User login
Management of Gastroenteropancreatic Neuroendocrine Tumors
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
A young man with psychosis whose heart is racing
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
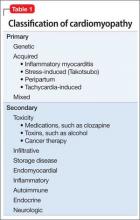
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
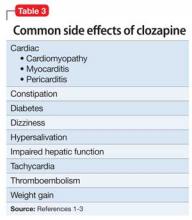
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
Primary Brain Tumors
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Primary central nervous system tumors are relatively rare, but they can cause significant morbidity. They are also among the most lethal of all neoplasms. Brain tumors are the second most common cause of death due to intracranial disease, second only to stroke. The estimated annual incidence of primary brain tumors is approximately 21 per 100,000 individuals in the United States. The incidence of brain tumors varies by gender, age, race, ethnicity, and geography and has increased over time. Gliomas and germ cell tumors are more common in men, whereas meningiomas are twice as common in women. The only validated environmental risk factor for primary brain tumors is exposure to ionizing radiation.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Primary central nervous system tumors are relatively rare, but they can cause significant morbidity. They are also among the most lethal of all neoplasms. Brain tumors are the second most common cause of death due to intracranial disease, second only to stroke. The estimated annual incidence of primary brain tumors is approximately 21 per 100,000 individuals in the United States. The incidence of brain tumors varies by gender, age, race, ethnicity, and geography and has increased over time. Gliomas and germ cell tumors are more common in men, whereas meningiomas are twice as common in women. The only validated environmental risk factor for primary brain tumors is exposure to ionizing radiation.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Primary central nervous system tumors are relatively rare, but they can cause significant morbidity. They are also among the most lethal of all neoplasms. Brain tumors are the second most common cause of death due to intracranial disease, second only to stroke. The estimated annual incidence of primary brain tumors is approximately 21 per 100,000 individuals in the United States. The incidence of brain tumors varies by gender, age, race, ethnicity, and geography and has increased over time. Gliomas and germ cell tumors are more common in men, whereas meningiomas are twice as common in women. The only validated environmental risk factor for primary brain tumors is exposure to ionizing radiation.
To read the full article in PDF:
Opioid use remits, depression remains
Case Forgetful and depressed
Mr. B, age 55, has been a patient at our clinic for 8 years, where he has been under our care for treatment-resistant depression and opioid addiction [read about earlier events in his case in “A life of drugs and ‘downtime’” Current Psychiatry, August 2007, p. 98-103].1 He reports feeling intermittently depressed since his teens and has had 3 near-fatal suicide attempts.
Three years ago, Mr. B reported severe depressive symptoms and short-term memory loss, which undermined his job performance and contributed to interpersonal conflict with his wife. The episode has been continuously severe for 10 months. He was taking sertraline, 150 mg/d, and duloxetine, 60 mg/d, for major depressive disorder (MDD) and sublingual buprenorphine/naloxone, 20 mg/d, for opioid dependence, which was in sustained full remission.2 Mr. B scored 24/30 in the Mini- Mental State Examination, indicating mild cognitive deficit. Negative results of a complete routine laboratory workup rule out an organic cause for his deteriorating cognition.
How would you diagnose Mr. B’s condition at this point?
a) treatment-resistant MDD
b) cognitive disorder not otherwise specified
c) opioid use disorder
d) a and c
The authors' observations
Relapse is a core feature of substance use disorders (SUDs) that contributes significantly to the longstanding functional impairment in patients with a mood disorder. With the relapse rate following substance use treatment estimated at more than 60%,3 SUDs often are described as chronic relapsing conditions. In chronic stress, corticotropin-releasing factor (CRF) is over-sensitized; we believe that acute stress can cause an unhealthy response to an over-expressed CRF system.
To prevent relapse in patients with an over-expressed CRF system, it is crucial to manage stress. One treatment option to consider in preventing relapse is mindfulness-based interventions (MBI). Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally.” In the event of a relapse, awareness and acceptance fostered by mindfulness may aid in recognizing and minimizing unhealthy responses, such as negative thinking that can increase the risk of relapse.
History Remission, then relapse
Mr. B was admitted to inpatient psychiatric unit after a near-fatal suicide attempt 8 years ago and given a diagnosis of MDD recurrent, severe without psychotic features. Trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
Before he presented to our clinic 8 years ago, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist added methylphenidate, 40 mg/d, to augment the antidepressants, but this did not alleviate Mr. B’s depression.
At age 40, he entered a methadone program, began working steadily, and got married. Five years later, he stopped methadone (it is unclear from the chart if his psychiatrist initiated this change). Mr. B’s depression persisted while using opioids and became worse after stopping methadone.
We considered electroconvulsive therapy (ECT) at the time, but switching the antidepressant or starting ECT would address only the persistent depression; buprenorphine/naloxone would target opioid craving. We started a trial of buprenorphine/ naloxone, a partial μ opioid agonist and ĸ opioid antagonist; ĸ receptor antagonism serves as an antidepressant. He responded well to augmentation of his current regimen (mirtazapine, 30 mg at bedtime, and venlafaxine, 225 mg/d) with buprenorphine/naloxone, 16 mg/d.4,5 he reported no anergia and said he felt more motivated and productive.
Mr. B took buprenorphine/naloxone, 32 mg/d, for 4 years until, because of concern for daytime sedation, his outpatient psychiatrist reduced the dose to 20 mg/d. With the lower dosage of buprenorphine/naloxone initiated 4 years ago, Mr. B reported irritability, anhedonia, insomnia, increased self-criticism, and decreased self-care.
How would you treat Mr. B’s depression at this point?
a) switch to a daytime antidepressant
b) adjust the dosage of buprenorphine/ naloxone
c) try ECT
d) try mindfulness-based cognitive therapy
The authors’ observations
Mindfulness meditation (MM) is a meditation practice that cultivates awareness. While learning MM, the practitioner intentionally focuses on awareness—a way of purposely paying attention to the present moment, non-judgmentally, to nurture calmness and self-acceptance. Being conscious of what the practitioner is doing while he is doing it is the core of mindfulness practice.6
Mindfulness-based interventions. We recommended the following forms of MBI to treat Mr. B:
• Mindfulness-based cognitive therapy (MBCT). MBCT is designed to help people who suffer repeated bouts of depression and chronic unhappiness. It combines the ideas of cognitive-behavioral therapy (CBT) with MM practices and attitudes based on cultivating mindfulness.7
• Mindfulness-based stress reduction (MBSR). MBSR brings together MM and physical/breathing exercises to relax body and mind.6
Chronic stress and drug addiction
The literature demonstrates a significant association between acute and chronic stress and motivation to abuse substances. Stress mobilizes the CRF system to stimulate the hypothalamic-pituitary-adrenal (HPA) axis, and extra-hypothalamic actions of CRF can kindle the neuronal circuits responsible for stress-induced anxiety, dysphoria, and drug abuse behaviors.8
A study to evaluate effects of mindfulness on young adult romantic partners’ HPA responses to conflict stress showed that MM has sex-specific effects on neuroendocrine response to interpersonal stress.9 Research has shown that MM practice can decrease stress, increase well-being, and affect brain structure and function.10 Meta-analysis of studies of animal models and humans described how specific interventions intended to encourage pro-social behavior and well-being might produce plasticity-related changes in the brain.11 This work concluded that, by taking responsibility for the mind and the brain by participating in regular mental exercise, plastic changes in the brain promoted could produce lasting beneficial consequences for social and emotional behavior.11
What could be perpetuating Mr. B’s depression?
a) psychosocial stressors
b) over-expression of CRF gene due to psychosocial stressors
c) a and b
Treatment Mindfulness practice
Mr. B was started on CBT to manage anxiety symptoms and cognitive distortions. After 2 months, he reports no improvements in anxiety, depression, or cognitive distortions.
We consider MBI for Mr. B, which was developed by Segal et al7 to help prevent relapse of depression and gain the benefits of MM. There is evidence that MBI can prevent relapse of SUDs.12 Mr. B’s MBI practice is based on MBCT, as outlined by Segal et al.7 He attends biweekly, 45-minute therapy sessions at our outpatient clinic. During these sessions, MM is practiced for 10 minutes under a psychiatrist’s supervision. The MBCT manual calls for 45 minutes of MM practice but, during the 10-minute session, we instruct Mr. B to independently practice MM at home. Mr. B is assessed for relapses, and drug cravings; a urine toxicology screen is performed every 6 months.
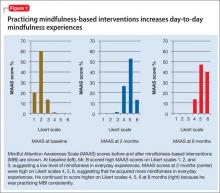
We score Mr. B’s day-to-day level of mindfulness experience, depression, and anxiety symptoms before starting MBI and after 8 weeks of practicing MBI (Figure 1). Mindfulness is scored with the Mindful Attention Awareness Scale (MAAS), a valid, reliable scale.13 The MAAS comprises 15 items designed to reflect mindfulness in everyday experiences, including awareness and attention to thoughts, emotions, actions, and physical states. Items are rated on a 6-point Likert-type scale of 1 (“almost never”) to 6 (“almost always”). A typical item on MAAS is “I find myself doing things without paying attention.”
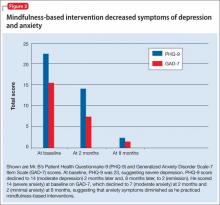
Depression and anxiety symptoms are measured using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Scale-7 (GAD-7) Item Scale. Mr. B scores a 23 on PHQ-9, indicating severe depression (he reports that he finds it ‘‘extremely difficult” to function) (Figure 2).
There is evidence to support the use of PHQ-9 for measurement-based care in the psychiatric population.14 PHQ-9 does not capture anxiety, which is a strong predicator of suicidal behavior; therefore, we use GAD-7 to measure the severity of Mr. B’s subjective anxiety.15 He scores a 14 on GAD-7 and reports that it is “very difficult” for him to function.
Mr. B is retested after 8 weeks. During those 8 weeks, he was instructed by audio guidance in body scan technique. He practices MBI techniques for 45 minutes every morning between 5 AM and 6 AM.6
After 3 months of MBI, Mr. B is promoted at work and reports that he is handling more responsibilities. He is stressed at his new job and, subsequently, experiences a relapse of anxiety symptoms and insomnia. Partly, this is because Mr. B is not able to consistently practice MBI and misses a few outpatient appointments. In the meantime, he has difficulties with sleep and concentration and anxiety symptoms.
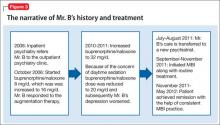
The treating psychiatrist reassures Mr. B and provides support to restart MBI. He manages to attend outpatient clinic appointments consistently and shows interest in practicing MBI daily. Later, he reports practicing MBI consistently along with his routine treatment at our clinic. The timeline of Mr. B’s history and treatment are summarized in Figure 3.
The authors’ observations
Mr. B’s CRF may have been down-regulated by MBI. This, in turn, decreased his depressive and anxiety symptoms, thereby helping to prevent relapse of depression and substance abuse. He benefited from MBI practices in several areas of his life, which can be described with the acronym FACES.10
Flexible. Mr. B became more cognitively flexible. He started to realize that “thoughts are not facts.”7 This change was reflected in his relationship with his wife. His wife came to one of our sessions because she noticed significant change in his attitude toward her. Their marriage of 15 years was riddled with conflict and his wife was excited to see the improvement he achieved within the short time of practicing MBI.
Adaptive. He became more adaptive to changes at the work place and reported that he is enjoying his work. This is a change from his feeling that his job was a burden, as he observed in our earlier sessions.
Coherent. He became more cognitively rational. He reported improvement in his memory and concentration. Five months after initiation of MBI and MM training, he was promoted and could cope with the stress at work.
Energized. Initially, he had said that he never wanted to be part of his extended family. During a session toward the end of the treatment, he mentioned that he made an effort to contact his extended family and reported that he found it more meaningful now to be reconnected with them.
Stable. He became more emotionally stable. He did not have the urge to use drugs and he did not relapse.
As we hypothesized, for Mr. B, practicing MBI was associated with abstinence from substance use, increased mindfulness, acceptance of mental health problems, and remission of psychiatric symptoms.
Bottom Line
Mindfulness-based interventions provide patients with tools to target symptoms such as poor affect regulation, poor impulse control, and rumination. Evidence supports that using MBI in addition to the usual treatment can prevent relapse of a substance use disorder.
Related Resources
• Sipe WE, Eisendrath SJ. Mindfulness-based cognitive therapy: theory and practice. Can J Psychiatry. 2012;57(2):63-69.• Lau MA, Grabovac AD. Mindfulness-based interventions: Effective for depression and anxiety. Current Psychiatry. 2009;8(12):39-55.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Buprenorphine/naloxone • Quetiapine • Seroquel
Suboxone
Bupropion • Wellbutrin Sertraline • Zoloft
Duloxetine • Cymbalta Trazodone • Desyrel
Methadone • Dolophine Venlafaxine • Effexor
Methylphenidate • Ritalin, Concerta
Acknowledgement
The manuscript preparation of Maju Mathew Koola, MD, DPM was supported by the NIMH T32 grant MH067533-07 (PI: William T. Carpenter, MD) and the American Psychiatric Association/Kempf Fund Award for Research Development in Psychobiological Psychiatry (PI: Koola). The treating Psychiatrist PGY-5 (2011-2012) Addiction Psychiatry fellow (Dr. Varghese) was supervised by Dr. Eiger. Drs. Koola and Varghese contributed equally with the manuscript preparation and are joint first authors. Dr. Varghese received a second prize for a poster presentation of this case report at the 34th Indo American Psychiatric Association meeting in San Francisco, CA, May 19, 2013. Christina Mathew, MD, also contributed with manuscript preparation.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufactures of competing products.
1. Tan EM, Eiger RI, Roth JD. A life of drugs and ‘downtime.’ Current Psychiatry. 2007;6(8):98-103.
2. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington, DC, American Psychiatric Association; 2000.
3. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
4. Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects—a possible opioid involvement in severe depression? J Mol Neurosci. 2002; 18(1-2):143-149.
5. Sikka P, Kaushik S, Kumar G, et al. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazepine) and their interaction with morphine and naloxone in mice. J Pharm Bioallied Sci. 2011;3(3):412-416.
6. Kabat-Zinn J. Full catastrophe living. 15th ed. New York, NY: Bantam Books; 1990.
7. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
8. Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3-14.
9. Laurent H, Laurent S, Hertz R, et al. Sex-specific effects of mindfulness on romantic partners’ cortisol responses to conflict and relations with psychological adjustment. Psychoneuroendocrinology. 2013;38(12):2905-2913.
10. Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: W.W. Norton & Company; 2007.
11. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689-695.
12. Bowen S, Chawla N, Collins SE, et al. Mindfulness-based prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295-305.
13. Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. (2001). Psychol Assess. 2011;23(4):1034-1040; discussion 1041-1046.
14. Koola MM, Fawcett JA, Kelly DL. Case report on the management of depression in schizoaffective disorder, bipolar type focusing on lithium levels and measurement-based care. J Nerv Ment Dis. 2011;199(12):989-990.
15. Nock MK, Hwang I, Sampson N, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6(8):e1000123. doi: 10.1371/journal.pmed.1000123.
Case Forgetful and depressed
Mr. B, age 55, has been a patient at our clinic for 8 years, where he has been under our care for treatment-resistant depression and opioid addiction [read about earlier events in his case in “A life of drugs and ‘downtime’” Current Psychiatry, August 2007, p. 98-103].1 He reports feeling intermittently depressed since his teens and has had 3 near-fatal suicide attempts.
Three years ago, Mr. B reported severe depressive symptoms and short-term memory loss, which undermined his job performance and contributed to interpersonal conflict with his wife. The episode has been continuously severe for 10 months. He was taking sertraline, 150 mg/d, and duloxetine, 60 mg/d, for major depressive disorder (MDD) and sublingual buprenorphine/naloxone, 20 mg/d, for opioid dependence, which was in sustained full remission.2 Mr. B scored 24/30 in the Mini- Mental State Examination, indicating mild cognitive deficit. Negative results of a complete routine laboratory workup rule out an organic cause for his deteriorating cognition.
How would you diagnose Mr. B’s condition at this point?
a) treatment-resistant MDD
b) cognitive disorder not otherwise specified
c) opioid use disorder
d) a and c
The authors' observations
Relapse is a core feature of substance use disorders (SUDs) that contributes significantly to the longstanding functional impairment in patients with a mood disorder. With the relapse rate following substance use treatment estimated at more than 60%,3 SUDs often are described as chronic relapsing conditions. In chronic stress, corticotropin-releasing factor (CRF) is over-sensitized; we believe that acute stress can cause an unhealthy response to an over-expressed CRF system.
To prevent relapse in patients with an over-expressed CRF system, it is crucial to manage stress. One treatment option to consider in preventing relapse is mindfulness-based interventions (MBI). Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally.” In the event of a relapse, awareness and acceptance fostered by mindfulness may aid in recognizing and minimizing unhealthy responses, such as negative thinking that can increase the risk of relapse.
History Remission, then relapse
Mr. B was admitted to inpatient psychiatric unit after a near-fatal suicide attempt 8 years ago and given a diagnosis of MDD recurrent, severe without psychotic features. Trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
Before he presented to our clinic 8 years ago, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist added methylphenidate, 40 mg/d, to augment the antidepressants, but this did not alleviate Mr. B’s depression.
At age 40, he entered a methadone program, began working steadily, and got married. Five years later, he stopped methadone (it is unclear from the chart if his psychiatrist initiated this change). Mr. B’s depression persisted while using opioids and became worse after stopping methadone.
We considered electroconvulsive therapy (ECT) at the time, but switching the antidepressant or starting ECT would address only the persistent depression; buprenorphine/naloxone would target opioid craving. We started a trial of buprenorphine/ naloxone, a partial μ opioid agonist and ĸ opioid antagonist; ĸ receptor antagonism serves as an antidepressant. He responded well to augmentation of his current regimen (mirtazapine, 30 mg at bedtime, and venlafaxine, 225 mg/d) with buprenorphine/naloxone, 16 mg/d.4,5 he reported no anergia and said he felt more motivated and productive.
Mr. B took buprenorphine/naloxone, 32 mg/d, for 4 years until, because of concern for daytime sedation, his outpatient psychiatrist reduced the dose to 20 mg/d. With the lower dosage of buprenorphine/naloxone initiated 4 years ago, Mr. B reported irritability, anhedonia, insomnia, increased self-criticism, and decreased self-care.
How would you treat Mr. B’s depression at this point?
a) switch to a daytime antidepressant
b) adjust the dosage of buprenorphine/ naloxone
c) try ECT
d) try mindfulness-based cognitive therapy
The authors’ observations
Mindfulness meditation (MM) is a meditation practice that cultivates awareness. While learning MM, the practitioner intentionally focuses on awareness—a way of purposely paying attention to the present moment, non-judgmentally, to nurture calmness and self-acceptance. Being conscious of what the practitioner is doing while he is doing it is the core of mindfulness practice.6
Mindfulness-based interventions. We recommended the following forms of MBI to treat Mr. B:
• Mindfulness-based cognitive therapy (MBCT). MBCT is designed to help people who suffer repeated bouts of depression and chronic unhappiness. It combines the ideas of cognitive-behavioral therapy (CBT) with MM practices and attitudes based on cultivating mindfulness.7
• Mindfulness-based stress reduction (MBSR). MBSR brings together MM and physical/breathing exercises to relax body and mind.6
Chronic stress and drug addiction
The literature demonstrates a significant association between acute and chronic stress and motivation to abuse substances. Stress mobilizes the CRF system to stimulate the hypothalamic-pituitary-adrenal (HPA) axis, and extra-hypothalamic actions of CRF can kindle the neuronal circuits responsible for stress-induced anxiety, dysphoria, and drug abuse behaviors.8
A study to evaluate effects of mindfulness on young adult romantic partners’ HPA responses to conflict stress showed that MM has sex-specific effects on neuroendocrine response to interpersonal stress.9 Research has shown that MM practice can decrease stress, increase well-being, and affect brain structure and function.10 Meta-analysis of studies of animal models and humans described how specific interventions intended to encourage pro-social behavior and well-being might produce plasticity-related changes in the brain.11 This work concluded that, by taking responsibility for the mind and the brain by participating in regular mental exercise, plastic changes in the brain promoted could produce lasting beneficial consequences for social and emotional behavior.11
What could be perpetuating Mr. B’s depression?
a) psychosocial stressors
b) over-expression of CRF gene due to psychosocial stressors
c) a and b
Treatment Mindfulness practice
Mr. B was started on CBT to manage anxiety symptoms and cognitive distortions. After 2 months, he reports no improvements in anxiety, depression, or cognitive distortions.
We consider MBI for Mr. B, which was developed by Segal et al7 to help prevent relapse of depression and gain the benefits of MM. There is evidence that MBI can prevent relapse of SUDs.12 Mr. B’s MBI practice is based on MBCT, as outlined by Segal et al.7 He attends biweekly, 45-minute therapy sessions at our outpatient clinic. During these sessions, MM is practiced for 10 minutes under a psychiatrist’s supervision. The MBCT manual calls for 45 minutes of MM practice but, during the 10-minute session, we instruct Mr. B to independently practice MM at home. Mr. B is assessed for relapses, and drug cravings; a urine toxicology screen is performed every 6 months.
We score Mr. B’s day-to-day level of mindfulness experience, depression, and anxiety symptoms before starting MBI and after 8 weeks of practicing MBI (Figure 1). Mindfulness is scored with the Mindful Attention Awareness Scale (MAAS), a valid, reliable scale.13 The MAAS comprises 15 items designed to reflect mindfulness in everyday experiences, including awareness and attention to thoughts, emotions, actions, and physical states. Items are rated on a 6-point Likert-type scale of 1 (“almost never”) to 6 (“almost always”). A typical item on MAAS is “I find myself doing things without paying attention.”
Depression and anxiety symptoms are measured using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Scale-7 (GAD-7) Item Scale. Mr. B scores a 23 on PHQ-9, indicating severe depression (he reports that he finds it ‘‘extremely difficult” to function) (Figure 2).
There is evidence to support the use of PHQ-9 for measurement-based care in the psychiatric population.14 PHQ-9 does not capture anxiety, which is a strong predicator of suicidal behavior; therefore, we use GAD-7 to measure the severity of Mr. B’s subjective anxiety.15 He scores a 14 on GAD-7 and reports that it is “very difficult” for him to function.
Mr. B is retested after 8 weeks. During those 8 weeks, he was instructed by audio guidance in body scan technique. He practices MBI techniques for 45 minutes every morning between 5 AM and 6 AM.6
After 3 months of MBI, Mr. B is promoted at work and reports that he is handling more responsibilities. He is stressed at his new job and, subsequently, experiences a relapse of anxiety symptoms and insomnia. Partly, this is because Mr. B is not able to consistently practice MBI and misses a few outpatient appointments. In the meantime, he has difficulties with sleep and concentration and anxiety symptoms.
The treating psychiatrist reassures Mr. B and provides support to restart MBI. He manages to attend outpatient clinic appointments consistently and shows interest in practicing MBI daily. Later, he reports practicing MBI consistently along with his routine treatment at our clinic. The timeline of Mr. B’s history and treatment are summarized in Figure 3.
The authors’ observations
Mr. B’s CRF may have been down-regulated by MBI. This, in turn, decreased his depressive and anxiety symptoms, thereby helping to prevent relapse of depression and substance abuse. He benefited from MBI practices in several areas of his life, which can be described with the acronym FACES.10
Flexible. Mr. B became more cognitively flexible. He started to realize that “thoughts are not facts.”7 This change was reflected in his relationship with his wife. His wife came to one of our sessions because she noticed significant change in his attitude toward her. Their marriage of 15 years was riddled with conflict and his wife was excited to see the improvement he achieved within the short time of practicing MBI.
Adaptive. He became more adaptive to changes at the work place and reported that he is enjoying his work. This is a change from his feeling that his job was a burden, as he observed in our earlier sessions.
Coherent. He became more cognitively rational. He reported improvement in his memory and concentration. Five months after initiation of MBI and MM training, he was promoted and could cope with the stress at work.
Energized. Initially, he had said that he never wanted to be part of his extended family. During a session toward the end of the treatment, he mentioned that he made an effort to contact his extended family and reported that he found it more meaningful now to be reconnected with them.
Stable. He became more emotionally stable. He did not have the urge to use drugs and he did not relapse.
As we hypothesized, for Mr. B, practicing MBI was associated with abstinence from substance use, increased mindfulness, acceptance of mental health problems, and remission of psychiatric symptoms.
Bottom Line
Mindfulness-based interventions provide patients with tools to target symptoms such as poor affect regulation, poor impulse control, and rumination. Evidence supports that using MBI in addition to the usual treatment can prevent relapse of a substance use disorder.
Related Resources
• Sipe WE, Eisendrath SJ. Mindfulness-based cognitive therapy: theory and practice. Can J Psychiatry. 2012;57(2):63-69.• Lau MA, Grabovac AD. Mindfulness-based interventions: Effective for depression and anxiety. Current Psychiatry. 2009;8(12):39-55.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Buprenorphine/naloxone • Quetiapine • Seroquel
Suboxone
Bupropion • Wellbutrin Sertraline • Zoloft
Duloxetine • Cymbalta Trazodone • Desyrel
Methadone • Dolophine Venlafaxine • Effexor
Methylphenidate • Ritalin, Concerta
Acknowledgement
The manuscript preparation of Maju Mathew Koola, MD, DPM was supported by the NIMH T32 grant MH067533-07 (PI: William T. Carpenter, MD) and the American Psychiatric Association/Kempf Fund Award for Research Development in Psychobiological Psychiatry (PI: Koola). The treating Psychiatrist PGY-5 (2011-2012) Addiction Psychiatry fellow (Dr. Varghese) was supervised by Dr. Eiger. Drs. Koola and Varghese contributed equally with the manuscript preparation and are joint first authors. Dr. Varghese received a second prize for a poster presentation of this case report at the 34th Indo American Psychiatric Association meeting in San Francisco, CA, May 19, 2013. Christina Mathew, MD, also contributed with manuscript preparation.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufactures of competing products.
Case Forgetful and depressed
Mr. B, age 55, has been a patient at our clinic for 8 years, where he has been under our care for treatment-resistant depression and opioid addiction [read about earlier events in his case in “A life of drugs and ‘downtime’” Current Psychiatry, August 2007, p. 98-103].1 He reports feeling intermittently depressed since his teens and has had 3 near-fatal suicide attempts.
Three years ago, Mr. B reported severe depressive symptoms and short-term memory loss, which undermined his job performance and contributed to interpersonal conflict with his wife. The episode has been continuously severe for 10 months. He was taking sertraline, 150 mg/d, and duloxetine, 60 mg/d, for major depressive disorder (MDD) and sublingual buprenorphine/naloxone, 20 mg/d, for opioid dependence, which was in sustained full remission.2 Mr. B scored 24/30 in the Mini- Mental State Examination, indicating mild cognitive deficit. Negative results of a complete routine laboratory workup rule out an organic cause for his deteriorating cognition.
How would you diagnose Mr. B’s condition at this point?
a) treatment-resistant MDD
b) cognitive disorder not otherwise specified
c) opioid use disorder
d) a and c
The authors' observations
Relapse is a core feature of substance use disorders (SUDs) that contributes significantly to the longstanding functional impairment in patients with a mood disorder. With the relapse rate following substance use treatment estimated at more than 60%,3 SUDs often are described as chronic relapsing conditions. In chronic stress, corticotropin-releasing factor (CRF) is over-sensitized; we believe that acute stress can cause an unhealthy response to an over-expressed CRF system.
To prevent relapse in patients with an over-expressed CRF system, it is crucial to manage stress. One treatment option to consider in preventing relapse is mindfulness-based interventions (MBI). Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally.” In the event of a relapse, awareness and acceptance fostered by mindfulness may aid in recognizing and minimizing unhealthy responses, such as negative thinking that can increase the risk of relapse.
History Remission, then relapse
Mr. B was admitted to inpatient psychiatric unit after a near-fatal suicide attempt 8 years ago and given a diagnosis of MDD recurrent, severe without psychotic features. Trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
Before he presented to our clinic 8 years ago, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist added methylphenidate, 40 mg/d, to augment the antidepressants, but this did not alleviate Mr. B’s depression.
At age 40, he entered a methadone program, began working steadily, and got married. Five years later, he stopped methadone (it is unclear from the chart if his psychiatrist initiated this change). Mr. B’s depression persisted while using opioids and became worse after stopping methadone.
We considered electroconvulsive therapy (ECT) at the time, but switching the antidepressant or starting ECT would address only the persistent depression; buprenorphine/naloxone would target opioid craving. We started a trial of buprenorphine/ naloxone, a partial μ opioid agonist and ĸ opioid antagonist; ĸ receptor antagonism serves as an antidepressant. He responded well to augmentation of his current regimen (mirtazapine, 30 mg at bedtime, and venlafaxine, 225 mg/d) with buprenorphine/naloxone, 16 mg/d.4,5 he reported no anergia and said he felt more motivated and productive.
Mr. B took buprenorphine/naloxone, 32 mg/d, for 4 years until, because of concern for daytime sedation, his outpatient psychiatrist reduced the dose to 20 mg/d. With the lower dosage of buprenorphine/naloxone initiated 4 years ago, Mr. B reported irritability, anhedonia, insomnia, increased self-criticism, and decreased self-care.
How would you treat Mr. B’s depression at this point?
a) switch to a daytime antidepressant
b) adjust the dosage of buprenorphine/ naloxone
c) try ECT
d) try mindfulness-based cognitive therapy
The authors’ observations
Mindfulness meditation (MM) is a meditation practice that cultivates awareness. While learning MM, the practitioner intentionally focuses on awareness—a way of purposely paying attention to the present moment, non-judgmentally, to nurture calmness and self-acceptance. Being conscious of what the practitioner is doing while he is doing it is the core of mindfulness practice.6
Mindfulness-based interventions. We recommended the following forms of MBI to treat Mr. B:
• Mindfulness-based cognitive therapy (MBCT). MBCT is designed to help people who suffer repeated bouts of depression and chronic unhappiness. It combines the ideas of cognitive-behavioral therapy (CBT) with MM practices and attitudes based on cultivating mindfulness.7
• Mindfulness-based stress reduction (MBSR). MBSR brings together MM and physical/breathing exercises to relax body and mind.6
Chronic stress and drug addiction
The literature demonstrates a significant association between acute and chronic stress and motivation to abuse substances. Stress mobilizes the CRF system to stimulate the hypothalamic-pituitary-adrenal (HPA) axis, and extra-hypothalamic actions of CRF can kindle the neuronal circuits responsible for stress-induced anxiety, dysphoria, and drug abuse behaviors.8
A study to evaluate effects of mindfulness on young adult romantic partners’ HPA responses to conflict stress showed that MM has sex-specific effects on neuroendocrine response to interpersonal stress.9 Research has shown that MM practice can decrease stress, increase well-being, and affect brain structure and function.10 Meta-analysis of studies of animal models and humans described how specific interventions intended to encourage pro-social behavior and well-being might produce plasticity-related changes in the brain.11 This work concluded that, by taking responsibility for the mind and the brain by participating in regular mental exercise, plastic changes in the brain promoted could produce lasting beneficial consequences for social and emotional behavior.11
What could be perpetuating Mr. B’s depression?
a) psychosocial stressors
b) over-expression of CRF gene due to psychosocial stressors
c) a and b
Treatment Mindfulness practice
Mr. B was started on CBT to manage anxiety symptoms and cognitive distortions. After 2 months, he reports no improvements in anxiety, depression, or cognitive distortions.
We consider MBI for Mr. B, which was developed by Segal et al7 to help prevent relapse of depression and gain the benefits of MM. There is evidence that MBI can prevent relapse of SUDs.12 Mr. B’s MBI practice is based on MBCT, as outlined by Segal et al.7 He attends biweekly, 45-minute therapy sessions at our outpatient clinic. During these sessions, MM is practiced for 10 minutes under a psychiatrist’s supervision. The MBCT manual calls for 45 minutes of MM practice but, during the 10-minute session, we instruct Mr. B to independently practice MM at home. Mr. B is assessed for relapses, and drug cravings; a urine toxicology screen is performed every 6 months.
We score Mr. B’s day-to-day level of mindfulness experience, depression, and anxiety symptoms before starting MBI and after 8 weeks of practicing MBI (Figure 1). Mindfulness is scored with the Mindful Attention Awareness Scale (MAAS), a valid, reliable scale.13 The MAAS comprises 15 items designed to reflect mindfulness in everyday experiences, including awareness and attention to thoughts, emotions, actions, and physical states. Items are rated on a 6-point Likert-type scale of 1 (“almost never”) to 6 (“almost always”). A typical item on MAAS is “I find myself doing things without paying attention.”
Depression and anxiety symptoms are measured using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Scale-7 (GAD-7) Item Scale. Mr. B scores a 23 on PHQ-9, indicating severe depression (he reports that he finds it ‘‘extremely difficult” to function) (Figure 2).
There is evidence to support the use of PHQ-9 for measurement-based care in the psychiatric population.14 PHQ-9 does not capture anxiety, which is a strong predicator of suicidal behavior; therefore, we use GAD-7 to measure the severity of Mr. B’s subjective anxiety.15 He scores a 14 on GAD-7 and reports that it is “very difficult” for him to function.
Mr. B is retested after 8 weeks. During those 8 weeks, he was instructed by audio guidance in body scan technique. He practices MBI techniques for 45 minutes every morning between 5 AM and 6 AM.6
After 3 months of MBI, Mr. B is promoted at work and reports that he is handling more responsibilities. He is stressed at his new job and, subsequently, experiences a relapse of anxiety symptoms and insomnia. Partly, this is because Mr. B is not able to consistently practice MBI and misses a few outpatient appointments. In the meantime, he has difficulties with sleep and concentration and anxiety symptoms.
The treating psychiatrist reassures Mr. B and provides support to restart MBI. He manages to attend outpatient clinic appointments consistently and shows interest in practicing MBI daily. Later, he reports practicing MBI consistently along with his routine treatment at our clinic. The timeline of Mr. B’s history and treatment are summarized in Figure 3.
The authors’ observations
Mr. B’s CRF may have been down-regulated by MBI. This, in turn, decreased his depressive and anxiety symptoms, thereby helping to prevent relapse of depression and substance abuse. He benefited from MBI practices in several areas of his life, which can be described with the acronym FACES.10
Flexible. Mr. B became more cognitively flexible. He started to realize that “thoughts are not facts.”7 This change was reflected in his relationship with his wife. His wife came to one of our sessions because she noticed significant change in his attitude toward her. Their marriage of 15 years was riddled with conflict and his wife was excited to see the improvement he achieved within the short time of practicing MBI.
Adaptive. He became more adaptive to changes at the work place and reported that he is enjoying his work. This is a change from his feeling that his job was a burden, as he observed in our earlier sessions.
Coherent. He became more cognitively rational. He reported improvement in his memory and concentration. Five months after initiation of MBI and MM training, he was promoted and could cope with the stress at work.
Energized. Initially, he had said that he never wanted to be part of his extended family. During a session toward the end of the treatment, he mentioned that he made an effort to contact his extended family and reported that he found it more meaningful now to be reconnected with them.
