User login
Rocky Mountain Spotted Fever Presenting as Febrile Pancytopenia
Cover
Tres Pasitos: "Three Little Steps" to Aldicarb Poisoning
Rhabdomyolysis: Evaluation and Emergent Management
2012 Update on Obstetrics
The authors report no financial relationships relevant to this article.
The year that has followed our inaugural “Update on Obstetrics” [OBG Management, January 2011, available at www.obgmanagement.com] saw a resurgence of interest in a number of aspects of obstetric care. We want to highlight four of them in this Update because we think they are particularly important—given the attention they’ve received in the medical literature and in the consumer media:
- the ever-increasing cesarean delivery rate
- home birth
- postpartum hemorrhage
- measurement of cervical length and the use of progesterone.
Taming the cesarean delivery rate—how can we
accomplish this?
No one should be surprised to learn that the cesarean delivery rate increased nearly sevenfold from 1970 to 2011—from a rate of approximately 5% in 1970 to nearly 35%. Recall that, in the 1990s, the US Public Health Service proposed, as part of Healthy People 2010, a target cesarean rate of 15%, with a vaginal birth after cesarean (VBAC) rate of 60%. Today, the cesarean delivery rate is, as we said, nearly 35% and the VBAC rate is less than 10%.
Many factors have been cited for the rise, including:
- the obesity pandemic
- delaying childbearing
- increasing use of assisted reproduction
- multiple gestation (although the incidence of higher-order multiple gestations is now decreasing, the rate of twin births remains quite high relative to past decades).
So, how did this happen? And what can we do?
For one, VBAC is not likely to gain in popularity. More than 60% of US hospitals that provide OB services handle a volume of fewer than 1,000 deliveries a year. Such low volumes generally will not be able to support (either with dollars or staffing) the resources needed to safely provide VBAC.
Other options have been proposed: Loosen the guidelines for VBAC, change the personnel requirements, gather community groups of doctors, attorneys, and patients to agree on guidelines that, if followed, would protect physicians from being sued1—and so on. The medicolegal reality, however, is that these options have not been shown to be viable. We have concluded that increasing VBAC utilization is not the answer. Rather, addressing ways to prevent primary cesarean delivery holds the most promise for, ultimately, reducing the current rising trend.
On a positive note: The most recent data available from the National Center for Health Statistics suggest that the cesarean delivery rate has dropped slightly: from 32.9% in 2009 to 32.8% in 2010. The drop is truly slight; we’ll watch with interest to see if a trend has begun.
Considering that the cesarean delivery rate in 1970 was 5%, and that the dictum at the time was “once a section, always a section,” it seems clear (to us, at least) that the solution to this problem lies in preventing first cesarean deliveries. How can the specialty and, in some ways, you, in your practice, work toward this goal? Here are possible strategies:
- Eliminate elective inductions of labor when the modified Bishop score is less than 8
- Return to defining “post-term” as 42—not 41—completed weeks’ gestation
- Eliminate all elective inductions before 39 weeks’ gestation
- Provide better and more standardized training of physicians in the interpretation of fetal heart-rate tracings
- Improve communication between obstetricians and anesthesiologists in regard to managing pain during labor
- Institute mandatory review of all cesarean deliveries that are performed in the latent phase of labor and all so-called “stat cesareans”
- Readjust the compensation scale for physicians and hospitals in such a way that successful vaginal delivery is rewarded.
Even if all these measures were implemented, we think it’s unlikely that we will ever see a 5% cesarean delivery rate again—although probably for good reason. But even a return to a more manageable 20% rate seems a reasonable goal.
Home birth: Consider where you stand
We suppose that one way to avoid cesarean delivery would be to deliver at home. The topic, and practice, of home birth has mushroomed in the past few years—for a number of social and economic reasons, probably. It seems to us that there are a few basic issues that must be addressed, however, before it’s possible to come to grips with home birth in the 21st century in an enlightened way:
- In 1935, the maternal mortality rate approached 500 to 600 for every 100,000 births; most of those deaths occurred at home. In 2009, the maternal mortality rate was approximately 8 for every 100,000 births. Both rates are very low, but the difference would be significant to the 492 to 592 women who met a potentially preventable death.
- Methods of identifying who might be an appropriate candidate for a home birth are, at best, imprecise.
- Infrastructure for rapidly transporting mother and baby to a hospital if matters go awry is inadequate.
Although evidence is limited, what data there are suggest that one significant outcome—neonatal death—occurs with higher frequency among home births than among hospital births, even after correcting for anomalies (odds ratio, 2.9 [95% confidence interval, 1.3–6.2]).2 Although women who delivered at home did have fewer episiotomies, fewer third- and fourth-degree perineal lacerations, fewer operative deliveries (vaginal and cesarean) and a lower rate of infection, those reductions seem inconsequential compared to the death of a newborn….
Bottom line? Home birth is legal; home birth may be appropriate for some women who are at low risk and willing to accept a legitimate amount of personal risk; and you, as an OB, are in no way required to participate in or endorse the practice.
Many institutions have addressed this matter by developing a family-centered health care model for obstetrics—so-called hospitals within hospitals—that allow for a less interventionist approach to childbirth within the safety net of a hospital facility, should unforeseeable complications arise. Consider your interest in affiliating with such a facility, based on your acceptance of the practice of home birth and your comfort with being part of this approach.
Formal, systematic planning is key to managing
postpartum hemorrhage
A question for mothers-to-be: What could be worse than having a cesarean delivery in your home?
Answer: Having an associated postpartum bleed.
Perhaps that isn’t the most elegant segue, but postpartum hemorrhage is a significant problem that remains a major contributor to maternal mortality in the United States. And, in fact, a prolonged and unsuccessful labor—the kind that could present to your hospital from an outside birthing facility or home—necessitating a cesarean delivery is a set-up for postpartum hemorrhage.
One of the tenets of emergency management in obstetrics is the three-pronged preparedness of 1) risk identification, 2) foreseeability, and 3) having a plan for taking action. Of late, many institutions have begun to develop a formal plan for managing OB emergencies—in particular, postpartum hemorrhage.
(A note about the potential role of interventional radiology in the management of postpartum hemorrhage: Our experience is limited, but we conjecture that, in most US hospitals that provide OB services, mobilizing an interventional radiology group in an emergency isn’t feasible. That makes it essential to have established medical and surgical management guidelines for such cases.)
To establish a plan on labor and delivery for managing postpartum bleeds, we recommend the following steps and direct you to ACOG’s “Practice Bulletin#76” for more specific information3:
- Establish a list of conditions that predispose a woman to postpartum hemorrhage and post that list throughout labor and delivery to heighten the awareness of team members
- Establish protocols for pharmacotherapeutic intervention—including oxytocin, methylergonovine, misoprostol, and prostaglandin F2a, with dosage and frequency guidelines and algorithms for use—and have those protocols readily available on labor and delivery, either on-line or posted
- Establish an “all-hands-on-deck” protocol for surgical emergencies—actual or potential—that includes what personnel to call and in what order to call them
- Use simulation to practice the all-hands-on-deck protocol and evaluate team and individual performance in managing hemorrhage
- Establish blood product replacement protocols, including order sets for products that are linked to particular diagnoses (e.g., typing and cross-matching for patients coming in to deliver who have a diagnosis of placenta previa; adding products such as fresh frozen plasma and platelets for patients who have complicating diagnoses, such as suspected placenta accreta or severe preeclampsia).
To prevent preterm birth: Cervical length screening
and progesterone
Preterm birth accounts for almost 13% of births in the United States, with spontaneous preterm labor and preterm rupture of membranes accounting for approximately 80% of those cases.4 Once preterm labor has begun, little in the way of successful intervention is possible, beyond short-term prolongation of pregnancy with tocolytic agents to allow for corticosteroid administration. Studies in recent years have, therefore, moved the focus back on prevention, using the same treatments that were used 60 years ago—progesterone supplementation and cerclage—with the addition of transvaginal ultrasonography (US) screening for cervical length.
Several large, randomized trials have examined the use of intramuscular injection or vaginal delivery of progesterone to prevent preterm birth in patients who are at high risk of preterm birth based on their obstetric history.5,6 Both 17a-hydroxyprogesterone caproate and vaginal progesterone suppositories are associated with a significant reduction in the risk of preterm birth in singleton pregnancies. ACOG reconfirmed the value of this finding in a 2011 Committee Opinion, which recommended the use of progesterone supplementation in singleton pregnancies in which there is a history of preterm labor or preterm rupture of membranes.7
There is mounting evidence that cervical length is inversely related to risk of preterm birth.The real question, however, is: What should be done about transvaginal cervical length: Should we be screening, or not? As recently as 2009, a Cochrane Review did not advocate universal screening for cervical length as a predictor for preterm birth4—despite mounting evidence that cervical length is inversely related to risk of preterm birth, with progressively shorter length (starting at <25 mm) associated with significantly higher risk of preterm birth.8,9 Keeping in mind that the decision to screen depends on your ability to treat the condition for which you are screening, what was needed was proof that intervention works.
2011 brought two studies that recommend screening for cervical length based on a successful reduction in preterm birth with a specific intervention. A large, randomized trial of vaginal progesterone gel for the prevention of preterm birth used universal screening for shortened cervical length (10 to 20 mm) as the criterion for randomization to treatment or placebo. The investigators demonstrated a 45% reduction in preterm birth of less than 33 weeks in the treatment arm.10
An interesting aspect of this study: The reduction in preterm birth was not, in fact, seen in patients who had a history of preterm birth, suggesting that this may be a different patient population that benefits from vaginal progesterone.
On the other hand, a recent meta-analysis concluded that patients who meet the criteria of 1) cervical length less than 25 mm and 2) a history of prior spontaneous preterm birth experience a significant reduction in preterm birth and a reduction in perinatal morbidity and mortality if they have cervical cerclage placed.11
Although these publications lead us to hope that there may be some benefit from preventive intervention for preterm birth, the question of how to screen for, and prevent, spontaneous preterm birth remains somewhat nebulous: It hasn’t been determined which patient population will benefit from which combination of screening and intervention. Larger trials for specific populations are still needed.
This is what we know, for now:
- Women who have a history of spontaneous preterm birth should have a thorough evaluation of their OB history to determine possible modifiable risk factors (e.g., smoking, short inter-pregnancy interval) and to determine, as definitively as possible, the likely cause of that preterm birth
- Women who have a singleton pregnancy and a history of either spontaneous preterm labor or preterm rupture of membranes can be offered progesterone supplementation as intramuscular 17a-hydroxyprogesterone or a vaginal preparation to reduce their risk of preterm birth
- Women who have an asymptomatic shortening of the cervix, as measured on transvaginal US at 18 to 24 weeks’ gestation, can be offered vaginal progesterone to reduce their risk of preterm birth
- Women who have a history of preterm birth and cervical shortening may see a reduction in their risk of preterm birth from cerclage placement
- The use of screening for cervical length or progesterone supplementation, or both, in a multiple gestation pregnancy are not recommended because their benefit in this population has not been demonstrated.
Until we fully understand the various etiologic pathways of spontaneous preterm birth, we won’t have a one-size-fits-all solution to this major cause of perinatal morbidity and mortality.
We want to hear from you! Tell us what you think.
1. Scott JR. Vaginal birth after cesarean delivery; a common sense approach. Obstet Gynecol. 2011;118(2 Pt 1):342-350.
2. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425-428.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039-1047.
4. Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery (Review). Cochrane Database Syst Rev. 2009;(3):CD007235.-
5. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419-424.
6. Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
7. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963-935.
8. Owen J, Yost N, Berghella V, et al:. National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340-1348.
9. Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193(3):1170-1174.
10. Hassan SS, Romero R, Vidyadhari D, et al. PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18-31.
11. Berghella V, Rafael TJ, Szychowski M, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth. Obstet Gynecol. 2011;117(3):663-771
The authors report no financial relationships relevant to this article.
The year that has followed our inaugural “Update on Obstetrics” [OBG Management, January 2011, available at www.obgmanagement.com] saw a resurgence of interest in a number of aspects of obstetric care. We want to highlight four of them in this Update because we think they are particularly important—given the attention they’ve received in the medical literature and in the consumer media:
- the ever-increasing cesarean delivery rate
- home birth
- postpartum hemorrhage
- measurement of cervical length and the use of progesterone.
Taming the cesarean delivery rate—how can we
accomplish this?
No one should be surprised to learn that the cesarean delivery rate increased nearly sevenfold from 1970 to 2011—from a rate of approximately 5% in 1970 to nearly 35%. Recall that, in the 1990s, the US Public Health Service proposed, as part of Healthy People 2010, a target cesarean rate of 15%, with a vaginal birth after cesarean (VBAC) rate of 60%. Today, the cesarean delivery rate is, as we said, nearly 35% and the VBAC rate is less than 10%.
Many factors have been cited for the rise, including:
- the obesity pandemic
- delaying childbearing
- increasing use of assisted reproduction
- multiple gestation (although the incidence of higher-order multiple gestations is now decreasing, the rate of twin births remains quite high relative to past decades).
So, how did this happen? And what can we do?
For one, VBAC is not likely to gain in popularity. More than 60% of US hospitals that provide OB services handle a volume of fewer than 1,000 deliveries a year. Such low volumes generally will not be able to support (either with dollars or staffing) the resources needed to safely provide VBAC.
Other options have been proposed: Loosen the guidelines for VBAC, change the personnel requirements, gather community groups of doctors, attorneys, and patients to agree on guidelines that, if followed, would protect physicians from being sued1—and so on. The medicolegal reality, however, is that these options have not been shown to be viable. We have concluded that increasing VBAC utilization is not the answer. Rather, addressing ways to prevent primary cesarean delivery holds the most promise for, ultimately, reducing the current rising trend.
On a positive note: The most recent data available from the National Center for Health Statistics suggest that the cesarean delivery rate has dropped slightly: from 32.9% in 2009 to 32.8% in 2010. The drop is truly slight; we’ll watch with interest to see if a trend has begun.
Considering that the cesarean delivery rate in 1970 was 5%, and that the dictum at the time was “once a section, always a section,” it seems clear (to us, at least) that the solution to this problem lies in preventing first cesarean deliveries. How can the specialty and, in some ways, you, in your practice, work toward this goal? Here are possible strategies:
- Eliminate elective inductions of labor when the modified Bishop score is less than 8
- Return to defining “post-term” as 42—not 41—completed weeks’ gestation
- Eliminate all elective inductions before 39 weeks’ gestation
- Provide better and more standardized training of physicians in the interpretation of fetal heart-rate tracings
- Improve communication between obstetricians and anesthesiologists in regard to managing pain during labor
- Institute mandatory review of all cesarean deliveries that are performed in the latent phase of labor and all so-called “stat cesareans”
- Readjust the compensation scale for physicians and hospitals in such a way that successful vaginal delivery is rewarded.
Even if all these measures were implemented, we think it’s unlikely that we will ever see a 5% cesarean delivery rate again—although probably for good reason. But even a return to a more manageable 20% rate seems a reasonable goal.
Home birth: Consider where you stand
We suppose that one way to avoid cesarean delivery would be to deliver at home. The topic, and practice, of home birth has mushroomed in the past few years—for a number of social and economic reasons, probably. It seems to us that there are a few basic issues that must be addressed, however, before it’s possible to come to grips with home birth in the 21st century in an enlightened way:
- In 1935, the maternal mortality rate approached 500 to 600 for every 100,000 births; most of those deaths occurred at home. In 2009, the maternal mortality rate was approximately 8 for every 100,000 births. Both rates are very low, but the difference would be significant to the 492 to 592 women who met a potentially preventable death.
- Methods of identifying who might be an appropriate candidate for a home birth are, at best, imprecise.
- Infrastructure for rapidly transporting mother and baby to a hospital if matters go awry is inadequate.
Although evidence is limited, what data there are suggest that one significant outcome—neonatal death—occurs with higher frequency among home births than among hospital births, even after correcting for anomalies (odds ratio, 2.9 [95% confidence interval, 1.3–6.2]).2 Although women who delivered at home did have fewer episiotomies, fewer third- and fourth-degree perineal lacerations, fewer operative deliveries (vaginal and cesarean) and a lower rate of infection, those reductions seem inconsequential compared to the death of a newborn….
Bottom line? Home birth is legal; home birth may be appropriate for some women who are at low risk and willing to accept a legitimate amount of personal risk; and you, as an OB, are in no way required to participate in or endorse the practice.
Many institutions have addressed this matter by developing a family-centered health care model for obstetrics—so-called hospitals within hospitals—that allow for a less interventionist approach to childbirth within the safety net of a hospital facility, should unforeseeable complications arise. Consider your interest in affiliating with such a facility, based on your acceptance of the practice of home birth and your comfort with being part of this approach.
Formal, systematic planning is key to managing
postpartum hemorrhage
A question for mothers-to-be: What could be worse than having a cesarean delivery in your home?
Answer: Having an associated postpartum bleed.
Perhaps that isn’t the most elegant segue, but postpartum hemorrhage is a significant problem that remains a major contributor to maternal mortality in the United States. And, in fact, a prolonged and unsuccessful labor—the kind that could present to your hospital from an outside birthing facility or home—necessitating a cesarean delivery is a set-up for postpartum hemorrhage.
One of the tenets of emergency management in obstetrics is the three-pronged preparedness of 1) risk identification, 2) foreseeability, and 3) having a plan for taking action. Of late, many institutions have begun to develop a formal plan for managing OB emergencies—in particular, postpartum hemorrhage.
(A note about the potential role of interventional radiology in the management of postpartum hemorrhage: Our experience is limited, but we conjecture that, in most US hospitals that provide OB services, mobilizing an interventional radiology group in an emergency isn’t feasible. That makes it essential to have established medical and surgical management guidelines for such cases.)
To establish a plan on labor and delivery for managing postpartum bleeds, we recommend the following steps and direct you to ACOG’s “Practice Bulletin#76” for more specific information3:
- Establish a list of conditions that predispose a woman to postpartum hemorrhage and post that list throughout labor and delivery to heighten the awareness of team members
- Establish protocols for pharmacotherapeutic intervention—including oxytocin, methylergonovine, misoprostol, and prostaglandin F2a, with dosage and frequency guidelines and algorithms for use—and have those protocols readily available on labor and delivery, either on-line or posted
- Establish an “all-hands-on-deck” protocol for surgical emergencies—actual or potential—that includes what personnel to call and in what order to call them
- Use simulation to practice the all-hands-on-deck protocol and evaluate team and individual performance in managing hemorrhage
- Establish blood product replacement protocols, including order sets for products that are linked to particular diagnoses (e.g., typing and cross-matching for patients coming in to deliver who have a diagnosis of placenta previa; adding products such as fresh frozen plasma and platelets for patients who have complicating diagnoses, such as suspected placenta accreta or severe preeclampsia).
To prevent preterm birth: Cervical length screening
and progesterone
Preterm birth accounts for almost 13% of births in the United States, with spontaneous preterm labor and preterm rupture of membranes accounting for approximately 80% of those cases.4 Once preterm labor has begun, little in the way of successful intervention is possible, beyond short-term prolongation of pregnancy with tocolytic agents to allow for corticosteroid administration. Studies in recent years have, therefore, moved the focus back on prevention, using the same treatments that were used 60 years ago—progesterone supplementation and cerclage—with the addition of transvaginal ultrasonography (US) screening for cervical length.
Several large, randomized trials have examined the use of intramuscular injection or vaginal delivery of progesterone to prevent preterm birth in patients who are at high risk of preterm birth based on their obstetric history.5,6 Both 17a-hydroxyprogesterone caproate and vaginal progesterone suppositories are associated with a significant reduction in the risk of preterm birth in singleton pregnancies. ACOG reconfirmed the value of this finding in a 2011 Committee Opinion, which recommended the use of progesterone supplementation in singleton pregnancies in which there is a history of preterm labor or preterm rupture of membranes.7

There is mounting evidence that cervical length is inversely related to risk of preterm birth.The real question, however, is: What should be done about transvaginal cervical length: Should we be screening, or not? As recently as 2009, a Cochrane Review did not advocate universal screening for cervical length as a predictor for preterm birth4—despite mounting evidence that cervical length is inversely related to risk of preterm birth, with progressively shorter length (starting at <25 mm) associated with significantly higher risk of preterm birth.8,9 Keeping in mind that the decision to screen depends on your ability to treat the condition for which you are screening, what was needed was proof that intervention works.
2011 brought two studies that recommend screening for cervical length based on a successful reduction in preterm birth with a specific intervention. A large, randomized trial of vaginal progesterone gel for the prevention of preterm birth used universal screening for shortened cervical length (10 to 20 mm) as the criterion for randomization to treatment or placebo. The investigators demonstrated a 45% reduction in preterm birth of less than 33 weeks in the treatment arm.10
An interesting aspect of this study: The reduction in preterm birth was not, in fact, seen in patients who had a history of preterm birth, suggesting that this may be a different patient population that benefits from vaginal progesterone.
On the other hand, a recent meta-analysis concluded that patients who meet the criteria of 1) cervical length less than 25 mm and 2) a history of prior spontaneous preterm birth experience a significant reduction in preterm birth and a reduction in perinatal morbidity and mortality if they have cervical cerclage placed.11
Although these publications lead us to hope that there may be some benefit from preventive intervention for preterm birth, the question of how to screen for, and prevent, spontaneous preterm birth remains somewhat nebulous: It hasn’t been determined which patient population will benefit from which combination of screening and intervention. Larger trials for specific populations are still needed.
This is what we know, for now:
- Women who have a history of spontaneous preterm birth should have a thorough evaluation of their OB history to determine possible modifiable risk factors (e.g., smoking, short inter-pregnancy interval) and to determine, as definitively as possible, the likely cause of that preterm birth
- Women who have a singleton pregnancy and a history of either spontaneous preterm labor or preterm rupture of membranes can be offered progesterone supplementation as intramuscular 17a-hydroxyprogesterone or a vaginal preparation to reduce their risk of preterm birth
- Women who have an asymptomatic shortening of the cervix, as measured on transvaginal US at 18 to 24 weeks’ gestation, can be offered vaginal progesterone to reduce their risk of preterm birth
- Women who have a history of preterm birth and cervical shortening may see a reduction in their risk of preterm birth from cerclage placement
- The use of screening for cervical length or progesterone supplementation, or both, in a multiple gestation pregnancy are not recommended because their benefit in this population has not been demonstrated.
Until we fully understand the various etiologic pathways of spontaneous preterm birth, we won’t have a one-size-fits-all solution to this major cause of perinatal morbidity and mortality.
We want to hear from you! Tell us what you think.
The authors report no financial relationships relevant to this article.
The year that has followed our inaugural “Update on Obstetrics” [OBG Management, January 2011, available at www.obgmanagement.com] saw a resurgence of interest in a number of aspects of obstetric care. We want to highlight four of them in this Update because we think they are particularly important—given the attention they’ve received in the medical literature and in the consumer media:
- the ever-increasing cesarean delivery rate
- home birth
- postpartum hemorrhage
- measurement of cervical length and the use of progesterone.
Taming the cesarean delivery rate—how can we
accomplish this?
No one should be surprised to learn that the cesarean delivery rate increased nearly sevenfold from 1970 to 2011—from a rate of approximately 5% in 1970 to nearly 35%. Recall that, in the 1990s, the US Public Health Service proposed, as part of Healthy People 2010, a target cesarean rate of 15%, with a vaginal birth after cesarean (VBAC) rate of 60%. Today, the cesarean delivery rate is, as we said, nearly 35% and the VBAC rate is less than 10%.
Many factors have been cited for the rise, including:
- the obesity pandemic
- delaying childbearing
- increasing use of assisted reproduction
- multiple gestation (although the incidence of higher-order multiple gestations is now decreasing, the rate of twin births remains quite high relative to past decades).
So, how did this happen? And what can we do?
For one, VBAC is not likely to gain in popularity. More than 60% of US hospitals that provide OB services handle a volume of fewer than 1,000 deliveries a year. Such low volumes generally will not be able to support (either with dollars or staffing) the resources needed to safely provide VBAC.
Other options have been proposed: Loosen the guidelines for VBAC, change the personnel requirements, gather community groups of doctors, attorneys, and patients to agree on guidelines that, if followed, would protect physicians from being sued1—and so on. The medicolegal reality, however, is that these options have not been shown to be viable. We have concluded that increasing VBAC utilization is not the answer. Rather, addressing ways to prevent primary cesarean delivery holds the most promise for, ultimately, reducing the current rising trend.
On a positive note: The most recent data available from the National Center for Health Statistics suggest that the cesarean delivery rate has dropped slightly: from 32.9% in 2009 to 32.8% in 2010. The drop is truly slight; we’ll watch with interest to see if a trend has begun.
Considering that the cesarean delivery rate in 1970 was 5%, and that the dictum at the time was “once a section, always a section,” it seems clear (to us, at least) that the solution to this problem lies in preventing first cesarean deliveries. How can the specialty and, in some ways, you, in your practice, work toward this goal? Here are possible strategies:
- Eliminate elective inductions of labor when the modified Bishop score is less than 8
- Return to defining “post-term” as 42—not 41—completed weeks’ gestation
- Eliminate all elective inductions before 39 weeks’ gestation
- Provide better and more standardized training of physicians in the interpretation of fetal heart-rate tracings
- Improve communication between obstetricians and anesthesiologists in regard to managing pain during labor
- Institute mandatory review of all cesarean deliveries that are performed in the latent phase of labor and all so-called “stat cesareans”
- Readjust the compensation scale for physicians and hospitals in such a way that successful vaginal delivery is rewarded.
Even if all these measures were implemented, we think it’s unlikely that we will ever see a 5% cesarean delivery rate again—although probably for good reason. But even a return to a more manageable 20% rate seems a reasonable goal.
Home birth: Consider where you stand
We suppose that one way to avoid cesarean delivery would be to deliver at home. The topic, and practice, of home birth has mushroomed in the past few years—for a number of social and economic reasons, probably. It seems to us that there are a few basic issues that must be addressed, however, before it’s possible to come to grips with home birth in the 21st century in an enlightened way:
- In 1935, the maternal mortality rate approached 500 to 600 for every 100,000 births; most of those deaths occurred at home. In 2009, the maternal mortality rate was approximately 8 for every 100,000 births. Both rates are very low, but the difference would be significant to the 492 to 592 women who met a potentially preventable death.
- Methods of identifying who might be an appropriate candidate for a home birth are, at best, imprecise.
- Infrastructure for rapidly transporting mother and baby to a hospital if matters go awry is inadequate.
Although evidence is limited, what data there are suggest that one significant outcome—neonatal death—occurs with higher frequency among home births than among hospital births, even after correcting for anomalies (odds ratio, 2.9 [95% confidence interval, 1.3–6.2]).2 Although women who delivered at home did have fewer episiotomies, fewer third- and fourth-degree perineal lacerations, fewer operative deliveries (vaginal and cesarean) and a lower rate of infection, those reductions seem inconsequential compared to the death of a newborn….
Bottom line? Home birth is legal; home birth may be appropriate for some women who are at low risk and willing to accept a legitimate amount of personal risk; and you, as an OB, are in no way required to participate in or endorse the practice.
Many institutions have addressed this matter by developing a family-centered health care model for obstetrics—so-called hospitals within hospitals—that allow for a less interventionist approach to childbirth within the safety net of a hospital facility, should unforeseeable complications arise. Consider your interest in affiliating with such a facility, based on your acceptance of the practice of home birth and your comfort with being part of this approach.
Formal, systematic planning is key to managing
postpartum hemorrhage
A question for mothers-to-be: What could be worse than having a cesarean delivery in your home?
Answer: Having an associated postpartum bleed.
Perhaps that isn’t the most elegant segue, but postpartum hemorrhage is a significant problem that remains a major contributor to maternal mortality in the United States. And, in fact, a prolonged and unsuccessful labor—the kind that could present to your hospital from an outside birthing facility or home—necessitating a cesarean delivery is a set-up for postpartum hemorrhage.
One of the tenets of emergency management in obstetrics is the three-pronged preparedness of 1) risk identification, 2) foreseeability, and 3) having a plan for taking action. Of late, many institutions have begun to develop a formal plan for managing OB emergencies—in particular, postpartum hemorrhage.
(A note about the potential role of interventional radiology in the management of postpartum hemorrhage: Our experience is limited, but we conjecture that, in most US hospitals that provide OB services, mobilizing an interventional radiology group in an emergency isn’t feasible. That makes it essential to have established medical and surgical management guidelines for such cases.)
To establish a plan on labor and delivery for managing postpartum bleeds, we recommend the following steps and direct you to ACOG’s “Practice Bulletin#76” for more specific information3:
- Establish a list of conditions that predispose a woman to postpartum hemorrhage and post that list throughout labor and delivery to heighten the awareness of team members
- Establish protocols for pharmacotherapeutic intervention—including oxytocin, methylergonovine, misoprostol, and prostaglandin F2a, with dosage and frequency guidelines and algorithms for use—and have those protocols readily available on labor and delivery, either on-line or posted
- Establish an “all-hands-on-deck” protocol for surgical emergencies—actual or potential—that includes what personnel to call and in what order to call them
- Use simulation to practice the all-hands-on-deck protocol and evaluate team and individual performance in managing hemorrhage
- Establish blood product replacement protocols, including order sets for products that are linked to particular diagnoses (e.g., typing and cross-matching for patients coming in to deliver who have a diagnosis of placenta previa; adding products such as fresh frozen plasma and platelets for patients who have complicating diagnoses, such as suspected placenta accreta or severe preeclampsia).
To prevent preterm birth: Cervical length screening
and progesterone
Preterm birth accounts for almost 13% of births in the United States, with spontaneous preterm labor and preterm rupture of membranes accounting for approximately 80% of those cases.4 Once preterm labor has begun, little in the way of successful intervention is possible, beyond short-term prolongation of pregnancy with tocolytic agents to allow for corticosteroid administration. Studies in recent years have, therefore, moved the focus back on prevention, using the same treatments that were used 60 years ago—progesterone supplementation and cerclage—with the addition of transvaginal ultrasonography (US) screening for cervical length.
