User login
MMSE vs MoCA: Is One Better Than the Other?
Featured Article: A Pain in the Neck--Nontraumatic Causes of Neck Pain
The English expression, “a pain in the neck” is said to have originated in the early 1900s as a euphemism for the less polite phrase, “a pain in the ass.”1 While one might wonder how the expressions of such disparate discomforts came to be idiomatically equivalent, the focus of this article is on etiology of the former. All wryness aside, since most ED presentations of neck pain are musculoskeletal in origin, one may easily fail to consider the myriad of less common, but possibly serious, causes.
Pain can originate from any part of the neck and occur as a result of inflammation (eg, infections and arthritides), vascular pathology (eg, cervical artery dissection [CAD]), spaceoccupying lesions (eg, hematomas, cysts, tumors), or even as referred pain from noncervical sources (eg, heart, diaphragm, lung apex). Any lesion encroaching on the limited space of the neck can quickly compromise the airway, compress nerves, or inhibit blood flow to the brain; therefore, knowledge of the causes of such conditions is critical. This article reviews some of the less common and generally atraumatic etiologies of nontraumatic neck pain of which the emergency physician should be familiar.
Vascular Disorders
Vascular-associated neck pain can originate from vessels within the neck or represent referred pain from a more distant structure. In both cases, however, the potential for morbidity is high and the need for consideration and timely recognition crucial.
Cervical Artery Dissection
The typical initial presenting symptom of CAD—ie, internal CAD (ICAD) or vertebral artery dissection (VAD)—is severe pain in the ipsilateral neck and/or head. Onset of pain may be sudden or gradual.2 CAD occurs in an estimated 2 or 3 of every 100,000 people per year, mostly in patients between ages 20 and 40 years, and it is considered the most common cause of stroke in patients younger than age 45 years.2 The pain associated with CAD generally follows trauma. While the precipitating trauma can be a major blunt or penetrating one, it is often caused by something seemingly trivial, such as “trauma” associated with coughing, painting a ceiling, yoga, or (classically and notoriously) chiropractic manipulation.3 There is frequently some rotational component to CAD-associated trauma,4 though dissection may occur spontaneously.5
The typical triad of symptoms is ipsilateral neck and/or head pain, partial Horner’s syndrome (ptosis and miosis without anhidrosis), and signs of cerebral ischemia. However, patients do not always present with all three of these symptoms, which can complicate the diagnosis. For example, in some patients, neck pain is the sole presenting symptom and can mimic the musculoskeletal pain expected from the mechanical strain that precipitated the dissection.6 In addition, partial Horner’s syndrome occurs in only 50% of cases, and ischemic symptoms might not present for hours to weeks after the onset of neck pain.6
In almost all cases of CAD, initial symptoms are otherwise unexplained pain described as a constant, steady aching. 7 Since cervical arteries are heavily invested with pain fibers,8 an intimal tear with dissecting intramural hematoma provokes pain. Pain associated with VAD is usually severe, unilateral, posterior neck, and/or occipital, while ICAD-associated pain is ipsilateral, anterolateral neck, head, and/or face. It is important to note that head or neck pain caused by a dissection normally precedes the ischemic manifestation as opposed to the more common stroke, in which the ischemia precedes or is simultaneous with the accompanying headache.9
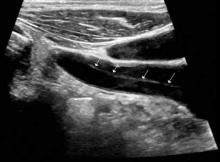
Ischemic neurological symptoms can arise from stenosis of the arterial lumen, secondary to an expanding intramural hematoma; a luminal thrombus developing at the intimal defect; or an embolization accompanied by ipsilateral Horner’s syndrome, any cranial nerve abnormality, or followed by cerebral or ocular ischemic symptoms (even if transient). A diagnosis is usually made through vascular ultrasound (Figure 1) and confirmed with computed tomography angiography (CTA) or magnetic resonance angiography. When requesting a CTA of the neck, the emergency physician should specifically make note of suspected CAD in the order. Immediate treatment includes a cervical collar and neurosurgical consultation even though treatment is essentially medical and surgery is rarely required. Anticoagulation therapy is routinely initiated to prevent thrombus propagation or embolization (unless there is brain hemorrhage). Antiplatelet therapy may be equally efficacious, 10 and can be initiated upon suspicions of CAD and while confirmatory studies are in progress. The prognosis for extracranial dissections is generally good.
Cervical Epidural Hematoma Cervical (spinal) epidural hematoma is an uncommon but potentially catastrophic event that can lead to permanent neurological deficits and death from respiratory failure. It presents as sudden and severe local neck pain with rapid development of radicular pain at the corresponding dermatomes. Motor and sensory deficits follow within minutes to days.12,13 Bleeding can occur spontaneously or secondary to trauma, surgery, or coagulopathy (which itself may be pathological—eg, hemophilia or iatrogenic in origin).14,15 Untreated, progressive cord compression can lead to permanent neurological deficits and death from respiratory failure. In the patient with acute neurological deficits, immediate correction of coagulation issues is required before decompressive surgery.
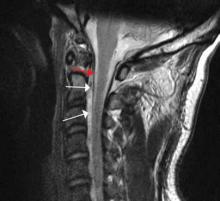
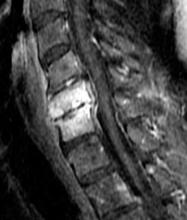
Diagnosis of cervical epidural hematoma is complicated by the rarity of the event and the lack of specific symptoms. When trauma is involved, cervical disc or nerve root injury is a more likely cause of sudden onset of neck pain, with rapid development of a radicular component. However, when symptoms occur following minor exertion (eg, sneezing, coitus, coughing) and in the presence of risk factors such as hematologic disorders, pregnancy, rheumatologic disorders, or liver dysfunction, epidural hematoma must be considered.16 Emergent magnetic resonance imaging (MRI) is the modality of choice for detecting this condition (Figure 2).
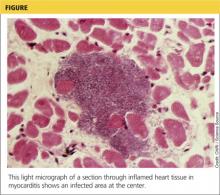
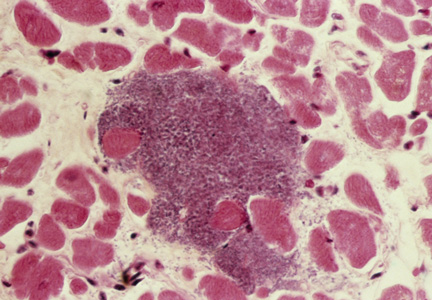
Coronary Ischemia Angina pectoris secondary to coronary ischemia is described as retrosternal “heaviness” or pressure, which may spread to either or both arms, the neck, or jaw. Pathology originating in the neck can be experienced as chest pain and may confound the diagnosis. Because cervical nerve roots C4-C8 contribute to the innervation of the anterior chest wall, irritation of any one of these nerves secondary to neck pathology can mimic true angina.17,18 Conversely, the likelihood that the only pain caused by coronary ischemia might be felt in the neck is low, but possible— especially in women.19,20 Coronary ischemia should be considered in patients with cardiac risk factors but no other obvious etiology for neck pain.21
Secondary Infection
Since emergency physicians are accustomed to dealing with infection, it is hard to imagine that we could fail to recognize infection as the etiology in a patient with a chief complaint of neck pain. Diagnosis in such cases is complicated by the anatomical location of deep neck-space infections, which limits the usefulness of standard physical examination. These sites are difficult to palpate and often impossible to visualize because they are covered with noninfected tissue. Unless specifically considered in the differential, more obscure causes of neck pain associated with infection may be missed, including retropharyngeal abscess, epiglottitis, Ludwig’s angina, vertebral osteomyelitis and discitis, cervical epidural abscess, and Lemierre’s syndrome.
Epiglottitis
Epiglottitis is inflammation of the epiglottis and adjacent supraglottic structures including the pharynx, uvula, and base of the tongue. The first recorded case is thought to have been that of George Washington, who is believed to have died from this disease.22 The high mortality rate (7% to 20% in the adult population) is a direct result of airway obstruction from inflammatory edema of the epiglottis and adjacent tissues.
Epiglottitis was originally considered a childhood disease; however, the widespread use of Haemophilus influenza vaccination has resulted in a decline in pediatric incidence. Most cases are now seen in adults (mean age of 46 years).23,24
Bacterial infection, especially from the genera Hemophilus, Streptococcus, Staphylococcus, and Klebsiella, is by far the most frequent cause of acute epiglottitis; viral and fungal-associated infections are rare. Thermal injury from swallowing hot foods or liquids, and even from inhaling crack cocaine,25 also has been implicated.
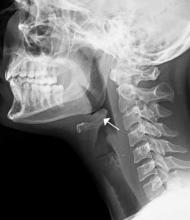
Clinical presentations of epiglottitis differ between children and adults. While children are typically dyspneic, drooling, stridorous and febrile, adults tend to present with a milder form of the disease and have painful swallowing, sore throat, and a muffled voice. In both children and adults, the larynx and upper trachea are tender to light palpation at the anterior neck.26 Although sore throat and odynophagia are more often symptoms of pharyngitis, suspicion should be aroused when pain is severe and/or there is dyspnea, severe pain with an unremarkable oropharynx examination, or anterior neck tenderness. When present, muffled voice and stridor indicate greater potential for airway compromise.27 In cases of significant airway obstruction, patients may assume the “tripod position,” leaning forward with neck extended and mouth open—panting. Since soft-tissue lateral neck radiographs are about 90% sensitive and specific for epiglottitis, a normalappearing film cannot reliably exclude the diagnosis.28 Evaluation for the classic “thumb sign” of epiglottic swelling27 (Figure 3) should be combined with the newly described “vallecula sign” for greatest accuracy.29 The vallecula sign is described as the partial or complete obliteration of a well-defined linear air pocket between the base of the tongue and the epiglottis seen on a closed mouth lateral neck X-ray.
Although CT is a useful modality for detecting epiglottic, peritonsillar, or deep neck space abscess, there are risks to patients with airway compromise; moreover, placing patients in a supine position for the study increases the likelihood of respiratory distress. Despite these risks, when indicated, CT is useful in differentiating these abscesses from similarly presenting entities such as lingual tonsillitis and upper airway foreign body.
Direct visualization via flexible oral or nasolaryngoscopy is the diagnostic gold standard but may be deferred in a stable patient. When absolutely indicated, it must be performed with caution, ideally by an anesthesiologist/otolaryngologist in a controlled setting, lest it precipitate further obstruction. Through the use of fiber optics, the need for emergent intubation can be more directly assessed and, if necessary, performed by “tube-over-scope” technique. In the ED, standby equipment for intubation and cricothyrotomy/needle cricothyrotomy should be immediately available and ready in the event of rapid deterioration, at the same time as intravenous (IV) infusion of third-generation cephalosporin or ampicillin/sulbactam, and methicillin-resistant Staphylococcus aureus (MRSA) coverage. Though the rationale for empirical use of antibiotics is evident, the role of corticosteroids and of nebulized racemic epinephrine is controversial.
Death, airway obstruction, epiglottic abscess, necrotizing epiglottitis, and secondary infections (eg, pneumonia, cervical adenitis, septic arthritis, meningitis) are the potential complications that make this source of neck pain one not to be missed. If epiglottitis is suspected, the patient must be admitted to an intensive care setting.
Retropharyngeal Abscess
The retropharyngeal space, immediately behind the posterior pharynx and esophagus, extends from the base of the skull to the mediastinum. It lies anterior to the deep cervical fascia and is bound laterally by the carotid sheaths.30 Because it is fused down the midline, abscesses in this area tend to be unilateral. The space cannot be directly assessed by physical examination, and infections in this area are rare. Timely diagnosis demands consideration of retropharyngeal abscess in patients presenting with fever, neck stiffness, and sore throat. The potential for serious morbidity and mortality is related to the host of vital structures immediately adjacent to the retropharyngeal space. Complications include mediastinitis, carotid artery erosion, jugular vein thrombosis, pericarditis, epidural abscess, sepsis, and airway compromise.
Most cases are typically observed in children younger than age 6 years. In this pediatric population, the retropharyngeal space has two parallel chains of lymph nodes draining the nose, sinuses, and pharynx; retropharyngeal abscesses usually occur as a suppurative extension from infections of these upper airway structures structures. Penetrating trauma, eg, from objects held in the mouth, is another possible cause. These nodes atrophy around 6 years of age; thereafter, the main cause of retropharyngeal abscess is purulent extension of an adjacent (frequently odontogenic) infection or posterior pharyngeal trauma (eg, from a fish bone or instrumentation).31 As befits its origin with oral flora, cultures are almost always polymicrobial (eg, Streptococci viridans and pyogenes, Staphylococcus, H influenza, Klebsiella, anaerobes).
Although retropharyngeal abscess is considered a disease of childhood, like epiglottitis, its incidence in adults is increasing. 32 Presenting symptoms are signs of respiratory distress, such as wheezing, stridor, and drooling with impending airway obstruction from the expanding posterior pharyngeal mass. Late signs of the illness are respiratory failure due to airway obstruction and septic shock, but an astute clinician should recognize the entity long before these symptoms present. Early symptoms include fever, sore throat, odynophagia, and neck pain and stiffness (typically manifesting as a reluctance to turn the neck).33 Patients may also complain of feeling a lump in the throat or pain in the posterior neck or shoulder with swallowing.34 Ninety-seven percent of pediatric patients present with neck pain,32 which could manifest dramatically as torticollis. Most likely, a child will have a subtle reluctance to move his or her neck during the course of the physical examination. In addition, there may be posterior pharyngeal edema and/or a visible unilateral posterior pharyngeal bulge, cervical adenopathy, and a “croupy” cry or cough resembling a duck’s quack—the “cri du canard.”35 Definitive diagnosis is made using X-ray and/or CT. A lateral soft-tissue neck X-ray will demonstrate widening of the prevertebral soft tissues. CT with contrast provides a more definitive diagnosis, and is also useful to differentiate abscess (ie, a hypodense lesion with ring enhancement) from cellulitis.
Regarding treatment, empiric IV antibiotics must be started immediately and may alone prevent progression if the diagnosis is made before cellulitis has progressed to abscess. Intravenous clindamycin is a reasonable first-line antibiotic; other suggested drugs include a penicillin/ beta lactamase inhibitor, penicillin G plus metronidazole, and cefoxitin.36 Airway protection is mandatory, and an otolaryngologist should be consulted early. Because of the potential for sudden airway deterioration, the emergency physician must be prepared to establish a surgical airway.
Ludwig’s Angina
Ludwig’s angina derives its name from the German physician Wilhelm Friedrich von Ludwig, who first described this deadly, rapidly progressive, fascial space/ connective tissue gangrenous cellulitis of the floor of the mouth and adjoining neck in 1836. In a curious twist of fate, it is believed that Dr Ludwig died from this very disease that bears his name.37
Ninety percent of cases of Ludwig’s angina are odontogenic, often due to periapical abscesses. This condition may result secondary to any oral or parapharyngeal infection that spreads by continuity from the submandibular space into the contiguous sublingual and submental spaces. The potential for airway obstruction comes from elevation and displacement of the tongue, resulting in a mortality rate greater than 50% if untreated. Causative organisms mirror normal, polymicrobial oral flora and include Staphylococcus, Streptococcus, Fusobacterium, and Bacteroides.38,23
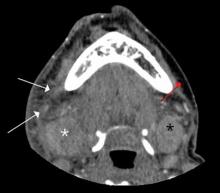
Diagnosis of Ludwig’s angina is primarily clinical. Neck pain and swelling, dental pain, dysphagia, malaise, and fever, along with a protruding or elevated tongue, are typical. Submandibular swelling, which is seen in 95% of patients, develops in advanced cases into an intense “woody” induration above the hyoid bone that portends the impending airway crisis.39 If the patient is sufficiently clinically stable and able to lie flat, definitive diagnosis can be made with a contrastenhanced, soft-tissue neck CT (Figure 4), which can also evaluate for a drainable abscess, soft-tissue gas, and mediastinal extension; this modality can also define the extent of soft-tissue swelling and airway patency.
Airway management is the primary consideration because of its potential for rapid deterioration. Traditional management has been aggressive and surgical, with the standard being early tracheostomy. More recent reports have encouraged more conservative management when possible.40 Impending or actual airway compromise, as manifest by significant trismus, inability to flex the neck without compromising the airway, inability to protrude the tongue, or actual resting dyspnea demand that a surgical airway be readied at bedside until fiber optic nasotracheal intubation is secured.
Antibiotics must be given early and include coverage for gram-positive, gramnegative, and anaerobic organisms. Intravenous metronidazole and penicillin (cefazolin or clindamycin if patient has an allergy to penicillin) are commonly prescribed.38,23 Although controversial, administration of IV dexamethasone (8 mg to 12 mg) and nebulized epinephrine (1:1000, 1 mL diluted to 5 mL with normal saline) to reduce edema has been advocated. 41
Lemierre’s Syndrome Lemierre’s syndrome, septic thrombophlebitis of the internal jugular vein, was first described in 1936 by André Lemierre, who published a series of cases of previously healthy young adults in whom oropharyngeal infections were followed by “anaerobic postanginal septicaemias.”42 Most of these patients presented with sore throat (referred to as “angina” in “old skool” speak) and worsening pain and tenderness at the anterolateral neck, with pulmonary symptoms manifesting several days to 2 weeks later. The causative organism, Fusobacterium necrophorum, is a gram-negative anaerobe that is part of the normal commensal oropharyngeal flora. It invades the internal jugular (IJ) vein via the lateral pharyngeal space and releases a hemagglutinin that promotes thrombus formation in the IJ and, ultimately, metastatic septic emboli. These emboli typically invade the lungs and cause multiple nodular infiltrates and small pleural effusions. Unfortunately, as each case is unique, diagnosis is often delayed. Septic emboli can migrate to other sites and cause arthritis (hip, knee, shoulder, sacroiliac, and other joints), osteomyelitis, young adult with a history of recent sore throat and fever who subsequently developed neck pain and tenderness (with or without swelling) over the IJ, rigors, pulmonary infiltrates, and possibly other signs of septic emboli.
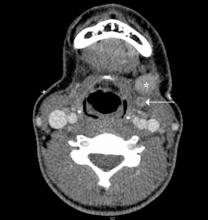
Doppler ultrasound or CT will show IJ thrombosis43 (Figure 5). Purulent discharge, if obtained, has a characteristic foul smell that has been likened to “limburger or overripe Camembert cheese.”44 Treatment is with high-dose IV penicillin and metronidazole or with clindamycin as single coverage. Heparin could potentially aid in dissemination of emboli, but it is used only when there is retrograde propagation of clot to the cavernous sinus.
With the routine antibiotic treatment of pharyngitis in the 1960s and 1970s, cases of Lemierre’s syndrome became so rare that it was referred to as the “forgotten disease.”45 Unfortunately, its incidence has increased over the past few years.43 It is unclear whether this rise is due to increasing antibiotic resistance or to an increasing resistance of clinicians to use antibiotics for “sore throats.”
Cervical Spinal Infections
Vertebral osteomyelitis, discitis, and spinal epidural abscess are rare in developed countries. Most cases stem from hematogenous seeding, skin abscesses, and urinary tract infections but can also originate from a host of other sites, including penetrating trauma and invasive spinal procedures (eg, lumbar punctures, epidural injections). 46,47 Cervical spine infections are associated with immune-compromising situations or conditions (eg, IV drug use, diabetes mellitus, malignancy, acquired immunodeficiency syndrome, renal insufficiency, long-term use of systemic corticosteroids).
All three of these conditions present similarly, often as localized neck pain that grows more intense over a period of days to weeks and worsens with neck movement. Neurological signs ordinarily appear late in the course of the illness. Fever is a classic symptom but is not always present.48 There is usually tenderness over the involved spinous process. The development of motor or sensory loss suggests formation of an abscess,49 which can rapidly lead to further compressive symptoms and sepsis.
Leukocytosis may be absent but erythrocyte sedimentation rate and C-reactive protein are often elevated. A CT scan with contrast is frequently required for diagnosis, though when available, MRI with IV gadolinium is the test of choice (Figure 6). Most cases are caused by S aureus, but antibiotic coverage for gram-positive organisms (including MRSA), gram-negative organisms, and anaerobes should be started as soon as blood cultures are drawn. Neurosurgery should be consulted emergently since, with cervical epidural abscess, neurological deterioration—even to the point of total paralysis—can develop in a matter of hours.50
Conclusion
Although most patients presenting to the ED with neck pain are musculoskeletal and associated with a traumatic event, other infrequent but potentially serious atraumatic causes may be present. Based on a patient’s symptoms, emergency physicians should also consider these conditions in the differential diagnosis to ensure rapid treatment to prevent further complications.
- Ammer C. The American heritage dictionary of idioms. Boston, MA: Houghton Mifflin Company; 1997:489.
- Fusco MR, Harrigan MR. Cerebrovascular dissections—a review part I: spontaneous dissections. Neurosurgery. 2011;68(1):242-257.
- Rubinstein SM, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke.2005;36(7):1575-1580.
- Bergin M, Bird P, Wright A. Internal carotid artery dissection following canalith repositioning procedure. J Laryngol Otol. 2010;124(5):575, 576.
- Brandt T, Grond-Ginsbach C. Spontaneous cervical artery dissection: from risk factors toward pathogenesis. Stroke. 2002;33(3):657,658.
- Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
- Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
- Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
- Engelter ST, Brandt T, Debette S; Cervical Artery Dissection in Ischemic Stroke Patients (CADISP) Study Group. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38(9):2605-2611.
- Arnold M, Bousser M, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
- Hsieh CT, Chang CF, Lin EY, Tsai TH, Chiang YH, Ju DT. Spontaneous spinal epidural hematomas of cervical spine: report of 4 cases and literature review. Am J Emerg Med. 2006;24(6):736-740.
