User login
Assessing the Need for a Pharmacist in the Emergency Department of an IHS Hospital
Promoting Tobacco Cessation During Substance Abuse Treatment
Autoimmune Thyroid Dysfunction: A Possible Effect of Mangosteen
Grand Rounds: Woman, 26, with Kidney Stones
A 26-year-old woman presented to a nephrology office in Virginia for a reevaluation and second opinion regarding her history of kidney stones. This condition had led to uremia and acute kidney failure, requiring hemodialysis.
Her history was significant for recurrent kidney stones and infections, beginning at age 12. Over the next six years, she passed at least five stones and underwent three lithotripsy procedures; according to the patient, however, neither she nor her parents were ever informed of any decrease in her kidney function. The patient said she had been told that her stones were composed of calcium oxalate, and she was placed on potassium citrate therapy but did not take the medication on a regular basis.
After high school, she left the area for college and for several years she frequently and spontaneously passed gravel and stones. She was a runner in high school and college and had two children without experiencing any hypertension, proteinuria, or stone problems during her pregnancies. She had been treated for numerous recurrent urinary tract infections in outpatient clinics and private offices during the 10 years leading up to her current presentation. She had a distant history of a cholecystectomy.
In May 2009, the patient was hospitalized for a kidney infection and underwent cystoscopy with a finding of left ureteral obstruction caused by a stone. A stent was placed, followed by lithotripsy. Her serum creatinine level was measured at 2.2 mg/dL at that time (normal range, 0.6 to 1.5 mg/dL). In August 2009, she was treated again for a kidney infection; a right-sided stone obstruction was noted at that time, and again a stent was placed and lithotripsy was performed. Her serum creatinine level was then 3.3 mg/dL. During these episodes, the patient’s calcium level ranged from 8.2 to 10.1 mg/dL (normal, 4.5 to 5.2 mg/dL). Her phosphorus level was noted to range from 2.6 to 9.5 mg/dL (normal, 2.5 to 4.5 mg/dL). Her intact parathyroid level was 354 pg/mL (normal, 10 to 60 pg/mL). Thus, she had documented secondary hyperparathyroidism, which was treated with paricalcitol and a phosphate binder.
In February 2010, the patient was “feeling poorly” and was taken to a local hospital in South Carolina. She was admitted in acute renal failure and started on dialysis. She did well on hemodialysis with little to no fluid gain and good urine volume. She returned to Virginia temporarily for treatment, to be closer to her family and to prepare for kidney transplantation. She had family members who were willing to donate an organ.
The patient’s family history was negative for gout, kidney disease, or kidney stones. No family member was known to have hypertension, diabetes, or enuresis.
Physical examination showed a thin white woman with a runner’s lean look. She denied laxative use. Her blood pressure was measured at 120/84 mm Hg, and her pulse, 96 beats/min. Findings in the skin/head/eyes/ears/nose/throat exam were within normal limits except for the presence of contact lenses and a subclavicular dialysis indwelling catheter. Neither thyroid enlargement nor supraclavicular adenopathy was noted. Her heart rate was regular without murmurs. The abdomen was soft and nontender without rebound. The extremities showed no edema. Neurologic and vascular findings were intact.
The most recent 24-hour urine study showed a urine creatinine clearance of 4 mL/min (normal, 85 to 125 mL/min), despite a very large urine volume. Renal ultrasonography revealed two small kidneys that were highly echogenic, with evidence of medullary nephrocalcinosis without obstruction bilaterally.
The presentation of a woman with a kidney stone load high enough to cause full kidney failure by age 26 led the nephrologist to suspect the presence of hyperoxaluria type 1 (primary) or type 2 (secondary). The patient’s urine oxalate level was 158 mcmol/L (normal, < 57 mcmol/L), and her plasma oxalate level was 73 mcmol/L (normal, < 10 mcmol/L).
