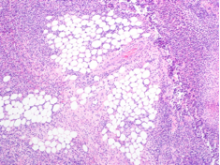
User login
Spontaneous Retrobulbar Hemorrhage
Most emergency physicians (EPs) encounter several patients a year with hemorrhages due to factor Xa (FXa) inhibitors. Such bleeding may occur in patterns not previously recognized with traditional anticoagulant therapy. Retrobulbar hemorrhage is typically associated with significant facial or orbital trauma, and spontaneous hemorrhage is a very rare cause of orbital compartment syndrome.1 Retrobulbar hemorrhage can lead to orbital compartment syndrome due to increased orbital pressure within a closed space. Because orbital compartment syndrome can compromise blood flow to the optic nerve or central retinal artery, it is extremely important to decrease orbital pressure as quickly as possible in affected patients. Therefore, canthotomy/cantholysis should be performed sooner rather than later, as 90 minutes of elevated intraocular pressure (IOP) can lead to permanent vision loss.2
Rivaroxaban, one of the relatively new oral anticoagulant agents that inhibit FXa, is used as an alternative therapy to vitamin K antagonists. The FXa agents have been approved to reduce the risk of stroke in patients with nonvalvular atrial fibrillation (AF).3 According to a meta-analysis of rivaroxaban and bleeding risk, rivaroxaban was shown to have no increased risk of major or clinically relevant nonmajor bleeding compared to vitamin K antagonists. Rivaroxaban was also associated with a significant decrease in fatal bleeding (relative risk, 0.48, 95%; confidence interval, 0.31 to 0.74).4
Case Report
A 79-year-old man with a medical history of hypertension, transient ischemic attacks (TIAs), and AF, for which he was taking rivaroxaban, was referred to our ED by a local rural ED for further evaluation and treatment of a retrobulbar hemorrhage. (The patient’s family refused emergency medical services transport from the rural ED.) The patient stated that upon awakening earlier that morning, he felt “pressure” in his right eye and experienced periorbital swelling that continued to worsen throughout the day. He denied any trauma, falls, or strikes to the face or head. The patient’s account and history were confirmed by the family members with whom he resided.
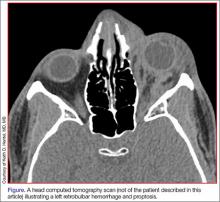
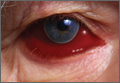
During the patient’s evaluation at the rural ED, a computed tomography (CT) scan of the head was performed, which demonstrated a retrobulbar hematoma on the right side (See the Figure for an example of a CT scan illustrating a retrobulbar hematoma with proptosis). Since the patient’s initial right IOP was 32 mm Hg (normal range, 12-22 mm Hg), ophthalmology services at this institution performed a lateral canthotomy. The patient’s right IOP postsurgery decreased but remained elevated at 27 mm Hg. In addition to surgical intervention, he was given oral acetazolamide and timolol. Then, because the patient was hemodynamically stable, he was referred to our institution for further evaluation.
Upon arrival at our ED, the patient reported slow bleeding from the canthotomy site. He denied any chest pain, shortness of breath, light-headedness, dizziness, or visual changes. Additional history revealed that in addition to taking rivaroxaban, the patient was also on a daily 81-mg aspirin regimen. His vital signs at presentation were: blood pressure (BP), 130/68 mm Hg; heart rate, 75 beats/minute; and respiratory rate, 16 breaths/minute; and temperature, afebrile. Oxygen saturation was 99% on room air.
Physical examination revealed blood oozing from the right eye at the canthotomy site. There was no other evidence of trauma to the eye or head, and IOP of the right eye was normal at 14 mm Hg. Laboratory studies revealed a hemoglobin value of 16.8 g/dL, a hematocrit of 48%, and a white blood cell (WBC) count of 8.8 x 109/L. The basic metabolic profile, including creatinine, was unremarkable. A type and screen blood pretransfusion compatibility test was also ordered.
Since the patient’s ocular hemorrhaging persisted, ophthalmology services were consulted. The ophthalmology examination measured a right IOP of 14 mm Hg and a visual acuity of 20/200. The patient’s pupils were equal, round, and reactive to light, and a subconjunctival hematoma was noted. The ophthalmologist recommended no further surgical interventions at that time.
Due to the continued ocular bleeding, hematology services were also consulted. The hematologist recommended 50 U/kg of intravenous (IV) prothrombin complex concentrate (PCC) to reverse the anticoagulatory effects of rivaroxaban. The patient was given one dose of PCC in the ED. Throughout his ED course, the patient did not experience any deterioration of visual acuity. However, during repeated IOP checks, he experienced one episode of vasovagal syncope with a systolic BP in the 70s. The syncope resolved promptly after the patient was placed in a supine position and was given an IV bolus of normal saline fluid. The patient still had oozing at the incision site, and was admitted to the general medicine floor.
During his inpatient stay, the patient remained hemodynamically stable and did not require transfusion of blood or platelet products. All home anticoagulant medications were discontinued. The patient continued to have some oozing the following morning, and was given an additional dose of IV PCC (50 U/kg), which resolved the bleeding. He remained hemostatic and, based on his history of AF, he was discharged home on warfarin without bridge therapy. Both rivaroxaban and daily aspirin therapy were discontinued. The lateral canthotomy and cantholysis healed without need for surgical intervention. An ophthalmology follow-up clinic visit 1 week after discharge from the hospital revealed an already self-healed incision without ectropion or retraction and with only mild laxity. Given the patient’s history of AF with TIAs while off anticoagulants, the ophthalmologist did not recommend any other surgical intervention that would have required discontinuing the warfarin.
Discussion
With any retrobulbar hematoma, one must be concerned for orbital compartment syndrome. Orbital hemorrhage is the most common cause of orbital compartment syndrome, usually occurring secondary to trauma, surgery, or retrobulbar injection. In this case, spontaneous hemorrhage due to anticoagulation was believed to be the cause—albeit a rare one—of orbital compartment syndrome. Because the orbital space is enclosed and cannot expand, it is vulnerable to compartment syndrome, and subsequent ischemia can lead to permanent vision impairment or complete loss of vision.5 Early recognition and treatment is imperative to preserve vision as an elevated intraorbital pressure for 60 to 100 minutes can lead to permanent visual sequelae.
Management
Treatment of retrobulbar hemorrhage includes lateral canthotomy and cantholysis, which have been shown to reduce IOP an average of 14.2 mm Hg.6 In our patient, IOP in the affected eye was reduced by 18 mm Hg. In addition to the patient’s high IOP at presentation, another concern was the continued hemorrhaging from both the incision site and its potential to exacerbate the underlying retrobulbar hematoma. Management of this condition posed a challenge because this patient was taking a newer anticoagulant, for which there is currently no specific reversal agent. After consultation with hematology services, the patient was given PCC because small studies have suggested that PCC may reverse rivaroxaban-induced anticoagulation.7 While more expensive than fresh frozen plasma, PCC has a high safety profile and should be considered in cases of life-threatening bleeding—especially in patients who have renal failure, as rivaroxaban is renally excreted. The half-life of rivaroxaban is 5 to 9 hours and its effects may last up to 12 hours. An IV dose of 50 U/kg PCC can be effective in reversing rivaroxaban; this dose can be repeated every 12 hours until hemorrhaging abates or until rivaroxaban is cleared.
Potential Factor Xa Reversal Agent
Phase IV trials are underway in the ANNEX-A (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Apixaban) and ANNEX-R (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Rivaroxaban) studies assessing andexanet alpha, an FXa inhibitor reversal agent and potential FXa inhibitor antidote. Andexanet alpha is a decoy protein that binds to FXa inhibitors in the active site, restoring endogenous FXa and reducing anticoagulant activity.8 This serves as another promising reversal agent for apixaban, edoxaban, and rivaroxaban. With the development of these new FXa reversal agents, EPs will have more options for reversal of anticoagulation in patients with unique hemorrhagic presentations.
Conclusion
Rivaroxaban has the potential to replace warfarin as a “novel” oral anticoagulant of choice for multiple indications, especially as more insurance companies cover the use of the FXa inhibitors. As a result of their increased use, the EP is likely to see an increasing number of patients who present with hemorrhagic consequences of the FXa inhibitors, and in turn must be familiar with the properties of this class of anticoagulants—including potential reversal strategies.
Our case of spontaneous retrobulbar hemorrhage may be one of these new patterns of bleeding to be expected from a novel FXa inhibitor. Therefore, it is imperative that EPs consider retrobulbar hemorrhage and other possible bleeding locations in patients on an FXa inhibitor.
1. McAllister AR, Sobel RK, Allen RC. Spontaneous retrobulbar hemorrhage with subsequent orbital compartment syndrome. University of Iowa Health Care Ophthalmology and Visual Sciences Web site. http://www.eyerounds.org/cases/168-orbital-compartment-syndrome.htm. Accessed June 14, 2016.
2. Winterton JV, Patel K, Mizen KD. Review of management options for a retrobulbar hemorrhage. J Oral Maxillofac Surg. 2007;65(2):296-299.
3. Wasserlauf G, Grandi SM, Filion KB, Eisenberg MJ. Meta-analysis of rivaroxaban and bleeding risk. Am J Cardiol. 2013;112(3):454-460.
4. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
5. Kloss BT, Patel R. Orbital compartment syndrome from retrobulbar hemorrhage. Int J Emerg Med. 2010;3(4):521-522.
6. Peak DA. Acute orbital compartment syndrome. Medscape. http://emedicine.medscape.com/article/799528-overview. Updated November 4, 2015. Accessed June 14, 2016.
7. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
8. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413-2424.
Most emergency physicians (EPs) encounter several patients a year with hemorrhages due to factor Xa (FXa) inhibitors. Such bleeding may occur in patterns not previously recognized with traditional anticoagulant therapy. Retrobulbar hemorrhage is typically associated with significant facial or orbital trauma, and spontaneous hemorrhage is a very rare cause of orbital compartment syndrome.1 Retrobulbar hemorrhage can lead to orbital compartment syndrome due to increased orbital pressure within a closed space. Because orbital compartment syndrome can compromise blood flow to the optic nerve or central retinal artery, it is extremely important to decrease orbital pressure as quickly as possible in affected patients. Therefore, canthotomy/cantholysis should be performed sooner rather than later, as 90 minutes of elevated intraocular pressure (IOP) can lead to permanent vision loss.2
Rivaroxaban, one of the relatively new oral anticoagulant agents that inhibit FXa, is used as an alternative therapy to vitamin K antagonists. The FXa agents have been approved to reduce the risk of stroke in patients with nonvalvular atrial fibrillation (AF).3 According to a meta-analysis of rivaroxaban and bleeding risk, rivaroxaban was shown to have no increased risk of major or clinically relevant nonmajor bleeding compared to vitamin K antagonists. Rivaroxaban was also associated with a significant decrease in fatal bleeding (relative risk, 0.48, 95%; confidence interval, 0.31 to 0.74).4
Case Report
A 79-year-old man with a medical history of hypertension, transient ischemic attacks (TIAs), and AF, for which he was taking rivaroxaban, was referred to our ED by a local rural ED for further evaluation and treatment of a retrobulbar hemorrhage. (The patient’s family refused emergency medical services transport from the rural ED.) The patient stated that upon awakening earlier that morning, he felt “pressure” in his right eye and experienced periorbital swelling that continued to worsen throughout the day. He denied any trauma, falls, or strikes to the face or head. The patient’s account and history were confirmed by the family members with whom he resided.
During the patient’s evaluation at the rural ED, a computed tomography (CT) scan of the head was performed, which demonstrated a retrobulbar hematoma on the right side (See the Figure for an example of a CT scan illustrating a retrobulbar hematoma with proptosis). Since the patient’s initial right IOP was 32 mm Hg (normal range, 12-22 mm Hg), ophthalmology services at this institution performed a lateral canthotomy. The patient’s right IOP postsurgery decreased but remained elevated at 27 mm Hg. In addition to surgical intervention, he was given oral acetazolamide and timolol. Then, because the patient was hemodynamically stable, he was referred to our institution for further evaluation.
Upon arrival at our ED, the patient reported slow bleeding from the canthotomy site. He denied any chest pain, shortness of breath, light-headedness, dizziness, or visual changes. Additional history revealed that in addition to taking rivaroxaban, the patient was also on a daily 81-mg aspirin regimen. His vital signs at presentation were: blood pressure (BP), 130/68 mm Hg; heart rate, 75 beats/minute; and respiratory rate, 16 breaths/minute; and temperature, afebrile. Oxygen saturation was 99% on room air.
Physical examination revealed blood oozing from the right eye at the canthotomy site. There was no other evidence of trauma to the eye or head, and IOP of the right eye was normal at 14 mm Hg. Laboratory studies revealed a hemoglobin value of 16.8 g/dL, a hematocrit of 48%, and a white blood cell (WBC) count of 8.8 x 109/L. The basic metabolic profile, including creatinine, was unremarkable. A type and screen blood pretransfusion compatibility test was also ordered.
Since the patient’s ocular hemorrhaging persisted, ophthalmology services were consulted. The ophthalmology examination measured a right IOP of 14 mm Hg and a visual acuity of 20/200. The patient’s pupils were equal, round, and reactive to light, and a subconjunctival hematoma was noted. The ophthalmologist recommended no further surgical interventions at that time.
Due to the continued ocular bleeding, hematology services were also consulted. The hematologist recommended 50 U/kg of intravenous (IV) prothrombin complex concentrate (PCC) to reverse the anticoagulatory effects of rivaroxaban. The patient was given one dose of PCC in the ED. Throughout his ED course, the patient did not experience any deterioration of visual acuity. However, during repeated IOP checks, he experienced one episode of vasovagal syncope with a systolic BP in the 70s. The syncope resolved promptly after the patient was placed in a supine position and was given an IV bolus of normal saline fluid. The patient still had oozing at the incision site, and was admitted to the general medicine floor.
During his inpatient stay, the patient remained hemodynamically stable and did not require transfusion of blood or platelet products. All home anticoagulant medications were discontinued. The patient continued to have some oozing the following morning, and was given an additional dose of IV PCC (50 U/kg), which resolved the bleeding. He remained hemostatic and, based on his history of AF, he was discharged home on warfarin without bridge therapy. Both rivaroxaban and daily aspirin therapy were discontinued. The lateral canthotomy and cantholysis healed without need for surgical intervention. An ophthalmology follow-up clinic visit 1 week after discharge from the hospital revealed an already self-healed incision without ectropion or retraction and with only mild laxity. Given the patient’s history of AF with TIAs while off anticoagulants, the ophthalmologist did not recommend any other surgical intervention that would have required discontinuing the warfarin.
Discussion
With any retrobulbar hematoma, one must be concerned for orbital compartment syndrome. Orbital hemorrhage is the most common cause of orbital compartment syndrome, usually occurring secondary to trauma, surgery, or retrobulbar injection. In this case, spontaneous hemorrhage due to anticoagulation was believed to be the cause—albeit a rare one—of orbital compartment syndrome. Because the orbital space is enclosed and cannot expand, it is vulnerable to compartment syndrome, and subsequent ischemia can lead to permanent vision impairment or complete loss of vision.5 Early recognition and treatment is imperative to preserve vision as an elevated intraorbital pressure for 60 to 100 minutes can lead to permanent visual sequelae.
Management
Treatment of retrobulbar hemorrhage includes lateral canthotomy and cantholysis, which have been shown to reduce IOP an average of 14.2 mm Hg.6 In our patient, IOP in the affected eye was reduced by 18 mm Hg. In addition to the patient’s high IOP at presentation, another concern was the continued hemorrhaging from both the incision site and its potential to exacerbate the underlying retrobulbar hematoma. Management of this condition posed a challenge because this patient was taking a newer anticoagulant, for which there is currently no specific reversal agent. After consultation with hematology services, the patient was given PCC because small studies have suggested that PCC may reverse rivaroxaban-induced anticoagulation.7 While more expensive than fresh frozen plasma, PCC has a high safety profile and should be considered in cases of life-threatening bleeding—especially in patients who have renal failure, as rivaroxaban is renally excreted. The half-life of rivaroxaban is 5 to 9 hours and its effects may last up to 12 hours. An IV dose of 50 U/kg PCC can be effective in reversing rivaroxaban; this dose can be repeated every 12 hours until hemorrhaging abates or until rivaroxaban is cleared.
Potential Factor Xa Reversal Agent
Phase IV trials are underway in the ANNEX-A (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Apixaban) and ANNEX-R (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Rivaroxaban) studies assessing andexanet alpha, an FXa inhibitor reversal agent and potential FXa inhibitor antidote. Andexanet alpha is a decoy protein that binds to FXa inhibitors in the active site, restoring endogenous FXa and reducing anticoagulant activity.8 This serves as another promising reversal agent for apixaban, edoxaban, and rivaroxaban. With the development of these new FXa reversal agents, EPs will have more options for reversal of anticoagulation in patients with unique hemorrhagic presentations.
Conclusion
Rivaroxaban has the potential to replace warfarin as a “novel” oral anticoagulant of choice for multiple indications, especially as more insurance companies cover the use of the FXa inhibitors. As a result of their increased use, the EP is likely to see an increasing number of patients who present with hemorrhagic consequences of the FXa inhibitors, and in turn must be familiar with the properties of this class of anticoagulants—including potential reversal strategies.
Our case of spontaneous retrobulbar hemorrhage may be one of these new patterns of bleeding to be expected from a novel FXa inhibitor. Therefore, it is imperative that EPs consider retrobulbar hemorrhage and other possible bleeding locations in patients on an FXa inhibitor.
Most emergency physicians (EPs) encounter several patients a year with hemorrhages due to factor Xa (FXa) inhibitors. Such bleeding may occur in patterns not previously recognized with traditional anticoagulant therapy. Retrobulbar hemorrhage is typically associated with significant facial or orbital trauma, and spontaneous hemorrhage is a very rare cause of orbital compartment syndrome.1 Retrobulbar hemorrhage can lead to orbital compartment syndrome due to increased orbital pressure within a closed space. Because orbital compartment syndrome can compromise blood flow to the optic nerve or central retinal artery, it is extremely important to decrease orbital pressure as quickly as possible in affected patients. Therefore, canthotomy/cantholysis should be performed sooner rather than later, as 90 minutes of elevated intraocular pressure (IOP) can lead to permanent vision loss.2
Rivaroxaban, one of the relatively new oral anticoagulant agents that inhibit FXa, is used as an alternative therapy to vitamin K antagonists. The FXa agents have been approved to reduce the risk of stroke in patients with nonvalvular atrial fibrillation (AF).3 According to a meta-analysis of rivaroxaban and bleeding risk, rivaroxaban was shown to have no increased risk of major or clinically relevant nonmajor bleeding compared to vitamin K antagonists. Rivaroxaban was also associated with a significant decrease in fatal bleeding (relative risk, 0.48, 95%; confidence interval, 0.31 to 0.74).4
Case Report
A 79-year-old man with a medical history of hypertension, transient ischemic attacks (TIAs), and AF, for which he was taking rivaroxaban, was referred to our ED by a local rural ED for further evaluation and treatment of a retrobulbar hemorrhage. (The patient’s family refused emergency medical services transport from the rural ED.) The patient stated that upon awakening earlier that morning, he felt “pressure” in his right eye and experienced periorbital swelling that continued to worsen throughout the day. He denied any trauma, falls, or strikes to the face or head. The patient’s account and history were confirmed by the family members with whom he resided.
During the patient’s evaluation at the rural ED, a computed tomography (CT) scan of the head was performed, which demonstrated a retrobulbar hematoma on the right side (See the Figure for an example of a CT scan illustrating a retrobulbar hematoma with proptosis). Since the patient’s initial right IOP was 32 mm Hg (normal range, 12-22 mm Hg), ophthalmology services at this institution performed a lateral canthotomy. The patient’s right IOP postsurgery decreased but remained elevated at 27 mm Hg. In addition to surgical intervention, he was given oral acetazolamide and timolol. Then, because the patient was hemodynamically stable, he was referred to our institution for further evaluation.
Upon arrival at our ED, the patient reported slow bleeding from the canthotomy site. He denied any chest pain, shortness of breath, light-headedness, dizziness, or visual changes. Additional history revealed that in addition to taking rivaroxaban, the patient was also on a daily 81-mg aspirin regimen. His vital signs at presentation were: blood pressure (BP), 130/68 mm Hg; heart rate, 75 beats/minute; and respiratory rate, 16 breaths/minute; and temperature, afebrile. Oxygen saturation was 99% on room air.
Physical examination revealed blood oozing from the right eye at the canthotomy site. There was no other evidence of trauma to the eye or head, and IOP of the right eye was normal at 14 mm Hg. Laboratory studies revealed a hemoglobin value of 16.8 g/dL, a hematocrit of 48%, and a white blood cell (WBC) count of 8.8 x 109/L. The basic metabolic profile, including creatinine, was unremarkable. A type and screen blood pretransfusion compatibility test was also ordered.
Since the patient’s ocular hemorrhaging persisted, ophthalmology services were consulted. The ophthalmology examination measured a right IOP of 14 mm Hg and a visual acuity of 20/200. The patient’s pupils were equal, round, and reactive to light, and a subconjunctival hematoma was noted. The ophthalmologist recommended no further surgical interventions at that time.
Due to the continued ocular bleeding, hematology services were also consulted. The hematologist recommended 50 U/kg of intravenous (IV) prothrombin complex concentrate (PCC) to reverse the anticoagulatory effects of rivaroxaban. The patient was given one dose of PCC in the ED. Throughout his ED course, the patient did not experience any deterioration of visual acuity. However, during repeated IOP checks, he experienced one episode of vasovagal syncope with a systolic BP in the 70s. The syncope resolved promptly after the patient was placed in a supine position and was given an IV bolus of normal saline fluid. The patient still had oozing at the incision site, and was admitted to the general medicine floor.
During his inpatient stay, the patient remained hemodynamically stable and did not require transfusion of blood or platelet products. All home anticoagulant medications were discontinued. The patient continued to have some oozing the following morning, and was given an additional dose of IV PCC (50 U/kg), which resolved the bleeding. He remained hemostatic and, based on his history of AF, he was discharged home on warfarin without bridge therapy. Both rivaroxaban and daily aspirin therapy were discontinued. The lateral canthotomy and cantholysis healed without need for surgical intervention. An ophthalmology follow-up clinic visit 1 week after discharge from the hospital revealed an already self-healed incision without ectropion or retraction and with only mild laxity. Given the patient’s history of AF with TIAs while off anticoagulants, the ophthalmologist did not recommend any other surgical intervention that would have required discontinuing the warfarin.
Discussion
With any retrobulbar hematoma, one must be concerned for orbital compartment syndrome. Orbital hemorrhage is the most common cause of orbital compartment syndrome, usually occurring secondary to trauma, surgery, or retrobulbar injection. In this case, spontaneous hemorrhage due to anticoagulation was believed to be the cause—albeit a rare one—of orbital compartment syndrome. Because the orbital space is enclosed and cannot expand, it is vulnerable to compartment syndrome, and subsequent ischemia can lead to permanent vision impairment or complete loss of vision.5 Early recognition and treatment is imperative to preserve vision as an elevated intraorbital pressure for 60 to 100 minutes can lead to permanent visual sequelae.
Management
Treatment of retrobulbar hemorrhage includes lateral canthotomy and cantholysis, which have been shown to reduce IOP an average of 14.2 mm Hg.6 In our patient, IOP in the affected eye was reduced by 18 mm Hg. In addition to the patient’s high IOP at presentation, another concern was the continued hemorrhaging from both the incision site and its potential to exacerbate the underlying retrobulbar hematoma. Management of this condition posed a challenge because this patient was taking a newer anticoagulant, for which there is currently no specific reversal agent. After consultation with hematology services, the patient was given PCC because small studies have suggested that PCC may reverse rivaroxaban-induced anticoagulation.7 While more expensive than fresh frozen plasma, PCC has a high safety profile and should be considered in cases of life-threatening bleeding—especially in patients who have renal failure, as rivaroxaban is renally excreted. The half-life of rivaroxaban is 5 to 9 hours and its effects may last up to 12 hours. An IV dose of 50 U/kg PCC can be effective in reversing rivaroxaban; this dose can be repeated every 12 hours until hemorrhaging abates or until rivaroxaban is cleared.
Potential Factor Xa Reversal Agent
Phase IV trials are underway in the ANNEX-A (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Apixaban) and ANNEX-R (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa inhibitors – Rivaroxaban) studies assessing andexanet alpha, an FXa inhibitor reversal agent and potential FXa inhibitor antidote. Andexanet alpha is a decoy protein that binds to FXa inhibitors in the active site, restoring endogenous FXa and reducing anticoagulant activity.8 This serves as another promising reversal agent for apixaban, edoxaban, and rivaroxaban. With the development of these new FXa reversal agents, EPs will have more options for reversal of anticoagulation in patients with unique hemorrhagic presentations.
Conclusion
Rivaroxaban has the potential to replace warfarin as a “novel” oral anticoagulant of choice for multiple indications, especially as more insurance companies cover the use of the FXa inhibitors. As a result of their increased use, the EP is likely to see an increasing number of patients who present with hemorrhagic consequences of the FXa inhibitors, and in turn must be familiar with the properties of this class of anticoagulants—including potential reversal strategies.
Our case of spontaneous retrobulbar hemorrhage may be one of these new patterns of bleeding to be expected from a novel FXa inhibitor. Therefore, it is imperative that EPs consider retrobulbar hemorrhage and other possible bleeding locations in patients on an FXa inhibitor.
1. McAllister AR, Sobel RK, Allen RC. Spontaneous retrobulbar hemorrhage with subsequent orbital compartment syndrome. University of Iowa Health Care Ophthalmology and Visual Sciences Web site. http://www.eyerounds.org/cases/168-orbital-compartment-syndrome.htm. Accessed June 14, 2016.
2. Winterton JV, Patel K, Mizen KD. Review of management options for a retrobulbar hemorrhage. J Oral Maxillofac Surg. 2007;65(2):296-299.
3. Wasserlauf G, Grandi SM, Filion KB, Eisenberg MJ. Meta-analysis of rivaroxaban and bleeding risk. Am J Cardiol. 2013;112(3):454-460.
4. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
5. Kloss BT, Patel R. Orbital compartment syndrome from retrobulbar hemorrhage. Int J Emerg Med. 2010;3(4):521-522.
6. Peak DA. Acute orbital compartment syndrome. Medscape. http://emedicine.medscape.com/article/799528-overview. Updated November 4, 2015. Accessed June 14, 2016.
7. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
8. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413-2424.
1. McAllister AR, Sobel RK, Allen RC. Spontaneous retrobulbar hemorrhage with subsequent orbital compartment syndrome. University of Iowa Health Care Ophthalmology and Visual Sciences Web site. http://www.eyerounds.org/cases/168-orbital-compartment-syndrome.htm. Accessed June 14, 2016.
2. Winterton JV, Patel K, Mizen KD. Review of management options for a retrobulbar hemorrhage. J Oral Maxillofac Surg. 2007;65(2):296-299.
3. Wasserlauf G, Grandi SM, Filion KB, Eisenberg MJ. Meta-analysis of rivaroxaban and bleeding risk. Am J Cardiol. 2013;112(3):454-460.
4. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
5. Kloss BT, Patel R. Orbital compartment syndrome from retrobulbar hemorrhage. Int J Emerg Med. 2010;3(4):521-522.
6. Peak DA. Acute orbital compartment syndrome. Medscape. http://emedicine.medscape.com/article/799528-overview. Updated November 4, 2015. Accessed June 14, 2016.
7. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
8. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413-2424.
Atypical Acute Myocardial Infarction and Concomitant Acute Cerebral Infarct
A 61-year-old woman without any known medical history presented with a chief complaint of right arm numbness and right-sided scalp numbness that had started 2 days earlier. She described a “pins and needles” sensation in her right upper extremity and right scalp, and said the numbness in her scalp was especially noticeable when she combed her hair. The patient denied any chest pain, shortness of breath, weakness in her arms or legs, headache, or blurred vision.
She said that 1 day prior to the onset of the paresthesias, she woke up fatigued and vomited once. Throughout that day, she had symptoms of nausea and fatigue, and sought treatment at an urgent care center that afternoon. At the urgent care center, she was diagnosed with a “stomach virus” and was given an antiemetic. The next day, her nausea improved, but the paresthesias began in her right hand and scalp. On the third day, the patient went to work, but the persistent paresthesias caused her to visit her primary care physician, who sent her to our ED for further work-up.
The patient said she had been in good health until 3 days ago. She reported no medical problems and was taking no medications. The patient denied smoking or using alcohol; her family history was significant only in that her father had a myocardial infarction (MI) while in his 50s.
On physical examination, the patient was alert, oriented, and in no apparent distress. Her body mass index was 28.3 kg/m2. Vital signs were: temperature, 99.2°F; blood pressure, 113/73 mm Hg; heart rate, 93 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 95% on room air.
Her head was normocephalic and atraumatic, and her eyes, ears, nose, and throat were normal. Her neck was supple and without jugular vein distension. The cardiac examination revealed normal heart sounds without murmurs, rubs, or gallops. Her lungs were clear without rales, wheezes, or rhonchi. Her abdomen was soft, without tenderness, guarding, or rebound, and she had normal bowel sounds.
Her musculoskeletal examination was normal, with +5/5 strength bilaterally in her upper and lower extremities. The patient’s skin examination also was normal. On neurological examination, her right upper extremity and right side of her face were noted to have decreased sensation via pinprick compared to the left side, but the examination was otherwise normal. The National Institutes of Health Stroke Scale score was 1.
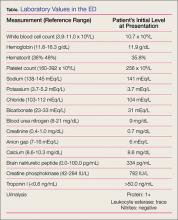
The patient’s electrocardiogram (ECG) showed a normal sinus rhythm (rate, 90 beats/min), a lateral infarct of undetermined age, and a left atrial abnormality. Laboratory evaluation was significant only for a brain natriuretic peptide level of 334 pg/mL, a creatine phosphokinase (CPK) level of 782 IU/L, and a troponin I level of >50 ng/mL (Table). Serial cardiac enzyme levels were obtained and showed a decline of CPK from 782 IU/L to 331 IU/L over the following 36 hours. However, the troponin I levels remained >50 ng/mL for 5 days and then declined to 31.6 ng/mL.
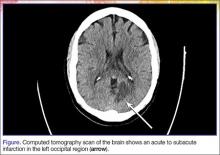
A computed tomography (CT) scan of the brain without contrast revealed an acute to subacute infarct in the left occipital and left thalamic regions (Figure). A stat transthoracic echocardiogram (ECHO) performed in the ED revealed a dilated left ventricle with an ejection fraction of 20% to 25%, along with a hypokinetic anterolateral wall and an akinetic inferolateral wall. No atrial thrombus was visible on the ECHO. Doppler studies of the patient’s lower extremities were negative for deep vein thrombosis. Magnetic resonance imaging of her brain showed an infarct in the posterior circulation distribution involving the left occipital lobes and small areas in the left thalamic and right parietal-occipital regions. Hemorrhagic conversion of the left occipital infarct without mass effect was also noted. The patient was admitted to the neurological intensive care unit for frequent neurological examinations and close monitoring for worsening cerebral hemorrhage.
When the patient had still been in the ED, cardiology services were consulted; the cardiologist initiated a heparin drip with close monitoring of the coagulation studies. Cardiac catheterization was not done immediately because the ECG did not show acute ST elevations. The day after her presentation to the ED, the patient underwent a primary percutaneous coronary catheterization and was found to have a small rudimentary left anterior descending artery, with only small branches supplying the septal region. The right circumflex artery was very large and was supplying the lateral wall. No stents were placed during this procedure. A transesophageal ECHO (TEE) showed no evidence of a left atrial appendage thrombus.
The patient experienced an episode of coffee ground emesis while undergoing the TEE. Her hemoglobin declined from 11.9 g/dL to 7.9 g/dL, which led to a transfusion of 2 U of packed red blood cells and platelets. Heparin was discontinued and a proton pump inhibitor was started; however, no endoscopy was done at that time.
Throughout her stay, the patient was continuously monitored, but no evidence of arrhythmia or atrial fibrillation was found. Upon discharge, the neurologist recommended the patient receive clopidogrel and aspirin therapy for 3 months with subsequent aspirin monotherapy afterward. The patient was discharged after 10 days in the hospital.
Discussion
Although she had an MI, the patient presented here did not experience any chest pain. Her chief complaint in the ED was paresthesias related to her concomitant stroke, and only on further probing did she describe the additional symptoms of fatigability and vomiting.
Since heart disease and stroke share common risk factors and pathophysiology, acute cerebral ischemic events may happen concurrently with MIs. In a review of studies that included approximately 2,900 patients who had an acute stroke, Kerr et al1 found that 20% had elevated troponin levels within 7 days of the stroke. In 2013, the American Heart Association and American Stroke Association published guidelines advising that all patients who present with acute cerebral ischemia have an emergent ECG and baseline troponin level.2 This was in response to evidence that even low positive troponin levels have been associated with an increased risk of mortality.3 Positive troponin levels are especially important because fatal and nonfatal stroke post-MI events have been found to be increasing in frequency for women, even though there has been a significant overall reduction in post-MI mortality.4 Patients who have an ischemic stroke concurrently with an acute MI or soon after have an overall poorer clinical prognosis.5
For emergency physicians (EPs), this is a “chicken or the egg” scenario. It is difficult to determine which came first: the MI or the cerebral ischemia. Similar risk factors can result in an acute embolic event from revascularization, atrial fibrillation without proper anticoagulation, or a poorly functioning left ventricle.6 It is important to remember that regardless of the order of occurrence, the incidence of ischemic stroke is markedly increased in conjunction with an acute MI.7 Several theories have been advanced regarding the relationship between ischemic stroke and acute MI. One theory proposes that elevated troponin levels could be related to a large catecholamine release after a cerebral ischemic stroke, resulting in subsequent myocardial injury or cardiomyopathy.7 However, this theory remains controversial.
