User login
Breast Cancer Tumor Board
Case Presentation
This case represents a composite of many different patients and is not meant to represent an individual. Any resemblance to an actual patient is coincidental.
A 32-year-old African American woman presented with a self-palpated left breast mass (axillary tail at 9 o’clock position). The patient was a nonsmoker, was otherwise healthy, and had no family history of breast or any other cancer. She had never used oral contraceptives or hormones, was never pregnant, her menarche was at age 12 years, and she had regular menstrual periods. On physical examination she had a 1-cm left breast mass and a palpable left axillary lymph node. A complete diagnostic workup revealed a 2-cm left breast mass. An ultrasound-guided biopsy of the axillary lymph node was positive for invasive ductal carcinoma (IDC). The final diagnosis was left breast cancer, stage IIB IDC, T1N1M0, ER+, PR+, HER2 2+ by immunohistochemistry, fluorescence in situ hybridization (FISH) was 2.4, confirming a HER2+ tumor.
Anita Aggarwal, DO, PhD. What is the role of genetic counseling and testing in this young patient who does not have a family history of breast cancer?
Vickie L. Venne, MS. This patient absolutely would be a candidate for counseling and testing. From a genetic counseling perspective, one of the first points has to do with what “no family history of cancer” means. Typically, in a fast-paced clinic, a patient will be asked “Does anybody else in your family have cancer?” And it’s not uncommon to get the answer “no.” Genetic counselors collect specific information on at least the first- and second-degree relatives, so we end up with 3 generations. This includes both the maternal and the paternal histories. We find that people who initially report no family history of cancer are often just thinking of breast cancer, even if the provider’s question is broad. When we start digging, we often find other cancers because cancer is common.
The other issue is that she was diagnosed at a young age. Clearly, 32 years is much younger than we typically see in breast cancer, and we know that individuals with hereditary cancers often have an earlier age of onset. With no other information, her a priori risk of having a BRCA1/2 mutation would be < 2.5%.
Regardless, based on current National Comprehensive Cancer Network (NCCN) guidelines, she would be a testing candidate. We would also recommend testing for more than just BRCA1/2. In the last few decades, there have been many genes identified that are associated with an increased susceptibility to cancer. Many of these genes are part of syndromes, so if you had a mutation, that also would increase the risk for a cancer in another organ. If this woman’s mother and father lived into their 70s or 80s and she had a number of aunts on both sides who never developed breast cancer, it would be less likely to be BRCA1/2. However, P53 also can present in young women and as a de novo mutation. Therefore, we would offer her a panel of actionable genes. Genes that if, in fact, we identified a mutation in one of them, would mean we could do something different for this young lady.
JoAnn Manning, MD. Let’s say she does have testing, and she comes back BRCA+. Then what would be the recommendations or guidance?
Ms. Venne. Women (and men as well!) with mutations have an increased risk for a second primary breast cancer as well as cancer in other organs. Focusing first on the breast story and all the media around BRCA1/2 mutations and surgery, this is a woman who may consider a more aggressive surgery, including prophylactic contralateral mastectomy, if she is concerned. She is young, so we also would explore her fertility plans. While her next few months will be filled with breast cancer treatment choices, women with BRCA mutations also are at an increased risk to develop ovarian cancer, so that might be a decision she makes as well. Her health care team may also eventually discuss chemotherapeutic options available specifically to women with mutations.
However, we often see young women who are extremely nervous because there is a sense that if you’re younger, your cancer must be inherited. Part of the pretest counseling is to explore psychosocial issues and help these young ladies understand that, especially if she does not have a family history of cancer and the only indication is her age, then it’s highly likely that we’re not going to find an identifiable mutation. And in that circumstance, she probably could consider a more conservative surgical decision.
Dr. Aggarwal. How common is a BRCA1 or BRCA2 mutation in African American females?
Ms. Venne. I have not paid attention to the prevalence of mutations based on ethnicity, so I don’t know. While many of the initial mutations were discovered in women of European ancestry, there are large cohorts of women with African ancestry whose specimens are now available for identifying genetic markers that will improve breast cancer risk assessment in them.1 However, because those mutations are still being characterized, it is more common to find a variant of uncertain significance (VUS) in African American women. A VUS is an alteration—a change in the gene—that we simply don’t know what it means yet. Clinicians don’t have enough information to know if that alteration is pathogenic or benign. The problem is that people try to make sense out of everything in their lives, so they also will try to make a VUS mean something. We try hard to help people understand that a VUS is really no more significant than if we had not tested in the first place, and they should not act on that information. They should use their family history, their age, their other psychosocial concerns about their experiences with cancer as they make their treatment decisions. But they also should check back periodically with their genetic counselor because VUSs can be reclassified. And if that happens, the information might be more useful for not only them, but their family members.
Dr. Manning. Would you consider this patient for any neoadjuvant chemotherapy?
Dr. Aggarwal. The patient is a young female with a small tumor that is HER2+. The indication for neoadjuvant chemotherapy is typically a big tumor or inoperable disease. Neoadjuvant chemotherapy is considered the standard of care for patients with inflammatory breast cancer and may confer a survival benefit in these patients. Of all the breast cancer subtypes, triple negative and HER2+ are considered the most chemosensitive and may benefit from neoadjuvant therapy. This patient has a small tumor, and I don’t think she’s a candidate for neoadjuvant chemotherapy unless the patient wants to see if her tumor is chemosensitive or not.
Dr. Manning, What’s the role and benefit of lumpectomy vs mastectomy?
Dr. Manning. Historically, mastectomy would have been considered the standard of care, but luckily, in the 1970s and the 1980s, we had a significant number of randomized controlled trials that demonstrated that certain women with particular characteristics would get the same overall survival if they chose mastectomy vs lumpectomy, the removal of the tumor with negative margin and whole-breast radiation. The key thing to understand is that breast-conserving surgery is now very well established with more than 20 years of data to support it. And that breast irradiation after breast-conserving surgery is essential to maximizing the local control and the overall survival (OS).
There have been a lot of major studies, but the one with the greatest follow-up now is the National Surgical Adjuvant Breast and Bowel Project (NSABP) B06 protocol, which was the only trial to compare mastectomy to lumpectomy and radiation or lumpectomy alone. It required negative margins. With 20 years of follow-up, the data still support that mastectomy or lumpectomy with radiation offers equivalent OS and local control. It’s really about patient preference if they are candidates.
Who is a candidate? Clearly, there are contraindications. We tend to look primarily at the size of the tumor. However, removing an average-sized tumor (< 2 cm) with a margin may not have a good cosmetic result for a patient with very small breasts. That patient may opt to go forward with a mastectomy instead. Young patients who are candidates must have to have negative margins. If they have persistently positive resection margins after excision or reexcision, then they need to go forward with mastectomy.
A patient who has imaging evidence of multicentric disease with 2 or more primary tumors in separate quadrants would not be a candidate for breast-conserving therapy. Diffuse malignant-appearing microcalcifications on a mammogram also would suggest multicentric disease. And a patient with a prior history of radiation therapy to the breast or chest wall cannot go through breast-conserving therapy.
In the case we are discussing, we also should make sure this young lady is not pregnant. If the patient is adamant about breast-conserving surgery and pregnant, especially in the third trimester, radiation could be deferred until after delivery. Another relative contraindication is for patients who have connective tissue disorders. Sometimes if they are given whole-breast radiation, the cosmetic result is poor. So if you’re doing this procedure to save the breast, then having a good cosmetic result is an important consideration for many patients.
When you look at the size of the tumor for this patient, she seems to be a good candidate for breast-conserving surgery. I would recommend that she go forward with lumpectomy followed by whole-breast radiation.
Ms. Venne. Although the numbers aren’t nearly as large as they were in the original trials looking at the lumpectomy vs mastectomy, there are now survival data for women with BRCA1/2 mutations. With all of the caveats that Dr. Manning mentioned, even if you have an identifiable mutation, you may not necessarily need that more aggressive surgery.2 Clearly, individuals with identifiable mutations would have a higher chance of a contralateral breast cancer, a second primary, so some individuals consider a prophylactic bilateral mastectomy. But from a survival perspective, there are a fair amount of data now available that say that lumpectomy vs mastectomy should really be the conversation based on all of the information that Dr. Manning outlines rather than using primarily the mutation status
Dr. Manning. I agree.
Dr. Aggarwal. This patient had a lumpectomy and axillary lymph node dissection. Pathology reported 1.5-cm mass, grade 3 IDC; the margins were negative. There was no skin involvement, 27 lymph nodes removed were all negative. Dr. Manning, can you please discuss the role of radiation in early stage breast cancer in patients like this case?
Dr. Manning. One of the questions that is always controversial for radiation in these early stage breast cancer cases is what do you do with the nodal irradiation? Previously, radiation oncologists based treatment plans on retrospective data, but in 2015, there were 2 major studies, 1 from Canada, and 1 from the European Organisation for Research and Treatment of Cancer (EORTC).3,4 Both studies tried to determine whether there was an advantage to doing regional nodal irradiation in early breast cancer cases. That encompassed axillary, supraclavicular, and internal mammary nodes. The studies showed that there was no survival advantage, but there was a statistically significant improvement in disease free survival and in local regional recurrence and distant mets.
Unfortunately, there are still a lot of unanswered questions, like what group potentially would benefit the most? In the MA.20 Study, some observers questioned that maybe the ER-/PR- women had the most benefit, but then, in the other study the benefit wasn’t clear.4,5 One question is which lymph node group is having the most impact? Was the benefit from radiating the supraclavicular nodes or was it from radiating the internal mammary nodes? Determining the answer is important from a technical point of view because when you radiate the internal mammary nodes, you have the potential to expose more heart and lung to radiation. You have to put all these together and make a recommendation.
Clearly, for a patient with negative nodes there is no question: You would not treat the regional nodes. However, for a patient with positive nodes you really have to individualize the approach and consider age, anatomy, tumor location, and burden of axillary disease.
I would sit down and have a discussion with this young woman to weigh the risks and the benefits. There is a slight increased risk of lymphedema in these patients, and radiation pneumonitis increases, but not significantly. A key concern is to minimize the total dose of radiation to the heart. There have been great developments in radiation oncology technology and capabilities, so the cardiac dose is now less. But when you think about a 32-year-old patient and weigh the benefit of a 2% to 3% decrease in the incidence of distant metastases and no OS advantage, then you really need to have a conversation about how to safely treat her. At a minimum, I would treat the high axilla and the supraclavicular nodes because she had a pretty extensive lymph node dissection with more than 20 nodes, and then with her getting systemic therapy, that should be more than adequate.
Dr. Aggarwal. Is there any cutoff for age or size of the tumor where you would not do any radiation to the breast?
Dr. Manning. In this particular patient absolutely not because of the lymph node. She had breast-conserving therapy, and she’s only 32-years-old. The PRIME 2 study offered lumpectomy alone vs lumpectomy and radiation for women aged ≥ 65 years with tumors ≤ 3 cm, low grade.6 The study participants had to have negative lymph nodes, be ER+, and low grade. It was a very select group. The lumpectomy patients had a recurrence rate around 4%, and the other was closer to 1.3%.
You have to look at the whole picture. Is this a healthy 70-year-old woman? Is it an inconvenience for her to get treatment? Is she going to get hormone therapy and will she be adherent? There’s a very small group of women who underwent breast-conserving surgery that I would feel safe about not offering radiation.
Dr. Aggarwal. About 15% to 20% of all breast cancers are HER2 over expressors, which used to be a poor prognostic characteristic. However, the development of anti-HER2 therapies has changed the picture of HER2 prognosis. After the initial discovery of activity, the pivotal study by Slamon et al showed benefit in terms of progression-free survival (PFS) and OS with chemotherapy and trastuzumab. The NCCN guideline recommends anti-HER2 antibody trastuzumab in combination with chemotherapy.7
Patients with tumor < 0.5 cm who are HER2+ and ER+ may not benefit from trastuzumab, but those who are ER- and HER2+ will still benefit from trastuzumab. The combination is adriamycin/cyclophosphamide followed by a taxane with trastuzumab and to complete 1 year of trastuzumab or trastuzumab in combination with carboplatin and taxanes.
Pertuzumab, in combination with trastuzumab and docetaxel (PHT) has been FDA-approved in neoadjuvant and metastatic HER2+ disease, but is not FDA approved yet in the adjuvant setting. However, these are expensive drugs, and we don’t know how long these drugs should be given.
Mr. Crawford, What are the adverse effects (AEs) of an anti-HER2 or trastuzumab treatment, and what is the cost of trastuzumab?
Russell Crawford, BPharm. The anti-HER2 antibodies have certainly changed treatment plans and outcomes for patients with breast cancer who test HER2+. There are actually 3 of these anti-HER2 drugs on the U.S. market, and they can be used in a variety of settings. Trastuzumab and pertuzumab are indicated in women or patients who have HER2+ disease, and they work by binding to the extracellular domain of the HER2 proteins and mediate antibody-dependent cellular toxicity by inhibiting proliferation of the cells that overexpress HER2.
In this patient, we would be looking at using adjuvant trastuzumab to complete a 1-year course of therapy while she’s getting her dose-dense doxorubicin and cyclophosphamide (AC) on a weekly basis for the first 12 weeks. Trastuzumab is dosed with an initial loading dose of 4 mg/kg as the first dose, and then it’s 2 mg/kg/wk until adjuvant chemotherapy is completed. We usually extend the dosing out to 6 mg/kg every 3 weeks to complete the year of treatment.
These drugs are fairly well tolerated. They are monoclonal proteins, so a lot of the AEs that patients experience are the things that we’re used to seeing with other monoclonal proteins like the infusion-related reactions and some flulike symptoms. The biggest concern with these patients is that being on the drug for a year, there is a risk of decreasing the left ventricular ejection fraction (LVEF) of the heart. That risk is increased when these drugs are combined with anthracyclines that we know are cardiotoxic. As a single agent, the impact on left ventricular function is not significant, but when it is combined with chemotherapy, it does become a problem. Usually, we recommend routine and periodic monitoring of the LVEF with a multiple-gated acquisition or an echocardiogram to make sure that we’re not causing harm related to this treatment.
The cost of these drugs depends on the frequency, is it every week, every 2 weeks, or every 3 weeks? There are different ways to give trastuzumab, but for most patients, we prefer the every 3-week dose. And it’s estimated that for a 70-kg patient, a dose of trastuzumab at 6 mg/kg at the rate of every 3 weeks costs about $2,500 per dose. The VA pays about $6 a milligram, but it’s certainly money well spent because it has changed the playing field and the outcomes for these patients.
The cost of pertuzumab is dosed a little bit differently. It’s a flat dose not a weight-based dose. Patients get an initial loading dose of 840 mg and a continuation dose of 420 mg every 3 weeks. The cost of the 420-mg dose of pertuzumab is just under $3,000, so that first-time loading dose would be a $6,000 dose, and the continuing doses are about $3,000 per dose every 3 weeks. The AE profile is no different from what you would expect with trastuzumab. There is a similar toxicity profile for these 2 drugs. It does not appear that there is any additional cardiotoxicity if you are using the combination in the neoadjuvant setting.
The third targeted agent that goes after the HER2 is ado-trastuzumab, but it is only used in the metastatic setting, so we’ll reserve that for down the road for this patient should we ever need it.
Dr. Aggarwal. The patient received adriamycin/cyclophosphamide followed by paclitaxel weekly for 12 weeks with trastuzumab. After the 12 weekly doses, she went on trastuzumab every 3 weeks. Because she was ER+, she was a candidate for additional endocrine ablation therapy. She was started on tamoxifen and leuprolide acetate for complete hormonal ablation.
Tamoxifen was the first targeted therapy for breast cancer. In women with ER+ breast cancer, with tamoxifen given for 5 years as adjuvant treatment, the odds of recurrences decreased by 39%, and death decreased by 30% in both pre- and postmenopausal women.8 Then the ATLAS data came, which randomly allocated patients to continue another 5 years of tamoxifen vs placebo, for a total of 10 years of treatment with tamoxifen. With a mean of 7.6 years of further follow-up after entry at year 5 in this trial showed that recurrence and breast cancer mortality during the second decade after diagnosis are reduced more effectively by 10 years of adjuvant tamoxifen than by 5 years.9 The current recommendation for pre- and postmenopausal is 10 years of tamoxifen.
In addition we have 3 aromatase inhibitors (AIs), anastrozole, letrozole, and exemestane, which block the production of estrogen in postmenopausal females. Anastrozole and letrozole are nonsteroidal, and exemestane is steroidal. There are countless big randomized trials using all of these drug in different combinations. In most of these trials, AIs are shown to be equal to tamoxifen when they are compared with each other, but their AE profile is different.
The recommendation by the American Society of Clinical Oncology and NCCN guidelines is to use only AIs for 5 years. There are different combinations: You can give tamoxifen for 2 to 3 years, followed by 5 years of an AI, or 5 years of tamoxifen and 5 years of an AI. Some patients wants to stop because of AEs, but others want to continue. Patients can develop osteoporosis and arthritis from an AI and hot flashes from tamoxifen.
Mr. Crawford, How would you manage of these AEs from these treatments?
Mr. Crawford. Because this woman is young, age 32, and premenopausal, tamoxifen would be the recommended endocrine therapy for her being ER+/PR+. But the role of the leuprolide acetate is to induce a chemical oophorectomy. We are putting her into ovarian ablation by using the leuprolide acetate.
The tamoxifen is relatively well tolerated, but as an ER blocker, it has a different AE profile than does an estrogen production decreaser. With tamoxifen patients tend to complain about hot flashes, edema, fluid retention, altered menses, spotting vaginal discharge, vaginal bleeding, and dryness. These medications also increase the risk of venous thromboembolism (VTE), and there is some concern about increased risk of developing endometrial cancers with these medications. We can give it either once or twice daily. There’s nothing that really says 10 mg twice daily vs 20 mg once daily is any different. So we may play with dosing to see if patients tolerate it better one way or the other.
There are medications that we can offer to help manage the hot flashes. These medications don’t necessarily make the hot flashes go away, but they can decrease the hot flash intensity or and/or frequency. Many medications have been evaluated for hot flashes. The best data are for venlafaxine, which is usually given once a day at bedtime (dosage 37.5-75.0 mg). There has been success with gabapentin titrated up to a dose of about 300 mg 3 times daily. They are fairly similar for decreasing hot flash scores and intensities, but the patient preferences were more favorable toward the venlafaxine than for the gabapentin.
The AIs, on the other hand, have a different AE profile. With tamoxifen we see vaginal discharges, bleeding, endometrial cancer risk, and VTE risk, but these are not significant problems with any of the AIs. The AE profiles for AIs include hot flashes, but more often it is complaints of bone pain, arthralgias, and myalgias. Probably the top reason why most patients discontinue taking AIs is arthralgia and myalgia.
Because we have shut off estrogen production with the AIs, and estrogen is an important component of maintaining good bone health and bone homeostasis, patients are at an increased risked of losing or declining bone mineral density (BMD). It is recommended that these patients get placed on routine calcium and vitamin D supplementation with routine dual-energy X-ray absorptiometry scans, so we know whether we will need to initiate osteoporosis treatment, whether with oral bisphosphonates, intravenous bisphosphonates, or subcutaneous rank ligand inhibitors.
With bisphosphonates there may be a slight increase in fracture rates. But we have to balance that with the BMD concerns. If the patient progresses into the metastatic setting and we know that there’s a fair chance that there’s going to be some skeletal involvement, those people are also at an increased risk of fracture. While there is a slight concern about the increased risk of fractures with bisphosphonates, I tend to believe that the benefits outweigh the risks.
Go to www.fedprac.com/AVAHO for a discussion of the next steps in the treatment for the patient after she returned 2 years later with nausea, vomiting, acute onset headache, and 2 brain lesions that were about 2 cm.
Click here to read the digital edition.
1. Feng Y, Rhie SK, Huo D, et al. Characterizing genetic susceptibility to breast cancer in women of african ancestry. Cancer Epidemiol Biomarkers. 2017;26(7):1016-1026.
2. Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169-180.
3. Poortmans PM, Collette S, Kirkove C, et al; EORTC Radiation Oncology and Breast Cancer Groups. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317-327.
4. Whelan TJ, Olivotto IA, Parulekar WR, et al; MA.20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307-316.
5. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-2135.
6. Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM; PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266-273.
7. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-182.
8. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717.
9. Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805-816.
Case Presentation
This case represents a composite of many different patients and is not meant to represent an individual. Any resemblance to an actual patient is coincidental.
A 32-year-old African American woman presented with a self-palpated left breast mass (axillary tail at 9 o’clock position). The patient was a nonsmoker, was otherwise healthy, and had no family history of breast or any other cancer. She had never used oral contraceptives or hormones, was never pregnant, her menarche was at age 12 years, and she had regular menstrual periods. On physical examination she had a 1-cm left breast mass and a palpable left axillary lymph node. A complete diagnostic workup revealed a 2-cm left breast mass. An ultrasound-guided biopsy of the axillary lymph node was positive for invasive ductal carcinoma (IDC). The final diagnosis was left breast cancer, stage IIB IDC, T1N1M0, ER+, PR+, HER2 2+ by immunohistochemistry, fluorescence in situ hybridization (FISH) was 2.4, confirming a HER2+ tumor.
Anita Aggarwal, DO, PhD. What is the role of genetic counseling and testing in this young patient who does not have a family history of breast cancer?
Vickie L. Venne, MS. This patient absolutely would be a candidate for counseling and testing. From a genetic counseling perspective, one of the first points has to do with what “no family history of cancer” means. Typically, in a fast-paced clinic, a patient will be asked “Does anybody else in your family have cancer?” And it’s not uncommon to get the answer “no.” Genetic counselors collect specific information on at least the first- and second-degree relatives, so we end up with 3 generations. This includes both the maternal and the paternal histories. We find that people who initially report no family history of cancer are often just thinking of breast cancer, even if the provider’s question is broad. When we start digging, we often find other cancers because cancer is common.
The other issue is that she was diagnosed at a young age. Clearly, 32 years is much younger than we typically see in breast cancer, and we know that individuals with hereditary cancers often have an earlier age of onset. With no other information, her a priori risk of having a BRCA1/2 mutation would be < 2.5%.
Regardless, based on current National Comprehensive Cancer Network (NCCN) guidelines, she would be a testing candidate. We would also recommend testing for more than just BRCA1/2. In the last few decades, there have been many genes identified that are associated with an increased susceptibility to cancer. Many of these genes are part of syndromes, so if you had a mutation, that also would increase the risk for a cancer in another organ. If this woman’s mother and father lived into their 70s or 80s and she had a number of aunts on both sides who never developed breast cancer, it would be less likely to be BRCA1/2. However, P53 also can present in young women and as a de novo mutation. Therefore, we would offer her a panel of actionable genes. Genes that if, in fact, we identified a mutation in one of them, would mean we could do something different for this young lady.
JoAnn Manning, MD. Let’s say she does have testing, and she comes back BRCA+. Then what would be the recommendations or guidance?
Ms. Venne. Women (and men as well!) with mutations have an increased risk for a second primary breast cancer as well as cancer in other organs. Focusing first on the breast story and all the media around BRCA1/2 mutations and surgery, this is a woman who may consider a more aggressive surgery, including prophylactic contralateral mastectomy, if she is concerned. She is young, so we also would explore her fertility plans. While her next few months will be filled with breast cancer treatment choices, women with BRCA mutations also are at an increased risk to develop ovarian cancer, so that might be a decision she makes as well. Her health care team may also eventually discuss chemotherapeutic options available specifically to women with mutations.
However, we often see young women who are extremely nervous because there is a sense that if you’re younger, your cancer must be inherited. Part of the pretest counseling is to explore psychosocial issues and help these young ladies understand that, especially if she does not have a family history of cancer and the only indication is her age, then it’s highly likely that we’re not going to find an identifiable mutation. And in that circumstance, she probably could consider a more conservative surgical decision.
Dr. Aggarwal. How common is a BRCA1 or BRCA2 mutation in African American females?
Ms. Venne. I have not paid attention to the prevalence of mutations based on ethnicity, so I don’t know. While many of the initial mutations were discovered in women of European ancestry, there are large cohorts of women with African ancestry whose specimens are now available for identifying genetic markers that will improve breast cancer risk assessment in them.1 However, because those mutations are still being characterized, it is more common to find a variant of uncertain significance (VUS) in African American women. A VUS is an alteration—a change in the gene—that we simply don’t know what it means yet. Clinicians don’t have enough information to know if that alteration is pathogenic or benign. The problem is that people try to make sense out of everything in their lives, so they also will try to make a VUS mean something. We try hard to help people understand that a VUS is really no more significant than if we had not tested in the first place, and they should not act on that information. They should use their family history, their age, their other psychosocial concerns about their experiences with cancer as they make their treatment decisions. But they also should check back periodically with their genetic counselor because VUSs can be reclassified. And if that happens, the information might be more useful for not only them, but their family members.
Dr. Manning. Would you consider this patient for any neoadjuvant chemotherapy?
Dr. Aggarwal. The patient is a young female with a small tumor that is HER2+. The indication for neoadjuvant chemotherapy is typically a big tumor or inoperable disease. Neoadjuvant chemotherapy is considered the standard of care for patients with inflammatory breast cancer and may confer a survival benefit in these patients. Of all the breast cancer subtypes, triple negative and HER2+ are considered the most chemosensitive and may benefit from neoadjuvant therapy. This patient has a small tumor, and I don’t think she’s a candidate for neoadjuvant chemotherapy unless the patient wants to see if her tumor is chemosensitive or not.
Dr. Manning, What’s the role and benefit of lumpectomy vs mastectomy?
Dr. Manning. Historically, mastectomy would have been considered the standard of care, but luckily, in the 1970s and the 1980s, we had a significant number of randomized controlled trials that demonstrated that certain women with particular characteristics would get the same overall survival if they chose mastectomy vs lumpectomy, the removal of the tumor with negative margin and whole-breast radiation. The key thing to understand is that breast-conserving surgery is now very well established with more than 20 years of data to support it. And that breast irradiation after breast-conserving surgery is essential to maximizing the local control and the overall survival (OS).
There have been a lot of major studies, but the one with the greatest follow-up now is the National Surgical Adjuvant Breast and Bowel Project (NSABP) B06 protocol, which was the only trial to compare mastectomy to lumpectomy and radiation or lumpectomy alone. It required negative margins. With 20 years of follow-up, the data still support that mastectomy or lumpectomy with radiation offers equivalent OS and local control. It’s really about patient preference if they are candidates.
Who is a candidate? Clearly, there are contraindications. We tend to look primarily at the size of the tumor. However, removing an average-sized tumor (< 2 cm) with a margin may not have a good cosmetic result for a patient with very small breasts. That patient may opt to go forward with a mastectomy instead. Young patients who are candidates must have to have negative margins. If they have persistently positive resection margins after excision or reexcision, then they need to go forward with mastectomy.
A patient who has imaging evidence of multicentric disease with 2 or more primary tumors in separate quadrants would not be a candidate for breast-conserving therapy. Diffuse malignant-appearing microcalcifications on a mammogram also would suggest multicentric disease. And a patient with a prior history of radiation therapy to the breast or chest wall cannot go through breast-conserving therapy.
In the case we are discussing, we also should make sure this young lady is not pregnant. If the patient is adamant about breast-conserving surgery and pregnant, especially in the third trimester, radiation could be deferred until after delivery. Another relative contraindication is for patients who have connective tissue disorders. Sometimes if they are given whole-breast radiation, the cosmetic result is poor. So if you’re doing this procedure to save the breast, then having a good cosmetic result is an important consideration for many patients.
When you look at the size of the tumor for this patient, she seems to be a good candidate for breast-conserving surgery. I would recommend that she go forward with lumpectomy followed by whole-breast radiation.
Ms. Venne. Although the numbers aren’t nearly as large as they were in the original trials looking at the lumpectomy vs mastectomy, there are now survival data for women with BRCA1/2 mutations. With all of the caveats that Dr. Manning mentioned, even if you have an identifiable mutation, you may not necessarily need that more aggressive surgery.2 Clearly, individuals with identifiable mutations would have a higher chance of a contralateral breast cancer, a second primary, so some individuals consider a prophylactic bilateral mastectomy. But from a survival perspective, there are a fair amount of data now available that say that lumpectomy vs mastectomy should really be the conversation based on all of the information that Dr. Manning outlines rather than using primarily the mutation status
Dr. Manning. I agree.
Dr. Aggarwal. This patient had a lumpectomy and axillary lymph node dissection. Pathology reported 1.5-cm mass, grade 3 IDC; the margins were negative. There was no skin involvement, 27 lymph nodes removed were all negative. Dr. Manning, can you please discuss the role of radiation in early stage breast cancer in patients like this case?
