpartum. The prescribing information for many of the medications used to treat bipolar disorder advise against breast-feeding, although there is little evidence of harm.
Drug dosages and levels in breast milk can be reported a few different ways:
• percentage of maternal dosage measured in the breast milk
• percentage weight-adjusted maternal dosage
• percentage of maternal plasma level, and milk-to-plasma ratio (M:P).
Daily infant dosage can be calculated by multiplying the average concentration of the drug in breast milk (mg/mL) by the average volume of milk the baby ingests in 24 hours (usually 150 mL).1 The relative infant dosage can be calculated as the percentage maternal dosage, which is the daily infant dosage (mg/kg/d) ÷ maternal dosage (mg/kg/d) × 100.1
According to the American Academy of Pediatrics, ≤10% of the maternal dosage is compatible with breast-feeding.1 Most psychotropics studied fall below this threshold. Keep in mind that all published research is for breast-feeding a full-term infant; exercise caution with premature or low birth weight infants. Infants born to mothers taking a psychotropic should be monitored for withdrawal symptoms, which might be associated with antidepressants and benzodiazepines, but otherwise are rare.
Lithium
Breast-feeding during lithium treatment has been considered contraindicated based on early reports that lithium was highly excreted in breast milk.2 A 2003 study2 of 11 women found that lithium was excreted in breast milk in amounts between zero and 30% of maternal dosage (mean, 12.2% ± 8.5%; median, 11.2%; 95% CI, 6.3% to 18.0%). Researchers measured serum concentrations in 2 infants and found that 1 received 17% to 20% of the maternal dosage, and the other showed 50%. None of the infants experienced adverse events. In a study of 10 mother-infant pairs, breast milk lithium concentration averaged 0.35 mEq/L (standard deviation [SD] = 0.10, range 0.19 to 0.48 mEq/L), with paired infant serum concentrations of 0.16 mEq/L (SD = 0.06, range 0.09 to 0.25 mEq/L).3 Some transient abnormalities were found in infant serum concentrations of thyroid-stimulating hormone (TSH), blood urea nitrogen, and creatinine; there were no adverse effects on development. The authors recommend monitoring for TSH abnormalities in infants.
Olanzapine
Olanzapine prescribing information cites a study reporting that 1.8% of the maternal dosage is transferred to breast milk.4 Yet the olanzapine prescribing information states, “It is recommended that women receiving olanzapine should not breast-feed.” Olanzapine use during breast-feeding has been studied more than many medications, in part because of a database maintained by the manufacturer. In a study using the manufacturer’s database (N = 102) adverse reactions were reported in 15.6% of the infants, with the most common being somnolence (3.9%), irritability (2%), tremor (2%), and insomnia (2%).5
Other second-generation antipsychotics
Aripiprazole. The only case report of aripiprazole excretion in human breast milk found a concentration of approximately 20% of the maternal plasma level and an M:P ratio of 0.18:0.2.6
Asenapine. According to asenapine prescribing information7 and a literature search, it is not known whether asenapine is excreted in breast milk of humans, although it is found in the milk of lactating rats.
Lurasidone. According to the lurasidone prescribing information8 and a literature search, it is not known whether lurasidone is excreted in human breast milk, although it is found in the milk of lactating rats.
Quetiapine. An initial study reported that 0.09% to 0.43% of the maternal dosage of quetiapine was excreted in breast milk.9 Further studies found excretion to be 0.09% of maternal dosage, with infant plasma levels reaching 6% of the maternal dosage.10 A case series found that one-third of babies exposed to quetiapine during breast-feeding showed some neurodevelopmental delay, although these mothers also were taking other psychotropics.11
Risperidone. A 2000 study12 of risperidone in lactation reported that 0.84% weight-adjusted maternal risperidone dosage and 3.46% of its metabolite 9-hydroxyrisperidone is transferred to the infant. A later study showed 2.3% to 4.7% of the maternal dosage is transferred, with no adverse events reported in infants.13 A case study reported no adverse events and normal neurodevelopment in a the child of a mother taking risperidone.14
Ziprasidone. According to the ziprasidone prescribing information15 and a literature search, is not known whether ziprasidone is excreted in human breast milk.
See the Table4,6-10,12,13,15 for a summary of the evidence levels of second-generation antipsychotics that are excreted in breast milk.
Other mood stabilizers
Carbamazepine has been measured in breast milk at 3.8% to 5.9% of the maternal dosage.16
Lamotrigine. In a study of 30 lactating women, the breast milk contained an average of 9.2% of the maternal dosage of lamotrigine.17 Mild thrombocytosis was detected in 7 of 8 infants; no other adverse effects were reported. A case study describes a woman who breast-fed while taking lamotrigine, 850 mg/d, and who experienced dizziness and visual disturbances. The infant had apnea episodes followed by a cyanotic crisis, which required resuscitation. The infant’s plasma lamotrigine level was 4.87 μg/mL. Symptoms disappeared when the mother stopped breast-feeding.18 Lamotrigine is considered to be moderately safe in breast-feeding patients with proper monitoring. The drug also has a known safety profile because of its use in children with epilepsy.
Valproic acid. Because of its high plasma protein binding, valproic acid does not pass readily into the breast milk. Newborns receive approximately 1.4% to 1.7% of the maternal dosage.16 Caution is advised, however, because of some reported adverse events. One case reported thrombocytopenic purpura and anemia in an infant.19 Valproic acid is considered to be compatible with breast-feeding with proper monitoring.
Benzodiazepines
Benzodiazepines can be helpful adjunctive medications to aid sleep, which is essential for the mother’s and infant’s health. In a prospectively recruited, retrospectively assessed cohort study that evaluated 124 women taking benzodiazepines while breast-feeding, adverse effects, specifically sedation, were noted in 1.6% of infants.20
Future developments in prescribing information
Under a 2008 FDA recommendation, the “Nursing Mothers” section of prescribing information would be replaced with a section entitled “Lactation.” This new heading would include the sub-headings Risk Summary, Clinical Considerations, and Data.1 It is expected that this new format will be more practical and will help clinicians and patients make informed decisions. The prescribing changes will be in effect on June 30, 2015.21
Related Resources
• Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
• LactMed. http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm.
• MOTHERISK. www.motherisk.org/women/ breastfeeding.jsp.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Quetiapine • Seroquel
Carbamazepine • Tegretol Risperidone • Risperdal
Lamotrigine • Lamictal Valproic acid • Depakene
Lithium • Eskalith, Lithobid Ziprasidone • Geodon
Lurasidone • Latuda
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sach HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3);e796-e809.
2. Moretti ME, Koren G, Verjee Z, et al. Monitoring lithium in breast milk: an individualized approach for breast-feeding mothers. Ther Drug Monit. 2003;25(3):364-366.
3. Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342-345.
4. Xyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
5. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38.
6. Schlotterbeck P, Leube D, Kircher T, et al. Aripiprazole in human milk. Int J Neuropsychopharmacol. 2007;10(3):433.
7. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2014.
8. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals; 2013.
9. Lee A, Giesbrecht E, Dunn E, et al. Excretion of quetiapine in breast milk. Am J Psychiatry. 2004;161(9):1715-1716.
10. Rampono J, Kristensen JH, Ilett KF, et al. Quetiapine and breastfeeding. Ann Pharmacother. 2007;41(4):711-714.
11. Misri S, Corral M, Wardrop AA, et al. Quetiapine augmentation in lactation: a series of case reports. J Clin Psychopharmacol. 2006;26(5):508-511.
12. Hill RC, McIvor RJ, Wojnar-Horton RE, et al. Risperidone distribution and excretion into human milk: case report and estimated infant exposure during breast-feeding. J Clin Psychopharmacol. 2000;20(2):285-286.
13. Ilett KF, Hackett LP, Kristensen JH, et al. Transfer of risperidone and 9-hydroxyrisperidone into human milk. Ann Pharmacother. 2004;38(2):273-276.
14. Aichhorn W, Stuppaek C, Whitworth AB. Risperidone and breast-feeding. J Psychopharmacol. 2005;19(2):211-213.
15. Geodon [package insert]. New York, NY: Pfizer; 2014.
16. Davanzo R, Dal Bo S, Bua J, et al. Antiepileptic drugs and breastfeeding. Ital J Pediatr. 2013;39:50.
17. Newport DJ, Pennell PB, Calamaras MR, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122(1):e223-e231.
18. Nordmo E, Aronsen L, Wasland K, et al. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43(11):1893-1897.
19. Stahl MM, Neiderud J, Vinge E. Thrombocytopenic purpura and anemia in a breast-fed infant whose mother was treated with valproic acid. J Pediatr. 1997;130(6):1001-1003.
20. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451.
21. U.S. Food and Drug Administration. FDA issues final rule on changes to pregnancy and lactation labeling information for prescription drug and biological products. http://www. fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm425317.htm. Published December 3. 2014. Accessed March 4, 2015.
Ms. K, age 35, soon will deliver her second child. She has a 12-year history of bipolar disorder, which was well controlled with lithium, 1,200 mg/d. During her first pregnancy 3 years ago, Ms. K stopped taking lithium because she was concerned about the risk of Ebstein’s anomaly. She experienced a bipolar relapse after her healthy baby was born, and developed postpartum psychosis that was treated by restarting lithium, 1,200 mg/d, and adding olanzapine, 10 mg/d.
Ms. K has continued these medications throughout her current pregnancy. She wants to breast-feed her infant and is concerned about the effects that psychotropics might have on her newborn.
Breast-feeding and medications
The benefits of breast-feeding for mother and infant are well-known. Despite this, some women with bipolar disorder are advised not to breast-feed or, worse, to discontinue their medications in order to breast-feed. Decisions about breast-feeding while taking medications should be based on evidence of benefits and risks to the infant, along with a discussion of the risks of untreated illness, which is high postpartum. The prescribing information for many of the medications used to treat bipolar disorder advise against breast-feeding, although there is little evidence of harm.
Drug dosages and levels in breast milk can be reported a few different ways:
• percentage of maternal dosage measured in the breast milk
• percentage weight-adjusted maternal dosage
• percentage of maternal plasma level, and milk-to-plasma ratio (M:P).
Daily infant dosage can be calculated by multiplying the average concentration of the drug in breast milk (mg/mL) by the average volume of milk the baby ingests in 24 hours (usually 150 mL).1 The relative infant dosage can be calculated as the percentage maternal dosage, which is the daily infant dosage (mg/kg/d) ÷ maternal dosage (mg/kg/d) × 100.1
According to the American Academy of Pediatrics, ≤10% of the maternal dosage is compatible with breast-feeding.1 Most psychotropics studied fall below this threshold. Keep in mind that all published research is for breast-feeding a full-term infant; exercise caution with premature or low birth weight infants. Infants born to mothers taking a psychotropic should be monitored for withdrawal symptoms, which might be associated with antidepressants and benzodiazepines, but otherwise are rare.
Lithium
Breast-feeding during lithium treatment has been considered contraindicated based on early reports that lithium was highly excreted in breast milk.2 A 2003 study2 of 11 women found that lithium was excreted in breast milk in amounts between zero and 30% of maternal dosage (mean, 12.2% ± 8.5%; median, 11.2%; 95% CI, 6.3% to 18.0%). Researchers measured serum concentrations in 2 infants and found that 1 received 17% to 20% of the maternal dosage, and the other showed 50%. None of the infants experienced adverse events. In a study of 10 mother-infant pairs, breast milk lithium concentration averaged 0.35 mEq/L (standard deviation [SD] = 0.10, range 0.19 to 0.48 mEq/L), with paired infant serum concentrations of 0.16 mEq/L (SD = 0.06, range 0.09 to 0.25 mEq/L).3 Some transient abnormalities were found in infant serum concentrations of thyroid-stimulating hormone (TSH), blood urea nitrogen, and creatinine; there were no adverse effects on development. The authors recommend monitoring for TSH abnormalities in infants.
Olanzapine
Olanzapine prescribing information cites a study reporting that 1.8% of the maternal dosage is transferred to breast milk.4 Yet the olanzapine prescribing information states, “It is recommended that women receiving olanzapine should not breast-feed.” Olanzapine use during breast-feeding has been studied more than many medications, in part because of a database maintained by the manufacturer. In a study using the manufacturer’s database (N = 102) adverse reactions were reported in 15.6% of the infants, with the most common being somnolence (3.9%), irritability (2%), tremor (2%), and insomnia (2%).5
Other second-generation antipsychotics
Aripiprazole. The only case report of aripiprazole excretion in human breast milk found a concentration of approximately 20% of the maternal plasma level and an M:P ratio of 0.18:0.2.6
Asenapine. According to asenapine prescribing information7 and a literature search, it is not known whether asenapine is excreted in breast milk of humans, although it is found in the milk of lactating rats.
Lurasidone. According to the lurasidone prescribing information8 and a literature search, it is not known whether lurasidone is excreted in human breast milk, although it is found in the milk of lactating rats.
Quetiapine. An initial study reported that 0.09% to 0.43% of the maternal dosage of quetiapine was excreted in breast milk.9 Further studies found excretion to be 0.09% of maternal dosage, with infant plasma levels reaching 6% of the maternal dosage.10 A case series found that one-third of babies exposed to quetiapine during breast-feeding showed some neurodevelopmental delay, although these mothers also were taking other psychotropics.11
Risperidone. A 2000 study12 of risperidone in lactation reported that 0.84% weight-adjusted maternal risperidone dosage and 3.46% of its metabolite 9-hydroxyrisperidone is transferred to the infant. A later study showed 2.3% to 4.7% of the maternal dosage is transferred, with no adverse events reported in infants.13 A case study reported no adverse events and normal neurodevelopment in a the child of a mother taking risperidone.14
Ziprasidone. According to the ziprasidone prescribing information15 and a literature search, is not known whether ziprasidone is excreted in human breast milk.
See the Table4,6-10,12,13,15 for a summary of the evidence levels of second-generation antipsychotics that are excreted in breast milk.
Other mood stabilizers
Carbamazepine has been measured in breast milk at 3.8% to 5.9% of the maternal dosage.16
Lamotrigine. In a study of 30 lactating women, the breast milk contained an average of 9.2% of the maternal dosage of lamotrigine.17 Mild thrombocytosis was detected in 7 of 8 infants; no other adverse effects were reported. A case study describes a woman who breast-fed while taking lamotrigine, 850 mg/d, and who experienced dizziness and visual disturbances. The infant had apnea episodes followed by a cyanotic crisis, which required resuscitation. The infant’s plasma lamotrigine level was 4.87 μg/mL. Symptoms disappeared when the mother stopped breast-feeding.18 Lamotrigine is considered to be moderately safe in breast-feeding patients with proper monitoring. The drug also has a known safety profile because of its use in children with epilepsy.
Valproic acid. Because of its high plasma protein binding, valproic acid does not pass readily into the breast milk. Newborns receive approximately 1.4% to 1.7% of the maternal dosage.16 Caution is advised, however, because of some reported adverse events. One case reported thrombocytopenic purpura and anemia in an infant.19 Valproic acid is considered to be compatible with breast-feeding with proper monitoring.
Benzodiazepines
Benzodiazepines can be helpful adjunctive medications to aid sleep, which is essential for the mother’s and infant’s health. In a prospectively recruited, retrospectively assessed cohort study that evaluated 124 women taking benzodiazepines while breast-feeding, adverse effects, specifically sedation, were noted in 1.6% of infants.20
Future developments in prescribing information
Under a 2008 FDA recommendation, the “Nursing Mothers” section of prescribing information would be replaced with a section entitled “Lactation.” This new heading would include the sub-headings Risk Summary, Clinical Considerations, and Data.1 It is expected that this new format will be more practical and will help clinicians and patients make informed decisions. The prescribing changes will be in effect on June 30, 2015.21
Related Resources
• Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
• LactMed. http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm.
• MOTHERISK. www.motherisk.org/women/ breastfeeding.jsp.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Quetiapine • Seroquel
Carbamazepine • Tegretol Risperidone • Risperdal
Lamotrigine • Lamictal Valproic acid • Depakene
Lithium • Eskalith, Lithobid Ziprasidone • Geodon
Lurasidone • Latuda
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. K, age 35, soon will deliver her second child. She has a 12-year history of bipolar disorder, which was well controlled with lithium, 1,200 mg/d. During her first pregnancy 3 years ago, Ms. K stopped taking lithium because she was concerned about the risk of Ebstein’s anomaly. She experienced a bipolar relapse after her healthy baby was born, and developed postpartum psychosis that was treated by restarting lithium, 1,200 mg/d, and adding olanzapine, 10 mg/d.
Ms. K has continued these medications throughout her current pregnancy. She wants to breast-feed her infant and is concerned about the effects that psychotropics might have on her newborn.
Breast-feeding and medications
The benefits of breast-feeding for mother and infant are well-known. Despite this, some women with bipolar disorder are advised not to breast-feed or, worse, to discontinue their medications in order to breast-feed. Decisions about breast-feeding while taking medications should be based on evidence of benefits and risks to the infant, along with a discussion of the risks of untreated illness, which is high postpartum. The prescribing information for many of the medications used to treat bipolar disorder advise against breast-feeding, although there is little evidence of harm.
Drug dosages and levels in breast milk can be reported a few different ways:
• percentage of maternal dosage measured in the breast milk
• percentage weight-adjusted maternal dosage
• percentage of maternal plasma level, and milk-to-plasma ratio (M:P).
Daily infant dosage can be calculated by multiplying the average concentration of the drug in breast milk (mg/mL) by the average volume of milk the baby ingests in 24 hours (usually 150 mL).1 The relative infant dosage can be calculated as the percentage maternal dosage, which is the daily infant dosage (mg/kg/d) ÷ maternal dosage (mg/kg/d) × 100.1
According to the American Academy of Pediatrics, ≤10% of the maternal dosage is compatible with breast-feeding.1 Most psychotropics studied fall below this threshold. Keep in mind that all published research is for breast-feeding a full-term infant; exercise caution with premature or low birth weight infants. Infants born to mothers taking a psychotropic should be monitored for withdrawal symptoms, which might be associated with antidepressants and benzodiazepines, but otherwise are rare.
Lithium
Breast-feeding during lithium treatment has been considered contraindicated based on early reports that lithium was highly excreted in breast milk.2 A 2003 study2 of 11 women found that lithium was excreted in breast milk in amounts between zero and 30% of maternal dosage (mean, 12.2% ± 8.5%; median, 11.2%; 95% CI, 6.3% to 18.0%). Researchers measured serum concentrations in 2 infants and found that 1 received 17% to 20% of the maternal dosage, and the other showed 50%. None of the infants experienced adverse events. In a study of 10 mother-infant pairs, breast milk lithium concentration averaged 0.35 mEq/L (standard deviation [SD] = 0.10, range 0.19 to 0.48 mEq/L), with paired infant serum concentrations of 0.16 mEq/L (SD = 0.06, range 0.09 to 0.25 mEq/L).3 Some transient abnormalities were found in infant serum concentrations of thyroid-stimulating hormone (TSH), blood urea nitrogen, and creatinine; there were no adverse effects on development. The authors recommend monitoring for TSH abnormalities in infants.
