User login
Which prophylactic therapies best prevent gout attacks?
Allopurinol and febuxostat reduce the frequency of gout attacks equally after 8 weeks of treatment (strength of recommendation [SOR]: B, multiple randomized control trials [RCTs] with limitations).
Intravenous pegloticase decreases serum uric acid and gout attacks and improves quality of life (QOL) (SOR: A, 2 RCTs).
Colchicine reduces gout attacks when combined with probenecid or allopurinol at the start of urate-lowering therapy (SOR: B, 1 high-quality and 1 low-quality RCT).
EVIDENCE SUMMARY
A 28-week RCT compared the effects of placebo, allopurinol (300 mg/d), and febuxostat (80 mg, 120 mg, and 240 mg) on serum uric acid levels (sUA) and gout attacks in 1067 patients with gout and hyperuricemia (94% male, 78% white, 18 to 85 years of age with mean age ranging from 51 to 54 years ± 12 years in each group).1 Patients also received prophylaxis with either colchicine or naproxen during the first 8 weeks of the study.
During Weeks 1 through 8, investigators found no statistically significant differences in the percentage of patients requiring treatment for gout attacks between the febuxostat 80 mg, allopurinol, and placebo groups (28%, 23%, and 20%, respectively). During Weeks 8 through 28, no statistically significant differences in gout attack rates occurred between the allopurinol and febuxostat groups, although the study didn’t report specific attack rates for this period.
Both allopurinol and all doses of febuxostat reduced sUA to <6 mg/dL more effectively than placebo; more patients treated with febuxostat than allopurinol achieved a uric acid level of less than <6 mg/dL.
Another RCT of 762 mostly white, male patients (mean age 52 years) with gout and sUA >8 mg/dL—35% of whom had renal impairment, defined as creatinine clearance <80 mL/min/1.73m2—also concluded that febuxostat and allopurinol are equally effective in reducing gout attacks (incidence of gout flares during Weeks 9 to 52 was 64% with both febuxostat 80 mg and allopurinol 300 mg).2 The percentage of patients with sUA <6 mg/dL at the last 3 monthly visits was 53% in the febuxostat 80 mg group compared with 21% in the allopurinol 300 mg group (P<.001; number needed to treat [NNT]=4]).
One significant limitation of both RCTs was the fixed dose of allopurinol (300 mg/d). US Food and Drug Administration-approved dosing for allopurinol allows for titration to a maximum of 800 mg/d to achieve serum uric acid <6 mg/dL.
IV pegloticase decreases gout attacks after 3 months, improves quality of life
Pegloticase is an intravenously administered, recombinant form of uricase, the natural enzyme that converts uric acid to more soluble allantoin. Two RCTs compared pegloticase with placebo in a total of 212 patients with gout (mean age 54 to 59 years; 70% to 90% male) intolerant or refractory to allopurinol (defined as baseline sUA of ≥8 mg/dL and at least one of the following: ≥3 self-reported gout flares during the previous 18 months, ≥1 tophi, or gouty arthropathy.
These trials found that treatment with 8 mg of pegloticase every 2 weeks for 6 months initially increased gout flares during Months 1 to 3 (75% with pegloticase, 53% with placebo; P=.02; number needed to harm [NNH]=5) but then decreased the incidence of acute gout attacks during Months 4 to 6 (41% with pegloticase, 67% with placebo; P=.007; NNT=4).3 In addition, pegloticase resulted in statistically significant improvements in QOL measured at the final visit using the Health Assessment Questionnaire (HAQ) pain scale, the HAQ-Disability Index, and the 36-item Short Form Health Survey.
Colchicine plus probenecid or allopurinol reduces gout attacks
One small, low-quality RCT (N=38) found that colchicine 0.5 mg administered 3 times daily effectively prevented gout attacks when administered concomitantly with probenecid initiated to lower urate (gout attacks per month in colchicine and placebo-treated patients, respectively, were 0.19±0.05 and 0.48±0.12; P<.05).4
Another RCT that compared allopurinol with and without colchicine showed that coadministration of colchicine 0.6 mg twice daily reduced gout attacks: 33% of patients treated with colchicine experienced a gout flare compared with 77% of placebo-treated patients (P=.008; NNT=3 over 6 months).5
We identified no RCTs that evaluated the uricosuric agent probenecid and no studies that assessed the use of nonsteroidal anti-inflammatory drugs (NSAIDs) to prevent recurrent gout attacks.
RECOMMENDATIONS
The American College of Rheumatology (ACR) guidelines on managing gout recommend allopurinol or febuxostat as first-line pharmacologic urate-lowering therapy, with a goal of reducing sUA to <6 mg/dL. They recommend probenecid as an alternative if contraindications exist or the patient is intolerant to allopurinol and febuxostat.6 The guidelines note that allopurinol doses may exceed 300 mg/d, even in patients with chronic kidney disease.
The ACR recommends anti-inflammatory prophylaxis with colchicine or NSAIDs upon initiation of urate-lowering therapy. Anti-inflammatory prophylaxis should be continued as long as clinical evidence of continuing gout disease exists and until the sUA target has been acheived.7
1. Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540-1548.
2. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450-2461.
3. Sundy JS, Baraf HSB, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306:711-720.
4. Paulus HE, Schlosstein LH, Godfrey RG, et al. Prophylactic colchicine therapy of intercritical gout: a placebo-controlled study of probenecid-treated patients. Arthritis Rheum. 1974;17:609-614.
5. Borstad GC, Bryant LR, Abel MP, et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31:2429-2432.
6. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431-1446.
7. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461.
Allopurinol and febuxostat reduce the frequency of gout attacks equally after 8 weeks of treatment (strength of recommendation [SOR]: B, multiple randomized control trials [RCTs] with limitations).
Intravenous pegloticase decreases serum uric acid and gout attacks and improves quality of life (QOL) (SOR: A, 2 RCTs).
Colchicine reduces gout attacks when combined with probenecid or allopurinol at the start of urate-lowering therapy (SOR: B, 1 high-quality and 1 low-quality RCT).
EVIDENCE SUMMARY
A 28-week RCT compared the effects of placebo, allopurinol (300 mg/d), and febuxostat (80 mg, 120 mg, and 240 mg) on serum uric acid levels (sUA) and gout attacks in 1067 patients with gout and hyperuricemia (94% male, 78% white, 18 to 85 years of age with mean age ranging from 51 to 54 years ± 12 years in each group).1 Patients also received prophylaxis with either colchicine or naproxen during the first 8 weeks of the study.
During Weeks 1 through 8, investigators found no statistically significant differences in the percentage of patients requiring treatment for gout attacks between the febuxostat 80 mg, allopurinol, and placebo groups (28%, 23%, and 20%, respectively). During Weeks 8 through 28, no statistically significant differences in gout attack rates occurred between the allopurinol and febuxostat groups, although the study didn’t report specific attack rates for this period.
Both allopurinol and all doses of febuxostat reduced sUA to <6 mg/dL more effectively than placebo; more patients treated with febuxostat than allopurinol achieved a uric acid level of less than <6 mg/dL.
Another RCT of 762 mostly white, male patients (mean age 52 years) with gout and sUA >8 mg/dL—35% of whom had renal impairment, defined as creatinine clearance <80 mL/min/1.73m2—also concluded that febuxostat and allopurinol are equally effective in reducing gout attacks (incidence of gout flares during Weeks 9 to 52 was 64% with both febuxostat 80 mg and allopurinol 300 mg).2 The percentage of patients with sUA <6 mg/dL at the last 3 monthly visits was 53% in the febuxostat 80 mg group compared with 21% in the allopurinol 300 mg group (P<.001; number needed to treat [NNT]=4]).
One significant limitation of both RCTs was the fixed dose of allopurinol (300 mg/d). US Food and Drug Administration-approved dosing for allopurinol allows for titration to a maximum of 800 mg/d to achieve serum uric acid <6 mg/dL.
IV pegloticase decreases gout attacks after 3 months, improves quality of life
Pegloticase is an intravenously administered, recombinant form of uricase, the natural enzyme that converts uric acid to more soluble allantoin. Two RCTs compared pegloticase with placebo in a total of 212 patients with gout (mean age 54 to 59 years; 70% to 90% male) intolerant or refractory to allopurinol (defined as baseline sUA of ≥8 mg/dL and at least one of the following: ≥3 self-reported gout flares during the previous 18 months, ≥1 tophi, or gouty arthropathy.
These trials found that treatment with 8 mg of pegloticase every 2 weeks for 6 months initially increased gout flares during Months 1 to 3 (75% with pegloticase, 53% with placebo; P=.02; number needed to harm [NNH]=5) but then decreased the incidence of acute gout attacks during Months 4 to 6 (41% with pegloticase, 67% with placebo; P=.007; NNT=4).3 In addition, pegloticase resulted in statistically significant improvements in QOL measured at the final visit using the Health Assessment Questionnaire (HAQ) pain scale, the HAQ-Disability Index, and the 36-item Short Form Health Survey.
Colchicine plus probenecid or allopurinol reduces gout attacks
One small, low-quality RCT (N=38) found that colchicine 0.5 mg administered 3 times daily effectively prevented gout attacks when administered concomitantly with probenecid initiated to lower urate (gout attacks per month in colchicine and placebo-treated patients, respectively, were 0.19±0.05 and 0.48±0.12; P<.05).4
Another RCT that compared allopurinol with and without colchicine showed that coadministration of colchicine 0.6 mg twice daily reduced gout attacks: 33% of patients treated with colchicine experienced a gout flare compared with 77% of placebo-treated patients (P=.008; NNT=3 over 6 months).5
We identified no RCTs that evaluated the uricosuric agent probenecid and no studies that assessed the use of nonsteroidal anti-inflammatory drugs (NSAIDs) to prevent recurrent gout attacks.
RECOMMENDATIONS
The American College of Rheumatology (ACR) guidelines on managing gout recommend allopurinol or febuxostat as first-line pharmacologic urate-lowering therapy, with a goal of reducing sUA to <6 mg/dL. They recommend probenecid as an alternative if contraindications exist or the patient is intolerant to allopurinol and febuxostat.6 The guidelines note that allopurinol doses may exceed 300 mg/d, even in patients with chronic kidney disease.
The ACR recommends anti-inflammatory prophylaxis with colchicine or NSAIDs upon initiation of urate-lowering therapy. Anti-inflammatory prophylaxis should be continued as long as clinical evidence of continuing gout disease exists and until the sUA target has been acheived.7
Allopurinol and febuxostat reduce the frequency of gout attacks equally after 8 weeks of treatment (strength of recommendation [SOR]: B, multiple randomized control trials [RCTs] with limitations).
Intravenous pegloticase decreases serum uric acid and gout attacks and improves quality of life (QOL) (SOR: A, 2 RCTs).
Colchicine reduces gout attacks when combined with probenecid or allopurinol at the start of urate-lowering therapy (SOR: B, 1 high-quality and 1 low-quality RCT).
EVIDENCE SUMMARY
A 28-week RCT compared the effects of placebo, allopurinol (300 mg/d), and febuxostat (80 mg, 120 mg, and 240 mg) on serum uric acid levels (sUA) and gout attacks in 1067 patients with gout and hyperuricemia (94% male, 78% white, 18 to 85 years of age with mean age ranging from 51 to 54 years ± 12 years in each group).1 Patients also received prophylaxis with either colchicine or naproxen during the first 8 weeks of the study.
During Weeks 1 through 8, investigators found no statistically significant differences in the percentage of patients requiring treatment for gout attacks between the febuxostat 80 mg, allopurinol, and placebo groups (28%, 23%, and 20%, respectively). During Weeks 8 through 28, no statistically significant differences in gout attack rates occurred between the allopurinol and febuxostat groups, although the study didn’t report specific attack rates for this period.
Both allopurinol and all doses of febuxostat reduced sUA to <6 mg/dL more effectively than placebo; more patients treated with febuxostat than allopurinol achieved a uric acid level of less than <6 mg/dL.
Another RCT of 762 mostly white, male patients (mean age 52 years) with gout and sUA >8 mg/dL—35% of whom had renal impairment, defined as creatinine clearance <80 mL/min/1.73m2—also concluded that febuxostat and allopurinol are equally effective in reducing gout attacks (incidence of gout flares during Weeks 9 to 52 was 64% with both febuxostat 80 mg and allopurinol 300 mg).2 The percentage of patients with sUA <6 mg/dL at the last 3 monthly visits was 53% in the febuxostat 80 mg group compared with 21% in the allopurinol 300 mg group (P<.001; number needed to treat [NNT]=4]).
One significant limitation of both RCTs was the fixed dose of allopurinol (300 mg/d). US Food and Drug Administration-approved dosing for allopurinol allows for titration to a maximum of 800 mg/d to achieve serum uric acid <6 mg/dL.
IV pegloticase decreases gout attacks after 3 months, improves quality of life
Pegloticase is an intravenously administered, recombinant form of uricase, the natural enzyme that converts uric acid to more soluble allantoin. Two RCTs compared pegloticase with placebo in a total of 212 patients with gout (mean age 54 to 59 years; 70% to 90% male) intolerant or refractory to allopurinol (defined as baseline sUA of ≥8 mg/dL and at least one of the following: ≥3 self-reported gout flares during the previous 18 months, ≥1 tophi, or gouty arthropathy.
These trials found that treatment with 8 mg of pegloticase every 2 weeks for 6 months initially increased gout flares during Months 1 to 3 (75% with pegloticase, 53% with placebo; P=.02; number needed to harm [NNH]=5) but then decreased the incidence of acute gout attacks during Months 4 to 6 (41% with pegloticase, 67% with placebo; P=.007; NNT=4).3 In addition, pegloticase resulted in statistically significant improvements in QOL measured at the final visit using the Health Assessment Questionnaire (HAQ) pain scale, the HAQ-Disability Index, and the 36-item Short Form Health Survey.
Colchicine plus probenecid or allopurinol reduces gout attacks
One small, low-quality RCT (N=38) found that colchicine 0.5 mg administered 3 times daily effectively prevented gout attacks when administered concomitantly with probenecid initiated to lower urate (gout attacks per month in colchicine and placebo-treated patients, respectively, were 0.19±0.05 and 0.48±0.12; P<.05).4
Another RCT that compared allopurinol with and without colchicine showed that coadministration of colchicine 0.6 mg twice daily reduced gout attacks: 33% of patients treated with colchicine experienced a gout flare compared with 77% of placebo-treated patients (P=.008; NNT=3 over 6 months).5
We identified no RCTs that evaluated the uricosuric agent probenecid and no studies that assessed the use of nonsteroidal anti-inflammatory drugs (NSAIDs) to prevent recurrent gout attacks.
RECOMMENDATIONS
The American College of Rheumatology (ACR) guidelines on managing gout recommend allopurinol or febuxostat as first-line pharmacologic urate-lowering therapy, with a goal of reducing sUA to <6 mg/dL. They recommend probenecid as an alternative if contraindications exist or the patient is intolerant to allopurinol and febuxostat.6 The guidelines note that allopurinol doses may exceed 300 mg/d, even in patients with chronic kidney disease.
The ACR recommends anti-inflammatory prophylaxis with colchicine or NSAIDs upon initiation of urate-lowering therapy. Anti-inflammatory prophylaxis should be continued as long as clinical evidence of continuing gout disease exists and until the sUA target has been acheived.7
1. Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540-1548.
2. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450-2461.
3. Sundy JS, Baraf HSB, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306:711-720.
4. Paulus HE, Schlosstein LH, Godfrey RG, et al. Prophylactic colchicine therapy of intercritical gout: a placebo-controlled study of probenecid-treated patients. Arthritis Rheum. 1974;17:609-614.
5. Borstad GC, Bryant LR, Abel MP, et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31:2429-2432.
6. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431-1446.
7. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461.
1. Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540-1548.
2. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450-2461.
3. Sundy JS, Baraf HSB, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306:711-720.
4. Paulus HE, Schlosstein LH, Godfrey RG, et al. Prophylactic colchicine therapy of intercritical gout: a placebo-controlled study of probenecid-treated patients. Arthritis Rheum. 1974;17:609-614.
5. Borstad GC, Bryant LR, Abel MP, et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31:2429-2432.
6. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431-1446.
7. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461.
Evidence-based answers from the Family Physicians Inquiries Network
How can we effectively treat stress urinary incontinence without drugs or surgery?
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
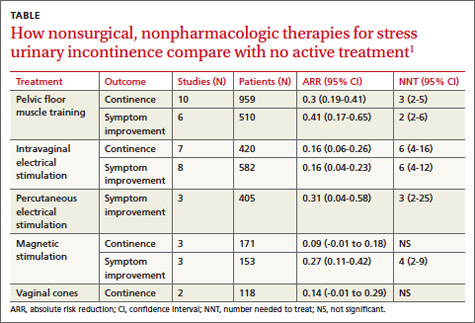
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1
Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1

Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1

Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
Evidence-based answers from the Family Physicians Inquiries Network
Is there a primary care tool to detect aberrant drug-related behaviors in patients on opioids?
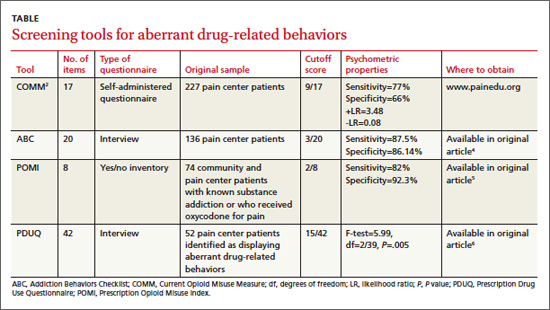
Yes. Of the several screening instruments developed and originally validated in patients in a pain center population (TABLE), one also has been validated in primary care. The Current Opioid Misuse Measure (COMM) predicts aberrant drug-related behaviors in primary care patients who have been prescribed opioids within the past 12 months with a sensitivity of 77% and specificity of 77% (strength of recommendation [SOR]: B, cohort studies).
