User login
Does any antidepressant besides bupropion help smokers quit?
EVIDENCE-BASED ANSWER:
Yes, nortriptyline approximately doubles smoking cessation rates, an effect comparable to bupropion. Adding nortriptyline to nicotine replacement therapy (NRT) doesn’t improve rates further (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Selective serotonin reuptake inhibitors (SSRIs; fluoxetine, paroxetine, sertraline, citalopram), venlafaxine, monoamine oxidase inhibitors (MAOIs; moclobemide, selegiline), doxepin, and St. John’s wort don’t improve smoking cessation rates (SOR: A, systematic reviews and RCTs).
EVIDENCE SUMMARY
Bupropion is the only Food and Drug Administration (FDA)-approved antidepressant recommended as a first-line pharmacologic agent to assist with smoking cessation, based in part on a meta-analysis of 44 placebo-controlled RCTs (13,728 patients), which found that bupropion had a relative risk (RR) of 1.62 for smoking cessation compared with placebo (95% confidence interval [CI], 1.49-1.76). Bupropion produced quit rates that were approximately double those of placebo rates (18% [range 4%-43%] for bupropion vs 9% [range 0%-18%] for placebo).1
Nortriptyline is also effective, other antidepressants not so much
A Cochrane systematic review of 10 antidepressants used for smoking cessation included 64 placebo-controlled trials, measuring at least 6-month abstinence rates as primary outcomes, and monitoring biochemical markers (such as breath carbon monoxide and urinary cotinine) to verify abstinence. Some trials included participants with previous depressive episodes, but most didn’t enroll patients with active major depression.1 The TABLE1 gives an overview of the studies and outcomes.

Nortriptyline, which was evaluated in 6 trials, was the only antidepressant besides bupropion that was superior to placebo.1 Two of the nortriptyline trials included participants with active depression and the other trials had participants with a history of depression. One trial found no difference in quit rates for patients taking nortriptyline with or without a history of major depression, although the subgroups were small. Two trials measured quit rates for 12 months whereas the other 4 trials used 6-month quit rates.
Four additional RCTs with 1644 patients that combined nortriptyline with NRT found no improvement in quit rates compared with NRT alone (RR=1.21; 95% CI, 0.94-1.55).1 Three RCTs with 417 patients compared bupropion with nortriptyline and found no difference (RR=1.3; 95% CI, 0.93-1.8).1
SSRIs. None of the 4 SSRIs investigated in the trials (fluoxetine, paroxetine, sertraline, citalopram) improved smoking cessation rates more than placebo.1 The 5 RCTs that studied the drugs followed participants for as long as a year. None of the participants were depressed at the time of the studies, although some had a history of depression.
The sertraline RCT used individual counseling sessions in conjunction with either sertraline or placebo. All participants had a history of major depression.
The paroxetine trial used NRT in all patients randomized to either paroxetine or placebo.
Venlafaxine. The serotonin-norepinephrine reuptake inhibitor venlafaxine didn’t improve smoking cessation rates over 12 months.1
MAOIs. Neither of the 2 MAOIs increased smoking cessation rates.1 The moclobemide RCT followed participants for 12 months; the 5 selegiline RCTs followed participants for as long as 6 months.
Other antidepressants. An RCT with 19 participants found that doxepin didn’t improve smoking cessation at 2 months.1 One RCT and one open, randomized trial of St. John’s wort found no benefit for smoking cessation.1,2
RECOMMENDATIONS
The United States Public Health Service (USPHS) and the University of Michigan Health System (UMHS) guidelines recommend the following FDA-approved pharmacotherapies as first-line agents for smoking cessation: sustained-release bupropion, NRT (gum, inhaler, lozenge, nasal spray, or patch), and varenicline.3,4 They say that clonidine and nortriptyline are also effective but recommend them as second-line agents because these drugs lack FDA approval for this purpose.
The USPHS also recommends combinations of NRT and bupropion for long-term use. Because of additional cost and limited benefit, UMHS recommends reserving NRT-bupropion combination therapy for highly addicted tobacco users who have several failed quit attempts.
The United States Preventive Services Task Force guideline emphasizes counseling and interventions to prevent tobacco use; it doesn’t provide recommendations for pharmacotherapy.5 It does cite the same agents recommended by USPHS and UMHS as effective.
1. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;1:CD000031.
2. Sood A, Ebbert JO, Prasad K, et al. A randomized clinical trial of St. John’s wort for smoking cessation. J Altern Complement Med. 2010;16:761-767.
3. Agency for Healthcare Research and Quality. Treating tobacco use and dependence: 2008 update. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Accessed October 9, 2014.
4. University of Michigan Health System. Tobacco treatment. University of Michigan Health System Web site. Available at: http://www.med.umich.edu/1info/fhp/practiceguides/smoking/smoking.pdf. Accessed October 9, 2014.
5. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2009;150:551-555.
EVIDENCE-BASED ANSWER:
Yes, nortriptyline approximately doubles smoking cessation rates, an effect comparable to bupropion. Adding nortriptyline to nicotine replacement therapy (NRT) doesn’t improve rates further (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Selective serotonin reuptake inhibitors (SSRIs; fluoxetine, paroxetine, sertraline, citalopram), venlafaxine, monoamine oxidase inhibitors (MAOIs; moclobemide, selegiline), doxepin, and St. John’s wort don’t improve smoking cessation rates (SOR: A, systematic reviews and RCTs).
EVIDENCE SUMMARY
Bupropion is the only Food and Drug Administration (FDA)-approved antidepressant recommended as a first-line pharmacologic agent to assist with smoking cessation, based in part on a meta-analysis of 44 placebo-controlled RCTs (13,728 patients), which found that bupropion had a relative risk (RR) of 1.62 for smoking cessation compared with placebo (95% confidence interval [CI], 1.49-1.76). Bupropion produced quit rates that were approximately double those of placebo rates (18% [range 4%-43%] for bupropion vs 9% [range 0%-18%] for placebo).1
Nortriptyline is also effective, other antidepressants not so much
A Cochrane systematic review of 10 antidepressants used for smoking cessation included 64 placebo-controlled trials, measuring at least 6-month abstinence rates as primary outcomes, and monitoring biochemical markers (such as breath carbon monoxide and urinary cotinine) to verify abstinence. Some trials included participants with previous depressive episodes, but most didn’t enroll patients with active major depression.1 The TABLE1 gives an overview of the studies and outcomes.

Nortriptyline, which was evaluated in 6 trials, was the only antidepressant besides bupropion that was superior to placebo.1 Two of the nortriptyline trials included participants with active depression and the other trials had participants with a history of depression. One trial found no difference in quit rates for patients taking nortriptyline with or without a history of major depression, although the subgroups were small. Two trials measured quit rates for 12 months whereas the other 4 trials used 6-month quit rates.
Four additional RCTs with 1644 patients that combined nortriptyline with NRT found no improvement in quit rates compared with NRT alone (RR=1.21; 95% CI, 0.94-1.55).1 Three RCTs with 417 patients compared bupropion with nortriptyline and found no difference (RR=1.3; 95% CI, 0.93-1.8).1
SSRIs. None of the 4 SSRIs investigated in the trials (fluoxetine, paroxetine, sertraline, citalopram) improved smoking cessation rates more than placebo.1 The 5 RCTs that studied the drugs followed participants for as long as a year. None of the participants were depressed at the time of the studies, although some had a history of depression.
The sertraline RCT used individual counseling sessions in conjunction with either sertraline or placebo. All participants had a history of major depression.
The paroxetine trial used NRT in all patients randomized to either paroxetine or placebo.
Venlafaxine. The serotonin-norepinephrine reuptake inhibitor venlafaxine didn’t improve smoking cessation rates over 12 months.1
MAOIs. Neither of the 2 MAOIs increased smoking cessation rates.1 The moclobemide RCT followed participants for 12 months; the 5 selegiline RCTs followed participants for as long as 6 months.
Other antidepressants. An RCT with 19 participants found that doxepin didn’t improve smoking cessation at 2 months.1 One RCT and one open, randomized trial of St. John’s wort found no benefit for smoking cessation.1,2
RECOMMENDATIONS
The United States Public Health Service (USPHS) and the University of Michigan Health System (UMHS) guidelines recommend the following FDA-approved pharmacotherapies as first-line agents for smoking cessation: sustained-release bupropion, NRT (gum, inhaler, lozenge, nasal spray, or patch), and varenicline.3,4 They say that clonidine and nortriptyline are also effective but recommend them as second-line agents because these drugs lack FDA approval for this purpose.
The USPHS also recommends combinations of NRT and bupropion for long-term use. Because of additional cost and limited benefit, UMHS recommends reserving NRT-bupropion combination therapy for highly addicted tobacco users who have several failed quit attempts.
The United States Preventive Services Task Force guideline emphasizes counseling and interventions to prevent tobacco use; it doesn’t provide recommendations for pharmacotherapy.5 It does cite the same agents recommended by USPHS and UMHS as effective.
EVIDENCE-BASED ANSWER:
Yes, nortriptyline approximately doubles smoking cessation rates, an effect comparable to bupropion. Adding nortriptyline to nicotine replacement therapy (NRT) doesn’t improve rates further (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Selective serotonin reuptake inhibitors (SSRIs; fluoxetine, paroxetine, sertraline, citalopram), venlafaxine, monoamine oxidase inhibitors (MAOIs; moclobemide, selegiline), doxepin, and St. John’s wort don’t improve smoking cessation rates (SOR: A, systematic reviews and RCTs).
EVIDENCE SUMMARY
Bupropion is the only Food and Drug Administration (FDA)-approved antidepressant recommended as a first-line pharmacologic agent to assist with smoking cessation, based in part on a meta-analysis of 44 placebo-controlled RCTs (13,728 patients), which found that bupropion had a relative risk (RR) of 1.62 for smoking cessation compared with placebo (95% confidence interval [CI], 1.49-1.76). Bupropion produced quit rates that were approximately double those of placebo rates (18% [range 4%-43%] for bupropion vs 9% [range 0%-18%] for placebo).1
Nortriptyline is also effective, other antidepressants not so much
A Cochrane systematic review of 10 antidepressants used for smoking cessation included 64 placebo-controlled trials, measuring at least 6-month abstinence rates as primary outcomes, and monitoring biochemical markers (such as breath carbon monoxide and urinary cotinine) to verify abstinence. Some trials included participants with previous depressive episodes, but most didn’t enroll patients with active major depression.1 The TABLE1 gives an overview of the studies and outcomes.

Nortriptyline, which was evaluated in 6 trials, was the only antidepressant besides bupropion that was superior to placebo.1 Two of the nortriptyline trials included participants with active depression and the other trials had participants with a history of depression. One trial found no difference in quit rates for patients taking nortriptyline with or without a history of major depression, although the subgroups were small. Two trials measured quit rates for 12 months whereas the other 4 trials used 6-month quit rates.
Four additional RCTs with 1644 patients that combined nortriptyline with NRT found no improvement in quit rates compared with NRT alone (RR=1.21; 95% CI, 0.94-1.55).1 Three RCTs with 417 patients compared bupropion with nortriptyline and found no difference (RR=1.3; 95% CI, 0.93-1.8).1
SSRIs. None of the 4 SSRIs investigated in the trials (fluoxetine, paroxetine, sertraline, citalopram) improved smoking cessation rates more than placebo.1 The 5 RCTs that studied the drugs followed participants for as long as a year. None of the participants were depressed at the time of the studies, although some had a history of depression.
The sertraline RCT used individual counseling sessions in conjunction with either sertraline or placebo. All participants had a history of major depression.
The paroxetine trial used NRT in all patients randomized to either paroxetine or placebo.
Venlafaxine. The serotonin-norepinephrine reuptake inhibitor venlafaxine didn’t improve smoking cessation rates over 12 months.1
MAOIs. Neither of the 2 MAOIs increased smoking cessation rates.1 The moclobemide RCT followed participants for 12 months; the 5 selegiline RCTs followed participants for as long as 6 months.
Other antidepressants. An RCT with 19 participants found that doxepin didn’t improve smoking cessation at 2 months.1 One RCT and one open, randomized trial of St. John’s wort found no benefit for smoking cessation.1,2
RECOMMENDATIONS
The United States Public Health Service (USPHS) and the University of Michigan Health System (UMHS) guidelines recommend the following FDA-approved pharmacotherapies as first-line agents for smoking cessation: sustained-release bupropion, NRT (gum, inhaler, lozenge, nasal spray, or patch), and varenicline.3,4 They say that clonidine and nortriptyline are also effective but recommend them as second-line agents because these drugs lack FDA approval for this purpose.
The USPHS also recommends combinations of NRT and bupropion for long-term use. Because of additional cost and limited benefit, UMHS recommends reserving NRT-bupropion combination therapy for highly addicted tobacco users who have several failed quit attempts.
The United States Preventive Services Task Force guideline emphasizes counseling and interventions to prevent tobacco use; it doesn’t provide recommendations for pharmacotherapy.5 It does cite the same agents recommended by USPHS and UMHS as effective.
1. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;1:CD000031.
2. Sood A, Ebbert JO, Prasad K, et al. A randomized clinical trial of St. John’s wort for smoking cessation. J Altern Complement Med. 2010;16:761-767.
3. Agency for Healthcare Research and Quality. Treating tobacco use and dependence: 2008 update. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Accessed October 9, 2014.
4. University of Michigan Health System. Tobacco treatment. University of Michigan Health System Web site. Available at: http://www.med.umich.edu/1info/fhp/practiceguides/smoking/smoking.pdf. Accessed October 9, 2014.
5. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2009;150:551-555.
1. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;1:CD000031.
2. Sood A, Ebbert JO, Prasad K, et al. A randomized clinical trial of St. John’s wort for smoking cessation. J Altern Complement Med. 2010;16:761-767.
3. Agency for Healthcare Research and Quality. Treating tobacco use and dependence: 2008 update. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Accessed October 9, 2014.
4. University of Michigan Health System. Tobacco treatment. University of Michigan Health System Web site. Available at: http://www.med.umich.edu/1info/fhp/practiceguides/smoking/smoking.pdf. Accessed October 9, 2014.
5. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2009;150:551-555.
Evidence-based answers from the Family Physicians Inquiries Network
What treatments relieve arthritis and fatigue associated with systemic lupus erythematosus?
EVIDENCE-BASED ANSWER:
Hydroxychloroquine and chloroquine improve the arthritis associated with mild systemic lupus erythematosus (SLE)—producing a 50% reduction in arthritis flares and articular involvement—and have few adverse effects (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Methotrexate reduces arthralgias by as much as 79%, but produces adverse effects in up to 70% of patients (SOR: B, systematic review of RCTs with limited patient-oriented evidence).
Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are often used for SLE joint pain (SOR: C, expert opinion).
Omega-3 fatty acids may reduce arthritis symptoms by about 35% (SOR: B, RCTs with inconsistent evidence).
Abatacept and dehydroepiandrosterone don’t produce clinically meaningful improvements in fatigue associated with SLE, and abatacept causes significant adverse effects (SOR: B, posthoc analysis of a single RCT).
Aerobic exercise may help fatigue (SOR: B, systematic review with inconsistent evidence).
EVIDENCE SUMMARY
A systematic review of pharmacotherapy for joint pain in patients with SLE found 4 poor-quality RCTs that evaluated hydroxychloroquine, chloroquine, and methotrexate.1 Of the 2 studies that examined the effect of hydroxychloroquine, one (47 patients) showed a statistically significant 50% reduction in SLE flares (including arthritis, pleuritis, and cutaneous symptoms) over 24 weeks in patients treated with hydroxychloroquine compared with placebo (TABLE1-8). The second study (71 subjects) found a nonquantified decrease in self-reported pain when hydroxychloroquine was compared with placebo, although some of the patients were also taking prednisone (10 mg/d).
An RCT that evaluated the effect of chloroquine showed a statistically significant reduction in unspecified “articular involvement” compared with placebo.
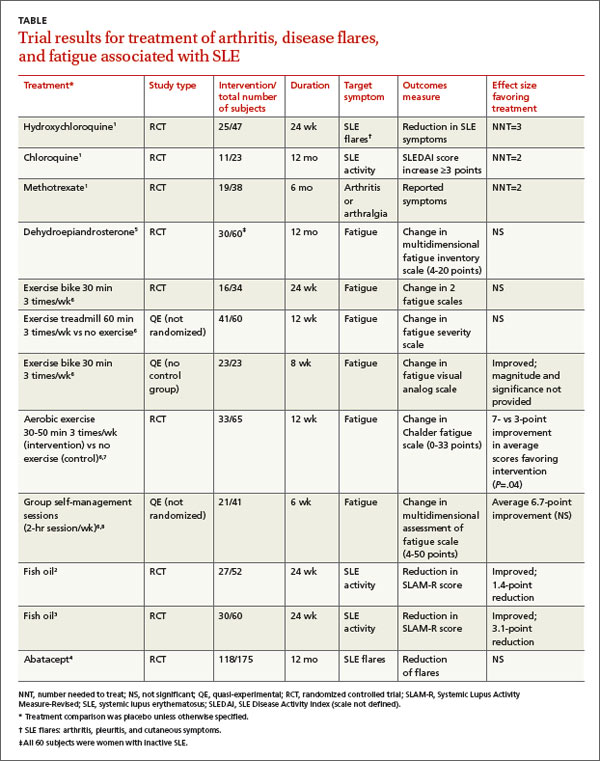
The fourth RCT, assessing methotrexate, found a statistically significant reduction by as much as 79% in patients with residual arthritis or arthralgia at 6 months compared with placebo, although 70% of patients taking methotrexate developed significant adverse effects, including infections, gastrointestinal symptoms, and elevated transaminases compared with 14% on placebo (number needed to harm [NNH]=2).
The authors of the review noted that consensus opinion holds that oral corticosteroids and NSAIDs reduce SLE-associated joint pain, but they found no studies that objectively evaluated either of these interventions.1
Fish oil also helps arthritis
Two RCTs on the effects of 3 g/d of omega-3 polyunsaturated fatty acids (fish oil) for 24 weeks in SLE patients with mild disease found a reduction in Systemic Lupus Activity Measure-Revised (SLAM-R) scores.2,3 SLAM-R is a validated measure of SLE disease activity, rated on a scale from 0 to 81, including 23 clinical and 7 laboratory manifestations of disease.
In the first study (52 subjects), disease activity decreased from an average SLAM-R score of 6.1 at baseline to 4.7 (P<.05). The second study (60 subjects) found a similar reduction in mean SLAM-R scores from 9.4 to 6.3 (P<.001) and joint pain scores from 1.27 to 0.83 (P=.047).
Drug treatments don’t significantly relieve fatigue
An industry-sponsored RCT that compared abatacept with placebo found improvements in fatigue that weren’t clinically meaningful in posthoc analysis (-9.45 points difference on a self-reported 0-to-100 visual analog scale; 95% confidence interval, -17.65 to -1.25, with a 10-point reduction considered to be clinically meaningful). Abatacept also had a high rate of serious adverse events, including facial edema, polyneuropathy, and serious infections (24/121 with abatacept vs 4/59 placebo; NNH=8).4
Another RCT found no effect of dehydroepiandrosterone on fatigue in women with inactive SLE.5
Nondrug treatments for fatigue produce mixed results
Studies of nondrug treatment of SLE-associated fatigue show inconsistent results. A systematic review of nonpharmacologic interventions for fatigue in several chronic diseases found 2 RCTs and 4 quasi-experimental studies that included 324 patients with SLE.6 Of 4 studies that evaluated the effect of exercise, 2 showed improvement and 2 didn’t. Neither group self-management nor relaxation therapy and telephone counseling significantly relieved fatigue.6-8 A small RCT (24 patients) found no benefit for acupuncture over sham needling in treating pain and fatigue in SLE.9
RECOMMENDATIONS
The American College of Rheumatology guideline for referral and management of SLE states that “NSAIDs are sometimes helpful for control of fever, arthritis, and mild serositis. Antimalarial agents (eg, hydroxychloroquine) are useful for skin and joint manifestations of SLE, for preventing flares, and for other constitutional symptoms of the disease. They may also reduce fatigue.”10
The European League Against Rheumatism recommends antimalarials or glucocorticoids to treat patients with SLE without major organ manifestations. They also say clinicians may try NSAIDs for limited periods of time in patients at low risk for the drugs’ complications.11
1. Madhok R, Wu O. Systemic lupus erythematosus. Clin Evid. 2009;7:1123.
2. Duffy EM, Meenagh GK, McMillan SA, et al. The clinical effect of dietary supplementation with omega-3 fish oils and/ or copper in systemic lupus erythematosus. J Rheumatol. 2004;31:1551-1556.