Olanzapine
Olanzapine prescribing information cites a study reporting that 1.8% of the maternal dosage is transferred to breast milk.4 Yet the olanzapine prescribing information states, “It is recommended that women receiving olanzapine should not breast-feed.” Olanzapine use during breast-feeding has been studied more than many medications, in part because of a database maintained by the manufacturer. In a study using the manufacturer’s database (N = 102) adverse reactions were reported in 15.6% of the infants, with the most common being somnolence (3.9%), irritability (2%), tremor (2%), and insomnia (2%).5
Other second-generation antipsychotics
Aripiprazole. The only case report of aripiprazole excretion in human breast milk found a concentration of approximately 20% of the maternal plasma level and an M:P ratio of 0.18:0.2.6
Asenapine. According to asenapine prescribing information7 and a literature search, it is not known whether asenapine is excreted in breast milk of humans, although it is found in the milk of lactating rats.
Lurasidone. According to the lurasidone prescribing information8 and a literature search, it is not known whether lurasidone is excreted in human breast milk, although it is found in the milk of lactating rats.
Quetiapine. An initial study reported that 0.09% to 0.43% of the maternal dosage of quetiapine was excreted in breast milk.9 Further studies found excretion to be 0.09% of maternal dosage, with infant plasma levels reaching 6% of the maternal dosage.10 A case series found that one-third of babies exposed to quetiapine during breast-feeding showed some neurodevelopmental delay, although these mothers also were taking other psychotropics.11
Risperidone. A 2000 study12 of risperidone in lactation reported that 0.84% weight-adjusted maternal risperidone dosage and 3.46% of its metabolite 9-hydroxyrisperidone is transferred to the infant. A later study showed 2.3% to 4.7% of the maternal dosage is transferred, with no adverse events reported in infants.13 A case study reported no adverse events and normal neurodevelopment in a the child of a mother taking risperidone.14
Ziprasidone. According to the ziprasidone prescribing information15 and a literature search, is not known whether ziprasidone is excreted in human breast milk.
See the Table4,6-10,12,13,15 for a summary of the evidence levels of second-generation antipsychotics that are excreted in breast milk.
Other mood stabilizers
Carbamazepine has been measured in breast milk at 3.8% to 5.9% of the maternal dosage.16
Lamotrigine. In a study of 30 lactating women, the breast milk contained an average of 9.2% of the maternal dosage of lamotrigine.17 Mild thrombocytosis was detected in 7 of 8 infants; no other adverse effects were reported. A case study describes a woman who breast-fed while taking lamotrigine, 850 mg/d, and who experienced dizziness and visual disturbances. The infant had apnea episodes followed by a cyanotic crisis, which required resuscitation. The infant’s plasma lamotrigine level was 4.87 μg/mL. Symptoms disappeared when the mother stopped breast-feeding.18 Lamotrigine is considered to be moderately safe in breast-feeding patients with proper monitoring. The drug also has a known safety profile because of its use in children with epilepsy.
Valproic acid. Because of its high plasma protein binding, valproic acid does not pass readily into the breast milk. Newborns receive approximately 1.4% to 1.7% of the maternal dosage.16 Caution is advised, however, because of some reported adverse events. One case reported thrombocytopenic purpura and anemia in an infant.19 Valproic acid is considered to be compatible with breast-feeding with proper monitoring.
Benzodiazepines
Benzodiazepines can be helpful adjunctive medications to aid sleep, which is essential for the mother’s and infant’s health. In a prospectively recruited, retrospectively assessed cohort study that evaluated 124 women taking benzodiazepines while breast-feeding, adverse effects, specifically sedation, were noted in 1.6% of infants.20
Future developments in prescribing information
Under a 2008 FDA recommendation, the “Nursing Mothers” section of prescribing information would be replaced with a section entitled “Lactation.” This new heading would include the sub-headings Risk Summary, Clinical Considerations, and Data.1 It is expected that this new format will be more practical and will help clinicians and patients make informed decisions. The prescribing changes will be in effect on June 30, 2015.21
Related Resources
• Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
• LactMed. http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm.
• MOTHERISK. www.motherisk.org/women/ breastfeeding.jsp.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Quetiapine • Seroquel
Carbamazepine • Tegretol Risperidone • Risperdal
Lamotrigine • Lamictal Valproic acid • Depakene
Lithium • Eskalith, Lithobid Ziprasidone • Geodon
Lurasidone • Latuda
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sach HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3);e796-e809.
2. Moretti ME, Koren G, Verjee Z, et al. Monitoring lithium in breast milk: an individualized approach for breast-feeding mothers. Ther Drug Monit. 2003;25(3):364-366.
3. Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342-345.
4. Xyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
5. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38.
6. Schlotterbeck P, Leube D, Kircher T, et al. Aripiprazole in human milk. Int J Neuropsychopharmacol. 2007;10(3):433.
7. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2014.
8. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals; 2013.
9. Lee A, Giesbrecht E, Dunn E, et al. Excretion of quetiapine in breast milk. Am J Psychiatry. 2004;161(9):1715-1716.
10. Rampono J, Kristensen JH, Ilett KF, et al. Quetiapine and breastfeeding. Ann Pharmacother. 2007;41(4):711-714.
11. Misri S, Corral M, Wardrop AA, et al. Quetiapine augmentation in lactation: a series of case reports. J Clin Psychopharmacol. 2006;26(5):508-511.
12. Hill RC, McIvor RJ, Wojnar-Horton RE, et al. Risperidone distribution and excretion into human milk: case report and estimated infant exposure during breast-feeding. J Clin Psychopharmacol. 2000;20(2):285-286.
13. Ilett KF, Hackett LP, Kristensen JH, et al. Transfer of risperidone and 9-hydroxyrisperidone into human milk. Ann Pharmacother. 2004;38(2):273-276.
14. Aichhorn W, Stuppaek C, Whitworth AB. Risperidone and breast-feeding. J Psychopharmacol. 2005;19(2):211-213.
15. Geodon [package insert]. New York, NY: Pfizer; 2014.
16. Davanzo R, Dal Bo S, Bua J, et al. Antiepileptic drugs and breastfeeding. Ital J Pediatr. 2013;39:50.
17. Newport DJ, Pennell PB, Calamaras MR, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122(1):e223-e231.
18. Nordmo E, Aronsen L, Wasland K, et al. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43(11):1893-1897.
19. Stahl MM, Neiderud J, Vinge E. Thrombocytopenic purpura and anemia in a breast-fed infant whose mother was treated with valproic acid. J Pediatr. 1997;130(6):1001-1003.
20. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451.
21. U.S. Food and Drug Administration. FDA issues final rule on changes to pregnancy and lactation labeling information for prescription drug and biological products. http://www. fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm425317.htm. Published December 3. 2014. Accessed March 4, 2015.
1. Sach HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3);e796-e809.
2. Moretti ME, Koren G, Verjee Z, et al. Monitoring lithium in breast milk: an individualized approach for breast-feeding mothers. Ther Drug Monit. 2003;25(3):364-366.
3. Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342-345.
4. Xyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
5. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38.
6. Schlotterbeck P, Leube D, Kircher T, et al. Aripiprazole in human milk. Int J Neuropsychopharmacol. 2007;10(3):433.
7. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2014.
8. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals; 2013.
9. Lee A, Giesbrecht E, Dunn E, et al. Excretion of quetiapine in breast milk. Am J Psychiatry. 2004;161(9):1715-1716.
10. Rampono J, Kristensen JH, Ilett KF, et al. Quetiapine and breastfeeding. Ann Pharmacother. 2007;41(4):711-714.
11. Misri S, Corral M, Wardrop AA, et al. Quetiapine augmentation in lactation: a series of case reports. J Clin Psychopharmacol. 2006;26(5):508-511.
12. Hill RC, McIvor RJ, Wojnar-Horton RE, et al. Risperidone distribution and excretion into human milk: case report and estimated infant exposure during breast-feeding. J Clin Psychopharmacol. 2000;20(2):285-286.
13. Ilett KF, Hackett LP, Kristensen JH, et al. Transfer of risperidone and 9-hydroxyrisperidone into human milk. Ann Pharmacother. 2004;38(2):273-276.
14. Aichhorn W, Stuppaek C, Whitworth AB. Risperidone and breast-feeding. J Psychopharmacol. 2005;19(2):211-213.
15. Geodon [package insert]. New York, NY: Pfizer; 2014.
16. Davanzo R, Dal Bo S, Bua J, et al. Antiepileptic drugs and breastfeeding. Ital J Pediatr. 2013;39:50.
17. Newport DJ, Pennell PB, Calamaras MR, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122(1):e223-e231.
18. Nordmo E, Aronsen L, Wasland K, et al. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43(11):1893-1897.
19. Stahl MM, Neiderud J, Vinge E. Thrombocytopenic purpura and anemia in a breast-fed infant whose mother was treated with valproic acid. J Pediatr. 1997;130(6):1001-1003.
20. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451.
21. U.S. Food and Drug Administration. FDA issues final rule on changes to pregnancy and lactation labeling information for prescription drug and biological products. http://www. fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm425317.htm. Published December 3. 2014. Accessed March 4, 2015.
Colorectal Cancer: Screening and Surveillance Recommendations
From the Boston University School of Medicine, Boston, MA.
Abstract
- Objective: To review recommendations for colorectal cancer (CRC) screening.
- Methods: Review of the literature.
- Results: In the United States, CRC is the third most commonly diagnosed cancer and the third leading cause of cancer death. CRC screening can reduce mortality through the detection of early-stage disease and the detection and removal of adenomatous polyps. There are several modalities for CRC screening, with current technology falling into 2 general categories: stool tests, which include tests for occult blood or exfoliated DNA; and structural exams, which include flexible sigmoidoscopy, colonoscopy, double-contrast barium enema, and CT colonography. The preferred CRC prevention test for average-risk individuals is colonoscopy starting at age 50 with subsequent examinations every 10 years. Patients unwilling to undergo screening colonoscopy may be offered flexible sigmoidoscopy, CT colonography, or fecal immunohistochemical test. Surveillance examinations should occur based on polyp findings on index colonoscopy. There is no recommendation to continue screening after age 75, though physicians can make a determination based on a patient’s health and risk/benefit profile. Current guidelines recommend against offering screening to patients over age 85.
- Conclusion: Increasing access to and utilization of CRC screening tests is likely to lead to improvements in mortality reduction, as only about half of people aged 50 or older report having received CRC testing consistent with current guidelines.
In the United States, colorectal cancer (CRC) is the third most commonly diagnosed cancer and the third leading cause of cancer death in both men and women [1]. In 2014, an estimated 136,830 people were diagnosed with CRC and about 50,310 people died of the disease [2]. Colorectal cancer usually develops slowly over a period of 10 to 15 years. The tumor typically begins as a noncancerous polyp, classically an adenomatous polyp or adenoma, though fewer than 10% of adenomas will progress to cancer [3]. Adenomas are common; an estimated one-third to one-half of all individuals will eventually develop 1 or more adenomas [4,5]. In the United States, the lifetime risk of being diagnosed with CRC is approximately 5% for both men and women [6]. Incidence rates for CRC increase with age, with an incidence rate more than 15 times higher in adults aged 50 years and older compared with those aged 20 to 49 years [7].
Certain demographic subgroups have been shown to be at higher risk. Overall, CRC incidence and mortality rates are about 35% to 40% higher in men than in women. The reasons for this are not completely understood but likely reflect complex interactions between gender-related differences in exposure to hormones and risk factors [8]. CRC incidence and mortality rates are highest in African-American men and women; incidence rates are 20% higher and mortality rates are about 45% higher than those in whites. Prior to 1989, incidence rates were predominantly higher in white men than in African American men and were similar for women of both races. Since that time, although incidence rates have declined as a whole [9], incidence rates have been higher for African Americans than whites in both men and women This crossover likely reflects a combination of greater access to and utilization of recommended screening tests among whites (resulting in detection and removal of precancerous polyps), as well as racial differences in trends for CRC risk factors [10].
CRC screening can reduce mortality through the detection of early-stage disease and the detection and removal of ademomatous polyps [11]. Increasing access to and utilization of CRC screening tests is likely to lead to improvements in mortality reduction, as only about half of people aged 50 or older report having received CRC testing consistent with current guidelines [1].
Case Study
Initial Presentation
A 55-year-old white male presents for a routine visit and asks about colon cancer screening. His father was diagnosed with colon cancer at the age of 78. Overall, he feels well and does not have any particular complaints. His bowel habits are normal and he denies melena and hematochezia. His past medical history is significant for diabetes, hypertension, and obesity. He was a previous smoker and has a few alcoholic drinks on the weekends. His physical exam is unremarkable. Results of recent blood work are normal and there is no evidence of anemia.
What are this patient’s risk factors for developing colon cancer?
Risk Factors for CRC
There are numerous factors that are thought to influence risk for CRC. Nonmodifiable risk factors include a personal or family history of CRC or adenomatous polyps, and a personal history of chronic inflammatory bowel disease. Modifiable risk factors that have been associated with an increased risk of CRC in epidemiologic studies include physical inactivity, obesity, high consumption of red or processed meats, smoking, and moderate-to-heavy alcohol consumption. In fact, a prospective study showed that up to 23% of colorectal cancers were considered to be potentially avoidable by adhering to multiple healthy lifestyle recommendations including maintaining a healthy weight, being physically active at least 30 minutes per day, eating a healthy diet, and avoiding smoking and drinking excessive amounts of alcohol [12].
People with a first-degree relative (parent, sibling, or offspring) who has had CRC have 2 to 3 times the risk of developing the disease compared with individuals with no family history; if the relative was diagnosed at a young age or if there is more than 1 affected relative, risk increases to 3 to 6 times that of the general population [13,14]. About 5% of patients with CRC have a well-defined genetic syndrome that causes the disease [15]. The most common of these is Lynch syndrome (also known as hereditary nonpolyposis colorectal cancer or HNPCC), which accounts for 2% to 4% of all CRC cases [16]. Although individuals with Lynch syndrome are predisposed to numerous types of cancer, risk of CRC is highest. A recent study of CRC in 147 Lynch syndrome families in the United States found lifetime risk of CRC to be 66% in men and 43% in women, with a median age at diagnosis of 42 years and 47 years, respectively [17]. Familial adenomatous polyposis (FAP) is the second most common predisposing genetic syndrome; for these individuals, the lifetime risk of CRC approaches 100% without intervention (eg, colectomy) [16].
People who have inflammatory bowel disease of the colon (both ulcerative colitis and Crohn’s disease) have an increased risk of developing CRC that correlates with the extent and the duration of the inflammation [18]. It is estimated that 18% of patients with a 30-year history of ulcerative colitis will develop CRC [19]. In addition, several studies have found an association between diabetes and increased risk of CRC [20,21]. Though adult-onset type 2 diabetes (the most common type) and CRC share similar risk factors, including physical inactivity and obesity, a positive association between diabetes and CRC has been found even after accounting for physical activity, body mass index, and waist circumference [22].
Being overweight or obese is also associated with a higher risk of CRC, with stronger associations more consistently observed in men than in women. Obesity increases the risk of CRC independent of physical activity. Abdominal obesity (measured by waist circumference) may be a more important risk factor for colon cancer than overall obesity in both men and women [23–25]. Diet and lifestyle strongly influence CRC risk; however, research on the role of specific dietary elements on CRC risk is still accumulating. Several studies, including one by the American Cancer Society, have found that high consumption of red and/or processed meat increases the risk of both colon and rectal cancer [23,26,27]. Further analyses indicate that the association between CRC and red meat may be related to the cooking process, because a higher risk of CRC is observed particularly among those individuals who consume meat that has been cooked at a high temperature for a long period of time [28]. In contrast to findings from earlier research, more recent large, prospective studies do not indicate a major relationship between CRC and vegetable, fruit, or fiber consumption [28,29]. However, some studies suggest that people with very low fruit and vegetable intake are at above-average risk for CRC [30,31]. Consumption of milk and calcium may decrease the risk of developing CRC [28,29,32].
In November 2009, the International Agency for Research on Cancer reported that there is now sufficient evidence to conclude that tobacco smoking causes CRC [33]. Colorectal cancer has been linked to even moderate alcohol use. Individuals who have a lifetime average of 2 to 4 alcoholic drinks per day have a 23% higher risk of CRC than those who consume less than 1 drink per day [34].
Protective Factors
One of the most consistently reported relationships between colon cancer risk and behavior is the protective effect of physical activity [35]. Based on these findings, as well as the numerous other health benefits of regular physical activity, the American Cancer Society recommends engaging in at least moderate activity for 30 minutes or more on 5 or more days per week.
Accumulating research suggests that aspirin-like drugs, postmenopausal hormones, and calcium supplements may help prevent CRC. Extensive evidence suggests that long-term, regular use of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) is asso-ciated with lower risk of CRC. The American Cancer Society does not currently recommend use of these drugs as chemoprevention because of the potential side effects of gastrointestinal bleeding from aspirin and other traditional NSAIDs and heart attacks from selective cyclooxygenase-2 (COX-2) inhibitors. However, people who are already taking NSAIDs for chronic arthritis or aspirin for heart disease prevention may have a lower risk of CRC as a positive side effect [36,37].
There is substantial evidence that women who use postmenopausal hormones have lower rates of CRC than those who do not. A decreased risk of CRC is especially evident in women who use hormones long-term, although the risk returns to that of nonusers within 3 years of cessation. Despite its positive effect on CRC risk, the use of postmenopausal hormones increases the risk of breast and other cancers as well as cardiovascular disease, and therefore it is not recommended for the prevention of CRC. At present, the American Cancer Society does not recommend any medications or supplements to prevent CRC because of uncertainties about their effectiveness, appropriate dosing, and potential toxicity [38–40].
Case Continued
The physician tells the patient that there are several environmental factors that may predispose him to developing CRC. He recommends that the patient follow a healthy lifestyle, including eating 5 servings of fruits and vegetables daily, minimizing consumption of red meats, exercising for 30 minutes at least 5 days per week, drinking only moderate amounts of alcohol, and continuing to take his aspirin in the setting of his diabetes. He also asks the patient if he would be interested in talking about weight loss and working together to make a plan.
The patient is appreciative of this information and wants to know what CRC creening test the physician recommends.
What screening test should be recommended?