Although not validated in primary care populations, 3 other instruments (the Addiction Behaviors Checklist [ABC], Prescription Opioid Misuse Index [POMI], and Prescription Drug Use Questionnaire [PDUQ]) detect aberrant drug-related behaviors in pain center patients with chronic pain with sensitivities of 82% to 87.5% and specificities of 86.14% to 92.3% (SOR: B, cohort studies).
EVIDENCE SUMMARY
The COMM—originally designed to detect recent aberrant drug-related behaviors in pain center patients—was validated by a cross-sectional study involving 238 primary care patients who had been prescribed an opioid within the previous 12 months.1
The study authors defined aberrant drug-related behaviors as meeting the criteria for prescription drug use disorder in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV). High COMM scores significantly predicted this diagnosis (P<.001). A COMM cutoff score >13 yielded a sensitivity of 77% and a specificity of 77% (positive predictive value=0.30; negative predictive value=0.96).
Development of the COMM. The authors of the COMM developed questions by expert consensus for use in a population of patients in a pain center. They established the validity of the questions by correlating COMM results from a cohort of pain center patients with 2 previously validated instruments: The Marlowe-Crowne Social Desirability Scale and the Aberrant Drug Behavior Index. They also tested COMM’s validity for monitoring changes in aberrant drug-related behaviors in a second cohort (sensitivity=94%; specificity=73%).2 They later cross-validated COMM with another group of 226 patients treated at pain management clinics, achieving similar results.3
Three additional tools have been validated only among pain clinic patients
The ABC was developed based on literature review and validated against the PDUQ and clinician judgment of opioid misuse. Scores on the ABC differed significantly between patients who were discontinued from opioid therapy (based on urine toxicology, for example) and patients who weren’t (P=.021).4
The authors of the POMI determined sensitivity and specificity by comparing the POMI with DSM-IV diagnostic criteria for opiate addiction. One weakness of this index is that it is based on a small, homogenous sample.5
Items in the PDUQ were based on a literature review and extracts from the charts of patients with chronic pain.6
Additional reviews
Two systematic reviews of screening tools used to predict aberrant behaviors in pain center populations included several studies with methodologic limitations.7,8
RECOMMENDATIONS
A guideline from the American Pain Society based on a systematic review concluded that the most predictive factor for aberrant drug-related behaviors is a personal or family history of drug or alcohol abuse.9,10 In 2009, APS and American Academy of Pain Medicine developed guidelines to assist in selecting, risk-stratifying, and monitoring patients on chronic pain medication.9,10 The American Society of Interventional Pain Physicians recommends evaluation of misuse risk, but considers screening tools an optional measure during initial assessment for opioid prescribing.11
1. Meltzer EC, Rybin D, Saitz R, et al. Identifying prescription opioid use disorder in primary care: diagnostic characteristics of the Current Opioid Misuse Measure (COMM). Pain. 2011;152:397-402.
2. Butler SF, Budham SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144-156.
3. Butler SF, Budman SH, Fanciullo GJ, et al. Cross validation of the current opioid misuse measure (COMM) to monitor chronic pain patients on opioid therapy. Clin J Pain. 2010;26:770-776.
4. Wu SM, Compton P, Bolus R, et. al. The addiction behaviors checklist: validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain Symptom Manage. 2006;32:342-351.
5. Knisely JS, Wunsch MJ, Cropsey KL, et al. Prescription Opioid Misuse Index: A brief questionnaire to assess misuse. J Subst Abuse Treat. 2008;35:380-386.
6. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
7. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician. 2012;15(3 suppl):ES67-ES92.
8. Solanki DR, Koyyalagunta D, Shah RV, et al. Monitoring opioid adherence in chronic pain patients: assessment of risk of substance misuse. Pain Physician. 2011;14:E119-E131.
9. Chou R. 2009 Clinical guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119:469-477.
10. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
11. Manchikanti L, Abdi S, Alturi S, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2—guidance. Pain Physician. 2012;15(3 suppl):S67-S116.
Yes. Of the several screening instruments developed and originally validated in patients in a pain center population (TABLE), one also has been validated in primary care. The Current Opioid Misuse Measure (COMM) predicts aberrant drug-related behaviors in primary care patients who have been prescribed opioids within the past 12 months with a sensitivity of 77% and specificity of 77% (strength of recommendation [SOR]: B, cohort studies).
Although not validated in primary care populations, 3 other instruments (the Addiction Behaviors Checklist [ABC], Prescription Opioid Misuse Index [POMI], and Prescription Drug Use Questionnaire [PDUQ]) detect aberrant drug-related behaviors in pain center patients with chronic pain with sensitivities of 82% to 87.5% and specificities of 86.14% to 92.3% (SOR: B, cohort studies).
EVIDENCE SUMMARY
The COMM—originally designed to detect recent aberrant drug-related behaviors in pain center patients—was validated by a cross-sectional study involving 238 primary care patients who had been prescribed an opioid within the previous 12 months.1
The study authors defined aberrant drug-related behaviors as meeting the criteria for prescription drug use disorder in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV). High COMM scores significantly predicted this diagnosis (P<.001). A COMM cutoff score >13 yielded a sensitivity of 77% and a specificity of 77% (positive predictive value=0.30; negative predictive value=0.96).
Development of the COMM. The authors of the COMM developed questions by expert consensus for use in a population of patients in a pain center. They established the validity of the questions by correlating COMM results from a cohort of pain center patients with 2 previously validated instruments: The Marlowe-Crowne Social Desirability Scale and the Aberrant Drug Behavior Index. They also tested COMM’s validity for monitoring changes in aberrant drug-related behaviors in a second cohort (sensitivity=94%; specificity=73%).2 They later cross-validated COMM with another group of 226 patients treated at pain management clinics, achieving similar results.3
Three additional tools have been validated only among pain clinic patients
The ABC was developed based on literature review and validated against the PDUQ and clinician judgment of opioid misuse. Scores on the ABC differed significantly between patients who were discontinued from opioid therapy (based on urine toxicology, for example) and patients who weren’t (P=.021).4
The authors of the POMI determined sensitivity and specificity by comparing the POMI with DSM-IV diagnostic criteria for opiate addiction. One weakness of this index is that it is based on a small, homogenous sample.5
Items in the PDUQ were based on a literature review and extracts from the charts of patients with chronic pain.6

Additional reviews
Two systematic reviews of screening tools used to predict aberrant behaviors in pain center populations included several studies with methodologic limitations.7,8
RECOMMENDATIONS
A guideline from the American Pain Society based on a systematic review concluded that the most predictive factor for aberrant drug-related behaviors is a personal or family history of drug or alcohol abuse.9,10 In 2009, APS and American Academy of Pain Medicine developed guidelines to assist in selecting, risk-stratifying, and monitoring patients on chronic pain medication.9,10 The American Society of Interventional Pain Physicians recommends evaluation of misuse risk, but considers screening tools an optional measure during initial assessment for opioid prescribing.11
Yes. Of the several screening instruments developed and originally validated in patients in a pain center population (TABLE), one also has been validated in primary care. The Current Opioid Misuse Measure (COMM) predicts aberrant drug-related behaviors in primary care patients who have been prescribed opioids within the past 12 months with a sensitivity of 77% and specificity of 77% (strength of recommendation [SOR]: B, cohort studies).
Although not validated in primary care populations, 3 other instruments (the Addiction Behaviors Checklist [ABC], Prescription Opioid Misuse Index [POMI], and Prescription Drug Use Questionnaire [PDUQ]) detect aberrant drug-related behaviors in pain center patients with chronic pain with sensitivities of 82% to 87.5% and specificities of 86.14% to 92.3% (SOR: B, cohort studies).
EVIDENCE SUMMARY
The COMM—originally designed to detect recent aberrant drug-related behaviors in pain center patients—was validated by a cross-sectional study involving 238 primary care patients who had been prescribed an opioid within the previous 12 months.1
The study authors defined aberrant drug-related behaviors as meeting the criteria for prescription drug use disorder in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV). High COMM scores significantly predicted this diagnosis (P<.001). A COMM cutoff score >13 yielded a sensitivity of 77% and a specificity of 77% (positive predictive value=0.30; negative predictive value=0.96).
Development of the COMM. The authors of the COMM developed questions by expert consensus for use in a population of patients in a pain center. They established the validity of the questions by correlating COMM results from a cohort of pain center patients with 2 previously validated instruments: The Marlowe-Crowne Social Desirability Scale and the Aberrant Drug Behavior Index. They also tested COMM’s validity for monitoring changes in aberrant drug-related behaviors in a second cohort (sensitivity=94%; specificity=73%).2 They later cross-validated COMM with another group of 226 patients treated at pain management clinics, achieving similar results.3
Three additional tools have been validated only among pain clinic patients
The ABC was developed based on literature review and validated against the PDUQ and clinician judgment of opioid misuse. Scores on the ABC differed significantly between patients who were discontinued from opioid therapy (based on urine toxicology, for example) and patients who weren’t (P=.021).4
The authors of the POMI determined sensitivity and specificity by comparing the POMI with DSM-IV diagnostic criteria for opiate addiction. One weakness of this index is that it is based on a small, homogenous sample.5
Items in the PDUQ were based on a literature review and extracts from the charts of patients with chronic pain.6

Additional reviews
Two systematic reviews of screening tools used to predict aberrant behaviors in pain center populations included several studies with methodologic limitations.7,8
RECOMMENDATIONS
A guideline from the American Pain Society based on a systematic review concluded that the most predictive factor for aberrant drug-related behaviors is a personal or family history of drug or alcohol abuse.9,10 In 2009, APS and American Academy of Pain Medicine developed guidelines to assist in selecting, risk-stratifying, and monitoring patients on chronic pain medication.9,10 The American Society of Interventional Pain Physicians recommends evaluation of misuse risk, but considers screening tools an optional measure during initial assessment for opioid prescribing.11
1. Meltzer EC, Rybin D, Saitz R, et al. Identifying prescription opioid use disorder in primary care: diagnostic characteristics of the Current Opioid Misuse Measure (COMM). Pain. 2011;152:397-402.
2. Butler SF, Budham SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144-156.
3. Butler SF, Budman SH, Fanciullo GJ, et al. Cross validation of the current opioid misuse measure (COMM) to monitor chronic pain patients on opioid therapy. Clin J Pain. 2010;26:770-776.
4. Wu SM, Compton P, Bolus R, et. al. The addiction behaviors checklist: validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain Symptom Manage. 2006;32:342-351.
5. Knisely JS, Wunsch MJ, Cropsey KL, et al. Prescription Opioid Misuse Index: A brief questionnaire to assess misuse. J Subst Abuse Treat. 2008;35:380-386.
6. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
7. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician. 2012;15(3 suppl):ES67-ES92.
8. Solanki DR, Koyyalagunta D, Shah RV, et al. Monitoring opioid adherence in chronic pain patients: assessment of risk of substance misuse. Pain Physician. 2011;14:E119-E131.
9. Chou R. 2009 Clinical guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119:469-477.
10. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
11. Manchikanti L, Abdi S, Alturi S, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2—guidance. Pain Physician. 2012;15(3 suppl):S67-S116.
1. Meltzer EC, Rybin D, Saitz R, et al. Identifying prescription opioid use disorder in primary care: diagnostic characteristics of the Current Opioid Misuse Measure (COMM). Pain. 2011;152:397-402.
2. Butler SF, Budham SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144-156.
3. Butler SF, Budman SH, Fanciullo GJ, et al. Cross validation of the current opioid misuse measure (COMM) to monitor chronic pain patients on opioid therapy. Clin J Pain. 2010;26:770-776.
4. Wu SM, Compton P, Bolus R, et. al. The addiction behaviors checklist: validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain Symptom Manage. 2006;32:342-351.
5. Knisely JS, Wunsch MJ, Cropsey KL, et al. Prescription Opioid Misuse Index: A brief questionnaire to assess misuse. J Subst Abuse Treat. 2008;35:380-386.
6. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
7. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician. 2012;15(3 suppl):ES67-ES92.
8. Solanki DR, Koyyalagunta D, Shah RV, et al. Monitoring opioid adherence in chronic pain patients: assessment of risk of substance misuse. Pain Physician. 2011;14:E119-E131.
9. Chou R. 2009 Clinical guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119:469-477.
10. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
11. Manchikanti L, Abdi S, Alturi S, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2—guidance. Pain Physician. 2012;15(3 suppl):S67-S116.
Evidence-based answers from the Family Physicians Inquiries Network
When you suspect ACS, which serologic marker is best?
Measurement of troponin levels provides the most sensitive and accurate serologic information in evaluating a patient with acute coronary syndrome (ACS); troponin elevations are more sensitive than elevations of creatine kinase-MB (CK-MB). Isolated elevation of troponin levels increases the likelihood of myocardial infarction (MI) or death, whereas isolated elevation of CK-MB levels doesn’t. (Strength of recommendation [SOR] for all statements: A, multiple, large prospective cohort studies.)
Repeated measurement of troponin levels at presentation and then 3 and 6 hours afterward increases the diagnostic sensitivity for acute myocardial infarction (AMI) (SOR: A, multiple, small prospective studies).
EVIDENCE SUMMARY
Troponin I and T proteins are specific to cardiac myocytes and, unlike CK-MB, aren’t elevated by damage to skeletal muscle.
Measuring troponin levels increased the number of patients diagnosed with AMI
A multinational prospective cohort study of patients with suspected ACS (N=10,719) found that measuring troponin levels in addition to CK-MB levels improved the diagnosis of AMI.1 Investigators used elevation of any biomarker (CK, CK-MB, or troponin I or T) above the upper limit of normal as their diagnostic criterion. They found that measuring troponin increased the number of patients diagnosed with AMI by 10.4% over patients diagnosed using CK and CK-MB levels. Elevated troponin levels were associated with an inpatient mortality rate 1.5 to 3 times higher, regardless of the patient’s CK-MB status.
Troponin levels are more sensitive and specific than CK-MB
A prospective cohort study of 718 patients with suspected AMI calculated the area under curve (AUC) of the receiver operator curve—a measure of diagnostic accuracy in which an AUC value of 1 indicates 100% sensitivity and specificity—for troponin and CK-MB levels at initial presentation.2 Two independent cardiologists reviewed all available medical records and made the final diagnosis. The AUCs for troponin levels ranged from 0.94 to 0.96 compared with 0.88 for CK-MB.
Troponin levels and odds of MI or death
A prospective study of 1852 patients with suspected ACS from 3 trial populations evaluated the prognostic value of increased troponin levels vs CK-MB levels at initial presentation, compared with a reference group with normal troponin and CK-MB levels.3 Patients with isolated troponin elevation had an increased odds of MI or death at 24 hours (odds ratio [OR]=5.2; 95% confidence interval [CI], 2.2-11.9) and 30 days (OR=2.1; 95% CI, 1.4-3.0), whereas patients with isolated CK-MB elevations didn't. At 30 days, patients with isolated CK-MB elevations equaled the reference group odds for MI and death (OR=1.0; 95% CI, 0.6-1.6).
Serial troponin assessment boosts diagnostic sensitivity
A prospective cohort study found that serial measurements of troponin increased the diagnostic sensitivity for AMI.4 Investigators evaluated 1818 consecutive patients with new onset chest pain in 3 German chest-pain units with troponin levels on admission and at 3 and 6 hours later. The gold standard was diagnosis of AMI by 2 independent cardiologists. Troponin measurement produced an AUC of 0.96 at admission, increasing to 0.98 and 0.99 at 3 and 6 hours after admission, respectively.
RECOMMENDATIONS
The American College of Cardiology and American Heart Association recommend measuring biomarkers of cardiac injury in all patients who present with chest discomfort consistent with ACS.5 A cardiac-specific troponin is the preferred marker and should be measured in all patients. If troponin is not available, CK-MB is the best alternative. Cardiac biomarkers should be repeated 6 to 9 hours after presentation and, in patients with a high clinical suspicion of AMI, at 12 to 24 hours.6,7
1. Goodman SG, Steg PG, Eagle KA, et al; GRACE Investigators. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: lessons from the global registry of acute coronary events (GRACE). Am Heart J. 2006;151:654-660.
2. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867.
3. Rao SV, Ohman EM, Granger CB, et al. Prognostic value of isolated troponin elevation across the spectrum of chest pain syndromes. Am J Cardiol. 2003;91:936-940.
4. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877.
5. Anderson JL, Adams CD, Antman EM, et al. American College of Cardiology, American Heart Association Task Force on Practice Guidelines (Writing Committee, American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Academic Emergency Medicine). ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology. J Am Coll Cardiol. 2007;50:e1-e157.
6. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53:552-574.
7. Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634-2653.
Measurement of troponin levels provides the most sensitive and accurate serologic information in evaluating a patient with acute coronary syndrome (ACS); troponin elevations are more sensitive than elevations of creatine kinase-MB (CK-MB). Isolated elevation of troponin levels increases the likelihood of myocardial infarction (MI) or death, whereas isolated elevation of CK-MB levels doesn’t. (Strength of recommendation [SOR] for all statements: A, multiple, large prospective cohort studies.)
Repeated measurement of troponin levels at presentation and then 3 and 6 hours afterward increases the diagnostic sensitivity for acute myocardial infarction (AMI) (SOR: A, multiple, small prospective studies).
EVIDENCE SUMMARY
Troponin I and T proteins are specific to cardiac myocytes and, unlike CK-MB, aren’t elevated by damage to skeletal muscle.
Measuring troponin levels increased the number of patients diagnosed with AMI
A multinational prospective cohort study of patients with suspected ACS (N=10,719) found that measuring troponin levels in addition to CK-MB levels improved the diagnosis of AMI.1 Investigators used elevation of any biomarker (CK, CK-MB, or troponin I or T) above the upper limit of normal as their diagnostic criterion. They found that measuring troponin increased the number of patients diagnosed with AMI by 10.4% over patients diagnosed using CK and CK-MB levels. Elevated troponin levels were associated with an inpatient mortality rate 1.5 to 3 times higher, regardless of the patient’s CK-MB status.