3. Wright SA, O’Prey FM, McHenry MT, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008;67:841-848.
4. Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077-3087.
5. Hartkamp A, Geenen R, Godaert GL, et al. Effects of dehydroepiandrosterone on fatigue and well-being in women with quiescent systemic lupus erythematosus: a randomized controlled trial. Ann Rheum Dis. 2010;69:1144-1147.
6. Neill J, Belan I, Reid K. Effectiveness of non-pharmacological interventions for fatigue in adults with multiple sclerosis, rheumatoid arthritis, or systemic lupus erythematosis: a systematic review. J Adv Nurs. 2006;56:617-635.
7. Tench CM, McCarthy J, McCurdie I, et al. Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology (Oxford). 2003;42:1050-1054.
8. Sohng KY. Effects of a self-management course for patients with systemic lupus erythematosus. J Adv Nurs. 2003;42:479-486.
9. Greco CM, Kao AH, Maksimowicz-McKinnon K, et al. Acupuncture for systemic lupus erythematosus: a pilot RCT feasibility and safety study. Lupus. 2008;17:1108-1116.
10. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Guidelines for referral and management of systemic lupus erythematosus in adults. Arthritis Rheum. 1999;42:1785-1796.
11. Bertsias G, Ioannidis JP, Boletis J, et al; Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195-205.
EVIDENCE-BASED ANSWER:
Hydroxychloroquine and chloroquine improve the arthritis associated with mild systemic lupus erythematosus (SLE)—producing a 50% reduction in arthritis flares and articular involvement—and have few adverse effects (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Methotrexate reduces arthralgias by as much as 79%, but produces adverse effects in up to 70% of patients (SOR: B, systematic review of RCTs with limited patient-oriented evidence).
Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are often used for SLE joint pain (SOR: C, expert opinion).
Omega-3 fatty acids may reduce arthritis symptoms by about 35% (SOR: B, RCTs with inconsistent evidence).
Abatacept and dehydroepiandrosterone don’t produce clinically meaningful improvements in fatigue associated with SLE, and abatacept causes significant adverse effects (SOR: B, posthoc analysis of a single RCT).
Aerobic exercise may help fatigue (SOR: B, systematic review with inconsistent evidence).
EVIDENCE SUMMARY
A systematic review of pharmacotherapy for joint pain in patients with SLE found 4 poor-quality RCTs that evaluated hydroxychloroquine, chloroquine, and methotrexate.1 Of the 2 studies that examined the effect of hydroxychloroquine, one (47 patients) showed a statistically significant 50% reduction in SLE flares (including arthritis, pleuritis, and cutaneous symptoms) over 24 weeks in patients treated with hydroxychloroquine compared with placebo (TABLE1-8). The second study (71 subjects) found a nonquantified decrease in self-reported pain when hydroxychloroquine was compared with placebo, although some of the patients were also taking prednisone (10 mg/d).
An RCT that evaluated the effect of chloroquine showed a statistically significant reduction in unspecified “articular involvement” compared with placebo.

The fourth RCT, assessing methotrexate, found a statistically significant reduction by as much as 79% in patients with residual arthritis or arthralgia at 6 months compared with placebo, although 70% of patients taking methotrexate developed significant adverse effects, including infections, gastrointestinal symptoms, and elevated transaminases compared with 14% on placebo (number needed to harm [NNH]=2).
The authors of the review noted that consensus opinion holds that oral corticosteroids and NSAIDs reduce SLE-associated joint pain, but they found no studies that objectively evaluated either of these interventions.1
Fish oil also helps arthritis
Two RCTs on the effects of 3 g/d of omega-3 polyunsaturated fatty acids (fish oil) for 24 weeks in SLE patients with mild disease found a reduction in Systemic Lupus Activity Measure-Revised (SLAM-R) scores.2,3 SLAM-R is a validated measure of SLE disease activity, rated on a scale from 0 to 81, including 23 clinical and 7 laboratory manifestations of disease.
In the first study (52 subjects), disease activity decreased from an average SLAM-R score of 6.1 at baseline to 4.7 (P<.05). The second study (60 subjects) found a similar reduction in mean SLAM-R scores from 9.4 to 6.3 (P<.001) and joint pain scores from 1.27 to 0.83 (P=.047).
Drug treatments don’t significantly relieve fatigue
An industry-sponsored RCT that compared abatacept with placebo found improvements in fatigue that weren’t clinically meaningful in posthoc analysis (-9.45 points difference on a self-reported 0-to-100 visual analog scale; 95% confidence interval, -17.65 to -1.25, with a 10-point reduction considered to be clinically meaningful). Abatacept also had a high rate of serious adverse events, including facial edema, polyneuropathy, and serious infections (24/121 with abatacept vs 4/59 placebo; NNH=8).4
Another RCT found no effect of dehydroepiandrosterone on fatigue in women with inactive SLE.5
Nondrug treatments for fatigue produce mixed results
Studies of nondrug treatment of SLE-associated fatigue show inconsistent results. A systematic review of nonpharmacologic interventions for fatigue in several chronic diseases found 2 RCTs and 4 quasi-experimental studies that included 324 patients with SLE.6 Of 4 studies that evaluated the effect of exercise, 2 showed improvement and 2 didn’t. Neither group self-management nor relaxation therapy and telephone counseling significantly relieved fatigue.6-8 A small RCT (24 patients) found no benefit for acupuncture over sham needling in treating pain and fatigue in SLE.9
RECOMMENDATIONS
The American College of Rheumatology guideline for referral and management of SLE states that “NSAIDs are sometimes helpful for control of fever, arthritis, and mild serositis. Antimalarial agents (eg, hydroxychloroquine) are useful for skin and joint manifestations of SLE, for preventing flares, and for other constitutional symptoms of the disease. They may also reduce fatigue.”10
The European League Against Rheumatism recommends antimalarials or glucocorticoids to treat patients with SLE without major organ manifestations. They also say clinicians may try NSAIDs for limited periods of time in patients at low risk for the drugs’ complications.11
EVIDENCE-BASED ANSWER:
Hydroxychloroquine and chloroquine improve the arthritis associated with mild systemic lupus erythematosus (SLE)—producing a 50% reduction in arthritis flares and articular involvement—and have few adverse effects (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Methotrexate reduces arthralgias by as much as 79%, but produces adverse effects in up to 70% of patients (SOR: B, systematic review of RCTs with limited patient-oriented evidence).
Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are often used for SLE joint pain (SOR: C, expert opinion).
Omega-3 fatty acids may reduce arthritis symptoms by about 35% (SOR: B, RCTs with inconsistent evidence).
Abatacept and dehydroepiandrosterone don’t produce clinically meaningful improvements in fatigue associated with SLE, and abatacept causes significant adverse effects (SOR: B, posthoc analysis of a single RCT).
Aerobic exercise may help fatigue (SOR: B, systematic review with inconsistent evidence).
EVIDENCE SUMMARY
A systematic review of pharmacotherapy for joint pain in patients with SLE found 4 poor-quality RCTs that evaluated hydroxychloroquine, chloroquine, and methotrexate.1 Of the 2 studies that examined the effect of hydroxychloroquine, one (47 patients) showed a statistically significant 50% reduction in SLE flares (including arthritis, pleuritis, and cutaneous symptoms) over 24 weeks in patients treated with hydroxychloroquine compared with placebo (TABLE1-8). The second study (71 subjects) found a nonquantified decrease in self-reported pain when hydroxychloroquine was compared with placebo, although some of the patients were also taking prednisone (10 mg/d).
An RCT that evaluated the effect of chloroquine showed a statistically significant reduction in unspecified “articular involvement” compared with placebo.

The fourth RCT, assessing methotrexate, found a statistically significant reduction by as much as 79% in patients with residual arthritis or arthralgia at 6 months compared with placebo, although 70% of patients taking methotrexate developed significant adverse effects, including infections, gastrointestinal symptoms, and elevated transaminases compared with 14% on placebo (number needed to harm [NNH]=2).
The authors of the review noted that consensus opinion holds that oral corticosteroids and NSAIDs reduce SLE-associated joint pain, but they found no studies that objectively evaluated either of these interventions.1
Fish oil also helps arthritis
Two RCTs on the effects of 3 g/d of omega-3 polyunsaturated fatty acids (fish oil) for 24 weeks in SLE patients with mild disease found a reduction in Systemic Lupus Activity Measure-Revised (SLAM-R) scores.2,3 SLAM-R is a validated measure of SLE disease activity, rated on a scale from 0 to 81, including 23 clinical and 7 laboratory manifestations of disease.
In the first study (52 subjects), disease activity decreased from an average SLAM-R score of 6.1 at baseline to 4.7 (P<.05). The second study (60 subjects) found a similar reduction in mean SLAM-R scores from 9.4 to 6.3 (P<.001) and joint pain scores from 1.27 to 0.83 (P=.047).
Drug treatments don’t significantly relieve fatigue
An industry-sponsored RCT that compared abatacept with placebo found improvements in fatigue that weren’t clinically meaningful in posthoc analysis (-9.45 points difference on a self-reported 0-to-100 visual analog scale; 95% confidence interval, -17.65 to -1.25, with a 10-point reduction considered to be clinically meaningful). Abatacept also had a high rate of serious adverse events, including facial edema, polyneuropathy, and serious infections (24/121 with abatacept vs 4/59 placebo; NNH=8).4
Another RCT found no effect of dehydroepiandrosterone on fatigue in women with inactive SLE.5
Nondrug treatments for fatigue produce mixed results
Studies of nondrug treatment of SLE-associated fatigue show inconsistent results. A systematic review of nonpharmacologic interventions for fatigue in several chronic diseases found 2 RCTs and 4 quasi-experimental studies that included 324 patients with SLE.6 Of 4 studies that evaluated the effect of exercise, 2 showed improvement and 2 didn’t. Neither group self-management nor relaxation therapy and telephone counseling significantly relieved fatigue.6-8 A small RCT (24 patients) found no benefit for acupuncture over sham needling in treating pain and fatigue in SLE.9
RECOMMENDATIONS
The American College of Rheumatology guideline for referral and management of SLE states that “NSAIDs are sometimes helpful for control of fever, arthritis, and mild serositis. Antimalarial agents (eg, hydroxychloroquine) are useful for skin and joint manifestations of SLE, for preventing flares, and for other constitutional symptoms of the disease. They may also reduce fatigue.”10
The European League Against Rheumatism recommends antimalarials or glucocorticoids to treat patients with SLE without major organ manifestations. They also say clinicians may try NSAIDs for limited periods of time in patients at low risk for the drugs’ complications.11
1. Madhok R, Wu O. Systemic lupus erythematosus. Clin Evid. 2009;7:1123.
2. Duffy EM, Meenagh GK, McMillan SA, et al. The clinical effect of dietary supplementation with omega-3 fish oils and/ or copper in systemic lupus erythematosus. J Rheumatol. 2004;31:1551-1556.
3. Wright SA, O’Prey FM, McHenry MT, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008;67:841-848.
4. Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077-3087.
5. Hartkamp A, Geenen R, Godaert GL, et al. Effects of dehydroepiandrosterone on fatigue and well-being in women with quiescent systemic lupus erythematosus: a randomized controlled trial. Ann Rheum Dis. 2010;69:1144-1147.
6. Neill J, Belan I, Reid K. Effectiveness of non-pharmacological interventions for fatigue in adults with multiple sclerosis, rheumatoid arthritis, or systemic lupus erythematosis: a systematic review. J Adv Nurs. 2006;56:617-635.
7. Tench CM, McCarthy J, McCurdie I, et al. Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology (Oxford). 2003;42:1050-1054.
8. Sohng KY. Effects of a self-management course for patients with systemic lupus erythematosus. J Adv Nurs. 2003;42:479-486.
9. Greco CM, Kao AH, Maksimowicz-McKinnon K, et al. Acupuncture for systemic lupus erythematosus: a pilot RCT feasibility and safety study. Lupus. 2008;17:1108-1116.
10. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Guidelines for referral and management of systemic lupus erythematosus in adults. Arthritis Rheum. 1999;42:1785-1796.
11. Bertsias G, Ioannidis JP, Boletis J, et al; Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195-205.
1. Madhok R, Wu O. Systemic lupus erythematosus. Clin Evid. 2009;7:1123.
2. Duffy EM, Meenagh GK, McMillan SA, et al. The clinical effect of dietary supplementation with omega-3 fish oils and/ or copper in systemic lupus erythematosus. J Rheumatol. 2004;31:1551-1556.
3. Wright SA, O’Prey FM, McHenry MT, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008;67:841-848.
4. Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077-3087.
5. Hartkamp A, Geenen R, Godaert GL, et al. Effects of dehydroepiandrosterone on fatigue and well-being in women with quiescent systemic lupus erythematosus: a randomized controlled trial. Ann Rheum Dis. 2010;69:1144-1147.
6. Neill J, Belan I, Reid K. Effectiveness of non-pharmacological interventions for fatigue in adults with multiple sclerosis, rheumatoid arthritis, or systemic lupus erythematosis: a systematic review. J Adv Nurs. 2006;56:617-635.
7. Tench CM, McCarthy J, McCurdie I, et al. Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology (Oxford). 2003;42:1050-1054.
8. Sohng KY. Effects of a self-management course for patients with systemic lupus erythematosus. J Adv Nurs. 2003;42:479-486.
9. Greco CM, Kao AH, Maksimowicz-McKinnon K, et al. Acupuncture for systemic lupus erythematosus: a pilot RCT feasibility and safety study. Lupus. 2008;17:1108-1116.
10. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Guidelines for referral and management of systemic lupus erythematosus in adults. Arthritis Rheum. 1999;42:1785-1796.
11. Bertsias G, Ioannidis JP, Boletis J, et al; Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195-205.
Evidence-based answers from the Family Physicians Inquiries Network
Do oral contraceptives put women with a family history of breast cancer at increased risk?
No. Modern combined oral contraceptive pills (OCPs) don’t increase breast cancer risk in women with a family history (strength of recommendation [SOR]: B, systematic review of cohort, case-control studies). However, older, higher-dose OCPs (in use before 1975) did increase breast cancer risk in these women (SOR: C, case-control study).
Similarly, modern OCPs don’t raise breast cancer risk in women with BRCA1/2 mutations, although higher-dose, pre-1975 OCPs did (SOR: B and C, a meta-analysis of cohort and case-control studies).
EVIDENCE SUMMARY
A systematic review of the effect of combined OCPs on women with a family history of breast cancer found no additional increase in risk.1 Investigators identified 3 retrospective cohort studies (N=66,500, with 8500 cases) and 7 case-control studies (total 10,500 cases) from the past 40 years, most including women from the United States and Canada, but one including women from 5 continents.
In most trials, women of reproductive age using combined OCPs had 1 or more first-degree female relatives with breast cancer, although a few trials also included second-degree relatives. Women ranged in age from 20 to 79 years at diagnosis, and most trials controlled for age, parity, menstrual and menopausal history, duration of OCP exposure, and age at first use. Follow-up intervals for the retrospective cohort studies ranged from 5 to 16 years. Investigators were unable to combine results because of heterogenous populations.
Three of the cohort studies found no significant difference in breast cancer risk between OCP users and nonusers, regardless of age or duration of use. One cohort study found an increased risk in women taking older, higher-dose OCPs from before 1975 (relative risk [RR]=3.3; 95% confidence interval [CI], 1.5-7.2). All of the case-control studies found no significant difference in breast cancer risk for any age of starting, duration of OCP use, or degree of relative with breast cancer.
A meta-analysis of 54 case-control studies (6757 cases), comprising approximately 90% of the epidemiologic information on this topic, also found no significant difference in breast cancer risk related to OCP use among women with one or more first-degree relatives with breast cancer.2 Investigators found that neither recent OCP use (<10 years, RR=0.77; 95% CI, 0.54-1.11) nor past OCP use (>10 years, RR=1.01; 95% CI, 0.80-1.28) affected risk of developing breast cancer.
Three additional case-control studies involving women with a family history of breast cancer also found no significant association for breast cancer incidence among OCP users compared with nonusers.3-5
Modern combined OCPs don’t raise risk in women with BRCA1/2 mutations
A meta-analysis of 5 studies (one retrospective cohort, 4 case-control, with a total of 2855 breast cancer cases and 2944 controls) evaluated whether combined OCPs increased the risk of breast cancer in women, all of whom were carrying BRCA1/2 mutations.6
Using modern combined OCPs didn’t raise the risk of breast cancer in BRCA1/2 carriers overall (RR=1.13; 95% CI, 0.88-1.45) or separately in BRCA1 carriers (5 studies, RR=1.09; 95% CI, 0.77-1.54) or BRCA2 carriers (3 studies, RR=1.15; 95% CI, 0.88-1.45).
However, pre-1975 (higher dose) combined OCPs produced significantly increased risk (RR=1.47; 95% CI, 1.06-2.04). Similarly, women who had used combined OCPs >10 years before the study (older women, likely to have been using pre-1975 OCPs) also had significantly increased risk (RR=1.46; 95% CI, 1.07-2.07).
A bit of good news: Combined OCPs reduce ovarian cancer risk
The analysis also determined that combined OCPs significantly reduced the risk of ovarian cancer in women carrying BRCA1/2 mutations (RR=0.50; 95% CI, 0.33-0.75), with an additional linear decrease in risk for each 10 years of OCP use (RR=0.64; 95% CI, 0.53-0.78).
RECOMMENDATIONS
The World Health Organization guidelines outlining criteria for contraceptive use state that OCPs don’t alter the risk of breast cancer among women with either a family history of breast cancer or breast cancer susceptibility genes.7
The American College of Obstetricians and Gynecologists (ACOG) says that a positive family history of breast cancer shouldn’t be regarded as a contraindication to OCP use.8 ACOG also says that women with the BRCA1 mutation have an increased risk of breast cancer if they used OCPs for longer than 5 years before age 30, but this risk may be more than balanced by the benefit of a greatly reduced risk of ovarian cancer.
1. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
2. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative re-analysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
3. Jernström H, Loman N, Johannsson OT, et al. Impact of teenage oral contraceptive use in a population-based series of early-onset breast cancer cases who have undergone BRCA mutation testing. Eur J Cancer. 2005;41:2312-2320.
4. Cibula D, Gompel A, Mueck AO, et al. Hormonal contraception and risk of cancer. Human Reprod Update. 2010;16: 631-650.
5. Long-term oral contraceptive use and the risk of breast cancer. The Centers for Disease Control Cancer and Steroid Hormone Study. JAMA. 1983;249:1591-1595.
6. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
7. World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 4th ed. Geneva, Switzerland: World Health Organization; 2009. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed September 24, 2013.
8. ACOG Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
No. Modern combined oral contraceptive pills (OCPs) don’t increase breast cancer risk in women with a family history (strength of recommendation [SOR]: B, systematic review of cohort, case-control studies). However, older, higher-dose OCPs (in use before 1975) did increase breast cancer risk in these women (SOR: C, case-control study).
Similarly, modern OCPs don’t raise breast cancer risk in women with BRCA1/2 mutations, although higher-dose, pre-1975 OCPs did (SOR: B and C, a meta-analysis of cohort and case-control studies).