- Sei A, Nakamura T, Hashimoto N, Mizuta H, Sasaki A, Takagi K. Cervical spinal epidural hematoma with spontaneous remission. J Spinal Disord. 1991;4(2):234-237.
- Williams JM, Allegra JR. Spontaneous cervical epidural hematoma. Ann Emerg Med. 1994;23(6):1368-1370.
- Demierre B, Unger PF, Bongioanni F. Sudden cervical pain: spontaneous cervical epidural hematoma. Am J Emerg Med. 1991;9(1):54-56.
- Broder J, L’Italien A. Evaluation and management of the patient with neck pain. In: Mattu A, Goyal DG eds. Emergency Medicine: Avoiding the Pitfalls and Improving the Outcomes. Malden, MA: Blackwell Publishing, Inc; 2007:46-54. http://onlinelibrary. wiley.com/book/10.1002/9780470755938. Accessed November 15, 2013.
- Brodsky AE. Cervical angina. A correlative study with emphasis on the use of coronary arteriography. Spine. 1985;10(8):699-709.
- Hanflig SS. Pain in the shoulder girdle, arm and precordium due to cervical arthritis. JAMA. 1936;106(7):523-526.
- Goldberg R, Goff D, Cooper L, et al. Age and sex differences in presentation of symptoms among patients with acute coronary disease: the REACT Trial. Rapid Early Action for Coronary Treatment. Coron Artery Dis. 2000;11(5):399-407.
- Coventry LL, Finn J, Bremner AP. Sex differences in symptom presentation in acute myocardial infarction: A systematic review and meta-analysis. Heart Lung. 2011;40(6):477-491.
- Lipetz JS, Ledon J, Silber J. Severe coronary artery disease presenting with a chief complaint of cervical pain. Am J Phys Med Rehabil. 2003;82(9):716-720.
- Morens DM. Death of a president. N Engl J Med. 1999;341(24):1845-1849.
- Winters M. Evidence-based diagnosis and management of ENT emergencies. Medscape. 2007. http://www.medscape.com/viewarticle/551650_1. Accessed November 15, 2013.
- Mayo-Smith MF, Spinale JW, Donskey CJ, Yukawa M, Li RH, Schiffman FJ. Acute epiglottitis: An 18-year experience in Rhode Island. Chest. 1995;108(6):1640-1670.
- Mayo-Smith MF, Spinale J. Thermal epiglottitis in adults: a new complication of illicit drug use. J Emerg Med. 1997;15(4):483-485.
- Bansal A, Miskoff J, Lis RJ. Otolaryngologic critical care. Crit Care Clin. 2003;19(1):55-72.
- Katori H, Tsukuda M. Acute epiglottitis: analysis of factors associated with airway intervention. J Laryngol Otol. 2005;119(12):967-972.
- Rothrock SG, Pignatiello GA, Howard RM. Radiologic diagnosis of epiglottitis: objective criteria for all ages. Ann Emerg Med. 1990;19(9):978-982.
- Ducic Y, Hébert PC, MacLachlan L, Neufeld K, Lamothe A. Description and evaluation of the vallecula sign: a new radiologic sign in the diagnosis of adult epiglottitis. Ann Emerg Med. 1997;30(1):1-6.
- Vieira F, Allen SM, Stocks RM, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483.
- Shores CG. Infections and disorders of the neck and upper airway. In: Tintinalli JE, Stapczynski JS, Kelen GD, eds. In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 6th ed. New York, NY: McGraw-Hill; 2004:1494-1499.
- Kahn JH. Retropharyngeal Abscess in Emergency Medicine. Medscape Review. 2008.
- Gibson CG. Do not rely on the presence of respiratory compromise to make the diagnosis of retropharyngeal abscess. In: Mattu A, Chanmugam AS, Swadron SP, Tibbles CD, Woolridge DP, eds. Avoiding Common Errors in the Emergency Department. New York, NY: Lippincott Williams & Wilkins; 2010:212.
- Greene JS, Asher IM. Retropharyngeal abscess: A previously unreported symptom. Ann Emerg Med. 1984;13(8):615-619.
- Melio FR. Upper respiratory tract infections. In: Marx J, Hockberger R, Walls R, eds. Rosen’s Emergency Medicine-Concepts and Clinical Practice 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:921-923.
- Sanford JP, Gilbert DN, Moellering RC, Sande MA, Eliopoulos GM, eds. The Sanford guide to Antimicrobial Therapy 2006-2007. 37th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2007:30.
- Murphy SC. The person behind the eponym: Wilhelm Frederick von Ludwig (1790-1865). J Oral Pathol Med. 1996;25(9):513-515.
- Hasan W, Leonard D, Russell J. Ludwig’s Angina—A controversial surgical emergency: How we do it. Int J Otolaryngol. 2011;2011:231816.
- Saifeldeen K, Evans R. Ludwig’s angina. Emerg Med J. 2004;21(2):242,243.
- Marple BF. Ludwig angina: a review of current airway management. Arch Otolaryngol Head Neck Surg. 1999;125(5):596-599.
- Buckley MF, O’Connor K. Ludwig’s angina in a 76-year-old man. Emerg Med J. 2009;26(9):679-680.
- Lemierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936;227(5874):701-703.
- Karkos PD, Asrani S, Karkos CD, et al. Lemierre’s syndrome: a systematic review. Laryngoscope. 2009;119(8):1552-1559.
- Alston JM. Necrobacillosis in Great Britain. Brit Med J. 1955;2(4955):1524-1528.
- Vargiami EG, Zafeiriou D. Eponym: The Lemierre syndrome. Eur J Pediatr. 2010;169(4):411-414.
- Martínez Hernández PL, Amer López M, Zamora Vargas F, et al. Spontaneous infectious spondylodiscitis in an internal medicine department: epidemiological and clinical study in 41 cases. Rev Clin Esp. 2008;208(7):347-352.
- Urrutia J, Bono CM, Mery P, Rojas C, Gana N, Campos M. Chronic liver failure and concomitant distant infections are associated with high rates of neurological involvement in pyogenic spinal infections. Spine. 2009;34(7):E240-E244.
- Buranapanitkit B, Lim A, Kiriratnikom T. Clinical manifestation of tuberculous and pyogenic spine infection. J Med Assoc Thai. 2001;84(11):1522-1526.
- Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15(2):110-117.
- Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012-2020.
The English expression, “a pain in the neck” is said to have originated in the early 1900s as a euphemism for the less polite phrase, “a pain in the ass.”1 While one might wonder how the expressions of such disparate discomforts came to be idiomatically equivalent, the focus of this article is on etiology of the former. All wryness aside, since most ED presentations of neck pain are musculoskeletal in origin, one may easily fail to consider the myriad of less common, but possibly serious, causes.
Pain can originate from any part of the neck and occur as a result of inflammation (eg, infections and arthritides), vascular pathology (eg, cervical artery dissection [CAD]), spaceoccupying lesions (eg, hematomas, cysts, tumors), or even as referred pain from noncervical sources (eg, heart, diaphragm, lung apex). Any lesion encroaching on the limited space of the neck can quickly compromise the airway, compress nerves, or inhibit blood flow to the brain; therefore, knowledge of the causes of such conditions is critical. This article reviews some of the less common and generally atraumatic etiologies of nontraumatic neck pain of which the emergency physician should be familiar.
Vascular Disorders
Vascular-associated neck pain can originate from vessels within the neck or represent referred pain from a more distant structure. In both cases, however, the potential for morbidity is high and the need for consideration and timely recognition crucial.
Cervical Artery Dissection
The typical initial presenting symptom of CAD—ie, internal CAD (ICAD) or vertebral artery dissection (VAD)—is severe pain in the ipsilateral neck and/or head. Onset of pain may be sudden or gradual.2 CAD occurs in an estimated 2 or 3 of every 100,000 people per year, mostly in patients between ages 20 and 40 years, and it is considered the most common cause of stroke in patients younger than age 45 years.2 The pain associated with CAD generally follows trauma. While the precipitating trauma can be a major blunt or penetrating one, it is often caused by something seemingly trivial, such as “trauma” associated with coughing, painting a ceiling, yoga, or (classically and notoriously) chiropractic manipulation.3 There is frequently some rotational component to CAD-associated trauma,4 though dissection may occur spontaneously.5
The typical triad of symptoms is ipsilateral neck and/or head pain, partial Horner’s syndrome (ptosis and miosis without anhidrosis), and signs of cerebral ischemia. However, patients do not always present with all three of these symptoms, which can complicate the diagnosis. For example, in some patients, neck pain is the sole presenting symptom and can mimic the musculoskeletal pain expected from the mechanical strain that precipitated the dissection.6 In addition, partial Horner’s syndrome occurs in only 50% of cases, and ischemic symptoms might not present for hours to weeks after the onset of neck pain.6
In almost all cases of CAD, initial symptoms are otherwise unexplained pain described as a constant, steady aching. 7 Since cervical arteries are heavily invested with pain fibers,8 an intimal tear with dissecting intramural hematoma provokes pain. Pain associated with VAD is usually severe, unilateral, posterior neck, and/or occipital, while ICAD-associated pain is ipsilateral, anterolateral neck, head, and/or face. It is important to note that head or neck pain caused by a dissection normally precedes the ischemic manifestation as opposed to the more common stroke, in which the ischemia precedes or is simultaneous with the accompanying headache.9
Ischemic neurological symptoms can arise from stenosis of the arterial lumen, secondary to an expanding intramural hematoma; a luminal thrombus developing at the intimal defect; or an embolization accompanied by ipsilateral Horner’s syndrome, any cranial nerve abnormality, or followed by cerebral or ocular ischemic symptoms (even if transient). A diagnosis is usually made through vascular ultrasound (Figure 1) and confirmed with computed tomography angiography (CTA) or magnetic resonance angiography. When requesting a CTA of the neck, the emergency physician should specifically make note of suspected CAD in the order. Immediate treatment includes a cervical collar and neurosurgical consultation even though treatment is essentially medical and surgery is rarely required. Anticoagulation therapy is routinely initiated to prevent thrombus propagation or embolization (unless there is brain hemorrhage). Antiplatelet therapy may be equally efficacious, 10 and can be initiated upon suspicions of CAD and while confirmatory studies are in progress. The prognosis for extracranial dissections is generally good.
Cervical Epidural Hematoma Cervical (spinal) epidural hematoma is an uncommon but potentially catastrophic event that can lead to permanent neurological deficits and death from respiratory failure. It presents as sudden and severe local neck pain with rapid development of radicular pain at the corresponding dermatomes. Motor and sensory deficits follow within minutes to days.12,13 Bleeding can occur spontaneously or secondary to trauma, surgery, or coagulopathy (which itself may be pathological—eg, hemophilia or iatrogenic in origin).14,15 Untreated, progressive cord compression can lead to permanent neurological deficits and death from respiratory failure. In the patient with acute neurological deficits, immediate correction of coagulation issues is required before decompressive surgery.
Diagnosis of cervical epidural hematoma is complicated by the rarity of the event and the lack of specific symptoms. When trauma is involved, cervical disc or nerve root injury is a more likely cause of sudden onset of neck pain, with rapid development of a radicular component. However, when symptoms occur following minor exertion (eg, sneezing, coitus, coughing) and in the presence of risk factors such as hematologic disorders, pregnancy, rheumatologic disorders, or liver dysfunction, epidural hematoma must be considered.16 Emergent magnetic resonance imaging (MRI) is the modality of choice for detecting this condition (Figure 2).
Coronary Ischemia Angina pectoris secondary to coronary ischemia is described as retrosternal “heaviness” or pressure, which may spread to either or both arms, the neck, or jaw. Pathology originating in the neck can be experienced as chest pain and may confound the diagnosis. Because cervical nerve roots C4-C8 contribute to the innervation of the anterior chest wall, irritation of any one of these nerves secondary to neck pathology can mimic true angina.17,18 Conversely, the likelihood that the only pain caused by coronary ischemia might be felt in the neck is low, but possible— especially in women.19,20 Coronary ischemia should be considered in patients with cardiac risk factors but no other obvious etiology for neck pain.21
Secondary Infection
Since emergency physicians are accustomed to dealing with infection, it is hard to imagine that we could fail to recognize infection as the etiology in a patient with a chief complaint of neck pain. Diagnosis in such cases is complicated by the anatomical location of deep neck-space infections, which limits the usefulness of standard physical examination. These sites are difficult to palpate and often impossible to visualize because they are covered with noninfected tissue. Unless specifically considered in the differential, more obscure causes of neck pain associated with infection may be missed, including retropharyngeal abscess, epiglottitis, Ludwig’s angina, vertebral osteomyelitis and discitis, cervical epidural abscess, and Lemierre’s syndrome.
Epiglottitis
Epiglottitis is inflammation of the epiglottis and adjacent supraglottic structures including the pharynx, uvula, and base of the tongue. The first recorded case is thought to have been that of George Washington, who is believed to have died from this disease.22 The high mortality rate (7% to 20% in the adult population) is a direct result of airway obstruction from inflammatory edema of the epiglottis and adjacent tissues.
Epiglottitis was originally considered a childhood disease; however, the widespread use of Haemophilus influenza vaccination has resulted in a decline in pediatric incidence. Most cases are now seen in adults (mean age of 46 years).23,24
Bacterial infection, especially from the genera Hemophilus, Streptococcus, Staphylococcus, and Klebsiella, is by far the most frequent cause of acute epiglottitis; viral and fungal-associated infections are rare. Thermal injury from swallowing hot foods or liquids, and even from inhaling crack cocaine,25 also has been implicated.
Clinical presentations of epiglottitis differ between children and adults. While children are typically dyspneic, drooling, stridorous and febrile, adults tend to present with a milder form of the disease and have painful swallowing, sore throat, and a muffled voice. In both children and adults, the larynx and upper trachea are tender to light palpation at the anterior neck.26 Although sore throat and odynophagia are more often symptoms of pharyngitis, suspicion should be aroused when pain is severe and/or there is dyspnea, severe pain with an unremarkable oropharynx examination, or anterior neck tenderness. When present, muffled voice and stridor indicate greater potential for airway compromise.27 In cases of significant airway obstruction, patients may assume the “tripod position,” leaning forward with neck extended and mouth open—panting. Since soft-tissue lateral neck radiographs are about 90% sensitive and specific for epiglottitis, a normalappearing film cannot reliably exclude the diagnosis.28 Evaluation for the classic “thumb sign” of epiglottic swelling27 (Figure 3) should be combined with the newly described “vallecula sign” for greatest accuracy.29 The vallecula sign is described as the partial or complete obliteration of a well-defined linear air pocket between the base of the tongue and the epiglottis seen on a closed mouth lateral neck X-ray.
Although CT is a useful modality for detecting epiglottic, peritonsillar, or deep neck space abscess, there are risks to patients with airway compromise; moreover, placing patients in a supine position for the study increases the likelihood of respiratory distress. Despite these risks, when indicated, CT is useful in differentiating these abscesses from similarly presenting entities such as lingual tonsillitis and upper airway foreign body.
Direct visualization via flexible oral or nasolaryngoscopy is the diagnostic gold standard but may be deferred in a stable patient. When absolutely indicated, it must be performed with caution, ideally by an anesthesiologist/otolaryngologist in a controlled setting, lest it precipitate further obstruction. Through the use of fiber optics, the need for emergent intubation can be more directly assessed and, if necessary, performed by “tube-over-scope” technique. In the ED, standby equipment for intubation and cricothyrotomy/needle cricothyrotomy should be immediately available and ready in the event of rapid deterioration, at the same time as intravenous (IV) infusion of third-generation cephalosporin or ampicillin/sulbactam, and methicillin-resistant Staphylococcus aureus (MRSA) coverage. Though the rationale for empirical use of antibiotics is evident, the role of corticosteroids and of nebulized racemic epinephrine is controversial.
Death, airway obstruction, epiglottic abscess, necrotizing epiglottitis, and secondary infections (eg, pneumonia, cervical adenitis, septic arthritis, meningitis) are the potential complications that make this source of neck pain one not to be missed. If epiglottitis is suspected, the patient must be admitted to an intensive care setting.
Retropharyngeal Abscess
The retropharyngeal space, immediately behind the posterior pharynx and esophagus, extends from the base of the skull to the mediastinum. It lies anterior to the deep cervical fascia and is bound laterally by the carotid sheaths.30 Because it is fused down the midline, abscesses in this area tend to be unilateral. The space cannot be directly assessed by physical examination, and infections in this area are rare. Timely diagnosis demands consideration of retropharyngeal abscess in patients presenting with fever, neck stiffness, and sore throat. The potential for serious morbidity and mortality is related to the host of vital structures immediately adjacent to the retropharyngeal space. Complications include mediastinitis, carotid artery erosion, jugular vein thrombosis, pericarditis, epidural abscess, sepsis, and airway compromise.
Most cases are typically observed in children younger than age 6 years. In this pediatric population, the retropharyngeal space has two parallel chains of lymph nodes draining the nose, sinuses, and pharynx; retropharyngeal abscesses usually occur as a suppurative extension from infections of these upper airway structures structures. Penetrating trauma, eg, from objects held in the mouth, is another possible cause. These nodes atrophy around 6 years of age; thereafter, the main cause of retropharyngeal abscess is purulent extension of an adjacent (frequently odontogenic) infection or posterior pharyngeal trauma (eg, from a fish bone or instrumentation).31 As befits its origin with oral flora, cultures are almost always polymicrobial (eg, Streptococci viridans and pyogenes, Staphylococcus, H influenza, Klebsiella, anaerobes).
Although retropharyngeal abscess is considered a disease of childhood, like epiglottitis, its incidence in adults is increasing. 32 Presenting symptoms are signs of respiratory distress, such as wheezing, stridor, and drooling with impending airway obstruction from the expanding posterior pharyngeal mass. Late signs of the illness are respiratory failure due to airway obstruction and septic shock, but an astute clinician should recognize the entity long before these symptoms present. Early symptoms include fever, sore throat, odynophagia, and neck pain and stiffness (typically manifesting as a reluctance to turn the neck).33 Patients may also complain of feeling a lump in the throat or pain in the posterior neck or shoulder with swallowing.34 Ninety-seven percent of pediatric patients present with neck pain,32 which could manifest dramatically as torticollis. Most likely, a child will have a subtle reluctance to move his or her neck during the course of the physical examination. In addition, there may be posterior pharyngeal edema and/or a visible unilateral posterior pharyngeal bulge, cervical adenopathy, and a “croupy” cry or cough resembling a duck’s quack—the “cri du canard.”35 Definitive diagnosis is made using X-ray and/or CT. A lateral soft-tissue neck X-ray will demonstrate widening of the prevertebral soft tissues. CT with contrast provides a more definitive diagnosis, and is also useful to differentiate abscess (ie, a hypodense lesion with ring enhancement) from cellulitis.
Regarding treatment, empiric IV antibiotics must be started immediately and may alone prevent progression if the diagnosis is made before cellulitis has progressed to abscess. Intravenous clindamycin is a reasonable first-line antibiotic; other suggested drugs include a penicillin/ beta lactamase inhibitor, penicillin G plus metronidazole, and cefoxitin.36 Airway protection is mandatory, and an otolaryngologist should be consulted early. Because of the potential for sudden airway deterioration, the emergency physician must be prepared to establish a surgical airway.
Ludwig’s Angina
Ludwig’s angina derives its name from the German physician Wilhelm Friedrich von Ludwig, who first described this deadly, rapidly progressive, fascial space/ connective tissue gangrenous cellulitis of the floor of the mouth and adjoining neck in 1836. In a curious twist of fate, it is believed that Dr Ludwig died from this very disease that bears his name.37
Ninety percent of cases of Ludwig’s angina are odontogenic, often due to periapical abscesses. This condition may result secondary to any oral or parapharyngeal infection that spreads by continuity from the submandibular space into the contiguous sublingual and submental spaces. The potential for airway obstruction comes from elevation and displacement of the tongue, resulting in a mortality rate greater than 50% if untreated. Causative organisms mirror normal, polymicrobial oral flora and include Staphylococcus, Streptococcus, Fusobacterium, and Bacteroides.38,23
Diagnosis of Ludwig’s angina is primarily clinical. Neck pain and swelling, dental pain, dysphagia, malaise, and fever, along with a protruding or elevated tongue, are typical. Submandibular swelling, which is seen in 95% of patients, develops in advanced cases into an intense “woody” induration above the hyoid bone that portends the impending airway crisis.39 If the patient is sufficiently clinically stable and able to lie flat, definitive diagnosis can be made with a contrastenhanced, soft-tissue neck CT (Figure 4), which can also evaluate for a drainable abscess, soft-tissue gas, and mediastinal extension; this modality can also define the extent of soft-tissue swelling and airway patency.
Airway management is the primary consideration because of its potential for rapid deterioration. Traditional management has been aggressive and surgical, with the standard being early tracheostomy. More recent reports have encouraged more conservative management when possible.40 Impending or actual airway compromise, as manifest by significant trismus, inability to flex the neck without compromising the airway, inability to protrude the tongue, or actual resting dyspnea demand that a surgical airway be readied at bedside until fiber optic nasotracheal intubation is secured.
