User login
Does blood pressure screening benefit children?
SCREENING MAY NOT SHOW BENEFITS in childhood but could pay off for adults. Although major professional organizations recommend measuring blood pressure (BP) at every clinic visit for all children older than 3 years (strength of recommendation [SOR]: C, expert opinion), scant evidence links earlier detection and treatment of childhood hypertension with improved patient-oriented outcomes.
However, detecting childhood hypertension may help identify adults who would benefit from earlier treatment. Children with elevated BP have a more than 60% chance of being hypertensive as young adults (SOR: B, prospective cohort study). Children with systolic BP above the 95th percentile had a more than 4-fold increase in coronary artery disease as adults compared with children below the 95th percentile (SOR: B, retrospective study).
Identifying hypertension in children is associated with a 15-fold greater likelihood of hypertension in their parents (SOR: B, case series).
Evidence summary
The US Preventive Services Task Force considers screening adults for hypertension a grade A recommendation because it’s known to improve patient outcomes through early diagnosis, treatment, and prevention of serious cardiovascular complications.1
The Fourth Task Force of the National High Blood Pressure Education Program Working Group, endorsed by the American Academy of Pediatrics, states that maintaining a large national database of BP values throughout childhood allows physicians to recognize children and adolescents with elevated BP.2 Data indicate that, in this population, the prevalence of prehypertension is 10% and the prevalence of hypertension is 4%.3
The Task Force suggests that detecting and treating childhood hypertension should be important because of increasing childhood obesity, the risk of developing left ventricular hypertrophy, and other intermediate cardiovascular effects in undiagnosed and untreated children. The Task Force acknowledges, however, that prospective longitudinal outcome studies in untreated children and adolescents are lacking.
Hypertensive children often grow up to be hypertensive adults
A prospective cohort study showed that children with elevated BP had a greater likelihood of adult hypertension than children with normal BP. Investigators followed 2445 children 7 to 18 years of age to determine whether elevated BP in childhood correlated with increased BP in adulthood.
Investigators obtained BP, height, and weight measurements biennially during the children’s school years and when they were young adults between 20 and 30 years of age. Twelve to 13 years later, 24% of children with BP above the 90th percentile still had BP above the 90th percentile (relative risk [RR]=2.4; P<.001) and 39% had BP above the 80th percentile (RR=1.9; P<.001). Ninety-four percent of children with more than 3 normal readings during the study were normotensive as young adults. Children with one or 2 abnormal readings had a 17% and 24% chance, respectively, of having hypertension as adults (P<.001).4
High childhood systolic BP may predict CAD in adulthood
A retrospective study evaluated 126 children 10 to 17 years of age who were admitted to the hospital for an elective surgical procedure between 1950 and 1967. Children with documented BP readings at admission were eligible for the study; children with preexisting cardiac and renal disease were excluded. Investigators reassessed patients as adults (age range 42-68 years); the mean follow-up period was 42 years.
Mean BP was 125/80 mm Hg at admission and 133/75 mm Hg at follow-up. Univariate logistic regression analysis showed a significant association between systolic BP in childhood and coronary artery disease at follow-up (odds ratio [OR]=1.052; 95% confidence interval [CI], 1.005-1.101; P=.027). Children with systolic BP at or above the 95th percentile had a 4-fold increase in coronary artery disease at follow-up compared with children whose systolic BP was below the 95th percentile (29% vs 7%, P=.03). Investigators also found an association between elevated BP in childhood and a diagnosis of hypertension at follow-up (P=.007).
Limitations of the study included small sample size, selection bias, changes in the definition of hypertension during the 4 decades since the study began, and limited childhood BP data (a single measurement at admission for surgery).5
Parents of hypertensive children are likely to be hypertensive themselves
Screening BP in children has the potential to identify families at increased risk for cardiovascular disease. A case series found a high incidence of hypertension among the parents of children with elevated BP. Investigators measured several risk factors, including BP in 141 children (mean age 10.5±3.4 years) and 108 parents (at least one a biological parent, mean age 38.5±7.5 years). They obtained 2 BP readings 15 to 30 minutes apart.
Parents of children with BPs at or above the 95th percentile had a 15-fold greater likelihood of hypertension themselves (OR=14.7; 95% CI, 3.02-71.56; P=.009, positive predictive value=75%; negative predictive value=81%).6 Limitations of the study included small sample size, high prevalence of obesity and black ethnicity in the study population (a population with a greater incidence of hypertension), and only 2 BP measurements in the same day, which isn’t diagnostic for hypertension.
Recommendations
The American College of Obstetricians and Gynecologists recommends screening girls for hypertension between 13 and 15 years of age.7
The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease.8
The European Society of Hypertension and European Society of Cardiology recommend that children older than 3 years have auscultatory BP measurements at each clinic visit.9
1. US Preventive Services Task Force. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed June 19, 2012.
2. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl):555-576.
3. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
4. Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633-641.
5. Erlingsdottir A, Indridason OS, Thorvaldsson O, et al. Blood pressure in children and target-organ damage later in life. Pediatr Nephrol. 2010;25:323-328.
6. Reis EC, Kip KE, Marroquin OC, et al. Screening children to identify families at increased risk for cardiovascular disease. Pediatrics. 2006;118:e1789-e1797.
7. American Congress of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 452: Primary and preventive care: periodic assessments. Obstet Gynecol. 2009;114:1444-1451.
8. American Academy of Family Physicians. Hypertension. Available at http://www.aafp.org/online/en/home/clinical/exam/hypertension.html. Accessed April 16, 2012.
9. Lurbe E, Cifkova R, Cruickshank J, et al. Management of high blood pressure in children and adolescents: recommendation of the European Society of Hypertension. J Hypertens. 2009;27:1719-1742.
SCREENING MAY NOT SHOW BENEFITS in childhood but could pay off for adults. Although major professional organizations recommend measuring blood pressure (BP) at every clinic visit for all children older than 3 years (strength of recommendation [SOR]: C, expert opinion), scant evidence links earlier detection and treatment of childhood hypertension with improved patient-oriented outcomes.
However, detecting childhood hypertension may help identify adults who would benefit from earlier treatment. Children with elevated BP have a more than 60% chance of being hypertensive as young adults (SOR: B, prospective cohort study). Children with systolic BP above the 95th percentile had a more than 4-fold increase in coronary artery disease as adults compared with children below the 95th percentile (SOR: B, retrospective study).
Identifying hypertension in children is associated with a 15-fold greater likelihood of hypertension in their parents (SOR: B, case series).
Evidence summary
The US Preventive Services Task Force considers screening adults for hypertension a grade A recommendation because it’s known to improve patient outcomes through early diagnosis, treatment, and prevention of serious cardiovascular complications.1
The Fourth Task Force of the National High Blood Pressure Education Program Working Group, endorsed by the American Academy of Pediatrics, states that maintaining a large national database of BP values throughout childhood allows physicians to recognize children and adolescents with elevated BP.2 Data indicate that, in this population, the prevalence of prehypertension is 10% and the prevalence of hypertension is 4%.3
The Task Force suggests that detecting and treating childhood hypertension should be important because of increasing childhood obesity, the risk of developing left ventricular hypertrophy, and other intermediate cardiovascular effects in undiagnosed and untreated children. The Task Force acknowledges, however, that prospective longitudinal outcome studies in untreated children and adolescents are lacking.
Hypertensive children often grow up to be hypertensive adults
A prospective cohort study showed that children with elevated BP had a greater likelihood of adult hypertension than children with normal BP. Investigators followed 2445 children 7 to 18 years of age to determine whether elevated BP in childhood correlated with increased BP in adulthood.
Investigators obtained BP, height, and weight measurements biennially during the children’s school years and when they were young adults between 20 and 30 years of age. Twelve to 13 years later, 24% of children with BP above the 90th percentile still had BP above the 90th percentile (relative risk [RR]=2.4; P<.001) and 39% had BP above the 80th percentile (RR=1.9; P<.001). Ninety-four percent of children with more than 3 normal readings during the study were normotensive as young adults. Children with one or 2 abnormal readings had a 17% and 24% chance, respectively, of having hypertension as adults (P<.001).4
High childhood systolic BP may predict CAD in adulthood
A retrospective study evaluated 126 children 10 to 17 years of age who were admitted to the hospital for an elective surgical procedure between 1950 and 1967. Children with documented BP readings at admission were eligible for the study; children with preexisting cardiac and renal disease were excluded. Investigators reassessed patients as adults (age range 42-68 years); the mean follow-up period was 42 years.
Mean BP was 125/80 mm Hg at admission and 133/75 mm Hg at follow-up. Univariate logistic regression analysis showed a significant association between systolic BP in childhood and coronary artery disease at follow-up (odds ratio [OR]=1.052; 95% confidence interval [CI], 1.005-1.101; P=.027). Children with systolic BP at or above the 95th percentile had a 4-fold increase in coronary artery disease at follow-up compared with children whose systolic BP was below the 95th percentile (29% vs 7%, P=.03). Investigators also found an association between elevated BP in childhood and a diagnosis of hypertension at follow-up (P=.007).
Limitations of the study included small sample size, selection bias, changes in the definition of hypertension during the 4 decades since the study began, and limited childhood BP data (a single measurement at admission for surgery).5
Parents of hypertensive children are likely to be hypertensive themselves
Screening BP in children has the potential to identify families at increased risk for cardiovascular disease. A case series found a high incidence of hypertension among the parents of children with elevated BP. Investigators measured several risk factors, including BP in 141 children (mean age 10.5±3.4 years) and 108 parents (at least one a biological parent, mean age 38.5±7.5 years). They obtained 2 BP readings 15 to 30 minutes apart.
Parents of children with BPs at or above the 95th percentile had a 15-fold greater likelihood of hypertension themselves (OR=14.7; 95% CI, 3.02-71.56; P=.009, positive predictive value=75%; negative predictive value=81%).6 Limitations of the study included small sample size, high prevalence of obesity and black ethnicity in the study population (a population with a greater incidence of hypertension), and only 2 BP measurements in the same day, which isn’t diagnostic for hypertension.
Recommendations
The American College of Obstetricians and Gynecologists recommends screening girls for hypertension between 13 and 15 years of age.7
The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease.8
The European Society of Hypertension and European Society of Cardiology recommend that children older than 3 years have auscultatory BP measurements at each clinic visit.9
SCREENING MAY NOT SHOW BENEFITS in childhood but could pay off for adults. Although major professional organizations recommend measuring blood pressure (BP) at every clinic visit for all children older than 3 years (strength of recommendation [SOR]: C, expert opinion), scant evidence links earlier detection and treatment of childhood hypertension with improved patient-oriented outcomes.
However, detecting childhood hypertension may help identify adults who would benefit from earlier treatment. Children with elevated BP have a more than 60% chance of being hypertensive as young adults (SOR: B, prospective cohort study). Children with systolic BP above the 95th percentile had a more than 4-fold increase in coronary artery disease as adults compared with children below the 95th percentile (SOR: B, retrospective study).
Identifying hypertension in children is associated with a 15-fold greater likelihood of hypertension in their parents (SOR: B, case series).
Evidence summary
The US Preventive Services Task Force considers screening adults for hypertension a grade A recommendation because it’s known to improve patient outcomes through early diagnosis, treatment, and prevention of serious cardiovascular complications.1
The Fourth Task Force of the National High Blood Pressure Education Program Working Group, endorsed by the American Academy of Pediatrics, states that maintaining a large national database of BP values throughout childhood allows physicians to recognize children and adolescents with elevated BP.2 Data indicate that, in this population, the prevalence of prehypertension is 10% and the prevalence of hypertension is 4%.3
The Task Force suggests that detecting and treating childhood hypertension should be important because of increasing childhood obesity, the risk of developing left ventricular hypertrophy, and other intermediate cardiovascular effects in undiagnosed and untreated children. The Task Force acknowledges, however, that prospective longitudinal outcome studies in untreated children and adolescents are lacking.
Hypertensive children often grow up to be hypertensive adults
A prospective cohort study showed that children with elevated BP had a greater likelihood of adult hypertension than children with normal BP. Investigators followed 2445 children 7 to 18 years of age to determine whether elevated BP in childhood correlated with increased BP in adulthood.
Investigators obtained BP, height, and weight measurements biennially during the children’s school years and when they were young adults between 20 and 30 years of age. Twelve to 13 years later, 24% of children with BP above the 90th percentile still had BP above the 90th percentile (relative risk [RR]=2.4; P<.001) and 39% had BP above the 80th percentile (RR=1.9; P<.001). Ninety-four percent of children with more than 3 normal readings during the study were normotensive as young adults. Children with one or 2 abnormal readings had a 17% and 24% chance, respectively, of having hypertension as adults (P<.001).4
High childhood systolic BP may predict CAD in adulthood
A retrospective study evaluated 126 children 10 to 17 years of age who were admitted to the hospital for an elective surgical procedure between 1950 and 1967. Children with documented BP readings at admission were eligible for the study; children with preexisting cardiac and renal disease were excluded. Investigators reassessed patients as adults (age range 42-68 years); the mean follow-up period was 42 years.
Mean BP was 125/80 mm Hg at admission and 133/75 mm Hg at follow-up. Univariate logistic regression analysis showed a significant association between systolic BP in childhood and coronary artery disease at follow-up (odds ratio [OR]=1.052; 95% confidence interval [CI], 1.005-1.101; P=.027). Children with systolic BP at or above the 95th percentile had a 4-fold increase in coronary artery disease at follow-up compared with children whose systolic BP was below the 95th percentile (29% vs 7%, P=.03). Investigators also found an association between elevated BP in childhood and a diagnosis of hypertension at follow-up (P=.007).
Limitations of the study included small sample size, selection bias, changes in the definition of hypertension during the 4 decades since the study began, and limited childhood BP data (a single measurement at admission for surgery).5
Parents of hypertensive children are likely to be hypertensive themselves
Screening BP in children has the potential to identify families at increased risk for cardiovascular disease. A case series found a high incidence of hypertension among the parents of children with elevated BP. Investigators measured several risk factors, including BP in 141 children (mean age 10.5±3.4 years) and 108 parents (at least one a biological parent, mean age 38.5±7.5 years). They obtained 2 BP readings 15 to 30 minutes apart.
Parents of children with BPs at or above the 95th percentile had a 15-fold greater likelihood of hypertension themselves (OR=14.7; 95% CI, 3.02-71.56; P=.009, positive predictive value=75%; negative predictive value=81%).6 Limitations of the study included small sample size, high prevalence of obesity and black ethnicity in the study population (a population with a greater incidence of hypertension), and only 2 BP measurements in the same day, which isn’t diagnostic for hypertension.
Recommendations
The American College of Obstetricians and Gynecologists recommends screening girls for hypertension between 13 and 15 years of age.7
The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease.8
The European Society of Hypertension and European Society of Cardiology recommend that children older than 3 years have auscultatory BP measurements at each clinic visit.9
1. US Preventive Services Task Force. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed June 19, 2012.
2. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl):555-576.
3. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
4. Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633-641.
5. Erlingsdottir A, Indridason OS, Thorvaldsson O, et al. Blood pressure in children and target-organ damage later in life. Pediatr Nephrol. 2010;25:323-328.
6. Reis EC, Kip KE, Marroquin OC, et al. Screening children to identify families at increased risk for cardiovascular disease. Pediatrics. 2006;118:e1789-e1797.
7. American Congress of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 452: Primary and preventive care: periodic assessments. Obstet Gynecol. 2009;114:1444-1451.
8. American Academy of Family Physicians. Hypertension. Available at http://www.aafp.org/online/en/home/clinical/exam/hypertension.html. Accessed April 16, 2012.
9. Lurbe E, Cifkova R, Cruickshank J, et al. Management of high blood pressure in children and adolescents: recommendation of the European Society of Hypertension. J Hypertens. 2009;27:1719-1742.
1. US Preventive Services Task Force. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed June 19, 2012.
2. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl):555-576.
3. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
4. Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633-641.
5. Erlingsdottir A, Indridason OS, Thorvaldsson O, et al. Blood pressure in children and target-organ damage later in life. Pediatr Nephrol. 2010;25:323-328.
6. Reis EC, Kip KE, Marroquin OC, et al. Screening children to identify families at increased risk for cardiovascular disease. Pediatrics. 2006;118:e1789-e1797.