Screening Options
There are several modalities for CRC screening, with current technology falling into 2 general categories: stool tests, which include tests for occult blood or exfoliated DNA; and structural exams, which include flexible sigmoidoscopy, colonoscopy, double-contrast barium enema (DCBE), and computed tomographic (CT) colonography. Stool tests are best suited for the detection of CRC, although they also will deliver positive findings for some advanced adenomas, while the structural exams can achieve both detection and prevention of CRC through identification and removal of adenomatous polyps [41]. These tests may be used alone or in combination to improve sensitivity or, in some instances, to ensure a complete examination of the colon if the initial test cannot be completed.
In principle, all adults should have access to the full range of options for CRC screening, and the availability of lower-cost, less invasive options in most practice settings is a public health advantage [11]. However, the availability of multiple testing options can overwhelm the primary care provider and presents challenges for practices in trying to support an office policy that can manage a broad range of testing choices, their follow-up requirements, and shared decision making related to the options. Shared decision making around CRC screening options is both demanding and time consuming and is complicated by the different characteristics of the tests and the test-specific requirements for individuals undergoing screening [42].
Recommended Tests
The joint guideline on screening for CRC from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology (the MSTF guideline) [11] is of the strong opinion that tests designed to detect early cancer and prevent cancer through the detection and removal of adenomatous polyps (the structural exams) should be encouraged if resources are available and patients are willing to undergo an invasive test [11]. In clinical settings in which economic issues preclude primary screening with colonoscopy, or for patients who decline invasive tests, clinicians may offer stool- based testing. However, providers and patients should understand that these tests are less likely to prevent cancer compared with the invasive tests, they must be repeated at regular intervals to be effective (ie, programmatic sensitivity), and if the test is abnormal, a colonoscopy will be needed to follow up. Therefore, if patients are not willing to have repeated testing or pursue colonoscopy if the test is abnormal, these programs will not be effective and should not be recommended [11].
Stool-Based Testing
Stool blood tests are conventionally known as fecal occult blood tests (FOBT) because they are designed to detect the presence of occult blood in stool. FOBT falls into 2 primary categories based on the detected analyte: guaiac-based and FIT. Blood in the stool is a nonspecific finding but may originate from CRC or larger (> 1 to 2 cm) polyps. Because small adenomatous polyps do not tend to bleed and bleeding from cancers or large polyps may be intermittent or undetectable in a single sample of stool, the proper use of stool blood tests requires annual testing that consists of collecting specimens (2 or 3, depending on the product) from consecutive bowel movements [45–47].
Guaiac-based FOBT
Guaiac-based FOBT (gFOBT) is the most common stool blood test for CRC screening and the only CRC screening test for which there is evidence of efficacy from randomized controlled trials [11]. The usual gFOBT protocol consists of collecting 2 samples from each of 3 consecutive bowel movements at home. Prior to testing with a sensitive guaiac-based test, individuals usually will be instructed to avoid aspirin and other NSAIDs, vitamin C, red meat, poultry, fish, and some raw vegetables because of diet-test interactions that can increase the risk of both false-positive and false-negative (specifically, vitamin C) results [48]. Collection of all 3 samples is important because test sensitivity improves with each additional stool sample [41]. Three large randomized controlled trials with gFOBT have demonstrated that screened patients have cancers detected at an early and more curable stage than unscreened patients. Over time (8 to 13 years), each of the trials demonstrated significant reductions in CRC mortality of 15% to 33% [49–51]. However, the reported sensitivity of a single gFOBT varies considerably [52].
FIT
FIT has several technological advantages when compared with gFOBT. FIT detects human globin, a protein that along with heme constitutes human hemoglobin. Thus, FIT is more specific for human blood than guaiac-based tests, which rely on detection of peroxidase in human blood and also react to the peroxidase that is present in dietary constituents such as rare red meat, cruciferous vegetables, and some fruits [53]. Furthermore, unlike gFOBT, FIT is not subject to false-negative results in the presence of high-dose vitamin C supplements, which block the peroxidase reaction. In addition, because globin is degraded by digestive enzymes in the upper gastrointestinal tract, FIT is also more specific for lower gastrointestinal bleeding, thus improving the specificity for CRC. Finally, the sample collection process for patients for some variants of FIT are less demanding than gFOBT, requiring fewer samples or less direct handling of stool, which may increase FIT’s appeal. Although FIT has superior performance characteristics when compared with older guaiac-based Hemoccult II cards [54–56], the spectrum of benefits, limitations, and harms is similar to a gFOBT with high sensitivity [41]. As for adherence with FIT, there were 10% and 12% gains in adherence with FIT in the first 2 randomized controlled trials comparing FIT with guaiac-based testing [57,58]. Therefore, FIT is preferred over Hemoccult Sensa and is the preferred annual cancer detection test when colonoscopy is not an option [43]. The American College of Gastroenterology supports the joint guideline recommendation [11] that older guaiac-based fecal occult blood testing be abandoned as a method for CRC screening.
sDNA
Fecal DNA testing uses knowledge of molecular genomics and provides the basis of a new method of CRC screening that tests stool for the presence of known DNA alterations in the adenoma-carcinoma sequence of colorectal carcinogenesis [11]. Three different types of fecal DNA testing kits have been evaluated. The sensitivity for cancer in each version was superior to traditional guaiac-based occult blood testing, but the sensitivities ranged from 52%–87%, with the specificities ranging from 82%–95%. Based on the accumulation of evidence since the last update of joint guideline, the joint guideline panel concluded that there now are sufficient data to include sDNA as an acceptable option for CRC screening [11].
As for overall recommendations for stool-based testing, the ACG supports the joint guideline recommendation that older guaiac-based fecal occult blood testing be abandoned as a method for CRC screening. Because of more extensive data (compared with Hemoccult Sensa), and the high cost of fecal DNA testing, the American College of Gastroenterology recommends FIT as the preferred cancer detection test in cases where colonoscopy is not an option [43].
Invasive Tests Other than Colonoscopy
The use of flexible sigmoidoscopy for CRC screening is supported by high-quality case-control and cohort studies [46]. The chief advantage of flexible sigmoidoscopy is that it can be performed with a simple preparation (2 enemas), without sedation, and by a variety of practitioners in diverse settings. The main limitation of the procedure is that it does not examine the entire colon but only the rectum, sigmoid, and descending colon. The effectiveness of a flexible sigmoidoscopy program is based on the assumption that if an adenoma is detected during the procedure, the patient would be referred for colonoscopy to examine the entire colon.
DCBE is an imaging modality which can evaluate the entire colon in almost all cases and can detect most cancers and the majority of significant polyps. However, the lower sensitivity for significant adenomas when compared with colonoscopy may result in less favorable outcomes regarding CRC morbidity and mortality. Double-contrast barium enema is no longer recommended as an alternative CRC prevention test because its use has declined dramatically and also as its effectiveness for polyp detection is less than CT colonography [43].
CT Colonography
CT colonography every 5 years is endorsed as an alternative to colonoscopy every 10 years because of its recent performance in the American College of Imaging Network Trial 6664 (also known as the National CT Colonography Trial) [59]. The principle performance feature that justifies inclusion of CT colonography as a viable alternative in patients who decline colonoscopy is that the sensitivity for polyps ≥ 1 cm in size was 90% in the most recent multicenter US trial [59]. In this study, 25% of radiologists who were tested for entry into the trial but performed poorly were excluded from participation, and thus lower sensitivity might be expected in actual clinical practice. CT colonography probably has a lower risk of perforation than colonoscopy in most settings, but for several reasons it is not considered the equivalent of colonoscopy as a screening strategy. First, the evidence to support an effect of endoscopic screening on prevention of incident CRC and mortality is overwhelming compared with that for CT colonography. Second, the inability of CT colonography to adequately detect polyps 5 mm and smaller, which constitutes 80% of colorectal neoplasms, and whose natural history is still not understood, necessitates performance of the test at 5-year rather than 10-year intervals [43]. Finally, false-positives are common, and the specificity for polyps ≥ 1 cm in size was only 86% in the National CT Colonography Trial, with a positive predictive value of 23% [59]. The American College of Gastroenterology recommends that asymptomatic patients be informed of the possibility of radiation risk associated with one or repeated CT colonography studies, though the exact risk associated with radiation is unclear [60,61].
The value of extracolonic findings detected by CT colonography is mixed, with substantial costs associated with incidental findings, but occasional important extracolonic findings are detected, such as asymptomatic cancers and large abdominal aortic aneurysms. As a final point, the ACG is also concerned about the potential impact of CT colonography on adherence with follow-up colonoscopy and thus on polypectomy rates. Thus, if CT colonography substantially improves adherence, it should improve polypectomy rates and thereby reduce CRC, even if only large polyps are detected and referred for colonoscopy. On the other hand, if CT colonography largely displaces patients who would otherwise be willing to undergo colonoscopy, then polypectomy rates will fall substantially, which could significantly increase the CRC incidence [62]. Thus, for multiple reasons and pending additional study, CT colonography should be offered to patients who decline colonoscopy. It should be noted that CT colonography should only be offered for the purposes of CRC screening and should not be used for diagnostic workup of symptoms (eg, patient with active bleeding or inflammatory bowel disease).
When should screening begin?
The American College of Gastroenterology continues to recommend that screening begin at age 50 years in average-risk persons (ie, those without a family history of colorectal neoplasia), except for African Americans, in whom it should begin at age 45 years [43]. The USPSTF does not currently provide specific recommendations based on race or ethnicity, but certain other subgroups of the average-risk population might warrant initiation of screening at an earlier or later age, depending on their risk. For example, the incident risk of CRC has been described to be greater in men than women [63]. In reviewing the literature, the writing committee also identified heavy cigarette smoking and obesity as linked to an increased risk of CRC and to the development of CRC at an earlier age.
For patients with a family history of CRC or adenomatous polyps, the 2008 MSTF guideline recommends initiation of screening at age 40 [11]. The American College of Gastroenterology recommendations for screening in patients with a family history are shown in Table 1. From a practical perspective, many clinicians have found that patients are often not aware of whether their first-degree relatives had advanced adenomas vs. small tubular adenomas, or whether their family members had non-neoplastic vs. neoplastic polyps. Given these difficulties, the American College of Gastroenterology now recommends that adenomas only be counted as equal to a family history of cancer when there is a clear history, or medical report containing evidence, or other evidence to indicate that family members had advanced adenomas (an adenoma ≥ 1 cm in size, or with high-grade dysplasia, or with villous elements) [43]. Continuation of the old recommendation to screen first-degree relatives of patients with only small tubular adenomas could result in most of the population being screened at age 40, with doubtful benefit.
What are screening considerations in patients with genetic syndromes?
Patients with features of an inherited CRC syndrome should be advised to pursue genetic counseling with a licensed genetic counselor and, if appropriate, genetic testing. Individuals with FAP should undergo adenomatous polyposis coli (APC) mutation testing and, if negative, MYH mutation testing. Patients with FAP or at risk of FAP based upon family history should undergo annual colonoscopy until colectomy is deemed by both physician and patient as the best treatment [64]. Patients with a retained rectum after total colectomy and ileorectal anastomosis, ileal pouch, after total proctocolectomy and ileal pouch anal anastomosis, or stoma after total proctocolectomy and end ileostomy, should undergo endoscopic assessment approximately every 6 to 12 months after surgery, depending on the polyp burden seen. Individuals with oligopolyposis (< 100 colorectal polyps) should be sent for genetic counseling, consideration of APC and MYH mutation testing, and individualized colonoscopy surveillance depending on the size, number, and pathology of polyps seen. Upper endoscopic surveillance is recommended in individuals with FAP, but there are no established guidelines for endoscopic surveillance in MAP (MYH-associated polyposis) [43].
Patients who meet the Bethesda criteria for HNPCC [65] can be screened by 2 different mechanisms. One is a DNA-based test for microsatellite instability of either the patient’s or a family member’s tumor. The other mechanism is to assess by immunohistochemical staining for evidence of mismatch repair proteins (eg, MLH1, MSH2, MSH6). In those patients in whom deleterious mutations are found, the affected individual should undergo colonoscopy every 2 years beginning at age 20 to 25 years until age 40 years, then annually thereafter [43]. If genetic testing is negative (ie, no deleterious mutation is found), but the patient is still felt to clinically have Lynch syndrome, then they should still be surveyed in the same way.
Case Continued
The physician recommends colonoscopy as the screening modality as it is the most efficient and accurate way of finding precancerous lesions and the most effective way of preventing CRC by removing precancerous lesions. He also explains that because the patient’s father developed CRC after the age of 60, this does not place the patient in a higher risk category and he can follow screening recommendations for “average-risk” individuals.
Screening
The patient undergoes colonoscopy. Two 5-mm adenomas in the transverse colon are detected and removed.
When should he have a repeat colonoscopy?
Surveillance Intervals
New data have recently emerged on the risk of interval cancer after colonoscopy. The overall rate of interval cancer is estimated to be 1.1–2.7 per 1000 person-years of follow-up. There are several reasons that may account for why patients develop interval cancers: (1) important lesions may be missed at baseline colonoscopy, (2) adenomas may be incompletely removed at the time of baseline colonoscopy, and (3) interval CRC may be biologically different or more aggressive than prevalent CRC. In order to minimize the risk of interval cancer development, it is important to perform a high-quality baseline screening colonoscopy examination as this is associated with lowering the risk of interval cancer [66]. A high-quality colonoscopy entails completion of the procedure to the cecum (with photodocumentation of the appendiceal orifice and ileocecal valve) with careful inspection of folds including adequate bowel cleanliness and a withdrawal time > 6 minutes.
Baseline Colonoscopy Findings
No Polyps
Several prospective observational studies in different populations have shown that the risk of advanced adenomas within 5 years after negative findings on colonoscopy is low (1.3%–2.4%) relative to the rate on initial screening examination (4%–10%) [68–73]. In these studies, interval cancers were rare within 5 years. A sigmoidoscopy randomized controlled trial performed in the United Kingdom demonstrated a reduction in CRC incidence and mortality at 10 years in patients who received one-time sigmoidoscopy compared with controls—a benefit limited to the distal colon [46]. This is the first randomized study to show the effectiveness of endoscopic screening, an effect that appears to have at least a 10-year duration [74]. Thus, in patients who have a baseline colonoscopic evaluation without any adenomas or polyps and are average-risk individuals, the recommendation for the next examination is in 10 years [66].
Distal Hyperplastic Polyps < 10 mm
There is considerable evidence that patients with only rectal or sigmoid hyperplastic polyps (HPs) appear to represent a low-risk cohort. Studies have focused on whether the finding in the distal colon was a marker of risk for advanced neoplasia elsewhere and most studies show no such relationship [67]. Prior and current evidence suggests that distal HPs <10 mm are benign without neoplastic potential. If the most advanced lesions at baseline colonoscopy are distal HPs <10 mm, the interval for colonoscopic follow-up should be 10 years [66].
1-2 Tubular Adenomas < 10 mm
Prior evidence suggested that patients with low-risk adenomas (<10 mm, no villous histology or high-grade dysplasia) had a lower risk of developing advanced adenomas during follow-up compared with patients with high risk adenomas (≥ 10mm, villous histology or high -grade dysplasia). At that time in 2006, consensus on the task force was that an interval of 5 years would be acceptable in this low-risk group [75]. Data published since 2006 endorse the assessment that patients with 1–2 tubular adenomas with low-grade dysplasia <10 mm represent a low-risk group. Three new studies suggest that this group may have only a small, nonsignificant increase in risk of advanced neoplasia within 5 years compared with individuals with no baseline neoplasia. The evidence now supports a surveillance interval of longer than 5 years for most patients and can be extended to 10 years based on the quality of the preparation and colonoscopy [66].
3–10 Tubular Adenomas
Two independent meta-analyses in 2006 found that patients with 3 or more adenomas at baseline had an increased RR for adenomas during surveillance, ranging from 1.7 to 4.8 [47,75]. New information from the VA study and the National Cancer Institute Pooling Project also support these prior findings. Patients with 3 or more adenomas have a level of risk for advanced neoplasia similar to other patients with advanced neoplasia (adenoma >10 mm, adenoma with high grade dysplasia) and thus, repeat examination should be performed in 3 years [66,68,76].
> 10 Adenomas
Only a small proportion of patients undergoing screening colonoscopy will have >10 adenomas. The 2006 guidelines for colonoscopy surveillance after polypectomy noted that such patients should be considered for evaluation of hereditary CRC syndromes [67]. Early follow-up surveillance colonoscopy is based on clinical judgment because there is little evidence to support a firm recommendation. At present, the recommendation is to consider follow-up in less than 3 years after a baseline colonoscopy [66].
1 or More Tubular Adenomas ≥ 10mm
The 2006 MSTF guideline reviewed data related to adenoma size, demonstrating that most studies showed a 2- to 5-fold increased risk of advanced neoplasia during follow-up if the baseline examination had one or more adenomas ≥ 10 mm [67]. Newer, additional data shows that patients with one or more adenomas ≥ 10 mm have an increased risk of advanced neoplasia during surveillance compared with those with no neoplasia or small (< 10 mm) adenomas [68,76]. Thus, the recommendations remains that repeat examination should be performed in 3 years [66]. If there is question about complete removal of an adenoma (ie, piecemeal resection), early follow-up colonoscopy is warranted [66].
1 or More Villous Adenomas
The 2006 MSTF guideline considers adenomas with villous histology to be high risk [67]. The NCI Pooling Project analyzed polyp histology as a risk factor for development of interval advanced neoplasia. Compared with patients with tubular adenomas, those with baseline polyp(s) showing adenomas with villous or tubulovillous histology (TVA) had increased risk of advanced neoplasia during follow-up (16.8% vs 9.7%; adjusted OR, 1.28; 95% CI, 1.07–1.52) [76]. Patients with one or more adenomas with villous histology were also found to have an increased risk of advanced neoplasia during surveillance compared with those with no neoplasia or small (<10 mm) tubular adenomas. Thus, the recommendation remains that repeat examination should be performed in 3 years [66].
Adenoma with High-Grade Dysplasia (HGD)
The 2006 MSTF guideline concluded that the presence of HGD in an adenoma was associated with both villous histology and larger size, which are both risk factors for advanced neoplasia during surveillance [67]. In a univariate analysis from the NCI Pooling Project, HGD was strongly associated with risk of advanced neoplasia during surveillance (OR, 1.77; 95% CI, 1.41–2.22) [76]. Thus, the recommendation remains that repeat examination should be performed in 3 years [66].
Serrated Lesions
A total of 20% to 30% of CRCs arise through a molecular pathway characterized by hypermethylation of genes, known as CgG Island Methylator Phenotype (CIMP) [77]. Precursors are believed to be serrated polyps. Tumors in this pathway have a high frequency of BRAF mutation, and up to 50% are microsatellite unstable. CIMP-positive tumors are overrepresented in interval cancers, particularly in the proximal colon. The principal precursor of hypermethylated cancers is probably the sessile serrated polyp (synonymous with sessile serrated adenoma). These polyps are difficult to detect at endoscopy. They may be the same color as surrounding colonic mucosa, have indiscrete edges, are nearly always flat or sessile, and may have a layer of adherent mucus and obscure the vascular pattern.
