User login
What is the best age to start vitamin D supplementation to prevent rickets in breastfed newborns?
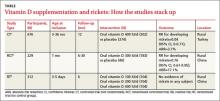
It’s unclear what age is best to start vitamin D supplementation because no comparison studies exist. That said, breastfed infants who take vitamin D beginning at 3 to 5 days of life don’t develop rickets (strength of recommendation [SOR]: B, randomized trial). Starting infants on vitamin D supplementation at one to 36 months of age reduces the risk of rickets (SOR: B, a controlled and a randomized controlled trial).
EVIDENCE SUMMARY
A Cochrane review of interventions for preventing rickets in children born at term found 2 studies, a controlled clinical trial and a cluster-randomized controlled trial, that included 905 breastfed infants.1 In these trials, oral vitamin D (300 or 400 IU per day) starting between one and 36 months of age reduced the risk of rickets when compared with no supplementation (TABLE). The authors concluded that it was reasonable to offer preventive measures (vitamin D or calcium) to all children 2 years or younger.
400 IU of vitamin D daily increases blood levels the most
A study done in north and south China investigated vitamin D dose by randomizing 312 infants to receive supplements of 100, 200, or 400 IU daily.2 Although no infant developed rickets, a dose of 400 IU per day achieved higher serum levels of 25-hydroxy vitamin D (25[OH]-D) than the lower doses.
In the northern location, doses of 100, 200, or 400 IU daily increased 25(OH)-D levels from 5 ng/mL at birth to an average of 12, 15, and 25 ng/mL, respectively, at 6 months. In the southern location, 25(OH)-D levels increased from 14 ng/mL at birth to 20, 22, and 25 ng/mL at 6 months for the 100, 200, and 400 IU per day doses, respectively.
Recommendations
The guidelines from the American Academy of Pediatrics (AAP) on preventing rickets and vitamin D deficiency in infants, children, and adolescents recommends that exclusively breastfed neonates receive 400 IU of vitamin D daily, starting in the first days of life—a revision of the previous recommendation of 200 IU daily beginning at 2 months. The AAP doesn’t recommend giving vitamin D supplements to formula-fed infants because formula contains 400 IU/L of vitamin D; infants who don’t drink at least 1 liter of formula per day should receive supplementation. The AAP advocates a serum 25(OH)-D level of at least 20 ng/mL.3
The Pediatric Endocrine Society (PES) defines vitamin D deficiency as a serum 25(OH)-D level below 15 ng/mL and recommends mends maintaining the level above 20 ng/mL to prevent rickets. The PES also recommends that breastfed infants receive 400 IU of vitamin D daily starting at birth.4
1. Lerch C, Meissner T. Interventions for the prevention of nutritional rickets in term born children. Cochrane Database Syst Rev. 2007;(4):CD006164.
2. Specker BL, Ho ML, Oestreich A, et al. Prospective study of vitamin D supplementation and rickets in China. J Pediatr. 1992;120:733-739.
3. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152.
4. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
It’s unclear what age is best to start vitamin D supplementation because no comparison studies exist. That said, breastfed infants who take vitamin D beginning at 3 to 5 days of life don’t develop rickets (strength of recommendation [SOR]: B, randomized trial). Starting infants on vitamin D supplementation at one to 36 months of age reduces the risk of rickets (SOR: B, a controlled and a randomized controlled trial).
EVIDENCE SUMMARY
A Cochrane review of interventions for preventing rickets in children born at term found 2 studies, a controlled clinical trial and a cluster-randomized controlled trial, that included 905 breastfed infants.1 In these trials, oral vitamin D (300 or 400 IU per day) starting between one and 36 months of age reduced the risk of rickets when compared with no supplementation (TABLE). The authors concluded that it was reasonable to offer preventive measures (vitamin D or calcium) to all children 2 years or younger.
400 IU of vitamin D daily increases blood levels the most
A study done in north and south China investigated vitamin D dose by randomizing 312 infants to receive supplements of 100, 200, or 400 IU daily.2 Although no infant developed rickets, a dose of 400 IU per day achieved higher serum levels of 25-hydroxy vitamin D (25[OH]-D) than the lower doses.
In the northern location, doses of 100, 200, or 400 IU daily increased 25(OH)-D levels from 5 ng/mL at birth to an average of 12, 15, and 25 ng/mL, respectively, at 6 months. In the southern location, 25(OH)-D levels increased from 14 ng/mL at birth to 20, 22, and 25 ng/mL at 6 months for the 100, 200, and 400 IU per day doses, respectively.
Recommendations
The guidelines from the American Academy of Pediatrics (AAP) on preventing rickets and vitamin D deficiency in infants, children, and adolescents recommends that exclusively breastfed neonates receive 400 IU of vitamin D daily, starting in the first days of life—a revision of the previous recommendation of 200 IU daily beginning at 2 months. The AAP doesn’t recommend giving vitamin D supplements to formula-fed infants because formula contains 400 IU/L of vitamin D; infants who don’t drink at least 1 liter of formula per day should receive supplementation. The AAP advocates a serum 25(OH)-D level of at least 20 ng/mL.3
The Pediatric Endocrine Society (PES) defines vitamin D deficiency as a serum 25(OH)-D level below 15 ng/mL and recommends mends maintaining the level above 20 ng/mL to prevent rickets. The PES also recommends that breastfed infants receive 400 IU of vitamin D daily starting at birth.4
It’s unclear what age is best to start vitamin D supplementation because no comparison studies exist. That said, breastfed infants who take vitamin D beginning at 3 to 5 days of life don’t develop rickets (strength of recommendation [SOR]: B, randomized trial). Starting infants on vitamin D supplementation at one to 36 months of age reduces the risk of rickets (SOR: B, a controlled and a randomized controlled trial).
EVIDENCE SUMMARY
A Cochrane review of interventions for preventing rickets in children born at term found 2 studies, a controlled clinical trial and a cluster-randomized controlled trial, that included 905 breastfed infants.1 In these trials, oral vitamin D (300 or 400 IU per day) starting between one and 36 months of age reduced the risk of rickets when compared with no supplementation (TABLE). The authors concluded that it was reasonable to offer preventive measures (vitamin D or calcium) to all children 2 years or younger.
400 IU of vitamin D daily increases blood levels the most
A study done in north and south China investigated vitamin D dose by randomizing 312 infants to receive supplements of 100, 200, or 400 IU daily.2 Although no infant developed rickets, a dose of 400 IU per day achieved higher serum levels of 25-hydroxy vitamin D (25[OH]-D) than the lower doses.
In the northern location, doses of 100, 200, or 400 IU daily increased 25(OH)-D levels from 5 ng/mL at birth to an average of 12, 15, and 25 ng/mL, respectively, at 6 months. In the southern location, 25(OH)-D levels increased from 14 ng/mL at birth to 20, 22, and 25 ng/mL at 6 months for the 100, 200, and 400 IU per day doses, respectively.
Recommendations
The guidelines from the American Academy of Pediatrics (AAP) on preventing rickets and vitamin D deficiency in infants, children, and adolescents recommends that exclusively breastfed neonates receive 400 IU of vitamin D daily, starting in the first days of life—a revision of the previous recommendation of 200 IU daily beginning at 2 months. The AAP doesn’t recommend giving vitamin D supplements to formula-fed infants because formula contains 400 IU/L of vitamin D; infants who don’t drink at least 1 liter of formula per day should receive supplementation. The AAP advocates a serum 25(OH)-D level of at least 20 ng/mL.3
The Pediatric Endocrine Society (PES) defines vitamin D deficiency as a serum 25(OH)-D level below 15 ng/mL and recommends mends maintaining the level above 20 ng/mL to prevent rickets. The PES also recommends that breastfed infants receive 400 IU of vitamin D daily starting at birth.4
1. Lerch C, Meissner T. Interventions for the prevention of nutritional rickets in term born children. Cochrane Database Syst Rev. 2007;(4):CD006164.
2. Specker BL, Ho ML, Oestreich A, et al. Prospective study of vitamin D supplementation and rickets in China. J Pediatr. 1992;120:733-739.
3. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152.
4. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
1. Lerch C, Meissner T. Interventions for the prevention of nutritional rickets in term born children. Cochrane Database Syst Rev. 2007;(4):CD006164.
2. Specker BL, Ho ML, Oestreich A, et al. Prospective study of vitamin D supplementation and rickets in China. J Pediatr. 1992;120:733-739.
3. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152.
4. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
Evidence-based answers from the Family Physicians Inquiries Network
What clinical clues differentiate migraine from sinus headaches?
Patients with sinus headaches have thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath (SOR: B, cross-sectional study).
The 5 symptoms that are most predictive of migraine are: pulsatile quality, duration of 4 to 72 hours, unilateral location, nausea or vomiting, and disabling intensity (SOR: B, retrospective cohort). As the number of these symptoms increases, so too, does the likelihood that the patient has a migraine (SOR: B, systematic review of retrospective cohort studies).
Most patients diagnosed with sinus headache actually have a migraine headache (SOR: B, 2 cross-sectional studies).
EVIDENCE SUMMARY
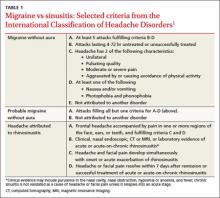
Clinical signs and symptoms define headache types. The International Headache Society (IHS)’s definition of migraine (which is considered the gold standard) includes many of the same symptoms associated with headaches attributed to rhinosinusitis (TABLE 1).1 In order for a headache to be attributed to rhinosinusitis, the patient must meet the definition of acute rhinosinusitis as defined by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS). The definition includes purulent discharge, nasal obstruction, and facial pain-pressure-fullness.
Migraine headache symptoms include nasal discharge but not purulent discharge or nasal obstruction. The IHS doesn’t accept chronic sinusitis as a cause of headaches unless the patient meets the criteria for acute rhinosinusitis.1,2
When a “sinus” headache isn’t
A cross-sectional study enrolled 100 patients (78 female, 22 male) 18 to 81 years of age who responded to an advertisement seeking people with self-diagnosed “sinus” headaches.3 A neurologist used the 2004 IHS criteria to classify the correct headache type. In 86% of patients, the investigators reclassified the patients with a migraine or probable migraine. Only 3 patients retained the sinus headache diagnosis. All 3 had at least one of the following: thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath. The remaining 11 patients suffered from other headache subtypes.
The big 5 migraine symptoms
A prospective cohort study of 166 French railway employees evaluated the sensitivity and specificity of individual components of the IHS criteria. Patients enrolled had an average age of 39 years and a female-to-male ratio of 1:2. A neurologist diagnosed the headache type and placed patients into either a migraine or nonmigraine cohort. Researchers asked participants about IHS defined migraine symptoms and then compared the frequency of positive responses from migraineurs vs nonmigraineurs.
Five specific IHS criteria were found to be useful in identifying patients with migraine: duration between 4 and 72 hours (odds ratio [OR]=2.5; P=.02), unilateral location (OR=2.3; P=.03), pulsating quality (OR=2.4; P=.02), disturbance of daily activity (OR=2.5; P=.02), and nausea or vomiting (OR=2.8; P=.009). Any 4 of these features indicated a probability of migraine headache of ≥70%. The presence of photophobia and phonophobia (OR=0.5; P=.11) and aggravation by physical activity (OR=1.7; P=.14) didn’t improve diagnostic accuracy.4
A systematic review of retrospective cohort studies later analyzed the data.5 The authors derived the POUND mnemonic (TABLE 2)5 to aid clinicians in using the 5 clinical features to determine the likelihood of migraine headache. When any 4 of the 5 screening questions are positively, the likelihood ratio (LR) for migraine is 24 (95% confidence interval [CI], 1.5-388). Conversely, when 2 or fewer screening questions elicit positive responses, migraine is less likely (LR=0.41; 95% CI, 0.32-0.52).5
Most “sinus” headaches found to be migraine
A multicenter cross-sectional study evaluated 2991 male and female primary care patients, 18 to 65 years of age, who reported at least 6 self-described or physician-diagnosed sinus headaches within the preceding 6 months.6 At baseline, patients reported the following symptoms: pulsing or throbbing pain (89%), pain that worsened with physical activity (85%), sinus pressure (84%), sinus pain (82%), nasal congestion (63%), photophobia (79%), nausea (73%), and phonophobia (67%). Patients were excluded if they had a previous diagnosis of migraine, used a triptan, had a radiologic diagnosis of sinusitis, or had purulent drainage or fever.
Using the patients’ headache histories, reported symptoms, and the IHS criteria, researchers reclassified 88% of these “sinus headache” patients as having migraine type headaches.
Recommendations
IHS recommends using strict criteria for diagnosing migraines and headaches attributed to rhinosinusitis.1
1. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd ed. Cephalalgia. 2004;24(1 suppl):S8-S160.
2. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–S31.
3. Eross E, Dodick D, Eross M. The sinus, allergy and migraine study (SAMS). Headache. 2007;47:213-224.
4. Michel P, Henry P, Letenneur L, et al. Diagnostic screen for assessment of the IHS criteria for migraine by general practitioners. Cephalagia.1993;13(12suppl):S54-S59.
5. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
6. Schreiber CP, Hutchinson S, Webster CJ, et al. Prevalence of migraine in patients with a history of self-reported or physician-diagnosed “sinus” headache. Arch Intern Med. 2004;164:1769-1772.
Patients with sinus headaches have thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath (SOR: B, cross-sectional study).
The 5 symptoms that are most predictive of migraine are: pulsatile quality, duration of 4 to 72 hours, unilateral location, nausea or vomiting, and disabling intensity (SOR: B, retrospective cohort). As the number of these symptoms increases, so too, does the likelihood that the patient has a migraine (SOR: B, systematic review of retrospective cohort studies).
Most patients diagnosed with sinus headache actually have a migraine headache (SOR: B, 2 cross-sectional studies).
EVIDENCE SUMMARY
Clinical signs and symptoms define headache types. The International Headache Society (IHS)’s definition of migraine (which is considered the gold standard) includes many of the same symptoms associated with headaches attributed to rhinosinusitis (TABLE 1).1 In order for a headache to be attributed to rhinosinusitis, the patient must meet the definition of acute rhinosinusitis as defined by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS). The definition includes purulent discharge, nasal obstruction, and facial pain-pressure-fullness.
Migraine headache symptoms include nasal discharge but not purulent discharge or nasal obstruction. The IHS doesn’t accept chronic sinusitis as a cause of headaches unless the patient meets the criteria for acute rhinosinusitis.1,2
When a “sinus” headache isn’t
A cross-sectional study enrolled 100 patients (78 female, 22 male) 18 to 81 years of age who responded to an advertisement seeking people with self-diagnosed “sinus” headaches.3 A neurologist used the 2004 IHS criteria to classify the correct headache type. In 86% of patients, the investigators reclassified the patients with a migraine or probable migraine. Only 3 patients retained the sinus headache diagnosis. All 3 had at least one of the following: thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath. The remaining 11 patients suffered from other headache subtypes.
The big 5 migraine symptoms
A prospective cohort study of 166 French railway employees evaluated the sensitivity and specificity of individual components of the IHS criteria. Patients enrolled had an average age of 39 years and a female-to-male ratio of 1:2. A neurologist diagnosed the headache type and placed patients into either a migraine or nonmigraine cohort. Researchers asked participants about IHS defined migraine symptoms and then compared the frequency of positive responses from migraineurs vs nonmigraineurs.
Five specific IHS criteria were found to be useful in identifying patients with migraine: duration between 4 and 72 hours (odds ratio [OR]=2.5; P=.02), unilateral location (OR=2.3; P=.03), pulsating quality (OR=2.4; P=.02), disturbance of daily activity (OR=2.5; P=.02), and nausea or vomiting (OR=2.8; P=.009). Any 4 of these features indicated a probability of migraine headache of ≥70%. The presence of photophobia and phonophobia (OR=0.5; P=.11) and aggravation by physical activity (OR=1.7; P=.14) didn’t improve diagnostic accuracy.4
A systematic review of retrospective cohort studies later analyzed the data.5 The authors derived the POUND mnemonic (TABLE 2)5 to aid clinicians in using the 5 clinical features to determine the likelihood of migraine headache. When any 4 of the 5 screening questions are positively, the likelihood ratio (LR) for migraine is 24 (95% confidence interval [CI], 1.5-388). Conversely, when 2 or fewer screening questions elicit positive responses, migraine is less likely (LR=0.41; 95% CI, 0.32-0.52).5
Most “sinus” headaches found to be migraine
A multicenter cross-sectional study evaluated 2991 male and female primary care patients, 18 to 65 years of age, who reported at least 6 self-described or physician-diagnosed sinus headaches within the preceding 6 months.6 At baseline, patients reported the following symptoms: pulsing or throbbing pain (89%), pain that worsened with physical activity (85%), sinus pressure (84%), sinus pain (82%), nasal congestion (63%), photophobia (79%), nausea (73%), and phonophobia (67%). Patients were excluded if they had a previous diagnosis of migraine, used a triptan, had a radiologic diagnosis of sinusitis, or had purulent drainage or fever.