Several large, randomized trials have examined the use of intramuscular injection or vaginal delivery of progesterone to prevent preterm birth in patients who are at high risk of preterm birth based on their obstetric history.5,6 Both 17a-hydroxyprogesterone caproate and vaginal progesterone suppositories are associated with a significant reduction in the risk of preterm birth in singleton pregnancies. ACOG reconfirmed the value of this finding in a 2011 Committee Opinion, which recommended the use of progesterone supplementation in singleton pregnancies in which there is a history of preterm labor or preterm rupture of membranes.7

There is mounting evidence that cervical length is inversely related to risk of preterm birth.The real question, however, is: What should be done about transvaginal cervical length: Should we be screening, or not? As recently as 2009, a Cochrane Review did not advocate universal screening for cervical length as a predictor for preterm birth4—despite mounting evidence that cervical length is inversely related to risk of preterm birth, with progressively shorter length (starting at <25 mm) associated with significantly higher risk of preterm birth.8,9 Keeping in mind that the decision to screen depends on your ability to treat the condition for which you are screening, what was needed was proof that intervention works.
2011 brought two studies that recommend screening for cervical length based on a successful reduction in preterm birth with a specific intervention. A large, randomized trial of vaginal progesterone gel for the prevention of preterm birth used universal screening for shortened cervical length (10 to 20 mm) as the criterion for randomization to treatment or placebo. The investigators demonstrated a 45% reduction in preterm birth of less than 33 weeks in the treatment arm.10
An interesting aspect of this study: The reduction in preterm birth was not, in fact, seen in patients who had a history of preterm birth, suggesting that this may be a different patient population that benefits from vaginal progesterone.
On the other hand, a recent meta-analysis concluded that patients who meet the criteria of 1) cervical length less than 25 mm and 2) a history of prior spontaneous preterm birth experience a significant reduction in preterm birth and a reduction in perinatal morbidity and mortality if they have cervical cerclage placed.11
Although these publications lead us to hope that there may be some benefit from preventive intervention for preterm birth, the question of how to screen for, and prevent, spontaneous preterm birth remains somewhat nebulous: It hasn’t been determined which patient population will benefit from which combination of screening and intervention. Larger trials for specific populations are still needed.
This is what we know, for now:
- Women who have a history of spontaneous preterm birth should have a thorough evaluation of their OB history to determine possible modifiable risk factors (e.g., smoking, short inter-pregnancy interval) and to determine, as definitively as possible, the likely cause of that preterm birth
- Women who have a singleton pregnancy and a history of either spontaneous preterm labor or preterm rupture of membranes can be offered progesterone supplementation as intramuscular 17a-hydroxyprogesterone or a vaginal preparation to reduce their risk of preterm birth
- Women who have an asymptomatic shortening of the cervix, as measured on transvaginal US at 18 to 24 weeks’ gestation, can be offered vaginal progesterone to reduce their risk of preterm birth
- Women who have a history of preterm birth and cervical shortening may see a reduction in their risk of preterm birth from cerclage placement
- The use of screening for cervical length or progesterone supplementation, or both, in a multiple gestation pregnancy are not recommended because their benefit in this population has not been demonstrated.
Until we fully understand the various etiologic pathways of spontaneous preterm birth, we won’t have a one-size-fits-all solution to this major cause of perinatal morbidity and mortality.
We want to hear from you! Tell us what you think.
1. Scott JR. Vaginal birth after cesarean delivery; a common sense approach. Obstet Gynecol. 2011;118(2 Pt 1):342-350.
2. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425-428.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039-1047.
4. Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery (Review). Cochrane Database Syst Rev. 2009;(3):CD007235.-
5. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419-424.
6. Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
7. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963-935.
8. Owen J, Yost N, Berghella V, et al:. National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340-1348.
9. Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193(3):1170-1174.
10. Hassan SS, Romero R, Vidyadhari D, et al. PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18-31.
11. Berghella V, Rafael TJ, Szychowski M, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth. Obstet Gynecol. 2011;117(3):663-771
1. Scott JR. Vaginal birth after cesarean delivery; a common sense approach. Obstet Gynecol. 2011;118(2 Pt 1):342-350.
2. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425-428.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039-1047.
4. Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery (Review). Cochrane Database Syst Rev. 2009;(3):CD007235.-
5. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419-424.
6. Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
7. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963-935.
8. Owen J, Yost N, Berghella V, et al:. National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340-1348.
9. Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193(3):1170-1174.
10. Hassan SS, Romero R, Vidyadhari D, et al. PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18-31.
11. Berghella V, Rafael TJ, Szychowski M, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth. Obstet Gynecol. 2011;117(3):663-771
Be vigilant for vulvar intraepithelial neoplasia— here is why
How should you evaluate a patient who has a cytologic diagnosis of atypical glandular cells (AGC)?
Charles J. Dunton, MD (Examining the Evidence; August 2011)
What is optimal surveillance after treatment for high-grade cervical intraepithelial neoplasia (CIN)?
Alan G. Waxman, MD, MPH (Examining the Evidence; June 2011)
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel (August 2010)
Dr. Massad reports no financial relationships relevant to this article.
The societal shifts of the 1960s generated many changes—among them, permanently altered sexual mores. That may be a primary reason why the incidence of vulvar intraepithelial neoplasia (VIN) increased more than 400% between 1973 and 2000, says L. Stewart Massad, Jr, MD, chairman of the Practice Committee of the American Society for Colposcopy and Cervical Pathology (ASCCP) and member of the ACOG Committee on Gynecologic Practice—and one of the authors of a new joint Committee Opinion on the management of VIN.1 This precancer is often associated with carcinogenic types of human papillomavirus (HPV), the most common sexually transmitted disease in the nation.
The 400% statistic caught the attention of OBG Management. The editors invited Dr. Massad to discuss the subject of VIN at length, elaborating on key issues such as its prevention, identification, treatment, and surveillance.
How to identify a VIN lesion
OBG Management: What is VIN? What does it look like?
Dr. Massad: VIN is a premalignant condition of the vulva that may present as unifocal or multifocal lesions. These lesions may be flesh-colored, hypopigmented, or hyperpigmented (FIGURE). They also may be erythematous, flat, or raised. They can be found on any part of the vulva. The dysplastic cells may extend into hair shafts or sweat glands; they don’t penetrate the basement membrane, however, so, by definition, they aren’t invasive.
Usual-type VIN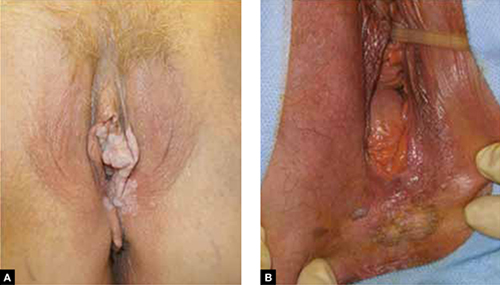
A. This warty lesion is hyperpigmented around the periphery, hypopigmented in the center. B. Another warty lesion. Both images reflect the application of acetic acid.OBG Management: Why has the incidence increased so considerably?
Dr. Massad: The data we have on incidence comes from the Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute, as reported by Judson and colleagues.2 Although better reporting of findings of VIN may play a role, the rising incidence seems to be attributable to changes in sexual behavior over the past half century. The incidence of vulvar cancer rose during the same period—about 20%.3 The much slower growth in the incidence of vulvar cancer suggests that treatment of VIN has blunted the risk of cancer.
OBG Management: Is VIN associated with any particular type of HPV?
Dr. Massad: Yes, more than 80% of VIN lesions are associated with HPV 16.
OBG Management: One study from 2005 noted that the mean age of women with VIN decreased from 50 years before 1980 to 39 years in subsequent years.4 Why are more younger women developing VIN?
Dr. Massad: The study that showed that age shift was from New Zealand. The authors speculated that the change was due to earlier sexual activity among women who smoke: HPV, especially HPV 16, and smoking are important risk factors for VIN. The question hasn’t been definitively answered.
OBG Management: What are the risk factors for VIN?
Dr. Massad: Smoking is a big one. More than 50% of women who have VIN are smokers. Thirty percent have concurrent or prior cervical intraepithelial neoplasia (CIN) or vaginal intraepithelial neoplasia (VAIN). The risk of invasion rises with age at the time of the initial diagnosis and with longer follow-up. I can talk about surveillance a little later.
Are some lesions more worrisome than others?
OBG Management: Are VIN lesions categorized similarly to CIN lesions—that is, using three different grades of severity?
Dr. Massad: Until recently, that was the case, but it is no longer so. Broadly, there are now two classes of VIN, according to the International Society for the Study of Vulvovaginal Disease (ISSVD): usual-type VIN and differentiated VIN.
ACOG and ASCCP have embraced this classification system, although not all pathologists have done so, and clinicians may still see reports using the old three-tier system.
Usual-type VIN is associated with infection with high-risk types of HPV—most notably, HPV 16, as I mentioned. Histologically, usual-type VIN can mimic common genital warts, and the warty subtype shows keratosis at the surface, a spiky or undulating surface, and vertical maturation of cells in the lesion but with pleomorphic cells filling half or more of the epithelial thickness. The basaloid subtype of usual VIN shows little maturation.
Differentiated VIN exhibits more subtle atypia, with keratin pearls and an eosinophilic cytoplasm.
Biologically, usual-type VIN is associated with HPV and linked to smoking and sexual activity, as we discussed. As its name suggests, it is found more frequently than differentiated VIN. It is most common in women in their late 30s to early 50s.
In contrast, differentiated VIN is not associated with HPV and is more common in postmenopausal women; it is frequently seen with lichen sclerosus.
OBG Management: When did this new way of classifying VIN—as usual-type and differentiated—originate?
Dr. Massad: The ISVVD classification system changed in 2004. Before then, it paralleled the CIN classification system, with three grades of intraepithelial neoplasia corresponding to the thickness of the epithelium filled by dysplastic cells: VIN 1, 2, and 3. However, VIN 1 was not really neoplastic. It reflected infection with HPV, and although it might progress to higher-grade dysplasia or cancer, the risk was minimal. So the ISVVD revised the classification system to include only high-grade VIN—the old VIN 2 and VIN 3. HPV-associated lesions with dysplastic cells confined to the lower third of the epithelium are managed like genital warts, with observation for spontaneous regression or treatment with topical therapy or surgery.
How to screen for VIN
OBG Management: What screening strategy is recommended for VIN?
Dr. Massad: There is no such recommendation. Screening for VIN hasn’t been implemented for several reasons. Most important, other than inspection of the vulva by a clinician, there is no good screening test. VIN isn’t very common, so mass inspection for lesions is unlikely to be cost-effective. The sensitivity and specificity of inspection by a clinician aren’t known. Most lesions are found by women, their partners, or clinicians before cancer develops. And most disease is treated before cancer arises.
OBG Management: Isn’t there a need for heightened scrutiny of the vulva?
Dr. Massad: Yes. Women should examine their genitalia several times a year and seek attention if anything changes. That’s especially true for women who have risk factors, such as smoking, immunosuppression, and a history of being treated for cervical dysplasia. It’s the same concept we employ when teaching women to identify early breast lesions through self-examination.
The biggest challenges in detecting VIN are educating women to report vulvar skin changes to their clinicians for assessment and educating clinicians to examine the vulva before inserting the speculum for cervical screening and vaginal inspection.
OBG Management: Is another challenge distinguishing some forms of VIN from genital warts?
Dr. Massad: It can be a challenge, but clinicians should recall that warts are most common among women around the time of the onset of sexual activity. Older women sometimes develop warts with a new sexual partner. However, when women in their 40s and older develop new warty lesions, always suspect VIN. A woman in her 60s or older who has a new, warty-appearing vulvar lesion should be assumed to have VIN or cancer.
OBG Management: Does VIN ever regress spontaneously?
Dr. Massad: Yes. There have been reports of spontaneous regression of VIN, especially in young women. Regrettably, there are also reports of progression to cancer during observation. There are no characteristics that allow us to distinguish lesions that are going to progress from those that will regress. The ACOG-ASCCP Committee Opinion recommends treatment of all VIN.1
Can VIN be prevented?
OBG Management: The Committee Opinion recommends that the quadrivalent HPV vaccine be offered to women “in target populations” because it can decrease the risk of VIN. What are those target populations?
Dr. Massad: The target population for HPV vaccination is 11- and 12-year-old girls, but catch-up vaccination is acceptable in patients as old as 26 years.
OBG Management: Why isn’t the bivalent vaccine recommended?
Dr. Massad: Only the quadrivalent vaccine has been approved by the US Food and Drug Administration (FDA) for prevention of VIN, although, in theory, the bivalent vaccine ought to be effective as well.
When to biopsy
OBG Management: Do you recommend that any suspect lesion on the vulva be biopsied?
Dr. Massad: The decision to biopsy should be individualized. However, women who have apparent warts that fail to respond to topical therapy should undergo biopsy, as should older women with warty lesions. Keep in mind that older women may develop verrucous carcinomas and may benefit from excision of enlarging warty lesions even if a biopsy is reported as only condylomata. Clinicians should not biopsy varicosities or obvious flat nevi.
OBG Management: Is colposcopy ever helpful in assessing vulvar lesions?
Dr. Massad: Most vulvar lesions can be identified without colposcopy, but colposcopy is useful in determining the extent of lesions. It often reveals subclinical disease not evident at the time of vulvar inspection.
OBG Management: When colposcopy is used, is the procedure the same as for cervical examination?
Dr. Massad: Not exactly. The clinician should apply 5% acetic acid for 5 minutes using a gauze sponge, but the magnification should be 63 to 103—not 153, as it is for cervical examination. It’s important to distinguish hyperplasia from VIN. In general, hyperplastic lesions are faint, gray, diffuse, and flat, whereas VIN lesions are raised and irregular in shape, with sharp borders.
OBG Management: What about toluidine blue? Is it useful in inspection of lesions?
Dr. Massad: Toluidine blue stains skin that is irritated. It isn’t very specific for VIN or vulvar cancer, and it can make colposcopy difficult, so experts no longer recommend it.
OBG Management: What are the treatment options for VIN?
Dr. Massad: They include surgical excision, laser ablation, and topical therapy with 5% imiquimod. All are potentially effective. The Committee Opinion doesn’t specify a preference, except to say that excision is advised when there is any suspicion of cancer to preserve a sample for pathologic analysis. Ablation destroys the lesion, making assessment of possible invasion impossible, and imiquimod may allow disease to progress during observation.
OBG Management: The Committee Opinion recommends wide local excision when cancer is suspected. What size of margin is optimal?
Dr. Massad: In general, a margin of 5 to 10 mm around the lesion is recommended. Vulvectomy isn’t needed because close follow-up usually identifies recurrence before invasion occurs.
OBG Management: When is laser ablation a good choice?
Dr. Massad: Whenever a biopsy shows VIN and cancer is not suspected. Laser ablation is ideal when lesions are multifocal or extensive, although repeated treatments may be required to resolve small foci of residual disease. Done with careful attention to power density and depth of ablation, laser therapy can be less disfiguring than excision.
OBG Management: You mentioned 5% imiquimod. Is there evidence that it’s effective in the treatment of VIN?
Dr. Massad: Multiple randomized, controlled trials have shown 5% imiquimod to be effective against VIN, although the agent does not have approval from the FDA for that indication.5,6 Lower concentrations of imiquimod have not been studied in the treatment of VIN. Women treated with this topical therapy should be followed every 4 weeks with colposcopy because progression to cancer has been reported during imiquimod therapy. Lesions that fail to respond completely after a full course of imiquimod should be treated with excision or laser ablation.
Surveillance is critical
OBG Management: According to the Committee Opinion, the recurrence rate of VIN can reach 30% to 50%.7 Why so high?
Dr. Massad: Usual-type VIN reflects exposure to carcinogenic HPV, and differentiated VIN arises from a vulvar dystrophy. In both situations, treatments destroy VIN and arrest progress to cancer, but the entire vulvar skin remains subject to the inciting condition.
OBG Management: Would skinning vulvectomy eliminate the risk of recurrence?
Dr. Massad: Full vulvectomy is crippling and usually unnecessary. Most patients and clinicians accept the risk of recurrence of VIN to avoid the side effects of radical treatment.
OBG Management: What kind of surveillance is recommended after treatment?
Dr. Massad: Patients should perform vulvar self-examination every few months. They should also be examined 6 and 12 months after initial treatment and annually thereafter because the risk of recurrence may persist for years.
Because VIN is associated with carcinogenic HPV, women with VIN should undergo an annual Pap test.
OBG Management: Thank you, Dr. Massad. Let’s hope the incidence of this precancer begins to decline.
- Recommend the quadrivalent HPV vaccine for girls in the target age range (11 and 12 years old) to reduce the risk of VIN.
- Encourage smoking cessation.
- Make it a practice to inspect the vulva before inserting the speculum for cervical examination.
- Biopsy most pigmented lesions on the vulva. Biopsy all warty lesions in postmenopausal women and in women who fail topical treatment for genital warts.
- Treat all VIN lesions. When cancer is suspected, use wide local excision with a margin of 5 to 10 mm.
- Keep in mind that dysplastic cells can extend into hair follicles and sweat glands.
- Closely follow up all women treated for VIN (6 and 12 months after treatment and annually thereafter) and encourage them to examine their vulva several times every year. Perform an annual Pap test for any woman found to have VIN.
We want to hear from you! Tell us what you think.
1. Committee on Gynecologic Practice; American College of Obstetricians and Gynecologists. Committee Opinion #509: Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2011;118(5):1192-1194.
2. Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018-1022.
3. Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1-24.
4. Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106(6):1319-1326.
5. Van Seters M, van Beurden M, ten Kate FJW, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358(14):1465-1473.
6. Terlou A, van Seters M, Ewing PC, et al. Treatment of vaginal intraepithelial neoplasia with topical imiquimod: seven years median follow-up of a randomized clinical trial. Gynecol Oncol. 2011;121(1):157-162.
7. Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO2 laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006;100(2):271-275.
How should you evaluate a patient who has a cytologic diagnosis of atypical glandular cells (AGC)?
Charles J. Dunton, MD (Examining the Evidence; August 2011)
What is optimal surveillance after treatment for high-grade cervical intraepithelial neoplasia (CIN)?
Alan G. Waxman, MD, MPH (Examining the Evidence; June 2011)
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel (August 2010)
Dr. Massad reports no financial relationships relevant to this article.
The societal shifts of the 1960s generated many changes—among them, permanently altered sexual mores. That may be a primary reason why the incidence of vulvar intraepithelial neoplasia (VIN) increased more than 400% between 1973 and 2000, says L. Stewart Massad, Jr, MD, chairman of the Practice Committee of the American Society for Colposcopy and Cervical Pathology (ASCCP) and member of the ACOG Committee on Gynecologic Practice—and one of the authors of a new joint Committee Opinion on the management of VIN.1 This precancer is often associated with carcinogenic types of human papillomavirus (HPV), the most common sexually transmitted disease in the nation.
The 400% statistic caught the attention of OBG Management. The editors invited Dr. Massad to discuss the subject of VIN at length, elaborating on key issues such as its prevention, identification, treatment, and surveillance.
How to identify a VIN lesion
OBG Management: What is VIN? What does it look like?
Dr. Massad: VIN is a premalignant condition of the vulva that may present as unifocal or multifocal lesions. These lesions may be flesh-colored, hypopigmented, or hyperpigmented (FIGURE). They also may be erythematous, flat, or raised. They can be found on any part of the vulva. The dysplastic cells may extend into hair shafts or sweat glands; they don’t penetrate the basement membrane, however, so, by definition, they aren’t invasive.
Usual-type VIN
A. This warty lesion is hyperpigmented around the periphery, hypopigmented in the center. B. Another warty lesion. Both images reflect the application of acetic acid.OBG Management: Why has the incidence increased so considerably?
Dr. Massad: The data we have on incidence comes from the Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute, as reported by Judson and colleagues.2 Although better reporting of findings of VIN may play a role, the rising incidence seems to be attributable to changes in sexual behavior over the past half century. The incidence of vulvar cancer rose during the same period—about 20%.3 The much slower growth in the incidence of vulvar cancer suggests that treatment of VIN has blunted the risk of cancer.
OBG Management: Is VIN associated with any particular type of HPV?
Dr. Massad: Yes, more than 80% of VIN lesions are associated with HPV 16.
OBG Management: One study from 2005 noted that the mean age of women with VIN decreased from 50 years before 1980 to 39 years in subsequent years.4 Why are more younger women developing VIN?
Dr. Massad: The study that showed that age shift was from New Zealand. The authors speculated that the change was due to earlier sexual activity among women who smoke: HPV, especially HPV 16, and smoking are important risk factors for VIN. The question hasn’t been definitively answered.
OBG Management: What are the risk factors for VIN?
Dr. Massad: Smoking is a big one. More than 50% of women who have VIN are smokers. Thirty percent have concurrent or prior cervical intraepithelial neoplasia (CIN) or vaginal intraepithelial neoplasia (VAIN). The risk of invasion rises with age at the time of the initial diagnosis and with longer follow-up. I can talk about surveillance a little later.
Are some lesions more worrisome than others?
OBG Management: Are VIN lesions categorized similarly to CIN lesions—that is, using three different grades of severity?
Dr. Massad: Until recently, that was the case, but it is no longer so. Broadly, there are now two classes of VIN, according to the International Society for the Study of Vulvovaginal Disease (ISSVD): usual-type VIN and differentiated VIN.
ACOG and ASCCP have embraced this classification system, although not all pathologists have done so, and clinicians may still see reports using the old three-tier system.
Usual-type VIN is associated with infection with high-risk types of HPV—most notably, HPV 16, as I mentioned. Histologically, usual-type VIN can mimic common genital warts, and the warty subtype shows keratosis at the surface, a spiky or undulating surface, and vertical maturation of cells in the lesion but with pleomorphic cells filling half or more of the epithelial thickness. The basaloid subtype of usual VIN shows little maturation.
Differentiated VIN exhibits more subtle atypia, with keratin pearls and an eosinophilic cytoplasm.
Biologically, usual-type VIN is associated with HPV and linked to smoking and sexual activity, as we discussed. As its name suggests, it is found more frequently than differentiated VIN. It is most common in women in their late 30s to early 50s.
In contrast, differentiated VIN is not associated with HPV and is more common in postmenopausal women; it is frequently seen with lichen sclerosus.
OBG Management: When did this new way of classifying VIN—as usual-type and differentiated—originate?
Dr. Massad: The ISVVD classification system changed in 2004. Before then, it paralleled the CIN classification system, with three grades of intraepithelial neoplasia corresponding to the thickness of the epithelium filled by dysplastic cells: VIN 1, 2, and 3. However, VIN 1 was not really neoplastic. It reflected infection with HPV, and although it might progress to higher-grade dysplasia or cancer, the risk was minimal. So the ISVVD revised the classification system to include only high-grade VIN—the old VIN 2 and VIN 3. HPV-associated lesions with dysplastic cells confined to the lower third of the epithelium are managed like genital warts, with observation for spontaneous regression or treatment with topical therapy or surgery.
How to screen for VIN
OBG Management: What screening strategy is recommended for VIN?
Dr. Massad: There is no such recommendation. Screening for VIN hasn’t been implemented for several reasons. Most important, other than inspection of the vulva by a clinician, there is no good screening test. VIN isn’t very common, so mass inspection for lesions is unlikely to be cost-effective. The sensitivity and specificity of inspection by a clinician aren’t known. Most lesions are found by women, their partners, or clinicians before cancer develops. And most disease is treated before cancer arises.
OBG Management: Isn’t there a need for heightened scrutiny of the vulva?
Dr. Massad: Yes. Women should examine their genitalia several times a year and seek attention if anything changes. That’s especially true for women who have risk factors, such as smoking, immunosuppression, and a history of being treated for cervical dysplasia. It’s the same concept we employ when teaching women to identify early breast lesions through self-examination.
The biggest challenges in detecting VIN are educating women to report vulvar skin changes to their clinicians for assessment and educating clinicians to examine the vulva before inserting the speculum for cervical screening and vaginal inspection.
OBG Management: Is another challenge distinguishing some forms of VIN from genital warts?
Dr. Massad: It can be a challenge, but clinicians should recall that warts are most common among women around the time of the onset of sexual activity. Older women sometimes develop warts with a new sexual partner. However, when women in their 40s and older develop new warty lesions, always suspect VIN. A woman in her 60s or older who has a new, warty-appearing vulvar lesion should be assumed to have VIN or cancer.
OBG Management: Does VIN ever regress spontaneously?
Dr. Massad: Yes. There have been reports of spontaneous regression of VIN, especially in young women. Regrettably, there are also reports of progression to cancer during observation. There are no characteristics that allow us to distinguish lesions that are going to progress from those that will regress. The ACOG-ASCCP Committee Opinion recommends treatment of all VIN.1
Can VIN be prevented?
OBG Management: The Committee Opinion recommends that the quadrivalent HPV vaccine be offered to women “in target populations” because it can decrease the risk of VIN. What are those target populations?
Dr. Massad: The target population for HPV vaccination is 11- and 12-year-old girls, but catch-up vaccination is acceptable in patients as old as 26 years.
OBG Management: Why isn’t the bivalent vaccine recommended?
Dr. Massad: Only the quadrivalent vaccine has been approved by the US Food and Drug Administration (FDA) for prevention of VIN, although, in theory, the bivalent vaccine ought to be effective as well.
When to biopsy
OBG Management: Do you recommend that any suspect lesion on the vulva be biopsied?
Dr. Massad: The decision to biopsy should be individualized. However, women who have apparent warts that fail to respond to topical therapy should undergo biopsy, as should older women with warty lesions. Keep in mind that older women may develop verrucous carcinomas and may benefit from excision of enlarging warty lesions even if a biopsy is reported as only condylomata. Clinicians should not biopsy varicosities or obvious flat nevi.
OBG Management: Is colposcopy ever helpful in assessing vulvar lesions?
Dr. Massad: Most vulvar lesions can be identified without colposcopy, but colposcopy is useful in determining the extent of lesions. It often reveals subclinical disease not evident at the time of vulvar inspection.
OBG Management: When colposcopy is used, is the procedure the same as for cervical examination?
Dr. Massad: Not exactly. The clinician should apply 5% acetic acid for 5 minutes using a gauze sponge, but the magnification should be 63 to 103—not 153, as it is for cervical examination. It’s important to distinguish hyperplasia from VIN. In general, hyperplastic lesions are faint, gray, diffuse, and flat, whereas VIN lesions are raised and irregular in shape, with sharp borders.
OBG Management: What about toluidine blue? Is it useful in inspection of lesions?
Dr. Massad: Toluidine blue stains skin that is irritated. It isn’t very specific for VIN or vulvar cancer, and it can make colposcopy difficult, so experts no longer recommend it.
OBG Management: What are the treatment options for VIN?
Dr. Massad: They include surgical excision, laser ablation, and topical therapy with 5% imiquimod. All are potentially effective. The Committee Opinion doesn’t specify a preference, except to say that excision is advised when there is any suspicion of cancer to preserve a sample for pathologic analysis. Ablation destroys the lesion, making assessment of possible invasion impossible, and imiquimod may allow disease to progress during observation.
OBG Management: The Committee Opinion recommends wide local excision when cancer is suspected. What size of margin is optimal?
Dr. Massad: In general, a margin of 5 to 10 mm around the lesion is recommended. Vulvectomy isn’t needed because close follow-up usually identifies recurrence before invasion occurs.
OBG Management: When is laser ablation a good choice?
Dr. Massad: Whenever a biopsy shows VIN and cancer is not suspected. Laser ablation is ideal when lesions are multifocal or extensive, although repeated treatments may be required to resolve small foci of residual disease. Done with careful attention to power density and depth of ablation, laser therapy can be less disfiguring than excision.
OBG Management: You mentioned 5% imiquimod. Is there evidence that it’s effective in the treatment of VIN?
Dr. Massad: Multiple randomized, controlled trials have shown 5% imiquimod to be effective against VIN, although the agent does not have approval from the FDA for that indication.5,6 Lower concentrations of imiquimod have not been studied in the treatment of VIN. Women treated with this topical therapy should be followed every 4 weeks with colposcopy because progression to cancer has been reported during imiquimod therapy. Lesions that fail to respond completely after a full course of imiquimod should be treated with excision or laser ablation.
Surveillance is critical
OBG Management: According to the Committee Opinion, the recurrence rate of VIN can reach 30% to 50%.7 Why so high?
Dr. Massad: Usual-type VIN reflects exposure to carcinogenic HPV, and differentiated VIN arises from a vulvar dystrophy. In both situations, treatments destroy VIN and arrest progress to cancer, but the entire vulvar skin remains subject to the inciting condition.
OBG Management: Would skinning vulvectomy eliminate the risk of recurrence?
Dr. Massad: Full vulvectomy is crippling and usually unnecessary. Most patients and clinicians accept the risk of recurrence of VIN to avoid the side effects of radical treatment.
OBG Management: What kind of surveillance is recommended after treatment?
Dr. Massad: Patients should perform vulvar self-examination every few months. They should also be examined 6 and 12 months after initial treatment and annually thereafter because the risk of recurrence may persist for years.
Because VIN is associated with carcinogenic HPV, women with VIN should undergo an annual Pap test.
OBG Management: Thank you, Dr. Massad. Let’s hope the incidence of this precancer begins to decline.
- Recommend the quadrivalent HPV vaccine for girls in the target age range (11 and 12 years old) to reduce the risk of VIN.
- Encourage smoking cessation.
- Make it a practice to inspect the vulva before inserting the speculum for cervical examination.
- Biopsy most pigmented lesions on the vulva. Biopsy all warty lesions in postmenopausal women and in women who fail topical treatment for genital warts.
- Treat all VIN lesions. When cancer is suspected, use wide local excision with a margin of 5 to 10 mm.
- Keep in mind that dysplastic cells can extend into hair follicles and sweat glands.
- Closely follow up all women treated for VIN (6 and 12 months after treatment and annually thereafter) and encourage them to examine their vulva several times every year. Perform an annual Pap test for any woman found to have VIN.
We want to hear from you! Tell us what you think.
How should you evaluate a patient who has a cytologic diagnosis of atypical glandular cells (AGC)?
Charles J. Dunton, MD (Examining the Evidence; August 2011)
What is optimal surveillance after treatment for high-grade cervical intraepithelial neoplasia (CIN)?