7. American Congress of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 452: Primary and preventive care: periodic assessments. Obstet Gynecol. 2009;114:1444-1451.
8. American Academy of Family Physicians. Hypertension. Available at http://www.aafp.org/online/en/home/clinical/exam/hypertension.html. Accessed April 16, 2012.
9. Lurbe E, Cifkova R, Cruickshank J, et al. Management of high blood pressure in children and adolescents: recommendation of the European Society of Hypertension. J Hypertens. 2009;27:1719-1742.
Evidence-based answers from the Family Physicians Inquiries Network
Can probiotics safely prevent recurrent vaginitis?
YES, using vaginal suppositories or eating yogurt with Lactobacillus may reduce recurrences of bacterial vaginosis (BV) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results).
Neither suppositories nor yogurt containing Lactobacillus are likely to prevent recurrences of vulvovaginal candidiasis (VVC) (SOR: B, RCTs with conflicting results).
Probiotic suppositories and yogurt don’t appear to have significant adverse effects (SOR: A, RCTs).
Evidence summary
A double-blind RCT found that probiotic vaginal suppositories reduce the incidence of recurrent BV. Investigators randomized 120 Chinese women, 18 to 55 years of age with a history of 2 or more episodes of BV in the previous year, to use suppositories containing either probiotics (Lactobacillus rhamnosus,L acidophilus, and Streptococcus thermophilus, total of 8×109 colony-forming units [cfu]) or placebo.1 All the women used suppositories daily for a week, stopped for a week, and then used them for another week.
Fewer women who used probiotic suppositories had recurrences of BV on examination during the following 2 months than women who used placebo (16% vs 45%; P<.001; number needed to treat [NNT]=3.4), and fewer reported recurrences in telephone interviews 2 to 11 months after treatment (11% vs 28%; P<.05; NNT=5.8). Interviewers recorded two-thirds fewer complaints of discharge and malodor among women who used probiotics than among women who used placebo (P<.05 for both comparisons).
But another RCT finds no effect on recurrent BV or VVC
Another RCT treated 95 women 18 to 45 years of age with clindamycin ovules (for BV) or clotrimazole suppositories (for VVC) and, after 5 days, randomized them to use probiotic suppositories (Lactobacillus species, 108-1010 cfu) or placebo for 5 more days.2
Probiotic suppositories after treatment didn’t reduce clinician-diagnosed recurrences of either BV or VVC compared with placebo (7% vs 17% after 2-3 days; 22% vs 29% after the first menstrual cycle; P=not significant for both). Probiotics did reduce self-reported malodorous discharge, however (P=.03). Probiotics didn’t produce adverse effects.
Probiotic yogurt decreases recurrent BV but not VVC in an RCT
An RCT that randomized 46 women, 20 to 39 years of age with a history of 4 or more episodes of BV or VVC in the previous year, to eat L acidophilus-enriched yogurt (108 cfu) or pasteurized yogurt daily for 2 months found that consuming probiotic-containing yogurt reduced the incidence of recurrent BV but not VVC.3
Women who ate L acidophilus yogurt had fewer episodes of clinician-diagnosed BV at 1 month than women who ate pasteurized yogurt (24% vs 53%; P<.05) and also at 2 months (4% vs 36%; P<.05). However, they didn’t have significantly fewer episodes of VVC (43% vs 37% at 1 month, 21% vs 29% at 2 months; P=not significant for both). Investigators reported no adverse effects.
Small, flawed trial finds fewer episodes of VVC with yogurt
An unblinded crossover trial found that daily consumption of probiotic yogurt reduced VVC recurrences in women with a history of the infection. Investigators randomized 33 women 24 to 50 years of age to eat either 8 ounces a day of yogurt (with L acidophilus, 108 cfu) or a yogurt-free diet.4 After 6 months, the groups switched. Investigators saw all patients monthly.
Women who ate yogurt had fewer episodes of VVC than women who didn’t (0.4 vs 2.5 over 6 months; P<.001) and reported no adverse effects. The study was flawed by small size and high attrition rates (only 13 women completed the trial).
Recommendations
The World Health Organization says some clinical evidence suggests that oral and vaginal administration of lactobacilli can eradicate asymptomatic and symptomatic BV. Supporting evidence for prevention of recurrent BV or VVC by probiotics is limited.5
A literature review by the Natural Standard Research Collaboration states that insufficient evidence exists to recommend probiotics for treating or preventing bacterial vaginosis and that preventing or treating vaginal yeast infections with probiotics hasn’t been adequately studied.6
1. Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. Am J Obstet Gynecol. 2010;203:120.e1-120.e6.
2. Ehrstrom S, Daroczy K, Rylander E, et al. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010;12:691-699.
3. Shalev E, Battino S, Weiner E, et al. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch Fam Med. 1996;5:593-596.
4. Hilton E, Isenberg HD, Alperstein P, et al. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med. 1992;116:353-357.
5. Food and Agriculture Organization of the United Nations and World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. October 1-4, 2001. Cordoba, Argentina.
6. National Standard Research Collaboration. Unclear if probiotics effective for bacterial vaginosis. October 2009. Available at: http://www.naturalstandard.com/news/news20091028.asp. Accessed September 1, 2011.
YES, using vaginal suppositories or eating yogurt with Lactobacillus may reduce recurrences of bacterial vaginosis (BV) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results).
Neither suppositories nor yogurt containing Lactobacillus are likely to prevent recurrences of vulvovaginal candidiasis (VVC) (SOR: B, RCTs with conflicting results).
Probiotic suppositories and yogurt don’t appear to have significant adverse effects (SOR: A, RCTs).
Evidence summary
A double-blind RCT found that probiotic vaginal suppositories reduce the incidence of recurrent BV. Investigators randomized 120 Chinese women, 18 to 55 years of age with a history of 2 or more episodes of BV in the previous year, to use suppositories containing either probiotics (Lactobacillus rhamnosus,L acidophilus, and Streptococcus thermophilus, total of 8×109 colony-forming units [cfu]) or placebo.1 All the women used suppositories daily for a week, stopped for a week, and then used them for another week.
Fewer women who used probiotic suppositories had recurrences of BV on examination during the following 2 months than women who used placebo (16% vs 45%; P<.001; number needed to treat [NNT]=3.4), and fewer reported recurrences in telephone interviews 2 to 11 months after treatment (11% vs 28%; P<.05; NNT=5.8). Interviewers recorded two-thirds fewer complaints of discharge and malodor among women who used probiotics than among women who used placebo (P<.05 for both comparisons).
But another RCT finds no effect on recurrent BV or VVC
Another RCT treated 95 women 18 to 45 years of age with clindamycin ovules (for BV) or clotrimazole suppositories (for VVC) and, after 5 days, randomized them to use probiotic suppositories (Lactobacillus species, 108-1010 cfu) or placebo for 5 more days.2
Probiotic suppositories after treatment didn’t reduce clinician-diagnosed recurrences of either BV or VVC compared with placebo (7% vs 17% after 2-3 days; 22% vs 29% after the first menstrual cycle; P=not significant for both). Probiotics did reduce self-reported malodorous discharge, however (P=.03). Probiotics didn’t produce adverse effects.
Probiotic yogurt decreases recurrent BV but not VVC in an RCT
An RCT that randomized 46 women, 20 to 39 years of age with a history of 4 or more episodes of BV or VVC in the previous year, to eat L acidophilus-enriched yogurt (108 cfu) or pasteurized yogurt daily for 2 months found that consuming probiotic-containing yogurt reduced the incidence of recurrent BV but not VVC.3
Women who ate L acidophilus yogurt had fewer episodes of clinician-diagnosed BV at 1 month than women who ate pasteurized yogurt (24% vs 53%; P<.05) and also at 2 months (4% vs 36%; P<.05). However, they didn’t have significantly fewer episodes of VVC (43% vs 37% at 1 month, 21% vs 29% at 2 months; P=not significant for both). Investigators reported no adverse effects.
Small, flawed trial finds fewer episodes of VVC with yogurt
An unblinded crossover trial found that daily consumption of probiotic yogurt reduced VVC recurrences in women with a history of the infection. Investigators randomized 33 women 24 to 50 years of age to eat either 8 ounces a day of yogurt (with L acidophilus, 108 cfu) or a yogurt-free diet.4 After 6 months, the groups switched. Investigators saw all patients monthly.
Women who ate yogurt had fewer episodes of VVC than women who didn’t (0.4 vs 2.5 over 6 months; P<.001) and reported no adverse effects. The study was flawed by small size and high attrition rates (only 13 women completed the trial).
Recommendations
The World Health Organization says some clinical evidence suggests that oral and vaginal administration of lactobacilli can eradicate asymptomatic and symptomatic BV. Supporting evidence for prevention of recurrent BV or VVC by probiotics is limited.5
A literature review by the Natural Standard Research Collaboration states that insufficient evidence exists to recommend probiotics for treating or preventing bacterial vaginosis and that preventing or treating vaginal yeast infections with probiotics hasn’t been adequately studied.6
YES, using vaginal suppositories or eating yogurt with Lactobacillus may reduce recurrences of bacterial vaginosis (BV) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results).
Neither suppositories nor yogurt containing Lactobacillus are likely to prevent recurrences of vulvovaginal candidiasis (VVC) (SOR: B, RCTs with conflicting results).
Probiotic suppositories and yogurt don’t appear to have significant adverse effects (SOR: A, RCTs).
Evidence summary
A double-blind RCT found that probiotic vaginal suppositories reduce the incidence of recurrent BV. Investigators randomized 120 Chinese women, 18 to 55 years of age with a history of 2 or more episodes of BV in the previous year, to use suppositories containing either probiotics (Lactobacillus rhamnosus,L acidophilus, and Streptococcus thermophilus, total of 8×109 colony-forming units [cfu]) or placebo.1 All the women used suppositories daily for a week, stopped for a week, and then used them for another week.
Fewer women who used probiotic suppositories had recurrences of BV on examination during the following 2 months than women who used placebo (16% vs 45%; P<.001; number needed to treat [NNT]=3.4), and fewer reported recurrences in telephone interviews 2 to 11 months after treatment (11% vs 28%; P<.05; NNT=5.8). Interviewers recorded two-thirds fewer complaints of discharge and malodor among women who used probiotics than among women who used placebo (P<.05 for both comparisons).
But another RCT finds no effect on recurrent BV or VVC
Another RCT treated 95 women 18 to 45 years of age with clindamycin ovules (for BV) or clotrimazole suppositories (for VVC) and, after 5 days, randomized them to use probiotic suppositories (Lactobacillus species, 108-1010 cfu) or placebo for 5 more days.2
Probiotic suppositories after treatment didn’t reduce clinician-diagnosed recurrences of either BV or VVC compared with placebo (7% vs 17% after 2-3 days; 22% vs 29% after the first menstrual cycle; P=not significant for both). Probiotics did reduce self-reported malodorous discharge, however (P=.03). Probiotics didn’t produce adverse effects.
Probiotic yogurt decreases recurrent BV but not VVC in an RCT
An RCT that randomized 46 women, 20 to 39 years of age with a history of 4 or more episodes of BV or VVC in the previous year, to eat L acidophilus-enriched yogurt (108 cfu) or pasteurized yogurt daily for 2 months found that consuming probiotic-containing yogurt reduced the incidence of recurrent BV but not VVC.3
Women who ate L acidophilus yogurt had fewer episodes of clinician-diagnosed BV at 1 month than women who ate pasteurized yogurt (24% vs 53%; P<.05) and also at 2 months (4% vs 36%; P<.05). However, they didn’t have significantly fewer episodes of VVC (43% vs 37% at 1 month, 21% vs 29% at 2 months; P=not significant for both). Investigators reported no adverse effects.
Small, flawed trial finds fewer episodes of VVC with yogurt
An unblinded crossover trial found that daily consumption of probiotic yogurt reduced VVC recurrences in women with a history of the infection. Investigators randomized 33 women 24 to 50 years of age to eat either 8 ounces a day of yogurt (with L acidophilus, 108 cfu) or a yogurt-free diet.4 After 6 months, the groups switched. Investigators saw all patients monthly.
Women who ate yogurt had fewer episodes of VVC than women who didn’t (0.4 vs 2.5 over 6 months; P<.001) and reported no adverse effects. The study was flawed by small size and high attrition rates (only 13 women completed the trial).
Recommendations
The World Health Organization says some clinical evidence suggests that oral and vaginal administration of lactobacilli can eradicate asymptomatic and symptomatic BV. Supporting evidence for prevention of recurrent BV or VVC by probiotics is limited.5
A literature review by the Natural Standard Research Collaboration states that insufficient evidence exists to recommend probiotics for treating or preventing bacterial vaginosis and that preventing or treating vaginal yeast infections with probiotics hasn’t been adequately studied.6
1. Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. Am J Obstet Gynecol. 2010;203:120.e1-120.e6.
2. Ehrstrom S, Daroczy K, Rylander E, et al. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010;12:691-699.
3. Shalev E, Battino S, Weiner E, et al. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch Fam Med. 1996;5:593-596.
4. Hilton E, Isenberg HD, Alperstein P, et al. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med. 1992;116:353-357.
5. Food and Agriculture Organization of the United Nations and World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. October 1-4, 2001. Cordoba, Argentina.
6. National Standard Research Collaboration. Unclear if probiotics effective for bacterial vaginosis. October 2009. Available at: http://www.naturalstandard.com/news/news20091028.asp. Accessed September 1, 2011.
1. Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. Am J Obstet Gynecol. 2010;203:120.e1-120.e6.
2. Ehrstrom S, Daroczy K, Rylander E, et al. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010;12:691-699.
3. Shalev E, Battino S, Weiner E, et al. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch Fam Med. 1996;5:593-596.
4. Hilton E, Isenberg HD, Alperstein P, et al. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med. 1992;116:353-357.
5. Food and Agriculture Organization of the United Nations and World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. October 1-4, 2001. Cordoba, Argentina.
6. National Standard Research Collaboration. Unclear if probiotics effective for bacterial vaginosis. October 2009. Available at: http://www.naturalstandard.com/news/news20091028.asp. Accessed September 1, 2011.
Evidence-based answers from the Family Physicians Inquiries Network
What drugs are effective for periodic limb movement disorder?
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
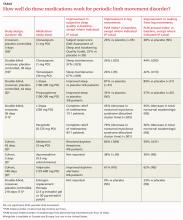
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
Evidence-based answers from the Family Physicians Inquiries Network
Medication vs radioablation for Graves’ disease: How do they compare?
THE BENEFITS ARE SIMILAR; the risks vary. Treating Graves’ disease initially with medication or radioablation (or surgery) produces comparable resolution of hyperthyroidism at 2 years (strength of recommendation [SOR]: B, a randomized clinical trial [RCT]). The goal of radio-ablation is lifelong hypothyroidism.
While radioablation doesn’t appear to increase the risk of neoplasia, “theoretical concerns” have led to the recommendation that it not be used for children younger than 5 years (SOR: C, expert opinion).
Radioablation carries a higher risk of thyroid-associated ophthalmopathy (TAO) than medical therapy (SOR: B, an RCT and a lower-quality meta-analysis).
Between 9% and 16% of patients are unable to tolerate medical therapy, mainly because of rash but also because of agranulocytosis (SOR: A, meta-analysis).
Evidence summary
A prospective RCT found that medical therapy, radioablation with iodine-131 (131I), and surgery produced similar control of Graves’ hyperthyroidism in 179 patients.1 Investigators stratified patients by age, assigning younger patients (20-34 years; N=60) to antithyroid medication (methimazole and a β-blocker) for 18 months or subtotal thyroidectomy and older patients (35-55 years; N=119) to 18 months of antithyroid medication, subtotal thyroidectomy, or 131I radioablation.
After 6 weeks, all therapies produced serum triiodothyronine levels of less than 2.5 nmol/L (data extracted from table; no comparison statistic given). Patients were followed for 48 to 121 months (average follow-up time not given). Investigators found no significant differences in sick leave (72 vs 83 days for medical treatment compared with radioablation; no comparison statistic given) or patient satisfaction (95% for both medical treatment and radioablation; no comparison statistic given).