Stable. He became more emotionally stable. He did not have the urge to use drugs and he did not relapse.
As we hypothesized, for Mr. B, practicing MBI was associated with abstinence from substance use, increased mindfulness, acceptance of mental health problems, and remission of psychiatric symptoms.
Bottom Line
Mindfulness-based interventions provide patients with tools to target symptoms such as poor affect regulation, poor impulse control, and rumination. Evidence supports that using MBI in addition to the usual treatment can prevent relapse of a substance use disorder.
Related Resources
• Sipe WE, Eisendrath SJ. Mindfulness-based cognitive therapy: theory and practice. Can J Psychiatry. 2012;57(2):63-69.• Lau MA, Grabovac AD. Mindfulness-based interventions: Effective for depression and anxiety. Current Psychiatry. 2009;8(12):39-55.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Buprenorphine/naloxone • Quetiapine • Seroquel
Suboxone
Bupropion • Wellbutrin Sertraline • Zoloft
Duloxetine • Cymbalta Trazodone • Desyrel
Methadone • Dolophine Venlafaxine • Effexor
Methylphenidate • Ritalin, Concerta
Acknowledgement
The manuscript preparation of Maju Mathew Koola, MD, DPM was supported by the NIMH T32 grant MH067533-07 (PI: William T. Carpenter, MD) and the American Psychiatric Association/Kempf Fund Award for Research Development in Psychobiological Psychiatry (PI: Koola). The treating Psychiatrist PGY-5 (2011-2012) Addiction Psychiatry fellow (Dr. Varghese) was supervised by Dr. Eiger. Drs. Koola and Varghese contributed equally with the manuscript preparation and are joint first authors. Dr. Varghese received a second prize for a poster presentation of this case report at the 34th Indo American Psychiatric Association meeting in San Francisco, CA, May 19, 2013. Christina Mathew, MD, also contributed with manuscript preparation.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufactures of competing products.
1. Tan EM, Eiger RI, Roth JD. A life of drugs and ‘downtime.’ Current Psychiatry. 2007;6(8):98-103.
2. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington, DC, American Psychiatric Association; 2000.
3. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
4. Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects—a possible opioid involvement in severe depression? J Mol Neurosci. 2002; 18(1-2):143-149.
5. Sikka P, Kaushik S, Kumar G, et al. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazepine) and their interaction with morphine and naloxone in mice. J Pharm Bioallied Sci. 2011;3(3):412-416.
6. Kabat-Zinn J. Full catastrophe living. 15th ed. New York, NY: Bantam Books; 1990.
7. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
8. Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3-14.
9. Laurent H, Laurent S, Hertz R, et al. Sex-specific effects of mindfulness on romantic partners’ cortisol responses to conflict and relations with psychological adjustment. Psychoneuroendocrinology. 2013;38(12):2905-2913.
10. Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: W.W. Norton & Company; 2007.
11. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689-695.
12. Bowen S, Chawla N, Collins SE, et al. Mindfulness-based prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295-305.
13. Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. (2001). Psychol Assess. 2011;23(4):1034-1040; discussion 1041-1046.
14. Koola MM, Fawcett JA, Kelly DL. Case report on the management of depression in schizoaffective disorder, bipolar type focusing on lithium levels and measurement-based care. J Nerv Ment Dis. 2011;199(12):989-990.
15. Nock MK, Hwang I, Sampson N, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6(8):e1000123. doi: 10.1371/journal.pmed.1000123.
1. Tan EM, Eiger RI, Roth JD. A life of drugs and ‘downtime.’ Current Psychiatry. 2007;6(8):98-103.
2. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington, DC, American Psychiatric Association; 2000.
3. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
4. Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects—a possible opioid involvement in severe depression? J Mol Neurosci. 2002; 18(1-2):143-149.
5. Sikka P, Kaushik S, Kumar G, et al. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazepine) and their interaction with morphine and naloxone in mice. J Pharm Bioallied Sci. 2011;3(3):412-416.
6. Kabat-Zinn J. Full catastrophe living. 15th ed. New York, NY: Bantam Books; 1990.
7. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
8. Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3-14.
9. Laurent H, Laurent S, Hertz R, et al. Sex-specific effects of mindfulness on romantic partners’ cortisol responses to conflict and relations with psychological adjustment. Psychoneuroendocrinology. 2013;38(12):2905-2913.
10. Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: W.W. Norton & Company; 2007.
11. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689-695.
12. Bowen S, Chawla N, Collins SE, et al. Mindfulness-based prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295-305.
13. Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. (2001). Psychol Assess. 2011;23(4):1034-1040; discussion 1041-1046.
14. Koola MM, Fawcett JA, Kelly DL. Case report on the management of depression in schizoaffective disorder, bipolar type focusing on lithium levels and measurement-based care. J Nerv Ment Dis. 2011;199(12):989-990.
15. Nock MK, Hwang I, Sampson N, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6(8):e1000123. doi: 10.1371/journal.pmed.1000123.
Adolescent Obesity and Its Risks: How to Screen and When to Refer
From the Department of Pediatrics, University of Wisconsin, Madison, WI.
Abstract
- Objective: To provide information that will assist clinicians in assessing and addressing risk for obesity-related comorbidities in adolescents.
- Methods: Review of the literature.
- Results: Childhood obesity is a major public health concern. Prevention of obesity or early detection of its health consequences are important responsibilities or opportunities for primary care clinicians. While body mass index (BMI) screening is valuable, insulin resistance and other obesity-related comorbidities can develop even when BMI falls below the 95th percentile threshold for obesity. Detailed history and physical examination can help identify comorbidities and guide diagnostic evaluation. Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention.
- Conclusion: For optimal health outcomes, management of adolescent obesity and associated comorbidities is should be adapted based on an individual’s overall risk rather than BMI alone.
Case Study
Initial Presentation
A 14-year-old Hispanic male presents for a well child check.
History and Physical Examination
The patient and his mother have no complaints or concerns. A comprehensive review of systems is positive for fatigue and snoring but is otherwise unremarkable. Past medical history is unremarkable except for mild intermittent asthma. Family history is positive for type 2 diabetes in paternal grandmother and a maternal uncle and cardiovascular disease and hypertension in multiple extended family members. Both maternal and paternal grandparents are from Mexico.
Vital signs are within normal limits. Height is 160 cm (30th percentile for age), weight is 58.4 kg (75th percentile for age), and body mass index (BMI) is 22.8 kg/m2 (85th percentile for age). Blood pressure is 127/81 mm Hg (95th percentile for age and gender). Physical exam is pertinent for acanthosis nigricans on neck and axilla and nonviolaceous striae on abdomen. Waist circumference is 88 cm (90th percentile for age and ethnicity). Otherwise, physical exam is within normal limits.
• Does this child’s physical examination findings pose a cause for concern?
Yes. A key concept is that while obesity is widespread, the adverse health complications of adiposity and overnutrition affect some children much earlier and more profoundly than others. Some children exhibit adiposity-associated comorbidities even prior to meeting obesity criteria defined by BMI. Careful history and examination can help identify those most at risk for developing adiposity-associated comorbidities, prompting earlier intervention and, when appropriate, subspecialty referral.
Obesity is caused by a complex interplay of genetic, environmental, and metabolic programming, especially early in life, and lifestyle habits [1,2]. The vast majority of obesity is due to excess nutrition leading to energy imbalance, while less than 1% is due to endocrine or syndromic causes [3]. Obesity is defined as excessive body fat and is often estimated indirectly by using a surrogate marker, BMI. Diagnostically, a BMI > 95th percentile for age on sex-specific CDC growth charts is defined as obese, while a BMI from the 85th to 94th percentile is defined as overweight [4]. Using these criteria, the prevalence of childhood obesity more than tripled in the past 3 decades [5], leading to its classification as an epidemic and public health crisis [2]. Today, an estimated 12.5 million American children are obese [5]. For adolescents specifically, the prevalence of obesity is 18.4%, with more than one-third overweight [6].
Childhood obesity is associated with both short- and long-term morbidities including insulin resistance and type 2 diabetes, hypertension, dyslipidemia, asthma, obstructive sleep apnea, psychosocial problems, and decreased quality of life [7,8]. Obese children, particularly older children and adolescents, are more likely become obese adults [2,7]. Obesity in adulthood is associated with both significant morbidity and premature death [9]. Individual characteristics such as lifestyle habits, fitness level, and genetic predisposition influence the likelihood of development of both obesity and associated comorbidities [10].
The burden of obesity and its associated comorbidities are not equally distributed among racial/ethnic and socioeconomic groups. Hispanic and non-Hispanic black children are much more likely to be obese and overweight than non-Hispanic white children [6]. Low socioeconomic status is associated with increased rates of obesity in certain subgroups, including adolescents [2]. In addition, certain ethnic/racial minorities are more likely to develop obesity-associated comorbidities, such as insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). With regard to insulin resistance and development of type 2 diabetes, the risk is greatest in Native Americans, but there is also increased risk in Hispanic/Latinos, non-Hispanic blacks, and Asian Americans as compared with non-Hispanic whites [11–13]. Collectively, these findings highlight the need for individualized assessment and the importance of obesity prevention and early intervention to improve long-term health outcomes. Primary care providers play a pivotal role in this process of preventing, identifying and treating childhood obesity and associated comorbidities [14]. In the case history, the child’s ethnicity, family history, and borderline overweight BMI indicate a high risk for future obesity-related morbidity and a critical opportunity for prevention intervention.
• What are the initial steps a practitioner can take to address overweight and obesity?
To encourage the development of healthy lifestyles and prevention of obesity, dietary and exercise counseling should be routinely provided as part of anticipatory guidance to all children and families regardless of weight status. It is critical to recognize individuals at high risk for becoming obese starting early in life. Risk factors for obesity in healthy weight children include rapid crossing of BMI percentiles, obese parent(s), maternal history of gestational diabetes during pregnancy, ethnicity, sedentary lifestyle, and excessive caloric intake [2]. Identification of these high-risk individuals can prompt more intensive counseling and early intervention with the goal of preventing the development of obesity and its complications. The use of automated BMI calculation and electronic medical records can facilitate identification of overweight and obesity status when already present and improve counseling rates [15].
Obesity due to excess nutrition is typically associated with linear growth acceleration that occurs subsequent to and to a lesser degree than the percentile shift in weight gain. A declining height velocity associated with obesity, therefore, is concerning and should prompt investigation for endocrine disease such as hypothyroidism, glucocorticoid excess, and growth hormone deficiency. Additional factors that warrant further investigation and/or referral include growth trajectory significantly below genetic potential, developmental delay, and dysmorphic features. A complete physical examination should be performed to evaluate for signs consistent with these conditions (eg, violaceous striae in glucocorticoid excess, microcephaly, and small hands/feet in Prader-Willi syndrome), and signs of obesity-associated comorbidities (eg, acanthosis nigricans). Accurate height, weight, BMI calculation, and blood pressure assessment using an appropriately sized cuff are essential.
While BMI screening is valuable, as noted above it is important to appreciate that insulin resistance (and other obesity-related comorbidities) can develop even when BMI is below the 95th percentile. Detailed history and physical examination can help identify these comorbidities of excess adiposity and guide diagnostic evaluation. Independent risk factors for insulin resistance and the development of type 2 diabetes include family history of diabetes, minority race/ethnicity, elevated waist circumference, and poor fitness level [18–20].
Further History
The patient reports skipping breakfast on most days, eats lunch at school, and snacks on chips and soda after school. Dinner is variable but usually contains carbohydrates and a protein and rarely includes vegetables. Family eats “take-out” about 3 times per week. Patient reports spending 3 hours a day watching television and playing on computer. He had gym last semester but currently reports very limited to no physical activity on most days.
• What are effective ways to raise the issue of obesity during an office visit?
Despite the strong connection of obesity with adverse health outcomes, discussion of obesity in routine office settings can be difficult and is often limited by many factors such as time, training, availability of support services, perceived lack of patient motivation, and low outcome expectations [21,22]. Perhaps most challenging is tactfully handling the stigma associated with obesity, which can make discussion awkward and difficult for patients, parents, and providers. To do this, efforts to choose words that convey a nonjudgmental message while maintaining focus on obesity as a health concern are helpful. For example, terms such as “fat” and “obese” are often perceived as stigmatizing and blaming while using the term “unhealthy weight” is less pejorative and can be motivating [23]. It can also be important to acknowledge and emphasize that some individuals are more susceptible to weight gain and its consequences than others and as a result can tolerate fewer calories without unwanted weight gain and health problems. These approaches shift the focus of the discussion toward the goal of restoring and preserving health rather than changing physical appearance without placing blame on the individual and/or family. Motivational interviewing techniques which can be performed effectively even in short office visits can help to actively engage families, reveal familial perception of obesity and assess readiness to change [2]. Their use may also improve the efficacy of other interventions [24].
Case Continued
The patient and his mother were asked if they had any concerns today, including concerns about future health. Mother expressed worry about the potential for diabetes given their family history. The clinician used this as an opportunity to discuss pertinent factors associated with insulin resistance and type 2 diabetes, including modifiable factors such as diet, fitness level, and weight.
• Should this non-obese adolescent be assessed for obesity comorbidities?
Yes. While there are multiple guidelines available for pediatric screening, all highlight the importance of obtaining individualized risk assessment to guide the extent of diagnostic workup. An Expert Committee comprised of representatives from 15 professional organizations appointed 3 writing groups to review the literature and recommend approaches to prevention, assessment, and treatment. Because effective strategies remain poorly defined, the writing groups used both available evidence and expert opinion to develop the recommendations [2]. In addition to routine blood pressure monitoring and universal lipid screening, the Expert Committee recommends obtaining additional laboratory assessment for obese children (BMI ≥ 95th percentile) including a fasting glucose and ALT/AST levels every 2 years starting at age 10 years. For overweight children (BMI > 85th percentile), the Expert Committee recommends obtaining these studies if additional risk factors are present [2]. The American Diabetes Association (ADA) recommends obtaining diabetes screening in all children classified as overweight (defined as either a BMI > 85th percentile for age and sex, weight for height > 85th percentile, or weight > 120% of ideal for height) once every 3 years beginning at age 10 or at pubertal onset (whichever is earliest) when 2 additional risk factors for diabetes are also present, including: (1) history of type 2 diabetes in a first- or second-degree relative, (2) race/ethnicity with increased risk for diabetes development (eg, Native American, African American, Latino, Asian American), (3) signs of insulin resistance or conditions associated with insulin resistance (eg, small for gestational age, polycystic ovary syndrome, hypertension) and (4) maternal history of gestational diabetes during pregnancy [25]. The ADA recommendations for diabetes screening test include either fasting plasma glucose, HgA1C, or oral glucose tolerance test [25].
With a BMI at the 85th percentile, on initial assessment our patient might be perceived as being at moderate or even low risk for obesity and its associated comorbidities. However, a more careful review has elicited several additional risk factors suggesting more appropriate classification in the high-risk category. First, family history of type 2 diabetes on both sides of his family suggests a degree of genetic predisposition. Second, Hispanic ethnicity is known to be independently associated with insulin resistance, type 2 diabetes, and NAFLD [26]. Moreover, physical exam findings of an elevated waist circumference (90th percentile for age and ethnicity [27]) and acanthosis nigricans are also supportive of insulin resistance. As a result, despite having a BMI at the 85th percentile, this adolescent is at high risk and further evaluation is warranted based on both Expert Committee and ADA guidelines. Detailed discussion of certain risk factors is outlined below.
Pattern of Adipose Tissue Distribution: Utility of BMI and Waist Circumference
BMI is a clinical tool that serves as a surrogate marker of adiposity, but since it does not directly measure body fat it provides a statistical, rather than inherent, description of risk. While it is a relatively specific marker (~95%) with moderately high sensitivity and positive predictive value (~70–80%) at BMI levels > 95th percentile, sensitivity and positive predictive value decrease substantially at lower BMI percentiles (PPV 18% in a sample of overweight children) [28]. Current CDC BMI percentile charts consider age and gender differences but do not take into account sexual maturation level or race/ethnicity, both of which are independently correlated with BMI [29]. That is, children with similar BMIs of the same age and sex may exhibit varying degrees of adiposity and risk attributable to their pubertal stage and/or ethnicity [30]. For example, many studies have demonstrated that at the same BMI percentile, Asian Americans tend to have more adiposity compared with non-Hispanic whites [31], whereas African Americans tend to have more fat-free mass compared with non-Hispanic whites [32]. As a result of these differences, some advocate for adjusting cut-offs for BMI based on ethnicity and/or utilizing alternative measures of adiposity such as waist circumference or waist to hip ratio. However, in order for these latter methods to be useful, standardized methods of measurement and normative reference values must be developed. In summary, though BMI can be a useful screening tool, it is an indirect measure of adiposity and cannot discern adipose distribution. Therefore, it is important to remember that when used alone, BMI may overlook children with high inherent risk for disease.
Abdominal adiposity is associated with increased metabolic risk, including insulin resistance, type 2 diabetes, hypertension, cardiovascular disease, and mortality [33]. Waist circumference, a marker of abdominal/truncal obesity, has been considered as a potential marker in place of or in combination with BMI to identify children with increased metabolic risk. In adults, it is well established that an elevated waist circumference is associated with increased health risk, even among those within a normal-weight BMI category [34], and it is recommended that waist circumference in addition to BMI be used to assess health risk [35]. Many studies have documented similar associations between increased waist circumference and metabolic risk factors in childhood and adolescence [36–38]. Specifically, waist circumference is an independent predictor of both insulin sensitivity and increased visceral adiposity tissue (VAT) in children and adolescents [39]. Waist circumference can provide valuable information beyond BMI alone and may be beneficial in the clinical setting in identifying adolescents at risk for obesity-associated comorbidities.
The use of waist circumference in routine clinical settings is complicated and limited by many factors. First, there is no universal method for waist circumference measurement. For example, the WHO recommends measurement at the midpoint between the superior iliac crest and inferior most rib, while the NIH and NHANES recommend measurement immediately above the iliac crest [40]. Since nationally representative data published by Fernandez et al [27] uses the latter method for waist circumference measurement, we recommend this method to allow for comparison of waist circumference percentile with available data for age, sex, and ethnicity. Second, while absolute waist circumference values are used as cut-offs in adulthood, in childhood use of waist circumference percentiles would be more appropriate to account for expected increases during childhood and changes related to pubertal stage. Unfortunately, a lack of standardized waist circumference percentile charts makes meaningful interpretation of waist circumference difficult. Moreover, even if standardized waist circumference percentile charts were developed, there are currently no accepted standards defining an abnormally elevated waist circumference percentile.
Many studies have identified increased metabolic risk factors associated with a waist circumference at or above the 90th percentile for age [41–43]. Based on these studies, the International Diabetes Federation uses waist circumference > 90th percentile as part of the criteria for metabolic syndrome in adolescents. While this ensures a high degree of specificity, use of waist circumference at the 75th percentile would allow for increased sensitivity. For example, Lee et al found that for insulin resistance use of waist circumference at the 75th percentile compared with the 90th percentile increased sensitivity from 61.3% to 86.1% while decreasing specificity from 91.4% to 71.5% [44]. Thus, for individuals at low risk based on history and clinical findings, a waist circumference threshold at the 90th percentile might be reasonable, while for individuals with additional risk factors for insulin resistance use of a lower waist circumference threshold (such as the 75th percentile) may be beneficial. Finally, since risk for insulin resistance and type 2 diabetes varies by race/ethnicity, which may correspond with visceral fat deposition, utilizing various threshold cut-offs based on race/ethnicity has been proposed by some. However, current data do not support this practice [44]. In summary, though there are many challenges to using waist circumference measurements in routine settings, if performed correctly determination of elevated waist circumference measurement can provide some additional information on an individual’s overall risk for complications of obesity.
Acanthosis Nigricans as an Indicator of Insulin Resistance
Insulin resistance, independent of adiposity, is associated with increased risk for type 2 diabetes, cardiovascular disease, ovarian hyperandrogenism, and certain forms of cancer [45]. Identification of insulin resistance in the clinical setting can lead to appropriate intervention (both lifestyle and, when warranted, pharmacologic) to reduce insulin resistance and improve health outcomes. Several risk factors for insulin resistance have been discussed above. Acanthosis nigricans, which is characterized by thick, velvety hyperpigmentation of the skin in intertriginous areas such as the neck and axilla, is an additional finding that is associated with insulin resistance. Its pathogenesis is felt to be related to activation of the IGF-1 receptor by high levels of circulating insulin [46]. Acanthosis nigricans is independently associated with fasting insulin levels and impaired glucose tolerance [47,48]. In addition to increased insulin resistance, one study found that 1 in 4 youths with acanthosis nigricans demonstrated abnormalities in glucose homeostasis and identified 2 individuals with diabetes who would not have been diagnosed based on fasting glucose levels alone [48]. The presence of acanthosis nigricans should alert the clinician to the likelihood of insulin resistance and prompt further investigation. Of note, the prevalence of acanthosis nigricans is increased among African American and Hispanic patients [49,50].
• What laboratory evaluation is warranted and practical in the office setting?
Laboratory evaluation is warranted when obesity or risk factors for comorbidities of obesity are present. At minimum, this should include lipid screening, liver enzymes (ALT and AST), and fasting glucose as outlined above. This approach, however, fails to identify all individuals with obesity-associated comorbidities. ALT is only moderately sensitive in detecting NAFLD [51], and fasting glucose levels only become abnormal when compensation for the degree of insulin resistance is inadequate to maintain normal fasting glucose homeostasis. As a result, while abnormal results on screening are suggestive of disease, normal results do not necessarily confer its absence. Thus, for high-risk subjects, additional testing and/or referral should be considered.
The hyperinsulinemic euglycemic clamp is the “gold standard” for measuring insulin sensitivity, but it is labor intensive and impractical in routine clinical settings. Alter-native approaches using surrogate markers have commonly been utilized, including fasting insulin and glucose levels and 2-hour oral glucose tolerance test (OGTT). The utility of these approaches in the clinical setting has been limited by several factors, including lack of a universal insulin assay. However, despite these limitations, obtaining fasting insulin in addition to fasting glucose or performing 2-hour OGTT can be useful in providing crude estimates of insulin resistance in certain high-risk subpopulations [52,53]. Recently, the ADA added HgA1C measurement as diagnostic criteria for pre-diabetes (5.7%–6.4%) and diabetes (> 6.5%) [54]. Benefits of HgA1C measurement include reliable measurements in nonfasting conditions and reflection of glucose over time. Studies in pediatric patients have shown the usefulness of HgA1C as a measure of future glucose intolerance or diabetes [55]. When fasting insulin or HgA1C are elevated and/or OGTT is abnormal, this suggests the presence of insulin resistance and need for intervention.
Proposed guideline criteria for the diagnosis of “metabolic syndrome” in adolescents include the following: (1) glucose intolerance, (2) elevated waist circumference or BMI, (3) hypertriglyceridemia, (4) low HDL, and 5) hypertension. There is no universal definition for metabolic syndrome in childhood and adolescence, and cut-off values in each category vary by study group [41–43,56]. When insulin resistance is present, it should alert the clinician to the increased likelihood for metabolic syndrome and NAFLD, and additional screening should be performed accordingly. NAFLD is present in about 25% of all overweight children and is strongly associated with insulin resistance and the metabolic syndrome [57]. Hispanic patients have an increased prevalence of NAFLD compared with patients of other ethnicities [58,59]. Elevated liver transaminases (AST and ALT) are commonly used to screen for NAFLD. However, since these markers are indicative of hepatocellular damage, they may remain within normal limits and correlate poorly with early steatosis [51]. Alternative approaches have been proposed in high-risk populations to detect early steatosis and improve long-term prognosis [60].
Case Continued
The patient underwent laboratory assessment that included fasting glucose and insulin, fasting lipid panel, and ALT. Results were suggestive of insulin resistance and metabolic syndrome and included the following: fasting glucose 108 mg/dL, fasting insulin 65 uIU/mL (reference range 3–25), HgA1C 5.9% (reference range 4.2–5.8), total cholesterol 178 mg/dL, HDL cholesterol 35 mg/dL, LDL cholesterol 110 mg/dL, triglycerides 157 mg/dL, and ALT 40 u/L. Blood pressure, as noted above, is at the 95th percentile for age and height.
• What is the recommended approach to intervention? When is referral warranted?
Staged Obesity Treatment
The initial stage, termed “Prevention Plus,” is similar to obesity prevention strategies and is focused on institution of healthy dietary and activity lifestyle habits tailored to the individual and family. Frequent follow-up and monitoring can be helpful and should be offered to families. Failure to demonstrate progress after 3 to 6 months warrants advancement to Stage 2, “Structured Weight Management,” which includes a planned diet with structured meals and snacks, reduction of screen time to 1 hour or less, 60 minutes of supervised physical activity, use of logs to document diet and activity levels, monthly follow-ups and positive reinforcement for achieving goals. Consultation with a dietician and health psychologist/counseling can be helpful at this level.
If no progress is noted after 3 to 6 months, progression to Stage 3, “Comprehensive Multidisciplinary Intervention,” is recommended. This stage emphasizes the importance of a multidisciplinary team including behavioral counselor, registered dietician and exercise specialist in addition to a medical provider. Current evidence suggests modest improvement of obesity and related comorbidities in adolescents participating in multidisciplinary weight management programs [62,63]. While these interventions can be implemented in community settings, coordination in this setting can be difficult and implementation more commonly involves weight management programs in tertiary care centers. Access to such programs can be limited by geographic accessibility, insurance coverage and physician awareness of available programs/resources [64]. Utilization of technology such as telemedicine visits is one way to overcome limited access [65]. Finally, Stage 4 “Tertiary Care Intervention”, involving discussion of pharmacologic or intensive/surgical weight loss options, can be considered for those who fail to show progression after successful intervention of previous stages.
Specialty Referral
Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention. Insulin resistance evidenced by impaired glucose tolerance (abnormal fasting or 2-hour glucose levels), HgA1C in the pre-diabetes range or higher (> 5.7%), or persistently elevated fasting insulin levels after 3 to 6 months of intensive lifestyle modification should prompt referral for consideration of metformin initiation. Metformin can reduce insulin resistance in children and may reduce progression from impaired glucose tolerance to diabetes [66]. For dyslipidemia related to metabolic syndrome, lifestyle interventions are most likely to be efficacious. Referral to preventative cardiology for consideration of pharmacologic intervention should be considered when severe hypertriglyceridemia is present (> 400 mg/dL) or LDL remains elevated after implementation of healthy lifestyle interventions. Elevations in ALT are highly specific for NAFLD and should prompt referral to gastroenterology. In addition, given the poor sensitivity of ALT for detection of early hepatic steatosis, referral might be considered when ALT is in the high normal ranges, especially in those with increased risk such as Hispanic patients [67]. Finally, when signs of obstructive sleep apnea are present, a sleep study should be performed. In summary, while specialty referral can aid targeted treatment of obesity-related morbidities, the central role of the primary care clinician in anticipating and preventing or minimizing their occurrence remains paramount.
Case Conclusion
The patient was referred to a multidisciplinary obesity clinic where he and his family met with dietician, exercise physiologist, health psychologist, and endocrinologist. Healthy lifestyle modifications with specific goals were instituted, including elimination of all calorie-containing beverages (except daily recommended intake of fat-free milk) and initiation of physical activity for 30 minutes a day 5 days per week. He was started on metformin due to glucose intolerance and increased risk for diabetes. Follow-up occurred at monthly intervals for the first 3 months. Additional goals and lifestyle interventions were implemented at each follow-up. At 6 months’ follow-up, the patient’s height was 164 cm, weight was stable at 58.4 kg and BMI was 21.7 (79th percentile). Blood pressure was slightly improved at 123/80 mm Hg. Repeat labs showed mild but consistent improvement in all areas. Specifically, fasting glucose 100 mg/dL, fasting insulin 40 uIU/mL, HgA1C 5.6%, total cholesterol 162 mg/dL, HDL cholesterol 40 mg/dL, LDL cholesterol 105 mg/dL, triglycerides 140 mg/dL, and ALT 38 u/L. The patient continues to be monitored closely with goal to improve metabolic health and long-term health outcomes.
Summary
Childhood obesity is a major public health concern. The health impact of obesity on children is broad and profound. Since treatment of obesity is often unsuccessful, prevention of obesity or early detection of its health consequences are crucial responsibilities and opportunities for primary care clinicians. While clinical guidelines can be instructive, application of clinical guidelines must be tailored to individual adolescent patients according to accompanying risk factors. This review aims to help clinicians stratify risk based on susceptibility to development of insulin resistance and other morbidities associated with adolescent obesity. While the enormity of the obesity epidemic can appear overwhelming to primary care providers, they remain in the best position to initiate early intervention strategies. Coordinating care between primary care providers and specialty clinics will continue to be an important partnership for the care of those experiencing health-threatening effects of adolescent obesity.
Corresponding author: Aaron L Carrel, MD, University of Wisconsin, 600 Highland Ave, H4-436, Madison, WI 53792.
Financial disclosures: Drs. Seibert and Carrel have received fellowship grants from Genentech.
1. CDC. Obesity task force report. 2010. Available at www.letsmove.gov/sites/letsmove.gov/files/TaskForce_on_Childhood_Obesity_May2010_FullReport.pdf. Accessed 4 Sept 2013.
2. Barlow SE, AAP Expert Committee. AAP Expert Committee Recommendations regarding prevention, assessment and treatment of child obesity. Pediatrics 2007;120:s164–92.
3. Dietz WH, Robinson TN. Overweight children and adolescents. N Engl J Med 2005;352:2100–9.
4. Centers for Disease Control and Prevention (CDC) 2012; Overweight and obesity. Available at www.cdc.gov/obesity/childhood/basics.html. Accessed 3 Sept 2013.
5. Centers for Disease Control and Prevention (CDC). Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2009–2010. Available at www.cdc.gov/nchs/data/hestat/obesity_child_09_10/obesity_child_09_10.pdf.
6. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90.
7. August GP, Caprio S, Fennoy I, et al; Endocrine Society. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008;93:4576–99.
8. Holmes ME, Eisenmann JC, Ekkekakis P, Gentile D. Physical activity, stress, and metabolic risk score in 8- to 18-year-old boys. J Phys Act Health 2008;5:294–307.
9. Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003;138:24–32.
10. Sharifi M, Marshall G, Marshall R, et al. Accelerating progress in reducing childhood obesity disparities: exploring best practices of positive outliers. J Health Care Poor Underserved 2013;24(2 Suppl):193–9.
11. Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 2004;89:2590–4.
12. Rosenbaum M, Fennoy I, Accacha S, et al. Racial/ethnic differences in clinical and biochemical type 2 diabetes mellitus risk factors in children. Obesity (Silver Spring) 2013;21:2081–90.
13. NIDDK. National diabetes statistics, 2011. Available at http://diabetes.niddk.nih.gov/dm/pubs/statistics/. Accessed 18 Sept 2013.
14. Janz KF, Butner KL, Pate RR. The role of pediatricians in increasing physical activity in youth. JAMA Pediatr 2013:1–2.
15. Coleman KJ, Hsii AC, Koebnick C, et al. Implementation of clinical practice guidelines for pediatric weight management. J Pediatrics 2012;160:918–22.
16. Ratcliff MB, Jenkins TM, Reiter-Purtill J, et al. Risk-taking behaviors of adolescents with extreme obesity: normative or not? Pediatrics 2011;127:827–34.
17. Goldenring J, Rosen D. Getting into adolescent heads: An essential update. Contemp Pediatr 2004;21:64.
18. Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc 2007;39:1251–6.
19. Weiss R, Shaw M, Savoye M, Caprio S. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Ped Diabetes 2009;10:360–7.
20. Rizzo NS, Ruiz JR, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: The European Youth Heart Study. J Pediatr 2007;150:388–94.
21. Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics 2002;110(1 Pt 2):210–4.
22. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
23. Puhl RM, Peterson JL, Luedicke J. Weight-based victimization: bullying experiences of weight loss treatment-seeking youth. Pediatrics 2013;131:e1–9.
24. Christie D, Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: a clinical review. Diabetes Obes Metab 2013. Aug 8 [Epub ahead of print].
25. Standards of medical care in diabetes--2010. Diabetes Care 2010;33 Suppl 1:S11–61.
26. Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 2010;95:4048–51.
27. Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatrics 2004;145:439–44.
28. Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009;124 Suppl 1:S23–34.
29. Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 1997;99:804–7.
30. Curtis VA, Carrel AL, Eickhoff JC, Allen DB. Gender and race influence metabolic benefits of fitness in children: a cross-sectional study. Int J Pediatr Endocrinol 2012;2012:4.
31. Nightingale CM, Rudnicka AR, Owen CG, et al. Influence of adiposity on insulin resistance and glycemia markers among U.K. Children of South Asian, black African-Caribbean, and white European origin: child heart and health study in England. Diabetes Care 2013;36:1712–9.
32. Gutin B, Yin Z, Humphries MC, Hoffman WH, et al. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr 2004;145:737–43.
33. Cook S. The metabolic syndrome: Antecedent of adult cardiovascular disease in pediatrics. J Pediatr 2004;145:427–30.
34. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002;162:2074–9.
35. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. September 1998. NIH Pub No. 98-4083. Available at www.ncbi.nlm.nih.gov/books/NBK2003/pdf/TOC.pdf. Accessed 29 Sept 2013.
36. Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005;115:1623–30.
37. Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 1999;69:308–17.
38. Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Rel Metab Disorders 2000;24:1453–8.
39. Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatrics 2006;148:188–94.
40. Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutrition 2003;77:379–84.
41. Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Ped Adol Med 2003;157:821–7.
42. Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 2005;28:878–81.
43. Cruz ML, Weigensberg MJ, Huang TT, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrin Metab 2004;89:108–13.
44. Lee JM, Davis MM, Woolford SJ, Gurney JG. Waist circumference percentile thresholds for identifying adolescents with insulin resistance in clinical practice. Pediatric Diabetes 2009;10:336–42.
45. Li S, Chen W, Srinivasan SR, et al. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol 2012;1:S142–9.
46. Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol 2002;147:1096–101.
47. Mukhtar Q, Cleverley G, Voorhees RE, McGrath JW. Prevalence of acanthosis nigricans and its association with hyperinsulinemia in New Mexico adolescents. J. Adolesc Health 2001;28:372–6.
48. Brickman WJ, Huang J, Silverman BL, Metzger BE. Acanthosis nigricans identifies youth at high risk for metabolic abnormalities. J Pediatrics 2010;156:87–92.
49. Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med 1989;87:269–72.
50. Brickman WJ, Binns HJ, Jovanovic BD, et al. Acanthosis nigricans: a common finding in overweight youth. Pediatr Dermatol 2007;24:601–6.
51. Yang HR, Kim HR, Kim MJ, et al. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:1525–30.
52. Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol 2008;159 Suppl 1:S67–74.
53. Adam TC, Hasson RE, Lane CJ, Goran MI. Fasting indicators of insulin sensitivity: effects of ethnicity and pubertal status. Diabetes Care 2011;34:994–9.
54. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36 Suppl 1:S67–74.
55. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
56. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74.
57. Martins C, Pizarro A, Aires L, et al. Fitness and metabolic syndrome in obese fatty liver children. Ann Hum Biol 2013;40:99–101.
58. Taveras EM, Gillman MW, Kleinman KP, et al. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013;167:731–8.
59. Wolfgram PM, Connor EL, Rehm JL, et al. Ethnic differences in the effects of hepatic fat deposition on insulin resistance in non-obese middle school girls. Obesity (Silver Spring) 2014;22:243–8.
60. Sowa JP, Heider D, Bechmann LP, et al. Novel algorithm for non-invasive assessment of fibrosis in NAFLD. PLoS One 2013;8:e62439.
61. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–192.
62. Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multidisciplinary weight management program. Clin Pediatrics 2011;50:187–91.
63. Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA 2007;297:2697–704.
64. Woolford SJ, Clark SJ, Gebremariam A, et al. Physicians’ perspectives on referring obese adolescents to pediatric multidisciplinary weight management programs. Clin Pediatrics 2010;49:871–5.
65. Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face-to-face care for pediatric obesity. Telemed J Ehealth 2013;19:806–8.
66. Park MH, Kinra S, Ward KJ, et al. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32:1743–5.
67. Urrutia-Rojas X, McConathy W, Willis B, et al. Abnormal glucose metabolism in Hispanic parents of children with acanthosis nigricans. ISRN Endocrinol 2011(Epub 2011 Dec 25.).
From the Department of Pediatrics, University of Wisconsin, Madison, WI.
Abstract
- Objective: To provide information that will assist clinicians in assessing and addressing risk for obesity-related comorbidities in adolescents.
- Methods: Review of the literature.
- Results: Childhood obesity is a major public health concern. Prevention of obesity or early detection of its health consequences are important responsibilities or opportunities for primary care clinicians. While body mass index (BMI) screening is valuable, insulin resistance and other obesity-related comorbidities can develop even when BMI falls below the 95th percentile threshold for obesity. Detailed history and physical examination can help identify comorbidities and guide diagnostic evaluation. Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention.
- Conclusion: For optimal health outcomes, management of adolescent obesity and associated comorbidities is should be adapted based on an individual’s overall risk rather than BMI alone.
Case Study
Initial Presentation
A 14-year-old Hispanic male presents for a well child check.
History and Physical Examination
The patient and his mother have no complaints or concerns. A comprehensive review of systems is positive for fatigue and snoring but is otherwise unremarkable. Past medical history is unremarkable except for mild intermittent asthma. Family history is positive for type 2 diabetes in paternal grandmother and a maternal uncle and cardiovascular disease and hypertension in multiple extended family members. Both maternal and paternal grandparents are from Mexico.
Vital signs are within normal limits. Height is 160 cm (30th percentile for age), weight is 58.4 kg (75th percentile for age), and body mass index (BMI) is 22.8 kg/m2 (85th percentile for age). Blood pressure is 127/81 mm Hg (95th percentile for age and gender). Physical exam is pertinent for acanthosis nigricans on neck and axilla and nonviolaceous striae on abdomen. Waist circumference is 88 cm (90th percentile for age and ethnicity). Otherwise, physical exam is within normal limits.
• Does this child’s physical examination findings pose a cause for concern?
Yes. A key concept is that while obesity is widespread, the adverse health complications of adiposity and overnutrition affect some children much earlier and more profoundly than others. Some children exhibit adiposity-associated comorbidities even prior to meeting obesity criteria defined by BMI. Careful history and examination can help identify those most at risk for developing adiposity-associated comorbidities, prompting earlier intervention and, when appropriate, subspecialty referral.
Obesity is caused by a complex interplay of genetic, environmental, and metabolic programming, especially early in life, and lifestyle habits [1,2]. The vast majority of obesity is due to excess nutrition leading to energy imbalance, while less than 1% is due to endocrine or syndromic causes [3]. Obesity is defined as excessive body fat and is often estimated indirectly by using a surrogate marker, BMI. Diagnostically, a BMI > 95th percentile for age on sex-specific CDC growth charts is defined as obese, while a BMI from the 85th to 94th percentile is defined as overweight [4]. Using these criteria, the prevalence of childhood obesity more than tripled in the past 3 decades [5], leading to its classification as an epidemic and public health crisis [2]. Today, an estimated 12.5 million American children are obese [5]. For adolescents specifically, the prevalence of obesity is 18.4%, with more than one-third overweight [6].
Childhood obesity is associated with both short- and long-term morbidities including insulin resistance and type 2 diabetes, hypertension, dyslipidemia, asthma, obstructive sleep apnea, psychosocial problems, and decreased quality of life [7,8]. Obese children, particularly older children and adolescents, are more likely become obese adults [2,7]. Obesity in adulthood is associated with both significant morbidity and premature death [9]. Individual characteristics such as lifestyle habits, fitness level, and genetic predisposition influence the likelihood of development of both obesity and associated comorbidities [10].
The burden of obesity and its associated comorbidities are not equally distributed among racial/ethnic and socioeconomic groups. Hispanic and non-Hispanic black children are much more likely to be obese and overweight than non-Hispanic white children [6]. Low socioeconomic status is associated with increased rates of obesity in certain subgroups, including adolescents [2]. In addition, certain ethnic/racial minorities are more likely to develop obesity-associated comorbidities, such as insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). With regard to insulin resistance and development of type 2 diabetes, the risk is greatest in Native Americans, but there is also increased risk in Hispanic/Latinos, non-Hispanic blacks, and Asian Americans as compared with non-Hispanic whites [11–13]. Collectively, these findings highlight the need for individualized assessment and the importance of obesity prevention and early intervention to improve long-term health outcomes. Primary care providers play a pivotal role in this process of preventing, identifying and treating childhood obesity and associated comorbidities [14]. In the case history, the child’s ethnicity, family history, and borderline overweight BMI indicate a high risk for future obesity-related morbidity and a critical opportunity for prevention intervention.
• What are the initial steps a practitioner can take to address overweight and obesity?
To encourage the development of healthy lifestyles and prevention of obesity, dietary and exercise counseling should be routinely provided as part of anticipatory guidance to all children and families regardless of weight status. It is critical to recognize individuals at high risk for becoming obese starting early in life. Risk factors for obesity in healthy weight children include rapid crossing of BMI percentiles, obese parent(s), maternal history of gestational diabetes during pregnancy, ethnicity, sedentary lifestyle, and excessive caloric intake [2]. Identification of these high-risk individuals can prompt more intensive counseling and early intervention with the goal of preventing the development of obesity and its complications. The use of automated BMI calculation and electronic medical records can facilitate identification of overweight and obesity status when already present and improve counseling rates [15].
Obesity due to excess nutrition is typically associated with linear growth acceleration that occurs subsequent to and to a lesser degree than the percentile shift in weight gain. A declining height velocity associated with obesity, therefore, is concerning and should prompt investigation for endocrine disease such as hypothyroidism, glucocorticoid excess, and growth hormone deficiency. Additional factors that warrant further investigation and/or referral include growth trajectory significantly below genetic potential, developmental delay, and dysmorphic features. A complete physical examination should be performed to evaluate for signs consistent with these conditions (eg, violaceous striae in glucocorticoid excess, microcephaly, and small hands/feet in Prader-Willi syndrome), and signs of obesity-associated comorbidities (eg, acanthosis nigricans). Accurate height, weight, BMI calculation, and blood pressure assessment using an appropriately sized cuff are essential.
While BMI screening is valuable, as noted above it is important to appreciate that insulin resistance (and other obesity-related comorbidities) can develop even when BMI is below the 95th percentile. Detailed history and physical examination can help identify these comorbidities of excess adiposity and guide diagnostic evaluation. Independent risk factors for insulin resistance and the development of type 2 diabetes include family history of diabetes, minority race/ethnicity, elevated waist circumference, and poor fitness level [18–20].
Further History
The patient reports skipping breakfast on most days, eats lunch at school, and snacks on chips and soda after school. Dinner is variable but usually contains carbohydrates and a protein and rarely includes vegetables. Family eats “take-out” about 3 times per week. Patient reports spending 3 hours a day watching television and playing on computer. He had gym last semester but currently reports very limited to no physical activity on most days.
• What are effective ways to raise the issue of obesity during an office visit?
Despite the strong connection of obesity with adverse health outcomes, discussion of obesity in routine office settings can be difficult and is often limited by many factors such as time, training, availability of support services, perceived lack of patient motivation, and low outcome expectations [21,22]. Perhaps most challenging is tactfully handling the stigma associated with obesity, which can make discussion awkward and difficult for patients, parents, and providers. To do this, efforts to choose words that convey a nonjudgmental message while maintaining focus on obesity as a health concern are helpful. For example, terms such as “fat” and “obese” are often perceived as stigmatizing and blaming while using the term “unhealthy weight” is less pejorative and can be motivating [23]. It can also be important to acknowledge and emphasize that some individuals are more susceptible to weight gain and its consequences than others and as a result can tolerate fewer calories without unwanted weight gain and health problems. These approaches shift the focus of the discussion toward the goal of restoring and preserving health rather than changing physical appearance without placing blame on the individual and/or family. Motivational interviewing techniques which can be performed effectively even in short office visits can help to actively engage families, reveal familial perception of obesity and assess readiness to change [2]. Their use may also improve the efficacy of other interventions [24].
Case Continued
The patient and his mother were asked if they had any concerns today, including concerns about future health. Mother expressed worry about the potential for diabetes given their family history. The clinician used this as an opportunity to discuss pertinent factors associated with insulin resistance and type 2 diabetes, including modifiable factors such as diet, fitness level, and weight.
• Should this non-obese adolescent be assessed for obesity comorbidities?
Yes. While there are multiple guidelines available for pediatric screening, all highlight the importance of obtaining individualized risk assessment to guide the extent of diagnostic workup. An Expert Committee comprised of representatives from 15 professional organizations appointed 3 writing groups to review the literature and recommend approaches to prevention, assessment, and treatment. Because effective strategies remain poorly defined, the writing groups used both available evidence and expert opinion to develop the recommendations [2]. In addition to routine blood pressure monitoring and universal lipid screening, the Expert Committee recommends obtaining additional laboratory assessment for obese children (BMI ≥ 95th percentile) including a fasting glucose and ALT/AST levels every 2 years starting at age 10 years. For overweight children (BMI > 85th percentile), the Expert Committee recommends obtaining these studies if additional risk factors are present [2]. The American Diabetes Association (ADA) recommends obtaining diabetes screening in all children classified as overweight (defined as either a BMI > 85th percentile for age and sex, weight for height > 85th percentile, or weight > 120% of ideal for height) once every 3 years beginning at age 10 or at pubertal onset (whichever is earliest) when 2 additional risk factors for diabetes are also present, including: (1) history of type 2 diabetes in a first- or second-degree relative, (2) race/ethnicity with increased risk for diabetes development (eg, Native American, African American, Latino, Asian American), (3) signs of insulin resistance or conditions associated with insulin resistance (eg, small for gestational age, polycystic ovary syndrome, hypertension) and (4) maternal history of gestational diabetes during pregnancy [25]. The ADA recommendations for diabetes screening test include either fasting plasma glucose, HgA1C, or oral glucose tolerance test [25].
With a BMI at the 85th percentile, on initial assessment our patient might be perceived as being at moderate or even low risk for obesity and its associated comorbidities. However, a more careful review has elicited several additional risk factors suggesting more appropriate classification in the high-risk category. First, family history of type 2 diabetes on both sides of his family suggests a degree of genetic predisposition. Second, Hispanic ethnicity is known to be independently associated with insulin resistance, type 2 diabetes, and NAFLD [26]. Moreover, physical exam findings of an elevated waist circumference (90th percentile for age and ethnicity [27]) and acanthosis nigricans are also supportive of insulin resistance. As a result, despite having a BMI at the 85th percentile, this adolescent is at high risk and further evaluation is warranted based on both Expert Committee and ADA guidelines. Detailed discussion of certain risk factors is outlined below.
Pattern of Adipose Tissue Distribution: Utility of BMI and Waist Circumference
BMI is a clinical tool that serves as a surrogate marker of adiposity, but since it does not directly measure body fat it provides a statistical, rather than inherent, description of risk. While it is a relatively specific marker (~95%) with moderately high sensitivity and positive predictive value (~70–80%) at BMI levels > 95th percentile, sensitivity and positive predictive value decrease substantially at lower BMI percentiles (PPV 18% in a sample of overweight children) [28]. Current CDC BMI percentile charts consider age and gender differences but do not take into account sexual maturation level or race/ethnicity, both of which are independently correlated with BMI [29]. That is, children with similar BMIs of the same age and sex may exhibit varying degrees of adiposity and risk attributable to their pubertal stage and/or ethnicity [30]. For example, many studies have demonstrated that at the same BMI percentile, Asian Americans tend to have more adiposity compared with non-Hispanic whites [31], whereas African Americans tend to have more fat-free mass compared with non-Hispanic whites [32]. As a result of these differences, some advocate for adjusting cut-offs for BMI based on ethnicity and/or utilizing alternative measures of adiposity such as waist circumference or waist to hip ratio. However, in order for these latter methods to be useful, standardized methods of measurement and normative reference values must be developed. In summary, though BMI can be a useful screening tool, it is an indirect measure of adiposity and cannot discern adipose distribution. Therefore, it is important to remember that when used alone, BMI may overlook children with high inherent risk for disease.
Abdominal adiposity is associated with increased metabolic risk, including insulin resistance, type 2 diabetes, hypertension, cardiovascular disease, and mortality [33]. Waist circumference, a marker of abdominal/truncal obesity, has been considered as a potential marker in place of or in combination with BMI to identify children with increased metabolic risk. In adults, it is well established that an elevated waist circumference is associated with increased health risk, even among those within a normal-weight BMI category [34], and it is recommended that waist circumference in addition to BMI be used to assess health risk [35]. Many studies have documented similar associations between increased waist circumference and metabolic risk factors in childhood and adolescence [36–38]. Specifically, waist circumference is an independent predictor of both insulin sensitivity and increased visceral adiposity tissue (VAT) in children and adolescents [39]. Waist circumference can provide valuable information beyond BMI alone and may be beneficial in the clinical setting in identifying adolescents at risk for obesity-associated comorbidities.
The use of waist circumference in routine clinical settings is complicated and limited by many factors. First, there is no universal method for waist circumference measurement. For example, the WHO recommends measurement at the midpoint between the superior iliac crest and inferior most rib, while the NIH and NHANES recommend measurement immediately above the iliac crest [40]. Since nationally representative data published by Fernandez et al [27] uses the latter method for waist circumference measurement, we recommend this method to allow for comparison of waist circumference percentile with available data for age, sex, and ethnicity. Second, while absolute waist circumference values are used as cut-offs in adulthood, in childhood use of waist circumference percentiles would be more appropriate to account for expected increases during childhood and changes related to pubertal stage. Unfortunately, a lack of standardized waist circumference percentile charts makes meaningful interpretation of waist circumference difficult. Moreover, even if standardized waist circumference percentile charts were developed, there are currently no accepted standards defining an abnormally elevated waist circumference percentile.
Many studies have identified increased metabolic risk factors associated with a waist circumference at or above the 90th percentile for age [41–43]. Based on these studies, the International Diabetes Federation uses waist circumference > 90th percentile as part of the criteria for metabolic syndrome in adolescents. While this ensures a high degree of specificity, use of waist circumference at the 75th percentile would allow for increased sensitivity. For example, Lee et al found that for insulin resistance use of waist circumference at the 75th percentile compared with the 90th percentile increased sensitivity from 61.3% to 86.1% while decreasing specificity from 91.4% to 71.5% [44]. Thus, for individuals at low risk based on history and clinical findings, a waist circumference threshold at the 90th percentile might be reasonable, while for individuals with additional risk factors for insulin resistance use of a lower waist circumference threshold (such as the 75th percentile) may be beneficial. Finally, since risk for insulin resistance and type 2 diabetes varies by race/ethnicity, which may correspond with visceral fat deposition, utilizing various threshold cut-offs based on race/ethnicity has been proposed by some. However, current data do not support this practice [44]. In summary, though there are many challenges to using waist circumference measurements in routine settings, if performed correctly determination of elevated waist circumference measurement can provide some additional information on an individual’s overall risk for complications of obesity.
Acanthosis Nigricans as an Indicator of Insulin Resistance
Insulin resistance, independent of adiposity, is associated with increased risk for type 2 diabetes, cardiovascular disease, ovarian hyperandrogenism, and certain forms of cancer [45]. Identification of insulin resistance in the clinical setting can lead to appropriate intervention (both lifestyle and, when warranted, pharmacologic) to reduce insulin resistance and improve health outcomes. Several risk factors for insulin resistance have been discussed above. Acanthosis nigricans, which is characterized by thick, velvety hyperpigmentation of the skin in intertriginous areas such as the neck and axilla, is an additional finding that is associated with insulin resistance. Its pathogenesis is felt to be related to activation of the IGF-1 receptor by high levels of circulating insulin [46]. Acanthosis nigricans is independently associated with fasting insulin levels and impaired glucose tolerance [47,48]. In addition to increased insulin resistance, one study found that 1 in 4 youths with acanthosis nigricans demonstrated abnormalities in glucose homeostasis and identified 2 individuals with diabetes who would not have been diagnosed based on fasting glucose levels alone [48]. The presence of acanthosis nigricans should alert the clinician to the likelihood of insulin resistance and prompt further investigation. Of note, the prevalence of acanthosis nigricans is increased among African American and Hispanic patients [49,50].
• What laboratory evaluation is warranted and practical in the office setting?
Laboratory evaluation is warranted when obesity or risk factors for comorbidities of obesity are present. At minimum, this should include lipid screening, liver enzymes (ALT and AST), and fasting glucose as outlined above. This approach, however, fails to identify all individuals with obesity-associated comorbidities. ALT is only moderately sensitive in detecting NAFLD [51], and fasting glucose levels only become abnormal when compensation for the degree of insulin resistance is inadequate to maintain normal fasting glucose homeostasis. As a result, while abnormal results on screening are suggestive of disease, normal results do not necessarily confer its absence. Thus, for high-risk subjects, additional testing and/or referral should be considered.
The hyperinsulinemic euglycemic clamp is the “gold standard” for measuring insulin sensitivity, but it is labor intensive and impractical in routine clinical settings. Alter-native approaches using surrogate markers have commonly been utilized, including fasting insulin and glucose levels and 2-hour oral glucose tolerance test (OGTT). The utility of these approaches in the clinical setting has been limited by several factors, including lack of a universal insulin assay. However, despite these limitations, obtaining fasting insulin in addition to fasting glucose or performing 2-hour OGTT can be useful in providing crude estimates of insulin resistance in certain high-risk subpopulations [52,53]. Recently, the ADA added HgA1C measurement as diagnostic criteria for pre-diabetes (5.7%–6.4%) and diabetes (> 6.5%) [54]. Benefits of HgA1C measurement include reliable measurements in nonfasting conditions and reflection of glucose over time. Studies in pediatric patients have shown the usefulness of HgA1C as a measure of future glucose intolerance or diabetes [55]. When fasting insulin or HgA1C are elevated and/or OGTT is abnormal, this suggests the presence of insulin resistance and need for intervention.
Proposed guideline criteria for the diagnosis of “metabolic syndrome” in adolescents include the following: (1) glucose intolerance, (2) elevated waist circumference or BMI, (3) hypertriglyceridemia, (4) low HDL, and 5) hypertension. There is no universal definition for metabolic syndrome in childhood and adolescence, and cut-off values in each category vary by study group [41–43,56]. When insulin resistance is present, it should alert the clinician to the increased likelihood for metabolic syndrome and NAFLD, and additional screening should be performed accordingly. NAFLD is present in about 25% of all overweight children and is strongly associated with insulin resistance and the metabolic syndrome [57]. Hispanic patients have an increased prevalence of NAFLD compared with patients of other ethnicities [58,59]. Elevated liver transaminases (AST and ALT) are commonly used to screen for NAFLD. However, since these markers are indicative of hepatocellular damage, they may remain within normal limits and correlate poorly with early steatosis [51]. Alternative approaches have been proposed in high-risk populations to detect early steatosis and improve long-term prognosis [60].
Case Continued
The patient underwent laboratory assessment that included fasting glucose and insulin, fasting lipid panel, and ALT. Results were suggestive of insulin resistance and metabolic syndrome and included the following: fasting glucose 108 mg/dL, fasting insulin 65 uIU/mL (reference range 3–25), HgA1C 5.9% (reference range 4.2–5.8), total cholesterol 178 mg/dL, HDL cholesterol 35 mg/dL, LDL cholesterol 110 mg/dL, triglycerides 157 mg/dL, and ALT 40 u/L. Blood pressure, as noted above, is at the 95th percentile for age and height.
• What is the recommended approach to intervention? When is referral warranted?
Staged Obesity Treatment
The initial stage, termed “Prevention Plus,” is similar to obesity prevention strategies and is focused on institution of healthy dietary and activity lifestyle habits tailored to the individual and family. Frequent follow-up and monitoring can be helpful and should be offered to families. Failure to demonstrate progress after 3 to 6 months warrants advancement to Stage 2, “Structured Weight Management,” which includes a planned diet with structured meals and snacks, reduction of screen time to 1 hour or less, 60 minutes of supervised physical activity, use of logs to document diet and activity levels, monthly follow-ups and positive reinforcement for achieving goals. Consultation with a dietician and health psychologist/counseling can be helpful at this level.
If no progress is noted after 3 to 6 months, progression to Stage 3, “Comprehensive Multidisciplinary Intervention,” is recommended. This stage emphasizes the importance of a multidisciplinary team including behavioral counselor, registered dietician and exercise specialist in addition to a medical provider. Current evidence suggests modest improvement of obesity and related comorbidities in adolescents participating in multidisciplinary weight management programs [62,63]. While these interventions can be implemented in community settings, coordination in this setting can be difficult and implementation more commonly involves weight management programs in tertiary care centers. Access to such programs can be limited by geographic accessibility, insurance coverage and physician awareness of available programs/resources [64]. Utilization of technology such as telemedicine visits is one way to overcome limited access [65]. Finally, Stage 4 “Tertiary Care Intervention”, involving discussion of pharmacologic or intensive/surgical weight loss options, can be considered for those who fail to show progression after successful intervention of previous stages.
Specialty Referral
Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention. Insulin resistance evidenced by impaired glucose tolerance (abnormal fasting or 2-hour glucose levels), HgA1C in the pre-diabetes range or higher (> 5.7%), or persistently elevated fasting insulin levels after 3 to 6 months of intensive lifestyle modification should prompt referral for consideration of metformin initiation. Metformin can reduce insulin resistance in children and may reduce progression from impaired glucose tolerance to diabetes [66]. For dyslipidemia related to metabolic syndrome, lifestyle interventions are most likely to be efficacious. Referral to preventative cardiology for consideration of pharmacologic intervention should be considered when severe hypertriglyceridemia is present (> 400 mg/dL) or LDL remains elevated after implementation of healthy lifestyle interventions. Elevations in ALT are highly specific for NAFLD and should prompt referral to gastroenterology. In addition, given the poor sensitivity of ALT for detection of early hepatic steatosis, referral might be considered when ALT is in the high normal ranges, especially in those with increased risk such as Hispanic patients [67]. Finally, when signs of obstructive sleep apnea are present, a sleep study should be performed. In summary, while specialty referral can aid targeted treatment of obesity-related morbidities, the central role of the primary care clinician in anticipating and preventing or minimizing their occurrence remains paramount.
Case Conclusion
The patient was referred to a multidisciplinary obesity clinic where he and his family met with dietician, exercise physiologist, health psychologist, and endocrinologist. Healthy lifestyle modifications with specific goals were instituted, including elimination of all calorie-containing beverages (except daily recommended intake of fat-free milk) and initiation of physical activity for 30 minutes a day 5 days per week. He was started on metformin due to glucose intolerance and increased risk for diabetes. Follow-up occurred at monthly intervals for the first 3 months. Additional goals and lifestyle interventions were implemented at each follow-up. At 6 months’ follow-up, the patient’s height was 164 cm, weight was stable at 58.4 kg and BMI was 21.7 (79th percentile). Blood pressure was slightly improved at 123/80 mm Hg. Repeat labs showed mild but consistent improvement in all areas. Specifically, fasting glucose 100 mg/dL, fasting insulin 40 uIU/mL, HgA1C 5.6%, total cholesterol 162 mg/dL, HDL cholesterol 40 mg/dL, LDL cholesterol 105 mg/dL, triglycerides 140 mg/dL, and ALT 38 u/L. The patient continues to be monitored closely with goal to improve metabolic health and long-term health outcomes.
Summary
Childhood obesity is a major public health concern. The health impact of obesity on children is broad and profound. Since treatment of obesity is often unsuccessful, prevention of obesity or early detection of its health consequences are crucial responsibilities and opportunities for primary care clinicians. While clinical guidelines can be instructive, application of clinical guidelines must be tailored to individual adolescent patients according to accompanying risk factors. This review aims to help clinicians stratify risk based on susceptibility to development of insulin resistance and other morbidities associated with adolescent obesity. While the enormity of the obesity epidemic can appear overwhelming to primary care providers, they remain in the best position to initiate early intervention strategies. Coordinating care between primary care providers and specialty clinics will continue to be an important partnership for the care of those experiencing health-threatening effects of adolescent obesity.
Corresponding author: Aaron L Carrel, MD, University of Wisconsin, 600 Highland Ave, H4-436, Madison, WI 53792.
Financial disclosures: Drs. Seibert and Carrel have received fellowship grants from Genentech.
From the Department of Pediatrics, University of Wisconsin, Madison, WI.
Abstract
- Objective: To provide information that will assist clinicians in assessing and addressing risk for obesity-related comorbidities in adolescents.
- Methods: Review of the literature.
- Results: Childhood obesity is a major public health concern. Prevention of obesity or early detection of its health consequences are important responsibilities or opportunities for primary care clinicians. While body mass index (BMI) screening is valuable, insulin resistance and other obesity-related comorbidities can develop even when BMI falls below the 95th percentile threshold for obesity. Detailed history and physical examination can help identify comorbidities and guide diagnostic evaluation. Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention.
- Conclusion: For optimal health outcomes, management of adolescent obesity and associated comorbidities is should be adapted based on an individual’s overall risk rather than BMI alone.
Case Study
Initial Presentation
A 14-year-old Hispanic male presents for a well child check.
History and Physical Examination
The patient and his mother have no complaints or concerns. A comprehensive review of systems is positive for fatigue and snoring but is otherwise unremarkable. Past medical history is unremarkable except for mild intermittent asthma. Family history is positive for type 2 diabetes in paternal grandmother and a maternal uncle and cardiovascular disease and hypertension in multiple extended family members. Both maternal and paternal grandparents are from Mexico.
Vital signs are within normal limits. Height is 160 cm (30th percentile for age), weight is 58.4 kg (75th percentile for age), and body mass index (BMI) is 22.8 kg/m2 (85th percentile for age). Blood pressure is 127/81 mm Hg (95th percentile for age and gender). Physical exam is pertinent for acanthosis nigricans on neck and axilla and nonviolaceous striae on abdomen. Waist circumference is 88 cm (90th percentile for age and ethnicity). Otherwise, physical exam is within normal limits.
• Does this child’s physical examination findings pose a cause for concern?
Yes. A key concept is that while obesity is widespread, the adverse health complications of adiposity and overnutrition affect some children much earlier and more profoundly than others. Some children exhibit adiposity-associated comorbidities even prior to meeting obesity criteria defined by BMI. Careful history and examination can help identify those most at risk for developing adiposity-associated comorbidities, prompting earlier intervention and, when appropriate, subspecialty referral.
Obesity is caused by a complex interplay of genetic, environmental, and metabolic programming, especially early in life, and lifestyle habits [1,2]. The vast majority of obesity is due to excess nutrition leading to energy imbalance, while less than 1% is due to endocrine or syndromic causes [3]. Obesity is defined as excessive body fat and is often estimated indirectly by using a surrogate marker, BMI. Diagnostically, a BMI > 95th percentile for age on sex-specific CDC growth charts is defined as obese, while a BMI from the 85th to 94th percentile is defined as overweight [4]. Using these criteria, the prevalence of childhood obesity more than tripled in the past 3 decades [5], leading to its classification as an epidemic and public health crisis [2]. Today, an estimated 12.5 million American children are obese [5]. For adolescents specifically, the prevalence of obesity is 18.4%, with more than one-third overweight [6].
Childhood obesity is associated with both short- and long-term morbidities including insulin resistance and type 2 diabetes, hypertension, dyslipidemia, asthma, obstructive sleep apnea, psychosocial problems, and decreased quality of life [7,8]. Obese children, particularly older children and adolescents, are more likely become obese adults [2,7]. Obesity in adulthood is associated with both significant morbidity and premature death [9]. Individual characteristics such as lifestyle habits, fitness level, and genetic predisposition influence the likelihood of development of both obesity and associated comorbidities [10].
The burden of obesity and its associated comorbidities are not equally distributed among racial/ethnic and socioeconomic groups. Hispanic and non-Hispanic black children are much more likely to be obese and overweight than non-Hispanic white children [6]. Low socioeconomic status is associated with increased rates of obesity in certain subgroups, including adolescents [2]. In addition, certain ethnic/racial minorities are more likely to develop obesity-associated comorbidities, such as insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). With regard to insulin resistance and development of type 2 diabetes, the risk is greatest in Native Americans, but there is also increased risk in Hispanic/Latinos, non-Hispanic blacks, and Asian Americans as compared with non-Hispanic whites [11–13]. Collectively, these findings highlight the need for individualized assessment and the importance of obesity prevention and early intervention to improve long-term health outcomes. Primary care providers play a pivotal role in this process of preventing, identifying and treating childhood obesity and associated comorbidities [14]. In the case history, the child’s ethnicity, family history, and borderline overweight BMI indicate a high risk for future obesity-related morbidity and a critical opportunity for prevention intervention.
• What are the initial steps a practitioner can take to address overweight and obesity?
To encourage the development of healthy lifestyles and prevention of obesity, dietary and exercise counseling should be routinely provided as part of anticipatory guidance to all children and families regardless of weight status. It is critical to recognize individuals at high risk for becoming obese starting early in life. Risk factors for obesity in healthy weight children include rapid crossing of BMI percentiles, obese parent(s), maternal history of gestational diabetes during pregnancy, ethnicity, sedentary lifestyle, and excessive caloric intake [2]. Identification of these high-risk individuals can prompt more intensive counseling and early intervention with the goal of preventing the development of obesity and its complications. The use of automated BMI calculation and electronic medical records can facilitate identification of overweight and obesity status when already present and improve counseling rates [15].
Obesity due to excess nutrition is typically associated with linear growth acceleration that occurs subsequent to and to a lesser degree than the percentile shift in weight gain. A declining height velocity associated with obesity, therefore, is concerning and should prompt investigation for endocrine disease such as hypothyroidism, glucocorticoid excess, and growth hormone deficiency. Additional factors that warrant further investigation and/or referral include growth trajectory significantly below genetic potential, developmental delay, and dysmorphic features. A complete physical examination should be performed to evaluate for signs consistent with these conditions (eg, violaceous striae in glucocorticoid excess, microcephaly, and small hands/feet in Prader-Willi syndrome), and signs of obesity-associated comorbidities (eg, acanthosis nigricans). Accurate height, weight, BMI calculation, and blood pressure assessment using an appropriately sized cuff are essential.
While BMI screening is valuable, as noted above it is important to appreciate that insulin resistance (and other obesity-related comorbidities) can develop even when BMI is below the 95th percentile. Detailed history and physical examination can help identify these comorbidities of excess adiposity and guide diagnostic evaluation. Independent risk factors for insulin resistance and the development of type 2 diabetes include family history of diabetes, minority race/ethnicity, elevated waist circumference, and poor fitness level [18–20].
Further History
The patient reports skipping breakfast on most days, eats lunch at school, and snacks on chips and soda after school. Dinner is variable but usually contains carbohydrates and a protein and rarely includes vegetables. Family eats “take-out” about 3 times per week. Patient reports spending 3 hours a day watching television and playing on computer. He had gym last semester but currently reports very limited to no physical activity on most days.
• What are effective ways to raise the issue of obesity during an office visit?
Despite the strong connection of obesity with adverse health outcomes, discussion of obesity in routine office settings can be difficult and is often limited by many factors such as time, training, availability of support services, perceived lack of patient motivation, and low outcome expectations [21,22]. Perhaps most challenging is tactfully handling the stigma associated with obesity, which can make discussion awkward and difficult for patients, parents, and providers. To do this, efforts to choose words that convey a nonjudgmental message while maintaining focus on obesity as a health concern are helpful. For example, terms such as “fat” and “obese” are often perceived as stigmatizing and blaming while using the term “unhealthy weight” is less pejorative and can be motivating [23]. It can also be important to acknowledge and emphasize that some individuals are more susceptible to weight gain and its consequences than others and as a result can tolerate fewer calories without unwanted weight gain and health problems. These approaches shift the focus of the discussion toward the goal of restoring and preserving health rather than changing physical appearance without placing blame on the individual and/or family. Motivational interviewing techniques which can be performed effectively even in short office visits can help to actively engage families, reveal familial perception of obesity and assess readiness to change [2]. Their use may also improve the efficacy of other interventions [24].
Case Continued
The patient and his mother were asked if they had any concerns today, including concerns about future health. Mother expressed worry about the potential for diabetes given their family history. The clinician used this as an opportunity to discuss pertinent factors associated with insulin resistance and type 2 diabetes, including modifiable factors such as diet, fitness level, and weight.
• Should this non-obese adolescent be assessed for obesity comorbidities?
Yes. While there are multiple guidelines available for pediatric screening, all highlight the importance of obtaining individualized risk assessment to guide the extent of diagnostic workup. An Expert Committee comprised of representatives from 15 professional organizations appointed 3 writing groups to review the literature and recommend approaches to prevention, assessment, and treatment. Because effective strategies remain poorly defined, the writing groups used both available evidence and expert opinion to develop the recommendations [2]. In addition to routine blood pressure monitoring and universal lipid screening, the Expert Committee recommends obtaining additional laboratory assessment for obese children (BMI ≥ 95th percentile) including a fasting glucose and ALT/AST levels every 2 years starting at age 10 years. For overweight children (BMI > 85th percentile), the Expert Committee recommends obtaining these studies if additional risk factors are present [2]. The American Diabetes Association (ADA) recommends obtaining diabetes screening in all children classified as overweight (defined as either a BMI > 85th percentile for age and sex, weight for height > 85th percentile, or weight > 120% of ideal for height) once every 3 years beginning at age 10 or at pubertal onset (whichever is earliest) when 2 additional risk factors for diabetes are also present, including: (1) history of type 2 diabetes in a first- or second-degree relative, (2) race/ethnicity with increased risk for diabetes development (eg, Native American, African American, Latino, Asian American), (3) signs of insulin resistance or conditions associated with insulin resistance (eg, small for gestational age, polycystic ovary syndrome, hypertension) and (4) maternal history of gestational diabetes during pregnancy [25]. The ADA recommendations for diabetes screening test include either fasting plasma glucose, HgA1C, or oral glucose tolerance test [25].