Alan G. Waxman, MD, MPH (Examining the Evidence; June 2011)
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel (August 2010)
Dr. Massad reports no financial relationships relevant to this article.
The societal shifts of the 1960s generated many changes—among them, permanently altered sexual mores. That may be a primary reason why the incidence of vulvar intraepithelial neoplasia (VIN) increased more than 400% between 1973 and 2000, says L. Stewart Massad, Jr, MD, chairman of the Practice Committee of the American Society for Colposcopy and Cervical Pathology (ASCCP) and member of the ACOG Committee on Gynecologic Practice—and one of the authors of a new joint Committee Opinion on the management of VIN.1 This precancer is often associated with carcinogenic types of human papillomavirus (HPV), the most common sexually transmitted disease in the nation.
The 400% statistic caught the attention of OBG Management. The editors invited Dr. Massad to discuss the subject of VIN at length, elaborating on key issues such as its prevention, identification, treatment, and surveillance.
How to identify a VIN lesion
OBG Management: What is VIN? What does it look like?
Dr. Massad: VIN is a premalignant condition of the vulva that may present as unifocal or multifocal lesions. These lesions may be flesh-colored, hypopigmented, or hyperpigmented (FIGURE). They also may be erythematous, flat, or raised. They can be found on any part of the vulva. The dysplastic cells may extend into hair shafts or sweat glands; they don’t penetrate the basement membrane, however, so, by definition, they aren’t invasive.
Usual-type VIN
A. This warty lesion is hyperpigmented around the periphery, hypopigmented in the center. B. Another warty lesion. Both images reflect the application of acetic acid.OBG Management: Why has the incidence increased so considerably?
Dr. Massad: The data we have on incidence comes from the Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute, as reported by Judson and colleagues.2 Although better reporting of findings of VIN may play a role, the rising incidence seems to be attributable to changes in sexual behavior over the past half century. The incidence of vulvar cancer rose during the same period—about 20%.3 The much slower growth in the incidence of vulvar cancer suggests that treatment of VIN has blunted the risk of cancer.
OBG Management: Is VIN associated with any particular type of HPV?
Dr. Massad: Yes, more than 80% of VIN lesions are associated with HPV 16.
OBG Management: One study from 2005 noted that the mean age of women with VIN decreased from 50 years before 1980 to 39 years in subsequent years.4 Why are more younger women developing VIN?
Dr. Massad: The study that showed that age shift was from New Zealand. The authors speculated that the change was due to earlier sexual activity among women who smoke: HPV, especially HPV 16, and smoking are important risk factors for VIN. The question hasn’t been definitively answered.
OBG Management: What are the risk factors for VIN?
Dr. Massad: Smoking is a big one. More than 50% of women who have VIN are smokers. Thirty percent have concurrent or prior cervical intraepithelial neoplasia (CIN) or vaginal intraepithelial neoplasia (VAIN). The risk of invasion rises with age at the time of the initial diagnosis and with longer follow-up. I can talk about surveillance a little later.
Are some lesions more worrisome than others?
OBG Management: Are VIN lesions categorized similarly to CIN lesions—that is, using three different grades of severity?
Dr. Massad: Until recently, that was the case, but it is no longer so. Broadly, there are now two classes of VIN, according to the International Society for the Study of Vulvovaginal Disease (ISSVD): usual-type VIN and differentiated VIN.
ACOG and ASCCP have embraced this classification system, although not all pathologists have done so, and clinicians may still see reports using the old three-tier system.
Usual-type VIN is associated with infection with high-risk types of HPV—most notably, HPV 16, as I mentioned. Histologically, usual-type VIN can mimic common genital warts, and the warty subtype shows keratosis at the surface, a spiky or undulating surface, and vertical maturation of cells in the lesion but with pleomorphic cells filling half or more of the epithelial thickness. The basaloid subtype of usual VIN shows little maturation.
Differentiated VIN exhibits more subtle atypia, with keratin pearls and an eosinophilic cytoplasm.
Biologically, usual-type VIN is associated with HPV and linked to smoking and sexual activity, as we discussed. As its name suggests, it is found more frequently than differentiated VIN. It is most common in women in their late 30s to early 50s.
In contrast, differentiated VIN is not associated with HPV and is more common in postmenopausal women; it is frequently seen with lichen sclerosus.
OBG Management: When did this new way of classifying VIN—as usual-type and differentiated—originate?
Dr. Massad: The ISVVD classification system changed in 2004. Before then, it paralleled the CIN classification system, with three grades of intraepithelial neoplasia corresponding to the thickness of the epithelium filled by dysplastic cells: VIN 1, 2, and 3. However, VIN 1 was not really neoplastic. It reflected infection with HPV, and although it might progress to higher-grade dysplasia or cancer, the risk was minimal. So the ISVVD revised the classification system to include only high-grade VIN—the old VIN 2 and VIN 3. HPV-associated lesions with dysplastic cells confined to the lower third of the epithelium are managed like genital warts, with observation for spontaneous regression or treatment with topical therapy or surgery.
How to screen for VIN
OBG Management: What screening strategy is recommended for VIN?
Dr. Massad: There is no such recommendation. Screening for VIN hasn’t been implemented for several reasons. Most important, other than inspection of the vulva by a clinician, there is no good screening test. VIN isn’t very common, so mass inspection for lesions is unlikely to be cost-effective. The sensitivity and specificity of inspection by a clinician aren’t known. Most lesions are found by women, their partners, or clinicians before cancer develops. And most disease is treated before cancer arises.
OBG Management: Isn’t there a need for heightened scrutiny of the vulva?
Dr. Massad: Yes. Women should examine their genitalia several times a year and seek attention if anything changes. That’s especially true for women who have risk factors, such as smoking, immunosuppression, and a history of being treated for cervical dysplasia. It’s the same concept we employ when teaching women to identify early breast lesions through self-examination.
The biggest challenges in detecting VIN are educating women to report vulvar skin changes to their clinicians for assessment and educating clinicians to examine the vulva before inserting the speculum for cervical screening and vaginal inspection.
OBG Management: Is another challenge distinguishing some forms of VIN from genital warts?
Dr. Massad: It can be a challenge, but clinicians should recall that warts are most common among women around the time of the onset of sexual activity. Older women sometimes develop warts with a new sexual partner. However, when women in their 40s and older develop new warty lesions, always suspect VIN. A woman in her 60s or older who has a new, warty-appearing vulvar lesion should be assumed to have VIN or cancer.
OBG Management: Does VIN ever regress spontaneously?
Dr. Massad: Yes. There have been reports of spontaneous regression of VIN, especially in young women. Regrettably, there are also reports of progression to cancer during observation. There are no characteristics that allow us to distinguish lesions that are going to progress from those that will regress. The ACOG-ASCCP Committee Opinion recommends treatment of all VIN.1
Can VIN be prevented?
OBG Management: The Committee Opinion recommends that the quadrivalent HPV vaccine be offered to women “in target populations” because it can decrease the risk of VIN. What are those target populations?
Dr. Massad: The target population for HPV vaccination is 11- and 12-year-old girls, but catch-up vaccination is acceptable in patients as old as 26 years.
OBG Management: Why isn’t the bivalent vaccine recommended?
Dr. Massad: Only the quadrivalent vaccine has been approved by the US Food and Drug Administration (FDA) for prevention of VIN, although, in theory, the bivalent vaccine ought to be effective as well.
When to biopsy
OBG Management: Do you recommend that any suspect lesion on the vulva be biopsied?
Dr. Massad: The decision to biopsy should be individualized. However, women who have apparent warts that fail to respond to topical therapy should undergo biopsy, as should older women with warty lesions. Keep in mind that older women may develop verrucous carcinomas and may benefit from excision of enlarging warty lesions even if a biopsy is reported as only condylomata. Clinicians should not biopsy varicosities or obvious flat nevi.
OBG Management: Is colposcopy ever helpful in assessing vulvar lesions?
Dr. Massad: Most vulvar lesions can be identified without colposcopy, but colposcopy is useful in determining the extent of lesions. It often reveals subclinical disease not evident at the time of vulvar inspection.
OBG Management: When colposcopy is used, is the procedure the same as for cervical examination?
Dr. Massad: Not exactly. The clinician should apply 5% acetic acid for 5 minutes using a gauze sponge, but the magnification should be 63 to 103—not 153, as it is for cervical examination. It’s important to distinguish hyperplasia from VIN. In general, hyperplastic lesions are faint, gray, diffuse, and flat, whereas VIN lesions are raised and irregular in shape, with sharp borders.
OBG Management: What about toluidine blue? Is it useful in inspection of lesions?
Dr. Massad: Toluidine blue stains skin that is irritated. It isn’t very specific for VIN or vulvar cancer, and it can make colposcopy difficult, so experts no longer recommend it.
OBG Management: What are the treatment options for VIN?
Dr. Massad: They include surgical excision, laser ablation, and topical therapy with 5% imiquimod. All are potentially effective. The Committee Opinion doesn’t specify a preference, except to say that excision is advised when there is any suspicion of cancer to preserve a sample for pathologic analysis. Ablation destroys the lesion, making assessment of possible invasion impossible, and imiquimod may allow disease to progress during observation.
OBG Management: The Committee Opinion recommends wide local excision when cancer is suspected. What size of margin is optimal?
Dr. Massad: In general, a margin of 5 to 10 mm around the lesion is recommended. Vulvectomy isn’t needed because close follow-up usually identifies recurrence before invasion occurs.
OBG Management: When is laser ablation a good choice?
Dr. Massad: Whenever a biopsy shows VIN and cancer is not suspected. Laser ablation is ideal when lesions are multifocal or extensive, although repeated treatments may be required to resolve small foci of residual disease. Done with careful attention to power density and depth of ablation, laser therapy can be less disfiguring than excision.
OBG Management: You mentioned 5% imiquimod. Is there evidence that it’s effective in the treatment of VIN?
Dr. Massad: Multiple randomized, controlled trials have shown 5% imiquimod to be effective against VIN, although the agent does not have approval from the FDA for that indication.5,6 Lower concentrations of imiquimod have not been studied in the treatment of VIN. Women treated with this topical therapy should be followed every 4 weeks with colposcopy because progression to cancer has been reported during imiquimod therapy. Lesions that fail to respond completely after a full course of imiquimod should be treated with excision or laser ablation.
Surveillance is critical
OBG Management: According to the Committee Opinion, the recurrence rate of VIN can reach 30% to 50%.7 Why so high?
Dr. Massad: Usual-type VIN reflects exposure to carcinogenic HPV, and differentiated VIN arises from a vulvar dystrophy. In both situations, treatments destroy VIN and arrest progress to cancer, but the entire vulvar skin remains subject to the inciting condition.
OBG Management: Would skinning vulvectomy eliminate the risk of recurrence?
Dr. Massad: Full vulvectomy is crippling and usually unnecessary. Most patients and clinicians accept the risk of recurrence of VIN to avoid the side effects of radical treatment.
OBG Management: What kind of surveillance is recommended after treatment?
Dr. Massad: Patients should perform vulvar self-examination every few months. They should also be examined 6 and 12 months after initial treatment and annually thereafter because the risk of recurrence may persist for years.
Because VIN is associated with carcinogenic HPV, women with VIN should undergo an annual Pap test.
OBG Management: Thank you, Dr. Massad. Let’s hope the incidence of this precancer begins to decline.
- Recommend the quadrivalent HPV vaccine for girls in the target age range (11 and 12 years old) to reduce the risk of VIN.
- Encourage smoking cessation.
- Make it a practice to inspect the vulva before inserting the speculum for cervical examination.
- Biopsy most pigmented lesions on the vulva. Biopsy all warty lesions in postmenopausal women and in women who fail topical treatment for genital warts.
- Treat all VIN lesions. When cancer is suspected, use wide local excision with a margin of 5 to 10 mm.
- Keep in mind that dysplastic cells can extend into hair follicles and sweat glands.
- Closely follow up all women treated for VIN (6 and 12 months after treatment and annually thereafter) and encourage them to examine their vulva several times every year. Perform an annual Pap test for any woman found to have VIN.
We want to hear from you! Tell us what you think.
1. Committee on Gynecologic Practice; American College of Obstetricians and Gynecologists. Committee Opinion #509: Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2011;118(5):1192-1194.
2. Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018-1022.
3. Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1-24.
4. Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106(6):1319-1326.
5. Van Seters M, van Beurden M, ten Kate FJW, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358(14):1465-1473.
6. Terlou A, van Seters M, Ewing PC, et al. Treatment of vaginal intraepithelial neoplasia with topical imiquimod: seven years median follow-up of a randomized clinical trial. Gynecol Oncol. 2011;121(1):157-162.
7. Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO2 laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006;100(2):271-275.
1. Committee on Gynecologic Practice; American College of Obstetricians and Gynecologists. Committee Opinion #509: Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2011;118(5):1192-1194.
2. Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018-1022.
3. Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1-24.
4. Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106(6):1319-1326.
5. Van Seters M, van Beurden M, ten Kate FJW, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358(14):1465-1473.
6. Terlou A, van Seters M, Ewing PC, et al. Treatment of vaginal intraepithelial neoplasia with topical imiquimod: seven years median follow-up of a randomized clinical trial. Gynecol Oncol. 2011;121(1):157-162.
7. Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO2 laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006;100(2):271-275.
How to prepare your patient for the many nuances of postpartum sexuality
CASE: Waiting for an OK to resume sex
L. L. is a 29-year-old woman, G1P1, who delivered a healthy infant 4 weeks ago by spontaneous vaginal birth. The delivery involved a 2-day induction of labor for preeclampsia and a second-degree tear that was repaired without complication. The patient also experienced postpartum hemorrhage that was managed with bimanual massage and uterotonics and for which she ultimately required transfusion of blood products. Her hospital course was otherwise unremarkable.
Before pregnancy, L. L. had a normal medical history and conceived spontaneously. Her antenatal course was uncomplicated.
Today, she returns for her postpartum visit. She reports being tired and says she still has some pain at the site of the tear, but reports no problems with urinary or fecal continence. She denies being depressed, and her Edinburgh Postnatal Depression Scale (EPDS) score is consistent with that report. She is breastfeeding and appears to be doing well on the progestin-only pill for contraception. She has not yet attempted intercourse because she is complying with instructions to wait until she sees you for her postpartum visit.
How should you counsel her about resuming sexual activity?

Childbirth is a central event in a woman’s life. Pregnancy and delivery are a time of psychological, biological, and physical transformation, and the postpartum period—the “fourth trimester”—is no exception. Sexual function may be affected. In fact, many women who seek assistance for sexual dissatisfaction note that their problem arose in the postpartum period.1
Postpartum sexuality involves considerably more than the physical act of genital stimulation—with or without intromission or penile penetration—and depends on more than the physical state of recovery of the vagina (after vaginal delivery). It also depends on:
- the woman’s sexual drive and motivation
- her general state of health and quality of life
- her emotional readiness to resume sexual intimacy with a partner
- her adaptation to the maternal role and ability to balance her identity as a mother with her identity as a sexual being
- her relationship with her partner.
Given all these contributing factors, many of which fall outside the scope of the clinical practice of obstetrics and gynecology, how do we go about counseling our patients about the resumption of sexual activity?
Other questions:
- How can we help patients manage expectations about the quality of their postpartum sexual function?
- What guidance can we provide regarding the interplay of psychosexual and physical aspects of the puerperium?
- Can we offer a method of screening for sexual dysfunction in the puerperium? If so, will it help prevent sexual problems or hasten their resolution?
This article addresses these issues. Ultimately, the answer to the question of when to resume sexual activity should reflect an awareness of cultural norms and taboos as well as familiarity with empirically based recommendations.
Traditional postpartum sexual education is not evidence-based and has limited effectiveness. More up-to-date strategies can be easily incorporated into even the busiest clinical practice. We offer the following counseling model for you to consider when addressing the sexual health of patients postpartum.
Educate, legitimize, and normalize
The first sexual encounter after childbirth can be an important step for couples to reclaim their intimate relationship.
Adaptation to the parental role, physical healing, hormonal changes, breastfeeding, and sleep deprivation contribute to a profound psychosocial challenge. The resumption of sexual activities and a satisfying postpartum sex life depend on many variables, many of which the patient may not even be aware.
First, do not assume that all patients are heterosexual and that intercourse is their only form of sexual activity.
Second, it is important to be proactive in antepartum and postpartum counseling and to offer anticipatory guidance. Counseling can take place any time during routine prenatal care, as well as at the time of hospital discharge and the postpartum visit.
Reassure the patient that, if sexual activity and frequency are lower during pregnancy and the postpartum period, it is likely a normal transition. Also give the patient time to talk about her expectations and perceptions. Explain to her the normal fluctuations and variability of sexual interest and enjoyment in pregnancy and the puerperium, and suggest that she consider alternative options for intimate expression, non-coital sexual activities, and mutual pleasure within her cultural context.
Be thorough
Take a comprehensive medical, obstetric, psychological, and social history as part of the sexual history. Also perform a physical intake and exam. Questions about urinary and fecal incontinence ought to be part of all postpartum assessment.
Other potential areas to address include the quality of the relationship, prepregnancy sexual function, the support network, planned or unplanned state of the pregnancy, previous pregnancy and delivery outcomes, the health status of current children, and present, previous, and future contraceptive use.29
Consider multiple visits
It is hard to know exactly when to evaluate a patient for postpartum sexual dysfunction, given the impact of pudendal nerve latency, fatigue, and breastfeeding. For this reason, assessment on multiple occasions may be appropriate. Numerous validated scales to assess sexual function can be easily incorporated into clinical practice.
Couples counseling and therapy may be needed in some cases; be aware of referral services in your area for sexual wellness specialists.
The bottom line: A “successful” sexual life does not necessarily mean adequate genital function (e.g., coital orgasm, improved clitoral blood flow, increased sexual frequency) but, rather, a sexual life that is intimate and satisfying to the individual patient.
A paucity of research
To date, research into sexuality during the postpartum period has focused primarily on the physical changes and constraints that affect the mechanics and frequency of intercourse and overall sexual satisfaction and desire.2 This perspective has begun to broaden to include the psychological aspects of sexuality.
TABLE 1
These validated tools can help you measure female sexual dysfunction
| Tool | Area assessed |
|---|---|
| Female Sexual Function Index (FSFI)30 | Desire, arousal, orgasm, and pain |
| Female Sexual Function Index 6-Item (FSFI-6)31 | Desire, arousal, orgasm, and pain |
| McCoy Female Sexual Function Questionnaire*32 | Presence of female sexual disorders |
| Brief Sexual Symptoms Checklist33 | Screener for sexual concerns |
| Female Sexual Distress Scale – Revised34 | Distress |
| Intimate Relationship Scale*35 | Changes in sexual relationship |
| Sexual Quality of Life – Female (SQol-F)36 | Quality of life in women with female sexual dysfunction |
| Golombok Rust Inventory of Sexual Satisfaction (GRISS)37 | Quality of sexual relationship |
| Decreased Sexual Desire Screener38 | Brief diagnostic tool for hypoactive sexual desire disorder |
| * Validated in pregnant and/or postpartum women | |
Women’s sexual health during the postpartum period has generally been under-researched. It wasn’t until the past decade that validated sexual function questionnaires were utilized. Although a number of these instruments are now available (TABLE 1, TABLE 2, FIGURE), it remains unclear whether they can accurately measure postpartum sexual function. Despite these limitations, significant information has been elicited that can be used to counsel patients struggling with postpartum sexual concerns.
TABLE 2
The 6-item Female Sexual Function Index*
| Question | Responses | |||||
|---|---|---|---|---|---|---|
| 0 points | 5 points | 4 points | 3 points | 2 points | 1 point | |
| How would you rate your level of sexual desire or interest? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How would you rate your level of sexual arousal (“turn on”) during sexual activity or intercourse? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How often did you become lubricated (“wet”) during sexual activity or intercourse? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| When you had sexual stimulation or intercourse, how often did you reach orgasm? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| How satisfied have you been overall with your sexual life? | No sexual activity | Very satisfied | Moderately satisfied | About equally satisfied and dissatisfied | Moderately dissatisfied | Very dissatisfied |
| How often did you experience discomfort or pain during vaginal penetration? | Did not attempt intercourse | Almost never or never | A few times | Sometimes | Most times | Almost always or always |
| *The components of this index are to be assessed over the past 4 weeks. The score is the sum of the ordinal responses to the 6 items and ranges from 2 to 30. A score of less than 19 indicates a need for further investigation, including the full-length Female Sexual Function Index. Source: Adapted from Isidori et al.31 | ||||||
Ideal period of abstinence is unknown
Although our knowledge of the female genital tract in the puerperium is based upon histologic evidence, there are no evidence-based policies to outline the ideal period of postpartum coital abstinence. It seems reasonable to assume that our traditional scientific recommendations developed in part to prevent uterine infection and disruption of sutured wounds. These concerns, combined with cultural and societal norms, have led to the routine discouragement of sexual activity until 4 to 6 weeks postpartum.
The possibility of shortening the period of postpartum abstinence was first suggested by the American College of Obstetricians and Gynecologists (ACOG) in 1984.1 In 1985, Pritchard and colleagues wrote about the individualization of postpartum prohibitions of sexual activity in Williams Obstetrics.1 The earliest time at which intercourse may be safely resumed is unknown, but the 23rd edition of Williams Obstetrics states that a woman can resume sexual intercourse as early as 2 weeks, based on her comfort and desire.3 The sixth edition of the American Academy of Pediatrics (AAP) and ACOG guidelines for perinatal care also states that the risks ought to be minimal at 2 weeks postpartum.4
BRIEF SEXUAL SYMPTOMS CHECKLIST FOR WOMEN (BSSC-W)

Reprinted from Hatzichristou et al. 33
Low desire is not unusual
Although a patient may be granted “permission” to engage in coital activity, other variables influence her decision. It is well known that sexual desire may fluctuate during pregnancy and typically decreases significantly during the third trimester.2 Many women enter the postpartum period with lower levels of sexual desire and satisfaction, and these depressed levels may continue for some time.2 Twenty-five percent of women report worsened sexual function, including diminished sexual satisfaction, during pregnancy that persists for 6 to 12 months postpartum.5 By 12 weeks postpartum, 80% to 93% of women have resumed intercourse, but as many as 83% report sexual problems during the first 3 months of the postpartum period. At 6 months, 18% to 30% of these women may still be experiencing sexual problems, including dyspareunia.5,6
In 1998, von Sydow performed a meta-content analysis of all existing studies on parental sexuality during pregnancy and the first 6 months postpartum.7 Using psychological and medical data banks, she brought together information from two branches of science and identified 59 relevant studies in English or German between 1950 and 1996. Although the majority of studies were retrospective and failed to utilize a validated instrument, von Sydow determined that, overall, sexual interest and activity were low or nonexistent during the first months after delivery. There was high variability between individuals, however, and levels of sexual interest and activity of individual women remained relatively constant from the time before pregnancy until 1 year postpartum.7 von Sydow determined that there is great variability in female sexuality during pregnancy and postpartum; this variability may represent fluctuations during this phase of life. She also determined that severe psychosexual and marital problems are much more prevalent in the postpartum period than during pregnancy and persist long after a physical cause can be used as an explanation.7
Fatigue and quality of the relationship have an impact on sexual function
De Judicibus and colleagues identified a broad range of variables that have a detrimental impact on sexuality at 12 weeks postpartum, most particularly:
- marital dissatisfaction
- dyspareunia
- fatigue
- depression
- breastfeeding.2
There is evidence to suggest that the addition of the first child reduces marital quality after the first month postpartum, and this decline in marital satisfaction continues for 6 to 18 months postpartum.2 Witting and coworkers suggested that this decline may represent a transitional phase of parenthood for some couples; data support the positive effects on overall marital satisfaction with the addition of children.8 Women who were more satisfied with their relationships reported higher sexual satisfaction and greater frequency of intercourse.2,8
Fatigue is one of the most common problems women experience during pregnancy and postpartum and is a common reason given for loss of sexual desire and interest, infrequent sexual activity, and lack of enjoyment.5 A high level of exhaustion is found during the first 8 weeks postpartum. Although it declines over the next 6 months, it does not appear to resolve completely in a good number of women.9
Don’t underestimate the impact of obstetric morbidity
Surprisingly, the long-term impact of severe obstetric events on postpartum maternal health is often overlooked. Waterstone and colleagues found that women who have severe obstetric morbidity, such as massive hemorrhage, preeclampsia, sepsis, and uterine rupture, experience significant changes in sexual health and well-being.10 They conducted a prospective cohort study of such women, measuring sexual activity, general health, and postpartum depression. They utilized two validated postnatal questionnaires—the Short Form 36 (SF-36) to measure general health and the EPDS. Women who had uncomplicated pregnancies and childbirth tended to perform well in most SF-36 categories, whereas women who had experienced severe morbidity scored worse in almost every category. These women also reported problems with intercourse. Thirteen percent of women had not resumed sexual relations by 6 to 12 months postpartum; of these women, more than half reported a fear of conceiving as a reason.
The female body undergoes dramatic physiologic, anatomic, and psychological changes immediately following delivery and throughout the restoration of its pre-pregnant state. This fourth trimester usually lasts 6 to 12 weeks.39
Uterus. The uterus undergoes rapid involution after separation of the placenta. By 2 to 4 weeks postpartum, it may no longer be palpable abdominally, and by 6 weeks, it usually has returned to its nonpregnant state and size. Seven to 14 days after delivery, a woman often experiences an episode of heavier vaginal bleeding that corresponds with the sloughing of the placental bed eschar. During this time of involution, myometrial vessels may be 5 mm or larger in diameter.40
Lochia. The postpartum lochia begins to change within days of birth, transitioning through its stages of lochia rubra, serosa, and alba. It decreases by 3 weeks postpartum and is likely completely resolved by 6 weeks.
Prolactin is responsible for lactogenesis. When the prolactin level is maintained through breastfeeding, it depresses ovarian production of estrogen by suppressing pituitary gonadotropin secretion, triggering a period of “steroid starvation” after the loss of estrogen and progesterone production from the placenta.1
Vagina. Early in the postpartum period, the vagina is typically edematous and lax and, as a result of parturition, there may be not only a spontaneous tear or episiotomy that must heal, but superficial small tears that do not require suturing. Ruggae begin to reappear by 3 weeks, and the vaginal epithelium will begin to mature under the influence of estrogen production. Much of this tissue damage is healed by 6 weeks postpartum.
The perception of pregnant and postpartum women’s sexuality varies, based on religious and cultural norms. In some religions and cultures, sexual activity is forbidden for 2 to 3 months postpartum; in others, it is prohibited until the child is weaned from the breast. The postpartum woman and lochia have traditionally been perceived as unclean, and many religions have specific proscriptions regarding the management of this time in a woman’s life.1 Although early cultures did not study these issues specifically, their doctrines suggest that they had some awareness of the natural physiologic transition of a woman’s body after she has given birth.
Exploring the role of body image
Paul and coworkers prospectively assessed female sexual function, body image, and pelvic symptoms from the first trimester until 6 months postpartum.11 They utilized the validated questionnaire instruments of the Female Sexual Function Index (FSFI), the Body Exposure during Sexual Activities Questionnaire (BESAQ), the short forms of the Urogenital Distress Inventory (UDI-6), the Incontinence Impact Questionnaire (IIQ-7), and the Fecal Incontinence Quality of Life Scale (FIQOL). They found that sexual activity and sexual function scores were highest before pregnancy, declined between the first and third trimesters, and did not return to pre-pregnancy baselines even by 6 months postpartum.11
Differences in sexual practices contributed to these patterns. Kissing, fondling, and vaginal intercourse remained stable across pregnancy, whereas oral sex, breast stimulation, and masturbation declined in the third trimester.
The decline of these activities during pregnancy and postpartum has been seen in other studies as well.12
Obstacles to sexual activity also changed across pregnancy and the postpartum period. Vaginal pain was more problematic in the third trimester and postpartum, whereas feelings of unattractiveness and issues of body image were present throughout pregnancy and at their worst in the postpartum period. Sexual function scores based on the FSFI declined during pregnancy and did not return to pre-pregnancy or first-trimester levels by 6 months postpartum. Urinary symptoms, as measured by the UDI-6, were associated with lower sexual function scores during the postpartum period. The association between urinary incontinence and sexual dysfunction has been seen in other studies.13,14
The enduring effects of perineal trauma
Childbirth may physically affect a woman’s sexual function through perineal trauma, pudendal neuropathy, and vaginal dryness associated with breastfeeding. There is an obvious connection between perineal laceration and perineal pain and problems with intercourse.5 Overall, dyspareunia is reported by 41% to 67% of women 2 to 3 months after delivery.15 Women who have an episiotomy complain of increased perineal pain and delayed return of sexual activity, compared with women who deliver with an intact perineum.16
Persistent dyspareunia is strongly associated with the severity of perineal trauma and operative vaginal delivery.3,17 Multiple studies have investigated this association and found a positive correlation 3 to 6 months postpartum,6,9,17 but the long-term effects and association remain unclear.18
Findings from research. Rogers and colleagues prospectively studied the effect of perineal trauma on postpartum sexual function in a midwifery population of women who had a low rate of episiotomy and operative vaginal delivery.6 They utilized the Intimate Relationship Scale (IRS), a validated questionnaire to measure postpartum sexual function in couples. Most women in this study had resumed sexual activity by 3 months postpartum and did not have postpartum inactivity or dysfunction, based on their IRS scores. However, women who were identified as having experienced major trauma (second-, third-, or fourth-degree laceration or a repaired first-degree laceration) had significantly less desire to engage in activities such as touching and stroking with their partner.6
Present-day limits on the routine use of episiotomy and operative vaginal delivery have yielded a lower rate of third- and fourth-degree laceration.19 Second-degree lacerations are common and constitute the majority of perineal trauma in births without episiotomy.20 There is evidence that the use of synthetic absorbable suture, such as polyglactin, rather than chromic suture, results in less postpartum perineal pain, as does leaving the well-approximated perineal skin edges unsutured.20
Signorello and coworkers found that second-, third-, and fourth-degree lacerations increased the risk of postpartum dyspareunia; operative vaginal delivery (forceps or vacuum) was also an independent risk factor for dyspareunia.21
The impact of route of delivery
Some researchers have concluded that the route of delivery has an impact on the long-term pelvic floor health of women.18 In 1986, Snooks and colleagues analyzed possible obstetric risk factors for damage to the innervation of the pelvic floor, which can lead to both stress urinary and anorectal incontinence.22 They found that the process of vaginal delivery causes a compression and stretch type of injury to the pudendal nerve, as well as the possibility of severe perineal lacerations. This injury may be less likely to occur when cesarean delivery is performed before labor, avoiding direct perineal trauma and possible pudendal neuropathy.15 Because the pudendal nerve mediates some of the reflex pathways in the female sexual response, it is plausible that damage to it could result in sexual dysfunction.