EVIDENCE SUMMARY
A systematic review of the effect of combined OCPs on women with a family history of breast cancer found no additional increase in risk.1 Investigators identified 3 retrospective cohort studies (N=66,500, with 8500 cases) and 7 case-control studies (total 10,500 cases) from the past 40 years, most including women from the United States and Canada, but one including women from 5 continents.
In most trials, women of reproductive age using combined OCPs had 1 or more first-degree female relatives with breast cancer, although a few trials also included second-degree relatives. Women ranged in age from 20 to 79 years at diagnosis, and most trials controlled for age, parity, menstrual and menopausal history, duration of OCP exposure, and age at first use. Follow-up intervals for the retrospective cohort studies ranged from 5 to 16 years. Investigators were unable to combine results because of heterogenous populations.
Three of the cohort studies found no significant difference in breast cancer risk between OCP users and nonusers, regardless of age or duration of use. One cohort study found an increased risk in women taking older, higher-dose OCPs from before 1975 (relative risk [RR]=3.3; 95% confidence interval [CI], 1.5-7.2). All of the case-control studies found no significant difference in breast cancer risk for any age of starting, duration of OCP use, or degree of relative with breast cancer.
A meta-analysis of 54 case-control studies (6757 cases), comprising approximately 90% of the epidemiologic information on this topic, also found no significant difference in breast cancer risk related to OCP use among women with one or more first-degree relatives with breast cancer.2 Investigators found that neither recent OCP use (<10 years, RR=0.77; 95% CI, 0.54-1.11) nor past OCP use (>10 years, RR=1.01; 95% CI, 0.80-1.28) affected risk of developing breast cancer.
Three additional case-control studies involving women with a family history of breast cancer also found no significant association for breast cancer incidence among OCP users compared with nonusers.3-5
Modern combined OCPs don’t raise risk in women with BRCA1/2 mutations
A meta-analysis of 5 studies (one retrospective cohort, 4 case-control, with a total of 2855 breast cancer cases and 2944 controls) evaluated whether combined OCPs increased the risk of breast cancer in women, all of whom were carrying BRCA1/2 mutations.6
Using modern combined OCPs didn’t raise the risk of breast cancer in BRCA1/2 carriers overall (RR=1.13; 95% CI, 0.88-1.45) or separately in BRCA1 carriers (5 studies, RR=1.09; 95% CI, 0.77-1.54) or BRCA2 carriers (3 studies, RR=1.15; 95% CI, 0.88-1.45).
However, pre-1975 (higher dose) combined OCPs produced significantly increased risk (RR=1.47; 95% CI, 1.06-2.04). Similarly, women who had used combined OCPs >10 years before the study (older women, likely to have been using pre-1975 OCPs) also had significantly increased risk (RR=1.46; 95% CI, 1.07-2.07).
A bit of good news: Combined OCPs reduce ovarian cancer risk
The analysis also determined that combined OCPs significantly reduced the risk of ovarian cancer in women carrying BRCA1/2 mutations (RR=0.50; 95% CI, 0.33-0.75), with an additional linear decrease in risk for each 10 years of OCP use (RR=0.64; 95% CI, 0.53-0.78).
RECOMMENDATIONS
The World Health Organization guidelines outlining criteria for contraceptive use state that OCPs don’t alter the risk of breast cancer among women with either a family history of breast cancer or breast cancer susceptibility genes.7
The American College of Obstetricians and Gynecologists (ACOG) says that a positive family history of breast cancer shouldn’t be regarded as a contraindication to OCP use.8 ACOG also says that women with the BRCA1 mutation have an increased risk of breast cancer if they used OCPs for longer than 5 years before age 30, but this risk may be more than balanced by the benefit of a greatly reduced risk of ovarian cancer.
No. Modern combined oral contraceptive pills (OCPs) don’t increase breast cancer risk in women with a family history (strength of recommendation [SOR]: B, systematic review of cohort, case-control studies). However, older, higher-dose OCPs (in use before 1975) did increase breast cancer risk in these women (SOR: C, case-control study).
Similarly, modern OCPs don’t raise breast cancer risk in women with BRCA1/2 mutations, although higher-dose, pre-1975 OCPs did (SOR: B and C, a meta-analysis of cohort and case-control studies).
EVIDENCE SUMMARY
A systematic review of the effect of combined OCPs on women with a family history of breast cancer found no additional increase in risk.1 Investigators identified 3 retrospective cohort studies (N=66,500, with 8500 cases) and 7 case-control studies (total 10,500 cases) from the past 40 years, most including women from the United States and Canada, but one including women from 5 continents.
In most trials, women of reproductive age using combined OCPs had 1 or more first-degree female relatives with breast cancer, although a few trials also included second-degree relatives. Women ranged in age from 20 to 79 years at diagnosis, and most trials controlled for age, parity, menstrual and menopausal history, duration of OCP exposure, and age at first use. Follow-up intervals for the retrospective cohort studies ranged from 5 to 16 years. Investigators were unable to combine results because of heterogenous populations.
Three of the cohort studies found no significant difference in breast cancer risk between OCP users and nonusers, regardless of age or duration of use. One cohort study found an increased risk in women taking older, higher-dose OCPs from before 1975 (relative risk [RR]=3.3; 95% confidence interval [CI], 1.5-7.2). All of the case-control studies found no significant difference in breast cancer risk for any age of starting, duration of OCP use, or degree of relative with breast cancer.
A meta-analysis of 54 case-control studies (6757 cases), comprising approximately 90% of the epidemiologic information on this topic, also found no significant difference in breast cancer risk related to OCP use among women with one or more first-degree relatives with breast cancer.2 Investigators found that neither recent OCP use (<10 years, RR=0.77; 95% CI, 0.54-1.11) nor past OCP use (>10 years, RR=1.01; 95% CI, 0.80-1.28) affected risk of developing breast cancer.
Three additional case-control studies involving women with a family history of breast cancer also found no significant association for breast cancer incidence among OCP users compared with nonusers.3-5
Modern combined OCPs don’t raise risk in women with BRCA1/2 mutations
A meta-analysis of 5 studies (one retrospective cohort, 4 case-control, with a total of 2855 breast cancer cases and 2944 controls) evaluated whether combined OCPs increased the risk of breast cancer in women, all of whom were carrying BRCA1/2 mutations.6
Using modern combined OCPs didn’t raise the risk of breast cancer in BRCA1/2 carriers overall (RR=1.13; 95% CI, 0.88-1.45) or separately in BRCA1 carriers (5 studies, RR=1.09; 95% CI, 0.77-1.54) or BRCA2 carriers (3 studies, RR=1.15; 95% CI, 0.88-1.45).
However, pre-1975 (higher dose) combined OCPs produced significantly increased risk (RR=1.47; 95% CI, 1.06-2.04). Similarly, women who had used combined OCPs >10 years before the study (older women, likely to have been using pre-1975 OCPs) also had significantly increased risk (RR=1.46; 95% CI, 1.07-2.07).
A bit of good news: Combined OCPs reduce ovarian cancer risk
The analysis also determined that combined OCPs significantly reduced the risk of ovarian cancer in women carrying BRCA1/2 mutations (RR=0.50; 95% CI, 0.33-0.75), with an additional linear decrease in risk for each 10 years of OCP use (RR=0.64; 95% CI, 0.53-0.78).
RECOMMENDATIONS
The World Health Organization guidelines outlining criteria for contraceptive use state that OCPs don’t alter the risk of breast cancer among women with either a family history of breast cancer or breast cancer susceptibility genes.7
The American College of Obstetricians and Gynecologists (ACOG) says that a positive family history of breast cancer shouldn’t be regarded as a contraindication to OCP use.8 ACOG also says that women with the BRCA1 mutation have an increased risk of breast cancer if they used OCPs for longer than 5 years before age 30, but this risk may be more than balanced by the benefit of a greatly reduced risk of ovarian cancer.
1. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
2. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative re-analysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
3. Jernström H, Loman N, Johannsson OT, et al. Impact of teenage oral contraceptive use in a population-based series of early-onset breast cancer cases who have undergone BRCA mutation testing. Eur J Cancer. 2005;41:2312-2320.
4. Cibula D, Gompel A, Mueck AO, et al. Hormonal contraception and risk of cancer. Human Reprod Update. 2010;16: 631-650.
5. Long-term oral contraceptive use and the risk of breast cancer. The Centers for Disease Control Cancer and Steroid Hormone Study. JAMA. 1983;249:1591-1595.
6. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
7. World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 4th ed. Geneva, Switzerland: World Health Organization; 2009. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed September 24, 2013.
8. ACOG Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
1. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
2. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative re-analysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
3. Jernström H, Loman N, Johannsson OT, et al. Impact of teenage oral contraceptive use in a population-based series of early-onset breast cancer cases who have undergone BRCA mutation testing. Eur J Cancer. 2005;41:2312-2320.
4. Cibula D, Gompel A, Mueck AO, et al. Hormonal contraception and risk of cancer. Human Reprod Update. 2010;16: 631-650.
5. Long-term oral contraceptive use and the risk of breast cancer. The Centers for Disease Control Cancer and Steroid Hormone Study. JAMA. 1983;249:1591-1595.
6. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
7. World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 4th ed. Geneva, Switzerland: World Health Organization; 2009. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed September 24, 2013.
8. ACOG Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best nonsurgical therapy for pelvic organ prolapse?
Pelvic floor muscle training (PFMT) and pessaries are equally effective in treating symptoms of pelvic organ prolapse (POP). PFMT transiently improves patient satisfaction and reduces urinary incontinence more than pessaries do (strength of recommendation [SOR]: B, a randomized controlled trial [RCT]).
PFMT moderately improves prolapse symptoms and severity, especially following 6 months of supervised intervention (SOR: B, a systematic review of randomized trials with some methodologic flaws).
Two pessaries (ring with support and Gellhorn) reduce symptoms in as many as 60% of patients (SOR: B, a systematic review of randomized trials).
Untreated postmenopausal women with mild grades of uterine prolapse are unlikely to develop more severe prolapse; 25% to 50% improve spontaneously (SOR: C, a prospective cohort study with methodologic flaws).
EVIDENCE SUMMARY
A 2010 multicenter RCT with 445 women (mean age 49.8 years) compared PFMT, pessary use, and combined treatment.1 Investigators used the Patient Global Impression of Improvement and the stress incontinence subscale of the Pelvic Floor Distress Inventory to measure patient satisfaction and urinary incontinence symptoms.
At 3 months, equivalent numbers of women using PFMT and a pessary (49% and 40%, respectively; P=.09) reported they were “much better” or “very much better.” More women in the PFMT cohort than women using a pessary reported resolution of incontinence symptoms at 3 months (49% vs 33%; P=.006), and satisfaction with treatment (75% vs 63%; P=.02), but these differences disappeared at 12 months. Combination therapy wasn’t superior to PFMT alone.
Pelvic floor muscle training improves symptoms, especially with perseverance
A 2011 Cochrane review that compared women receiving PFMT with a control group (observed but not treated) found that PFMT moderately improved prolapse symptoms and severity, especially following 6 months of supervised intervention.2 Investigators evalu-ated 4 trials, (N=857), including 3 with fewer than 25 women per arm.
Three studies found that PFMT improved symptom severity and manometric measures. Although the authors couldn’t pool the data because of different symptom scoring instruments, typical improvements ranged from 20% to 30%. Two trials found that PFMT increased the chance of improvement in POP stage by 17% (pooled data, relative risk=.83; 95% confidence interval [CI], .71-.96). PFMT also improved urinary outcomes (approximately 30% reduction in urinary frequency and stress incontinence symptoms) in 2 of 3 trials and improved bowel symptoms in one trial (approximately 25% to 30% reduction).
Pessaries also relieve symptoms
A 2013 Cochrane Review seeking to determine the effectiveness of pessaries in POP, identified one RCT (crossover, 3 month, multicenter, United States) that compared symptom relief and change in life impact over baseline for 134 women (parous, mean age 61 years, range 30-89 years) with POP stage II or greater who were treated with ring with support or Gellhorn pessaries.3 Sixty percent of patients who completed the study (the dropout rate was 37%) reported symptom relief with both types of pessary. Outcomes were measured by multiple questionnaires and Likert scales.
Patients reported improved symptoms on both the Pelvic Organ Prolapse Distress Inventory (POPDI) and Pelvic Organ Prolapse Impact Questionnaire (POPIQ) scales (P<.05 for difference from baseline on each scale, actual scores not reported). The ring with support and Gellhorn pessaries didn’t produce different scores on either scale (POPDI, P=.99; POPIQ, P=.29).
Untreated mild prolapse postmenopause usually doesn’t progress and may regress
A cohort of 412 postmenopausal women (ages ≥50 years) with POP who were observed, but not treated, found that mild POP was unlikely to progress and sometimes improved spontaneously.4 Over a mean follow-up of 5.7 years, few women with grade 1 POP (prolapsed pelvic organs remaining within the vagina) progressed to grade 2 or 3 (probability of progression for women with cystoceles=.095, 95% CI, .07-.13; women with rectoceles=.135, 95% CI, .09-.19; and women with uterine prolapse=.019, 95% CI, .0005-.099).
Some women with grade 1 POP regressed to grade 0 (probability of regression for women with cystoceles=.235, 95% CI, .19- .28; women with rectoceles=.22, 95% CI, .16-.28; and women with uterine prolapse=.48, 95% CI, 0.34-.62). Women with grades 2 and 3 POP were less likely to regress to grade 0 (probability of regression for women with cystoceles=.093, 95% CI, .05-.14; women with rectoceles=.033, 95% CI, .011-.075; and women with uterine prolapse=0, 95% CI, 0-.37).
One flaw of this study was that the women received hormone replacement therapy, which the investigators didn’t evaluate independently. However, a 2010 Cochrane review (2 small trials, one meta-analysis) found insufficient data to determine whether hormone replacement therapy alters POP.5
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists Practice Bulletin on POP recommends the following:6
- Pessaries can be fitted in most women with prolapse, regardless of prolapse stage (equivalent to grade) or site of predominant prolapse.
- Pessary use should be considered before surgical intervention in women with symptomatic prolapse.
- Women with prolapse who are asymptomatic or mildly symptomatic can be observed at regular intervals, unless new bothersome symptoms develop.
1. Richter HE, Burgio KL, Brubaker L, et al;Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
2. Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2011;12:CD003882.
3. Bugge C, Adams EJ, Gopinath D, et al. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;2:CD004010.
4. Handa VL, Garrett E, Hendrix S, et al. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27-32.
5. Ismail SI, Bain C, Hagen S. Oestrogens for treatment or prevention of pelvic organ prolapse in postmenopausal women. Cochrane Database Syst Rev. 2010;9:CD007063.
6. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110:717-729.
Pelvic floor muscle training (PFMT) and pessaries are equally effective in treating symptoms of pelvic organ prolapse (POP). PFMT transiently improves patient satisfaction and reduces urinary incontinence more than pessaries do (strength of recommendation [SOR]: B, a randomized controlled trial [RCT]).
PFMT moderately improves prolapse symptoms and severity, especially following 6 months of supervised intervention (SOR: B, a systematic review of randomized trials with some methodologic flaws).
Two pessaries (ring with support and Gellhorn) reduce symptoms in as many as 60% of patients (SOR: B, a systematic review of randomized trials).
Untreated postmenopausal women with mild grades of uterine prolapse are unlikely to develop more severe prolapse; 25% to 50% improve spontaneously (SOR: C, a prospective cohort study with methodologic flaws).
EVIDENCE SUMMARY
A 2010 multicenter RCT with 445 women (mean age 49.8 years) compared PFMT, pessary use, and combined treatment.1 Investigators used the Patient Global Impression of Improvement and the stress incontinence subscale of the Pelvic Floor Distress Inventory to measure patient satisfaction and urinary incontinence symptoms.
At 3 months, equivalent numbers of women using PFMT and a pessary (49% and 40%, respectively; P=.09) reported they were “much better” or “very much better.” More women in the PFMT cohort than women using a pessary reported resolution of incontinence symptoms at 3 months (49% vs 33%; P=.006), and satisfaction with treatment (75% vs 63%; P=.02), but these differences disappeared at 12 months. Combination therapy wasn’t superior to PFMT alone.
Pelvic floor muscle training improves symptoms, especially with perseverance
A 2011 Cochrane review that compared women receiving PFMT with a control group (observed but not treated) found that PFMT moderately improved prolapse symptoms and severity, especially following 6 months of supervised intervention.2 Investigators evalu-ated 4 trials, (N=857), including 3 with fewer than 25 women per arm.
Three studies found that PFMT improved symptom severity and manometric measures. Although the authors couldn’t pool the data because of different symptom scoring instruments, typical improvements ranged from 20% to 30%. Two trials found that PFMT increased the chance of improvement in POP stage by 17% (pooled data, relative risk=.83; 95% confidence interval [CI], .71-.96). PFMT also improved urinary outcomes (approximately 30% reduction in urinary frequency and stress incontinence symptoms) in 2 of 3 trials and improved bowel symptoms in one trial (approximately 25% to 30% reduction).
Pessaries also relieve symptoms
A 2013 Cochrane Review seeking to determine the effectiveness of pessaries in POP, identified one RCT (crossover, 3 month, multicenter, United States) that compared symptom relief and change in life impact over baseline for 134 women (parous, mean age 61 years, range 30-89 years) with POP stage II or greater who were treated with ring with support or Gellhorn pessaries.3 Sixty percent of patients who completed the study (the dropout rate was 37%) reported symptom relief with both types of pessary. Outcomes were measured by multiple questionnaires and Likert scales.
Patients reported improved symptoms on both the Pelvic Organ Prolapse Distress Inventory (POPDI) and Pelvic Organ Prolapse Impact Questionnaire (POPIQ) scales (P<.05 for difference from baseline on each scale, actual scores not reported). The ring with support and Gellhorn pessaries didn’t produce different scores on either scale (POPDI, P=.99; POPIQ, P=.29).
Untreated mild prolapse postmenopause usually doesn’t progress and may regress
A cohort of 412 postmenopausal women (ages ≥50 years) with POP who were observed, but not treated, found that mild POP was unlikely to progress and sometimes improved spontaneously.4 Over a mean follow-up of 5.7 years, few women with grade 1 POP (prolapsed pelvic organs remaining within the vagina) progressed to grade 2 or 3 (probability of progression for women with cystoceles=.095, 95% CI, .07-.13; women with rectoceles=.135, 95% CI, .09-.19; and women with uterine prolapse=.019, 95% CI, .0005-.099).
Some women with grade 1 POP regressed to grade 0 (probability of regression for women with cystoceles=.235, 95% CI, .19- .28; women with rectoceles=.22, 95% CI, .16-.28; and women with uterine prolapse=.48, 95% CI, 0.34-.62). Women with grades 2 and 3 POP were less likely to regress to grade 0 (probability of regression for women with cystoceles=.093, 95% CI, .05-.14; women with rectoceles=.033, 95% CI, .011-.075; and women with uterine prolapse=0, 95% CI, 0-.37).
One flaw of this study was that the women received hormone replacement therapy, which the investigators didn’t evaluate independently. However, a 2010 Cochrane review (2 small trials, one meta-analysis) found insufficient data to determine whether hormone replacement therapy alters POP.5
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists Practice Bulletin on POP recommends the following:6
- Pessaries can be fitted in most women with prolapse, regardless of prolapse stage (equivalent to grade) or site of predominant prolapse.
- Pessary use should be considered before surgical intervention in women with symptomatic prolapse.
- Women with prolapse who are asymptomatic or mildly symptomatic can be observed at regular intervals, unless new bothersome symptoms develop.
Pelvic floor muscle training (PFMT) and pessaries are equally effective in treating symptoms of pelvic organ prolapse (POP). PFMT transiently improves patient satisfaction and reduces urinary incontinence more than pessaries do (strength of recommendation [SOR]: B, a randomized controlled trial [RCT]).
PFMT moderately improves prolapse symptoms and severity, especially following 6 months of supervised intervention (SOR: B, a systematic review of randomized trials with some methodologic flaws).
