User login
Nephrogenic Systemic Fibrosis in a Patient With Multiple Inflammatory Disorders
First described in 2000 in a case series of 15 patients, nephrogenic systemic fibrosis (NSF) is a rare scleroderma-like fibrosing skin condition associated with gadolinium exposure in end stage renal disease (ESRD).1 Patients with advanced chronic kidney disease (CKD) or ESRD are at the highest risk for this condition when exposed to gadolinium-based contrast dyes.
Nephrogenic systemic fibrosis is a devastating and rapidly progressive condition, making its prevention in at-risk populations of utmost importance. In this article, the authors describe a case of a patient who developed NSF in the setting of gadolinium exposure and multiple inflammatory dermatologic conditions. This case illustrates the possible role of a pro-inflammatory state in predisposing to NSF, which may help further elucidate its mechanism of action.
Case Presentation
A 61-year-old Hispanic male with a history of IV heroin use with ESRD secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection presented to the West Los Angeles VAMC with fevers and night sweats that had persisted for 2 weeks. His physical examination was notable for diffuse tender palpable purpura and petechiae (including his palms and soles), altered mental status, and diffuse myoclonic jerks, which necessitated endotracheal intubation and mechanical ventilation for airway protection. Blood cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA). Laboratory results were notable for an elevated sedimentation rate of 53 mm/h (0-10 mm/h), C-reactive protein of 19.8 mg/L (< 0.744 mg/dL), and albumin of 1.2 g/dL (3.2-4.8 g/dL). An extensive rheumatologic workup was unrevealing, and a lumbar puncture was unremarkable. A biopsy of his skin lesions was consistent with leukocytoclastic vasculitis.
The patient’s prior hemodialysis access, a tunneled dialysis catheter in the right subclavian vein, was removed given concern for line infection and replaced with an internal jugular temporary hemodialysis line. Given his altered mental status and myoclonic jerks, the decision was made to pursue a magnetic resonance imaging (MRI) scan of the brain and spine with gadolinium contrast to evaluate for cerebral vasculitis and/or septic emboli to the brain.
The patient received 15 mL of gadoversetamide contrast in accordance with hospital imaging protocol. The MRI revealed only chronic ischemic changes. The patient underwent hemodialysis about 18 hours later. The patient was treated with a 6-week course of IV penicillin G. His altered mental status and myoclonic jerks resolved without intervention, and he was then discharged to an acute rehabilitation unit.
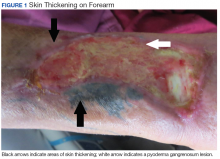
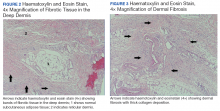
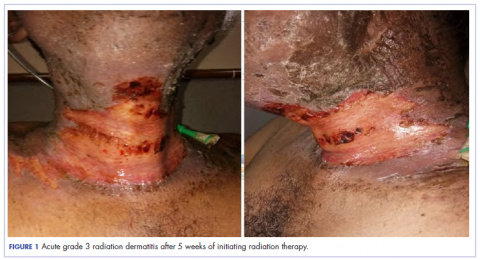
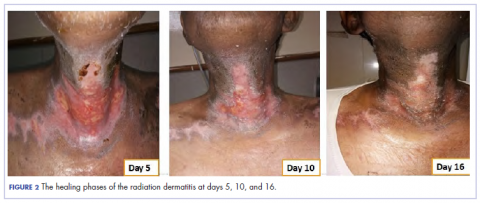
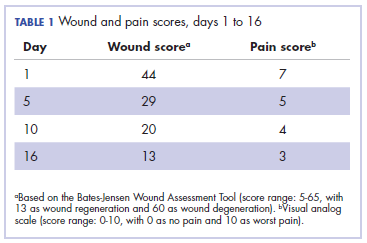
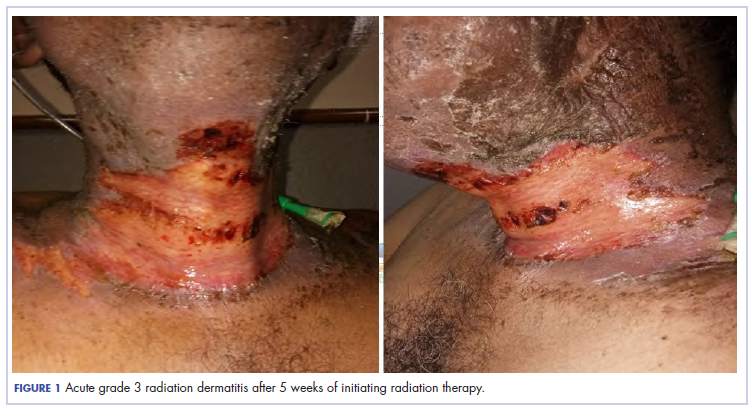
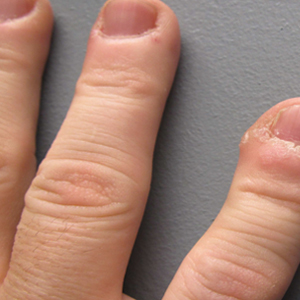
Eight weeks after his initial presentation the patient developed a purulent wound on his right forearm (Figure 1)
The patient was discharged to continue physical and occupational therapy to preserve his functional mobility, as no other treatment options were available.
Discussion
Nephrogenic systemic fibrosis is a poorly understood inflammatory condition that produces diffuse fibrosis of the skin. Typically, the disease begins with progressive skin induration of the extremities. Systemic involvement may occur, leading to fibrosis of skeletal muscle, fascia, and multiple organs. Flexion contractures may develop that limit physical function. Fibrosis can become apparent within days to months after exposure to gadolinium contrast.
Beyond renal insufficiency, it is unclear what other risk factors predispose patients to developing this condition. Only a minority of patients with CKD stages 1 through 4 will develop NSF on exposure to gadolinium contrast. However, the incidence of NSF among patients with CKD stage 5 who are exposed to gadolinium has been estimated to be about 13.4% in a prospective study involving 18 patients.2
In a 2015 meta-analysis by Zhang and colleagues, the only clear risk factor identified for the development of NSF, aside from gadolinium exposure, was severe renal insufficiency with a glomerular filtration rate of < 30 mL/min/1.75m2.3 Due to the limited number of patients identified with this disease, it is difficult to identify other risk factors associated with the development of NSF. Based on in vitro studies, it has been postulated that a pro-inflammatory state predisposes patients to develop NSF.4,5 The proposed mechanism for NSF involves extravasation of gadolinium in the setting of vascular endothelial permeability.5,6 Gadolinium then interacts with tissue macrophages, which induce the release of inflammatory cytokines and the secretion of smooth muscle actin by dermal fibroblasts.6,7
Treatment of NSF has been largely unsuccessful. Multiple modalities of treatment that included topical and oral steroids, immunosuppression, plasmapheresis, and ultraviolent therapy have been attempted, none of which have been proven to consistently limit progression of the disease.8 The most effective intervention is early physical therapy to preserve functionality and prevent contracture formation. For patients who are eligible, early renal transplantation may offer the best chance of improved mobility. In a case series review by Cuffy and colleagues, 5 of 6 patients who underwent renal transplantation after the development of NSF experienced softening of the involved skin, and 2 patients had improved mobility of joints.9
Conclusion
The case presented here illustrates a possible association between a pro-inflammatory state and the development of NSF. This patient had multiple inflammatory conditions, including MSSA bacteremia, leukocytoclastic vasculitis, and pyoderma gangrenosum (the latter 2 conditions were thought to be associated with his underlying chronic hepatitis C infection), which the authors believe predisposed him to endothelial permeability and risk for developing NSF. The risk of developing NSF in at-risk patients with each episode of gadolinium exposure is estimated around 2.4%, or an incidence of 4.3 cases per 1,000 patient-years, leading the American College of Radiologists to recommend against the administration of gadolinium-based contrast except in cases in which benefits clearly outweigh risks.10 However, an MRI with gadolinium contrast can offer high diagnostic yield in cases such as the one presented here in which a diagnosis remains elusive. Moreover, the use of linear gadolinium-based contrast agents such as gadoversetamide, as in this case, has been reported to be associated with higher incidence of NSF.5 Since this case, the West Los Angeles VAMC has switched to gadobutrol contrast for its MRI protocol, which has been purported to be a lower risk agent compared with that of linear gadolinium-based contrast agents (although several cases of NSF have been reported with gadobutrol in the literature).11
Providers weighing the decision to administer gadolinium contrast to patients with ESRD should discuss the risks and benefits thoroughly, especially in patients with preexisting inflammatory conditions. In addition, although it has not been shown to effectively reduce the risk of NSF after administration of gadolinium, hemodialysis is recommended 2 hours after contrast administration for individuals at risk (the study patient received hemodialysis approximately 18 hours after).12 Given the lack of effective treatment options for NSF, prevention is key. A deeper understanding of the pathophysiology of NSF and identification of its risk factors is paramount to the prevention of this devastating disease.
1. Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000-1001.
2. Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis. Arthritis Rheum. 2007;56(10):3433-3441.
3. Zhang B, Liang L, Chen W, Liang C, Zhang S. An updated study to determine association between gadolinium-based contrast agents and nephrogenic systemic fibrosis. PLoS One. 2015;10(6):e0129720.
4. Wermuth PJ, Del Galdo F, Jiménez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508-1518.
5. Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69(7):661-668.
6. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;31(1):F1-F11.
7. Idée JM, Fretellier N, Robic C, Corot C. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Rev Toxicol. 2014;44(10):895-913.
8. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-249.
9. Cuffy MC, Singh M, Formica R, et al. Renal transplantation for nephrogenic systemic fibrosis: a case report and review of the literature. Nephrol Dial Transplant. 2011;26(3):1099-1109.
10. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development of gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267
11. Elmholdt TR, Jørgensen B, Ramsing M, Pedersen M, Olesen AB. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287.
12. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18(3);188-198.
First described in 2000 in a case series of 15 patients, nephrogenic systemic fibrosis (NSF) is a rare scleroderma-like fibrosing skin condition associated with gadolinium exposure in end stage renal disease (ESRD).1 Patients with advanced chronic kidney disease (CKD) or ESRD are at the highest risk for this condition when exposed to gadolinium-based contrast dyes.
Nephrogenic systemic fibrosis is a devastating and rapidly progressive condition, making its prevention in at-risk populations of utmost importance. In this article, the authors describe a case of a patient who developed NSF in the setting of gadolinium exposure and multiple inflammatory dermatologic conditions. This case illustrates the possible role of a pro-inflammatory state in predisposing to NSF, which may help further elucidate its mechanism of action.
Case Presentation
A 61-year-old Hispanic male with a history of IV heroin use with ESRD secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection presented to the West Los Angeles VAMC with fevers and night sweats that had persisted for 2 weeks. His physical examination was notable for diffuse tender palpable purpura and petechiae (including his palms and soles), altered mental status, and diffuse myoclonic jerks, which necessitated endotracheal intubation and mechanical ventilation for airway protection. Blood cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA). Laboratory results were notable for an elevated sedimentation rate of 53 mm/h (0-10 mm/h), C-reactive protein of 19.8 mg/L (< 0.744 mg/dL), and albumin of 1.2 g/dL (3.2-4.8 g/dL). An extensive rheumatologic workup was unrevealing, and a lumbar puncture was unremarkable. A biopsy of his skin lesions was consistent with leukocytoclastic vasculitis.
The patient’s prior hemodialysis access, a tunneled dialysis catheter in the right subclavian vein, was removed given concern for line infection and replaced with an internal jugular temporary hemodialysis line. Given his altered mental status and myoclonic jerks, the decision was made to pursue a magnetic resonance imaging (MRI) scan of the brain and spine with gadolinium contrast to evaluate for cerebral vasculitis and/or septic emboli to the brain.
The patient received 15 mL of gadoversetamide contrast in accordance with hospital imaging protocol. The MRI revealed only chronic ischemic changes. The patient underwent hemodialysis about 18 hours later. The patient was treated with a 6-week course of IV penicillin G. His altered mental status and myoclonic jerks resolved without intervention, and he was then discharged to an acute rehabilitation unit.
Eight weeks after his initial presentation the patient developed a purulent wound on his right forearm (Figure 1)
The patient was discharged to continue physical and occupational therapy to preserve his functional mobility, as no other treatment options were available.
Discussion
Nephrogenic systemic fibrosis is a poorly understood inflammatory condition that produces diffuse fibrosis of the skin. Typically, the disease begins with progressive skin induration of the extremities. Systemic involvement may occur, leading to fibrosis of skeletal muscle, fascia, and multiple organs. Flexion contractures may develop that limit physical function. Fibrosis can become apparent within days to months after exposure to gadolinium contrast.
Beyond renal insufficiency, it is unclear what other risk factors predispose patients to developing this condition. Only a minority of patients with CKD stages 1 through 4 will develop NSF on exposure to gadolinium contrast. However, the incidence of NSF among patients with CKD stage 5 who are exposed to gadolinium has been estimated to be about 13.4% in a prospective study involving 18 patients.2
In a 2015 meta-analysis by Zhang and colleagues, the only clear risk factor identified for the development of NSF, aside from gadolinium exposure, was severe renal insufficiency with a glomerular filtration rate of < 30 mL/min/1.75m2.3 Due to the limited number of patients identified with this disease, it is difficult to identify other risk factors associated with the development of NSF. Based on in vitro studies, it has been postulated that a pro-inflammatory state predisposes patients to develop NSF.4,5 The proposed mechanism for NSF involves extravasation of gadolinium in the setting of vascular endothelial permeability.5,6 Gadolinium then interacts with tissue macrophages, which induce the release of inflammatory cytokines and the secretion of smooth muscle actin by dermal fibroblasts.6,7
Treatment of NSF has been largely unsuccessful. Multiple modalities of treatment that included topical and oral steroids, immunosuppression, plasmapheresis, and ultraviolent therapy have been attempted, none of which have been proven to consistently limit progression of the disease.8 The most effective intervention is early physical therapy to preserve functionality and prevent contracture formation. For patients who are eligible, early renal transplantation may offer the best chance of improved mobility. In a case series review by Cuffy and colleagues, 5 of 6 patients who underwent renal transplantation after the development of NSF experienced softening of the involved skin, and 2 patients had improved mobility of joints.9
Conclusion
The case presented here illustrates a possible association between a pro-inflammatory state and the development of NSF. This patient had multiple inflammatory conditions, including MSSA bacteremia, leukocytoclastic vasculitis, and pyoderma gangrenosum (the latter 2 conditions were thought to be associated with his underlying chronic hepatitis C infection), which the authors believe predisposed him to endothelial permeability and risk for developing NSF. The risk of developing NSF in at-risk patients with each episode of gadolinium exposure is estimated around 2.4%, or an incidence of 4.3 cases per 1,000 patient-years, leading the American College of Radiologists to recommend against the administration of gadolinium-based contrast except in cases in which benefits clearly outweigh risks.10 However, an MRI with gadolinium contrast can offer high diagnostic yield in cases such as the one presented here in which a diagnosis remains elusive. Moreover, the use of linear gadolinium-based contrast agents such as gadoversetamide, as in this case, has been reported to be associated with higher incidence of NSF.5 Since this case, the West Los Angeles VAMC has switched to gadobutrol contrast for its MRI protocol, which has been purported to be a lower risk agent compared with that of linear gadolinium-based contrast agents (although several cases of NSF have been reported with gadobutrol in the literature).11
Providers weighing the decision to administer gadolinium contrast to patients with ESRD should discuss the risks and benefits thoroughly, especially in patients with preexisting inflammatory conditions. In addition, although it has not been shown to effectively reduce the risk of NSF after administration of gadolinium, hemodialysis is recommended 2 hours after contrast administration for individuals at risk (the study patient received hemodialysis approximately 18 hours after).12 Given the lack of effective treatment options for NSF, prevention is key. A deeper understanding of the pathophysiology of NSF and identification of its risk factors is paramount to the prevention of this devastating disease.
First described in 2000 in a case series of 15 patients, nephrogenic systemic fibrosis (NSF) is a rare scleroderma-like fibrosing skin condition associated with gadolinium exposure in end stage renal disease (ESRD).1 Patients with advanced chronic kidney disease (CKD) or ESRD are at the highest risk for this condition when exposed to gadolinium-based contrast dyes.
Nephrogenic systemic fibrosis is a devastating and rapidly progressive condition, making its prevention in at-risk populations of utmost importance. In this article, the authors describe a case of a patient who developed NSF in the setting of gadolinium exposure and multiple inflammatory dermatologic conditions. This case illustrates the possible role of a pro-inflammatory state in predisposing to NSF, which may help further elucidate its mechanism of action.
Case Presentation
A 61-year-old Hispanic male with a history of IV heroin use with ESRD secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection presented to the West Los Angeles VAMC with fevers and night sweats that had persisted for 2 weeks. His physical examination was notable for diffuse tender palpable purpura and petechiae (including his palms and soles), altered mental status, and diffuse myoclonic jerks, which necessitated endotracheal intubation and mechanical ventilation for airway protection. Blood cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA). Laboratory results were notable for an elevated sedimentation rate of 53 mm/h (0-10 mm/h), C-reactive protein of 19.8 mg/L (< 0.744 mg/dL), and albumin of 1.2 g/dL (3.2-4.8 g/dL). An extensive rheumatologic workup was unrevealing, and a lumbar puncture was unremarkable. A biopsy of his skin lesions was consistent with leukocytoclastic vasculitis.
The patient’s prior hemodialysis access, a tunneled dialysis catheter in the right subclavian vein, was removed given concern for line infection and replaced with an internal jugular temporary hemodialysis line. Given his altered mental status and myoclonic jerks, the decision was made to pursue a magnetic resonance imaging (MRI) scan of the brain and spine with gadolinium contrast to evaluate for cerebral vasculitis and/or septic emboli to the brain.
The patient received 15 mL of gadoversetamide contrast in accordance with hospital imaging protocol. The MRI revealed only chronic ischemic changes. The patient underwent hemodialysis about 18 hours later. The patient was treated with a 6-week course of IV penicillin G. His altered mental status and myoclonic jerks resolved without intervention, and he was then discharged to an acute rehabilitation unit.
Eight weeks after his initial presentation the patient developed a purulent wound on his right forearm (Figure 1)
The patient was discharged to continue physical and occupational therapy to preserve his functional mobility, as no other treatment options were available.
Discussion
Nephrogenic systemic fibrosis is a poorly understood inflammatory condition that produces diffuse fibrosis of the skin. Typically, the disease begins with progressive skin induration of the extremities. Systemic involvement may occur, leading to fibrosis of skeletal muscle, fascia, and multiple organs. Flexion contractures may develop that limit physical function. Fibrosis can become apparent within days to months after exposure to gadolinium contrast.
Beyond renal insufficiency, it is unclear what other risk factors predispose patients to developing this condition. Only a minority of patients with CKD stages 1 through 4 will develop NSF on exposure to gadolinium contrast. However, the incidence of NSF among patients with CKD stage 5 who are exposed to gadolinium has been estimated to be about 13.4% in a prospective study involving 18 patients.2
In a 2015 meta-analysis by Zhang and colleagues, the only clear risk factor identified for the development of NSF, aside from gadolinium exposure, was severe renal insufficiency with a glomerular filtration rate of < 30 mL/min/1.75m2.3 Due to the limited number of patients identified with this disease, it is difficult to identify other risk factors associated with the development of NSF. Based on in vitro studies, it has been postulated that a pro-inflammatory state predisposes patients to develop NSF.4,5 The proposed mechanism for NSF involves extravasation of gadolinium in the setting of vascular endothelial permeability.5,6 Gadolinium then interacts with tissue macrophages, which induce the release of inflammatory cytokines and the secretion of smooth muscle actin by dermal fibroblasts.6,7
Treatment of NSF has been largely unsuccessful. Multiple modalities of treatment that included topical and oral steroids, immunosuppression, plasmapheresis, and ultraviolent therapy have been attempted, none of which have been proven to consistently limit progression of the disease.8 The most effective intervention is early physical therapy to preserve functionality and prevent contracture formation. For patients who are eligible, early renal transplantation may offer the best chance of improved mobility. In a case series review by Cuffy and colleagues, 5 of 6 patients who underwent renal transplantation after the development of NSF experienced softening of the involved skin, and 2 patients had improved mobility of joints.9
Conclusion
The case presented here illustrates a possible association between a pro-inflammatory state and the development of NSF. This patient had multiple inflammatory conditions, including MSSA bacteremia, leukocytoclastic vasculitis, and pyoderma gangrenosum (the latter 2 conditions were thought to be associated with his underlying chronic hepatitis C infection), which the authors believe predisposed him to endothelial permeability and risk for developing NSF. The risk of developing NSF in at-risk patients with each episode of gadolinium exposure is estimated around 2.4%, or an incidence of 4.3 cases per 1,000 patient-years, leading the American College of Radiologists to recommend against the administration of gadolinium-based contrast except in cases in which benefits clearly outweigh risks.10 However, an MRI with gadolinium contrast can offer high diagnostic yield in cases such as the one presented here in which a diagnosis remains elusive. Moreover, the use of linear gadolinium-based contrast agents such as gadoversetamide, as in this case, has been reported to be associated with higher incidence of NSF.5 Since this case, the West Los Angeles VAMC has switched to gadobutrol contrast for its MRI protocol, which has been purported to be a lower risk agent compared with that of linear gadolinium-based contrast agents (although several cases of NSF have been reported with gadobutrol in the literature).11
Providers weighing the decision to administer gadolinium contrast to patients with ESRD should discuss the risks and benefits thoroughly, especially in patients with preexisting inflammatory conditions. In addition, although it has not been shown to effectively reduce the risk of NSF after administration of gadolinium, hemodialysis is recommended 2 hours after contrast administration for individuals at risk (the study patient received hemodialysis approximately 18 hours after).12 Given the lack of effective treatment options for NSF, prevention is key. A deeper understanding of the pathophysiology of NSF and identification of its risk factors is paramount to the prevention of this devastating disease.
1. Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000-1001.
2. Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis. Arthritis Rheum. 2007;56(10):3433-3441.
3. Zhang B, Liang L, Chen W, Liang C, Zhang S. An updated study to determine association between gadolinium-based contrast agents and nephrogenic systemic fibrosis. PLoS One. 2015;10(6):e0129720.
4. Wermuth PJ, Del Galdo F, Jiménez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508-1518.
5. Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69(7):661-668.
6. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;31(1):F1-F11.
7. Idée JM, Fretellier N, Robic C, Corot C. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Rev Toxicol. 2014;44(10):895-913.
8. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-249.
9. Cuffy MC, Singh M, Formica R, et al. Renal transplantation for nephrogenic systemic fibrosis: a case report and review of the literature. Nephrol Dial Transplant. 2011;26(3):1099-1109.
10. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development of gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267
11. Elmholdt TR, Jørgensen B, Ramsing M, Pedersen M, Olesen AB. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287.
12. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18(3);188-198.
1. Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000-1001.
2. Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis. Arthritis Rheum. 2007;56(10):3433-3441.
3. Zhang B, Liang L, Chen W, Liang C, Zhang S. An updated study to determine association between gadolinium-based contrast agents and nephrogenic systemic fibrosis. PLoS One. 2015;10(6):e0129720.
4. Wermuth PJ, Del Galdo F, Jiménez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508-1518.
5. Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69(7):661-668.
6. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;31(1):F1-F11.
7. Idée JM, Fretellier N, Robic C, Corot C. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Rev Toxicol. 2014;44(10):895-913.
8. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-249.
9. Cuffy MC, Singh M, Formica R, et al. Renal transplantation for nephrogenic systemic fibrosis: a case report and review of the literature. Nephrol Dial Transplant. 2011;26(3):1099-1109.
10. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development of gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267
11. Elmholdt TR, Jørgensen B, Ramsing M, Pedersen M, Olesen AB. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287.
12. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18(3);188-198.
Recurrence of a small gastric gastrointestinal stromal tumor with high mitotic index
Gastrointestinal stromal tumor (GIST) is the most common soft tissue sarcoma of the gastrointestinal tract, usually arising from the interstitial cells of Cajal or similar cells in the outer wall of the gastrointestinal tract.1,2 Most GISTs have an activating mutation in KIT or platelet-derived growth factor receptor alpha (PDGFRA). Tumor size, mitotic rate, and anatomic site are the most common pathological features used to risk stratify GIST tumors.3-10 It is important to note when using such risk calculators that preoperative imatinib before determining tumor characteristics (such as mitoses per 50 high-power fields [hpf]) often changes the relevant parameters so that the same risk calculations may not apply. Tumors with a mitotic rate ≤5 mitoses per 50 hpf and a size ≤5 cm in greatest dimension have a lower recurrence rate after resection than tumors with a mitotic rate >5 mitoses per 50 hpf and a size >10 cm, and larger tumors can have a recurrence rate of up to 86%.11,12 Findings from a large observational study have suggested that the prognosis of gastric GIST in Korea and Japan may be more favorable compared with that in Western countries.13
The primary treatment of a localized primary GIST is surgical excision, but a cure is limited by recurrence.14,15 Imatinib is useful in the treatment of metastatic or recurrent GIST, and adjuvant treatment with imatinib after surgery has been shown to improve progression-free and overall survival in some cases.3,16-18 Responses to adjuvant imatinib depend on tumor sensitivity to the drug and the risk of recurrence. Drug sensitivity is largely dependent on the presence of mutations in KIT or PDGFRA.3,18 Recurrence risk is highly dependent on tumor size, tumor site, tumor rupture, and mitotic index.1,3,5,6,8,9,18,19 Findings on the use of gene expression patterns to predict recurrence risk have also been reported.20-27 However, recurrence risk is poorly understood for categories in which there are few cases with known outcomes, such as very small gastric GIST with a high mitotic index. For example, few cases of gastric GIST have been reported with a tumor size ≤2 cm, a mitotic rate >5 mitoses per 50 hpf, and adequate clinical follow-up. In such cases, it is difficult to assess the risk of recurrence.6 We report here the long-term outcome of a patient with a 1.8 cm gastric GIST with a mitotic index of 36 mitoses per 50 hpf and a KIT exon 11 mutation.
Case presentation and summary
A 69-year-old man presented with periumbilical and epigastric pain of 6-month duration. His medical history was notable for hyperlipidemia, hypertension, coronary angioplasty, and spinal surgery. He had a 40 pack-year smoking history and consumed 2 to 4 alcoholic drinks per day. The results of a physical examination were unremarkable. A computedtomographic (CT) scan showed no abnormalities. An esophagoduodenoscopy (EGD) revealed gastric ulcers. He was treated successfully with omeprazole 20 mg by mouth daily.
A month later, a follow-up EGD revealed a 1.8 × 1.5 cm submucosal mass 3 cm from the gastroesophageal junction. The patient underwent a fundus wedge resection, and a submucosal mass 1.8 cm in greatest dimension was removed. Pathologic examination revealed a GIST, spindle cell type, with a mitotic rate of 36 mitoses per 50 hpf with negative margins. Immunohistochemistry was positive for CD117. An exon 11 deletion (KVV558-560NV) was present in KIT. The patient’s risk of recurrence was unclear, and his follow-up included CT scans of the abdomen and pelvis every 3 to 4 months for the first 2 years, then every 6 months for the next 2.5 years.