Management
The major consideration for the EP is whether or not to give thrombolytics to a patient who presents with concomitant acute MI and ischemic stroke. An acute MI within the 3 months preceding an acute stroke is considered a relative contraindication for intravenous tissue plasminogen activator (tPA).8 It has also been found, albeit rarely, that there is an increased risk of cardiac rupture or tamponade due to the breakdown of the fibrin clot within the necrotic cardiac tissue.8
How should patients with stroke complicated by acute cardiac compromise be managed in the ED? One acute vascular event cannot be ignored while addressing the other. There are no evidence-based guidelines for the management of patients who present with this picture.8 In addition, no published clinical studies have focused on the decision-making process for these patients.8
Immediate percutaneous coronary intervention for the MI performed on such patients would prevent the use of tPA for the acute stroke. Though any anticoagulation increases the risk for postischemic cerebral hemorrhage, heparin is necessary to prevent the formation of a left ventricle thrombus.7 Alternately, mechanical thrombectomy and cardiac catheterization may be combined as emergent treatments for these patients, but performing these two procedures simultaneously is not widely available.
For a patient who presents to the ED within both the cardiac and stroke treatment windows, tPA might be a viable option, and the only one readily valuable.8 However, the EP must be mindful of the varying dosages of tPA and means of administration for different thrombosis sites. Also, care must be taken when treating a patient with dual or triple antiplatelet therapy because of the increased risk of hemorrhage.9 Currently, no safe standardized regimens have been established, and further trials need to be performed.10
If the patient in this case report had presented at our ED with only signs and symptoms of an MI, typically she would have been treated with heparin, aspirin, and an urgent cardiac catheterization. If she had presented with only signs and symptoms of a stroke, she would have been treated with full-dose aspirin and worked up from a neurological perspective. Because she had signs and symptoms of both, she presented a dilemma. She was initially treated with heparin to prevent a thrombus formation, but then later changed to only clopidogrel and aspirin to prevent further episodes of coffee ground emesis or worsening hemorrhagic conversion.
Conclusion
Common risk factors for cardiac and cerebral ischemic events may result in a patient presenting with both acute MI and an acute cerebral ischemic event. There have not been sufficient clinical studies to determine the best decision-making process for these patients. Therefore, patients with this complicated presentation must be assessed on an individual basis. Current treatment options are varied and are based according to history of the present illness, time of presentation to the ED, and the available resources within the hospital.
1. Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28(3):220-226.
2. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
3. Di Angelantonio E, Fiorelli M, Toni D, et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76(1):76-81.
4. Shiue I, Hristova K, Sharma J. Correspondence: gender and outcome from acute myocardial infarction and secondary stoke. Br J Cardiology. 2014;21:90.
5. Park S, Jung J. Risk factors for acute cardioembolic brain stroke in acute myocardial infarction. Korean Circulation J. 2005;35:353-356.
6. Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med. 2006;119(4):354.e1-e9.
7. Feher G, Tibold A, Kotlani K, Szapary L. The clinical importance of troponin elevation in ischaemic cerebrovascular events: a clinical review. Journal of Cardiology and Therapy. 2014;1(7):141-149.
8. Maciel R, Palma R, Sousa P, Ferreira F, Nzwalo H. Acute stroke with concomitant acute myocardial infarction: will you thrombolyse? J Stroke. 2015;17(1):84-86.
9. Toyoda K, Yasaka M, Iwade K, et al; Bleeding with Antithrombotic Therapy (BAT) Study Group. Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: a prospective, multicenter, observational study. Stroke. 2008;39(6):1740-1745.
10. Omar HR, Mangar D, Camporesi EM. Simultaneous thrombosis of 2 vascular territories: is thrombolytic therapy a better option? Am J Emerg Med. 2013;31(9):1412-1413.
A 61-year-old woman without any known medical history presented with a chief complaint of right arm numbness and right-sided scalp numbness that had started 2 days earlier. She described a “pins and needles” sensation in her right upper extremity and right scalp, and said the numbness in her scalp was especially noticeable when she combed her hair. The patient denied any chest pain, shortness of breath, weakness in her arms or legs, headache, or blurred vision.
She said that 1 day prior to the onset of the paresthesias, she woke up fatigued and vomited once. Throughout that day, she had symptoms of nausea and fatigue, and sought treatment at an urgent care center that afternoon. At the urgent care center, she was diagnosed with a “stomach virus” and was given an antiemetic. The next day, her nausea improved, but the paresthesias began in her right hand and scalp. On the third day, the patient went to work, but the persistent paresthesias caused her to visit her primary care physician, who sent her to our ED for further work-up.
The patient said she had been in good health until 3 days ago. She reported no medical problems and was taking no medications. The patient denied smoking or using alcohol; her family history was significant only in that her father had a myocardial infarction (MI) while in his 50s.
On physical examination, the patient was alert, oriented, and in no apparent distress. Her body mass index was 28.3 kg/m2. Vital signs were: temperature, 99.2°F; blood pressure, 113/73 mm Hg; heart rate, 93 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 95% on room air.
Her head was normocephalic and atraumatic, and her eyes, ears, nose, and throat were normal. Her neck was supple and without jugular vein distension. The cardiac examination revealed normal heart sounds without murmurs, rubs, or gallops. Her lungs were clear without rales, wheezes, or rhonchi. Her abdomen was soft, without tenderness, guarding, or rebound, and she had normal bowel sounds.
Her musculoskeletal examination was normal, with +5/5 strength bilaterally in her upper and lower extremities. The patient’s skin examination also was normal. On neurological examination, her right upper extremity and right side of her face were noted to have decreased sensation via pinprick compared to the left side, but the examination was otherwise normal. The National Institutes of Health Stroke Scale score was 1.
The patient’s electrocardiogram (ECG) showed a normal sinus rhythm (rate, 90 beats/min), a lateral infarct of undetermined age, and a left atrial abnormality. Laboratory evaluation was significant only for a brain natriuretic peptide level of 334 pg/mL, a creatine phosphokinase (CPK) level of 782 IU/L, and a troponin I level of >50 ng/mL (Table). Serial cardiac enzyme levels were obtained and showed a decline of CPK from 782 IU/L to 331 IU/L over the following 36 hours. However, the troponin I levels remained >50 ng/mL for 5 days and then declined to 31.6 ng/mL.
A computed tomography (CT) scan of the brain without contrast revealed an acute to subacute infarct in the left occipital and left thalamic regions (Figure). A stat transthoracic echocardiogram (ECHO) performed in the ED revealed a dilated left ventricle with an ejection fraction of 20% to 25%, along with a hypokinetic anterolateral wall and an akinetic inferolateral wall. No atrial thrombus was visible on the ECHO. Doppler studies of the patient’s lower extremities were negative for deep vein thrombosis. Magnetic resonance imaging of her brain showed an infarct in the posterior circulation distribution involving the left occipital lobes and small areas in the left thalamic and right parietal-occipital regions. Hemorrhagic conversion of the left occipital infarct without mass effect was also noted. The patient was admitted to the neurological intensive care unit for frequent neurological examinations and close monitoring for worsening cerebral hemorrhage.
When the patient had still been in the ED, cardiology services were consulted; the cardiologist initiated a heparin drip with close monitoring of the coagulation studies. Cardiac catheterization was not done immediately because the ECG did not show acute ST elevations. The day after her presentation to the ED, the patient underwent a primary percutaneous coronary catheterization and was found to have a small rudimentary left anterior descending artery, with only small branches supplying the septal region. The right circumflex artery was very large and was supplying the lateral wall. No stents were placed during this procedure. A transesophageal ECHO (TEE) showed no evidence of a left atrial appendage thrombus.
The patient experienced an episode of coffee ground emesis while undergoing the TEE. Her hemoglobin declined from 11.9 g/dL to 7.9 g/dL, which led to a transfusion of 2 U of packed red blood cells and platelets. Heparin was discontinued and a proton pump inhibitor was started; however, no endoscopy was done at that time.
Throughout her stay, the patient was continuously monitored, but no evidence of arrhythmia or atrial fibrillation was found. Upon discharge, the neurologist recommended the patient receive clopidogrel and aspirin therapy for 3 months with subsequent aspirin monotherapy afterward. The patient was discharged after 10 days in the hospital.
Discussion
Although she had an MI, the patient presented here did not experience any chest pain. Her chief complaint in the ED was paresthesias related to her concomitant stroke, and only on further probing did she describe the additional symptoms of fatigability and vomiting.
Since heart disease and stroke share common risk factors and pathophysiology, acute cerebral ischemic events may happen concurrently with MIs. In a review of studies that included approximately 2,900 patients who had an acute stroke, Kerr et al1 found that 20% had elevated troponin levels within 7 days of the stroke. In 2013, the American Heart Association and American Stroke Association published guidelines advising that all patients who present with acute cerebral ischemia have an emergent ECG and baseline troponin level.2 This was in response to evidence that even low positive troponin levels have been associated with an increased risk of mortality.3 Positive troponin levels are especially important because fatal and nonfatal stroke post-MI events have been found to be increasing in frequency for women, even though there has been a significant overall reduction in post-MI mortality.4 Patients who have an ischemic stroke concurrently with an acute MI or soon after have an overall poorer clinical prognosis.5
For emergency physicians (EPs), this is a “chicken or the egg” scenario. It is difficult to determine which came first: the MI or the cerebral ischemia. Similar risk factors can result in an acute embolic event from revascularization, atrial fibrillation without proper anticoagulation, or a poorly functioning left ventricle.6 It is important to remember that regardless of the order of occurrence, the incidence of ischemic stroke is markedly increased in conjunction with an acute MI.7 Several theories have been advanced regarding the relationship between ischemic stroke and acute MI. One theory proposes that elevated troponin levels could be related to a large catecholamine release after a cerebral ischemic stroke, resulting in subsequent myocardial injury or cardiomyopathy.7 However, this theory remains controversial.
Management
The major consideration for the EP is whether or not to give thrombolytics to a patient who presents with concomitant acute MI and ischemic stroke. An acute MI within the 3 months preceding an acute stroke is considered a relative contraindication for intravenous tissue plasminogen activator (tPA).8 It has also been found, albeit rarely, that there is an increased risk of cardiac rupture or tamponade due to the breakdown of the fibrin clot within the necrotic cardiac tissue.8
How should patients with stroke complicated by acute cardiac compromise be managed in the ED? One acute vascular event cannot be ignored while addressing the other. There are no evidence-based guidelines for the management of patients who present with this picture.8 In addition, no published clinical studies have focused on the decision-making process for these patients.8
Immediate percutaneous coronary intervention for the MI performed on such patients would prevent the use of tPA for the acute stroke. Though any anticoagulation increases the risk for postischemic cerebral hemorrhage, heparin is necessary to prevent the formation of a left ventricle thrombus.7 Alternately, mechanical thrombectomy and cardiac catheterization may be combined as emergent treatments for these patients, but performing these two procedures simultaneously is not widely available.
For a patient who presents to the ED within both the cardiac and stroke treatment windows, tPA might be a viable option, and the only one readily valuable.8 However, the EP must be mindful of the varying dosages of tPA and means of administration for different thrombosis sites. Also, care must be taken when treating a patient with dual or triple antiplatelet therapy because of the increased risk of hemorrhage.9 Currently, no safe standardized regimens have been established, and further trials need to be performed.10
If the patient in this case report had presented at our ED with only signs and symptoms of an MI, typically she would have been treated with heparin, aspirin, and an urgent cardiac catheterization. If she had presented with only signs and symptoms of a stroke, she would have been treated with full-dose aspirin and worked up from a neurological perspective. Because she had signs and symptoms of both, she presented a dilemma. She was initially treated with heparin to prevent a thrombus formation, but then later changed to only clopidogrel and aspirin to prevent further episodes of coffee ground emesis or worsening hemorrhagic conversion.
Conclusion
Common risk factors for cardiac and cerebral ischemic events may result in a patient presenting with both acute MI and an acute cerebral ischemic event. There have not been sufficient clinical studies to determine the best decision-making process for these patients. Therefore, patients with this complicated presentation must be assessed on an individual basis. Current treatment options are varied and are based according to history of the present illness, time of presentation to the ED, and the available resources within the hospital.
A 61-year-old woman without any known medical history presented with a chief complaint of right arm numbness and right-sided scalp numbness that had started 2 days earlier. She described a “pins and needles” sensation in her right upper extremity and right scalp, and said the numbness in her scalp was especially noticeable when she combed her hair. The patient denied any chest pain, shortness of breath, weakness in her arms or legs, headache, or blurred vision.
She said that 1 day prior to the onset of the paresthesias, she woke up fatigued and vomited once. Throughout that day, she had symptoms of nausea and fatigue, and sought treatment at an urgent care center that afternoon. At the urgent care center, she was diagnosed with a “stomach virus” and was given an antiemetic. The next day, her nausea improved, but the paresthesias began in her right hand and scalp. On the third day, the patient went to work, but the persistent paresthesias caused her to visit her primary care physician, who sent her to our ED for further work-up.
The patient said she had been in good health until 3 days ago. She reported no medical problems and was taking no medications. The patient denied smoking or using alcohol; her family history was significant only in that her father had a myocardial infarction (MI) while in his 50s.
On physical examination, the patient was alert, oriented, and in no apparent distress. Her body mass index was 28.3 kg/m2. Vital signs were: temperature, 99.2°F; blood pressure, 113/73 mm Hg; heart rate, 93 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 95% on room air.
Her head was normocephalic and atraumatic, and her eyes, ears, nose, and throat were normal. Her neck was supple and without jugular vein distension. The cardiac examination revealed normal heart sounds without murmurs, rubs, or gallops. Her lungs were clear without rales, wheezes, or rhonchi. Her abdomen was soft, without tenderness, guarding, or rebound, and she had normal bowel sounds.
Her musculoskeletal examination was normal, with +5/5 strength bilaterally in her upper and lower extremities. The patient’s skin examination also was normal. On neurological examination, her right upper extremity and right side of her face were noted to have decreased sensation via pinprick compared to the left side, but the examination was otherwise normal. The National Institutes of Health Stroke Scale score was 1.
The patient’s electrocardiogram (ECG) showed a normal sinus rhythm (rate, 90 beats/min), a lateral infarct of undetermined age, and a left atrial abnormality. Laboratory evaluation was significant only for a brain natriuretic peptide level of 334 pg/mL, a creatine phosphokinase (CPK) level of 782 IU/L, and a troponin I level of >50 ng/mL (Table). Serial cardiac enzyme levels were obtained and showed a decline of CPK from 782 IU/L to 331 IU/L over the following 36 hours. However, the troponin I levels remained >50 ng/mL for 5 days and then declined to 31.6 ng/mL.
A computed tomography (CT) scan of the brain without contrast revealed an acute to subacute infarct in the left occipital and left thalamic regions (Figure). A stat transthoracic echocardiogram (ECHO) performed in the ED revealed a dilated left ventricle with an ejection fraction of 20% to 25%, along with a hypokinetic anterolateral wall and an akinetic inferolateral wall. No atrial thrombus was visible on the ECHO. Doppler studies of the patient’s lower extremities were negative for deep vein thrombosis. Magnetic resonance imaging of her brain showed an infarct in the posterior circulation distribution involving the left occipital lobes and small areas in the left thalamic and right parietal-occipital regions. Hemorrhagic conversion of the left occipital infarct without mass effect was also noted. The patient was admitted to the neurological intensive care unit for frequent neurological examinations and close monitoring for worsening cerebral hemorrhage.
When the patient had still been in the ED, cardiology services were consulted; the cardiologist initiated a heparin drip with close monitoring of the coagulation studies. Cardiac catheterization was not done immediately because the ECG did not show acute ST elevations. The day after her presentation to the ED, the patient underwent a primary percutaneous coronary catheterization and was found to have a small rudimentary left anterior descending artery, with only small branches supplying the septal region. The right circumflex artery was very large and was supplying the lateral wall. No stents were placed during this procedure. A transesophageal ECHO (TEE) showed no evidence of a left atrial appendage thrombus.
The patient experienced an episode of coffee ground emesis while undergoing the TEE. Her hemoglobin declined from 11.9 g/dL to 7.9 g/dL, which led to a transfusion of 2 U of packed red blood cells and platelets. Heparin was discontinued and a proton pump inhibitor was started; however, no endoscopy was done at that time.
Throughout her stay, the patient was continuously monitored, but no evidence of arrhythmia or atrial fibrillation was found. Upon discharge, the neurologist recommended the patient receive clopidogrel and aspirin therapy for 3 months with subsequent aspirin monotherapy afterward. The patient was discharged after 10 days in the hospital.
Discussion
Although she had an MI, the patient presented here did not experience any chest pain. Her chief complaint in the ED was paresthesias related to her concomitant stroke, and only on further probing did she describe the additional symptoms of fatigability and vomiting.
Since heart disease and stroke share common risk factors and pathophysiology, acute cerebral ischemic events may happen concurrently with MIs. In a review of studies that included approximately 2,900 patients who had an acute stroke, Kerr et al1 found that 20% had elevated troponin levels within 7 days of the stroke. In 2013, the American Heart Association and American Stroke Association published guidelines advising that all patients who present with acute cerebral ischemia have an emergent ECG and baseline troponin level.2 This was in response to evidence that even low positive troponin levels have been associated with an increased risk of mortality.3 Positive troponin levels are especially important because fatal and nonfatal stroke post-MI events have been found to be increasing in frequency for women, even though there has been a significant overall reduction in post-MI mortality.4 Patients who have an ischemic stroke concurrently with an acute MI or soon after have an overall poorer clinical prognosis.5
For emergency physicians (EPs), this is a “chicken or the egg” scenario. It is difficult to determine which came first: the MI or the cerebral ischemia. Similar risk factors can result in an acute embolic event from revascularization, atrial fibrillation without proper anticoagulation, or a poorly functioning left ventricle.6 It is important to remember that regardless of the order of occurrence, the incidence of ischemic stroke is markedly increased in conjunction with an acute MI.7 Several theories have been advanced regarding the relationship between ischemic stroke and acute MI. One theory proposes that elevated troponin levels could be related to a large catecholamine release after a cerebral ischemic stroke, resulting in subsequent myocardial injury or cardiomyopathy.7 However, this theory remains controversial.
Management
The major consideration for the EP is whether or not to give thrombolytics to a patient who presents with concomitant acute MI and ischemic stroke. An acute MI within the 3 months preceding an acute stroke is considered a relative contraindication for intravenous tissue plasminogen activator (tPA).8 It has also been found, albeit rarely, that there is an increased risk of cardiac rupture or tamponade due to the breakdown of the fibrin clot within the necrotic cardiac tissue.8
How should patients with stroke complicated by acute cardiac compromise be managed in the ED? One acute vascular event cannot be ignored while addressing the other. There are no evidence-based guidelines for the management of patients who present with this picture.8 In addition, no published clinical studies have focused on the decision-making process for these patients.8
Immediate percutaneous coronary intervention for the MI performed on such patients would prevent the use of tPA for the acute stroke. Though any anticoagulation increases the risk for postischemic cerebral hemorrhage, heparin is necessary to prevent the formation of a left ventricle thrombus.7 Alternately, mechanical thrombectomy and cardiac catheterization may be combined as emergent treatments for these patients, but performing these two procedures simultaneously is not widely available.
For a patient who presents to the ED within both the cardiac and stroke treatment windows, tPA might be a viable option, and the only one readily valuable.8 However, the EP must be mindful of the varying dosages of tPA and means of administration for different thrombosis sites. Also, care must be taken when treating a patient with dual or triple antiplatelet therapy because of the increased risk of hemorrhage.9 Currently, no safe standardized regimens have been established, and further trials need to be performed.10
If the patient in this case report had presented at our ED with only signs and symptoms of an MI, typically she would have been treated with heparin, aspirin, and an urgent cardiac catheterization. If she had presented with only signs and symptoms of a stroke, she would have been treated with full-dose aspirin and worked up from a neurological perspective. Because she had signs and symptoms of both, she presented a dilemma. She was initially treated with heparin to prevent a thrombus formation, but then later changed to only clopidogrel and aspirin to prevent further episodes of coffee ground emesis or worsening hemorrhagic conversion.
Conclusion
Common risk factors for cardiac and cerebral ischemic events may result in a patient presenting with both acute MI and an acute cerebral ischemic event. There have not been sufficient clinical studies to determine the best decision-making process for these patients. Therefore, patients with this complicated presentation must be assessed on an individual basis. Current treatment options are varied and are based according to history of the present illness, time of presentation to the ED, and the available resources within the hospital.
1. Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28(3):220-226.
2. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
3. Di Angelantonio E, Fiorelli M, Toni D, et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76(1):76-81.
4. Shiue I, Hristova K, Sharma J. Correspondence: gender and outcome from acute myocardial infarction and secondary stoke. Br J Cardiology. 2014;21:90.
5. Park S, Jung J. Risk factors for acute cardioembolic brain stroke in acute myocardial infarction. Korean Circulation J. 2005;35:353-356.
6. Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med. 2006;119(4):354.e1-e9.
7. Feher G, Tibold A, Kotlani K, Szapary L. The clinical importance of troponin elevation in ischaemic cerebrovascular events: a clinical review. Journal of Cardiology and Therapy. 2014;1(7):141-149.
8. Maciel R, Palma R, Sousa P, Ferreira F, Nzwalo H. Acute stroke with concomitant acute myocardial infarction: will you thrombolyse? J Stroke. 2015;17(1):84-86.
9. Toyoda K, Yasaka M, Iwade K, et al; Bleeding with Antithrombotic Therapy (BAT) Study Group. Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: a prospective, multicenter, observational study. Stroke. 2008;39(6):1740-1745.
10. Omar HR, Mangar D, Camporesi EM. Simultaneous thrombosis of 2 vascular territories: is thrombolytic therapy a better option? Am J Emerg Med. 2013;31(9):1412-1413.
1. Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28(3):220-226.
2. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
3. Di Angelantonio E, Fiorelli M, Toni D, et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76(1):76-81.
4. Shiue I, Hristova K, Sharma J. Correspondence: gender and outcome from acute myocardial infarction and secondary stoke. Br J Cardiology. 2014;21:90.
5. Park S, Jung J. Risk factors for acute cardioembolic brain stroke in acute myocardial infarction. Korean Circulation J. 2005;35:353-356.
6. Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med. 2006;119(4):354.e1-e9.
7. Feher G, Tibold A, Kotlani K, Szapary L. The clinical importance of troponin elevation in ischaemic cerebrovascular events: a clinical review. Journal of Cardiology and Therapy. 2014;1(7):141-149.
8. Maciel R, Palma R, Sousa P, Ferreira F, Nzwalo H. Acute stroke with concomitant acute myocardial infarction: will you thrombolyse? J Stroke. 2015;17(1):84-86.
9. Toyoda K, Yasaka M, Iwade K, et al; Bleeding with Antithrombotic Therapy (BAT) Study Group. Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: a prospective, multicenter, observational study. Stroke. 2008;39(6):1740-1745.
10. Omar HR, Mangar D, Camporesi EM. Simultaneous thrombosis of 2 vascular territories: is thrombolytic therapy a better option? Am J Emerg Med. 2013;31(9):1412-1413.
A Spontaneous Internal Carotid Artery Dissection Presenting With Headache and Miosis
Internal carotid artery dissection (ICAD) is an uncommon cause of stroke that typically occurs in the setting of (often minor) trauma but can also occur spontaneously. Patients with ICAD typically present with ipsilateral head, face, or neck pain. In approximately half of ICAD cases, an acute partial, painful Horner syndrome is present on examination. Although computed tomography angiography (CTA) is currently the imaging study of choice, magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) and Doppler ultrasound can also be used. Management options include thrombolysis, antiplatelet or anticoagulation therapy, and endovascular or surgical intervention.
Case
A 56-year-old man with a history of migraines presented to the ED with a chief complaint of a 4-day history of right-sided headache. He stated that the pain felt different from his usual migraines and was located behind his right eye. Prior to presentation at the ED, the patient had initially visited an urgent care facility for evaluation. The physician who evaluated the patient at the urgent care facility noted the patient’s left eye appeared dilated and referred him to the ED for evaluation.
The patient further stated that the day prior to presentation, one of his friends had also remarked that the patient’s left eye appeared to be enlarged. The patient denied any visual disturbances, focal weakness, nausea, vomiting, neck pain, or stiffness. His medical history was significant for paroxysmal atrial flutter and hypertension. Regarding medications, the patient was taking dronedarone, clonazepam, omeprazole, and metoprolol.
On physical examination, the patient’s vital signs were: blood pressure, 162/109 mm Hg; heart rate, 85 beats/minute and regular; respiratory rate, 18 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 98% on room air. The patient appeared to be in no acute distress. Ocular examination revealed a dilated left pupil of approximately 5 to 6 mm, and a right pupil measuring approximately 3 mm. Both pupils reacted to light, and the extraocular muscles were intact. The patient’s face appeared symmetrical and had intact sensation. He had normal speech, midline tongue, and good bilateral shoulder shrug. The neck examination revealed normal range of motion with full flexion, without jugular vein distention, lymphadenopathy, or palpable thyroid. The cardiovascular, lung, and abdominal examinations were all normal. The neurological examination showed the patient to be awake, alert, and oriented to person, place, and time. He exhibited 5/5 motor strength in all four extremities, normal gait, and normal finger-to-nose performance; his reflexes were 2+ and symmetrical.
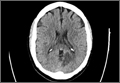
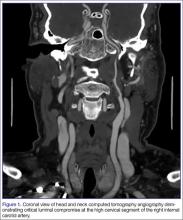
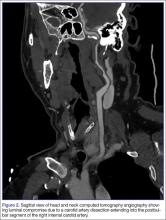
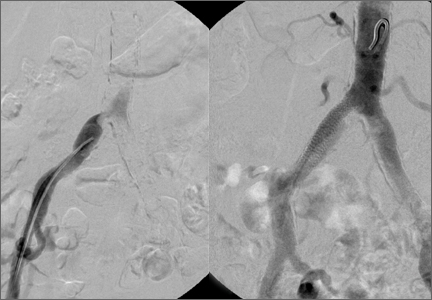
The emergency physician (EP) ordered a stat noncontrast CT scan of the head, complete blood count (CBC), and basic metabolic panel (BMP). The CT scan of the head was interpreted by the radiologist as “no acute intracranial abnormality,” and the CBC and the BMP were normal. Since the EP was concerned about a carotid artery aneurysm or dissection, he ordered a CTA of the head and neck (Figures 1 and 2). The CTA was read as:
Long segment dissection of postbulbar cervical segment of right internal carotid artery, very likely extending into the intracranial segment with critical stenosis at the junction of the high cervical segment and proximal petrous segment. Preserved runoff circulation to the anterior and middle cerebral arteries with robust capacity for collateral support in the context of patent anterior and posterior communicating arteries.
Based on the radiology report, the EP consulted with vascular surgery services, and ordered a Doppler study of the ICAs. The Doppler study demonstrated “arterial thrombus in the right distal extracranial internal carotid artery with hemodynamics suggestive of a distal occlusion/significant obstruction.” The patient was started on an intravenous (IV) heparin drip and admitted to the hospital. Three days later he experienced sudden onset of left arm weakness. An emergent CTA of the head and neck revealed an embolic occlusion of mid-to-distal M1 segment of the right middle cerebral artery. The patient was immediately evaluated by interventional radiology for possible clot removal; however, based on his rapid neurological improvement, he was instead treated medically with aspirin and clopidogrel and continued to show significant neurological improvement. He was discharged home on hospital day 8 on both antiplatelet agents with minimal neurological deficit.
Discussion
Even though ICAD accounts for only 1% to 2% of all strokes, it is responsible for 10% to 25% of strokes in young and middle-aged adults.1-3 The peak incidence for ICAD is in the fifth decade, and it affects men and women equally.4 The extracranial portion of the ICA is the most commonly affected vessel (>90%); dissections of the intracranial portion are associated with greater neurological deficits and have a poorer prognosis.2,5 Cerebral ischemia resulting from a dissection of the extracranial ICA may occur days to weeks after the onset of local symptoms such as head or neck pain, Horner syndrome, or tinnitus.5
In ICAD, a tear in the artery wall causes blood to enter the tunica media of the vessel, forming an intramural hematoma.2,6 This may result in either stenosis of the lumen of the vessel from the enlarging hematoma or an outward aneurysmal dilatation of the vessel that compresses surrounding structures.2,6 Subsequent cerebral ischemia is the result of either arterial embolism or hemodynamic compromise from vessel stenosis.2,3
Causes
Dissections often occur secondary to trauma, though the severity of the trauma may be quite minor.2,6 Seemingly trivial mechanisms that have been associated with dissections include nose blowing, coughing, sudden neck turning, and prolonged telephone conversations. Other known causes are motor vehicle accidents and chiropractic maneuvers. Inherited connective tissue disorders, including Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and osteogenesis imperfecta, are associated with spontaneous ICAD.2,6
Symptoms
The most common presenting symptom of carotid dissection in approximately two-thirds of patients is ipsilateral head, face, or neck pain,2,6 typically described as sharp, constant, and nonthrobbing. Patients report a subjective bruit in 21% to 39% of cases.7 An acute partial painful Horner syndrome is strongly associated with ICAD, but is present in fewer than half of such patients.6 It is characterized by miosis and ptosis and is the result of compression of the ascending sympathetic fibers that travel alongside the ICA within the carotid sheath. Anhidrosis is not present because the fibers for sweat function in the face travel along the external carotid artery.2
Differential Diagnosis
While there is overlap between strokes caused by ICAD versus plaque, some features can help guide the clinician. Symptomatic carotid disease will frequently present with a history of one or more transient ischemic attacks characterized by focal neurological dysfunction or transient monocular blindness—typically within the previous 6 months.8 This history is not usually present in patients with ICAD. Secondly, pain is a much more prominent symptom in ICAD compared to patients with severe carotid atherosclerosis. The history of trauma, even minor, should make dissection higher on the differential diagnosis. Fortunately, the imaging studies to evaluate for these two diseases are the same.
Assessing Pupillary Asymmetry and Ptosis
Careful attention must be paid to assessing a patient for pupillary asymmetry. In a patient with anisocoria, determining the abnormal pupil may require examination of the patient in both bright and dark lighting conditions. The first step is to examine the patient’s pupils under normal lighting conditions. The next step is to assess each pupil’s response to shining a bright light in each eye. The abnormal pupil is the pupil that does not respond well or at all to bright light shone directly in the eye. If the anisocoria is greatest in bright light, the larger pupil is the abnormal pupil. When the anisocoria is greater in dark conditions, the smaller pupil is the abnormal pupil. In this case, the patient’s abnormal pupil was incorrectly diagnosed as the contralateral larger pupil (ie, left)—highlighting the importance of performing a complete pupillary examination in all patients presenting with neurological symptoms.9
Furthermore, as demonstrated in this case, ptosis in a patient with Horner syndrome caused by an ICAD can be subtle. The ptosis is the result of paralysis of Müeller’s muscle, which is innervated by the sympathetic pathway. The levator palpebrae superioris, which causes the more profound ptosis seen in third nerve palsies, is unaffected.10
Imaging Studies
Once the diagnosis of ICAD is suspected, appropriate vascular imaging must be obtained. Digital subtraction angiography has historically been the gold standard for vascular imaging of the neck vessels, but it has largely been replaced by less invasive and more readily available imaging modalities such as CTA and MRI/MRA.11
Computed Tomography Angiography. This is a widely available, rapid imaging choice and has a sensitivity of 80% to 95% in the detection of ICAD.7 It has a greater ability than MRI to identify dissection features such as intimal flaps, pseudoaneurysms, and high-grade stenosis versus occlusion. One of its disadvantages is the need for iodinated contrast, which can limit the ability to obtain the test in those with renal disease or patients with true allergies to IV contrast material. In addition, a mural hematoma can be mistaken for a noncalcified atherosclerotic plaque in the vessel lumen.6
Magnetic Resonance Imaging and Magnetic Resonance Angiography. Both MRI and MRA are also frequently used to diagnose ICAD. The intramural hematoma displays a hyperdense signal on T1-weighted images and has a characteristic crescent shape adjacent to the lumen.11 Magnetic resonance imaging studies are also sensitive in detecting cerebral ischemia resulting from the dissection. However, the sensitivity of MRI/MRA is highest 2 days after the dissection has occurred.2
Doppler Ultrasound. This is another imaging modality used to detect ICAD—one that is noninvasive, less expensive, requires no contrast material, and is widely available. Limitations of Doppler ultrasound include the inability to scan the distal ICA and a lower sensitivity in detecting dissections that cause low-grade stenosis.2 It is more commonly used for follow-up monitoring of dissections.
