User login
Are inhaled steroids effective for a postviral cough?
No. Inhaled corticosteroids (ICS) don’t improve postviral cough in adults with subacute (3-8 weeks) or chronic (>8 weeks) cough, adolescents with a history of asthma but without recent asthma activity, or children with a history of episodic viral wheezing without asthma (strength of recommendation [SOR]: B, preponderance of small randomized controlled trials [RCTs]).
EVIDENCE SUMMARY
A systematic review of 7 RCTs with a total of 477 adults that examined the efficacy of ICS compared with placebo for treating subacute (3-8 weeks) and chronic (>8 weeks) cough found inconsistent, but mostly negative results.1 Most trials combined patients with nonspecific subacute and chronic cough.
The evaluated steroids included beclomethasone, budesonide, fluticasone, and mometasone; daily “budesonide equivalent” doses ranged from 320 mcg to 1600 mcg. Six of the 7 trials found that ICS didn’t improve cough. The seventh didn’t treat patients with postviral cough. The authors of the review couldn’t pool data because of heterogeneity.
Steroids don’t affect methacholine challenge in teens
A double-blind, placebo-controlled RCT of 56 adolescents found that giving ICS after viral upper respiratory infection didn’t change the methacholine dosing necessary to produce a 20% reduction in the forced expiratory volume in one second (FEV1).2 Investigators included patients if they had a previous diagnosis of asthma but no use of asthma medications in 2 years, a baseline FEV1 greater than 70% of predicted, and a concentration of methacholine that produced a 20% fall in FEV1 less than 8 mg/mL.
They randomized patients to inhaled budesonide (2 200-mcg puffs bid) or placebo (2 500-mcg puffs micronized lactose bid). Patients underwent spirometry and methacholine challenge testing every 3 months over a 9-month period. The groups didn’t differ in bronchial hyperresponsiveness or FEV1.
Lower respiratory symptoms don’t respond to ICS in nonasthmatic children
A systematic review of 5 RCTs with a total of 339 patients found that in 4 of the 5, ICS didn’t improve lower respiratory symptoms in children with episodic viral wheeze and no history of asthma.3 Investigators evaluated ICS efficacy using lower respiratory symptom scores (based primarily on cough and wheeze) and decreased use of oral steroids or reduced emergency room visits.
Four trials found no benefit from ICS; one trial (52 children with viral-induced wheeze) found that nebulized budesonide (400 mg qid for 2 days, then bid for 7 days) decreased respiratory symptom scores (weighted mean difference= -0.17; 95% confidence interval, -0.34 to -0.003) compared with placebo. Investigators didn’t assess cough separately from wheezing, however.
1. Johnstone KJ, Chang AB, Fong KM, et al. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev. 2013;3:CD009305.
2. Koh YY, Sun YH, Lim HS, et al. Effect of inhaled budesonide on bronchial hyperresponsiveness in adolescents with clinical remission of asthma. Chest. 2001;120:1140-1146.
3. McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev. 2000;(2):CD001107.
No. Inhaled corticosteroids (ICS) don’t improve postviral cough in adults with subacute (3-8 weeks) or chronic (>8 weeks) cough, adolescents with a history of asthma but without recent asthma activity, or children with a history of episodic viral wheezing without asthma (strength of recommendation [SOR]: B, preponderance of small randomized controlled trials [RCTs]).
EVIDENCE SUMMARY
A systematic review of 7 RCTs with a total of 477 adults that examined the efficacy of ICS compared with placebo for treating subacute (3-8 weeks) and chronic (>8 weeks) cough found inconsistent, but mostly negative results.1 Most trials combined patients with nonspecific subacute and chronic cough.
The evaluated steroids included beclomethasone, budesonide, fluticasone, and mometasone; daily “budesonide equivalent” doses ranged from 320 mcg to 1600 mcg. Six of the 7 trials found that ICS didn’t improve cough. The seventh didn’t treat patients with postviral cough. The authors of the review couldn’t pool data because of heterogeneity.
Steroids don’t affect methacholine challenge in teens
A double-blind, placebo-controlled RCT of 56 adolescents found that giving ICS after viral upper respiratory infection didn’t change the methacholine dosing necessary to produce a 20% reduction in the forced expiratory volume in one second (FEV1).2 Investigators included patients if they had a previous diagnosis of asthma but no use of asthma medications in 2 years, a baseline FEV1 greater than 70% of predicted, and a concentration of methacholine that produced a 20% fall in FEV1 less than 8 mg/mL.
They randomized patients to inhaled budesonide (2 200-mcg puffs bid) or placebo (2 500-mcg puffs micronized lactose bid). Patients underwent spirometry and methacholine challenge testing every 3 months over a 9-month period. The groups didn’t differ in bronchial hyperresponsiveness or FEV1.
Lower respiratory symptoms don’t respond to ICS in nonasthmatic children
A systematic review of 5 RCTs with a total of 339 patients found that in 4 of the 5, ICS didn’t improve lower respiratory symptoms in children with episodic viral wheeze and no history of asthma.3 Investigators evaluated ICS efficacy using lower respiratory symptom scores (based primarily on cough and wheeze) and decreased use of oral steroids or reduced emergency room visits.
Four trials found no benefit from ICS; one trial (52 children with viral-induced wheeze) found that nebulized budesonide (400 mg qid for 2 days, then bid for 7 days) decreased respiratory symptom scores (weighted mean difference= -0.17; 95% confidence interval, -0.34 to -0.003) compared with placebo. Investigators didn’t assess cough separately from wheezing, however.
No. Inhaled corticosteroids (ICS) don’t improve postviral cough in adults with subacute (3-8 weeks) or chronic (>8 weeks) cough, adolescents with a history of asthma but without recent asthma activity, or children with a history of episodic viral wheezing without asthma (strength of recommendation [SOR]: B, preponderance of small randomized controlled trials [RCTs]).
EVIDENCE SUMMARY
A systematic review of 7 RCTs with a total of 477 adults that examined the efficacy of ICS compared with placebo for treating subacute (3-8 weeks) and chronic (>8 weeks) cough found inconsistent, but mostly negative results.1 Most trials combined patients with nonspecific subacute and chronic cough.
The evaluated steroids included beclomethasone, budesonide, fluticasone, and mometasone; daily “budesonide equivalent” doses ranged from 320 mcg to 1600 mcg. Six of the 7 trials found that ICS didn’t improve cough. The seventh didn’t treat patients with postviral cough. The authors of the review couldn’t pool data because of heterogeneity.
Steroids don’t affect methacholine challenge in teens
A double-blind, placebo-controlled RCT of 56 adolescents found that giving ICS after viral upper respiratory infection didn’t change the methacholine dosing necessary to produce a 20% reduction in the forced expiratory volume in one second (FEV1).2 Investigators included patients if they had a previous diagnosis of asthma but no use of asthma medications in 2 years, a baseline FEV1 greater than 70% of predicted, and a concentration of methacholine that produced a 20% fall in FEV1 less than 8 mg/mL.
They randomized patients to inhaled budesonide (2 200-mcg puffs bid) or placebo (2 500-mcg puffs micronized lactose bid). Patients underwent spirometry and methacholine challenge testing every 3 months over a 9-month period. The groups didn’t differ in bronchial hyperresponsiveness or FEV1.
Lower respiratory symptoms don’t respond to ICS in nonasthmatic children
A systematic review of 5 RCTs with a total of 339 patients found that in 4 of the 5, ICS didn’t improve lower respiratory symptoms in children with episodic viral wheeze and no history of asthma.3 Investigators evaluated ICS efficacy using lower respiratory symptom scores (based primarily on cough and wheeze) and decreased use of oral steroids or reduced emergency room visits.
Four trials found no benefit from ICS; one trial (52 children with viral-induced wheeze) found that nebulized budesonide (400 mg qid for 2 days, then bid for 7 days) decreased respiratory symptom scores (weighted mean difference= -0.17; 95% confidence interval, -0.34 to -0.003) compared with placebo. Investigators didn’t assess cough separately from wheezing, however.
1. Johnstone KJ, Chang AB, Fong KM, et al. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev. 2013;3:CD009305.
2. Koh YY, Sun YH, Lim HS, et al. Effect of inhaled budesonide on bronchial hyperresponsiveness in adolescents with clinical remission of asthma. Chest. 2001;120:1140-1146.
3. McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev. 2000;(2):CD001107.
1. Johnstone KJ, Chang AB, Fong KM, et al. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev. 2013;3:CD009305.
2. Koh YY, Sun YH, Lim HS, et al. Effect of inhaled budesonide on bronchial hyperresponsiveness in adolescents with clinical remission of asthma. Chest. 2001;120:1140-1146.
3. McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev. 2000;(2):CD001107.
Evidence-based answers from the Family Physicians Inquiries Network
How well do antivirals shorten genital herpes pain duration?
Oral and intravenous (IV) acyclovir each shorten the duration of pain for a first primary outbreak of herpes by about 50%; topical acyclovir shortens it by about 25% (strength of recommendation [SOR]: B, small randomized controlled trials [RCTs] with some methodological flaws).
Oral valacyclovir and famciclovir are equivalent to oral acyclovir. Adding topical acyclovir to oral acyclovir doesn’t produce additional benefit (SOR: B, RCTs).
Patients with severe disease may require IV acyclovir (SOR: C, expert opinion).
General treatment measures that may improve patient comfort include keeping lesions clean and dry, avoiding tight clothing, taking analgesics, and using ice packs or taking warm baths (SOR: C, expert opinion).
Evidence for using complementary and alternative medicine to treat genital herpes is lacking or conflicting [SOR: C, narrative review of clinical trials].
EVIDENCE SUMMARY
A review of 3 double-blind, placebo-controlled RCTs compared topical, oral, and IV acyclovir in patients with a first episode of genital herpes.1 Researchers recruited a total of 138 patients and randomized them to receive either placebo or one of the following: oral acyclovir (200 mg 5 times daily for 10 days), IV acyclovir (5 mg/kg dose, 3 times daily for 5 days), or 5% topical acyclovir in polyethylene glycol (4 times daily for 6 days).
All treatments shortened duration of pain compared with placebo: oral (3 days vs 7 days, P<.01), IV (5 days vs 9 days, P<.05), and topical (5 days vs 7 days, P<.05).
A subsequent RCT with 50 patients found that adding topical acyclovir to oral acyclovir was no more effective than oral acyclovir alone.2
Oral acyclovir, valacyclovir, and famciclovir work equally well
Head-to-head trials comparing acyclovir with valacyclovir or famciclovir show no difference in decreased duration of pain caused by primary genital herpes. An RCT of 643 adults found valacyclovir (1000 mg twice daily for 10 days) to be as effective and well-tolerated as acyclovir (200 mg 5 times daily for 10 days).3 An RCT of 951 adults demonstrated that famciclovir (250 mg 3 times daily for either 5 or 10 days) worked as well as acyclovir (200 mg 5 times daily for 10 days).4
General treatment measures
Expert opinion recommends the following general treatment measures for genital herpes lesions: keeping the affected area clean and dry, wearing dry, loose-fitting clothing and cotton underwear, and not touching the lesions. Additional symptomatic treatments for local pain include ice packs, baking soda compresses, warm baths, oral analgesics, topical anesthetics, and drying the affected area with cool air.5-8
CAM approaches lack evidence of efficacy
A 2005 nonsystematic review of available scientific data on complementary and alternative medicine found a lack of evidence or conflicting evidence concerning the use of aloe vera, echinacea, L-lysine, bee products (honey pollen), zinc, and eleuthero for the treatment of pain in genital herpes.9
RECOMMENDATIONS
Clinical practice guidelines recommend prescribing oral antiviral therapy for patients with a first episode of genital herpes because patients with mild clinical findings at onset may develop severe or prolonged symptoms. Choices include a 7- to 10-day course of valacyclovir 1 g twice a day, famciclovir 250 mg 3 times a day, acyclovir 400 mg 3 times a day, or acyclovir 400 mg 5 times a day.5,10
The guidelines recommend treating patients with severe disease (such as disseminated infection, pneumonitis, hepatitis, or meningoencephalitis) with IV acyclovir (5-10 mg/kg every 8 hours for 2-7 days or until clinical improvement), followed by oral acyclovir for at least 10 days.
1. Corey L, Benedetti J, Critchlow C, et al. Treatment of primary first-episode genital herpes simplex virus infections with acyclovir: results of topical, intravenous and oral therapy. J Antimicrob Chemother. 1983;12(suppl B):79-88.
2. Kinghorn GR, Abeywickreme I, Jeavons M, et al. Efficacy of combined treatment with oral and topical acyclovir in first episode genital herpes. Genitourin Med. 1986;62:186-188.
3. Fife KH, Barabarash RA, Rudolph T, et al. Valaciclovir versus acyclovir in the treatment of first-episode genital herpes infection. Results of an international, multicenter, double-blind, randomized clinic trial. The Valaciclovir International Herpes Simplex Virus Study Group. Sex Transm Dis. 1997;24:481-486.
4. Loveless M, Sacks SL, Harris JRW. Famciclovir in the management of first-episode genital herpes. Infect Dis Clin Prac. 1997;6(suppl 1):S12-S16.
5. ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin: Clinical management guidelines for obstetrician-gynecologists, number 57, November 2004. Gynecologic herpes simplex virus infections. Obstet Gynecol. 2004;104(5 pt 1):1111-1118.
6. Beauman JG. Genital herpes: a review. Am Fam Physician. 2005;72:1527-1534.
7. Patel R. Progress in meeting today’s demands in genital herpes: an overview of current management. J Infect Dis. 2002;186(suppl 1):S847-S856.
8. Stanberry LR, Rosenthal SL. Genital herpes simplex virus infection in the adolescent: special considerations for management. Pediatr Drugs. 2002;4:291-297.
9. Perfect MM, Bourne N, Ebel C, et al. Use of complementary and alternative medicine for the treatment of genital herpes. Herpes. 2005;12:38-41.
10. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1-110.
Oral and intravenous (IV) acyclovir each shorten the duration of pain for a first primary outbreak of herpes by about 50%; topical acyclovir shortens it by about 25% (strength of recommendation [SOR]: B, small randomized controlled trials [RCTs] with some methodological flaws).
Oral valacyclovir and famciclovir are equivalent to oral acyclovir. Adding topical acyclovir to oral acyclovir doesn’t produce additional benefit (SOR: B, RCTs).
Patients with severe disease may require IV acyclovir (SOR: C, expert opinion).
General treatment measures that may improve patient comfort include keeping lesions clean and dry, avoiding tight clothing, taking analgesics, and using ice packs or taking warm baths (SOR: C, expert opinion).
Evidence for using complementary and alternative medicine to treat genital herpes is lacking or conflicting [SOR: C, narrative review of clinical trials].
EVIDENCE SUMMARY
A review of 3 double-blind, placebo-controlled RCTs compared topical, oral, and IV acyclovir in patients with a first episode of genital herpes.1 Researchers recruited a total of 138 patients and randomized them to receive either placebo or one of the following: oral acyclovir (200 mg 5 times daily for 10 days), IV acyclovir (5 mg/kg dose, 3 times daily for 5 days), or 5% topical acyclovir in polyethylene glycol (4 times daily for 6 days).
All treatments shortened duration of pain compared with placebo: oral (3 days vs 7 days, P<.01), IV (5 days vs 9 days, P<.05), and topical (5 days vs 7 days, P<.05).
A subsequent RCT with 50 patients found that adding topical acyclovir to oral acyclovir was no more effective than oral acyclovir alone.2
Oral acyclovir, valacyclovir, and famciclovir work equally well
Head-to-head trials comparing acyclovir with valacyclovir or famciclovir show no difference in decreased duration of pain caused by primary genital herpes. An RCT of 643 adults found valacyclovir (1000 mg twice daily for 10 days) to be as effective and well-tolerated as acyclovir (200 mg 5 times daily for 10 days).3 An RCT of 951 adults demonstrated that famciclovir (250 mg 3 times daily for either 5 or 10 days) worked as well as acyclovir (200 mg 5 times daily for 10 days).4
General treatment measures
Expert opinion recommends the following general treatment measures for genital herpes lesions: keeping the affected area clean and dry, wearing dry, loose-fitting clothing and cotton underwear, and not touching the lesions. Additional symptomatic treatments for local pain include ice packs, baking soda compresses, warm baths, oral analgesics, topical anesthetics, and drying the affected area with cool air.5-8
CAM approaches lack evidence of efficacy
A 2005 nonsystematic review of available scientific data on complementary and alternative medicine found a lack of evidence or conflicting evidence concerning the use of aloe vera, echinacea, L-lysine, bee products (honey pollen), zinc, and eleuthero for the treatment of pain in genital herpes.9
RECOMMENDATIONS
Clinical practice guidelines recommend prescribing oral antiviral therapy for patients with a first episode of genital herpes because patients with mild clinical findings at onset may develop severe or prolonged symptoms. Choices include a 7- to 10-day course of valacyclovir 1 g twice a day, famciclovir 250 mg 3 times a day, acyclovir 400 mg 3 times a day, or acyclovir 400 mg 5 times a day.5,10
The guidelines recommend treating patients with severe disease (such as disseminated infection, pneumonitis, hepatitis, or meningoencephalitis) with IV acyclovir (5-10 mg/kg every 8 hours for 2-7 days or until clinical improvement), followed by oral acyclovir for at least 10 days.