In response to the patient’s high blood and urine oxalate levels and her interest in kidney transplantation, genetic testing was performed to determine whether she had type 1 or type 2 hyperoxaluria. If she was found to have type 1 hyperoxaluria, she would need a liver transplant before her body showered a newly transplanted kidney with stones, causing recurrent kidney failure.
Discussion
Primary hyperoxaluria (PHO) type 1 is a very rare recessive hereditary disease with a prevalence of one to three cases per one million persons.1 Patients typically present with kidney stones at an early age (as did the case patient) or in full kidney failure. It is calculated that PHO is responsible for 1% of all end-stage renal disease among pediatric patients.2,3
Stones are caused by a deficiency of the liver enzyme alanine-glyoxylate aminotransferase (AGXT), which ordinarily converts glyoxylate to glycine.2,4 When AGXT is absent, glyoxylate is converted instead to oxalate, which forms insoluble salts that accumulate in the kidney as oxalate kidney stones. Most patients (ie, 80% to 90%) present in late childhood or early adolescence with systems of recurrent stones and urinary tract infections resulting from blockage.5,6 The natural history of the disease is progression to kidney failure and death from end-stage renal disease unless dialysis is initiated.
While testing of oxalate-to-creatinine molar ratio in a random urine sample may be helpful, this measurement does not stabilize until age 14 to 18—often after kidney damage has already occurred.7 Liver biopsy can confirm whether the enzyme AGXT is absent. Differentiation between PHO and type 2 hyperoxaluria can only be confirmed by genetic testing in which the AGXT gene is identified.8
There is an increased incidence of PHO in Tunisia and Kuwait9-11 and in the Arab and Druze families of Israel12 as a result of intermarriages in this population. Since AGXT is a recessive gene, the child of parents who are both carriers has a 25% chance of having the disease. If either parent carries the genetic variant, there is a 50% chance that the recessive gene will be passed on.
Diagnosis
Early diagnosis of PHO is critical. However, because the disease is so rare, more than 40% of affected patients do not receive a diagnosis until three years after symptoms develop, and 30% are diagnosed only upon presentation with end-stage renal disease.2,13
If PHO is detected early, the key management goal is to minimize renal and skeletal oxalate deposition. Components of medical management are shown in the table.2,14-17 It is important to note that these strategies are effective only if initiated early, that is, before the patient’s glomerular filtration rate drops below 25 mL/min.18
Treatment
Organ transplantation remains the only definitive treatment for PHO14,19—to prevent severe systemic oxalosis or to manage the patient who has progressed to end-stage renal disease. Researchers from the Mayo Clinic in Rochester, Minnesota (where, it should be noted, a National Oxalosis and Hyperoxaluria Registry is maintained under the direction of Dawn S. Milliner, MD), recently published an observational study of outcomes in transplant graft survival among 203 PHO patients. Bergstralh et al20 reported high rates of recurrent oxalosis in patients undergoing kidney transplantation alone, and significantly improved outcomes in patients who underwent both liver and kidney transplantation.
Before 1990, according to a report by the Rare Kidney Stone Consortium,18 the prognosis for PHO transplant patients in the United States was so poor that a donor kidney was considered wasted on these patients. Since the year 2000, however, survival after transplantation has improved greatly, with rates similar to those of all kidney transplant patients nationwide. The explanation for increased survival rates among PHO patients undergoing transplantation was twofold:
• Increased preoperative stone control
• Use of combined liver-kidney transplants.21,22
Since the liver is responsible for the cascade of calcium oxalate stones, the native liver must be fully removed prior to transplantation of a new liver and kidney. Postoperatively, stones will also emerge from where they have lodged in the skeletal tissue to shower the new kidney. Thus, medical management of this cascade of new stones is vital if the transplanted grafts are to survive.23 Calcium oxalate blood levels can remain high for one to two years posttransplantation,2,24 so long-term medical management of oxalate is essential.