Dr. Manning. One of the questions that is always controversial for radiation in these early stage breast cancer cases is what do you do with the nodal irradiation? Previously, radiation oncologists based treatment plans on retrospective data, but in 2015, there were 2 major studies, 1 from Canada, and 1 from the European Organisation for Research and Treatment of Cancer (EORTC).3,4 Both studies tried to determine whether there was an advantage to doing regional nodal irradiation in early breast cancer cases. That encompassed axillary, supraclavicular, and internal mammary nodes. The studies showed that there was no survival advantage, but there was a statistically significant improvement in disease free survival and in local regional recurrence and distant mets.
Unfortunately, there are still a lot of unanswered questions, like what group potentially would benefit the most? In the MA.20 Study, some observers questioned that maybe the ER-/PR- women had the most benefit, but then, in the other study the benefit wasn’t clear.4,5 One question is which lymph node group is having the most impact? Was the benefit from radiating the supraclavicular nodes or was it from radiating the internal mammary nodes? Determining the answer is important from a technical point of view because when you radiate the internal mammary nodes, you have the potential to expose more heart and lung to radiation. You have to put all these together and make a recommendation.
Clearly, for a patient with negative nodes there is no question: You would not treat the regional nodes. However, for a patient with positive nodes you really have to individualize the approach and consider age, anatomy, tumor location, and burden of axillary disease.
I would sit down and have a discussion with this young woman to weigh the risks and the benefits. There is a slight increased risk of lymphedema in these patients, and radiation pneumonitis increases, but not significantly. A key concern is to minimize the total dose of radiation to the heart. There have been great developments in radiation oncology technology and capabilities, so the cardiac dose is now less. But when you think about a 32-year-old patient and weigh the benefit of a 2% to 3% decrease in the incidence of distant metastases and no OS advantage, then you really need to have a conversation about how to safely treat her. At a minimum, I would treat the high axilla and the supraclavicular nodes because she had a pretty extensive lymph node dissection with more than 20 nodes, and then with her getting systemic therapy, that should be more than adequate.
Dr. Aggarwal. Is there any cutoff for age or size of the tumor where you would not do any radiation to the breast?
Dr. Manning. In this particular patient absolutely not because of the lymph node. She had breast-conserving therapy, and she’s only 32-years-old. The PRIME 2 study offered lumpectomy alone vs lumpectomy and radiation for women aged ≥ 65 years with tumors ≤ 3 cm, low grade.6 The study participants had to have negative lymph nodes, be ER+, and low grade. It was a very select group. The lumpectomy patients had a recurrence rate around 4%, and the other was closer to 1.3%.
You have to look at the whole picture. Is this a healthy 70-year-old woman? Is it an inconvenience for her to get treatment? Is she going to get hormone therapy and will she be adherent? There’s a very small group of women who underwent breast-conserving surgery that I would feel safe about not offering radiation.
Dr. Aggarwal. About 15% to 20% of all breast cancers are HER2 over expressors, which used to be a poor prognostic characteristic. However, the development of anti-HER2 therapies has changed the picture of HER2 prognosis. After the initial discovery of activity, the pivotal study by Slamon et al showed benefit in terms of progression-free survival (PFS) and OS with chemotherapy and trastuzumab. The NCCN guideline recommends anti-HER2 antibody trastuzumab in combination with chemotherapy.7
Patients with tumor < 0.5 cm who are HER2+ and ER+ may not benefit from trastuzumab, but those who are ER- and HER2+ will still benefit from trastuzumab. The combination is adriamycin/cyclophosphamide followed by a taxane with trastuzumab and to complete 1 year of trastuzumab or trastuzumab in combination with carboplatin and taxanes.
Pertuzumab, in combination with trastuzumab and docetaxel (PHT) has been FDA-approved in neoadjuvant and metastatic HER2+ disease, but is not FDA approved yet in the adjuvant setting. However, these are expensive drugs, and we don’t know how long these drugs should be given.
Mr. Crawford, What are the adverse effects (AEs) of an anti-HER2 or trastuzumab treatment, and what is the cost of trastuzumab?
Russell Crawford, BPharm. The anti-HER2 antibodies have certainly changed treatment plans and outcomes for patients with breast cancer who test HER2+. There are actually 3 of these anti-HER2 drugs on the U.S. market, and they can be used in a variety of settings. Trastuzumab and pertuzumab are indicated in women or patients who have HER2+ disease, and they work by binding to the extracellular domain of the HER2 proteins and mediate antibody-dependent cellular toxicity by inhibiting proliferation of the cells that overexpress HER2.
In this patient, we would be looking at using adjuvant trastuzumab to complete a 1-year course of therapy while she’s getting her dose-dense doxorubicin and cyclophosphamide (AC) on a weekly basis for the first 12 weeks. Trastuzumab is dosed with an initial loading dose of 4 mg/kg as the first dose, and then it’s 2 mg/kg/wk until adjuvant chemotherapy is completed. We usually extend the dosing out to 6 mg/kg every 3 weeks to complete the year of treatment.
These drugs are fairly well tolerated. They are monoclonal proteins, so a lot of the AEs that patients experience are the things that we’re used to seeing with other monoclonal proteins like the infusion-related reactions and some flulike symptoms. The biggest concern with these patients is that being on the drug for a year, there is a risk of decreasing the left ventricular ejection fraction (LVEF) of the heart. That risk is increased when these drugs are combined with anthracyclines that we know are cardiotoxic. As a single agent, the impact on left ventricular function is not significant, but when it is combined with chemotherapy, it does become a problem. Usually, we recommend routine and periodic monitoring of the LVEF with a multiple-gated acquisition or an echocardiogram to make sure that we’re not causing harm related to this treatment.
The cost of these drugs depends on the frequency, is it every week, every 2 weeks, or every 3 weeks? There are different ways to give trastuzumab, but for most patients, we prefer the every 3-week dose. And it’s estimated that for a 70-kg patient, a dose of trastuzumab at 6 mg/kg at the rate of every 3 weeks costs about $2,500 per dose. The VA pays about $6 a milligram, but it’s certainly money well spent because it has changed the playing field and the outcomes for these patients.
The cost of pertuzumab is dosed a little bit differently. It’s a flat dose not a weight-based dose. Patients get an initial loading dose of 840 mg and a continuation dose of 420 mg every 3 weeks. The cost of the 420-mg dose of pertuzumab is just under $3,000, so that first-time loading dose would be a $6,000 dose, and the continuing doses are about $3,000 per dose every 3 weeks. The AE profile is no different from what you would expect with trastuzumab. There is a similar toxicity profile for these 2 drugs. It does not appear that there is any additional cardiotoxicity if you are using the combination in the neoadjuvant setting.
The third targeted agent that goes after the HER2 is ado-trastuzumab, but it is only used in the metastatic setting, so we’ll reserve that for down the road for this patient should we ever need it.
Dr. Aggarwal. The patient received adriamycin/cyclophosphamide followed by paclitaxel weekly for 12 weeks with trastuzumab. After the 12 weekly doses, she went on trastuzumab every 3 weeks. Because she was ER+, she was a candidate for additional endocrine ablation therapy. She was started on tamoxifen and leuprolide acetate for complete hormonal ablation.
Tamoxifen was the first targeted therapy for breast cancer. In women with ER+ breast cancer, with tamoxifen given for 5 years as adjuvant treatment, the odds of recurrences decreased by 39%, and death decreased by 30% in both pre- and postmenopausal women.8 Then the ATLAS data came, which randomly allocated patients to continue another 5 years of tamoxifen vs placebo, for a total of 10 years of treatment with tamoxifen. With a mean of 7.6 years of further follow-up after entry at year 5 in this trial showed that recurrence and breast cancer mortality during the second decade after diagnosis are reduced more effectively by 10 years of adjuvant tamoxifen than by 5 years.9 The current recommendation for pre- and postmenopausal is 10 years of tamoxifen.
In addition we have 3 aromatase inhibitors (AIs), anastrozole, letrozole, and exemestane, which block the production of estrogen in postmenopausal females. Anastrozole and letrozole are nonsteroidal, and exemestane is steroidal. There are countless big randomized trials using all of these drug in different combinations. In most of these trials, AIs are shown to be equal to tamoxifen when they are compared with each other, but their AE profile is different.
The recommendation by the American Society of Clinical Oncology and NCCN guidelines is to use only AIs for 5 years. There are different combinations: You can give tamoxifen for 2 to 3 years, followed by 5 years of an AI, or 5 years of tamoxifen and 5 years of an AI. Some patients wants to stop because of AEs, but others want to continue. Patients can develop osteoporosis and arthritis from an AI and hot flashes from tamoxifen.
Mr. Crawford, How would you manage of these AEs from these treatments?
Mr. Crawford. Because this woman is young, age 32, and premenopausal, tamoxifen would be the recommended endocrine therapy for her being ER+/PR+. But the role of the leuprolide acetate is to induce a chemical oophorectomy. We are putting her into ovarian ablation by using the leuprolide acetate.
The tamoxifen is relatively well tolerated, but as an ER blocker, it has a different AE profile than does an estrogen production decreaser. With tamoxifen patients tend to complain about hot flashes, edema, fluid retention, altered menses, spotting vaginal discharge, vaginal bleeding, and dryness. These medications also increase the risk of venous thromboembolism (VTE), and there is some concern about increased risk of developing endometrial cancers with these medications. We can give it either once or twice daily. There’s nothing that really says 10 mg twice daily vs 20 mg once daily is any different. So we may play with dosing to see if patients tolerate it better one way or the other.
There are medications that we can offer to help manage the hot flashes. These medications don’t necessarily make the hot flashes go away, but they can decrease the hot flash intensity or and/or frequency. Many medications have been evaluated for hot flashes. The best data are for venlafaxine, which is usually given once a day at bedtime (dosage 37.5-75.0 mg). There has been success with gabapentin titrated up to a dose of about 300 mg 3 times daily. They are fairly similar for decreasing hot flash scores and intensities, but the patient preferences were more favorable toward the venlafaxine than for the gabapentin.
The AIs, on the other hand, have a different AE profile. With tamoxifen we see vaginal discharges, bleeding, endometrial cancer risk, and VTE risk, but these are not significant problems with any of the AIs. The AE profiles for AIs include hot flashes, but more often it is complaints of bone pain, arthralgias, and myalgias. Probably the top reason why most patients discontinue taking AIs is arthralgia and myalgia.
Because we have shut off estrogen production with the AIs, and estrogen is an important component of maintaining good bone health and bone homeostasis, patients are at an increased risked of losing or declining bone mineral density (BMD). It is recommended that these patients get placed on routine calcium and vitamin D supplementation with routine dual-energy X-ray absorptiometry scans, so we know whether we will need to initiate osteoporosis treatment, whether with oral bisphosphonates, intravenous bisphosphonates, or subcutaneous rank ligand inhibitors.
With bisphosphonates there may be a slight increase in fracture rates. But we have to balance that with the BMD concerns. If the patient progresses into the metastatic setting and we know that there’s a fair chance that there’s going to be some skeletal involvement, those people are also at an increased risk of fracture. While there is a slight concern about the increased risk of fractures with bisphosphonates, I tend to believe that the benefits outweigh the risks.
Go to www.fedprac.com/AVAHO for a discussion of the next steps in the treatment for the patient after she returned 2 years later with nausea, vomiting, acute onset headache, and 2 brain lesions that were about 2 cm.
Click here to read the digital edition.
Case Presentation
This case represents a composite of many different patients and is not meant to represent an individual. Any resemblance to an actual patient is coincidental.
A 32-year-old African American woman presented with a self-palpated left breast mass (axillary tail at 9 o’clock position). The patient was a nonsmoker, was otherwise healthy, and had no family history of breast or any other cancer. She had never used oral contraceptives or hormones, was never pregnant, her menarche was at age 12 years, and she had regular menstrual periods. On physical examination she had a 1-cm left breast mass and a palpable left axillary lymph node. A complete diagnostic workup revealed a 2-cm left breast mass. An ultrasound-guided biopsy of the axillary lymph node was positive for invasive ductal carcinoma (IDC). The final diagnosis was left breast cancer, stage IIB IDC, T1N1M0, ER+, PR+, HER2 2+ by immunohistochemistry, fluorescence in situ hybridization (FISH) was 2.4, confirming a HER2+ tumor.
Anita Aggarwal, DO, PhD. What is the role of genetic counseling and testing in this young patient who does not have a family history of breast cancer?
Vickie L. Venne, MS. This patient absolutely would be a candidate for counseling and testing. From a genetic counseling perspective, one of the first points has to do with what “no family history of cancer” means. Typically, in a fast-paced clinic, a patient will be asked “Does anybody else in your family have cancer?” And it’s not uncommon to get the answer “no.” Genetic counselors collect specific information on at least the first- and second-degree relatives, so we end up with 3 generations. This includes both the maternal and the paternal histories. We find that people who initially report no family history of cancer are often just thinking of breast cancer, even if the provider’s question is broad. When we start digging, we often find other cancers because cancer is common.
The other issue is that she was diagnosed at a young age. Clearly, 32 years is much younger than we typically see in breast cancer, and we know that individuals with hereditary cancers often have an earlier age of onset. With no other information, her a priori risk of having a BRCA1/2 mutation would be < 2.5%.
Regardless, based on current National Comprehensive Cancer Network (NCCN) guidelines, she would be a testing candidate. We would also recommend testing for more than just BRCA1/2. In the last few decades, there have been many genes identified that are associated with an increased susceptibility to cancer. Many of these genes are part of syndromes, so if you had a mutation, that also would increase the risk for a cancer in another organ. If this woman’s mother and father lived into their 70s or 80s and she had a number of aunts on both sides who never developed breast cancer, it would be less likely to be BRCA1/2. However, P53 also can present in young women and as a de novo mutation. Therefore, we would offer her a panel of actionable genes. Genes that if, in fact, we identified a mutation in one of them, would mean we could do something different for this young lady.
JoAnn Manning, MD. Let’s say she does have testing, and she comes back BRCA+. Then what would be the recommendations or guidance?
Ms. Venne. Women (and men as well!) with mutations have an increased risk for a second primary breast cancer as well as cancer in other organs. Focusing first on the breast story and all the media around BRCA1/2 mutations and surgery, this is a woman who may consider a more aggressive surgery, including prophylactic contralateral mastectomy, if she is concerned. She is young, so we also would explore her fertility plans. While her next few months will be filled with breast cancer treatment choices, women with BRCA mutations also are at an increased risk to develop ovarian cancer, so that might be a decision she makes as well. Her health care team may also eventually discuss chemotherapeutic options available specifically to women with mutations.
However, we often see young women who are extremely nervous because there is a sense that if you’re younger, your cancer must be inherited. Part of the pretest counseling is to explore psychosocial issues and help these young ladies understand that, especially if she does not have a family history of cancer and the only indication is her age, then it’s highly likely that we’re not going to find an identifiable mutation. And in that circumstance, she probably could consider a more conservative surgical decision.
Dr. Aggarwal. How common is a BRCA1 or BRCA2 mutation in African American females?
Ms. Venne. I have not paid attention to the prevalence of mutations based on ethnicity, so I don’t know. While many of the initial mutations were discovered in women of European ancestry, there are large cohorts of women with African ancestry whose specimens are now available for identifying genetic markers that will improve breast cancer risk assessment in them.1 However, because those mutations are still being characterized, it is more common to find a variant of uncertain significance (VUS) in African American women. A VUS is an alteration—a change in the gene—that we simply don’t know what it means yet. Clinicians don’t have enough information to know if that alteration is pathogenic or benign. The problem is that people try to make sense out of everything in their lives, so they also will try to make a VUS mean something. We try hard to help people understand that a VUS is really no more significant than if we had not tested in the first place, and they should not act on that information. They should use their family history, their age, their other psychosocial concerns about their experiences with cancer as they make their treatment decisions. But they also should check back periodically with their genetic counselor because VUSs can be reclassified. And if that happens, the information might be more useful for not only them, but their family members.
Dr. Manning. Would you consider this patient for any neoadjuvant chemotherapy?
Dr. Aggarwal. The patient is a young female with a small tumor that is HER2+. The indication for neoadjuvant chemotherapy is typically a big tumor or inoperable disease. Neoadjuvant chemotherapy is considered the standard of care for patients with inflammatory breast cancer and may confer a survival benefit in these patients. Of all the breast cancer subtypes, triple negative and HER2+ are considered the most chemosensitive and may benefit from neoadjuvant therapy. This patient has a small tumor, and I don’t think she’s a candidate for neoadjuvant chemotherapy unless the patient wants to see if her tumor is chemosensitive or not.
Dr. Manning, What’s the role and benefit of lumpectomy vs mastectomy?
Dr. Manning. Historically, mastectomy would have been considered the standard of care, but luckily, in the 1970s and the 1980s, we had a significant number of randomized controlled trials that demonstrated that certain women with particular characteristics would get the same overall survival if they chose mastectomy vs lumpectomy, the removal of the tumor with negative margin and whole-breast radiation. The key thing to understand is that breast-conserving surgery is now very well established with more than 20 years of data to support it. And that breast irradiation after breast-conserving surgery is essential to maximizing the local control and the overall survival (OS).
There have been a lot of major studies, but the one with the greatest follow-up now is the National Surgical Adjuvant Breast and Bowel Project (NSABP) B06 protocol, which was the only trial to compare mastectomy to lumpectomy and radiation or lumpectomy alone. It required negative margins. With 20 years of follow-up, the data still support that mastectomy or lumpectomy with radiation offers equivalent OS and local control. It’s really about patient preference if they are candidates.
Who is a candidate? Clearly, there are contraindications. We tend to look primarily at the size of the tumor. However, removing an average-sized tumor (< 2 cm) with a margin may not have a good cosmetic result for a patient with very small breasts. That patient may opt to go forward with a mastectomy instead. Young patients who are candidates must have to have negative margins. If they have persistently positive resection margins after excision or reexcision, then they need to go forward with mastectomy.
A patient who has imaging evidence of multicentric disease with 2 or more primary tumors in separate quadrants would not be a candidate for breast-conserving therapy. Diffuse malignant-appearing microcalcifications on a mammogram also would suggest multicentric disease. And a patient with a prior history of radiation therapy to the breast or chest wall cannot go through breast-conserving therapy.
In the case we are discussing, we also should make sure this young lady is not pregnant. If the patient is adamant about breast-conserving surgery and pregnant, especially in the third trimester, radiation could be deferred until after delivery. Another relative contraindication is for patients who have connective tissue disorders. Sometimes if they are given whole-breast radiation, the cosmetic result is poor. So if you’re doing this procedure to save the breast, then having a good cosmetic result is an important consideration for many patients.
When you look at the size of the tumor for this patient, she seems to be a good candidate for breast-conserving surgery. I would recommend that she go forward with lumpectomy followed by whole-breast radiation.
Ms. Venne. Although the numbers aren’t nearly as large as they were in the original trials looking at the lumpectomy vs mastectomy, there are now survival data for women with BRCA1/2 mutations. With all of the caveats that Dr. Manning mentioned, even if you have an identifiable mutation, you may not necessarily need that more aggressive surgery.2 Clearly, individuals with identifiable mutations would have a higher chance of a contralateral breast cancer, a second primary, so some individuals consider a prophylactic bilateral mastectomy. But from a survival perspective, there are a fair amount of data now available that say that lumpectomy vs mastectomy should really be the conversation based on all of the information that Dr. Manning outlines rather than using primarily the mutation status
Dr. Manning. I agree.
Dr. Aggarwal. This patient had a lumpectomy and axillary lymph node dissection. Pathology reported 1.5-cm mass, grade 3 IDC; the margins were negative. There was no skin involvement, 27 lymph nodes removed were all negative. Dr. Manning, can you please discuss the role of radiation in early stage breast cancer in patients like this case?
Dr. Manning. One of the questions that is always controversial for radiation in these early stage breast cancer cases is what do you do with the nodal irradiation? Previously, radiation oncologists based treatment plans on retrospective data, but in 2015, there were 2 major studies, 1 from Canada, and 1 from the European Organisation for Research and Treatment of Cancer (EORTC).3,4 Both studies tried to determine whether there was an advantage to doing regional nodal irradiation in early breast cancer cases. That encompassed axillary, supraclavicular, and internal mammary nodes. The studies showed that there was no survival advantage, but there was a statistically significant improvement in disease free survival and in local regional recurrence and distant mets.
Unfortunately, there are still a lot of unanswered questions, like what group potentially would benefit the most? In the MA.20 Study, some observers questioned that maybe the ER-/PR- women had the most benefit, but then, in the other study the benefit wasn’t clear.4,5 One question is which lymph node group is having the most impact? Was the benefit from radiating the supraclavicular nodes or was it from radiating the internal mammary nodes? Determining the answer is important from a technical point of view because when you radiate the internal mammary nodes, you have the potential to expose more heart and lung to radiation. You have to put all these together and make a recommendation.
Clearly, for a patient with negative nodes there is no question: You would not treat the regional nodes. However, for a patient with positive nodes you really have to individualize the approach and consider age, anatomy, tumor location, and burden of axillary disease.
I would sit down and have a discussion with this young woman to weigh the risks and the benefits. There is a slight increased risk of lymphedema in these patients, and radiation pneumonitis increases, but not significantly. A key concern is to minimize the total dose of radiation to the heart. There have been great developments in radiation oncology technology and capabilities, so the cardiac dose is now less. But when you think about a 32-year-old patient and weigh the benefit of a 2% to 3% decrease in the incidence of distant metastases and no OS advantage, then you really need to have a conversation about how to safely treat her. At a minimum, I would treat the high axilla and the supraclavicular nodes because she had a pretty extensive lymph node dissection with more than 20 nodes, and then with her getting systemic therapy, that should be more than adequate.
Dr. Aggarwal. Is there any cutoff for age or size of the tumor where you would not do any radiation to the breast?
Dr. Manning. In this particular patient absolutely not because of the lymph node. She had breast-conserving therapy, and she’s only 32-years-old. The PRIME 2 study offered lumpectomy alone vs lumpectomy and radiation for women aged ≥ 65 years with tumors ≤ 3 cm, low grade.6 The study participants had to have negative lymph nodes, be ER+, and low grade. It was a very select group. The lumpectomy patients had a recurrence rate around 4%, and the other was closer to 1.3%.
You have to look at the whole picture. Is this a healthy 70-year-old woman? Is it an inconvenience for her to get treatment? Is she going to get hormone therapy and will she be adherent? There’s a very small group of women who underwent breast-conserving surgery that I would feel safe about not offering radiation.
Dr. Aggarwal. About 15% to 20% of all breast cancers are HER2 over expressors, which used to be a poor prognostic characteristic. However, the development of anti-HER2 therapies has changed the picture of HER2 prognosis. After the initial discovery of activity, the pivotal study by Slamon et al showed benefit in terms of progression-free survival (PFS) and OS with chemotherapy and trastuzumab. The NCCN guideline recommends anti-HER2 antibody trastuzumab in combination with chemotherapy.7
Patients with tumor < 0.5 cm who are HER2+ and ER+ may not benefit from trastuzumab, but those who are ER- and HER2+ will still benefit from trastuzumab. The combination is adriamycin/cyclophosphamide followed by a taxane with trastuzumab and to complete 1 year of trastuzumab or trastuzumab in combination with carboplatin and taxanes.
Pertuzumab, in combination with trastuzumab and docetaxel (PHT) has been FDA-approved in neoadjuvant and metastatic HER2+ disease, but is not FDA approved yet in the adjuvant setting. However, these are expensive drugs, and we don’t know how long these drugs should be given.
Mr. Crawford, What are the adverse effects (AEs) of an anti-HER2 or trastuzumab treatment, and what is the cost of trastuzumab?
Russell Crawford, BPharm. The anti-HER2 antibodies have certainly changed treatment plans and outcomes for patients with breast cancer who test HER2+. There are actually 3 of these anti-HER2 drugs on the U.S. market, and they can be used in a variety of settings. Trastuzumab and pertuzumab are indicated in women or patients who have HER2+ disease, and they work by binding to the extracellular domain of the HER2 proteins and mediate antibody-dependent cellular toxicity by inhibiting proliferation of the cells that overexpress HER2.
In this patient, we would be looking at using adjuvant trastuzumab to complete a 1-year course of therapy while she’s getting her dose-dense doxorubicin and cyclophosphamide (AC) on a weekly basis for the first 12 weeks. Trastuzumab is dosed with an initial loading dose of 4 mg/kg as the first dose, and then it’s 2 mg/kg/wk until adjuvant chemotherapy is completed. We usually extend the dosing out to 6 mg/kg every 3 weeks to complete the year of treatment.
These drugs are fairly well tolerated. They are monoclonal proteins, so a lot of the AEs that patients experience are the things that we’re used to seeing with other monoclonal proteins like the infusion-related reactions and some flulike symptoms. The biggest concern with these patients is that being on the drug for a year, there is a risk of decreasing the left ventricular ejection fraction (LVEF) of the heart. That risk is increased when these drugs are combined with anthracyclines that we know are cardiotoxic. As a single agent, the impact on left ventricular function is not significant, but when it is combined with chemotherapy, it does become a problem. Usually, we recommend routine and periodic monitoring of the LVEF with a multiple-gated acquisition or an echocardiogram to make sure that we’re not causing harm related to this treatment.
The cost of these drugs depends on the frequency, is it every week, every 2 weeks, or every 3 weeks? There are different ways to give trastuzumab, but for most patients, we prefer the every 3-week dose. And it’s estimated that for a 70-kg patient, a dose of trastuzumab at 6 mg/kg at the rate of every 3 weeks costs about $2,500 per dose. The VA pays about $6 a milligram, but it’s certainly money well spent because it has changed the playing field and the outcomes for these patients.
The cost of pertuzumab is dosed a little bit differently. It’s a flat dose not a weight-based dose. Patients get an initial loading dose of 840 mg and a continuation dose of 420 mg every 3 weeks. The cost of the 420-mg dose of pertuzumab is just under $3,000, so that first-time loading dose would be a $6,000 dose, and the continuing doses are about $3,000 per dose every 3 weeks. The AE profile is no different from what you would expect with trastuzumab. There is a similar toxicity profile for these 2 drugs. It does not appear that there is any additional cardiotoxicity if you are using the combination in the neoadjuvant setting.
The third targeted agent that goes after the HER2 is ado-trastuzumab, but it is only used in the metastatic setting, so we’ll reserve that for down the road for this patient should we ever need it.
Dr. Aggarwal. The patient received adriamycin/cyclophosphamide followed by paclitaxel weekly for 12 weeks with trastuzumab. After the 12 weekly doses, she went on trastuzumab every 3 weeks. Because she was ER+, she was a candidate for additional endocrine ablation therapy. She was started on tamoxifen and leuprolide acetate for complete hormonal ablation.
Tamoxifen was the first targeted therapy for breast cancer. In women with ER+ breast cancer, with tamoxifen given for 5 years as adjuvant treatment, the odds of recurrences decreased by 39%, and death decreased by 30% in both pre- and postmenopausal women.8 Then the ATLAS data came, which randomly allocated patients to continue another 5 years of tamoxifen vs placebo, for a total of 10 years of treatment with tamoxifen. With a mean of 7.6 years of further follow-up after entry at year 5 in this trial showed that recurrence and breast cancer mortality during the second decade after diagnosis are reduced more effectively by 10 years of adjuvant tamoxifen than by 5 years.9 The current recommendation for pre- and postmenopausal is 10 years of tamoxifen.
In addition we have 3 aromatase inhibitors (AIs), anastrozole, letrozole, and exemestane, which block the production of estrogen in postmenopausal females. Anastrozole and letrozole are nonsteroidal, and exemestane is steroidal. There are countless big randomized trials using all of these drug in different combinations. In most of these trials, AIs are shown to be equal to tamoxifen when they are compared with each other, but their AE profile is different.
The recommendation by the American Society of Clinical Oncology and NCCN guidelines is to use only AIs for 5 years. There are different combinations: You can give tamoxifen for 2 to 3 years, followed by 5 years of an AI, or 5 years of tamoxifen and 5 years of an AI. Some patients wants to stop because of AEs, but others want to continue. Patients can develop osteoporosis and arthritis from an AI and hot flashes from tamoxifen.
Mr. Crawford, How would you manage of these AEs from these treatments?
Mr. Crawford. Because this woman is young, age 32, and premenopausal, tamoxifen would be the recommended endocrine therapy for her being ER+/PR+. But the role of the leuprolide acetate is to induce a chemical oophorectomy. We are putting her into ovarian ablation by using the leuprolide acetate.
The tamoxifen is relatively well tolerated, but as an ER blocker, it has a different AE profile than does an estrogen production decreaser. With tamoxifen patients tend to complain about hot flashes, edema, fluid retention, altered menses, spotting vaginal discharge, vaginal bleeding, and dryness. These medications also increase the risk of venous thromboembolism (VTE), and there is some concern about increased risk of developing endometrial cancers with these medications. We can give it either once or twice daily. There’s nothing that really says 10 mg twice daily vs 20 mg once daily is any different. So we may play with dosing to see if patients tolerate it better one way or the other.
There are medications that we can offer to help manage the hot flashes. These medications don’t necessarily make the hot flashes go away, but they can decrease the hot flash intensity or and/or frequency. Many medications have been evaluated for hot flashes. The best data are for venlafaxine, which is usually given once a day at bedtime (dosage 37.5-75.0 mg). There has been success with gabapentin titrated up to a dose of about 300 mg 3 times daily. They are fairly similar for decreasing hot flash scores and intensities, but the patient preferences were more favorable toward the venlafaxine than for the gabapentin.