Using the patients’ headache histories, reported symptoms, and the IHS criteria, researchers reclassified 88% of these “sinus headache” patients as having migraine type headaches.
Recommendations
IHS recommends using strict criteria for diagnosing migraines and headaches attributed to rhinosinusitis.1
Patients with sinus headaches have thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath (SOR: B, cross-sectional study).
The 5 symptoms that are most predictive of migraine are: pulsatile quality, duration of 4 to 72 hours, unilateral location, nausea or vomiting, and disabling intensity (SOR: B, retrospective cohort). As the number of these symptoms increases, so too, does the likelihood that the patient has a migraine (SOR: B, systematic review of retrospective cohort studies).
Most patients diagnosed with sinus headache actually have a migraine headache (SOR: B, 2 cross-sectional studies).
EVIDENCE SUMMARY
Clinical signs and symptoms define headache types. The International Headache Society (IHS)’s definition of migraine (which is considered the gold standard) includes many of the same symptoms associated with headaches attributed to rhinosinusitis (TABLE 1).1 In order for a headache to be attributed to rhinosinusitis, the patient must meet the definition of acute rhinosinusitis as defined by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS). The definition includes purulent discharge, nasal obstruction, and facial pain-pressure-fullness.
Migraine headache symptoms include nasal discharge but not purulent discharge or nasal obstruction. The IHS doesn’t accept chronic sinusitis as a cause of headaches unless the patient meets the criteria for acute rhinosinusitis.1,2
When a “sinus” headache isn’t
A cross-sectional study enrolled 100 patients (78 female, 22 male) 18 to 81 years of age who responded to an advertisement seeking people with self-diagnosed “sinus” headaches.3 A neurologist used the 2004 IHS criteria to classify the correct headache type. In 86% of patients, the investigators reclassified the patients with a migraine or probable migraine. Only 3 patients retained the sinus headache diagnosis. All 3 had at least one of the following: thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath. The remaining 11 patients suffered from other headache subtypes.
The big 5 migraine symptoms
A prospective cohort study of 166 French railway employees evaluated the sensitivity and specificity of individual components of the IHS criteria. Patients enrolled had an average age of 39 years and a female-to-male ratio of 1:2. A neurologist diagnosed the headache type and placed patients into either a migraine or nonmigraine cohort. Researchers asked participants about IHS defined migraine symptoms and then compared the frequency of positive responses from migraineurs vs nonmigraineurs.
Five specific IHS criteria were found to be useful in identifying patients with migraine: duration between 4 and 72 hours (odds ratio [OR]=2.5; P=.02), unilateral location (OR=2.3; P=.03), pulsating quality (OR=2.4; P=.02), disturbance of daily activity (OR=2.5; P=.02), and nausea or vomiting (OR=2.8; P=.009). Any 4 of these features indicated a probability of migraine headache of ≥70%. The presence of photophobia and phonophobia (OR=0.5; P=.11) and aggravation by physical activity (OR=1.7; P=.14) didn’t improve diagnostic accuracy.4
A systematic review of retrospective cohort studies later analyzed the data.5 The authors derived the POUND mnemonic (TABLE 2)5 to aid clinicians in using the 5 clinical features to determine the likelihood of migraine headache. When any 4 of the 5 screening questions are positively, the likelihood ratio (LR) for migraine is 24 (95% confidence interval [CI], 1.5-388). Conversely, when 2 or fewer screening questions elicit positive responses, migraine is less likely (LR=0.41; 95% CI, 0.32-0.52).5
Most “sinus” headaches found to be migraine
A multicenter cross-sectional study evaluated 2991 male and female primary care patients, 18 to 65 years of age, who reported at least 6 self-described or physician-diagnosed sinus headaches within the preceding 6 months.6 At baseline, patients reported the following symptoms: pulsing or throbbing pain (89%), pain that worsened with physical activity (85%), sinus pressure (84%), sinus pain (82%), nasal congestion (63%), photophobia (79%), nausea (73%), and phonophobia (67%). Patients were excluded if they had a previous diagnosis of migraine, used a triptan, had a radiologic diagnosis of sinusitis, or had purulent drainage or fever.
Using the patients’ headache histories, reported symptoms, and the IHS criteria, researchers reclassified 88% of these “sinus headache” patients as having migraine type headaches.
Recommendations
IHS recommends using strict criteria for diagnosing migraines and headaches attributed to rhinosinusitis.1
1. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd ed. Cephalalgia. 2004;24(1 suppl):S8-S160.
2. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–S31.
3. Eross E, Dodick D, Eross M. The sinus, allergy and migraine study (SAMS). Headache. 2007;47:213-224.
4. Michel P, Henry P, Letenneur L, et al. Diagnostic screen for assessment of the IHS criteria for migraine by general practitioners. Cephalagia.1993;13(12suppl):S54-S59.
5. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
6. Schreiber CP, Hutchinson S, Webster CJ, et al. Prevalence of migraine in patients with a history of self-reported or physician-diagnosed “sinus” headache. Arch Intern Med. 2004;164:1769-1772.
1. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd ed. Cephalalgia. 2004;24(1 suppl):S8-S160.
2. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–S31.
3. Eross E, Dodick D, Eross M. The sinus, allergy and migraine study (SAMS). Headache. 2007;47:213-224.
4. Michel P, Henry P, Letenneur L, et al. Diagnostic screen for assessment of the IHS criteria for migraine by general practitioners. Cephalagia.1993;13(12suppl):S54-S59.
5. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
6. Schreiber CP, Hutchinson S, Webster CJ, et al. Prevalence of migraine in patients with a history of self-reported or physician-diagnosed “sinus” headache. Arch Intern Med. 2004;164:1769-1772.
Evidence-based answers from the Family Physicians Inquiries Network
Do oral contraceptives carry a significant risk of stroke for women with migraines?
Perhaps. Estrogen-containing oral contraceptives may raise the risk of ischemic stroke in women with migraine, particularly migraine with aura (strength of recommendation [SOR]: C, small case-control studies with methodological flaws and conflicting results).
EVIDENCE SUMMARY
Women with probable migraine with visual aura (PMVA) have an increased risk for ischemic stroke (odds ratio [OR]=2.1) but not hemorrhagic stroke.1 Women with >12 PMVA episodes per year are at greatest risk (OR=2.2, compared with <12 PMVA episodes per year, OR=1.1).2 Women taking oral contraceptive pills (OCPs) also have an increased risk for stroke, depending on the estrogen dose (OR=4.8 for 50 mcg; OR=2.7 for 30-40 mcg; OR=1.7 for 20 mcg; and OR=1.0 for progestin-only pills).3
Women with migraines who smoke and take OCPs have the highest risk
Four case-control studies evaluated the risk of ischemic stroke in women with migraines who take OCPs. The first study compared the OR among 135 women 15 to 49 years of age with PMVA and a first ischemic stroke with 614 controls (no history of stroke, matched for age and ethnicity).2 Although women with PMVA overall had an increased risk of ischemic stroke (OR=1.5; 95% confidence interval [CI], 1.1-2.0), a subgroup of women with PMVA who also were taking OCPs didn’t have a significantly greater stroke risk than women with PMVA who were not taking OCPs (OR=1.6; 95% CI, not given but reported as not significant; P=.87). Investigators didn’t specify the type of OCPs.
Women with PMVA who smoked had a greater risk of ischemic stroke (OR=1.5; 95% CI, 1.1-2.3), and women with PMVA who both smoked and took OCPs had the highest risk of ischemic stroke (OR =7.0; 95% CI, 1.4-22.8).
In women younger than 45 years, OCPs are associated with higher stroke risk
The second study compared the odds ratio for ischemic stroke among 47 women younger than 45 years with PMVA who were taking combined OCPs with 63 controls.3 Most OCPs contained 30 to 40 mcg estrogen. Women with PMVA taking a combined OCP had a higher risk of ischemic stroke (OR=13.9; 95% CI, 5.5-35.1) than women with PMVA who didn’t take OCPs (OR=3.7; 95% CI, 1.5-9.1). Investigators didn’t report the number of PMVA episodes per year among the women.
The third study compared the odds ratio for ischemic stroke among 10 women 20 to 44 years of age with migraines who were taking combined OCPs with 23 controls.4 Investigators didn’t specify the type of migraine, although classic migraine was approximately twice as common as simple migraine among the women in the larger study population. Women with migraine taking OCPs were more likely to have an ischemic stroke overall (OR=16.9; 95% CI, 2.7-106), with the exception of those taking OCPs with <50 mcg estrogen (4 patients) (OR=0.59; 95 % CI, 0.79-54.8).
The fourth study compared the OR for ischemic stroke among 4 women 18 to 44 years old who had a history of migraine (type not specified) and used low-dose OCPs with 14 controls. Women with migraines taking OCPs had a higher risk of ischemic stroke (OR=2.08; 95% CI, 1.19-3.65).5
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists says that combined OCPs are contraindicated for women with migraine with focal neurologic symptoms such as aura.6 Although strokes are rare in women with migraine taking OCPs, the impact of a stroke is so devastating that clinicians should consider progestin-only, intrauterine, or barrier contraceptives for these women. However, physicians may consider combined OCPs for women younger than 35 years with migraine if they don’t have focal neurologic signs, don’t smoke, and are otherwise healthy.
The World Health Organization and Centers for Disease Control and Prevention state that women with a history of migraine who use combined OCPs are 2 to 4 times more likely to have an ischemic stroke than nonusers and conclude that combined OCP use in women older than 35 years with migraine, or migraine with aura at any age, represents an unacceptable health risk. However, the advantages of using combined OCPs in women younger than 35 years with migraine generally outweigh the theoretical or proven risks.7,8
1. Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systemic review and meta-analysis. BMJ. 2009;339:b3914.
2. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2445.
3. Tzourio C, Tehindrazanarivelo A, Iglésias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830-833.
4. Chang C, Donaghy M, Poulter N, et al. Migraine and stroke in young women: case-control study. The World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
5. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29:2277-2284.
6. ACOG Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 73. Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
7. US Medical Eligibility Criteria for Contraceptive Use, 2010. Adapted from the World Health Organization Medical Eligibility Criteria for Contraceptive Use, 4th edition. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a1.htm. Accessed July 15, 2012.
8. Appendix B: Classifications for combined hormonal contraceptives. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a3.htm. Accessed July 15, 2012.
Perhaps. Estrogen-containing oral contraceptives may raise the risk of ischemic stroke in women with migraine, particularly migraine with aura (strength of recommendation [SOR]: C, small case-control studies with methodological flaws and conflicting results).
EVIDENCE SUMMARY
Women with probable migraine with visual aura (PMVA) have an increased risk for ischemic stroke (odds ratio [OR]=2.1) but not hemorrhagic stroke.1 Women with >12 PMVA episodes per year are at greatest risk (OR=2.2, compared with <12 PMVA episodes per year, OR=1.1).2 Women taking oral contraceptive pills (OCPs) also have an increased risk for stroke, depending on the estrogen dose (OR=4.8 for 50 mcg; OR=2.7 for 30-40 mcg; OR=1.7 for 20 mcg; and OR=1.0 for progestin-only pills).3
Women with migraines who smoke and take OCPs have the highest risk
Four case-control studies evaluated the risk of ischemic stroke in women with migraines who take OCPs. The first study compared the OR among 135 women 15 to 49 years of age with PMVA and a first ischemic stroke with 614 controls (no history of stroke, matched for age and ethnicity).2 Although women with PMVA overall had an increased risk of ischemic stroke (OR=1.5; 95% confidence interval [CI], 1.1-2.0), a subgroup of women with PMVA who also were taking OCPs didn’t have a significantly greater stroke risk than women with PMVA who were not taking OCPs (OR=1.6; 95% CI, not given but reported as not significant; P=.87). Investigators didn’t specify the type of OCPs.
Women with PMVA who smoked had a greater risk of ischemic stroke (OR=1.5; 95% CI, 1.1-2.3), and women with PMVA who both smoked and took OCPs had the highest risk of ischemic stroke (OR =7.0; 95% CI, 1.4-22.8).
In women younger than 45 years, OCPs are associated with higher stroke risk
The second study compared the odds ratio for ischemic stroke among 47 women younger than 45 years with PMVA who were taking combined OCPs with 63 controls.3 Most OCPs contained 30 to 40 mcg estrogen. Women with PMVA taking a combined OCP had a higher risk of ischemic stroke (OR=13.9; 95% CI, 5.5-35.1) than women with PMVA who didn’t take OCPs (OR=3.7; 95% CI, 1.5-9.1). Investigators didn’t report the number of PMVA episodes per year among the women.
The third study compared the odds ratio for ischemic stroke among 10 women 20 to 44 years of age with migraines who were taking combined OCPs with 23 controls.4 Investigators didn’t specify the type of migraine, although classic migraine was approximately twice as common as simple migraine among the women in the larger study population. Women with migraine taking OCPs were more likely to have an ischemic stroke overall (OR=16.9; 95% CI, 2.7-106), with the exception of those taking OCPs with <50 mcg estrogen (4 patients) (OR=0.59; 95 % CI, 0.79-54.8).
The fourth study compared the OR for ischemic stroke among 4 women 18 to 44 years old who had a history of migraine (type not specified) and used low-dose OCPs with 14 controls. Women with migraines taking OCPs had a higher risk of ischemic stroke (OR=2.08; 95% CI, 1.19-3.65).5
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists says that combined OCPs are contraindicated for women with migraine with focal neurologic symptoms such as aura.6 Although strokes are rare in women with migraine taking OCPs, the impact of a stroke is so devastating that clinicians should consider progestin-only, intrauterine, or barrier contraceptives for these women. However, physicians may consider combined OCPs for women younger than 35 years with migraine if they don’t have focal neurologic signs, don’t smoke, and are otherwise healthy.
The World Health Organization and Centers for Disease Control and Prevention state that women with a history of migraine who use combined OCPs are 2 to 4 times more likely to have an ischemic stroke than nonusers and conclude that combined OCP use in women older than 35 years with migraine, or migraine with aura at any age, represents an unacceptable health risk. However, the advantages of using combined OCPs in women younger than 35 years with migraine generally outweigh the theoretical or proven risks.7,8
Perhaps. Estrogen-containing oral contraceptives may raise the risk of ischemic stroke in women with migraine, particularly migraine with aura (strength of recommendation [SOR]: C, small case-control studies with methodological flaws and conflicting results).
EVIDENCE SUMMARY
Women with probable migraine with visual aura (PMVA) have an increased risk for ischemic stroke (odds ratio [OR]=2.1) but not hemorrhagic stroke.1 Women with >12 PMVA episodes per year are at greatest risk (OR=2.2, compared with <12 PMVA episodes per year, OR=1.1).2 Women taking oral contraceptive pills (OCPs) also have an increased risk for stroke, depending on the estrogen dose (OR=4.8 for 50 mcg; OR=2.7 for 30-40 mcg; OR=1.7 for 20 mcg; and OR=1.0 for progestin-only pills).3
Women with migraines who smoke and take OCPs have the highest risk
Four case-control studies evaluated the risk of ischemic stroke in women with migraines who take OCPs. The first study compared the OR among 135 women 15 to 49 years of age with PMVA and a first ischemic stroke with 614 controls (no history of stroke, matched for age and ethnicity).2 Although women with PMVA overall had an increased risk of ischemic stroke (OR=1.5; 95% confidence interval [CI], 1.1-2.0), a subgroup of women with PMVA who also were taking OCPs didn’t have a significantly greater stroke risk than women with PMVA who were not taking OCPs (OR=1.6; 95% CI, not given but reported as not significant; P=.87). Investigators didn’t specify the type of OCPs.
Women with PMVA who smoked had a greater risk of ischemic stroke (OR=1.5; 95% CI, 1.1-2.3), and women with PMVA who both smoked and took OCPs had the highest risk of ischemic stroke (OR =7.0; 95% CI, 1.4-22.8).