The Case Patient
Clinicians engaged in a discussion with the patient and her family regarding a possible diagnosis of PHO. Blood was drawn and sent to the Mayo Clinic for genetic analysis. It was found that the patient had an abnormality in the AGXT gene; with the diagnosis of type 1 hyperoxaluria confirmed, she was flown to Rochester for a full workup.
The patient was the only member of her family with the defective AGXT gene, and her genetic counselors considered this a single mutation. She was accepted for the liver/kidney transplantation list.
Due to the increase in reported survival among patients if they undergo transplantation early in the natural history of stone deposition, the average wait time for PHO patients is only three to four months. The case patient returned to the dialysis unit in Virginia, where she was placed on a dialysis regimen of five-hour treatments, five times per week (nighttime and day); this was determined to be the peak treatment duration for most efficient stone removal, as determined by calcium oxalate measurement during her workup at the Mayo Clinic.
This regimen was continued for three months, at which time the patient was nearing the top of the transplant waiting list. She returned to the Mayo Clinic in September 2010 and underwent transplantation in October; since then, she has regained excellent kidney function and experienced an immediate drop in her calcium oxalate levels. She remained in Rochester until late November, then returned to her home in South Carolina, where she continues to undergo follow-up at a local transplantation center.
The case patient was fortunate that an attending nephrologist at the nephrology office in Virginia developed a high clinical suspicion for her actual condition and started the workup that led to a diagnosis of PHO. She could well have been among the 19% of patients with PHO in whom the correct diagnosis is not reached until after a newly transplanted kidney has been showered with stones again,18,25 necessitating a second kidney transplant following the essential liver transplantation.
Before her current presentation, the patient had been under the care of another nephrologist and had spent six months on a transplant waiting list. If she had proceeded with her original plan, the scheduled kidney transplant (unaccompanied by the essential liver transplant) would have been ineffective, and her donor would have undergone major surgery to no good result.
Conclusion
Type 1 hyperoxaluria is a rare diagnosis that is frequently missed. According to data from the Rare Kidney Stone Consortium,18 nearly one-fifth of patients with PHO do not receive a correct diagnosis until after an unsuccessful kidney transplantation, as liver transplantation is initially required.
The author wishes to extend special thanks to Stephen G. Goldberger, MD, “for being such a good detective.”
References
1. Ajzensztejn MJ, Sebire NJ, Trompeter RS, Marks SD. Primary hyperoxaluria type 1. Arch Dis Child. 2007; 92(3):197.
2. Niaudet P. Primary hyperoxaluria (2010). www.uptodate.com/contents/primary-hyperoxaluria?source=search_result& selectedTitle=1%7E39. Accessed February 17, 2011.
3. Latta K, Brodehl J. Primary hyperoxaluria type I. Eur J Pediatr. 1990;149(8):518-522.
4. Danpure CJ. Advances in the enzymology and molecular genetics of primary hyperoxaluria type 1: prospects for gene therapy. Nephrol Dial Transplant. 1995;10 suppl 8:24-29.
5. Lieske JC, Monico CG, Holmes WS, et al. International registry for primary hyperoxaluria. Am J Nephrol. 2005;25(3):290-296.
6. Genetics Home Reference. Primary hyperoxaluria. www.ghr.nlm.nih.gov/condition/primary-hyperoxaluria. Accessed February 17, 2011.
7. Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002;75(3):561-569.
8. Danpure CJ. Molecular and clinical heterogeneity in primary hyperoxaluria type 1. Am J Kidney Dis. 1991;17(4):366-369.
9. Kamoun A, Lakhoua R. End-stage renal disease of the Tunisian child: epidemiology, etiologies, and outcome. Pediatr Nephrol. 1996;10(4):479-482.
10. Al-Eisa AA, Samhan M, Naseef M. End-stage renal disease in Kuwaiti children: an 8-year experience. Transplant Proc. 2004;36(6):1788-1791.