The AIs, on the other hand, have a different AE profile. With tamoxifen we see vaginal discharges, bleeding, endometrial cancer risk, and VTE risk, but these are not significant problems with any of the AIs. The AE profiles for AIs include hot flashes, but more often it is complaints of bone pain, arthralgias, and myalgias. Probably the top reason why most patients discontinue taking AIs is arthralgia and myalgia.
Because we have shut off estrogen production with the AIs, and estrogen is an important component of maintaining good bone health and bone homeostasis, patients are at an increased risked of losing or declining bone mineral density (BMD). It is recommended that these patients get placed on routine calcium and vitamin D supplementation with routine dual-energy X-ray absorptiometry scans, so we know whether we will need to initiate osteoporosis treatment, whether with oral bisphosphonates, intravenous bisphosphonates, or subcutaneous rank ligand inhibitors.
With bisphosphonates there may be a slight increase in fracture rates. But we have to balance that with the BMD concerns. If the patient progresses into the metastatic setting and we know that there’s a fair chance that there’s going to be some skeletal involvement, those people are also at an increased risk of fracture. While there is a slight concern about the increased risk of fractures with bisphosphonates, I tend to believe that the benefits outweigh the risks.
Go to www.fedprac.com/AVAHO for a discussion of the next steps in the treatment for the patient after she returned 2 years later with nausea, vomiting, acute onset headache, and 2 brain lesions that were about 2 cm.
Click here to read the digital edition.
1. Feng Y, Rhie SK, Huo D, et al. Characterizing genetic susceptibility to breast cancer in women of african ancestry. Cancer Epidemiol Biomarkers. 2017;26(7):1016-1026.
2. Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169-180.
3. Poortmans PM, Collette S, Kirkove C, et al; EORTC Radiation Oncology and Breast Cancer Groups. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317-327.
4. Whelan TJ, Olivotto IA, Parulekar WR, et al; MA.20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307-316.
5. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-2135.
6. Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM; PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266-273.
7. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-182.
8. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717.
9. Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805-816.
1. Feng Y, Rhie SK, Huo D, et al. Characterizing genetic susceptibility to breast cancer in women of african ancestry. Cancer Epidemiol Biomarkers. 2017;26(7):1016-1026.
2. Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169-180.
3. Poortmans PM, Collette S, Kirkove C, et al; EORTC Radiation Oncology and Breast Cancer Groups. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317-327.
4. Whelan TJ, Olivotto IA, Parulekar WR, et al; MA.20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307-316.
5. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-2135.
6. Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM; PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266-273.
7. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-182.
8. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717.
9. Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805-816.
Raising More Than Moods: Escitalopram-Associated Priapism
Selective serotonin reuptake inhibitors are a common first choice medication for anxiety and depression treatment, but health care providers should be aware of priapism as a possible adverse effect.
Case Presentation
An 80-year-old white male was evaluated in a primary care clinic following a recent hospitalization for a suicide attempt. His past medical history included type 2 diabetes mellitus, chronic atrial fibrillation, essential hypertension and hyperlipidemia, and no prior psychiatric illness. Six weeks after his wife died of cancer, the patient attempted suicide by slitting his wrists, which resulted in significant blood loss and tendon damage.
After medical stabilization he was treated at an inpatient psychiatric facility for 10 days. There was no evidence of impaired memory nor psychosis during his hospitalization. He was prescribed doxazosin 1 mg twice daily and finasteride 5 mg daily for obstructive urinary symptoms, along with escitalopram 5 mg daily for depression and continuation of prior medications, including glipizide 10 mg twice daily, simvastatin 20 mg daily, metformin 500 mg twice daily, and lisinopril 20 mg daily. The patient’s estimated glomerular filtration rate was 85 at the time of these events.
He was evaluated by the mental health staff at the time of his primary care outpatient visit and noted to have a Patient Health Questionnaire (PHQ-9) score of 5 (mild depression symptoms) and a Generalized Anxiety Disorder 7 Item Scale (GAD-7) score of 1 (minimum anxiety symptoms). Eleven days later during his counseling appointment, he mentioned to staff that he had experienced a painful erection the day before, which lasted 4 hours. The primary care pharmacist was consulted for review of potential medication triggers. It was noted that there was a low frequency of priapism with both doxazosin and escitalopram, a selective serotonin reuptake inhibitor (SSRI). The provider team felt that the α blocker (doxazosin) was more likely than was the SSRI to cause the reported priapism event. Doxazosin was discontinued, and escitalopram 5 mg daily was maintained. His mood remained stable with no further suicidal ideation.
Eighteen days after discontinuation of doxazosin, the patient experienced a second priapism episode. He reported 2 days later that he experienced a prolonged, painful erection that lasted 4 hours and resolved without intervention. The patient’s mood continued without further suicidal thoughts, his appetite was normal, he had good social support and played cards with friends regularly. At that time, the decision was made to discontinue the escitalopram. The SSRI was felt to be a possible cause of priapism due to the length of time off doxazosin in relation to the second event.
The patient continued to do well 15 months after discontinuation of these medications. Unfortunately, he did not seek medical care during either episode of priapism, but he was felt to be reliable in his report based on a normal mental status exam. He does not have any of the other known risk factors for priapism, suggesting a possible association with his α blocker and SSRI.
Discussion
Priapism is a prolonged, painful erection lasting more than 4 hours and is considered a urologic emergency. It is divided into ischemic and nonischemic types. Ischemic priapism occurs with blood dyscrasias, such as sickle cell disease, thalassemia, leukemia, neurologic conditions affecting the spinal cord, and malignancies of bladder/prostate. The lifetime probability of priapism in patients affected by sickle cell disease is estimated at 29% to 42%.1 Medications associated with priapism include cocaine, ondansetron, antipsychotics, excessive use of erectile dysfunction drugs, and increasingly, antidepressants.2-8
Nonischemic priapism is usually associated with pelvic trauma. Cavernous blood gas obtained at the time of the event can help distinguish between the 2 types. The color of the aspirated blood sample is black in patients with ischemic priapism. Corporal blood gas analysis shows hypoxemia and acidemia. The color of blood is red in patients with nonischemic priapism and shows normal oxygen and pH. Priapism is a urologic emergency requiring aspiration of blood from the cavernous sinus to prevent ischemic tissue damage. At times surgical decompression may be required if aspiration is not successful.
Adrenergic α-blocking agents were developed for treatment of hypertension. They have become popular for management of lower urinary tract symptoms (LUTS) secondary to prostate enlargement. Doxazosin, prazosin, and terazosin are nonuroselective and have a higher risk of cardiac adverse effects (AEs), including dizziness and orthostatic hypotension. Lexicomp lists < 1% incidence of priapism associated with doxazosin.9 The drug is metabolized by CYP3A4 with secondary pathways, including CYP2D6 and 2C9 with a drug half-life of 22 hours. Newer agents (eg, tamsulosin, alfuzosin) are considered more uroselective, targeting the α-1b receptors. The older agents have more effect on the α-1a receptors, which are also present at higher level in the cardiovascular system.10 By blocking sympathetic stimuli responsible for penile detumescence, the nonselective α blockers have a higher propensity to cause priapism. There seems to be a direct correlation between higher doses and increased risk of priapism.11 Our patient was at a relatively low dose (1 mg twice daily) of the nonselective agent doxazosin for treatment of his LUTS.
Primary care providers and psychiatrists treating depression are familiar with common sexual AEs of the SSRI class of medications. Decreased sexual desire and delayed orgasm and ejaculation are all issues that lead patients to discontinue treatment. Although SSRIs are considered first-line treatment for depression, reports indicate that up to 60% of patients with prior normal sexual function started on paroxetine may experience sexual AEs.12 The exact frequency is difficult to estimate due to underreporting of these issues by patients.
A review of the literature for cases of priapism associated with SSRIs shows that often there is more than 1 possible drug trigger, and medications used in combination may be a risk factor. It is hypothesized that SSRI action on 5-HT3 receptors may be responsible for priapism occurring in patients treated with SSRIs.13 One study cites a case of priapism in a veteran being treated with escitalopram, prazosin, and trazodone for posttraumatic stress disorder.14 Trazodone inhibits the neuronal uptake of serotonin and is used to treat depression in addition to off-label use in treatment of insomnia. Trazodone is implicated in cases of priapism via its α-blocking properties. In the aforementioned case, trazodone was initially thought to be the causative agent and was discontinued. The patient had recurrent symptoms at which time his prazosin was discontinued, and he had no further events.
Another case cites citalopram-induced priapism that occurred with an accidental overdose of citalopram 80 mg, when a patient confused his antidepressant with 81-mg aspirin tablets.15 He also had a prior history of priapism while taking trazodone. We found only 1 case listing escitalopram as the probable causative agent of priapism.16 Similar to our patient, that case had no risk factors prior to escitalopram administration. Lexicomp notes < 1% incidence of priapism reported in postmarketing studies.
Our patient had been off doxazosin for 18 days when his second event of priapism occurred. It is less likely given the half-life of doxazosin (t ½ = 22 hours) that the α blocker was the causative agent, though a combination of the 2 agents cannot be excluded as a significant factor. The Naranjo Score is an algorithm for determining the likelihood of whether an adverse drug reaction is due to the drug or other factors.17 Scoring ranks the event as probable, possible, or doubtful. This case scored +3 (+2 = appeared after suspected drug given, +1 = improved when drug discontinued), indicating possible association of escitalopram and priapism.
Conclusion
In view of the frequent use of SSRIs in treatment of depression, it may be prudent to advise patients of this uncommon but serious medication AE. Recent use of α blockers may be a risk factor in combination with SSRI therapy. Patients should be counseled to seek emergency care in the event of prolonged erection when discussing potential AEs of SSRI therapy.
1. Manjunath AS, Hofer MD. Urologic emergencies. Med Clin North Am. 2018;102(2):373-385.
2. Altman AL, Seftel AD, Brown SL, et al. Cocaine associated priapism. J Urol. 1999;161(6):1817-1818.
3. Pivot D, Javot L, Swiegot D, et al. Two Cases of recurrent priapism during antineoplastic chemotherapy: think about ondansetron. Therapie. 2013;68(6):409-410.
4. Fu E, Kovach JG, Dubin WR. Priapism associate with antipsychotic medication use: case report. J Clin Pshychopharmacol. 2017;37(4):477-478.
5. Saghafi O, Kao A, Druck J. Recurrent priapism from therapeutic quetiapine. West J Emerg Med, 2014;15(1):114-116.
6. King SH, Hallock M, Strote J, et al. Tadalafil-associated priapism. Urology. 2005;66(2):432.
7. Giuliano F, Jackson G, Montorsi F, et al. Safety of sildenafil citrate: review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract. 2010;64(2):240-255.
8. Bhat IA, Shannon KD, Ara A, et al. Ninety-six hours ordeal of priapism induced by paroxetine: a case report and literature review. Int J Psychiatry Med. 2015;50(3):326-334.
9. Cardura [package insert]. New York, NY: Pfizer; 2009.
10. Spagnul SJ, Cabral PH, Verndl DO, Glina S. Adrenergic α-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23(3):95-98.
11. Avisrro MU, Fernandez IA, Sánchez AS, García-Pando AC, Arias LM, del Pozo JG. Doxazosin and priapism. J Urol. 2000;163(1): 238.
12. Higgins A, Nash M, Lynch AM. Antidepressant-associated sexual dysfunction: impact, effects, and treatments. Drug Healthc Patient Saf. 2010;2:141-150.
13. Bonnot O, Warot D, Cohen D. Priapism associated with sertraline. J Am Acad Child Adolesc Psychiatry. 2007;46(7):790-791.
14. Mann RA, George AK. Recurrent priapism in a military veteran receiving treatment for PTSD. Mil Med. 2017;182(11):e2014-e2017.
15. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
16. Tulachan P, Chapagain M, Ojha SP, Dhungana S. Escitalopram induced priapism. J Inst Med. 2014;36(1):118-120.
17. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
Selective serotonin reuptake inhibitors are a common first choice medication for anxiety and depression treatment, but health care providers should be aware of priapism as a possible adverse effect.
Selective serotonin reuptake inhibitors are a common first choice medication for anxiety and depression treatment, but health care providers should be aware of priapism as a possible adverse effect.
Case Presentation
An 80-year-old white male was evaluated in a primary care clinic following a recent hospitalization for a suicide attempt. His past medical history included type 2 diabetes mellitus, chronic atrial fibrillation, essential hypertension and hyperlipidemia, and no prior psychiatric illness. Six weeks after his wife died of cancer, the patient attempted suicide by slitting his wrists, which resulted in significant blood loss and tendon damage.
After medical stabilization he was treated at an inpatient psychiatric facility for 10 days. There was no evidence of impaired memory nor psychosis during his hospitalization. He was prescribed doxazosin 1 mg twice daily and finasteride 5 mg daily for obstructive urinary symptoms, along with escitalopram 5 mg daily for depression and continuation of prior medications, including glipizide 10 mg twice daily, simvastatin 20 mg daily, metformin 500 mg twice daily, and lisinopril 20 mg daily. The patient’s estimated glomerular filtration rate was 85 at the time of these events.
He was evaluated by the mental health staff at the time of his primary care outpatient visit and noted to have a Patient Health Questionnaire (PHQ-9) score of 5 (mild depression symptoms) and a Generalized Anxiety Disorder 7 Item Scale (GAD-7) score of 1 (minimum anxiety symptoms). Eleven days later during his counseling appointment, he mentioned to staff that he had experienced a painful erection the day before, which lasted 4 hours. The primary care pharmacist was consulted for review of potential medication triggers. It was noted that there was a low frequency of priapism with both doxazosin and escitalopram, a selective serotonin reuptake inhibitor (SSRI). The provider team felt that the α blocker (doxazosin) was more likely than was the SSRI to cause the reported priapism event. Doxazosin was discontinued, and escitalopram 5 mg daily was maintained. His mood remained stable with no further suicidal ideation.
Eighteen days after discontinuation of doxazosin, the patient experienced a second priapism episode. He reported 2 days later that he experienced a prolonged, painful erection that lasted 4 hours and resolved without intervention. The patient’s mood continued without further suicidal thoughts, his appetite was normal, he had good social support and played cards with friends regularly. At that time, the decision was made to discontinue the escitalopram. The SSRI was felt to be a possible cause of priapism due to the length of time off doxazosin in relation to the second event.
The patient continued to do well 15 months after discontinuation of these medications. Unfortunately, he did not seek medical care during either episode of priapism, but he was felt to be reliable in his report based on a normal mental status exam. He does not have any of the other known risk factors for priapism, suggesting a possible association with his α blocker and SSRI.
Discussion
Priapism is a prolonged, painful erection lasting more than 4 hours and is considered a urologic emergency. It is divided into ischemic and nonischemic types. Ischemic priapism occurs with blood dyscrasias, such as sickle cell disease, thalassemia, leukemia, neurologic conditions affecting the spinal cord, and malignancies of bladder/prostate. The lifetime probability of priapism in patients affected by sickle cell disease is estimated at 29% to 42%.1 Medications associated with priapism include cocaine, ondansetron, antipsychotics, excessive use of erectile dysfunction drugs, and increasingly, antidepressants.2-8
Nonischemic priapism is usually associated with pelvic trauma. Cavernous blood gas obtained at the time of the event can help distinguish between the 2 types. The color of the aspirated blood sample is black in patients with ischemic priapism. Corporal blood gas analysis shows hypoxemia and acidemia. The color of blood is red in patients with nonischemic priapism and shows normal oxygen and pH. Priapism is a urologic emergency requiring aspiration of blood from the cavernous sinus to prevent ischemic tissue damage. At times surgical decompression may be required if aspiration is not successful.
Adrenergic α-blocking agents were developed for treatment of hypertension. They have become popular for management of lower urinary tract symptoms (LUTS) secondary to prostate enlargement. Doxazosin, prazosin, and terazosin are nonuroselective and have a higher risk of cardiac adverse effects (AEs), including dizziness and orthostatic hypotension. Lexicomp lists < 1% incidence of priapism associated with doxazosin.9 The drug is metabolized by CYP3A4 with secondary pathways, including CYP2D6 and 2C9 with a drug half-life of 22 hours. Newer agents (eg, tamsulosin, alfuzosin) are considered more uroselective, targeting the α-1b receptors. The older agents have more effect on the α-1a receptors, which are also present at higher level in the cardiovascular system.10 By blocking sympathetic stimuli responsible for penile detumescence, the nonselective α blockers have a higher propensity to cause priapism. There seems to be a direct correlation between higher doses and increased risk of priapism.11 Our patient was at a relatively low dose (1 mg twice daily) of the nonselective agent doxazosin for treatment of his LUTS.
Primary care providers and psychiatrists treating depression are familiar with common sexual AEs of the SSRI class of medications. Decreased sexual desire and delayed orgasm and ejaculation are all issues that lead patients to discontinue treatment. Although SSRIs are considered first-line treatment for depression, reports indicate that up to 60% of patients with prior normal sexual function started on paroxetine may experience sexual AEs.12 The exact frequency is difficult to estimate due to underreporting of these issues by patients.
A review of the literature for cases of priapism associated with SSRIs shows that often there is more than 1 possible drug trigger, and medications used in combination may be a risk factor. It is hypothesized that SSRI action on 5-HT3 receptors may be responsible for priapism occurring in patients treated with SSRIs.13 One study cites a case of priapism in a veteran being treated with escitalopram, prazosin, and trazodone for posttraumatic stress disorder.14 Trazodone inhibits the neuronal uptake of serotonin and is used to treat depression in addition to off-label use in treatment of insomnia. Trazodone is implicated in cases of priapism via its α-blocking properties. In the aforementioned case, trazodone was initially thought to be the causative agent and was discontinued. The patient had recurrent symptoms at which time his prazosin was discontinued, and he had no further events.
Another case cites citalopram-induced priapism that occurred with an accidental overdose of citalopram 80 mg, when a patient confused his antidepressant with 81-mg aspirin tablets.15 He also had a prior history of priapism while taking trazodone. We found only 1 case listing escitalopram as the probable causative agent of priapism.16 Similar to our patient, that case had no risk factors prior to escitalopram administration. Lexicomp notes < 1% incidence of priapism reported in postmarketing studies.
Our patient had been off doxazosin for 18 days when his second event of priapism occurred. It is less likely given the half-life of doxazosin (t ½ = 22 hours) that the α blocker was the causative agent, though a combination of the 2 agents cannot be excluded as a significant factor. The Naranjo Score is an algorithm for determining the likelihood of whether an adverse drug reaction is due to the drug or other factors.17 Scoring ranks the event as probable, possible, or doubtful. This case scored +3 (+2 = appeared after suspected drug given, +1 = improved when drug discontinued), indicating possible association of escitalopram and priapism.
Conclusion
In view of the frequent use of SSRIs in treatment of depression, it may be prudent to advise patients of this uncommon but serious medication AE. Recent use of α blockers may be a risk factor in combination with SSRI therapy. Patients should be counseled to seek emergency care in the event of prolonged erection when discussing potential AEs of SSRI therapy.
Case Presentation
An 80-year-old white male was evaluated in a primary care clinic following a recent hospitalization for a suicide attempt. His past medical history included type 2 diabetes mellitus, chronic atrial fibrillation, essential hypertension and hyperlipidemia, and no prior psychiatric illness. Six weeks after his wife died of cancer, the patient attempted suicide by slitting his wrists, which resulted in significant blood loss and tendon damage.
After medical stabilization he was treated at an inpatient psychiatric facility for 10 days. There was no evidence of impaired memory nor psychosis during his hospitalization. He was prescribed doxazosin 1 mg twice daily and finasteride 5 mg daily for obstructive urinary symptoms, along with escitalopram 5 mg daily for depression and continuation of prior medications, including glipizide 10 mg twice daily, simvastatin 20 mg daily, metformin 500 mg twice daily, and lisinopril 20 mg daily. The patient’s estimated glomerular filtration rate was 85 at the time of these events.
He was evaluated by the mental health staff at the time of his primary care outpatient visit and noted to have a Patient Health Questionnaire (PHQ-9) score of 5 (mild depression symptoms) and a Generalized Anxiety Disorder 7 Item Scale (GAD-7) score of 1 (minimum anxiety symptoms). Eleven days later during his counseling appointment, he mentioned to staff that he had experienced a painful erection the day before, which lasted 4 hours. The primary care pharmacist was consulted for review of potential medication triggers. It was noted that there was a low frequency of priapism with both doxazosin and escitalopram, a selective serotonin reuptake inhibitor (SSRI). The provider team felt that the α blocker (doxazosin) was more likely than was the SSRI to cause the reported priapism event. Doxazosin was discontinued, and escitalopram 5 mg daily was maintained. His mood remained stable with no further suicidal ideation.
Eighteen days after discontinuation of doxazosin, the patient experienced a second priapism episode. He reported 2 days later that he experienced a prolonged, painful erection that lasted 4 hours and resolved without intervention. The patient’s mood continued without further suicidal thoughts, his appetite was normal, he had good social support and played cards with friends regularly. At that time, the decision was made to discontinue the escitalopram. The SSRI was felt to be a possible cause of priapism due to the length of time off doxazosin in relation to the second event.
The patient continued to do well 15 months after discontinuation of these medications. Unfortunately, he did not seek medical care during either episode of priapism, but he was felt to be reliable in his report based on a normal mental status exam. He does not have any of the other known risk factors for priapism, suggesting a possible association with his α blocker and SSRI.
Discussion
Priapism is a prolonged, painful erection lasting more than 4 hours and is considered a urologic emergency. It is divided into ischemic and nonischemic types. Ischemic priapism occurs with blood dyscrasias, such as sickle cell disease, thalassemia, leukemia, neurologic conditions affecting the spinal cord, and malignancies of bladder/prostate. The lifetime probability of priapism in patients affected by sickle cell disease is estimated at 29% to 42%.1 Medications associated with priapism include cocaine, ondansetron, antipsychotics, excessive use of erectile dysfunction drugs, and increasingly, antidepressants.2-8
Nonischemic priapism is usually associated with pelvic trauma. Cavernous blood gas obtained at the time of the event can help distinguish between the 2 types. The color of the aspirated blood sample is black in patients with ischemic priapism. Corporal blood gas analysis shows hypoxemia and acidemia. The color of blood is red in patients with nonischemic priapism and shows normal oxygen and pH. Priapism is a urologic emergency requiring aspiration of blood from the cavernous sinus to prevent ischemic tissue damage. At times surgical decompression may be required if aspiration is not successful.
Adrenergic α-blocking agents were developed for treatment of hypertension. They have become popular for management of lower urinary tract symptoms (LUTS) secondary to prostate enlargement. Doxazosin, prazosin, and terazosin are nonuroselective and have a higher risk of cardiac adverse effects (AEs), including dizziness and orthostatic hypotension. Lexicomp lists < 1% incidence of priapism associated with doxazosin.9 The drug is metabolized by CYP3A4 with secondary pathways, including CYP2D6 and 2C9 with a drug half-life of 22 hours. Newer agents (eg, tamsulosin, alfuzosin) are considered more uroselective, targeting the α-1b receptors. The older agents have more effect on the α-1a receptors, which are also present at higher level in the cardiovascular system.10 By blocking sympathetic stimuli responsible for penile detumescence, the nonselective α blockers have a higher propensity to cause priapism. There seems to be a direct correlation between higher doses and increased risk of priapism.11 Our patient was at a relatively low dose (1 mg twice daily) of the nonselective agent doxazosin for treatment of his LUTS.
Primary care providers and psychiatrists treating depression are familiar with common sexual AEs of the SSRI class of medications. Decreased sexual desire and delayed orgasm and ejaculation are all issues that lead patients to discontinue treatment. Although SSRIs are considered first-line treatment for depression, reports indicate that up to 60% of patients with prior normal sexual function started on paroxetine may experience sexual AEs.12 The exact frequency is difficult to estimate due to underreporting of these issues by patients.
A review of the literature for cases of priapism associated with SSRIs shows that often there is more than 1 possible drug trigger, and medications used in combination may be a risk factor. It is hypothesized that SSRI action on 5-HT3 receptors may be responsible for priapism occurring in patients treated with SSRIs.13 One study cites a case of priapism in a veteran being treated with escitalopram, prazosin, and trazodone for posttraumatic stress disorder.14 Trazodone inhibits the neuronal uptake of serotonin and is used to treat depression in addition to off-label use in treatment of insomnia. Trazodone is implicated in cases of priapism via its α-blocking properties. In the aforementioned case, trazodone was initially thought to be the causative agent and was discontinued. The patient had recurrent symptoms at which time his prazosin was discontinued, and he had no further events.
Another case cites citalopram-induced priapism that occurred with an accidental overdose of citalopram 80 mg, when a patient confused his antidepressant with 81-mg aspirin tablets.15 He also had a prior history of priapism while taking trazodone. We found only 1 case listing escitalopram as the probable causative agent of priapism.16 Similar to our patient, that case had no risk factors prior to escitalopram administration. Lexicomp notes < 1% incidence of priapism reported in postmarketing studies.
Our patient had been off doxazosin for 18 days when his second event of priapism occurred. It is less likely given the half-life of doxazosin (t ½ = 22 hours) that the α blocker was the causative agent, though a combination of the 2 agents cannot be excluded as a significant factor. The Naranjo Score is an algorithm for determining the likelihood of whether an adverse drug reaction is due to the drug or other factors.17 Scoring ranks the event as probable, possible, or doubtful. This case scored +3 (+2 = appeared after suspected drug given, +1 = improved when drug discontinued), indicating possible association of escitalopram and priapism.
Conclusion
In view of the frequent use of SSRIs in treatment of depression, it may be prudent to advise patients of this uncommon but serious medication AE. Recent use of α blockers may be a risk factor in combination with SSRI therapy. Patients should be counseled to seek emergency care in the event of prolonged erection when discussing potential AEs of SSRI therapy.
1. Manjunath AS, Hofer MD. Urologic emergencies. Med Clin North Am. 2018;102(2):373-385.
2. Altman AL, Seftel AD, Brown SL, et al. Cocaine associated priapism. J Urol. 1999;161(6):1817-1818.
3. Pivot D, Javot L, Swiegot D, et al. Two Cases of recurrent priapism during antineoplastic chemotherapy: think about ondansetron. Therapie. 2013;68(6):409-410.
4. Fu E, Kovach JG, Dubin WR. Priapism associate with antipsychotic medication use: case report. J Clin Pshychopharmacol. 2017;37(4):477-478.
5. Saghafi O, Kao A, Druck J. Recurrent priapism from therapeutic quetiapine. West J Emerg Med, 2014;15(1):114-116.
6. King SH, Hallock M, Strote J, et al. Tadalafil-associated priapism. Urology. 2005;66(2):432.
7. Giuliano F, Jackson G, Montorsi F, et al. Safety of sildenafil citrate: review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract. 2010;64(2):240-255.
8. Bhat IA, Shannon KD, Ara A, et al. Ninety-six hours ordeal of priapism induced by paroxetine: a case report and literature review. Int J Psychiatry Med. 2015;50(3):326-334.
9. Cardura [package insert]. New York, NY: Pfizer; 2009.
10. Spagnul SJ, Cabral PH, Verndl DO, Glina S. Adrenergic α-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23(3):95-98.
11. Avisrro MU, Fernandez IA, Sánchez AS, García-Pando AC, Arias LM, del Pozo JG. Doxazosin and priapism. J Urol. 2000;163(1): 238.
12. Higgins A, Nash M, Lynch AM. Antidepressant-associated sexual dysfunction: impact, effects, and treatments. Drug Healthc Patient Saf. 2010;2:141-150.
13. Bonnot O, Warot D, Cohen D. Priapism associated with sertraline. J Am Acad Child Adolesc Psychiatry. 2007;46(7):790-791.
14. Mann RA, George AK. Recurrent priapism in a military veteran receiving treatment for PTSD. Mil Med. 2017;182(11):e2014-e2017.
15. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
16. Tulachan P, Chapagain M, Ojha SP, Dhungana S. Escitalopram induced priapism. J Inst Med. 2014;36(1):118-120.
17. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
1. Manjunath AS, Hofer MD. Urologic emergencies. Med Clin North Am. 2018;102(2):373-385.
2. Altman AL, Seftel AD, Brown SL, et al. Cocaine associated priapism. J Urol. 1999;161(6):1817-1818.
3. Pivot D, Javot L, Swiegot D, et al. Two Cases of recurrent priapism during antineoplastic chemotherapy: think about ondansetron. Therapie. 2013;68(6):409-410.
4. Fu E, Kovach JG, Dubin WR. Priapism associate with antipsychotic medication use: case report. J Clin Pshychopharmacol. 2017;37(4):477-478.
5. Saghafi O, Kao A, Druck J. Recurrent priapism from therapeutic quetiapine. West J Emerg Med, 2014;15(1):114-116.
6. King SH, Hallock M, Strote J, et al. Tadalafil-associated priapism. Urology. 2005;66(2):432.
7. Giuliano F, Jackson G, Montorsi F, et al. Safety of sildenafil citrate: review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract. 2010;64(2):240-255.
8. Bhat IA, Shannon KD, Ara A, et al. Ninety-six hours ordeal of priapism induced by paroxetine: a case report and literature review. Int J Psychiatry Med. 2015;50(3):326-334.
9. Cardura [package insert]. New York, NY: Pfizer; 2009.
10. Spagnul SJ, Cabral PH, Verndl DO, Glina S. Adrenergic α-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23(3):95-98.
11. Avisrro MU, Fernandez IA, Sánchez AS, García-Pando AC, Arias LM, del Pozo JG. Doxazosin and priapism. J Urol. 2000;163(1): 238.
12. Higgins A, Nash M, Lynch AM. Antidepressant-associated sexual dysfunction: impact, effects, and treatments. Drug Healthc Patient Saf. 2010;2:141-150.
13. Bonnot O, Warot D, Cohen D. Priapism associated with sertraline. J Am Acad Child Adolesc Psychiatry. 2007;46(7):790-791.
14. Mann RA, George AK. Recurrent priapism in a military veteran receiving treatment for PTSD. Mil Med. 2017;182(11):e2014-e2017.
15. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
16. Tulachan P, Chapagain M, Ojha SP, Dhungana S. Escitalopram induced priapism. J Inst Med. 2014;36(1):118-120.
17. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
Clearance of Psoriasis After Ischemic Stroke
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
Practice Points
- Psoriasis is exacerbated in the presence of stress, and psoriatic lesions often have a symmetric distribution, which is evidence that the nervous system is involved in the pathophysiology of the condition.
- Various neuropeptides are involved in the pathophysiology of psoriasis, including substance P, nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide.
- Peripheral nerve damage results in decreased secretion of neuropeptides, which can lead to remission of psoriasis.
Psoriasis Treatment in Patients With Sickle Cell Disease
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Practice Points
• Tumor necrosis factor α contributes both to the vascular inflammatory state seen in sickle cell disease as well as the cycle of inflammation seen in the development of psoriasis.
• Tumor necrosis factor α inhibitors may be the drug of choice for patients with both psoriasis and sickle cell disease.
History of melanoma in situ • dyspnea • rib pain • Dx?
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.