Troponin levels are more sensitive and specific than CK-MB
A prospective cohort study of 718 patients with suspected AMI calculated the area under curve (AUC) of the receiver operator curve—a measure of diagnostic accuracy in which an AUC value of 1 indicates 100% sensitivity and specificity—for troponin and CK-MB levels at initial presentation.2 Two independent cardiologists reviewed all available medical records and made the final diagnosis. The AUCs for troponin levels ranged from 0.94 to 0.96 compared with 0.88 for CK-MB.
Troponin levels and odds of MI or death
A prospective study of 1852 patients with suspected ACS from 3 trial populations evaluated the prognostic value of increased troponin levels vs CK-MB levels at initial presentation, compared with a reference group with normal troponin and CK-MB levels.3 Patients with isolated troponin elevation had an increased odds of MI or death at 24 hours (odds ratio [OR]=5.2; 95% confidence interval [CI], 2.2-11.9) and 30 days (OR=2.1; 95% CI, 1.4-3.0), whereas patients with isolated CK-MB elevations didn't. At 30 days, patients with isolated CK-MB elevations equaled the reference group odds for MI and death (OR=1.0; 95% CI, 0.6-1.6).
Serial troponin assessment boosts diagnostic sensitivity
A prospective cohort study found that serial measurements of troponin increased the diagnostic sensitivity for AMI.4 Investigators evaluated 1818 consecutive patients with new onset chest pain in 3 German chest-pain units with troponin levels on admission and at 3 and 6 hours later. The gold standard was diagnosis of AMI by 2 independent cardiologists. Troponin measurement produced an AUC of 0.96 at admission, increasing to 0.98 and 0.99 at 3 and 6 hours after admission, respectively.
RECOMMENDATIONS
The American College of Cardiology and American Heart Association recommend measuring biomarkers of cardiac injury in all patients who present with chest discomfort consistent with ACS.5 A cardiac-specific troponin is the preferred marker and should be measured in all patients. If troponin is not available, CK-MB is the best alternative. Cardiac biomarkers should be repeated 6 to 9 hours after presentation and, in patients with a high clinical suspicion of AMI, at 12 to 24 hours.6,7
Measurement of troponin levels provides the most sensitive and accurate serologic information in evaluating a patient with acute coronary syndrome (ACS); troponin elevations are more sensitive than elevations of creatine kinase-MB (CK-MB). Isolated elevation of troponin levels increases the likelihood of myocardial infarction (MI) or death, whereas isolated elevation of CK-MB levels doesn’t. (Strength of recommendation [SOR] for all statements: A, multiple, large prospective cohort studies.)
Repeated measurement of troponin levels at presentation and then 3 and 6 hours afterward increases the diagnostic sensitivity for acute myocardial infarction (AMI) (SOR: A, multiple, small prospective studies).
EVIDENCE SUMMARY
Troponin I and T proteins are specific to cardiac myocytes and, unlike CK-MB, aren’t elevated by damage to skeletal muscle.
Measuring troponin levels increased the number of patients diagnosed with AMI
A multinational prospective cohort study of patients with suspected ACS (N=10,719) found that measuring troponin levels in addition to CK-MB levels improved the diagnosis of AMI.1 Investigators used elevation of any biomarker (CK, CK-MB, or troponin I or T) above the upper limit of normal as their diagnostic criterion. They found that measuring troponin increased the number of patients diagnosed with AMI by 10.4% over patients diagnosed using CK and CK-MB levels. Elevated troponin levels were associated with an inpatient mortality rate 1.5 to 3 times higher, regardless of the patient’s CK-MB status.
Troponin levels are more sensitive and specific than CK-MB
A prospective cohort study of 718 patients with suspected AMI calculated the area under curve (AUC) of the receiver operator curve—a measure of diagnostic accuracy in which an AUC value of 1 indicates 100% sensitivity and specificity—for troponin and CK-MB levels at initial presentation.2 Two independent cardiologists reviewed all available medical records and made the final diagnosis. The AUCs for troponin levels ranged from 0.94 to 0.96 compared with 0.88 for CK-MB.
Troponin levels and odds of MI or death
A prospective study of 1852 patients with suspected ACS from 3 trial populations evaluated the prognostic value of increased troponin levels vs CK-MB levels at initial presentation, compared with a reference group with normal troponin and CK-MB levels.3 Patients with isolated troponin elevation had an increased odds of MI or death at 24 hours (odds ratio [OR]=5.2; 95% confidence interval [CI], 2.2-11.9) and 30 days (OR=2.1; 95% CI, 1.4-3.0), whereas patients with isolated CK-MB elevations didn't. At 30 days, patients with isolated CK-MB elevations equaled the reference group odds for MI and death (OR=1.0; 95% CI, 0.6-1.6).
Serial troponin assessment boosts diagnostic sensitivity
A prospective cohort study found that serial measurements of troponin increased the diagnostic sensitivity for AMI.4 Investigators evaluated 1818 consecutive patients with new onset chest pain in 3 German chest-pain units with troponin levels on admission and at 3 and 6 hours later. The gold standard was diagnosis of AMI by 2 independent cardiologists. Troponin measurement produced an AUC of 0.96 at admission, increasing to 0.98 and 0.99 at 3 and 6 hours after admission, respectively.
RECOMMENDATIONS
The American College of Cardiology and American Heart Association recommend measuring biomarkers of cardiac injury in all patients who present with chest discomfort consistent with ACS.5 A cardiac-specific troponin is the preferred marker and should be measured in all patients. If troponin is not available, CK-MB is the best alternative. Cardiac biomarkers should be repeated 6 to 9 hours after presentation and, in patients with a high clinical suspicion of AMI, at 12 to 24 hours.6,7
1. Goodman SG, Steg PG, Eagle KA, et al; GRACE Investigators. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: lessons from the global registry of acute coronary events (GRACE). Am Heart J. 2006;151:654-660.
2. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867.
3. Rao SV, Ohman EM, Granger CB, et al. Prognostic value of isolated troponin elevation across the spectrum of chest pain syndromes. Am J Cardiol. 2003;91:936-940.
4. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877.
5. Anderson JL, Adams CD, Antman EM, et al. American College of Cardiology, American Heart Association Task Force on Practice Guidelines (Writing Committee, American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Academic Emergency Medicine). ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology. J Am Coll Cardiol. 2007;50:e1-e157.
6. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53:552-574.
7. Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634-2653.
1. Goodman SG, Steg PG, Eagle KA, et al; GRACE Investigators. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: lessons from the global registry of acute coronary events (GRACE). Am Heart J. 2006;151:654-660.
2. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867.
3. Rao SV, Ohman EM, Granger CB, et al. Prognostic value of isolated troponin elevation across the spectrum of chest pain syndromes. Am J Cardiol. 2003;91:936-940.
4. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877.
5. Anderson JL, Adams CD, Antman EM, et al. American College of Cardiology, American Heart Association Task Force on Practice Guidelines (Writing Committee, American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Academic Emergency Medicine). ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology. J Am Coll Cardiol. 2007;50:e1-e157.
6. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53:552-574.
7. Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634-2653.
Evidence-based answers from the Family Physicians Inquiries Network
Which drugs are most effective for recurrent herpes labialis?
Daily oral acyclovir or valacyclovir may help prevent herpes simplex labialis (HSL) recurrences (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs] with heterogeneous results).
No trials compare oral or topical treatments for HSL outbreaks against each other. Oral antivirals modestly reduce healing time and duration of pain, varying according to the agent used: valacyclovir reduces both healing time and duration of pain, famciclovir reduces both in one dosage form but not another, and acyclovir reduces only pain duration (SOR: B, single RCTs).
Several topical medications (acyclovir, penciclovir, docosanol) modestly decrease healing time and pain duration—typically by less than a day—and require multiple doses per day (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A systematic review and meta-analysis of the effectiveness of oral and topical nucleoside antiviral agents to prevent recurrent HSL in immunocompetent people found 11 RCTs with a total of 1250 patients that compared an active drug against placebo.1 The medications were topical 5% acyclovir, topical 1% penciclovir, and oral acyclovir, valacyclovir, or famciclovir in various doses. The primary outcome was recurrence of herpes simplex virus type 1 lesions during the treatment period. The relative risk (RR) of recurrence ranged from 0.22 to 1.22. Pooled results found a benefit favoring antiviral agents (RR of recurrence=0.70; 95% confidence interval [CI], 0.55-0.89).
Seven of the trials looked at acyclovir (5 oral, 2 topical). A subgroup analysis demonstrated that oral acyclovir (800-1000 mg/d) was more effective than placebo (RR=0.51; 95% CI, 0.29-0.88), whereas topical acyclovir wasn’t. Oral valacyclovir (2 studies; 500 mg/d for 4 months) also reduced recurrence (RR=0.65; 95% CI, 0.43-0.91). The authors of the meta-analysis noted that although 9 studies favored the use of an antiviral drug, only 4 showed statistically significant differences when compared with placebo, and none of them had a low risk of bias. They concluded that the review supported using oral acyclovir and valacyclovir to prevent recurrent HSL.1
Oral antivirals produce variable treatment results
Three RCTs evaluated oral antiviral medications against placebo to treat recurrent HSL, with mixed results. The largest RCT found that valacyclovir (2000 mg twice in 24 hours, with or without an additional 1000 mg twice in another 24 hours) modestly but significantly reduced both healing time and duration of pain (by 0.5-0.8 day).2 The second RCT showed that a higher, single dose of famciclovir (1500 mg) reduced healing time (by 1.8 days) and pain duration (by 1.2 days) and that a smaller, repeated dose (750 mg twice in 24 hours) reduced healing time alone (by 2.2 days).3
The third RCT demonstrated that acyclovir (400 mg 5 times a day for 5 days) reduced pain duration (by 0.9 day) but didn’t shorten healing time. If acyclovir was started during the prodrome, it decreased the time to disappearance of the lesion’s hard crust (2.1 days’ less time; P=.03), but the clinical significance of this finding is unclear.4
Topical treatment shows modest success
Two trials demonstrated that topical acyclovir (5% cream) modestly improved healing time and duration of pain (by as much as half a day). Patients in the first trial (paired RCTs reported together) began treatment within an hour of prodromal symptoms or signs, applying the medication 5 times daily for 4 days.5
Patients in the second trial used ME-609 cream (5% acyclovir plus 1% hydrocortisone), 5% acyclovir cream, or placebo, all applied 5 times daily for 5 days.6 Although the cream with acyclovir and hydrocortisone showed a slight benefit compared with placebo (lessening healing time by 0.8 day and pain duration by 1 day), it didn’t improve healing more than acyclovir alone. Other topical agents (penciclovir 1%; docosanol 10%) produced results similar to topical acyclovir.7,8
RECOMMENDATIONS
No national guidelines on this topic exist. An online resource notes that most patients don’t require treatment for mild self-limited HSL.9 For patients with prodromal symptoms, the authors recommend episodic oral antiviral therapy. Patients who have no prodome but multiple painful or disfiguring lesions may choose to use chronic suppressive therapy with an oral antiviral drug.
1. Rahimi H, Mara T, Costella J, et al. Effectiveness of antiviral agents for the prevention of recurrent herpes labialis: a systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:618-627.
2. Spruance SL, Jones TM, Blatter MM, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrob Agents Chemother. 2003;47:1072-1080.
3. Spruance SL, Bodsworth N, Resnick H, et al. Single-dose, patient-initiated famciclovir: a randomized, double-blind, placebo-controlled trial for episodic treatment of herpes labialis. J Am Acad Dermatol. 2006;55:47-53.
4. Spruance SL, Stewart JC, Rowe NH, et al. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis. 1990;161:185-190.
5. Spruance SL, Nett R, Marbury T, et al. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob Agents Chemother. 2002;46:2238-2243.
6. Hull CM, Harmenberg J, Arlander E, et al; ME-609 Studt Group. Early treatment of cold sores with topical ME-609 decreases the frequency of ulcerative lesions: a randomized, doubleblind, placebo-controlled, patient-initiated clinical trial. J Am Acad Dermatol. 2011;64:696.e1-696.e11.
7. Raborn GW, Martel AY, Lassonde M, et al; Worldwide Topical Penciclovir Collaborative Study Group. Effective treatment of herpes simplex labialis with penciclovir cream: combined results of two trials. J Am Dent Assoc. 2002;133:303-309.
8. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
9. Klein RS. Treatment of herpes simplex virus type 1 infection in immunocompetent patients. Waltham, MA: UpToDate; 2012. Available at: www.uptodate.com/contents/treatment-of-herpessimplex-virus-type-1-infection-in-immunocompetentpatients. Accessed January 19, 2012.
Daily oral acyclovir or valacyclovir may help prevent herpes simplex labialis (HSL) recurrences (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs] with heterogeneous results).
No trials compare oral or topical treatments for HSL outbreaks against each other. Oral antivirals modestly reduce healing time and duration of pain, varying according to the agent used: valacyclovir reduces both healing time and duration of pain, famciclovir reduces both in one dosage form but not another, and acyclovir reduces only pain duration (SOR: B, single RCTs).
Several topical medications (acyclovir, penciclovir, docosanol) modestly decrease healing time and pain duration—typically by less than a day—and require multiple doses per day (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A systematic review and meta-analysis of the effectiveness of oral and topical nucleoside antiviral agents to prevent recurrent HSL in immunocompetent people found 11 RCTs with a total of 1250 patients that compared an active drug against placebo.1 The medications were topical 5% acyclovir, topical 1% penciclovir, and oral acyclovir, valacyclovir, or famciclovir in various doses. The primary outcome was recurrence of herpes simplex virus type 1 lesions during the treatment period. The relative risk (RR) of recurrence ranged from 0.22 to 1.22. Pooled results found a benefit favoring antiviral agents (RR of recurrence=0.70; 95% confidence interval [CI], 0.55-0.89).
Seven of the trials looked at acyclovir (5 oral, 2 topical). A subgroup analysis demonstrated that oral acyclovir (800-1000 mg/d) was more effective than placebo (RR=0.51; 95% CI, 0.29-0.88), whereas topical acyclovir wasn’t. Oral valacyclovir (2 studies; 500 mg/d for 4 months) also reduced recurrence (RR=0.65; 95% CI, 0.43-0.91). The authors of the meta-analysis noted that although 9 studies favored the use of an antiviral drug, only 4 showed statistically significant differences when compared with placebo, and none of them had a low risk of bias. They concluded that the review supported using oral acyclovir and valacyclovir to prevent recurrent HSL.1
Oral antivirals produce variable treatment results
Three RCTs evaluated oral antiviral medications against placebo to treat recurrent HSL, with mixed results. The largest RCT found that valacyclovir (2000 mg twice in 24 hours, with or without an additional 1000 mg twice in another 24 hours) modestly but significantly reduced both healing time and duration of pain (by 0.5-0.8 day).2 The second RCT showed that a higher, single dose of famciclovir (1500 mg) reduced healing time (by 1.8 days) and pain duration (by 1.2 days) and that a smaller, repeated dose (750 mg twice in 24 hours) reduced healing time alone (by 2.2 days).3
The third RCT demonstrated that acyclovir (400 mg 5 times a day for 5 days) reduced pain duration (by 0.9 day) but didn’t shorten healing time. If acyclovir was started during the prodrome, it decreased the time to disappearance of the lesion’s hard crust (2.1 days’ less time; P=.03), but the clinical significance of this finding is unclear.4
Topical treatment shows modest success
Two trials demonstrated that topical acyclovir (5% cream) modestly improved healing time and duration of pain (by as much as half a day). Patients in the first trial (paired RCTs reported together) began treatment within an hour of prodromal symptoms or signs, applying the medication 5 times daily for 4 days.5
Patients in the second trial used ME-609 cream (5% acyclovir plus 1% hydrocortisone), 5% acyclovir cream, or placebo, all applied 5 times daily for 5 days.6 Although the cream with acyclovir and hydrocortisone showed a slight benefit compared with placebo (lessening healing time by 0.8 day and pain duration by 1 day), it didn’t improve healing more than acyclovir alone. Other topical agents (penciclovir 1%; docosanol 10%) produced results similar to topical acyclovir.7,8
RECOMMENDATIONS
No national guidelines on this topic exist. An online resource notes that most patients don’t require treatment for mild self-limited HSL.9 For patients with prodromal symptoms, the authors recommend episodic oral antiviral therapy. Patients who have no prodome but multiple painful or disfiguring lesions may choose to use chronic suppressive therapy with an oral antiviral drug.
Daily oral acyclovir or valacyclovir may help prevent herpes simplex labialis (HSL) recurrences (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs] with heterogeneous results).
No trials compare oral or topical treatments for HSL outbreaks against each other. Oral antivirals modestly reduce healing time and duration of pain, varying according to the agent used: valacyclovir reduces both healing time and duration of pain, famciclovir reduces both in one dosage form but not another, and acyclovir reduces only pain duration (SOR: B, single RCTs).
Several topical medications (acyclovir, penciclovir, docosanol) modestly decrease healing time and pain duration—typically by less than a day—and require multiple doses per day (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A systematic review and meta-analysis of the effectiveness of oral and topical nucleoside antiviral agents to prevent recurrent HSL in immunocompetent people found 11 RCTs with a total of 1250 patients that compared an active drug against placebo.1 The medications were topical 5% acyclovir, topical 1% penciclovir, and oral acyclovir, valacyclovir, or famciclovir in various doses. The primary outcome was recurrence of herpes simplex virus type 1 lesions during the treatment period. The relative risk (RR) of recurrence ranged from 0.22 to 1.22. Pooled results found a benefit favoring antiviral agents (RR of recurrence=0.70; 95% confidence interval [CI], 0.55-0.89).
