User login
What is appropriate fetal surveillance for women with diet-controlled gestational diabetes?
No evidence clearly supports the practice of increased fetal surveillance in the pregnancies of women with well-controlled (ie, fasting blood sugar <105 mg/dL) class A1 gestational diabetes (strength of recommendation [SOR]: B, consistent retrospective cohort studies). However, a number of guidelines recommend beginning surveillance of some kind between 32 and 40 weeks based on cumulative risk factors, including gestational diabetes (SOR: C, expert opinion).
Follow local standards of care and continue fetal surveillance
Julia Fashner, MD
Piqua, OH
Because malpractice issues weigh heavy in many states, a Family Physician who practices obstetrics may be liable even when a patient is at low risk. We know diabetes has devastating effects on patients. Why would there not be risk with gestational diabetes? The findings in this Clinical Inquiry provide practicing doctors little evidence for or against antenatal testing for women with gestational diabetes. I agree more research is needed to reassure physicians that increased fetal surveillance does not make a difference in fetal or maternal outcomes. Until that time, it would seem prudent to find out what your local standards of care would be—possibly non-stress testing or biophysical profiles during 32 to 40 weeks—and continue your fetal surveillance.
Evidence summary
Gestational diabetes mellitus is diagnosed when at least 2 of 4 values measured in a 3-hour glucose tolerance test are elevated; 2 different definitions of “elevated” are accepted (TABLE 1). White’s classification stratifies the risk of various types of diabetes during pregnancy (TABLE 2): Class A includes patients without a diagnosis of diabetes before pregnancy; classes B, C, and D include patients with pre-existing diabetes of increasing duration; and classes F, H, R, and T include patients with diabetes with various vascular complications.
Infants of mothers with pre-existing diabetes are at increased risk of pre- and neonatal complications (including stillbirth); it has been commonly assumed that type A1 gestational diabetes confers similar risks. However, 2 observational studies call this assumption into question. One study evaluated antepartum predictors of fetal distress requiring a cesarean delivery among 2134 pregnant women with gestational diabetes.4 Antepartum surveillance consisted of biweekly nonstress testing with amniotic fluid index determination starting at 34 weeks gestation. Of the 1501 eligible participants, the study included 810 and 580 class A1 and A2 patients, respectively; the remaining 111 were classes B–T. They considered women with A1 gestational diabetes with fasting plasma glucose levels <105 mg/dL to be well-controlled. Results of antepartum surveillance did not significantly differ among the different diabetic classes.
In univariate and multivariate analyses, the greatest indicator for cesarean section due to fetal distress was a nonreactive non-stress test with decelerations (odds ratio [OR]=5.63; 95% confidence interval [CI], 2.67–11.9). Routine amniotic fluid measurement was not significantly related to either the classification of diabetes or to cesarean delivery for fetal distress. No patients with normal surveillance testing within 4 days of delivery had a stillbirth. However, all 5 stillbirths in the study population occurred among those with A2 diabetes whose last non-stress test was >4 days prior.
An earlier retrospective study followed 97 pregnant patients with gestational diabetes, 69 of whom were diet-controlled (class A1, fasting glucose <105 mg/dL).5 Antepartum surveillance consisted of maternal monitoring and non-stress testing. At 28 weeks, pregnant patients assessed daily fetal activity; reassuring fetal well-being was defined as 10 fetal movements in a 12-hour period. At 40 weeks, a non-stress test was performed weekly. Contraction stress testing was performed for those with nonreactive non-stress tests. To observe for macrosomia, serial ultrasonography was performed every 4 to 6 weeks, starting at 28 weeks. Forty-four patients (64%) had spontaneous labors without intervention, while the rest required induction of labor or cesarean section (primary or failed induction). Five patients had primary cesarean section for suspected macrosomia, 3 patients had intervention for suspected intrauterine growth restriction, and only 4 (5.7%) patients were delivered due to fetal indications, defined as decreased fetal movement or a nonreactive nonstress test. No stillbirths or neonatal deaths occurred. Perinatal complications included hypoglycemia (n=13; 19%), hyperbilirubinemia (n=12; 17%), and macrosomia (n=11; 16%). The study did not compare complication rates between diet-controlled and insulin-requiring patients (SOR: B, retrospective study).
A Cochrane review found no evidence for or against increased surveillance in A1 gestational diabetes: “A lack of conclusive evidence has lead clinicians to equate the risk of adverse perinatal outcome with pre-existing diabetes. Consequently women are often managed with increased obstetrical monitoring, dietary regulation, and [pharmacological] treatment. However, no sound evidence base supports such intensive treatment.”6
TABLE
At least 2 of 4 measurements over 3 hours must be higher than these values to diagnose gestational diabetes mellitus
| STATUS | PLASMA OR SERUM GLUCOSE LEVEL (CARPENTER/COUSTAN CONVERSION AMERICAN DIABETES ASSOCIATION)1 | PLASMA LEVEL DATA (NATIONAL DIABETES GROUP CONVERSION AMERICAN COLLEGE OF OB/GYN)2 | ||
|---|---|---|---|---|
| MG/DL | MMOL/L | MG/DL | MMOL/L | |
| Fasting | 95 | 5.3 | 105 | 5.8 |
| 1 hour | 180 | 10.0 | 190 | 10.6 |
| 2 hours | 155 | 8.6 | 165 | 9.2 |
| 3 hours | 140 | 7.8 | 145 | 8.0 |
TABLE
White’s classification for gestational diabetes mellitus
| CLASS | DEFINITION |
|---|---|
| A1 | Diabetes diagnosed during pregnancy; non-insulin-dependent |
| A2 | Diabetes diagnosed during pregnancy; insulin-dependent |
| B | Diabetes diagnosed after age 20 years or duration less than 10 years; no vascular complications |
| C | Diabetes diagnosed between age 10 to 19 years or duration of 10 to 19 years; no vascular complications |
| D | Diabetes diagnosed before age of 10 years or duration greater than 20 years; vascular complications present |
| F | Diabetes with nephropathy |
| H | Diabetes with coronary artery or other heart disease |
| R | Diabetes with retinopathy |
| T | Diabetes status post–renal transplant |
Recommendations from others
The American College of Obstetricians and Gynecologists’ practice bulletin on gestational diabetes states that there is no consensus regarding fetal surveillance for women with diet-controlled gestational diabetes. However, local practice may include non-stress and contraction stress testing, amniotic fluid determination, and biophysical profile; this may start as early as 32 weeks to or as late as 40 weeks, based upon the total cumulative risk to the fetus from all potential complications.2 The American Diabetes Association states that increased fetal surveillance is appropriate but is not any more specific with this recommendation.1
1. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004;27(suppl 1):S88-S90.
2. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Gestational diabetes. Obstet Gynecol 2001;98:525-538.
3. White P. Classification of obstetric diabetes. Am J Obstet Gynecol 1978;130:228-230.
4. Kjos SL, Leung A, Henry OA, Victor MR, Paul RH, Medearis AL. Antepartum surveillance in diabetic pregnancies: Predictors of fetal distress in labor. Am J Obstet Gynecol 1995;173:1532-1539.
5. Landon MB, Gabbe SG. Antepartum fetal surveillance in gestational diabetes mellitus. Diabetes 1985;34(Suppl 2):50-54.
6. Tuffnell DJ, West J, Walkinshaw SA. Treatments for gestational diabetes and impaired glucose tolerance in pregnancy. Cochrane Database Syst Rev 2003;(3):CD003395.-
No evidence clearly supports the practice of increased fetal surveillance in the pregnancies of women with well-controlled (ie, fasting blood sugar <105 mg/dL) class A1 gestational diabetes (strength of recommendation [SOR]: B, consistent retrospective cohort studies). However, a number of guidelines recommend beginning surveillance of some kind between 32 and 40 weeks based on cumulative risk factors, including gestational diabetes (SOR: C, expert opinion).
Follow local standards of care and continue fetal surveillance
Julia Fashner, MD
Piqua, OH
Because malpractice issues weigh heavy in many states, a Family Physician who practices obstetrics may be liable even when a patient is at low risk. We know diabetes has devastating effects on patients. Why would there not be risk with gestational diabetes? The findings in this Clinical Inquiry provide practicing doctors little evidence for or against antenatal testing for women with gestational diabetes. I agree more research is needed to reassure physicians that increased fetal surveillance does not make a difference in fetal or maternal outcomes. Until that time, it would seem prudent to find out what your local standards of care would be—possibly non-stress testing or biophysical profiles during 32 to 40 weeks—and continue your fetal surveillance.
Evidence summary
Gestational diabetes mellitus is diagnosed when at least 2 of 4 values measured in a 3-hour glucose tolerance test are elevated; 2 different definitions of “elevated” are accepted (TABLE 1). White’s classification stratifies the risk of various types of diabetes during pregnancy (TABLE 2): Class A includes patients without a diagnosis of diabetes before pregnancy; classes B, C, and D include patients with pre-existing diabetes of increasing duration; and classes F, H, R, and T include patients with diabetes with various vascular complications.
Infants of mothers with pre-existing diabetes are at increased risk of pre- and neonatal complications (including stillbirth); it has been commonly assumed that type A1 gestational diabetes confers similar risks. However, 2 observational studies call this assumption into question. One study evaluated antepartum predictors of fetal distress requiring a cesarean delivery among 2134 pregnant women with gestational diabetes.4 Antepartum surveillance consisted of biweekly nonstress testing with amniotic fluid index determination starting at 34 weeks gestation. Of the 1501 eligible participants, the study included 810 and 580 class A1 and A2 patients, respectively; the remaining 111 were classes B–T. They considered women with A1 gestational diabetes with fasting plasma glucose levels <105 mg/dL to be well-controlled. Results of antepartum surveillance did not significantly differ among the different diabetic classes.
In univariate and multivariate analyses, the greatest indicator for cesarean section due to fetal distress was a nonreactive non-stress test with decelerations (odds ratio [OR]=5.63; 95% confidence interval [CI], 2.67–11.9). Routine amniotic fluid measurement was not significantly related to either the classification of diabetes or to cesarean delivery for fetal distress. No patients with normal surveillance testing within 4 days of delivery had a stillbirth. However, all 5 stillbirths in the study population occurred among those with A2 diabetes whose last non-stress test was >4 days prior.
An earlier retrospective study followed 97 pregnant patients with gestational diabetes, 69 of whom were diet-controlled (class A1, fasting glucose <105 mg/dL).5 Antepartum surveillance consisted of maternal monitoring and non-stress testing. At 28 weeks, pregnant patients assessed daily fetal activity; reassuring fetal well-being was defined as 10 fetal movements in a 12-hour period. At 40 weeks, a non-stress test was performed weekly. Contraction stress testing was performed for those with nonreactive non-stress tests. To observe for macrosomia, serial ultrasonography was performed every 4 to 6 weeks, starting at 28 weeks. Forty-four patients (64%) had spontaneous labors without intervention, while the rest required induction of labor or cesarean section (primary or failed induction). Five patients had primary cesarean section for suspected macrosomia, 3 patients had intervention for suspected intrauterine growth restriction, and only 4 (5.7%) patients were delivered due to fetal indications, defined as decreased fetal movement or a nonreactive nonstress test. No stillbirths or neonatal deaths occurred. Perinatal complications included hypoglycemia (n=13; 19%), hyperbilirubinemia (n=12; 17%), and macrosomia (n=11; 16%). The study did not compare complication rates between diet-controlled and insulin-requiring patients (SOR: B, retrospective study).
A Cochrane review found no evidence for or against increased surveillance in A1 gestational diabetes: “A lack of conclusive evidence has lead clinicians to equate the risk of adverse perinatal outcome with pre-existing diabetes. Consequently women are often managed with increased obstetrical monitoring, dietary regulation, and [pharmacological] treatment. However, no sound evidence base supports such intensive treatment.”6
TABLE
At least 2 of 4 measurements over 3 hours must be higher than these values to diagnose gestational diabetes mellitus
| STATUS | PLASMA OR SERUM GLUCOSE LEVEL (CARPENTER/COUSTAN CONVERSION AMERICAN DIABETES ASSOCIATION)1 | PLASMA LEVEL DATA (NATIONAL DIABETES GROUP CONVERSION AMERICAN COLLEGE OF OB/GYN)2 | ||
|---|---|---|---|---|
| MG/DL | MMOL/L | MG/DL | MMOL/L | |
| Fasting | 95 | 5.3 | 105 | 5.8 |
| 1 hour | 180 | 10.0 | 190 | 10.6 |
| 2 hours | 155 | 8.6 | 165 | 9.2 |
| 3 hours | 140 | 7.8 | 145 | 8.0 |
TABLE
White’s classification for gestational diabetes mellitus
| CLASS | DEFINITION |
|---|---|
| A1 | Diabetes diagnosed during pregnancy; non-insulin-dependent |
| A2 | Diabetes diagnosed during pregnancy; insulin-dependent |
| B | Diabetes diagnosed after age 20 years or duration less than 10 years; no vascular complications |
| C | Diabetes diagnosed between age 10 to 19 years or duration of 10 to 19 years; no vascular complications |
| D | Diabetes diagnosed before age of 10 years or duration greater than 20 years; vascular complications present |
| F | Diabetes with nephropathy |
| H | Diabetes with coronary artery or other heart disease |
| R | Diabetes with retinopathy |
| T | Diabetes status post–renal transplant |
Recommendations from others
The American College of Obstetricians and Gynecologists’ practice bulletin on gestational diabetes states that there is no consensus regarding fetal surveillance for women with diet-controlled gestational diabetes. However, local practice may include non-stress and contraction stress testing, amniotic fluid determination, and biophysical profile; this may start as early as 32 weeks to or as late as 40 weeks, based upon the total cumulative risk to the fetus from all potential complications.2 The American Diabetes Association states that increased fetal surveillance is appropriate but is not any more specific with this recommendation.1
No evidence clearly supports the practice of increased fetal surveillance in the pregnancies of women with well-controlled (ie, fasting blood sugar <105 mg/dL) class A1 gestational diabetes (strength of recommendation [SOR]: B, consistent retrospective cohort studies). However, a number of guidelines recommend beginning surveillance of some kind between 32 and 40 weeks based on cumulative risk factors, including gestational diabetes (SOR: C, expert opinion).
Follow local standards of care and continue fetal surveillance
Julia Fashner, MD
Piqua, OH
Because malpractice issues weigh heavy in many states, a Family Physician who practices obstetrics may be liable even when a patient is at low risk. We know diabetes has devastating effects on patients. Why would there not be risk with gestational diabetes? The findings in this Clinical Inquiry provide practicing doctors little evidence for or against antenatal testing for women with gestational diabetes. I agree more research is needed to reassure physicians that increased fetal surveillance does not make a difference in fetal or maternal outcomes. Until that time, it would seem prudent to find out what your local standards of care would be—possibly non-stress testing or biophysical profiles during 32 to 40 weeks—and continue your fetal surveillance.
Evidence summary
Gestational diabetes mellitus is diagnosed when at least 2 of 4 values measured in a 3-hour glucose tolerance test are elevated; 2 different definitions of “elevated” are accepted (TABLE 1). White’s classification stratifies the risk of various types of diabetes during pregnancy (TABLE 2): Class A includes patients without a diagnosis of diabetes before pregnancy; classes B, C, and D include patients with pre-existing diabetes of increasing duration; and classes F, H, R, and T include patients with diabetes with various vascular complications.
Infants of mothers with pre-existing diabetes are at increased risk of pre- and neonatal complications (including stillbirth); it has been commonly assumed that type A1 gestational diabetes confers similar risks. However, 2 observational studies call this assumption into question. One study evaluated antepartum predictors of fetal distress requiring a cesarean delivery among 2134 pregnant women with gestational diabetes.4 Antepartum surveillance consisted of biweekly nonstress testing with amniotic fluid index determination starting at 34 weeks gestation. Of the 1501 eligible participants, the study included 810 and 580 class A1 and A2 patients, respectively; the remaining 111 were classes B–T. They considered women with A1 gestational diabetes with fasting plasma glucose levels <105 mg/dL to be well-controlled. Results of antepartum surveillance did not significantly differ among the different diabetic classes.
In univariate and multivariate analyses, the greatest indicator for cesarean section due to fetal distress was a nonreactive non-stress test with decelerations (odds ratio [OR]=5.63; 95% confidence interval [CI], 2.67–11.9). Routine amniotic fluid measurement was not significantly related to either the classification of diabetes or to cesarean delivery for fetal distress. No patients with normal surveillance testing within 4 days of delivery had a stillbirth. However, all 5 stillbirths in the study population occurred among those with A2 diabetes whose last non-stress test was >4 days prior.
An earlier retrospective study followed 97 pregnant patients with gestational diabetes, 69 of whom were diet-controlled (class A1, fasting glucose <105 mg/dL).5 Antepartum surveillance consisted of maternal monitoring and non-stress testing. At 28 weeks, pregnant patients assessed daily fetal activity; reassuring fetal well-being was defined as 10 fetal movements in a 12-hour period. At 40 weeks, a non-stress test was performed weekly. Contraction stress testing was performed for those with nonreactive non-stress tests. To observe for macrosomia, serial ultrasonography was performed every 4 to 6 weeks, starting at 28 weeks. Forty-four patients (64%) had spontaneous labors without intervention, while the rest required induction of labor or cesarean section (primary or failed induction). Five patients had primary cesarean section for suspected macrosomia, 3 patients had intervention for suspected intrauterine growth restriction, and only 4 (5.7%) patients were delivered due to fetal indications, defined as decreased fetal movement or a nonreactive nonstress test. No stillbirths or neonatal deaths occurred. Perinatal complications included hypoglycemia (n=13; 19%), hyperbilirubinemia (n=12; 17%), and macrosomia (n=11; 16%). The study did not compare complication rates between diet-controlled and insulin-requiring patients (SOR: B, retrospective study).
A Cochrane review found no evidence for or against increased surveillance in A1 gestational diabetes: “A lack of conclusive evidence has lead clinicians to equate the risk of adverse perinatal outcome with pre-existing diabetes. Consequently women are often managed with increased obstetrical monitoring, dietary regulation, and [pharmacological] treatment. However, no sound evidence base supports such intensive treatment.”6
TABLE
At least 2 of 4 measurements over 3 hours must be higher than these values to diagnose gestational diabetes mellitus
| STATUS | PLASMA OR SERUM GLUCOSE LEVEL (CARPENTER/COUSTAN CONVERSION AMERICAN DIABETES ASSOCIATION)1 | PLASMA LEVEL DATA (NATIONAL DIABETES GROUP CONVERSION AMERICAN COLLEGE OF OB/GYN)2 | ||
|---|---|---|---|---|
| MG/DL | MMOL/L | MG/DL | MMOL/L | |
| Fasting | 95 | 5.3 | 105 | 5.8 |
| 1 hour | 180 | 10.0 | 190 | 10.6 |
| 2 hours | 155 | 8.6 | 165 | 9.2 |
| 3 hours | 140 | 7.8 | 145 | 8.0 |
TABLE
White’s classification for gestational diabetes mellitus
| CLASS | DEFINITION |
|---|---|
| A1 | Diabetes diagnosed during pregnancy; non-insulin-dependent |
| A2 | Diabetes diagnosed during pregnancy; insulin-dependent |
| B | Diabetes diagnosed after age 20 years or duration less than 10 years; no vascular complications |
| C | Diabetes diagnosed between age 10 to 19 years or duration of 10 to 19 years; no vascular complications |
| D | Diabetes diagnosed before age of 10 years or duration greater than 20 years; vascular complications present |
| F | Diabetes with nephropathy |
| H | Diabetes with coronary artery or other heart disease |
| R | Diabetes with retinopathy |
| T | Diabetes status post–renal transplant |
Recommendations from others
The American College of Obstetricians and Gynecologists’ practice bulletin on gestational diabetes states that there is no consensus regarding fetal surveillance for women with diet-controlled gestational diabetes. However, local practice may include non-stress and contraction stress testing, amniotic fluid determination, and biophysical profile; this may start as early as 32 weeks to or as late as 40 weeks, based upon the total cumulative risk to the fetus from all potential complications.2 The American Diabetes Association states that increased fetal surveillance is appropriate but is not any more specific with this recommendation.1
1. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004;27(suppl 1):S88-S90.
2. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Gestational diabetes. Obstet Gynecol 2001;98:525-538.
3. White P. Classification of obstetric diabetes. Am J Obstet Gynecol 1978;130:228-230.
4. Kjos SL, Leung A, Henry OA, Victor MR, Paul RH, Medearis AL. Antepartum surveillance in diabetic pregnancies: Predictors of fetal distress in labor. Am J Obstet Gynecol 1995;173:1532-1539.
5. Landon MB, Gabbe SG. Antepartum fetal surveillance in gestational diabetes mellitus. Diabetes 1985;34(Suppl 2):50-54.
6. Tuffnell DJ, West J, Walkinshaw SA. Treatments for gestational diabetes and impaired glucose tolerance in pregnancy. Cochrane Database Syst Rev 2003;(3):CD003395.-
1. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004;27(suppl 1):S88-S90.
2. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Gestational diabetes. Obstet Gynecol 2001;98:525-538.
3. White P. Classification of obstetric diabetes. Am J Obstet Gynecol 1978;130:228-230.
4. Kjos SL, Leung A, Henry OA, Victor MR, Paul RH, Medearis AL. Antepartum surveillance in diabetic pregnancies: Predictors of fetal distress in labor. Am J Obstet Gynecol 1995;173:1532-1539.
5. Landon MB, Gabbe SG. Antepartum fetal surveillance in gestational diabetes mellitus. Diabetes 1985;34(Suppl 2):50-54.
6. Tuffnell DJ, West J, Walkinshaw SA. Treatments for gestational diabetes and impaired glucose tolerance in pregnancy. Cochrane Database Syst Rev 2003;(3):CD003395.-
Evidence-based answers from the Family Physicians Inquiries Network
Does psychiatric treatment help patients with intractable chronic pain?