Medication (initially methimazole) was changed in 16% of patients because of adverse effects. More than a third of patients relapsed after medications were stopped (time to relapse 1-57 months); 21% relapsed after a single 131I treatment (time to relapse 5-16 months).
In another study, radioablation outperforms medication
A retrospective case series found that radioablation resolved hyperthyroidism more often than medical therapy among 194 consecutive Saudi Arabian patients (mean age 32 years) diagnosed with Graves’ disease and followed for an average of 50 months.2 One dose of radioiodine (13-15 mCi) cured hyperthyroidism in 83% of patients, whereas 18 months of medical therapy produced remission lasting at least 6 months past the end of therapy in only 26% of patients (no comparison statistic given).
The presence of TAO at diagnosis increased the likelihood of radioablation failure (odds ratio for failure to respond to single dose of radioiodine=6.4; 95% confidence interval [CI], 1.51-24.4; P<.01). A major weakness of the study was that the investigators didn’t describe the medication therapy clearly.
More patients develop TAO after radioablation than medical therapy
An RCT found that radioablation is more commonly associated with development of TAO than medical therapy.3 When investigators randomized 313 patients to receive 131I radioablation or medical therapy for 18 months and followed them for as long as 4 years, more patients receiving radioablation developed TAO (38% compared with 18% for medical therapy, using intention-to-treat analysis; P<.001; number needed to harm [NNH]=5). Twenty-five percent of patients initially receiving medical therapy later underwent radioablation, but these patients didn’t develop TAO at a higher rate.
An earlier meta-analysis of 2 RCTs (N=189) also found an increased risk of TAO with radioablation compared with medical therapy.4 Patients receiving radioablation were more likely to develop TAO (18% vs 4%; relative risk [RR]=4.2; 95% CI, 2.0-8.8; NNH=7) and more likely to develop severe TAO (10% vs 1.6%; RR=4.4; 95% CI, 1.3-15; NNH=12). Adjunctive use of steroids with radioablation didn’t alter the risk of new TAO. However, steroid prophylaxis in patients with preexisting TAO significantly reduced the risk of progression after radioablation (RR=0.03; 95% CI, 0.00-0.24). The authors of the meta-analysis didn’t evaluate the quality of the RCTs.
Despite low neoplasia risk, radioablation isn’t for young children
Expert guidelines state that the goal of radioablation is to induce lifelong hypothyroidism, which is managed with thyroid hormone replacement.5 The risk of neoplasia after radioablation is believed to be low with appropriate dosing. However, based on “theoretical concerns,” experts don’t recommend using radioiodine in children younger than 5 years and advise limited use in children 5 to 10 years of age.5
Medication adverse effects include rashes, transient agranulocytosis
A Cochrane review with 7 RCTs (N=620) describing withdrawal rates for patients receiving medication for Graves’ disease found that 9% to 16% of patients discontinued treatment because of adverse effects.6 Rashes were the most common adverse effect (6%-10% of patients), but as many as 3% of patients developed transient agranulocytosis. In addition, patients on medication need frequent blood tests to monitor for thyroid activity and potential toxicity.
Recommendations
The guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists state that overt Graves’ hyperthyroidism may be treated with any of the following: 131I radioablation, antithyroid medication, or thyroidectomy.3 Patient characteristics (pregnancy, mild disease, goiter compression symptoms) should help determine the appropriate option in any given case.
1. Törring O, Tallstedt L, Göran W, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine—a prospective, randomized study. J Clin Endocrinol Metab. 1996;81:2986-2993.
2. Alfadda A, Malabu UH, El-Desouki MI, et al. Treatment of Graves’ hyperthyroidism—prognostic factors for outcome. Saudi Med J. 2007;28:225-230.
3. Träisk F, Tallstedt L, Abraham-Nordling M, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
4. Acharya SH, Avenell A, Philip S, et al. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review (structured abstract). Clin Endocrinol. 2008;69:943-950.
5. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:456-520.
6. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;(1):CD003420.
THE BENEFITS ARE SIMILAR; the risks vary. Treating Graves’ disease initially with medication or radioablation (or surgery) produces comparable resolution of hyperthyroidism at 2 years (strength of recommendation [SOR]: B, a randomized clinical trial [RCT]). The goal of radio-ablation is lifelong hypothyroidism.
While radioablation doesn’t appear to increase the risk of neoplasia, “theoretical concerns” have led to the recommendation that it not be used for children younger than 5 years (SOR: C, expert opinion).
Radioablation carries a higher risk of thyroid-associated ophthalmopathy (TAO) than medical therapy (SOR: B, an RCT and a lower-quality meta-analysis).
Between 9% and 16% of patients are unable to tolerate medical therapy, mainly because of rash but also because of agranulocytosis (SOR: A, meta-analysis).
Evidence summary
A prospective RCT found that medical therapy, radioablation with iodine-131 (131I), and surgery produced similar control of Graves’ hyperthyroidism in 179 patients.1 Investigators stratified patients by age, assigning younger patients (20-34 years; N=60) to antithyroid medication (methimazole and a β-blocker) for 18 months or subtotal thyroidectomy and older patients (35-55 years; N=119) to 18 months of antithyroid medication, subtotal thyroidectomy, or 131I radioablation.
After 6 weeks, all therapies produced serum triiodothyronine levels of less than 2.5 nmol/L (data extracted from table; no comparison statistic given). Patients were followed for 48 to 121 months (average follow-up time not given). Investigators found no significant differences in sick leave (72 vs 83 days for medical treatment compared with radioablation; no comparison statistic given) or patient satisfaction (95% for both medical treatment and radioablation; no comparison statistic given).
Medication (initially methimazole) was changed in 16% of patients because of adverse effects. More than a third of patients relapsed after medications were stopped (time to relapse 1-57 months); 21% relapsed after a single 131I treatment (time to relapse 5-16 months).
In another study, radioablation outperforms medication
A retrospective case series found that radioablation resolved hyperthyroidism more often than medical therapy among 194 consecutive Saudi Arabian patients (mean age 32 years) diagnosed with Graves’ disease and followed for an average of 50 months.2 One dose of radioiodine (13-15 mCi) cured hyperthyroidism in 83% of patients, whereas 18 months of medical therapy produced remission lasting at least 6 months past the end of therapy in only 26% of patients (no comparison statistic given).
The presence of TAO at diagnosis increased the likelihood of radioablation failure (odds ratio for failure to respond to single dose of radioiodine=6.4; 95% confidence interval [CI], 1.51-24.4; P<.01). A major weakness of the study was that the investigators didn’t describe the medication therapy clearly.
More patients develop TAO after radioablation than medical therapy
An RCT found that radioablation is more commonly associated with development of TAO than medical therapy.3 When investigators randomized 313 patients to receive 131I radioablation or medical therapy for 18 months and followed them for as long as 4 years, more patients receiving radioablation developed TAO (38% compared with 18% for medical therapy, using intention-to-treat analysis; P<.001; number needed to harm [NNH]=5). Twenty-five percent of patients initially receiving medical therapy later underwent radioablation, but these patients didn’t develop TAO at a higher rate.
An earlier meta-analysis of 2 RCTs (N=189) also found an increased risk of TAO with radioablation compared with medical therapy.4 Patients receiving radioablation were more likely to develop TAO (18% vs 4%; relative risk [RR]=4.2; 95% CI, 2.0-8.8; NNH=7) and more likely to develop severe TAO (10% vs 1.6%; RR=4.4; 95% CI, 1.3-15; NNH=12). Adjunctive use of steroids with radioablation didn’t alter the risk of new TAO. However, steroid prophylaxis in patients with preexisting TAO significantly reduced the risk of progression after radioablation (RR=0.03; 95% CI, 0.00-0.24). The authors of the meta-analysis didn’t evaluate the quality of the RCTs.
Despite low neoplasia risk, radioablation isn’t for young children
Expert guidelines state that the goal of radioablation is to induce lifelong hypothyroidism, which is managed with thyroid hormone replacement.5 The risk of neoplasia after radioablation is believed to be low with appropriate dosing. However, based on “theoretical concerns,” experts don’t recommend using radioiodine in children younger than 5 years and advise limited use in children 5 to 10 years of age.5
Medication adverse effects include rashes, transient agranulocytosis
A Cochrane review with 7 RCTs (N=620) describing withdrawal rates for patients receiving medication for Graves’ disease found that 9% to 16% of patients discontinued treatment because of adverse effects.6 Rashes were the most common adverse effect (6%-10% of patients), but as many as 3% of patients developed transient agranulocytosis. In addition, patients on medication need frequent blood tests to monitor for thyroid activity and potential toxicity.
Recommendations
The guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists state that overt Graves’ hyperthyroidism may be treated with any of the following: 131I radioablation, antithyroid medication, or thyroidectomy.3 Patient characteristics (pregnancy, mild disease, goiter compression symptoms) should help determine the appropriate option in any given case.
THE BENEFITS ARE SIMILAR; the risks vary. Treating Graves’ disease initially with medication or radioablation (or surgery) produces comparable resolution of hyperthyroidism at 2 years (strength of recommendation [SOR]: B, a randomized clinical trial [RCT]). The goal of radio-ablation is lifelong hypothyroidism.
While radioablation doesn’t appear to increase the risk of neoplasia, “theoretical concerns” have led to the recommendation that it not be used for children younger than 5 years (SOR: C, expert opinion).
Radioablation carries a higher risk of thyroid-associated ophthalmopathy (TAO) than medical therapy (SOR: B, an RCT and a lower-quality meta-analysis).
Between 9% and 16% of patients are unable to tolerate medical therapy, mainly because of rash but also because of agranulocytosis (SOR: A, meta-analysis).
Evidence summary
A prospective RCT found that medical therapy, radioablation with iodine-131 (131I), and surgery produced similar control of Graves’ hyperthyroidism in 179 patients.1 Investigators stratified patients by age, assigning younger patients (20-34 years; N=60) to antithyroid medication (methimazole and a β-blocker) for 18 months or subtotal thyroidectomy and older patients (35-55 years; N=119) to 18 months of antithyroid medication, subtotal thyroidectomy, or 131I radioablation.
After 6 weeks, all therapies produced serum triiodothyronine levels of less than 2.5 nmol/L (data extracted from table; no comparison statistic given). Patients were followed for 48 to 121 months (average follow-up time not given). Investigators found no significant differences in sick leave (72 vs 83 days for medical treatment compared with radioablation; no comparison statistic given) or patient satisfaction (95% for both medical treatment and radioablation; no comparison statistic given).
Medication (initially methimazole) was changed in 16% of patients because of adverse effects. More than a third of patients relapsed after medications were stopped (time to relapse 1-57 months); 21% relapsed after a single 131I treatment (time to relapse 5-16 months).
In another study, radioablation outperforms medication
A retrospective case series found that radioablation resolved hyperthyroidism more often than medical therapy among 194 consecutive Saudi Arabian patients (mean age 32 years) diagnosed with Graves’ disease and followed for an average of 50 months.2 One dose of radioiodine (13-15 mCi) cured hyperthyroidism in 83% of patients, whereas 18 months of medical therapy produced remission lasting at least 6 months past the end of therapy in only 26% of patients (no comparison statistic given).
The presence of TAO at diagnosis increased the likelihood of radioablation failure (odds ratio for failure to respond to single dose of radioiodine=6.4; 95% confidence interval [CI], 1.51-24.4; P<.01). A major weakness of the study was that the investigators didn’t describe the medication therapy clearly.
More patients develop TAO after radioablation than medical therapy
An RCT found that radioablation is more commonly associated with development of TAO than medical therapy.3 When investigators randomized 313 patients to receive 131I radioablation or medical therapy for 18 months and followed them for as long as 4 years, more patients receiving radioablation developed TAO (38% compared with 18% for medical therapy, using intention-to-treat analysis; P<.001; number needed to harm [NNH]=5). Twenty-five percent of patients initially receiving medical therapy later underwent radioablation, but these patients didn’t develop TAO at a higher rate.
An earlier meta-analysis of 2 RCTs (N=189) also found an increased risk of TAO with radioablation compared with medical therapy.4 Patients receiving radioablation were more likely to develop TAO (18% vs 4%; relative risk [RR]=4.2; 95% CI, 2.0-8.8; NNH=7) and more likely to develop severe TAO (10% vs 1.6%; RR=4.4; 95% CI, 1.3-15; NNH=12). Adjunctive use of steroids with radioablation didn’t alter the risk of new TAO. However, steroid prophylaxis in patients with preexisting TAO significantly reduced the risk of progression after radioablation (RR=0.03; 95% CI, 0.00-0.24). The authors of the meta-analysis didn’t evaluate the quality of the RCTs.
Despite low neoplasia risk, radioablation isn’t for young children
Expert guidelines state that the goal of radioablation is to induce lifelong hypothyroidism, which is managed with thyroid hormone replacement.5 The risk of neoplasia after radioablation is believed to be low with appropriate dosing. However, based on “theoretical concerns,” experts don’t recommend using radioiodine in children younger than 5 years and advise limited use in children 5 to 10 years of age.5
Medication adverse effects include rashes, transient agranulocytosis
A Cochrane review with 7 RCTs (N=620) describing withdrawal rates for patients receiving medication for Graves’ disease found that 9% to 16% of patients discontinued treatment because of adverse effects.6 Rashes were the most common adverse effect (6%-10% of patients), but as many as 3% of patients developed transient agranulocytosis. In addition, patients on medication need frequent blood tests to monitor for thyroid activity and potential toxicity.
Recommendations
The guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists state that overt Graves’ hyperthyroidism may be treated with any of the following: 131I radioablation, antithyroid medication, or thyroidectomy.3 Patient characteristics (pregnancy, mild disease, goiter compression symptoms) should help determine the appropriate option in any given case.
1. Törring O, Tallstedt L, Göran W, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine—a prospective, randomized study. J Clin Endocrinol Metab. 1996;81:2986-2993.
2. Alfadda A, Malabu UH, El-Desouki MI, et al. Treatment of Graves’ hyperthyroidism—prognostic factors for outcome. Saudi Med J. 2007;28:225-230.
3. Träisk F, Tallstedt L, Abraham-Nordling M, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
4. Acharya SH, Avenell A, Philip S, et al. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review (structured abstract). Clin Endocrinol. 2008;69:943-950.
5. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:456-520.
6. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;(1):CD003420.
1. Törring O, Tallstedt L, Göran W, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine—a prospective, randomized study. J Clin Endocrinol Metab. 1996;81:2986-2993.
2. Alfadda A, Malabu UH, El-Desouki MI, et al. Treatment of Graves’ hyperthyroidism—prognostic factors for outcome. Saudi Med J. 2007;28:225-230.
3. Träisk F, Tallstedt L, Abraham-Nordling M, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
4. Acharya SH, Avenell A, Philip S, et al. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review (structured abstract). Clin Endocrinol. 2008;69:943-950.
5. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:456-520.
6. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;(1):CD003420.
Evidence-based answers from the Family Physicians Inquiries Network
How does smoking in the home affect children with asthma?
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
Evidence-based answers from the Family Physicians Inquiries Network
Do antibiotics shorten symptoms in patients with purulent nasal discharge?
NO. For most patients with purulent nasal discharge, antibiotics don’t decrease symptom duration; they do increase adverse events (strength of recommendation [SOR]: A, 3 meta-analyses and 2 randomized controlled trials [RCTs]).
Researchers in the field don’t recommend using antibiotics as routine treatment for purulent rhinorrhea associated with symptoms of upper respiratory infection ([SOR]: C, expert opinion).
Evidence summary
A Cochrane review of antibiotics for the common cold that included 5 RCTs with a total of 772 participants with purulent nasal discharge found no benefit from antibiotics.1 The relative risk (RR) for persistent acute purulent rhinitis with antibiotics compared with placebo was 0.63 (95% confidence interval [CI], 0.38-1.07; P=.087). The antibiotic groups showed an increase in adverse effects, with an RR of 1.46 (95% CI, 1.01-1.94; P=.047).
Benefits of antibiotics tempered by adverse effects
A meta-analysis of 6 RCTs with more than 1400 subjects showed persistent nasal discharge at 5 to 8 days, on average, in 23% of patients who received antibiotics compared with 46% of patients who received placebo (RR of benefits=1.18; 95% CI, 1.05-1.33; P=.05).2 Most subjects were between 12 and 50 years of age; 2 of the trials included children between 2 months and 16 years of age. All subjects had symptoms for fewer than 10 days.