Management
There are several options for managing ICAD. In patients with unstable lesions, progressing neurological deficit, or further strokes, endovascular stenting has been shown to have a technical success rate of 99% and a procedural complication rate of 1.3%.12 Similarly, if the patient exhibits symptoms of cerebral ischemia, severe narrowing of the arterial lumen, or an unstable plaque, IV heparin is frequently used, followed by warfarin.13 However, since the majority of carotid and vertebral artery dissections heal spontaneously,14 antithrombotic therapies, including aspirin, clopidogrel or warfarin, are often prescribed to prevent thromboembolic complications.
Conclusion
Diagnosing ICAD requires knowledge of the typical history and presenting features of the disease. Careful attention to the ocular examination must be undertaken in any patient presenting with headache or face or neck pain, because the findings can be subtle. In a patient in whom ICAD is suspected, imaging with CTA or MRI/MRA should be performed. Early consultation with vascular surgery services can help determine the most appropriate treatment strategy.
1. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
2. Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81(956):383-388.
3. Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29(12):2646-2648.
4. Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393-397.
5. Biousse V, D’Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995;26(2):235-239.
6. Kasravi N, Leung A, Silver I, Burneo JG. Dissection of the internal carotid artery causing Horner syndrome and palsy of cranial nerve XII. CMAJ. 2010;182(9):E373-E377.
1. Borgman CJ. Horner syndrome secondary to internal carotid artery dissection after a short-distance endurance run: a case study and review. J Optom. 2012;5:209-216.
2. Mohler ER III, Fairman RM. Management of symptomatic carotid atherosclerotic disease. UpToDate Web site. http://www.uptodate.com/contents/management-of-symptomatic-carotid-atherosclerotic-disease. Updated February 24, 2016. Accessed May 6, 2016.
3. Mann J. Anisocoria guidemap. Life in the Fastlane Web site. http://lifeinthefastlane.com/resources/jeff-manns-em-guidemaps/anisocoria-guidemap/. Accessed March 15, 2016.
10. Kedar S, Biousse V, Newman NJ. Horner syndrome. UpToDate Web site. http://www.uptodate.com/contents/horner-syndrome. Updated July 14, 2015. Accessed May 6, 2016.
11. Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
12. Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68(4):856-866.
13. Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
14. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
Internal carotid artery dissection (ICAD) is an uncommon cause of stroke that typically occurs in the setting of (often minor) trauma but can also occur spontaneously. Patients with ICAD typically present with ipsilateral head, face, or neck pain. In approximately half of ICAD cases, an acute partial, painful Horner syndrome is present on examination. Although computed tomography angiography (CTA) is currently the imaging study of choice, magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) and Doppler ultrasound can also be used. Management options include thrombolysis, antiplatelet or anticoagulation therapy, and endovascular or surgical intervention.
Case
A 56-year-old man with a history of migraines presented to the ED with a chief complaint of a 4-day history of right-sided headache. He stated that the pain felt different from his usual migraines and was located behind his right eye. Prior to presentation at the ED, the patient had initially visited an urgent care facility for evaluation. The physician who evaluated the patient at the urgent care facility noted the patient’s left eye appeared dilated and referred him to the ED for evaluation.
The patient further stated that the day prior to presentation, one of his friends had also remarked that the patient’s left eye appeared to be enlarged. The patient denied any visual disturbances, focal weakness, nausea, vomiting, neck pain, or stiffness. His medical history was significant for paroxysmal atrial flutter and hypertension. Regarding medications, the patient was taking dronedarone, clonazepam, omeprazole, and metoprolol.
On physical examination, the patient’s vital signs were: blood pressure, 162/109 mm Hg; heart rate, 85 beats/minute and regular; respiratory rate, 18 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 98% on room air. The patient appeared to be in no acute distress. Ocular examination revealed a dilated left pupil of approximately 5 to 6 mm, and a right pupil measuring approximately 3 mm. Both pupils reacted to light, and the extraocular muscles were intact. The patient’s face appeared symmetrical and had intact sensation. He had normal speech, midline tongue, and good bilateral shoulder shrug. The neck examination revealed normal range of motion with full flexion, without jugular vein distention, lymphadenopathy, or palpable thyroid. The cardiovascular, lung, and abdominal examinations were all normal. The neurological examination showed the patient to be awake, alert, and oriented to person, place, and time. He exhibited 5/5 motor strength in all four extremities, normal gait, and normal finger-to-nose performance; his reflexes were 2+ and symmetrical.
The emergency physician (EP) ordered a stat noncontrast CT scan of the head, complete blood count (CBC), and basic metabolic panel (BMP). The CT scan of the head was interpreted by the radiologist as “no acute intracranial abnormality,” and the CBC and the BMP were normal. Since the EP was concerned about a carotid artery aneurysm or dissection, he ordered a CTA of the head and neck (Figures 1 and 2). The CTA was read as:
Long segment dissection of postbulbar cervical segment of right internal carotid artery, very likely extending into the intracranial segment with critical stenosis at the junction of the high cervical segment and proximal petrous segment. Preserved runoff circulation to the anterior and middle cerebral arteries with robust capacity for collateral support in the context of patent anterior and posterior communicating arteries.
Based on the radiology report, the EP consulted with vascular surgery services, and ordered a Doppler study of the ICAs. The Doppler study demonstrated “arterial thrombus in the right distal extracranial internal carotid artery with hemodynamics suggestive of a distal occlusion/significant obstruction.” The patient was started on an intravenous (IV) heparin drip and admitted to the hospital. Three days later he experienced sudden onset of left arm weakness. An emergent CTA of the head and neck revealed an embolic occlusion of mid-to-distal M1 segment of the right middle cerebral artery. The patient was immediately evaluated by interventional radiology for possible clot removal; however, based on his rapid neurological improvement, he was instead treated medically with aspirin and clopidogrel and continued to show significant neurological improvement. He was discharged home on hospital day 8 on both antiplatelet agents with minimal neurological deficit.
Discussion
Even though ICAD accounts for only 1% to 2% of all strokes, it is responsible for 10% to 25% of strokes in young and middle-aged adults.1-3 The peak incidence for ICAD is in the fifth decade, and it affects men and women equally.4 The extracranial portion of the ICA is the most commonly affected vessel (>90%); dissections of the intracranial portion are associated with greater neurological deficits and have a poorer prognosis.2,5 Cerebral ischemia resulting from a dissection of the extracranial ICA may occur days to weeks after the onset of local symptoms such as head or neck pain, Horner syndrome, or tinnitus.5
In ICAD, a tear in the artery wall causes blood to enter the tunica media of the vessel, forming an intramural hematoma.2,6 This may result in either stenosis of the lumen of the vessel from the enlarging hematoma or an outward aneurysmal dilatation of the vessel that compresses surrounding structures.2,6 Subsequent cerebral ischemia is the result of either arterial embolism or hemodynamic compromise from vessel stenosis.2,3
Causes
Dissections often occur secondary to trauma, though the severity of the trauma may be quite minor.2,6 Seemingly trivial mechanisms that have been associated with dissections include nose blowing, coughing, sudden neck turning, and prolonged telephone conversations. Other known causes are motor vehicle accidents and chiropractic maneuvers. Inherited connective tissue disorders, including Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and osteogenesis imperfecta, are associated with spontaneous ICAD.2,6
Symptoms
The most common presenting symptom of carotid dissection in approximately two-thirds of patients is ipsilateral head, face, or neck pain,2,6 typically described as sharp, constant, and nonthrobbing. Patients report a subjective bruit in 21% to 39% of cases.7 An acute partial painful Horner syndrome is strongly associated with ICAD, but is present in fewer than half of such patients.6 It is characterized by miosis and ptosis and is the result of compression of the ascending sympathetic fibers that travel alongside the ICA within the carotid sheath. Anhidrosis is not present because the fibers for sweat function in the face travel along the external carotid artery.2
Differential Diagnosis
While there is overlap between strokes caused by ICAD versus plaque, some features can help guide the clinician. Symptomatic carotid disease will frequently present with a history of one or more transient ischemic attacks characterized by focal neurological dysfunction or transient monocular blindness—typically within the previous 6 months.8 This history is not usually present in patients with ICAD. Secondly, pain is a much more prominent symptom in ICAD compared to patients with severe carotid atherosclerosis. The history of trauma, even minor, should make dissection higher on the differential diagnosis. Fortunately, the imaging studies to evaluate for these two diseases are the same.
Assessing Pupillary Asymmetry and Ptosis
Careful attention must be paid to assessing a patient for pupillary asymmetry. In a patient with anisocoria, determining the abnormal pupil may require examination of the patient in both bright and dark lighting conditions. The first step is to examine the patient’s pupils under normal lighting conditions. The next step is to assess each pupil’s response to shining a bright light in each eye. The abnormal pupil is the pupil that does not respond well or at all to bright light shone directly in the eye. If the anisocoria is greatest in bright light, the larger pupil is the abnormal pupil. When the anisocoria is greater in dark conditions, the smaller pupil is the abnormal pupil. In this case, the patient’s abnormal pupil was incorrectly diagnosed as the contralateral larger pupil (ie, left)—highlighting the importance of performing a complete pupillary examination in all patients presenting with neurological symptoms.9
Furthermore, as demonstrated in this case, ptosis in a patient with Horner syndrome caused by an ICAD can be subtle. The ptosis is the result of paralysis of Müeller’s muscle, which is innervated by the sympathetic pathway. The levator palpebrae superioris, which causes the more profound ptosis seen in third nerve palsies, is unaffected.10
Imaging Studies
Once the diagnosis of ICAD is suspected, appropriate vascular imaging must be obtained. Digital subtraction angiography has historically been the gold standard for vascular imaging of the neck vessels, but it has largely been replaced by less invasive and more readily available imaging modalities such as CTA and MRI/MRA.11
Computed Tomography Angiography. This is a widely available, rapid imaging choice and has a sensitivity of 80% to 95% in the detection of ICAD.7 It has a greater ability than MRI to identify dissection features such as intimal flaps, pseudoaneurysms, and high-grade stenosis versus occlusion. One of its disadvantages is the need for iodinated contrast, which can limit the ability to obtain the test in those with renal disease or patients with true allergies to IV contrast material. In addition, a mural hematoma can be mistaken for a noncalcified atherosclerotic plaque in the vessel lumen.6
Magnetic Resonance Imaging and Magnetic Resonance Angiography. Both MRI and MRA are also frequently used to diagnose ICAD. The intramural hematoma displays a hyperdense signal on T1-weighted images and has a characteristic crescent shape adjacent to the lumen.11 Magnetic resonance imaging studies are also sensitive in detecting cerebral ischemia resulting from the dissection. However, the sensitivity of MRI/MRA is highest 2 days after the dissection has occurred.2
Doppler Ultrasound. This is another imaging modality used to detect ICAD—one that is noninvasive, less expensive, requires no contrast material, and is widely available. Limitations of Doppler ultrasound include the inability to scan the distal ICA and a lower sensitivity in detecting dissections that cause low-grade stenosis.2 It is more commonly used for follow-up monitoring of dissections.
Management
There are several options for managing ICAD. In patients with unstable lesions, progressing neurological deficit, or further strokes, endovascular stenting has been shown to have a technical success rate of 99% and a procedural complication rate of 1.3%.12 Similarly, if the patient exhibits symptoms of cerebral ischemia, severe narrowing of the arterial lumen, or an unstable plaque, IV heparin is frequently used, followed by warfarin.13 However, since the majority of carotid and vertebral artery dissections heal spontaneously,14 antithrombotic therapies, including aspirin, clopidogrel or warfarin, are often prescribed to prevent thromboembolic complications.
Conclusion
Diagnosing ICAD requires knowledge of the typical history and presenting features of the disease. Careful attention to the ocular examination must be undertaken in any patient presenting with headache or face or neck pain, because the findings can be subtle. In a patient in whom ICAD is suspected, imaging with CTA or MRI/MRA should be performed. Early consultation with vascular surgery services can help determine the most appropriate treatment strategy.
Internal carotid artery dissection (ICAD) is an uncommon cause of stroke that typically occurs in the setting of (often minor) trauma but can also occur spontaneously. Patients with ICAD typically present with ipsilateral head, face, or neck pain. In approximately half of ICAD cases, an acute partial, painful Horner syndrome is present on examination. Although computed tomography angiography (CTA) is currently the imaging study of choice, magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) and Doppler ultrasound can also be used. Management options include thrombolysis, antiplatelet or anticoagulation therapy, and endovascular or surgical intervention.
Case
A 56-year-old man with a history of migraines presented to the ED with a chief complaint of a 4-day history of right-sided headache. He stated that the pain felt different from his usual migraines and was located behind his right eye. Prior to presentation at the ED, the patient had initially visited an urgent care facility for evaluation. The physician who evaluated the patient at the urgent care facility noted the patient’s left eye appeared dilated and referred him to the ED for evaluation.
The patient further stated that the day prior to presentation, one of his friends had also remarked that the patient’s left eye appeared to be enlarged. The patient denied any visual disturbances, focal weakness, nausea, vomiting, neck pain, or stiffness. His medical history was significant for paroxysmal atrial flutter and hypertension. Regarding medications, the patient was taking dronedarone, clonazepam, omeprazole, and metoprolol.
On physical examination, the patient’s vital signs were: blood pressure, 162/109 mm Hg; heart rate, 85 beats/minute and regular; respiratory rate, 18 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 98% on room air. The patient appeared to be in no acute distress. Ocular examination revealed a dilated left pupil of approximately 5 to 6 mm, and a right pupil measuring approximately 3 mm. Both pupils reacted to light, and the extraocular muscles were intact. The patient’s face appeared symmetrical and had intact sensation. He had normal speech, midline tongue, and good bilateral shoulder shrug. The neck examination revealed normal range of motion with full flexion, without jugular vein distention, lymphadenopathy, or palpable thyroid. The cardiovascular, lung, and abdominal examinations were all normal. The neurological examination showed the patient to be awake, alert, and oriented to person, place, and time. He exhibited 5/5 motor strength in all four extremities, normal gait, and normal finger-to-nose performance; his reflexes were 2+ and symmetrical.
The emergency physician (EP) ordered a stat noncontrast CT scan of the head, complete blood count (CBC), and basic metabolic panel (BMP). The CT scan of the head was interpreted by the radiologist as “no acute intracranial abnormality,” and the CBC and the BMP were normal. Since the EP was concerned about a carotid artery aneurysm or dissection, he ordered a CTA of the head and neck (Figures 1 and 2). The CTA was read as:
Long segment dissection of postbulbar cervical segment of right internal carotid artery, very likely extending into the intracranial segment with critical stenosis at the junction of the high cervical segment and proximal petrous segment. Preserved runoff circulation to the anterior and middle cerebral arteries with robust capacity for collateral support in the context of patent anterior and posterior communicating arteries.
Based on the radiology report, the EP consulted with vascular surgery services, and ordered a Doppler study of the ICAs. The Doppler study demonstrated “arterial thrombus in the right distal extracranial internal carotid artery with hemodynamics suggestive of a distal occlusion/significant obstruction.” The patient was started on an intravenous (IV) heparin drip and admitted to the hospital. Three days later he experienced sudden onset of left arm weakness. An emergent CTA of the head and neck revealed an embolic occlusion of mid-to-distal M1 segment of the right middle cerebral artery. The patient was immediately evaluated by interventional radiology for possible clot removal; however, based on his rapid neurological improvement, he was instead treated medically with aspirin and clopidogrel and continued to show significant neurological improvement. He was discharged home on hospital day 8 on both antiplatelet agents with minimal neurological deficit.
Discussion
Even though ICAD accounts for only 1% to 2% of all strokes, it is responsible for 10% to 25% of strokes in young and middle-aged adults.1-3 The peak incidence for ICAD is in the fifth decade, and it affects men and women equally.4 The extracranial portion of the ICA is the most commonly affected vessel (>90%); dissections of the intracranial portion are associated with greater neurological deficits and have a poorer prognosis.2,5 Cerebral ischemia resulting from a dissection of the extracranial ICA may occur days to weeks after the onset of local symptoms such as head or neck pain, Horner syndrome, or tinnitus.5
In ICAD, a tear in the artery wall causes blood to enter the tunica media of the vessel, forming an intramural hematoma.2,6 This may result in either stenosis of the lumen of the vessel from the enlarging hematoma or an outward aneurysmal dilatation of the vessel that compresses surrounding structures.2,6 Subsequent cerebral ischemia is the result of either arterial embolism or hemodynamic compromise from vessel stenosis.2,3
Causes
Dissections often occur secondary to trauma, though the severity of the trauma may be quite minor.2,6 Seemingly trivial mechanisms that have been associated with dissections include nose blowing, coughing, sudden neck turning, and prolonged telephone conversations. Other known causes are motor vehicle accidents and chiropractic maneuvers. Inherited connective tissue disorders, including Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and osteogenesis imperfecta, are associated with spontaneous ICAD.2,6
Symptoms
The most common presenting symptom of carotid dissection in approximately two-thirds of patients is ipsilateral head, face, or neck pain,2,6 typically described as sharp, constant, and nonthrobbing. Patients report a subjective bruit in 21% to 39% of cases.7 An acute partial painful Horner syndrome is strongly associated with ICAD, but is present in fewer than half of such patients.6 It is characterized by miosis and ptosis and is the result of compression of the ascending sympathetic fibers that travel alongside the ICA within the carotid sheath. Anhidrosis is not present because the fibers for sweat function in the face travel along the external carotid artery.2
Differential Diagnosis
While there is overlap between strokes caused by ICAD versus plaque, some features can help guide the clinician. Symptomatic carotid disease will frequently present with a history of one or more transient ischemic attacks characterized by focal neurological dysfunction or transient monocular blindness—typically within the previous 6 months.8 This history is not usually present in patients with ICAD. Secondly, pain is a much more prominent symptom in ICAD compared to patients with severe carotid atherosclerosis. The history of trauma, even minor, should make dissection higher on the differential diagnosis. Fortunately, the imaging studies to evaluate for these two diseases are the same.
Assessing Pupillary Asymmetry and Ptosis
Careful attention must be paid to assessing a patient for pupillary asymmetry. In a patient with anisocoria, determining the abnormal pupil may require examination of the patient in both bright and dark lighting conditions. The first step is to examine the patient’s pupils under normal lighting conditions. The next step is to assess each pupil’s response to shining a bright light in each eye. The abnormal pupil is the pupil that does not respond well or at all to bright light shone directly in the eye. If the anisocoria is greatest in bright light, the larger pupil is the abnormal pupil. When the anisocoria is greater in dark conditions, the smaller pupil is the abnormal pupil. In this case, the patient’s abnormal pupil was incorrectly diagnosed as the contralateral larger pupil (ie, left)—highlighting the importance of performing a complete pupillary examination in all patients presenting with neurological symptoms.9
Furthermore, as demonstrated in this case, ptosis in a patient with Horner syndrome caused by an ICAD can be subtle. The ptosis is the result of paralysis of Müeller’s muscle, which is innervated by the sympathetic pathway. The levator palpebrae superioris, which causes the more profound ptosis seen in third nerve palsies, is unaffected.10
Imaging Studies
Once the diagnosis of ICAD is suspected, appropriate vascular imaging must be obtained. Digital subtraction angiography has historically been the gold standard for vascular imaging of the neck vessels, but it has largely been replaced by less invasive and more readily available imaging modalities such as CTA and MRI/MRA.11
Computed Tomography Angiography. This is a widely available, rapid imaging choice and has a sensitivity of 80% to 95% in the detection of ICAD.7 It has a greater ability than MRI to identify dissection features such as intimal flaps, pseudoaneurysms, and high-grade stenosis versus occlusion. One of its disadvantages is the need for iodinated contrast, which can limit the ability to obtain the test in those with renal disease or patients with true allergies to IV contrast material. In addition, a mural hematoma can be mistaken for a noncalcified atherosclerotic plaque in the vessel lumen.6
Magnetic Resonance Imaging and Magnetic Resonance Angiography. Both MRI and MRA are also frequently used to diagnose ICAD. The intramural hematoma displays a hyperdense signal on T1-weighted images and has a characteristic crescent shape adjacent to the lumen.11 Magnetic resonance imaging studies are also sensitive in detecting cerebral ischemia resulting from the dissection. However, the sensitivity of MRI/MRA is highest 2 days after the dissection has occurred.2
Doppler Ultrasound. This is another imaging modality used to detect ICAD—one that is noninvasive, less expensive, requires no contrast material, and is widely available. Limitations of Doppler ultrasound include the inability to scan the distal ICA and a lower sensitivity in detecting dissections that cause low-grade stenosis.2 It is more commonly used for follow-up monitoring of dissections.
Management
There are several options for managing ICAD. In patients with unstable lesions, progressing neurological deficit, or further strokes, endovascular stenting has been shown to have a technical success rate of 99% and a procedural complication rate of 1.3%.12 Similarly, if the patient exhibits symptoms of cerebral ischemia, severe narrowing of the arterial lumen, or an unstable plaque, IV heparin is frequently used, followed by warfarin.13 However, since the majority of carotid and vertebral artery dissections heal spontaneously,14 antithrombotic therapies, including aspirin, clopidogrel or warfarin, are often prescribed to prevent thromboembolic complications.
Conclusion
Diagnosing ICAD requires knowledge of the typical history and presenting features of the disease. Careful attention to the ocular examination must be undertaken in any patient presenting with headache or face or neck pain, because the findings can be subtle. In a patient in whom ICAD is suspected, imaging with CTA or MRI/MRA should be performed. Early consultation with vascular surgery services can help determine the most appropriate treatment strategy.
1. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
2. Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81(956):383-388.
3. Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29(12):2646-2648.
4. Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393-397.
5. Biousse V, D’Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995;26(2):235-239.
6. Kasravi N, Leung A, Silver I, Burneo JG. Dissection of the internal carotid artery causing Horner syndrome and palsy of cranial nerve XII. CMAJ. 2010;182(9):E373-E377.
1. Borgman CJ. Horner syndrome secondary to internal carotid artery dissection after a short-distance endurance run: a case study and review. J Optom. 2012;5:209-216.
2. Mohler ER III, Fairman RM. Management of symptomatic carotid atherosclerotic disease. UpToDate Web site. http://www.uptodate.com/contents/management-of-symptomatic-carotid-atherosclerotic-disease. Updated February 24, 2016. Accessed May 6, 2016.
3. Mann J. Anisocoria guidemap. Life in the Fastlane Web site. http://lifeinthefastlane.com/resources/jeff-manns-em-guidemaps/anisocoria-guidemap/. Accessed March 15, 2016.
10. Kedar S, Biousse V, Newman NJ. Horner syndrome. UpToDate Web site. http://www.uptodate.com/contents/horner-syndrome. Updated July 14, 2015. Accessed May 6, 2016.
11. Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
12. Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68(4):856-866.
13. Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
14. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
1. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
2. Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81(956):383-388.
3. Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29(12):2646-2648.
4. Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393-397.
5. Biousse V, D’Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995;26(2):235-239.
6. Kasravi N, Leung A, Silver I, Burneo JG. Dissection of the internal carotid artery causing Horner syndrome and palsy of cranial nerve XII. CMAJ. 2010;182(9):E373-E377.
1. Borgman CJ. Horner syndrome secondary to internal carotid artery dissection after a short-distance endurance run: a case study and review. J Optom. 2012;5:209-216.
2. Mohler ER III, Fairman RM. Management of symptomatic carotid atherosclerotic disease. UpToDate Web site. http://www.uptodate.com/contents/management-of-symptomatic-carotid-atherosclerotic-disease. Updated February 24, 2016. Accessed May 6, 2016.
3. Mann J. Anisocoria guidemap. Life in the Fastlane Web site. http://lifeinthefastlane.com/resources/jeff-manns-em-guidemaps/anisocoria-guidemap/. Accessed March 15, 2016.
10. Kedar S, Biousse V, Newman NJ. Horner syndrome. UpToDate Web site. http://www.uptodate.com/contents/horner-syndrome. Updated July 14, 2015. Accessed May 6, 2016.
11. Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
12. Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68(4):856-866.
13. Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
14. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
The Case for Case Reports
In The Case Report Issue, we feature four separate case reports presenting different conditions, much like patients may present in succession to a busy ED. Though considered of lesser importance than other types of peer-reviewed literature in this era of evidence-based medicine, case reports nevertheless fulfill an important role in clinical practice, medical education, and even medical research by identifying and tracking an important cause of a developing disease--especially one with a toxicologic or infectious etiology. In some instances, case reports also identify effective or ineffective treatments (though the latter is more rarely reported) and adverse effects of approved treatments, especially those of a newly introduced “Phase IV” medication.
Often, the ED is the initial setting for many reportable occurrences, and in recent years, patients first presenting to EDs have alerted the entire medical community to serious emerging illnesses such as Legionnaires’ disease, HIV and AIDS, anthrax, and Ebola. Most recently, firsthand reports by a pair of mother/daughter physicians in Brazil linked an alarming appearance of several new cases of microcephaly to a rash that followed a mosquito bite during pregnancy, and ultimately to identification of the mosquito-borne Zika virus.
Similarly, toxicologists at urban poison centers have been able to rapidly link cases of new and puzzling adverse effects and deaths reported by area emergency physicians to a dangerous new street drug or combination of drugs in that area, such as synthetic cannabinoid agonists, or heroin mixed with scopolamine, and then immediately alert other physicians and the public to these dangers.
As recently described by Florek and Dellavalle in Journal of Medical Case Reports (http://bit.ly/28PLi7w), case reports make meaningful contributions to the knowledge and education of medical students, residents, fellows, and (we would add) attendings. Written with the goal of sharing information for medical or scientific purposes, they often serve as a young physician’s first experience with medical writing and provide a solid foundation for manuscript preparation and publication.
Finally, a good ED case report that includes accurate descriptions of all relevant features along with any unique departures from classical presentations, followed by an up-to-date review of current treatments, presents most of us with a vivid means of identifying and remembering the salient features of a clinical problem or disease.
In The Case Report Issue, we feature four separate case reports presenting different conditions, much like patients may present in succession to a busy ED. Though considered of lesser importance than other types of peer-reviewed literature in this era of evidence-based medicine, case reports nevertheless fulfill an important role in clinical practice, medical education, and even medical research by identifying and tracking an important cause of a developing disease--especially one with a toxicologic or infectious etiology. In some instances, case reports also identify effective or ineffective treatments (though the latter is more rarely reported) and adverse effects of approved treatments, especially those of a newly introduced “Phase IV” medication.
Often, the ED is the initial setting for many reportable occurrences, and in recent years, patients first presenting to EDs have alerted the entire medical community to serious emerging illnesses such as Legionnaires’ disease, HIV and AIDS, anthrax, and Ebola. Most recently, firsthand reports by a pair of mother/daughter physicians in Brazil linked an alarming appearance of several new cases of microcephaly to a rash that followed a mosquito bite during pregnancy, and ultimately to identification of the mosquito-borne Zika virus.
Similarly, toxicologists at urban poison centers have been able to rapidly link cases of new and puzzling adverse effects and deaths reported by area emergency physicians to a dangerous new street drug or combination of drugs in that area, such as synthetic cannabinoid agonists, or heroin mixed with scopolamine, and then immediately alert other physicians and the public to these dangers.
As recently described by Florek and Dellavalle in Journal of Medical Case Reports (http://bit.ly/28PLi7w), case reports make meaningful contributions to the knowledge and education of medical students, residents, fellows, and (we would add) attendings. Written with the goal of sharing information for medical or scientific purposes, they often serve as a young physician’s first experience with medical writing and provide a solid foundation for manuscript preparation and publication.
Finally, a good ED case report that includes accurate descriptions of all relevant features along with any unique departures from classical presentations, followed by an up-to-date review of current treatments, presents most of us with a vivid means of identifying and remembering the salient features of a clinical problem or disease.
In The Case Report Issue, we feature four separate case reports presenting different conditions, much like patients may present in succession to a busy ED. Though considered of lesser importance than other types of peer-reviewed literature in this era of evidence-based medicine, case reports nevertheless fulfill an important role in clinical practice, medical education, and even medical research by identifying and tracking an important cause of a developing disease--especially one with a toxicologic or infectious etiology. In some instances, case reports also identify effective or ineffective treatments (though the latter is more rarely reported) and adverse effects of approved treatments, especially those of a newly introduced “Phase IV” medication.
Often, the ED is the initial setting for many reportable occurrences, and in recent years, patients first presenting to EDs have alerted the entire medical community to serious emerging illnesses such as Legionnaires’ disease, HIV and AIDS, anthrax, and Ebola. Most recently, firsthand reports by a pair of mother/daughter physicians in Brazil linked an alarming appearance of several new cases of microcephaly to a rash that followed a mosquito bite during pregnancy, and ultimately to identification of the mosquito-borne Zika virus.
Similarly, toxicologists at urban poison centers have been able to rapidly link cases of new and puzzling adverse effects and deaths reported by area emergency physicians to a dangerous new street drug or combination of drugs in that area, such as synthetic cannabinoid agonists, or heroin mixed with scopolamine, and then immediately alert other physicians and the public to these dangers.
As recently described by Florek and Dellavalle in Journal of Medical Case Reports (http://bit.ly/28PLi7w), case reports make meaningful contributions to the knowledge and education of medical students, residents, fellows, and (we would add) attendings. Written with the goal of sharing information for medical or scientific purposes, they often serve as a young physician’s first experience with medical writing and provide a solid foundation for manuscript preparation and publication.
Finally, a good ED case report that includes accurate descriptions of all relevant features along with any unique departures from classical presentations, followed by an up-to-date review of current treatments, presents most of us with a vivid means of identifying and remembering the salient features of a clinical problem or disease.
Tonsillectomy for this 35-year-old patient?
THE CASE
A 35-year-old woman sought care for a fever and sore throat that she’d had for 4 days. She denied symptoms of cough, rhinorrhea, or sputum production.
The patient’s medical history included severe recurrent streptococcal pharyngitis as a child and teenager. At the age of 17, she developed a fever of 105° F with associated delirium, dysphagia, nausea, and vomiting, and missed several days of school. She also lost 82 pounds, developed oral thrush, and continued to feel fatigued for approximately a year. After her primary care physician noted a heart murmur on physical exam, she was sent for echocardiography and diagnosed with rheumatic fever secondary to streptococcal pharyngitis.
Eighteen years (and numerous streptococcal infections) later, the patient was at our facility and we were ordering a rapid antigen detection test (RADT) for her current illness. The throat specimen was positive for group A ß-hemolytic streptococcus (GAS). The patient’s 8-year-old daughter also had a sore throat, fever, and positive RADT; her symptoms resolved with oral amoxicillin for 10 days. The patient’s husband was also treated successfully with oral amoxicillin/clavulanate for 10 days for similar symptoms. The patient herself, however, was unsuccessfully treated with oral amoxicillin 500 mg twice daily for 7 days.
She was then given oral amoxicillin/clavulanate 875 mg twice daily for 14 days, but received no relief. Even after receiving clindamycin 600 mg twice daily for 10 days, she had minimal relief and remained positive for GAS on repeat RADT. It was at this point that tonsillectomy was considered as a possible treatment modality for her refractory GAS pharyngitis.
The patient consented to the procedure and underwent a tonsillectomy. She has remained asymptomatic for 2 years and there have been no reported outbreaks of GAS infection in her household.
DISCUSSION
Streptococcal pharyngitis is an infection of the oropharynx and/or nasopharynx that is caused by Streptococcus pyogenes (also known as GAS). It is one of the most frequent illnesses encountered by primary care physicians, and primarily occurs in children ages 5 to 15 years.1,2 The signs and symptoms of GAS pharyngitis include an abrupt onset of a sore throat, tonsillar exudate, tender cervical adenopathy, and fever. (The classic presentation of GAS pharyngitis in a different patient can be seen in the FIGURE.)
Throat cultures are the gold standard for the diagnosis of GAS pharyngitis, but results take 24 to 48 hours, which can delay appropriate treatment. Therefore, the use of the RADT is often preferred clinically.1 RADT is not recommended for children and adults who show clinical symptoms that are highly suggestive of a viral illness, such as cough, rhinorrhea, hoarseness, or oral ulcers. A negative RADT in children and adolescents necessitates a throat culture to confirm the diagnosis.2
The antibiotics of choice are either penicillin 50 mg/kg/d in 4 divided doses or amoxicillin 40 mg/kg/d in 3 divided doses (maximum for both is 2000 mg/d) for 10 days. Options for patients with penicillin allergies include clindamycin or clarithromycin for 10 days or azithromycin for 5 days.2
The Infectious Diseases Society of America (IDSA) does not recommend routine testing or empiric treatment of asymptomatic carriers. However, it does recommend treatment of GAS carriers in certain situations, such as when: 2
- the carrier has acute rheumatic fever
- there is a family or personal history of acute rheumatic fever
- there is a post-streptococcal glomerulonephritis outbreak
- a family has excessive anxiety about GAS infections
- a tonsillectomy is being considered.
When—and for whom—is tonsillectomy beneficial?