Two pessaries (ring with support and Gellhorn) reduce symptoms in as many as 60% of patients (SOR: B, a systematic review of randomized trials).
Untreated postmenopausal women with mild grades of uterine prolapse are unlikely to develop more severe prolapse; 25% to 50% improve spontaneously (SOR: C, a prospective cohort study with methodologic flaws).
EVIDENCE SUMMARY
A 2010 multicenter RCT with 445 women (mean age 49.8 years) compared PFMT, pessary use, and combined treatment.1 Investigators used the Patient Global Impression of Improvement and the stress incontinence subscale of the Pelvic Floor Distress Inventory to measure patient satisfaction and urinary incontinence symptoms.
At 3 months, equivalent numbers of women using PFMT and a pessary (49% and 40%, respectively; P=.09) reported they were “much better” or “very much better.” More women in the PFMT cohort than women using a pessary reported resolution of incontinence symptoms at 3 months (49% vs 33%; P=.006), and satisfaction with treatment (75% vs 63%; P=.02), but these differences disappeared at 12 months. Combination therapy wasn’t superior to PFMT alone.
Pelvic floor muscle training improves symptoms, especially with perseverance
A 2011 Cochrane review that compared women receiving PFMT with a control group (observed but not treated) found that PFMT moderately improved prolapse symptoms and severity, especially following 6 months of supervised intervention.2 Investigators evalu-ated 4 trials, (N=857), including 3 with fewer than 25 women per arm.
Three studies found that PFMT improved symptom severity and manometric measures. Although the authors couldn’t pool the data because of different symptom scoring instruments, typical improvements ranged from 20% to 30%. Two trials found that PFMT increased the chance of improvement in POP stage by 17% (pooled data, relative risk=.83; 95% confidence interval [CI], .71-.96). PFMT also improved urinary outcomes (approximately 30% reduction in urinary frequency and stress incontinence symptoms) in 2 of 3 trials and improved bowel symptoms in one trial (approximately 25% to 30% reduction).
Pessaries also relieve symptoms
A 2013 Cochrane Review seeking to determine the effectiveness of pessaries in POP, identified one RCT (crossover, 3 month, multicenter, United States) that compared symptom relief and change in life impact over baseline for 134 women (parous, mean age 61 years, range 30-89 years) with POP stage II or greater who were treated with ring with support or Gellhorn pessaries.3 Sixty percent of patients who completed the study (the dropout rate was 37%) reported symptom relief with both types of pessary. Outcomes were measured by multiple questionnaires and Likert scales.
Patients reported improved symptoms on both the Pelvic Organ Prolapse Distress Inventory (POPDI) and Pelvic Organ Prolapse Impact Questionnaire (POPIQ) scales (P<.05 for difference from baseline on each scale, actual scores not reported). The ring with support and Gellhorn pessaries didn’t produce different scores on either scale (POPDI, P=.99; POPIQ, P=.29).
Untreated mild prolapse postmenopause usually doesn’t progress and may regress
A cohort of 412 postmenopausal women (ages ≥50 years) with POP who were observed, but not treated, found that mild POP was unlikely to progress and sometimes improved spontaneously.4 Over a mean follow-up of 5.7 years, few women with grade 1 POP (prolapsed pelvic organs remaining within the vagina) progressed to grade 2 or 3 (probability of progression for women with cystoceles=.095, 95% CI, .07-.13; women with rectoceles=.135, 95% CI, .09-.19; and women with uterine prolapse=.019, 95% CI, .0005-.099).
Some women with grade 1 POP regressed to grade 0 (probability of regression for women with cystoceles=.235, 95% CI, .19- .28; women with rectoceles=.22, 95% CI, .16-.28; and women with uterine prolapse=.48, 95% CI, 0.34-.62). Women with grades 2 and 3 POP were less likely to regress to grade 0 (probability of regression for women with cystoceles=.093, 95% CI, .05-.14; women with rectoceles=.033, 95% CI, .011-.075; and women with uterine prolapse=0, 95% CI, 0-.37).
One flaw of this study was that the women received hormone replacement therapy, which the investigators didn’t evaluate independently. However, a 2010 Cochrane review (2 small trials, one meta-analysis) found insufficient data to determine whether hormone replacement therapy alters POP.5
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists Practice Bulletin on POP recommends the following:6
- Pessaries can be fitted in most women with prolapse, regardless of prolapse stage (equivalent to grade) or site of predominant prolapse.
- Pessary use should be considered before surgical intervention in women with symptomatic prolapse.
- Women with prolapse who are asymptomatic or mildly symptomatic can be observed at regular intervals, unless new bothersome symptoms develop.
1. Richter HE, Burgio KL, Brubaker L, et al;Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
2. Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2011;12:CD003882.
3. Bugge C, Adams EJ, Gopinath D, et al. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;2:CD004010.
4. Handa VL, Garrett E, Hendrix S, et al. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27-32.
5. Ismail SI, Bain C, Hagen S. Oestrogens for treatment or prevention of pelvic organ prolapse in postmenopausal women. Cochrane Database Syst Rev. 2010;9:CD007063.
6. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110:717-729.
1. Richter HE, Burgio KL, Brubaker L, et al;Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
2. Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2011;12:CD003882.
3. Bugge C, Adams EJ, Gopinath D, et al. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;2:CD004010.
4. Handa VL, Garrett E, Hendrix S, et al. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27-32.
5. Ismail SI, Bain C, Hagen S. Oestrogens for treatment or prevention of pelvic organ prolapse in postmenopausal women. Cochrane Database Syst Rev. 2010;9:CD007063.
6. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110:717-729.
Evidence-based answers from the Family Physicians Inquiries Network
Are topical nitrates safe and effective for upper extremity tendinopathies?
Topical nitrates provide short-term relief with some side effects, especially headache. Topical nitroglycerin (NTG) patches improve subjective pain scores by about 30% and range of motion over 3 days in patients with acute shoulder tendinopathy (strength of recommendation [SOR]: C, small randomized controlled trial [RCT] with no methodologic flaws).
NTG patches, when combined with tendon rehabilitation, improve subjective pain ratings by about 30% and shoulder strength by about 10% in patients with chronic shoulder tendinopathy over 3 to 6 months, but not in the long term (SOR: C, RCTs with methodologic flaws). They improve pain and strength 15% to 50% for chronic extensor tendinosis of the elbow over a 6-month period (SOR: C, small RCT with methodologic flaws).
NTG patches used without tendon rehabilitation don’t improve pain or strength in chronic lateral epicondylitis over 8 weeks (SOR: C, RCT).
Topical NTG patches commonly produce headaches and rashes (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A small RCT found that NTG therapy improved short-term pain and joint mobility in patients with acute supraspinatus tendinitis.1 Investigators randomized 10 men and 10 women with acute shoulder tendonitis (fewer than 7 days’ duration) to use either 5-mg NTG patches or placebo patches daily for 3 days. Patients rated pain on a 10-point scale, and investigators measured joint mobility on a 4-point scale.
After 48 hours of treatment, NTG patches significantly reduced pain ratings from baseline (from 7 to 2 points; P<.001), whereas placebo didn’t (6 vs 6 points; P not significant). NTG patches also improved joint mobility from baseline (from 2 points “moderately restricted” to .1 points “not restricted”; P<.001), but placebo didn’t (1.2 points “mildly restricted” vs 1.2 points; P not significant). The placebo group had less pain and joint restriction than the NTG group at the start of the study. Two patients reported headache 24 hours after starting treatment.
NTG plus rehabilitation improves chronic shoulder pain, range of motion
A double-blind RCT evaluating NTG patches for 53 patients (57 shoulders) with chronic supraspinatus tendinopathy (shoulder pain lasting longer than 3 months) found that they improved pain, strength, and range of motion at 3 to 6 months.2 Investigators randomized patients to receive one-quarter of a 5-mg 24-hour NTG patch or placebo patch daily and enrolled all patients in a rehabilitation program. They assessed subjective pain (at night and with activity), strength, and external rotation at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved nighttime pain about 30% (at 12 and 24 weeks), pain with activity about 60% (at 24 weeks), strength about 10% (at 12 and 24 weeks), and range of motion about 20% (at 24 weeks; P<.05 for all comparisons). The placebo group initially had more pain, less strength, and less mobility than the NTG group. Investigators reported no adverse effects.
NTG and rehab improve elbow pain, but with side effects
Another RCT comparing topical NTG patches in patients with chronic extensor tendinosis of the elbow found that they improved most parameters.3 Investigators randomized 86 patients with elbow tendonitis (longer than 3 months) to NTG patches (one-quarter of a 5-mg 24-hour patch) or placebo patches and enrolled all patients in a tendon rehabilitation program. They assessed subjective pain, extensor tendon tenderness, and muscle strength at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved subjective pain, tendon tenderness, and strength significantly more than placebo at all follow-up points, by 15% to 50% (P<.05 for all comparisons). The study was flawed because the control group started with more pain, tenderness, and weakness than the NTG group. Five patients discontinued NTG because of adverse effects (headache, dermatitis, and facial flushing).
A follow-up study done 5 years after discontinuation of therapy found equal outcomes with NTG and placebo.4 Investigators evaluated, by phone or in person, 58 of the 86 patients in the original study. NTG and placebo therapy produced equivalent reductions in subjective 0 to 4 elbow pain scores over baseline (average pain 2.5 initially, 1.5 at 12 weeks, and 1.0 at 5 years; P<.01 for all comparisons with baseline, no significant difference between nitrates and placebo).
NTG without rehab works no better than placebo
Another RCT that evaluated 3 different doses of NTG patches for 8 weeks in 154 patients with chronic lateral epicondylosis found NTG treatment was no better than placebo for pain or strength.5 Investigators randomized patients with more than 3 months of symptoms to 3 NTG patch doses (.72 mg/24 h, 1.44 mg/ 24 h, or 3.6 mg/24 h) compared with placebo and evaluated subjective pain (at rest, with activity, and at night), grip strength, and force, at baseline and 8 weeks.
The study lacked a formal wrist strengthening rehabilitation program. Patients in the placebo group had lower baseline pain scores than the NTG groups. Seven patients dropped out of the study because of headaches.
RECOMMENDATIONS
We found no authoritative recommendations regarding the use of topical nitrates for upper extremity tendinopathies.
An online reference text doesn’t make a recommendation, but references the studies described previously.6 The authors state that headache is the most common adverse effect of topical nitrates, but it becomes less severe over the course of treatment. They recommend caution in patients with hypotension, pregnancy, or migraines, and those who take diuretics. The authors also note that nitrates are relatively contraindicated in patients with ischemic heart disease, anemia, phosphodiesterase inhibitor therapy (such as sildenafil), angle-closure glaucoma, and allergy to nitrates.
1. Berrazueta JR, Losada A, Poveda J, et al. Successful treatment of shoulder pain syndrome due to supraspinatus tendinitis with transdermal nitroglycerin. A double blind study. Pain. 1996;66:63-67.
2. Paoloni JA, Appleyard RC, Nelson J, et al. Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2005;33:806-813.
3. Paoloni JA, Appleyard RC, Nelson J, et al. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31:915-920.
4. McCallum SD, Paoloni JA, Murrell GA, et al. Five-year prospective comparison study of topical glyceryl trinitrate treatment of chronic lateral epicondylosis at the elbow. Br J Sports Med. 2011;45:416-420.
5. Paolini JA, Murrell GA, Burch RM, et al. Randomised, double-blind, placebo-controlled clinical trial of a new topical glyceryl trinitrate patch for chronic lateral epicondylosis. Br J Sports Med. 2009;43:299-302.
6. Simons SM, Kruse D. Rotator cuff tendinopathy. UpToDate Web site. Available at: www.uptodate.com/contents/rotator-cuff-tendinopathy. Accessed February 19, 2014.
Topical nitrates provide short-term relief with some side effects, especially headache. Topical nitroglycerin (NTG) patches improve subjective pain scores by about 30% and range of motion over 3 days in patients with acute shoulder tendinopathy (strength of recommendation [SOR]: C, small randomized controlled trial [RCT] with no methodologic flaws).
NTG patches, when combined with tendon rehabilitation, improve subjective pain ratings by about 30% and shoulder strength by about 10% in patients with chronic shoulder tendinopathy over 3 to 6 months, but not in the long term (SOR: C, RCTs with methodologic flaws). They improve pain and strength 15% to 50% for chronic extensor tendinosis of the elbow over a 6-month period (SOR: C, small RCT with methodologic flaws).
NTG patches used without tendon rehabilitation don’t improve pain or strength in chronic lateral epicondylitis over 8 weeks (SOR: C, RCT).
Topical NTG patches commonly produce headaches and rashes (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A small RCT found that NTG therapy improved short-term pain and joint mobility in patients with acute supraspinatus tendinitis.1 Investigators randomized 10 men and 10 women with acute shoulder tendonitis (fewer than 7 days’ duration) to use either 5-mg NTG patches or placebo patches daily for 3 days. Patients rated pain on a 10-point scale, and investigators measured joint mobility on a 4-point scale.
After 48 hours of treatment, NTG patches significantly reduced pain ratings from baseline (from 7 to 2 points; P<.001), whereas placebo didn’t (6 vs 6 points; P not significant). NTG patches also improved joint mobility from baseline (from 2 points “moderately restricted” to .1 points “not restricted”; P<.001), but placebo didn’t (1.2 points “mildly restricted” vs 1.2 points; P not significant). The placebo group had less pain and joint restriction than the NTG group at the start of the study. Two patients reported headache 24 hours after starting treatment.
NTG plus rehabilitation improves chronic shoulder pain, range of motion
A double-blind RCT evaluating NTG patches for 53 patients (57 shoulders) with chronic supraspinatus tendinopathy (shoulder pain lasting longer than 3 months) found that they improved pain, strength, and range of motion at 3 to 6 months.2 Investigators randomized patients to receive one-quarter of a 5-mg 24-hour NTG patch or placebo patch daily and enrolled all patients in a rehabilitation program. They assessed subjective pain (at night and with activity), strength, and external rotation at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved nighttime pain about 30% (at 12 and 24 weeks), pain with activity about 60% (at 24 weeks), strength about 10% (at 12 and 24 weeks), and range of motion about 20% (at 24 weeks; P<.05 for all comparisons). The placebo group initially had more pain, less strength, and less mobility than the NTG group. Investigators reported no adverse effects.
NTG and rehab improve elbow pain, but with side effects
Another RCT comparing topical NTG patches in patients with chronic extensor tendinosis of the elbow found that they improved most parameters.3 Investigators randomized 86 patients with elbow tendonitis (longer than 3 months) to NTG patches (one-quarter of a 5-mg 24-hour patch) or placebo patches and enrolled all patients in a tendon rehabilitation program. They assessed subjective pain, extensor tendon tenderness, and muscle strength at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved subjective pain, tendon tenderness, and strength significantly more than placebo at all follow-up points, by 15% to 50% (P<.05 for all comparisons). The study was flawed because the control group started with more pain, tenderness, and weakness than the NTG group. Five patients discontinued NTG because of adverse effects (headache, dermatitis, and facial flushing).
A follow-up study done 5 years after discontinuation of therapy found equal outcomes with NTG and placebo.4 Investigators evaluated, by phone or in person, 58 of the 86 patients in the original study. NTG and placebo therapy produced equivalent reductions in subjective 0 to 4 elbow pain scores over baseline (average pain 2.5 initially, 1.5 at 12 weeks, and 1.0 at 5 years; P<.01 for all comparisons with baseline, no significant difference between nitrates and placebo).
NTG without rehab works no better than placebo
Another RCT that evaluated 3 different doses of NTG patches for 8 weeks in 154 patients with chronic lateral epicondylosis found NTG treatment was no better than placebo for pain or strength.5 Investigators randomized patients with more than 3 months of symptoms to 3 NTG patch doses (.72 mg/24 h, 1.44 mg/ 24 h, or 3.6 mg/24 h) compared with placebo and evaluated subjective pain (at rest, with activity, and at night), grip strength, and force, at baseline and 8 weeks.
The study lacked a formal wrist strengthening rehabilitation program. Patients in the placebo group had lower baseline pain scores than the NTG groups. Seven patients dropped out of the study because of headaches.
RECOMMENDATIONS
We found no authoritative recommendations regarding the use of topical nitrates for upper extremity tendinopathies.
An online reference text doesn’t make a recommendation, but references the studies described previously.6 The authors state that headache is the most common adverse effect of topical nitrates, but it becomes less severe over the course of treatment. They recommend caution in patients with hypotension, pregnancy, or migraines, and those who take diuretics. The authors also note that nitrates are relatively contraindicated in patients with ischemic heart disease, anemia, phosphodiesterase inhibitor therapy (such as sildenafil), angle-closure glaucoma, and allergy to nitrates.
Topical nitrates provide short-term relief with some side effects, especially headache. Topical nitroglycerin (NTG) patches improve subjective pain scores by about 30% and range of motion over 3 days in patients with acute shoulder tendinopathy (strength of recommendation [SOR]: C, small randomized controlled trial [RCT] with no methodologic flaws).
NTG patches, when combined with tendon rehabilitation, improve subjective pain ratings by about 30% and shoulder strength by about 10% in patients with chronic shoulder tendinopathy over 3 to 6 months, but not in the long term (SOR: C, RCTs with methodologic flaws). They improve pain and strength 15% to 50% for chronic extensor tendinosis of the elbow over a 6-month period (SOR: C, small RCT with methodologic flaws).
NTG patches used without tendon rehabilitation don’t improve pain or strength in chronic lateral epicondylitis over 8 weeks (SOR: C, RCT).
Topical NTG patches commonly produce headaches and rashes (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A small RCT found that NTG therapy improved short-term pain and joint mobility in patients with acute supraspinatus tendinitis.1 Investigators randomized 10 men and 10 women with acute shoulder tendonitis (fewer than 7 days’ duration) to use either 5-mg NTG patches or placebo patches daily for 3 days. Patients rated pain on a 10-point scale, and investigators measured joint mobility on a 4-point scale.
After 48 hours of treatment, NTG patches significantly reduced pain ratings from baseline (from 7 to 2 points; P<.001), whereas placebo didn’t (6 vs 6 points; P not significant). NTG patches also improved joint mobility from baseline (from 2 points “moderately restricted” to .1 points “not restricted”; P<.001), but placebo didn’t (1.2 points “mildly restricted” vs 1.2 points; P not significant). The placebo group had less pain and joint restriction than the NTG group at the start of the study. Two patients reported headache 24 hours after starting treatment.
NTG plus rehabilitation improves chronic shoulder pain, range of motion
A double-blind RCT evaluating NTG patches for 53 patients (57 shoulders) with chronic supraspinatus tendinopathy (shoulder pain lasting longer than 3 months) found that they improved pain, strength, and range of motion at 3 to 6 months.2 Investigators randomized patients to receive one-quarter of a 5-mg 24-hour NTG patch or placebo patch daily and enrolled all patients in a rehabilitation program. They assessed subjective pain (at night and with activity), strength, and external rotation at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved nighttime pain about 30% (at 12 and 24 weeks), pain with activity about 60% (at 24 weeks), strength about 10% (at 12 and 24 weeks), and range of motion about 20% (at 24 weeks; P<.05 for all comparisons). The placebo group initially had more pain, less strength, and less mobility than the NTG group. Investigators reported no adverse effects.
NTG and rehab improve elbow pain, but with side effects
Another RCT comparing topical NTG patches in patients with chronic extensor tendinosis of the elbow found that they improved most parameters.3 Investigators randomized 86 patients with elbow tendonitis (longer than 3 months) to NTG patches (one-quarter of a 5-mg 24-hour patch) or placebo patches and enrolled all patients in a tendon rehabilitation program. They assessed subjective pain, extensor tendon tenderness, and muscle strength at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved subjective pain, tendon tenderness, and strength significantly more than placebo at all follow-up points, by 15% to 50% (P<.05 for all comparisons). The study was flawed because the control group started with more pain, tenderness, and weakness than the NTG group. Five patients discontinued NTG because of adverse effects (headache, dermatitis, and facial flushing).