Oral and intravenous (IV) acyclovir each shorten the duration of pain for a first primary outbreak of herpes by about 50%; topical acyclovir shortens it by about 25% (strength of recommendation [SOR]: B, small randomized controlled trials [RCTs] with some methodological flaws).
Oral valacyclovir and famciclovir are equivalent to oral acyclovir. Adding topical acyclovir to oral acyclovir doesn’t produce additional benefit (SOR: B, RCTs).
Patients with severe disease may require IV acyclovir (SOR: C, expert opinion).
General treatment measures that may improve patient comfort include keeping lesions clean and dry, avoiding tight clothing, taking analgesics, and using ice packs or taking warm baths (SOR: C, expert opinion).
Evidence for using complementary and alternative medicine to treat genital herpes is lacking or conflicting [SOR: C, narrative review of clinical trials].
EVIDENCE SUMMARY
A review of 3 double-blind, placebo-controlled RCTs compared topical, oral, and IV acyclovir in patients with a first episode of genital herpes.1 Researchers recruited a total of 138 patients and randomized them to receive either placebo or one of the following: oral acyclovir (200 mg 5 times daily for 10 days), IV acyclovir (5 mg/kg dose, 3 times daily for 5 days), or 5% topical acyclovir in polyethylene glycol (4 times daily for 6 days).
All treatments shortened duration of pain compared with placebo: oral (3 days vs 7 days, P<.01), IV (5 days vs 9 days, P<.05), and topical (5 days vs 7 days, P<.05).
A subsequent RCT with 50 patients found that adding topical acyclovir to oral acyclovir was no more effective than oral acyclovir alone.2
Oral acyclovir, valacyclovir, and famciclovir work equally well
Head-to-head trials comparing acyclovir with valacyclovir or famciclovir show no difference in decreased duration of pain caused by primary genital herpes. An RCT of 643 adults found valacyclovir (1000 mg twice daily for 10 days) to be as effective and well-tolerated as acyclovir (200 mg 5 times daily for 10 days).3 An RCT of 951 adults demonstrated that famciclovir (250 mg 3 times daily for either 5 or 10 days) worked as well as acyclovir (200 mg 5 times daily for 10 days).4
General treatment measures
Expert opinion recommends the following general treatment measures for genital herpes lesions: keeping the affected area clean and dry, wearing dry, loose-fitting clothing and cotton underwear, and not touching the lesions. Additional symptomatic treatments for local pain include ice packs, baking soda compresses, warm baths, oral analgesics, topical anesthetics, and drying the affected area with cool air.5-8
CAM approaches lack evidence of efficacy
A 2005 nonsystematic review of available scientific data on complementary and alternative medicine found a lack of evidence or conflicting evidence concerning the use of aloe vera, echinacea, L-lysine, bee products (honey pollen), zinc, and eleuthero for the treatment of pain in genital herpes.9
RECOMMENDATIONS
Clinical practice guidelines recommend prescribing oral antiviral therapy for patients with a first episode of genital herpes because patients with mild clinical findings at onset may develop severe or prolonged symptoms. Choices include a 7- to 10-day course of valacyclovir 1 g twice a day, famciclovir 250 mg 3 times a day, acyclovir 400 mg 3 times a day, or acyclovir 400 mg 5 times a day.5,10
The guidelines recommend treating patients with severe disease (such as disseminated infection, pneumonitis, hepatitis, or meningoencephalitis) with IV acyclovir (5-10 mg/kg every 8 hours for 2-7 days or until clinical improvement), followed by oral acyclovir for at least 10 days.
1. Corey L, Benedetti J, Critchlow C, et al. Treatment of primary first-episode genital herpes simplex virus infections with acyclovir: results of topical, intravenous and oral therapy. J Antimicrob Chemother. 1983;12(suppl B):79-88.
2. Kinghorn GR, Abeywickreme I, Jeavons M, et al. Efficacy of combined treatment with oral and topical acyclovir in first episode genital herpes. Genitourin Med. 1986;62:186-188.
3. Fife KH, Barabarash RA, Rudolph T, et al. Valaciclovir versus acyclovir in the treatment of first-episode genital herpes infection. Results of an international, multicenter, double-blind, randomized clinic trial. The Valaciclovir International Herpes Simplex Virus Study Group. Sex Transm Dis. 1997;24:481-486.
4. Loveless M, Sacks SL, Harris JRW. Famciclovir in the management of first-episode genital herpes. Infect Dis Clin Prac. 1997;6(suppl 1):S12-S16.
5. ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin: Clinical management guidelines for obstetrician-gynecologists, number 57, November 2004. Gynecologic herpes simplex virus infections. Obstet Gynecol. 2004;104(5 pt 1):1111-1118.
6. Beauman JG. Genital herpes: a review. Am Fam Physician. 2005;72:1527-1534.
7. Patel R. Progress in meeting today’s demands in genital herpes: an overview of current management. J Infect Dis. 2002;186(suppl 1):S847-S856.
8. Stanberry LR, Rosenthal SL. Genital herpes simplex virus infection in the adolescent: special considerations for management. Pediatr Drugs. 2002;4:291-297.
9. Perfect MM, Bourne N, Ebel C, et al. Use of complementary and alternative medicine for the treatment of genital herpes. Herpes. 2005;12:38-41.
10. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1-110.
1. Corey L, Benedetti J, Critchlow C, et al. Treatment of primary first-episode genital herpes simplex virus infections with acyclovir: results of topical, intravenous and oral therapy. J Antimicrob Chemother. 1983;12(suppl B):79-88.
2. Kinghorn GR, Abeywickreme I, Jeavons M, et al. Efficacy of combined treatment with oral and topical acyclovir in first episode genital herpes. Genitourin Med. 1986;62:186-188.
3. Fife KH, Barabarash RA, Rudolph T, et al. Valaciclovir versus acyclovir in the treatment of first-episode genital herpes infection. Results of an international, multicenter, double-blind, randomized clinic trial. The Valaciclovir International Herpes Simplex Virus Study Group. Sex Transm Dis. 1997;24:481-486.
4. Loveless M, Sacks SL, Harris JRW. Famciclovir in the management of first-episode genital herpes. Infect Dis Clin Prac. 1997;6(suppl 1):S12-S16.
5. ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin: Clinical management guidelines for obstetrician-gynecologists, number 57, November 2004. Gynecologic herpes simplex virus infections. Obstet Gynecol. 2004;104(5 pt 1):1111-1118.
6. Beauman JG. Genital herpes: a review. Am Fam Physician. 2005;72:1527-1534.
7. Patel R. Progress in meeting today’s demands in genital herpes: an overview of current management. J Infect Dis. 2002;186(suppl 1):S847-S856.
8. Stanberry LR, Rosenthal SL. Genital herpes simplex virus infection in the adolescent: special considerations for management. Pediatr Drugs. 2002;4:291-297.
9. Perfect MM, Bourne N, Ebel C, et al. Use of complementary and alternative medicine for the treatment of genital herpes. Herpes. 2005;12:38-41.
10. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1-110.
Evidence-based answers from the Family Physicians Inquiries Network
Does the presence of a trained support person during labor decrease C-section rates?
Sometimes. The continuous presence of a support person during labor slightly decreases (by about 2%) the likelihood of a cesarean section (C-section) but only when companions can’t be present and epidurals aren’t routine (strength of recommendation [SOR]: A, a well-done systematic review of randomized controlled trials [RCTs]). When the support person was neither hospital staff nor a member of the woman’s social network, C-section was significantly less likely (SOR A, a well-done systematic review of RCTs).
EVIDENCE SUMMARY
A 2012 Cochrane review of 22 multinational RCTs with a total of 15,288 patients investigated the effect of continuous support in labor on several outcomes, including C-section.1 All trials included pregnant women in labor. The study populations were heterogenous in terms of parity; most included only nulliparous women, but some included multiparous women. At least one study incorporated higher-risk groups such as mothers of twins, but several trials limited the study group to low-risk pregnancies.
The review found a small but significant decrease in risk of C-section in women receiving continuous support (absolute risk reduction [ARR]=2%; number needed to treat [NNT]=50; P=.0017).1 The average cost of trained childbirth support in 3 US metropolitan areas in October 2014 was about $875, according to a Web search of established businesses.
The effect only works in the absence of companions and epidurals…
A subgroup analysis of 22 studies investigated several variables to determine circumstances under which a support person decreased the risk of C-section.1 The support person’s presence was significant only when hospital policy prevented companions (such as the woman’s spouse) in the labor room and when epidurals were not routinely available. Eleven of the 22 studies (11,326 patients) permitted a companion; 11 studies (3849 patients) didn’t.
When policy allowed companions, the presence of a support person didn’t decrease C-section rates significantly (12.7% without support compared with 11.9% with support; P=.20).1 When the woman wasn’t permitted to have a companion, however, the presence of a support person significantly decreased C-section (ARR=5.4%; NNT=19; P<.01).
In 14 studies, with a total of 13,064 patients, epidurals were routinely available. In the other 8, with 2077 patients, epidurals weren’t available.1 These were older studies or studies conducted in developing countries. When epidurals were routinely available, the presence of a support person didn’t affect the C-section rate (13.8% rate without support, 12.9% with support; P=.12). But if epidural anesthesia wasn’t available, a support person decreased C-section (ARR=8.6%; NNT=12; P<.00001).
…And when the support person isn’t a hospital staffer or known to the patient
The Cochrane Review also evaluated different types of labor supporters: companions of the patient’s choice from her social network, hospital employees, and people who were neither. The support person conferred significant benefit only when that person was neither hospital staff nor a member of the woman’s social network.
Hospital staff members who provided support didn’t effectively decrease the C-section rate (12% rate in control group vs 11.3% in supported group; P=.28). Support people chosen by the patient likewise didn’t successfully reduce C-sections (19.4% control rate vs 15.5% supported rate; P=.062). When the support person was neither hospital staff nor someone well-known to the patient, the risk of C-section was significantly lower (ARR=6%; NNT=17; P=.0003).
RECOMMENDATIONS
In a Comparative Effectiveness Review published in October 2012, the Agency for Healthcare Research and Quality investigated 18 strategies to reduce C-section, one of which was psychosocial support from doulas and other providers. A trained support person was the only intervention that showed evidence of benefit in decreasing C-section, but the strength of evidence was low.2
An American College of Obstetricians and Gynecologists Practice Bulletin recommends continuous labor support, noting “the continuous presence of a support person may reduce the likelihood of…operative delivery” with no apparent harmful effects.3
1. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2012;(10):CD003766.
2. Agency for Healthcare Research and Quality. Strategies to reduce cesarean birth in low-risk women. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/263/1291/CER80_C-Section_ExecutiveSummary_20121018.pdf. Accessed February 22, 2015.
3. American College of Obstetrics and Gynecology Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
Sometimes. The continuous presence of a support person during labor slightly decreases (by about 2%) the likelihood of a cesarean section (C-section) but only when companions can’t be present and epidurals aren’t routine (strength of recommendation [SOR]: A, a well-done systematic review of randomized controlled trials [RCTs]). When the support person was neither hospital staff nor a member of the woman’s social network, C-section was significantly less likely (SOR A, a well-done systematic review of RCTs).
EVIDENCE SUMMARY
A 2012 Cochrane review of 22 multinational RCTs with a total of 15,288 patients investigated the effect of continuous support in labor on several outcomes, including C-section.1 All trials included pregnant women in labor. The study populations were heterogenous in terms of parity; most included only nulliparous women, but some included multiparous women. At least one study incorporated higher-risk groups such as mothers of twins, but several trials limited the study group to low-risk pregnancies.
The review found a small but significant decrease in risk of C-section in women receiving continuous support (absolute risk reduction [ARR]=2%; number needed to treat [NNT]=50; P=.0017).1 The average cost of trained childbirth support in 3 US metropolitan areas in October 2014 was about $875, according to a Web search of established businesses.
The effect only works in the absence of companions and epidurals…
A subgroup analysis of 22 studies investigated several variables to determine circumstances under which a support person decreased the risk of C-section.1 The support person’s presence was significant only when hospital policy prevented companions (such as the woman’s spouse) in the labor room and when epidurals were not routinely available. Eleven of the 22 studies (11,326 patients) permitted a companion; 11 studies (3849 patients) didn’t.
When policy allowed companions, the presence of a support person didn’t decrease C-section rates significantly (12.7% without support compared with 11.9% with support; P=.20).1 When the woman wasn’t permitted to have a companion, however, the presence of a support person significantly decreased C-section (ARR=5.4%; NNT=19; P<.01).
In 14 studies, with a total of 13,064 patients, epidurals were routinely available. In the other 8, with 2077 patients, epidurals weren’t available.1 These were older studies or studies conducted in developing countries. When epidurals were routinely available, the presence of a support person didn’t affect the C-section rate (13.8% rate without support, 12.9% with support; P=.12). But if epidural anesthesia wasn’t available, a support person decreased C-section (ARR=8.6%; NNT=12; P<.00001).
…And when the support person isn’t a hospital staffer or known to the patient
The Cochrane Review also evaluated different types of labor supporters: companions of the patient’s choice from her social network, hospital employees, and people who were neither. The support person conferred significant benefit only when that person was neither hospital staff nor a member of the woman’s social network.
Hospital staff members who provided support didn’t effectively decrease the C-section rate (12% rate in control group vs 11.3% in supported group; P=.28). Support people chosen by the patient likewise didn’t successfully reduce C-sections (19.4% control rate vs 15.5% supported rate; P=.062). When the support person was neither hospital staff nor someone well-known to the patient, the risk of C-section was significantly lower (ARR=6%; NNT=17; P=.0003).
RECOMMENDATIONS
In a Comparative Effectiveness Review published in October 2012, the Agency for Healthcare Research and Quality investigated 18 strategies to reduce C-section, one of which was psychosocial support from doulas and other providers. A trained support person was the only intervention that showed evidence of benefit in decreasing C-section, but the strength of evidence was low.2
An American College of Obstetricians and Gynecologists Practice Bulletin recommends continuous labor support, noting “the continuous presence of a support person may reduce the likelihood of…operative delivery” with no apparent harmful effects.3
Sometimes. The continuous presence of a support person during labor slightly decreases (by about 2%) the likelihood of a cesarean section (C-section) but only when companions can’t be present and epidurals aren’t routine (strength of recommendation [SOR]: A, a well-done systematic review of randomized controlled trials [RCTs]). When the support person was neither hospital staff nor a member of the woman’s social network, C-section was significantly less likely (SOR A, a well-done systematic review of RCTs).
EVIDENCE SUMMARY
A 2012 Cochrane review of 22 multinational RCTs with a total of 15,288 patients investigated the effect of continuous support in labor on several outcomes, including C-section.1 All trials included pregnant women in labor. The study populations were heterogenous in terms of parity; most included only nulliparous women, but some included multiparous women. At least one study incorporated higher-risk groups such as mothers of twins, but several trials limited the study group to low-risk pregnancies.
The review found a small but significant decrease in risk of C-section in women receiving continuous support (absolute risk reduction [ARR]=2%; number needed to treat [NNT]=50; P=.0017).1 The average cost of trained childbirth support in 3 US metropolitan areas in October 2014 was about $875, according to a Web search of established businesses.
The effect only works in the absence of companions and epidurals…
A subgroup analysis of 22 studies investigated several variables to determine circumstances under which a support person decreased the risk of C-section.1 The support person’s presence was significant only when hospital policy prevented companions (such as the woman’s spouse) in the labor room and when epidurals were not routinely available. Eleven of the 22 studies (11,326 patients) permitted a companion; 11 studies (3849 patients) didn’t.
When policy allowed companions, the presence of a support person didn’t decrease C-section rates significantly (12.7% without support compared with 11.9% with support; P=.20).1 When the woman wasn’t permitted to have a companion, however, the presence of a support person significantly decreased C-section (ARR=5.4%; NNT=19; P<.01).
In 14 studies, with a total of 13,064 patients, epidurals were routinely available. In the other 8, with 2077 patients, epidurals weren’t available.1 These were older studies or studies conducted in developing countries. When epidurals were routinely available, the presence of a support person didn’t affect the C-section rate (13.8% rate without support, 12.9% with support; P=.12). But if epidural anesthesia wasn’t available, a support person decreased C-section (ARR=8.6%; NNT=12; P<.00001).
…And when the support person isn’t a hospital staffer or known to the patient
The Cochrane Review also evaluated different types of labor supporters: companions of the patient’s choice from her social network, hospital employees, and people who were neither. The support person conferred significant benefit only when that person was neither hospital staff nor a member of the woman’s social network.
