User login
Transgender Care in the Primary Care Setting: A Review of Guidelines and Literature
Lesbian, gay, bisexual, and transgender (LGBT) individuals face significant difficulties in obtaining high-quality,compassionate medical care, much of which has been attributed to inadequate provider knowledge. In this article, the authors present a transgender patient seen in primary care and discuss the knowledge gleaned to inform future care of this patient as well as the care of other similar patients.
The following case discussion and review of the literature also seeks to improve the practice of other primary care providers (PCPs) who are inexperienced in this arena. This article aims to review the basics to permit PCPs to venture into transgender care, including a review of basic terminology; a few interactive tips; and basics in medical and hormonal treatment, follow-up, contraindications, and risk. More details can be obtained through electronic consultation (Transgender eConsult) in the VA.
Case Presentation
A 35-year-old patient who was assigned male sex at birth presented to the primary care clinic to discuss her desire to undergo male-to-female (MTF) transition. The patient stated that she had started taking female estrogen hormones 9 years previously purchased from craigslist without a prescription. She tried oral contraceptives as well as oral and injectable estradiol. While the patient was taking injectable estradiol she had breast growth, decreased anxiety, weight gain, and a feeling of peacefulness. The patient also reported that she had received several laser treatments for whole body hair removal, beginning 8 to 10 years before and more regularly in the past 2 to 3 years. She asked whether transition-related care could be provided, because she could no longer afford the hormones.
The patient wanted to transition because she felt that “Women are beautiful, the way they carry themselves, wear their hair, their nails, I want to be like that.” She also mentioned that when she watched TV, she envisioned herself as a woman. She reported that she enjoyed wearing her mother’s clothing since age 10, which made her feel more like herself. The patient noted that she had desired to remove her body hair since childhood but could not afford to do it until recently. She bought female clothing, shoes and makeup, and did her nails from a young age. The patient also reported that she did not “know what transgender was” until a decade ago.
The patient struggled with her identity growing up; however, she tried not to think about it or talk about it with anyone. She related that she was ashamed of her thoughts and that only recently had made peace with being transgender. Thus, she pursued talking to her medical provider about transitioning. The patient reported that she felt more energetic when taking female hormones and was better able to discuss the issue. Specifically, she noted that if she were not on estrogen now she would not be able to talk about transitioning.
The patient related that she has done extensive research about transitioning, including reading online about other transgender people. She noted that she was aware of “possible backlash with society,” but ultimately, she had decided that transitioning was the right decision for her.
She expressed a desire to have an orchiectomy and continue hormonal therapy to permit her “to have a more feminine face, soft skin, hairless body, big breasts, more fat around the hips, and a high-pitched voice.” She additionally related a desire to be in a stable relationship and be her true self. She also stated that she had not identified herself as a female to anyone yet but would like to soon. The patient reported a history of depression, especially during her military service when she wanted to be a woman but did not feel she understood what was going on or how to manage her feelings. She said that for the past 2 months she felt much happier since beginning to take estradiol 4 mg orally daily, which she had found online. She also tried to purchase anti-androgen medication but could not afford it. In addition, she said that she would like to eventually proceed with gender affirmation surgery.
She was currently having sex with men, primarily via anal receptive intercourse. She had no history of sexually transmitted infections but reported that she did not use condoms regularly. She had no history of physical or sexual abuse. The patient was offered referral to the HIV clinic to receive HIV preexposure prophylaxis therapy (emtricitabine + tenofovir), which she declined, but she was counseled on safe sex practice.
The patient was referred to psychiatry both for supportive mental health care and to clarify that her concomitant mental health issues would not preclude the prescription of gender-affirming hormone treatment. Based on the psychiatric evaluation, the patient was felt to be appropriate for treatment with feminizing hormone therapy. The psychiatric assessment also noted that although the patient had a history of psychosis, she was not exhibiting psychotic symptoms currently, and this would not be a contraindication to treatment.
After discussion of the risks and benefits of cross-sex hormone therapy, the patient was started on estradiol 4 mg orally daily, as well as spironolactone 50 mg daily. She was then switched to estradiol 10 mg intramuscular every 2 weeks with the aim of using a less thrombogenic route of administration.
Treatment Outcomes
The patient remains under care. She has had follow-up visits every 3 months to ensure appropriate signs of feminization and monitoring of adverse effects (AEs). The patient’s testosterone and estradiol levels are being checked every 3 months to ensure total testosterone is 1,2
After 12 months on therapy with estradiol and spironolactone, the patient notes that her mood has improved, she feels more energetic, she has gained some weight, and her skin is softer. Her voice pitch, with the help of speech therapy, is gradually changing to what she perceives as more feminine. Hormone levels and electrolytes are all in an acceptable range, and blood sugar and blood pressure (BP) are within normal range. The patient will be offered age-appropriate cancer screening at the appropriate time.
Discussion
The treatment of gender-nonconforming individuals has come a long way since Lili Elbe, the transgender artist depicted in The Danish Girl, underwent gender-affirmation surgery in the early 20th century. Lili and people like her paved the way for other transgender individuals by doggedly pursuing gender-affirming medical treatment although they faced rejection by society and forged a difficult path. In recent years, an increasing number of transgender individuals have begun to seek mainstream medical care; however, PCPs often lack the knowledge and training to properly interact with and care for transgender patients.3,4
Terminology
Although someone’s sex is typically assigned at birth based on the external appearance of their genitalia, gender identity refers to a person’s internal sense of self and how they fit in to the world. People often use these 2 terms interchangeably in everyday language, but these terms are different.1,2
Transgender refers to a person whose gender identity differs from the sex that was assigned at birth. A transgender man or transman, or female-to-male (FTM) transgender person, is an individual who is assigned female sex at birth but identifies as a male. A transgender woman, or transwoman or a male-to-female (MTF) transgender person, is an individual who is assigned male sex at birth but identifies as female. A nontransgender person may be referred to as cisgender.
Transsexual is a medical term and refers to a transgender individual who sought medical intervention to transition to their identified gender.
Sexual orientation describes sexual attraction only and is not related to gender identity. The sexual orientation of a transgender person is determined by emotional and/or physical attraction and not gender identity.
Gender dysphoria refers to the distress experienced by an individual when one’s gender identity and sex are not completely congruent.
Improving Patient Interaction
Transgender patients might avoid seeking care due to previous negative experiences or a fear of being judged. It is very important to create a safe environment where the patients feel comfortable. Meeting patients “where they are” without judgment will enhance the patient-physician relationship. It is necessary to train all clinic staff about the importance of transgender health issues. All staff should address the patient with the name, pronouns, and gender identity that the patient prefers. For patients with a gender identity that is not strictly male or female (nonbinary patients), gender-neutral pronouns, such as they/them/their, may be chosen. It is helpful to be direct in asking: What is your preferred name? When I speak about you to other providers, what pronouns do you prefer I use, he, she, they? This information can then be documented in the electronic health record (EHR) so that all staff know from visit to visit. Thank the patient for the clarification.
The physical examination can be uncomfortable for both the patient and the physician. Experience and familiarity with the current recommendations can help. The physical examination should be relevant to the anatomy that is present, regardless of the gender presentation. An anatomic survey of the organs currently present in an individual can be useful.1 The physician should be sensitive in examining and obtaining information from the patient, focusing on only those issues relevant to the presenting concern. Chest and genital examinations may be particularly distressing for patients. If a chest or genital examination is indicated, the provider and patient should have a discussion explaining the importance of the examination and how the patient’s comfort can be optimized.
Medical Treatment
Gender-affirmation treatment should be multidisciplinary and include some or all of the following: diagnostic assessment, psychotherapy or counseling, real-life experience (RLE), hormone therapy, and surgical therapy..1,2,5 The World Professional Association for Transgender Health (WPATH) has established internationally accepted Standards of Care (SOC) for the treatment of gender dysphoria that provide detailed expert opinion reviewing the background and guidance for care of transgender individuals. Most commonly, the diagnosis of gender dysphoria is made by a mental health professional (MHP) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) criteria for gender dysphoria.1,2 The involvement of a MHP can be crucial in assessing potential psychological and social risk factors for unfavorable outcomes of medical interventions. In case of severe psychopathology, which can interfere with diagnosis and treatment, the psychopathology should be addressed first.1,2 The MHP also can confirm that the patient has the capacity to make an informed decision.
The 2017 Endocrine Society guidelines for the treatment of gender-dysphoric/gender-incongruent persons emphasize the utility of evaluation of these patients by an expert MHP before starting the treatment.2 However, the guidelines from WPATH and the Center for Transgender Excellence at University of California, San Francisco (UCSF) have stipulated that any provider who feels comfortable assessing the informed decision-making process with a patient can make this determination.
The WPATH SOC states that RLE is essential to transition to the gender role that is congruent with the patient’s gender identity. The RLE is defined as the act of fully adopting a new or evolving gender role or gender presentation in everyday life. In the RLE, the person should fully experience life in the desired gender role before irreversible physical treatment is undertaken. Newer guidelines note that it may be too challenging to adopt the desired gender role without the benefit of feminizing or masculinizing treatment, and therefore, the treatment can be offered at the same time as adopting the new gender role.1
Medical treatment involves administration of masculinizing or feminizing hormone therapy. There are 2 major goals of this hormonal therapy.
For many transgender adults, genital reconstruction surgery and/or gonadectomy is a necessary step toward achieving their goal.
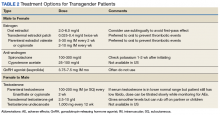
Pretreatment screening and appropriate medical monitoring is recommended for both FTM and MTF transgender patients during the endocrine transition and periodically thereafter.2 The physician should monitor the patient’s weight, BP, directed physical examinations, routine health questions focused on risk factors and medications, complete blood count, renal and liver functions, lipid and blood sugar.2
Physical Changes With Hormone Therapy
Transgender men. Physical changes that are expected to occur during the first 1 to 6 months of testosterone therapy include cessation of menses, increased sexual desire, increased facial and body hair, increased oiliness of skin, increased muscle, and redistribution of fat mass. Changes that occur within the first year of testosterone therapy include deepening of the voice, clitoromegaly, and male pattern hair loss (in some cases). Deepening of the voice, and clitoromegaly are not reversible with discontinuation of hormonal therapy.2
Transgender women. Physical changes that may occur in transgender females in the first 3 to 12 months of estrogen and anti-androgen therapy include decreased sexual desire, decreased spontaneous erections, decreased facial and body hair (usually mild), decreased oiliness of skin, increased breast tissue growth, and redistribution of fat mass. Breast development is generally maximal at 2 years after initiating estrogen, and it is irreversible.2 Effect on fertility may be permanent. Medical therapy has little effect on voice, and most transwomen will require speech therapy to achieve desired pitch.
Routine Health Maintenance
Breast Cancer Screening
Although there are limited data, it is thought that gender-affirming hormone therapy has similar risks as sex hormone replacement therapy in nontransgender males and females. Most AEs arise from use of supraphysiologic doses or inadequate doses.2 Therefore, regular clinical and laboratory monitoring is essential to cross-sex hormone therapy. Treatment with exogenous estrogen and anti-androgens result in transgender women developing breast tissue with ducts, lobules, and acini that is histologically identical to breast tissue in nontransgender females.6
Breast cancer is a concern in transgender women due to prolonged exposure to estrogen. However, the relationship between breast cancer and cross-sex hormone therapy is controversial.
Many factors contribute to breast cancer risk in patients of all genders. Studies of premenopausal and menopausal women taking exogenous estrogen alone have not shown an increase in breast cancer risk. However, the combination of estrogen and progesterone has shown an association with a significant increase in the incidence of breast cancer in postmenopausal women.2,7-10
A study of 5,136 veterans showed a statistically insignificant increased incidence of breast cancer in transgender women compared with data collected from the Surveillance, Epidemiology, and End Results database, although the sample size and duration of the observation were limiting factors.8 A European cohort study found decreased incidence of breast cancer in both MTF and FTM transgender patients, but these patients were an overall younger cohort with decreased risk in general. A cohort of 2,236 MTF individuals in the Netherlands in 1997 showed no increase in all-cause mortality related to hormone therapy at 30-year follow-up. Patients were exposed to exogenous estrogen from 2 months to 41 years.9 A follow-up of this study published in 2013, which included 2,307 MTF individuals taking estrogen for 5 years to > 30 years, revealed only 2 cases of breast cancer, which was the same incidence rate (4.1 per 100,000 person-years) as that of nontransgender women.10
In general, the incidence of breast cancer is rare in nontransgender men, and therefore there have not been a lot of clinical studies to assess risk factors and detection methods. The following risk factors can increase the risk of breast cancer in nontransgender patients: known presence of BRCA mutation, estrogen exposure/androgen insufficiency, Klinefelter syndrome, liver cirrhosis, and obesity.11
Guidelines from the Endocrine Society, WPATH, and UCSF suggest that MTF transgender individuals who have a known increased risk for breast cancer should follow screening guidelines recommended for nontransgender women if they are aged > 50 years and have had more than 5 years of hormone use.2 For FTM patients who have not had chest surgery, screening guidelines should follow those for nontransgender women. For those patients who have had chest reconstruction, small residual amounts of breast tissue may remain. Screening guidelines for these patients do not exist. For these patients, mammography can be technically difficult. Clinical chest wall examination, magnetic resonance imaging (MRI), and/or ultrasound may be helpful modalities. An individual risk vs benefit discussion with the patient is recommended.
Prostate Cancer Screening
Although the prostate gland will undergo atrophy with extended treatment with feminizing hormone therapy, there are case reports of prostate cancer in transgender women.12,13 Usually these patients have started hormone treatment after age 50 years. Therefore, prostate cancer screening is recommended in transgender women as per general guidelines. Because the prostate-specific antigen (PSA) level is expected to be reduced, a PSA > 1.0 should be considered abnormal.1
Cervical Cancer Screening
When a transgender man has a pap smear, it is essential to make it clear to the laboratory that the sample is a cervical pap smear (especially if the gender is marked as male) to avoid the sample being run incorrectly as an anal pap. Also, it is essential to indicate on the pap smear request form that the patient is on testosterone therapy and amenorrhea is present, because the lack of the female hormone can cause atrophy of cervix. This population has a high rate of inadequate specimens. Pretreatment with 1 to 2 weeks of vaginal estrogen can improve the success rate of inadequate specimens. Transgender women who have undergone vaginoplasty do not have a cervix, therefore, cervical cancer screening is not recommended. The anatomy of the neovagina has a more posterior orientation, and an anoscope is a more appropriate tool to examine the neovagina when necessary.
Hematology Health
Transgender women on cross-sex hormone therapy with estrogens may be at increased risk for a venous thromboembolism (VTE). In 2 European studies, patients treated with oral ethinyl estradiol as well as the anti-androgen cyproterone acetate were found to have up to 20 times increased risk of VTE. However, in later studies, oral ethinyl estradiol was changed to either oral conjugated estrogens or transdermal/intramuscular estradiol, and these studies did not show a significant increase in VTE risk.14-16 Tobacco use in combination with estrogen therapy is associated with an increased risk of deep vein thrombosis (DVT).1 All transgender women who smoke should be counseled on tobacco risks and cessation options at every visit.1 The transgender individuals who are not willing to quit smoking may be offered transdermal estrogen, which has lower risk of DVT.14-16
Sexual Health
Clinicians should assess the risks for sexually transmitted infection (STIs) or HIV for transgender patients based on current anatomy and sexual behaviors. Presentations of STIs can be atypical due to varied sexual practices and gender-affirming surgeries. Thus, providers must remain vigilant for symptoms consistent with common STIs and screen for asymptomatic STIs on the basis of behavior history and sexual practices.17 Preexposure prophylaxis for HIV should be considered when appropriate. Serologic screening recommendations for transgender people (eg, HIV, hepatitis B and C, syphilis) do not differ in recommendations from those for nontransgender people.
Cardiovascular Health
The effect of cross-hormone treatment on cardiovascular (CV) health is still unknown. There are no randomized controlled trials that have investigated the relationship between cross-hormone treatment and CV health. Evidence from several studies suggests that CV risk is unchanged among transgender men using testosterone compared with that of nontransgender women.18,19 There is conflicting evidence for transgender women with respect to CV risk and cross-sex hormone treatment.1,18,19 The current American College of Cardiology/American Heart Association guideline advises using the ASCVD risk calculator to determine the need for aspirin and statin treatment based on race, age, gender, and risk factors. There is no guideline on whether to use natal sex or affirmed gender while using the ASCVD calculator. It is reasonable to use the calculator based on natal sex if the cross-hormone treatment has started later in life, but if the cross-sex hormone treatment started at a young age, then one should consider using the affirmed gender to calculate the risk.
As with all patients, life style modifications, including smoking cessation, weight loss, physical activity, and management of BP and blood sugar, are important for CV health. For transgender women with CV risk factors or known CV disease, transdermal route of estrogen is preferred due to lower rates of VTE.18,19
Conclusion
In recent years, an increased number of transgender individuals are seeking mainstream medical care. However, PCPs often lack the knowledge and training to properly interact with and care for transgender patients. It is critical that clinicians understand the difference between sex, gender, and sexuality. For patients who desire transgender care, providers must be able to comfortably ask the patient about their preferred name and prior care, know the basics in cross-sex hormone therapy, including appropriate follow-up of hormonal levels as well as laboratory tests that delineate risk, and know possible complications and AEs. The VA offers significant resources, including electronic transgender care consultation for cases where the provider does not have adequate expertise in the care of these patients.
Both medical schools and residency training programs are starting to incorporate curricula regarding LGBT care. For those who have already completed training, this article serves as a brief guide to terminology, interactive tips, and management of this growing and underserved group of individuals.
1. Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. http://transhealth.ucsf.edu/protocols. Updated June 17, 2016. Accessed June 13, 2018.
2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoria/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903.
3. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
4. Sobralske M. Primary care needs of patients who have undergone gender reassignment. J Am Acad Nurse Pract. 2005;17(4):133-138.
5. Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5(6):877-884.
6. Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW. Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men. Am J Surg Pathol. 2000;24(1):74-80.
7. Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M. Cancer in transgender people: evidence and methodological consideration. Epidemiol Rev. 2017;39(1):93-107.
8. Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191-198.
9. Van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf). 1997;47(3):337-342.
10. Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med. 2013;10(12):3129-3134.
11. Johansen Taber KA, Morisy LR, Osbahr AJ III, Dickinson BD. Male breast cancer: risk factors, diagnosis and management (review). Oncol Rep. 2010;24(5):1115-1120.
12. Miksad RA, Bubley G, Church P, et al. Prostate cancer in a transgender woman, 41 years after initiation of feminization. JAMA. 2006;296(19):2316-2317.
13. Turo R, Jallad S, Prescott S, Cross WR. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7(7-8):E544-E546.
14. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 556: postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887-890.
15. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender treatment. Metabolism. 1989;38(9):869-873.
16. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635-642.
17. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
18. Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol. 2014;170(6):809-819.
19. Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Int Med. 2017;167(4):256-267.
Lesbian, gay, bisexual, and transgender (LGBT) individuals face significant difficulties in obtaining high-quality,compassionate medical care, much of which has been attributed to inadequate provider knowledge. In this article, the authors present a transgender patient seen in primary care and discuss the knowledge gleaned to inform future care of this patient as well as the care of other similar patients.
The following case discussion and review of the literature also seeks to improve the practice of other primary care providers (PCPs) who are inexperienced in this arena. This article aims to review the basics to permit PCPs to venture into transgender care, including a review of basic terminology; a few interactive tips; and basics in medical and hormonal treatment, follow-up, contraindications, and risk. More details can be obtained through electronic consultation (Transgender eConsult) in the VA.
Case Presentation
A 35-year-old patient who was assigned male sex at birth presented to the primary care clinic to discuss her desire to undergo male-to-female (MTF) transition. The patient stated that she had started taking female estrogen hormones 9 years previously purchased from craigslist without a prescription. She tried oral contraceptives as well as oral and injectable estradiol. While the patient was taking injectable estradiol she had breast growth, decreased anxiety, weight gain, and a feeling of peacefulness. The patient also reported that she had received several laser treatments for whole body hair removal, beginning 8 to 10 years before and more regularly in the past 2 to 3 years. She asked whether transition-related care could be provided, because she could no longer afford the hormones.