Women who deliver vaginally have a higher rate of fecal and urinary incontinence than women who deliver by cesarean.16,23 The presence of incontinence, however, does not always have a significant long-term effect on one’s sexual life.6
In the Term Breech Trial, the route of delivery had no impact on the resumption of intercourse, dyspareunia, or sexual satisfaction.23 Although the trial was randomized and controlled, it had many limitations that call its generalizability into question in regard to postpartum sexual dysfunction.
The National Institutes of Health (NIH) State-of-the-Science Conference on Cesarean Delivery on Maternal Request indicated that, by 6 months postpartum, there is no difference in sexual function based on the route of delivery.24 However, Lydon-Rochelle and colleagues used the SF-36 to assess reported general health status and found that women who had cesarean delivery or assisted vaginal delivery exhibited significantly poorer postpartum functional status than women who had spontaneous vaginal delivery in five areas at 7 weeks postpartum: physical functioning, mental health, general health perception, bodily pain, social functioning, and ability to perform daily activities.25 Women were more likely to be readmitted to the hospital and more likely to report fatigue during the first 2 months after cesarean delivery.9 It appears that women who undergo cesarean delivery have an elevated risk of nondyspareunia-related causes of sexual dysfunction. Any protective effect of cesarean on sexual function is limited to the early postnatal period and is related to the absence of perineal injury.18
How breastfeeding can affect sexual desire
Evidence is strong that breastfeeding reduces a woman’s sexual desire and the frequency of intercourse.1,5 A high level of prolactin suppresses ovarian production of estrogen, thereby reducing vaginal lubrication. Some women and their partner may identify this loss of lubrication as a lack of arousal. This type of vaginal dryness should be explained, and the use of a lubricant should be encouraged in breastfeeding women.
Nipple sensitivity may develop, making touching and foreplay uncomfortable in some women. One third to one half of mothers find breastfeeding to be an erotic experience, and one fourth feel guilty about this sexual excitement; others stop nursing or wean early due to these feelings.1,7 Women are often not educated about the relationship between the release of oxytocin, uterine contractions, milk ejection, sexual arousal, and orgasm; raising the subject can help to diminish any potential distress over this response.
Sleep disturbances from feeding on demand contribute to fatigue and exhaustion.
Many women may not realize that their loss of interest in sex may be because they are receiving sufficient physical contact or touching through their nurturing interactions with the baby. This may leave the partner feeling isolated and envious of the mother-baby relationship.
Couples should be encouraged to discuss these feelings to avoid misperceptions and to maintain the relationship dyad as a priority to prevent the development of relationship problems.
The majority of women will discuss contraception with a health provider, but only 15% will voluntarily discuss their sexual needs or dysfunction.17 This finding is alarming given that, during the postpartum period, two of every three new mothers will experience at least one problem related to sexual function, including dyspareunia, decreased libido, difficulty achieving orgasm, and vaginal dryness.41 This lack of discussion with a health-care provider may be the result of several variables: incomplete knowledge on the part of the provider about what affects sexual function, poor training in the taking of an effective sexual history, and uneasiness on the part of the patient about discussing the issue.5,42
Postnatal depression takes a toll
Depressed mood and emotional lability in the postpartum period are negatively associated with sexual interest, enjoyment, coital activity, and perceived tenderness of the partner.7 Conversely, reduced sexual interest, desire and satisfaction; a lower frequency of intercourse; and later resumption of intercourse are associated with a higher number of psychiatric symptoms in the postpartum period.2 Between 10% and 15% of women experience postpartum depression (PPD).26 Depression has been associated with a decreased frequency and interest in sexual activity at 8 to 12 weeks postpartum.2,5
Chivers and colleagues assessed sexual functioning and sexual behavior in women with and without symptoms of PPD using the FSFI and EPDS. Although theirs was a small study, they found that women who had depressive symptoms also reported poorer functioning in regard to sexual arousal, orgasm, pain, lubrication, and sexual satisfaction.26 Morof and coworkers found that women who had PPD were less likely to have resumed intercourse by 6 months postpartum; they were also less likely to engage in other sexual activities.27
Role of pharmacotherapy
Many women are started on antidepressant medication near the time of delivery or during the immediate postpartum period. Often, serotonin reuptake inhibitors (SRIs) are used because there is minimal transmission of this class of medication through breast milk. However, the potential sexual side effects of these medications should be discussed because they are the agents most commonly associated with female sexual dysfunction.28
For clinicians
American Association of Sex Educators, Counselors, and Therapists – A not-for-profit, interdisciplinary professional organization comprising sexuality educators, sexuality counselors, sex therapists, physicians, social workers, and other clinicians. Its home page links to a referral page and other resources. http://www.aasect.org" target="_blank">http://www.aasect.org
Association of Reproductive Health Professionals offers a resource for clinicians on postpartum counseling about sexuality. http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception" target="_blank">http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception
For patients
Mayo Clinic provides a fact sheet entitled “Sex after pregnancy: Set your own timeline.” http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146" target="_blank">http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146
Sex and a Healthier You – This site offers information for patients on sexuality and relationships. http://www.sexandahealthieryou.org/sex-health/index.html" target="_blank">http://www.sexandahealthieryou.org/sex-health/index.html
We want to hear from you! Tell us what you think.
1. Reamy KJ, White SE. Sexuality in the puerperium: a review. Arch Sex Behav. 1987;16(2):165-186.
2. De Judicibus MA, McCabe MP. Psychological factors and the sexuality of pregnant and postpartum women. J Sex Res. 2002;39(2):94-103.
3. The puerperium. In: Cunningham FG Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill Co.; 2010:646-660.
4. The American Academy of Pediatrics (AAP), American College of Obstetricians Gynecologists (ACOG) Guidelines for perinatal care. 6th ed. Washington DC: AAP, ACOG; 2008.
5. Glazener CM. Sexual function after childbirth: women’s experiences persistent morbidity and lack of professional recognition. Br J Obstet Gynaecol. 1997;104(3):330-335.
6. Rogers RG, Borders, N,, Leeman L, Albers L. Does spontaneous genital tract trauma impact postpartum sexual function? J Midwifery Womens Health. 2009;54(2):98-103.
7. von Sydow K. Sexuality during pregnancy and after childbirth: a metacontent analysis of 59 studies. J Psychosom Res. 1999;47(1):27-49.
8. Witting K, Santtila P, Alanko K, et al. Female sexual function and its associations with number of children, pregnancy, and relationship satisfaction. J Sex Marital Ther. 2008;34(2):89-106.
9. Thompson JF, Roberts CL, Currie M, Elwood DA. Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Birth. 2002;29(2):83-94.
10. Waterstone M, Wolfe C, Hooper R, Bewley S. Postnatal morbidity after childbirth and severe obstetric morbidity. BJOG. 2003;110(2):128-133.
11. Pauls RN, Occhino JA, Dryfhout VL. Effects of pregnancy on female sexual function and body image: A prospective study. J Sex Med. 2008;5(8):1915-1922.
12. von Sydow K, Ullmeyer M, Happ N. Sexual activity during pregnancy and after childbirth: Results from the Sexual P Questionnaire. J Psychosom Obstet Gynaecol. 2001;22(1):29-40.
13. Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Rsearch Group. Neurourol Urodyn. 1995;14(2):131-139.
14. Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC; Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99(2):281-289.
15. Handa VL. Sexual function and childbirth. Semin Perinatol. 2006;30(5):253-256.
16. Klein MC, Gauthier RJ, Robbins JM, et al. Relationship of episiotomy to perineal trauma and morbidity, sexual dysfunction, and pelvic floor relaxation. Am J Obstet Gynecol. 1994;171(3):591-598.
17. Barrett G, Pendry E, Peacock J, Victor C, Thakar, Manyonda I. Women’s sexual health after childbirth. BJOG. 2000;107(2):186-195.
18. Barrett G, Peacock J, Victor CR, Manyonda I. Cesarean section and postnatal sexual health. Birth. 2005;32(4):306-311.
19. Eason E, Labrecque M, Wells G, Feldman P. Preventing perineal trauma during childbirth: a systematic review. Obstet Gynecol. 2000;95(3):464-471.
20. Leeman LM, Rogers RG, Greulich B, Albers LL. Do unsutured second-degree perineal lacerations affect postpartum functional outcomes? J Am Board Fam Med. 2007;20(5):451-457.
21. Signorello L, Harlow BL, Chekos AK, Repke JT. Postpartum sexual functioning and its relationship to perineal trauma: A retrospective cohort study of primiparous women. Am J Obstet Gynecol. 2001;184(5):881-890.
22. Snooks SJ, Swash M, Henry MW, Setchell M. Risk factors in childbirth causing damage to the pelvic floor innervation. In J Colorect Dis. 1986;1(1):20-24.
23. Hannah ME, Whyte H, Hannah WJ, et al. Term Breech Trial Collaborative Group. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: the international randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191(3):917-927.
24. NIH State-of-the-Science Conference Statement on Cesarean Delivery on Maternal Request NIH Consens Sci Statements. 2006;23:1-29.http://consensus.nih.gov/2006/cesareanstatement.htm. Accessed December 6 2011.
25. Lydon-Rochelle MT, Holt VL, Martin DP. Delivery method and self-reported postpartum general health status among primiparous women. Paediatr Perinat Epidemiol. 2001;15(3):232-240.
26. Chivers ML, Pittini MD, Grigoriadis S, Villegas L, Ross LE. The relationship between sexual functioning and depressive symptomatology in postpartum women: a pilot study. J Sex Med. 2011;8(3):792-799.
27. Morof D, Barrett G, Peacock J, Victor CR, Manyonda I. Postnatal depression and sexual health after childbirth. Obstet Gynecol. 2003;102(6):1318-1325.
28. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No.119: Female sexual dysfunction. Obstet Gynecol. 2011;117(4):996-1007.
29. Read J. Sexual problems associated with infertility pregnancy and ageing. BMJ. 2004;329(7465):559-561.
30. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
31. Isidori AM, Pozza C, Esposito K, et al. The Female Sexual Function Index (FSFI): Development and validation of a 6-item version of the female sexual function index (FSFI) as a diagnostic tool for female sexual dysfunction. J Sex Med. 2010;7(3):1139-11.
32. McCoy NL. The McCoy Female Sexuality Questionnaire. Quality Life Res. 2000;9(suppl 6):739-745.
33. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 1):337-348.
34. DeRogatis LR, Allgood A, Rosen RC, Leiblum S, Zipfel L, Guo CY. Development and evaluation of the Women’s Sexual Interest Diagnostic Interview (WSID): a structured interview to diagnose hypoactive sexual desire disorder (HSDD) in standardized patients. J Sex Med. 2008;5(12):2827-2841.
35. Fischman SH, Rankin EA, Soeken KL, Lenz ER. Changes in sexual relationships in postpartum couples. J Obstet Gynecol Neonatal Nurs. 1986;15(1):58-63.
36. Sillis T, Wunderlich G, Pyke R, et al. The Sexual Interest and Desire Inventory-Female (SIDI-F): item response analyses of data from women diagnosed with hypoactive sexual desire disorder. J Sex Med. 2005;2(6):801-818.
37. Rust J, Golombok S. The Golombok-Rust Inventory of Sexual Satisfaction (GRISS). Br J Clin Psych. 1985;24(Pt 1):63-64.
38. Clayton AH, Balon R. The impact of mental illness and psychotropic medications on sexual functioning: the evidence and management. J Sex Med. 2009;6(5):1200-1213.
39. Jennings B, Edmundson M. The postpartum period: after confinement: the fourth trimester. Clin Obstet Gynecol. 1980;23(4):1093-1103.
40. Oppenheimer LS, Sheriff EA, Goodman JDS, shah D, James CE. The duration of lochia. Br J Obstet Gynaecol. 1986;93(7):754-757.
41. Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(4):263-267.
42. Pancholy AB, Goldenhar L, Fellner AN, Crisp C, Kleeman S, Pauls R. Resident education and training in female sexuality: results of a national survey. J Sex Med. 2011;8(2):361-366.
CASE: Waiting for an OK to resume sex
L. L. is a 29-year-old woman, G1P1, who delivered a healthy infant 4 weeks ago by spontaneous vaginal birth. The delivery involved a 2-day induction of labor for preeclampsia and a second-degree tear that was repaired without complication. The patient also experienced postpartum hemorrhage that was managed with bimanual massage and uterotonics and for which she ultimately required transfusion of blood products. Her hospital course was otherwise unremarkable.
Before pregnancy, L. L. had a normal medical history and conceived spontaneously. Her antenatal course was uncomplicated.
Today, she returns for her postpartum visit. She reports being tired and says she still has some pain at the site of the tear, but reports no problems with urinary or fecal continence. She denies being depressed, and her Edinburgh Postnatal Depression Scale (EPDS) score is consistent with that report. She is breastfeeding and appears to be doing well on the progestin-only pill for contraception. She has not yet attempted intercourse because she is complying with instructions to wait until she sees you for her postpartum visit.
How should you counsel her about resuming sexual activity?

Childbirth is a central event in a woman’s life. Pregnancy and delivery are a time of psychological, biological, and physical transformation, and the postpartum period—the “fourth trimester”—is no exception. Sexual function may be affected. In fact, many women who seek assistance for sexual dissatisfaction note that their problem arose in the postpartum period.1
Postpartum sexuality involves considerably more than the physical act of genital stimulation—with or without intromission or penile penetration—and depends on more than the physical state of recovery of the vagina (after vaginal delivery). It also depends on:
- the woman’s sexual drive and motivation
- her general state of health and quality of life
- her emotional readiness to resume sexual intimacy with a partner
- her adaptation to the maternal role and ability to balance her identity as a mother with her identity as a sexual being
- her relationship with her partner.
Given all these contributing factors, many of which fall outside the scope of the clinical practice of obstetrics and gynecology, how do we go about counseling our patients about the resumption of sexual activity?
Other questions:
- How can we help patients manage expectations about the quality of their postpartum sexual function?
- What guidance can we provide regarding the interplay of psychosexual and physical aspects of the puerperium?
- Can we offer a method of screening for sexual dysfunction in the puerperium? If so, will it help prevent sexual problems or hasten their resolution?
This article addresses these issues. Ultimately, the answer to the question of when to resume sexual activity should reflect an awareness of cultural norms and taboos as well as familiarity with empirically based recommendations.
Traditional postpartum sexual education is not evidence-based and has limited effectiveness. More up-to-date strategies can be easily incorporated into even the busiest clinical practice. We offer the following counseling model for you to consider when addressing the sexual health of patients postpartum.
Educate, legitimize, and normalize
The first sexual encounter after childbirth can be an important step for couples to reclaim their intimate relationship.
Adaptation to the parental role, physical healing, hormonal changes, breastfeeding, and sleep deprivation contribute to a profound psychosocial challenge. The resumption of sexual activities and a satisfying postpartum sex life depend on many variables, many of which the patient may not even be aware.
First, do not assume that all patients are heterosexual and that intercourse is their only form of sexual activity.
Second, it is important to be proactive in antepartum and postpartum counseling and to offer anticipatory guidance. Counseling can take place any time during routine prenatal care, as well as at the time of hospital discharge and the postpartum visit.
Reassure the patient that, if sexual activity and frequency are lower during pregnancy and the postpartum period, it is likely a normal transition. Also give the patient time to talk about her expectations and perceptions. Explain to her the normal fluctuations and variability of sexual interest and enjoyment in pregnancy and the puerperium, and suggest that she consider alternative options for intimate expression, non-coital sexual activities, and mutual pleasure within her cultural context.
Be thorough
Take a comprehensive medical, obstetric, psychological, and social history as part of the sexual history. Also perform a physical intake and exam. Questions about urinary and fecal incontinence ought to be part of all postpartum assessment.
Other potential areas to address include the quality of the relationship, prepregnancy sexual function, the support network, planned or unplanned state of the pregnancy, previous pregnancy and delivery outcomes, the health status of current children, and present, previous, and future contraceptive use.29
Consider multiple visits
It is hard to know exactly when to evaluate a patient for postpartum sexual dysfunction, given the impact of pudendal nerve latency, fatigue, and breastfeeding. For this reason, assessment on multiple occasions may be appropriate. Numerous validated scales to assess sexual function can be easily incorporated into clinical practice.
Couples counseling and therapy may be needed in some cases; be aware of referral services in your area for sexual wellness specialists.
The bottom line: A “successful” sexual life does not necessarily mean adequate genital function (e.g., coital orgasm, improved clitoral blood flow, increased sexual frequency) but, rather, a sexual life that is intimate and satisfying to the individual patient.
A paucity of research
To date, research into sexuality during the postpartum period has focused primarily on the physical changes and constraints that affect the mechanics and frequency of intercourse and overall sexual satisfaction and desire.2 This perspective has begun to broaden to include the psychological aspects of sexuality.
TABLE 1
These validated tools can help you measure female sexual dysfunction
| Tool | Area assessed |
|---|---|
| Female Sexual Function Index (FSFI)30 | Desire, arousal, orgasm, and pain |
| Female Sexual Function Index 6-Item (FSFI-6)31 | Desire, arousal, orgasm, and pain |
| McCoy Female Sexual Function Questionnaire*32 | Presence of female sexual disorders |
| Brief Sexual Symptoms Checklist33 | Screener for sexual concerns |
| Female Sexual Distress Scale – Revised34 | Distress |
| Intimate Relationship Scale*35 | Changes in sexual relationship |
| Sexual Quality of Life – Female (SQol-F)36 | Quality of life in women with female sexual dysfunction |
| Golombok Rust Inventory of Sexual Satisfaction (GRISS)37 | Quality of sexual relationship |
| Decreased Sexual Desire Screener38 | Brief diagnostic tool for hypoactive sexual desire disorder |
| * Validated in pregnant and/or postpartum women | |
Women’s sexual health during the postpartum period has generally been under-researched. It wasn’t until the past decade that validated sexual function questionnaires were utilized. Although a number of these instruments are now available (TABLE 1, TABLE 2, FIGURE), it remains unclear whether they can accurately measure postpartum sexual function. Despite these limitations, significant information has been elicited that can be used to counsel patients struggling with postpartum sexual concerns.
TABLE 2
The 6-item Female Sexual Function Index*
| Question | Responses | |||||
|---|---|---|---|---|---|---|
| 0 points | 5 points | 4 points | 3 points | 2 points | 1 point | |
| How would you rate your level of sexual desire or interest? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How would you rate your level of sexual arousal (“turn on”) during sexual activity or intercourse? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How often did you become lubricated (“wet”) during sexual activity or intercourse? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| When you had sexual stimulation or intercourse, how often did you reach orgasm? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| How satisfied have you been overall with your sexual life? | No sexual activity | Very satisfied | Moderately satisfied | About equally satisfied and dissatisfied | Moderately dissatisfied | Very dissatisfied |
| How often did you experience discomfort or pain during vaginal penetration? | Did not attempt intercourse | Almost never or never | A few times | Sometimes | Most times | Almost always or always |
| *The components of this index are to be assessed over the past 4 weeks. The score is the sum of the ordinal responses to the 6 items and ranges from 2 to 30. A score of less than 19 indicates a need for further investigation, including the full-length Female Sexual Function Index. Source: Adapted from Isidori et al.31 | ||||||
Ideal period of abstinence is unknown
Although our knowledge of the female genital tract in the puerperium is based upon histologic evidence, there are no evidence-based policies to outline the ideal period of postpartum coital abstinence. It seems reasonable to assume that our traditional scientific recommendations developed in part to prevent uterine infection and disruption of sutured wounds. These concerns, combined with cultural and societal norms, have led to the routine discouragement of sexual activity until 4 to 6 weeks postpartum.
The possibility of shortening the period of postpartum abstinence was first suggested by the American College of Obstetricians and Gynecologists (ACOG) in 1984.1 In 1985, Pritchard and colleagues wrote about the individualization of postpartum prohibitions of sexual activity in Williams Obstetrics.1 The earliest time at which intercourse may be safely resumed is unknown, but the 23rd edition of Williams Obstetrics states that a woman can resume sexual intercourse as early as 2 weeks, based on her comfort and desire.3 The sixth edition of the American Academy of Pediatrics (AAP) and ACOG guidelines for perinatal care also states that the risks ought to be minimal at 2 weeks postpartum.4
BRIEF SEXUAL SYMPTOMS CHECKLIST FOR WOMEN (BSSC-W)

Reprinted from Hatzichristou et al. 33
Low desire is not unusual
Although a patient may be granted “permission” to engage in coital activity, other variables influence her decision. It is well known that sexual desire may fluctuate during pregnancy and typically decreases significantly during the third trimester.2 Many women enter the postpartum period with lower levels of sexual desire and satisfaction, and these depressed levels may continue for some time.2 Twenty-five percent of women report worsened sexual function, including diminished sexual satisfaction, during pregnancy that persists for 6 to 12 months postpartum.5 By 12 weeks postpartum, 80% to 93% of women have resumed intercourse, but as many as 83% report sexual problems during the first 3 months of the postpartum period. At 6 months, 18% to 30% of these women may still be experiencing sexual problems, including dyspareunia.5,6
In 1998, von Sydow performed a meta-content analysis of all existing studies on parental sexuality during pregnancy and the first 6 months postpartum.7 Using psychological and medical data banks, she brought together information from two branches of science and identified 59 relevant studies in English or German between 1950 and 1996. Although the majority of studies were retrospective and failed to utilize a validated instrument, von Sydow determined that, overall, sexual interest and activity were low or nonexistent during the first months after delivery. There was high variability between individuals, however, and levels of sexual interest and activity of individual women remained relatively constant from the time before pregnancy until 1 year postpartum.7 von Sydow determined that there is great variability in female sexuality during pregnancy and postpartum; this variability may represent fluctuations during this phase of life. She also determined that severe psychosexual and marital problems are much more prevalent in the postpartum period than during pregnancy and persist long after a physical cause can be used as an explanation.7
Fatigue and quality of the relationship have an impact on sexual function
De Judicibus and colleagues identified a broad range of variables that have a detrimental impact on sexuality at 12 weeks postpartum, most particularly:
- marital dissatisfaction
- dyspareunia
- fatigue
- depression
- breastfeeding.2
There is evidence to suggest that the addition of the first child reduces marital quality after the first month postpartum, and this decline in marital satisfaction continues for 6 to 18 months postpartum.2 Witting and coworkers suggested that this decline may represent a transitional phase of parenthood for some couples; data support the positive effects on overall marital satisfaction with the addition of children.8 Women who were more satisfied with their relationships reported higher sexual satisfaction and greater frequency of intercourse.2,8
Fatigue is one of the most common problems women experience during pregnancy and postpartum and is a common reason given for loss of sexual desire and interest, infrequent sexual activity, and lack of enjoyment.5 A high level of exhaustion is found during the first 8 weeks postpartum. Although it declines over the next 6 months, it does not appear to resolve completely in a good number of women.9
Don’t underestimate the impact of obstetric morbidity
Surprisingly, the long-term impact of severe obstetric events on postpartum maternal health is often overlooked. Waterstone and colleagues found that women who have severe obstetric morbidity, such as massive hemorrhage, preeclampsia, sepsis, and uterine rupture, experience significant changes in sexual health and well-being.10 They conducted a prospective cohort study of such women, measuring sexual activity, general health, and postpartum depression. They utilized two validated postnatal questionnaires—the Short Form 36 (SF-36) to measure general health and the EPDS. Women who had uncomplicated pregnancies and childbirth tended to perform well in most SF-36 categories, whereas women who had experienced severe morbidity scored worse in almost every category. These women also reported problems with intercourse. Thirteen percent of women had not resumed sexual relations by 6 to 12 months postpartum; of these women, more than half reported a fear of conceiving as a reason.
The female body undergoes dramatic physiologic, anatomic, and psychological changes immediately following delivery and throughout the restoration of its pre-pregnant state. This fourth trimester usually lasts 6 to 12 weeks.39
Uterus. The uterus undergoes rapid involution after separation of the placenta. By 2 to 4 weeks postpartum, it may no longer be palpable abdominally, and by 6 weeks, it usually has returned to its nonpregnant state and size. Seven to 14 days after delivery, a woman often experiences an episode of heavier vaginal bleeding that corresponds with the sloughing of the placental bed eschar. During this time of involution, myometrial vessels may be 5 mm or larger in diameter.40
Lochia. The postpartum lochia begins to change within days of birth, transitioning through its stages of lochia rubra, serosa, and alba. It decreases by 3 weeks postpartum and is likely completely resolved by 6 weeks.
Prolactin is responsible for lactogenesis. When the prolactin level is maintained through breastfeeding, it depresses ovarian production of estrogen by suppressing pituitary gonadotropin secretion, triggering a period of “steroid starvation” after the loss of estrogen and progesterone production from the placenta.1
Vagina. Early in the postpartum period, the vagina is typically edematous and lax and, as a result of parturition, there may be not only a spontaneous tear or episiotomy that must heal, but superficial small tears that do not require suturing. Ruggae begin to reappear by 3 weeks, and the vaginal epithelium will begin to mature under the influence of estrogen production. Much of this tissue damage is healed by 6 weeks postpartum.
The perception of pregnant and postpartum women’s sexuality varies, based on religious and cultural norms. In some religions and cultures, sexual activity is forbidden for 2 to 3 months postpartum; in others, it is prohibited until the child is weaned from the breast. The postpartum woman and lochia have traditionally been perceived as unclean, and many religions have specific proscriptions regarding the management of this time in a woman’s life.1 Although early cultures did not study these issues specifically, their doctrines suggest that they had some awareness of the natural physiologic transition of a woman’s body after she has given birth.
Exploring the role of body image
Paul and coworkers prospectively assessed female sexual function, body image, and pelvic symptoms from the first trimester until 6 months postpartum.11 They utilized the validated questionnaire instruments of the Female Sexual Function Index (FSFI), the Body Exposure during Sexual Activities Questionnaire (BESAQ), the short forms of the Urogenital Distress Inventory (UDI-6), the Incontinence Impact Questionnaire (IIQ-7), and the Fecal Incontinence Quality of Life Scale (FIQOL). They found that sexual activity and sexual function scores were highest before pregnancy, declined between the first and third trimesters, and did not return to pre-pregnancy baselines even by 6 months postpartum.11
Differences in sexual practices contributed to these patterns. Kissing, fondling, and vaginal intercourse remained stable across pregnancy, whereas oral sex, breast stimulation, and masturbation declined in the third trimester.
The decline of these activities during pregnancy and postpartum has been seen in other studies as well.12
Obstacles to sexual activity also changed across pregnancy and the postpartum period. Vaginal pain was more problematic in the third trimester and postpartum, whereas feelings of unattractiveness and issues of body image were present throughout pregnancy and at their worst in the postpartum period. Sexual function scores based on the FSFI declined during pregnancy and did not return to pre-pregnancy or first-trimester levels by 6 months postpartum. Urinary symptoms, as measured by the UDI-6, were associated with lower sexual function scores during the postpartum period. The association between urinary incontinence and sexual dysfunction has been seen in other studies.13,14
The enduring effects of perineal trauma
Childbirth may physically affect a woman’s sexual function through perineal trauma, pudendal neuropathy, and vaginal dryness associated with breastfeeding. There is an obvious connection between perineal laceration and perineal pain and problems with intercourse.5 Overall, dyspareunia is reported by 41% to 67% of women 2 to 3 months after delivery.15 Women who have an episiotomy complain of increased perineal pain and delayed return of sexual activity, compared with women who deliver with an intact perineum.16
Persistent dyspareunia is strongly associated with the severity of perineal trauma and operative vaginal delivery.3,17 Multiple studies have investigated this association and found a positive correlation 3 to 6 months postpartum,6,9,17 but the long-term effects and association remain unclear.18
Findings from research. Rogers and colleagues prospectively studied the effect of perineal trauma on postpartum sexual function in a midwifery population of women who had a low rate of episiotomy and operative vaginal delivery.6 They utilized the Intimate Relationship Scale (IRS), a validated questionnaire to measure postpartum sexual function in couples. Most women in this study had resumed sexual activity by 3 months postpartum and did not have postpartum inactivity or dysfunction, based on their IRS scores. However, women who were identified as having experienced major trauma (second-, third-, or fourth-degree laceration or a repaired first-degree laceration) had significantly less desire to engage in activities such as touching and stroking with their partner.6
Present-day limits on the routine use of episiotomy and operative vaginal delivery have yielded a lower rate of third- and fourth-degree laceration.19 Second-degree lacerations are common and constitute the majority of perineal trauma in births without episiotomy.20 There is evidence that the use of synthetic absorbable suture, such as polyglactin, rather than chromic suture, results in less postpartum perineal pain, as does leaving the well-approximated perineal skin edges unsutured.20
Signorello and coworkers found that second-, third-, and fourth-degree lacerations increased the risk of postpartum dyspareunia; operative vaginal delivery (forceps or vacuum) was also an independent risk factor for dyspareunia.21
The impact of route of delivery
Some researchers have concluded that the route of delivery has an impact on the long-term pelvic floor health of women.18 In 1986, Snooks and colleagues analyzed possible obstetric risk factors for damage to the innervation of the pelvic floor, which can lead to both stress urinary and anorectal incontinence.22 They found that the process of vaginal delivery causes a compression and stretch type of injury to the pudendal nerve, as well as the possibility of severe perineal lacerations. This injury may be less likely to occur when cesarean delivery is performed before labor, avoiding direct perineal trauma and possible pudendal neuropathy.15 Because the pudendal nerve mediates some of the reflex pathways in the female sexual response, it is plausible that damage to it could result in sexual dysfunction.