Hospital staff members who provided support didn’t effectively decrease the C-section rate (12% rate in control group vs 11.3% in supported group; P=.28). Support people chosen by the patient likewise didn’t successfully reduce C-sections (19.4% control rate vs 15.5% supported rate; P=.062). When the support person was neither hospital staff nor someone well-known to the patient, the risk of C-section was significantly lower (ARR=6%; NNT=17; P=.0003).
RECOMMENDATIONS
In a Comparative Effectiveness Review published in October 2012, the Agency for Healthcare Research and Quality investigated 18 strategies to reduce C-section, one of which was psychosocial support from doulas and other providers. A trained support person was the only intervention that showed evidence of benefit in decreasing C-section, but the strength of evidence was low.2
An American College of Obstetricians and Gynecologists Practice Bulletin recommends continuous labor support, noting “the continuous presence of a support person may reduce the likelihood of…operative delivery” with no apparent harmful effects.3
1. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2012;(10):CD003766.
2. Agency for Healthcare Research and Quality. Strategies to reduce cesarean birth in low-risk women. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/263/1291/CER80_C-Section_ExecutiveSummary_20121018.pdf. Accessed February 22, 2015.
3. American College of Obstetrics and Gynecology Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
1. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2012;(10):CD003766.
2. Agency for Healthcare Research and Quality. Strategies to reduce cesarean birth in low-risk women. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/263/1291/CER80_C-Section_ExecutiveSummary_20121018.pdf. Accessed February 22, 2015.
3. American College of Obstetrics and Gynecology Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
Evidence-based answers from the Family Physicians Inquiries Network
Is red-yeast rice a safe and effective alternative to statins?
Yes, but perhaps not the red-yeast rice extracts available in the United States.
In patients with known coronary artery disease and dyslipidemia (secondary prevention), therapy with red-yeast rice extract containing naturally-occurring lovastatin is associated with a 30% reduction in coronary heart disease (CHD) mortality and a 60% reduction in myocardial infarction (MI), similar to the effect of statin medications (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] in China).
In patients older than 65 years with hypertension and a previous MI, the rate of adverse effects from lovastatin-containing red-yeast rice is 2.1% (SOR: B, RCT in China).
In patients with previous statin intolerance, the rates of myalgias and treatment discontinuation with lovastatin-containing red-yeast rice therapy are similar to either placebo or another statin (SOR: C, low-powered RCTs).
The US Food and Drug Administration (FDA) doesn’t allow lovastatin-containing red-yeast rice products on the US market; physicians should be aware that products purchased by patients online contain variable amounts of lovastatin.
EVIDENCE SUMMARY
Red-yeast rice is a Chinese dietary and medicinal product of yeast (Monascus purpureus) grown on rice. It contains a wide range of biologically active compounds, including lovastatin (monacolin K). The FDA has banned the sale of red-yeast rice products with more than trace amounts of lovastatin.1
Red-yeast rice beats placebo, similar to statins
A systematic review of 22 RCTs (N=6520), primarily conducted in China using 600 to 2400 mg red-yeast rice extract daily (lovastatin content 5-20 mg), assessed outcomes in patients with known CHD and dyslipidemia.2 In one trial of 4870 patients, users of red-yeast rice had significant reductions in CHD mortality (relative risk [RR]=0.69; 95% confidence interval [CI], 0.54-0.89), incidence of MI (RR=0.39; 95% CI, 0.28-0.55), and revascularization (RR=0.67; 95% CI, 0.50-0.89) compared with placebo users.
However, when compared with statin therapy, red-yeast rice didn’t yield statistically significant differences in CHD mortality (2 trials, N=220; RR=0.26; 95% CI, 0.06-1.21), incidence of MI (1 trial, N=84; RR=0.95; 95% CI, 0.30-3.05) or revascularization (1 trial, N=84; RR=1.14; 95% CI, 0.38-3.46).
Red-yeast rice outperforms placebo in CHD and MI—but not stroke
A secondary analysis of an RCT evaluated the impact of red-yeast rice extract (600 mg twice a day) for 4.5 years on cardiovascular events and mortality in 1530 Chinese patients 60 years of age and older with hypertension and a previous MI.3 The lovastatin content of the red-yeast rice was 5 to 6.4 mg/d.
Compared with placebo, red-yeast rice was associated with a lower incidence of CHD events (RR=0.63; 95% CI, 0.36-0.83), nonfatal MI (RR=0.48; 95% CI, 0.37-0.71), and all-cause mortality (RR=0.65; 95% CI, 0.49-0.83) but not with a statistically significant difference in stroke (RR=0.63; 95% CI, 0.47-1.09) or cardiac revascularization (RR=0.68; 95% CI, 0.52-1.19).
Total adverse events in this study were similar for red-yeast rice and placebo (2.1% vs 1.2%, respectively; P>.05). They included gastrointestinal discomfort, allergic reactions, myalgias, edema, erectile dysfunction, and neuropsychological symptoms.
Red-yeast rice is similar to placebo or another statin in statin-induced myalgia
In a small community-based trial of 62 adults with dyslipidemia and a history of statin-induced myalgia, investigators randomized patients to receive either red-yeast rice extract at 1800 mg (with 3.1 mg lovastatin) or placebo twice daily for 24 weeks.4 Patients’ weekly self-reports of pain (on a 10-point scale) were skewed at baseline (1.4 in the red-yeast rice group vs 2.6 in the placebo group; P=.026) but similar at 12 weeks (1.4 with red-yeast rice vs 1.9 with placebo; P=.30) and 24 weeks (1.2 with red-yeast rice vs 2.0 with placebo; P=.120).
An RCT of 43 adults with dyslipidemia and history of statin intolerance compared red-yeast rice extract (2400 mg, with 10 mg lovastatin) with pravastatin (20 mg) dosed twice a day.5 At the end of 12 weeks, mean self-reported pain scores (on a 10-point scale) were similar (1.4 with red-yeast rice vs 1.1 with pravastatin; P=.82), as were discontinuation rates because of myalgia (5% with red-yeast rice vs 9% with pravastatin; P=.99).
RECOMMENDATIONS
A narrative review of alternative therapies for heart failure and hypercholesterolemia states that red yeast rice may be a cost-saving option for hypercholesterolemia in patients who can’t afford other medications (purchased mostly online, cost $8-$20/month for a dosage equivalent to lovastatin 20 mg/d).6
A ConsumerLab review of red yeast rice products available since the FDA ban in 2011 tested products marketed in the United States and found variable amounts of lovastatin.1,7 The group determined that labeling was a poor guide to lovastatin content, which ranged from 0 to 20 mg per daily dose, and that the products may not have been standardized. The group concluded that therapeutic effects weren’t predictable.
1. National Institutes of Health. Red yeast rice: An introduction. National Center for Complementary and Integrative Health Web site. Available at: http://nccam.nih.gov/health/redyeastrice. Accessed October 28, 2013.
2. Shang Q, Liu Z, Chen K, et al. A systematic review of xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid Based Complement Alternat Med. 2012;2012:636547.
3. Li JJ, Lu ZL, Kou WR, et al. Beneficial impact of xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J Clin Pharmacol. 2009;49:947-956.
4. Becker DJ, Gordon RY, Halbert SC, et al. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150:830-839,
W147-W149.
5. Halbert SC, French B, Gordon RY, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105:198-204.
6. Morelli V, Zoorob RJ. Alternative therapies: Part II. Congestive heart failure and hypercholesterolemia. Am Fam Physician. 2000;62:1325-1330.
7. Consumerlab.com. Product Review: Red yeast rice supplements review. ConsumerLab Web site. Available at: https://www.consumerlab.com/reviews/Red-Yeast-Rice-Supplements-Review/Red_Yeast_Rice. Accessed January 20, 2015.
Yes, but perhaps not the red-yeast rice extracts available in the United States.
In patients with known coronary artery disease and dyslipidemia (secondary prevention), therapy with red-yeast rice extract containing naturally-occurring lovastatin is associated with a 30% reduction in coronary heart disease (CHD) mortality and a 60% reduction in myocardial infarction (MI), similar to the effect of statin medications (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] in China).
In patients older than 65 years with hypertension and a previous MI, the rate of adverse effects from lovastatin-containing red-yeast rice is 2.1% (SOR: B, RCT in China).
In patients with previous statin intolerance, the rates of myalgias and treatment discontinuation with lovastatin-containing red-yeast rice therapy are similar to either placebo or another statin (SOR: C, low-powered RCTs).
The US Food and Drug Administration (FDA) doesn’t allow lovastatin-containing red-yeast rice products on the US market; physicians should be aware that products purchased by patients online contain variable amounts of lovastatin.
EVIDENCE SUMMARY
Red-yeast rice is a Chinese dietary and medicinal product of yeast (Monascus purpureus) grown on rice. It contains a wide range of biologically active compounds, including lovastatin (monacolin K). The FDA has banned the sale of red-yeast rice products with more than trace amounts of lovastatin.1
Red-yeast rice beats placebo, similar to statins
A systematic review of 22 RCTs (N=6520), primarily conducted in China using 600 to 2400 mg red-yeast rice extract daily (lovastatin content 5-20 mg), assessed outcomes in patients with known CHD and dyslipidemia.2 In one trial of 4870 patients, users of red-yeast rice had significant reductions in CHD mortality (relative risk [RR]=0.69; 95% confidence interval [CI], 0.54-0.89), incidence of MI (RR=0.39; 95% CI, 0.28-0.55), and revascularization (RR=0.67; 95% CI, 0.50-0.89) compared with placebo users.
However, when compared with statin therapy, red-yeast rice didn’t yield statistically significant differences in CHD mortality (2 trials, N=220; RR=0.26; 95% CI, 0.06-1.21), incidence of MI (1 trial, N=84; RR=0.95; 95% CI, 0.30-3.05) or revascularization (1 trial, N=84; RR=1.14; 95% CI, 0.38-3.46).
Red-yeast rice outperforms placebo in CHD and MI—but not stroke
A secondary analysis of an RCT evaluated the impact of red-yeast rice extract (600 mg twice a day) for 4.5 years on cardiovascular events and mortality in 1530 Chinese patients 60 years of age and older with hypertension and a previous MI.3 The lovastatin content of the red-yeast rice was 5 to 6.4 mg/d.
Compared with placebo, red-yeast rice was associated with a lower incidence of CHD events (RR=0.63; 95% CI, 0.36-0.83), nonfatal MI (RR=0.48; 95% CI, 0.37-0.71), and all-cause mortality (RR=0.65; 95% CI, 0.49-0.83) but not with a statistically significant difference in stroke (RR=0.63; 95% CI, 0.47-1.09) or cardiac revascularization (RR=0.68; 95% CI, 0.52-1.19).
Total adverse events in this study were similar for red-yeast rice and placebo (2.1% vs 1.2%, respectively; P>.05). They included gastrointestinal discomfort, allergic reactions, myalgias, edema, erectile dysfunction, and neuropsychological symptoms.
Red-yeast rice is similar to placebo or another statin in statin-induced myalgia
In a small community-based trial of 62 adults with dyslipidemia and a history of statin-induced myalgia, investigators randomized patients to receive either red-yeast rice extract at 1800 mg (with 3.1 mg lovastatin) or placebo twice daily for 24 weeks.4 Patients’ weekly self-reports of pain (on a 10-point scale) were skewed at baseline (1.4 in the red-yeast rice group vs 2.6 in the placebo group; P=.026) but similar at 12 weeks (1.4 with red-yeast rice vs 1.9 with placebo; P=.30) and 24 weeks (1.2 with red-yeast rice vs 2.0 with placebo; P=.120).
An RCT of 43 adults with dyslipidemia and history of statin intolerance compared red-yeast rice extract (2400 mg, with 10 mg lovastatin) with pravastatin (20 mg) dosed twice a day.5 At the end of 12 weeks, mean self-reported pain scores (on a 10-point scale) were similar (1.4 with red-yeast rice vs 1.1 with pravastatin; P=.82), as were discontinuation rates because of myalgia (5% with red-yeast rice vs 9% with pravastatin; P=.99).
RECOMMENDATIONS
A narrative review of alternative therapies for heart failure and hypercholesterolemia states that red yeast rice may be a cost-saving option for hypercholesterolemia in patients who can’t afford other medications (purchased mostly online, cost $8-$20/month for a dosage equivalent to lovastatin 20 mg/d).6
A ConsumerLab review of red yeast rice products available since the FDA ban in 2011 tested products marketed in the United States and found variable amounts of lovastatin.1,7 The group determined that labeling was a poor guide to lovastatin content, which ranged from 0 to 20 mg per daily dose, and that the products may not have been standardized. The group concluded that therapeutic effects weren’t predictable.
Yes, but perhaps not the red-yeast rice extracts available in the United States.
In patients with known coronary artery disease and dyslipidemia (secondary prevention), therapy with red-yeast rice extract containing naturally-occurring lovastatin is associated with a 30% reduction in coronary heart disease (CHD) mortality and a 60% reduction in myocardial infarction (MI), similar to the effect of statin medications (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] in China).
In patients older than 65 years with hypertension and a previous MI, the rate of adverse effects from lovastatin-containing red-yeast rice is 2.1% (SOR: B, RCT in China).
In patients with previous statin intolerance, the rates of myalgias and treatment discontinuation with lovastatin-containing red-yeast rice therapy are similar to either placebo or another statin (SOR: C, low-powered RCTs).
The US Food and Drug Administration (FDA) doesn’t allow lovastatin-containing red-yeast rice products on the US market; physicians should be aware that products purchased by patients online contain variable amounts of lovastatin.
EVIDENCE SUMMARY
Red-yeast rice is a Chinese dietary and medicinal product of yeast (Monascus purpureus) grown on rice. It contains a wide range of biologically active compounds, including lovastatin (monacolin K). The FDA has banned the sale of red-yeast rice products with more than trace amounts of lovastatin.1
Red-yeast rice beats placebo, similar to statins
A systematic review of 22 RCTs (N=6520), primarily conducted in China using 600 to 2400 mg red-yeast rice extract daily (lovastatin content 5-20 mg), assessed outcomes in patients with known CHD and dyslipidemia.2 In one trial of 4870 patients, users of red-yeast rice had significant reductions in CHD mortality (relative risk [RR]=0.69; 95% confidence interval [CI], 0.54-0.89), incidence of MI (RR=0.39; 95% CI, 0.28-0.55), and revascularization (RR=0.67; 95% CI, 0.50-0.89) compared with placebo users.
However, when compared with statin therapy, red-yeast rice didn’t yield statistically significant differences in CHD mortality (2 trials, N=220; RR=0.26; 95% CI, 0.06-1.21), incidence of MI (1 trial, N=84; RR=0.95; 95% CI, 0.30-3.05) or revascularization (1 trial, N=84; RR=1.14; 95% CI, 0.38-3.46).
Red-yeast rice outperforms placebo in CHD and MI—but not stroke
A secondary analysis of an RCT evaluated the impact of red-yeast rice extract (600 mg twice a day) for 4.5 years on cardiovascular events and mortality in 1530 Chinese patients 60 years of age and older with hypertension and a previous MI.3 The lovastatin content of the red-yeast rice was 5 to 6.4 mg/d.
Compared with placebo, red-yeast rice was associated with a lower incidence of CHD events (RR=0.63; 95% CI, 0.36-0.83), nonfatal MI (RR=0.48; 95% CI, 0.37-0.71), and all-cause mortality (RR=0.65; 95% CI, 0.49-0.83) but not with a statistically significant difference in stroke (RR=0.63; 95% CI, 0.47-1.09) or cardiac revascularization (RR=0.68; 95% CI, 0.52-1.19).
Total adverse events in this study were similar for red-yeast rice and placebo (2.1% vs 1.2%, respectively; P>.05). They included gastrointestinal discomfort, allergic reactions, myalgias, edema, erectile dysfunction, and neuropsychological symptoms.
Red-yeast rice is similar to placebo or another statin in statin-induced myalgia
In a small community-based trial of 62 adults with dyslipidemia and a history of statin-induced myalgia, investigators randomized patients to receive either red-yeast rice extract at 1800 mg (with 3.1 mg lovastatin) or placebo twice daily for 24 weeks.4 Patients’ weekly self-reports of pain (on a 10-point scale) were skewed at baseline (1.4 in the red-yeast rice group vs 2.6 in the placebo group; P=.026) but similar at 12 weeks (1.4 with red-yeast rice vs 1.9 with placebo; P=.30) and 24 weeks (1.2 with red-yeast rice vs 2.0 with placebo; P=.120).
An RCT of 43 adults with dyslipidemia and history of statin intolerance compared red-yeast rice extract (2400 mg, with 10 mg lovastatin) with pravastatin (20 mg) dosed twice a day.5 At the end of 12 weeks, mean self-reported pain scores (on a 10-point scale) were similar (1.4 with red-yeast rice vs 1.1 with pravastatin; P=.82), as were discontinuation rates because of myalgia (5% with red-yeast rice vs 9% with pravastatin; P=.99).