Seven of the trials looked at acyclovir (5 oral, 2 topical). A subgroup analysis demonstrated that oral acyclovir (800-1000 mg/d) was more effective than placebo (RR=0.51; 95% CI, 0.29-0.88), whereas topical acyclovir wasn’t. Oral valacyclovir (2 studies; 500 mg/d for 4 months) also reduced recurrence (RR=0.65; 95% CI, 0.43-0.91). The authors of the meta-analysis noted that although 9 studies favored the use of an antiviral drug, only 4 showed statistically significant differences when compared with placebo, and none of them had a low risk of bias. They concluded that the review supported using oral acyclovir and valacyclovir to prevent recurrent HSL.1
Oral antivirals produce variable treatment results
Three RCTs evaluated oral antiviral medications against placebo to treat recurrent HSL, with mixed results. The largest RCT found that valacyclovir (2000 mg twice in 24 hours, with or without an additional 1000 mg twice in another 24 hours) modestly but significantly reduced both healing time and duration of pain (by 0.5-0.8 day).2 The second RCT showed that a higher, single dose of famciclovir (1500 mg) reduced healing time (by 1.8 days) and pain duration (by 1.2 days) and that a smaller, repeated dose (750 mg twice in 24 hours) reduced healing time alone (by 2.2 days).3
The third RCT demonstrated that acyclovir (400 mg 5 times a day for 5 days) reduced pain duration (by 0.9 day) but didn’t shorten healing time. If acyclovir was started during the prodrome, it decreased the time to disappearance of the lesion’s hard crust (2.1 days’ less time; P=.03), but the clinical significance of this finding is unclear.4
Topical treatment shows modest success
Two trials demonstrated that topical acyclovir (5% cream) modestly improved healing time and duration of pain (by as much as half a day). Patients in the first trial (paired RCTs reported together) began treatment within an hour of prodromal symptoms or signs, applying the medication 5 times daily for 4 days.5
Patients in the second trial used ME-609 cream (5% acyclovir plus 1% hydrocortisone), 5% acyclovir cream, or placebo, all applied 5 times daily for 5 days.6 Although the cream with acyclovir and hydrocortisone showed a slight benefit compared with placebo (lessening healing time by 0.8 day and pain duration by 1 day), it didn’t improve healing more than acyclovir alone. Other topical agents (penciclovir 1%; docosanol 10%) produced results similar to topical acyclovir.7,8
RECOMMENDATIONS
No national guidelines on this topic exist. An online resource notes that most patients don’t require treatment for mild self-limited HSL.9 For patients with prodromal symptoms, the authors recommend episodic oral antiviral therapy. Patients who have no prodome but multiple painful or disfiguring lesions may choose to use chronic suppressive therapy with an oral antiviral drug.
1. Rahimi H, Mara T, Costella J, et al. Effectiveness of antiviral agents for the prevention of recurrent herpes labialis: a systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:618-627.
2. Spruance SL, Jones TM, Blatter MM, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrob Agents Chemother. 2003;47:1072-1080.
3. Spruance SL, Bodsworth N, Resnick H, et al. Single-dose, patient-initiated famciclovir: a randomized, double-blind, placebo-controlled trial for episodic treatment of herpes labialis. J Am Acad Dermatol. 2006;55:47-53.
4. Spruance SL, Stewart JC, Rowe NH, et al. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis. 1990;161:185-190.
5. Spruance SL, Nett R, Marbury T, et al. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob Agents Chemother. 2002;46:2238-2243.
6. Hull CM, Harmenberg J, Arlander E, et al; ME-609 Studt Group. Early treatment of cold sores with topical ME-609 decreases the frequency of ulcerative lesions: a randomized, doubleblind, placebo-controlled, patient-initiated clinical trial. J Am Acad Dermatol. 2011;64:696.e1-696.e11.
7. Raborn GW, Martel AY, Lassonde M, et al; Worldwide Topical Penciclovir Collaborative Study Group. Effective treatment of herpes simplex labialis with penciclovir cream: combined results of two trials. J Am Dent Assoc. 2002;133:303-309.
8. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
9. Klein RS. Treatment of herpes simplex virus type 1 infection in immunocompetent patients. Waltham, MA: UpToDate; 2012. Available at: www.uptodate.com/contents/treatment-of-herpessimplex-virus-type-1-infection-in-immunocompetentpatients. Accessed January 19, 2012.
1. Rahimi H, Mara T, Costella J, et al. Effectiveness of antiviral agents for the prevention of recurrent herpes labialis: a systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:618-627.
2. Spruance SL, Jones TM, Blatter MM, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrob Agents Chemother. 2003;47:1072-1080.
3. Spruance SL, Bodsworth N, Resnick H, et al. Single-dose, patient-initiated famciclovir: a randomized, double-blind, placebo-controlled trial for episodic treatment of herpes labialis. J Am Acad Dermatol. 2006;55:47-53.
4. Spruance SL, Stewart JC, Rowe NH, et al. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis. 1990;161:185-190.
5. Spruance SL, Nett R, Marbury T, et al. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob Agents Chemother. 2002;46:2238-2243.
6. Hull CM, Harmenberg J, Arlander E, et al; ME-609 Studt Group. Early treatment of cold sores with topical ME-609 decreases the frequency of ulcerative lesions: a randomized, doubleblind, placebo-controlled, patient-initiated clinical trial. J Am Acad Dermatol. 2011;64:696.e1-696.e11.
7. Raborn GW, Martel AY, Lassonde M, et al; Worldwide Topical Penciclovir Collaborative Study Group. Effective treatment of herpes simplex labialis with penciclovir cream: combined results of two trials. J Am Dent Assoc. 2002;133:303-309.
8. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
9. Klein RS. Treatment of herpes simplex virus type 1 infection in immunocompetent patients. Waltham, MA: UpToDate; 2012. Available at: www.uptodate.com/contents/treatment-of-herpessimplex-virus-type-1-infection-in-immunocompetentpatients. Accessed January 19, 2012.
Evidence-based answers from the Family Physicians Inquiries Network
How best to treat UTIs in women who breastfeed?
It’s unclear, as no studies have specifically evaluated therapies for uncomplicated urinary tract infections (UTIs) in breastfeeding women. However, trimethoprim/sulfamethoxazole (TMP/ SMX), β-lactam antibiotics, nitrofurantoin, and fluoroquinolones all produce cure rates of 78% to 95% for uncomplicated UTIs in women who aren’t breastfeeding, and all appear to be equivalent (strength of recommendation [SOR]: A, a systematic review).
Women who take TMP/SMX develop drug concentrations in breast milk that are below recommended maximum safe levels for infants who don’t have glucose-6-phosphate dehydrogenase (G6PD) deficiency (SOR: B, a small observational study and expert opinion); treatment with nitrofurantoin and ciprofloxacin also produces low levels in breast milk (SOR: C, extrapolations from small observational studies and expert opinion). (Though in the case of nitrofurantoin, this does not include patients with G6PD deficiency.)
Some antibiotics taken by breastfeeding mothers may occasionally be associated with adverse effects in their infants: TMP/SMX may cause poor feeding; amoxicillin and cephalexin may cause diarrhea; nitrofurantoin may cause diarrhea or, in infants with G6PD deficiency, hemolytic anemia; and ciprofloxacin may cause pseudomembranous colitis in infants and green teeth in neonates (SOR: C, case reports and expert opinion).
EVIDENCE SUMMARY
Because no randomized controlled trials have evaluated the efficacy of UTI treatment in lactating women, recommendations are extrapolated from studies in other populations and case reports.
Antibiotics: Comparable and effective
A 2010 Cochrane review examined 21 good-quality randomized trials that compared the effectiveness of TMP-SMX, β-lactam antibiotics, nitrofurantoin, and fluoroquinolones for uncomplicated UTIs in 6016 women.1 The authors found no significant differences in short-term symptom cure rates: all antibiotics were very effective. Seven studies reported mixed (clinical and bacteriologic) cure rates.
Symptom cure rates for patients followed for as long as 2 weeks ranged from 78% to 95%; longer-term (as long as 8 weeks) symptom cure rates ranged from 82% to 91%. The review suggested that TMP-SMX may be slightly more likely to cause a rash than other antibiotics.1
Antibiotic concentrations in breast milk
In a case series, TMP/SMX, 160/800 mg, given to 50 lactating women 2 times (40 women) or 3 times (10 women) daily resulted in an average breast milk concentration of 2 μg/mL of TMP and 4.6 μg/mL of SMX, corresponding to respective doses of 0.3 and 0.7 mg/kg/d for infants taking 150 mL breast milk/kg/d.2 The authors state that this dose is safe for infants without G6PD deficiency. The study included only women with UTIs or other infections requiring antibiotic treatment.
A case series of 4 lactating mothers who received a single 100-mg oral dose of nitrofurantoin found that peak breast milk concentration occurred 4 hours later and averaged 2.4 μg/mL (standard deviation=1.7-3.2 μg/mL).3 The authors calculated a mean concentration over 12 hours of 1.3 μg nitrofurantoin/mL breast milk. This level would correspond to an estimated dose of 0.2 mg/kg/d for an infant consuming 150 mL/kg/d of breast milk whose mother takes 100 mg nitrofurantoin twice daily, much lower than the recommended pediatric dose of 5 to 7 mg/kg/d.
Data from a case series and a case report suggest the amount of ciprofloxacin transferred to breastfed infants is low. In the case series, researchers gave 10 lactating women 3 oral doses of ciprofloxacin, 750 mg, at 12-hour intervals and then measured ciprofloxacin levels in breast milk.4 The highest levels occurred 2 hours after the third dose and averaged 3.79 μg/mL. Average levels fell gradually to 0.02 μg/mL 24 hours after the third dose. Assuming a milk intake of 150 mL/kg/d, a breastfed infant would consume approximately 0.3 mg/kg/d, much lower than the 10 to 40 mg/kg/d dose recommended for treating sick infants.
A case report of a woman who took oral ciprofloxacin 500 mg/d for 10 days noted a breast milk ciprofloxacin concentration of 0.98 mg/L at 10.7 hours after the last dose.5 Ofloxacin, norfloxacin, and levofloxacin have been associated with lower milk concentrations than ciprofloxacin.6
Adverse effects
In a cohort study of 838 women from a program for pregnant and lactating women exposed to drugs and other substances, 2 of 12 mothers taking TMP/SMX reported poor feeding in their infants.7
The same program received reports of infants with diarrhea from mothers taking amoxicillin (3 of 25 infants), nitrofurantoin (2 of 6 infants), and cephalexin (2 of 7 infants), but no reports of other adverse effects. Another study demonstrated that nitrofurantoin is actively transported into the mother’s milk, making hemolytic anemia a possibility in G6PD-deficient infants.3
Studies indicate that adverse effects of fluoroquinolones in children are similar to those in adults despite a contraindication in children because of reports of arthropathy in young animals. One case of pseudomembranous colitis in a breastfeeding infant and 2 cases of green teeth in neonates have been reported with ciprofloxacin use.6,8,9
RECOMMENDATIONS
The Infectious Disease Society of America recommends nitrofurantoin, TMP/SMX, or fosfomycin for first-line treatment of uncomplicated UTIs in women, although fosfomycin appears to be inferior to other standard short-course antibiotics based on FDA data. Fluoroquinolones and β-lactams are recommended alternative treatments.10
The American Academy of Pediatrics’ Committee on Drugs says that TMP/SMX (unless G6PD deficiency is present), amoxicillin, nitrofurantoin, ciprofloxacin, and ofloxacin usually are compatible with breastfeeding.11
1. Zalmanovici Trestioreanu A, Green H, Paul M, et al. Antimicrobial agents for treating uncomplicated urinary tract infection in women. Cochrane Database of Syst Rev. 2010;(10):CD007182.
2. Miller RD, Salter AJ. The passage of trimethoprim/sulfamethoxazole into breast milk and its significance. Proceedings of the 8th International Congress of Chemotherapy, Athens. Hellenic Soc Chemother. 1974;1:687-691.
3. Gerk PM, Kuhn RJ, Desai NS, et al. Active transport of nitrofurantoin into human milk. Pharmacotherapy. 2001;21:669-675.
4. Giamarellou H, Kolokythas E, Petrikkos G, et al. Pharmacokinetics of three newer quinolones in pregnant and lactating women. Am J Med. 1989;87:49S-51S.
5. Gardner DK, Gabbe SG, Harter C. Simultaneous concentrations of ciprofloxacin in breast milk and in serum in mother and breast-fed infant. Clin Pharm. 1992;11:352-354.
6. Bar-Oz B, Bulkowstein M, Benyamini L, et al. Use of antibiotic and analgesic drugs during lactation. Drug Safety. 2003;26:925-935.
7. Ito S, Blajchman A, Stephenson M, et al. Prospective followup of breast-fed infants exposed to maternal medication. Am J Obstet Gynecol.1993;168:1393-1399.
8. Harmon T, Burkhart G, Applebaum H. Perforated pseudomembranous colitis in the breast-fed infant. J Pediatr Surg. 1992;27:744-746.
9. Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 8th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008.
10. Gupta K, Hooton TM, Naber KG, et al; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International Clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
11. American Academy of Pediatrics Committee on Drugs. The transfer of drugs and other chemical into human milk. Pediatrics. 2001;108:776-789.
It’s unclear, as no studies have specifically evaluated therapies for uncomplicated urinary tract infections (UTIs) in breastfeeding women. However, trimethoprim/sulfamethoxazole (TMP/ SMX), β-lactam antibiotics, nitrofurantoin, and fluoroquinolones all produce cure rates of 78% to 95% for uncomplicated UTIs in women who aren’t breastfeeding, and all appear to be equivalent (strength of recommendation [SOR]: A, a systematic review).
Women who take TMP/SMX develop drug concentrations in breast milk that are below recommended maximum safe levels for infants who don’t have glucose-6-phosphate dehydrogenase (G6PD) deficiency (SOR: B, a small observational study and expert opinion); treatment with nitrofurantoin and ciprofloxacin also produces low levels in breast milk (SOR: C, extrapolations from small observational studies and expert opinion). (Though in the case of nitrofurantoin, this does not include patients with G6PD deficiency.)
Some antibiotics taken by breastfeeding mothers may occasionally be associated with adverse effects in their infants: TMP/SMX may cause poor feeding; amoxicillin and cephalexin may cause diarrhea; nitrofurantoin may cause diarrhea or, in infants with G6PD deficiency, hemolytic anemia; and ciprofloxacin may cause pseudomembranous colitis in infants and green teeth in neonates (SOR: C, case reports and expert opinion).
EVIDENCE SUMMARY
Because no randomized controlled trials have evaluated the efficacy of UTI treatment in lactating women, recommendations are extrapolated from studies in other populations and case reports.
Antibiotics: Comparable and effective
A 2010 Cochrane review examined 21 good-quality randomized trials that compared the effectiveness of TMP-SMX, β-lactam antibiotics, nitrofurantoin, and fluoroquinolones for uncomplicated UTIs in 6016 women.1 The authors found no significant differences in short-term symptom cure rates: all antibiotics were very effective. Seven studies reported mixed (clinical and bacteriologic) cure rates.
Symptom cure rates for patients followed for as long as 2 weeks ranged from 78% to 95%; longer-term (as long as 8 weeks) symptom cure rates ranged from 82% to 91%. The review suggested that TMP-SMX may be slightly more likely to cause a rash than other antibiotics.1
Antibiotic concentrations in breast milk
In a case series, TMP/SMX, 160/800 mg, given to 50 lactating women 2 times (40 women) or 3 times (10 women) daily resulted in an average breast milk concentration of 2 μg/mL of TMP and 4.6 μg/mL of SMX, corresponding to respective doses of 0.3 and 0.7 mg/kg/d for infants taking 150 mL breast milk/kg/d.2 The authors state that this dose is safe for infants without G6PD deficiency. The study included only women with UTIs or other infections requiring antibiotic treatment.
A case series of 4 lactating mothers who received a single 100-mg oral dose of nitrofurantoin found that peak breast milk concentration occurred 4 hours later and averaged 2.4 μg/mL (standard deviation=1.7-3.2 μg/mL).3 The authors calculated a mean concentration over 12 hours of 1.3 μg nitrofurantoin/mL breast milk. This level would correspond to an estimated dose of 0.2 mg/kg/d for an infant consuming 150 mL/kg/d of breast milk whose mother takes 100 mg nitrofurantoin twice daily, much lower than the recommended pediatric dose of 5 to 7 mg/kg/d.
Data from a case series and a case report suggest the amount of ciprofloxacin transferred to breastfed infants is low. In the case series, researchers gave 10 lactating women 3 oral doses of ciprofloxacin, 750 mg, at 12-hour intervals and then measured ciprofloxacin levels in breast milk.4 The highest levels occurred 2 hours after the third dose and averaged 3.79 μg/mL. Average levels fell gradually to 0.02 μg/mL 24 hours after the third dose. Assuming a milk intake of 150 mL/kg/d, a breastfed infant would consume approximately 0.3 mg/kg/d, much lower than the 10 to 40 mg/kg/d dose recommended for treating sick infants.
A case report of a woman who took oral ciprofloxacin 500 mg/d for 10 days noted a breast milk ciprofloxacin concentration of 0.98 mg/L at 10.7 hours after the last dose.5 Ofloxacin, norfloxacin, and levofloxacin have been associated with lower milk concentrations than ciprofloxacin.6
Adverse effects
In a cohort study of 838 women from a program for pregnant and lactating women exposed to drugs and other substances, 2 of 12 mothers taking TMP/SMX reported poor feeding in their infants.7
The same program received reports of infants with diarrhea from mothers taking amoxicillin (3 of 25 infants), nitrofurantoin (2 of 6 infants), and cephalexin (2 of 7 infants), but no reports of other adverse effects. Another study demonstrated that nitrofurantoin is actively transported into the mother’s milk, making hemolytic anemia a possibility in G6PD-deficient infants.3
Studies indicate that adverse effects of fluoroquinolones in children are similar to those in adults despite a contraindication in children because of reports of arthropathy in young animals. One case of pseudomembranous colitis in a breastfeeding infant and 2 cases of green teeth in neonates have been reported with ciprofloxacin use.6,8,9
RECOMMENDATIONS
The Infectious Disease Society of America recommends nitrofurantoin, TMP/SMX, or fosfomycin for first-line treatment of uncomplicated UTIs in women, although fosfomycin appears to be inferior to other standard short-course antibiotics based on FDA data. Fluoroquinolones and β-lactams are recommended alternative treatments.10
The American Academy of Pediatrics’ Committee on Drugs says that TMP/SMX (unless G6PD deficiency is present), amoxicillin, nitrofurantoin, ciprofloxacin, and ofloxacin usually are compatible with breastfeeding.11
It’s unclear, as no studies have specifically evaluated therapies for uncomplicated urinary tract infections (UTIs) in breastfeeding women. However, trimethoprim/sulfamethoxazole (TMP/ SMX), β-lactam antibiotics, nitrofurantoin, and fluoroquinolones all produce cure rates of 78% to 95% for uncomplicated UTIs in women who aren’t breastfeeding, and all appear to be equivalent (strength of recommendation [SOR]: A, a systematic review).