Women who deliver vaginally have a higher rate of fecal and urinary incontinence than women who deliver by cesarean.16,23 The presence of incontinence, however, does not always have a significant long-term effect on one’s sexual life.6
In the Term Breech Trial, the route of delivery had no impact on the resumption of intercourse, dyspareunia, or sexual satisfaction.23 Although the trial was randomized and controlled, it had many limitations that call its generalizability into question in regard to postpartum sexual dysfunction.
The National Institutes of Health (NIH) State-of-the-Science Conference on Cesarean Delivery on Maternal Request indicated that, by 6 months postpartum, there is no difference in sexual function based on the route of delivery.24 However, Lydon-Rochelle and colleagues used the SF-36 to assess reported general health status and found that women who had cesarean delivery or assisted vaginal delivery exhibited significantly poorer postpartum functional status than women who had spontaneous vaginal delivery in five areas at 7 weeks postpartum: physical functioning, mental health, general health perception, bodily pain, social functioning, and ability to perform daily activities.25 Women were more likely to be readmitted to the hospital and more likely to report fatigue during the first 2 months after cesarean delivery.9 It appears that women who undergo cesarean delivery have an elevated risk of nondyspareunia-related causes of sexual dysfunction. Any protective effect of cesarean on sexual function is limited to the early postnatal period and is related to the absence of perineal injury.18
How breastfeeding can affect sexual desire
Evidence is strong that breastfeeding reduces a woman’s sexual desire and the frequency of intercourse.1,5 A high level of prolactin suppresses ovarian production of estrogen, thereby reducing vaginal lubrication. Some women and their partner may identify this loss of lubrication as a lack of arousal. This type of vaginal dryness should be explained, and the use of a lubricant should be encouraged in breastfeeding women.
Nipple sensitivity may develop, making touching and foreplay uncomfortable in some women. One third to one half of mothers find breastfeeding to be an erotic experience, and one fourth feel guilty about this sexual excitement; others stop nursing or wean early due to these feelings.1,7 Women are often not educated about the relationship between the release of oxytocin, uterine contractions, milk ejection, sexual arousal, and orgasm; raising the subject can help to diminish any potential distress over this response.
Sleep disturbances from feeding on demand contribute to fatigue and exhaustion.
Many women may not realize that their loss of interest in sex may be because they are receiving sufficient physical contact or touching through their nurturing interactions with the baby. This may leave the partner feeling isolated and envious of the mother-baby relationship.
Couples should be encouraged to discuss these feelings to avoid misperceptions and to maintain the relationship dyad as a priority to prevent the development of relationship problems.
The majority of women will discuss contraception with a health provider, but only 15% will voluntarily discuss their sexual needs or dysfunction.17 This finding is alarming given that, during the postpartum period, two of every three new mothers will experience at least one problem related to sexual function, including dyspareunia, decreased libido, difficulty achieving orgasm, and vaginal dryness.41 This lack of discussion with a health-care provider may be the result of several variables: incomplete knowledge on the part of the provider about what affects sexual function, poor training in the taking of an effective sexual history, and uneasiness on the part of the patient about discussing the issue.5,42
Postnatal depression takes a toll
Depressed mood and emotional lability in the postpartum period are negatively associated with sexual interest, enjoyment, coital activity, and perceived tenderness of the partner.7 Conversely, reduced sexual interest, desire and satisfaction; a lower frequency of intercourse; and later resumption of intercourse are associated with a higher number of psychiatric symptoms in the postpartum period.2 Between 10% and 15% of women experience postpartum depression (PPD).26 Depression has been associated with a decreased frequency and interest in sexual activity at 8 to 12 weeks postpartum.2,5
Chivers and colleagues assessed sexual functioning and sexual behavior in women with and without symptoms of PPD using the FSFI and EPDS. Although theirs was a small study, they found that women who had depressive symptoms also reported poorer functioning in regard to sexual arousal, orgasm, pain, lubrication, and sexual satisfaction.26 Morof and coworkers found that women who had PPD were less likely to have resumed intercourse by 6 months postpartum; they were also less likely to engage in other sexual activities.27
Role of pharmacotherapy
Many women are started on antidepressant medication near the time of delivery or during the immediate postpartum period. Often, serotonin reuptake inhibitors (SRIs) are used because there is minimal transmission of this class of medication through breast milk. However, the potential sexual side effects of these medications should be discussed because they are the agents most commonly associated with female sexual dysfunction.28
For clinicians
American Association of Sex Educators, Counselors, and Therapists – A not-for-profit, interdisciplinary professional organization comprising sexuality educators, sexuality counselors, sex therapists, physicians, social workers, and other clinicians. Its home page links to a referral page and other resources. http://www.aasect.org" target="_blank">http://www.aasect.org
Association of Reproductive Health Professionals offers a resource for clinicians on postpartum counseling about sexuality. http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception" target="_blank">http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception
For patients
Mayo Clinic provides a fact sheet entitled “Sex after pregnancy: Set your own timeline.” http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146" target="_blank">http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146
Sex and a Healthier You – This site offers information for patients on sexuality and relationships. http://www.sexandahealthieryou.org/sex-health/index.html" target="_blank">http://www.sexandahealthieryou.org/sex-health/index.html
We want to hear from you! Tell us what you think.
CASE: Waiting for an OK to resume sex
L. L. is a 29-year-old woman, G1P1, who delivered a healthy infant 4 weeks ago by spontaneous vaginal birth. The delivery involved a 2-day induction of labor for preeclampsia and a second-degree tear that was repaired without complication. The patient also experienced postpartum hemorrhage that was managed with bimanual massage and uterotonics and for which she ultimately required transfusion of blood products. Her hospital course was otherwise unremarkable.
Before pregnancy, L. L. had a normal medical history and conceived spontaneously. Her antenatal course was uncomplicated.
Today, she returns for her postpartum visit. She reports being tired and says she still has some pain at the site of the tear, but reports no problems with urinary or fecal continence. She denies being depressed, and her Edinburgh Postnatal Depression Scale (EPDS) score is consistent with that report. She is breastfeeding and appears to be doing well on the progestin-only pill for contraception. She has not yet attempted intercourse because she is complying with instructions to wait until she sees you for her postpartum visit.
How should you counsel her about resuming sexual activity?

Childbirth is a central event in a woman’s life. Pregnancy and delivery are a time of psychological, biological, and physical transformation, and the postpartum period—the “fourth trimester”—is no exception. Sexual function may be affected. In fact, many women who seek assistance for sexual dissatisfaction note that their problem arose in the postpartum period.1
Postpartum sexuality involves considerably more than the physical act of genital stimulation—with or without intromission or penile penetration—and depends on more than the physical state of recovery of the vagina (after vaginal delivery). It also depends on:
- the woman’s sexual drive and motivation
- her general state of health and quality of life
- her emotional readiness to resume sexual intimacy with a partner
- her adaptation to the maternal role and ability to balance her identity as a mother with her identity as a sexual being
- her relationship with her partner.
Given all these contributing factors, many of which fall outside the scope of the clinical practice of obstetrics and gynecology, how do we go about counseling our patients about the resumption of sexual activity?
Other questions:
- How can we help patients manage expectations about the quality of their postpartum sexual function?
- What guidance can we provide regarding the interplay of psychosexual and physical aspects of the puerperium?
- Can we offer a method of screening for sexual dysfunction in the puerperium? If so, will it help prevent sexual problems or hasten their resolution?
This article addresses these issues. Ultimately, the answer to the question of when to resume sexual activity should reflect an awareness of cultural norms and taboos as well as familiarity with empirically based recommendations.
Traditional postpartum sexual education is not evidence-based and has limited effectiveness. More up-to-date strategies can be easily incorporated into even the busiest clinical practice. We offer the following counseling model for you to consider when addressing the sexual health of patients postpartum.
Educate, legitimize, and normalize
The first sexual encounter after childbirth can be an important step for couples to reclaim their intimate relationship.
Adaptation to the parental role, physical healing, hormonal changes, breastfeeding, and sleep deprivation contribute to a profound psychosocial challenge. The resumption of sexual activities and a satisfying postpartum sex life depend on many variables, many of which the patient may not even be aware.
First, do not assume that all patients are heterosexual and that intercourse is their only form of sexual activity.
Second, it is important to be proactive in antepartum and postpartum counseling and to offer anticipatory guidance. Counseling can take place any time during routine prenatal care, as well as at the time of hospital discharge and the postpartum visit.
Reassure the patient that, if sexual activity and frequency are lower during pregnancy and the postpartum period, it is likely a normal transition. Also give the patient time to talk about her expectations and perceptions. Explain to her the normal fluctuations and variability of sexual interest and enjoyment in pregnancy and the puerperium, and suggest that she consider alternative options for intimate expression, non-coital sexual activities, and mutual pleasure within her cultural context.
Be thorough
Take a comprehensive medical, obstetric, psychological, and social history as part of the sexual history. Also perform a physical intake and exam. Questions about urinary and fecal incontinence ought to be part of all postpartum assessment.
Other potential areas to address include the quality of the relationship, prepregnancy sexual function, the support network, planned or unplanned state of the pregnancy, previous pregnancy and delivery outcomes, the health status of current children, and present, previous, and future contraceptive use.29
Consider multiple visits
It is hard to know exactly when to evaluate a patient for postpartum sexual dysfunction, given the impact of pudendal nerve latency, fatigue, and breastfeeding. For this reason, assessment on multiple occasions may be appropriate. Numerous validated scales to assess sexual function can be easily incorporated into clinical practice.
Couples counseling and therapy may be needed in some cases; be aware of referral services in your area for sexual wellness specialists.
The bottom line: A “successful” sexual life does not necessarily mean adequate genital function (e.g., coital orgasm, improved clitoral blood flow, increased sexual frequency) but, rather, a sexual life that is intimate and satisfying to the individual patient.
A paucity of research
To date, research into sexuality during the postpartum period has focused primarily on the physical changes and constraints that affect the mechanics and frequency of intercourse and overall sexual satisfaction and desire.2 This perspective has begun to broaden to include the psychological aspects of sexuality.
TABLE 1
These validated tools can help you measure female sexual dysfunction
| Tool | Area assessed |
|---|---|
| Female Sexual Function Index (FSFI)30 | Desire, arousal, orgasm, and pain |
| Female Sexual Function Index 6-Item (FSFI-6)31 | Desire, arousal, orgasm, and pain |
| McCoy Female Sexual Function Questionnaire*32 | Presence of female sexual disorders |
| Brief Sexual Symptoms Checklist33 | Screener for sexual concerns |
| Female Sexual Distress Scale – Revised34 | Distress |
| Intimate Relationship Scale*35 | Changes in sexual relationship |
| Sexual Quality of Life – Female (SQol-F)36 | Quality of life in women with female sexual dysfunction |
| Golombok Rust Inventory of Sexual Satisfaction (GRISS)37 | Quality of sexual relationship |
| Decreased Sexual Desire Screener38 | Brief diagnostic tool for hypoactive sexual desire disorder |
| * Validated in pregnant and/or postpartum women | |
Women’s sexual health during the postpartum period has generally been under-researched. It wasn’t until the past decade that validated sexual function questionnaires were utilized. Although a number of these instruments are now available (TABLE 1, TABLE 2, FIGURE), it remains unclear whether they can accurately measure postpartum sexual function. Despite these limitations, significant information has been elicited that can be used to counsel patients struggling with postpartum sexual concerns.
TABLE 2
The 6-item Female Sexual Function Index*
| Question | Responses | |||||
|---|---|---|---|---|---|---|
| 0 points | 5 points | 4 points | 3 points | 2 points | 1 point | |
| How would you rate your level of sexual desire or interest? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How would you rate your level of sexual arousal (“turn on”) during sexual activity or intercourse? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How often did you become lubricated (“wet”) during sexual activity or intercourse? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| When you had sexual stimulation or intercourse, how often did you reach orgasm? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| How satisfied have you been overall with your sexual life? | No sexual activity | Very satisfied | Moderately satisfied | About equally satisfied and dissatisfied | Moderately dissatisfied | Very dissatisfied |
| How often did you experience discomfort or pain during vaginal penetration? | Did not attempt intercourse | Almost never or never | A few times | Sometimes | Most times | Almost always or always |
| *The components of this index are to be assessed over the past 4 weeks. The score is the sum of the ordinal responses to the 6 items and ranges from 2 to 30. A score of less than 19 indicates a need for further investigation, including the full-length Female Sexual Function Index. Source: Adapted from Isidori et al.31 | ||||||
Ideal period of abstinence is unknown
Although our knowledge of the female genital tract in the puerperium is based upon histologic evidence, there are no evidence-based policies to outline the ideal period of postpartum coital abstinence. It seems reasonable to assume that our traditional scientific recommendations developed in part to prevent uterine infection and disruption of sutured wounds. These concerns, combined with cultural and societal norms, have led to the routine discouragement of sexual activity until 4 to 6 weeks postpartum.
The possibility of shortening the period of postpartum abstinence was first suggested by the American College of Obstetricians and Gynecologists (ACOG) in 1984.1 In 1985, Pritchard and colleagues wrote about the individualization of postpartum prohibitions of sexual activity in Williams Obstetrics.1 The earliest time at which intercourse may be safely resumed is unknown, but the 23rd edition of Williams Obstetrics states that a woman can resume sexual intercourse as early as 2 weeks, based on her comfort and desire.3 The sixth edition of the American Academy of Pediatrics (AAP) and ACOG guidelines for perinatal care also states that the risks ought to be minimal at 2 weeks postpartum.4
BRIEF SEXUAL SYMPTOMS CHECKLIST FOR WOMEN (BSSC-W)

Reprinted from Hatzichristou et al. 33
Low desire is not unusual
Although a patient may be granted “permission” to engage in coital activity, other variables influence her decision. It is well known that sexual desire may fluctuate during pregnancy and typically decreases significantly during the third trimester.2 Many women enter the postpartum period with lower levels of sexual desire and satisfaction, and these depressed levels may continue for some time.2 Twenty-five percent of women report worsened sexual function, including diminished sexual satisfaction, during pregnancy that persists for 6 to 12 months postpartum.5 By 12 weeks postpartum, 80% to 93% of women have resumed intercourse, but as many as 83% report sexual problems during the first 3 months of the postpartum period. At 6 months, 18% to 30% of these women may still be experiencing sexual problems, including dyspareunia.5,6
In 1998, von Sydow performed a meta-content analysis of all existing studies on parental sexuality during pregnancy and the first 6 months postpartum.7 Using psychological and medical data banks, she brought together information from two branches of science and identified 59 relevant studies in English or German between 1950 and 1996. Although the majority of studies were retrospective and failed to utilize a validated instrument, von Sydow determined that, overall, sexual interest and activity were low or nonexistent during the first months after delivery. There was high variability between individuals, however, and levels of sexual interest and activity of individual women remained relatively constant from the time before pregnancy until 1 year postpartum.7 von Sydow determined that there is great variability in female sexuality during pregnancy and postpartum; this variability may represent fluctuations during this phase of life. She also determined that severe psychosexual and marital problems are much more prevalent in the postpartum period than during pregnancy and persist long after a physical cause can be used as an explanation.7
Fatigue and quality of the relationship have an impact on sexual function
De Judicibus and colleagues identified a broad range of variables that have a detrimental impact on sexuality at 12 weeks postpartum, most particularly:
- marital dissatisfaction
- dyspareunia
- fatigue
- depression
- breastfeeding.2
There is evidence to suggest that the addition of the first child reduces marital quality after the first month postpartum, and this decline in marital satisfaction continues for 6 to 18 months postpartum.2 Witting and coworkers suggested that this decline may represent a transitional phase of parenthood for some couples; data support the positive effects on overall marital satisfaction with the addition of children.8 Women who were more satisfied with their relationships reported higher sexual satisfaction and greater frequency of intercourse.2,8
Fatigue is one of the most common problems women experience during pregnancy and postpartum and is a common reason given for loss of sexual desire and interest, infrequent sexual activity, and lack of enjoyment.5 A high level of exhaustion is found during the first 8 weeks postpartum. Although it declines over the next 6 months, it does not appear to resolve completely in a good number of women.9
Don’t underestimate the impact of obstetric morbidity
Surprisingly, the long-term impact of severe obstetric events on postpartum maternal health is often overlooked. Waterstone and colleagues found that women who have severe obstetric morbidity, such as massive hemorrhage, preeclampsia, sepsis, and uterine rupture, experience significant changes in sexual health and well-being.10 They conducted a prospective cohort study of such women, measuring sexual activity, general health, and postpartum depression. They utilized two validated postnatal questionnaires—the Short Form 36 (SF-36) to measure general health and the EPDS. Women who had uncomplicated pregnancies and childbirth tended to perform well in most SF-36 categories, whereas women who had experienced severe morbidity scored worse in almost every category. These women also reported problems with intercourse. Thirteen percent of women had not resumed sexual relations by 6 to 12 months postpartum; of these women, more than half reported a fear of conceiving as a reason.
The female body undergoes dramatic physiologic, anatomic, and psychological changes immediately following delivery and throughout the restoration of its pre-pregnant state. This fourth trimester usually lasts 6 to 12 weeks.39
Uterus. The uterus undergoes rapid involution after separation of the placenta. By 2 to 4 weeks postpartum, it may no longer be palpable abdominally, and by 6 weeks, it usually has returned to its nonpregnant state and size. Seven to 14 days after delivery, a woman often experiences an episode of heavier vaginal bleeding that corresponds with the sloughing of the placental bed eschar. During this time of involution, myometrial vessels may be 5 mm or larger in diameter.40
Lochia. The postpartum lochia begins to change within days of birth, transitioning through its stages of lochia rubra, serosa, and alba. It decreases by 3 weeks postpartum and is likely completely resolved by 6 weeks.
Prolactin is responsible for lactogenesis. When the prolactin level is maintained through breastfeeding, it depresses ovarian production of estrogen by suppressing pituitary gonadotropin secretion, triggering a period of “steroid starvation” after the loss of estrogen and progesterone production from the placenta.1
Vagina. Early in the postpartum period, the vagina is typically edematous and lax and, as a result of parturition, there may be not only a spontaneous tear or episiotomy that must heal, but superficial small tears that do not require suturing. Ruggae begin to reappear by 3 weeks, and the vaginal epithelium will begin to mature under the influence of estrogen production. Much of this tissue damage is healed by 6 weeks postpartum.
The perception of pregnant and postpartum women’s sexuality varies, based on religious and cultural norms. In some religions and cultures, sexual activity is forbidden for 2 to 3 months postpartum; in others, it is prohibited until the child is weaned from the breast. The postpartum woman and lochia have traditionally been perceived as unclean, and many religions have specific proscriptions regarding the management of this time in a woman’s life.1 Although early cultures did not study these issues specifically, their doctrines suggest that they had some awareness of the natural physiologic transition of a woman’s body after she has given birth.
Exploring the role of body image
Paul and coworkers prospectively assessed female sexual function, body image, and pelvic symptoms from the first trimester until 6 months postpartum.11 They utilized the validated questionnaire instruments of the Female Sexual Function Index (FSFI), the Body Exposure during Sexual Activities Questionnaire (BESAQ), the short forms of the Urogenital Distress Inventory (UDI-6), the Incontinence Impact Questionnaire (IIQ-7), and the Fecal Incontinence Quality of Life Scale (FIQOL). They found that sexual activity and sexual function scores were highest before pregnancy, declined between the first and third trimesters, and did not return to pre-pregnancy baselines even by 6 months postpartum.11
Differences in sexual practices contributed to these patterns. Kissing, fondling, and vaginal intercourse remained stable across pregnancy, whereas oral sex, breast stimulation, and masturbation declined in the third trimester.
The decline of these activities during pregnancy and postpartum has been seen in other studies as well.12
Obstacles to sexual activity also changed across pregnancy and the postpartum period. Vaginal pain was more problematic in the third trimester and postpartum, whereas feelings of unattractiveness and issues of body image were present throughout pregnancy and at their worst in the postpartum period. Sexual function scores based on the FSFI declined during pregnancy and did not return to pre-pregnancy or first-trimester levels by 6 months postpartum. Urinary symptoms, as measured by the UDI-6, were associated with lower sexual function scores during the postpartum period. The association between urinary incontinence and sexual dysfunction has been seen in other studies.13,14
The enduring effects of perineal trauma
Childbirth may physically affect a woman’s sexual function through perineal trauma, pudendal neuropathy, and vaginal dryness associated with breastfeeding. There is an obvious connection between perineal laceration and perineal pain and problems with intercourse.5 Overall, dyspareunia is reported by 41% to 67% of women 2 to 3 months after delivery.15 Women who have an episiotomy complain of increased perineal pain and delayed return of sexual activity, compared with women who deliver with an intact perineum.16
Persistent dyspareunia is strongly associated with the severity of perineal trauma and operative vaginal delivery.3,17 Multiple studies have investigated this association and found a positive correlation 3 to 6 months postpartum,6,9,17 but the long-term effects and association remain unclear.18
Findings from research. Rogers and colleagues prospectively studied the effect of perineal trauma on postpartum sexual function in a midwifery population of women who had a low rate of episiotomy and operative vaginal delivery.6 They utilized the Intimate Relationship Scale (IRS), a validated questionnaire to measure postpartum sexual function in couples. Most women in this study had resumed sexual activity by 3 months postpartum and did not have postpartum inactivity or dysfunction, based on their IRS scores. However, women who were identified as having experienced major trauma (second-, third-, or fourth-degree laceration or a repaired first-degree laceration) had significantly less desire to engage in activities such as touching and stroking with their partner.6
Present-day limits on the routine use of episiotomy and operative vaginal delivery have yielded a lower rate of third- and fourth-degree laceration.19 Second-degree lacerations are common and constitute the majority of perineal trauma in births without episiotomy.20 There is evidence that the use of synthetic absorbable suture, such as polyglactin, rather than chromic suture, results in less postpartum perineal pain, as does leaving the well-approximated perineal skin edges unsutured.20
Signorello and coworkers found that second-, third-, and fourth-degree lacerations increased the risk of postpartum dyspareunia; operative vaginal delivery (forceps or vacuum) was also an independent risk factor for dyspareunia.21
The impact of route of delivery
Some researchers have concluded that the route of delivery has an impact on the long-term pelvic floor health of women.18 In 1986, Snooks and colleagues analyzed possible obstetric risk factors for damage to the innervation of the pelvic floor, which can lead to both stress urinary and anorectal incontinence.22 They found that the process of vaginal delivery causes a compression and stretch type of injury to the pudendal nerve, as well as the possibility of severe perineal lacerations. This injury may be less likely to occur when cesarean delivery is performed before labor, avoiding direct perineal trauma and possible pudendal neuropathy.15 Because the pudendal nerve mediates some of the reflex pathways in the female sexual response, it is plausible that damage to it could result in sexual dysfunction.
Women who deliver vaginally have a higher rate of fecal and urinary incontinence than women who deliver by cesarean.16,23 The presence of incontinence, however, does not always have a significant long-term effect on one’s sexual life.6
In the Term Breech Trial, the route of delivery had no impact on the resumption of intercourse, dyspareunia, or sexual satisfaction.23 Although the trial was randomized and controlled, it had many limitations that call its generalizability into question in regard to postpartum sexual dysfunction.
The National Institutes of Health (NIH) State-of-the-Science Conference on Cesarean Delivery on Maternal Request indicated that, by 6 months postpartum, there is no difference in sexual function based on the route of delivery.24 However, Lydon-Rochelle and colleagues used the SF-36 to assess reported general health status and found that women who had cesarean delivery or assisted vaginal delivery exhibited significantly poorer postpartum functional status than women who had spontaneous vaginal delivery in five areas at 7 weeks postpartum: physical functioning, mental health, general health perception, bodily pain, social functioning, and ability to perform daily activities.25 Women were more likely to be readmitted to the hospital and more likely to report fatigue during the first 2 months after cesarean delivery.9 It appears that women who undergo cesarean delivery have an elevated risk of nondyspareunia-related causes of sexual dysfunction. Any protective effect of cesarean on sexual function is limited to the early postnatal period and is related to the absence of perineal injury.18
How breastfeeding can affect sexual desire
Evidence is strong that breastfeeding reduces a woman’s sexual desire and the frequency of intercourse.1,5 A high level of prolactin suppresses ovarian production of estrogen, thereby reducing vaginal lubrication. Some women and their partner may identify this loss of lubrication as a lack of arousal. This type of vaginal dryness should be explained, and the use of a lubricant should be encouraged in breastfeeding women.
Nipple sensitivity may develop, making touching and foreplay uncomfortable in some women. One third to one half of mothers find breastfeeding to be an erotic experience, and one fourth feel guilty about this sexual excitement; others stop nursing or wean early due to these feelings.1,7 Women are often not educated about the relationship between the release of oxytocin, uterine contractions, milk ejection, sexual arousal, and orgasm; raising the subject can help to diminish any potential distress over this response.
Sleep disturbances from feeding on demand contribute to fatigue and exhaustion.
Many women may not realize that their loss of interest in sex may be because they are receiving sufficient physical contact or touching through their nurturing interactions with the baby. This may leave the partner feeling isolated and envious of the mother-baby relationship.
Couples should be encouraged to discuss these feelings to avoid misperceptions and to maintain the relationship dyad as a priority to prevent the development of relationship problems.
The majority of women will discuss contraception with a health provider, but only 15% will voluntarily discuss their sexual needs or dysfunction.17 This finding is alarming given that, during the postpartum period, two of every three new mothers will experience at least one problem related to sexual function, including dyspareunia, decreased libido, difficulty achieving orgasm, and vaginal dryness.41 This lack of discussion with a health-care provider may be the result of several variables: incomplete knowledge on the part of the provider about what affects sexual function, poor training in the taking of an effective sexual history, and uneasiness on the part of the patient about discussing the issue.5,42
Postnatal depression takes a toll
Depressed mood and emotional lability in the postpartum period are negatively associated with sexual interest, enjoyment, coital activity, and perceived tenderness of the partner.7 Conversely, reduced sexual interest, desire and satisfaction; a lower frequency of intercourse; and later resumption of intercourse are associated with a higher number of psychiatric symptoms in the postpartum period.2 Between 10% and 15% of women experience postpartum depression (PPD).26 Depression has been associated with a decreased frequency and interest in sexual activity at 8 to 12 weeks postpartum.2,5
Chivers and colleagues assessed sexual functioning and sexual behavior in women with and without symptoms of PPD using the FSFI and EPDS. Although theirs was a small study, they found that women who had depressive symptoms also reported poorer functioning in regard to sexual arousal, orgasm, pain, lubrication, and sexual satisfaction.26 Morof and coworkers found that women who had PPD were less likely to have resumed intercourse by 6 months postpartum; they were also less likely to engage in other sexual activities.27
Role of pharmacotherapy
Many women are started on antidepressant medication near the time of delivery or during the immediate postpartum period. Often, serotonin reuptake inhibitors (SRIs) are used because there is minimal transmission of this class of medication through breast milk. However, the potential sexual side effects of these medications should be discussed because they are the agents most commonly associated with female sexual dysfunction.28
For clinicians
American Association of Sex Educators, Counselors, and Therapists – A not-for-profit, interdisciplinary professional organization comprising sexuality educators, sexuality counselors, sex therapists, physicians, social workers, and other clinicians. Its home page links to a referral page and other resources. http://www.aasect.org" target="_blank">http://www.aasect.org
Association of Reproductive Health Professionals offers a resource for clinicians on postpartum counseling about sexuality. http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception" target="_blank">http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception
For patients
Mayo Clinic provides a fact sheet entitled “Sex after pregnancy: Set your own timeline.” http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146" target="_blank">http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146
Sex and a Healthier You – This site offers information for patients on sexuality and relationships. http://www.sexandahealthieryou.org/sex-health/index.html" target="_blank">http://www.sexandahealthieryou.org/sex-health/index.html
We want to hear from you! Tell us what you think.
1. Reamy KJ, White SE. Sexuality in the puerperium: a review. Arch Sex Behav. 1987;16(2):165-186.
2. De Judicibus MA, McCabe MP. Psychological factors and the sexuality of pregnant and postpartum women. J Sex Res. 2002;39(2):94-103.
3. The puerperium. In: Cunningham FG Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill Co.; 2010:646-660.
4. The American Academy of Pediatrics (AAP), American College of Obstetricians Gynecologists (ACOG) Guidelines for perinatal care. 6th ed. Washington DC: AAP, ACOG; 2008.
5. Glazener CM. Sexual function after childbirth: women’s experiences persistent morbidity and lack of professional recognition. Br J Obstet Gynaecol. 1997;104(3):330-335.
6. Rogers RG, Borders, N,, Leeman L, Albers L. Does spontaneous genital tract trauma impact postpartum sexual function? J Midwifery Womens Health. 2009;54(2):98-103.
7. von Sydow K. Sexuality during pregnancy and after childbirth: a metacontent analysis of 59 studies. J Psychosom Res. 1999;47(1):27-49.
8. Witting K, Santtila P, Alanko K, et al. Female sexual function and its associations with number of children, pregnancy, and relationship satisfaction. J Sex Marital Ther. 2008;34(2):89-106.
9. Thompson JF, Roberts CL, Currie M, Elwood DA. Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Birth. 2002;29(2):83-94.
10. Waterstone M, Wolfe C, Hooper R, Bewley S. Postnatal morbidity after childbirth and severe obstetric morbidity. BJOG. 2003;110(2):128-133.
11. Pauls RN, Occhino JA, Dryfhout VL. Effects of pregnancy on female sexual function and body image: A prospective study. J Sex Med. 2008;5(8):1915-1922.
12. von Sydow K, Ullmeyer M, Happ N. Sexual activity during pregnancy and after childbirth: Results from the Sexual P Questionnaire. J Psychosom Obstet Gynaecol. 2001;22(1):29-40.
13. Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Rsearch Group. Neurourol Urodyn. 1995;14(2):131-139.
14. Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC; Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99(2):281-289.
15. Handa VL. Sexual function and childbirth. Semin Perinatol. 2006;30(5):253-256.
16. Klein MC, Gauthier RJ, Robbins JM, et al. Relationship of episiotomy to perineal trauma and morbidity, sexual dysfunction, and pelvic floor relaxation. Am J Obstet Gynecol. 1994;171(3):591-598.