RECOMMENDATIONS
A narrative review of alternative therapies for heart failure and hypercholesterolemia states that red yeast rice may be a cost-saving option for hypercholesterolemia in patients who can’t afford other medications (purchased mostly online, cost $8-$20/month for a dosage equivalent to lovastatin 20 mg/d).6
A ConsumerLab review of red yeast rice products available since the FDA ban in 2011 tested products marketed in the United States and found variable amounts of lovastatin.1,7 The group determined that labeling was a poor guide to lovastatin content, which ranged from 0 to 20 mg per daily dose, and that the products may not have been standardized. The group concluded that therapeutic effects weren’t predictable.
1. National Institutes of Health. Red yeast rice: An introduction. National Center for Complementary and Integrative Health Web site. Available at: http://nccam.nih.gov/health/redyeastrice. Accessed October 28, 2013.
2. Shang Q, Liu Z, Chen K, et al. A systematic review of xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid Based Complement Alternat Med. 2012;2012:636547.
3. Li JJ, Lu ZL, Kou WR, et al. Beneficial impact of xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J Clin Pharmacol. 2009;49:947-956.
4. Becker DJ, Gordon RY, Halbert SC, et al. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150:830-839,
W147-W149.
5. Halbert SC, French B, Gordon RY, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105:198-204.
6. Morelli V, Zoorob RJ. Alternative therapies: Part II. Congestive heart failure and hypercholesterolemia. Am Fam Physician. 2000;62:1325-1330.
7. Consumerlab.com. Product Review: Red yeast rice supplements review. ConsumerLab Web site. Available at: https://www.consumerlab.com/reviews/Red-Yeast-Rice-Supplements-Review/Red_Yeast_Rice. Accessed January 20, 2015.
1. National Institutes of Health. Red yeast rice: An introduction. National Center for Complementary and Integrative Health Web site. Available at: http://nccam.nih.gov/health/redyeastrice. Accessed October 28, 2013.
2. Shang Q, Liu Z, Chen K, et al. A systematic review of xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid Based Complement Alternat Med. 2012;2012:636547.
3. Li JJ, Lu ZL, Kou WR, et al. Beneficial impact of xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J Clin Pharmacol. 2009;49:947-956.
4. Becker DJ, Gordon RY, Halbert SC, et al. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150:830-839,
W147-W149.
5. Halbert SC, French B, Gordon RY, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105:198-204.
6. Morelli V, Zoorob RJ. Alternative therapies: Part II. Congestive heart failure and hypercholesterolemia. Am Fam Physician. 2000;62:1325-1330.
7. Consumerlab.com. Product Review: Red yeast rice supplements review. ConsumerLab Web site. Available at: https://www.consumerlab.com/reviews/Red-Yeast-Rice-Supplements-Review/Red_Yeast_Rice. Accessed January 20, 2015.
Evidence-based answers from the Family Physicians Inquiries Network
Does frenotomy help infants with tongue-tie overcome breastfeeding difficulties?
Probably not. No evidence exists for improved latching after frenotomy, and evidence concerning improvements in maternal comfort is conflicting. At best, frenotomy improves maternal nipple pain by 10% and maternal subjective sense of improvement over the short term (0 to 2 weeks) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results for maternal nipple pain and overall feeding).
No studies have evaluated outcomes such as infant weight gain following frenotomy.
Experts don’t recommend frenotomy unless a clear association exists between ankyloglossia (tongue-tie) and breastfeeding problems. Frenotomy should be performed with anesthesia by an experienced clinician to minimize the risk of complications (SOR: C, a practice guideline.)
EVIDENCE SUMMARY
Two RCTs found short-term (0-14 days) improvement in breastfeeding after frenotomy. One, which evaluated the effect of frenotomy on infants with significant ankyloglossia and breastfeeding difficulties, found short-term improvement in maternal nipple pain. Investigators randomized 58 infants (mean age 6 days) with ankyloglossia (rated 8 out of 10 on a standardized severity scale) to receive either frenotomy or no intervention.1 They used the 50-point Short Form McGill Pain Questionnaire to measure maternal nipple pain at baseline, immediately after, and at 2, 4, 8, and 52 weeks.
Mothers in the intervention group reported a 10% greater reduction in nipple pain after frenotomy compared with the control group (11 points vs 6 points; P=.001). The improvement persisted at 2 weeks (graphic representation in study, P value not supplied) but not at 4 weeks or beyond.
An earlier, unblinded RCT randomized 40 infants (mean age 14 days) with ankyloglossia and breastfeeding problems to frenotomy or lactation support.2 It found maternal subjective ratings of “improvement” (not quantified) by telephone interview at 24 hours (85% vs 3%; P<.01). Investigators performed frenotomy on all 19 of the unimproved control infants at 48 hours.
Frenotomy doesn’t improve breastfeeding overall
Two newer RCTs evaluating frenotomy and LATCH (Latch, Audible swallowing, nipple Type, Comfort, and Hold) scores, which include a component measuring maternal comfort, found no breastfeeding improvements. (LATCH is a validated 10-point score with moderate predictive value for identifying mothers at risk for early weaning because of sore nipples.3)
A double-blind RCT that assessed frenotomy in 57 infants (mean age 32 days) with ankyloglossia and breastfeeding problems (severity of both unspecified) found no improvement in breastfeeding overall or nipple pain.4 Investigators randomized infants to frenotomy or sham frenotomy and used independent observers to measure outcomes with the LATCH score and the Infant Breastfeeding Assessment Tool (IBFAT), a standardized method of assessing overall feeding.
They observed no significant differences in LATCH or IBFAT scores between groups. More mothers in the frenotomy group reported improved breastfeeding, but most were able to determine whether their baby had undergone frenotomy.
A single-blinded, RCT of early frenotomy in 107 younger infants with breastfeeding difficulties and mild to moderate ankyloglossia also found no improvement in LATCH scores.5 Researchers randomized infants younger than 2 weeks (blinded to researchers and unblinded to mothers) to either immediate frenotomy or standard care. They measured LATCH scores at baseline and after 5 days (by intention to treat).
Investigators found no difference in LATCH scores at 5 days postfrenotomy (pretreatment score 6.4 ± 2.3, posttreatment 6.8 ± 2.0; not significant).
RECOMMENDATIONS
A 2011 position statement from the Community Paediatrics Committee of the Canadian Paediatric Society notes that ankyloglossia is a relatively uncommon congenital anomaly, and associations between ankyloglossia and breastfeeding problems in infants have been inconsistent.6 For these reasons, the Committee doesn’t recommend frenotomy.
However, if the clinician deems surgical intervention necessary based on a clear association between significant tongue-tie and major breastfeeding problems, then frenotomy should be performed by a clinician experienced in the procedure and with appropriate analgesia. The Committee states that although ankyloglossia release appears to be a minor procedure, it may cause complications such as bleeding, infection, or injury to the Wharton’s duct.
The Academy of Breastfeeding Medicine, a worldwide organization of physicians dedicated to the promotion, protection, and support of breastfeeding and human lactation, is currently revising its guidelines on neonatal ankyloglossia.7
1. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
2. Hogan M, Westcott C, Griffiths M. Randomized, controlled trial of division of tongue-tie in infants with feeding problems. Paediatr Child Health. 2005;41:246-250.
3. Emond A, Ingram J, Johnson D, et al. Randomised controlled trial of early frenotomy in breastfed infants with mild-moderate tongue-tie. Arch Dis Child Fetal Neonatal Ed. 2014;99:F189-F195.
4. Berry J, Griffiths M, Westcott C. A double-blind, randomized, controlled trial of tongue-tie division and its immediate effect on breastfeeding. Breastfeed Med. 2012;7:189-193.
5. Riordan J, Bibb D, Miller M, et al. Predicting breastfeeding duration using the LATCH breastfeeding assessment tool. J Hum Lact. 2001;17:20-23.
6. Rowan-Legg A. Ankyloglossia and breastfeeding. Paediatr Child Health. 2011;16:222.
7. Academy of Breastfeeding Medicine. Statements. Academy of Breastfeeding Medicine Web site. Available at: www.bfmed.org/Resources/Protocols.aspx. Accessed January 10, 2015.
Probably not. No evidence exists for improved latching after frenotomy, and evidence concerning improvements in maternal comfort is conflicting. At best, frenotomy improves maternal nipple pain by 10% and maternal subjective sense of improvement over the short term (0 to 2 weeks) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results for maternal nipple pain and overall feeding).
No studies have evaluated outcomes such as infant weight gain following frenotomy.
Experts don’t recommend frenotomy unless a clear association exists between ankyloglossia (tongue-tie) and breastfeeding problems. Frenotomy should be performed with anesthesia by an experienced clinician to minimize the risk of complications (SOR: C, a practice guideline.)
EVIDENCE SUMMARY
Two RCTs found short-term (0-14 days) improvement in breastfeeding after frenotomy. One, which evaluated the effect of frenotomy on infants with significant ankyloglossia and breastfeeding difficulties, found short-term improvement in maternal nipple pain. Investigators randomized 58 infants (mean age 6 days) with ankyloglossia (rated 8 out of 10 on a standardized severity scale) to receive either frenotomy or no intervention.1 They used the 50-point Short Form McGill Pain Questionnaire to measure maternal nipple pain at baseline, immediately after, and at 2, 4, 8, and 52 weeks.
Mothers in the intervention group reported a 10% greater reduction in nipple pain after frenotomy compared with the control group (11 points vs 6 points; P=.001). The improvement persisted at 2 weeks (graphic representation in study, P value not supplied) but not at 4 weeks or beyond.
An earlier, unblinded RCT randomized 40 infants (mean age 14 days) with ankyloglossia and breastfeeding problems to frenotomy or lactation support.2 It found maternal subjective ratings of “improvement” (not quantified) by telephone interview at 24 hours (85% vs 3%; P<.01). Investigators performed frenotomy on all 19 of the unimproved control infants at 48 hours.
Frenotomy doesn’t improve breastfeeding overall
Two newer RCTs evaluating frenotomy and LATCH (Latch, Audible swallowing, nipple Type, Comfort, and Hold) scores, which include a component measuring maternal comfort, found no breastfeeding improvements. (LATCH is a validated 10-point score with moderate predictive value for identifying mothers at risk for early weaning because of sore nipples.3)
A double-blind RCT that assessed frenotomy in 57 infants (mean age 32 days) with ankyloglossia and breastfeeding problems (severity of both unspecified) found no improvement in breastfeeding overall or nipple pain.4 Investigators randomized infants to frenotomy or sham frenotomy and used independent observers to measure outcomes with the LATCH score and the Infant Breastfeeding Assessment Tool (IBFAT), a standardized method of assessing overall feeding.
They observed no significant differences in LATCH or IBFAT scores between groups. More mothers in the frenotomy group reported improved breastfeeding, but most were able to determine whether their baby had undergone frenotomy.
A single-blinded, RCT of early frenotomy in 107 younger infants with breastfeeding difficulties and mild to moderate ankyloglossia also found no improvement in LATCH scores.5 Researchers randomized infants younger than 2 weeks (blinded to researchers and unblinded to mothers) to either immediate frenotomy or standard care. They measured LATCH scores at baseline and after 5 days (by intention to treat).
Investigators found no difference in LATCH scores at 5 days postfrenotomy (pretreatment score 6.4 ± 2.3, posttreatment 6.8 ± 2.0; not significant).
RECOMMENDATIONS
A 2011 position statement from the Community Paediatrics Committee of the Canadian Paediatric Society notes that ankyloglossia is a relatively uncommon congenital anomaly, and associations between ankyloglossia and breastfeeding problems in infants have been inconsistent.6 For these reasons, the Committee doesn’t recommend frenotomy.
However, if the clinician deems surgical intervention necessary based on a clear association between significant tongue-tie and major breastfeeding problems, then frenotomy should be performed by a clinician experienced in the procedure and with appropriate analgesia. The Committee states that although ankyloglossia release appears to be a minor procedure, it may cause complications such as bleeding, infection, or injury to the Wharton’s duct.
The Academy of Breastfeeding Medicine, a worldwide organization of physicians dedicated to the promotion, protection, and support of breastfeeding and human lactation, is currently revising its guidelines on neonatal ankyloglossia.7
Probably not. No evidence exists for improved latching after frenotomy, and evidence concerning improvements in maternal comfort is conflicting. At best, frenotomy improves maternal nipple pain by 10% and maternal subjective sense of improvement over the short term (0 to 2 weeks) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results for maternal nipple pain and overall feeding).
No studies have evaluated outcomes such as infant weight gain following frenotomy.
Experts don’t recommend frenotomy unless a clear association exists between ankyloglossia (tongue-tie) and breastfeeding problems. Frenotomy should be performed with anesthesia by an experienced clinician to minimize the risk of complications (SOR: C, a practice guideline.)
EVIDENCE SUMMARY
Two RCTs found short-term (0-14 days) improvement in breastfeeding after frenotomy. One, which evaluated the effect of frenotomy on infants with significant ankyloglossia and breastfeeding difficulties, found short-term improvement in maternal nipple pain. Investigators randomized 58 infants (mean age 6 days) with ankyloglossia (rated 8 out of 10 on a standardized severity scale) to receive either frenotomy or no intervention.1 They used the 50-point Short Form McGill Pain Questionnaire to measure maternal nipple pain at baseline, immediately after, and at 2, 4, 8, and 52 weeks.
Mothers in the intervention group reported a 10% greater reduction in nipple pain after frenotomy compared with the control group (11 points vs 6 points; P=.001). The improvement persisted at 2 weeks (graphic representation in study, P value not supplied) but not at 4 weeks or beyond.
An earlier, unblinded RCT randomized 40 infants (mean age 14 days) with ankyloglossia and breastfeeding problems to frenotomy or lactation support.2 It found maternal subjective ratings of “improvement” (not quantified) by telephone interview at 24 hours (85% vs 3%; P<.01). Investigators performed frenotomy on all 19 of the unimproved control infants at 48 hours.
Frenotomy doesn’t improve breastfeeding overall
Two newer RCTs evaluating frenotomy and LATCH (Latch, Audible swallowing, nipple Type, Comfort, and Hold) scores, which include a component measuring maternal comfort, found no breastfeeding improvements. (LATCH is a validated 10-point score with moderate predictive value for identifying mothers at risk for early weaning because of sore nipples.3)
A double-blind RCT that assessed frenotomy in 57 infants (mean age 32 days) with ankyloglossia and breastfeeding problems (severity of both unspecified) found no improvement in breastfeeding overall or nipple pain.4 Investigators randomized infants to frenotomy or sham frenotomy and used independent observers to measure outcomes with the LATCH score and the Infant Breastfeeding Assessment Tool (IBFAT), a standardized method of assessing overall feeding.
They observed no significant differences in LATCH or IBFAT scores between groups. More mothers in the frenotomy group reported improved breastfeeding, but most were able to determine whether their baby had undergone frenotomy.
A single-blinded, RCT of early frenotomy in 107 younger infants with breastfeeding difficulties and mild to moderate ankyloglossia also found no improvement in LATCH scores.5 Researchers randomized infants younger than 2 weeks (blinded to researchers and unblinded to mothers) to either immediate frenotomy or standard care. They measured LATCH scores at baseline and after 5 days (by intention to treat).
Investigators found no difference in LATCH scores at 5 days postfrenotomy (pretreatment score 6.4 ± 2.3, posttreatment 6.8 ± 2.0; not significant).
RECOMMENDATIONS
A 2011 position statement from the Community Paediatrics Committee of the Canadian Paediatric Society notes that ankyloglossia is a relatively uncommon congenital anomaly, and associations between ankyloglossia and breastfeeding problems in infants have been inconsistent.6 For these reasons, the Committee doesn’t recommend frenotomy.
However, if the clinician deems surgical intervention necessary based on a clear association between significant tongue-tie and major breastfeeding problems, then frenotomy should be performed by a clinician experienced in the procedure and with appropriate analgesia. The Committee states that although ankyloglossia release appears to be a minor procedure, it may cause complications such as bleeding, infection, or injury to the Wharton’s duct.
The Academy of Breastfeeding Medicine, a worldwide organization of physicians dedicated to the promotion, protection, and support of breastfeeding and human lactation, is currently revising its guidelines on neonatal ankyloglossia.7
1. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
2. Hogan M, Westcott C, Griffiths M. Randomized, controlled trial of division of tongue-tie in infants with feeding problems. Paediatr Child Health. 2005;41:246-250.
3. Emond A, Ingram J, Johnson D, et al. Randomised controlled trial of early frenotomy in breastfed infants with mild-moderate tongue-tie. Arch Dis Child Fetal Neonatal Ed. 2014;99:F189-F195.
4. Berry J, Griffiths M, Westcott C. A double-blind, randomized, controlled trial of tongue-tie division and its immediate effect on breastfeeding. Breastfeed Med. 2012;7:189-193.
5. Riordan J, Bibb D, Miller M, et al. Predicting breastfeeding duration using the LATCH breastfeeding assessment tool. J Hum Lact. 2001;17:20-23.