With a BMI at the 85th percentile, on initial assessment our patient might be perceived as being at moderate or even low risk for obesity and its associated comorbidities. However, a more careful review has elicited several additional risk factors suggesting more appropriate classification in the high-risk category. First, family history of type 2 diabetes on both sides of his family suggests a degree of genetic predisposition. Second, Hispanic ethnicity is known to be independently associated with insulin resistance, type 2 diabetes, and NAFLD [26]. Moreover, physical exam findings of an elevated waist circumference (90th percentile for age and ethnicity [27]) and acanthosis nigricans are also supportive of insulin resistance. As a result, despite having a BMI at the 85th percentile, this adolescent is at high risk and further evaluation is warranted based on both Expert Committee and ADA guidelines. Detailed discussion of certain risk factors is outlined below.
Pattern of Adipose Tissue Distribution: Utility of BMI and Waist Circumference
BMI is a clinical tool that serves as a surrogate marker of adiposity, but since it does not directly measure body fat it provides a statistical, rather than inherent, description of risk. While it is a relatively specific marker (~95%) with moderately high sensitivity and positive predictive value (~70–80%) at BMI levels > 95th percentile, sensitivity and positive predictive value decrease substantially at lower BMI percentiles (PPV 18% in a sample of overweight children) [28]. Current CDC BMI percentile charts consider age and gender differences but do not take into account sexual maturation level or race/ethnicity, both of which are independently correlated with BMI [29]. That is, children with similar BMIs of the same age and sex may exhibit varying degrees of adiposity and risk attributable to their pubertal stage and/or ethnicity [30]. For example, many studies have demonstrated that at the same BMI percentile, Asian Americans tend to have more adiposity compared with non-Hispanic whites [31], whereas African Americans tend to have more fat-free mass compared with non-Hispanic whites [32]. As a result of these differences, some advocate for adjusting cut-offs for BMI based on ethnicity and/or utilizing alternative measures of adiposity such as waist circumference or waist to hip ratio. However, in order for these latter methods to be useful, standardized methods of measurement and normative reference values must be developed. In summary, though BMI can be a useful screening tool, it is an indirect measure of adiposity and cannot discern adipose distribution. Therefore, it is important to remember that when used alone, BMI may overlook children with high inherent risk for disease.
Abdominal adiposity is associated with increased metabolic risk, including insulin resistance, type 2 diabetes, hypertension, cardiovascular disease, and mortality [33]. Waist circumference, a marker of abdominal/truncal obesity, has been considered as a potential marker in place of or in combination with BMI to identify children with increased metabolic risk. In adults, it is well established that an elevated waist circumference is associated with increased health risk, even among those within a normal-weight BMI category [34], and it is recommended that waist circumference in addition to BMI be used to assess health risk [35]. Many studies have documented similar associations between increased waist circumference and metabolic risk factors in childhood and adolescence [36–38]. Specifically, waist circumference is an independent predictor of both insulin sensitivity and increased visceral adiposity tissue (VAT) in children and adolescents [39]. Waist circumference can provide valuable information beyond BMI alone and may be beneficial in the clinical setting in identifying adolescents at risk for obesity-associated comorbidities.
The use of waist circumference in routine clinical settings is complicated and limited by many factors. First, there is no universal method for waist circumference measurement. For example, the WHO recommends measurement at the midpoint between the superior iliac crest and inferior most rib, while the NIH and NHANES recommend measurement immediately above the iliac crest [40]. Since nationally representative data published by Fernandez et al [27] uses the latter method for waist circumference measurement, we recommend this method to allow for comparison of waist circumference percentile with available data for age, sex, and ethnicity. Second, while absolute waist circumference values are used as cut-offs in adulthood, in childhood use of waist circumference percentiles would be more appropriate to account for expected increases during childhood and changes related to pubertal stage. Unfortunately, a lack of standardized waist circumference percentile charts makes meaningful interpretation of waist circumference difficult. Moreover, even if standardized waist circumference percentile charts were developed, there are currently no accepted standards defining an abnormally elevated waist circumference percentile.
Many studies have identified increased metabolic risk factors associated with a waist circumference at or above the 90th percentile for age [41–43]. Based on these studies, the International Diabetes Federation uses waist circumference > 90th percentile as part of the criteria for metabolic syndrome in adolescents. While this ensures a high degree of specificity, use of waist circumference at the 75th percentile would allow for increased sensitivity. For example, Lee et al found that for insulin resistance use of waist circumference at the 75th percentile compared with the 90th percentile increased sensitivity from 61.3% to 86.1% while decreasing specificity from 91.4% to 71.5% [44]. Thus, for individuals at low risk based on history and clinical findings, a waist circumference threshold at the 90th percentile might be reasonable, while for individuals with additional risk factors for insulin resistance use of a lower waist circumference threshold (such as the 75th percentile) may be beneficial. Finally, since risk for insulin resistance and type 2 diabetes varies by race/ethnicity, which may correspond with visceral fat deposition, utilizing various threshold cut-offs based on race/ethnicity has been proposed by some. However, current data do not support this practice [44]. In summary, though there are many challenges to using waist circumference measurements in routine settings, if performed correctly determination of elevated waist circumference measurement can provide some additional information on an individual’s overall risk for complications of obesity.
Acanthosis Nigricans as an Indicator of Insulin Resistance
Insulin resistance, independent of adiposity, is associated with increased risk for type 2 diabetes, cardiovascular disease, ovarian hyperandrogenism, and certain forms of cancer [45]. Identification of insulin resistance in the clinical setting can lead to appropriate intervention (both lifestyle and, when warranted, pharmacologic) to reduce insulin resistance and improve health outcomes. Several risk factors for insulin resistance have been discussed above. Acanthosis nigricans, which is characterized by thick, velvety hyperpigmentation of the skin in intertriginous areas such as the neck and axilla, is an additional finding that is associated with insulin resistance. Its pathogenesis is felt to be related to activation of the IGF-1 receptor by high levels of circulating insulin [46]. Acanthosis nigricans is independently associated with fasting insulin levels and impaired glucose tolerance [47,48]. In addition to increased insulin resistance, one study found that 1 in 4 youths with acanthosis nigricans demonstrated abnormalities in glucose homeostasis and identified 2 individuals with diabetes who would not have been diagnosed based on fasting glucose levels alone [48]. The presence of acanthosis nigricans should alert the clinician to the likelihood of insulin resistance and prompt further investigation. Of note, the prevalence of acanthosis nigricans is increased among African American and Hispanic patients [49,50].
• What laboratory evaluation is warranted and practical in the office setting?
Laboratory evaluation is warranted when obesity or risk factors for comorbidities of obesity are present. At minimum, this should include lipid screening, liver enzymes (ALT and AST), and fasting glucose as outlined above. This approach, however, fails to identify all individuals with obesity-associated comorbidities. ALT is only moderately sensitive in detecting NAFLD [51], and fasting glucose levels only become abnormal when compensation for the degree of insulin resistance is inadequate to maintain normal fasting glucose homeostasis. As a result, while abnormal results on screening are suggestive of disease, normal results do not necessarily confer its absence. Thus, for high-risk subjects, additional testing and/or referral should be considered.
The hyperinsulinemic euglycemic clamp is the “gold standard” for measuring insulin sensitivity, but it is labor intensive and impractical in routine clinical settings. Alter-native approaches using surrogate markers have commonly been utilized, including fasting insulin and glucose levels and 2-hour oral glucose tolerance test (OGTT). The utility of these approaches in the clinical setting has been limited by several factors, including lack of a universal insulin assay. However, despite these limitations, obtaining fasting insulin in addition to fasting glucose or performing 2-hour OGTT can be useful in providing crude estimates of insulin resistance in certain high-risk subpopulations [52,53]. Recently, the ADA added HgA1C measurement as diagnostic criteria for pre-diabetes (5.7%–6.4%) and diabetes (> 6.5%) [54]. Benefits of HgA1C measurement include reliable measurements in nonfasting conditions and reflection of glucose over time. Studies in pediatric patients have shown the usefulness of HgA1C as a measure of future glucose intolerance or diabetes [55]. When fasting insulin or HgA1C are elevated and/or OGTT is abnormal, this suggests the presence of insulin resistance and need for intervention.
Proposed guideline criteria for the diagnosis of “metabolic syndrome” in adolescents include the following: (1) glucose intolerance, (2) elevated waist circumference or BMI, (3) hypertriglyceridemia, (4) low HDL, and 5) hypertension. There is no universal definition for metabolic syndrome in childhood and adolescence, and cut-off values in each category vary by study group [41–43,56]. When insulin resistance is present, it should alert the clinician to the increased likelihood for metabolic syndrome and NAFLD, and additional screening should be performed accordingly. NAFLD is present in about 25% of all overweight children and is strongly associated with insulin resistance and the metabolic syndrome [57]. Hispanic patients have an increased prevalence of NAFLD compared with patients of other ethnicities [58,59]. Elevated liver transaminases (AST and ALT) are commonly used to screen for NAFLD. However, since these markers are indicative of hepatocellular damage, they may remain within normal limits and correlate poorly with early steatosis [51]. Alternative approaches have been proposed in high-risk populations to detect early steatosis and improve long-term prognosis [60].
Case Continued
The patient underwent laboratory assessment that included fasting glucose and insulin, fasting lipid panel, and ALT. Results were suggestive of insulin resistance and metabolic syndrome and included the following: fasting glucose 108 mg/dL, fasting insulin 65 uIU/mL (reference range 3–25), HgA1C 5.9% (reference range 4.2–5.8), total cholesterol 178 mg/dL, HDL cholesterol 35 mg/dL, LDL cholesterol 110 mg/dL, triglycerides 157 mg/dL, and ALT 40 u/L. Blood pressure, as noted above, is at the 95th percentile for age and height.
• What is the recommended approach to intervention? When is referral warranted?
Staged Obesity Treatment
The initial stage, termed “Prevention Plus,” is similar to obesity prevention strategies and is focused on institution of healthy dietary and activity lifestyle habits tailored to the individual and family. Frequent follow-up and monitoring can be helpful and should be offered to families. Failure to demonstrate progress after 3 to 6 months warrants advancement to Stage 2, “Structured Weight Management,” which includes a planned diet with structured meals and snacks, reduction of screen time to 1 hour or less, 60 minutes of supervised physical activity, use of logs to document diet and activity levels, monthly follow-ups and positive reinforcement for achieving goals. Consultation with a dietician and health psychologist/counseling can be helpful at this level.
If no progress is noted after 3 to 6 months, progression to Stage 3, “Comprehensive Multidisciplinary Intervention,” is recommended. This stage emphasizes the importance of a multidisciplinary team including behavioral counselor, registered dietician and exercise specialist in addition to a medical provider. Current evidence suggests modest improvement of obesity and related comorbidities in adolescents participating in multidisciplinary weight management programs [62,63]. While these interventions can be implemented in community settings, coordination in this setting can be difficult and implementation more commonly involves weight management programs in tertiary care centers. Access to such programs can be limited by geographic accessibility, insurance coverage and physician awareness of available programs/resources [64]. Utilization of technology such as telemedicine visits is one way to overcome limited access [65]. Finally, Stage 4 “Tertiary Care Intervention”, involving discussion of pharmacologic or intensive/surgical weight loss options, can be considered for those who fail to show progression after successful intervention of previous stages.
Specialty Referral
Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention. Insulin resistance evidenced by impaired glucose tolerance (abnormal fasting or 2-hour glucose levels), HgA1C in the pre-diabetes range or higher (> 5.7%), or persistently elevated fasting insulin levels after 3 to 6 months of intensive lifestyle modification should prompt referral for consideration of metformin initiation. Metformin can reduce insulin resistance in children and may reduce progression from impaired glucose tolerance to diabetes [66]. For dyslipidemia related to metabolic syndrome, lifestyle interventions are most likely to be efficacious. Referral to preventative cardiology for consideration of pharmacologic intervention should be considered when severe hypertriglyceridemia is present (> 400 mg/dL) or LDL remains elevated after implementation of healthy lifestyle interventions. Elevations in ALT are highly specific for NAFLD and should prompt referral to gastroenterology. In addition, given the poor sensitivity of ALT for detection of early hepatic steatosis, referral might be considered when ALT is in the high normal ranges, especially in those with increased risk such as Hispanic patients [67]. Finally, when signs of obstructive sleep apnea are present, a sleep study should be performed. In summary, while specialty referral can aid targeted treatment of obesity-related morbidities, the central role of the primary care clinician in anticipating and preventing or minimizing their occurrence remains paramount.
Case Conclusion
The patient was referred to a multidisciplinary obesity clinic where he and his family met with dietician, exercise physiologist, health psychologist, and endocrinologist. Healthy lifestyle modifications with specific goals were instituted, including elimination of all calorie-containing beverages (except daily recommended intake of fat-free milk) and initiation of physical activity for 30 minutes a day 5 days per week. He was started on metformin due to glucose intolerance and increased risk for diabetes. Follow-up occurred at monthly intervals for the first 3 months. Additional goals and lifestyle interventions were implemented at each follow-up. At 6 months’ follow-up, the patient’s height was 164 cm, weight was stable at 58.4 kg and BMI was 21.7 (79th percentile). Blood pressure was slightly improved at 123/80 mm Hg. Repeat labs showed mild but consistent improvement in all areas. Specifically, fasting glucose 100 mg/dL, fasting insulin 40 uIU/mL, HgA1C 5.6%, total cholesterol 162 mg/dL, HDL cholesterol 40 mg/dL, LDL cholesterol 105 mg/dL, triglycerides 140 mg/dL, and ALT 38 u/L. The patient continues to be monitored closely with goal to improve metabolic health and long-term health outcomes.
Summary
Childhood obesity is a major public health concern. The health impact of obesity on children is broad and profound. Since treatment of obesity is often unsuccessful, prevention of obesity or early detection of its health consequences are crucial responsibilities and opportunities for primary care clinicians. While clinical guidelines can be instructive, application of clinical guidelines must be tailored to individual adolescent patients according to accompanying risk factors. This review aims to help clinicians stratify risk based on susceptibility to development of insulin resistance and other morbidities associated with adolescent obesity. While the enormity of the obesity epidemic can appear overwhelming to primary care providers, they remain in the best position to initiate early intervention strategies. Coordinating care between primary care providers and specialty clinics will continue to be an important partnership for the care of those experiencing health-threatening effects of adolescent obesity.
Corresponding author: Aaron L Carrel, MD, University of Wisconsin, 600 Highland Ave, H4-436, Madison, WI 53792.
Financial disclosures: Drs. Seibert and Carrel have received fellowship grants from Genentech.
1. CDC. Obesity task force report. 2010. Available at www.letsmove.gov/sites/letsmove.gov/files/TaskForce_on_Childhood_Obesity_May2010_FullReport.pdf. Accessed 4 Sept 2013.
2. Barlow SE, AAP Expert Committee. AAP Expert Committee Recommendations regarding prevention, assessment and treatment of child obesity. Pediatrics 2007;120:s164–92.
3. Dietz WH, Robinson TN. Overweight children and adolescents. N Engl J Med 2005;352:2100–9.
4. Centers for Disease Control and Prevention (CDC) 2012; Overweight and obesity. Available at www.cdc.gov/obesity/childhood/basics.html. Accessed 3 Sept 2013.
5. Centers for Disease Control and Prevention (CDC). Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2009–2010. Available at www.cdc.gov/nchs/data/hestat/obesity_child_09_10/obesity_child_09_10.pdf.
6. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90.
7. August GP, Caprio S, Fennoy I, et al; Endocrine Society. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008;93:4576–99.
8. Holmes ME, Eisenmann JC, Ekkekakis P, Gentile D. Physical activity, stress, and metabolic risk score in 8- to 18-year-old boys. J Phys Act Health 2008;5:294–307.
9. Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003;138:24–32.
10. Sharifi M, Marshall G, Marshall R, et al. Accelerating progress in reducing childhood obesity disparities: exploring best practices of positive outliers. J Health Care Poor Underserved 2013;24(2 Suppl):193–9.
11. Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 2004;89:2590–4.
12. Rosenbaum M, Fennoy I, Accacha S, et al. Racial/ethnic differences in clinical and biochemical type 2 diabetes mellitus risk factors in children. Obesity (Silver Spring) 2013;21:2081–90.
13. NIDDK. National diabetes statistics, 2011. Available at http://diabetes.niddk.nih.gov/dm/pubs/statistics/. Accessed 18 Sept 2013.
14. Janz KF, Butner KL, Pate RR. The role of pediatricians in increasing physical activity in youth. JAMA Pediatr 2013:1–2.
15. Coleman KJ, Hsii AC, Koebnick C, et al. Implementation of clinical practice guidelines for pediatric weight management. J Pediatrics 2012;160:918–22.
16. Ratcliff MB, Jenkins TM, Reiter-Purtill J, et al. Risk-taking behaviors of adolescents with extreme obesity: normative or not? Pediatrics 2011;127:827–34.
17. Goldenring J, Rosen D. Getting into adolescent heads: An essential update. Contemp Pediatr 2004;21:64.
18. Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc 2007;39:1251–6.
19. Weiss R, Shaw M, Savoye M, Caprio S. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Ped Diabetes 2009;10:360–7.
20. Rizzo NS, Ruiz JR, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: The European Youth Heart Study. J Pediatr 2007;150:388–94.
21. Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics 2002;110(1 Pt 2):210–4.
22. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
23. Puhl RM, Peterson JL, Luedicke J. Weight-based victimization: bullying experiences of weight loss treatment-seeking youth. Pediatrics 2013;131:e1–9.
24. Christie D, Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: a clinical review. Diabetes Obes Metab 2013. Aug 8 [Epub ahead of print].
25. Standards of medical care in diabetes--2010. Diabetes Care 2010;33 Suppl 1:S11–61.
26. Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 2010;95:4048–51.
27. Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatrics 2004;145:439–44.
28. Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009;124 Suppl 1:S23–34.
29. Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 1997;99:804–7.
30. Curtis VA, Carrel AL, Eickhoff JC, Allen DB. Gender and race influence metabolic benefits of fitness in children: a cross-sectional study. Int J Pediatr Endocrinol 2012;2012:4.
31. Nightingale CM, Rudnicka AR, Owen CG, et al. Influence of adiposity on insulin resistance and glycemia markers among U.K. Children of South Asian, black African-Caribbean, and white European origin: child heart and health study in England. Diabetes Care 2013;36:1712–9.
32. Gutin B, Yin Z, Humphries MC, Hoffman WH, et al. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr 2004;145:737–43.
33. Cook S. The metabolic syndrome: Antecedent of adult cardiovascular disease in pediatrics. J Pediatr 2004;145:427–30.
34. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002;162:2074–9.
35. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. September 1998. NIH Pub No. 98-4083. Available at www.ncbi.nlm.nih.gov/books/NBK2003/pdf/TOC.pdf. Accessed 29 Sept 2013.
36. Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005;115:1623–30.
37. Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 1999;69:308–17.
38. Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Rel Metab Disorders 2000;24:1453–8.
39. Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatrics 2006;148:188–94.
40. Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutrition 2003;77:379–84.
41. Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Ped Adol Med 2003;157:821–7.
42. Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 2005;28:878–81.
43. Cruz ML, Weigensberg MJ, Huang TT, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrin Metab 2004;89:108–13.
44. Lee JM, Davis MM, Woolford SJ, Gurney JG. Waist circumference percentile thresholds for identifying adolescents with insulin resistance in clinical practice. Pediatric Diabetes 2009;10:336–42.
45. Li S, Chen W, Srinivasan SR, et al. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol 2012;1:S142–9.
46. Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol 2002;147:1096–101.
47. Mukhtar Q, Cleverley G, Voorhees RE, McGrath JW. Prevalence of acanthosis nigricans and its association with hyperinsulinemia in New Mexico adolescents. J. Adolesc Health 2001;28:372–6.
48. Brickman WJ, Huang J, Silverman BL, Metzger BE. Acanthosis nigricans identifies youth at high risk for metabolic abnormalities. J Pediatrics 2010;156:87–92.
49. Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med 1989;87:269–72.
50. Brickman WJ, Binns HJ, Jovanovic BD, et al. Acanthosis nigricans: a common finding in overweight youth. Pediatr Dermatol 2007;24:601–6.
51. Yang HR, Kim HR, Kim MJ, et al. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:1525–30.
52. Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol 2008;159 Suppl 1:S67–74.
53. Adam TC, Hasson RE, Lane CJ, Goran MI. Fasting indicators of insulin sensitivity: effects of ethnicity and pubertal status. Diabetes Care 2011;34:994–9.
54. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36 Suppl 1:S67–74.
55. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
56. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74.
57. Martins C, Pizarro A, Aires L, et al. Fitness and metabolic syndrome in obese fatty liver children. Ann Hum Biol 2013;40:99–101.
58. Taveras EM, Gillman MW, Kleinman KP, et al. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013;167:731–8.
59. Wolfgram PM, Connor EL, Rehm JL, et al. Ethnic differences in the effects of hepatic fat deposition on insulin resistance in non-obese middle school girls. Obesity (Silver Spring) 2014;22:243–8.
60. Sowa JP, Heider D, Bechmann LP, et al. Novel algorithm for non-invasive assessment of fibrosis in NAFLD. PLoS One 2013;8:e62439.
61. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–192.
62. Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multidisciplinary weight management program. Clin Pediatrics 2011;50:187–91.
63. Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA 2007;297:2697–704.
64. Woolford SJ, Clark SJ, Gebremariam A, et al. Physicians’ perspectives on referring obese adolescents to pediatric multidisciplinary weight management programs. Clin Pediatrics 2010;49:871–5.
65. Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face-to-face care for pediatric obesity. Telemed J Ehealth 2013;19:806–8.
66. Park MH, Kinra S, Ward KJ, et al. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32:1743–5.
67. Urrutia-Rojas X, McConathy W, Willis B, et al. Abnormal glucose metabolism in Hispanic parents of children with acanthosis nigricans. ISRN Endocrinol 2011(Epub 2011 Dec 25.).
1. CDC. Obesity task force report. 2010. Available at www.letsmove.gov/sites/letsmove.gov/files/TaskForce_on_Childhood_Obesity_May2010_FullReport.pdf. Accessed 4 Sept 2013.
2. Barlow SE, AAP Expert Committee. AAP Expert Committee Recommendations regarding prevention, assessment and treatment of child obesity. Pediatrics 2007;120:s164–92.
3. Dietz WH, Robinson TN. Overweight children and adolescents. N Engl J Med 2005;352:2100–9.
4. Centers for Disease Control and Prevention (CDC) 2012; Overweight and obesity. Available at www.cdc.gov/obesity/childhood/basics.html. Accessed 3 Sept 2013.
5. Centers for Disease Control and Prevention (CDC). Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2009–2010. Available at www.cdc.gov/nchs/data/hestat/obesity_child_09_10/obesity_child_09_10.pdf.
6. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90.
7. August GP, Caprio S, Fennoy I, et al; Endocrine Society. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008;93:4576–99.
8. Holmes ME, Eisenmann JC, Ekkekakis P, Gentile D. Physical activity, stress, and metabolic risk score in 8- to 18-year-old boys. J Phys Act Health 2008;5:294–307.
9. Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003;138:24–32.
10. Sharifi M, Marshall G, Marshall R, et al. Accelerating progress in reducing childhood obesity disparities: exploring best practices of positive outliers. J Health Care Poor Underserved 2013;24(2 Suppl):193–9.
11. Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 2004;89:2590–4.
12. Rosenbaum M, Fennoy I, Accacha S, et al. Racial/ethnic differences in clinical and biochemical type 2 diabetes mellitus risk factors in children. Obesity (Silver Spring) 2013;21:2081–90.
13. NIDDK. National diabetes statistics, 2011. Available at http://diabetes.niddk.nih.gov/dm/pubs/statistics/. Accessed 18 Sept 2013.
14. Janz KF, Butner KL, Pate RR. The role of pediatricians in increasing physical activity in youth. JAMA Pediatr 2013:1–2.
15. Coleman KJ, Hsii AC, Koebnick C, et al. Implementation of clinical practice guidelines for pediatric weight management. J Pediatrics 2012;160:918–22.
16. Ratcliff MB, Jenkins TM, Reiter-Purtill J, et al. Risk-taking behaviors of adolescents with extreme obesity: normative or not? Pediatrics 2011;127:827–34.
17. Goldenring J, Rosen D. Getting into adolescent heads: An essential update. Contemp Pediatr 2004;21:64.
18. Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc 2007;39:1251–6.
19. Weiss R, Shaw M, Savoye M, Caprio S. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Ped Diabetes 2009;10:360–7.
20. Rizzo NS, Ruiz JR, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: The European Youth Heart Study. J Pediatr 2007;150:388–94.
21. Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics 2002;110(1 Pt 2):210–4.
22. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
23. Puhl RM, Peterson JL, Luedicke J. Weight-based victimization: bullying experiences of weight loss treatment-seeking youth. Pediatrics 2013;131:e1–9.
24. Christie D, Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: a clinical review. Diabetes Obes Metab 2013. Aug 8 [Epub ahead of print].
25. Standards of medical care in diabetes--2010. Diabetes Care 2010;33 Suppl 1:S11–61.
26. Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 2010;95:4048–51.
27. Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatrics 2004;145:439–44.
28. Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009;124 Suppl 1:S23–34.
29. Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 1997;99:804–7.
30. Curtis VA, Carrel AL, Eickhoff JC, Allen DB. Gender and race influence metabolic benefits of fitness in children: a cross-sectional study. Int J Pediatr Endocrinol 2012;2012:4.
31. Nightingale CM, Rudnicka AR, Owen CG, et al. Influence of adiposity on insulin resistance and glycemia markers among U.K. Children of South Asian, black African-Caribbean, and white European origin: child heart and health study in England. Diabetes Care 2013;36:1712–9.
32. Gutin B, Yin Z, Humphries MC, Hoffman WH, et al. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr 2004;145:737–43.
33. Cook S. The metabolic syndrome: Antecedent of adult cardiovascular disease in pediatrics. J Pediatr 2004;145:427–30.
34. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002;162:2074–9.
35. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. September 1998. NIH Pub No. 98-4083. Available at www.ncbi.nlm.nih.gov/books/NBK2003/pdf/TOC.pdf. Accessed 29 Sept 2013.
36. Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005;115:1623–30.
37. Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 1999;69:308–17.
38. Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Rel Metab Disorders 2000;24:1453–8.
39. Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatrics 2006;148:188–94.
40. Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutrition 2003;77:379–84.
41. Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Ped Adol Med 2003;157:821–7.
42. Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 2005;28:878–81.
43. Cruz ML, Weigensberg MJ, Huang TT, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrin Metab 2004;89:108–13.
44. Lee JM, Davis MM, Woolford SJ, Gurney JG. Waist circumference percentile thresholds for identifying adolescents with insulin resistance in clinical practice. Pediatric Diabetes 2009;10:336–42.
45. Li S, Chen W, Srinivasan SR, et al. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol 2012;1:S142–9.
46. Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol 2002;147:1096–101.
47. Mukhtar Q, Cleverley G, Voorhees RE, McGrath JW. Prevalence of acanthosis nigricans and its association with hyperinsulinemia in New Mexico adolescents. J. Adolesc Health 2001;28:372–6.
48. Brickman WJ, Huang J, Silverman BL, Metzger BE. Acanthosis nigricans identifies youth at high risk for metabolic abnormalities. J Pediatrics 2010;156:87–92.
49. Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med 1989;87:269–72.
50. Brickman WJ, Binns HJ, Jovanovic BD, et al. Acanthosis nigricans: a common finding in overweight youth. Pediatr Dermatol 2007;24:601–6.
51. Yang HR, Kim HR, Kim MJ, et al. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:1525–30.
52. Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol 2008;159 Suppl 1:S67–74.
53. Adam TC, Hasson RE, Lane CJ, Goran MI. Fasting indicators of insulin sensitivity: effects of ethnicity and pubertal status. Diabetes Care 2011;34:994–9.
54. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36 Suppl 1:S67–74.
55. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
56. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74.
57. Martins C, Pizarro A, Aires L, et al. Fitness and metabolic syndrome in obese fatty liver children. Ann Hum Biol 2013;40:99–101.
58. Taveras EM, Gillman MW, Kleinman KP, et al. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013;167:731–8.
59. Wolfgram PM, Connor EL, Rehm JL, et al. Ethnic differences in the effects of hepatic fat deposition on insulin resistance in non-obese middle school girls. Obesity (Silver Spring) 2014;22:243–8.
60. Sowa JP, Heider D, Bechmann LP, et al. Novel algorithm for non-invasive assessment of fibrosis in NAFLD. PLoS One 2013;8:e62439.
61. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–192.
62. Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multidisciplinary weight management program. Clin Pediatrics 2011;50:187–91.
63. Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA 2007;297:2697–704.
64. Woolford SJ, Clark SJ, Gebremariam A, et al. Physicians’ perspectives on referring obese adolescents to pediatric multidisciplinary weight management programs. Clin Pediatrics 2010;49:871–5.
65. Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face-to-face care for pediatric obesity. Telemed J Ehealth 2013;19:806–8.
66. Park MH, Kinra S, Ward KJ, et al. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32:1743–5.
67. Urrutia-Rojas X, McConathy W, Willis B, et al. Abnormal glucose metabolism in Hispanic parents of children with acanthosis nigricans. ISRN Endocrinol 2011(Epub 2011 Dec 25.).
Cutaneous Melanoma
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Melanoma is the sixth most common cancer in the United States and the leading cause of deaths among all cutaneous malignancies. In 2012, it was estimated that approximately 75,000 individuals were diagnosed with melanoma and more than 9000 died. The incidence of melanoma is rising the fastest among all major malignancies, and the lifetime risk of melanoma among men and women now exceeds 1 in 68, as compared with 1:1500 in 1930.4 The incidence of melanoma is predicted to continue increasing, and there has been no corresponding decrease in mortality. This case-based review summarizes the etiology, risk factors, clinical presentation, and management of cutaneous melanomas, which comprise the majority of melanoma cases. The biology and management for other noncutaneous melanomas (such as mucosal or ocular melanomas) are beyond the scope of this review.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Melanoma is the sixth most common cancer in the United States and the leading cause of deaths among all cutaneous malignancies. In 2012, it was estimated that approximately 75,000 individuals were diagnosed with melanoma and more than 9000 died. The incidence of melanoma is rising the fastest among all major malignancies, and the lifetime risk of melanoma among men and women now exceeds 1 in 68, as compared with 1:1500 in 1930.4 The incidence of melanoma is predicted to continue increasing, and there has been no corresponding decrease in mortality. This case-based review summarizes the etiology, risk factors, clinical presentation, and management of cutaneous melanomas, which comprise the majority of melanoma cases. The biology and management for other noncutaneous melanomas (such as mucosal or ocular melanomas) are beyond the scope of this review.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Melanoma is the sixth most common cancer in the United States and the leading cause of deaths among all cutaneous malignancies. In 2012, it was estimated that approximately 75,000 individuals were diagnosed with melanoma and more than 9000 died. The incidence of melanoma is rising the fastest among all major malignancies, and the lifetime risk of melanoma among men and women now exceeds 1 in 68, as compared with 1:1500 in 1930.4 The incidence of melanoma is predicted to continue increasing, and there has been no corresponding decrease in mortality. This case-based review summarizes the etiology, risk factors, clinical presentation, and management of cutaneous melanomas, which comprise the majority of melanoma cases. The biology and management for other noncutaneous melanomas (such as mucosal or ocular melanomas) are beyond the scope of this review.
To read the full article in PDF:
Confused and nearly naked after going on spending sprees
CASE Nearly naked
Mr. A, age 68, is found sitting in his car, wearing only a jacket, underpants, and boots. He speaks of spreading a message about Osama bin Laden and “taking a census.” Police officers bring him to a hospital emergency department for evaluation.
The examining clinician determines that Mr. A is a danger to himself and others because of mental illness, leading to admission to our state psychiatric hospital.
Mr. A’s wife describes recent spending sprees with large purchases. She had obtained a restraining order against her husband because of his threatening remarks and behaviors. Within days of the order issuance, he got a home equity loan and purchased a $300,000 house.
The medical history is notable for type 2 diabetes mellitus. Although he is not taking medications, his blood sugar is well controlled. Other than an initial resting heart rate of 116 beats per minute, vital signs are stable and within normal limits. Physical examination is unremarkable. Screening laboratory studies are notable for mildly elevated hepatic function, which approaches normal range several days after admission.
Mr. A reports a remote history of alcohol abuse but says he had not been drinking recently, and does not detail his pattern of use. Urine toxicology screen is negative for all substances of abuse.
Mental status examination reveals disheveled appearance, motor agitation, pressured speech, labile affect, loosening of associations, grandiose delusions, and auditory hallucinations. Mr. A’s thought processes are grossly disorganized, such that we could not gather a meaningful history. He believes God is speaking directly to him about plans to build a carousel at Disney World. He makes strange gestures with his hands throughout the interview, as if attempting to trace the shapes of letters and numbers. He frequently speaks of seeing an array of colors. Cognitive examination reveals a score of 5 of 30 on the Montreal Cognitive Assessment (Figure 1), indicating a severe impairment in neurocognitive functioning. He demonstrates limited insight and markedly impaired judgment, and denies having a mental illness.
What should be the next step in managing Mr. A?
a) obtain records from other facilities and collateral history
b) start an antipsychotic
c) order a brain MRI
d) start an alcohol withdrawal protocol
The authors’ observations
Mr. A showed elements of mania, psychosis, and delirium. We considered a broad differential diagnosis (Table). Mr. A initially could not provide reliable or accurate information. The least invasive next step was to obtain additional history from his wife and other medical records to refine the differential diagnosis.
HISTORY Bizarre behavior
Mr. A allows staff to speak with his wife and obtain records from a psychiatric hospitalization 3 years earlier. Mrs. A reports significant and rapid changes in her husband’s behavior and personality over 3 months, but does not describe a recent alcohol relapse. Mr. A sleeps very little, remaining awake and active throughout the night. He frequently rearranges the furniture in their home for no clear reason. Once, he knocked on the door of a young female neighbor asking if she found him attractive.
Mr. A has a significant criminal history. Approximately 30 years ago, he was charged with attempted murder of his ex-wife and he had faced charges of attempted kidnapping and assaulting a police officer. However, he has no recent legal issues.
Mr. A has a history of episodes that are similar to this presentation. Seven years ago, he impulsively purchased a $650,000 house after his fourth wife died. He then had a $90,000 heart-shaped pool installed. He also drove a tractor through his stepdaughter’s car for no apparent reason. Also, 3 years ago, he displayed symptoms similar to his current presentation, including insomnia, irritability, and grandiosity. He engaged in strange behaviors, such as dressing up and imitating homeless people at his church.
During the hospitalization 3 years ago, clinicians gave Mr. A a diagnosis of bipolar disorder, current episode manic, and delirium of an unclear cause. A medical workup, including brain MRI, did not uncover a basis for his delirium. Antipsychotics (risperidone and perphenazine) and mood stabilizers (lithium and valproic acid), stabilized his condition; after 7 weeks, Mr. A was discharged, but he did not pursue outpatient psychiatric care.
