User login
Man, 54, With Delusions and Seizures
A 54-year-old African-American man was brought by police officers to the emergency department (ED) after he called 911 several times to report seeing a Rottweiler looking into his second-story window. At the scene, the police were unable to confirm his story, thought the man seemed intoxicated, and brought him to the ED for evaluation.
The patient reported that he had been drinking the previous evening but denied current intoxication or illicit drug use. He denied experiencing symptoms of alcohol withdrawal.
Regarding his medical history, the patient admitted to having had seizures, including two episodes that he said required hospitalization. He described these episodes as right-hand “tingling” (paresthesias), accompanied by right-facial numbness and aphasia. The patient said his physician had instructed him to take “a few phenytoin pills” whenever these episodes occurred. He reported that the medication usually helped resolve his symptoms. He said he had taken phenytoin shortly before his current presentation.
According to friends of the patient who were questioned, he had had noticeable memory problems during the previous six to eight months. They said that he often told the same joke, day after day. His speech had become increasingly slurred, even when he was not drinking.
Once the patient’s medical records were retrieved, it was revealed that he had been hospitalized twice for witnessed grand mal seizures about six months before his current admission; he had been drinking alcohol prior to both episodes. He underwent electroencephalography (EEG) during one of these hospitalizations, with results reported as normal. On both occasions, the patient was discharged with phenytoin and was instructed to follow up with his primary care provider and neurologist.
The patient, who reported working in customer service, had no known allergies. He claimed to drink one or two 40-ounce beers twice per week and admitted to occasional cocaine use. Of significance in his family history was a fatal MI in his mother. Although the patient denied any history of rashes or lesions, his current delirium made it impossible to obtain a reliable sexual history; a friend who was questioned, however, described the patient as promiscuous.
On initial physical examination, the man was afebrile, tachycardic, and somewhat combative with the ED staff. He was fully oriented to self but only partially to place and time.
His right pupil was 3+ and his left pupil was 2+, with neither reactive to light. He spoke with tangential speech and his gait was unsteady, but no other significant abnormalities were noted. A full assessment revealed no rashes or other lesions.
Significant laboratory findings included a low level of phenytoin, a negative blood alcohol level, presence of cocaine on urine drug screening, and normal levels of thyroid-stimulating hormone (TSH), vitamin B12, and folate. The patient’s serum VDRL (venereal disease research laboratory) titer was positive at 1:256.
Electroencephalography showed diffuse slowing, and brain CT performed in the ED showed atrophy that was mild but appropriate for a person of the patient’s age, with no evidence of a cerebrovascular accident (CVA). Aneurysm was ruled out by CT angiography of the brain. MRI revealed persistent increased signal in the subarachnoid space.
The patient was admitted with an initial diagnosis of paranoid delusional psychosis and monitored for alcohol withdrawal. He was given lorazepam as needed for agitation. Consultations were arranged with the psychiatry service regarding his delusions, and with neurology to determine whether to continue phenytoin.
The patient showed little response during the next several days. Based on positive results on serum VDRL with high titer, the presence of Argyll-Robertson pupils on exam, and his history of dementia-like symptoms, a lumbar puncture was performed to rule out neurosyphilis. In the patient’s cerebral spinal fluid (CSF) analysis, the first tube was clear and colorless, with 72 cells (28% neutrophils, 59% lymphocytes); glucose, 64 mg/dL; and total protein, 117 mg/dL. The fourth tube had 34 cells (17% neutrophils, 65% lymphocytes) and a positive VDRL titer at 1:128. Results from a serum syphilis immunoglobulin G (IgG) test were positive, and HIV antibody testing was nonreactive, confirming the diagnosis of neurosyphilis.
The hospital’s infectious disease (ID) team recommended treatment with IV penicillin for 14 days. Once this was completed, the patient was discharged with instructions to follow up at the ID clinic in three months for a repeat CSF VDRL titer to monitor for resolution of the disease. His prescription for phenytoin was discontinued.
At the time of discharge, it was noted that the patient showed no evidence of having regained cognitive function. He was deemed by the psychiatry service to lack decision-making capacity—a likely sequelae of untreated neurosyphilis of unknown duration.
He did return to the ID clinic six months after his discharge. At that visit, a VDRL serum titer was drawn with a result of 1:64, a decrease from 1:128. His syphilis IgG remained positive, however.
Discussion
Definition and Epidemiology
Syphilis is commonly known as a sexually transmitted disease with primary, secondary, and tertiary (early and late latent) stages.1 Neurosyphilis is defined as a manifestation of the inflammatory response to invasion over decades by the Treponema pallidum spirochete in the CSF as a result of untreated primary and/or secondary syphilis.2 About one in 10 patients with untreated syphilis will experience neurologic involvement.3,4 Before 2005, neurosyphilis was required to be reported as a specific stage of syphilis (ie, a manifestation of tertiary syphilis4), but now should be reported as syphilis with neurologic manifestations.5
A reportable infectious disease, syphilis was widespread until the advent of penicillin. According to CDC statistics,6 the number of reported cases of primary and secondary syphilis has declined steadily since 1943. In the late 1970s and early 1980s, the number of tertiary cases also began to plateau, likely as a result of earlier diagnosis and more widespread use of penicillin. Recent case reports suggest greater prevalence of syphilis among men than women and increased incidence among men who have sex with men.7
Pathogenesis
Syphilis is most commonly spread by sexual contact or contact with an infected primary lesion (chancre). Less likely routes of transmission are placental passage or blood transfusion. Infectivity is greatest in the early disease stages.8
Primary syphilis is marked by transmission of the spirochete, ending with development of secondary syphilis (usually two to 12 weeks after transmission). A chancre commonly develops but is often missed by patients because it is painless and can heal spontaneously.7 The chancre is also often confused with two other sources of genital lesions, herpes simplex (genital herpes) and Haemophilus ducreyi (chancroid). In two-thirds of cases of untreated primary syphilis, the infection clears spontaneously, but in the remaining one-third, the disease progresses.8
Secondary syphilis, with or without presence of a chancre, manifests with constitutional symptoms, including lymphadenopathy, fever, headache, and malaise. Patients in this disease phase may also present with a generalized, nonpruritic, macular to maculopapular or pustular rash. The rash can affect the skin of the trunk, the proximal extremities, and the palms and soles. Ocular involvement may occur, especially in patients who are coinfected with HIV.8 In either primary or secondary syphilis, infection can invade the central nervous system.1
During latent syphilis, patients show serologic conversion without overt symptoms. Early latent syphilis is defined as infection within the previous year, as demonstrated by conversion from negative to positive testing, or an increase in titers within the previous year. Any case occurring after one year is defined as late or unknown latent syphilis.8
Tertiary syphilis is marked by complications resulting from untreated syphilis; affected patients commonly experience central nervous system and cardiovascular involvement. Gummatous disease is seen in 15% of patients.1
The early stages of neurosyphilis may be asymptomatic, acute meningeal, and meningovascular.1,4,8,9 Only 5% of patients with early neurosyphilis are symptomatic, with the added potential for cranial neuritis or ocular involvement.1 The late stages of neurosyphilis are detailed in the table.1,4,8
Diagnosis
A diagnosis of syphilis is made by testing blood samples or scrapings from a lesion. In patients with suspected syphilis, rapid plasma reagin (RPR) testing or a VDRL titer is commonly ordered. When results are positive, a serum treponemal test is recommended to confirm a diagnosis of syphilis. Options include the fluorescent treponemal antibody absorption test (FTA-ABS) and the microhemagglutinin assay for antibody to T pallidum (MHA-TP).5
If neurologic symptoms are present, a CSF sample should be obtained, followed by the same testing. A confirmed diagnosis of neurosyphilis is defined by the CDC as syphilis at any stage that meets laboratory criteria for neurosyphilis5; these include increased CSF protein or an elevated CSF leukocyte count with no other known cause, and clinical signs or symptoms without other known causes.7
Treatment
Treatment of syphilis generally consists of penicillin, administered intramuscularly (IM) or IV, depending on the stage. According to 2006 guidelines from the CDC,10,11 treatment for adults with primary and secondary syphilis is a single dose of IM penicillin G, 2.4 million units. If neurosyphilis is suspected, recommended treatment is IV penicillin G, 18 to 24 million units per day divided into six doses (ie, 3 to 4 million units every four hours) or continuous pump infusion for 10 to 14 days.10-12 Follow-up is recommended by monitoring CSF titers to ensure clearance of infection; retreatment may be required if CSF abnormalities persist after two years.11
Patients with a penicillin allergy should undergo desensitization, as penicillin is the preferred agent; the potential exists for cross-reactivity with ceftriaxone, a possible alternative for patients with neurosyphilis.11 All patients diagnosed with syphilis should also be tested for HIV and other sexually transmitted diseases.10-12
The prognosis of patients treated for neurosyphilis is generally good if the condition is diagnosed and treated early. In patients with cerebral atrophy, frontal lesions, dementia, or tabes dorsalis, the potential for recovery decreases.2,13,14
Teaching Points
There are several teaching points to take away from this case:
• Remember to rule out a CVA in any patient who presents with numbness, paresthesias, or slurred speech. In this case, a brain CT and CT angiography of the brain were both obtained in the ED before the patient was admitted. They both yielded negative results; because the patient’s history was consistent with alcohol and drug use and he had a history of seizures, he was monitored closely for signs of withdrawal or further seizure.
• Phenytoin is an antiepileptic agent whose use requires proper patient education and drug level monitoring. Appropriate follow-up must be ensured before phenytoin therapy is begun, as toxicity can result in nystagmus, ataxia, slurred speech, decreased coordination, mental confusion, and possibly death.15,16
• For patients with a suspected acute change in mental status, a workup is required and should be tailored appropriately, based on findings. This should include, but not be limited to, a thorough history and physical exam, CT of the brain (to rule out an acute brain injury17), and, if warranted, MRI of the brain. Also, a urine drug screen and alcohol level, a complete blood count, a TSH level (to evaluate for altered thyroid function that may explain mental status changes), comprehensive panel, RPR testing and/or a VDRL titer should be obtained, depending on the facility’s protocol18,19; at some facilities, a treponemal test, rather than VDRL, is being obtained at the outset.20 Levels of vitamin B12 (as part of the dementia workup), folate, thiamine, and ammonia (in patients with suspected liver disease) can also be obtained in patients with change in mental status.18,19 Urinalysis should not be overlooked to check for a urinary tract infection, especially in elderly patients.21
• If primary syphilis is suspected, treatment must be undertaken.20
Conclusion
Despite the decline seen since the 1940s in cases of primary and secondary syphilis, and the effectiveness of penicillin in treating the infection early, patients with late-stage syphilis, including those with neurosyphilis, may still present to the emergency care, urgent care, or primary care setting. Immediate treatment with penicillin is recommended to achieve an optimal prognosis for the affected patient.
1. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
2. Simon RP. Chapter 20. Neurosyphilis. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. USA: The McGraw-Hill Companies; 2007:130-137.
3. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48:440-445.
4. Marra CM. Neurosyphilis. Curr Neurol Neurosci Rep. 2004;4(6):435-440.
5. CDC. Sexually transmitted diseases surveillance, 2007: STD surveillance case definitions. www.cdc.gov/std/stats07/app-casedef.htm. Accessed March 23, 2011.
6. CDC. 2008 Sexually Transmitted Diseases Surveillance: Table 1. Cases of sexually transmitted diseases reported by state health departments and rates per 100,000 population: United States, 1941-2008. www.cdc.gov/std/stats08/tables/1.htm. Accessed March 23, 2011.
7. CDC. Sexually transmitted diseases (STDs): Syphilis: CDC fact sheet. www.cdc.gov/std/syphilis/STDfact-syphilis.htm. Accessed March 23, 2011.
8. Tramont EC. Chapter 238. Treponema pallidum (syphilis). In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier Churchill Livingstone; 2009.
9. Ghanem KG. Neurosyphilis: a historical perspective and review. CNS Neurosci Ther. 2010; 16(5):e157-e168.
10. Workowski KA, Berman SM; CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
11. CDC. Sexually transmitted diseases: treatment guidelines 2006. www.cdc.gov/std/treatment/2006/genital-ulcers.htm#genulc6. Accessed March 29, 2011.
12. Drugs for sexually transmitted infections. Treatment Guidelines from the Medical Letter. 2010;95:95a. http://secure.medicalletter.org. Accessed March 23, 2011.
13. Russouw HG, Roberts MC, Emsley RA, et al. Psychiatric manifestations and magnetic resonance imaging in HIV-negative neurosyphilis. Biol Psychiatry. 1997;41(4):467-473.
14. Hooshmand H, Escobar MR, Kopf SW. Neurosyphylis: a study of 241 patients. JAMA. 1972;219 (6):726-729.
15. Miller CA, Joyce DM. Toxicity, phenytoin. http://emedicine.medscape.com/article/816447-overview. Accessed March 23, 2011.
16. Earnest MP, Marx JA, Drury LR. Complications of intravenous phenytoin for acute treatment of seizures: recommendations for usage. JAMA. 1983; 246(6):762-765.
17. Geschwind MD, Shu H, Haman A, et al. Rapidly progressive dementia. Ann Neurol. 2008;64(1): 97-108.
18. Mechem CC. Chapter 143. Altered mental status and coma. In: Ma J, Cline DM, Tintinalli JE, et al, eds. Emergency Medicine Manual, 6e. www.access emergencymedicine.com/content.aspx?aID=2020. Accessed March 23, 2011.
19. Knopman DS, DeKosky ST, Cummings JL, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis of dementia (an evidence-based review). Neurology. 2001;56(9):1143-1153.
20. CDC. Syphilis testing algorithms using treponemal tests for initial screening—four laboratories, New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57(32):872-875.
21. Anderson CA, Filley CM. Chapter 33. Behavioral presentations of medical and neurologic disorders. In: Jacobson JL, Jacobson AM, eds. Psychiatric Secrets. 2nd ed. St. Louis, MO: Hanley & Belfus; 2001.
A 54-year-old African-American man was brought by police officers to the emergency department (ED) after he called 911 several times to report seeing a Rottweiler looking into his second-story window. At the scene, the police were unable to confirm his story, thought the man seemed intoxicated, and brought him to the ED for evaluation.
The patient reported that he had been drinking the previous evening but denied current intoxication or illicit drug use. He denied experiencing symptoms of alcohol withdrawal.
Regarding his medical history, the patient admitted to having had seizures, including two episodes that he said required hospitalization. He described these episodes as right-hand “tingling” (paresthesias), accompanied by right-facial numbness and aphasia. The patient said his physician had instructed him to take “a few phenytoin pills” whenever these episodes occurred. He reported that the medication usually helped resolve his symptoms. He said he had taken phenytoin shortly before his current presentation.
According to friends of the patient who were questioned, he had had noticeable memory problems during the previous six to eight months. They said that he often told the same joke, day after day. His speech had become increasingly slurred, even when he was not drinking.
Once the patient’s medical records were retrieved, it was revealed that he had been hospitalized twice for witnessed grand mal seizures about six months before his current admission; he had been drinking alcohol prior to both episodes. He underwent electroencephalography (EEG) during one of these hospitalizations, with results reported as normal. On both occasions, the patient was discharged with phenytoin and was instructed to follow up with his primary care provider and neurologist.
The patient, who reported working in customer service, had no known allergies. He claimed to drink one or two 40-ounce beers twice per week and admitted to occasional cocaine use. Of significance in his family history was a fatal MI in his mother. Although the patient denied any history of rashes or lesions, his current delirium made it impossible to obtain a reliable sexual history; a friend who was questioned, however, described the patient as promiscuous.
On initial physical examination, the man was afebrile, tachycardic, and somewhat combative with the ED staff. He was fully oriented to self but only partially to place and time.
His right pupil was 3+ and his left pupil was 2+, with neither reactive to light. He spoke with tangential speech and his gait was unsteady, but no other significant abnormalities were noted. A full assessment revealed no rashes or other lesions.
Significant laboratory findings included a low level of phenytoin, a negative blood alcohol level, presence of cocaine on urine drug screening, and normal levels of thyroid-stimulating hormone (TSH), vitamin B12, and folate. The patient’s serum VDRL (venereal disease research laboratory) titer was positive at 1:256.
Electroencephalography showed diffuse slowing, and brain CT performed in the ED showed atrophy that was mild but appropriate for a person of the patient’s age, with no evidence of a cerebrovascular accident (CVA). Aneurysm was ruled out by CT angiography of the brain. MRI revealed persistent increased signal in the subarachnoid space.
The patient was admitted with an initial diagnosis of paranoid delusional psychosis and monitored for alcohol withdrawal. He was given lorazepam as needed for agitation. Consultations were arranged with the psychiatry service regarding his delusions, and with neurology to determine whether to continue phenytoin.
The patient showed little response during the next several days. Based on positive results on serum VDRL with high titer, the presence of Argyll-Robertson pupils on exam, and his history of dementia-like symptoms, a lumbar puncture was performed to rule out neurosyphilis. In the patient’s cerebral spinal fluid (CSF) analysis, the first tube was clear and colorless, with 72 cells (28% neutrophils, 59% lymphocytes); glucose, 64 mg/dL; and total protein, 117 mg/dL. The fourth tube had 34 cells (17% neutrophils, 65% lymphocytes) and a positive VDRL titer at 1:128. Results from a serum syphilis immunoglobulin G (IgG) test were positive, and HIV antibody testing was nonreactive, confirming the diagnosis of neurosyphilis.
The hospital’s infectious disease (ID) team recommended treatment with IV penicillin for 14 days. Once this was completed, the patient was discharged with instructions to follow up at the ID clinic in three months for a repeat CSF VDRL titer to monitor for resolution of the disease. His prescription for phenytoin was discontinued.
At the time of discharge, it was noted that the patient showed no evidence of having regained cognitive function. He was deemed by the psychiatry service to lack decision-making capacity—a likely sequelae of untreated neurosyphilis of unknown duration.
He did return to the ID clinic six months after his discharge. At that visit, a VDRL serum titer was drawn with a result of 1:64, a decrease from 1:128. His syphilis IgG remained positive, however.
Discussion
Definition and Epidemiology
Syphilis is commonly known as a sexually transmitted disease with primary, secondary, and tertiary (early and late latent) stages.1 Neurosyphilis is defined as a manifestation of the inflammatory response to invasion over decades by the Treponema pallidum spirochete in the CSF as a result of untreated primary and/or secondary syphilis.2 About one in 10 patients with untreated syphilis will experience neurologic involvement.3,4 Before 2005, neurosyphilis was required to be reported as a specific stage of syphilis (ie, a manifestation of tertiary syphilis4), but now should be reported as syphilis with neurologic manifestations.5
A reportable infectious disease, syphilis was widespread until the advent of penicillin. According to CDC statistics,6 the number of reported cases of primary and secondary syphilis has declined steadily since 1943. In the late 1970s and early 1980s, the number of tertiary cases also began to plateau, likely as a result of earlier diagnosis and more widespread use of penicillin. Recent case reports suggest greater prevalence of syphilis among men than women and increased incidence among men who have sex with men.7
Pathogenesis
Syphilis is most commonly spread by sexual contact or contact with an infected primary lesion (chancre). Less likely routes of transmission are placental passage or blood transfusion. Infectivity is greatest in the early disease stages.8
Primary syphilis is marked by transmission of the spirochete, ending with development of secondary syphilis (usually two to 12 weeks after transmission). A chancre commonly develops but is often missed by patients because it is painless and can heal spontaneously.7 The chancre is also often confused with two other sources of genital lesions, herpes simplex (genital herpes) and Haemophilus ducreyi (chancroid). In two-thirds of cases of untreated primary syphilis, the infection clears spontaneously, but in the remaining one-third, the disease progresses.8
Secondary syphilis, with or without presence of a chancre, manifests with constitutional symptoms, including lymphadenopathy, fever, headache, and malaise. Patients in this disease phase may also present with a generalized, nonpruritic, macular to maculopapular or pustular rash. The rash can affect the skin of the trunk, the proximal extremities, and the palms and soles. Ocular involvement may occur, especially in patients who are coinfected with HIV.8 In either primary or secondary syphilis, infection can invade the central nervous system.1
During latent syphilis, patients show serologic conversion without overt symptoms. Early latent syphilis is defined as infection within the previous year, as demonstrated by conversion from negative to positive testing, or an increase in titers within the previous year. Any case occurring after one year is defined as late or unknown latent syphilis.8
Tertiary syphilis is marked by complications resulting from untreated syphilis; affected patients commonly experience central nervous system and cardiovascular involvement. Gummatous disease is seen in 15% of patients.1
The early stages of neurosyphilis may be asymptomatic, acute meningeal, and meningovascular.1,4,8,9 Only 5% of patients with early neurosyphilis are symptomatic, with the added potential for cranial neuritis or ocular involvement.1 The late stages of neurosyphilis are detailed in the table.1,4,8
Diagnosis
A diagnosis of syphilis is made by testing blood samples or scrapings from a lesion. In patients with suspected syphilis, rapid plasma reagin (RPR) testing or a VDRL titer is commonly ordered. When results are positive, a serum treponemal test is recommended to confirm a diagnosis of syphilis. Options include the fluorescent treponemal antibody absorption test (FTA-ABS) and the microhemagglutinin assay for antibody to T pallidum (MHA-TP).5
If neurologic symptoms are present, a CSF sample should be obtained, followed by the same testing. A confirmed diagnosis of neurosyphilis is defined by the CDC as syphilis at any stage that meets laboratory criteria for neurosyphilis5; these include increased CSF protein or an elevated CSF leukocyte count with no other known cause, and clinical signs or symptoms without other known causes.7
Treatment
Treatment of syphilis generally consists of penicillin, administered intramuscularly (IM) or IV, depending on the stage. According to 2006 guidelines from the CDC,10,11 treatment for adults with primary and secondary syphilis is a single dose of IM penicillin G, 2.4 million units. If neurosyphilis is suspected, recommended treatment is IV penicillin G, 18 to 24 million units per day divided into six doses (ie, 3 to 4 million units every four hours) or continuous pump infusion for 10 to 14 days.10-12 Follow-up is recommended by monitoring CSF titers to ensure clearance of infection; retreatment may be required if CSF abnormalities persist after two years.11
Patients with a penicillin allergy should undergo desensitization, as penicillin is the preferred agent; the potential exists for cross-reactivity with ceftriaxone, a possible alternative for patients with neurosyphilis.11 All patients diagnosed with syphilis should also be tested for HIV and other sexually transmitted diseases.10-12
The prognosis of patients treated for neurosyphilis is generally good if the condition is diagnosed and treated early. In patients with cerebral atrophy, frontal lesions, dementia, or tabes dorsalis, the potential for recovery decreases.2,13,14
Teaching Points
There are several teaching points to take away from this case:
• Remember to rule out a CVA in any patient who presents with numbness, paresthesias, or slurred speech. In this case, a brain CT and CT angiography of the brain were both obtained in the ED before the patient was admitted. They both yielded negative results; because the patient’s history was consistent with alcohol and drug use and he had a history of seizures, he was monitored closely for signs of withdrawal or further seizure.