Tonsillectomy is a treatment option for patients with recurrent episodes of GAS pharyngitis. Indications include patients with 7 GAS infections in a year, 5 episodes in 2 years, or 3 episodes in 3 years.3,4 In select patient populations, tonsillectomy has been shown to decrease missed work days and medical expenses caused by recurrent pharyngitis.5,6
Alho et al demonstrated that adults with recurrent episodes of GAS pharyngitis benefit from tonsillectomy in terms of fewer repeat infections and more days without throat pain.7 A randomized controlled trial conducted by Koskenkorva et al found that the overall rates of pharyngitis, throat pain, rhinitis, and cough were significantly lower in adults who received a tonsillectomy vs those who did not.5 Still, whether tonsillectomy is worthwhile in adults is debatable; Burton et al found no evidence that tonsillectomy is effective for chronic or recurrent acute tonsillitis in adults.8
Overall meta-analysis results indicate that tonsillectomy results in a 43% reduction in the incidence of pharyngitis in children between the ages of 4 and 16.8,9 One study found that children without tonsillectomy were 3.1 times more likely to develop subsequent GAS pharyngitis than children who underwent tonsillectomy.9 Another study found that children who received tonsillectomy demonstrated a decrease in sore throat episodes by 1.2 episodes per year and a decrease in school absenteeism by 2.8 days per year.6 Tonsillectomy does carry a risk of intraoperative and postoperative bleeding in children and adults, which may make it a less desirable option for some patients.6
THE TAKEAWAY
Recurrent GAS pharyngitis poses a significant challenge for clinicians. When episodes recur, it may be prudent to treat asymptomatic carriers in the patient’s household. Tonsillectomy should be considered in refractory cases since recurrent GAS pharyngitis directly impacts the wellness and productivity of patients. Our patient certainly benefited from the surgery: She has not missed any work days or had to visit her primary care physician because of a GAS infection since her tonsillectomy.
1. Gurol Y, Akan H, Izbirak G, et al. The sensitivity and the specificity of rapid antigen test in streptococcal upper respiratory tract infections. Int J Pediatr Otorhinolaryngol. 2010;74:591-593.
2. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
3. Stuck BA, Götte K, Windfuhr JP, et al. Tonsillectomy in children. Dtsch Arztebl Int. 2008;105:852-860.
4. Baugh RF, Archer SM, Mitchell RB, et al; American Academy of Otolaryngology-Head and Neck Surgery Foundation. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144:S1-S30.
5. Koskenkorva T, Koivunen P, Koskela M, et al. Short-term outcomes of tonsillectomy in adult patients with recurrent pharyngitis: a randomized controlled trial. CMAJ. 2013;185:E331-E336.
6. van Staaij BK, van den Akker EH, van der Heijden GJ, et al. Adenotonsillectomy for upper respiratory infections: evidence based? Arch Dis Child. 2005;90:19-25.
7. Alho OP, Koivunen P, Penna T, et al. Tonsillectomy versus watchful waiting in recurrent streptococcal pharyngitis in adults: randomised controlled trial. BMJ. 2007;334:939.
8. Burton MJ, Towler B, Glasziou P. Tonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev. 2000;(2):CD001802.
9. Orvidas LJ, St Sauver JL, Weaver AL. Efficacy of tonsillectomy in treatment of recurrent group A beta-hemolytic streptococcal pharyngitis. Laryngoscope. 2006;116:1946-1950.
THE CASE
A 35-year-old woman sought care for a fever and sore throat that she’d had for 4 days. She denied symptoms of cough, rhinorrhea, or sputum production.
The patient’s medical history included severe recurrent streptococcal pharyngitis as a child and teenager. At the age of 17, she developed a fever of 105° F with associated delirium, dysphagia, nausea, and vomiting, and missed several days of school. She also lost 82 pounds, developed oral thrush, and continued to feel fatigued for approximately a year. After her primary care physician noted a heart murmur on physical exam, she was sent for echocardiography and diagnosed with rheumatic fever secondary to streptococcal pharyngitis.
Eighteen years (and numerous streptococcal infections) later, the patient was at our facility and we were ordering a rapid antigen detection test (RADT) for her current illness. The throat specimen was positive for group A ß-hemolytic streptococcus (GAS). The patient’s 8-year-old daughter also had a sore throat, fever, and positive RADT; her symptoms resolved with oral amoxicillin for 10 days. The patient’s husband was also treated successfully with oral amoxicillin/clavulanate for 10 days for similar symptoms. The patient herself, however, was unsuccessfully treated with oral amoxicillin 500 mg twice daily for 7 days.
She was then given oral amoxicillin/clavulanate 875 mg twice daily for 14 days, but received no relief. Even after receiving clindamycin 600 mg twice daily for 10 days, she had minimal relief and remained positive for GAS on repeat RADT. It was at this point that tonsillectomy was considered as a possible treatment modality for her refractory GAS pharyngitis.
The patient consented to the procedure and underwent a tonsillectomy. She has remained asymptomatic for 2 years and there have been no reported outbreaks of GAS infection in her household.
DISCUSSION
Streptococcal pharyngitis is an infection of the oropharynx and/or nasopharynx that is caused by Streptococcus pyogenes (also known as GAS). It is one of the most frequent illnesses encountered by primary care physicians, and primarily occurs in children ages 5 to 15 years.1,2 The signs and symptoms of GAS pharyngitis include an abrupt onset of a sore throat, tonsillar exudate, tender cervical adenopathy, and fever. (The classic presentation of GAS pharyngitis in a different patient can be seen in the FIGURE.)
Throat cultures are the gold standard for the diagnosis of GAS pharyngitis, but results take 24 to 48 hours, which can delay appropriate treatment. Therefore, the use of the RADT is often preferred clinically.1 RADT is not recommended for children and adults who show clinical symptoms that are highly suggestive of a viral illness, such as cough, rhinorrhea, hoarseness, or oral ulcers. A negative RADT in children and adolescents necessitates a throat culture to confirm the diagnosis.2
The antibiotics of choice are either penicillin 50 mg/kg/d in 4 divided doses or amoxicillin 40 mg/kg/d in 3 divided doses (maximum for both is 2000 mg/d) for 10 days. Options for patients with penicillin allergies include clindamycin or clarithromycin for 10 days or azithromycin for 5 days.2
The Infectious Diseases Society of America (IDSA) does not recommend routine testing or empiric treatment of asymptomatic carriers. However, it does recommend treatment of GAS carriers in certain situations, such as when: 2
- the carrier has acute rheumatic fever
- there is a family or personal history of acute rheumatic fever
- there is a post-streptococcal glomerulonephritis outbreak
- a family has excessive anxiety about GAS infections
- a tonsillectomy is being considered.
When—and for whom—is tonsillectomy beneficial?
Tonsillectomy is a treatment option for patients with recurrent episodes of GAS pharyngitis. Indications include patients with 7 GAS infections in a year, 5 episodes in 2 years, or 3 episodes in 3 years.3,4 In select patient populations, tonsillectomy has been shown to decrease missed work days and medical expenses caused by recurrent pharyngitis.5,6
Alho et al demonstrated that adults with recurrent episodes of GAS pharyngitis benefit from tonsillectomy in terms of fewer repeat infections and more days without throat pain.7 A randomized controlled trial conducted by Koskenkorva et al found that the overall rates of pharyngitis, throat pain, rhinitis, and cough were significantly lower in adults who received a tonsillectomy vs those who did not.5 Still, whether tonsillectomy is worthwhile in adults is debatable; Burton et al found no evidence that tonsillectomy is effective for chronic or recurrent acute tonsillitis in adults.8
Overall meta-analysis results indicate that tonsillectomy results in a 43% reduction in the incidence of pharyngitis in children between the ages of 4 and 16.8,9 One study found that children without tonsillectomy were 3.1 times more likely to develop subsequent GAS pharyngitis than children who underwent tonsillectomy.9 Another study found that children who received tonsillectomy demonstrated a decrease in sore throat episodes by 1.2 episodes per year and a decrease in school absenteeism by 2.8 days per year.6 Tonsillectomy does carry a risk of intraoperative and postoperative bleeding in children and adults, which may make it a less desirable option for some patients.6
THE TAKEAWAY
Recurrent GAS pharyngitis poses a significant challenge for clinicians. When episodes recur, it may be prudent to treat asymptomatic carriers in the patient’s household. Tonsillectomy should be considered in refractory cases since recurrent GAS pharyngitis directly impacts the wellness and productivity of patients. Our patient certainly benefited from the surgery: She has not missed any work days or had to visit her primary care physician because of a GAS infection since her tonsillectomy.
THE CASE
A 35-year-old woman sought care for a fever and sore throat that she’d had for 4 days. She denied symptoms of cough, rhinorrhea, or sputum production.
The patient’s medical history included severe recurrent streptococcal pharyngitis as a child and teenager. At the age of 17, she developed a fever of 105° F with associated delirium, dysphagia, nausea, and vomiting, and missed several days of school. She also lost 82 pounds, developed oral thrush, and continued to feel fatigued for approximately a year. After her primary care physician noted a heart murmur on physical exam, she was sent for echocardiography and diagnosed with rheumatic fever secondary to streptococcal pharyngitis.
Eighteen years (and numerous streptococcal infections) later, the patient was at our facility and we were ordering a rapid antigen detection test (RADT) for her current illness. The throat specimen was positive for group A ß-hemolytic streptococcus (GAS). The patient’s 8-year-old daughter also had a sore throat, fever, and positive RADT; her symptoms resolved with oral amoxicillin for 10 days. The patient’s husband was also treated successfully with oral amoxicillin/clavulanate for 10 days for similar symptoms. The patient herself, however, was unsuccessfully treated with oral amoxicillin 500 mg twice daily for 7 days.
She was then given oral amoxicillin/clavulanate 875 mg twice daily for 14 days, but received no relief. Even after receiving clindamycin 600 mg twice daily for 10 days, she had minimal relief and remained positive for GAS on repeat RADT. It was at this point that tonsillectomy was considered as a possible treatment modality for her refractory GAS pharyngitis.
The patient consented to the procedure and underwent a tonsillectomy. She has remained asymptomatic for 2 years and there have been no reported outbreaks of GAS infection in her household.
DISCUSSION
Streptococcal pharyngitis is an infection of the oropharynx and/or nasopharynx that is caused by Streptococcus pyogenes (also known as GAS). It is one of the most frequent illnesses encountered by primary care physicians, and primarily occurs in children ages 5 to 15 years.1,2 The signs and symptoms of GAS pharyngitis include an abrupt onset of a sore throat, tonsillar exudate, tender cervical adenopathy, and fever. (The classic presentation of GAS pharyngitis in a different patient can be seen in the FIGURE.)
Throat cultures are the gold standard for the diagnosis of GAS pharyngitis, but results take 24 to 48 hours, which can delay appropriate treatment. Therefore, the use of the RADT is often preferred clinically.1 RADT is not recommended for children and adults who show clinical symptoms that are highly suggestive of a viral illness, such as cough, rhinorrhea, hoarseness, or oral ulcers. A negative RADT in children and adolescents necessitates a throat culture to confirm the diagnosis.2
The antibiotics of choice are either penicillin 50 mg/kg/d in 4 divided doses or amoxicillin 40 mg/kg/d in 3 divided doses (maximum for both is 2000 mg/d) for 10 days. Options for patients with penicillin allergies include clindamycin or clarithromycin for 10 days or azithromycin for 5 days.2
The Infectious Diseases Society of America (IDSA) does not recommend routine testing or empiric treatment of asymptomatic carriers. However, it does recommend treatment of GAS carriers in certain situations, such as when: 2
- the carrier has acute rheumatic fever
- there is a family or personal history of acute rheumatic fever
- there is a post-streptococcal glomerulonephritis outbreak
- a family has excessive anxiety about GAS infections
- a tonsillectomy is being considered.
When—and for whom—is tonsillectomy beneficial?
Tonsillectomy is a treatment option for patients with recurrent episodes of GAS pharyngitis. Indications include patients with 7 GAS infections in a year, 5 episodes in 2 years, or 3 episodes in 3 years.3,4 In select patient populations, tonsillectomy has been shown to decrease missed work days and medical expenses caused by recurrent pharyngitis.5,6
Alho et al demonstrated that adults with recurrent episodes of GAS pharyngitis benefit from tonsillectomy in terms of fewer repeat infections and more days without throat pain.7 A randomized controlled trial conducted by Koskenkorva et al found that the overall rates of pharyngitis, throat pain, rhinitis, and cough were significantly lower in adults who received a tonsillectomy vs those who did not.5 Still, whether tonsillectomy is worthwhile in adults is debatable; Burton et al found no evidence that tonsillectomy is effective for chronic or recurrent acute tonsillitis in adults.8
Overall meta-analysis results indicate that tonsillectomy results in a 43% reduction in the incidence of pharyngitis in children between the ages of 4 and 16.8,9 One study found that children without tonsillectomy were 3.1 times more likely to develop subsequent GAS pharyngitis than children who underwent tonsillectomy.9 Another study found that children who received tonsillectomy demonstrated a decrease in sore throat episodes by 1.2 episodes per year and a decrease in school absenteeism by 2.8 days per year.6 Tonsillectomy does carry a risk of intraoperative and postoperative bleeding in children and adults, which may make it a less desirable option for some patients.6
THE TAKEAWAY
Recurrent GAS pharyngitis poses a significant challenge for clinicians. When episodes recur, it may be prudent to treat asymptomatic carriers in the patient’s household. Tonsillectomy should be considered in refractory cases since recurrent GAS pharyngitis directly impacts the wellness and productivity of patients. Our patient certainly benefited from the surgery: She has not missed any work days or had to visit her primary care physician because of a GAS infection since her tonsillectomy.
1. Gurol Y, Akan H, Izbirak G, et al. The sensitivity and the specificity of rapid antigen test in streptococcal upper respiratory tract infections. Int J Pediatr Otorhinolaryngol. 2010;74:591-593.
2. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
3. Stuck BA, Götte K, Windfuhr JP, et al. Tonsillectomy in children. Dtsch Arztebl Int. 2008;105:852-860.
4. Baugh RF, Archer SM, Mitchell RB, et al; American Academy of Otolaryngology-Head and Neck Surgery Foundation. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144:S1-S30.
5. Koskenkorva T, Koivunen P, Koskela M, et al. Short-term outcomes of tonsillectomy in adult patients with recurrent pharyngitis: a randomized controlled trial. CMAJ. 2013;185:E331-E336.
6. van Staaij BK, van den Akker EH, van der Heijden GJ, et al. Adenotonsillectomy for upper respiratory infections: evidence based? Arch Dis Child. 2005;90:19-25.
7. Alho OP, Koivunen P, Penna T, et al. Tonsillectomy versus watchful waiting in recurrent streptococcal pharyngitis in adults: randomised controlled trial. BMJ. 2007;334:939.
8. Burton MJ, Towler B, Glasziou P. Tonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev. 2000;(2):CD001802.
9. Orvidas LJ, St Sauver JL, Weaver AL. Efficacy of tonsillectomy in treatment of recurrent group A beta-hemolytic streptococcal pharyngitis. Laryngoscope. 2006;116:1946-1950.
1. Gurol Y, Akan H, Izbirak G, et al. The sensitivity and the specificity of rapid antigen test in streptococcal upper respiratory tract infections. Int J Pediatr Otorhinolaryngol. 2010;74:591-593.
2. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
3. Stuck BA, Götte K, Windfuhr JP, et al. Tonsillectomy in children. Dtsch Arztebl Int. 2008;105:852-860.
4. Baugh RF, Archer SM, Mitchell RB, et al; American Academy of Otolaryngology-Head and Neck Surgery Foundation. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144:S1-S30.
5. Koskenkorva T, Koivunen P, Koskela M, et al. Short-term outcomes of tonsillectomy in adult patients with recurrent pharyngitis: a randomized controlled trial. CMAJ. 2013;185:E331-E336.
6. van Staaij BK, van den Akker EH, van der Heijden GJ, et al. Adenotonsillectomy for upper respiratory infections: evidence based? Arch Dis Child. 2005;90:19-25.
7. Alho OP, Koivunen P, Penna T, et al. Tonsillectomy versus watchful waiting in recurrent streptococcal pharyngitis in adults: randomised controlled trial. BMJ. 2007;334:939.
8. Burton MJ, Towler B, Glasziou P. Tonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev. 2000;(2):CD001802.
9. Orvidas LJ, St Sauver JL, Weaver AL. Efficacy of tonsillectomy in treatment of recurrent group A beta-hemolytic streptococcal pharyngitis. Laryngoscope. 2006;116:1946-1950.
Don’t Forget the Pulses! Aortoiliac Peripheral Artery Disease Masquerading as Lumbar Radiculopathy—A Report of 3 Cases
Lumbar radiculopathy is a common problem encountered by orthopedic surgeons, and typically presents with lower back or buttock pain radiating down the leg.1 While the most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis, the differential diagnosis for lower extremity pain is broad and can be musculoskeletal, vascular, neurologic, or inflammatory in nature.1,2 Differentiating between orthopedic, neurologic, and vascular causes of leg pain, such as peripheral artery disease (PAD), can sometimes be challenging. This is especially true in aortoiliac PAD, which can present with hip, buttock, and thigh pain. Dorsalis pedis pulses can be palpable due to collateral circulation. A careful history and physical examination is crucial to the correct diagnosis. The history should clearly document the nature of the pain, details of walking impairment, and the alleviating effects of standing still or positional changes. A complete neurovascular examination should include observations regarding the skin, hair, and nails, examination of dorsal pedis, popliteal, and femoral pulses in comparison to the contralateral side, and documentation of dural tension signs. Misdiagnoses can send the patient down a path of unnecessary tests, unindicated procedures, and ultimately, a delay in definitive diagnosis and treatment.1
To our knowledge, this is the first report on a series of patients with thigh pain initially diagnosed as radiculopathy who underwent unproductive diagnostic tests and procedures, and ultimately were given delayed diagnoses of aortoiliac PAD. The patients provided written informed consent for print and electronic publication of these case reports.
Case 1
An 81-year-old woman with a medical history notable for hypertension, hyperlipidemia, and stroke initially presented to an outside orthopedic institution with complaints of several months of lower back and right hip, thigh, and leg pain when walking. She did not report any history of night pain, weakness, or numbness. Examination at the time was notable for painful back extension, 4/5 hip flexion strength on the right compared to 5/5 on the left, but symmetric reflexes and negative dural tension signs. X-rays showed multilevel degenerative disc disease of the lumbar spine, and magnetic resonance imaging (MRI) showed a small L3/4 disc protrusion causing impingement of the L4 nerve root.
A transforaminal epidural steroid injection at the L4 level was performed with minimal resolution of symptoms. Several months later, right-sided intra-articular facet injections were performed at the L4/5 and L5/S1 levels, again with minimal relief of symptoms. At this point, the patient was sent for further physical therapy.
Over a year after symptom onset, the patient presented to our institution and was evaluated by a vascular surgeon. Physical examination was notable for 1+ femoral artery and dorsal pedis pulses on the right side, compared to 2+ on the left. An aortoiliac duplex ultrasound showed severe significant stenosis of the right common iliac artery (>75%).
The patient underwent a right common iliac artery angioplasty and stenting (Figures 1A, 1B), which resolved her symptoms.
Case 2
A 65-year-old man, who is a former smoker with a medical history notable for hyperlipidemia and coronary artery disease status post myocardial infarction, presented with a long history of right leg pain. He underwent a L5/S1 anterior posterior fusion at an outside institution and did well for about 5 years after the procedure (Figures 2A, 2B). The pain returned and he underwent several years of physical therapy, epidural steroid injections, and implantation of a spinal cord stimulator with no improvement. He reported right leg pain with minimal back pain, primarily in the thigh and not radiating to the feet and toes. The pain limited him from walking more than 1 block. On examination, strength was 5/5 bilaterally. Pulse examination was notable for lack of dorsalis pedis/posterior tibial pulses bilaterally. He had no bowel or bladder dysfunction.
Computed tomography myelogram showed a moderate amount of stenosis at L3/4 and L4/5. He was sent for evaluation by a vascular surgeon. Arterial duplex ultrasound showed significant stenosis of the right common iliac artery.
Angioplasty was attempted but vascular surgery was unable to cross the lesion (Figures 3A, 3B), and the patient ultimately had a femoral-femoral bypass, which resolved his leg pain.
Case 3
A 78-year-old woman, nonsmoker, presented with a 1-year history of left buttock and thigh pain exacerbated by ambulation. Ambulation was limited to 2 blocks. The patient was being worked up for spinal and hip etiologies of pain at an outside hospital. MRI revealed a mild posterior disc herniation at L3/4 and L4/5 and moderate narrowing of the spinal canal. She underwent 2 epidural steroid injections with no improvement. The patient’s relative, a physician, suggested that the patient receive a vascular surgery consultation, and the patient ultimately presented to our institution for evaluation by vascular surgery.
The physical examination was significant for a 1+ dorsal pedis pulse on the left compared to 2+ on the right. Moreover, the patient only demonstrated trace L femoral pulse compared to the right. Strength was 5/5 bilaterally.
The patient was taken to the operating room for angioplasty and stenting of the left common iliac artery (Figures 4A, 4B). This provided immediate symptom relief, and she has remained asymptomatic.
Discussion
Lumbar radiculopathy is a common diagnosis encountered by orthopedic surgeons. Although the diagnosis can appear to be straightforward in a patient presenting with lower back and leg pain, the etiology of lower back and leg pain can be extremely varied, and can be musculoskeletal, neurologic, vascular, rheumatologic, or oncologic in origin.1 In particular, differentiating between radiculopathy and vascular claudication can sometimes be challenging.
The 2 most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis.1 Lumbar disc herniation results from tear in the annulus of the intervertebral disc, resulting in herniation of disc material into the spinal canal causing compression and irritation of spinal nerve roots.1 Spinal stenosis is narrowing of the spinal canal that produces compression of neural elements before they exit the neural foramen.3 Adult degenerative spinal stenosis is most often caused by osteophytes from the facet joints or hypertrophy of the ligamentum flavum, and can be broadly categorized into central spinal stenosis or lateral spinal stenosis.
PAD is defined as progressive stenosis or occlusion, or aneurysmal dilation of noncoronary arteries.2 When PAD affects the vessels of the lower extremities, the symptoms typically manifest as intermittent claudication, which is exercise-induced ischemic pain in the lower extremity that is relieved by rest.2 As the disease progresses, symptoms can progress to rest pain, ulceration, and, eventually, gangrene. The most common cause of PAD is atherosclerosis, and the risk factors include smoking, hypertension, diabetes, and hyperlipidemia. The prevalence of PAD rises sharply with age, starting from <3% in ages less than 60 years to >20% in ages 75 years and older.4
A detailed and pertinent history from the patient provides important information for differentiating radiculopathy and neurogenic claudication from vascular claudication. Patients with lumbar radiculopathy typically report pain in the lower back radiating down the leg past the knee in a dermatomal distribution. The pain often begins soon if not immediately after activity, but often takes time for relief onset after rest. Positional changes in the back such as flexion can provide relief.2 Patients with neurogenic claudication from central spinal stenosis can present with bilateral thigh pain from prolonged standing and activity that is alleviated with flexion or stooping.3 Patients may admit to a positive “shopping cart sign,” with increased walking comfort stooped forward with hands on a shopping cart.
In contrast, patients with vascular claudication often report pain in the calf, thigh, or hip, but rarely in the foot. The location of pain varies with area of stenosis; generally, patients with superficial femoral artery occlusion present with calf claudication, while patients with aortoiliac disease present with buttock and thigh pain. The pain typically occurs after a very reproducible length of walking, and is relieved by cessation of walking, often even if the patient remains standing. Back positioning should have no effect on the pain.2-5
Physical examination should begin with observation of the patient’s gait and posture, which may be hunched over in the setting of spinal stenosis. Examination of the patient’s skin may show loss of hair, shiny skin, or atrophic changes suggestive of vascular disease (Figure 5).1 Prior to proceeding to a spine examination, palpating the trochanteric bursa and testing for hip range of motion is important to rule out intra-articular hip pathology and trochanteric bursitis as common causes of pain in the area. Patients with radiculopathy may show sensory disturbances in a dermatomal distribution, muscular weakness at the corresponding spinal level, and decreased deep tendon reflexes. The straight leg raise test can elicit signs of nerve root tension. A careful examination of bilateral lower extremity pulses at the dorsal pedis, popliteal, and femoral levels can help identify any asymmetric or decreased pulses that would indicate peripheral vascular disease. With chronic aortoiliac disease, it is important to check for femoral pulses, given the dorsal pedis pulse can be present due to collateral circulation. And finally, the ankle brachial index (ABI), measured as the ratio of the systolic pressure at the ankle divided by the systolic pressure at the arm, is a good screening test for PAD.6 A normal ABI is >1.
A thorough history and physical examination can elicit important information that is helpful in evaluating orthopedic patients, especially to differentiate between spinal and vascular causes of leg pain. This can help avoid misdiagnoses, which result in unnecessary tests, procedures, and wasted time. Don’t forget the pulses!
1. Grimm BD, Blessinger BJ, Darden BV, Brigham CD, Kneisl JS, Laxer EB. Mimickers of lumbar radiculopathy. J Am Acad Orthop Surg. 2015;23(1):7-17.
2. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17(9):1383-1397.
3. Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998;80(7):1053-1066.
4. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510-515.
5. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
6. Jeon CH, Han SH, Chung NS, Hyun HS. The validity of ankle-brachial index for the differential diagnosis of peripheral arterial disease and lumbar spinal stenosis in patients with atypical claudication. Eur Spine J. 2012;21(6):1165-1170.
Lumbar radiculopathy is a common problem encountered by orthopedic surgeons, and typically presents with lower back or buttock pain radiating down the leg.1 While the most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis, the differential diagnosis for lower extremity pain is broad and can be musculoskeletal, vascular, neurologic, or inflammatory in nature.1,2 Differentiating between orthopedic, neurologic, and vascular causes of leg pain, such as peripheral artery disease (PAD), can sometimes be challenging. This is especially true in aortoiliac PAD, which can present with hip, buttock, and thigh pain. Dorsalis pedis pulses can be palpable due to collateral circulation. A careful history and physical examination is crucial to the correct diagnosis. The history should clearly document the nature of the pain, details of walking impairment, and the alleviating effects of standing still or positional changes. A complete neurovascular examination should include observations regarding the skin, hair, and nails, examination of dorsal pedis, popliteal, and femoral pulses in comparison to the contralateral side, and documentation of dural tension signs. Misdiagnoses can send the patient down a path of unnecessary tests, unindicated procedures, and ultimately, a delay in definitive diagnosis and treatment.1
To our knowledge, this is the first report on a series of patients with thigh pain initially diagnosed as radiculopathy who underwent unproductive diagnostic tests and procedures, and ultimately were given delayed diagnoses of aortoiliac PAD. The patients provided written informed consent for print and electronic publication of these case reports.
Case 1
An 81-year-old woman with a medical history notable for hypertension, hyperlipidemia, and stroke initially presented to an outside orthopedic institution with complaints of several months of lower back and right hip, thigh, and leg pain when walking. She did not report any history of night pain, weakness, or numbness. Examination at the time was notable for painful back extension, 4/5 hip flexion strength on the right compared to 5/5 on the left, but symmetric reflexes and negative dural tension signs. X-rays showed multilevel degenerative disc disease of the lumbar spine, and magnetic resonance imaging (MRI) showed a small L3/4 disc protrusion causing impingement of the L4 nerve root.
A transforaminal epidural steroid injection at the L4 level was performed with minimal resolution of symptoms. Several months later, right-sided intra-articular facet injections were performed at the L4/5 and L5/S1 levels, again with minimal relief of symptoms. At this point, the patient was sent for further physical therapy.
Over a year after symptom onset, the patient presented to our institution and was evaluated by a vascular surgeon. Physical examination was notable for 1+ femoral artery and dorsal pedis pulses on the right side, compared to 2+ on the left. An aortoiliac duplex ultrasound showed severe significant stenosis of the right common iliac artery (>75%).
The patient underwent a right common iliac artery angioplasty and stenting (Figures 1A, 1B), which resolved her symptoms.
Case 2
A 65-year-old man, who is a former smoker with a medical history notable for hyperlipidemia and coronary artery disease status post myocardial infarction, presented with a long history of right leg pain. He underwent a L5/S1 anterior posterior fusion at an outside institution and did well for about 5 years after the procedure (Figures 2A, 2B). The pain returned and he underwent several years of physical therapy, epidural steroid injections, and implantation of a spinal cord stimulator with no improvement. He reported right leg pain with minimal back pain, primarily in the thigh and not radiating to the feet and toes. The pain limited him from walking more than 1 block. On examination, strength was 5/5 bilaterally. Pulse examination was notable for lack of dorsalis pedis/posterior tibial pulses bilaterally. He had no bowel or bladder dysfunction.
Computed tomography myelogram showed a moderate amount of stenosis at L3/4 and L4/5. He was sent for evaluation by a vascular surgeon. Arterial duplex ultrasound showed significant stenosis of the right common iliac artery.
Angioplasty was attempted but vascular surgery was unable to cross the lesion (Figures 3A, 3B), and the patient ultimately had a femoral-femoral bypass, which resolved his leg pain.
Case 3
A 78-year-old woman, nonsmoker, presented with a 1-year history of left buttock and thigh pain exacerbated by ambulation. Ambulation was limited to 2 blocks. The patient was being worked up for spinal and hip etiologies of pain at an outside hospital. MRI revealed a mild posterior disc herniation at L3/4 and L4/5 and moderate narrowing of the spinal canal. She underwent 2 epidural steroid injections with no improvement. The patient’s relative, a physician, suggested that the patient receive a vascular surgery consultation, and the patient ultimately presented to our institution for evaluation by vascular surgery.
The physical examination was significant for a 1+ dorsal pedis pulse on the left compared to 2+ on the right. Moreover, the patient only demonstrated trace L femoral pulse compared to the right. Strength was 5/5 bilaterally.
The patient was taken to the operating room for angioplasty and stenting of the left common iliac artery (Figures 4A, 4B). This provided immediate symptom relief, and she has remained asymptomatic.
Discussion
Lumbar radiculopathy is a common diagnosis encountered by orthopedic surgeons. Although the diagnosis can appear to be straightforward in a patient presenting with lower back and leg pain, the etiology of lower back and leg pain can be extremely varied, and can be musculoskeletal, neurologic, vascular, rheumatologic, or oncologic in origin.1 In particular, differentiating between radiculopathy and vascular claudication can sometimes be challenging.
The 2 most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis.1 Lumbar disc herniation results from tear in the annulus of the intervertebral disc, resulting in herniation of disc material into the spinal canal causing compression and irritation of spinal nerve roots.1 Spinal stenosis is narrowing of the spinal canal that produces compression of neural elements before they exit the neural foramen.3 Adult degenerative spinal stenosis is most often caused by osteophytes from the facet joints or hypertrophy of the ligamentum flavum, and can be broadly categorized into central spinal stenosis or lateral spinal stenosis.
PAD is defined as progressive stenosis or occlusion, or aneurysmal dilation of noncoronary arteries.2 When PAD affects the vessels of the lower extremities, the symptoms typically manifest as intermittent claudication, which is exercise-induced ischemic pain in the lower extremity that is relieved by rest.2 As the disease progresses, symptoms can progress to rest pain, ulceration, and, eventually, gangrene. The most common cause of PAD is atherosclerosis, and the risk factors include smoking, hypertension, diabetes, and hyperlipidemia. The prevalence of PAD rises sharply with age, starting from <3% in ages less than 60 years to >20% in ages 75 years and older.4
A detailed and pertinent history from the patient provides important information for differentiating radiculopathy and neurogenic claudication from vascular claudication. Patients with lumbar radiculopathy typically report pain in the lower back radiating down the leg past the knee in a dermatomal distribution. The pain often begins soon if not immediately after activity, but often takes time for relief onset after rest. Positional changes in the back such as flexion can provide relief.2 Patients with neurogenic claudication from central spinal stenosis can present with bilateral thigh pain from prolonged standing and activity that is alleviated with flexion or stooping.3 Patients may admit to a positive “shopping cart sign,” with increased walking comfort stooped forward with hands on a shopping cart.
In contrast, patients with vascular claudication often report pain in the calf, thigh, or hip, but rarely in the foot. The location of pain varies with area of stenosis; generally, patients with superficial femoral artery occlusion present with calf claudication, while patients with aortoiliac disease present with buttock and thigh pain. The pain typically occurs after a very reproducible length of walking, and is relieved by cessation of walking, often even if the patient remains standing. Back positioning should have no effect on the pain.2-5
Physical examination should begin with observation of the patient’s gait and posture, which may be hunched over in the setting of spinal stenosis. Examination of the patient’s skin may show loss of hair, shiny skin, or atrophic changes suggestive of vascular disease (Figure 5).1 Prior to proceeding to a spine examination, palpating the trochanteric bursa and testing for hip range of motion is important to rule out intra-articular hip pathology and trochanteric bursitis as common causes of pain in the area. Patients with radiculopathy may show sensory disturbances in a dermatomal distribution, muscular weakness at the corresponding spinal level, and decreased deep tendon reflexes. The straight leg raise test can elicit signs of nerve root tension. A careful examination of bilateral lower extremity pulses at the dorsal pedis, popliteal, and femoral levels can help identify any asymmetric or decreased pulses that would indicate peripheral vascular disease. With chronic aortoiliac disease, it is important to check for femoral pulses, given the dorsal pedis pulse can be present due to collateral circulation. And finally, the ankle brachial index (ABI), measured as the ratio of the systolic pressure at the ankle divided by the systolic pressure at the arm, is a good screening test for PAD.6 A normal ABI is >1.