The adverse effects of antibiotic treatment, primarily rash and diarrhea, were also addressed (RR of adverse effects=1.46; 95% CI, 1.10-1.94; P=.028). Given the overlap of the number needed to treat (7-15) and number needed to harm (12-78), the authors concluded that most patients get better without antibiotics, supporting “no antibiotic as first line” treatment advice.
Other studies show minimal benefit for antibiotics
A meta-analysis of 9 placebo-controlled RCTs (2640 adult subjects with rhinosinusitis-like complaints) found that antibiotics provided minimal benefit. For patients with visible purulent drainage in the pharynx, the NNT overlapped with the NNH; patients without visible purulent discharge showed even less benefit from antibiotics.3
Clinical improvement is insufficient to recommend antibiotic treatment
Three double-blinded RCTs studied patients older than 12 years who presented to a family practice clinic complaining of purulent rhinitis.4-6 All 3 studies compared amoxicillin treatment with placebo; outcomes were based primarily on patient diaries that recorded symptoms, including nasal discharge.
The first study randomized 135 patients to either amoxicillin (n=67) or placebo (n=68) for 10 days.4 At the end of 2 weeks, both groups had similar rates of symptom improvement—although in a subgroup of 57 patients who had complete symptom resolution at 2 weeks, the median number of days until resolution of purulent nasal discharge was 8 in the amoxicillin group compared with 12 days for the placebo group (P=.039). The authors could not identify clinical characteristics favoring antibiotic treatment.
In the second study, 207 patients received amoxicillin and 209 placebo.5 After 10 days of therapy, symptom resolution rates were not significantly different (35% for amoxicillin vs 29% for placebo). However, patients in the amoxicillin group had quicker resolution of purulent nasal discharge (9 vs 14 days for 75% of patients to be free of that symptom; P=.007).5
The third study (240 adults) didn’t find a significant decrease in duration of purulent nasal discharge in the antibiotic group compared with the placebo group.6
Despite the findings of decreased duration of purulent nasal discharge in the first 2 studies, the authors of all 3 studies concluded that the clinical difference in improvement between antibiotic and placebo groups was not enough to recommend treatment with antibiotics. Although the trials didn’t measure adverse outcomes, the authors advised clinicians to consider the potential for adverse reactions before recommending antibiotic treatment.
Recommendations
Both the American Academy of Otolaryngology and the American Academy of Allergy, Asthma, and Immunology recommend watchful waiting without antibiotics for acute sinusitis with mild pain or temperature lower than 101°F and consideration of antibiotics only if symptoms worsen or fail to improve by 7 days after diagnosis. Neither group offers specific recommendations regarding patients with purulent discharge.7,8
The Centers for Disease Control and Prevention recommend reserving antibiotic treatment of acute bacterial rhinosinusitis for patients with symptoms lasting longer than 7 days and patients who have unilateral symptoms with purulent nasal discharge.9
1. Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2005;(3):CD000247.-
2. Arroll B, Kenealy T. Are antibiotics effective for acute purulent rhinitis? Systematic review and meta-analysis of placebo controlled randomised trials. BMJ. 2006;333:279.-
3. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
4. Merenstein D, Whittaker C, Chadwell T, et al. Are antibiotics beneficial for patients with sinusitis complaints? A randomized double-blind clinical trial. J Fam Pract. 2005;54:144-151.
5. De Sutter AI, De Meyere MJ, Christiaens TC, et al. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract. 2002;51:317-323.
6. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
7. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
8. Slavin RG, Spector SL, Bernstein IL, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116(6 suppl):S13-S47.
9. Hickner JM, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Emerg Med. 2001;37:703-710.
NO. For most patients with purulent nasal discharge, antibiotics don’t decrease symptom duration; they do increase adverse events (strength of recommendation [SOR]: A, 3 meta-analyses and 2 randomized controlled trials [RCTs]).
Researchers in the field don’t recommend using antibiotics as routine treatment for purulent rhinorrhea associated with symptoms of upper respiratory infection ([SOR]: C, expert opinion).
Evidence summary
A Cochrane review of antibiotics for the common cold that included 5 RCTs with a total of 772 participants with purulent nasal discharge found no benefit from antibiotics.1 The relative risk (RR) for persistent acute purulent rhinitis with antibiotics compared with placebo was 0.63 (95% confidence interval [CI], 0.38-1.07; P=.087). The antibiotic groups showed an increase in adverse effects, with an RR of 1.46 (95% CI, 1.01-1.94; P=.047).
Benefits of antibiotics tempered by adverse effects
A meta-analysis of 6 RCTs with more than 1400 subjects showed persistent nasal discharge at 5 to 8 days, on average, in 23% of patients who received antibiotics compared with 46% of patients who received placebo (RR of benefits=1.18; 95% CI, 1.05-1.33; P=.05).2 Most subjects were between 12 and 50 years of age; 2 of the trials included children between 2 months and 16 years of age. All subjects had symptoms for fewer than 10 days.
The adverse effects of antibiotic treatment, primarily rash and diarrhea, were also addressed (RR of adverse effects=1.46; 95% CI, 1.10-1.94; P=.028). Given the overlap of the number needed to treat (7-15) and number needed to harm (12-78), the authors concluded that most patients get better without antibiotics, supporting “no antibiotic as first line” treatment advice.
Other studies show minimal benefit for antibiotics
A meta-analysis of 9 placebo-controlled RCTs (2640 adult subjects with rhinosinusitis-like complaints) found that antibiotics provided minimal benefit. For patients with visible purulent drainage in the pharynx, the NNT overlapped with the NNH; patients without visible purulent discharge showed even less benefit from antibiotics.3
Clinical improvement is insufficient to recommend antibiotic treatment
Three double-blinded RCTs studied patients older than 12 years who presented to a family practice clinic complaining of purulent rhinitis.4-6 All 3 studies compared amoxicillin treatment with placebo; outcomes were based primarily on patient diaries that recorded symptoms, including nasal discharge.
The first study randomized 135 patients to either amoxicillin (n=67) or placebo (n=68) for 10 days.4 At the end of 2 weeks, both groups had similar rates of symptom improvement—although in a subgroup of 57 patients who had complete symptom resolution at 2 weeks, the median number of days until resolution of purulent nasal discharge was 8 in the amoxicillin group compared with 12 days for the placebo group (P=.039). The authors could not identify clinical characteristics favoring antibiotic treatment.
In the second study, 207 patients received amoxicillin and 209 placebo.5 After 10 days of therapy, symptom resolution rates were not significantly different (35% for amoxicillin vs 29% for placebo). However, patients in the amoxicillin group had quicker resolution of purulent nasal discharge (9 vs 14 days for 75% of patients to be free of that symptom; P=.007).5
The third study (240 adults) didn’t find a significant decrease in duration of purulent nasal discharge in the antibiotic group compared with the placebo group.6
Despite the findings of decreased duration of purulent nasal discharge in the first 2 studies, the authors of all 3 studies concluded that the clinical difference in improvement between antibiotic and placebo groups was not enough to recommend treatment with antibiotics. Although the trials didn’t measure adverse outcomes, the authors advised clinicians to consider the potential for adverse reactions before recommending antibiotic treatment.
Recommendations
Both the American Academy of Otolaryngology and the American Academy of Allergy, Asthma, and Immunology recommend watchful waiting without antibiotics for acute sinusitis with mild pain or temperature lower than 101°F and consideration of antibiotics only if symptoms worsen or fail to improve by 7 days after diagnosis. Neither group offers specific recommendations regarding patients with purulent discharge.7,8
The Centers for Disease Control and Prevention recommend reserving antibiotic treatment of acute bacterial rhinosinusitis for patients with symptoms lasting longer than 7 days and patients who have unilateral symptoms with purulent nasal discharge.9
NO. For most patients with purulent nasal discharge, antibiotics don’t decrease symptom duration; they do increase adverse events (strength of recommendation [SOR]: A, 3 meta-analyses and 2 randomized controlled trials [RCTs]).
Researchers in the field don’t recommend using antibiotics as routine treatment for purulent rhinorrhea associated with symptoms of upper respiratory infection ([SOR]: C, expert opinion).
Evidence summary
A Cochrane review of antibiotics for the common cold that included 5 RCTs with a total of 772 participants with purulent nasal discharge found no benefit from antibiotics.1 The relative risk (RR) for persistent acute purulent rhinitis with antibiotics compared with placebo was 0.63 (95% confidence interval [CI], 0.38-1.07; P=.087). The antibiotic groups showed an increase in adverse effects, with an RR of 1.46 (95% CI, 1.01-1.94; P=.047).
Benefits of antibiotics tempered by adverse effects
A meta-analysis of 6 RCTs with more than 1400 subjects showed persistent nasal discharge at 5 to 8 days, on average, in 23% of patients who received antibiotics compared with 46% of patients who received placebo (RR of benefits=1.18; 95% CI, 1.05-1.33; P=.05).2 Most subjects were between 12 and 50 years of age; 2 of the trials included children between 2 months and 16 years of age. All subjects had symptoms for fewer than 10 days.
The adverse effects of antibiotic treatment, primarily rash and diarrhea, were also addressed (RR of adverse effects=1.46; 95% CI, 1.10-1.94; P=.028). Given the overlap of the number needed to treat (7-15) and number needed to harm (12-78), the authors concluded that most patients get better without antibiotics, supporting “no antibiotic as first line” treatment advice.
Other studies show minimal benefit for antibiotics
A meta-analysis of 9 placebo-controlled RCTs (2640 adult subjects with rhinosinusitis-like complaints) found that antibiotics provided minimal benefit. For patients with visible purulent drainage in the pharynx, the NNT overlapped with the NNH; patients without visible purulent discharge showed even less benefit from antibiotics.3
Clinical improvement is insufficient to recommend antibiotic treatment
Three double-blinded RCTs studied patients older than 12 years who presented to a family practice clinic complaining of purulent rhinitis.4-6 All 3 studies compared amoxicillin treatment with placebo; outcomes were based primarily on patient diaries that recorded symptoms, including nasal discharge.
The first study randomized 135 patients to either amoxicillin (n=67) or placebo (n=68) for 10 days.4 At the end of 2 weeks, both groups had similar rates of symptom improvement—although in a subgroup of 57 patients who had complete symptom resolution at 2 weeks, the median number of days until resolution of purulent nasal discharge was 8 in the amoxicillin group compared with 12 days for the placebo group (P=.039). The authors could not identify clinical characteristics favoring antibiotic treatment.
In the second study, 207 patients received amoxicillin and 209 placebo.5 After 10 days of therapy, symptom resolution rates were not significantly different (35% for amoxicillin vs 29% for placebo). However, patients in the amoxicillin group had quicker resolution of purulent nasal discharge (9 vs 14 days for 75% of patients to be free of that symptom; P=.007).5
The third study (240 adults) didn’t find a significant decrease in duration of purulent nasal discharge in the antibiotic group compared with the placebo group.6
Despite the findings of decreased duration of purulent nasal discharge in the first 2 studies, the authors of all 3 studies concluded that the clinical difference in improvement between antibiotic and placebo groups was not enough to recommend treatment with antibiotics. Although the trials didn’t measure adverse outcomes, the authors advised clinicians to consider the potential for adverse reactions before recommending antibiotic treatment.
Recommendations
Both the American Academy of Otolaryngology and the American Academy of Allergy, Asthma, and Immunology recommend watchful waiting without antibiotics for acute sinusitis with mild pain or temperature lower than 101°F and consideration of antibiotics only if symptoms worsen or fail to improve by 7 days after diagnosis. Neither group offers specific recommendations regarding patients with purulent discharge.7,8
The Centers for Disease Control and Prevention recommend reserving antibiotic treatment of acute bacterial rhinosinusitis for patients with symptoms lasting longer than 7 days and patients who have unilateral symptoms with purulent nasal discharge.9
1. Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2005;(3):CD000247.-
2. Arroll B, Kenealy T. Are antibiotics effective for acute purulent rhinitis? Systematic review and meta-analysis of placebo controlled randomised trials. BMJ. 2006;333:279.-
3. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
4. Merenstein D, Whittaker C, Chadwell T, et al. Are antibiotics beneficial for patients with sinusitis complaints? A randomized double-blind clinical trial. J Fam Pract. 2005;54:144-151.
5. De Sutter AI, De Meyere MJ, Christiaens TC, et al. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract. 2002;51:317-323.
6. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
7. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
8. Slavin RG, Spector SL, Bernstein IL, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116(6 suppl):S13-S47.
9. Hickner JM, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Emerg Med. 2001;37:703-710.
1. Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2005;(3):CD000247.-
2. Arroll B, Kenealy T. Are antibiotics effective for acute purulent rhinitis? Systematic review and meta-analysis of placebo controlled randomised trials. BMJ. 2006;333:279.-
3. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
4. Merenstein D, Whittaker C, Chadwell T, et al. Are antibiotics beneficial for patients with sinusitis complaints? A randomized double-blind clinical trial. J Fam Pract. 2005;54:144-151.
5. De Sutter AI, De Meyere MJ, Christiaens TC, et al. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract. 2002;51:317-323.
6. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
7. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
8. Slavin RG, Spector SL, Bernstein IL, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116(6 suppl):S13-S47.
9. Hickner JM, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Emerg Med. 2001;37:703-710.
Evidence-based answers from the Family Physicians Inquiries Network
What nutritional deficiencies and toxic exposures are associated with nail changes?
INFANTS WITH IRON DEFICIENCY have a higher rate of koilonychia—concavity of the outer surface of the nail—(strength of recommendation [SOR]: C, one case-control study).
Vitamin B12 deficiency is associated with various nail pigment changes that are reversible with treatment (SOR: C, case reports).
Muehrcke’s lines (transverse white bands that run parallel to the lunula) occur in a minority of patients with hypoalbuminemia. (SOR: C, one cross-sectional study).
Fingernail clubbing has been found in most patients with kwashiorkor, or protein malnutrition (SOR: C, one cross-sectional study).
Transplacental exposure to polychlorinated biphenyls (PCBs) and polychlorinated dibenzofurans (PCDFs) has been associated with nail deformities and color changes (SOR: C, one case-control study).
Evidence summary
The evidence linking nail changes with nutritional deficiencies and toxic exposures is sparse, dated, and of low quality. Conditions associated with nail changes include iron deficiency, B12 deficiency, hypoalbuminemia, protein malnutrition, and PCB/PCDF exposures.
Iron deficiency is associated with koilonychia
A case-control study of 400 infants in a low-income well-baby clinic found 22 with koilonychia, a prevalence of approximately 5%. Randomly selected age-matched infants without koilonychia served as controls.
Infants with koilonychia had significantly lower hematocrit (30% vs 34%; P<.005), hemoglobin (9.4 vs 10.7 g/dL; P<.02), and serum iron (50 vs 84 mcg/dL; P<.001) than controls.1
B12 deficiency can discolor nails, but changes are reversible
Four articles describe 5 case reports of pigment changes to nails associated with B12 deficiency, all of which resolved with B12 therapy. Nail changes included brownish reticulate pigmentation,2 longitudinal hyperpigmented streaks,3 bluish-black pigment of all nails with transverse longitudinal hyperpigmented streaks,4 and entirely blue nails.5 B12 levels ranged from undetectable to 113 pg/mL.
Hypoalbuminemia linked to Muehrcke’s lines
A cross-sectional study of 72 patients selected on the basis of general cachectic appearance found 44 to have low serum albumin. Of those, 10 (23%) had Muehrcke’s lines. All of the patients with Muehreke’s lines had albumin levels <2.7 g/dL. None of the 28 patients with normal albumin had Muehrcke’s lines.6
Kwashiorkor can cause fingernail clubbing
In a cross-sectional study of 60 children 1 to 4 years of age diagnosed with kwashiorkor without evidence of tuberculosis infection, fingernail clubbing was found in 46 (76.7%). Clubbing was mild in 26 (43.3%) children, moderate in 19 (37.7%), and severe in one (1.7%).7 Kwashiorkor is extremely rare in children in the United States and other developed countries.