Tricyclic antidepressants and intensive multi-disciplinary programs are moderately effective for reducing chronic back pain; tricyclics are also effective for diabetic neuropathy and irritable bowel syndrome (strength of recommendation [SOR]: A, meta-analyses and multiple small randomized controlled trials).
Cognitive therapies are modestly effective for reducing pain in the following: chronic back pain, other chronic musculoskeletal disorders including rheumatoid arthritis (SOR: B, multiple meta-analyses with significant heterogeneity), and for chronic cancer pain (SOR: B, 1 meta-analysis of various quality studies).
Consider tricyclics for all chronic pain sufferers without a contraindication
Stan Sherman, MD
Oklahoma State University, Tulsa
Dealing with issues of chronic pain is frustrating for both clinicians and patients. With inability to relieve the patient’s pain, confounding factors of medication overuse, noncompliance, and secondary gain or malingering often cloud the clinical picture. Add to this the high rate of comorbid depression, and it makes sense to use behavioral services in treating patient’s pain.
But does it really help? The evidence indicates that behavioral treatment helps some, but it depends who is doing the treating, and the intensity of the therapy. By far the easiest evidence to put into practice is the use of tricyclic antidepressants, which should probably be prescribed to all chronic pain sufferers who do not have a medical contraindication, such as suicide risk or heart disease.
Evidence summary
Amitriptyline and other tricyclic and tetracyclic antidepressants moderately improve pain control for patients with chronic back pain.1,2 The pain reduction was independent of the presence of depression, although patients who were depressed had a significant improvement in mood. The outcome on chronic pain of antidepressants with serotonin and norepinephrine reuptake inhibitory activity is still being evaluated. It appears that those with only SSRI activity are not effective improving chronic pain.2
Tricyclics are effective for diabetic neuropathy (number needed to treat [NNT]=3.5 for 50% reduction of pain),3 and they are effective for reducing pain but not for global symptoms in irritable bowel syndrome.4 Amitriptyline reduces the pain of diabetic peripheral neuropathy in a dose related manner up to 150 mg/d, although much lower doses are often effective and cause fewer anticholinergic side-effects.
For chronic back pain, a Cochrane review including 1964 patients found strong evidence for pain reduction and modest evidence for functional improvement from intensive (>100 hours) multidisciplinary biopsychosocial rehabilitation. Less intense and less comprehensive psychophysical programs did not reduce pain or improve function.5 It was unclear if the intensive programs were generalizable. Another review found that cognitive and progressive relaxation therapy had a moderate effect on short-term pain control vs waiting-list controls for chronic back pain. However, only a third of the studies were of “high quality,” and the total number of patients in the relaxation analysis was 39.6
A systematic review of 25 studies (1672 patients) found significant effect sizes for cognitive therapies in reducing pain and other symptoms in chronic musculoskeletal pain, including rheumatoid arthritis, fibromyalgia, back, and other pain syndromes.7 However, many of the trials were small or taken from “samples of convenience” from rehabilitation and pain clinics, and most lacked documentation of randomization. For rheumatoid arthritis alone, a systematic review of 19 studies found cognitive therapies had a small but statistically significant effect on pain, functional disability, depression, coping, and self-efficacy for 1298 patients at initial follow-up. However, only “tender points” and coping remained improved at subsequent follow-ups averaging 8.5 months.8
In adults with cancer pain, a recent meta-analysis of 1723 patients showed modest but significant effects on pain from psycho-educational interventions in 25 studies.9 Although just 3 of the studies lasted 52 weeks or longer, effects were found from good-quality studies for “relaxation-promoting,” educational, and supportive counseling plus content therapies.
A significant confounder in many of these studies may be that some treatments seem more effective in secondary care than in primary care settings, as based on a systemic review of interventions for somatic symptoms in primary care.10
Recommendations from others
The NIH states that antidepressants are effective adjuvants in pain management, and that cognitive-behavioral treatments may be beneficial.11 The American Society of Anesthesiology states that “the literature supports the use of antidepressants for reducing chronic pain without notable adverse effects.”12 The Arthritis Foundation lists amitriptyline, duloxetine, fluoxetine, and paroxetine as treatment options for pain and for helping sleep in fibromyalgia.13
1. Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002;162:19-24.
2. Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine 2003;28:2540-2545.
3. Newton WP, Collins L, Fotinos C. Clinical inquiries. What is the best treatment for diabetic neuropathy? J Fam Pract 2004;53:403-408.
4. Holten KB. Irritable bowel syndrome: minimize testing, let symptoms guide treatment. J Fam Pract 2003;52:942-950.
5. Guzman J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary bio-psycho-social rehabilitation for chronic low-back pain. Cochrane Database Syst Rev 2002;(1):CD000963.
6. Ostelo RW, van Tulder MW, Vlaeyen JW, Linton SJ, Morley SJ, Assendelft WJ. Behavioural treatment for chronic low back pain. Cochrane Database Syst Rev 2005;(1):CD002014.
7. Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13.
8. Astin JA, Beckner W, Soeken K, Hochberg MC, Berman B. Psychological interventions for rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum 2002;47:291-302.
9. Devine EC. Meta-analysis of the effect of psychoeducational interventions on pain in adults with cancer. Oncol Nurs Forum 2003;30:75-89.
10. Raine R, Haines A, Sensky T, Hutchings A, Larkin K, Black N. Systematic review of mental health interventions for patients with common somatic symptoms: can research from secondary care be extrapolated to primary care? BMJ 2002;325:1082-1085.
11. The National Institutes of Health State-of-the-Science Conference on Symptom Management in Cancer: Pain Depression and Fatigue. Bethesda, Md: Oxford University Press, 2004.
12. Practice guidelines for chronic pain management. A report by the American Society of Anesthesiologists Task Force on Pain Management, Chronic Pain Section. Anesthesiology 1997;86:995-1004.
13. Fibromyalgia drugs. Arthritis Today’s Drug Guide 2005. Available at: www.arthritis.org/conditions/DrugGuide/about_fibromyalgia.asp. Accessed on February 9, 2006.
Tricyclic antidepressants and intensive multi-disciplinary programs are moderately effective for reducing chronic back pain; tricyclics are also effective for diabetic neuropathy and irritable bowel syndrome (strength of recommendation [SOR]: A, meta-analyses and multiple small randomized controlled trials).
Cognitive therapies are modestly effective for reducing pain in the following: chronic back pain, other chronic musculoskeletal disorders including rheumatoid arthritis (SOR: B, multiple meta-analyses with significant heterogeneity), and for chronic cancer pain (SOR: B, 1 meta-analysis of various quality studies).
Consider tricyclics for all chronic pain sufferers without a contraindication
Stan Sherman, MD
Oklahoma State University, Tulsa
Dealing with issues of chronic pain is frustrating for both clinicians and patients. With inability to relieve the patient’s pain, confounding factors of medication overuse, noncompliance, and secondary gain or malingering often cloud the clinical picture. Add to this the high rate of comorbid depression, and it makes sense to use behavioral services in treating patient’s pain.
But does it really help? The evidence indicates that behavioral treatment helps some, but it depends who is doing the treating, and the intensity of the therapy. By far the easiest evidence to put into practice is the use of tricyclic antidepressants, which should probably be prescribed to all chronic pain sufferers who do not have a medical contraindication, such as suicide risk or heart disease.
Evidence summary
Amitriptyline and other tricyclic and tetracyclic antidepressants moderately improve pain control for patients with chronic back pain.1,2 The pain reduction was independent of the presence of depression, although patients who were depressed had a significant improvement in mood. The outcome on chronic pain of antidepressants with serotonin and norepinephrine reuptake inhibitory activity is still being evaluated. It appears that those with only SSRI activity are not effective improving chronic pain.2
Tricyclics are effective for diabetic neuropathy (number needed to treat [NNT]=3.5 for 50% reduction of pain),3 and they are effective for reducing pain but not for global symptoms in irritable bowel syndrome.4 Amitriptyline reduces the pain of diabetic peripheral neuropathy in a dose related manner up to 150 mg/d, although much lower doses are often effective and cause fewer anticholinergic side-effects.
For chronic back pain, a Cochrane review including 1964 patients found strong evidence for pain reduction and modest evidence for functional improvement from intensive (>100 hours) multidisciplinary biopsychosocial rehabilitation. Less intense and less comprehensive psychophysical programs did not reduce pain or improve function.5 It was unclear if the intensive programs were generalizable. Another review found that cognitive and progressive relaxation therapy had a moderate effect on short-term pain control vs waiting-list controls for chronic back pain. However, only a third of the studies were of “high quality,” and the total number of patients in the relaxation analysis was 39.6
A systematic review of 25 studies (1672 patients) found significant effect sizes for cognitive therapies in reducing pain and other symptoms in chronic musculoskeletal pain, including rheumatoid arthritis, fibromyalgia, back, and other pain syndromes.7 However, many of the trials were small or taken from “samples of convenience” from rehabilitation and pain clinics, and most lacked documentation of randomization. For rheumatoid arthritis alone, a systematic review of 19 studies found cognitive therapies had a small but statistically significant effect on pain, functional disability, depression, coping, and self-efficacy for 1298 patients at initial follow-up. However, only “tender points” and coping remained improved at subsequent follow-ups averaging 8.5 months.8
In adults with cancer pain, a recent meta-analysis of 1723 patients showed modest but significant effects on pain from psycho-educational interventions in 25 studies.9 Although just 3 of the studies lasted 52 weeks or longer, effects were found from good-quality studies for “relaxation-promoting,” educational, and supportive counseling plus content therapies.
A significant confounder in many of these studies may be that some treatments seem more effective in secondary care than in primary care settings, as based on a systemic review of interventions for somatic symptoms in primary care.10
Recommendations from others
The NIH states that antidepressants are effective adjuvants in pain management, and that cognitive-behavioral treatments may be beneficial.11 The American Society of Anesthesiology states that “the literature supports the use of antidepressants for reducing chronic pain without notable adverse effects.”12 The Arthritis Foundation lists amitriptyline, duloxetine, fluoxetine, and paroxetine as treatment options for pain and for helping sleep in fibromyalgia.13
Tricyclic antidepressants and intensive multi-disciplinary programs are moderately effective for reducing chronic back pain; tricyclics are also effective for diabetic neuropathy and irritable bowel syndrome (strength of recommendation [SOR]: A, meta-analyses and multiple small randomized controlled trials).
Cognitive therapies are modestly effective for reducing pain in the following: chronic back pain, other chronic musculoskeletal disorders including rheumatoid arthritis (SOR: B, multiple meta-analyses with significant heterogeneity), and for chronic cancer pain (SOR: B, 1 meta-analysis of various quality studies).
Consider tricyclics for all chronic pain sufferers without a contraindication
Stan Sherman, MD
Oklahoma State University, Tulsa
Dealing with issues of chronic pain is frustrating for both clinicians and patients. With inability to relieve the patient’s pain, confounding factors of medication overuse, noncompliance, and secondary gain or malingering often cloud the clinical picture. Add to this the high rate of comorbid depression, and it makes sense to use behavioral services in treating patient’s pain.
But does it really help? The evidence indicates that behavioral treatment helps some, but it depends who is doing the treating, and the intensity of the therapy. By far the easiest evidence to put into practice is the use of tricyclic antidepressants, which should probably be prescribed to all chronic pain sufferers who do not have a medical contraindication, such as suicide risk or heart disease.
Evidence summary
Amitriptyline and other tricyclic and tetracyclic antidepressants moderately improve pain control for patients with chronic back pain.1,2 The pain reduction was independent of the presence of depression, although patients who were depressed had a significant improvement in mood. The outcome on chronic pain of antidepressants with serotonin and norepinephrine reuptake inhibitory activity is still being evaluated. It appears that those with only SSRI activity are not effective improving chronic pain.2
Tricyclics are effective for diabetic neuropathy (number needed to treat [NNT]=3.5 for 50% reduction of pain),3 and they are effective for reducing pain but not for global symptoms in irritable bowel syndrome.4 Amitriptyline reduces the pain of diabetic peripheral neuropathy in a dose related manner up to 150 mg/d, although much lower doses are often effective and cause fewer anticholinergic side-effects.
For chronic back pain, a Cochrane review including 1964 patients found strong evidence for pain reduction and modest evidence for functional improvement from intensive (>100 hours) multidisciplinary biopsychosocial rehabilitation. Less intense and less comprehensive psychophysical programs did not reduce pain or improve function.5 It was unclear if the intensive programs were generalizable. Another review found that cognitive and progressive relaxation therapy had a moderate effect on short-term pain control vs waiting-list controls for chronic back pain. However, only a third of the studies were of “high quality,” and the total number of patients in the relaxation analysis was 39.6
A systematic review of 25 studies (1672 patients) found significant effect sizes for cognitive therapies in reducing pain and other symptoms in chronic musculoskeletal pain, including rheumatoid arthritis, fibromyalgia, back, and other pain syndromes.7 However, many of the trials were small or taken from “samples of convenience” from rehabilitation and pain clinics, and most lacked documentation of randomization. For rheumatoid arthritis alone, a systematic review of 19 studies found cognitive therapies had a small but statistically significant effect on pain, functional disability, depression, coping, and self-efficacy for 1298 patients at initial follow-up. However, only “tender points” and coping remained improved at subsequent follow-ups averaging 8.5 months.8
In adults with cancer pain, a recent meta-analysis of 1723 patients showed modest but significant effects on pain from psycho-educational interventions in 25 studies.9 Although just 3 of the studies lasted 52 weeks or longer, effects were found from good-quality studies for “relaxation-promoting,” educational, and supportive counseling plus content therapies.
A significant confounder in many of these studies may be that some treatments seem more effective in secondary care than in primary care settings, as based on a systemic review of interventions for somatic symptoms in primary care.10
Recommendations from others
The NIH states that antidepressants are effective adjuvants in pain management, and that cognitive-behavioral treatments may be beneficial.11 The American Society of Anesthesiology states that “the literature supports the use of antidepressants for reducing chronic pain without notable adverse effects.”12 The Arthritis Foundation lists amitriptyline, duloxetine, fluoxetine, and paroxetine as treatment options for pain and for helping sleep in fibromyalgia.13
1. Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002;162:19-24.
2. Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine 2003;28:2540-2545.
3. Newton WP, Collins L, Fotinos C. Clinical inquiries. What is the best treatment for diabetic neuropathy? J Fam Pract 2004;53:403-408.
4. Holten KB. Irritable bowel syndrome: minimize testing, let symptoms guide treatment. J Fam Pract 2003;52:942-950.
5. Guzman J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary bio-psycho-social rehabilitation for chronic low-back pain. Cochrane Database Syst Rev 2002;(1):CD000963.
6. Ostelo RW, van Tulder MW, Vlaeyen JW, Linton SJ, Morley SJ, Assendelft WJ. Behavioural treatment for chronic low back pain. Cochrane Database Syst Rev 2005;(1):CD002014.
7. Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13.
8. Astin JA, Beckner W, Soeken K, Hochberg MC, Berman B. Psychological interventions for rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum 2002;47:291-302.
9. Devine EC. Meta-analysis of the effect of psychoeducational interventions on pain in adults with cancer. Oncol Nurs Forum 2003;30:75-89.
10. Raine R, Haines A, Sensky T, Hutchings A, Larkin K, Black N. Systematic review of mental health interventions for patients with common somatic symptoms: can research from secondary care be extrapolated to primary care? BMJ 2002;325:1082-1085.
11. The National Institutes of Health State-of-the-Science Conference on Symptom Management in Cancer: Pain Depression and Fatigue. Bethesda, Md: Oxford University Press, 2004.
12. Practice guidelines for chronic pain management. A report by the American Society of Anesthesiologists Task Force on Pain Management, Chronic Pain Section. Anesthesiology 1997;86:995-1004.
13. Fibromyalgia drugs. Arthritis Today’s Drug Guide 2005. Available at: www.arthritis.org/conditions/DrugGuide/about_fibromyalgia.asp. Accessed on February 9, 2006.
1. Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002;162:19-24.
2. Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine 2003;28:2540-2545.
3. Newton WP, Collins L, Fotinos C. Clinical inquiries. What is the best treatment for diabetic neuropathy? J Fam Pract 2004;53:403-408.
4. Holten KB. Irritable bowel syndrome: minimize testing, let symptoms guide treatment. J Fam Pract 2003;52:942-950.
5. Guzman J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary bio-psycho-social rehabilitation for chronic low-back pain. Cochrane Database Syst Rev 2002;(1):CD000963.
6. Ostelo RW, van Tulder MW, Vlaeyen JW, Linton SJ, Morley SJ, Assendelft WJ. Behavioural treatment for chronic low back pain. Cochrane Database Syst Rev 2005;(1):CD002014.
7. Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13.
8. Astin JA, Beckner W, Soeken K, Hochberg MC, Berman B. Psychological interventions for rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum 2002;47:291-302.
9. Devine EC. Meta-analysis of the effect of psychoeducational interventions on pain in adults with cancer. Oncol Nurs Forum 2003;30:75-89.
10. Raine R, Haines A, Sensky T, Hutchings A, Larkin K, Black N. Systematic review of mental health interventions for patients with common somatic symptoms: can research from secondary care be extrapolated to primary care? BMJ 2002;325:1082-1085.
11. The National Institutes of Health State-of-the-Science Conference on Symptom Management in Cancer: Pain Depression and Fatigue. Bethesda, Md: Oxford University Press, 2004.
12. Practice guidelines for chronic pain management. A report by the American Society of Anesthesiologists Task Force on Pain Management, Chronic Pain Section. Anesthesiology 1997;86:995-1004.
13. Fibromyalgia drugs. Arthritis Today’s Drug Guide 2005. Available at: www.arthritis.org/conditions/DrugGuide/about_fibromyalgia.asp. Accessed on February 9, 2006.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best duration of steroid therapy for contact dermatitis (rhus)?
Scant evidence exists for the best duration of steroid therapy for contact dermatitis due to plants (rhus). Review articles recommend 10 to 21 days of treatment with topical or oral corticosteroids for moderate to severe contact dermatitis due to plants (strength of recommendation [SOR]: C, based on review articles). The primary reason given for the duration of 2 to 3 weeks is to prevent rebound dermatitis.
Prescribe oral steroids for severe cases
Brian Crownover, MD, FAAFP, Lt Col, USAF, MC
US Air Force, Eglin Family Medicine Residency, Eglin Air Force Base, Eglin, Fla
Evidence for the best treatment of rhus dermatitis is negligible. Most recommendations stem from review articles and expert opinion. Rhus dermatitis is one example of a disorder for which we must fall back on our logic and personal experience. Since the painful itchy blisters and erythema from the oleoresin may take up to 1 week to appear, and because the rash may persist for more than 2 weeks, it makes sense to prescribe oral steroids in severe cases for longer than the usual 5- to 7-day burst. Habif, a popular dermatology text, suggests gradually tapering steroids from 60 to 10 mg over a 14-day course.1 In the absence of any randomized controlled trials (and remembering my patient who bounced back after I only gave 1 week of steroids), I will continue to prescribe 14 days of oral steroids.
Evidence summary
No published studies compare varying durations of treatment with steroids for contact dermatitis due to plants, including rhus. Many review articles refer to rebound dermatitis when using courses of oral steroids (such as Medrol dosepaks) for fewer than 14 days. One case report noted failure of a tapering dose over 5 days of oral methylprednisolone for treatment of poison ivy contact dermatitis.2
Most review articles recommend systemic steroids for severe poison ivy contact dermatitis, but these articles do not define “severe,” describe the taper, or give a definite length of treatment.3-5 One review recommends a tapering dose of oral prednisone to prevent rebound recurrence if the rash affects >25% of the body surface area, has severe blistering or itching, or significantly involves the face, hands, or genital area. That review suggests starting with oral prednisone 60 mg/d for 4 days, followed by a 10-day taper (50 mg/d for 2 days, 40 mg/d for 2 days, 30 mg/d for 2 days, 20 mg/d for 2 days, then 10 mg/d for 2 days).3
Another review recommends using systemic steroids for severe cases, defined as involvement of greater than 20% of total body surface area, bullae formation, or extensive facial involvement. That review recommends a starting dose of 1 mg/kg/d, or 40 to 60 mg/d in adults, followed by a 2- to 3-week taper of oral prednisone.4 A third review recommends using prednisone for children with allergic contact dermatitis involving more than 10% of the total body surface area.5
Recommendations from others
Guidelines for treatment of contact dermatitis published by the American Academy of Dermatology recommend topical treatment alone for mild cases of contact dermatitis, defined as “limited site of involvement, acute contact dermatitis when the offending agent has been removed, or chronic contact dermatitis with limited symptoms.” The guideline states that systemic treatment may be indicated to control itching or edema, or for moderate to severe cases. The systemic treatments listed include oral or intramuscular corticosteroids, but no discussion of duration is mentioned.6
UpToDate discusses avoidance of the offending substance for 2 to 4 weeks, use of topical corticosteroids of medium to strong potency for a limited time (without defining the duration), and use of systemic corticosteroids in severe cases, prescribing a course of prednisone at 40 mg daily for 4 to 6 days followed by 20 mg for 4 to 6 days.7
eMedicine states that although oral systemic steroids, with a taper of prednisone over 10 to 14 days, are the standard for severe toxicodendron dermatitis, some authors suggest high-potency steroid creams twice daily for a week, then daily for a week.8
ACP Medicine states that most cases of allergic contact dermatitis are “effectively managed without use of systemic corticosteroids,” but that “short courses of systemic corticosteroids are indicated for patients with severe vesiculobullous eruptions of the hands and feet or face,” without describing duration or dose.9
1. Habif TP. Clinical Dermatology. 4th ed. St. Louis, Mo: Mosby; 2004.
2. Ives TJ, Tepper RS. Failure of a tapering dose of oral methylprednisolone to treat reactions to poison ivy. JAMA 1991;266:1362.-
3. Brodell RT, Williams L. Taking the itch out of poison ivy: are you prescribing the right medication? Postgrad Med 1999;106:69-70.