A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).
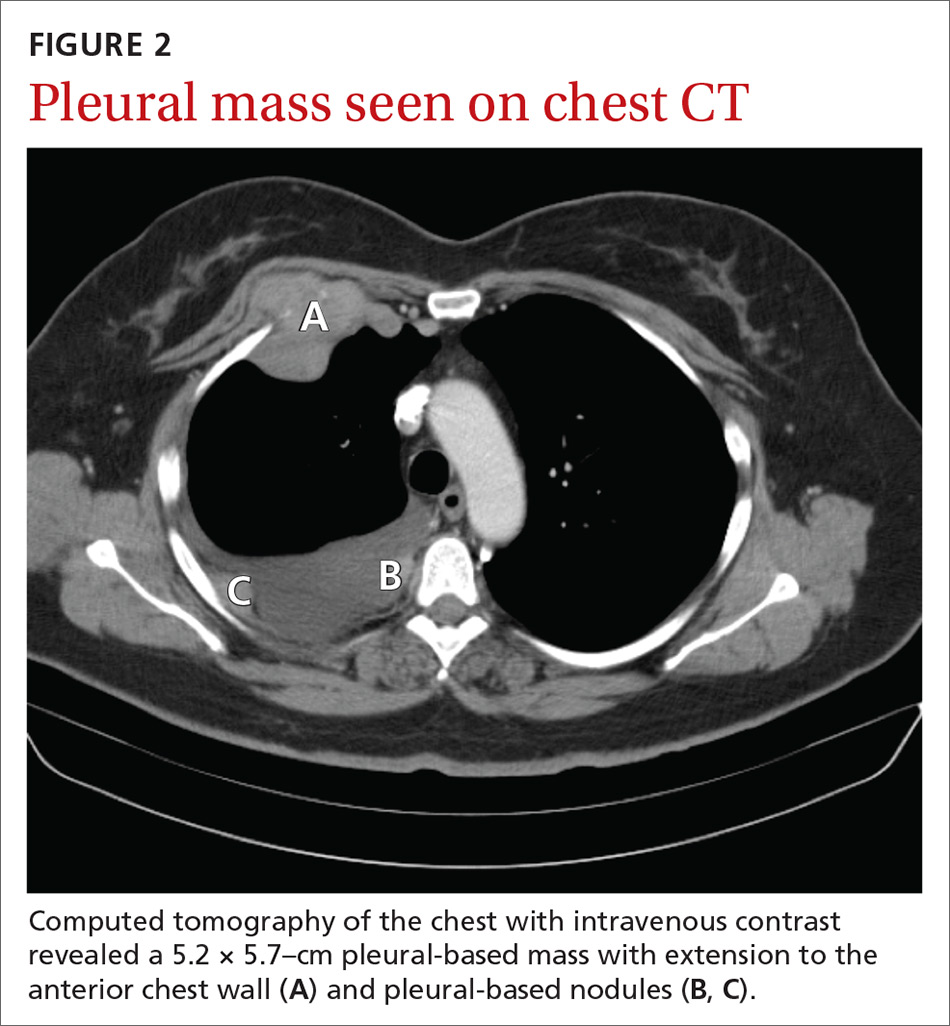
A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.
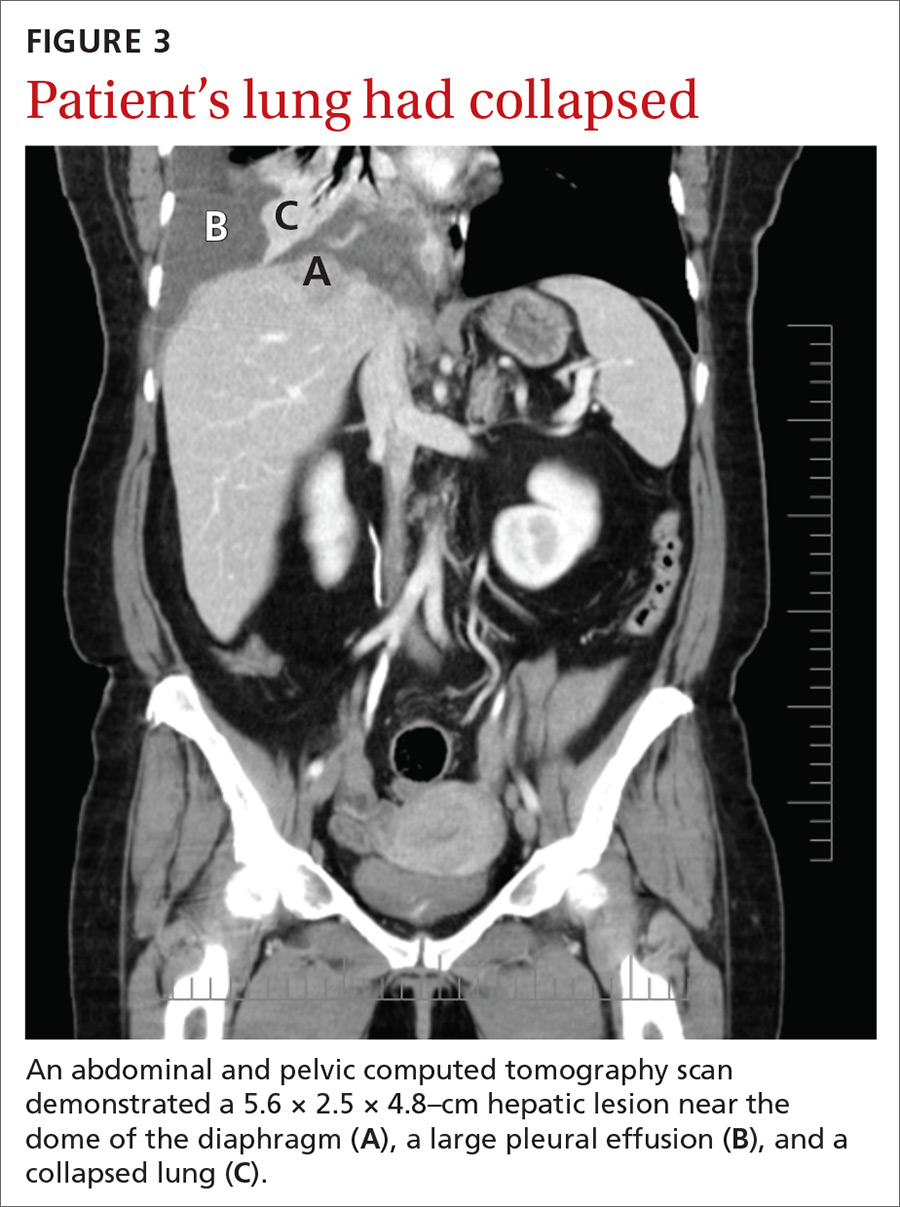
THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; [email protected]
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.