Recent studies show that proximal colon location or size ≥ 10 mm may be markers of risk for synchronous advanced adenomas elsewhere in the colon [78,79]. Surveillance after colonoscopy was evaluated in one study, which found that coexisting serrated polyps and high-risk adenomas (HRA; ie, size ≥ 10 mm, villous histology, or presence of HGD) is associated with a higher risk of advanced neoplasia at surveillance [78]. This study also found that if small proximal serrated polyps are the only finding at baseline, the risk of adenomas during surveillance is similar to that of patients with low-risk adenomas (LRA; ie, 1–2 small adenomas).
The current evidence suggests that size (>10 mm), histology (a sessile serrated polyp is a more significant lesion than an HP; a sessile serrated polyp with cytological dysplasia is more advanced than a sessile serrated polyp without dysplasia), and location (proximal to the sigmoid colon) are risk factors that might be associated with higher risk of CRC. A sessile serrated polyp ≥ 10 mm and a sessile serrated polyp with cytological dysplasia should be managed like a HRA with repeat colonoscopy occurring in 3 years. Serrated polyps that are <10 mm in size and do not have cytological dysplasia may have lower risk and can be managed like LRA with repeat colonoscopy occurring in 5 years [66].
Follow-up After Surveillance
In a 2009 study, 564 participants underwent 2 surveillance colonoscopies after an index procedure and 10.3% had high-risk findings at the third study examination. If the second examination showed high-risk findings, then results from the first examination added no significant information about the probability of high-risk findings on the third examination (18.2% for high-risk findings on the first examination vs. 20.0% for low-risk findings on the first examination; P = 0.78). If the second examination showed no adenomas, then the results from the first examination added significant information about the probability of high-risk findings on the third exam-ination (12.3% if the first examination had high-risk findings vs. 4.9% if the first examination had low-risk findings; P = 0.015) [80]. Thus, information from 2 previous colonoscopies appears to be helpful in defining the risk of neoplasia for individual patients and in the future, guidelines might consider accounting for the results of 2 exams to tailor surveillance intervals for patients.
When should screening / surveillance be stopped?
There is considerable new evidence that the risks of colonoscopy increase with advancing age [81,82]. Neither surveillance nor screening colonoscopy should be performed when the risk of the preparation, sedation, or procedure outweighs the potential benefit. For patients aged 75–85 years, the USPSTF recommends against routine screening but argues for individualization based on comorbidities and findings on any prior colonoscopy. The USPSTF recommends against continued screening after age 85 years because risk could exceed potential benefit [44].
In terms of surveillance of prior adenomas, the 75-85 year age group may still benefit from surveillance because patients with prior HRA are at higher risk for developing advanced neoplasia compared with average-risk screenees. However, the decision to continue surveillance in this population should be individualized and based on an assessment of benefit and risk in the context of the person’s estimated life expectancy [66]. More importantly, it should be noted that an individual’s most important and impactful screening colonoscopy is his or her first one and therefore, from a public health standpoint, great effort should be taken to increase the number of people in a population who undergo screening rather than simply targeting those who need surveillance for prior polyps. This is ever true in settings with limited resources.
Case Conclusion
The physician discusses the findings from the colonoscopy (2 small adenomas) with the patient and recommends a repeat colonoscopy in 5 to 10 years.
Summary
Colorectal cancer is one of the leading causes of cancer-related death in the United States. Since the advent of colonoscopy and the implementation of CRC screening efforts, the rates of CRC have started to decline. There are several environmental factors which have been associated with the development of CRC including obesity, dietary intake, physical activity and smoking. At present, there are multiple tools available for CRC prevention, but the most accurate and effective method is currently colonoscopy. Stool-based tests like FIT should be offered when a patient declines colonoscopy. For those interested in colonoscopy, average-risk individuals should be screened starting at the age of 50 with subsequent examinations every 10 years. Surveillance examinations should occur based on polyp findings on index colonoscopy. There is no recommendation to continue screening after the age of 75, though physicians can determine this based on patients health and risk/benefit profile. Current guidelines recommend against offering any screening to patients over the age of 85. Despite these recommendations, almost half of the eligible screening population has yet to undergo appropriate CRC screening. Future work should include public health efforts to improve access and appeal of widespread CRC screening regardless of modality. While colonoscopy is considered the most effective screening test, the best test is still the one the patient gets.
Corresponding author: Audrey H. Calderwood, MD, MS, 85 E. Concord St., Rm. 7724, Boston, MA 02118, [email protected].
Financial disclosures: None.
1. American Cancer Society. Colorectal cancer facts & figures 2014–2016. Atlanta: American Cancer Society; 2014.
2. Ries L, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014.
3. Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med 2006;355:2551–7.
4. Bond JH. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 2000;95:3053–63.
5. Schatzkin A, Freedman LS, Dawsey SM, Lanza E. Interpreting precursor studies: what polyp trials tell us about large-bowel cancer. J Natl Cancer Inst 1994;86:1053–7.
6. DevCan: Probability of developing or dying of cancer software, version 6.5.0; Statistical Research and Applications Branch, National Cancer Institute, 2005. http://srab.cancer.gov/devcan [computer program].
7. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2010, based on the November 2009 submission.
8. Murphy G, Devesa SS, Cross AJ, et al. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer 2011;128:1668–7.
9. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544–73.
10. Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975-2002). Cancer Epidemiol Biomarkers Prev 2006;15:792–7.
11. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58:130–60.
12. Kirkegaard H, Johnsen NF, Christensen J, et al. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. BMJ 2010;341:c5504.
13. Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer 2006;42:216–27.
14. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol 2001;96:2992–3003.
15. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919–32.
16. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology 2010;138:2044–58.
17. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology 2009;137:1621–7.
18. Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001;91:854–62.
19. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35.
20. Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005;97:1679–87.
21. Campbell PT, Deka A, Jacobs EJ, et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology 2010;139:1138–46.
22. Larsson SC, Giovannucci E, Wolk A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care 2005;28:1805–7.
23. Huxley RR, Ansary-Moghaddam A, Clifton P, et al. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer 2009;125:171–80.
24. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556–65.
25. Wang Y, Jacobs EJ, Patel AV, et al. A prospective study of waist circumference and body mass index in relation to colorectal cancer incidence. Cancer Causes Control 2008;19:783–92.
26. Chao A, Thun MJ, Connell CJ, et al. Meat consumption and risk of colorectal cancer. JAMA 2005;293:172–82.
27. Cross AJ, Ferrucci LM, Risch A, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res 2010;70:2406–14.
28. Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology 2010;138:2029–43.
29. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: World Cancer Research Fund/American Institute for Cancer Research; 2007.
30. McCullough ML, Robertson AS, Chao A, et al. A prospective study of whole grains, fruits, vegetables and colon cancer risk. Cancer Causes Control 2003;14:959–70.
31. Terry P, Giovannucci E, Michels KB, et al. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst 2001;93:525–33.
32. Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst 2004;96:1015–22.
33. Secretan B, Straif K, Baan R, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009;10:1033–4.
34. Ferrari P, Jenab M, Norat T, et al. Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 2007;121:2065–72.
35. Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis 2005;7:204–13.
36. Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 2007;369:1603–13.
37. Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741–50.
38. Hildebrand JS, Jacobs EJ, Campbell PT, et al. Colorectal cancer incidence and postmenopausal hormone use by type, recency, and duration in cancer prevention study II. Cancer Epidemiol Biomarkers Prev 2009;18:2835–41.
39. Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA 2008;299:1036–45.
40. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–33.
41. Lieberman DA,Weiss DG. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med 2001;345:555–60.
42. Lafata JE, Divine G, Moon C,Williams LK. Patient-physician colorectal cancer screening discussions and screening use. Am J Prev Med 2006;31:202–9.
43. Rex DK, Johnson DA, Andersone JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol 2009;104:739–50.
44. U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149:627–37.
45. Smith RA, von Eschenbach AC,Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001—testing for early lung cancer detection. CA Cancer J Clin 2001;51:38–75.
46. Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology 2003;124:544–60.
47. Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2006;130:1865–71.
48. Ransohoff DF, Lang CA. Screening for colorectal cancer with the fecal occult blood test: a background paper. American College of Physicians. Ann Intern Med 1997;126:811–22.
49. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult blood screening for colorectal cancer. Lancet 1996;348:1472–7.
50. Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult blood test. Lancet 1996;348:1467–71.
51. Wilson JMG, Junger G. Principles and practice of screening for disease. Geneva: World Health Organization; 1968.
52. Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 1996;334:155–9.
53. Caligiore P, Macrae FA, St John DJ, et al. Peroxidase levels in food: relevance to colorectal cancer screening. Am J Clin Nutr 1982;35:1487–9.
54. Nakajima M, Saito H, Soma Y, et al. Prevention of advanced colorectal cancer by screening using the immunochemical faecal occult blood test: a case-control study. Br J Cancer 2003;89:23–8.
55. Lee KJ, Inoue M, Otani T, et al. Colorectal cancer screening using fecal occult blood test and subsequent risk of colorectal cancer: a prospective cohort study in Japan. Cancer Detect Prev 2007;31:3–11.
56. Zappa M, Csatiglione G, Gazzini G, et al. Effect of faecal occult blood testing on colorectal mortality: results of a population-based case-control study in the district of Florence, Italy. Int J Cancer 1997;73:208–10.
57. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008;135:82–90.
58. Hol L, van Leerdam ME, van Ballegooijen M, et al. Attendance to screening for colorectal cancer in the Netherlands; randomized controlled trial comparing two different forms of faecal occult blood tests and sigmoidoscopy. Gastroenterology 2008;134:A87.
59. Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers.
N Engl J Med 2008;359:1207–17.
60. Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology 2005;129:328–37.
61. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med 2007;35: 2277–84.
62. Hur C, Chung DC, Schoen RE, et al. The management of small polyps found by virtual colonoscopy: results of a decision analysis. Clin Gastroenterol Hepatol 2007;5:237–44.
63. Chu KC, Tarone RE, Chow WH, et al. Temporal patterns in colorectal cancer incidence, survival, and mortality from 1950 through 1990. J Natl Cancer Inst 1994;86:997–1006.
64. Vasen HF, Moslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008;57:704–13.
65. Umar A, Boland CR, Terdiman PJ, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal Cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261–8.
66. Lieberman DA, Rex DR, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–57.
67. Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on colorectal cancer and the American Cancer Society. Gastroenterology 2006;130:1872–85.
68. Lieberman DA, Weiss DG, Harford WV, et al. Five year colon surveillance after screening colonoscopy. Gastroenterology 2007;133:1077–85.
69. Imperiale TF, Glowinski EA, Lin-Cooper C, et al. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med 2008;359:1218–24.
70. Leung WK, Lau JYW, Suen BY, et al. Repeat screening colonoscopy 5 years after normal baseline screening colonoscopy in average-risk Chinese: a prospective study. Am J Gastroenterol 2009;104:2028–34.
71. Brenner H, Haug U, Arndt V, et al. Low risk of colorectal cancer and advanced adenomas more than 10 years after negative colonoscopy. Gastroenterology 2010;138:870–6.
72. Miller H, Mukherjee R, Tian J, et al. Colonoscopy surveillance after polypectomy may be extended beyond five years. J Clin Gastroenterol 2010;44:e162–e166.
73. Chung SJ, Kim YS, Yang SY, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut 2011;60:1537–43.
74. Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33.
75. Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc 2006;64:614–26.
76. Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses following colonoscopic polypectomy. Gastroenterology 2009;136:832–41.
77. Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010;138:
2088–100.
78. Schreiner MA, Weiss DG, Lieberman DA. Proximal and large nonneoplastic serrated polyps: association with synchronous neoplasia at screening colonoscopy and with interval neoplasia at follow- up colonoscopy. Gastroenterology 2010;139:1497–502.
79. Hiraoka S, Kato J, Fujiki S, et al. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology 2010;139:1503–10.
80. Robertson DJ, Burke CA, Welch HG, et al. Using the results of a baseline and a surveillance colonoscopy to predict recurrent adenomas with high-risk characteristics. Ann Intern Med 2009;151:103–9.
81. Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med 2009;150:849–57.
82. Ko CW, Riffle S, Michaels L, et al. Serious complications within 30 days of screening and surveillance colonoscopy: a multicenter study. Clin Gastroenterol Hepatol 2010;8:166–73.
From the Boston University School of Medicine, Boston, MA.
Abstract
- Objective: To review recommendations for colorectal cancer (CRC) screening.
- Methods: Review of the literature.
- Results: In the United States, CRC is the third most commonly diagnosed cancer and the third leading cause of cancer death. CRC screening can reduce mortality through the detection of early-stage disease and the detection and removal of adenomatous polyps. There are several modalities for CRC screening, with current technology falling into 2 general categories: stool tests, which include tests for occult blood or exfoliated DNA; and structural exams, which include flexible sigmoidoscopy, colonoscopy, double-contrast barium enema, and CT colonography. The preferred CRC prevention test for average-risk individuals is colonoscopy starting at age 50 with subsequent examinations every 10 years. Patients unwilling to undergo screening colonoscopy may be offered flexible sigmoidoscopy, CT colonography, or fecal immunohistochemical test. Surveillance examinations should occur based on polyp findings on index colonoscopy. There is no recommendation to continue screening after age 75, though physicians can make a determination based on a patient’s health and risk/benefit profile. Current guidelines recommend against offering screening to patients over age 85.
- Conclusion: Increasing access to and utilization of CRC screening tests is likely to lead to improvements in mortality reduction, as only about half of people aged 50 or older report having received CRC testing consistent with current guidelines.
In the United States, colorectal cancer (CRC) is the third most commonly diagnosed cancer and the third leading cause of cancer death in both men and women [1]. In 2014, an estimated 136,830 people were diagnosed with CRC and about 50,310 people died of the disease [2]. Colorectal cancer usually develops slowly over a period of 10 to 15 years. The tumor typically begins as a noncancerous polyp, classically an adenomatous polyp or adenoma, though fewer than 10% of adenomas will progress to cancer [3]. Adenomas are common; an estimated one-third to one-half of all individuals will eventually develop 1 or more adenomas [4,5]. In the United States, the lifetime risk of being diagnosed with CRC is approximately 5% for both men and women [6]. Incidence rates for CRC increase with age, with an incidence rate more than 15 times higher in adults aged 50 years and older compared with those aged 20 to 49 years [7].
Certain demographic subgroups have been shown to be at higher risk. Overall, CRC incidence and mortality rates are about 35% to 40% higher in men than in women. The reasons for this are not completely understood but likely reflect complex interactions between gender-related differences in exposure to hormones and risk factors [8]. CRC incidence and mortality rates are highest in African-American men and women; incidence rates are 20% higher and mortality rates are about 45% higher than those in whites. Prior to 1989, incidence rates were predominantly higher in white men than in African American men and were similar for women of both races. Since that time, although incidence rates have declined as a whole [9], incidence rates have been higher for African Americans than whites in both men and women This crossover likely reflects a combination of greater access to and utilization of recommended screening tests among whites (resulting in detection and removal of precancerous polyps), as well as racial differences in trends for CRC risk factors [10].
CRC screening can reduce mortality through the detection of early-stage disease and the detection and removal of ademomatous polyps [11]. Increasing access to and utilization of CRC screening tests is likely to lead to improvements in mortality reduction, as only about half of people aged 50 or older report having received CRC testing consistent with current guidelines [1].
Case Study
Initial Presentation
A 55-year-old white male presents for a routine visit and asks about colon cancer screening. His father was diagnosed with colon cancer at the age of 78. Overall, he feels well and does not have any particular complaints. His bowel habits are normal and he denies melena and hematochezia. His past medical history is significant for diabetes, hypertension, and obesity. He was a previous smoker and has a few alcoholic drinks on the weekends. His physical exam is unremarkable. Results of recent blood work are normal and there is no evidence of anemia.
What are this patient’s risk factors for developing colon cancer?
Risk Factors for CRC
There are numerous factors that are thought to influence risk for CRC. Nonmodifiable risk factors include a personal or family history of CRC or adenomatous polyps, and a personal history of chronic inflammatory bowel disease. Modifiable risk factors that have been associated with an increased risk of CRC in epidemiologic studies include physical inactivity, obesity, high consumption of red or processed meats, smoking, and moderate-to-heavy alcohol consumption. In fact, a prospective study showed that up to 23% of colorectal cancers were considered to be potentially avoidable by adhering to multiple healthy lifestyle recommendations including maintaining a healthy weight, being physically active at least 30 minutes per day, eating a healthy diet, and avoiding smoking and drinking excessive amounts of alcohol [12].
People with a first-degree relative (parent, sibling, or offspring) who has had CRC have 2 to 3 times the risk of developing the disease compared with individuals with no family history; if the relative was diagnosed at a young age or if there is more than 1 affected relative, risk increases to 3 to 6 times that of the general population [13,14]. About 5% of patients with CRC have a well-defined genetic syndrome that causes the disease [15]. The most common of these is Lynch syndrome (also known as hereditary nonpolyposis colorectal cancer or HNPCC), which accounts for 2% to 4% of all CRC cases [16]. Although individuals with Lynch syndrome are predisposed to numerous types of cancer, risk of CRC is highest. A recent study of CRC in 147 Lynch syndrome families in the United States found lifetime risk of CRC to be 66% in men and 43% in women, with a median age at diagnosis of 42 years and 47 years, respectively [17]. Familial adenomatous polyposis (FAP) is the second most common predisposing genetic syndrome; for these individuals, the lifetime risk of CRC approaches 100% without intervention (eg, colectomy) [16].
People who have inflammatory bowel disease of the colon (both ulcerative colitis and Crohn’s disease) have an increased risk of developing CRC that correlates with the extent and the duration of the inflammation [18]. It is estimated that 18% of patients with a 30-year history of ulcerative colitis will develop CRC [19]. In addition, several studies have found an association between diabetes and increased risk of CRC [20,21]. Though adult-onset type 2 diabetes (the most common type) and CRC share similar risk factors, including physical inactivity and obesity, a positive association between diabetes and CRC has been found even after accounting for physical activity, body mass index, and waist circumference [22].