17. Barrett G, Pendry E, Peacock J, Victor C, Thakar, Manyonda I. Women’s sexual health after childbirth. BJOG. 2000;107(2):186-195.
18. Barrett G, Peacock J, Victor CR, Manyonda I. Cesarean section and postnatal sexual health. Birth. 2005;32(4):306-311.
19. Eason E, Labrecque M, Wells G, Feldman P. Preventing perineal trauma during childbirth: a systematic review. Obstet Gynecol. 2000;95(3):464-471.
20. Leeman LM, Rogers RG, Greulich B, Albers LL. Do unsutured second-degree perineal lacerations affect postpartum functional outcomes? J Am Board Fam Med. 2007;20(5):451-457.
21. Signorello L, Harlow BL, Chekos AK, Repke JT. Postpartum sexual functioning and its relationship to perineal trauma: A retrospective cohort study of primiparous women. Am J Obstet Gynecol. 2001;184(5):881-890.
22. Snooks SJ, Swash M, Henry MW, Setchell M. Risk factors in childbirth causing damage to the pelvic floor innervation. In J Colorect Dis. 1986;1(1):20-24.
23. Hannah ME, Whyte H, Hannah WJ, et al. Term Breech Trial Collaborative Group. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: the international randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191(3):917-927.
24. NIH State-of-the-Science Conference Statement on Cesarean Delivery on Maternal Request NIH Consens Sci Statements. 2006;23:1-29.http://consensus.nih.gov/2006/cesareanstatement.htm. Accessed December 6 2011.
25. Lydon-Rochelle MT, Holt VL, Martin DP. Delivery method and self-reported postpartum general health status among primiparous women. Paediatr Perinat Epidemiol. 2001;15(3):232-240.
26. Chivers ML, Pittini MD, Grigoriadis S, Villegas L, Ross LE. The relationship between sexual functioning and depressive symptomatology in postpartum women: a pilot study. J Sex Med. 2011;8(3):792-799.
27. Morof D, Barrett G, Peacock J, Victor CR, Manyonda I. Postnatal depression and sexual health after childbirth. Obstet Gynecol. 2003;102(6):1318-1325.
28. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No.119: Female sexual dysfunction. Obstet Gynecol. 2011;117(4):996-1007.
29. Read J. Sexual problems associated with infertility pregnancy and ageing. BMJ. 2004;329(7465):559-561.
30. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
31. Isidori AM, Pozza C, Esposito K, et al. The Female Sexual Function Index (FSFI): Development and validation of a 6-item version of the female sexual function index (FSFI) as a diagnostic tool for female sexual dysfunction. J Sex Med. 2010;7(3):1139-11.
32. McCoy NL. The McCoy Female Sexuality Questionnaire. Quality Life Res. 2000;9(suppl 6):739-745.
33. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 1):337-348.
34. DeRogatis LR, Allgood A, Rosen RC, Leiblum S, Zipfel L, Guo CY. Development and evaluation of the Women’s Sexual Interest Diagnostic Interview (WSID): a structured interview to diagnose hypoactive sexual desire disorder (HSDD) in standardized patients. J Sex Med. 2008;5(12):2827-2841.
35. Fischman SH, Rankin EA, Soeken KL, Lenz ER. Changes in sexual relationships in postpartum couples. J Obstet Gynecol Neonatal Nurs. 1986;15(1):58-63.
36. Sillis T, Wunderlich G, Pyke R, et al. The Sexual Interest and Desire Inventory-Female (SIDI-F): item response analyses of data from women diagnosed with hypoactive sexual desire disorder. J Sex Med. 2005;2(6):801-818.
37. Rust J, Golombok S. The Golombok-Rust Inventory of Sexual Satisfaction (GRISS). Br J Clin Psych. 1985;24(Pt 1):63-64.
38. Clayton AH, Balon R. The impact of mental illness and psychotropic medications on sexual functioning: the evidence and management. J Sex Med. 2009;6(5):1200-1213.
39. Jennings B, Edmundson M. The postpartum period: after confinement: the fourth trimester. Clin Obstet Gynecol. 1980;23(4):1093-1103.
40. Oppenheimer LS, Sheriff EA, Goodman JDS, shah D, James CE. The duration of lochia. Br J Obstet Gynaecol. 1986;93(7):754-757.
41. Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(4):263-267.
42. Pancholy AB, Goldenhar L, Fellner AN, Crisp C, Kleeman S, Pauls R. Resident education and training in female sexuality: results of a national survey. J Sex Med. 2011;8(2):361-366.
1. Reamy KJ, White SE. Sexuality in the puerperium: a review. Arch Sex Behav. 1987;16(2):165-186.
2. De Judicibus MA, McCabe MP. Psychological factors and the sexuality of pregnant and postpartum women. J Sex Res. 2002;39(2):94-103.
3. The puerperium. In: Cunningham FG Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill Co.; 2010:646-660.
4. The American Academy of Pediatrics (AAP), American College of Obstetricians Gynecologists (ACOG) Guidelines for perinatal care. 6th ed. Washington DC: AAP, ACOG; 2008.
5. Glazener CM. Sexual function after childbirth: women’s experiences persistent morbidity and lack of professional recognition. Br J Obstet Gynaecol. 1997;104(3):330-335.
6. Rogers RG, Borders, N,, Leeman L, Albers L. Does spontaneous genital tract trauma impact postpartum sexual function? J Midwifery Womens Health. 2009;54(2):98-103.
7. von Sydow K. Sexuality during pregnancy and after childbirth: a metacontent analysis of 59 studies. J Psychosom Res. 1999;47(1):27-49.
8. Witting K, Santtila P, Alanko K, et al. Female sexual function and its associations with number of children, pregnancy, and relationship satisfaction. J Sex Marital Ther. 2008;34(2):89-106.
9. Thompson JF, Roberts CL, Currie M, Elwood DA. Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Birth. 2002;29(2):83-94.
10. Waterstone M, Wolfe C, Hooper R, Bewley S. Postnatal morbidity after childbirth and severe obstetric morbidity. BJOG. 2003;110(2):128-133.
11. Pauls RN, Occhino JA, Dryfhout VL. Effects of pregnancy on female sexual function and body image: A prospective study. J Sex Med. 2008;5(8):1915-1922.
12. von Sydow K, Ullmeyer M, Happ N. Sexual activity during pregnancy and after childbirth: Results from the Sexual P Questionnaire. J Psychosom Obstet Gynaecol. 2001;22(1):29-40.
13. Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Rsearch Group. Neurourol Urodyn. 1995;14(2):131-139.
14. Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC; Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99(2):281-289.
15. Handa VL. Sexual function and childbirth. Semin Perinatol. 2006;30(5):253-256.
16. Klein MC, Gauthier RJ, Robbins JM, et al. Relationship of episiotomy to perineal trauma and morbidity, sexual dysfunction, and pelvic floor relaxation. Am J Obstet Gynecol. 1994;171(3):591-598.
17. Barrett G, Pendry E, Peacock J, Victor C, Thakar, Manyonda I. Women’s sexual health after childbirth. BJOG. 2000;107(2):186-195.
18. Barrett G, Peacock J, Victor CR, Manyonda I. Cesarean section and postnatal sexual health. Birth. 2005;32(4):306-311.
19. Eason E, Labrecque M, Wells G, Feldman P. Preventing perineal trauma during childbirth: a systematic review. Obstet Gynecol. 2000;95(3):464-471.
20. Leeman LM, Rogers RG, Greulich B, Albers LL. Do unsutured second-degree perineal lacerations affect postpartum functional outcomes? J Am Board Fam Med. 2007;20(5):451-457.
21. Signorello L, Harlow BL, Chekos AK, Repke JT. Postpartum sexual functioning and its relationship to perineal trauma: A retrospective cohort study of primiparous women. Am J Obstet Gynecol. 2001;184(5):881-890.
22. Snooks SJ, Swash M, Henry MW, Setchell M. Risk factors in childbirth causing damage to the pelvic floor innervation. In J Colorect Dis. 1986;1(1):20-24.
23. Hannah ME, Whyte H, Hannah WJ, et al. Term Breech Trial Collaborative Group. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: the international randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191(3):917-927.
24. NIH State-of-the-Science Conference Statement on Cesarean Delivery on Maternal Request NIH Consens Sci Statements. 2006;23:1-29.http://consensus.nih.gov/2006/cesareanstatement.htm. Accessed December 6 2011.
25. Lydon-Rochelle MT, Holt VL, Martin DP. Delivery method and self-reported postpartum general health status among primiparous women. Paediatr Perinat Epidemiol. 2001;15(3):232-240.
26. Chivers ML, Pittini MD, Grigoriadis S, Villegas L, Ross LE. The relationship between sexual functioning and depressive symptomatology in postpartum women: a pilot study. J Sex Med. 2011;8(3):792-799.
27. Morof D, Barrett G, Peacock J, Victor CR, Manyonda I. Postnatal depression and sexual health after childbirth. Obstet Gynecol. 2003;102(6):1318-1325.
28. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No.119: Female sexual dysfunction. Obstet Gynecol. 2011;117(4):996-1007.
29. Read J. Sexual problems associated with infertility pregnancy and ageing. BMJ. 2004;329(7465):559-561.
30. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
31. Isidori AM, Pozza C, Esposito K, et al. The Female Sexual Function Index (FSFI): Development and validation of a 6-item version of the female sexual function index (FSFI) as a diagnostic tool for female sexual dysfunction. J Sex Med. 2010;7(3):1139-11.
32. McCoy NL. The McCoy Female Sexuality Questionnaire. Quality Life Res. 2000;9(suppl 6):739-745.
33. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 1):337-348.
34. DeRogatis LR, Allgood A, Rosen RC, Leiblum S, Zipfel L, Guo CY. Development and evaluation of the Women’s Sexual Interest Diagnostic Interview (WSID): a structured interview to diagnose hypoactive sexual desire disorder (HSDD) in standardized patients. J Sex Med. 2008;5(12):2827-2841.
35. Fischman SH, Rankin EA, Soeken KL, Lenz ER. Changes in sexual relationships in postpartum couples. J Obstet Gynecol Neonatal Nurs. 1986;15(1):58-63.
36. Sillis T, Wunderlich G, Pyke R, et al. The Sexual Interest and Desire Inventory-Female (SIDI-F): item response analyses of data from women diagnosed with hypoactive sexual desire disorder. J Sex Med. 2005;2(6):801-818.
37. Rust J, Golombok S. The Golombok-Rust Inventory of Sexual Satisfaction (GRISS). Br J Clin Psych. 1985;24(Pt 1):63-64.
38. Clayton AH, Balon R. The impact of mental illness and psychotropic medications on sexual functioning: the evidence and management. J Sex Med. 2009;6(5):1200-1213.
39. Jennings B, Edmundson M. The postpartum period: after confinement: the fourth trimester. Clin Obstet Gynecol. 1980;23(4):1093-1103.
40. Oppenheimer LS, Sheriff EA, Goodman JDS, shah D, James CE. The duration of lochia. Br J Obstet Gynaecol. 1986;93(7):754-757.
41. Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(4):263-267.
42. Pancholy AB, Goldenhar L, Fellner AN, Crisp C, Kleeman S, Pauls R. Resident education and training in female sexuality: results of a national survey. J Sex Med. 2011;8(2):361-366.
Clostridium difficile Infection
Clostridium difficile, a causative pathogen in antibiotic-associated colitis,1 is a slow-growing, spore-forming, gram-positive anaerobic bacillus,2 named difficile because it was so difficult to culture. Although this pathogenic spore was first identified in the 1930s,3 its vegetative, toxin-producing form was not recognized as a causative organism in diarrhea and colitis until the late 1970s.4,5 Since that time, C difficile has become a growing challenge to health care providers for accurate diagnosis, treatment, and containment of the spread of disease. C difficile infection (CDI) often takes a virulent course with associated morbidity, mortality, and health care costs.6
EPIDEMIOLOGY
In healthy adult patient populations, 2% to 3% are colonized with C difficile.2 The colonization rate among healthy infants is significantly higher, between 60% and 70%, but clinical infection is uncommon.7 As the colon becomes populated with flora, between ages 18 and 24 months, the carrier state disappears.8
In the hospital setting, 20% to 30% of patients become colonized with the organism by the fecal-oral route, which is facilitated when antibiotic therapy disrupts normal flora in the gut, enabling C difficile spores to proliferate; most patients are asymptomatic.5 In 2005, in US acute care hospitals, the incidence of CDI was reported at 84 cases per 100,000 patients.9C difficile remains the leading pathogen associated with inpatient antibiotic-associated diarrhea (AAD). It can be identified as the causative organism in 15% to 25% of cases of AAD in hospitalized patients.2
The mortality rate among hospitalized patients rises significantly in those identified with C difficile: 20.6%, compared with 7.0% of matched inpatient controls.10 ICU admission poses a significant burden of disease: The overall incidence of infection in the ICU population is about 4%. ICU patients who contract CDI have up to a 20% rate of fulminant colitis, a severe form of the disease often necessitating surgery. In this segment of the inpatient population, the mortality rate can approach 60%.8
Data analyzed by Zilberberg11 have demonstrated a significant increase in C difficile–associated infections in US hospitals in recent years. In 2001, the number of discharged patients with documented CDI was approximately 134,000, compared with 291,000 in 2005. The rising incidence of CDI has been attributed to increased antibiotic use, aging of the population, an increasing rate of comorbid conditions, fluoroquinolone resistance, and increased suspicion for the illness, which has led to increased use of testing.12,13
Of Significant Concern
Recurrent disease poses a particular challenge to the health care system. It has been reported that recurrence rates for CDI are between 15% and 35% after a first bout and between 33% and 65% after subsequent episodes of infection.2,14
A hypervirulent strain, the North American pulsed-field gel electrophoresis type 1 (NAP1/B1/027) has been implicated in several C difficile outbreaks.15 This subtype, which is especially resistant to fluoroquinolones,1 produces toxins earlier and in much greater quantities, including toxins A and B at levels 15 to 20 times greater than those seen in less virulent subtypes.4,12 Thus, the NAP1 strain is implicated in more severe disease and is more lethal.16 Affected patients have a 30-day mortality rate twice as high as that among patients with other strains of C difficile.12
The calculated cost of CDI adds between $2,454 and $7,179 in nonreimbursable costs per hospitalized patient, and an additional 3.6 to 7.0 days to their hospital stays.17 Estimates for CDI treatment in the US range from $436 million to $3 billion per year.6,17
Community-Acquired CDI
Community-acquired C difficile infection (CA-CDI) is a subtype that develops in patients who have not been hospitalized in the previous year,18 with incidence recently reported at 11.16 cases per 100,000 person-years.19 Affected patients tend to be younger than those with hospital-acquired infection and to have a less severe disease course. To meet the criteria of this subgroup, according to recent clinical practice guidelines jointly issued by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America (SHEA-IDSA),1 a patient may have developed symptoms no sooner than 12 weeks after hospital discharge (if any hospitalization occurred).
It is always important to perform a thorough history in patients with suspected CA-CDI to assess for recent hospitalizations and antibiotic use. In a retrospective study published in 2011, Kuntz et al19 found that CA-CDI–affected patients were six times as likely as healthy controls to have taken antimicrobials within 180 days before illness (including beta-lactam/beta-lactamase inhibitors, cephalosporins, clindamycin, fluoroquinolones, and penicillin) and twice as likely to have used gastric acid suppressants in that time.
One emerging theory is that CA-CDI is spread through food-borne illness. Unlike the vegetative form that resides in the bowel, C difficile spores are resistant to temperatures at which food is cooked (as they are to alcohol and many other disinfectant agents).5 Several studies have shown that livestock can harbor C difficile.12
PATHOPHYSIOLOGY
Normal gastrointestinal flora resist colonization and proliferation of C difficile; colonization of nontoxigenic strains appears to be protective against toxigenic strains.1 Alteration in the colonic bacterial environment, including the suppression of normal flora proliferation that can occur when a patient receives antibiotics or antineoplastic agents, is thought to allow the overgrowth of C difficile.2
Only C difficile strains that produce exotoxins (the enterotoxin toxin A, and the cytotoxin toxin B) are pathogenic. The organism produces a variety of adhesion proteins (with production accelerated by the presence of antibiotics, such as ampicillin and clindamycin), leading the toxins to bind to specific receptors in the intestinal mucosal cells. C difficile also produces proteases that trigger degradation of the intestinal extracellular matrix and disruption of epithelial cell signaling.16 The toxins activate proinflammatory cytokines, including interleukin (IL)-1, tumor necrosis factor (TNF)-, and IL-8. The result is an intestinal inflammatory response that is clinically apparent in the form of diarrhea and pseudomembranous colitis (PMC).20
RISK FACTORS
Among several known risk factors for CDI (see Table 11,2,4,18,21-24), perhaps the most widely recognized is the use of nearly any antimicrobial agent. Previous administration of antibiotics has been documented in about 95% of inpatients with CDI. Broad-spectrum antibiotics with anti-anaerobic coverage appear to pose the greatest risk.1,4 These include clindamycin, along with cephalosporins, fluoroquinolones, and beta-lactams.1,13 Fluoroquinolone use has been implicated in the development of the NAP1 subtype of the disease.25 In patients who receive multiple antibiotics or prolonged courses, an even greater risk for CDI is incurred.4 Even single-dose administration of an antibiotic carries a CDI risk of about 1.5%.21
A prolonged hospital stay or residence in a long-term care facility increases the risk for CDI, as does advanced age.1 Patients 65 or older have a 20-fold higher risk for CDI than their younger counterparts.1,2 Patients with inflammatory bowel disease (IBD) have an increased risk for CDI.23
It has been theorized that the use of gastric acid–suppressive medications can increase the risk for CDI to between 2.5 and 2.9 times that among persons who do not take these agents.18,22 Results from other trials have refuted this theory, however.13,26
Additional risk factors include ICU admission, recent gastrointestinal surgery or manipulation, immunosuppression, and serious underlying illnesses.24 Postpyloric enteral tube feeding has also been implicated as a risk factor; this route bypasses the stomach, where a very acidic environment ordinarily helps to kill the organism.4
CLINICAL PRESENTATION
Watery diarrhea, occurring 10 to 15 times in a day, is the most common manifestation of CDI. It may present during administration of an antimicrobial agent or within a few days afterward; rarely, it can develop as long as two months after cessation of such treatment.2 In most studies, median onset of diarrhea is about two days.20 Descriptive characteristics of the stool, including odor and color, may vary. Diarrhea may be accompanied by lower abdominal pain, cramping, low-grade fever, and leukocytosis.
In some patients, particularly those taking narcotics, diarrhea may be absent. This manifestation may signal a more severe disease course, including the possibility of fulminant infection. Endoscopic evaluation of the colon may reveal the classic pseudomembranes (ie, adherent yellow plaques)2; see figure.
Stratifying Disease Severity
The severity of CDI-associated colitis increases as systemic symptoms worsen and the clinical picture deteriorates.4 In the 2010 SHEA-IDSA guidelines,1 CDI is stratified, both in the initial episode and the first recurrence, as mild-to-moderate, severe, or severe complicated. The following data may be used to appropriately stage disease severity in the initial episode:
• Mild-to-moderate disease is characterized by nonbloody diarrhea (fewer than 10 to 12 bowel movements per day), possibly accompanied by mild, crampy abdominal pain. Typically, affected patients do not exhibit significant systemic symptoms or marked abdominal tenderness. Leukocytosis is usually represented by fewer than 15,000 cells/L, and the serum creatinine level is less than 1.5 times the baseline level.1,2,5,8
• Severe disease, which should always be a consideration in older patients,1 includes profuse, watery diarrhea, significant abdominal pain and distension, fever, nausea and vomiting, and clinical volume depletion. Significant leukocytosis (≥ 15,000 cells/L) and serum creatinine ≥ 1.5 times baseline or leukopenia with possible bandemia may occur.1,27 Occult blood may be present, but frank blood is rare. A history of ICU admission alone increases the classification to severe due to the aforementioned poor outcomes associated with CDI in ICU patients.8,24
• Severe, complicated CDI may involve hypotension, shock, or a paralytic ileus1; a paradoxical decrease or absence of diarrhea may occur.2 At the severe end of the disease spectrum, toxic megacolon can develop and progress to colonic perforation.2,5,8,28 Hypoalbuminemia may be present due to the large protein losses in the course of the disease.28
The Most Severe Manifestations
Fulminant CDI can occur in 3% to 8% of patients with CDI.27,29 Fulminant disease carries a mortality rate between 35% and 80%.23 The patient’s clinical picture may resemble that seen in severe disease, with the addition of an acute abdomen indicating peritonitis; lethargy, hypotension, oliguria (including renal failure), and/or tachycardia due to a severe systemic inflammatory response induced by toxin production from C difficile. Up to 20% of patients with fulminant C difficile do not have diarrhea due to reasons explained previously.2,5,23
Bacteremia occurs rarely in patients with CDI.1,30 Risk factors for this severe condition include failure of medical treatment, leukocytosis exceeding 16,000 cells/L, surgery in the previous 30 days, a history of IBD, and previous administration of IV immunoglobulin.23
DIAGNOSIS
According to recommendations from the SHEA-IDSA expert panel,1 only unformed stool from symptomatic patients should be tested for C difficile or its toxins. In spite of slow turnaround time, stool culture (followed by toxigenic culture to identify a toxigenic isolate) is currently considered standard testing for C difficile. Cell cytotoxin assay has 98% sensitivity and 99% specificity, but turnaround time is 24 to 48 hours,28 potentially delaying treatment for patients who test positive.
Enzyme immunoassay (EIA) testing for C difficile toxin A and toxin B yields results within hours but is not as sensitive as the cell cytotoxin assay.1 Because toxin testing lacks sensitivity, a two-step strategy has been proposed and is called an “interim recommendation” by the SHEA-IDSA guideline authors: EIA testing for glutamate dehydrogenase (GDH), an enzyme produced by C difficile; then, in patients with positive results, confirmation by cell cytotoxin assay or toxigenic culture.1,4,28 (EIA testing for GDH can be a rapid, inexpensive method for ruling out CDI.28)
Polymerase chain reaction testing, recently developed for the detection of pathogenic C difficile, is rapid, sensitive, and specific.1 However, this method has not yet gained wide acceptance due to its relatively high cost.6
Imaging Options
Endoscopic visualization confirming the presence of PMC is also considered diagnostic of CDI; although half of patients with CDI lack this finding on endoscopy, CDI is present in 95% of patients with confirmed PMC.1,28 Colonoscopy is advantageous over sigmoidoscopy because up to one-third of patients have only right-sided colonic involvement.28 To its disadvantage, endoscopy carries a risk for perforation (particularly in patients with fulminant disease), as well as the inherent risks of sedation required to perform the procedure.
Though not specific, CT can be used as an adjunct to the diagnosis; features such as colonic wall thickening, pericolic stranding, ascites, pneumatosis, and free air resulting from perforation may suggest CDI and help determine the extent of disease.4,20 The accordion sign (high-attenuation oral contrast in the colon lumen, alternating with low attenuation of inflamed mucosa) and the double halo sign (IV contrast having varying degrees of attenuation due to submucosal inflammation and hyperemia) have also been reported in patients with CDI and may indicate PMC or fulminant CDI.28
The small intestine is typically not involved in CDI except in the setting of ileus and the rare entity of C difficile enteritis.2 Plain radiography is only helpful in cases of ileus or megacolon.28
PHARMACOLOGIC TREATMENT
Discontinuation of the offending drug (usually an antibiotic), whenever possible, is the first step; up to 25% of CDI patients recover without any further therapies. Limiting management to antibiotic withdrawal, however, is currently recommended only in patients with the mildest form of CDI due to the risk for subsequent fulminant disease and clinical deterioration.14 In patients with suspected severe (but unconfirmed) CDI, empiric treatment with one of the antimicrobial agents listed below may be appropriate5 (see also Table 21,8,14). For patients with confirmed illness who require continued antimicrobial therapy, an agent not associated with CDI may be substituted (eg, sulfamethoxazole, macrolides, aminoglycosides).14
Metronidazole is the first-line agent for mild-to-moderate initial disease.1 The mechanism of action is through DNA disruption and inhibition of nucleic acid synthesis, a process that induces cell death. Metronidazole also appears to have anti-inflammatory, antioxidant, and immunomodulating properties that assist in overcoming the disease. It should not be used in women who are pregnant.8
Dosage of the drug is 250 mg four times per day or 500 mg three times a day, given orally; or parenterally, if the patient cannot take it orally. The duration is 10 to 14 days (or longer in patients with underlying infection),1 and the cost is much lower than that of other appropriate antimicrobial agents (ie, less than $1/day).2,31
In patients with severe or refractory disease, vancomycin should be used. Vancomycin has shown superiority in severe disease compared with metronidazole, with clinical cure rates of 97% and 76%, respectively.1,4,32 The typical oral dosage is 125 mg four times per day for 10 days.1
Vancomycin is effective only when given enterally; the drug is not absorbed by the gastrointestinal tract, allowing it to achieve high concentrations in the colon. In the patient who cannot receive standard enteral therapy, vancomycin can be instilled directly into the colon by enema or colonic catheter. With this route of administration, there is a small risk for iatrogenic perforation.2 There has been advocacy in very severe or fulminant disease to use vancomycin (orally or rectally) combined with IV metronidazole.1,8 The cost for 10 to 14 days of treatment ranges from $1,000 to $1,500.31
Earlier this year, fidaxomicin, a macrocyclic antibiotic, was approved for treatment of CDI in adults. This agent has minimal systemic absorption and works in the intestinal lumen by inhibiting the bacterial RNA polymerase.33 In a randomized, controlled trial involving 629 patients, fidaxomicin’s effectiveness was found comparable to that of vancomycin for treatment of CDI (clinical cure rates in the intention-to-treat analysis, 88.2% vs 85.8%, respectively), and fidaxomicin-treated patients had a lower rate of recurrence after initial use (15.4% vs 25.3%, respectively; patients with fulminant disease were not included).33 Dosage is 200 mg every 12 hours for 10 days,33,34 at a reported cost of $2,800.31
Neither oral bacitracin nor fusidic acid has been shown to eliminate CDI or reduce recurrence.1,5,33
Clinical resolution reveals adequate response to treatment and need not be confirmed by laboratory testing. Asymptomatic carriers do not require treatment.5
Less Conventional Agents
Several nonantibiotic treatment regimens have been proposed for CDI. Use of probiotics has been controversial. In a systematic review, Dendukuri et al35 found insufficient evidence in the routine use of probiotics to prevent or treat CDI. There have even been reports of Saccharomyces boulardii–associated fungemia and lactobacillus-associated bacteremia resulting from probiotics use.36
An anion-exchange resin, cholestyramine, is thought to help bind toxins; when studied, however, it failed to show promising results in improving patients’ clinical course.1,37 Additionally, it can bind to other drugs, such as vancomycin, resulting in decreased pharmacologic efficacy of this and other agents.1,2 Therefore, it is not recommended.
Intravenous immunoglobulin can provide an option for treatment of severe and/or recurrent disease as a last resort.1,38 Thus far, only results from small observational or retrospective studies have supported its use.
SURGICAL OPTIONS
Early surgical consultation is warranted in severe or refractory disease9 and in patients with specific manifestations detected via abdominal/pelvic CT: ileus, perforation, obstruction, thickening of the colonic wall, toxic megacolon, ascites, necrotizing colitis, or a systemic inflammatory response that could lead to multiorgan system failure.14,27 Colectomy is undertaken in 0.4% to 3.5% of patients with CDI,2 with a goal of resecting the involved bowel and diverting the fecal stream. In the past, near-total colectomy was the treatment of choice.2
There have been published reports of successful segmental resection for fulminant CDI.23 The mortality rate is approximately 50% in patients who undergo colectomy.2 Survival rates are noted to improve with early and prompt surgical management of severe disease,8,29 which can be life threatening.14
A recently developed procedure for fulminant disease involves creating a diverting loop ileostomy. Through the ostomy, 8 L of propylene glycol electrolyte solution is instilled to reduce the colonic C difficile count; the patient is also administered a vancomycin enema. In a literature review of recent studies involving patients who underwent the procedure, Olivas et al29 reported a 30-day mortality rate of 19%, and 93% of surviving patients did not require a colectomy. Further investigation to reduce surgical mortality in patients with fulminant disease is ongoing.
INVESTIGATIVE TREATMENT OPTIONS
Fecal transplantation from healthy donors to those infected with pathogenic C difficile via nasogastric tube or enema have been studied.1,39 The theory is to help reconstitute normal colonic flora with the transplanted stool. This treatment option has only very limited data and acceptance.8
Promising research has been published regarding the infusion of combined monoclonal antibodies against toxin A and toxin B. Among 200 patients who were randomized to receive the study therapy or placebo, the rate of recurrence was 7% versus 25%, respectively; recurrence rates among patients infected with the virulent NAP1/B1/027 strain were 8% and 32%, respectively.40 Length of stay did not improve in patients taking monoclonal antibodies, and adverse events were reported in both patient groups.
In preliminary trials, a parenteral vaccine containing inactivated toxins A and B was reported safe and capable of triggering a “vigorous” serum antitoxin A response in healthy adults.9,14
ADDITIONAL MANAGEMENT RECOMMENDATIONS
Medications that slow gastrointestinal motility should not be used, as the slowing of peristalsis may allow toxins to accumulate in the colon, leading to worsening disease.18,22 The use of opiates and anti-diarrheal medications should be limited.24
Aggressive fluid and electrolyte replacement should be administered until diarrhea has been resolved (usually within three to six days). Patients may require vasoactive medications to support hemodynamics.2,5,14,24
Patients with mild disease can eat as they normally would. Those with severe disease, including those who may require surgery, fare best with bowel rest and possibly enteral nutrition.
Monitoring the patient for signs of improvement during the first 24 to 48 hours is an important component of management. The patient’s white blood cell count and temperature, the number and frequency of bowel movements, and the overall clinical picture should be evaluated daily. Patients who show improvement should complete the current regimen.