A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).

A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.

THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; [email protected]
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.

A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).

A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.

THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; [email protected]
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
Leg-length discrepancy • asymmetric gluteal folds and popliteal fossae • positive Galeazzi test • Dx?
THE CASE
A healthy 6-month-old girl born via spontaneous vaginal delivery to a 33-year-old mother presented to her family physician (FP) for a routine well-child examination. The mother’s prenatal anatomy scan, delivery, and personal and family history were unremarkable. The patient was not firstborn or breech, and there was no family history of hip dysplasia. On prior infant well-child examinations, Ortolani and Barlow maneuvers were negative, and the patient demonstrated spontaneous movement of both legs. There was no evidence of hip dysplasia, lower extremity weakness, musculoskeletal abnormalities, or abnormal skin markings. The patient had normal growth and development (50th percentile for height and weight, average Ages & Stages Questionnaire scores) and no history of infection or trauma.
At the current presentation, the FP noted a leg-length discrepancy while palpating the bony (patellar and malleolar) landmarks of the lower extremities, but the right and left anterior superior iliac spine was symmetrical. The gluteal folds and popliteal fossae were asymmetric, a Galeazzi test was positive, and the right leg measured approximately 2 cm shorter than the left leg. There was no evidence of scoliosis or pelvic abnormalities. Physical examination revealed no ecchymosis or trauma. Orthopedic evaluation by the FP of the hips, knees, and ankles was normal, including negative repeat Ortolani and Barlow maneuvers and normal range of motion. We obtained x-rays of the lower extremities and ordered an orthopedic consultation.
THE DIAGNOSIS
The differential diagnosis included congenital, traumatic, infectious, inflammatory, idiopathic, and neurologic causes.1-3 The most common etiologies of leg-length discrepancies are summarized in TABLE 1.1-3 Radiographic imaging showed a femur length discrepancy, which was determined to be congenital without indication of trauma or disease; therefore, a diagnosis of congenital femoral bowing was made.
Initial orthopedic evaluation revealed a femur length discrepancy of approximately 2 cm. Plain films showed lateral femoral bowing (FIGURE 1A).
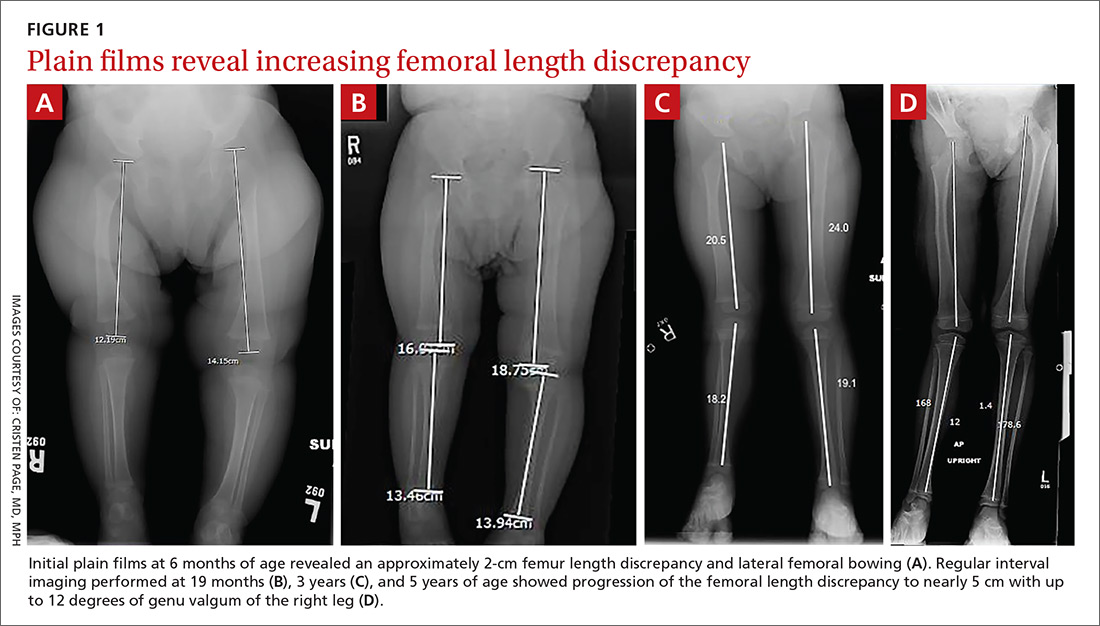
DISCUSSION
Congenital femoral bowing, which can present as a leg-length discrepancy in infants, is a relatively rare finding with an incidence of 1 per 52,000 births.4 Our patient presented with an isolated limb deformity, but congenital femoral bowing is recognized as a clinical feature of several skeletal dysplasias (TABLE 2).5
What’s recommended
The American Academy of Pediatrics recommends routine age-appropriate physical examination without specifying leg-length assessment.6 There is insufficient evidence, according to
Congenital femoral bowing requires plain film diagnosis
Following physical examination, diagnosis of congenital femoral bowing should be confirmed by plain films. Plain radiography remains the main imaging modality for proximal focal femoral deficiency and fibular hemimelia, and appropriate identification of the osseous abnormalities seen on radiographs allows for accurate classification of congenital femoral bowing, prognosis, and surgical planning.
Continue to: Early diagnosis can improve treatment outcome
Early diagnosis can improve treatment outcome
Both early diagnosis of congenital femoral bowing and prediction of leg-length discrepancy at skeletal maturity can influence potential treatment options, which range from conservative management (eg, watchful waiting, physical therapy, shoe lifts, orthotics, bracing) to surgical intervention. Several models have been used to predict skeletal growth, including the Moseley straight line graph, Green and Anderson growth curve, Amstutz method, and Paley’s multiplier method.4,9-14
Intervention for leg-length discrepancy generally is dictated by the magnitude of the inequality and the presence of functional deficits and/or pain.2 If the degree of femur angulation begins to affect structural development, surgical intervention should be considered to align development and/or correct the discrepancy. Physical therapy, shoe lifts, orthotics, and bracing are treatment options for managing smaller discrepancies.2,15
Our patient. The physician (CP) reviewed treatment options with the family that included watchful waiting, use of a shoe lift and/or orthotics, and bracing. The family chose watchful waiting due to the structural integrity of the patient’s other major joints and her relatively preserved function.
Surgery. Ultimately the patient underwent medial distal femoral hemiepiphysiodesis of the right lower extremity at 6 years of age due to increasing leg-length discrepancy and lateralization of the patella from the valgus deformity. The patient’s mother reported that she did well postoperatively, with increased range of motion, improved physical capabilities, and reduced discomfort in the right leg.
A second surgery. At approximately 8 years and 9 months of age, the orthopedist noted that the patient’s leg-length discrepancy had increased, and she had right extensor mechanism malalignment and severe patellar subluxation. The patient subsequently underwent surgery to remove the existing hardware, including right extensor mechanism realignment via a Roux-Goldthwait procedure (with reconstruction of the medial patellofemoral ligament and anterior cruciate ligament), as well as left distal femoral epiphysiodesis. She did very well postoperatively and continues to participate in physical therapy approximately once weekly. She has had an improvement in her gait and stability using shoe lifts.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A routine well-child examination can be an opportunity to identify congenital musculoskeletal problems. Congenital femoral bowing is a relatively rare finding4 that may present as a leg-length discrepancy. With proper evaluation, including visual inspection, palpation, range-of-motion testing, and special tests as needed (eg, Galeazzi test, Ortolani and Barlow maneuvers), early intervention is possible if a leg-length discrepancy is noted. Close monitoring of gait abnormalities at routine well-child visits is essential.
Physical therapy, shoe lift therapy, and surgical approaches are treatment options for leg-length discrepancy,2 and early intervention can improve treatment outcomes.14 Understanding how to manage congenital femoral bowing over time is important in providing options and counselling patients and their families.15
Treatment of leg-length discrepancy in pediatric patients requires long-term management with a team approach that includes patients and their families. The goal of intervention is to reduce physical and emotional trauma, while addressing complications and maintaining function of the affected limb, as well as the whole body.15
CORRESPONDENCE
Beth P. Davis, DPT, MBA, FNAP, Emory University School of Medicine, Department of Rehabilitation Medicine, Division of Physical Therapy, 1462 Clifton Road NE, Suite 312, Atlanta, GA 30342; [email protected].
1. Shailam R, Jaramillo D, Kan JH. Growth arrest and leg-length discrepancy. Pediatr Radiol. 2013:43(suppl 1):S155-S165.
2. Brady RJ, Dean JB, Skinner TM, et al. Limb length inequality: clinical implications for assessment and intervention. J Orthop Sports Phys Ther. 2003;33:221-234.
3. Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7:143-153.
4. Bedoya MA, Chauvin NA, Jaramillo D, et al. Common patterns of congenital lower extremity shortening: diagnosis, classification, and follow-up. Radiographics. 2015;35:1191-1207.
5. Alanay Y, Krakow D, Rimoin DL, et al. Angulated femurs and the skeletal dysplasias: experience of the International Skeletal Dysplasia Registry (1988-2006). Am J Med Genet. 2007;143A:1159-1168.
6. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017.
7. Shipman SA, Hefland M, Moyer VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117:557-576.
8. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117:898-902.
9. Paley D, Bhave A, Herzenberg JE, et al. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A:1432-1446.
10. Castaneda P, Urquhart B, Sullivan E, et al. Hemiepiphysiodesis for the correcting of angular deformity about the knee. J Pediatr Orthop. 2008;28:188-191.
11. Mosley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg Am. 1977; 59:174-179.
12. Anderson M, Messner MB, Green WT. Distribution of lengths of the normal femur and tibia in children from one to eighteen years of age. J Bone Joint Surg Am. 1964;46:1197-1202.
13. Amstutz HC. The morphology, natural history, and treatment of proximal femoral focal deficiency. In: GT Aitken, ed. Proximal Femoral Focal Deficiency: A Congenital Anomaly. Washington, DC: National Academy of Sciences; 1969: 50-76.
14. Amstutz HC. Natural history and treatment of congenital absence of the fibula. J Bone and Joint Surg Am. 1972;54(A):1349.
15. Guidera KJ, Helal AA, Zuern KA. Management of pediatric limb length inequality. Adv Pediatr. 1995;42:501-543.
THE CASE
A healthy 6-month-old girl born via spontaneous vaginal delivery to a 33-year-old mother presented to her family physician (FP) for a routine well-child examination. The mother’s prenatal anatomy scan, delivery, and personal and family history were unremarkable. The patient was not firstborn or breech, and there was no family history of hip dysplasia. On prior infant well-child examinations, Ortolani and Barlow maneuvers were negative, and the patient demonstrated spontaneous movement of both legs. There was no evidence of hip dysplasia, lower extremity weakness, musculoskeletal abnormalities, or abnormal skin markings. The patient had normal growth and development (50th percentile for height and weight, average Ages & Stages Questionnaire scores) and no history of infection or trauma.
At the current presentation, the FP noted a leg-length discrepancy while palpating the bony (patellar and malleolar) landmarks of the lower extremities, but the right and left anterior superior iliac spine was symmetrical. The gluteal folds and popliteal fossae were asymmetric, a Galeazzi test was positive, and the right leg measured approximately 2 cm shorter than the left leg. There was no evidence of scoliosis or pelvic abnormalities. Physical examination revealed no ecchymosis or trauma. Orthopedic evaluation by the FP of the hips, knees, and ankles was normal, including negative repeat Ortolani and Barlow maneuvers and normal range of motion. We obtained x-rays of the lower extremities and ordered an orthopedic consultation.
THE DIAGNOSIS
The differential diagnosis included congenital, traumatic, infectious, inflammatory, idiopathic, and neurologic causes.1-3 The most common etiologies of leg-length discrepancies are summarized in TABLE 1.1-3 Radiographic imaging showed a femur length discrepancy, which was determined to be congenital without indication of trauma or disease; therefore, a diagnosis of congenital femoral bowing was made.

Initial orthopedic evaluation revealed a femur length discrepancy of approximately 2 cm. Plain films showed lateral femoral bowing (FIGURE 1A).

DISCUSSION
Congenital femoral bowing, which can present as a leg-length discrepancy in infants, is a relatively rare finding with an incidence of 1 per 52,000 births.4 Our patient presented with an isolated limb deformity, but congenital femoral bowing is recognized as a clinical feature of several skeletal dysplasias (TABLE 2).5

What’s recommended
The American Academy of Pediatrics recommends routine age-appropriate physical examination without specifying leg-length assessment.6 There is insufficient evidence, according to
Congenital femoral bowing requires plain film diagnosis
Following physical examination, diagnosis of congenital femoral bowing should be confirmed by plain films. Plain radiography remains the main imaging modality for proximal focal femoral deficiency and fibular hemimelia, and appropriate identification of the osseous abnormalities seen on radiographs allows for accurate classification of congenital femoral bowing, prognosis, and surgical planning.
Continue to: Early diagnosis can improve treatment outcome
Early diagnosis can improve treatment outcome
Both early diagnosis of congenital femoral bowing and prediction of leg-length discrepancy at skeletal maturity can influence potential treatment options, which range from conservative management (eg, watchful waiting, physical therapy, shoe lifts, orthotics, bracing) to surgical intervention. Several models have been used to predict skeletal growth, including the Moseley straight line graph, Green and Anderson growth curve, Amstutz method, and Paley’s multiplier method.4,9-14
Intervention for leg-length discrepancy generally is dictated by the magnitude of the inequality and the presence of functional deficits and/or pain.2 If the degree of femur angulation begins to affect structural development, surgical intervention should be considered to align development and/or correct the discrepancy. Physical therapy, shoe lifts, orthotics, and bracing are treatment options for managing smaller discrepancies.2,15
Our patient. The physician (CP) reviewed treatment options with the family that included watchful waiting, use of a shoe lift and/or orthotics, and bracing. The family chose watchful waiting due to the structural integrity of the patient’s other major joints and her relatively preserved function.
Surgery. Ultimately the patient underwent medial distal femoral hemiepiphysiodesis of the right lower extremity at 6 years of age due to increasing leg-length discrepancy and lateralization of the patella from the valgus deformity. The patient’s mother reported that she did well postoperatively, with increased range of motion, improved physical capabilities, and reduced discomfort in the right leg.
A second surgery. At approximately 8 years and 9 months of age, the orthopedist noted that the patient’s leg-length discrepancy had increased, and she had right extensor mechanism malalignment and severe patellar subluxation. The patient subsequently underwent surgery to remove the existing hardware, including right extensor mechanism realignment via a Roux-Goldthwait procedure (with reconstruction of the medial patellofemoral ligament and anterior cruciate ligament), as well as left distal femoral epiphysiodesis. She did very well postoperatively and continues to participate in physical therapy approximately once weekly. She has had an improvement in her gait and stability using shoe lifts.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A routine well-child examination can be an opportunity to identify congenital musculoskeletal problems. Congenital femoral bowing is a relatively rare finding4 that may present as a leg-length discrepancy. With proper evaluation, including visual inspection, palpation, range-of-motion testing, and special tests as needed (eg, Galeazzi test, Ortolani and Barlow maneuvers), early intervention is possible if a leg-length discrepancy is noted. Close monitoring of gait abnormalities at routine well-child visits is essential.
Physical therapy, shoe lift therapy, and surgical approaches are treatment options for leg-length discrepancy,2 and early intervention can improve treatment outcomes.14 Understanding how to manage congenital femoral bowing over time is important in providing options and counselling patients and their families.15
Treatment of leg-length discrepancy in pediatric patients requires long-term management with a team approach that includes patients and their families. The goal of intervention is to reduce physical and emotional trauma, while addressing complications and maintaining function of the affected limb, as well as the whole body.15
CORRESPONDENCE
Beth P. Davis, DPT, MBA, FNAP, Emory University School of Medicine, Department of Rehabilitation Medicine, Division of Physical Therapy, 1462 Clifton Road NE, Suite 312, Atlanta, GA 30342; [email protected].
THE CASE
A healthy 6-month-old girl born via spontaneous vaginal delivery to a 33-year-old mother presented to her family physician (FP) for a routine well-child examination. The mother’s prenatal anatomy scan, delivery, and personal and family history were unremarkable. The patient was not firstborn or breech, and there was no family history of hip dysplasia. On prior infant well-child examinations, Ortolani and Barlow maneuvers were negative, and the patient demonstrated spontaneous movement of both legs. There was no evidence of hip dysplasia, lower extremity weakness, musculoskeletal abnormalities, or abnormal skin markings. The patient had normal growth and development (50th percentile for height and weight, average Ages & Stages Questionnaire scores) and no history of infection or trauma.
At the current presentation, the FP noted a leg-length discrepancy while palpating the bony (patellar and malleolar) landmarks of the lower extremities, but the right and left anterior superior iliac spine was symmetrical. The gluteal folds and popliteal fossae were asymmetric, a Galeazzi test was positive, and the right leg measured approximately 2 cm shorter than the left leg. There was no evidence of scoliosis or pelvic abnormalities. Physical examination revealed no ecchymosis or trauma. Orthopedic evaluation by the FP of the hips, knees, and ankles was normal, including negative repeat Ortolani and Barlow maneuvers and normal range of motion. We obtained x-rays of the lower extremities and ordered an orthopedic consultation.
THE DIAGNOSIS
The differential diagnosis included congenital, traumatic, infectious, inflammatory, idiopathic, and neurologic causes.1-3 The most common etiologies of leg-length discrepancies are summarized in TABLE 1.1-3 Radiographic imaging showed a femur length discrepancy, which was determined to be congenital without indication of trauma or disease; therefore, a diagnosis of congenital femoral bowing was made.

Initial orthopedic evaluation revealed a femur length discrepancy of approximately 2 cm. Plain films showed lateral femoral bowing (FIGURE 1A).

DISCUSSION
Congenital femoral bowing, which can present as a leg-length discrepancy in infants, is a relatively rare finding with an incidence of 1 per 52,000 births.4 Our patient presented with an isolated limb deformity, but congenital femoral bowing is recognized as a clinical feature of several skeletal dysplasias (TABLE 2).5

What’s recommended
The American Academy of Pediatrics recommends routine age-appropriate physical examination without specifying leg-length assessment.6 There is insufficient evidence, according to
Congenital femoral bowing requires plain film diagnosis
Following physical examination, diagnosis of congenital femoral bowing should be confirmed by plain films. Plain radiography remains the main imaging modality for proximal focal femoral deficiency and fibular hemimelia, and appropriate identification of the osseous abnormalities seen on radiographs allows for accurate classification of congenital femoral bowing, prognosis, and surgical planning.
Continue to: Early diagnosis can improve treatment outcome
Early diagnosis can improve treatment outcome
Both early diagnosis of congenital femoral bowing and prediction of leg-length discrepancy at skeletal maturity can influence potential treatment options, which range from conservative management (eg, watchful waiting, physical therapy, shoe lifts, orthotics, bracing) to surgical intervention. Several models have been used to predict skeletal growth, including the Moseley straight line graph, Green and Anderson growth curve, Amstutz method, and Paley’s multiplier method.4,9-14
Intervention for leg-length discrepancy generally is dictated by the magnitude of the inequality and the presence of functional deficits and/or pain.2 If the degree of femur angulation begins to affect structural development, surgical intervention should be considered to align development and/or correct the discrepancy. Physical therapy, shoe lifts, orthotics, and bracing are treatment options for managing smaller discrepancies.2,15
Our patient. The physician (CP) reviewed treatment options with the family that included watchful waiting, use of a shoe lift and/or orthotics, and bracing. The family chose watchful waiting due to the structural integrity of the patient’s other major joints and her relatively preserved function.
Surgery. Ultimately the patient underwent medial distal femoral hemiepiphysiodesis of the right lower extremity at 6 years of age due to increasing leg-length discrepancy and lateralization of the patella from the valgus deformity. The patient’s mother reported that she did well postoperatively, with increased range of motion, improved physical capabilities, and reduced discomfort in the right leg.
A second surgery. At approximately 8 years and 9 months of age, the orthopedist noted that the patient’s leg-length discrepancy had increased, and she had right extensor mechanism malalignment and severe patellar subluxation. The patient subsequently underwent surgery to remove the existing hardware, including right extensor mechanism realignment via a Roux-Goldthwait procedure (with reconstruction of the medial patellofemoral ligament and anterior cruciate ligament), as well as left distal femoral epiphysiodesis. She did very well postoperatively and continues to participate in physical therapy approximately once weekly. She has had an improvement in her gait and stability using shoe lifts.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A routine well-child examination can be an opportunity to identify congenital musculoskeletal problems. Congenital femoral bowing is a relatively rare finding4 that may present as a leg-length discrepancy. With proper evaluation, including visual inspection, palpation, range-of-motion testing, and special tests as needed (eg, Galeazzi test, Ortolani and Barlow maneuvers), early intervention is possible if a leg-length discrepancy is noted. Close monitoring of gait abnormalities at routine well-child visits is essential.
Physical therapy, shoe lift therapy, and surgical approaches are treatment options for leg-length discrepancy,2 and early intervention can improve treatment outcomes.14 Understanding how to manage congenital femoral bowing over time is important in providing options and counselling patients and their families.15
Treatment of leg-length discrepancy in pediatric patients requires long-term management with a team approach that includes patients and their families. The goal of intervention is to reduce physical and emotional trauma, while addressing complications and maintaining function of the affected limb, as well as the whole body.15
CORRESPONDENCE
Beth P. Davis, DPT, MBA, FNAP, Emory University School of Medicine, Department of Rehabilitation Medicine, Division of Physical Therapy, 1462 Clifton Road NE, Suite 312, Atlanta, GA 30342; [email protected].
1. Shailam R, Jaramillo D, Kan JH. Growth arrest and leg-length discrepancy. Pediatr Radiol. 2013:43(suppl 1):S155-S165.
2. Brady RJ, Dean JB, Skinner TM, et al. Limb length inequality: clinical implications for assessment and intervention. J Orthop Sports Phys Ther. 2003;33:221-234.
3. Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7:143-153.
4. Bedoya MA, Chauvin NA, Jaramillo D, et al. Common patterns of congenital lower extremity shortening: diagnosis, classification, and follow-up. Radiographics. 2015;35:1191-1207.
5. Alanay Y, Krakow D, Rimoin DL, et al. Angulated femurs and the skeletal dysplasias: experience of the International Skeletal Dysplasia Registry (1988-2006). Am J Med Genet. 2007;143A:1159-1168.
6. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017.
7. Shipman SA, Hefland M, Moyer VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117:557-576.
8. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117:898-902.
9. Paley D, Bhave A, Herzenberg JE, et al. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A:1432-1446.
10. Castaneda P, Urquhart B, Sullivan E, et al. Hemiepiphysiodesis for the correcting of angular deformity about the knee. J Pediatr Orthop. 2008;28:188-191.
11. Mosley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg Am. 1977; 59:174-179.
12. Anderson M, Messner MB, Green WT. Distribution of lengths of the normal femur and tibia in children from one to eighteen years of age. J Bone Joint Surg Am. 1964;46:1197-1202.
13. Amstutz HC. The morphology, natural history, and treatment of proximal femoral focal deficiency. In: GT Aitken, ed. Proximal Femoral Focal Deficiency: A Congenital Anomaly. Washington, DC: National Academy of Sciences; 1969: 50-76.
14. Amstutz HC. Natural history and treatment of congenital absence of the fibula. J Bone and Joint Surg Am. 1972;54(A):1349.
15. Guidera KJ, Helal AA, Zuern KA. Management of pediatric limb length inequality. Adv Pediatr. 1995;42:501-543.
1. Shailam R, Jaramillo D, Kan JH. Growth arrest and leg-length discrepancy. Pediatr Radiol. 2013:43(suppl 1):S155-S165.
2. Brady RJ, Dean JB, Skinner TM, et al. Limb length inequality: clinical implications for assessment and intervention. J Orthop Sports Phys Ther. 2003;33:221-234.
3. Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7:143-153.
4. Bedoya MA, Chauvin NA, Jaramillo D, et al. Common patterns of congenital lower extremity shortening: diagnosis, classification, and follow-up. Radiographics. 2015;35:1191-1207.
5. Alanay Y, Krakow D, Rimoin DL, et al. Angulated femurs and the skeletal dysplasias: experience of the International Skeletal Dysplasia Registry (1988-2006). Am J Med Genet. 2007;143A:1159-1168.
6. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017.
7. Shipman SA, Hefland M, Moyer VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117:557-576.
8. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117:898-902.
9. Paley D, Bhave A, Herzenberg JE, et al. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A:1432-1446.
10. Castaneda P, Urquhart B, Sullivan E, et al. Hemiepiphysiodesis for the correcting of angular deformity about the knee. J Pediatr Orthop. 2008;28:188-191.
11. Mosley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg Am. 1977; 59:174-179.
12. Anderson M, Messner MB, Green WT. Distribution of lengths of the normal femur and tibia in children from one to eighteen years of age. J Bone Joint Surg Am. 1964;46:1197-1202.
13. Amstutz HC. The morphology, natural history, and treatment of proximal femoral focal deficiency. In: GT Aitken, ed. Proximal Femoral Focal Deficiency: A Congenital Anomaly. Washington, DC: National Academy of Sciences; 1969: 50-76.
14. Amstutz HC. Natural history and treatment of congenital absence of the fibula. J Bone and Joint Surg Am. 1972;54(A):1349.
15. Guidera KJ, Helal AA, Zuern KA. Management of pediatric limb length inequality. Adv Pediatr. 1995;42:501-543.
Pain in right shoulder • recent influenza vaccination • history of hypertension and myocardial infarction • Dx?
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).
The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; [email protected]
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).

The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; [email protected]
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).