Antibiotics must be given early and include coverage for gram-positive, gramnegative, and anaerobic organisms. Intravenous metronidazole and penicillin (cefazolin or clindamycin if patient has an allergy to penicillin) are commonly prescribed.38,23 Although controversial, administration of IV dexamethasone (8 mg to 12 mg) and nebulized epinephrine (1:1000, 1 mL diluted to 5 mL with normal saline) to reduce edema has been advocated. 41
Lemierre’s Syndrome Lemierre’s syndrome, septic thrombophlebitis of the internal jugular vein, was first described in 1936 by André Lemierre, who published a series of cases of previously healthy young adults in whom oropharyngeal infections were followed by “anaerobic postanginal septicaemias.”42 Most of these patients presented with sore throat (referred to as “angina” in “old skool” speak) and worsening pain and tenderness at the anterolateral neck, with pulmonary symptoms manifesting several days to 2 weeks later. The causative organism, Fusobacterium necrophorum, is a gram-negative anaerobe that is part of the normal commensal oropharyngeal flora. It invades the internal jugular (IJ) vein via the lateral pharyngeal space and releases a hemagglutinin that promotes thrombus formation in the IJ and, ultimately, metastatic septic emboli. These emboli typically invade the lungs and cause multiple nodular infiltrates and small pleural effusions. Unfortunately, as each case is unique, diagnosis is often delayed. Septic emboli can migrate to other sites and cause arthritis (hip, knee, shoulder, sacroiliac, and other joints), osteomyelitis, young adult with a history of recent sore throat and fever who subsequently developed neck pain and tenderness (with or without swelling) over the IJ, rigors, pulmonary infiltrates, and possibly other signs of septic emboli.
Doppler ultrasound or CT will show IJ thrombosis43 (Figure 5). Purulent discharge, if obtained, has a characteristic foul smell that has been likened to “limburger or overripe Camembert cheese.”44 Treatment is with high-dose IV penicillin and metronidazole or with clindamycin as single coverage. Heparin could potentially aid in dissemination of emboli, but it is used only when there is retrograde propagation of clot to the cavernous sinus.
With the routine antibiotic treatment of pharyngitis in the 1960s and 1970s, cases of Lemierre’s syndrome became so rare that it was referred to as the “forgotten disease.”45 Unfortunately, its incidence has increased over the past few years.43 It is unclear whether this rise is due to increasing antibiotic resistance or to an increasing resistance of clinicians to use antibiotics for “sore throats.”
Cervical Spinal Infections
Vertebral osteomyelitis, discitis, and spinal epidural abscess are rare in developed countries. Most cases stem from hematogenous seeding, skin abscesses, and urinary tract infections but can also originate from a host of other sites, including penetrating trauma and invasive spinal procedures (eg, lumbar punctures, epidural injections). 46,47 Cervical spine infections are associated with immune-compromising situations or conditions (eg, IV drug use, diabetes mellitus, malignancy, acquired immunodeficiency syndrome, renal insufficiency, long-term use of systemic corticosteroids).
All three of these conditions present similarly, often as localized neck pain that grows more intense over a period of days to weeks and worsens with neck movement. Neurological signs ordinarily appear late in the course of the illness. Fever is a classic symptom but is not always present.48 There is usually tenderness over the involved spinous process. The development of motor or sensory loss suggests formation of an abscess,49 which can rapidly lead to further compressive symptoms and sepsis.
Leukocytosis may be absent but erythrocyte sedimentation rate and C-reactive protein are often elevated. A CT scan with contrast is frequently required for diagnosis, though when available, MRI with IV gadolinium is the test of choice (Figure 6). Most cases are caused by S aureus, but antibiotic coverage for gram-positive organisms (including MRSA), gram-negative organisms, and anaerobes should be started as soon as blood cultures are drawn. Neurosurgery should be consulted emergently since, with cervical epidural abscess, neurological deterioration—even to the point of total paralysis—can develop in a matter of hours.50
Conclusion
Although most patients presenting to the ED with neck pain are musculoskeletal and associated with a traumatic event, other infrequent but potentially serious atraumatic causes may be present. Based on a patient’s symptoms, emergency physicians should also consider these conditions in the differential diagnosis to ensure rapid treatment to prevent further complications.
The English expression, “a pain in the neck” is said to have originated in the early 1900s as a euphemism for the less polite phrase, “a pain in the ass.”1 While one might wonder how the expressions of such disparate discomforts came to be idiomatically equivalent, the focus of this article is on etiology of the former. All wryness aside, since most ED presentations of neck pain are musculoskeletal in origin, one may easily fail to consider the myriad of less common, but possibly serious, causes.
Pain can originate from any part of the neck and occur as a result of inflammation (eg, infections and arthritides), vascular pathology (eg, cervical artery dissection [CAD]), spaceoccupying lesions (eg, hematomas, cysts, tumors), or even as referred pain from noncervical sources (eg, heart, diaphragm, lung apex). Any lesion encroaching on the limited space of the neck can quickly compromise the airway, compress nerves, or inhibit blood flow to the brain; therefore, knowledge of the causes of such conditions is critical. This article reviews some of the less common and generally atraumatic etiologies of nontraumatic neck pain of which the emergency physician should be familiar.
Vascular Disorders
Vascular-associated neck pain can originate from vessels within the neck or represent referred pain from a more distant structure. In both cases, however, the potential for morbidity is high and the need for consideration and timely recognition crucial.
Cervical Artery Dissection
The typical initial presenting symptom of CAD—ie, internal CAD (ICAD) or vertebral artery dissection (VAD)—is severe pain in the ipsilateral neck and/or head. Onset of pain may be sudden or gradual.2 CAD occurs in an estimated 2 or 3 of every 100,000 people per year, mostly in patients between ages 20 and 40 years, and it is considered the most common cause of stroke in patients younger than age 45 years.2 The pain associated with CAD generally follows trauma. While the precipitating trauma can be a major blunt or penetrating one, it is often caused by something seemingly trivial, such as “trauma” associated with coughing, painting a ceiling, yoga, or (classically and notoriously) chiropractic manipulation.3 There is frequently some rotational component to CAD-associated trauma,4 though dissection may occur spontaneously.5
The typical triad of symptoms is ipsilateral neck and/or head pain, partial Horner’s syndrome (ptosis and miosis without anhidrosis), and signs of cerebral ischemia. However, patients do not always present with all three of these symptoms, which can complicate the diagnosis. For example, in some patients, neck pain is the sole presenting symptom and can mimic the musculoskeletal pain expected from the mechanical strain that precipitated the dissection.6 In addition, partial Horner’s syndrome occurs in only 50% of cases, and ischemic symptoms might not present for hours to weeks after the onset of neck pain.6
In almost all cases of CAD, initial symptoms are otherwise unexplained pain described as a constant, steady aching. 7 Since cervical arteries are heavily invested with pain fibers,8 an intimal tear with dissecting intramural hematoma provokes pain. Pain associated with VAD is usually severe, unilateral, posterior neck, and/or occipital, while ICAD-associated pain is ipsilateral, anterolateral neck, head, and/or face. It is important to note that head or neck pain caused by a dissection normally precedes the ischemic manifestation as opposed to the more common stroke, in which the ischemia precedes or is simultaneous with the accompanying headache.9
Ischemic neurological symptoms can arise from stenosis of the arterial lumen, secondary to an expanding intramural hematoma; a luminal thrombus developing at the intimal defect; or an embolization accompanied by ipsilateral Horner’s syndrome, any cranial nerve abnormality, or followed by cerebral or ocular ischemic symptoms (even if transient). A diagnosis is usually made through vascular ultrasound (Figure 1) and confirmed with computed tomography angiography (CTA) or magnetic resonance angiography. When requesting a CTA of the neck, the emergency physician should specifically make note of suspected CAD in the order. Immediate treatment includes a cervical collar and neurosurgical consultation even though treatment is essentially medical and surgery is rarely required. Anticoagulation therapy is routinely initiated to prevent thrombus propagation or embolization (unless there is brain hemorrhage). Antiplatelet therapy may be equally efficacious, 10 and can be initiated upon suspicions of CAD and while confirmatory studies are in progress. The prognosis for extracranial dissections is generally good.
Cervical Epidural Hematoma Cervical (spinal) epidural hematoma is an uncommon but potentially catastrophic event that can lead to permanent neurological deficits and death from respiratory failure. It presents as sudden and severe local neck pain with rapid development of radicular pain at the corresponding dermatomes. Motor and sensory deficits follow within minutes to days.12,13 Bleeding can occur spontaneously or secondary to trauma, surgery, or coagulopathy (which itself may be pathological—eg, hemophilia or iatrogenic in origin).14,15 Untreated, progressive cord compression can lead to permanent neurological deficits and death from respiratory failure. In the patient with acute neurological deficits, immediate correction of coagulation issues is required before decompressive surgery.
Diagnosis of cervical epidural hematoma is complicated by the rarity of the event and the lack of specific symptoms. When trauma is involved, cervical disc or nerve root injury is a more likely cause of sudden onset of neck pain, with rapid development of a radicular component. However, when symptoms occur following minor exertion (eg, sneezing, coitus, coughing) and in the presence of risk factors such as hematologic disorders, pregnancy, rheumatologic disorders, or liver dysfunction, epidural hematoma must be considered.16 Emergent magnetic resonance imaging (MRI) is the modality of choice for detecting this condition (Figure 2).
Coronary Ischemia Angina pectoris secondary to coronary ischemia is described as retrosternal “heaviness” or pressure, which may spread to either or both arms, the neck, or jaw. Pathology originating in the neck can be experienced as chest pain and may confound the diagnosis. Because cervical nerve roots C4-C8 contribute to the innervation of the anterior chest wall, irritation of any one of these nerves secondary to neck pathology can mimic true angina.17,18 Conversely, the likelihood that the only pain caused by coronary ischemia might be felt in the neck is low, but possible— especially in women.19,20 Coronary ischemia should be considered in patients with cardiac risk factors but no other obvious etiology for neck pain.21
Secondary Infection
Since emergency physicians are accustomed to dealing with infection, it is hard to imagine that we could fail to recognize infection as the etiology in a patient with a chief complaint of neck pain. Diagnosis in such cases is complicated by the anatomical location of deep neck-space infections, which limits the usefulness of standard physical examination. These sites are difficult to palpate and often impossible to visualize because they are covered with noninfected tissue. Unless specifically considered in the differential, more obscure causes of neck pain associated with infection may be missed, including retropharyngeal abscess, epiglottitis, Ludwig’s angina, vertebral osteomyelitis and discitis, cervical epidural abscess, and Lemierre’s syndrome.
Epiglottitis
Epiglottitis is inflammation of the epiglottis and adjacent supraglottic structures including the pharynx, uvula, and base of the tongue. The first recorded case is thought to have been that of George Washington, who is believed to have died from this disease.22 The high mortality rate (7% to 20% in the adult population) is a direct result of airway obstruction from inflammatory edema of the epiglottis and adjacent tissues.
Epiglottitis was originally considered a childhood disease; however, the widespread use of Haemophilus influenza vaccination has resulted in a decline in pediatric incidence. Most cases are now seen in adults (mean age of 46 years).23,24
Bacterial infection, especially from the genera Hemophilus, Streptococcus, Staphylococcus, and Klebsiella, is by far the most frequent cause of acute epiglottitis; viral and fungal-associated infections are rare. Thermal injury from swallowing hot foods or liquids, and even from inhaling crack cocaine,25 also has been implicated.
Clinical presentations of epiglottitis differ between children and adults. While children are typically dyspneic, drooling, stridorous and febrile, adults tend to present with a milder form of the disease and have painful swallowing, sore throat, and a muffled voice. In both children and adults, the larynx and upper trachea are tender to light palpation at the anterior neck.26 Although sore throat and odynophagia are more often symptoms of pharyngitis, suspicion should be aroused when pain is severe and/or there is dyspnea, severe pain with an unremarkable oropharynx examination, or anterior neck tenderness. When present, muffled voice and stridor indicate greater potential for airway compromise.27 In cases of significant airway obstruction, patients may assume the “tripod position,” leaning forward with neck extended and mouth open—panting. Since soft-tissue lateral neck radiographs are about 90% sensitive and specific for epiglottitis, a normalappearing film cannot reliably exclude the diagnosis.28 Evaluation for the classic “thumb sign” of epiglottic swelling27 (Figure 3) should be combined with the newly described “vallecula sign” for greatest accuracy.29 The vallecula sign is described as the partial or complete obliteration of a well-defined linear air pocket between the base of the tongue and the epiglottis seen on a closed mouth lateral neck X-ray.
Although CT is a useful modality for detecting epiglottic, peritonsillar, or deep neck space abscess, there are risks to patients with airway compromise; moreover, placing patients in a supine position for the study increases the likelihood of respiratory distress. Despite these risks, when indicated, CT is useful in differentiating these abscesses from similarly presenting entities such as lingual tonsillitis and upper airway foreign body.
Direct visualization via flexible oral or nasolaryngoscopy is the diagnostic gold standard but may be deferred in a stable patient. When absolutely indicated, it must be performed with caution, ideally by an anesthesiologist/otolaryngologist in a controlled setting, lest it precipitate further obstruction. Through the use of fiber optics, the need for emergent intubation can be more directly assessed and, if necessary, performed by “tube-over-scope” technique. In the ED, standby equipment for intubation and cricothyrotomy/needle cricothyrotomy should be immediately available and ready in the event of rapid deterioration, at the same time as intravenous (IV) infusion of third-generation cephalosporin or ampicillin/sulbactam, and methicillin-resistant Staphylococcus aureus (MRSA) coverage. Though the rationale for empirical use of antibiotics is evident, the role of corticosteroids and of nebulized racemic epinephrine is controversial.
Death, airway obstruction, epiglottic abscess, necrotizing epiglottitis, and secondary infections (eg, pneumonia, cervical adenitis, septic arthritis, meningitis) are the potential complications that make this source of neck pain one not to be missed. If epiglottitis is suspected, the patient must be admitted to an intensive care setting.
Retropharyngeal Abscess
The retropharyngeal space, immediately behind the posterior pharynx and esophagus, extends from the base of the skull to the mediastinum. It lies anterior to the deep cervical fascia and is bound laterally by the carotid sheaths.30 Because it is fused down the midline, abscesses in this area tend to be unilateral. The space cannot be directly assessed by physical examination, and infections in this area are rare. Timely diagnosis demands consideration of retropharyngeal abscess in patients presenting with fever, neck stiffness, and sore throat. The potential for serious morbidity and mortality is related to the host of vital structures immediately adjacent to the retropharyngeal space. Complications include mediastinitis, carotid artery erosion, jugular vein thrombosis, pericarditis, epidural abscess, sepsis, and airway compromise.
Most cases are typically observed in children younger than age 6 years. In this pediatric population, the retropharyngeal space has two parallel chains of lymph nodes draining the nose, sinuses, and pharynx; retropharyngeal abscesses usually occur as a suppurative extension from infections of these upper airway structures structures. Penetrating trauma, eg, from objects held in the mouth, is another possible cause. These nodes atrophy around 6 years of age; thereafter, the main cause of retropharyngeal abscess is purulent extension of an adjacent (frequently odontogenic) infection or posterior pharyngeal trauma (eg, from a fish bone or instrumentation).31 As befits its origin with oral flora, cultures are almost always polymicrobial (eg, Streptococci viridans and pyogenes, Staphylococcus, H influenza, Klebsiella, anaerobes).
Although retropharyngeal abscess is considered a disease of childhood, like epiglottitis, its incidence in adults is increasing. 32 Presenting symptoms are signs of respiratory distress, such as wheezing, stridor, and drooling with impending airway obstruction from the expanding posterior pharyngeal mass. Late signs of the illness are respiratory failure due to airway obstruction and septic shock, but an astute clinician should recognize the entity long before these symptoms present. Early symptoms include fever, sore throat, odynophagia, and neck pain and stiffness (typically manifesting as a reluctance to turn the neck).33 Patients may also complain of feeling a lump in the throat or pain in the posterior neck or shoulder with swallowing.34 Ninety-seven percent of pediatric patients present with neck pain,32 which could manifest dramatically as torticollis. Most likely, a child will have a subtle reluctance to move his or her neck during the course of the physical examination. In addition, there may be posterior pharyngeal edema and/or a visible unilateral posterior pharyngeal bulge, cervical adenopathy, and a “croupy” cry or cough resembling a duck’s quack—the “cri du canard.”35 Definitive diagnosis is made using X-ray and/or CT. A lateral soft-tissue neck X-ray will demonstrate widening of the prevertebral soft tissues. CT with contrast provides a more definitive diagnosis, and is also useful to differentiate abscess (ie, a hypodense lesion with ring enhancement) from cellulitis.
Regarding treatment, empiric IV antibiotics must be started immediately and may alone prevent progression if the diagnosis is made before cellulitis has progressed to abscess. Intravenous clindamycin is a reasonable first-line antibiotic; other suggested drugs include a penicillin/ beta lactamase inhibitor, penicillin G plus metronidazole, and cefoxitin.36 Airway protection is mandatory, and an otolaryngologist should be consulted early. Because of the potential for sudden airway deterioration, the emergency physician must be prepared to establish a surgical airway.
Ludwig’s Angina
Ludwig’s angina derives its name from the German physician Wilhelm Friedrich von Ludwig, who first described this deadly, rapidly progressive, fascial space/ connective tissue gangrenous cellulitis of the floor of the mouth and adjoining neck in 1836. In a curious twist of fate, it is believed that Dr Ludwig died from this very disease that bears his name.37
Ninety percent of cases of Ludwig’s angina are odontogenic, often due to periapical abscesses. This condition may result secondary to any oral or parapharyngeal infection that spreads by continuity from the submandibular space into the contiguous sublingual and submental spaces. The potential for airway obstruction comes from elevation and displacement of the tongue, resulting in a mortality rate greater than 50% if untreated. Causative organisms mirror normal, polymicrobial oral flora and include Staphylococcus, Streptococcus, Fusobacterium, and Bacteroides.38,23
Diagnosis of Ludwig’s angina is primarily clinical. Neck pain and swelling, dental pain, dysphagia, malaise, and fever, along with a protruding or elevated tongue, are typical. Submandibular swelling, which is seen in 95% of patients, develops in advanced cases into an intense “woody” induration above the hyoid bone that portends the impending airway crisis.39 If the patient is sufficiently clinically stable and able to lie flat, definitive diagnosis can be made with a contrastenhanced, soft-tissue neck CT (Figure 4), which can also evaluate for a drainable abscess, soft-tissue gas, and mediastinal extension; this modality can also define the extent of soft-tissue swelling and airway patency.
Airway management is the primary consideration because of its potential for rapid deterioration. Traditional management has been aggressive and surgical, with the standard being early tracheostomy. More recent reports have encouraged more conservative management when possible.40 Impending or actual airway compromise, as manifest by significant trismus, inability to flex the neck without compromising the airway, inability to protrude the tongue, or actual resting dyspnea demand that a surgical airway be readied at bedside until fiber optic nasotracheal intubation is secured.
Antibiotics must be given early and include coverage for gram-positive, gramnegative, and anaerobic organisms. Intravenous metronidazole and penicillin (cefazolin or clindamycin if patient has an allergy to penicillin) are commonly prescribed.38,23 Although controversial, administration of IV dexamethasone (8 mg to 12 mg) and nebulized epinephrine (1:1000, 1 mL diluted to 5 mL with normal saline) to reduce edema has been advocated. 41
Lemierre’s Syndrome Lemierre’s syndrome, septic thrombophlebitis of the internal jugular vein, was first described in 1936 by André Lemierre, who published a series of cases of previously healthy young adults in whom oropharyngeal infections were followed by “anaerobic postanginal septicaemias.”42 Most of these patients presented with sore throat (referred to as “angina” in “old skool” speak) and worsening pain and tenderness at the anterolateral neck, with pulmonary symptoms manifesting several days to 2 weeks later. The causative organism, Fusobacterium necrophorum, is a gram-negative anaerobe that is part of the normal commensal oropharyngeal flora. It invades the internal jugular (IJ) vein via the lateral pharyngeal space and releases a hemagglutinin that promotes thrombus formation in the IJ and, ultimately, metastatic septic emboli. These emboli typically invade the lungs and cause multiple nodular infiltrates and small pleural effusions. Unfortunately, as each case is unique, diagnosis is often delayed. Septic emboli can migrate to other sites and cause arthritis (hip, knee, shoulder, sacroiliac, and other joints), osteomyelitis, young adult with a history of recent sore throat and fever who subsequently developed neck pain and tenderness (with or without swelling) over the IJ, rigors, pulmonary infiltrates, and possibly other signs of septic emboli.
Doppler ultrasound or CT will show IJ thrombosis43 (Figure 5). Purulent discharge, if obtained, has a characteristic foul smell that has been likened to “limburger or overripe Camembert cheese.”44 Treatment is with high-dose IV penicillin and metronidazole or with clindamycin as single coverage. Heparin could potentially aid in dissemination of emboli, but it is used only when there is retrograde propagation of clot to the cavernous sinus.
With the routine antibiotic treatment of pharyngitis in the 1960s and 1970s, cases of Lemierre’s syndrome became so rare that it was referred to as the “forgotten disease.”45 Unfortunately, its incidence has increased over the past few years.43 It is unclear whether this rise is due to increasing antibiotic resistance or to an increasing resistance of clinicians to use antibiotics for “sore throats.”
Cervical Spinal Infections
Vertebral osteomyelitis, discitis, and spinal epidural abscess are rare in developed countries. Most cases stem from hematogenous seeding, skin abscesses, and urinary tract infections but can also originate from a host of other sites, including penetrating trauma and invasive spinal procedures (eg, lumbar punctures, epidural injections). 46,47 Cervical spine infections are associated with immune-compromising situations or conditions (eg, IV drug use, diabetes mellitus, malignancy, acquired immunodeficiency syndrome, renal insufficiency, long-term use of systemic corticosteroids).
All three of these conditions present similarly, often as localized neck pain that grows more intense over a period of days to weeks and worsens with neck movement. Neurological signs ordinarily appear late in the course of the illness. Fever is a classic symptom but is not always present.48 There is usually tenderness over the involved spinous process. The development of motor or sensory loss suggests formation of an abscess,49 which can rapidly lead to further compressive symptoms and sepsis.