11. Cochat P, Liutkus A, Fargue S, et al. Primary hyperoxaluria type 1: still challenging! Pediatr Nephrol. 2006;21(8):1075-1081.
12. Rinat C, Wanders RJ, Drukker A, et al. Primary hyperoxaluria type I: a model for multiple mutations in a monogenic disease within a distinct ethnic group. J Am Soc Nephrol. 1999;10(11):2352-2358.
13. Hoppe B, Langman CB. A United States survey on diagnosis, treatment, and outcome of primary hyperoxaluria. Pediatr Nephrol. 2003;18(10):986-991.
14. Watts RW. Primary hyperoxaluria type I. QJM. 1994;87(10):593-600.
15. Hoppe B, Latta K, von Schnakenburg C, Kemper MJ. Primary hyperoxaluria: the German experience. Am J Nephrol. 2005;25(3):276-281.
16. Milliner DS, Eickholt JT, Bergstralh EJ, et al. Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N Engl J Med. 1994;331(23):1553-1558.
17. Danpure CJ. Primary hyperoxaluria: from gene defects to designer drugs? Nephrol Dial Transplant. 2005;20(8):1525-1529.
18. Rare Kidney Stone Consortium. Primary hyperoxaluria. www.rarekidneystones.org/hyperoxaluria. Accessed February 9, 2011.
19. Brinkert F, Ganschow R, Helmke, K, et al. Transplantation procedures in children with primary hyperoxaluria type 1: outcome and longitudinal growth. Transplantation. 2009;87(9):1415:1421.
20. Bergstralh EJ, Monico CG, Lieske JC, et al; IPHR Investigators. Transplantation outcomes in primary hyperoxaluria. Am J Transplant. 2010;10(11):2493-2501.
21. Millan MT, Berquist WE, So SK, et al. One hundred percent patient and kidney allograft survival with simultaneous liver and kidney transplantation in infants with primary hyperoxaluria: a single-center experience. Transplantation. 2003;76(10):1458-1463.
22. Watts RWE, Danpure CJ, De Pauw L, Toussaint C; European Study Group on Transplantation in Hyperoxaluria Type 1. Combined liver-kidney and isolated liver transplantations for primary hyperoxaluria type 1: the European experience. Nephrol Dial Transplant. 1991;6(7):502-511.
23. Broyer M, Jouvet P, Niaudet P, et al. Management of oxalosis. Kidney Int Suppl. 1996;53:S93-S98.
24. de Pauw L, Gelin M, Danpure CJ, et al. Combined liver-kidney transplantation in primary hyperoxaluria type 1. Transplantation. 1990;50(5):886-887.
25. Broyer M, Brunner FP, Brynger H, et al. Kidney transplantation in primary oxalosis: data from the EDTA Registry. Nephrol Dial Transplant. 1990;5(5):332-336.
A 26-year-old woman presented to a nephrology office in Virginia for a reevaluation and second opinion regarding her history of kidney stones. This condition had led to uremia and acute kidney failure, requiring hemodialysis.
Her history was significant for recurrent kidney stones and infections, beginning at age 12. Over the next six years, she passed at least five stones and underwent three lithotripsy procedures; according to the patient, however, neither she nor her parents were ever informed of any decrease in her kidney function. The patient said she had been told that her stones were composed of calcium oxalate, and she was placed on potassium citrate therapy but did not take the medication on a regular basis.
After high school, she left the area for college and for several years she frequently and spontaneously passed gravel and stones. She was a runner in high school and college and had two children without experiencing any hypertension, proteinuria, or stone problems during her pregnancies. She had been treated for numerous recurrent urinary tract infections in outpatient clinics and private offices during the 10 years leading up to her current presentation. She had a distant history of a cholecystectomy.