Women who take TMP/SMX develop drug concentrations in breast milk that are below recommended maximum safe levels for infants who don’t have glucose-6-phosphate dehydrogenase (G6PD) deficiency (SOR: B, a small observational study and expert opinion); treatment with nitrofurantoin and ciprofloxacin also produces low levels in breast milk (SOR: C, extrapolations from small observational studies and expert opinion). (Though in the case of nitrofurantoin, this does not include patients with G6PD deficiency.)
Some antibiotics taken by breastfeeding mothers may occasionally be associated with adverse effects in their infants: TMP/SMX may cause poor feeding; amoxicillin and cephalexin may cause diarrhea; nitrofurantoin may cause diarrhea or, in infants with G6PD deficiency, hemolytic anemia; and ciprofloxacin may cause pseudomembranous colitis in infants and green teeth in neonates (SOR: C, case reports and expert opinion).
EVIDENCE SUMMARY
Because no randomized controlled trials have evaluated the efficacy of UTI treatment in lactating women, recommendations are extrapolated from studies in other populations and case reports.
Antibiotics: Comparable and effective
A 2010 Cochrane review examined 21 good-quality randomized trials that compared the effectiveness of TMP-SMX, β-lactam antibiotics, nitrofurantoin, and fluoroquinolones for uncomplicated UTIs in 6016 women.1 The authors found no significant differences in short-term symptom cure rates: all antibiotics were very effective. Seven studies reported mixed (clinical and bacteriologic) cure rates.
Symptom cure rates for patients followed for as long as 2 weeks ranged from 78% to 95%; longer-term (as long as 8 weeks) symptom cure rates ranged from 82% to 91%. The review suggested that TMP-SMX may be slightly more likely to cause a rash than other antibiotics.1
Antibiotic concentrations in breast milk
In a case series, TMP/SMX, 160/800 mg, given to 50 lactating women 2 times (40 women) or 3 times (10 women) daily resulted in an average breast milk concentration of 2 μg/mL of TMP and 4.6 μg/mL of SMX, corresponding to respective doses of 0.3 and 0.7 mg/kg/d for infants taking 150 mL breast milk/kg/d.2 The authors state that this dose is safe for infants without G6PD deficiency. The study included only women with UTIs or other infections requiring antibiotic treatment.
A case series of 4 lactating mothers who received a single 100-mg oral dose of nitrofurantoin found that peak breast milk concentration occurred 4 hours later and averaged 2.4 μg/mL (standard deviation=1.7-3.2 μg/mL).3 The authors calculated a mean concentration over 12 hours of 1.3 μg nitrofurantoin/mL breast milk. This level would correspond to an estimated dose of 0.2 mg/kg/d for an infant consuming 150 mL/kg/d of breast milk whose mother takes 100 mg nitrofurantoin twice daily, much lower than the recommended pediatric dose of 5 to 7 mg/kg/d.
Data from a case series and a case report suggest the amount of ciprofloxacin transferred to breastfed infants is low. In the case series, researchers gave 10 lactating women 3 oral doses of ciprofloxacin, 750 mg, at 12-hour intervals and then measured ciprofloxacin levels in breast milk.4 The highest levels occurred 2 hours after the third dose and averaged 3.79 μg/mL. Average levels fell gradually to 0.02 μg/mL 24 hours after the third dose. Assuming a milk intake of 150 mL/kg/d, a breastfed infant would consume approximately 0.3 mg/kg/d, much lower than the 10 to 40 mg/kg/d dose recommended for treating sick infants.
A case report of a woman who took oral ciprofloxacin 500 mg/d for 10 days noted a breast milk ciprofloxacin concentration of 0.98 mg/L at 10.7 hours after the last dose.5 Ofloxacin, norfloxacin, and levofloxacin have been associated with lower milk concentrations than ciprofloxacin.6
Adverse effects
In a cohort study of 838 women from a program for pregnant and lactating women exposed to drugs and other substances, 2 of 12 mothers taking TMP/SMX reported poor feeding in their infants.7
The same program received reports of infants with diarrhea from mothers taking amoxicillin (3 of 25 infants), nitrofurantoin (2 of 6 infants), and cephalexin (2 of 7 infants), but no reports of other adverse effects. Another study demonstrated that nitrofurantoin is actively transported into the mother’s milk, making hemolytic anemia a possibility in G6PD-deficient infants.3
Studies indicate that adverse effects of fluoroquinolones in children are similar to those in adults despite a contraindication in children because of reports of arthropathy in young animals. One case of pseudomembranous colitis in a breastfeeding infant and 2 cases of green teeth in neonates have been reported with ciprofloxacin use.6,8,9
RECOMMENDATIONS
The Infectious Disease Society of America recommends nitrofurantoin, TMP/SMX, or fosfomycin for first-line treatment of uncomplicated UTIs in women, although fosfomycin appears to be inferior to other standard short-course antibiotics based on FDA data. Fluoroquinolones and β-lactams are recommended alternative treatments.10
The American Academy of Pediatrics’ Committee on Drugs says that TMP/SMX (unless G6PD deficiency is present), amoxicillin, nitrofurantoin, ciprofloxacin, and ofloxacin usually are compatible with breastfeeding.11
1. Zalmanovici Trestioreanu A, Green H, Paul M, et al. Antimicrobial agents for treating uncomplicated urinary tract infection in women. Cochrane Database of Syst Rev. 2010;(10):CD007182.
2. Miller RD, Salter AJ. The passage of trimethoprim/sulfamethoxazole into breast milk and its significance. Proceedings of the 8th International Congress of Chemotherapy, Athens. Hellenic Soc Chemother. 1974;1:687-691.
3. Gerk PM, Kuhn RJ, Desai NS, et al. Active transport of nitrofurantoin into human milk. Pharmacotherapy. 2001;21:669-675.
4. Giamarellou H, Kolokythas E, Petrikkos G, et al. Pharmacokinetics of three newer quinolones in pregnant and lactating women. Am J Med. 1989;87:49S-51S.
5. Gardner DK, Gabbe SG, Harter C. Simultaneous concentrations of ciprofloxacin in breast milk and in serum in mother and breast-fed infant. Clin Pharm. 1992;11:352-354.
6. Bar-Oz B, Bulkowstein M, Benyamini L, et al. Use of antibiotic and analgesic drugs during lactation. Drug Safety. 2003;26:925-935.
7. Ito S, Blajchman A, Stephenson M, et al. Prospective followup of breast-fed infants exposed to maternal medication. Am J Obstet Gynecol.1993;168:1393-1399.
8. Harmon T, Burkhart G, Applebaum H. Perforated pseudomembranous colitis in the breast-fed infant. J Pediatr Surg. 1992;27:744-746.
9. Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 8th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008.
10. Gupta K, Hooton TM, Naber KG, et al; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International Clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
11. American Academy of Pediatrics Committee on Drugs. The transfer of drugs and other chemical into human milk. Pediatrics. 2001;108:776-789.
1. Zalmanovici Trestioreanu A, Green H, Paul M, et al. Antimicrobial agents for treating uncomplicated urinary tract infection in women. Cochrane Database of Syst Rev. 2010;(10):CD007182.
2. Miller RD, Salter AJ. The passage of trimethoprim/sulfamethoxazole into breast milk and its significance. Proceedings of the 8th International Congress of Chemotherapy, Athens. Hellenic Soc Chemother. 1974;1:687-691.
3. Gerk PM, Kuhn RJ, Desai NS, et al. Active transport of nitrofurantoin into human milk. Pharmacotherapy. 2001;21:669-675.
4. Giamarellou H, Kolokythas E, Petrikkos G, et al. Pharmacokinetics of three newer quinolones in pregnant and lactating women. Am J Med. 1989;87:49S-51S.
5. Gardner DK, Gabbe SG, Harter C. Simultaneous concentrations of ciprofloxacin in breast milk and in serum in mother and breast-fed infant. Clin Pharm. 1992;11:352-354.
6. Bar-Oz B, Bulkowstein M, Benyamini L, et al. Use of antibiotic and analgesic drugs during lactation. Drug Safety. 2003;26:925-935.
7. Ito S, Blajchman A, Stephenson M, et al. Prospective followup of breast-fed infants exposed to maternal medication. Am J Obstet Gynecol.1993;168:1393-1399.
8. Harmon T, Burkhart G, Applebaum H. Perforated pseudomembranous colitis in the breast-fed infant. J Pediatr Surg. 1992;27:744-746.
9. Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 8th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008.
10. Gupta K, Hooton TM, Naber KG, et al; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International Clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
11. American Academy of Pediatrics Committee on Drugs. The transfer of drugs and other chemical into human milk. Pediatrics. 2001;108:776-789.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best approach to goiter in euthyroid patients?
IN THE ABSENCE OF OUTCOME STUDIES, experts recommend ultrasound evaluation of nontoxic mulinodular goiters (MNG) followed by fine-needle aspiration (FNA) of suspicious nodules (strength of recommendation [SOR]: C, consensus-based guidelines).
Thyroid hormone suppression therapy reduces the size of MNG (SOR: A, systematic review of randomized controlled trials [RCTs]), but it risks inducing hyperthyroidism (SOR: C, expert opinion).
Experts recommend thyroidectomy for compressive symptoms, progressive growth, or ultrasound or FNA results indicating thyroid cancer (SOR: C, consensus based guidelines).
Expert guidelines recommend repeat ultrasound at 6 to 18 months to follow up benign nodules or nonendemic MNG in patients at low risk of malignancy and subsequent follow-up of stable nodules every 3 to 5 years (SOR: C, consensus-based guidelines).
EVIDENCE SUMMARY
This summary updates the 2007 Clinical Inquiry, “What is the best approach to goiter for euthyroid patients?”1
Initial evaluation of palpable goiter with a normal thyrotropin
In the United States, MNG is generally nonendemic and unrelated to iodine deficiency, as distinguished from endemic goiter caused by iodine deficiency in other parts of the world.
Our structured search of the literature found no randomized trials or prospective cohort studies comparing diagnostic approaches. The American Association of Clinical Endocrinologists’ (AACE) 2010 guidelines and American Thyroid Association (ATA) guidelines recommend ultrasound for all MNG.2,3 The AACE guidelines recommend thyroid scintigraphy when clinicians suspect retrosternal MNG.2
Ultrasound findings can change management, avoid biopsy
In a retrospective analysis of 223 patients with nodular thyroid disease, thyroid ultrasound altered clinical management of 63% of patients with abnormal thyroid exams.4 A single center retrospective cohort study of 650 FNA biopsies identified 4 morphologic patterns on ultrasound that predicted benign cytology with 100% specificity. The authors concluded that using ultrasound pattern to determine which patients require FNA could have obviated more than 60% of thyroid biopsies.5
Thyroid hormone suppression therapy risks hyperthyroidis
A systematic review of 9 RCTs of 18-month or shorter duration found that thyroid hormone suppression therapy reduced benign thyroid nodule volume (relative risk=1.88 compared with placebo or no treatment; 95% confidence interval [CI], 1.18-3.01; P=.008). The number needed to treat was 8 to reduce volume by >50% (risk difference=0.13; 95% CI, 0.06-0.19; P=.0003).6 However, thyroid hormone suppression therapy risks inducing hyperthyroidism and is not routinely recommended by the AACE or the ATA.2,3
Thyroidectomy: The treatment of choice
Thyroidectomy is the definitive therapy for MNG. A narrative review of 15 mostly retrospective cohort studies demonstrated MNG recurrence rates of 0% to 0.3% after total thyroidectomy, with follow-up intervals of 4.8 to 30 years.7
AACE consensus opinion recommends thyroidectomy for compressive symptoms, progressive growth, or when ultrasound or FNA results indicate thyroid cancer.2
A retrospective cohort study of 462 thyroidectomies for MNG found incidental thyroid carcinomas in 8.9% (41 patients). Risk factors included neck irradiation (odds ratio [OR]=21.64; 95% CI, 3.28-143), parenchymal calcifications on imaging (OR=2.30; 95% CI, 0.85-6.23), and family history of thyroid disease (OR=8.2; 95% CI, 2.15-29.87). Living in a goiter-endemic area was protective (OR=0.24; 95% CI, 0.07-0.83).8
Follow-up of patients with initial benign evaluation
Consensus opinion regarding follow-up of MNG is based on observational studies of the natural history of the condition. Benign MNG rarely progresses to malignancy. A review of 6 cohort studies, including 1265 patients with untreated nontoxic MNG who were followed for 60 to 130 months from 1990 to 2007, yielded an annual incidence range of 1.3 to 3.7 new cases of thyroid carcinoma per 1000 patients.9
Some goiters are more likely to enlarge. A retrospective cohort study of 488 patients treated surgically for MNG identified risk factors for enlargement: African American (OR=3.3; 95% CI, 2.0-5.4), age >40 years (OR=2.1; 95% CI, 1.2-3.8), and body mass index >30 (OR=2.5; 95% CI, 1.5-4.0).10
RECOMMENDATIONS
The AACE and the ATA recommend that patients with MNG with benign nodules have a repeat examination, TSH, and ultrasound in 6 to 18 months. Follow-up of stable nodules can then be done in 3 to 5 years.
An enlarging nodule requires repeat FNA.2 If palpation or ultrasound reveal evidence of nodule growth (more than a 50% change in volume or a 20% increase in at least 2 nodule dimensions, with a minimal increase of 2 mm in solid nodules or the solid portion of mixed cystic-solid nodules), the AACE and ATA recommend FNA, preferably with ultrasound guidance.3 Low TSH suggests autonomous nodules and the ATA recommends radionuclide scanning with FNA of hypofunctioning nodules with suspicious US features.3
1. Hoffman MR, Meadows SE, Langlois JP. Clinical inquiries. What is the best approach to goiter for euthyroid patients? J Fam Pract. 2007;56:479-480.
2. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract. 2010;16(suppl 1):S1-S43.
3. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
4. Marqusee E, Benson CB, Frates MC, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696-700.
5. Bonavita JA, Mayo J, Babb J, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009;193:207-213.
6. Sdano MT, Falciglia M, Welge JA, et al. Efficacy of thyroid hormone suppression for benign thyroid nodules: metaanalysis of randomized trials. Otolaryngol Head Neck Surg. 2005;133:391-396.
7. Moalem J, Suh I, Duh QY. Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature. World J Surg. 2008;32:1301-1312.
8. Botrugno I, Lovisetto F, Cobianchi L, et al. Incidental carcinoma in multinodular goiter: risk factors. Am Surg. 2011;77:1553-1558.
9. Winbladh A, Järhult J. Fate of the non-operated, non-toxic goiter in a defined population. Brit J Surg. 2008;95:338-343.
10. Phitayakorn R, Super DM, McHenry CR. An investigation of epidemiologic factors associated with large nodular goiter. J Surg Res. 2006;133:16-21.
IN THE ABSENCE OF OUTCOME STUDIES, experts recommend ultrasound evaluation of nontoxic mulinodular goiters (MNG) followed by fine-needle aspiration (FNA) of suspicious nodules (strength of recommendation [SOR]: C, consensus-based guidelines).
Thyroid hormone suppression therapy reduces the size of MNG (SOR: A, systematic review of randomized controlled trials [RCTs]), but it risks inducing hyperthyroidism (SOR: C, expert opinion).
Experts recommend thyroidectomy for compressive symptoms, progressive growth, or ultrasound or FNA results indicating thyroid cancer (SOR: C, consensus based guidelines).
Expert guidelines recommend repeat ultrasound at 6 to 18 months to follow up benign nodules or nonendemic MNG in patients at low risk of malignancy and subsequent follow-up of stable nodules every 3 to 5 years (SOR: C, consensus-based guidelines).
EVIDENCE SUMMARY
This summary updates the 2007 Clinical Inquiry, “What is the best approach to goiter for euthyroid patients?”1
Initial evaluation of palpable goiter with a normal thyrotropin
In the United States, MNG is generally nonendemic and unrelated to iodine deficiency, as distinguished from endemic goiter caused by iodine deficiency in other parts of the world.