Being overweight or obese is also associated with a higher risk of CRC, with stronger associations more consistently observed in men than in women. Obesity increases the risk of CRC independent of physical activity. Abdominal obesity (measured by waist circumference) may be a more important risk factor for colon cancer than overall obesity in both men and women [23–25]. Diet and lifestyle strongly influence CRC risk; however, research on the role of specific dietary elements on CRC risk is still accumulating. Several studies, including one by the American Cancer Society, have found that high consumption of red and/or processed meat increases the risk of both colon and rectal cancer [23,26,27]. Further analyses indicate that the association between CRC and red meat may be related to the cooking process, because a higher risk of CRC is observed particularly among those individuals who consume meat that has been cooked at a high temperature for a long period of time [28]. In contrast to findings from earlier research, more recent large, prospective studies do not indicate a major relationship between CRC and vegetable, fruit, or fiber consumption [28,29]. However, some studies suggest that people with very low fruit and vegetable intake are at above-average risk for CRC [30,31]. Consumption of milk and calcium may decrease the risk of developing CRC [28,29,32].
In November 2009, the International Agency for Research on Cancer reported that there is now sufficient evidence to conclude that tobacco smoking causes CRC [33]. Colorectal cancer has been linked to even moderate alcohol use. Individuals who have a lifetime average of 2 to 4 alcoholic drinks per day have a 23% higher risk of CRC than those who consume less than 1 drink per day [34].
Protective Factors
One of the most consistently reported relationships between colon cancer risk and behavior is the protective effect of physical activity [35]. Based on these findings, as well as the numerous other health benefits of regular physical activity, the American Cancer Society recommends engaging in at least moderate activity for 30 minutes or more on 5 or more days per week.
Accumulating research suggests that aspirin-like drugs, postmenopausal hormones, and calcium supplements may help prevent CRC. Extensive evidence suggests that long-term, regular use of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) is asso-ciated with lower risk of CRC. The American Cancer Society does not currently recommend use of these drugs as chemoprevention because of the potential side effects of gastrointestinal bleeding from aspirin and other traditional NSAIDs and heart attacks from selective cyclooxygenase-2 (COX-2) inhibitors. However, people who are already taking NSAIDs for chronic arthritis or aspirin for heart disease prevention may have a lower risk of CRC as a positive side effect [36,37].
There is substantial evidence that women who use postmenopausal hormones have lower rates of CRC than those who do not. A decreased risk of CRC is especially evident in women who use hormones long-term, although the risk returns to that of nonusers within 3 years of cessation. Despite its positive effect on CRC risk, the use of postmenopausal hormones increases the risk of breast and other cancers as well as cardiovascular disease, and therefore it is not recommended for the prevention of CRC. At present, the American Cancer Society does not recommend any medications or supplements to prevent CRC because of uncertainties about their effectiveness, appropriate dosing, and potential toxicity [38–40].
Case Continued
The physician tells the patient that there are several environmental factors that may predispose him to developing CRC. He recommends that the patient follow a healthy lifestyle, including eating 5 servings of fruits and vegetables daily, minimizing consumption of red meats, exercising for 30 minutes at least 5 days per week, drinking only moderate amounts of alcohol, and continuing to take his aspirin in the setting of his diabetes. He also asks the patient if he would be interested in talking about weight loss and working together to make a plan.
The patient is appreciative of this information and wants to know what CRC creening test the physician recommends.
What screening test should be recommended?
Screening Options
There are several modalities for CRC screening, with current technology falling into 2 general categories: stool tests, which include tests for occult blood or exfoliated DNA; and structural exams, which include flexible sigmoidoscopy, colonoscopy, double-contrast barium enema (DCBE), and computed tomographic (CT) colonography. Stool tests are best suited for the detection of CRC, although they also will deliver positive findings for some advanced adenomas, while the structural exams can achieve both detection and prevention of CRC through identification and removal of adenomatous polyps [41]. These tests may be used alone or in combination to improve sensitivity or, in some instances, to ensure a complete examination of the colon if the initial test cannot be completed.
In principle, all adults should have access to the full range of options for CRC screening, and the availability of lower-cost, less invasive options in most practice settings is a public health advantage [11]. However, the availability of multiple testing options can overwhelm the primary care provider and presents challenges for practices in trying to support an office policy that can manage a broad range of testing choices, their follow-up requirements, and shared decision making related to the options. Shared decision making around CRC screening options is both demanding and time consuming and is complicated by the different characteristics of the tests and the test-specific requirements for individuals undergoing screening [42].
Recommended Tests
The joint guideline on screening for CRC from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology (the MSTF guideline) [11] is of the strong opinion that tests designed to detect early cancer and prevent cancer through the detection and removal of adenomatous polyps (the structural exams) should be encouraged if resources are available and patients are willing to undergo an invasive test [11]. In clinical settings in which economic issues preclude primary screening with colonoscopy, or for patients who decline invasive tests, clinicians may offer stool- based testing. However, providers and patients should understand that these tests are less likely to prevent cancer compared with the invasive tests, they must be repeated at regular intervals to be effective (ie, programmatic sensitivity), and if the test is abnormal, a colonoscopy will be needed to follow up. Therefore, if patients are not willing to have repeated testing or pursue colonoscopy if the test is abnormal, these programs will not be effective and should not be recommended [11].
Stool-Based Testing
Stool blood tests are conventionally known as fecal occult blood tests (FOBT) because they are designed to detect the presence of occult blood in stool. FOBT falls into 2 primary categories based on the detected analyte: guaiac-based and FIT. Blood in the stool is a nonspecific finding but may originate from CRC or larger (> 1 to 2 cm) polyps. Because small adenomatous polyps do not tend to bleed and bleeding from cancers or large polyps may be intermittent or undetectable in a single sample of stool, the proper use of stool blood tests requires annual testing that consists of collecting specimens (2 or 3, depending on the product) from consecutive bowel movements [45–47].
Guaiac-based FOBT
Guaiac-based FOBT (gFOBT) is the most common stool blood test for CRC screening and the only CRC screening test for which there is evidence of efficacy from randomized controlled trials [11]. The usual gFOBT protocol consists of collecting 2 samples from each of 3 consecutive bowel movements at home. Prior to testing with a sensitive guaiac-based test, individuals usually will be instructed to avoid aspirin and other NSAIDs, vitamin C, red meat, poultry, fish, and some raw vegetables because of diet-test interactions that can increase the risk of both false-positive and false-negative (specifically, vitamin C) results [48]. Collection of all 3 samples is important because test sensitivity improves with each additional stool sample [41]. Three large randomized controlled trials with gFOBT have demonstrated that screened patients have cancers detected at an early and more curable stage than unscreened patients. Over time (8 to 13 years), each of the trials demonstrated significant reductions in CRC mortality of 15% to 33% [49–51]. However, the reported sensitivity of a single gFOBT varies considerably [52].
FIT
FIT has several technological advantages when compared with gFOBT. FIT detects human globin, a protein that along with heme constitutes human hemoglobin. Thus, FIT is more specific for human blood than guaiac-based tests, which rely on detection of peroxidase in human blood and also react to the peroxidase that is present in dietary constituents such as rare red meat, cruciferous vegetables, and some fruits [53]. Furthermore, unlike gFOBT, FIT is not subject to false-negative results in the presence of high-dose vitamin C supplements, which block the peroxidase reaction. In addition, because globin is degraded by digestive enzymes in the upper gastrointestinal tract, FIT is also more specific for lower gastrointestinal bleeding, thus improving the specificity for CRC. Finally, the sample collection process for patients for some variants of FIT are less demanding than gFOBT, requiring fewer samples or less direct handling of stool, which may increase FIT’s appeal. Although FIT has superior performance characteristics when compared with older guaiac-based Hemoccult II cards [54–56], the spectrum of benefits, limitations, and harms is similar to a gFOBT with high sensitivity [41]. As for adherence with FIT, there were 10% and 12% gains in adherence with FIT in the first 2 randomized controlled trials comparing FIT with guaiac-based testing [57,58]. Therefore, FIT is preferred over Hemoccult Sensa and is the preferred annual cancer detection test when colonoscopy is not an option [43]. The American College of Gastroenterology supports the joint guideline recommendation [11] that older guaiac-based fecal occult blood testing be abandoned as a method for CRC screening.
sDNA
Fecal DNA testing uses knowledge of molecular genomics and provides the basis of a new method of CRC screening that tests stool for the presence of known DNA alterations in the adenoma-carcinoma sequence of colorectal carcinogenesis [11]. Three different types of fecal DNA testing kits have been evaluated. The sensitivity for cancer in each version was superior to traditional guaiac-based occult blood testing, but the sensitivities ranged from 52%–87%, with the specificities ranging from 82%–95%. Based on the accumulation of evidence since the last update of joint guideline, the joint guideline panel concluded that there now are sufficient data to include sDNA as an acceptable option for CRC screening [11].
As for overall recommendations for stool-based testing, the ACG supports the joint guideline recommendation that older guaiac-based fecal occult blood testing be abandoned as a method for CRC screening. Because of more extensive data (compared with Hemoccult Sensa), and the high cost of fecal DNA testing, the American College of Gastroenterology recommends FIT as the preferred cancer detection test in cases where colonoscopy is not an option [43].
Invasive Tests Other than Colonoscopy
The use of flexible sigmoidoscopy for CRC screening is supported by high-quality case-control and cohort studies [46]. The chief advantage of flexible sigmoidoscopy is that it can be performed with a simple preparation (2 enemas), without sedation, and by a variety of practitioners in diverse settings. The main limitation of the procedure is that it does not examine the entire colon but only the rectum, sigmoid, and descending colon. The effectiveness of a flexible sigmoidoscopy program is based on the assumption that if an adenoma is detected during the procedure, the patient would be referred for colonoscopy to examine the entire colon.
DCBE is an imaging modality which can evaluate the entire colon in almost all cases and can detect most cancers and the majority of significant polyps. However, the lower sensitivity for significant adenomas when compared with colonoscopy may result in less favorable outcomes regarding CRC morbidity and mortality. Double-contrast barium enema is no longer recommended as an alternative CRC prevention test because its use has declined dramatically and also as its effectiveness for polyp detection is less than CT colonography [43].
CT Colonography
CT colonography every 5 years is endorsed as an alternative to colonoscopy every 10 years because of its recent performance in the American College of Imaging Network Trial 6664 (also known as the National CT Colonography Trial) [59]. The principle performance feature that justifies inclusion of CT colonography as a viable alternative in patients who decline colonoscopy is that the sensitivity for polyps ≥ 1 cm in size was 90% in the most recent multicenter US trial [59]. In this study, 25% of radiologists who were tested for entry into the trial but performed poorly were excluded from participation, and thus lower sensitivity might be expected in actual clinical practice. CT colonography probably has a lower risk of perforation than colonoscopy in most settings, but for several reasons it is not considered the equivalent of colonoscopy as a screening strategy. First, the evidence to support an effect of endoscopic screening on prevention of incident CRC and mortality is overwhelming compared with that for CT colonography. Second, the inability of CT colonography to adequately detect polyps 5 mm and smaller, which constitutes 80% of colorectal neoplasms, and whose natural history is still not understood, necessitates performance of the test at 5-year rather than 10-year intervals [43]. Finally, false-positives are common, and the specificity for polyps ≥ 1 cm in size was only 86% in the National CT Colonography Trial, with a positive predictive value of 23% [59]. The American College of Gastroenterology recommends that asymptomatic patients be informed of the possibility of radiation risk associated with one or repeated CT colonography studies, though the exact risk associated with radiation is unclear [60,61].
The value of extracolonic findings detected by CT colonography is mixed, with substantial costs associated with incidental findings, but occasional important extracolonic findings are detected, such as asymptomatic cancers and large abdominal aortic aneurysms. As a final point, the ACG is also concerned about the potential impact of CT colonography on adherence with follow-up colonoscopy and thus on polypectomy rates. Thus, if CT colonography substantially improves adherence, it should improve polypectomy rates and thereby reduce CRC, even if only large polyps are detected and referred for colonoscopy. On the other hand, if CT colonography largely displaces patients who would otherwise be willing to undergo colonoscopy, then polypectomy rates will fall substantially, which could significantly increase the CRC incidence [62]. Thus, for multiple reasons and pending additional study, CT colonography should be offered to patients who decline colonoscopy. It should be noted that CT colonography should only be offered for the purposes of CRC screening and should not be used for diagnostic workup of symptoms (eg, patient with active bleeding or inflammatory bowel disease).
When should screening begin?
The American College of Gastroenterology continues to recommend that screening begin at age 50 years in average-risk persons (ie, those without a family history of colorectal neoplasia), except for African Americans, in whom it should begin at age 45 years [43]. The USPSTF does not currently provide specific recommendations based on race or ethnicity, but certain other subgroups of the average-risk population might warrant initiation of screening at an earlier or later age, depending on their risk. For example, the incident risk of CRC has been described to be greater in men than women [63]. In reviewing the literature, the writing committee also identified heavy cigarette smoking and obesity as linked to an increased risk of CRC and to the development of CRC at an earlier age.
For patients with a family history of CRC or adenomatous polyps, the 2008 MSTF guideline recommends initiation of screening at age 40 [11]. The American College of Gastroenterology recommendations for screening in patients with a family history are shown in Table 1. From a practical perspective, many clinicians have found that patients are often not aware of whether their first-degree relatives had advanced adenomas vs. small tubular adenomas, or whether their family members had non-neoplastic vs. neoplastic polyps. Given these difficulties, the American College of Gastroenterology now recommends that adenomas only be counted as equal to a family history of cancer when there is a clear history, or medical report containing evidence, or other evidence to indicate that family members had advanced adenomas (an adenoma ≥ 1 cm in size, or with high-grade dysplasia, or with villous elements) [43]. Continuation of the old recommendation to screen first-degree relatives of patients with only small tubular adenomas could result in most of the population being screened at age 40, with doubtful benefit.
What are screening considerations in patients with genetic syndromes?
Patients with features of an inherited CRC syndrome should be advised to pursue genetic counseling with a licensed genetic counselor and, if appropriate, genetic testing. Individuals with FAP should undergo adenomatous polyposis coli (APC) mutation testing and, if negative, MYH mutation testing. Patients with FAP or at risk of FAP based upon family history should undergo annual colonoscopy until colectomy is deemed by both physician and patient as the best treatment [64]. Patients with a retained rectum after total colectomy and ileorectal anastomosis, ileal pouch, after total proctocolectomy and ileal pouch anal anastomosis, or stoma after total proctocolectomy and end ileostomy, should undergo endoscopic assessment approximately every 6 to 12 months after surgery, depending on the polyp burden seen. Individuals with oligopolyposis (< 100 colorectal polyps) should be sent for genetic counseling, consideration of APC and MYH mutation testing, and individualized colonoscopy surveillance depending on the size, number, and pathology of polyps seen. Upper endoscopic surveillance is recommended in individuals with FAP, but there are no established guidelines for endoscopic surveillance in MAP (MYH-associated polyposis) [43].
Patients who meet the Bethesda criteria for HNPCC [65] can be screened by 2 different mechanisms. One is a DNA-based test for microsatellite instability of either the patient’s or a family member’s tumor. The other mechanism is to assess by immunohistochemical staining for evidence of mismatch repair proteins (eg, MLH1, MSH2, MSH6). In those patients in whom deleterious mutations are found, the affected individual should undergo colonoscopy every 2 years beginning at age 20 to 25 years until age 40 years, then annually thereafter [43]. If genetic testing is negative (ie, no deleterious mutation is found), but the patient is still felt to clinically have Lynch syndrome, then they should still be surveyed in the same way.
Case Continued
The physician recommends colonoscopy as the screening modality as it is the most efficient and accurate way of finding precancerous lesions and the most effective way of preventing CRC by removing precancerous lesions. He also explains that because the patient’s father developed CRC after the age of 60, this does not place the patient in a higher risk category and he can follow screening recommendations for “average-risk” individuals.
Screening
The patient undergoes colonoscopy. Two 5-mm adenomas in the transverse colon are detected and removed.
When should he have a repeat colonoscopy?
Surveillance Intervals
New data have recently emerged on the risk of interval cancer after colonoscopy. The overall rate of interval cancer is estimated to be 1.1–2.7 per 1000 person-years of follow-up. There are several reasons that may account for why patients develop interval cancers: (1) important lesions may be missed at baseline colonoscopy, (2) adenomas may be incompletely removed at the time of baseline colonoscopy, and (3) interval CRC may be biologically different or more aggressive than prevalent CRC. In order to minimize the risk of interval cancer development, it is important to perform a high-quality baseline screening colonoscopy examination as this is associated with lowering the risk of interval cancer [66]. A high-quality colonoscopy entails completion of the procedure to the cecum (with photodocumentation of the appendiceal orifice and ileocecal valve) with careful inspection of folds including adequate bowel cleanliness and a withdrawal time > 6 minutes.
Baseline Colonoscopy Findings
No Polyps
Several prospective observational studies in different populations have shown that the risk of advanced adenomas within 5 years after negative findings on colonoscopy is low (1.3%–2.4%) relative to the rate on initial screening examination (4%–10%) [68–73]. In these studies, interval cancers were rare within 5 years. A sigmoidoscopy randomized controlled trial performed in the United Kingdom demonstrated a reduction in CRC incidence and mortality at 10 years in patients who received one-time sigmoidoscopy compared with controls—a benefit limited to the distal colon [46]. This is the first randomized study to show the effectiveness of endoscopic screening, an effect that appears to have at least a 10-year duration [74]. Thus, in patients who have a baseline colonoscopic evaluation without any adenomas or polyps and are average-risk individuals, the recommendation for the next examination is in 10 years [66].
Distal Hyperplastic Polyps < 10 mm
There is considerable evidence that patients with only rectal or sigmoid hyperplastic polyps (HPs) appear to represent a low-risk cohort. Studies have focused on whether the finding in the distal colon was a marker of risk for advanced neoplasia elsewhere and most studies show no such relationship [67]. Prior and current evidence suggests that distal HPs <10 mm are benign without neoplastic potential. If the most advanced lesions at baseline colonoscopy are distal HPs <10 mm, the interval for colonoscopic follow-up should be 10 years [66].
1-2 Tubular Adenomas < 10 mm
Prior evidence suggested that patients with low-risk adenomas (<10 mm, no villous histology or high-grade dysplasia) had a lower risk of developing advanced adenomas during follow-up compared with patients with high risk adenomas (≥ 10mm, villous histology or high -grade dysplasia). At that time in 2006, consensus on the task force was that an interval of 5 years would be acceptable in this low-risk group [75]. Data published since 2006 endorse the assessment that patients with 1–2 tubular adenomas with low-grade dysplasia <10 mm represent a low-risk group. Three new studies suggest that this group may have only a small, nonsignificant increase in risk of advanced neoplasia within 5 years compared with individuals with no baseline neoplasia. The evidence now supports a surveillance interval of longer than 5 years for most patients and can be extended to 10 years based on the quality of the preparation and colonoscopy [66].