6. Rowan-Legg A. Ankyloglossia and breastfeeding. Paediatr Child Health. 2011;16:222.
7. Academy of Breastfeeding Medicine. Statements. Academy of Breastfeeding Medicine Web site. Available at: www.bfmed.org/Resources/Protocols.aspx. Accessed January 10, 2015.
1. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
2. Hogan M, Westcott C, Griffiths M. Randomized, controlled trial of division of tongue-tie in infants with feeding problems. Paediatr Child Health. 2005;41:246-250.
3. Emond A, Ingram J, Johnson D, et al. Randomised controlled trial of early frenotomy in breastfed infants with mild-moderate tongue-tie. Arch Dis Child Fetal Neonatal Ed. 2014;99:F189-F195.
4. Berry J, Griffiths M, Westcott C. A double-blind, randomized, controlled trial of tongue-tie division and its immediate effect on breastfeeding. Breastfeed Med. 2012;7:189-193.
5. Riordan J, Bibb D, Miller M, et al. Predicting breastfeeding duration using the LATCH breastfeeding assessment tool. J Hum Lact. 2001;17:20-23.
6. Rowan-Legg A. Ankyloglossia and breastfeeding. Paediatr Child Health. 2011;16:222.
7. Academy of Breastfeeding Medicine. Statements. Academy of Breastfeeding Medicine Web site. Available at: www.bfmed.org/Resources/Protocols.aspx. Accessed January 10, 2015.
Evidence-based answers from the Family Physicians Inquiries Network
Does topical diclofenac relieve osteoarthritis pain?
Yes, at least in the short term. Topical diclofenac, with and without dimethyl sulfoxide (DMSO), modestly improves pain and function scores (by 4%-8%) for as long as 12 weeks in patients with osteoarthritis (OA) of the knee (strength of recommendation [SOR]: A, meta-analyses of multiple randomized controlled trials [RCTs]).
Topical diclofenac modestly decreases pain scores in patients with OA of the hand in the short term (by 9% at 6 weeks) but no more than placebo at 8 weeks (SOR: B, RCT).
Both topical diclofenac with DMSO and oral diclofenac produce similar pain and function scores in patients with OA of the knee. In addition to minor skin dryness, topical diclofenac causes gastrointestinal (GI) adverse effects in about a third of patients (SOR: B, RCT).
EVIDENCE SUMMARY
Diclofenac gel ($260-$330 per 150-mL bottle) and diclofenac with DMSO solution are the only topical nonsteroidal anti-inflammatory drugs (NSAIDs) available in the United States.
Topical diclofenac with DMSO beats placebo
In a meta-analysis of 3 RCTs (697 patients, mean age 63.2, 37% male) with knee OA, topical diclofenac solution with DMSO (Pennsaid, 40 drops applied 4 times daily) demonstrated superiority to vehicle-controlled placebo at 4 to 12 weeks (mean 8.5 weeks) using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index.1 The WOMAC is a standardized patient questionnaire measuring 5 items for pain (score range 0-20), 2 for stiffness (score range 0-8), and 17 for functional limitation (score range 0-68).
Compared with placebo, topical diclofenac with DMSO resulted in 1.6 units greater reduction in pain (8% difference), 0.6 units greater reduction in stiffness (7.5% difference), and 5.5 units greater improvement in physical function (8% difference). Patients using diclofenac reported more minor skin dryness than patients using placebo (number needed to harm [NNH]=6).
Diclofenac gel is also effective, but may cause dermatitis
A pooled analysis of 3 12-week randomized, double-blind, parallel-group, multicenter trials of 1426 patients with OA of the knee compared topical diclofenac gel (4 g applied 4 times a day) with vehicle placebo for patients older than 25 years and patients older than 65 years.2 Investigators evaluated 972 patients who suffered a symptom flare at 1, 4, 8, and 12 weeks after a one-week washout period.
Diclofenac demonstrated statistical superiority across all age groups when compared with placebo, based on pain and physical function measured on the WOMAC Index. Its effects were modest, however.
At 12 weeks, patients younger than 65 years showed pain improvement of -5.8 vs -4.7 for placebo (a 5.5% improvement on the 20-point scale) and improvement in physical function of -17.9 vs -14.2 (a 5.4% improvement on the 68-point scale). Patients older than 65 years demonstrated pain improvement of -5.3 vs -4.1 for placebo (6% improvement on the 20-point scale) and physical function improvement of -15.5 vs -11.0 for placebo (6.6% improvement on the 68-point scale).
Dermatitis was more common in the diclofenac groups, with a NNH of 30 in patients younger than 65 years and 19 in patients older than 65 years.
Diclofenac gel effectively treated hand OA for as long as 6 weeks in a randomized, double-blind, placebo-controlled trial of 809 men and women older than 39 years. Pain scores on a 100-point visual analog scale improved when compared with placebo alone.3 At 6 weeks, topical diclofenac reduced pain scores by 45% compared with 36% for placebo (P=.023). Pain reductions also were greater in the diclofenac group at 8 weeks, although not statistically different.
Oral diclofenac works well, too, but has more GI adverse effects
An RCT of 622 patients (40-85 years of age) with symptomatic and radiographically diagnosed OA of the knee compared topical diclofenac solution (75 mg/d) with oral diclofenac (50 mg 3 times a day) and found similar efficacy at 12 weeks, with no significant difference between oral and topical preparations for pain, physical function, and stiffness measured with the WOMAC Index (P=.23, .06, .24, respectively).4 Oral diclofenac produced more adverse GI side effects than the topical solution (48% vs 35%; P=.0006).
RECOMMENDATIONS
The Agency for Healthcare Research and Quality states that topical and oral NSAIDs reduce knee OA pain equally.5
The Guidelines of the American Academy of Orthopaedic Surgeons, American College of Rheumatology, European League Against Rheumatism, Osteoarthritis Research Society International, and National Institute for Health and Care Excellence all state that clinicians may consider topical NSAIDs for patients with mild to moderate OA of the knee or hand, particularly in patients with few affected joints or a history of sensitivity to oral NSAIDs.6
1. Towheed TE. Pennsaid therapy for osteoarthritis of the knee: a systematic review and meta-analysis of randomized controlled trials. J Rheumatol. 2006;33:567-573.
2. Baraf HS, Gloth FM, Barthel HR, et al. Safety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trials. Drugs Aging. 2011;28:27-40.
3. Altman RD, Dreiser RL, Fisher CL, et al. Diclofenac sodium gel in patients with primary hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:1991-1999.
4. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (Pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
5. Chou R, McDonagh MS, Nakamoto E, et al. Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review. Rockville, MD: Agency for Healthcare Research and Quality; 2011. AHRQ Publication No. 11(12)-EHC076-EF.
6. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis. part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476-499
Yes, at least in the short term. Topical diclofenac, with and without dimethyl sulfoxide (DMSO), modestly improves pain and function scores (by 4%-8%) for as long as 12 weeks in patients with osteoarthritis (OA) of the knee (strength of recommendation [SOR]: A, meta-analyses of multiple randomized controlled trials [RCTs]).
Topical diclofenac modestly decreases pain scores in patients with OA of the hand in the short term (by 9% at 6 weeks) but no more than placebo at 8 weeks (SOR: B, RCT).
Both topical diclofenac with DMSO and oral diclofenac produce similar pain and function scores in patients with OA of the knee. In addition to minor skin dryness, topical diclofenac causes gastrointestinal (GI) adverse effects in about a third of patients (SOR: B, RCT).
EVIDENCE SUMMARY
Diclofenac gel ($260-$330 per 150-mL bottle) and diclofenac with DMSO solution are the only topical nonsteroidal anti-inflammatory drugs (NSAIDs) available in the United States.
Topical diclofenac with DMSO beats placebo
In a meta-analysis of 3 RCTs (697 patients, mean age 63.2, 37% male) with knee OA, topical diclofenac solution with DMSO (Pennsaid, 40 drops applied 4 times daily) demonstrated superiority to vehicle-controlled placebo at 4 to 12 weeks (mean 8.5 weeks) using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index.1 The WOMAC is a standardized patient questionnaire measuring 5 items for pain (score range 0-20), 2 for stiffness (score range 0-8), and 17 for functional limitation (score range 0-68).
Compared with placebo, topical diclofenac with DMSO resulted in 1.6 units greater reduction in pain (8% difference), 0.6 units greater reduction in stiffness (7.5% difference), and 5.5 units greater improvement in physical function (8% difference). Patients using diclofenac reported more minor skin dryness than patients using placebo (number needed to harm [NNH]=6).
Diclofenac gel is also effective, but may cause dermatitis
A pooled analysis of 3 12-week randomized, double-blind, parallel-group, multicenter trials of 1426 patients with OA of the knee compared topical diclofenac gel (4 g applied 4 times a day) with vehicle placebo for patients older than 25 years and patients older than 65 years.2 Investigators evaluated 972 patients who suffered a symptom flare at 1, 4, 8, and 12 weeks after a one-week washout period.
Diclofenac demonstrated statistical superiority across all age groups when compared with placebo, based on pain and physical function measured on the WOMAC Index. Its effects were modest, however.
At 12 weeks, patients younger than 65 years showed pain improvement of -5.8 vs -4.7 for placebo (a 5.5% improvement on the 20-point scale) and improvement in physical function of -17.9 vs -14.2 (a 5.4% improvement on the 68-point scale). Patients older than 65 years demonstrated pain improvement of -5.3 vs -4.1 for placebo (6% improvement on the 20-point scale) and physical function improvement of -15.5 vs -11.0 for placebo (6.6% improvement on the 68-point scale).
Dermatitis was more common in the diclofenac groups, with a NNH of 30 in patients younger than 65 years and 19 in patients older than 65 years.
Diclofenac gel effectively treated hand OA for as long as 6 weeks in a randomized, double-blind, placebo-controlled trial of 809 men and women older than 39 years. Pain scores on a 100-point visual analog scale improved when compared with placebo alone.3 At 6 weeks, topical diclofenac reduced pain scores by 45% compared with 36% for placebo (P=.023). Pain reductions also were greater in the diclofenac group at 8 weeks, although not statistically different.
Oral diclofenac works well, too, but has more GI adverse effects
An RCT of 622 patients (40-85 years of age) with symptomatic and radiographically diagnosed OA of the knee compared topical diclofenac solution (75 mg/d) with oral diclofenac (50 mg 3 times a day) and found similar efficacy at 12 weeks, with no significant difference between oral and topical preparations for pain, physical function, and stiffness measured with the WOMAC Index (P=.23, .06, .24, respectively).4 Oral diclofenac produced more adverse GI side effects than the topical solution (48% vs 35%; P=.0006).
RECOMMENDATIONS
The Agency for Healthcare Research and Quality states that topical and oral NSAIDs reduce knee OA pain equally.5
The Guidelines of the American Academy of Orthopaedic Surgeons, American College of Rheumatology, European League Against Rheumatism, Osteoarthritis Research Society International, and National Institute for Health and Care Excellence all state that clinicians may consider topical NSAIDs for patients with mild to moderate OA of the knee or hand, particularly in patients with few affected joints or a history of sensitivity to oral NSAIDs.6
Yes, at least in the short term. Topical diclofenac, with and without dimethyl sulfoxide (DMSO), modestly improves pain and function scores (by 4%-8%) for as long as 12 weeks in patients with osteoarthritis (OA) of the knee (strength of recommendation [SOR]: A, meta-analyses of multiple randomized controlled trials [RCTs]).
Topical diclofenac modestly decreases pain scores in patients with OA of the hand in the short term (by 9% at 6 weeks) but no more than placebo at 8 weeks (SOR: B, RCT).
Both topical diclofenac with DMSO and oral diclofenac produce similar pain and function scores in patients with OA of the knee. In addition to minor skin dryness, topical diclofenac causes gastrointestinal (GI) adverse effects in about a third of patients (SOR: B, RCT).
EVIDENCE SUMMARY
Diclofenac gel ($260-$330 per 150-mL bottle) and diclofenac with DMSO solution are the only topical nonsteroidal anti-inflammatory drugs (NSAIDs) available in the United States.
Topical diclofenac with DMSO beats placebo
In a meta-analysis of 3 RCTs (697 patients, mean age 63.2, 37% male) with knee OA, topical diclofenac solution with DMSO (Pennsaid, 40 drops applied 4 times daily) demonstrated superiority to vehicle-controlled placebo at 4 to 12 weeks (mean 8.5 weeks) using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index.1 The WOMAC is a standardized patient questionnaire measuring 5 items for pain (score range 0-20), 2 for stiffness (score range 0-8), and 17 for functional limitation (score range 0-68).
Compared with placebo, topical diclofenac with DMSO resulted in 1.6 units greater reduction in pain (8% difference), 0.6 units greater reduction in stiffness (7.5% difference), and 5.5 units greater improvement in physical function (8% difference). Patients using diclofenac reported more minor skin dryness than patients using placebo (number needed to harm [NNH]=6).
Diclofenac gel is also effective, but may cause dermatitis
A pooled analysis of 3 12-week randomized, double-blind, parallel-group, multicenter trials of 1426 patients with OA of the knee compared topical diclofenac gel (4 g applied 4 times a day) with vehicle placebo for patients older than 25 years and patients older than 65 years.2 Investigators evaluated 972 patients who suffered a symptom flare at 1, 4, 8, and 12 weeks after a one-week washout period.
Diclofenac demonstrated statistical superiority across all age groups when compared with placebo, based on pain and physical function measured on the WOMAC Index. Its effects were modest, however.
At 12 weeks, patients younger than 65 years showed pain improvement of -5.8 vs -4.7 for placebo (a 5.5% improvement on the 20-point scale) and improvement in physical function of -17.9 vs -14.2 (a 5.4% improvement on the 68-point scale). Patients older than 65 years demonstrated pain improvement of -5.3 vs -4.1 for placebo (6% improvement on the 20-point scale) and physical function improvement of -15.5 vs -11.0 for placebo (6.6% improvement on the 68-point scale).
Dermatitis was more common in the diclofenac groups, with a NNH of 30 in patients younger than 65 years and 19 in patients older than 65 years.
Diclofenac gel effectively treated hand OA for as long as 6 weeks in a randomized, double-blind, placebo-controlled trial of 809 men and women older than 39 years. Pain scores on a 100-point visual analog scale improved when compared with placebo alone.3 At 6 weeks, topical diclofenac reduced pain scores by 45% compared with 36% for placebo (P=.023). Pain reductions also were greater in the diclofenac group at 8 weeks, although not statistically different.
Oral diclofenac works well, too, but has more GI adverse effects
An RCT of 622 patients (40-85 years of age) with symptomatic and radiographically diagnosed OA of the knee compared topical diclofenac solution (75 mg/d) with oral diclofenac (50 mg 3 times a day) and found similar efficacy at 12 weeks, with no significant difference between oral and topical preparations for pain, physical function, and stiffness measured with the WOMAC Index (P=.23, .06, .24, respectively).4 Oral diclofenac produced more adverse GI side effects than the topical solution (48% vs 35%; P=.0006).
RECOMMENDATIONS
The Agency for Healthcare Research and Quality states that topical and oral NSAIDs reduce knee OA pain equally.5
The Guidelines of the American Academy of Orthopaedic Surgeons, American College of Rheumatology, European League Against Rheumatism, Osteoarthritis Research Society International, and National Institute for Health and Care Excellence all state that clinicians may consider topical NSAIDs for patients with mild to moderate OA of the knee or hand, particularly in patients with few affected joints or a history of sensitivity to oral NSAIDs.6
1. Towheed TE. Pennsaid therapy for osteoarthritis of the knee: a systematic review and meta-analysis of randomized controlled trials. J Rheumatol. 2006;33:567-573.
2. Baraf HS, Gloth FM, Barthel HR, et al. Safety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trials. Drugs Aging. 2011;28:27-40.
3. Altman RD, Dreiser RL, Fisher CL, et al. Diclofenac sodium gel in patients with primary hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:1991-1999.
4. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (Pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
5. Chou R, McDonagh MS, Nakamoto E, et al. Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review. Rockville, MD: Agency for Healthcare Research and Quality; 2011. AHRQ Publication No. 11(12)-EHC076-EF.
6. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis. part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476-499
1. Towheed TE. Pennsaid therapy for osteoarthritis of the knee: a systematic review and meta-analysis of randomized controlled trials. J Rheumatol. 2006;33:567-573.
2. Baraf HS, Gloth FM, Barthel HR, et al. Safety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trials. Drugs Aging. 2011;28:27-40.
3. Altman RD, Dreiser RL, Fisher CL, et al. Diclofenac sodium gel in patients with primary hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:1991-1999.
4. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (Pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
5. Chou R, McDonagh MS, Nakamoto E, et al. Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review. Rockville, MD: Agency for Healthcare Research and Quality; 2011. AHRQ Publication No. 11(12)-EHC076-EF.
6. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis. part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476-499
Evidence-based answers from the Family Physicians Inquiries Network
What is the best beta-blocker for systolic heart failure?