• Phenytoin is an antiepileptic agent whose use requires proper patient education and drug level monitoring. Appropriate follow-up must be ensured before phenytoin therapy is begun, as toxicity can result in nystagmus, ataxia, slurred speech, decreased coordination, mental confusion, and possibly death.15,16
• For patients with a suspected acute change in mental status, a workup is required and should be tailored appropriately, based on findings. This should include, but not be limited to, a thorough history and physical exam, CT of the brain (to rule out an acute brain injury17), and, if warranted, MRI of the brain. Also, a urine drug screen and alcohol level, a complete blood count, a TSH level (to evaluate for altered thyroid function that may explain mental status changes), comprehensive panel, RPR testing and/or a VDRL titer should be obtained, depending on the facility’s protocol18,19; at some facilities, a treponemal test, rather than VDRL, is being obtained at the outset.20 Levels of vitamin B12 (as part of the dementia workup), folate, thiamine, and ammonia (in patients with suspected liver disease) can also be obtained in patients with change in mental status.18,19 Urinalysis should not be overlooked to check for a urinary tract infection, especially in elderly patients.21
• If primary syphilis is suspected, treatment must be undertaken.20
Conclusion
Despite the decline seen since the 1940s in cases of primary and secondary syphilis, and the effectiveness of penicillin in treating the infection early, patients with late-stage syphilis, including those with neurosyphilis, may still present to the emergency care, urgent care, or primary care setting. Immediate treatment with penicillin is recommended to achieve an optimal prognosis for the affected patient.
A 54-year-old African-American man was brought by police officers to the emergency department (ED) after he called 911 several times to report seeing a Rottweiler looking into his second-story window. At the scene, the police were unable to confirm his story, thought the man seemed intoxicated, and brought him to the ED for evaluation.
The patient reported that he had been drinking the previous evening but denied current intoxication or illicit drug use. He denied experiencing symptoms of alcohol withdrawal.
Regarding his medical history, the patient admitted to having had seizures, including two episodes that he said required hospitalization. He described these episodes as right-hand “tingling” (paresthesias), accompanied by right-facial numbness and aphasia. The patient said his physician had instructed him to take “a few phenytoin pills” whenever these episodes occurred. He reported that the medication usually helped resolve his symptoms. He said he had taken phenytoin shortly before his current presentation.
According to friends of the patient who were questioned, he had had noticeable memory problems during the previous six to eight months. They said that he often told the same joke, day after day. His speech had become increasingly slurred, even when he was not drinking.
Once the patient’s medical records were retrieved, it was revealed that he had been hospitalized twice for witnessed grand mal seizures about six months before his current admission; he had been drinking alcohol prior to both episodes. He underwent electroencephalography (EEG) during one of these hospitalizations, with results reported as normal. On both occasions, the patient was discharged with phenytoin and was instructed to follow up with his primary care provider and neurologist.
The patient, who reported working in customer service, had no known allergies. He claimed to drink one or two 40-ounce beers twice per week and admitted to occasional cocaine use. Of significance in his family history was a fatal MI in his mother. Although the patient denied any history of rashes or lesions, his current delirium made it impossible to obtain a reliable sexual history; a friend who was questioned, however, described the patient as promiscuous.
On initial physical examination, the man was afebrile, tachycardic, and somewhat combative with the ED staff. He was fully oriented to self but only partially to place and time.
His right pupil was 3+ and his left pupil was 2+, with neither reactive to light. He spoke with tangential speech and his gait was unsteady, but no other significant abnormalities were noted. A full assessment revealed no rashes or other lesions.
Significant laboratory findings included a low level of phenytoin, a negative blood alcohol level, presence of cocaine on urine drug screening, and normal levels of thyroid-stimulating hormone (TSH), vitamin B12, and folate. The patient’s serum VDRL (venereal disease research laboratory) titer was positive at 1:256.
Electroencephalography showed diffuse slowing, and brain CT performed in the ED showed atrophy that was mild but appropriate for a person of the patient’s age, with no evidence of a cerebrovascular accident (CVA). Aneurysm was ruled out by CT angiography of the brain. MRI revealed persistent increased signal in the subarachnoid space.
The patient was admitted with an initial diagnosis of paranoid delusional psychosis and monitored for alcohol withdrawal. He was given lorazepam as needed for agitation. Consultations were arranged with the psychiatry service regarding his delusions, and with neurology to determine whether to continue phenytoin.
The patient showed little response during the next several days. Based on positive results on serum VDRL with high titer, the presence of Argyll-Robertson pupils on exam, and his history of dementia-like symptoms, a lumbar puncture was performed to rule out neurosyphilis. In the patient’s cerebral spinal fluid (CSF) analysis, the first tube was clear and colorless, with 72 cells (28% neutrophils, 59% lymphocytes); glucose, 64 mg/dL; and total protein, 117 mg/dL. The fourth tube had 34 cells (17% neutrophils, 65% lymphocytes) and a positive VDRL titer at 1:128. Results from a serum syphilis immunoglobulin G (IgG) test were positive, and HIV antibody testing was nonreactive, confirming the diagnosis of neurosyphilis.
The hospital’s infectious disease (ID) team recommended treatment with IV penicillin for 14 days. Once this was completed, the patient was discharged with instructions to follow up at the ID clinic in three months for a repeat CSF VDRL titer to monitor for resolution of the disease. His prescription for phenytoin was discontinued.
At the time of discharge, it was noted that the patient showed no evidence of having regained cognitive function. He was deemed by the psychiatry service to lack decision-making capacity—a likely sequelae of untreated neurosyphilis of unknown duration.
He did return to the ID clinic six months after his discharge. At that visit, a VDRL serum titer was drawn with a result of 1:64, a decrease from 1:128. His syphilis IgG remained positive, however.
Discussion
Definition and Epidemiology
Syphilis is commonly known as a sexually transmitted disease with primary, secondary, and tertiary (early and late latent) stages.1 Neurosyphilis is defined as a manifestation of the inflammatory response to invasion over decades by the Treponema pallidum spirochete in the CSF as a result of untreated primary and/or secondary syphilis.2 About one in 10 patients with untreated syphilis will experience neurologic involvement.3,4 Before 2005, neurosyphilis was required to be reported as a specific stage of syphilis (ie, a manifestation of tertiary syphilis4), but now should be reported as syphilis with neurologic manifestations.5
A reportable infectious disease, syphilis was widespread until the advent of penicillin. According to CDC statistics,6 the number of reported cases of primary and secondary syphilis has declined steadily since 1943. In the late 1970s and early 1980s, the number of tertiary cases also began to plateau, likely as a result of earlier diagnosis and more widespread use of penicillin. Recent case reports suggest greater prevalence of syphilis among men than women and increased incidence among men who have sex with men.7
Pathogenesis
Syphilis is most commonly spread by sexual contact or contact with an infected primary lesion (chancre). Less likely routes of transmission are placental passage or blood transfusion. Infectivity is greatest in the early disease stages.8
Primary syphilis is marked by transmission of the spirochete, ending with development of secondary syphilis (usually two to 12 weeks after transmission). A chancre commonly develops but is often missed by patients because it is painless and can heal spontaneously.7 The chancre is also often confused with two other sources of genital lesions, herpes simplex (genital herpes) and Haemophilus ducreyi (chancroid). In two-thirds of cases of untreated primary syphilis, the infection clears spontaneously, but in the remaining one-third, the disease progresses.8
Secondary syphilis, with or without presence of a chancre, manifests with constitutional symptoms, including lymphadenopathy, fever, headache, and malaise. Patients in this disease phase may also present with a generalized, nonpruritic, macular to maculopapular or pustular rash. The rash can affect the skin of the trunk, the proximal extremities, and the palms and soles. Ocular involvement may occur, especially in patients who are coinfected with HIV.8 In either primary or secondary syphilis, infection can invade the central nervous system.1
During latent syphilis, patients show serologic conversion without overt symptoms. Early latent syphilis is defined as infection within the previous year, as demonstrated by conversion from negative to positive testing, or an increase in titers within the previous year. Any case occurring after one year is defined as late or unknown latent syphilis.8
Tertiary syphilis is marked by complications resulting from untreated syphilis; affected patients commonly experience central nervous system and cardiovascular involvement. Gummatous disease is seen in 15% of patients.1
The early stages of neurosyphilis may be asymptomatic, acute meningeal, and meningovascular.1,4,8,9 Only 5% of patients with early neurosyphilis are symptomatic, with the added potential for cranial neuritis or ocular involvement.1 The late stages of neurosyphilis are detailed in the table.1,4,8
Diagnosis
A diagnosis of syphilis is made by testing blood samples or scrapings from a lesion. In patients with suspected syphilis, rapid plasma reagin (RPR) testing or a VDRL titer is commonly ordered. When results are positive, a serum treponemal test is recommended to confirm a diagnosis of syphilis. Options include the fluorescent treponemal antibody absorption test (FTA-ABS) and the microhemagglutinin assay for antibody to T pallidum (MHA-TP).5
If neurologic symptoms are present, a CSF sample should be obtained, followed by the same testing. A confirmed diagnosis of neurosyphilis is defined by the CDC as syphilis at any stage that meets laboratory criteria for neurosyphilis5; these include increased CSF protein or an elevated CSF leukocyte count with no other known cause, and clinical signs or symptoms without other known causes.7
Treatment
Treatment of syphilis generally consists of penicillin, administered intramuscularly (IM) or IV, depending on the stage. According to 2006 guidelines from the CDC,10,11 treatment for adults with primary and secondary syphilis is a single dose of IM penicillin G, 2.4 million units. If neurosyphilis is suspected, recommended treatment is IV penicillin G, 18 to 24 million units per day divided into six doses (ie, 3 to 4 million units every four hours) or continuous pump infusion for 10 to 14 days.10-12 Follow-up is recommended by monitoring CSF titers to ensure clearance of infection; retreatment may be required if CSF abnormalities persist after two years.11
Patients with a penicillin allergy should undergo desensitization, as penicillin is the preferred agent; the potential exists for cross-reactivity with ceftriaxone, a possible alternative for patients with neurosyphilis.11 All patients diagnosed with syphilis should also be tested for HIV and other sexually transmitted diseases.10-12
The prognosis of patients treated for neurosyphilis is generally good if the condition is diagnosed and treated early. In patients with cerebral atrophy, frontal lesions, dementia, or tabes dorsalis, the potential for recovery decreases.2,13,14
Teaching Points
There are several teaching points to take away from this case:
• Remember to rule out a CVA in any patient who presents with numbness, paresthesias, or slurred speech. In this case, a brain CT and CT angiography of the brain were both obtained in the ED before the patient was admitted. They both yielded negative results; because the patient’s history was consistent with alcohol and drug use and he had a history of seizures, he was monitored closely for signs of withdrawal or further seizure.
• Phenytoin is an antiepileptic agent whose use requires proper patient education and drug level monitoring. Appropriate follow-up must be ensured before phenytoin therapy is begun, as toxicity can result in nystagmus, ataxia, slurred speech, decreased coordination, mental confusion, and possibly death.15,16
• For patients with a suspected acute change in mental status, a workup is required and should be tailored appropriately, based on findings. This should include, but not be limited to, a thorough history and physical exam, CT of the brain (to rule out an acute brain injury17), and, if warranted, MRI of the brain. Also, a urine drug screen and alcohol level, a complete blood count, a TSH level (to evaluate for altered thyroid function that may explain mental status changes), comprehensive panel, RPR testing and/or a VDRL titer should be obtained, depending on the facility’s protocol18,19; at some facilities, a treponemal test, rather than VDRL, is being obtained at the outset.20 Levels of vitamin B12 (as part of the dementia workup), folate, thiamine, and ammonia (in patients with suspected liver disease) can also be obtained in patients with change in mental status.18,19 Urinalysis should not be overlooked to check for a urinary tract infection, especially in elderly patients.21
• If primary syphilis is suspected, treatment must be undertaken.20
Conclusion
Despite the decline seen since the 1940s in cases of primary and secondary syphilis, and the effectiveness of penicillin in treating the infection early, patients with late-stage syphilis, including those with neurosyphilis, may still present to the emergency care, urgent care, or primary care setting. Immediate treatment with penicillin is recommended to achieve an optimal prognosis for the affected patient.
1. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
2. Simon RP. Chapter 20. Neurosyphilis. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. USA: The McGraw-Hill Companies; 2007:130-137.
3. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48:440-445.
4. Marra CM. Neurosyphilis. Curr Neurol Neurosci Rep. 2004;4(6):435-440.
5. CDC. Sexually transmitted diseases surveillance, 2007: STD surveillance case definitions. www.cdc.gov/std/stats07/app-casedef.htm. Accessed March 23, 2011.
6. CDC. 2008 Sexually Transmitted Diseases Surveillance: Table 1. Cases of sexually transmitted diseases reported by state health departments and rates per 100,000 population: United States, 1941-2008. www.cdc.gov/std/stats08/tables/1.htm. Accessed March 23, 2011.
7. CDC. Sexually transmitted diseases (STDs): Syphilis: CDC fact sheet. www.cdc.gov/std/syphilis/STDfact-syphilis.htm. Accessed March 23, 2011.
8. Tramont EC. Chapter 238. Treponema pallidum (syphilis). In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier Churchill Livingstone; 2009.
9. Ghanem KG. Neurosyphilis: a historical perspective and review. CNS Neurosci Ther. 2010; 16(5):e157-e168.
10. Workowski KA, Berman SM; CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
11. CDC. Sexually transmitted diseases: treatment guidelines 2006. www.cdc.gov/std/treatment/2006/genital-ulcers.htm#genulc6. Accessed March 29, 2011.
12. Drugs for sexually transmitted infections. Treatment Guidelines from the Medical Letter. 2010;95:95a. http://secure.medicalletter.org. Accessed March 23, 2011.
13. Russouw HG, Roberts MC, Emsley RA, et al. Psychiatric manifestations and magnetic resonance imaging in HIV-negative neurosyphilis. Biol Psychiatry. 1997;41(4):467-473.
14. Hooshmand H, Escobar MR, Kopf SW. Neurosyphylis: a study of 241 patients. JAMA. 1972;219 (6):726-729.
15. Miller CA, Joyce DM. Toxicity, phenytoin. http://emedicine.medscape.com/article/816447-overview. Accessed March 23, 2011.
16. Earnest MP, Marx JA, Drury LR. Complications of intravenous phenytoin for acute treatment of seizures: recommendations for usage. JAMA. 1983; 246(6):762-765.
17. Geschwind MD, Shu H, Haman A, et al. Rapidly progressive dementia. Ann Neurol. 2008;64(1): 97-108.
18. Mechem CC. Chapter 143. Altered mental status and coma. In: Ma J, Cline DM, Tintinalli JE, et al, eds. Emergency Medicine Manual, 6e. www.access emergencymedicine.com/content.aspx?aID=2020. Accessed March 23, 2011.
19. Knopman DS, DeKosky ST, Cummings JL, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis of dementia (an evidence-based review). Neurology. 2001;56(9):1143-1153.
20. CDC. Syphilis testing algorithms using treponemal tests for initial screening—four laboratories, New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57(32):872-875.
21. Anderson CA, Filley CM. Chapter 33. Behavioral presentations of medical and neurologic disorders. In: Jacobson JL, Jacobson AM, eds. Psychiatric Secrets. 2nd ed. St. Louis, MO: Hanley & Belfus; 2001.
1. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
2. Simon RP. Chapter 20. Neurosyphilis. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. USA: The McGraw-Hill Companies; 2007:130-137.
3. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48:440-445.
4. Marra CM. Neurosyphilis. Curr Neurol Neurosci Rep. 2004;4(6):435-440.
5. CDC. Sexually transmitted diseases surveillance, 2007: STD surveillance case definitions. www.cdc.gov/std/stats07/app-casedef.htm. Accessed March 23, 2011.
6. CDC. 2008 Sexually Transmitted Diseases Surveillance: Table 1. Cases of sexually transmitted diseases reported by state health departments and rates per 100,000 population: United States, 1941-2008. www.cdc.gov/std/stats08/tables/1.htm. Accessed March 23, 2011.
7. CDC. Sexually transmitted diseases (STDs): Syphilis: CDC fact sheet. www.cdc.gov/std/syphilis/STDfact-syphilis.htm. Accessed March 23, 2011.
8. Tramont EC. Chapter 238. Treponema pallidum (syphilis). In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier Churchill Livingstone; 2009.
9. Ghanem KG. Neurosyphilis: a historical perspective and review. CNS Neurosci Ther. 2010; 16(5):e157-e168.
10. Workowski KA, Berman SM; CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
11. CDC. Sexually transmitted diseases: treatment guidelines 2006. www.cdc.gov/std/treatment/2006/genital-ulcers.htm#genulc6. Accessed March 29, 2011.
12. Drugs for sexually transmitted infections. Treatment Guidelines from the Medical Letter. 2010;95:95a. http://secure.medicalletter.org. Accessed March 23, 2011.
13. Russouw HG, Roberts MC, Emsley RA, et al. Psychiatric manifestations and magnetic resonance imaging in HIV-negative neurosyphilis. Biol Psychiatry. 1997;41(4):467-473.
14. Hooshmand H, Escobar MR, Kopf SW. Neurosyphylis: a study of 241 patients. JAMA. 1972;219 (6):726-729.
15. Miller CA, Joyce DM. Toxicity, phenytoin. http://emedicine.medscape.com/article/816447-overview. Accessed March 23, 2011.
16. Earnest MP, Marx JA, Drury LR. Complications of intravenous phenytoin for acute treatment of seizures: recommendations for usage. JAMA. 1983; 246(6):762-765.
17. Geschwind MD, Shu H, Haman A, et al. Rapidly progressive dementia. Ann Neurol. 2008;64(1): 97-108.
18. Mechem CC. Chapter 143. Altered mental status and coma. In: Ma J, Cline DM, Tintinalli JE, et al, eds. Emergency Medicine Manual, 6e. www.access emergencymedicine.com/content.aspx?aID=2020. Accessed March 23, 2011.
19. Knopman DS, DeKosky ST, Cummings JL, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis of dementia (an evidence-based review). Neurology. 2001;56(9):1143-1153.
20. CDC. Syphilis testing algorithms using treponemal tests for initial screening—four laboratories, New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57(32):872-875.
21. Anderson CA, Filley CM. Chapter 33. Behavioral presentations of medical and neurologic disorders. In: Jacobson JL, Jacobson AM, eds. Psychiatric Secrets. 2nd ed. St. Louis, MO: Hanley & Belfus; 2001.
A Toxic Swimming Pool Hazard
Procedural Sedation and Analgesia in the ED: Focusing on Deep Sedation
Tibial Stress Injuries in Athletes
Women's Musculoskeletal Foot Conditions Exacerbated by Shoe Wear: An Imaging Perspective
Diagnostics in Suspicion of Ankle Syndesmotic Injury
UPDATE ON MINIMALLY INVASIVE SURGERY
- Applying single-incision laparoscopic surgery to gyn practice: What’s involved
Russell P. Atkin, MD; Michael L. Nimaroff, MD; Vrunda Bhavsar, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The uterine leiomyoma is the most common tumor of the female genital tract. Seventy percent of white women and 80% of black women develop one or more of these tumors by the time they reach 50 years, and the myomas are clinically apparent in 25% of patients.1,2 When a fibroid is submucosal, it is often associated with menorrhagia, abnormal uterine bleeding, and infertility.2-4
In this article, I describe three aspects of managing leiomyomata:
- ways of classifying the tumor to better predict the blood loss, operative time and morbidity associated with removal
- the indications for hysteroscopic myomectomy and polypectomy
- new tools for the removal of polyps and myomas.
Preoperative assessment of submucosal myomas is essential
Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: a new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—preliminary report. J Minim Invasive Gynecol. 2005;12(4):308–311.
Wamsteker and colleagues were the first to propose a system for classifying myoma position within the uterine cavity as a means of estimating the degree of difficulty of resectoscopic removal.5 The European Society for Gynaecological Endoscopy (ESGE) later adopted this system, which is now known by its acronym. According to the ESGE system, myomas that lie entirely within the uterine cavity (Type 0) are easier to remove, require less operative time, and involve less fluid deficit and blood loss than myomas that invade the myometrium to varying degrees (FIGURE 1).
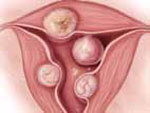
FIGURE 1 ESGE classification
Submucosal myomas are classified as Type 0, Type I, or Type II, according to the degree of myometrial penetration.When more than 50% of a tumor penetrates the myometrium (Type II), the risk of excessive intraoperative fluid absorption is elevated, along with the risk of bleeding and the likelihood of electrolyte abnormalities with the use of non-electrolyte fluid media. Type II tumors also increase operative time and the likelihood that additional procedures will be needed because of incomplete resection—even in the hands of expert hysteroscopic surgeons.5
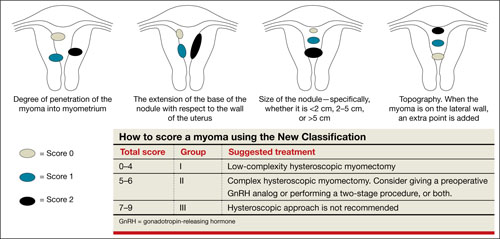
FIGURE 2 New classification
New classification system increases accuracy
Lasmar and colleagues devised a new system for preoperative assessment of submucosal myomas, hoping to estimate more precisely the likelihood of successful removal via resectoscopy. They call their system the New Classification (NC). Besides taking into account the degree of penetration into the myometrium, they consider the percentage of uterine wall encompassed by the myoma and the location of the myoma within the uterus (i.e., fundus, body, or lower segment) (FIGURE 2). The total score is used to categorize the tumor into Group I, II, or III to estimate the likelihood of successful removal.
In devising the system, Lasmar and colleagues used the NC and ESGE systems to analyze 55 myomectomy cases involving 57 myomas. They found that the NC more accurately predicts differences between Groups I and II in regard to completed procedures, fluid deficit, and operative time.