In women younger than 45 years, OCPs are associated with higher stroke risk
The second study compared the odds ratio for ischemic stroke among 47 women younger than 45 years with PMVA who were taking combined OCPs with 63 controls.3 Most OCPs contained 30 to 40 mcg estrogen. Women with PMVA taking a combined OCP had a higher risk of ischemic stroke (OR=13.9; 95% CI, 5.5-35.1) than women with PMVA who didn’t take OCPs (OR=3.7; 95% CI, 1.5-9.1). Investigators didn’t report the number of PMVA episodes per year among the women.
The third study compared the odds ratio for ischemic stroke among 10 women 20 to 44 years of age with migraines who were taking combined OCPs with 23 controls.4 Investigators didn’t specify the type of migraine, although classic migraine was approximately twice as common as simple migraine among the women in the larger study population. Women with migraine taking OCPs were more likely to have an ischemic stroke overall (OR=16.9; 95% CI, 2.7-106), with the exception of those taking OCPs with <50 mcg estrogen (4 patients) (OR=0.59; 95 % CI, 0.79-54.8).
The fourth study compared the OR for ischemic stroke among 4 women 18 to 44 years old who had a history of migraine (type not specified) and used low-dose OCPs with 14 controls. Women with migraines taking OCPs had a higher risk of ischemic stroke (OR=2.08; 95% CI, 1.19-3.65).5
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists says that combined OCPs are contraindicated for women with migraine with focal neurologic symptoms such as aura.6 Although strokes are rare in women with migraine taking OCPs, the impact of a stroke is so devastating that clinicians should consider progestin-only, intrauterine, or barrier contraceptives for these women. However, physicians may consider combined OCPs for women younger than 35 years with migraine if they don’t have focal neurologic signs, don’t smoke, and are otherwise healthy.
The World Health Organization and Centers for Disease Control and Prevention state that women with a history of migraine who use combined OCPs are 2 to 4 times more likely to have an ischemic stroke than nonusers and conclude that combined OCP use in women older than 35 years with migraine, or migraine with aura at any age, represents an unacceptable health risk. However, the advantages of using combined OCPs in women younger than 35 years with migraine generally outweigh the theoretical or proven risks.7,8
1. Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systemic review and meta-analysis. BMJ. 2009;339:b3914.
2. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2445.
3. Tzourio C, Tehindrazanarivelo A, Iglésias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830-833.
4. Chang C, Donaghy M, Poulter N, et al. Migraine and stroke in young women: case-control study. The World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
5. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29:2277-2284.
6. ACOG Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 73. Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
7. US Medical Eligibility Criteria for Contraceptive Use, 2010. Adapted from the World Health Organization Medical Eligibility Criteria for Contraceptive Use, 4th edition. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a1.htm. Accessed July 15, 2012.
8. Appendix B: Classifications for combined hormonal contraceptives. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a3.htm. Accessed July 15, 2012.
1. Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systemic review and meta-analysis. BMJ. 2009;339:b3914.
2. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2445.
3. Tzourio C, Tehindrazanarivelo A, Iglésias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830-833.
4. Chang C, Donaghy M, Poulter N, et al. Migraine and stroke in young women: case-control study. The World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
5. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29:2277-2284.
6. ACOG Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 73. Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
7. US Medical Eligibility Criteria for Contraceptive Use, 2010. Adapted from the World Health Organization Medical Eligibility Criteria for Contraceptive Use, 4th edition. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a1.htm. Accessed July 15, 2012.
8. Appendix B: Classifications for combined hormonal contraceptives. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a3.htm. Accessed July 15, 2012.
Evidence-based answers from the Family Physicians Inquiries Network
How do antidepressants affect sexual function?
Patients treated with selective serotonin reuptake inhibitors (SSRIs) and the serotonin/norepinephrine reuptake inhibitor (SNRI) venlafaxine have significantly higher rates of overall sexual dysfunction—including desire, arousal, and orgasm—than patients treated with placebo (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with heterogeneous results). Patients treated with bupropion, a norepinephrine-dopamine reuptake inhibitor (NDRI), have rates of overall sexual dysfunction comparable to placebo (SOR: B, RCTs with heterogeneous results).
EVIDENCE SUMMARY
In a meta-analysis of 31 studies with 10,130 patients, researchers reported that the total rate of sexual dysfunction (SD) associated with selective serotonin reuptake inhibitors (SSRIs) was significantly higher than the placebo rate of 14.2% (TABLE).1 The SSRIs citalopram, fluoxetine, paroxetine, and sertraline as well as the SNRI venlafaxine, had significantly greater rates (70%-80%) of reported total sexual dysfunction, including desire, arousal, and orgasm, than placebo.
Bupropion has sexual dysfunction rates comparable to placebo
Other SSRIs (fluvoxamine, escitalopram), the tricyclic antidepressant imipramine, and the SNRI duloxetine also had total SD rates significantly greater than placebo. However, the rates of dysfunction with these agents are often lower than the dysfunction rates of SSRIs such as sertraline and citalopram, and thus, may be viewed as falling into an intermediate risk category. The total SD rates for the NDRI bupropion were comparable to the placebo rate.1
With few exceptions, all drugs associated with overall SD were associated with significant dysfunction affecting the sexual components of desire, arousal, and orgasm. The results of this meta-analysis should be interpreted with some degree of caution because methods of assessing SD varied within individual studies.
AHRQ weighs in
An Agency for Healthcare Research and Quality (AHRQ) review of antidepressants found that paroxetine, citalopram, and venlafaxine, when compared with other antidepressants (fluoxetine, fluvoxamine, nefazodone, sertraline), generally were associated with more reports of SD, specifically complaints of erectile dysfunction in men and decreased vaginal lubrication in women. 2 The number needed to treat one additional person with general sexual functioning satisfaction was 6 (95% CI, 4-9) with buproprion.2
RECOMMENDATIONS
The American College of Physicians’ clinical practice guidelines suggest that although SD is likely underreported, the NDRI bupropion has consistently shown lower rates of associated dysfunction than the SSRIs fluoxetine and sertraline.3 Conversely, the SSRI paroxetine has shown higher rates of adverse sexual events than other SSRIs, such as fluoxetine and fluvoxamine, and the serotonin reuptake inhibitor/antagonist nefazodone.3
1. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29:259-266.
2. Garlehner G, Hansen R, Thieda P, et al. Comparative Effectiveness of Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: Comparative Effectiveness Review Number 7. Rockville, MD; Agency for Healthcare Research and Quality; 2007. Available at: www.effectivehealthcare.ahrq.gov/ehc/products/7/59/Antidepressants_Final_Report.pdf. Accessed: March 5, 2012.
3. Qaseem A, Snow V, Denberg TD, et al; Clinical Efficacy Assessment Subcomittee of Physicians. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:725-733.
Patients treated with selective serotonin reuptake inhibitors (SSRIs) and the serotonin/norepinephrine reuptake inhibitor (SNRI) venlafaxine have significantly higher rates of overall sexual dysfunction—including desire, arousal, and orgasm—than patients treated with placebo (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with heterogeneous results). Patients treated with bupropion, a norepinephrine-dopamine reuptake inhibitor (NDRI), have rates of overall sexual dysfunction comparable to placebo (SOR: B, RCTs with heterogeneous results).
EVIDENCE SUMMARY
In a meta-analysis of 31 studies with 10,130 patients, researchers reported that the total rate of sexual dysfunction (SD) associated with selective serotonin reuptake inhibitors (SSRIs) was significantly higher than the placebo rate of 14.2% (TABLE).1 The SSRIs citalopram, fluoxetine, paroxetine, and sertraline as well as the SNRI venlafaxine, had significantly greater rates (70%-80%) of reported total sexual dysfunction, including desire, arousal, and orgasm, than placebo.
Bupropion has sexual dysfunction rates comparable to placebo
Other SSRIs (fluvoxamine, escitalopram), the tricyclic antidepressant imipramine, and the SNRI duloxetine also had total SD rates significantly greater than placebo. However, the rates of dysfunction with these agents are often lower than the dysfunction rates of SSRIs such as sertraline and citalopram, and thus, may be viewed as falling into an intermediate risk category. The total SD rates for the NDRI bupropion were comparable to the placebo rate.1
With few exceptions, all drugs associated with overall SD were associated with significant dysfunction affecting the sexual components of desire, arousal, and orgasm. The results of this meta-analysis should be interpreted with some degree of caution because methods of assessing SD varied within individual studies.
AHRQ weighs in
An Agency for Healthcare Research and Quality (AHRQ) review of antidepressants found that paroxetine, citalopram, and venlafaxine, when compared with other antidepressants (fluoxetine, fluvoxamine, nefazodone, sertraline), generally were associated with more reports of SD, specifically complaints of erectile dysfunction in men and decreased vaginal lubrication in women. 2 The number needed to treat one additional person with general sexual functioning satisfaction was 6 (95% CI, 4-9) with buproprion.2
RECOMMENDATIONS
The American College of Physicians’ clinical practice guidelines suggest that although SD is likely underreported, the NDRI bupropion has consistently shown lower rates of associated dysfunction than the SSRIs fluoxetine and sertraline.3 Conversely, the SSRI paroxetine has shown higher rates of adverse sexual events than other SSRIs, such as fluoxetine and fluvoxamine, and the serotonin reuptake inhibitor/antagonist nefazodone.3
Patients treated with selective serotonin reuptake inhibitors (SSRIs) and the serotonin/norepinephrine reuptake inhibitor (SNRI) venlafaxine have significantly higher rates of overall sexual dysfunction—including desire, arousal, and orgasm—than patients treated with placebo (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with heterogeneous results). Patients treated with bupropion, a norepinephrine-dopamine reuptake inhibitor (NDRI), have rates of overall sexual dysfunction comparable to placebo (SOR: B, RCTs with heterogeneous results).
EVIDENCE SUMMARY
In a meta-analysis of 31 studies with 10,130 patients, researchers reported that the total rate of sexual dysfunction (SD) associated with selective serotonin reuptake inhibitors (SSRIs) was significantly higher than the placebo rate of 14.2% (TABLE).1 The SSRIs citalopram, fluoxetine, paroxetine, and sertraline as well as the SNRI venlafaxine, had significantly greater rates (70%-80%) of reported total sexual dysfunction, including desire, arousal, and orgasm, than placebo.
Bupropion has sexual dysfunction rates comparable to placebo
Other SSRIs (fluvoxamine, escitalopram), the tricyclic antidepressant imipramine, and the SNRI duloxetine also had total SD rates significantly greater than placebo. However, the rates of dysfunction with these agents are often lower than the dysfunction rates of SSRIs such as sertraline and citalopram, and thus, may be viewed as falling into an intermediate risk category. The total SD rates for the NDRI bupropion were comparable to the placebo rate.1
With few exceptions, all drugs associated with overall SD were associated with significant dysfunction affecting the sexual components of desire, arousal, and orgasm. The results of this meta-analysis should be interpreted with some degree of caution because methods of assessing SD varied within individual studies.
AHRQ weighs in
An Agency for Healthcare Research and Quality (AHRQ) review of antidepressants found that paroxetine, citalopram, and venlafaxine, when compared with other antidepressants (fluoxetine, fluvoxamine, nefazodone, sertraline), generally were associated with more reports of SD, specifically complaints of erectile dysfunction in men and decreased vaginal lubrication in women. 2 The number needed to treat one additional person with general sexual functioning satisfaction was 6 (95% CI, 4-9) with buproprion.2
RECOMMENDATIONS
The American College of Physicians’ clinical practice guidelines suggest that although SD is likely underreported, the NDRI bupropion has consistently shown lower rates of associated dysfunction than the SSRIs fluoxetine and sertraline.3 Conversely, the SSRI paroxetine has shown higher rates of adverse sexual events than other SSRIs, such as fluoxetine and fluvoxamine, and the serotonin reuptake inhibitor/antagonist nefazodone.3
1. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29:259-266.
2. Garlehner G, Hansen R, Thieda P, et al. Comparative Effectiveness of Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: Comparative Effectiveness Review Number 7. Rockville, MD; Agency for Healthcare Research and Quality; 2007. Available at: www.effectivehealthcare.ahrq.gov/ehc/products/7/59/Antidepressants_Final_Report.pdf. Accessed: March 5, 2012.
3. Qaseem A, Snow V, Denberg TD, et al; Clinical Efficacy Assessment Subcomittee of Physicians. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:725-733.
1. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29:259-266.
2. Garlehner G, Hansen R, Thieda P, et al. Comparative Effectiveness of Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: Comparative Effectiveness Review Number 7. Rockville, MD; Agency for Healthcare Research and Quality; 2007. Available at: www.effectivehealthcare.ahrq.gov/ehc/products/7/59/Antidepressants_Final_Report.pdf. Accessed: March 5, 2012.
3. Qaseem A, Snow V, Denberg TD, et al; Clinical Efficacy Assessment Subcomittee of Physicians. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:725-733.
Evidence-based answers from the Family Physicians Inquiries Network
Elevated troponin but no CVD: What’s the prognosis?
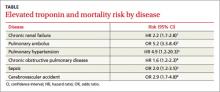
Patients with elevated troponin levels and chronic renal disease, pulmonary hypertension, pulmonary embolism, chronic obstructive pulmonary disease, sepsis, or acute ischemic stroke have a 2- to 5-fold increased risk of death, even in the absence of known cardiovascular disease (TABLE)1-6 (strength of recommendation: B, meta-analysis, multiple prospective and retrospective observational studies.)
EVIDENCE SUMMARY
To investigate the prognostic value of troponin on overall mortality, a multicenter prospective study followed 847 patients 18 years and older (mean age 59 years) with end-stage renal disease whose troponin T levels were measured 3 months from the start of peritoneal dialysis or hemodialysis until transplantation or death.1 At enrollment, 566 patients had a troponin level of ≤0.04 ng/dL, 188 had a value between 0.05 and 0.10 ng/dL, and 93 had a level of more than 0.10 ng/dL.
Using Cox regression, patients whose troponin levels were more than 0.10 ng/dL had an increased hazard ratio (HR) for all-cause mortality of 2.2 (95% confidence interval [CI], 1.7-2.8) compared with patients who had levels ≤0.04 ng/dL. Cardiovascular mortality also was higher (HR=1.9; 95% CI, 0.9-3.7) with troponin elevations, but didn’t reach statistical significance. Investigators found no significant differences in mortality risk between patients on peritoneal or hemodialysis, patients with or without a history of acute myocardial infarction, or patients who suffered cerebrovascular accidents.
Elevated troponin raises risk of death 5-fold in pulmonary embolism patients
A meta-analysis of 20 trials with a total of 1985 patients assessed the prognostic value of troponin for short-term mortality in patients admitted with acute pulmonary embolism.2 Sixteen studies (1527 patients) were prospective trials and the remainder (458 patients) were retrospective trials. Investigators obtained troponin levels for all patients at admission. They used several different troponin assays (both I and T), but most of the studies used the assay manufacturers’ cutoff points (exceeding the 99th percentile).
High troponin levels were associated with a 5-fold increased risk of short-term death, defined as in-hospital death up to 30 days after discharge (19.7% with elevated troponin vs 3.7% with normal troponin; odds ratio [OR]=5.24; 95% CI, 3.3-8.4).
Increased risk of death among those with pulmonary hypertension, COPD A prospective single-center study of 56 patients with chronic pulmonary hypertension found that the 14% of those whose troponin T was elevated (≥0.01 ng/mL) had a lower survival rate than the other patients. Patients who either had a positive troponin on initial assessment or developed troponin elevation within the 2-year follow-up period had a cumulative 24-month survival rate of 29%, compared with 81% for their troponin T-negative counterparts (P=.001).3
Patients with elevated troponin levels and certain conditions have a 2- to 5-fold increased risk of death, even without known cardiovascular disease.
Elevated troponin I is an independent predictor of mortality in severe sepsis
A double-blind, placebo-controlled, phase 3 trial evaluated the effect of drotrecogin alfa (activated)—withdrawn from the market in 2011—on survival of patients with severe sepsis.5 Investigators used positive troponin I levels (≥0.06 ng/mL) as a prognostic indicator of mortality. Patients who were troponin-positive had a 28-day mortality rate of 32%, compared with 14% in the troponin-negative group (P<.0001).
A bias of this study is that the patients with positive troponin levels tended to be older and more critically ill. However, in a multivariate model, troponin I still remained an independent predictor of mortality.
Elevated troponin predicts increased death risk in up to 20% of stroke patients
A systematic review of 15 trials with a total of 2901 patients evaluated the relationship between troponin levels and stroke.6 Investigators assessed the prevalence of elevated troponin in acute stroke patients, the association of elevated troponin levels with electrocardiographic changes, and the overall morbidity and mortality associated with troponin levels. Thirteen of the 15 studies used a troponin T or I level obtained within 72 hours of admission and a cut-off level of 0.1 ng/mL. The remaining 2 studies used troponin I cut-off levels >0.2 and 0.4 ng/mL.