TREATMENT FAILURE AND RECURRENT CDI
If a patient’s condition does not improve or worsens at any point during therapy for CDI, a change to another antimicrobial agent is warranted. Also, surgical, gastroenterological, and/or infectious disease consult may be needed if no improvement is evident after five days of seemingly appropriate therapy.14,24
Recurrent CDI, which occurs at least once in 6% to 25% of treated patients, is most likely to occur 7 to 14 days after treatment completion.1,14 Seldom caused by resistant strains of C difficile, it is more likely to result from inadequate adherence to treatment, the presence of residual spores in the colon after treatment, or reinfection—although relapse is considered more common than reinfection.14,24 However, since a patient’s symptoms may have other causes, confirmation of recurrence should be sought through laboratory testing.
Recurrent illness is managed in the same way as successful initial therapy, based on the severity of disease; vancomycin is recommended for the first recurrence in a patient with a rising white blood cell count or serum creatinine level. Otherwise, metronidazole use may be considered.1
In patients who experience a second recurrence of confirmed CDI, a tapered or pulsed-dose regimen of oral vancomycin over a six-week period has been advocated1,8,14 (for details, see Table 2).
Results from small studies of patients with several recurrences of CDI suggest that oral rifaximin therapy can reduce subsequent recurrences if administered immediately after the conclusion of a course of vancomycin.1,41
PREVENTION OF C DIFFICILE INFECTION
Although “research gaps” exist regarding the optimal strategies to prevent CDI,1 decreased prescribing of nonessential antibiotics is key. Without the alteration in colonic flora caused by antimicrobial use, gut colonization cannot occur, and C difficile typically cannot proliferate.2
Preventing transmission of the pathogen is challenging in health care facilities, where C difficile spores have been cultured from staff members’ hands and from beds, floors, windowsills, and other areas; the spores can survive in hospital rooms for as long as 40 days after a patient with CDI has been discharged.24 Appropriate isolation precautions are essential, including single-patient–use equipment (eg, disposable rectal thermometers) and caregivers’ use of gowns, vinyl gloves, and cleaning agents that are effective against the spores, particularly bleach.1 Alcohol-based products are not effective against C difficile spores; diligent handwashing with soap or chlorhexidine is imperative to prevent the spread of CDI.1,2
CONCLUSION
Clinicians and patients alike face the clinical challenge of Clostridium difficile infection. Mild to moderate disease can be treated medically with excellent success rates. Severe disease carries a significant risk to life, and a multidisciplinary approach including early surgical consultation is warranted.
With early recognition and appropriate treatment, along with strict adherence to isolation policies, the health care community can help limit the spread of this insidious illness and its associated morbidity and mortality.
REFERENCES
1. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
2. Efron PA, Mazuski JE. Clostridium difficile colitis. Surg Clin North Am. 2009;89(2):483-500.
3. Hall IC, O’Toole E. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390-402.
4. Riddle DJ, Dubberke ER. Clostridium difficile infection in the intensive care unit. Infect Dis Clin North Am. 2009;23(3):727-743.
5. Sunenshine RH, McDonald LC. Clostridium difficile–associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006; 73(2):187-197.
6. Currie B. Improved testing methods are improving diagnosis of Clostridium difficile infections. Advance Administrators Laboratory. 2009;21:10. http://laboratory-manager.advance web.com/Article/PCR-for-C-diff.aspx. Accessed November 8, 2011.
7. Larson HE, Barclay FE, Honour P, Hill ID. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146(6):727-733.
8. Leffler DA, Lamont JT. Treatment of Clostridium difficile–associated disease. Gastroenterology. 2009;136(6):1899-1912.
9. Kelly CP, Lamont JT. Clostridium difficile: more difficult than ever. N Engl J Med. 2008;359(18): 1932-1940.
10. Pépin J, Valiquette L, Cossette B. Mortality attributed to nosocomial Clostridium difficile–associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005; 173(9):1037-1042.
11. Zilberberg MD. Clostridium difficile–related hospitalizations among US adults, 2006. Emerg Infect Dis. 2009;15(1):122-124.
12. Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in the inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4(4):409-416.
13. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile–associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260.
14. Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46 suppl 1:S32-S42.
15. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23): 2433-2441.
16. Dawson LF, Valiente E, Wren BW. Clostridium difficile: a continually evolving and problematic pathogen. Infect Genet Evol. 2009;9(6):
1410-1417.
17. Dubberke ER, Reske KA, Olsen MA, et al. Short- and long-term attributable costs of Clostridium difficile–associated disease in nonsurgical patients. Clin Infect Dis. 2008;46(4):497-504.
18. Dial S, Delaney JAC, Barkun An, et al. Use of gastric acid–suppression agents and the risk of community-acquired Clostridium difficile disease. JAMA. 2005;294(23):2989-2995.
19. Kuntz JL, Chrischilles EA, Pendergast JF, et al. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect Dis. 2011 Jul 15;11:194.
20. Kelly CP, Lamont JT. Antibiotic-associated diarrhea, pseudomembranous enterocolitis, and Clostridium difficile–associated diarrhea and colitis. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 9th ed. Philadelphia, PA: WB Saunders; 2010:1889-1902.
21. Carignan A, Allard C, Pépin J, et al. Risk of Clostridium difficile infection after perioperative antibacterial prophylaxis before and during an outbreak of infection due to a hypervirulent strain. Clin Infect Dis. 2008;46(12):1838-1843.
22. Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003; 54(3):243-245.
23. Butala P, Divino CM. Surgical aspects of fulminant Clostridium difficile colitis. Am J Surg. 2010;200(1):131-135.
24. Schroeder MS. Clostridium difficile–associated diarrhea. Am Fam Physician. 2005;71(5): 921-928.
25. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile–associated disease? A review of the evidence. Curr Med Res Opin. 2008;24(2):329-333.
26. Lowe DO, Mamdani MM, Kopp A, et al. Proton pump inhibitors and hospitalization for Clostridium difficile–associated disease: a population-based study. Clin Infect Dis. 2006;43(10): 1272-1276.
27. Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009;144(5):433-439.
28. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile. Clin Infect Dis. 2008;46 suppl 1:S12-S18.
29. Olivas AP, Umanskiy K, Zuckerbraun B, Alverdy JC. Avoiding colectomy during surgical management of fulminant Clostridium difficile colitis. Surg Infect. 2010;11(3):299-305.
30. Feldman RJ, Kallich M, Weinstein MP. Bacteremia due to Clostridium difficile: case report and review of extraintestinal C difficile infections. Clin Infect Dis. 1995;20(6):1560-1562.
31. BioPharm Physicians. What next for Dificid (fidaxomicin)? Jun 6, 2011. www.biopharmphysi cians.com/what-next-for-dificid-fidaxomicin. Accessed November 7, 2011.
32. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
33. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
34. Sullivan KM, Spooner LM. Fidaxomicin: a macrocyclic antibiotic for the management of Clostridium difficile infection. Ann Pharmacother. 2010;44(2):352-359.
35. Dendukuri N, Costa V, McGregor M, Brophy JM. Probiotic therapy for the prevention and treatment of Clostridium difficile–associated diarrhea: a systematic review. CMAJ. 2005;173(2): 167-170.
36. Segarra-Newnham M. Probiotics for Clostridium difficile–associated diarrhea: focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii. Ann Pharmacother. 2007;41(7): 1212-1221.
37. Lagrotteria D, Holmes S, Smieja M, et al. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile–associated diarrhea. Clin Infect Dis. 2006;43(5):547-552.
38. McPherson S, Rees CJ, Ellis R, et al. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum. 2006;49(5): 640-645.
39. Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003; 36(5):580-585.
40. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362(3): 197-205.
41. Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile–associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007; 44(6):846-848.
Clostridium difficile, a causative pathogen in antibiotic-associated colitis,1 is a slow-growing, spore-forming, gram-positive anaerobic bacillus,2 named difficile because it was so difficult to culture. Although this pathogenic spore was first identified in the 1930s,3 its vegetative, toxin-producing form was not recognized as a causative organism in diarrhea and colitis until the late 1970s.4,5 Since that time, C difficile has become a growing challenge to health care providers for accurate diagnosis, treatment, and containment of the spread of disease. C difficile infection (CDI) often takes a virulent course with associated morbidity, mortality, and health care costs.6
EPIDEMIOLOGY
In healthy adult patient populations, 2% to 3% are colonized with C difficile.2 The colonization rate among healthy infants is significantly higher, between 60% and 70%, but clinical infection is uncommon.7 As the colon becomes populated with flora, between ages 18 and 24 months, the carrier state disappears.8
In the hospital setting, 20% to 30% of patients become colonized with the organism by the fecal-oral route, which is facilitated when antibiotic therapy disrupts normal flora in the gut, enabling C difficile spores to proliferate; most patients are asymptomatic.5 In 2005, in US acute care hospitals, the incidence of CDI was reported at 84 cases per 100,000 patients.9C difficile remains the leading pathogen associated with inpatient antibiotic-associated diarrhea (AAD). It can be identified as the causative organism in 15% to 25% of cases of AAD in hospitalized patients.2
The mortality rate among hospitalized patients rises significantly in those identified with C difficile: 20.6%, compared with 7.0% of matched inpatient controls.10 ICU admission poses a significant burden of disease: The overall incidence of infection in the ICU population is about 4%. ICU patients who contract CDI have up to a 20% rate of fulminant colitis, a severe form of the disease often necessitating surgery. In this segment of the inpatient population, the mortality rate can approach 60%.8
Data analyzed by Zilberberg11 have demonstrated a significant increase in C difficile–associated infections in US hospitals in recent years. In 2001, the number of discharged patients with documented CDI was approximately 134,000, compared with 291,000 in 2005. The rising incidence of CDI has been attributed to increased antibiotic use, aging of the population, an increasing rate of comorbid conditions, fluoroquinolone resistance, and increased suspicion for the illness, which has led to increased use of testing.12,13
Of Significant Concern
Recurrent disease poses a particular challenge to the health care system. It has been reported that recurrence rates for CDI are between 15% and 35% after a first bout and between 33% and 65% after subsequent episodes of infection.2,14
A hypervirulent strain, the North American pulsed-field gel electrophoresis type 1 (NAP1/B1/027) has been implicated in several C difficile outbreaks.15 This subtype, which is especially resistant to fluoroquinolones,1 produces toxins earlier and in much greater quantities, including toxins A and B at levels 15 to 20 times greater than those seen in less virulent subtypes.4,12 Thus, the NAP1 strain is implicated in more severe disease and is more lethal.16 Affected patients have a 30-day mortality rate twice as high as that among patients with other strains of C difficile.12
The calculated cost of CDI adds between $2,454 and $7,179 in nonreimbursable costs per hospitalized patient, and an additional 3.6 to 7.0 days to their hospital stays.17 Estimates for CDI treatment in the US range from $436 million to $3 billion per year.6,17
Community-Acquired CDI
Community-acquired C difficile infection (CA-CDI) is a subtype that develops in patients who have not been hospitalized in the previous year,18 with incidence recently reported at 11.16 cases per 100,000 person-years.19 Affected patients tend to be younger than those with hospital-acquired infection and to have a less severe disease course. To meet the criteria of this subgroup, according to recent clinical practice guidelines jointly issued by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America (SHEA-IDSA),1 a patient may have developed symptoms no sooner than 12 weeks after hospital discharge (if any hospitalization occurred).
It is always important to perform a thorough history in patients with suspected CA-CDI to assess for recent hospitalizations and antibiotic use. In a retrospective study published in 2011, Kuntz et al19 found that CA-CDI–affected patients were six times as likely as healthy controls to have taken antimicrobials within 180 days before illness (including beta-lactam/beta-lactamase inhibitors, cephalosporins, clindamycin, fluoroquinolones, and penicillin) and twice as likely to have used gastric acid suppressants in that time.
One emerging theory is that CA-CDI is spread through food-borne illness. Unlike the vegetative form that resides in the bowel, C difficile spores are resistant to temperatures at which food is cooked (as they are to alcohol and many other disinfectant agents).5 Several studies have shown that livestock can harbor C difficile.12
PATHOPHYSIOLOGY
Normal gastrointestinal flora resist colonization and proliferation of C difficile; colonization of nontoxigenic strains appears to be protective against toxigenic strains.1 Alteration in the colonic bacterial environment, including the suppression of normal flora proliferation that can occur when a patient receives antibiotics or antineoplastic agents, is thought to allow the overgrowth of C difficile.2
Only C difficile strains that produce exotoxins (the enterotoxin toxin A, and the cytotoxin toxin B) are pathogenic. The organism produces a variety of adhesion proteins (with production accelerated by the presence of antibiotics, such as ampicillin and clindamycin), leading the toxins to bind to specific receptors in the intestinal mucosal cells. C difficile also produces proteases that trigger degradation of the intestinal extracellular matrix and disruption of epithelial cell signaling.16 The toxins activate proinflammatory cytokines, including interleukin (IL)-1, tumor necrosis factor (TNF)-, and IL-8. The result is an intestinal inflammatory response that is clinically apparent in the form of diarrhea and pseudomembranous colitis (PMC).20
RISK FACTORS
Among several known risk factors for CDI (see Table 11,2,4,18,21-24), perhaps the most widely recognized is the use of nearly any antimicrobial agent. Previous administration of antibiotics has been documented in about 95% of inpatients with CDI. Broad-spectrum antibiotics with anti-anaerobic coverage appear to pose the greatest risk.1,4 These include clindamycin, along with cephalosporins, fluoroquinolones, and beta-lactams.1,13 Fluoroquinolone use has been implicated in the development of the NAP1 subtype of the disease.25 In patients who receive multiple antibiotics or prolonged courses, an even greater risk for CDI is incurred.4 Even single-dose administration of an antibiotic carries a CDI risk of about 1.5%.21
A prolonged hospital stay or residence in a long-term care facility increases the risk for CDI, as does advanced age.1 Patients 65 or older have a 20-fold higher risk for CDI than their younger counterparts.1,2 Patients with inflammatory bowel disease (IBD) have an increased risk for CDI.23
It has been theorized that the use of gastric acid–suppressive medications can increase the risk for CDI to between 2.5 and 2.9 times that among persons who do not take these agents.18,22 Results from other trials have refuted this theory, however.13,26
Additional risk factors include ICU admission, recent gastrointestinal surgery or manipulation, immunosuppression, and serious underlying illnesses.24 Postpyloric enteral tube feeding has also been implicated as a risk factor; this route bypasses the stomach, where a very acidic environment ordinarily helps to kill the organism.4
CLINICAL PRESENTATION
Watery diarrhea, occurring 10 to 15 times in a day, is the most common manifestation of CDI. It may present during administration of an antimicrobial agent or within a few days afterward; rarely, it can develop as long as two months after cessation of such treatment.2 In most studies, median onset of diarrhea is about two days.20 Descriptive characteristics of the stool, including odor and color, may vary. Diarrhea may be accompanied by lower abdominal pain, cramping, low-grade fever, and leukocytosis.
In some patients, particularly those taking narcotics, diarrhea may be absent. This manifestation may signal a more severe disease course, including the possibility of fulminant infection. Endoscopic evaluation of the colon may reveal the classic pseudomembranes (ie, adherent yellow plaques)2; see figure.
Stratifying Disease Severity
The severity of CDI-associated colitis increases as systemic symptoms worsen and the clinical picture deteriorates.4 In the 2010 SHEA-IDSA guidelines,1 CDI is stratified, both in the initial episode and the first recurrence, as mild-to-moderate, severe, or severe complicated. The following data may be used to appropriately stage disease severity in the initial episode:
• Mild-to-moderate disease is characterized by nonbloody diarrhea (fewer than 10 to 12 bowel movements per day), possibly accompanied by mild, crampy abdominal pain. Typically, affected patients do not exhibit significant systemic symptoms or marked abdominal tenderness. Leukocytosis is usually represented by fewer than 15,000 cells/L, and the serum creatinine level is less than 1.5 times the baseline level.1,2,5,8
• Severe disease, which should always be a consideration in older patients,1 includes profuse, watery diarrhea, significant abdominal pain and distension, fever, nausea and vomiting, and clinical volume depletion. Significant leukocytosis (≥ 15,000 cells/L) and serum creatinine ≥ 1.5 times baseline or leukopenia with possible bandemia may occur.1,27 Occult blood may be present, but frank blood is rare. A history of ICU admission alone increases the classification to severe due to the aforementioned poor outcomes associated with CDI in ICU patients.8,24
• Severe, complicated CDI may involve hypotension, shock, or a paralytic ileus1; a paradoxical decrease or absence of diarrhea may occur.2 At the severe end of the disease spectrum, toxic megacolon can develop and progress to colonic perforation.2,5,8,28 Hypoalbuminemia may be present due to the large protein losses in the course of the disease.28
The Most Severe Manifestations
Fulminant CDI can occur in 3% to 8% of patients with CDI.27,29 Fulminant disease carries a mortality rate between 35% and 80%.23 The patient’s clinical picture may resemble that seen in severe disease, with the addition of an acute abdomen indicating peritonitis; lethargy, hypotension, oliguria (including renal failure), and/or tachycardia due to a severe systemic inflammatory response induced by toxin production from C difficile. Up to 20% of patients with fulminant C difficile do not have diarrhea due to reasons explained previously.2,5,23
Bacteremia occurs rarely in patients with CDI.1,30 Risk factors for this severe condition include failure of medical treatment, leukocytosis exceeding 16,000 cells/L, surgery in the previous 30 days, a history of IBD, and previous administration of IV immunoglobulin.23
DIAGNOSIS
According to recommendations from the SHEA-IDSA expert panel,1 only unformed stool from symptomatic patients should be tested for C difficile or its toxins. In spite of slow turnaround time, stool culture (followed by toxigenic culture to identify a toxigenic isolate) is currently considered standard testing for C difficile. Cell cytotoxin assay has 98% sensitivity and 99% specificity, but turnaround time is 24 to 48 hours,28 potentially delaying treatment for patients who test positive.
Enzyme immunoassay (EIA) testing for C difficile toxin A and toxin B yields results within hours but is not as sensitive as the cell cytotoxin assay.1 Because toxin testing lacks sensitivity, a two-step strategy has been proposed and is called an “interim recommendation” by the SHEA-IDSA guideline authors: EIA testing for glutamate dehydrogenase (GDH), an enzyme produced by C difficile; then, in patients with positive results, confirmation by cell cytotoxin assay or toxigenic culture.1,4,28 (EIA testing for GDH can be a rapid, inexpensive method for ruling out CDI.28)
Polymerase chain reaction testing, recently developed for the detection of pathogenic C difficile, is rapid, sensitive, and specific.1 However, this method has not yet gained wide acceptance due to its relatively high cost.6
Imaging Options
Endoscopic visualization confirming the presence of PMC is also considered diagnostic of CDI; although half of patients with CDI lack this finding on endoscopy, CDI is present in 95% of patients with confirmed PMC.1,28 Colonoscopy is advantageous over sigmoidoscopy because up to one-third of patients have only right-sided colonic involvement.28 To its disadvantage, endoscopy carries a risk for perforation (particularly in patients with fulminant disease), as well as the inherent risks of sedation required to perform the procedure.
Though not specific, CT can be used as an adjunct to the diagnosis; features such as colonic wall thickening, pericolic stranding, ascites, pneumatosis, and free air resulting from perforation may suggest CDI and help determine the extent of disease.4,20 The accordion sign (high-attenuation oral contrast in the colon lumen, alternating with low attenuation of inflamed mucosa) and the double halo sign (IV contrast having varying degrees of attenuation due to submucosal inflammation and hyperemia) have also been reported in patients with CDI and may indicate PMC or fulminant CDI.28
The small intestine is typically not involved in CDI except in the setting of ileus and the rare entity of C difficile enteritis.2 Plain radiography is only helpful in cases of ileus or megacolon.28
PHARMACOLOGIC TREATMENT
Discontinuation of the offending drug (usually an antibiotic), whenever possible, is the first step; up to 25% of CDI patients recover without any further therapies. Limiting management to antibiotic withdrawal, however, is currently recommended only in patients with the mildest form of CDI due to the risk for subsequent fulminant disease and clinical deterioration.14 In patients with suspected severe (but unconfirmed) CDI, empiric treatment with one of the antimicrobial agents listed below may be appropriate5 (see also Table 21,8,14). For patients with confirmed illness who require continued antimicrobial therapy, an agent not associated with CDI may be substituted (eg, sulfamethoxazole, macrolides, aminoglycosides).14
Metronidazole is the first-line agent for mild-to-moderate initial disease.1 The mechanism of action is through DNA disruption and inhibition of nucleic acid synthesis, a process that induces cell death. Metronidazole also appears to have anti-inflammatory, antioxidant, and immunomodulating properties that assist in overcoming the disease. It should not be used in women who are pregnant.8
Dosage of the drug is 250 mg four times per day or 500 mg three times a day, given orally; or parenterally, if the patient cannot take it orally. The duration is 10 to 14 days (or longer in patients with underlying infection),1 and the cost is much lower than that of other appropriate antimicrobial agents (ie, less than $1/day).2,31
In patients with severe or refractory disease, vancomycin should be used. Vancomycin has shown superiority in severe disease compared with metronidazole, with clinical cure rates of 97% and 76%, respectively.1,4,32 The typical oral dosage is 125 mg four times per day for 10 days.1
Vancomycin is effective only when given enterally; the drug is not absorbed by the gastrointestinal tract, allowing it to achieve high concentrations in the colon. In the patient who cannot receive standard enteral therapy, vancomycin can be instilled directly into the colon by enema or colonic catheter. With this route of administration, there is a small risk for iatrogenic perforation.2 There has been advocacy in very severe or fulminant disease to use vancomycin (orally or rectally) combined with IV metronidazole.1,8 The cost for 10 to 14 days of treatment ranges from $1,000 to $1,500.31
Earlier this year, fidaxomicin, a macrocyclic antibiotic, was approved for treatment of CDI in adults. This agent has minimal systemic absorption and works in the intestinal lumen by inhibiting the bacterial RNA polymerase.33 In a randomized, controlled trial involving 629 patients, fidaxomicin’s effectiveness was found comparable to that of vancomycin for treatment of CDI (clinical cure rates in the intention-to-treat analysis, 88.2% vs 85.8%, respectively), and fidaxomicin-treated patients had a lower rate of recurrence after initial use (15.4% vs 25.3%, respectively; patients with fulminant disease were not included).33 Dosage is 200 mg every 12 hours for 10 days,33,34 at a reported cost of $2,800.31
Neither oral bacitracin nor fusidic acid has been shown to eliminate CDI or reduce recurrence.1,5,33
Clinical resolution reveals adequate response to treatment and need not be confirmed by laboratory testing. Asymptomatic carriers do not require treatment.5
Less Conventional Agents
Several nonantibiotic treatment regimens have been proposed for CDI. Use of probiotics has been controversial. In a systematic review, Dendukuri et al35 found insufficient evidence in the routine use of probiotics to prevent or treat CDI. There have even been reports of Saccharomyces boulardii–associated fungemia and lactobacillus-associated bacteremia resulting from probiotics use.36
An anion-exchange resin, cholestyramine, is thought to help bind toxins; when studied, however, it failed to show promising results in improving patients’ clinical course.1,37 Additionally, it can bind to other drugs, such as vancomycin, resulting in decreased pharmacologic efficacy of this and other agents.1,2 Therefore, it is not recommended.
Intravenous immunoglobulin can provide an option for treatment of severe and/or recurrent disease as a last resort.1,38 Thus far, only results from small observational or retrospective studies have supported its use.
SURGICAL OPTIONS
Early surgical consultation is warranted in severe or refractory disease9 and in patients with specific manifestations detected via abdominal/pelvic CT: ileus, perforation, obstruction, thickening of the colonic wall, toxic megacolon, ascites, necrotizing colitis, or a systemic inflammatory response that could lead to multiorgan system failure.14,27 Colectomy is undertaken in 0.4% to 3.5% of patients with CDI,2 with a goal of resecting the involved bowel and diverting the fecal stream. In the past, near-total colectomy was the treatment of choice.2
There have been published reports of successful segmental resection for fulminant CDI.23 The mortality rate is approximately 50% in patients who undergo colectomy.2 Survival rates are noted to improve with early and prompt surgical management of severe disease,8,29 which can be life threatening.14
A recently developed procedure for fulminant disease involves creating a diverting loop ileostomy. Through the ostomy, 8 L of propylene glycol electrolyte solution is instilled to reduce the colonic C difficile count; the patient is also administered a vancomycin enema. In a literature review of recent studies involving patients who underwent the procedure, Olivas et al29 reported a 30-day mortality rate of 19%, and 93% of surviving patients did not require a colectomy. Further investigation to reduce surgical mortality in patients with fulminant disease is ongoing.
INVESTIGATIVE TREATMENT OPTIONS
Fecal transplantation from healthy donors to those infected with pathogenic C difficile via nasogastric tube or enema have been studied.1,39 The theory is to help reconstitute normal colonic flora with the transplanted stool. This treatment option has only very limited data and acceptance.8
Promising research has been published regarding the infusion of combined monoclonal antibodies against toxin A and toxin B. Among 200 patients who were randomized to receive the study therapy or placebo, the rate of recurrence was 7% versus 25%, respectively; recurrence rates among patients infected with the virulent NAP1/B1/027 strain were 8% and 32%, respectively.40 Length of stay did not improve in patients taking monoclonal antibodies, and adverse events were reported in both patient groups.
In preliminary trials, a parenteral vaccine containing inactivated toxins A and B was reported safe and capable of triggering a “vigorous” serum antitoxin A response in healthy adults.9,14
ADDITIONAL MANAGEMENT RECOMMENDATIONS
Medications that slow gastrointestinal motility should not be used, as the slowing of peristalsis may allow toxins to accumulate in the colon, leading to worsening disease.18,22 The use of opiates and anti-diarrheal medications should be limited.24
Aggressive fluid and electrolyte replacement should be administered until diarrhea has been resolved (usually within three to six days). Patients may require vasoactive medications to support hemodynamics.2,5,14,24
Patients with mild disease can eat as they normally would. Those with severe disease, including those who may require surgery, fare best with bowel rest and possibly enteral nutrition.
Monitoring the patient for signs of improvement during the first 24 to 48 hours is an important component of management. The patient’s white blood cell count and temperature, the number and frequency of bowel movements, and the overall clinical picture should be evaluated daily. Patients who show improvement should complete the current regimen.
TREATMENT FAILURE AND RECURRENT CDI
If a patient’s condition does not improve or worsens at any point during therapy for CDI, a change to another antimicrobial agent is warranted. Also, surgical, gastroenterological, and/or infectious disease consult may be needed if no improvement is evident after five days of seemingly appropriate therapy.14,24
Recurrent CDI, which occurs at least once in 6% to 25% of treated patients, is most likely to occur 7 to 14 days after treatment completion.1,14 Seldom caused by resistant strains of C difficile, it is more likely to result from inadequate adherence to treatment, the presence of residual spores in the colon after treatment, or reinfection—although relapse is considered more common than reinfection.14,24 However, since a patient’s symptoms may have other causes, confirmation of recurrence should be sought through laboratory testing.
Recurrent illness is managed in the same way as successful initial therapy, based on the severity of disease; vancomycin is recommended for the first recurrence in a patient with a rising white blood cell count or serum creatinine level. Otherwise, metronidazole use may be considered.1
In patients who experience a second recurrence of confirmed CDI, a tapered or pulsed-dose regimen of oral vancomycin over a six-week period has been advocated1,8,14 (for details, see Table 2).
Results from small studies of patients with several recurrences of CDI suggest that oral rifaximin therapy can reduce subsequent recurrences if administered immediately after the conclusion of a course of vancomycin.1,41
PREVENTION OF C DIFFICILE INFECTION
Although “research gaps” exist regarding the optimal strategies to prevent CDI,1 decreased prescribing of nonessential antibiotics is key. Without the alteration in colonic flora caused by antimicrobial use, gut colonization cannot occur, and C difficile typically cannot proliferate.2
Preventing transmission of the pathogen is challenging in health care facilities, where C difficile spores have been cultured from staff members’ hands and from beds, floors, windowsills, and other areas; the spores can survive in hospital rooms for as long as 40 days after a patient with CDI has been discharged.24 Appropriate isolation precautions are essential, including single-patient–use equipment (eg, disposable rectal thermometers) and caregivers’ use of gowns, vinyl gloves, and cleaning agents that are effective against the spores, particularly bleach.1 Alcohol-based products are not effective against C difficile spores; diligent handwashing with soap or chlorhexidine is imperative to prevent the spread of CDI.1,2
CONCLUSION
Clinicians and patients alike face the clinical challenge of Clostridium difficile infection. Mild to moderate disease can be treated medically with excellent success rates. Severe disease carries a significant risk to life, and a multidisciplinary approach including early surgical consultation is warranted.
With early recognition and appropriate treatment, along with strict adherence to isolation policies, the health care community can help limit the spread of this insidious illness and its associated morbidity and mortality.
REFERENCES
1. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
2. Efron PA, Mazuski JE. Clostridium difficile colitis. Surg Clin North Am. 2009;89(2):483-500.
3. Hall IC, O’Toole E. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390-402.
4. Riddle DJ, Dubberke ER. Clostridium difficile infection in the intensive care unit. Infect Dis Clin North Am. 2009;23(3):727-743.
5. Sunenshine RH, McDonald LC. Clostridium difficile–associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006; 73(2):187-197.
6. Currie B. Improved testing methods are improving diagnosis of Clostridium difficile infections. Advance Administrators Laboratory. 2009;21:10. http://laboratory-manager.advance web.com/Article/PCR-for-C-diff.aspx. Accessed November 8, 2011.
7. Larson HE, Barclay FE, Honour P, Hill ID. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146(6):727-733.
8. Leffler DA, Lamont JT. Treatment of Clostridium difficile–associated disease. Gastroenterology. 2009;136(6):1899-1912.
9. Kelly CP, Lamont JT. Clostridium difficile: more difficult than ever. N Engl J Med. 2008;359(18): 1932-1940.
10. Pépin J, Valiquette L, Cossette B. Mortality attributed to nosocomial Clostridium difficile–associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005; 173(9):1037-1042.