Preoperative hysteroscopic evaluation of submucosal myomas is essential and reliable using the New Classification system.
Hysteroscopic removal of myomas and polyps
yields multiple benefits
Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724–729.
Rackow BW, Jorgensen E, Taylor HS. Endometrial polyps affect uterine receptivity [published online ahead of print January 24, 2011]. Fertil Steril. doi 10.1016/j. fertnstert.2010.12.034.
Afifi K, Anand S. Nallapeta S, Gelbaya TA. Management of endometrial polyps in subfertile women: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):117–121.
Studies evaluating the association between infertility and submucosal fibroids have been controversial because the exact mechanism has not been identified. However, new evidence suggests a molecular causal relationship, and Pritts and colleagues demonstrated improved fertility after submucosal myomectomy.3,6
More recently, Shokeir and coworkers conducted a prospective, randomized, age-matched, controlled trial to explore the effects of hysteroscopic myomectomy on otherwise unexplained primary infertility. They enrolled 215 women who had infertility longer than 12 months and who had their fibroids assessed by means of ultrasonography and classified according to the ESGE system.
Women who underwent myomectomy were twice as likely as women in the control group to become pregnant (relative risk = 2.1; 95% confidence interval = 1.5–2.9). Women who had Type 0 and Type I myomas removed had significantly higher pregnancy rates than women in the control group (P < .001). No statistically significant difference in the pregnancy rate between groups was found for Type II myomas.
Polyps may also affect fertility
Rackow and coworkers demonstrated that endometrial polyps affect uterine receptivity on the molecular level, suggesting a relationship between endometrial polyps and infertility. And after a systematic review of endometrial polyps in women who had subfertility, Afifi and colleagues concluded that polypectomy can improve fertility, especially when assisted reproductive technologies are planned.
Myomas, polyps also contribute to bleeding abnormalities
Submucosal myomas have been associated with bleeding abnormalities, such as heavy menstrual bleeding and menopausal bleeding. Although the precise mechanism is unknown by which these bleeding abnormalities arise in the presence of submucosal fibroids, abnormalities within the endometrium or myometrium may play a role at the genetic and molecular level.7,8 There is clear evidence supporting hysteroscopic removal of submucosal fibroids to improve bleeding abnormalities.9,10
Hysteroscopic removal of eSge type 0 and type i submucosal myomas improves the pregnancy rate for patients who have otherwise unexplained primary infertility. Removal of endometrial polyps is also recommended to improve fertility.
Besides improving fertility, hysteroscopic removal of submucosal myomas and endometrial polyps improves menorrhagia and irregular and abnormal uterine bleeding.
Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. They allow resection using saline, operate without electrical energy, and utilize vacuum suction to remove tissue fragments from the uterine cavity.
Hysteroscopic morcellators ease the task of myomectomy
Hysteroscopic removal of submucosal myomas and polyps is an effective treatment for women who experience bleeding abnormalities or infertility, but the potential for complications deters many gynecologists from performing resectoscopic myomectomy.
Use of a monopolar loop electrode (VIDEO 1) requires an electrolyte-free distention medium, such as 1.5% glycine or 3% sorbitol, and intravasation of these fluids must be limited to minimize the risk of complications such as hyponatremia, cardiovascular compromise, cerebral edema, and, even, death.12 Although the use of normal saline with bipolar resectoscopic instrumentation (VIDEO 2) and automated fluid-management systems reduces the risk of fluid overload, it does not eliminate it entirely, and fluid balance must be carefully scrutinized.13
Intrauterine electrosurgery can burn pelvic organs if an activated electrode perforates the uterine wall and makes contact with bowel or other organs. Burns to the cervix, vagina, and vulva have also been reported when monopolar resectoscopic insulation fails or monopolar electrical current is inadvertently diverted.12
In addition, unless one uses tissue-vaporizing electrodes (VIDEO 3) or is equipped
with newer instrumentation that allows tissue to be removed through the operative sheath of the resectoscope, the myoma must be extracted in pieces, often with repeated removal and reinsertion of the resectoscope and grasping instruments, increasing the risk of cervical injury or uterine perforation with each placement.
Another variable that deters hysteroscopic myomectomy is the lack of training at the residency level. The typical ObGyn resident graduating between 2002 and 2007 had performed a median of only 40 to 51 operative hysteroscopic procedures by the time of graduation.14 This statistic suggests that few residency programs provide adequate training for more demanding hysteroscopic surgeries.
Mechanical morcellators facilitate tissue removal
Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. These morcellators allow resection of a myoma using saline, minimizing the hazards of fluid overload. Because they are mechanical devices that do not require electrical energy, the potential for thermal injury is eliminated.
Mechanical morcellators utilize vacuum suction to remove tissue fragments from the uterine cavity, maintaining a tissue-free operative environment and eliminating the need for repeated manual removal. This feature also reduces the risks of perforation, creation of a false passageway, and gas embolus that have been linked to instrument reinsertion and manual removal of tissue fragments.12
Furthermore, mechanical morcellators are easy to use, reducing operative time and fluid deficit.
Removing Type II myomas with a hysteroscopic morcellator may pose a challenge, however, because of significant myometrial penetration. In addition, bleeding is more likely during removal of a Type II myoma than during removal of other types of tumors, necessitating the use of electric current to address it appropriately. Surgeons who are experienced using the morcellator can overcome these challenges by avoiding the myometrial interface and allowing uterine expulsive contractions to push the myoma into the cavity, making it unnecessary to penetrate the myometrium with the instrument. Thorough preoperative evaluation of Type II myomas is recommended, keeping in mind that removal may be safer and more effective using electrosurgical loop resection.
Option 1: TRUCLEAR morcellator
The TRUCLEAR Hysteroscopic Morcellator (Smith & Nephew) was FDA-approved in 2005 as the first intrauterine mechanical morcellator (VIDEO 4). It requires a dedicated fluid pump and has different instrumentation for myomas and polyps. For myomas, the instrument consists of a rotating tube that reciprocates within an outer 4-mm tube. Both tubes have windows at the end with cutting edges. A vacuum connected to the inner tube provides controlled suction that pulls the tissue into the window on the outer tube and cuts it as the inner tube rotates (VIDEO 5).
For polyps, both inner and outer tubes have oscillating serrated edges on each window (VIDEO 6).
Both instruments are used through a 9-mm offset rod-lens continuous-flow hysteroscope.
In a retrospective analysis, the TRUCLEAR morcellator reduced operative time by about two thirds for polyps and one half for Type 0 and Type I myomas, compared with monopolar loop resection.15 A later study of inexperienced ObGyn residents demonstrated shorter operative times and lower total fluid deficits for the TRUCLEAR morcellator, compared with resectoscopic procedures overall, during polypectomy and myomectomy of Type 0 and Type I myomas.16
Smith & Nephew recently introduced a smaller set of instruments, including a 2.9-mm blade for removal of polyps through a 5.6-mm continuous-flow hysteroscope. However, the new instruments have not yet been approved by the FDA and are unavailable within the United States.
Option 2: MyoSure
The MyoSure Tissue Removal System (Hologic) was FDA-approved in 2009. The hand piece is a rotating and reciprocating 2-mm blade within a 3-mm outer tube. The cutter is connected to a vacuum source that aspirates resected tissue through a side-facing cutting window in the outer tube. The system utilizes standard hysteroscopy set-up for fluid inflow and suction. The instrument is placed through an offset lens continuous-flow hysteroscope with an outer diameter of
6.25 mm. The smaller diameter reduces the amount of cervical dilation required, as well as the risk of uterine perforation.
The smaller size of the instrument renders it ideal for an office setting. Miller and colleagues demonstrated its safety and efficacy for office removal of polyps and myomas (VIDEO 7; VIDEO 8).17
Inadequate reimbursement?
Although both morcellators simplify hysteroscopic myomectomy and polypectomy, insurance reimbursement does not yet differentiate between places of service—unlike other in-office procedures that take into account the cost of the procedural device (see “Reimbursement is limited for hysteroscopic myomectomy in an office setting”). Until the relative value unit (RVU) is modified to reflect this cost, office use of the hysteroscopic morcellator for myomectomy and polypectomy will be financially restrictive to the gynecologist in private practice. Nevertheless, both instruments are easy to use and offer improved safety, increasing access to uterine-preserving surgery.
Thanks to Dr. Andrew I. Brill and Dr. William H. Parker for their thoughtful review of this article.
Since the inception of the resource-based relative value scale, the Centers for Medicare and Medicaid Services (CMS) have provided for different levels of payment to physicians, depending on the place of service and the extent of work involved. The relative value units (RVUs) established for each clinical service are based on three components:
- physician work
- practice expense
- malpractice expense.
The practice expense includes supplies, equipment, clinical and administrative staff, and renting and leasing of space.
When a physician provides a service in a hospital setting or outpatient clinic or surgery unit, the practice expense is lower because the hospital or outpatient facility shoulders those costs. In an office setting, however, the physician practice incurs the full expense of providing the service. In most cases, therefore, the practice is reimbursed at a higher total RVU for office procedures.
The “place of service” code required on your claim form lets the payer know whether the service was rendered in your office (code 11) or a facility such as a hospital or outpatient surgery center (codes 21–24). Physicians who work out of a hospital-owned facility—i.e., physicians who are employed by a hospital—would bill for a facility place of service rather than an office.
The difference in RVUs can be significant. For example, hysteroscopic sterilization (CPT code 58565) has two different RVUs, depending on whether the service is performed in a facility or office (TABLE). However, although hysteroscopic myomectomy can now be safely performed in the office setting for small, less invasive myomas, CMS has not yet assigned a place of service differential for this procedure (CPT code 58561). In other words, CMS has determined that hysteroscopic myomectomy—by definition or practice—is rarely or never performed outside a hospital or outpatient facility.
Medicare reimbursement for hysteroscopic procedures
| Procedure | CPT code | Relative value units | |
|---|---|---|---|
| Facility | Office | ||
| Sterilization | 58565 | 12.90 | 56.66 |
| Endometrial ablation | 58563 | 10.23 | 52.05 |
| Cryoablation | 58356 | 10.34 | 58.92 |
| Myomectomy | 58561 | 16.33 | NA |
| Polypectomy (with dilation and curettage, biopsy) | 58558 | 7.95 | 10.60 |
| To determine reimbursement, multiply the RVU by the Medicare conversion factor, which is $33.9764 | |||
When contracting with a private payer, be sure to ask how the payer reimburses for hysteroscopic myomectomy in an office setting. Payers that do not include a place of service differential may be amenable to negotiation if you can demonstrate that extra compensation can actually save them money and maintain high-quality patient care.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
We want to hear from you! Tell us what you think.
1. Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188(1):100-107.
2. Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: A new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—Preliminary report. J Minim Invasive Gynecol. 2010;12(4):308-311.
3. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215-1223.
4. Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724-729.
5. Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol. 1993;82(5):736-740.
6. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027-2034.
7. Stewart EA, Nowak RA. Leiomyoma-related bleeding: a classic hypothesis updated for the molecular era. Human Repro Update. 1996;2(4):295-306.
8. Laughlin SK, Stewart EA. Uterine leiomyomas. Individualizing the approach to a heterogeneous condition. Obstet Gynecol. 2011;117(2 pt 1):396-403.
9. Loffer FD. Improving results of hysteroscopic submucosal myomectomy for menorrhagia by concomitant endometrial ablation. J Minim Invasive Gynecol. 2005;12(3):254-260.
10. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93(5 pt 1):743-748.
11. Nathani F, Clark TJ. Uterine polypectomy in the management of abnormal uterine bleeding: a systematic review. J Minim Invasive Gynecol. 2006;13(4):260-268.
12. Munro MG. Complications of hysteroscopic and uterine resectoscopic surgery. Obstet Gynecol Clin N Am. 2010;37(3):399-425.
13. Kung RC, Vilos GA, Thomas B, Penkin P, Zaltz AP, Stabinsky SA. A new bipolar system for performing operative hysteroscopy in normal saline. J Am Assoc Gynecol Laparosc. 1999;6(3):331-336.
14. Miller CE. Training in minimally iInvasive surgery—you say you want a revolution. J Minim Invasive Gynecol. 2009;16(2):113-120.
15. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic moperating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62-66.
16. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466-471.
17. Miller CE, Glazerman L, Roy K, Lukes A. Clinical evaluation of a new hysteroscopic morcellator—retrospective case review. J Med. 2009;2(3):163-166.
- Applying single-incision laparoscopic surgery to gyn practice: What’s involved
Russell P. Atkin, MD; Michael L. Nimaroff, MD; Vrunda Bhavsar, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The uterine leiomyoma is the most common tumor of the female genital tract. Seventy percent of white women and 80% of black women develop one or more of these tumors by the time they reach 50 years, and the myomas are clinically apparent in 25% of patients.1,2 When a fibroid is submucosal, it is often associated with menorrhagia, abnormal uterine bleeding, and infertility.2-4
In this article, I describe three aspects of managing leiomyomata:
- ways of classifying the tumor to better predict the blood loss, operative time and morbidity associated with removal
- the indications for hysteroscopic myomectomy and polypectomy
- new tools for the removal of polyps and myomas.
Preoperative assessment of submucosal myomas is essential
Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: a new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—preliminary report. J Minim Invasive Gynecol. 2005;12(4):308–311.
Wamsteker and colleagues were the first to propose a system for classifying myoma position within the uterine cavity as a means of estimating the degree of difficulty of resectoscopic removal.5 The European Society for Gynaecological Endoscopy (ESGE) later adopted this system, which is now known by its acronym. According to the ESGE system, myomas that lie entirely within the uterine cavity (Type 0) are easier to remove, require less operative time, and involve less fluid deficit and blood loss than myomas that invade the myometrium to varying degrees (FIGURE 1).

FIGURE 1 ESGE classification
Submucosal myomas are classified as Type 0, Type I, or Type II, according to the degree of myometrial penetration.When more than 50% of a tumor penetrates the myometrium (Type II), the risk of excessive intraoperative fluid absorption is elevated, along with the risk of bleeding and the likelihood of electrolyte abnormalities with the use of non-electrolyte fluid media. Type II tumors also increase operative time and the likelihood that additional procedures will be needed because of incomplete resection—even in the hands of expert hysteroscopic surgeons.5

FIGURE 2 New classification
New classification system increases accuracy
Lasmar and colleagues devised a new system for preoperative assessment of submucosal myomas, hoping to estimate more precisely the likelihood of successful removal via resectoscopy. They call their system the New Classification (NC). Besides taking into account the degree of penetration into the myometrium, they consider the percentage of uterine wall encompassed by the myoma and the location of the myoma within the uterus (i.e., fundus, body, or lower segment) (FIGURE 2). The total score is used to categorize the tumor into Group I, II, or III to estimate the likelihood of successful removal.
In devising the system, Lasmar and colleagues used the NC and ESGE systems to analyze 55 myomectomy cases involving 57 myomas. They found that the NC more accurately predicts differences between Groups I and II in regard to completed procedures, fluid deficit, and operative time.
Preoperative hysteroscopic evaluation of submucosal myomas is essential and reliable using the New Classification system.
Hysteroscopic removal of myomas and polyps
yields multiple benefits
Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724–729.
Rackow BW, Jorgensen E, Taylor HS. Endometrial polyps affect uterine receptivity [published online ahead of print January 24, 2011]. Fertil Steril. doi 10.1016/j. fertnstert.2010.12.034.
Afifi K, Anand S. Nallapeta S, Gelbaya TA. Management of endometrial polyps in subfertile women: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):117–121.
Studies evaluating the association between infertility and submucosal fibroids have been controversial because the exact mechanism has not been identified. However, new evidence suggests a molecular causal relationship, and Pritts and colleagues demonstrated improved fertility after submucosal myomectomy.3,6
More recently, Shokeir and coworkers conducted a prospective, randomized, age-matched, controlled trial to explore the effects of hysteroscopic myomectomy on otherwise unexplained primary infertility. They enrolled 215 women who had infertility longer than 12 months and who had their fibroids assessed by means of ultrasonography and classified according to the ESGE system.
Women who underwent myomectomy were twice as likely as women in the control group to become pregnant (relative risk = 2.1; 95% confidence interval = 1.5–2.9). Women who had Type 0 and Type I myomas removed had significantly higher pregnancy rates than women in the control group (P < .001). No statistically significant difference in the pregnancy rate between groups was found for Type II myomas.
Polyps may also affect fertility
Rackow and coworkers demonstrated that endometrial polyps affect uterine receptivity on the molecular level, suggesting a relationship between endometrial polyps and infertility. And after a systematic review of endometrial polyps in women who had subfertility, Afifi and colleagues concluded that polypectomy can improve fertility, especially when assisted reproductive technologies are planned.
Myomas, polyps also contribute to bleeding abnormalities
Submucosal myomas have been associated with bleeding abnormalities, such as heavy menstrual bleeding and menopausal bleeding. Although the precise mechanism is unknown by which these bleeding abnormalities arise in the presence of submucosal fibroids, abnormalities within the endometrium or myometrium may play a role at the genetic and molecular level.7,8 There is clear evidence supporting hysteroscopic removal of submucosal fibroids to improve bleeding abnormalities.9,10
Hysteroscopic removal of eSge type 0 and type i submucosal myomas improves the pregnancy rate for patients who have otherwise unexplained primary infertility. Removal of endometrial polyps is also recommended to improve fertility.
Besides improving fertility, hysteroscopic removal of submucosal myomas and endometrial polyps improves menorrhagia and irregular and abnormal uterine bleeding.

Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. They allow resection using saline, operate without electrical energy, and utilize vacuum suction to remove tissue fragments from the uterine cavity.
Hysteroscopic morcellators ease the task of myomectomy
Hysteroscopic removal of submucosal myomas and polyps is an effective treatment for women who experience bleeding abnormalities or infertility, but the potential for complications deters many gynecologists from performing resectoscopic myomectomy.
Use of a monopolar loop electrode (VIDEO 1) requires an electrolyte-free distention medium, such as 1.5% glycine or 3% sorbitol, and intravasation of these fluids must be limited to minimize the risk of complications such as hyponatremia, cardiovascular compromise, cerebral edema, and, even, death.12 Although the use of normal saline with bipolar resectoscopic instrumentation (VIDEO 2) and automated fluid-management systems reduces the risk of fluid overload, it does not eliminate it entirely, and fluid balance must be carefully scrutinized.13
Intrauterine electrosurgery can burn pelvic organs if an activated electrode perforates the uterine wall and makes contact with bowel or other organs. Burns to the cervix, vagina, and vulva have also been reported when monopolar resectoscopic insulation fails or monopolar electrical current is inadvertently diverted.12
In addition, unless one uses tissue-vaporizing electrodes (VIDEO 3) or is equipped
with newer instrumentation that allows tissue to be removed through the operative sheath of the resectoscope, the myoma must be extracted in pieces, often with repeated removal and reinsertion of the resectoscope and grasping instruments, increasing the risk of cervical injury or uterine perforation with each placement.
Another variable that deters hysteroscopic myomectomy is the lack of training at the residency level. The typical ObGyn resident graduating between 2002 and 2007 had performed a median of only 40 to 51 operative hysteroscopic procedures by the time of graduation.14 This statistic suggests that few residency programs provide adequate training for more demanding hysteroscopic surgeries.
Mechanical morcellators facilitate tissue removal
Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. These morcellators allow resection of a myoma using saline, minimizing the hazards of fluid overload. Because they are mechanical devices that do not require electrical energy, the potential for thermal injury is eliminated.
Mechanical morcellators utilize vacuum suction to remove tissue fragments from the uterine cavity, maintaining a tissue-free operative environment and eliminating the need for repeated manual removal. This feature also reduces the risks of perforation, creation of a false passageway, and gas embolus that have been linked to instrument reinsertion and manual removal of tissue fragments.12
Furthermore, mechanical morcellators are easy to use, reducing operative time and fluid deficit.
Removing Type II myomas with a hysteroscopic morcellator may pose a challenge, however, because of significant myometrial penetration. In addition, bleeding is more likely during removal of a Type II myoma than during removal of other types of tumors, necessitating the use of electric current to address it appropriately. Surgeons who are experienced using the morcellator can overcome these challenges by avoiding the myometrial interface and allowing uterine expulsive contractions to push the myoma into the cavity, making it unnecessary to penetrate the myometrium with the instrument. Thorough preoperative evaluation of Type II myomas is recommended, keeping in mind that removal may be safer and more effective using electrosurgical loop resection.
Option 1: TRUCLEAR morcellator
The TRUCLEAR Hysteroscopic Morcellator (Smith & Nephew) was FDA-approved in 2005 as the first intrauterine mechanical morcellator (VIDEO 4). It requires a dedicated fluid pump and has different instrumentation for myomas and polyps. For myomas, the instrument consists of a rotating tube that reciprocates within an outer 4-mm tube. Both tubes have windows at the end with cutting edges. A vacuum connected to the inner tube provides controlled suction that pulls the tissue into the window on the outer tube and cuts it as the inner tube rotates (VIDEO 5).
For polyps, both inner and outer tubes have oscillating serrated edges on each window (VIDEO 6).
Both instruments are used through a 9-mm offset rod-lens continuous-flow hysteroscope.
In a retrospective analysis, the TRUCLEAR morcellator reduced operative time by about two thirds for polyps and one half for Type 0 and Type I myomas, compared with monopolar loop resection.15 A later study of inexperienced ObGyn residents demonstrated shorter operative times and lower total fluid deficits for the TRUCLEAR morcellator, compared with resectoscopic procedures overall, during polypectomy and myomectomy of Type 0 and Type I myomas.16
Smith & Nephew recently introduced a smaller set of instruments, including a 2.9-mm blade for removal of polyps through a 5.6-mm continuous-flow hysteroscope. However, the new instruments have not yet been approved by the FDA and are unavailable within the United States.
Option 2: MyoSure
The MyoSure Tissue Removal System (Hologic) was FDA-approved in 2009. The hand piece is a rotating and reciprocating 2-mm blade within a 3-mm outer tube. The cutter is connected to a vacuum source that aspirates resected tissue through a side-facing cutting window in the outer tube. The system utilizes standard hysteroscopy set-up for fluid inflow and suction. The instrument is placed through an offset lens continuous-flow hysteroscope with an outer diameter of
6.25 mm. The smaller diameter reduces the amount of cervical dilation required, as well as the risk of uterine perforation.