The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; [email protected]
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.
Five-day fever • elevated creatinine levels • kidney transplant 10 months prior • Dx?
THE CASE
On examination, the patient appeared to be in mild distress. His vital signs included: temperature 38.5°C, blood pressure 136/94 mm Hg, pulse 89 beats/min, and respiratory rate 18 breaths/min. Cardiopulmonary, abdominal, and genitourinary examinations were unremarkable. A well-healed scar was seen in the right lower quadrant at the site of the renal allograft and was nontender to palpation.
Laboratory values showed a white blood cell (WBC) count of 4.3 × 109/L and an elevated creatinine of 1.16 mg/dL. Six months prior to presentation, his creatinine was 0.98 mg/dL. Blood cultures were obtained, and ceftriaxone (1 g) was given prior to obtaining a urine specimen. A urine dipstick revealed moderate leukocyte esterase, small blood, and 30 mg/dL of protein. Urine microscopy showed >50 WBCs per high power field (hpf), 6-10 red blood cells (RBCs), 30 mg/dL of protein, and an absence of bacteria.
THE DIAGNOSIS
Fever and urinary symptoms in a renal transplant patient may be due to acute graft pyelonephritis (AGP) or acute renal allograft rejection. Initial assessment should be focused on empiric treatment for infection while also evaluating for the possibility of rejection.
Patients with AGP present with lower urinary tract symptoms suggestive of cystitis (frequency, urgency, dysuria, hematuria, suprapubic pain) along with upper urinary tract symptoms (fever, chills, pain at graft site). However, since the kidney graft is denervated, lack of tenderness over graft site does not rule out pyelonephritis.1
This patient was hospitalized and continued on ceftriaxone. Renal ultrasound showed an 11-cm transplanted kidney without hydronephrosis and normal Doppler flow at the anastomotic sites of the renal artery and vein. On hospital Day 2, his urine and blood cultures were negative, but ceftriaxone was continued since it had been given prior to obtaining urine culture. The patient’s tacrolimus level was slightly elevated at 15.6 mcg/L (therapeutic range: 5-15 mcg/L). He also tested negative for chlamydia and gonorrhea; a urine Wright stain was negative for eosinophils.
On hospital Day 4, the patient remained febrile, urinary symptoms persisted, and creatinine increased to 1.5 mg/dL. Tacrolimus was stopped and mycophenolate mofetil dosing was decreased to 500 mg PO bid, then 250 mg PO bid, and then stopped on hospital Day 5. Tacrolimus was reinitated on hospital Day 6 at 1 mg PO bid.
Continue to: Computed tomography (CT) of the abdomen...
Computed tomography (CT) of the abdomen and pelvis without contrast evaluating for a perinephric or renal abscess was negative. Antibiotic coverage was broadened to meropenem 1 g every 8 hours and vancomycin 1500 mg once, with levels to follow. Repeat urinalysis showed persistent pyuria and worsened hematuria and proteinuria. Urine protein to creatinine ratio was elevated at 1.3 mg/mg. Cystoscopy showed a normal urethra and multiple areas of erythema and edema in the bladder, which was consistent with cystitis.
Due to the lack of clinical improvement on broad-spectrum antibiotic coverage, other urinary pathogens, including BK virus, cytomegalovirus (CMV), fungi, and Mycobacterium tuberculosis (MTB), were considered. Serum qualitative polymerase chain reaction (PCR) for BK virus and CMV were negative. Quantitative PCR for BK virus revealed presence of <500 copies/mL of BK virus. Quantiferon gold, urine MTB PCR, and urine fungal culture were negative.
The presence of worsening hematuria raised suspicion for hemorrhagic cystitis due to adenovirus. Urine adenovirus PCR confirmed the diagnosis of AGP due to adenovirus.
DISCUSSION
Acute graft pyelonephritis, also known as pyelonephritis of the renal allograft, can be categorized as early-onset (<6 months after transplant) or late onset (>6 months after transplant). Early-onset AGP is associated with bacteremia, pyelonephritis, and high rate of relapse,1-3 whereas late-onset AGP is associated with increased risk of graft loss.4
In a renal transplant patient, UTIs are usually caused by the same gram-negative bacteria that cause UTIs in patients without a transplant.5 Additionally, Pseudomonas aeruginosa and gram-positive bacteria such as those within the Enterococcus species should be considered. Candida albicans is the most common fungal cause and is associated with urinary obstruction.6
Continue to: Fungal culture...
Fungal culture, CMV PCR, and BK virus PCR should be considered in a patient who does not improve despite appropriate antibiotic coverage. Hematuria should raise concern for BK virus7 and adenovirus. BK virus should be considered when treating patients on high doses of immune suppression, as there is an association between this infection and graft failure.7 Rarely, MTB can cause AGP.8
Empiric antibiotic coverage includes broad-spectrum antibiotics against gram-negative enteric organisms, including Pseudomonas aeruginosa, and gram-positive organisms, including Enterococcus species. Although optimal duration of antibiotics for AGP is unknown, most nephrologists treat graft pyelonephritis due to a bacterial etiology for 10 to 14 days.1 Complications include poor graft outcome and decreased long-term survival.
Adenovirus infection in a renal transplant patient is uncommon and typically presents with hemorrhagic cystitis. In rare cases, this infection can cause disseminated infection. Management is mostly supportive. Reduction of immunosuppression may be associated with viral clearance.9 Cidofovir and intravenous immune globulin may be considered for patients with life-threatening adenovirus infection10; however, there are no large trials that show a clear benefit for patients with AGP due to adenovirus.
Our patient’s urinary symptoms and fever resolved after 1 week of hospitalization with supportive measures and a reduction in immunosuppression, namely a reduction of the dosing of mycophenolate mofetil and tacrolimus. (Optimal changes in the dosing of immunosuppressive agents should be carried out under consultation with a transplant nephrologist.) However, our patient’s creatinine remained elevated at 1.5 mg/dL. Given the low suspicion for graft rejection, biopsy of the kidney transplant was not performed. He returned to the nephrology clinic 3 months later with an improved creatinine of 1.1 mg/dL.
THE TAKEAWAY
Fever and urinary symptoms in a renal transplant patient suggest either graft pyelonephritis or graft rejection. In addition to the usual gram-negative enteric organisms associated with pyelonephritis in a patient with native kidneys, clinicians should consider low-virulence gram-positive organisms, viruses, fungi, and mycobacteria as potential etiologies. The risks and benefits of reducing or discontinuing immunosuppressive medications in the setting of AGP should be discussed with a nephrologist.
CORRESPONDENCE
Pruthul Patel, MD, Los Angeles County + University of Southern California Medical Center, IRD Building, 2020 Zonal Ave, Rm. 115 Los Angeles, CA 90033; [email protected]
1. de Souza RM, Olsburgh J. Urinary tract infection in the renal transplant patient. Nat Clin Pract Nephrol. 2008;4:252-264.
2. Schmaldienst S, Dittrich E, Hörl WH. Urinary tract infections after renal transplantation. Curr Opin Urol. 2002;12:125-130.
3. Brown PD. Urinary tract infections in renal transplant recipients. Curr Infect Dis Rep. 2002;4:525-528.
4. Abbott KC, Swanson SJ, Richter ER, et al. Late urinary tract infection after renal transplantation in the United States. Am J Kidney Dis. 2004;44:353-362.
5. Pellé F, Vimont S, Levy PP, et al. Acute pyelonephritis represents risk factor impairing long-term kidney graft function. Am J Transplant. 2007;7:899-907.
6. Alangaden GJ, Thyagarajan R, Gruber SA, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant. 2006;20:401-409.
7. Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2:25-30.
8. Wagener MM, Yu VL. Bacteremia in transplant recipients: a prospective study of demographics, etiologic agents, risk factors, and outcomes. Am J Infect Control. 1992;20:239-247.
9. Asim M, Chong-Lopez A, Nickeleit V. Adenovirus infection of a renal allograft. Am J Kidney Dis. 2003;41:696-701.
10. Barraclough K, Oliver K, Playford EG, et al. Life-threatening adenovirus infection in a kidney transplant recipient. Clin Kidney J. 2010;3:388-392.
THE CASE
On examination, the patient appeared to be in mild distress. His vital signs included: temperature 38.5°C, blood pressure 136/94 mm Hg, pulse 89 beats/min, and respiratory rate 18 breaths/min. Cardiopulmonary, abdominal, and genitourinary examinations were unremarkable. A well-healed scar was seen in the right lower quadrant at the site of the renal allograft and was nontender to palpation.
Laboratory values showed a white blood cell (WBC) count of 4.3 × 109/L and an elevated creatinine of 1.16 mg/dL. Six months prior to presentation, his creatinine was 0.98 mg/dL. Blood cultures were obtained, and ceftriaxone (1 g) was given prior to obtaining a urine specimen. A urine dipstick revealed moderate leukocyte esterase, small blood, and 30 mg/dL of protein. Urine microscopy showed >50 WBCs per high power field (hpf), 6-10 red blood cells (RBCs), 30 mg/dL of protein, and an absence of bacteria.
THE DIAGNOSIS
Fever and urinary symptoms in a renal transplant patient may be due to acute graft pyelonephritis (AGP) or acute renal allograft rejection. Initial assessment should be focused on empiric treatment for infection while also evaluating for the possibility of rejection.
Patients with AGP present with lower urinary tract symptoms suggestive of cystitis (frequency, urgency, dysuria, hematuria, suprapubic pain) along with upper urinary tract symptoms (fever, chills, pain at graft site). However, since the kidney graft is denervated, lack of tenderness over graft site does not rule out pyelonephritis.1
This patient was hospitalized and continued on ceftriaxone. Renal ultrasound showed an 11-cm transplanted kidney without hydronephrosis and normal Doppler flow at the anastomotic sites of the renal artery and vein. On hospital Day 2, his urine and blood cultures were negative, but ceftriaxone was continued since it had been given prior to obtaining urine culture. The patient’s tacrolimus level was slightly elevated at 15.6 mcg/L (therapeutic range: 5-15 mcg/L). He also tested negative for chlamydia and gonorrhea; a urine Wright stain was negative for eosinophils.
On hospital Day 4, the patient remained febrile, urinary symptoms persisted, and creatinine increased to 1.5 mg/dL. Tacrolimus was stopped and mycophenolate mofetil dosing was decreased to 500 mg PO bid, then 250 mg PO bid, and then stopped on hospital Day 5. Tacrolimus was reinitated on hospital Day 6 at 1 mg PO bid.
Continue to: Computed tomography (CT) of the abdomen...
Computed tomography (CT) of the abdomen and pelvis without contrast evaluating for a perinephric or renal abscess was negative. Antibiotic coverage was broadened to meropenem 1 g every 8 hours and vancomycin 1500 mg once, with levels to follow. Repeat urinalysis showed persistent pyuria and worsened hematuria and proteinuria. Urine protein to creatinine ratio was elevated at 1.3 mg/mg. Cystoscopy showed a normal urethra and multiple areas of erythema and edema in the bladder, which was consistent with cystitis.
Due to the lack of clinical improvement on broad-spectrum antibiotic coverage, other urinary pathogens, including BK virus, cytomegalovirus (CMV), fungi, and Mycobacterium tuberculosis (MTB), were considered. Serum qualitative polymerase chain reaction (PCR) for BK virus and CMV were negative. Quantitative PCR for BK virus revealed presence of <500 copies/mL of BK virus. Quantiferon gold, urine MTB PCR, and urine fungal culture were negative.
The presence of worsening hematuria raised suspicion for hemorrhagic cystitis due to adenovirus. Urine adenovirus PCR confirmed the diagnosis of AGP due to adenovirus.
DISCUSSION
Acute graft pyelonephritis, also known as pyelonephritis of the renal allograft, can be categorized as early-onset (<6 months after transplant) or late onset (>6 months after transplant). Early-onset AGP is associated with bacteremia, pyelonephritis, and high rate of relapse,1-3 whereas late-onset AGP is associated with increased risk of graft loss.4
In a renal transplant patient, UTIs are usually caused by the same gram-negative bacteria that cause UTIs in patients without a transplant.5 Additionally, Pseudomonas aeruginosa and gram-positive bacteria such as those within the Enterococcus species should be considered. Candida albicans is the most common fungal cause and is associated with urinary obstruction.6
Continue to: Fungal culture...
Fungal culture, CMV PCR, and BK virus PCR should be considered in a patient who does not improve despite appropriate antibiotic coverage. Hematuria should raise concern for BK virus7 and adenovirus. BK virus should be considered when treating patients on high doses of immune suppression, as there is an association between this infection and graft failure.7 Rarely, MTB can cause AGP.8
Empiric antibiotic coverage includes broad-spectrum antibiotics against gram-negative enteric organisms, including Pseudomonas aeruginosa, and gram-positive organisms, including Enterococcus species. Although optimal duration of antibiotics for AGP is unknown, most nephrologists treat graft pyelonephritis due to a bacterial etiology for 10 to 14 days.1 Complications include poor graft outcome and decreased long-term survival.
Adenovirus infection in a renal transplant patient is uncommon and typically presents with hemorrhagic cystitis. In rare cases, this infection can cause disseminated infection. Management is mostly supportive. Reduction of immunosuppression may be associated with viral clearance.9 Cidofovir and intravenous immune globulin may be considered for patients with life-threatening adenovirus infection10; however, there are no large trials that show a clear benefit for patients with AGP due to adenovirus.
Our patient’s urinary symptoms and fever resolved after 1 week of hospitalization with supportive measures and a reduction in immunosuppression, namely a reduction of the dosing of mycophenolate mofetil and tacrolimus. (Optimal changes in the dosing of immunosuppressive agents should be carried out under consultation with a transplant nephrologist.) However, our patient’s creatinine remained elevated at 1.5 mg/dL. Given the low suspicion for graft rejection, biopsy of the kidney transplant was not performed. He returned to the nephrology clinic 3 months later with an improved creatinine of 1.1 mg/dL.
THE TAKEAWAY
Fever and urinary symptoms in a renal transplant patient suggest either graft pyelonephritis or graft rejection. In addition to the usual gram-negative enteric organisms associated with pyelonephritis in a patient with native kidneys, clinicians should consider low-virulence gram-positive organisms, viruses, fungi, and mycobacteria as potential etiologies. The risks and benefits of reducing or discontinuing immunosuppressive medications in the setting of AGP should be discussed with a nephrologist.
CORRESPONDENCE
Pruthul Patel, MD, Los Angeles County + University of Southern California Medical Center, IRD Building, 2020 Zonal Ave, Rm. 115 Los Angeles, CA 90033; [email protected]
THE CASE
On examination, the patient appeared to be in mild distress. His vital signs included: temperature 38.5°C, blood pressure 136/94 mm Hg, pulse 89 beats/min, and respiratory rate 18 breaths/min. Cardiopulmonary, abdominal, and genitourinary examinations were unremarkable. A well-healed scar was seen in the right lower quadrant at the site of the renal allograft and was nontender to palpation.
Laboratory values showed a white blood cell (WBC) count of 4.3 × 109/L and an elevated creatinine of 1.16 mg/dL. Six months prior to presentation, his creatinine was 0.98 mg/dL. Blood cultures were obtained, and ceftriaxone (1 g) was given prior to obtaining a urine specimen. A urine dipstick revealed moderate leukocyte esterase, small blood, and 30 mg/dL of protein. Urine microscopy showed >50 WBCs per high power field (hpf), 6-10 red blood cells (RBCs), 30 mg/dL of protein, and an absence of bacteria.
THE DIAGNOSIS
Fever and urinary symptoms in a renal transplant patient may be due to acute graft pyelonephritis (AGP) or acute renal allograft rejection. Initial assessment should be focused on empiric treatment for infection while also evaluating for the possibility of rejection.
Patients with AGP present with lower urinary tract symptoms suggestive of cystitis (frequency, urgency, dysuria, hematuria, suprapubic pain) along with upper urinary tract symptoms (fever, chills, pain at graft site). However, since the kidney graft is denervated, lack of tenderness over graft site does not rule out pyelonephritis.1
This patient was hospitalized and continued on ceftriaxone. Renal ultrasound showed an 11-cm transplanted kidney without hydronephrosis and normal Doppler flow at the anastomotic sites of the renal artery and vein. On hospital Day 2, his urine and blood cultures were negative, but ceftriaxone was continued since it had been given prior to obtaining urine culture. The patient’s tacrolimus level was slightly elevated at 15.6 mcg/L (therapeutic range: 5-15 mcg/L). He also tested negative for chlamydia and gonorrhea; a urine Wright stain was negative for eosinophils.
On hospital Day 4, the patient remained febrile, urinary symptoms persisted, and creatinine increased to 1.5 mg/dL. Tacrolimus was stopped and mycophenolate mofetil dosing was decreased to 500 mg PO bid, then 250 mg PO bid, and then stopped on hospital Day 5. Tacrolimus was reinitated on hospital Day 6 at 1 mg PO bid.
Continue to: Computed tomography (CT) of the abdomen...
Computed tomography (CT) of the abdomen and pelvis without contrast evaluating for a perinephric or renal abscess was negative. Antibiotic coverage was broadened to meropenem 1 g every 8 hours and vancomycin 1500 mg once, with levels to follow. Repeat urinalysis showed persistent pyuria and worsened hematuria and proteinuria. Urine protein to creatinine ratio was elevated at 1.3 mg/mg. Cystoscopy showed a normal urethra and multiple areas of erythema and edema in the bladder, which was consistent with cystitis.
Due to the lack of clinical improvement on broad-spectrum antibiotic coverage, other urinary pathogens, including BK virus, cytomegalovirus (CMV), fungi, and Mycobacterium tuberculosis (MTB), were considered. Serum qualitative polymerase chain reaction (PCR) for BK virus and CMV were negative. Quantitative PCR for BK virus revealed presence of <500 copies/mL of BK virus. Quantiferon gold, urine MTB PCR, and urine fungal culture were negative.
The presence of worsening hematuria raised suspicion for hemorrhagic cystitis due to adenovirus. Urine adenovirus PCR confirmed the diagnosis of AGP due to adenovirus.
DISCUSSION
Acute graft pyelonephritis, also known as pyelonephritis of the renal allograft, can be categorized as early-onset (<6 months after transplant) or late onset (>6 months after transplant). Early-onset AGP is associated with bacteremia, pyelonephritis, and high rate of relapse,1-3 whereas late-onset AGP is associated with increased risk of graft loss.4
In a renal transplant patient, UTIs are usually caused by the same gram-negative bacteria that cause UTIs in patients without a transplant.5 Additionally, Pseudomonas aeruginosa and gram-positive bacteria such as those within the Enterococcus species should be considered. Candida albicans is the most common fungal cause and is associated with urinary obstruction.6
Continue to: Fungal culture...
Fungal culture, CMV PCR, and BK virus PCR should be considered in a patient who does not improve despite appropriate antibiotic coverage. Hematuria should raise concern for BK virus7 and adenovirus. BK virus should be considered when treating patients on high doses of immune suppression, as there is an association between this infection and graft failure.7 Rarely, MTB can cause AGP.8
Empiric antibiotic coverage includes broad-spectrum antibiotics against gram-negative enteric organisms, including Pseudomonas aeruginosa, and gram-positive organisms, including Enterococcus species. Although optimal duration of antibiotics for AGP is unknown, most nephrologists treat graft pyelonephritis due to a bacterial etiology for 10 to 14 days.1 Complications include poor graft outcome and decreased long-term survival.
Adenovirus infection in a renal transplant patient is uncommon and typically presents with hemorrhagic cystitis. In rare cases, this infection can cause disseminated infection. Management is mostly supportive. Reduction of immunosuppression may be associated with viral clearance.9 Cidofovir and intravenous immune globulin may be considered for patients with life-threatening adenovirus infection10; however, there are no large trials that show a clear benefit for patients with AGP due to adenovirus.
Our patient’s urinary symptoms and fever resolved after 1 week of hospitalization with supportive measures and a reduction in immunosuppression, namely a reduction of the dosing of mycophenolate mofetil and tacrolimus. (Optimal changes in the dosing of immunosuppressive agents should be carried out under consultation with a transplant nephrologist.) However, our patient’s creatinine remained elevated at 1.5 mg/dL. Given the low suspicion for graft rejection, biopsy of the kidney transplant was not performed. He returned to the nephrology clinic 3 months later with an improved creatinine of 1.1 mg/dL.
THE TAKEAWAY
Fever and urinary symptoms in a renal transplant patient suggest either graft pyelonephritis or graft rejection. In addition to the usual gram-negative enteric organisms associated with pyelonephritis in a patient with native kidneys, clinicians should consider low-virulence gram-positive organisms, viruses, fungi, and mycobacteria as potential etiologies. The risks and benefits of reducing or discontinuing immunosuppressive medications in the setting of AGP should be discussed with a nephrologist.
CORRESPONDENCE
Pruthul Patel, MD, Los Angeles County + University of Southern California Medical Center, IRD Building, 2020 Zonal Ave, Rm. 115 Los Angeles, CA 90033; [email protected]
1. de Souza RM, Olsburgh J. Urinary tract infection in the renal transplant patient. Nat Clin Pract Nephrol. 2008;4:252-264.
2. Schmaldienst S, Dittrich E, Hörl WH. Urinary tract infections after renal transplantation. Curr Opin Urol. 2002;12:125-130.
3. Brown PD. Urinary tract infections in renal transplant recipients. Curr Infect Dis Rep. 2002;4:525-528.
4. Abbott KC, Swanson SJ, Richter ER, et al. Late urinary tract infection after renal transplantation in the United States. Am J Kidney Dis. 2004;44:353-362.
5. Pellé F, Vimont S, Levy PP, et al. Acute pyelonephritis represents risk factor impairing long-term kidney graft function. Am J Transplant. 2007;7:899-907.
6. Alangaden GJ, Thyagarajan R, Gruber SA, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant. 2006;20:401-409.
7. Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2:25-30.
8. Wagener MM, Yu VL. Bacteremia in transplant recipients: a prospective study of demographics, etiologic agents, risk factors, and outcomes. Am J Infect Control. 1992;20:239-247.
9. Asim M, Chong-Lopez A, Nickeleit V. Adenovirus infection of a renal allograft. Am J Kidney Dis. 2003;41:696-701.
10. Barraclough K, Oliver K, Playford EG, et al. Life-threatening adenovirus infection in a kidney transplant recipient. Clin Kidney J. 2010;3:388-392.
1. de Souza RM, Olsburgh J. Urinary tract infection in the renal transplant patient. Nat Clin Pract Nephrol. 2008;4:252-264.
2. Schmaldienst S, Dittrich E, Hörl WH. Urinary tract infections after renal transplantation. Curr Opin Urol. 2002;12:125-130.
3. Brown PD. Urinary tract infections in renal transplant recipients. Curr Infect Dis Rep. 2002;4:525-528.
4. Abbott KC, Swanson SJ, Richter ER, et al. Late urinary tract infection after renal transplantation in the United States. Am J Kidney Dis. 2004;44:353-362.
5. Pellé F, Vimont S, Levy PP, et al. Acute pyelonephritis represents risk factor impairing long-term kidney graft function. Am J Transplant. 2007;7:899-907.
6. Alangaden GJ, Thyagarajan R, Gruber SA, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant. 2006;20:401-409.
7. Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2:25-30.
8. Wagener MM, Yu VL. Bacteremia in transplant recipients: a prospective study of demographics, etiologic agents, risk factors, and outcomes. Am J Infect Control. 1992;20:239-247.
9. Asim M, Chong-Lopez A, Nickeleit V. Adenovirus infection of a renal allograft. Am J Kidney Dis. 2003;41:696-701.
10. Barraclough K, Oliver K, Playford EG, et al. Life-threatening adenovirus infection in a kidney transplant recipient. Clin Kidney J. 2010;3:388-392.
Glucocorticoid Treatment of Symptomatic Sarcoidosis in 2 Morbidly Obese Patients
Corticosteroid management for patients with sarcoidosis requires the need for close monitoring to detect and manage any complications that may arise during treatment.
Sarcoidosis is a systemic inflammatory condition with pulmonary and extrapulmonary manifestations. The etiology of sarcoidosis remains unknown. Iannuzzi and colleagues hypothesize that an unknown antigen sets off a cycle of chronic granulomatous inflammation in a genetically susceptible host.1
Diagnosis
A diagnosis of sarcoidosis is typically based on a patient having an appropriate clinical presentation and a biopsy, often of lungs or skin, showing noncaseating granulomas.
Symptoms
Of the protean manifestations of sarcoidosis, respiratory symptoms are the most common and typically include subacute or chronic cough and progressive dyspnea on exertion.2 Chest imaging may show only hilar or mediastinal lymphadenopathy, diffuse micronodular lung disease, or signs of chronic inflammation and fibrosis.2 Upper airway involvement and progressive lung disease may lead to increased risk of sleep-disordered breathing, particularly obstructive sleep apnea (OSA).3
Sarcoidosis also can develop in the skin, neurologic system, heart, and other systems. It typically presents as areas of patchy, infiltrative inflammation. In the heart, this can lead to heart failure, often with reduced ejection fraction (EF) and ventricular arrhythmias.1 Pulmonary hypertension (PH) may result from multiple possible mechanisms, including left-heart disease, parenchymal lung disease, sleep-disordered breathing, and possibly direct inflammation and compression of the pulmonary vasculature.2-4
Sarcoidosis in Obese Patients
Emerging evidence shows that sarcoidosis occurs at higher rates in obese patients, suggesting that obesity may be a risk factor for the disease.5-7 Rates of morbid obesity are increasing in the US. From 2000 to 2010, the prevalence of morbid obesity, defined as body mass index (BMI) > 40, increased by 70%, with even larger relative increases in the number of patients with BMI > 50.8 Among veterans who receive health care at the US Department of Veterans Affairs (VA) medical centers, 28% are obese.9 As a result, VA physicians will encounter more patients with morbid obesity and another significant comorbid condition.