Our structured search of the literature found no randomized trials or prospective cohort studies comparing diagnostic approaches. The American Association of Clinical Endocrinologists’ (AACE) 2010 guidelines and American Thyroid Association (ATA) guidelines recommend ultrasound for all MNG.2,3 The AACE guidelines recommend thyroid scintigraphy when clinicians suspect retrosternal MNG.2
Ultrasound findings can change management, avoid biopsy
In a retrospective analysis of 223 patients with nodular thyroid disease, thyroid ultrasound altered clinical management of 63% of patients with abnormal thyroid exams.4 A single center retrospective cohort study of 650 FNA biopsies identified 4 morphologic patterns on ultrasound that predicted benign cytology with 100% specificity. The authors concluded that using ultrasound pattern to determine which patients require FNA could have obviated more than 60% of thyroid biopsies.5
Thyroid hormone suppression therapy risks hyperthyroidis
A systematic review of 9 RCTs of 18-month or shorter duration found that thyroid hormone suppression therapy reduced benign thyroid nodule volume (relative risk=1.88 compared with placebo or no treatment; 95% confidence interval [CI], 1.18-3.01; P=.008). The number needed to treat was 8 to reduce volume by >50% (risk difference=0.13; 95% CI, 0.06-0.19; P=.0003).6 However, thyroid hormone suppression therapy risks inducing hyperthyroidism and is not routinely recommended by the AACE or the ATA.2,3
Thyroidectomy: The treatment of choice
Thyroidectomy is the definitive therapy for MNG. A narrative review of 15 mostly retrospective cohort studies demonstrated MNG recurrence rates of 0% to 0.3% after total thyroidectomy, with follow-up intervals of 4.8 to 30 years.7
AACE consensus opinion recommends thyroidectomy for compressive symptoms, progressive growth, or when ultrasound or FNA results indicate thyroid cancer.2
A retrospective cohort study of 462 thyroidectomies for MNG found incidental thyroid carcinomas in 8.9% (41 patients). Risk factors included neck irradiation (odds ratio [OR]=21.64; 95% CI, 3.28-143), parenchymal calcifications on imaging (OR=2.30; 95% CI, 0.85-6.23), and family history of thyroid disease (OR=8.2; 95% CI, 2.15-29.87). Living in a goiter-endemic area was protective (OR=0.24; 95% CI, 0.07-0.83).8
Follow-up of patients with initial benign evaluation
Consensus opinion regarding follow-up of MNG is based on observational studies of the natural history of the condition. Benign MNG rarely progresses to malignancy. A review of 6 cohort studies, including 1265 patients with untreated nontoxic MNG who were followed for 60 to 130 months from 1990 to 2007, yielded an annual incidence range of 1.3 to 3.7 new cases of thyroid carcinoma per 1000 patients.9
Some goiters are more likely to enlarge. A retrospective cohort study of 488 patients treated surgically for MNG identified risk factors for enlargement: African American (OR=3.3; 95% CI, 2.0-5.4), age >40 years (OR=2.1; 95% CI, 1.2-3.8), and body mass index >30 (OR=2.5; 95% CI, 1.5-4.0).10
RECOMMENDATIONS
The AACE and the ATA recommend that patients with MNG with benign nodules have a repeat examination, TSH, and ultrasound in 6 to 18 months. Follow-up of stable nodules can then be done in 3 to 5 years.
An enlarging nodule requires repeat FNA.2 If palpation or ultrasound reveal evidence of nodule growth (more than a 50% change in volume or a 20% increase in at least 2 nodule dimensions, with a minimal increase of 2 mm in solid nodules or the solid portion of mixed cystic-solid nodules), the AACE and ATA recommend FNA, preferably with ultrasound guidance.3 Low TSH suggests autonomous nodules and the ATA recommends radionuclide scanning with FNA of hypofunctioning nodules with suspicious US features.3
IN THE ABSENCE OF OUTCOME STUDIES, experts recommend ultrasound evaluation of nontoxic mulinodular goiters (MNG) followed by fine-needle aspiration (FNA) of suspicious nodules (strength of recommendation [SOR]: C, consensus-based guidelines).
Thyroid hormone suppression therapy reduces the size of MNG (SOR: A, systematic review of randomized controlled trials [RCTs]), but it risks inducing hyperthyroidism (SOR: C, expert opinion).
Experts recommend thyroidectomy for compressive symptoms, progressive growth, or ultrasound or FNA results indicating thyroid cancer (SOR: C, consensus based guidelines).
Expert guidelines recommend repeat ultrasound at 6 to 18 months to follow up benign nodules or nonendemic MNG in patients at low risk of malignancy and subsequent follow-up of stable nodules every 3 to 5 years (SOR: C, consensus-based guidelines).
EVIDENCE SUMMARY
This summary updates the 2007 Clinical Inquiry, “What is the best approach to goiter for euthyroid patients?”1
Initial evaluation of palpable goiter with a normal thyrotropin
In the United States, MNG is generally nonendemic and unrelated to iodine deficiency, as distinguished from endemic goiter caused by iodine deficiency in other parts of the world.
Our structured search of the literature found no randomized trials or prospective cohort studies comparing diagnostic approaches. The American Association of Clinical Endocrinologists’ (AACE) 2010 guidelines and American Thyroid Association (ATA) guidelines recommend ultrasound for all MNG.2,3 The AACE guidelines recommend thyroid scintigraphy when clinicians suspect retrosternal MNG.2
Ultrasound findings can change management, avoid biopsy
In a retrospective analysis of 223 patients with nodular thyroid disease, thyroid ultrasound altered clinical management of 63% of patients with abnormal thyroid exams.4 A single center retrospective cohort study of 650 FNA biopsies identified 4 morphologic patterns on ultrasound that predicted benign cytology with 100% specificity. The authors concluded that using ultrasound pattern to determine which patients require FNA could have obviated more than 60% of thyroid biopsies.5
Thyroid hormone suppression therapy risks hyperthyroidis
A systematic review of 9 RCTs of 18-month or shorter duration found that thyroid hormone suppression therapy reduced benign thyroid nodule volume (relative risk=1.88 compared with placebo or no treatment; 95% confidence interval [CI], 1.18-3.01; P=.008). The number needed to treat was 8 to reduce volume by >50% (risk difference=0.13; 95% CI, 0.06-0.19; P=.0003).6 However, thyroid hormone suppression therapy risks inducing hyperthyroidism and is not routinely recommended by the AACE or the ATA.2,3
Thyroidectomy: The treatment of choice
Thyroidectomy is the definitive therapy for MNG. A narrative review of 15 mostly retrospective cohort studies demonstrated MNG recurrence rates of 0% to 0.3% after total thyroidectomy, with follow-up intervals of 4.8 to 30 years.7
AACE consensus opinion recommends thyroidectomy for compressive symptoms, progressive growth, or when ultrasound or FNA results indicate thyroid cancer.2
A retrospective cohort study of 462 thyroidectomies for MNG found incidental thyroid carcinomas in 8.9% (41 patients). Risk factors included neck irradiation (odds ratio [OR]=21.64; 95% CI, 3.28-143), parenchymal calcifications on imaging (OR=2.30; 95% CI, 0.85-6.23), and family history of thyroid disease (OR=8.2; 95% CI, 2.15-29.87). Living in a goiter-endemic area was protective (OR=0.24; 95% CI, 0.07-0.83).8
Follow-up of patients with initial benign evaluation
Consensus opinion regarding follow-up of MNG is based on observational studies of the natural history of the condition. Benign MNG rarely progresses to malignancy. A review of 6 cohort studies, including 1265 patients with untreated nontoxic MNG who were followed for 60 to 130 months from 1990 to 2007, yielded an annual incidence range of 1.3 to 3.7 new cases of thyroid carcinoma per 1000 patients.9
Some goiters are more likely to enlarge. A retrospective cohort study of 488 patients treated surgically for MNG identified risk factors for enlargement: African American (OR=3.3; 95% CI, 2.0-5.4), age >40 years (OR=2.1; 95% CI, 1.2-3.8), and body mass index >30 (OR=2.5; 95% CI, 1.5-4.0).10
RECOMMENDATIONS
The AACE and the ATA recommend that patients with MNG with benign nodules have a repeat examination, TSH, and ultrasound in 6 to 18 months. Follow-up of stable nodules can then be done in 3 to 5 years.
An enlarging nodule requires repeat FNA.2 If palpation or ultrasound reveal evidence of nodule growth (more than a 50% change in volume or a 20% increase in at least 2 nodule dimensions, with a minimal increase of 2 mm in solid nodules or the solid portion of mixed cystic-solid nodules), the AACE and ATA recommend FNA, preferably with ultrasound guidance.3 Low TSH suggests autonomous nodules and the ATA recommends radionuclide scanning with FNA of hypofunctioning nodules with suspicious US features.3
1. Hoffman MR, Meadows SE, Langlois JP. Clinical inquiries. What is the best approach to goiter for euthyroid patients? J Fam Pract. 2007;56:479-480.
2. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract. 2010;16(suppl 1):S1-S43.
3. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
4. Marqusee E, Benson CB, Frates MC, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696-700.
5. Bonavita JA, Mayo J, Babb J, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009;193:207-213.
6. Sdano MT, Falciglia M, Welge JA, et al. Efficacy of thyroid hormone suppression for benign thyroid nodules: metaanalysis of randomized trials. Otolaryngol Head Neck Surg. 2005;133:391-396.
7. Moalem J, Suh I, Duh QY. Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature. World J Surg. 2008;32:1301-1312.
8. Botrugno I, Lovisetto F, Cobianchi L, et al. Incidental carcinoma in multinodular goiter: risk factors. Am Surg. 2011;77:1553-1558.
9. Winbladh A, Järhult J. Fate of the non-operated, non-toxic goiter in a defined population. Brit J Surg. 2008;95:338-343.
10. Phitayakorn R, Super DM, McHenry CR. An investigation of epidemiologic factors associated with large nodular goiter. J Surg Res. 2006;133:16-21.
1. Hoffman MR, Meadows SE, Langlois JP. Clinical inquiries. What is the best approach to goiter for euthyroid patients? J Fam Pract. 2007;56:479-480.
2. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract. 2010;16(suppl 1):S1-S43.
3. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
4. Marqusee E, Benson CB, Frates MC, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696-700.
5. Bonavita JA, Mayo J, Babb J, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009;193:207-213.
6. Sdano MT, Falciglia M, Welge JA, et al. Efficacy of thyroid hormone suppression for benign thyroid nodules: metaanalysis of randomized trials. Otolaryngol Head Neck Surg. 2005;133:391-396.
7. Moalem J, Suh I, Duh QY. Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature. World J Surg. 2008;32:1301-1312.
8. Botrugno I, Lovisetto F, Cobianchi L, et al. Incidental carcinoma in multinodular goiter: risk factors. Am Surg. 2011;77:1553-1558.
9. Winbladh A, Järhult J. Fate of the non-operated, non-toxic goiter in a defined population. Brit J Surg. 2008;95:338-343.
10. Phitayakorn R, Super DM, McHenry CR. An investigation of epidemiologic factors associated with large nodular goiter. J Surg Res. 2006;133:16-21.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best imaging method for patients with a presumed acute stroke?
It depends on whether the stroke is schemic or hemorrhagic. For early detection of ischemic stroke, magnetic resonance imaging (MRI) using diffusion-weighted imaging (DWI) is highly sensitive and specific, whereas computed tomography (CT) is less sensitive but about as specific (strength of recommendation [SOR]: B, a meta-analysis of lower quality RCTs). MRI using DWI and CT are probably comparable for detecting acute hemorrhagic stroke (SOR: B, a cohort study).
When thrombolysis is being considered and hemorrhage must be ruled out rapidly, either test is acceptable if it can be performed and interpreted within 45 minutes of patient arrival, although MRI typically costs about twice as much as CT (SOR: C, expert opinion).
EVIDENCE SUMMARY
Clinical A Cochrane review identified 7 studies that compared MRI with CT for detecting ischemic stroke in a total of 226 patients, average age 65 years, with stroke-like symptoms.1 Investigators performed imaging within 12 hours of symptom onset in all patients, including those whose final diagnosis was transient ischemic attack (TIA). They identified 161 patients with ischemic stroke based on a combination of imaging and clinical examination. MRI with DWI was more sensitive than CT (0.99; 95% confidence interval [CI], 0.23-1.00 vs 0.39; 95% CI, 0.16-0.69); both techniques had comparable specificity (0.92; 95% CI, 0.83-0.97 and 1.00; 95% CI, 0.94-1.00, respectively).
Many issues could have affected the ischemic stroke analysis: All studies included some retrospective data collection; in all but one study, the MRI was performed a mean of one hour after the CT; and in 4 studies, the physicians reading the scans weren’t blinded to the clinical outcome. The Cochrane authors also found evidence of “prescreening” that appeared to select for patients with middle-cerebral artery infarcts. They concluded that the reliability and generalizability of the results “were questionable.”
MRI and CT have similar sensitivity and specificity for hemorrhagic stroke
A prospective cohort study of 27 patients (mean age 76 years) who had an acute hemorrhagic stroke that was imaged using both MRI with DWI and CT within 3 hours of symptom onset found that both imaging studies had comparable sensitivity (0.81; 95% CI, 0.61-0.93 vs 0.89; 95% CI, 0.70-0.97, respectively) and specificity (1.0; 95% CI, 0.98-1.0 for both).2
A retrospective case-control study evaluated the ability of DWI to detect hemorrhagic stroke in 86 patients who presented with symptoms consistent with acute stroke.3 Investigators compared the sensitivity and specificity of DWI against the pooled results of 5 different MRI sequences. Both case and control imaging was performed within 6 hours of symptom onset. Half of the patients in the study had hemorrhagic strokes (43); the rest had ischemic strokes (41) or a TIA and postictal deficit (2). The sensitivity and specificity of DWI for hemorrhagic stroke were both 1.0. However, there was no independent reference standard.
MRI costs more than CT
Although costs vary widely, one textbook put the national average charge for a head CT at about $1000.4 MRI neuroimaging charges ranged from $1000 to $4700, with an average of about $2300. Medicare reimbursements were significantly less, although the cost of MRIs was still about double that of CTs.
Recommendations
The Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology says that DWI is more useful than noncontrast CT for diagnosing acute ischemic stroke in patients presenting within 12 hours of symptom onset.5 The subcommittee made no recommendation for imaging hemorrhagic stroke.
American Heart Association and American Stroke Association guidelines for early management of adults with ischemic stroke recommend neuroimaging with either DWI or CT within 45 minutes of arrival in candidates for tissue plasminogen activator.6 They also recommend neuroimaging with either CT or MRI to distinguish ischemic from hemorrhagic stroke.7 The guidelines state that other imaging methods (including CT angiography, contrast-enhanced MRI, and magnetic resonance angiography) “may be considered” to evaluate for clinically suspected underlying structural lesions, including vascular malformations and tumors.
1. Brazeli M, Sandercock PA, Chappell FM, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev. 2009;(4):CD007424.
2. Chelela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
3. Oppenheim C, Touzé E, Hernalsteen D, et al. Comparison of five MR sequences for the detection of acute intracranial hemorrhage. Cerebrovasc Dis. 2005;20:388-394.
4. Broder J, Preston R. Imaging the head and brain. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, Pa: Elsevier/Saunders; 2011:26-27.
5. Shellinger PD, Bryan RN, Caplan LR, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75:177-185.
6. Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
7. Morgenstern LB, Hemphill III JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for health care professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
It depends on whether the stroke is schemic or hemorrhagic. For early detection of ischemic stroke, magnetic resonance imaging (MRI) using diffusion-weighted imaging (DWI) is highly sensitive and specific, whereas computed tomography (CT) is less sensitive but about as specific (strength of recommendation [SOR]: B, a meta-analysis of lower quality RCTs). MRI using DWI and CT are probably comparable for detecting acute hemorrhagic stroke (SOR: B, a cohort study).
When thrombolysis is being considered and hemorrhage must be ruled out rapidly, either test is acceptable if it can be performed and interpreted within 45 minutes of patient arrival, although MRI typically costs about twice as much as CT (SOR: C, expert opinion).
EVIDENCE SUMMARY
Clinical A Cochrane review identified 7 studies that compared MRI with CT for detecting ischemic stroke in a total of 226 patients, average age 65 years, with stroke-like symptoms.1 Investigators performed imaging within 12 hours of symptom onset in all patients, including those whose final diagnosis was transient ischemic attack (TIA). They identified 161 patients with ischemic stroke based on a combination of imaging and clinical examination. MRI with DWI was more sensitive than CT (0.99; 95% confidence interval [CI], 0.23-1.00 vs 0.39; 95% CI, 0.16-0.69); both techniques had comparable specificity (0.92; 95% CI, 0.83-0.97 and 1.00; 95% CI, 0.94-1.00, respectively).
Many issues could have affected the ischemic stroke analysis: All studies included some retrospective data collection; in all but one study, the MRI was performed a mean of one hour after the CT; and in 4 studies, the physicians reading the scans weren’t blinded to the clinical outcome. The Cochrane authors also found evidence of “prescreening” that appeared to select for patients with middle-cerebral artery infarcts. They concluded that the reliability and generalizability of the results “were questionable.”
MRI and CT have similar sensitivity and specificity for hemorrhagic stroke
A prospective cohort study of 27 patients (mean age 76 years) who had an acute hemorrhagic stroke that was imaged using both MRI with DWI and CT within 3 hours of symptom onset found that both imaging studies had comparable sensitivity (0.81; 95% CI, 0.61-0.93 vs 0.89; 95% CI, 0.70-0.97, respectively) and specificity (1.0; 95% CI, 0.98-1.0 for both).2
A retrospective case-control study evaluated the ability of DWI to detect hemorrhagic stroke in 86 patients who presented with symptoms consistent with acute stroke.3 Investigators compared the sensitivity and specificity of DWI against the pooled results of 5 different MRI sequences. Both case and control imaging was performed within 6 hours of symptom onset. Half of the patients in the study had hemorrhagic strokes (43); the rest had ischemic strokes (41) or a TIA and postictal deficit (2). The sensitivity and specificity of DWI for hemorrhagic stroke were both 1.0. However, there was no independent reference standard.
MRI costs more than CT
Although costs vary widely, one textbook put the national average charge for a head CT at about $1000.4 MRI neuroimaging charges ranged from $1000 to $4700, with an average of about $2300. Medicare reimbursements were significantly less, although the cost of MRIs was still about double that of CTs.
Recommendations
The Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology says that DWI is more useful than noncontrast CT for diagnosing acute ischemic stroke in patients presenting within 12 hours of symptom onset.5 The subcommittee made no recommendation for imaging hemorrhagic stroke.
American Heart Association and American Stroke Association guidelines for early management of adults with ischemic stroke recommend neuroimaging with either DWI or CT within 45 minutes of arrival in candidates for tissue plasminogen activator.6 They also recommend neuroimaging with either CT or MRI to distinguish ischemic from hemorrhagic stroke.7 The guidelines state that other imaging methods (including CT angiography, contrast-enhanced MRI, and magnetic resonance angiography) “may be considered” to evaluate for clinically suspected underlying structural lesions, including vascular malformations and tumors.