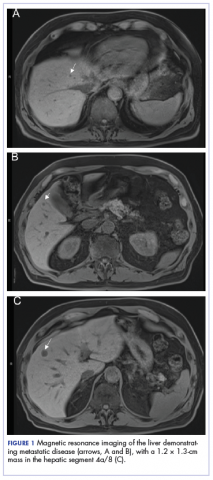
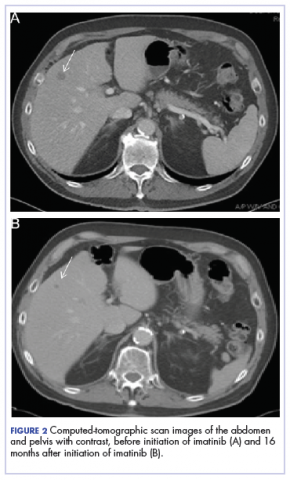
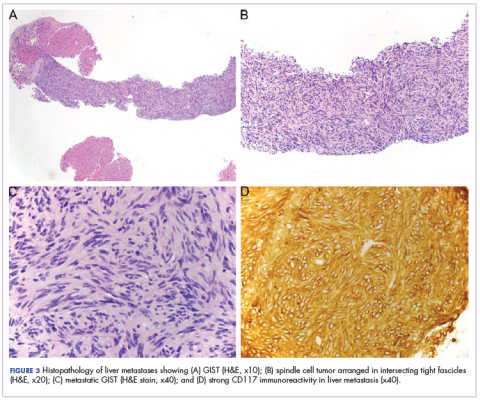
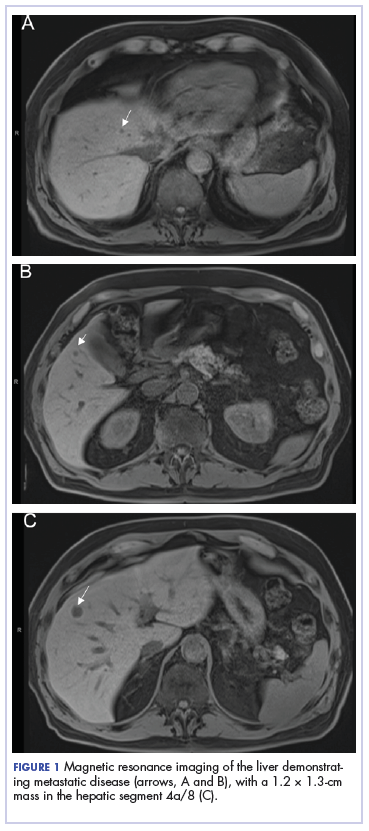
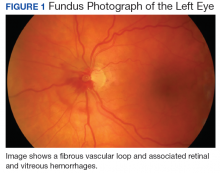
A CT scan about 3.5 years after primary resection revealed small nonspecific liver hypodensities that became more prominent during the next year. About 5 years after primary resection, magnetic resonance imaging (MRI) revealed several liver lesions, the largest of which measured at 1.3 cm in greatest dimension. The patient’s liver metastases were readily identified by MRI (Figure 1) and CT imaging (Figure 2A).
Discussion
Small gastric GISTs are sometimes found by endoscopy performed for unrelated reasons. Recent data suggest that the incidence of gastric GIST may be higher than previously thought. In a Japanese study of patients with gastric cancer in which 100 stomachs were systematically examined pathologically, 50 microscopic GISTs were found in 35 patients.28 Most small gastric GISTs have a low mitotic index. Few cases have been described with a high mitotic index. In a study of 1765 cases of GIST of the stomach, 8 patients had a tumor size less than 2 cm and a mitotic index greater than 5. Of those, only 6 patients had long-term follow-up, and 3 were alive without disease at 2, 17, and 20 years of follow-up.7 These limited data make it impossible to predict outcomes in patients with small gastric GIST with a high mitotic index.
For patients who are at high risk of recurrence after surgery, 3 years of adjuvant imatinib treatment compared with 1 year has been shown to improve overall survival and is the current standard of care.10,17 A study comparing 5 and 3 years of imatinib is ongoing to establish whether a longer period of adjuvant treatment is warranted. In patients with metastatic GIST, lifelong imatinib until lack of benefit is considered optimal treatment.10 All patients should undergo KIT mutation analysis. Those with the PDGFRA D842V mutation, SDH (succinate dehydrogenase) deficiency, or neurofibromatosis-related GIST should not receive adjuvant imatinib.
This case has several unusual features. The small tumor size with a very high mitotic rate is rare. Such cases have not been reported in large numbers and have therefore not been reliably incorporated into risk prediction algorithms. In addition, despite a high mitotic index, the tumor was not FDG avid on PET imaging. The diagnosis of GIST is strongly supported by the KIT mutation and response to imatinib. This particular KIT mutation in larger GISTs is associated with aggressive disease. The present case adds to the data on the biology of small gastric GISTs with a high mitotic index and suggests the mitotic index in these tumors may be a more important predictor than size.
Acknowledgment
The authors thank Michael Franklin, MS, for editorial assistance, and Sabrina Porter for media edits.
1. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865-878.
2. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-580.
3. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563-1570.
4. Huang J, Zheng DL, Qin FS, et al. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120(1):223-241.
5. Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265-274.
6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466-1478.
7. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52-68.
8. Patel S. Navigating risk stratification systems for the management of patients with GIST. Ann Surg Oncol. 2011;18(6):1698-1704.
9. Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35(11):1646-1656.
10. National Comprehensive Cancer Network. Sarcoma. https://www.nccn.org. Accessed March 27, 2018.
11. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81-89.
12. Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141(6):748-756.
13. Kim MC, Yook JH, Yang HK, et al. Long-term surgical outcome of 1057 gastric GISTs according to 7th UICC/AJCC TNM system: multicenter observational study From Korea and Japan. Medicine (Baltimore). 2015;94(41):e1526.
14. Casali PG, Blay JY; ESMO/CONTICANET/EUROBONET Consensus Panel of experts. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v198-v203.
15. Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247-258.
16. Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104.
17. Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272.
18. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33(6):634-642.
19. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459-465.
20. Antonescu CR, Viale A, Sarran L, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10(10):3282-3290.
21. Arne G, Kristiansson E, Nerman O, et al. Expression profiling of GIST: CD133 is associated with KIT exon 11 mutations, gastric location and poor prognosis. Int J Cancer. 2011;129(5):1149-1161.
22. Bertucci F, Finetti P, Ostrowski J, et al. Genomic Grade Index predicts postoperative clinical outcome of GIST. Br J Cancer. 2012;107(8):1433-1441.
23. Koon N, Schneider-Stock R, Sarlomo-Rikala M, et al. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 2004;53(2):235-240.
24. Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18(3):826-838.
25. Skubitz KM, Geschwind K, Xu WW, Koopmeiners JS, Skubitz AP. Gene expression identifies heterogeneity of metastatic behavior among gastrointestinal stromal tumors. J Transl Med. 2016;14:51.
26. Yamaguchi U, Nakayama R, Honda K, et al. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J Clin Oncol. 2008;26(25):4100-4108.
27. Ylipaa A, Hunt KK, Yang J, et al. Integrative genomic characterization and a genomic staging system for gastrointestinal stromal tumors. Cancer. 2011;117(2):380-389.
28. Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37(12):1527-1535.
Gastrointestinal stromal tumor (GIST) is the most common soft tissue sarcoma of the gastrointestinal tract, usually arising from the interstitial cells of Cajal or similar cells in the outer wall of the gastrointestinal tract.1,2 Most GISTs have an activating mutation in KIT or platelet-derived growth factor receptor alpha (PDGFRA). Tumor size, mitotic rate, and anatomic site are the most common pathological features used to risk stratify GIST tumors.3-10 It is important to note when using such risk calculators that preoperative imatinib before determining tumor characteristics (such as mitoses per 50 high-power fields [hpf]) often changes the relevant parameters so that the same risk calculations may not apply. Tumors with a mitotic rate ≤5 mitoses per 50 hpf and a size ≤5 cm in greatest dimension have a lower recurrence rate after resection than tumors with a mitotic rate >5 mitoses per 50 hpf and a size >10 cm, and larger tumors can have a recurrence rate of up to 86%.11,12 Findings from a large observational study have suggested that the prognosis of gastric GIST in Korea and Japan may be more favorable compared with that in Western countries.13
The primary treatment of a localized primary GIST is surgical excision, but a cure is limited by recurrence.14,15 Imatinib is useful in the treatment of metastatic or recurrent GIST, and adjuvant treatment with imatinib after surgery has been shown to improve progression-free and overall survival in some cases.3,16-18 Responses to adjuvant imatinib depend on tumor sensitivity to the drug and the risk of recurrence. Drug sensitivity is largely dependent on the presence of mutations in KIT or PDGFRA.3,18 Recurrence risk is highly dependent on tumor size, tumor site, tumor rupture, and mitotic index.1,3,5,6,8,9,18,19 Findings on the use of gene expression patterns to predict recurrence risk have also been reported.20-27 However, recurrence risk is poorly understood for categories in which there are few cases with known outcomes, such as very small gastric GIST with a high mitotic index. For example, few cases of gastric GIST have been reported with a tumor size ≤2 cm, a mitotic rate >5 mitoses per 50 hpf, and adequate clinical follow-up. In such cases, it is difficult to assess the risk of recurrence.6 We report here the long-term outcome of a patient with a 1.8 cm gastric GIST with a mitotic index of 36 mitoses per 50 hpf and a KIT exon 11 mutation.
Case presentation and summary
A 69-year-old man presented with periumbilical and epigastric pain of 6-month duration. His medical history was notable for hyperlipidemia, hypertension, coronary angioplasty, and spinal surgery. He had a 40 pack-year smoking history and consumed 2 to 4 alcoholic drinks per day. The results of a physical examination were unremarkable. A computedtomographic (CT) scan showed no abnormalities. An esophagoduodenoscopy (EGD) revealed gastric ulcers. He was treated successfully with omeprazole 20 mg by mouth daily.
A month later, a follow-up EGD revealed a 1.8 × 1.5 cm submucosal mass 3 cm from the gastroesophageal junction. The patient underwent a fundus wedge resection, and a submucosal mass 1.8 cm in greatest dimension was removed. Pathologic examination revealed a GIST, spindle cell type, with a mitotic rate of 36 mitoses per 50 hpf with negative margins. Immunohistochemistry was positive for CD117. An exon 11 deletion (KVV558-560NV) was present in KIT. The patient’s risk of recurrence was unclear, and his follow-up included CT scans of the abdomen and pelvis every 3 to 4 months for the first 2 years, then every 6 months for the next 2.5 years.
A CT scan about 3.5 years after primary resection revealed small nonspecific liver hypodensities that became more prominent during the next year. About 5 years after primary resection, magnetic resonance imaging (MRI) revealed several liver lesions, the largest of which measured at 1.3 cm in greatest dimension. The patient’s liver metastases were readily identified by MRI (Figure 1) and CT imaging (Figure 2A).
Discussion
Small gastric GISTs are sometimes found by endoscopy performed for unrelated reasons. Recent data suggest that the incidence of gastric GIST may be higher than previously thought. In a Japanese study of patients with gastric cancer in which 100 stomachs were systematically examined pathologically, 50 microscopic GISTs were found in 35 patients.28 Most small gastric GISTs have a low mitotic index. Few cases have been described with a high mitotic index. In a study of 1765 cases of GIST of the stomach, 8 patients had a tumor size less than 2 cm and a mitotic index greater than 5. Of those, only 6 patients had long-term follow-up, and 3 were alive without disease at 2, 17, and 20 years of follow-up.7 These limited data make it impossible to predict outcomes in patients with small gastric GIST with a high mitotic index.
For patients who are at high risk of recurrence after surgery, 3 years of adjuvant imatinib treatment compared with 1 year has been shown to improve overall survival and is the current standard of care.10,17 A study comparing 5 and 3 years of imatinib is ongoing to establish whether a longer period of adjuvant treatment is warranted. In patients with metastatic GIST, lifelong imatinib until lack of benefit is considered optimal treatment.10 All patients should undergo KIT mutation analysis. Those with the PDGFRA D842V mutation, SDH (succinate dehydrogenase) deficiency, or neurofibromatosis-related GIST should not receive adjuvant imatinib.
This case has several unusual features. The small tumor size with a very high mitotic rate is rare. Such cases have not been reported in large numbers and have therefore not been reliably incorporated into risk prediction algorithms. In addition, despite a high mitotic index, the tumor was not FDG avid on PET imaging. The diagnosis of GIST is strongly supported by the KIT mutation and response to imatinib. This particular KIT mutation in larger GISTs is associated with aggressive disease. The present case adds to the data on the biology of small gastric GISTs with a high mitotic index and suggests the mitotic index in these tumors may be a more important predictor than size.
Acknowledgment
The authors thank Michael Franklin, MS, for editorial assistance, and Sabrina Porter for media edits.
Gastrointestinal stromal tumor (GIST) is the most common soft tissue sarcoma of the gastrointestinal tract, usually arising from the interstitial cells of Cajal or similar cells in the outer wall of the gastrointestinal tract.1,2 Most GISTs have an activating mutation in KIT or platelet-derived growth factor receptor alpha (PDGFRA). Tumor size, mitotic rate, and anatomic site are the most common pathological features used to risk stratify GIST tumors.3-10 It is important to note when using such risk calculators that preoperative imatinib before determining tumor characteristics (such as mitoses per 50 high-power fields [hpf]) often changes the relevant parameters so that the same risk calculations may not apply. Tumors with a mitotic rate ≤5 mitoses per 50 hpf and a size ≤5 cm in greatest dimension have a lower recurrence rate after resection than tumors with a mitotic rate >5 mitoses per 50 hpf and a size >10 cm, and larger tumors can have a recurrence rate of up to 86%.11,12 Findings from a large observational study have suggested that the prognosis of gastric GIST in Korea and Japan may be more favorable compared with that in Western countries.13
The primary treatment of a localized primary GIST is surgical excision, but a cure is limited by recurrence.14,15 Imatinib is useful in the treatment of metastatic or recurrent GIST, and adjuvant treatment with imatinib after surgery has been shown to improve progression-free and overall survival in some cases.3,16-18 Responses to adjuvant imatinib depend on tumor sensitivity to the drug and the risk of recurrence. Drug sensitivity is largely dependent on the presence of mutations in KIT or PDGFRA.3,18 Recurrence risk is highly dependent on tumor size, tumor site, tumor rupture, and mitotic index.1,3,5,6,8,9,18,19 Findings on the use of gene expression patterns to predict recurrence risk have also been reported.20-27 However, recurrence risk is poorly understood for categories in which there are few cases with known outcomes, such as very small gastric GIST with a high mitotic index. For example, few cases of gastric GIST have been reported with a tumor size ≤2 cm, a mitotic rate >5 mitoses per 50 hpf, and adequate clinical follow-up. In such cases, it is difficult to assess the risk of recurrence.6 We report here the long-term outcome of a patient with a 1.8 cm gastric GIST with a mitotic index of 36 mitoses per 50 hpf and a KIT exon 11 mutation.
Case presentation and summary
A 69-year-old man presented with periumbilical and epigastric pain of 6-month duration. His medical history was notable for hyperlipidemia, hypertension, coronary angioplasty, and spinal surgery. He had a 40 pack-year smoking history and consumed 2 to 4 alcoholic drinks per day. The results of a physical examination were unremarkable. A computedtomographic (CT) scan showed no abnormalities. An esophagoduodenoscopy (EGD) revealed gastric ulcers. He was treated successfully with omeprazole 20 mg by mouth daily.
A month later, a follow-up EGD revealed a 1.8 × 1.5 cm submucosal mass 3 cm from the gastroesophageal junction. The patient underwent a fundus wedge resection, and a submucosal mass 1.8 cm in greatest dimension was removed. Pathologic examination revealed a GIST, spindle cell type, with a mitotic rate of 36 mitoses per 50 hpf with negative margins. Immunohistochemistry was positive for CD117. An exon 11 deletion (KVV558-560NV) was present in KIT. The patient’s risk of recurrence was unclear, and his follow-up included CT scans of the abdomen and pelvis every 3 to 4 months for the first 2 years, then every 6 months for the next 2.5 years.
A CT scan about 3.5 years after primary resection revealed small nonspecific liver hypodensities that became more prominent during the next year. About 5 years after primary resection, magnetic resonance imaging (MRI) revealed several liver lesions, the largest of which measured at 1.3 cm in greatest dimension. The patient’s liver metastases were readily identified by MRI (Figure 1) and CT imaging (Figure 2A).
Discussion
Small gastric GISTs are sometimes found by endoscopy performed for unrelated reasons. Recent data suggest that the incidence of gastric GIST may be higher than previously thought. In a Japanese study of patients with gastric cancer in which 100 stomachs were systematically examined pathologically, 50 microscopic GISTs were found in 35 patients.28 Most small gastric GISTs have a low mitotic index. Few cases have been described with a high mitotic index. In a study of 1765 cases of GIST of the stomach, 8 patients had a tumor size less than 2 cm and a mitotic index greater than 5. Of those, only 6 patients had long-term follow-up, and 3 were alive without disease at 2, 17, and 20 years of follow-up.7 These limited data make it impossible to predict outcomes in patients with small gastric GIST with a high mitotic index.
For patients who are at high risk of recurrence after surgery, 3 years of adjuvant imatinib treatment compared with 1 year has been shown to improve overall survival and is the current standard of care.10,17 A study comparing 5 and 3 years of imatinib is ongoing to establish whether a longer period of adjuvant treatment is warranted. In patients with metastatic GIST, lifelong imatinib until lack of benefit is considered optimal treatment.10 All patients should undergo KIT mutation analysis. Those with the PDGFRA D842V mutation, SDH (succinate dehydrogenase) deficiency, or neurofibromatosis-related GIST should not receive adjuvant imatinib.
This case has several unusual features. The small tumor size with a very high mitotic rate is rare. Such cases have not been reported in large numbers and have therefore not been reliably incorporated into risk prediction algorithms. In addition, despite a high mitotic index, the tumor was not FDG avid on PET imaging. The diagnosis of GIST is strongly supported by the KIT mutation and response to imatinib. This particular KIT mutation in larger GISTs is associated with aggressive disease. The present case adds to the data on the biology of small gastric GISTs with a high mitotic index and suggests the mitotic index in these tumors may be a more important predictor than size.
Acknowledgment
The authors thank Michael Franklin, MS, for editorial assistance, and Sabrina Porter for media edits.
1. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865-878.
2. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-580.
3. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563-1570.
4. Huang J, Zheng DL, Qin FS, et al. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120(1):223-241.
5. Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265-274.
6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466-1478.
7. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52-68.
8. Patel S. Navigating risk stratification systems for the management of patients with GIST. Ann Surg Oncol. 2011;18(6):1698-1704.
9. Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35(11):1646-1656.
10. National Comprehensive Cancer Network. Sarcoma. https://www.nccn.org. Accessed March 27, 2018.
11. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81-89.
12. Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141(6):748-756.
13. Kim MC, Yook JH, Yang HK, et al. Long-term surgical outcome of 1057 gastric GISTs according to 7th UICC/AJCC TNM system: multicenter observational study From Korea and Japan. Medicine (Baltimore). 2015;94(41):e1526.
14. Casali PG, Blay JY; ESMO/CONTICANET/EUROBONET Consensus Panel of experts. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v198-v203.
15. Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247-258.
16. Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104.
17. Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272.
18. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33(6):634-642.
19. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459-465.
20. Antonescu CR, Viale A, Sarran L, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10(10):3282-3290.
21. Arne G, Kristiansson E, Nerman O, et al. Expression profiling of GIST: CD133 is associated with KIT exon 11 mutations, gastric location and poor prognosis. Int J Cancer. 2011;129(5):1149-1161.
22. Bertucci F, Finetti P, Ostrowski J, et al. Genomic Grade Index predicts postoperative clinical outcome of GIST. Br J Cancer. 2012;107(8):1433-1441.
23. Koon N, Schneider-Stock R, Sarlomo-Rikala M, et al. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 2004;53(2):235-240.
24. Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18(3):826-838.
25. Skubitz KM, Geschwind K, Xu WW, Koopmeiners JS, Skubitz AP. Gene expression identifies heterogeneity of metastatic behavior among gastrointestinal stromal tumors. J Transl Med. 2016;14:51.
26. Yamaguchi U, Nakayama R, Honda K, et al. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J Clin Oncol. 2008;26(25):4100-4108.
27. Ylipaa A, Hunt KK, Yang J, et al. Integrative genomic characterization and a genomic staging system for gastrointestinal stromal tumors. Cancer. 2011;117(2):380-389.
28. Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37(12):1527-1535.
1. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865-878.
2. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-580.
3. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563-1570.
4. Huang J, Zheng DL, Qin FS, et al. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120(1):223-241.
5. Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265-274.
6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466-1478.
7. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52-68.
8. Patel S. Navigating risk stratification systems for the management of patients with GIST. Ann Surg Oncol. 2011;18(6):1698-1704.
9. Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35(11):1646-1656.
10. National Comprehensive Cancer Network. Sarcoma. https://www.nccn.org. Accessed March 27, 2018.
11. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81-89.
12. Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141(6):748-756.
13. Kim MC, Yook JH, Yang HK, et al. Long-term surgical outcome of 1057 gastric GISTs according to 7th UICC/AJCC TNM system: multicenter observational study From Korea and Japan. Medicine (Baltimore). 2015;94(41):e1526.
14. Casali PG, Blay JY; ESMO/CONTICANET/EUROBONET Consensus Panel of experts. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v198-v203.
15. Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247-258.
16. Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104.
17. Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272.
18. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33(6):634-642.
19. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459-465.
20. Antonescu CR, Viale A, Sarran L, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10(10):3282-3290.
21. Arne G, Kristiansson E, Nerman O, et al. Expression profiling of GIST: CD133 is associated with KIT exon 11 mutations, gastric location and poor prognosis. Int J Cancer. 2011;129(5):1149-1161.
22. Bertucci F, Finetti P, Ostrowski J, et al. Genomic Grade Index predicts postoperative clinical outcome of GIST. Br J Cancer. 2012;107(8):1433-1441.
23. Koon N, Schneider-Stock R, Sarlomo-Rikala M, et al. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 2004;53(2):235-240.
24. Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18(3):826-838.
25. Skubitz KM, Geschwind K, Xu WW, Koopmeiners JS, Skubitz AP. Gene expression identifies heterogeneity of metastatic behavior among gastrointestinal stromal tumors. J Transl Med. 2016;14:51.
26. Yamaguchi U, Nakayama R, Honda K, et al. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J Clin Oncol. 2008;26(25):4100-4108.
27. Ylipaa A, Hunt KK, Yang J, et al. Integrative genomic characterization and a genomic staging system for gastrointestinal stromal tumors. Cancer. 2011;117(2):380-389.
28. Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37(12):1527-1535.
Striking rash in a patient with lung cancer on a checkpoint inhibitor
Lung cancer remains the most common cause of cancer death in the United States and worldwide.1 Despite advances in the treatment of the disease and development of targeted therapy, the 5-year overall survival in stage IV non–small-cell lung cancer remains poor, ranging from 6% to 10%.2 More recently, checkpoint inhibitors have had a major impact on the treatment of lung cancer. Nivolumab was the first program cell death protein-1 (PD-1) inhibitor approved for malignant melanoma.3 In July 2015, it was approved as a second-line treatment of squamous cell carcinoma of the lung.4 Since then, the use of nivolumab has extended to other malignancies such as head and neck cancer, renal cell carcinoma, and the list continues to expand. In lung cancer, it demonstrated superior overall survival of 9 months, compared with 6 months with docetaxel.4 Other checkpoint inhibitors such as pembrolizumab5 and atezolizumab6 were subsequently developed, and are also used in the treatment of lung cancer.
Serious potential autoimmune complications arise in up to 30% of patients treated with PD-1 inhibitors. Dermatologic toxicity is the most common immune-related adverse event in these patients. In addition to vitiligo, most common is a reticular maculopapular rash on the trunk and extremities. Other adverse events, such as photosensitivity, alopecia, xerosis, and hair color changes, are reported less frequently.7 We report here a case of rash at an unusual location (auricular and periauricular) with skin exfoliation mimicking other common skin conditions such as eczema and psoriasis.
Case presentation and summary
A 57-year-old woman with a history of cerebrovascular accident with residual left lower-leg paresis presented for acute onset expressive aphasia in the absence of other constitutional or neurological findings. Magnetic resonance imaging of the brain showed a posterior, left parietal lobe lesion of 1.6 cm with intralesional hemorrhage and surrounding edema suggestive of brain metastasis. The patient had a 35 pack-year history of smoking. A staging work-up with computed-tomographic (CT) scans showed a spiculated enhancing nodule in the superior segment of the right lower lobe plus mediastinal adenopathy.
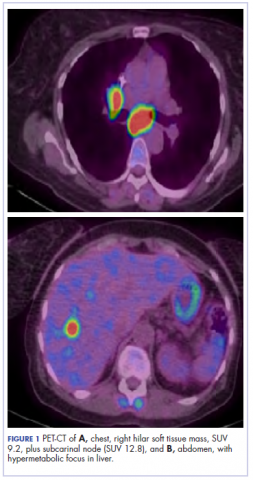
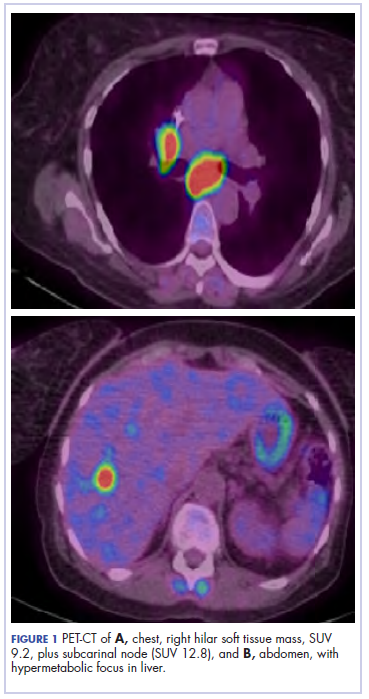
The patient underwent a CT-guided core biopsy of the spiculated nodule, which was found to be consistent with adenocarcinoma of the lung. It was negative for EGFR mutation or ALK rearrangement. She received stereotactic radiosurgery to the left posterior parietal lesion, and after completion of radiation, was started on systemic chemotherapy with cisplatin plus pemetrexed for adenocarcinoma of the lung. She received 4 cycles of chemotherapy. Repeat imaging with a PET-CT showed interval increase of the mediastinal hypermetabolic lymphadenopathy with new hypermetabolic pretracheal lymph nodes and interval development of multiple liver metastases in the right and left lobes of the liver (Figure 1). She was started on second-line therapy with nivolumab at a dose of 240 mg every 2 weeks. The treatment was complicated initially by new onset grade 2 papular pruritic rash after cycle 2 of therapy. The rash involved the upper and lower extremities, sparing the palms, soles, trunk, abdomen, and the back. It resolved with treatment delay and topical steroids.
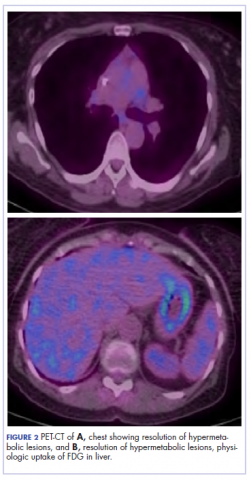
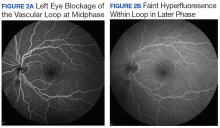
The patient resumed treatment with nivolumab after complete resolution of the rash. However, she developed grade 2 nephritis after cycle 5 with a creatinine level of 1.98 mg/dL (reference range, 0.6-1.2 mg/ dL). This was resolved after treatment with oral prednisone, at a starting dose of 1 mg/kg and tapered over 4 weeks. PET CT scans obtained after cycles 5 and 11 showed no metabolic activity in the mediastinum or the liver and markedly decreased uptake in the right lower lobe nodule, down to an SUV of 1.7 with no new nodules. An MRI of the brain was stable (Figure 2).
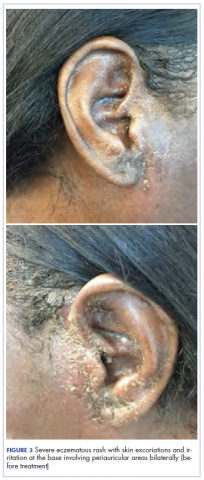
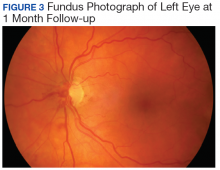
After cycle 16 of nivolumab, the patient developed a severe eczematous rash with excoriations at the base of both ears involving the periauricular and auricular areas bilaterally (Figure 3).
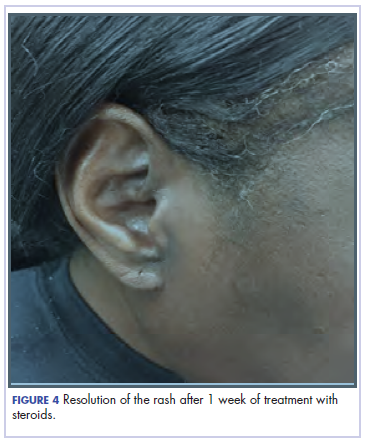
She completed 4 weeks of steroid therapy on a tapering schedule. Treatment with nivolumab was resumed afterward with no adverse autoimmune complications. At her last visit (25 months after initiating a PD-1 inhibitor), there was no clinical or radiologic evidence of lung cancer nor any of autoimmune adverse effects.