Managing symptomatic sarcoidosis in patients with morbid obesity poses a dilemma. Typical treatment for symptomatic pulmonary sarcoidosis is prednisone 20 mg to 40 mg daily.10,11 Higher doses are suggested for involvement of other organs, such as the heart.2,12 Associated weight gain from corticosteroid treatment with possible sleep-disordered breathing increases an already high risk of metabolic complications in morbidly obese patients.13 No clear consensus exists on how corticosteroid doses should be adjusted. We present 2 cases that highlight the complexity of corticosteroid management in the obese sarcoidosis patient.
Case 1: Pulmonary Sarcoidosis
A 43-year-old morbidly obese man presented to his primary care provider with subacute onset of dyspnea. He had a history of OSA that was diagnosed empirically at another institution without polysomnogram and treated with autotitrating continuous positive airway pressure (CPAP).
The patient was admitted for expedited evaluation. His BMI was 63.2 with declining exercise tolerance and hypoxemia on ambulation. His oxyhemoglobin saturation rate was 85% after walking a short distance. Ongoing CPAP therapy for sleep-disordered breathing made laboratory evaluation for obesity hypoventilation syndrome (OHS) challenging. The patient’s serum bicarbonate test result was normal. Serum markers as well as induced sputum stains and cultures were negative for evidence of mycobacterial or fungal infections. A chest radiograph showed bilateral hilar adenopathy and miliary nodularity. Pulmonary function testing revealed severe obstruction and restriction as well as a moderate diffusion impairment. Bronchoscopy with biopsy revealed noncaseating granulomas consistent with sarcoidosis. An electrocardiogram (ECG) was normal. Transthoracic echocardiogram showed evidence of diastolic dysfunction and a mildly dilated right ventricle with normal function, suggestive of possible PH. We were unable to assess his pulmonary artery pressure.
Upon release, the patient began a course of 50 mg (0.24 mg/kg actual body weight) oral prednisone daily and home oxygen.
Six weeks after initiation of steroids, the patient reported that his dyspnea had improved. However, after 6 months of steroid treatment, his weight increased from 462 pounds to 503 pounds. He was evaluated for possible neurosarcoidosis with hypothalamic or pituitary involvement as a possible cause for the weight gain. Brain magnetic resonance imaging and hormonal testing were normal. We considered starting him on a steroid-sparing agent. However, after early efficacy, prednisone was gradually tapered and, after 1 year of treatment, discontinued. At that time, symptoms had substantially improved: His pulmonary function tests had normalized, and he was weaned off oxygen; repeat chest imaging showed only residual enlargement of the hilar lymph nodes. After cessation of steroids, the patient was able to lose 20 pounds.
Case 2: Cardiac Sarcoidosis
A 57-year-old morbidly obese man presented to the emergency department with subacute increasing dyspnea on exertion. He had a known history of sarcoidosis diagnosed by skin biopsy 28 years earlier but had been without treatment for decades. His history also included prediabetes, heart failure with preserved ejection fraction (HFpEF), OSA with an apnea hypopnea index (AHI) of 114.7 per hour, PH diagnosed by prior echocardiogram, and paroxysmal atrial fibrillation (AF). He required 2 L/m home oxygen and bilevel positive airway pressure (PAP) of 22/17 cm H2O while sleeping.
On physical examination, the patient’s BMI was 54.6. He was tachycardic and hypoxemic on his usual oxygen flow rate. His serum bicarbonate, arterial blood pH, and PaCO2 blood levels were normal. We heard bibasilar crackles over the lungs. Chest radiograph revealed an enlarged cardiac silhouette and bilateral infiltrates concerning for cardiogenic pulmonary edema. An echocardiogram showed a restrictive filling pattern with preserved EF and moderate dilation and dysfunction of the right ventricle, consistent with PH. A positron emission tomography (PET)/computed tomography scan, the preferred study for cardiac sarcoidosis, suggested active infiltrative septal cardiac disease and active hilar and mediastinal adenopathy. This was concerning for both cardiac and pulmonary sarcoidosis. Ongoing treatment of sleep-disordered breathing made laboratory assessment for OHS challenging. Given his intact EF, the absence of ventricular arrhythmias, and improvement with diuretics and bilevel PAP, specific treatment of sarcoidosis was not initiated. He was discharged home with a plan to re-evaluate sarcoidosis symptoms and initiate treatment as an outpatient.
The patient was readmitted 2 weeks later with worsened dyspnea, hypoxemia, and volume overload. A right heart catheterization confirmed PH with a mean pulmonary artery pressure of 44 mm Hg (68/32 mm Hg) and pulmonary vascular resistance of 4.6 Wood units. We also found evidence of left-heart dysfunction with a pulmonary capillary wedge pressure of 16 mm Hg.
Given his recurrent symptoms, evidence of active myocardial inflammation on recent PET, and prior biopsy-proven sarcoidosis, we made the decision to pursue treatment for symptomatic sarcoidosis. He began a course of 40 mg (0.20 mg/kg actual body weight) oral prednisone daily. He now required 6 L/m supplemental oxygen. After IV diuretic therapy during his hospitalization, the patient was discharged on his preadmission oral diuretic dose. Pulmonary vasodilator therapy was not initiated for PH as left heart disease and sleep-disordered breathing needed to be managed first.
One month after steroid initiation, the patient reported that the dyspnea and hypoxemia had markedly improved. His oxygen flow rate was reduced to 2 L/m. He remained normotensive and had no further difficulties with fluid retention or volume overload on a stable dose of oral diuretics. He had elevated blood glucose with a glycated hemoglobin (HbA1c) of 6.4%. He began treatment with glipizide 5 mg daily.
After 3 months, he returned to the emergency department with hyperosmolar nonketotic hyperglycemia due to steroid-induced diabetes mellitus (DM). His HbA1c was now 17.1%. The patient was started on a home insulin regimen, and his blood sugar values subsequently improved. He remained symptomatically better and lost 40 pounds with a guided weight management program and a stable diuretic regimen. He underwent arrhythmia evaluation with a Holter monitor that showed AF without ventricular arrhythmias.
Unfortunately, he did not return for cardiac or pulmonary reevaluation, and was lost to follow-up. Nine months after initiation of treatment, the patient died after an out-of-hospital cardiac arrest.
Discussion
These 2 cases highlight therapeutic challenges that may arise in the management of sarcoidosis with symptomatic vital organ involvement and coexistent extreme obesity. Both patients showed symptom improvement with moderate doses of prednisone (40 mg to 50 mg daily), but serious treatment-related complications developed: further weight gain in the first patient, and severe DM in the second. Although DM may have been a direct treatment complication in our second patient, his HFpEF and PH were high-risk comorbidities; he did not present with acute symptomatic worsening after treatment initiation. His symptoms were never reassessed when he was lost to follow-up.
Sarcoidosis/Obesity Relationship
Recent evidence suggests that patients with obesity are at increased risk of developing sarcoidosis.5-7 Although the mechanism of association is unclear, several possibilities have been proposed.
Neurosarcoidosis. One known but rare cause of obesity is neurosarcoidosis of the hypothalamus or pituitary.14 This was investigated in one of our patients.
Proinflammatory responses. Another possible mechanism for the association of sarcoidosis and obesity is the proinflammatory properties of increased fat and adipose tissue.15 Obesity has been linked to an aberrant expansion of inflammatory cells and mediators, including macrophages, proinflammatory cytokines, T cells, and B cells.15 Leptin, produced primarily by adipocytes, also is higher in obese patients and has been found to be proinflammatory.16 These seem to underlie the link between obesity and other inflammatory diseases, including type 2 DM, gout, and atherosclerosis.15
Behavioral link. There also is a possible behavioral link between sarcoidosis and obesity: A patient might develop symptomatic sarcoidosis and later become less active due to dyspnea, which could predispose to weight gain.5
Management of Comorbid Sarcoidosis and Obesity
Regardless of the exact mechanism of this association, management of the co-occurrence of sarcoidosis and obesity poses a clinical problem, especially in cases of extreme obesity. Corticosteroids are generally considered the treatment of choice for symptomatic sarcoidosis. The initial treatment of symptomatic pulmonary sarcoidosis is 20 mg to 40 mg prednisone daily.10,11 Higher daily doses such as 60 mg to 80 mg or 0.5 mg/kg are typically used to treat cardiac sarcoidosis, although no clear consensus exists on the appropriate dose.12,17 One recent study showed no difference in cardiac outcomes in patients treated with high- and low-dose prednisone.18
For patients who are obese and require steroids to treat a medical condition, there is conflicting evidence on whether steroid doses should be increased in proportion to total body weight. Milsap and colleagues found clearance of prednisolone correlated strongly with degree of obesity, suggesting steroid dose should be increased in accordance with actual weight.19 In contrast, Dunn and colleagues found decreased clearance of methylprednisolone in obese patients, suggesting that ideal body weight dosing is appropriate.20
Identifying the appropriate steroid dose is important because corticosteroids place obese patients at higher risk of developing complications. Treatment-related comorbidities include DM, hypertension, fluid retention, osteoporosis, and infection. Further weight gain due to steroid use is a risk for progressive OSA and, even though not generally associated with sarcoidosis alone, OHS. For patients with sarcoidosis, these complications (DM, fluid retention, hypertension, sleep-disordered breathing) may increase the risk of cardiovascular disease and PH.21-23 Cardiomyopathy, especially with reduced EF and increased PH, can be associated with a poor prognosis in sarcoidosis.4,24-26 PH also can be challenging to treat patients with sarcoidosis because the response of PH to steroids is unclear.27 Small trials have shown the benefit of pulmonary vasodilators on hemodynamics, but these have generally been used in patients with stable sarcoidosis who do not have left-heart disease.28-30
Our Prescription Model
We empirically prescribed moderate total doses of prednisone—although low on a mg/kg basis—to balance efficacy and the risk of adverse effects in these 2 morbidly obese patients. We also managed treatment-related complications with guided weight-management programs, CPAP, or noninvasive ventilation for sleep-disordered breathing, and DM treatment.
Our cases demonstrate the need for close monitoring of weight, blood pressure, and blood glucose to detect and treat any complications that may arise during corticosteroid treatment. Aggressive treatment of hyperglycemia with insulin or oral alternatives associated with weight loss such as metformin, sulfonylureas, dipeptidyl peptidase 4 inhibitors, or glucagon-like peptide 1 receptor agonists, may help prevent further DM complications. Sleep-disordered breathing should be assessed and treated. Bariatric surgery may be an option to treat obesity and minimize resultant complications. In our patients, and likely many others, the degree of respiratory and cardiac disease coupled with poor wound healing due to chronic prednisone, may increase the procedural risks.
Conclusion
Our experiences with these patients illustrate that symptomatic and objective improvement in sarcoidosis may be achieved in morbidly obese patients with doses of prednisone that are generally considered moderate, though quite low on a mg/kg basis.
We believe ours is the first report to describe the use of corticosteroids for the treatment of sarcoidosis in patients with morbid obesity. That 2 patients were treated at a single VA medical center within 1-year likely reflects the rising incidence of morbid obesity in the US veteran population and suggests that other federal practitioners might encounter similar patients.
Further study may show that, as an alternative to initial moderate-dose prednisone, patients with symptomatic sarcoidosis and extreme obesity might be started on antimetabolite or antitumor necrosis factor medication or on low-dose prednisone and a second steroid-sparing agent.
1. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165.
2. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2014;383 (9923):1155-1167.
3. Lal C, Medarov BI, Judson MA. Interrelationship between sleep-disordered breathing and sarcoidosis. Chest. 2015;148(4):1105-1114.
4. Dobarro D, Schreiber BE, Handler C, Beynon H, Denton CP, Coghlan JG. Clinical characteristics, haemodynamics and treatment of pulmonary hypertension in sarcoidosis in a single centre, and meta-analysis of the published data. Am J Cardiol. 2013;111(2):278-285.
5. Cozier YC, Coogan PF, Govender P, Berman JS, Palmer JR, Rosenberg L. Obesity and weight gain in relation to incidence of sarcoidosis in US black women: data from the Black Women’s Health Study. Chest. 2015;147(4):1086-1093.
6. Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43(3):843-855.
7. Ungprasert P, Crowson CS, Matteson EL. Smoking, obesity and risk of sarcoidosis: a population-based nested case-control study. Respir Med. 2016;120:87-90.
8. Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond). 2013;37(6):889-891.
9. Nelson KM. The burden of obesity among a national probability sample of veterans. J Gen Intern Med. 2006; 21(9):915-919.
10. Moller DR, Chen ES. Systemic sarcoidosis. In: Grippi MA, Elias JA, Fishman et al, eds. Fishman’s Pulmonary Diseases and Disorders. 5th ed. New York, NY: McGraw-Hill; 2015: 823-841
11. Judson MA, Morgenthau AS, Baughman RP. Sarcoidosis. In: Broaddus VC, Mason RJ, Ernst JD, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:1188-1206.
12. Patel D, Hamzeh NY. Immunosuppressive management of cardiac sarcoidosis. In: Freeman AM, Weinberger HD, eds. Cardiac Sarcoidosis. New York, NY: Springer; 2015:103-112.
13. Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89(3):309-319.
14. Anthony J, Esper GJ, Ioachimescu A. Hypothalamic-pituitary sarcoidosis with vision loss and hypopituitarism: case series and literature review. Pituitary. 2016;19(1):19-29.
15. Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707-712.
16. Matarese G, Leiter EH, La Cava A. Leptin in autoimmunity: many questions, some answers. Tissue Antigens. 2007;70(2):87-95.
17. Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92(2):282-288.
18. Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006-1010.
19. Milsap RL, Plaisance KI, Jusko WJ. Prednisolone disposition in obese men. Clin Pharmacol Ther. 1984;36(6):824-831.
20. Dunn TE, Ludwig EA, Slaughter RL, Camara DS, Jusko WJ. Pharmacokinetics and pharmacodynamics of methylprednisolone in obesity. Clin Pharmacol Ther. 1991;49(5):536-549.
21. Eastwood PR, Malhotra A, Palmer LJ, et al. Obstructive sleep apnoea: from pathogenesis to treatment: current controversies and future directions. Respirology. 2010;15(4):587-595.
22. Wong HS, Williams AJ, Mok Y. The relationship between pulmonary hypertension and obstructive sleep apnea. Curr Opin Pulm Med. 2017;23(6):517-521.
23. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82-93.
24. Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129(5):1246-1252.
25. Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest. 2010;138(5):1078-1085.
26. Birnie DH, Kandolin R, Nery PB, Kupari M. Cardiac manifestations of sarcoidosis: diagnosis and management. Eur Heart J. 2017;38(35):2663-2670.
27. Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61(1):68-74.
28. Judson MA, Highland KB, Kwon S, et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(2):139-145.
29. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest. 2014;145(4):810-817.
30. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26(2):110-120.
Corticosteroid management for patients with sarcoidosis requires the need for close monitoring to detect and manage any complications that may arise during treatment.
Corticosteroid management for patients with sarcoidosis requires the need for close monitoring to detect and manage any complications that may arise during treatment.
Sarcoidosis is a systemic inflammatory condition with pulmonary and extrapulmonary manifestations. The etiology of sarcoidosis remains unknown. Iannuzzi and colleagues hypothesize that an unknown antigen sets off a cycle of chronic granulomatous inflammation in a genetically susceptible host.1
Diagnosis
A diagnosis of sarcoidosis is typically based on a patient having an appropriate clinical presentation and a biopsy, often of lungs or skin, showing noncaseating granulomas.
Symptoms
Of the protean manifestations of sarcoidosis, respiratory symptoms are the most common and typically include subacute or chronic cough and progressive dyspnea on exertion.2 Chest imaging may show only hilar or mediastinal lymphadenopathy, diffuse micronodular lung disease, or signs of chronic inflammation and fibrosis.2 Upper airway involvement and progressive lung disease may lead to increased risk of sleep-disordered breathing, particularly obstructive sleep apnea (OSA).3
Sarcoidosis also can develop in the skin, neurologic system, heart, and other systems. It typically presents as areas of patchy, infiltrative inflammation. In the heart, this can lead to heart failure, often with reduced ejection fraction (EF) and ventricular arrhythmias.1 Pulmonary hypertension (PH) may result from multiple possible mechanisms, including left-heart disease, parenchymal lung disease, sleep-disordered breathing, and possibly direct inflammation and compression of the pulmonary vasculature.2-4
Sarcoidosis in Obese Patients
Emerging evidence shows that sarcoidosis occurs at higher rates in obese patients, suggesting that obesity may be a risk factor for the disease.5-7 Rates of morbid obesity are increasing in the US. From 2000 to 2010, the prevalence of morbid obesity, defined as body mass index (BMI) > 40, increased by 70%, with even larger relative increases in the number of patients with BMI > 50.8 Among veterans who receive health care at the US Department of Veterans Affairs (VA) medical centers, 28% are obese.9 As a result, VA physicians will encounter more patients with morbid obesity and another significant comorbid condition.
Managing symptomatic sarcoidosis in patients with morbid obesity poses a dilemma. Typical treatment for symptomatic pulmonary sarcoidosis is prednisone 20 mg to 40 mg daily.10,11 Higher doses are suggested for involvement of other organs, such as the heart.2,12 Associated weight gain from corticosteroid treatment with possible sleep-disordered breathing increases an already high risk of metabolic complications in morbidly obese patients.13 No clear consensus exists on how corticosteroid doses should be adjusted. We present 2 cases that highlight the complexity of corticosteroid management in the obese sarcoidosis patient.
Case 1: Pulmonary Sarcoidosis
A 43-year-old morbidly obese man presented to his primary care provider with subacute onset of dyspnea. He had a history of OSA that was diagnosed empirically at another institution without polysomnogram and treated with autotitrating continuous positive airway pressure (CPAP).
The patient was admitted for expedited evaluation. His BMI was 63.2 with declining exercise tolerance and hypoxemia on ambulation. His oxyhemoglobin saturation rate was 85% after walking a short distance. Ongoing CPAP therapy for sleep-disordered breathing made laboratory evaluation for obesity hypoventilation syndrome (OHS) challenging. The patient’s serum bicarbonate test result was normal. Serum markers as well as induced sputum stains and cultures were negative for evidence of mycobacterial or fungal infections. A chest radiograph showed bilateral hilar adenopathy and miliary nodularity. Pulmonary function testing revealed severe obstruction and restriction as well as a moderate diffusion impairment. Bronchoscopy with biopsy revealed noncaseating granulomas consistent with sarcoidosis. An electrocardiogram (ECG) was normal. Transthoracic echocardiogram showed evidence of diastolic dysfunction and a mildly dilated right ventricle with normal function, suggestive of possible PH. We were unable to assess his pulmonary artery pressure.
Upon release, the patient began a course of 50 mg (0.24 mg/kg actual body weight) oral prednisone daily and home oxygen.
Six weeks after initiation of steroids, the patient reported that his dyspnea had improved. However, after 6 months of steroid treatment, his weight increased from 462 pounds to 503 pounds. He was evaluated for possible neurosarcoidosis with hypothalamic or pituitary involvement as a possible cause for the weight gain. Brain magnetic resonance imaging and hormonal testing were normal. We considered starting him on a steroid-sparing agent. However, after early efficacy, prednisone was gradually tapered and, after 1 year of treatment, discontinued. At that time, symptoms had substantially improved: His pulmonary function tests had normalized, and he was weaned off oxygen; repeat chest imaging showed only residual enlargement of the hilar lymph nodes. After cessation of steroids, the patient was able to lose 20 pounds.
Case 2: Cardiac Sarcoidosis
A 57-year-old morbidly obese man presented to the emergency department with subacute increasing dyspnea on exertion. He had a known history of sarcoidosis diagnosed by skin biopsy 28 years earlier but had been without treatment for decades. His history also included prediabetes, heart failure with preserved ejection fraction (HFpEF), OSA with an apnea hypopnea index (AHI) of 114.7 per hour, PH diagnosed by prior echocardiogram, and paroxysmal atrial fibrillation (AF). He required 2 L/m home oxygen and bilevel positive airway pressure (PAP) of 22/17 cm H2O while sleeping.
On physical examination, the patient’s BMI was 54.6. He was tachycardic and hypoxemic on his usual oxygen flow rate. His serum bicarbonate, arterial blood pH, and PaCO2 blood levels were normal. We heard bibasilar crackles over the lungs. Chest radiograph revealed an enlarged cardiac silhouette and bilateral infiltrates concerning for cardiogenic pulmonary edema. An echocardiogram showed a restrictive filling pattern with preserved EF and moderate dilation and dysfunction of the right ventricle, consistent with PH. A positron emission tomography (PET)/computed tomography scan, the preferred study for cardiac sarcoidosis, suggested active infiltrative septal cardiac disease and active hilar and mediastinal adenopathy. This was concerning for both cardiac and pulmonary sarcoidosis. Ongoing treatment of sleep-disordered breathing made laboratory assessment for OHS challenging. Given his intact EF, the absence of ventricular arrhythmias, and improvement with diuretics and bilevel PAP, specific treatment of sarcoidosis was not initiated. He was discharged home with a plan to re-evaluate sarcoidosis symptoms and initiate treatment as an outpatient.
The patient was readmitted 2 weeks later with worsened dyspnea, hypoxemia, and volume overload. A right heart catheterization confirmed PH with a mean pulmonary artery pressure of 44 mm Hg (68/32 mm Hg) and pulmonary vascular resistance of 4.6 Wood units. We also found evidence of left-heart dysfunction with a pulmonary capillary wedge pressure of 16 mm Hg.
Given his recurrent symptoms, evidence of active myocardial inflammation on recent PET, and prior biopsy-proven sarcoidosis, we made the decision to pursue treatment for symptomatic sarcoidosis. He began a course of 40 mg (0.20 mg/kg actual body weight) oral prednisone daily. He now required 6 L/m supplemental oxygen. After IV diuretic therapy during his hospitalization, the patient was discharged on his preadmission oral diuretic dose. Pulmonary vasodilator therapy was not initiated for PH as left heart disease and sleep-disordered breathing needed to be managed first.
One month after steroid initiation, the patient reported that the dyspnea and hypoxemia had markedly improved. His oxygen flow rate was reduced to 2 L/m. He remained normotensive and had no further difficulties with fluid retention or volume overload on a stable dose of oral diuretics. He had elevated blood glucose with a glycated hemoglobin (HbA1c) of 6.4%. He began treatment with glipizide 5 mg daily.
After 3 months, he returned to the emergency department with hyperosmolar nonketotic hyperglycemia due to steroid-induced diabetes mellitus (DM). His HbA1c was now 17.1%. The patient was started on a home insulin regimen, and his blood sugar values subsequently improved. He remained symptomatically better and lost 40 pounds with a guided weight management program and a stable diuretic regimen. He underwent arrhythmia evaluation with a Holter monitor that showed AF without ventricular arrhythmias.
Unfortunately, he did not return for cardiac or pulmonary reevaluation, and was lost to follow-up. Nine months after initiation of treatment, the patient died after an out-of-hospital cardiac arrest.
Discussion
These 2 cases highlight therapeutic challenges that may arise in the management of sarcoidosis with symptomatic vital organ involvement and coexistent extreme obesity. Both patients showed symptom improvement with moderate doses of prednisone (40 mg to 50 mg daily), but serious treatment-related complications developed: further weight gain in the first patient, and severe DM in the second. Although DM may have been a direct treatment complication in our second patient, his HFpEF and PH were high-risk comorbidities; he did not present with acute symptomatic worsening after treatment initiation. His symptoms were never reassessed when he was lost to follow-up.
Sarcoidosis/Obesity Relationship
Recent evidence suggests that patients with obesity are at increased risk of developing sarcoidosis.5-7 Although the mechanism of association is unclear, several possibilities have been proposed.
Neurosarcoidosis. One known but rare cause of obesity is neurosarcoidosis of the hypothalamus or pituitary.14 This was investigated in one of our patients.