A follow-up study done 5 years after discontinuation of therapy found equal outcomes with NTG and placebo.4 Investigators evaluated, by phone or in person, 58 of the 86 patients in the original study. NTG and placebo therapy produced equivalent reductions in subjective 0 to 4 elbow pain scores over baseline (average pain 2.5 initially, 1.5 at 12 weeks, and 1.0 at 5 years; P<.01 for all comparisons with baseline, no significant difference between nitrates and placebo).
NTG without rehab works no better than placebo
Another RCT that evaluated 3 different doses of NTG patches for 8 weeks in 154 patients with chronic lateral epicondylosis found NTG treatment was no better than placebo for pain or strength.5 Investigators randomized patients with more than 3 months of symptoms to 3 NTG patch doses (.72 mg/24 h, 1.44 mg/ 24 h, or 3.6 mg/24 h) compared with placebo and evaluated subjective pain (at rest, with activity, and at night), grip strength, and force, at baseline and 8 weeks.
The study lacked a formal wrist strengthening rehabilitation program. Patients in the placebo group had lower baseline pain scores than the NTG groups. Seven patients dropped out of the study because of headaches.
RECOMMENDATIONS
We found no authoritative recommendations regarding the use of topical nitrates for upper extremity tendinopathies.
An online reference text doesn’t make a recommendation, but references the studies described previously.6 The authors state that headache is the most common adverse effect of topical nitrates, but it becomes less severe over the course of treatment. They recommend caution in patients with hypotension, pregnancy, or migraines, and those who take diuretics. The authors also note that nitrates are relatively contraindicated in patients with ischemic heart disease, anemia, phosphodiesterase inhibitor therapy (such as sildenafil), angle-closure glaucoma, and allergy to nitrates.
1. Berrazueta JR, Losada A, Poveda J, et al. Successful treatment of shoulder pain syndrome due to supraspinatus tendinitis with transdermal nitroglycerin. A double blind study. Pain. 1996;66:63-67.
2. Paoloni JA, Appleyard RC, Nelson J, et al. Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2005;33:806-813.
3. Paoloni JA, Appleyard RC, Nelson J, et al. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31:915-920.
4. McCallum SD, Paoloni JA, Murrell GA, et al. Five-year prospective comparison study of topical glyceryl trinitrate treatment of chronic lateral epicondylosis at the elbow. Br J Sports Med. 2011;45:416-420.
5. Paolini JA, Murrell GA, Burch RM, et al. Randomised, double-blind, placebo-controlled clinical trial of a new topical glyceryl trinitrate patch for chronic lateral epicondylosis. Br J Sports Med. 2009;43:299-302.
6. Simons SM, Kruse D. Rotator cuff tendinopathy. UpToDate Web site. Available at: www.uptodate.com/contents/rotator-cuff-tendinopathy. Accessed February 19, 2014.
1. Berrazueta JR, Losada A, Poveda J, et al. Successful treatment of shoulder pain syndrome due to supraspinatus tendinitis with transdermal nitroglycerin. A double blind study. Pain. 1996;66:63-67.
2. Paoloni JA, Appleyard RC, Nelson J, et al. Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2005;33:806-813.
3. Paoloni JA, Appleyard RC, Nelson J, et al. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31:915-920.
4. McCallum SD, Paoloni JA, Murrell GA, et al. Five-year prospective comparison study of topical glyceryl trinitrate treatment of chronic lateral epicondylosis at the elbow. Br J Sports Med. 2011;45:416-420.
5. Paolini JA, Murrell GA, Burch RM, et al. Randomised, double-blind, placebo-controlled clinical trial of a new topical glyceryl trinitrate patch for chronic lateral epicondylosis. Br J Sports Med. 2009;43:299-302.
6. Simons SM, Kruse D. Rotator cuff tendinopathy. UpToDate Web site. Available at: www.uptodate.com/contents/rotator-cuff-tendinopathy. Accessed February 19, 2014.
Evidence-based answers from the Family Physicians Inquiries Network
Can yoga reduce symptoms of anxiety and depression?
Yes, yoga can reduce symptoms of anxiety and depression (strength of recommendation [SOR]: B, systematic reviews of randomized controlled trials [RCTs] with significant heterogeneity). Across multiple RCTs using varied yoga interventions and diverse study populations, yoga typically improves overall symptom scores for anxiety and depression by about 40%, both by itself and as an adjunctive treatment. It produces no reported harmful side effects.
EVIDENCE SUMMARY
Across 3 systematic reviews of yoga for depression, anxiety, and stress, yoga produced overall reductions of symptoms between 12% and 76%, with an average of 39% net reduction in symptom scores across measures (TABLE).1-3 The RCTs included in the systematic reviews were too heterogeneous to allow quantitative analyses of effect sizes.
Yoga found to significantly reduce depression symptoms
Two 2012 systematic reviews of yoga for depression evaluated 13 RCTs with a total of 782 participants, ages 18 to 80 years with mild to moderate depression. In the 12 RCTs that reported gender, 82% of participants were female; in 6 RCTs a total of 313 patients had cancer.1,2
The RCTs compared yoga to wait-list controls, counseling, education, exercise, or usual care. They evaluated yoga both as a stand-alone intervention and an adjunct to usual care. Yoga sessions varied from 1 hour weekly to 90 minutes daily over 2 to 24 weeks and included physical postures, relaxation, and breathing techniques.
Eight moderate- to high-quality RCTs with a total of 483 participants reported statistically significant reductions in depression symptoms in the yoga groups compared with control groups. In 3 RCTs, yoga was equivalent to wait-list controls; 2 RCTs showed results equivalent to exercise and superior to wait-list controls.
Yoga alleviates anxiety and stress without adverse effects
A 2012 systematic review of yoga for stress and anxiety evaluated 10 RCTs with a total of 813 heterogeneous participants, ages 18 to 76 years, including pregnant women, breast cancer patients, flood survivors, healthy volunteers, patients with chronic illnesses, perimenopausal women, adults with metabolic syndrome, and people working in finance, all with a range of anxiety and stress symptoms.3 The RCTs compared yoga, as an adjunctive or stand-alone treatment, with wait-list controls, relaxation, therapy, anxiety education, rest, or exercise. Yoga regimens varied from a single 20-minute session to 16 weeks of daily 1-hour sessions, with most regimens lasting 6 to 10 weeks.
Of the 10 RCTs reviewed, 7 moderate- to high-quality studies with a total of 627 participants found statistically significant reductions in anxiety and stress in yoga groups compared with control groups. Of the remaining 3 studies, 1 found yoga equivalent to cognitive therapy; 1 found a nonsignificant benefit for yoga compared with wait-list controls; and 1 found no improvement with either yoga or relaxation.
Study limitations included a range of symptom severity, variable type and length of yoga, lack of participant blinding, wait-list rather than active-treatment controls, and a lack of consistent long-term follow-up data. The RCTs didn’t report any adverse effects of yoga, and yoga is considered safe when taught by a competent instructor.3,4
RECOMMENDATIONS
The Institute for Clinical Systems Improvement and the Canadian Network for Mood and Anxiety Treatments recommend yoga as an effective adjunctive treatment to decrease the severity of depression symptoms.5,6
The Veterans Health Administration and the US Department of Defense recommend yoga as a potential adjunctive treatment to manage the hyperarousal symptoms of post-traumatic stress disorder (PTSD).7
The Work Loss Data Institute recommends yoga as an intervention for workers compensation conditions including occupational stress, major depressive disorder, PTSD, and other mental disorders.8
1. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2012;3:117.
2. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
3. Li AW, Goldsmith CA. The effects of yoga on anxiety and stress. Altern Med Rev. 2012;17:21-35.
4. Brown RP, Gerbarg PL. Sudarshan Kriya Yogic breathing in the treatment of stress, anxiety, and depression. Part II—clinical applications and guidelines. J Altern Complement Med. 2005;11:711-717.
5. Mitchell J, Trangle M, Degnan B, et al. Institute for Clinical Systems Improvement. Adult depression primary care. Available at: https://www.icsi.org/_asset/fnhdm3/Depr-Interactive0512b.pdf. Updated September 2013. Accessed March 6, 2014.
6. Ravindran AV, Lam RW, Filteau MJ, et al; Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. V. Complementary and alternative medicine treatments. J Affect Disord. 2009;117(suppl 1):S54-S64.
7. US Department of Veterans Affairs. VA/DoD clinical practice guideline for management of post-traumatic stress disorder and acute stress reaction. US Department of Veterans Affairs Web site. Available at: http://www.healthquality.va.gov/ptsd/. Accessed March 6, 2014.
8. Agency for Healthcare Research and Quality. Mental illness & stress. Agency for Healthcare Research and Quality Web site. Available at: http://www.guideline.gov/content.aspx?id=47588. Updated May 2011. Accessed June 17, 2014
Yes, yoga can reduce symptoms of anxiety and depression (strength of recommendation [SOR]: B, systematic reviews of randomized controlled trials [RCTs] with significant heterogeneity). Across multiple RCTs using varied yoga interventions and diverse study populations, yoga typically improves overall symptom scores for anxiety and depression by about 40%, both by itself and as an adjunctive treatment. It produces no reported harmful side effects.
EVIDENCE SUMMARY
Across 3 systematic reviews of yoga for depression, anxiety, and stress, yoga produced overall reductions of symptoms between 12% and 76%, with an average of 39% net reduction in symptom scores across measures (TABLE).1-3 The RCTs included in the systematic reviews were too heterogeneous to allow quantitative analyses of effect sizes.
Yoga found to significantly reduce depression symptoms
Two 2012 systematic reviews of yoga for depression evaluated 13 RCTs with a total of 782 participants, ages 18 to 80 years with mild to moderate depression. In the 12 RCTs that reported gender, 82% of participants were female; in 6 RCTs a total of 313 patients had cancer.1,2
The RCTs compared yoga to wait-list controls, counseling, education, exercise, or usual care. They evaluated yoga both as a stand-alone intervention and an adjunct to usual care. Yoga sessions varied from 1 hour weekly to 90 minutes daily over 2 to 24 weeks and included physical postures, relaxation, and breathing techniques.
Eight moderate- to high-quality RCTs with a total of 483 participants reported statistically significant reductions in depression symptoms in the yoga groups compared with control groups. In 3 RCTs, yoga was equivalent to wait-list controls; 2 RCTs showed results equivalent to exercise and superior to wait-list controls.
Yoga alleviates anxiety and stress without adverse effects
A 2012 systematic review of yoga for stress and anxiety evaluated 10 RCTs with a total of 813 heterogeneous participants, ages 18 to 76 years, including pregnant women, breast cancer patients, flood survivors, healthy volunteers, patients with chronic illnesses, perimenopausal women, adults with metabolic syndrome, and people working in finance, all with a range of anxiety and stress symptoms.3 The RCTs compared yoga, as an adjunctive or stand-alone treatment, with wait-list controls, relaxation, therapy, anxiety education, rest, or exercise. Yoga regimens varied from a single 20-minute session to 16 weeks of daily 1-hour sessions, with most regimens lasting 6 to 10 weeks.
Of the 10 RCTs reviewed, 7 moderate- to high-quality studies with a total of 627 participants found statistically significant reductions in anxiety and stress in yoga groups compared with control groups. Of the remaining 3 studies, 1 found yoga equivalent to cognitive therapy; 1 found a nonsignificant benefit for yoga compared with wait-list controls; and 1 found no improvement with either yoga or relaxation.

Study limitations included a range of symptom severity, variable type and length of yoga, lack of participant blinding, wait-list rather than active-treatment controls, and a lack of consistent long-term follow-up data. The RCTs didn’t report any adverse effects of yoga, and yoga is considered safe when taught by a competent instructor.3,4
RECOMMENDATIONS
The Institute for Clinical Systems Improvement and the Canadian Network for Mood and Anxiety Treatments recommend yoga as an effective adjunctive treatment to decrease the severity of depression symptoms.5,6
The Veterans Health Administration and the US Department of Defense recommend yoga as a potential adjunctive treatment to manage the hyperarousal symptoms of post-traumatic stress disorder (PTSD).7
The Work Loss Data Institute recommends yoga as an intervention for workers compensation conditions including occupational stress, major depressive disorder, PTSD, and other mental disorders.8
Yes, yoga can reduce symptoms of anxiety and depression (strength of recommendation [SOR]: B, systematic reviews of randomized controlled trials [RCTs] with significant heterogeneity). Across multiple RCTs using varied yoga interventions and diverse study populations, yoga typically improves overall symptom scores for anxiety and depression by about 40%, both by itself and as an adjunctive treatment. It produces no reported harmful side effects.
EVIDENCE SUMMARY
Across 3 systematic reviews of yoga for depression, anxiety, and stress, yoga produced overall reductions of symptoms between 12% and 76%, with an average of 39% net reduction in symptom scores across measures (TABLE).1-3 The RCTs included in the systematic reviews were too heterogeneous to allow quantitative analyses of effect sizes.
Yoga found to significantly reduce depression symptoms
Two 2012 systematic reviews of yoga for depression evaluated 13 RCTs with a total of 782 participants, ages 18 to 80 years with mild to moderate depression. In the 12 RCTs that reported gender, 82% of participants were female; in 6 RCTs a total of 313 patients had cancer.1,2
The RCTs compared yoga to wait-list controls, counseling, education, exercise, or usual care. They evaluated yoga both as a stand-alone intervention and an adjunct to usual care. Yoga sessions varied from 1 hour weekly to 90 minutes daily over 2 to 24 weeks and included physical postures, relaxation, and breathing techniques.
Eight moderate- to high-quality RCTs with a total of 483 participants reported statistically significant reductions in depression symptoms in the yoga groups compared with control groups. In 3 RCTs, yoga was equivalent to wait-list controls; 2 RCTs showed results equivalent to exercise and superior to wait-list controls.
Yoga alleviates anxiety and stress without adverse effects
A 2012 systematic review of yoga for stress and anxiety evaluated 10 RCTs with a total of 813 heterogeneous participants, ages 18 to 76 years, including pregnant women, breast cancer patients, flood survivors, healthy volunteers, patients with chronic illnesses, perimenopausal women, adults with metabolic syndrome, and people working in finance, all with a range of anxiety and stress symptoms.3 The RCTs compared yoga, as an adjunctive or stand-alone treatment, with wait-list controls, relaxation, therapy, anxiety education, rest, or exercise. Yoga regimens varied from a single 20-minute session to 16 weeks of daily 1-hour sessions, with most regimens lasting 6 to 10 weeks.
Of the 10 RCTs reviewed, 7 moderate- to high-quality studies with a total of 627 participants found statistically significant reductions in anxiety and stress in yoga groups compared with control groups. Of the remaining 3 studies, 1 found yoga equivalent to cognitive therapy; 1 found a nonsignificant benefit for yoga compared with wait-list controls; and 1 found no improvement with either yoga or relaxation.

Study limitations included a range of symptom severity, variable type and length of yoga, lack of participant blinding, wait-list rather than active-treatment controls, and a lack of consistent long-term follow-up data. The RCTs didn’t report any adverse effects of yoga, and yoga is considered safe when taught by a competent instructor.3,4
RECOMMENDATIONS
The Institute for Clinical Systems Improvement and the Canadian Network for Mood and Anxiety Treatments recommend yoga as an effective adjunctive treatment to decrease the severity of depression symptoms.5,6
The Veterans Health Administration and the US Department of Defense recommend yoga as a potential adjunctive treatment to manage the hyperarousal symptoms of post-traumatic stress disorder (PTSD).7
The Work Loss Data Institute recommends yoga as an intervention for workers compensation conditions including occupational stress, major depressive disorder, PTSD, and other mental disorders.8
1. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2012;3:117.
2. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
3. Li AW, Goldsmith CA. The effects of yoga on anxiety and stress. Altern Med Rev. 2012;17:21-35.
4. Brown RP, Gerbarg PL. Sudarshan Kriya Yogic breathing in the treatment of stress, anxiety, and depression. Part II—clinical applications and guidelines. J Altern Complement Med. 2005;11:711-717.
5. Mitchell J, Trangle M, Degnan B, et al. Institute for Clinical Systems Improvement. Adult depression primary care. Available at: https://www.icsi.org/_asset/fnhdm3/Depr-Interactive0512b.pdf. Updated September 2013. Accessed March 6, 2014.
6. Ravindran AV, Lam RW, Filteau MJ, et al; Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. V. Complementary and alternative medicine treatments. J Affect Disord. 2009;117(suppl 1):S54-S64.
7. US Department of Veterans Affairs. VA/DoD clinical practice guideline for management of post-traumatic stress disorder and acute stress reaction. US Department of Veterans Affairs Web site. Available at: http://www.healthquality.va.gov/ptsd/. Accessed March 6, 2014.
8. Agency for Healthcare Research and Quality. Mental illness & stress. Agency for Healthcare Research and Quality Web site. Available at: http://www.guideline.gov/content.aspx?id=47588. Updated May 2011. Accessed June 17, 2014
1. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2012;3:117.
2. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
3. Li AW, Goldsmith CA. The effects of yoga on anxiety and stress. Altern Med Rev. 2012;17:21-35.
4. Brown RP, Gerbarg PL. Sudarshan Kriya Yogic breathing in the treatment of stress, anxiety, and depression. Part II—clinical applications and guidelines. J Altern Complement Med. 2005;11:711-717.
5. Mitchell J, Trangle M, Degnan B, et al. Institute for Clinical Systems Improvement. Adult depression primary care. Available at: https://www.icsi.org/_asset/fnhdm3/Depr-Interactive0512b.pdf. Updated September 2013. Accessed March 6, 2014.
6. Ravindran AV, Lam RW, Filteau MJ, et al; Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. V. Complementary and alternative medicine treatments. J Affect Disord. 2009;117(suppl 1):S54-S64.
7. US Department of Veterans Affairs. VA/DoD clinical practice guideline for management of post-traumatic stress disorder and acute stress reaction. US Department of Veterans Affairs Web site. Available at: http://www.healthquality.va.gov/ptsd/. Accessed March 6, 2014.
8. Agency for Healthcare Research and Quality. Mental illness & stress. Agency for Healthcare Research and Quality Web site. Available at: http://www.guideline.gov/content.aspx?id=47588. Updated May 2011. Accessed June 17, 2014
Evidence-based answers from the Family Physicians Inquiries Network
What is the best treatment for impetigo?
Although evidence is lacking to support a single best treatment for impetigo, topical mupirocin, fusidic acid, gentamicin, and retapamulin are all at least 20% more likely than placebo to produce cure or improvement (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and a single RCT of retapamulin).
Topical bacitracin and fusidic acid are 15% more likely than disinfectant solutions to cure or improve impetigo (SOR: A, systematic review of RCTs).
Oral antibiotics may be as effective as topical antibiotics (SOR: B, RCTs with different results).
EVIDENCE SUMMARY
Most data on the effectiveness of topical antibiotics focus on bacitracin, fusidic acid (not available in the United States), and mupirocin. Retapamulin 1% ointment, a topical antibiotic in the pleuromutilin class, is approved by the US Food and Drug Administration (FDA) for use in adults and children older than 9 months to treat impetigo caused by methicillin-susceptible Staphylococcus aureus and Streptococcus pyogenes.1
Topical antibiotics outperform placebo
A 2003 meta-analysis of 16 studies (1944 patients) evaluated treatments for impetigo in both adults and children.2 Investigators conducted most of the studies in outpatient settings in the United States, United Kingdom, Northern Europe, and Canada. They expressed outcomes in terms of cure or clinical improvement within 7 to 14 days of starting treatment.
Topical agents, including mupirocin, fusidic acid, and gentamicin, resulted in cure or improvement in more patients at 7 to 14 days than placebo (absolute benefit increase=20%; number needed to treat [NNT]=5; 95% confidence interval [CI], 1.49-4.86). Definitions of cure or improvement varied among the included studies, however.