A thorough history and physical examination can elicit important information that is helpful in evaluating orthopedic patients, especially to differentiate between spinal and vascular causes of leg pain. This can help avoid misdiagnoses, which result in unnecessary tests, procedures, and wasted time. Don’t forget the pulses!
Lumbar radiculopathy is a common problem encountered by orthopedic surgeons, and typically presents with lower back or buttock pain radiating down the leg.1 While the most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis, the differential diagnosis for lower extremity pain is broad and can be musculoskeletal, vascular, neurologic, or inflammatory in nature.1,2 Differentiating between orthopedic, neurologic, and vascular causes of leg pain, such as peripheral artery disease (PAD), can sometimes be challenging. This is especially true in aortoiliac PAD, which can present with hip, buttock, and thigh pain. Dorsalis pedis pulses can be palpable due to collateral circulation. A careful history and physical examination is crucial to the correct diagnosis. The history should clearly document the nature of the pain, details of walking impairment, and the alleviating effects of standing still or positional changes. A complete neurovascular examination should include observations regarding the skin, hair, and nails, examination of dorsal pedis, popliteal, and femoral pulses in comparison to the contralateral side, and documentation of dural tension signs. Misdiagnoses can send the patient down a path of unnecessary tests, unindicated procedures, and ultimately, a delay in definitive diagnosis and treatment.1
To our knowledge, this is the first report on a series of patients with thigh pain initially diagnosed as radiculopathy who underwent unproductive diagnostic tests and procedures, and ultimately were given delayed diagnoses of aortoiliac PAD. The patients provided written informed consent for print and electronic publication of these case reports.
Case 1
An 81-year-old woman with a medical history notable for hypertension, hyperlipidemia, and stroke initially presented to an outside orthopedic institution with complaints of several months of lower back and right hip, thigh, and leg pain when walking. She did not report any history of night pain, weakness, or numbness. Examination at the time was notable for painful back extension, 4/5 hip flexion strength on the right compared to 5/5 on the left, but symmetric reflexes and negative dural tension signs. X-rays showed multilevel degenerative disc disease of the lumbar spine, and magnetic resonance imaging (MRI) showed a small L3/4 disc protrusion causing impingement of the L4 nerve root.
A transforaminal epidural steroid injection at the L4 level was performed with minimal resolution of symptoms. Several months later, right-sided intra-articular facet injections were performed at the L4/5 and L5/S1 levels, again with minimal relief of symptoms. At this point, the patient was sent for further physical therapy.
Over a year after symptom onset, the patient presented to our institution and was evaluated by a vascular surgeon. Physical examination was notable for 1+ femoral artery and dorsal pedis pulses on the right side, compared to 2+ on the left. An aortoiliac duplex ultrasound showed severe significant stenosis of the right common iliac artery (>75%).
The patient underwent a right common iliac artery angioplasty and stenting (Figures 1A, 1B), which resolved her symptoms.
Case 2
A 65-year-old man, who is a former smoker with a medical history notable for hyperlipidemia and coronary artery disease status post myocardial infarction, presented with a long history of right leg pain. He underwent a L5/S1 anterior posterior fusion at an outside institution and did well for about 5 years after the procedure (Figures 2A, 2B). The pain returned and he underwent several years of physical therapy, epidural steroid injections, and implantation of a spinal cord stimulator with no improvement. He reported right leg pain with minimal back pain, primarily in the thigh and not radiating to the feet and toes. The pain limited him from walking more than 1 block. On examination, strength was 5/5 bilaterally. Pulse examination was notable for lack of dorsalis pedis/posterior tibial pulses bilaterally. He had no bowel or bladder dysfunction.
Computed tomography myelogram showed a moderate amount of stenosis at L3/4 and L4/5. He was sent for evaluation by a vascular surgeon. Arterial duplex ultrasound showed significant stenosis of the right common iliac artery.
Angioplasty was attempted but vascular surgery was unable to cross the lesion (Figures 3A, 3B), and the patient ultimately had a femoral-femoral bypass, which resolved his leg pain.
Case 3
A 78-year-old woman, nonsmoker, presented with a 1-year history of left buttock and thigh pain exacerbated by ambulation. Ambulation was limited to 2 blocks. The patient was being worked up for spinal and hip etiologies of pain at an outside hospital. MRI revealed a mild posterior disc herniation at L3/4 and L4/5 and moderate narrowing of the spinal canal. She underwent 2 epidural steroid injections with no improvement. The patient’s relative, a physician, suggested that the patient receive a vascular surgery consultation, and the patient ultimately presented to our institution for evaluation by vascular surgery.
The physical examination was significant for a 1+ dorsal pedis pulse on the left compared to 2+ on the right. Moreover, the patient only demonstrated trace L femoral pulse compared to the right. Strength was 5/5 bilaterally.
The patient was taken to the operating room for angioplasty and stenting of the left common iliac artery (Figures 4A, 4B). This provided immediate symptom relief, and she has remained asymptomatic.
Discussion
Lumbar radiculopathy is a common diagnosis encountered by orthopedic surgeons. Although the diagnosis can appear to be straightforward in a patient presenting with lower back and leg pain, the etiology of lower back and leg pain can be extremely varied, and can be musculoskeletal, neurologic, vascular, rheumatologic, or oncologic in origin.1 In particular, differentiating between radiculopathy and vascular claudication can sometimes be challenging.
The 2 most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis.1 Lumbar disc herniation results from tear in the annulus of the intervertebral disc, resulting in herniation of disc material into the spinal canal causing compression and irritation of spinal nerve roots.1 Spinal stenosis is narrowing of the spinal canal that produces compression of neural elements before they exit the neural foramen.3 Adult degenerative spinal stenosis is most often caused by osteophytes from the facet joints or hypertrophy of the ligamentum flavum, and can be broadly categorized into central spinal stenosis or lateral spinal stenosis.
PAD is defined as progressive stenosis or occlusion, or aneurysmal dilation of noncoronary arteries.2 When PAD affects the vessels of the lower extremities, the symptoms typically manifest as intermittent claudication, which is exercise-induced ischemic pain in the lower extremity that is relieved by rest.2 As the disease progresses, symptoms can progress to rest pain, ulceration, and, eventually, gangrene. The most common cause of PAD is atherosclerosis, and the risk factors include smoking, hypertension, diabetes, and hyperlipidemia. The prevalence of PAD rises sharply with age, starting from <3% in ages less than 60 years to >20% in ages 75 years and older.4
A detailed and pertinent history from the patient provides important information for differentiating radiculopathy and neurogenic claudication from vascular claudication. Patients with lumbar radiculopathy typically report pain in the lower back radiating down the leg past the knee in a dermatomal distribution. The pain often begins soon if not immediately after activity, but often takes time for relief onset after rest. Positional changes in the back such as flexion can provide relief.2 Patients with neurogenic claudication from central spinal stenosis can present with bilateral thigh pain from prolonged standing and activity that is alleviated with flexion or stooping.3 Patients may admit to a positive “shopping cart sign,” with increased walking comfort stooped forward with hands on a shopping cart.
In contrast, patients with vascular claudication often report pain in the calf, thigh, or hip, but rarely in the foot. The location of pain varies with area of stenosis; generally, patients with superficial femoral artery occlusion present with calf claudication, while patients with aortoiliac disease present with buttock and thigh pain. The pain typically occurs after a very reproducible length of walking, and is relieved by cessation of walking, often even if the patient remains standing. Back positioning should have no effect on the pain.2-5
Physical examination should begin with observation of the patient’s gait and posture, which may be hunched over in the setting of spinal stenosis. Examination of the patient’s skin may show loss of hair, shiny skin, or atrophic changes suggestive of vascular disease (Figure 5).1 Prior to proceeding to a spine examination, palpating the trochanteric bursa and testing for hip range of motion is important to rule out intra-articular hip pathology and trochanteric bursitis as common causes of pain in the area. Patients with radiculopathy may show sensory disturbances in a dermatomal distribution, muscular weakness at the corresponding spinal level, and decreased deep tendon reflexes. The straight leg raise test can elicit signs of nerve root tension. A careful examination of bilateral lower extremity pulses at the dorsal pedis, popliteal, and femoral levels can help identify any asymmetric or decreased pulses that would indicate peripheral vascular disease. With chronic aortoiliac disease, it is important to check for femoral pulses, given the dorsal pedis pulse can be present due to collateral circulation. And finally, the ankle brachial index (ABI), measured as the ratio of the systolic pressure at the ankle divided by the systolic pressure at the arm, is a good screening test for PAD.6 A normal ABI is >1.
A thorough history and physical examination can elicit important information that is helpful in evaluating orthopedic patients, especially to differentiate between spinal and vascular causes of leg pain. This can help avoid misdiagnoses, which result in unnecessary tests, procedures, and wasted time. Don’t forget the pulses!
1. Grimm BD, Blessinger BJ, Darden BV, Brigham CD, Kneisl JS, Laxer EB. Mimickers of lumbar radiculopathy. J Am Acad Orthop Surg. 2015;23(1):7-17.
2. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17(9):1383-1397.
3. Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998;80(7):1053-1066.
4. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510-515.
5. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
6. Jeon CH, Han SH, Chung NS, Hyun HS. The validity of ankle-brachial index for the differential diagnosis of peripheral arterial disease and lumbar spinal stenosis in patients with atypical claudication. Eur Spine J. 2012;21(6):1165-1170.
1. Grimm BD, Blessinger BJ, Darden BV, Brigham CD, Kneisl JS, Laxer EB. Mimickers of lumbar radiculopathy. J Am Acad Orthop Surg. 2015;23(1):7-17.
2. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17(9):1383-1397.
3. Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998;80(7):1053-1066.
4. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510-515.
5. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
6. Jeon CH, Han SH, Chung NS, Hyun HS. The validity of ankle-brachial index for the differential diagnosis of peripheral arterial disease and lumbar spinal stenosis in patients with atypical claudication. Eur Spine J. 2012;21(6):1165-1170.
A Physician With Thigh Pain
Necrotizing soft-tissue infection (NSTI) often is difficult to distinguish from a superficial soft-tissue infection like cellulitis. Both conditions present with pain, edema, and erythema and can be accompanied by fever and malaise. The diagnosis of NSTI must be made quickly because successful treatment requires early surgical debridement and broad-spectrum antibiotics. The following case demonstrates the challenge of diagnosing NSTI.
Case Presentation
A 50-year-old physician developed a sore throat with subjective fevers, night sweats, and chills. After 2 days, his symptoms resolved. The next day he developed right thigh pain while playing tennis and limped off the court. That night he had fevers, chills, and sweats. For the next 3 days, his right thigh pain persisted with waxing and waning fevers.
The patient’s medical history included gastroesophageal reflux disease, vitamin D deficiency, and a positive purified protein derivative test for which he had completed 1 year of isoniazid therapy. The patient was married and in a monogamous relationship with his wife. He had traveled to the Sierra National Forest and Yosemite Park during the preceding winter. He did not swim in a lake or recall a tick bite. He had not consumed raw food, imported meats, or dairy products. He recently started oral fluconazole for tinea corporis.
The patient’s temperature was 39.5°C, heart rate was 115 beats per minute, blood pressure (BP) was 142/88 mm Hg, and respiratory rate was 18 breaths per minute with an oxygen saturation of 95% while breathing ambient air. He was drenched in sweat yet remained comfortable throughout the interview. The oropharyngeal mucosa was moist without lesions or erythema. There was no rash or lymphadenopathy. The lungs were clear to auscultation. The cardiac exam revealed tachycardia. There was point tenderness to deep palpation of the mid-anterior right thigh without crepitus, erythema, or edema.
The patient’s sodium level was 129 mmol/L (normal range 135-145 mmol/L), bicarbonate was 20 mmol/L (normal range 22-32 mmol/L), creatinine was 1.1 mg/dL (normal range 0.7-1.2 mg/dL), and glucose was 194 mg/dL. The white blood cell count (WBC) was 12,900 cells/mm3 (normal range 3,400-10,000 cells/mm3) with 96% neutrophils. The hematocrit was 41% (normal range 41-53%), and the platelet count was 347,000 cells/mm3 (normal range 140,000-450,000 cells/mm3). The lactate level was 2.2 mmol/L (normal range 0-2 mmol/L). The creatine kinase level was 347 U/L (normal range 50-388 U/L), and the lactate dehydrogenase level was 254 U/L (normal range 102-199 U/L). A rapid group A streptococcal (GAS) antigen test was negative. A radiograph of the right femur revealed mildly edematous soft tissue. On ultrasound the right quadriceps appeared mildly edematous, but there was no evidence of abscess or discrete fluid collection (eFigure 1).
eFigure 1. Ultrasound of the Right Anterior Thigh Ultrasound revealed heterogeneous, mildly edematous quadriceps muscle. There was no abscess or discrete fluid collection. There was trace fluid along the fascia of the quadriceps muscle.
Four liters of normal saline, acetaminophen, ceftriaxone, and doxycycline were administered to the patient. Overnight he was afebrile, tachycardic, and normotensive. The following morning his BP decreased to 81/53 mm Hg. His WBC count was 33,000 cells/mm3 with 96% neutrophils. A peripheral blood smear showed immature granulocytes. The sodium and creatinine increased to 135 mmol/L and 1.3 mg/dL, respectively. The erythrocyte sedimentation rate was 20 mm/h (normal range 0-10 mm/h), and the C-reactive protein level was 174 mg/L (normal range < 6.3 mg/L).The right thigh became erythematous and edematous.
Given concern for necrotizing fasciitis, antibiotics were changed to vancomycin, piperacillin-tazobactam, and clindamycin. The patient was taken to the operating room (OR). The right quadriceps muscle was markedly edematous with overlying necrotic fibrofatty tissue with easy separation of the fascia from the anterolateral rectus femoris and rectus lateralis muscles. Necrotizing fasciitis was diagnosed.
The tissue was debrided, and surgical pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation (eFigure 2). After the anterolateral space between the fascia and underlying thigh muscle was drained, a Penrose drain was placed, and the wound was left open with plans for a second-look operation within 24 hours.
eFigure 2. Surgical Pathology of Debrided Right Thigh
Pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation.
eFigure 3. Right Anterior Thigh
Two Penrose drains inserted after second operation.
In the ensuing hours erythema extended proximal to the operative site. The patient was emergently taken to the OR. The focus of necrotizing fasciitis along the anterolateral aspect of the thigh had extended posteriorly and superiorly. This area was irrigated, all loculations were disrupted, and a second Penrose drain was placed.
The wound was left open for 6 more days. On hospital day 9, operative exploration revealed no necrotizing fasciitis. The fascia and skin wound were then closed (eFigure 3).
Cultures from the fascia grew the GAS bacteria Streptococcus pyogenes (S pyogenes), which was sensitive to penicillin. The blood cultures from admission were sterile. A test for Epstein-Barr virus immunoglobulin M antibody was negative. The patient was discharged after 10 days in the hospital to complete a 2-week course of IV penicillin. Two months later he resumed playing tennis and returned to his clinical duties.
Discussion
In the U.S., there are approximately 3.5 cases of invasive GAS infection per 100,000 persons.1 Type I NSTI is polymicrobial (aerobic and anaerobic organisms). Risk factors include recent surgery, immunocompromised states, drug use, diabetes mellitus, and traumatic wounds.2 Type II NSTI is caused by GAS or other β-hemolytic streptococci either alone or in association with another organism, most commonly Staphylococcus aureus. Type II NSTI is classically found on the extremities and occurs in young, healthy, immunocompetent patients—such as this patient.3
The portal of entry in nearly half of type II NSTI is unknown; minor local trauma is often suspected.4 However, cases have been reported in which the only identifiable source was a preceding sore throat.4 The origin of this patient’s GAS remains unknown, but perhaps his pharyngitis led to transient bacteremia, which then seeded his injured thigh muscle. An in vitro model demonstrated that injured muscles increase surface expression of the cytoskeletal protein vimentin, which binds GAS.5 Exotoxins and endotoxins produced by S pyogenes may lead to microvascular thrombosis, tissue ischemia, liquefactive necrosis, and systemic release of cytokines followed by systemic illness, multiorgan dysfunction, and death.6
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was developed to aid in early diagnosis of NSTI.7 It was derived from a series of 2,555 patients admitted with cellulitis or abscesses at a single institution. Scores > 8 have a positive predictive value of 93% for NSTI. This patient had a LRINEC score of 9. Radiographs or computed tomography scans may demonstrate soft-tissue air collections but lack sensitivity and are often nondiagnostic.8,9 T1-weighted magnetic resonance imaging can delineate the anatomic extent of soft-tissue infections but is time consuming and may delay treatment.10 When the pretest probability is high, proceeding directly to the OR for direct visualization and possible debridement is advisable. Histologic features of necrotizing fasciitis include inflammation with polymorphonuclear cells and necrosis of the subcutaneous fat and fascia with relative sparing of the muscle.11Necrotizing soft-tissue infection requires early surgical debridement and broad-spectrum antibiotic coverage. Without surgical debridement, the mortality rate approaches 100%.2 Antibiotics should include activity against Gram-positive, Gram-negative, and anaerobic organisms. The duration of antibiotic therapy has not been defined and is dependent on the patient’s clinical status. Adjunctive treatment options may include IV immunoglobulin and hyperbaric oxygen therapy, although the data supporting their utility are limited.12,13
Conclusion
Despite the LRINEC scoring systems and advanced imaging, necrotizing fasciitis remains challenging to diagnose in a timely manner. In this case, close monitoring of the patient facilitated timely evaluation and treatment of a fatal disease.
1. O'Loughlin RE, Roberson A, Cieslak PR, et al; Active Bacterial Core Surveillance Team. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853-857.
2. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705-710.
3. Naqvi GA, Malik SA, Jan W. Necrotizing fasciitis of the lower extremity: a case report and current concept of diagnosis and management. Scand J Trauma Resusc Emerg Med. 2009;17:28.
4. Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1195;1(3):69-78.
5. Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685-1692.
6. Cainzos M, Gonzalez-Rodriguez FJ. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13(4):433-439.
7. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
8. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):119-125.
9. Lancerotto L, Tocco I, Salmaso R, Vindigni V, Basetto F. Necrotizing fasciitis: classification, diagnosis and management. J Trauma Acute Care Surg. 2012;72(3):560-566.
10. Brothers TE, Tagge DU, Stutley JE, Conway WF, Del Schutte H Jr, Byrne TK. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg. 1998;187(4):416-421.
11. Bakleh M, Wold LE, Mandrekar JN, Harmsen WS, Dimashkieh HH, Baddour LM. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40(3):410-414.
12. Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267(24):3315-3316.
13. Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg. 2004;139(12):1339-1345.
Necrotizing soft-tissue infection (NSTI) often is difficult to distinguish from a superficial soft-tissue infection like cellulitis. Both conditions present with pain, edema, and erythema and can be accompanied by fever and malaise. The diagnosis of NSTI must be made quickly because successful treatment requires early surgical debridement and broad-spectrum antibiotics. The following case demonstrates the challenge of diagnosing NSTI.
Case Presentation
A 50-year-old physician developed a sore throat with subjective fevers, night sweats, and chills. After 2 days, his symptoms resolved. The next day he developed right thigh pain while playing tennis and limped off the court. That night he had fevers, chills, and sweats. For the next 3 days, his right thigh pain persisted with waxing and waning fevers.
The patient’s medical history included gastroesophageal reflux disease, vitamin D deficiency, and a positive purified protein derivative test for which he had completed 1 year of isoniazid therapy. The patient was married and in a monogamous relationship with his wife. He had traveled to the Sierra National Forest and Yosemite Park during the preceding winter. He did not swim in a lake or recall a tick bite. He had not consumed raw food, imported meats, or dairy products. He recently started oral fluconazole for tinea corporis.
The patient’s temperature was 39.5°C, heart rate was 115 beats per minute, blood pressure (BP) was 142/88 mm Hg, and respiratory rate was 18 breaths per minute with an oxygen saturation of 95% while breathing ambient air. He was drenched in sweat yet remained comfortable throughout the interview. The oropharyngeal mucosa was moist without lesions or erythema. There was no rash or lymphadenopathy. The lungs were clear to auscultation. The cardiac exam revealed tachycardia. There was point tenderness to deep palpation of the mid-anterior right thigh without crepitus, erythema, or edema.
The patient’s sodium level was 129 mmol/L (normal range 135-145 mmol/L), bicarbonate was 20 mmol/L (normal range 22-32 mmol/L), creatinine was 1.1 mg/dL (normal range 0.7-1.2 mg/dL), and glucose was 194 mg/dL. The white blood cell count (WBC) was 12,900 cells/mm3 (normal range 3,400-10,000 cells/mm3) with 96% neutrophils. The hematocrit was 41% (normal range 41-53%), and the platelet count was 347,000 cells/mm3 (normal range 140,000-450,000 cells/mm3). The lactate level was 2.2 mmol/L (normal range 0-2 mmol/L). The creatine kinase level was 347 U/L (normal range 50-388 U/L), and the lactate dehydrogenase level was 254 U/L (normal range 102-199 U/L). A rapid group A streptococcal (GAS) antigen test was negative. A radiograph of the right femur revealed mildly edematous soft tissue. On ultrasound the right quadriceps appeared mildly edematous, but there was no evidence of abscess or discrete fluid collection (eFigure 1).
eFigure 1. Ultrasound of the Right Anterior Thigh Ultrasound revealed heterogeneous, mildly edematous quadriceps muscle. There was no abscess or discrete fluid collection. There was trace fluid along the fascia of the quadriceps muscle.
Four liters of normal saline, acetaminophen, ceftriaxone, and doxycycline were administered to the patient. Overnight he was afebrile, tachycardic, and normotensive. The following morning his BP decreased to 81/53 mm Hg. His WBC count was 33,000 cells/mm3 with 96% neutrophils. A peripheral blood smear showed immature granulocytes. The sodium and creatinine increased to 135 mmol/L and 1.3 mg/dL, respectively. The erythrocyte sedimentation rate was 20 mm/h (normal range 0-10 mm/h), and the C-reactive protein level was 174 mg/L (normal range < 6.3 mg/L).The right thigh became erythematous and edematous.
Given concern for necrotizing fasciitis, antibiotics were changed to vancomycin, piperacillin-tazobactam, and clindamycin. The patient was taken to the operating room (OR). The right quadriceps muscle was markedly edematous with overlying necrotic fibrofatty tissue with easy separation of the fascia from the anterolateral rectus femoris and rectus lateralis muscles. Necrotizing fasciitis was diagnosed.
The tissue was debrided, and surgical pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation (eFigure 2). After the anterolateral space between the fascia and underlying thigh muscle was drained, a Penrose drain was placed, and the wound was left open with plans for a second-look operation within 24 hours.
eFigure 2. Surgical Pathology of Debrided Right Thigh
Pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation.
eFigure 3. Right Anterior Thigh
Two Penrose drains inserted after second operation.
In the ensuing hours erythema extended proximal to the operative site. The patient was emergently taken to the OR. The focus of necrotizing fasciitis along the anterolateral aspect of the thigh had extended posteriorly and superiorly. This area was irrigated, all loculations were disrupted, and a second Penrose drain was placed.
The wound was left open for 6 more days. On hospital day 9, operative exploration revealed no necrotizing fasciitis. The fascia and skin wound were then closed (eFigure 3).
Cultures from the fascia grew the GAS bacteria Streptococcus pyogenes (S pyogenes), which was sensitive to penicillin. The blood cultures from admission were sterile. A test for Epstein-Barr virus immunoglobulin M antibody was negative. The patient was discharged after 10 days in the hospital to complete a 2-week course of IV penicillin. Two months later he resumed playing tennis and returned to his clinical duties.
Discussion
In the U.S., there are approximately 3.5 cases of invasive GAS infection per 100,000 persons.1 Type I NSTI is polymicrobial (aerobic and anaerobic organisms). Risk factors include recent surgery, immunocompromised states, drug use, diabetes mellitus, and traumatic wounds.2 Type II NSTI is caused by GAS or other β-hemolytic streptococci either alone or in association with another organism, most commonly Staphylococcus aureus. Type II NSTI is classically found on the extremities and occurs in young, healthy, immunocompetent patients—such as this patient.3
The portal of entry in nearly half of type II NSTI is unknown; minor local trauma is often suspected.4 However, cases have been reported in which the only identifiable source was a preceding sore throat.4 The origin of this patient’s GAS remains unknown, but perhaps his pharyngitis led to transient bacteremia, which then seeded his injured thigh muscle. An in vitro model demonstrated that injured muscles increase surface expression of the cytoskeletal protein vimentin, which binds GAS.5 Exotoxins and endotoxins produced by S pyogenes may lead to microvascular thrombosis, tissue ischemia, liquefactive necrosis, and systemic release of cytokines followed by systemic illness, multiorgan dysfunction, and death.6
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was developed to aid in early diagnosis of NSTI.7 It was derived from a series of 2,555 patients admitted with cellulitis or abscesses at a single institution. Scores > 8 have a positive predictive value of 93% for NSTI. This patient had a LRINEC score of 9. Radiographs or computed tomography scans may demonstrate soft-tissue air collections but lack sensitivity and are often nondiagnostic.8,9 T1-weighted magnetic resonance imaging can delineate the anatomic extent of soft-tissue infections but is time consuming and may delay treatment.10 When the pretest probability is high, proceeding directly to the OR for direct visualization and possible debridement is advisable. Histologic features of necrotizing fasciitis include inflammation with polymorphonuclear cells and necrosis of the subcutaneous fat and fascia with relative sparing of the muscle.11Necrotizing soft-tissue infection requires early surgical debridement and broad-spectrum antibiotic coverage. Without surgical debridement, the mortality rate approaches 100%.2 Antibiotics should include activity against Gram-positive, Gram-negative, and anaerobic organisms. The duration of antibiotic therapy has not been defined and is dependent on the patient’s clinical status. Adjunctive treatment options may include IV immunoglobulin and hyperbaric oxygen therapy, although the data supporting their utility are limited.12,13
Conclusion
Despite the LRINEC scoring systems and advanced imaging, necrotizing fasciitis remains challenging to diagnose in a timely manner. In this case, close monitoring of the patient facilitated timely evaluation and treatment of a fatal disease.
Necrotizing soft-tissue infection (NSTI) often is difficult to distinguish from a superficial soft-tissue infection like cellulitis. Both conditions present with pain, edema, and erythema and can be accompanied by fever and malaise. The diagnosis of NSTI must be made quickly because successful treatment requires early surgical debridement and broad-spectrum antibiotics. The following case demonstrates the challenge of diagnosing NSTI.
Case Presentation
A 50-year-old physician developed a sore throat with subjective fevers, night sweats, and chills. After 2 days, his symptoms resolved. The next day he developed right thigh pain while playing tennis and limped off the court. That night he had fevers, chills, and sweats. For the next 3 days, his right thigh pain persisted with waxing and waning fevers.
The patient’s medical history included gastroesophageal reflux disease, vitamin D deficiency, and a positive purified protein derivative test for which he had completed 1 year of isoniazid therapy. The patient was married and in a monogamous relationship with his wife. He had traveled to the Sierra National Forest and Yosemite Park during the preceding winter. He did not swim in a lake or recall a tick bite. He had not consumed raw food, imported meats, or dairy products. He recently started oral fluconazole for tinea corporis.
The patient’s temperature was 39.5°C, heart rate was 115 beats per minute, blood pressure (BP) was 142/88 mm Hg, and respiratory rate was 18 breaths per minute with an oxygen saturation of 95% while breathing ambient air. He was drenched in sweat yet remained comfortable throughout the interview. The oropharyngeal mucosa was moist without lesions or erythema. There was no rash or lymphadenopathy. The lungs were clear to auscultation. The cardiac exam revealed tachycardia. There was point tenderness to deep palpation of the mid-anterior right thigh without crepitus, erythema, or edema.
The patient’s sodium level was 129 mmol/L (normal range 135-145 mmol/L), bicarbonate was 20 mmol/L (normal range 22-32 mmol/L), creatinine was 1.1 mg/dL (normal range 0.7-1.2 mg/dL), and glucose was 194 mg/dL. The white blood cell count (WBC) was 12,900 cells/mm3 (normal range 3,400-10,000 cells/mm3) with 96% neutrophils. The hematocrit was 41% (normal range 41-53%), and the platelet count was 347,000 cells/mm3 (normal range 140,000-450,000 cells/mm3). The lactate level was 2.2 mmol/L (normal range 0-2 mmol/L). The creatine kinase level was 347 U/L (normal range 50-388 U/L), and the lactate dehydrogenase level was 254 U/L (normal range 102-199 U/L). A rapid group A streptococcal (GAS) antigen test was negative. A radiograph of the right femur revealed mildly edematous soft tissue. On ultrasound the right quadriceps appeared mildly edematous, but there was no evidence of abscess or discrete fluid collection (eFigure 1).
eFigure 1. Ultrasound of the Right Anterior Thigh Ultrasound revealed heterogeneous, mildly edematous quadriceps muscle. There was no abscess or discrete fluid collection. There was trace fluid along the fascia of the quadriceps muscle.
Four liters of normal saline, acetaminophen, ceftriaxone, and doxycycline were administered to the patient. Overnight he was afebrile, tachycardic, and normotensive. The following morning his BP decreased to 81/53 mm Hg. His WBC count was 33,000 cells/mm3 with 96% neutrophils. A peripheral blood smear showed immature granulocytes. The sodium and creatinine increased to 135 mmol/L and 1.3 mg/dL, respectively. The erythrocyte sedimentation rate was 20 mm/h (normal range 0-10 mm/h), and the C-reactive protein level was 174 mg/L (normal range < 6.3 mg/L).The right thigh became erythematous and edematous.
Given concern for necrotizing fasciitis, antibiotics were changed to vancomycin, piperacillin-tazobactam, and clindamycin. The patient was taken to the operating room (OR). The right quadriceps muscle was markedly edematous with overlying necrotic fibrofatty tissue with easy separation of the fascia from the anterolateral rectus femoris and rectus lateralis muscles. Necrotizing fasciitis was diagnosed.
The tissue was debrided, and surgical pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation (eFigure 2). After the anterolateral space between the fascia and underlying thigh muscle was drained, a Penrose drain was placed, and the wound was left open with plans for a second-look operation within 24 hours.
eFigure 2. Surgical Pathology of Debrided Right Thigh
Pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation.
eFigure 3. Right Anterior Thigh
Two Penrose drains inserted after second operation.
In the ensuing hours erythema extended proximal to the operative site. The patient was emergently taken to the OR. The focus of necrotizing fasciitis along the anterolateral aspect of the thigh had extended posteriorly and superiorly. This area was irrigated, all loculations were disrupted, and a second Penrose drain was placed.
The wound was left open for 6 more days. On hospital day 9, operative exploration revealed no necrotizing fasciitis. The fascia and skin wound were then closed (eFigure 3).
Cultures from the fascia grew the GAS bacteria Streptococcus pyogenes (S pyogenes), which was sensitive to penicillin. The blood cultures from admission were sterile. A test for Epstein-Barr virus immunoglobulin M antibody was negative. The patient was discharged after 10 days in the hospital to complete a 2-week course of IV penicillin. Two months later he resumed playing tennis and returned to his clinical duties.
Discussion
In the U.S., there are approximately 3.5 cases of invasive GAS infection per 100,000 persons.1 Type I NSTI is polymicrobial (aerobic and anaerobic organisms). Risk factors include recent surgery, immunocompromised states, drug use, diabetes mellitus, and traumatic wounds.2 Type II NSTI is caused by GAS or other β-hemolytic streptococci either alone or in association with another organism, most commonly Staphylococcus aureus. Type II NSTI is classically found on the extremities and occurs in young, healthy, immunocompetent patients—such as this patient.3
The portal of entry in nearly half of type II NSTI is unknown; minor local trauma is often suspected.4 However, cases have been reported in which the only identifiable source was a preceding sore throat.4 The origin of this patient’s GAS remains unknown, but perhaps his pharyngitis led to transient bacteremia, which then seeded his injured thigh muscle. An in vitro model demonstrated that injured muscles increase surface expression of the cytoskeletal protein vimentin, which binds GAS.5 Exotoxins and endotoxins produced by S pyogenes may lead to microvascular thrombosis, tissue ischemia, liquefactive necrosis, and systemic release of cytokines followed by systemic illness, multiorgan dysfunction, and death.6
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was developed to aid in early diagnosis of NSTI.7 It was derived from a series of 2,555 patients admitted with cellulitis or abscesses at a single institution. Scores > 8 have a positive predictive value of 93% for NSTI. This patient had a LRINEC score of 9. Radiographs or computed tomography scans may demonstrate soft-tissue air collections but lack sensitivity and are often nondiagnostic.8,9 T1-weighted magnetic resonance imaging can delineate the anatomic extent of soft-tissue infections but is time consuming and may delay treatment.10 When the pretest probability is high, proceeding directly to the OR for direct visualization and possible debridement is advisable. Histologic features of necrotizing fasciitis include inflammation with polymorphonuclear cells and necrosis of the subcutaneous fat and fascia with relative sparing of the muscle.11Necrotizing soft-tissue infection requires early surgical debridement and broad-spectrum antibiotic coverage. Without surgical debridement, the mortality rate approaches 100%.2 Antibiotics should include activity against Gram-positive, Gram-negative, and anaerobic organisms. The duration of antibiotic therapy has not been defined and is dependent on the patient’s clinical status. Adjunctive treatment options may include IV immunoglobulin and hyperbaric oxygen therapy, although the data supporting their utility are limited.12,13
Conclusion
Despite the LRINEC scoring systems and advanced imaging, necrotizing fasciitis remains challenging to diagnose in a timely manner. In this case, close monitoring of the patient facilitated timely evaluation and treatment of a fatal disease.