11. Zilberberg MD. Clostridium difficile–related hospitalizations among US adults, 2006. Emerg Infect Dis. 2009;15(1):122-124.
12. Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in the inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4(4):409-416.
13. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile–associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260.
14. Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46 suppl 1:S32-S42.
15. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23): 2433-2441.
16. Dawson LF, Valiente E, Wren BW. Clostridium difficile: a continually evolving and problematic pathogen. Infect Genet Evol. 2009;9(6):
1410-1417.
17. Dubberke ER, Reske KA, Olsen MA, et al. Short- and long-term attributable costs of Clostridium difficile–associated disease in nonsurgical patients. Clin Infect Dis. 2008;46(4):497-504.
18. Dial S, Delaney JAC, Barkun An, et al. Use of gastric acid–suppression agents and the risk of community-acquired Clostridium difficile disease. JAMA. 2005;294(23):2989-2995.
19. Kuntz JL, Chrischilles EA, Pendergast JF, et al. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect Dis. 2011 Jul 15;11:194.
20. Kelly CP, Lamont JT. Antibiotic-associated diarrhea, pseudomembranous enterocolitis, and Clostridium difficile–associated diarrhea and colitis. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 9th ed. Philadelphia, PA: WB Saunders; 2010:1889-1902.
21. Carignan A, Allard C, Pépin J, et al. Risk of Clostridium difficile infection after perioperative antibacterial prophylaxis before and during an outbreak of infection due to a hypervirulent strain. Clin Infect Dis. 2008;46(12):1838-1843.
22. Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003; 54(3):243-245.
23. Butala P, Divino CM. Surgical aspects of fulminant Clostridium difficile colitis. Am J Surg. 2010;200(1):131-135.
24. Schroeder MS. Clostridium difficile–associated diarrhea. Am Fam Physician. 2005;71(5): 921-928.
25. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile–associated disease? A review of the evidence. Curr Med Res Opin. 2008;24(2):329-333.
26. Lowe DO, Mamdani MM, Kopp A, et al. Proton pump inhibitors and hospitalization for Clostridium difficile–associated disease: a population-based study. Clin Infect Dis. 2006;43(10): 1272-1276.
27. Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009;144(5):433-439.
28. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile. Clin Infect Dis. 2008;46 suppl 1:S12-S18.
29. Olivas AP, Umanskiy K, Zuckerbraun B, Alverdy JC. Avoiding colectomy during surgical management of fulminant Clostridium difficile colitis. Surg Infect. 2010;11(3):299-305.
30. Feldman RJ, Kallich M, Weinstein MP. Bacteremia due to Clostridium difficile: case report and review of extraintestinal C difficile infections. Clin Infect Dis. 1995;20(6):1560-1562.
31. BioPharm Physicians. What next for Dificid (fidaxomicin)? Jun 6, 2011. www.biopharmphysi cians.com/what-next-for-dificid-fidaxomicin. Accessed November 7, 2011.
32. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
33. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
34. Sullivan KM, Spooner LM. Fidaxomicin: a macrocyclic antibiotic for the management of Clostridium difficile infection. Ann Pharmacother. 2010;44(2):352-359.
35. Dendukuri N, Costa V, McGregor M, Brophy JM. Probiotic therapy for the prevention and treatment of Clostridium difficile–associated diarrhea: a systematic review. CMAJ. 2005;173(2): 167-170.
36. Segarra-Newnham M. Probiotics for Clostridium difficile–associated diarrhea: focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii. Ann Pharmacother. 2007;41(7): 1212-1221.
37. Lagrotteria D, Holmes S, Smieja M, et al. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile–associated diarrhea. Clin Infect Dis. 2006;43(5):547-552.
38. McPherson S, Rees CJ, Ellis R, et al. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum. 2006;49(5): 640-645.
39. Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003; 36(5):580-585.
40. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362(3): 197-205.
41. Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile–associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007; 44(6):846-848.
Clostridium difficile, a causative pathogen in antibiotic-associated colitis,1 is a slow-growing, spore-forming, gram-positive anaerobic bacillus,2 named difficile because it was so difficult to culture. Although this pathogenic spore was first identified in the 1930s,3 its vegetative, toxin-producing form was not recognized as a causative organism in diarrhea and colitis until the late 1970s.4,5 Since that time, C difficile has become a growing challenge to health care providers for accurate diagnosis, treatment, and containment of the spread of disease. C difficile infection (CDI) often takes a virulent course with associated morbidity, mortality, and health care costs.6
EPIDEMIOLOGY
In healthy adult patient populations, 2% to 3% are colonized with C difficile.2 The colonization rate among healthy infants is significantly higher, between 60% and 70%, but clinical infection is uncommon.7 As the colon becomes populated with flora, between ages 18 and 24 months, the carrier state disappears.8
In the hospital setting, 20% to 30% of patients become colonized with the organism by the fecal-oral route, which is facilitated when antibiotic therapy disrupts normal flora in the gut, enabling C difficile spores to proliferate; most patients are asymptomatic.5 In 2005, in US acute care hospitals, the incidence of CDI was reported at 84 cases per 100,000 patients.9C difficile remains the leading pathogen associated with inpatient antibiotic-associated diarrhea (AAD). It can be identified as the causative organism in 15% to 25% of cases of AAD in hospitalized patients.2
The mortality rate among hospitalized patients rises significantly in those identified with C difficile: 20.6%, compared with 7.0% of matched inpatient controls.10 ICU admission poses a significant burden of disease: The overall incidence of infection in the ICU population is about 4%. ICU patients who contract CDI have up to a 20% rate of fulminant colitis, a severe form of the disease often necessitating surgery. In this segment of the inpatient population, the mortality rate can approach 60%.8
Data analyzed by Zilberberg11 have demonstrated a significant increase in C difficile–associated infections in US hospitals in recent years. In 2001, the number of discharged patients with documented CDI was approximately 134,000, compared with 291,000 in 2005. The rising incidence of CDI has been attributed to increased antibiotic use, aging of the population, an increasing rate of comorbid conditions, fluoroquinolone resistance, and increased suspicion for the illness, which has led to increased use of testing.12,13
Of Significant Concern
Recurrent disease poses a particular challenge to the health care system. It has been reported that recurrence rates for CDI are between 15% and 35% after a first bout and between 33% and 65% after subsequent episodes of infection.2,14
A hypervirulent strain, the North American pulsed-field gel electrophoresis type 1 (NAP1/B1/027) has been implicated in several C difficile outbreaks.15 This subtype, which is especially resistant to fluoroquinolones,1 produces toxins earlier and in much greater quantities, including toxins A and B at levels 15 to 20 times greater than those seen in less virulent subtypes.4,12 Thus, the NAP1 strain is implicated in more severe disease and is more lethal.16 Affected patients have a 30-day mortality rate twice as high as that among patients with other strains of C difficile.12
The calculated cost of CDI adds between $2,454 and $7,179 in nonreimbursable costs per hospitalized patient, and an additional 3.6 to 7.0 days to their hospital stays.17 Estimates for CDI treatment in the US range from $436 million to $3 billion per year.6,17
Community-Acquired CDI
Community-acquired C difficile infection (CA-CDI) is a subtype that develops in patients who have not been hospitalized in the previous year,18 with incidence recently reported at 11.16 cases per 100,000 person-years.19 Affected patients tend to be younger than those with hospital-acquired infection and to have a less severe disease course. To meet the criteria of this subgroup, according to recent clinical practice guidelines jointly issued by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America (SHEA-IDSA),1 a patient may have developed symptoms no sooner than 12 weeks after hospital discharge (if any hospitalization occurred).
It is always important to perform a thorough history in patients with suspected CA-CDI to assess for recent hospitalizations and antibiotic use. In a retrospective study published in 2011, Kuntz et al19 found that CA-CDI–affected patients were six times as likely as healthy controls to have taken antimicrobials within 180 days before illness (including beta-lactam/beta-lactamase inhibitors, cephalosporins, clindamycin, fluoroquinolones, and penicillin) and twice as likely to have used gastric acid suppressants in that time.
One emerging theory is that CA-CDI is spread through food-borne illness. Unlike the vegetative form that resides in the bowel, C difficile spores are resistant to temperatures at which food is cooked (as they are to alcohol and many other disinfectant agents).5 Several studies have shown that livestock can harbor C difficile.12
PATHOPHYSIOLOGY
Normal gastrointestinal flora resist colonization and proliferation of C difficile; colonization of nontoxigenic strains appears to be protective against toxigenic strains.1 Alteration in the colonic bacterial environment, including the suppression of normal flora proliferation that can occur when a patient receives antibiotics or antineoplastic agents, is thought to allow the overgrowth of C difficile.2
Only C difficile strains that produce exotoxins (the enterotoxin toxin A, and the cytotoxin toxin B) are pathogenic. The organism produces a variety of adhesion proteins (with production accelerated by the presence of antibiotics, such as ampicillin and clindamycin), leading the toxins to bind to specific receptors in the intestinal mucosal cells. C difficile also produces proteases that trigger degradation of the intestinal extracellular matrix and disruption of epithelial cell signaling.16 The toxins activate proinflammatory cytokines, including interleukin (IL)-1, tumor necrosis factor (TNF)-, and IL-8. The result is an intestinal inflammatory response that is clinically apparent in the form of diarrhea and pseudomembranous colitis (PMC).20
RISK FACTORS
Among several known risk factors for CDI (see Table 11,2,4,18,21-24), perhaps the most widely recognized is the use of nearly any antimicrobial agent. Previous administration of antibiotics has been documented in about 95% of inpatients with CDI. Broad-spectrum antibiotics with anti-anaerobic coverage appear to pose the greatest risk.1,4 These include clindamycin, along with cephalosporins, fluoroquinolones, and beta-lactams.1,13 Fluoroquinolone use has been implicated in the development of the NAP1 subtype of the disease.25 In patients who receive multiple antibiotics or prolonged courses, an even greater risk for CDI is incurred.4 Even single-dose administration of an antibiotic carries a CDI risk of about 1.5%.21
A prolonged hospital stay or residence in a long-term care facility increases the risk for CDI, as does advanced age.1 Patients 65 or older have a 20-fold higher risk for CDI than their younger counterparts.1,2 Patients with inflammatory bowel disease (IBD) have an increased risk for CDI.23
It has been theorized that the use of gastric acid–suppressive medications can increase the risk for CDI to between 2.5 and 2.9 times that among persons who do not take these agents.18,22 Results from other trials have refuted this theory, however.13,26
Additional risk factors include ICU admission, recent gastrointestinal surgery or manipulation, immunosuppression, and serious underlying illnesses.24 Postpyloric enteral tube feeding has also been implicated as a risk factor; this route bypasses the stomach, where a very acidic environment ordinarily helps to kill the organism.4
CLINICAL PRESENTATION
Watery diarrhea, occurring 10 to 15 times in a day, is the most common manifestation of CDI. It may present during administration of an antimicrobial agent or within a few days afterward; rarely, it can develop as long as two months after cessation of such treatment.2 In most studies, median onset of diarrhea is about two days.20 Descriptive characteristics of the stool, including odor and color, may vary. Diarrhea may be accompanied by lower abdominal pain, cramping, low-grade fever, and leukocytosis.
In some patients, particularly those taking narcotics, diarrhea may be absent. This manifestation may signal a more severe disease course, including the possibility of fulminant infection. Endoscopic evaluation of the colon may reveal the classic pseudomembranes (ie, adherent yellow plaques)2; see figure.
Stratifying Disease Severity
The severity of CDI-associated colitis increases as systemic symptoms worsen and the clinical picture deteriorates.4 In the 2010 SHEA-IDSA guidelines,1 CDI is stratified, both in the initial episode and the first recurrence, as mild-to-moderate, severe, or severe complicated. The following data may be used to appropriately stage disease severity in the initial episode:
• Mild-to-moderate disease is characterized by nonbloody diarrhea (fewer than 10 to 12 bowel movements per day), possibly accompanied by mild, crampy abdominal pain. Typically, affected patients do not exhibit significant systemic symptoms or marked abdominal tenderness. Leukocytosis is usually represented by fewer than 15,000 cells/L, and the serum creatinine level is less than 1.5 times the baseline level.1,2,5,8
• Severe disease, which should always be a consideration in older patients,1 includes profuse, watery diarrhea, significant abdominal pain and distension, fever, nausea and vomiting, and clinical volume depletion. Significant leukocytosis (≥ 15,000 cells/L) and serum creatinine ≥ 1.5 times baseline or leukopenia with possible bandemia may occur.1,27 Occult blood may be present, but frank blood is rare. A history of ICU admission alone increases the classification to severe due to the aforementioned poor outcomes associated with CDI in ICU patients.8,24
• Severe, complicated CDI may involve hypotension, shock, or a paralytic ileus1; a paradoxical decrease or absence of diarrhea may occur.2 At the severe end of the disease spectrum, toxic megacolon can develop and progress to colonic perforation.2,5,8,28 Hypoalbuminemia may be present due to the large protein losses in the course of the disease.28
The Most Severe Manifestations
Fulminant CDI can occur in 3% to 8% of patients with CDI.27,29 Fulminant disease carries a mortality rate between 35% and 80%.23 The patient’s clinical picture may resemble that seen in severe disease, with the addition of an acute abdomen indicating peritonitis; lethargy, hypotension, oliguria (including renal failure), and/or tachycardia due to a severe systemic inflammatory response induced by toxin production from C difficile. Up to 20% of patients with fulminant C difficile do not have diarrhea due to reasons explained previously.2,5,23
Bacteremia occurs rarely in patients with CDI.1,30 Risk factors for this severe condition include failure of medical treatment, leukocytosis exceeding 16,000 cells/L, surgery in the previous 30 days, a history of IBD, and previous administration of IV immunoglobulin.23
DIAGNOSIS
According to recommendations from the SHEA-IDSA expert panel,1 only unformed stool from symptomatic patients should be tested for C difficile or its toxins. In spite of slow turnaround time, stool culture (followed by toxigenic culture to identify a toxigenic isolate) is currently considered standard testing for C difficile. Cell cytotoxin assay has 98% sensitivity and 99% specificity, but turnaround time is 24 to 48 hours,28 potentially delaying treatment for patients who test positive.
Enzyme immunoassay (EIA) testing for C difficile toxin A and toxin B yields results within hours but is not as sensitive as the cell cytotoxin assay.1 Because toxin testing lacks sensitivity, a two-step strategy has been proposed and is called an “interim recommendation” by the SHEA-IDSA guideline authors: EIA testing for glutamate dehydrogenase (GDH), an enzyme produced by C difficile; then, in patients with positive results, confirmation by cell cytotoxin assay or toxigenic culture.1,4,28 (EIA testing for GDH can be a rapid, inexpensive method for ruling out CDI.28)
Polymerase chain reaction testing, recently developed for the detection of pathogenic C difficile, is rapid, sensitive, and specific.1 However, this method has not yet gained wide acceptance due to its relatively high cost.6
Imaging Options
Endoscopic visualization confirming the presence of PMC is also considered diagnostic of CDI; although half of patients with CDI lack this finding on endoscopy, CDI is present in 95% of patients with confirmed PMC.1,28 Colonoscopy is advantageous over sigmoidoscopy because up to one-third of patients have only right-sided colonic involvement.28 To its disadvantage, endoscopy carries a risk for perforation (particularly in patients with fulminant disease), as well as the inherent risks of sedation required to perform the procedure.
Though not specific, CT can be used as an adjunct to the diagnosis; features such as colonic wall thickening, pericolic stranding, ascites, pneumatosis, and free air resulting from perforation may suggest CDI and help determine the extent of disease.4,20 The accordion sign (high-attenuation oral contrast in the colon lumen, alternating with low attenuation of inflamed mucosa) and the double halo sign (IV contrast having varying degrees of attenuation due to submucosal inflammation and hyperemia) have also been reported in patients with CDI and may indicate PMC or fulminant CDI.28
The small intestine is typically not involved in CDI except in the setting of ileus and the rare entity of C difficile enteritis.2 Plain radiography is only helpful in cases of ileus or megacolon.28
PHARMACOLOGIC TREATMENT
Discontinuation of the offending drug (usually an antibiotic), whenever possible, is the first step; up to 25% of CDI patients recover without any further therapies. Limiting management to antibiotic withdrawal, however, is currently recommended only in patients with the mildest form of CDI due to the risk for subsequent fulminant disease and clinical deterioration.14 In patients with suspected severe (but unconfirmed) CDI, empiric treatment with one of the antimicrobial agents listed below may be appropriate5 (see also Table 21,8,14). For patients with confirmed illness who require continued antimicrobial therapy, an agent not associated with CDI may be substituted (eg, sulfamethoxazole, macrolides, aminoglycosides).14
Metronidazole is the first-line agent for mild-to-moderate initial disease.1 The mechanism of action is through DNA disruption and inhibition of nucleic acid synthesis, a process that induces cell death. Metronidazole also appears to have anti-inflammatory, antioxidant, and immunomodulating properties that assist in overcoming the disease. It should not be used in women who are pregnant.8
Dosage of the drug is 250 mg four times per day or 500 mg three times a day, given orally; or parenterally, if the patient cannot take it orally. The duration is 10 to 14 days (or longer in patients with underlying infection),1 and the cost is much lower than that of other appropriate antimicrobial agents (ie, less than $1/day).2,31
In patients with severe or refractory disease, vancomycin should be used. Vancomycin has shown superiority in severe disease compared with metronidazole, with clinical cure rates of 97% and 76%, respectively.1,4,32 The typical oral dosage is 125 mg four times per day for 10 days.1
Vancomycin is effective only when given enterally; the drug is not absorbed by the gastrointestinal tract, allowing it to achieve high concentrations in the colon. In the patient who cannot receive standard enteral therapy, vancomycin can be instilled directly into the colon by enema or colonic catheter. With this route of administration, there is a small risk for iatrogenic perforation.2 There has been advocacy in very severe or fulminant disease to use vancomycin (orally or rectally) combined with IV metronidazole.1,8 The cost for 10 to 14 days of treatment ranges from $1,000 to $1,500.31
Earlier this year, fidaxomicin, a macrocyclic antibiotic, was approved for treatment of CDI in adults. This agent has minimal systemic absorption and works in the intestinal lumen by inhibiting the bacterial RNA polymerase.33 In a randomized, controlled trial involving 629 patients, fidaxomicin’s effectiveness was found comparable to that of vancomycin for treatment of CDI (clinical cure rates in the intention-to-treat analysis, 88.2% vs 85.8%, respectively), and fidaxomicin-treated patients had a lower rate of recurrence after initial use (15.4% vs 25.3%, respectively; patients with fulminant disease were not included).33 Dosage is 200 mg every 12 hours for 10 days,33,34 at a reported cost of $2,800.31
Neither oral bacitracin nor fusidic acid has been shown to eliminate CDI or reduce recurrence.1,5,33
Clinical resolution reveals adequate response to treatment and need not be confirmed by laboratory testing. Asymptomatic carriers do not require treatment.5
Less Conventional Agents
Several nonantibiotic treatment regimens have been proposed for CDI. Use of probiotics has been controversial. In a systematic review, Dendukuri et al35 found insufficient evidence in the routine use of probiotics to prevent or treat CDI. There have even been reports of Saccharomyces boulardii–associated fungemia and lactobacillus-associated bacteremia resulting from probiotics use.36
An anion-exchange resin, cholestyramine, is thought to help bind toxins; when studied, however, it failed to show promising results in improving patients’ clinical course.1,37 Additionally, it can bind to other drugs, such as vancomycin, resulting in decreased pharmacologic efficacy of this and other agents.1,2 Therefore, it is not recommended.
Intravenous immunoglobulin can provide an option for treatment of severe and/or recurrent disease as a last resort.1,38 Thus far, only results from small observational or retrospective studies have supported its use.
SURGICAL OPTIONS
Early surgical consultation is warranted in severe or refractory disease9 and in patients with specific manifestations detected via abdominal/pelvic CT: ileus, perforation, obstruction, thickening of the colonic wall, toxic megacolon, ascites, necrotizing colitis, or a systemic inflammatory response that could lead to multiorgan system failure.14,27 Colectomy is undertaken in 0.4% to 3.5% of patients with CDI,2 with a goal of resecting the involved bowel and diverting the fecal stream. In the past, near-total colectomy was the treatment of choice.2
There have been published reports of successful segmental resection for fulminant CDI.23 The mortality rate is approximately 50% in patients who undergo colectomy.2 Survival rates are noted to improve with early and prompt surgical management of severe disease,8,29 which can be life threatening.14
A recently developed procedure for fulminant disease involves creating a diverting loop ileostomy. Through the ostomy, 8 L of propylene glycol electrolyte solution is instilled to reduce the colonic C difficile count; the patient is also administered a vancomycin enema. In a literature review of recent studies involving patients who underwent the procedure, Olivas et al29 reported a 30-day mortality rate of 19%, and 93% of surviving patients did not require a colectomy. Further investigation to reduce surgical mortality in patients with fulminant disease is ongoing.
INVESTIGATIVE TREATMENT OPTIONS
Fecal transplantation from healthy donors to those infected with pathogenic C difficile via nasogastric tube or enema have been studied.1,39 The theory is to help reconstitute normal colonic flora with the transplanted stool. This treatment option has only very limited data and acceptance.8
Promising research has been published regarding the infusion of combined monoclonal antibodies against toxin A and toxin B. Among 200 patients who were randomized to receive the study therapy or placebo, the rate of recurrence was 7% versus 25%, respectively; recurrence rates among patients infected with the virulent NAP1/B1/027 strain were 8% and 32%, respectively.40 Length of stay did not improve in patients taking monoclonal antibodies, and adverse events were reported in both patient groups.
In preliminary trials, a parenteral vaccine containing inactivated toxins A and B was reported safe and capable of triggering a “vigorous” serum antitoxin A response in healthy adults.9,14
ADDITIONAL MANAGEMENT RECOMMENDATIONS
Medications that slow gastrointestinal motility should not be used, as the slowing of peristalsis may allow toxins to accumulate in the colon, leading to worsening disease.18,22 The use of opiates and anti-diarrheal medications should be limited.24
Aggressive fluid and electrolyte replacement should be administered until diarrhea has been resolved (usually within three to six days). Patients may require vasoactive medications to support hemodynamics.2,5,14,24
Patients with mild disease can eat as they normally would. Those with severe disease, including those who may require surgery, fare best with bowel rest and possibly enteral nutrition.
Monitoring the patient for signs of improvement during the first 24 to 48 hours is an important component of management. The patient’s white blood cell count and temperature, the number and frequency of bowel movements, and the overall clinical picture should be evaluated daily. Patients who show improvement should complete the current regimen.
TREATMENT FAILURE AND RECURRENT CDI
If a patient’s condition does not improve or worsens at any point during therapy for CDI, a change to another antimicrobial agent is warranted. Also, surgical, gastroenterological, and/or infectious disease consult may be needed if no improvement is evident after five days of seemingly appropriate therapy.14,24
Recurrent CDI, which occurs at least once in 6% to 25% of treated patients, is most likely to occur 7 to 14 days after treatment completion.1,14 Seldom caused by resistant strains of C difficile, it is more likely to result from inadequate adherence to treatment, the presence of residual spores in the colon after treatment, or reinfection—although relapse is considered more common than reinfection.14,24 However, since a patient’s symptoms may have other causes, confirmation of recurrence should be sought through laboratory testing.
Recurrent illness is managed in the same way as successful initial therapy, based on the severity of disease; vancomycin is recommended for the first recurrence in a patient with a rising white blood cell count or serum creatinine level. Otherwise, metronidazole use may be considered.1
In patients who experience a second recurrence of confirmed CDI, a tapered or pulsed-dose regimen of oral vancomycin over a six-week period has been advocated1,8,14 (for details, see Table 2).
Results from small studies of patients with several recurrences of CDI suggest that oral rifaximin therapy can reduce subsequent recurrences if administered immediately after the conclusion of a course of vancomycin.1,41
PREVENTION OF C DIFFICILE INFECTION
Although “research gaps” exist regarding the optimal strategies to prevent CDI,1 decreased prescribing of nonessential antibiotics is key. Without the alteration in colonic flora caused by antimicrobial use, gut colonization cannot occur, and C difficile typically cannot proliferate.2
Preventing transmission of the pathogen is challenging in health care facilities, where C difficile spores have been cultured from staff members’ hands and from beds, floors, windowsills, and other areas; the spores can survive in hospital rooms for as long as 40 days after a patient with CDI has been discharged.24 Appropriate isolation precautions are essential, including single-patient–use equipment (eg, disposable rectal thermometers) and caregivers’ use of gowns, vinyl gloves, and cleaning agents that are effective against the spores, particularly bleach.1 Alcohol-based products are not effective against C difficile spores; diligent handwashing with soap or chlorhexidine is imperative to prevent the spread of CDI.1,2
CONCLUSION
Clinicians and patients alike face the clinical challenge of Clostridium difficile infection. Mild to moderate disease can be treated medically with excellent success rates. Severe disease carries a significant risk to life, and a multidisciplinary approach including early surgical consultation is warranted.
With early recognition and appropriate treatment, along with strict adherence to isolation policies, the health care community can help limit the spread of this insidious illness and its associated morbidity and mortality.
REFERENCES
1. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
2. Efron PA, Mazuski JE. Clostridium difficile colitis. Surg Clin North Am. 2009;89(2):483-500.
3. Hall IC, O’Toole E. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390-402.
4. Riddle DJ, Dubberke ER. Clostridium difficile infection in the intensive care unit. Infect Dis Clin North Am. 2009;23(3):727-743.
5. Sunenshine RH, McDonald LC. Clostridium difficile–associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006; 73(2):187-197.
6. Currie B. Improved testing methods are improving diagnosis of Clostridium difficile infections. Advance Administrators Laboratory. 2009;21:10. http://laboratory-manager.advance web.com/Article/PCR-for-C-diff.aspx. Accessed November 8, 2011.
7. Larson HE, Barclay FE, Honour P, Hill ID. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146(6):727-733.
8. Leffler DA, Lamont JT. Treatment of Clostridium difficile–associated disease. Gastroenterology. 2009;136(6):1899-1912.
9. Kelly CP, Lamont JT. Clostridium difficile: more difficult than ever. N Engl J Med. 2008;359(18): 1932-1940.
10. Pépin J, Valiquette L, Cossette B. Mortality attributed to nosocomial Clostridium difficile–associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005; 173(9):1037-1042.
11. Zilberberg MD. Clostridium difficile–related hospitalizations among US adults, 2006. Emerg Infect Dis. 2009;15(1):122-124.
12. Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in the inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4(4):409-416.
13. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile–associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260.
14. Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46 suppl 1:S32-S42.
15. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23): 2433-2441.
16. Dawson LF, Valiente E, Wren BW. Clostridium difficile: a continually evolving and problematic pathogen. Infect Genet Evol. 2009;9(6):
1410-1417.
17. Dubberke ER, Reske KA, Olsen MA, et al. Short- and long-term attributable costs of Clostridium difficile–associated disease in nonsurgical patients. Clin Infect Dis. 2008;46(4):497-504.
18. Dial S, Delaney JAC, Barkun An, et al. Use of gastric acid–suppression agents and the risk of community-acquired Clostridium difficile disease. JAMA. 2005;294(23):2989-2995.
19. Kuntz JL, Chrischilles EA, Pendergast JF, et al. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect Dis. 2011 Jul 15;11:194.
20. Kelly CP, Lamont JT. Antibiotic-associated diarrhea, pseudomembranous enterocolitis, and Clostridium difficile–associated diarrhea and colitis. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 9th ed. Philadelphia, PA: WB Saunders; 2010:1889-1902.
21. Carignan A, Allard C, Pépin J, et al. Risk of Clostridium difficile infection after perioperative antibacterial prophylaxis before and during an outbreak of infection due to a hypervirulent strain. Clin Infect Dis. 2008;46(12):1838-1843.
22. Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003; 54(3):243-245.
23. Butala P, Divino CM. Surgical aspects of fulminant Clostridium difficile colitis. Am J Surg. 2010;200(1):131-135.
24. Schroeder MS. Clostridium difficile–associated diarrhea. Am Fam Physician. 2005;71(5): 921-928.
25. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile–associated disease? A review of the evidence. Curr Med Res Opin. 2008;24(2):329-333.
26. Lowe DO, Mamdani MM, Kopp A, et al. Proton pump inhibitors and hospitalization for Clostridium difficile–associated disease: a population-based study. Clin Infect Dis. 2006;43(10): 1272-1276.
27. Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009;144(5):433-439.
28. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile. Clin Infect Dis. 2008;46 suppl 1:S12-S18.
29. Olivas AP, Umanskiy K, Zuckerbraun B, Alverdy JC. Avoiding colectomy during surgical management of fulminant Clostridium difficile colitis. Surg Infect. 2010;11(3):299-305.
30. Feldman RJ, Kallich M, Weinstein MP. Bacteremia due to Clostridium difficile: case report and review of extraintestinal C difficile infections. Clin Infect Dis. 1995;20(6):1560-1562.
31. BioPharm Physicians. What next for Dificid (fidaxomicin)? Jun 6, 2011. www.biopharmphysi cians.com/what-next-for-dificid-fidaxomicin. Accessed November 7, 2011.
32. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
33. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
34. Sullivan KM, Spooner LM. Fidaxomicin: a macrocyclic antibiotic for the management of Clostridium difficile infection. Ann Pharmacother. 2010;44(2):352-359.
35. Dendukuri N, Costa V, McGregor M, Brophy JM. Probiotic therapy for the prevention and treatment of Clostridium difficile–associated diarrhea: a systematic review. CMAJ. 2005;173(2): 167-170.
36. Segarra-Newnham M. Probiotics for Clostridium difficile–associated diarrhea: focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii. Ann Pharmacother. 2007;41(7): 1212-1221.
37. Lagrotteria D, Holmes S, Smieja M, et al. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile–associated diarrhea. Clin Infect Dis. 2006;43(5):547-552.
38. McPherson S, Rees CJ, Ellis R, et al. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum. 2006;49(5): 640-645.
39. Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003; 36(5):580-585.
40. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362(3): 197-205.
41. Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile–associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007; 44(6):846-848.