3–10 Tubular Adenomas
Two independent meta-analyses in 2006 found that patients with 3 or more adenomas at baseline had an increased RR for adenomas during surveillance, ranging from 1.7 to 4.8 [47,75]. New information from the VA study and the National Cancer Institute Pooling Project also support these prior findings. Patients with 3 or more adenomas have a level of risk for advanced neoplasia similar to other patients with advanced neoplasia (adenoma >10 mm, adenoma with high grade dysplasia) and thus, repeat examination should be performed in 3 years [66,68,76].
> 10 Adenomas
Only a small proportion of patients undergoing screening colonoscopy will have >10 adenomas. The 2006 guidelines for colonoscopy surveillance after polypectomy noted that such patients should be considered for evaluation of hereditary CRC syndromes [67]. Early follow-up surveillance colonoscopy is based on clinical judgment because there is little evidence to support a firm recommendation. At present, the recommendation is to consider follow-up in less than 3 years after a baseline colonoscopy [66].
1 or More Tubular Adenomas ≥ 10mm
The 2006 MSTF guideline reviewed data related to adenoma size, demonstrating that most studies showed a 2- to 5-fold increased risk of advanced neoplasia during follow-up if the baseline examination had one or more adenomas ≥ 10 mm [67]. Newer, additional data shows that patients with one or more adenomas ≥ 10 mm have an increased risk of advanced neoplasia during surveillance compared with those with no neoplasia or small (< 10 mm) adenomas [68,76]. Thus, the recommendations remains that repeat examination should be performed in 3 years [66]. If there is question about complete removal of an adenoma (ie, piecemeal resection), early follow-up colonoscopy is warranted [66].
1 or More Villous Adenomas
The 2006 MSTF guideline considers adenomas with villous histology to be high risk [67]. The NCI Pooling Project analyzed polyp histology as a risk factor for development of interval advanced neoplasia. Compared with patients with tubular adenomas, those with baseline polyp(s) showing adenomas with villous or tubulovillous histology (TVA) had increased risk of advanced neoplasia during follow-up (16.8% vs 9.7%; adjusted OR, 1.28; 95% CI, 1.07–1.52) [76]. Patients with one or more adenomas with villous histology were also found to have an increased risk of advanced neoplasia during surveillance compared with those with no neoplasia or small (<10 mm) tubular adenomas. Thus, the recommendation remains that repeat examination should be performed in 3 years [66].
Adenoma with High-Grade Dysplasia (HGD)
The 2006 MSTF guideline concluded that the presence of HGD in an adenoma was associated with both villous histology and larger size, which are both risk factors for advanced neoplasia during surveillance [67]. In a univariate analysis from the NCI Pooling Project, HGD was strongly associated with risk of advanced neoplasia during surveillance (OR, 1.77; 95% CI, 1.41–2.22) [76]. Thus, the recommendation remains that repeat examination should be performed in 3 years [66].
Serrated Lesions
A total of 20% to 30% of CRCs arise through a molecular pathway characterized by hypermethylation of genes, known as CgG Island Methylator Phenotype (CIMP) [77]. Precursors are believed to be serrated polyps. Tumors in this pathway have a high frequency of BRAF mutation, and up to 50% are microsatellite unstable. CIMP-positive tumors are overrepresented in interval cancers, particularly in the proximal colon. The principal precursor of hypermethylated cancers is probably the sessile serrated polyp (synonymous with sessile serrated adenoma). These polyps are difficult to detect at endoscopy. They may be the same color as surrounding colonic mucosa, have indiscrete edges, are nearly always flat or sessile, and may have a layer of adherent mucus and obscure the vascular pattern.
Recent studies show that proximal colon location or size ≥ 10 mm may be markers of risk for synchronous advanced adenomas elsewhere in the colon [78,79]. Surveillance after colonoscopy was evaluated in one study, which found that coexisting serrated polyps and high-risk adenomas (HRA; ie, size ≥ 10 mm, villous histology, or presence of HGD) is associated with a higher risk of advanced neoplasia at surveillance [78]. This study also found that if small proximal serrated polyps are the only finding at baseline, the risk of adenomas during surveillance is similar to that of patients with low-risk adenomas (LRA; ie, 1–2 small adenomas).
The current evidence suggests that size (>10 mm), histology (a sessile serrated polyp is a more significant lesion than an HP; a sessile serrated polyp with cytological dysplasia is more advanced than a sessile serrated polyp without dysplasia), and location (proximal to the sigmoid colon) are risk factors that might be associated with higher risk of CRC. A sessile serrated polyp ≥ 10 mm and a sessile serrated polyp with cytological dysplasia should be managed like a HRA with repeat colonoscopy occurring in 3 years. Serrated polyps that are <10 mm in size and do not have cytological dysplasia may have lower risk and can be managed like LRA with repeat colonoscopy occurring in 5 years [66].
Follow-up After Surveillance
In a 2009 study, 564 participants underwent 2 surveillance colonoscopies after an index procedure and 10.3% had high-risk findings at the third study examination. If the second examination showed high-risk findings, then results from the first examination added no significant information about the probability of high-risk findings on the third examination (18.2% for high-risk findings on the first examination vs. 20.0% for low-risk findings on the first examination; P = 0.78). If the second examination showed no adenomas, then the results from the first examination added significant information about the probability of high-risk findings on the third exam-ination (12.3% if the first examination had high-risk findings vs. 4.9% if the first examination had low-risk findings; P = 0.015) [80]. Thus, information from 2 previous colonoscopies appears to be helpful in defining the risk of neoplasia for individual patients and in the future, guidelines might consider accounting for the results of 2 exams to tailor surveillance intervals for patients.
When should screening / surveillance be stopped?
There is considerable new evidence that the risks of colonoscopy increase with advancing age [81,82]. Neither surveillance nor screening colonoscopy should be performed when the risk of the preparation, sedation, or procedure outweighs the potential benefit. For patients aged 75–85 years, the USPSTF recommends against routine screening but argues for individualization based on comorbidities and findings on any prior colonoscopy. The USPSTF recommends against continued screening after age 85 years because risk could exceed potential benefit [44].
In terms of surveillance of prior adenomas, the 75-85 year age group may still benefit from surveillance because patients with prior HRA are at higher risk for developing advanced neoplasia compared with average-risk screenees. However, the decision to continue surveillance in this population should be individualized and based on an assessment of benefit and risk in the context of the person’s estimated life expectancy [66]. More importantly, it should be noted that an individual’s most important and impactful screening colonoscopy is his or her first one and therefore, from a public health standpoint, great effort should be taken to increase the number of people in a population who undergo screening rather than simply targeting those who need surveillance for prior polyps. This is ever true in settings with limited resources.
Case Conclusion
The physician discusses the findings from the colonoscopy (2 small adenomas) with the patient and recommends a repeat colonoscopy in 5 to 10 years.
Summary
Colorectal cancer is one of the leading causes of cancer-related death in the United States. Since the advent of colonoscopy and the implementation of CRC screening efforts, the rates of CRC have started to decline. There are several environmental factors which have been associated with the development of CRC including obesity, dietary intake, physical activity and smoking. At present, there are multiple tools available for CRC prevention, but the most accurate and effective method is currently colonoscopy. Stool-based tests like FIT should be offered when a patient declines colonoscopy. For those interested in colonoscopy, average-risk individuals should be screened starting at the age of 50 with subsequent examinations every 10 years. Surveillance examinations should occur based on polyp findings on index colonoscopy. There is no recommendation to continue screening after the age of 75, though physicians can determine this based on patients health and risk/benefit profile. Current guidelines recommend against offering any screening to patients over the age of 85. Despite these recommendations, almost half of the eligible screening population has yet to undergo appropriate CRC screening. Future work should include public health efforts to improve access and appeal of widespread CRC screening regardless of modality. While colonoscopy is considered the most effective screening test, the best test is still the one the patient gets.
Corresponding author: Audrey H. Calderwood, MD, MS, 85 E. Concord St., Rm. 7724, Boston, MA 02118, [email protected].
Financial disclosures: None.
From the Boston University School of Medicine, Boston, MA.
Abstract
- Objective: To review recommendations for colorectal cancer (CRC) screening.
- Methods: Review of the literature.
- Results: In the United States, CRC is the third most commonly diagnosed cancer and the third leading cause of cancer death. CRC screening can reduce mortality through the detection of early-stage disease and the detection and removal of adenomatous polyps. There are several modalities for CRC screening, with current technology falling into 2 general categories: stool tests, which include tests for occult blood or exfoliated DNA; and structural exams, which include flexible sigmoidoscopy, colonoscopy, double-contrast barium enema, and CT colonography. The preferred CRC prevention test for average-risk individuals is colonoscopy starting at age 50 with subsequent examinations every 10 years. Patients unwilling to undergo screening colonoscopy may be offered flexible sigmoidoscopy, CT colonography, or fecal immunohistochemical test. Surveillance examinations should occur based on polyp findings on index colonoscopy. There is no recommendation to continue screening after age 75, though physicians can make a determination based on a patient’s health and risk/benefit profile. Current guidelines recommend against offering screening to patients over age 85.
- Conclusion: Increasing access to and utilization of CRC screening tests is likely to lead to improvements in mortality reduction, as only about half of people aged 50 or older report having received CRC testing consistent with current guidelines.
In the United States, colorectal cancer (CRC) is the third most commonly diagnosed cancer and the third leading cause of cancer death in both men and women [1]. In 2014, an estimated 136,830 people were diagnosed with CRC and about 50,310 people died of the disease [2]. Colorectal cancer usually develops slowly over a period of 10 to 15 years. The tumor typically begins as a noncancerous polyp, classically an adenomatous polyp or adenoma, though fewer than 10% of adenomas will progress to cancer [3]. Adenomas are common; an estimated one-third to one-half of all individuals will eventually develop 1 or more adenomas [4,5]. In the United States, the lifetime risk of being diagnosed with CRC is approximately 5% for both men and women [6]. Incidence rates for CRC increase with age, with an incidence rate more than 15 times higher in adults aged 50 years and older compared with those aged 20 to 49 years [7].
Certain demographic subgroups have been shown to be at higher risk. Overall, CRC incidence and mortality rates are about 35% to 40% higher in men than in women. The reasons for this are not completely understood but likely reflect complex interactions between gender-related differences in exposure to hormones and risk factors [8]. CRC incidence and mortality rates are highest in African-American men and women; incidence rates are 20% higher and mortality rates are about 45% higher than those in whites. Prior to 1989, incidence rates were predominantly higher in white men than in African American men and were similar for women of both races. Since that time, although incidence rates have declined as a whole [9], incidence rates have been higher for African Americans than whites in both men and women This crossover likely reflects a combination of greater access to and utilization of recommended screening tests among whites (resulting in detection and removal of precancerous polyps), as well as racial differences in trends for CRC risk factors [10].
CRC screening can reduce mortality through the detection of early-stage disease and the detection and removal of ademomatous polyps [11]. Increasing access to and utilization of CRC screening tests is likely to lead to improvements in mortality reduction, as only about half of people aged 50 or older report having received CRC testing consistent with current guidelines [1].
Case Study
Initial Presentation
A 55-year-old white male presents for a routine visit and asks about colon cancer screening. His father was diagnosed with colon cancer at the age of 78. Overall, he feels well and does not have any particular complaints. His bowel habits are normal and he denies melena and hematochezia. His past medical history is significant for diabetes, hypertension, and obesity. He was a previous smoker and has a few alcoholic drinks on the weekends. His physical exam is unremarkable. Results of recent blood work are normal and there is no evidence of anemia.
What are this patient’s risk factors for developing colon cancer?
Risk Factors for CRC
There are numerous factors that are thought to influence risk for CRC. Nonmodifiable risk factors include a personal or family history of CRC or adenomatous polyps, and a personal history of chronic inflammatory bowel disease. Modifiable risk factors that have been associated with an increased risk of CRC in epidemiologic studies include physical inactivity, obesity, high consumption of red or processed meats, smoking, and moderate-to-heavy alcohol consumption. In fact, a prospective study showed that up to 23% of colorectal cancers were considered to be potentially avoidable by adhering to multiple healthy lifestyle recommendations including maintaining a healthy weight, being physically active at least 30 minutes per day, eating a healthy diet, and avoiding smoking and drinking excessive amounts of alcohol [12].
People with a first-degree relative (parent, sibling, or offspring) who has had CRC have 2 to 3 times the risk of developing the disease compared with individuals with no family history; if the relative was diagnosed at a young age or if there is more than 1 affected relative, risk increases to 3 to 6 times that of the general population [13,14]. About 5% of patients with CRC have a well-defined genetic syndrome that causes the disease [15]. The most common of these is Lynch syndrome (also known as hereditary nonpolyposis colorectal cancer or HNPCC), which accounts for 2% to 4% of all CRC cases [16]. Although individuals with Lynch syndrome are predisposed to numerous types of cancer, risk of CRC is highest. A recent study of CRC in 147 Lynch syndrome families in the United States found lifetime risk of CRC to be 66% in men and 43% in women, with a median age at diagnosis of 42 years and 47 years, respectively [17]. Familial adenomatous polyposis (FAP) is the second most common predisposing genetic syndrome; for these individuals, the lifetime risk of CRC approaches 100% without intervention (eg, colectomy) [16].
People who have inflammatory bowel disease of the colon (both ulcerative colitis and Crohn’s disease) have an increased risk of developing CRC that correlates with the extent and the duration of the inflammation [18]. It is estimated that 18% of patients with a 30-year history of ulcerative colitis will develop CRC [19]. In addition, several studies have found an association between diabetes and increased risk of CRC [20,21]. Though adult-onset type 2 diabetes (the most common type) and CRC share similar risk factors, including physical inactivity and obesity, a positive association between diabetes and CRC has been found even after accounting for physical activity, body mass index, and waist circumference [22].
Being overweight or obese is also associated with a higher risk of CRC, with stronger associations more consistently observed in men than in women. Obesity increases the risk of CRC independent of physical activity. Abdominal obesity (measured by waist circumference) may be a more important risk factor for colon cancer than overall obesity in both men and women [23–25]. Diet and lifestyle strongly influence CRC risk; however, research on the role of specific dietary elements on CRC risk is still accumulating. Several studies, including one by the American Cancer Society, have found that high consumption of red and/or processed meat increases the risk of both colon and rectal cancer [23,26,27]. Further analyses indicate that the association between CRC and red meat may be related to the cooking process, because a higher risk of CRC is observed particularly among those individuals who consume meat that has been cooked at a high temperature for a long period of time [28]. In contrast to findings from earlier research, more recent large, prospective studies do not indicate a major relationship between CRC and vegetable, fruit, or fiber consumption [28,29]. However, some studies suggest that people with very low fruit and vegetable intake are at above-average risk for CRC [30,31]. Consumption of milk and calcium may decrease the risk of developing CRC [28,29,32].
In November 2009, the International Agency for Research on Cancer reported that there is now sufficient evidence to conclude that tobacco smoking causes CRC [33]. Colorectal cancer has been linked to even moderate alcohol use. Individuals who have a lifetime average of 2 to 4 alcoholic drinks per day have a 23% higher risk of CRC than those who consume less than 1 drink per day [34].
Protective Factors
One of the most consistently reported relationships between colon cancer risk and behavior is the protective effect of physical activity [35]. Based on these findings, as well as the numerous other health benefits of regular physical activity, the American Cancer Society recommends engaging in at least moderate activity for 30 minutes or more on 5 or more days per week.
Accumulating research suggests that aspirin-like drugs, postmenopausal hormones, and calcium supplements may help prevent CRC. Extensive evidence suggests that long-term, regular use of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) is asso-ciated with lower risk of CRC. The American Cancer Society does not currently recommend use of these drugs as chemoprevention because of the potential side effects of gastrointestinal bleeding from aspirin and other traditional NSAIDs and heart attacks from selective cyclooxygenase-2 (COX-2) inhibitors. However, people who are already taking NSAIDs for chronic arthritis or aspirin for heart disease prevention may have a lower risk of CRC as a positive side effect [36,37].
There is substantial evidence that women who use postmenopausal hormones have lower rates of CRC than those who do not. A decreased risk of CRC is especially evident in women who use hormones long-term, although the risk returns to that of nonusers within 3 years of cessation. Despite its positive effect on CRC risk, the use of postmenopausal hormones increases the risk of breast and other cancers as well as cardiovascular disease, and therefore it is not recommended for the prevention of CRC. At present, the American Cancer Society does not recommend any medications or supplements to prevent CRC because of uncertainties about their effectiveness, appropriate dosing, and potential toxicity [38–40].
Case Continued
The physician tells the patient that there are several environmental factors that may predispose him to developing CRC. He recommends that the patient follow a healthy lifestyle, including eating 5 servings of fruits and vegetables daily, minimizing consumption of red meats, exercising for 30 minutes at least 5 days per week, drinking only moderate amounts of alcohol, and continuing to take his aspirin in the setting of his diabetes. He also asks the patient if he would be interested in talking about weight loss and working together to make a plan.
The patient is appreciative of this information and wants to know what CRC creening test the physician recommends.
What screening test should be recommended?
Screening Options
There are several modalities for CRC screening, with current technology falling into 2 general categories: stool tests, which include tests for occult blood or exfoliated DNA; and structural exams, which include flexible sigmoidoscopy, colonoscopy, double-contrast barium enema (DCBE), and computed tomographic (CT) colonography. Stool tests are best suited for the detection of CRC, although they also will deliver positive findings for some advanced adenomas, while the structural exams can achieve both detection and prevention of CRC through identification and removal of adenomatous polyps [41]. These tests may be used alone or in combination to improve sensitivity or, in some instances, to ensure a complete examination of the colon if the initial test cannot be completed.
In principle, all adults should have access to the full range of options for CRC screening, and the availability of lower-cost, less invasive options in most practice settings is a public health advantage [11]. However, the availability of multiple testing options can overwhelm the primary care provider and presents challenges for practices in trying to support an office policy that can manage a broad range of testing choices, their follow-up requirements, and shared decision making related to the options. Shared decision making around CRC screening options is both demanding and time consuming and is complicated by the different characteristics of the tests and the test-specific requirements for individuals undergoing screening [42].
Recommended Tests
The joint guideline on screening for CRC from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology (the MSTF guideline) [11] is of the strong opinion that tests designed to detect early cancer and prevent cancer through the detection and removal of adenomatous polyps (the structural exams) should be encouraged if resources are available and patients are willing to undergo an invasive test [11]. In clinical settings in which economic issues preclude primary screening with colonoscopy, or for patients who decline invasive tests, clinicians may offer stool- based testing. However, providers and patients should understand that these tests are less likely to prevent cancer compared with the invasive tests, they must be repeated at regular intervals to be effective (ie, programmatic sensitivity), and if the test is abnormal, a colonoscopy will be needed to follow up. Therefore, if patients are not willing to have repeated testing or pursue colonoscopy if the test is abnormal, these programs will not be effective and should not be recommended [11].