1. O'Loughlin RE, Roberson A, Cieslak PR, et al; Active Bacterial Core Surveillance Team. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853-857.
2. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705-710.
3. Naqvi GA, Malik SA, Jan W. Necrotizing fasciitis of the lower extremity: a case report and current concept of diagnosis and management. Scand J Trauma Resusc Emerg Med. 2009;17:28.
4. Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1195;1(3):69-78.
5. Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685-1692.
6. Cainzos M, Gonzalez-Rodriguez FJ. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13(4):433-439.
7. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
8. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):119-125.
9. Lancerotto L, Tocco I, Salmaso R, Vindigni V, Basetto F. Necrotizing fasciitis: classification, diagnosis and management. J Trauma Acute Care Surg. 2012;72(3):560-566.
10. Brothers TE, Tagge DU, Stutley JE, Conway WF, Del Schutte H Jr, Byrne TK. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg. 1998;187(4):416-421.
11. Bakleh M, Wold LE, Mandrekar JN, Harmsen WS, Dimashkieh HH, Baddour LM. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40(3):410-414.
12. Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267(24):3315-3316.
13. Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg. 2004;139(12):1339-1345.
1. O'Loughlin RE, Roberson A, Cieslak PR, et al; Active Bacterial Core Surveillance Team. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853-857.
2. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705-710.
3. Naqvi GA, Malik SA, Jan W. Necrotizing fasciitis of the lower extremity: a case report and current concept of diagnosis and management. Scand J Trauma Resusc Emerg Med. 2009;17:28.
4. Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1195;1(3):69-78.
5. Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685-1692.
6. Cainzos M, Gonzalez-Rodriguez FJ. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13(4):433-439.
7. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
8. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):119-125.
9. Lancerotto L, Tocco I, Salmaso R, Vindigni V, Basetto F. Necrotizing fasciitis: classification, diagnosis and management. J Trauma Acute Care Surg. 2012;72(3):560-566.
10. Brothers TE, Tagge DU, Stutley JE, Conway WF, Del Schutte H Jr, Byrne TK. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg. 1998;187(4):416-421.
11. Bakleh M, Wold LE, Mandrekar JN, Harmsen WS, Dimashkieh HH, Baddour LM. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40(3):410-414.
12. Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267(24):3315-3316.
13. Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg. 2004;139(12):1339-1345.
Lichen Planus Pemphigoides Associated With Pregnancy Mimicking Pemphigoid Gestationis
Case Report
A 25-year-old woman with a 5-month history of severe lichen planus (LP) on the arms, legs, and trunk presented to the emergency department with generalized blisters and erythema over the entire body, including the face and soles, of 2 days’ duration. She was evaluated for the LP 1 week prior in a referral dermatology clinic, and in addition to topical corticosteroids, she received 1 injection of 40 mg intramuscular triamcinolone acetonide. Hours following the injection she developed nausea, vomiting, and fever. The patient reported that her last menstrual period was 3 weeks prior to the current presentation.
Physical examination revealed numerous lichenified, flat-topped, pink-violaceous, hyperpigmented, scaly papules and plaques (Figure 1), as well as tense, yellow, fluid-filled vesicles and bullae of various sizes on the neck, arms (Figure 2), legs, trunk, and dorsal aspect of the feet. The vesicles occurred on both normal skin and the lichenified plaques with a negative Nikolsky sign. There also were urticarial erythematous papules and plaques on the arms, trunk, neck, and face, some of which had vesicles or a violaceous dusky central hue (Figure 3). Vesicles were noted within both nostrils (nasal mucosa), and there were extremely tender erythematous patches and thick sheets of scales on the soles.
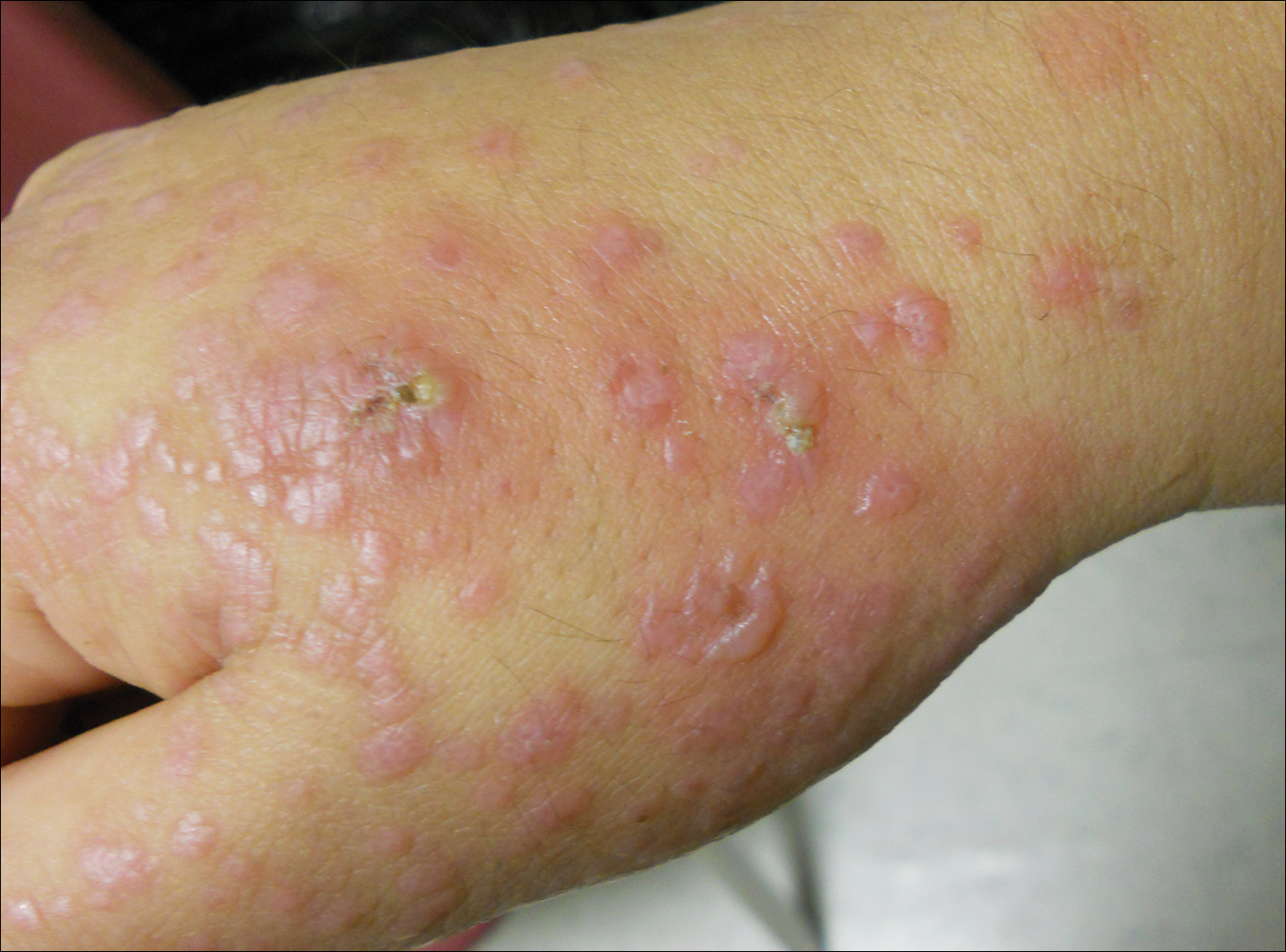
An elevated β human chorionic gonadotropin level and transvaginal ultrasonography confirmed an intrauterine pregnancy of 12 weeks’ gestation despite the patient’s report of the last menstrual period.
Histologic examination of a vesicle on the right arm revealed hyperkeratosis with hypergranulosis, vacuolar alteration of the basal layer with a paucicellular subepidermal vesicle, and melanophages in the superficial dermis consistent with vesicular LP (Figure 4). Histologic examination of an erythematous edematous plaque on the right upper leg revealed edema in the upper dermis with a perivascular and interstitial lymphocytic infiltrate with eosinophils. A third biopsy of a lichenoid flat-topped papule on the left arm revealed a mild bandlike infiltrate of lymphocytes and scattered eosinophils, eosinophilic colloid bodies and edema in the papillary dermis, and subepidermal vesicles and vacuolar alteration of the basal layer consistent with a vesicular lichenoid dermatitis (Figure 5). Direct immunofluorescence (DIF) of perilesional skin showed linear deposition of C3 and IgM along the basement membrane zone (BMZ) in addition to a shaggy pattern with cytoid bodies (Figure 6). There also was a faint linear deposit of IgA along the BMZ with cytoid bodies but negative for IgG. These results were interpreted as consistent with LP pemphigoides (LPP). Neither an enzyme-linked immunosorbent assay nor an immunoblot analysis was performed.
Because the patient was pregnant and had failed to respond to topical and intramuscular corticosteroids, she was started on intravenous methylprednisolone in the emergency department until new lesions stopped appearing. She was then discharged home on oral prednisone 50 mg (0.5 mg/kg/d), with close observation by her obstetrician. She also used clobetasol propionate ointment 0.05% for more severe lesions and triamcinolone acetonide cream 0.1% for less severe lesions until lesions resolved.
During treatment, the patient developed cellulitis on the leg that presented as pustules and erythema at a site of an eroded bulla, inframammary and axillary cutaneous candidiasis, and hyperglycemia at 19 weeks’ gestation. The cutaneous infections resolved with oral clindamycin 300 mg 3 times daily for 10 days. Topical mupirocin was used to treat the cellulitis and a mixture of zinc oxide, econazole cream, and desonide cream twice daily treated the candidiasis. Her obstetrician managed the hyperglycemia.
The bullous lesions and LP completely resolved after 2 months of treatment with oral prednisone 50 mg daily. The patient tolerated a corticosteroid taper (dose decreased by 5 mg every 2 weeks) until arriving at 10 mg, which was then decreased to 7.5 mg until delivery. A cesarean delivery was performed due to a large-for-gestational-age fetus, and an internist was consulted for the necessary precautions to increase the steroid dose during delivery due to the stress of the surgery and the risk for a hypothalamic crisis. There were no peripartum complications, and the baby was born without cutaneous lesions and remains healthy 1 year later. The patient remained disease free over 2 months postpartum, until new LP lesions developed without vesicles or bullae, which were then controlled with topical therapy. She was subsequently lost to follow-up.
Comment
Kaposi first described LPP in 1892 and used the term lichen ruber pemphigoides to describe a case of typical LP together with a widespread bullous eruption. Lichen planus pemphigoides is characterized by tense blisters that arise on lesions of LP as well as on skin unaffected by LP. In contrast, bullous LP blisters are confined to LP lesions only and occur from intense lichenoid inflammation and extensive liquefactive degeneration of basal keratinocytes. The vesicle formation in LPP is a result of autoantibodies to the bullous pemphigoid (BP) antigen BPAg2, which can be explained by the epitope spreading epiphenomenon whereby epidermotropic cytotoxic T cells damage the basal keratinocytes in LP by targeting unknown epidermal antigens, resulting in the exposure of BP180 and therefore instigating the autoimmune response.1 The process of epitope spreading takes months to develop; the mean duration of LP before LPP is 8 weeks in children and 12 weeks in adults,2 which is comparable to the current case.
Pathogenesis
Lichen planus pemphigoides usually is idiopathic; however, there have been cases reported in association with various medications including calcium channel blockers such as diltiazem, Chinese herbs,3 simvastatin,4 ramipril,5,6 captopril,7 psoralen plus UVA phototherapy,8 and cinnarizine.9 In addition, in a case-controlled study, the use of neuroleptics or diuretics was found to be a risk factor for LPP development.10
This case is unique because it shows an association of LPP with an intrauterine pregnancy. Despite the fact that we did not perform the required studies to determine the exact cause, there probably exists an association between LPP and the pregnancy, as the patient presented with a 5-month history of severe LP prior to vesicle formation. The patient only developed the vesicular lesions during pregnancy, which were later controlled with systemic steroids and then recurred postpartum only as LP lesions, suggesting that the patient’s pregnancy may have contributed in the pathogenesis as an inducing factor. We suspect that the LP was aggravated by the pregnancy and continued to worsen, so much as to cause epitope spreading and lead to the bullous eruption at the end of the first trimester.2
Differential Diagnosis
Initially, we suspected a diagnosis of pemphigoid gestationis (PG), previously known as herpes gestationis. The classic presentation of PG starts with an intense pruritus followed by the emergence of pruritic urticarial papules and plaques in the umbilical or periumbilical areas. The lesions may become targetlike or polycyclic and may spread to other areas of the trunk, arms, and legs, often including the palms and soles.11-15 Just as in our case, vesicles and bullous lesions appear at both the site of the urticarial plaques as well as on normal skin.16 The clinical features noted in our patient that were not typical of PG included the multiple lesions on the face and inside the nostrils. Only 20% of PG cases are associated with mucosal involvement,11,12,15 and there are no documented reports of PG occurring in a patient with LP, according to a PubMed search of articles indexed for MEDLINE using the search terms pemphigoid gestationis, herpes gestationis, and lichen planus.
Lichen planus pemphigoides can be easily differentiated from BP. Lichen planus pemphigoides occurs in younger patients, with a mean age of 35 years, unlike BP, which commonly affects elderly men.17 Lichen planus pemphigoides also is less severe and has a better response to treatment than BP. It also affects the palms and soles, which are rarely affected in BP. There are no reports in the literature of BP developing during pregnancy, according to a PubMed search using the terms bullous pemphigoid and pregnancy. However, LPP and BP share a common antibody, the BP180 antigen, and differences exist in the epitope where the antibody binds in each condition.18,19
Diagnosing LPP
In LPP, DIF typically shows linear deposits of IgG, IgM, IgA, fibrinogen, and C3 along the BMZ, of which IgG and C3 are most commonly seen.3 Our patient had linear deposition of C3, IgM, and IgA along the BMZ, which excluded bullous LP from the differential diagnosis. Bullous LP is not an autoimmune condition but rather is on the severe spectrum of LP where Max Joseph spaces become so large so as to lead to vesicle and bullae formation. In addition to the linear deposit at the BMZ, LPP typically reveals immunoglobulin (mainly IgM but also IgA), C3, and fibrinogen staining of colloid bodies in the papillary dermis on DIF; however, some cases of LPP only present with a linear deposition of C3 along the BMZ, which is why, similar to PG, these diagnoses by DIF are similar. Direct immunofluorescence of PG reveals linear IgG1 and IgG3 along the BMZ. IgG1 and IgG3 immunoglobulins are known to fix complement better than other immunoglobulins, thus linear C3 along the BMZ is the most consistently positive immunoreactant. Less common positive immunoreactivity with the same pattern has been seen with IgA, IgM, C1, and C4 (Table).14,15,18 The lack of linear IgG and the presence of IgM is more suggestive of LPP.
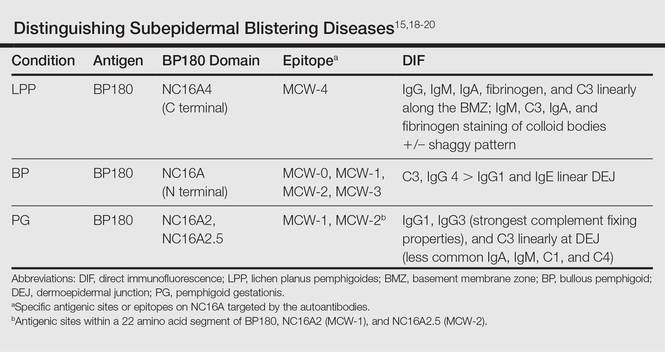
The differential diagnosis of the subepidermal autoimmune blistering diseases associated with antibodies against BP180, including BP, LPP, and PG, often is challenging.15 However, LPP can now be distinguished by immunological studies including immunoblot analysis of the immunodominant region of NC16A of the BP180 antigen and the immunoglobulin subclass that reacts to 180-, 200-,20 and 230-kDa antigens within the BMZ (Table).15,18-20 The Table summarizes the different autoantibodies, antigens, and epitopes to distinguish subepidermal autoimmune blistering diseases.
Despite not performing these studies in our patient, we concluded that the clinical, histological, and DIF findings of this case are more consistent with LPP than with the other subepidermal blistering diseases. However, we cannot exclude the possibility of the patient having a new entity with a unique antibody from epitope spreading.
Conclusion
We present a case of lichenoid papules and plaques consistent with LP, with the development of vesicles and bullae after the first trimester of pregnancy. The clinical, pathologic, and DIF findings were highly suggestive of LPP. Although the exact pathogenic mechanism is not fully known, we suspect that pregnancy may have contributed to the origin of the disease. Further evaluation of pregnant patients with lichenoid lesions who develop blisters are needed for the elucidation of the mechanism, which may be secondary to epitope spreading that led to new autoantibody formation.
- Stingl G, Holubar K. Coexistence of lichen planus and bullous pemphigoid. an immunopathological study. Br J Dermatol. 1975;93:313-320.
- Paige DG, Bhogal BS, Black MM, et al. Lichen planus pemphigoides in a child—immunopathological findings. Clin Exp Dermatol. 1993;18:552-554.
- Xu HH, Xiao T, He CD, et al. Lichen planus pemphigoides associated with Chinese herbs. Clin Exp Dermatol. 2009;34:329-332.
- Stoebner PE, Michot C, Ligeron C, et al. Simvastatin induced lichen planus pemphigoides. Ann Dermatol Venereol. 2003;130:187-190.
- Zhu YI, Fitzpatrick JE, Kornfeld BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Ogg GS, Bhogal BS, Hashimoto T, et al. Ramipril-associated lichen planus pemphigoides. Br J Dermatol. 1997;136:412-414.
- Flageul B, Foldes C, Wallach D, et al. Captopril-induced lichen planus pemphigoides with pemphigus-like features. a case report. Dermatologica. 1986;173:248-255.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Miyagawa S, Ohi H, Muramatsu T, et al. Lichen planus pemphigoides-like lesions induced by Cinnarizine. Br J Dermatol. 1985;112:607-613.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Ambros-Rudolph CM. Dermatoses of pregnancy-clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23:265-275.
- DiZenzo G, Calabresi V, Grosso F, et al. The intracellular and extracellular domains of BP180 antigen comprise novel epitopes targeted by pemphigoid gestationis autoantibodies. J Invest Dermatol. 2006;127:864-873.
- Jenkis RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigus gestationis. Clin Exp Dermatol. 1999;24:255-259.
- Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid diseases: pathogenesis, diagnosis, and treatment. Autoimmunity. 2012;45:55-70.
- Cobo MF, Santi CG, Maruta CW, et al. Pemphigoid gestationis: clinical and laboratory evaluation. Clinics. 2009;64:1042-1047.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180 kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Harjai B, Mendiratta V, Kakkar S, et al. Childhood lichen planus pemphigoides—a rare entity. J Eur Acad Dermatol Venereol. 2006;20:117-118.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med. 2002;51:21-28.
- Davis AL, Bhogal BS, Whitehead P, et al. Lichen planus pemphigoides: its relationship to bullous pemphigoid. Br J Dermatol. 1991;125:263-271.
Case Report
A 25-year-old woman with a 5-month history of severe lichen planus (LP) on the arms, legs, and trunk presented to the emergency department with generalized blisters and erythema over the entire body, including the face and soles, of 2 days’ duration. She was evaluated for the LP 1 week prior in a referral dermatology clinic, and in addition to topical corticosteroids, she received 1 injection of 40 mg intramuscular triamcinolone acetonide. Hours following the injection she developed nausea, vomiting, and fever. The patient reported that her last menstrual period was 3 weeks prior to the current presentation.
Physical examination revealed numerous lichenified, flat-topped, pink-violaceous, hyperpigmented, scaly papules and plaques (Figure 1), as well as tense, yellow, fluid-filled vesicles and bullae of various sizes on the neck, arms (Figure 2), legs, trunk, and dorsal aspect of the feet. The vesicles occurred on both normal skin and the lichenified plaques with a negative Nikolsky sign. There also were urticarial erythematous papules and plaques on the arms, trunk, neck, and face, some of which had vesicles or a violaceous dusky central hue (Figure 3). Vesicles were noted within both nostrils (nasal mucosa), and there were extremely tender erythematous patches and thick sheets of scales on the soles.



An elevated β human chorionic gonadotropin level and transvaginal ultrasonography confirmed an intrauterine pregnancy of 12 weeks’ gestation despite the patient’s report of the last menstrual period.
Histologic examination of a vesicle on the right arm revealed hyperkeratosis with hypergranulosis, vacuolar alteration of the basal layer with a paucicellular subepidermal vesicle, and melanophages in the superficial dermis consistent with vesicular LP (Figure 4). Histologic examination of an erythematous edematous plaque on the right upper leg revealed edema in the upper dermis with a perivascular and interstitial lymphocytic infiltrate with eosinophils. A third biopsy of a lichenoid flat-topped papule on the left arm revealed a mild bandlike infiltrate of lymphocytes and scattered eosinophils, eosinophilic colloid bodies and edema in the papillary dermis, and subepidermal vesicles and vacuolar alteration of the basal layer consistent with a vesicular lichenoid dermatitis (Figure 5). Direct immunofluorescence (DIF) of perilesional skin showed linear deposition of C3 and IgM along the basement membrane zone (BMZ) in addition to a shaggy pattern with cytoid bodies (Figure 6). There also was a faint linear deposit of IgA along the BMZ with cytoid bodies but negative for IgG. These results were interpreted as consistent with LP pemphigoides (LPP). Neither an enzyme-linked immunosorbent assay nor an immunoblot analysis was performed.



Because the patient was pregnant and had failed to respond to topical and intramuscular corticosteroids, she was started on intravenous methylprednisolone in the emergency department until new lesions stopped appearing. She was then discharged home on oral prednisone 50 mg (0.5 mg/kg/d), with close observation by her obstetrician. She also used clobetasol propionate ointment 0.05% for more severe lesions and triamcinolone acetonide cream 0.1% for less severe lesions until lesions resolved.
During treatment, the patient developed cellulitis on the leg that presented as pustules and erythema at a site of an eroded bulla, inframammary and axillary cutaneous candidiasis, and hyperglycemia at 19 weeks’ gestation. The cutaneous infections resolved with oral clindamycin 300 mg 3 times daily for 10 days. Topical mupirocin was used to treat the cellulitis and a mixture of zinc oxide, econazole cream, and desonide cream twice daily treated the candidiasis. Her obstetrician managed the hyperglycemia.
The bullous lesions and LP completely resolved after 2 months of treatment with oral prednisone 50 mg daily. The patient tolerated a corticosteroid taper (dose decreased by 5 mg every 2 weeks) until arriving at 10 mg, which was then decreased to 7.5 mg until delivery. A cesarean delivery was performed due to a large-for-gestational-age fetus, and an internist was consulted for the necessary precautions to increase the steroid dose during delivery due to the stress of the surgery and the risk for a hypothalamic crisis. There were no peripartum complications, and the baby was born without cutaneous lesions and remains healthy 1 year later. The patient remained disease free over 2 months postpartum, until new LP lesions developed without vesicles or bullae, which were then controlled with topical therapy. She was subsequently lost to follow-up.
Comment
Kaposi first described LPP in 1892 and used the term lichen ruber pemphigoides to describe a case of typical LP together with a widespread bullous eruption. Lichen planus pemphigoides is characterized by tense blisters that arise on lesions of LP as well as on skin unaffected by LP. In contrast, bullous LP blisters are confined to LP lesions only and occur from intense lichenoid inflammation and extensive liquefactive degeneration of basal keratinocytes. The vesicle formation in LPP is a result of autoantibodies to the bullous pemphigoid (BP) antigen BPAg2, which can be explained by the epitope spreading epiphenomenon whereby epidermotropic cytotoxic T cells damage the basal keratinocytes in LP by targeting unknown epidermal antigens, resulting in the exposure of BP180 and therefore instigating the autoimmune response.1 The process of epitope spreading takes months to develop; the mean duration of LP before LPP is 8 weeks in children and 12 weeks in adults,2 which is comparable to the current case.
Pathogenesis
Lichen planus pemphigoides usually is idiopathic; however, there have been cases reported in association with various medications including calcium channel blockers such as diltiazem, Chinese herbs,3 simvastatin,4 ramipril,5,6 captopril,7 psoralen plus UVA phototherapy,8 and cinnarizine.9 In addition, in a case-controlled study, the use of neuroleptics or diuretics was found to be a risk factor for LPP development.10
This case is unique because it shows an association of LPP with an intrauterine pregnancy. Despite the fact that we did not perform the required studies to determine the exact cause, there probably exists an association between LPP and the pregnancy, as the patient presented with a 5-month history of severe LP prior to vesicle formation. The patient only developed the vesicular lesions during pregnancy, which were later controlled with systemic steroids and then recurred postpartum only as LP lesions, suggesting that the patient’s pregnancy may have contributed in the pathogenesis as an inducing factor. We suspect that the LP was aggravated by the pregnancy and continued to worsen, so much as to cause epitope spreading and lead to the bullous eruption at the end of the first trimester.2
Differential Diagnosis
Initially, we suspected a diagnosis of pemphigoid gestationis (PG), previously known as herpes gestationis. The classic presentation of PG starts with an intense pruritus followed by the emergence of pruritic urticarial papules and plaques in the umbilical or periumbilical areas. The lesions may become targetlike or polycyclic and may spread to other areas of the trunk, arms, and legs, often including the palms and soles.11-15 Just as in our case, vesicles and bullous lesions appear at both the site of the urticarial plaques as well as on normal skin.16 The clinical features noted in our patient that were not typical of PG included the multiple lesions on the face and inside the nostrils. Only 20% of PG cases are associated with mucosal involvement,11,12,15 and there are no documented reports of PG occurring in a patient with LP, according to a PubMed search of articles indexed for MEDLINE using the search terms pemphigoid gestationis, herpes gestationis, and lichen planus.
Lichen planus pemphigoides can be easily differentiated from BP. Lichen planus pemphigoides occurs in younger patients, with a mean age of 35 years, unlike BP, which commonly affects elderly men.17 Lichen planus pemphigoides also is less severe and has a better response to treatment than BP. It also affects the palms and soles, which are rarely affected in BP. There are no reports in the literature of BP developing during pregnancy, according to a PubMed search using the terms bullous pemphigoid and pregnancy. However, LPP and BP share a common antibody, the BP180 antigen, and differences exist in the epitope where the antibody binds in each condition.18,19
Diagnosing LPP
In LPP, DIF typically shows linear deposits of IgG, IgM, IgA, fibrinogen, and C3 along the BMZ, of which IgG and C3 are most commonly seen.3 Our patient had linear deposition of C3, IgM, and IgA along the BMZ, which excluded bullous LP from the differential diagnosis. Bullous LP is not an autoimmune condition but rather is on the severe spectrum of LP where Max Joseph spaces become so large so as to lead to vesicle and bullae formation. In addition to the linear deposit at the BMZ, LPP typically reveals immunoglobulin (mainly IgM but also IgA), C3, and fibrinogen staining of colloid bodies in the papillary dermis on DIF; however, some cases of LPP only present with a linear deposition of C3 along the BMZ, which is why, similar to PG, these diagnoses by DIF are similar. Direct immunofluorescence of PG reveals linear IgG1 and IgG3 along the BMZ. IgG1 and IgG3 immunoglobulins are known to fix complement better than other immunoglobulins, thus linear C3 along the BMZ is the most consistently positive immunoreactant. Less common positive immunoreactivity with the same pattern has been seen with IgA, IgM, C1, and C4 (Table).14,15,18 The lack of linear IgG and the presence of IgM is more suggestive of LPP.

The differential diagnosis of the subepidermal autoimmune blistering diseases associated with antibodies against BP180, including BP, LPP, and PG, often is challenging.15 However, LPP can now be distinguished by immunological studies including immunoblot analysis of the immunodominant region of NC16A of the BP180 antigen and the immunoglobulin subclass that reacts to 180-, 200-,20 and 230-kDa antigens within the BMZ (Table).15,18-20 The Table summarizes the different autoantibodies, antigens, and epitopes to distinguish subepidermal autoimmune blistering diseases.
Despite not performing these studies in our patient, we concluded that the clinical, histological, and DIF findings of this case are more consistent with LPP than with the other subepidermal blistering diseases. However, we cannot exclude the possibility of the patient having a new entity with a unique antibody from epitope spreading.
Conclusion
We present a case of lichenoid papules and plaques consistent with LP, with the development of vesicles and bullae after the first trimester of pregnancy. The clinical, pathologic, and DIF findings were highly suggestive of LPP. Although the exact pathogenic mechanism is not fully known, we suspect that pregnancy may have contributed to the origin of the disease. Further evaluation of pregnant patients with lichenoid lesions who develop blisters are needed for the elucidation of the mechanism, which may be secondary to epitope spreading that led to new autoantibody formation.
Case Report
A 25-year-old woman with a 5-month history of severe lichen planus (LP) on the arms, legs, and trunk presented to the emergency department with generalized blisters and erythema over the entire body, including the face and soles, of 2 days’ duration. She was evaluated for the LP 1 week prior in a referral dermatology clinic, and in addition to topical corticosteroids, she received 1 injection of 40 mg intramuscular triamcinolone acetonide. Hours following the injection she developed nausea, vomiting, and fever. The patient reported that her last menstrual period was 3 weeks prior to the current presentation.
Physical examination revealed numerous lichenified, flat-topped, pink-violaceous, hyperpigmented, scaly papules and plaques (Figure 1), as well as tense, yellow, fluid-filled vesicles and bullae of various sizes on the neck, arms (Figure 2), legs, trunk, and dorsal aspect of the feet. The vesicles occurred on both normal skin and the lichenified plaques with a negative Nikolsky sign. There also were urticarial erythematous papules and plaques on the arms, trunk, neck, and face, some of which had vesicles or a violaceous dusky central hue (Figure 3). Vesicles were noted within both nostrils (nasal mucosa), and there were extremely tender erythematous patches and thick sheets of scales on the soles.



An elevated β human chorionic gonadotropin level and transvaginal ultrasonography confirmed an intrauterine pregnancy of 12 weeks’ gestation despite the patient’s report of the last menstrual period.
Histologic examination of a vesicle on the right arm revealed hyperkeratosis with hypergranulosis, vacuolar alteration of the basal layer with a paucicellular subepidermal vesicle, and melanophages in the superficial dermis consistent with vesicular LP (Figure 4). Histologic examination of an erythematous edematous plaque on the right upper leg revealed edema in the upper dermis with a perivascular and interstitial lymphocytic infiltrate with eosinophils. A third biopsy of a lichenoid flat-topped papule on the left arm revealed a mild bandlike infiltrate of lymphocytes and scattered eosinophils, eosinophilic colloid bodies and edema in the papillary dermis, and subepidermal vesicles and vacuolar alteration of the basal layer consistent with a vesicular lichenoid dermatitis (Figure 5). Direct immunofluorescence (DIF) of perilesional skin showed linear deposition of C3 and IgM along the basement membrane zone (BMZ) in addition to a shaggy pattern with cytoid bodies (Figure 6). There also was a faint linear deposit of IgA along the BMZ with cytoid bodies but negative for IgG. These results were interpreted as consistent with LP pemphigoides (LPP). Neither an enzyme-linked immunosorbent assay nor an immunoblot analysis was performed.



Because the patient was pregnant and had failed to respond to topical and intramuscular corticosteroids, she was started on intravenous methylprednisolone in the emergency department until new lesions stopped appearing. She was then discharged home on oral prednisone 50 mg (0.5 mg/kg/d), with close observation by her obstetrician. She also used clobetasol propionate ointment 0.05% for more severe lesions and triamcinolone acetonide cream 0.1% for less severe lesions until lesions resolved.
During treatment, the patient developed cellulitis on the leg that presented as pustules and erythema at a site of an eroded bulla, inframammary and axillary cutaneous candidiasis, and hyperglycemia at 19 weeks’ gestation. The cutaneous infections resolved with oral clindamycin 300 mg 3 times daily for 10 days. Topical mupirocin was used to treat the cellulitis and a mixture of zinc oxide, econazole cream, and desonide cream twice daily treated the candidiasis. Her obstetrician managed the hyperglycemia.
The bullous lesions and LP completely resolved after 2 months of treatment with oral prednisone 50 mg daily. The patient tolerated a corticosteroid taper (dose decreased by 5 mg every 2 weeks) until arriving at 10 mg, which was then decreased to 7.5 mg until delivery. A cesarean delivery was performed due to a large-for-gestational-age fetus, and an internist was consulted for the necessary precautions to increase the steroid dose during delivery due to the stress of the surgery and the risk for a hypothalamic crisis. There were no peripartum complications, and the baby was born without cutaneous lesions and remains healthy 1 year later. The patient remained disease free over 2 months postpartum, until new LP lesions developed without vesicles or bullae, which were then controlled with topical therapy. She was subsequently lost to follow-up.