The patient wanted to transition because she felt that “Women are beautiful, the way they carry themselves, wear their hair, their nails, I want to be like that.” She also mentioned that when she watched TV, she envisioned herself as a woman. She reported that she enjoyed wearing her mother’s clothing since age 10, which made her feel more like herself. The patient noted that she had desired to remove her body hair since childhood but could not afford to do it until recently. She bought female clothing, shoes and makeup, and did her nails from a young age. The patient also reported that she did not “know what transgender was” until a decade ago.
The patient struggled with her identity growing up; however, she tried not to think about it or talk about it with anyone. She related that she was ashamed of her thoughts and that only recently had made peace with being transgender. Thus, she pursued talking to her medical provider about transitioning. The patient reported that she felt more energetic when taking female hormones and was better able to discuss the issue. Specifically, she noted that if she were not on estrogen now she would not be able to talk about transitioning.
The patient related that she has done extensive research about transitioning, including reading online about other transgender people. She noted that she was aware of “possible backlash with society,” but ultimately, she had decided that transitioning was the right decision for her.
She expressed a desire to have an orchiectomy and continue hormonal therapy to permit her “to have a more feminine face, soft skin, hairless body, big breasts, more fat around the hips, and a high-pitched voice.” She additionally related a desire to be in a stable relationship and be her true self. She also stated that she had not identified herself as a female to anyone yet but would like to soon. The patient reported a history of depression, especially during her military service when she wanted to be a woman but did not feel she understood what was going on or how to manage her feelings. She said that for the past 2 months she felt much happier since beginning to take estradiol 4 mg orally daily, which she had found online. She also tried to purchase anti-androgen medication but could not afford it. In addition, she said that she would like to eventually proceed with gender affirmation surgery.
She was currently having sex with men, primarily via anal receptive intercourse. She had no history of sexually transmitted infections but reported that she did not use condoms regularly. She had no history of physical or sexual abuse. The patient was offered referral to the HIV clinic to receive HIV preexposure prophylaxis therapy (emtricitabine + tenofovir), which she declined, but she was counseled on safe sex practice.
The patient was referred to psychiatry both for supportive mental health care and to clarify that her concomitant mental health issues would not preclude the prescription of gender-affirming hormone treatment. Based on the psychiatric evaluation, the patient was felt to be appropriate for treatment with feminizing hormone therapy. The psychiatric assessment also noted that although the patient had a history of psychosis, she was not exhibiting psychotic symptoms currently, and this would not be a contraindication to treatment.
After discussion of the risks and benefits of cross-sex hormone therapy, the patient was started on estradiol 4 mg orally daily, as well as spironolactone 50 mg daily. She was then switched to estradiol 10 mg intramuscular every 2 weeks with the aim of using a less thrombogenic route of administration.
Treatment Outcomes
The patient remains under care. She has had follow-up visits every 3 months to ensure appropriate signs of feminization and monitoring of adverse effects (AEs). The patient’s testosterone and estradiol levels are being checked every 3 months to ensure total testosterone is 1,2
After 12 months on therapy with estradiol and spironolactone, the patient notes that her mood has improved, she feels more energetic, she has gained some weight, and her skin is softer. Her voice pitch, with the help of speech therapy, is gradually changing to what she perceives as more feminine. Hormone levels and electrolytes are all in an acceptable range, and blood sugar and blood pressure (BP) are within normal range. The patient will be offered age-appropriate cancer screening at the appropriate time.
Discussion
The treatment of gender-nonconforming individuals has come a long way since Lili Elbe, the transgender artist depicted in The Danish Girl, underwent gender-affirmation surgery in the early 20th century. Lili and people like her paved the way for other transgender individuals by doggedly pursuing gender-affirming medical treatment although they faced rejection by society and forged a difficult path. In recent years, an increasing number of transgender individuals have begun to seek mainstream medical care; however, PCPs often lack the knowledge and training to properly interact with and care for transgender patients.3,4
Terminology
Although someone’s sex is typically assigned at birth based on the external appearance of their genitalia, gender identity refers to a person’s internal sense of self and how they fit in to the world. People often use these 2 terms interchangeably in everyday language, but these terms are different.1,2
Transgender refers to a person whose gender identity differs from the sex that was assigned at birth. A transgender man or transman, or female-to-male (FTM) transgender person, is an individual who is assigned female sex at birth but identifies as a male. A transgender woman, or transwoman or a male-to-female (MTF) transgender person, is an individual who is assigned male sex at birth but identifies as female. A nontransgender person may be referred to as cisgender.
Transsexual is a medical term and refers to a transgender individual who sought medical intervention to transition to their identified gender.
Sexual orientation describes sexual attraction only and is not related to gender identity. The sexual orientation of a transgender person is determined by emotional and/or physical attraction and not gender identity.
Gender dysphoria refers to the distress experienced by an individual when one’s gender identity and sex are not completely congruent.
Improving Patient Interaction
Transgender patients might avoid seeking care due to previous negative experiences or a fear of being judged. It is very important to create a safe environment where the patients feel comfortable. Meeting patients “where they are” without judgment will enhance the patient-physician relationship. It is necessary to train all clinic staff about the importance of transgender health issues. All staff should address the patient with the name, pronouns, and gender identity that the patient prefers. For patients with a gender identity that is not strictly male or female (nonbinary patients), gender-neutral pronouns, such as they/them/their, may be chosen. It is helpful to be direct in asking: What is your preferred name? When I speak about you to other providers, what pronouns do you prefer I use, he, she, they? This information can then be documented in the electronic health record (EHR) so that all staff know from visit to visit. Thank the patient for the clarification.
The physical examination can be uncomfortable for both the patient and the physician. Experience and familiarity with the current recommendations can help. The physical examination should be relevant to the anatomy that is present, regardless of the gender presentation. An anatomic survey of the organs currently present in an individual can be useful.1 The physician should be sensitive in examining and obtaining information from the patient, focusing on only those issues relevant to the presenting concern. Chest and genital examinations may be particularly distressing for patients. If a chest or genital examination is indicated, the provider and patient should have a discussion explaining the importance of the examination and how the patient’s comfort can be optimized.
Medical Treatment
Gender-affirmation treatment should be multidisciplinary and include some or all of the following: diagnostic assessment, psychotherapy or counseling, real-life experience (RLE), hormone therapy, and surgical therapy..1,2,5 The World Professional Association for Transgender Health (WPATH) has established internationally accepted Standards of Care (SOC) for the treatment of gender dysphoria that provide detailed expert opinion reviewing the background and guidance for care of transgender individuals. Most commonly, the diagnosis of gender dysphoria is made by a mental health professional (MHP) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) criteria for gender dysphoria.1,2 The involvement of a MHP can be crucial in assessing potential psychological and social risk factors for unfavorable outcomes of medical interventions. In case of severe psychopathology, which can interfere with diagnosis and treatment, the psychopathology should be addressed first.1,2 The MHP also can confirm that the patient has the capacity to make an informed decision.
The 2017 Endocrine Society guidelines for the treatment of gender-dysphoric/gender-incongruent persons emphasize the utility of evaluation of these patients by an expert MHP before starting the treatment.2 However, the guidelines from WPATH and the Center for Transgender Excellence at University of California, San Francisco (UCSF) have stipulated that any provider who feels comfortable assessing the informed decision-making process with a patient can make this determination.
The WPATH SOC states that RLE is essential to transition to the gender role that is congruent with the patient’s gender identity. The RLE is defined as the act of fully adopting a new or evolving gender role or gender presentation in everyday life. In the RLE, the person should fully experience life in the desired gender role before irreversible physical treatment is undertaken. Newer guidelines note that it may be too challenging to adopt the desired gender role without the benefit of feminizing or masculinizing treatment, and therefore, the treatment can be offered at the same time as adopting the new gender role.1
Medical treatment involves administration of masculinizing or feminizing hormone therapy. There are 2 major goals of this hormonal therapy.
For many transgender adults, genital reconstruction surgery and/or gonadectomy is a necessary step toward achieving their goal.
Pretreatment screening and appropriate medical monitoring is recommended for both FTM and MTF transgender patients during the endocrine transition and periodically thereafter.2 The physician should monitor the patient’s weight, BP, directed physical examinations, routine health questions focused on risk factors and medications, complete blood count, renal and liver functions, lipid and blood sugar.2
Physical Changes With Hormone Therapy
Transgender men. Physical changes that are expected to occur during the first 1 to 6 months of testosterone therapy include cessation of menses, increased sexual desire, increased facial and body hair, increased oiliness of skin, increased muscle, and redistribution of fat mass. Changes that occur within the first year of testosterone therapy include deepening of the voice, clitoromegaly, and male pattern hair loss (in some cases). Deepening of the voice, and clitoromegaly are not reversible with discontinuation of hormonal therapy.2
Transgender women. Physical changes that may occur in transgender females in the first 3 to 12 months of estrogen and anti-androgen therapy include decreased sexual desire, decreased spontaneous erections, decreased facial and body hair (usually mild), decreased oiliness of skin, increased breast tissue growth, and redistribution of fat mass. Breast development is generally maximal at 2 years after initiating estrogen, and it is irreversible.2 Effect on fertility may be permanent. Medical therapy has little effect on voice, and most transwomen will require speech therapy to achieve desired pitch.
Routine Health Maintenance
Breast Cancer Screening
Although there are limited data, it is thought that gender-affirming hormone therapy has similar risks as sex hormone replacement therapy in nontransgender males and females. Most AEs arise from use of supraphysiologic doses or inadequate doses.2 Therefore, regular clinical and laboratory monitoring is essential to cross-sex hormone therapy. Treatment with exogenous estrogen and anti-androgens result in transgender women developing breast tissue with ducts, lobules, and acini that is histologically identical to breast tissue in nontransgender females.6
Breast cancer is a concern in transgender women due to prolonged exposure to estrogen. However, the relationship between breast cancer and cross-sex hormone therapy is controversial.
Many factors contribute to breast cancer risk in patients of all genders. Studies of premenopausal and menopausal women taking exogenous estrogen alone have not shown an increase in breast cancer risk. However, the combination of estrogen and progesterone has shown an association with a significant increase in the incidence of breast cancer in postmenopausal women.2,7-10
A study of 5,136 veterans showed a statistically insignificant increased incidence of breast cancer in transgender women compared with data collected from the Surveillance, Epidemiology, and End Results database, although the sample size and duration of the observation were limiting factors.8 A European cohort study found decreased incidence of breast cancer in both MTF and FTM transgender patients, but these patients were an overall younger cohort with decreased risk in general. A cohort of 2,236 MTF individuals in the Netherlands in 1997 showed no increase in all-cause mortality related to hormone therapy at 30-year follow-up. Patients were exposed to exogenous estrogen from 2 months to 41 years.9 A follow-up of this study published in 2013, which included 2,307 MTF individuals taking estrogen for 5 years to > 30 years, revealed only 2 cases of breast cancer, which was the same incidence rate (4.1 per 100,000 person-years) as that of nontransgender women.10
In general, the incidence of breast cancer is rare in nontransgender men, and therefore there have not been a lot of clinical studies to assess risk factors and detection methods. The following risk factors can increase the risk of breast cancer in nontransgender patients: known presence of BRCA mutation, estrogen exposure/androgen insufficiency, Klinefelter syndrome, liver cirrhosis, and obesity.11
Guidelines from the Endocrine Society, WPATH, and UCSF suggest that MTF transgender individuals who have a known increased risk for breast cancer should follow screening guidelines recommended for nontransgender women if they are aged > 50 years and have had more than 5 years of hormone use.2 For FTM patients who have not had chest surgery, screening guidelines should follow those for nontransgender women. For those patients who have had chest reconstruction, small residual amounts of breast tissue may remain. Screening guidelines for these patients do not exist. For these patients, mammography can be technically difficult. Clinical chest wall examination, magnetic resonance imaging (MRI), and/or ultrasound may be helpful modalities. An individual risk vs benefit discussion with the patient is recommended.
Prostate Cancer Screening
Although the prostate gland will undergo atrophy with extended treatment with feminizing hormone therapy, there are case reports of prostate cancer in transgender women.12,13 Usually these patients have started hormone treatment after age 50 years. Therefore, prostate cancer screening is recommended in transgender women as per general guidelines. Because the prostate-specific antigen (PSA) level is expected to be reduced, a PSA > 1.0 should be considered abnormal.1
Cervical Cancer Screening
When a transgender man has a pap smear, it is essential to make it clear to the laboratory that the sample is a cervical pap smear (especially if the gender is marked as male) to avoid the sample being run incorrectly as an anal pap. Also, it is essential to indicate on the pap smear request form that the patient is on testosterone therapy and amenorrhea is present, because the lack of the female hormone can cause atrophy of cervix. This population has a high rate of inadequate specimens. Pretreatment with 1 to 2 weeks of vaginal estrogen can improve the success rate of inadequate specimens. Transgender women who have undergone vaginoplasty do not have a cervix, therefore, cervical cancer screening is not recommended. The anatomy of the neovagina has a more posterior orientation, and an anoscope is a more appropriate tool to examine the neovagina when necessary.
Hematology Health
Transgender women on cross-sex hormone therapy with estrogens may be at increased risk for a venous thromboembolism (VTE). In 2 European studies, patients treated with oral ethinyl estradiol as well as the anti-androgen cyproterone acetate were found to have up to 20 times increased risk of VTE. However, in later studies, oral ethinyl estradiol was changed to either oral conjugated estrogens or transdermal/intramuscular estradiol, and these studies did not show a significant increase in VTE risk.14-16 Tobacco use in combination with estrogen therapy is associated with an increased risk of deep vein thrombosis (DVT).1 All transgender women who smoke should be counseled on tobacco risks and cessation options at every visit.1 The transgender individuals who are not willing to quit smoking may be offered transdermal estrogen, which has lower risk of DVT.14-16
Sexual Health
Clinicians should assess the risks for sexually transmitted infection (STIs) or HIV for transgender patients based on current anatomy and sexual behaviors. Presentations of STIs can be atypical due to varied sexual practices and gender-affirming surgeries. Thus, providers must remain vigilant for symptoms consistent with common STIs and screen for asymptomatic STIs on the basis of behavior history and sexual practices.17 Preexposure prophylaxis for HIV should be considered when appropriate. Serologic screening recommendations for transgender people (eg, HIV, hepatitis B and C, syphilis) do not differ in recommendations from those for nontransgender people.
Cardiovascular Health
The effect of cross-hormone treatment on cardiovascular (CV) health is still unknown. There are no randomized controlled trials that have investigated the relationship between cross-hormone treatment and CV health. Evidence from several studies suggests that CV risk is unchanged among transgender men using testosterone compared with that of nontransgender women.18,19 There is conflicting evidence for transgender women with respect to CV risk and cross-sex hormone treatment.1,18,19 The current American College of Cardiology/American Heart Association guideline advises using the ASCVD risk calculator to determine the need for aspirin and statin treatment based on race, age, gender, and risk factors. There is no guideline on whether to use natal sex or affirmed gender while using the ASCVD calculator. It is reasonable to use the calculator based on natal sex if the cross-hormone treatment has started later in life, but if the cross-sex hormone treatment started at a young age, then one should consider using the affirmed gender to calculate the risk.
As with all patients, life style modifications, including smoking cessation, weight loss, physical activity, and management of BP and blood sugar, are important for CV health. For transgender women with CV risk factors or known CV disease, transdermal route of estrogen is preferred due to lower rates of VTE.18,19
Conclusion
In recent years, an increased number of transgender individuals are seeking mainstream medical care. However, PCPs often lack the knowledge and training to properly interact with and care for transgender patients. It is critical that clinicians understand the difference between sex, gender, and sexuality. For patients who desire transgender care, providers must be able to comfortably ask the patient about their preferred name and prior care, know the basics in cross-sex hormone therapy, including appropriate follow-up of hormonal levels as well as laboratory tests that delineate risk, and know possible complications and AEs. The VA offers significant resources, including electronic transgender care consultation for cases where the provider does not have adequate expertise in the care of these patients.
Both medical schools and residency training programs are starting to incorporate curricula regarding LGBT care. For those who have already completed training, this article serves as a brief guide to terminology, interactive tips, and management of this growing and underserved group of individuals.
Lesbian, gay, bisexual, and transgender (LGBT) individuals face significant difficulties in obtaining high-quality,compassionate medical care, much of which has been attributed to inadequate provider knowledge. In this article, the authors present a transgender patient seen in primary care and discuss the knowledge gleaned to inform future care of this patient as well as the care of other similar patients.
The following case discussion and review of the literature also seeks to improve the practice of other primary care providers (PCPs) who are inexperienced in this arena. This article aims to review the basics to permit PCPs to venture into transgender care, including a review of basic terminology; a few interactive tips; and basics in medical and hormonal treatment, follow-up, contraindications, and risk. More details can be obtained through electronic consultation (Transgender eConsult) in the VA.
Case Presentation
A 35-year-old patient who was assigned male sex at birth presented to the primary care clinic to discuss her desire to undergo male-to-female (MTF) transition. The patient stated that she had started taking female estrogen hormones 9 years previously purchased from craigslist without a prescription. She tried oral contraceptives as well as oral and injectable estradiol. While the patient was taking injectable estradiol she had breast growth, decreased anxiety, weight gain, and a feeling of peacefulness. The patient also reported that she had received several laser treatments for whole body hair removal, beginning 8 to 10 years before and more regularly in the past 2 to 3 years. She asked whether transition-related care could be provided, because she could no longer afford the hormones.
The patient wanted to transition because she felt that “Women are beautiful, the way they carry themselves, wear their hair, their nails, I want to be like that.” She also mentioned that when she watched TV, she envisioned herself as a woman. She reported that she enjoyed wearing her mother’s clothing since age 10, which made her feel more like herself. The patient noted that she had desired to remove her body hair since childhood but could not afford to do it until recently. She bought female clothing, shoes and makeup, and did her nails from a young age. The patient also reported that she did not “know what transgender was” until a decade ago.
The patient struggled with her identity growing up; however, she tried not to think about it or talk about it with anyone. She related that she was ashamed of her thoughts and that only recently had made peace with being transgender. Thus, she pursued talking to her medical provider about transitioning. The patient reported that she felt more energetic when taking female hormones and was better able to discuss the issue. Specifically, she noted that if she were not on estrogen now she would not be able to talk about transitioning.
The patient related that she has done extensive research about transitioning, including reading online about other transgender people. She noted that she was aware of “possible backlash with society,” but ultimately, she had decided that transitioning was the right decision for her.
She expressed a desire to have an orchiectomy and continue hormonal therapy to permit her “to have a more feminine face, soft skin, hairless body, big breasts, more fat around the hips, and a high-pitched voice.” She additionally related a desire to be in a stable relationship and be her true self. She also stated that she had not identified herself as a female to anyone yet but would like to soon. The patient reported a history of depression, especially during her military service when she wanted to be a woman but did not feel she understood what was going on or how to manage her feelings. She said that for the past 2 months she felt much happier since beginning to take estradiol 4 mg orally daily, which she had found online. She also tried to purchase anti-androgen medication but could not afford it. In addition, she said that she would like to eventually proceed with gender affirmation surgery.
She was currently having sex with men, primarily via anal receptive intercourse. She had no history of sexually transmitted infections but reported that she did not use condoms regularly. She had no history of physical or sexual abuse. The patient was offered referral to the HIV clinic to receive HIV preexposure prophylaxis therapy (emtricitabine + tenofovir), which she declined, but she was counseled on safe sex practice.
The patient was referred to psychiatry both for supportive mental health care and to clarify that her concomitant mental health issues would not preclude the prescription of gender-affirming hormone treatment. Based on the psychiatric evaluation, the patient was felt to be appropriate for treatment with feminizing hormone therapy. The psychiatric assessment also noted that although the patient had a history of psychosis, she was not exhibiting psychotic symptoms currently, and this would not be a contraindication to treatment.
After discussion of the risks and benefits of cross-sex hormone therapy, the patient was started on estradiol 4 mg orally daily, as well as spironolactone 50 mg daily. She was then switched to estradiol 10 mg intramuscular every 2 weeks with the aim of using a less thrombogenic route of administration.
Treatment Outcomes
The patient remains under care. She has had follow-up visits every 3 months to ensure appropriate signs of feminization and monitoring of adverse effects (AEs). The patient’s testosterone and estradiol levels are being checked every 3 months to ensure total testosterone is 1,2
After 12 months on therapy with estradiol and spironolactone, the patient notes that her mood has improved, she feels more energetic, she has gained some weight, and her skin is softer. Her voice pitch, with the help of speech therapy, is gradually changing to what she perceives as more feminine. Hormone levels and electrolytes are all in an acceptable range, and blood sugar and blood pressure (BP) are within normal range. The patient will be offered age-appropriate cancer screening at the appropriate time.