Overall, 18% of acute stroke patients had elevated troponin levels. Studies that excluded patients with known cardiac disease had a lower prevalence of elevated levels (10% vs 22%). Patients with elevated troponin levels had an associated overall increased risk of death (OR=2.9; 95% CI, 1.7-4.8) and were 3 times more likely to have ischemic changes on electrocardiogram (OR=3.0; 95% CI, 1.5-6.2). Investigators concluded that elevated troponin levels occur in as many as one in 5 patients and are associated with an increased risk of death.
Troponin elevations may be observed in congestive heart failure, chest wall trauma, cardioversion/defibrillator shocks, rhabdomyolysis, and ultra-endurance activities.7 However, this analysis didn’t address prognostic implications of elevated troponins.
RECOMMENDATIONS
No recommendation exists for biochemical testing of troponins in various medical conditions except in the presence of signs and symptoms consistent with acute coronary syndrome. The American College of Cardiology and American Heart Association recommend routine testing of cardiac troponins in patients hospitalized for worsening congestive heart failure symptoms.8
The European Society of Cardiology recommends measuring troponin levels to further stratify risk in non-high-risk patients with confirmed pulmonary embolus.9
The National Academy of Clinical Biochemistry recommends using cardiac troponins to help define mortality risk in end-stage renal disease and critically ill patients.10
1. Havekes B, van Manen J, Krediet R, et al. Serum troponin T concentration as a predictor of mortality in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2006;47:823-829.
2. Becattini C, Vedovati MC, Agnelli G. Prognostic value of tropo- nins in acute pulmonary embolism. Circulation. 2007;116:427- 433.
3. Torbicki A, Kurzyna M, Kuca P, et al. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844-848.
4. Brekke PH, Omland T, Holmedal SH, et al. Troponin T eleva- tion and long-term mortality after chronic obstructive pulmo- nary disease exacerbation. Eur Respir J. 2008;31:563-570.
5. John J, Woodward DB, Wang Y, et al. Troponin I as a prog- nosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270-275.
6. Kerr G, Ray G, Wu O, et al. Elevated troponin after stroke: a sys- tematic review. Cerebrovasc Dis. 2009;28:220-226.
7. Korff S, Katus HA, Giannitsis E. Differential diagnosis of el- evated troponins. Heart. 2006;92:987-993.
8. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diag- nosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines devel- oped in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
9. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Eur Heart J. 2008;29:2276-2315.
10. Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086-2096.
Patients with elevated troponin levels and chronic renal disease, pulmonary hypertension, pulmonary embolism, chronic obstructive pulmonary disease, sepsis, or acute ischemic stroke have a 2- to 5-fold increased risk of death, even in the absence of known cardiovascular disease (TABLE)1-6 (strength of recommendation: B, meta-analysis, multiple prospective and retrospective observational studies.)
EVIDENCE SUMMARY
To investigate the prognostic value of troponin on overall mortality, a multicenter prospective study followed 847 patients 18 years and older (mean age 59 years) with end-stage renal disease whose troponin T levels were measured 3 months from the start of peritoneal dialysis or hemodialysis until transplantation or death.1 At enrollment, 566 patients had a troponin level of ≤0.04 ng/dL, 188 had a value between 0.05 and 0.10 ng/dL, and 93 had a level of more than 0.10 ng/dL.
Using Cox regression, patients whose troponin levels were more than 0.10 ng/dL had an increased hazard ratio (HR) for all-cause mortality of 2.2 (95% confidence interval [CI], 1.7-2.8) compared with patients who had levels ≤0.04 ng/dL. Cardiovascular mortality also was higher (HR=1.9; 95% CI, 0.9-3.7) with troponin elevations, but didn’t reach statistical significance. Investigators found no significant differences in mortality risk between patients on peritoneal or hemodialysis, patients with or without a history of acute myocardial infarction, or patients who suffered cerebrovascular accidents.
Elevated troponin raises risk of death 5-fold in pulmonary embolism patients
A meta-analysis of 20 trials with a total of 1985 patients assessed the prognostic value of troponin for short-term mortality in patients admitted with acute pulmonary embolism.2 Sixteen studies (1527 patients) were prospective trials and the remainder (458 patients) were retrospective trials. Investigators obtained troponin levels for all patients at admission. They used several different troponin assays (both I and T), but most of the studies used the assay manufacturers’ cutoff points (exceeding the 99th percentile).
High troponin levels were associated with a 5-fold increased risk of short-term death, defined as in-hospital death up to 30 days after discharge (19.7% with elevated troponin vs 3.7% with normal troponin; odds ratio [OR]=5.24; 95% CI, 3.3-8.4).
Increased risk of death among those with pulmonary hypertension, COPD A prospective single-center study of 56 patients with chronic pulmonary hypertension found that the 14% of those whose troponin T was elevated (≥0.01 ng/mL) had a lower survival rate than the other patients. Patients who either had a positive troponin on initial assessment or developed troponin elevation within the 2-year follow-up period had a cumulative 24-month survival rate of 29%, compared with 81% for their troponin T-negative counterparts (P=.001).3
Patients with elevated troponin levels and certain conditions have a 2- to 5-fold increased risk of death, even without known cardiovascular disease.
Elevated troponin I is an independent predictor of mortality in severe sepsis
A double-blind, placebo-controlled, phase 3 trial evaluated the effect of drotrecogin alfa (activated)—withdrawn from the market in 2011—on survival of patients with severe sepsis.5 Investigators used positive troponin I levels (≥0.06 ng/mL) as a prognostic indicator of mortality. Patients who were troponin-positive had a 28-day mortality rate of 32%, compared with 14% in the troponin-negative group (P<.0001).
A bias of this study is that the patients with positive troponin levels tended to be older and more critically ill. However, in a multivariate model, troponin I still remained an independent predictor of mortality.
Elevated troponin predicts increased death risk in up to 20% of stroke patients
A systematic review of 15 trials with a total of 2901 patients evaluated the relationship between troponin levels and stroke.6 Investigators assessed the prevalence of elevated troponin in acute stroke patients, the association of elevated troponin levels with electrocardiographic changes, and the overall morbidity and mortality associated with troponin levels. Thirteen of the 15 studies used a troponin T or I level obtained within 72 hours of admission and a cut-off level of 0.1 ng/mL. The remaining 2 studies used troponin I cut-off levels >0.2 and 0.4 ng/mL.
Overall, 18% of acute stroke patients had elevated troponin levels. Studies that excluded patients with known cardiac disease had a lower prevalence of elevated levels (10% vs 22%). Patients with elevated troponin levels had an associated overall increased risk of death (OR=2.9; 95% CI, 1.7-4.8) and were 3 times more likely to have ischemic changes on electrocardiogram (OR=3.0; 95% CI, 1.5-6.2). Investigators concluded that elevated troponin levels occur in as many as one in 5 patients and are associated with an increased risk of death.
Troponin elevations may be observed in congestive heart failure, chest wall trauma, cardioversion/defibrillator shocks, rhabdomyolysis, and ultra-endurance activities.7 However, this analysis didn’t address prognostic implications of elevated troponins.
RECOMMENDATIONS
No recommendation exists for biochemical testing of troponins in various medical conditions except in the presence of signs and symptoms consistent with acute coronary syndrome. The American College of Cardiology and American Heart Association recommend routine testing of cardiac troponins in patients hospitalized for worsening congestive heart failure symptoms.8
The European Society of Cardiology recommends measuring troponin levels to further stratify risk in non-high-risk patients with confirmed pulmonary embolus.9
The National Academy of Clinical Biochemistry recommends using cardiac troponins to help define mortality risk in end-stage renal disease and critically ill patients.10
Patients with elevated troponin levels and chronic renal disease, pulmonary hypertension, pulmonary embolism, chronic obstructive pulmonary disease, sepsis, or acute ischemic stroke have a 2- to 5-fold increased risk of death, even in the absence of known cardiovascular disease (TABLE)1-6 (strength of recommendation: B, meta-analysis, multiple prospective and retrospective observational studies.)
EVIDENCE SUMMARY
To investigate the prognostic value of troponin on overall mortality, a multicenter prospective study followed 847 patients 18 years and older (mean age 59 years) with end-stage renal disease whose troponin T levels were measured 3 months from the start of peritoneal dialysis or hemodialysis until transplantation or death.1 At enrollment, 566 patients had a troponin level of ≤0.04 ng/dL, 188 had a value between 0.05 and 0.10 ng/dL, and 93 had a level of more than 0.10 ng/dL.
Using Cox regression, patients whose troponin levels were more than 0.10 ng/dL had an increased hazard ratio (HR) for all-cause mortality of 2.2 (95% confidence interval [CI], 1.7-2.8) compared with patients who had levels ≤0.04 ng/dL. Cardiovascular mortality also was higher (HR=1.9; 95% CI, 0.9-3.7) with troponin elevations, but didn’t reach statistical significance. Investigators found no significant differences in mortality risk between patients on peritoneal or hemodialysis, patients with or without a history of acute myocardial infarction, or patients who suffered cerebrovascular accidents.
Elevated troponin raises risk of death 5-fold in pulmonary embolism patients
A meta-analysis of 20 trials with a total of 1985 patients assessed the prognostic value of troponin for short-term mortality in patients admitted with acute pulmonary embolism.2 Sixteen studies (1527 patients) were prospective trials and the remainder (458 patients) were retrospective trials. Investigators obtained troponin levels for all patients at admission. They used several different troponin assays (both I and T), but most of the studies used the assay manufacturers’ cutoff points (exceeding the 99th percentile).
High troponin levels were associated with a 5-fold increased risk of short-term death, defined as in-hospital death up to 30 days after discharge (19.7% with elevated troponin vs 3.7% with normal troponin; odds ratio [OR]=5.24; 95% CI, 3.3-8.4).
Increased risk of death among those with pulmonary hypertension, COPD A prospective single-center study of 56 patients with chronic pulmonary hypertension found that the 14% of those whose troponin T was elevated (≥0.01 ng/mL) had a lower survival rate than the other patients. Patients who either had a positive troponin on initial assessment or developed troponin elevation within the 2-year follow-up period had a cumulative 24-month survival rate of 29%, compared with 81% for their troponin T-negative counterparts (P=.001).3
Patients with elevated troponin levels and certain conditions have a 2- to 5-fold increased risk of death, even without known cardiovascular disease.
Elevated troponin I is an independent predictor of mortality in severe sepsis
A double-blind, placebo-controlled, phase 3 trial evaluated the effect of drotrecogin alfa (activated)—withdrawn from the market in 2011—on survival of patients with severe sepsis.5 Investigators used positive troponin I levels (≥0.06 ng/mL) as a prognostic indicator of mortality. Patients who were troponin-positive had a 28-day mortality rate of 32%, compared with 14% in the troponin-negative group (P<.0001).
A bias of this study is that the patients with positive troponin levels tended to be older and more critically ill. However, in a multivariate model, troponin I still remained an independent predictor of mortality.
Elevated troponin predicts increased death risk in up to 20% of stroke patients
A systematic review of 15 trials with a total of 2901 patients evaluated the relationship between troponin levels and stroke.6 Investigators assessed the prevalence of elevated troponin in acute stroke patients, the association of elevated troponin levels with electrocardiographic changes, and the overall morbidity and mortality associated with troponin levels. Thirteen of the 15 studies used a troponin T or I level obtained within 72 hours of admission and a cut-off level of 0.1 ng/mL. The remaining 2 studies used troponin I cut-off levels >0.2 and 0.4 ng/mL.
Overall, 18% of acute stroke patients had elevated troponin levels. Studies that excluded patients with known cardiac disease had a lower prevalence of elevated levels (10% vs 22%). Patients with elevated troponin levels had an associated overall increased risk of death (OR=2.9; 95% CI, 1.7-4.8) and were 3 times more likely to have ischemic changes on electrocardiogram (OR=3.0; 95% CI, 1.5-6.2). Investigators concluded that elevated troponin levels occur in as many as one in 5 patients and are associated with an increased risk of death.
Troponin elevations may be observed in congestive heart failure, chest wall trauma, cardioversion/defibrillator shocks, rhabdomyolysis, and ultra-endurance activities.7 However, this analysis didn’t address prognostic implications of elevated troponins.
RECOMMENDATIONS
No recommendation exists for biochemical testing of troponins in various medical conditions except in the presence of signs and symptoms consistent with acute coronary syndrome. The American College of Cardiology and American Heart Association recommend routine testing of cardiac troponins in patients hospitalized for worsening congestive heart failure symptoms.8
The European Society of Cardiology recommends measuring troponin levels to further stratify risk in non-high-risk patients with confirmed pulmonary embolus.9
The National Academy of Clinical Biochemistry recommends using cardiac troponins to help define mortality risk in end-stage renal disease and critically ill patients.10
1. Havekes B, van Manen J, Krediet R, et al. Serum troponin T concentration as a predictor of mortality in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2006;47:823-829.
2. Becattini C, Vedovati MC, Agnelli G. Prognostic value of tropo- nins in acute pulmonary embolism. Circulation. 2007;116:427- 433.
3. Torbicki A, Kurzyna M, Kuca P, et al. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844-848.
4. Brekke PH, Omland T, Holmedal SH, et al. Troponin T eleva- tion and long-term mortality after chronic obstructive pulmo- nary disease exacerbation. Eur Respir J. 2008;31:563-570.
5. John J, Woodward DB, Wang Y, et al. Troponin I as a prog- nosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270-275.
6. Kerr G, Ray G, Wu O, et al. Elevated troponin after stroke: a sys- tematic review. Cerebrovasc Dis. 2009;28:220-226.
7. Korff S, Katus HA, Giannitsis E. Differential diagnosis of el- evated troponins. Heart. 2006;92:987-993.
8. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diag- nosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines devel- oped in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
9. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Eur Heart J. 2008;29:2276-2315.
10. Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086-2096.
1. Havekes B, van Manen J, Krediet R, et al. Serum troponin T concentration as a predictor of mortality in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2006;47:823-829.
2. Becattini C, Vedovati MC, Agnelli G. Prognostic value of tropo- nins in acute pulmonary embolism. Circulation. 2007;116:427- 433.
3. Torbicki A, Kurzyna M, Kuca P, et al. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844-848.
4. Brekke PH, Omland T, Holmedal SH, et al. Troponin T eleva- tion and long-term mortality after chronic obstructive pulmo- nary disease exacerbation. Eur Respir J. 2008;31:563-570.
5. John J, Woodward DB, Wang Y, et al. Troponin I as a prog- nosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270-275.
6. Kerr G, Ray G, Wu O, et al. Elevated troponin after stroke: a sys- tematic review. Cerebrovasc Dis. 2009;28:220-226.
7. Korff S, Katus HA, Giannitsis E. Differential diagnosis of el- evated troponins. Heart. 2006;92:987-993.
8. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diag- nosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines devel- oped in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
9. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Eur Heart J. 2008;29:2276-2315.
10. Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086-2096.
Evidence-based answers from the Family Physicians Inquiries Network
Do dietary choices alone alter the risk of developing metabolic syndrome?
YES, but not in the short term. In studies of patient populations controlled for differences in dietary content alone, independent of weight loss or exercise changes, diets with high glycemic index foods, low whole grain and fiber content, and low fruit and vegetable content are associated with an increased incidence of metabolic syndrome (strength of recommendation [SOR]: B, multiple large cohort studies).
In the short term, however, switching patients at high risk for metabolic syndrome from a high- to low-glycemic index diet doesn’t improve serum markers of metabolic syndrome (SOR: C, a small randomized controlled trial).
Evidence summary
Six studies (5 cohort studies and one randomized crossover study) attempted to isolate specific dietary components as risk factors for metabolic syndrome, by performing multivariate analyses to control for weight and exercise habits. The cohort studies all used the National Cholesterol Education Program Adult Treatment Panel III definition of metabolic syndrome. Overall, consumption of foods with a high glycemic index was associated with an increased risk of metabolic syndrome.
A cohort study that evaluated the diet, body habitus, and serum metabolic parameters of 2834 US adults using a validated, interviewer-administered food frequency questionnaire found that the rate of metabolic syndrome was significantly higher in patients with the highest glycemic index diets (highest vs lowest quintile adjusted odds ratio [aOR]=1.4; 95% confidence interval [CI], 1.04-1.9).1 Conversely, metabolic syndrome was less common in subjects who ate diets rich in whole grains (aOR=0.67; 95% CI, 0.48-0.91) and cereal fiber (aOR=0.62; 95% CI, 0.45-0.86).