A 2012 Cochrane review of various interventions included 68 RCTs with a total of 5708 participants, primarily from pediatric or dermatology hospital outpatient clinics in North America and Europe.3 Clinical cure (defined as clearance of crusts, blisters, and redness as determined by investigators) or improvement at one week were the primary outcomes (TABLE).3,4 Mupirocin (relative risk [RR]=2.21; 95% CI, 1.16-3.13), fusidic acid (RR=4.42; 95% CI, 2.39-8.17), and retapamulin (RR=1.64; 95% CI, 1.30-2.07) all demonstrated higher rates of cure or improvement than placebo.
Retapamulin produces greater clinical response than placebo in an RCT
A 2008 randomized, double-blind, multicenter, industry-funded, placebo-controlled trial of 213 patients evaluated the effectiveness of retapamulin to treat uncomplicated impetigo with an outcome of clinical response at 7 days.4 Clinical response was defined as total absence of lesions, drying of treated lesions without crusts or erythema, decrease in the size of the affected area or decrease in the number of lesions. Retapamulin ointment produced a higher rate of clinical response than placebo (absolute risk reduction=33.5%; 95% CI, 20.5-46.5; NNT=3, P<.001).
TABLE
How well do impetigo treatments work?3,4
Comparison | Number of patients | ARR for cure or improvement | NNT | Cost of treatment* |
Topical antibiotics vs placebo | 575 | 41.2% | 2 |
|
Retapamulin vs placebo | 213 | 33.5% | 3 | Retapamulin 1% ointment (15 g): $130.12 |
Topical antibiotics vs disinfectant solution | 292 | 11.4% | 9 |
|
Mupirocin vs fusidic acid | 440 | NS | NS | Mupirocin ointment 2% (22 g): $42.75 Fusidic acid is not available in the United States |
Mupirocin vs oral erythromycin | 581 | 5.1% | 20 | Erythromycin 100 tabs: $295.01 (250 mg), $314.23 (333 mg), $338.93 (500 mg) Erythromycin ethylsuccinate solution (100 mL): $170.50 (200 mg/5 mL), $218.14 (400 mg/5 mL) |
Mupirocin vs dicloxacillin | 53 | NS | NS | Dicloxacillin 100 tabs (250 mg):$66 |
Mupirocin vs ampicillin | 13 | NS | NS | Ampicillin 100 tabs (500 mg): $39.88 Ampicillin suspension 100 mL: $9.54 (125 mg/5 mL), $14.08 (250 mg/5 mL) |
Bacitracin vs oral erythromycin | 30 | NS | NS | Bacitracin ointment 500 units/g (28.4 g): $3.47 |
Bacitracin vs penicillin | 34 | NS | NS | Penicillin V oral 100 tabs (500 mg): $77.77 Penicillin V suspension 100 mL: $3.84 (125 mg/5 mL), $4.31 (250 mg/5 mL) |
Cephalexin vs bacitracin | 19 | 56.7% | 2 | Cephalexin 100 tabs (500 mg): $526.13 Cephalexin oral suspension 100 mL: $8.93 (125 mg/5 mL), $18.90 |
Erythromycin vs penicillin | 79 | 22.4% | 4 | See above |
Cloxacillin vs penicillin | 166 | 35.9% | 3 | Cloxacillin is not available in the United States |
ARR, absolute risk reduction; NNT, number needed to treat; NS, not significant.
*Cost data obtained from Medi-Span at www.uptodate.com. Accessed December 5, 2013.
Topical antibiotics work slightly better than disinfectant solutions
In a pooled analysis from the 2012 Cochrane review, topical bacitracin and fusidic acid demonstrated slightly higher rates of cure or improvement than disinfectant solutions (RR=1.15; 95% CI, 1.01-1.32).3 Oral antibiotics may work as well as, or better than, topicals The 2012 Cochrane review found better rates of cure or improvement for topical mupirocin than oral erythromycin (RR=1.07; 95% CI, 1.01-1.13).3 Investigators noted no significant differences between topical mupirocin and bacitracin and oral antibiotics other than erythromycin, although in one small study (10 patients), oral cephalexin resulted in a higher rate of cure or improvement than topical bacitracin (absolute risk reduction [ARR]=56.7%; NNT=2).
Studies comparing oral antibiotics found that both erythromycin and cloxacillin (not available in the United States) produced higher rates of cure or improvement than penicillin (erythromycin, RR=1.29; 95% CI, 1.07-1.56; cloxacillin, RR=1.14; 95% CI, 0.80-1.62).
RECOMMENDATIONS
The Infectious Diseases Society of America recommends topical mupirocin as first-line therapy for impetigo, although resistance to the drug exists. Patients with numerous lesions or who fail to respond to topical treatment should be treated with oral antibiotics active against S pyogenes and S aureus. Recommended oral antibiotics include dicloxacillin, amoxicillin/clavulanate, cephalexin, erythromycin, and clindamycin.5
1. Altabax. Med Library Web site. Available at: http://medlibrary.org/lib/rx/meds/altabax-3/. Accessed May 12, 2014.
2. George A, Rubin G. A systematic review and meta-analysis of treatments for impetigo. Br J Gen Practice. 2003;53:480-487.
3. Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
4. Koning S, van der Wouden JC, Chosidow O, et al. Efficacy and safety of retapamulin ointment as treatment of impetigo: randomized double-blind multicentre placebo-controlled trial. Br J Dermatol. 2008;158:1077-1082.
5. Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406.
Although evidence is lacking to support a single best treatment for impetigo, topical mupirocin, fusidic acid, gentamicin, and retapamulin are all at least 20% more likely than placebo to produce cure or improvement (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and a single RCT of retapamulin).
Topical bacitracin and fusidic acid are 15% more likely than disinfectant solutions to cure or improve impetigo (SOR: A, systematic review of RCTs).
Oral antibiotics may be as effective as topical antibiotics (SOR: B, RCTs with different results).
EVIDENCE SUMMARY
Most data on the effectiveness of topical antibiotics focus on bacitracin, fusidic acid (not available in the United States), and mupirocin. Retapamulin 1% ointment, a topical antibiotic in the pleuromutilin class, is approved by the US Food and Drug Administration (FDA) for use in adults and children older than 9 months to treat impetigo caused by methicillin-susceptible Staphylococcus aureus and Streptococcus pyogenes.1
Topical antibiotics outperform placebo
A 2003 meta-analysis of 16 studies (1944 patients) evaluated treatments for impetigo in both adults and children.2 Investigators conducted most of the studies in outpatient settings in the United States, United Kingdom, Northern Europe, and Canada. They expressed outcomes in terms of cure or clinical improvement within 7 to 14 days of starting treatment.
Topical agents, including mupirocin, fusidic acid, and gentamicin, resulted in cure or improvement in more patients at 7 to 14 days than placebo (absolute benefit increase=20%; number needed to treat [NNT]=5; 95% confidence interval [CI], 1.49-4.86). Definitions of cure or improvement varied among the included studies, however.
A 2012 Cochrane review of various interventions included 68 RCTs with a total of 5708 participants, primarily from pediatric or dermatology hospital outpatient clinics in North America and Europe.3 Clinical cure (defined as clearance of crusts, blisters, and redness as determined by investigators) or improvement at one week were the primary outcomes (TABLE).3,4 Mupirocin (relative risk [RR]=2.21; 95% CI, 1.16-3.13), fusidic acid (RR=4.42; 95% CI, 2.39-8.17), and retapamulin (RR=1.64; 95% CI, 1.30-2.07) all demonstrated higher rates of cure or improvement than placebo.
Retapamulin produces greater clinical response than placebo in an RCT
A 2008 randomized, double-blind, multicenter, industry-funded, placebo-controlled trial of 213 patients evaluated the effectiveness of retapamulin to treat uncomplicated impetigo with an outcome of clinical response at 7 days.4 Clinical response was defined as total absence of lesions, drying of treated lesions without crusts or erythema, decrease in the size of the affected area or decrease in the number of lesions. Retapamulin ointment produced a higher rate of clinical response than placebo (absolute risk reduction=33.5%; 95% CI, 20.5-46.5; NNT=3, P<.001).
TABLE
How well do impetigo treatments work?3,4
Comparison | Number of patients | ARR for cure or improvement | NNT | Cost of treatment* |
Topical antibiotics vs placebo | 575 | 41.2% | 2 |
|
Retapamulin vs placebo | 213 | 33.5% | 3 | Retapamulin 1% ointment (15 g): $130.12 |
Topical antibiotics vs disinfectant solution | 292 | 11.4% | 9 |
|
Mupirocin vs fusidic acid | 440 | NS | NS | Mupirocin ointment 2% (22 g): $42.75 Fusidic acid is not available in the United States |
Mupirocin vs oral erythromycin | 581 | 5.1% | 20 | Erythromycin 100 tabs: $295.01 (250 mg), $314.23 (333 mg), $338.93 (500 mg) Erythromycin ethylsuccinate solution (100 mL): $170.50 (200 mg/5 mL), $218.14 (400 mg/5 mL) |
Mupirocin vs dicloxacillin | 53 | NS | NS | Dicloxacillin 100 tabs (250 mg):$66 |
Mupirocin vs ampicillin | 13 | NS | NS | Ampicillin 100 tabs (500 mg): $39.88 Ampicillin suspension 100 mL: $9.54 (125 mg/5 mL), $14.08 (250 mg/5 mL) |
Bacitracin vs oral erythromycin | 30 | NS | NS | Bacitracin ointment 500 units/g (28.4 g): $3.47 |
Bacitracin vs penicillin | 34 | NS | NS | Penicillin V oral 100 tabs (500 mg): $77.77 Penicillin V suspension 100 mL: $3.84 (125 mg/5 mL), $4.31 (250 mg/5 mL) |
Cephalexin vs bacitracin | 19 | 56.7% | 2 | Cephalexin 100 tabs (500 mg): $526.13 Cephalexin oral suspension 100 mL: $8.93 (125 mg/5 mL), $18.90 |
Erythromycin vs penicillin | 79 | 22.4% | 4 | See above |
Cloxacillin vs penicillin | 166 | 35.9% | 3 | Cloxacillin is not available in the United States |
ARR, absolute risk reduction; NNT, number needed to treat; NS, not significant.
*Cost data obtained from Medi-Span at www.uptodate.com. Accessed December 5, 2013.
Topical antibiotics work slightly better than disinfectant solutions
In a pooled analysis from the 2012 Cochrane review, topical bacitracin and fusidic acid demonstrated slightly higher rates of cure or improvement than disinfectant solutions (RR=1.15; 95% CI, 1.01-1.32).3 Oral antibiotics may work as well as, or better than, topicals The 2012 Cochrane review found better rates of cure or improvement for topical mupirocin than oral erythromycin (RR=1.07; 95% CI, 1.01-1.13).3 Investigators noted no significant differences between topical mupirocin and bacitracin and oral antibiotics other than erythromycin, although in one small study (10 patients), oral cephalexin resulted in a higher rate of cure or improvement than topical bacitracin (absolute risk reduction [ARR]=56.7%; NNT=2).
Studies comparing oral antibiotics found that both erythromycin and cloxacillin (not available in the United States) produced higher rates of cure or improvement than penicillin (erythromycin, RR=1.29; 95% CI, 1.07-1.56; cloxacillin, RR=1.14; 95% CI, 0.80-1.62).
RECOMMENDATIONS
The Infectious Diseases Society of America recommends topical mupirocin as first-line therapy for impetigo, although resistance to the drug exists. Patients with numerous lesions or who fail to respond to topical treatment should be treated with oral antibiotics active against S pyogenes and S aureus. Recommended oral antibiotics include dicloxacillin, amoxicillin/clavulanate, cephalexin, erythromycin, and clindamycin.5
Although evidence is lacking to support a single best treatment for impetigo, topical mupirocin, fusidic acid, gentamicin, and retapamulin are all at least 20% more likely than placebo to produce cure or improvement (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and a single RCT of retapamulin).
Topical bacitracin and fusidic acid are 15% more likely than disinfectant solutions to cure or improve impetigo (SOR: A, systematic review of RCTs).
Oral antibiotics may be as effective as topical antibiotics (SOR: B, RCTs with different results).
EVIDENCE SUMMARY
Most data on the effectiveness of topical antibiotics focus on bacitracin, fusidic acid (not available in the United States), and mupirocin. Retapamulin 1% ointment, a topical antibiotic in the pleuromutilin class, is approved by the US Food and Drug Administration (FDA) for use in adults and children older than 9 months to treat impetigo caused by methicillin-susceptible Staphylococcus aureus and Streptococcus pyogenes.1
Topical antibiotics outperform placebo
A 2003 meta-analysis of 16 studies (1944 patients) evaluated treatments for impetigo in both adults and children.2 Investigators conducted most of the studies in outpatient settings in the United States, United Kingdom, Northern Europe, and Canada. They expressed outcomes in terms of cure or clinical improvement within 7 to 14 days of starting treatment.
Topical agents, including mupirocin, fusidic acid, and gentamicin, resulted in cure or improvement in more patients at 7 to 14 days than placebo (absolute benefit increase=20%; number needed to treat [NNT]=5; 95% confidence interval [CI], 1.49-4.86). Definitions of cure or improvement varied among the included studies, however.
A 2012 Cochrane review of various interventions included 68 RCTs with a total of 5708 participants, primarily from pediatric or dermatology hospital outpatient clinics in North America and Europe.3 Clinical cure (defined as clearance of crusts, blisters, and redness as determined by investigators) or improvement at one week were the primary outcomes (TABLE).3,4 Mupirocin (relative risk [RR]=2.21; 95% CI, 1.16-3.13), fusidic acid (RR=4.42; 95% CI, 2.39-8.17), and retapamulin (RR=1.64; 95% CI, 1.30-2.07) all demonstrated higher rates of cure or improvement than placebo.
Retapamulin produces greater clinical response than placebo in an RCT
A 2008 randomized, double-blind, multicenter, industry-funded, placebo-controlled trial of 213 patients evaluated the effectiveness of retapamulin to treat uncomplicated impetigo with an outcome of clinical response at 7 days.4 Clinical response was defined as total absence of lesions, drying of treated lesions without crusts or erythema, decrease in the size of the affected area or decrease in the number of lesions. Retapamulin ointment produced a higher rate of clinical response than placebo (absolute risk reduction=33.5%; 95% CI, 20.5-46.5; NNT=3, P<.001).
TABLE
How well do impetigo treatments work?3,4
Comparison | Number of patients | ARR for cure or improvement | NNT | Cost of treatment* |
Topical antibiotics vs placebo | 575 | 41.2% | 2 |
|
Retapamulin vs placebo | 213 | 33.5% | 3 | Retapamulin 1% ointment (15 g): $130.12 |
Topical antibiotics vs disinfectant solution | 292 | 11.4% | 9 |
|
Mupirocin vs fusidic acid | 440 | NS | NS | Mupirocin ointment 2% (22 g): $42.75 Fusidic acid is not available in the United States |
Mupirocin vs oral erythromycin | 581 | 5.1% | 20 | Erythromycin 100 tabs: $295.01 (250 mg), $314.23 (333 mg), $338.93 (500 mg) Erythromycin ethylsuccinate solution (100 mL): $170.50 (200 mg/5 mL), $218.14 (400 mg/5 mL) |
Mupirocin vs dicloxacillin | 53 | NS | NS | Dicloxacillin 100 tabs (250 mg):$66 |
Mupirocin vs ampicillin | 13 | NS | NS | Ampicillin 100 tabs (500 mg): $39.88 Ampicillin suspension 100 mL: $9.54 (125 mg/5 mL), $14.08 (250 mg/5 mL) |
Bacitracin vs oral erythromycin | 30 | NS | NS | Bacitracin ointment 500 units/g (28.4 g): $3.47 |
Bacitracin vs penicillin | 34 | NS | NS | Penicillin V oral 100 tabs (500 mg): $77.77 Penicillin V suspension 100 mL: $3.84 (125 mg/5 mL), $4.31 (250 mg/5 mL) |
Cephalexin vs bacitracin | 19 | 56.7% | 2 | Cephalexin 100 tabs (500 mg): $526.13 Cephalexin oral suspension 100 mL: $8.93 (125 mg/5 mL), $18.90 |
Erythromycin vs penicillin | 79 | 22.4% | 4 | See above |
Cloxacillin vs penicillin | 166 | 35.9% | 3 | Cloxacillin is not available in the United States |
ARR, absolute risk reduction; NNT, number needed to treat; NS, not significant.
*Cost data obtained from Medi-Span at www.uptodate.com. Accessed December 5, 2013.
Topical antibiotics work slightly better than disinfectant solutions
In a pooled analysis from the 2012 Cochrane review, topical bacitracin and fusidic acid demonstrated slightly higher rates of cure or improvement than disinfectant solutions (RR=1.15; 95% CI, 1.01-1.32).3 Oral antibiotics may work as well as, or better than, topicals The 2012 Cochrane review found better rates of cure or improvement for topical mupirocin than oral erythromycin (RR=1.07; 95% CI, 1.01-1.13).3 Investigators noted no significant differences between topical mupirocin and bacitracin and oral antibiotics other than erythromycin, although in one small study (10 patients), oral cephalexin resulted in a higher rate of cure or improvement than topical bacitracin (absolute risk reduction [ARR]=56.7%; NNT=2).
Studies comparing oral antibiotics found that both erythromycin and cloxacillin (not available in the United States) produced higher rates of cure or improvement than penicillin (erythromycin, RR=1.29; 95% CI, 1.07-1.56; cloxacillin, RR=1.14; 95% CI, 0.80-1.62).
RECOMMENDATIONS
The Infectious Diseases Society of America recommends topical mupirocin as first-line therapy for impetigo, although resistance to the drug exists. Patients with numerous lesions or who fail to respond to topical treatment should be treated with oral antibiotics active against S pyogenes and S aureus. Recommended oral antibiotics include dicloxacillin, amoxicillin/clavulanate, cephalexin, erythromycin, and clindamycin.5
1. Altabax. Med Library Web site. Available at: http://medlibrary.org/lib/rx/meds/altabax-3/. Accessed May 12, 2014.
2. George A, Rubin G. A systematic review and meta-analysis of treatments for impetigo. Br J Gen Practice. 2003;53:480-487.
3. Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
4. Koning S, van der Wouden JC, Chosidow O, et al. Efficacy and safety of retapamulin ointment as treatment of impetigo: randomized double-blind multicentre placebo-controlled trial. Br J Dermatol. 2008;158:1077-1082.
5. Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406.
1. Altabax. Med Library Web site. Available at: http://medlibrary.org/lib/rx/meds/altabax-3/. Accessed May 12, 2014.
2. George A, Rubin G. A systematic review and meta-analysis of treatments for impetigo. Br J Gen Practice. 2003;53:480-487.
3. Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
4. Koning S, van der Wouden JC, Chosidow O, et al. Efficacy and safety of retapamulin ointment as treatment of impetigo: randomized double-blind multicentre placebo-controlled trial. Br J Dermatol. 2008;158:1077-1082.
5. Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406.
Evidence-based answers from the Family Physicians Inquiries Network
Do complementary agents lower HbA1c when used with standard type 2 diabetes therapy?
No, there is no high-quality evidence that supports using complementary or alternative agents to lower hemoglobin A1c (HbA1c) in patients with noninsulin-dependent type 2 diabetes. Oral chromium in widely varying doses reduces HbA1c a small amount (strength of recommendation [SOR]: C, meta-analysis of low-quality randomized, controlled trials [RCTs] of disease-oriented outcomes, with inconsistent results).
Oral cinnamon 1 to 3 g/d causes a small (<0.1%) drop in HbA1c (SOR: C, meta-analysis of low-quality RCTs of disease-oriented outcomes).
Fenugreek, milk thistle, safflower oil, and sweet potato extract may also reduce HbA1c (SOR: C, small, low-quality RCTs of disease-oriented outcomes).