4. Li LY, Cruz PD. Allergic contact dermatitis: Pathophysiology applied to future therapy. Dermatologic Therapy 2004;17:219-223.
5. Bruckner AL, Weston WL. Allergic contact dermatitis in children: A practical approach to management. Skin Therapy Letter 2002;7:3-5.
6. Rietschel RL, Adams RM, Daily AD. Guidelines of care for contact dermatitis. J Am Acad Dermatol 1995;32:109-113.
7. Overview of dermatitis. UpToDate [database]. Available at: www.uptodateonline.com/application/topic.asp?file=pri_derm/15769&type=A&selectedTitle=1~4. Accessed on January 10, 2006.
8. Stephanides SL. Plant poisoning, toxicodendron. E-Medicine [website]. Updated September 25, 2001. Available at www.emedicine.com/emerg/topic452.htm. Accessed on January 10, 2006.
9. Taylor JS. Contact dermatitis and related disorders. ACP Medicine. Updated September, 2005. Available at: www.acpmedicine.com/cgi-bin/publiccgi.pl?loginOP. Accessed on January 10, 2006.
Scant evidence exists for the best duration of steroid therapy for contact dermatitis due to plants (rhus). Review articles recommend 10 to 21 days of treatment with topical or oral corticosteroids for moderate to severe contact dermatitis due to plants (strength of recommendation [SOR]: C, based on review articles). The primary reason given for the duration of 2 to 3 weeks is to prevent rebound dermatitis.
Prescribe oral steroids for severe cases
Brian Crownover, MD, FAAFP, Lt Col, USAF, MC
US Air Force, Eglin Family Medicine Residency, Eglin Air Force Base, Eglin, Fla
Evidence for the best treatment of rhus dermatitis is negligible. Most recommendations stem from review articles and expert opinion. Rhus dermatitis is one example of a disorder for which we must fall back on our logic and personal experience. Since the painful itchy blisters and erythema from the oleoresin may take up to 1 week to appear, and because the rash may persist for more than 2 weeks, it makes sense to prescribe oral steroids in severe cases for longer than the usual 5- to 7-day burst. Habif, a popular dermatology text, suggests gradually tapering steroids from 60 to 10 mg over a 14-day course.1 In the absence of any randomized controlled trials (and remembering my patient who bounced back after I only gave 1 week of steroids), I will continue to prescribe 14 days of oral steroids.
Evidence summary
No published studies compare varying durations of treatment with steroids for contact dermatitis due to plants, including rhus. Many review articles refer to rebound dermatitis when using courses of oral steroids (such as Medrol dosepaks) for fewer than 14 days. One case report noted failure of a tapering dose over 5 days of oral methylprednisolone for treatment of poison ivy contact dermatitis.2
Most review articles recommend systemic steroids for severe poison ivy contact dermatitis, but these articles do not define “severe,” describe the taper, or give a definite length of treatment.3-5 One review recommends a tapering dose of oral prednisone to prevent rebound recurrence if the rash affects >25% of the body surface area, has severe blistering or itching, or significantly involves the face, hands, or genital area. That review suggests starting with oral prednisone 60 mg/d for 4 days, followed by a 10-day taper (50 mg/d for 2 days, 40 mg/d for 2 days, 30 mg/d for 2 days, 20 mg/d for 2 days, then 10 mg/d for 2 days).3
Another review recommends using systemic steroids for severe cases, defined as involvement of greater than 20% of total body surface area, bullae formation, or extensive facial involvement. That review recommends a starting dose of 1 mg/kg/d, or 40 to 60 mg/d in adults, followed by a 2- to 3-week taper of oral prednisone.4 A third review recommends using prednisone for children with allergic contact dermatitis involving more than 10% of the total body surface area.5
Recommendations from others
Guidelines for treatment of contact dermatitis published by the American Academy of Dermatology recommend topical treatment alone for mild cases of contact dermatitis, defined as “limited site of involvement, acute contact dermatitis when the offending agent has been removed, or chronic contact dermatitis with limited symptoms.” The guideline states that systemic treatment may be indicated to control itching or edema, or for moderate to severe cases. The systemic treatments listed include oral or intramuscular corticosteroids, but no discussion of duration is mentioned.6
UpToDate discusses avoidance of the offending substance for 2 to 4 weeks, use of topical corticosteroids of medium to strong potency for a limited time (without defining the duration), and use of systemic corticosteroids in severe cases, prescribing a course of prednisone at 40 mg daily for 4 to 6 days followed by 20 mg for 4 to 6 days.7
eMedicine states that although oral systemic steroids, with a taper of prednisone over 10 to 14 days, are the standard for severe toxicodendron dermatitis, some authors suggest high-potency steroid creams twice daily for a week, then daily for a week.8
ACP Medicine states that most cases of allergic contact dermatitis are “effectively managed without use of systemic corticosteroids,” but that “short courses of systemic corticosteroids are indicated for patients with severe vesiculobullous eruptions of the hands and feet or face,” without describing duration or dose.9
Scant evidence exists for the best duration of steroid therapy for contact dermatitis due to plants (rhus). Review articles recommend 10 to 21 days of treatment with topical or oral corticosteroids for moderate to severe contact dermatitis due to plants (strength of recommendation [SOR]: C, based on review articles). The primary reason given for the duration of 2 to 3 weeks is to prevent rebound dermatitis.
Prescribe oral steroids for severe cases
Brian Crownover, MD, FAAFP, Lt Col, USAF, MC
US Air Force, Eglin Family Medicine Residency, Eglin Air Force Base, Eglin, Fla
Evidence for the best treatment of rhus dermatitis is negligible. Most recommendations stem from review articles and expert opinion. Rhus dermatitis is one example of a disorder for which we must fall back on our logic and personal experience. Since the painful itchy blisters and erythema from the oleoresin may take up to 1 week to appear, and because the rash may persist for more than 2 weeks, it makes sense to prescribe oral steroids in severe cases for longer than the usual 5- to 7-day burst. Habif, a popular dermatology text, suggests gradually tapering steroids from 60 to 10 mg over a 14-day course.1 In the absence of any randomized controlled trials (and remembering my patient who bounced back after I only gave 1 week of steroids), I will continue to prescribe 14 days of oral steroids.
Evidence summary
No published studies compare varying durations of treatment with steroids for contact dermatitis due to plants, including rhus. Many review articles refer to rebound dermatitis when using courses of oral steroids (such as Medrol dosepaks) for fewer than 14 days. One case report noted failure of a tapering dose over 5 days of oral methylprednisolone for treatment of poison ivy contact dermatitis.2
Most review articles recommend systemic steroids for severe poison ivy contact dermatitis, but these articles do not define “severe,” describe the taper, or give a definite length of treatment.3-5 One review recommends a tapering dose of oral prednisone to prevent rebound recurrence if the rash affects >25% of the body surface area, has severe blistering or itching, or significantly involves the face, hands, or genital area. That review suggests starting with oral prednisone 60 mg/d for 4 days, followed by a 10-day taper (50 mg/d for 2 days, 40 mg/d for 2 days, 30 mg/d for 2 days, 20 mg/d for 2 days, then 10 mg/d for 2 days).3
Another review recommends using systemic steroids for severe cases, defined as involvement of greater than 20% of total body surface area, bullae formation, or extensive facial involvement. That review recommends a starting dose of 1 mg/kg/d, or 40 to 60 mg/d in adults, followed by a 2- to 3-week taper of oral prednisone.4 A third review recommends using prednisone for children with allergic contact dermatitis involving more than 10% of the total body surface area.5
Recommendations from others
Guidelines for treatment of contact dermatitis published by the American Academy of Dermatology recommend topical treatment alone for mild cases of contact dermatitis, defined as “limited site of involvement, acute contact dermatitis when the offending agent has been removed, or chronic contact dermatitis with limited symptoms.” The guideline states that systemic treatment may be indicated to control itching or edema, or for moderate to severe cases. The systemic treatments listed include oral or intramuscular corticosteroids, but no discussion of duration is mentioned.6
UpToDate discusses avoidance of the offending substance for 2 to 4 weeks, use of topical corticosteroids of medium to strong potency for a limited time (without defining the duration), and use of systemic corticosteroids in severe cases, prescribing a course of prednisone at 40 mg daily for 4 to 6 days followed by 20 mg for 4 to 6 days.7
eMedicine states that although oral systemic steroids, with a taper of prednisone over 10 to 14 days, are the standard for severe toxicodendron dermatitis, some authors suggest high-potency steroid creams twice daily for a week, then daily for a week.8
ACP Medicine states that most cases of allergic contact dermatitis are “effectively managed without use of systemic corticosteroids,” but that “short courses of systemic corticosteroids are indicated for patients with severe vesiculobullous eruptions of the hands and feet or face,” without describing duration or dose.9
1. Habif TP. Clinical Dermatology. 4th ed. St. Louis, Mo: Mosby; 2004.
2. Ives TJ, Tepper RS. Failure of a tapering dose of oral methylprednisolone to treat reactions to poison ivy. JAMA 1991;266:1362.-
3. Brodell RT, Williams L. Taking the itch out of poison ivy: are you prescribing the right medication? Postgrad Med 1999;106:69-70.
4. Li LY, Cruz PD. Allergic contact dermatitis: Pathophysiology applied to future therapy. Dermatologic Therapy 2004;17:219-223.
5. Bruckner AL, Weston WL. Allergic contact dermatitis in children: A practical approach to management. Skin Therapy Letter 2002;7:3-5.
6. Rietschel RL, Adams RM, Daily AD. Guidelines of care for contact dermatitis. J Am Acad Dermatol 1995;32:109-113.
7. Overview of dermatitis. UpToDate [database]. Available at: www.uptodateonline.com/application/topic.asp?file=pri_derm/15769&type=A&selectedTitle=1~4. Accessed on January 10, 2006.
8. Stephanides SL. Plant poisoning, toxicodendron. E-Medicine [website]. Updated September 25, 2001. Available at www.emedicine.com/emerg/topic452.htm. Accessed on January 10, 2006.
9. Taylor JS. Contact dermatitis and related disorders. ACP Medicine. Updated September, 2005. Available at: www.acpmedicine.com/cgi-bin/publiccgi.pl?loginOP. Accessed on January 10, 2006.
1. Habif TP. Clinical Dermatology. 4th ed. St. Louis, Mo: Mosby; 2004.
2. Ives TJ, Tepper RS. Failure of a tapering dose of oral methylprednisolone to treat reactions to poison ivy. JAMA 1991;266:1362.-
3. Brodell RT, Williams L. Taking the itch out of poison ivy: are you prescribing the right medication? Postgrad Med 1999;106:69-70.
4. Li LY, Cruz PD. Allergic contact dermatitis: Pathophysiology applied to future therapy. Dermatologic Therapy 2004;17:219-223.
5. Bruckner AL, Weston WL. Allergic contact dermatitis in children: A practical approach to management. Skin Therapy Letter 2002;7:3-5.
6. Rietschel RL, Adams RM, Daily AD. Guidelines of care for contact dermatitis. J Am Acad Dermatol 1995;32:109-113.
7. Overview of dermatitis. UpToDate [database]. Available at: www.uptodateonline.com/application/topic.asp?file=pri_derm/15769&type=A&selectedTitle=1~4. Accessed on January 10, 2006.
8. Stephanides SL. Plant poisoning, toxicodendron. E-Medicine [website]. Updated September 25, 2001. Available at www.emedicine.com/emerg/topic452.htm. Accessed on January 10, 2006.
9. Taylor JS. Contact dermatitis and related disorders. ACP Medicine. Updated September, 2005. Available at: www.acpmedicine.com/cgi-bin/publiccgi.pl?loginOP. Accessed on January 10, 2006.
Evidence-based answers from the Family Physicians Inquiries Network
What is the recommended approach to asymptomatic patients who develop a reactive PPD?
Clinical evaluation and chest x-ray are recommended for asymptomatic patients with a positive purified protein derivative (PPD) test result, to exclude the slight possibility of active tuberculosis (TB). Patients with radiographic evidence of old (healed) TB infection should also undergo sputum testing (strength of recommendation [SOR]: C, expert opinion).
Treatment with isoniazid (INH) monotherapy (300 mg/d) reduces progression of latent tuberculosis to active disease (SOR: A, large randomized controlled trials [RCT]), with 9 months as the optimal treatment length (SOR: B, derivation from RCTs). A 3-month course of combined rifampin (600 mg/d) and INH (300 mg/d) is equivalent in efficacy to INH monotherapy and is associated with similar rates of toxicity (SOR: A, meta-analysis of RCTs), but this regimen is not included in Centers for Disease Control and Prevention recommendations.
Address patient concerns about TB and treatment side effects
Richard Guthmann, MD
University of Illinois at Chicago/Advocate Illinois Masonic Family Medicine Residency, Chicago
Patients’ understanding of tuberculosis—the disease, the treatment, and the outcome—poses an important challenge in the care of an asymptomatic PPD-positive patient. These patients may ask, “Will I get sick? Do I have to take the medicine? Are there side effects? And would you take the medicine?” We need to be prepared to answer these questions.
Most patients with a positive PPD will not get active tuberculosis, but when they do it can be serious and it can spread easily. The medication significantly decreases the risk of developing active tuberculosis. The medication side effects are uncommon but can be severe. These side effects are reversible if the medication is stopped promptly. Under the supervision of my physician, I would take the medicine.
Evidence summary
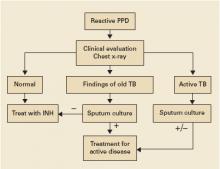
Clinical evaluation with medical history and physical exam, chest radiography, and selected sputum sampling to exclude active tuberculosis are part of the recommended algorithm for all patients who develop a positive PPD (FIGURE).1-3 These recommendations are derived from expert opinion, and their usefulness has not been evaluated in any population-based study of asymptomatic PPD-positive patients.
A comprehensive review of RCTs from the 1950s and 1960s demonstrated that INH treatment of patients with latent tuberculosis infection is effective in decreasing the progression to active tuberculosis.4 A series of double-blinded RCTs performed by the US Public Health Service included 25,923 patients with latent tuberculosis who were randomized to receive either daily INH or placebo for 1 year with 6- to 10-year follow-up. Groups studied included household contacts of patients with active tuberculosis (rate of progression to active disease in placebo group [baseline rate]=27/1000, relative risk with INH [RR]=0.4, number needed to treat [NNT]=63), patients in mental institutions (baseline rate=12/1000, RR=0.3, NNT=121), and patients with x-ray findings of healed tuberculosis (baseline rate=69/1000, RR=0.4, NNT=23).
The optimal length of treatment for PPD-positive patients without active disease was evaluated through 1 double-blinded RCT enrolling 28,000 patients with 5-year follow-up after 12, 24, or 52 weeks of INH or placebo. Active TB developed in 0.35% (24/6919) after 52 weeks of INH compared with 0.49% (34/6965) after 24 weeks (RR=1.4, NNT=708).5 Incidence in the placebo group was 1.4%. Subgroup analysis determined that maximum efficacy with fewest side effects was achieved at 9 months.6 Nine months of INH is also recommended for HIV-positive patients, based on extrapolations from these and other studies.3
INH monotherapy was compared with combination INH and rifampin in a 2005 meta-analysis of 5 RCTs of variable quality involving 1926 patients.7 This meta-analysis found equivalency in risk of active TB and mortality between INH monotherapy for 6 to 12 months and the combination of rifampin and INH for 3 months (pooled risk difference=0%; 95% confidence interval [CI], –1% to 2%). This study also showed similar rates of adverse events in both groups (pooled risk difference=–1%; 95% CI, –7% to 5%). Short-course combination rifampin and pyrazinamide is no longer recommended after an open-label RCT with 589 patients demonstrated severe hepatoxicity in 7.7% (16/207) on a 2-month course of pyrazinamide and rifampin, compared with 1% (2/204) on 6 months of INH (RR=7.9, number needed to harm=15).8 Rifampin monotherapy has only been studied in patients with silicosis in a RCT enrolling 652 participants with latent tuberculosis. A 12-week course of rifampin (600 mg daily) was as effective as 6 months of INH in preventing development of active TB over the next 5 years.9
FIGURE
Suggested workup of asymptomatic, HIV-negative patients with a positive PPD
Source: Am J Respir Crit Care Med 2000;2 Jasmer et al, N Engl J Med 2002.3
Recommendations from others
Centers for Disease Control and Prevention, American Thoracic Society, and Infectious Disease Society of America guidelines recommend targeted screening of high-risk persons followed by further clinical evaluation of all those with a reactive PPD (FIGURE).2,10 The recommended treatment regimen for latent TB is daily INH for 9 months. Less preferable regimens are daily INH for 6 months, or daily rifampin for 4 months in patients who cannot tolerate INH. A 2-month course of rifampin and pyrazinamide is no longer recommended. The recent meta-analysis supporting a 3-month regimen of combination INH and rifampin has not been incorporated into expert guidelines.7
1. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-1395.
2. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-S247.
3. Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med 2002;347:1860-1866.
4. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc 1970;26:28-106.
5. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 1982;60:555-564.
6. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999;3:847-850.
7. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005;40:670-676.
8. Jasmer RM, Saukkonen JJ, Blumberg HM, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med 2002;137:640-647.
9. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36-41.
10. Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. MMWR Recomm Rep 2005;54(RR-12):1-81.
Clinical evaluation and chest x-ray are recommended for asymptomatic patients with a positive purified protein derivative (PPD) test result, to exclude the slight possibility of active tuberculosis (TB). Patients with radiographic evidence of old (healed) TB infection should also undergo sputum testing (strength of recommendation [SOR]: C, expert opinion).
Treatment with isoniazid (INH) monotherapy (300 mg/d) reduces progression of latent tuberculosis to active disease (SOR: A, large randomized controlled trials [RCT]), with 9 months as the optimal treatment length (SOR: B, derivation from RCTs). A 3-month course of combined rifampin (600 mg/d) and INH (300 mg/d) is equivalent in efficacy to INH monotherapy and is associated with similar rates of toxicity (SOR: A, meta-analysis of RCTs), but this regimen is not included in Centers for Disease Control and Prevention recommendations.
Address patient concerns about TB and treatment side effects
Richard Guthmann, MD
University of Illinois at Chicago/Advocate Illinois Masonic Family Medicine Residency, Chicago
Patients’ understanding of tuberculosis—the disease, the treatment, and the outcome—poses an important challenge in the care of an asymptomatic PPD-positive patient. These patients may ask, “Will I get sick? Do I have to take the medicine? Are there side effects? And would you take the medicine?” We need to be prepared to answer these questions.
Most patients with a positive PPD will not get active tuberculosis, but when they do it can be serious and it can spread easily. The medication significantly decreases the risk of developing active tuberculosis. The medication side effects are uncommon but can be severe. These side effects are reversible if the medication is stopped promptly. Under the supervision of my physician, I would take the medicine.
Evidence summary
Clinical evaluation with medical history and physical exam, chest radiography, and selected sputum sampling to exclude active tuberculosis are part of the recommended algorithm for all patients who develop a positive PPD (FIGURE).1-3 These recommendations are derived from expert opinion, and their usefulness has not been evaluated in any population-based study of asymptomatic PPD-positive patients.
A comprehensive review of RCTs from the 1950s and 1960s demonstrated that INH treatment of patients with latent tuberculosis infection is effective in decreasing the progression to active tuberculosis.4 A series of double-blinded RCTs performed by the US Public Health Service included 25,923 patients with latent tuberculosis who were randomized to receive either daily INH or placebo for 1 year with 6- to 10-year follow-up. Groups studied included household contacts of patients with active tuberculosis (rate of progression to active disease in placebo group [baseline rate]=27/1000, relative risk with INH [RR]=0.4, number needed to treat [NNT]=63), patients in mental institutions (baseline rate=12/1000, RR=0.3, NNT=121), and patients with x-ray findings of healed tuberculosis (baseline rate=69/1000, RR=0.4, NNT=23).
The optimal length of treatment for PPD-positive patients without active disease was evaluated through 1 double-blinded RCT enrolling 28,000 patients with 5-year follow-up after 12, 24, or 52 weeks of INH or placebo. Active TB developed in 0.35% (24/6919) after 52 weeks of INH compared with 0.49% (34/6965) after 24 weeks (RR=1.4, NNT=708).5 Incidence in the placebo group was 1.4%. Subgroup analysis determined that maximum efficacy with fewest side effects was achieved at 9 months.6 Nine months of INH is also recommended for HIV-positive patients, based on extrapolations from these and other studies.3
INH monotherapy was compared with combination INH and rifampin in a 2005 meta-analysis of 5 RCTs of variable quality involving 1926 patients.7 This meta-analysis found equivalency in risk of active TB and mortality between INH monotherapy for 6 to 12 months and the combination of rifampin and INH for 3 months (pooled risk difference=0%; 95% confidence interval [CI], –1% to 2%). This study also showed similar rates of adverse events in both groups (pooled risk difference=–1%; 95% CI, –7% to 5%). Short-course combination rifampin and pyrazinamide is no longer recommended after an open-label RCT with 589 patients demonstrated severe hepatoxicity in 7.7% (16/207) on a 2-month course of pyrazinamide and rifampin, compared with 1% (2/204) on 6 months of INH (RR=7.9, number needed to harm=15).8 Rifampin monotherapy has only been studied in patients with silicosis in a RCT enrolling 652 participants with latent tuberculosis. A 12-week course of rifampin (600 mg daily) was as effective as 6 months of INH in preventing development of active TB over the next 5 years.9
FIGURE
Suggested workup of asymptomatic, HIV-negative patients with a positive PPD
Source: Am J Respir Crit Care Med 2000;2 Jasmer et al, N Engl J Med 2002.3
Recommendations from others
Centers for Disease Control and Prevention, American Thoracic Society, and Infectious Disease Society of America guidelines recommend targeted screening of high-risk persons followed by further clinical evaluation of all those with a reactive PPD (FIGURE).2,10 The recommended treatment regimen for latent TB is daily INH for 9 months. Less preferable regimens are daily INH for 6 months, or daily rifampin for 4 months in patients who cannot tolerate INH. A 2-month course of rifampin and pyrazinamide is no longer recommended. The recent meta-analysis supporting a 3-month regimen of combination INH and rifampin has not been incorporated into expert guidelines.7
Clinical evaluation and chest x-ray are recommended for asymptomatic patients with a positive purified protein derivative (PPD) test result, to exclude the slight possibility of active tuberculosis (TB). Patients with radiographic evidence of old (healed) TB infection should also undergo sputum testing (strength of recommendation [SOR]: C, expert opinion).