A second cohort study evaluated the diet, body habitus, and metabolic parameters in 2043 Asian women using the same food frequency questionnaire to obtain dietary history.2 Metabolic syndrome was significantly more common among the women with a high refined carbohydrate intake (highest vs lowest quartile aOR=7.8; 95% CI, 4.7-13).
“Western” diet, lack of diversity associated with metabolic syndrome
Two studies from Iran evaluated the rates of metabolic syndrome according to different dietary patterns. The first evaluated a cohort of 486 female teachers 40 to 60 years of age.3 Investigators characterized dietary patterns as “healthy” (rich in fruits, vegetables, and whole grains) or “Western” (more meat and refined grains). The more “Western” the dietary pattern became, the more often metabolic syndrome was diagnosed (highest vs lowest quintile aOR=1.7; 95% CI, 1.1-1.9).
In the second study, 581 healthy adults received dietary surveys and were tested for metabolic syndrome.4 Diets were assessed and scored for their diversity. High levels of dietary diversity were inversely associated with metabolic syndrome (highest vs lowest quartile aOR=0.77; 95% CI, 0.59-0.93).
No short-term gain in switching to foods with low glycemic index
Switching to foods with a low glycemic index, however, may not provide much benefit, at least in the short term. An 11-week prospective, double-blind, crossover trial in which 15 overweight patients at risk of developing metabolic syndrome alternated eating foods with high and low glycemic indexes found no significant difference in the serum markers associated with metabolic syndrome (fasting glucose, insulin, and triglyceride levels).5
Recommendations
The 2010 Dietary Guidelines for Americans, jointly issued by the US Department of Agriculture and Health and Human Services, recommend increasing fruit and vegetable intake, eating a variety of vegetables, and consuming at least half of all grains as whole grains.6 The guidelines further recommend limiting consumption of foods that contain refined grains, “especially refined grain foods that contain solid fats, added sugars, and sodium.”
The American Diabetes Association (ADA) encourages consumption of low-glycemic index foods, especially foods rich in fiber and other nutrients. However, the ADA also states that there are “not sufficient, consistent” data to conclude that low-glycemic index diets reduce the risk of diabetes.7
1. McKown NM, Meigs JB, Liu S, et al. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham offspring cohort. Diabetes Care. 2004;27:538-546.
2. Radhika G, Van Dam RM, Sudha V, et al. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai urban rural epidemiology study 57). Metabolism. 2009;58:675-681.
3. Esmaillzadeh A, Kimiagar M, Mehrabi Y, et al. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr. 2007;85:910-918.
4. Azadbakht L, Mirmiran P, Azizi F. Dietary diversity score is favorably associated with the metabolic syndrome in Tehranian adults. Int J Obes (Lond). 2005;29:1361-1367.
5. Vrolix R, Mensink RP. Effects of glycemic load on metabolic risk markers in subjects at increased risk of developing metabolic syndrome. Am J Clin Nutr. 2010;92:366-374.
6. US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: US Government Printing Office; 2010. Available at: http://www.cnpp.usda.gov/dietaryguidelines. htm. Accessed August 19, 2013.
7. American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(suppl 1): S61-S78.
YES, but not in the short term. In studies of patient populations controlled for differences in dietary content alone, independent of weight loss or exercise changes, diets with high glycemic index foods, low whole grain and fiber content, and low fruit and vegetable content are associated with an increased incidence of metabolic syndrome (strength of recommendation [SOR]: B, multiple large cohort studies).
In the short term, however, switching patients at high risk for metabolic syndrome from a high- to low-glycemic index diet doesn’t improve serum markers of metabolic syndrome (SOR: C, a small randomized controlled trial).
Evidence summary
Six studies (5 cohort studies and one randomized crossover study) attempted to isolate specific dietary components as risk factors for metabolic syndrome, by performing multivariate analyses to control for weight and exercise habits. The cohort studies all used the National Cholesterol Education Program Adult Treatment Panel III definition of metabolic syndrome. Overall, consumption of foods with a high glycemic index was associated with an increased risk of metabolic syndrome.
A cohort study that evaluated the diet, body habitus, and serum metabolic parameters of 2834 US adults using a validated, interviewer-administered food frequency questionnaire found that the rate of metabolic syndrome was significantly higher in patients with the highest glycemic index diets (highest vs lowest quintile adjusted odds ratio [aOR]=1.4; 95% confidence interval [CI], 1.04-1.9).1 Conversely, metabolic syndrome was less common in subjects who ate diets rich in whole grains (aOR=0.67; 95% CI, 0.48-0.91) and cereal fiber (aOR=0.62; 95% CI, 0.45-0.86).
A second cohort study evaluated the diet, body habitus, and metabolic parameters in 2043 Asian women using the same food frequency questionnaire to obtain dietary history.2 Metabolic syndrome was significantly more common among the women with a high refined carbohydrate intake (highest vs lowest quartile aOR=7.8; 95% CI, 4.7-13).
“Western” diet, lack of diversity associated with metabolic syndrome
Two studies from Iran evaluated the rates of metabolic syndrome according to different dietary patterns. The first evaluated a cohort of 486 female teachers 40 to 60 years of age.3 Investigators characterized dietary patterns as “healthy” (rich in fruits, vegetables, and whole grains) or “Western” (more meat and refined grains). The more “Western” the dietary pattern became, the more often metabolic syndrome was diagnosed (highest vs lowest quintile aOR=1.7; 95% CI, 1.1-1.9).
In the second study, 581 healthy adults received dietary surveys and were tested for metabolic syndrome.4 Diets were assessed and scored for their diversity. High levels of dietary diversity were inversely associated with metabolic syndrome (highest vs lowest quartile aOR=0.77; 95% CI, 0.59-0.93).
No short-term gain in switching to foods with low glycemic index
Switching to foods with a low glycemic index, however, may not provide much benefit, at least in the short term. An 11-week prospective, double-blind, crossover trial in which 15 overweight patients at risk of developing metabolic syndrome alternated eating foods with high and low glycemic indexes found no significant difference in the serum markers associated with metabolic syndrome (fasting glucose, insulin, and triglyceride levels).5
Recommendations
The 2010 Dietary Guidelines for Americans, jointly issued by the US Department of Agriculture and Health and Human Services, recommend increasing fruit and vegetable intake, eating a variety of vegetables, and consuming at least half of all grains as whole grains.6 The guidelines further recommend limiting consumption of foods that contain refined grains, “especially refined grain foods that contain solid fats, added sugars, and sodium.”
The American Diabetes Association (ADA) encourages consumption of low-glycemic index foods, especially foods rich in fiber and other nutrients. However, the ADA also states that there are “not sufficient, consistent” data to conclude that low-glycemic index diets reduce the risk of diabetes.7
YES, but not in the short term. In studies of patient populations controlled for differences in dietary content alone, independent of weight loss or exercise changes, diets with high glycemic index foods, low whole grain and fiber content, and low fruit and vegetable content are associated with an increased incidence of metabolic syndrome (strength of recommendation [SOR]: B, multiple large cohort studies).
In the short term, however, switching patients at high risk for metabolic syndrome from a high- to low-glycemic index diet doesn’t improve serum markers of metabolic syndrome (SOR: C, a small randomized controlled trial).
Evidence summary
Six studies (5 cohort studies and one randomized crossover study) attempted to isolate specific dietary components as risk factors for metabolic syndrome, by performing multivariate analyses to control for weight and exercise habits. The cohort studies all used the National Cholesterol Education Program Adult Treatment Panel III definition of metabolic syndrome. Overall, consumption of foods with a high glycemic index was associated with an increased risk of metabolic syndrome.
A cohort study that evaluated the diet, body habitus, and serum metabolic parameters of 2834 US adults using a validated, interviewer-administered food frequency questionnaire found that the rate of metabolic syndrome was significantly higher in patients with the highest glycemic index diets (highest vs lowest quintile adjusted odds ratio [aOR]=1.4; 95% confidence interval [CI], 1.04-1.9).1 Conversely, metabolic syndrome was less common in subjects who ate diets rich in whole grains (aOR=0.67; 95% CI, 0.48-0.91) and cereal fiber (aOR=0.62; 95% CI, 0.45-0.86).
A second cohort study evaluated the diet, body habitus, and metabolic parameters in 2043 Asian women using the same food frequency questionnaire to obtain dietary history.2 Metabolic syndrome was significantly more common among the women with a high refined carbohydrate intake (highest vs lowest quartile aOR=7.8; 95% CI, 4.7-13).
“Western” diet, lack of diversity associated with metabolic syndrome
Two studies from Iran evaluated the rates of metabolic syndrome according to different dietary patterns. The first evaluated a cohort of 486 female teachers 40 to 60 years of age.3 Investigators characterized dietary patterns as “healthy” (rich in fruits, vegetables, and whole grains) or “Western” (more meat and refined grains). The more “Western” the dietary pattern became, the more often metabolic syndrome was diagnosed (highest vs lowest quintile aOR=1.7; 95% CI, 1.1-1.9).
In the second study, 581 healthy adults received dietary surveys and were tested for metabolic syndrome.4 Diets were assessed and scored for their diversity. High levels of dietary diversity were inversely associated with metabolic syndrome (highest vs lowest quartile aOR=0.77; 95% CI, 0.59-0.93).
No short-term gain in switching to foods with low glycemic index
Switching to foods with a low glycemic index, however, may not provide much benefit, at least in the short term. An 11-week prospective, double-blind, crossover trial in which 15 overweight patients at risk of developing metabolic syndrome alternated eating foods with high and low glycemic indexes found no significant difference in the serum markers associated with metabolic syndrome (fasting glucose, insulin, and triglyceride levels).5
Recommendations
The 2010 Dietary Guidelines for Americans, jointly issued by the US Department of Agriculture and Health and Human Services, recommend increasing fruit and vegetable intake, eating a variety of vegetables, and consuming at least half of all grains as whole grains.6 The guidelines further recommend limiting consumption of foods that contain refined grains, “especially refined grain foods that contain solid fats, added sugars, and sodium.”
The American Diabetes Association (ADA) encourages consumption of low-glycemic index foods, especially foods rich in fiber and other nutrients. However, the ADA also states that there are “not sufficient, consistent” data to conclude that low-glycemic index diets reduce the risk of diabetes.7
1. McKown NM, Meigs JB, Liu S, et al. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham offspring cohort. Diabetes Care. 2004;27:538-546.
2. Radhika G, Van Dam RM, Sudha V, et al. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai urban rural epidemiology study 57). Metabolism. 2009;58:675-681.
3. Esmaillzadeh A, Kimiagar M, Mehrabi Y, et al. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr. 2007;85:910-918.
4. Azadbakht L, Mirmiran P, Azizi F. Dietary diversity score is favorably associated with the metabolic syndrome in Tehranian adults. Int J Obes (Lond). 2005;29:1361-1367.
5. Vrolix R, Mensink RP. Effects of glycemic load on metabolic risk markers in subjects at increased risk of developing metabolic syndrome. Am J Clin Nutr. 2010;92:366-374.
6. US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: US Government Printing Office; 2010. Available at: http://www.cnpp.usda.gov/dietaryguidelines. htm. Accessed August 19, 2013.
7. American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(suppl 1): S61-S78.
1. McKown NM, Meigs JB, Liu S, et al. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham offspring cohort. Diabetes Care. 2004;27:538-546.
2. Radhika G, Van Dam RM, Sudha V, et al. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai urban rural epidemiology study 57). Metabolism. 2009;58:675-681.
3. Esmaillzadeh A, Kimiagar M, Mehrabi Y, et al. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr. 2007;85:910-918.
4. Azadbakht L, Mirmiran P, Azizi F. Dietary diversity score is favorably associated with the metabolic syndrome in Tehranian adults. Int J Obes (Lond). 2005;29:1361-1367.
5. Vrolix R, Mensink RP. Effects of glycemic load on metabolic risk markers in subjects at increased risk of developing metabolic syndrome. Am J Clin Nutr. 2010;92:366-374.
6. US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: US Government Printing Office; 2010. Available at: http://www.cnpp.usda.gov/dietaryguidelines. htm. Accessed August 19, 2013.
7. American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(suppl 1): S61-S78.
Evidence-based answers from the Family Physicians Inquiries Network
Do asymptomatic adults need screening EKGs?
PROBABLY NOT. Although certain electrocardiogram (EKG) findings in asymptomatic adults are associated with increased mortality (strength of recommendation [SOR]: A, high-quality cohort studies), no randomized trials demonstrate that any intervention based on abnormal screening EKGs improves outcomes in this group of patients. Comparison to a baseline EKG has a minimal effect on emergency department (ED) management.(SOR: B, 2 prospective studies and one retrospective study).
Evidence summary
The US Pooling Project divided EKG abnormalities into major and minor findings.1 A number of large cohort studies have shown that both major and minor findings are associated with an elevated odds ratio for mortality (TABLE).1-5 However, these studies, completed before the development of modern medical management of acute coronary syndrome and stable coronary artery disease, may no longer estimate mortality accurately. Moreover, no studies have examined the effect of screening EKGs on coronary heart disease (CHD) outcomes.
Neither major nor minor EKG abnormalities linked to higher mortality
A 2012 cohort study—which included Q-waves as major criteria and examined fewer minor abnormalities than previous studies—followed 2192 patients 70 to 79 years of age for 8 years.6 The study enrolled a higher percentage of women and blacks than earlier investigations had.
Major EKG abnormalities predicted an increase in CHD events (hazard ratio [HR]=1.51; 95% confidence interval [CI], 1.20-1.90) as did minor abnormalities (HR=1.35; 95% CI, 1.02-1.81). In contrast to earlier studies, which tended to enroll younger patients, neither type of abnormality was associated with a significantly increased risk of all-cause mortality.6
Including EKG abnormalities in a regression model of traditional risk factors improved stratification (overall net reclassification improvement [NRI]=7.4%; 95% CI, 3.1%-19.0%).6 No low-risk patients were reclassified as high risk and no high-risk patients were reclassified as low risk. Overall, 156 intermediate risk patients were correctly reclassified and an equal number were incorrectly reclassified. Adding EKG abnormalities to the Framingham Risk Score (which hasn’t been validated in adults >75 years) didn’t significantly improve stratification (NRI=5.7%; 95% CI, −0.4% to 11.8%).6
Comparing ED with baseline EKGs has little effect on management
A 1980 retrospective study looked at 236 patients with acute chest pain and no known CHD who were seen in the ED. Comparing routine baseline EKGs obtained before ED presentation for 6 of 41 patients with equivocal EKGs in the ED—including T-wave inversions, nonspecific T-wave and ST-segment abnormalities, and bundle branch blocks—prevented 2 admissions (no EKG change from baseline) and caused 4 unnecessary admissions (EKG changed from baseline with no subsequent evidence of acute coronary syndromes).7
A 1985 prospective study of 84 ED patients, in which treating physicians were given baseline EKGs after committing to an initial disposition plan, showed that the baseline EKG altered the decision to admit or discharge in only one case.8
A 1990 prospective multicenter study of 5673 patients older than 30 years—41% of whom had known CHD—reported that when the current EKG was consistent with ischemia or infarction, a baseline EKG showing the changes to be old (10% of study population) increased the likelihood that the patient would be discharged from the ED to home (26% vs 12%; risk difference=14%; 95% CI, 7%-23%). Unlike previous studies, however, the exact role of the baseline EKG in the admission decision was isolated not by study design but rather by multivariate logistic regression modeling.9
Recommendations
The 2010 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guideline for assessment of cardiovascular risk in asymptomatic adults states that a resting EKG is probably indicated in patients with diabetes and hypertension and that its usefulness in patients without these conditions isn’t well established.2
The US Preventive Services Task Force recommends against screening EKGs in adults at low risk for CHD events (grade D recommendation).10
1. Sosenko J, Gardner L. Relationship of blood pressure, serum cholesterol, smoking habit, relative weight and ECG abnormalities to incidence of major coronary events: final report of the pooling project. J Chronic Dis. 1978;31:201-306.
2. Greenland P, Alpert J, Beller G, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50-e103.
3. Sox HC Jr, Garber AM, Littenberg B. The resting electrocardiogram as a screening test. A clinical analysis. Ann Intern Med. 1989;111:489-502.
4. DeBaquer D, De Backer G, Kornitzer M, et al. Prognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and women. Heart. 1998;80: 570-577.