What is the most likely DSM-5 diagnosis?
a) major neurocognitive disorder (dementia)
b) alcohol use disorder (eg, Wernicke- Korsakoff syndrome)
c) delirium secondary to mania
d) psychotic disorder
The authors’ observations
DSM-51 suggests a stepwise approach to diagnosis, with consideration of:
• signs and symptoms
• substance use
• general medical condition
• developmental conflict or stage
• whether a mental disorder is present.
Mr. A’s age and severe cognitive impairment raise the possibility of dementia. Rapid onset, history of similar episodes, and apparent inter-episode recovery make dementia unlikely. The history of alcohol abuse and mildly elevated hepatic function tests suggest a substance use disorder such as Wernicke-Korsakoff syndrome or a withdrawal syndrome. However, there is no evidence of excessive alcohol use over the past several months, toxicology studies were negative, and vital signs were stable. General medical causes for Mr. A’s presentation, such as hypoglycemia, head trauma, intracranial infection, and metabolic disturbance were considered, but physical examination and laboratory studies did not suggest any condition that would explain his condition.
Mr. A’s previous psychiatric hospitalization is critical in clarifying the more likely diagnosis. A similar presentation yielded the diagnosis of bipolar disorder, manic phase. Our working diagnosis, therefore, was bipolar disorder with features of delirious mania.
Delirious mania
Delirious mania was first described by Luther Bell in 1849 and is characterized by an acute and simultaneous onset of mania— severe insomnia, poor judgment, grandiosity, excitement, emotional lability, bizarre hallucinations, and delusions—and delirium—altered consciousness, disorientation, and confusion.2,3 Although there are no diagnostic criteria, some authors suggest that delirious mania is characterized by inappropriate toileting, denudation, profound lack of sleep, and episodic memory impairment that can last hours or days.4 Catatonia frequently is seen with delirious mania.5 Initial case descriptions described a high mortality rate, approaching 75% of patients.6 There is little published literature and no classification of delirious mania in DSM-5.1 Estimates are that delirium is concomitant in 20% to 33% of patients with mania.7,8
Several theories try to clarify the underlying etiology of delirious mania. Jacobowski et al9 summarized the etiology and proposed that it is:
• 1 of 3 types of mania, including: acute and delusional manias, as initially proposed by Kraeplin
• a severe form of catatonia
• a condition akin to, but distinct from, delirium with similar underlying medical causes
• a primary psychiatric disorder underlying the cause of delirium.
EVALUATION Brain changes
For several days, Mr. A continues to engage in strange behavior. He tries to take patients’ belongings, is denudative, crawls on floors, licks walls, is unable to feed himself, and exhibits odd motor movements with purposeless motor activity.
We consult our internal medicine team to identify treatable, medical causes. Results of serum B12, thyroid-stimulating hormone, and rapid plasma reagin studies are within normal limits. Urinalysis is negative. A brain MRI reveals numerous white-matter T2-weighted and FLAIR hyperintensities, indicating small-vessel ischemic changes that are consistent with the findings of an MRI 3 years ago. A sleep-deprived EEG with temporal leads obtained on Day 4 of hospitalization demonstrates a diffusely slow and marginally to poorly organized background, believed to indicate global cerebral dysfunction that is most consistent with nonfocal global encephalopathy. There is no seizure activity. We do not perform a lumbar puncture because of Mr. A’s absence of focal neurologic deficits, lack of fever, and normal white blood cell count.
What is the most appropriate treatment?
a) electroconvulsive therapy (ECT)
b) high-dose benzodiazepine
c) mood stabilizer
d) antipsychotic
The authors’ observations
We strongly suspect that Mr. A has delirious mania. Symptoms and signs of mania include labile mood, excessive spending, grandiosity, insomnia, and psychosis together with delirium (marked disorientation, confusion). We ascribed Mr. A’s odd motor behaviors to catatonia, a hallmark of delirious mania. The literature has little description of EEG findings in suspected cases of delirious mania; however, abnormal EEG tracings have been reported.10 We also speculated that Mr. A’s EEG reflected effects produced by his prescribed antipsychotic regimen.
Treatment
There is no clear consensus on treating delirious mania. Because catatonia is a key feature of delirious mania—whether etiologically or as a prominent sign of the condition—ECT and benzodiazepines are proposed as primary treatments. In a study of 16 patients with delirious mania, Karmacharya et al4 found ECT to be effective, with patients showing improvement after 1 to 4 treatments. Lee et al10 reported similar findings. Although a high-dose benzodiazepine is not as effective as ECT, a 1-time oral dose of 3 to 4 mg of lorazepam has been used to treat delirious mania.
The efficacy of antipsychotic and mood-stabilizing pharmacotherapy is not clear. Bond3 described 3 cases in which patients were treated effectively with a typical antipsychotic (haloperidol or chlorpromazine) and lithium. Jung and Lee11demonstrated the efficacy of atypical antipsychotics, with a marked improvement in symptoms within 1 week. However, other studies do not support these findings. Karmacharya et al4 found that typical antipsychotics 1) make the clinical picture worse by increasing extrapyramidal symptoms and 2) produce inconsistent effects. Mood stabilizers sometimes proved beneficial.
Karmacharya et al4 further argued that the delay in improvement seen with any antipsychotics and mood stabilizers suggest they should not be considered a first-line treatment. These discordant findings are the result of a small number of studies and a lack of understanding of the exact nature of delirious mania.
TREATMENT Quick Response
Mr. A’s symptoms rapidly resolve with a combination of quetiapine, 800 mg/d, haloperidol, 10 mg/d, and lithium, 1,200 mg/d. His mood returns to euthymia and his psychotic symptoms abate. He is able to attend to all activities of daily living. Mental status clears and he is fully oriented and able to hold a logical conversation. He scores 28 out of 30 on a subsequent Montreal Cognitive Assessment, administered 11 days after the initial assessment (Figure 2), indicating normal neurocognitive function. He returns to his baseline level of functioning and is discharged in psychiatrically stable condition. Mr. A has no recollection of the bizarre behaviors he displayed earlier in his hospitalization.
The authors’ observations
We started Mr. A on antipsychotics because of his initial level of agitation. In reviewing pharmacotherapy options for Mr. A’s mania and delirium, we contemplated several options. Quetiapine and lithium were chosen after a review of outside hospital records demonstrated a combination of a mood stabilizer and an antipsychotic was effective in treating a previous similar episode, which led to remission of Mr. A’s symptoms. We chose quetiapine because of it highly sedating properties, suspecting that it would help treat his insomnia. We thought that the risk that lithium would make delirium worse was mitigated by Mr. A’s previous therapeutic response to it. Haloperidol was added for treating delirium, given its more potent D2 antagonism. Mr. A responded quickly to these interventions.
We did not consider ECT at the beginning of Mr. A’s admission, and we avoided sedative-hypnotic agents because we were concerned that a benzodiazepine might make his delirium worse. In light of available data suggesting that ECT and benzodiazepines are preferred treatments for delirious mania, it is noteworthy that Mr. A responded so robustly and rapidly to an antipsychotic and a mood stabilizer.
Bottom Line
Consider delirious mania in any patient who has a history of bipolar disorder presenting with co-occuring symptoms of mania and delirium. Collateral information is vital to establishing a diagnosis. With suspected delirium, rule out concomitant reversible medical problems. Electroconvulsive therapy, high-dose benzodiazepines, antipsychotics, and mood stabilizers have shown efficacy.
Related Resources
• Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
• Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
Drug Brand Names
Chlorpromazine • Thorazine Perphenazine • Trilafon
Haloperidol • Haldol Quetiapine • Seroquel
Lithium • Eskalith Risperidone • Risperdal
Lorazepam • Ativan Valproic acid • Depakene
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Nearly naked
Mr. A, age 68, is found sitting in his car, wearing only a jacket, underpants, and boots. He speaks of spreading a message about Osama bin Laden and “taking a census.” Police officers bring him to a hospital emergency department for evaluation.
The examining clinician determines that Mr. A is a danger to himself and others because of mental illness, leading to admission to our state psychiatric hospital.
Mr. A’s wife describes recent spending sprees with large purchases. She had obtained a restraining order against her husband because of his threatening remarks and behaviors. Within days of the order issuance, he got a home equity loan and purchased a $300,000 house.
The medical history is notable for type 2 diabetes mellitus. Although he is not taking medications, his blood sugar is well controlled. Other than an initial resting heart rate of 116 beats per minute, vital signs are stable and within normal limits. Physical examination is unremarkable. Screening laboratory studies are notable for mildly elevated hepatic function, which approaches normal range several days after admission.
Mr. A reports a remote history of alcohol abuse but says he had not been drinking recently, and does not detail his pattern of use. Urine toxicology screen is negative for all substances of abuse.
Mental status examination reveals disheveled appearance, motor agitation, pressured speech, labile affect, loosening of associations, grandiose delusions, and auditory hallucinations. Mr. A’s thought processes are grossly disorganized, such that we could not gather a meaningful history. He believes God is speaking directly to him about plans to build a carousel at Disney World. He makes strange gestures with his hands throughout the interview, as if attempting to trace the shapes of letters and numbers. He frequently speaks of seeing an array of colors. Cognitive examination reveals a score of 5 of 30 on the Montreal Cognitive Assessment (Figure 1), indicating a severe impairment in neurocognitive functioning. He demonstrates limited insight and markedly impaired judgment, and denies having a mental illness.
What should be the next step in managing Mr. A?
a) obtain records from other facilities and collateral history
b) start an antipsychotic
c) order a brain MRI
d) start an alcohol withdrawal protocol
The authors’ observations
Mr. A showed elements of mania, psychosis, and delirium. We considered a broad differential diagnosis (Table). Mr. A initially could not provide reliable or accurate information. The least invasive next step was to obtain additional history from his wife and other medical records to refine the differential diagnosis.
HISTORY Bizarre behavior
Mr. A allows staff to speak with his wife and obtain records from a psychiatric hospitalization 3 years earlier. Mrs. A reports significant and rapid changes in her husband’s behavior and personality over 3 months, but does not describe a recent alcohol relapse. Mr. A sleeps very little, remaining awake and active throughout the night. He frequently rearranges the furniture in their home for no clear reason. Once, he knocked on the door of a young female neighbor asking if she found him attractive.
Mr. A has a significant criminal history. Approximately 30 years ago, he was charged with attempted murder of his ex-wife and he had faced charges of attempted kidnapping and assaulting a police officer. However, he has no recent legal issues.
Mr. A has a history of episodes that are similar to this presentation. Seven years ago, he impulsively purchased a $650,000 house after his fourth wife died. He then had a $90,000 heart-shaped pool installed. He also drove a tractor through his stepdaughter’s car for no apparent reason. Also, 3 years ago, he displayed symptoms similar to his current presentation, including insomnia, irritability, and grandiosity. He engaged in strange behaviors, such as dressing up and imitating homeless people at his church.
During the hospitalization 3 years ago, clinicians gave Mr. A a diagnosis of bipolar disorder, current episode manic, and delirium of an unclear cause. A medical workup, including brain MRI, did not uncover a basis for his delirium. Antipsychotics (risperidone and perphenazine) and mood stabilizers (lithium and valproic acid), stabilized his condition; after 7 weeks, Mr. A was discharged, but he did not pursue outpatient psychiatric care.
What is the most likely DSM-5 diagnosis?
a) major neurocognitive disorder (dementia)
b) alcohol use disorder (eg, Wernicke- Korsakoff syndrome)
c) delirium secondary to mania
d) psychotic disorder
The authors’ observations
DSM-51 suggests a stepwise approach to diagnosis, with consideration of:
• signs and symptoms
• substance use
• general medical condition
• developmental conflict or stage
• whether a mental disorder is present.
Mr. A’s age and severe cognitive impairment raise the possibility of dementia. Rapid onset, history of similar episodes, and apparent inter-episode recovery make dementia unlikely. The history of alcohol abuse and mildly elevated hepatic function tests suggest a substance use disorder such as Wernicke-Korsakoff syndrome or a withdrawal syndrome. However, there is no evidence of excessive alcohol use over the past several months, toxicology studies were negative, and vital signs were stable. General medical causes for Mr. A’s presentation, such as hypoglycemia, head trauma, intracranial infection, and metabolic disturbance were considered, but physical examination and laboratory studies did not suggest any condition that would explain his condition.
Mr. A’s previous psychiatric hospitalization is critical in clarifying the more likely diagnosis. A similar presentation yielded the diagnosis of bipolar disorder, manic phase. Our working diagnosis, therefore, was bipolar disorder with features of delirious mania.
Delirious mania
Delirious mania was first described by Luther Bell in 1849 and is characterized by an acute and simultaneous onset of mania— severe insomnia, poor judgment, grandiosity, excitement, emotional lability, bizarre hallucinations, and delusions—and delirium—altered consciousness, disorientation, and confusion.2,3 Although there are no diagnostic criteria, some authors suggest that delirious mania is characterized by inappropriate toileting, denudation, profound lack of sleep, and episodic memory impairment that can last hours or days.4 Catatonia frequently is seen with delirious mania.5 Initial case descriptions described a high mortality rate, approaching 75% of patients.6 There is little published literature and no classification of delirious mania in DSM-5.1 Estimates are that delirium is concomitant in 20% to 33% of patients with mania.7,8
Several theories try to clarify the underlying etiology of delirious mania. Jacobowski et al9 summarized the etiology and proposed that it is:
• 1 of 3 types of mania, including: acute and delusional manias, as initially proposed by Kraeplin
• a severe form of catatonia
• a condition akin to, but distinct from, delirium with similar underlying medical causes
• a primary psychiatric disorder underlying the cause of delirium.
EVALUATION Brain changes
For several days, Mr. A continues to engage in strange behavior. He tries to take patients’ belongings, is denudative, crawls on floors, licks walls, is unable to feed himself, and exhibits odd motor movements with purposeless motor activity.
We consult our internal medicine team to identify treatable, medical causes. Results of serum B12, thyroid-stimulating hormone, and rapid plasma reagin studies are within normal limits. Urinalysis is negative. A brain MRI reveals numerous white-matter T2-weighted and FLAIR hyperintensities, indicating small-vessel ischemic changes that are consistent with the findings of an MRI 3 years ago. A sleep-deprived EEG with temporal leads obtained on Day 4 of hospitalization demonstrates a diffusely slow and marginally to poorly organized background, believed to indicate global cerebral dysfunction that is most consistent with nonfocal global encephalopathy. There is no seizure activity. We do not perform a lumbar puncture because of Mr. A’s absence of focal neurologic deficits, lack of fever, and normal white blood cell count.
What is the most appropriate treatment?
a) electroconvulsive therapy (ECT)
b) high-dose benzodiazepine
c) mood stabilizer
d) antipsychotic
The authors’ observations
We strongly suspect that Mr. A has delirious mania. Symptoms and signs of mania include labile mood, excessive spending, grandiosity, insomnia, and psychosis together with delirium (marked disorientation, confusion). We ascribed Mr. A’s odd motor behaviors to catatonia, a hallmark of delirious mania. The literature has little description of EEG findings in suspected cases of delirious mania; however, abnormal EEG tracings have been reported.10 We also speculated that Mr. A’s EEG reflected effects produced by his prescribed antipsychotic regimen.
Treatment
There is no clear consensus on treating delirious mania. Because catatonia is a key feature of delirious mania—whether etiologically or as a prominent sign of the condition—ECT and benzodiazepines are proposed as primary treatments. In a study of 16 patients with delirious mania, Karmacharya et al4 found ECT to be effective, with patients showing improvement after 1 to 4 treatments. Lee et al10 reported similar findings. Although a high-dose benzodiazepine is not as effective as ECT, a 1-time oral dose of 3 to 4 mg of lorazepam has been used to treat delirious mania.
The efficacy of antipsychotic and mood-stabilizing pharmacotherapy is not clear. Bond3 described 3 cases in which patients were treated effectively with a typical antipsychotic (haloperidol or chlorpromazine) and lithium. Jung and Lee11demonstrated the efficacy of atypical antipsychotics, with a marked improvement in symptoms within 1 week. However, other studies do not support these findings. Karmacharya et al4 found that typical antipsychotics 1) make the clinical picture worse by increasing extrapyramidal symptoms and 2) produce inconsistent effects. Mood stabilizers sometimes proved beneficial.
Karmacharya et al4 further argued that the delay in improvement seen with any antipsychotics and mood stabilizers suggest they should not be considered a first-line treatment. These discordant findings are the result of a small number of studies and a lack of understanding of the exact nature of delirious mania.
TREATMENT Quick Response
Mr. A’s symptoms rapidly resolve with a combination of quetiapine, 800 mg/d, haloperidol, 10 mg/d, and lithium, 1,200 mg/d. His mood returns to euthymia and his psychotic symptoms abate. He is able to attend to all activities of daily living. Mental status clears and he is fully oriented and able to hold a logical conversation. He scores 28 out of 30 on a subsequent Montreal Cognitive Assessment, administered 11 days after the initial assessment (Figure 2), indicating normal neurocognitive function. He returns to his baseline level of functioning and is discharged in psychiatrically stable condition. Mr. A has no recollection of the bizarre behaviors he displayed earlier in his hospitalization.
The authors’ observations
We started Mr. A on antipsychotics because of his initial level of agitation. In reviewing pharmacotherapy options for Mr. A’s mania and delirium, we contemplated several options. Quetiapine and lithium were chosen after a review of outside hospital records demonstrated a combination of a mood stabilizer and an antipsychotic was effective in treating a previous similar episode, which led to remission of Mr. A’s symptoms. We chose quetiapine because of it highly sedating properties, suspecting that it would help treat his insomnia. We thought that the risk that lithium would make delirium worse was mitigated by Mr. A’s previous therapeutic response to it. Haloperidol was added for treating delirium, given its more potent D2 antagonism. Mr. A responded quickly to these interventions.
We did not consider ECT at the beginning of Mr. A’s admission, and we avoided sedative-hypnotic agents because we were concerned that a benzodiazepine might make his delirium worse. In light of available data suggesting that ECT and benzodiazepines are preferred treatments for delirious mania, it is noteworthy that Mr. A responded so robustly and rapidly to an antipsychotic and a mood stabilizer.
Bottom Line
Consider delirious mania in any patient who has a history of bipolar disorder presenting with co-occuring symptoms of mania and delirium. Collateral information is vital to establishing a diagnosis. With suspected delirium, rule out concomitant reversible medical problems. Electroconvulsive therapy, high-dose benzodiazepines, antipsychotics, and mood stabilizers have shown efficacy.
Related Resources
• Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
• Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
Drug Brand Names
Chlorpromazine • Thorazine Perphenazine • Trilafon
Haloperidol • Haldol Quetiapine • Seroquel
Lithium • Eskalith Risperidone • Risperdal
Lorazepam • Ativan Valproic acid • Depakene
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Nearly naked
Mr. A, age 68, is found sitting in his car, wearing only a jacket, underpants, and boots. He speaks of spreading a message about Osama bin Laden and “taking a census.” Police officers bring him to a hospital emergency department for evaluation.
The examining clinician determines that Mr. A is a danger to himself and others because of mental illness, leading to admission to our state psychiatric hospital.
Mr. A’s wife describes recent spending sprees with large purchases. She had obtained a restraining order against her husband because of his threatening remarks and behaviors. Within days of the order issuance, he got a home equity loan and purchased a $300,000 house.
The medical history is notable for type 2 diabetes mellitus. Although he is not taking medications, his blood sugar is well controlled. Other than an initial resting heart rate of 116 beats per minute, vital signs are stable and within normal limits. Physical examination is unremarkable. Screening laboratory studies are notable for mildly elevated hepatic function, which approaches normal range several days after admission.
Mr. A reports a remote history of alcohol abuse but says he had not been drinking recently, and does not detail his pattern of use. Urine toxicology screen is negative for all substances of abuse.
Mental status examination reveals disheveled appearance, motor agitation, pressured speech, labile affect, loosening of associations, grandiose delusions, and auditory hallucinations. Mr. A’s thought processes are grossly disorganized, such that we could not gather a meaningful history. He believes God is speaking directly to him about plans to build a carousel at Disney World. He makes strange gestures with his hands throughout the interview, as if attempting to trace the shapes of letters and numbers. He frequently speaks of seeing an array of colors. Cognitive examination reveals a score of 5 of 30 on the Montreal Cognitive Assessment (Figure 1), indicating a severe impairment in neurocognitive functioning. He demonstrates limited insight and markedly impaired judgment, and denies having a mental illness.
What should be the next step in managing Mr. A?
a) obtain records from other facilities and collateral history
b) start an antipsychotic
c) order a brain MRI
d) start an alcohol withdrawal protocol
The authors’ observations
Mr. A showed elements of mania, psychosis, and delirium. We considered a broad differential diagnosis (Table). Mr. A initially could not provide reliable or accurate information. The least invasive next step was to obtain additional history from his wife and other medical records to refine the differential diagnosis.
HISTORY Bizarre behavior
Mr. A allows staff to speak with his wife and obtain records from a psychiatric hospitalization 3 years earlier. Mrs. A reports significant and rapid changes in her husband’s behavior and personality over 3 months, but does not describe a recent alcohol relapse. Mr. A sleeps very little, remaining awake and active throughout the night. He frequently rearranges the furniture in their home for no clear reason. Once, he knocked on the door of a young female neighbor asking if she found him attractive.
Mr. A has a significant criminal history. Approximately 30 years ago, he was charged with attempted murder of his ex-wife and he had faced charges of attempted kidnapping and assaulting a police officer. However, he has no recent legal issues.
Mr. A has a history of episodes that are similar to this presentation. Seven years ago, he impulsively purchased a $650,000 house after his fourth wife died. He then had a $90,000 heart-shaped pool installed. He also drove a tractor through his stepdaughter’s car for no apparent reason. Also, 3 years ago, he displayed symptoms similar to his current presentation, including insomnia, irritability, and grandiosity. He engaged in strange behaviors, such as dressing up and imitating homeless people at his church.
During the hospitalization 3 years ago, clinicians gave Mr. A a diagnosis of bipolar disorder, current episode manic, and delirium of an unclear cause. A medical workup, including brain MRI, did not uncover a basis for his delirium. Antipsychotics (risperidone and perphenazine) and mood stabilizers (lithium and valproic acid), stabilized his condition; after 7 weeks, Mr. A was discharged, but he did not pursue outpatient psychiatric care.
What is the most likely DSM-5 diagnosis?
a) major neurocognitive disorder (dementia)
b) alcohol use disorder (eg, Wernicke- Korsakoff syndrome)
c) delirium secondary to mania
d) psychotic disorder
The authors’ observations
DSM-51 suggests a stepwise approach to diagnosis, with consideration of:
• signs and symptoms
• substance use
• general medical condition
• developmental conflict or stage
• whether a mental disorder is present.
Mr. A’s age and severe cognitive impairment raise the possibility of dementia. Rapid onset, history of similar episodes, and apparent inter-episode recovery make dementia unlikely. The history of alcohol abuse and mildly elevated hepatic function tests suggest a substance use disorder such as Wernicke-Korsakoff syndrome or a withdrawal syndrome. However, there is no evidence of excessive alcohol use over the past several months, toxicology studies were negative, and vital signs were stable. General medical causes for Mr. A’s presentation, such as hypoglycemia, head trauma, intracranial infection, and metabolic disturbance were considered, but physical examination and laboratory studies did not suggest any condition that would explain his condition.
Mr. A’s previous psychiatric hospitalization is critical in clarifying the more likely diagnosis. A similar presentation yielded the diagnosis of bipolar disorder, manic phase. Our working diagnosis, therefore, was bipolar disorder with features of delirious mania.
Delirious mania
Delirious mania was first described by Luther Bell in 1849 and is characterized by an acute and simultaneous onset of mania— severe insomnia, poor judgment, grandiosity, excitement, emotional lability, bizarre hallucinations, and delusions—and delirium—altered consciousness, disorientation, and confusion.2,3 Although there are no diagnostic criteria, some authors suggest that delirious mania is characterized by inappropriate toileting, denudation, profound lack of sleep, and episodic memory impairment that can last hours or days.4 Catatonia frequently is seen with delirious mania.5 Initial case descriptions described a high mortality rate, approaching 75% of patients.6 There is little published literature and no classification of delirious mania in DSM-5.1 Estimates are that delirium is concomitant in 20% to 33% of patients with mania.7,8
Several theories try to clarify the underlying etiology of delirious mania. Jacobowski et al9 summarized the etiology and proposed that it is:
• 1 of 3 types of mania, including: acute and delusional manias, as initially proposed by Kraeplin
• a severe form of catatonia
• a condition akin to, but distinct from, delirium with similar underlying medical causes
• a primary psychiatric disorder underlying the cause of delirium.
EVALUATION Brain changes
For several days, Mr. A continues to engage in strange behavior. He tries to take patients’ belongings, is denudative, crawls on floors, licks walls, is unable to feed himself, and exhibits odd motor movements with purposeless motor activity.
We consult our internal medicine team to identify treatable, medical causes. Results of serum B12, thyroid-stimulating hormone, and rapid plasma reagin studies are within normal limits. Urinalysis is negative. A brain MRI reveals numerous white-matter T2-weighted and FLAIR hyperintensities, indicating small-vessel ischemic changes that are consistent with the findings of an MRI 3 years ago. A sleep-deprived EEG with temporal leads obtained on Day 4 of hospitalization demonstrates a diffusely slow and marginally to poorly organized background, believed to indicate global cerebral dysfunction that is most consistent with nonfocal global encephalopathy. There is no seizure activity. We do not perform a lumbar puncture because of Mr. A’s absence of focal neurologic deficits, lack of fever, and normal white blood cell count.
What is the most appropriate treatment?
a) electroconvulsive therapy (ECT)
b) high-dose benzodiazepine
c) mood stabilizer
d) antipsychotic
The authors’ observations
We strongly suspect that Mr. A has delirious mania. Symptoms and signs of mania include labile mood, excessive spending, grandiosity, insomnia, and psychosis together with delirium (marked disorientation, confusion). We ascribed Mr. A’s odd motor behaviors to catatonia, a hallmark of delirious mania. The literature has little description of EEG findings in suspected cases of delirious mania; however, abnormal EEG tracings have been reported.10 We also speculated that Mr. A’s EEG reflected effects produced by his prescribed antipsychotic regimen.
Treatment
There is no clear consensus on treating delirious mania. Because catatonia is a key feature of delirious mania—whether etiologically or as a prominent sign of the condition—ECT and benzodiazepines are proposed as primary treatments. In a study of 16 patients with delirious mania, Karmacharya et al4 found ECT to be effective, with patients showing improvement after 1 to 4 treatments. Lee et al10 reported similar findings. Although a high-dose benzodiazepine is not as effective as ECT, a 1-time oral dose of 3 to 4 mg of lorazepam has been used to treat delirious mania.
The efficacy of antipsychotic and mood-stabilizing pharmacotherapy is not clear. Bond3 described 3 cases in which patients were treated effectively with a typical antipsychotic (haloperidol or chlorpromazine) and lithium. Jung and Lee11demonstrated the efficacy of atypical antipsychotics, with a marked improvement in symptoms within 1 week. However, other studies do not support these findings. Karmacharya et al4 found that typical antipsychotics 1) make the clinical picture worse by increasing extrapyramidal symptoms and 2) produce inconsistent effects. Mood stabilizers sometimes proved beneficial.
Karmacharya et al4 further argued that the delay in improvement seen with any antipsychotics and mood stabilizers suggest they should not be considered a first-line treatment. These discordant findings are the result of a small number of studies and a lack of understanding of the exact nature of delirious mania.
TREATMENT Quick Response
Mr. A’s symptoms rapidly resolve with a combination of quetiapine, 800 mg/d, haloperidol, 10 mg/d, and lithium, 1,200 mg/d. His mood returns to euthymia and his psychotic symptoms abate. He is able to attend to all activities of daily living. Mental status clears and he is fully oriented and able to hold a logical conversation. He scores 28 out of 30 on a subsequent Montreal Cognitive Assessment, administered 11 days after the initial assessment (Figure 2), indicating normal neurocognitive function. He returns to his baseline level of functioning and is discharged in psychiatrically stable condition. Mr. A has no recollection of the bizarre behaviors he displayed earlier in his hospitalization.
The authors’ observations
We started Mr. A on antipsychotics because of his initial level of agitation. In reviewing pharmacotherapy options for Mr. A’s mania and delirium, we contemplated several options. Quetiapine and lithium were chosen after a review of outside hospital records demonstrated a combination of a mood stabilizer and an antipsychotic was effective in treating a previous similar episode, which led to remission of Mr. A’s symptoms. We chose quetiapine because of it highly sedating properties, suspecting that it would help treat his insomnia. We thought that the risk that lithium would make delirium worse was mitigated by Mr. A’s previous therapeutic response to it. Haloperidol was added for treating delirium, given its more potent D2 antagonism. Mr. A responded quickly to these interventions.
We did not consider ECT at the beginning of Mr. A’s admission, and we avoided sedative-hypnotic agents because we were concerned that a benzodiazepine might make his delirium worse. In light of available data suggesting that ECT and benzodiazepines are preferred treatments for delirious mania, it is noteworthy that Mr. A responded so robustly and rapidly to an antipsychotic and a mood stabilizer.
Bottom Line
Consider delirious mania in any patient who has a history of bipolar disorder presenting with co-occuring symptoms of mania and delirium. Collateral information is vital to establishing a diagnosis. With suspected delirium, rule out concomitant reversible medical problems. Electroconvulsive therapy, high-dose benzodiazepines, antipsychotics, and mood stabilizers have shown efficacy.
Related Resources
• Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
• Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
Drug Brand Names
Chlorpromazine • Thorazine Perphenazine • Trilafon
Haloperidol • Haldol Quetiapine • Seroquel
Lithium • Eskalith Risperidone • Risperdal
Lorazepam • Ativan Valproic acid • Depakene
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
How should you use the lab to monitor patients taking a mood stabilizer?
Ms. W, age 27, presents with a chief concern of “depression.” She describes a history of several hypomanic episodes as well as the current depressive episode, prompting a bipolar II disorder diagnosis. She is naïve to all psychotropics. You plan to initiate a mood-stabilizing agent. What would you include in your initial workup before starting treatment and how would you monitor her as she continues treatment?
Mood stabilizers are employed to treat bipolar spectrum disorders (bipolar I, bipolar II, and cyclothymic disorder) and schizoaffective disorder, bipolar type. Some evidence suggests that mood stabilizers also can be used for treatment-resistant depressive disorders and borderline personality disorder.1 Mood stabilizers include lithium, valproate, carbamazepine, oxcarbazepine, and lamotrigine.2-5
This review focuses on applications and monitoring of mood stabilizers for bipolar I and II disorders. We also will briefly review atypical antipsychotics because they also are used to treat bipolar spectrum disorders (see the September 2013 issue of Current Psychiatry at CurrentPsychiatry.com for a more detailed article on monitoring of antipsychotics).6
There are several well-researched guidelines used to guide clinical practice.2-5 Many guidelines recommend baseline and routine monitoring parameters based on the characteristics of the agent used. However, the International Society for Bipolar Disorders (ISBD) guidelines highlight the importance of monitoring medical comorbidities, which are common among patients with bipolar disorder and can affect pharmacotherapy and clinical outcomes. These recommendations are similar to metabolic monitoring guidelines for antipsychotics.5
Reviews of therapeutic monitoring show that only one-third to one-half of patien
taking a mood stabilizer are appropriately monitored. Poor adherence to guideline recommendations often is observed because of patients’ lack of insight or medication adherence and because psychiatric care generally is segregated from other medical care.7-9
Baseline testing
The ISBD guidelines recommend an initial workup for all patients that includes:
• waist circumference or body mass index (BMI), or both
• blood pressure
• complete blood count (CBC)
• electrolytes
• blood urea nitrogen (BUN) and creatinine
• liver function tests (LFTs)
• fasting glucose
• fasting lipid profile.
In addition, medical history, cigarette smoking status, alcohol intake, and family history of cardiovascular disease, cerebrovascular disease, hypertension, dyslipidemia, and diabetes mellitus should be documented. Rule out pregnancy in women of childbearing potential.2 The Figure describes monitoring parameters based on selected agent.
Agent-specific monitoring
Lithium. Patients beginning lithium therapy should undergo thyroid function testing and, for patients age >40, ECG monitoring. Educate patients about potential side effects of lithium, signs and symptoms of lithium toxicity, and the importance of avoiding dehydration. Adding or changing certain medications could elevate the serum lithium level (eg, diuretics, angiotensin-converting enzyme [ACE]-inhibitors, nonsteroidal anti-inflammatory drugs [NSAIDs], COX-2 inhibitors).
Lithium can cause weight gain and adverse effects in several organ systems, including:
• gastrointestinal (GI) (nausea, vomiting, abdominal pain, loss of appetite, diarrhea)
• renal (nephrogenic diabetes insipidus, tubulointerstitial renal disease)
• neurologic (tremors, cognitive dulling, raised intracranial pressure)
• endocrine (thyroid and parathyroid dysfunction)
• cardiac (benign electrocardiographic changes, conduction abnormalities)
• dermatologic (acne, psoriasis, hair loss)
• hematologic (benign leukocytosis).
Lithium has a narrow therapeutic index (0.5 to 1.2 mEq/L), which means that small changes in the serum level can result in therapeutic inefficacy or toxicity. Lithium toxicity can cause irreversible organ damage or death. Serum lithium levels, symptomatic response, emergence and evolution of adverse drug reactions (ADRs), and the recognition of patient risk factors for toxicity can help guide dosing. From a safety monitoring viewpoint, lithium toxicity, renal and endocrine adverse effects, and potential drug interactions are foremost concerns.
Lithium usually is started at a low, divided dosages to minimize side effects, and titrated according to response. Check lithium levels before and after each dose increase. Serum levels reach steady state 5 days after dosage adjustment, but might need to be checked sooner if a rapid increase is necessary, such as when treating acute mania, or if you suspect toxicity.
If the patient has renal insufficiency, it may take longer for the lithium to reach steady state; therefore, delaying a blood level beyond 5 days may be necessary to gauge a true steady state. Also, anytime a medication that interferes with lithium renal elimination, such as diuretics, ACE inhibitors, NSAIDs, COX-2 inhibitors, is added or the dosage is changed, a new lithium level will need to be obtained to reassess the level in 5 days, assuming adequate renal function. In general, renal function and thyroid function should be evaluated once or twice during the first 6 months of lithium treatment.
Subsequently, renal and thyroid function can be checked every 6 months to 1 year in stable patients or when clinically indicated. Check a patient’s weight after 6 months of therapy, then at least annually.2
Valproic acid (VPA) and its derivatives. The most important initial monitoring for VPA therapy includes LFTs and CBC. Before initiating VPA treatment, take a medical history, with special attention to hepatic, hematologic, and bleeding abnormalities. Therapeutic blood monitoring can be conducted once steady state is achieved and as clinically necessary thereafter.