Transplacental chemical exposures found to deform and discolor nails
A case-control study compared finger- and toenail findings from more than 100 Taiwanese children exposed transplacentally to high levels of PCBs and PCDFs with nail findings from a comparable number of controls. The investigators looked at parental reports and physical examination results.8
Although the rates of nail abnormalities reported by parents of exposed children differed slightly from rates documented by physical examination, children exposed to PCBs and PCDFs had consistently higher rates of nail deformity than controls. The researchers examined 117 cases and 106 controls. They identified dystrophic fingernails in 15% of exposed children and 1% of controls (odds ratio [OR]=15.4; 95% confidence interval [CI], 2-119); dystrophic toenails occurred in 32% of exposed children and 18% of controls (OR=2.2; 95% CI, 1.2-4.1). The most common fingernail deformities were grooves and ridges. The most common toenail deformities were koilonychias, ridges, thickening, and pigmentation changes.8
PCB exposure is an issue in the United States and other developed countries, but at much lower levels than the accidental contamination. Whether lower levels of exposure cause nail changes isn’t known.
Recommendations
No recommendations are available. A dermatology textbook lists several nail changes associated with nutritional deficiencies and toxic exposures (TABLE).9
Table
Nail changes linked to nutrition deficits and toxins9
| Nail change | Associated with |
|---|---|
| Beau’s lines (transverse depressions of all the nails) | Zinc deficiency |
| Diffuse white nail | Zinc deficiency, anemia |
| Koilonychia (concave nails) | Iron deficiency |
| Diffuse brown, black, or white bands | Malnutrition |
| Diffuse brown nail | Photographic developer |
| Variable white | Hyopcalcemia, thallium toxicity (rat poison) |
| Muehrcke’s lines (transverse, stationary, paired white bands) | Hypoalbuminemia |
| Mee’s lines (transverse white bands) | Arsenic |
| Longitudinal pigmentation | B12 or folate deficiency |
1. Hogan GR, Jones B. The relationship of koilonychia and iron deficiency in infants. J Pediatr. 1970;77:1054-1057.
2. Ridley CM. Pigmentation of fingertips and nails in vitamin B12 deficiency. Br J Dermatol. 1977;97:105-106.
3. Niiyama S, Mukai H. Reversible cutaneous hyperpigmentation and nails with white hair due to vitamin B12 deficiency. Eur J Dermatol. 2007;17:551-552.
4. Noppakun N, Swasdikul D. Reversible hyperpigmentation of skin and nails with white hair due to vitamin B12 deficiency. Arch Dermatol. 1986;122:896-899.
5. Carmel R. Hair and fingernail changes in acquired and congenital pernicious anemia. Arch Intern Med. 1985;145:484-485.
6. Conn RD, Smith RH. Malnutrition, myoedema, and Muehrcke’s lines. Arch Intern Med. 1965;116:875-878.
7. Amla I, Narayan JV. Finger nail clubbing in Kwashiorkor. Indian J Pediatr. 1968;35:19-22.
8. Gladen BC, Taylor JS, Wu YC, et al. Dermatological findings in children exposed transplacentally to heat-degraded polychlorinated biphenyls in Taiwan. Br J Dermatol. 1990;122:799-808.
9. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Edinburgh: Mosby; 2010:947–973.
INFANTS WITH IRON DEFICIENCY have a higher rate of koilonychia—concavity of the outer surface of the nail—(strength of recommendation [SOR]: C, one case-control study).
Vitamin B12 deficiency is associated with various nail pigment changes that are reversible with treatment (SOR: C, case reports).
Muehrcke’s lines (transverse white bands that run parallel to the lunula) occur in a minority of patients with hypoalbuminemia. (SOR: C, one cross-sectional study).
Fingernail clubbing has been found in most patients with kwashiorkor, or protein malnutrition (SOR: C, one cross-sectional study).
Transplacental exposure to polychlorinated biphenyls (PCBs) and polychlorinated dibenzofurans (PCDFs) has been associated with nail deformities and color changes (SOR: C, one case-control study).
Evidence summary
The evidence linking nail changes with nutritional deficiencies and toxic exposures is sparse, dated, and of low quality. Conditions associated with nail changes include iron deficiency, B12 deficiency, hypoalbuminemia, protein malnutrition, and PCB/PCDF exposures.
Iron deficiency is associated with koilonychia
A case-control study of 400 infants in a low-income well-baby clinic found 22 with koilonychia, a prevalence of approximately 5%. Randomly selected age-matched infants without koilonychia served as controls.
Infants with koilonychia had significantly lower hematocrit (30% vs 34%; P<.005), hemoglobin (9.4 vs 10.7 g/dL; P<.02), and serum iron (50 vs 84 mcg/dL; P<.001) than controls.1
B12 deficiency can discolor nails, but changes are reversible
Four articles describe 5 case reports of pigment changes to nails associated with B12 deficiency, all of which resolved with B12 therapy. Nail changes included brownish reticulate pigmentation,2 longitudinal hyperpigmented streaks,3 bluish-black pigment of all nails with transverse longitudinal hyperpigmented streaks,4 and entirely blue nails.5 B12 levels ranged from undetectable to 113 pg/mL.
Hypoalbuminemia linked to Muehrcke’s lines
A cross-sectional study of 72 patients selected on the basis of general cachectic appearance found 44 to have low serum albumin. Of those, 10 (23%) had Muehrcke’s lines. All of the patients with Muehreke’s lines had albumin levels <2.7 g/dL. None of the 28 patients with normal albumin had Muehrcke’s lines.6
Kwashiorkor can cause fingernail clubbing
In a cross-sectional study of 60 children 1 to 4 years of age diagnosed with kwashiorkor without evidence of tuberculosis infection, fingernail clubbing was found in 46 (76.7%). Clubbing was mild in 26 (43.3%) children, moderate in 19 (37.7%), and severe in one (1.7%).7 Kwashiorkor is extremely rare in children in the United States and other developed countries.
Transplacental chemical exposures found to deform and discolor nails
A case-control study compared finger- and toenail findings from more than 100 Taiwanese children exposed transplacentally to high levels of PCBs and PCDFs with nail findings from a comparable number of controls. The investigators looked at parental reports and physical examination results.8
Although the rates of nail abnormalities reported by parents of exposed children differed slightly from rates documented by physical examination, children exposed to PCBs and PCDFs had consistently higher rates of nail deformity than controls. The researchers examined 117 cases and 106 controls. They identified dystrophic fingernails in 15% of exposed children and 1% of controls (odds ratio [OR]=15.4; 95% confidence interval [CI], 2-119); dystrophic toenails occurred in 32% of exposed children and 18% of controls (OR=2.2; 95% CI, 1.2-4.1). The most common fingernail deformities were grooves and ridges. The most common toenail deformities were koilonychias, ridges, thickening, and pigmentation changes.8
PCB exposure is an issue in the United States and other developed countries, but at much lower levels than the accidental contamination. Whether lower levels of exposure cause nail changes isn’t known.
Recommendations
No recommendations are available. A dermatology textbook lists several nail changes associated with nutritional deficiencies and toxic exposures (TABLE).9
Table
Nail changes linked to nutrition deficits and toxins9
| Nail change | Associated with |
|---|---|
| Beau’s lines (transverse depressions of all the nails) | Zinc deficiency |
| Diffuse white nail | Zinc deficiency, anemia |
| Koilonychia (concave nails) | Iron deficiency |
| Diffuse brown, black, or white bands | Malnutrition |
| Diffuse brown nail | Photographic developer |
| Variable white | Hyopcalcemia, thallium toxicity (rat poison) |
| Muehrcke’s lines (transverse, stationary, paired white bands) | Hypoalbuminemia |
| Mee’s lines (transverse white bands) | Arsenic |
| Longitudinal pigmentation | B12 or folate deficiency |
INFANTS WITH IRON DEFICIENCY have a higher rate of koilonychia—concavity of the outer surface of the nail—(strength of recommendation [SOR]: C, one case-control study).
Vitamin B12 deficiency is associated with various nail pigment changes that are reversible with treatment (SOR: C, case reports).
Muehrcke’s lines (transverse white bands that run parallel to the lunula) occur in a minority of patients with hypoalbuminemia. (SOR: C, one cross-sectional study).
Fingernail clubbing has been found in most patients with kwashiorkor, or protein malnutrition (SOR: C, one cross-sectional study).
Transplacental exposure to polychlorinated biphenyls (PCBs) and polychlorinated dibenzofurans (PCDFs) has been associated with nail deformities and color changes (SOR: C, one case-control study).
Evidence summary
The evidence linking nail changes with nutritional deficiencies and toxic exposures is sparse, dated, and of low quality. Conditions associated with nail changes include iron deficiency, B12 deficiency, hypoalbuminemia, protein malnutrition, and PCB/PCDF exposures.
Iron deficiency is associated with koilonychia
A case-control study of 400 infants in a low-income well-baby clinic found 22 with koilonychia, a prevalence of approximately 5%. Randomly selected age-matched infants without koilonychia served as controls.
Infants with koilonychia had significantly lower hematocrit (30% vs 34%; P<.005), hemoglobin (9.4 vs 10.7 g/dL; P<.02), and serum iron (50 vs 84 mcg/dL; P<.001) than controls.1
B12 deficiency can discolor nails, but changes are reversible
Four articles describe 5 case reports of pigment changes to nails associated with B12 deficiency, all of which resolved with B12 therapy. Nail changes included brownish reticulate pigmentation,2 longitudinal hyperpigmented streaks,3 bluish-black pigment of all nails with transverse longitudinal hyperpigmented streaks,4 and entirely blue nails.5 B12 levels ranged from undetectable to 113 pg/mL.
Hypoalbuminemia linked to Muehrcke’s lines
A cross-sectional study of 72 patients selected on the basis of general cachectic appearance found 44 to have low serum albumin. Of those, 10 (23%) had Muehrcke’s lines. All of the patients with Muehreke’s lines had albumin levels <2.7 g/dL. None of the 28 patients with normal albumin had Muehrcke’s lines.6
Kwashiorkor can cause fingernail clubbing
In a cross-sectional study of 60 children 1 to 4 years of age diagnosed with kwashiorkor without evidence of tuberculosis infection, fingernail clubbing was found in 46 (76.7%). Clubbing was mild in 26 (43.3%) children, moderate in 19 (37.7%), and severe in one (1.7%).7 Kwashiorkor is extremely rare in children in the United States and other developed countries.
Transplacental chemical exposures found to deform and discolor nails
A case-control study compared finger- and toenail findings from more than 100 Taiwanese children exposed transplacentally to high levels of PCBs and PCDFs with nail findings from a comparable number of controls. The investigators looked at parental reports and physical examination results.8
Although the rates of nail abnormalities reported by parents of exposed children differed slightly from rates documented by physical examination, children exposed to PCBs and PCDFs had consistently higher rates of nail deformity than controls. The researchers examined 117 cases and 106 controls. They identified dystrophic fingernails in 15% of exposed children and 1% of controls (odds ratio [OR]=15.4; 95% confidence interval [CI], 2-119); dystrophic toenails occurred in 32% of exposed children and 18% of controls (OR=2.2; 95% CI, 1.2-4.1). The most common fingernail deformities were grooves and ridges. The most common toenail deformities were koilonychias, ridges, thickening, and pigmentation changes.8
PCB exposure is an issue in the United States and other developed countries, but at much lower levels than the accidental contamination. Whether lower levels of exposure cause nail changes isn’t known.
Recommendations
No recommendations are available. A dermatology textbook lists several nail changes associated with nutritional deficiencies and toxic exposures (TABLE).9
Table
Nail changes linked to nutrition deficits and toxins9
| Nail change | Associated with |
|---|---|
| Beau’s lines (transverse depressions of all the nails) | Zinc deficiency |
| Diffuse white nail | Zinc deficiency, anemia |
| Koilonychia (concave nails) | Iron deficiency |
| Diffuse brown, black, or white bands | Malnutrition |
| Diffuse brown nail | Photographic developer |
| Variable white | Hyopcalcemia, thallium toxicity (rat poison) |
| Muehrcke’s lines (transverse, stationary, paired white bands) | Hypoalbuminemia |
| Mee’s lines (transverse white bands) | Arsenic |
| Longitudinal pigmentation | B12 or folate deficiency |
1. Hogan GR, Jones B. The relationship of koilonychia and iron deficiency in infants. J Pediatr. 1970;77:1054-1057.
2. Ridley CM. Pigmentation of fingertips and nails in vitamin B12 deficiency. Br J Dermatol. 1977;97:105-106.
3. Niiyama S, Mukai H. Reversible cutaneous hyperpigmentation and nails with white hair due to vitamin B12 deficiency. Eur J Dermatol. 2007;17:551-552.
4. Noppakun N, Swasdikul D. Reversible hyperpigmentation of skin and nails with white hair due to vitamin B12 deficiency. Arch Dermatol. 1986;122:896-899.
5. Carmel R. Hair and fingernail changes in acquired and congenital pernicious anemia. Arch Intern Med. 1985;145:484-485.
6. Conn RD, Smith RH. Malnutrition, myoedema, and Muehrcke’s lines. Arch Intern Med. 1965;116:875-878.
7. Amla I, Narayan JV. Finger nail clubbing in Kwashiorkor. Indian J Pediatr. 1968;35:19-22.
8. Gladen BC, Taylor JS, Wu YC, et al. Dermatological findings in children exposed transplacentally to heat-degraded polychlorinated biphenyls in Taiwan. Br J Dermatol. 1990;122:799-808.
9. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Edinburgh: Mosby; 2010:947–973.
1. Hogan GR, Jones B. The relationship of koilonychia and iron deficiency in infants. J Pediatr. 1970;77:1054-1057.
2. Ridley CM. Pigmentation of fingertips and nails in vitamin B12 deficiency. Br J Dermatol. 1977;97:105-106.
3. Niiyama S, Mukai H. Reversible cutaneous hyperpigmentation and nails with white hair due to vitamin B12 deficiency. Eur J Dermatol. 2007;17:551-552.
4. Noppakun N, Swasdikul D. Reversible hyperpigmentation of skin and nails with white hair due to vitamin B12 deficiency. Arch Dermatol. 1986;122:896-899.
5. Carmel R. Hair and fingernail changes in acquired and congenital pernicious anemia. Arch Intern Med. 1985;145:484-485.
6. Conn RD, Smith RH. Malnutrition, myoedema, and Muehrcke’s lines. Arch Intern Med. 1965;116:875-878.
7. Amla I, Narayan JV. Finger nail clubbing in Kwashiorkor. Indian J Pediatr. 1968;35:19-22.
8. Gladen BC, Taylor JS, Wu YC, et al. Dermatological findings in children exposed transplacentally to heat-degraded polychlorinated biphenyls in Taiwan. Br J Dermatol. 1990;122:799-808.
9. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Edinburgh: Mosby; 2010:947–973.
Evidence-based answers from the Family Physicians Inquiries Network
Is high-dose oral B12 a safe and effective alternative to a B12 injection?
YES. Both high-dose oral B12 and injected B12 raised low vitamin B12 levels and improved hematologic parameters and neurologic symptoms in short-term studies (3-4 months) predominantly involving patients with conditions associated with intestinal malabsorption (strength of recommendation: A, randomized controlled trials [RCTs]).
Both forms are well tolerated. Oral B12 is less expensive.
Evidence summary
Two open-label RCTs compared oral and intramuscular (IM) therapy for vitamin B12 deficiency.1,2 Both studies enrolled patients from hospital-based clinics—not primary care centers. Most patients (63 of 93 total) had conditions associated with intestinal malabsorption, including 7 patients with pernicious anemia and 3 with ileal resection. Both trials excluded patients with celiac and inflammatory bowel disease.
Oral therapy works as well as injections and costs less
One RCT compared the effects of oral B12 with IM therapy in 60 patients (mean age 62 years) with B12 deficiency and megaloblastic anemia.1 Investigators gave patients in each group equivalent doses of cobalamin: 1000 mcg daily for 10 days, weekly for 4 weeks, and then monthly to complete a 90-day course.