The smaller size of the instrument renders it ideal for an office setting. Miller and colleagues demonstrated its safety and efficacy for office removal of polyps and myomas (VIDEO 7; VIDEO 8).17
Inadequate reimbursement?
Although both morcellators simplify hysteroscopic myomectomy and polypectomy, insurance reimbursement does not yet differentiate between places of service—unlike other in-office procedures that take into account the cost of the procedural device (see “Reimbursement is limited for hysteroscopic myomectomy in an office setting”). Until the relative value unit (RVU) is modified to reflect this cost, office use of the hysteroscopic morcellator for myomectomy and polypectomy will be financially restrictive to the gynecologist in private practice. Nevertheless, both instruments are easy to use and offer improved safety, increasing access to uterine-preserving surgery.
Thanks to Dr. Andrew I. Brill and Dr. William H. Parker for their thoughtful review of this article.
Since the inception of the resource-based relative value scale, the Centers for Medicare and Medicaid Services (CMS) have provided for different levels of payment to physicians, depending on the place of service and the extent of work involved. The relative value units (RVUs) established for each clinical service are based on three components:
- physician work
- practice expense
- malpractice expense.
The practice expense includes supplies, equipment, clinical and administrative staff, and renting and leasing of space.
When a physician provides a service in a hospital setting or outpatient clinic or surgery unit, the practice expense is lower because the hospital or outpatient facility shoulders those costs. In an office setting, however, the physician practice incurs the full expense of providing the service. In most cases, therefore, the practice is reimbursed at a higher total RVU for office procedures.
The “place of service” code required on your claim form lets the payer know whether the service was rendered in your office (code 11) or a facility such as a hospital or outpatient surgery center (codes 21–24). Physicians who work out of a hospital-owned facility—i.e., physicians who are employed by a hospital—would bill for a facility place of service rather than an office.
The difference in RVUs can be significant. For example, hysteroscopic sterilization (CPT code 58565) has two different RVUs, depending on whether the service is performed in a facility or office (TABLE). However, although hysteroscopic myomectomy can now be safely performed in the office setting for small, less invasive myomas, CMS has not yet assigned a place of service differential for this procedure (CPT code 58561). In other words, CMS has determined that hysteroscopic myomectomy—by definition or practice—is rarely or never performed outside a hospital or outpatient facility.
Medicare reimbursement for hysteroscopic procedures
| Procedure | CPT code | Relative value units | |
|---|---|---|---|
| Facility | Office | ||
| Sterilization | 58565 | 12.90 | 56.66 |
| Endometrial ablation | 58563 | 10.23 | 52.05 |
| Cryoablation | 58356 | 10.34 | 58.92 |
| Myomectomy | 58561 | 16.33 | NA |
| Polypectomy (with dilation and curettage, biopsy) | 58558 | 7.95 | 10.60 |
| To determine reimbursement, multiply the RVU by the Medicare conversion factor, which is $33.9764 | |||
When contracting with a private payer, be sure to ask how the payer reimburses for hysteroscopic myomectomy in an office setting. Payers that do not include a place of service differential may be amenable to negotiation if you can demonstrate that extra compensation can actually save them money and maintain high-quality patient care.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
We want to hear from you! Tell us what you think.
- Applying single-incision laparoscopic surgery to gyn practice: What’s involved
Russell P. Atkin, MD; Michael L. Nimaroff, MD; Vrunda Bhavsar, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The uterine leiomyoma is the most common tumor of the female genital tract. Seventy percent of white women and 80% of black women develop one or more of these tumors by the time they reach 50 years, and the myomas are clinically apparent in 25% of patients.1,2 When a fibroid is submucosal, it is often associated with menorrhagia, abnormal uterine bleeding, and infertility.2-4
In this article, I describe three aspects of managing leiomyomata:
- ways of classifying the tumor to better predict the blood loss, operative time and morbidity associated with removal
- the indications for hysteroscopic myomectomy and polypectomy
- new tools for the removal of polyps and myomas.
Preoperative assessment of submucosal myomas is essential
Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: a new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—preliminary report. J Minim Invasive Gynecol. 2005;12(4):308–311.
Wamsteker and colleagues were the first to propose a system for classifying myoma position within the uterine cavity as a means of estimating the degree of difficulty of resectoscopic removal.5 The European Society for Gynaecological Endoscopy (ESGE) later adopted this system, which is now known by its acronym. According to the ESGE system, myomas that lie entirely within the uterine cavity (Type 0) are easier to remove, require less operative time, and involve less fluid deficit and blood loss than myomas that invade the myometrium to varying degrees (FIGURE 1).

FIGURE 1 ESGE classification
Submucosal myomas are classified as Type 0, Type I, or Type II, according to the degree of myometrial penetration.When more than 50% of a tumor penetrates the myometrium (Type II), the risk of excessive intraoperative fluid absorption is elevated, along with the risk of bleeding and the likelihood of electrolyte abnormalities with the use of non-electrolyte fluid media. Type II tumors also increase operative time and the likelihood that additional procedures will be needed because of incomplete resection—even in the hands of expert hysteroscopic surgeons.5

FIGURE 2 New classification
New classification system increases accuracy
Lasmar and colleagues devised a new system for preoperative assessment of submucosal myomas, hoping to estimate more precisely the likelihood of successful removal via resectoscopy. They call their system the New Classification (NC). Besides taking into account the degree of penetration into the myometrium, they consider the percentage of uterine wall encompassed by the myoma and the location of the myoma within the uterus (i.e., fundus, body, or lower segment) (FIGURE 2). The total score is used to categorize the tumor into Group I, II, or III to estimate the likelihood of successful removal.
In devising the system, Lasmar and colleagues used the NC and ESGE systems to analyze 55 myomectomy cases involving 57 myomas. They found that the NC more accurately predicts differences between Groups I and II in regard to completed procedures, fluid deficit, and operative time.
Preoperative hysteroscopic evaluation of submucosal myomas is essential and reliable using the New Classification system.
Hysteroscopic removal of myomas and polyps
yields multiple benefits
Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724–729.
Rackow BW, Jorgensen E, Taylor HS. Endometrial polyps affect uterine receptivity [published online ahead of print January 24, 2011]. Fertil Steril. doi 10.1016/j. fertnstert.2010.12.034.
Afifi K, Anand S. Nallapeta S, Gelbaya TA. Management of endometrial polyps in subfertile women: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):117–121.
Studies evaluating the association between infertility and submucosal fibroids have been controversial because the exact mechanism has not been identified. However, new evidence suggests a molecular causal relationship, and Pritts and colleagues demonstrated improved fertility after submucosal myomectomy.3,6
More recently, Shokeir and coworkers conducted a prospective, randomized, age-matched, controlled trial to explore the effects of hysteroscopic myomectomy on otherwise unexplained primary infertility. They enrolled 215 women who had infertility longer than 12 months and who had their fibroids assessed by means of ultrasonography and classified according to the ESGE system.
Women who underwent myomectomy were twice as likely as women in the control group to become pregnant (relative risk = 2.1; 95% confidence interval = 1.5–2.9). Women who had Type 0 and Type I myomas removed had significantly higher pregnancy rates than women in the control group (P < .001). No statistically significant difference in the pregnancy rate between groups was found for Type II myomas.
Polyps may also affect fertility
Rackow and coworkers demonstrated that endometrial polyps affect uterine receptivity on the molecular level, suggesting a relationship between endometrial polyps and infertility. And after a systematic review of endometrial polyps in women who had subfertility, Afifi and colleagues concluded that polypectomy can improve fertility, especially when assisted reproductive technologies are planned.
Myomas, polyps also contribute to bleeding abnormalities
Submucosal myomas have been associated with bleeding abnormalities, such as heavy menstrual bleeding and menopausal bleeding. Although the precise mechanism is unknown by which these bleeding abnormalities arise in the presence of submucosal fibroids, abnormalities within the endometrium or myometrium may play a role at the genetic and molecular level.7,8 There is clear evidence supporting hysteroscopic removal of submucosal fibroids to improve bleeding abnormalities.9,10
Hysteroscopic removal of eSge type 0 and type i submucosal myomas improves the pregnancy rate for patients who have otherwise unexplained primary infertility. Removal of endometrial polyps is also recommended to improve fertility.
Besides improving fertility, hysteroscopic removal of submucosal myomas and endometrial polyps improves menorrhagia and irregular and abnormal uterine bleeding.

Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. They allow resection using saline, operate without electrical energy, and utilize vacuum suction to remove tissue fragments from the uterine cavity.
Hysteroscopic morcellators ease the task of myomectomy
Hysteroscopic removal of submucosal myomas and polyps is an effective treatment for women who experience bleeding abnormalities or infertility, but the potential for complications deters many gynecologists from performing resectoscopic myomectomy.
Use of a monopolar loop electrode (VIDEO 1) requires an electrolyte-free distention medium, such as 1.5% glycine or 3% sorbitol, and intravasation of these fluids must be limited to minimize the risk of complications such as hyponatremia, cardiovascular compromise, cerebral edema, and, even, death.12 Although the use of normal saline with bipolar resectoscopic instrumentation (VIDEO 2) and automated fluid-management systems reduces the risk of fluid overload, it does not eliminate it entirely, and fluid balance must be carefully scrutinized.13
Intrauterine electrosurgery can burn pelvic organs if an activated electrode perforates the uterine wall and makes contact with bowel or other organs. Burns to the cervix, vagina, and vulva have also been reported when monopolar resectoscopic insulation fails or monopolar electrical current is inadvertently diverted.12
In addition, unless one uses tissue-vaporizing electrodes (VIDEO 3) or is equipped
with newer instrumentation that allows tissue to be removed through the operative sheath of the resectoscope, the myoma must be extracted in pieces, often with repeated removal and reinsertion of the resectoscope and grasping instruments, increasing the risk of cervical injury or uterine perforation with each placement.
Another variable that deters hysteroscopic myomectomy is the lack of training at the residency level. The typical ObGyn resident graduating between 2002 and 2007 had performed a median of only 40 to 51 operative hysteroscopic procedures by the time of graduation.14 This statistic suggests that few residency programs provide adequate training for more demanding hysteroscopic surgeries.
Mechanical morcellators facilitate tissue removal
Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. These morcellators allow resection of a myoma using saline, minimizing the hazards of fluid overload. Because they are mechanical devices that do not require electrical energy, the potential for thermal injury is eliminated.
Mechanical morcellators utilize vacuum suction to remove tissue fragments from the uterine cavity, maintaining a tissue-free operative environment and eliminating the need for repeated manual removal. This feature also reduces the risks of perforation, creation of a false passageway, and gas embolus that have been linked to instrument reinsertion and manual removal of tissue fragments.12
Furthermore, mechanical morcellators are easy to use, reducing operative time and fluid deficit.
Removing Type II myomas with a hysteroscopic morcellator may pose a challenge, however, because of significant myometrial penetration. In addition, bleeding is more likely during removal of a Type II myoma than during removal of other types of tumors, necessitating the use of electric current to address it appropriately. Surgeons who are experienced using the morcellator can overcome these challenges by avoiding the myometrial interface and allowing uterine expulsive contractions to push the myoma into the cavity, making it unnecessary to penetrate the myometrium with the instrument. Thorough preoperative evaluation of Type II myomas is recommended, keeping in mind that removal may be safer and more effective using electrosurgical loop resection.
Option 1: TRUCLEAR morcellator
The TRUCLEAR Hysteroscopic Morcellator (Smith & Nephew) was FDA-approved in 2005 as the first intrauterine mechanical morcellator (VIDEO 4). It requires a dedicated fluid pump and has different instrumentation for myomas and polyps. For myomas, the instrument consists of a rotating tube that reciprocates within an outer 4-mm tube. Both tubes have windows at the end with cutting edges. A vacuum connected to the inner tube provides controlled suction that pulls the tissue into the window on the outer tube and cuts it as the inner tube rotates (VIDEO 5).
For polyps, both inner and outer tubes have oscillating serrated edges on each window (VIDEO 6).
Both instruments are used through a 9-mm offset rod-lens continuous-flow hysteroscope.
In a retrospective analysis, the TRUCLEAR morcellator reduced operative time by about two thirds for polyps and one half for Type 0 and Type I myomas, compared with monopolar loop resection.15 A later study of inexperienced ObGyn residents demonstrated shorter operative times and lower total fluid deficits for the TRUCLEAR morcellator, compared with resectoscopic procedures overall, during polypectomy and myomectomy of Type 0 and Type I myomas.16
Smith & Nephew recently introduced a smaller set of instruments, including a 2.9-mm blade for removal of polyps through a 5.6-mm continuous-flow hysteroscope. However, the new instruments have not yet been approved by the FDA and are unavailable within the United States.
Option 2: MyoSure
The MyoSure Tissue Removal System (Hologic) was FDA-approved in 2009. The hand piece is a rotating and reciprocating 2-mm blade within a 3-mm outer tube. The cutter is connected to a vacuum source that aspirates resected tissue through a side-facing cutting window in the outer tube. The system utilizes standard hysteroscopy set-up for fluid inflow and suction. The instrument is placed through an offset lens continuous-flow hysteroscope with an outer diameter of
6.25 mm. The smaller diameter reduces the amount of cervical dilation required, as well as the risk of uterine perforation.
The smaller size of the instrument renders it ideal for an office setting. Miller and colleagues demonstrated its safety and efficacy for office removal of polyps and myomas (VIDEO 7; VIDEO 8).17
Inadequate reimbursement?
Although both morcellators simplify hysteroscopic myomectomy and polypectomy, insurance reimbursement does not yet differentiate between places of service—unlike other in-office procedures that take into account the cost of the procedural device (see “Reimbursement is limited for hysteroscopic myomectomy in an office setting”). Until the relative value unit (RVU) is modified to reflect this cost, office use of the hysteroscopic morcellator for myomectomy and polypectomy will be financially restrictive to the gynecologist in private practice. Nevertheless, both instruments are easy to use and offer improved safety, increasing access to uterine-preserving surgery.
Thanks to Dr. Andrew I. Brill and Dr. William H. Parker for their thoughtful review of this article.
Since the inception of the resource-based relative value scale, the Centers for Medicare and Medicaid Services (CMS) have provided for different levels of payment to physicians, depending on the place of service and the extent of work involved. The relative value units (RVUs) established for each clinical service are based on three components:
- physician work
- practice expense
- malpractice expense.
The practice expense includes supplies, equipment, clinical and administrative staff, and renting and leasing of space.
When a physician provides a service in a hospital setting or outpatient clinic or surgery unit, the practice expense is lower because the hospital or outpatient facility shoulders those costs. In an office setting, however, the physician practice incurs the full expense of providing the service. In most cases, therefore, the practice is reimbursed at a higher total RVU for office procedures.
The “place of service” code required on your claim form lets the payer know whether the service was rendered in your office (code 11) or a facility such as a hospital or outpatient surgery center (codes 21–24). Physicians who work out of a hospital-owned facility—i.e., physicians who are employed by a hospital—would bill for a facility place of service rather than an office.
The difference in RVUs can be significant. For example, hysteroscopic sterilization (CPT code 58565) has two different RVUs, depending on whether the service is performed in a facility or office (TABLE). However, although hysteroscopic myomectomy can now be safely performed in the office setting for small, less invasive myomas, CMS has not yet assigned a place of service differential for this procedure (CPT code 58561). In other words, CMS has determined that hysteroscopic myomectomy—by definition or practice—is rarely or never performed outside a hospital or outpatient facility.
Medicare reimbursement for hysteroscopic procedures
| Procedure | CPT code | Relative value units | |
|---|---|---|---|
| Facility | Office | ||
| Sterilization | 58565 | 12.90 | 56.66 |
| Endometrial ablation | 58563 | 10.23 | 52.05 |
| Cryoablation | 58356 | 10.34 | 58.92 |
| Myomectomy | 58561 | 16.33 | NA |
| Polypectomy (with dilation and curettage, biopsy) | 58558 | 7.95 | 10.60 |
| To determine reimbursement, multiply the RVU by the Medicare conversion factor, which is $33.9764 | |||
When contracting with a private payer, be sure to ask how the payer reimburses for hysteroscopic myomectomy in an office setting. Payers that do not include a place of service differential may be amenable to negotiation if you can demonstrate that extra compensation can actually save them money and maintain high-quality patient care.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
We want to hear from you! Tell us what you think.
1. Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188(1):100-107.
2. Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: A new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—Preliminary report. J Minim Invasive Gynecol. 2010;12(4):308-311.
3. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215-1223.
4. Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724-729.
5. Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol. 1993;82(5):736-740.
6. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027-2034.
7. Stewart EA, Nowak RA. Leiomyoma-related bleeding: a classic hypothesis updated for the molecular era. Human Repro Update. 1996;2(4):295-306.
8. Laughlin SK, Stewart EA. Uterine leiomyomas. Individualizing the approach to a heterogeneous condition. Obstet Gynecol. 2011;117(2 pt 1):396-403.
9. Loffer FD. Improving results of hysteroscopic submucosal myomectomy for menorrhagia by concomitant endometrial ablation. J Minim Invasive Gynecol. 2005;12(3):254-260.
10. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93(5 pt 1):743-748.
11. Nathani F, Clark TJ. Uterine polypectomy in the management of abnormal uterine bleeding: a systematic review. J Minim Invasive Gynecol. 2006;13(4):260-268.
12. Munro MG. Complications of hysteroscopic and uterine resectoscopic surgery. Obstet Gynecol Clin N Am. 2010;37(3):399-425.
13. Kung RC, Vilos GA, Thomas B, Penkin P, Zaltz AP, Stabinsky SA. A new bipolar system for performing operative hysteroscopy in normal saline. J Am Assoc Gynecol Laparosc. 1999;6(3):331-336.
14. Miller CE. Training in minimally iInvasive surgery—you say you want a revolution. J Minim Invasive Gynecol. 2009;16(2):113-120.
15. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic moperating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62-66.
16. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466-471.
17. Miller CE, Glazerman L, Roy K, Lukes A. Clinical evaluation of a new hysteroscopic morcellator—retrospective case review. J Med. 2009;2(3):163-166.
1. Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188(1):100-107.
2. Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: A new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—Preliminary report. J Minim Invasive Gynecol. 2010;12(4):308-311.
3. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215-1223.
4. Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724-729.
5. Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol. 1993;82(5):736-740.
6. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027-2034.
7. Stewart EA, Nowak RA. Leiomyoma-related bleeding: a classic hypothesis updated for the molecular era. Human Repro Update. 1996;2(4):295-306.
8. Laughlin SK, Stewart EA. Uterine leiomyomas. Individualizing the approach to a heterogeneous condition. Obstet Gynecol. 2011;117(2 pt 1):396-403.
9. Loffer FD. Improving results of hysteroscopic submucosal myomectomy for menorrhagia by concomitant endometrial ablation. J Minim Invasive Gynecol. 2005;12(3):254-260.
10. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93(5 pt 1):743-748.
11. Nathani F, Clark TJ. Uterine polypectomy in the management of abnormal uterine bleeding: a systematic review. J Minim Invasive Gynecol. 2006;13(4):260-268.
12. Munro MG. Complications of hysteroscopic and uterine resectoscopic surgery. Obstet Gynecol Clin N Am. 2010;37(3):399-425.
13. Kung RC, Vilos GA, Thomas B, Penkin P, Zaltz AP, Stabinsky SA. A new bipolar system for performing operative hysteroscopy in normal saline. J Am Assoc Gynecol Laparosc. 1999;6(3):331-336.
14. Miller CE. Training in minimally iInvasive surgery—you say you want a revolution. J Minim Invasive Gynecol. 2009;16(2):113-120.
15. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic moperating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62-66.
16. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466-471.
17. Miller CE, Glazerman L, Roy K, Lukes A. Clinical evaluation of a new hysteroscopic morcellator—retrospective case review. J Med. 2009;2(3):163-166.
Health Care for Refugees Resettled in the US
It is estimated that three million refugees from all over the world—forced to flee their native countries for various reasons—have entered the United States since 1980.1,2 Prior to resettling in the US, refugees undergo health screenings for high-risk infectious diseases that preclude emigration; those free of such diseases may enter. However, the civil surgeon who conducts a refugee’s medical examination does not screen for chronic diseases that are not considered a threat to public health. Other infectious illnesses, previous traumatic injuries, and mental health issues may also go undetected at this exam.
Refugees may have had little or no access to health care before their arrival in the US, lived in conditions that increased their risk for exposure to various illnesses, and experienced traumatic events before fleeing their native lands. After their arrival, refugees may face access issues, including language and cultural barriers, health care ineligibility, and lack of transportation. This article seeks to increase awareness among primary care practitioners of the needs and issues of refugees who may be seen in their practices for conditions that developed before their arrival in the US and others emerging since their resettlement.
This activity will begin with an overview of who refugees are, how they come to reside in the US, and the medical process they undergo before resettlement. Next follows a discussion of medical issues that practitioners should be aware of among refugees, including conditions not commonly seen in the US. Finally, language and cultural issues will be addressed, including an explanatory model3 to help bridge discrepancies between practitioners of Western medicine and patients of non-Western traditions.
Man, 58, from Burundi
At age 55, the patient was resettled to the US with his family. Since then, he has had trouble holding a job, and his difficulties have been attributed to the stress of transition to life in the US. His current employer has sent him for an occupational examination. Findings are within normal limits except for visual acuity, which is tested at 20/25 in his right eye and 20/200 in his left. When asked, the patient reports having had “river blindness,” that is, onchocerciasis, as a child. Onchocerciasis is an uncommon cause of permanent blindness.
BACKGROUND AND DEFINITIONS
Refugees are defined by US Citizenship and Immigration Services4 as “people who have been persecuted or fear they will be persecuted on account of race, religion, nationality, and/or membership in a particular social group or political opinion.” The United Nations High Commission for Refugees (UNHCR)5 adds that a refugee “is outside the country of his nationality, and is unable to, or owing to such fear, is unwilling to, avail himself of the protection of that country.”
The man from Burundi came to the US through a long, complicated process. His family fled genocide in their native country to a refugee camp in Tanzania. Once there, they attained official refugee status, an essential part of the resettlement process. Refugees must fall into one of three processing priority categories:
(1) those referred by UNHCR, a US embassy, or a designated voluntary agency
(2) persons designated by a US refugee program as belonging to a “special humanitarian concern” group
(3) certain family members of refugees who currently reside in the US.6
The application generally involves biographical information and a family tree.7 Because they had fled their home, the family from Burundi had limited paperwork but were referred by UNHCR to the US for resettlement.