VPA can be administered at an initial starting dosage of 20 to 30 mg/kg/d in inpatients. In outpatients it is given in low, divided doses or as once-daily dosing using an extended-release formulation to minimize GI and neurologic toxicity and titrated every few days. Target serum level is 50 to 125 μg/mL.
Side effects of VPA include GI distress (eg, anorexia, nausea, dyspepsia, vomiting, diarrhea), hematologic effects (reversible leukopenia, thrombocytopenia), hair loss, weight gain, tremor, hepatic effects (benign LFT elevations, hepatotoxicity), osteoporosis, and sedation. Patients with prior or current hepatic disease may be at greater risk for hepatotoxicity. There is an association between VPA and polycystic ovarian syndrome. Rare, idiosyncratic, but potentially fatal adverse events with valproate include irreversible hepatic failure, hemorrhagic pancreatitis, and agranulocytosis.
Older monitoring standards indicated taking LFTs and CBC every 6 months and serum VPA level as clinically indicated. According to ISBD guidelines, weight, CBC, LFTs, and menstrual history should be monitored every 3 months for the first year and then annually; blood pressure, bone status (densitometry), fasting glucose, and fasting lipids should be monitored only in patients with related risk factors. Routine ammonia levels are not recommended but might be indicated if a patient has sudden mental status changes or change in condition.2
Carbamazepine and oxcarbazepine. The most important initial monitoring for carbamazepine therapy includes LFTs, renal function, electrolytes, and CBC. Before treatment, take a medical history, with special emphasis on history of blood dyscrasias or liver disease. After initiating carbamazepine, CBC, LFTs, electrolytes, and renal function should be done monthly for 3 months, then repeated annually.
Carbamazepine is a substrate and an inducer of the cytochrome P450 (CYP) system, so it can reduce levels of many other drugs including other antiepileptics, warfarin, and oral contraceptives. Serum level of carbamazepine can be measured at trough after 5 days, with a target level of 4 to 12 μg/mL. Two levels should be drawn, 4 weeks apart, to establish therapeutic dosage secondary to autoinduction of the CYP450 system.2
As many as one-half of patients experience side effects with carbamazepine. The most common side effects include fatigue, nausea, and neurologic symptoms (diplopia, blurred vision, and ataxia). Less frequent side effects include skin rashes, leukopenia, liver enzyme elevations, thrombocytopenia, hyponatremia, and hypo-osmolality. Rare, potentially fatal side effects include agranulocytosis, aplastic anemia, thrombocytopenia, hepatic failure, and exfoliative dermatitis (eg, Stevens-Johnson syndrome).
Patients of Asian descent who are taking carbamazepine should undergo genetic testing for the HLA-B*1502 enzyme because persons with this allele are at higher risk of developing Stevens-Johnson syndrome. Also, patients should be educated about the signs and symptoms of these rare adverse reactions so that medical treatment is not delayed should these adverse events present.
Lamotrigine does not require further laboratory monitoring beyond the initial recommended workup. The most important variables to consider are interactions with other medications (especially other antiepileptics, such as VPA and carbamazepine) and observing for rash. Titration takes several weeks to minimize risk of developing a rash.2 Similar to carbamazepine, the patient should be educated on the signs and symptoms of exfoliative dermatitis (eg, Stevens-Johnson syndrome) so that medical treatment is sought out should this reaction occur.
Atypical antipsychotics. Baseline workup includes the general monitoring parameters described above. Atypical antipsychotics have a lower incidence of extrapyramidal side effects than typical antipsychotics, but are associated with an increased risk of metabolic complications. Other major ADRs to consider are cardiac effects and hyperprolactinemia; clinicians should therefore inquire about a personal or family history of cardiac problems, including congenital long QT syndrome. Patients should be screened for any medications that can prolong the QTc interval or interact with the metabolism of medications known to cause QTc prolongation.
Measure weight monthly for the first 3 months, then every 3 months to monitor for metabolic side effects during ongoing treatment. Obtain blood pressure and fasting glucose every 3 months for the first year, then annually. Repeat a fasting lipid profile 3 months after initiating treatment, then annually. Cardiac effects and prolactin levels can be monitored as needed if clinically indicated.2
CASE CONTINUED
You discuss with Ms. W choices of a mood stabilizing agent to treat her bipolar II disorder; she agrees to start lithium. Before initiating treatment, you obtain her weight (and calculate her BMI), blood pressure, CBC, electrolyte levels, BUN and creatinine levels, liver function tests, fasting glucose, fasting lipid profile, and thyroid panel. You also review her medical history, lifestyle factors (cigarette smoking status, alcohol intake), and family history. A urine pregnancy screen is negative. The pharmacist assists in screening for potential drug-drug interactions, including over-the-counter medications that Ms. W occasionally takes as needed. She is counseled on the use of NSAIDS because these drugs can increase the lithium level.
Ms. W tolerates and responds well to lithium. No further dosing recommendations are made, based on clinical response. You measure her weight at 6 months, then annually. Renal function and thyroid function are monitored at 3 and 6 months after lithium is initiated, and then annually. One year after starting lithium, she continues to tolerate the medication and has minimal metabolic side effects.
Related Resources
• McInnis MG. Lithium for bipolar disorder: A re-emerging treatment for mood instability. Current Psychiatry. 2014; 13(6):38-44.
• Stahl SM. Stahl’s illustrated mood stabilizers. New York, NY: Cambridge University Press; 2009.
Drug Brand Names
Carbamazepine • Tegretol Valproic acid • Depacon, Depakote
Lamotrigine • Lamictal Warfarin • Coumadin
Lithium • Lithobid, Eskalith
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review No. 43. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.effectivehealthcare.ahrq.gov/ehc/products/150/778/CER43_Off-LabelAntipsychotics_20110928.pdf. Published September 2011. Accessed June 6, 2014.
2. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
3. Ng F, Mammen OK, Wilting I, et al; International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
4. National Institute for Health and Clinical Excellence. Bipolar disorder (CG38). The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. http://www.nice.org.uk/CG038. Updated February 13, 2014. Accessed June 6, 2014.
5. Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord. 2006;8(6):721-739.
6. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013; 12(9):51-54.
7. Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8.
8. Kilbourne AM, Post EP, Bauer MS, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord. 2007;102(1-3):145-151.
9. Marcus SC, Olfson M, Pincus HA, et al. Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. Am J Psychiatry. 1999;156(7):1014-1018.
Ms. W, age 27, presents with a chief concern of “depression.” She describes a history of several hypomanic episodes as well as the current depressive episode, prompting a bipolar II disorder diagnosis. She is naïve to all psychotropics. You plan to initiate a mood-stabilizing agent. What would you include in your initial workup before starting treatment and how would you monitor her as she continues treatment?
Mood stabilizers are employed to treat bipolar spectrum disorders (bipolar I, bipolar II, and cyclothymic disorder) and schizoaffective disorder, bipolar type. Some evidence suggests that mood stabilizers also can be used for treatment-resistant depressive disorders and borderline personality disorder.1 Mood stabilizers include lithium, valproate, carbamazepine, oxcarbazepine, and lamotrigine.2-5
This review focuses on applications and monitoring of mood stabilizers for bipolar I and II disorders. We also will briefly review atypical antipsychotics because they also are used to treat bipolar spectrum disorders (see the September 2013 issue of Current Psychiatry at CurrentPsychiatry.com for a more detailed article on monitoring of antipsychotics).6
There are several well-researched guidelines used to guide clinical practice.2-5 Many guidelines recommend baseline and routine monitoring parameters based on the characteristics of the agent used. However, the International Society for Bipolar Disorders (ISBD) guidelines highlight the importance of monitoring medical comorbidities, which are common among patients with bipolar disorder and can affect pharmacotherapy and clinical outcomes. These recommendations are similar to metabolic monitoring guidelines for antipsychotics.5
Reviews of therapeutic monitoring show that only one-third to one-half of patien
taking a mood stabilizer are appropriately monitored. Poor adherence to guideline recommendations often is observed because of patients’ lack of insight or medication adherence and because psychiatric care generally is segregated from other medical care.7-9
Baseline testing
The ISBD guidelines recommend an initial workup for all patients that includes:
• waist circumference or body mass index (BMI), or both
• blood pressure
• complete blood count (CBC)
• electrolytes
• blood urea nitrogen (BUN) and creatinine
• liver function tests (LFTs)
• fasting glucose
• fasting lipid profile.
In addition, medical history, cigarette smoking status, alcohol intake, and family history of cardiovascular disease, cerebrovascular disease, hypertension, dyslipidemia, and diabetes mellitus should be documented. Rule out pregnancy in women of childbearing potential.2 The Figure describes monitoring parameters based on selected agent.
Agent-specific monitoring
Lithium. Patients beginning lithium therapy should undergo thyroid function testing and, for patients age >40, ECG monitoring. Educate patients about potential side effects of lithium, signs and symptoms of lithium toxicity, and the importance of avoiding dehydration. Adding or changing certain medications could elevate the serum lithium level (eg, diuretics, angiotensin-converting enzyme [ACE]-inhibitors, nonsteroidal anti-inflammatory drugs [NSAIDs], COX-2 inhibitors).
Lithium can cause weight gain and adverse effects in several organ systems, including:
• gastrointestinal (GI) (nausea, vomiting, abdominal pain, loss of appetite, diarrhea)
• renal (nephrogenic diabetes insipidus, tubulointerstitial renal disease)
• neurologic (tremors, cognitive dulling, raised intracranial pressure)
• endocrine (thyroid and parathyroid dysfunction)
• cardiac (benign electrocardiographic changes, conduction abnormalities)
• dermatologic (acne, psoriasis, hair loss)
• hematologic (benign leukocytosis).
Lithium has a narrow therapeutic index (0.5 to 1.2 mEq/L), which means that small changes in the serum level can result in therapeutic inefficacy or toxicity. Lithium toxicity can cause irreversible organ damage or death. Serum lithium levels, symptomatic response, emergence and evolution of adverse drug reactions (ADRs), and the recognition of patient risk factors for toxicity can help guide dosing. From a safety monitoring viewpoint, lithium toxicity, renal and endocrine adverse effects, and potential drug interactions are foremost concerns.
Lithium usually is started at a low, divided dosages to minimize side effects, and titrated according to response. Check lithium levels before and after each dose increase. Serum levels reach steady state 5 days after dosage adjustment, but might need to be checked sooner if a rapid increase is necessary, such as when treating acute mania, or if you suspect toxicity.
If the patient has renal insufficiency, it may take longer for the lithium to reach steady state; therefore, delaying a blood level beyond 5 days may be necessary to gauge a true steady state. Also, anytime a medication that interferes with lithium renal elimination, such as diuretics, ACE inhibitors, NSAIDs, COX-2 inhibitors, is added or the dosage is changed, a new lithium level will need to be obtained to reassess the level in 5 days, assuming adequate renal function. In general, renal function and thyroid function should be evaluated once or twice during the first 6 months of lithium treatment.
Subsequently, renal and thyroid function can be checked every 6 months to 1 year in stable patients or when clinically indicated. Check a patient’s weight after 6 months of therapy, then at least annually.2
Valproic acid (VPA) and its derivatives. The most important initial monitoring for VPA therapy includes LFTs and CBC. Before initiating VPA treatment, take a medical history, with special attention to hepatic, hematologic, and bleeding abnormalities. Therapeutic blood monitoring can be conducted once steady state is achieved and as clinically necessary thereafter.
VPA can be administered at an initial starting dosage of 20 to 30 mg/kg/d in inpatients. In outpatients it is given in low, divided doses or as once-daily dosing using an extended-release formulation to minimize GI and neurologic toxicity and titrated every few days. Target serum level is 50 to 125 μg/mL.
Side effects of VPA include GI distress (eg, anorexia, nausea, dyspepsia, vomiting, diarrhea), hematologic effects (reversible leukopenia, thrombocytopenia), hair loss, weight gain, tremor, hepatic effects (benign LFT elevations, hepatotoxicity), osteoporosis, and sedation. Patients with prior or current hepatic disease may be at greater risk for hepatotoxicity. There is an association between VPA and polycystic ovarian syndrome. Rare, idiosyncratic, but potentially fatal adverse events with valproate include irreversible hepatic failure, hemorrhagic pancreatitis, and agranulocytosis.
Older monitoring standards indicated taking LFTs and CBC every 6 months and serum VPA level as clinically indicated. According to ISBD guidelines, weight, CBC, LFTs, and menstrual history should be monitored every 3 months for the first year and then annually; blood pressure, bone status (densitometry), fasting glucose, and fasting lipids should be monitored only in patients with related risk factors. Routine ammonia levels are not recommended but might be indicated if a patient has sudden mental status changes or change in condition.2
Carbamazepine and oxcarbazepine. The most important initial monitoring for carbamazepine therapy includes LFTs, renal function, electrolytes, and CBC. Before treatment, take a medical history, with special emphasis on history of blood dyscrasias or liver disease. After initiating carbamazepine, CBC, LFTs, electrolytes, and renal function should be done monthly for 3 months, then repeated annually.
Carbamazepine is a substrate and an inducer of the cytochrome P450 (CYP) system, so it can reduce levels of many other drugs including other antiepileptics, warfarin, and oral contraceptives. Serum level of carbamazepine can be measured at trough after 5 days, with a target level of 4 to 12 μg/mL. Two levels should be drawn, 4 weeks apart, to establish therapeutic dosage secondary to autoinduction of the CYP450 system.2
As many as one-half of patients experience side effects with carbamazepine. The most common side effects include fatigue, nausea, and neurologic symptoms (diplopia, blurred vision, and ataxia). Less frequent side effects include skin rashes, leukopenia, liver enzyme elevations, thrombocytopenia, hyponatremia, and hypo-osmolality. Rare, potentially fatal side effects include agranulocytosis, aplastic anemia, thrombocytopenia, hepatic failure, and exfoliative dermatitis (eg, Stevens-Johnson syndrome).
Patients of Asian descent who are taking carbamazepine should undergo genetic testing for the HLA-B*1502 enzyme because persons with this allele are at higher risk of developing Stevens-Johnson syndrome. Also, patients should be educated about the signs and symptoms of these rare adverse reactions so that medical treatment is not delayed should these adverse events present.
Lamotrigine does not require further laboratory monitoring beyond the initial recommended workup. The most important variables to consider are interactions with other medications (especially other antiepileptics, such as VPA and carbamazepine) and observing for rash. Titration takes several weeks to minimize risk of developing a rash.2 Similar to carbamazepine, the patient should be educated on the signs and symptoms of exfoliative dermatitis (eg, Stevens-Johnson syndrome) so that medical treatment is sought out should this reaction occur.
Atypical antipsychotics. Baseline workup includes the general monitoring parameters described above. Atypical antipsychotics have a lower incidence of extrapyramidal side effects than typical antipsychotics, but are associated with an increased risk of metabolic complications. Other major ADRs to consider are cardiac effects and hyperprolactinemia; clinicians should therefore inquire about a personal or family history of cardiac problems, including congenital long QT syndrome. Patients should be screened for any medications that can prolong the QTc interval or interact with the metabolism of medications known to cause QTc prolongation.
Measure weight monthly for the first 3 months, then every 3 months to monitor for metabolic side effects during ongoing treatment. Obtain blood pressure and fasting glucose every 3 months for the first year, then annually. Repeat a fasting lipid profile 3 months after initiating treatment, then annually. Cardiac effects and prolactin levels can be monitored as needed if clinically indicated.2
CASE CONTINUED
You discuss with Ms. W choices of a mood stabilizing agent to treat her bipolar II disorder; she agrees to start lithium. Before initiating treatment, you obtain her weight (and calculate her BMI), blood pressure, CBC, electrolyte levels, BUN and creatinine levels, liver function tests, fasting glucose, fasting lipid profile, and thyroid panel. You also review her medical history, lifestyle factors (cigarette smoking status, alcohol intake), and family history. A urine pregnancy screen is negative. The pharmacist assists in screening for potential drug-drug interactions, including over-the-counter medications that Ms. W occasionally takes as needed. She is counseled on the use of NSAIDS because these drugs can increase the lithium level.
Ms. W tolerates and responds well to lithium. No further dosing recommendations are made, based on clinical response. You measure her weight at 6 months, then annually. Renal function and thyroid function are monitored at 3 and 6 months after lithium is initiated, and then annually. One year after starting lithium, she continues to tolerate the medication and has minimal metabolic side effects.
Related Resources
• McInnis MG. Lithium for bipolar disorder: A re-emerging treatment for mood instability. Current Psychiatry. 2014; 13(6):38-44.
• Stahl SM. Stahl’s illustrated mood stabilizers. New York, NY: Cambridge University Press; 2009.
Drug Brand Names
Carbamazepine • Tegretol Valproic acid • Depacon, Depakote
Lamotrigine • Lamictal Warfarin • Coumadin
Lithium • Lithobid, Eskalith
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. W, age 27, presents with a chief concern of “depression.” She describes a history of several hypomanic episodes as well as the current depressive episode, prompting a bipolar II disorder diagnosis. She is naïve to all psychotropics. You plan to initiate a mood-stabilizing agent. What would you include in your initial workup before starting treatment and how would you monitor her as she continues treatment?
Mood stabilizers are employed to treat bipolar spectrum disorders (bipolar I, bipolar II, and cyclothymic disorder) and schizoaffective disorder, bipolar type. Some evidence suggests that mood stabilizers also can be used for treatment-resistant depressive disorders and borderline personality disorder.1 Mood stabilizers include lithium, valproate, carbamazepine, oxcarbazepine, and lamotrigine.2-5
This review focuses on applications and monitoring of mood stabilizers for bipolar I and II disorders. We also will briefly review atypical antipsychotics because they also are used to treat bipolar spectrum disorders (see the September 2013 issue of Current Psychiatry at CurrentPsychiatry.com for a more detailed article on monitoring of antipsychotics).6
There are several well-researched guidelines used to guide clinical practice.2-5 Many guidelines recommend baseline and routine monitoring parameters based on the characteristics of the agent used. However, the International Society for Bipolar Disorders (ISBD) guidelines highlight the importance of monitoring medical comorbidities, which are common among patients with bipolar disorder and can affect pharmacotherapy and clinical outcomes. These recommendations are similar to metabolic monitoring guidelines for antipsychotics.5
Reviews of therapeutic monitoring show that only one-third to one-half of patien
taking a mood stabilizer are appropriately monitored. Poor adherence to guideline recommendations often is observed because of patients’ lack of insight or medication adherence and because psychiatric care generally is segregated from other medical care.7-9
Baseline testing
The ISBD guidelines recommend an initial workup for all patients that includes:
• waist circumference or body mass index (BMI), or both
• blood pressure
• complete blood count (CBC)
• electrolytes
• blood urea nitrogen (BUN) and creatinine
• liver function tests (LFTs)
• fasting glucose
• fasting lipid profile.
In addition, medical history, cigarette smoking status, alcohol intake, and family history of cardiovascular disease, cerebrovascular disease, hypertension, dyslipidemia, and diabetes mellitus should be documented. Rule out pregnancy in women of childbearing potential.2 The Figure describes monitoring parameters based on selected agent.
Agent-specific monitoring
Lithium. Patients beginning lithium therapy should undergo thyroid function testing and, for patients age >40, ECG monitoring. Educate patients about potential side effects of lithium, signs and symptoms of lithium toxicity, and the importance of avoiding dehydration. Adding or changing certain medications could elevate the serum lithium level (eg, diuretics, angiotensin-converting enzyme [ACE]-inhibitors, nonsteroidal anti-inflammatory drugs [NSAIDs], COX-2 inhibitors).
Lithium can cause weight gain and adverse effects in several organ systems, including:
• gastrointestinal (GI) (nausea, vomiting, abdominal pain, loss of appetite, diarrhea)
• renal (nephrogenic diabetes insipidus, tubulointerstitial renal disease)
• neurologic (tremors, cognitive dulling, raised intracranial pressure)
• endocrine (thyroid and parathyroid dysfunction)
• cardiac (benign electrocardiographic changes, conduction abnormalities)
• dermatologic (acne, psoriasis, hair loss)
• hematologic (benign leukocytosis).
Lithium has a narrow therapeutic index (0.5 to 1.2 mEq/L), which means that small changes in the serum level can result in therapeutic inefficacy or toxicity. Lithium toxicity can cause irreversible organ damage or death. Serum lithium levels, symptomatic response, emergence and evolution of adverse drug reactions (ADRs), and the recognition of patient risk factors for toxicity can help guide dosing. From a safety monitoring viewpoint, lithium toxicity, renal and endocrine adverse effects, and potential drug interactions are foremost concerns.
Lithium usually is started at a low, divided dosages to minimize side effects, and titrated according to response. Check lithium levels before and after each dose increase. Serum levels reach steady state 5 days after dosage adjustment, but might need to be checked sooner if a rapid increase is necessary, such as when treating acute mania, or if you suspect toxicity.
If the patient has renal insufficiency, it may take longer for the lithium to reach steady state; therefore, delaying a blood level beyond 5 days may be necessary to gauge a true steady state. Also, anytime a medication that interferes with lithium renal elimination, such as diuretics, ACE inhibitors, NSAIDs, COX-2 inhibitors, is added or the dosage is changed, a new lithium level will need to be obtained to reassess the level in 5 days, assuming adequate renal function. In general, renal function and thyroid function should be evaluated once or twice during the first 6 months of lithium treatment.
Subsequently, renal and thyroid function can be checked every 6 months to 1 year in stable patients or when clinically indicated. Check a patient’s weight after 6 months of therapy, then at least annually.2
Valproic acid (VPA) and its derivatives. The most important initial monitoring for VPA therapy includes LFTs and CBC. Before initiating VPA treatment, take a medical history, with special attention to hepatic, hematologic, and bleeding abnormalities. Therapeutic blood monitoring can be conducted once steady state is achieved and as clinically necessary thereafter.
VPA can be administered at an initial starting dosage of 20 to 30 mg/kg/d in inpatients. In outpatients it is given in low, divided doses or as once-daily dosing using an extended-release formulation to minimize GI and neurologic toxicity and titrated every few days. Target serum level is 50 to 125 μg/mL.
Side effects of VPA include GI distress (eg, anorexia, nausea, dyspepsia, vomiting, diarrhea), hematologic effects (reversible leukopenia, thrombocytopenia), hair loss, weight gain, tremor, hepatic effects (benign LFT elevations, hepatotoxicity), osteoporosis, and sedation. Patients with prior or current hepatic disease may be at greater risk for hepatotoxicity. There is an association between VPA and polycystic ovarian syndrome. Rare, idiosyncratic, but potentially fatal adverse events with valproate include irreversible hepatic failure, hemorrhagic pancreatitis, and agranulocytosis.
Older monitoring standards indicated taking LFTs and CBC every 6 months and serum VPA level as clinically indicated. According to ISBD guidelines, weight, CBC, LFTs, and menstrual history should be monitored every 3 months for the first year and then annually; blood pressure, bone status (densitometry), fasting glucose, and fasting lipids should be monitored only in patients with related risk factors. Routine ammonia levels are not recommended but might be indicated if a patient has sudden mental status changes or change in condition.2
Carbamazepine and oxcarbazepine. The most important initial monitoring for carbamazepine therapy includes LFTs, renal function, electrolytes, and CBC. Before treatment, take a medical history, with special emphasis on history of blood dyscrasias or liver disease. After initiating carbamazepine, CBC, LFTs, electrolytes, and renal function should be done monthly for 3 months, then repeated annually.
Carbamazepine is a substrate and an inducer of the cytochrome P450 (CYP) system, so it can reduce levels of many other drugs including other antiepileptics, warfarin, and oral contraceptives. Serum level of carbamazepine can be measured at trough after 5 days, with a target level of 4 to 12 μg/mL. Two levels should be drawn, 4 weeks apart, to establish therapeutic dosage secondary to autoinduction of the CYP450 system.2
As many as one-half of patients experience side effects with carbamazepine. The most common side effects include fatigue, nausea, and neurologic symptoms (diplopia, blurred vision, and ataxia). Less frequent side effects include skin rashes, leukopenia, liver enzyme elevations, thrombocytopenia, hyponatremia, and hypo-osmolality. Rare, potentially fatal side effects include agranulocytosis, aplastic anemia, thrombocytopenia, hepatic failure, and exfoliative dermatitis (eg, Stevens-Johnson syndrome).
Patients of Asian descent who are taking carbamazepine should undergo genetic testing for the HLA-B*1502 enzyme because persons with this allele are at higher risk of developing Stevens-Johnson syndrome. Also, patients should be educated about the signs and symptoms of these rare adverse reactions so that medical treatment is not delayed should these adverse events present.
Lamotrigine does not require further laboratory monitoring beyond the initial recommended workup. The most important variables to consider are interactions with other medications (especially other antiepileptics, such as VPA and carbamazepine) and observing for rash. Titration takes several weeks to minimize risk of developing a rash.2 Similar to carbamazepine, the patient should be educated on the signs and symptoms of exfoliative dermatitis (eg, Stevens-Johnson syndrome) so that medical treatment is sought out should this reaction occur.
Atypical antipsychotics. Baseline workup includes the general monitoring parameters described above. Atypical antipsychotics have a lower incidence of extrapyramidal side effects than typical antipsychotics, but are associated with an increased risk of metabolic complications. Other major ADRs to consider are cardiac effects and hyperprolactinemia; clinicians should therefore inquire about a personal or family history of cardiac problems, including congenital long QT syndrome. Patients should be screened for any medications that can prolong the QTc interval or interact with the metabolism of medications known to cause QTc prolongation.
Measure weight monthly for the first 3 months, then every 3 months to monitor for metabolic side effects during ongoing treatment. Obtain blood pressure and fasting glucose every 3 months for the first year, then annually. Repeat a fasting lipid profile 3 months after initiating treatment, then annually. Cardiac effects and prolactin levels can be monitored as needed if clinically indicated.2
CASE CONTINUED
You discuss with Ms. W choices of a mood stabilizing agent to treat her bipolar II disorder; she agrees to start lithium. Before initiating treatment, you obtain her weight (and calculate her BMI), blood pressure, CBC, electrolyte levels, BUN and creatinine levels, liver function tests, fasting glucose, fasting lipid profile, and thyroid panel. You also review her medical history, lifestyle factors (cigarette smoking status, alcohol intake), and family history. A urine pregnancy screen is negative. The pharmacist assists in screening for potential drug-drug interactions, including over-the-counter medications that Ms. W occasionally takes as needed. She is counseled on the use of NSAIDS because these drugs can increase the lithium level.
Ms. W tolerates and responds well to lithium. No further dosing recommendations are made, based on clinical response. You measure her weight at 6 months, then annually. Renal function and thyroid function are monitored at 3 and 6 months after lithium is initiated, and then annually. One year after starting lithium, she continues to tolerate the medication and has minimal metabolic side effects.
Related Resources
• McInnis MG. Lithium for bipolar disorder: A re-emerging treatment for mood instability. Current Psychiatry. 2014; 13(6):38-44.
• Stahl SM. Stahl’s illustrated mood stabilizers. New York, NY: Cambridge University Press; 2009.
Drug Brand Names
Carbamazepine • Tegretol Valproic acid • Depacon, Depakote
Lamotrigine • Lamictal Warfarin • Coumadin
Lithium • Lithobid, Eskalith
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review No. 43. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.effectivehealthcare.ahrq.gov/ehc/products/150/778/CER43_Off-LabelAntipsychotics_20110928.pdf. Published September 2011. Accessed June 6, 2014.
2. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
3. Ng F, Mammen OK, Wilting I, et al; International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
4. National Institute for Health and Clinical Excellence. Bipolar disorder (CG38). The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. http://www.nice.org.uk/CG038. Updated February 13, 2014. Accessed June 6, 2014.
5. Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord. 2006;8(6):721-739.
6. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013; 12(9):51-54.
7. Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8.
8. Kilbourne AM, Post EP, Bauer MS, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord. 2007;102(1-3):145-151.
9. Marcus SC, Olfson M, Pincus HA, et al. Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. Am J Psychiatry. 1999;156(7):1014-1018.
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review No. 43. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.effectivehealthcare.ahrq.gov/ehc/products/150/778/CER43_Off-LabelAntipsychotics_20110928.pdf. Published September 2011. Accessed June 6, 2014.
2. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
3. Ng F, Mammen OK, Wilting I, et al; International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
4. National Institute for Health and Clinical Excellence. Bipolar disorder (CG38). The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. http://www.nice.org.uk/CG038. Updated February 13, 2014. Accessed June 6, 2014.
5. Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord. 2006;8(6):721-739.
6. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013; 12(9):51-54.
7. Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8.
8. Kilbourne AM, Post EP, Bauer MS, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord. 2007;102(1-3):145-151.
9. Marcus SC, Olfson M, Pincus HA, et al. Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. Am J Psychiatry. 1999;156(7):1014-1018.
Aggressive and delusional about his alien origins, but refusing treatment
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
Management of Papillary Thyroid Cancer: An Overview for the Primary Care Physician
From the Yale School of Medicine, New Haven, CT.
ABSTRACT
• Objective: To review management of papillary thyroid cancer.
• Methods: Review of the literature.
• Results: Papillary thyroid cancer is the most common endocrine malignancy. The standard treatment for papillary thyroid cancer is thyroidectomy. Adjuvant therapy includes lifelong thyroid-stimulating hormone suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event.
• Conclusion: The incidence of papillary thyroid cancer is rapidly increasing. Surgery remains the cornerstone of treatment.
Papillary thyroid cancer is the most common endocrine malignancy and accounts for the majority of cancers of the thyroid. The incidence of papillary thyroid cancer is rapidly increasing [1]. Although increasing detection has been proposed as a possible factor [2], some studies reject this hypothesis, reporting increase in the incidence of larger tumors [3]. Papillary thyroid cancer is characterized by a low mortality but a high recurrence rate [1], posing challenges not only to the endocrinologist and oncologist but also to the general practitioner.
The most frequent presentation of papillary thyroid cancer is a palpable thyroid nodule, cervical lymphadenopathy, or incidental detection on imaging. Locally advanced disease can present with hoarseness or voice alteration. Common risks factors include history of radiation exposure during childhood (the most important risk factor), thyroid cancer in a first-degree relative, family history of a thyroid cancer syndrome (such as Werner syndrome, Cowden syndrome, Carney complex, or familial polyposis), and female sex (2.5:1). Thyroid nodules in the context of an autoimmune thyroiditis may have a higher risk of malignancy [4].
CASE STUDY
Initial Presentation
A 49-year-old man with no significant past medical history presents with a painless mass in the anterior part of his neck.
History, Physical Examination, and Initial Investigations
He has no other symptoms, no weight changes, no history of radiation exposure to the neck, and no family history of malignancy. Physical exam shows a mass in the left thyroid lobe. There is no evidence of cardiac arrhythmias, tremors, or ophthalmologic abnormalities. Thyroid-stimulating hormone (TSH) level is 2.8 mIU/L (normal range, 0.4–4.5 mIU/L) and free thyroxine (T4) level is 1.1 ng/dL (normal range, 0.8–1.5 ng/dL). An ultrasound scan of the neck shows enlargement of the left lobe of thyroid gland, containing multiple complex lesions, the largest measuring 2 x 3 cm, with calcification as well as 3 enlarged lymph nodes in the left level IV. Fine-needle aspiration of the thyroid mass is positive for papillary carcinoma.
• What is the approach to the initial evaluation of a thyroid nodule?
Initial diagnostic evaluation includes history, physical examination, and TSH measurement; nonfunctioning nodules, associated with normal or high values of TSH, carry a higher risk of malignancy [5]. Cervical ultrasound should be performed in all patients with nodules. Fine-needle aspiration (FNA) should be used to evaluate nonfunctioning nodules > 1 cm or subcentimeter nodules with suspicious ultrasound features or if the patient has major risk factors (history of ionizing radiation exposure, external beam radiation exposure, family or personal history of papillary thyroid cancer, or FDG-PET [fluorinated glucose positron emission tomography]–positive thyroid nodules). Scintigraphy can be used to evaluate the need for ultrasound and FNA in patients with low TSH values [6,7]; hyperfunctioning nodules are at low risk for malignancy and do not require biopsy.
• What is initial treatment of papillary thyroid cancer?
Surgery is the primary treatment for papillary thyroid cancer. Unlike for many cancers, surgical removal of the primary tumor is indicated even in the presence of metastatic disease [8]. Total or near-total thyroidectomy is used to treat patients with tumors > 1 cm or with tumors < 1 cm and associated risk factors (eg, contralateral nodules, affected lymph nodes, metastasis, history of radiation, first-degree family history of papillary thyroid cancer, or age > 45 years) [6]. There is a lower risk of recurrence in patients treated with total thyroidectomy versus lobectomy in papillary thyroid cancer [9,10]. Thyroid lobectomy may be used in small (< 1 cm) unifocal tumors without the presence of the associated risk factors listed above.
Patients with central or lateral neck lymph node involvement should also undergo central-compartment (level VI) neck dissection. Therapeutic lateral neck compartmental lymph node dissection is recommended in patients with biopsy-proven metastatic lateral cervical adenopathy [6,7]. The role of unilateral or bilateral prophylactic central-compartment nodal dissection (PCND), that is, lymph node dissection in the level VI compartment of neck in patients without evidence of lymphadenopathy, is controversial. The data for the possible benefit of PCND are inconclusive [11] although the ATA recommends the procedure for locally invasive T3 and T4 tumors [6].
The American Thyroid Association (ATA) and National Comprehensive Cancer Network (NCCN) guidelines [6,7] recommend a preoperative cervical ultrasound in patients with biopsy-proven papillary thyroid cancer to evaluate the presence of disease in the cervical lymph nodes, especially in the lateral and central compartments, and in the contralateral thyroid lobe. If suspicious lymph nodes are found, FNA confirmation is necessary only if this would change management. Systematic use of other preoperative imaging studies, such as CT or MRI, is not recommended [6,7].
Surgical Treatment
The patient underwent a total thyroidectomy with bilateral central neck dissection and selective supraclavicular left-sided lateral neck dissection. Lymph nodes on both sides of the neck (paratracheal nodes) as well as the left supraclavicular nodes were removed. Pathology showed multifocal papillary cancer with extracapsular extension to the paratracheal soft tissue, 14/14 lymph nodes affected, stage IVA T4N1bM0.
• How is papillary thyroid cancer staged?
• How should this patient be treated after surgery? Is any adjuvant therapy indicated?
TSH Suppression
In an effort to reduce risk of recurrence, patients should receive lifelong suppression of TSH using supraphysiologic doses of levothyroxine after total thyroidectomy. This is based upon the hypothesis that TSH is a growth factor for thyroid cancer cells [12,13]. Although a meta-analysis [14] supports the efficacy of TSH suppression therapy, some authors have questioned its widespread use, especially in light of the adverse effects of its use over the long term [15]. Many support its use only in high-risk patients [16], arguing that there is no evidence of benefit for low-risk patients [17]. This view is reflected in the ATA guidelines, which recommend TSH suppression below 0.1 mU/L for high-risk and intermediate-risk patients, while normal or slightly below normal TSH levels are recommended for low-risk patients [6].