The mean hemoglobin increased significantly in both the oral and IM groups (from 8.4 to 13.8 g/dL, P<.001 for oral therapy; from 8.3 to 13.7 g/dL, P<.001 for IM therapy), as did mean serum B12 levels (from 73 to 214 pg/mL, P<.001, oral; and from 70 to 226 pg/mL, P<.001, IM). Neurologic symptoms (sensitive peripheral neuropathy, alteration of cognitive function, loss of sense of vibration) either cleared or improved markedly in both groups within one month (7 of 9 patients with oral therapy and 9 of 12 patients with IM treatment; P value not given).
Oral therapy cost less ($80 vs $220 per patient) and neither group reported adverse effects.1
B12 therapy changes hematologic parameters
The second RCT compared oral with IM B12 therapy in 33 patients (mean age 72 years) with newly diagnosed B12 deficiency.2 Investigators randomized patients to receive either oral cyanocobalamin (2000 mcg daily) for 120 days or IM cobalamin (1000 mcg) on Days 1, 3, 7, 10, 14, 21, 30, 60, and 90.
At 4 months, both groups had improved significantly from baseline in all metabolite measures and achieved a normal serum cobalamin level. The higher-dose oral therapy raised cobalamin levels more than IM therapy (from 93 to 1005 pg/mL, P<.0005 with oral therapy vs from 95 to 325 pg/mL, P<.0005 with IM treatment). Oral therapy increased cobalamin levels above 300 pg/mL in all patients; only half the patients treated with injections reached that level.
B12 therapy also significantly changed hematologic parameters from baseline even though the patients in the study didn’t have anemia. Mean corpuscular volume, for example, decreased from 100 to 90 fL with oral therapy and 102 to 97 fL with IM therapy (P<.005 for each). Neurologic symptoms (memory loss, paresthesias, ataxia) either cleared or improved markedly in all patients. Investigators compared all parameters against baseline values but didn’t directly compare oral with IM therapy. The trial didn’t assess safety outcomes.2
Recommendations
Canada’s British Columbia Medical Association and Ministry of Health recommend oral replacement of B12 (1000-2000 mcg/d) for most cases of vitamin B12 deficiency, including pernicious anemia. For patients with neurologic symptoms, they recommend an initial B12 injection (1000 mcg IM) followed by oral replacement.3
The US Centers for Disease Control and Prevention recommends either oral (1000 mcg daily) or parenteral B12 replacement. They advise giving parenteral therapy either subcutaneously (to reduce the burning sensation) or IM (1000 mcg per week for 8 weeks, then monthly for life).4
1. Bolaman Z, Kadikoylu G, Yukselen, et al. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center prospective, randomized, open-label study. Clin Ther. 2003;25:3124-3134.
2. Kuzminski AM, Del Giacco EJ, Allen RH, et al. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92:1191-1198.
3. British Columbia Ministry of Health and the Guidelines and Protocols Advisory Committee. B12 deficiency: investigation and management of vitamin B12 and folate deficiency. Clinical Practice Guidelines and Protocols in British Columbia. December 15, 2006. Available at: www.bcguidelines.ca/gpac/guideline_b12.html. Accessed May 27, 2010.
4. Centers for Disease Control and Prevention Managing patients with evidence of vitamin B12 deficiency. June 29, 2009. Available at: http://cdc.gov/ncbddd/b12/patients.html. Accessed May 27, 2010.
YES. Both high-dose oral B12 and injected B12 raised low vitamin B12 levels and improved hematologic parameters and neurologic symptoms in short-term studies (3-4 months) predominantly involving patients with conditions associated with intestinal malabsorption (strength of recommendation: A, randomized controlled trials [RCTs]).
Both forms are well tolerated. Oral B12 is less expensive.
Evidence summary
Two open-label RCTs compared oral and intramuscular (IM) therapy for vitamin B12 deficiency.1,2 Both studies enrolled patients from hospital-based clinics—not primary care centers. Most patients (63 of 93 total) had conditions associated with intestinal malabsorption, including 7 patients with pernicious anemia and 3 with ileal resection. Both trials excluded patients with celiac and inflammatory bowel disease.
Oral therapy works as well as injections and costs less
One RCT compared the effects of oral B12 with IM therapy in 60 patients (mean age 62 years) with B12 deficiency and megaloblastic anemia.1 Investigators gave patients in each group equivalent doses of cobalamin: 1000 mcg daily for 10 days, weekly for 4 weeks, and then monthly to complete a 90-day course.
The mean hemoglobin increased significantly in both the oral and IM groups (from 8.4 to 13.8 g/dL, P<.001 for oral therapy; from 8.3 to 13.7 g/dL, P<.001 for IM therapy), as did mean serum B12 levels (from 73 to 214 pg/mL, P<.001, oral; and from 70 to 226 pg/mL, P<.001, IM). Neurologic symptoms (sensitive peripheral neuropathy, alteration of cognitive function, loss of sense of vibration) either cleared or improved markedly in both groups within one month (7 of 9 patients with oral therapy and 9 of 12 patients with IM treatment; P value not given).
Oral therapy cost less ($80 vs $220 per patient) and neither group reported adverse effects.1
B12 therapy changes hematologic parameters
The second RCT compared oral with IM B12 therapy in 33 patients (mean age 72 years) with newly diagnosed B12 deficiency.2 Investigators randomized patients to receive either oral cyanocobalamin (2000 mcg daily) for 120 days or IM cobalamin (1000 mcg) on Days 1, 3, 7, 10, 14, 21, 30, 60, and 90.
At 4 months, both groups had improved significantly from baseline in all metabolite measures and achieved a normal serum cobalamin level. The higher-dose oral therapy raised cobalamin levels more than IM therapy (from 93 to 1005 pg/mL, P<.0005 with oral therapy vs from 95 to 325 pg/mL, P<.0005 with IM treatment). Oral therapy increased cobalamin levels above 300 pg/mL in all patients; only half the patients treated with injections reached that level.
B12 therapy also significantly changed hematologic parameters from baseline even though the patients in the study didn’t have anemia. Mean corpuscular volume, for example, decreased from 100 to 90 fL with oral therapy and 102 to 97 fL with IM therapy (P<.005 for each). Neurologic symptoms (memory loss, paresthesias, ataxia) either cleared or improved markedly in all patients. Investigators compared all parameters against baseline values but didn’t directly compare oral with IM therapy. The trial didn’t assess safety outcomes.2
Recommendations
Canada’s British Columbia Medical Association and Ministry of Health recommend oral replacement of B12 (1000-2000 mcg/d) for most cases of vitamin B12 deficiency, including pernicious anemia. For patients with neurologic symptoms, they recommend an initial B12 injection (1000 mcg IM) followed by oral replacement.3
The US Centers for Disease Control and Prevention recommends either oral (1000 mcg daily) or parenteral B12 replacement. They advise giving parenteral therapy either subcutaneously (to reduce the burning sensation) or IM (1000 mcg per week for 8 weeks, then monthly for life).4
YES. Both high-dose oral B12 and injected B12 raised low vitamin B12 levels and improved hematologic parameters and neurologic symptoms in short-term studies (3-4 months) predominantly involving patients with conditions associated with intestinal malabsorption (strength of recommendation: A, randomized controlled trials [RCTs]).
Both forms are well tolerated. Oral B12 is less expensive.
Evidence summary
Two open-label RCTs compared oral and intramuscular (IM) therapy for vitamin B12 deficiency.1,2 Both studies enrolled patients from hospital-based clinics—not primary care centers. Most patients (63 of 93 total) had conditions associated with intestinal malabsorption, including 7 patients with pernicious anemia and 3 with ileal resection. Both trials excluded patients with celiac and inflammatory bowel disease.
Oral therapy works as well as injections and costs less
One RCT compared the effects of oral B12 with IM therapy in 60 patients (mean age 62 years) with B12 deficiency and megaloblastic anemia.1 Investigators gave patients in each group equivalent doses of cobalamin: 1000 mcg daily for 10 days, weekly for 4 weeks, and then monthly to complete a 90-day course.
The mean hemoglobin increased significantly in both the oral and IM groups (from 8.4 to 13.8 g/dL, P<.001 for oral therapy; from 8.3 to 13.7 g/dL, P<.001 for IM therapy), as did mean serum B12 levels (from 73 to 214 pg/mL, P<.001, oral; and from 70 to 226 pg/mL, P<.001, IM). Neurologic symptoms (sensitive peripheral neuropathy, alteration of cognitive function, loss of sense of vibration) either cleared or improved markedly in both groups within one month (7 of 9 patients with oral therapy and 9 of 12 patients with IM treatment; P value not given).
Oral therapy cost less ($80 vs $220 per patient) and neither group reported adverse effects.1
B12 therapy changes hematologic parameters
The second RCT compared oral with IM B12 therapy in 33 patients (mean age 72 years) with newly diagnosed B12 deficiency.2 Investigators randomized patients to receive either oral cyanocobalamin (2000 mcg daily) for 120 days or IM cobalamin (1000 mcg) on Days 1, 3, 7, 10, 14, 21, 30, 60, and 90.
At 4 months, both groups had improved significantly from baseline in all metabolite measures and achieved a normal serum cobalamin level. The higher-dose oral therapy raised cobalamin levels more than IM therapy (from 93 to 1005 pg/mL, P<.0005 with oral therapy vs from 95 to 325 pg/mL, P<.0005 with IM treatment). Oral therapy increased cobalamin levels above 300 pg/mL in all patients; only half the patients treated with injections reached that level.
B12 therapy also significantly changed hematologic parameters from baseline even though the patients in the study didn’t have anemia. Mean corpuscular volume, for example, decreased from 100 to 90 fL with oral therapy and 102 to 97 fL with IM therapy (P<.005 for each). Neurologic symptoms (memory loss, paresthesias, ataxia) either cleared or improved markedly in all patients. Investigators compared all parameters against baseline values but didn’t directly compare oral with IM therapy. The trial didn’t assess safety outcomes.2
Recommendations
Canada’s British Columbia Medical Association and Ministry of Health recommend oral replacement of B12 (1000-2000 mcg/d) for most cases of vitamin B12 deficiency, including pernicious anemia. For patients with neurologic symptoms, they recommend an initial B12 injection (1000 mcg IM) followed by oral replacement.3
The US Centers for Disease Control and Prevention recommends either oral (1000 mcg daily) or parenteral B12 replacement. They advise giving parenteral therapy either subcutaneously (to reduce the burning sensation) or IM (1000 mcg per week for 8 weeks, then monthly for life).4
1. Bolaman Z, Kadikoylu G, Yukselen, et al. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center prospective, randomized, open-label study. Clin Ther. 2003;25:3124-3134.
2. Kuzminski AM, Del Giacco EJ, Allen RH, et al. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92:1191-1198.
3. British Columbia Ministry of Health and the Guidelines and Protocols Advisory Committee. B12 deficiency: investigation and management of vitamin B12 and folate deficiency. Clinical Practice Guidelines and Protocols in British Columbia. December 15, 2006. Available at: www.bcguidelines.ca/gpac/guideline_b12.html. Accessed May 27, 2010.
4. Centers for Disease Control and Prevention Managing patients with evidence of vitamin B12 deficiency. June 29, 2009. Available at: http://cdc.gov/ncbddd/b12/patients.html. Accessed May 27, 2010.
1. Bolaman Z, Kadikoylu G, Yukselen, et al. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center prospective, randomized, open-label study. Clin Ther. 2003;25:3124-3134.
2. Kuzminski AM, Del Giacco EJ, Allen RH, et al. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92:1191-1198.
3. British Columbia Ministry of Health and the Guidelines and Protocols Advisory Committee. B12 deficiency: investigation and management of vitamin B12 and folate deficiency. Clinical Practice Guidelines and Protocols in British Columbia. December 15, 2006. Available at: www.bcguidelines.ca/gpac/guideline_b12.html. Accessed May 27, 2010.
4. Centers for Disease Control and Prevention Managing patients with evidence of vitamin B12 deficiency. June 29, 2009. Available at: http://cdc.gov/ncbddd/b12/patients.html. Accessed May 27, 2010.
Evidence-based answers from the Family Physicians Inquiries Network
How best to diagnose iron-deficiency anemia in patients with inflammatory disease?
THE SERUM FERRITIN LEVEL is the most sensitive and specific initial laboratory test for iron-deficiency anemia (IDA) in patients with inflammation. Serum ferritin levels <45 ng/dL confirm IDA, and levels of ≥100 ng/dL essentially rule it out (strength of recommendation [SOR]: B, systematic review of prospective validating cohort studies with heterogeneity).
For patients with intermediate serum ferritin levels (45-99 ng/dL), the soluble transferrin receptor (sTfR) level and the sTfR-ferritin index (ratio of sTfR to log ferritin) are highly sensitive and specific. An sTfR-ferritin index of ≥1.5 is diagnostic of IDA, even in the presence of acute or chronic inflammation (SOR: B, systematic review of prospective validating cohort studies that lacked standardized reference values).
Evidence summary
A systematic review of 55 studies that included 45 validating prospective studies (total 2579 patients, of whom 919 had inflammatory, liver, or neoplastic disease) found that a low serum ferritin level was the most sensitive and specific initial test to diagnose IDA, even in the presence of inflammation.1 More than 80% of patients studied had confirmatory bone marrow aspiration.
Serum ferritin <45 ng/dL performed well for identifying IDA in all patients combined (sensitivity=85%; specificity=92%; positive likelihood ratio=11), although the authors didn’t calculate sensitivity and specificity for the subgroup of patients with an inflammatory disease process.
A serum ferritin level >100 ng/dL made IDA unlikely (sensitivity=5.5%; specificity=29%; negative likelihood ratio=0.08). Serum ferritin levels between 45 and 99 ng/dL weren’t useful in evaluating IDA. The subgroup analysis of the patients with inflammatory, liver, or neoplastic disease found that serum ferritin outperformed measurements of mean cell volume, transferrin saturation, red cell protoporphyrin, and red cell volume for diagnosing IDA.1
Follow up with the sTfR-ferritin index if necessary
The sTfR-ferritin index is sensitive and specific for diagnosing IDA in patients with concomitant inflammation, including those with intermediate serum ferritin levels of 45 to 99 ng/dL. A systematic review of 9 prospective validating cohort studies (total 818 patients, of whom 678 had inflammatory disease) compared sTfR levels and sTfR-ferritin index (sTfR-to-log ferritin ratio) values against bone marrow biopsy for identifying IDA.2
A mean sTfR level ≥2.5 mg/L diagnosed IDA with a sensitivity of 68% to 97% and a specificity of 47% to 100%. In patients with acute and chronic inflammation, a mean sTfR-ferritin index ≥1.5 mg/L had a higher sensitivity (88%-100%) and specificity (93%-100%). The studies were limited by heterogenous populations, small sample sizes, and nonstandardized techniques and reference ranges for sTfR levels.
Recommendations
No major professional organizations in the United States have issued recommendations or clinical guidelines for diagnosing iron deficiency in patients with chronic inflammation.
The British Society of Gastroenterology suggests that a serum ferritin level <50 ng/dL is consistent with iron deficiency but lists the use of sTfR as promising, but unproven, in the clinical setting.3
1. Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7:145-153.
2. Koulaouzidis A, Said E, Cottier R, et al. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009;18:345-352.
3. Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency anaemia. British Society of Gastroenterology. 2005. Available at: www.bsg.org.uk/images/stories/docs/clinical/guidelines/sbn/iron_def.pdf. Accessed February 7, 2011.
THE SERUM FERRITIN LEVEL is the most sensitive and specific initial laboratory test for iron-deficiency anemia (IDA) in patients with inflammation. Serum ferritin levels <45 ng/dL confirm IDA, and levels of ≥100 ng/dL essentially rule it out (strength of recommendation [SOR]: B, systematic review of prospective validating cohort studies with heterogeneity).
For patients with intermediate serum ferritin levels (45-99 ng/dL), the soluble transferrin receptor (sTfR) level and the sTfR-ferritin index (ratio of sTfR to log ferritin) are highly sensitive and specific. An sTfR-ferritin index of ≥1.5 is diagnostic of IDA, even in the presence of acute or chronic inflammation (SOR: B, systematic review of prospective validating cohort studies that lacked standardized reference values).
Evidence summary
A systematic review of 55 studies that included 45 validating prospective studies (total 2579 patients, of whom 919 had inflammatory, liver, or neoplastic disease) found that a low serum ferritin level was the most sensitive and specific initial test to diagnose IDA, even in the presence of inflammation.1 More than 80% of patients studied had confirmatory bone marrow aspiration.