Discussion
Among multiple autoimmune complications, dermatologic toxicity is the most common immune-related adverse event, occuring in about 30% to 40% of patients7,8 and with an average onset of 3-4 weeks after initiating treatment with checkpoint inhibitors.9 In addition to vitiligo, the most common type of rash described is a reticular maculopapular rash on the trunk and extremities.10 Other findings, such as photosensitivity, alopecia, xerosis, and hair color changes, have been reported in smaller numbers. Skin exfoliation, as seen in the present case, has been reported in fewer than 1% of the cases.4 Perivascular lymphocytic infiltrates extending deep into the dermis are most likely to be seen if the lesions are biopsied. Both the location of the rash in our patient and its relapsing nature are rare and make it more interesting as it presents a diagnostic dilemma for treating physicians. Ear, nose, and throat surgeons are more likely to encounter such a complication with the expanded use of PD-1 and PD-ligand 1 inhibitors in advanced head and neck cancers. The differential diagnosis includes localized eczema, psoriatic rash, skin infection, or an autoimmune phenomenon.
The location of the rash was also of concern because there have been reports of autoimmune inner-ear disease related to immunotherapy.11 After the failure of treatment with empiric antibiotics and topical steroids, in addition to the development of a new rash on her abdomen, we concluded that this case might represent an unusual autoimmune skin complication. The resolution of the skin lesions in both locations (the ears and the abdomen) with the oral steroid therapy, supported our suspected diagnosis of autoimmune dermatitis.
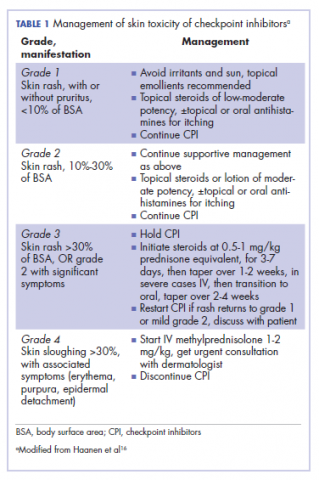
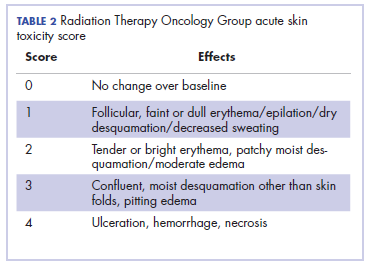
It is essential that these complications are detected early and misdiagnosis is avoided because timely treatment with steroids will prevent progression to more severe problems such as Steven-Johnson syndrome, toxic epidermal necrolysis,12 or extension into the inner ear.11This case is part of a growing spectrum of other unusual cases seen with immunotherapy treatment, such as erythema nodosum-like reactions,13 bullous dermatitis,14 and psoriasiform eruptions.15 It highlights the need for an awareness of expanding dermatologic complications from immunotherapy beyond the reported common manifestations. Established guidelines and algorithms for the management of immune-related dermatologic toxicity are available to assist the physician in treatment (Table 1).16 Skin biopsy should be considered if the diagnosis remains uncertain, although starting empiric treatment with steroids is a widely acceptable approach. Reassessing the skin rash in 48 hours to 1 week after treatment initiation is crucial because steroid-refractory cases will need additional immunosuppression. Early termination of steroids is associated with higher recurrence rate, therefore tapering steroids over 4 weeks is highly recommended before resuming treatment with checkpoint inhibitors.
In summary, increased awareness among health care professionals of the common and unusual complications of immunotherapy agents is important and essential in patient care. In addition to oncologists, head and neck surgeons, pulmonologists, urologists, dermatologists, and general internists will encounter patients with immunotherapy-related complications. Patient education should be emphasized to ensure prompt investigation and treatment of complications. Finally, it is not yet clear whether the development of autoimmune reactions predicts disease response to treatment. In a series of 134 patients with lung cancer, the occurrence of autoimmune adverse events correlated with improved survival.17 More research is needed to identify prognostic and predictive biomarkers for response to immunotherapy.
Conclusion
This pattern of autoimmune dermatitis localizing to the ears is rare (<1% of cases of dermatitis). Nevertheless, it raises the awareness for dermatologic complications of immunotherapy beyond the classical reported manifestations. Prompt diagnosis and treatment is essential to avoid serious complications such as Steven-Johnson syndrome, toxic epidermal necrolysis, and potentially damage to the inner ear.
1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39-51.
3. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123-135.
5. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemo-therapy for PD- L1- positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823- 1833.
6. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265.
7. Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse events of the immune checkpoint inhibitors. Curr Prob Cancer. 2017;41:125-128.
8. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375.
9. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
10. Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
11. Zibelman M, Pollak N, Olszanski AJ. Autoimmune inner ear disease in a melanoma patient treated with pembrolizumab. J Immunother Cancer. 2016;4:8.
12. Nayar N, Briscoe K, Penas PF. Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. 2016;39(3):149-152.
13. Tetzlaff MT, Jazaeri AA, Torres-Cabala CA, et al. Erythema nodosum-like panniculitis mimicking disease recurrence: a novel toxicity from immune checkpoint blockade therapy - report of 2 patients. J Cutan Pathol. 2017;44(12):1080-1086.
14. Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383-389.
15. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
16. Haanen JBAG, Carbonnel F, Robert C, et al; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119-iv142.
17. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374-378.
Lung cancer remains the most common cause of cancer death in the United States and worldwide.1 Despite advances in the treatment of the disease and development of targeted therapy, the 5-year overall survival in stage IV non–small-cell lung cancer remains poor, ranging from 6% to 10%.2 More recently, checkpoint inhibitors have had a major impact on the treatment of lung cancer. Nivolumab was the first program cell death protein-1 (PD-1) inhibitor approved for malignant melanoma.3 In July 2015, it was approved as a second-line treatment of squamous cell carcinoma of the lung.4 Since then, the use of nivolumab has extended to other malignancies such as head and neck cancer, renal cell carcinoma, and the list continues to expand. In lung cancer, it demonstrated superior overall survival of 9 months, compared with 6 months with docetaxel.4 Other checkpoint inhibitors such as pembrolizumab5 and atezolizumab6 were subsequently developed, and are also used in the treatment of lung cancer.
Serious potential autoimmune complications arise in up to 30% of patients treated with PD-1 inhibitors. Dermatologic toxicity is the most common immune-related adverse event in these patients. In addition to vitiligo, most common is a reticular maculopapular rash on the trunk and extremities. Other adverse events, such as photosensitivity, alopecia, xerosis, and hair color changes, are reported less frequently.7 We report here a case of rash at an unusual location (auricular and periauricular) with skin exfoliation mimicking other common skin conditions such as eczema and psoriasis.
Case presentation and summary
A 57-year-old woman with a history of cerebrovascular accident with residual left lower-leg paresis presented for acute onset expressive aphasia in the absence of other constitutional or neurological findings. Magnetic resonance imaging of the brain showed a posterior, left parietal lobe lesion of 1.6 cm with intralesional hemorrhage and surrounding edema suggestive of brain metastasis. The patient had a 35 pack-year history of smoking. A staging work-up with computed-tomographic (CT) scans showed a spiculated enhancing nodule in the superior segment of the right lower lobe plus mediastinal adenopathy.
The patient underwent a CT-guided core biopsy of the spiculated nodule, which was found to be consistent with adenocarcinoma of the lung. It was negative for EGFR mutation or ALK rearrangement. She received stereotactic radiosurgery to the left posterior parietal lesion, and after completion of radiation, was started on systemic chemotherapy with cisplatin plus pemetrexed for adenocarcinoma of the lung. She received 4 cycles of chemotherapy. Repeat imaging with a PET-CT showed interval increase of the mediastinal hypermetabolic lymphadenopathy with new hypermetabolic pretracheal lymph nodes and interval development of multiple liver metastases in the right and left lobes of the liver (Figure 1). She was started on second-line therapy with nivolumab at a dose of 240 mg every 2 weeks. The treatment was complicated initially by new onset grade 2 papular pruritic rash after cycle 2 of therapy. The rash involved the upper and lower extremities, sparing the palms, soles, trunk, abdomen, and the back. It resolved with treatment delay and topical steroids.
The patient resumed treatment with nivolumab after complete resolution of the rash. However, she developed grade 2 nephritis after cycle 5 with a creatinine level of 1.98 mg/dL (reference range, 0.6-1.2 mg/ dL). This was resolved after treatment with oral prednisone, at a starting dose of 1 mg/kg and tapered over 4 weeks. PET CT scans obtained after cycles 5 and 11 showed no metabolic activity in the mediastinum or the liver and markedly decreased uptake in the right lower lobe nodule, down to an SUV of 1.7 with no new nodules. An MRI of the brain was stable (Figure 2).
After cycle 16 of nivolumab, the patient developed a severe eczematous rash with excoriations at the base of both ears involving the periauricular and auricular areas bilaterally (Figure 3).
She completed 4 weeks of steroid therapy on a tapering schedule. Treatment with nivolumab was resumed afterward with no adverse autoimmune complications. At her last visit (25 months after initiating a PD-1 inhibitor), there was no clinical or radiologic evidence of lung cancer nor any of autoimmune adverse effects.
Discussion
Among multiple autoimmune complications, dermatologic toxicity is the most common immune-related adverse event, occuring in about 30% to 40% of patients7,8 and with an average onset of 3-4 weeks after initiating treatment with checkpoint inhibitors.9 In addition to vitiligo, the most common type of rash described is a reticular maculopapular rash on the trunk and extremities.10 Other findings, such as photosensitivity, alopecia, xerosis, and hair color changes, have been reported in smaller numbers. Skin exfoliation, as seen in the present case, has been reported in fewer than 1% of the cases.4 Perivascular lymphocytic infiltrates extending deep into the dermis are most likely to be seen if the lesions are biopsied. Both the location of the rash in our patient and its relapsing nature are rare and make it more interesting as it presents a diagnostic dilemma for treating physicians. Ear, nose, and throat surgeons are more likely to encounter such a complication with the expanded use of PD-1 and PD-ligand 1 inhibitors in advanced head and neck cancers. The differential diagnosis includes localized eczema, psoriatic rash, skin infection, or an autoimmune phenomenon.
The location of the rash was also of concern because there have been reports of autoimmune inner-ear disease related to immunotherapy.11 After the failure of treatment with empiric antibiotics and topical steroids, in addition to the development of a new rash on her abdomen, we concluded that this case might represent an unusual autoimmune skin complication. The resolution of the skin lesions in both locations (the ears and the abdomen) with the oral steroid therapy, supported our suspected diagnosis of autoimmune dermatitis.
It is essential that these complications are detected early and misdiagnosis is avoided because timely treatment with steroids will prevent progression to more severe problems such as Steven-Johnson syndrome, toxic epidermal necrolysis,12 or extension into the inner ear.11This case is part of a growing spectrum of other unusual cases seen with immunotherapy treatment, such as erythema nodosum-like reactions,13 bullous dermatitis,14 and psoriasiform eruptions.15 It highlights the need for an awareness of expanding dermatologic complications from immunotherapy beyond the reported common manifestations. Established guidelines and algorithms for the management of immune-related dermatologic toxicity are available to assist the physician in treatment (Table 1).16 Skin biopsy should be considered if the diagnosis remains uncertain, although starting empiric treatment with steroids is a widely acceptable approach. Reassessing the skin rash in 48 hours to 1 week after treatment initiation is crucial because steroid-refractory cases will need additional immunosuppression. Early termination of steroids is associated with higher recurrence rate, therefore tapering steroids over 4 weeks is highly recommended before resuming treatment with checkpoint inhibitors.
In summary, increased awareness among health care professionals of the common and unusual complications of immunotherapy agents is important and essential in patient care. In addition to oncologists, head and neck surgeons, pulmonologists, urologists, dermatologists, and general internists will encounter patients with immunotherapy-related complications. Patient education should be emphasized to ensure prompt investigation and treatment of complications. Finally, it is not yet clear whether the development of autoimmune reactions predicts disease response to treatment. In a series of 134 patients with lung cancer, the occurrence of autoimmune adverse events correlated with improved survival.17 More research is needed to identify prognostic and predictive biomarkers for response to immunotherapy.
Conclusion
This pattern of autoimmune dermatitis localizing to the ears is rare (<1% of cases of dermatitis). Nevertheless, it raises the awareness for dermatologic complications of immunotherapy beyond the classical reported manifestations. Prompt diagnosis and treatment is essential to avoid serious complications such as Steven-Johnson syndrome, toxic epidermal necrolysis, and potentially damage to the inner ear.
Lung cancer remains the most common cause of cancer death in the United States and worldwide.1 Despite advances in the treatment of the disease and development of targeted therapy, the 5-year overall survival in stage IV non–small-cell lung cancer remains poor, ranging from 6% to 10%.2 More recently, checkpoint inhibitors have had a major impact on the treatment of lung cancer. Nivolumab was the first program cell death protein-1 (PD-1) inhibitor approved for malignant melanoma.3 In July 2015, it was approved as a second-line treatment of squamous cell carcinoma of the lung.4 Since then, the use of nivolumab has extended to other malignancies such as head and neck cancer, renal cell carcinoma, and the list continues to expand. In lung cancer, it demonstrated superior overall survival of 9 months, compared with 6 months with docetaxel.4 Other checkpoint inhibitors such as pembrolizumab5 and atezolizumab6 were subsequently developed, and are also used in the treatment of lung cancer.
Serious potential autoimmune complications arise in up to 30% of patients treated with PD-1 inhibitors. Dermatologic toxicity is the most common immune-related adverse event in these patients. In addition to vitiligo, most common is a reticular maculopapular rash on the trunk and extremities. Other adverse events, such as photosensitivity, alopecia, xerosis, and hair color changes, are reported less frequently.7 We report here a case of rash at an unusual location (auricular and periauricular) with skin exfoliation mimicking other common skin conditions such as eczema and psoriasis.
Case presentation and summary
A 57-year-old woman with a history of cerebrovascular accident with residual left lower-leg paresis presented for acute onset expressive aphasia in the absence of other constitutional or neurological findings. Magnetic resonance imaging of the brain showed a posterior, left parietal lobe lesion of 1.6 cm with intralesional hemorrhage and surrounding edema suggestive of brain metastasis. The patient had a 35 pack-year history of smoking. A staging work-up with computed-tomographic (CT) scans showed a spiculated enhancing nodule in the superior segment of the right lower lobe plus mediastinal adenopathy.
The patient underwent a CT-guided core biopsy of the spiculated nodule, which was found to be consistent with adenocarcinoma of the lung. It was negative for EGFR mutation or ALK rearrangement. She received stereotactic radiosurgery to the left posterior parietal lesion, and after completion of radiation, was started on systemic chemotherapy with cisplatin plus pemetrexed for adenocarcinoma of the lung. She received 4 cycles of chemotherapy. Repeat imaging with a PET-CT showed interval increase of the mediastinal hypermetabolic lymphadenopathy with new hypermetabolic pretracheal lymph nodes and interval development of multiple liver metastases in the right and left lobes of the liver (Figure 1). She was started on second-line therapy with nivolumab at a dose of 240 mg every 2 weeks. The treatment was complicated initially by new onset grade 2 papular pruritic rash after cycle 2 of therapy. The rash involved the upper and lower extremities, sparing the palms, soles, trunk, abdomen, and the back. It resolved with treatment delay and topical steroids.
The patient resumed treatment with nivolumab after complete resolution of the rash. However, she developed grade 2 nephritis after cycle 5 with a creatinine level of 1.98 mg/dL (reference range, 0.6-1.2 mg/ dL). This was resolved after treatment with oral prednisone, at a starting dose of 1 mg/kg and tapered over 4 weeks. PET CT scans obtained after cycles 5 and 11 showed no metabolic activity in the mediastinum or the liver and markedly decreased uptake in the right lower lobe nodule, down to an SUV of 1.7 with no new nodules. An MRI of the brain was stable (Figure 2).
After cycle 16 of nivolumab, the patient developed a severe eczematous rash with excoriations at the base of both ears involving the periauricular and auricular areas bilaterally (Figure 3).
She completed 4 weeks of steroid therapy on a tapering schedule. Treatment with nivolumab was resumed afterward with no adverse autoimmune complications. At her last visit (25 months after initiating a PD-1 inhibitor), there was no clinical or radiologic evidence of lung cancer nor any of autoimmune adverse effects.
Discussion
Among multiple autoimmune complications, dermatologic toxicity is the most common immune-related adverse event, occuring in about 30% to 40% of patients7,8 and with an average onset of 3-4 weeks after initiating treatment with checkpoint inhibitors.9 In addition to vitiligo, the most common type of rash described is a reticular maculopapular rash on the trunk and extremities.10 Other findings, such as photosensitivity, alopecia, xerosis, and hair color changes, have been reported in smaller numbers. Skin exfoliation, as seen in the present case, has been reported in fewer than 1% of the cases.4 Perivascular lymphocytic infiltrates extending deep into the dermis are most likely to be seen if the lesions are biopsied. Both the location of the rash in our patient and its relapsing nature are rare and make it more interesting as it presents a diagnostic dilemma for treating physicians. Ear, nose, and throat surgeons are more likely to encounter such a complication with the expanded use of PD-1 and PD-ligand 1 inhibitors in advanced head and neck cancers. The differential diagnosis includes localized eczema, psoriatic rash, skin infection, or an autoimmune phenomenon.
The location of the rash was also of concern because there have been reports of autoimmune inner-ear disease related to immunotherapy.11 After the failure of treatment with empiric antibiotics and topical steroids, in addition to the development of a new rash on her abdomen, we concluded that this case might represent an unusual autoimmune skin complication. The resolution of the skin lesions in both locations (the ears and the abdomen) with the oral steroid therapy, supported our suspected diagnosis of autoimmune dermatitis.
It is essential that these complications are detected early and misdiagnosis is avoided because timely treatment with steroids will prevent progression to more severe problems such as Steven-Johnson syndrome, toxic epidermal necrolysis,12 or extension into the inner ear.11This case is part of a growing spectrum of other unusual cases seen with immunotherapy treatment, such as erythema nodosum-like reactions,13 bullous dermatitis,14 and psoriasiform eruptions.15 It highlights the need for an awareness of expanding dermatologic complications from immunotherapy beyond the reported common manifestations. Established guidelines and algorithms for the management of immune-related dermatologic toxicity are available to assist the physician in treatment (Table 1).16 Skin biopsy should be considered if the diagnosis remains uncertain, although starting empiric treatment with steroids is a widely acceptable approach. Reassessing the skin rash in 48 hours to 1 week after treatment initiation is crucial because steroid-refractory cases will need additional immunosuppression. Early termination of steroids is associated with higher recurrence rate, therefore tapering steroids over 4 weeks is highly recommended before resuming treatment with checkpoint inhibitors.
In summary, increased awareness among health care professionals of the common and unusual complications of immunotherapy agents is important and essential in patient care. In addition to oncologists, head and neck surgeons, pulmonologists, urologists, dermatologists, and general internists will encounter patients with immunotherapy-related complications. Patient education should be emphasized to ensure prompt investigation and treatment of complications. Finally, it is not yet clear whether the development of autoimmune reactions predicts disease response to treatment. In a series of 134 patients with lung cancer, the occurrence of autoimmune adverse events correlated with improved survival.17 More research is needed to identify prognostic and predictive biomarkers for response to immunotherapy.
Conclusion
This pattern of autoimmune dermatitis localizing to the ears is rare (<1% of cases of dermatitis). Nevertheless, it raises the awareness for dermatologic complications of immunotherapy beyond the classical reported manifestations. Prompt diagnosis and treatment is essential to avoid serious complications such as Steven-Johnson syndrome, toxic epidermal necrolysis, and potentially damage to the inner ear.
1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39-51.
3. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123-135.
5. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemo-therapy for PD- L1- positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823- 1833.
6. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265.
7. Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse events of the immune checkpoint inhibitors. Curr Prob Cancer. 2017;41:125-128.
8. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375.
9. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
10. Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
11. Zibelman M, Pollak N, Olszanski AJ. Autoimmune inner ear disease in a melanoma patient treated with pembrolizumab. J Immunother Cancer. 2016;4:8.
12. Nayar N, Briscoe K, Penas PF. Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. 2016;39(3):149-152.
13. Tetzlaff MT, Jazaeri AA, Torres-Cabala CA, et al. Erythema nodosum-like panniculitis mimicking disease recurrence: a novel toxicity from immune checkpoint blockade therapy - report of 2 patients. J Cutan Pathol. 2017;44(12):1080-1086.
14. Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383-389.
15. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
16. Haanen JBAG, Carbonnel F, Robert C, et al; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119-iv142.
17. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374-378.
1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39-51.
3. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123-135.
5. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemo-therapy for PD- L1- positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823- 1833.
6. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265.
7. Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse events of the immune checkpoint inhibitors. Curr Prob Cancer. 2017;41:125-128.
8. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375.
9. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
10. Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
11. Zibelman M, Pollak N, Olszanski AJ. Autoimmune inner ear disease in a melanoma patient treated with pembrolizumab. J Immunother Cancer. 2016;4:8.
12. Nayar N, Briscoe K, Penas PF. Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. 2016;39(3):149-152.
13. Tetzlaff MT, Jazaeri AA, Torres-Cabala CA, et al. Erythema nodosum-like panniculitis mimicking disease recurrence: a novel toxicity from immune checkpoint blockade therapy - report of 2 patients. J Cutan Pathol. 2017;44(12):1080-1086.
14. Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383-389.
15. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
16. Haanen JBAG, Carbonnel F, Robert C, et al; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119-iv142.
17. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374-378.
Effective management of severe radiation dermatitis after head and neck radiotherapy
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
When the Poisoned Risk Poisoning Others: Fatal Sodium Azide Overdose
Case
A 24-year-old man in cardiac arrest was brought to the ED via emergency medical services (EMS). Unfortunately, resuscitation efforts were unsuccessful. Little was known about the patient, but the emergency physician was informed that the patient had ingested sodium azide (NaN3), which he had ordered online. The patient collapsed shortly after ingesting the sodium azide, approximately the same time police officers arrived at the patient’s home.
No specific details were known about the patient’s ingestion. Upon learning of the exposure to sodium azide, a member of the ED staff contacted the local poison control center for information on the proper course of action to ensure staff safety and limit exposure. Shortly thereafter, several of emergency medical technicians and police officers, who had responded to the emergency assistance call for this patient, presented to the ED with concerns of exposure.
What is sodium azide?
Sodium azide is a colorless, odorless crystalline water-soluble solid that has a pK of 4.8.1 When sodium azide is dissolved in an acid, it liberates hydrazoic acid (HN3), which has a pungent odor, high vapor pressure (484 mm Hg), and a relatively low-boiling point of 37°C (98°F).2
The most common industrial use of sodium azide is as a propellant in air bags. In this capacity, sodium azide rapidly decomposes to nitrogen gas when it reaches a temperature of 300°C (572°F), causing rapid expansion of the air bag. In addition to air bags, sodium azide is used in research laboratories as a preservative and in agriculture as a pesticide. The main nontoxicological concern with all azide agents is the potential for explosion when they react with metals, such as lead, copper, silver, and mercury, to form metal azides that are sensitive to shock.3 An example of the explosive nature of these azides was demonstrated in a report wherein diluted sodium azide was poured down a drain, causing an explosion as a worker was fixing the pipe.4
In addition to industrial and commercial use, sodium azide is occasionally used in suicide attempts because it is rapidly fatal, has no specific antidote, and can be purchased online.3
What is the toxicity of sodium azide?
The lethal dose for both oral and dermal exposure to sodium azide is approximately 10 to 20 mg/kg.3,5 Therefore, ingestion of 700 mg of sodium azide, a volume approximately the size of a penny, is likely to be fatal.3
Sodium azide is primarily a mitochondrial toxin, which binds the electron transport chain, inhibiting oxidative phosphorylation. The resulting reduction in adenosine triphosphate (ATP) production, even in the presence of oxygen, results in metabolic failure.6 This mechanism of action is similar to that of cyanide, although sodium azide causes more pronounced vasodilation due to the in vivo conversion of some azide to the vasodilator nitric oxide.7 Some reports suggest that azide lethality is due to enhanced excitatory transmission from nitric oxide in the central nervous system.8
What are the clinical manifestations of azide poisoning, and what is the treatment?
The early clinical findings of a patient with azide poisoning include hypotension, dizziness, headache, nausea, vomiting, palpitations, tachycardia, dyspnea, and restlessness. Inhalation of hydrazoic acid can also produce wheezing and coughing. The most common effect is hypotension, which can occur within 1 minute of exposure. Following depletion of cellular ATP, anaerobic glycolysis generates lactate and produces acidemia. More severe findings of azide poisoning include seizures, cardiac arrhythmia, loss of consciousness, pulmonary edema, and cardiopulmonary failure.3
Currently, there is no specific antidote for azide poisoning, and treatment mainly consists of supportive care. Cyanide antidote treatments are generally ineffective in reducing azide-related death in animal models.3,8Early aggressive supportive care can improve survival rates.9 Some authors suggest that administration of oral activated charcoal, orogastric lavage, hemodialysis, and plasma exchange reduce azide concentrations, while others believe these treatments have little effect.3,9 More research is needed to identify effective therapeutic measures and to control for dose, time, and patient population.
What are the safety concerns for emergency medical technicians and hospital staff following exposure to sodium azide?
The most probable routes of exposure for prehospital and hospital staff include dermal contact with sodium azide or inhalation of gaseous hydrazoic acid; inhalational exposure is most concerning.1 In one case, hospital-staff members developed headaches, light-headedness, and nausea while treating a patient for azide poisoning; however, staff exposure was not confirmed and no sequelae were evident.10
More objectively, workers at an azide plant exposed to azide concentrations above the occupational exposure limit developed headaches, hypotension, and palpitations.11 Another study found no evidence of kidney, heart, or liver damage after patients were given sodium azide for more than a year during a clinical trial.12 Not unexpectedly, there is little risk of exposure when proper safety precautions are taken.
Emergency response personnel should carefully inspect the scene for the presence of any sodium azide powder, and should also question bystanders and family members to determine if anyone performed mouth-to-mouth resuscitation on the patient. Standard universal precautions, along with attentiveness to one’s surroundings, should be sufficient to prevent dermal exposure. If small amounts of sodium azide residue are found on the patient, his or her clothes should be cautiously removed and placed in a plastic bag to prevent dispersion of particles. If large quantities of sodium azide are present on a patient, the hazardous materials response team should be called, in accordance with institutional and regional protocols. To avoid explosion, every attempt should be made to prevent azide salt (eg, from emesis) from contact with any metal surfaces (eg, oxygen tanks, metal stretcher).13Vomit from patients who have ingested sodium azide can cause liberation of hydrazoic acid, which can escape through the esophagus. A pungent ambient odor may provide a warning, which is particularly concerning in a confined space such as an ambulance. As a precaution, EMS personnel should open windows and maximize ventilation. After the call, EMS and hospital personnel should thoroughly wash their hands with soap and water, and change their uniform if they believe it has been contaminated. There is no risk of delayed exposure following exposure to hydrazoic acid.
During autopsy, medical examiners must exercise caution due to the potential for liberation of hydrazoic acids from the stomach.14Unless it is absolutely necessary, the medical examiner should avoid opening the stomach. If this is unavoidable, the autopsy should occur in a well-ventilated setting with the examiner wearing a supplied air respirator to limit exposure in a high-risk scenario.
Case Conclusion
None of the exposed first responders experienced dizziness, light-headedness, or irritation, and after a period of observation in the ED, they were discharged home without further sequelae. All hospital staff involved in the patient’s care, including those who performed cardiopulmonary resuscitation on the patient and cleaned his room, were advised to use protective equipment when handling the patient and bodily secretions. None of the health care workers developed abnormal clinical findings. Given the hazard in conducting a full postmortem examination, the medical examiner opted to send blood, bile, urine, and vitreous humor out for analysis, but did not conduct a full postmortem examination. Notably, the stomach was not opened, and its contents were not exposed.
1. Compound summary for CID 33557 (sodium azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/sodium_azide. Accessed May 10, 2018.
2. Compound summary for CID 24530 (hydrogen azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/hydrazoic_acid. Accessed May 10, 2018.
3. Chang S, Lamm SH. Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol. 2003;22(3):175-186. doi:10.1080/10915810305109.