Adverse effects of TSH suppression therapy are derived from the induced mild thyrotoxicosis, including cardiovascular and skeletal manifestations. Notably, elderly patients have a higher risk of cardiovascular side effects [18] such as atrial fibrillation, diastolic dysfunction, tachyarrhythmias, increased heart rate or increased left ventricular mass. Likewise, postmenopausal women are most susceptible for skeletal effects such as decreased mineral bone density and fractures [19].
Radioiodine Ablative Therapy
Radioactive iodine (RAI or radioiodine) therapy is based on the capacity of thyroid tissue to take up and retain iodine, specifically, radioiodine. This capacity is present but reduced in papillary and follicular cancer cells.
Radioiodine remnant ablation is performed after surgery, acting as adjuvant therapy by destroying remnant pathological or normal thyroid tissue. The destruction of normal thyroid tissue is useful as it increases the reliability of thyroglobulin testing and radioiodine scanning in the detection of recurrent or metastatic disease. Moreover, remnant ablation has been shown to prevent new thyroid neoplasias in high-risk patients (ie, those with history of radiation exposure). Radioiodine ablative therapy has been shown to reduce recurrence and cause-specific mortality [20] in certain subgroups; however, patients with low mortality risk do not seem to benefit from this therapy [21,22]. Its use is recommended in patients with distant metastases, tumors > 4 cm, or with extrathyroidal extension. It is also recommended for selected patients with tumors 1–4 cm who have high-risk features (such as lymph node involvement, history of radiation, or others previously mentioned) when there is an intermediate to high risk of recurrence or death from thyroid cancer [6]. Lymph node involvement can occur in up to 50% of cases [39] and normally responds to radioiodine therapy.
Since TSH increases radioiodine uptake by normal or pathological thyroid cells, TSH stimulation is required for radioiodine therapy. This can be done by endogenous TSH elevation or by recombinant human TSH (rhTSH). The former can be achieved by either stopping thyroxine 2 to 3 weeks prior to the remnant ablation, or by withdrawing thyroxine and switching to liothyronine for 2 to 3 weeks followed by a discontinuation of liothyronine for 2 weeks. Both approaches seem to produce the same incidence of hypothyroid symptoms [23]. Thyroxine therapy can be resumed 2 to 3 days after radioiodine ablative therapy. Recombinant human TSH can be used with equal efficacy in place of thyroxine withdrawal [24], with the advantage of not producing transitory hypothyroidism. It is especially recommended for patients who are unable to tolerate hypothyroidism or who cannot achieve an adequate TSH level. Short-term recurrence rates are similar in patients treated with rhTSH or thyroxine withdrawal [25].
In addition, a low-iodine diet for 1 or 2 weeks is recommended for patients undergoing radioiodine remnant ablation. The rationale is that a high-iodine diet or iodine exposure (ie, amiodarone treatment or intravenous contrast) can decrease radioiodine uptake by papillary cancer cells due to further dilution of radioactive iodine in an expanded endogenous non-radioactive iodine pool. Patients with suspected high iodine levels can be screened using spot urinary levels [26].
Commonly, a diagnostic scan using low activities of iodine-131 is performed prior to radioablation to avoid the controversial “stunning effect” [27] from any exposure to sublethal radiation in a diagnostic dose. In stunning, the diagnostic RAI dose decreases uptake of a subsequent therapeutic dose. Alternatively, we use [I-123] radioiodine at very low dose (1.4 mCi) in pre-ablation patients. Uptake in the thyroid bed occurs in 75% to 100% of patients, commonly due to remnant normal thyroid tissue [28].
The typical activity used for RAI ablative therapy is 30–100 mCi. The administration of high activities (150–200 mCi) of [I-131] radioiodine has been used to treat recurrent or metastatic disease. This treatment can be very effective, especially in young patients [29].
Side Effects and Contraindications
Common side effects of radioiodine treatment include sialadenitis, radiation thyroiditis, tumor hemorrhage or edema, nausea, transient oligospermia or amenorrhea and nasolacrimal duct obstruction. Moreover, patients treated with radioiodine have a modest increased risk of developing other malignancies [30].
[I-131]Radioiodine must be avoided in pregnancy and in breastfeeding [31]. Indeed, breast tissue has a strong tendency to uptake iodine so breastfeeding should be stopped 5 to 8 weeks before radioiodine treatment, otherwise it can lead to a false-positive radioiodine scan in the chest [32], or worse, deliver radioiodine to the baby with detrimental effects and potential ablation to the baby’s thyroid gland.
Patients treated with radioiodine are advised to drink abundant water after the treatment in order to increase its renal elimination. If no stool elimination occurs in 14 to 24 hours, laxatives may be indicated to eliminate radioiodine from the gastrointestinal track. In addition, patients are advised to avoid sexual contact, avoid sharing bed, utensils, towels, toothbrushes, razors, and avoid public transportation and public places among other measures to avoid exposing the population to radiation [33]. The duration of this restriction depends on the dose administered.
Adjuvant Treatment in this Patient
As the patient was at high risk for recurrence, he received TSH suppression therapy to levels < 0.1 mIU/L. He was referred to nuclear medicine for I-131 treatment. However, at 3 months following thyroidectomy, thyroglobulin measurement showed an elevation (40.5 ng/mL). Ultrasound showed enlarged lymph nodes at level II at the right and at level II at the left. A FNA of left neck node was positive for papillary thyroid cancer.
• How should the patient be treated now?
Treatment of Locoregional Metastatic Disease
The best treatment for residual disease or local recurrences is surgery. ATA guidelines recommend compartmental lateral and/or central neck dissection for patients with persistent or recurrent disease confined to the neck [6]. Radioiodine can be an alternative when recurrent disease is not visible on imaging. Other treatments that can be used for local recurrences or isolated metastases when surgery is not possible are radiofrequency ablation [34], chemo-embolization [35], or ethanol ablation [36]. External beam radiotherapy, which is discussed later, could also be used in selected cases.
Further Treatment
The patient underwent a bilateral modified radical neck dissection followed by adjunctive radioiodine therapy. His initial radioiodine scan showed mild uptake in the neck at the site of his prior surgery. He received treatment with 215 mCi, then 6 months later he was treated with 250 mCi, as his scan showed continued mild uptake. Eleven months later his radioiodine scan showed no uptake and thyroglobulin levels remained stable at 14.4 ng/mL.
One year later, in a follow-up blood analysis he was found to have an elevated thyroglobulin level (90.4 ng/mL). A PET/CT scan showed multiple bone metastases. A neck ultrasound revealed enlarged lymph nodes in the right thyroid bed.
• How common is radioiodine-refractory thyroid cancer?
Radioiodine-refractory thyroid cancer in patients with progression of disease despite radioiodine therapy, or with non-radioiodine-avid lesions [37], is uncommon. It has a poor prognosis with a median survival of 3 to 6 years after diagnosis. It is more frequent in older patients. These lesions are often hypermetabolic and hence [F-18]FDG-avid [38], with a worse prognosis. In one study of patients with metastatic differentiated thyroid cancer, the 10-year overall survival rate was 56% in patients with radioiodine-avid lesions but only 10% in patients with non-radioiodine-avid lesions [38].
• Is the bone a common place for metastasis? Where else should we expect to find a lesion?
Metastatic Pattern
The most common sites for distant metastasis of papillary thyroid cancer are the lungs and the bone. The 10-year survival rate of papillary thyroid cancer patients with lung metastases is between 30% and 50% [38,39]; the prog-nosis is better in patients < 45 years and with radiodine uptake [40]; indeed, patients with pulmonary metastasis seen only in 131-I scans and not on CT or chest x-ray have a longer survival [41]. Pulmonary metastasis can be treated with radioiodine if they are radioiodine-avid. With this treatment complete remission is possible, although it is extremely difficult to achieve in macronodular metastasis.
Bones are the second most common place for distant metastases. Bone metastases seem to have a worse response to treatment with an unfavorable prognosis [42]. Pamidronate (a biphosphonate) and denosumab (a RANK ligand inhibitor) have been used to prevent skeletal related events, including pathologic fractures and cord compression, in bone metastases from other cancers such as breast and prostate, and may also be useful in thyroid cancer, although this has not yet been studied [43,44]. Moreover, surgical resection of isolated bone metastasis seems to improve survival [45].
Skin, liver, and brain metastasis, although uncommon, can also occur. There are also reported rare cases of metastasis in the breast, parotid, larynx, pharynx, adrenal glands, pituitary, kidney, liver, orbit, the sphenoid sinus, choroid plexus, pancreas, and skeletal muscles [46].
• Which treatments can we offer to a patient with metastatic disease refractory to radioiodine?
Chemotherapy and Treatment of Radioiodine-Resistant Disease
Therapeutic options for patients with metastatic papillary thyroid cancer resistant to radioiodine and TSH suppression are limited. Cytotoxic drugs do not play a major role in the treatment of refractory metastatic papillary thyroid cancer, and new research is mainly focused on tyrosine kinase inhibitors (TKIs) with a considerable number of clinical trials either completed or ongoing.
Tyrosine kinases are enzymes that transfer phosphate groups from adenosine triphosphate to proteins. In tumor cells their signaling paths promote proliferation, avoidance of apoptosis, invasion, angiogenesis, and metastasis. TKIs are small molecules that are able to inhibit tyrosine kinase function even at very low intracellullar concentrations. Some of them inhibit various tyrosine kinases and are known as multi-kinase inhibitors (MKIs).
Sorafenib
Sorafenib (400 mg twice daily) is an oral MKI that targets RAF, platelet-derived growth factor receptor, vascular endothelial growth factor receptors 2 and 3, RET and c-Kit [47]. It was approved in November 2013 for patients with radioiodine-refractory differentiated thyroid cancer [48]. Three phase II studies had previously evaluated sorafenib in papillary thyroid cancer, showing a partial response in 15% to 31% of patients and a progression-free survival up to 79 weeks [49–51]. Common adverse effects included weight loss, fatigue, rash, hypertension and the main dose-limiting toxicity—a hand-foot syndrome consisting of swelling, reddening, numbness, and desquamation on palms and soles [52].
Approval of the drug was based on the DECISION trial [52]. A total of 417 patients were randomized (207 to sorafenib and 210 to placebo), of which 57% had papillary thyroid cancer. The primary endpoint of progression-free survival (PFS) was significantly higher in the sorafenib arm, (median, 10.8 months) compared with placebo (median, 5.8 months) (hazard ratio [HR] 0.58, 95% confidence interval [CI] 0.45–0.75, P < 0.001). Median overall survival had not been reached in either arm [52]. The PFS of 5.8 months in the placebo arm confirmed that the group of patients in this study had a rapidly progressing disease, unlike the majority of patients with RAI-sensitive disease.
Selumetinib
Radioiodine re-sensitization was addressed in a study using selumitinib, an inhibitor of mitogen-activated protein kinase kinase (MAPK kinase or MEK). Preclinical models had shown that radioiodine-refractory tumors exposed to inhibitors of this enzyme were able to uptake radioiodine again. Twenty patients with radioiodine-refractory thyroid cancers were treated with selumetinib for 4 weeks and 12 showed increased radioiodine uptake following the treatment. Furthermore, 8 of these patients went on to show responses clinically to retreatment with radioiodine [53].Further studies with this agent will be needed to determine its place in treating patients with differentiated thyroid cancer.
External Beam Radiotherapy and Local Treatment for Metastases
The role of external beam radiotherapy in papillary thyroid cancer is mainly for symptom management. Local radiation can be used in patients with refractory metastatic disease or in lesions that do not uptake radioiodine. Examples include painful bone metastasis or brain metastasis that cannot be treated with surgery. In addition, radiofrequency ablation, chemo-embolization, or ethanol ablation can be used in certain patients.
Sequence of Treatments
In the setting of symptomatic metastatic, radioiodine-resistant disease, we prefer to use a TKI, normally sorafenib, as a first-line treatment. For second-line treatments, enrollment in a clinical trial is an option. Over 70% of patients with metastatic papillary thyroid cancer have mutations of the enzyme BRAF kinase. Vemurafenib is an inhibitor of this enzyme and appears to have some activity in patients with RAI-refractory thyroid cancer in early clinical trials [54–58]. Other TKIs such as sunitinib can also be used. Doxorubicin is only used in cases when a patient is not eligible for a trial and the off-label use of another TKI is contraindicated.
Further Treatment in this Patient
The patient received a trial of sorafenib. He showed disease stabilization that lasted 5 months. The treatment was stopped due to adverse effects (loss of weight and vomiting) and progression of the disease. He was then enrolled in a trial of vemurafenib. He stopped treatment because of adverse events related to the medication and currently has stable disease.
Summary
Papillary thyroid cancer is the most common endocrine malignancy. It is characterized by low mortality but high recurrence rate and can have a considerable impact on quality of life. Any anterior neck nodule, especially in a patient with a history of neck irradiation, should raise concern for this disease. Surgery remains the cornerstone of treatment. Adjuvant therapy includes lifelong TSH suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event. Thyroid cancer has a tendency to metastasize to the bones and lungs. Metastatic radioiodine-resistant disease is often treated with TKIs such as sorafenib. Enrollment in clinical trials is recommended as second-line therapy in radioiodine-resistant metastatic disease.
Corresponding author: Hari A. Deshpande, MD, Yale Cancer Center, FMP 124, 333 Cedar St., New Haven, CT 06520, [email protected]
Financial disclosures: Dr. Deshpande reports that he is on the advisory board of Bayer/Onyx.
Author contributions: conception and design, PT, EHH, GGC, HAD; drafting of article, PT, EHH, GGC, HAD; critical revision of the article, EHH, GGC, HAD.
REFERENCES
1. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2010.
2. Katoh R, Sasaki J, Kurihara H, et al. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma: a clinicopathologic study of 105 consecutive patients. Cancer 1992;70:1585–90.
3. Morris LG, Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 2010;200:454–61.
4. Fiore E, Rago T, Latrofa F, et al. Hashimoto’s thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with Lthyroxine. Endocr Relat Cancer 2011;18:429–37.
5. Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 2008;93:809–14.
6. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214.
7. National Comprehensive Cancer Network guidelines. Available at www.nccn.org/professionals/physician_gls/pdf/thyroid. pdf.
8. Stephenson BM, Wheeler MH, Clark OH. The role of total thyroidectomy in the management of differentiated thyroid cancer. Curr Opin Gen Surg 1994 53–9.
9. Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 2007;246:375–81.
10. Hay ID, Grant CS, Bergstralh EJ, et al. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery 1998;124:958–64.
11. McLeod DS, Sawka AM, Cooper DS. Controversies in primarytreatment of low-risk papillary thyroid cancer. Lancet 2013;381:1046–57.
12. Brabant G. 2008 Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J Clin Endocrinol Metab 2008;93:1167–9.
13. Kim HK, Yoon JH, Kim SJ, Cho JS. Higher TSH level is a risk factor for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2013;78:472–7.
14. McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med 2002;34:554–64.
15. Zafón C. TSH-suppressive treatment in differentiated thyroid cancer. A dogma under review. Endocrin Nutr 2012;59:125–30.
16. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998;8:737-44.
17. Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006;16:1229–42.
18. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 1994;33:1249–52.
19. Kung AW, Yeung SS. Prevention of bone loss induced by thyroxine suppressive therapy in postmenopausal women: the effect of calcium and calcitonin. J Clin Endocrinol Metab 1996;81:1232–36.
20. Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab 1992;75:714–20.
21. Sugitani I, Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J 1999;46:209–16.
22. Kim S, Wei JP, Braveman JM, Brams DM. Predictingoutcome and directing therapy for papillary thyroid carcinoma. Arch Surg 2004;139:390–4.
23. Leboeuf R, Perron P, Carpentier AC, et al. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clin Endocrinol (Oxf ) 2007;67:839–44.
24. Pacini F, Ladenson PW, Schlumberger M, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab 2006;91:926–32.
25. Tuttle RM, Brokhin M, Omry G, et al. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal. J Nucl Med 2008;49:764–70.
26. Pluijmen MJ, Eustatia-Rutten C, Goslings BM, et al. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf ) 2003;58:428–35.
27. Park HM. Stunned thyroid after high-dose I-131 imaging. Clin Nucl Med 1992; 17:501–2.
28. Salvatori M, Raffaelli M, Castaldi P, et al. Evaluation of the surgical completeness after total thyroidectomy for differentiated thyroid carcinoma. Eur J Surg Oncol 2007;33:648–54.
29. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–9.
30. Lang BH, Wong IO, Wong KP, et al. Risk of second primary malignancy in differentiated thyroid carcinoma treated with radioactive iodine therapy. Surgery 2012;151:844–50.
31. Rubow S, Klopper J. Excretion of radioiodine in human milk following a therapeutic dose of I-131. Eur J Nucl Med 1988;14:632–3.
32. Bakheet SM, Hammami MM. Patterns of radioiodine uptake by the lactating breast. Eur J Nucl Med 1994;21:604–8.
33. American Thyroid Association Taskforce on Radioiodine Safety, Sisson JC, Freitas J, et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: practice recommendations of the American Thyroid Association. Thyroid 2011;21:335–46.
34. Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from welldifferentiated thyroid malignancy. Surgery 2001;130:971–7.
35. Eustatia-Rutten CF, Romijn JA, Guijt MJ, et al. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J Clin Endocrinol Metab 2003;88:3184–9.
36. Lewis BD, Hay ID, Charboneau JW, et al. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. Am J Roentgenol 2002;178:699–704.
37. Xing MM, Haugen B, Schlumberger M. Progress in molecular based management of differentiated thyroid cancer. Lancet 2013;381:1058–69.
38. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–99.
39. Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome Clin Endoc 2005;63:87–93.
40. Ronga G, Filesi M, Montesano T, et al. Lung metastases from differentiated thyroid carcinoma. A 40 years’ experience. Q J Nucl Med Mol Imaging 2004;48:12–19.
41. Samaan NA, Schultz PN, Haynie TP, Ordonez NG. Pulmonary metastasis of differentiated thyroid carcinoma: treatment results in 101 patients. J Clin Endocrinol Metab 1985;60:376–80.
42. Lang BH, Wong KP, Cheung CY, et al. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 2013;20:1329–35.
43. Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med 1996;335:1785–92.
44. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebocontrolled trial. Lancet 379:39–46.
45. Zettinig G, Fueger BJ, Passler C, et al. Long-term follow-up of patients with bone metastases from differentiated thyroid carcinoma—surgery or conventional therapy? Clin Endocrinol (Oxf ) 2002;56:377–82.
46. Song H-J, Xue Y-L, Xu Y-H, et al. Rare metastases of differentiated thyroid carcinoma: pictorial review Endocr Relat Cancer 2011;18:R165–R174.
47. Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–109.
48. www.fda.gov/NewsEvents/Newsroom/PresAnnouncements/ucm376443.htm.
49. Kloos RT, Ringel MD, Knopp MV et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 2009;27:1675–84.
50. Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 2008;26:4714–9.
51. Schneider TC, Abdulrahman RM, Corssmit EP, et al. Longterm analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol 2012;167:643–50.
52. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine refractory differentiated thyroid cancer: The phase III DECISION trial. J Clin Oncol 2013;31(Suppl, abstr 4).
53. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 2013;368:623–32.
54. Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res 2009;15:485–91.
55. Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364–8.
56. Elisei R, Ugolini C, Viola D, et al. BRAF (V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 2008;93:3943–9.
57. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAFV600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2103;309:1493–501.
58. Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring
From the Yale School of Medicine, New Haven, CT.
ABSTRACT
• Objective: To review management of papillary thyroid cancer.
• Methods: Review of the literature.
• Results: Papillary thyroid cancer is the most common endocrine malignancy. The standard treatment for papillary thyroid cancer is thyroidectomy. Adjuvant therapy includes lifelong thyroid-stimulating hormone suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event.
• Conclusion: The incidence of papillary thyroid cancer is rapidly increasing. Surgery remains the cornerstone of treatment.
Papillary thyroid cancer is the most common endocrine malignancy and accounts for the majority of cancers of the thyroid. The incidence of papillary thyroid cancer is rapidly increasing [1]. Although increasing detection has been proposed as a possible factor [2], some studies reject this hypothesis, reporting increase in the incidence of larger tumors [3]. Papillary thyroid cancer is characterized by a low mortality but a high recurrence rate [1], posing challenges not only to the endocrinologist and oncologist but also to the general practitioner.
The most frequent presentation of papillary thyroid cancer is a palpable thyroid nodule, cervical lymphadenopathy, or incidental detection on imaging. Locally advanced disease can present with hoarseness or voice alteration. Common risks factors include history of radiation exposure during childhood (the most important risk factor), thyroid cancer in a first-degree relative, family history of a thyroid cancer syndrome (such as Werner syndrome, Cowden syndrome, Carney complex, or familial polyposis), and female sex (2.5:1). Thyroid nodules in the context of an autoimmune thyroiditis may have a higher risk of malignancy [4].
CASE STUDY
Initial Presentation
A 49-year-old man with no significant past medical history presents with a painless mass in the anterior part of his neck.
History, Physical Examination, and Initial Investigations
He has no other symptoms, no weight changes, no history of radiation exposure to the neck, and no family history of malignancy. Physical exam shows a mass in the left thyroid lobe. There is no evidence of cardiac arrhythmias, tremors, or ophthalmologic abnormalities. Thyroid-stimulating hormone (TSH) level is 2.8 mIU/L (normal range, 0.4–4.5 mIU/L) and free thyroxine (T4) level is 1.1 ng/dL (normal range, 0.8–1.5 ng/dL). An ultrasound scan of the neck shows enlargement of the left lobe of thyroid gland, containing multiple complex lesions, the largest measuring 2 x 3 cm, with calcification as well as 3 enlarged lymph nodes in the left level IV. Fine-needle aspiration of the thyroid mass is positive for papillary carcinoma.
• What is the approach to the initial evaluation of a thyroid nodule?
Initial diagnostic evaluation includes history, physical examination, and TSH measurement; nonfunctioning nodules, associated with normal or high values of TSH, carry a higher risk of malignancy [5]. Cervical ultrasound should be performed in all patients with nodules. Fine-needle aspiration (FNA) should be used to evaluate nonfunctioning nodules > 1 cm or subcentimeter nodules with suspicious ultrasound features or if the patient has major risk factors (history of ionizing radiation exposure, external beam radiation exposure, family or personal history of papillary thyroid cancer, or FDG-PET [fluorinated glucose positron emission tomography]–positive thyroid nodules). Scintigraphy can be used to evaluate the need for ultrasound and FNA in patients with low TSH values [6,7]; hyperfunctioning nodules are at low risk for malignancy and do not require biopsy.
• What is initial treatment of papillary thyroid cancer?
Surgery is the primary treatment for papillary thyroid cancer. Unlike for many cancers, surgical removal of the primary tumor is indicated even in the presence of metastatic disease [8]. Total or near-total thyroidectomy is used to treat patients with tumors > 1 cm or with tumors < 1 cm and associated risk factors (eg, contralateral nodules, affected lymph nodes, metastasis, history of radiation, first-degree family history of papillary thyroid cancer, or age > 45 years) [6]. There is a lower risk of recurrence in patients treated with total thyroidectomy versus lobectomy in papillary thyroid cancer [9,10]. Thyroid lobectomy may be used in small (< 1 cm) unifocal tumors without the presence of the associated risk factors listed above.
Patients with central or lateral neck lymph node involvement should also undergo central-compartment (level VI) neck dissection. Therapeutic lateral neck compartmental lymph node dissection is recommended in patients with biopsy-proven metastatic lateral cervical adenopathy [6,7]. The role of unilateral or bilateral prophylactic central-compartment nodal dissection (PCND), that is, lymph node dissection in the level VI compartment of neck in patients without evidence of lymphadenopathy, is controversial. The data for the possible benefit of PCND are inconclusive [11] although the ATA recommends the procedure for locally invasive T3 and T4 tumors [6].
The American Thyroid Association (ATA) and National Comprehensive Cancer Network (NCCN) guidelines [6,7] recommend a preoperative cervical ultrasound in patients with biopsy-proven papillary thyroid cancer to evaluate the presence of disease in the cervical lymph nodes, especially in the lateral and central compartments, and in the contralateral thyroid lobe. If suspicious lymph nodes are found, FNA confirmation is necessary only if this would change management. Systematic use of other preoperative imaging studies, such as CT or MRI, is not recommended [6,7].
Surgical Treatment
The patient underwent a total thyroidectomy with bilateral central neck dissection and selective supraclavicular left-sided lateral neck dissection. Lymph nodes on both sides of the neck (paratracheal nodes) as well as the left supraclavicular nodes were removed. Pathology showed multifocal papillary cancer with extracapsular extension to the paratracheal soft tissue, 14/14 lymph nodes affected, stage IVA T4N1bM0.
• How is papillary thyroid cancer staged?
• How should this patient be treated after surgery? Is any adjuvant therapy indicated?
TSH Suppression
In an effort to reduce risk of recurrence, patients should receive lifelong suppression of TSH using supraphysiologic doses of levothyroxine after total thyroidectomy. This is based upon the hypothesis that TSH is a growth factor for thyroid cancer cells [12,13]. Although a meta-analysis [14] supports the efficacy of TSH suppression therapy, some authors have questioned its widespread use, especially in light of the adverse effects of its use over the long term [15]. Many support its use only in high-risk patients [16], arguing that there is no evidence of benefit for low-risk patients [17]. This view is reflected in the ATA guidelines, which recommend TSH suppression below 0.1 mU/L for high-risk and intermediate-risk patients, while normal or slightly below normal TSH levels are recommended for low-risk patients [6].
Adverse effects of TSH suppression therapy are derived from the induced mild thyrotoxicosis, including cardiovascular and skeletal manifestations. Notably, elderly patients have a higher risk of cardiovascular side effects [18] such as atrial fibrillation, diastolic dysfunction, tachyarrhythmias, increased heart rate or increased left ventricular mass. Likewise, postmenopausal women are most susceptible for skeletal effects such as decreased mineral bone density and fractures [19].
Radioiodine Ablative Therapy
Radioactive iodine (RAI or radioiodine) therapy is based on the capacity of thyroid tissue to take up and retain iodine, specifically, radioiodine. This capacity is present but reduced in papillary and follicular cancer cells.
Radioiodine remnant ablation is performed after surgery, acting as adjuvant therapy by destroying remnant pathological or normal thyroid tissue. The destruction of normal thyroid tissue is useful as it increases the reliability of thyroglobulin testing and radioiodine scanning in the detection of recurrent or metastatic disease. Moreover, remnant ablation has been shown to prevent new thyroid neoplasias in high-risk patients (ie, those with history of radiation exposure). Radioiodine ablative therapy has been shown to reduce recurrence and cause-specific mortality [20] in certain subgroups; however, patients with low mortality risk do not seem to benefit from this therapy [21,22]. Its use is recommended in patients with distant metastases, tumors > 4 cm, or with extrathyroidal extension. It is also recommended for selected patients with tumors 1–4 cm who have high-risk features (such as lymph node involvement, history of radiation, or others previously mentioned) when there is an intermediate to high risk of recurrence or death from thyroid cancer [6]. Lymph node involvement can occur in up to 50% of cases [39] and normally responds to radioiodine therapy.
Since TSH increases radioiodine uptake by normal or pathological thyroid cells, TSH stimulation is required for radioiodine therapy. This can be done by endogenous TSH elevation or by recombinant human TSH (rhTSH). The former can be achieved by either stopping thyroxine 2 to 3 weeks prior to the remnant ablation, or by withdrawing thyroxine and switching to liothyronine for 2 to 3 weeks followed by a discontinuation of liothyronine for 2 weeks. Both approaches seem to produce the same incidence of hypothyroid symptoms [23]. Thyroxine therapy can be resumed 2 to 3 days after radioiodine ablative therapy. Recombinant human TSH can be used with equal efficacy in place of thyroxine withdrawal [24], with the advantage of not producing transitory hypothyroidism. It is especially recommended for patients who are unable to tolerate hypothyroidism or who cannot achieve an adequate TSH level. Short-term recurrence rates are similar in patients treated with rhTSH or thyroxine withdrawal [25].
In addition, a low-iodine diet for 1 or 2 weeks is recommended for patients undergoing radioiodine remnant ablation. The rationale is that a high-iodine diet or iodine exposure (ie, amiodarone treatment or intravenous contrast) can decrease radioiodine uptake by papillary cancer cells due to further dilution of radioactive iodine in an expanded endogenous non-radioactive iodine pool. Patients with suspected high iodine levels can be screened using spot urinary levels [26].
Commonly, a diagnostic scan using low activities of iodine-131 is performed prior to radioablation to avoid the controversial “stunning effect” [27] from any exposure to sublethal radiation in a diagnostic dose. In stunning, the diagnostic RAI dose decreases uptake of a subsequent therapeutic dose. Alternatively, we use [I-123] radioiodine at very low dose (1.4 mCi) in pre-ablation patients. Uptake in the thyroid bed occurs in 75% to 100% of patients, commonly due to remnant normal thyroid tissue [28].
The typical activity used for RAI ablative therapy is 30–100 mCi. The administration of high activities (150–200 mCi) of [I-131] radioiodine has been used to treat recurrent or metastatic disease. This treatment can be very effective, especially in young patients [29].
Side Effects and Contraindications
Common side effects of radioiodine treatment include sialadenitis, radiation thyroiditis, tumor hemorrhage or edema, nausea, transient oligospermia or amenorrhea and nasolacrimal duct obstruction. Moreover, patients treated with radioiodine have a modest increased risk of developing other malignancies [30].
[I-131]Radioiodine must be avoided in pregnancy and in breastfeeding [31]. Indeed, breast tissue has a strong tendency to uptake iodine so breastfeeding should be stopped 5 to 8 weeks before radioiodine treatment, otherwise it can lead to a false-positive radioiodine scan in the chest [32], or worse, deliver radioiodine to the baby with detrimental effects and potential ablation to the baby’s thyroid gland.
Patients treated with radioiodine are advised to drink abundant water after the treatment in order to increase its renal elimination. If no stool elimination occurs in 14 to 24 hours, laxatives may be indicated to eliminate radioiodine from the gastrointestinal track. In addition, patients are advised to avoid sexual contact, avoid sharing bed, utensils, towels, toothbrushes, razors, and avoid public transportation and public places among other measures to avoid exposing the population to radiation [33]. The duration of this restriction depends on the dose administered.
Adjuvant Treatment in this Patient
As the patient was at high risk for recurrence, he received TSH suppression therapy to levels < 0.1 mIU/L. He was referred to nuclear medicine for I-131 treatment. However, at 3 months following thyroidectomy, thyroglobulin measurement showed an elevation (40.5 ng/mL). Ultrasound showed enlarged lymph nodes at level II at the right and at level II at the left. A FNA of left neck node was positive for papillary thyroid cancer.
• How should the patient be treated now?
Treatment of Locoregional Metastatic Disease
The best treatment for residual disease or local recurrences is surgery. ATA guidelines recommend compartmental lateral and/or central neck dissection for patients with persistent or recurrent disease confined to the neck [6]. Radioiodine can be an alternative when recurrent disease is not visible on imaging. Other treatments that can be used for local recurrences or isolated metastases when surgery is not possible are radiofrequency ablation [34], chemo-embolization [35], or ethanol ablation [36]. External beam radiotherapy, which is discussed later, could also be used in selected cases.
Further Treatment
The patient underwent a bilateral modified radical neck dissection followed by adjunctive radioiodine therapy. His initial radioiodine scan showed mild uptake in the neck at the site of his prior surgery. He received treatment with 215 mCi, then 6 months later he was treated with 250 mCi, as his scan showed continued mild uptake. Eleven months later his radioiodine scan showed no uptake and thyroglobulin levels remained stable at 14.4 ng/mL.
One year later, in a follow-up blood analysis he was found to have an elevated thyroglobulin level (90.4 ng/mL). A PET/CT scan showed multiple bone metastases. A neck ultrasound revealed enlarged lymph nodes in the right thyroid bed.
• How common is radioiodine-refractory thyroid cancer?
Radioiodine-refractory thyroid cancer in patients with progression of disease despite radioiodine therapy, or with non-radioiodine-avid lesions [37], is uncommon. It has a poor prognosis with a median survival of 3 to 6 years after diagnosis. It is more frequent in older patients. These lesions are often hypermetabolic and hence [F-18]FDG-avid [38], with a worse prognosis. In one study of patients with metastatic differentiated thyroid cancer, the 10-year overall survival rate was 56% in patients with radioiodine-avid lesions but only 10% in patients with non-radioiodine-avid lesions [38].
• Is the bone a common place for metastasis? Where else should we expect to find a lesion?
Metastatic Pattern
The most common sites for distant metastasis of papillary thyroid cancer are the lungs and the bone. The 10-year survival rate of papillary thyroid cancer patients with lung metastases is between 30% and 50% [38,39]; the prog-nosis is better in patients < 45 years and with radiodine uptake [40]; indeed, patients with pulmonary metastasis seen only in 131-I scans and not on CT or chest x-ray have a longer survival [41]. Pulmonary metastasis can be treated with radioiodine if they are radioiodine-avid. With this treatment complete remission is possible, although it is extremely difficult to achieve in macronodular metastasis.
Bones are the second most common place for distant metastases. Bone metastases seem to have a worse response to treatment with an unfavorable prognosis [42]. Pamidronate (a biphosphonate) and denosumab (a RANK ligand inhibitor) have been used to prevent skeletal related events, including pathologic fractures and cord compression, in bone metastases from other cancers such as breast and prostate, and may also be useful in thyroid cancer, although this has not yet been studied [43,44]. Moreover, surgical resection of isolated bone metastasis seems to improve survival [45].
Skin, liver, and brain metastasis, although uncommon, can also occur. There are also reported rare cases of metastasis in the breast, parotid, larynx, pharynx, adrenal glands, pituitary, kidney, liver, orbit, the sphenoid sinus, choroid plexus, pancreas, and skeletal muscles [46].
• Which treatments can we offer to a patient with metastatic disease refractory to radioiodine?
Chemotherapy and Treatment of Radioiodine-Resistant Disease
Therapeutic options for patients with metastatic papillary thyroid cancer resistant to radioiodine and TSH suppression are limited. Cytotoxic drugs do not play a major role in the treatment of refractory metastatic papillary thyroid cancer, and new research is mainly focused on tyrosine kinase inhibitors (TKIs) with a considerable number of clinical trials either completed or ongoing.
Tyrosine kinases are enzymes that transfer phosphate groups from adenosine triphosphate to proteins. In tumor cells their signaling paths promote proliferation, avoidance of apoptosis, invasion, angiogenesis, and metastasis. TKIs are small molecules that are able to inhibit tyrosine kinase function even at very low intracellullar concentrations. Some of them inhibit various tyrosine kinases and are known as multi-kinase inhibitors (MKIs).
Sorafenib
Sorafenib (400 mg twice daily) is an oral MKI that targets RAF, platelet-derived growth factor receptor, vascular endothelial growth factor receptors 2 and 3, RET and c-Kit [47]. It was approved in November 2013 for patients with radioiodine-refractory differentiated thyroid cancer [48]. Three phase II studies had previously evaluated sorafenib in papillary thyroid cancer, showing a partial response in 15% to 31% of patients and a progression-free survival up to 79 weeks [49–51]. Common adverse effects included weight loss, fatigue, rash, hypertension and the main dose-limiting toxicity—a hand-foot syndrome consisting of swelling, reddening, numbness, and desquamation on palms and soles [52].