Leukocytosis may be absent but erythrocyte sedimentation rate and C-reactive protein are often elevated. A CT scan with contrast is frequently required for diagnosis, though when available, MRI with IV gadolinium is the test of choice (Figure 6). Most cases are caused by S aureus, but antibiotic coverage for gram-positive organisms (including MRSA), gram-negative organisms, and anaerobes should be started as soon as blood cultures are drawn. Neurosurgery should be consulted emergently since, with cervical epidural abscess, neurological deterioration—even to the point of total paralysis—can develop in a matter of hours.50
Conclusion
Although most patients presenting to the ED with neck pain are musculoskeletal and associated with a traumatic event, other infrequent but potentially serious atraumatic causes may be present. Based on a patient’s symptoms, emergency physicians should also consider these conditions in the differential diagnosis to ensure rapid treatment to prevent further complications.
- Ammer C. The American heritage dictionary of idioms. Boston, MA: Houghton Mifflin Company; 1997:489.
- Fusco MR, Harrigan MR. Cerebrovascular dissections—a review part I: spontaneous dissections. Neurosurgery. 2011;68(1):242-257.
- Rubinstein SM, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke.2005;36(7):1575-1580.
- Bergin M, Bird P, Wright A. Internal carotid artery dissection following canalith repositioning procedure. J Laryngol Otol. 2010;124(5):575, 576.
- Brandt T, Grond-Ginsbach C. Spontaneous cervical artery dissection: from risk factors toward pathogenesis. Stroke. 2002;33(3):657,658.
- Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
- Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
- Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
- Engelter ST, Brandt T, Debette S; Cervical Artery Dissection in Ischemic Stroke Patients (CADISP) Study Group. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38(9):2605-2611.
- Arnold M, Bousser M, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
- Hsieh CT, Chang CF, Lin EY, Tsai TH, Chiang YH, Ju DT. Spontaneous spinal epidural hematomas of cervical spine: report of 4 cases and literature review. Am J Emerg Med. 2006;24(6):736-740.
- Sei A, Nakamura T, Hashimoto N, Mizuta H, Sasaki A, Takagi K. Cervical spinal epidural hematoma with spontaneous remission. J Spinal Disord. 1991;4(2):234-237.
- Williams JM, Allegra JR. Spontaneous cervical epidural hematoma. Ann Emerg Med. 1994;23(6):1368-1370.
- Demierre B, Unger PF, Bongioanni F. Sudden cervical pain: spontaneous cervical epidural hematoma. Am J Emerg Med. 1991;9(1):54-56.
- Broder J, L’Italien A. Evaluation and management of the patient with neck pain. In: Mattu A, Goyal DG eds. Emergency Medicine: Avoiding the Pitfalls and Improving the Outcomes. Malden, MA: Blackwell Publishing, Inc; 2007:46-54. http://onlinelibrary. wiley.com/book/10.1002/9780470755938. Accessed November 15, 2013.
- Brodsky AE. Cervical angina. A correlative study with emphasis on the use of coronary arteriography. Spine. 1985;10(8):699-709.
- Hanflig SS. Pain in the shoulder girdle, arm and precordium due to cervical arthritis. JAMA. 1936;106(7):523-526.
- Goldberg R, Goff D, Cooper L, et al. Age and sex differences in presentation of symptoms among patients with acute coronary disease: the REACT Trial. Rapid Early Action for Coronary Treatment. Coron Artery Dis. 2000;11(5):399-407.
- Coventry LL, Finn J, Bremner AP. Sex differences in symptom presentation in acute myocardial infarction: A systematic review and meta-analysis. Heart Lung. 2011;40(6):477-491.
- Lipetz JS, Ledon J, Silber J. Severe coronary artery disease presenting with a chief complaint of cervical pain. Am J Phys Med Rehabil. 2003;82(9):716-720.
- Morens DM. Death of a president. N Engl J Med. 1999;341(24):1845-1849.
- Winters M. Evidence-based diagnosis and management of ENT emergencies. Medscape. 2007. http://www.medscape.com/viewarticle/551650_1. Accessed November 15, 2013.
- Mayo-Smith MF, Spinale JW, Donskey CJ, Yukawa M, Li RH, Schiffman FJ. Acute epiglottitis: An 18-year experience in Rhode Island. Chest. 1995;108(6):1640-1670.
- Mayo-Smith MF, Spinale J. Thermal epiglottitis in adults: a new complication of illicit drug use. J Emerg Med. 1997;15(4):483-485.
- Bansal A, Miskoff J, Lis RJ. Otolaryngologic critical care. Crit Care Clin. 2003;19(1):55-72.
- Katori H, Tsukuda M. Acute epiglottitis: analysis of factors associated with airway intervention. J Laryngol Otol. 2005;119(12):967-972.
- Rothrock SG, Pignatiello GA, Howard RM. Radiologic diagnosis of epiglottitis: objective criteria for all ages. Ann Emerg Med. 1990;19(9):978-982.
- Ducic Y, Hébert PC, MacLachlan L, Neufeld K, Lamothe A. Description and evaluation of the vallecula sign: a new radiologic sign in the diagnosis of adult epiglottitis. Ann Emerg Med. 1997;30(1):1-6.
- Vieira F, Allen SM, Stocks RM, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483.
- Shores CG. Infections and disorders of the neck and upper airway. In: Tintinalli JE, Stapczynski JS, Kelen GD, eds. In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 6th ed. New York, NY: McGraw-Hill; 2004:1494-1499.
- Kahn JH. Retropharyngeal Abscess in Emergency Medicine. Medscape Review. 2008.
- Gibson CG. Do not rely on the presence of respiratory compromise to make the diagnosis of retropharyngeal abscess. In: Mattu A, Chanmugam AS, Swadron SP, Tibbles CD, Woolridge DP, eds. Avoiding Common Errors in the Emergency Department. New York, NY: Lippincott Williams & Wilkins; 2010:212.
- Greene JS, Asher IM. Retropharyngeal abscess: A previously unreported symptom. Ann Emerg Med. 1984;13(8):615-619.
- Melio FR. Upper respiratory tract infections. In: Marx J, Hockberger R, Walls R, eds. Rosen’s Emergency Medicine-Concepts and Clinical Practice 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:921-923.
- Sanford JP, Gilbert DN, Moellering RC, Sande MA, Eliopoulos GM, eds. The Sanford guide to Antimicrobial Therapy 2006-2007. 37th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2007:30.
- Murphy SC. The person behind the eponym: Wilhelm Frederick von Ludwig (1790-1865). J Oral Pathol Med. 1996;25(9):513-515.
- Hasan W, Leonard D, Russell J. Ludwig’s Angina—A controversial surgical emergency: How we do it. Int J Otolaryngol. 2011;2011:231816.
- Saifeldeen K, Evans R. Ludwig’s angina. Emerg Med J. 2004;21(2):242,243.
- Marple BF. Ludwig angina: a review of current airway management. Arch Otolaryngol Head Neck Surg. 1999;125(5):596-599.
- Buckley MF, O’Connor K. Ludwig’s angina in a 76-year-old man. Emerg Med J. 2009;26(9):679-680.
- Lemierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936;227(5874):701-703.
- Karkos PD, Asrani S, Karkos CD, et al. Lemierre’s syndrome: a systematic review. Laryngoscope. 2009;119(8):1552-1559.
- Alston JM. Necrobacillosis in Great Britain. Brit Med J. 1955;2(4955):1524-1528.
- Vargiami EG, Zafeiriou D. Eponym: The Lemierre syndrome. Eur J Pediatr. 2010;169(4):411-414.
- Martínez Hernández PL, Amer López M, Zamora Vargas F, et al. Spontaneous infectious spondylodiscitis in an internal medicine department: epidemiological and clinical study in 41 cases. Rev Clin Esp. 2008;208(7):347-352.
- Urrutia J, Bono CM, Mery P, Rojas C, Gana N, Campos M. Chronic liver failure and concomitant distant infections are associated with high rates of neurological involvement in pyogenic spinal infections. Spine. 2009;34(7):E240-E244.
- Buranapanitkit B, Lim A, Kiriratnikom T. Clinical manifestation of tuberculous and pyogenic spine infection. J Med Assoc Thai. 2001;84(11):1522-1526.
- Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15(2):110-117.
- Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012-2020.
- Ammer C. The American heritage dictionary of idioms. Boston, MA: Houghton Mifflin Company; 1997:489.
- Fusco MR, Harrigan MR. Cerebrovascular dissections—a review part I: spontaneous dissections. Neurosurgery. 2011;68(1):242-257.
- Rubinstein SM, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke.2005;36(7):1575-1580.
- Bergin M, Bird P, Wright A. Internal carotid artery dissection following canalith repositioning procedure. J Laryngol Otol. 2010;124(5):575, 576.
- Brandt T, Grond-Ginsbach C. Spontaneous cervical artery dissection: from risk factors toward pathogenesis. Stroke. 2002;33(3):657,658.
- Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
- Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
- Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
- Engelter ST, Brandt T, Debette S; Cervical Artery Dissection in Ischemic Stroke Patients (CADISP) Study Group. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38(9):2605-2611.
- Arnold M, Bousser M, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
- Hsieh CT, Chang CF, Lin EY, Tsai TH, Chiang YH, Ju DT. Spontaneous spinal epidural hematomas of cervical spine: report of 4 cases and literature review. Am J Emerg Med. 2006;24(6):736-740.
- Sei A, Nakamura T, Hashimoto N, Mizuta H, Sasaki A, Takagi K. Cervical spinal epidural hematoma with spontaneous remission. J Spinal Disord. 1991;4(2):234-237.
- Williams JM, Allegra JR. Spontaneous cervical epidural hematoma. Ann Emerg Med. 1994;23(6):1368-1370.
- Demierre B, Unger PF, Bongioanni F. Sudden cervical pain: spontaneous cervical epidural hematoma. Am J Emerg Med. 1991;9(1):54-56.
- Broder J, L’Italien A. Evaluation and management of the patient with neck pain. In: Mattu A, Goyal DG eds. Emergency Medicine: Avoiding the Pitfalls and Improving the Outcomes. Malden, MA: Blackwell Publishing, Inc; 2007:46-54. http://onlinelibrary. wiley.com/book/10.1002/9780470755938. Accessed November 15, 2013.
- Brodsky AE. Cervical angina. A correlative study with emphasis on the use of coronary arteriography. Spine. 1985;10(8):699-709.
- Hanflig SS. Pain in the shoulder girdle, arm and precordium due to cervical arthritis. JAMA. 1936;106(7):523-526.
- Goldberg R, Goff D, Cooper L, et al. Age and sex differences in presentation of symptoms among patients with acute coronary disease: the REACT Trial. Rapid Early Action for Coronary Treatment. Coron Artery Dis. 2000;11(5):399-407.
- Coventry LL, Finn J, Bremner AP. Sex differences in symptom presentation in acute myocardial infarction: A systematic review and meta-analysis. Heart Lung. 2011;40(6):477-491.
- Lipetz JS, Ledon J, Silber J. Severe coronary artery disease presenting with a chief complaint of cervical pain. Am J Phys Med Rehabil. 2003;82(9):716-720.
- Morens DM. Death of a president. N Engl J Med. 1999;341(24):1845-1849.
- Winters M. Evidence-based diagnosis and management of ENT emergencies. Medscape. 2007. http://www.medscape.com/viewarticle/551650_1. Accessed November 15, 2013.
- Mayo-Smith MF, Spinale JW, Donskey CJ, Yukawa M, Li RH, Schiffman FJ. Acute epiglottitis: An 18-year experience in Rhode Island. Chest. 1995;108(6):1640-1670.
- Mayo-Smith MF, Spinale J. Thermal epiglottitis in adults: a new complication of illicit drug use. J Emerg Med. 1997;15(4):483-485.
- Bansal A, Miskoff J, Lis RJ. Otolaryngologic critical care. Crit Care Clin. 2003;19(1):55-72.
- Katori H, Tsukuda M. Acute epiglottitis: analysis of factors associated with airway intervention. J Laryngol Otol. 2005;119(12):967-972.
- Rothrock SG, Pignatiello GA, Howard RM. Radiologic diagnosis of epiglottitis: objective criteria for all ages. Ann Emerg Med. 1990;19(9):978-982.
- Ducic Y, Hébert PC, MacLachlan L, Neufeld K, Lamothe A. Description and evaluation of the vallecula sign: a new radiologic sign in the diagnosis of adult epiglottitis. Ann Emerg Med. 1997;30(1):1-6.
- Vieira F, Allen SM, Stocks RM, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483.
- Shores CG. Infections and disorders of the neck and upper airway. In: Tintinalli JE, Stapczynski JS, Kelen GD, eds. In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 6th ed. New York, NY: McGraw-Hill; 2004:1494-1499.
- Kahn JH. Retropharyngeal Abscess in Emergency Medicine. Medscape Review. 2008.
- Gibson CG. Do not rely on the presence of respiratory compromise to make the diagnosis of retropharyngeal abscess. In: Mattu A, Chanmugam AS, Swadron SP, Tibbles CD, Woolridge DP, eds. Avoiding Common Errors in the Emergency Department. New York, NY: Lippincott Williams & Wilkins; 2010:212.
- Greene JS, Asher IM. Retropharyngeal abscess: A previously unreported symptom. Ann Emerg Med. 1984;13(8):615-619.
- Melio FR. Upper respiratory tract infections. In: Marx J, Hockberger R, Walls R, eds. Rosen’s Emergency Medicine-Concepts and Clinical Practice 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:921-923.
- Sanford JP, Gilbert DN, Moellering RC, Sande MA, Eliopoulos GM, eds. The Sanford guide to Antimicrobial Therapy 2006-2007. 37th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2007:30.
- Murphy SC. The person behind the eponym: Wilhelm Frederick von Ludwig (1790-1865). J Oral Pathol Med. 1996;25(9):513-515.
- Hasan W, Leonard D, Russell J. Ludwig’s Angina—A controversial surgical emergency: How we do it. Int J Otolaryngol. 2011;2011:231816.
- Saifeldeen K, Evans R. Ludwig’s angina. Emerg Med J. 2004;21(2):242,243.
- Marple BF. Ludwig angina: a review of current airway management. Arch Otolaryngol Head Neck Surg. 1999;125(5):596-599.
- Buckley MF, O’Connor K. Ludwig’s angina in a 76-year-old man. Emerg Med J. 2009;26(9):679-680.
- Lemierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936;227(5874):701-703.
- Karkos PD, Asrani S, Karkos CD, et al. Lemierre’s syndrome: a systematic review. Laryngoscope. 2009;119(8):1552-1559.
- Alston JM. Necrobacillosis in Great Britain. Brit Med J. 1955;2(4955):1524-1528.
- Vargiami EG, Zafeiriou D. Eponym: The Lemierre syndrome. Eur J Pediatr. 2010;169(4):411-414.
- Martínez Hernández PL, Amer López M, Zamora Vargas F, et al. Spontaneous infectious spondylodiscitis in an internal medicine department: epidemiological and clinical study in 41 cases. Rev Clin Esp. 2008;208(7):347-352.
- Urrutia J, Bono CM, Mery P, Rojas C, Gana N, Campos M. Chronic liver failure and concomitant distant infections are associated with high rates of neurological involvement in pyogenic spinal infections. Spine. 2009;34(7):E240-E244.
- Buranapanitkit B, Lim A, Kiriratnikom T. Clinical manifestation of tuberculous and pyogenic spine infection. J Med Assoc Thai. 2001;84(11):1522-1526.
- Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15(2):110-117.
- Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012-2020.
Venous Thromboembolism
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 develop a VTE in the Western countries. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of patients diagnosed with thrombotic events will die within the first month after diagnosis. PE is a common consequence of DVT; 40% of patients who are diagnosed with a DVT will be subsequently found to have a PE upon further imaging. The high rate of association is also seen in those who present with a PE, 70% of whom will also be found to have a concomitant DVT.
To read the full article in PDF:
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 develop a VTE in the Western countries. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of patients diagnosed with thrombotic events will die within the first month after diagnosis. PE is a common consequence of DVT; 40% of patients who are diagnosed with a DVT will be subsequently found to have a PE upon further imaging. The high rate of association is also seen in those who present with a PE, 70% of whom will also be found to have a concomitant DVT.
To read the full article in PDF:
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 develop a VTE in the Western countries. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of patients diagnosed with thrombotic events will die within the first month after diagnosis. PE is a common consequence of DVT; 40% of patients who are diagnosed with a DVT will be subsequently found to have a PE upon further imaging. The high rate of association is also seen in those who present with a PE, 70% of whom will also be found to have a concomitant DVT.
To read the full article in PDF:
Update on Osteoporosis
Because the low bone mass and deterioration of bone microarchitecture and quality that characterize osteoporosis can lead to fragility fracture, it is vital that we intervene in our patients’ health in a timely manner to reduce this risk. One way to accomplish this goal is to understand the role of age in determining a woman’s fracture risk. For example, an 80-year-old woman and a 50-year-old woman with a T-score of –2.5, as measured by dual x-ray absorptiometry (DXA), will have dramatically different fracture risks. According to the World Health Organization’s fracture-risk assessment tool (http://www.shef.ac.uk/FRAX/), the older woman has a 10-year probability of hip fracture approximately five times greater than the younger woman.
Although no new therapies have been approved during the past year, several important findings were published that affect clinical management of menopausal patients or suggest changes likely in the future.
In this article, I review:
- the latest guidance on osteoporosis from the American College of Obstetricians and Gynecologists (ACOG)
- the most recent indications for bone mineral density (BMD) testing from the International Society for Clinical Densitometry (ISCD)
- a study exploring the effect of oral hormonal contraception on the acquisition of peak BMD in adolescents and young women
- results of a randomized trial of the experimental agent odanacatib in postmenopausal women
- a pilot study of teriparatide (Forteo) for idiopathic osteoporosis in premenopausal women.
ACOG ISSUES RECOMMENDATIONS ON SCREENING, TREATMENT, AND LIFESTYLE
Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin #129: Osteoporosis. Obstet Gynecol. 2012;120(3):718–734.
This comprehensive review of management guidelines for ObGyns deserves “top billing” in this update. It offers recommendations on important interventions, from BMD measurement and subsequent monitoring to calcium and vitamin D supplementation.
When to initiate screening
- Begin BMD screening using DXA at age 65. DXA also may be appropriate for younger women if they are postmenopausal and have other significant risk factors for osteoporosis or fracture (Level A evidence – based on good and consistent scientific evidence).
- In the absence of new risk factors, do not perform DXA screening more frequently than every 2 years (Level B evidence – based on limited or inconsistent scientific evidence).
Which patients should be treated?
Treatment is recommended for:
- women with a T-score of –2.5 or lower
- women who have had a low-trauma fracture
- women with a T-score between –1 and –2.5 and a 10-year FRAX hip-fracture risk of 3% or higher or a 10-year FRAX risk of major osteoporotic fracture of 20% or higher, or both. A major osteoporotic fracture involves the forearm, hip, or shoulder, or a clinical vertebral fracture (Level A evidence).
Only therapies approved by the US FDA should be used for medical treatment. They are raloxifene (Evista), bisphosphonates (Actonel, Boniva, Fosamax, Reclast), parathyroid hormone, denosumab (Prolia), and calcitonin (Fortical, Miacalcin) (Level A evidence).
Monitoring of therapy
In the absence of new risk factors, do not repeat DXA monitoring of therapy once BMD has been determined to be stable or improved (Level B evidence).
Lifestyle recommendations
- Counsel women about lifestyle factors that may affect BMD and fracture risk, which include smoking, poor nutrition and excessive weight loss, weight-bearing and muscle-strengthening exercise, and fall prevention (Level B evidence).
- Advise patients of current recommendations for calcium and vitamin D intake from the Institute of Medicine, which are calcium 1,200 mg/day and vitamin D 600 IU/day for women aged 51 to 70 years (Level B evidence).
- Counsel girls and women of all ages about the effects of lifestyle on bone health (Level C evidence – based on consensus and expert opinion).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
By utilizing the FRAX risk-assessment tool, we can determine which patients truly require treatment. In the process, we should be able to reduce the overtreatment of younger women with low bone mass as well as the undertreatment of older women who appear to have less deranged bone mass.
ACOG also emphasizes the need to avoid the overutilization of DXA scans in various groups, as well as the importance of lifestyle adjustments to promote bone health in all age groups.
Related Article: STOP performing DXA scans in healthy, perimenopausal women Lisa Larkin, MD, and Andrew M. Kaunitz, MD (Stop/Start, Januaray 2013)
CLINICAL DENSITOMETRISTS WEIGH IN ON INDICATIONS FOR BMD ASSESSMENT
International Society for Clinical Densitometry (ISCD). Indications for bone mineral density (BMD) testing. http://www.iscd.org/official-positions/2013-iscd-official-positions-adult/. Updated August 15, 2013. Accessed November 7, 2013.
In its comprehensive review of BMD assessment, the ISCD elucidates the process, which typically involves DXA imaging.
Indications for BMD assessment
- The female patient is age 65 or older
- The postmenopausal patient is younger than age 65 but has a risk factor for low bone mass, such as low body weight, a history of fracture, use of a high-risk medication, or a disease or condition associated with bone loss
- The perimenopausal woman has clinical risk factors for fracture, such as low body weight, history of fracture, or use of a high-risk medication
- The adult sustains a fragility fracture
- The adult has a disease or condition associated with low bone mass or bone loss
- The adult is taking a medication associated with low bone mass or bone loss
- The patient is being considered for pharmacologic therapy
- The patient is being treated, to monitor effect
- The patient is not receiving therapy, but evidence of bone loss would lead to treatment.
When serial BMD assessment is appropriate
- When it is used to determine whether treatment should be initiated (in untreated patients) because of significant bone loss
- To monitor response to therapy by identifying an increase or stabilization of BMD
- To identify nonresponse by documenting a loss of BMD, suggesting the need for treatment re-evaluation and assessment for a secondary cause of osteoporosis
- To follow-up earlier assessment when the expected change in BMD equals or exceeds the least significant change
- When the interval is appropriate for the patient’s clinical status. (In general, BMD assessment is performed 1 year after initiation or change of therapy, with longer intervals once a therapeutic effect has been established.)