4. Sodium azide explosion hazard. Washington State Department of Labor & Industries. Division of Occupational Safety and Health. https://www.lni.wa.gov/safety/hazardalerts/SodiumAzide.pdf. August 11, 2011. Accessed May 10, 2018.
5. Safety data sheet: sodium azide. ThermoFischer Scientific. https://www.fishersci.com/store/msds?partNumber=S227I1&productDescription=SODIUM+AZIDE+GRAN+PURIF+1+KG&vendorId=VN00033897&countryCode=US&language=en. Updated January 17, 2018. Accessed May 10, 2018.
6. Bogucka K, Wojtczak L. Effect of sodium azide on oxidation and phosphorylation processes in rat-liver mitochondria. Biochim Biophys Acta. 1966;122(3):381-392. doi:10.1016/0926-6593(66)90031-2.
7. Kruszyna H, Kruszyna R, Smith RP, Wilcox DE. Red blood cells generate nitric oxide from directly acting, nitrogenous vasodilators. Toxicol Appl Pharmacol. 1987;91(3):429-438. doi:10.1016/0041-008x(87)90064-0.
8. Smith RP, Louis CA, Kruszyna R, Kruszyna H. Acute neurotoxicity of sodium azide and nitric oxide. Fundam Appl Toxicol. 1991;17(1):120-127. doi:10.1093/toxsci/17.1.120.
9. Watanabe K, Hirasawa H, Oda S, et al. A case of survival following high-dose sodium azide poisoning. Clin Toxicol (Phila). 2007;45(7):810-811.
10. Abrams J, el-Mallakh RS, Meyer R. Suicidal sodium azide ingestion. Ann Emerg Med. 1987;16(12):1378-1380. doi:10.1016/s0196-0644(87)80423-7
11. Trout D, Esswein EJ, Hales T, Brown K, Solomon G, Miller M. Exposures and health effects: an evaluation of workers at a sodium azide production plant. Am J Ind Med. 1996;30(3):343-350.
12. Black, MM, Zweifach BW, Speer FD. Comparison of hypotensive action of sodium azide in normotensive and hypertensive patients. Exper Biol Med. 1954;85(1):11-16. doi:10.3181/00379727-85-20770.
13. Emergency preparedness and response. Facts about sodium azide. Centers for Disease Control and Prevention. Office of Public Health Preparedness and Response. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp. Updated April 10, 2018. Accessed May 10, 2018.
14. Le Blanc-Louvry I, Laburthe-Tolra P, Massol V, et al. Suicidal sodium azide intoxication: An analytical challenge based on a rare case. Forensic Sci Int. 2012;221(1-3):e17-20. doi:10.1016/j.forsciint.2012.04.006.
Case
A 24-year-old man in cardiac arrest was brought to the ED via emergency medical services (EMS). Unfortunately, resuscitation efforts were unsuccessful. Little was known about the patient, but the emergency physician was informed that the patient had ingested sodium azide (NaN3), which he had ordered online. The patient collapsed shortly after ingesting the sodium azide, approximately the same time police officers arrived at the patient’s home.
No specific details were known about the patient’s ingestion. Upon learning of the exposure to sodium azide, a member of the ED staff contacted the local poison control center for information on the proper course of action to ensure staff safety and limit exposure. Shortly thereafter, several of emergency medical technicians and police officers, who had responded to the emergency assistance call for this patient, presented to the ED with concerns of exposure.
What is sodium azide?
Sodium azide is a colorless, odorless crystalline water-soluble solid that has a pK of 4.8.1 When sodium azide is dissolved in an acid, it liberates hydrazoic acid (HN3), which has a pungent odor, high vapor pressure (484 mm Hg), and a relatively low-boiling point of 37°C (98°F).2
The most common industrial use of sodium azide is as a propellant in air bags. In this capacity, sodium azide rapidly decomposes to nitrogen gas when it reaches a temperature of 300°C (572°F), causing rapid expansion of the air bag. In addition to air bags, sodium azide is used in research laboratories as a preservative and in agriculture as a pesticide. The main nontoxicological concern with all azide agents is the potential for explosion when they react with metals, such as lead, copper, silver, and mercury, to form metal azides that are sensitive to shock.3 An example of the explosive nature of these azides was demonstrated in a report wherein diluted sodium azide was poured down a drain, causing an explosion as a worker was fixing the pipe.4
In addition to industrial and commercial use, sodium azide is occasionally used in suicide attempts because it is rapidly fatal, has no specific antidote, and can be purchased online.3
What is the toxicity of sodium azide?
The lethal dose for both oral and dermal exposure to sodium azide is approximately 10 to 20 mg/kg.3,5 Therefore, ingestion of 700 mg of sodium azide, a volume approximately the size of a penny, is likely to be fatal.3
Sodium azide is primarily a mitochondrial toxin, which binds the electron transport chain, inhibiting oxidative phosphorylation. The resulting reduction in adenosine triphosphate (ATP) production, even in the presence of oxygen, results in metabolic failure.6 This mechanism of action is similar to that of cyanide, although sodium azide causes more pronounced vasodilation due to the in vivo conversion of some azide to the vasodilator nitric oxide.7 Some reports suggest that azide lethality is due to enhanced excitatory transmission from nitric oxide in the central nervous system.8
What are the clinical manifestations of azide poisoning, and what is the treatment?
The early clinical findings of a patient with azide poisoning include hypotension, dizziness, headache, nausea, vomiting, palpitations, tachycardia, dyspnea, and restlessness. Inhalation of hydrazoic acid can also produce wheezing and coughing. The most common effect is hypotension, which can occur within 1 minute of exposure. Following depletion of cellular ATP, anaerobic glycolysis generates lactate and produces acidemia. More severe findings of azide poisoning include seizures, cardiac arrhythmia, loss of consciousness, pulmonary edema, and cardiopulmonary failure.3
Currently, there is no specific antidote for azide poisoning, and treatment mainly consists of supportive care. Cyanide antidote treatments are generally ineffective in reducing azide-related death in animal models.3,8Early aggressive supportive care can improve survival rates.9 Some authors suggest that administration of oral activated charcoal, orogastric lavage, hemodialysis, and plasma exchange reduce azide concentrations, while others believe these treatments have little effect.3,9 More research is needed to identify effective therapeutic measures and to control for dose, time, and patient population.
What are the safety concerns for emergency medical technicians and hospital staff following exposure to sodium azide?
The most probable routes of exposure for prehospital and hospital staff include dermal contact with sodium azide or inhalation of gaseous hydrazoic acid; inhalational exposure is most concerning.1 In one case, hospital-staff members developed headaches, light-headedness, and nausea while treating a patient for azide poisoning; however, staff exposure was not confirmed and no sequelae were evident.10
More objectively, workers at an azide plant exposed to azide concentrations above the occupational exposure limit developed headaches, hypotension, and palpitations.11 Another study found no evidence of kidney, heart, or liver damage after patients were given sodium azide for more than a year during a clinical trial.12 Not unexpectedly, there is little risk of exposure when proper safety precautions are taken.
Emergency response personnel should carefully inspect the scene for the presence of any sodium azide powder, and should also question bystanders and family members to determine if anyone performed mouth-to-mouth resuscitation on the patient. Standard universal precautions, along with attentiveness to one’s surroundings, should be sufficient to prevent dermal exposure. If small amounts of sodium azide residue are found on the patient, his or her clothes should be cautiously removed and placed in a plastic bag to prevent dispersion of particles. If large quantities of sodium azide are present on a patient, the hazardous materials response team should be called, in accordance with institutional and regional protocols. To avoid explosion, every attempt should be made to prevent azide salt (eg, from emesis) from contact with any metal surfaces (eg, oxygen tanks, metal stretcher).13Vomit from patients who have ingested sodium azide can cause liberation of hydrazoic acid, which can escape through the esophagus. A pungent ambient odor may provide a warning, which is particularly concerning in a confined space such as an ambulance. As a precaution, EMS personnel should open windows and maximize ventilation. After the call, EMS and hospital personnel should thoroughly wash their hands with soap and water, and change their uniform if they believe it has been contaminated. There is no risk of delayed exposure following exposure to hydrazoic acid.
During autopsy, medical examiners must exercise caution due to the potential for liberation of hydrazoic acids from the stomach.14Unless it is absolutely necessary, the medical examiner should avoid opening the stomach. If this is unavoidable, the autopsy should occur in a well-ventilated setting with the examiner wearing a supplied air respirator to limit exposure in a high-risk scenario.
Case Conclusion
None of the exposed first responders experienced dizziness, light-headedness, or irritation, and after a period of observation in the ED, they were discharged home without further sequelae. All hospital staff involved in the patient’s care, including those who performed cardiopulmonary resuscitation on the patient and cleaned his room, were advised to use protective equipment when handling the patient and bodily secretions. None of the health care workers developed abnormal clinical findings. Given the hazard in conducting a full postmortem examination, the medical examiner opted to send blood, bile, urine, and vitreous humor out for analysis, but did not conduct a full postmortem examination. Notably, the stomach was not opened, and its contents were not exposed.
Case
A 24-year-old man in cardiac arrest was brought to the ED via emergency medical services (EMS). Unfortunately, resuscitation efforts were unsuccessful. Little was known about the patient, but the emergency physician was informed that the patient had ingested sodium azide (NaN3), which he had ordered online. The patient collapsed shortly after ingesting the sodium azide, approximately the same time police officers arrived at the patient’s home.
No specific details were known about the patient’s ingestion. Upon learning of the exposure to sodium azide, a member of the ED staff contacted the local poison control center for information on the proper course of action to ensure staff safety and limit exposure. Shortly thereafter, several of emergency medical technicians and police officers, who had responded to the emergency assistance call for this patient, presented to the ED with concerns of exposure.
What is sodium azide?
Sodium azide is a colorless, odorless crystalline water-soluble solid that has a pK of 4.8.1 When sodium azide is dissolved in an acid, it liberates hydrazoic acid (HN3), which has a pungent odor, high vapor pressure (484 mm Hg), and a relatively low-boiling point of 37°C (98°F).2
The most common industrial use of sodium azide is as a propellant in air bags. In this capacity, sodium azide rapidly decomposes to nitrogen gas when it reaches a temperature of 300°C (572°F), causing rapid expansion of the air bag. In addition to air bags, sodium azide is used in research laboratories as a preservative and in agriculture as a pesticide. The main nontoxicological concern with all azide agents is the potential for explosion when they react with metals, such as lead, copper, silver, and mercury, to form metal azides that are sensitive to shock.3 An example of the explosive nature of these azides was demonstrated in a report wherein diluted sodium azide was poured down a drain, causing an explosion as a worker was fixing the pipe.4
In addition to industrial and commercial use, sodium azide is occasionally used in suicide attempts because it is rapidly fatal, has no specific antidote, and can be purchased online.3
What is the toxicity of sodium azide?
The lethal dose for both oral and dermal exposure to sodium azide is approximately 10 to 20 mg/kg.3,5 Therefore, ingestion of 700 mg of sodium azide, a volume approximately the size of a penny, is likely to be fatal.3
Sodium azide is primarily a mitochondrial toxin, which binds the electron transport chain, inhibiting oxidative phosphorylation. The resulting reduction in adenosine triphosphate (ATP) production, even in the presence of oxygen, results in metabolic failure.6 This mechanism of action is similar to that of cyanide, although sodium azide causes more pronounced vasodilation due to the in vivo conversion of some azide to the vasodilator nitric oxide.7 Some reports suggest that azide lethality is due to enhanced excitatory transmission from nitric oxide in the central nervous system.8
What are the clinical manifestations of azide poisoning, and what is the treatment?
The early clinical findings of a patient with azide poisoning include hypotension, dizziness, headache, nausea, vomiting, palpitations, tachycardia, dyspnea, and restlessness. Inhalation of hydrazoic acid can also produce wheezing and coughing. The most common effect is hypotension, which can occur within 1 minute of exposure. Following depletion of cellular ATP, anaerobic glycolysis generates lactate and produces acidemia. More severe findings of azide poisoning include seizures, cardiac arrhythmia, loss of consciousness, pulmonary edema, and cardiopulmonary failure.3
Currently, there is no specific antidote for azide poisoning, and treatment mainly consists of supportive care. Cyanide antidote treatments are generally ineffective in reducing azide-related death in animal models.3,8Early aggressive supportive care can improve survival rates.9 Some authors suggest that administration of oral activated charcoal, orogastric lavage, hemodialysis, and plasma exchange reduce azide concentrations, while others believe these treatments have little effect.3,9 More research is needed to identify effective therapeutic measures and to control for dose, time, and patient population.
What are the safety concerns for emergency medical technicians and hospital staff following exposure to sodium azide?
The most probable routes of exposure for prehospital and hospital staff include dermal contact with sodium azide or inhalation of gaseous hydrazoic acid; inhalational exposure is most concerning.1 In one case, hospital-staff members developed headaches, light-headedness, and nausea while treating a patient for azide poisoning; however, staff exposure was not confirmed and no sequelae were evident.10
More objectively, workers at an azide plant exposed to azide concentrations above the occupational exposure limit developed headaches, hypotension, and palpitations.11 Another study found no evidence of kidney, heart, or liver damage after patients were given sodium azide for more than a year during a clinical trial.12 Not unexpectedly, there is little risk of exposure when proper safety precautions are taken.
Emergency response personnel should carefully inspect the scene for the presence of any sodium azide powder, and should also question bystanders and family members to determine if anyone performed mouth-to-mouth resuscitation on the patient. Standard universal precautions, along with attentiveness to one’s surroundings, should be sufficient to prevent dermal exposure. If small amounts of sodium azide residue are found on the patient, his or her clothes should be cautiously removed and placed in a plastic bag to prevent dispersion of particles. If large quantities of sodium azide are present on a patient, the hazardous materials response team should be called, in accordance with institutional and regional protocols. To avoid explosion, every attempt should be made to prevent azide salt (eg, from emesis) from contact with any metal surfaces (eg, oxygen tanks, metal stretcher).13Vomit from patients who have ingested sodium azide can cause liberation of hydrazoic acid, which can escape through the esophagus. A pungent ambient odor may provide a warning, which is particularly concerning in a confined space such as an ambulance. As a precaution, EMS personnel should open windows and maximize ventilation. After the call, EMS and hospital personnel should thoroughly wash their hands with soap and water, and change their uniform if they believe it has been contaminated. There is no risk of delayed exposure following exposure to hydrazoic acid.
During autopsy, medical examiners must exercise caution due to the potential for liberation of hydrazoic acids from the stomach.14Unless it is absolutely necessary, the medical examiner should avoid opening the stomach. If this is unavoidable, the autopsy should occur in a well-ventilated setting with the examiner wearing a supplied air respirator to limit exposure in a high-risk scenario.
Case Conclusion
None of the exposed first responders experienced dizziness, light-headedness, or irritation, and after a period of observation in the ED, they were discharged home without further sequelae. All hospital staff involved in the patient’s care, including those who performed cardiopulmonary resuscitation on the patient and cleaned his room, were advised to use protective equipment when handling the patient and bodily secretions. None of the health care workers developed abnormal clinical findings. Given the hazard in conducting a full postmortem examination, the medical examiner opted to send blood, bile, urine, and vitreous humor out for analysis, but did not conduct a full postmortem examination. Notably, the stomach was not opened, and its contents were not exposed.
1. Compound summary for CID 33557 (sodium azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/sodium_azide. Accessed May 10, 2018.
2. Compound summary for CID 24530 (hydrogen azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/hydrazoic_acid. Accessed May 10, 2018.
3. Chang S, Lamm SH. Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol. 2003;22(3):175-186. doi:10.1080/10915810305109.
4. Sodium azide explosion hazard. Washington State Department of Labor & Industries. Division of Occupational Safety and Health. https://www.lni.wa.gov/safety/hazardalerts/SodiumAzide.pdf. August 11, 2011. Accessed May 10, 2018.
5. Safety data sheet: sodium azide. ThermoFischer Scientific. https://www.fishersci.com/store/msds?partNumber=S227I1&productDescription=SODIUM+AZIDE+GRAN+PURIF+1+KG&vendorId=VN00033897&countryCode=US&language=en. Updated January 17, 2018. Accessed May 10, 2018.
6. Bogucka K, Wojtczak L. Effect of sodium azide on oxidation and phosphorylation processes in rat-liver mitochondria. Biochim Biophys Acta. 1966;122(3):381-392. doi:10.1016/0926-6593(66)90031-2.
7. Kruszyna H, Kruszyna R, Smith RP, Wilcox DE. Red blood cells generate nitric oxide from directly acting, nitrogenous vasodilators. Toxicol Appl Pharmacol. 1987;91(3):429-438. doi:10.1016/0041-008x(87)90064-0.
8. Smith RP, Louis CA, Kruszyna R, Kruszyna H. Acute neurotoxicity of sodium azide and nitric oxide. Fundam Appl Toxicol. 1991;17(1):120-127. doi:10.1093/toxsci/17.1.120.
9. Watanabe K, Hirasawa H, Oda S, et al. A case of survival following high-dose sodium azide poisoning. Clin Toxicol (Phila). 2007;45(7):810-811.
10. Abrams J, el-Mallakh RS, Meyer R. Suicidal sodium azide ingestion. Ann Emerg Med. 1987;16(12):1378-1380. doi:10.1016/s0196-0644(87)80423-7
11. Trout D, Esswein EJ, Hales T, Brown K, Solomon G, Miller M. Exposures and health effects: an evaluation of workers at a sodium azide production plant. Am J Ind Med. 1996;30(3):343-350.
12. Black, MM, Zweifach BW, Speer FD. Comparison of hypotensive action of sodium azide in normotensive and hypertensive patients. Exper Biol Med. 1954;85(1):11-16. doi:10.3181/00379727-85-20770.
13. Emergency preparedness and response. Facts about sodium azide. Centers for Disease Control and Prevention. Office of Public Health Preparedness and Response. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp. Updated April 10, 2018. Accessed May 10, 2018.
14. Le Blanc-Louvry I, Laburthe-Tolra P, Massol V, et al. Suicidal sodium azide intoxication: An analytical challenge based on a rare case. Forensic Sci Int. 2012;221(1-3):e17-20. doi:10.1016/j.forsciint.2012.04.006.
1. Compound summary for CID 33557 (sodium azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/sodium_azide. Accessed May 10, 2018.
2. Compound summary for CID 24530 (hydrogen azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/hydrazoic_acid. Accessed May 10, 2018.
3. Chang S, Lamm SH. Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol. 2003;22(3):175-186. doi:10.1080/10915810305109.
4. Sodium azide explosion hazard. Washington State Department of Labor & Industries. Division of Occupational Safety and Health. https://www.lni.wa.gov/safety/hazardalerts/SodiumAzide.pdf. August 11, 2011. Accessed May 10, 2018.
5. Safety data sheet: sodium azide. ThermoFischer Scientific. https://www.fishersci.com/store/msds?partNumber=S227I1&productDescription=SODIUM+AZIDE+GRAN+PURIF+1+KG&vendorId=VN00033897&countryCode=US&language=en. Updated January 17, 2018. Accessed May 10, 2018.
6. Bogucka K, Wojtczak L. Effect of sodium azide on oxidation and phosphorylation processes in rat-liver mitochondria. Biochim Biophys Acta. 1966;122(3):381-392. doi:10.1016/0926-6593(66)90031-2.
7. Kruszyna H, Kruszyna R, Smith RP, Wilcox DE. Red blood cells generate nitric oxide from directly acting, nitrogenous vasodilators. Toxicol Appl Pharmacol. 1987;91(3):429-438. doi:10.1016/0041-008x(87)90064-0.
8. Smith RP, Louis CA, Kruszyna R, Kruszyna H. Acute neurotoxicity of sodium azide and nitric oxide. Fundam Appl Toxicol. 1991;17(1):120-127. doi:10.1093/toxsci/17.1.120.
9. Watanabe K, Hirasawa H, Oda S, et al. A case of survival following high-dose sodium azide poisoning. Clin Toxicol (Phila). 2007;45(7):810-811.
10. Abrams J, el-Mallakh RS, Meyer R. Suicidal sodium azide ingestion. Ann Emerg Med. 1987;16(12):1378-1380. doi:10.1016/s0196-0644(87)80423-7
11. Trout D, Esswein EJ, Hales T, Brown K, Solomon G, Miller M. Exposures and health effects: an evaluation of workers at a sodium azide production plant. Am J Ind Med. 1996;30(3):343-350.
12. Black, MM, Zweifach BW, Speer FD. Comparison of hypotensive action of sodium azide in normotensive and hypertensive patients. Exper Biol Med. 1954;85(1):11-16. doi:10.3181/00379727-85-20770.
13. Emergency preparedness and response. Facts about sodium azide. Centers for Disease Control and Prevention. Office of Public Health Preparedness and Response. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp. Updated April 10, 2018. Accessed May 10, 2018.
14. Le Blanc-Louvry I, Laburthe-Tolra P, Massol V, et al. Suicidal sodium azide intoxication: An analytical challenge based on a rare case. Forensic Sci Int. 2012;221(1-3):e17-20. doi:10.1016/j.forsciint.2012.04.006.
Vitreous Hemorrhage in the Setting of a Vascular Loop
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
Imiquimod-Induced Hypopigmentation Following Treatment of Periungual Verruca Vulgaris
Imiquimod is derived from the imidazoquinoline family and works by activating both innate and adaptive immune pathways. Imiquimod binds to toll-like receptor 7 located on monocytes, macrophages, and dendritic cells,1 which allows nuclear factor κβ light chain enhancer of activated B cells to induce production of proinflammatory cytokines, including IFN-α and tumor necrosis factor α, as well as IL-1, IL-6, IL-8, IL-10, and IL-12.2 These proinflammatory cytokines play a role in the innate immunity, triggering upregulation of the adaptive immune pathway and activating type 1 helper T cells, cytotoxic T cells, and natural killer cells. These cells have antiviral and antitumoral effects that lend to their significance in coordinating innate and adaptive immune mechanisms.3 More specifically, imiquimod enhances dendritic cell migration to regional lymph nodes and induces apoptosis via activation of proapoptotic B-cell lymphoma 2 proteins.1,2 Imiquimod has been approved by the US Food and Drug Administration (FDA) to treat external genitalia and perianal condyloma acuminata, actinic keratoses (AKs), and superficial basal cell carcinoma (BCC). It often is used off label for antiviral or antitumoral therapy in Bowen disease, squamous cell carcinoma, lentigo maligna, vulvar intraepithelial neoplasia, molluscum contagiosum, common warts, and leishmaniasis.1,2 Imiquimod is generally well tolerated; erythema and irritation at the application site are the most common side effects, with pigmentary change being less common.
Case Report
A 51-year-old man with a medical history of vitamin D deficiency, vitamin B12 deficiency, tinea pedis, and BCC presented with periungual verruca vulgaris on the right fifth digit and left thumb (Figure 1). The patient was prescribed imiquimod cream 5% to be applied 3 times weekly for 3 months. At 5-month follow-up the patient reported new-onset vitiligolike patches of depigmentation on the hands and feet that abruptly began 3 months after initiating treatment with imiquimod. On examination he had several depigmented patches with well-defined irregular borders on the bilateral dorsal hands and right foot as well as the right elbow (Figure 2). There was no personal or family history of vitiligo, thyroid disease, or autoimmune disease. Thyroid function studies and autoimmune panel were unremarkable. The patient also denied applying imiquimod to areas other than the periungual region of the right fifth digit and left thumb. He declined a biopsy of the lesions and was given a prescription for tacrolimus ointment 0.1% for twice-daily application. At 3-month follow-up the depigmented patches had spread. The patient is currently on 5-fluorouracil cream 5%. Despite loss of pigmentation, the periungual verruca vulgaris has persisted as well as depigmentation.
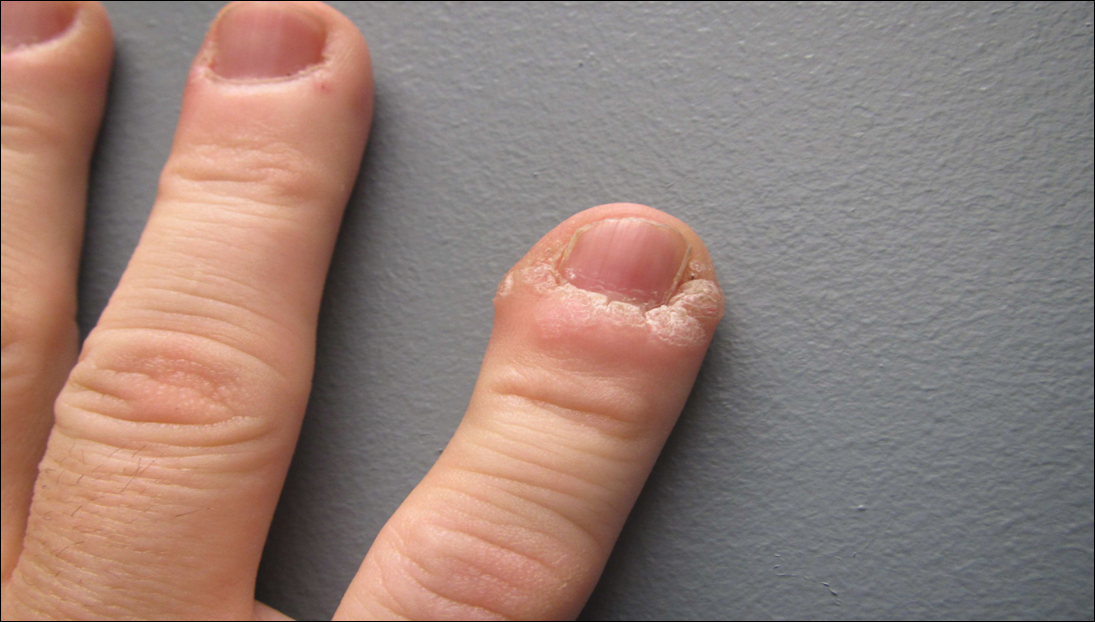
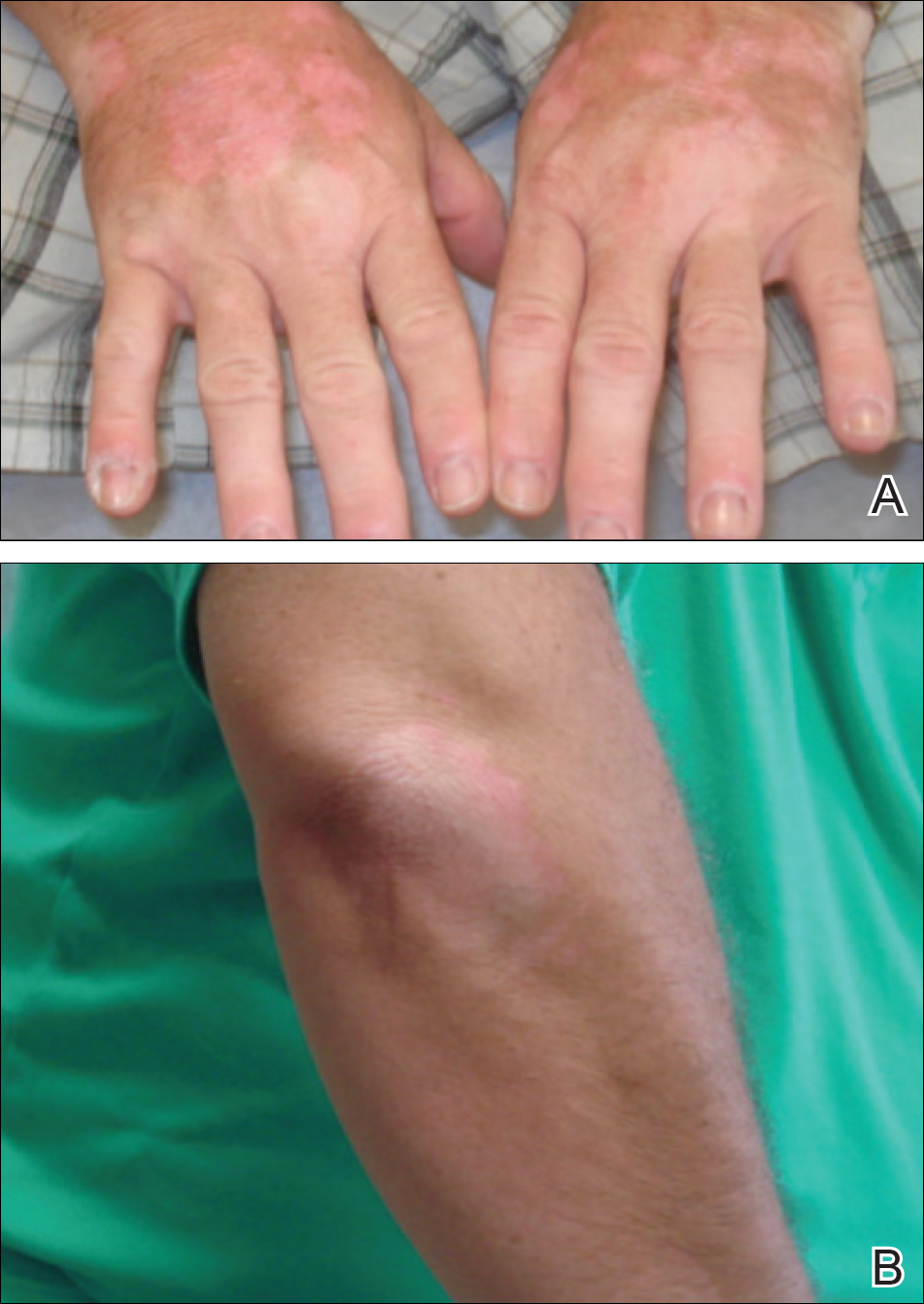
Comment
Imiquimod therapy is commonly used to treat conditions for which an antiviral or antitumor immune response is necessary for treatment and full resolution of skin conditions. It can yield positive results in conditions that are difficult to treat, such as periungual verruca vulgaris.4 The most common adverse effects of imiquimod include localized inflammation and application-site reactions. Pigment changes, though less common, also have been reported. From 1997 to 2003, 1257 cases of imiquimod adverse effects were reported to the FDA. There were 68 reported cases of pigmentary change, of which 51 documented vitiligo, hypopigmentation, or depigmentation. The others reported hyperpigmentation following imiquimod use.4 The imiquimod package insert lists application-site hypopigmentation as a possible adverse effect.5 Imiquimod-induced hypopigmentation and depigmentation have been reported in the peer-reviewed literature.4,6-14 Pigment loss has been reported in imiquimod treatment of condyloma acuminata, superficial BCC, nodular BCC, and extramammary Paget disease.6-8 Duration of therapy to onset of pigment loss ranged from 7 to 28 weeks.9 Imiquimod dosing varied among reported cases, ranging from 3 times weekly to daily application. Interestingly, hypopigmentation or depigmentation are not commonly associated with imiquimod use for the treatment of AKs, which Burnett and Kouba9 proposed may be due to the twice weekly imiquimod dosing regimen recommended by the FDA for the treatment of AK (below the minimum threshold for pigment loss). Our patient applied imiquimod cream 5% to periungual verruca vulgaris 3 times weekly for 3 months and may have developed vitiligolike depigmentation because he met this theoretical dosage threshold. Further research is necessary to confirm a dosage-related threshold for the development of depigmentation. Imiquimod-induced pigment loss has mainly been limited to the site of application.