In May 2009, the patient was hospitalized for a kidney infection and underwent cystoscopy with a finding of left ureteral obstruction caused by a stone. A stent was placed, followed by lithotripsy. Her serum creatinine level was measured at 2.2 mg/dL at that time (normal range, 0.6 to 1.5 mg/dL). In August 2009, she was treated again for a kidney infection; a right-sided stone obstruction was noted at that time, and again a stent was placed and lithotripsy was performed. Her serum creatinine level was then 3.3 mg/dL. During these episodes, the patient’s calcium level ranged from 8.2 to 10.1 mg/dL (normal, 4.5 to 5.2 mg/dL). Her phosphorus level was noted to range from 2.6 to 9.5 mg/dL (normal, 2.5 to 4.5 mg/dL). Her intact parathyroid level was 354 pg/mL (normal, 10 to 60 pg/mL). Thus, she had documented secondary hyperparathyroidism, which was treated with paricalcitol and a phosphate binder.
In February 2010, the patient was “feeling poorly” and was taken to a local hospital in South Carolina. She was admitted in acute renal failure and started on dialysis. She did well on hemodialysis with little to no fluid gain and good urine volume. She returned to Virginia temporarily for treatment, to be closer to her family and to prepare for kidney transplantation. She had family members who were willing to donate an organ.
The patient’s family history was negative for gout, kidney disease, or kidney stones. No family member was known to have hypertension, diabetes, or enuresis.
Physical examination showed a thin white woman with a runner’s lean look. She denied laxative use. Her blood pressure was measured at 120/84 mm Hg, and her pulse, 96 beats/min. Findings in the skin/head/eyes/ears/nose/throat exam were within normal limits except for the presence of contact lenses and a subclavicular dialysis indwelling catheter. Neither thyroid enlargement nor supraclavicular adenopathy was noted. Her heart rate was regular without murmurs. The abdomen was soft and nontender without rebound. The extremities showed no edema. Neurologic and vascular findings were intact.
The most recent 24-hour urine study showed a urine creatinine clearance of 4 mL/min (normal, 85 to 125 mL/min), despite a very large urine volume. Renal ultrasonography revealed two small kidneys that were highly echogenic, with evidence of medullary nephrocalcinosis without obstruction bilaterally.
The presentation of a woman with a kidney stone load high enough to cause full kidney failure by age 26 led the nephrologist to suspect the presence of hyperoxaluria type 1 (primary) or type 2 (secondary). The patient’s urine oxalate level was 158 mcmol/L (normal, < 57 mcmol/L), and her plasma oxalate level was 73 mcmol/L (normal, < 10 mcmol/L).
In response to the patient’s high blood and urine oxalate levels and her interest in kidney transplantation, genetic testing was performed to determine whether she had type 1 or type 2 hyperoxaluria. If she was found to have type 1 hyperoxaluria, she would need a liver transplant before her body showered a newly transplanted kidney with stones, causing recurrent kidney failure.
Discussion
Primary hyperoxaluria (PHO) type 1 is a very rare recessive hereditary disease with a prevalence of one to three cases per one million persons.1 Patients typically present with kidney stones at an early age (as did the case patient) or in full kidney failure. It is calculated that PHO is responsible for 1% of all end-stage renal disease among pediatric patients.2,3
Stones are caused by a deficiency of the liver enzyme alanine-glyoxylate aminotransferase (AGXT), which ordinarily converts glyoxylate to glycine.2,4 When AGXT is absent, glyoxylate is converted instead to oxalate, which forms insoluble salts that accumulate in the kidney as oxalate kidney stones. Most patients (ie, 80% to 90%) present in late childhood or early adolescence with systems of recurrent stones and urinary tract infections resulting from blockage.5,6 The natural history of the disease is progression to kidney failure and death from end-stage renal disease unless dialysis is initiated.