Serum ferritin <45 ng/dL performed well for identifying IDA in all patients combined (sensitivity=85%; specificity=92%; positive likelihood ratio=11), although the authors didn’t calculate sensitivity and specificity for the subgroup of patients with an inflammatory disease process.
A serum ferritin level >100 ng/dL made IDA unlikely (sensitivity=5.5%; specificity=29%; negative likelihood ratio=0.08). Serum ferritin levels between 45 and 99 ng/dL weren’t useful in evaluating IDA. The subgroup analysis of the patients with inflammatory, liver, or neoplastic disease found that serum ferritin outperformed measurements of mean cell volume, transferrin saturation, red cell protoporphyrin, and red cell volume for diagnosing IDA.1
Follow up with the sTfR-ferritin index if necessary
The sTfR-ferritin index is sensitive and specific for diagnosing IDA in patients with concomitant inflammation, including those with intermediate serum ferritin levels of 45 to 99 ng/dL. A systematic review of 9 prospective validating cohort studies (total 818 patients, of whom 678 had inflammatory disease) compared sTfR levels and sTfR-ferritin index (sTfR-to-log ferritin ratio) values against bone marrow biopsy for identifying IDA.2
A mean sTfR level ≥2.5 mg/L diagnosed IDA with a sensitivity of 68% to 97% and a specificity of 47% to 100%. In patients with acute and chronic inflammation, a mean sTfR-ferritin index ≥1.5 mg/L had a higher sensitivity (88%-100%) and specificity (93%-100%). The studies were limited by heterogenous populations, small sample sizes, and nonstandardized techniques and reference ranges for sTfR levels.
Recommendations
No major professional organizations in the United States have issued recommendations or clinical guidelines for diagnosing iron deficiency in patients with chronic inflammation.
The British Society of Gastroenterology suggests that a serum ferritin level <50 ng/dL is consistent with iron deficiency but lists the use of sTfR as promising, but unproven, in the clinical setting.3
THE SERUM FERRITIN LEVEL is the most sensitive and specific initial laboratory test for iron-deficiency anemia (IDA) in patients with inflammation. Serum ferritin levels <45 ng/dL confirm IDA, and levels of ≥100 ng/dL essentially rule it out (strength of recommendation [SOR]: B, systematic review of prospective validating cohort studies with heterogeneity).
For patients with intermediate serum ferritin levels (45-99 ng/dL), the soluble transferrin receptor (sTfR) level and the sTfR-ferritin index (ratio of sTfR to log ferritin) are highly sensitive and specific. An sTfR-ferritin index of ≥1.5 is diagnostic of IDA, even in the presence of acute or chronic inflammation (SOR: B, systematic review of prospective validating cohort studies that lacked standardized reference values).
Evidence summary
A systematic review of 55 studies that included 45 validating prospective studies (total 2579 patients, of whom 919 had inflammatory, liver, or neoplastic disease) found that a low serum ferritin level was the most sensitive and specific initial test to diagnose IDA, even in the presence of inflammation.1 More than 80% of patients studied had confirmatory bone marrow aspiration.
Serum ferritin <45 ng/dL performed well for identifying IDA in all patients combined (sensitivity=85%; specificity=92%; positive likelihood ratio=11), although the authors didn’t calculate sensitivity and specificity for the subgroup of patients with an inflammatory disease process.
A serum ferritin level >100 ng/dL made IDA unlikely (sensitivity=5.5%; specificity=29%; negative likelihood ratio=0.08). Serum ferritin levels between 45 and 99 ng/dL weren’t useful in evaluating IDA. The subgroup analysis of the patients with inflammatory, liver, or neoplastic disease found that serum ferritin outperformed measurements of mean cell volume, transferrin saturation, red cell protoporphyrin, and red cell volume for diagnosing IDA.1
Follow up with the sTfR-ferritin index if necessary
The sTfR-ferritin index is sensitive and specific for diagnosing IDA in patients with concomitant inflammation, including those with intermediate serum ferritin levels of 45 to 99 ng/dL. A systematic review of 9 prospective validating cohort studies (total 818 patients, of whom 678 had inflammatory disease) compared sTfR levels and sTfR-ferritin index (sTfR-to-log ferritin ratio) values against bone marrow biopsy for identifying IDA.2
A mean sTfR level ≥2.5 mg/L diagnosed IDA with a sensitivity of 68% to 97% and a specificity of 47% to 100%. In patients with acute and chronic inflammation, a mean sTfR-ferritin index ≥1.5 mg/L had a higher sensitivity (88%-100%) and specificity (93%-100%). The studies were limited by heterogenous populations, small sample sizes, and nonstandardized techniques and reference ranges for sTfR levels.
Recommendations
No major professional organizations in the United States have issued recommendations or clinical guidelines for diagnosing iron deficiency in patients with chronic inflammation.
The British Society of Gastroenterology suggests that a serum ferritin level <50 ng/dL is consistent with iron deficiency but lists the use of sTfR as promising, but unproven, in the clinical setting.3
1. Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7:145-153.
2. Koulaouzidis A, Said E, Cottier R, et al. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009;18:345-352.
3. Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency anaemia. British Society of Gastroenterology. 2005. Available at: www.bsg.org.uk/images/stories/docs/clinical/guidelines/sbn/iron_def.pdf. Accessed February 7, 2011.
1. Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7:145-153.
2. Koulaouzidis A, Said E, Cottier R, et al. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009;18:345-352.
3. Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency anaemia. British Society of Gastroenterology. 2005. Available at: www.bsg.org.uk/images/stories/docs/clinical/guidelines/sbn/iron_def.pdf. Accessed February 7, 2011.
Evidence-based answers from the Family Physicians Inquiries Network
Which drugs work best for early Parkinson’s disease?
LEVODOPA/CARBIDOPA is the most effective medical therapy for Parkinson’s disease, but it’s associated with dyskinesia (strength of recommendation [SOR]: A, Cochrane reviews and randomized controlled trials [RCTs]). Treating early Parkinson’s disease with dopamine agonists such as bromocriptine can improve symptoms (SOR: B, Cochrane reviews, RCTs with heterogeneity).
Evidence summary
Levodopa/carbidopa is the most commonly prescribed medication for Parkinson’s disease. Although its efficacy is established, it can cause dyskinesia and dystonia.1 Recent studies (TABLE) have evaluated the use of other medications early in the course of Parkinson’s disease in hopes of delaying the waning effectiveness of levodopa over time.
TABLE
Medications commonly used to treat Parkinson’s disease
| Medication class brand (generic)9 | Advantages | Disadvantages | Approximate monthly cost at usual dosage (in US $) for generic (brand name prices cited if no generic available)10 |
|---|---|---|---|
| Carbidopa/levodopa Sinemet (carbidopa/ levodopa) Sinemet CR (carbidopa/levodopa controlled-release) | First-line therapy; most effective at improving motor disability1 | Dyskinesia, dystonia, hallucinations No documented benefit of long-acting form1,8 | $34.99-$101.98 $80.99-$295.97 (Highly variable due to dose range) |
| COMT inhibitor Comtan (entacapone) Stalevo (carbidopa/levodopa/entacapone) | Augments levodopa, may improve activities of daily living6 | Same side effects as above plus possible increased nausea, vomiting, diarrhea6 Possible increased cardiovascular risk and prostate cancer | $310.97-$414.62 $318.00-$1043.97 |
| Dopamine agonist Mirapex (pramipexole) Requip (ropinirole) Parlodel (bromocriptine) | Reduced dyskinesias, dystonia, and motor complications2 | Nausea, dizziness, constipation, somnolence, hallucinations, edema2 | $239.99 $71.99-$143.98 $385.97-$1133.92 |
| MAO-B inhibitor Eldepryl (selegiline) | Mild improved motor symptoms of disease, decreased motor fluctuations of treatment, possible “levosparing effect”5 | Limited efficacy and multiple adverse effects leading to high dropout rate; not recommended by Cochrane review5 | $101.99 |
| Anticholinergic Cogentin (benztropine mesylate) | Improved symptoms, mostly tremor7 | Confusion, memory loss, hallucinations, restlessness; contraindicated in dementia7 | $13.99-$22.99 |
| Other Symmetrel (amantadine) | No good updated studies, unproven long-term benefit, nausea, dizziness, insomnia, can cause psychosis9 | $43.17 | |
| COMT, catechol-O-methyltransferase; MAO-B, monoamine oxidase type B. | |||
Dopamine agonists: Dyskinesia reduction, but at a price
A Cochrane review of 29 trials with 5247 patients compared dopamine agonists with levodopa.2 Levodopa controlled symptoms better than dopamine agonists, but inconsistent data reporting prevented quantifying this result.
Compared with the group taking levodopa, patients taking dopamine agonists demonstrated a significant reduction in dyskinesia (odds ratio [OR]=0.45; 95% CI, 0.37-0.54), dystonia (OR=0.64; 95% CI, 0.51-0.81), and motor fluctuations (OR= 0.71; 95% CI, 0.58-0.87).
However, patients taking dopamine agonists with or without levodopa experienced significantly more adverse effects than patients taking levodopa alone. Side effects included increased edema (OR=3.68; 95% CI, 2.62-5.18), somnolence (OR=1.49; 95% CI, 1.12-2.00), constipation (OR=1.59; 95% CI, 1.11-2.28), dizziness (OR=1.45; 95% CI, 1.09-1.92), hallucinations (OR=1.69; 95% CI, 1.13-2.52), and nausea (OR=1.32; 95% CI, 1.05-1.66). Patients treated with dopamine agonists were also significantly more likely to discontinue treatment because of adverse events (OR=2.49; 95% CI, 2.08-2.98; P<.00001).
Bromocriptine studies hampered by poor quality
Two Cochrane reviews specifically evaluated the dopamine agonist bromocriptine.3,4 The first focused on 6 head-to-head trials with levodopa that enrolled 850 patients.3 The studies were of poor quality, marred by methodological flaws and clinical heterogeneity. Problems included inadequate power, high variability in study duration (23 weeks to 5 years), differences in reporting, and lack of description of the randomization method in 3 of the 6 trials. Although bromocriptine showed a trend toward lower incidence of motor complications, many patients dropped out of the studies because of increased non-motor adverse effects and inadequate response to treatment.
The second review, of 7 trials with a total of 1100 patients, compared bromocriptine plus levodopa with levodopa alone.4 The studies were of poor quality for reasons similar to the studies in the first review. Researchers found no statistically significant or consistent evidence to determine whether bromocriptine plus levodopa prevents or delays motor complications.
MAO-B inhibitors: Minimally effective with troubling side effects
A Cochrane review of monoamine oxidase type B (MAO-B) inhibitors included 10 trials with 2422 participants.5 The review found statistically, but not clinically, significant improvements in scores on 2 sections of the United Parkinson Disease Rating Scale (UPDRS), a standardized assessment tool that facilitates accurate documentation of disease progression and treatment response.
Compared with the control groups (either placebo or levodopa at study onset), the MAO-B group (either alone or with levodopa) showed significant improvement on the motor section (weighted mean difference [WMD]=–3.81 on a 108-point scale; 95% CI, –5.36 to –2.27) and activities of daily living section (WMD=–1.50 on a 52-point scale; 95% CI, –2.53 to –0.48). Fewer motor complications occurred in the MAO-B group (OR=0.75; 95% CI, 0.59-0.94) than the control group. Lower doses and shorter treatment with levodopa were necessary to control symptoms in the MAO-B group.
The clinical impact of MAO-B inhibitors on Parkinson’s symptoms was small, and almost all patients required the addition of levodopa to the treatment regimen after 3 or 4 years. Withdrawals because of medication side effects were significantly higher in the MAO-B inhibitor group than controls (OR=2.36; 95% CI, 1.32-4.20). Side effects included nausea, confusion, hallucinations, and postural hypotension. Concerns about cardiovascular adverse effects raised in previous studies, especially with selegiline, weren’t found to be significant (OR=1.15; 95% CI, 0.92-1.44). Because of their minimal effectiveness and worrisome adverse effects, MAO-B inhibitors aren’t recommended for routine use in early Parkinson’s disease.
COMT inhibitors may boost levodopa/carbidopa’s effects
A randomized double-blinded trial followed 423 patients for 39 weeks to compare the combination of the catechol-O-methyltransferase (COMT) inhibitor entacapone and levodopa/carbidopa (LCE) with levodopa/carbidopa alone (LC).6 The researchers found statistically significant improvements with LCE in UPDRS scores for activities of daily living (mean change from baseline=3.0 for LCE vs 2.3 for LC on a 52-point scale; P=.025) but not mentation or motor symptoms.
Dyskinesia and wearing-off symptoms (motor fluctuations) didn’t differ significantly between the 2 groups. LCE was associated with a higher incidence of adverse effects than LC, and involved mostly nausea (26.6% vs 13.5%) and diarrhea (8.7% vs 2.8%).
Anticholinergics may help, but cause adverse mental effects
Another Cochrane review compared anticholinergic agents with placebo or no treatment in 9 studies that included 221 patients.7 Meta-analysis wasn’t possible because of heterogeneity in patient populations, outcomes, and measurements and incomplete reporting. Compared with placebo, anticholinergic agents may improve Parkinson’s-related motor symptoms but have significant mental adverse effects, including confusion, memory problems, restlessness, and hallucinations.
Recommendations
The most recent guidelines (2002) from the American Academy of Neurology recommend levodopa and dopamine agonists as first-line therapies.8 Levodopa is more effective at improving the motor symptoms of Parkinson’s disease but is associated with a higher risk of dyskinesia than dopamine agonists. No compelling evidence suggests a difference in efficacy between long- and short-acting levodopa.
1. Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1-8.
2. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;(2):CD006564.-
3. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine versus levodopa in early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD002258.-
4. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine/levodopa combined versus levodopa alone for early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD003634.-
5. Macleod AD, Counsell CE, Ives N, et al. Monoamine oxidase B inhibitors for early Parkinson’s disease. Cochrane Database Syst Rev. 2005;(3):CD004898.-
6. Hauser RA, Panisset M, Abbruzzese G, et al. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/ carbidopa in early Parkinson’s disease. Mov Disord. 2009;24:541-550.
7. Katzenschlager R, Sampaio C, Costa J, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735.-
8. Miyasaki JM, Martin W, Suchowersky O, et al. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11-17.
9. Drugs for Parkinson’s disease Treat Guidl Med Lett. 2011;9:1-6
10. Drugstore.com Online Pharmacy. Pharmacy drug costs. Available at http://www.drugstore.com. Accessed August 30, 2011.
LEVODOPA/CARBIDOPA is the most effective medical therapy for Parkinson’s disease, but it’s associated with dyskinesia (strength of recommendation [SOR]: A, Cochrane reviews and randomized controlled trials [RCTs]). Treating early Parkinson’s disease with dopamine agonists such as bromocriptine can improve symptoms (SOR: B, Cochrane reviews, RCTs with heterogeneity).
Evidence summary
Levodopa/carbidopa is the most commonly prescribed medication for Parkinson’s disease. Although its efficacy is established, it can cause dyskinesia and dystonia.1 Recent studies (TABLE) have evaluated the use of other medications early in the course of Parkinson’s disease in hopes of delaying the waning effectiveness of levodopa over time.