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3
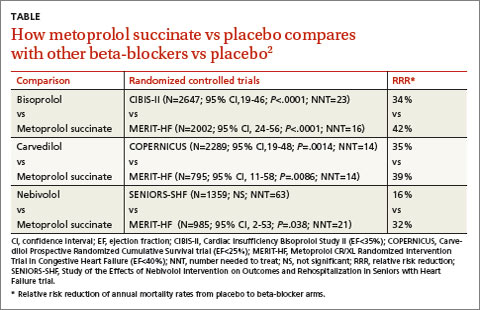
RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3

RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3

RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
Evidence-based answers from the Family Physicians Inquiries Network
Do annual pelvic exams benefit asymptomatic women who receive regular Pap smears?
No evidence exists to support a clinical benefit from annual pelvic examinations for asymptomatic women who receive Pap smears every 3 to 5 years. However, the American College of Obstetricians and Gynecologists (ACOG) committee on gynecologic practice recommends annual pelvic exams (strength of recommendation [SOR]: C, expert opinion).
Urine testing alone reliably diagnoses gonorrhea and chlamydia (SOR: A, systematic review of cohort studies).
Pelvic examinations unreliably detect adnexal masses (SOR: B, single cohort study); pelvic exams accompanied by ultrasound fail to affect outcomes in ovarian cancer screening (SOR: B, cohort studies).
Pelvic exams aren’t necessary before prescribing oral contraceptive pills (OCPs) (SOR: C, expert opinion).
Vulvar carcinoma has a low prevalence and is usually symptomatic (SOR: B, ecologic study and a case series).
EVIDENCE SUMMARY
A systematic review and meta-analysis included 29 studies that compared the sensitivity and specificity of nucleic acid amplification tests on specimens collected invasively from the cervix or urethra with noninvasively collected urine specimens.1 Studies included both asymptomatic and symptomatic patients. Reference standards varied and included cervical culture, enzyme immunoassay, direct fluorescent antibody, ligase chain reaction, and positive results on 2 of 3 nucleic acid amplification assays.
The sensitivity and specificity of chlamydia and gonorrhea detection didn’t differ between urine and cervical specimens. The pooled sensitivity and specificity for polymerase chain reaction urine samples were 83.3% (95% confidence interval [CI], 77.7%-88.9%) and 99.5% (CI, 99.3%-99.8%), respectively, and for cervical samples 85.5% (CI, 80.3%-90.6%) and 99.6% (CI, 99.4%-99.8%), respectively.1
Pelvic exams detect adnexal masses, but not reliably
A prospective cohort of 127 women undergoing pelvic surgery had preoperative bimanual exams under anesthesia to detect an adnexal mass.2 The gold standard for detection was findings at surgery. The woman had a high prevalence (20%) of ovarian masses. Indications for surgery included diagnosis, sterilization, and suspected malignancy.
When the preoperative bimanual examination detected a left adnexal mass, the odds of finding one at surgery increased 2.8 times, whereas when the exam was normal the odds decreased by 0.8 (positive predictive value [PPV]=0.64; 95% CI, 0.45-0.83). Conversely, the preoperative examination failed to correctly predict a right adnexal mass regardless of the result; the likelihood ratio for both normal and abnormal right adnexal examinations was 1 (PPV=0.26; 95% CI, 0.12-0.47).
What about pelvic exams with ultrasound?
An investigation of transvaginal ultrasonography (TVUS) from November 1987 to January 1991 screened a cohort of 1300 asymptomatic postmenopausal women for an ovarian tumor.3 To be eligible for the study, subjects had to have been without menses for at least 6 months and have no history of a pelvic tumor. Each woman underwent both a pelvic exam and TVUS.
TVUS found that 33 of the women had abnormal ovarian size and morphology when compared with normal standards. Twenty-seven of the 33, who had abnormalities that persisted longer than 1 month, underwent exploratory laparotomy. Ovarian enlargement also was apparent on clinical examination in 10 patients.
Of the 27 patients who underwent surgery, 2 had primary ovarian carcinomas. Significantly, both women had documented normal pelvic examinations on screening.
Another cohort trial conducted between October 1984 and July 1987 studied 801 women ages 40 to 70 years who were at high risk for ovarian cancer.4 Risk factors included nulliparity; symptoms such as abdominal pain, urinary frequency, or irregular bleeding; a personal history of cancer; and a family history of ovarian, breast, or endometrial cancer.
The women underwent both pelvic examination and abdominal ultrasound scanning. Fifty-one patients had abnormal pelvic examinations but normal sonograms. None of the 51 patients, who were followed to the end of the study, developed evidence of ovarian carcinoma. Abnormal abdominal ultrasound scans in 163 patients resulted in 3 diagnoses of malignancy. The 3 patients had normal pelvic examinations.
A pelvic exam isn’t needed before prescribing hormonal contraception
A 2001 JAMA literature review addressed pelvic exams as a prerequisite for administering hormonal contraceptives.5 Investigators identified consensus statements, policy statements, and reviews on the subject and contacted major health associations such as the World Health Organization for their recommendations.
Despite a lack of evidence, these expert sources concluded that a pelvic exam isn’t necessary to identify conditions in which OCPs are contraindicated (pregnancy, breast cancer, hypertension, and thromboembolic disease). Medical history and blood pressure measurement provide adequate screening.
Vulvar cancer is rare and usually symptomatic
Vulvar disease is uncommon and almost always symptomatic. The United Kingdom national cancer registry found an incidence of 3.7 per 100,000.6 A prospective study of 102 women presenting with squamous cell carcinoma of the vulva showed that 94% reported a history of symptomatic vulvar irritation.7 Eighty-eight percent had had symptoms for longer than 6 months.
RECOMMENDATIONS
Regarding screening for gonorrhea and chlamydia, the United States Preventive Services Task Force (USPSTF) states that newer tests, including nucleic acid amplification tests of urine, have improved sensitivity and comparable specificity when compared with cervical culture.8,9
The USPSTF recommends against screening for ovarian cancer in general, (Grade D recommendation: no net benefit or the harms outweigh the benefits). The Task Force states that the sensitivity of pelvic examination in detecting ovarian cancer is unknown based on several ultrasound studies.10
A 2012 ACOG committee opinion recommends that an annual pelvic examination remain a part of the well-woman visit even though the committee found no evidence in support of an annual exam for asymptomatic, low-risk patients.11 The committee notes that patients and providers should discuss the decision to perform a pelvic exam annually.
1. Cook RL, Hutchison SL, østergarrd L, et al. Systemic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrheoeae. Ann Intern Med. 2005;142:914-925.
2. Padilla LA, Radosevich DM, Milad MP. Accuracy of the pelvic examination in detecting adnexal masses. Obset Gynecol. 2000;96:593-598.
3. Van Nagell JR Jr, DePriest PD, Puls LE, et al. Ovarian cancer screening in asymptomatic postmenopausal women by transvaginal sonography. Cancer. 1991;68:458-462.
4. Andolf E, Jørgensen C, Astedt B. Ultrasound examination for detection of ovarian carcinoma in risk groups. Obstet Gyenocol. 1990;75:106-109.
5. Stewart FH, Harper CC, Ellerston CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
6. CancerResearchUK. Vulval cancer incidence statistics. Cancer Research UK Web site. Available at: http://info.cancerresearchuk.org/cancerstats/types/vulva/incidence/. Accessed October 30, 2013.
7. Jones RW, Joura EA. Analyzing prior clinical events at presentation in 102 women with vulvar carcinoma. Evidence of diagnostic delays. J Reprod Med. 1999;44:766-768.
8. US Preventive Services Task Force. Screening for gonorrhea: recommendation statement. Ann Fam Med. 2005;3:263-267.
9. US Preventive Services Task Force. Screening for chlamydial infection: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2007;147:128-134.
10. US Preventive Services Task Force. Screening for ovarian cancer: recommendation statement. Ann Fam Med. 2004;2:260-262.
11. Committee on Gyencologic Practice. Committee opinion no. 534: well-woman visit. Obstet Gynecol. 2012;120:421-424.
No evidence exists to support a clinical benefit from annual pelvic examinations for asymptomatic women who receive Pap smears every 3 to 5 years. However, the American College of Obstetricians and Gynecologists (ACOG) committee on gynecologic practice recommends annual pelvic exams (strength of recommendation [SOR]: C, expert opinion).
Urine testing alone reliably diagnoses gonorrhea and chlamydia (SOR: A, systematic review of cohort studies).
Pelvic examinations unreliably detect adnexal masses (SOR: B, single cohort study); pelvic exams accompanied by ultrasound fail to affect outcomes in ovarian cancer screening (SOR: B, cohort studies).
Pelvic exams aren’t necessary before prescribing oral contraceptive pills (OCPs) (SOR: C, expert opinion).
Vulvar carcinoma has a low prevalence and is usually symptomatic (SOR: B, ecologic study and a case series).
EVIDENCE SUMMARY
A systematic review and meta-analysis included 29 studies that compared the sensitivity and specificity of nucleic acid amplification tests on specimens collected invasively from the cervix or urethra with noninvasively collected urine specimens.1 Studies included both asymptomatic and symptomatic patients. Reference standards varied and included cervical culture, enzyme immunoassay, direct fluorescent antibody, ligase chain reaction, and positive results on 2 of 3 nucleic acid amplification assays.
The sensitivity and specificity of chlamydia and gonorrhea detection didn’t differ between urine and cervical specimens. The pooled sensitivity and specificity for polymerase chain reaction urine samples were 83.3% (95% confidence interval [CI], 77.7%-88.9%) and 99.5% (CI, 99.3%-99.8%), respectively, and for cervical samples 85.5% (CI, 80.3%-90.6%) and 99.6% (CI, 99.4%-99.8%), respectively.1
Pelvic exams detect adnexal masses, but not reliably
A prospective cohort of 127 women undergoing pelvic surgery had preoperative bimanual exams under anesthesia to detect an adnexal mass.2 The gold standard for detection was findings at surgery. The woman had a high prevalence (20%) of ovarian masses. Indications for surgery included diagnosis, sterilization, and suspected malignancy.
When the preoperative bimanual examination detected a left adnexal mass, the odds of finding one at surgery increased 2.8 times, whereas when the exam was normal the odds decreased by 0.8 (positive predictive value [PPV]=0.64; 95% CI, 0.45-0.83). Conversely, the preoperative examination failed to correctly predict a right adnexal mass regardless of the result; the likelihood ratio for both normal and abnormal right adnexal examinations was 1 (PPV=0.26; 95% CI, 0.12-0.47).
What about pelvic exams with ultrasound?
An investigation of transvaginal ultrasonography (TVUS) from November 1987 to January 1991 screened a cohort of 1300 asymptomatic postmenopausal women for an ovarian tumor.3 To be eligible for the study, subjects had to have been without menses for at least 6 months and have no history of a pelvic tumor. Each woman underwent both a pelvic exam and TVUS.
TVUS found that 33 of the women had abnormal ovarian size and morphology when compared with normal standards. Twenty-seven of the 33, who had abnormalities that persisted longer than 1 month, underwent exploratory laparotomy. Ovarian enlargement also was apparent on clinical examination in 10 patients.
Of the 27 patients who underwent surgery, 2 had primary ovarian carcinomas. Significantly, both women had documented normal pelvic examinations on screening.
Another cohort trial conducted between October 1984 and July 1987 studied 801 women ages 40 to 70 years who were at high risk for ovarian cancer.4 Risk factors included nulliparity; symptoms such as abdominal pain, urinary frequency, or irregular bleeding; a personal history of cancer; and a family history of ovarian, breast, or endometrial cancer.
The women underwent both pelvic examination and abdominal ultrasound scanning. Fifty-one patients had abnormal pelvic examinations but normal sonograms. None of the 51 patients, who were followed to the end of the study, developed evidence of ovarian carcinoma. Abnormal abdominal ultrasound scans in 163 patients resulted in 3 diagnoses of malignancy. The 3 patients had normal pelvic examinations.
A pelvic exam isn’t needed before prescribing hormonal contraception
A 2001 JAMA literature review addressed pelvic exams as a prerequisite for administering hormonal contraceptives.5 Investigators identified consensus statements, policy statements, and reviews on the subject and contacted major health associations such as the World Health Organization for their recommendations.
Despite a lack of evidence, these expert sources concluded that a pelvic exam isn’t necessary to identify conditions in which OCPs are contraindicated (pregnancy, breast cancer, hypertension, and thromboembolic disease). Medical history and blood pressure measurement provide adequate screening.
Vulvar cancer is rare and usually symptomatic
Vulvar disease is uncommon and almost always symptomatic. The United Kingdom national cancer registry found an incidence of 3.7 per 100,000.6 A prospective study of 102 women presenting with squamous cell carcinoma of the vulva showed that 94% reported a history of symptomatic vulvar irritation.7 Eighty-eight percent had had symptoms for longer than 6 months.
RECOMMENDATIONS
Regarding screening for gonorrhea and chlamydia, the United States Preventive Services Task Force (USPSTF) states that newer tests, including nucleic acid amplification tests of urine, have improved sensitivity and comparable specificity when compared with cervical culture.8,9
The USPSTF recommends against screening for ovarian cancer in general, (Grade D recommendation: no net benefit or the harms outweigh the benefits). The Task Force states that the sensitivity of pelvic examination in detecting ovarian cancer is unknown based on several ultrasound studies.10
A 2012 ACOG committee opinion recommends that an annual pelvic examination remain a part of the well-woman visit even though the committee found no evidence in support of an annual exam for asymptomatic, low-risk patients.11 The committee notes that patients and providers should discuss the decision to perform a pelvic exam annually.
No evidence exists to support a clinical benefit from annual pelvic examinations for asymptomatic women who receive Pap smears every 3 to 5 years. However, the American College of Obstetricians and Gynecologists (ACOG) committee on gynecologic practice recommends annual pelvic exams (strength of recommendation [SOR]: C, expert opinion).
Urine testing alone reliably diagnoses gonorrhea and chlamydia (SOR: A, systematic review of cohort studies).
Pelvic examinations unreliably detect adnexal masses (SOR: B, single cohort study); pelvic exams accompanied by ultrasound fail to affect outcomes in ovarian cancer screening (SOR: B, cohort studies).
Pelvic exams aren’t necessary before prescribing oral contraceptive pills (OCPs) (SOR: C, expert opinion).
Vulvar carcinoma has a low prevalence and is usually symptomatic (SOR: B, ecologic study and a case series).
EVIDENCE SUMMARY
A systematic review and meta-analysis included 29 studies that compared the sensitivity and specificity of nucleic acid amplification tests on specimens collected invasively from the cervix or urethra with noninvasively collected urine specimens.1 Studies included both asymptomatic and symptomatic patients. Reference standards varied and included cervical culture, enzyme immunoassay, direct fluorescent antibody, ligase chain reaction, and positive results on 2 of 3 nucleic acid amplification assays.
The sensitivity and specificity of chlamydia and gonorrhea detection didn’t differ between urine and cervical specimens. The pooled sensitivity and specificity for polymerase chain reaction urine samples were 83.3% (95% confidence interval [CI], 77.7%-88.9%) and 99.5% (CI, 99.3%-99.8%), respectively, and for cervical samples 85.5% (CI, 80.3%-90.6%) and 99.6% (CI, 99.4%-99.8%), respectively.1
Pelvic exams detect adnexal masses, but not reliably
A prospective cohort of 127 women undergoing pelvic surgery had preoperative bimanual exams under anesthesia to detect an adnexal mass.2 The gold standard for detection was findings at surgery. The woman had a high prevalence (20%) of ovarian masses. Indications for surgery included diagnosis, sterilization, and suspected malignancy.
When the preoperative bimanual examination detected a left adnexal mass, the odds of finding one at surgery increased 2.8 times, whereas when the exam was normal the odds decreased by 0.8 (positive predictive value [PPV]=0.64; 95% CI, 0.45-0.83). Conversely, the preoperative examination failed to correctly predict a right adnexal mass regardless of the result; the likelihood ratio for both normal and abnormal right adnexal examinations was 1 (PPV=0.26; 95% CI, 0.12-0.47).
What about pelvic exams with ultrasound?
An investigation of transvaginal ultrasonography (TVUS) from November 1987 to January 1991 screened a cohort of 1300 asymptomatic postmenopausal women for an ovarian tumor.3 To be eligible for the study, subjects had to have been without menses for at least 6 months and have no history of a pelvic tumor. Each woman underwent both a pelvic exam and TVUS.
TVUS found that 33 of the women had abnormal ovarian size and morphology when compared with normal standards. Twenty-seven of the 33, who had abnormalities that persisted longer than 1 month, underwent exploratory laparotomy. Ovarian enlargement also was apparent on clinical examination in 10 patients.
Of the 27 patients who underwent surgery, 2 had primary ovarian carcinomas. Significantly, both women had documented normal pelvic examinations on screening.