Stool-Based Testing
Stool blood tests are conventionally known as fecal occult blood tests (FOBT) because they are designed to detect the presence of occult blood in stool. FOBT falls into 2 primary categories based on the detected analyte: guaiac-based and FIT. Blood in the stool is a nonspecific finding but may originate from CRC or larger (> 1 to 2 cm) polyps. Because small adenomatous polyps do not tend to bleed and bleeding from cancers or large polyps may be intermittent or undetectable in a single sample of stool, the proper use of stool blood tests requires annual testing that consists of collecting specimens (2 or 3, depending on the product) from consecutive bowel movements [45–47].
Guaiac-based FOBT
Guaiac-based FOBT (gFOBT) is the most common stool blood test for CRC screening and the only CRC screening test for which there is evidence of efficacy from randomized controlled trials [11]. The usual gFOBT protocol consists of collecting 2 samples from each of 3 consecutive bowel movements at home. Prior to testing with a sensitive guaiac-based test, individuals usually will be instructed to avoid aspirin and other NSAIDs, vitamin C, red meat, poultry, fish, and some raw vegetables because of diet-test interactions that can increase the risk of both false-positive and false-negative (specifically, vitamin C) results [48]. Collection of all 3 samples is important because test sensitivity improves with each additional stool sample [41]. Three large randomized controlled trials with gFOBT have demonstrated that screened patients have cancers detected at an early and more curable stage than unscreened patients. Over time (8 to 13 years), each of the trials demonstrated significant reductions in CRC mortality of 15% to 33% [49–51]. However, the reported sensitivity of a single gFOBT varies considerably [52].
FIT
FIT has several technological advantages when compared with gFOBT. FIT detects human globin, a protein that along with heme constitutes human hemoglobin. Thus, FIT is more specific for human blood than guaiac-based tests, which rely on detection of peroxidase in human blood and also react to the peroxidase that is present in dietary constituents such as rare red meat, cruciferous vegetables, and some fruits [53]. Furthermore, unlike gFOBT, FIT is not subject to false-negative results in the presence of high-dose vitamin C supplements, which block the peroxidase reaction. In addition, because globin is degraded by digestive enzymes in the upper gastrointestinal tract, FIT is also more specific for lower gastrointestinal bleeding, thus improving the specificity for CRC. Finally, the sample collection process for patients for some variants of FIT are less demanding than gFOBT, requiring fewer samples or less direct handling of stool, which may increase FIT’s appeal. Although FIT has superior performance characteristics when compared with older guaiac-based Hemoccult II cards [54–56], the spectrum of benefits, limitations, and harms is similar to a gFOBT with high sensitivity [41]. As for adherence with FIT, there were 10% and 12% gains in adherence with FIT in the first 2 randomized controlled trials comparing FIT with guaiac-based testing [57,58]. Therefore, FIT is preferred over Hemoccult Sensa and is the preferred annual cancer detection test when colonoscopy is not an option [43]. The American College of Gastroenterology supports the joint guideline recommendation [11] that older guaiac-based fecal occult blood testing be abandoned as a method for CRC screening.
sDNA
Fecal DNA testing uses knowledge of molecular genomics and provides the basis of a new method of CRC screening that tests stool for the presence of known DNA alterations in the adenoma-carcinoma sequence of colorectal carcinogenesis [11]. Three different types of fecal DNA testing kits have been evaluated. The sensitivity for cancer in each version was superior to traditional guaiac-based occult blood testing, but the sensitivities ranged from 52%–87%, with the specificities ranging from 82%–95%. Based on the accumulation of evidence since the last update of joint guideline, the joint guideline panel concluded that there now are sufficient data to include sDNA as an acceptable option for CRC screening [11].
As for overall recommendations for stool-based testing, the ACG supports the joint guideline recommendation that older guaiac-based fecal occult blood testing be abandoned as a method for CRC screening. Because of more extensive data (compared with Hemoccult Sensa), and the high cost of fecal DNA testing, the American College of Gastroenterology recommends FIT as the preferred cancer detection test in cases where colonoscopy is not an option [43].
Invasive Tests Other than Colonoscopy
The use of flexible sigmoidoscopy for CRC screening is supported by high-quality case-control and cohort studies [46]. The chief advantage of flexible sigmoidoscopy is that it can be performed with a simple preparation (2 enemas), without sedation, and by a variety of practitioners in diverse settings. The main limitation of the procedure is that it does not examine the entire colon but only the rectum, sigmoid, and descending colon. The effectiveness of a flexible sigmoidoscopy program is based on the assumption that if an adenoma is detected during the procedure, the patient would be referred for colonoscopy to examine the entire colon.
DCBE is an imaging modality which can evaluate the entire colon in almost all cases and can detect most cancers and the majority of significant polyps. However, the lower sensitivity for significant adenomas when compared with colonoscopy may result in less favorable outcomes regarding CRC morbidity and mortality. Double-contrast barium enema is no longer recommended as an alternative CRC prevention test because its use has declined dramatically and also as its effectiveness for polyp detection is less than CT colonography [43].
CT Colonography
CT colonography every 5 years is endorsed as an alternative to colonoscopy every 10 years because of its recent performance in the American College of Imaging Network Trial 6664 (also known as the National CT Colonography Trial) [59]. The principle performance feature that justifies inclusion of CT colonography as a viable alternative in patients who decline colonoscopy is that the sensitivity for polyps ≥ 1 cm in size was 90% in the most recent multicenter US trial [59]. In this study, 25% of radiologists who were tested for entry into the trial but performed poorly were excluded from participation, and thus lower sensitivity might be expected in actual clinical practice. CT colonography probably has a lower risk of perforation than colonoscopy in most settings, but for several reasons it is not considered the equivalent of colonoscopy as a screening strategy. First, the evidence to support an effect of endoscopic screening on prevention of incident CRC and mortality is overwhelming compared with that for CT colonography. Second, the inability of CT colonography to adequately detect polyps 5 mm and smaller, which constitutes 80% of colorectal neoplasms, and whose natural history is still not understood, necessitates performance of the test at 5-year rather than 10-year intervals [43]. Finally, false-positives are common, and the specificity for polyps ≥ 1 cm in size was only 86% in the National CT Colonography Trial, with a positive predictive value of 23% [59]. The American College of Gastroenterology recommends that asymptomatic patients be informed of the possibility of radiation risk associated with one or repeated CT colonography studies, though the exact risk associated with radiation is unclear [60,61].
The value of extracolonic findings detected by CT colonography is mixed, with substantial costs associated with incidental findings, but occasional important extracolonic findings are detected, such as asymptomatic cancers and large abdominal aortic aneurysms. As a final point, the ACG is also concerned about the potential impact of CT colonography on adherence with follow-up colonoscopy and thus on polypectomy rates. Thus, if CT colonography substantially improves adherence, it should improve polypectomy rates and thereby reduce CRC, even if only large polyps are detected and referred for colonoscopy. On the other hand, if CT colonography largely displaces patients who would otherwise be willing to undergo colonoscopy, then polypectomy rates will fall substantially, which could significantly increase the CRC incidence [62]. Thus, for multiple reasons and pending additional study, CT colonography should be offered to patients who decline colonoscopy. It should be noted that CT colonography should only be offered for the purposes of CRC screening and should not be used for diagnostic workup of symptoms (eg, patient with active bleeding or inflammatory bowel disease).
When should screening begin?
The American College of Gastroenterology continues to recommend that screening begin at age 50 years in average-risk persons (ie, those without a family history of colorectal neoplasia), except for African Americans, in whom it should begin at age 45 years [43]. The USPSTF does not currently provide specific recommendations based on race or ethnicity, but certain other subgroups of the average-risk population might warrant initiation of screening at an earlier or later age, depending on their risk. For example, the incident risk of CRC has been described to be greater in men than women [63]. In reviewing the literature, the writing committee also identified heavy cigarette smoking and obesity as linked to an increased risk of CRC and to the development of CRC at an earlier age.
For patients with a family history of CRC or adenomatous polyps, the 2008 MSTF guideline recommends initiation of screening at age 40 [11]. The American College of Gastroenterology recommendations for screening in patients with a family history are shown in Table 1. From a practical perspective, many clinicians have found that patients are often not aware of whether their first-degree relatives had advanced adenomas vs. small tubular adenomas, or whether their family members had non-neoplastic vs. neoplastic polyps. Given these difficulties, the American College of Gastroenterology now recommends that adenomas only be counted as equal to a family history of cancer when there is a clear history, or medical report containing evidence, or other evidence to indicate that family members had advanced adenomas (an adenoma ≥ 1 cm in size, or with high-grade dysplasia, or with villous elements) [43]. Continuation of the old recommendation to screen first-degree relatives of patients with only small tubular adenomas could result in most of the population being screened at age 40, with doubtful benefit.
What are screening considerations in patients with genetic syndromes?
Patients with features of an inherited CRC syndrome should be advised to pursue genetic counseling with a licensed genetic counselor and, if appropriate, genetic testing. Individuals with FAP should undergo adenomatous polyposis coli (APC) mutation testing and, if negative, MYH mutation testing. Patients with FAP or at risk of FAP based upon family history should undergo annual colonoscopy until colectomy is deemed by both physician and patient as the best treatment [64]. Patients with a retained rectum after total colectomy and ileorectal anastomosis, ileal pouch, after total proctocolectomy and ileal pouch anal anastomosis, or stoma after total proctocolectomy and end ileostomy, should undergo endoscopic assessment approximately every 6 to 12 months after surgery, depending on the polyp burden seen. Individuals with oligopolyposis (< 100 colorectal polyps) should be sent for genetic counseling, consideration of APC and MYH mutation testing, and individualized colonoscopy surveillance depending on the size, number, and pathology of polyps seen. Upper endoscopic surveillance is recommended in individuals with FAP, but there are no established guidelines for endoscopic surveillance in MAP (MYH-associated polyposis) [43].
Patients who meet the Bethesda criteria for HNPCC [65] can be screened by 2 different mechanisms. One is a DNA-based test for microsatellite instability of either the patient’s or a family member’s tumor. The other mechanism is to assess by immunohistochemical staining for evidence of mismatch repair proteins (eg, MLH1, MSH2, MSH6). In those patients in whom deleterious mutations are found, the affected individual should undergo colonoscopy every 2 years beginning at age 20 to 25 years until age 40 years, then annually thereafter [43]. If genetic testing is negative (ie, no deleterious mutation is found), but the patient is still felt to clinically have Lynch syndrome, then they should still be surveyed in the same way.
Case Continued
The physician recommends colonoscopy as the screening modality as it is the most efficient and accurate way of finding precancerous lesions and the most effective way of preventing CRC by removing precancerous lesions. He also explains that because the patient’s father developed CRC after the age of 60, this does not place the patient in a higher risk category and he can follow screening recommendations for “average-risk” individuals.
Screening
The patient undergoes colonoscopy. Two 5-mm adenomas in the transverse colon are detected and removed.
When should he have a repeat colonoscopy?
Surveillance Intervals
New data have recently emerged on the risk of interval cancer after colonoscopy. The overall rate of interval cancer is estimated to be 1.1–2.7 per 1000 person-years of follow-up. There are several reasons that may account for why patients develop interval cancers: (1) important lesions may be missed at baseline colonoscopy, (2) adenomas may be incompletely removed at the time of baseline colonoscopy, and (3) interval CRC may be biologically different or more aggressive than prevalent CRC. In order to minimize the risk of interval cancer development, it is important to perform a high-quality baseline screening colonoscopy examination as this is associated with lowering the risk of interval cancer [66]. A high-quality colonoscopy entails completion of the procedure to the cecum (with photodocumentation of the appendiceal orifice and ileocecal valve) with careful inspection of folds including adequate bowel cleanliness and a withdrawal time > 6 minutes.
Baseline Colonoscopy Findings
No Polyps
Several prospective observational studies in different populations have shown that the risk of advanced adenomas within 5 years after negative findings on colonoscopy is low (1.3%–2.4%) relative to the rate on initial screening examination (4%–10%) [68–73]. In these studies, interval cancers were rare within 5 years. A sigmoidoscopy randomized controlled trial performed in the United Kingdom demonstrated a reduction in CRC incidence and mortality at 10 years in patients who received one-time sigmoidoscopy compared with controls—a benefit limited to the distal colon [46]. This is the first randomized study to show the effectiveness of endoscopic screening, an effect that appears to have at least a 10-year duration [74]. Thus, in patients who have a baseline colonoscopic evaluation without any adenomas or polyps and are average-risk individuals, the recommendation for the next examination is in 10 years [66].
Distal Hyperplastic Polyps < 10 mm
There is considerable evidence that patients with only rectal or sigmoid hyperplastic polyps (HPs) appear to represent a low-risk cohort. Studies have focused on whether the finding in the distal colon was a marker of risk for advanced neoplasia elsewhere and most studies show no such relationship [67]. Prior and current evidence suggests that distal HPs <10 mm are benign without neoplastic potential. If the most advanced lesions at baseline colonoscopy are distal HPs <10 mm, the interval for colonoscopic follow-up should be 10 years [66].
1-2 Tubular Adenomas < 10 mm
Prior evidence suggested that patients with low-risk adenomas (<10 mm, no villous histology or high-grade dysplasia) had a lower risk of developing advanced adenomas during follow-up compared with patients with high risk adenomas (≥ 10mm, villous histology or high -grade dysplasia). At that time in 2006, consensus on the task force was that an interval of 5 years would be acceptable in this low-risk group [75]. Data published since 2006 endorse the assessment that patients with 1–2 tubular adenomas with low-grade dysplasia <10 mm represent a low-risk group. Three new studies suggest that this group may have only a small, nonsignificant increase in risk of advanced neoplasia within 5 years compared with individuals with no baseline neoplasia. The evidence now supports a surveillance interval of longer than 5 years for most patients and can be extended to 10 years based on the quality of the preparation and colonoscopy [66].
3–10 Tubular Adenomas
Two independent meta-analyses in 2006 found that patients with 3 or more adenomas at baseline had an increased RR for adenomas during surveillance, ranging from 1.7 to 4.8 [47,75]. New information from the VA study and the National Cancer Institute Pooling Project also support these prior findings. Patients with 3 or more adenomas have a level of risk for advanced neoplasia similar to other patients with advanced neoplasia (adenoma >10 mm, adenoma with high grade dysplasia) and thus, repeat examination should be performed in 3 years [66,68,76].
> 10 Adenomas
Only a small proportion of patients undergoing screening colonoscopy will have >10 adenomas. The 2006 guidelines for colonoscopy surveillance after polypectomy noted that such patients should be considered for evaluation of hereditary CRC syndromes [67]. Early follow-up surveillance colonoscopy is based on clinical judgment because there is little evidence to support a firm recommendation. At present, the recommendation is to consider follow-up in less than 3 years after a baseline colonoscopy [66].
1 or More Tubular Adenomas ≥ 10mm
The 2006 MSTF guideline reviewed data related to adenoma size, demonstrating that most studies showed a 2- to 5-fold increased risk of advanced neoplasia during follow-up if the baseline examination had one or more adenomas ≥ 10 mm [67]. Newer, additional data shows that patients with one or more adenomas ≥ 10 mm have an increased risk of advanced neoplasia during surveillance compared with those with no neoplasia or small (< 10 mm) adenomas [68,76]. Thus, the recommendations remains that repeat examination should be performed in 3 years [66]. If there is question about complete removal of an adenoma (ie, piecemeal resection), early follow-up colonoscopy is warranted [66].
1 or More Villous Adenomas
The 2006 MSTF guideline considers adenomas with villous histology to be high risk [67]. The NCI Pooling Project analyzed polyp histology as a risk factor for development of interval advanced neoplasia. Compared with patients with tubular adenomas, those with baseline polyp(s) showing adenomas with villous or tubulovillous histology (TVA) had increased risk of advanced neoplasia during follow-up (16.8% vs 9.7%; adjusted OR, 1.28; 95% CI, 1.07–1.52) [76]. Patients with one or more adenomas with villous histology were also found to have an increased risk of advanced neoplasia during surveillance compared with those with no neoplasia or small (<10 mm) tubular adenomas. Thus, the recommendation remains that repeat examination should be performed in 3 years [66].
Adenoma with High-Grade Dysplasia (HGD)
The 2006 MSTF guideline concluded that the presence of HGD in an adenoma was associated with both villous histology and larger size, which are both risk factors for advanced neoplasia during surveillance [67]. In a univariate analysis from the NCI Pooling Project, HGD was strongly associated with risk of advanced neoplasia during surveillance (OR, 1.77; 95% CI, 1.41–2.22) [76]. Thus, the recommendation remains that repeat examination should be performed in 3 years [66].
Serrated Lesions
A total of 20% to 30% of CRCs arise through a molecular pathway characterized by hypermethylation of genes, known as CgG Island Methylator Phenotype (CIMP) [77]. Precursors are believed to be serrated polyps. Tumors in this pathway have a high frequency of BRAF mutation, and up to 50% are microsatellite unstable. CIMP-positive tumors are overrepresented in interval cancers, particularly in the proximal colon. The principal precursor of hypermethylated cancers is probably the sessile serrated polyp (synonymous with sessile serrated adenoma). These polyps are difficult to detect at endoscopy. They may be the same color as surrounding colonic mucosa, have indiscrete edges, are nearly always flat or sessile, and may have a layer of adherent mucus and obscure the vascular pattern.
Recent studies show that proximal colon location or size ≥ 10 mm may be markers of risk for synchronous advanced adenomas elsewhere in the colon [78,79]. Surveillance after colonoscopy was evaluated in one study, which found that coexisting serrated polyps and high-risk adenomas (HRA; ie, size ≥ 10 mm, villous histology, or presence of HGD) is associated with a higher risk of advanced neoplasia at surveillance [78]. This study also found that if small proximal serrated polyps are the only finding at baseline, the risk of adenomas during surveillance is similar to that of patients with low-risk adenomas (LRA; ie, 1–2 small adenomas).
The current evidence suggests that size (>10 mm), histology (a sessile serrated polyp is a more significant lesion than an HP; a sessile serrated polyp with cytological dysplasia is more advanced than a sessile serrated polyp without dysplasia), and location (proximal to the sigmoid colon) are risk factors that might be associated with higher risk of CRC. A sessile serrated polyp ≥ 10 mm and a sessile serrated polyp with cytological dysplasia should be managed like a HRA with repeat colonoscopy occurring in 3 years. Serrated polyps that are <10 mm in size and do not have cytological dysplasia may have lower risk and can be managed like LRA with repeat colonoscopy occurring in 5 years [66].