- When the patient is using a medication associated with rapid bone loss, such as glucocorticoid therapy. In such a patient, more frequent testing may be appropriate.
- Note that these recommendations differ slightly from ACOG’s statements regarding the use of DXA.
Diagnosis of osteoporosis
- According to the WHO international reference standard, osteoporosis can be diagnosed when a patient has a T-score of –2.5 or below at the femoral neck. The reference standard from which the T-score is calculated is the white female population aged 20 to 29 years in the National Health and Nutrition Examination Survey (NHANES) III database.
- Osteoporosis also may be diagnosed in postmenopausal women and men aged 50 or older when the T-score of the lumbar spine, total hip, or femoral neck is –2.5 or below. In some circumstances, the
33% radius (also called the 1/3 radius) may be utilized. - Other hip regions of interest, including Ward’s area and the greater trochanter, should not be used for diagnosis.
A move away from use of the term “osteopenia”
- The term may be retained, but “low bone mass” or “low bone density” is preferred
- People with low bone mass or low bone density do not necessarily have a high risk of fracture.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
DXA testing remains the cornerstone of diagnosis for patients at risk for fragility fracture. It also is the optimal method to determine the need for pharmacotherapy. In some instances, however, overutilization of DXA imaging has led to overtreatment, especially in younger women with low bone mass, when treatment is based on variables other than diminished bone quality (including, “small-boned” women, genetics, and failure to achieve peak bone mass as high as one’s peer group prior to menopause).
These recommendations help to clarify the rationale for follow-up DXA imaging for patients on therapy, an area in which scientific unanimity is lacking.
Related Article: What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis treatment initiation? Steven R. Goldstein, MD (Examining the Evidence, August 2012)
IN ADOLESCENTS AND YOUNG WOMEN, CONSIDER THE BONE EFFECTS OF ORAL CONTRACEPTIVES
Ziglar S, Hunter TS. The effect of hormonal oral contraception on acquisition of peak bone mineral density of adolescents and young women. J Pharm Pract. 2012;25(3):331–340.
The bone loss observed in adolescents and young women who use depot medroxyprogesterone acetate (Depo-Provera) for contraception led to an FDA-mandated boxed warning on the medication’s package insert. The effect of oral contraceptives (OCs) on bone growth has received little publicity, however.
The best strategy to offset the natural loss of bone associated with aging and the menopausal transition is to ensure the development of maximal bone mass in youth. When maximal BMD is not achieved, the risk of osteoporosis is increased.
Adolescence is a critical period of bone mineralization, which is mediated by endogenous estradiol. The highest rate of bone mass accrual occurs 1 year before and 3 years after menarche. Young women who consume a diet low in calcium or who have an eating disorder, who fail to exercise, who smoke, or who have low estrogen status are most likely to have low peak bone mass.
OCs suppress endogenous estradiol production by interrupting the hypothalamic-pituitary-ovarian axis. By replacing endogenous estradiol with ethinyl estradiol (EE), OCs establish and maintain new hormone levels. Early initiation and use of very-low-dose EE increases the likelihood that the accrual of bone mass will be jeopardized at a critical time of bone mineralization.
Details of this meta-analysis
Ziglar and Hunter reviewed 11 prospective trials that showed a decrease in bone mass in adolescents and young women who used low-dose OCs, six trials that showed a neutral effect, and one trial that found an increase in bone mass. This last study involved only members of the US military whose level of daily exercise may not be representative of the general population of women the same age. Investigators also theorized that the use of norethindrone acetate as an androgenic progestin in this study may have exerted a positive effect on bone accrual.
Ziglar and Hunter concluded that the use of OCs containing 20 µg EE prevents adolescents and young women from attaining peak BMD. Evidence on the effect of contraceptives formulated with 30 to 35 µg EE is less definitive, but this dose may also impede BMD acquisition in adolescents.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
To ensure breast health and reduce the risk of venous thromboembolic events, drug makers have developed OCs with lower and lower doses of EE. In the process, however, the beneficial effects of endogenous estradiol on bone acquisition have been suppressed. Therefore, the lowest-dose OC may not necessarily be the most appropriate clinical choice for adolescents and young women seeking contraception.
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
EXPERIMENTAL DRUG REDUCES BONE RESORPTION WITHOUT IMPEDING BONE FORMATION
Brixen K, Chapurlat R, Cheung AM, et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab. 2013;98(2):571–580.
Current treatments for osteoporosis include antiresorptive agents, such as bisphosphonates and denosumab, that preserve bone mass by reducing the rate of bone turnover. These drugs reduce the number or activity (or both) of bone-resorbing osteoclasts. Because osteoclasts play a role in stimulating bone formation by osteoblasts, these treatments indirectly lower bone formation.
Odanacatib is a drug in Phase 3 development for the treatment of postmenopausal osteoporosis. It is a highly selective and reversible oral inhibitor of the collagenase activity of cathepsin K, which is secreted by osteoclasts. Odanacatib reduces bone resorption without reducing the number of osteoclasts and, thus, appears to preserve bone formation.
Details of the trial
Brixen and colleagues conducted a randomized, double-blind, international, 2-year, Phase 3 trial comparing odancatib 50 mg once weekly with placebo in postmenopausal women treated with calcium and vitamin D. The primary endpoint was the change from baseline BMD at the lumbar spine at 1 year, as assessed by DXA. Secondary endpoints included the change from baseline BMD at the hip (total hip, femoral neck, and trochanter) at 1 year, the change from baseline BMD at the spine and hip at 2 years, and 1- and 2-year changes in bone-turnover markers. A total of 214 women were enrolled (average age: 64 years; average T-score of 1.8 at the lumbar spine, –1.8 at the femoral neck, and –1.3 at the total hip).
At 1 year, the change from baseline BMD at the lumbar spine was significantly higher (P <.001) in women receiving odanacatib, compared with placebo (treatment difference: 3.5%). At 2 years, the treatment difference was even higher (5.4%). The mean changes in BMD at the femoral neck, total hip, and trochanter also were significantly greater (P <.001) in women receiving odanacatib, with treatment differences at 2 years of 3.8%, 3.3%, and 5.5%, respectively.
During the first 6 months of the trial, serum concentrations of bone-turnover markers (CTX and P1NP) decreased significantly (P <.001) in odanacatib-treated women, compared with those given placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although no new agents for the treatment of osteoporosis have been introduced over the past year, cathepsin K inhibitors appear to offer great promise for the future. As clinicians, we need to keep abreast of new developments that may be of potential value to our patients.
PILOT STUDY: TERIPARATIDE WAS EFFECTIVE IN 81% OF PREMENOPAUSAL WOMEN WITH IDIOPATHIC OSTEOPOROSIS
Cohen A, Stein EM, Recker RR, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: A pilot study. J Clin Endocrinol Metab. 2013;98(5):1971–1981.
Idiopathic osteoporosis (IOP) affects young, otherwise healthy men and women with intact gonadal function and no secondary cause of bone loss or fragility. Women with IOP have abnormal bone microarchitecture with thinner cortices; fewer, thinner, and more widely separated and heterogeneously distributed trabeculae; more rod-like trabecular structures; less trabecular stiffness; and a higher level of marrow fat.
The osteoanabolic agent teriperatide increases BMD and reduces the incidence of fracture in postmenopausal women and in patients with glucocorticoid-induced osteoporosis, and it increases BMD in men with IOP. This study explored its effect in premenopausal women with IOP.
Details of the study
Cohen and colleagues recruited premenopausal women aged 20 to 48 years who had one or both of the following traits:
- a history of at least one low-trauma fracture more than 6 months before enrollment
- low BMD of the spine or hip (Z-score of –2.0 or below), as assessed by DXA.
All participants had regular menses and early follicular-phase follicle-stimulating hormone (FSH) levels below 20 mIU/mL; none were using hormonal contraception. Women who had secondary osteoporosis related to estrogen deficiency, an eating disorder, an endocrinopathy, celiac or gastrointestinal disease, hyperparathyroidism, marked hypercalciuria, a low serum level of 25-hydroxyvitamin D (<20 ng/mL), and drug exposures were excluded.
All participants (n = 21) received teriparatide 20 µg daily in the morning or evening, according to preference. They also were given calcium 630 mg and vitamin D 800 IU daily.
BMD increased at the spine by 10.8% (standard deviation: 8.3%), total hip by 6.2% (5.6%), and femoral neck by 7.6% (3.4%) (all P <.001). Transiliac biopsies demonstrated significant increases in cortical width and porosity, and trabecular bone volume and number increased as well. Four women had no increase in BMD.
Overall, Cohen and colleagues concluded that teriparatide was associated with increased BMD at the spine and hip and improved trabecular microarchitecture and stiffness at the iliac crest in the majority of women with IOP.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although ObGyns rarely prescribe teriparatide, often leaving this option for metabolic bone experts to offer, we should keep premenopausal IOP in mind when younger patients sustain low-trauma fractures of the hip or vertebrae, as well as fracture of an upper or lower extremity or the ribs.
Teriparatide appears to be an excellent choice for the majority of premenopausal patients with IOP (81% of patients in this pilot study).
Because the low bone mass and deterioration of bone microarchitecture and quality that characterize osteoporosis can lead to fragility fracture, it is vital that we intervene in our patients’ health in a timely manner to reduce this risk. One way to accomplish this goal is to understand the role of age in determining a woman’s fracture risk. For example, an 80-year-old woman and a 50-year-old woman with a T-score of –2.5, as measured by dual x-ray absorptiometry (DXA), will have dramatically different fracture risks. According to the World Health Organization’s fracture-risk assessment tool (http://www.shef.ac.uk/FRAX/), the older woman has a 10-year probability of hip fracture approximately five times greater than the younger woman.
Although no new therapies have been approved during the past year, several important findings were published that affect clinical management of menopausal patients or suggest changes likely in the future.
In this article, I review:
- the latest guidance on osteoporosis from the American College of Obstetricians and Gynecologists (ACOG)
- the most recent indications for bone mineral density (BMD) testing from the International Society for Clinical Densitometry (ISCD)
- a study exploring the effect of oral hormonal contraception on the acquisition of peak BMD in adolescents and young women
- results of a randomized trial of the experimental agent odanacatib in postmenopausal women
- a pilot study of teriparatide (Forteo) for idiopathic osteoporosis in premenopausal women.
ACOG ISSUES RECOMMENDATIONS ON SCREENING, TREATMENT, AND LIFESTYLE
Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin #129: Osteoporosis. Obstet Gynecol. 2012;120(3):718–734.
This comprehensive review of management guidelines for ObGyns deserves “top billing” in this update. It offers recommendations on important interventions, from BMD measurement and subsequent monitoring to calcium and vitamin D supplementation.
When to initiate screening
- Begin BMD screening using DXA at age 65. DXA also may be appropriate for younger women if they are postmenopausal and have other significant risk factors for osteoporosis or fracture (Level A evidence – based on good and consistent scientific evidence).
- In the absence of new risk factors, do not perform DXA screening more frequently than every 2 years (Level B evidence – based on limited or inconsistent scientific evidence).
Which patients should be treated?
Treatment is recommended for:
- women with a T-score of –2.5 or lower
- women who have had a low-trauma fracture
- women with a T-score between –1 and –2.5 and a 10-year FRAX hip-fracture risk of 3% or higher or a 10-year FRAX risk of major osteoporotic fracture of 20% or higher, or both. A major osteoporotic fracture involves the forearm, hip, or shoulder, or a clinical vertebral fracture (Level A evidence).
Only therapies approved by the US FDA should be used for medical treatment. They are raloxifene (Evista), bisphosphonates (Actonel, Boniva, Fosamax, Reclast), parathyroid hormone, denosumab (Prolia), and calcitonin (Fortical, Miacalcin) (Level A evidence).
Monitoring of therapy
In the absence of new risk factors, do not repeat DXA monitoring of therapy once BMD has been determined to be stable or improved (Level B evidence).
Lifestyle recommendations
- Counsel women about lifestyle factors that may affect BMD and fracture risk, which include smoking, poor nutrition and excessive weight loss, weight-bearing and muscle-strengthening exercise, and fall prevention (Level B evidence).
- Advise patients of current recommendations for calcium and vitamin D intake from the Institute of Medicine, which are calcium 1,200 mg/day and vitamin D 600 IU/day for women aged 51 to 70 years (Level B evidence).
- Counsel girls and women of all ages about the effects of lifestyle on bone health (Level C evidence – based on consensus and expert opinion).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
By utilizing the FRAX risk-assessment tool, we can determine which patients truly require treatment. In the process, we should be able to reduce the overtreatment of younger women with low bone mass as well as the undertreatment of older women who appear to have less deranged bone mass.
ACOG also emphasizes the need to avoid the overutilization of DXA scans in various groups, as well as the importance of lifestyle adjustments to promote bone health in all age groups.
Related Article: STOP performing DXA scans in healthy, perimenopausal women Lisa Larkin, MD, and Andrew M. Kaunitz, MD (Stop/Start, Januaray 2013)
CLINICAL DENSITOMETRISTS WEIGH IN ON INDICATIONS FOR BMD ASSESSMENT
International Society for Clinical Densitometry (ISCD). Indications for bone mineral density (BMD) testing. http://www.iscd.org/official-positions/2013-iscd-official-positions-adult/. Updated August 15, 2013. Accessed November 7, 2013.
In its comprehensive review of BMD assessment, the ISCD elucidates the process, which typically involves DXA imaging.
Indications for BMD assessment
- The female patient is age 65 or older
- The postmenopausal patient is younger than age 65 but has a risk factor for low bone mass, such as low body weight, a history of fracture, use of a high-risk medication, or a disease or condition associated with bone loss
- The perimenopausal woman has clinical risk factors for fracture, such as low body weight, history of fracture, or use of a high-risk medication
- The adult sustains a fragility fracture
- The adult has a disease or condition associated with low bone mass or bone loss
- The adult is taking a medication associated with low bone mass or bone loss
- The patient is being considered for pharmacologic therapy
- The patient is being treated, to monitor effect
- The patient is not receiving therapy, but evidence of bone loss would lead to treatment.
When serial BMD assessment is appropriate
- When it is used to determine whether treatment should be initiated (in untreated patients) because of significant bone loss
- To monitor response to therapy by identifying an increase or stabilization of BMD
- To identify nonresponse by documenting a loss of BMD, suggesting the need for treatment re-evaluation and assessment for a secondary cause of osteoporosis
- To follow-up earlier assessment when the expected change in BMD equals or exceeds the least significant change
- When the interval is appropriate for the patient’s clinical status. (In general, BMD assessment is performed 1 year after initiation or change of therapy, with longer intervals once a therapeutic effect has been established.)
- When the patient is using a medication associated with rapid bone loss, such as glucocorticoid therapy. In such a patient, more frequent testing may be appropriate.
- Note that these recommendations differ slightly from ACOG’s statements regarding the use of DXA.
Diagnosis of osteoporosis
- According to the WHO international reference standard, osteoporosis can be diagnosed when a patient has a T-score of –2.5 or below at the femoral neck. The reference standard from which the T-score is calculated is the white female population aged 20 to 29 years in the National Health and Nutrition Examination Survey (NHANES) III database.
- Osteoporosis also may be diagnosed in postmenopausal women and men aged 50 or older when the T-score of the lumbar spine, total hip, or femoral neck is –2.5 or below. In some circumstances, the
33% radius (also called the 1/3 radius) may be utilized. - Other hip regions of interest, including Ward’s area and the greater trochanter, should not be used for diagnosis.
A move away from use of the term “osteopenia”
- The term may be retained, but “low bone mass” or “low bone density” is preferred
- People with low bone mass or low bone density do not necessarily have a high risk of fracture.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
DXA testing remains the cornerstone of diagnosis for patients at risk for fragility fracture. It also is the optimal method to determine the need for pharmacotherapy. In some instances, however, overutilization of DXA imaging has led to overtreatment, especially in younger women with low bone mass, when treatment is based on variables other than diminished bone quality (including, “small-boned” women, genetics, and failure to achieve peak bone mass as high as one’s peer group prior to menopause).
These recommendations help to clarify the rationale for follow-up DXA imaging for patients on therapy, an area in which scientific unanimity is lacking.
Related Article: What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis treatment initiation? Steven R. Goldstein, MD (Examining the Evidence, August 2012)
IN ADOLESCENTS AND YOUNG WOMEN, CONSIDER THE BONE EFFECTS OF ORAL CONTRACEPTIVES
Ziglar S, Hunter TS. The effect of hormonal oral contraception on acquisition of peak bone mineral density of adolescents and young women. J Pharm Pract. 2012;25(3):331–340.
The bone loss observed in adolescents and young women who use depot medroxyprogesterone acetate (Depo-Provera) for contraception led to an FDA-mandated boxed warning on the medication’s package insert. The effect of oral contraceptives (OCs) on bone growth has received little publicity, however.
The best strategy to offset the natural loss of bone associated with aging and the menopausal transition is to ensure the development of maximal bone mass in youth. When maximal BMD is not achieved, the risk of osteoporosis is increased.
Adolescence is a critical period of bone mineralization, which is mediated by endogenous estradiol. The highest rate of bone mass accrual occurs 1 year before and 3 years after menarche. Young women who consume a diet low in calcium or who have an eating disorder, who fail to exercise, who smoke, or who have low estrogen status are most likely to have low peak bone mass.
OCs suppress endogenous estradiol production by interrupting the hypothalamic-pituitary-ovarian axis. By replacing endogenous estradiol with ethinyl estradiol (EE), OCs establish and maintain new hormone levels. Early initiation and use of very-low-dose EE increases the likelihood that the accrual of bone mass will be jeopardized at a critical time of bone mineralization.
Details of this meta-analysis
Ziglar and Hunter reviewed 11 prospective trials that showed a decrease in bone mass in adolescents and young women who used low-dose OCs, six trials that showed a neutral effect, and one trial that found an increase in bone mass. This last study involved only members of the US military whose level of daily exercise may not be representative of the general population of women the same age. Investigators also theorized that the use of norethindrone acetate as an androgenic progestin in this study may have exerted a positive effect on bone accrual.
Ziglar and Hunter concluded that the use of OCs containing 20 µg EE prevents adolescents and young women from attaining peak BMD. Evidence on the effect of contraceptives formulated with 30 to 35 µg EE is less definitive, but this dose may also impede BMD acquisition in adolescents.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
To ensure breast health and reduce the risk of venous thromboembolic events, drug makers have developed OCs with lower and lower doses of EE. In the process, however, the beneficial effects of endogenous estradiol on bone acquisition have been suppressed. Therefore, the lowest-dose OC may not necessarily be the most appropriate clinical choice for adolescents and young women seeking contraception.
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
EXPERIMENTAL DRUG REDUCES BONE RESORPTION WITHOUT IMPEDING BONE FORMATION
Brixen K, Chapurlat R, Cheung AM, et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab. 2013;98(2):571–580.
Current treatments for osteoporosis include antiresorptive agents, such as bisphosphonates and denosumab, that preserve bone mass by reducing the rate of bone turnover. These drugs reduce the number or activity (or both) of bone-resorbing osteoclasts. Because osteoclasts play a role in stimulating bone formation by osteoblasts, these treatments indirectly lower bone formation.
Odanacatib is a drug in Phase 3 development for the treatment of postmenopausal osteoporosis. It is a highly selective and reversible oral inhibitor of the collagenase activity of cathepsin K, which is secreted by osteoclasts. Odanacatib reduces bone resorption without reducing the number of osteoclasts and, thus, appears to preserve bone formation.
Details of the trial
Brixen and colleagues conducted a randomized, double-blind, international, 2-year, Phase 3 trial comparing odancatib 50 mg once weekly with placebo in postmenopausal women treated with calcium and vitamin D. The primary endpoint was the change from baseline BMD at the lumbar spine at 1 year, as assessed by DXA. Secondary endpoints included the change from baseline BMD at the hip (total hip, femoral neck, and trochanter) at 1 year, the change from baseline BMD at the spine and hip at 2 years, and 1- and 2-year changes in bone-turnover markers. A total of 214 women were enrolled (average age: 64 years; average T-score of 1.8 at the lumbar spine, –1.8 at the femoral neck, and –1.3 at the total hip).
At 1 year, the change from baseline BMD at the lumbar spine was significantly higher (P <.001) in women receiving odanacatib, compared with placebo (treatment difference: 3.5%). At 2 years, the treatment difference was even higher (5.4%). The mean changes in BMD at the femoral neck, total hip, and trochanter also were significantly greater (P <.001) in women receiving odanacatib, with treatment differences at 2 years of 3.8%, 3.3%, and 5.5%, respectively.
During the first 6 months of the trial, serum concentrations of bone-turnover markers (CTX and P1NP) decreased significantly (P <.001) in odanacatib-treated women, compared with those given placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although no new agents for the treatment of osteoporosis have been introduced over the past year, cathepsin K inhibitors appear to offer great promise for the future. As clinicians, we need to keep abreast of new developments that may be of potential value to our patients.
PILOT STUDY: TERIPARATIDE WAS EFFECTIVE IN 81% OF PREMENOPAUSAL WOMEN WITH IDIOPATHIC OSTEOPOROSIS
Cohen A, Stein EM, Recker RR, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: A pilot study. J Clin Endocrinol Metab. 2013;98(5):1971–1981.
Idiopathic osteoporosis (IOP) affects young, otherwise healthy men and women with intact gonadal function and no secondary cause of bone loss or fragility. Women with IOP have abnormal bone microarchitecture with thinner cortices; fewer, thinner, and more widely separated and heterogeneously distributed trabeculae; more rod-like trabecular structures; less trabecular stiffness; and a higher level of marrow fat.