Another cohort trial conducted between October 1984 and July 1987 studied 801 women ages 40 to 70 years who were at high risk for ovarian cancer.4 Risk factors included nulliparity; symptoms such as abdominal pain, urinary frequency, or irregular bleeding; a personal history of cancer; and a family history of ovarian, breast, or endometrial cancer.
The women underwent both pelvic examination and abdominal ultrasound scanning. Fifty-one patients had abnormal pelvic examinations but normal sonograms. None of the 51 patients, who were followed to the end of the study, developed evidence of ovarian carcinoma. Abnormal abdominal ultrasound scans in 163 patients resulted in 3 diagnoses of malignancy. The 3 patients had normal pelvic examinations.
A pelvic exam isn’t needed before prescribing hormonal contraception
A 2001 JAMA literature review addressed pelvic exams as a prerequisite for administering hormonal contraceptives.5 Investigators identified consensus statements, policy statements, and reviews on the subject and contacted major health associations such as the World Health Organization for their recommendations.
Despite a lack of evidence, these expert sources concluded that a pelvic exam isn’t necessary to identify conditions in which OCPs are contraindicated (pregnancy, breast cancer, hypertension, and thromboembolic disease). Medical history and blood pressure measurement provide adequate screening.
Vulvar cancer is rare and usually symptomatic
Vulvar disease is uncommon and almost always symptomatic. The United Kingdom national cancer registry found an incidence of 3.7 per 100,000.6 A prospective study of 102 women presenting with squamous cell carcinoma of the vulva showed that 94% reported a history of symptomatic vulvar irritation.7 Eighty-eight percent had had symptoms for longer than 6 months.
RECOMMENDATIONS
Regarding screening for gonorrhea and chlamydia, the United States Preventive Services Task Force (USPSTF) states that newer tests, including nucleic acid amplification tests of urine, have improved sensitivity and comparable specificity when compared with cervical culture.8,9
The USPSTF recommends against screening for ovarian cancer in general, (Grade D recommendation: no net benefit or the harms outweigh the benefits). The Task Force states that the sensitivity of pelvic examination in detecting ovarian cancer is unknown based on several ultrasound studies.10
A 2012 ACOG committee opinion recommends that an annual pelvic examination remain a part of the well-woman visit even though the committee found no evidence in support of an annual exam for asymptomatic, low-risk patients.11 The committee notes that patients and providers should discuss the decision to perform a pelvic exam annually.
1. Cook RL, Hutchison SL, østergarrd L, et al. Systemic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrheoeae. Ann Intern Med. 2005;142:914-925.
2. Padilla LA, Radosevich DM, Milad MP. Accuracy of the pelvic examination in detecting adnexal masses. Obset Gynecol. 2000;96:593-598.
3. Van Nagell JR Jr, DePriest PD, Puls LE, et al. Ovarian cancer screening in asymptomatic postmenopausal women by transvaginal sonography. Cancer. 1991;68:458-462.
4. Andolf E, Jørgensen C, Astedt B. Ultrasound examination for detection of ovarian carcinoma in risk groups. Obstet Gyenocol. 1990;75:106-109.
5. Stewart FH, Harper CC, Ellerston CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
6. CancerResearchUK. Vulval cancer incidence statistics. Cancer Research UK Web site. Available at: http://info.cancerresearchuk.org/cancerstats/types/vulva/incidence/. Accessed October 30, 2013.
7. Jones RW, Joura EA. Analyzing prior clinical events at presentation in 102 women with vulvar carcinoma. Evidence of diagnostic delays. J Reprod Med. 1999;44:766-768.
8. US Preventive Services Task Force. Screening for gonorrhea: recommendation statement. Ann Fam Med. 2005;3:263-267.
9. US Preventive Services Task Force. Screening for chlamydial infection: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2007;147:128-134.
10. US Preventive Services Task Force. Screening for ovarian cancer: recommendation statement. Ann Fam Med. 2004;2:260-262.
11. Committee on Gyencologic Practice. Committee opinion no. 534: well-woman visit. Obstet Gynecol. 2012;120:421-424.
1. Cook RL, Hutchison SL, østergarrd L, et al. Systemic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrheoeae. Ann Intern Med. 2005;142:914-925.
2. Padilla LA, Radosevich DM, Milad MP. Accuracy of the pelvic examination in detecting adnexal masses. Obset Gynecol. 2000;96:593-598.
3. Van Nagell JR Jr, DePriest PD, Puls LE, et al. Ovarian cancer screening in asymptomatic postmenopausal women by transvaginal sonography. Cancer. 1991;68:458-462.
4. Andolf E, Jørgensen C, Astedt B. Ultrasound examination for detection of ovarian carcinoma in risk groups. Obstet Gyenocol. 1990;75:106-109.
5. Stewart FH, Harper CC, Ellerston CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
6. CancerResearchUK. Vulval cancer incidence statistics. Cancer Research UK Web site. Available at: http://info.cancerresearchuk.org/cancerstats/types/vulva/incidence/. Accessed October 30, 2013.
7. Jones RW, Joura EA. Analyzing prior clinical events at presentation in 102 women with vulvar carcinoma. Evidence of diagnostic delays. J Reprod Med. 1999;44:766-768.
8. US Preventive Services Task Force. Screening for gonorrhea: recommendation statement. Ann Fam Med. 2005;3:263-267.
9. US Preventive Services Task Force. Screening for chlamydial infection: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2007;147:128-134.
10. US Preventive Services Task Force. Screening for ovarian cancer: recommendation statement. Ann Fam Med. 2004;2:260-262.
11. Committee on Gyencologic Practice. Committee opinion no. 534: well-woman visit. Obstet Gynecol. 2012;120:421-424.
Evidence-based answers from the Family Physicians Inquiries Network
Which risk factors and signs and symptoms are associated with coccidioidomycosis?
EVIDENCE-BASED ANSWER:
Risk factors for coccidioidomycosis, or valley fever, include lower respiratory tract symptoms lasting longer than 14 days, chest pain, rash, having lived in endemic areas fewer than 10 years, and diabetes mellitus or immunosuppressive conditions (strength of recommendation [SOR]: B, several prospective cohort and case-control studies).
The most common signs and symptoms include cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), dyspnea (27%), weight loss (21%), and rash (14%) (SOR: B, retrospective cohort study).
EVIDENCE SUMMARY
A 2013 surveillance report by the Centers for Disease Control and Prevention that included 111,717 patients in 28 states and the District of Columbia found an 8-fold increase in reported coccidioidomycosis in endemic areas from 1998 to 2011 (age-adjusted incidence rates: 5.3 per 100,000 in 1998 and 42.6 per 100,000 in 2011). Cases in nonendemic states increased 40-fold in the same time period, from 6 cases to 240.1 The disease is endemic in the southwest United States and northwest Mexico.
Risk factors include persistent symptoms, chest pain, diabetes, immunosuppression
A 2008 case-control study of 136 patients in Phoenix, Arizona (an endemic area) found that 15% of the patients diagnosed with community-acquired pneumonia (CAP) had coccidioidomycosis on serologic testing. Risk factors for CAP caused by coccidioidomycosis in this population were symptom duration longer than 14 days (odds ratio [OR]=5.0; 95% confidence interval [CI], 2.1-15.7), age younger than 18 years (OR=5.5; 95% CI, 2.1-15.3), chest pain (OR=4.6; 95% CI, 1.8-11.8), and diabetes mellitus or an immunosuppressive condition (OR=3.8; 95% CI, 1.0-16.5).2
Abnormal chest X-rays, myalgia— and a rash
A 2006 prospective cohort study of 55 patients in Tucson, Arizona, which is part of the endemic area, found that 29% of patients diagnosed with CAP tested serologically positive for coccidioidomycosis. Risk factors included fewer than 10 years of exposure to an endemic area (OR=4.11; 95% CI, 1.01-16.8). Chest radiograph abnormalities were more common in patients with CAP caused by coccidioidomycosis than patients without coccidioidomycosis (75% vs 25%, P=.005). Myalgia is more common when coccidioidal pneumonia is present (69% vs 23%, P=.0022).3
A 2009 prospective cohort study of 35 patients with CAP in Phoenix, Arizona found that 6 patients (17%) tested positive for coccidioidomycosis. Only 1 statistically significant risk factor was identified—half of patients with coccidioidomycosis exhibited a rash, while there were no rashes in the group without the disease (P=.002).4
Other common signs and symptoms
A retrospective cohort study in San Diego, California in 2004 evaluated and stratified 223 patients with known coccidioidomycosis for presenting symptoms, exam findings, and radiographic findings. The most common signs and symptoms at time of seropositive testing were cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), weight loss (21%), rash (14%), and arthralgia or myalgia (13% and 12%, respectively).5
Airspace opacity was the most common radiographic abnormality (58.8%); the second most common was pulmonary nodules (22.8%).5 The study didn’t compare the frequency of these findings with noncoccidioidal pneumonia.
RECOMMENDATIONS
In 2005 guidelines, the Infectious Diseases Society of America (IDSA) stated that the “management of coccidioidomycosis first involves recognizing that a coccidioidal infection exists, defining the extent of infection, and identifying host factors that predispose to disease severity.”6 The IDSA didn’t give specific recommendations regarding how to diagnose or differentiate coccidioidal infection from CAP.
1. Centers for Disease Control and Prevention (CDC). Increase in reported coccidioidomycosis—United States, 1998-2011. MMWR Morb Mortal Wkly Rep. 2013;62:217-221.
2. Chang DC, Anderson S, Wannemuehler K, et al. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis. 2008;14: 1053-1059.
3. Valdivia L, Nix D, Wright M, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006;12:958-962.
4. Kim MM, Blair JE, Carey EJ, et al. Coccidioidal pneumonia, Phoenix, Arizona, USA, 2000-2004. Emerg Infect Dis. 2009;15:397-401.
5. Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
6. Galgiani JN, Ampel NM, Blair JE, et al; Infectious Disease Society of America. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217-1223.
EVIDENCE-BASED ANSWER:
Risk factors for coccidioidomycosis, or valley fever, include lower respiratory tract symptoms lasting longer than 14 days, chest pain, rash, having lived in endemic areas fewer than 10 years, and diabetes mellitus or immunosuppressive conditions (strength of recommendation [SOR]: B, several prospective cohort and case-control studies).
The most common signs and symptoms include cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), dyspnea (27%), weight loss (21%), and rash (14%) (SOR: B, retrospective cohort study).
EVIDENCE SUMMARY
A 2013 surveillance report by the Centers for Disease Control and Prevention that included 111,717 patients in 28 states and the District of Columbia found an 8-fold increase in reported coccidioidomycosis in endemic areas from 1998 to 2011 (age-adjusted incidence rates: 5.3 per 100,000 in 1998 and 42.6 per 100,000 in 2011). Cases in nonendemic states increased 40-fold in the same time period, from 6 cases to 240.1 The disease is endemic in the southwest United States and northwest Mexico.
Risk factors include persistent symptoms, chest pain, diabetes, immunosuppression
A 2008 case-control study of 136 patients in Phoenix, Arizona (an endemic area) found that 15% of the patients diagnosed with community-acquired pneumonia (CAP) had coccidioidomycosis on serologic testing. Risk factors for CAP caused by coccidioidomycosis in this population were symptom duration longer than 14 days (odds ratio [OR]=5.0; 95% confidence interval [CI], 2.1-15.7), age younger than 18 years (OR=5.5; 95% CI, 2.1-15.3), chest pain (OR=4.6; 95% CI, 1.8-11.8), and diabetes mellitus or an immunosuppressive condition (OR=3.8; 95% CI, 1.0-16.5).2
Abnormal chest X-rays, myalgia— and a rash
A 2006 prospective cohort study of 55 patients in Tucson, Arizona, which is part of the endemic area, found that 29% of patients diagnosed with CAP tested serologically positive for coccidioidomycosis. Risk factors included fewer than 10 years of exposure to an endemic area (OR=4.11; 95% CI, 1.01-16.8). Chest radiograph abnormalities were more common in patients with CAP caused by coccidioidomycosis than patients without coccidioidomycosis (75% vs 25%, P=.005). Myalgia is more common when coccidioidal pneumonia is present (69% vs 23%, P=.0022).3
A 2009 prospective cohort study of 35 patients with CAP in Phoenix, Arizona found that 6 patients (17%) tested positive for coccidioidomycosis. Only 1 statistically significant risk factor was identified—half of patients with coccidioidomycosis exhibited a rash, while there were no rashes in the group without the disease (P=.002).4
Other common signs and symptoms
A retrospective cohort study in San Diego, California in 2004 evaluated and stratified 223 patients with known coccidioidomycosis for presenting symptoms, exam findings, and radiographic findings. The most common signs and symptoms at time of seropositive testing were cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), weight loss (21%), rash (14%), and arthralgia or myalgia (13% and 12%, respectively).5
Airspace opacity was the most common radiographic abnormality (58.8%); the second most common was pulmonary nodules (22.8%).5 The study didn’t compare the frequency of these findings with noncoccidioidal pneumonia.
RECOMMENDATIONS
In 2005 guidelines, the Infectious Diseases Society of America (IDSA) stated that the “management of coccidioidomycosis first involves recognizing that a coccidioidal infection exists, defining the extent of infection, and identifying host factors that predispose to disease severity.”6 The IDSA didn’t give specific recommendations regarding how to diagnose or differentiate coccidioidal infection from CAP.
EVIDENCE-BASED ANSWER:
Risk factors for coccidioidomycosis, or valley fever, include lower respiratory tract symptoms lasting longer than 14 days, chest pain, rash, having lived in endemic areas fewer than 10 years, and diabetes mellitus or immunosuppressive conditions (strength of recommendation [SOR]: B, several prospective cohort and case-control studies).
The most common signs and symptoms include cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), dyspnea (27%), weight loss (21%), and rash (14%) (SOR: B, retrospective cohort study).
EVIDENCE SUMMARY
A 2013 surveillance report by the Centers for Disease Control and Prevention that included 111,717 patients in 28 states and the District of Columbia found an 8-fold increase in reported coccidioidomycosis in endemic areas from 1998 to 2011 (age-adjusted incidence rates: 5.3 per 100,000 in 1998 and 42.6 per 100,000 in 2011). Cases in nonendemic states increased 40-fold in the same time period, from 6 cases to 240.1 The disease is endemic in the southwest United States and northwest Mexico.
Risk factors include persistent symptoms, chest pain, diabetes, immunosuppression
A 2008 case-control study of 136 patients in Phoenix, Arizona (an endemic area) found that 15% of the patients diagnosed with community-acquired pneumonia (CAP) had coccidioidomycosis on serologic testing. Risk factors for CAP caused by coccidioidomycosis in this population were symptom duration longer than 14 days (odds ratio [OR]=5.0; 95% confidence interval [CI], 2.1-15.7), age younger than 18 years (OR=5.5; 95% CI, 2.1-15.3), chest pain (OR=4.6; 95% CI, 1.8-11.8), and diabetes mellitus or an immunosuppressive condition (OR=3.8; 95% CI, 1.0-16.5).2
Abnormal chest X-rays, myalgia— and a rash
A 2006 prospective cohort study of 55 patients in Tucson, Arizona, which is part of the endemic area, found that 29% of patients diagnosed with CAP tested serologically positive for coccidioidomycosis. Risk factors included fewer than 10 years of exposure to an endemic area (OR=4.11; 95% CI, 1.01-16.8). Chest radiograph abnormalities were more common in patients with CAP caused by coccidioidomycosis than patients without coccidioidomycosis (75% vs 25%, P=.005). Myalgia is more common when coccidioidal pneumonia is present (69% vs 23%, P=.0022).3
A 2009 prospective cohort study of 35 patients with CAP in Phoenix, Arizona found that 6 patients (17%) tested positive for coccidioidomycosis. Only 1 statistically significant risk factor was identified—half of patients with coccidioidomycosis exhibited a rash, while there were no rashes in the group without the disease (P=.002).4
Other common signs and symptoms
A retrospective cohort study in San Diego, California in 2004 evaluated and stratified 223 patients with known coccidioidomycosis for presenting symptoms, exam findings, and radiographic findings. The most common signs and symptoms at time of seropositive testing were cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), weight loss (21%), rash (14%), and arthralgia or myalgia (13% and 12%, respectively).5
Airspace opacity was the most common radiographic abnormality (58.8%); the second most common was pulmonary nodules (22.8%).5 The study didn’t compare the frequency of these findings with noncoccidioidal pneumonia.
RECOMMENDATIONS
In 2005 guidelines, the Infectious Diseases Society of America (IDSA) stated that the “management of coccidioidomycosis first involves recognizing that a coccidioidal infection exists, defining the extent of infection, and identifying host factors that predispose to disease severity.”6 The IDSA didn’t give specific recommendations regarding how to diagnose or differentiate coccidioidal infection from CAP.
1. Centers for Disease Control and Prevention (CDC). Increase in reported coccidioidomycosis—United States, 1998-2011. MMWR Morb Mortal Wkly Rep. 2013;62:217-221.