Follow-up After Surveillance
In a 2009 study, 564 participants underwent 2 surveillance colonoscopies after an index procedure and 10.3% had high-risk findings at the third study examination. If the second examination showed high-risk findings, then results from the first examination added no significant information about the probability of high-risk findings on the third examination (18.2% for high-risk findings on the first examination vs. 20.0% for low-risk findings on the first examination; P = 0.78). If the second examination showed no adenomas, then the results from the first examination added significant information about the probability of high-risk findings on the third exam-ination (12.3% if the first examination had high-risk findings vs. 4.9% if the first examination had low-risk findings; P = 0.015) [80]. Thus, information from 2 previous colonoscopies appears to be helpful in defining the risk of neoplasia for individual patients and in the future, guidelines might consider accounting for the results of 2 exams to tailor surveillance intervals for patients.
When should screening / surveillance be stopped?
There is considerable new evidence that the risks of colonoscopy increase with advancing age [81,82]. Neither surveillance nor screening colonoscopy should be performed when the risk of the preparation, sedation, or procedure outweighs the potential benefit. For patients aged 75–85 years, the USPSTF recommends against routine screening but argues for individualization based on comorbidities and findings on any prior colonoscopy. The USPSTF recommends against continued screening after age 85 years because risk could exceed potential benefit [44].
In terms of surveillance of prior adenomas, the 75-85 year age group may still benefit from surveillance because patients with prior HRA are at higher risk for developing advanced neoplasia compared with average-risk screenees. However, the decision to continue surveillance in this population should be individualized and based on an assessment of benefit and risk in the context of the person’s estimated life expectancy [66]. More importantly, it should be noted that an individual’s most important and impactful screening colonoscopy is his or her first one and therefore, from a public health standpoint, great effort should be taken to increase the number of people in a population who undergo screening rather than simply targeting those who need surveillance for prior polyps. This is ever true in settings with limited resources.
Case Conclusion
The physician discusses the findings from the colonoscopy (2 small adenomas) with the patient and recommends a repeat colonoscopy in 5 to 10 years.
Summary
Colorectal cancer is one of the leading causes of cancer-related death in the United States. Since the advent of colonoscopy and the implementation of CRC screening efforts, the rates of CRC have started to decline. There are several environmental factors which have been associated with the development of CRC including obesity, dietary intake, physical activity and smoking. At present, there are multiple tools available for CRC prevention, but the most accurate and effective method is currently colonoscopy. Stool-based tests like FIT should be offered when a patient declines colonoscopy. For those interested in colonoscopy, average-risk individuals should be screened starting at the age of 50 with subsequent examinations every 10 years. Surveillance examinations should occur based on polyp findings on index colonoscopy. There is no recommendation to continue screening after the age of 75, though physicians can determine this based on patients health and risk/benefit profile. Current guidelines recommend against offering any screening to patients over the age of 85. Despite these recommendations, almost half of the eligible screening population has yet to undergo appropriate CRC screening. Future work should include public health efforts to improve access and appeal of widespread CRC screening regardless of modality. While colonoscopy is considered the most effective screening test, the best test is still the one the patient gets.
Corresponding author: Audrey H. Calderwood, MD, MS, 85 E. Concord St., Rm. 7724, Boston, MA 02118, [email protected].
Financial disclosures: None.
1. American Cancer Society. Colorectal cancer facts & figures 2014–2016. Atlanta: American Cancer Society; 2014.
2. Ries L, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014.
3. Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med 2006;355:2551–7.
4. Bond JH. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 2000;95:3053–63.
5. Schatzkin A, Freedman LS, Dawsey SM, Lanza E. Interpreting precursor studies: what polyp trials tell us about large-bowel cancer. J Natl Cancer Inst 1994;86:1053–7.
6. DevCan: Probability of developing or dying of cancer software, version 6.5.0; Statistical Research and Applications Branch, National Cancer Institute, 2005. http://srab.cancer.gov/devcan [computer program].
7. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2010, based on the November 2009 submission.
8. Murphy G, Devesa SS, Cross AJ, et al. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer 2011;128:1668–7.
9. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544–73.
10. Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975-2002). Cancer Epidemiol Biomarkers Prev 2006;15:792–7.
11. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58:130–60.
12. Kirkegaard H, Johnsen NF, Christensen J, et al. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. BMJ 2010;341:c5504.
13. Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer 2006;42:216–27.
14. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol 2001;96:2992–3003.
15. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919–32.
16. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology 2010;138:2044–58.
17. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology 2009;137:1621–7.
18. Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001;91:854–62.
19. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35.
20. Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005;97:1679–87.
21. Campbell PT, Deka A, Jacobs EJ, et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology 2010;139:1138–46.
22. Larsson SC, Giovannucci E, Wolk A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care 2005;28:1805–7.
23. Huxley RR, Ansary-Moghaddam A, Clifton P, et al. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer 2009;125:171–80.
24. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556–65.
25. Wang Y, Jacobs EJ, Patel AV, et al. A prospective study of waist circumference and body mass index in relation to colorectal cancer incidence. Cancer Causes Control 2008;19:783–92.
26. Chao A, Thun MJ, Connell CJ, et al. Meat consumption and risk of colorectal cancer. JAMA 2005;293:172–82.
27. Cross AJ, Ferrucci LM, Risch A, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res 2010;70:2406–14.
28. Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology 2010;138:2029–43.
29. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: World Cancer Research Fund/American Institute for Cancer Research; 2007.
30. McCullough ML, Robertson AS, Chao A, et al. A prospective study of whole grains, fruits, vegetables and colon cancer risk. Cancer Causes Control 2003;14:959–70.
31. Terry P, Giovannucci E, Michels KB, et al. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst 2001;93:525–33.
32. Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst 2004;96:1015–22.
33. Secretan B, Straif K, Baan R, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009;10:1033–4.
34. Ferrari P, Jenab M, Norat T, et al. Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 2007;121:2065–72.
35. Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis 2005;7:204–13.
36. Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 2007;369:1603–13.
37. Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741–50.
38. Hildebrand JS, Jacobs EJ, Campbell PT, et al. Colorectal cancer incidence and postmenopausal hormone use by type, recency, and duration in cancer prevention study II. Cancer Epidemiol Biomarkers Prev 2009;18:2835–41.
39. Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA 2008;299:1036–45.
40. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–33.
41. Lieberman DA,Weiss DG. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med 2001;345:555–60.
42. Lafata JE, Divine G, Moon C,Williams LK. Patient-physician colorectal cancer screening discussions and screening use. Am J Prev Med 2006;31:202–9.
43. Rex DK, Johnson DA, Andersone JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol 2009;104:739–50.
44. U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149:627–37.
45. Smith RA, von Eschenbach AC,Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001—testing for early lung cancer detection. CA Cancer J Clin 2001;51:38–75.
46. Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology 2003;124:544–60.
47. Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2006;130:1865–71.
48. Ransohoff DF, Lang CA. Screening for colorectal cancer with the fecal occult blood test: a background paper. American College of Physicians. Ann Intern Med 1997;126:811–22.
49. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult blood screening for colorectal cancer. Lancet 1996;348:1472–7.
50. Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult blood test. Lancet 1996;348:1467–71.
51. Wilson JMG, Junger G. Principles and practice of screening for disease. Geneva: World Health Organization; 1968.
52. Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 1996;334:155–9.
53. Caligiore P, Macrae FA, St John DJ, et al. Peroxidase levels in food: relevance to colorectal cancer screening. Am J Clin Nutr 1982;35:1487–9.
54. Nakajima M, Saito H, Soma Y, et al. Prevention of advanced colorectal cancer by screening using the immunochemical faecal occult blood test: a case-control study. Br J Cancer 2003;89:23–8.
55. Lee KJ, Inoue M, Otani T, et al. Colorectal cancer screening using fecal occult blood test and subsequent risk of colorectal cancer: a prospective cohort study in Japan. Cancer Detect Prev 2007;31:3–11.
56. Zappa M, Csatiglione G, Gazzini G, et al. Effect of faecal occult blood testing on colorectal mortality: results of a population-based case-control study in the district of Florence, Italy. Int J Cancer 1997;73:208–10.
57. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008;135:82–90.
58. Hol L, van Leerdam ME, van Ballegooijen M, et al. Attendance to screening for colorectal cancer in the Netherlands; randomized controlled trial comparing two different forms of faecal occult blood tests and sigmoidoscopy. Gastroenterology 2008;134:A87.
59. Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers.
N Engl J Med 2008;359:1207–17.
60. Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology 2005;129:328–37.
61. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med 2007;35: 2277–84.
62. Hur C, Chung DC, Schoen RE, et al. The management of small polyps found by virtual colonoscopy: results of a decision analysis. Clin Gastroenterol Hepatol 2007;5:237–44.
63. Chu KC, Tarone RE, Chow WH, et al. Temporal patterns in colorectal cancer incidence, survival, and mortality from 1950 through 1990. J Natl Cancer Inst 1994;86:997–1006.
64. Vasen HF, Moslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008;57:704–13.
65. Umar A, Boland CR, Terdiman PJ, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal Cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261–8.
66. Lieberman DA, Rex DR, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–57.
67. Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on colorectal cancer and the American Cancer Society. Gastroenterology 2006;130:1872–85.
68. Lieberman DA, Weiss DG, Harford WV, et al. Five year colon surveillance after screening colonoscopy. Gastroenterology 2007;133:1077–85.
69. Imperiale TF, Glowinski EA, Lin-Cooper C, et al. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med 2008;359:1218–24.
70. Leung WK, Lau JYW, Suen BY, et al. Repeat screening colonoscopy 5 years after normal baseline screening colonoscopy in average-risk Chinese: a prospective study. Am J Gastroenterol 2009;104:2028–34.
71. Brenner H, Haug U, Arndt V, et al. Low risk of colorectal cancer and advanced adenomas more than 10 years after negative colonoscopy. Gastroenterology 2010;138:870–6.
72. Miller H, Mukherjee R, Tian J, et al. Colonoscopy surveillance after polypectomy may be extended beyond five years. J Clin Gastroenterol 2010;44:e162–e166.
73. Chung SJ, Kim YS, Yang SY, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut 2011;60:1537–43.
74. Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33.
75. Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc 2006;64:614–26.
76. Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses following colonoscopic polypectomy. Gastroenterology 2009;136:832–41.
77. Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010;138:
2088–100.
78. Schreiner MA, Weiss DG, Lieberman DA. Proximal and large nonneoplastic serrated polyps: association with synchronous neoplasia at screening colonoscopy and with interval neoplasia at follow- up colonoscopy. Gastroenterology 2010;139:1497–502.
79. Hiraoka S, Kato J, Fujiki S, et al. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology 2010;139:1503–10.
80. Robertson DJ, Burke CA, Welch HG, et al. Using the results of a baseline and a surveillance colonoscopy to predict recurrent adenomas with high-risk characteristics. Ann Intern Med 2009;151:103–9.
81. Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med 2009;150:849–57.
82. Ko CW, Riffle S, Michaels L, et al. Serious complications within 30 days of screening and surveillance colonoscopy: a multicenter study. Clin Gastroenterol Hepatol 2010;8:166–73.
1. American Cancer Society. Colorectal cancer facts & figures 2014–2016. Atlanta: American Cancer Society; 2014.
2. Ries L, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014.
3. Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med 2006;355:2551–7.
4. Bond JH. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 2000;95:3053–63.
5. Schatzkin A, Freedman LS, Dawsey SM, Lanza E. Interpreting precursor studies: what polyp trials tell us about large-bowel cancer. J Natl Cancer Inst 1994;86:1053–7.
6. DevCan: Probability of developing or dying of cancer software, version 6.5.0; Statistical Research and Applications Branch, National Cancer Institute, 2005. http://srab.cancer.gov/devcan [computer program].
7. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2010, based on the November 2009 submission.
8. Murphy G, Devesa SS, Cross AJ, et al. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer 2011;128:1668–7.
9. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544–73.
10. Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975-2002). Cancer Epidemiol Biomarkers Prev 2006;15:792–7.
11. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58:130–60.
12. Kirkegaard H, Johnsen NF, Christensen J, et al. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. BMJ 2010;341:c5504.
13. Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer 2006;42:216–27.
14. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol 2001;96:2992–3003.
15. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919–32.
16. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology 2010;138:2044–58.
17. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology 2009;137:1621–7.
18. Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001;91:854–62.
19. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35.
20. Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005;97:1679–87.
21. Campbell PT, Deka A, Jacobs EJ, et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology 2010;139:1138–46.
22. Larsson SC, Giovannucci E, Wolk A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care 2005;28:1805–7.
23. Huxley RR, Ansary-Moghaddam A, Clifton P, et al. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer 2009;125:171–80.
24. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556–65.
25. Wang Y, Jacobs EJ, Patel AV, et al. A prospective study of waist circumference and body mass index in relation to colorectal cancer incidence. Cancer Causes Control 2008;19:783–92.
26. Chao A, Thun MJ, Connell CJ, et al. Meat consumption and risk of colorectal cancer. JAMA 2005;293:172–82.
27. Cross AJ, Ferrucci LM, Risch A, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res 2010;70:2406–14.
28. Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology 2010;138:2029–43.
29. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: World Cancer Research Fund/American Institute for Cancer Research; 2007.
30. McCullough ML, Robertson AS, Chao A, et al. A prospective study of whole grains, fruits, vegetables and colon cancer risk. Cancer Causes Control 2003;14:959–70.
31. Terry P, Giovannucci E, Michels KB, et al. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst 2001;93:525–33.
32. Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst 2004;96:1015–22.
33. Secretan B, Straif K, Baan R, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009;10:1033–4.
34. Ferrari P, Jenab M, Norat T, et al. Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 2007;121:2065–72.
35. Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis 2005;7:204–13.
36. Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 2007;369:1603–13.
37. Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741–50.
38. Hildebrand JS, Jacobs EJ, Campbell PT, et al. Colorectal cancer incidence and postmenopausal hormone use by type, recency, and duration in cancer prevention study II. Cancer Epidemiol Biomarkers Prev 2009;18:2835–41.
39. Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA 2008;299:1036–45.
40. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–33.
41. Lieberman DA,Weiss DG. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med 2001;345:555–60.
42. Lafata JE, Divine G, Moon C,Williams LK. Patient-physician colorectal cancer screening discussions and screening use. Am J Prev Med 2006;31:202–9.
43. Rex DK, Johnson DA, Andersone JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol 2009;104:739–50.
44. U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149:627–37.
45. Smith RA, von Eschenbach AC,Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001—testing for early lung cancer detection. CA Cancer J Clin 2001;51:38–75.
46. Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology 2003;124:544–60.
47. Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2006;130:1865–71.
48. Ransohoff DF, Lang CA. Screening for colorectal cancer with the fecal occult blood test: a background paper. American College of Physicians. Ann Intern Med 1997;126:811–22.
49. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult blood screening for colorectal cancer. Lancet 1996;348:1472–7.
50. Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult blood test. Lancet 1996;348:1467–71.
51. Wilson JMG, Junger G. Principles and practice of screening for disease. Geneva: World Health Organization; 1968.
52. Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 1996;334:155–9.
53. Caligiore P, Macrae FA, St John DJ, et al. Peroxidase levels in food: relevance to colorectal cancer screening. Am J Clin Nutr 1982;35:1487–9.
54. Nakajima M, Saito H, Soma Y, et al. Prevention of advanced colorectal cancer by screening using the immunochemical faecal occult blood test: a case-control study. Br J Cancer 2003;89:23–8.
55. Lee KJ, Inoue M, Otani T, et al. Colorectal cancer screening using fecal occult blood test and subsequent risk of colorectal cancer: a prospective cohort study in Japan. Cancer Detect Prev 2007;31:3–11.
56. Zappa M, Csatiglione G, Gazzini G, et al. Effect of faecal occult blood testing on colorectal mortality: results of a population-based case-control study in the district of Florence, Italy. Int J Cancer 1997;73:208–10.
57. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008;135:82–90.
58. Hol L, van Leerdam ME, van Ballegooijen M, et al. Attendance to screening for colorectal cancer in the Netherlands; randomized controlled trial comparing two different forms of faecal occult blood tests and sigmoidoscopy. Gastroenterology 2008;134:A87.
59. Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers.
N Engl J Med 2008;359:1207–17.
60. Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology 2005;129:328–37.
61. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med 2007;35: 2277–84.
62. Hur C, Chung DC, Schoen RE, et al. The management of small polyps found by virtual colonoscopy: results of a decision analysis. Clin Gastroenterol Hepatol 2007;5:237–44.
63. Chu KC, Tarone RE, Chow WH, et al. Temporal patterns in colorectal cancer incidence, survival, and mortality from 1950 through 1990. J Natl Cancer Inst 1994;86:997–1006.
64. Vasen HF, Moslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008;57:704–13.
65. Umar A, Boland CR, Terdiman PJ, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal Cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261–8.
66. Lieberman DA, Rex DR, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–57.
67. Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on colorectal cancer and the American Cancer Society. Gastroenterology 2006;130:1872–85.
68. Lieberman DA, Weiss DG, Harford WV, et al. Five year colon surveillance after screening colonoscopy. Gastroenterology 2007;133:1077–85.
69. Imperiale TF, Glowinski EA, Lin-Cooper C, et al. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med 2008;359:1218–24.
70. Leung WK, Lau JYW, Suen BY, et al. Repeat screening colonoscopy 5 years after normal baseline screening colonoscopy in average-risk Chinese: a prospective study. Am J Gastroenterol 2009;104:2028–34.
71. Brenner H, Haug U, Arndt V, et al. Low risk of colorectal cancer and advanced adenomas more than 10 years after negative colonoscopy. Gastroenterology 2010;138:870–6.
72. Miller H, Mukherjee R, Tian J, et al. Colonoscopy surveillance after polypectomy may be extended beyond five years. J Clin Gastroenterol 2010;44:e162–e166.
73. Chung SJ, Kim YS, Yang SY, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut 2011;60:1537–43.
74. Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33.
75. Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc 2006;64:614–26.
76. Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses following colonoscopic polypectomy. Gastroenterology 2009;136:832–41.
77. Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010;138:
2088–100.
78. Schreiner MA, Weiss DG, Lieberman DA. Proximal and large nonneoplastic serrated polyps: association with synchronous neoplasia at screening colonoscopy and with interval neoplasia at follow- up colonoscopy. Gastroenterology 2010;139:1497–502.
79. Hiraoka S, Kato J, Fujiki S, et al. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology 2010;139:1503–10.
80. Robertson DJ, Burke CA, Welch HG, et al. Using the results of a baseline and a surveillance colonoscopy to predict recurrent adenomas with high-risk characteristics. Ann Intern Med 2009;151:103–9.
81. Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med 2009;150:849–57.
82. Ko CW, Riffle S, Michaels L, et al. Serious complications within 30 days of screening and surveillance colonoscopy: a multicenter study. Clin Gastroenterol Hepatol 2010;8:166–73.