The osteoanabolic agent teriperatide increases BMD and reduces the incidence of fracture in postmenopausal women and in patients with glucocorticoid-induced osteoporosis, and it increases BMD in men with IOP. This study explored its effect in premenopausal women with IOP.
Details of the study
Cohen and colleagues recruited premenopausal women aged 20 to 48 years who had one or both of the following traits:
- a history of at least one low-trauma fracture more than 6 months before enrollment
- low BMD of the spine or hip (Z-score of –2.0 or below), as assessed by DXA.
All participants had regular menses and early follicular-phase follicle-stimulating hormone (FSH) levels below 20 mIU/mL; none were using hormonal contraception. Women who had secondary osteoporosis related to estrogen deficiency, an eating disorder, an endocrinopathy, celiac or gastrointestinal disease, hyperparathyroidism, marked hypercalciuria, a low serum level of 25-hydroxyvitamin D (<20 ng/mL), and drug exposures were excluded.
All participants (n = 21) received teriparatide 20 µg daily in the morning or evening, according to preference. They also were given calcium 630 mg and vitamin D 800 IU daily.
BMD increased at the spine by 10.8% (standard deviation: 8.3%), total hip by 6.2% (5.6%), and femoral neck by 7.6% (3.4%) (all P <.001). Transiliac biopsies demonstrated significant increases in cortical width and porosity, and trabecular bone volume and number increased as well. Four women had no increase in BMD.
Overall, Cohen and colleagues concluded that teriparatide was associated with increased BMD at the spine and hip and improved trabecular microarchitecture and stiffness at the iliac crest in the majority of women with IOP.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although ObGyns rarely prescribe teriparatide, often leaving this option for metabolic bone experts to offer, we should keep premenopausal IOP in mind when younger patients sustain low-trauma fractures of the hip or vertebrae, as well as fracture of an upper or lower extremity or the ribs.
Teriparatide appears to be an excellent choice for the majority of premenopausal patients with IOP (81% of patients in this pilot study).
Because the low bone mass and deterioration of bone microarchitecture and quality that characterize osteoporosis can lead to fragility fracture, it is vital that we intervene in our patients’ health in a timely manner to reduce this risk. One way to accomplish this goal is to understand the role of age in determining a woman’s fracture risk. For example, an 80-year-old woman and a 50-year-old woman with a T-score of –2.5, as measured by dual x-ray absorptiometry (DXA), will have dramatically different fracture risks. According to the World Health Organization’s fracture-risk assessment tool (http://www.shef.ac.uk/FRAX/), the older woman has a 10-year probability of hip fracture approximately five times greater than the younger woman.
Although no new therapies have been approved during the past year, several important findings were published that affect clinical management of menopausal patients or suggest changes likely in the future.
In this article, I review:
- the latest guidance on osteoporosis from the American College of Obstetricians and Gynecologists (ACOG)
- the most recent indications for bone mineral density (BMD) testing from the International Society for Clinical Densitometry (ISCD)
- a study exploring the effect of oral hormonal contraception on the acquisition of peak BMD in adolescents and young women
- results of a randomized trial of the experimental agent odanacatib in postmenopausal women
- a pilot study of teriparatide (Forteo) for idiopathic osteoporosis in premenopausal women.
ACOG ISSUES RECOMMENDATIONS ON SCREENING, TREATMENT, AND LIFESTYLE
Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin #129: Osteoporosis. Obstet Gynecol. 2012;120(3):718–734.
This comprehensive review of management guidelines for ObGyns deserves “top billing” in this update. It offers recommendations on important interventions, from BMD measurement and subsequent monitoring to calcium and vitamin D supplementation.
When to initiate screening
- Begin BMD screening using DXA at age 65. DXA also may be appropriate for younger women if they are postmenopausal and have other significant risk factors for osteoporosis or fracture (Level A evidence – based on good and consistent scientific evidence).
- In the absence of new risk factors, do not perform DXA screening more frequently than every 2 years (Level B evidence – based on limited or inconsistent scientific evidence).
Which patients should be treated?
Treatment is recommended for:
- women with a T-score of –2.5 or lower
- women who have had a low-trauma fracture
- women with a T-score between –1 and –2.5 and a 10-year FRAX hip-fracture risk of 3% or higher or a 10-year FRAX risk of major osteoporotic fracture of 20% or higher, or both. A major osteoporotic fracture involves the forearm, hip, or shoulder, or a clinical vertebral fracture (Level A evidence).
Only therapies approved by the US FDA should be used for medical treatment. They are raloxifene (Evista), bisphosphonates (Actonel, Boniva, Fosamax, Reclast), parathyroid hormone, denosumab (Prolia), and calcitonin (Fortical, Miacalcin) (Level A evidence).
Monitoring of therapy
In the absence of new risk factors, do not repeat DXA monitoring of therapy once BMD has been determined to be stable or improved (Level B evidence).
Lifestyle recommendations
- Counsel women about lifestyle factors that may affect BMD and fracture risk, which include smoking, poor nutrition and excessive weight loss, weight-bearing and muscle-strengthening exercise, and fall prevention (Level B evidence).
- Advise patients of current recommendations for calcium and vitamin D intake from the Institute of Medicine, which are calcium 1,200 mg/day and vitamin D 600 IU/day for women aged 51 to 70 years (Level B evidence).
- Counsel girls and women of all ages about the effects of lifestyle on bone health (Level C evidence – based on consensus and expert opinion).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
By utilizing the FRAX risk-assessment tool, we can determine which patients truly require treatment. In the process, we should be able to reduce the overtreatment of younger women with low bone mass as well as the undertreatment of older women who appear to have less deranged bone mass.
ACOG also emphasizes the need to avoid the overutilization of DXA scans in various groups, as well as the importance of lifestyle adjustments to promote bone health in all age groups.
Related Article: STOP performing DXA scans in healthy, perimenopausal women Lisa Larkin, MD, and Andrew M. Kaunitz, MD (Stop/Start, Januaray 2013)
CLINICAL DENSITOMETRISTS WEIGH IN ON INDICATIONS FOR BMD ASSESSMENT
International Society for Clinical Densitometry (ISCD). Indications for bone mineral density (BMD) testing. http://www.iscd.org/official-positions/2013-iscd-official-positions-adult/. Updated August 15, 2013. Accessed November 7, 2013.
In its comprehensive review of BMD assessment, the ISCD elucidates the process, which typically involves DXA imaging.
Indications for BMD assessment
- The female patient is age 65 or older
- The postmenopausal patient is younger than age 65 but has a risk factor for low bone mass, such as low body weight, a history of fracture, use of a high-risk medication, or a disease or condition associated with bone loss
- The perimenopausal woman has clinical risk factors for fracture, such as low body weight, history of fracture, or use of a high-risk medication
- The adult sustains a fragility fracture
- The adult has a disease or condition associated with low bone mass or bone loss
- The adult is taking a medication associated with low bone mass or bone loss
- The patient is being considered for pharmacologic therapy
- The patient is being treated, to monitor effect
- The patient is not receiving therapy, but evidence of bone loss would lead to treatment.
When serial BMD assessment is appropriate
- When it is used to determine whether treatment should be initiated (in untreated patients) because of significant bone loss
- To monitor response to therapy by identifying an increase or stabilization of BMD
- To identify nonresponse by documenting a loss of BMD, suggesting the need for treatment re-evaluation and assessment for a secondary cause of osteoporosis
- To follow-up earlier assessment when the expected change in BMD equals or exceeds the least significant change
- When the interval is appropriate for the patient’s clinical status. (In general, BMD assessment is performed 1 year after initiation or change of therapy, with longer intervals once a therapeutic effect has been established.)
- When the patient is using a medication associated with rapid bone loss, such as glucocorticoid therapy. In such a patient, more frequent testing may be appropriate.
- Note that these recommendations differ slightly from ACOG’s statements regarding the use of DXA.
Diagnosis of osteoporosis
- According to the WHO international reference standard, osteoporosis can be diagnosed when a patient has a T-score of –2.5 or below at the femoral neck. The reference standard from which the T-score is calculated is the white female population aged 20 to 29 years in the National Health and Nutrition Examination Survey (NHANES) III database.
- Osteoporosis also may be diagnosed in postmenopausal women and men aged 50 or older when the T-score of the lumbar spine, total hip, or femoral neck is –2.5 or below. In some circumstances, the
33% radius (also called the 1/3 radius) may be utilized. - Other hip regions of interest, including Ward’s area and the greater trochanter, should not be used for diagnosis.
A move away from use of the term “osteopenia”
- The term may be retained, but “low bone mass” or “low bone density” is preferred
- People with low bone mass or low bone density do not necessarily have a high risk of fracture.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
DXA testing remains the cornerstone of diagnosis for patients at risk for fragility fracture. It also is the optimal method to determine the need for pharmacotherapy. In some instances, however, overutilization of DXA imaging has led to overtreatment, especially in younger women with low bone mass, when treatment is based on variables other than diminished bone quality (including, “small-boned” women, genetics, and failure to achieve peak bone mass as high as one’s peer group prior to menopause).
These recommendations help to clarify the rationale for follow-up DXA imaging for patients on therapy, an area in which scientific unanimity is lacking.
Related Article: What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis treatment initiation? Steven R. Goldstein, MD (Examining the Evidence, August 2012)
IN ADOLESCENTS AND YOUNG WOMEN, CONSIDER THE BONE EFFECTS OF ORAL CONTRACEPTIVES
Ziglar S, Hunter TS. The effect of hormonal oral contraception on acquisition of peak bone mineral density of adolescents and young women. J Pharm Pract. 2012;25(3):331–340.
The bone loss observed in adolescents and young women who use depot medroxyprogesterone acetate (Depo-Provera) for contraception led to an FDA-mandated boxed warning on the medication’s package insert. The effect of oral contraceptives (OCs) on bone growth has received little publicity, however.
The best strategy to offset the natural loss of bone associated with aging and the menopausal transition is to ensure the development of maximal bone mass in youth. When maximal BMD is not achieved, the risk of osteoporosis is increased.
Adolescence is a critical period of bone mineralization, which is mediated by endogenous estradiol. The highest rate of bone mass accrual occurs 1 year before and 3 years after menarche. Young women who consume a diet low in calcium or who have an eating disorder, who fail to exercise, who smoke, or who have low estrogen status are most likely to have low peak bone mass.
OCs suppress endogenous estradiol production by interrupting the hypothalamic-pituitary-ovarian axis. By replacing endogenous estradiol with ethinyl estradiol (EE), OCs establish and maintain new hormone levels. Early initiation and use of very-low-dose EE increases the likelihood that the accrual of bone mass will be jeopardized at a critical time of bone mineralization.
Details of this meta-analysis
Ziglar and Hunter reviewed 11 prospective trials that showed a decrease in bone mass in adolescents and young women who used low-dose OCs, six trials that showed a neutral effect, and one trial that found an increase in bone mass. This last study involved only members of the US military whose level of daily exercise may not be representative of the general population of women the same age. Investigators also theorized that the use of norethindrone acetate as an androgenic progestin in this study may have exerted a positive effect on bone accrual.
Ziglar and Hunter concluded that the use of OCs containing 20 µg EE prevents adolescents and young women from attaining peak BMD. Evidence on the effect of contraceptives formulated with 30 to 35 µg EE is less definitive, but this dose may also impede BMD acquisition in adolescents.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
To ensure breast health and reduce the risk of venous thromboembolic events, drug makers have developed OCs with lower and lower doses of EE. In the process, however, the beneficial effects of endogenous estradiol on bone acquisition have been suppressed. Therefore, the lowest-dose OC may not necessarily be the most appropriate clinical choice for adolescents and young women seeking contraception.
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
EXPERIMENTAL DRUG REDUCES BONE RESORPTION WITHOUT IMPEDING BONE FORMATION
Brixen K, Chapurlat R, Cheung AM, et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab. 2013;98(2):571–580.
Current treatments for osteoporosis include antiresorptive agents, such as bisphosphonates and denosumab, that preserve bone mass by reducing the rate of bone turnover. These drugs reduce the number or activity (or both) of bone-resorbing osteoclasts. Because osteoclasts play a role in stimulating bone formation by osteoblasts, these treatments indirectly lower bone formation.
Odanacatib is a drug in Phase 3 development for the treatment of postmenopausal osteoporosis. It is a highly selective and reversible oral inhibitor of the collagenase activity of cathepsin K, which is secreted by osteoclasts. Odanacatib reduces bone resorption without reducing the number of osteoclasts and, thus, appears to preserve bone formation.
Details of the trial
Brixen and colleagues conducted a randomized, double-blind, international, 2-year, Phase 3 trial comparing odancatib 50 mg once weekly with placebo in postmenopausal women treated with calcium and vitamin D. The primary endpoint was the change from baseline BMD at the lumbar spine at 1 year, as assessed by DXA. Secondary endpoints included the change from baseline BMD at the hip (total hip, femoral neck, and trochanter) at 1 year, the change from baseline BMD at the spine and hip at 2 years, and 1- and 2-year changes in bone-turnover markers. A total of 214 women were enrolled (average age: 64 years; average T-score of 1.8 at the lumbar spine, –1.8 at the femoral neck, and –1.3 at the total hip).
At 1 year, the change from baseline BMD at the lumbar spine was significantly higher (P <.001) in women receiving odanacatib, compared with placebo (treatment difference: 3.5%). At 2 years, the treatment difference was even higher (5.4%). The mean changes in BMD at the femoral neck, total hip, and trochanter also were significantly greater (P <.001) in women receiving odanacatib, with treatment differences at 2 years of 3.8%, 3.3%, and 5.5%, respectively.
During the first 6 months of the trial, serum concentrations of bone-turnover markers (CTX and P1NP) decreased significantly (P <.001) in odanacatib-treated women, compared with those given placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although no new agents for the treatment of osteoporosis have been introduced over the past year, cathepsin K inhibitors appear to offer great promise for the future. As clinicians, we need to keep abreast of new developments that may be of potential value to our patients.
PILOT STUDY: TERIPARATIDE WAS EFFECTIVE IN 81% OF PREMENOPAUSAL WOMEN WITH IDIOPATHIC OSTEOPOROSIS
Cohen A, Stein EM, Recker RR, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: A pilot study. J Clin Endocrinol Metab. 2013;98(5):1971–1981.
Idiopathic osteoporosis (IOP) affects young, otherwise healthy men and women with intact gonadal function and no secondary cause of bone loss or fragility. Women with IOP have abnormal bone microarchitecture with thinner cortices; fewer, thinner, and more widely separated and heterogeneously distributed trabeculae; more rod-like trabecular structures; less trabecular stiffness; and a higher level of marrow fat.
The osteoanabolic agent teriperatide increases BMD and reduces the incidence of fracture in postmenopausal women and in patients with glucocorticoid-induced osteoporosis, and it increases BMD in men with IOP. This study explored its effect in premenopausal women with IOP.
Details of the study
Cohen and colleagues recruited premenopausal women aged 20 to 48 years who had one or both of the following traits:
- a history of at least one low-trauma fracture more than 6 months before enrollment
- low BMD of the spine or hip (Z-score of –2.0 or below), as assessed by DXA.
All participants had regular menses and early follicular-phase follicle-stimulating hormone (FSH) levels below 20 mIU/mL; none were using hormonal contraception. Women who had secondary osteoporosis related to estrogen deficiency, an eating disorder, an endocrinopathy, celiac or gastrointestinal disease, hyperparathyroidism, marked hypercalciuria, a low serum level of 25-hydroxyvitamin D (<20 ng/mL), and drug exposures were excluded.
All participants (n = 21) received teriparatide 20 µg daily in the morning or evening, according to preference. They also were given calcium 630 mg and vitamin D 800 IU daily.
BMD increased at the spine by 10.8% (standard deviation: 8.3%), total hip by 6.2% (5.6%), and femoral neck by 7.6% (3.4%) (all P <.001). Transiliac biopsies demonstrated significant increases in cortical width and porosity, and trabecular bone volume and number increased as well. Four women had no increase in BMD.
Overall, Cohen and colleagues concluded that teriparatide was associated with increased BMD at the spine and hip and improved trabecular microarchitecture and stiffness at the iliac crest in the majority of women with IOP.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although ObGyns rarely prescribe teriparatide, often leaving this option for metabolic bone experts to offer, we should keep premenopausal IOP in mind when younger patients sustain low-trauma fractures of the hip or vertebrae, as well as fracture of an upper or lower extremity or the ribs.
Teriparatide appears to be an excellent choice for the majority of premenopausal patients with IOP (81% of patients in this pilot study).
Man, 55, With Mild Chest Discomfort
A 55-year-old white man with controlled hypertension and hypercholesterolemia awoke with mild chest discomfort that he believed was mild gastroesophageal reflux. He denied radiation of pain to the shoulders, arms, back, or neck; dyspnea; palpitations; diaphoresis; nausea/vomiting; cough; or fever, during the first 30 hours of discomfort. There was no change in discomfort with deep breath, palpation of the chest, or administration of antacids. Minimal, short-lived improvement was noted with belching.
The patient had no trouble sleeping in the prone position and did not notice an increase in discomfort or unusual difficulty during his daily vigorous 30-minute aerobic workout. In fact, his symptoms seemed to improve or disappear during exercise. The patient denied any recent illness or exposure to sick people, had not traveled outside the United States, and had not been exposed to radiation of the chest wall. At the end of the second day of discomfort, the patient noted irregular palpitations with mild shortness of breath and was transported to the hospital for evaluation. He denied being a cigarette smoker or illicit drug user.
The patient had no history of MI or diabetes. The patient’s father had an MI in his 80s, and two uncles died suddenly in their 50s of “massive heart attacks.” His mother, who had died of sepsis of uncertain etiology approximately 10 days earlier, also had hypertension and hypercholesterolemia but no history of coronary artery disease (CAD). Both of the patient’s adult daughters had been diagnosed with celiac disease in the preceding three years. His elder daughter had also been diagnosed with type 1 diabetes within the past two years.
On examination, the patient was afebrile, with a blood pressure of 143/87 mm Hg; pulse, 53 beats/min; and respiratory rate, 17 breaths/min. The patient’s weight was 204 lb and his height, 75 in (BMI, 25.5). The patient was in no apparent distress. Head, eyes, ears, nose, and throat were unremarkable. There was no significant jugular venous distention. The carotid pulses were full, and no bruits were appreciated. S1 and S2 sounds were within normal limits. No murmurs or S3 or S4 gallops were appreciated. The chest was clear on auscultation. Results of the abdominal exam were negative, no edema was noted in the extremities, and pulses were symmetrical.
ECG demonstrated subtle ST-segment elevation in leads I and aVL with a prominent R wave in lead V1. This pattern was interpreted as consistent with an acute inferolateral MI. A baseline ECG, previously obtained by the patient’s internist, had been interpreted as normal.
Peak troponin level was 55 ng/mL (normal, < 0.03 ng/mL); total creatine kinase (CK), 807 U/L (reference range, 20 to 259 U/L); and mass CK-MB fraction, 44 ng/mL (0.1 to 6.6 ng/mL). Total cholesterol was 105 mg/dL, with both LDL- and HDL-cholesterol fractions at 46 mg/dL. A complete blood count without differential revealed a total white blood cell count of 53,000/µL. Hemoglobin and hematocrit were both low (12.3 g/dL and 34.5%, respectively). All indices were within normal limits, as was the platelet count. Glucose, blood urea nitrogen, creatinine, potassium chloride bicarbonate, and calcium were all within normal limits. The sodium level was slightly low (132 mEq/L). Emergency catheterization revealed an ejection fraction of 45% (reference range, 55% to 70%), with mild-to-moderate diffuse hypokinesis but normal coronary arteries.
The patient was diagnosed with myocarditis, likely of viral origin.
DISCUSSION
Although the incidence of myocarditis in the US is difficult to assess, autopsy reports implicate it in 8.6% to 12% of cases of sudden cardiac death in young adults,1,2 and a large prospective series implicated myocarditis in 9% of cases of dilated cardiomyopathy.3 Myocarditis is considered to be at one extreme of a spectrum of perimyocardial processes that result in inflammation of the myocardium (see figure), pericardium, or both.4
The underlying pathology involves an acute injury to the myocyte. This activates the innate and humoral immune systems, resulting in severe inflammation. The immune reaction eventually subsides, and the myocardium recovers. In certain patients, however, myocardial inflammation persists, resulting in ongoing myocyte damage, relentless symptomatic heart failure, or even death.5
Although a variety of diagnostic criteria have been developed and employed, the diagnosis of myocarditis is often one of exclusion. First proposed in 1986, the Dallas criteria—a histopathologic classification for myocarditis diagnosis—are based on endomyocardial biopsy, with inflammatory cellular infiltrate (with or without associated myocyte necrosis) visible on conventionally stained myocardial tissue sections.5 However, this method poses significant practical limitations, including low sensitivity (43% to 64%) and complication and death rates of 6% and 0.4%, respectively.5,6
An empiric diagnosis of myocarditis is often based on a combination of clinical findings including altered ECG, increase in myocardial enzymes, and lack of significant CAD.6 The recommended diagnostic cardiac magnetic resonance (CMR) imaging criteria for clinically suspected myocardial inflammation (ie, the Lake Louise Criteria) include at least two of the following:7
• Regional or global myocardial signal intensity increase in T2-weighted images.
• Increased global myocardial early gadolinium enhancement ratio between myocardium and skeletal muscle in gadolinium-enhanced T1-weighted images.
• At least one focal lesion with nonischemic regional distribution in inversion recovery–prepared gadolinium-enhanced T1-weighted images (“late gadolinium enhancement”).
Because of its reported high sensitivity and specificity (100% and 90%, respectively), CMR was used in the case patient to confirm the diagnosis of myocarditis.8 Specifically, CMR with contrast demonstrated normal left ventricular cavity size and mild reduction in overall left ventricular systolic function, with a visually estimated left ventricular ejection fraction of 45% to 50%. Regional hypokinesis of the mid-inferior wall and apical inferior septum was noted. Delayed contrast imaging demonstrated extensive non-CAD scarring and fibrosis, involving the basal anterior wall, basal inferior wall, and basal and midlateral wall in a pattern consistent with acute myocarditis.