2. Chang DC, Anderson S, Wannemuehler K, et al. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis. 2008;14: 1053-1059.
3. Valdivia L, Nix D, Wright M, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006;12:958-962.
4. Kim MM, Blair JE, Carey EJ, et al. Coccidioidal pneumonia, Phoenix, Arizona, USA, 2000-2004. Emerg Infect Dis. 2009;15:397-401.
5. Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
6. Galgiani JN, Ampel NM, Blair JE, et al; Infectious Disease Society of America. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217-1223.
1. Centers for Disease Control and Prevention (CDC). Increase in reported coccidioidomycosis—United States, 1998-2011. MMWR Morb Mortal Wkly Rep. 2013;62:217-221.
2. Chang DC, Anderson S, Wannemuehler K, et al. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis. 2008;14: 1053-1059.
3. Valdivia L, Nix D, Wright M, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006;12:958-962.
4. Kim MM, Blair JE, Carey EJ, et al. Coccidioidal pneumonia, Phoenix, Arizona, USA, 2000-2004. Emerg Infect Dis. 2009;15:397-401.
5. Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
6. Galgiani JN, Ampel NM, Blair JE, et al; Infectious Disease Society of America. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217-1223.
Evidence-based answers from the Family Physicians Inquiries Network
Is immediate-release topiramate an effective treatment for adult obesity?
EVIDENCE-BASED ANSWER:
Yes. Topiramate (at daily doses of 64-400 mg) produces an average 5.34 kg of additional weight loss compared with placebo (95% confidence interval [CI], -6.12 to -4.56) in overweight to obese adults for periods of 16 to 60 weeks (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs]).
Topiramate increases the chances of losing 5% or more of baseline body weight (BBW) with a number needed to treat (NNT) of 3 (95% CI, 2-3) and 10% or more of BBW with an NNT of 4 (95% CI, 3-4). However, approximately 17% of patients discontinue the drug because of adverse effects, including paresthesia, hypoesthesia, taste perversion, and psychomotor impairment (SOR: A, meta-analyses of RCTs).
EVIDENCE SUMMARY
A meta-analysis of 10 well-done RCTs with a total of 3320 patients found that topiramate produced more weight loss than placebo.1 Studies included men and women ages 18 to 75 years, with a body mass index (BMI) of 27 to 50. Several studies included patients with hypertension, dyslipidemia, and diabetes mellitus; one study included patients with binge eating disorder. Investigators recruited subjects from sites in Europe, North America, Australia, and South Africa. The studies lasted 16 to 60 weeks and used variable doses of topiramate (64-400 mg daily). Most incorporated a structured lifestyle intervention program for both the treatment and control groups.
Patients taking topiramate lost 5.34 kg (95% CI, -6.12 to -4.56) more than subjects taking placebo. All studies showed significantly greater weight loss in the topiramate groups, regardless of dose and duration, although there was some heterogeneity among the results. The NNTs to achieve weight loss of 5% or more of BBW and 10% or more of BBW were 3 (95% CI, 2-3) and 4 (95% CI, 3-4), respectively.
No major adverse events, but some unpleasant effects
A safety analysis on 6620 subjects found no major adverse events.1 Subjects in the topiramate group were more likely to withdraw because of adverse effects (odds ratio=1.97; 95% CI, 1.64-2.29; number needed to harm=14; 95% CI, 11-18). The most common adverse effects were paresthesia, hypoesthesia, taste perversion, and psychomotor impairment, and these effects were most likely to lead to discontinuation at daily doses >96 mg.
Two formulas are effective in patients with diabetes
Investigators stopped 6 studies early because the sponsor wanted to pursue development of a controlled-release formulation of topiramate. The meta-analysis includes a single study of controlled-release topiramate, 175 mg daily in patients with diabetes, that showed equivalent efficacy and similar tolerability to immediate-release topiramate.2
Three other RCTs included in the meta-analysis specifically examined obese patients with type 2 diabetes, a population deemed more resistant to typical weight loss regimens, treated with immediate-release topiramate in dosages of 96 mg and 192 mg daily.3-5 These patients also experienced greater weight loss than patients taking placebo, comparable to what was seen in the overall meta-analysis.
FDA approval and cost of therapy
Topiramate monotherapy isn’t approved by the US Food and Drug Administration (FDA) for obesity treatment. In 2012, the FDA approved phentermine/topiramate extended-release (Qsymia) for long-term treatment of obesity; the monthly cost for a maintenance dose of 7.5 mg/46 mg daily is approximately $185.6 Topiramate immediate-release tablets cost approximately $25 per month for twice daily doses of 50 to 100 mg.7
RECOMMENDATIONS
The US Preventive Services Task Force recommends screening all adults for obesity by measuring BMI and referring patients with a BMI ≥30 for high-intensity, comprehensive behavioral interventions. It makes no recommendation for pharmacologic management.8
The Institute for Clinical Systems Improvement concludes that pharmacotherapy should be used only as part of a comprehensive obesity treatment plan. Pharmacotherapy should be considered if obese patients are unable to lose 1 pound per week with diet, physical activity, and behavior modification.9
1. Kramer CK, Leitão CB, Pinto LC, et al. Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials. Obes Rev. 2011;12:e338-e347.
2. Rosenstock J, Hollander P, Gadde KM, et al; OBD-202 Study Group. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30:1480-1486.
3. Stenlöf K, Rössner S, Vercruysse F, et al. Topiramate in the treatment of obese subjects with drug-naive type 2 diabetes. Diabetes Obes Metab. 2007;9:360-368.
4. Toplak H, Hamann A, Moore R, et al. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond). 2007;31:138-146.
5. Eliasson B, Gudbjörnsdottir S, Cederholm J, et al. Weight loss and metabolic effects of topiramate in overweight and obese type 2 diabetic patients: randomized double-blind placebo-controlled trial. Int J Obes (Lond). 2007;31: 1140-1147.
6. Drugs.com. Qsymia. Drugs.com Web site. Available at: www.drugs.com/pro/qsymia.html. Accessed September 26, 2014.
7. Drugs.com. Topirimate prices, coupons and patient assistance programs. Drugs.com Web site. Available at: www.drugs.com/price-guide/topiramate. Accessed September 26, 2014.
8. US Preventive Services Task Force. Obesity in Adults: Screening and management. US Preventive Services Task Force Web site. Available at: www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed September 30, 2014.
9. Fitch A, Everling L, Fox C, et al. Prevention and management of obesity for adults. Institute for Clinical Systems Improvement Web site. Available at: www.icsi.org/_asset/s935hy/Obesity-Adults.pdf. Accessed September 30, 2014.
EVIDENCE-BASED ANSWER:
Yes. Topiramate (at daily doses of 64-400 mg) produces an average 5.34 kg of additional weight loss compared with placebo (95% confidence interval [CI], -6.12 to -4.56) in overweight to obese adults for periods of 16 to 60 weeks (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs]).
Topiramate increases the chances of losing 5% or more of baseline body weight (BBW) with a number needed to treat (NNT) of 3 (95% CI, 2-3) and 10% or more of BBW with an NNT of 4 (95% CI, 3-4). However, approximately 17% of patients discontinue the drug because of adverse effects, including paresthesia, hypoesthesia, taste perversion, and psychomotor impairment (SOR: A, meta-analyses of RCTs).
EVIDENCE SUMMARY
A meta-analysis of 10 well-done RCTs with a total of 3320 patients found that topiramate produced more weight loss than placebo.1 Studies included men and women ages 18 to 75 years, with a body mass index (BMI) of 27 to 50. Several studies included patients with hypertension, dyslipidemia, and diabetes mellitus; one study included patients with binge eating disorder. Investigators recruited subjects from sites in Europe, North America, Australia, and South Africa. The studies lasted 16 to 60 weeks and used variable doses of topiramate (64-400 mg daily). Most incorporated a structured lifestyle intervention program for both the treatment and control groups.
Patients taking topiramate lost 5.34 kg (95% CI, -6.12 to -4.56) more than subjects taking placebo. All studies showed significantly greater weight loss in the topiramate groups, regardless of dose and duration, although there was some heterogeneity among the results. The NNTs to achieve weight loss of 5% or more of BBW and 10% or more of BBW were 3 (95% CI, 2-3) and 4 (95% CI, 3-4), respectively.
No major adverse events, but some unpleasant effects
A safety analysis on 6620 subjects found no major adverse events.1 Subjects in the topiramate group were more likely to withdraw because of adverse effects (odds ratio=1.97; 95% CI, 1.64-2.29; number needed to harm=14; 95% CI, 11-18). The most common adverse effects were paresthesia, hypoesthesia, taste perversion, and psychomotor impairment, and these effects were most likely to lead to discontinuation at daily doses >96 mg.
Two formulas are effective in patients with diabetes
Investigators stopped 6 studies early because the sponsor wanted to pursue development of a controlled-release formulation of topiramate. The meta-analysis includes a single study of controlled-release topiramate, 175 mg daily in patients with diabetes, that showed equivalent efficacy and similar tolerability to immediate-release topiramate.2
Three other RCTs included in the meta-analysis specifically examined obese patients with type 2 diabetes, a population deemed more resistant to typical weight loss regimens, treated with immediate-release topiramate in dosages of 96 mg and 192 mg daily.3-5 These patients also experienced greater weight loss than patients taking placebo, comparable to what was seen in the overall meta-analysis.
FDA approval and cost of therapy
Topiramate monotherapy isn’t approved by the US Food and Drug Administration (FDA) for obesity treatment. In 2012, the FDA approved phentermine/topiramate extended-release (Qsymia) for long-term treatment of obesity; the monthly cost for a maintenance dose of 7.5 mg/46 mg daily is approximately $185.6 Topiramate immediate-release tablets cost approximately $25 per month for twice daily doses of 50 to 100 mg.7
RECOMMENDATIONS
The US Preventive Services Task Force recommends screening all adults for obesity by measuring BMI and referring patients with a BMI ≥30 for high-intensity, comprehensive behavioral interventions. It makes no recommendation for pharmacologic management.8
The Institute for Clinical Systems Improvement concludes that pharmacotherapy should be used only as part of a comprehensive obesity treatment plan. Pharmacotherapy should be considered if obese patients are unable to lose 1 pound per week with diet, physical activity, and behavior modification.9
EVIDENCE-BASED ANSWER:
Yes. Topiramate (at daily doses of 64-400 mg) produces an average 5.34 kg of additional weight loss compared with placebo (95% confidence interval [CI], -6.12 to -4.56) in overweight to obese adults for periods of 16 to 60 weeks (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs]).
Topiramate increases the chances of losing 5% or more of baseline body weight (BBW) with a number needed to treat (NNT) of 3 (95% CI, 2-3) and 10% or more of BBW with an NNT of 4 (95% CI, 3-4). However, approximately 17% of patients discontinue the drug because of adverse effects, including paresthesia, hypoesthesia, taste perversion, and psychomotor impairment (SOR: A, meta-analyses of RCTs).
EVIDENCE SUMMARY
A meta-analysis of 10 well-done RCTs with a total of 3320 patients found that topiramate produced more weight loss than placebo.1 Studies included men and women ages 18 to 75 years, with a body mass index (BMI) of 27 to 50. Several studies included patients with hypertension, dyslipidemia, and diabetes mellitus; one study included patients with binge eating disorder. Investigators recruited subjects from sites in Europe, North America, Australia, and South Africa. The studies lasted 16 to 60 weeks and used variable doses of topiramate (64-400 mg daily). Most incorporated a structured lifestyle intervention program for both the treatment and control groups.
Patients taking topiramate lost 5.34 kg (95% CI, -6.12 to -4.56) more than subjects taking placebo. All studies showed significantly greater weight loss in the topiramate groups, regardless of dose and duration, although there was some heterogeneity among the results. The NNTs to achieve weight loss of 5% or more of BBW and 10% or more of BBW were 3 (95% CI, 2-3) and 4 (95% CI, 3-4), respectively.
No major adverse events, but some unpleasant effects
A safety analysis on 6620 subjects found no major adverse events.1 Subjects in the topiramate group were more likely to withdraw because of adverse effects (odds ratio=1.97; 95% CI, 1.64-2.29; number needed to harm=14; 95% CI, 11-18). The most common adverse effects were paresthesia, hypoesthesia, taste perversion, and psychomotor impairment, and these effects were most likely to lead to discontinuation at daily doses >96 mg.
Two formulas are effective in patients with diabetes
Investigators stopped 6 studies early because the sponsor wanted to pursue development of a controlled-release formulation of topiramate. The meta-analysis includes a single study of controlled-release topiramate, 175 mg daily in patients with diabetes, that showed equivalent efficacy and similar tolerability to immediate-release topiramate.2
Three other RCTs included in the meta-analysis specifically examined obese patients with type 2 diabetes, a population deemed more resistant to typical weight loss regimens, treated with immediate-release topiramate in dosages of 96 mg and 192 mg daily.3-5 These patients also experienced greater weight loss than patients taking placebo, comparable to what was seen in the overall meta-analysis.
FDA approval and cost of therapy
Topiramate monotherapy isn’t approved by the US Food and Drug Administration (FDA) for obesity treatment. In 2012, the FDA approved phentermine/topiramate extended-release (Qsymia) for long-term treatment of obesity; the monthly cost for a maintenance dose of 7.5 mg/46 mg daily is approximately $185.6 Topiramate immediate-release tablets cost approximately $25 per month for twice daily doses of 50 to 100 mg.7
RECOMMENDATIONS
The US Preventive Services Task Force recommends screening all adults for obesity by measuring BMI and referring patients with a BMI ≥30 for high-intensity, comprehensive behavioral interventions. It makes no recommendation for pharmacologic management.8
The Institute for Clinical Systems Improvement concludes that pharmacotherapy should be used only as part of a comprehensive obesity treatment plan. Pharmacotherapy should be considered if obese patients are unable to lose 1 pound per week with diet, physical activity, and behavior modification.9
1. Kramer CK, Leitão CB, Pinto LC, et al. Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials. Obes Rev. 2011;12:e338-e347.
2. Rosenstock J, Hollander P, Gadde KM, et al; OBD-202 Study Group. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30:1480-1486.
3. Stenlöf K, Rössner S, Vercruysse F, et al. Topiramate in the treatment of obese subjects with drug-naive type 2 diabetes. Diabetes Obes Metab. 2007;9:360-368.
4. Toplak H, Hamann A, Moore R, et al. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond). 2007;31:138-146.
5. Eliasson B, Gudbjörnsdottir S, Cederholm J, et al. Weight loss and metabolic effects of topiramate in overweight and obese type 2 diabetic patients: randomized double-blind placebo-controlled trial. Int J Obes (Lond). 2007;31: 1140-1147.
6. Drugs.com. Qsymia. Drugs.com Web site. Available at: www.drugs.com/pro/qsymia.html. Accessed September 26, 2014.
7. Drugs.com. Topirimate prices, coupons and patient assistance programs. Drugs.com Web site. Available at: www.drugs.com/price-guide/topiramate. Accessed September 26, 2014.
8. US Preventive Services Task Force. Obesity in Adults: Screening and management. US Preventive Services Task Force Web site. Available at: www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed September 30, 2014.
9. Fitch A, Everling L, Fox C, et al. Prevention and management of obesity for adults. Institute for Clinical Systems Improvement Web site. Available at: www.icsi.org/_asset/s935hy/Obesity-Adults.pdf. Accessed September 30, 2014.
1. Kramer CK, Leitão CB, Pinto LC, et al. Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials. Obes Rev. 2011;12:e338-e347.
2. Rosenstock J, Hollander P, Gadde KM, et al; OBD-202 Study Group. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30:1480-1486.
3. Stenlöf K, Rössner S, Vercruysse F, et al. Topiramate in the treatment of obese subjects with drug-naive type 2 diabetes. Diabetes Obes Metab. 2007;9:360-368.
4. Toplak H, Hamann A, Moore R, et al. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond). 2007;31:138-146.
5. Eliasson B, Gudbjörnsdottir S, Cederholm J, et al. Weight loss and metabolic effects of topiramate in overweight and obese type 2 diabetic patients: randomized double-blind placebo-controlled trial. Int J Obes (Lond). 2007;31: 1140-1147.
6. Drugs.com. Qsymia. Drugs.com Web site. Available at: www.drugs.com/pro/qsymia.html. Accessed September 26, 2014.
7. Drugs.com. Topirimate prices, coupons and patient assistance programs. Drugs.com Web site. Available at: www.drugs.com/price-guide/topiramate. Accessed September 26, 2014.
8. US Preventive Services Task Force. Obesity in Adults: Screening and management. US Preventive Services Task Force Web site. Available at: www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed September 30, 2014.
9. Fitch A, Everling L, Fox C, et al. Prevention and management of obesity for adults. Institute for Clinical Systems Improvement Web site. Available at: www.icsi.org/_asset/s935hy/Obesity-Adults.pdf. Accessed September 30, 2014.
Evidence-based answers from the Family Physicians Inquiries Network