5. Whinnicup P, Goya W, Marcarlane P, et al. Resting electrocardiogram and risk of coronary heart disease in middle-aged British men. J Cardiovasc Risk. 1995;2:533-543.
6. Auer R, Bauer DC, Marques-Vidal P, et al. Association of major and minor ECG abnormalities with coronary heart disease events. JAMA. 2012;307:1497-1505.
7. Rubenstein LZ, Greenfield S. The baseline ECG in the evaluation of acute cardiac complaints. JAMA. 1980;244:2536-2539.
8. Hoffman JR, Igarashi E. Influence of electrocardiographic findings on admission decisions in patients with acute chest pain. Am J Med. 1985;79:699-707.
9. Lee TH, Cook EF, Weisberg MC, et al. Impact of the availability of a prior electrocardiogram on the triage of the patient with acute chest pain. J Gen Intern Med. 1990;5:381-388.
10. Chou R, Arora B, Dana T, et al. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2011;155:375-385.
PROBABLY NOT. Although certain electrocardiogram (EKG) findings in asymptomatic adults are associated with increased mortality (strength of recommendation [SOR]: A, high-quality cohort studies), no randomized trials demonstrate that any intervention based on abnormal screening EKGs improves outcomes in this group of patients. Comparison to a baseline EKG has a minimal effect on emergency department (ED) management.(SOR: B, 2 prospective studies and one retrospective study).
Evidence summary
The US Pooling Project divided EKG abnormalities into major and minor findings.1 A number of large cohort studies have shown that both major and minor findings are associated with an elevated odds ratio for mortality (TABLE).1-5 However, these studies, completed before the development of modern medical management of acute coronary syndrome and stable coronary artery disease, may no longer estimate mortality accurately. Moreover, no studies have examined the effect of screening EKGs on coronary heart disease (CHD) outcomes.
Neither major nor minor EKG abnormalities linked to higher mortality
A 2012 cohort study—which included Q-waves as major criteria and examined fewer minor abnormalities than previous studies—followed 2192 patients 70 to 79 years of age for 8 years.6 The study enrolled a higher percentage of women and blacks than earlier investigations had.
Major EKG abnormalities predicted an increase in CHD events (hazard ratio [HR]=1.51; 95% confidence interval [CI], 1.20-1.90) as did minor abnormalities (HR=1.35; 95% CI, 1.02-1.81). In contrast to earlier studies, which tended to enroll younger patients, neither type of abnormality was associated with a significantly increased risk of all-cause mortality.6
Including EKG abnormalities in a regression model of traditional risk factors improved stratification (overall net reclassification improvement [NRI]=7.4%; 95% CI, 3.1%-19.0%).6 No low-risk patients were reclassified as high risk and no high-risk patients were reclassified as low risk. Overall, 156 intermediate risk patients were correctly reclassified and an equal number were incorrectly reclassified. Adding EKG abnormalities to the Framingham Risk Score (which hasn’t been validated in adults >75 years) didn’t significantly improve stratification (NRI=5.7%; 95% CI, −0.4% to 11.8%).6
Comparing ED with baseline EKGs has little effect on management
A 1980 retrospective study looked at 236 patients with acute chest pain and no known CHD who were seen in the ED. Comparing routine baseline EKGs obtained before ED presentation for 6 of 41 patients with equivocal EKGs in the ED—including T-wave inversions, nonspecific T-wave and ST-segment abnormalities, and bundle branch blocks—prevented 2 admissions (no EKG change from baseline) and caused 4 unnecessary admissions (EKG changed from baseline with no subsequent evidence of acute coronary syndromes).7
A 1985 prospective study of 84 ED patients, in which treating physicians were given baseline EKGs after committing to an initial disposition plan, showed that the baseline EKG altered the decision to admit or discharge in only one case.8
A 1990 prospective multicenter study of 5673 patients older than 30 years—41% of whom had known CHD—reported that when the current EKG was consistent with ischemia or infarction, a baseline EKG showing the changes to be old (10% of study population) increased the likelihood that the patient would be discharged from the ED to home (26% vs 12%; risk difference=14%; 95% CI, 7%-23%). Unlike previous studies, however, the exact role of the baseline EKG in the admission decision was isolated not by study design but rather by multivariate logistic regression modeling.9
Recommendations
The 2010 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guideline for assessment of cardiovascular risk in asymptomatic adults states that a resting EKG is probably indicated in patients with diabetes and hypertension and that its usefulness in patients without these conditions isn’t well established.2
The US Preventive Services Task Force recommends against screening EKGs in adults at low risk for CHD events (grade D recommendation).10
PROBABLY NOT. Although certain electrocardiogram (EKG) findings in asymptomatic adults are associated with increased mortality (strength of recommendation [SOR]: A, high-quality cohort studies), no randomized trials demonstrate that any intervention based on abnormal screening EKGs improves outcomes in this group of patients. Comparison to a baseline EKG has a minimal effect on emergency department (ED) management.(SOR: B, 2 prospective studies and one retrospective study).
Evidence summary
The US Pooling Project divided EKG abnormalities into major and minor findings.1 A number of large cohort studies have shown that both major and minor findings are associated with an elevated odds ratio for mortality (TABLE).1-5 However, these studies, completed before the development of modern medical management of acute coronary syndrome and stable coronary artery disease, may no longer estimate mortality accurately. Moreover, no studies have examined the effect of screening EKGs on coronary heart disease (CHD) outcomes.
Neither major nor minor EKG abnormalities linked to higher mortality
A 2012 cohort study—which included Q-waves as major criteria and examined fewer minor abnormalities than previous studies—followed 2192 patients 70 to 79 years of age for 8 years.6 The study enrolled a higher percentage of women and blacks than earlier investigations had.
Major EKG abnormalities predicted an increase in CHD events (hazard ratio [HR]=1.51; 95% confidence interval [CI], 1.20-1.90) as did minor abnormalities (HR=1.35; 95% CI, 1.02-1.81). In contrast to earlier studies, which tended to enroll younger patients, neither type of abnormality was associated with a significantly increased risk of all-cause mortality.6
Including EKG abnormalities in a regression model of traditional risk factors improved stratification (overall net reclassification improvement [NRI]=7.4%; 95% CI, 3.1%-19.0%).6 No low-risk patients were reclassified as high risk and no high-risk patients were reclassified as low risk. Overall, 156 intermediate risk patients were correctly reclassified and an equal number were incorrectly reclassified. Adding EKG abnormalities to the Framingham Risk Score (which hasn’t been validated in adults >75 years) didn’t significantly improve stratification (NRI=5.7%; 95% CI, −0.4% to 11.8%).6
Comparing ED with baseline EKGs has little effect on management
A 1980 retrospective study looked at 236 patients with acute chest pain and no known CHD who were seen in the ED. Comparing routine baseline EKGs obtained before ED presentation for 6 of 41 patients with equivocal EKGs in the ED—including T-wave inversions, nonspecific T-wave and ST-segment abnormalities, and bundle branch blocks—prevented 2 admissions (no EKG change from baseline) and caused 4 unnecessary admissions (EKG changed from baseline with no subsequent evidence of acute coronary syndromes).7
A 1985 prospective study of 84 ED patients, in which treating physicians were given baseline EKGs after committing to an initial disposition plan, showed that the baseline EKG altered the decision to admit or discharge in only one case.8
A 1990 prospective multicenter study of 5673 patients older than 30 years—41% of whom had known CHD—reported that when the current EKG was consistent with ischemia or infarction, a baseline EKG showing the changes to be old (10% of study population) increased the likelihood that the patient would be discharged from the ED to home (26% vs 12%; risk difference=14%; 95% CI, 7%-23%). Unlike previous studies, however, the exact role of the baseline EKG in the admission decision was isolated not by study design but rather by multivariate logistic regression modeling.9
Recommendations
The 2010 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guideline for assessment of cardiovascular risk in asymptomatic adults states that a resting EKG is probably indicated in patients with diabetes and hypertension and that its usefulness in patients without these conditions isn’t well established.2
The US Preventive Services Task Force recommends against screening EKGs in adults at low risk for CHD events (grade D recommendation).10
1. Sosenko J, Gardner L. Relationship of blood pressure, serum cholesterol, smoking habit, relative weight and ECG abnormalities to incidence of major coronary events: final report of the pooling project. J Chronic Dis. 1978;31:201-306.
2. Greenland P, Alpert J, Beller G, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50-e103.
3. Sox HC Jr, Garber AM, Littenberg B. The resting electrocardiogram as a screening test. A clinical analysis. Ann Intern Med. 1989;111:489-502.
4. DeBaquer D, De Backer G, Kornitzer M, et al. Prognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and women. Heart. 1998;80: 570-577.
5. Whinnicup P, Goya W, Marcarlane P, et al. Resting electrocardiogram and risk of coronary heart disease in middle-aged British men. J Cardiovasc Risk. 1995;2:533-543.
6. Auer R, Bauer DC, Marques-Vidal P, et al. Association of major and minor ECG abnormalities with coronary heart disease events. JAMA. 2012;307:1497-1505.
7. Rubenstein LZ, Greenfield S. The baseline ECG in the evaluation of acute cardiac complaints. JAMA. 1980;244:2536-2539.
8. Hoffman JR, Igarashi E. Influence of electrocardiographic findings on admission decisions in patients with acute chest pain. Am J Med. 1985;79:699-707.
9. Lee TH, Cook EF, Weisberg MC, et al. Impact of the availability of a prior electrocardiogram on the triage of the patient with acute chest pain. J Gen Intern Med. 1990;5:381-388.
10. Chou R, Arora B, Dana T, et al. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2011;155:375-385.
1. Sosenko J, Gardner L. Relationship of blood pressure, serum cholesterol, smoking habit, relative weight and ECG abnormalities to incidence of major coronary events: final report of the pooling project. J Chronic Dis. 1978;31:201-306.
2. Greenland P, Alpert J, Beller G, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50-e103.
3. Sox HC Jr, Garber AM, Littenberg B. The resting electrocardiogram as a screening test. A clinical analysis. Ann Intern Med. 1989;111:489-502.
4. DeBaquer D, De Backer G, Kornitzer M, et al. Prognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and women. Heart. 1998;80: 570-577.
5. Whinnicup P, Goya W, Marcarlane P, et al. Resting electrocardiogram and risk of coronary heart disease in middle-aged British men. J Cardiovasc Risk. 1995;2:533-543.
6. Auer R, Bauer DC, Marques-Vidal P, et al. Association of major and minor ECG abnormalities with coronary heart disease events. JAMA. 2012;307:1497-1505.
7. Rubenstein LZ, Greenfield S. The baseline ECG in the evaluation of acute cardiac complaints. JAMA. 1980;244:2536-2539.
8. Hoffman JR, Igarashi E. Influence of electrocardiographic findings on admission decisions in patients with acute chest pain. Am J Med. 1985;79:699-707.
9. Lee TH, Cook EF, Weisberg MC, et al. Impact of the availability of a prior electrocardiogram on the triage of the patient with acute chest pain. J Gen Intern Med. 1990;5:381-388.
10. Chou R, Arora B, Dana T, et al. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2011;155:375-385.
Evidence-based answers from the Family Physicians Inquiries Network
Does metformin prevent diabetes in at-risk adults?
Yes. Metformin therapy reduces the risk of developing diabetes for adults with one or more risk factors for at least 5 years (strength of recommendation[SOR]: A, consistent meta-analyses) and perhaps as long as 10 years (SOR: B, randomized clinical trial[RCT]).
Lifestyle modification with diet and exercise is approximately twice as effective as metformin for preventing diabetes, especially in older patients (SOR:B,RCTs).
Evidence summary
Three meta-analyses of studies lasting from 2 months to 5 years found that metformin reduced the risk of developing overt diabetes in at-risk adults when compared with placebo (TABLE).1-3
Metformin is likely effective for as long as 10 years, based on long-term follow-up of patients in the Diabetes Prevention Program (DPP). In this trial, investigators randomized 3234 at-risk patients to 3 groups: metformin 850 mg twice daily; lifestyle modification (7% weight loss, 150 minutes of physical activity per week, and a one-to-one 16-lesson curriculum covering diet, exercise, and behavior modification); or placebo.4 At a mean 2.8-year follow-up, the incidence of diabetes was 31% lower in the metformin group (95% confidence interval [CI], 17%-43%) and 58% lower in the lifestyle modification group than in the placebo group (95% CI, 48%-66%; P<.001 for both comparisons).
At the close of the DPP trial, investigators offered lifestyle intervention to all 3 groups. Patients in the original metformin group continued to take metformin (with participants unblinded to assignment); patients in the original lifestyle intervention group were offered additional lifestyle support.5 At a median follow-up of 10 years after initial enrollment in the DPP trial, metformin reduced the incidence of overt diabetes by 18% compared with placebo (95% CI, 7%-28%), and lifestyle intervention reduced it by 34% (95% CI, 24%-42%; no statistic of comparison supplied).
Lifestyle modification works better than metformin in older adults
Another analysis found that metformin was equally effective in preventing diabetes in older and younger patients, whereas lifestyle modification was more effective in older patients. Investigators followed the patients in the DPP trial for an additional 5 months and stratified the effect of metformin and lifestyle modification by age.6
Metformin’s effectiveness didn’t change significantly in older adults compared with younger adults (hazard ratio [HR] for developing diabetes at age 60-85 years vs 25-44 years=1.45; 95% CI, 0.98-2.16; P=.06). In contrast, lifestyle modification worked better in older adults than younger adults (HR at age 60-85 years vs 25-44 years=0.47; 95% CI, 0.28- 0.78;P<.01).
Recommendations
The American Diabetes Association says that physicians may consider using metformin to prevent type 2 diabetes in patients at the highest risk, such as patients with multiple risk factors, especially if they show progression of hyperglycemia (HbA1c ≥6%, for example) despite lifestyle intervention.7
1. Lilly M, Godwin M. Treating prediabetes with metformin. Can Fam Physician. 2009;55:363-369.
2. Phung OJ, Sood A, Sill BE, et al. Oral anti-diabetic drugs for the prevention of type 2 diabetes. Diabet Med. 2011;28:948-964.
3. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: met- formin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121:149-157.
4. Knowler W, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
5. Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow- up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet. 2009;374: 1677-1686.
6. Crandall J, Schade D, Ma Y, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61:1075-1081.
7. American Diabetes Association. Executive summary: stan- dards of medical care in diabetes—2011. Diabetes Care. 2011;34(suppl 1):S4-S10.
Yes. Metformin therapy reduces the risk of developing diabetes for adults with one or more risk factors for at least 5 years (strength of recommendation[SOR]: A, consistent meta-analyses) and perhaps as long as 10 years (SOR: B, randomized clinical trial[RCT]).
Lifestyle modification with diet and exercise is approximately twice as effective as metformin for preventing diabetes, especially in older patients (SOR:B,RCTs).
Evidence summary
Three meta-analyses of studies lasting from 2 months to 5 years found that metformin reduced the risk of developing overt diabetes in at-risk adults when compared with placebo (TABLE).1-3
Metformin is likely effective for as long as 10 years, based on long-term follow-up of patients in the Diabetes Prevention Program (DPP). In this trial, investigators randomized 3234 at-risk patients to 3 groups: metformin 850 mg twice daily; lifestyle modification (7% weight loss, 150 minutes of physical activity per week, and a one-to-one 16-lesson curriculum covering diet, exercise, and behavior modification); or placebo.4 At a mean 2.8-year follow-up, the incidence of diabetes was 31% lower in the metformin group (95% confidence interval [CI], 17%-43%) and 58% lower in the lifestyle modification group than in the placebo group (95% CI, 48%-66%; P<.001 for both comparisons).
At the close of the DPP trial, investigators offered lifestyle intervention to all 3 groups. Patients in the original metformin group continued to take metformin (with participants unblinded to assignment); patients in the original lifestyle intervention group were offered additional lifestyle support.5 At a median follow-up of 10 years after initial enrollment in the DPP trial, metformin reduced the incidence of overt diabetes by 18% compared with placebo (95% CI, 7%-28%), and lifestyle intervention reduced it by 34% (95% CI, 24%-42%; no statistic of comparison supplied).
Lifestyle modification works better than metformin in older adults
Another analysis found that metformin was equally effective in preventing diabetes in older and younger patients, whereas lifestyle modification was more effective in older patients. Investigators followed the patients in the DPP trial for an additional 5 months and stratified the effect of metformin and lifestyle modification by age.6
Metformin’s effectiveness didn’t change significantly in older adults compared with younger adults (hazard ratio [HR] for developing diabetes at age 60-85 years vs 25-44 years=1.45; 95% CI, 0.98-2.16; P=.06). In contrast, lifestyle modification worked better in older adults than younger adults (HR at age 60-85 years vs 25-44 years=0.47; 95% CI, 0.28- 0.78;P<.01).