While testing of oxalate-to-creatinine molar ratio in a random urine sample may be helpful, this measurement does not stabilize until age 14 to 18—often after kidney damage has already occurred.7 Liver biopsy can confirm whether the enzyme AGXT is absent. Differentiation between PHO and type 2 hyperoxaluria can only be confirmed by genetic testing in which the AGXT gene is identified.8
There is an increased incidence of PHO in Tunisia and Kuwait9-11 and in the Arab and Druze families of Israel12 as a result of intermarriages in this population. Since AGXT is a recessive gene, the child of parents who are both carriers has a 25% chance of having the disease. If either parent carries the genetic variant, there is a 50% chance that the recessive gene will be passed on.
Diagnosis
Early diagnosis of PHO is critical. However, because the disease is so rare, more than 40% of affected patients do not receive a diagnosis until three years after symptoms develop, and 30% are diagnosed only upon presentation with end-stage renal disease.2,13
If PHO is detected early, the key management goal is to minimize renal and skeletal oxalate deposition. Components of medical management are shown in the table.2,14-17 It is important to note that these strategies are effective only if initiated early, that is, before the patient’s glomerular filtration rate drops below 25 mL/min.18
Treatment
Organ transplantation remains the only definitive treatment for PHO14,19—to prevent severe systemic oxalosis or to manage the patient who has progressed to end-stage renal disease. Researchers from the Mayo Clinic in Rochester, Minnesota (where, it should be noted, a National Oxalosis and Hyperoxaluria Registry is maintained under the direction of Dawn S. Milliner, MD), recently published an observational study of outcomes in transplant graft survival among 203 PHO patients. Bergstralh et al20 reported high rates of recurrent oxalosis in patients undergoing kidney transplantation alone, and significantly improved outcomes in patients who underwent both liver and kidney transplantation.
Before 1990, according to a report by the Rare Kidney Stone Consortium,18 the prognosis for PHO transplant patients in the United States was so poor that a donor kidney was considered wasted on these patients. Since the year 2000, however, survival after transplantation has improved greatly, with rates similar to those of all kidney transplant patients nationwide. The explanation for increased survival rates among PHO patients undergoing transplantation was twofold:
• Increased preoperative stone control
• Use of combined liver-kidney transplants.21,22
Since the liver is responsible for the cascade of calcium oxalate stones, the native liver must be fully removed prior to transplantation of a new liver and kidney. Postoperatively, stones will also emerge from where they have lodged in the skeletal tissue to shower the new kidney. Thus, medical management of this cascade of new stones is vital if the transplanted grafts are to survive.23 Calcium oxalate blood levels can remain high for one to two years posttransplantation,2,24 so long-term medical management of oxalate is essential.
The Case Patient
Clinicians engaged in a discussion with the patient and her family regarding a possible diagnosis of PHO. Blood was drawn and sent to the Mayo Clinic for genetic analysis. It was found that the patient had an abnormality in the AGXT gene; with the diagnosis of type 1 hyperoxaluria confirmed, she was flown to Rochester for a full workup.
The patient was the only member of her family with the defective AGXT gene, and her genetic counselors considered this a single mutation. She was accepted for the liver/kidney transplantation list.
Due to the increase in reported survival among patients if they undergo transplantation early in the natural history of stone deposition, the average wait time for PHO patients is only three to four months. The case patient returned to the dialysis unit in Virginia, where she was placed on a dialysis regimen of five-hour treatments, five times per week (nighttime and day); this was determined to be the peak treatment duration for most efficient stone removal, as determined by calcium oxalate measurement during her workup at the Mayo Clinic.
This regimen was continued for three months, at which time the patient was nearing the top of the transplant waiting list. She returned to the Mayo Clinic in September 2010 and underwent transplantation in October; since then, she has regained excellent kidney function and experienced an immediate drop in her calcium oxalate levels. She remained in Rochester until late November, then returned to her home in South Carolina, where she continues to undergo follow-up at a local transplantation center.