Treatment with isoniazid (INH) monotherapy (300 mg/d) reduces progression of latent tuberculosis to active disease (SOR: A, large randomized controlled trials [RCT]), with 9 months as the optimal treatment length (SOR: B, derivation from RCTs). A 3-month course of combined rifampin (600 mg/d) and INH (300 mg/d) is equivalent in efficacy to INH monotherapy and is associated with similar rates of toxicity (SOR: A, meta-analysis of RCTs), but this regimen is not included in Centers for Disease Control and Prevention recommendations.
Address patient concerns about TB and treatment side effects
Richard Guthmann, MD
University of Illinois at Chicago/Advocate Illinois Masonic Family Medicine Residency, Chicago
Patients’ understanding of tuberculosis—the disease, the treatment, and the outcome—poses an important challenge in the care of an asymptomatic PPD-positive patient. These patients may ask, “Will I get sick? Do I have to take the medicine? Are there side effects? And would you take the medicine?” We need to be prepared to answer these questions.
Most patients with a positive PPD will not get active tuberculosis, but when they do it can be serious and it can spread easily. The medication significantly decreases the risk of developing active tuberculosis. The medication side effects are uncommon but can be severe. These side effects are reversible if the medication is stopped promptly. Under the supervision of my physician, I would take the medicine.
Evidence summary
Clinical evaluation with medical history and physical exam, chest radiography, and selected sputum sampling to exclude active tuberculosis are part of the recommended algorithm for all patients who develop a positive PPD (FIGURE).1-3 These recommendations are derived from expert opinion, and their usefulness has not been evaluated in any population-based study of asymptomatic PPD-positive patients.
A comprehensive review of RCTs from the 1950s and 1960s demonstrated that INH treatment of patients with latent tuberculosis infection is effective in decreasing the progression to active tuberculosis.4 A series of double-blinded RCTs performed by the US Public Health Service included 25,923 patients with latent tuberculosis who were randomized to receive either daily INH or placebo for 1 year with 6- to 10-year follow-up. Groups studied included household contacts of patients with active tuberculosis (rate of progression to active disease in placebo group [baseline rate]=27/1000, relative risk with INH [RR]=0.4, number needed to treat [NNT]=63), patients in mental institutions (baseline rate=12/1000, RR=0.3, NNT=121), and patients with x-ray findings of healed tuberculosis (baseline rate=69/1000, RR=0.4, NNT=23).
The optimal length of treatment for PPD-positive patients without active disease was evaluated through 1 double-blinded RCT enrolling 28,000 patients with 5-year follow-up after 12, 24, or 52 weeks of INH or placebo. Active TB developed in 0.35% (24/6919) after 52 weeks of INH compared with 0.49% (34/6965) after 24 weeks (RR=1.4, NNT=708).5 Incidence in the placebo group was 1.4%. Subgroup analysis determined that maximum efficacy with fewest side effects was achieved at 9 months.6 Nine months of INH is also recommended for HIV-positive patients, based on extrapolations from these and other studies.3
INH monotherapy was compared with combination INH and rifampin in a 2005 meta-analysis of 5 RCTs of variable quality involving 1926 patients.7 This meta-analysis found equivalency in risk of active TB and mortality between INH monotherapy for 6 to 12 months and the combination of rifampin and INH for 3 months (pooled risk difference=0%; 95% confidence interval [CI], –1% to 2%). This study also showed similar rates of adverse events in both groups (pooled risk difference=–1%; 95% CI, –7% to 5%). Short-course combination rifampin and pyrazinamide is no longer recommended after an open-label RCT with 589 patients demonstrated severe hepatoxicity in 7.7% (16/207) on a 2-month course of pyrazinamide and rifampin, compared with 1% (2/204) on 6 months of INH (RR=7.9, number needed to harm=15).8 Rifampin monotherapy has only been studied in patients with silicosis in a RCT enrolling 652 participants with latent tuberculosis. A 12-week course of rifampin (600 mg daily) was as effective as 6 months of INH in preventing development of active TB over the next 5 years.9
FIGURE
Suggested workup of asymptomatic, HIV-negative patients with a positive PPD
Source: Am J Respir Crit Care Med 2000;2 Jasmer et al, N Engl J Med 2002.3
Recommendations from others
Centers for Disease Control and Prevention, American Thoracic Society, and Infectious Disease Society of America guidelines recommend targeted screening of high-risk persons followed by further clinical evaluation of all those with a reactive PPD (FIGURE).2,10 The recommended treatment regimen for latent TB is daily INH for 9 months. Less preferable regimens are daily INH for 6 months, or daily rifampin for 4 months in patients who cannot tolerate INH. A 2-month course of rifampin and pyrazinamide is no longer recommended. The recent meta-analysis supporting a 3-month regimen of combination INH and rifampin has not been incorporated into expert guidelines.7
1. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-1395.
2. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-S247.
3. Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med 2002;347:1860-1866.
4. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc 1970;26:28-106.
5. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 1982;60:555-564.
6. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999;3:847-850.
7. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005;40:670-676.
8. Jasmer RM, Saukkonen JJ, Blumberg HM, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med 2002;137:640-647.
9. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36-41.
10. Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. MMWR Recomm Rep 2005;54(RR-12):1-81.
1. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-1395.
2. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-S247.
3. Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med 2002;347:1860-1866.
4. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc 1970;26:28-106.
5. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 1982;60:555-564.
6. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999;3:847-850.
7. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005;40:670-676.
8. Jasmer RM, Saukkonen JJ, Blumberg HM, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med 2002;137:640-647.
9. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36-41.
10. Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. MMWR Recomm Rep 2005;54(RR-12):1-81.
Evidence-based answers from the Family Physicians Inquiries Network
Are major bleeding events from falls more likely in patients on warfarin?
There is no evidence of increased risk for major bleeding as a result of falls in hospitalized patients taking warfarin (strength of recommendation [SOR]: B, based on retrospective cohort studies). In the average patient taking warfarin for atrial fibrillation, the risk of intracranial hemorrhage from a fall is much smaller than the benefit gained from reducing risk of stroke (SOR: A, based on decision analysis of systematic reviews with sensitivity analysis).
Major bleeding infrequent in fall patients with a therapeutic INR; more common with higher INR
Paul Crawford, MD
Eglin Air Force Base Family Practice Residency, Eglin AFB, Fla
Decisions to initiate or withhold anticoagulation can be difficult to make, but this Clinical Inquiry should simplify matters. Clearly, for patients with atrial fibrillation, the risk of stroke while not taking warfarin is greater than the risk of major bleeding from a fall while on it. Also, major bleeding from a fall occurs infrequently in patients with a therapeutic internal normalized ratio (INR). However, bleeding is more common in patients with a supratherapeutic INR, so remain alert to possible uncontrolled anticoagulation either from medication interactions or from impaired cognition.
This Clinical Inquiry should also help physicians considering an inferior vena cava filter instead of warfarin. Complications with inferior vena cava filters include death (0.82%), filter migration (3%–69%), and penetration (9%–24%) or obstruction (6%–30%) of the inferior vena cava.6
Evidence summary
Increased risk of falling is often given as a reason for not recommending anticoagulation for atrial fibrillation in frail or elderly patients. However, no studies directly address the risk for major bleeding in anticoagulated patients who fall.
One retrospective study of 2633 falls in 1861 hospital inpatients compared the rate of major hemorrhage between those taking anticoagulation therapy with those not taking it.1 Major hemorrhage was defined as bruising or cuts requiring immediate attention from a physician. The rate of major hemorrhage was 6.2% for patients taking warfarin and 11.3% for patients receiving no therapy. Patients with INR=2–3 had a major hemorrhage rate of 6.9% compared with 10.1% for those with INR <1.3. Criteria for using warfarin were not reported; there may have been selection bias in favor of prescribing warfarin for patients judged less likely to fall.
A smaller study of 400 consecutive falls among 264 post-stroke patients in a rehab hospital found no difference in minor injury rates (19% vs 18%, NS); no major hemorrhagic complications were seen following 131 falls in the anticoagulation group (93 patients) and 269 falls in the group not on anticoagulation (175 patients).2 Patients on anticoagulation had an average protime of 16.1 seconds (INR was not reported). The calculated risk of major hemorrhage in an anticoagulated patient from a single fall was 2.3% or less. The study was limited because most falls were from a seated position or partially controlled by an attendant; few patients fell from a standing position.
Another study presented a Markov decision analysis (comparison of risk estimates in separate disease states) evaluating whether risk from falls should influence choice of anticoagulation therapy in elderly patients with atrial fibrillation.3 Risk of intracranial bleeding from falls was calculated from prospective cohort studies and retrospective case series from anticoagulation clinics, and stroke reduction benefit from anticoagulation was taken from a meta-analysis of 5 randomized controlled trials. Sensitivity analyses were performed to test the results of the decision analysis. The calculated risk of subdural hematoma from falling was such that a patient with a 5% annual stroke risk from atrial fibrillation would need to fall 295 times in a year for the fall risk to outweigh the stroke reduction benefit of warfarin.
Recommendations from others
Guidelines from the American Heart Association and the American College of Chest Physicians do not include fall risk in the decision to use anticoagulation.4
Guidelines from the Institute for Clinical Systems Improvement note that patients with 3 falls in the previous year or with recurrent, injurious falls were excluded from trials evaluating efficacy and safety of anticoagulation in patients with nonvalvular atrial fibrillation.5
1. Bond AJ, Molnar FJ, Li M, Mackey M, Man-Son-Hing M. The risk of hemorrhagic complications in hospital in-patients who fall while receiving antithrombotic therapy. Thromb J 2005;3:1-6.
2. Stein J, Viramontes BS, Kerrigan DC. Fall-related injuries in anticoagulated stroke patients during inpatient rehabilitation. Arch Phys Med Rehabil 1995;76:840-843.
3. Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999;159:677-685.
4. Institute for Clinical Systems Improvement. Anticoagulation therapy supplement. April 2005. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=151t. Accessed on January 9, 2006.
5. Singer D, Albers G. Antithrombotic therapy in atrial fibrillation—the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:429s-456s.
6. Kinney TB. Update on inferior vena cava filters. J Vasc Inter Rad 2003;14:425.
There is no evidence of increased risk for major bleeding as a result of falls in hospitalized patients taking warfarin (strength of recommendation [SOR]: B, based on retrospective cohort studies). In the average patient taking warfarin for atrial fibrillation, the risk of intracranial hemorrhage from a fall is much smaller than the benefit gained from reducing risk of stroke (SOR: A, based on decision analysis of systematic reviews with sensitivity analysis).
Major bleeding infrequent in fall patients with a therapeutic INR; more common with higher INR
Paul Crawford, MD
Eglin Air Force Base Family Practice Residency, Eglin AFB, Fla
Decisions to initiate or withhold anticoagulation can be difficult to make, but this Clinical Inquiry should simplify matters. Clearly, for patients with atrial fibrillation, the risk of stroke while not taking warfarin is greater than the risk of major bleeding from a fall while on it. Also, major bleeding from a fall occurs infrequently in patients with a therapeutic internal normalized ratio (INR). However, bleeding is more common in patients with a supratherapeutic INR, so remain alert to possible uncontrolled anticoagulation either from medication interactions or from impaired cognition.
This Clinical Inquiry should also help physicians considering an inferior vena cava filter instead of warfarin. Complications with inferior vena cava filters include death (0.82%), filter migration (3%–69%), and penetration (9%–24%) or obstruction (6%–30%) of the inferior vena cava.6
Evidence summary
Increased risk of falling is often given as a reason for not recommending anticoagulation for atrial fibrillation in frail or elderly patients. However, no studies directly address the risk for major bleeding in anticoagulated patients who fall.
One retrospective study of 2633 falls in 1861 hospital inpatients compared the rate of major hemorrhage between those taking anticoagulation therapy with those not taking it.1 Major hemorrhage was defined as bruising or cuts requiring immediate attention from a physician. The rate of major hemorrhage was 6.2% for patients taking warfarin and 11.3% for patients receiving no therapy. Patients with INR=2–3 had a major hemorrhage rate of 6.9% compared with 10.1% for those with INR <1.3. Criteria for using warfarin were not reported; there may have been selection bias in favor of prescribing warfarin for patients judged less likely to fall.
A smaller study of 400 consecutive falls among 264 post-stroke patients in a rehab hospital found no difference in minor injury rates (19% vs 18%, NS); no major hemorrhagic complications were seen following 131 falls in the anticoagulation group (93 patients) and 269 falls in the group not on anticoagulation (175 patients).2 Patients on anticoagulation had an average protime of 16.1 seconds (INR was not reported). The calculated risk of major hemorrhage in an anticoagulated patient from a single fall was 2.3% or less. The study was limited because most falls were from a seated position or partially controlled by an attendant; few patients fell from a standing position.
Another study presented a Markov decision analysis (comparison of risk estimates in separate disease states) evaluating whether risk from falls should influence choice of anticoagulation therapy in elderly patients with atrial fibrillation.3 Risk of intracranial bleeding from falls was calculated from prospective cohort studies and retrospective case series from anticoagulation clinics, and stroke reduction benefit from anticoagulation was taken from a meta-analysis of 5 randomized controlled trials. Sensitivity analyses were performed to test the results of the decision analysis. The calculated risk of subdural hematoma from falling was such that a patient with a 5% annual stroke risk from atrial fibrillation would need to fall 295 times in a year for the fall risk to outweigh the stroke reduction benefit of warfarin.
Recommendations from others
Guidelines from the American Heart Association and the American College of Chest Physicians do not include fall risk in the decision to use anticoagulation.4
Guidelines from the Institute for Clinical Systems Improvement note that patients with 3 falls in the previous year or with recurrent, injurious falls were excluded from trials evaluating efficacy and safety of anticoagulation in patients with nonvalvular atrial fibrillation.5
There is no evidence of increased risk for major bleeding as a result of falls in hospitalized patients taking warfarin (strength of recommendation [SOR]: B, based on retrospective cohort studies). In the average patient taking warfarin for atrial fibrillation, the risk of intracranial hemorrhage from a fall is much smaller than the benefit gained from reducing risk of stroke (SOR: A, based on decision analysis of systematic reviews with sensitivity analysis).
Major bleeding infrequent in fall patients with a therapeutic INR; more common with higher INR
Paul Crawford, MD
Eglin Air Force Base Family Practice Residency, Eglin AFB, Fla
Decisions to initiate or withhold anticoagulation can be difficult to make, but this Clinical Inquiry should simplify matters. Clearly, for patients with atrial fibrillation, the risk of stroke while not taking warfarin is greater than the risk of major bleeding from a fall while on it. Also, major bleeding from a fall occurs infrequently in patients with a therapeutic internal normalized ratio (INR). However, bleeding is more common in patients with a supratherapeutic INR, so remain alert to possible uncontrolled anticoagulation either from medication interactions or from impaired cognition.
This Clinical Inquiry should also help physicians considering an inferior vena cava filter instead of warfarin. Complications with inferior vena cava filters include death (0.82%), filter migration (3%–69%), and penetration (9%–24%) or obstruction (6%–30%) of the inferior vena cava.6
Evidence summary
Increased risk of falling is often given as a reason for not recommending anticoagulation for atrial fibrillation in frail or elderly patients. However, no studies directly address the risk for major bleeding in anticoagulated patients who fall.
One retrospective study of 2633 falls in 1861 hospital inpatients compared the rate of major hemorrhage between those taking anticoagulation therapy with those not taking it.1 Major hemorrhage was defined as bruising or cuts requiring immediate attention from a physician. The rate of major hemorrhage was 6.2% for patients taking warfarin and 11.3% for patients receiving no therapy. Patients with INR=2–3 had a major hemorrhage rate of 6.9% compared with 10.1% for those with INR <1.3. Criteria for using warfarin were not reported; there may have been selection bias in favor of prescribing warfarin for patients judged less likely to fall.
A smaller study of 400 consecutive falls among 264 post-stroke patients in a rehab hospital found no difference in minor injury rates (19% vs 18%, NS); no major hemorrhagic complications were seen following 131 falls in the anticoagulation group (93 patients) and 269 falls in the group not on anticoagulation (175 patients).2 Patients on anticoagulation had an average protime of 16.1 seconds (INR was not reported). The calculated risk of major hemorrhage in an anticoagulated patient from a single fall was 2.3% or less. The study was limited because most falls were from a seated position or partially controlled by an attendant; few patients fell from a standing position.
Another study presented a Markov decision analysis (comparison of risk estimates in separate disease states) evaluating whether risk from falls should influence choice of anticoagulation therapy in elderly patients with atrial fibrillation.3 Risk of intracranial bleeding from falls was calculated from prospective cohort studies and retrospective case series from anticoagulation clinics, and stroke reduction benefit from anticoagulation was taken from a meta-analysis of 5 randomized controlled trials. Sensitivity analyses were performed to test the results of the decision analysis. The calculated risk of subdural hematoma from falling was such that a patient with a 5% annual stroke risk from atrial fibrillation would need to fall 295 times in a year for the fall risk to outweigh the stroke reduction benefit of warfarin.
Recommendations from others
Guidelines from the American Heart Association and the American College of Chest Physicians do not include fall risk in the decision to use anticoagulation.4
Guidelines from the Institute for Clinical Systems Improvement note that patients with 3 falls in the previous year or with recurrent, injurious falls were excluded from trials evaluating efficacy and safety of anticoagulation in patients with nonvalvular atrial fibrillation.5
1. Bond AJ, Molnar FJ, Li M, Mackey M, Man-Son-Hing M. The risk of hemorrhagic complications in hospital in-patients who fall while receiving antithrombotic therapy. Thromb J 2005;3:1-6.
2. Stein J, Viramontes BS, Kerrigan DC. Fall-related injuries in anticoagulated stroke patients during inpatient rehabilitation. Arch Phys Med Rehabil 1995;76:840-843.
3. Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999;159:677-685.
4. Institute for Clinical Systems Improvement. Anticoagulation therapy supplement. April 2005. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=151t. Accessed on January 9, 2006.
5. Singer D, Albers G. Antithrombotic therapy in atrial fibrillation—the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:429s-456s.
6. Kinney TB. Update on inferior vena cava filters. J Vasc Inter Rad 2003;14:425.
1. Bond AJ, Molnar FJ, Li M, Mackey M, Man-Son-Hing M. The risk of hemorrhagic complications in hospital in-patients who fall while receiving antithrombotic therapy. Thromb J 2005;3:1-6.
2. Stein J, Viramontes BS, Kerrigan DC. Fall-related injuries in anticoagulated stroke patients during inpatient rehabilitation. Arch Phys Med Rehabil 1995;76:840-843.
3. Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999;159:677-685.
4. Institute for Clinical Systems Improvement. Anticoagulation therapy supplement. April 2005. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=151t. Accessed on January 9, 2006.
5. Singer D, Albers G. Antithrombotic therapy in atrial fibrillation—the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:429s-456s.
6. Kinney TB. Update on inferior vena cava filters. J Vasc Inter Rad 2003;14:425.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best surveillance for hepatocellular carcinoma in chronic carriers of hepatitis B?
Screening patients with chronic hepatitis B infection (HBsAg+) for hepatocellular carcinoma by alpha-fetoprotein (AFP) or by AFP plus ultrasound (AFP/US) detects hepatocellular carcinoma tumors at earlier stages and increases resection rates (strength of recommendation [SOR]: B, based on a systematic review of fair-quality randomized controlled trials). It is unclear whether screening with AFP or AFP/US improves disease-specific or all-cause mortality (SOR: B).
Offer screening to all with chronic hepatitis B infection, but stratify risk for HCC first
Michael Mendoza, MD, MPH
ACCESS Community Health Network and Department of Family Medicine, University of Chicago
Because no mortality benefit to screening for hepatocellular carcinoma has been shown, we should give added consideration to how we counsel our patients before offering screening, particularly since positive screening results can lead to further invasive studies. An important consideration for me is whether a patient has, or is at risk, for cirrhosis, because the incidence of hepatocellular carcinoma is higher if cirrhosis is present. Screening for coinfection with hepatitis C or a history of alcohol abuse becomes especially critical in this situation. Biochemical evidence of chronic active liver inflammation, whatever the cause, should also be an important factor in deciding whether to screen. While I still offer screening to all patients with chronic hepatitis B infection, it helps to have stratified a patient’s underlying risk for hepatocellular carcinoma first and counseling him or her accordingly.