Once refugees become eligible for resettlement in the US, they undergo medical screening, security clearance, and cultural orientation. They are then placed by one of the sponsoring resettlement agencies listed in Table 1.8 This process can take from two months to several years.9 The medical screening may be performed by a panel physician, an overseas practitioner who examines refugees prior to their resettlement; or by a civil surgeon, who examines refugees after their arrival in the US—generally when the refugee applies for status adjustment.10
The man from Burundi represents a relatively common issue among refugees in the US. Many chronic conditions go untreated within this population because follow-up may be inadequate or absent, patients’ access to health care is insufficient, or the health care provider is unfamiliar with refugee issues. In this case, evaluation of the man’s vision was not part of the routine examination conducted in all refugees. Because he was never offered a subsequent vision check, his blindness went unnoticed, and his work difficulties were attributed to language and adjustment issues.
His visual problem could not be corrected, but once it was identified, accommodations were made in the workplace that facilitated his adjustment to the new work environment.
Girl, 16, from Liberia
This patient, who is being seen in your office for a sports physical, arrived in the US three years ago. Her medical screening at the time of immigration indicated “good health,” and she has had no health problems since then. Her exam seems unremarkable except for a low-pitched, rumbling, diastolic murmur best heard with the bell of the stethoscope near the apex when she lies in the left lateral decubitus position.
HEALTH SCREENINGS
Before coming to the US, refugees must undergo a health evaluation that includes a thorough medical history, full physical examination, chest x-ray, if indicated, for tuberculosis (TB), vaccination verification, and laboratory work as needed to identify specific infectious diseases.11 Screenings included in this evaluation target problems that are considered important from a public health perspective, including TB and certain sexually transmitted illnesses. Many infectious diseases are not considered a threat to public health because the requisite vector is not present in the US (eg, malaria, schistosomiasis).12
Public health conditions are categorized as Class A or Class B (see Table 2,11,13). Typically, refugees with a Class A condition are considered ineligible for admission to the US. Presence of a Class B condition must be brought to the attention of consular authorities, as it may indicate future disability or need for medical treatment.13,14
It is important for practitioners to know that if an illness or medical condition is discovered during the panel physician’s examination but is not considered “relevant to the visa medical examination,” the panel physician does not treat the patient. Rather, he or she recommends that the patient seek care from a medical provider.15 Essentially, refugees with a condition that is not considered a public health threat are allowed into the US, whether or not the condition has been addressed—as in the case of the diastolic heart murmur in the girl from Liberia.
This heart murmur could be a complication of rheumatic fever secondary to untreated group A streptococcal infection. In developing nations, rheumatic heart disease is the most commonly acquired heart condition, frequently exceeding congenital heart disease as a cause of hospitalization among children, adolescents, and teens.16 The risks associated with pregnancy in women with rheumatic heart disease have long been known,17 but as rheumatic heart disease has become less common in the developed nations, so has the sequela of mitral valve stenosis.
Clinicians need to become familiar with other illnesses commonly found in the developing nations, such as malaria and measles.12 Furthermore, practitioners should be aware that refugees may be immunologically susceptible to pathogens endemic to the countries of resettlement.
Woman, 65, from Bhutan
Forcibly deported from Bhutan to Nepal, this patient resettled to the US four years ago. She neither reads nor writes in her first language (Nepali) nor in English. She has been attending English language classes since her arrival in the US but has made little progress. The woman visits a health fair, bringing with her nine medications, including two bottles of insulin and three empty bottles for three different antihypertensive medications. Through an interpreter, you learn that the woman is not taking any of these medications regularly. She is also unaware that she should neither reuse needles nor share her medications with her son, who is also diabetic. She cannot afford to refill her prescriptions.
THE PROCESS, THE BARRIERS
Upon their arrival in the US, refugees receive 30 to 90 days’ support for “housing, essential furnishings, food, clothing, community orientation, and referral to other social, medical, and employment services.”8 In addition to the predeparture medical screening, refugees receive assistance for travel arrangements and a loan to pay for travel to the US.18 Refugees must sign a promissory note indicating that they will begin repayment of the loan within six months of their arrival and will complete repayment within 42months.19
At the time of their arrival, refugees apply for a Social Security number, register their children for school, undergo another medical evaluation, and receive English language training, if needed.8 Within six months of their arrival, refugees of working age are expected to have obtained employment.7 After one year in the US, refugees must apply for Legal Permanent Resident Status; after five years, they can apply for citizenship.8,20,21
In the case of the woman from Bhutan, neither diabetes nor hypertension precluded her resettlement; however, several factors now complicate her health care. Although she has access to health care providers, she cannot afford copayments for her medications, nor does she understand the instructions for their use. That she is not literate in any language complicates the challenge for her to learn to speak English. Her lack of linguistic and health literacy adds to the financial burden of the medications she needs.
The use of formally trained medical interpreters can alleviate some, but not all, of these problems. The refugee who does not become a citizen within seven years risks losing all benefits, including Medicare and Medicaid. This is particularly problematic for older refugees with multiple health problems. The process of becoming a US citizen is arduous, including lessons in civics and a required level of language fluency that is difficult to attain, particularly for those who are not literate in any language.
The Office of Refugee Resettlement22 recommends that refugees undergo a second medical screening after their arrival to identify any conditions that were not addressed by the panel physician. This examination, according to the CDC, “provides an opportunity to identify important causes of morbidity among resettled refugees that might not have been discovered previously, and enables early referral for treatment and follow-up care.”1 This screening also offers refugees the chance to establish a medical home and to begin to become familiar with the US health care system, potential barriers notwithstanding.
Girl, 15, from Sudan
A cough is the presenting symptom in this girl, who resettled to the US three years ago. She has no other upper respiratory symptoms. Results of laboratory testing indicate eosinophilia and mild anemia.
COMMON HEALTH ISSUES
Health conditions that refugees face but that may not be found in other immigrants or nonimmigrants include TB, hepatitis, parasites, HIV and other sexually transmitted infections, and mental health issues.23,24 For some refugees, female genital mutilation/cutting (FGM/C)23 or lead exposure25 may be a significant concern. Additionally, the traumatic events that led refugees to flee their homes may have resulted in musculoskeletal or neurologic injuries with a wide array of manifestations.
Parasites
Parasitic infections are common among refugees, and these can lead to anemia resulting from blood loss and iron deficiency, malnutrition, growth retardation, invasive illnesses, and death.26,27 According to Carlsten and Jackson,28 immigrants can be infected by multiple pathogens simultaneously, and some parasites may survive for as long as decades.
The most common parasitic infections among refugees are hookworm, whipworm, roundworm, and Giardia lamblia.23 In a screening performed five years after the arrival in the US of the “Lost Boys and Girls of Sudan,” 64% of a cohort of the boys living in Atlanta tested positive for Schistosoma mansoni or Schistosoma haematobium, and 25% tested positive for Strongyloides stercoralis—the organisms responsible for schistosomiasis and strongyloidiasis, respectively.26 In 2005, the CDC recommended presumptive treatment for both illnesses in Sudanese refugees who were not treated for these infections before their resettlement.29
Despite treatment and prophylaxis prior to refugees’ departure for the US, parasitic infections remain common in the refugee population.23 Practitioners should be aware that a cough could indicate the presence of roundworms that have entered the body through the skin and spread to the lungs via the blood.30,31 All refugees should be screened for eosinophilia to detect parasitic infections.23 An absolute eosinophil count exceeding 400 cells/L warrants further investigation.27
Because of presumptive treatment for malaria given to nonpregnant refugees, this disease is rarely seen after refugees’ arrival in the US.23 However, practitioners should not overlook the possibility of malaria when they examine refugee patients, as malaria may take time to manifest clinically.24
Tuberculosis
While refugees cannot be admitted into the US with active, infectious TB (a Class A disease), the majority of cases of TB in the US occur among foreign-born individuals, with prevalence 10 times that in the US-born population.32 Refugees are at particular risk for TB.33
When examining refugee patients, especially those recently arrived in the US, clinicians should be aware of the potential for extrapulmonary TB, which accounted for 20% of TB cases in the US in 2008.32 Extrapulmonary TB can be found anywhere in the body, with more common sites including the lymph nodes, pleura, and osteoarticular areas. Skeletal TB accounts for 35% of extrapulmonary TB cases—most commonly Pott’s disease, or spinal TB.34
Use of bacille Calmette-Guerin (BCG), a vaccine given in various countries to prevent childhood tuberculous meningitis and miliary disease, often leads to confusion when the tuberculin skin test (TST, previously known as the purified protein derivative, or PPD) is used to screen for TB.35 While BCG can increase the number of false-positive TST results, TST reaction following BCG decreases with time and generally is not seen longer than 10 years postvaccination.36
Furthermore, the immunity produced by BCG weakens over time; thus, an adult, though immunized as an infant, is at risk for TB infection. The CDC currently recommends the same testing for TB, whether or not patients have undergone BCG vaccination. Similarly, TST results should be interpreted in the same way for BCG-vaccinated patients and nonvaccinated patients alike.35
Finally, BCG does not affect results of blood tests for TB. However, these tests are new, expensive, and not available everywhere.35
Hepatitis
Among the forms of hepatitis, hepatitis B virus (HBV) is of greatest concern within the refugee community, as it is endemic to much of the world.37 Between 2003 and 2007, 10.7% of refugees screened in DeKalb County, Georgia, for HBsAg (the hepatitis B surface antigen that indicates exposure to the virus) tested positive, accounting for 43.3% of HBsAg-positive test results in the county during that period. Chronic HBV infection can lead to end-stage liver disease, cirrhosis, and hepatocellular carcinoma.37
Museru et al37 recommend that health care providers ascertain the hepatitis B–serological status of resettled refugees from areas that are highly endemic for HBV infection. In addition, Adams and colleagues23 recommend screening patients who have undergone blood transfusions, female genital surgery, or other surgical procedures in their countries of origin, as well as patients from Africa or southeast Asia, for hepatitis C.23
Mutilation or Cutting of the Female Genitalia
Ritual FGM/C is the practice of injuring or removing part or all of the external female genitalia for cultural and other nonmedical reasons.38 FGM/C is primarily practiced in Africa (see Figure,39,40) but may occur also in Asia, the Middle East, and Central and South America.38 It is often practiced by informally trained individuals, with “inexact surgical outcomes.”23 FGM/C has been outlawed in the US and other countries with large immigrant populations; some nations grant asylum to women who fear being subjected to FGM/C if they return to their country of origin.39
Practitioners who care for female refugees should be aware of both the short-term sequelae (pain, bleeding, trauma, sepsis) and additional long-term sequelae (dyspareunia, urinary retention and recurrent urinary tract infections, chronic pelvic inflammatory disease, keloid scar formation, childbirth complications) of FGM/C, in addition to psychological sequelae.23,38,41 It is important to approach affected patients with sympathy and without judgment, as the decision to undergo FGM/C may not have been theirs.41
The Royal College of Obstetricians and Gynaecologists in the United Kingdom has produced a helpful set of guidelines, Female Genital Mutilation and its Management,41 for clinicians working with patients who have undergone FGM/C.
Sexually Transmitted Infections
In light of a new law allowing refugees with HIV to be resettled in the US,42 practitioners must now be aware of the possibility of HIV infection in a refugee patient, whether documented or not. Practitioners should follow the same guidelines for refugees as they do for all patients regarding HIV screening and counseling, including allowing patients the opportunity to decline testing.43 However, they should also be aware of countries in which HIV prevalence rates are high.43
Additionally, while HIV-1 is the world’s predominant strain of the disease, refugees from West African countries have been at increased risk for exposure to HIV-2 and should be tested accordingly.43,44 Refugees may also be at increased risk for HIV and other sexually transmitted infections attributable to physical or sexual violence.43,45
All screening for HIV and other sexually transmitted infections should be performed in a culturally appropriate manner, with the use of trained interpreters as needed, to ensure that all patients receive accurate information and counseling.43
Exposure to Lead
Refugee children are at high risk for lead exposure both before and after their arrival in the US—the latter as a result of their families’ living conditions after resettlement, despite the ban on lead-based paint.25 A study in Minnesota from 2000 to 2002 showed that among refugee children younger than 6, prevalence of lead poisoning was 14 times that found in American children in their age-group.25 In New York City, Asian children have been shown to be at particular risk for lead poisoning—including the case of a year-old Cambodian child who was evidently exposed to an amulet with leaded beads. Sources of lead other than paint may include imported food, spices, cosmetics, pottery, and health remedies.46
Where children were born and where they have lived throughout their lives appear to be the greatest predictors of lead poisoning risk.25 One primary risk factor for lead poisoning is malnutrition, associated with increased absorption of lead in the intestines and the resulting micronutrient deficiencies.25
The CDC recommends:
• Screening for lead in all children from age 6 months to 16 years at the time of their arrival in the US
• Follow-up blood lead testing of children ages 6 months to 6 years, 3 to 6 months after they have moved into a permanent residence25
• Nutritional assessments for children younger than 6 years, as well as measurement of hemoglobin/hematocrit levels, including at least one of the following measurements: mean corpuscular volume with red-cell distribution width, ferritin, transferrin saturation, or reticulocyte hemoglobin content
• Daily multivitamins with iron for refugee children ages 6 months to approximately 5 years.
Lead poisoning, as indicated by a blood lead level (BLL) exceeding 10 g/dL, is known to have neurodevelopmental and cognitive sequelae. In children with a significantly higher BLL, manifestations may include headaches, abdominal pain, anorexia, constipation, clumsiness, agitation, and lethargy in the acute phase.25
Woman, 48, from Afghanistan
Through an interpreter, you learn that this visitor to a local health fair has been experiencing left arm pain since she and her family fled Kabul. When her house there was hit by a rocket, she ran back in, despite a fire, to save her infant daughter. Although she received care as a refugee in Pakistan for burns to her arms and body, she has scarring and strictures the length of her arm. Thanks to the efforts of a volunteer physical therapist, use of the woman’s injured arm has been preserved. She is diagnosed with posttraumatic stress syndrome and referred to a local mental health clinic whose staff specializes in working with survivors of trauma and torture.
Mental Health Issues
Mental health issues are a significant component of refugee health. MacDuff et al47 report that 36% of complementary and alternative medicine use among refugees targets mental health issues resulting from trauma. Because refugees were forced by dangerous conditions to flee their home countries, they are particularly susceptible to mental health concerns. They may have witnessed violence, undergone torture, or been subjected to unsafe or unsanitary conditions in refugee camps. Many have had trouble adjusting to their new culture.23,24,28
As a result, refugees are at increased risk for depression, posttraumatic stress disorder, substance abuse, somatization, psychosis, and suicide.23,28 Mental health issues among refugees are also complicated by the cultural and communication barriers that often exist between refugees and practitioners.23 Thus, practitioners need to take careful histories with sensitivity to their patients’ previous experiences.
While the acuity of these issues begins to decrease around three years after refugees’ arrival in their country of resettlement, the burden of mental health problems often persists for many years.28 Adams et al23 recommend that refugees be referred to social workers, cultural case mediators, and community organizations. Clinicians who do not feel comfortable managing mental health conditions should refer refugee patients to appropriate mental health practitioners and follow up to make sure that patients’ needs are being met.
Musculoskeletal and Neurologic Injuries
Traumatic events can lead to a wide variety of musculoskeletal and neurologic injuries—for example, wounds inflicted by weapons, amputations following land mine injuries, crush injuries from collapsing buildings, or burns sustained in rocket attacks. The array of possibilities necessitates a thorough history and complete musculoskeletal and neurologic assessments.
LANGUAGE AND CULTURAL ISSUES
In addition to being aware of the potential health issues that arise within the refugee population, primary care providers need to be prepared to confront language and cultural issues that may arise. The National Standards on Culturally and Linguistically Appropriate Services (CLAS)48 offer appropriate guidance in 14 key areas.
Refugees frequently have limited or no working knowledge of English. Whenever possible, practitioners should use medically trained interpreters to help them receive and convey accurate information and thus provide comprehensive care. When professional interpreters are not available, telephone interpreter services are available for purchase by the facility or practice. Children or other family members should not be relied on for accurate interpretation.48
As for cultural differences, it is important to note that a refugee’s concept of family may differ from that found in Western culture.49 For example, it is not uncommon to find extended families living together, with members referring to nieces and nephews as their children, or aunts and uncles as their parents. A thorough exploration of the relationships among patients and their families is important, particularly during the family history.
Regardless of where resettled refugees come from, it is probable that their cultural and personal beliefs about medicine differ from those of practitioners with years of Western medicine training. In addition to implementing CLAS guidelines,48 practitioners should be familiar with Kleinman’s3explanatory model, which explores the differences between the patient and practitioner models—not necessarily differences in levels of knowledge, but rather of “values and interests.”3 Thus, people unfamiliar with or resistant to the Western model of medicine are often seen as ignorant, whereas an issue of values may be at play.
Identifying the differences between the patient’s and the clinician’s explanatory models allows the clinician to anticipate and address potential misunderstandings, understand patients’ perceived needs, and involve patients in management strategies that will motivate them to comply with treatment. To help clinicians assess the explanatory models of their patients, Kleinman provides eight questions (shown in Table 3,3,50).
CONCLUSION
As the world becomes more of a global village, increasing numbers of primary care providers will see refugee patients. Practitioners need to be aware of the physical, socioeconomic, and psychological issues that affect refugees during and after resettlement in the US. Refugees may have conditions that could not be addressed in their home country or refugee camp. They may have illnesses with which US practitioners are ordinarily unfamiliar, poorly treated or untreated traumatic injuries, or psychological trauma resulting from conditions that forced them to flee their native lands.
Clinicians who work with refugees should be familiar with the resettlement process and perform a thorough examination after the refugee’s resettlement, managing previously unaddressed health issues. Complete histories must be taken and physical examinations performed in a culturally appropriate manner and an atmosphere of mutual trust.
Finally, it is vital for providers to explore the explanatory models from which patients view their illnesses. Such an understanding facilitates culturally appropriate care with patient participation, and ultimately more positive clinical outcomes.
Authors’ note: The patients portrayed in this article are all composites. While the issues described are real, these “patients” were developed from multiple cases to protect individual patient privacy. None is real, and any resemblance to any real persons is purely accidental.
REFERENCES
1. Ramos M, Orozovich P, Moser K, et al. Health of resettled Iraqi refugees: San Diego County, California, October 2007–September 2009. MMWR Morb Mortal Wkly Rep. 2010;59(49):1614-1618.
2. US Department of State. FY 2010 cumulative summary of refugee admissions. www.wrapsnet .org/reports/archives/tabid/215/language/en-us/default.aspx. Accessed February 17, 2011.
3. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88(2):251-258.
4. US Citizenship and Immigration Services. Refugees and asylum. www.uscis.gov/portal/site/uscis/menuitem.eb1d4c2a3e5b9ac89243c6a7543f6d1a/?vgnextoid=1f1c3e4d77d73210VgnVCM100000082ca60aRCRD&vgnextchannel=1f1c3e4d77d73210VgnVCM100000082ca60aRCRD. Accessed February 17, 2011.
5. United Nations High Commissioner for Refugees. Refugees: flowing across borders. www .unhcr.org/pages/49c3646c125.html. Accessed February 17, 2011.
6. US Citizenship and Immigration Services. The United States Refugee Admissions Program (USRAP) consultation and worldwide processing priorities. www.uscis.gov/portal/site/uscis/menuitem.5af9bb95919f35e66f614176543f6d1a/?vgnextoid=796b0eb389683210VgnVCM100000082ca60aRCRD&vgnextchannel=385d3e4d77d73210VgnVCM100000082ca60aRCRD. Accessed February 17, 2011.
7. US Committee for Refugees and Immigrants. Frequently asked questions. www.refugees.org/about-us/faqs.html. Accessed February 17, 2011.
8. US Department of State. Refugee Admissions Reception and Placement Program. www.state
.gov/g/prm/rls/125478.htm. Accessed February 17, 2011.
9. Office of Refugee Resettlement. Report to Congress: FY 2007. www.acf.hhs.gov/programs/orr/data/ORR_2007_report.pdf. Accessed February 17, 2011.
10. CDC. Medical examination: frequently asked questions (FAQs). www.cdc.gov/immigrant
refugeehealth/exams/medical-examination-faqs .html#9. Accessed February 17, 2011.
11. US Department of State. Medical Examination for Immigrant or Refugee Applicant (DS-2053, OMB No. 1405-0113, expiration date 04/30/2012. http://bangkok.usembassy.gov/root/pdfs/med forms_043012.pdf. Accessed February 17, 2011.
12. Cohen J, Powderly WG, Opal SM, eds. Infectious Diseases. 3rd ed. Philadelphia, PA: Elsevier; 2010.
13. CDC. Domestic Refugee Health Program: Frequently Asked Questions (2010). www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/domestic-refugee-questions.html. Accessed February 17, 2011.
14. CDC. Technical Instructions: guidance for HIV for panel physicians and civil surgeons (2010). www.cdc.gov/immigrantrefugeehealth/exams/ti/hiv-guidance-panel-civil.html. Accessed February 17, 2011.
15. CDC. Medical history and physical examination: technical instructions for medical examination of aliens. www.cdc.gov/immigrantrefugee health/exams/ti/panel/technical-instructions/panel-physicians/medical-history-physical-exam .html. Accessed February 17, 2011.
16. Lee JL, Naguwa SM, Cheema GS, Gershwin ME. Acute rheumatic fever and its consequences: a persistent threat to developing nations in the 21st century. Autoimmun Rev. 2009;9(2):117-123.
17. Henderson DN. Pregnancy complicated by rheumatic heart disease. Can Med Assoc J. 1936; 35(4):394-398.
18. US Citizenship and Immigration Services. Refugees. www.uscis.gov/portal/site/uscis/menu item.eb1d4c2a3e5b9ac89243c6a7543f6d1a/?vgnextoid=385d3e4d77d73210VgnVCM100000082ca60aRCRD&vgnextchannel=385d3e4d77d73210VgnVCM100000082ca60aRCRD. Accessed February 17, 2011.
19. US Department of State. 9 FAM appendix O, exhibit II: Promissory note (2007). In: Foreign Affairs Manual Volume 9: Visas. www.state.gov/documents/organization/88070.pdf. Accessed February 17, 2011.
20. United States Department of State. US Refugee Admissions Program. www.state.gov/g/prm/c26471.htm. Accessed February 17, 2011.
21. J. Kernan, Community Relations Officer, US Citizenship and Immigration Services, personal communication, March 29, 2010.