Discussion
The treatment of gender-nonconforming individuals has come a long way since Lili Elbe, the transgender artist depicted in The Danish Girl, underwent gender-affirmation surgery in the early 20th century. Lili and people like her paved the way for other transgender individuals by doggedly pursuing gender-affirming medical treatment although they faced rejection by society and forged a difficult path. In recent years, an increasing number of transgender individuals have begun to seek mainstream medical care; however, PCPs often lack the knowledge and training to properly interact with and care for transgender patients.3,4
Terminology
Although someone’s sex is typically assigned at birth based on the external appearance of their genitalia, gender identity refers to a person’s internal sense of self and how they fit in to the world. People often use these 2 terms interchangeably in everyday language, but these terms are different.1,2
Transgender refers to a person whose gender identity differs from the sex that was assigned at birth. A transgender man or transman, or female-to-male (FTM) transgender person, is an individual who is assigned female sex at birth but identifies as a male. A transgender woman, or transwoman or a male-to-female (MTF) transgender person, is an individual who is assigned male sex at birth but identifies as female. A nontransgender person may be referred to as cisgender.
Transsexual is a medical term and refers to a transgender individual who sought medical intervention to transition to their identified gender.
Sexual orientation describes sexual attraction only and is not related to gender identity. The sexual orientation of a transgender person is determined by emotional and/or physical attraction and not gender identity.
Gender dysphoria refers to the distress experienced by an individual when one’s gender identity and sex are not completely congruent.
Improving Patient Interaction
Transgender patients might avoid seeking care due to previous negative experiences or a fear of being judged. It is very important to create a safe environment where the patients feel comfortable. Meeting patients “where they are” without judgment will enhance the patient-physician relationship. It is necessary to train all clinic staff about the importance of transgender health issues. All staff should address the patient with the name, pronouns, and gender identity that the patient prefers. For patients with a gender identity that is not strictly male or female (nonbinary patients), gender-neutral pronouns, such as they/them/their, may be chosen. It is helpful to be direct in asking: What is your preferred name? When I speak about you to other providers, what pronouns do you prefer I use, he, she, they? This information can then be documented in the electronic health record (EHR) so that all staff know from visit to visit. Thank the patient for the clarification.
The physical examination can be uncomfortable for both the patient and the physician. Experience and familiarity with the current recommendations can help. The physical examination should be relevant to the anatomy that is present, regardless of the gender presentation. An anatomic survey of the organs currently present in an individual can be useful.1 The physician should be sensitive in examining and obtaining information from the patient, focusing on only those issues relevant to the presenting concern. Chest and genital examinations may be particularly distressing for patients. If a chest or genital examination is indicated, the provider and patient should have a discussion explaining the importance of the examination and how the patient’s comfort can be optimized.
Medical Treatment
Gender-affirmation treatment should be multidisciplinary and include some or all of the following: diagnostic assessment, psychotherapy or counseling, real-life experience (RLE), hormone therapy, and surgical therapy..1,2,5 The World Professional Association for Transgender Health (WPATH) has established internationally accepted Standards of Care (SOC) for the treatment of gender dysphoria that provide detailed expert opinion reviewing the background and guidance for care of transgender individuals. Most commonly, the diagnosis of gender dysphoria is made by a mental health professional (MHP) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) criteria for gender dysphoria.1,2 The involvement of a MHP can be crucial in assessing potential psychological and social risk factors for unfavorable outcomes of medical interventions. In case of severe psychopathology, which can interfere with diagnosis and treatment, the psychopathology should be addressed first.1,2 The MHP also can confirm that the patient has the capacity to make an informed decision.
The 2017 Endocrine Society guidelines for the treatment of gender-dysphoric/gender-incongruent persons emphasize the utility of evaluation of these patients by an expert MHP before starting the treatment.2 However, the guidelines from WPATH and the Center for Transgender Excellence at University of California, San Francisco (UCSF) have stipulated that any provider who feels comfortable assessing the informed decision-making process with a patient can make this determination.
The WPATH SOC states that RLE is essential to transition to the gender role that is congruent with the patient’s gender identity. The RLE is defined as the act of fully adopting a new or evolving gender role or gender presentation in everyday life. In the RLE, the person should fully experience life in the desired gender role before irreversible physical treatment is undertaken. Newer guidelines note that it may be too challenging to adopt the desired gender role without the benefit of feminizing or masculinizing treatment, and therefore, the treatment can be offered at the same time as adopting the new gender role.1
Medical treatment involves administration of masculinizing or feminizing hormone therapy. There are 2 major goals of this hormonal therapy.
For many transgender adults, genital reconstruction surgery and/or gonadectomy is a necessary step toward achieving their goal.
Pretreatment screening and appropriate medical monitoring is recommended for both FTM and MTF transgender patients during the endocrine transition and periodically thereafter.2 The physician should monitor the patient’s weight, BP, directed physical examinations, routine health questions focused on risk factors and medications, complete blood count, renal and liver functions, lipid and blood sugar.2
Physical Changes With Hormone Therapy
Transgender men. Physical changes that are expected to occur during the first 1 to 6 months of testosterone therapy include cessation of menses, increased sexual desire, increased facial and body hair, increased oiliness of skin, increased muscle, and redistribution of fat mass. Changes that occur within the first year of testosterone therapy include deepening of the voice, clitoromegaly, and male pattern hair loss (in some cases). Deepening of the voice, and clitoromegaly are not reversible with discontinuation of hormonal therapy.2
Transgender women. Physical changes that may occur in transgender females in the first 3 to 12 months of estrogen and anti-androgen therapy include decreased sexual desire, decreased spontaneous erections, decreased facial and body hair (usually mild), decreased oiliness of skin, increased breast tissue growth, and redistribution of fat mass. Breast development is generally maximal at 2 years after initiating estrogen, and it is irreversible.2 Effect on fertility may be permanent. Medical therapy has little effect on voice, and most transwomen will require speech therapy to achieve desired pitch.
Routine Health Maintenance
Breast Cancer Screening
Although there are limited data, it is thought that gender-affirming hormone therapy has similar risks as sex hormone replacement therapy in nontransgender males and females. Most AEs arise from use of supraphysiologic doses or inadequate doses.2 Therefore, regular clinical and laboratory monitoring is essential to cross-sex hormone therapy. Treatment with exogenous estrogen and anti-androgens result in transgender women developing breast tissue with ducts, lobules, and acini that is histologically identical to breast tissue in nontransgender females.6
Breast cancer is a concern in transgender women due to prolonged exposure to estrogen. However, the relationship between breast cancer and cross-sex hormone therapy is controversial.
Many factors contribute to breast cancer risk in patients of all genders. Studies of premenopausal and menopausal women taking exogenous estrogen alone have not shown an increase in breast cancer risk. However, the combination of estrogen and progesterone has shown an association with a significant increase in the incidence of breast cancer in postmenopausal women.2,7-10
A study of 5,136 veterans showed a statistically insignificant increased incidence of breast cancer in transgender women compared with data collected from the Surveillance, Epidemiology, and End Results database, although the sample size and duration of the observation were limiting factors.8 A European cohort study found decreased incidence of breast cancer in both MTF and FTM transgender patients, but these patients were an overall younger cohort with decreased risk in general. A cohort of 2,236 MTF individuals in the Netherlands in 1997 showed no increase in all-cause mortality related to hormone therapy at 30-year follow-up. Patients were exposed to exogenous estrogen from 2 months to 41 years.9 A follow-up of this study published in 2013, which included 2,307 MTF individuals taking estrogen for 5 years to > 30 years, revealed only 2 cases of breast cancer, which was the same incidence rate (4.1 per 100,000 person-years) as that of nontransgender women.10
In general, the incidence of breast cancer is rare in nontransgender men, and therefore there have not been a lot of clinical studies to assess risk factors and detection methods. The following risk factors can increase the risk of breast cancer in nontransgender patients: known presence of BRCA mutation, estrogen exposure/androgen insufficiency, Klinefelter syndrome, liver cirrhosis, and obesity.11
Guidelines from the Endocrine Society, WPATH, and UCSF suggest that MTF transgender individuals who have a known increased risk for breast cancer should follow screening guidelines recommended for nontransgender women if they are aged > 50 years and have had more than 5 years of hormone use.2 For FTM patients who have not had chest surgery, screening guidelines should follow those for nontransgender women. For those patients who have had chest reconstruction, small residual amounts of breast tissue may remain. Screening guidelines for these patients do not exist. For these patients, mammography can be technically difficult. Clinical chest wall examination, magnetic resonance imaging (MRI), and/or ultrasound may be helpful modalities. An individual risk vs benefit discussion with the patient is recommended.
Prostate Cancer Screening
Although the prostate gland will undergo atrophy with extended treatment with feminizing hormone therapy, there are case reports of prostate cancer in transgender women.12,13 Usually these patients have started hormone treatment after age 50 years. Therefore, prostate cancer screening is recommended in transgender women as per general guidelines. Because the prostate-specific antigen (PSA) level is expected to be reduced, a PSA > 1.0 should be considered abnormal.1
Cervical Cancer Screening
When a transgender man has a pap smear, it is essential to make it clear to the laboratory that the sample is a cervical pap smear (especially if the gender is marked as male) to avoid the sample being run incorrectly as an anal pap. Also, it is essential to indicate on the pap smear request form that the patient is on testosterone therapy and amenorrhea is present, because the lack of the female hormone can cause atrophy of cervix. This population has a high rate of inadequate specimens. Pretreatment with 1 to 2 weeks of vaginal estrogen can improve the success rate of inadequate specimens. Transgender women who have undergone vaginoplasty do not have a cervix, therefore, cervical cancer screening is not recommended. The anatomy of the neovagina has a more posterior orientation, and an anoscope is a more appropriate tool to examine the neovagina when necessary.
Hematology Health
Transgender women on cross-sex hormone therapy with estrogens may be at increased risk for a venous thromboembolism (VTE). In 2 European studies, patients treated with oral ethinyl estradiol as well as the anti-androgen cyproterone acetate were found to have up to 20 times increased risk of VTE. However, in later studies, oral ethinyl estradiol was changed to either oral conjugated estrogens or transdermal/intramuscular estradiol, and these studies did not show a significant increase in VTE risk.14-16 Tobacco use in combination with estrogen therapy is associated with an increased risk of deep vein thrombosis (DVT).1 All transgender women who smoke should be counseled on tobacco risks and cessation options at every visit.1 The transgender individuals who are not willing to quit smoking may be offered transdermal estrogen, which has lower risk of DVT.14-16
Sexual Health
Clinicians should assess the risks for sexually transmitted infection (STIs) or HIV for transgender patients based on current anatomy and sexual behaviors. Presentations of STIs can be atypical due to varied sexual practices and gender-affirming surgeries. Thus, providers must remain vigilant for symptoms consistent with common STIs and screen for asymptomatic STIs on the basis of behavior history and sexual practices.17 Preexposure prophylaxis for HIV should be considered when appropriate. Serologic screening recommendations for transgender people (eg, HIV, hepatitis B and C, syphilis) do not differ in recommendations from those for nontransgender people.
Cardiovascular Health
The effect of cross-hormone treatment on cardiovascular (CV) health is still unknown. There are no randomized controlled trials that have investigated the relationship between cross-hormone treatment and CV health. Evidence from several studies suggests that CV risk is unchanged among transgender men using testosterone compared with that of nontransgender women.18,19 There is conflicting evidence for transgender women with respect to CV risk and cross-sex hormone treatment.1,18,19 The current American College of Cardiology/American Heart Association guideline advises using the ASCVD risk calculator to determine the need for aspirin and statin treatment based on race, age, gender, and risk factors. There is no guideline on whether to use natal sex or affirmed gender while using the ASCVD calculator. It is reasonable to use the calculator based on natal sex if the cross-hormone treatment has started later in life, but if the cross-sex hormone treatment started at a young age, then one should consider using the affirmed gender to calculate the risk.
As with all patients, life style modifications, including smoking cessation, weight loss, physical activity, and management of BP and blood sugar, are important for CV health. For transgender women with CV risk factors or known CV disease, transdermal route of estrogen is preferred due to lower rates of VTE.18,19
Conclusion
In recent years, an increased number of transgender individuals are seeking mainstream medical care. However, PCPs often lack the knowledge and training to properly interact with and care for transgender patients. It is critical that clinicians understand the difference between sex, gender, and sexuality. For patients who desire transgender care, providers must be able to comfortably ask the patient about their preferred name and prior care, know the basics in cross-sex hormone therapy, including appropriate follow-up of hormonal levels as well as laboratory tests that delineate risk, and know possible complications and AEs. The VA offers significant resources, including electronic transgender care consultation for cases where the provider does not have adequate expertise in the care of these patients.
Both medical schools and residency training programs are starting to incorporate curricula regarding LGBT care. For those who have already completed training, this article serves as a brief guide to terminology, interactive tips, and management of this growing and underserved group of individuals.
1. Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. http://transhealth.ucsf.edu/protocols. Updated June 17, 2016. Accessed June 13, 2018.
2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoria/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903.
3. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
4. Sobralske M. Primary care needs of patients who have undergone gender reassignment. J Am Acad Nurse Pract. 2005;17(4):133-138.
5. Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5(6):877-884.
6. Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW. Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men. Am J Surg Pathol. 2000;24(1):74-80.
7. Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M. Cancer in transgender people: evidence and methodological consideration. Epidemiol Rev. 2017;39(1):93-107.
8. Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191-198.
9. Van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf). 1997;47(3):337-342.
10. Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med. 2013;10(12):3129-3134.
11. Johansen Taber KA, Morisy LR, Osbahr AJ III, Dickinson BD. Male breast cancer: risk factors, diagnosis and management (review). Oncol Rep. 2010;24(5):1115-1120.
12. Miksad RA, Bubley G, Church P, et al. Prostate cancer in a transgender woman, 41 years after initiation of feminization. JAMA. 2006;296(19):2316-2317.
13. Turo R, Jallad S, Prescott S, Cross WR. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7(7-8):E544-E546.
14. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 556: postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887-890.
15. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender treatment. Metabolism. 1989;38(9):869-873.
16. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635-642.
17. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
18. Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol. 2014;170(6):809-819.
19. Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Int Med. 2017;167(4):256-267.
1. Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. http://transhealth.ucsf.edu/protocols. Updated June 17, 2016. Accessed June 13, 2018.
2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoria/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903.
3. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
4. Sobralske M. Primary care needs of patients who have undergone gender reassignment. J Am Acad Nurse Pract. 2005;17(4):133-138.
5. Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5(6):877-884.
6. Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW. Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men. Am J Surg Pathol. 2000;24(1):74-80.
7. Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M. Cancer in transgender people: evidence and methodological consideration. Epidemiol Rev. 2017;39(1):93-107.
8. Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191-198.
9. Van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf). 1997;47(3):337-342.
10. Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med. 2013;10(12):3129-3134.
11. Johansen Taber KA, Morisy LR, Osbahr AJ III, Dickinson BD. Male breast cancer: risk factors, diagnosis and management (review). Oncol Rep. 2010;24(5):1115-1120.
12. Miksad RA, Bubley G, Church P, et al. Prostate cancer in a transgender woman, 41 years after initiation of feminization. JAMA. 2006;296(19):2316-2317.
13. Turo R, Jallad S, Prescott S, Cross WR. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7(7-8):E544-E546.
14. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 556: postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887-890.
15. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender treatment. Metabolism. 1989;38(9):869-873.
16. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635-642.
17. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
18. Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol. 2014;170(6):809-819.
19. Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Int Med. 2017;167(4):256-267.
Not Another Missed Spinal Epidural Abscess
A 55-year-old man presented for evaluation of a 2-day history of worsening left lower back pain.
Delay in the diagnosis and treatment of spinal epidural abscess (SEA) increases the likelihood of permanent disability (eg, residual motor weakness) or even death.1-5 Studies suggest that the incidence of SEA may be on the rise,6,7 which is especially troubling in an era of emerging antibiotic resistance.8 The pervasive theme among the medical literature stresses the challenges with early recognition; however, missed SEA is a theoretical mishap in a manner akin to Schrödinger’s cat or Heisenberg’s uncertainty principle.9,10
One will recall Erwin Schrödinger’s thought experiment of 1935 when he challenged the theory of quantum mechanics by asking whether or not the cat in a box is still alive if there is a 50/50 chance poisonous gas has been released. He suggested that before one looks in the box, the cat is both alive and dead—a state of superposition.
Unfortunately, medical diagnoses do not exist in dual states. Tests are either positive or negative; disease is either present or absent; and in medicine, the cat is either alive or dead. Moreover, when SEA is diagnosed after the initial presentation and workup, (ie, the “bounce-back”), the clinician cannot categorically assume the condition was present, but missed, at the initial evaluation. We present the following case, not as a miraculous catch, or a “zebra-hunting guide” but rather as a rare glimpse into the evolution of a disease process.
Case
A 55-year-old man with history of type 2 diabetes mellitus (DM), hypertension, and hyperlipidemia presented to the ED with a 2-day history of progressively worsening left lower back pain. Although the patient denied a recent history of trauma, he did state that he helped one of his friends move furniture 1 day prior to presentation and had attributed the worsening pain to this event. The patient described his pain as mild and dull when he was at rest, rating it as a 2 on a pain scale of 1 to 10; and sharp-feeling and at its worst upon movement, rating it as a 9 on a pain scale of 1 to 10. The patient noted experiencing only mild relief when he shifted to certain positions.
The patient’s pain was nonradiating and associated with dull pain in the left anterior proximal thigh. The patient denied any numbness or weakness in any of his extremities. He also denied any perineal numbness or urinary or bowel incontinence; however, he did note experiencing a sense of incomplete evacuation of stools over the past 5 mornings.
The patient denied any recent history of fever, chills, numbness, weakness, difficulty with balance, direct trauma, instrumentation or chiropractic manipulation, or unexplained or unintentional weight loss. He had no history of malignancy and vehemently denied intravenous (IV) drug use.
On physical examination, the patient’s vital signs were: blood pressure, 143/93 mm Hg; heart rate, 108 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.6°F. Oxygen saturation was 95% on room air. Upon examination, the patient was in no acute distress and was resting comfortably and quietly. Pertinent findings included a supple neck examination, without lymphadenopathy or meningismus. There was no midline tenderness to palpation of the cervical spine and no step-off deformities. The lungs were clear to auscultation bilaterally and without wheezing, rhonchi, or rales. Examination of the heart revealed a regular rhythm with borderline tachycardia, but without murmurs, rubs, or gallops. The patient had 2+ pulses in all four extremities, and capillary refill was less than 2 seconds. The abdomen was soft and nontender, without rebound, guarding, or rigidity. There were no pulsatile abdominal masses or bruits, and bowel sounds were present. The patient had no costovertebral angle tenderness on percussion, and had full range of motion of all four extremities, with no tenderness to palpation and no bony deformities.
Examination of the back revealed a positive straight leg raise on the right, but there was no midline tenderness to palpation or step-off deformity of the thoracic or lumbar spine. The patient exhibited mild left-sided upper lumbar paraspinal tenderness to palpation, but had no associated muscle spasm or overlying skin changes. On neurological assessment, the patient was alert and oriented with cranial nerves II-XII intact. He had 5/5 motor strength in all four extremities, with careful attention to hip flexion and extension, knee flexion and extension, and dorsiflexion and plantar flexion at the ankle. The sensory examination was normal, as were patella and ankle reflexes. The patient was able to ambulate with a steady gait.
Laboratory evaluation included a complete blood count, basic metabolic profile (BMP), urinalysis, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level. The urinalysis was negative for blood or signs of infection. The BMP demonstrated hyperglycemia without acidosis, but no additional electrolyte abnormalities or renal insufficiency. The patient did have leukocytosis (white blood cell [WBC], 19.8×109/L) with a left shift. The ESR was within normal limits (14 mm/h), but the CRP was mildly elevated (37.33 nmol/L).
Blood cultures were ordered, and the patient was given 2,500 mg vancomycin and 2,000 mg ceftriaxone IV. Given the patient’s abnormal back examination and the presence of a leukocytosis, a magnetic resonance imaging (MRI) study of the thoracic and lumbar spine was ordered. Radiology services reported the following findings from the MRI:
- Unremarkable thoracic spine MRI. No evidence of thoracic spine infection or significant degenerative changes.
- No evidence of infection involving the lumbar spine.
- L3-4 and L4-5 disc bulges and posterior element degenerative changes with moderate canal stenoses.
- Edema in the posterior paraspinous musculature on the left at the L3 and L4 levels.
The patient had DM with multiple systemic inflammatory response syndrome criteria and was admitted to the hospital for undifferentiated sepsis, clinical uncertainty, and pain control. Within 24 hours, blood cultures were positive for gram-positive cocci, later identified as methicillin-sensitive Staphylococcus aureus. However, despite treatment with antibiotics and analgesics, the patient’s back pain persisted. A repeat MRI of the lumbar spine obtained on hospital day 4 revealed the following:
At L3-4, since the comparison study, there has been development of two epidural abscesses with abnormal peripheral enhancement, one located dorsally measuring 6.8 x 8.1 x 14 mm and another located in the left lateral recess measuring 8.1 x 9.7 x 10.9 mm. The combination of the broad-based disk bulge, epidural abscesses, and hypertrophic facets resulted in severe spinal canal stenosis.
After receiving this report, the hospitalist contacted neurosurgery services. Shortly thereafter the patient underwent unilateral laminotomy with bilateral canal decompression on hospital day 5. He was discharged home on hospital day 10 without any neurological deficits, and continued IV antibiotics as an outpatient for an additional 5 weeks.
Discussion
Only a minority of patients with SEA present with the classic triad of back pain, fever, and progressive neurological findings associated with this condition.1Careful history-taking therefore is essential to identify high-risk patients. Risk factors for SEA include diabetes, IV drug abuse, immunosuppression, chronic renal failure, liver disease, alcoholism, indwelling catheter, recent invasive spinal procedure, recent vertebral fracture, cancer, and distant site of infection.1-3,5,7,11
Leukocytosis (WBC >10×109/L) is only found in two-thirds or less of patients with SEA at the time of admission.1,3,12 Inflammatory markers such as CRP and ESR are more sensitive but not specific to SEAs.1,2,5,7,11-13
An MRI study with gadolinium is the diagnostic modality of choice over computed tomography myelography to assess for SEAs due to its noninvasive nature and ability to better delineate the extent of disease.5,7,14 An MRI of the entire spine is recommended to delineate longitudinal and paraspinal extension as SEA can traverse multiple vertebral levels.15 While awaiting the results of blood cultures, patients should be treated with broad spectrum antibiotics that include coverage of the most common etiology of SEA, S aureus.1,3,4,7,11,13 While some cases of SEA may be managed medically, the emergency physician should always treat SEA as a neurosurgical emergency and obtain consultation with the appropriate services (eg, neurosurgery, infectious disease, neurology radiology).1,5
Our patient represents an unusual case of SEAs in that he presented with S aureus bacteremia while afebrile, along with back pain and tachycardia. He subsequently developed SEA, which was recognized only through serial MRI studies. The patient’s tachycardia alone could have been easily attributed to pain and anxiety associated with the ED environment. As such, he could have easily been discharged home with a prescription [for] nonsteroidal anti-inflammatory drugs and/or muscle relaxers for pain management—though it is likely that he would have returned to the ED 3 days later with persistent and even worsening symptoms, during which he would have undergone additional testing, possibly MRI, which would have revealed the missed SEA.
Our case clearly demonstrates that no SEA was present at the time of the
Studies show that half of all patients with SEAs are not diagnosed until after two or more visits to the ED.1,11 The literature posits that most cases are misdiagnosed at the time of initial evaluation. It has even been postulated that “misdiagnosis of spinal epidural abscess is the rule rather than the exception.”1 Although our patient was eventually diagnosed with a SEA, it was not present on the first MRI taken during the initial evaluation.
Summary
Unlike the rules of quantum mechanics and the paradox of Schrödinger’s cat, SEA follows a progression of disease.3,7,16 There is no superposition—the MRI is either positive or negative. However, excellent care requires the practitioner to know the risk factors of SEA, apply the appropriate screening tests, obtain MRI when necessary, and if diagnostic uncertainty remains, discuss with the patient or family signs and symptoms to monitor as well as reasons to return for re-evaluation.
1. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291.
2. Bhise V, Meyer A, Singh H, et al. errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi:10.1016/j.amjmed.2017.03.009.
3. Darouiche RO, Hamill RJ, Greenberg SB, Weathers SW, Musher DM. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore). 1992;71(6):369-385.
4. Baker AS, Ojemann RG, Swartz MN, Richardson EP Jr. Spinal epidural abscess. N Engl J Med. 1975;293(10):463-468. doi:10.1056/NEJM197509042931001.
5. Nussbaum ES, Rigamonti D, Standiford H, Numaguchi Y, Wolf AL, Robinson WL. Spinal epidural abscess: a report of 40 cases and review. Surg Neurol. 1992;38(3):225-231.
6. Vakili M, Crum-Cianflone NF. Spinal epidural abscess: a series of 101 cases. Am J Med. 2017;130(12):1458-1463. doi:10.1016/j.amjmed.2017.07.017.
7. Rigamonti D, Liem L, Sampath P, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52(2):189-196; discussion 197.
8. The World Health Organization. Antimicrobial resistance: global report on surveillance. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1. Accessed June 12, 2018.
9. Schrödinger E. Die gegenwärtige situation in der quantenmechanik. Maturwissenschaften. 1935;23(48):807-812; 823-828; 844-849. doi:10.1007/BF01491891.
10. Heisenberg H. Über quantentheoretische umdeutung kinematischer und mechanischer beziehungen. Zeitschrift für Physik. 1925;33(1):879-893. doi:10.1007/BF01328377.
11. Tang HJ, Lin HJ, Liu YC, Li CM. Spinal epidural abscess—experience with 46 patients and evaluation of prognostic factors. J Infect. 2002;45(2):76-81.
12. Soehle M, Wallenfang T. Spinal epidural abscesses: clinical manifestations, prognostic factors, and outcomes. Neurosurgery. 2002;51(1):79-85; discussion 86-87.
13. Del Curling O Jr, Gower DJ, McWhorter JM. Changing concepts in spinal epidural abscess: a report of 29 cases. Neurosurgery. 1990;27(2):185-192.
14. Hlavin ML, Kaminski HJ, Ross JS, Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurgery. 1990;27(2):177-184.
15. Parkinson JF, Sekhon LH. Spinal epidural abscess: appearance on magnetic resonance imaging as a guide to surgical management. Report of five cases. Neurosurg Focus. 2004;17(6):E12.
16. Heusner AP. Nontuberculous spinal epidural infections. N Engl J Med. 1948;239(23):845-854. doi:10.1056/NEJM194812022392301.
A 55-year-old man presented for evaluation of a 2-day history of worsening left lower back pain.
A 55-year-old man presented for evaluation of a 2-day history of worsening left lower back pain.
Delay in the diagnosis and treatment of spinal epidural abscess (SEA) increases the likelihood of permanent disability (eg, residual motor weakness) or even death.1-5 Studies suggest that the incidence of SEA may be on the rise,6,7 which is especially troubling in an era of emerging antibiotic resistance.8 The pervasive theme among the medical literature stresses the challenges with early recognition; however, missed SEA is a theoretical mishap in a manner akin to Schrödinger’s cat or Heisenberg’s uncertainty principle.9,10
One will recall Erwin Schrödinger’s thought experiment of 1935 when he challenged the theory of quantum mechanics by asking whether or not the cat in a box is still alive if there is a 50/50 chance poisonous gas has been released. He suggested that before one looks in the box, the cat is both alive and dead—a state of superposition.
Unfortunately, medical diagnoses do not exist in dual states. Tests are either positive or negative; disease is either present or absent; and in medicine, the cat is either alive or dead. Moreover, when SEA is diagnosed after the initial presentation and workup, (ie, the “bounce-back”), the clinician cannot categorically assume the condition was present, but missed, at the initial evaluation. We present the following case, not as a miraculous catch, or a “zebra-hunting guide” but rather as a rare glimpse into the evolution of a disease process.
Case
A 55-year-old man with history of type 2 diabetes mellitus (DM), hypertension, and hyperlipidemia presented to the ED with a 2-day history of progressively worsening left lower back pain. Although the patient denied a recent history of trauma, he did state that he helped one of his friends move furniture 1 day prior to presentation and had attributed the worsening pain to this event. The patient described his pain as mild and dull when he was at rest, rating it as a 2 on a pain scale of 1 to 10; and sharp-feeling and at its worst upon movement, rating it as a 9 on a pain scale of 1 to 10. The patient noted experiencing only mild relief when he shifted to certain positions.
The patient’s pain was nonradiating and associated with dull pain in the left anterior proximal thigh. The patient denied any numbness or weakness in any of his extremities. He also denied any perineal numbness or urinary or bowel incontinence; however, he did note experiencing a sense of incomplete evacuation of stools over the past 5 mornings.
The patient denied any recent history of fever, chills, numbness, weakness, difficulty with balance, direct trauma, instrumentation or chiropractic manipulation, or unexplained or unintentional weight loss. He had no history of malignancy and vehemently denied intravenous (IV) drug use.
On physical examination, the patient’s vital signs were: blood pressure, 143/93 mm Hg; heart rate, 108 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.6°F. Oxygen saturation was 95% on room air. Upon examination, the patient was in no acute distress and was resting comfortably and quietly. Pertinent findings included a supple neck examination, without lymphadenopathy or meningismus. There was no midline tenderness to palpation of the cervical spine and no step-off deformities. The lungs were clear to auscultation bilaterally and without wheezing, rhonchi, or rales. Examination of the heart revealed a regular rhythm with borderline tachycardia, but without murmurs, rubs, or gallops. The patient had 2+ pulses in all four extremities, and capillary refill was less than 2 seconds. The abdomen was soft and nontender, without rebound, guarding, or rigidity. There were no pulsatile abdominal masses or bruits, and bowel sounds were present. The patient had no costovertebral angle tenderness on percussion, and had full range of motion of all four extremities, with no tenderness to palpation and no bony deformities.
Examination of the back revealed a positive straight leg raise on the right, but there was no midline tenderness to palpation or step-off deformity of the thoracic or lumbar spine. The patient exhibited mild left-sided upper lumbar paraspinal tenderness to palpation, but had no associated muscle spasm or overlying skin changes. On neurological assessment, the patient was alert and oriented with cranial nerves II-XII intact. He had 5/5 motor strength in all four extremities, with careful attention to hip flexion and extension, knee flexion and extension, and dorsiflexion and plantar flexion at the ankle. The sensory examination was normal, as were patella and ankle reflexes. The patient was able to ambulate with a steady gait.
Laboratory evaluation included a complete blood count, basic metabolic profile (BMP), urinalysis, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level. The urinalysis was negative for blood or signs of infection. The BMP demonstrated hyperglycemia without acidosis, but no additional electrolyte abnormalities or renal insufficiency. The patient did have leukocytosis (white blood cell [WBC], 19.8×109/L) with a left shift. The ESR was within normal limits (14 mm/h), but the CRP was mildly elevated (37.33 nmol/L).
Blood cultures were ordered, and the patient was given 2,500 mg vancomycin and 2,000 mg ceftriaxone IV. Given the patient’s abnormal back examination and the presence of a leukocytosis, a magnetic resonance imaging (MRI) study of the thoracic and lumbar spine was ordered. Radiology services reported the following findings from the MRI:
- Unremarkable thoracic spine MRI. No evidence of thoracic spine infection or significant degenerative changes.
- No evidence of infection involving the lumbar spine.
- L3-4 and L4-5 disc bulges and posterior element degenerative changes with moderate canal stenoses.
- Edema in the posterior paraspinous musculature on the left at the L3 and L4 levels.
The patient had DM with multiple systemic inflammatory response syndrome criteria and was admitted to the hospital for undifferentiated sepsis, clinical uncertainty, and pain control. Within 24 hours, blood cultures were positive for gram-positive cocci, later identified as methicillin-sensitive Staphylococcus aureus. However, despite treatment with antibiotics and analgesics, the patient’s back pain persisted. A repeat MRI of the lumbar spine obtained on hospital day 4 revealed the following:
At L3-4, since the comparison study, there has been development of two epidural abscesses with abnormal peripheral enhancement, one located dorsally measuring 6.8 x 8.1 x 14 mm and another located in the left lateral recess measuring 8.1 x 9.7 x 10.9 mm. The combination of the broad-based disk bulge, epidural abscesses, and hypertrophic facets resulted in severe spinal canal stenosis.
After receiving this report, the hospitalist contacted neurosurgery services. Shortly thereafter the patient underwent unilateral laminotomy with bilateral canal decompression on hospital day 5. He was discharged home on hospital day 10 without any neurological deficits, and continued IV antibiotics as an outpatient for an additional 5 weeks.
Discussion
Only a minority of patients with SEA present with the classic triad of back pain, fever, and progressive neurological findings associated with this condition.1Careful history-taking therefore is essential to identify high-risk patients. Risk factors for SEA include diabetes, IV drug abuse, immunosuppression, chronic renal failure, liver disease, alcoholism, indwelling catheter, recent invasive spinal procedure, recent vertebral fracture, cancer, and distant site of infection.1-3,5,7,11
Leukocytosis (WBC >10×109/L) is only found in two-thirds or less of patients with SEA at the time of admission.1,3,12 Inflammatory markers such as CRP and ESR are more sensitive but not specific to SEAs.1,2,5,7,11-13
An MRI study with gadolinium is the diagnostic modality of choice over computed tomography myelography to assess for SEAs due to its noninvasive nature and ability to better delineate the extent of disease.5,7,14 An MRI of the entire spine is recommended to delineate longitudinal and paraspinal extension as SEA can traverse multiple vertebral levels.15 While awaiting the results of blood cultures, patients should be treated with broad spectrum antibiotics that include coverage of the most common etiology of SEA, S aureus.1,3,4,7,11,13 While some cases of SEA may be managed medically, the emergency physician should always treat SEA as a neurosurgical emergency and obtain consultation with the appropriate services (eg, neurosurgery, infectious disease, neurology radiology).1,5
Our patient represents an unusual case of SEAs in that he presented with S aureus bacteremia while afebrile, along with back pain and tachycardia. He subsequently developed SEA, which was recognized only through serial MRI studies. The patient’s tachycardia alone could have been easily attributed to pain and anxiety associated with the ED environment. As such, he could have easily been discharged home with a prescription [for] nonsteroidal anti-inflammatory drugs and/or muscle relaxers for pain management—though it is likely that he would have returned to the ED 3 days later with persistent and even worsening symptoms, during which he would have undergone additional testing, possibly MRI, which would have revealed the missed SEA.
Our case clearly demonstrates that no SEA was present at the time of the
Studies show that half of all patients with SEAs are not diagnosed until after two or more visits to the ED.1,11 The literature posits that most cases are misdiagnosed at the time of initial evaluation. It has even been postulated that “misdiagnosis of spinal epidural abscess is the rule rather than the exception.”1 Although our patient was eventually diagnosed with a SEA, it was not present on the first MRI taken during the initial evaluation.
Summary
Unlike the rules of quantum mechanics and the paradox of Schrödinger’s cat, SEA follows a progression of disease.3,7,16 There is no superposition—the MRI is either positive or negative. However, excellent care requires the practitioner to know the risk factors of SEA, apply the appropriate screening tests, obtain MRI when necessary, and if diagnostic uncertainty remains, discuss with the patient or family signs and symptoms to monitor as well as reasons to return for re-evaluation.
Delay in the diagnosis and treatment of spinal epidural abscess (SEA) increases the likelihood of permanent disability (eg, residual motor weakness) or even death.1-5 Studies suggest that the incidence of SEA may be on the rise,6,7 which is especially troubling in an era of emerging antibiotic resistance.8 The pervasive theme among the medical literature stresses the challenges with early recognition; however, missed SEA is a theoretical mishap in a manner akin to Schrödinger’s cat or Heisenberg’s uncertainty principle.9,10
One will recall Erwin Schrödinger’s thought experiment of 1935 when he challenged the theory of quantum mechanics by asking whether or not the cat in a box is still alive if there is a 50/50 chance poisonous gas has been released. He suggested that before one looks in the box, the cat is both alive and dead—a state of superposition.
Unfortunately, medical diagnoses do not exist in dual states. Tests are either positive or negative; disease is either present or absent; and in medicine, the cat is either alive or dead. Moreover, when SEA is diagnosed after the initial presentation and workup, (ie, the “bounce-back”), the clinician cannot categorically assume the condition was present, but missed, at the initial evaluation. We present the following case, not as a miraculous catch, or a “zebra-hunting guide” but rather as a rare glimpse into the evolution of a disease process.
Case
A 55-year-old man with history of type 2 diabetes mellitus (DM), hypertension, and hyperlipidemia presented to the ED with a 2-day history of progressively worsening left lower back pain. Although the patient denied a recent history of trauma, he did state that he helped one of his friends move furniture 1 day prior to presentation and had attributed the worsening pain to this event. The patient described his pain as mild and dull when he was at rest, rating it as a 2 on a pain scale of 1 to 10; and sharp-feeling and at its worst upon movement, rating it as a 9 on a pain scale of 1 to 10. The patient noted experiencing only mild relief when he shifted to certain positions.
The patient’s pain was nonradiating and associated with dull pain in the left anterior proximal thigh. The patient denied any numbness or weakness in any of his extremities. He also denied any perineal numbness or urinary or bowel incontinence; however, he did note experiencing a sense of incomplete evacuation of stools over the past 5 mornings.
The patient denied any recent history of fever, chills, numbness, weakness, difficulty with balance, direct trauma, instrumentation or chiropractic manipulation, or unexplained or unintentional weight loss. He had no history of malignancy and vehemently denied intravenous (IV) drug use.
On physical examination, the patient’s vital signs were: blood pressure, 143/93 mm Hg; heart rate, 108 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.6°F. Oxygen saturation was 95% on room air. Upon examination, the patient was in no acute distress and was resting comfortably and quietly. Pertinent findings included a supple neck examination, without lymphadenopathy or meningismus. There was no midline tenderness to palpation of the cervical spine and no step-off deformities. The lungs were clear to auscultation bilaterally and without wheezing, rhonchi, or rales. Examination of the heart revealed a regular rhythm with borderline tachycardia, but without murmurs, rubs, or gallops. The patient had 2+ pulses in all four extremities, and capillary refill was less than 2 seconds. The abdomen was soft and nontender, without rebound, guarding, or rigidity. There were no pulsatile abdominal masses or bruits, and bowel sounds were present. The patient had no costovertebral angle tenderness on percussion, and had full range of motion of all four extremities, with no tenderness to palpation and no bony deformities.
Examination of the back revealed a positive straight leg raise on the right, but there was no midline tenderness to palpation or step-off deformity of the thoracic or lumbar spine. The patient exhibited mild left-sided upper lumbar paraspinal tenderness to palpation, but had no associated muscle spasm or overlying skin changes. On neurological assessment, the patient was alert and oriented with cranial nerves II-XII intact. He had 5/5 motor strength in all four extremities, with careful attention to hip flexion and extension, knee flexion and extension, and dorsiflexion and plantar flexion at the ankle. The sensory examination was normal, as were patella and ankle reflexes. The patient was able to ambulate with a steady gait.
Laboratory evaluation included a complete blood count, basic metabolic profile (BMP), urinalysis, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level. The urinalysis was negative for blood or signs of infection. The BMP demonstrated hyperglycemia without acidosis, but no additional electrolyte abnormalities or renal insufficiency. The patient did have leukocytosis (white blood cell [WBC], 19.8×109/L) with a left shift. The ESR was within normal limits (14 mm/h), but the CRP was mildly elevated (37.33 nmol/L).
Blood cultures were ordered, and the patient was given 2,500 mg vancomycin and 2,000 mg ceftriaxone IV. Given the patient’s abnormal back examination and the presence of a leukocytosis, a magnetic resonance imaging (MRI) study of the thoracic and lumbar spine was ordered. Radiology services reported the following findings from the MRI:
- Unremarkable thoracic spine MRI. No evidence of thoracic spine infection or significant degenerative changes.
- No evidence of infection involving the lumbar spine.
- L3-4 and L4-5 disc bulges and posterior element degenerative changes with moderate canal stenoses.
- Edema in the posterior paraspinous musculature on the left at the L3 and L4 levels.
The patient had DM with multiple systemic inflammatory response syndrome criteria and was admitted to the hospital for undifferentiated sepsis, clinical uncertainty, and pain control. Within 24 hours, blood cultures were positive for gram-positive cocci, later identified as methicillin-sensitive Staphylococcus aureus. However, despite treatment with antibiotics and analgesics, the patient’s back pain persisted. A repeat MRI of the lumbar spine obtained on hospital day 4 revealed the following:
At L3-4, since the comparison study, there has been development of two epidural abscesses with abnormal peripheral enhancement, one located dorsally measuring 6.8 x 8.1 x 14 mm and another located in the left lateral recess measuring 8.1 x 9.7 x 10.9 mm. The combination of the broad-based disk bulge, epidural abscesses, and hypertrophic facets resulted in severe spinal canal stenosis.
After receiving this report, the hospitalist contacted neurosurgery services. Shortly thereafter the patient underwent unilateral laminotomy with bilateral canal decompression on hospital day 5. He was discharged home on hospital day 10 without any neurological deficits, and continued IV antibiotics as an outpatient for an additional 5 weeks.
Discussion
Only a minority of patients with SEA present with the classic triad of back pain, fever, and progressive neurological findings associated with this condition.1Careful history-taking therefore is essential to identify high-risk patients. Risk factors for SEA include diabetes, IV drug abuse, immunosuppression, chronic renal failure, liver disease, alcoholism, indwelling catheter, recent invasive spinal procedure, recent vertebral fracture, cancer, and distant site of infection.1-3,5,7,11
Leukocytosis (WBC >10×109/L) is only found in two-thirds or less of patients with SEA at the time of admission.1,3,12 Inflammatory markers such as CRP and ESR are more sensitive but not specific to SEAs.1,2,5,7,11-13
An MRI study with gadolinium is the diagnostic modality of choice over computed tomography myelography to assess for SEAs due to its noninvasive nature and ability to better delineate the extent of disease.5,7,14 An MRI of the entire spine is recommended to delineate longitudinal and paraspinal extension as SEA can traverse multiple vertebral levels.15 While awaiting the results of blood cultures, patients should be treated with broad spectrum antibiotics that include coverage of the most common etiology of SEA, S aureus.1,3,4,7,11,13 While some cases of SEA may be managed medically, the emergency physician should always treat SEA as a neurosurgical emergency and obtain consultation with the appropriate services (eg, neurosurgery, infectious disease, neurology radiology).1,5
Our patient represents an unusual case of SEAs in that he presented with S aureus bacteremia while afebrile, along with back pain and tachycardia. He subsequently developed SEA, which was recognized only through serial MRI studies. The patient’s tachycardia alone could have been easily attributed to pain and anxiety associated with the ED environment. As such, he could have easily been discharged home with a prescription [for] nonsteroidal anti-inflammatory drugs and/or muscle relaxers for pain management—though it is likely that he would have returned to the ED 3 days later with persistent and even worsening symptoms, during which he would have undergone additional testing, possibly MRI, which would have revealed the missed SEA.
Our case clearly demonstrates that no SEA was present at the time of the
Studies show that half of all patients with SEAs are not diagnosed until after two or more visits to the ED.1,11 The literature posits that most cases are misdiagnosed at the time of initial evaluation. It has even been postulated that “misdiagnosis of spinal epidural abscess is the rule rather than the exception.”1 Although our patient was eventually diagnosed with a SEA, it was not present on the first MRI taken during the initial evaluation.
Summary
Unlike the rules of quantum mechanics and the paradox of Schrödinger’s cat, SEA follows a progression of disease.3,7,16 There is no superposition—the MRI is either positive or negative. However, excellent care requires the practitioner to know the risk factors of SEA, apply the appropriate screening tests, obtain MRI when necessary, and if diagnostic uncertainty remains, discuss with the patient or family signs and symptoms to monitor as well as reasons to return for re-evaluation.
1. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291.
2. Bhise V, Meyer A, Singh H, et al. errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi:10.1016/j.amjmed.2017.03.009.
3. Darouiche RO, Hamill RJ, Greenberg SB, Weathers SW, Musher DM. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore). 1992;71(6):369-385.
4. Baker AS, Ojemann RG, Swartz MN, Richardson EP Jr. Spinal epidural abscess. N Engl J Med. 1975;293(10):463-468. doi:10.1056/NEJM197509042931001.
5. Nussbaum ES, Rigamonti D, Standiford H, Numaguchi Y, Wolf AL, Robinson WL. Spinal epidural abscess: a report of 40 cases and review. Surg Neurol. 1992;38(3):225-231.
6. Vakili M, Crum-Cianflone NF. Spinal epidural abscess: a series of 101 cases. Am J Med. 2017;130(12):1458-1463. doi:10.1016/j.amjmed.2017.07.017.
7. Rigamonti D, Liem L, Sampath P, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52(2):189-196; discussion 197.
8. The World Health Organization. Antimicrobial resistance: global report on surveillance. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1. Accessed June 12, 2018.
9. Schrödinger E. Die gegenwärtige situation in der quantenmechanik. Maturwissenschaften. 1935;23(48):807-812; 823-828; 844-849. doi:10.1007/BF01491891.
10. Heisenberg H. Über quantentheoretische umdeutung kinematischer und mechanischer beziehungen. Zeitschrift für Physik. 1925;33(1):879-893. doi:10.1007/BF01328377.
11. Tang HJ, Lin HJ, Liu YC, Li CM. Spinal epidural abscess—experience with 46 patients and evaluation of prognostic factors. J Infect. 2002;45(2):76-81.
12. Soehle M, Wallenfang T. Spinal epidural abscesses: clinical manifestations, prognostic factors, and outcomes. Neurosurgery. 2002;51(1):79-85; discussion 86-87.
13. Del Curling O Jr, Gower DJ, McWhorter JM. Changing concepts in spinal epidural abscess: a report of 29 cases. Neurosurgery. 1990;27(2):185-192.
14. Hlavin ML, Kaminski HJ, Ross JS, Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurgery. 1990;27(2):177-184.
15. Parkinson JF, Sekhon LH. Spinal epidural abscess: appearance on magnetic resonance imaging as a guide to surgical management. Report of five cases. Neurosurg Focus. 2004;17(6):E12.
16. Heusner AP. Nontuberculous spinal epidural infections. N Engl J Med. 1948;239(23):845-854. doi:10.1056/NEJM194812022392301.
1. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291.
2. Bhise V, Meyer A, Singh H, et al. errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi:10.1016/j.amjmed.2017.03.009.
3. Darouiche RO, Hamill RJ, Greenberg SB, Weathers SW, Musher DM. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore). 1992;71(6):369-385.
4. Baker AS, Ojemann RG, Swartz MN, Richardson EP Jr. Spinal epidural abscess. N Engl J Med. 1975;293(10):463-468. doi:10.1056/NEJM197509042931001.
5. Nussbaum ES, Rigamonti D, Standiford H, Numaguchi Y, Wolf AL, Robinson WL. Spinal epidural abscess: a report of 40 cases and review. Surg Neurol. 1992;38(3):225-231.
6. Vakili M, Crum-Cianflone NF. Spinal epidural abscess: a series of 101 cases. Am J Med. 2017;130(12):1458-1463. doi:10.1016/j.amjmed.2017.07.017.
7. Rigamonti D, Liem L, Sampath P, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52(2):189-196; discussion 197.
8. The World Health Organization. Antimicrobial resistance: global report on surveillance. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1. Accessed June 12, 2018.
9. Schrödinger E. Die gegenwärtige situation in der quantenmechanik. Maturwissenschaften. 1935;23(48):807-812; 823-828; 844-849. doi:10.1007/BF01491891.
10. Heisenberg H. Über quantentheoretische umdeutung kinematischer und mechanischer beziehungen. Zeitschrift für Physik. 1925;33(1):879-893. doi:10.1007/BF01328377.
11. Tang HJ, Lin HJ, Liu YC, Li CM. Spinal epidural abscess—experience with 46 patients and evaluation of prognostic factors. J Infect. 2002;45(2):76-81.
12. Soehle M, Wallenfang T. Spinal epidural abscesses: clinical manifestations, prognostic factors, and outcomes. Neurosurgery. 2002;51(1):79-85; discussion 86-87.
13. Del Curling O Jr, Gower DJ, McWhorter JM. Changing concepts in spinal epidural abscess: a report of 29 cases. Neurosurgery. 1990;27(2):185-192.
14. Hlavin ML, Kaminski HJ, Ross JS, Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurgery. 1990;27(2):177-184.
15. Parkinson JF, Sekhon LH. Spinal epidural abscess: appearance on magnetic resonance imaging as a guide to surgical management. Report of five cases. Neurosurg Focus. 2004;17(6):E12.
16. Heusner AP. Nontuberculous spinal epidural infections. N Engl J Med. 1948;239(23):845-854. doi:10.1056/NEJM194812022392301.
Antegrade Femoral Nail Distal Interlocking Screw Causing Rupture of the Medial Patellofemoral Ligament and Patellar Instability
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.
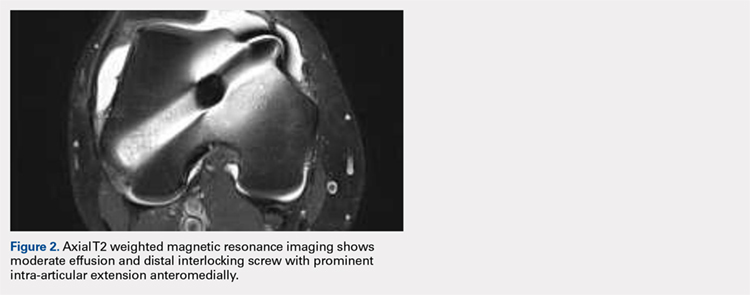
Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).
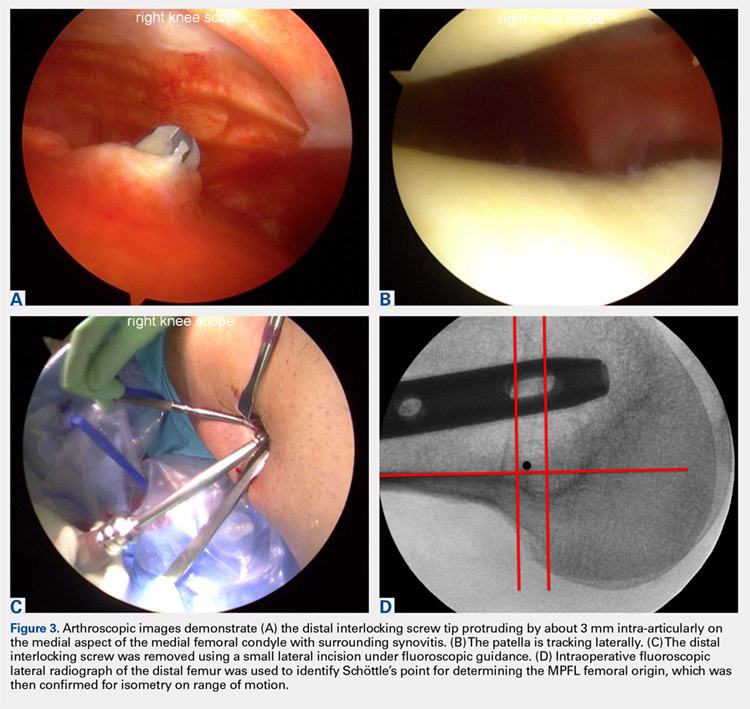
Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).
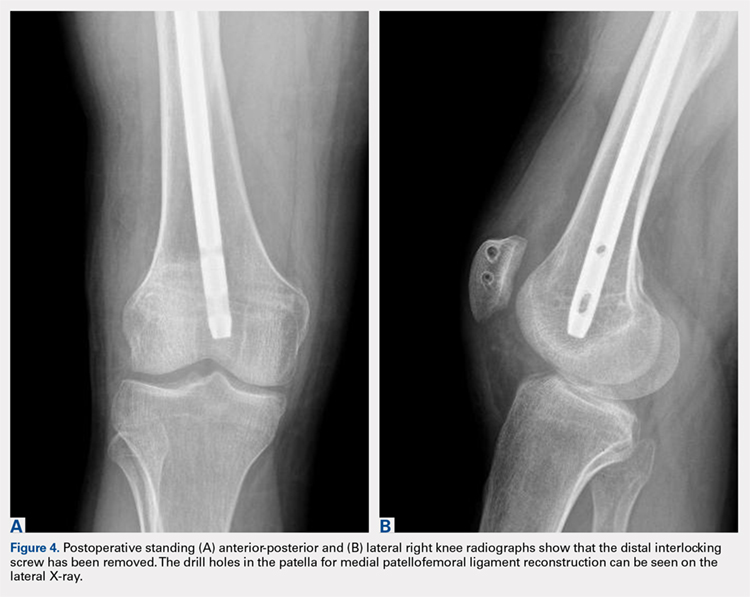
DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.


Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).

Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).

DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.


Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).

Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).

DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
TAKE-HOME POINTS
- Anterograde intramedullary nailing is the gold standard for fixation of diaphyseal femur fractures.
- Damage to the MPFL can be caused by the distal interlocking screw of an anterograde intramedullary nail.
- The trajectory of the distal interlocking screw from posterolateral to anteromedial, and a prominent screw tip, likely contributed to the injury to the MPFL observed in this case.
- Surgeons treating these conditions should pursue advanced imaging if patients present with effusion and patellar instability after femoral intramedullary nail placement.
- Distal interlocking screw removal and arthroscopic MPFL reconstruction can result in successful return of function and normal activities.
Scalp Psoriasis With Increased Hair Density
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Practice Points
- Scalp psoriasis may present with hair loss or increased hair density.
- Psoriasis with increased hair density may make topical medications more difficult to apply.
Reflectance Confocal Microscopy as a First-Line Diagnostic Technique for Mycosis Fungoides
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.
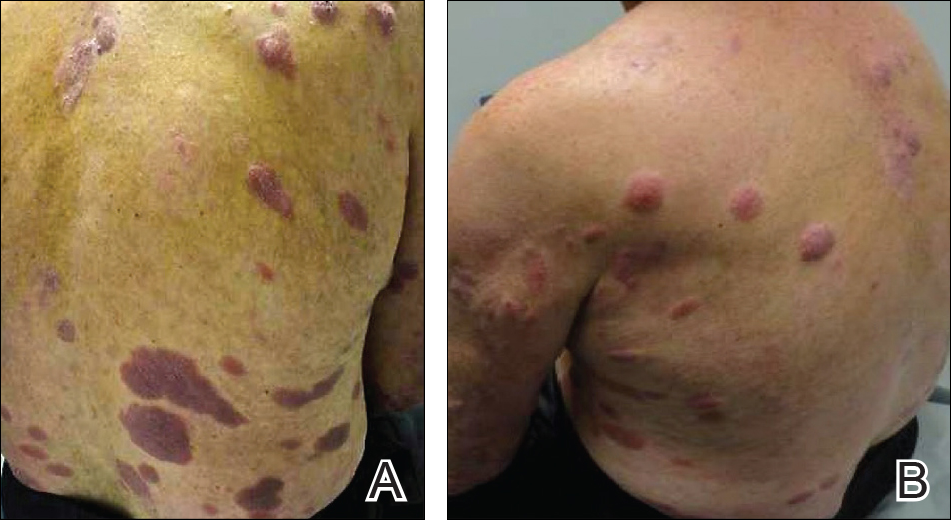
A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.


A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.


A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
Practice Points
- Mycosis fungoides (MF) can be a challenging diagnosis to establish and often requires multiple biopsies.
- Reflectance confocal microscopy (RCM) may be helpful as a bedside noninvasive diagnostic technique.
- In suspected MF cases, RCM may assist in selecting the optimal biopsy site for better yield of histopathologic results.
Nonscarring Alopecia Associated With Vitamin D Deficiency
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.
On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.
Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.

On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.

Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.

On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.

Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
Practice Points
- The evaluation of vitamin D levels is important in the management of nonscarring alopecia.
- Vitamin D deficiency can present as nonscarring alopecia not associated with alopecia areata, androgenetic alopecia, or telogen effluvium.
Vertebral Artery Dissection in Active-Duty Soldier Due to Mixed Martial Arts Choke Hold
Knowledge of the potential dangers of mixed martial arts is valuable for Department of Defense (DoD) health care providers as the military continues to implement combatives training into regular military instruction. This case study presents an active-duty service member who developed a spontaneous vertebral artery dissection (sVAD) during mixed martial arts training, which led to a cerebellar stroke.
To the authors’ knowledge this is the first documented case of a sVAD with associated stroke related to a mixed martial arts choke hold. Understanding the diagnosis, management, and prognosis of this condition will remain important as hand-to-hand combat instruction continues to be a part of regular military training.
Case Presentation
A 39-year-old active-duty male without significant past medical history presented to the emergency department (ED) at the San Antonio Military Medical Center in Texas for evaluation of severe vertigo with associated nausea and vomiting. He had participated in a Jiu-Jitsu match the evening prior to his presentation and reported that he was placed in a choke hold within the last 12 seconds of the match. He denied losing consciousness during this hold.
Once released, he attempted to stand and developed sudden onset vertigo with severe nausea, leading to multiple bouts of emesis. He additionally developed a throbbing, left-sided headache radiating down the left side of his neck. While the vertigo resolved within an hour, he continued to experience bouts of nausea and emesis, prompting him to present to the ED for further evaluation. The patient’s past medical history was remarkable only for multiple prior concussions, and his only medication was occasional ibuprofen. He denied the usage of recreational drugs.
Upon presentation to the ED, the patient’s vital signs were 139/93 mm Hg blood pressure, 73 beats per minute heart rate, 16 breaths per minute respiration, 100% oxygen saturation on room air, and 97.7° F temperature.
The patient demonstrated normal balance and exhibited no nystagmus or limb/truncal ataxia as evaluated with finger-to-nose/heel-to-shin testing and gait exam. Complete blood count, comprehensive metabolic panel, and coagulation panel all demonstrated no abnormalities.
The patient was admitted to the hospital for symptom control and further monitoring. His headache and nausea were managed with medications, and he began antiplatelet therapy with aspirin 325 mg daily. Given the size of his cerebellar infarction, it was decided that he would be monitored in the hospital for 72 hours for the development of significant cerebellar edema. He remained stable throughout his hospitalization and had only a mild headache at the time of discharge.
The patient was last seen 3 months postinjury with no subjective complaints and a completely normal neurologic exam. The treatment plan for the patient is to continue aspirin for 6 months postinjury at which time a repeat CT will be performed to ensure resolution. He has been counseled to avoid heavy lifting and any activity with potential for sudden movement/force of the neck (grappling/wrestling, chiropractic manipulation, roller coasters, or sit-ups) until the repeat CT has been completed.
Discussion
Spontaneous vertebral artery and carotid artery dissections are collectively referred to as sCADs. Spontaneous cervical artery dissections are a rare condition with a higher incidence of internal carotid dissections than are VADs (1.72 vs 0.97 per 100,000 people).1 In contrast to the general stroke population, patients with sCADs are typically younger (mean age 45.3 years); and more than half of the patients are male.1,2
Spontaneous cervical artery dissections are typically characterized by subintimal tears of the vertebral artery leading to the accumulation of an intramural hematoma and creation of a “false lumen” in the arterial wall.3 A sVAD is more often found in the pars transversaria (V2; 35%) or atlas loop (V3; 34%) segments of the vertebral artery than in the prevertebral (V1; 20%) or intracranial (V4; 11%) segments.3-5 The etiology of these injuries is thought to be minor trauma to the neck in the context of a likely underlying connective tissue disease, though no direct association with a particular disease has been shown.
Biopsy evaluation of the superficial temporal arteries of patients with sCADs have revealed pathologic changes of the media and adventitial layers, including vacuolar degeneration and capillary neoangiogenesis, which are not found in the arteries of control patients.5 Although definitive association with a known connective tissue disease is rare, angiographic evidence of fibromuscular dysplasia, a nonspecific marker of connective tissue disease, is noted in as many as 15% to 20% of patients.6 Consequently, routine connective tissue disease screening is not recommended in these patients. One study found that about 40% of sCAD patients can recall minor cervical trauma in the preceding month in comparison to only 10% of other patients with stroke, leading to the moniker of “bottoms-up” or “beauty-parlor strokes” for these injuries. The most common mechanisms of minor neck trauma causing sCADs include tennis and golf swings, yoga, and roller-coaster rides.7,8
Usually symptomatic at presentation, the most frequently encountered sCAD symptoms are head or neck pain (80%), brain ischemia (56%), and Horner syndrome (25%).1 A study of 161 consecutive patients with internal carotid (n = 135) or vertebral artery (n = 26) dissections revealed that headache was reported by 69% of those with sVADs, and when present, was the initial manifestation in 33%. Headaches typically were ipsilateral to the dissection, located posteriorly in 83% of patients, and lasted an average duration of 72 hours. Neck pain, which was noted in 46% of sVAD patients, was predominantly posterior and ipsilateral in location as well.9 Ischemic symptoms of sVAD may include posterior circulation symptoms, such as vertigo, ataxia, diplopia, and leg weakness as well as lateral medullary (Wallenberg) syndrome characterized by dizziness, postural instability, limb hypotonia/ataxia, blurred vision, and nystagmus.
In a study of 169 patients with sCAD, brain ischemia occurred in 77% (131 patients) including 67% (n = 114) with ischemic stroke and 10% (n = 17) with transient ischemic attack. Head and/or neck pain was noted in 88% of those with brain ischemia.4 Etiologies for infarction included thromboembolic (85%), hemodynamic (12%), and mixed (3%).10 Isolated local symptoms are rare with one study of 245 patients with sCAD revealing only 20 (8%) presenting with pain only. Of those with pain only, 6 presented with headache, 2 with neck pain, and 12 with both.11
Diagnosis of sVAD requires a high index of suspicion and is confirmed by diagnostic testing. Previously, invasive angiography was the diagnostic gold standard, but with the improvement in quality of CT and MR angiography, these noninvasive modalities have become the tests of choice. There have been no studies to date revealing a definitive benefit of one modality over the other. A meta-analysis of 25 articles that compared the use of CT and MR angiography for the diagnosis of carotid and VAD revealed similar sensitivity and specificity.12 In contrast, a study involving 10 patients with confirmed sVAD who had both CT and MR angiographies during evaluation showed more total findings consistent with dissection on CT than with MR angiography when graded by 2 neuroradiologists. Additionally, the neuroradiologists subjectively rated CT angiography as preferential to MR in showing the imaging findings of dissection in 8 of 10 cases of vertebral dissection.13
Treatment for sCAD remains heavily debated. The use of IV thrombolysis within the standard time window for acute ischemic stroke is advocated for these patients. A meta-analysis of patients with sCAD vs matched patients with stroke from other causes treated with IV thrombolysis showed no difference in mortality at 3 months (9.0% vs 8.8%) or symptomatic intracranial hemorrhage (3.3% vs 3.0%). Additionally, similar percentages of patients had excellent (30.9% vs 37.4%) and favorable (58.2% vs 52.2%) 3-month functional statuses as expressed by the Modified Rankin Score (mRS).14,15
Debate remains regarding subacute therapy for sCAD with either antiplatelet or anticoagulant therapy. A randomized study of 250 patients with cervical artery dissection (118 carotid, 132 vertebral) in which 126 patients were assigned to antiplatelet therapy and 124 patients were assigned to anticoagulant therapy showed an overall low rate of recurrent stroke (2%). There was no significant difference in efficacy between the therapy groups with stroke or death occurring in 3 antiplatelet patients and 1 anticoagulated patient. Adverse effects were very low in both groups with no deaths and only 1 major bleed in the anticoagulation group. Of note, stroke rates were lower in this study than prior observational studies.16
A nonrandomized study of 88 patients with extracranial sCAD showed overall low rates of recurrent ischemic stroke at 3 months with 1/59 (1.7%) in the antiplatelet group and 1/28 (3.6%) in the anticoagulation group (P 17 Given this low overall rate of recurrent stroke in prior studies, a guideline recommendation for antiplatelet or anticoagulant therapy cannot be made at this time.
The overall prognosis for this condition is fair. Functional status and recurrence risk are favorable, with one study finding a mRS score of 1 Additionally, a historic cohort study of 432 patients with first event of sCAD revealed that after a mean follow-up of 31 months, only 4 (0.9%) patients had a recurrent ischemic stroke either due to incomplete recanalization of the artery (n = 2) or recurrent sCAD (n = 2), and only 4 (0.9%) total recurrences of sCAD were report (2 without associated ischemic strokes).18 Further, a prospective study of 61 patients with confirmed sVAD revealed complete recanalization of 45.9% at 3 months, 62.3% at 6 months, and 63.9% at 12 months, suggesting that recanalization occurs mostly during the initial 6 months. There was no identified association between outcome and complete recanalization with favorable outcomes observed in 55 (90.2%) of patients and no further ischemic symptoms during follow-up.19
Neck maneuvers have been cited as a more common cause of sCAD in several previous studies. One retrospective study found chiropractic neck manipulation to be the etiology in 12 of 141 patients with CT- or MR- confirmed sCAD.20 As noted previously, to the authors’ knowledge this is the first reported case of a sVAD occurring after a mixed martial arts choke hold. While sports-related strokes are rare, one evaluation of 70 published cases found that 80% were due to sCAD. Commonly associated sports in this study included football, yoga, wrestling, tennis, golf, and swimming.21 Grappling-related neck manipulation has been noted as an etiology in a few case reports.
Hyperextension of the neck was deemed to be the etiology in boys aged 11 years and 17 years who developed a sCAD while participating in Judo and backyard wrestling, respectively.22,23 In the martial arts realm, there is a case report of a 26-year-old male who developed a sVAD after rapid head turning during a solo Kung Fu maneuver as well as a report of a 41-year-old male experiencing a right VAD complicated by a posterior infarction several days after straining his neck during a mixed martial arts competition.24,25 The patient denied any choke hold or direct blow to the neck.
The present case is different in that it is the first reported case of a sVAD occurring after a submission maneuver. Prior grappling-related sVADs were associated with hyperextension or rapid acceleration/deceleration forces on the neck. Isometric force to the neck is a rarely described mechanism for development of this injury. Although there are isolated and infrequent forensic case reports of carotid dissection with strangulation injuries, the authors believe this is the first documented case of a sVAD attributed to a combatives submission.
In the context of the military health system, it is important to be aware of this potential complication of combatives as instruction in close-quarters combat continues to be an important part of military training.
1. Lee VH, Brown RD Jr, Mandrekar J, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809-1812.
2. Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67(6):1050-1052.
3. Schvienk W. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
4. Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
5. Völker W, Dittrich R, Grewe S, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. 2011;76(17):1463-1471.
6. Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40(6):459-466.
7. DeBehnke D, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12(1):27-31.
8. Engelter ST, Grond-Ginsbach C, Metso TM, et al; Cervical Artery Dissection and Ischemic Stroke Patients Study Group. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology. 2013;80(21):1950-1957.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
10. Morel A, Naggara O, Touzé E, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. 2012;43(5):1354-1361.
11. Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
12. Provenzale J, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;1939(4):1167-1174.
13. Vertinsky AT, Schwartz NE, Fishbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
14. Zinkstok SM, Vergouwen MD, Engelter ST, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. 2011;42(9):2515-2520.
15. Engelter S, Rutgers M, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. 2009;40(12):3772-3776.
16. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomized trial. Lancet Neurol. 2015;14(4):361-367.
17. Kennedy F, Lanfranconi S, Hicks C, et al; CADISS Investigators. Antiplatelets vs. anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology. 2012;79(7):686-689.
18. Touze E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL; Multicenter Survey on Natural History of Cervical Artery Dissection. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61(10):1347-1351.
19. Arauz A, Marquez J, Artigas C, Balderrama J, Orrego H. Recanalization of vertebral artery dissection. Stroke. 2010;41(4):717-721.
20. Kennell KA, Daghfal MM, Patel SG, et DeSanto JR, Waterman GS, Bertino RE. Cervical artery dissection related to chiropractic manipulation: one institution’s experience. J Fam Pract. 2017;66(9):556-562.
21. McCrory P. Vertebral artery dissection causing stroke in sport. J Clin Neurosci. 2000;7(4):298-300.
22. Lannuzel A, Moulin T, Amsallem D, Galmiche J, Rumbach L. Vertebral artery dissection following a judo session: a case report. Neuropediatrics. 1994;25(2):106-108.
23. Gupta V, Dhawan N, Bahl J. Minor trauma causing stroke in a young athlete. Case Rep Neurol Med. 2015;2015: 182875.
24. Pacei F, Valvasorri L, Bet L. Vertebral artery dissection during Kung-Fu training. Neurol Sci. 2014;35(2):331-332.
25. Slowey M, Maw G, Furyk J. Case report on vertebral artery dissection in mixed martial arts. Emerg Med Australas. 2012;24(2):203-206.
Knowledge of the potential dangers of mixed martial arts is valuable for Department of Defense (DoD) health care providers as the military continues to implement combatives training into regular military instruction. This case study presents an active-duty service member who developed a spontaneous vertebral artery dissection (sVAD) during mixed martial arts training, which led to a cerebellar stroke.
To the authors’ knowledge this is the first documented case of a sVAD with associated stroke related to a mixed martial arts choke hold. Understanding the diagnosis, management, and prognosis of this condition will remain important as hand-to-hand combat instruction continues to be a part of regular military training.
Case Presentation
A 39-year-old active-duty male without significant past medical history presented to the emergency department (ED) at the San Antonio Military Medical Center in Texas for evaluation of severe vertigo with associated nausea and vomiting. He had participated in a Jiu-Jitsu match the evening prior to his presentation and reported that he was placed in a choke hold within the last 12 seconds of the match. He denied losing consciousness during this hold.
Once released, he attempted to stand and developed sudden onset vertigo with severe nausea, leading to multiple bouts of emesis. He additionally developed a throbbing, left-sided headache radiating down the left side of his neck. While the vertigo resolved within an hour, he continued to experience bouts of nausea and emesis, prompting him to present to the ED for further evaluation. The patient’s past medical history was remarkable only for multiple prior concussions, and his only medication was occasional ibuprofen. He denied the usage of recreational drugs.
Upon presentation to the ED, the patient’s vital signs were 139/93 mm Hg blood pressure, 73 beats per minute heart rate, 16 breaths per minute respiration, 100% oxygen saturation on room air, and 97.7° F temperature.
The patient demonstrated normal balance and exhibited no nystagmus or limb/truncal ataxia as evaluated with finger-to-nose/heel-to-shin testing and gait exam. Complete blood count, comprehensive metabolic panel, and coagulation panel all demonstrated no abnormalities.
The patient was admitted to the hospital for symptom control and further monitoring. His headache and nausea were managed with medications, and he began antiplatelet therapy with aspirin 325 mg daily. Given the size of his cerebellar infarction, it was decided that he would be monitored in the hospital for 72 hours for the development of significant cerebellar edema. He remained stable throughout his hospitalization and had only a mild headache at the time of discharge.
The patient was last seen 3 months postinjury with no subjective complaints and a completely normal neurologic exam. The treatment plan for the patient is to continue aspirin for 6 months postinjury at which time a repeat CT will be performed to ensure resolution. He has been counseled to avoid heavy lifting and any activity with potential for sudden movement/force of the neck (grappling/wrestling, chiropractic manipulation, roller coasters, or sit-ups) until the repeat CT has been completed.
Discussion
Spontaneous vertebral artery and carotid artery dissections are collectively referred to as sCADs. Spontaneous cervical artery dissections are a rare condition with a higher incidence of internal carotid dissections than are VADs (1.72 vs 0.97 per 100,000 people).1 In contrast to the general stroke population, patients with sCADs are typically younger (mean age 45.3 years); and more than half of the patients are male.1,2
Spontaneous cervical artery dissections are typically characterized by subintimal tears of the vertebral artery leading to the accumulation of an intramural hematoma and creation of a “false lumen” in the arterial wall.3 A sVAD is more often found in the pars transversaria (V2; 35%) or atlas loop (V3; 34%) segments of the vertebral artery than in the prevertebral (V1; 20%) or intracranial (V4; 11%) segments.3-5 The etiology of these injuries is thought to be minor trauma to the neck in the context of a likely underlying connective tissue disease, though no direct association with a particular disease has been shown.
Biopsy evaluation of the superficial temporal arteries of patients with sCADs have revealed pathologic changes of the media and adventitial layers, including vacuolar degeneration and capillary neoangiogenesis, which are not found in the arteries of control patients.5 Although definitive association with a known connective tissue disease is rare, angiographic evidence of fibromuscular dysplasia, a nonspecific marker of connective tissue disease, is noted in as many as 15% to 20% of patients.6 Consequently, routine connective tissue disease screening is not recommended in these patients. One study found that about 40% of sCAD patients can recall minor cervical trauma in the preceding month in comparison to only 10% of other patients with stroke, leading to the moniker of “bottoms-up” or “beauty-parlor strokes” for these injuries. The most common mechanisms of minor neck trauma causing sCADs include tennis and golf swings, yoga, and roller-coaster rides.7,8
Usually symptomatic at presentation, the most frequently encountered sCAD symptoms are head or neck pain (80%), brain ischemia (56%), and Horner syndrome (25%).1 A study of 161 consecutive patients with internal carotid (n = 135) or vertebral artery (n = 26) dissections revealed that headache was reported by 69% of those with sVADs, and when present, was the initial manifestation in 33%. Headaches typically were ipsilateral to the dissection, located posteriorly in 83% of patients, and lasted an average duration of 72 hours. Neck pain, which was noted in 46% of sVAD patients, was predominantly posterior and ipsilateral in location as well.9 Ischemic symptoms of sVAD may include posterior circulation symptoms, such as vertigo, ataxia, diplopia, and leg weakness as well as lateral medullary (Wallenberg) syndrome characterized by dizziness, postural instability, limb hypotonia/ataxia, blurred vision, and nystagmus.
In a study of 169 patients with sCAD, brain ischemia occurred in 77% (131 patients) including 67% (n = 114) with ischemic stroke and 10% (n = 17) with transient ischemic attack. Head and/or neck pain was noted in 88% of those with brain ischemia.4 Etiologies for infarction included thromboembolic (85%), hemodynamic (12%), and mixed (3%).10 Isolated local symptoms are rare with one study of 245 patients with sCAD revealing only 20 (8%) presenting with pain only. Of those with pain only, 6 presented with headache, 2 with neck pain, and 12 with both.11
Diagnosis of sVAD requires a high index of suspicion and is confirmed by diagnostic testing. Previously, invasive angiography was the diagnostic gold standard, but with the improvement in quality of CT and MR angiography, these noninvasive modalities have become the tests of choice. There have been no studies to date revealing a definitive benefit of one modality over the other. A meta-analysis of 25 articles that compared the use of CT and MR angiography for the diagnosis of carotid and VAD revealed similar sensitivity and specificity.12 In contrast, a study involving 10 patients with confirmed sVAD who had both CT and MR angiographies during evaluation showed more total findings consistent with dissection on CT than with MR angiography when graded by 2 neuroradiologists. Additionally, the neuroradiologists subjectively rated CT angiography as preferential to MR in showing the imaging findings of dissection in 8 of 10 cases of vertebral dissection.13
Treatment for sCAD remains heavily debated. The use of IV thrombolysis within the standard time window for acute ischemic stroke is advocated for these patients. A meta-analysis of patients with sCAD vs matched patients with stroke from other causes treated with IV thrombolysis showed no difference in mortality at 3 months (9.0% vs 8.8%) or symptomatic intracranial hemorrhage (3.3% vs 3.0%). Additionally, similar percentages of patients had excellent (30.9% vs 37.4%) and favorable (58.2% vs 52.2%) 3-month functional statuses as expressed by the Modified Rankin Score (mRS).14,15
Debate remains regarding subacute therapy for sCAD with either antiplatelet or anticoagulant therapy. A randomized study of 250 patients with cervical artery dissection (118 carotid, 132 vertebral) in which 126 patients were assigned to antiplatelet therapy and 124 patients were assigned to anticoagulant therapy showed an overall low rate of recurrent stroke (2%). There was no significant difference in efficacy between the therapy groups with stroke or death occurring in 3 antiplatelet patients and 1 anticoagulated patient. Adverse effects were very low in both groups with no deaths and only 1 major bleed in the anticoagulation group. Of note, stroke rates were lower in this study than prior observational studies.16
A nonrandomized study of 88 patients with extracranial sCAD showed overall low rates of recurrent ischemic stroke at 3 months with 1/59 (1.7%) in the antiplatelet group and 1/28 (3.6%) in the anticoagulation group (P 17 Given this low overall rate of recurrent stroke in prior studies, a guideline recommendation for antiplatelet or anticoagulant therapy cannot be made at this time.
The overall prognosis for this condition is fair. Functional status and recurrence risk are favorable, with one study finding a mRS score of 1 Additionally, a historic cohort study of 432 patients with first event of sCAD revealed that after a mean follow-up of 31 months, only 4 (0.9%) patients had a recurrent ischemic stroke either due to incomplete recanalization of the artery (n = 2) or recurrent sCAD (n = 2), and only 4 (0.9%) total recurrences of sCAD were report (2 without associated ischemic strokes).18 Further, a prospective study of 61 patients with confirmed sVAD revealed complete recanalization of 45.9% at 3 months, 62.3% at 6 months, and 63.9% at 12 months, suggesting that recanalization occurs mostly during the initial 6 months. There was no identified association between outcome and complete recanalization with favorable outcomes observed in 55 (90.2%) of patients and no further ischemic symptoms during follow-up.19
Neck maneuvers have been cited as a more common cause of sCAD in several previous studies. One retrospective study found chiropractic neck manipulation to be the etiology in 12 of 141 patients with CT- or MR- confirmed sCAD.20 As noted previously, to the authors’ knowledge this is the first reported case of a sVAD occurring after a mixed martial arts choke hold. While sports-related strokes are rare, one evaluation of 70 published cases found that 80% were due to sCAD. Commonly associated sports in this study included football, yoga, wrestling, tennis, golf, and swimming.21 Grappling-related neck manipulation has been noted as an etiology in a few case reports.
Hyperextension of the neck was deemed to be the etiology in boys aged 11 years and 17 years who developed a sCAD while participating in Judo and backyard wrestling, respectively.22,23 In the martial arts realm, there is a case report of a 26-year-old male who developed a sVAD after rapid head turning during a solo Kung Fu maneuver as well as a report of a 41-year-old male experiencing a right VAD complicated by a posterior infarction several days after straining his neck during a mixed martial arts competition.24,25 The patient denied any choke hold or direct blow to the neck.
The present case is different in that it is the first reported case of a sVAD occurring after a submission maneuver. Prior grappling-related sVADs were associated with hyperextension or rapid acceleration/deceleration forces on the neck. Isometric force to the neck is a rarely described mechanism for development of this injury. Although there are isolated and infrequent forensic case reports of carotid dissection with strangulation injuries, the authors believe this is the first documented case of a sVAD attributed to a combatives submission.
In the context of the military health system, it is important to be aware of this potential complication of combatives as instruction in close-quarters combat continues to be an important part of military training.
Knowledge of the potential dangers of mixed martial arts is valuable for Department of Defense (DoD) health care providers as the military continues to implement combatives training into regular military instruction. This case study presents an active-duty service member who developed a spontaneous vertebral artery dissection (sVAD) during mixed martial arts training, which led to a cerebellar stroke.
To the authors’ knowledge this is the first documented case of a sVAD with associated stroke related to a mixed martial arts choke hold. Understanding the diagnosis, management, and prognosis of this condition will remain important as hand-to-hand combat instruction continues to be a part of regular military training.
Case Presentation
A 39-year-old active-duty male without significant past medical history presented to the emergency department (ED) at the San Antonio Military Medical Center in Texas for evaluation of severe vertigo with associated nausea and vomiting. He had participated in a Jiu-Jitsu match the evening prior to his presentation and reported that he was placed in a choke hold within the last 12 seconds of the match. He denied losing consciousness during this hold.
Once released, he attempted to stand and developed sudden onset vertigo with severe nausea, leading to multiple bouts of emesis. He additionally developed a throbbing, left-sided headache radiating down the left side of his neck. While the vertigo resolved within an hour, he continued to experience bouts of nausea and emesis, prompting him to present to the ED for further evaluation. The patient’s past medical history was remarkable only for multiple prior concussions, and his only medication was occasional ibuprofen. He denied the usage of recreational drugs.
Upon presentation to the ED, the patient’s vital signs were 139/93 mm Hg blood pressure, 73 beats per minute heart rate, 16 breaths per minute respiration, 100% oxygen saturation on room air, and 97.7° F temperature.
The patient demonstrated normal balance and exhibited no nystagmus or limb/truncal ataxia as evaluated with finger-to-nose/heel-to-shin testing and gait exam. Complete blood count, comprehensive metabolic panel, and coagulation panel all demonstrated no abnormalities.
The patient was admitted to the hospital for symptom control and further monitoring. His headache and nausea were managed with medications, and he began antiplatelet therapy with aspirin 325 mg daily. Given the size of his cerebellar infarction, it was decided that he would be monitored in the hospital for 72 hours for the development of significant cerebellar edema. He remained stable throughout his hospitalization and had only a mild headache at the time of discharge.
The patient was last seen 3 months postinjury with no subjective complaints and a completely normal neurologic exam. The treatment plan for the patient is to continue aspirin for 6 months postinjury at which time a repeat CT will be performed to ensure resolution. He has been counseled to avoid heavy lifting and any activity with potential for sudden movement/force of the neck (grappling/wrestling, chiropractic manipulation, roller coasters, or sit-ups) until the repeat CT has been completed.
Discussion
Spontaneous vertebral artery and carotid artery dissections are collectively referred to as sCADs. Spontaneous cervical artery dissections are a rare condition with a higher incidence of internal carotid dissections than are VADs (1.72 vs 0.97 per 100,000 people).1 In contrast to the general stroke population, patients with sCADs are typically younger (mean age 45.3 years); and more than half of the patients are male.1,2
Spontaneous cervical artery dissections are typically characterized by subintimal tears of the vertebral artery leading to the accumulation of an intramural hematoma and creation of a “false lumen” in the arterial wall.3 A sVAD is more often found in the pars transversaria (V2; 35%) or atlas loop (V3; 34%) segments of the vertebral artery than in the prevertebral (V1; 20%) or intracranial (V4; 11%) segments.3-5 The etiology of these injuries is thought to be minor trauma to the neck in the context of a likely underlying connective tissue disease, though no direct association with a particular disease has been shown.
Biopsy evaluation of the superficial temporal arteries of patients with sCADs have revealed pathologic changes of the media and adventitial layers, including vacuolar degeneration and capillary neoangiogenesis, which are not found in the arteries of control patients.5 Although definitive association with a known connective tissue disease is rare, angiographic evidence of fibromuscular dysplasia, a nonspecific marker of connective tissue disease, is noted in as many as 15% to 20% of patients.6 Consequently, routine connective tissue disease screening is not recommended in these patients. One study found that about 40% of sCAD patients can recall minor cervical trauma in the preceding month in comparison to only 10% of other patients with stroke, leading to the moniker of “bottoms-up” or “beauty-parlor strokes” for these injuries. The most common mechanisms of minor neck trauma causing sCADs include tennis and golf swings, yoga, and roller-coaster rides.7,8
Usually symptomatic at presentation, the most frequently encountered sCAD symptoms are head or neck pain (80%), brain ischemia (56%), and Horner syndrome (25%).1 A study of 161 consecutive patients with internal carotid (n = 135) or vertebral artery (n = 26) dissections revealed that headache was reported by 69% of those with sVADs, and when present, was the initial manifestation in 33%. Headaches typically were ipsilateral to the dissection, located posteriorly in 83% of patients, and lasted an average duration of 72 hours. Neck pain, which was noted in 46% of sVAD patients, was predominantly posterior and ipsilateral in location as well.9 Ischemic symptoms of sVAD may include posterior circulation symptoms, such as vertigo, ataxia, diplopia, and leg weakness as well as lateral medullary (Wallenberg) syndrome characterized by dizziness, postural instability, limb hypotonia/ataxia, blurred vision, and nystagmus.
In a study of 169 patients with sCAD, brain ischemia occurred in 77% (131 patients) including 67% (n = 114) with ischemic stroke and 10% (n = 17) with transient ischemic attack. Head and/or neck pain was noted in 88% of those with brain ischemia.4 Etiologies for infarction included thromboembolic (85%), hemodynamic (12%), and mixed (3%).10 Isolated local symptoms are rare with one study of 245 patients with sCAD revealing only 20 (8%) presenting with pain only. Of those with pain only, 6 presented with headache, 2 with neck pain, and 12 with both.11
Diagnosis of sVAD requires a high index of suspicion and is confirmed by diagnostic testing. Previously, invasive angiography was the diagnostic gold standard, but with the improvement in quality of CT and MR angiography, these noninvasive modalities have become the tests of choice. There have been no studies to date revealing a definitive benefit of one modality over the other. A meta-analysis of 25 articles that compared the use of CT and MR angiography for the diagnosis of carotid and VAD revealed similar sensitivity and specificity.12 In contrast, a study involving 10 patients with confirmed sVAD who had both CT and MR angiographies during evaluation showed more total findings consistent with dissection on CT than with MR angiography when graded by 2 neuroradiologists. Additionally, the neuroradiologists subjectively rated CT angiography as preferential to MR in showing the imaging findings of dissection in 8 of 10 cases of vertebral dissection.13
Treatment for sCAD remains heavily debated. The use of IV thrombolysis within the standard time window for acute ischemic stroke is advocated for these patients. A meta-analysis of patients with sCAD vs matched patients with stroke from other causes treated with IV thrombolysis showed no difference in mortality at 3 months (9.0% vs 8.8%) or symptomatic intracranial hemorrhage (3.3% vs 3.0%). Additionally, similar percentages of patients had excellent (30.9% vs 37.4%) and favorable (58.2% vs 52.2%) 3-month functional statuses as expressed by the Modified Rankin Score (mRS).14,15
Debate remains regarding subacute therapy for sCAD with either antiplatelet or anticoagulant therapy. A randomized study of 250 patients with cervical artery dissection (118 carotid, 132 vertebral) in which 126 patients were assigned to antiplatelet therapy and 124 patients were assigned to anticoagulant therapy showed an overall low rate of recurrent stroke (2%). There was no significant difference in efficacy between the therapy groups with stroke or death occurring in 3 antiplatelet patients and 1 anticoagulated patient. Adverse effects were very low in both groups with no deaths and only 1 major bleed in the anticoagulation group. Of note, stroke rates were lower in this study than prior observational studies.16
A nonrandomized study of 88 patients with extracranial sCAD showed overall low rates of recurrent ischemic stroke at 3 months with 1/59 (1.7%) in the antiplatelet group and 1/28 (3.6%) in the anticoagulation group (P 17 Given this low overall rate of recurrent stroke in prior studies, a guideline recommendation for antiplatelet or anticoagulant therapy cannot be made at this time.
The overall prognosis for this condition is fair. Functional status and recurrence risk are favorable, with one study finding a mRS score of 1 Additionally, a historic cohort study of 432 patients with first event of sCAD revealed that after a mean follow-up of 31 months, only 4 (0.9%) patients had a recurrent ischemic stroke either due to incomplete recanalization of the artery (n = 2) or recurrent sCAD (n = 2), and only 4 (0.9%) total recurrences of sCAD were report (2 without associated ischemic strokes).18 Further, a prospective study of 61 patients with confirmed sVAD revealed complete recanalization of 45.9% at 3 months, 62.3% at 6 months, and 63.9% at 12 months, suggesting that recanalization occurs mostly during the initial 6 months. There was no identified association between outcome and complete recanalization with favorable outcomes observed in 55 (90.2%) of patients and no further ischemic symptoms during follow-up.19
Neck maneuvers have been cited as a more common cause of sCAD in several previous studies. One retrospective study found chiropractic neck manipulation to be the etiology in 12 of 141 patients with CT- or MR- confirmed sCAD.20 As noted previously, to the authors’ knowledge this is the first reported case of a sVAD occurring after a mixed martial arts choke hold. While sports-related strokes are rare, one evaluation of 70 published cases found that 80% were due to sCAD. Commonly associated sports in this study included football, yoga, wrestling, tennis, golf, and swimming.21 Grappling-related neck manipulation has been noted as an etiology in a few case reports.
Hyperextension of the neck was deemed to be the etiology in boys aged 11 years and 17 years who developed a sCAD while participating in Judo and backyard wrestling, respectively.22,23 In the martial arts realm, there is a case report of a 26-year-old male who developed a sVAD after rapid head turning during a solo Kung Fu maneuver as well as a report of a 41-year-old male experiencing a right VAD complicated by a posterior infarction several days after straining his neck during a mixed martial arts competition.24,25 The patient denied any choke hold or direct blow to the neck.
The present case is different in that it is the first reported case of a sVAD occurring after a submission maneuver. Prior grappling-related sVADs were associated with hyperextension or rapid acceleration/deceleration forces on the neck. Isometric force to the neck is a rarely described mechanism for development of this injury. Although there are isolated and infrequent forensic case reports of carotid dissection with strangulation injuries, the authors believe this is the first documented case of a sVAD attributed to a combatives submission.
In the context of the military health system, it is important to be aware of this potential complication of combatives as instruction in close-quarters combat continues to be an important part of military training.
1. Lee VH, Brown RD Jr, Mandrekar J, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809-1812.
2. Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67(6):1050-1052.
3. Schvienk W. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
4. Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
5. Völker W, Dittrich R, Grewe S, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. 2011;76(17):1463-1471.
6. Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40(6):459-466.
7. DeBehnke D, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12(1):27-31.
8. Engelter ST, Grond-Ginsbach C, Metso TM, et al; Cervical Artery Dissection and Ischemic Stroke Patients Study Group. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology. 2013;80(21):1950-1957.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
10. Morel A, Naggara O, Touzé E, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. 2012;43(5):1354-1361.
11. Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
12. Provenzale J, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;1939(4):1167-1174.
13. Vertinsky AT, Schwartz NE, Fishbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
14. Zinkstok SM, Vergouwen MD, Engelter ST, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. 2011;42(9):2515-2520.
15. Engelter S, Rutgers M, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. 2009;40(12):3772-3776.
16. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomized trial. Lancet Neurol. 2015;14(4):361-367.
17. Kennedy F, Lanfranconi S, Hicks C, et al; CADISS Investigators. Antiplatelets vs. anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology. 2012;79(7):686-689.
18. Touze E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL; Multicenter Survey on Natural History of Cervical Artery Dissection. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61(10):1347-1351.
19. Arauz A, Marquez J, Artigas C, Balderrama J, Orrego H. Recanalization of vertebral artery dissection. Stroke. 2010;41(4):717-721.
20. Kennell KA, Daghfal MM, Patel SG, et DeSanto JR, Waterman GS, Bertino RE. Cervical artery dissection related to chiropractic manipulation: one institution’s experience. J Fam Pract. 2017;66(9):556-562.
21. McCrory P. Vertebral artery dissection causing stroke in sport. J Clin Neurosci. 2000;7(4):298-300.
22. Lannuzel A, Moulin T, Amsallem D, Galmiche J, Rumbach L. Vertebral artery dissection following a judo session: a case report. Neuropediatrics. 1994;25(2):106-108.
23. Gupta V, Dhawan N, Bahl J. Minor trauma causing stroke in a young athlete. Case Rep Neurol Med. 2015;2015: 182875.
24. Pacei F, Valvasorri L, Bet L. Vertebral artery dissection during Kung-Fu training. Neurol Sci. 2014;35(2):331-332.
25. Slowey M, Maw G, Furyk J. Case report on vertebral artery dissection in mixed martial arts. Emerg Med Australas. 2012;24(2):203-206.
1. Lee VH, Brown RD Jr, Mandrekar J, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809-1812.
2. Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67(6):1050-1052.
3. Schvienk W. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
4. Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
5. Völker W, Dittrich R, Grewe S, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. 2011;76(17):1463-1471.
6. Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40(6):459-466.
7. DeBehnke D, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12(1):27-31.
8. Engelter ST, Grond-Ginsbach C, Metso TM, et al; Cervical Artery Dissection and Ischemic Stroke Patients Study Group. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology. 2013;80(21):1950-1957.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
10. Morel A, Naggara O, Touzé E, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. 2012;43(5):1354-1361.
11. Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
12. Provenzale J, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;1939(4):1167-1174.
13. Vertinsky AT, Schwartz NE, Fishbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
14. Zinkstok SM, Vergouwen MD, Engelter ST, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. 2011;42(9):2515-2520.
15. Engelter S, Rutgers M, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. 2009;40(12):3772-3776.
16. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomized trial. Lancet Neurol. 2015;14(4):361-367.
17. Kennedy F, Lanfranconi S, Hicks C, et al; CADISS Investigators. Antiplatelets vs. anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology. 2012;79(7):686-689.
18. Touze E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL; Multicenter Survey on Natural History of Cervical Artery Dissection. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61(10):1347-1351.
19. Arauz A, Marquez J, Artigas C, Balderrama J, Orrego H. Recanalization of vertebral artery dissection. Stroke. 2010;41(4):717-721.
20. Kennell KA, Daghfal MM, Patel SG, et DeSanto JR, Waterman GS, Bertino RE. Cervical artery dissection related to chiropractic manipulation: one institution’s experience. J Fam Pract. 2017;66(9):556-562.
21. McCrory P. Vertebral artery dissection causing stroke in sport. J Clin Neurosci. 2000;7(4):298-300.
22. Lannuzel A, Moulin T, Amsallem D, Galmiche J, Rumbach L. Vertebral artery dissection following a judo session: a case report. Neuropediatrics. 1994;25(2):106-108.
23. Gupta V, Dhawan N, Bahl J. Minor trauma causing stroke in a young athlete. Case Rep Neurol Med. 2015;2015: 182875.
24. Pacei F, Valvasorri L, Bet L. Vertebral artery dissection during Kung-Fu training. Neurol Sci. 2014;35(2):331-332.
25. Slowey M, Maw G, Furyk J. Case report on vertebral artery dissection in mixed martial arts. Emerg Med Australas. 2012;24(2):203-206.
Body-wide, pruritic, papular rash • scalp lesion • excoriation • Dx?
THE CASE
A 7-year-old boy presented with a one-week history of a pruritic rash, which first appeared on his back and continued to spread across his entire body. The patient’s medical history was significant for a scalp lesion (FIGURE 1) that was being treated with oral griseofulvin (started 3 days earlier). He had no history of seasonal allergies, asthma, recent illness, or recent immunizations.
The physical exam was significant for a body-wide, nonerythematous, papular rash (FIGURE 2). There was evidence of excoriation due to itching. No mucosal involvement was appreciated. The remainder of the examination was unremarkable.
QUESTION
Based on the patient’s history and physical exam, which of the following is the most likely diagnosis?
A. Gianotti-Crosti syndrome
B. Atopic dermatitis
C. Dermatophytid reaction
D. Morbilliform drug eruption.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
The answer is C, dermatophytid reaction.
DISCUSSION
A dermatophytid reaction is a type of id reaction, or autoeczematization. An id reaction is when a localized dermatitis becomes a generalized pruritic eruption.1 In this case, the patient’s dermatitis was the result of a dermatophyte infection (tinea capitis), but an id reaction can also occur in response to noninfectious dermatitides and may be of an atopic, contact, or seborrheic nature.1
Dermatophytid reactions occur in up to 5% of all dermatophyte infections (most commonly tinea pedis) and are proposed to be type IV hypersensitivity reactions to the release of fungal antigens.1 These reactions can occur either before or after the initiation of antifungal treatment. They manifest as symmetric, pruritic, papulovesicular eruptions with fine scaling and commonly affect the face, trunk, extremities, palms, and interdigital spaces.1
What about other possible diagnoses?
Gianotti-Crosti syndrome is an asymptomatic, symmetric, papulovesicular dermatosis that involves the face, limbs, and buttocks of children 2 to 6 years of age.2 The lesions develop in response to a respiratory or gastrointestinal illness.2 They are typically associated with Epstein-Barr virus, hepatitis B, cytomegalovirus, respiratory syncytial virus, and coxsackievirus, but can occur with bacterial infections or following administration of routine immunizations.2
The lesions are self-limited and resolve within 2 months.2 Symptomatic lesions may be treated with oral antihistamines or steroids (topical or systemic).2
Continue to: Atopic dermatitis
Atopic dermatitis is characterized by symmetric involvement of the flexural surfaces of the body with a pruritic, erythematous rash that may have a fine scale.3 It usually manifests prior to 2 years of age, is recurrent, and is commonly associated with allergic rhinitis and asthma.3 Treatment involves trigger avoidance, topical emollients, topical corticosteroids, dilute bleach baths, and topical calcineurin inhibitors.3,4 For patients with significant nocturnal symptoms and sleep loss, oral antihistamines may be helpful.4
Morbilliform drug eruptions are the most common type of dermatologic drug reaction.5 These rashes occur approximately one to 2 weeks after exposure to a causative drug; they consist of pruritic, erythematous papules or macules that start centrally and may spread to the proximal extremities.5 Treatment involves discontinuation of the offending agent. Symptomatic relief may be achieved with oral antihistamines or topical or systemic corticosteroids.5
Treatment of dermatophytid reactions
While the initial impulse in the treatment of a dermatophytid reaction may be to discontinue oral antifungals, these treatments actually help resolve the underlying dermatophyte infection and should be continued. For children with tinea capitis, at least 6 weeks of treatment with an oral antifungal agent is warranted. Medications approved by the US Food and Drug Administration include terbinafine (for patients >4 years of age) and griseofulvin (for patients >2 years of age). Dosages are weight-based. (Fluconazole and itraconazole are not approved for this indication.) Lubricants, topical corticosteroids, and oral antihistamines can be used for acute management of pruritus.1
Our patient was treated successfully with griseofulvin and an oral antihistamine. However, he experienced headaches attributed to griseofulvin and was switched to terbinafine 5 mg/kg/d for 4 weeks. His tinea capitis was resolved at 8 weeks.
CORRESPONDENCE
Richard Temple, MD, CAPT, MC, USN. Department of Family Medicine, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547; [email protected].
1. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
2. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
3. Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86:35-42.
4. Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015;136:554-565.
5. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
THE CASE
A 7-year-old boy presented with a one-week history of a pruritic rash, which first appeared on his back and continued to spread across his entire body. The patient’s medical history was significant for a scalp lesion (FIGURE 1) that was being treated with oral griseofulvin (started 3 days earlier). He had no history of seasonal allergies, asthma, recent illness, or recent immunizations.

The physical exam was significant for a body-wide, nonerythematous, papular rash (FIGURE 2). There was evidence of excoriation due to itching. No mucosal involvement was appreciated. The remainder of the examination was unremarkable.
QUESTION
Based on the patient’s history and physical exam, which of the following is the most likely diagnosis?
A. Gianotti-Crosti syndrome
B. Atopic dermatitis
C. Dermatophytid reaction
D. Morbilliform drug eruption.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
The answer is C, dermatophytid reaction.
DISCUSSION
A dermatophytid reaction is a type of id reaction, or autoeczematization. An id reaction is when a localized dermatitis becomes a generalized pruritic eruption.1 In this case, the patient’s dermatitis was the result of a dermatophyte infection (tinea capitis), but an id reaction can also occur in response to noninfectious dermatitides and may be of an atopic, contact, or seborrheic nature.1
Dermatophytid reactions occur in up to 5% of all dermatophyte infections (most commonly tinea pedis) and are proposed to be type IV hypersensitivity reactions to the release of fungal antigens.1 These reactions can occur either before or after the initiation of antifungal treatment. They manifest as symmetric, pruritic, papulovesicular eruptions with fine scaling and commonly affect the face, trunk, extremities, palms, and interdigital spaces.1
What about other possible diagnoses?
Gianotti-Crosti syndrome is an asymptomatic, symmetric, papulovesicular dermatosis that involves the face, limbs, and buttocks of children 2 to 6 years of age.2 The lesions develop in response to a respiratory or gastrointestinal illness.2 They are typically associated with Epstein-Barr virus, hepatitis B, cytomegalovirus, respiratory syncytial virus, and coxsackievirus, but can occur with bacterial infections or following administration of routine immunizations.2
The lesions are self-limited and resolve within 2 months.2 Symptomatic lesions may be treated with oral antihistamines or steroids (topical or systemic).2
Continue to: Atopic dermatitis
Atopic dermatitis is characterized by symmetric involvement of the flexural surfaces of the body with a pruritic, erythematous rash that may have a fine scale.3 It usually manifests prior to 2 years of age, is recurrent, and is commonly associated with allergic rhinitis and asthma.3 Treatment involves trigger avoidance, topical emollients, topical corticosteroids, dilute bleach baths, and topical calcineurin inhibitors.3,4 For patients with significant nocturnal symptoms and sleep loss, oral antihistamines may be helpful.4
Morbilliform drug eruptions are the most common type of dermatologic drug reaction.5 These rashes occur approximately one to 2 weeks after exposure to a causative drug; they consist of pruritic, erythematous papules or macules that start centrally and may spread to the proximal extremities.5 Treatment involves discontinuation of the offending agent. Symptomatic relief may be achieved with oral antihistamines or topical or systemic corticosteroids.5
Treatment of dermatophytid reactions
While the initial impulse in the treatment of a dermatophytid reaction may be to discontinue oral antifungals, these treatments actually help resolve the underlying dermatophyte infection and should be continued. For children with tinea capitis, at least 6 weeks of treatment with an oral antifungal agent is warranted. Medications approved by the US Food and Drug Administration include terbinafine (for patients >4 years of age) and griseofulvin (for patients >2 years of age). Dosages are weight-based. (Fluconazole and itraconazole are not approved for this indication.) Lubricants, topical corticosteroids, and oral antihistamines can be used for acute management of pruritus.1
Our patient was treated successfully with griseofulvin and an oral antihistamine. However, he experienced headaches attributed to griseofulvin and was switched to terbinafine 5 mg/kg/d for 4 weeks. His tinea capitis was resolved at 8 weeks.
CORRESPONDENCE
Richard Temple, MD, CAPT, MC, USN. Department of Family Medicine, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547; [email protected].
THE CASE
A 7-year-old boy presented with a one-week history of a pruritic rash, which first appeared on his back and continued to spread across his entire body. The patient’s medical history was significant for a scalp lesion (FIGURE 1) that was being treated with oral griseofulvin (started 3 days earlier). He had no history of seasonal allergies, asthma, recent illness, or recent immunizations.

The physical exam was significant for a body-wide, nonerythematous, papular rash (FIGURE 2). There was evidence of excoriation due to itching. No mucosal involvement was appreciated. The remainder of the examination was unremarkable.
QUESTION
Based on the patient’s history and physical exam, which of the following is the most likely diagnosis?
A. Gianotti-Crosti syndrome
B. Atopic dermatitis
C. Dermatophytid reaction
D. Morbilliform drug eruption.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
The answer is C, dermatophytid reaction.
DISCUSSION
A dermatophytid reaction is a type of id reaction, or autoeczematization. An id reaction is when a localized dermatitis becomes a generalized pruritic eruption.1 In this case, the patient’s dermatitis was the result of a dermatophyte infection (tinea capitis), but an id reaction can also occur in response to noninfectious dermatitides and may be of an atopic, contact, or seborrheic nature.1
Dermatophytid reactions occur in up to 5% of all dermatophyte infections (most commonly tinea pedis) and are proposed to be type IV hypersensitivity reactions to the release of fungal antigens.1 These reactions can occur either before or after the initiation of antifungal treatment. They manifest as symmetric, pruritic, papulovesicular eruptions with fine scaling and commonly affect the face, trunk, extremities, palms, and interdigital spaces.1
What about other possible diagnoses?
Gianotti-Crosti syndrome is an asymptomatic, symmetric, papulovesicular dermatosis that involves the face, limbs, and buttocks of children 2 to 6 years of age.2 The lesions develop in response to a respiratory or gastrointestinal illness.2 They are typically associated with Epstein-Barr virus, hepatitis B, cytomegalovirus, respiratory syncytial virus, and coxsackievirus, but can occur with bacterial infections or following administration of routine immunizations.2
The lesions are self-limited and resolve within 2 months.2 Symptomatic lesions may be treated with oral antihistamines or steroids (topical or systemic).2
Continue to: Atopic dermatitis
Atopic dermatitis is characterized by symmetric involvement of the flexural surfaces of the body with a pruritic, erythematous rash that may have a fine scale.3 It usually manifests prior to 2 years of age, is recurrent, and is commonly associated with allergic rhinitis and asthma.3 Treatment involves trigger avoidance, topical emollients, topical corticosteroids, dilute bleach baths, and topical calcineurin inhibitors.3,4 For patients with significant nocturnal symptoms and sleep loss, oral antihistamines may be helpful.4
Morbilliform drug eruptions are the most common type of dermatologic drug reaction.5 These rashes occur approximately one to 2 weeks after exposure to a causative drug; they consist of pruritic, erythematous papules or macules that start centrally and may spread to the proximal extremities.5 Treatment involves discontinuation of the offending agent. Symptomatic relief may be achieved with oral antihistamines or topical or systemic corticosteroids.5
Treatment of dermatophytid reactions
While the initial impulse in the treatment of a dermatophytid reaction may be to discontinue oral antifungals, these treatments actually help resolve the underlying dermatophyte infection and should be continued. For children with tinea capitis, at least 6 weeks of treatment with an oral antifungal agent is warranted. Medications approved by the US Food and Drug Administration include terbinafine (for patients >4 years of age) and griseofulvin (for patients >2 years of age). Dosages are weight-based. (Fluconazole and itraconazole are not approved for this indication.) Lubricants, topical corticosteroids, and oral antihistamines can be used for acute management of pruritus.1
Our patient was treated successfully with griseofulvin and an oral antihistamine. However, he experienced headaches attributed to griseofulvin and was switched to terbinafine 5 mg/kg/d for 4 weeks. His tinea capitis was resolved at 8 weeks.
CORRESPONDENCE
Richard Temple, MD, CAPT, MC, USN. Department of Family Medicine, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547; [email protected].
1. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
2. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
3. Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86:35-42.
4. Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015;136:554-565.
5. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
1. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
2. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
3. Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86:35-42.
4. Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015;136:554-565.
5. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
Clostridium difficile Colitis in a Patient With Abdominal Distention, Pain, and Severe Constipation
A 66-year-old man with steroid-dependent asthma, well-controlled diabetes mellitus (DM), and chronic pain on hospice presented to Georg
On presentation, the patient reported taking the following medications: daily oxycodone 20 to 30 mg, tramadol 200 mg, gabapentin 1,200 mg, and frequent doses of morphine concentrate. Due to episodes of constipation and diarrhea, the veteran had recently self-discontinued taking stool softener (Senna plus). One month prior to this admission, the patient was enrolled in hospice service by his primary physician for severe COPD due to chronic hypoxic respiratory failure and worsening frailty. His baseline oxygen requirement was 4 to 5 L of supplemental oxygen with continued dyspnea upon any ambulation. The patient reported frequent falls prior to admission. Despite chronic steroid use, the patient’s DM was well controlled with metformin His hemoglobin A1c ranged from 6.0 to 7.8.
The patient was supine and appeared to be uncomfortable but not in acute distress on exam. His body habitus was Cushingoid, and he appeared much older than his stated age. His vitals were as follows: temperature 100.2°F, heart rate of 104 beats per minute, blood pressure of 98/56 mm Hg, and 95% oxygen on 4L nasal cannula (baseline 4-5L). A respiratory exam revealed distant breath sounds without wheeze, rhonchi, or rales, and a cardiac exam revealed no murmurs. He was in sinus rhythm with tachycardia. The abdomen was obese with purple straie and markedly distended. On percussion, his abdomen was tympanic with tinkling bowel sounds. He had no rebound tenderness, peritoneal signs, or fluid wave.
Laboratory results revealed a white blood cell (WBC) count of 13,790 cells/μL with a neutrophilic shift of 82.0, and an elevated creatinine of 2.16 mg/dL up from a baseline of 1.12 mg/dL. The chemistry panel was abnormal with a 125 mmol/L sodium (reference range 137-145 mmol/L).
Diagnosis
On admission, the authors’ differential diagnosis included fecal impaction with large bowel obstruction, colitis, narcotic induced ileus, dehydration leading to severe constipation, and delayed gastric emptying secondary to long-standing DM. Ciprofloxacin and metronidazole antibiotics were initiated out of concern for possible colitis and potential bacterial translocation. Intravenous fluids were initiated, and the patient was instructed to have nothing by mouth (NPO) aside from the antibiotics. All opioids, including tramadol, were held. Out of concern for narcotic-induced constipation, a dose of methylnaltrexone to induce stooling was administered but had no effect on the constipation.
The gastroenterology department was consulted for a possible endoscopy to aid in decompression of the sigmoid. However, given the amount of distention and concern for perforation with endoscopy, the patient did not undergo endoscopy on admission. The patient remained afebrile on hospital day 3, and all antibiotics were discontinued. His WBC count normalized with complete resolution of the kidney injury. Antibiotic stewardship and infectious disease consults at George E. Wahlen VAMC reviewed the case and supported the decision to stop all antibiotics since it was not clear whether or not the patient was infected. Despite aggressive bowel care that included a nasogastric tube for large-volume polyethylene glycol and lactulose, various enemas and suppositories, the patient remained constipated.
On hospital day 5, still NPO, the patient had several bilious liquid stools that appeared to have a sediment quality to them. His abdomen remained distended, tympanic, and uncomfortable to palpation., He was examined frequently due to concern for possible perforation. On hospital day 8, gastroenterology reevaluated the need for endoscopy and proceeded with a flexible sigmoidoscopy.
Polymerase chain reaction analysis of the colonoscopy stool samples were positive for Clostridium difficile (C difficile). The patient was started on IV metronidazole and oral vancomycin. His diet advanced and over the next few days he began stooling. He was subsequently discharged back to an extended care facility for rehabilitation. During this hospitalization, he made it clear he wished to be discharged from hospice services. He wanted to regain his strength through aggressive physical and occupational therapies.
Conclusion
Typical clinical manifestations of fulminant colitis include fever, diarrhea, abdominal pain, distention, and frequently WBC counts > 20,000 cells/μL. However, C difficile colitis, also known as pseudomembranous colitis, occasionally can present as an acute ileus, with little or no diarrhea.1 This veteran had several risk factors for C difficile infection, which included long-term residence in an extended care facility, frequent asthma exacerbations that required antibiotics, severe chronic disease, aged > 65 years,and ciprofloxacin given the first 3 days of this hospitalization.2 Until the endoscopy results were presented, no one on the patient’s care team, including gastroenterology and infectious disease, had included an infectious etiology in the differential diagnosis. This case reinforces the need to broaden differential diagnoses and look beyond assumptions that opioids without an adequate bowel regime were the cause. Avoiding anchoring heuristics can be a challenge as this case demonstrates.
1. Kawsar HI, Gopal KV, Shahnewaz J, Daw HA. Constipation in Clostridium difficile infection. BMJ Case Rep. 2012;2012: pii: bcr0220125938.
2. Leffler D, Lamont T. Clostridium difficile infection. N Engl J Med. 2015;372(16)1539-1548.
A 66-year-old man with steroid-dependent asthma, well-controlled diabetes mellitus (DM), and chronic pain on hospice presented to Georg
On presentation, the patient reported taking the following medications: daily oxycodone 20 to 30 mg, tramadol 200 mg, gabapentin 1,200 mg, and frequent doses of morphine concentrate. Due to episodes of constipation and diarrhea, the veteran had recently self-discontinued taking stool softener (Senna plus). One month prior to this admission, the patient was enrolled in hospice service by his primary physician for severe COPD due to chronic hypoxic respiratory failure and worsening frailty. His baseline oxygen requirement was 4 to 5 L of supplemental oxygen with continued dyspnea upon any ambulation. The patient reported frequent falls prior to admission. Despite chronic steroid use, the patient’s DM was well controlled with metformin His hemoglobin A1c ranged from 6.0 to 7.8.
The patient was supine and appeared to be uncomfortable but not in acute distress on exam. His body habitus was Cushingoid, and he appeared much older than his stated age. His vitals were as follows: temperature 100.2°F, heart rate of 104 beats per minute, blood pressure of 98/56 mm Hg, and 95% oxygen on 4L nasal cannula (baseline 4-5L). A respiratory exam revealed distant breath sounds without wheeze, rhonchi, or rales, and a cardiac exam revealed no murmurs. He was in sinus rhythm with tachycardia. The abdomen was obese with purple straie and markedly distended. On percussion, his abdomen was tympanic with tinkling bowel sounds. He had no rebound tenderness, peritoneal signs, or fluid wave.
Laboratory results revealed a white blood cell (WBC) count of 13,790 cells/μL with a neutrophilic shift of 82.0, and an elevated creatinine of 2.16 mg/dL up from a baseline of 1.12 mg/dL. The chemistry panel was abnormal with a 125 mmol/L sodium (reference range 137-145 mmol/L).
Diagnosis
On admission, the authors’ differential diagnosis included fecal impaction with large bowel obstruction, colitis, narcotic induced ileus, dehydration leading to severe constipation, and delayed gastric emptying secondary to long-standing DM. Ciprofloxacin and metronidazole antibiotics were initiated out of concern for possible colitis and potential bacterial translocation. Intravenous fluids were initiated, and the patient was instructed to have nothing by mouth (NPO) aside from the antibiotics. All opioids, including tramadol, were held. Out of concern for narcotic-induced constipation, a dose of methylnaltrexone to induce stooling was administered but had no effect on the constipation.
The gastroenterology department was consulted for a possible endoscopy to aid in decompression of the sigmoid. However, given the amount of distention and concern for perforation with endoscopy, the patient did not undergo endoscopy on admission. The patient remained afebrile on hospital day 3, and all antibiotics were discontinued. His WBC count normalized with complete resolution of the kidney injury. Antibiotic stewardship and infectious disease consults at George E. Wahlen VAMC reviewed the case and supported the decision to stop all antibiotics since it was not clear whether or not the patient was infected. Despite aggressive bowel care that included a nasogastric tube for large-volume polyethylene glycol and lactulose, various enemas and suppositories, the patient remained constipated.
On hospital day 5, still NPO, the patient had several bilious liquid stools that appeared to have a sediment quality to them. His abdomen remained distended, tympanic, and uncomfortable to palpation., He was examined frequently due to concern for possible perforation. On hospital day 8, gastroenterology reevaluated the need for endoscopy and proceeded with a flexible sigmoidoscopy.
Polymerase chain reaction analysis of the colonoscopy stool samples were positive for Clostridium difficile (C difficile). The patient was started on IV metronidazole and oral vancomycin. His diet advanced and over the next few days he began stooling. He was subsequently discharged back to an extended care facility for rehabilitation. During this hospitalization, he made it clear he wished to be discharged from hospice services. He wanted to regain his strength through aggressive physical and occupational therapies.
Conclusion
Typical clinical manifestations of fulminant colitis include fever, diarrhea, abdominal pain, distention, and frequently WBC counts > 20,000 cells/μL. However, C difficile colitis, also known as pseudomembranous colitis, occasionally can present as an acute ileus, with little or no diarrhea.1 This veteran had several risk factors for C difficile infection, which included long-term residence in an extended care facility, frequent asthma exacerbations that required antibiotics, severe chronic disease, aged > 65 years,and ciprofloxacin given the first 3 days of this hospitalization.2 Until the endoscopy results were presented, no one on the patient’s care team, including gastroenterology and infectious disease, had included an infectious etiology in the differential diagnosis. This case reinforces the need to broaden differential diagnoses and look beyond assumptions that opioids without an adequate bowel regime were the cause. Avoiding anchoring heuristics can be a challenge as this case demonstrates.
A 66-year-old man with steroid-dependent asthma, well-controlled diabetes mellitus (DM), and chronic pain on hospice presented to Georg
On presentation, the patient reported taking the following medications: daily oxycodone 20 to 30 mg, tramadol 200 mg, gabapentin 1,200 mg, and frequent doses of morphine concentrate. Due to episodes of constipation and diarrhea, the veteran had recently self-discontinued taking stool softener (Senna plus). One month prior to this admission, the patient was enrolled in hospice service by his primary physician for severe COPD due to chronic hypoxic respiratory failure and worsening frailty. His baseline oxygen requirement was 4 to 5 L of supplemental oxygen with continued dyspnea upon any ambulation. The patient reported frequent falls prior to admission. Despite chronic steroid use, the patient’s DM was well controlled with metformin His hemoglobin A1c ranged from 6.0 to 7.8.
The patient was supine and appeared to be uncomfortable but not in acute distress on exam. His body habitus was Cushingoid, and he appeared much older than his stated age. His vitals were as follows: temperature 100.2°F, heart rate of 104 beats per minute, blood pressure of 98/56 mm Hg, and 95% oxygen on 4L nasal cannula (baseline 4-5L). A respiratory exam revealed distant breath sounds without wheeze, rhonchi, or rales, and a cardiac exam revealed no murmurs. He was in sinus rhythm with tachycardia. The abdomen was obese with purple straie and markedly distended. On percussion, his abdomen was tympanic with tinkling bowel sounds. He had no rebound tenderness, peritoneal signs, or fluid wave.
Laboratory results revealed a white blood cell (WBC) count of 13,790 cells/μL with a neutrophilic shift of 82.0, and an elevated creatinine of 2.16 mg/dL up from a baseline of 1.12 mg/dL. The chemistry panel was abnormal with a 125 mmol/L sodium (reference range 137-145 mmol/L).
Diagnosis
On admission, the authors’ differential diagnosis included fecal impaction with large bowel obstruction, colitis, narcotic induced ileus, dehydration leading to severe constipation, and delayed gastric emptying secondary to long-standing DM. Ciprofloxacin and metronidazole antibiotics were initiated out of concern for possible colitis and potential bacterial translocation. Intravenous fluids were initiated, and the patient was instructed to have nothing by mouth (NPO) aside from the antibiotics. All opioids, including tramadol, were held. Out of concern for narcotic-induced constipation, a dose of methylnaltrexone to induce stooling was administered but had no effect on the constipation.
The gastroenterology department was consulted for a possible endoscopy to aid in decompression of the sigmoid. However, given the amount of distention and concern for perforation with endoscopy, the patient did not undergo endoscopy on admission. The patient remained afebrile on hospital day 3, and all antibiotics were discontinued. His WBC count normalized with complete resolution of the kidney injury. Antibiotic stewardship and infectious disease consults at George E. Wahlen VAMC reviewed the case and supported the decision to stop all antibiotics since it was not clear whether or not the patient was infected. Despite aggressive bowel care that included a nasogastric tube for large-volume polyethylene glycol and lactulose, various enemas and suppositories, the patient remained constipated.
On hospital day 5, still NPO, the patient had several bilious liquid stools that appeared to have a sediment quality to them. His abdomen remained distended, tympanic, and uncomfortable to palpation., He was examined frequently due to concern for possible perforation. On hospital day 8, gastroenterology reevaluated the need for endoscopy and proceeded with a flexible sigmoidoscopy.
Polymerase chain reaction analysis of the colonoscopy stool samples were positive for Clostridium difficile (C difficile). The patient was started on IV metronidazole and oral vancomycin. His diet advanced and over the next few days he began stooling. He was subsequently discharged back to an extended care facility for rehabilitation. During this hospitalization, he made it clear he wished to be discharged from hospice services. He wanted to regain his strength through aggressive physical and occupational therapies.
Conclusion
Typical clinical manifestations of fulminant colitis include fever, diarrhea, abdominal pain, distention, and frequently WBC counts > 20,000 cells/μL. However, C difficile colitis, also known as pseudomembranous colitis, occasionally can present as an acute ileus, with little or no diarrhea.1 This veteran had several risk factors for C difficile infection, which included long-term residence in an extended care facility, frequent asthma exacerbations that required antibiotics, severe chronic disease, aged > 65 years,and ciprofloxacin given the first 3 days of this hospitalization.2 Until the endoscopy results were presented, no one on the patient’s care team, including gastroenterology and infectious disease, had included an infectious etiology in the differential diagnosis. This case reinforces the need to broaden differential diagnoses and look beyond assumptions that opioids without an adequate bowel regime were the cause. Avoiding anchoring heuristics can be a challenge as this case demonstrates.
1. Kawsar HI, Gopal KV, Shahnewaz J, Daw HA. Constipation in Clostridium difficile infection. BMJ Case Rep. 2012;2012: pii: bcr0220125938.
2. Leffler D, Lamont T. Clostridium difficile infection. N Engl J Med. 2015;372(16)1539-1548.
1. Kawsar HI, Gopal KV, Shahnewaz J, Daw HA. Constipation in Clostridium difficile infection. BMJ Case Rep. 2012;2012: pii: bcr0220125938.
2. Leffler D, Lamont T. Clostridium difficile infection. N Engl J Med. 2015;372(16)1539-1548.