Comment
Kaposi first described LPP in 1892 and used the term lichen ruber pemphigoides to describe a case of typical LP together with a widespread bullous eruption. Lichen planus pemphigoides is characterized by tense blisters that arise on lesions of LP as well as on skin unaffected by LP. In contrast, bullous LP blisters are confined to LP lesions only and occur from intense lichenoid inflammation and extensive liquefactive degeneration of basal keratinocytes. The vesicle formation in LPP is a result of autoantibodies to the bullous pemphigoid (BP) antigen BPAg2, which can be explained by the epitope spreading epiphenomenon whereby epidermotropic cytotoxic T cells damage the basal keratinocytes in LP by targeting unknown epidermal antigens, resulting in the exposure of BP180 and therefore instigating the autoimmune response.1 The process of epitope spreading takes months to develop; the mean duration of LP before LPP is 8 weeks in children and 12 weeks in adults,2 which is comparable to the current case.
Pathogenesis
Lichen planus pemphigoides usually is idiopathic; however, there have been cases reported in association with various medications including calcium channel blockers such as diltiazem, Chinese herbs,3 simvastatin,4 ramipril,5,6 captopril,7 psoralen plus UVA phototherapy,8 and cinnarizine.9 In addition, in a case-controlled study, the use of neuroleptics or diuretics was found to be a risk factor for LPP development.10
This case is unique because it shows an association of LPP with an intrauterine pregnancy. Despite the fact that we did not perform the required studies to determine the exact cause, there probably exists an association between LPP and the pregnancy, as the patient presented with a 5-month history of severe LP prior to vesicle formation. The patient only developed the vesicular lesions during pregnancy, which were later controlled with systemic steroids and then recurred postpartum only as LP lesions, suggesting that the patient’s pregnancy may have contributed in the pathogenesis as an inducing factor. We suspect that the LP was aggravated by the pregnancy and continued to worsen, so much as to cause epitope spreading and lead to the bullous eruption at the end of the first trimester.2
Differential Diagnosis
Initially, we suspected a diagnosis of pemphigoid gestationis (PG), previously known as herpes gestationis. The classic presentation of PG starts with an intense pruritus followed by the emergence of pruritic urticarial papules and plaques in the umbilical or periumbilical areas. The lesions may become targetlike or polycyclic and may spread to other areas of the trunk, arms, and legs, often including the palms and soles.11-15 Just as in our case, vesicles and bullous lesions appear at both the site of the urticarial plaques as well as on normal skin.16 The clinical features noted in our patient that were not typical of PG included the multiple lesions on the face and inside the nostrils. Only 20% of PG cases are associated with mucosal involvement,11,12,15 and there are no documented reports of PG occurring in a patient with LP, according to a PubMed search of articles indexed for MEDLINE using the search terms pemphigoid gestationis, herpes gestationis, and lichen planus.
Lichen planus pemphigoides can be easily differentiated from BP. Lichen planus pemphigoides occurs in younger patients, with a mean age of 35 years, unlike BP, which commonly affects elderly men.17 Lichen planus pemphigoides also is less severe and has a better response to treatment than BP. It also affects the palms and soles, which are rarely affected in BP. There are no reports in the literature of BP developing during pregnancy, according to a PubMed search using the terms bullous pemphigoid and pregnancy. However, LPP and BP share a common antibody, the BP180 antigen, and differences exist in the epitope where the antibody binds in each condition.18,19
Diagnosing LPP
In LPP, DIF typically shows linear deposits of IgG, IgM, IgA, fibrinogen, and C3 along the BMZ, of which IgG and C3 are most commonly seen.3 Our patient had linear deposition of C3, IgM, and IgA along the BMZ, which excluded bullous LP from the differential diagnosis. Bullous LP is not an autoimmune condition but rather is on the severe spectrum of LP where Max Joseph spaces become so large so as to lead to vesicle and bullae formation. In addition to the linear deposit at the BMZ, LPP typically reveals immunoglobulin (mainly IgM but also IgA), C3, and fibrinogen staining of colloid bodies in the papillary dermis on DIF; however, some cases of LPP only present with a linear deposition of C3 along the BMZ, which is why, similar to PG, these diagnoses by DIF are similar. Direct immunofluorescence of PG reveals linear IgG1 and IgG3 along the BMZ. IgG1 and IgG3 immunoglobulins are known to fix complement better than other immunoglobulins, thus linear C3 along the BMZ is the most consistently positive immunoreactant. Less common positive immunoreactivity with the same pattern has been seen with IgA, IgM, C1, and C4 (Table).14,15,18 The lack of linear IgG and the presence of IgM is more suggestive of LPP.

The differential diagnosis of the subepidermal autoimmune blistering diseases associated with antibodies against BP180, including BP, LPP, and PG, often is challenging.15 However, LPP can now be distinguished by immunological studies including immunoblot analysis of the immunodominant region of NC16A of the BP180 antigen and the immunoglobulin subclass that reacts to 180-, 200-,20 and 230-kDa antigens within the BMZ (Table).15,18-20 The Table summarizes the different autoantibodies, antigens, and epitopes to distinguish subepidermal autoimmune blistering diseases.
Despite not performing these studies in our patient, we concluded that the clinical, histological, and DIF findings of this case are more consistent with LPP than with the other subepidermal blistering diseases. However, we cannot exclude the possibility of the patient having a new entity with a unique antibody from epitope spreading.
Conclusion
We present a case of lichenoid papules and plaques consistent with LP, with the development of vesicles and bullae after the first trimester of pregnancy. The clinical, pathologic, and DIF findings were highly suggestive of LPP. Although the exact pathogenic mechanism is not fully known, we suspect that pregnancy may have contributed to the origin of the disease. Further evaluation of pregnant patients with lichenoid lesions who develop blisters are needed for the elucidation of the mechanism, which may be secondary to epitope spreading that led to new autoantibody formation.
- Stingl G, Holubar K. Coexistence of lichen planus and bullous pemphigoid. an immunopathological study. Br J Dermatol. 1975;93:313-320.
- Paige DG, Bhogal BS, Black MM, et al. Lichen planus pemphigoides in a child—immunopathological findings. Clin Exp Dermatol. 1993;18:552-554.
- Xu HH, Xiao T, He CD, et al. Lichen planus pemphigoides associated with Chinese herbs. Clin Exp Dermatol. 2009;34:329-332.
- Stoebner PE, Michot C, Ligeron C, et al. Simvastatin induced lichen planus pemphigoides. Ann Dermatol Venereol. 2003;130:187-190.
- Zhu YI, Fitzpatrick JE, Kornfeld BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Ogg GS, Bhogal BS, Hashimoto T, et al. Ramipril-associated lichen planus pemphigoides. Br J Dermatol. 1997;136:412-414.
- Flageul B, Foldes C, Wallach D, et al. Captopril-induced lichen planus pemphigoides with pemphigus-like features. a case report. Dermatologica. 1986;173:248-255.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Miyagawa S, Ohi H, Muramatsu T, et al. Lichen planus pemphigoides-like lesions induced by Cinnarizine. Br J Dermatol. 1985;112:607-613.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Ambros-Rudolph CM. Dermatoses of pregnancy-clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23:265-275.
- DiZenzo G, Calabresi V, Grosso F, et al. The intracellular and extracellular domains of BP180 antigen comprise novel epitopes targeted by pemphigoid gestationis autoantibodies. J Invest Dermatol. 2006;127:864-873.
- Jenkis RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigus gestationis. Clin Exp Dermatol. 1999;24:255-259.
- Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid diseases: pathogenesis, diagnosis, and treatment. Autoimmunity. 2012;45:55-70.
- Cobo MF, Santi CG, Maruta CW, et al. Pemphigoid gestationis: clinical and laboratory evaluation. Clinics. 2009;64:1042-1047.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180 kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Harjai B, Mendiratta V, Kakkar S, et al. Childhood lichen planus pemphigoides—a rare entity. J Eur Acad Dermatol Venereol. 2006;20:117-118.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med. 2002;51:21-28.
- Davis AL, Bhogal BS, Whitehead P, et al. Lichen planus pemphigoides: its relationship to bullous pemphigoid. Br J Dermatol. 1991;125:263-271.
- Stingl G, Holubar K. Coexistence of lichen planus and bullous pemphigoid. an immunopathological study. Br J Dermatol. 1975;93:313-320.
- Paige DG, Bhogal BS, Black MM, et al. Lichen planus pemphigoides in a child—immunopathological findings. Clin Exp Dermatol. 1993;18:552-554.
- Xu HH, Xiao T, He CD, et al. Lichen planus pemphigoides associated with Chinese herbs. Clin Exp Dermatol. 2009;34:329-332.
- Stoebner PE, Michot C, Ligeron C, et al. Simvastatin induced lichen planus pemphigoides. Ann Dermatol Venereol. 2003;130:187-190.
- Zhu YI, Fitzpatrick JE, Kornfeld BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Ogg GS, Bhogal BS, Hashimoto T, et al. Ramipril-associated lichen planus pemphigoides. Br J Dermatol. 1997;136:412-414.
- Flageul B, Foldes C, Wallach D, et al. Captopril-induced lichen planus pemphigoides with pemphigus-like features. a case report. Dermatologica. 1986;173:248-255.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Miyagawa S, Ohi H, Muramatsu T, et al. Lichen planus pemphigoides-like lesions induced by Cinnarizine. Br J Dermatol. 1985;112:607-613.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Ambros-Rudolph CM. Dermatoses of pregnancy-clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23:265-275.
- DiZenzo G, Calabresi V, Grosso F, et al. The intracellular and extracellular domains of BP180 antigen comprise novel epitopes targeted by pemphigoid gestationis autoantibodies. J Invest Dermatol. 2006;127:864-873.
- Jenkis RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigus gestationis. Clin Exp Dermatol. 1999;24:255-259.
- Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid diseases: pathogenesis, diagnosis, and treatment. Autoimmunity. 2012;45:55-70.
- Cobo MF, Santi CG, Maruta CW, et al. Pemphigoid gestationis: clinical and laboratory evaluation. Clinics. 2009;64:1042-1047.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180 kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Harjai B, Mendiratta V, Kakkar S, et al. Childhood lichen planus pemphigoides—a rare entity. J Eur Acad Dermatol Venereol. 2006;20:117-118.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med. 2002;51:21-28.
- Davis AL, Bhogal BS, Whitehead P, et al. Lichen planus pemphigoides: its relationship to bullous pemphigoid. Br J Dermatol. 1991;125:263-271.
Practice Points
- Lichen planus pemphigoides (LPP) is characterized by tense blisters that arise not only on lichen planus lesions such as bullous lichen planus but also on skin unaffected by lichen planus.
- In LPP, the autoantibodies specifically target the MCW-4 epitope of the NC16A4 domain of the bullous pemphigoid antigen BPAg2, distinguishing it from other autoimmune blistering diseases against the NC16A domain.
Strangulation of Radial Nerve Within Nondisplaced Fracture Component of Humeral Shaft Fracture
A radial nerve injury in association with a humeral shaft fracture is not an infrequent occurrence.1,2 The nerve injury typically is thought to be a neurapraxia caused by a contusion, as spontaneous recovery rates range from 70% to 90%.2-4 In cases in which acute nerve exploration and open reduction and internal fixation (ORIF) are not indicated, patient and clinician wait months for the nerve to recover. In some conservatively treated cases, the nerve is lacerated or entrapped. Patients with a lacerated or entrapped nerve may have better outcomes with early operative management.
We report on a rare case of the radial nerve entrapped within a nondisplaced segment of a closed humeral shaft fracture and describe the clinical outcome of early operative management. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An intoxicated, restrained 18-year-old driver in a motor vehicle collision sustained multiple injuries, including rib fracture, apical pneumothorax with pulmonary contusion, and corneal abrasion. Orthopedic injuries included right subtrochanteric femur fracture and midshaft right humeral shaft fracture (Figure 1).
Initial orthopedic evaluation of the right arm revealed decreased sensation in the radial nerve distribution. Motor function in the radial nerve was absent; the patient was incapable of active wrist extension or finger extension. Median and ulnar nerves were motor- and sensory-intact. Radiographs showed a displaced transverse midshaft humeral shaft fracture with a minimally displaced vertical fracture line extending from the fracture site about 3 cm into the proximal segment. The patient was placed in a coaptation splint. The femur fracture was treated with an antegrade piriformis entry intramedullary nail.
ORIF of the humerus was performed to facilitate mobilization of this polytrauma patient. He was positioned prone on a flat-top table with his right arm over a radiolucent extension. The arm was abducted at the shoulder and the elbow flexed. A posterior midline skin incision was made to reflect the triceps in a lateral-to-medial direction, facilitating dissection of the lateral brachial cutaneous nerve on the lateral aspect of the triceps, with resultant localization of the radial nerve. At that time, the radial nerve was noted to be entrapped in the fracture site (Figure 2). In the proximal segment was a sagittal split, displaced about 1 mm, and it was in this interval the nerve was held. This sagittal fracture appeared incomplete as it was followed more proximally. A unicortical Kirschner wire was placed in a posterior-to-anterior direction in each fragment alongside the nerve. A lamina spreader engaged the wires and distracted the fracture site as the tines were spread apart, releasing the nerve (Figure 3). The nerve was in continuity but was severely contused at that location. After the sagittal split was reduced, two 2.7-mm lag screws were used in lag fashion, and the transverse midshaft component was fixed with a 10-hole, 4.5-mm narrow locking compression plate. The radial nerve lay on the posterior aspect of the plate, between holes 4 and 5 (Figures 4, 5). The wound was closed, and the patient was made weight-bearing as tolerated in the right upper extremity. He was sent to occupational therapy, and static and dynamic splints were made for his wrist and hand.
Two months after injury, radial nerve examination findings were unchanged: decreased sensation on dorsum of hand and no motor function. At 3 months, electrodiagnostic testing showed neurophysiologic evidence of severe right radial neuropathy proximal to the innervation of the right brachioradialis. There were electrodiagnostic signs of ongoing axonal loss and no signs of ongoing reinnervation. At 4 months, only motor strength in wrist extension was improved (2/5). At 5 months, the patient had 4–/5 wrist extension, 3/5 metacarpophalangeal (MCP) extension of fingers, and 0/5 MCP/interphalangeal extension of thumb. Sensation in the radial nerve distribution was still decreased. At 7 months, strength in wrist extension and finger MCP extension was 4+/5. The fracture was now well healed, with maintained alignment and no changes in hardware appearance.
Discussion
In most cases, closed treatment of a humeral shaft fracture with an associated radial nerve injury has a successful outcome.5 The etiology of the neurapraxia likely is nerve contusion after the fracture. A neurapraxia is by definition a temporary injury to the myelin sheath with an intact nerve; the nerve function recovers rapidly.
Some humeral shaft fractures, however, have been associated with radial nerve injuries more severe than contusions, resulting in axonotmesis or neurotmesis. These more severe injuries make up 10% to 30% of humeral shaft fractures, including those with a frank laceration of the nerve and those with an entrapped nerve.2,3 Shao and colleagues2 reported a 90% recovery rate for patients who delayed extrication of the entrapped radial nerve. Although there is no consensus on timing of surgical exploration, motor and sensory function of the nerve is temporally related, which may indicate that earlier diagnosis and treatment lead to improved outcome.6,7 Loss of radial nerve function can have devastating effects on upper extremity function. Often, patients lose all or some extension of the wrist and fingers and abduction and extension of the thumb.
In a standard history or physical examination, there are no particular features indicating nerve entrapment. Absolute indications for humeral shaft fractures with radial palsy are limited to open fractures, vascular injuries, and unacceptable fracture alignment. Relative indications are polytrauma and secondary palsy after attempted fracture reduction. For all other humeral shaft fractures with radial nerve palsy, observation is still the mainstay of treatment, with spontaneous recovery occurring in up to 90% of patients.2,8-12 Our patient did not have an absolute indication for operative treatment; surgery was nevertheless performed to address the polytrauma and to facilitate earlier mobilization.
Electromyelogram (EMG) studies typically are not useful after acute injury. EMG studies are better used serially to evaluate reinnervation after the acute phase. Bodner and colleagues13,14 used ultrasonography to identify the radial nerve in a patient with unimproved radial nerve palsy 6 weeks after humeral shaft fracture. They found the nerve within the fracture site, whereas magnetic resonance imaging (MRI) could not follow its course. Neither ultrasonography nor MRI would likely be used after acute injury. More research is needed to improve evaluation of patients with continued palsy after nonoperative treatment.
In the case of our patient’s humeral shaft fracture, surgery was performed early because of polytrauma and radial nerve entrapment. If left interposed between 2 fracture fragments, the nerve would have been subjected to continued ischemia and likely would not have recovered spontaneously. Ikeda and Osamura7 reported on a case of radial nerve palsy that occurred after humerus shaft fracture. The nerve, entrapped between fracture fragments, was explored later, after function failed to return. As it was found within callus, the nerve was cut and then repaired end-to-end. In our patient’s case, early exploration led to release of the radial nerve from the fracture site—preventing irreversible nerve damage and allowing for spontaneous recovery over subsequent months.
Surgery for polytrauma patients with a humeral shaft fracture and radial nerve palsy may also be beneficial with respect to early nerve exploration and early mobilization. Although our patient’s fracture was well aligned and as an isolated injury would not have required surgery, the polytrauma called for early surgical management, which revealed radial nerve entrapment and led to early recovery of nerve function.
1. Ekholm R, Adami J, Tidermark J, Hansson K, Törnkvist H, Ponzer S. Fractures of the shaft of the humerus. An epidemiological study of 401 fractures. J Bone Joint Surg Br. 2006;88(11):1469-1473.
2. Shao YC, Harwood P, Grotz MR, Limb D, Giannoudis PV. Radial nerve palsy associated with fractures of the shaft of the humerus: a systematic review. J Bone Joint Surg Br. 2005;87(12):1647-1652.
3. Shah JJ, Bhatti NA. Radial nerve paralysis associated with fractures of the humerus. A review of 62 cases. Clin Orthop Relat Res. 1983;(172):171-176.
4. Ring D, Chin K, Jupiter JB. Radial nerve palsy associated with high-energy humeral shaft fractures. J Hand Surg. 2004;29(1):144-147.
5. Sarmiento A, Zagorski JB, Zych GA, Latta LL, Capps CA. Functional bracing for the treatment of fractures of the humeral diaphysis. J Bone Joint Surg Am. 2000;82(4):478-486.
6. Hugon S, Daubresse F, Depierreux L. Radial nerve entrapment in a humeral fracture callus. Acta Orthop Belg. 2008;74(1):118-121.
7. Ikeda K, Osamura N. The radial nerve palsy caused by embedding in the humeral shaft fracture—a case report. Hand Surg. 2014;19(1):91-93.
8. Green DP, Hotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. 2 vols. 5th ed. Philadelphia, PA: Elsevier/Churchill Livingstone; 2005.
9. Kettelkamp DB, Alexander H. Clinical review of radial nerve injury. J Trauma. 1967;7(3):424-432.
10. Pollock FH, Drake D, Bovill EG, Day L, Trafton PG. Treatment of radial neuropathy associated with fractures of the humerus. J Bone Joint Surg Am. 1981;63(2):239-243.
11. Li Y, Ning G, Wu Q, Wu Q, Li Y, Feng S. Review of literature of radial nerve injuries associated with humeral fractures—an integrated management strategy. PloS One. 2013;8(11):e78576.
12. DeFranco MJ, Lawton JN. Radial nerve injuries associated with humeral fractures. J Hand Surg. 2006;31(4):655-663.
13. Bodner G, Huber B, Schwabegger A, Lutz M, Waldenberger P. Sonographic detection of radial nerve entrapment within a humerus fracture. J Ultrasound Med. 1999;18(10):703-706.
14. Bodner G, Buchberger W, Schocke M, et al. Radial nerve palsy associated with humeral shaft fracture: evaluation with US—initial experience. Radiology. 2001;219(3):811-816.
A radial nerve injury in association with a humeral shaft fracture is not an infrequent occurrence.1,2 The nerve injury typically is thought to be a neurapraxia caused by a contusion, as spontaneous recovery rates range from 70% to 90%.2-4 In cases in which acute nerve exploration and open reduction and internal fixation (ORIF) are not indicated, patient and clinician wait months for the nerve to recover. In some conservatively treated cases, the nerve is lacerated or entrapped. Patients with a lacerated or entrapped nerve may have better outcomes with early operative management.
We report on a rare case of the radial nerve entrapped within a nondisplaced segment of a closed humeral shaft fracture and describe the clinical outcome of early operative management. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An intoxicated, restrained 18-year-old driver in a motor vehicle collision sustained multiple injuries, including rib fracture, apical pneumothorax with pulmonary contusion, and corneal abrasion. Orthopedic injuries included right subtrochanteric femur fracture and midshaft right humeral shaft fracture (Figure 1).
Initial orthopedic evaluation of the right arm revealed decreased sensation in the radial nerve distribution. Motor function in the radial nerve was absent; the patient was incapable of active wrist extension or finger extension. Median and ulnar nerves were motor- and sensory-intact. Radiographs showed a displaced transverse midshaft humeral shaft fracture with a minimally displaced vertical fracture line extending from the fracture site about 3 cm into the proximal segment. The patient was placed in a coaptation splint. The femur fracture was treated with an antegrade piriformis entry intramedullary nail.
ORIF of the humerus was performed to facilitate mobilization of this polytrauma patient. He was positioned prone on a flat-top table with his right arm over a radiolucent extension. The arm was abducted at the shoulder and the elbow flexed. A posterior midline skin incision was made to reflect the triceps in a lateral-to-medial direction, facilitating dissection of the lateral brachial cutaneous nerve on the lateral aspect of the triceps, with resultant localization of the radial nerve. At that time, the radial nerve was noted to be entrapped in the fracture site (Figure 2). In the proximal segment was a sagittal split, displaced about 1 mm, and it was in this interval the nerve was held. This sagittal fracture appeared incomplete as it was followed more proximally. A unicortical Kirschner wire was placed in a posterior-to-anterior direction in each fragment alongside the nerve. A lamina spreader engaged the wires and distracted the fracture site as the tines were spread apart, releasing the nerve (Figure 3). The nerve was in continuity but was severely contused at that location. After the sagittal split was reduced, two 2.7-mm lag screws were used in lag fashion, and the transverse midshaft component was fixed with a 10-hole, 4.5-mm narrow locking compression plate. The radial nerve lay on the posterior aspect of the plate, between holes 4 and 5 (Figures 4, 5). The wound was closed, and the patient was made weight-bearing as tolerated in the right upper extremity. He was sent to occupational therapy, and static and dynamic splints were made for his wrist and hand.
Two months after injury, radial nerve examination findings were unchanged: decreased sensation on dorsum of hand and no motor function. At 3 months, electrodiagnostic testing showed neurophysiologic evidence of severe right radial neuropathy proximal to the innervation of the right brachioradialis. There were electrodiagnostic signs of ongoing axonal loss and no signs of ongoing reinnervation. At 4 months, only motor strength in wrist extension was improved (2/5). At 5 months, the patient had 4–/5 wrist extension, 3/5 metacarpophalangeal (MCP) extension of fingers, and 0/5 MCP/interphalangeal extension of thumb. Sensation in the radial nerve distribution was still decreased. At 7 months, strength in wrist extension and finger MCP extension was 4+/5. The fracture was now well healed, with maintained alignment and no changes in hardware appearance.
Discussion
In most cases, closed treatment of a humeral shaft fracture with an associated radial nerve injury has a successful outcome.5 The etiology of the neurapraxia likely is nerve contusion after the fracture. A neurapraxia is by definition a temporary injury to the myelin sheath with an intact nerve; the nerve function recovers rapidly.
Some humeral shaft fractures, however, have been associated with radial nerve injuries more severe than contusions, resulting in axonotmesis or neurotmesis. These more severe injuries make up 10% to 30% of humeral shaft fractures, including those with a frank laceration of the nerve and those with an entrapped nerve.2,3 Shao and colleagues2 reported a 90% recovery rate for patients who delayed extrication of the entrapped radial nerve. Although there is no consensus on timing of surgical exploration, motor and sensory function of the nerve is temporally related, which may indicate that earlier diagnosis and treatment lead to improved outcome.6,7 Loss of radial nerve function can have devastating effects on upper extremity function. Often, patients lose all or some extension of the wrist and fingers and abduction and extension of the thumb.
In a standard history or physical examination, there are no particular features indicating nerve entrapment. Absolute indications for humeral shaft fractures with radial palsy are limited to open fractures, vascular injuries, and unacceptable fracture alignment. Relative indications are polytrauma and secondary palsy after attempted fracture reduction. For all other humeral shaft fractures with radial nerve palsy, observation is still the mainstay of treatment, with spontaneous recovery occurring in up to 90% of patients.2,8-12 Our patient did not have an absolute indication for operative treatment; surgery was nevertheless performed to address the polytrauma and to facilitate earlier mobilization.
Electromyelogram (EMG) studies typically are not useful after acute injury. EMG studies are better used serially to evaluate reinnervation after the acute phase. Bodner and colleagues13,14 used ultrasonography to identify the radial nerve in a patient with unimproved radial nerve palsy 6 weeks after humeral shaft fracture. They found the nerve within the fracture site, whereas magnetic resonance imaging (MRI) could not follow its course. Neither ultrasonography nor MRI would likely be used after acute injury. More research is needed to improve evaluation of patients with continued palsy after nonoperative treatment.
In the case of our patient’s humeral shaft fracture, surgery was performed early because of polytrauma and radial nerve entrapment. If left interposed between 2 fracture fragments, the nerve would have been subjected to continued ischemia and likely would not have recovered spontaneously. Ikeda and Osamura7 reported on a case of radial nerve palsy that occurred after humerus shaft fracture. The nerve, entrapped between fracture fragments, was explored later, after function failed to return. As it was found within callus, the nerve was cut and then repaired end-to-end. In our patient’s case, early exploration led to release of the radial nerve from the fracture site—preventing irreversible nerve damage and allowing for spontaneous recovery over subsequent months.
Surgery for polytrauma patients with a humeral shaft fracture and radial nerve palsy may also be beneficial with respect to early nerve exploration and early mobilization. Although our patient’s fracture was well aligned and as an isolated injury would not have required surgery, the polytrauma called for early surgical management, which revealed radial nerve entrapment and led to early recovery of nerve function.
A radial nerve injury in association with a humeral shaft fracture is not an infrequent occurrence.1,2 The nerve injury typically is thought to be a neurapraxia caused by a contusion, as spontaneous recovery rates range from 70% to 90%.2-4 In cases in which acute nerve exploration and open reduction and internal fixation (ORIF) are not indicated, patient and clinician wait months for the nerve to recover. In some conservatively treated cases, the nerve is lacerated or entrapped. Patients with a lacerated or entrapped nerve may have better outcomes with early operative management.
We report on a rare case of the radial nerve entrapped within a nondisplaced segment of a closed humeral shaft fracture and describe the clinical outcome of early operative management. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An intoxicated, restrained 18-year-old driver in a motor vehicle collision sustained multiple injuries, including rib fracture, apical pneumothorax with pulmonary contusion, and corneal abrasion. Orthopedic injuries included right subtrochanteric femur fracture and midshaft right humeral shaft fracture (Figure 1).
Initial orthopedic evaluation of the right arm revealed decreased sensation in the radial nerve distribution. Motor function in the radial nerve was absent; the patient was incapable of active wrist extension or finger extension. Median and ulnar nerves were motor- and sensory-intact. Radiographs showed a displaced transverse midshaft humeral shaft fracture with a minimally displaced vertical fracture line extending from the fracture site about 3 cm into the proximal segment. The patient was placed in a coaptation splint. The femur fracture was treated with an antegrade piriformis entry intramedullary nail.
ORIF of the humerus was performed to facilitate mobilization of this polytrauma patient. He was positioned prone on a flat-top table with his right arm over a radiolucent extension. The arm was abducted at the shoulder and the elbow flexed. A posterior midline skin incision was made to reflect the triceps in a lateral-to-medial direction, facilitating dissection of the lateral brachial cutaneous nerve on the lateral aspect of the triceps, with resultant localization of the radial nerve. At that time, the radial nerve was noted to be entrapped in the fracture site (Figure 2). In the proximal segment was a sagittal split, displaced about 1 mm, and it was in this interval the nerve was held. This sagittal fracture appeared incomplete as it was followed more proximally. A unicortical Kirschner wire was placed in a posterior-to-anterior direction in each fragment alongside the nerve. A lamina spreader engaged the wires and distracted the fracture site as the tines were spread apart, releasing the nerve (Figure 3). The nerve was in continuity but was severely contused at that location. After the sagittal split was reduced, two 2.7-mm lag screws were used in lag fashion, and the transverse midshaft component was fixed with a 10-hole, 4.5-mm narrow locking compression plate. The radial nerve lay on the posterior aspect of the plate, between holes 4 and 5 (Figures 4, 5). The wound was closed, and the patient was made weight-bearing as tolerated in the right upper extremity. He was sent to occupational therapy, and static and dynamic splints were made for his wrist and hand.
Two months after injury, radial nerve examination findings were unchanged: decreased sensation on dorsum of hand and no motor function. At 3 months, electrodiagnostic testing showed neurophysiologic evidence of severe right radial neuropathy proximal to the innervation of the right brachioradialis. There were electrodiagnostic signs of ongoing axonal loss and no signs of ongoing reinnervation. At 4 months, only motor strength in wrist extension was improved (2/5). At 5 months, the patient had 4–/5 wrist extension, 3/5 metacarpophalangeal (MCP) extension of fingers, and 0/5 MCP/interphalangeal extension of thumb. Sensation in the radial nerve distribution was still decreased. At 7 months, strength in wrist extension and finger MCP extension was 4+/5. The fracture was now well healed, with maintained alignment and no changes in hardware appearance.
Discussion
In most cases, closed treatment of a humeral shaft fracture with an associated radial nerve injury has a successful outcome.5 The etiology of the neurapraxia likely is nerve contusion after the fracture. A neurapraxia is by definition a temporary injury to the myelin sheath with an intact nerve; the nerve function recovers rapidly.
Some humeral shaft fractures, however, have been associated with radial nerve injuries more severe than contusions, resulting in axonotmesis or neurotmesis. These more severe injuries make up 10% to 30% of humeral shaft fractures, including those with a frank laceration of the nerve and those with an entrapped nerve.2,3 Shao and colleagues2 reported a 90% recovery rate for patients who delayed extrication of the entrapped radial nerve. Although there is no consensus on timing of surgical exploration, motor and sensory function of the nerve is temporally related, which may indicate that earlier diagnosis and treatment lead to improved outcome.6,7 Loss of radial nerve function can have devastating effects on upper extremity function. Often, patients lose all or some extension of the wrist and fingers and abduction and extension of the thumb.
In a standard history or physical examination, there are no particular features indicating nerve entrapment. Absolute indications for humeral shaft fractures with radial palsy are limited to open fractures, vascular injuries, and unacceptable fracture alignment. Relative indications are polytrauma and secondary palsy after attempted fracture reduction. For all other humeral shaft fractures with radial nerve palsy, observation is still the mainstay of treatment, with spontaneous recovery occurring in up to 90% of patients.2,8-12 Our patient did not have an absolute indication for operative treatment; surgery was nevertheless performed to address the polytrauma and to facilitate earlier mobilization.
Electromyelogram (EMG) studies typically are not useful after acute injury. EMG studies are better used serially to evaluate reinnervation after the acute phase. Bodner and colleagues13,14 used ultrasonography to identify the radial nerve in a patient with unimproved radial nerve palsy 6 weeks after humeral shaft fracture. They found the nerve within the fracture site, whereas magnetic resonance imaging (MRI) could not follow its course. Neither ultrasonography nor MRI would likely be used after acute injury. More research is needed to improve evaluation of patients with continued palsy after nonoperative treatment.
In the case of our patient’s humeral shaft fracture, surgery was performed early because of polytrauma and radial nerve entrapment. If left interposed between 2 fracture fragments, the nerve would have been subjected to continued ischemia and likely would not have recovered spontaneously. Ikeda and Osamura7 reported on a case of radial nerve palsy that occurred after humerus shaft fracture. The nerve, entrapped between fracture fragments, was explored later, after function failed to return. As it was found within callus, the nerve was cut and then repaired end-to-end. In our patient’s case, early exploration led to release of the radial nerve from the fracture site—preventing irreversible nerve damage and allowing for spontaneous recovery over subsequent months.
Surgery for polytrauma patients with a humeral shaft fracture and radial nerve palsy may also be beneficial with respect to early nerve exploration and early mobilization. Although our patient’s fracture was well aligned and as an isolated injury would not have required surgery, the polytrauma called for early surgical management, which revealed radial nerve entrapment and led to early recovery of nerve function.
1. Ekholm R, Adami J, Tidermark J, Hansson K, Törnkvist H, Ponzer S. Fractures of the shaft of the humerus. An epidemiological study of 401 fractures. J Bone Joint Surg Br. 2006;88(11):1469-1473.