Recommendations
The American Diabetes Association says that physicians may consider using metformin to prevent type 2 diabetes in patients at the highest risk, such as patients with multiple risk factors, especially if they show progression of hyperglycemia (HbA1c ≥6%, for example) despite lifestyle intervention.7
Yes. Metformin therapy reduces the risk of developing diabetes for adults with one or more risk factors for at least 5 years (strength of recommendation[SOR]: A, consistent meta-analyses) and perhaps as long as 10 years (SOR: B, randomized clinical trial[RCT]).
Lifestyle modification with diet and exercise is approximately twice as effective as metformin for preventing diabetes, especially in older patients (SOR:B,RCTs).
Evidence summary
Three meta-analyses of studies lasting from 2 months to 5 years found that metformin reduced the risk of developing overt diabetes in at-risk adults when compared with placebo (TABLE).1-3
Metformin is likely effective for as long as 10 years, based on long-term follow-up of patients in the Diabetes Prevention Program (DPP). In this trial, investigators randomized 3234 at-risk patients to 3 groups: metformin 850 mg twice daily; lifestyle modification (7% weight loss, 150 minutes of physical activity per week, and a one-to-one 16-lesson curriculum covering diet, exercise, and behavior modification); or placebo.4 At a mean 2.8-year follow-up, the incidence of diabetes was 31% lower in the metformin group (95% confidence interval [CI], 17%-43%) and 58% lower in the lifestyle modification group than in the placebo group (95% CI, 48%-66%; P<.001 for both comparisons).
At the close of the DPP trial, investigators offered lifestyle intervention to all 3 groups. Patients in the original metformin group continued to take metformin (with participants unblinded to assignment); patients in the original lifestyle intervention group were offered additional lifestyle support.5 At a median follow-up of 10 years after initial enrollment in the DPP trial, metformin reduced the incidence of overt diabetes by 18% compared with placebo (95% CI, 7%-28%), and lifestyle intervention reduced it by 34% (95% CI, 24%-42%; no statistic of comparison supplied).
Lifestyle modification works better than metformin in older adults
Another analysis found that metformin was equally effective in preventing diabetes in older and younger patients, whereas lifestyle modification was more effective in older patients. Investigators followed the patients in the DPP trial for an additional 5 months and stratified the effect of metformin and lifestyle modification by age.6
Metformin’s effectiveness didn’t change significantly in older adults compared with younger adults (hazard ratio [HR] for developing diabetes at age 60-85 years vs 25-44 years=1.45; 95% CI, 0.98-2.16; P=.06). In contrast, lifestyle modification worked better in older adults than younger adults (HR at age 60-85 years vs 25-44 years=0.47; 95% CI, 0.28- 0.78;P<.01).
Recommendations
The American Diabetes Association says that physicians may consider using metformin to prevent type 2 diabetes in patients at the highest risk, such as patients with multiple risk factors, especially if they show progression of hyperglycemia (HbA1c ≥6%, for example) despite lifestyle intervention.7
1. Lilly M, Godwin M. Treating prediabetes with metformin. Can Fam Physician. 2009;55:363-369.
2. Phung OJ, Sood A, Sill BE, et al. Oral anti-diabetic drugs for the prevention of type 2 diabetes. Diabet Med. 2011;28:948-964.
3. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: met- formin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121:149-157.
4. Knowler W, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
5. Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow- up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet. 2009;374: 1677-1686.
6. Crandall J, Schade D, Ma Y, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61:1075-1081.
7. American Diabetes Association. Executive summary: stan- dards of medical care in diabetes—2011. Diabetes Care. 2011;34(suppl 1):S4-S10.
1. Lilly M, Godwin M. Treating prediabetes with metformin. Can Fam Physician. 2009;55:363-369.
2. Phung OJ, Sood A, Sill BE, et al. Oral anti-diabetic drugs for the prevention of type 2 diabetes. Diabet Med. 2011;28:948-964.
3. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: met- formin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121:149-157.
4. Knowler W, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
5. Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow- up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet. 2009;374: 1677-1686.
6. Crandall J, Schade D, Ma Y, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61:1075-1081.
7. American Diabetes Association. Executive summary: stan- dards of medical care in diabetes—2011. Diabetes Care. 2011;34(suppl 1):S4-S10.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best initial treatment for venous stasis ulcers?
THE MAINSTAY OF INITIAL TREATMENT of venous stasis ulcers is compression therapy (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]). Multicomponent compression therapy is slightly superior to single-component therapy (SOR: B, systematic review of RCTs with inconsistent results). The various types of dressings available for managing venous stasis ulcers aresimilarly efficacious (SOR: A, systematic review of RCTs).
Systemic therapies such as aspirin (SOR: B, single RCT) and pentoxifylline (SOR: A, systematic review of RCTs) improve healing rates whereas antibiotics don’t (SOR: A, systematic review of RCTs). Cadexomer iodine, a topical antiseptic, improves ulcer healing but may not be feasible in most clinical settings because of the frequent dressing changes required (SOR: B, single RCT).
Evidence summary
A systematic review found 7 RCTs with a total of 686 subjects that compared compression with no compression for venous leg ulcers.1 Although the outcome data were too heterogeneous for a meta-analysis, the 4 studies in which statistical analysis was possible showed that compression healed venous leg ulcers faster than no compression (relative risk [RR] range=1.2 to 4 in favor of compression), as detailed in the TABLE. Notably, only 2 of the studies achieved statistical significance (P<.05).
Two other studies in this review, with 20 and 245 patients, compared 4-component with single-component compression. In the smaller study, multicomponent compression produced more completely healed ulcers at 12 weeks, but the difference wasn’t statistically significant.
In the larger study, nonhealing at 24 weeks was much less common among patients treated with multicomponent compression than single-component therapy (RR=0.74; 95% confidence interval [CI], 0.59-0.92; number needed to treat [NNT]=5.7; P=.009), and median time to healing was shorter(78 vs 168 days; statistical significance not reported).
The reviewers concluded that compression increases ulcer healing rates compared with no compression and that multicomponent systems are more effective than single component systems.1
Similar results, different costs among dressing types
A systematic review and meta-analysis of 42 RCTs that included 3001 patients compared multiple dressing types, including hydrocolloid, foam, alginate, and low-adherent dressings, used beneath compression.2 The study found no significant differences in healing rates among the dressings, although costs varied widely.
Systemic therapy: Aspirin and pentoxifylline help
Systemic or topical treatments are an alternative for patients with contraindications or intolerance to compression. An RCT of 20 patients that compared aspirin 300 mg/day with placebo found higher ulcer healing rates in the aspirin group after 4 months (38% vs 0%; NNT=2.6; P<.007). Improvement, defined as reduction in ulcer size, occurred in 52%of patients treated with aspirin but only 26%treated with placebo(NNT=3.8;P<.007).3
A systematic review of 11 trials (N=841) found that pentoxifylline accelerated healing rates vs placebo (NNT=4; 95% CI, 3-6); the authors recommended its use in conjunction with compression therapy when possible.4
Another systematic review of 5 RCTs (N=232) found that systemic antibiotics didn’t improve outcomes significantly more than placebo.5
Topical cadexomer iodine: Effective, but is it feasible?
One of the 5 reviewed RCTs (60 patients) found that topical cadexomer iodine produced more frequent healing than standard care at 6 weeks (NNT=3;95%CI,2-19).5 However, the cadexomer regimen involved daily dressing changes, which might limit feasibility in many clinical settings.
Systemic aspirin and pentoxifylline improve healing rates, but systemic antibiotics don’t. Other interventions to consider for venous ulcers include hyperbaric oxygen and venous surgery.
Recommendations
The Association for the Advancement of Wound Care recommends compression therapy and limb elevation to reduce edema. They also recommend cleaning the ulcer with a safe cleanser, debriding nonvital tissue, maintaining a moist wound environment, and managing pain and odor.6
The Wound, Ostomy, and Continence Nurses Society and the American Society of Plastic Surgeons make similar recommendations: ulcer debridement, edema management, infection control, and pain management.7,8
1. O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2009;(1):CD000265.
2. Palfreyman SJ, Nelson EA, Lochiel R, et al. Dressings for healing venous leg ulcers. Cochrane Database Syst Rev. 2006;(3):CD001103.
3. Layton AM, Ibbotson SH, Davies JA, et al. Randomized trial of oral aspirin for chronic venous leg ulcers. Lancet. 1994;344:164-165.
4. JullAB,ArrollB, ParagV, et al. Pentoxifylline fortreating venous leg ulcers. Cochrane Database Syst Rev. 2007;(3):CD001733.
5. O’Meara S, Al-Kurdi D, Ovington LG, et al. Antibiotics and antiseptics for venous leg ulcers. CochraneDatabase Syst Rev. 2010;(1):CD003557.
6. Association fortheAdvancement of WoundCare (AAWC) venous ulcer guideline. 2010. Available at: http://aawconline.org/wpcontent/uploads/2012/03/AAWC-Venous-UlcerGuideline-Update+Algorithm-v28.pdf. Accessed November 16, 2012.
7. Wound, Ostomy, and Continence Nurses Society. Guideline for management of wounds in patients with lower-extremity venous disease. 2011. Available at: http://guideline.gov/content.aspx?id=38249. Accessed November 16, 2012.
8. American Society of Plastic Surgeons. Evidence-based clinicalpracticeguideline:Chronicwoundsofthelowerextremity. 2007. Available at: http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/evidencepractice/Evidence-based-Clinical-Practice-GuidelineChronic-Wounds-of-the-Lower-Extremity.pdf. Accessed November 16, 2012.
THE MAINSTAY OF INITIAL TREATMENT of venous stasis ulcers is compression therapy (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]). Multicomponent compression therapy is slightly superior to single-component therapy (SOR: B, systematic review of RCTs with inconsistent results). The various types of dressings available for managing venous stasis ulcers aresimilarly efficacious (SOR: A, systematic review of RCTs).
Systemic therapies such as aspirin (SOR: B, single RCT) and pentoxifylline (SOR: A, systematic review of RCTs) improve healing rates whereas antibiotics don’t (SOR: A, systematic review of RCTs). Cadexomer iodine, a topical antiseptic, improves ulcer healing but may not be feasible in most clinical settings because of the frequent dressing changes required (SOR: B, single RCT).
Evidence summary
A systematic review found 7 RCTs with a total of 686 subjects that compared compression with no compression for venous leg ulcers.1 Although the outcome data were too heterogeneous for a meta-analysis, the 4 studies in which statistical analysis was possible showed that compression healed venous leg ulcers faster than no compression (relative risk [RR] range=1.2 to 4 in favor of compression), as detailed in the TABLE. Notably, only 2 of the studies achieved statistical significance (P<.05).
Two other studies in this review, with 20 and 245 patients, compared 4-component with single-component compression. In the smaller study, multicomponent compression produced more completely healed ulcers at 12 weeks, but the difference wasn’t statistically significant.
In the larger study, nonhealing at 24 weeks was much less common among patients treated with multicomponent compression than single-component therapy (RR=0.74; 95% confidence interval [CI], 0.59-0.92; number needed to treat [NNT]=5.7; P=.009), and median time to healing was shorter(78 vs 168 days; statistical significance not reported).
The reviewers concluded that compression increases ulcer healing rates compared with no compression and that multicomponent systems are more effective than single component systems.1
Similar results, different costs among dressing types
A systematic review and meta-analysis of 42 RCTs that included 3001 patients compared multiple dressing types, including hydrocolloid, foam, alginate, and low-adherent dressings, used beneath compression.2 The study found no significant differences in healing rates among the dressings, although costs varied widely.
Systemic therapy: Aspirin and pentoxifylline help
Systemic or topical treatments are an alternative for patients with contraindications or intolerance to compression. An RCT of 20 patients that compared aspirin 300 mg/day with placebo found higher ulcer healing rates in the aspirin group after 4 months (38% vs 0%; NNT=2.6; P<.007). Improvement, defined as reduction in ulcer size, occurred in 52%of patients treated with aspirin but only 26%treated with placebo(NNT=3.8;P<.007).3
A systematic review of 11 trials (N=841) found that pentoxifylline accelerated healing rates vs placebo (NNT=4; 95% CI, 3-6); the authors recommended its use in conjunction with compression therapy when possible.4
Another systematic review of 5 RCTs (N=232) found that systemic antibiotics didn’t improve outcomes significantly more than placebo.5
Topical cadexomer iodine: Effective, but is it feasible?
One of the 5 reviewed RCTs (60 patients) found that topical cadexomer iodine produced more frequent healing than standard care at 6 weeks (NNT=3;95%CI,2-19).5 However, the cadexomer regimen involved daily dressing changes, which might limit feasibility in many clinical settings.
Systemic aspirin and pentoxifylline improve healing rates, but systemic antibiotics don’t. Other interventions to consider for venous ulcers include hyperbaric oxygen and venous surgery.
Recommendations
The Association for the Advancement of Wound Care recommends compression therapy and limb elevation to reduce edema. They also recommend cleaning the ulcer with a safe cleanser, debriding nonvital tissue, maintaining a moist wound environment, and managing pain and odor.6
The Wound, Ostomy, and Continence Nurses Society and the American Society of Plastic Surgeons make similar recommendations: ulcer debridement, edema management, infection control, and pain management.7,8
THE MAINSTAY OF INITIAL TREATMENT of venous stasis ulcers is compression therapy (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]). Multicomponent compression therapy is slightly superior to single-component therapy (SOR: B, systematic review of RCTs with inconsistent results). The various types of dressings available for managing venous stasis ulcers aresimilarly efficacious (SOR: A, systematic review of RCTs).
Systemic therapies such as aspirin (SOR: B, single RCT) and pentoxifylline (SOR: A, systematic review of RCTs) improve healing rates whereas antibiotics don’t (SOR: A, systematic review of RCTs). Cadexomer iodine, a topical antiseptic, improves ulcer healing but may not be feasible in most clinical settings because of the frequent dressing changes required (SOR: B, single RCT).
Evidence summary
A systematic review found 7 RCTs with a total of 686 subjects that compared compression with no compression for venous leg ulcers.1 Although the outcome data were too heterogeneous for a meta-analysis, the 4 studies in which statistical analysis was possible showed that compression healed venous leg ulcers faster than no compression (relative risk [RR] range=1.2 to 4 in favor of compression), as detailed in the TABLE. Notably, only 2 of the studies achieved statistical significance (P<.05).
Two other studies in this review, with 20 and 245 patients, compared 4-component with single-component compression. In the smaller study, multicomponent compression produced more completely healed ulcers at 12 weeks, but the difference wasn’t statistically significant.
In the larger study, nonhealing at 24 weeks was much less common among patients treated with multicomponent compression than single-component therapy (RR=0.74; 95% confidence interval [CI], 0.59-0.92; number needed to treat [NNT]=5.7; P=.009), and median time to healing was shorter(78 vs 168 days; statistical significance not reported).
The reviewers concluded that compression increases ulcer healing rates compared with no compression and that multicomponent systems are more effective than single component systems.1
Similar results, different costs among dressing types
A systematic review and meta-analysis of 42 RCTs that included 3001 patients compared multiple dressing types, including hydrocolloid, foam, alginate, and low-adherent dressings, used beneath compression.2 The study found no significant differences in healing rates among the dressings, although costs varied widely.
Systemic therapy: Aspirin and pentoxifylline help
Systemic or topical treatments are an alternative for patients with contraindications or intolerance to compression. An RCT of 20 patients that compared aspirin 300 mg/day with placebo found higher ulcer healing rates in the aspirin group after 4 months (38% vs 0%; NNT=2.6; P<.007). Improvement, defined as reduction in ulcer size, occurred in 52%of patients treated with aspirin but only 26%treated with placebo(NNT=3.8;P<.007).3
A systematic review of 11 trials (N=841) found that pentoxifylline accelerated healing rates vs placebo (NNT=4; 95% CI, 3-6); the authors recommended its use in conjunction with compression therapy when possible.4
Another systematic review of 5 RCTs (N=232) found that systemic antibiotics didn’t improve outcomes significantly more than placebo.5
Topical cadexomer iodine: Effective, but is it feasible?
One of the 5 reviewed RCTs (60 patients) found that topical cadexomer iodine produced more frequent healing than standard care at 6 weeks (NNT=3;95%CI,2-19).5 However, the cadexomer regimen involved daily dressing changes, which might limit feasibility in many clinical settings.