Proinflammatory responses. Another possible mechanism for the association of sarcoidosis and obesity is the proinflammatory properties of increased fat and adipose tissue.15 Obesity has been linked to an aberrant expansion of inflammatory cells and mediators, including macrophages, proinflammatory cytokines, T cells, and B cells.15 Leptin, produced primarily by adipocytes, also is higher in obese patients and has been found to be proinflammatory.16 These seem to underlie the link between obesity and other inflammatory diseases, including type 2 DM, gout, and atherosclerosis.15
Behavioral link. There also is a possible behavioral link between sarcoidosis and obesity: A patient might develop symptomatic sarcoidosis and later become less active due to dyspnea, which could predispose to weight gain.5
Management of Comorbid Sarcoidosis and Obesity
Regardless of the exact mechanism of this association, management of the co-occurrence of sarcoidosis and obesity poses a clinical problem, especially in cases of extreme obesity. Corticosteroids are generally considered the treatment of choice for symptomatic sarcoidosis. The initial treatment of symptomatic pulmonary sarcoidosis is 20 mg to 40 mg prednisone daily.10,11 Higher daily doses such as 60 mg to 80 mg or 0.5 mg/kg are typically used to treat cardiac sarcoidosis, although no clear consensus exists on the appropriate dose.12,17 One recent study showed no difference in cardiac outcomes in patients treated with high- and low-dose prednisone.18
For patients who are obese and require steroids to treat a medical condition, there is conflicting evidence on whether steroid doses should be increased in proportion to total body weight. Milsap and colleagues found clearance of prednisolone correlated strongly with degree of obesity, suggesting steroid dose should be increased in accordance with actual weight.19 In contrast, Dunn and colleagues found decreased clearance of methylprednisolone in obese patients, suggesting that ideal body weight dosing is appropriate.20
Identifying the appropriate steroid dose is important because corticosteroids place obese patients at higher risk of developing complications. Treatment-related comorbidities include DM, hypertension, fluid retention, osteoporosis, and infection. Further weight gain due to steroid use is a risk for progressive OSA and, even though not generally associated with sarcoidosis alone, OHS. For patients with sarcoidosis, these complications (DM, fluid retention, hypertension, sleep-disordered breathing) may increase the risk of cardiovascular disease and PH.21-23 Cardiomyopathy, especially with reduced EF and increased PH, can be associated with a poor prognosis in sarcoidosis.4,24-26 PH also can be challenging to treat patients with sarcoidosis because the response of PH to steroids is unclear.27 Small trials have shown the benefit of pulmonary vasodilators on hemodynamics, but these have generally been used in patients with stable sarcoidosis who do not have left-heart disease.28-30
Our Prescription Model
We empirically prescribed moderate total doses of prednisone—although low on a mg/kg basis—to balance efficacy and the risk of adverse effects in these 2 morbidly obese patients. We also managed treatment-related complications with guided weight-management programs, CPAP, or noninvasive ventilation for sleep-disordered breathing, and DM treatment.
Our cases demonstrate the need for close monitoring of weight, blood pressure, and blood glucose to detect and treat any complications that may arise during corticosteroid treatment. Aggressive treatment of hyperglycemia with insulin or oral alternatives associated with weight loss such as metformin, sulfonylureas, dipeptidyl peptidase 4 inhibitors, or glucagon-like peptide 1 receptor agonists, may help prevent further DM complications. Sleep-disordered breathing should be assessed and treated. Bariatric surgery may be an option to treat obesity and minimize resultant complications. In our patients, and likely many others, the degree of respiratory and cardiac disease coupled with poor wound healing due to chronic prednisone, may increase the procedural risks.
Conclusion
Our experiences with these patients illustrate that symptomatic and objective improvement in sarcoidosis may be achieved in morbidly obese patients with doses of prednisone that are generally considered moderate, though quite low on a mg/kg basis.
We believe ours is the first report to describe the use of corticosteroids for the treatment of sarcoidosis in patients with morbid obesity. That 2 patients were treated at a single VA medical center within 1-year likely reflects the rising incidence of morbid obesity in the US veteran population and suggests that other federal practitioners might encounter similar patients.
Further study may show that, as an alternative to initial moderate-dose prednisone, patients with symptomatic sarcoidosis and extreme obesity might be started on antimetabolite or antitumor necrosis factor medication or on low-dose prednisone and a second steroid-sparing agent.
Sarcoidosis is a systemic inflammatory condition with pulmonary and extrapulmonary manifestations. The etiology of sarcoidosis remains unknown. Iannuzzi and colleagues hypothesize that an unknown antigen sets off a cycle of chronic granulomatous inflammation in a genetically susceptible host.1
Diagnosis
A diagnosis of sarcoidosis is typically based on a patient having an appropriate clinical presentation and a biopsy, often of lungs or skin, showing noncaseating granulomas.
Symptoms
Of the protean manifestations of sarcoidosis, respiratory symptoms are the most common and typically include subacute or chronic cough and progressive dyspnea on exertion.2 Chest imaging may show only hilar or mediastinal lymphadenopathy, diffuse micronodular lung disease, or signs of chronic inflammation and fibrosis.2 Upper airway involvement and progressive lung disease may lead to increased risk of sleep-disordered breathing, particularly obstructive sleep apnea (OSA).3
Sarcoidosis also can develop in the skin, neurologic system, heart, and other systems. It typically presents as areas of patchy, infiltrative inflammation. In the heart, this can lead to heart failure, often with reduced ejection fraction (EF) and ventricular arrhythmias.1 Pulmonary hypertension (PH) may result from multiple possible mechanisms, including left-heart disease, parenchymal lung disease, sleep-disordered breathing, and possibly direct inflammation and compression of the pulmonary vasculature.2-4
Sarcoidosis in Obese Patients
Emerging evidence shows that sarcoidosis occurs at higher rates in obese patients, suggesting that obesity may be a risk factor for the disease.5-7 Rates of morbid obesity are increasing in the US. From 2000 to 2010, the prevalence of morbid obesity, defined as body mass index (BMI) > 40, increased by 70%, with even larger relative increases in the number of patients with BMI > 50.8 Among veterans who receive health care at the US Department of Veterans Affairs (VA) medical centers, 28% are obese.9 As a result, VA physicians will encounter more patients with morbid obesity and another significant comorbid condition.
Managing symptomatic sarcoidosis in patients with morbid obesity poses a dilemma. Typical treatment for symptomatic pulmonary sarcoidosis is prednisone 20 mg to 40 mg daily.10,11 Higher doses are suggested for involvement of other organs, such as the heart.2,12 Associated weight gain from corticosteroid treatment with possible sleep-disordered breathing increases an already high risk of metabolic complications in morbidly obese patients.13 No clear consensus exists on how corticosteroid doses should be adjusted. We present 2 cases that highlight the complexity of corticosteroid management in the obese sarcoidosis patient.
Case 1: Pulmonary Sarcoidosis
A 43-year-old morbidly obese man presented to his primary care provider with subacute onset of dyspnea. He had a history of OSA that was diagnosed empirically at another institution without polysomnogram and treated with autotitrating continuous positive airway pressure (CPAP).
The patient was admitted for expedited evaluation. His BMI was 63.2 with declining exercise tolerance and hypoxemia on ambulation. His oxyhemoglobin saturation rate was 85% after walking a short distance. Ongoing CPAP therapy for sleep-disordered breathing made laboratory evaluation for obesity hypoventilation syndrome (OHS) challenging. The patient’s serum bicarbonate test result was normal. Serum markers as well as induced sputum stains and cultures were negative for evidence of mycobacterial or fungal infections. A chest radiograph showed bilateral hilar adenopathy and miliary nodularity. Pulmonary function testing revealed severe obstruction and restriction as well as a moderate diffusion impairment. Bronchoscopy with biopsy revealed noncaseating granulomas consistent with sarcoidosis. An electrocardiogram (ECG) was normal. Transthoracic echocardiogram showed evidence of diastolic dysfunction and a mildly dilated right ventricle with normal function, suggestive of possible PH. We were unable to assess his pulmonary artery pressure.
Upon release, the patient began a course of 50 mg (0.24 mg/kg actual body weight) oral prednisone daily and home oxygen.
Six weeks after initiation of steroids, the patient reported that his dyspnea had improved. However, after 6 months of steroid treatment, his weight increased from 462 pounds to 503 pounds. He was evaluated for possible neurosarcoidosis with hypothalamic or pituitary involvement as a possible cause for the weight gain. Brain magnetic resonance imaging and hormonal testing were normal. We considered starting him on a steroid-sparing agent. However, after early efficacy, prednisone was gradually tapered and, after 1 year of treatment, discontinued. At that time, symptoms had substantially improved: His pulmonary function tests had normalized, and he was weaned off oxygen; repeat chest imaging showed only residual enlargement of the hilar lymph nodes. After cessation of steroids, the patient was able to lose 20 pounds.
Case 2: Cardiac Sarcoidosis
A 57-year-old morbidly obese man presented to the emergency department with subacute increasing dyspnea on exertion. He had a known history of sarcoidosis diagnosed by skin biopsy 28 years earlier but had been without treatment for decades. His history also included prediabetes, heart failure with preserved ejection fraction (HFpEF), OSA with an apnea hypopnea index (AHI) of 114.7 per hour, PH diagnosed by prior echocardiogram, and paroxysmal atrial fibrillation (AF). He required 2 L/m home oxygen and bilevel positive airway pressure (PAP) of 22/17 cm H2O while sleeping.
On physical examination, the patient’s BMI was 54.6. He was tachycardic and hypoxemic on his usual oxygen flow rate. His serum bicarbonate, arterial blood pH, and PaCO2 blood levels were normal. We heard bibasilar crackles over the lungs. Chest radiograph revealed an enlarged cardiac silhouette and bilateral infiltrates concerning for cardiogenic pulmonary edema. An echocardiogram showed a restrictive filling pattern with preserved EF and moderate dilation and dysfunction of the right ventricle, consistent with PH. A positron emission tomography (PET)/computed tomography scan, the preferred study for cardiac sarcoidosis, suggested active infiltrative septal cardiac disease and active hilar and mediastinal adenopathy. This was concerning for both cardiac and pulmonary sarcoidosis. Ongoing treatment of sleep-disordered breathing made laboratory assessment for OHS challenging. Given his intact EF, the absence of ventricular arrhythmias, and improvement with diuretics and bilevel PAP, specific treatment of sarcoidosis was not initiated. He was discharged home with a plan to re-evaluate sarcoidosis symptoms and initiate treatment as an outpatient.
The patient was readmitted 2 weeks later with worsened dyspnea, hypoxemia, and volume overload. A right heart catheterization confirmed PH with a mean pulmonary artery pressure of 44 mm Hg (68/32 mm Hg) and pulmonary vascular resistance of 4.6 Wood units. We also found evidence of left-heart dysfunction with a pulmonary capillary wedge pressure of 16 mm Hg.
Given his recurrent symptoms, evidence of active myocardial inflammation on recent PET, and prior biopsy-proven sarcoidosis, we made the decision to pursue treatment for symptomatic sarcoidosis. He began a course of 40 mg (0.20 mg/kg actual body weight) oral prednisone daily. He now required 6 L/m supplemental oxygen. After IV diuretic therapy during his hospitalization, the patient was discharged on his preadmission oral diuretic dose. Pulmonary vasodilator therapy was not initiated for PH as left heart disease and sleep-disordered breathing needed to be managed first.
One month after steroid initiation, the patient reported that the dyspnea and hypoxemia had markedly improved. His oxygen flow rate was reduced to 2 L/m. He remained normotensive and had no further difficulties with fluid retention or volume overload on a stable dose of oral diuretics. He had elevated blood glucose with a glycated hemoglobin (HbA1c) of 6.4%. He began treatment with glipizide 5 mg daily.
After 3 months, he returned to the emergency department with hyperosmolar nonketotic hyperglycemia due to steroid-induced diabetes mellitus (DM). His HbA1c was now 17.1%. The patient was started on a home insulin regimen, and his blood sugar values subsequently improved. He remained symptomatically better and lost 40 pounds with a guided weight management program and a stable diuretic regimen. He underwent arrhythmia evaluation with a Holter monitor that showed AF without ventricular arrhythmias.
Unfortunately, he did not return for cardiac or pulmonary reevaluation, and was lost to follow-up. Nine months after initiation of treatment, the patient died after an out-of-hospital cardiac arrest.
Discussion
These 2 cases highlight therapeutic challenges that may arise in the management of sarcoidosis with symptomatic vital organ involvement and coexistent extreme obesity. Both patients showed symptom improvement with moderate doses of prednisone (40 mg to 50 mg daily), but serious treatment-related complications developed: further weight gain in the first patient, and severe DM in the second. Although DM may have been a direct treatment complication in our second patient, his HFpEF and PH were high-risk comorbidities; he did not present with acute symptomatic worsening after treatment initiation. His symptoms were never reassessed when he was lost to follow-up.
Sarcoidosis/Obesity Relationship
Recent evidence suggests that patients with obesity are at increased risk of developing sarcoidosis.5-7 Although the mechanism of association is unclear, several possibilities have been proposed.
Neurosarcoidosis. One known but rare cause of obesity is neurosarcoidosis of the hypothalamus or pituitary.14 This was investigated in one of our patients.
Proinflammatory responses. Another possible mechanism for the association of sarcoidosis and obesity is the proinflammatory properties of increased fat and adipose tissue.15 Obesity has been linked to an aberrant expansion of inflammatory cells and mediators, including macrophages, proinflammatory cytokines, T cells, and B cells.15 Leptin, produced primarily by adipocytes, also is higher in obese patients and has been found to be proinflammatory.16 These seem to underlie the link between obesity and other inflammatory diseases, including type 2 DM, gout, and atherosclerosis.15
Behavioral link. There also is a possible behavioral link between sarcoidosis and obesity: A patient might develop symptomatic sarcoidosis and later become less active due to dyspnea, which could predispose to weight gain.5
Management of Comorbid Sarcoidosis and Obesity
Regardless of the exact mechanism of this association, management of the co-occurrence of sarcoidosis and obesity poses a clinical problem, especially in cases of extreme obesity. Corticosteroids are generally considered the treatment of choice for symptomatic sarcoidosis. The initial treatment of symptomatic pulmonary sarcoidosis is 20 mg to 40 mg prednisone daily.10,11 Higher daily doses such as 60 mg to 80 mg or 0.5 mg/kg are typically used to treat cardiac sarcoidosis, although no clear consensus exists on the appropriate dose.12,17 One recent study showed no difference in cardiac outcomes in patients treated with high- and low-dose prednisone.18
For patients who are obese and require steroids to treat a medical condition, there is conflicting evidence on whether steroid doses should be increased in proportion to total body weight. Milsap and colleagues found clearance of prednisolone correlated strongly with degree of obesity, suggesting steroid dose should be increased in accordance with actual weight.19 In contrast, Dunn and colleagues found decreased clearance of methylprednisolone in obese patients, suggesting that ideal body weight dosing is appropriate.20
Identifying the appropriate steroid dose is important because corticosteroids place obese patients at higher risk of developing complications. Treatment-related comorbidities include DM, hypertension, fluid retention, osteoporosis, and infection. Further weight gain due to steroid use is a risk for progressive OSA and, even though not generally associated with sarcoidosis alone, OHS. For patients with sarcoidosis, these complications (DM, fluid retention, hypertension, sleep-disordered breathing) may increase the risk of cardiovascular disease and PH.21-23 Cardiomyopathy, especially with reduced EF and increased PH, can be associated with a poor prognosis in sarcoidosis.4,24-26 PH also can be challenging to treat patients with sarcoidosis because the response of PH to steroids is unclear.27 Small trials have shown the benefit of pulmonary vasodilators on hemodynamics, but these have generally been used in patients with stable sarcoidosis who do not have left-heart disease.28-30
Our Prescription Model
We empirically prescribed moderate total doses of prednisone—although low on a mg/kg basis—to balance efficacy and the risk of adverse effects in these 2 morbidly obese patients. We also managed treatment-related complications with guided weight-management programs, CPAP, or noninvasive ventilation for sleep-disordered breathing, and DM treatment.
Our cases demonstrate the need for close monitoring of weight, blood pressure, and blood glucose to detect and treat any complications that may arise during corticosteroid treatment. Aggressive treatment of hyperglycemia with insulin or oral alternatives associated with weight loss such as metformin, sulfonylureas, dipeptidyl peptidase 4 inhibitors, or glucagon-like peptide 1 receptor agonists, may help prevent further DM complications. Sleep-disordered breathing should be assessed and treated. Bariatric surgery may be an option to treat obesity and minimize resultant complications. In our patients, and likely many others, the degree of respiratory and cardiac disease coupled with poor wound healing due to chronic prednisone, may increase the procedural risks.
Conclusion
Our experiences with these patients illustrate that symptomatic and objective improvement in sarcoidosis may be achieved in morbidly obese patients with doses of prednisone that are generally considered moderate, though quite low on a mg/kg basis.
We believe ours is the first report to describe the use of corticosteroids for the treatment of sarcoidosis in patients with morbid obesity. That 2 patients were treated at a single VA medical center within 1-year likely reflects the rising incidence of morbid obesity in the US veteran population and suggests that other federal practitioners might encounter similar patients.
Further study may show that, as an alternative to initial moderate-dose prednisone, patients with symptomatic sarcoidosis and extreme obesity might be started on antimetabolite or antitumor necrosis factor medication or on low-dose prednisone and a second steroid-sparing agent.
1. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165.
2. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2014;383 (9923):1155-1167.
3. Lal C, Medarov BI, Judson MA. Interrelationship between sleep-disordered breathing and sarcoidosis. Chest. 2015;148(4):1105-1114.
4. Dobarro D, Schreiber BE, Handler C, Beynon H, Denton CP, Coghlan JG. Clinical characteristics, haemodynamics and treatment of pulmonary hypertension in sarcoidosis in a single centre, and meta-analysis of the published data. Am J Cardiol. 2013;111(2):278-285.
5. Cozier YC, Coogan PF, Govender P, Berman JS, Palmer JR, Rosenberg L. Obesity and weight gain in relation to incidence of sarcoidosis in US black women: data from the Black Women’s Health Study. Chest. 2015;147(4):1086-1093.
6. Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43(3):843-855.
7. Ungprasert P, Crowson CS, Matteson EL. Smoking, obesity and risk of sarcoidosis: a population-based nested case-control study. Respir Med. 2016;120:87-90.
8. Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond). 2013;37(6):889-891.
9. Nelson KM. The burden of obesity among a national probability sample of veterans. J Gen Intern Med. 2006; 21(9):915-919.
10. Moller DR, Chen ES. Systemic sarcoidosis. In: Grippi MA, Elias JA, Fishman et al, eds. Fishman’s Pulmonary Diseases and Disorders. 5th ed. New York, NY: McGraw-Hill; 2015: 823-841
11. Judson MA, Morgenthau AS, Baughman RP. Sarcoidosis. In: Broaddus VC, Mason RJ, Ernst JD, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:1188-1206.
12. Patel D, Hamzeh NY. Immunosuppressive management of cardiac sarcoidosis. In: Freeman AM, Weinberger HD, eds. Cardiac Sarcoidosis. New York, NY: Springer; 2015:103-112.
13. Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89(3):309-319.
14. Anthony J, Esper GJ, Ioachimescu A. Hypothalamic-pituitary sarcoidosis with vision loss and hypopituitarism: case series and literature review. Pituitary. 2016;19(1):19-29.
15. Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707-712.
16. Matarese G, Leiter EH, La Cava A. Leptin in autoimmunity: many questions, some answers. Tissue Antigens. 2007;70(2):87-95.
17. Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92(2):282-288.
18. Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006-1010.
19. Milsap RL, Plaisance KI, Jusko WJ. Prednisolone disposition in obese men. Clin Pharmacol Ther. 1984;36(6):824-831.
20. Dunn TE, Ludwig EA, Slaughter RL, Camara DS, Jusko WJ. Pharmacokinetics and pharmacodynamics of methylprednisolone in obesity. Clin Pharmacol Ther. 1991;49(5):536-549.
21. Eastwood PR, Malhotra A, Palmer LJ, et al. Obstructive sleep apnoea: from pathogenesis to treatment: current controversies and future directions. Respirology. 2010;15(4):587-595.
22. Wong HS, Williams AJ, Mok Y. The relationship between pulmonary hypertension and obstructive sleep apnea. Curr Opin Pulm Med. 2017;23(6):517-521.
23. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82-93.
24. Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129(5):1246-1252.
25. Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest. 2010;138(5):1078-1085.
26. Birnie DH, Kandolin R, Nery PB, Kupari M. Cardiac manifestations of sarcoidosis: diagnosis and management. Eur Heart J. 2017;38(35):2663-2670.
27. Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61(1):68-74.
28. Judson MA, Highland KB, Kwon S, et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(2):139-145.
29. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest. 2014;145(4):810-817.
30. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26(2):110-120.
1. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165.
2. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2014;383 (9923):1155-1167.
3. Lal C, Medarov BI, Judson MA. Interrelationship between sleep-disordered breathing and sarcoidosis. Chest. 2015;148(4):1105-1114.
4. Dobarro D, Schreiber BE, Handler C, Beynon H, Denton CP, Coghlan JG. Clinical characteristics, haemodynamics and treatment of pulmonary hypertension in sarcoidosis in a single centre, and meta-analysis of the published data. Am J Cardiol. 2013;111(2):278-285.
5. Cozier YC, Coogan PF, Govender P, Berman JS, Palmer JR, Rosenberg L. Obesity and weight gain in relation to incidence of sarcoidosis in US black women: data from the Black Women’s Health Study. Chest. 2015;147(4):1086-1093.
6. Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43(3):843-855.
7. Ungprasert P, Crowson CS, Matteson EL. Smoking, obesity and risk of sarcoidosis: a population-based nested case-control study. Respir Med. 2016;120:87-90.
8. Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond). 2013;37(6):889-891.
9. Nelson KM. The burden of obesity among a national probability sample of veterans. J Gen Intern Med. 2006; 21(9):915-919.
10. Moller DR, Chen ES. Systemic sarcoidosis. In: Grippi MA, Elias JA, Fishman et al, eds. Fishman’s Pulmonary Diseases and Disorders. 5th ed. New York, NY: McGraw-Hill; 2015: 823-841
11. Judson MA, Morgenthau AS, Baughman RP. Sarcoidosis. In: Broaddus VC, Mason RJ, Ernst JD, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:1188-1206.
12. Patel D, Hamzeh NY. Immunosuppressive management of cardiac sarcoidosis. In: Freeman AM, Weinberger HD, eds. Cardiac Sarcoidosis. New York, NY: Springer; 2015:103-112.
13. Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89(3):309-319.
14. Anthony J, Esper GJ, Ioachimescu A. Hypothalamic-pituitary sarcoidosis with vision loss and hypopituitarism: case series and literature review. Pituitary. 2016;19(1):19-29.
15. Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707-712.
16. Matarese G, Leiter EH, La Cava A. Leptin in autoimmunity: many questions, some answers. Tissue Antigens. 2007;70(2):87-95.
17. Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92(2):282-288.
18. Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006-1010.
19. Milsap RL, Plaisance KI, Jusko WJ. Prednisolone disposition in obese men. Clin Pharmacol Ther. 1984;36(6):824-831.
20. Dunn TE, Ludwig EA, Slaughter RL, Camara DS, Jusko WJ. Pharmacokinetics and pharmacodynamics of methylprednisolone in obesity. Clin Pharmacol Ther. 1991;49(5):536-549.
21. Eastwood PR, Malhotra A, Palmer LJ, et al. Obstructive sleep apnoea: from pathogenesis to treatment: current controversies and future directions. Respirology. 2010;15(4):587-595.
22. Wong HS, Williams AJ, Mok Y. The relationship between pulmonary hypertension and obstructive sleep apnea. Curr Opin Pulm Med. 2017;23(6):517-521.
23. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82-93.
24. Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129(5):1246-1252.
25. Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest. 2010;138(5):1078-1085.
26. Birnie DH, Kandolin R, Nery PB, Kupari M. Cardiac manifestations of sarcoidosis: diagnosis and management. Eur Heart J. 2017;38(35):2663-2670.
27. Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61(1):68-74.
28. Judson MA, Highland KB, Kwon S, et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(2):139-145.
29. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest. 2014;145(4):810-817.
30. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26(2):110-120.