It depends on whether the stroke is schemic or hemorrhagic. For early detection of ischemic stroke, magnetic resonance imaging (MRI) using diffusion-weighted imaging (DWI) is highly sensitive and specific, whereas computed tomography (CT) is less sensitive but about as specific (strength of recommendation [SOR]: B, a meta-analysis of lower quality RCTs). MRI using DWI and CT are probably comparable for detecting acute hemorrhagic stroke (SOR: B, a cohort study).
When thrombolysis is being considered and hemorrhage must be ruled out rapidly, either test is acceptable if it can be performed and interpreted within 45 minutes of patient arrival, although MRI typically costs about twice as much as CT (SOR: C, expert opinion).
EVIDENCE SUMMARY
Clinical A Cochrane review identified 7 studies that compared MRI with CT for detecting ischemic stroke in a total of 226 patients, average age 65 years, with stroke-like symptoms.1 Investigators performed imaging within 12 hours of symptom onset in all patients, including those whose final diagnosis was transient ischemic attack (TIA). They identified 161 patients with ischemic stroke based on a combination of imaging and clinical examination. MRI with DWI was more sensitive than CT (0.99; 95% confidence interval [CI], 0.23-1.00 vs 0.39; 95% CI, 0.16-0.69); both techniques had comparable specificity (0.92; 95% CI, 0.83-0.97 and 1.00; 95% CI, 0.94-1.00, respectively).
Many issues could have affected the ischemic stroke analysis: All studies included some retrospective data collection; in all but one study, the MRI was performed a mean of one hour after the CT; and in 4 studies, the physicians reading the scans weren’t blinded to the clinical outcome. The Cochrane authors also found evidence of “prescreening” that appeared to select for patients with middle-cerebral artery infarcts. They concluded that the reliability and generalizability of the results “were questionable.”
MRI and CT have similar sensitivity and specificity for hemorrhagic stroke
A prospective cohort study of 27 patients (mean age 76 years) who had an acute hemorrhagic stroke that was imaged using both MRI with DWI and CT within 3 hours of symptom onset found that both imaging studies had comparable sensitivity (0.81; 95% CI, 0.61-0.93 vs 0.89; 95% CI, 0.70-0.97, respectively) and specificity (1.0; 95% CI, 0.98-1.0 for both).2
A retrospective case-control study evaluated the ability of DWI to detect hemorrhagic stroke in 86 patients who presented with symptoms consistent with acute stroke.3 Investigators compared the sensitivity and specificity of DWI against the pooled results of 5 different MRI sequences. Both case and control imaging was performed within 6 hours of symptom onset. Half of the patients in the study had hemorrhagic strokes (43); the rest had ischemic strokes (41) or a TIA and postictal deficit (2). The sensitivity and specificity of DWI for hemorrhagic stroke were both 1.0. However, there was no independent reference standard.
MRI costs more than CT
Although costs vary widely, one textbook put the national average charge for a head CT at about $1000.4 MRI neuroimaging charges ranged from $1000 to $4700, with an average of about $2300. Medicare reimbursements were significantly less, although the cost of MRIs was still about double that of CTs.
Recommendations
The Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology says that DWI is more useful than noncontrast CT for diagnosing acute ischemic stroke in patients presenting within 12 hours of symptom onset.5 The subcommittee made no recommendation for imaging hemorrhagic stroke.
American Heart Association and American Stroke Association guidelines for early management of adults with ischemic stroke recommend neuroimaging with either DWI or CT within 45 minutes of arrival in candidates for tissue plasminogen activator.6 They also recommend neuroimaging with either CT or MRI to distinguish ischemic from hemorrhagic stroke.7 The guidelines state that other imaging methods (including CT angiography, contrast-enhanced MRI, and magnetic resonance angiography) “may be considered” to evaluate for clinically suspected underlying structural lesions, including vascular malformations and tumors.
1. Brazeli M, Sandercock PA, Chappell FM, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev. 2009;(4):CD007424.
2. Chelela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
3. Oppenheim C, Touzé E, Hernalsteen D, et al. Comparison of five MR sequences for the detection of acute intracranial hemorrhage. Cerebrovasc Dis. 2005;20:388-394.
4. Broder J, Preston R. Imaging the head and brain. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, Pa: Elsevier/Saunders; 2011:26-27.
5. Shellinger PD, Bryan RN, Caplan LR, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75:177-185.
6. Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
7. Morgenstern LB, Hemphill III JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for health care professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
1. Brazeli M, Sandercock PA, Chappell FM, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev. 2009;(4):CD007424.
2. Chelela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
3. Oppenheim C, Touzé E, Hernalsteen D, et al. Comparison of five MR sequences for the detection of acute intracranial hemorrhage. Cerebrovasc Dis. 2005;20:388-394.
4. Broder J, Preston R. Imaging the head and brain. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, Pa: Elsevier/Saunders; 2011:26-27.
5. Shellinger PD, Bryan RN, Caplan LR, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75:177-185.
6. Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
7. Morgenstern LB, Hemphill III JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for health care professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
Evidence-based answers from the Family Physicians Inquiries Network
Whom should you test for secondary causes of hypertension?
IT'S RECOMMENDED that all children and adolescents with a new diagnosis of hypertension undergo renal ultrasound and laboratory evaluation for renal pathology (strength of recommendation [SOR]: C, consensus-based guidelines).
Specific diagnostic tests are recommended for newly diagnosed patients who have suspicious clinical findings suggestive of a secondary cause of hypertension based on the initial history (excess daytime sleepiness, palpitations, tremor, sweating); physical examination (abdominal bruit, thyromegaly, malar rash); or laboratory analysis (elevated serum creatinine, low thyroid-stimulating hormone) (SOR: C, consensus-based guidelines).
Patients with undifferentiated resistant hypertension should receive further directed evaluation for secondary causes (SOR: C, consensus-based guidelines).
EVIDENCE SUMMARY
The evidence for selecting which patients should undergo additional testing for potentially correctable secondary causes of hypertension is based on the prevalence of these causes in different age groups, case series of reversal of hypertension with effective treatment of the underlying cause, and clinical suspicion of a secondary cause that may be reversible. We found no prospective cohort studies or randomized trials evaluating diagnostic approaches or outcomes associated with particular selection criteria for conducting additional diagnostic evaluations in search of secondary causes. Therefore, our recommendations are based primarily on expert guidelines, which we summarize here.
When caring for children and adolescents with newly diagnosed hypertension...
Secondary hypertension is more prevalent in younger children and in children and adolescents with stage 2 hypertension (blood pressure [BP] >99th percentile for age and height plus 5 mm Hg).1 Renoparenchymal and renovascular disease account for most cases of secondary hypertension in these children.2
The National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents recommends that all children and adolescents with hypertension have an additional diagnostic work-up. This is based on the observation that 70% to 85% of children <12 years and 10% to 15% of adolescents 12 to 18 years with hypertension have an underlying cause, most commonly renoparenchymal and renovascular disease.3
According to the National Institutes of Health (NIH), “the possibility that some underlying disorder may be the cause of the hypertension should be considered in every child or adolescent” with elevated BP, but the evaluation itself should be individualized.3
The NIH recommends more extensive evaluation for very young children, children with stage 2 hypertension, and children or adolescents who show clinical signs suggesting hypertension-linked systemic conditions. Such evaluation should include a renal ultrasound and laboratory testing (creatinine, urinalysis, and urine culture) to look for structural or functional anomalies.3
What about newly diagnosed adults with suspected secondary causes?
Secondary hypertension reportedly occurs in 5% to 10% of hypertensive patients.4,5 The only prospective study completed in a primary care setting evaluated 1020 patients at a general outpatient clinic in Yokohama, Japan. The investigators reported that 9.1% of the patients had an endocrinologic or renovascular cause contributing to their hypertension.6 The 5 most common causes were primary aldosteronism (6%), Cushing syndrome (1%), preclinical Cushing syndrome (1%), pheochromocytoma (0.6%), and renovascular disease (0.5 %).6
According to the Institute for Clinical Systems Improvement (ICSI), patients at highest risk for secondary hypertension have no family history of hypertension; abrupt onset, symptomatic, or crisis hypertension; stage 2 hypertension; sudden loss of hypertensive control; and drug-resistant hypertension.7
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommends that patients with the following characteristics undergo further directed evaluation for a secondary cause:8
• younger than 30 years with no family history of hypertension
• older than 55 years with new hypertension
• abdominal bruit with diastolic component
• sudden worsening of BP control
• recurrent flash pulmonary edema
• renal failure with abnormal urinary sediment or proteinuria
• acute renal failure after administration of an ACE inhibitor or ARB.
These patients should receive particular scrutiny
Patients with resistant hypertension (BP>140/90 mm Hg despite taking optimal doses of 3 antihypertensive medications, one of which is a diuretic) should receive particular scrutiny for an identifiable secondary cause, according to the ICSI.7
In a retrospective analysis of 141 patients with resistant hypertension referred to a university hypertension center in Chicago in 2005, 5% of patients had an identifiable secondary cause.9 A chart review of 436 patients presenting to a tertiary hypertension clinic in Japan identified 91 with resistant hypertension. A secondary cause was identified in 9.1%.10
Careful history and examination should identify patients suffering from uncontrolled hypertension because of noncompliance, suboptimal antihypertensive regimen, inaccurate BP readings, antagonizing substances, and white coat hypertension.11 The TABLE summarizes common presentations of, and workup for, secondary causes of hypertension.12-14
1. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Rockville, MD: National Heart, Lung, and Blood Institute, US Department of Health and Human Services; May 2005. NIH Publication No. 05-5267.
2. Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874-879.
3. Brady TM, Feld LG. Pediatric approach to hypertension. Semin Nephrol. 2009;29:379-388.
4. Taler SJ. Secondary causes of hypertension. Prim Care Clin Office Pract. 2008;35:489-500.
5. Chiong JR, Aronow WS, Khan IA, et al. Secondary hypertension: current diagnosis and treatment. Int J Cardiol. 2008;124:6-21.
6. Omura M, Saito J, Yamaguchi K, et al. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27:193-202.
7. Luehr D, Woolley T, Burke R, et al. Institute for Clinical Systems Improvement. Hypertension Diagnosis and Treatment. Available at: http://bit.ly/Hypertension1112. Updated November 2012. Accessed January 7, 2010.
8. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206-1252.
9. Garg JP, Elliott WJ, Folker A, et al; Rush University Hypertension Service. Resistant hypertension revisisted: a comparison of two university-based cohorts. Am J Hypertens. 2005;185:619-626.
10. Yakovlevitch M, Black HR. Resistant hypertension in a tertiary care clinic. Arch Intern Med. 1991;151:1786-1792.
11. O’Rorke JE, Richardson WS. What to do when hypertension is difficult to control. BMJ. 2001;322:1229-1232.
12. Rossi GP, Seccia TM, Pessina AC. Clinical use of laboratory tests for the identification of secondary forms of arterial hypertension. Crit Rev Clin Lab Sci. 2007;44:1-85.
13. Riley M, Bluhm B. High blood pressure in children and adolescents. Am Fam Physician. 2012;85:693-700.
14. Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age based approach. Am Fam Physician. 2010;82:1471-1478.
IT'S RECOMMENDED that all children and adolescents with a new diagnosis of hypertension undergo renal ultrasound and laboratory evaluation for renal pathology (strength of recommendation [SOR]: C, consensus-based guidelines).
Specific diagnostic tests are recommended for newly diagnosed patients who have suspicious clinical findings suggestive of a secondary cause of hypertension based on the initial history (excess daytime sleepiness, palpitations, tremor, sweating); physical examination (abdominal bruit, thyromegaly, malar rash); or laboratory analysis (elevated serum creatinine, low thyroid-stimulating hormone) (SOR: C, consensus-based guidelines).
Patients with undifferentiated resistant hypertension should receive further directed evaluation for secondary causes (SOR: C, consensus-based guidelines).
EVIDENCE SUMMARY
The evidence for selecting which patients should undergo additional testing for potentially correctable secondary causes of hypertension is based on the prevalence of these causes in different age groups, case series of reversal of hypertension with effective treatment of the underlying cause, and clinical suspicion of a secondary cause that may be reversible. We found no prospective cohort studies or randomized trials evaluating diagnostic approaches or outcomes associated with particular selection criteria for conducting additional diagnostic evaluations in search of secondary causes. Therefore, our recommendations are based primarily on expert guidelines, which we summarize here.
When caring for children and adolescents with newly diagnosed hypertension...
Secondary hypertension is more prevalent in younger children and in children and adolescents with stage 2 hypertension (blood pressure [BP] >99th percentile for age and height plus 5 mm Hg).1 Renoparenchymal and renovascular disease account for most cases of secondary hypertension in these children.2
The National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents recommends that all children and adolescents with hypertension have an additional diagnostic work-up. This is based on the observation that 70% to 85% of children <12 years and 10% to 15% of adolescents 12 to 18 years with hypertension have an underlying cause, most commonly renoparenchymal and renovascular disease.3
According to the National Institutes of Health (NIH), “the possibility that some underlying disorder may be the cause of the hypertension should be considered in every child or adolescent” with elevated BP, but the evaluation itself should be individualized.3
The NIH recommends more extensive evaluation for very young children, children with stage 2 hypertension, and children or adolescents who show clinical signs suggesting hypertension-linked systemic conditions. Such evaluation should include a renal ultrasound and laboratory testing (creatinine, urinalysis, and urine culture) to look for structural or functional anomalies.3
What about newly diagnosed adults with suspected secondary causes?
Secondary hypertension reportedly occurs in 5% to 10% of hypertensive patients.4,5 The only prospective study completed in a primary care setting evaluated 1020 patients at a general outpatient clinic in Yokohama, Japan. The investigators reported that 9.1% of the patients had an endocrinologic or renovascular cause contributing to their hypertension.6 The 5 most common causes were primary aldosteronism (6%), Cushing syndrome (1%), preclinical Cushing syndrome (1%), pheochromocytoma (0.6%), and renovascular disease (0.5 %).6
According to the Institute for Clinical Systems Improvement (ICSI), patients at highest risk for secondary hypertension have no family history of hypertension; abrupt onset, symptomatic, or crisis hypertension; stage 2 hypertension; sudden loss of hypertensive control; and drug-resistant hypertension.7
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommends that patients with the following characteristics undergo further directed evaluation for a secondary cause:8
• younger than 30 years with no family history of hypertension
• older than 55 years with new hypertension
• abdominal bruit with diastolic component
• sudden worsening of BP control
• recurrent flash pulmonary edema
• renal failure with abnormal urinary sediment or proteinuria
• acute renal failure after administration of an ACE inhibitor or ARB.
These patients should receive particular scrutiny
Patients with resistant hypertension (BP>140/90 mm Hg despite taking optimal doses of 3 antihypertensive medications, one of which is a diuretic) should receive particular scrutiny for an identifiable secondary cause, according to the ICSI.7
In a retrospective analysis of 141 patients with resistant hypertension referred to a university hypertension center in Chicago in 2005, 5% of patients had an identifiable secondary cause.9 A chart review of 436 patients presenting to a tertiary hypertension clinic in Japan identified 91 with resistant hypertension. A secondary cause was identified in 9.1%.10
Careful history and examination should identify patients suffering from uncontrolled hypertension because of noncompliance, suboptimal antihypertensive regimen, inaccurate BP readings, antagonizing substances, and white coat hypertension.11 The TABLE summarizes common presentations of, and workup for, secondary causes of hypertension.12-14
IT'S RECOMMENDED that all children and adolescents with a new diagnosis of hypertension undergo renal ultrasound and laboratory evaluation for renal pathology (strength of recommendation [SOR]: C, consensus-based guidelines).
Specific diagnostic tests are recommended for newly diagnosed patients who have suspicious clinical findings suggestive of a secondary cause of hypertension based on the initial history (excess daytime sleepiness, palpitations, tremor, sweating); physical examination (abdominal bruit, thyromegaly, malar rash); or laboratory analysis (elevated serum creatinine, low thyroid-stimulating hormone) (SOR: C, consensus-based guidelines).
Patients with undifferentiated resistant hypertension should receive further directed evaluation for secondary causes (SOR: C, consensus-based guidelines).
EVIDENCE SUMMARY
The evidence for selecting which patients should undergo additional testing for potentially correctable secondary causes of hypertension is based on the prevalence of these causes in different age groups, case series of reversal of hypertension with effective treatment of the underlying cause, and clinical suspicion of a secondary cause that may be reversible. We found no prospective cohort studies or randomized trials evaluating diagnostic approaches or outcomes associated with particular selection criteria for conducting additional diagnostic evaluations in search of secondary causes. Therefore, our recommendations are based primarily on expert guidelines, which we summarize here.
When caring for children and adolescents with newly diagnosed hypertension...
Secondary hypertension is more prevalent in younger children and in children and adolescents with stage 2 hypertension (blood pressure [BP] >99th percentile for age and height plus 5 mm Hg).1 Renoparenchymal and renovascular disease account for most cases of secondary hypertension in these children.2
The National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents recommends that all children and adolescents with hypertension have an additional diagnostic work-up. This is based on the observation that 70% to 85% of children <12 years and 10% to 15% of adolescents 12 to 18 years with hypertension have an underlying cause, most commonly renoparenchymal and renovascular disease.3
According to the National Institutes of Health (NIH), “the possibility that some underlying disorder may be the cause of the hypertension should be considered in every child or adolescent” with elevated BP, but the evaluation itself should be individualized.3
The NIH recommends more extensive evaluation for very young children, children with stage 2 hypertension, and children or adolescents who show clinical signs suggesting hypertension-linked systemic conditions. Such evaluation should include a renal ultrasound and laboratory testing (creatinine, urinalysis, and urine culture) to look for structural or functional anomalies.3
What about newly diagnosed adults with suspected secondary causes?