Evidence summary
Many serum markers and screening methods have been proposed to detect hepatocellular carcinoma at a treatable stage, but only 2—AFP and US—are in clinical use.1
A Cochrane systematic review on screening for hepatocellular carcinoma in the HBsAg+ population was published in 2003 and updated May 2004.2 Our literature search did not find any subsequent relevant trials. The Cochrane review included 2 randomized control trials. The larger trial was performed in Shanghai, China and included 18,816 HBsAg+ patients aged 35 to 55 years.3 Subjects were recruited from their place of employment and randomized to either AFP/US every 6 months (n=9373) or to no screening (n=9443).
Fifty-one hepatocellular carcinomas were diagnosed in the control group and 86 in screened group. Screened subjects had a significantly higher percentage of tumors that were less than 5 cm at the time of diagnosis and a higher number of patients who underwent resection. While the 5-year survival for those with hepatocellular carcinoma in the screened group was higher, the disease-specific mortality rate was not statistically different between the 2 groups.
Additional data became available in 2002. The original study authors claimed the new data showed a statistically significant disease-specific mortality rate ratio of 0.63, favoring the screened group.4 However, the Cochrane group performed their own analysis on the same data and determined that no statistically significant difference in the disease-specific mortality rates existed between the 2 groups.2 Therefore, it is not clear whether these new data definitively demonstrate that screening provides any benefit.
The other randomized control trial took place in Toronto, and included 1069 patients, 71% of whom were of Asian ancestry. Subjects had AFP testing every 6 months, and half were randomly assigned to have US performed every 6 months.5 Eight of the 11 incident tumors would have been diagnosed based on AFP levels alone, and 3 would have been missed with US alone. The authors conclude that for AFP, sensitivity=64.3% and specificity=91.4%; for US, sensitivity=78.8% and specificity=93.8%. However, their study was too small to determine if AFP/US is superior to AFP for hepatocellular carcinoma screening in a HBsAg+ population. They estimate that detecting such a difference would take a sample size of 10,000 or more.
Both studies have important flaws. Neither study applied a reference standard test (such as a computed tomography scan or magnetic resonance imaging) to both study arms. Carcinomas may have been undetected by either AFP or US. Without knowing the real prevalence of hepatocellular carcinoma, the true sensitivity and specificity for AFP, US, and AFP/US in these studies cannot be determined. Both studies included prevalent tumors (tumors diagnosed during the very first screening cycle) in their analysis. Approximately 20% of detected carcinomas in both studies were present at the start of the studies and did not represent newly incident tumors detected by regular screening.3,5
Both of these trials would be improved if they started with cohorts known to be disease-free at baseline. Additionally, the Shanghai study randomized patients in clusters. The only English-language report of this study did not describe whether adjustments for this were made in analysis;5 failing to do so could overestimate the benefit of screening.
Recommendations from others
The American Association for the Study of Liver Disease recommends that carriers of the hepatitis B virus who are at high risk for developing hepatocellular carcinoma—men aged >45 years, those with cirrhosis or a family history of hepatocellular carcinoma—should be screened periodically with AFP/US. Also consider periodic screening for low-risk HBsAg+ patients who are from an area where hepatocellular carcinoma is endemic (SOR: C, based on expert opinion or descriptive epidemiology).6
1. Sherman M. Screening for hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 2005;19:101-118.
2. Wun YT, Dickinson JA. Alpha-fetoprotein and/or ultrasonography for liver cancer screening in patients with chronic hepatitis B. Cochrane Database Syst Rev 2003;(2):CD002799.
3. Yang B, Zhang B, Xu Y, et al. Prospective study of early detection for primary liver cancer. J Cancer Res Clin Oncol 1997;123:357-360.
4. Zhang B, Yang B, Tang Z. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417-422.
5. Sherman M, Peltekian K, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a north American urban population. Hepatology 1995;22:432-438.
6. Lok A, McMahon B. AASLD practice guidelines: chronic Hepatitis B. American Association for the Study of Liver Disease web site. Available at: https://www.aasld.org/eweb/docs/chronichep_B.pdf. Accessed on January 9, 2006.
Screening patients with chronic hepatitis B infection (HBsAg+) for hepatocellular carcinoma by alpha-fetoprotein (AFP) or by AFP plus ultrasound (AFP/US) detects hepatocellular carcinoma tumors at earlier stages and increases resection rates (strength of recommendation [SOR]: B, based on a systematic review of fair-quality randomized controlled trials). It is unclear whether screening with AFP or AFP/US improves disease-specific or all-cause mortality (SOR: B).
Offer screening to all with chronic hepatitis B infection, but stratify risk for HCC first
Michael Mendoza, MD, MPH
ACCESS Community Health Network and Department of Family Medicine, University of Chicago
Because no mortality benefit to screening for hepatocellular carcinoma has been shown, we should give added consideration to how we counsel our patients before offering screening, particularly since positive screening results can lead to further invasive studies. An important consideration for me is whether a patient has, or is at risk, for cirrhosis, because the incidence of hepatocellular carcinoma is higher if cirrhosis is present. Screening for coinfection with hepatitis C or a history of alcohol abuse becomes especially critical in this situation. Biochemical evidence of chronic active liver inflammation, whatever the cause, should also be an important factor in deciding whether to screen. While I still offer screening to all patients with chronic hepatitis B infection, it helps to have stratified a patient’s underlying risk for hepatocellular carcinoma first and counseling him or her accordingly.
Evidence summary
Many serum markers and screening methods have been proposed to detect hepatocellular carcinoma at a treatable stage, but only 2—AFP and US—are in clinical use.1
A Cochrane systematic review on screening for hepatocellular carcinoma in the HBsAg+ population was published in 2003 and updated May 2004.2 Our literature search did not find any subsequent relevant trials. The Cochrane review included 2 randomized control trials. The larger trial was performed in Shanghai, China and included 18,816 HBsAg+ patients aged 35 to 55 years.3 Subjects were recruited from their place of employment and randomized to either AFP/US every 6 months (n=9373) or to no screening (n=9443).
Fifty-one hepatocellular carcinomas were diagnosed in the control group and 86 in screened group. Screened subjects had a significantly higher percentage of tumors that were less than 5 cm at the time of diagnosis and a higher number of patients who underwent resection. While the 5-year survival for those with hepatocellular carcinoma in the screened group was higher, the disease-specific mortality rate was not statistically different between the 2 groups.
Additional data became available in 2002. The original study authors claimed the new data showed a statistically significant disease-specific mortality rate ratio of 0.63, favoring the screened group.4 However, the Cochrane group performed their own analysis on the same data and determined that no statistically significant difference in the disease-specific mortality rates existed between the 2 groups.2 Therefore, it is not clear whether these new data definitively demonstrate that screening provides any benefit.
The other randomized control trial took place in Toronto, and included 1069 patients, 71% of whom were of Asian ancestry. Subjects had AFP testing every 6 months, and half were randomly assigned to have US performed every 6 months.5 Eight of the 11 incident tumors would have been diagnosed based on AFP levels alone, and 3 would have been missed with US alone. The authors conclude that for AFP, sensitivity=64.3% and specificity=91.4%; for US, sensitivity=78.8% and specificity=93.8%. However, their study was too small to determine if AFP/US is superior to AFP for hepatocellular carcinoma screening in a HBsAg+ population. They estimate that detecting such a difference would take a sample size of 10,000 or more.
Both studies have important flaws. Neither study applied a reference standard test (such as a computed tomography scan or magnetic resonance imaging) to both study arms. Carcinomas may have been undetected by either AFP or US. Without knowing the real prevalence of hepatocellular carcinoma, the true sensitivity and specificity for AFP, US, and AFP/US in these studies cannot be determined. Both studies included prevalent tumors (tumors diagnosed during the very first screening cycle) in their analysis. Approximately 20% of detected carcinomas in both studies were present at the start of the studies and did not represent newly incident tumors detected by regular screening.3,5
Both of these trials would be improved if they started with cohorts known to be disease-free at baseline. Additionally, the Shanghai study randomized patients in clusters. The only English-language report of this study did not describe whether adjustments for this were made in analysis;5 failing to do so could overestimate the benefit of screening.
Recommendations from others
The American Association for the Study of Liver Disease recommends that carriers of the hepatitis B virus who are at high risk for developing hepatocellular carcinoma—men aged >45 years, those with cirrhosis or a family history of hepatocellular carcinoma—should be screened periodically with AFP/US. Also consider periodic screening for low-risk HBsAg+ patients who are from an area where hepatocellular carcinoma is endemic (SOR: C, based on expert opinion or descriptive epidemiology).6
Screening patients with chronic hepatitis B infection (HBsAg+) for hepatocellular carcinoma by alpha-fetoprotein (AFP) or by AFP plus ultrasound (AFP/US) detects hepatocellular carcinoma tumors at earlier stages and increases resection rates (strength of recommendation [SOR]: B, based on a systematic review of fair-quality randomized controlled trials). It is unclear whether screening with AFP or AFP/US improves disease-specific or all-cause mortality (SOR: B).
Offer screening to all with chronic hepatitis B infection, but stratify risk for HCC first
Michael Mendoza, MD, MPH
ACCESS Community Health Network and Department of Family Medicine, University of Chicago
Because no mortality benefit to screening for hepatocellular carcinoma has been shown, we should give added consideration to how we counsel our patients before offering screening, particularly since positive screening results can lead to further invasive studies. An important consideration for me is whether a patient has, or is at risk, for cirrhosis, because the incidence of hepatocellular carcinoma is higher if cirrhosis is present. Screening for coinfection with hepatitis C or a history of alcohol abuse becomes especially critical in this situation. Biochemical evidence of chronic active liver inflammation, whatever the cause, should also be an important factor in deciding whether to screen. While I still offer screening to all patients with chronic hepatitis B infection, it helps to have stratified a patient’s underlying risk for hepatocellular carcinoma first and counseling him or her accordingly.
Evidence summary
Many serum markers and screening methods have been proposed to detect hepatocellular carcinoma at a treatable stage, but only 2—AFP and US—are in clinical use.1
A Cochrane systematic review on screening for hepatocellular carcinoma in the HBsAg+ population was published in 2003 and updated May 2004.2 Our literature search did not find any subsequent relevant trials. The Cochrane review included 2 randomized control trials. The larger trial was performed in Shanghai, China and included 18,816 HBsAg+ patients aged 35 to 55 years.3 Subjects were recruited from their place of employment and randomized to either AFP/US every 6 months (n=9373) or to no screening (n=9443).
Fifty-one hepatocellular carcinomas were diagnosed in the control group and 86 in screened group. Screened subjects had a significantly higher percentage of tumors that were less than 5 cm at the time of diagnosis and a higher number of patients who underwent resection. While the 5-year survival for those with hepatocellular carcinoma in the screened group was higher, the disease-specific mortality rate was not statistically different between the 2 groups.
Additional data became available in 2002. The original study authors claimed the new data showed a statistically significant disease-specific mortality rate ratio of 0.63, favoring the screened group.4 However, the Cochrane group performed their own analysis on the same data and determined that no statistically significant difference in the disease-specific mortality rates existed between the 2 groups.2 Therefore, it is not clear whether these new data definitively demonstrate that screening provides any benefit.
The other randomized control trial took place in Toronto, and included 1069 patients, 71% of whom were of Asian ancestry. Subjects had AFP testing every 6 months, and half were randomly assigned to have US performed every 6 months.5 Eight of the 11 incident tumors would have been diagnosed based on AFP levels alone, and 3 would have been missed with US alone. The authors conclude that for AFP, sensitivity=64.3% and specificity=91.4%; for US, sensitivity=78.8% and specificity=93.8%. However, their study was too small to determine if AFP/US is superior to AFP for hepatocellular carcinoma screening in a HBsAg+ population. They estimate that detecting such a difference would take a sample size of 10,000 or more.
Both studies have important flaws. Neither study applied a reference standard test (such as a computed tomography scan or magnetic resonance imaging) to both study arms. Carcinomas may have been undetected by either AFP or US. Without knowing the real prevalence of hepatocellular carcinoma, the true sensitivity and specificity for AFP, US, and AFP/US in these studies cannot be determined. Both studies included prevalent tumors (tumors diagnosed during the very first screening cycle) in their analysis. Approximately 20% of detected carcinomas in both studies were present at the start of the studies and did not represent newly incident tumors detected by regular screening.3,5
Both of these trials would be improved if they started with cohorts known to be disease-free at baseline. Additionally, the Shanghai study randomized patients in clusters. The only English-language report of this study did not describe whether adjustments for this were made in analysis;5 failing to do so could overestimate the benefit of screening.
Recommendations from others
The American Association for the Study of Liver Disease recommends that carriers of the hepatitis B virus who are at high risk for developing hepatocellular carcinoma—men aged >45 years, those with cirrhosis or a family history of hepatocellular carcinoma—should be screened periodically with AFP/US. Also consider periodic screening for low-risk HBsAg+ patients who are from an area where hepatocellular carcinoma is endemic (SOR: C, based on expert opinion or descriptive epidemiology).6
1. Sherman M. Screening for hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 2005;19:101-118.
2. Wun YT, Dickinson JA. Alpha-fetoprotein and/or ultrasonography for liver cancer screening in patients with chronic hepatitis B. Cochrane Database Syst Rev 2003;(2):CD002799.
3. Yang B, Zhang B, Xu Y, et al. Prospective study of early detection for primary liver cancer. J Cancer Res Clin Oncol 1997;123:357-360.
4. Zhang B, Yang B, Tang Z. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417-422.
5. Sherman M, Peltekian K, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a north American urban population. Hepatology 1995;22:432-438.
6. Lok A, McMahon B. AASLD practice guidelines: chronic Hepatitis B. American Association for the Study of Liver Disease web site. Available at: https://www.aasld.org/eweb/docs/chronichep_B.pdf. Accessed on January 9, 2006.
1. Sherman M. Screening for hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 2005;19:101-118.
2. Wun YT, Dickinson JA. Alpha-fetoprotein and/or ultrasonography for liver cancer screening in patients with chronic hepatitis B. Cochrane Database Syst Rev 2003;(2):CD002799.
3. Yang B, Zhang B, Xu Y, et al. Prospective study of early detection for primary liver cancer. J Cancer Res Clin Oncol 1997;123:357-360.
4. Zhang B, Yang B, Tang Z. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417-422.
5. Sherman M, Peltekian K, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a north American urban population. Hepatology 1995;22:432-438.
6. Lok A, McMahon B. AASLD practice guidelines: chronic Hepatitis B. American Association for the Study of Liver Disease web site. Available at: https://www.aasld.org/eweb/docs/chronichep_B.pdf. Accessed on January 9, 2006.
Evidence-based answers from the Family Physicians Inquiries Network
How safe is vaginal birth after cesarean section for the mother and fetus?
Compared with planned repeat low-transverse cesarean section, vaginal birth after cesarean section (VBAC) is not associated with increased risk of maternal or neonatal mortality (strength of recommendation [SOR]: B). Morbidity is slightly increased, as evidenced by higher uterine rupture rates and some neonatal outcome measures (SOR: B).
Risks of C-section and labor must be considered when counseling regarding route of delivery
Lynda DeArmond, MD
Waco Family Practice Residency Program, Waco, Texas
Another question to pose is: how safe is repeat cesarean section for the mother and fetus? How much do morbidity and mortality increase with each new intra-abdominal procedure? Each time the belly is opened there is new scar, with increased likelihood of adhesions and potential for future bowel obstruction. Consider these risks when counseling regarding route of delivery. Risk of uterine rupture appears to be higher in trials of labor (and confers a statistically significant but small increase in morbidity but not mortality). However, the uterine scar can silently fail without labor—as is sometimes discovered at a scheduled repeat section, usually without untoward effects on mother or fetus.
Remember that you are sending a young woman home with a new baby to care for (along with other children) and a major abdominal procedure (through an old scar) to recover from, which one could certainly define as morbidity. Cesarean section is an important tool, but we must be careful to practice best possible care and consider all patient factors and preferences. And data are still lacking to support the notion that VBAC is unsafe.
Evidence summary
Contrary to the goals of Healthy People 2010, the rate of cesarean sections is increasing.1 The repeat cesarean rate for low-risk women of all ages and racial groups is now 88.7%, the highest rate since the Centers for Disease Control and Prevention (CDC) began tracking the statistic in 1989. Is VBAC safe, or is a trial of labor no longer supported by the data?
The most recent Cochrane Review found that both VBAC and repeat lowtransverse cesarean section have benefits and risks associated with them; however, after reviewing the limited data, they concluded that no trial exists to adequately help women and their caregivers make an informed decision between the two.2 A strong theme in the Cochrane Review, echoed in most reviews, was the absence of high-quality prospective randomized data.
In an attempt to quantify the risks of VBAC, a systematic review determined that attempted VBAC, compared with repeat low-transverse cesarean section, increased the risk of uterine rupture by 2.7 per 1000 cases (95% confidence interval [CI], 0.73–4.73).3 This additional risk rate is often quoted in VBAC reviews and was cited in the Agency for Healthcare Research and Quality evidence report; it is based on 1 prospective, nonrandomized cohort trial and 1 retrospective cohort study.4,5
No randomized controlled trials exist for determining maternal safety of VBAC, although another recent systematic review found 2 nonrandomized prospective trials of sufficient quality to analyze. The authors concluded there were “no statistically significant differences between planned elective repeat cesarean section and planned VBAC.”6 Upon closer review in PubMed, one of the cited studies did not study 312 patients for VBAC outcomes as alleged; rather, it investigated patient attitudes towards VBAC.7
Since publication of that review, a large, multicenter, prospective, nonrandomized trial involving 33,699 patients found no significant difference between VBAC and planned cesarean for hysterectomy (0.2% vs 0.3%; odds ratio [OR]=0.77; 95% CI, 0.51–1.17), maternal death (0.02% vs 0.04%; OR=0.38; 95% CI, 0.1–1.46), and neonatal death (0.08% vs 0.05%; OR=1.82; 95% CI, 0.73–4.57).8 Significant associations were found for uterine rupture rates in spontaneous labor (24/6685 [0.4%] vs no cases; number needed to harm [NNH]=279) and neonatal hypoxic-ischemic encephalopathy (0.46 cases per 1000 vs no cases; NNH=2174).8
A retrospective Canadian cohort trial of 308,755 women also demonstrated an association of VBAC with uterine rupture(0.65% of trial-of-labor cases; OR=2.38; 95% CI, 2.12–2.67), and a trend towards higher maternal mortality in the cesarean group (1.6 per 100,000 for VBAC vs 5.6 per 100,000 for planned cesarean; OR=0.32; 95% CI, 0.07–1.47).9
The effect of VBAC on neonatal morbidity and mortality is unclear. In contrast to the negative larger trial,8 a smaller retrospective cohort of 24,529 births found a higher association of perinatal death for trial of labor (adjusted OR=11.7; 95% CI, 1.4–101.6).10 The perinatal death rate was similar to rates in nulliparous women. Regarding morbidity, one retrospective cohort trial showed VBAC was associated with an increase in neonatal sepsis (1% vs 0%; CI not given) compared with planned cesarean, but VBAC resulted in less transient tachypnea (5% vs 7%) and hyper-bilirubinemia (2% vs 6%).11
Recommendations from others
Both the American College of Obstetricians and Gynecologists and the Society of Obstetricians and Gynecologists of Canada state that women with 1 previous low-transverse cesarean section should be offered a trial of labor after appropriate counseling of the risks and benefits.12,13 Furthermore, induction with oxytocin is allowed, but the use of prostaglandins is not recommended. Based on expert opinion, both organizations encourage VBAC only in institutions staffed with surgeons and anesthesiologists immediately available to provide emergent cesarean.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and are not to be construed as official, or as reflecting the views of the US Air Force medical department or the US Air Force at large.
1. Menacker F. Trends in cesarean rates for first births and repeat cesarean rates for low-risk women: United States, 1990-2003. Natl Vital Stat Rep 2005;54:1-8.
2. Dodd J, Crowther C, Huertas E, Guise J, Horey D. Planned elective repeat caesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev 2005;(4):CD004224.
3. Guise J, McDonagh M, Osterweil P, Nygren P, Chan B, Helfand M. Systematic review of the incidence and consequences of uterine rupture in women with previous caesarean section. BMJ 2004;329:19-25.
4. Vaginal Birth After Cesarean (VBAC). Summary. Evidence Report/Technology Assessment, No. 71. AHRQ publication 03-E017. Rockville, Md: Agency for Healthcare Research and Quality; 2003. Available at: www.ahrq.gov/clinic/epcsums/vbacsum.htm. Accessed on January 9, 2006.
5. McMahon MJ, Luther ER, Bowes WA, Olshan AF. Comparison of a trial of labor with an elective second cesarean section. N Engl J Med 1996;335:689-695.
6. Dodd J, Crowther C. Vaginal birth after Caesarean versus elective repeat Caesarean for women with a single prior Caesarean birth: a systematic review of the literature. Aust N Z J Obstet Gynaecol 2004;44:387-391.
7. Abitbol MM, Castillo I, Taylor UB, Rochelson BL, Shmoys S, Monheit AG. Vaginal birth after cesarean section: the patient’s point of view. Am Fam Physician 1993;47:129-134.
8. Landon M, Hauth J, Leveno K, et al. for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med 2004;351:2581-2589.
9. Wen S, Rusen I, Walker M, et al. Comparison of maternal mortality and morbidity between trial of labor and elective cesarean section among women with previous cesarean delivery. Am J Obstet Gynecol 2004;191:1263-1269.
10. Smith G, Pell J, Cameron A, Dobbie R. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA 2002;287:2684-2690.
11. Hook B, Kiwi R, Amini S, Fanaroff A, Hack M. Neonatal morbidity after elective repeat cesarean section and trial of labor. Pediatrics 1997;100:348-353.
12. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Vaginal Birth After Cesarean Delivery. Obstet Gynecol 2004;104:203-212.
13. Martel M, MacKinnon C, and the Clinical Practice Obstetrics Committee, Society of Obstetricians and Gynaecologists of Canada. Guidelines for vaginal birth after previous Caesarean birth. J Obstet Gynaecol Can 2005;27:164-188.
Compared with planned repeat low-transverse cesarean section, vaginal birth after cesarean section (VBAC) is not associated with increased risk of maternal or neonatal mortality (strength of recommendation [SOR]: B). Morbidity is slightly increased, as evidenced by higher uterine rupture rates and some neonatal outcome measures (SOR: B).
Risks of C-section and labor must be considered when counseling regarding route of delivery
Lynda DeArmond, MD
Waco Family Practice Residency Program, Waco, Texas
Another question to pose is: how safe is repeat cesarean section for the mother and fetus? How much do morbidity and mortality increase with each new intra-abdominal procedure? Each time the belly is opened there is new scar, with increased likelihood of adhesions and potential for future bowel obstruction. Consider these risks when counseling regarding route of delivery. Risk of uterine rupture appears to be higher in trials of labor (and confers a statistically significant but small increase in morbidity but not mortality). However, the uterine scar can silently fail without labor—as is sometimes discovered at a scheduled repeat section, usually without untoward effects on mother or fetus.
Remember that you are sending a young woman home with a new baby to care for (along with other children) and a major abdominal procedure (through an old scar) to recover from, which one could certainly define as morbidity. Cesarean section is an important tool, but we must be careful to practice best possible care and consider all patient factors and preferences. And data are still lacking to support the notion that VBAC is unsafe.
Evidence summary
Contrary to the goals of Healthy People 2010, the rate of cesarean sections is increasing.1 The repeat cesarean rate for low-risk women of all ages and racial groups is now 88.7%, the highest rate since the Centers for Disease Control and Prevention (CDC) began tracking the statistic in 1989. Is VBAC safe, or is a trial of labor no longer supported by the data?
The most recent Cochrane Review found that both VBAC and repeat lowtransverse cesarean section have benefits and risks associated with them; however, after reviewing the limited data, they concluded that no trial exists to adequately help women and their caregivers make an informed decision between the two.2 A strong theme in the Cochrane Review, echoed in most reviews, was the absence of high-quality prospective randomized data.
In an attempt to quantify the risks of VBAC, a systematic review determined that attempted VBAC, compared with repeat low-transverse cesarean section, increased the risk of uterine rupture by 2.7 per 1000 cases (95% confidence interval [CI], 0.73–4.73).3 This additional risk rate is often quoted in VBAC reviews and was cited in the Agency for Healthcare Research and Quality evidence report; it is based on 1 prospective, nonrandomized cohort trial and 1 retrospective cohort study.4,5
No randomized controlled trials exist for determining maternal safety of VBAC, although another recent systematic review found 2 nonrandomized prospective trials of sufficient quality to analyze. The authors concluded there were “no statistically significant differences between planned elective repeat cesarean section and planned VBAC.”6 Upon closer review in PubMed, one of the cited studies did not study 312 patients for VBAC outcomes as alleged; rather, it investigated patient attitudes towards VBAC.7
Since publication of that review, a large, multicenter, prospective, nonrandomized trial involving 33,699 patients found no significant difference between VBAC and planned cesarean for hysterectomy (0.2% vs 0.3%; odds ratio [OR]=0.77; 95% CI, 0.51–1.17), maternal death (0.02% vs 0.04%; OR=0.38; 95% CI, 0.1–1.46), and neonatal death (0.08% vs 0.05%; OR=1.82; 95% CI, 0.73–4.57).8 Significant associations were found for uterine rupture rates in spontaneous labor (24/6685 [0.4%] vs no cases; number needed to harm [NNH]=279) and neonatal hypoxic-ischemic encephalopathy (0.46 cases per 1000 vs no cases; NNH=2174).8
A retrospective Canadian cohort trial of 308,755 women also demonstrated an association of VBAC with uterine rupture(0.65% of trial-of-labor cases; OR=2.38; 95% CI, 2.12–2.67), and a trend towards higher maternal mortality in the cesarean group (1.6 per 100,000 for VBAC vs 5.6 per 100,000 for planned cesarean; OR=0.32; 95% CI, 0.07–1.47).9
The effect of VBAC on neonatal morbidity and mortality is unclear. In contrast to the negative larger trial,8 a smaller retrospective cohort of 24,529 births found a higher association of perinatal death for trial of labor (adjusted OR=11.7; 95% CI, 1.4–101.6).10 The perinatal death rate was similar to rates in nulliparous women. Regarding morbidity, one retrospective cohort trial showed VBAC was associated with an increase in neonatal sepsis (1% vs 0%; CI not given) compared with planned cesarean, but VBAC resulted in less transient tachypnea (5% vs 7%) and hyper-bilirubinemia (2% vs 6%).11
Recommendations from others
Both the American College of Obstetricians and Gynecologists and the Society of Obstetricians and Gynecologists of Canada state that women with 1 previous low-transverse cesarean section should be offered a trial of labor after appropriate counseling of the risks and benefits.12,13 Furthermore, induction with oxytocin is allowed, but the use of prostaglandins is not recommended. Based on expert opinion, both organizations encourage VBAC only in institutions staffed with surgeons and anesthesiologists immediately available to provide emergent cesarean.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and are not to be construed as official, or as reflecting the views of the US Air Force medical department or the US Air Force at large.
Compared with planned repeat low-transverse cesarean section, vaginal birth after cesarean section (VBAC) is not associated with increased risk of maternal or neonatal mortality (strength of recommendation [SOR]: B). Morbidity is slightly increased, as evidenced by higher uterine rupture rates and some neonatal outcome measures (SOR: B).
Risks of C-section and labor must be considered when counseling regarding route of delivery
Lynda DeArmond, MD
Waco Family Practice Residency Program, Waco, Texas
Another question to pose is: how safe is repeat cesarean section for the mother and fetus? How much do morbidity and mortality increase with each new intra-abdominal procedure? Each time the belly is opened there is new scar, with increased likelihood of adhesions and potential for future bowel obstruction. Consider these risks when counseling regarding route of delivery. Risk of uterine rupture appears to be higher in trials of labor (and confers a statistically significant but small increase in morbidity but not mortality). However, the uterine scar can silently fail without labor—as is sometimes discovered at a scheduled repeat section, usually without untoward effects on mother or fetus.
Remember that you are sending a young woman home with a new baby to care for (along with other children) and a major abdominal procedure (through an old scar) to recover from, which one could certainly define as morbidity. Cesarean section is an important tool, but we must be careful to practice best possible care and consider all patient factors and preferences. And data are still lacking to support the notion that VBAC is unsafe.
Evidence summary
Contrary to the goals of Healthy People 2010, the rate of cesarean sections is increasing.1 The repeat cesarean rate for low-risk women of all ages and racial groups is now 88.7%, the highest rate since the Centers for Disease Control and Prevention (CDC) began tracking the statistic in 1989. Is VBAC safe, or is a trial of labor no longer supported by the data?
The most recent Cochrane Review found that both VBAC and repeat lowtransverse cesarean section have benefits and risks associated with them; however, after reviewing the limited data, they concluded that no trial exists to adequately help women and their caregivers make an informed decision between the two.2 A strong theme in the Cochrane Review, echoed in most reviews, was the absence of high-quality prospective randomized data.
In an attempt to quantify the risks of VBAC, a systematic review determined that attempted VBAC, compared with repeat low-transverse cesarean section, increased the risk of uterine rupture by 2.7 per 1000 cases (95% confidence interval [CI], 0.73–4.73).3 This additional risk rate is often quoted in VBAC reviews and was cited in the Agency for Healthcare Research and Quality evidence report; it is based on 1 prospective, nonrandomized cohort trial and 1 retrospective cohort study.4,5
No randomized controlled trials exist for determining maternal safety of VBAC, although another recent systematic review found 2 nonrandomized prospective trials of sufficient quality to analyze. The authors concluded there were “no statistically significant differences between planned elective repeat cesarean section and planned VBAC.”6 Upon closer review in PubMed, one of the cited studies did not study 312 patients for VBAC outcomes as alleged; rather, it investigated patient attitudes towards VBAC.7
Since publication of that review, a large, multicenter, prospective, nonrandomized trial involving 33,699 patients found no significant difference between VBAC and planned cesarean for hysterectomy (0.2% vs 0.3%; odds ratio [OR]=0.77; 95% CI, 0.51–1.17), maternal death (0.02% vs 0.04%; OR=0.38; 95% CI, 0.1–1.46), and neonatal death (0.08% vs 0.05%; OR=1.82; 95% CI, 0.73–4.57).8 Significant associations were found for uterine rupture rates in spontaneous labor (24/6685 [0.4%] vs no cases; number needed to harm [NNH]=279) and neonatal hypoxic-ischemic encephalopathy (0.46 cases per 1000 vs no cases; NNH=2174).8
A retrospective Canadian cohort trial of 308,755 women also demonstrated an association of VBAC with uterine rupture(0.65% of trial-of-labor cases; OR=2.38; 95% CI, 2.12–2.67), and a trend towards higher maternal mortality in the cesarean group (1.6 per 100,000 for VBAC vs 5.6 per 100,000 for planned cesarean; OR=0.32; 95% CI, 0.07–1.47).9
The effect of VBAC on neonatal morbidity and mortality is unclear. In contrast to the negative larger trial,8 a smaller retrospective cohort of 24,529 births found a higher association of perinatal death for trial of labor (adjusted OR=11.7; 95% CI, 1.4–101.6).10 The perinatal death rate was similar to rates in nulliparous women. Regarding morbidity, one retrospective cohort trial showed VBAC was associated with an increase in neonatal sepsis (1% vs 0%; CI not given) compared with planned cesarean, but VBAC resulted in less transient tachypnea (5% vs 7%) and hyper-bilirubinemia (2% vs 6%).11
Recommendations from others
Both the American College of Obstetricians and Gynecologists and the Society of Obstetricians and Gynecologists of Canada state that women with 1 previous low-transverse cesarean section should be offered a trial of labor after appropriate counseling of the risks and benefits.12,13 Furthermore, induction with oxytocin is allowed, but the use of prostaglandins is not recommended. Based on expert opinion, both organizations encourage VBAC only in institutions staffed with surgeons and anesthesiologists immediately available to provide emergent cesarean.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and are not to be construed as official, or as reflecting the views of the US Air Force medical department or the US Air Force at large.
1. Menacker F. Trends in cesarean rates for first births and repeat cesarean rates for low-risk women: United States, 1990-2003. Natl Vital Stat Rep 2005;54:1-8.
2. Dodd J, Crowther C, Huertas E, Guise J, Horey D. Planned elective repeat caesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev 2005;(4):CD004224.
3. Guise J, McDonagh M, Osterweil P, Nygren P, Chan B, Helfand M. Systematic review of the incidence and consequences of uterine rupture in women with previous caesarean section. BMJ 2004;329:19-25.
4. Vaginal Birth After Cesarean (VBAC). Summary. Evidence Report/Technology Assessment, No. 71. AHRQ publication 03-E017. Rockville, Md: Agency for Healthcare Research and Quality; 2003. Available at: www.ahrq.gov/clinic/epcsums/vbacsum.htm. Accessed on January 9, 2006.
5. McMahon MJ, Luther ER, Bowes WA, Olshan AF. Comparison of a trial of labor with an elective second cesarean section. N Engl J Med 1996;335:689-695.
6. Dodd J, Crowther C. Vaginal birth after Caesarean versus elective repeat Caesarean for women with a single prior Caesarean birth: a systematic review of the literature. Aust N Z J Obstet Gynaecol 2004;44:387-391.
7. Abitbol MM, Castillo I, Taylor UB, Rochelson BL, Shmoys S, Monheit AG. Vaginal birth after cesarean section: the patient’s point of view. Am Fam Physician 1993;47:129-134.
8. Landon M, Hauth J, Leveno K, et al. for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med 2004;351:2581-2589.
9. Wen S, Rusen I, Walker M, et al. Comparison of maternal mortality and morbidity between trial of labor and elective cesarean section among women with previous cesarean delivery. Am J Obstet Gynecol 2004;191:1263-1269.
10. Smith G, Pell J, Cameron A, Dobbie R. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA 2002;287:2684-2690.
11. Hook B, Kiwi R, Amini S, Fanaroff A, Hack M. Neonatal morbidity after elective repeat cesarean section and trial of labor. Pediatrics 1997;100:348-353.
12. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Vaginal Birth After Cesarean Delivery. Obstet Gynecol 2004;104:203-212.
13. Martel M, MacKinnon C, and the Clinical Practice Obstetrics Committee, Society of Obstetricians and Gynaecologists of Canada. Guidelines for vaginal birth after previous Caesarean birth. J Obstet Gynaecol Can 2005;27:164-188.
1. Menacker F. Trends in cesarean rates for first births and repeat cesarean rates for low-risk women: United States, 1990-2003. Natl Vital Stat Rep 2005;54:1-8.
2. Dodd J, Crowther C, Huertas E, Guise J, Horey D. Planned elective repeat caesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev 2005;(4):CD004224.
3. Guise J, McDonagh M, Osterweil P, Nygren P, Chan B, Helfand M. Systematic review of the incidence and consequences of uterine rupture in women with previous caesarean section. BMJ 2004;329:19-25.
4. Vaginal Birth After Cesarean (VBAC). Summary. Evidence Report/Technology Assessment, No. 71. AHRQ publication 03-E017. Rockville, Md: Agency for Healthcare Research and Quality; 2003. Available at: www.ahrq.gov/clinic/epcsums/vbacsum.htm. Accessed on January 9, 2006.
5. McMahon MJ, Luther ER, Bowes WA, Olshan AF. Comparison of a trial of labor with an elective second cesarean section. N Engl J Med 1996;335:689-695.
6. Dodd J, Crowther C. Vaginal birth after Caesarean versus elective repeat Caesarean for women with a single prior Caesarean birth: a systematic review of the literature. Aust N Z J Obstet Gynaecol 2004;44:387-391.
7. Abitbol MM, Castillo I, Taylor UB, Rochelson BL, Shmoys S, Monheit AG. Vaginal birth after cesarean section: the patient’s point of view. Am Fam Physician 1993;47:129-134.
8. Landon M, Hauth J, Leveno K, et al. for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med 2004;351:2581-2589.
9. Wen S, Rusen I, Walker M, et al. Comparison of maternal mortality and morbidity between trial of labor and elective cesarean section among women with previous cesarean delivery. Am J Obstet Gynecol 2004;191:1263-1269.
10. Smith G, Pell J, Cameron A, Dobbie R. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA 2002;287:2684-2690.
11. Hook B, Kiwi R, Amini S, Fanaroff A, Hack M. Neonatal morbidity after elective repeat cesarean section and trial of labor. Pediatrics 1997;100:348-353.
12. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Vaginal Birth After Cesarean Delivery. Obstet Gynecol 2004;104:203-212.
13. Martel M, MacKinnon C, and the Clinical Practice Obstetrics Committee, Society of Obstetricians and Gynaecologists of Canada. Guidelines for vaginal birth after previous Caesarean birth. J Obstet Gynaecol Can 2005;27:164-188.
Evidence-based answers from the Family Physicians Inquiries Network
What is the appropriate management for a patient with CIN1 on colposcopy?
Of the different strategies available for managing cervical intraepithelial neoplasia grade 1 (CIN1), testing for high-risk subtypes of the human papillomavirus (hr-HPV) DNA at 12 months has the highest sensitivity for predicting the development of CIN2 or CIN3 and leads to the lowest rate of referral to repeat colposcopy (TABLE 1). If the hr-HPV DNA test result is negative at 12 months, then the patient may return to routine cytology screening. If the hr-HPV DNA test result is positive, the patient should undergo repeat colposcopy.1,2
Base follow-up testing on patient preference, cost, and convenience
Patients with a colposcopic biopsy revealing mild dysplasia (HPV effect or CIN1) need follow-up due to the 12% risk of progression to CIN2 or CIN3 over 2 years.1 The decision to follow the patient with either a single HPV test at 12 months or cytologic testing at 6 and 12 months should be based on patient preference, cost, and convenience. One of the 2 follow-up strategies should be selected and a notification system implemented to ensure patients return at the appropriate follow-up interval.
Evidence summary
One study was found that attempted to determine the appropriate follow-up for women diagnosed with CIN1 on adequate colposcopic biopsy (FIGURE). This 2-year prospective study examined a subpopulation of 1539 women from the ASCUS/LSIL (atypical squamous cells of uncertain significance)/low-grade squamous intraepithelial lesion) Triage Study (ALTS) to determine the ideal follow-up strategy for women diagnosed with CIN1 or HPV effect on colposcopic biopsy-obtained histology. This study randomly assigned these women with CIN1 or HPV effect to either HPV testing or colposcopic examination at 6, 12, and 18 months. Every woman under-went an exit colposcopy at 24 months.
The study found that hr-HPV testing 12 months after the initial colposcopic exam had the highest sensitivity for detecting advanced disease (92.2%) and lowest referral rate to repeat colposcopy (55%). Follow-up of these patients with repeat cytology alone at 6 and 12 months had a lower sensitivity for detection of advanced disease (85%) and greater referral rate to repeat colposcopy (60%) when compared with HPV testing at 12 months alone. Three cytologic evaluations at 6, 12, and 18 months without HPV testing increased sensitivity (95%), although this increase was not statistically significant. A much higher percentage of patients were referred to colposcopy with this strategy. Combining both hr-HPV testing and cytology at 12 months did not significantly increase the identification of advanced disease and resulted in a higher re-referral rate to colposcopy.1-3
TABLE
Strategies for managing CIN1
| FOLLOW-UP/INTERVENTION AFTER DIAGNOSIS OF CIN1 AT COLPOSCOPY | SENSITIVITY FOR DETECTING CIN1 OR HIGHER | REFERRAL RATE FOR REPEAT COLPOSCOPY |
|---|---|---|
| hr-HPV test at 12 months | 92.2% | 55% |
| hr-HPV test at 6 months | 90.9% | 62.4% |
| Cytology at 6 and 12 months | 85% | 60% |
| Cytology at 6, 12, 18 months | 95% | Not available |
| hr-HPV test and cytology at 12 months | 94.8% | 64.1% |
| CIN1, cervical intraepithelial neoplasia grade 1; hr-HPV, high-risk subtypes of human papillomavirus. | ||
| Source: Guido et al, Am J Obstet Gynecol 20031; Guido et al, J Lower Genital Tract Dis 2002.2 | ||
Recommendations from others
American Society for Colposcopy and Cervical Pathology consensus guidelines for the management of women with CIN were published in 2003. Their recommendation now states that follow-up after adequate colposcopy with a biopsy revealing CIN1 or HPV effect may include either repeat cervical cytology tests at 6 and 12 months or HPV testing at 12 months. After a negative test for high-risk HPV types or 2 consecutive negative cervical cytology tests, the patient may return to annual cytologic screening. If the HPV test is positive for high-risk viral types or the cytology is reported as atypical squamous cells (ASC) or higher, the patient should undergo repeat colposcopy.4
1. Guido R, Schiffman M, Solomon D, Burke L. ASCUS LSIL Triage Study (ALTS) Group. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two year prospective study. Am J Obstet Gynecol 2003;188:1401-1405.
2. Guido R, Solomon D, Schiffman M, Burke L. Comparison of management strategies for women diagnosed as CIN 1 or less postcolposcopic evaluation: data from the ASCUS and LSIL triage study (ALTS), a multicenter randomized trial. J Lower Genital Tract Dis 2002;6:176.-
3. Schiffman M, Adrianza ME. ALTS Group. ASCUS-LSIL Triage Study. Design, methods, and characteristics of trial participants. Acta Cytol 2000;44:726-742
4. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. American Society for Colposcopy and Cervical Pathology 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol 2003;189:295-304.
Of the different strategies available for managing cervical intraepithelial neoplasia grade 1 (CIN1), testing for high-risk subtypes of the human papillomavirus (hr-HPV) DNA at 12 months has the highest sensitivity for predicting the development of CIN2 or CIN3 and leads to the lowest rate of referral to repeat colposcopy (TABLE 1). If the hr-HPV DNA test result is negative at 12 months, then the patient may return to routine cytology screening. If the hr-HPV DNA test result is positive, the patient should undergo repeat colposcopy.1,2
Base follow-up testing on patient preference, cost, and convenience
Patients with a colposcopic biopsy revealing mild dysplasia (HPV effect or CIN1) need follow-up due to the 12% risk of progression to CIN2 or CIN3 over 2 years.1 The decision to follow the patient with either a single HPV test at 12 months or cytologic testing at 6 and 12 months should be based on patient preference, cost, and convenience. One of the 2 follow-up strategies should be selected and a notification system implemented to ensure patients return at the appropriate follow-up interval.
Evidence summary
One study was found that attempted to determine the appropriate follow-up for women diagnosed with CIN1 on adequate colposcopic biopsy (FIGURE). This 2-year prospective study examined a subpopulation of 1539 women from the ASCUS/LSIL (atypical squamous cells of uncertain significance)/low-grade squamous intraepithelial lesion) Triage Study (ALTS) to determine the ideal follow-up strategy for women diagnosed with CIN1 or HPV effect on colposcopic biopsy-obtained histology. This study randomly assigned these women with CIN1 or HPV effect to either HPV testing or colposcopic examination at 6, 12, and 18 months. Every woman under-went an exit colposcopy at 24 months.