2. Shao YC, Harwood P, Grotz MR, Limb D, Giannoudis PV. Radial nerve palsy associated with fractures of the shaft of the humerus: a systematic review. J Bone Joint Surg Br. 2005;87(12):1647-1652.
3. Shah JJ, Bhatti NA. Radial nerve paralysis associated with fractures of the humerus. A review of 62 cases. Clin Orthop Relat Res. 1983;(172):171-176.
4. Ring D, Chin K, Jupiter JB. Radial nerve palsy associated with high-energy humeral shaft fractures. J Hand Surg. 2004;29(1):144-147.
5. Sarmiento A, Zagorski JB, Zych GA, Latta LL, Capps CA. Functional bracing for the treatment of fractures of the humeral diaphysis. J Bone Joint Surg Am. 2000;82(4):478-486.
6. Hugon S, Daubresse F, Depierreux L. Radial nerve entrapment in a humeral fracture callus. Acta Orthop Belg. 2008;74(1):118-121.
7. Ikeda K, Osamura N. The radial nerve palsy caused by embedding in the humeral shaft fracture—a case report. Hand Surg. 2014;19(1):91-93.
8. Green DP, Hotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. 2 vols. 5th ed. Philadelphia, PA: Elsevier/Churchill Livingstone; 2005.
9. Kettelkamp DB, Alexander H. Clinical review of radial nerve injury. J Trauma. 1967;7(3):424-432.
10. Pollock FH, Drake D, Bovill EG, Day L, Trafton PG. Treatment of radial neuropathy associated with fractures of the humerus. J Bone Joint Surg Am. 1981;63(2):239-243.
11. Li Y, Ning G, Wu Q, Wu Q, Li Y, Feng S. Review of literature of radial nerve injuries associated with humeral fractures—an integrated management strategy. PloS One. 2013;8(11):e78576.
12. DeFranco MJ, Lawton JN. Radial nerve injuries associated with humeral fractures. J Hand Surg. 2006;31(4):655-663.
13. Bodner G, Huber B, Schwabegger A, Lutz M, Waldenberger P. Sonographic detection of radial nerve entrapment within a humerus fracture. J Ultrasound Med. 1999;18(10):703-706.
14. Bodner G, Buchberger W, Schocke M, et al. Radial nerve palsy associated with humeral shaft fracture: evaluation with US—initial experience. Radiology. 2001;219(3):811-816.
1. Ekholm R, Adami J, Tidermark J, Hansson K, Törnkvist H, Ponzer S. Fractures of the shaft of the humerus. An epidemiological study of 401 fractures. J Bone Joint Surg Br. 2006;88(11):1469-1473.
2. Shao YC, Harwood P, Grotz MR, Limb D, Giannoudis PV. Radial nerve palsy associated with fractures of the shaft of the humerus: a systematic review. J Bone Joint Surg Br. 2005;87(12):1647-1652.
3. Shah JJ, Bhatti NA. Radial nerve paralysis associated with fractures of the humerus. A review of 62 cases. Clin Orthop Relat Res. 1983;(172):171-176.
4. Ring D, Chin K, Jupiter JB. Radial nerve palsy associated with high-energy humeral shaft fractures. J Hand Surg. 2004;29(1):144-147.
5. Sarmiento A, Zagorski JB, Zych GA, Latta LL, Capps CA. Functional bracing for the treatment of fractures of the humeral diaphysis. J Bone Joint Surg Am. 2000;82(4):478-486.
6. Hugon S, Daubresse F, Depierreux L. Radial nerve entrapment in a humeral fracture callus. Acta Orthop Belg. 2008;74(1):118-121.
7. Ikeda K, Osamura N. The radial nerve palsy caused by embedding in the humeral shaft fracture—a case report. Hand Surg. 2014;19(1):91-93.
8. Green DP, Hotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. 2 vols. 5th ed. Philadelphia, PA: Elsevier/Churchill Livingstone; 2005.
9. Kettelkamp DB, Alexander H. Clinical review of radial nerve injury. J Trauma. 1967;7(3):424-432.
10. Pollock FH, Drake D, Bovill EG, Day L, Trafton PG. Treatment of radial neuropathy associated with fractures of the humerus. J Bone Joint Surg Am. 1981;63(2):239-243.
11. Li Y, Ning G, Wu Q, Wu Q, Li Y, Feng S. Review of literature of radial nerve injuries associated with humeral fractures—an integrated management strategy. PloS One. 2013;8(11):e78576.
12. DeFranco MJ, Lawton JN. Radial nerve injuries associated with humeral fractures. J Hand Surg. 2006;31(4):655-663.
13. Bodner G, Huber B, Schwabegger A, Lutz M, Waldenberger P. Sonographic detection of radial nerve entrapment within a humerus fracture. J Ultrasound Med. 1999;18(10):703-706.
14. Bodner G, Buchberger W, Schocke M, et al. Radial nerve palsy associated with humeral shaft fracture: evaluation with US—initial experience. Radiology. 2001;219(3):811-816.
He Huffed and He Puffed and He Got Frostbite
Case
A 27-year-old man presented to an ED after experiencing a syncopal episode. His vital signs at presentation were normal. Physical examination was generally normal except that there were blisters on the patient’s abdomen, left hand, and right arm, as well as a hypertrophic nodule on the right elbow (Figure) and hard growths on the digits of the right hand. The patient stated the growths started 5 months ago and had been increasing in size. On further questioning, the patient admitted to “huffing” (ie, inhaling) at least six cans of pressurized dust-removal keyboard cleaning spray daily for the past 11 months.
Why do patients abuse keyboard cleaning spray?
The propellant used in certain liquefied compressed gas products is 1,1-difluoroethane (1,1-DFE), a fluorinated hydrocarbon. It is a member of a broad class of related compounds that are present in spray paints, glues, nail polish removers, fuels, hair sprays, and air-freshening products. These 1,1-DFE-containing products are abused for their rapid and short-acting central nervous system (CNS) depressant effects—not unlike that of ethanol. Typically, the vapor of a volatile hydrocarbon is inhaled directly from the open container (“sniffing”), from a bag (“bagging”), or from a soaked rag (huffing). Not only are such hydrocarbon-containing products easy to conceal, they are also highly accessible and inexpensive. Moreover, there are generally no direct legal consequences resulting from abuse of these substances.
All of the aforementioned factors make hydrocarbons a popular drug of abuse among adolescents. Approximately 75% of the population abusing hydrocarbons is younger than age 18 years, half of whom reported first use prior to age 13 years.1,2 Though inhalant abuse rarely continues into adulthood, 0.1% of individuals between the ages of 18 and 30 years report having an inhalant-use disorder.
Hydrocarbons and their halogenated derivatives are lipophilic compounds that are rapidly absorbed after inhalation and rapidly distributed to CNS and cardiac tissue. The brain concentration of 1,1-DFE likely peaks higher than concentrations in other organs and is cleared more rapidly.3 Hydrocarbons produce CNS depression secondary to multiple mechanisms, including gamma-aminobutyric acid agonism, dopamine modulation, and N-methyl-D-aspartate-receptor antagonism.4,5
What causes skin lesions on the abdomen and arms?
The lesions on the patient’s abdomen and extremities were consistent with frostbite. The liquefied compressed gas in computer-cleaning and related products is housed in a pressurized canister. The pressure is released when the spray nozzle is depressed; this causes the liquid to rapidly expand to a gas as it is released, resulting in a quick decrease in the temperature of the metal canister. This process, referred to as adiabatic cooling, demonstrates the first law of thermodynamics. The cold temperature of both the liquid and the canister can cause frostbite in the digits and other parts of the body with which the canister or liquid comes into contact.6
Why did the patient have syncope?
Halogenated hydrocarbons inhibit the cardiac delayed rectifier potassium channels involved in the repolarization of cardiac myocytes, causing a delay in repolarization that is manifested as prolongation of the QT interval on an electrocardiogram. This condition places patients at an increased risk of developing torsades de pointes (TdP).7 In most cases, TdP is self-terminating; however, if TdP persists, degeneration to ventricular fibrillation will result. Deaths caused in this fashion have been referred to as “sudden sniffing death syndrome,” and account for half of all hydrocarbon-related deaths.6,8 In addition to the cardiac effects, hydrocarbons are simple asphyxiants that act by displacing oxygen from inspired air, which also contributes to syncope.
It is important to note that epinephrine and other catecholamines increase the risk for dysrhythmias such as TdP in the setting of hydrocarbon abuse.9 For this reason, epinephrine should be used with caution in the setting of a hydrocarbon-induced arrhythmia. Beta-adrenergic antagonists such as esmolol and propranolol are preferable because they reduce the incidence of ectopia that may trigger TdP.10
What is the significance of the masses noted on the examination and radiograph?
Fluorosis is associated with abnormalities of skeletal and dental tissue. Skeletal fluorosis causes osteosclerosis of the axial skeleton, periosteal new bone formation, ligamentous and tendinous ossification, and osteophyte formation. Dental fluorosis causes a yellow/brown discoloration of the teeth with horizontal streaking (mottling), pitting, and chipping.11 Fluorosis is well-described in regions where water fluoride concentrations are high due to industrial exposure; from consumption of fluorinated wine or chronic overconsumption of tea (especially green or black tea); or from fluoridated toothpaste.12-14 More recently, fluorosis has been described in patients treated for an extended duration of time with voriconazole, a fluorinated antifungal agent.15 Unlike other hydrocarbon products, fluorinated hydrocarbons such as 1,1-DFE can significantly increase systemic fluoride concentrations with excessive use. Rapid skeletal fluorosis is not well described, but has been reported after chronic abuse of fluorinated hydrocarbons.16
How is fluorosis diagnosed and managed?
The lack of rapid laboratory testing available for serum, urine, and bone fluoride concentrations makes the initial diagnosis of fluorosis a clinical one. Imaging studies are generally highly suggestive of fluorosis and can be used to support the diagnosis. A dual energy X-ray absorptiometry scan of the spine, hip, femur, and distal portions of the radii can reveal elevated T-scores consistent with osteosclerosis.14 These findings, in conjunction with bone or joint pain, reduced range of motion, or kyphosis, should prompt clinicians to conduct further testing—even without a confirmed fluoride source. A serum fluoride (reference range, 0.2-3.2 mg/L) and 24-hour urine fluoride (reference range, 0.2-3.2 mg/dL) and creatinine evaluation can be used to diagnose fluorosis. However, a bone biopsy with quantitative bone ash fluoride analysis remains the gold standard for the diagnosis of skeletal fluorosis.16 Laboratory evaluation should also include an assessment of electrolytes, specifically calcium, 25-hydroxyvitamin D, and alkaline phosphatase. The differential diagnosis should include hemoglobinopathies, renal osteodystrophy, Paget disease, hypothyroidism, and skeletal metastases.16
Treatment of fluorosis is largely symptomatic and supportive, with identification and discontinuation of the fluoride source. Patients should be referred to an orthopedist for evaluation and management as needed. Evaluation by an endocrinologist should also be considered because patients may have chronic vitamin D and calcium deficiencies as a result of systemic fluorosis.
Case Conclusion
The patient’s laboratory assessment was notable for the following: alkaline phosphatase, 624 U/L (reference range, 44-147 IU/L); vitamin D, 10 ng/mL (reference range, 20-40 ng/mL); serum fluoride, 0.3 mg/L (reference range, 0.2-3.2 mg/L); urine fluoride, 52 mg/dL (0.2-3.2 mg/dL); and urine creatinine, 1 g/L (reference range, 0.3-3 g/L). Imaging studies noted periosteal bone formation on the lateral epicondyle of the distal right humerus, as well as similar osseous abnormalities in other locations. A bone biopsy was scheduled. The patient was treated with oral vitamin D and educated about the importance of discontinuing the huffing of all hydrocarbons.
1. Williams JF, Storck M; American Academy of Pediatrics Committee on Substance Abuse; American Academy of Pediatrics Committee on Native American Child Health. Inhalant abuse. Pediatrics. 2007;119(5):1009-1017.
2. Wu LT, Pilowsky DJ, Schlenger WE. Inhalant abuse and dependence among adolescents in the United States. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1206-1214.
3. Avella J, Kunaparaju N, Kumar S, Lehrer M, Zito SW, Barletta M. Uptake and distribution of the abused inhalant 1,1-difluoroethane in the rat. J Anal Toxicol. 2010;34(7):381-388.
4. Tormoehlen LM, Tekulve KJ, Nañagas KA. Hydrocarbon toxicity: A review. Clin Toxicol (Phila). 2014;52(5):479-489.
5. Duncan JR, Lawrence AJ. Conventional concepts and new perspectives for understanding the addictive properties of inhalants. J Pharmacol Sci. 2013;122(4):237-243.
6. Sakai K, Maruyama-Maebashi K, Takatsu A, et al. Sudden death involving inhalation of 1,1-difluoroethane (HFC-152a) with spray cleaner: three case reports. Forensic Sci Int. 2011;206(1-3):e58-e61.
7. Himmel HM. Mechanisms involved in cardiac sensitization by volatile anesthetics: general applicability to halogenated hydrocarbons? Crit Rev Toxicol. 2008;38(9):773-803.
8. Avella J, Wilson JC, Lehrer M. Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE). Am J Forensic Med Pathol. 2006;27(1):58-60.
9. Nelson LS. Toxicologic myocardial sensitization. J Toxicol Clin Toxicol. 2002;40(7):867-879.
10. Mortiz F, de La Chapelle A, Bauer F, Leroy JP, Goullé JP, Bonmarchand G. Esmolol in the treatment of severe arrhythmia after acute trichloroethylene poisoning. Intensive Care Med. 2000;26(2):256.
11. Majumdar KK. Health impact of supplying safe drinking water containing fluoride below permissible level on flourosis patients in a fluoride-endemic rural area of West Bengal. Indian J Public Health. 2011;55(4):303-308.
12. Kakumanu N, Rao SD. Images in clinical medicine. Skeletal fluorosis due to excessive tea drinking. N Engl J Med 2013;368(12):1140.
13. Soriano M, Manchón F. Radiological aspects of a new type of bone fluorosis, periostitis deformans. Radiology 1966;87(6):1089-1094.
14. Tamer MN, Kale Köroğlu B, Arslan C, et al. Osteosclerosis due to endemic fluorosis. Sci Total Environ. 2007;373(1):43-48.
15. Bucknor MD, Gross AJ, Link TM. Voriconazole-induced periostitis in two post-transplant patients. J Radiol Case Rep. 2013;7(8):10-17.
16. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connector. 2014;4(4):e108.
Case
A 27-year-old man presented to an ED after experiencing a syncopal episode. His vital signs at presentation were normal. Physical examination was generally normal except that there were blisters on the patient’s abdomen, left hand, and right arm, as well as a hypertrophic nodule on the right elbow (Figure) and hard growths on the digits of the right hand. The patient stated the growths started 5 months ago and had been increasing in size. On further questioning, the patient admitted to “huffing” (ie, inhaling) at least six cans of pressurized dust-removal keyboard cleaning spray daily for the past 11 months.
Why do patients abuse keyboard cleaning spray?
The propellant used in certain liquefied compressed gas products is 1,1-difluoroethane (1,1-DFE), a fluorinated hydrocarbon. It is a member of a broad class of related compounds that are present in spray paints, glues, nail polish removers, fuels, hair sprays, and air-freshening products. These 1,1-DFE-containing products are abused for their rapid and short-acting central nervous system (CNS) depressant effects—not unlike that of ethanol. Typically, the vapor of a volatile hydrocarbon is inhaled directly from the open container (“sniffing”), from a bag (“bagging”), or from a soaked rag (huffing). Not only are such hydrocarbon-containing products easy to conceal, they are also highly accessible and inexpensive. Moreover, there are generally no direct legal consequences resulting from abuse of these substances.
All of the aforementioned factors make hydrocarbons a popular drug of abuse among adolescents. Approximately 75% of the population abusing hydrocarbons is younger than age 18 years, half of whom reported first use prior to age 13 years.1,2 Though inhalant abuse rarely continues into adulthood, 0.1% of individuals between the ages of 18 and 30 years report having an inhalant-use disorder.
Hydrocarbons and their halogenated derivatives are lipophilic compounds that are rapidly absorbed after inhalation and rapidly distributed to CNS and cardiac tissue. The brain concentration of 1,1-DFE likely peaks higher than concentrations in other organs and is cleared more rapidly.3 Hydrocarbons produce CNS depression secondary to multiple mechanisms, including gamma-aminobutyric acid agonism, dopamine modulation, and N-methyl-D-aspartate-receptor antagonism.4,5
What causes skin lesions on the abdomen and arms?
The lesions on the patient’s abdomen and extremities were consistent with frostbite. The liquefied compressed gas in computer-cleaning and related products is housed in a pressurized canister. The pressure is released when the spray nozzle is depressed; this causes the liquid to rapidly expand to a gas as it is released, resulting in a quick decrease in the temperature of the metal canister. This process, referred to as adiabatic cooling, demonstrates the first law of thermodynamics. The cold temperature of both the liquid and the canister can cause frostbite in the digits and other parts of the body with which the canister or liquid comes into contact.6
Why did the patient have syncope?
Halogenated hydrocarbons inhibit the cardiac delayed rectifier potassium channels involved in the repolarization of cardiac myocytes, causing a delay in repolarization that is manifested as prolongation of the QT interval on an electrocardiogram. This condition places patients at an increased risk of developing torsades de pointes (TdP).7 In most cases, TdP is self-terminating; however, if TdP persists, degeneration to ventricular fibrillation will result. Deaths caused in this fashion have been referred to as “sudden sniffing death syndrome,” and account for half of all hydrocarbon-related deaths.6,8 In addition to the cardiac effects, hydrocarbons are simple asphyxiants that act by displacing oxygen from inspired air, which also contributes to syncope.
It is important to note that epinephrine and other catecholamines increase the risk for dysrhythmias such as TdP in the setting of hydrocarbon abuse.9 For this reason, epinephrine should be used with caution in the setting of a hydrocarbon-induced arrhythmia. Beta-adrenergic antagonists such as esmolol and propranolol are preferable because they reduce the incidence of ectopia that may trigger TdP.10
What is the significance of the masses noted on the examination and radiograph?
Fluorosis is associated with abnormalities of skeletal and dental tissue. Skeletal fluorosis causes osteosclerosis of the axial skeleton, periosteal new bone formation, ligamentous and tendinous ossification, and osteophyte formation. Dental fluorosis causes a yellow/brown discoloration of the teeth with horizontal streaking (mottling), pitting, and chipping.11 Fluorosis is well-described in regions where water fluoride concentrations are high due to industrial exposure; from consumption of fluorinated wine or chronic overconsumption of tea (especially green or black tea); or from fluoridated toothpaste.12-14 More recently, fluorosis has been described in patients treated for an extended duration of time with voriconazole, a fluorinated antifungal agent.15 Unlike other hydrocarbon products, fluorinated hydrocarbons such as 1,1-DFE can significantly increase systemic fluoride concentrations with excessive use. Rapid skeletal fluorosis is not well described, but has been reported after chronic abuse of fluorinated hydrocarbons.16
How is fluorosis diagnosed and managed?
The lack of rapid laboratory testing available for serum, urine, and bone fluoride concentrations makes the initial diagnosis of fluorosis a clinical one. Imaging studies are generally highly suggestive of fluorosis and can be used to support the diagnosis. A dual energy X-ray absorptiometry scan of the spine, hip, femur, and distal portions of the radii can reveal elevated T-scores consistent with osteosclerosis.14 These findings, in conjunction with bone or joint pain, reduced range of motion, or kyphosis, should prompt clinicians to conduct further testing—even without a confirmed fluoride source. A serum fluoride (reference range, 0.2-3.2 mg/L) and 24-hour urine fluoride (reference range, 0.2-3.2 mg/dL) and creatinine evaluation can be used to diagnose fluorosis. However, a bone biopsy with quantitative bone ash fluoride analysis remains the gold standard for the diagnosis of skeletal fluorosis.16 Laboratory evaluation should also include an assessment of electrolytes, specifically calcium, 25-hydroxyvitamin D, and alkaline phosphatase. The differential diagnosis should include hemoglobinopathies, renal osteodystrophy, Paget disease, hypothyroidism, and skeletal metastases.16
Treatment of fluorosis is largely symptomatic and supportive, with identification and discontinuation of the fluoride source. Patients should be referred to an orthopedist for evaluation and management as needed. Evaluation by an endocrinologist should also be considered because patients may have chronic vitamin D and calcium deficiencies as a result of systemic fluorosis.
Case Conclusion
The patient’s laboratory assessment was notable for the following: alkaline phosphatase, 624 U/L (reference range, 44-147 IU/L); vitamin D, 10 ng/mL (reference range, 20-40 ng/mL); serum fluoride, 0.3 mg/L (reference range, 0.2-3.2 mg/L); urine fluoride, 52 mg/dL (0.2-3.2 mg/dL); and urine creatinine, 1 g/L (reference range, 0.3-3 g/L). Imaging studies noted periosteal bone formation on the lateral epicondyle of the distal right humerus, as well as similar osseous abnormalities in other locations. A bone biopsy was scheduled. The patient was treated with oral vitamin D and educated about the importance of discontinuing the huffing of all hydrocarbons.
Case
A 27-year-old man presented to an ED after experiencing a syncopal episode. His vital signs at presentation were normal. Physical examination was generally normal except that there were blisters on the patient’s abdomen, left hand, and right arm, as well as a hypertrophic nodule on the right elbow (Figure) and hard growths on the digits of the right hand. The patient stated the growths started 5 months ago and had been increasing in size. On further questioning, the patient admitted to “huffing” (ie, inhaling) at least six cans of pressurized dust-removal keyboard cleaning spray daily for the past 11 months.
Why do patients abuse keyboard cleaning spray?
The propellant used in certain liquefied compressed gas products is 1,1-difluoroethane (1,1-DFE), a fluorinated hydrocarbon. It is a member of a broad class of related compounds that are present in spray paints, glues, nail polish removers, fuels, hair sprays, and air-freshening products. These 1,1-DFE-containing products are abused for their rapid and short-acting central nervous system (CNS) depressant effects—not unlike that of ethanol. Typically, the vapor of a volatile hydrocarbon is inhaled directly from the open container (“sniffing”), from a bag (“bagging”), or from a soaked rag (huffing). Not only are such hydrocarbon-containing products easy to conceal, they are also highly accessible and inexpensive. Moreover, there are generally no direct legal consequences resulting from abuse of these substances.
All of the aforementioned factors make hydrocarbons a popular drug of abuse among adolescents. Approximately 75% of the population abusing hydrocarbons is younger than age 18 years, half of whom reported first use prior to age 13 years.1,2 Though inhalant abuse rarely continues into adulthood, 0.1% of individuals between the ages of 18 and 30 years report having an inhalant-use disorder.
Hydrocarbons and their halogenated derivatives are lipophilic compounds that are rapidly absorbed after inhalation and rapidly distributed to CNS and cardiac tissue. The brain concentration of 1,1-DFE likely peaks higher than concentrations in other organs and is cleared more rapidly.3 Hydrocarbons produce CNS depression secondary to multiple mechanisms, including gamma-aminobutyric acid agonism, dopamine modulation, and N-methyl-D-aspartate-receptor antagonism.4,5
What causes skin lesions on the abdomen and arms?
The lesions on the patient’s abdomen and extremities were consistent with frostbite. The liquefied compressed gas in computer-cleaning and related products is housed in a pressurized canister. The pressure is released when the spray nozzle is depressed; this causes the liquid to rapidly expand to a gas as it is released, resulting in a quick decrease in the temperature of the metal canister. This process, referred to as adiabatic cooling, demonstrates the first law of thermodynamics. The cold temperature of both the liquid and the canister can cause frostbite in the digits and other parts of the body with which the canister or liquid comes into contact.6
Why did the patient have syncope?
Halogenated hydrocarbons inhibit the cardiac delayed rectifier potassium channels involved in the repolarization of cardiac myocytes, causing a delay in repolarization that is manifested as prolongation of the QT interval on an electrocardiogram. This condition places patients at an increased risk of developing torsades de pointes (TdP).7 In most cases, TdP is self-terminating; however, if TdP persists, degeneration to ventricular fibrillation will result. Deaths caused in this fashion have been referred to as “sudden sniffing death syndrome,” and account for half of all hydrocarbon-related deaths.6,8 In addition to the cardiac effects, hydrocarbons are simple asphyxiants that act by displacing oxygen from inspired air, which also contributes to syncope.
It is important to note that epinephrine and other catecholamines increase the risk for dysrhythmias such as TdP in the setting of hydrocarbon abuse.9 For this reason, epinephrine should be used with caution in the setting of a hydrocarbon-induced arrhythmia. Beta-adrenergic antagonists such as esmolol and propranolol are preferable because they reduce the incidence of ectopia that may trigger TdP.10
What is the significance of the masses noted on the examination and radiograph?
Fluorosis is associated with abnormalities of skeletal and dental tissue. Skeletal fluorosis causes osteosclerosis of the axial skeleton, periosteal new bone formation, ligamentous and tendinous ossification, and osteophyte formation. Dental fluorosis causes a yellow/brown discoloration of the teeth with horizontal streaking (mottling), pitting, and chipping.11 Fluorosis is well-described in regions where water fluoride concentrations are high due to industrial exposure; from consumption of fluorinated wine or chronic overconsumption of tea (especially green or black tea); or from fluoridated toothpaste.12-14 More recently, fluorosis has been described in patients treated for an extended duration of time with voriconazole, a fluorinated antifungal agent.15 Unlike other hydrocarbon products, fluorinated hydrocarbons such as 1,1-DFE can significantly increase systemic fluoride concentrations with excessive use. Rapid skeletal fluorosis is not well described, but has been reported after chronic abuse of fluorinated hydrocarbons.16
How is fluorosis diagnosed and managed?
The lack of rapid laboratory testing available for serum, urine, and bone fluoride concentrations makes the initial diagnosis of fluorosis a clinical one. Imaging studies are generally highly suggestive of fluorosis and can be used to support the diagnosis. A dual energy X-ray absorptiometry scan of the spine, hip, femur, and distal portions of the radii can reveal elevated T-scores consistent with osteosclerosis.14 These findings, in conjunction with bone or joint pain, reduced range of motion, or kyphosis, should prompt clinicians to conduct further testing—even without a confirmed fluoride source. A serum fluoride (reference range, 0.2-3.2 mg/L) and 24-hour urine fluoride (reference range, 0.2-3.2 mg/dL) and creatinine evaluation can be used to diagnose fluorosis. However, a bone biopsy with quantitative bone ash fluoride analysis remains the gold standard for the diagnosis of skeletal fluorosis.16 Laboratory evaluation should also include an assessment of electrolytes, specifically calcium, 25-hydroxyvitamin D, and alkaline phosphatase. The differential diagnosis should include hemoglobinopathies, renal osteodystrophy, Paget disease, hypothyroidism, and skeletal metastases.16
Treatment of fluorosis is largely symptomatic and supportive, with identification and discontinuation of the fluoride source. Patients should be referred to an orthopedist for evaluation and management as needed. Evaluation by an endocrinologist should also be considered because patients may have chronic vitamin D and calcium deficiencies as a result of systemic fluorosis.
Case Conclusion
The patient’s laboratory assessment was notable for the following: alkaline phosphatase, 624 U/L (reference range, 44-147 IU/L); vitamin D, 10 ng/mL (reference range, 20-40 ng/mL); serum fluoride, 0.3 mg/L (reference range, 0.2-3.2 mg/L); urine fluoride, 52 mg/dL (0.2-3.2 mg/dL); and urine creatinine, 1 g/L (reference range, 0.3-3 g/L). Imaging studies noted periosteal bone formation on the lateral epicondyle of the distal right humerus, as well as similar osseous abnormalities in other locations. A bone biopsy was scheduled. The patient was treated with oral vitamin D and educated about the importance of discontinuing the huffing of all hydrocarbons.
1. Williams JF, Storck M; American Academy of Pediatrics Committee on Substance Abuse; American Academy of Pediatrics Committee on Native American Child Health. Inhalant abuse. Pediatrics. 2007;119(5):1009-1017.
2. Wu LT, Pilowsky DJ, Schlenger WE. Inhalant abuse and dependence among adolescents in the United States. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1206-1214.
3. Avella J, Kunaparaju N, Kumar S, Lehrer M, Zito SW, Barletta M. Uptake and distribution of the abused inhalant 1,1-difluoroethane in the rat. J Anal Toxicol. 2010;34(7):381-388.
4. Tormoehlen LM, Tekulve KJ, Nañagas KA. Hydrocarbon toxicity: A review. Clin Toxicol (Phila). 2014;52(5):479-489.
5. Duncan JR, Lawrence AJ. Conventional concepts and new perspectives for understanding the addictive properties of inhalants. J Pharmacol Sci. 2013;122(4):237-243.
6. Sakai K, Maruyama-Maebashi K, Takatsu A, et al. Sudden death involving inhalation of 1,1-difluoroethane (HFC-152a) with spray cleaner: three case reports. Forensic Sci Int. 2011;206(1-3):e58-e61.
7. Himmel HM. Mechanisms involved in cardiac sensitization by volatile anesthetics: general applicability to halogenated hydrocarbons? Crit Rev Toxicol. 2008;38(9):773-803.
8. Avella J, Wilson JC, Lehrer M. Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE). Am J Forensic Med Pathol. 2006;27(1):58-60.
9. Nelson LS. Toxicologic myocardial sensitization. J Toxicol Clin Toxicol. 2002;40(7):867-879.
10. Mortiz F, de La Chapelle A, Bauer F, Leroy JP, Goullé JP, Bonmarchand G. Esmolol in the treatment of severe arrhythmia after acute trichloroethylene poisoning. Intensive Care Med. 2000;26(2):256.
11. Majumdar KK. Health impact of supplying safe drinking water containing fluoride below permissible level on flourosis patients in a fluoride-endemic rural area of West Bengal. Indian J Public Health. 2011;55(4):303-308.
12. Kakumanu N, Rao SD. Images in clinical medicine. Skeletal fluorosis due to excessive tea drinking. N Engl J Med 2013;368(12):1140.
13. Soriano M, Manchón F. Radiological aspects of a new type of bone fluorosis, periostitis deformans. Radiology 1966;87(6):1089-1094.
14. Tamer MN, Kale Köroğlu B, Arslan C, et al. Osteosclerosis due to endemic fluorosis. Sci Total Environ. 2007;373(1):43-48.
15. Bucknor MD, Gross AJ, Link TM. Voriconazole-induced periostitis in two post-transplant patients. J Radiol Case Rep. 2013;7(8):10-17.
16. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connector. 2014;4(4):e108.
1. Williams JF, Storck M; American Academy of Pediatrics Committee on Substance Abuse; American Academy of Pediatrics Committee on Native American Child Health. Inhalant abuse. Pediatrics. 2007;119(5):1009-1017.
2. Wu LT, Pilowsky DJ, Schlenger WE. Inhalant abuse and dependence among adolescents in the United States. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1206-1214.
3. Avella J, Kunaparaju N, Kumar S, Lehrer M, Zito SW, Barletta M. Uptake and distribution of the abused inhalant 1,1-difluoroethane in the rat. J Anal Toxicol. 2010;34(7):381-388.
4. Tormoehlen LM, Tekulve KJ, Nañagas KA. Hydrocarbon toxicity: A review. Clin Toxicol (Phila). 2014;52(5):479-489.
5. Duncan JR, Lawrence AJ. Conventional concepts and new perspectives for understanding the addictive properties of inhalants. J Pharmacol Sci. 2013;122(4):237-243.
6. Sakai K, Maruyama-Maebashi K, Takatsu A, et al. Sudden death involving inhalation of 1,1-difluoroethane (HFC-152a) with spray cleaner: three case reports. Forensic Sci Int. 2011;206(1-3):e58-e61.
7. Himmel HM. Mechanisms involved in cardiac sensitization by volatile anesthetics: general applicability to halogenated hydrocarbons? Crit Rev Toxicol. 2008;38(9):773-803.
8. Avella J, Wilson JC, Lehrer M. Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE). Am J Forensic Med Pathol. 2006;27(1):58-60.
9. Nelson LS. Toxicologic myocardial sensitization. J Toxicol Clin Toxicol. 2002;40(7):867-879.
10. Mortiz F, de La Chapelle A, Bauer F, Leroy JP, Goullé JP, Bonmarchand G. Esmolol in the treatment of severe arrhythmia after acute trichloroethylene poisoning. Intensive Care Med. 2000;26(2):256.
11. Majumdar KK. Health impact of supplying safe drinking water containing fluoride below permissible level on flourosis patients in a fluoride-endemic rural area of West Bengal. Indian J Public Health. 2011;55(4):303-308.
12. Kakumanu N, Rao SD. Images in clinical medicine. Skeletal fluorosis due to excessive tea drinking. N Engl J Med 2013;368(12):1140.
13. Soriano M, Manchón F. Radiological aspects of a new type of bone fluorosis, periostitis deformans. Radiology 1966;87(6):1089-1094.
14. Tamer MN, Kale Köroğlu B, Arslan C, et al. Osteosclerosis due to endemic fluorosis. Sci Total Environ. 2007;373(1):43-48.
15. Bucknor MD, Gross AJ, Link TM. Voriconazole-induced periostitis in two post-transplant patients. J Radiol Case Rep. 2013;7(8):10-17.
16. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connector. 2014;4(4):e108.