Secondary hypertension reportedly occurs in 5% to 10% of hypertensive patients.4,5 The only prospective study completed in a primary care setting evaluated 1020 patients at a general outpatient clinic in Yokohama, Japan. The investigators reported that 9.1% of the patients had an endocrinologic or renovascular cause contributing to their hypertension.6 The 5 most common causes were primary aldosteronism (6%), Cushing syndrome (1%), preclinical Cushing syndrome (1%), pheochromocytoma (0.6%), and renovascular disease (0.5 %).6
According to the Institute for Clinical Systems Improvement (ICSI), patients at highest risk for secondary hypertension have no family history of hypertension; abrupt onset, symptomatic, or crisis hypertension; stage 2 hypertension; sudden loss of hypertensive control; and drug-resistant hypertension.7
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommends that patients with the following characteristics undergo further directed evaluation for a secondary cause:8
• younger than 30 years with no family history of hypertension
• older than 55 years with new hypertension
• abdominal bruit with diastolic component
• sudden worsening of BP control
• recurrent flash pulmonary edema
• renal failure with abnormal urinary sediment or proteinuria
• acute renal failure after administration of an ACE inhibitor or ARB.
These patients should receive particular scrutiny
Patients with resistant hypertension (BP>140/90 mm Hg despite taking optimal doses of 3 antihypertensive medications, one of which is a diuretic) should receive particular scrutiny for an identifiable secondary cause, according to the ICSI.7
In a retrospective analysis of 141 patients with resistant hypertension referred to a university hypertension center in Chicago in 2005, 5% of patients had an identifiable secondary cause.9 A chart review of 436 patients presenting to a tertiary hypertension clinic in Japan identified 91 with resistant hypertension. A secondary cause was identified in 9.1%.10
Careful history and examination should identify patients suffering from uncontrolled hypertension because of noncompliance, suboptimal antihypertensive regimen, inaccurate BP readings, antagonizing substances, and white coat hypertension.11 The TABLE summarizes common presentations of, and workup for, secondary causes of hypertension.12-14
1. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Rockville, MD: National Heart, Lung, and Blood Institute, US Department of Health and Human Services; May 2005. NIH Publication No. 05-5267.
2. Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874-879.
3. Brady TM, Feld LG. Pediatric approach to hypertension. Semin Nephrol. 2009;29:379-388.
4. Taler SJ. Secondary causes of hypertension. Prim Care Clin Office Pract. 2008;35:489-500.
5. Chiong JR, Aronow WS, Khan IA, et al. Secondary hypertension: current diagnosis and treatment. Int J Cardiol. 2008;124:6-21.
6. Omura M, Saito J, Yamaguchi K, et al. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27:193-202.
7. Luehr D, Woolley T, Burke R, et al. Institute for Clinical Systems Improvement. Hypertension Diagnosis and Treatment. Available at: http://bit.ly/Hypertension1112. Updated November 2012. Accessed January 7, 2010.
8. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206-1252.
9. Garg JP, Elliott WJ, Folker A, et al; Rush University Hypertension Service. Resistant hypertension revisisted: a comparison of two university-based cohorts. Am J Hypertens. 2005;185:619-626.
10. Yakovlevitch M, Black HR. Resistant hypertension in a tertiary care clinic. Arch Intern Med. 1991;151:1786-1792.
11. O’Rorke JE, Richardson WS. What to do when hypertension is difficult to control. BMJ. 2001;322:1229-1232.
12. Rossi GP, Seccia TM, Pessina AC. Clinical use of laboratory tests for the identification of secondary forms of arterial hypertension. Crit Rev Clin Lab Sci. 2007;44:1-85.
13. Riley M, Bluhm B. High blood pressure in children and adolescents. Am Fam Physician. 2012;85:693-700.
14. Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age based approach. Am Fam Physician. 2010;82:1471-1478.
1. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Rockville, MD: National Heart, Lung, and Blood Institute, US Department of Health and Human Services; May 2005. NIH Publication No. 05-5267.
2. Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874-879.
3. Brady TM, Feld LG. Pediatric approach to hypertension. Semin Nephrol. 2009;29:379-388.
4. Taler SJ. Secondary causes of hypertension. Prim Care Clin Office Pract. 2008;35:489-500.
5. Chiong JR, Aronow WS, Khan IA, et al. Secondary hypertension: current diagnosis and treatment. Int J Cardiol. 2008;124:6-21.
6. Omura M, Saito J, Yamaguchi K, et al. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27:193-202.
7. Luehr D, Woolley T, Burke R, et al. Institute for Clinical Systems Improvement. Hypertension Diagnosis and Treatment. Available at: http://bit.ly/Hypertension1112. Updated November 2012. Accessed January 7, 2010.
8. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206-1252.
9. Garg JP, Elliott WJ, Folker A, et al; Rush University Hypertension Service. Resistant hypertension revisisted: a comparison of two university-based cohorts. Am J Hypertens. 2005;185:619-626.
10. Yakovlevitch M, Black HR. Resistant hypertension in a tertiary care clinic. Arch Intern Med. 1991;151:1786-1792.
11. O’Rorke JE, Richardson WS. What to do when hypertension is difficult to control. BMJ. 2001;322:1229-1232.
12. Rossi GP, Seccia TM, Pessina AC. Clinical use of laboratory tests for the identification of secondary forms of arterial hypertension. Crit Rev Clin Lab Sci. 2007;44:1-85.
13. Riley M, Bluhm B. High blood pressure in children and adolescents. Am Fam Physician. 2012;85:693-700.
14. Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age based approach. Am Fam Physician. 2010;82:1471-1478.
Evidence-based answers from the Family Physicians Inquiries Network
Does ultrasound guidance improve outcomes for steroid joint injections?
A Patients yes, at least in the short term. Ultrasound-guided (USG) injections of triamcinolone into the shoulder improve function more than palpation-guided (PG) steroid injections over 6 weeks (strength of recommendation [SOR]: B, 2 small randomized, controlled trials [RCTs]).
USG steroid injections are also less painful than PG injections (SOR: A, multiple RCTs). They reduce pain more than PG injections in arthritic joints (shoulder, elbow, wrist, hand, hip, knee, or ankle) over 2 weeks (SOR: B, lower quality RCTs with some inconsistent results) but possibly not at 6 weeks (SOR: B, multiple RCTs with conflicting results).
EVIDENCE SUMMARY
A prospective RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with shoulder pain unresponsive to nonsteroidal anti-inflammatory drugs (NSAIDs).1 Investigators randomized 60 patients (mean age 52.5 years) to either USG or PG injections of triamcinolone 40 mg given by a rheumatologist. They used a 10-point visual analog scale (VAS) to assess pain and evaluated joint function at 6 weeks using a validated 100-point scale for shoulder function,2 with high scores indicating better function.
The USG group showed greater improvement from baseline in pain (TABLE)1,3-7 and function scores than the PG group (32 vs 12 points; P<.05).1 Investigators didn’t control for a possible placebo effect from ultrasound in this trial (or any trial described here). Another RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with rheumatoid arthritis and at least one month of shoulder pain unresponsive to NSAIDs.3 Investigators randomized 41 rheumatology clinic patients (mean age 52.4 years) to USG or PG injections of 20 mg triamcinolone.
They assessed function at 6 weeks with a validated 70-point shoulder function assessment tool designed for patients with rheumatoid arthritis,8 which evaluates pain with motion, range of motion, and activities of daily living (higher scores indicate better shoulder function), and used a 100-point VAS to assess pain.3 Function scores showed greater improvement from baseline in the USG group than the PG group (15 vs 6 points; P=.012), as did pain scores (TABLE).
Ultrasound injections hurt less than palpation-guided injections
Three RCTs, all using triamcinolone, found that USG joint injections were less painful than PG joint injections (TABLE).4-6 Three of 4 studies found that USG injections also were associated with lower pain scores 2 weeks after injection, as measured with a standardized VAS.1,3-7 A common weakness of the 3 studies demonstrating a difference at 2 weeks was that they compared end scores rather than the magnitude of change from baseline between groups.
Two of 3 RCTs found that USG injections produced a greater reduction in the VAS pain score at 6 weeks, although the negative study was larger than the other 2 combined—184 patients, compared with a total of 285 patients for all 3 studies.1,3,7
Recommendations
The American College of Radiology’s practice guidelines for musculoskeletal ultrasound examination recommend using ultrasound to guide interventional procedures.9 However, no consensus statements comment on the use of ultrasound as opposed to palpation for guiding steroid joint injections.
1. Ucuncu F, Capkin E, Karkucak M, et al. A comparison of the effectiveness of landmark-guided injections and ultrasonography-guided injections for shoulder pain. Clin J Pain. 2009;25:786-789.
2. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160-164.
3. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31:308-314.
4. Sibbitt WL Jr, Kettwich LG, Band PA, et al. Does ultrasound guidance improve the outcomes of arthrocentesis and corticosteroid injection of the knee? Scand J Rheumatol. 2012;41:66-72.
5. Sibbitt W Jr, Band PA, Kettwich LG, et al. A randomized controlled trial evaluating the cost-effectiveness of sonographic guidance for intra-articular injection of the osteoarthritic knee. J Clin Rheumatol. 2011;17:409-415.
6. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36:1892-1902.
7. Cunnington J, Marshall N, Hide G, et al. A randomized, double-blind, controlled study of ultrasound-guided corticosteroid injection into the joints of patients with inflammatory arthritis. Arthritis Rheum. 2010;62;1862-1869.
8. van Den Ende CH, Rozing PM, Dijkmans BA, et al. Assessment of shoulder function in rheumatoid arthritis. J Rheumatol. 1996;23:2043-2048.
9. ACR-AIUM-SPR-SRU practice guideline for the performance of the musculoskeletal ultrasound examination. Updated 2012. American College of Radiology; 2007, updated 2012. Available at: http://amclc.acr.org/LinkClick.aspx?fileticket=z6ih9CEE6_w%3D&tabid=61. Accessed August 3, 2012.
A Patients yes, at least in the short term. Ultrasound-guided (USG) injections of triamcinolone into the shoulder improve function more than palpation-guided (PG) steroid injections over 6 weeks (strength of recommendation [SOR]: B, 2 small randomized, controlled trials [RCTs]).
USG steroid injections are also less painful than PG injections (SOR: A, multiple RCTs). They reduce pain more than PG injections in arthritic joints (shoulder, elbow, wrist, hand, hip, knee, or ankle) over 2 weeks (SOR: B, lower quality RCTs with some inconsistent results) but possibly not at 6 weeks (SOR: B, multiple RCTs with conflicting results).
EVIDENCE SUMMARY
A prospective RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with shoulder pain unresponsive to nonsteroidal anti-inflammatory drugs (NSAIDs).1 Investigators randomized 60 patients (mean age 52.5 years) to either USG or PG injections of triamcinolone 40 mg given by a rheumatologist. They used a 10-point visual analog scale (VAS) to assess pain and evaluated joint function at 6 weeks using a validated 100-point scale for shoulder function,2 with high scores indicating better function.
The USG group showed greater improvement from baseline in pain (TABLE)1,3-7 and function scores than the PG group (32 vs 12 points; P<.05).1 Investigators didn’t control for a possible placebo effect from ultrasound in this trial (or any trial described here). Another RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with rheumatoid arthritis and at least one month of shoulder pain unresponsive to NSAIDs.3 Investigators randomized 41 rheumatology clinic patients (mean age 52.4 years) to USG or PG injections of 20 mg triamcinolone.
They assessed function at 6 weeks with a validated 70-point shoulder function assessment tool designed for patients with rheumatoid arthritis,8 which evaluates pain with motion, range of motion, and activities of daily living (higher scores indicate better shoulder function), and used a 100-point VAS to assess pain.3 Function scores showed greater improvement from baseline in the USG group than the PG group (15 vs 6 points; P=.012), as did pain scores (TABLE).
Ultrasound injections hurt less than palpation-guided injections
Three RCTs, all using triamcinolone, found that USG joint injections were less painful than PG joint injections (TABLE).4-6 Three of 4 studies found that USG injections also were associated with lower pain scores 2 weeks after injection, as measured with a standardized VAS.1,3-7 A common weakness of the 3 studies demonstrating a difference at 2 weeks was that they compared end scores rather than the magnitude of change from baseline between groups.
Two of 3 RCTs found that USG injections produced a greater reduction in the VAS pain score at 6 weeks, although the negative study was larger than the other 2 combined—184 patients, compared with a total of 285 patients for all 3 studies.1,3,7
Recommendations
The American College of Radiology’s practice guidelines for musculoskeletal ultrasound examination recommend using ultrasound to guide interventional procedures.9 However, no consensus statements comment on the use of ultrasound as opposed to palpation for guiding steroid joint injections.
A Patients yes, at least in the short term. Ultrasound-guided (USG) injections of triamcinolone into the shoulder improve function more than palpation-guided (PG) steroid injections over 6 weeks (strength of recommendation [SOR]: B, 2 small randomized, controlled trials [RCTs]).
USG steroid injections are also less painful than PG injections (SOR: A, multiple RCTs). They reduce pain more than PG injections in arthritic joints (shoulder, elbow, wrist, hand, hip, knee, or ankle) over 2 weeks (SOR: B, lower quality RCTs with some inconsistent results) but possibly not at 6 weeks (SOR: B, multiple RCTs with conflicting results).
EVIDENCE SUMMARY
A prospective RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with shoulder pain unresponsive to nonsteroidal anti-inflammatory drugs (NSAIDs).1 Investigators randomized 60 patients (mean age 52.5 years) to either USG or PG injections of triamcinolone 40 mg given by a rheumatologist. They used a 10-point visual analog scale (VAS) to assess pain and evaluated joint function at 6 weeks using a validated 100-point scale for shoulder function,2 with high scores indicating better function.
The USG group showed greater improvement from baseline in pain (TABLE)1,3-7 and function scores than the PG group (32 vs 12 points; P<.05).1 Investigators didn’t control for a possible placebo effect from ultrasound in this trial (or any trial described here). Another RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with rheumatoid arthritis and at least one month of shoulder pain unresponsive to NSAIDs.3 Investigators randomized 41 rheumatology clinic patients (mean age 52.4 years) to USG or PG injections of 20 mg triamcinolone.
They assessed function at 6 weeks with a validated 70-point shoulder function assessment tool designed for patients with rheumatoid arthritis,8 which evaluates pain with motion, range of motion, and activities of daily living (higher scores indicate better shoulder function), and used a 100-point VAS to assess pain.3 Function scores showed greater improvement from baseline in the USG group than the PG group (15 vs 6 points; P=.012), as did pain scores (TABLE).
Ultrasound injections hurt less than palpation-guided injections
Three RCTs, all using triamcinolone, found that USG joint injections were less painful than PG joint injections (TABLE).4-6 Three of 4 studies found that USG injections also were associated with lower pain scores 2 weeks after injection, as measured with a standardized VAS.1,3-7 A common weakness of the 3 studies demonstrating a difference at 2 weeks was that they compared end scores rather than the magnitude of change from baseline between groups.
Two of 3 RCTs found that USG injections produced a greater reduction in the VAS pain score at 6 weeks, although the negative study was larger than the other 2 combined—184 patients, compared with a total of 285 patients for all 3 studies.1,3,7
Recommendations
The American College of Radiology’s practice guidelines for musculoskeletal ultrasound examination recommend using ultrasound to guide interventional procedures.9 However, no consensus statements comment on the use of ultrasound as opposed to palpation for guiding steroid joint injections.
1. Ucuncu F, Capkin E, Karkucak M, et al. A comparison of the effectiveness of landmark-guided injections and ultrasonography-guided injections for shoulder pain. Clin J Pain. 2009;25:786-789.
2. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160-164.
3. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31:308-314.
4. Sibbitt WL Jr, Kettwich LG, Band PA, et al. Does ultrasound guidance improve the outcomes of arthrocentesis and corticosteroid injection of the knee? Scand J Rheumatol. 2012;41:66-72.
5. Sibbitt W Jr, Band PA, Kettwich LG, et al. A randomized controlled trial evaluating the cost-effectiveness of sonographic guidance for intra-articular injection of the osteoarthritic knee. J Clin Rheumatol. 2011;17:409-415.
6. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36:1892-1902.
7. Cunnington J, Marshall N, Hide G, et al. A randomized, double-blind, controlled study of ultrasound-guided corticosteroid injection into the joints of patients with inflammatory arthritis. Arthritis Rheum. 2010;62;1862-1869.
8. van Den Ende CH, Rozing PM, Dijkmans BA, et al. Assessment of shoulder function in rheumatoid arthritis. J Rheumatol. 1996;23:2043-2048.
9. ACR-AIUM-SPR-SRU practice guideline for the performance of the musculoskeletal ultrasound examination. Updated 2012. American College of Radiology; 2007, updated 2012. Available at: http://amclc.acr.org/LinkClick.aspx?fileticket=z6ih9CEE6_w%3D&tabid=61. Accessed August 3, 2012.
1. Ucuncu F, Capkin E, Karkucak M, et al. A comparison of the effectiveness of landmark-guided injections and ultrasonography-guided injections for shoulder pain. Clin J Pain. 2009;25:786-789.
2. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160-164.
3. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31:308-314.
4. Sibbitt WL Jr, Kettwich LG, Band PA, et al. Does ultrasound guidance improve the outcomes of arthrocentesis and corticosteroid injection of the knee? Scand J Rheumatol. 2012;41:66-72.
5. Sibbitt W Jr, Band PA, Kettwich LG, et al. A randomized controlled trial evaluating the cost-effectiveness of sonographic guidance for intra-articular injection of the osteoarthritic knee. J Clin Rheumatol. 2011;17:409-415.
6. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36:1892-1902.
7. Cunnington J, Marshall N, Hide G, et al. A randomized, double-blind, controlled study of ultrasound-guided corticosteroid injection into the joints of patients with inflammatory arthritis. Arthritis Rheum. 2010;62;1862-1869.
8. van Den Ende CH, Rozing PM, Dijkmans BA, et al. Assessment of shoulder function in rheumatoid arthritis. J Rheumatol. 1996;23:2043-2048.
9. ACR-AIUM-SPR-SRU practice guideline for the performance of the musculoskeletal ultrasound examination. Updated 2012. American College of Radiology; 2007, updated 2012. Available at: http://amclc.acr.org/LinkClick.aspx?fileticket=z6ih9CEE6_w%3D&tabid=61. Accessed August 3, 2012.
Evidence-based answers from the Family Physicians Inquiries Network