The study found that hr-HPV testing 12 months after the initial colposcopic exam had the highest sensitivity for detecting advanced disease (92.2%) and lowest referral rate to repeat colposcopy (55%). Follow-up of these patients with repeat cytology alone at 6 and 12 months had a lower sensitivity for detection of advanced disease (85%) and greater referral rate to repeat colposcopy (60%) when compared with HPV testing at 12 months alone. Three cytologic evaluations at 6, 12, and 18 months without HPV testing increased sensitivity (95%), although this increase was not statistically significant. A much higher percentage of patients were referred to colposcopy with this strategy. Combining both hr-HPV testing and cytology at 12 months did not significantly increase the identification of advanced disease and resulted in a higher re-referral rate to colposcopy.1-3
TABLE
Strategies for managing CIN1
| FOLLOW-UP/INTERVENTION AFTER DIAGNOSIS OF CIN1 AT COLPOSCOPY | SENSITIVITY FOR DETECTING CIN1 OR HIGHER | REFERRAL RATE FOR REPEAT COLPOSCOPY |
|---|---|---|
| hr-HPV test at 12 months | 92.2% | 55% |
| hr-HPV test at 6 months | 90.9% | 62.4% |
| Cytology at 6 and 12 months | 85% | 60% |
| Cytology at 6, 12, 18 months | 95% | Not available |
| hr-HPV test and cytology at 12 months | 94.8% | 64.1% |
| CIN1, cervical intraepithelial neoplasia grade 1; hr-HPV, high-risk subtypes of human papillomavirus. | ||
| Source: Guido et al, Am J Obstet Gynecol 20031; Guido et al, J Lower Genital Tract Dis 2002.2 | ||
Recommendations from others
American Society for Colposcopy and Cervical Pathology consensus guidelines for the management of women with CIN were published in 2003. Their recommendation now states that follow-up after adequate colposcopy with a biopsy revealing CIN1 or HPV effect may include either repeat cervical cytology tests at 6 and 12 months or HPV testing at 12 months. After a negative test for high-risk HPV types or 2 consecutive negative cervical cytology tests, the patient may return to annual cytologic screening. If the HPV test is positive for high-risk viral types or the cytology is reported as atypical squamous cells (ASC) or higher, the patient should undergo repeat colposcopy.4
Of the different strategies available for managing cervical intraepithelial neoplasia grade 1 (CIN1), testing for high-risk subtypes of the human papillomavirus (hr-HPV) DNA at 12 months has the highest sensitivity for predicting the development of CIN2 or CIN3 and leads to the lowest rate of referral to repeat colposcopy (TABLE 1). If the hr-HPV DNA test result is negative at 12 months, then the patient may return to routine cytology screening. If the hr-HPV DNA test result is positive, the patient should undergo repeat colposcopy.1,2
Base follow-up testing on patient preference, cost, and convenience
Patients with a colposcopic biopsy revealing mild dysplasia (HPV effect or CIN1) need follow-up due to the 12% risk of progression to CIN2 or CIN3 over 2 years.1 The decision to follow the patient with either a single HPV test at 12 months or cytologic testing at 6 and 12 months should be based on patient preference, cost, and convenience. One of the 2 follow-up strategies should be selected and a notification system implemented to ensure patients return at the appropriate follow-up interval.
Evidence summary
One study was found that attempted to determine the appropriate follow-up for women diagnosed with CIN1 on adequate colposcopic biopsy (FIGURE). This 2-year prospective study examined a subpopulation of 1539 women from the ASCUS/LSIL (atypical squamous cells of uncertain significance)/low-grade squamous intraepithelial lesion) Triage Study (ALTS) to determine the ideal follow-up strategy for women diagnosed with CIN1 or HPV effect on colposcopic biopsy-obtained histology. This study randomly assigned these women with CIN1 or HPV effect to either HPV testing or colposcopic examination at 6, 12, and 18 months. Every woman under-went an exit colposcopy at 24 months.
The study found that hr-HPV testing 12 months after the initial colposcopic exam had the highest sensitivity for detecting advanced disease (92.2%) and lowest referral rate to repeat colposcopy (55%). Follow-up of these patients with repeat cytology alone at 6 and 12 months had a lower sensitivity for detection of advanced disease (85%) and greater referral rate to repeat colposcopy (60%) when compared with HPV testing at 12 months alone. Three cytologic evaluations at 6, 12, and 18 months without HPV testing increased sensitivity (95%), although this increase was not statistically significant. A much higher percentage of patients were referred to colposcopy with this strategy. Combining both hr-HPV testing and cytology at 12 months did not significantly increase the identification of advanced disease and resulted in a higher re-referral rate to colposcopy.1-3
TABLE
Strategies for managing CIN1
| FOLLOW-UP/INTERVENTION AFTER DIAGNOSIS OF CIN1 AT COLPOSCOPY | SENSITIVITY FOR DETECTING CIN1 OR HIGHER | REFERRAL RATE FOR REPEAT COLPOSCOPY |
|---|---|---|
| hr-HPV test at 12 months | 92.2% | 55% |
| hr-HPV test at 6 months | 90.9% | 62.4% |
| Cytology at 6 and 12 months | 85% | 60% |
| Cytology at 6, 12, 18 months | 95% | Not available |
| hr-HPV test and cytology at 12 months | 94.8% | 64.1% |
| CIN1, cervical intraepithelial neoplasia grade 1; hr-HPV, high-risk subtypes of human papillomavirus. | ||
| Source: Guido et al, Am J Obstet Gynecol 20031; Guido et al, J Lower Genital Tract Dis 2002.2 | ||
Recommendations from others
American Society for Colposcopy and Cervical Pathology consensus guidelines for the management of women with CIN were published in 2003. Their recommendation now states that follow-up after adequate colposcopy with a biopsy revealing CIN1 or HPV effect may include either repeat cervical cytology tests at 6 and 12 months or HPV testing at 12 months. After a negative test for high-risk HPV types or 2 consecutive negative cervical cytology tests, the patient may return to annual cytologic screening. If the HPV test is positive for high-risk viral types or the cytology is reported as atypical squamous cells (ASC) or higher, the patient should undergo repeat colposcopy.4
1. Guido R, Schiffman M, Solomon D, Burke L. ASCUS LSIL Triage Study (ALTS) Group. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two year prospective study. Am J Obstet Gynecol 2003;188:1401-1405.
2. Guido R, Solomon D, Schiffman M, Burke L. Comparison of management strategies for women diagnosed as CIN 1 or less postcolposcopic evaluation: data from the ASCUS and LSIL triage study (ALTS), a multicenter randomized trial. J Lower Genital Tract Dis 2002;6:176.-
3. Schiffman M, Adrianza ME. ALTS Group. ASCUS-LSIL Triage Study. Design, methods, and characteristics of trial participants. Acta Cytol 2000;44:726-742
4. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. American Society for Colposcopy and Cervical Pathology 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol 2003;189:295-304.
1. Guido R, Schiffman M, Solomon D, Burke L. ASCUS LSIL Triage Study (ALTS) Group. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two year prospective study. Am J Obstet Gynecol 2003;188:1401-1405.
2. Guido R, Solomon D, Schiffman M, Burke L. Comparison of management strategies for women diagnosed as CIN 1 or less postcolposcopic evaluation: data from the ASCUS and LSIL triage study (ALTS), a multicenter randomized trial. J Lower Genital Tract Dis 2002;6:176.-
3. Schiffman M, Adrianza ME. ALTS Group. ASCUS-LSIL Triage Study. Design, methods, and characteristics of trial participants. Acta Cytol 2000;44:726-742
4. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. American Society for Colposcopy and Cervical Pathology 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol 2003;189:295-304.
Evidence-based answers from the Family Physicians Inquiries Network
What precautions should we use with statins for women of childbearing age?
Statins are contraindicated for women who are pregnant or breastfeeding. Data evaluating statin use for women of childbearing age is limited; however, they may be used cautiously with adequate contraception. Pravastatin may be preferred based on its low tissue-penetration properties. Cholesterol-lowering with simvastatin 40 mg/d did not disrupt menstrual cycles or effect luteal phase duration (strength of recommendation: C).
Use statins only as a last resort for women of childbearing age
Ariel Smits, MD
Department of Family Medicine, Oregon Health & Science University, Portland
I try to follow the USPSTF recommendations and not screen women aged <45 years without coronary artery disease risk factors for hyperlipidemia. When a woman of any age needs treatment, my first-line therapy is lifestyle modification. Given the risks of statin drugs to the developing fetus, women with childbearing potential should give fully informed consent and be offered reliable contraception before stating statin therapy.
Before reading this review, I had not been aware of the serious effects of statin medications on the developing fetus. In conversations with my colleagues, I found that the adverse effects of statins during pregnancy are not readily known. Such information needs to be more widely disseminated.
Evidence summary
Hydroxymethyl glutaryl coenzyme A (HMG CoA) reductase inhibitors, commonly called statins, have been on the market since the late 1980s. Statins are primarily used to treat hypercholesterolemia, and in recent years have been shown to reduce the risks of coronary events, stroke, and cardiovascular mortality.1
Use of statins is contraindicated during pregnancy based on pre-marketing animal studies showing developmental toxicities in animal fetuses; consequently they are pregnancy category X.2 To date, no controlled studies demonstrate teratogenic effects in humans; however, numerous case reports have documented congenital anomalies, including vertebral, anal, cardiac, tracheal, esophageal, renal, and limb deficiency (VACTERL association), intrauterine growth retardation (IUGR), and demise in fetuses exposed during pregnancy, especially in the first trimester. It is thought that adverse events are under-reported and likely biased toward severe outcomes, thereby limiting actual reported exposures. Despite this limitation, the likelihood of observing specific anomalies has been predicted based upon prescription data and birth rates. The overall birth prevalence of any isolated lower-limb defect or VACTERL anomaly is estimated as 1:100,000 and ranges from 1:50,000 for simvastatin (Zocor) to 1:500,000 for lovastatin (Mevacor).3 These congenital anomaly frequencies do not exceed general population rates.
One study suggests that short-term use of simvastatin does not affect menstruation or ovulation of premenopausal women. This double-blind, randomized, placebo-controlled trial enrolled 86 normally cycling women. Mean age of women completing the study was 35. Simvastatin 40 mg/d was studied for cholesterol effects and female reproductive effects. Urinary luteinizing hormone (LH) and pregnanediol glucuronide (PDG), a progesterone metabolite, were assessed to determine if treatment with simvastatin adversely affects luteal function. Simvastatin lowered low-density lipoprotein (LDL) cholesterol by 34.3% (P<.001). Normal luteal phase duration and peak were confirmed by urinary PDG and LH levels. This study demonstrated that treatment with simvastatin for 4 months had no significant clinical changes on reproductive gonadal function compared with placebo.4
Although ovulation may not be affected by simvastatin, do statins provide a reward worth the risk of other adverse effects? A recent meta-analysis evaluated the benefits of lipid-lowering medication in trials of at least 1 year duration that included women. Total and coronary heart disease (CHD) mortality, nonfatal myocardial infarction, revascularization, and total CHD events were assessed among women with and without cardiovascular disease (CVD). Ten trials included statins. Of the 5 studies that reported age, the average was 61 years. For women without CVD, lipid-lowering treatment was not shown to affect total or CHD mortality. For women with known CVD, hyperlipidemia treatment did not affect total mortality, but was shown effective in reducing CHD events, CHD mortality, nonfatal myocardial infarction, and revascularization; the relative risk of CHD events for statin users was 0.80 (95% confidence interval [CI], 0.71–0.91). The number of women needed to treat (NNT) to prevent an initial CHD event was 140. For secondary prevention, the NNT to prevent 1 CHD event was 26. Since women of child-bearing potential have lower probability of CHD events compared to the older women studied in this meta-analysis, the expected benefit for younger women is likely to be substantially lower.5
Consider initial pregnancy tests and inform all women of childbearing age of the possibility of fetal injury before starting statin therapy.2 Highly lipophilic statins—such as simvastatin, atorvastatin (Lipitor), and lovastatin—achieve embryoplacental concentrations similar to those of maternal plasma. For this reason, if statin therapy is needed, these agents should be avoided. Pravastatin (Pravachol) is the most hydrophilic statin and has no reports of abnormal pregnancy outcomes, even in animal research.3
Recommendations from others
The National Cholesterol Education Program Expert Panel and the American Heart Association make no specific recommendations regarding precautions with statin use for women of childbearing age who require treatment for hypercholesterolemia or coronary heart disease.6,7 The American College of Obstetrics and Gynecologists makes no distinction regarding recommendations for pharmacological treatment of hyperlipidemia for women aged 20 to 45 years.8
The US Preventive Services Task Force makes no recommendations on treatment with statins; they only address screening for hypercholesterolemia.9 The Food and Drug Administration has given statin agents a pregnancy category of X (risks involved in use of the drug by pregnant women clearly outweigh potential benefits).
1. Moore TH, Bartlett C, Burke MA, Davey Smith G, Ebrahim SB. Statins for preventing cardiovascular disease. Cochrane Database Syst Rev 2004;(2):CD004816.
2. Draft summary of reproductive toxicology studies on Mevacor NDA 21-213: Joint Meeting of the Nonprescription Drugs Advisory Committee and Endocrinologic and Metabolic Drugs Advisory Committee of the Federal Drug Administration, Merck & Co (July 13, 2000). Available at: www.fda.gov/ohrms/dockets/ac/00/backgrd/3622b1b_summary.pdf. Accessed on December 7, 2005.
3. Edison RJ, Muenke M. Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am J Med Genet A 2004;131:287-298.
4. Plotkin D, Miller S, Nakajima S, et al. Lowering low density lipoprotein cholesterol with simvastatin, a hydroxyl-3-methylglutaryl-coenzyme a reductase inhibitor, does not affect luteal function in premenopausal women. J Clin Endocrinol Metabol 2002;87:3155-3161.
5. Walsh JME, Pignone M. Drug treatment of hyperlipidemia in women. JAMA 2004;291:2243-2252.
6. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-2497.
7. Mosca L, Appel JA, Benjamin EJ, et al. AHA guidelines: Evidence-based Guidelines for Cardiovascular Disease Prevention in Women. Circulation 2004;109:672-693.
8. Herbert WNP, Braly PS, Barss VA, et al. ACOG: Guidelines for Women’s Health Care. 2nd ed. Washington, DC: ACOG; 2002;209-211.
9. US Preventive Services Task Force. Screening for lipid disorder in adults: recommendations and rationale. Internet J Intern Med 2002;3(2).-Available at: www.ispub.com/ostia/index.php?xmlFilePath=journals/ijim/vol3n2/lipid.xml. Accessed on December 8, 2005.
Statins are contraindicated for women who are pregnant or breastfeeding. Data evaluating statin use for women of childbearing age is limited; however, they may be used cautiously with adequate contraception. Pravastatin may be preferred based on its low tissue-penetration properties. Cholesterol-lowering with simvastatin 40 mg/d did not disrupt menstrual cycles or effect luteal phase duration (strength of recommendation: C).
Use statins only as a last resort for women of childbearing age
Ariel Smits, MD
Department of Family Medicine, Oregon Health & Science University, Portland
I try to follow the USPSTF recommendations and not screen women aged <45 years without coronary artery disease risk factors for hyperlipidemia. When a woman of any age needs treatment, my first-line therapy is lifestyle modification. Given the risks of statin drugs to the developing fetus, women with childbearing potential should give fully informed consent and be offered reliable contraception before stating statin therapy.
Before reading this review, I had not been aware of the serious effects of statin medications on the developing fetus. In conversations with my colleagues, I found that the adverse effects of statins during pregnancy are not readily known. Such information needs to be more widely disseminated.
Evidence summary
Hydroxymethyl glutaryl coenzyme A (HMG CoA) reductase inhibitors, commonly called statins, have been on the market since the late 1980s. Statins are primarily used to treat hypercholesterolemia, and in recent years have been shown to reduce the risks of coronary events, stroke, and cardiovascular mortality.1
Use of statins is contraindicated during pregnancy based on pre-marketing animal studies showing developmental toxicities in animal fetuses; consequently they are pregnancy category X.2 To date, no controlled studies demonstrate teratogenic effects in humans; however, numerous case reports have documented congenital anomalies, including vertebral, anal, cardiac, tracheal, esophageal, renal, and limb deficiency (VACTERL association), intrauterine growth retardation (IUGR), and demise in fetuses exposed during pregnancy, especially in the first trimester. It is thought that adverse events are under-reported and likely biased toward severe outcomes, thereby limiting actual reported exposures. Despite this limitation, the likelihood of observing specific anomalies has been predicted based upon prescription data and birth rates. The overall birth prevalence of any isolated lower-limb defect or VACTERL anomaly is estimated as 1:100,000 and ranges from 1:50,000 for simvastatin (Zocor) to 1:500,000 for lovastatin (Mevacor).3 These congenital anomaly frequencies do not exceed general population rates.
One study suggests that short-term use of simvastatin does not affect menstruation or ovulation of premenopausal women. This double-blind, randomized, placebo-controlled trial enrolled 86 normally cycling women. Mean age of women completing the study was 35. Simvastatin 40 mg/d was studied for cholesterol effects and female reproductive effects. Urinary luteinizing hormone (LH) and pregnanediol glucuronide (PDG), a progesterone metabolite, were assessed to determine if treatment with simvastatin adversely affects luteal function. Simvastatin lowered low-density lipoprotein (LDL) cholesterol by 34.3% (P<.001). Normal luteal phase duration and peak were confirmed by urinary PDG and LH levels. This study demonstrated that treatment with simvastatin for 4 months had no significant clinical changes on reproductive gonadal function compared with placebo.4
Although ovulation may not be affected by simvastatin, do statins provide a reward worth the risk of other adverse effects? A recent meta-analysis evaluated the benefits of lipid-lowering medication in trials of at least 1 year duration that included women. Total and coronary heart disease (CHD) mortality, nonfatal myocardial infarction, revascularization, and total CHD events were assessed among women with and without cardiovascular disease (CVD). Ten trials included statins. Of the 5 studies that reported age, the average was 61 years. For women without CVD, lipid-lowering treatment was not shown to affect total or CHD mortality. For women with known CVD, hyperlipidemia treatment did not affect total mortality, but was shown effective in reducing CHD events, CHD mortality, nonfatal myocardial infarction, and revascularization; the relative risk of CHD events for statin users was 0.80 (95% confidence interval [CI], 0.71–0.91). The number of women needed to treat (NNT) to prevent an initial CHD event was 140. For secondary prevention, the NNT to prevent 1 CHD event was 26. Since women of child-bearing potential have lower probability of CHD events compared to the older women studied in this meta-analysis, the expected benefit for younger women is likely to be substantially lower.5
Consider initial pregnancy tests and inform all women of childbearing age of the possibility of fetal injury before starting statin therapy.2 Highly lipophilic statins—such as simvastatin, atorvastatin (Lipitor), and lovastatin—achieve embryoplacental concentrations similar to those of maternal plasma. For this reason, if statin therapy is needed, these agents should be avoided. Pravastatin (Pravachol) is the most hydrophilic statin and has no reports of abnormal pregnancy outcomes, even in animal research.3
Recommendations from others
The National Cholesterol Education Program Expert Panel and the American Heart Association make no specific recommendations regarding precautions with statin use for women of childbearing age who require treatment for hypercholesterolemia or coronary heart disease.6,7 The American College of Obstetrics and Gynecologists makes no distinction regarding recommendations for pharmacological treatment of hyperlipidemia for women aged 20 to 45 years.8
The US Preventive Services Task Force makes no recommendations on treatment with statins; they only address screening for hypercholesterolemia.9 The Food and Drug Administration has given statin agents a pregnancy category of X (risks involved in use of the drug by pregnant women clearly outweigh potential benefits).
Statins are contraindicated for women who are pregnant or breastfeeding. Data evaluating statin use for women of childbearing age is limited; however, they may be used cautiously with adequate contraception. Pravastatin may be preferred based on its low tissue-penetration properties. Cholesterol-lowering with simvastatin 40 mg/d did not disrupt menstrual cycles or effect luteal phase duration (strength of recommendation: C).
Use statins only as a last resort for women of childbearing age
Ariel Smits, MD
Department of Family Medicine, Oregon Health & Science University, Portland
I try to follow the USPSTF recommendations and not screen women aged <45 years without coronary artery disease risk factors for hyperlipidemia. When a woman of any age needs treatment, my first-line therapy is lifestyle modification. Given the risks of statin drugs to the developing fetus, women with childbearing potential should give fully informed consent and be offered reliable contraception before stating statin therapy.
Before reading this review, I had not been aware of the serious effects of statin medications on the developing fetus. In conversations with my colleagues, I found that the adverse effects of statins during pregnancy are not readily known. Such information needs to be more widely disseminated.
Evidence summary
Hydroxymethyl glutaryl coenzyme A (HMG CoA) reductase inhibitors, commonly called statins, have been on the market since the late 1980s. Statins are primarily used to treat hypercholesterolemia, and in recent years have been shown to reduce the risks of coronary events, stroke, and cardiovascular mortality.1
Use of statins is contraindicated during pregnancy based on pre-marketing animal studies showing developmental toxicities in animal fetuses; consequently they are pregnancy category X.2 To date, no controlled studies demonstrate teratogenic effects in humans; however, numerous case reports have documented congenital anomalies, including vertebral, anal, cardiac, tracheal, esophageal, renal, and limb deficiency (VACTERL association), intrauterine growth retardation (IUGR), and demise in fetuses exposed during pregnancy, especially in the first trimester. It is thought that adverse events are under-reported and likely biased toward severe outcomes, thereby limiting actual reported exposures. Despite this limitation, the likelihood of observing specific anomalies has been predicted based upon prescription data and birth rates. The overall birth prevalence of any isolated lower-limb defect or VACTERL anomaly is estimated as 1:100,000 and ranges from 1:50,000 for simvastatin (Zocor) to 1:500,000 for lovastatin (Mevacor).3 These congenital anomaly frequencies do not exceed general population rates.