Depigmentation was limited to the application site the majority of the time; however, depigmentation at adjacent sites has been reported.10 This finding was consistent with the proposed notion that cytokines induced by imiquimod have localized paracrine activity.11 Our patient was unique in that his depigmentation was present at the site of application, adjacent to the site of application, and at distant sites. He applied imiquimod only to the periungual area of the right fifth digit and left thumb but experienced depigmentation at several other sites. Although it is possible that our patient unintentionally spread imiquimod on the distant sites, it is less likely that the application would have been sufficient to cause depigmentation. Although systemic absorption of topical medications varies depending on multiple factors, the systemic absorption of imiquimod is minimal with mild systemic side effects reported, including headache, myalgia, and influenzalike symptoms.5 Thus, it is possible that our patient developed distant vitiligolike depigmentation as a systemic side effect of imiquimod therapy. Although our patient declined to have a biopsy performed, Gowda et al15 reported biopsy-proven vitiligo, demonstrating the absence of melanin and melanocytes following the use of imiquimod.
Several mechanisms have been proposed for imiquimod-induced depigmentation. For example, imiquimod may induce melanocyte apoptosis by increasing the levels of several proinflammatory and proapoptotic cytokines.16 Imiquimod-induced melanocyte apoptosis appears to involve elevated caspase-3, decreased B-cell lymphoma 2, altered mitogen-activated protein kinase expression, and ubiquitin-mediated proteolysis.13,17 Additionally, increased levels of IL-6 appear to increase melanocyte-binding molecules and increase melanocyte-leukocyte interactions. Another proposed theory targets toll-like receptor 7 on melanocytes that are acted on directly by imiquimod.11,17 In contrast, development of vitiligo following trauma (Koebner phenomenon) is not uncommon, and the immune effects induced by imiquimod may mimic those seen with trauma.14 Further research is needed to elucidate the mechanism by which imiquimod causes vitiligolike depigmentation.
Unfortunately, the depigmentation seen with imiquimod generally is permanent. Stefanaki et al10 showed repigmentation on cessation of imiquimod use. Our patient’s depigmentation remains unchanged despite treatment with tacrolimus ointment. Although it is possible for vitiligo to occur de novo without obvious inciting event or laboratory abnormality, the timeline and number of other cases in the literature make ours highly suspect for imiquimod-induced depigmentation.
Conclusion
Imiquimod is a commonly used immune-enhancing medication with an increasing list of off-label uses. Prior to prescribing imiquimod for a benign skin condition, clinicians should be cognizant of the potential for localized or possibly even distant depigmentation. We report a case of distant depigmentation following the use of imiquimod for periungual verruca vulgaris.
- Ganjian S, Ourian AJ, Shamtoub G, et al. Off-label indications for imiquimod. Dermatol Online J. 2009;15:4.
- Skinner RB Jr. Imiquimod. Dermatol Clin. 2003;21:291-300.
- Murphy K, Travers P, Walport M. Innate immunity. In: Murphy K, Travers P, Walport M, eds. Janeway’s Immunobiology. 7th ed. New York, NY: Garland Science. 2008:39-108.
- Brown T, Zirvi M, Cotsarelis G, et al. Vitiligo-like hypopigmentation associated with imiquimod treatment of genital warts. J Am Acad Dermatol. 2005;52:715-716.
- Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2007.
- Kwon HH, Cho KH. Induction of vitiligo-like hypopigmentation after imiquimod treatment of extramammary Paget’s disease. Ann Dermatol. 2012;24:482-484.
- Mendonca CO, Yates VM. Permanent facial hypopigmentation following treatment with imiquimod. Clin Exp Dermatol. 2006;31:721-722.
- Zhang R, Zhu W. Genital vitiligo following use of imiquimod 5% cream. Indian J Dermatol. 2011;56:335-336.
- Burnett CT, Kouba DJ. Imiquimod-induced depigmentation: report of two cases and review of the literature. Dermatol Surg. 2012;38:1872-1875.
- Stefanaki C, Nicolaidou E, Hadjivassiliou M. Imiquimod-induced vitiligo in a patient with genital warts. J Eur Acad Dermatol Venereol. 2006;20:755-756.
- Al-Dujaili Z, Hsu S. Imiquimod-induced vitiligo. Dermatol Online J. 2007;13:10.
- Mashiah J, Brenner S. Possible mechanisms in the induction of vitiligo-like hypopigmentation by topical imiquimod. Clin Exp Dermatol. 2007;33:74-76.
- Grahovac M, Ehmann LM, Flaig M, et al. Giant basal cell carcinoma. Improvement and vitiligo-like hypopigmentation after intermittent treatment with 5% imiquimod. Acta Dermatovenerol Croat. 2012;20:275-278.
- Serrão VV, Páris FR, Feio AB. Genital vitiligo-like depigmentation following use of imiquimod 5% cream. Eur J Dermatol. 2008;18:342-343.
- Gowda S, Tillman DK, Fitzpatrick JE, et al. Imiquimod-induced vitiligo after treatment of nodular basal cell carcinoma. J Cutan Pathol. 2009;36:878-881.
- Kim CH, Ahn JH, Kang SU, et al. Imiquimod induces apoptosis of human melanocytes. Arch Dermatol Res. 2010;302:301-306.
- Eapen BR. Vitiligo, psoriasis, and imiquimod: fitting all into the same pathway. Indian J Dermatol Venereol Leprol. 2008;74:169.
Imiquimod is derived from the imidazoquinoline family and works by activating both innate and adaptive immune pathways. Imiquimod binds to toll-like receptor 7 located on monocytes, macrophages, and dendritic cells,1 which allows nuclear factor κβ light chain enhancer of activated B cells to induce production of proinflammatory cytokines, including IFN-α and tumor necrosis factor α, as well as IL-1, IL-6, IL-8, IL-10, and IL-12.2 These proinflammatory cytokines play a role in the innate immunity, triggering upregulation of the adaptive immune pathway and activating type 1 helper T cells, cytotoxic T cells, and natural killer cells. These cells have antiviral and antitumoral effects that lend to their significance in coordinating innate and adaptive immune mechanisms.3 More specifically, imiquimod enhances dendritic cell migration to regional lymph nodes and induces apoptosis via activation of proapoptotic B-cell lymphoma 2 proteins.1,2 Imiquimod has been approved by the US Food and Drug Administration (FDA) to treat external genitalia and perianal condyloma acuminata, actinic keratoses (AKs), and superficial basal cell carcinoma (BCC). It often is used off label for antiviral or antitumoral therapy in Bowen disease, squamous cell carcinoma, lentigo maligna, vulvar intraepithelial neoplasia, molluscum contagiosum, common warts, and leishmaniasis.1,2 Imiquimod is generally well tolerated; erythema and irritation at the application site are the most common side effects, with pigmentary change being less common.
Case Report
A 51-year-old man with a medical history of vitamin D deficiency, vitamin B12 deficiency, tinea pedis, and BCC presented with periungual verruca vulgaris on the right fifth digit and left thumb (Figure 1). The patient was prescribed imiquimod cream 5% to be applied 3 times weekly for 3 months. At 5-month follow-up the patient reported new-onset vitiligolike patches of depigmentation on the hands and feet that abruptly began 3 months after initiating treatment with imiquimod. On examination he had several depigmented patches with well-defined irregular borders on the bilateral dorsal hands and right foot as well as the right elbow (Figure 2). There was no personal or family history of vitiligo, thyroid disease, or autoimmune disease. Thyroid function studies and autoimmune panel were unremarkable. The patient also denied applying imiquimod to areas other than the periungual region of the right fifth digit and left thumb. He declined a biopsy of the lesions and was given a prescription for tacrolimus ointment 0.1% for twice-daily application. At 3-month follow-up the depigmented patches had spread. The patient is currently on 5-fluorouracil cream 5%. Despite loss of pigmentation, the periungual verruca vulgaris has persisted as well as depigmentation.


Comment
Imiquimod therapy is commonly used to treat conditions for which an antiviral or antitumor immune response is necessary for treatment and full resolution of skin conditions. It can yield positive results in conditions that are difficult to treat, such as periungual verruca vulgaris.4 The most common adverse effects of imiquimod include localized inflammation and application-site reactions. Pigment changes, though less common, also have been reported. From 1997 to 2003, 1257 cases of imiquimod adverse effects were reported to the FDA. There were 68 reported cases of pigmentary change, of which 51 documented vitiligo, hypopigmentation, or depigmentation. The others reported hyperpigmentation following imiquimod use.4 The imiquimod package insert lists application-site hypopigmentation as a possible adverse effect.5 Imiquimod-induced hypopigmentation and depigmentation have been reported in the peer-reviewed literature.4,6-14 Pigment loss has been reported in imiquimod treatment of condyloma acuminata, superficial BCC, nodular BCC, and extramammary Paget disease.6-8 Duration of therapy to onset of pigment loss ranged from 7 to 28 weeks.9 Imiquimod dosing varied among reported cases, ranging from 3 times weekly to daily application. Interestingly, hypopigmentation or depigmentation are not commonly associated with imiquimod use for the treatment of AKs, which Burnett and Kouba9 proposed may be due to the twice weekly imiquimod dosing regimen recommended by the FDA for the treatment of AK (below the minimum threshold for pigment loss). Our patient applied imiquimod cream 5% to periungual verruca vulgaris 3 times weekly for 3 months and may have developed vitiligolike depigmentation because he met this theoretical dosage threshold. Further research is necessary to confirm a dosage-related threshold for the development of depigmentation. Imiquimod-induced pigment loss has mainly been limited to the site of application.
Depigmentation was limited to the application site the majority of the time; however, depigmentation at adjacent sites has been reported.10 This finding was consistent with the proposed notion that cytokines induced by imiquimod have localized paracrine activity.11 Our patient was unique in that his depigmentation was present at the site of application, adjacent to the site of application, and at distant sites. He applied imiquimod only to the periungual area of the right fifth digit and left thumb but experienced depigmentation at several other sites. Although it is possible that our patient unintentionally spread imiquimod on the distant sites, it is less likely that the application would have been sufficient to cause depigmentation. Although systemic absorption of topical medications varies depending on multiple factors, the systemic absorption of imiquimod is minimal with mild systemic side effects reported, including headache, myalgia, and influenzalike symptoms.5 Thus, it is possible that our patient developed distant vitiligolike depigmentation as a systemic side effect of imiquimod therapy. Although our patient declined to have a biopsy performed, Gowda et al15 reported biopsy-proven vitiligo, demonstrating the absence of melanin and melanocytes following the use of imiquimod.
Several mechanisms have been proposed for imiquimod-induced depigmentation. For example, imiquimod may induce melanocyte apoptosis by increasing the levels of several proinflammatory and proapoptotic cytokines.16 Imiquimod-induced melanocyte apoptosis appears to involve elevated caspase-3, decreased B-cell lymphoma 2, altered mitogen-activated protein kinase expression, and ubiquitin-mediated proteolysis.13,17 Additionally, increased levels of IL-6 appear to increase melanocyte-binding molecules and increase melanocyte-leukocyte interactions. Another proposed theory targets toll-like receptor 7 on melanocytes that are acted on directly by imiquimod.11,17 In contrast, development of vitiligo following trauma (Koebner phenomenon) is not uncommon, and the immune effects induced by imiquimod may mimic those seen with trauma.14 Further research is needed to elucidate the mechanism by which imiquimod causes vitiligolike depigmentation.
Unfortunately, the depigmentation seen with imiquimod generally is permanent. Stefanaki et al10 showed repigmentation on cessation of imiquimod use. Our patient’s depigmentation remains unchanged despite treatment with tacrolimus ointment. Although it is possible for vitiligo to occur de novo without obvious inciting event or laboratory abnormality, the timeline and number of other cases in the literature make ours highly suspect for imiquimod-induced depigmentation.
Conclusion
Imiquimod is a commonly used immune-enhancing medication with an increasing list of off-label uses. Prior to prescribing imiquimod for a benign skin condition, clinicians should be cognizant of the potential for localized or possibly even distant depigmentation. We report a case of distant depigmentation following the use of imiquimod for periungual verruca vulgaris.
Imiquimod is derived from the imidazoquinoline family and works by activating both innate and adaptive immune pathways. Imiquimod binds to toll-like receptor 7 located on monocytes, macrophages, and dendritic cells,1 which allows nuclear factor κβ light chain enhancer of activated B cells to induce production of proinflammatory cytokines, including IFN-α and tumor necrosis factor α, as well as IL-1, IL-6, IL-8, IL-10, and IL-12.2 These proinflammatory cytokines play a role in the innate immunity, triggering upregulation of the adaptive immune pathway and activating type 1 helper T cells, cytotoxic T cells, and natural killer cells. These cells have antiviral and antitumoral effects that lend to their significance in coordinating innate and adaptive immune mechanisms.3 More specifically, imiquimod enhances dendritic cell migration to regional lymph nodes and induces apoptosis via activation of proapoptotic B-cell lymphoma 2 proteins.1,2 Imiquimod has been approved by the US Food and Drug Administration (FDA) to treat external genitalia and perianal condyloma acuminata, actinic keratoses (AKs), and superficial basal cell carcinoma (BCC). It often is used off label for antiviral or antitumoral therapy in Bowen disease, squamous cell carcinoma, lentigo maligna, vulvar intraepithelial neoplasia, molluscum contagiosum, common warts, and leishmaniasis.1,2 Imiquimod is generally well tolerated; erythema and irritation at the application site are the most common side effects, with pigmentary change being less common.
Case Report
A 51-year-old man with a medical history of vitamin D deficiency, vitamin B12 deficiency, tinea pedis, and BCC presented with periungual verruca vulgaris on the right fifth digit and left thumb (Figure 1). The patient was prescribed imiquimod cream 5% to be applied 3 times weekly for 3 months. At 5-month follow-up the patient reported new-onset vitiligolike patches of depigmentation on the hands and feet that abruptly began 3 months after initiating treatment with imiquimod. On examination he had several depigmented patches with well-defined irregular borders on the bilateral dorsal hands and right foot as well as the right elbow (Figure 2). There was no personal or family history of vitiligo, thyroid disease, or autoimmune disease. Thyroid function studies and autoimmune panel were unremarkable. The patient also denied applying imiquimod to areas other than the periungual region of the right fifth digit and left thumb. He declined a biopsy of the lesions and was given a prescription for tacrolimus ointment 0.1% for twice-daily application. At 3-month follow-up the depigmented patches had spread. The patient is currently on 5-fluorouracil cream 5%. Despite loss of pigmentation, the periungual verruca vulgaris has persisted as well as depigmentation.


Comment
Imiquimod therapy is commonly used to treat conditions for which an antiviral or antitumor immune response is necessary for treatment and full resolution of skin conditions. It can yield positive results in conditions that are difficult to treat, such as periungual verruca vulgaris.4 The most common adverse effects of imiquimod include localized inflammation and application-site reactions. Pigment changes, though less common, also have been reported. From 1997 to 2003, 1257 cases of imiquimod adverse effects were reported to the FDA. There were 68 reported cases of pigmentary change, of which 51 documented vitiligo, hypopigmentation, or depigmentation. The others reported hyperpigmentation following imiquimod use.4 The imiquimod package insert lists application-site hypopigmentation as a possible adverse effect.5 Imiquimod-induced hypopigmentation and depigmentation have been reported in the peer-reviewed literature.4,6-14 Pigment loss has been reported in imiquimod treatment of condyloma acuminata, superficial BCC, nodular BCC, and extramammary Paget disease.6-8 Duration of therapy to onset of pigment loss ranged from 7 to 28 weeks.9 Imiquimod dosing varied among reported cases, ranging from 3 times weekly to daily application. Interestingly, hypopigmentation or depigmentation are not commonly associated with imiquimod use for the treatment of AKs, which Burnett and Kouba9 proposed may be due to the twice weekly imiquimod dosing regimen recommended by the FDA for the treatment of AK (below the minimum threshold for pigment loss). Our patient applied imiquimod cream 5% to periungual verruca vulgaris 3 times weekly for 3 months and may have developed vitiligolike depigmentation because he met this theoretical dosage threshold. Further research is necessary to confirm a dosage-related threshold for the development of depigmentation. Imiquimod-induced pigment loss has mainly been limited to the site of application.
Depigmentation was limited to the application site the majority of the time; however, depigmentation at adjacent sites has been reported.10 This finding was consistent with the proposed notion that cytokines induced by imiquimod have localized paracrine activity.11 Our patient was unique in that his depigmentation was present at the site of application, adjacent to the site of application, and at distant sites. He applied imiquimod only to the periungual area of the right fifth digit and left thumb but experienced depigmentation at several other sites. Although it is possible that our patient unintentionally spread imiquimod on the distant sites, it is less likely that the application would have been sufficient to cause depigmentation. Although systemic absorption of topical medications varies depending on multiple factors, the systemic absorption of imiquimod is minimal with mild systemic side effects reported, including headache, myalgia, and influenzalike symptoms.5 Thus, it is possible that our patient developed distant vitiligolike depigmentation as a systemic side effect of imiquimod therapy. Although our patient declined to have a biopsy performed, Gowda et al15 reported biopsy-proven vitiligo, demonstrating the absence of melanin and melanocytes following the use of imiquimod.
Several mechanisms have been proposed for imiquimod-induced depigmentation. For example, imiquimod may induce melanocyte apoptosis by increasing the levels of several proinflammatory and proapoptotic cytokines.16 Imiquimod-induced melanocyte apoptosis appears to involve elevated caspase-3, decreased B-cell lymphoma 2, altered mitogen-activated protein kinase expression, and ubiquitin-mediated proteolysis.13,17 Additionally, increased levels of IL-6 appear to increase melanocyte-binding molecules and increase melanocyte-leukocyte interactions. Another proposed theory targets toll-like receptor 7 on melanocytes that are acted on directly by imiquimod.11,17 In contrast, development of vitiligo following trauma (Koebner phenomenon) is not uncommon, and the immune effects induced by imiquimod may mimic those seen with trauma.14 Further research is needed to elucidate the mechanism by which imiquimod causes vitiligolike depigmentation.
Unfortunately, the depigmentation seen with imiquimod generally is permanent. Stefanaki et al10 showed repigmentation on cessation of imiquimod use. Our patient’s depigmentation remains unchanged despite treatment with tacrolimus ointment. Although it is possible for vitiligo to occur de novo without obvious inciting event or laboratory abnormality, the timeline and number of other cases in the literature make ours highly suspect for imiquimod-induced depigmentation.
Conclusion
Imiquimod is a commonly used immune-enhancing medication with an increasing list of off-label uses. Prior to prescribing imiquimod for a benign skin condition, clinicians should be cognizant of the potential for localized or possibly even distant depigmentation. We report a case of distant depigmentation following the use of imiquimod for periungual verruca vulgaris.
- Ganjian S, Ourian AJ, Shamtoub G, et al. Off-label indications for imiquimod. Dermatol Online J. 2009;15:4.
- Skinner RB Jr. Imiquimod. Dermatol Clin. 2003;21:291-300.
- Murphy K, Travers P, Walport M. Innate immunity. In: Murphy K, Travers P, Walport M, eds. Janeway’s Immunobiology. 7th ed. New York, NY: Garland Science. 2008:39-108.
- Brown T, Zirvi M, Cotsarelis G, et al. Vitiligo-like hypopigmentation associated with imiquimod treatment of genital warts. J Am Acad Dermatol. 2005;52:715-716.
- Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2007.
- Kwon HH, Cho KH. Induction of vitiligo-like hypopigmentation after imiquimod treatment of extramammary Paget’s disease. Ann Dermatol. 2012;24:482-484.
- Mendonca CO, Yates VM. Permanent facial hypopigmentation following treatment with imiquimod. Clin Exp Dermatol. 2006;31:721-722.
- Zhang R, Zhu W. Genital vitiligo following use of imiquimod 5% cream. Indian J Dermatol. 2011;56:335-336.
- Burnett CT, Kouba DJ. Imiquimod-induced depigmentation: report of two cases and review of the literature. Dermatol Surg. 2012;38:1872-1875.
- Stefanaki C, Nicolaidou E, Hadjivassiliou M. Imiquimod-induced vitiligo in a patient with genital warts. J Eur Acad Dermatol Venereol. 2006;20:755-756.
- Al-Dujaili Z, Hsu S. Imiquimod-induced vitiligo. Dermatol Online J. 2007;13:10.
- Mashiah J, Brenner S. Possible mechanisms in the induction of vitiligo-like hypopigmentation by topical imiquimod. Clin Exp Dermatol. 2007;33:74-76.
- Grahovac M, Ehmann LM, Flaig M, et al. Giant basal cell carcinoma. Improvement and vitiligo-like hypopigmentation after intermittent treatment with 5% imiquimod. Acta Dermatovenerol Croat. 2012;20:275-278.
- Serrão VV, Páris FR, Feio AB. Genital vitiligo-like depigmentation following use of imiquimod 5% cream. Eur J Dermatol. 2008;18:342-343.
- Gowda S, Tillman DK, Fitzpatrick JE, et al. Imiquimod-induced vitiligo after treatment of nodular basal cell carcinoma. J Cutan Pathol. 2009;36:878-881.
- Kim CH, Ahn JH, Kang SU, et al. Imiquimod induces apoptosis of human melanocytes. Arch Dermatol Res. 2010;302:301-306.
- Eapen BR. Vitiligo, psoriasis, and imiquimod: fitting all into the same pathway. Indian J Dermatol Venereol Leprol. 2008;74:169.
- Ganjian S, Ourian AJ, Shamtoub G, et al. Off-label indications for imiquimod. Dermatol Online J. 2009;15:4.
- Skinner RB Jr. Imiquimod. Dermatol Clin. 2003;21:291-300.
- Murphy K, Travers P, Walport M. Innate immunity. In: Murphy K, Travers P, Walport M, eds. Janeway’s Immunobiology. 7th ed. New York, NY: Garland Science. 2008:39-108.
- Brown T, Zirvi M, Cotsarelis G, et al. Vitiligo-like hypopigmentation associated with imiquimod treatment of genital warts. J Am Acad Dermatol. 2005;52:715-716.
- Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2007.
- Kwon HH, Cho KH. Induction of vitiligo-like hypopigmentation after imiquimod treatment of extramammary Paget’s disease. Ann Dermatol. 2012;24:482-484.
- Mendonca CO, Yates VM. Permanent facial hypopigmentation following treatment with imiquimod. Clin Exp Dermatol. 2006;31:721-722.
- Zhang R, Zhu W. Genital vitiligo following use of imiquimod 5% cream. Indian J Dermatol. 2011;56:335-336.
- Burnett CT, Kouba DJ. Imiquimod-induced depigmentation: report of two cases and review of the literature. Dermatol Surg. 2012;38:1872-1875.
- Stefanaki C, Nicolaidou E, Hadjivassiliou M. Imiquimod-induced vitiligo in a patient with genital warts. J Eur Acad Dermatol Venereol. 2006;20:755-756.
- Al-Dujaili Z, Hsu S. Imiquimod-induced vitiligo. Dermatol Online J. 2007;13:10.
- Mashiah J, Brenner S. Possible mechanisms in the induction of vitiligo-like hypopigmentation by topical imiquimod. Clin Exp Dermatol. 2007;33:74-76.
- Grahovac M, Ehmann LM, Flaig M, et al. Giant basal cell carcinoma. Improvement and vitiligo-like hypopigmentation after intermittent treatment with 5% imiquimod. Acta Dermatovenerol Croat. 2012;20:275-278.
- Serrão VV, Páris FR, Feio AB. Genital vitiligo-like depigmentation following use of imiquimod 5% cream. Eur J Dermatol. 2008;18:342-343.
- Gowda S, Tillman DK, Fitzpatrick JE, et al. Imiquimod-induced vitiligo after treatment of nodular basal cell carcinoma. J Cutan Pathol. 2009;36:878-881.
- Kim CH, Ahn JH, Kang SU, et al. Imiquimod induces apoptosis of human melanocytes. Arch Dermatol Res. 2010;302:301-306.
- Eapen BR. Vitiligo, psoriasis, and imiquimod: fitting all into the same pathway. Indian J Dermatol Venereol Leprol. 2008;74:169.
Practice Points
- Imiquimod commonly is used off label to treat viral and neoplastic processes.
- Clinicians should be aware of the potential for dyspigmentation or depigmentation as a side effect from treatment.
Unusual Presentation of Erythema Elevatum Diutinum With Underlying Hepatitis B Infection
Erythema elevatum diutinum (EED) manifests on a clinicopathologic spectrum of chronic cutaneous small vessel vasculitis. The lesions typically present as persistent, symmetric, firm, red to purple papules or nodules on the extensor arms and dorsal hands.1,2 Underlying infectious, malignant, or autoimmune processes are commonly associated with the disease, notably Streptococcus infection and IgA monoclonal gammopathy.2,3 Hepatitis virus also is often implicated in association with EED. Cases of EED have been seen with concomitant human immunodeficiency virus (HIV) infection.4-6 We report a case of EED presenting in various stages of evolution associated with underlying hepatitis B infection alone.
Case Report
A 57-year-old man originally presented to an outpatient dermatology practice with a nodular, painful, episodic rash on the trunk and upper and lower extremities. A biopsy revealed leukocytoclastic vasculitis (LCV) with prominent eosinophils. At the time, the skin findings were believed to be a manifestation of drug hypersensitivity, likely to opioid use. The patient was lost to follow-up.