Approval of the drug was based on the DECISION trial [52]. A total of 417 patients were randomized (207 to sorafenib and 210 to placebo), of which 57% had papillary thyroid cancer. The primary endpoint of progression-free survival (PFS) was significantly higher in the sorafenib arm, (median, 10.8 months) compared with placebo (median, 5.8 months) (hazard ratio [HR] 0.58, 95% confidence interval [CI] 0.45–0.75, P < 0.001). Median overall survival had not been reached in either arm [52]. The PFS of 5.8 months in the placebo arm confirmed that the group of patients in this study had a rapidly progressing disease, unlike the majority of patients with RAI-sensitive disease.
Selumetinib
Radioiodine re-sensitization was addressed in a study using selumitinib, an inhibitor of mitogen-activated protein kinase kinase (MAPK kinase or MEK). Preclinical models had shown that radioiodine-refractory tumors exposed to inhibitors of this enzyme were able to uptake radioiodine again. Twenty patients with radioiodine-refractory thyroid cancers were treated with selumetinib for 4 weeks and 12 showed increased radioiodine uptake following the treatment. Furthermore, 8 of these patients went on to show responses clinically to retreatment with radioiodine [53].Further studies with this agent will be needed to determine its place in treating patients with differentiated thyroid cancer.
External Beam Radiotherapy and Local Treatment for Metastases
The role of external beam radiotherapy in papillary thyroid cancer is mainly for symptom management. Local radiation can be used in patients with refractory metastatic disease or in lesions that do not uptake radioiodine. Examples include painful bone metastasis or brain metastasis that cannot be treated with surgery. In addition, radiofrequency ablation, chemo-embolization, or ethanol ablation can be used in certain patients.
Sequence of Treatments
In the setting of symptomatic metastatic, radioiodine-resistant disease, we prefer to use a TKI, normally sorafenib, as a first-line treatment. For second-line treatments, enrollment in a clinical trial is an option. Over 70% of patients with metastatic papillary thyroid cancer have mutations of the enzyme BRAF kinase. Vemurafenib is an inhibitor of this enzyme and appears to have some activity in patients with RAI-refractory thyroid cancer in early clinical trials [54–58]. Other TKIs such as sunitinib can also be used. Doxorubicin is only used in cases when a patient is not eligible for a trial and the off-label use of another TKI is contraindicated.
Further Treatment in this Patient
The patient received a trial of sorafenib. He showed disease stabilization that lasted 5 months. The treatment was stopped due to adverse effects (loss of weight and vomiting) and progression of the disease. He was then enrolled in a trial of vemurafenib. He stopped treatment because of adverse events related to the medication and currently has stable disease.
Summary
Papillary thyroid cancer is the most common endocrine malignancy. It is characterized by low mortality but high recurrence rate and can have a considerable impact on quality of life. Any anterior neck nodule, especially in a patient with a history of neck irradiation, should raise concern for this disease. Surgery remains the cornerstone of treatment. Adjuvant therapy includes lifelong TSH suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event. Thyroid cancer has a tendency to metastasize to the bones and lungs. Metastatic radioiodine-resistant disease is often treated with TKIs such as sorafenib. Enrollment in clinical trials is recommended as second-line therapy in radioiodine-resistant metastatic disease.
Corresponding author: Hari A. Deshpande, MD, Yale Cancer Center, FMP 124, 333 Cedar St., New Haven, CT 06520, [email protected]
Financial disclosures: Dr. Deshpande reports that he is on the advisory board of Bayer/Onyx.
Author contributions: conception and design, PT, EHH, GGC, HAD; drafting of article, PT, EHH, GGC, HAD; critical revision of the article, EHH, GGC, HAD.
REFERENCES
1. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2010.
2. Katoh R, Sasaki J, Kurihara H, et al. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma: a clinicopathologic study of 105 consecutive patients. Cancer 1992;70:1585–90.
3. Morris LG, Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 2010;200:454–61.
4. Fiore E, Rago T, Latrofa F, et al. Hashimoto’s thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with Lthyroxine. Endocr Relat Cancer 2011;18:429–37.
5. Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 2008;93:809–14.
6. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214.
7. National Comprehensive Cancer Network guidelines. Available at www.nccn.org/professionals/physician_gls/pdf/thyroid. pdf.
8. Stephenson BM, Wheeler MH, Clark OH. The role of total thyroidectomy in the management of differentiated thyroid cancer. Curr Opin Gen Surg 1994 53–9.
9. Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 2007;246:375–81.
10. Hay ID, Grant CS, Bergstralh EJ, et al. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery 1998;124:958–64.
11. McLeod DS, Sawka AM, Cooper DS. Controversies in primarytreatment of low-risk papillary thyroid cancer. Lancet 2013;381:1046–57.
12. Brabant G. 2008 Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J Clin Endocrinol Metab 2008;93:1167–9.
13. Kim HK, Yoon JH, Kim SJ, Cho JS. Higher TSH level is a risk factor for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2013;78:472–7.
14. McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med 2002;34:554–64.
15. Zafón C. TSH-suppressive treatment in differentiated thyroid cancer. A dogma under review. Endocrin Nutr 2012;59:125–30.
16. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998;8:737-44.
17. Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006;16:1229–42.
18. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 1994;33:1249–52.
19. Kung AW, Yeung SS. Prevention of bone loss induced by thyroxine suppressive therapy in postmenopausal women: the effect of calcium and calcitonin. J Clin Endocrinol Metab 1996;81:1232–36.
20. Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab 1992;75:714–20.
21. Sugitani I, Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J 1999;46:209–16.
22. Kim S, Wei JP, Braveman JM, Brams DM. Predictingoutcome and directing therapy for papillary thyroid carcinoma. Arch Surg 2004;139:390–4.
23. Leboeuf R, Perron P, Carpentier AC, et al. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clin Endocrinol (Oxf ) 2007;67:839–44.
24. Pacini F, Ladenson PW, Schlumberger M, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab 2006;91:926–32.
25. Tuttle RM, Brokhin M, Omry G, et al. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal. J Nucl Med 2008;49:764–70.
26. Pluijmen MJ, Eustatia-Rutten C, Goslings BM, et al. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf ) 2003;58:428–35.
27. Park HM. Stunned thyroid after high-dose I-131 imaging. Clin Nucl Med 1992; 17:501–2.
28. Salvatori M, Raffaelli M, Castaldi P, et al. Evaluation of the surgical completeness after total thyroidectomy for differentiated thyroid carcinoma. Eur J Surg Oncol 2007;33:648–54.
29. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–9.
30. Lang BH, Wong IO, Wong KP, et al. Risk of second primary malignancy in differentiated thyroid carcinoma treated with radioactive iodine therapy. Surgery 2012;151:844–50.
31. Rubow S, Klopper J. Excretion of radioiodine in human milk following a therapeutic dose of I-131. Eur J Nucl Med 1988;14:632–3.
32. Bakheet SM, Hammami MM. Patterns of radioiodine uptake by the lactating breast. Eur J Nucl Med 1994;21:604–8.
33. American Thyroid Association Taskforce on Radioiodine Safety, Sisson JC, Freitas J, et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: practice recommendations of the American Thyroid Association. Thyroid 2011;21:335–46.
34. Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from welldifferentiated thyroid malignancy. Surgery 2001;130:971–7.
35. Eustatia-Rutten CF, Romijn JA, Guijt MJ, et al. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J Clin Endocrinol Metab 2003;88:3184–9.
36. Lewis BD, Hay ID, Charboneau JW, et al. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. Am J Roentgenol 2002;178:699–704.
37. Xing MM, Haugen B, Schlumberger M. Progress in molecular based management of differentiated thyroid cancer. Lancet 2013;381:1058–69.
38. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–99.
39. Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome Clin Endoc 2005;63:87–93.
40. Ronga G, Filesi M, Montesano T, et al. Lung metastases from differentiated thyroid carcinoma. A 40 years’ experience. Q J Nucl Med Mol Imaging 2004;48:12–19.
41. Samaan NA, Schultz PN, Haynie TP, Ordonez NG. Pulmonary metastasis of differentiated thyroid carcinoma: treatment results in 101 patients. J Clin Endocrinol Metab 1985;60:376–80.
42. Lang BH, Wong KP, Cheung CY, et al. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 2013;20:1329–35.
43. Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med 1996;335:1785–92.
44. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebocontrolled trial. Lancet 379:39–46.
45. Zettinig G, Fueger BJ, Passler C, et al. Long-term follow-up of patients with bone metastases from differentiated thyroid carcinoma—surgery or conventional therapy? Clin Endocrinol (Oxf ) 2002;56:377–82.
46. Song H-J, Xue Y-L, Xu Y-H, et al. Rare metastases of differentiated thyroid carcinoma: pictorial review Endocr Relat Cancer 2011;18:R165–R174.
47. Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–109.
48. www.fda.gov/NewsEvents/Newsroom/PresAnnouncements/ucm376443.htm.
49. Kloos RT, Ringel MD, Knopp MV et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 2009;27:1675–84.
50. Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 2008;26:4714–9.
51. Schneider TC, Abdulrahman RM, Corssmit EP, et al. Longterm analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol 2012;167:643–50.
52. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine refractory differentiated thyroid cancer: The phase III DECISION trial. J Clin Oncol 2013;31(Suppl, abstr 4).
53. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 2013;368:623–32.
54. Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res 2009;15:485–91.
55. Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364–8.
56. Elisei R, Ugolini C, Viola D, et al. BRAF (V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 2008;93:3943–9.
57. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAFV600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2103;309:1493–501.
58. Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring
From the Yale School of Medicine, New Haven, CT.
ABSTRACT
• Objective: To review management of papillary thyroid cancer.
• Methods: Review of the literature.
• Results: Papillary thyroid cancer is the most common endocrine malignancy. The standard treatment for papillary thyroid cancer is thyroidectomy. Adjuvant therapy includes lifelong thyroid-stimulating hormone suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event.
• Conclusion: The incidence of papillary thyroid cancer is rapidly increasing. Surgery remains the cornerstone of treatment.
Papillary thyroid cancer is the most common endocrine malignancy and accounts for the majority of cancers of the thyroid. The incidence of papillary thyroid cancer is rapidly increasing [1]. Although increasing detection has been proposed as a possible factor [2], some studies reject this hypothesis, reporting increase in the incidence of larger tumors [3]. Papillary thyroid cancer is characterized by a low mortality but a high recurrence rate [1], posing challenges not only to the endocrinologist and oncologist but also to the general practitioner.
The most frequent presentation of papillary thyroid cancer is a palpable thyroid nodule, cervical lymphadenopathy, or incidental detection on imaging. Locally advanced disease can present with hoarseness or voice alteration. Common risks factors include history of radiation exposure during childhood (the most important risk factor), thyroid cancer in a first-degree relative, family history of a thyroid cancer syndrome (such as Werner syndrome, Cowden syndrome, Carney complex, or familial polyposis), and female sex (2.5:1). Thyroid nodules in the context of an autoimmune thyroiditis may have a higher risk of malignancy [4].
CASE STUDY
Initial Presentation
A 49-year-old man with no significant past medical history presents with a painless mass in the anterior part of his neck.
History, Physical Examination, and Initial Investigations
He has no other symptoms, no weight changes, no history of radiation exposure to the neck, and no family history of malignancy. Physical exam shows a mass in the left thyroid lobe. There is no evidence of cardiac arrhythmias, tremors, or ophthalmologic abnormalities. Thyroid-stimulating hormone (TSH) level is 2.8 mIU/L (normal range, 0.4–4.5 mIU/L) and free thyroxine (T4) level is 1.1 ng/dL (normal range, 0.8–1.5 ng/dL). An ultrasound scan of the neck shows enlargement of the left lobe of thyroid gland, containing multiple complex lesions, the largest measuring 2 x 3 cm, with calcification as well as 3 enlarged lymph nodes in the left level IV. Fine-needle aspiration of the thyroid mass is positive for papillary carcinoma.
• What is the approach to the initial evaluation of a thyroid nodule?
Initial diagnostic evaluation includes history, physical examination, and TSH measurement; nonfunctioning nodules, associated with normal or high values of TSH, carry a higher risk of malignancy [5]. Cervical ultrasound should be performed in all patients with nodules. Fine-needle aspiration (FNA) should be used to evaluate nonfunctioning nodules > 1 cm or subcentimeter nodules with suspicious ultrasound features or if the patient has major risk factors (history of ionizing radiation exposure, external beam radiation exposure, family or personal history of papillary thyroid cancer, or FDG-PET [fluorinated glucose positron emission tomography]–positive thyroid nodules). Scintigraphy can be used to evaluate the need for ultrasound and FNA in patients with low TSH values [6,7]; hyperfunctioning nodules are at low risk for malignancy and do not require biopsy.
• What is initial treatment of papillary thyroid cancer?
Surgery is the primary treatment for papillary thyroid cancer. Unlike for many cancers, surgical removal of the primary tumor is indicated even in the presence of metastatic disease [8]. Total or near-total thyroidectomy is used to treat patients with tumors > 1 cm or with tumors < 1 cm and associated risk factors (eg, contralateral nodules, affected lymph nodes, metastasis, history of radiation, first-degree family history of papillary thyroid cancer, or age > 45 years) [6]. There is a lower risk of recurrence in patients treated with total thyroidectomy versus lobectomy in papillary thyroid cancer [9,10]. Thyroid lobectomy may be used in small (< 1 cm) unifocal tumors without the presence of the associated risk factors listed above.
Patients with central or lateral neck lymph node involvement should also undergo central-compartment (level VI) neck dissection. Therapeutic lateral neck compartmental lymph node dissection is recommended in patients with biopsy-proven metastatic lateral cervical adenopathy [6,7]. The role of unilateral or bilateral prophylactic central-compartment nodal dissection (PCND), that is, lymph node dissection in the level VI compartment of neck in patients without evidence of lymphadenopathy, is controversial. The data for the possible benefit of PCND are inconclusive [11] although the ATA recommends the procedure for locally invasive T3 and T4 tumors [6].
The American Thyroid Association (ATA) and National Comprehensive Cancer Network (NCCN) guidelines [6,7] recommend a preoperative cervical ultrasound in patients with biopsy-proven papillary thyroid cancer to evaluate the presence of disease in the cervical lymph nodes, especially in the lateral and central compartments, and in the contralateral thyroid lobe. If suspicious lymph nodes are found, FNA confirmation is necessary only if this would change management. Systematic use of other preoperative imaging studies, such as CT or MRI, is not recommended [6,7].
Surgical Treatment
The patient underwent a total thyroidectomy with bilateral central neck dissection and selective supraclavicular left-sided lateral neck dissection. Lymph nodes on both sides of the neck (paratracheal nodes) as well as the left supraclavicular nodes were removed. Pathology showed multifocal papillary cancer with extracapsular extension to the paratracheal soft tissue, 14/14 lymph nodes affected, stage IVA T4N1bM0.
• How is papillary thyroid cancer staged?
• How should this patient be treated after surgery? Is any adjuvant therapy indicated?
TSH Suppression
In an effort to reduce risk of recurrence, patients should receive lifelong suppression of TSH using supraphysiologic doses of levothyroxine after total thyroidectomy. This is based upon the hypothesis that TSH is a growth factor for thyroid cancer cells [12,13]. Although a meta-analysis [14] supports the efficacy of TSH suppression therapy, some authors have questioned its widespread use, especially in light of the adverse effects of its use over the long term [15]. Many support its use only in high-risk patients [16], arguing that there is no evidence of benefit for low-risk patients [17]. This view is reflected in the ATA guidelines, which recommend TSH suppression below 0.1 mU/L for high-risk and intermediate-risk patients, while normal or slightly below normal TSH levels are recommended for low-risk patients [6].
Adverse effects of TSH suppression therapy are derived from the induced mild thyrotoxicosis, including cardiovascular and skeletal manifestations. Notably, elderly patients have a higher risk of cardiovascular side effects [18] such as atrial fibrillation, diastolic dysfunction, tachyarrhythmias, increased heart rate or increased left ventricular mass. Likewise, postmenopausal women are most susceptible for skeletal effects such as decreased mineral bone density and fractures [19].
Radioiodine Ablative Therapy
Radioactive iodine (RAI or radioiodine) therapy is based on the capacity of thyroid tissue to take up and retain iodine, specifically, radioiodine. This capacity is present but reduced in papillary and follicular cancer cells.
Radioiodine remnant ablation is performed after surgery, acting as adjuvant therapy by destroying remnant pathological or normal thyroid tissue. The destruction of normal thyroid tissue is useful as it increases the reliability of thyroglobulin testing and radioiodine scanning in the detection of recurrent or metastatic disease. Moreover, remnant ablation has been shown to prevent new thyroid neoplasias in high-risk patients (ie, those with history of radiation exposure). Radioiodine ablative therapy has been shown to reduce recurrence and cause-specific mortality [20] in certain subgroups; however, patients with low mortality risk do not seem to benefit from this therapy [21,22]. Its use is recommended in patients with distant metastases, tumors > 4 cm, or with extrathyroidal extension. It is also recommended for selected patients with tumors 1–4 cm who have high-risk features (such as lymph node involvement, history of radiation, or others previously mentioned) when there is an intermediate to high risk of recurrence or death from thyroid cancer [6]. Lymph node involvement can occur in up to 50% of cases [39] and normally responds to radioiodine therapy.
Since TSH increases radioiodine uptake by normal or pathological thyroid cells, TSH stimulation is required for radioiodine therapy. This can be done by endogenous TSH elevation or by recombinant human TSH (rhTSH). The former can be achieved by either stopping thyroxine 2 to 3 weeks prior to the remnant ablation, or by withdrawing thyroxine and switching to liothyronine for 2 to 3 weeks followed by a discontinuation of liothyronine for 2 weeks. Both approaches seem to produce the same incidence of hypothyroid symptoms [23]. Thyroxine therapy can be resumed 2 to 3 days after radioiodine ablative therapy. Recombinant human TSH can be used with equal efficacy in place of thyroxine withdrawal [24], with the advantage of not producing transitory hypothyroidism. It is especially recommended for patients who are unable to tolerate hypothyroidism or who cannot achieve an adequate TSH level. Short-term recurrence rates are similar in patients treated with rhTSH or thyroxine withdrawal [25].
In addition, a low-iodine diet for 1 or 2 weeks is recommended for patients undergoing radioiodine remnant ablation. The rationale is that a high-iodine diet or iodine exposure (ie, amiodarone treatment or intravenous contrast) can decrease radioiodine uptake by papillary cancer cells due to further dilution of radioactive iodine in an expanded endogenous non-radioactive iodine pool. Patients with suspected high iodine levels can be screened using spot urinary levels [26].
Commonly, a diagnostic scan using low activities of iodine-131 is performed prior to radioablation to avoid the controversial “stunning effect” [27] from any exposure to sublethal radiation in a diagnostic dose. In stunning, the diagnostic RAI dose decreases uptake of a subsequent therapeutic dose. Alternatively, we use [I-123] radioiodine at very low dose (1.4 mCi) in pre-ablation patients. Uptake in the thyroid bed occurs in 75% to 100% of patients, commonly due to remnant normal thyroid tissue [28].
The typical activity used for RAI ablative therapy is 30–100 mCi. The administration of high activities (150–200 mCi) of [I-131] radioiodine has been used to treat recurrent or metastatic disease. This treatment can be very effective, especially in young patients [29].
Side Effects and Contraindications
Common side effects of radioiodine treatment include sialadenitis, radiation thyroiditis, tumor hemorrhage or edema, nausea, transient oligospermia or amenorrhea and nasolacrimal duct obstruction. Moreover, patients treated with radioiodine have a modest increased risk of developing other malignancies [30].
[I-131]Radioiodine must be avoided in pregnancy and in breastfeeding [31]. Indeed, breast tissue has a strong tendency to uptake iodine so breastfeeding should be stopped 5 to 8 weeks before radioiodine treatment, otherwise it can lead to a false-positive radioiodine scan in the chest [32], or worse, deliver radioiodine to the baby with detrimental effects and potential ablation to the baby’s thyroid gland.
Patients treated with radioiodine are advised to drink abundant water after the treatment in order to increase its renal elimination. If no stool elimination occurs in 14 to 24 hours, laxatives may be indicated to eliminate radioiodine from the gastrointestinal track. In addition, patients are advised to avoid sexual contact, avoid sharing bed, utensils, towels, toothbrushes, razors, and avoid public transportation and public places among other measures to avoid exposing the population to radiation [33]. The duration of this restriction depends on the dose administered.
Adjuvant Treatment in this Patient
As the patient was at high risk for recurrence, he received TSH suppression therapy to levels < 0.1 mIU/L. He was referred to nuclear medicine for I-131 treatment. However, at 3 months following thyroidectomy, thyroglobulin measurement showed an elevation (40.5 ng/mL). Ultrasound showed enlarged lymph nodes at level II at the right and at level II at the left. A FNA of left neck node was positive for papillary thyroid cancer.
• How should the patient be treated now?
Treatment of Locoregional Metastatic Disease
The best treatment for residual disease or local recurrences is surgery. ATA guidelines recommend compartmental lateral and/or central neck dissection for patients with persistent or recurrent disease confined to the neck [6]. Radioiodine can be an alternative when recurrent disease is not visible on imaging. Other treatments that can be used for local recurrences or isolated metastases when surgery is not possible are radiofrequency ablation [34], chemo-embolization [35], or ethanol ablation [36]. External beam radiotherapy, which is discussed later, could also be used in selected cases.
Further Treatment
The patient underwent a bilateral modified radical neck dissection followed by adjunctive radioiodine therapy. His initial radioiodine scan showed mild uptake in the neck at the site of his prior surgery. He received treatment with 215 mCi, then 6 months later he was treated with 250 mCi, as his scan showed continued mild uptake. Eleven months later his radioiodine scan showed no uptake and thyroglobulin levels remained stable at 14.4 ng/mL.
One year later, in a follow-up blood analysis he was found to have an elevated thyroglobulin level (90.4 ng/mL). A PET/CT scan showed multiple bone metastases. A neck ultrasound revealed enlarged lymph nodes in the right thyroid bed.
• How common is radioiodine-refractory thyroid cancer?
Radioiodine-refractory thyroid cancer in patients with progression of disease despite radioiodine therapy, or with non-radioiodine-avid lesions [37], is uncommon. It has a poor prognosis with a median survival of 3 to 6 years after diagnosis. It is more frequent in older patients. These lesions are often hypermetabolic and hence [F-18]FDG-avid [38], with a worse prognosis. In one study of patients with metastatic differentiated thyroid cancer, the 10-year overall survival rate was 56% in patients with radioiodine-avid lesions but only 10% in patients with non-radioiodine-avid lesions [38].
• Is the bone a common place for metastasis? Where else should we expect to find a lesion?
Metastatic Pattern
The most common sites for distant metastasis of papillary thyroid cancer are the lungs and the bone. The 10-year survival rate of papillary thyroid cancer patients with lung metastases is between 30% and 50% [38,39]; the prog-nosis is better in patients < 45 years and with radiodine uptake [40]; indeed, patients with pulmonary metastasis seen only in 131-I scans and not on CT or chest x-ray have a longer survival [41]. Pulmonary metastasis can be treated with radioiodine if they are radioiodine-avid. With this treatment complete remission is possible, although it is extremely difficult to achieve in macronodular metastasis.
Bones are the second most common place for distant metastases. Bone metastases seem to have a worse response to treatment with an unfavorable prognosis [42]. Pamidronate (a biphosphonate) and denosumab (a RANK ligand inhibitor) have been used to prevent skeletal related events, including pathologic fractures and cord compression, in bone metastases from other cancers such as breast and prostate, and may also be useful in thyroid cancer, although this has not yet been studied [43,44]. Moreover, surgical resection of isolated bone metastasis seems to improve survival [45].
Skin, liver, and brain metastasis, although uncommon, can also occur. There are also reported rare cases of metastasis in the breast, parotid, larynx, pharynx, adrenal glands, pituitary, kidney, liver, orbit, the sphenoid sinus, choroid plexus, pancreas, and skeletal muscles [46].
• Which treatments can we offer to a patient with metastatic disease refractory to radioiodine?
Chemotherapy and Treatment of Radioiodine-Resistant Disease
Therapeutic options for patients with metastatic papillary thyroid cancer resistant to radioiodine and TSH suppression are limited. Cytotoxic drugs do not play a major role in the treatment of refractory metastatic papillary thyroid cancer, and new research is mainly focused on tyrosine kinase inhibitors (TKIs) with a considerable number of clinical trials either completed or ongoing.
Tyrosine kinases are enzymes that transfer phosphate groups from adenosine triphosphate to proteins. In tumor cells their signaling paths promote proliferation, avoidance of apoptosis, invasion, angiogenesis, and metastasis. TKIs are small molecules that are able to inhibit tyrosine kinase function even at very low intracellullar concentrations. Some of them inhibit various tyrosine kinases and are known as multi-kinase inhibitors (MKIs).
Sorafenib
Sorafenib (400 mg twice daily) is an oral MKI that targets RAF, platelet-derived growth factor receptor, vascular endothelial growth factor receptors 2 and 3, RET and c-Kit [47]. It was approved in November 2013 for patients with radioiodine-refractory differentiated thyroid cancer [48]. Three phase II studies had previously evaluated sorafenib in papillary thyroid cancer, showing a partial response in 15% to 31% of patients and a progression-free survival up to 79 weeks [49–51]. Common adverse effects included weight loss, fatigue, rash, hypertension and the main dose-limiting toxicity—a hand-foot syndrome consisting of swelling, reddening, numbness, and desquamation on palms and soles [52].
Approval of the drug was based on the DECISION trial [52]. A total of 417 patients were randomized (207 to sorafenib and 210 to placebo), of which 57% had papillary thyroid cancer. The primary endpoint of progression-free survival (PFS) was significantly higher in the sorafenib arm, (median, 10.8 months) compared with placebo (median, 5.8 months) (hazard ratio [HR] 0.58, 95% confidence interval [CI] 0.45–0.75, P < 0.001). Median overall survival had not been reached in either arm [52]. The PFS of 5.8 months in the placebo arm confirmed that the group of patients in this study had a rapidly progressing disease, unlike the majority of patients with RAI-sensitive disease.
Selumetinib
Radioiodine re-sensitization was addressed in a study using selumitinib, an inhibitor of mitogen-activated protein kinase kinase (MAPK kinase or MEK). Preclinical models had shown that radioiodine-refractory tumors exposed to inhibitors of this enzyme were able to uptake radioiodine again. Twenty patients with radioiodine-refractory thyroid cancers were treated with selumetinib for 4 weeks and 12 showed increased radioiodine uptake following the treatment. Furthermore, 8 of these patients went on to show responses clinically to retreatment with radioiodine [53].Further studies with this agent will be needed to determine its place in treating patients with differentiated thyroid cancer.
External Beam Radiotherapy and Local Treatment for Metastases
The role of external beam radiotherapy in papillary thyroid cancer is mainly for symptom management. Local radiation can be used in patients with refractory metastatic disease or in lesions that do not uptake radioiodine. Examples include painful bone metastasis or brain metastasis that cannot be treated with surgery. In addition, radiofrequency ablation, chemo-embolization, or ethanol ablation can be used in certain patients.
Sequence of Treatments
In the setting of symptomatic metastatic, radioiodine-resistant disease, we prefer to use a TKI, normally sorafenib, as a first-line treatment. For second-line treatments, enrollment in a clinical trial is an option. Over 70% of patients with metastatic papillary thyroid cancer have mutations of the enzyme BRAF kinase. Vemurafenib is an inhibitor of this enzyme and appears to have some activity in patients with RAI-refractory thyroid cancer in early clinical trials [54–58]. Other TKIs such as sunitinib can also be used. Doxorubicin is only used in cases when a patient is not eligible for a trial and the off-label use of another TKI is contraindicated.
Further Treatment in this Patient
The patient received a trial of sorafenib. He showed disease stabilization that lasted 5 months. The treatment was stopped due to adverse effects (loss of weight and vomiting) and progression of the disease. He was then enrolled in a trial of vemurafenib. He stopped treatment because of adverse events related to the medication and currently has stable disease.
Summary
Papillary thyroid cancer is the most common endocrine malignancy. It is characterized by low mortality but high recurrence rate and can have a considerable impact on quality of life. Any anterior neck nodule, especially in a patient with a history of neck irradiation, should raise concern for this disease. Surgery remains the cornerstone of treatment. Adjuvant therapy includes lifelong TSH suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event. Thyroid cancer has a tendency to metastasize to the bones and lungs. Metastatic radioiodine-resistant disease is often treated with TKIs such as sorafenib. Enrollment in clinical trials is recommended as second-line therapy in radioiodine-resistant metastatic disease.
Corresponding author: Hari A. Deshpande, MD, Yale Cancer Center, FMP 124, 333 Cedar St., New Haven, CT 06520, [email protected]
Financial disclosures: Dr. Deshpande reports that he is on the advisory board of Bayer/Onyx.
Author contributions: conception and design, PT, EHH, GGC, HAD; drafting of article, PT, EHH, GGC, HAD; critical revision of the article, EHH, GGC, HAD.
REFERENCES
1. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2010.
2. Katoh R, Sasaki J, Kurihara H, et al. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma: a clinicopathologic study of 105 consecutive patients. Cancer 1992;70:1585–90.
3. Morris LG, Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 2010;200:454–61.
4. Fiore E, Rago T, Latrofa F, et al. Hashimoto’s thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with Lthyroxine. Endocr Relat Cancer 2011;18:429–37.
5. Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 2008;93:809–14.
6. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214.
7. National Comprehensive Cancer Network guidelines. Available at www.nccn.org/professionals/physician_gls/pdf/thyroid. pdf.
8. Stephenson BM, Wheeler MH, Clark OH. The role of total thyroidectomy in the management of differentiated thyroid cancer. Curr Opin Gen Surg 1994 53–9.
9. Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 2007;246:375–81.
10. Hay ID, Grant CS, Bergstralh EJ, et al. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery 1998;124:958–64.
11. McLeod DS, Sawka AM, Cooper DS. Controversies in primarytreatment of low-risk papillary thyroid cancer. Lancet 2013;381:1046–57.
12. Brabant G. 2008 Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J Clin Endocrinol Metab 2008;93:1167–9.
13. Kim HK, Yoon JH, Kim SJ, Cho JS. Higher TSH level is a risk factor for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2013;78:472–7.
14. McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med 2002;34:554–64.
15. Zafón C. TSH-suppressive treatment in differentiated thyroid cancer. A dogma under review. Endocrin Nutr 2012;59:125–30.
16. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998;8:737-44.
17. Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006;16:1229–42.
18. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 1994;33:1249–52.
19. Kung AW, Yeung SS. Prevention of bone loss induced by thyroxine suppressive therapy in postmenopausal women: the effect of calcium and calcitonin. J Clin Endocrinol Metab 1996;81:1232–36.
20. Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab 1992;75:714–20.
21. Sugitani I, Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J 1999;46:209–16.
22. Kim S, Wei JP, Braveman JM, Brams DM. Predictingoutcome and directing therapy for papillary thyroid carcinoma. Arch Surg 2004;139:390–4.
23. Leboeuf R, Perron P, Carpentier AC, et al. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clin Endocrinol (Oxf ) 2007;67:839–44.
24. Pacini F, Ladenson PW, Schlumberger M, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab 2006;91:926–32.
25. Tuttle RM, Brokhin M, Omry G, et al. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal. J Nucl Med 2008;49:764–70.
26. Pluijmen MJ, Eustatia-Rutten C, Goslings BM, et al. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf ) 2003;58:428–35.
27. Park HM. Stunned thyroid after high-dose I-131 imaging. Clin Nucl Med 1992; 17:501–2.
28. Salvatori M, Raffaelli M, Castaldi P, et al. Evaluation of the surgical completeness after total thyroidectomy for differentiated thyroid carcinoma. Eur J Surg Oncol 2007;33:648–54.
29. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–9.
30. Lang BH, Wong IO, Wong KP, et al. Risk of second primary malignancy in differentiated thyroid carcinoma treated with radioactive iodine therapy. Surgery 2012;151:844–50.
31. Rubow S, Klopper J. Excretion of radioiodine in human milk following a therapeutic dose of I-131. Eur J Nucl Med 1988;14:632–3.
32. Bakheet SM, Hammami MM. Patterns of radioiodine uptake by the lactating breast. Eur J Nucl Med 1994;21:604–8.
33. American Thyroid Association Taskforce on Radioiodine Safety, Sisson JC, Freitas J, et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: practice recommendations of the American Thyroid Association. Thyroid 2011;21:335–46.
34. Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from welldifferentiated thyroid malignancy. Surgery 2001;130:971–7.
35. Eustatia-Rutten CF, Romijn JA, Guijt MJ, et al. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J Clin Endocrinol Metab 2003;88:3184–9.
36. Lewis BD, Hay ID, Charboneau JW, et al. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. Am J Roentgenol 2002;178:699–704.
37. Xing MM, Haugen B, Schlumberger M. Progress in molecular based management of differentiated thyroid cancer. Lancet 2013;381:1058–69.
38. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–99.
39. Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome Clin Endoc 2005;63:87–93.
40. Ronga G, Filesi M, Montesano T, et al. Lung metastases from differentiated thyroid carcinoma. A 40 years’ experience. Q J Nucl Med Mol Imaging 2004;48:12–19.
41. Samaan NA, Schultz PN, Haynie TP, Ordonez NG. Pulmonary metastasis of differentiated thyroid carcinoma: treatment results in 101 patients. J Clin Endocrinol Metab 1985;60:376–80.
42. Lang BH, Wong KP, Cheung CY, et al. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 2013;20:1329–35.
43. Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med 1996;335:1785–92.
44. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebocontrolled trial. Lancet 379:39–46.
45. Zettinig G, Fueger BJ, Passler C, et al. Long-term follow-up of patients with bone metastases from differentiated thyroid carcinoma—surgery or conventional therapy? Clin Endocrinol (Oxf ) 2002;56:377–82.
46. Song H-J, Xue Y-L, Xu Y-H, et al. Rare metastases of differentiated thyroid carcinoma: pictorial review Endocr Relat Cancer 2011;18:R165–R174.
47. Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–109.
48. www.fda.gov/NewsEvents/Newsroom/PresAnnouncements/ucm376443.htm.
49. Kloos RT, Ringel MD, Knopp MV et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 2009;27:1675–84.
50. Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 2008;26:4714–9.
51. Schneider TC, Abdulrahman RM, Corssmit EP, et al. Longterm analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol 2012;167:643–50.
52. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine refractory differentiated thyroid cancer: The phase III DECISION trial. J Clin Oncol 2013;31(Suppl, abstr 4).
53. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 2013;368:623–32.
54. Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res 2009;15:485–91.
55. Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364–8.
56. Elisei R, Ugolini C, Viola D, et al. BRAF (V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 2008;93:3943–9.
57. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAFV600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2103;309:1493–501.
58. Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring