User login
Tuberculous Cellulitis: Diseases Behind Cellulitislike Erythema
Local tender erythema is a typical manifestation of cellulitis, which is commonly seen by dermatologists; however, cutaneous manifestations of other diseases may bear resemblance to the more banal cellulitis. We present the case of a patient with tuberculous cellulitis, a rare variant of cutaneous tuberculosis.
Case Report
An 89-year-old man presented to a local primary care physician with a fever (temperature, 38°C). Infectious disease was suspected. Antibiotic therapy with oral cefaclor and intravenous cefotiam hydrochloride was started, but the patient’s fever did not subside. Six days after initiation of treatment, he was referred to our dermatology department for evaluation of a painful erythematous rash on the left thigh that had suddenly appeared. The patient had a history of pulmonary tuberculosis 71 years prior. He also underwent surgical treatment of pancreatic cancer 14 years prior. Additionally, he had chronic kidney disease (CKD) and polymyalgia rheumatica, which was currently being treated with oral prednisolone 5 mg once daily.
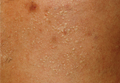
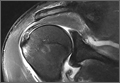
Physical examination revealed a hot and tender erythematous plaque on the left thigh (Figure 1). The edge of the lesion was not well defined and there was no regional lymphadenopathy.

A complete blood cell count revealed anemia (white blood cell count, 8070/μL [reference range, 4000–9,000/μL]; neutrophils, 77.1% [reference range, 44%–74%]; lymphocytes, 13.8% [reference range, 20%–50%]; hemoglobin, 9.3 g/dL [reference range, 13.0–17.0 g/dL]; and platelet count, 329×103/μL [reference range, 150–400×103/μL]). The C-reactive protein level was 7.3 mg/dL (reference range, 0.08–0.3 mg/dL). The creatinine level was 2.93 mg/dL (reference range, 0.6–1.2 mg/dL). There were no signs of liver dysfunction.
A blood culture was negative. A purified protein derivative (tuberculin) skin test was negative (6×7 mm [reference range, ≤9 mm). A chest computed tomography (CT) scan showed small centrilobular nodules that had not changed in number or size since evaluation 3 months prior.
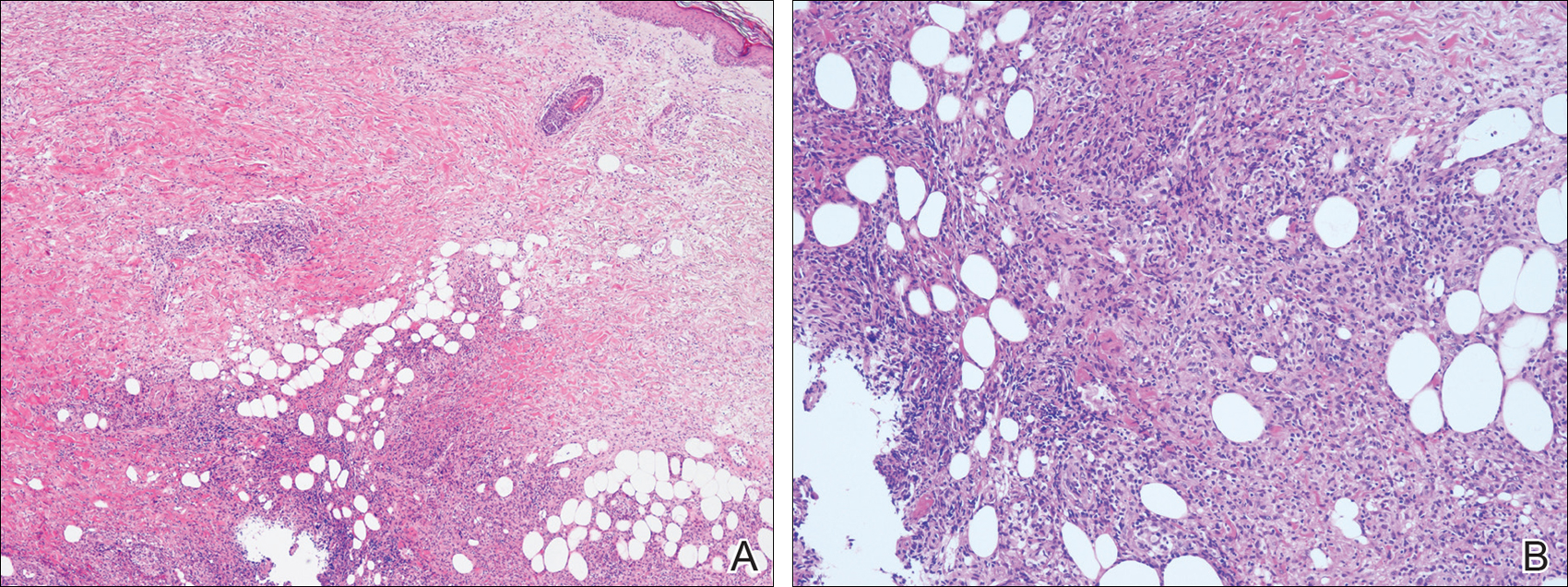
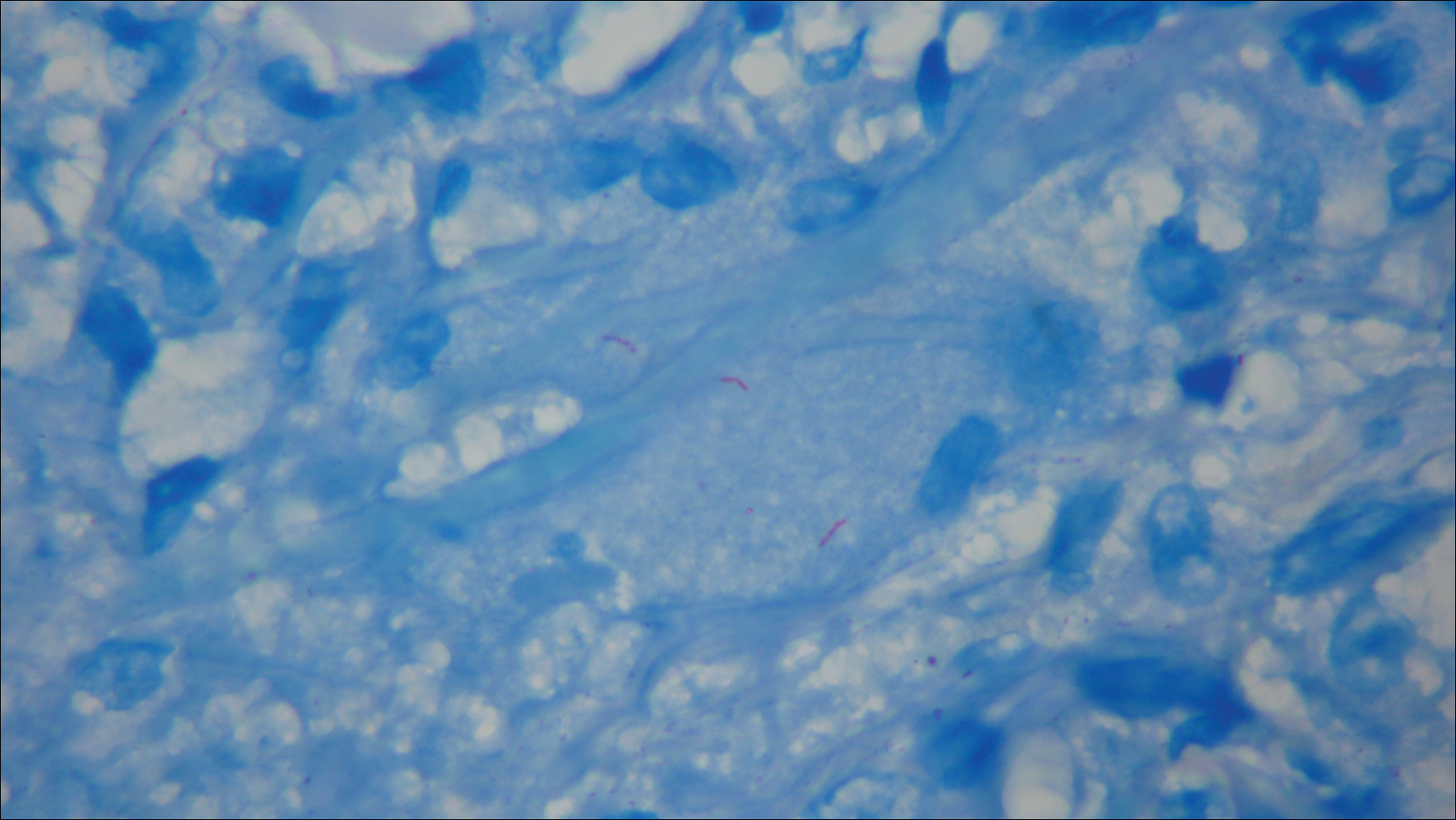
The antibiotics were changed to meropenem hydrate 0.5 g and clindamycin 300 mg twice daily for presumed bacterial cellulitis, then meropenem hydrate 1 g and clindamycin 600 mg daily, but there was still no improvement after about 1 week. Therefore, a skin biopsy was performed on the left thigh. The specimen showed epithelioid cell granulomas throughout the dermis and subcutis (Figure 2). Ziehl-Neelsen stain revealed numerous acid-fast bacilli (Figure 3). Polymerase chain reaction was positive for Mycobacterium tuberculosis in the skin biopsy specimen and gastric fluid. Additionally, M tuberculosis was isolated from the skin biopsy specimen, gastric fluid, and sputum culture. After the series of treatments described above, a remarkable increase in nodule size and number was observed in a follow-up chest CT scan compared with the prior examination. These pulmonary lesions showed bronchogenic spread.
A diagnosis of tuberculous cellulitis with pulmonary tuberculosis was made. Treatment with isoniazid 200 mg once daily, rifampin 300 mg once daily, and ethambutol 500 mg once every other day was started; the dosages were reduced from the standard dose due to the patient’s CKD.1 Four days after initiation of these medications, the patient was transferred to a hospital specifically for the treatment of tuberculosis. Approximately 8 months after treatment with isoniazid, rifampin, and ethambutol, M tuberculosis could not be detected in the sputum and a chest CT revealed that the pulmonary lesions were remarkably improved. However, polymerase chain reaction of the skin biopsy specimen was still positive for M tuberculosis. It was determined that debridement of the skin lesion was needed, but the patient died from complications of deteriorating CKD 10 months after the initiation of the antituberculosis medications.
Comment
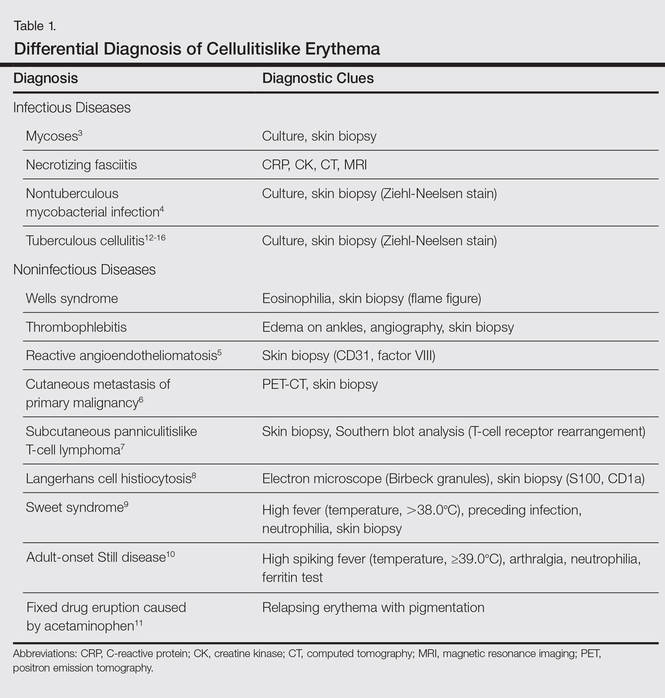
Cellulitis is a suppurative inflammation involving the subcutis.2 Local tender erythema, malaise, chills, and fever may be present at the onset. Cellulitis is commonly seen by dermatologists, and it is well known that other infectious diseases such as necrotizing fasciitis, cutaneous and subcutaneous mycoses,3 and nontuberculous mycobacterial infections4 sometimes present as cellulitislike skin lesions. Moreover, noninfectious diseases, such as Wells syndrome, thrombophlebitis, reactive angioendotheliomatosis,5 cutaneous metastasis of a primary malignancy,6 subcutaneous panniculitislike T-cell lymphoma,7 Langerhans cell histiocytosis,8 Sweet syndrome,9 adult-onset Still disease,10 and fixed drug eruption caused by acetaminophen11 should be excluded. These differential diagnoses and diagnostic clues of cellulitislike erythema are summarized in Table 1.3-16
Cutaneous tuberculosis presenting as cellulitis, so-called tuberculous cellulitis, also is characterized as a clinical mimicker of cellulitis. On the other hand, histologically, it has features of cutaneous tuberculosis (eg, necrotic granuloma).12,14,15 Tuberculous cellulitis is rare and therefore may often be misdiagnosed even in highly endemic areas. We summarized the clinical information of 5 well-documented cases of tuberculous cellulitis along with the current case in Table 2.12-16 All of these cases had an associated disease and involved patients who were currently taking oral corticosteroids. If a patient undergoing immunosuppressive therapy develops cellulitislike erythema, tuberculous cellulitis should be considered in the differential diagnosis.

Cutaneous tuberculosis generally is classified into 4 types according to the mechanism of disease acquisition: (1) inoculation from an exogenous source, (2) endogenous cutaneous spread contiguously or by autoinoculation, (3) hematogenous spread to the skin, and (4) tuberculids. In our case, it was suspected that the cellulitislike erythema may have been caused by hematogenous spread from pulmonary tuberculosis. Considering that negative reactions to purified protein derivative (tuberculin) skin tests often are observed in cases of miliary tuberculosis (widespread dissemination of M tuberculosis to 2 or more organs via hematogenous spread), we suspected that our patient could proceed to miliary tuberculosis; in fact, a case was reported in which miliary tuberculosis emerged approximately 3 weeks after the onset of erythema,13 as observed in the present case. Therefore, erythema in the setting of tuberculosis may be a predictor of miliary tuberculosis. The types of cutaneous lesions caused by tuberculosis infection also are dependent on multiple host factors.2 Cutaneous tuberculosis with an atypical clinical appearance has become more common because of the increasing number of immunocompromised patients.17
In addition, most cases of cutaneous tuberculosis are not associated with pain. Generally, tuberculous cellulitis also causes nontender erythematous plaques or nodules.2 However, in some cases of tuberculous cellulitis, including our case, tender skin lesions have been reported.12-14 Therefore, this symptom is not a sensitive factor for differential diagnosis.
We suggest that tuberculous cellulitis should always be included in the differential diagnosis of a cellulitislike rash with or without pain if the skin lesion is not improved despite antibiotic therapy.
- Daido-Horiuchi Y, Kikuchi Y, Kobayashi S, et al. Tuberculous cellulitis in a patient with chronic kidney disease and polymyalgia rheumatica. Intern Med. 2012;51:3203-3206.
- James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders Elsevier; 2011:322-329.
- Schupbach CW, Wheeler CE Jr, Briggaman RA, et al. Cutaneous manifestations of disseminated cryptococcosis. Arch Dermatol. 1976;112:1734-1740.
- Hsu PY, Yang YH, Hsiao CH, et al. Mycobacterium kansasii infection presenting as cellulitis in a patient with systemic lupus erythematosus. J Formos Med Assoc. 2002;101:581-584.
- Aguayo-Leiva I, Vano-Galván S, Salguero I, et al. Reactive angioendotheliomatosis in a patient with myelodysplastic syndrome presenting as a cellulitis-like plaque. Eur J Dermatol. 2009;19:182-183.
- Yang HI, Lee MC, Kuo TT, et al. Cellulitis-like cutaneous metastasis of uterine cervical carcinoma. J Am Acad Dermatol. 2007;56:S26-S28.
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitis-like T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106:S55-S59.
- Sharma PK, Sabhnani S, Bhardwaj M, et al. Acral, pure cutaneous, self-healing, late-onset, cellulitis-like Langerhans cell histiocytosis. J Cutan Med Surg. 2009;13:43-47.
- Tercedor J, Ródenas JM, Henraz MT, et al. Facial cellulitis-like Sweet’s syndrome in acute myelogenous leukemia. Int J Dermatol. 1992;31:598-599.
- Inaoki M, Nishijima C, Kumada S, et al. Adult-onset Still disease with a cellulitis-like eruption. Eur J Dermatol. 2009;19:80-81.
- Prabhu MM, Prabhu S, Mishra P, et al. Cellulitis-like fixed drug eruption attributed to paracetamol (acetaminophen). Dermatol Online J. 2005;11:24.
- Lee NH, Choi EH, Lee WS, et al. Tuberculous cellulitis. Clin Exp Dermatol. 2000;25:222-223.
- Kim JE, Ko JY, Bae SC, et al. Tuberculous cellulitis as a manifestation of miliary tuberculosis in a patient with malignancy-associated dermatomyositis. J Am Acad Dermatol. 2011;65:450-452.
- Chin PW, Koh CK, Wong KT. Cutaneous tuberculosis mimicking cellulitis in an immunosuppressed patient. Singapore Med J. 1999;40:44-45.
- Seyahi N, Apaydin S, Kahveci A, et al. Cellulitis as a manifestation of miliary tuberculosis in a renal transplant recipient. Transpl Infect Dis. 2005;7:80-85.
- Kato G, Watanabe K, Shibuya Y, et al. A case of cutaneous tuberculosis with cellulitis-like appearance [in Japanese]. Rinsho Hifuka (Jpn J Clin Dermatol). 2010;64:1055-1059.
- Fariña MC, Gegundez MI, Piqué E, et al. Cutaneous tuberculosis: a clinical, histopathologic, and bacteriologic study. J Am Acad Dermatol. 1995;33:433-440.
Local tender erythema is a typical manifestation of cellulitis, which is commonly seen by dermatologists; however, cutaneous manifestations of other diseases may bear resemblance to the more banal cellulitis. We present the case of a patient with tuberculous cellulitis, a rare variant of cutaneous tuberculosis.
Case Report
An 89-year-old man presented to a local primary care physician with a fever (temperature, 38°C). Infectious disease was suspected. Antibiotic therapy with oral cefaclor and intravenous cefotiam hydrochloride was started, but the patient’s fever did not subside. Six days after initiation of treatment, he was referred to our dermatology department for evaluation of a painful erythematous rash on the left thigh that had suddenly appeared. The patient had a history of pulmonary tuberculosis 71 years prior. He also underwent surgical treatment of pancreatic cancer 14 years prior. Additionally, he had chronic kidney disease (CKD) and polymyalgia rheumatica, which was currently being treated with oral prednisolone 5 mg once daily.
Physical examination revealed a hot and tender erythematous plaque on the left thigh (Figure 1). The edge of the lesion was not well defined and there was no regional lymphadenopathy.

A complete blood cell count revealed anemia (white blood cell count, 8070/μL [reference range, 4000–9,000/μL]; neutrophils, 77.1% [reference range, 44%–74%]; lymphocytes, 13.8% [reference range, 20%–50%]; hemoglobin, 9.3 g/dL [reference range, 13.0–17.0 g/dL]; and platelet count, 329×103/μL [reference range, 150–400×103/μL]). The C-reactive protein level was 7.3 mg/dL (reference range, 0.08–0.3 mg/dL). The creatinine level was 2.93 mg/dL (reference range, 0.6–1.2 mg/dL). There were no signs of liver dysfunction.
A blood culture was negative. A purified protein derivative (tuberculin) skin test was negative (6×7 mm [reference range, ≤9 mm). A chest computed tomography (CT) scan showed small centrilobular nodules that had not changed in number or size since evaluation 3 months prior.
The antibiotics were changed to meropenem hydrate 0.5 g and clindamycin 300 mg twice daily for presumed bacterial cellulitis, then meropenem hydrate 1 g and clindamycin 600 mg daily, but there was still no improvement after about 1 week. Therefore, a skin biopsy was performed on the left thigh. The specimen showed epithelioid cell granulomas throughout the dermis and subcutis (Figure 2). Ziehl-Neelsen stain revealed numerous acid-fast bacilli (Figure 3). Polymerase chain reaction was positive for Mycobacterium tuberculosis in the skin biopsy specimen and gastric fluid. Additionally, M tuberculosis was isolated from the skin biopsy specimen, gastric fluid, and sputum culture. After the series of treatments described above, a remarkable increase in nodule size and number was observed in a follow-up chest CT scan compared with the prior examination. These pulmonary lesions showed bronchogenic spread.


A diagnosis of tuberculous cellulitis with pulmonary tuberculosis was made. Treatment with isoniazid 200 mg once daily, rifampin 300 mg once daily, and ethambutol 500 mg once every other day was started; the dosages were reduced from the standard dose due to the patient’s CKD.1 Four days after initiation of these medications, the patient was transferred to a hospital specifically for the treatment of tuberculosis. Approximately 8 months after treatment with isoniazid, rifampin, and ethambutol, M tuberculosis could not be detected in the sputum and a chest CT revealed that the pulmonary lesions were remarkably improved. However, polymerase chain reaction of the skin biopsy specimen was still positive for M tuberculosis. It was determined that debridement of the skin lesion was needed, but the patient died from complications of deteriorating CKD 10 months after the initiation of the antituberculosis medications.
Comment
Cellulitis is a suppurative inflammation involving the subcutis.2 Local tender erythema, malaise, chills, and fever may be present at the onset. Cellulitis is commonly seen by dermatologists, and it is well known that other infectious diseases such as necrotizing fasciitis, cutaneous and subcutaneous mycoses,3 and nontuberculous mycobacterial infections4 sometimes present as cellulitislike skin lesions. Moreover, noninfectious diseases, such as Wells syndrome, thrombophlebitis, reactive angioendotheliomatosis,5 cutaneous metastasis of a primary malignancy,6 subcutaneous panniculitislike T-cell lymphoma,7 Langerhans cell histiocytosis,8 Sweet syndrome,9 adult-onset Still disease,10 and fixed drug eruption caused by acetaminophen11 should be excluded. These differential diagnoses and diagnostic clues of cellulitislike erythema are summarized in Table 1.3-16

Cutaneous tuberculosis presenting as cellulitis, so-called tuberculous cellulitis, also is characterized as a clinical mimicker of cellulitis. On the other hand, histologically, it has features of cutaneous tuberculosis (eg, necrotic granuloma).12,14,15 Tuberculous cellulitis is rare and therefore may often be misdiagnosed even in highly endemic areas. We summarized the clinical information of 5 well-documented cases of tuberculous cellulitis along with the current case in Table 2.12-16 All of these cases had an associated disease and involved patients who were currently taking oral corticosteroids. If a patient undergoing immunosuppressive therapy develops cellulitislike erythema, tuberculous cellulitis should be considered in the differential diagnosis.

Cutaneous tuberculosis generally is classified into 4 types according to the mechanism of disease acquisition: (1) inoculation from an exogenous source, (2) endogenous cutaneous spread contiguously or by autoinoculation, (3) hematogenous spread to the skin, and (4) tuberculids. In our case, it was suspected that the cellulitislike erythema may have been caused by hematogenous spread from pulmonary tuberculosis. Considering that negative reactions to purified protein derivative (tuberculin) skin tests often are observed in cases of miliary tuberculosis (widespread dissemination of M tuberculosis to 2 or more organs via hematogenous spread), we suspected that our patient could proceed to miliary tuberculosis; in fact, a case was reported in which miliary tuberculosis emerged approximately 3 weeks after the onset of erythema,13 as observed in the present case. Therefore, erythema in the setting of tuberculosis may be a predictor of miliary tuberculosis. The types of cutaneous lesions caused by tuberculosis infection also are dependent on multiple host factors.2 Cutaneous tuberculosis with an atypical clinical appearance has become more common because of the increasing number of immunocompromised patients.17
In addition, most cases of cutaneous tuberculosis are not associated with pain. Generally, tuberculous cellulitis also causes nontender erythematous plaques or nodules.2 However, in some cases of tuberculous cellulitis, including our case, tender skin lesions have been reported.12-14 Therefore, this symptom is not a sensitive factor for differential diagnosis.
We suggest that tuberculous cellulitis should always be included in the differential diagnosis of a cellulitislike rash with or without pain if the skin lesion is not improved despite antibiotic therapy.
Local tender erythema is a typical manifestation of cellulitis, which is commonly seen by dermatologists; however, cutaneous manifestations of other diseases may bear resemblance to the more banal cellulitis. We present the case of a patient with tuberculous cellulitis, a rare variant of cutaneous tuberculosis.
Case Report
An 89-year-old man presented to a local primary care physician with a fever (temperature, 38°C). Infectious disease was suspected. Antibiotic therapy with oral cefaclor and intravenous cefotiam hydrochloride was started, but the patient’s fever did not subside. Six days after initiation of treatment, he was referred to our dermatology department for evaluation of a painful erythematous rash on the left thigh that had suddenly appeared. The patient had a history of pulmonary tuberculosis 71 years prior. He also underwent surgical treatment of pancreatic cancer 14 years prior. Additionally, he had chronic kidney disease (CKD) and polymyalgia rheumatica, which was currently being treated with oral prednisolone 5 mg once daily.
Physical examination revealed a hot and tender erythematous plaque on the left thigh (Figure 1). The edge of the lesion was not well defined and there was no regional lymphadenopathy.

A complete blood cell count revealed anemia (white blood cell count, 8070/μL [reference range, 4000–9,000/μL]; neutrophils, 77.1% [reference range, 44%–74%]; lymphocytes, 13.8% [reference range, 20%–50%]; hemoglobin, 9.3 g/dL [reference range, 13.0–17.0 g/dL]; and platelet count, 329×103/μL [reference range, 150–400×103/μL]). The C-reactive protein level was 7.3 mg/dL (reference range, 0.08–0.3 mg/dL). The creatinine level was 2.93 mg/dL (reference range, 0.6–1.2 mg/dL). There were no signs of liver dysfunction.
A blood culture was negative. A purified protein derivative (tuberculin) skin test was negative (6×7 mm [reference range, ≤9 mm). A chest computed tomography (CT) scan showed small centrilobular nodules that had not changed in number or size since evaluation 3 months prior.
The antibiotics were changed to meropenem hydrate 0.5 g and clindamycin 300 mg twice daily for presumed bacterial cellulitis, then meropenem hydrate 1 g and clindamycin 600 mg daily, but there was still no improvement after about 1 week. Therefore, a skin biopsy was performed on the left thigh. The specimen showed epithelioid cell granulomas throughout the dermis and subcutis (Figure 2). Ziehl-Neelsen stain revealed numerous acid-fast bacilli (Figure 3). Polymerase chain reaction was positive for Mycobacterium tuberculosis in the skin biopsy specimen and gastric fluid. Additionally, M tuberculosis was isolated from the skin biopsy specimen, gastric fluid, and sputum culture. After the series of treatments described above, a remarkable increase in nodule size and number was observed in a follow-up chest CT scan compared with the prior examination. These pulmonary lesions showed bronchogenic spread.


A diagnosis of tuberculous cellulitis with pulmonary tuberculosis was made. Treatment with isoniazid 200 mg once daily, rifampin 300 mg once daily, and ethambutol 500 mg once every other day was started; the dosages were reduced from the standard dose due to the patient’s CKD.1 Four days after initiation of these medications, the patient was transferred to a hospital specifically for the treatment of tuberculosis. Approximately 8 months after treatment with isoniazid, rifampin, and ethambutol, M tuberculosis could not be detected in the sputum and a chest CT revealed that the pulmonary lesions were remarkably improved. However, polymerase chain reaction of the skin biopsy specimen was still positive for M tuberculosis. It was determined that debridement of the skin lesion was needed, but the patient died from complications of deteriorating CKD 10 months after the initiation of the antituberculosis medications.
Comment
Cellulitis is a suppurative inflammation involving the subcutis.2 Local tender erythema, malaise, chills, and fever may be present at the onset. Cellulitis is commonly seen by dermatologists, and it is well known that other infectious diseases such as necrotizing fasciitis, cutaneous and subcutaneous mycoses,3 and nontuberculous mycobacterial infections4 sometimes present as cellulitislike skin lesions. Moreover, noninfectious diseases, such as Wells syndrome, thrombophlebitis, reactive angioendotheliomatosis,5 cutaneous metastasis of a primary malignancy,6 subcutaneous panniculitislike T-cell lymphoma,7 Langerhans cell histiocytosis,8 Sweet syndrome,9 adult-onset Still disease,10 and fixed drug eruption caused by acetaminophen11 should be excluded. These differential diagnoses and diagnostic clues of cellulitislike erythema are summarized in Table 1.3-16

Cutaneous tuberculosis presenting as cellulitis, so-called tuberculous cellulitis, also is characterized as a clinical mimicker of cellulitis. On the other hand, histologically, it has features of cutaneous tuberculosis (eg, necrotic granuloma).12,14,15 Tuberculous cellulitis is rare and therefore may often be misdiagnosed even in highly endemic areas. We summarized the clinical information of 5 well-documented cases of tuberculous cellulitis along with the current case in Table 2.12-16 All of these cases had an associated disease and involved patients who were currently taking oral corticosteroids. If a patient undergoing immunosuppressive therapy develops cellulitislike erythema, tuberculous cellulitis should be considered in the differential diagnosis.

Cutaneous tuberculosis generally is classified into 4 types according to the mechanism of disease acquisition: (1) inoculation from an exogenous source, (2) endogenous cutaneous spread contiguously or by autoinoculation, (3) hematogenous spread to the skin, and (4) tuberculids. In our case, it was suspected that the cellulitislike erythema may have been caused by hematogenous spread from pulmonary tuberculosis. Considering that negative reactions to purified protein derivative (tuberculin) skin tests often are observed in cases of miliary tuberculosis (widespread dissemination of M tuberculosis to 2 or more organs via hematogenous spread), we suspected that our patient could proceed to miliary tuberculosis; in fact, a case was reported in which miliary tuberculosis emerged approximately 3 weeks after the onset of erythema,13 as observed in the present case. Therefore, erythema in the setting of tuberculosis may be a predictor of miliary tuberculosis. The types of cutaneous lesions caused by tuberculosis infection also are dependent on multiple host factors.2 Cutaneous tuberculosis with an atypical clinical appearance has become more common because of the increasing number of immunocompromised patients.17
In addition, most cases of cutaneous tuberculosis are not associated with pain. Generally, tuberculous cellulitis also causes nontender erythematous plaques or nodules.2 However, in some cases of tuberculous cellulitis, including our case, tender skin lesions have been reported.12-14 Therefore, this symptom is not a sensitive factor for differential diagnosis.
We suggest that tuberculous cellulitis should always be included in the differential diagnosis of a cellulitislike rash with or without pain if the skin lesion is not improved despite antibiotic therapy.
- Daido-Horiuchi Y, Kikuchi Y, Kobayashi S, et al. Tuberculous cellulitis in a patient with chronic kidney disease and polymyalgia rheumatica. Intern Med. 2012;51:3203-3206.
- James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders Elsevier; 2011:322-329.
- Schupbach CW, Wheeler CE Jr, Briggaman RA, et al. Cutaneous manifestations of disseminated cryptococcosis. Arch Dermatol. 1976;112:1734-1740.
- Hsu PY, Yang YH, Hsiao CH, et al. Mycobacterium kansasii infection presenting as cellulitis in a patient with systemic lupus erythematosus. J Formos Med Assoc. 2002;101:581-584.
- Aguayo-Leiva I, Vano-Galván S, Salguero I, et al. Reactive angioendotheliomatosis in a patient with myelodysplastic syndrome presenting as a cellulitis-like plaque. Eur J Dermatol. 2009;19:182-183.
- Yang HI, Lee MC, Kuo TT, et al. Cellulitis-like cutaneous metastasis of uterine cervical carcinoma. J Am Acad Dermatol. 2007;56:S26-S28.
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitis-like T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106:S55-S59.
- Sharma PK, Sabhnani S, Bhardwaj M, et al. Acral, pure cutaneous, self-healing, late-onset, cellulitis-like Langerhans cell histiocytosis. J Cutan Med Surg. 2009;13:43-47.
- Tercedor J, Ródenas JM, Henraz MT, et al. Facial cellulitis-like Sweet’s syndrome in acute myelogenous leukemia. Int J Dermatol. 1992;31:598-599.
- Inaoki M, Nishijima C, Kumada S, et al. Adult-onset Still disease with a cellulitis-like eruption. Eur J Dermatol. 2009;19:80-81.
- Prabhu MM, Prabhu S, Mishra P, et al. Cellulitis-like fixed drug eruption attributed to paracetamol (acetaminophen). Dermatol Online J. 2005;11:24.
- Lee NH, Choi EH, Lee WS, et al. Tuberculous cellulitis. Clin Exp Dermatol. 2000;25:222-223.
- Kim JE, Ko JY, Bae SC, et al. Tuberculous cellulitis as a manifestation of miliary tuberculosis in a patient with malignancy-associated dermatomyositis. J Am Acad Dermatol. 2011;65:450-452.
- Chin PW, Koh CK, Wong KT. Cutaneous tuberculosis mimicking cellulitis in an immunosuppressed patient. Singapore Med J. 1999;40:44-45.
- Seyahi N, Apaydin S, Kahveci A, et al. Cellulitis as a manifestation of miliary tuberculosis in a renal transplant recipient. Transpl Infect Dis. 2005;7:80-85.
- Kato G, Watanabe K, Shibuya Y, et al. A case of cutaneous tuberculosis with cellulitis-like appearance [in Japanese]. Rinsho Hifuka (Jpn J Clin Dermatol). 2010;64:1055-1059.
- Fariña MC, Gegundez MI, Piqué E, et al. Cutaneous tuberculosis: a clinical, histopathologic, and bacteriologic study. J Am Acad Dermatol. 1995;33:433-440.
- Daido-Horiuchi Y, Kikuchi Y, Kobayashi S, et al. Tuberculous cellulitis in a patient with chronic kidney disease and polymyalgia rheumatica. Intern Med. 2012;51:3203-3206.
- James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders Elsevier; 2011:322-329.
- Schupbach CW, Wheeler CE Jr, Briggaman RA, et al. Cutaneous manifestations of disseminated cryptococcosis. Arch Dermatol. 1976;112:1734-1740.
- Hsu PY, Yang YH, Hsiao CH, et al. Mycobacterium kansasii infection presenting as cellulitis in a patient with systemic lupus erythematosus. J Formos Med Assoc. 2002;101:581-584.
- Aguayo-Leiva I, Vano-Galván S, Salguero I, et al. Reactive angioendotheliomatosis in a patient with myelodysplastic syndrome presenting as a cellulitis-like plaque. Eur J Dermatol. 2009;19:182-183.
- Yang HI, Lee MC, Kuo TT, et al. Cellulitis-like cutaneous metastasis of uterine cervical carcinoma. J Am Acad Dermatol. 2007;56:S26-S28.
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitis-like T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106:S55-S59.
- Sharma PK, Sabhnani S, Bhardwaj M, et al. Acral, pure cutaneous, self-healing, late-onset, cellulitis-like Langerhans cell histiocytosis. J Cutan Med Surg. 2009;13:43-47.
- Tercedor J, Ródenas JM, Henraz MT, et al. Facial cellulitis-like Sweet’s syndrome in acute myelogenous leukemia. Int J Dermatol. 1992;31:598-599.
- Inaoki M, Nishijima C, Kumada S, et al. Adult-onset Still disease with a cellulitis-like eruption. Eur J Dermatol. 2009;19:80-81.
- Prabhu MM, Prabhu S, Mishra P, et al. Cellulitis-like fixed drug eruption attributed to paracetamol (acetaminophen). Dermatol Online J. 2005;11:24.
- Lee NH, Choi EH, Lee WS, et al. Tuberculous cellulitis. Clin Exp Dermatol. 2000;25:222-223.
- Kim JE, Ko JY, Bae SC, et al. Tuberculous cellulitis as a manifestation of miliary tuberculosis in a patient with malignancy-associated dermatomyositis. J Am Acad Dermatol. 2011;65:450-452.
- Chin PW, Koh CK, Wong KT. Cutaneous tuberculosis mimicking cellulitis in an immunosuppressed patient. Singapore Med J. 1999;40:44-45.
- Seyahi N, Apaydin S, Kahveci A, et al. Cellulitis as a manifestation of miliary tuberculosis in a renal transplant recipient. Transpl Infect Dis. 2005;7:80-85.
- Kato G, Watanabe K, Shibuya Y, et al. A case of cutaneous tuberculosis with cellulitis-like appearance [in Japanese]. Rinsho Hifuka (Jpn J Clin Dermatol). 2010;64:1055-1059.
- Fariña MC, Gegundez MI, Piqué E, et al. Cutaneous tuberculosis: a clinical, histopathologic, and bacteriologic study. J Am Acad Dermatol. 1995;33:433-440.
Diabetic Peripheral Neuropathy: The Learning Curve
When R was a 19-year-old sailor heading out to sea, he had no idea of the forthcoming medical diagnosis that would change his life. R was like any other young seaman: ready to do his assigned tasks and ready to serve his country. He was stationed on a Los Angeles class, nuclear-powered, fast attack submarine. R was living his lifelong dream of serving in the U.S. Navy. The submarine was conducting sound trials and tactical readiness exams off the southeast U.S. coast near Bermuda. The days were long, but he loved what he was doing, so he ignored his symptoms of fatigue, attributing it to his busy schedule. He was enjoying his time in the navy and looking forward to a long career.
Diagnosis
R was assigned watch duty during the day, but he couldn’t understand why he felt so fatigued during his watch or his ability to fall asleep while standing. R didn’t complain because he knew everyone was working hard, long hours. He knew he was not sleeping well, mainly due to the frequent trips to the bathroom to urinate, and at first attributed it to drinking large amounts of coffee and sugar to stay awake during the drills. He also knew he was constantly hungry, thirsty, and tired. After falling asleep while on duty during the exercise, R found himself facing possible disciplinary action. He had no idea what was happening but realized it was not in his nature to fail at a task and certainly not to fall asleep on duty. Having a chronic disease that would affect him for the rest of his life was certainly not on his mind. He recalled, “At the time I didn’t even know what diabetes was.”
R finally admitted his array of symptoms to one of the corpsman. He often urinated every 20 minutes and at times did not make it to the bathroom. His vision was blurred to the point he could not make out faces just a few feet away from him, and the lethargy was overwhelming.
The corpsman immediately knew something was wrong with R and instructed R to report to the boat’s sick bay. Fortunately for R, the classic symptoms of hunger, thirst, frequent urination, and fatigue struck a chord with the corpsman who also noticed that R had lost a great amount of weight, a fact R had not noticed. Labs were drawn, and a urine specimen was obtained. R had a blood glucose level of > 1,000 mg/dL, was in severe ketoacidosis, and was diagnosed with type 1 diabetes mellitus (T1DM). The corpsman was surprised he was even coherent at this point. He was given IV infusions in both arms. The boat’s mission was halted. The immediate thought was to send for a medical evacuation helicopter. The weather conditions were too severe at the time to arrange for air evacuation, so the captain decided to head back to port and transfer R to the Portsmouth Naval Medical Center. R will never forget that day; however, the days and weeks following became somewhat of a blur. R recalls, “Time seemed to standstill some days, then others were on fast forward.” He was hospitalized for the next 2 weeks. His condition was stabilized, and he learned how to care for himself.
Learning About Diabetes
The following weeks and months while on medical hold and being processed for discharge, R was assigned a variety of duties. He felt well prepared to manage his disease on a daily basis and at first had hopes of continuing his navy career. He recalls now, he had no clue what the diagnosis would mean in the years to come. R learned he would eventually be medically retired from the navy and rejoined civilian life.
Initial Complications
After leaving the navy, R decided to become a law enforcement officer. He joined a local police department and quickly rose through the ranks. He began to settle into a routine, learning to manage his insulin, control his diet, and enjoy his new career. For the next several years, he experienced few complications, although he never regained the 50 pounds he had lost when he was first diagnosed. Around 25 years old, he began to notice pain in the bottom of his feet. He was still able to run, had great balance, and didn’t think his symptoms of sore feet were attributable to his diabetes. He did notice that without shoes on, his feet were extremely sensitive to any texture.
Over the next year, R experienced worsening pain and increased sensitivity in his feet. He started to spend more time in his patrol car instead of on foot patrol because of the pain. He was no longer able to enjoy one of his favorite pastimes, walking barefoot on the beach. During the next several years, R would gradually begin to realize he had no sensation in his feet. He noted this affected his balance and gait. He loved his career in law enforcement, but often the complications of his disease would impact his daily work. He felt he was no longer fulfilling his responsibilities as an officer because of his inability to complete daily assignments due to the neuropathy in his feet. He left his law enforcement career and spent most of his time in an office, which was much less taxing on his body.
Foot Ulcers
In 2011, 15 years after the T1DM diagnosis, R experienced his first foot wound. After a day of hiking and walking in creek beds, he realized he had essentially rubbed off the skin on the ball of his foot. He cleaned it like he normally would; however, the area failed to heal. He developed a hard callus around the wound, but the center remained open. At the time, he did not realize the significance of this type of wound for a diabetic patient.
The foot ulcer was discovered while in the emergency department for an unrelated issue. It was then he was referred to the Greenville VA Outpatient Clinic wound healing center in South Carolina for further treatment. At 36 years old, he was far younger than most of the veterans being treated for diabetic foot ulcers. Per the CDC Report Card, about 90% to 95% of patients with diabetes have type 2 diabetes mellitus (T2DM).1,2 Most persons diagnosed with diabetes are in the fifth and sixth decades of life.1,2 For R, patient education had consisted of learning to manage his diet and insulin therapy. He has no recollection of education about future complications and reported feeling “clueless” about the potential complications of foot ulcerations.
During the patient’s first visit to the wound healing center, R was educated about diabetic foot health, complications, the healing process, and the importance of diabetes management. The center is staffed by a nurse practitioner (NP) certified in wound care with extensive experience in diabetic foot ulcers and by several wound care nurses. Each staff member incorporates patient education and positive reinforcement into every patient visit. According to Jeffrey Frenchman, DPM, director of limb preservation at the Atlanta VAMC in Georgia, “Patient education and positive reinforcement cost nothing to provide and offer great return on patient adherence.” (Jeffrey Frenchman, April 12, 2014, oral interview).
R visited the center once or twice weekly, depending on the appearance of the wound and the type of treatment he was receiving. He noted that having frequent contact with the wound center staff made him feel as though he was making progress. For the staff, ensuring R could adhere to the treatment regimen was paramount. If a patient is unable to follow home care instructions or lacks understanding of the importance of following wound care instructions, then the likelihood of adherence is less.
Continued Complications
R was unprepared for the months of healing. He learned about the importance of offloading (the reduction of pressure), noting that during the weeks he spent more time on his feet, ulcer healing failed to progress or worsened.3 Eventually, the ulcer healed, and he felt better prepared to prevent future problems as a result of having been educated about foot care. Unfortunately, he experienced his next complication a few months later after wearing new boots. When removing his boots at the end of the workday, he noticed blood on his sock. He realized the boots had caused blisters that had ruptured on the third, fourth, and fifth toes. Once again, having T1DM and totally insensate feet caused further problems with delayed healing. Since his first foot ulcer in 2011, R continued to have problems with foot ulcers. Some ulcers were caused by shoe pressure, blisters from hot beach sand, or from a typical neuropathic foot ulcer, which first develops as a preulcerative callus and rapidly progresses to an ulcer. Despite his daily astute monitoring of his feet he noted, “Problems just seem to occur overnight.”
Quality of Life
The greatest impact of diabetes for R was on his quality of life (QOL). He noted that the frustrations of dealing with foot wounds had a profound negative impact on QOL. As an avid outdoor enthusiast, the months he spent on crutches, wearing off-loading shoes, attending numerous wound clinic visits, and being unable to take part in the activities he loved greatly impacted his mental and physical well-being. “Having to change my daily routine such as bathing, driving, and even going out to dinner is hard enough. Having to give up hiking, camping, and swimming changes my entire outlook on life.” R also noted the unintended isolation from friends had a profound impact on his feelings. “They want to include you, but know they can’t. You want to go, but know you can’t keep up. Sometimes being alone is the worst feeling.”
Receiving care from wound care professionals offered R hope that his wounds would heal and he would return to the activities he enjoyed. He noted that the education and support he received from the wound center staff made him feel more confident not only in caring for current wounds, but also in preventing wounds in the future. He also realized that prompt treatment for even the smallest of wounds was essential.
R was able to contact the wound center staff either by phone or by secure messaging e-mail anytime he had a concern or question. When he developed new foot wounds, he could contact the staff and be evaluated within 72 hours of notification. He noted that being able to talk with the staff as soon as a problem developed offered him reassurance that he was properly taking care of his feet.
During his treatment, R needed to wear offloading shoes to minimize the weight-bearing pressure.3 The wound center staff took care to ensure that R could ambulate safely with these shoes and avoid further injury. They also reinforced the importance of wearing these shoes, despite their unfashionable appearance.
Given the depth of some of R’s foot ulcers, the staff used negative pressure dressings to enhance healing. Negative pressure dressings provide a vacuum source to create continuous or intermittent negative pressure inside a wound to remove fluid, exudates, and infectious materials and prepare the wound for healing and closure. A mechanically powered, negative pressure dressing with a 125 mm Hg cartridge device was used during R’s treatment. This type of negative pressure dressing offered the benefit of dressing changes twice weekly vs 3 times weekly with other electric-powered negative pressure devices.4
Another important aspect of R’s care was the use of human amniotic tissue allografts. When R’s wounds did not show healing progression during the first 4 weeks of traditional treatment, amniotic tissue allografts were added to his plan of care. This type of product for the wound bed provides critical growth factors and collagen to promote effective, enhanced wound healing. Patient education again is critical when using human amniotic tissue allografts so that the patient learns to keep dressings intact and undisturbed.5
Future Implications
Diabetic foot ulcers are a preventable complication of diabetic peripheral neuropathy. Patient education about foot health should not only be incorporated in diabetic education, but also reinforced by the health care staff at each visit. When a patient presents with a diabetic foot ulcer, early, prompt treatment is vital to ensure a favorable outcome.
For health care providers, cognizance of the impact that wounds have on patients’ QOL is an essential aspect of care. Identification of factors that promote expedient and effective wound healing is vital. Patient education that is focused on engaging the patient to actively participate in the healing process is paramount. Involving R in every aspect of his care was the focus of the wound center staff. Explaining the purpose of each product used and why it was chosen was not only interesting for R, but also allowed him to actively participate in his appointments and care. As the leader of the wound treatment team, the NP may order, guide, and direct care, but empowering patients to be active participants in their care enhances adherence to the plan of care.6
Conclusion
Focusing on these critical aspects of patient-centered wound healing must be at the forefront when treating patients with diabetic foot ulcers. Although a price cannot be placed on QOL, the cost of diabetic foot ulcers and its complications is astounding. In 2007, nearly $116 billion was spent on diabetes treatment, and more than one-third was for the care of diabetic foot ulcers and complications from those ulcers.7
Finally, the incidence of T2DM is rising: The average age of patients at the onset of T2DM is becoming younger, the development of T2DM in children is rising, and treatment costs are rising.1 Given the alarming statistics of T2DM and its complications in the U.S., focusing on prevention, patient education, and effective treatment of diabetic foot ulcers is important.
1. Diabetes Report Card 2014. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf. Published 2012. Accessed June 1, 2016.
2. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Published 2012. Accessed June 1, 2016.
3. Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31(11):2118-2119. Armstrong DG, Marston WA, Reyzelman AM. Kirsner RS.
4. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20(3):332-341.
5. Zelen CM, Serena TE, Fetterolf DE. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: a long-term follow-up study. Wound Med. 2014;4:1-4.
6. Boulton AJ, Kirsner RS, Vileikyte L. Clinical Practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004;351(1):48-55.
7. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3)(suppl):17S-22S.
When R was a 19-year-old sailor heading out to sea, he had no idea of the forthcoming medical diagnosis that would change his life. R was like any other young seaman: ready to do his assigned tasks and ready to serve his country. He was stationed on a Los Angeles class, nuclear-powered, fast attack submarine. R was living his lifelong dream of serving in the U.S. Navy. The submarine was conducting sound trials and tactical readiness exams off the southeast U.S. coast near Bermuda. The days were long, but he loved what he was doing, so he ignored his symptoms of fatigue, attributing it to his busy schedule. He was enjoying his time in the navy and looking forward to a long career.
Diagnosis
R was assigned watch duty during the day, but he couldn’t understand why he felt so fatigued during his watch or his ability to fall asleep while standing. R didn’t complain because he knew everyone was working hard, long hours. He knew he was not sleeping well, mainly due to the frequent trips to the bathroom to urinate, and at first attributed it to drinking large amounts of coffee and sugar to stay awake during the drills. He also knew he was constantly hungry, thirsty, and tired. After falling asleep while on duty during the exercise, R found himself facing possible disciplinary action. He had no idea what was happening but realized it was not in his nature to fail at a task and certainly not to fall asleep on duty. Having a chronic disease that would affect him for the rest of his life was certainly not on his mind. He recalled, “At the time I didn’t even know what diabetes was.”
R finally admitted his array of symptoms to one of the corpsman. He often urinated every 20 minutes and at times did not make it to the bathroom. His vision was blurred to the point he could not make out faces just a few feet away from him, and the lethargy was overwhelming.
The corpsman immediately knew something was wrong with R and instructed R to report to the boat’s sick bay. Fortunately for R, the classic symptoms of hunger, thirst, frequent urination, and fatigue struck a chord with the corpsman who also noticed that R had lost a great amount of weight, a fact R had not noticed. Labs were drawn, and a urine specimen was obtained. R had a blood glucose level of > 1,000 mg/dL, was in severe ketoacidosis, and was diagnosed with type 1 diabetes mellitus (T1DM). The corpsman was surprised he was even coherent at this point. He was given IV infusions in both arms. The boat’s mission was halted. The immediate thought was to send for a medical evacuation helicopter. The weather conditions were too severe at the time to arrange for air evacuation, so the captain decided to head back to port and transfer R to the Portsmouth Naval Medical Center. R will never forget that day; however, the days and weeks following became somewhat of a blur. R recalls, “Time seemed to standstill some days, then others were on fast forward.” He was hospitalized for the next 2 weeks. His condition was stabilized, and he learned how to care for himself.
Learning About Diabetes
The following weeks and months while on medical hold and being processed for discharge, R was assigned a variety of duties. He felt well prepared to manage his disease on a daily basis and at first had hopes of continuing his navy career. He recalls now, he had no clue what the diagnosis would mean in the years to come. R learned he would eventually be medically retired from the navy and rejoined civilian life.
Initial Complications
After leaving the navy, R decided to become a law enforcement officer. He joined a local police department and quickly rose through the ranks. He began to settle into a routine, learning to manage his insulin, control his diet, and enjoy his new career. For the next several years, he experienced few complications, although he never regained the 50 pounds he had lost when he was first diagnosed. Around 25 years old, he began to notice pain in the bottom of his feet. He was still able to run, had great balance, and didn’t think his symptoms of sore feet were attributable to his diabetes. He did notice that without shoes on, his feet were extremely sensitive to any texture.
Over the next year, R experienced worsening pain and increased sensitivity in his feet. He started to spend more time in his patrol car instead of on foot patrol because of the pain. He was no longer able to enjoy one of his favorite pastimes, walking barefoot on the beach. During the next several years, R would gradually begin to realize he had no sensation in his feet. He noted this affected his balance and gait. He loved his career in law enforcement, but often the complications of his disease would impact his daily work. He felt he was no longer fulfilling his responsibilities as an officer because of his inability to complete daily assignments due to the neuropathy in his feet. He left his law enforcement career and spent most of his time in an office, which was much less taxing on his body.
Foot Ulcers
In 2011, 15 years after the T1DM diagnosis, R experienced his first foot wound. After a day of hiking and walking in creek beds, he realized he had essentially rubbed off the skin on the ball of his foot. He cleaned it like he normally would; however, the area failed to heal. He developed a hard callus around the wound, but the center remained open. At the time, he did not realize the significance of this type of wound for a diabetic patient.
The foot ulcer was discovered while in the emergency department for an unrelated issue. It was then he was referred to the Greenville VA Outpatient Clinic wound healing center in South Carolina for further treatment. At 36 years old, he was far younger than most of the veterans being treated for diabetic foot ulcers. Per the CDC Report Card, about 90% to 95% of patients with diabetes have type 2 diabetes mellitus (T2DM).1,2 Most persons diagnosed with diabetes are in the fifth and sixth decades of life.1,2 For R, patient education had consisted of learning to manage his diet and insulin therapy. He has no recollection of education about future complications and reported feeling “clueless” about the potential complications of foot ulcerations.
During the patient’s first visit to the wound healing center, R was educated about diabetic foot health, complications, the healing process, and the importance of diabetes management. The center is staffed by a nurse practitioner (NP) certified in wound care with extensive experience in diabetic foot ulcers and by several wound care nurses. Each staff member incorporates patient education and positive reinforcement into every patient visit. According to Jeffrey Frenchman, DPM, director of limb preservation at the Atlanta VAMC in Georgia, “Patient education and positive reinforcement cost nothing to provide and offer great return on patient adherence.” (Jeffrey Frenchman, April 12, 2014, oral interview).
R visited the center once or twice weekly, depending on the appearance of the wound and the type of treatment he was receiving. He noted that having frequent contact with the wound center staff made him feel as though he was making progress. For the staff, ensuring R could adhere to the treatment regimen was paramount. If a patient is unable to follow home care instructions or lacks understanding of the importance of following wound care instructions, then the likelihood of adherence is less.
Continued Complications
R was unprepared for the months of healing. He learned about the importance of offloading (the reduction of pressure), noting that during the weeks he spent more time on his feet, ulcer healing failed to progress or worsened.3 Eventually, the ulcer healed, and he felt better prepared to prevent future problems as a result of having been educated about foot care. Unfortunately, he experienced his next complication a few months later after wearing new boots. When removing his boots at the end of the workday, he noticed blood on his sock. He realized the boots had caused blisters that had ruptured on the third, fourth, and fifth toes. Once again, having T1DM and totally insensate feet caused further problems with delayed healing. Since his first foot ulcer in 2011, R continued to have problems with foot ulcers. Some ulcers were caused by shoe pressure, blisters from hot beach sand, or from a typical neuropathic foot ulcer, which first develops as a preulcerative callus and rapidly progresses to an ulcer. Despite his daily astute monitoring of his feet he noted, “Problems just seem to occur overnight.”
Quality of Life
The greatest impact of diabetes for R was on his quality of life (QOL). He noted that the frustrations of dealing with foot wounds had a profound negative impact on QOL. As an avid outdoor enthusiast, the months he spent on crutches, wearing off-loading shoes, attending numerous wound clinic visits, and being unable to take part in the activities he loved greatly impacted his mental and physical well-being. “Having to change my daily routine such as bathing, driving, and even going out to dinner is hard enough. Having to give up hiking, camping, and swimming changes my entire outlook on life.” R also noted the unintended isolation from friends had a profound impact on his feelings. “They want to include you, but know they can’t. You want to go, but know you can’t keep up. Sometimes being alone is the worst feeling.”
Receiving care from wound care professionals offered R hope that his wounds would heal and he would return to the activities he enjoyed. He noted that the education and support he received from the wound center staff made him feel more confident not only in caring for current wounds, but also in preventing wounds in the future. He also realized that prompt treatment for even the smallest of wounds was essential.
R was able to contact the wound center staff either by phone or by secure messaging e-mail anytime he had a concern or question. When he developed new foot wounds, he could contact the staff and be evaluated within 72 hours of notification. He noted that being able to talk with the staff as soon as a problem developed offered him reassurance that he was properly taking care of his feet.
During his treatment, R needed to wear offloading shoes to minimize the weight-bearing pressure.3 The wound center staff took care to ensure that R could ambulate safely with these shoes and avoid further injury. They also reinforced the importance of wearing these shoes, despite their unfashionable appearance.
Given the depth of some of R’s foot ulcers, the staff used negative pressure dressings to enhance healing. Negative pressure dressings provide a vacuum source to create continuous or intermittent negative pressure inside a wound to remove fluid, exudates, and infectious materials and prepare the wound for healing and closure. A mechanically powered, negative pressure dressing with a 125 mm Hg cartridge device was used during R’s treatment. This type of negative pressure dressing offered the benefit of dressing changes twice weekly vs 3 times weekly with other electric-powered negative pressure devices.4
Another important aspect of R’s care was the use of human amniotic tissue allografts. When R’s wounds did not show healing progression during the first 4 weeks of traditional treatment, amniotic tissue allografts were added to his plan of care. This type of product for the wound bed provides critical growth factors and collagen to promote effective, enhanced wound healing. Patient education again is critical when using human amniotic tissue allografts so that the patient learns to keep dressings intact and undisturbed.5
Future Implications
Diabetic foot ulcers are a preventable complication of diabetic peripheral neuropathy. Patient education about foot health should not only be incorporated in diabetic education, but also reinforced by the health care staff at each visit. When a patient presents with a diabetic foot ulcer, early, prompt treatment is vital to ensure a favorable outcome.
For health care providers, cognizance of the impact that wounds have on patients’ QOL is an essential aspect of care. Identification of factors that promote expedient and effective wound healing is vital. Patient education that is focused on engaging the patient to actively participate in the healing process is paramount. Involving R in every aspect of his care was the focus of the wound center staff. Explaining the purpose of each product used and why it was chosen was not only interesting for R, but also allowed him to actively participate in his appointments and care. As the leader of the wound treatment team, the NP may order, guide, and direct care, but empowering patients to be active participants in their care enhances adherence to the plan of care.6
Conclusion
Focusing on these critical aspects of patient-centered wound healing must be at the forefront when treating patients with diabetic foot ulcers. Although a price cannot be placed on QOL, the cost of diabetic foot ulcers and its complications is astounding. In 2007, nearly $116 billion was spent on diabetes treatment, and more than one-third was for the care of diabetic foot ulcers and complications from those ulcers.7
Finally, the incidence of T2DM is rising: The average age of patients at the onset of T2DM is becoming younger, the development of T2DM in children is rising, and treatment costs are rising.1 Given the alarming statistics of T2DM and its complications in the U.S., focusing on prevention, patient education, and effective treatment of diabetic foot ulcers is important.
When R was a 19-year-old sailor heading out to sea, he had no idea of the forthcoming medical diagnosis that would change his life. R was like any other young seaman: ready to do his assigned tasks and ready to serve his country. He was stationed on a Los Angeles class, nuclear-powered, fast attack submarine. R was living his lifelong dream of serving in the U.S. Navy. The submarine was conducting sound trials and tactical readiness exams off the southeast U.S. coast near Bermuda. The days were long, but he loved what he was doing, so he ignored his symptoms of fatigue, attributing it to his busy schedule. He was enjoying his time in the navy and looking forward to a long career.
Diagnosis
R was assigned watch duty during the day, but he couldn’t understand why he felt so fatigued during his watch or his ability to fall asleep while standing. R didn’t complain because he knew everyone was working hard, long hours. He knew he was not sleeping well, mainly due to the frequent trips to the bathroom to urinate, and at first attributed it to drinking large amounts of coffee and sugar to stay awake during the drills. He also knew he was constantly hungry, thirsty, and tired. After falling asleep while on duty during the exercise, R found himself facing possible disciplinary action. He had no idea what was happening but realized it was not in his nature to fail at a task and certainly not to fall asleep on duty. Having a chronic disease that would affect him for the rest of his life was certainly not on his mind. He recalled, “At the time I didn’t even know what diabetes was.”
R finally admitted his array of symptoms to one of the corpsman. He often urinated every 20 minutes and at times did not make it to the bathroom. His vision was blurred to the point he could not make out faces just a few feet away from him, and the lethargy was overwhelming.
The corpsman immediately knew something was wrong with R and instructed R to report to the boat’s sick bay. Fortunately for R, the classic symptoms of hunger, thirst, frequent urination, and fatigue struck a chord with the corpsman who also noticed that R had lost a great amount of weight, a fact R had not noticed. Labs were drawn, and a urine specimen was obtained. R had a blood glucose level of > 1,000 mg/dL, was in severe ketoacidosis, and was diagnosed with type 1 diabetes mellitus (T1DM). The corpsman was surprised he was even coherent at this point. He was given IV infusions in both arms. The boat’s mission was halted. The immediate thought was to send for a medical evacuation helicopter. The weather conditions were too severe at the time to arrange for air evacuation, so the captain decided to head back to port and transfer R to the Portsmouth Naval Medical Center. R will never forget that day; however, the days and weeks following became somewhat of a blur. R recalls, “Time seemed to standstill some days, then others were on fast forward.” He was hospitalized for the next 2 weeks. His condition was stabilized, and he learned how to care for himself.
Learning About Diabetes
The following weeks and months while on medical hold and being processed for discharge, R was assigned a variety of duties. He felt well prepared to manage his disease on a daily basis and at first had hopes of continuing his navy career. He recalls now, he had no clue what the diagnosis would mean in the years to come. R learned he would eventually be medically retired from the navy and rejoined civilian life.
Initial Complications
After leaving the navy, R decided to become a law enforcement officer. He joined a local police department and quickly rose through the ranks. He began to settle into a routine, learning to manage his insulin, control his diet, and enjoy his new career. For the next several years, he experienced few complications, although he never regained the 50 pounds he had lost when he was first diagnosed. Around 25 years old, he began to notice pain in the bottom of his feet. He was still able to run, had great balance, and didn’t think his symptoms of sore feet were attributable to his diabetes. He did notice that without shoes on, his feet were extremely sensitive to any texture.
Over the next year, R experienced worsening pain and increased sensitivity in his feet. He started to spend more time in his patrol car instead of on foot patrol because of the pain. He was no longer able to enjoy one of his favorite pastimes, walking barefoot on the beach. During the next several years, R would gradually begin to realize he had no sensation in his feet. He noted this affected his balance and gait. He loved his career in law enforcement, but often the complications of his disease would impact his daily work. He felt he was no longer fulfilling his responsibilities as an officer because of his inability to complete daily assignments due to the neuropathy in his feet. He left his law enforcement career and spent most of his time in an office, which was much less taxing on his body.
Foot Ulcers
In 2011, 15 years after the T1DM diagnosis, R experienced his first foot wound. After a day of hiking and walking in creek beds, he realized he had essentially rubbed off the skin on the ball of his foot. He cleaned it like he normally would; however, the area failed to heal. He developed a hard callus around the wound, but the center remained open. At the time, he did not realize the significance of this type of wound for a diabetic patient.
The foot ulcer was discovered while in the emergency department for an unrelated issue. It was then he was referred to the Greenville VA Outpatient Clinic wound healing center in South Carolina for further treatment. At 36 years old, he was far younger than most of the veterans being treated for diabetic foot ulcers. Per the CDC Report Card, about 90% to 95% of patients with diabetes have type 2 diabetes mellitus (T2DM).1,2 Most persons diagnosed with diabetes are in the fifth and sixth decades of life.1,2 For R, patient education had consisted of learning to manage his diet and insulin therapy. He has no recollection of education about future complications and reported feeling “clueless” about the potential complications of foot ulcerations.
During the patient’s first visit to the wound healing center, R was educated about diabetic foot health, complications, the healing process, and the importance of diabetes management. The center is staffed by a nurse practitioner (NP) certified in wound care with extensive experience in diabetic foot ulcers and by several wound care nurses. Each staff member incorporates patient education and positive reinforcement into every patient visit. According to Jeffrey Frenchman, DPM, director of limb preservation at the Atlanta VAMC in Georgia, “Patient education and positive reinforcement cost nothing to provide and offer great return on patient adherence.” (Jeffrey Frenchman, April 12, 2014, oral interview).
R visited the center once or twice weekly, depending on the appearance of the wound and the type of treatment he was receiving. He noted that having frequent contact with the wound center staff made him feel as though he was making progress. For the staff, ensuring R could adhere to the treatment regimen was paramount. If a patient is unable to follow home care instructions or lacks understanding of the importance of following wound care instructions, then the likelihood of adherence is less.
Continued Complications
R was unprepared for the months of healing. He learned about the importance of offloading (the reduction of pressure), noting that during the weeks he spent more time on his feet, ulcer healing failed to progress or worsened.3 Eventually, the ulcer healed, and he felt better prepared to prevent future problems as a result of having been educated about foot care. Unfortunately, he experienced his next complication a few months later after wearing new boots. When removing his boots at the end of the workday, he noticed blood on his sock. He realized the boots had caused blisters that had ruptured on the third, fourth, and fifth toes. Once again, having T1DM and totally insensate feet caused further problems with delayed healing. Since his first foot ulcer in 2011, R continued to have problems with foot ulcers. Some ulcers were caused by shoe pressure, blisters from hot beach sand, or from a typical neuropathic foot ulcer, which first develops as a preulcerative callus and rapidly progresses to an ulcer. Despite his daily astute monitoring of his feet he noted, “Problems just seem to occur overnight.”
Quality of Life
The greatest impact of diabetes for R was on his quality of life (QOL). He noted that the frustrations of dealing with foot wounds had a profound negative impact on QOL. As an avid outdoor enthusiast, the months he spent on crutches, wearing off-loading shoes, attending numerous wound clinic visits, and being unable to take part in the activities he loved greatly impacted his mental and physical well-being. “Having to change my daily routine such as bathing, driving, and even going out to dinner is hard enough. Having to give up hiking, camping, and swimming changes my entire outlook on life.” R also noted the unintended isolation from friends had a profound impact on his feelings. “They want to include you, but know they can’t. You want to go, but know you can’t keep up. Sometimes being alone is the worst feeling.”
Receiving care from wound care professionals offered R hope that his wounds would heal and he would return to the activities he enjoyed. He noted that the education and support he received from the wound center staff made him feel more confident not only in caring for current wounds, but also in preventing wounds in the future. He also realized that prompt treatment for even the smallest of wounds was essential.
R was able to contact the wound center staff either by phone or by secure messaging e-mail anytime he had a concern or question. When he developed new foot wounds, he could contact the staff and be evaluated within 72 hours of notification. He noted that being able to talk with the staff as soon as a problem developed offered him reassurance that he was properly taking care of his feet.
During his treatment, R needed to wear offloading shoes to minimize the weight-bearing pressure.3 The wound center staff took care to ensure that R could ambulate safely with these shoes and avoid further injury. They also reinforced the importance of wearing these shoes, despite their unfashionable appearance.
Given the depth of some of R’s foot ulcers, the staff used negative pressure dressings to enhance healing. Negative pressure dressings provide a vacuum source to create continuous or intermittent negative pressure inside a wound to remove fluid, exudates, and infectious materials and prepare the wound for healing and closure. A mechanically powered, negative pressure dressing with a 125 mm Hg cartridge device was used during R’s treatment. This type of negative pressure dressing offered the benefit of dressing changes twice weekly vs 3 times weekly with other electric-powered negative pressure devices.4
Another important aspect of R’s care was the use of human amniotic tissue allografts. When R’s wounds did not show healing progression during the first 4 weeks of traditional treatment, amniotic tissue allografts were added to his plan of care. This type of product for the wound bed provides critical growth factors and collagen to promote effective, enhanced wound healing. Patient education again is critical when using human amniotic tissue allografts so that the patient learns to keep dressings intact and undisturbed.5
Future Implications
Diabetic foot ulcers are a preventable complication of diabetic peripheral neuropathy. Patient education about foot health should not only be incorporated in diabetic education, but also reinforced by the health care staff at each visit. When a patient presents with a diabetic foot ulcer, early, prompt treatment is vital to ensure a favorable outcome.
For health care providers, cognizance of the impact that wounds have on patients’ QOL is an essential aspect of care. Identification of factors that promote expedient and effective wound healing is vital. Patient education that is focused on engaging the patient to actively participate in the healing process is paramount. Involving R in every aspect of his care was the focus of the wound center staff. Explaining the purpose of each product used and why it was chosen was not only interesting for R, but also allowed him to actively participate in his appointments and care. As the leader of the wound treatment team, the NP may order, guide, and direct care, but empowering patients to be active participants in their care enhances adherence to the plan of care.6
Conclusion
Focusing on these critical aspects of patient-centered wound healing must be at the forefront when treating patients with diabetic foot ulcers. Although a price cannot be placed on QOL, the cost of diabetic foot ulcers and its complications is astounding. In 2007, nearly $116 billion was spent on diabetes treatment, and more than one-third was for the care of diabetic foot ulcers and complications from those ulcers.7
Finally, the incidence of T2DM is rising: The average age of patients at the onset of T2DM is becoming younger, the development of T2DM in children is rising, and treatment costs are rising.1 Given the alarming statistics of T2DM and its complications in the U.S., focusing on prevention, patient education, and effective treatment of diabetic foot ulcers is important.
1. Diabetes Report Card 2014. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf. Published 2012. Accessed June 1, 2016.
2. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Published 2012. Accessed June 1, 2016.
3. Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31(11):2118-2119. Armstrong DG, Marston WA, Reyzelman AM. Kirsner RS.
4. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20(3):332-341.
5. Zelen CM, Serena TE, Fetterolf DE. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: a long-term follow-up study. Wound Med. 2014;4:1-4.
6. Boulton AJ, Kirsner RS, Vileikyte L. Clinical Practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004;351(1):48-55.
7. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3)(suppl):17S-22S.
1. Diabetes Report Card 2014. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf. Published 2012. Accessed June 1, 2016.
2. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Published 2012. Accessed June 1, 2016.
3. Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31(11):2118-2119. Armstrong DG, Marston WA, Reyzelman AM. Kirsner RS.
4. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20(3):332-341.
5. Zelen CM, Serena TE, Fetterolf DE. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: a long-term follow-up study. Wound Med. 2014;4:1-4.
6. Boulton AJ, Kirsner RS, Vileikyte L. Clinical Practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004;351(1):48-55.
7. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3)(suppl):17S-22S.
Primary Cutaneous Dermal Mucinosis on Herpes Zoster Scars
Mucin is an amorphous gelatinous substance that is found in a large variety of tissues. There are 2 types of cutaneous mucin: dermal and epithelial. Both types appear as basophilic shreds and granules with hematoxylin and eosin stain.1 Epithelial mucin (sialomucin) is found mainly in the gastrointestinal tract and lungs. In the skin, it is present in the cytoplasm of the dark cells of the eccrine glands and in the apocrine secretory cells. Epithelial mucin contains both neutral and acid glycosaminoglycans, stains positive with Alcian blue (pH 2.5) and periodic acid–Schiff, is resistant to hyaluronidase, and does not stain metachromatically with toluidine blue. Dermal mucin is composed of acid glycosaminoglycans (eg, dermatan sulfate, chondroitin 6-sulfate, chondroitin 4-sulfate, hyaluronic acid) and normally is produced by dermal fibroblasts. Dermal mucin stains positive with Alcian blue (pH 2.5); is periodic acid–Schiff negative and sensitive to hyaluronidase; and shows metachromasia with toluidine blue, methylene blue, and thionine.
Cutaneous mucinosis comprises a heterogeneous group of skin disorders characterized by the deposition of mucin in the interstices of the dermis. These diseases may be classified as primary mucinosis with the mucin deposition as the main histologic feature resulting in clinically distinctive lesions and secondary mucinosis with the mucin deposition as an additional histologic finding within the context of an independent skin disease or lesion (eg, basal cell carcinoma) with deposits of mucin in the stroma. Primary cutaneous mucinosis may be subclassified into 2 groups: degenerative-inflammatory mucinoses and neoplastic-hamartomatous mucinoses. According to the histologic features, the degenerative-inflammatory mucinoses are better divided into dermal and follicular mucinoses.2 We describe a case of primary cutaneous dermal mucinosis on herpes zoster (HZ) scars as an isotopic response.
Case Report
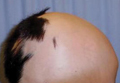
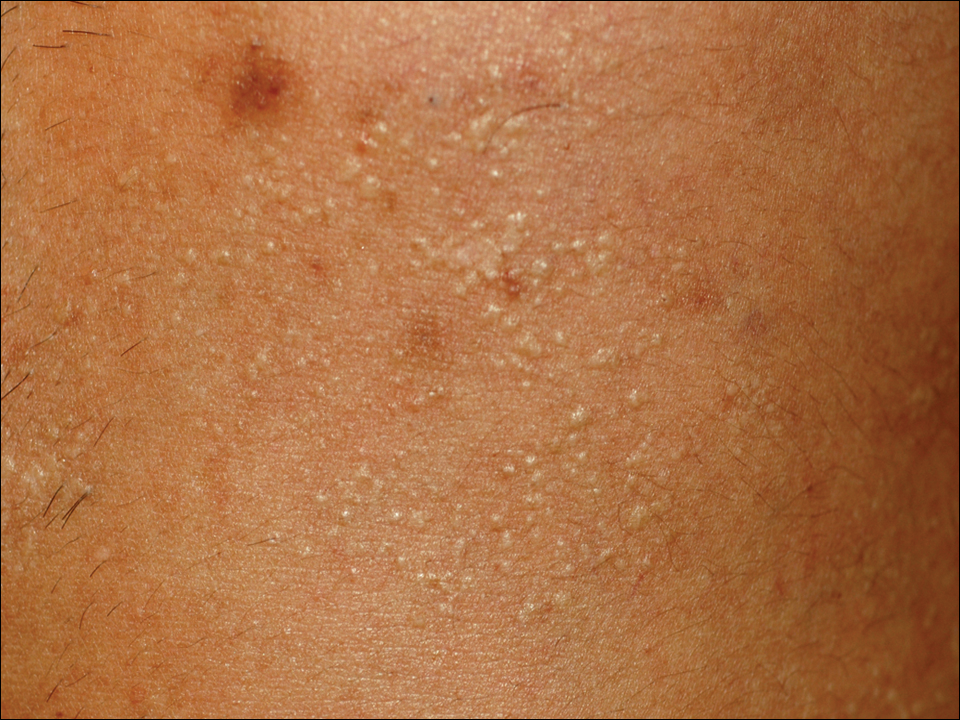
A 33-year-old man presented to the dermatology department with slightly pruritic lesions on the left side of the chest and back that had appeared progressively at the site of HZ scars that had healed without treatment 9 months prior. Dermatologic examination revealed sharply defined whitish papules (Figure 1) measuring 2 to 4 mm in diameter with a smooth surface and linear distribution over the area of the left T8 and T9 dermatomes. The patient reported no postherpetic neuralgia and was otherwise healthy. Laboratory tests including a complete blood cell count, biochemistry, urinalysis, and determination of free thyroid hormones were within reference range. Serologic tests for human immunodeficiency virus, hepatitis B and C viruses, and syphilis were negative. Antinuclear antibodies also were negative.
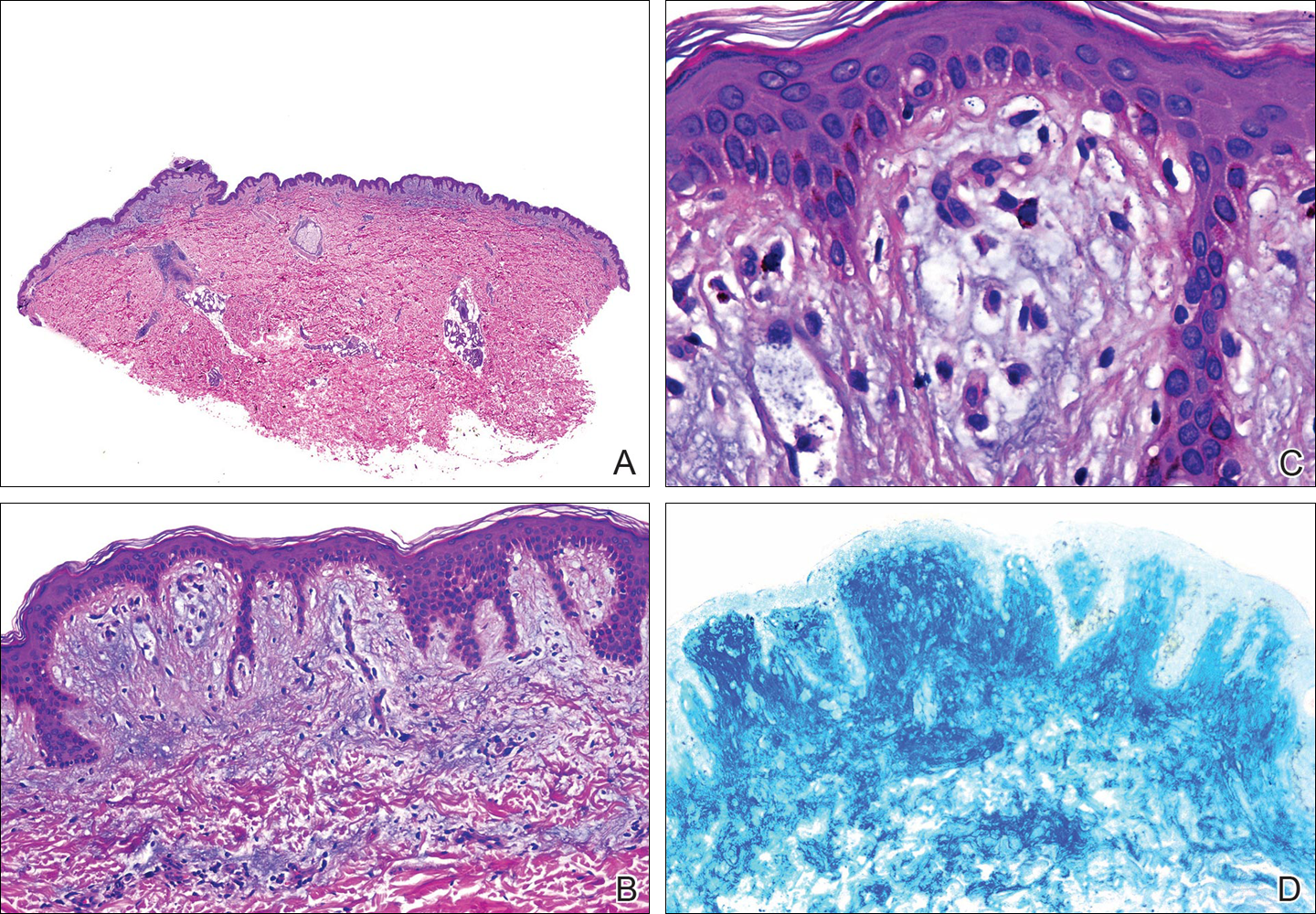
Histopathology demonstrated abundant bluish granular material between collagen bundles of the papillary dermis (Figure 2). No cytopathologic signs of active herpetic infection were seen. The Alcian blue stain at pH 2.5 was strongly positive for mucin, which confirmed the diagnosis of primary cutaneous dermal mucinosis.
Topical corticosteroids were applied for 2 months with no notable improvement. The lesions gradually improved without any other therapy during the subsequent 6 months.
Comment
The occurrence of a new skin disease at the exact site of a prior unrelated cutaneous disorder that had already resolved was first reported by Wyburn-Mason3 in 1955. Forty years later, the term isotopic response was coined by Wolf et al4 to describe this phenomenon. Diverse types of skin diseases such as herpes simplex virus,5 varicella-zoster infections,4 and thrombophlebitis4 have been implicated in cases of isotopic response, but the most frequently associated primary disorder by far is cutaneous HZ.
Several benign and malignant disorders may occur at sites of resolved HZ lesions, including granulomatous dermatitis,6 granuloma annulare,7 fungal granuloma,8 fungal folliculitis,9 psoriasis,10 morphea,11 lichen sclerosus,12 Kaposi sarcoma,13 the lichenoid variant of chronic graft-versus-host disease,14 cutaneous sarcoidosis,15 granulomatous folliculitis,16 comedones,17 furuncles,18 erythema annulare centrifugum,19 eosinophilic dermatosis,20 cutaneous pseudolymphoma,21 granulomatous vasculitis,22 Rosai-Dorfman disease,12 xanthomatous changes,23 tuberculoid granulomas,24 acneform eruption,25 lichen planus,26 acquired reactive perforating collagenosis,27 lymphoma,28 leukemia,29 angiosarcoma,30 basal cell carcinoma,31 squamous cell carcinoma, and cutaneous metastasis from internal carcinoma.32 The interval between the acute HZ episode and presentation of the second disease is quite variable, ranging from days to several months. Postzoster isotopic response has been described in individuals with varying degrees of immune response, affecting both immunocompetent12 and immunocompromised patients.14 There is no predilection for age, sex, or race. It also seems that antiviral treatment during the active episode does not prevent the development of secondary reactions.Kim et al33 reported a 59-year-old woman who developed flesh-colored or erythematous papules on HZ scars over the area of the left T1 and T2 dermatomes 1 week after the active viral process. Histopathologic study demonstrated deposition of mucin between collagen bundles in the dermis. The authors established the diagnosis of secondary cutaneous mucinosis as an isotopic response.33 Nevertheless, we believe that based on the aforementioned classification of cutaneous mucinosis,2 both this case and our case are better considered as primary cutaneous dermal mucinosis, as the mucin deposition in the dermis was the main histologic finding resulting in a distinctive cutaneous disorder. In the case reported by Kim et al,33 a possible relationship between cutaneous mucinosis and postherpetic neuralgia was suggested based on the slow regression of skin lesions in accordance with the improvement of the neuralgic pain; however, our patient did not have postherpetic neuralgia and the lesions persisted unchanged several months after the acute HZ episode. In the literature, there are reports of primary cutaneous dermal mucinosis associated with altered thyroid function34; autoimmune connective tissue diseases, mostly lupus erythematosus35; monoclonal gammopathy36; and human immunodeficiency virus infection,37 but these possibilities were ruled out in our patient by pertinent laboratory studies.
The pathogenesis of the postherpetic isotopic response remains unknown, but several mechanisms have been proposed. Some authors have suggested that postzoster dermatoses may represent isomorphic response of Köbner phenomenon.13,15 Although isomorphic and isotopic responses share some similarities, these terms describe 2 different phenomena: the first refers to the appearance of the same cutaneous disorder at a different site favored by trauma, while the second manifests a new and unrelated disease at the same location.38 Local anatomic changes such as altered microcirculation, collagen rearrangement, and an imperfect skin barrier may promote a prolonged local inflammatory response. Moreover, the destruction of nerve fibers by the varicella-zoster virus may indirectly influence the local immune system through the release of specific neuropeptides in the skin.39 It has been speculated that some secondary reactions may be the result of type III and type IV hypersensitivity reactions40 to viral antigens or to tissue antigens modified by the virus, inducing either immune hypersensitivity or local immune suppression.41 Some authors have documented the presence of varicella-zoster DNA within early postzoster lesions6,7 by using polymerase chain reaction in early lesions but not in late-stage and residual lesions.12,22 Nikkels et al42 studied early granulomatous lesions by immunohistochemistry and in situ hybridization techniques and concluded that major viral envelope glycoproteins (glycoproteins I and II) rather than complete viral particles could be responsible for delayed-type hypersensitivity reactions. All these findings suggest that secondary reactions presenting on HZ scars are mainly the result of atypical immune reactions to local antigenic stimuli.
The pathogenesis of our case is unknown. From a theoretical point of view, it is possible that varicella-zoster virus may induce fibroblastic proliferation and mucin production on HZ scars; however, if HZ is a frequent process and the virus may induce mucin production, then focal dermal mucinosis in an HZ scar should be a common finding. In our patient, there was no associated disease favoring the development of the cutaneous mucinosis. These localized variants of primary cutaneous mucinosis usually do not require therapy, and a wait-and-see approach is recommended. Topical applications of corticosteroids, pimecrolimus, or tacrolimus, as well as oral isotretinoin, may have some benefit,43 but spontaneous resolution may occur.44 In our patient, topical corticosteroids were applied 2 months following initial presentation without any benefit and the cutaneous lesions gradually improved without any therapy during the subsequent 6 months. Focal dermal mucinosis should be added to the list of cutaneous reactions that may develop in HZ scars.
- Truhan AP, Roenigk HH Jr. The cutaneous mucinoses. J Am Acad Dermatol. 1986;14:1-18.
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. BMJ. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Ruocco E. Genital warts at the site of healed herpes progenitalis: the isotopic response. Int J Dermatol. 2000;39:705-706.
- Serfling U, Penneys NS, Zhu WY, et al. Varicella-zoster virus DNA in granulomatous skin lesions following herpes zoster. a study by the polymerase chain reaction. J Cutan Pathol. 1993;20:28-33.
- Gibney MD, Nahass GT, Leonardi CL. Cutaneous reactions following herpes zoster infections: report of three cases and a review of the literature. Br J Dermatol. 1996;134:504-509.
- Huang CW, Tu ME, Wu YH, et al. Isotopic response of fungal granuloma following facial herpes zoster infections-report of three cases. Int J Dermatol. 2007;46:1141-1145.
- Tüzün Y, Işçimen A, Göksügür N, et al. Wolf’s isotopic response: Trichophyton rubrum folliculitis appearing on a herpes zoster scar. Int J Dermatol. 2000;39:766-768.
- Allegue F, Fachal C, Romo M, et al. Psoriasis at the site of healed herpes zoster: Wolf’s isotopic response. Actas Dermosifiliogr. 2007;98:576-578.
- Forschner A, Metzler G, Rassner G, et al. Morphea with features of lichen sclerosus et atrophicus at the site of a herpes zoster scar: another case of an isotopic response. Int J Dermatol. 2005;44:524-525.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Niedt GW, Prioleau PG. Kaposi’s sarcoma occurring in a dermatome previously involved by herpes zoster. J Am Acad Dermatol. 1988;18:448-451.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Cecchi R, Giomi A. Scar sarcoidosis following herpes zoster. J Eur Acad Dermatol Venereol. 1999;12:280-282.
- Fernández-Redondo V, Amrouni B, Varela E, et al. Granulomatous folliculitis at sites of herpes zoster scars: Wolf’s isotopic response. J Eur Acad Dermatol Venereol. 2002;16:628-630.
- Sanchez-Salas MP. Appearance of comedones at the site of healed herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2011;50:633-634.
- Ghorpade A. Wolf’s isotopic response—furuncles at the site of healed herpes zoster in an Indian male. Int J Dermatol. 2010;49:105-107.
- Lee HW, Lee DK, Rhee DY, et al. Erythema annulare centrifugum following herpes zoster infection: Wolf’s isotopic response? Br J Dermatol. 2005;153:1241-1243.
- Mitsuhashi Y, Kondo S. Post-zoster eosinophilic dermatosis. Br J Dermatol. 1997;136:465-466.
- Roo E, Villegas C, Lopez-Bran E, et al. Postzoster cutaneous pseudolymphoma. Arch Dermatol. 1994;130:661-663.
- Langenberg A, Yen TS, LeBoit PE. Granulomatous vasculitis occurring after cutaneous herpes zoster despite absence of viral genome. J Am Acad Dermatol. 1991;24:429-433.
- Weidman F, Boston LN. Generalized xanthoma tuberosum with xantomathous changes in fresh scars of intercurrent zoster. Arch Intern Med. 1937;59:793-822.
- Olalquiaga J, Minaño R, Barrio J. Granuloma tuberculoide post-herpético en un paciente con leucemia linfocítica crónica. Med Cutan ILA. 1995;23:113-115.
- Stubbings JM, Goodfield MJ. An unusual distribution of an acneiform rash due to herpes zoster infection. Clin Exp Dermatol. 1993;18:92-93.
- Shemer A, Weiss G, Trau H. Wolf’s isotopic response: a case of zosteriform lichen planus on the site of healed herpes zoster. J Eur Acad Dermatol Venereol. 2001;15:445-447.
- Bang SW, Kim YK, Whang KU. Acquired reactive perforating collagenosis: unilateral umbilicated papules along the lesions of herpes zoster. J Am Acad Dermatol. 1997;36:778-779.
- Paydaş S, Sahin B, Yavuz S, et al. Lymphomatous skin infiltration at the site of previous varicella zoster virus infection in a patient with T cell lymphoma. Leuk Lymphoma. 2000;37:229-232.
- Cerroni L, Kerl H. Cutaneous localization of B-cell chronic lymphocytic leukemia at the site of varicella/herpes virus eruptions. J Am Acad Dermatol. 1997;37:1022.
- Hudson CP, Hanno R, Callen JP. Cutaneous angiosarcoma in a site of healed herpes zoster. Int J Dermatol. 1984;23:404-407.
- Wyburn-Mason R. Visceral lesions in herpes zoster. Br Med J. 1957;1:678-681.
- Caroti A. Metastasi cutanee di a adenocarcinoma papillifero ovarico in sede di herpes zoster. Chron Dermatol. 1987;18:769-773.
- Kim MB, Jwa SW, Ko HC, et al. A case of secondary cutaneous mucinosis following herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2009;48:212-214.
- Burman KD, McKinley-Grant L. Dermatologic aspects of thyroid disease. Clin Dermatol. 2006;24:247-255.
- Shekari AM, Ghiasi M, Ghasemi E, et al. Papulonodular mucinosis indicating systemic lupus erythematosus. Clin Exp Dermatol. 2009;34:558-560.
- Dinneen AM, Dicken CH. Scleromyxedema. J Am Acad Dermatol. 1995;33:37-43.
- Rongioletti F, Ghigliotti G, De Marchi R, et al. Cutaneous mucinoses and HIV infection. Br J Dermatol. 1998;139:1077-1080.
- Krahl D, Hartschuh W, Tilgen W. Granuloma annulare perforans in herpes zoster scars. J Am Acad Dermatol. 1993;29:859-862.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- Fisher G, Jaworski R. Granuloma formation in herpes zoster scars. J Am Acad Dermatol. 1987;16:1261-1263.
- Ruocco V, Grimaldi Filioli F. La risposta isotopica post-erpetica: possibile sequela di un locus minoris resistentiae acquisito. G Ital Dermatol Venereol. 1999;134:547-552.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;5:530-532.
- Kwon OS, Moon SE, Kim JA, et al. Lichen myxodematosus with rapid spontaneous regression. Br J Dermatol. 1997;136:295-296.
Mucin is an amorphous gelatinous substance that is found in a large variety of tissues. There are 2 types of cutaneous mucin: dermal and epithelial. Both types appear as basophilic shreds and granules with hematoxylin and eosin stain.1 Epithelial mucin (sialomucin) is found mainly in the gastrointestinal tract and lungs. In the skin, it is present in the cytoplasm of the dark cells of the eccrine glands and in the apocrine secretory cells. Epithelial mucin contains both neutral and acid glycosaminoglycans, stains positive with Alcian blue (pH 2.5) and periodic acid–Schiff, is resistant to hyaluronidase, and does not stain metachromatically with toluidine blue. Dermal mucin is composed of acid glycosaminoglycans (eg, dermatan sulfate, chondroitin 6-sulfate, chondroitin 4-sulfate, hyaluronic acid) and normally is produced by dermal fibroblasts. Dermal mucin stains positive with Alcian blue (pH 2.5); is periodic acid–Schiff negative and sensitive to hyaluronidase; and shows metachromasia with toluidine blue, methylene blue, and thionine.
Cutaneous mucinosis comprises a heterogeneous group of skin disorders characterized by the deposition of mucin in the interstices of the dermis. These diseases may be classified as primary mucinosis with the mucin deposition as the main histologic feature resulting in clinically distinctive lesions and secondary mucinosis with the mucin deposition as an additional histologic finding within the context of an independent skin disease or lesion (eg, basal cell carcinoma) with deposits of mucin in the stroma. Primary cutaneous mucinosis may be subclassified into 2 groups: degenerative-inflammatory mucinoses and neoplastic-hamartomatous mucinoses. According to the histologic features, the degenerative-inflammatory mucinoses are better divided into dermal and follicular mucinoses.2 We describe a case of primary cutaneous dermal mucinosis on herpes zoster (HZ) scars as an isotopic response.
Case Report
A 33-year-old man presented to the dermatology department with slightly pruritic lesions on the left side of the chest and back that had appeared progressively at the site of HZ scars that had healed without treatment 9 months prior. Dermatologic examination revealed sharply defined whitish papules (Figure 1) measuring 2 to 4 mm in diameter with a smooth surface and linear distribution over the area of the left T8 and T9 dermatomes. The patient reported no postherpetic neuralgia and was otherwise healthy. Laboratory tests including a complete blood cell count, biochemistry, urinalysis, and determination of free thyroid hormones were within reference range. Serologic tests for human immunodeficiency virus, hepatitis B and C viruses, and syphilis were negative. Antinuclear antibodies also were negative.

Histopathology demonstrated abundant bluish granular material between collagen bundles of the papillary dermis (Figure 2). No cytopathologic signs of active herpetic infection were seen. The Alcian blue stain at pH 2.5 was strongly positive for mucin, which confirmed the diagnosis of primary cutaneous dermal mucinosis.
Topical corticosteroids were applied for 2 months with no notable improvement. The lesions gradually improved without any other therapy during the subsequent 6 months.

Comment
The occurrence of a new skin disease at the exact site of a prior unrelated cutaneous disorder that had already resolved was first reported by Wyburn-Mason3 in 1955. Forty years later, the term isotopic response was coined by Wolf et al4 to describe this phenomenon. Diverse types of skin diseases such as herpes simplex virus,5 varicella-zoster infections,4 and thrombophlebitis4 have been implicated in cases of isotopic response, but the most frequently associated primary disorder by far is cutaneous HZ.
Several benign and malignant disorders may occur at sites of resolved HZ lesions, including granulomatous dermatitis,6 granuloma annulare,7 fungal granuloma,8 fungal folliculitis,9 psoriasis,10 morphea,11 lichen sclerosus,12 Kaposi sarcoma,13 the lichenoid variant of chronic graft-versus-host disease,14 cutaneous sarcoidosis,15 granulomatous folliculitis,16 comedones,17 furuncles,18 erythema annulare centrifugum,19 eosinophilic dermatosis,20 cutaneous pseudolymphoma,21 granulomatous vasculitis,22 Rosai-Dorfman disease,12 xanthomatous changes,23 tuberculoid granulomas,24 acneform eruption,25 lichen planus,26 acquired reactive perforating collagenosis,27 lymphoma,28 leukemia,29 angiosarcoma,30 basal cell carcinoma,31 squamous cell carcinoma, and cutaneous metastasis from internal carcinoma.32 The interval between the acute HZ episode and presentation of the second disease is quite variable, ranging from days to several months. Postzoster isotopic response has been described in individuals with varying degrees of immune response, affecting both immunocompetent12 and immunocompromised patients.14 There is no predilection for age, sex, or race. It also seems that antiviral treatment during the active episode does not prevent the development of secondary reactions.Kim et al33 reported a 59-year-old woman who developed flesh-colored or erythematous papules on HZ scars over the area of the left T1 and T2 dermatomes 1 week after the active viral process. Histopathologic study demonstrated deposition of mucin between collagen bundles in the dermis. The authors established the diagnosis of secondary cutaneous mucinosis as an isotopic response.33 Nevertheless, we believe that based on the aforementioned classification of cutaneous mucinosis,2 both this case and our case are better considered as primary cutaneous dermal mucinosis, as the mucin deposition in the dermis was the main histologic finding resulting in a distinctive cutaneous disorder. In the case reported by Kim et al,33 a possible relationship between cutaneous mucinosis and postherpetic neuralgia was suggested based on the slow regression of skin lesions in accordance with the improvement of the neuralgic pain; however, our patient did not have postherpetic neuralgia and the lesions persisted unchanged several months after the acute HZ episode. In the literature, there are reports of primary cutaneous dermal mucinosis associated with altered thyroid function34; autoimmune connective tissue diseases, mostly lupus erythematosus35; monoclonal gammopathy36; and human immunodeficiency virus infection,37 but these possibilities were ruled out in our patient by pertinent laboratory studies.
The pathogenesis of the postherpetic isotopic response remains unknown, but several mechanisms have been proposed. Some authors have suggested that postzoster dermatoses may represent isomorphic response of Köbner phenomenon.13,15 Although isomorphic and isotopic responses share some similarities, these terms describe 2 different phenomena: the first refers to the appearance of the same cutaneous disorder at a different site favored by trauma, while the second manifests a new and unrelated disease at the same location.38 Local anatomic changes such as altered microcirculation, collagen rearrangement, and an imperfect skin barrier may promote a prolonged local inflammatory response. Moreover, the destruction of nerve fibers by the varicella-zoster virus may indirectly influence the local immune system through the release of specific neuropeptides in the skin.39 It has been speculated that some secondary reactions may be the result of type III and type IV hypersensitivity reactions40 to viral antigens or to tissue antigens modified by the virus, inducing either immune hypersensitivity or local immune suppression.41 Some authors have documented the presence of varicella-zoster DNA within early postzoster lesions6,7 by using polymerase chain reaction in early lesions but not in late-stage and residual lesions.12,22 Nikkels et al42 studied early granulomatous lesions by immunohistochemistry and in situ hybridization techniques and concluded that major viral envelope glycoproteins (glycoproteins I and II) rather than complete viral particles could be responsible for delayed-type hypersensitivity reactions. All these findings suggest that secondary reactions presenting on HZ scars are mainly the result of atypical immune reactions to local antigenic stimuli.
The pathogenesis of our case is unknown. From a theoretical point of view, it is possible that varicella-zoster virus may induce fibroblastic proliferation and mucin production on HZ scars; however, if HZ is a frequent process and the virus may induce mucin production, then focal dermal mucinosis in an HZ scar should be a common finding. In our patient, there was no associated disease favoring the development of the cutaneous mucinosis. These localized variants of primary cutaneous mucinosis usually do not require therapy, and a wait-and-see approach is recommended. Topical applications of corticosteroids, pimecrolimus, or tacrolimus, as well as oral isotretinoin, may have some benefit,43 but spontaneous resolution may occur.44 In our patient, topical corticosteroids were applied 2 months following initial presentation without any benefit and the cutaneous lesions gradually improved without any therapy during the subsequent 6 months. Focal dermal mucinosis should be added to the list of cutaneous reactions that may develop in HZ scars.
Mucin is an amorphous gelatinous substance that is found in a large variety of tissues. There are 2 types of cutaneous mucin: dermal and epithelial. Both types appear as basophilic shreds and granules with hematoxylin and eosin stain.1 Epithelial mucin (sialomucin) is found mainly in the gastrointestinal tract and lungs. In the skin, it is present in the cytoplasm of the dark cells of the eccrine glands and in the apocrine secretory cells. Epithelial mucin contains both neutral and acid glycosaminoglycans, stains positive with Alcian blue (pH 2.5) and periodic acid–Schiff, is resistant to hyaluronidase, and does not stain metachromatically with toluidine blue. Dermal mucin is composed of acid glycosaminoglycans (eg, dermatan sulfate, chondroitin 6-sulfate, chondroitin 4-sulfate, hyaluronic acid) and normally is produced by dermal fibroblasts. Dermal mucin stains positive with Alcian blue (pH 2.5); is periodic acid–Schiff negative and sensitive to hyaluronidase; and shows metachromasia with toluidine blue, methylene blue, and thionine.
Cutaneous mucinosis comprises a heterogeneous group of skin disorders characterized by the deposition of mucin in the interstices of the dermis. These diseases may be classified as primary mucinosis with the mucin deposition as the main histologic feature resulting in clinically distinctive lesions and secondary mucinosis with the mucin deposition as an additional histologic finding within the context of an independent skin disease or lesion (eg, basal cell carcinoma) with deposits of mucin in the stroma. Primary cutaneous mucinosis may be subclassified into 2 groups: degenerative-inflammatory mucinoses and neoplastic-hamartomatous mucinoses. According to the histologic features, the degenerative-inflammatory mucinoses are better divided into dermal and follicular mucinoses.2 We describe a case of primary cutaneous dermal mucinosis on herpes zoster (HZ) scars as an isotopic response.
Case Report
A 33-year-old man presented to the dermatology department with slightly pruritic lesions on the left side of the chest and back that had appeared progressively at the site of HZ scars that had healed without treatment 9 months prior. Dermatologic examination revealed sharply defined whitish papules (Figure 1) measuring 2 to 4 mm in diameter with a smooth surface and linear distribution over the area of the left T8 and T9 dermatomes. The patient reported no postherpetic neuralgia and was otherwise healthy. Laboratory tests including a complete blood cell count, biochemistry, urinalysis, and determination of free thyroid hormones were within reference range. Serologic tests for human immunodeficiency virus, hepatitis B and C viruses, and syphilis were negative. Antinuclear antibodies also were negative.

Histopathology demonstrated abundant bluish granular material between collagen bundles of the papillary dermis (Figure 2). No cytopathologic signs of active herpetic infection were seen. The Alcian blue stain at pH 2.5 was strongly positive for mucin, which confirmed the diagnosis of primary cutaneous dermal mucinosis.
Topical corticosteroids were applied for 2 months with no notable improvement. The lesions gradually improved without any other therapy during the subsequent 6 months.

Comment
The occurrence of a new skin disease at the exact site of a prior unrelated cutaneous disorder that had already resolved was first reported by Wyburn-Mason3 in 1955. Forty years later, the term isotopic response was coined by Wolf et al4 to describe this phenomenon. Diverse types of skin diseases such as herpes simplex virus,5 varicella-zoster infections,4 and thrombophlebitis4 have been implicated in cases of isotopic response, but the most frequently associated primary disorder by far is cutaneous HZ.
Several benign and malignant disorders may occur at sites of resolved HZ lesions, including granulomatous dermatitis,6 granuloma annulare,7 fungal granuloma,8 fungal folliculitis,9 psoriasis,10 morphea,11 lichen sclerosus,12 Kaposi sarcoma,13 the lichenoid variant of chronic graft-versus-host disease,14 cutaneous sarcoidosis,15 granulomatous folliculitis,16 comedones,17 furuncles,18 erythema annulare centrifugum,19 eosinophilic dermatosis,20 cutaneous pseudolymphoma,21 granulomatous vasculitis,22 Rosai-Dorfman disease,12 xanthomatous changes,23 tuberculoid granulomas,24 acneform eruption,25 lichen planus,26 acquired reactive perforating collagenosis,27 lymphoma,28 leukemia,29 angiosarcoma,30 basal cell carcinoma,31 squamous cell carcinoma, and cutaneous metastasis from internal carcinoma.32 The interval between the acute HZ episode and presentation of the second disease is quite variable, ranging from days to several months. Postzoster isotopic response has been described in individuals with varying degrees of immune response, affecting both immunocompetent12 and immunocompromised patients.14 There is no predilection for age, sex, or race. It also seems that antiviral treatment during the active episode does not prevent the development of secondary reactions.Kim et al33 reported a 59-year-old woman who developed flesh-colored or erythematous papules on HZ scars over the area of the left T1 and T2 dermatomes 1 week after the active viral process. Histopathologic study demonstrated deposition of mucin between collagen bundles in the dermis. The authors established the diagnosis of secondary cutaneous mucinosis as an isotopic response.33 Nevertheless, we believe that based on the aforementioned classification of cutaneous mucinosis,2 both this case and our case are better considered as primary cutaneous dermal mucinosis, as the mucin deposition in the dermis was the main histologic finding resulting in a distinctive cutaneous disorder. In the case reported by Kim et al,33 a possible relationship between cutaneous mucinosis and postherpetic neuralgia was suggested based on the slow regression of skin lesions in accordance with the improvement of the neuralgic pain; however, our patient did not have postherpetic neuralgia and the lesions persisted unchanged several months after the acute HZ episode. In the literature, there are reports of primary cutaneous dermal mucinosis associated with altered thyroid function34; autoimmune connective tissue diseases, mostly lupus erythematosus35; monoclonal gammopathy36; and human immunodeficiency virus infection,37 but these possibilities were ruled out in our patient by pertinent laboratory studies.
The pathogenesis of the postherpetic isotopic response remains unknown, but several mechanisms have been proposed. Some authors have suggested that postzoster dermatoses may represent isomorphic response of Köbner phenomenon.13,15 Although isomorphic and isotopic responses share some similarities, these terms describe 2 different phenomena: the first refers to the appearance of the same cutaneous disorder at a different site favored by trauma, while the second manifests a new and unrelated disease at the same location.38 Local anatomic changes such as altered microcirculation, collagen rearrangement, and an imperfect skin barrier may promote a prolonged local inflammatory response. Moreover, the destruction of nerve fibers by the varicella-zoster virus may indirectly influence the local immune system through the release of specific neuropeptides in the skin.39 It has been speculated that some secondary reactions may be the result of type III and type IV hypersensitivity reactions40 to viral antigens or to tissue antigens modified by the virus, inducing either immune hypersensitivity or local immune suppression.41 Some authors have documented the presence of varicella-zoster DNA within early postzoster lesions6,7 by using polymerase chain reaction in early lesions but not in late-stage and residual lesions.12,22 Nikkels et al42 studied early granulomatous lesions by immunohistochemistry and in situ hybridization techniques and concluded that major viral envelope glycoproteins (glycoproteins I and II) rather than complete viral particles could be responsible for delayed-type hypersensitivity reactions. All these findings suggest that secondary reactions presenting on HZ scars are mainly the result of atypical immune reactions to local antigenic stimuli.
The pathogenesis of our case is unknown. From a theoretical point of view, it is possible that varicella-zoster virus may induce fibroblastic proliferation and mucin production on HZ scars; however, if HZ is a frequent process and the virus may induce mucin production, then focal dermal mucinosis in an HZ scar should be a common finding. In our patient, there was no associated disease favoring the development of the cutaneous mucinosis. These localized variants of primary cutaneous mucinosis usually do not require therapy, and a wait-and-see approach is recommended. Topical applications of corticosteroids, pimecrolimus, or tacrolimus, as well as oral isotretinoin, may have some benefit,43 but spontaneous resolution may occur.44 In our patient, topical corticosteroids were applied 2 months following initial presentation without any benefit and the cutaneous lesions gradually improved without any therapy during the subsequent 6 months. Focal dermal mucinosis should be added to the list of cutaneous reactions that may develop in HZ scars.
- Truhan AP, Roenigk HH Jr. The cutaneous mucinoses. J Am Acad Dermatol. 1986;14:1-18.
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. BMJ. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Ruocco E. Genital warts at the site of healed herpes progenitalis: the isotopic response. Int J Dermatol. 2000;39:705-706.
- Serfling U, Penneys NS, Zhu WY, et al. Varicella-zoster virus DNA in granulomatous skin lesions following herpes zoster. a study by the polymerase chain reaction. J Cutan Pathol. 1993;20:28-33.
- Gibney MD, Nahass GT, Leonardi CL. Cutaneous reactions following herpes zoster infections: report of three cases and a review of the literature. Br J Dermatol. 1996;134:504-509.
- Huang CW, Tu ME, Wu YH, et al. Isotopic response of fungal granuloma following facial herpes zoster infections-report of three cases. Int J Dermatol. 2007;46:1141-1145.
- Tüzün Y, Işçimen A, Göksügür N, et al. Wolf’s isotopic response: Trichophyton rubrum folliculitis appearing on a herpes zoster scar. Int J Dermatol. 2000;39:766-768.
- Allegue F, Fachal C, Romo M, et al. Psoriasis at the site of healed herpes zoster: Wolf’s isotopic response. Actas Dermosifiliogr. 2007;98:576-578.
- Forschner A, Metzler G, Rassner G, et al. Morphea with features of lichen sclerosus et atrophicus at the site of a herpes zoster scar: another case of an isotopic response. Int J Dermatol. 2005;44:524-525.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Niedt GW, Prioleau PG. Kaposi’s sarcoma occurring in a dermatome previously involved by herpes zoster. J Am Acad Dermatol. 1988;18:448-451.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Cecchi R, Giomi A. Scar sarcoidosis following herpes zoster. J Eur Acad Dermatol Venereol. 1999;12:280-282.
- Fernández-Redondo V, Amrouni B, Varela E, et al. Granulomatous folliculitis at sites of herpes zoster scars: Wolf’s isotopic response. J Eur Acad Dermatol Venereol. 2002;16:628-630.
- Sanchez-Salas MP. Appearance of comedones at the site of healed herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2011;50:633-634.
- Ghorpade A. Wolf’s isotopic response—furuncles at the site of healed herpes zoster in an Indian male. Int J Dermatol. 2010;49:105-107.
- Lee HW, Lee DK, Rhee DY, et al. Erythema annulare centrifugum following herpes zoster infection: Wolf’s isotopic response? Br J Dermatol. 2005;153:1241-1243.
- Mitsuhashi Y, Kondo S. Post-zoster eosinophilic dermatosis. Br J Dermatol. 1997;136:465-466.
- Roo E, Villegas C, Lopez-Bran E, et al. Postzoster cutaneous pseudolymphoma. Arch Dermatol. 1994;130:661-663.
- Langenberg A, Yen TS, LeBoit PE. Granulomatous vasculitis occurring after cutaneous herpes zoster despite absence of viral genome. J Am Acad Dermatol. 1991;24:429-433.
- Weidman F, Boston LN. Generalized xanthoma tuberosum with xantomathous changes in fresh scars of intercurrent zoster. Arch Intern Med. 1937;59:793-822.
- Olalquiaga J, Minaño R, Barrio J. Granuloma tuberculoide post-herpético en un paciente con leucemia linfocítica crónica. Med Cutan ILA. 1995;23:113-115.
- Stubbings JM, Goodfield MJ. An unusual distribution of an acneiform rash due to herpes zoster infection. Clin Exp Dermatol. 1993;18:92-93.
- Shemer A, Weiss G, Trau H. Wolf’s isotopic response: a case of zosteriform lichen planus on the site of healed herpes zoster. J Eur Acad Dermatol Venereol. 2001;15:445-447.
- Bang SW, Kim YK, Whang KU. Acquired reactive perforating collagenosis: unilateral umbilicated papules along the lesions of herpes zoster. J Am Acad Dermatol. 1997;36:778-779.
- Paydaş S, Sahin B, Yavuz S, et al. Lymphomatous skin infiltration at the site of previous varicella zoster virus infection in a patient with T cell lymphoma. Leuk Lymphoma. 2000;37:229-232.
- Cerroni L, Kerl H. Cutaneous localization of B-cell chronic lymphocytic leukemia at the site of varicella/herpes virus eruptions. J Am Acad Dermatol. 1997;37:1022.
- Hudson CP, Hanno R, Callen JP. Cutaneous angiosarcoma in a site of healed herpes zoster. Int J Dermatol. 1984;23:404-407.
- Wyburn-Mason R. Visceral lesions in herpes zoster. Br Med J. 1957;1:678-681.
- Caroti A. Metastasi cutanee di a adenocarcinoma papillifero ovarico in sede di herpes zoster. Chron Dermatol. 1987;18:769-773.
- Kim MB, Jwa SW, Ko HC, et al. A case of secondary cutaneous mucinosis following herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2009;48:212-214.
- Burman KD, McKinley-Grant L. Dermatologic aspects of thyroid disease. Clin Dermatol. 2006;24:247-255.
- Shekari AM, Ghiasi M, Ghasemi E, et al. Papulonodular mucinosis indicating systemic lupus erythematosus. Clin Exp Dermatol. 2009;34:558-560.
- Dinneen AM, Dicken CH. Scleromyxedema. J Am Acad Dermatol. 1995;33:37-43.
- Rongioletti F, Ghigliotti G, De Marchi R, et al. Cutaneous mucinoses and HIV infection. Br J Dermatol. 1998;139:1077-1080.
- Krahl D, Hartschuh W, Tilgen W. Granuloma annulare perforans in herpes zoster scars. J Am Acad Dermatol. 1993;29:859-862.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- Fisher G, Jaworski R. Granuloma formation in herpes zoster scars. J Am Acad Dermatol. 1987;16:1261-1263.
- Ruocco V, Grimaldi Filioli F. La risposta isotopica post-erpetica: possibile sequela di un locus minoris resistentiae acquisito. G Ital Dermatol Venereol. 1999;134:547-552.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;5:530-532.
- Kwon OS, Moon SE, Kim JA, et al. Lichen myxodematosus with rapid spontaneous regression. Br J Dermatol. 1997;136:295-296.
- Truhan AP, Roenigk HH Jr. The cutaneous mucinoses. J Am Acad Dermatol. 1986;14:1-18.
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. BMJ. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Ruocco E. Genital warts at the site of healed herpes progenitalis: the isotopic response. Int J Dermatol. 2000;39:705-706.
- Serfling U, Penneys NS, Zhu WY, et al. Varicella-zoster virus DNA in granulomatous skin lesions following herpes zoster. a study by the polymerase chain reaction. J Cutan Pathol. 1993;20:28-33.
- Gibney MD, Nahass GT, Leonardi CL. Cutaneous reactions following herpes zoster infections: report of three cases and a review of the literature. Br J Dermatol. 1996;134:504-509.
- Huang CW, Tu ME, Wu YH, et al. Isotopic response of fungal granuloma following facial herpes zoster infections-report of three cases. Int J Dermatol. 2007;46:1141-1145.
- Tüzün Y, Işçimen A, Göksügür N, et al. Wolf’s isotopic response: Trichophyton rubrum folliculitis appearing on a herpes zoster scar. Int J Dermatol. 2000;39:766-768.
- Allegue F, Fachal C, Romo M, et al. Psoriasis at the site of healed herpes zoster: Wolf’s isotopic response. Actas Dermosifiliogr. 2007;98:576-578.
- Forschner A, Metzler G, Rassner G, et al. Morphea with features of lichen sclerosus et atrophicus at the site of a herpes zoster scar: another case of an isotopic response. Int J Dermatol. 2005;44:524-525.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Niedt GW, Prioleau PG. Kaposi’s sarcoma occurring in a dermatome previously involved by herpes zoster. J Am Acad Dermatol. 1988;18:448-451.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Cecchi R, Giomi A. Scar sarcoidosis following herpes zoster. J Eur Acad Dermatol Venereol. 1999;12:280-282.
- Fernández-Redondo V, Amrouni B, Varela E, et al. Granulomatous folliculitis at sites of herpes zoster scars: Wolf’s isotopic response. J Eur Acad Dermatol Venereol. 2002;16:628-630.
- Sanchez-Salas MP. Appearance of comedones at the site of healed herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2011;50:633-634.
- Ghorpade A. Wolf’s isotopic response—furuncles at the site of healed herpes zoster in an Indian male. Int J Dermatol. 2010;49:105-107.
- Lee HW, Lee DK, Rhee DY, et al. Erythema annulare centrifugum following herpes zoster infection: Wolf’s isotopic response? Br J Dermatol. 2005;153:1241-1243.
- Mitsuhashi Y, Kondo S. Post-zoster eosinophilic dermatosis. Br J Dermatol. 1997;136:465-466.
- Roo E, Villegas C, Lopez-Bran E, et al. Postzoster cutaneous pseudolymphoma. Arch Dermatol. 1994;130:661-663.
- Langenberg A, Yen TS, LeBoit PE. Granulomatous vasculitis occurring after cutaneous herpes zoster despite absence of viral genome. J Am Acad Dermatol. 1991;24:429-433.
- Weidman F, Boston LN. Generalized xanthoma tuberosum with xantomathous changes in fresh scars of intercurrent zoster. Arch Intern Med. 1937;59:793-822.
- Olalquiaga J, Minaño R, Barrio J. Granuloma tuberculoide post-herpético en un paciente con leucemia linfocítica crónica. Med Cutan ILA. 1995;23:113-115.
- Stubbings JM, Goodfield MJ. An unusual distribution of an acneiform rash due to herpes zoster infection. Clin Exp Dermatol. 1993;18:92-93.
- Shemer A, Weiss G, Trau H. Wolf’s isotopic response: a case of zosteriform lichen planus on the site of healed herpes zoster. J Eur Acad Dermatol Venereol. 2001;15:445-447.
- Bang SW, Kim YK, Whang KU. Acquired reactive perforating collagenosis: unilateral umbilicated papules along the lesions of herpes zoster. J Am Acad Dermatol. 1997;36:778-779.
- Paydaş S, Sahin B, Yavuz S, et al. Lymphomatous skin infiltration at the site of previous varicella zoster virus infection in a patient with T cell lymphoma. Leuk Lymphoma. 2000;37:229-232.
- Cerroni L, Kerl H. Cutaneous localization of B-cell chronic lymphocytic leukemia at the site of varicella/herpes virus eruptions. J Am Acad Dermatol. 1997;37:1022.
- Hudson CP, Hanno R, Callen JP. Cutaneous angiosarcoma in a site of healed herpes zoster. Int J Dermatol. 1984;23:404-407.
- Wyburn-Mason R. Visceral lesions in herpes zoster. Br Med J. 1957;1:678-681.
- Caroti A. Metastasi cutanee di a adenocarcinoma papillifero ovarico in sede di herpes zoster. Chron Dermatol. 1987;18:769-773.
- Kim MB, Jwa SW, Ko HC, et al. A case of secondary cutaneous mucinosis following herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2009;48:212-214.
- Burman KD, McKinley-Grant L. Dermatologic aspects of thyroid disease. Clin Dermatol. 2006;24:247-255.
- Shekari AM, Ghiasi M, Ghasemi E, et al. Papulonodular mucinosis indicating systemic lupus erythematosus. Clin Exp Dermatol. 2009;34:558-560.
- Dinneen AM, Dicken CH. Scleromyxedema. J Am Acad Dermatol. 1995;33:37-43.
- Rongioletti F, Ghigliotti G, De Marchi R, et al. Cutaneous mucinoses and HIV infection. Br J Dermatol. 1998;139:1077-1080.
- Krahl D, Hartschuh W, Tilgen W. Granuloma annulare perforans in herpes zoster scars. J Am Acad Dermatol. 1993;29:859-862.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- Fisher G, Jaworski R. Granuloma formation in herpes zoster scars. J Am Acad Dermatol. 1987;16:1261-1263.
- Ruocco V, Grimaldi Filioli F. La risposta isotopica post-erpetica: possibile sequela di un locus minoris resistentiae acquisito. G Ital Dermatol Venereol. 1999;134:547-552.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;5:530-532.
- Kwon OS, Moon SE, Kim JA, et al. Lichen myxodematosus with rapid spontaneous regression. Br J Dermatol. 1997;136:295-296.
Practice Points
- Focal mucinosis is a histopathologic finding that may be seen in different cutaneous disorders. It is an exceptional histopathologic finding that has rarely been described in herpes zoster scars.
- In most cases, focal mucinosis is just a histopathologic finding with no therapeutic consequences.
Successful Nonoperative Management of HAGL (Humeral Avulsion of Glenohumeral Ligament) Lesion With Concurrent Axillary Nerve Injury in an Active-Duty US Navy SEAL
The humeral avulsion of glenohumeral ligament (HAGL) lesion has been recognized as a cause of recurrent shoulder instability. In 1942, Nicola1 was the first to describe this lesion, in a small case series of avulsions of the anterior band of the inferior glenohumeral ligament from the humeral neck secondary to a dislocation injury. In 1988, Bach and colleagues2 described it in 2 patients with recurrent anterior dislocations. Wolf and colleagues3 were the first to apply the term HAGL to the injury, in 1995.
HAGL lesion incidence ranges from 1% to 9%, but many authors think the lesion is underdiagnosed.3-5 It occurs in isolation or in combination with other injuries, and it is commonly identified on recurrence of instability. Bui-Mansfield and colleagues6 found that 11% of patients with a diagnosis of HAGL lesion previously had surgery on the same shoulder, whereas for 62% the lesion was associated with other, concurrent lesions, including labral tears (18, 25%), rotator cuff tears (16, 23%), and Hill-Sachs deformities (12, 17%).
Most young athletes who undergo nonoperative therapy for a HAGL lesion continue to experience pain and/or instability that then requires surgical intervention.4 To our knowledge, there are no reports of return to full function in young competitive athletes or return to manual labor after nonoperative management of a HAGL lesion.
In this article, we report the case of a US Navy SEAL who sustained a traction injury causing an axillary nerve injury and a HAGL lesion. Successful nonoperative management allowed him to return to full duty. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 26-year-old Navy SEAL presented with pain and significant weakness in the right (dominant) upper extremity after an injury in a training exercise. The shoulder sustained a traction injury when the man’s fast-moving marine attack craft was in a collision and he was trying not to be thrown off. He reported having a sense of dislocation yet never required a reduction.
Physical examination revealed severe weakness with shoulder abduction, external rotation, and forward flexion; inability to contract the deltoid muscle; and complete numbness along the cutaneous distribution of the axillary nerve. On neurovascular examination, the right upper extremity was otherwise intact. The patient had complete passive range of motion (ROM) with apprehension in abduction with external rotation along with anterior laxity and normal posterior stability.
Standard shoulder radiographs showed no bony abnormalities and a concentrically reduced glenohumeral joint. Magnetic resonance imaging (MRI), reviewed by a staff musculoskeletal radiologist and a sports fellowship–trained orthopedic surgeon, showed a greater tuberosity contusion, a partial tear of the infraspinatus, and a HAGL lesion (Figure 1).
The patient was counseled toward surgical intervention to prevent symptoms of recurrent instability. A detailed discussion ensued about whether to proceed with surgery immediately or to pursue temporary nonoperative treatment to allow for assessment and return of deltoid function. Patient and surgeon decided to delay operative intervention because of concerns about the patient’s ability to effectively rehabilitate while still having a compromised axillary nerve after surgery. The recommendation was to delay initial electromyographic (EMG) and nerve conduction velocity testing at least 4 weeks to allow for completion of Wallerian degeneration and more accurate assessment of the axillary nerve.7 Physical therapy for gentle ROM (excluding external rotation) and isometric rotator cuff exercises were initiated.
Five weeks after injury, the patient left the area to attend a 2-month nonphysical training course and continued rehabilitation and orthopedic follow-up at another military medical facility. Six weeks after injury, initial EMG testing revealed the expected axillary neuropraxia. In addition, some marginal improvement in ROM was noted, but deltoid function was still very limited.
Ten weeks after injury, clinical inspection revealed deltoid wasting. Active shoulder ROM was limited, and deltoid strength was 3/5, though the patient was able to perform a standard push-up without difficulty and showed no sign of laxity or apprehension on shoulder examination. Repeat EMG testing revealed axillary nerve denervation with no sign of regeneration. Twelve weeks after injury, MRA showed reorganization and partial healing of the HAGL lesion relative to the prior study (Figure 2).
On the patient’s return from training, 15 weeks after injury, he had improved active ROM and 4+/5 deltoid strength. Axillary nerve sensation was still decreased but markedly improved. Physical examination revealed no significant shoulder laxity or apprehension, and the patient denied feelings of instability. Activities were advanced to include an organized strengthening program.
Six months after injury, the patient was cleared to return to his unit with only mild physical restriction. Function continued to steadily improve. After 9 months, he was cleared for full, unrestricted duty. Although he still demonstrated slight asymmetric weakness in the right deltoid with continued muscular atrophy, examination findings were otherwise normal, and he was back to full activities without significant symptoms.
Eleven months after injury, MRI showed healing of the HAGL lesion (Figure 3). At 17 months, EMG testing revealed significant interval improvement in axillary motor unit potentials but still about a 50% decrement compared with the noninjured side. The patient denied any motor or sensory deficits and any instability events since his injury. He continued with full function as an active-duty Navy SEAL.
Discussion
Nonoperative management has been used for injuries to the inferior glenohumeral ligament complex when there is no humeral detachment but generally has been reserved for low-demand patients and patients who cannot tolerate surgical intervention.4 Detached lesions may initially be managed nonoperatively with physical therapy and rehabilitation, but the rate of recurrent instability after nonoperative management of a known HAGL lesion remains unknown.4 Most active young people are expected to have persistent pain and/or instability and require surgical intervention. Both arthroscopic and open methods have been used successfully.3,8-15 Persistent instability is often the primary complaint leading to a diagnosis of a HAGL lesion.4 The patient in this case report neither demonstrated nor reported any instability event after his 6-month period of nonoperative management, despite his young age and elite physical requirements.
To our knowledge, there are no reports of successful nonoperative management of a known symptomatic HAGL lesion in a high-demand athlete. Although we do not routinely recommend nonoperative treatment for cases such as the one reported here, the decision to delay this Navy SEAL’s surgical management was made out of concern about potential complications of postoperative rehabilitation given the concurrent axillary nerve injury.
With anterior shoulder dislocations, multiple concomitant shoulder injuries, including a HAGL lesion, are not uncommon.6,16 With HAGL lesions, associated rotator cuff injuries occur at a rate as high as 23%.6 Our patient had a concurrent partial rotator cuff tear but also an axillary nerve traction injury. To our knowledge, the literature has not described axillary nerve injury specifically in association with a HAGL lesion, though it is well documented and maintained as a possible concurrent injury with anterior shoulder instability events.17 Robinson and colleagues16 found a 5.8% incidence of a clinically apparent neurologic deficit after traumatic anterior shoulder dislocation in 3633 dislocations, about 75% of which were isolated axillary nerve injuries. They also reported a 25.7% rate of rotator cuff tear or greater tuberosity fracture, either of which significantly increased the likelihood of a neurologic deficit in their study.
When nerve continuity remains, functional recovery occurs after 3 to 6 months, or within weeks in some cases.18-20 Nerve injuries in continuity but with persistent, severe clinical deficits may require surgical exploration with subsequent neurolysis and/or repair.19-21 Our patient showed gradual axillary nerve recovery from a clinical standpoint. By 6 months after injury, despite continued muscle atrophy and decreased axillary nerve sensation, he had returned to full duty as a Navy SEAL. By 17 months, atrophy was markedly improved, and strength and ROM had subjectively returned, despite there being significant conduction amplitude losses, up to 50% of the contralateral side, on EMG testing.
This case represents a scenario in which likely initial surgical management was precluded by a concomitant injury, and the patient had a serendipitous outcome. It is possible the axillary neuropraxia and subsequent temporary deltoid dysfunction provided a unique environment that was conducive to the healing of the HAGL lesion. With classic Bankart lesions, many surgeons prefer to use aggressive early surgical treatment in first-time dislocators, especially elite athletes, in an attempt to avoid recurrent instability.22-26 However, some have suggested that initial immobilization after acute injury may lead to successful nonoperative management.27 Perhaps our case report raises the question as to whether a prolonged period of initial immobilization can prove successful in management of a HAGL lesion. Prospective studies comparing early surgical and nonoperative treatment of these challenging lesions are warranted.
We have reported a case of successful nonoperative management of a HAGL lesion in an active-duty Navy SEAL with concomitant shoulder injuries. This case could suggest that a trial of initial nonoperative management should be considered for injuries that involve a HAGL lesion when there are concerns about the patient’s ability to complete functional rehabilitation because of the combined injuries of the shoulder.
1. Nicola T. Anterior dislocation of the shoulder: the role of the articular capsule. J Bone Joint Surg. 1942;25:614-616.
2. Bach BR, Warren RF, Fronek J. Disruption of the lateral capsule of the shoulder. A cause of recurrent dislocation. J Bone Joint Surg Br. 1988;70(2):274-276.
3. Wolf EM, Cheng JC, Dickson K. Humeral avulsion of glenohumeral ligaments as a cause of anterior shoulder instability. Arthroscopy. 1995;11(5):600-607.
4. George MS, Khazzam M, Kuhn JE. Humeral avulsion of glenohumeral ligaments. J Am Acad Orthop Surg. 2011;19(3):127-133.
5. Tirman PF, Steinbach LS, Feller JF, Stauffer AE. Humeral avulsion of the anterior shoulder stabilizing structures after anterior shoulder dislocation: demonstration by MRI and MR arthrography. Skeletal Radiol. 1996;25(8):743-748.
6. Bui-Mansfield LT, Banks KP, Taylor DC. Humeral avulsion of the glenohumeral ligaments: the HAGL lesion. Am J Sports Med. 2007;35(11):1960-1966.
7. Dumitru D, Zwarts MJ. Needle electromyography. In: Dumitru D, Amato AA, Zwarts MJ, eds. Electrodiagnostic Medicine. 3rd ed. Philadelphia, PA: Hanley & Belfus; 2005:257-292.
8. Parameswaran AD, Provencher MT, Bach BR Jr, Verma N, Romeo AA. Humeral avulsion of the glenohumeral ligament. Injury pattern and arthroscopic repair techniques. Orthopedics. 2008;31(8):773-779.
9. Kon Y, Shiozaki H, Sugaya H. Arthroscopic repair of a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2005;21(5):632.
10. Bokor DJ, Conboy VB, Olson C. Anterior instability of the glenohumeral joint with humeral avulsion of the glenohumeral ligament: a review of 41 cases. J Bone Joint Surg Br. 1999;81(1):93-96.
11. Field LD, Bokor DJ, Savoie FH 3rd. Humeral and glenoid detachment of the anterior inferior glenohumeral ligament: a cause of anterior shoulder instability. J Shoulder Elbow Surg. 1997;6(1):6-10.
12. Arciero RA, Mazzocca AD. Mini-open repair technique of HAGL (humeral avulsion of the glenohumeral ligament) lesion. Arthroscopy. 2005;21(9):1152.
13. Bhatia DN, DeBeer JF, van Rooyen KS. The “subscapularis-sparing” approach: a new mini-open technique to repair a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2009;25(6):686-690.
14. Huberty D, Burkhart S. Arthroscopic repair of anterior humeral avulsion of the glenohumeral ligaments. Tech Shoulder Elbow Surg. 2006;7(4):186-190.
15. Richards DP, Burkhart SS. Arthroscopic humeral avulsion of the glenohumeral ligaments (HAGL) repair. Arthroscopy. 2004;20(suppl 2):134-141.
16. Robinson CM, Shur N, Sharpe T, Ray A, Murray IR. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94(1):18-26.
17. Visser CP, Coene LN, Brand R, Tavy DL. The incidence of nerve injury in anterior dislocation of the shoulder and its influence on functional recovery. A prospective clinical and EMG study. J Bone Joint Surg Br. 1999;81(4):679-685.
18. Gumina S, Bertino A, Di Giorgio G, Postacchini F. Injury of the axillary nerve subsequent to recurrence of shoulder dislocation. Clinical and electromyographic study. Chir Organi Mov. 2005;90(2):153-158.
19. Perlmutter GS. Axillary nerve injury. Clin Orthop Relat Res. 1999;(368):28-36.
20. Saragaglia D, Picard F, Le Bredonchel T, Moncenis C, Sardo M, Tourne Y. Acute anterior instability of the shoulder: short- and mid-term outcome after conservative treatment [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2001;87(3):215-220.
21. Kline DG, Kim DH. Axillary nerve repair in 99 patients with 101 stretch injuries. J Neurosurg. 2003;99(4):630-636.
22. Kralinger FS, Golser K, Wischatta R, Wambacher M, Sperner G. Predicting recurrence after primary anterior shoulder dislocation. Am J Sports Med. 2002;30(1):116-120.
23. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
24. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
25. Jakobsen BW, Johannsen HV, Suder P, Søjbjerg JO. Primary repair versus conservative treatment of first-time traumatic anterior dislocation of the shoulder: a randomized study with 10-year follow-up. Arthroscopy. 2007;23(2):118-123.
26. Kirkley A, Griffin S, Richards C, Miniaci A, Mohtadi N. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder. Arthroscopy. 1999;15(5):507-514.
27. Paterson WH, Throckmorton TW, Koester M, Azar FM, Kuhn JE. Position and duration of immobilization after primary anterior shoulder dislocation: a systematic review and meta-analysis of the literature. J Bone Joint Surg Am. 2010;92(18):2924-2933.
The humeral avulsion of glenohumeral ligament (HAGL) lesion has been recognized as a cause of recurrent shoulder instability. In 1942, Nicola1 was the first to describe this lesion, in a small case series of avulsions of the anterior band of the inferior glenohumeral ligament from the humeral neck secondary to a dislocation injury. In 1988, Bach and colleagues2 described it in 2 patients with recurrent anterior dislocations. Wolf and colleagues3 were the first to apply the term HAGL to the injury, in 1995.
HAGL lesion incidence ranges from 1% to 9%, but many authors think the lesion is underdiagnosed.3-5 It occurs in isolation or in combination with other injuries, and it is commonly identified on recurrence of instability. Bui-Mansfield and colleagues6 found that 11% of patients with a diagnosis of HAGL lesion previously had surgery on the same shoulder, whereas for 62% the lesion was associated with other, concurrent lesions, including labral tears (18, 25%), rotator cuff tears (16, 23%), and Hill-Sachs deformities (12, 17%).
Most young athletes who undergo nonoperative therapy for a HAGL lesion continue to experience pain and/or instability that then requires surgical intervention.4 To our knowledge, there are no reports of return to full function in young competitive athletes or return to manual labor after nonoperative management of a HAGL lesion.
In this article, we report the case of a US Navy SEAL who sustained a traction injury causing an axillary nerve injury and a HAGL lesion. Successful nonoperative management allowed him to return to full duty. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 26-year-old Navy SEAL presented with pain and significant weakness in the right (dominant) upper extremity after an injury in a training exercise. The shoulder sustained a traction injury when the man’s fast-moving marine attack craft was in a collision and he was trying not to be thrown off. He reported having a sense of dislocation yet never required a reduction.
Physical examination revealed severe weakness with shoulder abduction, external rotation, and forward flexion; inability to contract the deltoid muscle; and complete numbness along the cutaneous distribution of the axillary nerve. On neurovascular examination, the right upper extremity was otherwise intact. The patient had complete passive range of motion (ROM) with apprehension in abduction with external rotation along with anterior laxity and normal posterior stability.
Standard shoulder radiographs showed no bony abnormalities and a concentrically reduced glenohumeral joint. Magnetic resonance imaging (MRI), reviewed by a staff musculoskeletal radiologist and a sports fellowship–trained orthopedic surgeon, showed a greater tuberosity contusion, a partial tear of the infraspinatus, and a HAGL lesion (Figure 1).
The patient was counseled toward surgical intervention to prevent symptoms of recurrent instability. A detailed discussion ensued about whether to proceed with surgery immediately or to pursue temporary nonoperative treatment to allow for assessment and return of deltoid function. Patient and surgeon decided to delay operative intervention because of concerns about the patient’s ability to effectively rehabilitate while still having a compromised axillary nerve after surgery. The recommendation was to delay initial electromyographic (EMG) and nerve conduction velocity testing at least 4 weeks to allow for completion of Wallerian degeneration and more accurate assessment of the axillary nerve.7 Physical therapy for gentle ROM (excluding external rotation) and isometric rotator cuff exercises were initiated.
Five weeks after injury, the patient left the area to attend a 2-month nonphysical training course and continued rehabilitation and orthopedic follow-up at another military medical facility. Six weeks after injury, initial EMG testing revealed the expected axillary neuropraxia. In addition, some marginal improvement in ROM was noted, but deltoid function was still very limited.
Ten weeks after injury, clinical inspection revealed deltoid wasting. Active shoulder ROM was limited, and deltoid strength was 3/5, though the patient was able to perform a standard push-up without difficulty and showed no sign of laxity or apprehension on shoulder examination. Repeat EMG testing revealed axillary nerve denervation with no sign of regeneration. Twelve weeks after injury, MRA showed reorganization and partial healing of the HAGL lesion relative to the prior study (Figure 2).
On the patient’s return from training, 15 weeks after injury, he had improved active ROM and 4+/5 deltoid strength. Axillary nerve sensation was still decreased but markedly improved. Physical examination revealed no significant shoulder laxity or apprehension, and the patient denied feelings of instability. Activities were advanced to include an organized strengthening program.
Six months after injury, the patient was cleared to return to his unit with only mild physical restriction. Function continued to steadily improve. After 9 months, he was cleared for full, unrestricted duty. Although he still demonstrated slight asymmetric weakness in the right deltoid with continued muscular atrophy, examination findings were otherwise normal, and he was back to full activities without significant symptoms.
Eleven months after injury, MRI showed healing of the HAGL lesion (Figure 3). At 17 months, EMG testing revealed significant interval improvement in axillary motor unit potentials but still about a 50% decrement compared with the noninjured side. The patient denied any motor or sensory deficits and any instability events since his injury. He continued with full function as an active-duty Navy SEAL.
Discussion
Nonoperative management has been used for injuries to the inferior glenohumeral ligament complex when there is no humeral detachment but generally has been reserved for low-demand patients and patients who cannot tolerate surgical intervention.4 Detached lesions may initially be managed nonoperatively with physical therapy and rehabilitation, but the rate of recurrent instability after nonoperative management of a known HAGL lesion remains unknown.4 Most active young people are expected to have persistent pain and/or instability and require surgical intervention. Both arthroscopic and open methods have been used successfully.3,8-15 Persistent instability is often the primary complaint leading to a diagnosis of a HAGL lesion.4 The patient in this case report neither demonstrated nor reported any instability event after his 6-month period of nonoperative management, despite his young age and elite physical requirements.
To our knowledge, there are no reports of successful nonoperative management of a known symptomatic HAGL lesion in a high-demand athlete. Although we do not routinely recommend nonoperative treatment for cases such as the one reported here, the decision to delay this Navy SEAL’s surgical management was made out of concern about potential complications of postoperative rehabilitation given the concurrent axillary nerve injury.
With anterior shoulder dislocations, multiple concomitant shoulder injuries, including a HAGL lesion, are not uncommon.6,16 With HAGL lesions, associated rotator cuff injuries occur at a rate as high as 23%.6 Our patient had a concurrent partial rotator cuff tear but also an axillary nerve traction injury. To our knowledge, the literature has not described axillary nerve injury specifically in association with a HAGL lesion, though it is well documented and maintained as a possible concurrent injury with anterior shoulder instability events.17 Robinson and colleagues16 found a 5.8% incidence of a clinically apparent neurologic deficit after traumatic anterior shoulder dislocation in 3633 dislocations, about 75% of which were isolated axillary nerve injuries. They also reported a 25.7% rate of rotator cuff tear or greater tuberosity fracture, either of which significantly increased the likelihood of a neurologic deficit in their study.
When nerve continuity remains, functional recovery occurs after 3 to 6 months, or within weeks in some cases.18-20 Nerve injuries in continuity but with persistent, severe clinical deficits may require surgical exploration with subsequent neurolysis and/or repair.19-21 Our patient showed gradual axillary nerve recovery from a clinical standpoint. By 6 months after injury, despite continued muscle atrophy and decreased axillary nerve sensation, he had returned to full duty as a Navy SEAL. By 17 months, atrophy was markedly improved, and strength and ROM had subjectively returned, despite there being significant conduction amplitude losses, up to 50% of the contralateral side, on EMG testing.
This case represents a scenario in which likely initial surgical management was precluded by a concomitant injury, and the patient had a serendipitous outcome. It is possible the axillary neuropraxia and subsequent temporary deltoid dysfunction provided a unique environment that was conducive to the healing of the HAGL lesion. With classic Bankart lesions, many surgeons prefer to use aggressive early surgical treatment in first-time dislocators, especially elite athletes, in an attempt to avoid recurrent instability.22-26 However, some have suggested that initial immobilization after acute injury may lead to successful nonoperative management.27 Perhaps our case report raises the question as to whether a prolonged period of initial immobilization can prove successful in management of a HAGL lesion. Prospective studies comparing early surgical and nonoperative treatment of these challenging lesions are warranted.
We have reported a case of successful nonoperative management of a HAGL lesion in an active-duty Navy SEAL with concomitant shoulder injuries. This case could suggest that a trial of initial nonoperative management should be considered for injuries that involve a HAGL lesion when there are concerns about the patient’s ability to complete functional rehabilitation because of the combined injuries of the shoulder.
The humeral avulsion of glenohumeral ligament (HAGL) lesion has been recognized as a cause of recurrent shoulder instability. In 1942, Nicola1 was the first to describe this lesion, in a small case series of avulsions of the anterior band of the inferior glenohumeral ligament from the humeral neck secondary to a dislocation injury. In 1988, Bach and colleagues2 described it in 2 patients with recurrent anterior dislocations. Wolf and colleagues3 were the first to apply the term HAGL to the injury, in 1995.
HAGL lesion incidence ranges from 1% to 9%, but many authors think the lesion is underdiagnosed.3-5 It occurs in isolation or in combination with other injuries, and it is commonly identified on recurrence of instability. Bui-Mansfield and colleagues6 found that 11% of patients with a diagnosis of HAGL lesion previously had surgery on the same shoulder, whereas for 62% the lesion was associated with other, concurrent lesions, including labral tears (18, 25%), rotator cuff tears (16, 23%), and Hill-Sachs deformities (12, 17%).
Most young athletes who undergo nonoperative therapy for a HAGL lesion continue to experience pain and/or instability that then requires surgical intervention.4 To our knowledge, there are no reports of return to full function in young competitive athletes or return to manual labor after nonoperative management of a HAGL lesion.
In this article, we report the case of a US Navy SEAL who sustained a traction injury causing an axillary nerve injury and a HAGL lesion. Successful nonoperative management allowed him to return to full duty. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 26-year-old Navy SEAL presented with pain and significant weakness in the right (dominant) upper extremity after an injury in a training exercise. The shoulder sustained a traction injury when the man’s fast-moving marine attack craft was in a collision and he was trying not to be thrown off. He reported having a sense of dislocation yet never required a reduction.
Physical examination revealed severe weakness with shoulder abduction, external rotation, and forward flexion; inability to contract the deltoid muscle; and complete numbness along the cutaneous distribution of the axillary nerve. On neurovascular examination, the right upper extremity was otherwise intact. The patient had complete passive range of motion (ROM) with apprehension in abduction with external rotation along with anterior laxity and normal posterior stability.
Standard shoulder radiographs showed no bony abnormalities and a concentrically reduced glenohumeral joint. Magnetic resonance imaging (MRI), reviewed by a staff musculoskeletal radiologist and a sports fellowship–trained orthopedic surgeon, showed a greater tuberosity contusion, a partial tear of the infraspinatus, and a HAGL lesion (Figure 1).
The patient was counseled toward surgical intervention to prevent symptoms of recurrent instability. A detailed discussion ensued about whether to proceed with surgery immediately or to pursue temporary nonoperative treatment to allow for assessment and return of deltoid function. Patient and surgeon decided to delay operative intervention because of concerns about the patient’s ability to effectively rehabilitate while still having a compromised axillary nerve after surgery. The recommendation was to delay initial electromyographic (EMG) and nerve conduction velocity testing at least 4 weeks to allow for completion of Wallerian degeneration and more accurate assessment of the axillary nerve.7 Physical therapy for gentle ROM (excluding external rotation) and isometric rotator cuff exercises were initiated.
Five weeks after injury, the patient left the area to attend a 2-month nonphysical training course and continued rehabilitation and orthopedic follow-up at another military medical facility. Six weeks after injury, initial EMG testing revealed the expected axillary neuropraxia. In addition, some marginal improvement in ROM was noted, but deltoid function was still very limited.
Ten weeks after injury, clinical inspection revealed deltoid wasting. Active shoulder ROM was limited, and deltoid strength was 3/5, though the patient was able to perform a standard push-up without difficulty and showed no sign of laxity or apprehension on shoulder examination. Repeat EMG testing revealed axillary nerve denervation with no sign of regeneration. Twelve weeks after injury, MRA showed reorganization and partial healing of the HAGL lesion relative to the prior study (Figure 2).
On the patient’s return from training, 15 weeks after injury, he had improved active ROM and 4+/5 deltoid strength. Axillary nerve sensation was still decreased but markedly improved. Physical examination revealed no significant shoulder laxity or apprehension, and the patient denied feelings of instability. Activities were advanced to include an organized strengthening program.
Six months after injury, the patient was cleared to return to his unit with only mild physical restriction. Function continued to steadily improve. After 9 months, he was cleared for full, unrestricted duty. Although he still demonstrated slight asymmetric weakness in the right deltoid with continued muscular atrophy, examination findings were otherwise normal, and he was back to full activities without significant symptoms.
Eleven months after injury, MRI showed healing of the HAGL lesion (Figure 3). At 17 months, EMG testing revealed significant interval improvement in axillary motor unit potentials but still about a 50% decrement compared with the noninjured side. The patient denied any motor or sensory deficits and any instability events since his injury. He continued with full function as an active-duty Navy SEAL.
Discussion
Nonoperative management has been used for injuries to the inferior glenohumeral ligament complex when there is no humeral detachment but generally has been reserved for low-demand patients and patients who cannot tolerate surgical intervention.4 Detached lesions may initially be managed nonoperatively with physical therapy and rehabilitation, but the rate of recurrent instability after nonoperative management of a known HAGL lesion remains unknown.4 Most active young people are expected to have persistent pain and/or instability and require surgical intervention. Both arthroscopic and open methods have been used successfully.3,8-15 Persistent instability is often the primary complaint leading to a diagnosis of a HAGL lesion.4 The patient in this case report neither demonstrated nor reported any instability event after his 6-month period of nonoperative management, despite his young age and elite physical requirements.
To our knowledge, there are no reports of successful nonoperative management of a known symptomatic HAGL lesion in a high-demand athlete. Although we do not routinely recommend nonoperative treatment for cases such as the one reported here, the decision to delay this Navy SEAL’s surgical management was made out of concern about potential complications of postoperative rehabilitation given the concurrent axillary nerve injury.
With anterior shoulder dislocations, multiple concomitant shoulder injuries, including a HAGL lesion, are not uncommon.6,16 With HAGL lesions, associated rotator cuff injuries occur at a rate as high as 23%.6 Our patient had a concurrent partial rotator cuff tear but also an axillary nerve traction injury. To our knowledge, the literature has not described axillary nerve injury specifically in association with a HAGL lesion, though it is well documented and maintained as a possible concurrent injury with anterior shoulder instability events.17 Robinson and colleagues16 found a 5.8% incidence of a clinically apparent neurologic deficit after traumatic anterior shoulder dislocation in 3633 dislocations, about 75% of which were isolated axillary nerve injuries. They also reported a 25.7% rate of rotator cuff tear or greater tuberosity fracture, either of which significantly increased the likelihood of a neurologic deficit in their study.
When nerve continuity remains, functional recovery occurs after 3 to 6 months, or within weeks in some cases.18-20 Nerve injuries in continuity but with persistent, severe clinical deficits may require surgical exploration with subsequent neurolysis and/or repair.19-21 Our patient showed gradual axillary nerve recovery from a clinical standpoint. By 6 months after injury, despite continued muscle atrophy and decreased axillary nerve sensation, he had returned to full duty as a Navy SEAL. By 17 months, atrophy was markedly improved, and strength and ROM had subjectively returned, despite there being significant conduction amplitude losses, up to 50% of the contralateral side, on EMG testing.
This case represents a scenario in which likely initial surgical management was precluded by a concomitant injury, and the patient had a serendipitous outcome. It is possible the axillary neuropraxia and subsequent temporary deltoid dysfunction provided a unique environment that was conducive to the healing of the HAGL lesion. With classic Bankart lesions, many surgeons prefer to use aggressive early surgical treatment in first-time dislocators, especially elite athletes, in an attempt to avoid recurrent instability.22-26 However, some have suggested that initial immobilization after acute injury may lead to successful nonoperative management.27 Perhaps our case report raises the question as to whether a prolonged period of initial immobilization can prove successful in management of a HAGL lesion. Prospective studies comparing early surgical and nonoperative treatment of these challenging lesions are warranted.
We have reported a case of successful nonoperative management of a HAGL lesion in an active-duty Navy SEAL with concomitant shoulder injuries. This case could suggest that a trial of initial nonoperative management should be considered for injuries that involve a HAGL lesion when there are concerns about the patient’s ability to complete functional rehabilitation because of the combined injuries of the shoulder.
1. Nicola T. Anterior dislocation of the shoulder: the role of the articular capsule. J Bone Joint Surg. 1942;25:614-616.
2. Bach BR, Warren RF, Fronek J. Disruption of the lateral capsule of the shoulder. A cause of recurrent dislocation. J Bone Joint Surg Br. 1988;70(2):274-276.
3. Wolf EM, Cheng JC, Dickson K. Humeral avulsion of glenohumeral ligaments as a cause of anterior shoulder instability. Arthroscopy. 1995;11(5):600-607.
4. George MS, Khazzam M, Kuhn JE. Humeral avulsion of glenohumeral ligaments. J Am Acad Orthop Surg. 2011;19(3):127-133.
5. Tirman PF, Steinbach LS, Feller JF, Stauffer AE. Humeral avulsion of the anterior shoulder stabilizing structures after anterior shoulder dislocation: demonstration by MRI and MR arthrography. Skeletal Radiol. 1996;25(8):743-748.
6. Bui-Mansfield LT, Banks KP, Taylor DC. Humeral avulsion of the glenohumeral ligaments: the HAGL lesion. Am J Sports Med. 2007;35(11):1960-1966.
7. Dumitru D, Zwarts MJ. Needle electromyography. In: Dumitru D, Amato AA, Zwarts MJ, eds. Electrodiagnostic Medicine. 3rd ed. Philadelphia, PA: Hanley & Belfus; 2005:257-292.
8. Parameswaran AD, Provencher MT, Bach BR Jr, Verma N, Romeo AA. Humeral avulsion of the glenohumeral ligament. Injury pattern and arthroscopic repair techniques. Orthopedics. 2008;31(8):773-779.
9. Kon Y, Shiozaki H, Sugaya H. Arthroscopic repair of a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2005;21(5):632.
10. Bokor DJ, Conboy VB, Olson C. Anterior instability of the glenohumeral joint with humeral avulsion of the glenohumeral ligament: a review of 41 cases. J Bone Joint Surg Br. 1999;81(1):93-96.
11. Field LD, Bokor DJ, Savoie FH 3rd. Humeral and glenoid detachment of the anterior inferior glenohumeral ligament: a cause of anterior shoulder instability. J Shoulder Elbow Surg. 1997;6(1):6-10.
12. Arciero RA, Mazzocca AD. Mini-open repair technique of HAGL (humeral avulsion of the glenohumeral ligament) lesion. Arthroscopy. 2005;21(9):1152.
13. Bhatia DN, DeBeer JF, van Rooyen KS. The “subscapularis-sparing” approach: a new mini-open technique to repair a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2009;25(6):686-690.
14. Huberty D, Burkhart S. Arthroscopic repair of anterior humeral avulsion of the glenohumeral ligaments. Tech Shoulder Elbow Surg. 2006;7(4):186-190.
15. Richards DP, Burkhart SS. Arthroscopic humeral avulsion of the glenohumeral ligaments (HAGL) repair. Arthroscopy. 2004;20(suppl 2):134-141.
16. Robinson CM, Shur N, Sharpe T, Ray A, Murray IR. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94(1):18-26.
17. Visser CP, Coene LN, Brand R, Tavy DL. The incidence of nerve injury in anterior dislocation of the shoulder and its influence on functional recovery. A prospective clinical and EMG study. J Bone Joint Surg Br. 1999;81(4):679-685.
18. Gumina S, Bertino A, Di Giorgio G, Postacchini F. Injury of the axillary nerve subsequent to recurrence of shoulder dislocation. Clinical and electromyographic study. Chir Organi Mov. 2005;90(2):153-158.
19. Perlmutter GS. Axillary nerve injury. Clin Orthop Relat Res. 1999;(368):28-36.
20. Saragaglia D, Picard F, Le Bredonchel T, Moncenis C, Sardo M, Tourne Y. Acute anterior instability of the shoulder: short- and mid-term outcome after conservative treatment [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2001;87(3):215-220.
21. Kline DG, Kim DH. Axillary nerve repair in 99 patients with 101 stretch injuries. J Neurosurg. 2003;99(4):630-636.
22. Kralinger FS, Golser K, Wischatta R, Wambacher M, Sperner G. Predicting recurrence after primary anterior shoulder dislocation. Am J Sports Med. 2002;30(1):116-120.
23. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
24. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
25. Jakobsen BW, Johannsen HV, Suder P, Søjbjerg JO. Primary repair versus conservative treatment of first-time traumatic anterior dislocation of the shoulder: a randomized study with 10-year follow-up. Arthroscopy. 2007;23(2):118-123.
26. Kirkley A, Griffin S, Richards C, Miniaci A, Mohtadi N. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder. Arthroscopy. 1999;15(5):507-514.
27. Paterson WH, Throckmorton TW, Koester M, Azar FM, Kuhn JE. Position and duration of immobilization after primary anterior shoulder dislocation: a systematic review and meta-analysis of the literature. J Bone Joint Surg Am. 2010;92(18):2924-2933.
1. Nicola T. Anterior dislocation of the shoulder: the role of the articular capsule. J Bone Joint Surg. 1942;25:614-616.
2. Bach BR, Warren RF, Fronek J. Disruption of the lateral capsule of the shoulder. A cause of recurrent dislocation. J Bone Joint Surg Br. 1988;70(2):274-276.
3. Wolf EM, Cheng JC, Dickson K. Humeral avulsion of glenohumeral ligaments as a cause of anterior shoulder instability. Arthroscopy. 1995;11(5):600-607.
4. George MS, Khazzam M, Kuhn JE. Humeral avulsion of glenohumeral ligaments. J Am Acad Orthop Surg. 2011;19(3):127-133.
5. Tirman PF, Steinbach LS, Feller JF, Stauffer AE. Humeral avulsion of the anterior shoulder stabilizing structures after anterior shoulder dislocation: demonstration by MRI and MR arthrography. Skeletal Radiol. 1996;25(8):743-748.
6. Bui-Mansfield LT, Banks KP, Taylor DC. Humeral avulsion of the glenohumeral ligaments: the HAGL lesion. Am J Sports Med. 2007;35(11):1960-1966.
7. Dumitru D, Zwarts MJ. Needle electromyography. In: Dumitru D, Amato AA, Zwarts MJ, eds. Electrodiagnostic Medicine. 3rd ed. Philadelphia, PA: Hanley & Belfus; 2005:257-292.
8. Parameswaran AD, Provencher MT, Bach BR Jr, Verma N, Romeo AA. Humeral avulsion of the glenohumeral ligament. Injury pattern and arthroscopic repair techniques. Orthopedics. 2008;31(8):773-779.
9. Kon Y, Shiozaki H, Sugaya H. Arthroscopic repair of a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2005;21(5):632.
10. Bokor DJ, Conboy VB, Olson C. Anterior instability of the glenohumeral joint with humeral avulsion of the glenohumeral ligament: a review of 41 cases. J Bone Joint Surg Br. 1999;81(1):93-96.
11. Field LD, Bokor DJ, Savoie FH 3rd. Humeral and glenoid detachment of the anterior inferior glenohumeral ligament: a cause of anterior shoulder instability. J Shoulder Elbow Surg. 1997;6(1):6-10.
12. Arciero RA, Mazzocca AD. Mini-open repair technique of HAGL (humeral avulsion of the glenohumeral ligament) lesion. Arthroscopy. 2005;21(9):1152.
13. Bhatia DN, DeBeer JF, van Rooyen KS. The “subscapularis-sparing” approach: a new mini-open technique to repair a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2009;25(6):686-690.
14. Huberty D, Burkhart S. Arthroscopic repair of anterior humeral avulsion of the glenohumeral ligaments. Tech Shoulder Elbow Surg. 2006;7(4):186-190.
15. Richards DP, Burkhart SS. Arthroscopic humeral avulsion of the glenohumeral ligaments (HAGL) repair. Arthroscopy. 2004;20(suppl 2):134-141.
16. Robinson CM, Shur N, Sharpe T, Ray A, Murray IR. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94(1):18-26.
17. Visser CP, Coene LN, Brand R, Tavy DL. The incidence of nerve injury in anterior dislocation of the shoulder and its influence on functional recovery. A prospective clinical and EMG study. J Bone Joint Surg Br. 1999;81(4):679-685.
18. Gumina S, Bertino A, Di Giorgio G, Postacchini F. Injury of the axillary nerve subsequent to recurrence of shoulder dislocation. Clinical and electromyographic study. Chir Organi Mov. 2005;90(2):153-158.
19. Perlmutter GS. Axillary nerve injury. Clin Orthop Relat Res. 1999;(368):28-36.
20. Saragaglia D, Picard F, Le Bredonchel T, Moncenis C, Sardo M, Tourne Y. Acute anterior instability of the shoulder: short- and mid-term outcome after conservative treatment [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2001;87(3):215-220.
21. Kline DG, Kim DH. Axillary nerve repair in 99 patients with 101 stretch injuries. J Neurosurg. 2003;99(4):630-636.
22. Kralinger FS, Golser K, Wischatta R, Wambacher M, Sperner G. Predicting recurrence after primary anterior shoulder dislocation. Am J Sports Med. 2002;30(1):116-120.
23. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
24. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
25. Jakobsen BW, Johannsen HV, Suder P, Søjbjerg JO. Primary repair versus conservative treatment of first-time traumatic anterior dislocation of the shoulder: a randomized study with 10-year follow-up. Arthroscopy. 2007;23(2):118-123.
26. Kirkley A, Griffin S, Richards C, Miniaci A, Mohtadi N. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder. Arthroscopy. 1999;15(5):507-514.
27. Paterson WH, Throckmorton TW, Koester M, Azar FM, Kuhn JE. Position and duration of immobilization after primary anterior shoulder dislocation: a systematic review and meta-analysis of the literature. J Bone Joint Surg Am. 2010;92(18):2924-2933.
Clinical Characteristics and HLA Alleles of a Family With Simultaneously Occurring Alopecia Areata
Alopecia areata (AA) presents as sudden, nonscarring, recurrent hair loss characterized by well-circumscribed hairless patches. Although AA may be observed on any hair-bearing areas of the body, the most commonly affected sites are the scalp, beard area, eyebrows, and eyelashes.1 The incidence of AA is 1% to 2% in the general population and it is more common in males than females younger than 40 years.2 Although the majority of patients present with self-limited and well-circumscribed hairless patches that resolve within 2 years, 7% to 10% display a chronic and severe prognosis.3
The etiopathogenesis of AA is not clearly understood, but its occurrence and progression can involve immune dysfunction, genetic predisposition, infections, and physical and psychological trauma.2 Alopecia areata is observed to occur sporadically in most patients. Family history has been found in 3% to 42% of cases, but simultaneous occurrence of AA in family members is rare.4 In this case series, we present 4 cases of active AA lesions occurring simultaneously in a family who also had associated psychologic disorders.
Case Series
Patient 1 (Proband)
An 11-year-old boy presented with a 6-year history of ongoing AA with recurrent improvement and relapses on the scalp, eyebrows, and eyelashes. Various topical and oral medications had been prescribed by several outside dermatologists; however, these treatments provided minimal benefit and resulted in the recurrence of AA. Dermatologic examination revealed hair loss on the entire frontal, parietal, and temporal regions of the scalp, as well as half of the occipital region and one-third of the lateral side of the eyebrows (Figure 1). Psychological evaluation revealed introvert personality characteristics, lack of self-confidence, and signs of depression and anxiety.
Patient 2 (Proband’s Father)
A 38-year-old man presented with a 16-year history of recurrent loss and regrowth of hair on the scalp and beard area and white spots on the penis and arms. He previously had not undergone any treatments. Dermatologic examination revealed well-circumscribed, 1- to 4-cm, hairless patches on the occipital region of the scalp and in the beard area (Figure 2A) and multiple, 2- to 10-mm, vitiliginous lesions on both forearms (Figure 2B) and the penis. The patient had been unemployed for 6 months. Psychological evaluation revealed obsessive-compulsive disorder and obsessive-compulsive personality disorder.
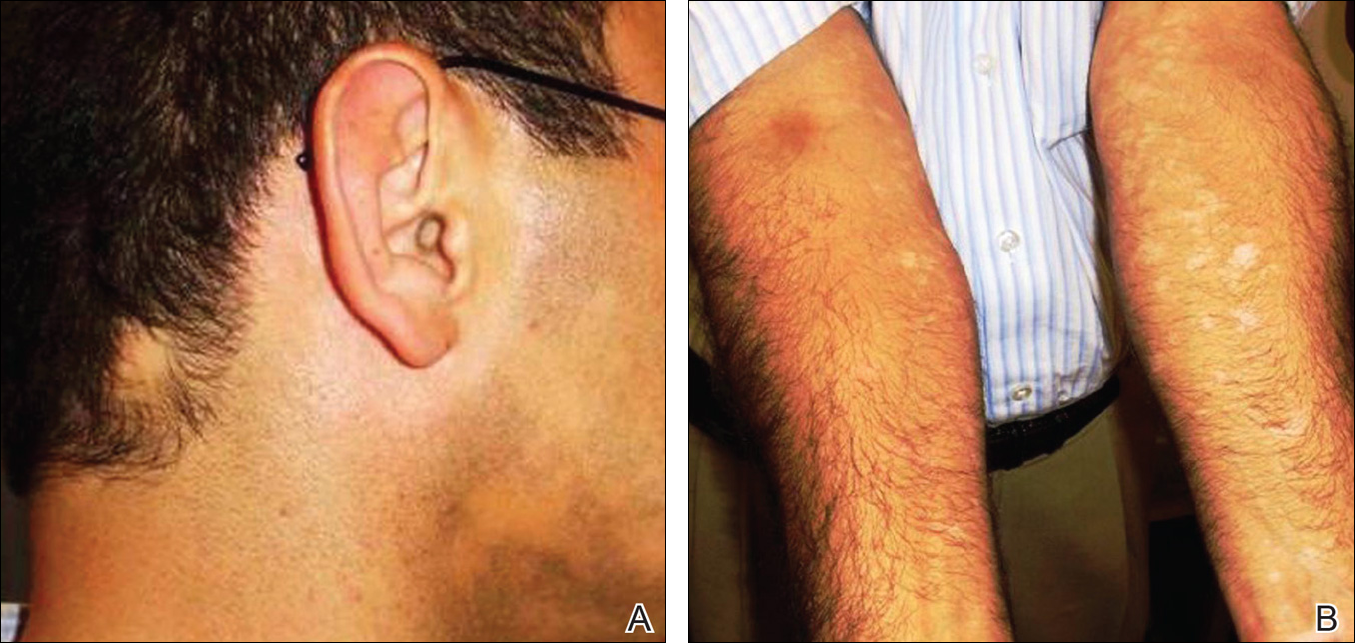
Patient 3 (Proband’s Mother)
A 32-year-old woman presented with a 3-year history of chronic AA. She previously had not undergone any treatments. Dermatologic examination revealed 2 well-circumscribed, 3- to 4-cm patches of hair loss on the occipital and left temporal regions of the scalp (Figure 3). Psychological evaluation revealed obsessive-compulsive personality disorder and depression. The patient did not have any autoimmune diseases.

Patient 4 (Proband’s Sister)
A 10-year-old girl presented with a 6-year history of recurrent, self-limited AA on various areas of scalp. She previously had not undergone any treatments. Dermatologic examination revealed a 3-cm hairless patch on the occipital region of the scalp (Figure 4). Psychiatric evaluation revealed narcissistic personality disorder, anxiety, and lack of self-confidence.
Laboratory Evaluation and HLA Antigen DNA Typing
Laboratory testing including complete blood cell count; liver, kidney, and thyroid function; and vitamin B12, zinc, folic acid, and fasting blood sugar levels were performed in all patients.
HLA antigen DNA typing was performed by polymerase chain reaction with sequence-specific primers in all patients after informed consent was obtained.
Clinical and laboratory examinations revealed no symptoms or findings of Epstein-Barr virus and cytomegalovirus infections, cicatricial alopecia, or connective tissue diseases in any of the patients. HLA antigen DNA typing revealed the following HLA alleles: B*35/40, C*04/15, DRB1*08/10, and DQB1*03/05 in patient 1; B*04/13, C*06/15, DRB1*07/10, and DQB1*02/05 in patient 2; B*33/37, C*04/06, DRB1*08/15, and DQ*06/06 in patient 3; B*13/37, C*06/06, DRB1*07/15, and DQB1*02/06 in patient 4.
Laboratory testing revealed vitamin B12 deficiency in patient 2 and iron deficiency anemia in patient 3; all other laboratory tests were within reference range. Antithyroglobulin and antithyroid peroxidase autoantibodies were all negative. Clinical features and laboratory analyses for all patients are summarized in the Table.
Treatment
All patients were recommended psychiatric therapy and started on dermatologic treatments. Topical corticosteroids, intralesional triamcinolone acetonide (8 mg/mL) injections into areas of hair loss, 8 total sessions of cryotherapy administered at 3-week intervals, and minoxidil solution 2% were administered respectively to all 4 patients. Alopecia areata in patients 3 and 4 completely regressed; however, no benefit was observed in patients 1 and 2 after 1 year of treatment. Because there was no response to the prior interventions, patient 1 was started on treatment with cyclosporine 2.5 mg/kg twice daily. However, therapy was discontinued after 1 month and treatment with narrowband UVB (3 times per week for 7 months [total of 57 sessions]) and topical corticosteroids were initiated (Table). The patient partially benefited from these regimens and recurrence was observed during the course of the treatment.
Although it was recommended that all 4 patients undergo psychiatric treatment and follow-up regularly with a psychiatrist, the patients declined. After approximately 1 year of dermatologic treatment, all 4 patients were lost to follow-up.
Comment
The etiopathogenesis of AA is unclear, but there is strong evidence suggesting that it is a T-cell–mediated autoimmune disease targeting the hair follicles. Common association of AA with autoimmune diseases such as vitiligo and thyroiditis support the immunological origin of the disease.3 In our case, patient 2 had AA along with vitiligo, but no associated autoimmune diseases (eg, vitiligo, diabetes mellitus, pernicious anemia, thyroid diseases) were noted in the other patients. Genetic and environmental factors are known to be influential as much as immune dysfunction in the etiology of AA.2
The presence of family history in 20% of patients supports the genetic predisposition of AA.4 In a genetic study by Martinez-Mir et al,5 susceptibility loci for AA were demonstrated on chromosomes 6, 10, 16, and 18. HLA antigen alleles, which provide predisposition to AA, have been investigated and associations with many different HLA antigens have been described for AA. In these studies, a relationship between AA and HLA class I antigens was not determined. Notable results mainly focused on HLA class II antigens.6-8 Colombe et al7 and Marques Da Costa et al8 demonstrated that long-lasting alopecia totalis or alopecia universalis (AT/AU) patients had a strong relationship with HLA-DRB1*1104; DRB1*04/05 was reported to be the most frequent HLA group among all patients with AA.6-10 In contrast, we did not detect these alleles in our patients. Colombe et al7,11 noted that HLA-DQB1*03 is a marker for both patch-type AA and AT/AU. Colombe et al10 showed that HLA-DQB1*03 was present in more than 80% of patients (N=286) with long-lasting AA. Barahmani et al9 confirmed a strong association between HLA-DQB1*0301, DRB1*1104, and AT/AU. In our patients, we detected HLA-DQB1*03/05 in patient 1 who had the earliest onset and most severe presentation of AA. In some studies, HLA-DRB1*03 was found to be less frequent in patients with AA, and this allele was suggested to be a protective factor.6,12 However, this allele was not detected in any of our patients.
The association of HLA alleles and AA has been investigated in Turkish patients with AA.13-15 Akar et al13 and Kavak et al14 detected that the frequency of HLA-DQB1*03 allele was remarkably higher in patients with AA than in healthy controls. These results were consistent with Colombe et al.10 On the other hand, Kavak et al14 reported that the frequency of HLA-DR16 was decreased in the patient group with AA. In another study, the frequency of HLA-B62 was increased in patients with AA compared to healthy controls.15 The HLA-DQB1*03 allele was found to be associated with AA in only patient 1 in our case series, and HLA alleles were not commonly shared among the 4 patients. Additionally, lack of consanguinity between patients 2 and 3 (the parents) also suggested that genetic factors were not involved in our familial cases.
Blaumeiser et al16 reported a lifetime risk of 7.4% in parents and 7.1% in siblings of 206 AA patients; however, because these studies investigated the presence of AA in any given life period of the family members, their results do not reflect frequency of simultaneous AA presence within one family. In a literature search using PubMed, Google Scholar, and other national databases for the terms alopecia areata as well as family, sibling, concurrently, concomitant, co-existent, and simultaneously, only 2 cases involving a husband and wife and 1 case of 2 siblings who concurrently had AA have been previously reported.17,18 Simultaneous presence of AA in more than 3 members of the same family is rare, and these cases have been observed in different generations and time periods.19 Among our patients, despite different age of onset and duration, AA was simultaneously present in the entire family.
Moreover, Rodriguez et al20 reported that the concordance rate of AA in identical twins was 42% and dizygotic twins was 10%. Environmental factors and infections also have been implicated in the etiology of AA. Infections caused by viruses such as cytomegalovirus and Epstein-Barr virus have been thought to be potential triggering factors; however, no evidence has been found.21,22 The clinical and laboratory examinations in our study did not reveal any presence and/or history of any known infectious disease, and there was no history of contact with water infected by acrylamide or a similar chemical.
Various life events and intense psychological stress may play an important role in triggering AA. Depression, hysteria, psychopathic deviance, psychasthenia, schizophrenia, anxiety, health concerns, bizarre thoughts, and family problems were found to be more frequent in patients with AA than healthy controls.23 The most common psychological disorders associated with AA are generalized anxiety disorder, major depressive disorder, adjustment disorders, and phobias.1,24 Ruiz-Doblado et al25 determined the presence of psychiatric comorbidities in 66% (21/32) of AA cases. Chu et al26 reported that the differences in ages of onset of AA revealed differences in psychiatric comorbidities. The risk for depression was higher in patients with AA younger than 20 years. An increased rate of anxiety was detected with patients with an onset of AA between the ages of 20 and 39 years. Obsessive-compulsive disorder and anxiety were more common in patients aged 40 to 59 years. Interestingly, the investigators also observed that approximately 50% of psychiatric disorders occurred prior to onset of AA.26 One study showed higher rates of stressful life events in children than in controls.27 Ghanizadeh24 reported at least 1 psychiatric disorder in 78% (11/14) of children and adolescents with AA. In the same study, obsessive-compulsive disorder was found to be the second common condition following major depression in AA.24
In our patients, psychiatric evaluations revealed obsessive-compulsive personality disorder in patients 2 and 3, depression in patient 3, and symptoms of anxiety with a lack of self-confidence in patients 1 and 4. Psychiatric disorders affecting the entire family may stem from unemployment of the father. Similar to the results noted in prior studies, depression, the most commonly associated psychiatric disorder of AA, was present in 2 of 4 patients. Obsessive-compulsive disorder, the second most common psychiatric disorder among AA patients, was present in patients 2 and 3. These results indicate that AA may be associated with shared stressful events and psychiatric disorders. Therefore, in addition to dermatologic treatment, it was recommended that all patients undergo psychiatric treatment and follow-up regularly with a psychiatrist; however, the patients declined. At the end of a 1-year treatment period and follow-up, resistance to therapy with minimal recovery followed by a rapid recurrence was determined in patients 1 and 2.
Conclusion
This report demonstrated that familial AA was strongly associated with psychological disorders that were detected in all patients. In our patients, HLA alleles did not seem to have a role in the development of familial AA. These results suggest that HLA was not associated with AA triggered by psychological stress. We believe that psychological disorders and stressful life events may play an important role in the occurrence of AA and lead to the development of resistance against treatment in familial and resistant AA cases.
- García-Hernández MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, et al. Alopecia areata, stress and psychiatric disorders: a review. J Dermatol. 1999;26:625-632.
- Bhat YJ, Manzoor S, Khan AR, et al. Trace element levels in alopecia areata. Indian J Dermatol Venereol Leprol. 2009;75:29-31.
- Alexis AF, Dudda-Subramanya R, Sinha AA. Alopecia areata: autoimmune basis of hair loss. Eur J Dermatol. 2004;14:364-370.
- Green J, Sinclair RD. Genetics of alopecia areata. Australas J Dermatol. 2000;41:213-218.
- Martinez-Mir A, Zlotogorski A, Gordon D, et al.Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80:316-328.
- Entz P, Blaumeiser B, Betz RC, et al. Investigation of the HLA-DRB1 locus in alopecia areata. Eur J Dermatol. 2006;16:363-367.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II alleles in long-standing alopecia totalis/alopecia universalis and long-standing patchy alopecia areata differentiate these two clinical groups. J Invest Dermatol. 1995;104(suppl 5):4-5.
- Marques Da Costa C, Dupont E, Van der Cruys M, et al. Earlier occurrence of severe alopecia areata in HLA-DRB1*11-positive patients. Dermatology. 2006;213:12-14.
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Colombe BW, Lou CD, Price VH. The genetic basis of alopecia areata: HLA associations with patchy alopecia areata versus alopecia totalis and alopecia universalis. J Investig Dermatol Symp Proc. 1999;4:216-219.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II antigen associations help to define two types of alopecia areata. J Am Acad Dermatol. 1995;33(5, pt 1):757-764.
- Broniarczyk-Dyła G, Prusińska-Bratoś M, Dubla-Berner M, et al. The protective role of the HLA-DR locus in patients with various clinical types of alopecia areata. Arch Immunol Ther Exp (Warsz). 2002;50:333-336.
- Akar A, Orkunuglu E, Sengul A, et al. HLA class II alleles in patients with alopecia areata. Eur J Dermatol. 2002;12:236-239.
- Kavak A, Baykal C, Ozarmagan G, et al. HLA in alopecia areata. Int J Dermatol. 2000;30:589-592.
- Aliagaoglu C, Pirim I, Atasoy M, et al. Association between alopecia areata and HLA class I and II in Turkey. J Dermatol. 2005;32:711-714.
- Blaumeiser B, Goot I, Fimmers R, et al. Familial aggregation of alopecia areata. J Am Acad Dermatol. 2006;54:627-632.
- Zalka AD, Byarlay JA, Goldsmith LA. Alopecia a deux: simultaneous occurrence of alopecia in a husband and wife. Arch Dermatol. 1994;130:390-392.
- Menon R, Kiran C. Concomitant presentation of alopecia areata in siblings: a rare occurrence. Int J Trichology. 2012;4:86-88.
- Valsecchi R, Vicari O, Frigeni A, et al. Familial alopecia areata-genetic susceptibility or coincidence? Acta Derm Venereol (Stockh). 1985;65:175-177.
- Rodriguez TA, Fernandes KE, Dresser KL, et al. Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J Am Acad Dermatol. 2010;62:525-527.
- Rodriguez TA, Duvic M. Onset of alopecia areata after Epstein Barr virus infectious mononucleosis. J Am Acad Dermatol. 2008;59:137-139.
- Offidani A, Amerio P, Bernardini ML, et al. Role of cytomegalovirus replication in alopecia areata pathogenesis. J Cutan Med Surg. 2000;4:63-65.
- Alfani S, Antinone V, Mozzetta A, et al. Psychological status of patients with alopecia areata. Acta Derm Venereol. 2012;92:304-306.
- Ghanizadeh A. Comorbidity of psychiatric disorders in children and adolescents with alopecia areata in a child and adolescent psychiatry clinical sample. Int J Dermatol. 2008;47:1118-1120.
- Ruiz-Doblado S, Carrizosa A, Garcia-Hernandez MJ. Alopecia areata: psychiatric comorbidity and adjustment to illness. Int J Dermatol. 2003;42:434-437.
- Chu SY, Chen YJ, Tseng WC, et al. Psychiatric comorbidities in patients with alopecia areata in Taiwan: a case-control study. Br J Dermatol. 2012;166:525-531.
- Manolache L, Petrescu-Seceleanu D, Benea V. Alopecia areata and stressful events in children. J Eur Acad Dermatol Venereol. 2009;23:107-109.
Alopecia areata (AA) presents as sudden, nonscarring, recurrent hair loss characterized by well-circumscribed hairless patches. Although AA may be observed on any hair-bearing areas of the body, the most commonly affected sites are the scalp, beard area, eyebrows, and eyelashes.1 The incidence of AA is 1% to 2% in the general population and it is more common in males than females younger than 40 years.2 Although the majority of patients present with self-limited and well-circumscribed hairless patches that resolve within 2 years, 7% to 10% display a chronic and severe prognosis.3
The etiopathogenesis of AA is not clearly understood, but its occurrence and progression can involve immune dysfunction, genetic predisposition, infections, and physical and psychological trauma.2 Alopecia areata is observed to occur sporadically in most patients. Family history has been found in 3% to 42% of cases, but simultaneous occurrence of AA in family members is rare.4 In this case series, we present 4 cases of active AA lesions occurring simultaneously in a family who also had associated psychologic disorders.
Case Series
Patient 1 (Proband)
An 11-year-old boy presented with a 6-year history of ongoing AA with recurrent improvement and relapses on the scalp, eyebrows, and eyelashes. Various topical and oral medications had been prescribed by several outside dermatologists; however, these treatments provided minimal benefit and resulted in the recurrence of AA. Dermatologic examination revealed hair loss on the entire frontal, parietal, and temporal regions of the scalp, as well as half of the occipital region and one-third of the lateral side of the eyebrows (Figure 1). Psychological evaluation revealed introvert personality characteristics, lack of self-confidence, and signs of depression and anxiety.

Patient 2 (Proband’s Father)
A 38-year-old man presented with a 16-year history of recurrent loss and regrowth of hair on the scalp and beard area and white spots on the penis and arms. He previously had not undergone any treatments. Dermatologic examination revealed well-circumscribed, 1- to 4-cm, hairless patches on the occipital region of the scalp and in the beard area (Figure 2A) and multiple, 2- to 10-mm, vitiliginous lesions on both forearms (Figure 2B) and the penis. The patient had been unemployed for 6 months. Psychological evaluation revealed obsessive-compulsive disorder and obsessive-compulsive personality disorder.

Patient 3 (Proband’s Mother)
A 32-year-old woman presented with a 3-year history of chronic AA. She previously had not undergone any treatments. Dermatologic examination revealed 2 well-circumscribed, 3- to 4-cm patches of hair loss on the occipital and left temporal regions of the scalp (Figure 3). Psychological evaluation revealed obsessive-compulsive personality disorder and depression. The patient did not have any autoimmune diseases.

Patient 4 (Proband’s Sister)
A 10-year-old girl presented with a 6-year history of recurrent, self-limited AA on various areas of scalp. She previously had not undergone any treatments. Dermatologic examination revealed a 3-cm hairless patch on the occipital region of the scalp (Figure 4). Psychiatric evaluation revealed narcissistic personality disorder, anxiety, and lack of self-confidence.

Laboratory Evaluation and HLA Antigen DNA Typing
Laboratory testing including complete blood cell count; liver, kidney, and thyroid function; and vitamin B12, zinc, folic acid, and fasting blood sugar levels were performed in all patients.
HLA antigen DNA typing was performed by polymerase chain reaction with sequence-specific primers in all patients after informed consent was obtained.
Clinical and laboratory examinations revealed no symptoms or findings of Epstein-Barr virus and cytomegalovirus infections, cicatricial alopecia, or connective tissue diseases in any of the patients. HLA antigen DNA typing revealed the following HLA alleles: B*35/40, C*04/15, DRB1*08/10, and DQB1*03/05 in patient 1; B*04/13, C*06/15, DRB1*07/10, and DQB1*02/05 in patient 2; B*33/37, C*04/06, DRB1*08/15, and DQ*06/06 in patient 3; B*13/37, C*06/06, DRB1*07/15, and DQB1*02/06 in patient 4.
Laboratory testing revealed vitamin B12 deficiency in patient 2 and iron deficiency anemia in patient 3; all other laboratory tests were within reference range. Antithyroglobulin and antithyroid peroxidase autoantibodies were all negative. Clinical features and laboratory analyses for all patients are summarized in the Table.
Treatment
All patients were recommended psychiatric therapy and started on dermatologic treatments. Topical corticosteroids, intralesional triamcinolone acetonide (8 mg/mL) injections into areas of hair loss, 8 total sessions of cryotherapy administered at 3-week intervals, and minoxidil solution 2% were administered respectively to all 4 patients. Alopecia areata in patients 3 and 4 completely regressed; however, no benefit was observed in patients 1 and 2 after 1 year of treatment. Because there was no response to the prior interventions, patient 1 was started on treatment with cyclosporine 2.5 mg/kg twice daily. However, therapy was discontinued after 1 month and treatment with narrowband UVB (3 times per week for 7 months [total of 57 sessions]) and topical corticosteroids were initiated (Table). The patient partially benefited from these regimens and recurrence was observed during the course of the treatment.

Although it was recommended that all 4 patients undergo psychiatric treatment and follow-up regularly with a psychiatrist, the patients declined. After approximately 1 year of dermatologic treatment, all 4 patients were lost to follow-up.
Comment
The etiopathogenesis of AA is unclear, but there is strong evidence suggesting that it is a T-cell–mediated autoimmune disease targeting the hair follicles. Common association of AA with autoimmune diseases such as vitiligo and thyroiditis support the immunological origin of the disease.3 In our case, patient 2 had AA along with vitiligo, but no associated autoimmune diseases (eg, vitiligo, diabetes mellitus, pernicious anemia, thyroid diseases) were noted in the other patients. Genetic and environmental factors are known to be influential as much as immune dysfunction in the etiology of AA.2
The presence of family history in 20% of patients supports the genetic predisposition of AA.4 In a genetic study by Martinez-Mir et al,5 susceptibility loci for AA were demonstrated on chromosomes 6, 10, 16, and 18. HLA antigen alleles, which provide predisposition to AA, have been investigated and associations with many different HLA antigens have been described for AA. In these studies, a relationship between AA and HLA class I antigens was not determined. Notable results mainly focused on HLA class II antigens.6-8 Colombe et al7 and Marques Da Costa et al8 demonstrated that long-lasting alopecia totalis or alopecia universalis (AT/AU) patients had a strong relationship with HLA-DRB1*1104; DRB1*04/05 was reported to be the most frequent HLA group among all patients with AA.6-10 In contrast, we did not detect these alleles in our patients. Colombe et al7,11 noted that HLA-DQB1*03 is a marker for both patch-type AA and AT/AU. Colombe et al10 showed that HLA-DQB1*03 was present in more than 80% of patients (N=286) with long-lasting AA. Barahmani et al9 confirmed a strong association between HLA-DQB1*0301, DRB1*1104, and AT/AU. In our patients, we detected HLA-DQB1*03/05 in patient 1 who had the earliest onset and most severe presentation of AA. In some studies, HLA-DRB1*03 was found to be less frequent in patients with AA, and this allele was suggested to be a protective factor.6,12 However, this allele was not detected in any of our patients.
The association of HLA alleles and AA has been investigated in Turkish patients with AA.13-15 Akar et al13 and Kavak et al14 detected that the frequency of HLA-DQB1*03 allele was remarkably higher in patients with AA than in healthy controls. These results were consistent with Colombe et al.10 On the other hand, Kavak et al14 reported that the frequency of HLA-DR16 was decreased in the patient group with AA. In another study, the frequency of HLA-B62 was increased in patients with AA compared to healthy controls.15 The HLA-DQB1*03 allele was found to be associated with AA in only patient 1 in our case series, and HLA alleles were not commonly shared among the 4 patients. Additionally, lack of consanguinity between patients 2 and 3 (the parents) also suggested that genetic factors were not involved in our familial cases.
Blaumeiser et al16 reported a lifetime risk of 7.4% in parents and 7.1% in siblings of 206 AA patients; however, because these studies investigated the presence of AA in any given life period of the family members, their results do not reflect frequency of simultaneous AA presence within one family. In a literature search using PubMed, Google Scholar, and other national databases for the terms alopecia areata as well as family, sibling, concurrently, concomitant, co-existent, and simultaneously, only 2 cases involving a husband and wife and 1 case of 2 siblings who concurrently had AA have been previously reported.17,18 Simultaneous presence of AA in more than 3 members of the same family is rare, and these cases have been observed in different generations and time periods.19 Among our patients, despite different age of onset and duration, AA was simultaneously present in the entire family.
Moreover, Rodriguez et al20 reported that the concordance rate of AA in identical twins was 42% and dizygotic twins was 10%. Environmental factors and infections also have been implicated in the etiology of AA. Infections caused by viruses such as cytomegalovirus and Epstein-Barr virus have been thought to be potential triggering factors; however, no evidence has been found.21,22 The clinical and laboratory examinations in our study did not reveal any presence and/or history of any known infectious disease, and there was no history of contact with water infected by acrylamide or a similar chemical.
Various life events and intense psychological stress may play an important role in triggering AA. Depression, hysteria, psychopathic deviance, psychasthenia, schizophrenia, anxiety, health concerns, bizarre thoughts, and family problems were found to be more frequent in patients with AA than healthy controls.23 The most common psychological disorders associated with AA are generalized anxiety disorder, major depressive disorder, adjustment disorders, and phobias.1,24 Ruiz-Doblado et al25 determined the presence of psychiatric comorbidities in 66% (21/32) of AA cases. Chu et al26 reported that the differences in ages of onset of AA revealed differences in psychiatric comorbidities. The risk for depression was higher in patients with AA younger than 20 years. An increased rate of anxiety was detected with patients with an onset of AA between the ages of 20 and 39 years. Obsessive-compulsive disorder and anxiety were more common in patients aged 40 to 59 years. Interestingly, the investigators also observed that approximately 50% of psychiatric disorders occurred prior to onset of AA.26 One study showed higher rates of stressful life events in children than in controls.27 Ghanizadeh24 reported at least 1 psychiatric disorder in 78% (11/14) of children and adolescents with AA. In the same study, obsessive-compulsive disorder was found to be the second common condition following major depression in AA.24
In our patients, psychiatric evaluations revealed obsessive-compulsive personality disorder in patients 2 and 3, depression in patient 3, and symptoms of anxiety with a lack of self-confidence in patients 1 and 4. Psychiatric disorders affecting the entire family may stem from unemployment of the father. Similar to the results noted in prior studies, depression, the most commonly associated psychiatric disorder of AA, was present in 2 of 4 patients. Obsessive-compulsive disorder, the second most common psychiatric disorder among AA patients, was present in patients 2 and 3. These results indicate that AA may be associated with shared stressful events and psychiatric disorders. Therefore, in addition to dermatologic treatment, it was recommended that all patients undergo psychiatric treatment and follow-up regularly with a psychiatrist; however, the patients declined. At the end of a 1-year treatment period and follow-up, resistance to therapy with minimal recovery followed by a rapid recurrence was determined in patients 1 and 2.
Conclusion
This report demonstrated that familial AA was strongly associated with psychological disorders that were detected in all patients. In our patients, HLA alleles did not seem to have a role in the development of familial AA. These results suggest that HLA was not associated with AA triggered by psychological stress. We believe that psychological disorders and stressful life events may play an important role in the occurrence of AA and lead to the development of resistance against treatment in familial and resistant AA cases.
Alopecia areata (AA) presents as sudden, nonscarring, recurrent hair loss characterized by well-circumscribed hairless patches. Although AA may be observed on any hair-bearing areas of the body, the most commonly affected sites are the scalp, beard area, eyebrows, and eyelashes.1 The incidence of AA is 1% to 2% in the general population and it is more common in males than females younger than 40 years.2 Although the majority of patients present with self-limited and well-circumscribed hairless patches that resolve within 2 years, 7% to 10% display a chronic and severe prognosis.3
The etiopathogenesis of AA is not clearly understood, but its occurrence and progression can involve immune dysfunction, genetic predisposition, infections, and physical and psychological trauma.2 Alopecia areata is observed to occur sporadically in most patients. Family history has been found in 3% to 42% of cases, but simultaneous occurrence of AA in family members is rare.4 In this case series, we present 4 cases of active AA lesions occurring simultaneously in a family who also had associated psychologic disorders.
Case Series
Patient 1 (Proband)
An 11-year-old boy presented with a 6-year history of ongoing AA with recurrent improvement and relapses on the scalp, eyebrows, and eyelashes. Various topical and oral medications had been prescribed by several outside dermatologists; however, these treatments provided minimal benefit and resulted in the recurrence of AA. Dermatologic examination revealed hair loss on the entire frontal, parietal, and temporal regions of the scalp, as well as half of the occipital region and one-third of the lateral side of the eyebrows (Figure 1). Psychological evaluation revealed introvert personality characteristics, lack of self-confidence, and signs of depression and anxiety.

Patient 2 (Proband’s Father)
A 38-year-old man presented with a 16-year history of recurrent loss and regrowth of hair on the scalp and beard area and white spots on the penis and arms. He previously had not undergone any treatments. Dermatologic examination revealed well-circumscribed, 1- to 4-cm, hairless patches on the occipital region of the scalp and in the beard area (Figure 2A) and multiple, 2- to 10-mm, vitiliginous lesions on both forearms (Figure 2B) and the penis. The patient had been unemployed for 6 months. Psychological evaluation revealed obsessive-compulsive disorder and obsessive-compulsive personality disorder.

Patient 3 (Proband’s Mother)
A 32-year-old woman presented with a 3-year history of chronic AA. She previously had not undergone any treatments. Dermatologic examination revealed 2 well-circumscribed, 3- to 4-cm patches of hair loss on the occipital and left temporal regions of the scalp (Figure 3). Psychological evaluation revealed obsessive-compulsive personality disorder and depression. The patient did not have any autoimmune diseases.

Patient 4 (Proband’s Sister)
A 10-year-old girl presented with a 6-year history of recurrent, self-limited AA on various areas of scalp. She previously had not undergone any treatments. Dermatologic examination revealed a 3-cm hairless patch on the occipital region of the scalp (Figure 4). Psychiatric evaluation revealed narcissistic personality disorder, anxiety, and lack of self-confidence.

Laboratory Evaluation and HLA Antigen DNA Typing
Laboratory testing including complete blood cell count; liver, kidney, and thyroid function; and vitamin B12, zinc, folic acid, and fasting blood sugar levels were performed in all patients.
HLA antigen DNA typing was performed by polymerase chain reaction with sequence-specific primers in all patients after informed consent was obtained.
Clinical and laboratory examinations revealed no symptoms or findings of Epstein-Barr virus and cytomegalovirus infections, cicatricial alopecia, or connective tissue diseases in any of the patients. HLA antigen DNA typing revealed the following HLA alleles: B*35/40, C*04/15, DRB1*08/10, and DQB1*03/05 in patient 1; B*04/13, C*06/15, DRB1*07/10, and DQB1*02/05 in patient 2; B*33/37, C*04/06, DRB1*08/15, and DQ*06/06 in patient 3; B*13/37, C*06/06, DRB1*07/15, and DQB1*02/06 in patient 4.
Laboratory testing revealed vitamin B12 deficiency in patient 2 and iron deficiency anemia in patient 3; all other laboratory tests were within reference range. Antithyroglobulin and antithyroid peroxidase autoantibodies were all negative. Clinical features and laboratory analyses for all patients are summarized in the Table.
Treatment
All patients were recommended psychiatric therapy and started on dermatologic treatments. Topical corticosteroids, intralesional triamcinolone acetonide (8 mg/mL) injections into areas of hair loss, 8 total sessions of cryotherapy administered at 3-week intervals, and minoxidil solution 2% were administered respectively to all 4 patients. Alopecia areata in patients 3 and 4 completely regressed; however, no benefit was observed in patients 1 and 2 after 1 year of treatment. Because there was no response to the prior interventions, patient 1 was started on treatment with cyclosporine 2.5 mg/kg twice daily. However, therapy was discontinued after 1 month and treatment with narrowband UVB (3 times per week for 7 months [total of 57 sessions]) and topical corticosteroids were initiated (Table). The patient partially benefited from these regimens and recurrence was observed during the course of the treatment.

Although it was recommended that all 4 patients undergo psychiatric treatment and follow-up regularly with a psychiatrist, the patients declined. After approximately 1 year of dermatologic treatment, all 4 patients were lost to follow-up.
Comment
The etiopathogenesis of AA is unclear, but there is strong evidence suggesting that it is a T-cell–mediated autoimmune disease targeting the hair follicles. Common association of AA with autoimmune diseases such as vitiligo and thyroiditis support the immunological origin of the disease.3 In our case, patient 2 had AA along with vitiligo, but no associated autoimmune diseases (eg, vitiligo, diabetes mellitus, pernicious anemia, thyroid diseases) were noted in the other patients. Genetic and environmental factors are known to be influential as much as immune dysfunction in the etiology of AA.2
The presence of family history in 20% of patients supports the genetic predisposition of AA.4 In a genetic study by Martinez-Mir et al,5 susceptibility loci for AA were demonstrated on chromosomes 6, 10, 16, and 18. HLA antigen alleles, which provide predisposition to AA, have been investigated and associations with many different HLA antigens have been described for AA. In these studies, a relationship between AA and HLA class I antigens was not determined. Notable results mainly focused on HLA class II antigens.6-8 Colombe et al7 and Marques Da Costa et al8 demonstrated that long-lasting alopecia totalis or alopecia universalis (AT/AU) patients had a strong relationship with HLA-DRB1*1104; DRB1*04/05 was reported to be the most frequent HLA group among all patients with AA.6-10 In contrast, we did not detect these alleles in our patients. Colombe et al7,11 noted that HLA-DQB1*03 is a marker for both patch-type AA and AT/AU. Colombe et al10 showed that HLA-DQB1*03 was present in more than 80% of patients (N=286) with long-lasting AA. Barahmani et al9 confirmed a strong association between HLA-DQB1*0301, DRB1*1104, and AT/AU. In our patients, we detected HLA-DQB1*03/05 in patient 1 who had the earliest onset and most severe presentation of AA. In some studies, HLA-DRB1*03 was found to be less frequent in patients with AA, and this allele was suggested to be a protective factor.6,12 However, this allele was not detected in any of our patients.
The association of HLA alleles and AA has been investigated in Turkish patients with AA.13-15 Akar et al13 and Kavak et al14 detected that the frequency of HLA-DQB1*03 allele was remarkably higher in patients with AA than in healthy controls. These results were consistent with Colombe et al.10 On the other hand, Kavak et al14 reported that the frequency of HLA-DR16 was decreased in the patient group with AA. In another study, the frequency of HLA-B62 was increased in patients with AA compared to healthy controls.15 The HLA-DQB1*03 allele was found to be associated with AA in only patient 1 in our case series, and HLA alleles were not commonly shared among the 4 patients. Additionally, lack of consanguinity between patients 2 and 3 (the parents) also suggested that genetic factors were not involved in our familial cases.
Blaumeiser et al16 reported a lifetime risk of 7.4% in parents and 7.1% in siblings of 206 AA patients; however, because these studies investigated the presence of AA in any given life period of the family members, their results do not reflect frequency of simultaneous AA presence within one family. In a literature search using PubMed, Google Scholar, and other national databases for the terms alopecia areata as well as family, sibling, concurrently, concomitant, co-existent, and simultaneously, only 2 cases involving a husband and wife and 1 case of 2 siblings who concurrently had AA have been previously reported.17,18 Simultaneous presence of AA in more than 3 members of the same family is rare, and these cases have been observed in different generations and time periods.19 Among our patients, despite different age of onset and duration, AA was simultaneously present in the entire family.
Moreover, Rodriguez et al20 reported that the concordance rate of AA in identical twins was 42% and dizygotic twins was 10%. Environmental factors and infections also have been implicated in the etiology of AA. Infections caused by viruses such as cytomegalovirus and Epstein-Barr virus have been thought to be potential triggering factors; however, no evidence has been found.21,22 The clinical and laboratory examinations in our study did not reveal any presence and/or history of any known infectious disease, and there was no history of contact with water infected by acrylamide or a similar chemical.
Various life events and intense psychological stress may play an important role in triggering AA. Depression, hysteria, psychopathic deviance, psychasthenia, schizophrenia, anxiety, health concerns, bizarre thoughts, and family problems were found to be more frequent in patients with AA than healthy controls.23 The most common psychological disorders associated with AA are generalized anxiety disorder, major depressive disorder, adjustment disorders, and phobias.1,24 Ruiz-Doblado et al25 determined the presence of psychiatric comorbidities in 66% (21/32) of AA cases. Chu et al26 reported that the differences in ages of onset of AA revealed differences in psychiatric comorbidities. The risk for depression was higher in patients with AA younger than 20 years. An increased rate of anxiety was detected with patients with an onset of AA between the ages of 20 and 39 years. Obsessive-compulsive disorder and anxiety were more common in patients aged 40 to 59 years. Interestingly, the investigators also observed that approximately 50% of psychiatric disorders occurred prior to onset of AA.26 One study showed higher rates of stressful life events in children than in controls.27 Ghanizadeh24 reported at least 1 psychiatric disorder in 78% (11/14) of children and adolescents with AA. In the same study, obsessive-compulsive disorder was found to be the second common condition following major depression in AA.24
In our patients, psychiatric evaluations revealed obsessive-compulsive personality disorder in patients 2 and 3, depression in patient 3, and symptoms of anxiety with a lack of self-confidence in patients 1 and 4. Psychiatric disorders affecting the entire family may stem from unemployment of the father. Similar to the results noted in prior studies, depression, the most commonly associated psychiatric disorder of AA, was present in 2 of 4 patients. Obsessive-compulsive disorder, the second most common psychiatric disorder among AA patients, was present in patients 2 and 3. These results indicate that AA may be associated with shared stressful events and psychiatric disorders. Therefore, in addition to dermatologic treatment, it was recommended that all patients undergo psychiatric treatment and follow-up regularly with a psychiatrist; however, the patients declined. At the end of a 1-year treatment period and follow-up, resistance to therapy with minimal recovery followed by a rapid recurrence was determined in patients 1 and 2.
Conclusion
This report demonstrated that familial AA was strongly associated with psychological disorders that were detected in all patients. In our patients, HLA alleles did not seem to have a role in the development of familial AA. These results suggest that HLA was not associated with AA triggered by psychological stress. We believe that psychological disorders and stressful life events may play an important role in the occurrence of AA and lead to the development of resistance against treatment in familial and resistant AA cases.
- García-Hernández MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, et al. Alopecia areata, stress and psychiatric disorders: a review. J Dermatol. 1999;26:625-632.
- Bhat YJ, Manzoor S, Khan AR, et al. Trace element levels in alopecia areata. Indian J Dermatol Venereol Leprol. 2009;75:29-31.
- Alexis AF, Dudda-Subramanya R, Sinha AA. Alopecia areata: autoimmune basis of hair loss. Eur J Dermatol. 2004;14:364-370.
- Green J, Sinclair RD. Genetics of alopecia areata. Australas J Dermatol. 2000;41:213-218.
- Martinez-Mir A, Zlotogorski A, Gordon D, et al.Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80:316-328.
- Entz P, Blaumeiser B, Betz RC, et al. Investigation of the HLA-DRB1 locus in alopecia areata. Eur J Dermatol. 2006;16:363-367.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II alleles in long-standing alopecia totalis/alopecia universalis and long-standing patchy alopecia areata differentiate these two clinical groups. J Invest Dermatol. 1995;104(suppl 5):4-5.
- Marques Da Costa C, Dupont E, Van der Cruys M, et al. Earlier occurrence of severe alopecia areata in HLA-DRB1*11-positive patients. Dermatology. 2006;213:12-14.
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Colombe BW, Lou CD, Price VH. The genetic basis of alopecia areata: HLA associations with patchy alopecia areata versus alopecia totalis and alopecia universalis. J Investig Dermatol Symp Proc. 1999;4:216-219.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II antigen associations help to define two types of alopecia areata. J Am Acad Dermatol. 1995;33(5, pt 1):757-764.
- Broniarczyk-Dyła G, Prusińska-Bratoś M, Dubla-Berner M, et al. The protective role of the HLA-DR locus in patients with various clinical types of alopecia areata. Arch Immunol Ther Exp (Warsz). 2002;50:333-336.
- Akar A, Orkunuglu E, Sengul A, et al. HLA class II alleles in patients with alopecia areata. Eur J Dermatol. 2002;12:236-239.
- Kavak A, Baykal C, Ozarmagan G, et al. HLA in alopecia areata. Int J Dermatol. 2000;30:589-592.
- Aliagaoglu C, Pirim I, Atasoy M, et al. Association between alopecia areata and HLA class I and II in Turkey. J Dermatol. 2005;32:711-714.
- Blaumeiser B, Goot I, Fimmers R, et al. Familial aggregation of alopecia areata. J Am Acad Dermatol. 2006;54:627-632.
- Zalka AD, Byarlay JA, Goldsmith LA. Alopecia a deux: simultaneous occurrence of alopecia in a husband and wife. Arch Dermatol. 1994;130:390-392.
- Menon R, Kiran C. Concomitant presentation of alopecia areata in siblings: a rare occurrence. Int J Trichology. 2012;4:86-88.
- Valsecchi R, Vicari O, Frigeni A, et al. Familial alopecia areata-genetic susceptibility or coincidence? Acta Derm Venereol (Stockh). 1985;65:175-177.
- Rodriguez TA, Fernandes KE, Dresser KL, et al. Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J Am Acad Dermatol. 2010;62:525-527.
- Rodriguez TA, Duvic M. Onset of alopecia areata after Epstein Barr virus infectious mononucleosis. J Am Acad Dermatol. 2008;59:137-139.
- Offidani A, Amerio P, Bernardini ML, et al. Role of cytomegalovirus replication in alopecia areata pathogenesis. J Cutan Med Surg. 2000;4:63-65.
- Alfani S, Antinone V, Mozzetta A, et al. Psychological status of patients with alopecia areata. Acta Derm Venereol. 2012;92:304-306.
- Ghanizadeh A. Comorbidity of psychiatric disorders in children and adolescents with alopecia areata in a child and adolescent psychiatry clinical sample. Int J Dermatol. 2008;47:1118-1120.
- Ruiz-Doblado S, Carrizosa A, Garcia-Hernandez MJ. Alopecia areata: psychiatric comorbidity and adjustment to illness. Int J Dermatol. 2003;42:434-437.
- Chu SY, Chen YJ, Tseng WC, et al. Psychiatric comorbidities in patients with alopecia areata in Taiwan: a case-control study. Br J Dermatol. 2012;166:525-531.
- Manolache L, Petrescu-Seceleanu D, Benea V. Alopecia areata and stressful events in children. J Eur Acad Dermatol Venereol. 2009;23:107-109.
- García-Hernández MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, et al. Alopecia areata, stress and psychiatric disorders: a review. J Dermatol. 1999;26:625-632.
- Bhat YJ, Manzoor S, Khan AR, et al. Trace element levels in alopecia areata. Indian J Dermatol Venereol Leprol. 2009;75:29-31.
- Alexis AF, Dudda-Subramanya R, Sinha AA. Alopecia areata: autoimmune basis of hair loss. Eur J Dermatol. 2004;14:364-370.
- Green J, Sinclair RD. Genetics of alopecia areata. Australas J Dermatol. 2000;41:213-218.
- Martinez-Mir A, Zlotogorski A, Gordon D, et al.Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80:316-328.
- Entz P, Blaumeiser B, Betz RC, et al. Investigation of the HLA-DRB1 locus in alopecia areata. Eur J Dermatol. 2006;16:363-367.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II alleles in long-standing alopecia totalis/alopecia universalis and long-standing patchy alopecia areata differentiate these two clinical groups. J Invest Dermatol. 1995;104(suppl 5):4-5.
- Marques Da Costa C, Dupont E, Van der Cruys M, et al. Earlier occurrence of severe alopecia areata in HLA-DRB1*11-positive patients. Dermatology. 2006;213:12-14.
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Colombe BW, Lou CD, Price VH. The genetic basis of alopecia areata: HLA associations with patchy alopecia areata versus alopecia totalis and alopecia universalis. J Investig Dermatol Symp Proc. 1999;4:216-219.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II antigen associations help to define two types of alopecia areata. J Am Acad Dermatol. 1995;33(5, pt 1):757-764.
- Broniarczyk-Dyła G, Prusińska-Bratoś M, Dubla-Berner M, et al. The protective role of the HLA-DR locus in patients with various clinical types of alopecia areata. Arch Immunol Ther Exp (Warsz). 2002;50:333-336.
- Akar A, Orkunuglu E, Sengul A, et al. HLA class II alleles in patients with alopecia areata. Eur J Dermatol. 2002;12:236-239.
- Kavak A, Baykal C, Ozarmagan G, et al. HLA in alopecia areata. Int J Dermatol. 2000;30:589-592.
- Aliagaoglu C, Pirim I, Atasoy M, et al. Association between alopecia areata and HLA class I and II in Turkey. J Dermatol. 2005;32:711-714.
- Blaumeiser B, Goot I, Fimmers R, et al. Familial aggregation of alopecia areata. J Am Acad Dermatol. 2006;54:627-632.
- Zalka AD, Byarlay JA, Goldsmith LA. Alopecia a deux: simultaneous occurrence of alopecia in a husband and wife. Arch Dermatol. 1994;130:390-392.
- Menon R, Kiran C. Concomitant presentation of alopecia areata in siblings: a rare occurrence. Int J Trichology. 2012;4:86-88.
- Valsecchi R, Vicari O, Frigeni A, et al. Familial alopecia areata-genetic susceptibility or coincidence? Acta Derm Venereol (Stockh). 1985;65:175-177.
- Rodriguez TA, Fernandes KE, Dresser KL, et al. Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J Am Acad Dermatol. 2010;62:525-527.
- Rodriguez TA, Duvic M. Onset of alopecia areata after Epstein Barr virus infectious mononucleosis. J Am Acad Dermatol. 2008;59:137-139.
- Offidani A, Amerio P, Bernardini ML, et al. Role of cytomegalovirus replication in alopecia areata pathogenesis. J Cutan Med Surg. 2000;4:63-65.
- Alfani S, Antinone V, Mozzetta A, et al. Psychological status of patients with alopecia areata. Acta Derm Venereol. 2012;92:304-306.
- Ghanizadeh A. Comorbidity of psychiatric disorders in children and adolescents with alopecia areata in a child and adolescent psychiatry clinical sample. Int J Dermatol. 2008;47:1118-1120.
- Ruiz-Doblado S, Carrizosa A, Garcia-Hernandez MJ. Alopecia areata: psychiatric comorbidity and adjustment to illness. Int J Dermatol. 2003;42:434-437.
- Chu SY, Chen YJ, Tseng WC, et al. Psychiatric comorbidities in patients with alopecia areata in Taiwan: a case-control study. Br J Dermatol. 2012;166:525-531.
- Manolache L, Petrescu-Seceleanu D, Benea V. Alopecia areata and stressful events in children. J Eur Acad Dermatol Venereol. 2009;23:107-109.
Practice Points
- The etiopathogenesis of alopecia areata (AA) is not clearly understood, but its occurrence and progression can involve immune dysfunction, genetic predisposition, infections, and physical and psychological trauma.
- Alopecia areata is observed to occur sporadically in most patients. Simultaneous presence of AA in more than 3 members of the same family is rare, and these cases have been observed in different generations and time periods.
- HLA antigen alleles, which provide predisposition to AA, have been investigated, and associations with many different HLA antigens have been described for AA. In previous studies, HLA-DQB1*03 allele was reported as the most common HLA allele in patients with AA.
- Psychological disorders and shared stressful life events may play an important role in the occurrence of AA and lead to the development of resistance against treatment in familial and resistant AA cases.
Invasive Salmonellosis in a 45-Day-Old Infant
The management of a febrile infant is complex and requires obtaining a detailed history of all possible exposures. Published guidelines alone are not always completely accurate for diagnosing or excluding serious illness, and are not a substitute for a thorough examination and history.
Case
The parents of a 45-day-old girl were referred to our regional pediatric hospital by a local community hospital for emergent evaluation of their infant. The day prior, they had taken the infant to the referring ED because of persistent fussiness and subjective fever. They were neither sure of the tests that were obtained during that visit nor why they were instructed to take their daughter to our pediatric facility. They did, however, recall that during the visit to the community ED, the patient had a rectal temperature of 102.7°F, was given an antibiotic injection, and was well appearing and acting normally. Also, at discharge, the infant’s parents were instructed to follow up with the patient’s pediatrician within 24 hours.
Since their daughter’s discharge from the community ED, both parents noted that she seemed more irritable, felt warm, and had not been feeding well. They confirmed that she was an otherwise healthy infant who had been born full term via normal vaginal delivery and without complications.
On initial assessment at our ED, the patient was fussy and had mottled extremities and dusky nail beds. Her vital signs at presentation were: heart rate, 223 beats/minute; respiratory rate, 36 breaths/minute; and rectal temperature, 103.6°F. Oxygen saturation was 96% on room air. The infant was resuscitated with 20 mL/kg of intravenous (IV) normal saline and given oral acetaminophen. Laboratory studies were obtained, and a lumbar puncture (LP) was performed.
She was treated with IV acyclovir, ceftriaxone, and vancomycin. Her complete blood count (CBC) was notable for a white blood cell count (WBC) of 22.60 x 109/L; cerebrospinal fluid (CSF) analysis revealed a WBC of 4.52 x 109/L with 75% neutrophils, and serum glucose of 63 mg/dL.
Since the patient’s parents did not have any paperwork or information from the prior ED visit, our ED contacted the community ED by phone, and a representative provided the following information: the patient had appeared well but was febrile at presentation; laboratory evaluation was obtained, but no LP was performed; she was treated with intramuscular (IM) ceftriaxone and acetaminophen; and she was discharged home in the care of her parents. Regarding laboratory studies performed at the community ED, the only test result made available by phone was the preliminary blood culture report that revealed growth of gram-negative rods with speciation pending, which prompted the referral to our facility.
Based on the information provided by the community ED and our evaluation and work-up, the patient was admitted to the pediatric intensive care unit. Magnetic resonance imaging (MRI) of the brain was performed, which showed debris in the lateral ventricles consistent with ventriculitis—likely secondary to meningitis. The blood, urine, and cerebrospinal fluid (CSF) cultures collected during our evaluation produced no growth; however, blood cultures from the rural ED eventually grew Salmonella.
A further detailed history revealed that the infant and her parents had been living with a family friend who owned an iguana. According to reports, the iguana had free run of the home and often crawled around and across the infant while she was lying on a blanket on the floor. The patient’s parents were not aware of the diseases associated with reptile contact. Due to concerns over the social situation, the patient was kept in the hospital for the entire recommended 21-day course of antibiotic therapy, during which time the parents received assistance finding alternate living arrangements.
Discussion
Current Practice Guidelines for Managing Febrile Infants
Current guidelines from the American Academy of Pediatrics (AAP) and the American College of Emergency Physicians (ACEP) recommend a full sepsis work-up for all neonates (ie, ages 0 to 28 days) who present with a fever (defined as a rectal temperature ≥100.4°F).1,2 The probability of a serious bacterial infection (SBI) in patients in this age group who present with fever is approximately 12%.3 A full sepsis work-up generally includes a CBC, blood cultures, urinalysis with culture, CSF analysis with culture, and stool cultures if diarrhea is present.
Current guidelines for infants 29 to 90 days of age who present with fever differ between professional associations. The AAP and the American Academy of Family Physicians recommend the following for children in this age range: laboratory evaluation with CBC, blood cultures, CSF analysis, urinalysis, and culture. If laboratory evaluation reveals a WBC of less than 15 x 109/L with an absolute neutrophil count of less than 10 x 109/L, along with a normal CSF and urinalysis, the patient can be given IM ceftriaxone and follow-up arranged in 24 hours. This approach is recommended for patients who are otherwise healthy, nontoxic at presentation, and under the care of a responsible adult.4,5 By comparison, the Philadelphia protocol, though suggesting an identical work-up, recommends against the use of antibiotics in infants deemed at low risk for SBI.6
The ACEP does not specifically endorse a management strategy for febrile infants in the 29- to 90-day age group, but instead acknowledges that no age cut-off within this group can be considered absolute when determining management strategy, and suggests that children up to 60 days old should be managed in a manner similar to neonates.2 The published guidelines do not include consideration of specific history exposure in the management recommendations.
Typhoidal Serotypes
Salmonella can be divided into typhoidal (including S typhi and S paratyphi) and nontyphoidal serotypes (NTS), with the two groups manifesting as very different diseases.7 Typhoidal serotypes lead to the disease process known as typhoid, which typically presents with fever, chills, abdominal pain, nonbloody diarrhea or constipation, nausea, anorexia, headache, hepatosplenomegaly, and rose spots.8 These symptoms typically present after a 14-day incubation period and persist for 21 days.9 Humans are the only known infected source of these species, which are spread via the fecal-oral route.10
In contrast, disease from NTS manifests within 12 hours of exposure with watery diarrhea, nausea, vomiting, and fever, with symptoms lasting up to 10 days.11 Both groups cause disease by invading the intestinal epithelium12; however, typhoidal species induce less intestinal inflammation, facilitating bacterial invasion and making systemic disease more likely.13
Transmission
Many animals are known to carry NTS, including reptiles, where Salmonella occurs naturally in their gastrointestinal tract.14 Twenty-five percent of Salmonella infections in children younger than age 5 years have been attributed to contact with a pet,15 with small turtles (shell diameter <4 inches) accounting for 42% of all pet-related Salmonella infections.
Though gastroenteritis is the most common clinical manifestation of infection with NTS, approximately 5% of patients will develop invasive disease, including bacteremia, meningitis, septic arthritis, or osteomyelitis.16 Children with invasive disease are more likely to have been exposed to an iguana, snake, or bearded dragon than to a turtle. If the pet is kept indoors, the risk of invasive disease is more likely. The average age of patients with invasive disease is 62 days, versus 2 years for noninvasive disease.17
Diagnosis
Growth of Salmonella on cultures of stool, blood, urine, or CSF dishes is the mainstay of diagnosis of typhoid and nontyphoidal disease, but bacterial concentrations are higher in bone marrow aspirate, making it superior to blood cultures.18 Biopsy of the rose spots of typhoid may also provide the diagnosis.
Management
Since Salmonella gastroenteritis is usually a self-limited disease, current recommendations reserve treatment with antibiotics for patients with severe disease or who are immunocompromised. When necessary, treatment consists of 7 to 10 days of a fluoroquinolone or third-generation cephalosporin, which is the same regimen suggested for typhoid. Treatment of central nervous system (CNS) salmonellosis consists of at least 3 weeks of a third-generation cephalosporin; the AAP recommends at least 4 weeks of treatment.19
Case Conclusion
Prior to the patient’s transfer to our facility, she was treated empirically with ceftriaxone without prior CSF analysis—an approach that does not follow any current guidelines for the treatment of a febrile infant. Though an LP was not performed until approximately 24 hours after the initial antibiotic was given, the patient demonstrated CSF pleocytosis with no organisms on gram stain and no growth on culture. Given this pleocytosis and Salmonella bacteremia in the context of prior antibiotic treatment, and MRI consistent with CNS involvement, the patient was treated for 21 days for presumed Salmonella meningitis. A CSF analysis performed on her initial visit could have more accurately directed the type and duration of treatment if the findings on subsequent imaging studies and CSF analysis were ambiguous.
Summary
Emergency physicians may underestimate the likelihood of SBI in otherwise well-appearing febrile infants. While certain aspects of the history and physical examination in a febrile, well-appearing infant have been shown to correlate with an increased risk of SBI, no single finding can definitively rule in or rule out the disease.20 Opinions differ as to optimal management strategies for febrile, well-appearing infants outside the neonatal period. However, an appropriate level of clinical suspicion, within the context of a thorough investigation into the infant’s health history and social situation, can aid the clinician and guide treatment and disposition.
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. Erratum in Ann Emerg Med. 1993;22(9):1490.
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545.
3. Kadish HA, Loveridge B, Tobey J, Bolte RG, Corneli HM. Applying outpatient protocols in febrile infants 1-28 days of age: can the threshold be lowered? Clin Pediatr (Phila). 2000;39(2):81-88.
4. Sur DK, Bukont EL. Evaluating fever of unidentifiable source in young children. Am Fam Physician. 2007;75(12):1805-1811.
5. Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J. 1993;12(5):389-394.
6. Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Eng J Med. 1993;329(20):1437-1441.
7. Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front in Microbiol. 2014;5:391.
8. Stuart BM, Pullen RL. Typhoid: clinical analysis of 360 cases. Arch Intern Med (Chic). 1946;78(6):
629-661.
9. Olsen SJ, Bleasdale SC, Magnano AR, et al. Outbreaks of typhoid fever in the United States, 1960-99. Epidemiol Infect. 2003;130(1):13-21.
10. Newton AE, Routh JA, Mahon BE. Typhoid and Paratyphoid Fever. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/typhoid-paratyphoid-fever. Updated July 10, 2015. Accessed June 13, 2016.
11. McGovern VJ, Slavutin LJ. Pathology of Salmonella colitis. Am J Surg Pathol. 1979;3(6):483-490.
12. Liu SL, Ezaki T, Miura H, Matsui K, Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infect Immun. 1988;56(8):1967-1973.
13. House D, Wain J, Ho VA, et al. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J Clin Microbiol. 2001;39(3):1002-1007.
14. Hoelzer K, Moreno Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42(1):34.
15. Murphy D, Oshin F. Reptile-associated salmonellosis in children aged under 5 years in South West England. Arch Dis Child. 2015;100(4):364-365.
16. Iwamoto M. Infectious diseases related to travel. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/salmonellosis-nontyphoidal. Updated July 10, 2015. Accessed June 13, 2016.
17. Meyer Sauteur PM, Relly C, Hug M, Wittenbrink MM, Berger C. Risk factors for invasive reptile-associated salmonellosis in children. Vector-Borne and Zoonotic Dis. 2013;13(6):419-421.
18. Gasem MH, Keuter M, Dolmans WM, Van Der Ven-Jongekrijg J, Djokomoeljanto R, Van Der Meer JW. Persistence of Salmonellae in blood and bone marrow: randomized controlled trial comparing ciprofloxacin and chloramphenicol treatments against enteric fever. Antimicrob Agents Chemother. 2003;47(5):1727-1731.
19. Price EH, de Louvois J, Workman MR. Antibiotics for Salmonella meningitis in children. J Antimicrob Chemother. 2000;46(5) 653-655.
20. Craig JC, Williams GJ, Jones M, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. BMJ. 2010;340:c1594.
The management of a febrile infant is complex and requires obtaining a detailed history of all possible exposures. Published guidelines alone are not always completely accurate for diagnosing or excluding serious illness, and are not a substitute for a thorough examination and history.
Case
The parents of a 45-day-old girl were referred to our regional pediatric hospital by a local community hospital for emergent evaluation of their infant. The day prior, they had taken the infant to the referring ED because of persistent fussiness and subjective fever. They were neither sure of the tests that were obtained during that visit nor why they were instructed to take their daughter to our pediatric facility. They did, however, recall that during the visit to the community ED, the patient had a rectal temperature of 102.7°F, was given an antibiotic injection, and was well appearing and acting normally. Also, at discharge, the infant’s parents were instructed to follow up with the patient’s pediatrician within 24 hours.
Since their daughter’s discharge from the community ED, both parents noted that she seemed more irritable, felt warm, and had not been feeding well. They confirmed that she was an otherwise healthy infant who had been born full term via normal vaginal delivery and without complications.
On initial assessment at our ED, the patient was fussy and had mottled extremities and dusky nail beds. Her vital signs at presentation were: heart rate, 223 beats/minute; respiratory rate, 36 breaths/minute; and rectal temperature, 103.6°F. Oxygen saturation was 96% on room air. The infant was resuscitated with 20 mL/kg of intravenous (IV) normal saline and given oral acetaminophen. Laboratory studies were obtained, and a lumbar puncture (LP) was performed.
She was treated with IV acyclovir, ceftriaxone, and vancomycin. Her complete blood count (CBC) was notable for a white blood cell count (WBC) of 22.60 x 109/L; cerebrospinal fluid (CSF) analysis revealed a WBC of 4.52 x 109/L with 75% neutrophils, and serum glucose of 63 mg/dL.
Since the patient’s parents did not have any paperwork or information from the prior ED visit, our ED contacted the community ED by phone, and a representative provided the following information: the patient had appeared well but was febrile at presentation; laboratory evaluation was obtained, but no LP was performed; she was treated with intramuscular (IM) ceftriaxone and acetaminophen; and she was discharged home in the care of her parents. Regarding laboratory studies performed at the community ED, the only test result made available by phone was the preliminary blood culture report that revealed growth of gram-negative rods with speciation pending, which prompted the referral to our facility.
Based on the information provided by the community ED and our evaluation and work-up, the patient was admitted to the pediatric intensive care unit. Magnetic resonance imaging (MRI) of the brain was performed, which showed debris in the lateral ventricles consistent with ventriculitis—likely secondary to meningitis. The blood, urine, and cerebrospinal fluid (CSF) cultures collected during our evaluation produced no growth; however, blood cultures from the rural ED eventually grew Salmonella.
A further detailed history revealed that the infant and her parents had been living with a family friend who owned an iguana. According to reports, the iguana had free run of the home and often crawled around and across the infant while she was lying on a blanket on the floor. The patient’s parents were not aware of the diseases associated with reptile contact. Due to concerns over the social situation, the patient was kept in the hospital for the entire recommended 21-day course of antibiotic therapy, during which time the parents received assistance finding alternate living arrangements.
Discussion
Current Practice Guidelines for Managing Febrile Infants
Current guidelines from the American Academy of Pediatrics (AAP) and the American College of Emergency Physicians (ACEP) recommend a full sepsis work-up for all neonates (ie, ages 0 to 28 days) who present with a fever (defined as a rectal temperature ≥100.4°F).1,2 The probability of a serious bacterial infection (SBI) in patients in this age group who present with fever is approximately 12%.3 A full sepsis work-up generally includes a CBC, blood cultures, urinalysis with culture, CSF analysis with culture, and stool cultures if diarrhea is present.
Current guidelines for infants 29 to 90 days of age who present with fever differ between professional associations. The AAP and the American Academy of Family Physicians recommend the following for children in this age range: laboratory evaluation with CBC, blood cultures, CSF analysis, urinalysis, and culture. If laboratory evaluation reveals a WBC of less than 15 x 109/L with an absolute neutrophil count of less than 10 x 109/L, along with a normal CSF and urinalysis, the patient can be given IM ceftriaxone and follow-up arranged in 24 hours. This approach is recommended for patients who are otherwise healthy, nontoxic at presentation, and under the care of a responsible adult.4,5 By comparison, the Philadelphia protocol, though suggesting an identical work-up, recommends against the use of antibiotics in infants deemed at low risk for SBI.6
The ACEP does not specifically endorse a management strategy for febrile infants in the 29- to 90-day age group, but instead acknowledges that no age cut-off within this group can be considered absolute when determining management strategy, and suggests that children up to 60 days old should be managed in a manner similar to neonates.2 The published guidelines do not include consideration of specific history exposure in the management recommendations.
Typhoidal Serotypes
Salmonella can be divided into typhoidal (including S typhi and S paratyphi) and nontyphoidal serotypes (NTS), with the two groups manifesting as very different diseases.7 Typhoidal serotypes lead to the disease process known as typhoid, which typically presents with fever, chills, abdominal pain, nonbloody diarrhea or constipation, nausea, anorexia, headache, hepatosplenomegaly, and rose spots.8 These symptoms typically present after a 14-day incubation period and persist for 21 days.9 Humans are the only known infected source of these species, which are spread via the fecal-oral route.10
In contrast, disease from NTS manifests within 12 hours of exposure with watery diarrhea, nausea, vomiting, and fever, with symptoms lasting up to 10 days.11 Both groups cause disease by invading the intestinal epithelium12; however, typhoidal species induce less intestinal inflammation, facilitating bacterial invasion and making systemic disease more likely.13
Transmission
Many animals are known to carry NTS, including reptiles, where Salmonella occurs naturally in their gastrointestinal tract.14 Twenty-five percent of Salmonella infections in children younger than age 5 years have been attributed to contact with a pet,15 with small turtles (shell diameter <4 inches) accounting for 42% of all pet-related Salmonella infections.
Though gastroenteritis is the most common clinical manifestation of infection with NTS, approximately 5% of patients will develop invasive disease, including bacteremia, meningitis, septic arthritis, or osteomyelitis.16 Children with invasive disease are more likely to have been exposed to an iguana, snake, or bearded dragon than to a turtle. If the pet is kept indoors, the risk of invasive disease is more likely. The average age of patients with invasive disease is 62 days, versus 2 years for noninvasive disease.17
Diagnosis
Growth of Salmonella on cultures of stool, blood, urine, or CSF dishes is the mainstay of diagnosis of typhoid and nontyphoidal disease, but bacterial concentrations are higher in bone marrow aspirate, making it superior to blood cultures.18 Biopsy of the rose spots of typhoid may also provide the diagnosis.
Management
Since Salmonella gastroenteritis is usually a self-limited disease, current recommendations reserve treatment with antibiotics for patients with severe disease or who are immunocompromised. When necessary, treatment consists of 7 to 10 days of a fluoroquinolone or third-generation cephalosporin, which is the same regimen suggested for typhoid. Treatment of central nervous system (CNS) salmonellosis consists of at least 3 weeks of a third-generation cephalosporin; the AAP recommends at least 4 weeks of treatment.19
Case Conclusion
Prior to the patient’s transfer to our facility, she was treated empirically with ceftriaxone without prior CSF analysis—an approach that does not follow any current guidelines for the treatment of a febrile infant. Though an LP was not performed until approximately 24 hours after the initial antibiotic was given, the patient demonstrated CSF pleocytosis with no organisms on gram stain and no growth on culture. Given this pleocytosis and Salmonella bacteremia in the context of prior antibiotic treatment, and MRI consistent with CNS involvement, the patient was treated for 21 days for presumed Salmonella meningitis. A CSF analysis performed on her initial visit could have more accurately directed the type and duration of treatment if the findings on subsequent imaging studies and CSF analysis were ambiguous.
Summary
Emergency physicians may underestimate the likelihood of SBI in otherwise well-appearing febrile infants. While certain aspects of the history and physical examination in a febrile, well-appearing infant have been shown to correlate with an increased risk of SBI, no single finding can definitively rule in or rule out the disease.20 Opinions differ as to optimal management strategies for febrile, well-appearing infants outside the neonatal period. However, an appropriate level of clinical suspicion, within the context of a thorough investigation into the infant’s health history and social situation, can aid the clinician and guide treatment and disposition.
The management of a febrile infant is complex and requires obtaining a detailed history of all possible exposures. Published guidelines alone are not always completely accurate for diagnosing or excluding serious illness, and are not a substitute for a thorough examination and history.
Case
The parents of a 45-day-old girl were referred to our regional pediatric hospital by a local community hospital for emergent evaluation of their infant. The day prior, they had taken the infant to the referring ED because of persistent fussiness and subjective fever. They were neither sure of the tests that were obtained during that visit nor why they were instructed to take their daughter to our pediatric facility. They did, however, recall that during the visit to the community ED, the patient had a rectal temperature of 102.7°F, was given an antibiotic injection, and was well appearing and acting normally. Also, at discharge, the infant’s parents were instructed to follow up with the patient’s pediatrician within 24 hours.
Since their daughter’s discharge from the community ED, both parents noted that she seemed more irritable, felt warm, and had not been feeding well. They confirmed that she was an otherwise healthy infant who had been born full term via normal vaginal delivery and without complications.
On initial assessment at our ED, the patient was fussy and had mottled extremities and dusky nail beds. Her vital signs at presentation were: heart rate, 223 beats/minute; respiratory rate, 36 breaths/minute; and rectal temperature, 103.6°F. Oxygen saturation was 96% on room air. The infant was resuscitated with 20 mL/kg of intravenous (IV) normal saline and given oral acetaminophen. Laboratory studies were obtained, and a lumbar puncture (LP) was performed.
She was treated with IV acyclovir, ceftriaxone, and vancomycin. Her complete blood count (CBC) was notable for a white blood cell count (WBC) of 22.60 x 109/L; cerebrospinal fluid (CSF) analysis revealed a WBC of 4.52 x 109/L with 75% neutrophils, and serum glucose of 63 mg/dL.
Since the patient’s parents did not have any paperwork or information from the prior ED visit, our ED contacted the community ED by phone, and a representative provided the following information: the patient had appeared well but was febrile at presentation; laboratory evaluation was obtained, but no LP was performed; she was treated with intramuscular (IM) ceftriaxone and acetaminophen; and she was discharged home in the care of her parents. Regarding laboratory studies performed at the community ED, the only test result made available by phone was the preliminary blood culture report that revealed growth of gram-negative rods with speciation pending, which prompted the referral to our facility.
Based on the information provided by the community ED and our evaluation and work-up, the patient was admitted to the pediatric intensive care unit. Magnetic resonance imaging (MRI) of the brain was performed, which showed debris in the lateral ventricles consistent with ventriculitis—likely secondary to meningitis. The blood, urine, and cerebrospinal fluid (CSF) cultures collected during our evaluation produced no growth; however, blood cultures from the rural ED eventually grew Salmonella.
A further detailed history revealed that the infant and her parents had been living with a family friend who owned an iguana. According to reports, the iguana had free run of the home and often crawled around and across the infant while she was lying on a blanket on the floor. The patient’s parents were not aware of the diseases associated with reptile contact. Due to concerns over the social situation, the patient was kept in the hospital for the entire recommended 21-day course of antibiotic therapy, during which time the parents received assistance finding alternate living arrangements.
Discussion
Current Practice Guidelines for Managing Febrile Infants
Current guidelines from the American Academy of Pediatrics (AAP) and the American College of Emergency Physicians (ACEP) recommend a full sepsis work-up for all neonates (ie, ages 0 to 28 days) who present with a fever (defined as a rectal temperature ≥100.4°F).1,2 The probability of a serious bacterial infection (SBI) in patients in this age group who present with fever is approximately 12%.3 A full sepsis work-up generally includes a CBC, blood cultures, urinalysis with culture, CSF analysis with culture, and stool cultures if diarrhea is present.
Current guidelines for infants 29 to 90 days of age who present with fever differ between professional associations. The AAP and the American Academy of Family Physicians recommend the following for children in this age range: laboratory evaluation with CBC, blood cultures, CSF analysis, urinalysis, and culture. If laboratory evaluation reveals a WBC of less than 15 x 109/L with an absolute neutrophil count of less than 10 x 109/L, along with a normal CSF and urinalysis, the patient can be given IM ceftriaxone and follow-up arranged in 24 hours. This approach is recommended for patients who are otherwise healthy, nontoxic at presentation, and under the care of a responsible adult.4,5 By comparison, the Philadelphia protocol, though suggesting an identical work-up, recommends against the use of antibiotics in infants deemed at low risk for SBI.6
The ACEP does not specifically endorse a management strategy for febrile infants in the 29- to 90-day age group, but instead acknowledges that no age cut-off within this group can be considered absolute when determining management strategy, and suggests that children up to 60 days old should be managed in a manner similar to neonates.2 The published guidelines do not include consideration of specific history exposure in the management recommendations.
Typhoidal Serotypes
Salmonella can be divided into typhoidal (including S typhi and S paratyphi) and nontyphoidal serotypes (NTS), with the two groups manifesting as very different diseases.7 Typhoidal serotypes lead to the disease process known as typhoid, which typically presents with fever, chills, abdominal pain, nonbloody diarrhea or constipation, nausea, anorexia, headache, hepatosplenomegaly, and rose spots.8 These symptoms typically present after a 14-day incubation period and persist for 21 days.9 Humans are the only known infected source of these species, which are spread via the fecal-oral route.10
In contrast, disease from NTS manifests within 12 hours of exposure with watery diarrhea, nausea, vomiting, and fever, with symptoms lasting up to 10 days.11 Both groups cause disease by invading the intestinal epithelium12; however, typhoidal species induce less intestinal inflammation, facilitating bacterial invasion and making systemic disease more likely.13
Transmission
Many animals are known to carry NTS, including reptiles, where Salmonella occurs naturally in their gastrointestinal tract.14 Twenty-five percent of Salmonella infections in children younger than age 5 years have been attributed to contact with a pet,15 with small turtles (shell diameter <4 inches) accounting for 42% of all pet-related Salmonella infections.
Though gastroenteritis is the most common clinical manifestation of infection with NTS, approximately 5% of patients will develop invasive disease, including bacteremia, meningitis, septic arthritis, or osteomyelitis.16 Children with invasive disease are more likely to have been exposed to an iguana, snake, or bearded dragon than to a turtle. If the pet is kept indoors, the risk of invasive disease is more likely. The average age of patients with invasive disease is 62 days, versus 2 years for noninvasive disease.17
Diagnosis
Growth of Salmonella on cultures of stool, blood, urine, or CSF dishes is the mainstay of diagnosis of typhoid and nontyphoidal disease, but bacterial concentrations are higher in bone marrow aspirate, making it superior to blood cultures.18 Biopsy of the rose spots of typhoid may also provide the diagnosis.
Management
Since Salmonella gastroenteritis is usually a self-limited disease, current recommendations reserve treatment with antibiotics for patients with severe disease or who are immunocompromised. When necessary, treatment consists of 7 to 10 days of a fluoroquinolone or third-generation cephalosporin, which is the same regimen suggested for typhoid. Treatment of central nervous system (CNS) salmonellosis consists of at least 3 weeks of a third-generation cephalosporin; the AAP recommends at least 4 weeks of treatment.19
Case Conclusion
Prior to the patient’s transfer to our facility, she was treated empirically with ceftriaxone without prior CSF analysis—an approach that does not follow any current guidelines for the treatment of a febrile infant. Though an LP was not performed until approximately 24 hours after the initial antibiotic was given, the patient demonstrated CSF pleocytosis with no organisms on gram stain and no growth on culture. Given this pleocytosis and Salmonella bacteremia in the context of prior antibiotic treatment, and MRI consistent with CNS involvement, the patient was treated for 21 days for presumed Salmonella meningitis. A CSF analysis performed on her initial visit could have more accurately directed the type and duration of treatment if the findings on subsequent imaging studies and CSF analysis were ambiguous.
Summary
Emergency physicians may underestimate the likelihood of SBI in otherwise well-appearing febrile infants. While certain aspects of the history and physical examination in a febrile, well-appearing infant have been shown to correlate with an increased risk of SBI, no single finding can definitively rule in or rule out the disease.20 Opinions differ as to optimal management strategies for febrile, well-appearing infants outside the neonatal period. However, an appropriate level of clinical suspicion, within the context of a thorough investigation into the infant’s health history and social situation, can aid the clinician and guide treatment and disposition.
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. Erratum in Ann Emerg Med. 1993;22(9):1490.
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545.
3. Kadish HA, Loveridge B, Tobey J, Bolte RG, Corneli HM. Applying outpatient protocols in febrile infants 1-28 days of age: can the threshold be lowered? Clin Pediatr (Phila). 2000;39(2):81-88.
4. Sur DK, Bukont EL. Evaluating fever of unidentifiable source in young children. Am Fam Physician. 2007;75(12):1805-1811.
5. Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J. 1993;12(5):389-394.
6. Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Eng J Med. 1993;329(20):1437-1441.
7. Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front in Microbiol. 2014;5:391.
8. Stuart BM, Pullen RL. Typhoid: clinical analysis of 360 cases. Arch Intern Med (Chic). 1946;78(6):
629-661.
9. Olsen SJ, Bleasdale SC, Magnano AR, et al. Outbreaks of typhoid fever in the United States, 1960-99. Epidemiol Infect. 2003;130(1):13-21.
10. Newton AE, Routh JA, Mahon BE. Typhoid and Paratyphoid Fever. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/typhoid-paratyphoid-fever. Updated July 10, 2015. Accessed June 13, 2016.
11. McGovern VJ, Slavutin LJ. Pathology of Salmonella colitis. Am J Surg Pathol. 1979;3(6):483-490.
12. Liu SL, Ezaki T, Miura H, Matsui K, Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infect Immun. 1988;56(8):1967-1973.
13. House D, Wain J, Ho VA, et al. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J Clin Microbiol. 2001;39(3):1002-1007.
14. Hoelzer K, Moreno Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42(1):34.
15. Murphy D, Oshin F. Reptile-associated salmonellosis in children aged under 5 years in South West England. Arch Dis Child. 2015;100(4):364-365.
16. Iwamoto M. Infectious diseases related to travel. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/salmonellosis-nontyphoidal. Updated July 10, 2015. Accessed June 13, 2016.
17. Meyer Sauteur PM, Relly C, Hug M, Wittenbrink MM, Berger C. Risk factors for invasive reptile-associated salmonellosis in children. Vector-Borne and Zoonotic Dis. 2013;13(6):419-421.
18. Gasem MH, Keuter M, Dolmans WM, Van Der Ven-Jongekrijg J, Djokomoeljanto R, Van Der Meer JW. Persistence of Salmonellae in blood and bone marrow: randomized controlled trial comparing ciprofloxacin and chloramphenicol treatments against enteric fever. Antimicrob Agents Chemother. 2003;47(5):1727-1731.
19. Price EH, de Louvois J, Workman MR. Antibiotics for Salmonella meningitis in children. J Antimicrob Chemother. 2000;46(5) 653-655.
20. Craig JC, Williams GJ, Jones M, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. BMJ. 2010;340:c1594.
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. Erratum in Ann Emerg Med. 1993;22(9):1490.
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545.
3. Kadish HA, Loveridge B, Tobey J, Bolte RG, Corneli HM. Applying outpatient protocols in febrile infants 1-28 days of age: can the threshold be lowered? Clin Pediatr (Phila). 2000;39(2):81-88.
4. Sur DK, Bukont EL. Evaluating fever of unidentifiable source in young children. Am Fam Physician. 2007;75(12):1805-1811.
5. Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J. 1993;12(5):389-394.
6. Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Eng J Med. 1993;329(20):1437-1441.
7. Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front in Microbiol. 2014;5:391.
8. Stuart BM, Pullen RL. Typhoid: clinical analysis of 360 cases. Arch Intern Med (Chic). 1946;78(6):
629-661.
9. Olsen SJ, Bleasdale SC, Magnano AR, et al. Outbreaks of typhoid fever in the United States, 1960-99. Epidemiol Infect. 2003;130(1):13-21.
10. Newton AE, Routh JA, Mahon BE. Typhoid and Paratyphoid Fever. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/typhoid-paratyphoid-fever. Updated July 10, 2015. Accessed June 13, 2016.
11. McGovern VJ, Slavutin LJ. Pathology of Salmonella colitis. Am J Surg Pathol. 1979;3(6):483-490.
12. Liu SL, Ezaki T, Miura H, Matsui K, Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infect Immun. 1988;56(8):1967-1973.
13. House D, Wain J, Ho VA, et al. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J Clin Microbiol. 2001;39(3):1002-1007.
14. Hoelzer K, Moreno Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42(1):34.
15. Murphy D, Oshin F. Reptile-associated salmonellosis in children aged under 5 years in South West England. Arch Dis Child. 2015;100(4):364-365.
16. Iwamoto M. Infectious diseases related to travel. In: Brunette GW, Kozarsky PE, Cohen NJ, et al, eds. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2016. http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/salmonellosis-nontyphoidal. Updated July 10, 2015. Accessed June 13, 2016.
17. Meyer Sauteur PM, Relly C, Hug M, Wittenbrink MM, Berger C. Risk factors for invasive reptile-associated salmonellosis in children. Vector-Borne and Zoonotic Dis. 2013;13(6):419-421.
18. Gasem MH, Keuter M, Dolmans WM, Van Der Ven-Jongekrijg J, Djokomoeljanto R, Van Der Meer JW. Persistence of Salmonellae in blood and bone marrow: randomized controlled trial comparing ciprofloxacin and chloramphenicol treatments against enteric fever. Antimicrob Agents Chemother. 2003;47(5):1727-1731.
19. Price EH, de Louvois J, Workman MR. Antibiotics for Salmonella meningitis in children. J Antimicrob Chemother. 2000;46(5) 653-655.
20. Craig JC, Williams GJ, Jones M, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. BMJ. 2010;340:c1594.