Seven years later, the patient was admitted to the hospital with new-onset burning and stinging red nodules on the dorsum of the hands and persistence of the original episodic rash over the lower legs and bilateral flanks. In the interim, he was briefly treated with an oral prednisone taper and topical corticosteroids including triamcinolone cream 0.1% and clobetasol cream 0.05% without improvement.
On examination deep red to violaceous discrete nodules and plaques with overlying hyperkeratosis involving all distal and proximal interphalangeal joints of the hands and extensor elbows were seen (Figure 1A). On the bilateral posterior arms (Figure 1B), anterior legs, and periumbilical area were deeply erythematous papules and plaques with background hyperpigmentation. Across his lower back and bilateral flanks were erythematous papules with central hemorrhagic crusting (Figure 1C).
Pertinent laboratory findings included a positive hepatitis B surface antigen with hepatitis B DNA value 4,313,876 IU/mL and a hepatitis B virus quantitative polymerase chain reaction value of 6.64 U. The etiology was suspected to be intravenous drug abuse; however, the patient denied recreational drug use.
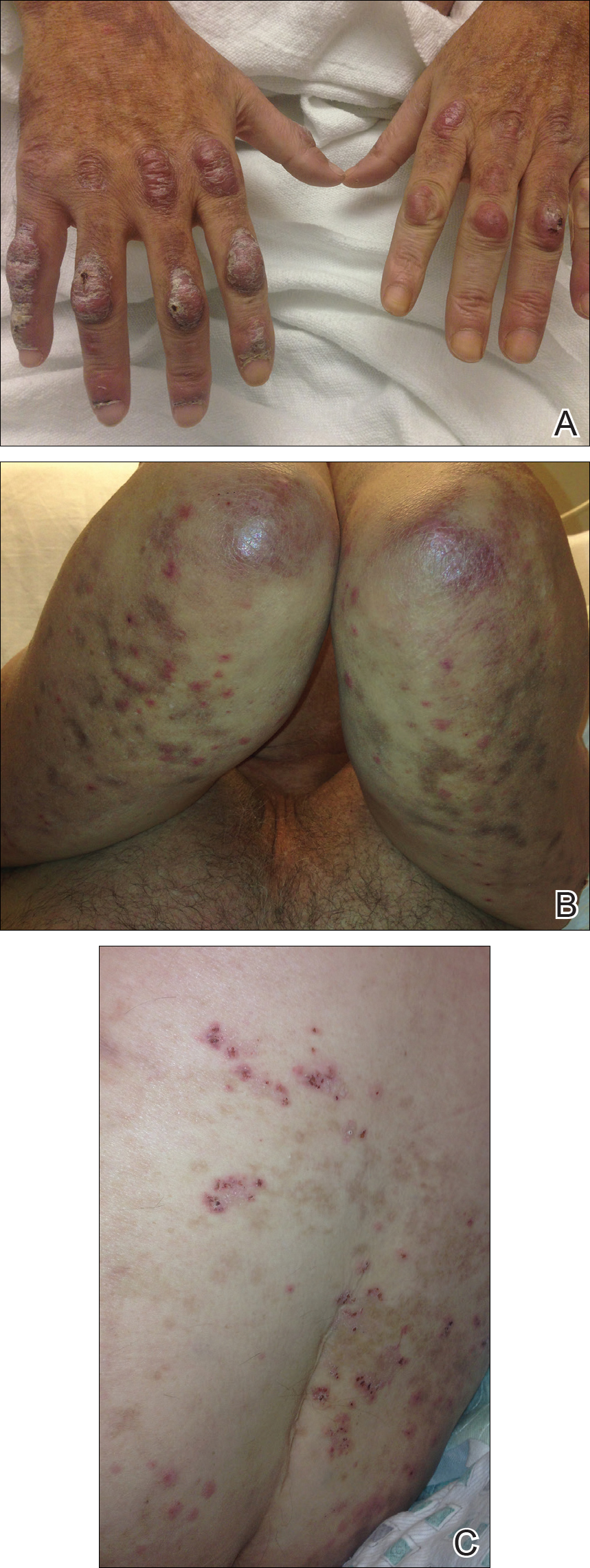
An additional infectious workup was negative for hepatitis C, streptococcus, syphilis, tuberculosis, and HIV. A complete blood cell count, complete metabolic panel, urinalysis, complement, cryoglobulins, and serum protein electrophoresis were within reference range. Autoimmune serologies were negative including antinuclear antibody, rheumatoid factor, anti-Sjögren syndrome–related antigen A and B, anticyclic citrullinated peptide, anti-Smith, and antineutrophilic cytoplasmic antibodies. Peripheral blood immunophenotyping, lactate dehydrogenase, quantitative immunoglobulins, and age-appropriate cancer screens did not demonstrate evidence for malignancy underlying the disease. Bilateral hand radiographs showed mild periostitis of the proximal phalanges without obvious erosions.
Three 4-mm punch biopsies were performed from the left fifth digit, left posterior arm, and left flank. Tissue of the left fifth digit showed an intradermal vascular proliferation with a concentric pattern resembling onion skin in a background of increased fibrosis. The blood vessels showed focal fibrinoid necrosis (Figure 2A). The biopsy of the left posterior arm showed an intradermal vascular proliferation with an associated mild acute and chronic perivascular inflammation (Figure 2B). The left flank biopsy showed LCV with focal epidermal necrosis (Figure 2C).
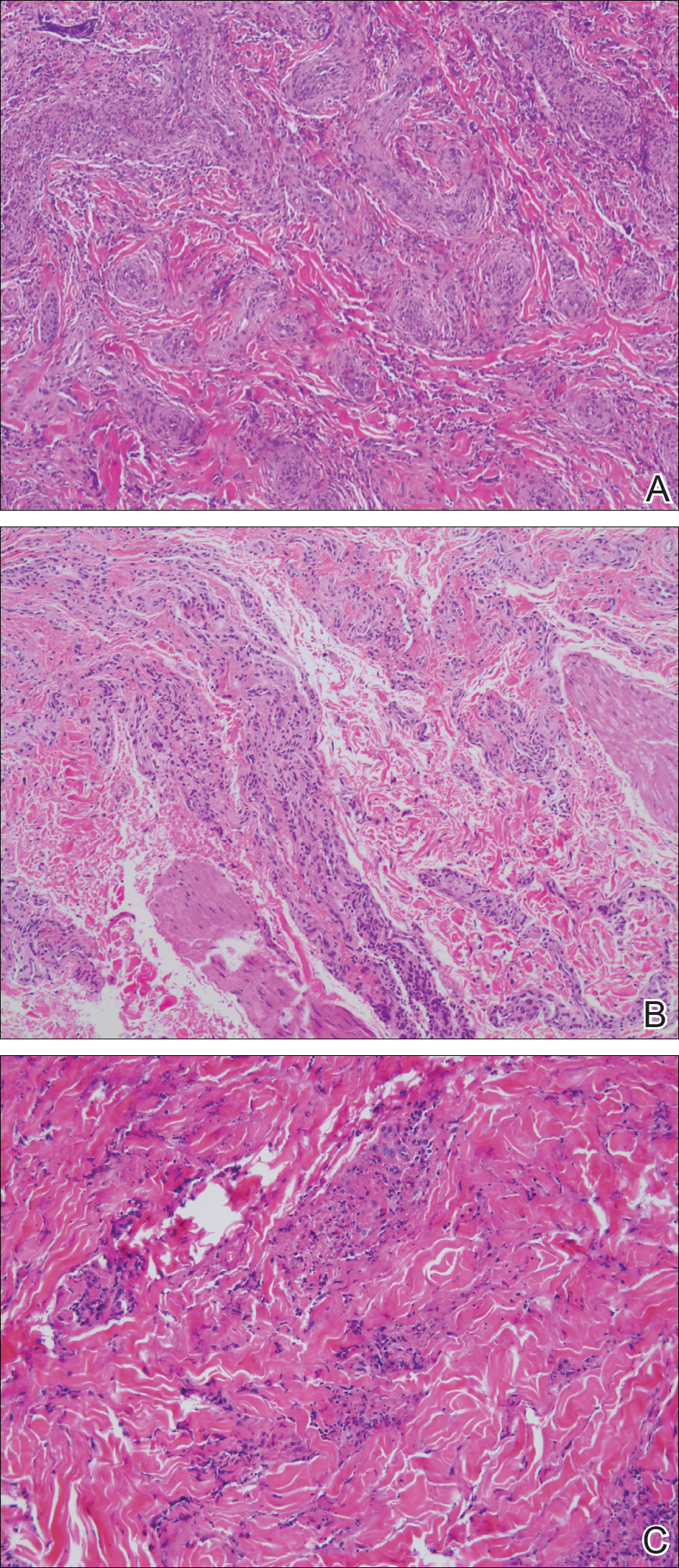
The constellation of clinical findings together with the histopathologic changes represented EED in various stages of evolution. The patient was started on dapsone 100 mg daily and referred to the infectious disease service for treatment of chronic hepatitis B; however, he was subsequently lost to follow-up.
Comment
Overview of EED
Erythema elevatum diutinum represents a rare form of chronic cutaneous small vessel vasculitis. Originally described by Hutchinson7 and Bury8 as symmetric purpuric nodules of the skin, it was later named by Crocker and Williams9 in 1894. The disease classically presents as firm, fixed, red-brown to violaceous papules, plaques, and nodules affecting the extensor upper or lower extremities.1 Lesions are most commonly found symmetrically overlying joints of the hands, feet, elbows, and knees, as well as the Achilles tendon and buttocks.3 Less common locations include the palms and soles, face,10,11 trunk,12 and periauricular region.1 Although they are typically asymptomatic, sensations such as burning, stinging, and pruritus have been noted.1 Our patient was unique because in addition to typical lesions of EED, he presented with crusted papules on the flanks and violaceous papules of the lower legs and periumbilicus.
Etiology
Originally associated with Streptococcus as isolated from EED lesions,3,13 additional infectious etiologies include viral hepatitis,4-6 human herpesvirus 6,14 and rarely HIV.1,15 Hepatitis B and C are well known to be associated with EED, with only rare reports in patients with concomitant HIV infection. Erythema elevatum diutinum also has been described in relationship to myeloproliferative disorders and hematologic malignancies such as IgA myeloma,16 non-Hodgkin lymphoma,17 chronic lymphocytic leukemia,18 and hypergammaglobulinemia.19 In a study of 13 patients with EED, 4 had associated underlying IgA monoclonal gammopathy.2 Autoimmune conditions such as rheumatoid arthritis,20 ulcerative colitis,21 relapsing polychondritis,22 and systemic lupus erythematosus23 also have been implicated.
Pathogenesis
Although the precise pathogenesis of EED remains unknown, it has been suggested that a complement cascade initiated by immune-complex deposition in postcapillary venules induces an LCV.24,25 Chronic antigenic exposure or high antibody levels26 in the face of infections, autoimmune disease, or malignancy may incite this immune-complex reaction. Skin lesions seen in association with hepatitis reflect circulating immune-complex deposition in vessel walls causing destruction. It has been postulated that the duration of immune complexemia may be sufficient to account for the differences in the type of vascular injury seen in acute versus chronic infection.27
Histopathology
Erythema elevatum diutinum may present on a histopathologic spectrum of LCV, as manifested in our patient. Early lesions show predominantly polymorphonuclear cells with nuclear dust pattern in a wedge-shaped infiltrate with fibrin deposition in the superficial and mid dermis.2,3 Later lesions show vasculitis in addition to dermal aggregates of lymphocytes, neutrophils, fibrosis, and areas of granulation tissue. The fibrosis may be dense and comprised of fibroblasts and myofibroblasts.28 Newly formed vessels within the granulation tissue have been postulated to be more susceptible to immune-complex deposition, thus potentiating the process.1,29
Management
Spontaneous resolution of EED may occur, albeit after a prolonged and recurrent course of up to 5 to 10 years.30 Treatment of the underlying cause, when identified, remains paramount. First-line therapy includes dapsone, shown to be effective in reducing lesion size to complete resolution in 80% of the 47 cases reviewed by Momen et al.31 Dapsone monotherapy tends to be less effective in treating nodular lesions associated with HIV-positivity, likely due to the extensive fibrosis.4,31 Combination therapy with dapsone and a sulfonamide,32 niacinamide and tetracycline,33 colchicine,34 or surgical excision35 may be necessary in more resistant cases.
Conclusion
Our case exemplifies the clinical histologic spectrum that EED can present. The constellation of clinical findings was histologically confirmed to be manifestations of the disease in various stages of evolution. When typical lesions of EED present along with cutaneous findings in less common locations, performing multiple biopsies can be helpful. The clinician should retain a high index of suspicion for an underlying etiology and perform a complete workup for infection, malignancy, or autoimmune disease.
- Gibson LE, el-Azhary RA. Erythema elevatum diutinum. Clin Dermatol. 2000;18:295-299.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Wilkinson SM, English JS, Smith NP, et al. Erythema elevatum diutinum: a clinicopathological study. Clin Exp Dermatol. 1992;17:87-93.
- Fakheri A, Gupta SM, White SM, et al. Erythema elevatum diutinum in a patient with human immunodeficiency virus. Cutis. 2001;68:41-42, 55.
- Kim H. Erythema elevatum diutinum in an HIV-positive patient. J Drugs Dermatol. 2003;2:411-412.
- Revenga F, Vera A, Muñoz A, et al. Erythema elevatum diutinum and AIDS: are they related? Clin Exp Dermatol. 1997;22:250-251.
- Hutchinson J. On two remarkable cases of symmetrieal purple congestion of the skin in patches, with induration. Br J Dermatol. 1888;1:10-15.
- Bury JS. A case of erythema with remarkable nodular thickening and induration of the skin associated with intermittent albuminuria. Illustrated Medical News. 1889;3:145-149.
- Crocker HR, Williams C. Erythema elevatum diutinum. Br J Dermatol. 1894;6:33-38.
- Barzegar M, Davatchi CC, Akhyani M, et al. An atypical presentation of erythema elevatum diutinum involving palms and soles. Int J Dermatol. 2009;48:73-75.
- Futei Y, Konohana I. A case of erythema elevatum diutinum associated with B-cell lymphoma: a rare distribution involving palms, soles and nails. Br J Dermatol. 2000;142:116-119.
- Ben-Zvi GT, Bardsley V, Burrows NP. An atypical distribution of erythema elevatum diutinum. Clin Exp Dermatol. 2014;39:269-270.
- Weidman FD, Besancon JH. Erythema elevatum diutinum. role of streptococci, and relationship to other rheumatic dermatoses. Arch Dermatol Syphilol. 1929;20:593-620.
- Drago F, Semino M, Rampini P, et al. Erythema elevatum diutinum in a patient with human herpesvirus 6 infection. Acta Derm Venereol. 1999;79:91-92.
- Muratori S, Carrera C, Gorani A, et al. Erythema elevatum diutinum and HIV infection: a report of five cases. Br J Dermatol. 1999;141:335-338.
- Archimandritis AJ, Fertakis A, Alegakis G, et al. Erythema elevatum diutinum and IgA myeloma: an interesting association. Br Med J. 1977;2:613-614.
- Hatzitolios A, Tzellos TG, Savopoulos C, et al. Erythema elevatum diutinum with rare distribution as a first clinical sign of non-Hodgkin’s lymphoma: a novel association? J Dermatol. 2008;35:297-300.
- Delaporte E, Alfandari S, Fenaux P, et al. Erythema elevatum diutinum and chronic lymphocytic leukaemia. Clin Exp Dermatol. 1994;19:188-189.
- Miyagawa S, Kitamura W, Morita K, et al. Association of hyperimmunoglobulinaemia D syndrome with erythema elevatum diutinum. Br J Dermatol. 1993;128:572-574.
- Collier PM, Neill SM, Branfoot AC, et al. Erythema elevatum diutinum—a solitary lesion in a patient with rheumatoid arthritis. Clin Exp Dermatol. 1990;15:394-395.
- Buahene K, Hudson M, Mowat A, et al. Erythema elevatum diutinum—an unusual association with ulcerative colitis. Clin Exp Dermatol. 1991;16:204-206.
- Bernard P, Bedane C, Delrous JL, et al. Erythema elevatum diutinum in a patient with relapsing polychondritis. J Am Acad Dermatol. 1992;26:312-315.
- Hancox JG, Wallace CA, Sangueza OP, et al. Erythema elevatum diutinum associated with lupus panniculitis in a patient with discoid lesions of chronic cutaneous lupus erythematosus. J Am Acad Dermatol. 2004;50:652-653.
- Haber H. Erythema elevatum diutinum. Br J Dermatol. 1955;67:121-145.
- Katz SI, Gallin JL, Hertz KC, et al. Erythema elevatum diutinum: skin and systemic manifestations, immunologic studies, and successful treatment with dapsone. Medicine (Baltimore). 1977;56:443-455.
- Walker KD, Badame AJ. Erythema elevatum diutinum in a patient with Crohn’s disease. J Am Acad Dermatol. 1990;22:948-952.
- Popp JW, Harrist T, Dienstag JL, et al. Cutaneous vasculitis associated with acute and chronic hepatitis. Arch Intern Med. 1981;141:623-629.
- Lee AY, Nakagawa H, Nogita T, et al. Erythema elevatum diutinum: an ultrastructural case study. J Cutan Pathol. 1989;16:211-217.
- LeBoit PE, Yen TS, Wintroub B. The evolution of lesions in erythema elevatum diutinum. Am J Dermatopathol. 1986;8:392-402.
- Soubeiran E, Wacker J, Hausser I, et al. Erythema elevatum diutinum with unusual clinical appearance. J Dtsch Dermatol Ges. 2008;6:303-305.
- Momen SE, Jorizzo J, Al-Niaimi F. Erythema elevatum diutinum: a review of presentation and treatment. J Eur Acad Dermatol Venereol. 2014;28:1594-1602.
- Vollum DI. Erythema elevatum diutinum—vesicular lesions and sulfone response. Br J Dermatol. 1968;80:178-183.
- Kohler IK, Lorincz AL. Erythema elevatum diutinum treated with niacinamide and tetracycline. Arch Dermatol. 1980;116:693-695.
- Henriksson R, Hofor PA, Hörngvist R. Erythema elevatum diutinum—a case successfully treated with colchicine. Clin Exp Dermatol. 1989;14:451-453.
- Zacaron LH, Gonçalves JC, Curty VM, et al. Clinical and surgical therapeutic approach in erythema elevatum diutinum—case report. An Bras Dermatol. 2013;88(6, suppl 1):15-18.
Erythema elevatum diutinum (EED) manifests on a clinicopathologic spectrum of chronic cutaneous small vessel vasculitis. The lesions typically present as persistent, symmetric, firm, red to purple papules or nodules on the extensor arms and dorsal hands.1,2 Underlying infectious, malignant, or autoimmune processes are commonly associated with the disease, notably Streptococcus infection and IgA monoclonal gammopathy.2,3 Hepatitis virus also is often implicated in association with EED. Cases of EED have been seen with concomitant human immunodeficiency virus (HIV) infection.4-6 We report a case of EED presenting in various stages of evolution associated with underlying hepatitis B infection alone.
Case Report
A 57-year-old man originally presented to an outpatient dermatology practice with a nodular, painful, episodic rash on the trunk and upper and lower extremities. A biopsy revealed leukocytoclastic vasculitis (LCV) with prominent eosinophils. At the time, the skin findings were believed to be a manifestation of drug hypersensitivity, likely to opioid use. The patient was lost to follow-up.
Seven years later, the patient was admitted to the hospital with new-onset burning and stinging red nodules on the dorsum of the hands and persistence of the original episodic rash over the lower legs and bilateral flanks. In the interim, he was briefly treated with an oral prednisone taper and topical corticosteroids including triamcinolone cream 0.1% and clobetasol cream 0.05% without improvement.
On examination deep red to violaceous discrete nodules and plaques with overlying hyperkeratosis involving all distal and proximal interphalangeal joints of the hands and extensor elbows were seen (Figure 1A). On the bilateral posterior arms (Figure 1B), anterior legs, and periumbilical area were deeply erythematous papules and plaques with background hyperpigmentation. Across his lower back and bilateral flanks were erythematous papules with central hemorrhagic crusting (Figure 1C).
Pertinent laboratory findings included a positive hepatitis B surface antigen with hepatitis B DNA value 4,313,876 IU/mL and a hepatitis B virus quantitative polymerase chain reaction value of 6.64 U. The etiology was suspected to be intravenous drug abuse; however, the patient denied recreational drug use.

An additional infectious workup was negative for hepatitis C, streptococcus, syphilis, tuberculosis, and HIV. A complete blood cell count, complete metabolic panel, urinalysis, complement, cryoglobulins, and serum protein electrophoresis were within reference range. Autoimmune serologies were negative including antinuclear antibody, rheumatoid factor, anti-Sjögren syndrome–related antigen A and B, anticyclic citrullinated peptide, anti-Smith, and antineutrophilic cytoplasmic antibodies. Peripheral blood immunophenotyping, lactate dehydrogenase, quantitative immunoglobulins, and age-appropriate cancer screens did not demonstrate evidence for malignancy underlying the disease. Bilateral hand radiographs showed mild periostitis of the proximal phalanges without obvious erosions.
Three 4-mm punch biopsies were performed from the left fifth digit, left posterior arm, and left flank. Tissue of the left fifth digit showed an intradermal vascular proliferation with a concentric pattern resembling onion skin in a background of increased fibrosis. The blood vessels showed focal fibrinoid necrosis (Figure 2A). The biopsy of the left posterior arm showed an intradermal vascular proliferation with an associated mild acute and chronic perivascular inflammation (Figure 2B). The left flank biopsy showed LCV with focal epidermal necrosis (Figure 2C).

The constellation of clinical findings together with the histopathologic changes represented EED in various stages of evolution. The patient was started on dapsone 100 mg daily and referred to the infectious disease service for treatment of chronic hepatitis B; however, he was subsequently lost to follow-up.
Comment
Overview of EED
Erythema elevatum diutinum represents a rare form of chronic cutaneous small vessel vasculitis. Originally described by Hutchinson7 and Bury8 as symmetric purpuric nodules of the skin, it was later named by Crocker and Williams9 in 1894. The disease classically presents as firm, fixed, red-brown to violaceous papules, plaques, and nodules affecting the extensor upper or lower extremities.1 Lesions are most commonly found symmetrically overlying joints of the hands, feet, elbows, and knees, as well as the Achilles tendon and buttocks.3 Less common locations include the palms and soles, face,10,11 trunk,12 and periauricular region.1 Although they are typically asymptomatic, sensations such as burning, stinging, and pruritus have been noted.1 Our patient was unique because in addition to typical lesions of EED, he presented with crusted papules on the flanks and violaceous papules of the lower legs and periumbilicus.
Etiology
Originally associated with Streptococcus as isolated from EED lesions,3,13 additional infectious etiologies include viral hepatitis,4-6 human herpesvirus 6,14 and rarely HIV.1,15 Hepatitis B and C are well known to be associated with EED, with only rare reports in patients with concomitant HIV infection. Erythema elevatum diutinum also has been described in relationship to myeloproliferative disorders and hematologic malignancies such as IgA myeloma,16 non-Hodgkin lymphoma,17 chronic lymphocytic leukemia,18 and hypergammaglobulinemia.19 In a study of 13 patients with EED, 4 had associated underlying IgA monoclonal gammopathy.2 Autoimmune conditions such as rheumatoid arthritis,20 ulcerative colitis,21 relapsing polychondritis,22 and systemic lupus erythematosus23 also have been implicated.
Pathogenesis
Although the precise pathogenesis of EED remains unknown, it has been suggested that a complement cascade initiated by immune-complex deposition in postcapillary venules induces an LCV.24,25 Chronic antigenic exposure or high antibody levels26 in the face of infections, autoimmune disease, or malignancy may incite this immune-complex reaction. Skin lesions seen in association with hepatitis reflect circulating immune-complex deposition in vessel walls causing destruction. It has been postulated that the duration of immune complexemia may be sufficient to account for the differences in the type of vascular injury seen in acute versus chronic infection.27
Histopathology
Erythema elevatum diutinum may present on a histopathologic spectrum of LCV, as manifested in our patient. Early lesions show predominantly polymorphonuclear cells with nuclear dust pattern in a wedge-shaped infiltrate with fibrin deposition in the superficial and mid dermis.2,3 Later lesions show vasculitis in addition to dermal aggregates of lymphocytes, neutrophils, fibrosis, and areas of granulation tissue. The fibrosis may be dense and comprised of fibroblasts and myofibroblasts.28 Newly formed vessels within the granulation tissue have been postulated to be more susceptible to immune-complex deposition, thus potentiating the process.1,29
Management
Spontaneous resolution of EED may occur, albeit after a prolonged and recurrent course of up to 5 to 10 years.30 Treatment of the underlying cause, when identified, remains paramount. First-line therapy includes dapsone, shown to be effective in reducing lesion size to complete resolution in 80% of the 47 cases reviewed by Momen et al.31 Dapsone monotherapy tends to be less effective in treating nodular lesions associated with HIV-positivity, likely due to the extensive fibrosis.4,31 Combination therapy with dapsone and a sulfonamide,32 niacinamide and tetracycline,33 colchicine,34 or surgical excision35 may be necessary in more resistant cases.
Conclusion
Our case exemplifies the clinical histologic spectrum that EED can present. The constellation of clinical findings was histologically confirmed to be manifestations of the disease in various stages of evolution. When typical lesions of EED present along with cutaneous findings in less common locations, performing multiple biopsies can be helpful. The clinician should retain a high index of suspicion for an underlying etiology and perform a complete workup for infection, malignancy, or autoimmune disease.
Erythema elevatum diutinum (EED) manifests on a clinicopathologic spectrum of chronic cutaneous small vessel vasculitis. The lesions typically present as persistent, symmetric, firm, red to purple papules or nodules on the extensor arms and dorsal hands.1,2 Underlying infectious, malignant, or autoimmune processes are commonly associated with the disease, notably Streptococcus infection and IgA monoclonal gammopathy.2,3 Hepatitis virus also is often implicated in association with EED. Cases of EED have been seen with concomitant human immunodeficiency virus (HIV) infection.4-6 We report a case of EED presenting in various stages of evolution associated with underlying hepatitis B infection alone.
Case Report
A 57-year-old man originally presented to an outpatient dermatology practice with a nodular, painful, episodic rash on the trunk and upper and lower extremities. A biopsy revealed leukocytoclastic vasculitis (LCV) with prominent eosinophils. At the time, the skin findings were believed to be a manifestation of drug hypersensitivity, likely to opioid use. The patient was lost to follow-up.
Seven years later, the patient was admitted to the hospital with new-onset burning and stinging red nodules on the dorsum of the hands and persistence of the original episodic rash over the lower legs and bilateral flanks. In the interim, he was briefly treated with an oral prednisone taper and topical corticosteroids including triamcinolone cream 0.1% and clobetasol cream 0.05% without improvement.
On examination deep red to violaceous discrete nodules and plaques with overlying hyperkeratosis involving all distal and proximal interphalangeal joints of the hands and extensor elbows were seen (Figure 1A). On the bilateral posterior arms (Figure 1B), anterior legs, and periumbilical area were deeply erythematous papules and plaques with background hyperpigmentation. Across his lower back and bilateral flanks were erythematous papules with central hemorrhagic crusting (Figure 1C).
Pertinent laboratory findings included a positive hepatitis B surface antigen with hepatitis B DNA value 4,313,876 IU/mL and a hepatitis B virus quantitative polymerase chain reaction value of 6.64 U. The etiology was suspected to be intravenous drug abuse; however, the patient denied recreational drug use.

An additional infectious workup was negative for hepatitis C, streptococcus, syphilis, tuberculosis, and HIV. A complete blood cell count, complete metabolic panel, urinalysis, complement, cryoglobulins, and serum protein electrophoresis were within reference range. Autoimmune serologies were negative including antinuclear antibody, rheumatoid factor, anti-Sjögren syndrome–related antigen A and B, anticyclic citrullinated peptide, anti-Smith, and antineutrophilic cytoplasmic antibodies. Peripheral blood immunophenotyping, lactate dehydrogenase, quantitative immunoglobulins, and age-appropriate cancer screens did not demonstrate evidence for malignancy underlying the disease. Bilateral hand radiographs showed mild periostitis of the proximal phalanges without obvious erosions.
Three 4-mm punch biopsies were performed from the left fifth digit, left posterior arm, and left flank. Tissue of the left fifth digit showed an intradermal vascular proliferation with a concentric pattern resembling onion skin in a background of increased fibrosis. The blood vessels showed focal fibrinoid necrosis (Figure 2A). The biopsy of the left posterior arm showed an intradermal vascular proliferation with an associated mild acute and chronic perivascular inflammation (Figure 2B). The left flank biopsy showed LCV with focal epidermal necrosis (Figure 2C).

The constellation of clinical findings together with the histopathologic changes represented EED in various stages of evolution. The patient was started on dapsone 100 mg daily and referred to the infectious disease service for treatment of chronic hepatitis B; however, he was subsequently lost to follow-up.
Comment
Overview of EED
Erythema elevatum diutinum represents a rare form of chronic cutaneous small vessel vasculitis. Originally described by Hutchinson7 and Bury8 as symmetric purpuric nodules of the skin, it was later named by Crocker and Williams9 in 1894. The disease classically presents as firm, fixed, red-brown to violaceous papules, plaques, and nodules affecting the extensor upper or lower extremities.1 Lesions are most commonly found symmetrically overlying joints of the hands, feet, elbows, and knees, as well as the Achilles tendon and buttocks.3 Less common locations include the palms and soles, face,10,11 trunk,12 and periauricular region.1 Although they are typically asymptomatic, sensations such as burning, stinging, and pruritus have been noted.1 Our patient was unique because in addition to typical lesions of EED, he presented with crusted papules on the flanks and violaceous papules of the lower legs and periumbilicus.
Etiology
Originally associated with Streptococcus as isolated from EED lesions,3,13 additional infectious etiologies include viral hepatitis,4-6 human herpesvirus 6,14 and rarely HIV.1,15 Hepatitis B and C are well known to be associated with EED, with only rare reports in patients with concomitant HIV infection. Erythema elevatum diutinum also has been described in relationship to myeloproliferative disorders and hematologic malignancies such as IgA myeloma,16 non-Hodgkin lymphoma,17 chronic lymphocytic leukemia,18 and hypergammaglobulinemia.19 In a study of 13 patients with EED, 4 had associated underlying IgA monoclonal gammopathy.2 Autoimmune conditions such as rheumatoid arthritis,20 ulcerative colitis,21 relapsing polychondritis,22 and systemic lupus erythematosus23 also have been implicated.
Pathogenesis
Although the precise pathogenesis of EED remains unknown, it has been suggested that a complement cascade initiated by immune-complex deposition in postcapillary venules induces an LCV.24,25 Chronic antigenic exposure or high antibody levels26 in the face of infections, autoimmune disease, or malignancy may incite this immune-complex reaction. Skin lesions seen in association with hepatitis reflect circulating immune-complex deposition in vessel walls causing destruction. It has been postulated that the duration of immune complexemia may be sufficient to account for the differences in the type of vascular injury seen in acute versus chronic infection.27
Histopathology
Erythema elevatum diutinum may present on a histopathologic spectrum of LCV, as manifested in our patient. Early lesions show predominantly polymorphonuclear cells with nuclear dust pattern in a wedge-shaped infiltrate with fibrin deposition in the superficial and mid dermis.2,3 Later lesions show vasculitis in addition to dermal aggregates of lymphocytes, neutrophils, fibrosis, and areas of granulation tissue. The fibrosis may be dense and comprised of fibroblasts and myofibroblasts.28 Newly formed vessels within the granulation tissue have been postulated to be more susceptible to immune-complex deposition, thus potentiating the process.1,29
Management
Spontaneous resolution of EED may occur, albeit after a prolonged and recurrent course of up to 5 to 10 years.30 Treatment of the underlying cause, when identified, remains paramount. First-line therapy includes dapsone, shown to be effective in reducing lesion size to complete resolution in 80% of the 47 cases reviewed by Momen et al.31 Dapsone monotherapy tends to be less effective in treating nodular lesions associated with HIV-positivity, likely due to the extensive fibrosis.4,31 Combination therapy with dapsone and a sulfonamide,32 niacinamide and tetracycline,33 colchicine,34 or surgical excision35 may be necessary in more resistant cases.
Conclusion
Our case exemplifies the clinical histologic spectrum that EED can present. The constellation of clinical findings was histologically confirmed to be manifestations of the disease in various stages of evolution. When typical lesions of EED present along with cutaneous findings in less common locations, performing multiple biopsies can be helpful. The clinician should retain a high index of suspicion for an underlying etiology and perform a complete workup for infection, malignancy, or autoimmune disease.
- Gibson LE, el-Azhary RA. Erythema elevatum diutinum. Clin Dermatol. 2000;18:295-299.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Wilkinson SM, English JS, Smith NP, et al. Erythema elevatum diutinum: a clinicopathological study. Clin Exp Dermatol. 1992;17:87-93.
- Fakheri A, Gupta SM, White SM, et al. Erythema elevatum diutinum in a patient with human immunodeficiency virus. Cutis. 2001;68:41-42, 55.
- Kim H. Erythema elevatum diutinum in an HIV-positive patient. J Drugs Dermatol. 2003;2:411-412.
- Revenga F, Vera A, Muñoz A, et al. Erythema elevatum diutinum and AIDS: are they related? Clin Exp Dermatol. 1997;22:250-251.
- Hutchinson J. On two remarkable cases of symmetrieal purple congestion of the skin in patches, with induration. Br J Dermatol. 1888;1:10-15.
- Bury JS. A case of erythema with remarkable nodular thickening and induration of the skin associated with intermittent albuminuria. Illustrated Medical News. 1889;3:145-149.
- Crocker HR, Williams C. Erythema elevatum diutinum. Br J Dermatol. 1894;6:33-38.
- Barzegar M, Davatchi CC, Akhyani M, et al. An atypical presentation of erythema elevatum diutinum involving palms and soles. Int J Dermatol. 2009;48:73-75.
- Futei Y, Konohana I. A case of erythema elevatum diutinum associated with B-cell lymphoma: a rare distribution involving palms, soles and nails. Br J Dermatol. 2000;142:116-119.
- Ben-Zvi GT, Bardsley V, Burrows NP. An atypical distribution of erythema elevatum diutinum. Clin Exp Dermatol. 2014;39:269-270.
- Weidman FD, Besancon JH. Erythema elevatum diutinum. role of streptococci, and relationship to other rheumatic dermatoses. Arch Dermatol Syphilol. 1929;20:593-620.
- Drago F, Semino M, Rampini P, et al. Erythema elevatum diutinum in a patient with human herpesvirus 6 infection. Acta Derm Venereol. 1999;79:91-92.
- Muratori S, Carrera C, Gorani A, et al. Erythema elevatum diutinum and HIV infection: a report of five cases. Br J Dermatol. 1999;141:335-338.
- Archimandritis AJ, Fertakis A, Alegakis G, et al. Erythema elevatum diutinum and IgA myeloma: an interesting association. Br Med J. 1977;2:613-614.
- Hatzitolios A, Tzellos TG, Savopoulos C, et al. Erythema elevatum diutinum with rare distribution as a first clinical sign of non-Hodgkin’s lymphoma: a novel association? J Dermatol. 2008;35:297-300.
- Delaporte E, Alfandari S, Fenaux P, et al. Erythema elevatum diutinum and chronic lymphocytic leukaemia. Clin Exp Dermatol. 1994;19:188-189.
- Miyagawa S, Kitamura W, Morita K, et al. Association of hyperimmunoglobulinaemia D syndrome with erythema elevatum diutinum. Br J Dermatol. 1993;128:572-574.
- Collier PM, Neill SM, Branfoot AC, et al. Erythema elevatum diutinum—a solitary lesion in a patient with rheumatoid arthritis. Clin Exp Dermatol. 1990;15:394-395.
- Buahene K, Hudson M, Mowat A, et al. Erythema elevatum diutinum—an unusual association with ulcerative colitis. Clin Exp Dermatol. 1991;16:204-206.
- Bernard P, Bedane C, Delrous JL, et al. Erythema elevatum diutinum in a patient with relapsing polychondritis. J Am Acad Dermatol. 1992;26:312-315.
- Hancox JG, Wallace CA, Sangueza OP, et al. Erythema elevatum diutinum associated with lupus panniculitis in a patient with discoid lesions of chronic cutaneous lupus erythematosus. J Am Acad Dermatol. 2004;50:652-653.
- Haber H. Erythema elevatum diutinum. Br J Dermatol. 1955;67:121-145.
- Katz SI, Gallin JL, Hertz KC, et al. Erythema elevatum diutinum: skin and systemic manifestations, immunologic studies, and successful treatment with dapsone. Medicine (Baltimore). 1977;56:443-455.
- Walker KD, Badame AJ. Erythema elevatum diutinum in a patient with Crohn’s disease. J Am Acad Dermatol. 1990;22:948-952.
- Popp JW, Harrist T, Dienstag JL, et al. Cutaneous vasculitis associated with acute and chronic hepatitis. Arch Intern Med. 1981;141:623-629.
- Lee AY, Nakagawa H, Nogita T, et al. Erythema elevatum diutinum: an ultrastructural case study. J Cutan Pathol. 1989;16:211-217.
- LeBoit PE, Yen TS, Wintroub B. The evolution of lesions in erythema elevatum diutinum. Am J Dermatopathol. 1986;8:392-402.
- Soubeiran E, Wacker J, Hausser I, et al. Erythema elevatum diutinum with unusual clinical appearance. J Dtsch Dermatol Ges. 2008;6:303-305.
- Momen SE, Jorizzo J, Al-Niaimi F. Erythema elevatum diutinum: a review of presentation and treatment. J Eur Acad Dermatol Venereol. 2014;28:1594-1602.
- Vollum DI. Erythema elevatum diutinum—vesicular lesions and sulfone response. Br J Dermatol. 1968;80:178-183.
- Kohler IK, Lorincz AL. Erythema elevatum diutinum treated with niacinamide and tetracycline. Arch Dermatol. 1980;116:693-695.
- Henriksson R, Hofor PA, Hörngvist R. Erythema elevatum diutinum—a case successfully treated with colchicine. Clin Exp Dermatol. 1989;14:451-453.
- Zacaron LH, Gonçalves JC, Curty VM, et al. Clinical and surgical therapeutic approach in erythema elevatum diutinum—case report. An Bras Dermatol. 2013;88(6, suppl 1):15-18.
- Gibson LE, el-Azhary RA. Erythema elevatum diutinum. Clin Dermatol. 2000;18:295-299.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Wilkinson SM, English JS, Smith NP, et al. Erythema elevatum diutinum: a clinicopathological study. Clin Exp Dermatol. 1992;17:87-93.
- Fakheri A, Gupta SM, White SM, et al. Erythema elevatum diutinum in a patient with human immunodeficiency virus. Cutis. 2001;68:41-42, 55.
- Kim H. Erythema elevatum diutinum in an HIV-positive patient. J Drugs Dermatol. 2003;2:411-412.
- Revenga F, Vera A, Muñoz A, et al. Erythema elevatum diutinum and AIDS: are they related? Clin Exp Dermatol. 1997;22:250-251.
- Hutchinson J. On two remarkable cases of symmetrieal purple congestion of the skin in patches, with induration. Br J Dermatol. 1888;1:10-15.
- Bury JS. A case of erythema with remarkable nodular thickening and induration of the skin associated with intermittent albuminuria. Illustrated Medical News. 1889;3:145-149.
- Crocker HR, Williams C. Erythema elevatum diutinum. Br J Dermatol. 1894;6:33-38.
- Barzegar M, Davatchi CC, Akhyani M, et al. An atypical presentation of erythema elevatum diutinum involving palms and soles. Int J Dermatol. 2009;48:73-75.
- Futei Y, Konohana I. A case of erythema elevatum diutinum associated with B-cell lymphoma: a rare distribution involving palms, soles and nails. Br J Dermatol. 2000;142:116-119.
- Ben-Zvi GT, Bardsley V, Burrows NP. An atypical distribution of erythema elevatum diutinum. Clin Exp Dermatol. 2014;39:269-270.
- Weidman FD, Besancon JH. Erythema elevatum diutinum. role of streptococci, and relationship to other rheumatic dermatoses. Arch Dermatol Syphilol. 1929;20:593-620.
- Drago F, Semino M, Rampini P, et al. Erythema elevatum diutinum in a patient with human herpesvirus 6 infection. Acta Derm Venereol. 1999;79:91-92.
- Muratori S, Carrera C, Gorani A, et al. Erythema elevatum diutinum and HIV infection: a report of five cases. Br J Dermatol. 1999;141:335-338.
- Archimandritis AJ, Fertakis A, Alegakis G, et al. Erythema elevatum diutinum and IgA myeloma: an interesting association. Br Med J. 1977;2:613-614.
- Hatzitolios A, Tzellos TG, Savopoulos C, et al. Erythema elevatum diutinum with rare distribution as a first clinical sign of non-Hodgkin’s lymphoma: a novel association? J Dermatol. 2008;35:297-300.
- Delaporte E, Alfandari S, Fenaux P, et al. Erythema elevatum diutinum and chronic lymphocytic leukaemia. Clin Exp Dermatol. 1994;19:188-189.
- Miyagawa S, Kitamura W, Morita K, et al. Association of hyperimmunoglobulinaemia D syndrome with erythema elevatum diutinum. Br J Dermatol. 1993;128:572-574.
- Collier PM, Neill SM, Branfoot AC, et al. Erythema elevatum diutinum—a solitary lesion in a patient with rheumatoid arthritis. Clin Exp Dermatol. 1990;15:394-395.
- Buahene K, Hudson M, Mowat A, et al. Erythema elevatum diutinum—an unusual association with ulcerative colitis. Clin Exp Dermatol. 1991;16:204-206.
- Bernard P, Bedane C, Delrous JL, et al. Erythema elevatum diutinum in a patient with relapsing polychondritis. J Am Acad Dermatol. 1992;26:312-315.
- Hancox JG, Wallace CA, Sangueza OP, et al. Erythema elevatum diutinum associated with lupus panniculitis in a patient with discoid lesions of chronic cutaneous lupus erythematosus. J Am Acad Dermatol. 2004;50:652-653.
- Haber H. Erythema elevatum diutinum. Br J Dermatol. 1955;67:121-145.
- Katz SI, Gallin JL, Hertz KC, et al. Erythema elevatum diutinum: skin and systemic manifestations, immunologic studies, and successful treatment with dapsone. Medicine (Baltimore). 1977;56:443-455.
- Walker KD, Badame AJ. Erythema elevatum diutinum in a patient with Crohn’s disease. J Am Acad Dermatol. 1990;22:948-952.
- Popp JW, Harrist T, Dienstag JL, et al. Cutaneous vasculitis associated with acute and chronic hepatitis. Arch Intern Med. 1981;141:623-629.
- Lee AY, Nakagawa H, Nogita T, et al. Erythema elevatum diutinum: an ultrastructural case study. J Cutan Pathol. 1989;16:211-217.
- LeBoit PE, Yen TS, Wintroub B. The evolution of lesions in erythema elevatum diutinum. Am J Dermatopathol. 1986;8:392-402.
- Soubeiran E, Wacker J, Hausser I, et al. Erythema elevatum diutinum with unusual clinical appearance. J Dtsch Dermatol Ges. 2008;6:303-305.
- Momen SE, Jorizzo J, Al-Niaimi F. Erythema elevatum diutinum: a review of presentation and treatment. J Eur Acad Dermatol Venereol. 2014;28:1594-1602.
- Vollum DI. Erythema elevatum diutinum—vesicular lesions and sulfone response. Br J Dermatol. 1968;80:178-183.
- Kohler IK, Lorincz AL. Erythema elevatum diutinum treated with niacinamide and tetracycline. Arch Dermatol. 1980;116:693-695.
- Henriksson R, Hofor PA, Hörngvist R. Erythema elevatum diutinum—a case successfully treated with colchicine. Clin Exp Dermatol. 1989;14:451-453.
- Zacaron LH, Gonçalves JC, Curty VM, et al. Clinical and surgical therapeutic approach in erythema elevatum diutinum—case report. An Bras Dermatol. 2013;88(6, suppl 1):15-18.
Practice Points
- Erythema elevatum diutinum (EED) often is associated with an underlying infectious process, including hepatitis B and hepatitis C, or a hematologic or autoimmune condition.
- If EED is suspected clinically, it may be beneficial to perform multiple biopsies from lesions at different stages of evolution to establish the diagnosis.
- First-line therapy includes treatment of any underlying condition and dapsone.
Eosinophilic Pustular Folliculitis With Underlying Mantle Cell Lymphoma
Eosinophilic pustular folliculitis (EPF) was originally described in 1965 and has since evolved into 3 distinct subtypes: classic, immunosuppressed (IS), and infantile types. Immunosuppressed EPF can be further subdivided into human immunodeficiency virus (HIV) associated (IS-HIV) and non-HIV associated. Human immunodeficiency virus–seronegative cases have been associated with underlying malignancies (IS-heme) or chronic immunosuppression, such as that seen in transplant patients.
Case Report
A 52-year-old man with a medical history limited to prostate adenocarcinoma treated with a robotic prostatectomy presented with a pruritic red rash on the face, neck, shoulders, and chest of 1 month’s duration. The patient previously completed a course of azithromycin 250 mg, intramuscular triamcinolone, and oral prednisone with only minor improvement. Physical examination demonstrated multiple pink folliculocentric papules and pustules scattered on the head (Figure 1A), neck, and chest (Figure 1B), as well as edematous pink papules and plaques on the forehead (Figures 1C and 1D). The palms, soles, and oral mucosa were clear.
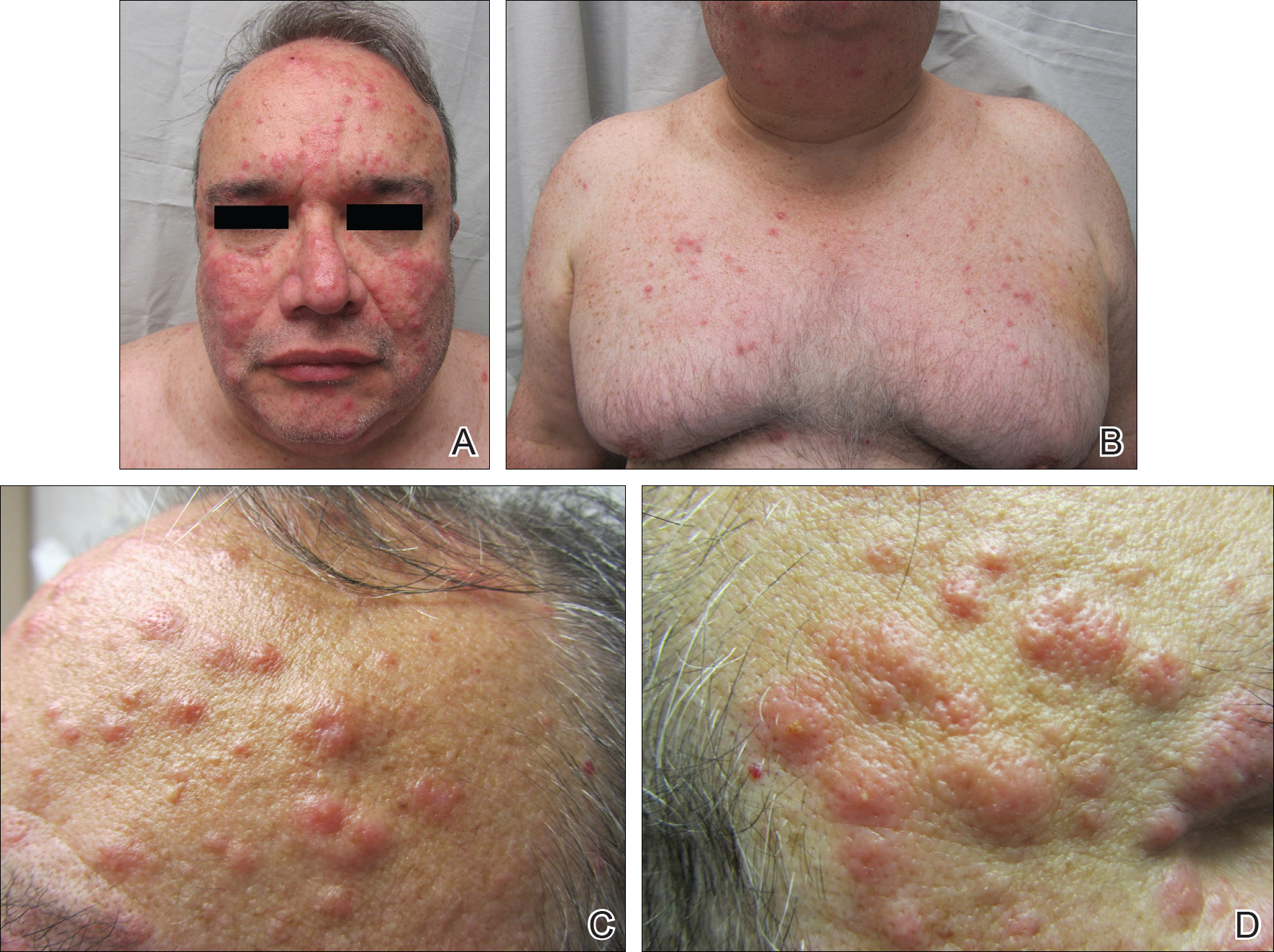
Initial biopsy of the right side of the chest was nonspecific and most consistent with a reaction to an arthropod bite. The patient was started on oral doxycycline 100 mg twice daily for 2 weeks. With no improvement seen, additional biopsies were obtained from the left side of the chest and forehead. The biopsy of the chest showed ruptured folliculitis with evidence of acute and chronic inflammation. The biopsy of the forehead demonstrated eosinophilic follicular spongiosis with intrafollicular Langerhans cell microgranulomas along with abundant eosinophils adjacent to follicles, consistent with EPF (Figure 2). Serum HIV testing was negative. Serum white blood cell count was normal at 6400/µL (reference range, 4500–11,000/µL) with mild elevation of eosinophils (8%). The remaining complete blood cell count and comprehensive metabolic panel were within reference range. The patient was subsequently started on oral indomethacin 25 mg twice daily and triamcinolone cream 0.1%. Within a few days he experienced initial improvement in his symptoms of pruritus and diminution in the number of inflammatory follicular papules.
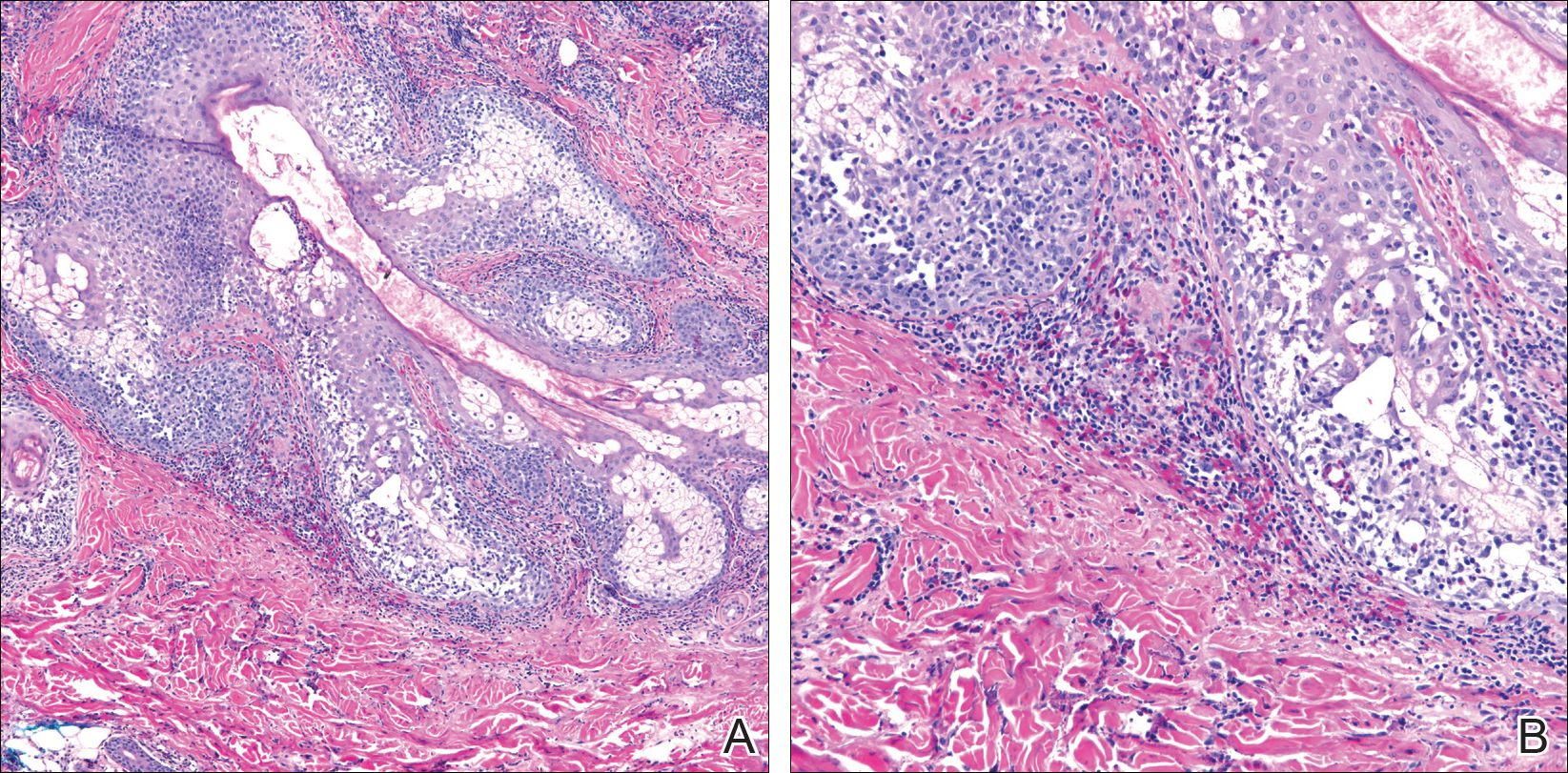
Approximately 1 month after presentation, he began to experience symptoms of dysphagia and fatigue. In addition, tonsillar hypertrophy and palpable neck and axillary lymphadenopathy were present. Computed tomography of the neck, chest, and abdomen showed diffuse lymphadenopathy. Full-body positron emission tomography–computed tomography demonstrated extensive metabolically active lymphoma in multiple nodal groups above and below the diaphragm. There also was lymphomatous involvement of the spleen. An axillary lymph node biopsy was diagnostic for mantle cell lymphoma (CD4:CD8, 1:1; CD45 negative; CD20 positive; CD5 positive). He
Comment
Subtypes of EPF
Eosinophilic pustular folliculitis was first described in a Japanese female presenting with folliculocentric pustules distributed on the face, torso, and arms.1 This noninfectious eosinophilic infiltration of hair follicles predominantly seen in the Japanese population is now regarded as the classic form. Three distinct subtypes of EPF now exist, including the originally described classic variant (Ofuji disease), an IS variant, and a rare infantile form.1
All 3 subtypes of EPF are more commonly seen in men than women. The classic form has a peak incidence between the third and fourth decades of life. It presents as chronic annular papules and sterile pustules exhibiting peripheral extension, with individual lesions lasting for approximately 7 to 10 days with frequent relapses. The face is the most common area of involvement, followed by the trunk, extremities, and more rarely the palmoplantar surfaces. Concomitant leukocytosis with eosinophilia is seen in up to 35% of patients.1 The infantile type represents the rarest EPF form. The average age of onset is 5 months, with most cases resolving by 14 months of age.1
Clinically, EPF is characterized by recurrent papules and pustules predominantly on the scalp without annular or polycyclic ring formation, as seen in the classic type. The palms and soles may be involved, which can clinically mimic infantile acropustulosis and scabies infection. Most patients exhibit a concomitant peripheral eosinophilia.1,2
In the late 1980s, the IS variant of EPF was recognized in HIV-positive (IS-HIV) and HIV-negative malignancy-associated (IS-heme) populations.1,3 This newly characterized form differs morphologically and biologically from the classic and infantile subtypes. The IS subtype has a unique presentation including intensely pruritic, discrete, erythematous, follicular papules with palmoplantar sparing and infrequent annular or circinate plaque forms.1 Frequently, with the IS-HIV form, CD4+ T-cell counts are below 300 cells/mL, and 25% to 50% of patients have lymphopenia with eosinophilia.3 Highly active antiretroviral therapy has been associated with EPF resolution in HIV-positive individuals; however, it also has been shown to induce transient EPF during the first 3 to 6 months of initiation.1,3,4
Unlike the IS-HIV form, the IS-heme form has occurred solely in males and is predominantly associated with hematologic malignancies (eg, non-Hodgkin lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndrome) 30 to 90 days following bone marrow transplant, peripheral blood stem cell transplant, or chemotherapy treatment.5,6 Unlike the chronic and persistent IS-HIV form, prior cases of IS-heme EPF have been predominantly self-limited. Interestingly, only 2 reported cases of EPF have occurred prior to the diagnosis of malignancy including B-cell leukemia and myelodysplastic syndrome.5
Histopathology
All 3 identified forms of EPF histopathologically show acute and chronic lymphoeosinophilic infiltrate concentrated at the follicular isthmus, which can lead to follicular destruction. Scattered mononuclear cells, eosinophils, and neutrophils are found within the pilar outer root sheath, sebaceous glands, and ducts. Approximately 40% of cases demonstrate follicular mucinosis.1 Histopathology of lesional palmar skin in classic-type EPF demonstrates intraepidermal pustule formation with abundant eosinophils and neutrophils adjacent to the acrosyringium.7,8
Pathogenesis
Although the pathophysiology of EPF is largely unknown, it is thought to represent a helper T cell (TH2) response involving IL-4, IL-5, and IL-13 cytokines.9 Chemoattractant receptor homologous molecule 2, which is expressed on eosinophils and lymphocytes, is believed to play a role in the pruritus, edema, and inflammatory response seen adjacent to pilosebaceous units in EPF.10 Moreover, immunohistochemical and flow cytometry analysis has revealed a prevalence of prostaglandin D2 within the perisebocyte infiltrate in EPF.9 Prostaglandin D2 induces eotaxin-3 production within sebocytes via peroxisome proliferator-activated receptor γ, which enhances chemoattraction of eosinophils. This pathogenesis represents a prostaglandin-based mechanism and potentially explains the efficacy of indomethacin treatment of EPF through its cyclooxygenase inhibition and reduction of chemoattractant receptor homologous molecule 2 expression.9-11
Treatment
Multiple therapeutic modalities have been reported for the treatment of EPF. For all 3 subtypes, moderate- to high-potency topical corticosteroids are considered first-line therapy. UVB phototherapy 2 to 3 times weekly remains the gold standard, given its consistent efficacy.1,12 Indomethacin (50–75 mg daily) remains first-line treatment of classic EPF.4,12 Previously reported cases of classic EPF and IS-EPF have responded well to oral prednisone (1 mg/kg daily).12,13 In a retrospective review of EPF treatment data, the following treatments also have been reported to be successful: psoralen plus UVA, oral cetirizine (20–40 mg daily, particularly for IS-EPF cases), metronidazole (250 mg 3 times daily), minocycline (150 mg daily), itraconazole (200–400 mg daily, dapsone (50–200 mg daily), systemic retinoids, tacrolimus ointment 0.1%, and permethrin cream.4,12
Malignancy
Although the entity of IS-heme EPF is rare, the morphology and treatment are unique and can potentially unmask an underlying hematologic malignancy. In patients with EPF and associated malignancy, such as our patient, a differential diagnosis to consider is eosinophilic dermatosis of hematologic malignancy (EDHM). Eosinophilic dermatosis of hematologic malignancy is most commonly associated with chronic lymphocytic leukemia and can be differentiated from EPF clinically, histopathologically, and by treatment response. Eosinophilic dermatosis of hematologic malignancy clinically presents with nonspecific papules, pustules, and/or vesicles on the head, trunk, and extremities. On histopathology, EDHM shows a superficial and deep perivascular and interstitial lymphoeosinophilic infiltration. Furthermore, EDHM patients typically exhibit a poor treatment response to oral indomethacin.14
Conclusion
Eosinophilic pustular folliculitis is a noninfectious folliculocentric process comprised of 3 distinct types. The histopathology shows follicular spongiosis with increased eosinophils. The pathogenesis is most likely related to a multifactorial immune system dysregulation involving TH2 T cells, prostaglandin D2, and eotaxin-3. The treatment of EPF may involve topical corticosteroids, UVB phototherapy, or most notably oral indomethacin. In patients with EPF and malignancy, EDHM is a differential diagnosis to consider. Our case serves as a reminder that rare eosinophilic dermatoses may represent manifestations of underlying hematopoietic malignancy and, when investigated early, can lead to appropriate life-saving treatment.
- Nervi J, Stephen. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- Hernández-Martín Á, Nuño-González A, Colmenero I, et al. Eosinophilic pustular folliculitis of infancy: a series of 15 cases and review of the literature [published online July 21, 2012]. J Am Acad Dermatol. 2013;68:150-155.
- Soepr
ono F, Schinella R. Eosinophilic pustular folliculitis in patients with acquired immunodeficiency syndrome. report of three cases. J Am Acad Dermatol. 1986;14:1020-1022. - Katoh
M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. - Keida
T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplant. J Dermatol. 2004;31:21-26. - Goiriz R, Gul-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36.
- Satoh T, Ikeda H, Yokozeki H. Acrosyringeal involvement of palmoplantar lesions of eosinophilic pustular folliculitis. Acta Derm Venereol. 2013;93:99.
- Tsuboi H, Wakita K, Fujimura T, et al. Acral variant of eosinophilic pustular folliculitis (Ofuji’s disease). Clin Exp Dermatol. 2003;28:321-324.
- Nakahig
ashi K, Doi H, Otsuka A, et al. PGD2 induces eotaxin-3 via PPARgamma from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;129:536-543. - Satoh
T, Shimura C, Miyagishi C, et al. Indomethacin-induced reduction in CRTH2 in eosinophilic pustular folliculitis (Ofuji’s disease): a proposed mechanism of action. Acta Derm Venereol. 2010;90:18-22. - Hagiwara A, Fujimura T, Furudate S, et al. Induction of CD163(+)M2 macrophages in the lesional skin of eosinophilic pustular folliculitis. Acta Derm Venereol. 2014;94:104-106.
- Ellis
E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197. - Bull R
H, Harland CA, Fallowfield ME, et al. Eosinophilic folliculitis: a self-limiting illness in patients being treated for haematological malignancy. Br J Dermatol. 1993;129:178-182. - Farber M, Forgia S, Sahu J, et al. Eosinophilic dermatosis of hematologic malignancy. J Cutan Pathol. 2012;39:690-695.
Eosinophilic pustular folliculitis (EPF) was originally described in 1965 and has since evolved into 3 distinct subtypes: classic, immunosuppressed (IS), and infantile types. Immunosuppressed EPF can be further subdivided into human immunodeficiency virus (HIV) associated (IS-HIV) and non-HIV associated. Human immunodeficiency virus–seronegative cases have been associated with underlying malignancies (IS-heme) or chronic immunosuppression, such as that seen in transplant patients.
Case Report
A 52-year-old man with a medical history limited to prostate adenocarcinoma treated with a robotic prostatectomy presented with a pruritic red rash on the face, neck, shoulders, and chest of 1 month’s duration. The patient previously completed a course of azithromycin 250 mg, intramuscular triamcinolone, and oral prednisone with only minor improvement. Physical examination demonstrated multiple pink folliculocentric papules and pustules scattered on the head (Figure 1A), neck, and chest (Figure 1B), as well as edematous pink papules and plaques on the forehead (Figures 1C and 1D). The palms, soles, and oral mucosa were clear.

Initial biopsy of the right side of the chest was nonspecific and most consistent with a reaction to an arthropod bite. The patient was started on oral doxycycline 100 mg twice daily for 2 weeks. With no improvement seen, additional biopsies were obtained from the left side of the chest and forehead. The biopsy of the chest showed ruptured folliculitis with evidence of acute and chronic inflammation. The biopsy of the forehead demonstrated eosinophilic follicular spongiosis with intrafollicular Langerhans cell microgranulomas along with abundant eosinophils adjacent to follicles, consistent with EPF (Figure 2). Serum HIV testing was negative. Serum white blood cell count was normal at 6400/µL (reference range, 4500–11,000/µL) with mild elevation of eosinophils (8%). The remaining complete blood cell count and comprehensive metabolic panel were within reference range. The patient was subsequently started on oral indomethacin 25 mg twice daily and triamcinolone cream 0.1%. Within a few days he experienced initial improvement in his symptoms of pruritus and diminution in the number of inflammatory follicular papules.

Approximately 1 month after presentation, he began to experience symptoms of dysphagia and fatigue. In addition, tonsillar hypertrophy and palpable neck and axillary lymphadenopathy were present. Computed tomography of the neck, chest, and abdomen showed diffuse lymphadenopathy. Full-body positron emission tomography–computed tomography demonstrated extensive metabolically active lymphoma in multiple nodal groups above and below the diaphragm. There also was lymphomatous involvement of the spleen. An axillary lymph node biopsy was diagnostic for mantle cell lymphoma (CD4:CD8, 1:1; CD45 negative; CD20 positive; CD5 positive). He
Comment
Subtypes of EPF
Eosinophilic pustular folliculitis was first described in a Japanese female presenting with folliculocentric pustules distributed on the face, torso, and arms.1 This noninfectious eosinophilic infiltration of hair follicles predominantly seen in the Japanese population is now regarded as the classic form. Three distinct subtypes of EPF now exist, including the originally described classic variant (Ofuji disease), an IS variant, and a rare infantile form.1
All 3 subtypes of EPF are more commonly seen in men than women. The classic form has a peak incidence between the third and fourth decades of life. It presents as chronic annular papules and sterile pustules exhibiting peripheral extension, with individual lesions lasting for approximately 7 to 10 days with frequent relapses. The face is the most common area of involvement, followed by the trunk, extremities, and more rarely the palmoplantar surfaces. Concomitant leukocytosis with eosinophilia is seen in up to 35% of patients.1 The infantile type represents the rarest EPF form. The average age of onset is 5 months, with most cases resolving by 14 months of age.1
Clinically, EPF is characterized by recurrent papules and pustules predominantly on the scalp without annular or polycyclic ring formation, as seen in the classic type. The palms and soles may be involved, which can clinically mimic infantile acropustulosis and scabies infection. Most patients exhibit a concomitant peripheral eosinophilia.1,2
In the late 1980s, the IS variant of EPF was recognized in HIV-positive (IS-HIV) and HIV-negative malignancy-associated (IS-heme) populations.1,3 This newly characterized form differs morphologically and biologically from the classic and infantile subtypes. The IS subtype has a unique presentation including intensely pruritic, discrete, erythematous, follicular papules with palmoplantar sparing and infrequent annular or circinate plaque forms.1 Frequently, with the IS-HIV form, CD4+ T-cell counts are below 300 cells/mL, and 25% to 50% of patients have lymphopenia with eosinophilia.3 Highly active antiretroviral therapy has been associated with EPF resolution in HIV-positive individuals; however, it also has been shown to induce transient EPF during the first 3 to 6 months of initiation.1,3,4
Unlike the IS-HIV form, the IS-heme form has occurred solely in males and is predominantly associated with hematologic malignancies (eg, non-Hodgkin lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndrome) 30 to 90 days following bone marrow transplant, peripheral blood stem cell transplant, or chemotherapy treatment.5,6 Unlike the chronic and persistent IS-HIV form, prior cases of IS-heme EPF have been predominantly self-limited. Interestingly, only 2 reported cases of EPF have occurred prior to the diagnosis of malignancy including B-cell leukemia and myelodysplastic syndrome.5
Histopathology
All 3 identified forms of EPF histopathologically show acute and chronic lymphoeosinophilic infiltrate concentrated at the follicular isthmus, which can lead to follicular destruction. Scattered mononuclear cells, eosinophils, and neutrophils are found within the pilar outer root sheath, sebaceous glands, and ducts. Approximately 40% of cases demonstrate follicular mucinosis.1 Histopathology of lesional palmar skin in classic-type EPF demonstrates intraepidermal pustule formation with abundant eosinophils and neutrophils adjacent to the acrosyringium.7,8
Pathogenesis
Although the pathophysiology of EPF is largely unknown, it is thought to represent a helper T cell (TH2) response involving IL-4, IL-5, and IL-13 cytokines.9 Chemoattractant receptor homologous molecule 2, which is expressed on eosinophils and lymphocytes, is believed to play a role in the pruritus, edema, and inflammatory response seen adjacent to pilosebaceous units in EPF.10 Moreover, immunohistochemical and flow cytometry analysis has revealed a prevalence of prostaglandin D2 within the perisebocyte infiltrate in EPF.9 Prostaglandin D2 induces eotaxin-3 production within sebocytes via peroxisome proliferator-activated receptor γ, which enhances chemoattraction of eosinophils. This pathogenesis represents a prostaglandin-based mechanism and potentially explains the efficacy of indomethacin treatment of EPF through its cyclooxygenase inhibition and reduction of chemoattractant receptor homologous molecule 2 expression.9-11
Treatment
Multiple therapeutic modalities have been reported for the treatment of EPF. For all 3 subtypes, moderate- to high-potency topical corticosteroids are considered first-line therapy. UVB phototherapy 2 to 3 times weekly remains the gold standard, given its consistent efficacy.1,12 Indomethacin (50–75 mg daily) remains first-line treatment of classic EPF.4,12 Previously reported cases of classic EPF and IS-EPF have responded well to oral prednisone (1 mg/kg daily).12,13 In a retrospective review of EPF treatment data, the following treatments also have been reported to be successful: psoralen plus UVA, oral cetirizine (20–40 mg daily, particularly for IS-EPF cases), metronidazole (250 mg 3 times daily), minocycline (150 mg daily), itraconazole (200–400 mg daily, dapsone (50–200 mg daily), systemic retinoids, tacrolimus ointment 0.1%, and permethrin cream.4,12
Malignancy
Although the entity of IS-heme EPF is rare, the morphology and treatment are unique and can potentially unmask an underlying hematologic malignancy. In patients with EPF and associated malignancy, such as our patient, a differential diagnosis to consider is eosinophilic dermatosis of hematologic malignancy (EDHM). Eosinophilic dermatosis of hematologic malignancy is most commonly associated with chronic lymphocytic leukemia and can be differentiated from EPF clinically, histopathologically, and by treatment response. Eosinophilic dermatosis of hematologic malignancy clinically presents with nonspecific papules, pustules, and/or vesicles on the head, trunk, and extremities. On histopathology, EDHM shows a superficial and deep perivascular and interstitial lymphoeosinophilic infiltration. Furthermore, EDHM patients typically exhibit a poor treatment response to oral indomethacin.14
Conclusion
Eosinophilic pustular folliculitis is a noninfectious folliculocentric process comprised of 3 distinct types. The histopathology shows follicular spongiosis with increased eosinophils. The pathogenesis is most likely related to a multifactorial immune system dysregulation involving TH2 T cells, prostaglandin D2, and eotaxin-3. The treatment of EPF may involve topical corticosteroids, UVB phototherapy, or most notably oral indomethacin. In patients with EPF and malignancy, EDHM is a differential diagnosis to consider. Our case serves as a reminder that rare eosinophilic dermatoses may represent manifestations of underlying hematopoietic malignancy and, when investigated early, can lead to appropriate life-saving treatment.
Eosinophilic pustular folliculitis (EPF) was originally described in 1965 and has since evolved into 3 distinct subtypes: classic, immunosuppressed (IS), and infantile types. Immunosuppressed EPF can be further subdivided into human immunodeficiency virus (HIV) associated (IS-HIV) and non-HIV associated. Human immunodeficiency virus–seronegative cases have been associated with underlying malignancies (IS-heme) or chronic immunosuppression, such as that seen in transplant patients.
Case Report
A 52-year-old man with a medical history limited to prostate adenocarcinoma treated with a robotic prostatectomy presented with a pruritic red rash on the face, neck, shoulders, and chest of 1 month’s duration. The patient previously completed a course of azithromycin 250 mg, intramuscular triamcinolone, and oral prednisone with only minor improvement. Physical examination demonstrated multiple pink folliculocentric papules and pustules scattered on the head (Figure 1A), neck, and chest (Figure 1B), as well as edematous pink papules and plaques on the forehead (Figures 1C and 1D). The palms, soles, and oral mucosa were clear.

Initial biopsy of the right side of the chest was nonspecific and most consistent with a reaction to an arthropod bite. The patient was started on oral doxycycline 100 mg twice daily for 2 weeks. With no improvement seen, additional biopsies were obtained from the left side of the chest and forehead. The biopsy of the chest showed ruptured folliculitis with evidence of acute and chronic inflammation. The biopsy of the forehead demonstrated eosinophilic follicular spongiosis with intrafollicular Langerhans cell microgranulomas along with abundant eosinophils adjacent to follicles, consistent with EPF (Figure 2). Serum HIV testing was negative. Serum white blood cell count was normal at 6400/µL (reference range, 4500–11,000/µL) with mild elevation of eosinophils (8%). The remaining complete blood cell count and comprehensive metabolic panel were within reference range. The patient was subsequently started on oral indomethacin 25 mg twice daily and triamcinolone cream 0.1%. Within a few days he experienced initial improvement in his symptoms of pruritus and diminution in the number of inflammatory follicular papules.

Approximately 1 month after presentation, he began to experience symptoms of dysphagia and fatigue. In addition, tonsillar hypertrophy and palpable neck and axillary lymphadenopathy were present. Computed tomography of the neck, chest, and abdomen showed diffuse lymphadenopathy. Full-body positron emission tomography–computed tomography demonstrated extensive metabolically active lymphoma in multiple nodal groups above and below the diaphragm. There also was lymphomatous involvement of the spleen. An axillary lymph node biopsy was diagnostic for mantle cell lymphoma (CD4:CD8, 1:1; CD45 negative; CD20 positive; CD5 positive). He
Comment
Subtypes of EPF
Eosinophilic pustular folliculitis was first described in a Japanese female presenting with folliculocentric pustules distributed on the face, torso, and arms.1 This noninfectious eosinophilic infiltration of hair follicles predominantly seen in the Japanese population is now regarded as the classic form. Three distinct subtypes of EPF now exist, including the originally described classic variant (Ofuji disease), an IS variant, and a rare infantile form.1
All 3 subtypes of EPF are more commonly seen in men than women. The classic form has a peak incidence between the third and fourth decades of life. It presents as chronic annular papules and sterile pustules exhibiting peripheral extension, with individual lesions lasting for approximately 7 to 10 days with frequent relapses. The face is the most common area of involvement, followed by the trunk, extremities, and more rarely the palmoplantar surfaces. Concomitant leukocytosis with eosinophilia is seen in up to 35% of patients.1 The infantile type represents the rarest EPF form. The average age of onset is 5 months, with most cases resolving by 14 months of age.1
Clinically, EPF is characterized by recurrent papules and pustules predominantly on the scalp without annular or polycyclic ring formation, as seen in the classic type. The palms and soles may be involved, which can clinically mimic infantile acropustulosis and scabies infection. Most patients exhibit a concomitant peripheral eosinophilia.1,2
In the late 1980s, the IS variant of EPF was recognized in HIV-positive (IS-HIV) and HIV-negative malignancy-associated (IS-heme) populations.1,3 This newly characterized form differs morphologically and biologically from the classic and infantile subtypes. The IS subtype has a unique presentation including intensely pruritic, discrete, erythematous, follicular papules with palmoplantar sparing and infrequent annular or circinate plaque forms.1 Frequently, with the IS-HIV form, CD4+ T-cell counts are below 300 cells/mL, and 25% to 50% of patients have lymphopenia with eosinophilia.3 Highly active antiretroviral therapy has been associated with EPF resolution in HIV-positive individuals; however, it also has been shown to induce transient EPF during the first 3 to 6 months of initiation.1,3,4
Unlike the IS-HIV form, the IS-heme form has occurred solely in males and is predominantly associated with hematologic malignancies (eg, non-Hodgkin lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndrome) 30 to 90 days following bone marrow transplant, peripheral blood stem cell transplant, or chemotherapy treatment.5,6 Unlike the chronic and persistent IS-HIV form, prior cases of IS-heme EPF have been predominantly self-limited. Interestingly, only 2 reported cases of EPF have occurred prior to the diagnosis of malignancy including B-cell leukemia and myelodysplastic syndrome.5
Histopathology
All 3 identified forms of EPF histopathologically show acute and chronic lymphoeosinophilic infiltrate concentrated at the follicular isthmus, which can lead to follicular destruction. Scattered mononuclear cells, eosinophils, and neutrophils are found within the pilar outer root sheath, sebaceous glands, and ducts. Approximately 40% of cases demonstrate follicular mucinosis.1 Histopathology of lesional palmar skin in classic-type EPF demonstrates intraepidermal pustule formation with abundant eosinophils and neutrophils adjacent to the acrosyringium.7,8
Pathogenesis
Although the pathophysiology of EPF is largely unknown, it is thought to represent a helper T cell (TH2) response involving IL-4, IL-5, and IL-13 cytokines.9 Chemoattractant receptor homologous molecule 2, which is expressed on eosinophils and lymphocytes, is believed to play a role in the pruritus, edema, and inflammatory response seen adjacent to pilosebaceous units in EPF.10 Moreover, immunohistochemical and flow cytometry analysis has revealed a prevalence of prostaglandin D2 within the perisebocyte infiltrate in EPF.9 Prostaglandin D2 induces eotaxin-3 production within sebocytes via peroxisome proliferator-activated receptor γ, which enhances chemoattraction of eosinophils. This pathogenesis represents a prostaglandin-based mechanism and potentially explains the efficacy of indomethacin treatment of EPF through its cyclooxygenase inhibition and reduction of chemoattractant receptor homologous molecule 2 expression.9-11
Treatment
Multiple therapeutic modalities have been reported for the treatment of EPF. For all 3 subtypes, moderate- to high-potency topical corticosteroids are considered first-line therapy. UVB phototherapy 2 to 3 times weekly remains the gold standard, given its consistent efficacy.1,12 Indomethacin (50–75 mg daily) remains first-line treatment of classic EPF.4,12 Previously reported cases of classic EPF and IS-EPF have responded well to oral prednisone (1 mg/kg daily).12,13 In a retrospective review of EPF treatment data, the following treatments also have been reported to be successful: psoralen plus UVA, oral cetirizine (20–40 mg daily, particularly for IS-EPF cases), metronidazole (250 mg 3 times daily), minocycline (150 mg daily), itraconazole (200–400 mg daily, dapsone (50–200 mg daily), systemic retinoids, tacrolimus ointment 0.1%, and permethrin cream.4,12
Malignancy
Although the entity of IS-heme EPF is rare, the morphology and treatment are unique and can potentially unmask an underlying hematologic malignancy. In patients with EPF and associated malignancy, such as our patient, a differential diagnosis to consider is eosinophilic dermatosis of hematologic malignancy (EDHM). Eosinophilic dermatosis of hematologic malignancy is most commonly associated with chronic lymphocytic leukemia and can be differentiated from EPF clinically, histopathologically, and by treatment response. Eosinophilic dermatosis of hematologic malignancy clinically presents with nonspecific papules, pustules, and/or vesicles on the head, trunk, and extremities. On histopathology, EDHM shows a superficial and deep perivascular and interstitial lymphoeosinophilic infiltration. Furthermore, EDHM patients typically exhibit a poor treatment response to oral indomethacin.14
Conclusion
Eosinophilic pustular folliculitis is a noninfectious folliculocentric process comprised of 3 distinct types. The histopathology shows follicular spongiosis with increased eosinophils. The pathogenesis is most likely related to a multifactorial immune system dysregulation involving TH2 T cells, prostaglandin D2, and eotaxin-3. The treatment of EPF may involve topical corticosteroids, UVB phototherapy, or most notably oral indomethacin. In patients with EPF and malignancy, EDHM is a differential diagnosis to consider. Our case serves as a reminder that rare eosinophilic dermatoses may represent manifestations of underlying hematopoietic malignancy and, when investigated early, can lead to appropriate life-saving treatment.
- Nervi J, Stephen. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- Hernández-Martín Á, Nuño-González A, Colmenero I, et al. Eosinophilic pustular folliculitis of infancy: a series of 15 cases and review of the literature [published online July 21, 2012]. J Am Acad Dermatol. 2013;68:150-155.
- Soepr
ono F, Schinella R. Eosinophilic pustular folliculitis in patients with acquired immunodeficiency syndrome. report of three cases. J Am Acad Dermatol. 1986;14:1020-1022. - Katoh
M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. - Keida
T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplant. J Dermatol. 2004;31:21-26. - Goiriz R, Gul-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36.
- Satoh T, Ikeda H, Yokozeki H. Acrosyringeal involvement of palmoplantar lesions of eosinophilic pustular folliculitis. Acta Derm Venereol. 2013;93:99.
- Tsuboi H, Wakita K, Fujimura T, et al. Acral variant of eosinophilic pustular folliculitis (Ofuji’s disease). Clin Exp Dermatol. 2003;28:321-324.
- Nakahig
ashi K, Doi H, Otsuka A, et al. PGD2 induces eotaxin-3 via PPARgamma from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;129:536-543. - Satoh
T, Shimura C, Miyagishi C, et al. Indomethacin-induced reduction in CRTH2 in eosinophilic pustular folliculitis (Ofuji’s disease): a proposed mechanism of action. Acta Derm Venereol. 2010;90:18-22. - Hagiwara A, Fujimura T, Furudate S, et al. Induction of CD163(+)M2 macrophages in the lesional skin of eosinophilic pustular folliculitis. Acta Derm Venereol. 2014;94:104-106.
- Ellis
E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197. - Bull R
H, Harland CA, Fallowfield ME, et al. Eosinophilic folliculitis: a self-limiting illness in patients being treated for haematological malignancy. Br J Dermatol. 1993;129:178-182. - Farber M, Forgia S, Sahu J, et al. Eosinophilic dermatosis of hematologic malignancy. J Cutan Pathol. 2012;39:690-695.
- Nervi J, Stephen. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- Hernández-Martín Á, Nuño-González A, Colmenero I, et al. Eosinophilic pustular folliculitis of infancy: a series of 15 cases and review of the literature [published online July 21, 2012]. J Am Acad Dermatol. 2013;68:150-155.
- Soepr
ono F, Schinella R. Eosinophilic pustular folliculitis in patients with acquired immunodeficiency syndrome. report of three cases. J Am Acad Dermatol. 1986;14:1020-1022. - Katoh
M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. - Keida
T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplant. J Dermatol. 2004;31:21-26. - Goiriz R, Gul-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36.
- Satoh T, Ikeda H, Yokozeki H. Acrosyringeal involvement of palmoplantar lesions of eosinophilic pustular folliculitis. Acta Derm Venereol. 2013;93:99.
- Tsuboi H, Wakita K, Fujimura T, et al. Acral variant of eosinophilic pustular folliculitis (Ofuji’s disease). Clin Exp Dermatol. 2003;28:321-324.
- Nakahig
ashi K, Doi H, Otsuka A, et al. PGD2 induces eotaxin-3 via PPARgamma from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;129:536-543. - Satoh
T, Shimura C, Miyagishi C, et al. Indomethacin-induced reduction in CRTH2 in eosinophilic pustular folliculitis (Ofuji’s disease): a proposed mechanism of action. Acta Derm Venereol. 2010;90:18-22. - Hagiwara A, Fujimura T, Furudate S, et al. Induction of CD163(+)M2 macrophages in the lesional skin of eosinophilic pustular folliculitis. Acta Derm Venereol. 2014;94:104-106.
- Ellis
E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197. - Bull R
H, Harland CA, Fallowfield ME, et al. Eosinophilic folliculitis: a self-limiting illness in patients being treated for haematological malignancy. Br J Dermatol. 1993;129:178-182. - Farber M, Forgia S, Sahu J, et al. Eosinophilic dermatosis of hematologic malignancy. J Cutan Pathol. 2012;39:690-695.
Practice Points
- Recalcitrant folliculocentric papules and pustules involving the head, trunk, arms, and legs should raise suspicion of possible eosinophilic pustular folliculitis (EPF).
- Underlying hematopoietic malignancy may be associated with cases of EPF.