One study suggests that short-term use of simvastatin does not affect menstruation or ovulation of premenopausal women. This double-blind, randomized, placebo-controlled trial enrolled 86 normally cycling women. Mean age of women completing the study was 35. Simvastatin 40 mg/d was studied for cholesterol effects and female reproductive effects. Urinary luteinizing hormone (LH) and pregnanediol glucuronide (PDG), a progesterone metabolite, were assessed to determine if treatment with simvastatin adversely affects luteal function. Simvastatin lowered low-density lipoprotein (LDL) cholesterol by 34.3% (P<.001). Normal luteal phase duration and peak were confirmed by urinary PDG and LH levels. This study demonstrated that treatment with simvastatin for 4 months had no significant clinical changes on reproductive gonadal function compared with placebo.4
Although ovulation may not be affected by simvastatin, do statins provide a reward worth the risk of other adverse effects? A recent meta-analysis evaluated the benefits of lipid-lowering medication in trials of at least 1 year duration that included women. Total and coronary heart disease (CHD) mortality, nonfatal myocardial infarction, revascularization, and total CHD events were assessed among women with and without cardiovascular disease (CVD). Ten trials included statins. Of the 5 studies that reported age, the average was 61 years. For women without CVD, lipid-lowering treatment was not shown to affect total or CHD mortality. For women with known CVD, hyperlipidemia treatment did not affect total mortality, but was shown effective in reducing CHD events, CHD mortality, nonfatal myocardial infarction, and revascularization; the relative risk of CHD events for statin users was 0.80 (95% confidence interval [CI], 0.71–0.91). The number of women needed to treat (NNT) to prevent an initial CHD event was 140. For secondary prevention, the NNT to prevent 1 CHD event was 26. Since women of child-bearing potential have lower probability of CHD events compared to the older women studied in this meta-analysis, the expected benefit for younger women is likely to be substantially lower.5
Consider initial pregnancy tests and inform all women of childbearing age of the possibility of fetal injury before starting statin therapy.2 Highly lipophilic statins—such as simvastatin, atorvastatin (Lipitor), and lovastatin—achieve embryoplacental concentrations similar to those of maternal plasma. For this reason, if statin therapy is needed, these agents should be avoided. Pravastatin (Pravachol) is the most hydrophilic statin and has no reports of abnormal pregnancy outcomes, even in animal research.3
Recommendations from others
The National Cholesterol Education Program Expert Panel and the American Heart Association make no specific recommendations regarding precautions with statin use for women of childbearing age who require treatment for hypercholesterolemia or coronary heart disease.6,7 The American College of Obstetrics and Gynecologists makes no distinction regarding recommendations for pharmacological treatment of hyperlipidemia for women aged 20 to 45 years.8
The US Preventive Services Task Force makes no recommendations on treatment with statins; they only address screening for hypercholesterolemia.9 The Food and Drug Administration has given statin agents a pregnancy category of X (risks involved in use of the drug by pregnant women clearly outweigh potential benefits).
1. Moore TH, Bartlett C, Burke MA, Davey Smith G, Ebrahim SB. Statins for preventing cardiovascular disease. Cochrane Database Syst Rev 2004;(2):CD004816.
2. Draft summary of reproductive toxicology studies on Mevacor NDA 21-213: Joint Meeting of the Nonprescription Drugs Advisory Committee and Endocrinologic and Metabolic Drugs Advisory Committee of the Federal Drug Administration, Merck & Co (July 13, 2000). Available at: www.fda.gov/ohrms/dockets/ac/00/backgrd/3622b1b_summary.pdf. Accessed on December 7, 2005.
3. Edison RJ, Muenke M. Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am J Med Genet A 2004;131:287-298.
4. Plotkin D, Miller S, Nakajima S, et al. Lowering low density lipoprotein cholesterol with simvastatin, a hydroxyl-3-methylglutaryl-coenzyme a reductase inhibitor, does not affect luteal function in premenopausal women. J Clin Endocrinol Metabol 2002;87:3155-3161.
5. Walsh JME, Pignone M. Drug treatment of hyperlipidemia in women. JAMA 2004;291:2243-2252.
6. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-2497.
7. Mosca L, Appel JA, Benjamin EJ, et al. AHA guidelines: Evidence-based Guidelines for Cardiovascular Disease Prevention in Women. Circulation 2004;109:672-693.
8. Herbert WNP, Braly PS, Barss VA, et al. ACOG: Guidelines for Women’s Health Care. 2nd ed. Washington, DC: ACOG; 2002;209-211.
9. US Preventive Services Task Force. Screening for lipid disorder in adults: recommendations and rationale. Internet J Intern Med 2002;3(2).-Available at: www.ispub.com/ostia/index.php?xmlFilePath=journals/ijim/vol3n2/lipid.xml. Accessed on December 8, 2005.
1. Moore TH, Bartlett C, Burke MA, Davey Smith G, Ebrahim SB. Statins for preventing cardiovascular disease. Cochrane Database Syst Rev 2004;(2):CD004816.
2. Draft summary of reproductive toxicology studies on Mevacor NDA 21-213: Joint Meeting of the Nonprescription Drugs Advisory Committee and Endocrinologic and Metabolic Drugs Advisory Committee of the Federal Drug Administration, Merck & Co (July 13, 2000). Available at: www.fda.gov/ohrms/dockets/ac/00/backgrd/3622b1b_summary.pdf. Accessed on December 7, 2005.
3. Edison RJ, Muenke M. Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am J Med Genet A 2004;131:287-298.
4. Plotkin D, Miller S, Nakajima S, et al. Lowering low density lipoprotein cholesterol with simvastatin, a hydroxyl-3-methylglutaryl-coenzyme a reductase inhibitor, does not affect luteal function in premenopausal women. J Clin Endocrinol Metabol 2002;87:3155-3161.
5. Walsh JME, Pignone M. Drug treatment of hyperlipidemia in women. JAMA 2004;291:2243-2252.
6. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-2497.
7. Mosca L, Appel JA, Benjamin EJ, et al. AHA guidelines: Evidence-based Guidelines for Cardiovascular Disease Prevention in Women. Circulation 2004;109:672-693.
8. Herbert WNP, Braly PS, Barss VA, et al. ACOG: Guidelines for Women’s Health Care. 2nd ed. Washington, DC: ACOG; 2002;209-211.
9. US Preventive Services Task Force. Screening for lipid disorder in adults: recommendations and rationale. Internet J Intern Med 2002;3(2).-Available at: www.ispub.com/ostia/index.php?xmlFilePath=journals/ijim/vol3n2/lipid.xml. Accessed on December 8, 2005.
Evidence-based answers from the Family Physicians Inquiries Network
How effective are lifestyle changes for controlling hypertension?
Regular aerobic exercise, weight loss of 3% to 9% of body weight, reduced dietary salt, the DASH diet, and moderation of alcohol intake are all lifestyle interventions that lower blood pressure. Average blood pressure decreases range from 3 to 11 mm Hg systolic and 2.5 to 5.5 mm Hg diastolic, depending on the particular intervention (strength of recommendation [SOR]: A, based on systematic reviews of randomized controlled trials [RCTs]). Studies of community-based interventions advocating combinations of the above have had mixed results with less reduction in blood pressure noted than for the individual interventions described above (SOR: B, RCTs with inconsistent results).
Lifestyle modifications plus drug therapy is the best treatment for patients with hypertension
Joseph Saseen, PharmD, FCCP, BCPS
Departments of Clinical Pharmacy and Family Medicine, University of Colorado Health Sciences Center
Most Americans with hypertension are not at their goal blood pressure, so the value of lifestyle modifications cannot be ignored. While some clinicians argue that these modifications are unreliable, this review should serve to reinforce the substantial impact of lifestyle modifications. Clinicians should remember that drug therapy is the only treatment modality proven to lower blood pressure and cardiovascular morbidity and mortality due to hypertension, based on evidence from outcome-based studies. Reducing cardiovascular morbidity and mortality is the ultimate goal of treating hypertension. Therefore, lifestyle modifications with antihypertensive drug therapy are the best treatments to reduce cardiovascular risk and attain goal blood pressure values for patients with hypertension.
Evidence summary
Lifestyle changes are advocated as first-line therapy for hypertension. This review examines the evidence on exercise, dietary interventions, weight loss, alcohol moderation, and smoking cessation. Average systolic blood pressure (SBP) and diastolic blood pressure (DBP) changes are reported in the TABLE.
Exercise. A well-done systematic review and meta-analysis from 2002 (including 15 studies with 770 participants) concluded that for hypertensive patients, aerobic exercise with at least one 40-minute session of moderate intensity per week is associated with a drop in SBP of about 5 mm Hg and a drop in DBP of about 4 mm Hg.1
DASH diet. The Dietary Approaches to Stop Hypertension (DASH) diet is a diet rich in fish, chicken, lean meat, low-fat dairy, fruits, vegetables, whole grains, legumes, nuts, and seeds. In a high-quality RCT, the DASH diet lowered SBP for hypertensive patients by an average of 11 mm Hg and DBP by an average of 5.5 mm Hg compared with the control group.2 Participants were provided with all food during the entire 8-week length of the trial.
Weight loss. A Cochrane review of 18 trials with 2611 participants concluded that for overweight hypertensive patients, weight loss of 3% to 9% of body weight is associated with 3 mm Hg decreases in both SBP and DBP.3
Salt reduction. A Cochrane review of 17 trials with 734 participants concluded that for individuals with hypertension, a reduced-salt diet results in a mean SBP and DBP reductions of 5 mm Hg and 3 mm Hg, respectively.4
Alcohol moderation. A well-done meta-analysis of alcohol reduction and blood pressure included 7 studies with 415 hypertensive patients.5 Mean baseline alcohol consumption was 3 to 6 alcoholic drinks per day, and the mean reduction in consumption was 67%. For this patient population, the average improvement was almost 4 mm Hg for SBP and nearly 2.5 mm Hg for DBP.
Smoking cessation. No high-quality studies show a long-term effect of smoking cessation on blood pressure. Smoking cessation has other well-documented health benefits and should still be recommended for patients with hypertension.
Multifactorial interventions. Thirteen randomized controlled trials of community-based interventions involving various combinations of lifestyle change advice show mixed results. In general, studies of interventions that were more intensive (ie, longer in duration, larger number of sessions, small group or one-on-one as opposed to large group lectures) and studies with shorter follow-up periods showed more positive results. The magnitude of the blood pressure improvements tended to be lower than for each individual intervention described above. (References are located in the APPENDIX on our web site at www.jfponline.com.
TABLE
Average effect on blood pressure from lifestyle interventions
| LIFESTYLE INTERVENTION | AVERAGE EFFECT ON SBP (MM HG) | AVERAGE EFFECT ON DBP (MM HG) |
|---|---|---|
| Regular aerobic exercise | –5 | –4 |
| DASH diet | –11 | –5.5 |
| Weight loss of 3% to 9% of body weight in overweight patients | –3 | –3 |
| Reduced salt diet | –5 | –3 |
| Alcohol moderation | –4 | –2.5 |
| SBP, systolic blood pressure; DBP, diastolic blood pressure | ||
Recommendations from others
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommends lifestyle modifications for all patients with hypertension.6 They point out that DASH diet plan with 1600 mg sodium had average blood pressure effects similar to single-drug therapy.
1. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann Intern Med 2002;136:493-503.
2. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N Engl J Med 1997;336:1117-1124.
3. Mulrow CD, Chiquette E, Angel L, et al. Dieting to reduce body weight for controlling hypertension in adults. Cochrane Database Syst Rev 2000;(2):000484.-
4. He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;(3):004937.-
5. Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension 2001;38:1112-1117.
6. Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 2003;289:2560-2572.
Regular aerobic exercise, weight loss of 3% to 9% of body weight, reduced dietary salt, the DASH diet, and moderation of alcohol intake are all lifestyle interventions that lower blood pressure. Average blood pressure decreases range from 3 to 11 mm Hg systolic and 2.5 to 5.5 mm Hg diastolic, depending on the particular intervention (strength of recommendation [SOR]: A, based on systematic reviews of randomized controlled trials [RCTs]). Studies of community-based interventions advocating combinations of the above have had mixed results with less reduction in blood pressure noted than for the individual interventions described above (SOR: B, RCTs with inconsistent results).
Lifestyle modifications plus drug therapy is the best treatment for patients with hypertension
Joseph Saseen, PharmD, FCCP, BCPS
Departments of Clinical Pharmacy and Family Medicine, University of Colorado Health Sciences Center
Most Americans with hypertension are not at their goal blood pressure, so the value of lifestyle modifications cannot be ignored. While some clinicians argue that these modifications are unreliable, this review should serve to reinforce the substantial impact of lifestyle modifications. Clinicians should remember that drug therapy is the only treatment modality proven to lower blood pressure and cardiovascular morbidity and mortality due to hypertension, based on evidence from outcome-based studies. Reducing cardiovascular morbidity and mortality is the ultimate goal of treating hypertension. Therefore, lifestyle modifications with antihypertensive drug therapy are the best treatments to reduce cardiovascular risk and attain goal blood pressure values for patients with hypertension.
Evidence summary
Lifestyle changes are advocated as first-line therapy for hypertension. This review examines the evidence on exercise, dietary interventions, weight loss, alcohol moderation, and smoking cessation. Average systolic blood pressure (SBP) and diastolic blood pressure (DBP) changes are reported in the TABLE.
Exercise. A well-done systematic review and meta-analysis from 2002 (including 15 studies with 770 participants) concluded that for hypertensive patients, aerobic exercise with at least one 40-minute session of moderate intensity per week is associated with a drop in SBP of about 5 mm Hg and a drop in DBP of about 4 mm Hg.1
DASH diet. The Dietary Approaches to Stop Hypertension (DASH) diet is a diet rich in fish, chicken, lean meat, low-fat dairy, fruits, vegetables, whole grains, legumes, nuts, and seeds. In a high-quality RCT, the DASH diet lowered SBP for hypertensive patients by an average of 11 mm Hg and DBP by an average of 5.5 mm Hg compared with the control group.2 Participants were provided with all food during the entire 8-week length of the trial.
Weight loss. A Cochrane review of 18 trials with 2611 participants concluded that for overweight hypertensive patients, weight loss of 3% to 9% of body weight is associated with 3 mm Hg decreases in both SBP and DBP.3
Salt reduction. A Cochrane review of 17 trials with 734 participants concluded that for individuals with hypertension, a reduced-salt diet results in a mean SBP and DBP reductions of 5 mm Hg and 3 mm Hg, respectively.4
Alcohol moderation. A well-done meta-analysis of alcohol reduction and blood pressure included 7 studies with 415 hypertensive patients.5 Mean baseline alcohol consumption was 3 to 6 alcoholic drinks per day, and the mean reduction in consumption was 67%. For this patient population, the average improvement was almost 4 mm Hg for SBP and nearly 2.5 mm Hg for DBP.
Smoking cessation. No high-quality studies show a long-term effect of smoking cessation on blood pressure. Smoking cessation has other well-documented health benefits and should still be recommended for patients with hypertension.
Multifactorial interventions. Thirteen randomized controlled trials of community-based interventions involving various combinations of lifestyle change advice show mixed results. In general, studies of interventions that were more intensive (ie, longer in duration, larger number of sessions, small group or one-on-one as opposed to large group lectures) and studies with shorter follow-up periods showed more positive results. The magnitude of the blood pressure improvements tended to be lower than for each individual intervention described above. (References are located in the APPENDIX on our web site at www.jfponline.com.
TABLE
Average effect on blood pressure from lifestyle interventions
| LIFESTYLE INTERVENTION | AVERAGE EFFECT ON SBP (MM HG) | AVERAGE EFFECT ON DBP (MM HG) |
|---|---|---|
| Regular aerobic exercise | –5 | –4 |
| DASH diet | –11 | –5.5 |
| Weight loss of 3% to 9% of body weight in overweight patients | –3 | –3 |
| Reduced salt diet | –5 | –3 |
| Alcohol moderation | –4 | –2.5 |
| SBP, systolic blood pressure; DBP, diastolic blood pressure | ||
Recommendations from others
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommends lifestyle modifications for all patients with hypertension.6 They point out that DASH diet plan with 1600 mg sodium had average blood pressure effects similar to single-drug therapy.
Regular aerobic exercise, weight loss of 3% to 9% of body weight, reduced dietary salt, the DASH diet, and moderation of alcohol intake are all lifestyle interventions that lower blood pressure. Average blood pressure decreases range from 3 to 11 mm Hg systolic and 2.5 to 5.5 mm Hg diastolic, depending on the particular intervention (strength of recommendation [SOR]: A, based on systematic reviews of randomized controlled trials [RCTs]). Studies of community-based interventions advocating combinations of the above have had mixed results with less reduction in blood pressure noted than for the individual interventions described above (SOR: B, RCTs with inconsistent results).
Lifestyle modifications plus drug therapy is the best treatment for patients with hypertension
Joseph Saseen, PharmD, FCCP, BCPS
Departments of Clinical Pharmacy and Family Medicine, University of Colorado Health Sciences Center
Most Americans with hypertension are not at their goal blood pressure, so the value of lifestyle modifications cannot be ignored. While some clinicians argue that these modifications are unreliable, this review should serve to reinforce the substantial impact of lifestyle modifications. Clinicians should remember that drug therapy is the only treatment modality proven to lower blood pressure and cardiovascular morbidity and mortality due to hypertension, based on evidence from outcome-based studies. Reducing cardiovascular morbidity and mortality is the ultimate goal of treating hypertension. Therefore, lifestyle modifications with antihypertensive drug therapy are the best treatments to reduce cardiovascular risk and attain goal blood pressure values for patients with hypertension.
Evidence summary
Lifestyle changes are advocated as first-line therapy for hypertension. This review examines the evidence on exercise, dietary interventions, weight loss, alcohol moderation, and smoking cessation. Average systolic blood pressure (SBP) and diastolic blood pressure (DBP) changes are reported in the TABLE.
Exercise. A well-done systematic review and meta-analysis from 2002 (including 15 studies with 770 participants) concluded that for hypertensive patients, aerobic exercise with at least one 40-minute session of moderate intensity per week is associated with a drop in SBP of about 5 mm Hg and a drop in DBP of about 4 mm Hg.1
DASH diet. The Dietary Approaches to Stop Hypertension (DASH) diet is a diet rich in fish, chicken, lean meat, low-fat dairy, fruits, vegetables, whole grains, legumes, nuts, and seeds. In a high-quality RCT, the DASH diet lowered SBP for hypertensive patients by an average of 11 mm Hg and DBP by an average of 5.5 mm Hg compared with the control group.2 Participants were provided with all food during the entire 8-week length of the trial.
Weight loss. A Cochrane review of 18 trials with 2611 participants concluded that for overweight hypertensive patients, weight loss of 3% to 9% of body weight is associated with 3 mm Hg decreases in both SBP and DBP.3
Salt reduction. A Cochrane review of 17 trials with 734 participants concluded that for individuals with hypertension, a reduced-salt diet results in a mean SBP and DBP reductions of 5 mm Hg and 3 mm Hg, respectively.4
Alcohol moderation. A well-done meta-analysis of alcohol reduction and blood pressure included 7 studies with 415 hypertensive patients.5 Mean baseline alcohol consumption was 3 to 6 alcoholic drinks per day, and the mean reduction in consumption was 67%. For this patient population, the average improvement was almost 4 mm Hg for SBP and nearly 2.5 mm Hg for DBP.
Smoking cessation. No high-quality studies show a long-term effect of smoking cessation on blood pressure. Smoking cessation has other well-documented health benefits and should still be recommended for patients with hypertension.
Multifactorial interventions. Thirteen randomized controlled trials of community-based interventions involving various combinations of lifestyle change advice show mixed results. In general, studies of interventions that were more intensive (ie, longer in duration, larger number of sessions, small group or one-on-one as opposed to large group lectures) and studies with shorter follow-up periods showed more positive results. The magnitude of the blood pressure improvements tended to be lower than for each individual intervention described above. (References are located in the APPENDIX on our web site at www.jfponline.com.
TABLE
Average effect on blood pressure from lifestyle interventions
| LIFESTYLE INTERVENTION | AVERAGE EFFECT ON SBP (MM HG) | AVERAGE EFFECT ON DBP (MM HG) |
|---|---|---|
| Regular aerobic exercise | –5 | –4 |
| DASH diet | –11 | –5.5 |
| Weight loss of 3% to 9% of body weight in overweight patients | –3 | –3 |
| Reduced salt diet | –5 | –3 |
| Alcohol moderation | –4 | –2.5 |
| SBP, systolic blood pressure; DBP, diastolic blood pressure | ||
Recommendations from others
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommends lifestyle modifications for all patients with hypertension.6 They point out that DASH diet plan with 1600 mg sodium had average blood pressure effects similar to single-drug therapy.
1. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann Intern Med 2002;136:493-503.
2. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N Engl J Med 1997;336:1117-1124.
3. Mulrow CD, Chiquette E, Angel L, et al. Dieting to reduce body weight for controlling hypertension in adults. Cochrane Database Syst Rev 2000;(2):000484.-
4. He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;(3):004937.-
5. Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension 2001;38:1112-1117.
6. Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 2003;289:2560-2572.
1. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann Intern Med 2002;136:493-503.
2. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N Engl J Med 1997;336:1117-1124.
3. Mulrow CD, Chiquette E, Angel L, et al. Dieting to reduce body weight for controlling hypertension in adults. Cochrane Database Syst Rev 2000;(2):000484.-
4. He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;(3):004937.-
5. Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension 2001;38:1112-1117.
6. Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 2003;289:2560-2572.
Evidence-based answers from the Family Physicians Inquiries Network