Just as there is variability in the specific criteria by which the diagnosis of myocarditis can be made, the array of clinical findings with which it can manifest range from fatigue and other nonspecific symptoms to fulminant congestive heart failure and sudden death.6 Often, but not always, a viral prodrome precedes the onset of “cardiac symptoms” (eg, chest pain, dyspnea, palpitations, or syncope).5 This patient’s multiple risk factors for CAD and a suggestive, albeit atypical, history of chest discomfort, palpitations, and shortness of breath helped to focus the clinicians’ evaluation on the heart.
Potential Causes
Once a diagnosis of myocarditis is rendered, the next challenge is distinguishing its specific source from a plethora of potential etiologies, including infection, toxic exposure, or hypersensitivity/autoimmune reaction. Viral infections (mostly herpes, parvovirus, and cytomegalovirus) are thought to cause most cases of myocarditis in developed countries.5,9
Viral myocarditis results when viruses enter cardiac myocytes and incite a cytotoxic effect with activation of the immune response, including expression of interferon , natural killer cells, and release of nitric oxide. The majority of patients recover, but some develop an adaptive immune response, which further causes cardiac damage. In this response, antibodies to viral and to some cardiac proteins are produced, and effector T lymphocytes proliferate. Viral genome or inflammatory mechanisms may persist, contributing to ventricular dysfunction leading to heart failure and arrhythmias.10
Celiac disease is a chronic gastroenterologic disease caused by an immune response to a gluten protein. Damage to the brush border of the small intestine results in an inability to absorb fat, protein, vitamins, and minerals. Intermittent diarrhea, abdominal pain, and bloating are most commonly reported, but celiac disease may also manifest less obviously with iron deficiency anemia, joint pain, muscle cramps, osteoporosis, and neuropathy.11 Iron deficiency anemia that is refractory to iron replacement may offer insight into diagnosing myocarditis due to celiac disease.12 Although studies have found that more than 4% of patients with myocarditis also had celiac disease, none had the classic GI symptoms of celiac disease.12
Takotsubo cardiomyopathy is a transient left ventricular apical ballooning syndrome of unknown etiology. (For more information, see Fasolino T. Takotsubo cardiomyopathy: a clinical overview). Patients who have experienced emotional or physiologic stress and postmenopausal women appear to be at greatest risk. The clinical symptoms mimic MI, including chest pain with ST-segment elevation in the precordial leads on ECG13 and minor elevation of the cardiac enzyme and biomarker levels.14 However, patients experiencing this stress cardiomyopathy lack evidence of atherosclerotic CAD.15 An echocardiogram or CMR imaging reveals characteristic wall motion hypokinesis, akinesis, or dyskinesis of the left ventricular apex and mid-ventricle that help to differentiate it from other forms of myocarditis.15,16 Patient prognosis is favorable, with 95% of patients experiencing a full recovery; left ventricular dysfunction usually begins to improve in a few weeks.13,14
Sarcoidosis is a systemic disease resulting in noncaseating granulomas in multiple organs.17 Initial presentation typically includes bilateral hilar adenopathy, pulmonary reticular opacities, and/or skin, joint, or eye lesions.18 Patients with cardiac sarcoidosis most commonly present with conduction disturbances and ventricular arrhythmias.17 Although frequently absent, clinical symptoms may include palpitations, syncope, dizziness, or chest pain and clinical heart failure.17,18 It is difficult to distinguish cardiac sarcoidosis from other forms of myocarditis unless signs of systemic sarcoidosis are evident. A patient with suspected cardiac sarcoidosis should have an ECG to detect subclinical conduction abnormalities.17 The patient should wear a Holter monitor for 24 hours to screen for cardiac involvement, and echocardiography should be performed to define cardiac abnormalities.19
Giant-cell myocarditis (GCM) is a rare, rapidly progressive, and frequently fatal myocardial disease. Based on endomyocardial or surgical biopsy, GCM is histologically defined by multinucleated giant cells, a lymphocytic inflammatory infiltrate, and myocyte necrosis. It is often found in association with various immune-related systemic disorders.20 Patients present with heart failure, ventricular arrhythmias, and atrioventricular block that fails to improve with standard therapy.21
Treatment and Management
The typical management of acute myocarditis includes supportive care for left ventricular dysfunction and arrhythmia control.22 Many of the standard heart failure therapies—β-blockers, ACE inhibitors, angiotensin receptor blockers, and aldosterone antagonists—are efficacious; several, at least in animal models, appear to exert anti-inflammatory as well as the standard cardiovascular effects.23
Caution is advised regarding the selection of specific therapies. For example, in one study, metoprolol produced deleterious effects in acute murine Coxsackie virus myocarditis; inflammation, necrosis, and mortality significantly increased in the treatment group, compared with the placebo group.23
Information on the effects of particular therapies for specific etiologies of myocarditis are limited, but some evidence supports immunosuppressive and immune-modulating therapies for chronic, virus-negative inflammatory cardiomyopathy. Immunosuppressive therapy is also beneficial for acute GCM and sarcoidosis.23 For patients with myocarditis associated with celiac disease, a gluten-free diet alone or in combination with immunosuppressive agents can significantly improve clinical outcomes.12
OUTCOME FOR THE CASE PATIENT
Because the patient was already taking a statin and an ACE inhibitor for hypercholesterolemia and hypertension, respectively, as well as one baby aspirin per day, only a β-blocker was added to his discharge medication regimen.
Three months after hospital discharge, the patient underwent repeat CMR imaging. The ejection fraction had markedly improved to the 55%-to-60% range, although extensive midmyocardial-to-epicardial scarring in a multifocal pattern, primarily involving the basilar anterior and anterolateral wall, was still present, as was a small focus of an active (albeit healing) process in the inferior wall. Clinically, the patient was doing reasonably well and was vigorously exercising daily without dizziness, syncope, chest discomfort, or shortness of breath.
However, within several weeks of discharge, the patient reported having one two-hour episode of frequent palpitations at rest. Since that episode, palpitations have occurred infrequently. A 48-hour Holter monitor was ordered to better evaluate the palpitations and showed only rare premature ventricular contractions and isolated premature atrial contractions; no complex ectopy was noted. A follow-up stress echocardiogram was scheduled for 12 months, assuming the patient was free of clinical signs and symptoms of heart failure and arrhythmias at that time.
CONCLUSION
Myocarditis can manifest with a broad spectrum of signs and symptoms that may make its identification difficult, especially if a cardiac source is not initially considered in the differential diagnosis. However, for patients who present with elevated biomarkers and normal coronary artery anatomy, the identification of myocarditis is relatively easy; the difficulty in this circumstance relates to the identification of the specific etiology of the myocarditis.
The long-term prognosis for myocarditis is frequently good and the treatment straightforward, using medications that are modeled after standard heart failure therapy. However, depending on the etiology, specific treatment may be advisable—or required—in order to improve outcomes.
References
1. Fabre A, Sheppard MN. Sudden adult death syndrome and other nonischaemic causes of sudden cardiac death: a UK experience. Heart. 2006;92:316-320.
2. Doolan A, Semsarian C, Langlois N. Causes of sudden cardiac death in young Australians. Med J Aust. 2004;180:110-112.
3. Felker GM, Hu W, Hare JM, Hruban RH, et al. The spectrum of dilated cardiomyopathy: the Johns Hopkins experience with 1,278 patients. Medicine (Baltimore). 1999;78:270-283.
4. Leitman M, Tyomkin V, Peleg E, et al. Left ventricular function in acute inflammatory peri-myocardial diseases—new insights and long-term follow-up. Cardiovasc Ultrasound. 2012;10:42.
5. Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52:274-288.
6. Testani JM, Kolansky DM, Litt H, Gerstenfeld EP. Focal myocarditis mimicking acute ST-elevation myocardial infarction: diagnosis using cardiac magnetic resonance imaging. Tex Heart Inst J. 2006;33:256-259.
7. Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53: 1475-1487.
8. Olimulder MA, van Es J, Galjee MA. The importance of cardiac MRI as a diagnostic tool in viral myocarditis-induced cardiomyopathy. Neth Heart J. 2009;17:481-486.
9. Mavrogeni S, Bratis K, Markussis V, et al. The diagnostic role of cardiac magnetic resonance imaging in detecting myocardial inflammation in systemic lupus erythematosus. Differentiation from viral myocarditis. Lupus. 2013;22:34-43.
10. Schultz JC, Hilliard AA, Cooper LT, Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clin Proc. 2009;84:1001-1009.
11. Schuppan D, Dieterich W. Pathogenesis, epidemiology, and clinical manifestations of celiac disease in adults (2013). www.uptodate.com/contents/pathogenesis-epidemiology-and-clinical-manifestations-of-celiac-disease-in-adults. Accessed November 14, 2013.
12. Frustaci A, Cuoco L, Chimenti C, et al. Celiac disease associated with autoimmune myocarditis. Circulation. 2002;105:2611-2618.
13. Thakar S, Chandra P, Hollander G, Lichstein E. Electrocardiographic changes in Takotsubo cardiomyopathy. Pacing Clin Electrophysiol. 2011;34:
1278-1282.
14. Fefer P, Chelvanathan A, Dick A, et al. Takotsubo cardiomyopathy and left ventricular outflow tract obstruction. J Interv Cardiol. 2009;22:444-452.
15. Stensaeth KH, Fossum E, Hoffmann P, et al. Takotsubo cardiomyopathy in acute coronary syndrome; clinical features and contribution of cardiac magnetic resonance during the acute and convalescent phase. Scand Cardiovasc J. 2011;45:77-85.
16. Omerovic E. How to think about stress-induced cardiomyopathy?—Think “out of the box”! Scand Cardiovasc J. 2011;45:67-71.
17. McKenna WJ. Cardiac sarcoidosis (2013). www.uptodate.com/contents/cardiac-sarcoidosis. Accessed November 14, 2013.
18. King TE Jr. Clinical manifestations and diagnosis of sarcoidosis (2013). www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-sarcoidosis. Accessed November 14, 2013.
19. Bussinguer M, Danielian A, Sharma O. Cardiac sarcoidosis: diagnosis and management. Curr Treat Options Cardiovasc Med. 2012;14:652-664.
20. Cooper LT Jr, Berry GJ, Shabetai R; Multicenter Giant Cell Myocarditis Study Group Investigators. Idiopathic giant-cell myocarditis—natural history and treatment. N Engl J Med. 1997;336(26):1860-1866.
21. Kandolin R, Lehtonen J, Salmenkivi K, et al. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail. 2013;6:15-22.
22. Htwe TH, Khardori NM. Cardiac emergencies: infective endocarditis, pericarditis, and myocarditis. Med Clin North Am. 2012;96:1149-1169.
23. Kindermann I, Barth C, Mahfoud F, et al. Update on myocarditis. J Am Coll Cardiol. 2012;59:779-792.
A 55-year-old white man with controlled hypertension and hypercholesterolemia awoke with mild chest discomfort that he believed was mild gastroesophageal reflux. He denied radiation of pain to the shoulders, arms, back, or neck; dyspnea; palpitations; diaphoresis; nausea/vomiting; cough; or fever, during the first 30 hours of discomfort. There was no change in discomfort with deep breath, palpation of the chest, or administration of antacids. Minimal, short-lived improvement was noted with belching.
The patient had no trouble sleeping in the prone position and did not notice an increase in discomfort or unusual difficulty during his daily vigorous 30-minute aerobic workout. In fact, his symptoms seemed to improve or disappear during exercise. The patient denied any recent illness or exposure to sick people, had not traveled outside the United States, and had not been exposed to radiation of the chest wall. At the end of the second day of discomfort, the patient noted irregular palpitations with mild shortness of breath and was transported to the hospital for evaluation. He denied being a cigarette smoker or illicit drug user.
The patient had no history of MI or diabetes. The patient’s father had an MI in his 80s, and two uncles died suddenly in their 50s of “massive heart attacks.” His mother, who had died of sepsis of uncertain etiology approximately 10 days earlier, also had hypertension and hypercholesterolemia but no history of coronary artery disease (CAD). Both of the patient’s adult daughters had been diagnosed with celiac disease in the preceding three years. His elder daughter had also been diagnosed with type 1 diabetes within the past two years.
On examination, the patient was afebrile, with a blood pressure of 143/87 mm Hg; pulse, 53 beats/min; and respiratory rate, 17 breaths/min. The patient’s weight was 204 lb and his height, 75 in (BMI, 25.5). The patient was in no apparent distress. Head, eyes, ears, nose, and throat were unremarkable. There was no significant jugular venous distention. The carotid pulses were full, and no bruits were appreciated. S1 and S2 sounds were within normal limits. No murmurs or S3 or S4 gallops were appreciated. The chest was clear on auscultation. Results of the abdominal exam were negative, no edema was noted in the extremities, and pulses were symmetrical.
ECG demonstrated subtle ST-segment elevation in leads I and aVL with a prominent R wave in lead V1. This pattern was interpreted as consistent with an acute inferolateral MI. A baseline ECG, previously obtained by the patient’s internist, had been interpreted as normal.
Peak troponin level was 55 ng/mL (normal, < 0.03 ng/mL); total creatine kinase (CK), 807 U/L (reference range, 20 to 259 U/L); and mass CK-MB fraction, 44 ng/mL (0.1 to 6.6 ng/mL). Total cholesterol was 105 mg/dL, with both LDL- and HDL-cholesterol fractions at 46 mg/dL. A complete blood count without differential revealed a total white blood cell count of 53,000/µL. Hemoglobin and hematocrit were both low (12.3 g/dL and 34.5%, respectively). All indices were within normal limits, as was the platelet count. Glucose, blood urea nitrogen, creatinine, potassium chloride bicarbonate, and calcium were all within normal limits. The sodium level was slightly low (132 mEq/L). Emergency catheterization revealed an ejection fraction of 45% (reference range, 55% to 70%), with mild-to-moderate diffuse hypokinesis but normal coronary arteries.
The patient was diagnosed with myocarditis, likely of viral origin.
DISCUSSION
Although the incidence of myocarditis in the US is difficult to assess, autopsy reports implicate it in 8.6% to 12% of cases of sudden cardiac death in young adults,1,2 and a large prospective series implicated myocarditis in 9% of cases of dilated cardiomyopathy.3 Myocarditis is considered to be at one extreme of a spectrum of perimyocardial processes that result in inflammation of the myocardium (see figure), pericardium, or both.4
The underlying pathology involves an acute injury to the myocyte. This activates the innate and humoral immune systems, resulting in severe inflammation. The immune reaction eventually subsides, and the myocardium recovers. In certain patients, however, myocardial inflammation persists, resulting in ongoing myocyte damage, relentless symptomatic heart failure, or even death.5
Although a variety of diagnostic criteria have been developed and employed, the diagnosis of myocarditis is often one of exclusion. First proposed in 1986, the Dallas criteria—a histopathologic classification for myocarditis diagnosis—are based on endomyocardial biopsy, with inflammatory cellular infiltrate (with or without associated myocyte necrosis) visible on conventionally stained myocardial tissue sections.5 However, this method poses significant practical limitations, including low sensitivity (43% to 64%) and complication and death rates of 6% and 0.4%, respectively.5,6
An empiric diagnosis of myocarditis is often based on a combination of clinical findings including altered ECG, increase in myocardial enzymes, and lack of significant CAD.6 The recommended diagnostic cardiac magnetic resonance (CMR) imaging criteria for clinically suspected myocardial inflammation (ie, the Lake Louise Criteria) include at least two of the following:7
• Regional or global myocardial signal intensity increase in T2-weighted images.
• Increased global myocardial early gadolinium enhancement ratio between myocardium and skeletal muscle in gadolinium-enhanced T1-weighted images.
• At least one focal lesion with nonischemic regional distribution in inversion recovery–prepared gadolinium-enhanced T1-weighted images (“late gadolinium enhancement”).
Because of its reported high sensitivity and specificity (100% and 90%, respectively), CMR was used in the case patient to confirm the diagnosis of myocarditis.8 Specifically, CMR with contrast demonstrated normal left ventricular cavity size and mild reduction in overall left ventricular systolic function, with a visually estimated left ventricular ejection fraction of 45% to 50%. Regional hypokinesis of the mid-inferior wall and apical inferior septum was noted. Delayed contrast imaging demonstrated extensive non-CAD scarring and fibrosis, involving the basal anterior wall, basal inferior wall, and basal and midlateral wall in a pattern consistent with acute myocarditis.
Just as there is variability in the specific criteria by which the diagnosis of myocarditis can be made, the array of clinical findings with which it can manifest range from fatigue and other nonspecific symptoms to fulminant congestive heart failure and sudden death.6 Often, but not always, a viral prodrome precedes the onset of “cardiac symptoms” (eg, chest pain, dyspnea, palpitations, or syncope).5 This patient’s multiple risk factors for CAD and a suggestive, albeit atypical, history of chest discomfort, palpitations, and shortness of breath helped to focus the clinicians’ evaluation on the heart.
Potential Causes
Once a diagnosis of myocarditis is rendered, the next challenge is distinguishing its specific source from a plethora of potential etiologies, including infection, toxic exposure, or hypersensitivity/autoimmune reaction. Viral infections (mostly herpes, parvovirus, and cytomegalovirus) are thought to cause most cases of myocarditis in developed countries.5,9
Viral myocarditis results when viruses enter cardiac myocytes and incite a cytotoxic effect with activation of the immune response, including expression of interferon , natural killer cells, and release of nitric oxide. The majority of patients recover, but some develop an adaptive immune response, which further causes cardiac damage. In this response, antibodies to viral and to some cardiac proteins are produced, and effector T lymphocytes proliferate. Viral genome or inflammatory mechanisms may persist, contributing to ventricular dysfunction leading to heart failure and arrhythmias.10
Celiac disease is a chronic gastroenterologic disease caused by an immune response to a gluten protein. Damage to the brush border of the small intestine results in an inability to absorb fat, protein, vitamins, and minerals. Intermittent diarrhea, abdominal pain, and bloating are most commonly reported, but celiac disease may also manifest less obviously with iron deficiency anemia, joint pain, muscle cramps, osteoporosis, and neuropathy.11 Iron deficiency anemia that is refractory to iron replacement may offer insight into diagnosing myocarditis due to celiac disease.12 Although studies have found that more than 4% of patients with myocarditis also had celiac disease, none had the classic GI symptoms of celiac disease.12
Takotsubo cardiomyopathy is a transient left ventricular apical ballooning syndrome of unknown etiology. (For more information, see Fasolino T. Takotsubo cardiomyopathy: a clinical overview). Patients who have experienced emotional or physiologic stress and postmenopausal women appear to be at greatest risk. The clinical symptoms mimic MI, including chest pain with ST-segment elevation in the precordial leads on ECG13 and minor elevation of the cardiac enzyme and biomarker levels.14 However, patients experiencing this stress cardiomyopathy lack evidence of atherosclerotic CAD.15 An echocardiogram or CMR imaging reveals characteristic wall motion hypokinesis, akinesis, or dyskinesis of the left ventricular apex and mid-ventricle that help to differentiate it from other forms of myocarditis.15,16 Patient prognosis is favorable, with 95% of patients experiencing a full recovery; left ventricular dysfunction usually begins to improve in a few weeks.13,14
Sarcoidosis is a systemic disease resulting in noncaseating granulomas in multiple organs.17 Initial presentation typically includes bilateral hilar adenopathy, pulmonary reticular opacities, and/or skin, joint, or eye lesions.18 Patients with cardiac sarcoidosis most commonly present with conduction disturbances and ventricular arrhythmias.17 Although frequently absent, clinical symptoms may include palpitations, syncope, dizziness, or chest pain and clinical heart failure.17,18 It is difficult to distinguish cardiac sarcoidosis from other forms of myocarditis unless signs of systemic sarcoidosis are evident. A patient with suspected cardiac sarcoidosis should have an ECG to detect subclinical conduction abnormalities.17 The patient should wear a Holter monitor for 24 hours to screen for cardiac involvement, and echocardiography should be performed to define cardiac abnormalities.19
Giant-cell myocarditis (GCM) is a rare, rapidly progressive, and frequently fatal myocardial disease. Based on endomyocardial or surgical biopsy, GCM is histologically defined by multinucleated giant cells, a lymphocytic inflammatory infiltrate, and myocyte necrosis. It is often found in association with various immune-related systemic disorders.20 Patients present with heart failure, ventricular arrhythmias, and atrioventricular block that fails to improve with standard therapy.21
Treatment and Management
The typical management of acute myocarditis includes supportive care for left ventricular dysfunction and arrhythmia control.22 Many of the standard heart failure therapies—β-blockers, ACE inhibitors, angiotensin receptor blockers, and aldosterone antagonists—are efficacious; several, at least in animal models, appear to exert anti-inflammatory as well as the standard cardiovascular effects.23
Caution is advised regarding the selection of specific therapies. For example, in one study, metoprolol produced deleterious effects in acute murine Coxsackie virus myocarditis; inflammation, necrosis, and mortality significantly increased in the treatment group, compared with the placebo group.23
Information on the effects of particular therapies for specific etiologies of myocarditis are limited, but some evidence supports immunosuppressive and immune-modulating therapies for chronic, virus-negative inflammatory cardiomyopathy. Immunosuppressive therapy is also beneficial for acute GCM and sarcoidosis.23 For patients with myocarditis associated with celiac disease, a gluten-free diet alone or in combination with immunosuppressive agents can significantly improve clinical outcomes.12
OUTCOME FOR THE CASE PATIENT
Because the patient was already taking a statin and an ACE inhibitor for hypercholesterolemia and hypertension, respectively, as well as one baby aspirin per day, only a β-blocker was added to his discharge medication regimen.