22. Office of Refugee Resettlement. Health. www.acf.hhs.gov/programs/orr/benefits/health .htm. Accessed February 17, 2011.
23. Adams KM, Gardiner LD, Assefi N. Healthcare challenges from the developing world: post-immigration refugee medicine. BMJ. 2004;328(7455): 1548-1552.
24. Walker PF, Jaranson J. Refugee and immigrant health care. Med Clin North Am. 1999; 83(4):1103-1120.
25. CDC. Screening for lead at the domestic refugee medical examination (2005). www.cdc.gov/immigrantrefugeehealth/pdf/lead.pdf. Accessed February 17, 2011.
26. Franco-Paredes C, Dismukes R, Nicolls D, et al. Short report: persistent and untreated tropical infectious diseases among Sudanese refugees in the United States. Am J Trop Med Hyg. 2007;77 (4):633-635.
27. CDC. Immigrant and Refugee Health: Domestic Refugee Health Guidelines: Intestinal Parasites. www.cdc.gov/immigrantrefugeehealth/guidelines/ip/intestinal-parasites-domestic.html#asympto matic2. Accessed February 17, 2011.
28. Carlsten C, Jackson C. Refugee and immigrant health care. EthnoMed. http://ethnomed.org/clinical/refugee-health/carlsten.pdf. Accessed February 17, 2011.
29. Conly JM, Johnston BL. The infectious diseases implications of the “Lost Boys and Girls of Sudan.” Can J Infect Dis Med Microbiol. 2008;19 (3):215-216.
30. Aggarwal B, Sharma M, Singh T. Acute eosinophilic pneumonia due to round worm infestation. Indian J Pediatr. 2008;75(3):296-297.
31. Tsai HC, Lee SS, Liu YC, et al. Clinical manifestations of strongyloidiasis in southern Taiwan.
J Microbiol Immunol Infect. 2002;35(1):29-36.
32. CDC. Reported tuberculosis in the United States, 2008. www.cdc.gov/tb/statistics/reports/2008/pdf/2008report.pdf. Accessed February 17, 2011.
33. Oeltmann JE, Varma JK, Ortega L, et al. Multidrug-resistant tuberculosis outbreak among US-bound Hmong refugees, Thailand, 2005. Emerg Infect Dis. 2008;14(11):1715-1721.
34. Golden MP, Vikram H. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005; 72(9):1761-1768.
35. CDC. Tuberculosis (TB) Fact Sheets: BCG Vaccine. www.cdc.gov/tb/publications/factsheets/prevention/BCG.htm. Accessed February 17, 2011.
36. The role of BCG vaccine in the prevention and control of tuberculosis in the United States: a joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45(RR-4):1-18.
37. Museru OI, Vargas M, Kinyua M, et al. Hepatitis B virus infection among refugees resettled in the US: high prevalence and challenges in access to health care. J Immigr Minor Health. 2010;12 (6):823-827.
38. World Health Organization, UNAIDS, UNDP, UNECA, UNESO, UNFPA, UNHCHR, UNHCR, UNICEF, UNIFEM. Eliminating Female Genital Mutilation: An Interagency Statement (2008). http://whqlibdoc.who.int/publications/2008/ 9789241596442_eng.pdf. Accessed February 17, 2010.
39. United Nations Children’s Fund. Female Genital Mutilation/Cutting: A Statistical Exploration (2005). www.unicef.org/publications/files/FGM-C_final_10_October.pdf. Accessed February 17, 2011.
40. Yoder PS, Abderrahim N, Zhuzhuni A. DHS Comparative Reports No. 7: Female Genital Cutting in the Demographic and Health Surveys: A Critical and Comparative Analysis. Calverton, MD: ORC Macro. September 2004.
41. Royal College of Obstetricians and Gynaecologists. Female Genital Mutilation and its Management (Green-top Guideline No. 53; 2009). www .rcog.org.uk/files/rcog-corp/GreenTop53Female GenitalMutilation.pdf. Accessed February 17, 2011.
42. CDC. Final Rule Removing HIV Infection from U.S. Immigration Screening: Revision of 42 CFR Part 34 (Medical Examination of Aliens) Removal of Human Immunodeficiency Virus (HIV) from Definition of Communicable Disease of Public Health Significance—Final Rule. www.cdc.gov/immigrantrefugeehealth/laws-regs/hiv-ban-removal/final-rule.html. Accessed February 17, 2011.
43. CDC. Immigrant and Refugee Health: Screening for HIV-infection during the refugee domestic medical examination. www.cdc.gov/immigrant
refugeehealth/guidelines/domestic/screening-
hiv-infection-domestic.html. Accessed February 17, 2011.
44. CDC. Human Immunodeficiency Virus Type 2: HIV/AIDS Fact Sheets. Atlanta: Centers for Disease Control and Prevention; 2007.
45. Mills EJ, Nachega JB. HIV infection as a weapon of war. Lancet Infect Dis. 2006;6(12):752-753.
46. CDC. Lead poisoning of a child associated with use of a Cambodian amulet—New York City, 2009. MMWR Morb Mortal Wkly Rep. 2011;60(3): 69-71.
47. MacDuff S, Grodin MA, Gardiner P. The use of complementary and alternative medicine among refugees: a systematic review. J Immigr Minor Health. 2010 Mar 12 [Epub ahead of print].
48. US Department of Health and Human Services, Office of Minority Health. National standards on culturally and linguistically appropriate services (CLAS). http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15. Accessed February 17, 2011.
49. Haviland WA, Prins HEL, Walrath D, McBride B, eds. Cultural Anthropology: The Human Challenge. 12th ed. Belmont, CA: Wadsworth Publishing; 2008.
50. Fadiman A. The Spirit Catches You and You Fall Down: A Hmong Child, Her American Doctors, and the Collision of Two Cultures. New York, NY: Farrar, Straus and Giroux; 1997.
It is estimated that three million refugees from all over the world—forced to flee their native countries for various reasons—have entered the United States since 1980.1,2 Prior to resettling in the US, refugees undergo health screenings for high-risk infectious diseases that preclude emigration; those free of such diseases may enter. However, the civil surgeon who conducts a refugee’s medical examination does not screen for chronic diseases that are not considered a threat to public health. Other infectious illnesses, previous traumatic injuries, and mental health issues may also go undetected at this exam.
Refugees may have had little or no access to health care before their arrival in the US, lived in conditions that increased their risk for exposure to various illnesses, and experienced traumatic events before fleeing their native lands. After their arrival, refugees may face access issues, including language and cultural barriers, health care ineligibility, and lack of transportation. This article seeks to increase awareness among primary care practitioners of the needs and issues of refugees who may be seen in their practices for conditions that developed before their arrival in the US and others emerging since their resettlement.
This activity will begin with an overview of who refugees are, how they come to reside in the US, and the medical process they undergo before resettlement. Next follows a discussion of medical issues that practitioners should be aware of among refugees, including conditions not commonly seen in the US. Finally, language and cultural issues will be addressed, including an explanatory model3 to help bridge discrepancies between practitioners of Western medicine and patients of non-Western traditions.
Man, 58, from Burundi
At age 55, the patient was resettled to the US with his family. Since then, he has had trouble holding a job, and his difficulties have been attributed to the stress of transition to life in the US. His current employer has sent him for an occupational examination. Findings are within normal limits except for visual acuity, which is tested at 20/25 in his right eye and 20/200 in his left. When asked, the patient reports having had “river blindness,” that is, onchocerciasis, as a child. Onchocerciasis is an uncommon cause of permanent blindness.
BACKGROUND AND DEFINITIONS
Refugees are defined by US Citizenship and Immigration Services4 as “people who have been persecuted or fear they will be persecuted on account of race, religion, nationality, and/or membership in a particular social group or political opinion.” The United Nations High Commission for Refugees (UNHCR)5 adds that a refugee “is outside the country of his nationality, and is unable to, or owing to such fear, is unwilling to, avail himself of the protection of that country.”
The man from Burundi came to the US through a long, complicated process. His family fled genocide in their native country to a refugee camp in Tanzania. Once there, they attained official refugee status, an essential part of the resettlement process. Refugees must fall into one of three processing priority categories:
(1) those referred by UNHCR, a US embassy, or a designated voluntary agency
(2) persons designated by a US refugee program as belonging to a “special humanitarian concern” group
(3) certain family members of refugees who currently reside in the US.6
The application generally involves biographical information and a family tree.7 Because they had fled their home, the family from Burundi had limited paperwork but were referred by UNHCR to the US for resettlement.
Once refugees become eligible for resettlement in the US, they undergo medical screening, security clearance, and cultural orientation. They are then placed by one of the sponsoring resettlement agencies listed in Table 1.8 This process can take from two months to several years.9 The medical screening may be performed by a panel physician, an overseas practitioner who examines refugees prior to their resettlement; or by a civil surgeon, who examines refugees after their arrival in the US—generally when the refugee applies for status adjustment.10
The man from Burundi represents a relatively common issue among refugees in the US. Many chronic conditions go untreated within this population because follow-up may be inadequate or absent, patients’ access to health care is insufficient, or the health care provider is unfamiliar with refugee issues. In this case, evaluation of the man’s vision was not part of the routine examination conducted in all refugees. Because he was never offered a subsequent vision check, his blindness went unnoticed, and his work difficulties were attributed to language and adjustment issues.
His visual problem could not be corrected, but once it was identified, accommodations were made in the workplace that facilitated his adjustment to the new work environment.
Girl, 16, from Liberia
This patient, who is being seen in your office for a sports physical, arrived in the US three years ago. Her medical screening at the time of immigration indicated “good health,” and she has had no health problems since then. Her exam seems unremarkable except for a low-pitched, rumbling, diastolic murmur best heard with the bell of the stethoscope near the apex when she lies in the left lateral decubitus position.
HEALTH SCREENINGS
Before coming to the US, refugees must undergo a health evaluation that includes a thorough medical history, full physical examination, chest x-ray, if indicated, for tuberculosis (TB), vaccination verification, and laboratory work as needed to identify specific infectious diseases.11 Screenings included in this evaluation target problems that are considered important from a public health perspective, including TB and certain sexually transmitted illnesses. Many infectious diseases are not considered a threat to public health because the requisite vector is not present in the US (eg, malaria, schistosomiasis).12
Public health conditions are categorized as Class A or Class B (see Table 2,11,13). Typically, refugees with a Class A condition are considered ineligible for admission to the US. Presence of a Class B condition must be brought to the attention of consular authorities, as it may indicate future disability or need for medical treatment.13,14
It is important for practitioners to know that if an illness or medical condition is discovered during the panel physician’s examination but is not considered “relevant to the visa medical examination,” the panel physician does not treat the patient. Rather, he or she recommends that the patient seek care from a medical provider.15 Essentially, refugees with a condition that is not considered a public health threat are allowed into the US, whether or not the condition has been addressed—as in the case of the diastolic heart murmur in the girl from Liberia.
This heart murmur could be a complication of rheumatic fever secondary to untreated group A streptococcal infection. In developing nations, rheumatic heart disease is the most commonly acquired heart condition, frequently exceeding congenital heart disease as a cause of hospitalization among children, adolescents, and teens.16 The risks associated with pregnancy in women with rheumatic heart disease have long been known,17 but as rheumatic heart disease has become less common in the developed nations, so has the sequela of mitral valve stenosis.
Clinicians need to become familiar with other illnesses commonly found in the developing nations, such as malaria and measles.12 Furthermore, practitioners should be aware that refugees may be immunologically susceptible to pathogens endemic to the countries of resettlement.
Woman, 65, from Bhutan
Forcibly deported from Bhutan to Nepal, this patient resettled to the US four years ago. She neither reads nor writes in her first language (Nepali) nor in English. She has been attending English language classes since her arrival in the US but has made little progress. The woman visits a health fair, bringing with her nine medications, including two bottles of insulin and three empty bottles for three different antihypertensive medications. Through an interpreter, you learn that the woman is not taking any of these medications regularly. She is also unaware that she should neither reuse needles nor share her medications with her son, who is also diabetic. She cannot afford to refill her prescriptions.
THE PROCESS, THE BARRIERS
Upon their arrival in the US, refugees receive 30 to 90 days’ support for “housing, essential furnishings, food, clothing, community orientation, and referral to other social, medical, and employment services.”8 In addition to the predeparture medical screening, refugees receive assistance for travel arrangements and a loan to pay for travel to the US.18 Refugees must sign a promissory note indicating that they will begin repayment of the loan within six months of their arrival and will complete repayment within 42months.19
At the time of their arrival, refugees apply for a Social Security number, register their children for school, undergo another medical evaluation, and receive English language training, if needed.8 Within six months of their arrival, refugees of working age are expected to have obtained employment.7 After one year in the US, refugees must apply for Legal Permanent Resident Status; after five years, they can apply for citizenship.8,20,21
In the case of the woman from Bhutan, neither diabetes nor hypertension precluded her resettlement; however, several factors now complicate her health care. Although she has access to health care providers, she cannot afford copayments for her medications, nor does she understand the instructions for their use. That she is not literate in any language complicates the challenge for her to learn to speak English. Her lack of linguistic and health literacy adds to the financial burden of the medications she needs.
The use of formally trained medical interpreters can alleviate some, but not all, of these problems. The refugee who does not become a citizen within seven years risks losing all benefits, including Medicare and Medicaid. This is particularly problematic for older refugees with multiple health problems. The process of becoming a US citizen is arduous, including lessons in civics and a required level of language fluency that is difficult to attain, particularly for those who are not literate in any language.
The Office of Refugee Resettlement22 recommends that refugees undergo a second medical screening after their arrival to identify any conditions that were not addressed by the panel physician. This examination, according to the CDC, “provides an opportunity to identify important causes of morbidity among resettled refugees that might not have been discovered previously, and enables early referral for treatment and follow-up care.”1 This screening also offers refugees the chance to establish a medical home and to begin to become familiar with the US health care system, potential barriers notwithstanding.
Girl, 15, from Sudan
A cough is the presenting symptom in this girl, who resettled to the US three years ago. She has no other upper respiratory symptoms. Results of laboratory testing indicate eosinophilia and mild anemia.
COMMON HEALTH ISSUES
Health conditions that refugees face but that may not be found in other immigrants or nonimmigrants include TB, hepatitis, parasites, HIV and other sexually transmitted infections, and mental health issues.23,24 For some refugees, female genital mutilation/cutting (FGM/C)23 or lead exposure25 may be a significant concern. Additionally, the traumatic events that led refugees to flee their homes may have resulted in musculoskeletal or neurologic injuries with a wide array of manifestations.
Parasites
Parasitic infections are common among refugees, and these can lead to anemia resulting from blood loss and iron deficiency, malnutrition, growth retardation, invasive illnesses, and death.26,27 According to Carlsten and Jackson,28 immigrants can be infected by multiple pathogens simultaneously, and some parasites may survive for as long as decades.
The most common parasitic infections among refugees are hookworm, whipworm, roundworm, and Giardia lamblia.23 In a screening performed five years after the arrival in the US of the “Lost Boys and Girls of Sudan,” 64% of a cohort of the boys living in Atlanta tested positive for Schistosoma mansoni or Schistosoma haematobium, and 25% tested positive for Strongyloides stercoralis—the organisms responsible for schistosomiasis and strongyloidiasis, respectively.26 In 2005, the CDC recommended presumptive treatment for both illnesses in Sudanese refugees who were not treated for these infections before their resettlement.29
Despite treatment and prophylaxis prior to refugees’ departure for the US, parasitic infections remain common in the refugee population.23 Practitioners should be aware that a cough could indicate the presence of roundworms that have entered the body through the skin and spread to the lungs via the blood.30,31 All refugees should be screened for eosinophilia to detect parasitic infections.23 An absolute eosinophil count exceeding 400 cells/L warrants further investigation.27
Because of presumptive treatment for malaria given to nonpregnant refugees, this disease is rarely seen after refugees’ arrival in the US.23 However, practitioners should not overlook the possibility of malaria when they examine refugee patients, as malaria may take time to manifest clinically.24
Tuberculosis
While refugees cannot be admitted into the US with active, infectious TB (a Class A disease), the majority of cases of TB in the US occur among foreign-born individuals, with prevalence 10 times that in the US-born population.32 Refugees are at particular risk for TB.33
When examining refugee patients, especially those recently arrived in the US, clinicians should be aware of the potential for extrapulmonary TB, which accounted for 20% of TB cases in the US in 2008.32 Extrapulmonary TB can be found anywhere in the body, with more common sites including the lymph nodes, pleura, and osteoarticular areas. Skeletal TB accounts for 35% of extrapulmonary TB cases—most commonly Pott’s disease, or spinal TB.34
Use of bacille Calmette-Guerin (BCG), a vaccine given in various countries to prevent childhood tuberculous meningitis and miliary disease, often leads to confusion when the tuberculin skin test (TST, previously known as the purified protein derivative, or PPD) is used to screen for TB.35 While BCG can increase the number of false-positive TST results, TST reaction following BCG decreases with time and generally is not seen longer than 10 years postvaccination.36
Furthermore, the immunity produced by BCG weakens over time; thus, an adult, though immunized as an infant, is at risk for TB infection. The CDC currently recommends the same testing for TB, whether or not patients have undergone BCG vaccination. Similarly, TST results should be interpreted in the same way for BCG-vaccinated patients and nonvaccinated patients alike.35
Finally, BCG does not affect results of blood tests for TB. However, these tests are new, expensive, and not available everywhere.35
Hepatitis
Among the forms of hepatitis, hepatitis B virus (HBV) is of greatest concern within the refugee community, as it is endemic to much of the world.37 Between 2003 and 2007, 10.7% of refugees screened in DeKalb County, Georgia, for HBsAg (the hepatitis B surface antigen that indicates exposure to the virus) tested positive, accounting for 43.3% of HBsAg-positive test results in the county during that period. Chronic HBV infection can lead to end-stage liver disease, cirrhosis, and hepatocellular carcinoma.37
Museru et al37 recommend that health care providers ascertain the hepatitis B–serological status of resettled refugees from areas that are highly endemic for HBV infection. In addition, Adams and colleagues23 recommend screening patients who have undergone blood transfusions, female genital surgery, or other surgical procedures in their countries of origin, as well as patients from Africa or southeast Asia, for hepatitis C.23
Mutilation or Cutting of the Female Genitalia
Ritual FGM/C is the practice of injuring or removing part or all of the external female genitalia for cultural and other nonmedical reasons.38 FGM/C is primarily practiced in Africa (see Figure,39,40) but may occur also in Asia, the Middle East, and Central and South America.38 It is often practiced by informally trained individuals, with “inexact surgical outcomes.”23 FGM/C has been outlawed in the US and other countries with large immigrant populations; some nations grant asylum to women who fear being subjected to FGM/C if they return to their country of origin.39
Practitioners who care for female refugees should be aware of both the short-term sequelae (pain, bleeding, trauma, sepsis) and additional long-term sequelae (dyspareunia, urinary retention and recurrent urinary tract infections, chronic pelvic inflammatory disease, keloid scar formation, childbirth complications) of FGM/C, in addition to psychological sequelae.23,38,41 It is important to approach affected patients with sympathy and without judgment, as the decision to undergo FGM/C may not have been theirs.41
The Royal College of Obstetricians and Gynaecologists in the United Kingdom has produced a helpful set of guidelines, Female Genital Mutilation and its Management,41 for clinicians working with patients who have undergone FGM/C.
Sexually Transmitted Infections
In light of a new law allowing refugees with HIV to be resettled in the US,42 practitioners must now be aware of the possibility of HIV infection in a refugee patient, whether documented or not. Practitioners should follow the same guidelines for refugees as they do for all patients regarding HIV screening and counseling, including allowing patients the opportunity to decline testing.43 However, they should also be aware of countries in which HIV prevalence rates are high.43
Additionally, while HIV-1 is the world’s predominant strain of the disease, refugees from West African countries have been at increased risk for exposure to HIV-2 and should be tested accordingly.43,44 Refugees may also be at increased risk for HIV and other sexually transmitted infections attributable to physical or sexual violence.43,45
All screening for HIV and other sexually transmitted infections should be performed in a culturally appropriate manner, with the use of trained interpreters as needed, to ensure that all patients receive accurate information and counseling.43
Exposure to Lead
Refugee children are at high risk for lead exposure both before and after their arrival in the US—the latter as a result of their families’ living conditions after resettlement, despite the ban on lead-based paint.25 A study in Minnesota from 2000 to 2002 showed that among refugee children younger than 6, prevalence of lead poisoning was 14 times that found in American children in their age-group.25 In New York City, Asian children have been shown to be at particular risk for lead poisoning—including the case of a year-old Cambodian child who was evidently exposed to an amulet with leaded beads. Sources of lead other than paint may include imported food, spices, cosmetics, pottery, and health remedies.46
Where children were born and where they have lived throughout their lives appear to be the greatest predictors of lead poisoning risk.25 One primary risk factor for lead poisoning is malnutrition, associated with increased absorption of lead in the intestines and the resulting micronutrient deficiencies.25
The CDC recommends:
• Screening for lead in all children from age 6 months to 16 years at the time of their arrival in the US
• Follow-up blood lead testing of children ages 6 months to 6 years, 3 to 6 months after they have moved into a permanent residence25
• Nutritional assessments for children younger than 6 years, as well as measurement of hemoglobin/hematocrit levels, including at least one of the following measurements: mean corpuscular volume with red-cell distribution width, ferritin, transferrin saturation, or reticulocyte hemoglobin content
• Daily multivitamins with iron for refugee children ages 6 months to approximately 5 years.
Lead poisoning, as indicated by a blood lead level (BLL) exceeding 10 g/dL, is known to have neurodevelopmental and cognitive sequelae. In children with a significantly higher BLL, manifestations may include headaches, abdominal pain, anorexia, constipation, clumsiness, agitation, and lethargy in the acute phase.25
Woman, 48, from Afghanistan
Through an interpreter, you learn that this visitor to a local health fair has been experiencing left arm pain since she and her family fled Kabul. When her house there was hit by a rocket, she ran back in, despite a fire, to save her infant daughter. Although she received care as a refugee in Pakistan for burns to her arms and body, she has scarring and strictures the length of her arm. Thanks to the efforts of a volunteer physical therapist, use of the woman’s injured arm has been preserved. She is diagnosed with posttraumatic stress syndrome and referred to a local mental health clinic whose staff specializes in working with survivors of trauma and torture.