EVIDENCE SUMMARY
Almost all complementary and alternative agents reviewed here were tested against placebo, and most were used in combination with standard therapy, usually identified as diet with or without oral hypoglycemic agents (TABLE).1-8
Meta-analyses evaluate effects of chromium and cinnamon
A meta-analysis of 13 RCTs evaluating the effect of oral chromium in patients with type 2 diabetes (age range not given) found a small improvement in HbA1c.1 Limitations of the meta-analysis included a wide range of chromium dosages and preparations. Ten studies showed no benefit, and of the 3 showing improvement, the researchers rated 2 as poor-quality.
A meta-analysis of 5 RCTs assessing the effect of oral cinnamon in patients with type 2 diabetes, 42 to 71 years of age, found that cinnamon produced a clinically irrelevant but statistically significant decrease in mean HbA1c.2 After analyzing the 2 RCTs with the largest effects, the researchers concluded that cinnamon might have a greater effect in patients with poorly controlled diabetes (baseline HbA1c>8.2%).
When they evaluated these RCTs for study homogeneity, they found significant differences among the studies in subject age, gender, ethnicity, body mass index, disease duration, concurrent medications, and baseline HbA1c levels, as well as variations in cinnamon dose, preparation, and therapy duration. Furthermore, only one of the studies reported randomization methods and whether allocation was concealed.
What about caiapo, fenugreek, milk thistle, and safflower oil?
Two small, moderate-quality RCTs of caiapo (sweet potato skin extract) in diet-controlled patients with diabetes demonstrated small but possibly clinically significant reductions in HbA1c between the intervention and control groups.3,4
TABLE
Effect of complementary or alternative agents on HbA1c in type 2 diabetes
CAA* | Dose/day | Concurrent diabetes therapy | Study type | Study size | Study duration | Difference in HbA1c (in HbA1c units) | 95% CI or P value |
Chromium1 | 1.28-1000 mcg | Not given | Meta-analysis of 13 RCTs | 381 | 3 wk-8 mo | -0.6† | -0.9 to -0.2 |
Cinnamon2 | 1-3 g | Various oral hypoglycemic agents‡ | Meta-analysis of 5 RCTs | 315 | 1.5-4 mo | -0.09 (WMD)† | -0.14 to -0.04 |
Caiapo3 | 4 g | Diet only | RCT | 61 | 5 mo | -0.21 (caiapo)§ +0.25 (placebo)§ | P=.08
P=.0001 |
Caiapo4 | 4 g | Diet only | RCT | 61 | 3 mo | -0.53 (caiapo)§ +0.06 (placebo)§ | P<.001
P=.23 |
Trigonella foenum-graecum (fenugreek)5 | 6.84 g | Sulfonylurea | RCT | 69 | 3 mo | -1.46 (fenugreek)§ -0.41 (placebo)§ | P<.05
P<.05 |
Silybum marianum (milk thistle)6 | 200 mg | Metformin and sulfonylurea | RCT | 51 | 4 mo | -1.0 (milk thistle)§ +1.2 (placebo)§ | P<.001
P<.0001 |
Silybum marianum (milk thistle)7 | 200 mg | Sulfonylurea | RCT | 38 | 4 mo | -1.5 (milk thistle)§ -0.5 (placebo)§ | P<.05
P=NS |
Safflower oil vs conjugated linoleic acid8 | 8 g | Various oral hypoglycemic agents‡ | DBRCD | 35 | 4 mo | -0.6 (safflower oil)§ +0.1 (conjugated linoleic acid)§ | P=.0007
P=NS |
CAA, complementary or alternative agents; CI, confidence interval; DBRCD, double-blind, randomized, crossover design; HbA1c, glycosylated hemoglobin A1c; NS, not significant; RCT, randomized controlled trial; WMD, weighted mean difference.
*All CAAs were compared against placebo, with the exception of safflower oil, which was compared against conjugated linoleic acid supplementation.
† Change in HbA1c means at study endpoint; the difference in HbA1c in intervention vs placebo groups.
‡ Oral hypoglycemic agents included a-glucosidase inhibitors, biguanides, glinides, glitazones, sulfonylureas, and thiazolidinediones.
§ Change in HbA1c means at study endpoint; the change in HbA1c from baseline.
Four small, placebo-controlled RCTs of fenugreek, milk thistle, and safflower oil found statistically and clinically significant reductions in HbA1c, but all these studies were of poor quality with unclear methods of randomization, threats to blinding, and a lack of baseline demographics.5-8
RECOMMENDATIONS
Both the American Diabetes Association (ADA) and the Diabetes UK Nutrition Working Group state that, “there is no clear evidence of benefit from vitamin or mineral supplementation in people with diabetes (compared with the general population), who do not have underlying deficiencies.”9,10 The ADA specifically states that chromium cannot be recommended because it lacks any clear benefit.9
1. Balk ME, Tatsioni A, Lichtenstein AH, et al. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154-2163.
2. Akilen R, Tsiami A, Devendra D, et al. Cinnamon in glycaemic control: Systematic review and meta analysis. Clin Nutr. 2012;31:609-615.
3. Ludvik B, Hanefeld M, Pacini G. Improved metabolic control by Ipomoea batatas (Caiapo) is associated with increased adiponectin and decreased fibrinogen levels in type 2 diabetic subjects. Diabetes Obes Metab. 2008;10:586-592.
4. Ludvik, B, Neuffer, B, Pacini G. Efficacy of Ipomoea batatas (Caiapo) on diabetes control in type 2 diabetic subjects treated with diet. Diabetes Care. 2004;27:436-440.
5. Lu FR, Shen L, Qin Y, et al. Clinical observation on trigonella foenum-graecum L. total saponins in combination with sulfonylureas in the treatment of type 2 diabetes mellitus. Chin J Integr Med. 2008;14:56-60.
6. Huseini HF, Larijani B, Heshmat R, et al. The efficacy of Silybummarianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2006;20:1036-1039.
7. Hussain SA. Silymarin as an adjunct to glibenclamide therapy improves long-term and postprandial glycemic control and body mass index in type 2 diabetes. J Med Food. 2007;10:543-547.
8. Asp ML, Collene AL, Norris LE, et al. Time-dependent effects of safflower oil to improve glycemia, inflammation and blood lipids in obese, post-menopausal women with type 2 diabetes: a randomized,double-masked, crossover study. Clin Nutr. 2011;30:443-449.
9. American Diabetes Association; Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31 suppl 1:S61-S78.
10. Diabetes UK Nutrition Working Group, Dyson PA, Kelly T, Deakin T, et al. Evidence-Based Nutrition Guidelines for the Prevention and Management of Diabetes. Diabetes UK Web site. Available at: www.diabetes.org.uk/Documents/Reports/nutritional-guidelines-2013-amendment-0413.pdf. Accessed October 2, 2013.
No, there is no high-quality evidence that supports using complementary or alternative agents to lower hemoglobin A1c (HbA1c) in patients with noninsulin-dependent type 2 diabetes. Oral chromium in widely varying doses reduces HbA1c a small amount (strength of recommendation [SOR]: C, meta-analysis of low-quality randomized, controlled trials [RCTs] of disease-oriented outcomes, with inconsistent results).
Oral cinnamon 1 to 3 g/d causes a small (<0.1%) drop in HbA1c (SOR: C, meta-analysis of low-quality RCTs of disease-oriented outcomes).
Fenugreek, milk thistle, safflower oil, and sweet potato extract may also reduce HbA1c (SOR: C, small, low-quality RCTs of disease-oriented outcomes).
EVIDENCE SUMMARY
Almost all complementary and alternative agents reviewed here were tested against placebo, and most were used in combination with standard therapy, usually identified as diet with or without oral hypoglycemic agents (TABLE).1-8
Meta-analyses evaluate effects of chromium and cinnamon
A meta-analysis of 13 RCTs evaluating the effect of oral chromium in patients with type 2 diabetes (age range not given) found a small improvement in HbA1c.1 Limitations of the meta-analysis included a wide range of chromium dosages and preparations. Ten studies showed no benefit, and of the 3 showing improvement, the researchers rated 2 as poor-quality.
A meta-analysis of 5 RCTs assessing the effect of oral cinnamon in patients with type 2 diabetes, 42 to 71 years of age, found that cinnamon produced a clinically irrelevant but statistically significant decrease in mean HbA1c.2 After analyzing the 2 RCTs with the largest effects, the researchers concluded that cinnamon might have a greater effect in patients with poorly controlled diabetes (baseline HbA1c>8.2%).
When they evaluated these RCTs for study homogeneity, they found significant differences among the studies in subject age, gender, ethnicity, body mass index, disease duration, concurrent medications, and baseline HbA1c levels, as well as variations in cinnamon dose, preparation, and therapy duration. Furthermore, only one of the studies reported randomization methods and whether allocation was concealed.
What about caiapo, fenugreek, milk thistle, and safflower oil?
Two small, moderate-quality RCTs of caiapo (sweet potato skin extract) in diet-controlled patients with diabetes demonstrated small but possibly clinically significant reductions in HbA1c between the intervention and control groups.3,4
TABLE
Effect of complementary or alternative agents on HbA1c in type 2 diabetes
CAA* | Dose/day | Concurrent diabetes therapy | Study type | Study size | Study duration | Difference in HbA1c (in HbA1c units) | 95% CI or P value |
Chromium1 | 1.28-1000 mcg | Not given | Meta-analysis of 13 RCTs | 381 | 3 wk-8 mo | -0.6† | -0.9 to -0.2 |
Cinnamon2 | 1-3 g | Various oral hypoglycemic agents‡ | Meta-analysis of 5 RCTs | 315 | 1.5-4 mo | -0.09 (WMD)† | -0.14 to -0.04 |
Caiapo3 | 4 g | Diet only | RCT | 61 | 5 mo | -0.21 (caiapo)§ +0.25 (placebo)§ | P=.08
P=.0001 |
Caiapo4 | 4 g | Diet only | RCT | 61 | 3 mo | -0.53 (caiapo)§ +0.06 (placebo)§ | P<.001
P=.23 |
Trigonella foenum-graecum (fenugreek)5 | 6.84 g | Sulfonylurea | RCT | 69 | 3 mo | -1.46 (fenugreek)§ -0.41 (placebo)§ | P<.05
P<.05 |
Silybum marianum (milk thistle)6 | 200 mg | Metformin and sulfonylurea | RCT | 51 | 4 mo | -1.0 (milk thistle)§ +1.2 (placebo)§ | P<.001
P<.0001 |
Silybum marianum (milk thistle)7 | 200 mg | Sulfonylurea | RCT | 38 | 4 mo | -1.5 (milk thistle)§ -0.5 (placebo)§ | P<.05
P=NS |
Safflower oil vs conjugated linoleic acid8 | 8 g | Various oral hypoglycemic agents‡ | DBRCD | 35 | 4 mo | -0.6 (safflower oil)§ +0.1 (conjugated linoleic acid)§ | P=.0007
P=NS |
CAA, complementary or alternative agents; CI, confidence interval; DBRCD, double-blind, randomized, crossover design; HbA1c, glycosylated hemoglobin A1c; NS, not significant; RCT, randomized controlled trial; WMD, weighted mean difference.
*All CAAs were compared against placebo, with the exception of safflower oil, which was compared against conjugated linoleic acid supplementation.
† Change in HbA1c means at study endpoint; the difference in HbA1c in intervention vs placebo groups.
‡ Oral hypoglycemic agents included a-glucosidase inhibitors, biguanides, glinides, glitazones, sulfonylureas, and thiazolidinediones.
§ Change in HbA1c means at study endpoint; the change in HbA1c from baseline.
Four small, placebo-controlled RCTs of fenugreek, milk thistle, and safflower oil found statistically and clinically significant reductions in HbA1c, but all these studies were of poor quality with unclear methods of randomization, threats to blinding, and a lack of baseline demographics.5-8
RECOMMENDATIONS
Both the American Diabetes Association (ADA) and the Diabetes UK Nutrition Working Group state that, “there is no clear evidence of benefit from vitamin or mineral supplementation in people with diabetes (compared with the general population), who do not have underlying deficiencies.”9,10 The ADA specifically states that chromium cannot be recommended because it lacks any clear benefit.9
No, there is no high-quality evidence that supports using complementary or alternative agents to lower hemoglobin A1c (HbA1c) in patients with noninsulin-dependent type 2 diabetes. Oral chromium in widely varying doses reduces HbA1c a small amount (strength of recommendation [SOR]: C, meta-analysis of low-quality randomized, controlled trials [RCTs] of disease-oriented outcomes, with inconsistent results).
Oral cinnamon 1 to 3 g/d causes a small (<0.1%) drop in HbA1c (SOR: C, meta-analysis of low-quality RCTs of disease-oriented outcomes).
Fenugreek, milk thistle, safflower oil, and sweet potato extract may also reduce HbA1c (SOR: C, small, low-quality RCTs of disease-oriented outcomes).
EVIDENCE SUMMARY
Almost all complementary and alternative agents reviewed here were tested against placebo, and most were used in combination with standard therapy, usually identified as diet with or without oral hypoglycemic agents (TABLE).1-8
Meta-analyses evaluate effects of chromium and cinnamon
A meta-analysis of 13 RCTs evaluating the effect of oral chromium in patients with type 2 diabetes (age range not given) found a small improvement in HbA1c.1 Limitations of the meta-analysis included a wide range of chromium dosages and preparations. Ten studies showed no benefit, and of the 3 showing improvement, the researchers rated 2 as poor-quality.
A meta-analysis of 5 RCTs assessing the effect of oral cinnamon in patients with type 2 diabetes, 42 to 71 years of age, found that cinnamon produced a clinically irrelevant but statistically significant decrease in mean HbA1c.2 After analyzing the 2 RCTs with the largest effects, the researchers concluded that cinnamon might have a greater effect in patients with poorly controlled diabetes (baseline HbA1c>8.2%).
When they evaluated these RCTs for study homogeneity, they found significant differences among the studies in subject age, gender, ethnicity, body mass index, disease duration, concurrent medications, and baseline HbA1c levels, as well as variations in cinnamon dose, preparation, and therapy duration. Furthermore, only one of the studies reported randomization methods and whether allocation was concealed.
What about caiapo, fenugreek, milk thistle, and safflower oil?
Two small, moderate-quality RCTs of caiapo (sweet potato skin extract) in diet-controlled patients with diabetes demonstrated small but possibly clinically significant reductions in HbA1c between the intervention and control groups.3,4
TABLE
Effect of complementary or alternative agents on HbA1c in type 2 diabetes
CAA* | Dose/day | Concurrent diabetes therapy | Study type | Study size | Study duration | Difference in HbA1c (in HbA1c units) | 95% CI or P value |
Chromium1 | 1.28-1000 mcg | Not given | Meta-analysis of 13 RCTs | 381 | 3 wk-8 mo | -0.6† | -0.9 to -0.2 |
Cinnamon2 | 1-3 g | Various oral hypoglycemic agents‡ | Meta-analysis of 5 RCTs | 315 | 1.5-4 mo | -0.09 (WMD)† | -0.14 to -0.04 |
Caiapo3 | 4 g | Diet only | RCT | 61 | 5 mo | -0.21 (caiapo)§ +0.25 (placebo)§ | P=.08
P=.0001 |
Caiapo4 | 4 g | Diet only | RCT | 61 | 3 mo | -0.53 (caiapo)§ +0.06 (placebo)§ | P<.001
P=.23 |
Trigonella foenum-graecum (fenugreek)5 | 6.84 g | Sulfonylurea | RCT | 69 | 3 mo | -1.46 (fenugreek)§ -0.41 (placebo)§ | P<.05
P<.05 |
Silybum marianum (milk thistle)6 | 200 mg | Metformin and sulfonylurea | RCT | 51 | 4 mo | -1.0 (milk thistle)§ +1.2 (placebo)§ | P<.001
P<.0001 |
Silybum marianum (milk thistle)7 | 200 mg | Sulfonylurea | RCT | 38 | 4 mo | -1.5 (milk thistle)§ -0.5 (placebo)§ | P<.05
P=NS |
Safflower oil vs conjugated linoleic acid8 | 8 g | Various oral hypoglycemic agents‡ | DBRCD | 35 | 4 mo | -0.6 (safflower oil)§ +0.1 (conjugated linoleic acid)§ | P=.0007
P=NS |
CAA, complementary or alternative agents; CI, confidence interval; DBRCD, double-blind, randomized, crossover design; HbA1c, glycosylated hemoglobin A1c; NS, not significant; RCT, randomized controlled trial; WMD, weighted mean difference.
*All CAAs were compared against placebo, with the exception of safflower oil, which was compared against conjugated linoleic acid supplementation.
† Change in HbA1c means at study endpoint; the difference in HbA1c in intervention vs placebo groups.
‡ Oral hypoglycemic agents included a-glucosidase inhibitors, biguanides, glinides, glitazones, sulfonylureas, and thiazolidinediones.
§ Change in HbA1c means at study endpoint; the change in HbA1c from baseline.
Four small, placebo-controlled RCTs of fenugreek, milk thistle, and safflower oil found statistically and clinically significant reductions in HbA1c, but all these studies were of poor quality with unclear methods of randomization, threats to blinding, and a lack of baseline demographics.5-8
RECOMMENDATIONS
Both the American Diabetes Association (ADA) and the Diabetes UK Nutrition Working Group state that, “there is no clear evidence of benefit from vitamin or mineral supplementation in people with diabetes (compared with the general population), who do not have underlying deficiencies.”9,10 The ADA specifically states that chromium cannot be recommended because it lacks any clear benefit.9
1. Balk ME, Tatsioni A, Lichtenstein AH, et al. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154-2163.
2. Akilen R, Tsiami A, Devendra D, et al. Cinnamon in glycaemic control: Systematic review and meta analysis. Clin Nutr. 2012;31:609-615.
3. Ludvik B, Hanefeld M, Pacini G. Improved metabolic control by Ipomoea batatas (Caiapo) is associated with increased adiponectin and decreased fibrinogen levels in type 2 diabetic subjects. Diabetes Obes Metab. 2008;10:586-592.
4. Ludvik, B, Neuffer, B, Pacini G. Efficacy of Ipomoea batatas (Caiapo) on diabetes control in type 2 diabetic subjects treated with diet. Diabetes Care. 2004;27:436-440.
5. Lu FR, Shen L, Qin Y, et al. Clinical observation on trigonella foenum-graecum L. total saponins in combination with sulfonylureas in the treatment of type 2 diabetes mellitus. Chin J Integr Med. 2008;14:56-60.
6. Huseini HF, Larijani B, Heshmat R, et al. The efficacy of Silybummarianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2006;20:1036-1039.
7. Hussain SA. Silymarin as an adjunct to glibenclamide therapy improves long-term and postprandial glycemic control and body mass index in type 2 diabetes. J Med Food. 2007;10:543-547.
8. Asp ML, Collene AL, Norris LE, et al. Time-dependent effects of safflower oil to improve glycemia, inflammation and blood lipids in obese, post-menopausal women with type 2 diabetes: a randomized,double-masked, crossover study. Clin Nutr. 2011;30:443-449.
9. American Diabetes Association; Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31 suppl 1:S61-S78.
10. Diabetes UK Nutrition Working Group, Dyson PA, Kelly T, Deakin T, et al. Evidence-Based Nutrition Guidelines for the Prevention and Management of Diabetes. Diabetes UK Web site. Available at: www.diabetes.org.uk/Documents/Reports/nutritional-guidelines-2013-amendment-0413.pdf. Accessed October 2, 2013.
1. Balk ME, Tatsioni A, Lichtenstein AH, et al. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154-2163.
2. Akilen R, Tsiami A, Devendra D, et al. Cinnamon in glycaemic control: Systematic review and meta analysis. Clin Nutr. 2012;31:609-615.
3. Ludvik B, Hanefeld M, Pacini G. Improved metabolic control by Ipomoea batatas (Caiapo) is associated with increased adiponectin and decreased fibrinogen levels in type 2 diabetic subjects. Diabetes Obes Metab. 2008;10:586-592.
4. Ludvik, B, Neuffer, B, Pacini G. Efficacy of Ipomoea batatas (Caiapo) on diabetes control in type 2 diabetic subjects treated with diet. Diabetes Care. 2004;27:436-440.
5. Lu FR, Shen L, Qin Y, et al. Clinical observation on trigonella foenum-graecum L. total saponins in combination with sulfonylureas in the treatment of type 2 diabetes mellitus. Chin J Integr Med. 2008;14:56-60.
6. Huseini HF, Larijani B, Heshmat R, et al. The efficacy of Silybummarianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2006;20:1036-1039.
7. Hussain SA. Silymarin as an adjunct to glibenclamide therapy improves long-term and postprandial glycemic control and body mass index in type 2 diabetes. J Med Food. 2007;10:543-547.
8. Asp ML, Collene AL, Norris LE, et al. Time-dependent effects of safflower oil to improve glycemia, inflammation and blood lipids in obese, post-menopausal women with type 2 diabetes: a randomized,double-masked, crossover study. Clin Nutr. 2011;30:443-449.
9. American Diabetes Association; Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31 suppl 1:S61-S78.
10. Diabetes UK Nutrition Working Group, Dyson PA, Kelly T, Deakin T, et al. Evidence-Based Nutrition Guidelines for the Prevention and Management of Diabetes. Diabetes UK Web site. Available at: www.diabetes.org.uk/Documents/Reports/nutritional-guidelines-2013-amendment-0413.pdf. Accessed October 2, 2013.
Evidence-based answers from the Family Physicians Inquiries Network
What are the benefits and risks of inhaled corticosteroids for COPD?
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.
Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
RECOMMENDATIONS
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.

Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
RECOMMENDATIONS
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.

Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
RECOMMENDATIONS
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
Evidence-based answers from the Family Physicians Inquiries Network
How do hydrochlorothiazide and chlorthalidone compare for treating hypertension?
Both medications reduce theincidence of cardiovascular events in patients with hypertension, but chlorthalidone may confer additional cardiovascular risk reduction (strength of recommendation [SOR]: B, conflicting network meta-analysis and cohort studies). (No head-to-head studies of hydrochlorothiazide [HCTZ] and chlorthalidone have been done.)
Serious hypokalemia and hyponatremia can occur with either medication; it is unclear if the rates of these adverse effects are the same at equivalent doses. Patients taking chlorthalidone are less likely to need a second antihypertensive medication but more likely to be nonadherent than patients taking HCTZ (SOR: B, cohort studies).
EVIDENCE SUMMARY
A network meta-analysis—designed to compare 2 interventions that haven’t been studied head-to-head—examined 9 trials that evaluated cardiovascular outcomes in 18,000 patients taking HCTZ and 60,000 patients taking chlorthalidone against outcomes for placebo or other antihypertensive agents.1 Daily doses ranged from 12.5 to 25 mg for HCTZ and 12.5 to 100 mg for chlorthalidone (although most patients taking chlorthalidone were on 12.5-25 mg).
In a drug-adjusted analysis using shared comparator medications, chlorthalidone proved superior to HCTZ in reducing the risk of both heart failure (relative risk [RR]=0.77; 95% confidence interval [CI], 0.61-0.98) and combined cardiovascular events—myocardial infarction (MI), stroke, a new diagnosis of coronary artery disease, and new-onset congestive heart failure (RR=0.79; 95% CI, 0.72-0.88).
After adjusting for achieved blood pressure, chlorthalidone was still associated with lower rates of cardiovascular events than HCTZ (RR=0.82; 95% CI, 0.70-0.97). Relative to HCTZ, the number needed to treat with chlorthalidone to prevent 1 additional cardiovascular event over 5 years was 27. Because network meta-analyses draw from a wider body of research than standard meta-analyses, they may be weakened by increased variability in study design and patient demographics.
But another study shows no significant difference in cardiovascular outcomes
A subsequent retrospective cohort study didn’t find a significant difference in cardiovascular outcomes between HCTZ and chlorthalidone. The study compared pooled cardiovascular outcomes (MI, heart failure, and stroke) in 10,400 patients recently started on chlorthalidone and 19,500 started on HCTZ.2 Initial doses were typically either 25 mg chlorthalidone (70% of patients on chlorthalidone) or 12.5 mg HCTZ (67% of patients on HCTZ). The median follow-up was about a year, but lasted as long as 5 years in some cases.
The 2 groups showed no significant difference in cardiovascular events (3.2 events per 100 person-years for chlorthalidone compared with 3.4 for HCTZ; adjusted hazard ratio [aHR]=0.93; 95% CI, 0.81-1.06).
Serious hypokalemia and hyponatremia are risks
Patients taking chlorthalidone were more likely to be hospitalized for hypokalemia (0.69 per 100 person-years vs 0.27 for HCTZ; aHR=3.1; 95% CI, 2.0-4.6; number needed to harm [NNH]=238 in 1 year) or hyponatremia (0.69 per 100 person-years vs 0.49 for HCTZ; aHR=1.7; 95% CI, 1.2-2.3; NNH=434 in 1 year).2 However, the all-cause hospitalization rates for the 2 drugs were the same (aHR=1.0; 95% CI, 0.93-1.07).
Lower systolic BP and serum potassium found with chlorthalidone
A smaller retrospective cohort analysis (6441 participants who received either chlorthalidone or HCTZ starting at 50 mg and stepped once to 100 mg) also assessed the difference in cardiovascular events between patients taking the 2 drugs.3 (Cardiovascular events were defined as pooled MIs, onset of angina or peripheral artery occlusive disease, or need for coronary artery bypass.) Although significant reductions in pooled events occurred in both groups over the 7-year study, these reductions were significantly lower in the chlorthalidone group than in the HCTZ group (aHR=0.79; 95% CI, 0.68-0.92).
Systolic blood pressures were statistically lower in the chlorthalidone group during Years 1 through 5 but not in Years 6 and 7 (difference 2-4 mm Hg). Serum potassium was also lower in patients taking chlorthalidone (3.8 mEq/L on chlorthalidone vs 4.0 mEq/L on HCTZ after 7 years; P<.05).
Chlorthalidone users more responsive, but less adherent than HCTZ users
A retrospective cohort study investigated medication tolerance in veterans who had recently started either HCTZ (120,000 patients) or chlorthalidone (2200 patients) and were followed for a year.4 Most received doses between 12.5 and 25 mg of active drug.
One primary outcome was “nonpersistence,” defined as failure to refill the medication after double the number of days as the initial prescription. The other was “insufficient response,” defined as the need to start another antihypertensive medication. Chlorthalidone users were less likely than HCTZ users to have an insufficient response (odds ratio [OR]=0.71; 95% CI, 0.63-0.80) but more likely to exhibit nonpersistence (OR=1.6; 95% CI, 1.5-1.8).
RECOMMENDATIONS
For primary hypertension, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommends diuretic monotherapy in patients older than 55 years who are poor candidates for calcium channel blockers.5 If a diuretic is to be initiated or changed, NICE recommends chlorthalidone (12.5-25 mg daily) or indapamide (1.5-2.5 mg daily) in preference to HCTZ. The guideline set forth in the eighth annual report of the United States Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure makes no distinction between chlorthalidone and HCTZ; it refers only to “thiazidetype diuretics.” Thiazide-type diuretics are listed as one option (along with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers) for initial monotherapy in nonblack patients.6
1. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59:1110–1117.
2. Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013;158:447–455.
3. Dorsh MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689–694.
4. Lund BC, Ernst ME. The comparative effectiveness of hydrochlorothiazide and chlorthalidone in an observational cohort of veterans. J Clin Hypertension. 2012;14:623–629.
5. Hypertension: clinical management of primary hypertension in adults. (NICE Clinical Guideline 127). National Institute for Health and Care Excellence Web site. London, UK: National Institute for Health and Care Excellence; 2011. Available at: www.nice.org.UK/guidance/CG127. Accessed December 16, 2013.
6. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
Both medications reduce theincidence of cardiovascular events in patients with hypertension, but chlorthalidone may confer additional cardiovascular risk reduction (strength of recommendation [SOR]: B, conflicting network meta-analysis and cohort studies). (No head-to-head studies of hydrochlorothiazide [HCTZ] and chlorthalidone have been done.)
Serious hypokalemia and hyponatremia can occur with either medication; it is unclear if the rates of these adverse effects are the same at equivalent doses. Patients taking chlorthalidone are less likely to need a second antihypertensive medication but more likely to be nonadherent than patients taking HCTZ (SOR: B, cohort studies).
EVIDENCE SUMMARY
A network meta-analysis—designed to compare 2 interventions that haven’t been studied head-to-head—examined 9 trials that evaluated cardiovascular outcomes in 18,000 patients taking HCTZ and 60,000 patients taking chlorthalidone against outcomes for placebo or other antihypertensive agents.1 Daily doses ranged from 12.5 to 25 mg for HCTZ and 12.5 to 100 mg for chlorthalidone (although most patients taking chlorthalidone were on 12.5-25 mg).
In a drug-adjusted analysis using shared comparator medications, chlorthalidone proved superior to HCTZ in reducing the risk of both heart failure (relative risk [RR]=0.77; 95% confidence interval [CI], 0.61-0.98) and combined cardiovascular events—myocardial infarction (MI), stroke, a new diagnosis of coronary artery disease, and new-onset congestive heart failure (RR=0.79; 95% CI, 0.72-0.88).
After adjusting for achieved blood pressure, chlorthalidone was still associated with lower rates of cardiovascular events than HCTZ (RR=0.82; 95% CI, 0.70-0.97). Relative to HCTZ, the number needed to treat with chlorthalidone to prevent 1 additional cardiovascular event over 5 years was 27. Because network meta-analyses draw from a wider body of research than standard meta-analyses, they may be weakened by increased variability in study design and patient demographics.
But another study shows no significant difference in cardiovascular outcomes
A subsequent retrospective cohort study didn’t find a significant difference in cardiovascular outcomes between HCTZ and chlorthalidone. The study compared pooled cardiovascular outcomes (MI, heart failure, and stroke) in 10,400 patients recently started on chlorthalidone and 19,500 started on HCTZ.2 Initial doses were typically either 25 mg chlorthalidone (70% of patients on chlorthalidone) or 12.5 mg HCTZ (67% of patients on HCTZ). The median follow-up was about a year, but lasted as long as 5 years in some cases.
The 2 groups showed no significant difference in cardiovascular events (3.2 events per 100 person-years for chlorthalidone compared with 3.4 for HCTZ; adjusted hazard ratio [aHR]=0.93; 95% CI, 0.81-1.06).
Serious hypokalemia and hyponatremia are risks
Patients taking chlorthalidone were more likely to be hospitalized for hypokalemia (0.69 per 100 person-years vs 0.27 for HCTZ; aHR=3.1; 95% CI, 2.0-4.6; number needed to harm [NNH]=238 in 1 year) or hyponatremia (0.69 per 100 person-years vs 0.49 for HCTZ; aHR=1.7; 95% CI, 1.2-2.3; NNH=434 in 1 year).2 However, the all-cause hospitalization rates for the 2 drugs were the same (aHR=1.0; 95% CI, 0.93-1.07).
Lower systolic BP and serum potassium found with chlorthalidone
A smaller retrospective cohort analysis (6441 participants who received either chlorthalidone or HCTZ starting at 50 mg and stepped once to 100 mg) also assessed the difference in cardiovascular events between patients taking the 2 drugs.3 (Cardiovascular events were defined as pooled MIs, onset of angina or peripheral artery occlusive disease, or need for coronary artery bypass.) Although significant reductions in pooled events occurred in both groups over the 7-year study, these reductions were significantly lower in the chlorthalidone group than in the HCTZ group (aHR=0.79; 95% CI, 0.68-0.92).
Systolic blood pressures were statistically lower in the chlorthalidone group during Years 1 through 5 but not in Years 6 and 7 (difference 2-4 mm Hg). Serum potassium was also lower in patients taking chlorthalidone (3.8 mEq/L on chlorthalidone vs 4.0 mEq/L on HCTZ after 7 years; P<.05).
Chlorthalidone users more responsive, but less adherent than HCTZ users
A retrospective cohort study investigated medication tolerance in veterans who had recently started either HCTZ (120,000 patients) or chlorthalidone (2200 patients) and were followed for a year.4 Most received doses between 12.5 and 25 mg of active drug.
One primary outcome was “nonpersistence,” defined as failure to refill the medication after double the number of days as the initial prescription. The other was “insufficient response,” defined as the need to start another antihypertensive medication. Chlorthalidone users were less likely than HCTZ users to have an insufficient response (odds ratio [OR]=0.71; 95% CI, 0.63-0.80) but more likely to exhibit nonpersistence (OR=1.6; 95% CI, 1.5-1.8).
RECOMMENDATIONS
For primary hypertension, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommends diuretic monotherapy in patients older than 55 years who are poor candidates for calcium channel blockers.5 If a diuretic is to be initiated or changed, NICE recommends chlorthalidone (12.5-25 mg daily) or indapamide (1.5-2.5 mg daily) in preference to HCTZ. The guideline set forth in the eighth annual report of the United States Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure makes no distinction between chlorthalidone and HCTZ; it refers only to “thiazidetype diuretics.” Thiazide-type diuretics are listed as one option (along with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers) for initial monotherapy in nonblack patients.6
Both medications reduce theincidence of cardiovascular events in patients with hypertension, but chlorthalidone may confer additional cardiovascular risk reduction (strength of recommendation [SOR]: B, conflicting network meta-analysis and cohort studies). (No head-to-head studies of hydrochlorothiazide [HCTZ] and chlorthalidone have been done.)
Serious hypokalemia and hyponatremia can occur with either medication; it is unclear if the rates of these adverse effects are the same at equivalent doses. Patients taking chlorthalidone are less likely to need a second antihypertensive medication but more likely to be nonadherent than patients taking HCTZ (SOR: B, cohort studies).
EVIDENCE SUMMARY
A network meta-analysis—designed to compare 2 interventions that haven’t been studied head-to-head—examined 9 trials that evaluated cardiovascular outcomes in 18,000 patients taking HCTZ and 60,000 patients taking chlorthalidone against outcomes for placebo or other antihypertensive agents.1 Daily doses ranged from 12.5 to 25 mg for HCTZ and 12.5 to 100 mg for chlorthalidone (although most patients taking chlorthalidone were on 12.5-25 mg).
In a drug-adjusted analysis using shared comparator medications, chlorthalidone proved superior to HCTZ in reducing the risk of both heart failure (relative risk [RR]=0.77; 95% confidence interval [CI], 0.61-0.98) and combined cardiovascular events—myocardial infarction (MI), stroke, a new diagnosis of coronary artery disease, and new-onset congestive heart failure (RR=0.79; 95% CI, 0.72-0.88).
After adjusting for achieved blood pressure, chlorthalidone was still associated with lower rates of cardiovascular events than HCTZ (RR=0.82; 95% CI, 0.70-0.97). Relative to HCTZ, the number needed to treat with chlorthalidone to prevent 1 additional cardiovascular event over 5 years was 27. Because network meta-analyses draw from a wider body of research than standard meta-analyses, they may be weakened by increased variability in study design and patient demographics.
But another study shows no significant difference in cardiovascular outcomes
A subsequent retrospective cohort study didn’t find a significant difference in cardiovascular outcomes between HCTZ and chlorthalidone. The study compared pooled cardiovascular outcomes (MI, heart failure, and stroke) in 10,400 patients recently started on chlorthalidone and 19,500 started on HCTZ.2 Initial doses were typically either 25 mg chlorthalidone (70% of patients on chlorthalidone) or 12.5 mg HCTZ (67% of patients on HCTZ). The median follow-up was about a year, but lasted as long as 5 years in some cases.
The 2 groups showed no significant difference in cardiovascular events (3.2 events per 100 person-years for chlorthalidone compared with 3.4 for HCTZ; adjusted hazard ratio [aHR]=0.93; 95% CI, 0.81-1.06).
Serious hypokalemia and hyponatremia are risks
Patients taking chlorthalidone were more likely to be hospitalized for hypokalemia (0.69 per 100 person-years vs 0.27 for HCTZ; aHR=3.1; 95% CI, 2.0-4.6; number needed to harm [NNH]=238 in 1 year) or hyponatremia (0.69 per 100 person-years vs 0.49 for HCTZ; aHR=1.7; 95% CI, 1.2-2.3; NNH=434 in 1 year).2 However, the all-cause hospitalization rates for the 2 drugs were the same (aHR=1.0; 95% CI, 0.93-1.07).
Lower systolic BP and serum potassium found with chlorthalidone
A smaller retrospective cohort analysis (6441 participants who received either chlorthalidone or HCTZ starting at 50 mg and stepped once to 100 mg) also assessed the difference in cardiovascular events between patients taking the 2 drugs.3 (Cardiovascular events were defined as pooled MIs, onset of angina or peripheral artery occlusive disease, or need for coronary artery bypass.) Although significant reductions in pooled events occurred in both groups over the 7-year study, these reductions were significantly lower in the chlorthalidone group than in the HCTZ group (aHR=0.79; 95% CI, 0.68-0.92).
Systolic blood pressures were statistically lower in the chlorthalidone group during Years 1 through 5 but not in Years 6 and 7 (difference 2-4 mm Hg). Serum potassium was also lower in patients taking chlorthalidone (3.8 mEq/L on chlorthalidone vs 4.0 mEq/L on HCTZ after 7 years; P<.05).
Chlorthalidone users more responsive, but less adherent than HCTZ users
A retrospective cohort study investigated medication tolerance in veterans who had recently started either HCTZ (120,000 patients) or chlorthalidone (2200 patients) and were followed for a year.4 Most received doses between 12.5 and 25 mg of active drug.
One primary outcome was “nonpersistence,” defined as failure to refill the medication after double the number of days as the initial prescription. The other was “insufficient response,” defined as the need to start another antihypertensive medication. Chlorthalidone users were less likely than HCTZ users to have an insufficient response (odds ratio [OR]=0.71; 95% CI, 0.63-0.80) but more likely to exhibit nonpersistence (OR=1.6; 95% CI, 1.5-1.8).
RECOMMENDATIONS
For primary hypertension, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommends diuretic monotherapy in patients older than 55 years who are poor candidates for calcium channel blockers.5 If a diuretic is to be initiated or changed, NICE recommends chlorthalidone (12.5-25 mg daily) or indapamide (1.5-2.5 mg daily) in preference to HCTZ. The guideline set forth in the eighth annual report of the United States Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure makes no distinction between chlorthalidone and HCTZ; it refers only to “thiazidetype diuretics.” Thiazide-type diuretics are listed as one option (along with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers) for initial monotherapy in nonblack patients.6
1. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59:1110–1117.
2. Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013;158:447–455.
3. Dorsh MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689–694.
4. Lund BC, Ernst ME. The comparative effectiveness of hydrochlorothiazide and chlorthalidone in an observational cohort of veterans. J Clin Hypertension. 2012;14:623–629.
5. Hypertension: clinical management of primary hypertension in adults. (NICE Clinical Guideline 127). National Institute for Health and Care Excellence Web site. London, UK: National Institute for Health and Care Excellence; 2011. Available at: www.nice.org.UK/guidance/CG127. Accessed December 16, 2013.
6. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
1. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59:1110–1117.
2. Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013;158:447–455.
3. Dorsh MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689–694.
4. Lund BC, Ernst ME. The comparative effectiveness of hydrochlorothiazide and chlorthalidone in an observational cohort of veterans. J Clin Hypertension. 2012;14:623–629.
5. Hypertension: clinical management of primary hypertension in adults. (NICE Clinical Guideline 127). National Institute for Health and Care Excellence Web site. London, UK: National Institute for Health and Care Excellence; 2011. Available at: www.nice.org.UK/guidance/CG127. Accessed December 16, 2013.
6. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
Evidence-based answers from the Family Physicians Inquiries Network