Three months after hospital discharge, the patient underwent repeat CMR imaging. The ejection fraction had markedly improved to the 55%-to-60% range, although extensive midmyocardial-to-epicardial scarring in a multifocal pattern, primarily involving the basilar anterior and anterolateral wall, was still present, as was a small focus of an active (albeit healing) process in the inferior wall. Clinically, the patient was doing reasonably well and was vigorously exercising daily without dizziness, syncope, chest discomfort, or shortness of breath.
However, within several weeks of discharge, the patient reported having one two-hour episode of frequent palpitations at rest. Since that episode, palpitations have occurred infrequently. A 48-hour Holter monitor was ordered to better evaluate the palpitations and showed only rare premature ventricular contractions and isolated premature atrial contractions; no complex ectopy was noted. A follow-up stress echocardiogram was scheduled for 12 months, assuming the patient was free of clinical signs and symptoms of heart failure and arrhythmias at that time.
CONCLUSION
Myocarditis can manifest with a broad spectrum of signs and symptoms that may make its identification difficult, especially if a cardiac source is not initially considered in the differential diagnosis. However, for patients who present with elevated biomarkers and normal coronary artery anatomy, the identification of myocarditis is relatively easy; the difficulty in this circumstance relates to the identification of the specific etiology of the myocarditis.
The long-term prognosis for myocarditis is frequently good and the treatment straightforward, using medications that are modeled after standard heart failure therapy. However, depending on the etiology, specific treatment may be advisable—or required—in order to improve outcomes.
References
1. Fabre A, Sheppard MN. Sudden adult death syndrome and other nonischaemic causes of sudden cardiac death: a UK experience. Heart. 2006;92:316-320.
2. Doolan A, Semsarian C, Langlois N. Causes of sudden cardiac death in young Australians. Med J Aust. 2004;180:110-112.
3. Felker GM, Hu W, Hare JM, Hruban RH, et al. The spectrum of dilated cardiomyopathy: the Johns Hopkins experience with 1,278 patients. Medicine (Baltimore). 1999;78:270-283.
4. Leitman M, Tyomkin V, Peleg E, et al. Left ventricular function in acute inflammatory peri-myocardial diseases—new insights and long-term follow-up. Cardiovasc Ultrasound. 2012;10:42.
5. Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52:274-288.
6. Testani JM, Kolansky DM, Litt H, Gerstenfeld EP. Focal myocarditis mimicking acute ST-elevation myocardial infarction: diagnosis using cardiac magnetic resonance imaging. Tex Heart Inst J. 2006;33:256-259.
7. Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53: 1475-1487.
8. Olimulder MA, van Es J, Galjee MA. The importance of cardiac MRI as a diagnostic tool in viral myocarditis-induced cardiomyopathy. Neth Heart J. 2009;17:481-486.
9. Mavrogeni S, Bratis K, Markussis V, et al. The diagnostic role of cardiac magnetic resonance imaging in detecting myocardial inflammation in systemic lupus erythematosus. Differentiation from viral myocarditis. Lupus. 2013;22:34-43.
10. Schultz JC, Hilliard AA, Cooper LT, Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clin Proc. 2009;84:1001-1009.
11. Schuppan D, Dieterich W. Pathogenesis, epidemiology, and clinical manifestations of celiac disease in adults (2013). www.uptodate.com/contents/pathogenesis-epidemiology-and-clinical-manifestations-of-celiac-disease-in-adults. Accessed November 14, 2013.
12. Frustaci A, Cuoco L, Chimenti C, et al. Celiac disease associated with autoimmune myocarditis. Circulation. 2002;105:2611-2618.
13. Thakar S, Chandra P, Hollander G, Lichstein E. Electrocardiographic changes in Takotsubo cardiomyopathy. Pacing Clin Electrophysiol. 2011;34:
1278-1282.
14. Fefer P, Chelvanathan A, Dick A, et al. Takotsubo cardiomyopathy and left ventricular outflow tract obstruction. J Interv Cardiol. 2009;22:444-452.
15. Stensaeth KH, Fossum E, Hoffmann P, et al. Takotsubo cardiomyopathy in acute coronary syndrome; clinical features and contribution of cardiac magnetic resonance during the acute and convalescent phase. Scand Cardiovasc J. 2011;45:77-85.
16. Omerovic E. How to think about stress-induced cardiomyopathy?—Think “out of the box”! Scand Cardiovasc J. 2011;45:67-71.
17. McKenna WJ. Cardiac sarcoidosis (2013). www.uptodate.com/contents/cardiac-sarcoidosis. Accessed November 14, 2013.
18. King TE Jr. Clinical manifestations and diagnosis of sarcoidosis (2013). www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-sarcoidosis. Accessed November 14, 2013.
19. Bussinguer M, Danielian A, Sharma O. Cardiac sarcoidosis: diagnosis and management. Curr Treat Options Cardiovasc Med. 2012;14:652-664.
20. Cooper LT Jr, Berry GJ, Shabetai R; Multicenter Giant Cell Myocarditis Study Group Investigators. Idiopathic giant-cell myocarditis—natural history and treatment. N Engl J Med. 1997;336(26):1860-1866.
21. Kandolin R, Lehtonen J, Salmenkivi K, et al. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail. 2013;6:15-22.
22. Htwe TH, Khardori NM. Cardiac emergencies: infective endocarditis, pericarditis, and myocarditis. Med Clin North Am. 2012;96:1149-1169.
23. Kindermann I, Barth C, Mahfoud F, et al. Update on myocarditis. J Am Coll Cardiol. 2012;59:779-792.
A 55-year-old white man with controlled hypertension and hypercholesterolemia awoke with mild chest discomfort that he believed was mild gastroesophageal reflux. He denied radiation of pain to the shoulders, arms, back, or neck; dyspnea; palpitations; diaphoresis; nausea/vomiting; cough; or fever, during the first 30 hours of discomfort. There was no change in discomfort with deep breath, palpation of the chest, or administration of antacids. Minimal, short-lived improvement was noted with belching.
The patient had no trouble sleeping in the prone position and did not notice an increase in discomfort or unusual difficulty during his daily vigorous 30-minute aerobic workout. In fact, his symptoms seemed to improve or disappear during exercise. The patient denied any recent illness or exposure to sick people, had not traveled outside the United States, and had not been exposed to radiation of the chest wall. At the end of the second day of discomfort, the patient noted irregular palpitations with mild shortness of breath and was transported to the hospital for evaluation. He denied being a cigarette smoker or illicit drug user.
The patient had no history of MI or diabetes. The patient’s father had an MI in his 80s, and two uncles died suddenly in their 50s of “massive heart attacks.” His mother, who had died of sepsis of uncertain etiology approximately 10 days earlier, also had hypertension and hypercholesterolemia but no history of coronary artery disease (CAD). Both of the patient’s adult daughters had been diagnosed with celiac disease in the preceding three years. His elder daughter had also been diagnosed with type 1 diabetes within the past two years.
On examination, the patient was afebrile, with a blood pressure of 143/87 mm Hg; pulse, 53 beats/min; and respiratory rate, 17 breaths/min. The patient’s weight was 204 lb and his height, 75 in (BMI, 25.5). The patient was in no apparent distress. Head, eyes, ears, nose, and throat were unremarkable. There was no significant jugular venous distention. The carotid pulses were full, and no bruits were appreciated. S1 and S2 sounds were within normal limits. No murmurs or S3 or S4 gallops were appreciated. The chest was clear on auscultation. Results of the abdominal exam were negative, no edema was noted in the extremities, and pulses were symmetrical.
ECG demonstrated subtle ST-segment elevation in leads I and aVL with a prominent R wave in lead V1. This pattern was interpreted as consistent with an acute inferolateral MI. A baseline ECG, previously obtained by the patient’s internist, had been interpreted as normal.
Peak troponin level was 55 ng/mL (normal, < 0.03 ng/mL); total creatine kinase (CK), 807 U/L (reference range, 20 to 259 U/L); and mass CK-MB fraction, 44 ng/mL (0.1 to 6.6 ng/mL). Total cholesterol was 105 mg/dL, with both LDL- and HDL-cholesterol fractions at 46 mg/dL. A complete blood count without differential revealed a total white blood cell count of 53,000/µL. Hemoglobin and hematocrit were both low (12.3 g/dL and 34.5%, respectively). All indices were within normal limits, as was the platelet count. Glucose, blood urea nitrogen, creatinine, potassium chloride bicarbonate, and calcium were all within normal limits. The sodium level was slightly low (132 mEq/L). Emergency catheterization revealed an ejection fraction of 45% (reference range, 55% to 70%), with mild-to-moderate diffuse hypokinesis but normal coronary arteries.
The patient was diagnosed with myocarditis, likely of viral origin.
DISCUSSION
Although the incidence of myocarditis in the US is difficult to assess, autopsy reports implicate it in 8.6% to 12% of cases of sudden cardiac death in young adults,1,2 and a large prospective series implicated myocarditis in 9% of cases of dilated cardiomyopathy.3 Myocarditis is considered to be at one extreme of a spectrum of perimyocardial processes that result in inflammation of the myocardium (see figure), pericardium, or both.4
The underlying pathology involves an acute injury to the myocyte. This activates the innate and humoral immune systems, resulting in severe inflammation. The immune reaction eventually subsides, and the myocardium recovers. In certain patients, however, myocardial inflammation persists, resulting in ongoing myocyte damage, relentless symptomatic heart failure, or even death.5
Although a variety of diagnostic criteria have been developed and employed, the diagnosis of myocarditis is often one of exclusion. First proposed in 1986, the Dallas criteria—a histopathologic classification for myocarditis diagnosis—are based on endomyocardial biopsy, with inflammatory cellular infiltrate (with or without associated myocyte necrosis) visible on conventionally stained myocardial tissue sections.5 However, this method poses significant practical limitations, including low sensitivity (43% to 64%) and complication and death rates of 6% and 0.4%, respectively.5,6
An empiric diagnosis of myocarditis is often based on a combination of clinical findings including altered ECG, increase in myocardial enzymes, and lack of significant CAD.6 The recommended diagnostic cardiac magnetic resonance (CMR) imaging criteria for clinically suspected myocardial inflammation (ie, the Lake Louise Criteria) include at least two of the following:7
• Regional or global myocardial signal intensity increase in T2-weighted images.
• Increased global myocardial early gadolinium enhancement ratio between myocardium and skeletal muscle in gadolinium-enhanced T1-weighted images.
• At least one focal lesion with nonischemic regional distribution in inversion recovery–prepared gadolinium-enhanced T1-weighted images (“late gadolinium enhancement”).
Because of its reported high sensitivity and specificity (100% and 90%, respectively), CMR was used in the case patient to confirm the diagnosis of myocarditis.8 Specifically, CMR with contrast demonstrated normal left ventricular cavity size and mild reduction in overall left ventricular systolic function, with a visually estimated left ventricular ejection fraction of 45% to 50%. Regional hypokinesis of the mid-inferior wall and apical inferior septum was noted. Delayed contrast imaging demonstrated extensive non-CAD scarring and fibrosis, involving the basal anterior wall, basal inferior wall, and basal and midlateral wall in a pattern consistent with acute myocarditis.
Just as there is variability in the specific criteria by which the diagnosis of myocarditis can be made, the array of clinical findings with which it can manifest range from fatigue and other nonspecific symptoms to fulminant congestive heart failure and sudden death.6 Often, but not always, a viral prodrome precedes the onset of “cardiac symptoms” (eg, chest pain, dyspnea, palpitations, or syncope).5 This patient’s multiple risk factors for CAD and a suggestive, albeit atypical, history of chest discomfort, palpitations, and shortness of breath helped to focus the clinicians’ evaluation on the heart.
Potential Causes
Once a diagnosis of myocarditis is rendered, the next challenge is distinguishing its specific source from a plethora of potential etiologies, including infection, toxic exposure, or hypersensitivity/autoimmune reaction. Viral infections (mostly herpes, parvovirus, and cytomegalovirus) are thought to cause most cases of myocarditis in developed countries.5,9
Viral myocarditis results when viruses enter cardiac myocytes and incite a cytotoxic effect with activation of the immune response, including expression of interferon , natural killer cells, and release of nitric oxide. The majority of patients recover, but some develop an adaptive immune response, which further causes cardiac damage. In this response, antibodies to viral and to some cardiac proteins are produced, and effector T lymphocytes proliferate. Viral genome or inflammatory mechanisms may persist, contributing to ventricular dysfunction leading to heart failure and arrhythmias.10
Celiac disease is a chronic gastroenterologic disease caused by an immune response to a gluten protein. Damage to the brush border of the small intestine results in an inability to absorb fat, protein, vitamins, and minerals. Intermittent diarrhea, abdominal pain, and bloating are most commonly reported, but celiac disease may also manifest less obviously with iron deficiency anemia, joint pain, muscle cramps, osteoporosis, and neuropathy.11 Iron deficiency anemia that is refractory to iron replacement may offer insight into diagnosing myocarditis due to celiac disease.12 Although studies have found that more than 4% of patients with myocarditis also had celiac disease, none had the classic GI symptoms of celiac disease.12
Takotsubo cardiomyopathy is a transient left ventricular apical ballooning syndrome of unknown etiology. (For more information, see Fasolino T. Takotsubo cardiomyopathy: a clinical overview). Patients who have experienced emotional or physiologic stress and postmenopausal women appear to be at greatest risk. The clinical symptoms mimic MI, including chest pain with ST-segment elevation in the precordial leads on ECG13 and minor elevation of the cardiac enzyme and biomarker levels.14 However, patients experiencing this stress cardiomyopathy lack evidence of atherosclerotic CAD.15 An echocardiogram or CMR imaging reveals characteristic wall motion hypokinesis, akinesis, or dyskinesis of the left ventricular apex and mid-ventricle that help to differentiate it from other forms of myocarditis.15,16 Patient prognosis is favorable, with 95% of patients experiencing a full recovery; left ventricular dysfunction usually begins to improve in a few weeks.13,14
Sarcoidosis is a systemic disease resulting in noncaseating granulomas in multiple organs.17 Initial presentation typically includes bilateral hilar adenopathy, pulmonary reticular opacities, and/or skin, joint, or eye lesions.18 Patients with cardiac sarcoidosis most commonly present with conduction disturbances and ventricular arrhythmias.17 Although frequently absent, clinical symptoms may include palpitations, syncope, dizziness, or chest pain and clinical heart failure.17,18 It is difficult to distinguish cardiac sarcoidosis from other forms of myocarditis unless signs of systemic sarcoidosis are evident. A patient with suspected cardiac sarcoidosis should have an ECG to detect subclinical conduction abnormalities.17 The patient should wear a Holter monitor for 24 hours to screen for cardiac involvement, and echocardiography should be performed to define cardiac abnormalities.19
Giant-cell myocarditis (GCM) is a rare, rapidly progressive, and frequently fatal myocardial disease. Based on endomyocardial or surgical biopsy, GCM is histologically defined by multinucleated giant cells, a lymphocytic inflammatory infiltrate, and myocyte necrosis. It is often found in association with various immune-related systemic disorders.20 Patients present with heart failure, ventricular arrhythmias, and atrioventricular block that fails to improve with standard therapy.21
Treatment and Management
The typical management of acute myocarditis includes supportive care for left ventricular dysfunction and arrhythmia control.22 Many of the standard heart failure therapies—β-blockers, ACE inhibitors, angiotensin receptor blockers, and aldosterone antagonists—are efficacious; several, at least in animal models, appear to exert anti-inflammatory as well as the standard cardiovascular effects.23
Caution is advised regarding the selection of specific therapies. For example, in one study, metoprolol produced deleterious effects in acute murine Coxsackie virus myocarditis; inflammation, necrosis, and mortality significantly increased in the treatment group, compared with the placebo group.23
Information on the effects of particular therapies for specific etiologies of myocarditis are limited, but some evidence supports immunosuppressive and immune-modulating therapies for chronic, virus-negative inflammatory cardiomyopathy. Immunosuppressive therapy is also beneficial for acute GCM and sarcoidosis.23 For patients with myocarditis associated with celiac disease, a gluten-free diet alone or in combination with immunosuppressive agents can significantly improve clinical outcomes.12
OUTCOME FOR THE CASE PATIENT
Because the patient was already taking a statin and an ACE inhibitor for hypercholesterolemia and hypertension, respectively, as well as one baby aspirin per day, only a β-blocker was added to his discharge medication regimen.
Three months after hospital discharge, the patient underwent repeat CMR imaging. The ejection fraction had markedly improved to the 55%-to-60% range, although extensive midmyocardial-to-epicardial scarring in a multifocal pattern, primarily involving the basilar anterior and anterolateral wall, was still present, as was a small focus of an active (albeit healing) process in the inferior wall. Clinically, the patient was doing reasonably well and was vigorously exercising daily without dizziness, syncope, chest discomfort, or shortness of breath.
However, within several weeks of discharge, the patient reported having one two-hour episode of frequent palpitations at rest. Since that episode, palpitations have occurred infrequently. A 48-hour Holter monitor was ordered to better evaluate the palpitations and showed only rare premature ventricular contractions and isolated premature atrial contractions; no complex ectopy was noted. A follow-up stress echocardiogram was scheduled for 12 months, assuming the patient was free of clinical signs and symptoms of heart failure and arrhythmias at that time.
CONCLUSION
Myocarditis can manifest with a broad spectrum of signs and symptoms that may make its identification difficult, especially if a cardiac source is not initially considered in the differential diagnosis. However, for patients who present with elevated biomarkers and normal coronary artery anatomy, the identification of myocarditis is relatively easy; the difficulty in this circumstance relates to the identification of the specific etiology of the myocarditis.
The long-term prognosis for myocarditis is frequently good and the treatment straightforward, using medications that are modeled after standard heart failure therapy. However, depending on the etiology, specific treatment may be advisable—or required—in order to improve outcomes.
References
1. Fabre A, Sheppard MN. Sudden adult death syndrome and other nonischaemic causes of sudden cardiac death: a UK experience. Heart. 2006;92:316-320.
2. Doolan A, Semsarian C, Langlois N. Causes of sudden cardiac death in young Australians. Med J Aust. 2004;180:110-112.
3. Felker GM, Hu W, Hare JM, Hruban RH, et al. The spectrum of dilated cardiomyopathy: the Johns Hopkins experience with 1,278 patients. Medicine (Baltimore). 1999;78:270-283.
4. Leitman M, Tyomkin V, Peleg E, et al. Left ventricular function in acute inflammatory peri-myocardial diseases—new insights and long-term follow-up. Cardiovasc Ultrasound. 2012;10:42.
5. Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52:274-288.
6. Testani JM, Kolansky DM, Litt H, Gerstenfeld EP. Focal myocarditis mimicking acute ST-elevation myocardial infarction: diagnosis using cardiac magnetic resonance imaging. Tex Heart Inst J. 2006;33:256-259.
7. Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53: 1475-1487.
8. Olimulder MA, van Es J, Galjee MA. The importance of cardiac MRI as a diagnostic tool in viral myocarditis-induced cardiomyopathy. Neth Heart J. 2009;17:481-486.
9. Mavrogeni S, Bratis K, Markussis V, et al. The diagnostic role of cardiac magnetic resonance imaging in detecting myocardial inflammation in systemic lupus erythematosus. Differentiation from viral myocarditis. Lupus. 2013;22:34-43.
10. Schultz JC, Hilliard AA, Cooper LT, Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clin Proc. 2009;84:1001-1009.
11. Schuppan D, Dieterich W. Pathogenesis, epidemiology, and clinical manifestations of celiac disease in adults (2013). www.uptodate.com/contents/pathogenesis-epidemiology-and-clinical-manifestations-of-celiac-disease-in-adults. Accessed November 14, 2013.
12. Frustaci A, Cuoco L, Chimenti C, et al. Celiac disease associated with autoimmune myocarditis. Circulation. 2002;105:2611-2618.
13. Thakar S, Chandra P, Hollander G, Lichstein E. Electrocardiographic changes in Takotsubo cardiomyopathy. Pacing Clin Electrophysiol. 2011;34:
1278-1282.
14. Fefer P, Chelvanathan A, Dick A, et al. Takotsubo cardiomyopathy and left ventricular outflow tract obstruction. J Interv Cardiol. 2009;22:444-452.
15. Stensaeth KH, Fossum E, Hoffmann P, et al. Takotsubo cardiomyopathy in acute coronary syndrome; clinical features and contribution of cardiac magnetic resonance during the acute and convalescent phase. Scand Cardiovasc J. 2011;45:77-85.
16. Omerovic E. How to think about stress-induced cardiomyopathy?—Think “out of the box”! Scand Cardiovasc J. 2011;45:67-71.
17. McKenna WJ. Cardiac sarcoidosis (2013). www.uptodate.com/contents/cardiac-sarcoidosis. Accessed November 14, 2013.
18. King TE Jr. Clinical manifestations and diagnosis of sarcoidosis (2013). www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-sarcoidosis. Accessed November 14, 2013.
19. Bussinguer M, Danielian A, Sharma O. Cardiac sarcoidosis: diagnosis and management. Curr Treat Options Cardiovasc Med. 2012;14:652-664.
20. Cooper LT Jr, Berry GJ, Shabetai R; Multicenter Giant Cell Myocarditis Study Group Investigators. Idiopathic giant-cell myocarditis—natural history and treatment. N Engl J Med. 1997;336(26):1860-1866.
21. Kandolin R, Lehtonen J, Salmenkivi K, et al. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail. 2013;6:15-22.
22. Htwe TH, Khardori NM. Cardiac emergencies: infective endocarditis, pericarditis, and myocarditis. Med Clin North Am. 2012;96:1149-1169.
23. Kindermann I, Barth C, Mahfoud F, et al. Update on myocarditis. J Am Coll Cardiol. 2012;59:779-792.