Mental Health Issues
Mental health issues are a significant component of refugee health. MacDuff et al47 report that 36% of complementary and alternative medicine use among refugees targets mental health issues resulting from trauma. Because refugees were forced by dangerous conditions to flee their home countries, they are particularly susceptible to mental health concerns. They may have witnessed violence, undergone torture, or been subjected to unsafe or unsanitary conditions in refugee camps. Many have had trouble adjusting to their new culture.23,24,28
As a result, refugees are at increased risk for depression, posttraumatic stress disorder, substance abuse, somatization, psychosis, and suicide.23,28 Mental health issues among refugees are also complicated by the cultural and communication barriers that often exist between refugees and practitioners.23 Thus, practitioners need to take careful histories with sensitivity to their patients’ previous experiences.
While the acuity of these issues begins to decrease around three years after refugees’ arrival in their country of resettlement, the burden of mental health problems often persists for many years.28 Adams et al23 recommend that refugees be referred to social workers, cultural case mediators, and community organizations. Clinicians who do not feel comfortable managing mental health conditions should refer refugee patients to appropriate mental health practitioners and follow up to make sure that patients’ needs are being met.
Musculoskeletal and Neurologic Injuries
Traumatic events can lead to a wide variety of musculoskeletal and neurologic injuries—for example, wounds inflicted by weapons, amputations following land mine injuries, crush injuries from collapsing buildings, or burns sustained in rocket attacks. The array of possibilities necessitates a thorough history and complete musculoskeletal and neurologic assessments.
LANGUAGE AND CULTURAL ISSUES
In addition to being aware of the potential health issues that arise within the refugee population, primary care providers need to be prepared to confront language and cultural issues that may arise. The National Standards on Culturally and Linguistically Appropriate Services (CLAS)48 offer appropriate guidance in 14 key areas.
Refugees frequently have limited or no working knowledge of English. Whenever possible, practitioners should use medically trained interpreters to help them receive and convey accurate information and thus provide comprehensive care. When professional interpreters are not available, telephone interpreter services are available for purchase by the facility or practice. Children or other family members should not be relied on for accurate interpretation.48
As for cultural differences, it is important to note that a refugee’s concept of family may differ from that found in Western culture.49 For example, it is not uncommon to find extended families living together, with members referring to nieces and nephews as their children, or aunts and uncles as their parents. A thorough exploration of the relationships among patients and their families is important, particularly during the family history.
Regardless of where resettled refugees come from, it is probable that their cultural and personal beliefs about medicine differ from those of practitioners with years of Western medicine training. In addition to implementing CLAS guidelines,48 practitioners should be familiar with Kleinman’s3explanatory model, which explores the differences between the patient and practitioner models—not necessarily differences in levels of knowledge, but rather of “values and interests.”3 Thus, people unfamiliar with or resistant to the Western model of medicine are often seen as ignorant, whereas an issue of values may be at play.
Identifying the differences between the patient’s and the clinician’s explanatory models allows the clinician to anticipate and address potential misunderstandings, understand patients’ perceived needs, and involve patients in management strategies that will motivate them to comply with treatment. To help clinicians assess the explanatory models of their patients, Kleinman provides eight questions (shown in Table 3,3,50).
CONCLUSION
As the world becomes more of a global village, increasing numbers of primary care providers will see refugee patients. Practitioners need to be aware of the physical, socioeconomic, and psychological issues that affect refugees during and after resettlement in the US. Refugees may have conditions that could not be addressed in their home country or refugee camp. They may have illnesses with which US practitioners are ordinarily unfamiliar, poorly treated or untreated traumatic injuries, or psychological trauma resulting from conditions that forced them to flee their native lands.
Clinicians who work with refugees should be familiar with the resettlement process and perform a thorough examination after the refugee’s resettlement, managing previously unaddressed health issues. Complete histories must be taken and physical examinations performed in a culturally appropriate manner and an atmosphere of mutual trust.
Finally, it is vital for providers to explore the explanatory models from which patients view their illnesses. Such an understanding facilitates culturally appropriate care with patient participation, and ultimately more positive clinical outcomes.
Authors’ note: The patients portrayed in this article are all composites. While the issues described are real, these “patients” were developed from multiple cases to protect individual patient privacy. None is real, and any resemblance to any real persons is purely accidental.
REFERENCES
1. Ramos M, Orozovich P, Moser K, et al. Health of resettled Iraqi refugees: San Diego County, California, October 2007–September 2009. MMWR Morb Mortal Wkly Rep. 2010;59(49):1614-1618.
2. US Department of State. FY 2010 cumulative summary of refugee admissions. www.wrapsnet .org/reports/archives/tabid/215/language/en-us/default.aspx. Accessed February 17, 2011.
3. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88(2):251-258.
4. US Citizenship and Immigration Services. Refugees and asylum. www.uscis.gov/portal/site/uscis/menuitem.eb1d4c2a3e5b9ac89243c6a7543f6d1a/?vgnextoid=1f1c3e4d77d73210VgnVCM100000082ca60aRCRD&vgnextchannel=1f1c3e4d77d73210VgnVCM100000082ca60aRCRD. Accessed February 17, 2011.
5. United Nations High Commissioner for Refugees. Refugees: flowing across borders. www .unhcr.org/pages/49c3646c125.html. Accessed February 17, 2011.
6. US Citizenship and Immigration Services. The United States Refugee Admissions Program (USRAP) consultation and worldwide processing priorities. www.uscis.gov/portal/site/uscis/menuitem.5af9bb95919f35e66f614176543f6d1a/?vgnextoid=796b0eb389683210VgnVCM100000082ca60aRCRD&vgnextchannel=385d3e4d77d73210VgnVCM100000082ca60aRCRD. Accessed February 17, 2011.
7. US Committee for Refugees and Immigrants. Frequently asked questions. www.refugees.org/about-us/faqs.html. Accessed February 17, 2011.
8. US Department of State. Refugee Admissions Reception and Placement Program. www.state
.gov/g/prm/rls/125478.htm. Accessed February 17, 2011.
9. Office of Refugee Resettlement. Report to Congress: FY 2007. www.acf.hhs.gov/programs/orr/data/ORR_2007_report.pdf. Accessed February 17, 2011.
10. CDC. Medical examination: frequently asked questions (FAQs). www.cdc.gov/immigrant
refugeehealth/exams/medical-examination-faqs .html#9. Accessed February 17, 2011.
11. US Department of State. Medical Examination for Immigrant or Refugee Applicant (DS-2053, OMB No. 1405-0113, expiration date 04/30/2012. http://bangkok.usembassy.gov/root/pdfs/med forms_043012.pdf. Accessed February 17, 2011.
12. Cohen J, Powderly WG, Opal SM, eds. Infectious Diseases. 3rd ed. Philadelphia, PA: Elsevier; 2010.
13. CDC. Domestic Refugee Health Program: Frequently Asked Questions (2010). www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/domestic-refugee-questions.html. Accessed February 17, 2011.
14. CDC. Technical Instructions: guidance for HIV for panel physicians and civil surgeons (2010). www.cdc.gov/immigrantrefugeehealth/exams/ti/hiv-guidance-panel-civil.html. Accessed February 17, 2011.
15. CDC. Medical history and physical examination: technical instructions for medical examination of aliens. www.cdc.gov/immigrantrefugee health/exams/ti/panel/technical-instructions/panel-physicians/medical-history-physical-exam .html. Accessed February 17, 2011.
16. Lee JL, Naguwa SM, Cheema GS, Gershwin ME. Acute rheumatic fever and its consequences: a persistent threat to developing nations in the 21st century. Autoimmun Rev. 2009;9(2):117-123.
17. Henderson DN. Pregnancy complicated by rheumatic heart disease. Can Med Assoc J. 1936; 35(4):394-398.
18. US Citizenship and Immigration Services. Refugees. www.uscis.gov/portal/site/uscis/menu item.eb1d4c2a3e5b9ac89243c6a7543f6d1a/?vgnextoid=385d3e4d77d73210VgnVCM100000082ca60aRCRD&vgnextchannel=385d3e4d77d73210VgnVCM100000082ca60aRCRD. Accessed February 17, 2011.
19. US Department of State. 9 FAM appendix O, exhibit II: Promissory note (2007). In: Foreign Affairs Manual Volume 9: Visas. www.state.gov/documents/organization/88070.pdf. Accessed February 17, 2011.
20. United States Department of State. US Refugee Admissions Program. www.state.gov/g/prm/c26471.htm. Accessed February 17, 2011.
21. J. Kernan, Community Relations Officer, US Citizenship and Immigration Services, personal communication, March 29, 2010.
22. Office of Refugee Resettlement. Health. www.acf.hhs.gov/programs/orr/benefits/health .htm. Accessed February 17, 2011.
23. Adams KM, Gardiner LD, Assefi N. Healthcare challenges from the developing world: post-immigration refugee medicine. BMJ. 2004;328(7455): 1548-1552.
24. Walker PF, Jaranson J. Refugee and immigrant health care. Med Clin North Am. 1999; 83(4):1103-1120.
25. CDC. Screening for lead at the domestic refugee medical examination (2005). www.cdc.gov/immigrantrefugeehealth/pdf/lead.pdf. Accessed February 17, 2011.
26. Franco-Paredes C, Dismukes R, Nicolls D, et al. Short report: persistent and untreated tropical infectious diseases among Sudanese refugees in the United States. Am J Trop Med Hyg. 2007;77 (4):633-635.
27. CDC. Immigrant and Refugee Health: Domestic Refugee Health Guidelines: Intestinal Parasites. www.cdc.gov/immigrantrefugeehealth/guidelines/ip/intestinal-parasites-domestic.html#asympto matic2. Accessed February 17, 2011.
28. Carlsten C, Jackson C. Refugee and immigrant health care. EthnoMed. http://ethnomed.org/clinical/refugee-health/carlsten.pdf. Accessed February 17, 2011.
29. Conly JM, Johnston BL. The infectious diseases implications of the “Lost Boys and Girls of Sudan.” Can J Infect Dis Med Microbiol. 2008;19 (3):215-216.
30. Aggarwal B, Sharma M, Singh T. Acute eosinophilic pneumonia due to round worm infestation. Indian J Pediatr. 2008;75(3):296-297.
31. Tsai HC, Lee SS, Liu YC, et al. Clinical manifestations of strongyloidiasis in southern Taiwan.
J Microbiol Immunol Infect. 2002;35(1):29-36.
32. CDC. Reported tuberculosis in the United States, 2008. www.cdc.gov/tb/statistics/reports/2008/pdf/2008report.pdf. Accessed February 17, 2011.
33. Oeltmann JE, Varma JK, Ortega L, et al. Multidrug-resistant tuberculosis outbreak among US-bound Hmong refugees, Thailand, 2005. Emerg Infect Dis. 2008;14(11):1715-1721.
34. Golden MP, Vikram H. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005; 72(9):1761-1768.
35. CDC. Tuberculosis (TB) Fact Sheets: BCG Vaccine. www.cdc.gov/tb/publications/factsheets/prevention/BCG.htm. Accessed February 17, 2011.
36. The role of BCG vaccine in the prevention and control of tuberculosis in the United States: a joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45(RR-4):1-18.
37. Museru OI, Vargas M, Kinyua M, et al. Hepatitis B virus infection among refugees resettled in the US: high prevalence and challenges in access to health care. J Immigr Minor Health. 2010;12 (6):823-827.
38. World Health Organization, UNAIDS, UNDP, UNECA, UNESO, UNFPA, UNHCHR, UNHCR, UNICEF, UNIFEM. Eliminating Female Genital Mutilation: An Interagency Statement (2008). http://whqlibdoc.who.int/publications/2008/ 9789241596442_eng.pdf. Accessed February 17, 2010.
39. United Nations Children’s Fund. Female Genital Mutilation/Cutting: A Statistical Exploration (2005). www.unicef.org/publications/files/FGM-C_final_10_October.pdf. Accessed February 17, 2011.
40. Yoder PS, Abderrahim N, Zhuzhuni A. DHS Comparative Reports No. 7: Female Genital Cutting in the Demographic and Health Surveys: A Critical and Comparative Analysis. Calverton, MD: ORC Macro. September 2004.
41. Royal College of Obstetricians and Gynaecologists. Female Genital Mutilation and its Management (Green-top Guideline No. 53; 2009). www .rcog.org.uk/files/rcog-corp/GreenTop53Female GenitalMutilation.pdf. Accessed February 17, 2011.
42. CDC. Final Rule Removing HIV Infection from U.S. Immigration Screening: Revision of 42 CFR Part 34 (Medical Examination of Aliens) Removal of Human Immunodeficiency Virus (HIV) from Definition of Communicable Disease of Public Health Significance—Final Rule. www.cdc.gov/immigrantrefugeehealth/laws-regs/hiv-ban-removal/final-rule.html. Accessed February 17, 2011.
43. CDC. Immigrant and Refugee Health: Screening for HIV-infection during the refugee domestic medical examination. www.cdc.gov/immigrant
refugeehealth/guidelines/domestic/screening-
hiv-infection-domestic.html. Accessed February 17, 2011.
44. CDC. Human Immunodeficiency Virus Type 2: HIV/AIDS Fact Sheets. Atlanta: Centers for Disease Control and Prevention; 2007.
45. Mills EJ, Nachega JB. HIV infection as a weapon of war. Lancet Infect Dis. 2006;6(12):752-753.
46. CDC. Lead poisoning of a child associated with use of a Cambodian amulet—New York City, 2009. MMWR Morb Mortal Wkly Rep. 2011;60(3): 69-71.
47. MacDuff S, Grodin MA, Gardiner P. The use of complementary and alternative medicine among refugees: a systematic review. J Immigr Minor Health. 2010 Mar 12 [Epub ahead of print].
48. US Department of Health and Human Services, Office of Minority Health. National standards on culturally and linguistically appropriate services (CLAS). http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15. Accessed February 17, 2011.
49. Haviland WA, Prins HEL, Walrath D, McBride B, eds. Cultural Anthropology: The Human Challenge. 12th ed. Belmont, CA: Wadsworth Publishing; 2008.
50. Fadiman A. The Spirit Catches You and You Fall Down: A Hmong Child, Her American Doctors, and the Collision of Two Cultures. New York, NY: Farrar, Straus and Giroux; 1997.
It is estimated that three million refugees from all over the world—forced to flee their native countries for various reasons—have entered the United States since 1980.1,2 Prior to resettling in the US, refugees undergo health screenings for high-risk infectious diseases that preclude emigration; those free of such diseases may enter. However, the civil surgeon who conducts a refugee’s medical examination does not screen for chronic diseases that are not considered a threat to public health. Other infectious illnesses, previous traumatic injuries, and mental health issues may also go undetected at this exam.
Refugees may have had little or no access to health care before their arrival in the US, lived in conditions that increased their risk for exposure to various illnesses, and experienced traumatic events before fleeing their native lands. After their arrival, refugees may face access issues, including language and cultural barriers, health care ineligibility, and lack of transportation. This article seeks to increase awareness among primary care practitioners of the needs and issues of refugees who may be seen in their practices for conditions that developed before their arrival in the US and others emerging since their resettlement.
This activity will begin with an overview of who refugees are, how they come to reside in the US, and the medical process they undergo before resettlement. Next follows a discussion of medical issues that practitioners should be aware of among refugees, including conditions not commonly seen in the US. Finally, language and cultural issues will be addressed, including an explanatory model3 to help bridge discrepancies between practitioners of Western medicine and patients of non-Western traditions.
Man, 58, from Burundi
At age 55, the patient was resettled to the US with his family. Since then, he has had trouble holding a job, and his difficulties have been attributed to the stress of transition to life in the US. His current employer has sent him for an occupational examination. Findings are within normal limits except for visual acuity, which is tested at 20/25 in his right eye and 20/200 in his left. When asked, the patient reports having had “river blindness,” that is, onchocerciasis, as a child. Onchocerciasis is an uncommon cause of permanent blindness.
BACKGROUND AND DEFINITIONS
Refugees are defined by US Citizenship and Immigration Services4 as “people who have been persecuted or fear they will be persecuted on account of race, religion, nationality, and/or membership in a particular social group or political opinion.” The United Nations High Commission for Refugees (UNHCR)5 adds that a refugee “is outside the country of his nationality, and is unable to, or owing to such fear, is unwilling to, avail himself of the protection of that country.”
The man from Burundi came to the US through a long, complicated process. His family fled genocide in their native country to a refugee camp in Tanzania. Once there, they attained official refugee status, an essential part of the resettlement process. Refugees must fall into one of three processing priority categories:
(1) those referred by UNHCR, a US embassy, or a designated voluntary agency
(2) persons designated by a US refugee program as belonging to a “special humanitarian concern” group
(3) certain family members of refugees who currently reside in the US.6
The application generally involves biographical information and a family tree.7 Because they had fled their home, the family from Burundi had limited paperwork but were referred by UNHCR to the US for resettlement.
Once refugees become eligible for resettlement in the US, they undergo medical screening, security clearance, and cultural orientation. They are then placed by one of the sponsoring resettlement agencies listed in Table 1.8 This process can take from two months to several years.9 The medical screening may be performed by a panel physician, an overseas practitioner who examines refugees prior to their resettlement; or by a civil surgeon, who examines refugees after their arrival in the US—generally when the refugee applies for status adjustment.10
The man from Burundi represents a relatively common issue among refugees in the US. Many chronic conditions go untreated within this population because follow-up may be inadequate or absent, patients’ access to health care is insufficient, or the health care provider is unfamiliar with refugee issues. In this case, evaluation of the man’s vision was not part of the routine examination conducted in all refugees. Because he was never offered a subsequent vision check, his blindness went unnoticed, and his work difficulties were attributed to language and adjustment issues.
His visual problem could not be corrected, but once it was identified, accommodations were made in the workplace that facilitated his adjustment to the new work environment.
Girl, 16, from Liberia
This patient, who is being seen in your office for a sports physical, arrived in the US three years ago. Her medical screening at the time of immigration indicated “good health,” and she has had no health problems since then. Her exam seems unremarkable except for a low-pitched, rumbling, diastolic murmur best heard with the bell of the stethoscope near the apex when she lies in the left lateral decubitus position.
HEALTH SCREENINGS
Before coming to the US, refugees must undergo a health evaluation that includes a thorough medical history, full physical examination, chest x-ray, if indicated, for tuberculosis (TB), vaccination verification, and laboratory work as needed to identify specific infectious diseases.11 Screenings included in this evaluation target problems that are considered important from a public health perspective, including TB and certain sexually transmitted illnesses. Many infectious diseases are not considered a threat to public health because the requisite vector is not present in the US (eg, malaria, schistosomiasis).12
Public health conditions are categorized as Class A or Class B (see Table 2,11,13). Typically, refugees with a Class A condition are considered ineligible for admission to the US. Presence of a Class B condition must be brought to the attention of consular authorities, as it may indicate future disability or need for medical treatment.13,14
It is important for practitioners to know that if an illness or medical condition is discovered during the panel physician’s examination but is not considered “relevant to the visa medical examination,” the panel physician does not treat the patient. Rather, he or she recommends that the patient seek care from a medical provider.15 Essentially, refugees with a condition that is not considered a public health threat are allowed into the US, whether or not the condition has been addressed—as in the case of the diastolic heart murmur in the girl from Liberia.
This heart murmur could be a complication of rheumatic fever secondary to untreated group A streptococcal infection. In developing nations, rheumatic heart disease is the most commonly acquired heart condition, frequently exceeding congenital heart disease as a cause of hospitalization among children, adolescents, and teens.16 The risks associated with pregnancy in women with rheumatic heart disease have long been known,17 but as rheumatic heart disease has become less common in the developed nations, so has the sequela of mitral valve stenosis.
Clinicians need to become familiar with other illnesses commonly found in the developing nations, such as malaria and measles.12 Furthermore, practitioners should be aware that refugees may be immunologically susceptible to pathogens endemic to the countries of resettlement.
Woman, 65, from Bhutan
Forcibly deported from Bhutan to Nepal, this patient resettled to the US four years ago. She neither reads nor writes in her first language (Nepali) nor in English. She has been attending English language classes since her arrival in the US but has made little progress. The woman visits a health fair, bringing with her nine medications, including two bottles of insulin and three empty bottles for three different antihypertensive medications. Through an interpreter, you learn that the woman is not taking any of these medications regularly. She is also unaware that she should neither reuse needles nor share her medications with her son, who is also diabetic. She cannot afford to refill her prescriptions.
THE PROCESS, THE BARRIERS
Upon their arrival in the US, refugees receive 30 to 90 days’ support for “housing, essential furnishings, food, clothing, community orientation, and referral to other social, medical, and employment services.”8 In addition to the predeparture medical screening, refugees receive assistance for travel arrangements and a loan to pay for travel to the US.18 Refugees must sign a promissory note indicating that they will begin repayment of the loan within six months of their arrival and will complete repayment within 42months.19
At the time of their arrival, refugees apply for a Social Security number, register their children for school, undergo another medical evaluation, and receive English language training, if needed.8 Within six months of their arrival, refugees of working age are expected to have obtained employment.7 After one year in the US, refugees must apply for Legal Permanent Resident Status; after five years, they can apply for citizenship.8,20,21
In the case of the woman from Bhutan, neither diabetes nor hypertension precluded her resettlement; however, several factors now complicate her health care. Although she has access to health care providers, she cannot afford copayments for her medications, nor does she understand the instructions for their use. That she is not literate in any language complicates the challenge for her to learn to speak English. Her lack of linguistic and health literacy adds to the financial burden of the medications she needs.
The use of formally trained medical interpreters can alleviate some, but not all, of these problems. The refugee who does not become a citizen within seven years risks losing all benefits, including Medicare and Medicaid. This is particularly problematic for older refugees with multiple health problems. The process of becoming a US citizen is arduous, including lessons in civics and a required level of language fluency that is difficult to attain, particularly for those who are not literate in any language.
The Office of Refugee Resettlement22 recommends that refugees undergo a second medical screening after their arrival to identify any conditions that were not addressed by the panel physician. This examination, according to the CDC, “provides an opportunity to identify important causes of morbidity among resettled refugees that might not have been discovered previously, and enables early referral for treatment and follow-up care.”1 This screening also offers refugees the chance to establish a medical home and to begin to become familiar with the US health care system, potential barriers notwithstanding.
Girl, 15, from Sudan
A cough is the presenting symptom in this girl, who resettled to the US three years ago. She has no other upper respiratory symptoms. Results of laboratory testing indicate eosinophilia and mild anemia.