TABLE
Medications commonly used to treat Parkinson’s disease
| Medication class brand (generic)9 | Advantages | Disadvantages | Approximate monthly cost at usual dosage (in US $) for generic (brand name prices cited if no generic available)10 |
|---|---|---|---|
| Carbidopa/levodopa Sinemet (carbidopa/ levodopa) Sinemet CR (carbidopa/levodopa controlled-release) | First-line therapy; most effective at improving motor disability1 | Dyskinesia, dystonia, hallucinations No documented benefit of long-acting form1,8 | $34.99-$101.98 $80.99-$295.97 (Highly variable due to dose range) |
| COMT inhibitor Comtan (entacapone) Stalevo (carbidopa/levodopa/entacapone) | Augments levodopa, may improve activities of daily living6 | Same side effects as above plus possible increased nausea, vomiting, diarrhea6 Possible increased cardiovascular risk and prostate cancer | $310.97-$414.62 $318.00-$1043.97 |
| Dopamine agonist Mirapex (pramipexole) Requip (ropinirole) Parlodel (bromocriptine) | Reduced dyskinesias, dystonia, and motor complications2 | Nausea, dizziness, constipation, somnolence, hallucinations, edema2 | $239.99 $71.99-$143.98 $385.97-$1133.92 |
| MAO-B inhibitor Eldepryl (selegiline) | Mild improved motor symptoms of disease, decreased motor fluctuations of treatment, possible “levosparing effect”5 | Limited efficacy and multiple adverse effects leading to high dropout rate; not recommended by Cochrane review5 | $101.99 |
| Anticholinergic Cogentin (benztropine mesylate) | Improved symptoms, mostly tremor7 | Confusion, memory loss, hallucinations, restlessness; contraindicated in dementia7 | $13.99-$22.99 |
| Other Symmetrel (amantadine) | No good updated studies, unproven long-term benefit, nausea, dizziness, insomnia, can cause psychosis9 | $43.17 | |
| COMT, catechol-O-methyltransferase; MAO-B, monoamine oxidase type B. | |||
Dopamine agonists: Dyskinesia reduction, but at a price
A Cochrane review of 29 trials with 5247 patients compared dopamine agonists with levodopa.2 Levodopa controlled symptoms better than dopamine agonists, but inconsistent data reporting prevented quantifying this result.
Compared with the group taking levodopa, patients taking dopamine agonists demonstrated a significant reduction in dyskinesia (odds ratio [OR]=0.45; 95% CI, 0.37-0.54), dystonia (OR=0.64; 95% CI, 0.51-0.81), and motor fluctuations (OR= 0.71; 95% CI, 0.58-0.87).
However, patients taking dopamine agonists with or without levodopa experienced significantly more adverse effects than patients taking levodopa alone. Side effects included increased edema (OR=3.68; 95% CI, 2.62-5.18), somnolence (OR=1.49; 95% CI, 1.12-2.00), constipation (OR=1.59; 95% CI, 1.11-2.28), dizziness (OR=1.45; 95% CI, 1.09-1.92), hallucinations (OR=1.69; 95% CI, 1.13-2.52), and nausea (OR=1.32; 95% CI, 1.05-1.66). Patients treated with dopamine agonists were also significantly more likely to discontinue treatment because of adverse events (OR=2.49; 95% CI, 2.08-2.98; P<.00001).
Bromocriptine studies hampered by poor quality
Two Cochrane reviews specifically evaluated the dopamine agonist bromocriptine.3,4 The first focused on 6 head-to-head trials with levodopa that enrolled 850 patients.3 The studies were of poor quality, marred by methodological flaws and clinical heterogeneity. Problems included inadequate power, high variability in study duration (23 weeks to 5 years), differences in reporting, and lack of description of the randomization method in 3 of the 6 trials. Although bromocriptine showed a trend toward lower incidence of motor complications, many patients dropped out of the studies because of increased non-motor adverse effects and inadequate response to treatment.
The second review, of 7 trials with a total of 1100 patients, compared bromocriptine plus levodopa with levodopa alone.4 The studies were of poor quality for reasons similar to the studies in the first review. Researchers found no statistically significant or consistent evidence to determine whether bromocriptine plus levodopa prevents or delays motor complications.
MAO-B inhibitors: Minimally effective with troubling side effects
A Cochrane review of monoamine oxidase type B (MAO-B) inhibitors included 10 trials with 2422 participants.5 The review found statistically, but not clinically, significant improvements in scores on 2 sections of the United Parkinson Disease Rating Scale (UPDRS), a standardized assessment tool that facilitates accurate documentation of disease progression and treatment response.
Compared with the control groups (either placebo or levodopa at study onset), the MAO-B group (either alone or with levodopa) showed significant improvement on the motor section (weighted mean difference [WMD]=–3.81 on a 108-point scale; 95% CI, –5.36 to –2.27) and activities of daily living section (WMD=–1.50 on a 52-point scale; 95% CI, –2.53 to –0.48). Fewer motor complications occurred in the MAO-B group (OR=0.75; 95% CI, 0.59-0.94) than the control group. Lower doses and shorter treatment with levodopa were necessary to control symptoms in the MAO-B group.
The clinical impact of MAO-B inhibitors on Parkinson’s symptoms was small, and almost all patients required the addition of levodopa to the treatment regimen after 3 or 4 years. Withdrawals because of medication side effects were significantly higher in the MAO-B inhibitor group than controls (OR=2.36; 95% CI, 1.32-4.20). Side effects included nausea, confusion, hallucinations, and postural hypotension. Concerns about cardiovascular adverse effects raised in previous studies, especially with selegiline, weren’t found to be significant (OR=1.15; 95% CI, 0.92-1.44). Because of their minimal effectiveness and worrisome adverse effects, MAO-B inhibitors aren’t recommended for routine use in early Parkinson’s disease.
COMT inhibitors may boost levodopa/carbidopa’s effects
A randomized double-blinded trial followed 423 patients for 39 weeks to compare the combination of the catechol-O-methyltransferase (COMT) inhibitor entacapone and levodopa/carbidopa (LCE) with levodopa/carbidopa alone (LC).6 The researchers found statistically significant improvements with LCE in UPDRS scores for activities of daily living (mean change from baseline=3.0 for LCE vs 2.3 for LC on a 52-point scale; P=.025) but not mentation or motor symptoms.
Dyskinesia and wearing-off symptoms (motor fluctuations) didn’t differ significantly between the 2 groups. LCE was associated with a higher incidence of adverse effects than LC, and involved mostly nausea (26.6% vs 13.5%) and diarrhea (8.7% vs 2.8%).
Anticholinergics may help, but cause adverse mental effects
Another Cochrane review compared anticholinergic agents with placebo or no treatment in 9 studies that included 221 patients.7 Meta-analysis wasn’t possible because of heterogeneity in patient populations, outcomes, and measurements and incomplete reporting. Compared with placebo, anticholinergic agents may improve Parkinson’s-related motor symptoms but have significant mental adverse effects, including confusion, memory problems, restlessness, and hallucinations.
Recommendations
The most recent guidelines (2002) from the American Academy of Neurology recommend levodopa and dopamine agonists as first-line therapies.8 Levodopa is more effective at improving the motor symptoms of Parkinson’s disease but is associated with a higher risk of dyskinesia than dopamine agonists. No compelling evidence suggests a difference in efficacy between long- and short-acting levodopa.
LEVODOPA/CARBIDOPA is the most effective medical therapy for Parkinson’s disease, but it’s associated with dyskinesia (strength of recommendation [SOR]: A, Cochrane reviews and randomized controlled trials [RCTs]). Treating early Parkinson’s disease with dopamine agonists such as bromocriptine can improve symptoms (SOR: B, Cochrane reviews, RCTs with heterogeneity).
Evidence summary
Levodopa/carbidopa is the most commonly prescribed medication for Parkinson’s disease. Although its efficacy is established, it can cause dyskinesia and dystonia.1 Recent studies (TABLE) have evaluated the use of other medications early in the course of Parkinson’s disease in hopes of delaying the waning effectiveness of levodopa over time.
TABLE
Medications commonly used to treat Parkinson’s disease
| Medication class brand (generic)9 | Advantages | Disadvantages | Approximate monthly cost at usual dosage (in US $) for generic (brand name prices cited if no generic available)10 |
|---|---|---|---|
| Carbidopa/levodopa Sinemet (carbidopa/ levodopa) Sinemet CR (carbidopa/levodopa controlled-release) | First-line therapy; most effective at improving motor disability1 | Dyskinesia, dystonia, hallucinations No documented benefit of long-acting form1,8 | $34.99-$101.98 $80.99-$295.97 (Highly variable due to dose range) |
| COMT inhibitor Comtan (entacapone) Stalevo (carbidopa/levodopa/entacapone) | Augments levodopa, may improve activities of daily living6 | Same side effects as above plus possible increased nausea, vomiting, diarrhea6 Possible increased cardiovascular risk and prostate cancer | $310.97-$414.62 $318.00-$1043.97 |
| Dopamine agonist Mirapex (pramipexole) Requip (ropinirole) Parlodel (bromocriptine) | Reduced dyskinesias, dystonia, and motor complications2 | Nausea, dizziness, constipation, somnolence, hallucinations, edema2 | $239.99 $71.99-$143.98 $385.97-$1133.92 |
| MAO-B inhibitor Eldepryl (selegiline) | Mild improved motor symptoms of disease, decreased motor fluctuations of treatment, possible “levosparing effect”5 | Limited efficacy and multiple adverse effects leading to high dropout rate; not recommended by Cochrane review5 | $101.99 |
| Anticholinergic Cogentin (benztropine mesylate) | Improved symptoms, mostly tremor7 | Confusion, memory loss, hallucinations, restlessness; contraindicated in dementia7 | $13.99-$22.99 |
| Other Symmetrel (amantadine) | No good updated studies, unproven long-term benefit, nausea, dizziness, insomnia, can cause psychosis9 | $43.17 | |
| COMT, catechol-O-methyltransferase; MAO-B, monoamine oxidase type B. | |||
Dopamine agonists: Dyskinesia reduction, but at a price
A Cochrane review of 29 trials with 5247 patients compared dopamine agonists with levodopa.2 Levodopa controlled symptoms better than dopamine agonists, but inconsistent data reporting prevented quantifying this result.
Compared with the group taking levodopa, patients taking dopamine agonists demonstrated a significant reduction in dyskinesia (odds ratio [OR]=0.45; 95% CI, 0.37-0.54), dystonia (OR=0.64; 95% CI, 0.51-0.81), and motor fluctuations (OR= 0.71; 95% CI, 0.58-0.87).
However, patients taking dopamine agonists with or without levodopa experienced significantly more adverse effects than patients taking levodopa alone. Side effects included increased edema (OR=3.68; 95% CI, 2.62-5.18), somnolence (OR=1.49; 95% CI, 1.12-2.00), constipation (OR=1.59; 95% CI, 1.11-2.28), dizziness (OR=1.45; 95% CI, 1.09-1.92), hallucinations (OR=1.69; 95% CI, 1.13-2.52), and nausea (OR=1.32; 95% CI, 1.05-1.66). Patients treated with dopamine agonists were also significantly more likely to discontinue treatment because of adverse events (OR=2.49; 95% CI, 2.08-2.98; P<.00001).
Bromocriptine studies hampered by poor quality
Two Cochrane reviews specifically evaluated the dopamine agonist bromocriptine.3,4 The first focused on 6 head-to-head trials with levodopa that enrolled 850 patients.3 The studies were of poor quality, marred by methodological flaws and clinical heterogeneity. Problems included inadequate power, high variability in study duration (23 weeks to 5 years), differences in reporting, and lack of description of the randomization method in 3 of the 6 trials. Although bromocriptine showed a trend toward lower incidence of motor complications, many patients dropped out of the studies because of increased non-motor adverse effects and inadequate response to treatment.
The second review, of 7 trials with a total of 1100 patients, compared bromocriptine plus levodopa with levodopa alone.4 The studies were of poor quality for reasons similar to the studies in the first review. Researchers found no statistically significant or consistent evidence to determine whether bromocriptine plus levodopa prevents or delays motor complications.
MAO-B inhibitors: Minimally effective with troubling side effects
A Cochrane review of monoamine oxidase type B (MAO-B) inhibitors included 10 trials with 2422 participants.5 The review found statistically, but not clinically, significant improvements in scores on 2 sections of the United Parkinson Disease Rating Scale (UPDRS), a standardized assessment tool that facilitates accurate documentation of disease progression and treatment response.
Compared with the control groups (either placebo or levodopa at study onset), the MAO-B group (either alone or with levodopa) showed significant improvement on the motor section (weighted mean difference [WMD]=–3.81 on a 108-point scale; 95% CI, –5.36 to –2.27) and activities of daily living section (WMD=–1.50 on a 52-point scale; 95% CI, –2.53 to –0.48). Fewer motor complications occurred in the MAO-B group (OR=0.75; 95% CI, 0.59-0.94) than the control group. Lower doses and shorter treatment with levodopa were necessary to control symptoms in the MAO-B group.
The clinical impact of MAO-B inhibitors on Parkinson’s symptoms was small, and almost all patients required the addition of levodopa to the treatment regimen after 3 or 4 years. Withdrawals because of medication side effects were significantly higher in the MAO-B inhibitor group than controls (OR=2.36; 95% CI, 1.32-4.20). Side effects included nausea, confusion, hallucinations, and postural hypotension. Concerns about cardiovascular adverse effects raised in previous studies, especially with selegiline, weren’t found to be significant (OR=1.15; 95% CI, 0.92-1.44). Because of their minimal effectiveness and worrisome adverse effects, MAO-B inhibitors aren’t recommended for routine use in early Parkinson’s disease.
COMT inhibitors may boost levodopa/carbidopa’s effects
A randomized double-blinded trial followed 423 patients for 39 weeks to compare the combination of the catechol-O-methyltransferase (COMT) inhibitor entacapone and levodopa/carbidopa (LCE) with levodopa/carbidopa alone (LC).6 The researchers found statistically significant improvements with LCE in UPDRS scores for activities of daily living (mean change from baseline=3.0 for LCE vs 2.3 for LC on a 52-point scale; P=.025) but not mentation or motor symptoms.
Dyskinesia and wearing-off symptoms (motor fluctuations) didn’t differ significantly between the 2 groups. LCE was associated with a higher incidence of adverse effects than LC, and involved mostly nausea (26.6% vs 13.5%) and diarrhea (8.7% vs 2.8%).
Anticholinergics may help, but cause adverse mental effects
Another Cochrane review compared anticholinergic agents with placebo or no treatment in 9 studies that included 221 patients.7 Meta-analysis wasn’t possible because of heterogeneity in patient populations, outcomes, and measurements and incomplete reporting. Compared with placebo, anticholinergic agents may improve Parkinson’s-related motor symptoms but have significant mental adverse effects, including confusion, memory problems, restlessness, and hallucinations.
Recommendations
The most recent guidelines (2002) from the American Academy of Neurology recommend levodopa and dopamine agonists as first-line therapies.8 Levodopa is more effective at improving the motor symptoms of Parkinson’s disease but is associated with a higher risk of dyskinesia than dopamine agonists. No compelling evidence suggests a difference in efficacy between long- and short-acting levodopa.
1. Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1-8.
2. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;(2):CD006564.-
3. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine versus levodopa in early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD002258.-
4. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine/levodopa combined versus levodopa alone for early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD003634.-
5. Macleod AD, Counsell CE, Ives N, et al. Monoamine oxidase B inhibitors for early Parkinson’s disease. Cochrane Database Syst Rev. 2005;(3):CD004898.-
6. Hauser RA, Panisset M, Abbruzzese G, et al. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/ carbidopa in early Parkinson’s disease. Mov Disord. 2009;24:541-550.
7. Katzenschlager R, Sampaio C, Costa J, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735.-
8. Miyasaki JM, Martin W, Suchowersky O, et al. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11-17.
9. Drugs for Parkinson’s disease Treat Guidl Med Lett. 2011;9:1-6
10. Drugstore.com Online Pharmacy. Pharmacy drug costs. Available at http://www.drugstore.com. Accessed August 30, 2011.
1. Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1-8.
2. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;(2):CD006564.-
3. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine versus levodopa in early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD002258.-
4. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine/levodopa combined versus levodopa alone for early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD003634.-
5. Macleod AD, Counsell CE, Ives N, et al. Monoamine oxidase B inhibitors for early Parkinson’s disease. Cochrane Database Syst Rev. 2005;(3):CD004898.-
6. Hauser RA, Panisset M, Abbruzzese G, et al. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/ carbidopa in early Parkinson’s disease. Mov Disord. 2009;24:541-550.
7. Katzenschlager R, Sampaio C, Costa J, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735.-
8. Miyasaki JM, Martin W, Suchowersky O, et al. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11-17.
9. Drugs for Parkinson’s disease Treat Guidl Med Lett. 2011;9:1-6
10. Drugstore.com Online Pharmacy. Pharmacy drug costs. Available at http://www.drugstore.com. Accessed August 30, 2011.
Evidence-based answers from the Family Physicians Inquiries Network