The case patient was fortunate that an attending nephrologist at the nephrology office in Virginia developed a high clinical suspicion for her actual condition and started the workup that led to a diagnosis of PHO. She could well have been among the 19% of patients with PHO in whom the correct diagnosis is not reached until after a newly transplanted kidney has been showered with stones again,18,25 necessitating a second kidney transplant following the essential liver transplantation.
Before her current presentation, the patient had been under the care of another nephrologist and had spent six months on a transplant waiting list. If she had proceeded with her original plan, the scheduled kidney transplant (unaccompanied by the essential liver transplant) would have been ineffective, and her donor would have undergone major surgery to no good result.
Conclusion
Type 1 hyperoxaluria is a rare diagnosis that is frequently missed. According to data from the Rare Kidney Stone Consortium,18 nearly one-fifth of patients with PHO do not receive a correct diagnosis until after an unsuccessful kidney transplantation, as liver transplantation is initially required.
The author wishes to extend special thanks to Stephen G. Goldberger, MD, “for being such a good detective.”
References
1. Ajzensztejn MJ, Sebire NJ, Trompeter RS, Marks SD. Primary hyperoxaluria type 1. Arch Dis Child. 2007; 92(3):197.
2. Niaudet P. Primary hyperoxaluria (2010). www.uptodate.com/contents/primary-hyperoxaluria?source=search_result& selectedTitle=1%7E39. Accessed February 17, 2011.
3. Latta K, Brodehl J. Primary hyperoxaluria type I. Eur J Pediatr. 1990;149(8):518-522.
4. Danpure CJ. Advances in the enzymology and molecular genetics of primary hyperoxaluria type 1: prospects for gene therapy. Nephrol Dial Transplant. 1995;10 suppl 8:24-29.
5. Lieske JC, Monico CG, Holmes WS, et al. International registry for primary hyperoxaluria. Am J Nephrol. 2005;25(3):290-296.
6. Genetics Home Reference. Primary hyperoxaluria. www.ghr.nlm.nih.gov/condition/primary-hyperoxaluria. Accessed February 17, 2011.
7. Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002;75(3):561-569.
8. Danpure CJ. Molecular and clinical heterogeneity in primary hyperoxaluria type 1. Am J Kidney Dis. 1991;17(4):366-369.
9. Kamoun A, Lakhoua R. End-stage renal disease of the Tunisian child: epidemiology, etiologies, and outcome. Pediatr Nephrol. 1996;10(4):479-482.
10. Al-Eisa AA, Samhan M, Naseef M. End-stage renal disease in Kuwaiti children: an 8-year experience. Transplant Proc. 2004;36(6):1788-1791.
11. Cochat P, Liutkus A, Fargue S, et al. Primary hyperoxaluria type 1: still challenging! Pediatr Nephrol. 2006;21(8):1075-1081.
12. Rinat C, Wanders RJ, Drukker A, et al. Primary hyperoxaluria type I: a model for multiple mutations in a monogenic disease within a distinct ethnic group. J Am Soc Nephrol. 1999;10(11):2352-2358.
13. Hoppe B, Langman CB. A United States survey on diagnosis, treatment, and outcome of primary hyperoxaluria. Pediatr Nephrol. 2003;18(10):986-991.
14. Watts RW. Primary hyperoxaluria type I. QJM. 1994;87(10):593-600.
15. Hoppe B, Latta K, von Schnakenburg C, Kemper MJ. Primary hyperoxaluria: the German experience. Am J Nephrol. 2005;25(3):276-281.
16. Milliner DS, Eickholt JT, Bergstralh EJ, et al. Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N Engl J Med. 1994;331(23):1553-1558.
17. Danpure CJ. Primary hyperoxaluria: from gene defects to designer drugs? Nephrol Dial Transplant. 2005;20(8):1525-1529.
18. Rare Kidney Stone Consortium. Primary hyperoxaluria. www.rarekidneystones.org/hyperoxaluria. Accessed February 9, 2011.