COMMON HEALTH ISSUES
Health conditions that refugees face but that may not be found in other immigrants or nonimmigrants include TB, hepatitis, parasites, HIV and other sexually transmitted infections, and mental health issues.23,24 For some refugees, female genital mutilation/cutting (FGM/C)23 or lead exposure25 may be a significant concern. Additionally, the traumatic events that led refugees to flee their homes may have resulted in musculoskeletal or neurologic injuries with a wide array of manifestations.
Parasites
Parasitic infections are common among refugees, and these can lead to anemia resulting from blood loss and iron deficiency, malnutrition, growth retardation, invasive illnesses, and death.26,27 According to Carlsten and Jackson,28 immigrants can be infected by multiple pathogens simultaneously, and some parasites may survive for as long as decades.
The most common parasitic infections among refugees are hookworm, whipworm, roundworm, and Giardia lamblia.23 In a screening performed five years after the arrival in the US of the “Lost Boys and Girls of Sudan,” 64% of a cohort of the boys living in Atlanta tested positive for Schistosoma mansoni or Schistosoma haematobium, and 25% tested positive for Strongyloides stercoralis—the organisms responsible for schistosomiasis and strongyloidiasis, respectively.26 In 2005, the CDC recommended presumptive treatment for both illnesses in Sudanese refugees who were not treated for these infections before their resettlement.29
Despite treatment and prophylaxis prior to refugees’ departure for the US, parasitic infections remain common in the refugee population.23 Practitioners should be aware that a cough could indicate the presence of roundworms that have entered the body through the skin and spread to the lungs via the blood.30,31 All refugees should be screened for eosinophilia to detect parasitic infections.23 An absolute eosinophil count exceeding 400 cells/L warrants further investigation.27
Because of presumptive treatment for malaria given to nonpregnant refugees, this disease is rarely seen after refugees’ arrival in the US.23 However, practitioners should not overlook the possibility of malaria when they examine refugee patients, as malaria may take time to manifest clinically.24
Tuberculosis
While refugees cannot be admitted into the US with active, infectious TB (a Class A disease), the majority of cases of TB in the US occur among foreign-born individuals, with prevalence 10 times that in the US-born population.32 Refugees are at particular risk for TB.33
When examining refugee patients, especially those recently arrived in the US, clinicians should be aware of the potential for extrapulmonary TB, which accounted for 20% of TB cases in the US in 2008.32 Extrapulmonary TB can be found anywhere in the body, with more common sites including the lymph nodes, pleura, and osteoarticular areas. Skeletal TB accounts for 35% of extrapulmonary TB cases—most commonly Pott’s disease, or spinal TB.34
Use of bacille Calmette-Guerin (BCG), a vaccine given in various countries to prevent childhood tuberculous meningitis and miliary disease, often leads to confusion when the tuberculin skin test (TST, previously known as the purified protein derivative, or PPD) is used to screen for TB.35 While BCG can increase the number of false-positive TST results, TST reaction following BCG decreases with time and generally is not seen longer than 10 years postvaccination.36
Furthermore, the immunity produced by BCG weakens over time; thus, an adult, though immunized as an infant, is at risk for TB infection. The CDC currently recommends the same testing for TB, whether or not patients have undergone BCG vaccination. Similarly, TST results should be interpreted in the same way for BCG-vaccinated patients and nonvaccinated patients alike.35
Finally, BCG does not affect results of blood tests for TB. However, these tests are new, expensive, and not available everywhere.35
Hepatitis
Among the forms of hepatitis, hepatitis B virus (HBV) is of greatest concern within the refugee community, as it is endemic to much of the world.37 Between 2003 and 2007, 10.7% of refugees screened in DeKalb County, Georgia, for HBsAg (the hepatitis B surface antigen that indicates exposure to the virus) tested positive, accounting for 43.3% of HBsAg-positive test results in the county during that period. Chronic HBV infection can lead to end-stage liver disease, cirrhosis, and hepatocellular carcinoma.37
Museru et al37 recommend that health care providers ascertain the hepatitis B–serological status of resettled refugees from areas that are highly endemic for HBV infection. In addition, Adams and colleagues23 recommend screening patients who have undergone blood transfusions, female genital surgery, or other surgical procedures in their countries of origin, as well as patients from Africa or southeast Asia, for hepatitis C.23
Mutilation or Cutting of the Female Genitalia
Ritual FGM/C is the practice of injuring or removing part or all of the external female genitalia for cultural and other nonmedical reasons.38 FGM/C is primarily practiced in Africa (see Figure,39,40) but may occur also in Asia, the Middle East, and Central and South America.38 It is often practiced by informally trained individuals, with “inexact surgical outcomes.”23 FGM/C has been outlawed in the US and other countries with large immigrant populations; some nations grant asylum to women who fear being subjected to FGM/C if they return to their country of origin.39
Practitioners who care for female refugees should be aware of both the short-term sequelae (pain, bleeding, trauma, sepsis) and additional long-term sequelae (dyspareunia, urinary retention and recurrent urinary tract infections, chronic pelvic inflammatory disease, keloid scar formation, childbirth complications) of FGM/C, in addition to psychological sequelae.23,38,41 It is important to approach affected patients with sympathy and without judgment, as the decision to undergo FGM/C may not have been theirs.41
The Royal College of Obstetricians and Gynaecologists in the United Kingdom has produced a helpful set of guidelines, Female Genital Mutilation and its Management,41 for clinicians working with patients who have undergone FGM/C.
Sexually Transmitted Infections
In light of a new law allowing refugees with HIV to be resettled in the US,42 practitioners must now be aware of the possibility of HIV infection in a refugee patient, whether documented or not. Practitioners should follow the same guidelines for refugees as they do for all patients regarding HIV screening and counseling, including allowing patients the opportunity to decline testing.43 However, they should also be aware of countries in which HIV prevalence rates are high.43
Additionally, while HIV-1 is the world’s predominant strain of the disease, refugees from West African countries have been at increased risk for exposure to HIV-2 and should be tested accordingly.43,44 Refugees may also be at increased risk for HIV and other sexually transmitted infections attributable to physical or sexual violence.43,45
All screening for HIV and other sexually transmitted infections should be performed in a culturally appropriate manner, with the use of trained interpreters as needed, to ensure that all patients receive accurate information and counseling.43
Exposure to Lead
Refugee children are at high risk for lead exposure both before and after their arrival in the US—the latter as a result of their families’ living conditions after resettlement, despite the ban on lead-based paint.25 A study in Minnesota from 2000 to 2002 showed that among refugee children younger than 6, prevalence of lead poisoning was 14 times that found in American children in their age-group.25 In New York City, Asian children have been shown to be at particular risk for lead poisoning—including the case of a year-old Cambodian child who was evidently exposed to an amulet with leaded beads. Sources of lead other than paint may include imported food, spices, cosmetics, pottery, and health remedies.46
Where children were born and where they have lived throughout their lives appear to be the greatest predictors of lead poisoning risk.25 One primary risk factor for lead poisoning is malnutrition, associated with increased absorption of lead in the intestines and the resulting micronutrient deficiencies.25
The CDC recommends:
• Screening for lead in all children from age 6 months to 16 years at the time of their arrival in the US
• Follow-up blood lead testing of children ages 6 months to 6 years, 3 to 6 months after they have moved into a permanent residence25
• Nutritional assessments for children younger than 6 years, as well as measurement of hemoglobin/hematocrit levels, including at least one of the following measurements: mean corpuscular volume with red-cell distribution width, ferritin, transferrin saturation, or reticulocyte hemoglobin content
• Daily multivitamins with iron for refugee children ages 6 months to approximately 5 years.
Lead poisoning, as indicated by a blood lead level (BLL) exceeding 10 g/dL, is known to have neurodevelopmental and cognitive sequelae. In children with a significantly higher BLL, manifestations may include headaches, abdominal pain, anorexia, constipation, clumsiness, agitation, and lethargy in the acute phase.25
Woman, 48, from Afghanistan
Through an interpreter, you learn that this visitor to a local health fair has been experiencing left arm pain since she and her family fled Kabul. When her house there was hit by a rocket, she ran back in, despite a fire, to save her infant daughter. Although she received care as a refugee in Pakistan for burns to her arms and body, she has scarring and strictures the length of her arm. Thanks to the efforts of a volunteer physical therapist, use of the woman’s injured arm has been preserved. She is diagnosed with posttraumatic stress syndrome and referred to a local mental health clinic whose staff specializes in working with survivors of trauma and torture.
Mental Health Issues
Mental health issues are a significant component of refugee health. MacDuff et al47 report that 36% of complementary and alternative medicine use among refugees targets mental health issues resulting from trauma. Because refugees were forced by dangerous conditions to flee their home countries, they are particularly susceptible to mental health concerns. They may have witnessed violence, undergone torture, or been subjected to unsafe or unsanitary conditions in refugee camps. Many have had trouble adjusting to their new culture.23,24,28
As a result, refugees are at increased risk for depression, posttraumatic stress disorder, substance abuse, somatization, psychosis, and suicide.23,28 Mental health issues among refugees are also complicated by the cultural and communication barriers that often exist between refugees and practitioners.23 Thus, practitioners need to take careful histories with sensitivity to their patients’ previous experiences.
While the acuity of these issues begins to decrease around three years after refugees’ arrival in their country of resettlement, the burden of mental health problems often persists for many years.28 Adams et al23 recommend that refugees be referred to social workers, cultural case mediators, and community organizations. Clinicians who do not feel comfortable managing mental health conditions should refer refugee patients to appropriate mental health practitioners and follow up to make sure that patients’ needs are being met.
Musculoskeletal and Neurologic Injuries
Traumatic events can lead to a wide variety of musculoskeletal and neurologic injuries—for example, wounds inflicted by weapons, amputations following land mine injuries, crush injuries from collapsing buildings, or burns sustained in rocket attacks. The array of possibilities necessitates a thorough history and complete musculoskeletal and neurologic assessments.
LANGUAGE AND CULTURAL ISSUES
In addition to being aware of the potential health issues that arise within the refugee population, primary care providers need to be prepared to confront language and cultural issues that may arise. The National Standards on Culturally and Linguistically Appropriate Services (CLAS)48 offer appropriate guidance in 14 key areas.
Refugees frequently have limited or no working knowledge of English. Whenever possible, practitioners should use medically trained interpreters to help them receive and convey accurate information and thus provide comprehensive care. When professional interpreters are not available, telephone interpreter services are available for purchase by the facility or practice. Children or other family members should not be relied on for accurate interpretation.48
As for cultural differences, it is important to note that a refugee’s concept of family may differ from that found in Western culture.49 For example, it is not uncommon to find extended families living together, with members referring to nieces and nephews as their children, or aunts and uncles as their parents. A thorough exploration of the relationships among patients and their families is important, particularly during the family history.
Regardless of where resettled refugees come from, it is probable that their cultural and personal beliefs about medicine differ from those of practitioners with years of Western medicine training. In addition to implementing CLAS guidelines,48 practitioners should be familiar with Kleinman’s3explanatory model, which explores the differences between the patient and practitioner models—not necessarily differences in levels of knowledge, but rather of “values and interests.”3 Thus, people unfamiliar with or resistant to the Western model of medicine are often seen as ignorant, whereas an issue of values may be at play.
Identifying the differences between the patient’s and the clinician’s explanatory models allows the clinician to anticipate and address potential misunderstandings, understand patients’ perceived needs, and involve patients in management strategies that will motivate them to comply with treatment. To help clinicians assess the explanatory models of their patients, Kleinman provides eight questions (shown in Table 3,3,50).
CONCLUSION
As the world becomes more of a global village, increasing numbers of primary care providers will see refugee patients. Practitioners need to be aware of the physical, socioeconomic, and psychological issues that affect refugees during and after resettlement in the US. Refugees may have conditions that could not be addressed in their home country or refugee camp. They may have illnesses with which US practitioners are ordinarily unfamiliar, poorly treated or untreated traumatic injuries, or psychological trauma resulting from conditions that forced them to flee their native lands.
Clinicians who work with refugees should be familiar with the resettlement process and perform a thorough examination after the refugee’s resettlement, managing previously unaddressed health issues. Complete histories must be taken and physical examinations performed in a culturally appropriate manner and an atmosphere of mutual trust.
Finally, it is vital for providers to explore the explanatory models from which patients view their illnesses. Such an understanding facilitates culturally appropriate care with patient participation, and ultimately more positive clinical outcomes.
Authors’ note: The patients portrayed in this article are all composites. While the issues described are real, these “patients” were developed from multiple cases to protect individual patient privacy. None is real, and any resemblance to any real persons is purely accidental.
REFERENCES
1. Ramos M, Orozovich P, Moser K, et al. Health of resettled Iraqi refugees: San Diego County, California, October 2007–September 2009. MMWR Morb Mortal Wkly Rep. 2010;59(49):1614-1618.
2. US Department of State. FY 2010 cumulative summary of refugee admissions. www.wrapsnet .org/reports/archives/tabid/215/language/en-us/default.aspx. Accessed February 17, 2011.
3. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88(2):251-258.
4. US Citizenship and Immigration Services. Refugees and asylum. www.uscis.gov/portal/site/uscis/menuitem.eb1d4c2a3e5b9ac89243c6a7543f6d1a/?vgnextoid=1f1c3e4d77d73210VgnVCM100000082ca60aRCRD&vgnextchannel=1f1c3e4d77d73210VgnVCM100000082ca60aRCRD. Accessed February 17, 2011.
5. United Nations High Commissioner for Refugees. Refugees: flowing across borders. www .unhcr.org/pages/49c3646c125.html. Accessed February 17, 2011.
6. US Citizenship and Immigration Services. The United States Refugee Admissions Program (USRAP) consultation and worldwide processing priorities. www.uscis.gov/portal/site/uscis/menuitem.5af9bb95919f35e66f614176543f6d1a/?vgnextoid=796b0eb389683210VgnVCM100000082ca60aRCRD&vgnextchannel=385d3e4d77d73210VgnVCM100000082ca60aRCRD. Accessed February 17, 2011.
7. US Committee for Refugees and Immigrants. Frequently asked questions. www.refugees.org/about-us/faqs.html. Accessed February 17, 2011.
8. US Department of State. Refugee Admissions Reception and Placement Program. www.state
.gov/g/prm/rls/125478.htm. Accessed February 17, 2011.
9. Office of Refugee Resettlement. Report to Congress: FY 2007. www.acf.hhs.gov/programs/orr/data/ORR_2007_report.pdf. Accessed February 17, 2011.
10. CDC. Medical examination: frequently asked questions (FAQs). www.cdc.gov/immigrant
refugeehealth/exams/medical-examination-faqs .html#9. Accessed February 17, 2011.
11. US Department of State. Medical Examination for Immigrant or Refugee Applicant (DS-2053, OMB No. 1405-0113, expiration date 04/30/2012. http://bangkok.usembassy.gov/root/pdfs/med forms_043012.pdf. Accessed February 17, 2011.
12. Cohen J, Powderly WG, Opal SM, eds. Infectious Diseases. 3rd ed. Philadelphia, PA: Elsevier; 2010.
13. CDC. Domestic Refugee Health Program: Frequently Asked Questions (2010). www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/domestic-refugee-questions.html. Accessed February 17, 2011.
14. CDC. Technical Instructions: guidance for HIV for panel physicians and civil surgeons (2010). www.cdc.gov/immigrantrefugeehealth/exams/ti/hiv-guidance-panel-civil.html. Accessed February 17, 2011.
15. CDC. Medical history and physical examination: technical instructions for medical examination of aliens. www.cdc.gov/immigrantrefugee health/exams/ti/panel/technical-instructions/panel-physicians/medical-history-physical-exam .html. Accessed February 17, 2011.
16. Lee JL, Naguwa SM, Cheema GS, Gershwin ME. Acute rheumatic fever and its consequences: a persistent threat to developing nations in the 21st century. Autoimmun Rev. 2009;9(2):117-123.
17. Henderson DN. Pregnancy complicated by rheumatic heart disease. Can Med Assoc J. 1936; 35(4):394-398.
18. US Citizenship and Immigration Services. Refugees. www.uscis.gov/portal/site/uscis/menu item.eb1d4c2a3e5b9ac89243c6a7543f6d1a/?vgnextoid=385d3e4d77d73210VgnVCM100000082ca60aRCRD&vgnextchannel=385d3e4d77d73210VgnVCM100000082ca60aRCRD. Accessed February 17, 2011.
19. US Department of State. 9 FAM appendix O, exhibit II: Promissory note (2007). In: Foreign Affairs Manual Volume 9: Visas. www.state.gov/documents/organization/88070.pdf. Accessed February 17, 2011.
20. United States Department of State. US Refugee Admissions Program. www.state.gov/g/prm/c26471.htm. Accessed February 17, 2011.
21. J. Kernan, Community Relations Officer, US Citizenship and Immigration Services, personal communication, March 29, 2010.
22. Office of Refugee Resettlement. Health. www.acf.hhs.gov/programs/orr/benefits/health .htm. Accessed February 17, 2011.
23. Adams KM, Gardiner LD, Assefi N. Healthcare challenges from the developing world: post-immigration refugee medicine. BMJ. 2004;328(7455): 1548-1552.
24. Walker PF, Jaranson J. Refugee and immigrant health care. Med Clin North Am. 1999; 83(4):1103-1120.
25. CDC. Screening for lead at the domestic refugee medical examination (2005). www.cdc.gov/immigrantrefugeehealth/pdf/lead.pdf. Accessed February 17, 2011.
26. Franco-Paredes C, Dismukes R, Nicolls D, et al. Short report: persistent and untreated tropical infectious diseases among Sudanese refugees in the United States. Am J Trop Med Hyg. 2007;77 (4):633-635.
27. CDC. Immigrant and Refugee Health: Domestic Refugee Health Guidelines: Intestinal Parasites. www.cdc.gov/immigrantrefugeehealth/guidelines/ip/intestinal-parasites-domestic.html#asympto matic2. Accessed February 17, 2011.
28. Carlsten C, Jackson C. Refugee and immigrant health care. EthnoMed. http://ethnomed.org/clinical/refugee-health/carlsten.pdf. Accessed February 17, 2011.
29. Conly JM, Johnston BL. The infectious diseases implications of the “Lost Boys and Girls of Sudan.” Can J Infect Dis Med Microbiol. 2008;19 (3):215-216.
30. Aggarwal B, Sharma M, Singh T. Acute eosinophilic pneumonia due to round worm infestation. Indian J Pediatr. 2008;75(3):296-297.
31. Tsai HC, Lee SS, Liu YC, et al. Clinical manifestations of strongyloidiasis in southern Taiwan.
J Microbiol Immunol Infect. 2002;35(1):29-36.
32. CDC. Reported tuberculosis in the United States, 2008. www.cdc.gov/tb/statistics/reports/2008/pdf/2008report.pdf. Accessed February 17, 2011.
33. Oeltmann JE, Varma JK, Ortega L, et al. Multidrug-resistant tuberculosis outbreak among US-bound Hmong refugees, Thailand, 2005. Emerg Infect Dis. 2008;14(11):1715-1721.
34. Golden MP, Vikram H. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005; 72(9):1761-1768.
35. CDC. Tuberculosis (TB) Fact Sheets: BCG Vaccine. www.cdc.gov/tb/publications/factsheets/prevention/BCG.htm. Accessed February 17, 2011.
36. The role of BCG vaccine in the prevention and control of tuberculosis in the United States: a joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45(RR-4):1-18.
37. Museru OI, Vargas M, Kinyua M, et al. Hepatitis B virus infection among refugees resettled in the US: high prevalence and challenges in access to health care. J Immigr Minor Health. 2010;12 (6):823-827.
38. World Health Organization, UNAIDS, UNDP, UNECA, UNESO, UNFPA, UNHCHR, UNHCR, UNICEF, UNIFEM. Eliminating Female Genital Mutilation: An Interagency Statement (2008). http://whqlibdoc.who.int/publications/2008/ 9789241596442_eng.pdf. Accessed February 17, 2010.
39. United Nations Children’s Fund. Female Genital Mutilation/Cutting: A Statistical Exploration (2005). www.unicef.org/publications/files/FGM-C_final_10_October.pdf. Accessed February 17, 2011.
40. Yoder PS, Abderrahim N, Zhuzhuni A. DHS Comparative Reports No. 7: Female Genital Cutting in the Demographic and Health Surveys: A Critical and Comparative Analysis. Calverton, MD: ORC Macro. September 2004.
41. Royal College of Obstetricians and Gynaecologists. Female Genital Mutilation and its Management (Green-top Guideline No. 53; 2009). www .rcog.org.uk/files/rcog-corp/GreenTop53Female GenitalMutilation.pdf. Accessed February 17, 2011.
42. CDC. Final Rule Removing HIV Infection from U.S. Immigration Screening: Revision of 42 CFR Part 34 (Medical Examination of Aliens) Removal of Human Immunodeficiency Virus (HIV) from Definition of Communicable Disease of Public Health Significance—Final Rule. www.cdc.gov/immigrantrefugeehealth/laws-regs/hiv-ban-removal/final-rule.html. Accessed February 17, 2011.
43. CDC. Immigrant and Refugee Health: Screening for HIV-infection during the refugee domestic medical examination. www.cdc.gov/immigrant
refugeehealth/guidelines/domestic/screening-
hiv-infection-domestic.html. Accessed February 17, 2011.
44. CDC. Human Immunodeficiency Virus Type 2: HIV/AIDS Fact Sheets. Atlanta: Centers for Disease Control and Prevention; 2007.
45. Mills EJ, Nachega JB. HIV infection as a weapon of war. Lancet Infect Dis. 2006;6(12):752-753.
46. CDC. Lead poisoning of a child associated with use of a Cambodian amulet—New York City, 2009. MMWR Morb Mortal Wkly Rep. 2011;60(3): 69-71.
47. MacDuff S, Grodin MA, Gardiner P. The use of complementary and alternative medicine among refugees: a systematic review. J Immigr Minor Health. 2010 Mar 12 [Epub ahead of print].
48. US Department of Health and Human Services, Office of Minority Health. National standards on culturally and linguistically appropriate services (CLAS). http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15. Accessed February 17, 2011.
49. Haviland WA, Prins HEL, Walrath D, McBride B, eds. Cultural Anthropology: The Human Challenge. 12th ed. Belmont, CA: Wadsworth Publishing; 2008.
50. Fadiman A. The Spirit Catches You and You Fall Down: A Hmong Child, Her American Doctors, and the Collision of Two Cultures. New York, NY: Farrar, Straus and Giroux; 1997.