Systemic aspirin and pentoxifylline improve healing rates, but systemic antibiotics don’t. Other interventions to consider for venous ulcers include hyperbaric oxygen and venous surgery.
Recommendations
The Association for the Advancement of Wound Care recommends compression therapy and limb elevation to reduce edema. They also recommend cleaning the ulcer with a safe cleanser, debriding nonvital tissue, maintaining a moist wound environment, and managing pain and odor.6
The Wound, Ostomy, and Continence Nurses Society and the American Society of Plastic Surgeons make similar recommendations: ulcer debridement, edema management, infection control, and pain management.7,8
1. O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2009;(1):CD000265.
2. Palfreyman SJ, Nelson EA, Lochiel R, et al. Dressings for healing venous leg ulcers. Cochrane Database Syst Rev. 2006;(3):CD001103.
3. Layton AM, Ibbotson SH, Davies JA, et al. Randomized trial of oral aspirin for chronic venous leg ulcers. Lancet. 1994;344:164-165.
4. JullAB,ArrollB, ParagV, et al. Pentoxifylline fortreating venous leg ulcers. Cochrane Database Syst Rev. 2007;(3):CD001733.
5. O’Meara S, Al-Kurdi D, Ovington LG, et al. Antibiotics and antiseptics for venous leg ulcers. CochraneDatabase Syst Rev. 2010;(1):CD003557.
6. Association fortheAdvancement of WoundCare (AAWC) venous ulcer guideline. 2010. Available at: http://aawconline.org/wpcontent/uploads/2012/03/AAWC-Venous-UlcerGuideline-Update+Algorithm-v28.pdf. Accessed November 16, 2012.
7. Wound, Ostomy, and Continence Nurses Society. Guideline for management of wounds in patients with lower-extremity venous disease. 2011. Available at: http://guideline.gov/content.aspx?id=38249. Accessed November 16, 2012.
8. American Society of Plastic Surgeons. Evidence-based clinicalpracticeguideline:Chronicwoundsofthelowerextremity. 2007. Available at: http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/evidencepractice/Evidence-based-Clinical-Practice-GuidelineChronic-Wounds-of-the-Lower-Extremity.pdf. Accessed November 16, 2012.
1. O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2009;(1):CD000265.
2. Palfreyman SJ, Nelson EA, Lochiel R, et al. Dressings for healing venous leg ulcers. Cochrane Database Syst Rev. 2006;(3):CD001103.
3. Layton AM, Ibbotson SH, Davies JA, et al. Randomized trial of oral aspirin for chronic venous leg ulcers. Lancet. 1994;344:164-165.
4. JullAB,ArrollB, ParagV, et al. Pentoxifylline fortreating venous leg ulcers. Cochrane Database Syst Rev. 2007;(3):CD001733.
5. O’Meara S, Al-Kurdi D, Ovington LG, et al. Antibiotics and antiseptics for venous leg ulcers. CochraneDatabase Syst Rev. 2010;(1):CD003557.
6. Association fortheAdvancement of WoundCare (AAWC) venous ulcer guideline. 2010. Available at: http://aawconline.org/wpcontent/uploads/2012/03/AAWC-Venous-UlcerGuideline-Update+Algorithm-v28.pdf. Accessed November 16, 2012.
7. Wound, Ostomy, and Continence Nurses Society. Guideline for management of wounds in patients with lower-extremity venous disease. 2011. Available at: http://guideline.gov/content.aspx?id=38249. Accessed November 16, 2012.
8. American Society of Plastic Surgeons. Evidence-based clinicalpracticeguideline:Chronicwoundsofthelowerextremity. 2007. Available at: http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/evidencepractice/Evidence-based-Clinical-Practice-GuidelineChronic-Wounds-of-the-Lower-Extremity.pdf. Accessed November 16, 2012.
Evidence-based answers from the Family Physicians Inquiries Network
How best to treat agitation in patients with irreversible dementia?
Atypical antipsychotics modestly reduce agitation compared with placebo but have significant adverse effects (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Haloperidol doesn’t reduce symptoms and has serious adverse effects (SOR: A, systematic reviews of RCTs).
Selective serotonin reuptake inhibitors (SSRIs) and melatonin—although well tolerated—don’t reduce agitation (SOR: B, extrapolated data from systematic reviews of RCTs).
Evidence summary
A meta-analysis by the Agency for Healthcare Research and Quality of 37 RCTs examined off-label use of atypical antipsychotics in a total of 5364 patients.1 Pooled results from 17 RCTs showed a statistically significant but clinically modest difference between atypical antipsychotics and placebo for agitation; the standard mean difference was 0.22 (95% confidence interval [CI], 0.09-0.35). Investigators found statistically significant but small effect sizes for aripiprazole, olanzapine, and risperidone.
Atypical antipsychotics are associated with serious adverse cerebrovascular events and extrapyramidal symptoms. A meta-analysis of 17 RCTs (N= 5106) demonstrated that patients who received antipsychotics had higher mortality than patients who received placebo (3.5% vs 2.3%).2
Haloperidol has significant adverse effects without significant results
A systematic review of 5 RCTs compared haloperidol with placebo over 3 to 16 weeks in 856 patients ages 72 to 81 years with dementia and agitation.3 When investigators pooled results from 3 RCTs (N=690) using an intention-to-treat analysis and 3 assessment tools, they found that haloperidol produced a statistically significant, but not clinically meaningful, standard mean difference in aggression.
Adverse effects included extrapyramidal symptoms (odds ratio [OR]=2.34; 95% CI, 1.25-4.38; number needed to harm [NNH]=6), somnolence (OR=4.20; 95% CI, 1.78-9.91; NNH=8), and fatigue (OR=5.39; 95% CI, 2.04-14.22; NNH=3). Most studies were underpowered, didn’t document randomization, and had dropout rates as high as 20%.
Antidepressants have no effect
A systematic review of 9 RCTs involving 692 patients with dementia compared antidepressants with placebo, other antidepressants, and antipsychotics using various neuropsychiatric symptom scales.4 Investigators performed meta-analyses for numerous outcomes but found none of clinical or statistical significance.
Pooled analysis of 2 RCTs that examined a total of 250 outpatients with Alzheimer’s disease found that sertraline and fluoxetine produced a statistically, but not clinically, significant difference in the Cohen Mansfield Agitation Inventory total score. One RCT (N=52) demonstrated that citalopram improved the Neurobehavioral Rating Scale total score after adjusting for baseline severity.
Investigators found no difference in withdrawal rates between SSRIs and placebo (relative risk=1.07; 95% CI, 0.55-2.11). All studies had multiple methodological limitations.
Melatonin has no adverse effects, but no benefit either
A systematic review that included 2 RCTs compared melatonin with placebo for agitation in 121 patients ages 77 to 79 years with dementia.5 Investigators prescribed melatonin for periods of 4 to 7 weeks and found reductions in agitation that were statistically significant, but not clinically meaningful. They reported no adverse events. The studies had a low risk of bias.
Recommendations
The American Psychiatric Association (APA) advocates evaluating and treating secondary causes of agitation and using environmental and behavioral measures to reduce agitation.6 The APA advocates using the lowest effective dosages of antipsychotics after considering adverse effect profiles and the risks of not treating.
The APA recommends benzodiazepines to treat prominent anxiety or infrequent agitation, preferably lorazepam and oxazepam rather than diazepam or clonazepam and suggests trazodone or SSRIs as alternative therapy for agitation in patients without psychosis or those who are intolerant to antipsychotics.6
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review Number 43. Executive Summary. Rockville, Md: Agency for Healthcare Research and Quality; 2011.
2. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo controlled trials. JAMA. 2005;295:1934-1943.
3. Lonergan E, Luxenberg J, Colford JM, et al. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
4. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2):CD008191.
5. Jansen SL, Forbes D, Duncan V, et al. Melatonin for the treatment of dementia. Cochrane Database Syst Rev. 2006;(1):CD003802.
6. American Psychiatric Association Work Group on Alzheimer’s Disease and Other Dementias. Practice guidelines for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. Available at: http://psychiatryonline.org/
pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed April 3, 2013.
Atypical antipsychotics modestly reduce agitation compared with placebo but have significant adverse effects (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Haloperidol doesn’t reduce symptoms and has serious adverse effects (SOR: A, systematic reviews of RCTs).
Selective serotonin reuptake inhibitors (SSRIs) and melatonin—although well tolerated—don’t reduce agitation (SOR: B, extrapolated data from systematic reviews of RCTs).
Evidence summary
A meta-analysis by the Agency for Healthcare Research and Quality of 37 RCTs examined off-label use of atypical antipsychotics in a total of 5364 patients.1 Pooled results from 17 RCTs showed a statistically significant but clinically modest difference between atypical antipsychotics and placebo for agitation; the standard mean difference was 0.22 (95% confidence interval [CI], 0.09-0.35). Investigators found statistically significant but small effect sizes for aripiprazole, olanzapine, and risperidone.
Atypical antipsychotics are associated with serious adverse cerebrovascular events and extrapyramidal symptoms. A meta-analysis of 17 RCTs (N= 5106) demonstrated that patients who received antipsychotics had higher mortality than patients who received placebo (3.5% vs 2.3%).2
Haloperidol has significant adverse effects without significant results
A systematic review of 5 RCTs compared haloperidol with placebo over 3 to 16 weeks in 856 patients ages 72 to 81 years with dementia and agitation.3 When investigators pooled results from 3 RCTs (N=690) using an intention-to-treat analysis and 3 assessment tools, they found that haloperidol produced a statistically significant, but not clinically meaningful, standard mean difference in aggression.
Adverse effects included extrapyramidal symptoms (odds ratio [OR]=2.34; 95% CI, 1.25-4.38; number needed to harm [NNH]=6), somnolence (OR=4.20; 95% CI, 1.78-9.91; NNH=8), and fatigue (OR=5.39; 95% CI, 2.04-14.22; NNH=3). Most studies were underpowered, didn’t document randomization, and had dropout rates as high as 20%.
Antidepressants have no effect
A systematic review of 9 RCTs involving 692 patients with dementia compared antidepressants with placebo, other antidepressants, and antipsychotics using various neuropsychiatric symptom scales.4 Investigators performed meta-analyses for numerous outcomes but found none of clinical or statistical significance.
Pooled analysis of 2 RCTs that examined a total of 250 outpatients with Alzheimer’s disease found that sertraline and fluoxetine produced a statistically, but not clinically, significant difference in the Cohen Mansfield Agitation Inventory total score. One RCT (N=52) demonstrated that citalopram improved the Neurobehavioral Rating Scale total score after adjusting for baseline severity.
Investigators found no difference in withdrawal rates between SSRIs and placebo (relative risk=1.07; 95% CI, 0.55-2.11). All studies had multiple methodological limitations.
Melatonin has no adverse effects, but no benefit either
A systematic review that included 2 RCTs compared melatonin with placebo for agitation in 121 patients ages 77 to 79 years with dementia.5 Investigators prescribed melatonin for periods of 4 to 7 weeks and found reductions in agitation that were statistically significant, but not clinically meaningful. They reported no adverse events. The studies had a low risk of bias.
Recommendations
The American Psychiatric Association (APA) advocates evaluating and treating secondary causes of agitation and using environmental and behavioral measures to reduce agitation.6 The APA advocates using the lowest effective dosages of antipsychotics after considering adverse effect profiles and the risks of not treating.
The APA recommends benzodiazepines to treat prominent anxiety or infrequent agitation, preferably lorazepam and oxazepam rather than diazepam or clonazepam and suggests trazodone or SSRIs as alternative therapy for agitation in patients without psychosis or those who are intolerant to antipsychotics.6
Atypical antipsychotics modestly reduce agitation compared with placebo but have significant adverse effects (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Haloperidol doesn’t reduce symptoms and has serious adverse effects (SOR: A, systematic reviews of RCTs).
Selective serotonin reuptake inhibitors (SSRIs) and melatonin—although well tolerated—don’t reduce agitation (SOR: B, extrapolated data from systematic reviews of RCTs).
Evidence summary
A meta-analysis by the Agency for Healthcare Research and Quality of 37 RCTs examined off-label use of atypical antipsychotics in a total of 5364 patients.1 Pooled results from 17 RCTs showed a statistically significant but clinically modest difference between atypical antipsychotics and placebo for agitation; the standard mean difference was 0.22 (95% confidence interval [CI], 0.09-0.35). Investigators found statistically significant but small effect sizes for aripiprazole, olanzapine, and risperidone.
Atypical antipsychotics are associated with serious adverse cerebrovascular events and extrapyramidal symptoms. A meta-analysis of 17 RCTs (N= 5106) demonstrated that patients who received antipsychotics had higher mortality than patients who received placebo (3.5% vs 2.3%).2
Haloperidol has significant adverse effects without significant results
A systematic review of 5 RCTs compared haloperidol with placebo over 3 to 16 weeks in 856 patients ages 72 to 81 years with dementia and agitation.3 When investigators pooled results from 3 RCTs (N=690) using an intention-to-treat analysis and 3 assessment tools, they found that haloperidol produced a statistically significant, but not clinically meaningful, standard mean difference in aggression.
Adverse effects included extrapyramidal symptoms (odds ratio [OR]=2.34; 95% CI, 1.25-4.38; number needed to harm [NNH]=6), somnolence (OR=4.20; 95% CI, 1.78-9.91; NNH=8), and fatigue (OR=5.39; 95% CI, 2.04-14.22; NNH=3). Most studies were underpowered, didn’t document randomization, and had dropout rates as high as 20%.
Antidepressants have no effect
A systematic review of 9 RCTs involving 692 patients with dementia compared antidepressants with placebo, other antidepressants, and antipsychotics using various neuropsychiatric symptom scales.4 Investigators performed meta-analyses for numerous outcomes but found none of clinical or statistical significance.
Pooled analysis of 2 RCTs that examined a total of 250 outpatients with Alzheimer’s disease found that sertraline and fluoxetine produced a statistically, but not clinically, significant difference in the Cohen Mansfield Agitation Inventory total score. One RCT (N=52) demonstrated that citalopram improved the Neurobehavioral Rating Scale total score after adjusting for baseline severity.
Investigators found no difference in withdrawal rates between SSRIs and placebo (relative risk=1.07; 95% CI, 0.55-2.11). All studies had multiple methodological limitations.
Melatonin has no adverse effects, but no benefit either
A systematic review that included 2 RCTs compared melatonin with placebo for agitation in 121 patients ages 77 to 79 years with dementia.5 Investigators prescribed melatonin for periods of 4 to 7 weeks and found reductions in agitation that were statistically significant, but not clinically meaningful. They reported no adverse events. The studies had a low risk of bias.
Recommendations
The American Psychiatric Association (APA) advocates evaluating and treating secondary causes of agitation and using environmental and behavioral measures to reduce agitation.6 The APA advocates using the lowest effective dosages of antipsychotics after considering adverse effect profiles and the risks of not treating.
The APA recommends benzodiazepines to treat prominent anxiety or infrequent agitation, preferably lorazepam and oxazepam rather than diazepam or clonazepam and suggests trazodone or SSRIs as alternative therapy for agitation in patients without psychosis or those who are intolerant to antipsychotics.6
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review Number 43. Executive Summary. Rockville, Md: Agency for Healthcare Research and Quality; 2011.
2. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo controlled trials. JAMA. 2005;295:1934-1943.
3. Lonergan E, Luxenberg J, Colford JM, et al. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
4. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2):CD008191.
5. Jansen SL, Forbes D, Duncan V, et al. Melatonin for the treatment of dementia. Cochrane Database Syst Rev. 2006;(1):CD003802.
6. American Psychiatric Association Work Group on Alzheimer’s Disease and Other Dementias. Practice guidelines for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. Available at: http://psychiatryonline.org/
pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed April 3, 2013.
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review Number 43. Executive Summary. Rockville, Md: Agency for Healthcare Research and Quality; 2011.
2. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo controlled trials. JAMA. 2005;295:1934-1943.
3. Lonergan E, Luxenberg J, Colford JM, et al. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
4. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2):CD008191.
5. Jansen SL, Forbes D, Duncan V, et al. Melatonin for the treatment of dementia. Cochrane Database Syst Rev. 2006;(1):CD003802.
6. American Psychiatric Association Work Group on Alzheimer’s Disease and Other Dementias. Practice guidelines for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. Available at: http://psychiatryonline.org/
pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed April 3, 2013.
Evidence-based answers from the Family Physicians Inquiries Network