19. Brinkert F, Ganschow R, Helmke, K, et al. Transplantation procedures in children with primary hyperoxaluria type 1: outcome and longitudinal growth. Transplantation. 2009;87(9):1415:1421.
20. Bergstralh EJ, Monico CG, Lieske JC, et al; IPHR Investigators. Transplantation outcomes in primary hyperoxaluria. Am J Transplant. 2010;10(11):2493-2501.
21. Millan MT, Berquist WE, So SK, et al. One hundred percent patient and kidney allograft survival with simultaneous liver and kidney transplantation in infants with primary hyperoxaluria: a single-center experience. Transplantation. 2003;76(10):1458-1463.
22. Watts RWE, Danpure CJ, De Pauw L, Toussaint C; European Study Group on Transplantation in Hyperoxaluria Type 1. Combined liver-kidney and isolated liver transplantations for primary hyperoxaluria type 1: the European experience. Nephrol Dial Transplant. 1991;6(7):502-511.
23. Broyer M, Jouvet P, Niaudet P, et al. Management of oxalosis. Kidney Int Suppl. 1996;53:S93-S98.
24. de Pauw L, Gelin M, Danpure CJ, et al. Combined liver-kidney transplantation in primary hyperoxaluria type 1. Transplantation. 1990;50(5):886-887.
25. Broyer M, Brunner FP, Brynger H, et al. Kidney transplantation in primary oxalosis: data from the EDTA Registry. Nephrol Dial Transplant. 1990;5(5):332-336.
A 26-year-old woman presented to a nephrology office in Virginia for a reevaluation and second opinion regarding her history of kidney stones. This condition had led to uremia and acute kidney failure, requiring hemodialysis.
Her history was significant for recurrent kidney stones and infections, beginning at age 12. Over the next six years, she passed at least five stones and underwent three lithotripsy procedures; according to the patient, however, neither she nor her parents were ever informed of any decrease in her kidney function. The patient said she had been told that her stones were composed of calcium oxalate, and she was placed on potassium citrate therapy but did not take the medication on a regular basis.
After high school, she left the area for college and for several years she frequently and spontaneously passed gravel and stones. She was a runner in high school and college and had two children without experiencing any hypertension, proteinuria, or stone problems during her pregnancies. She had been treated for numerous recurrent urinary tract infections in outpatient clinics and private offices during the 10 years leading up to her current presentation. She had a distant history of a cholecystectomy.
In May 2009, the patient was hospitalized for a kidney infection and underwent cystoscopy with a finding of left ureteral obstruction caused by a stone. A stent was placed, followed by lithotripsy. Her serum creatinine level was measured at 2.2 mg/dL at that time (normal range, 0.6 to 1.5 mg/dL). In August 2009, she was treated again for a kidney infection; a right-sided stone obstruction was noted at that time, and again a stent was placed and lithotripsy was performed. Her serum creatinine level was then 3.3 mg/dL. During these episodes, the patient’s calcium level ranged from 8.2 to 10.1 mg/dL (normal, 4.5 to 5.2 mg/dL). Her phosphorus level was noted to range from 2.6 to 9.5 mg/dL (normal, 2.5 to 4.5 mg/dL). Her intact parathyroid level was 354 pg/mL (normal, 10 to 60 pg/mL). Thus, she had documented secondary hyperparathyroidism, which was treated with paricalcitol and a phosphate binder.
In February 2010, the patient was “feeling poorly” and was taken to a local hospital in South Carolina. She was admitted in acute renal failure and started on dialysis. She did well on hemodialysis with little to no fluid gain and good urine volume. She returned to Virginia temporarily for treatment, to be closer to her family and to prepare for kidney transplantation. She had family members who were willing to donate an organ.
The patient’s family history was negative for gout, kidney disease, or kidney stones. No family member was known to have hypertension, diabetes, or enuresis.
Physical examination showed a thin white woman with a runner’s lean look. She denied laxative use. Her blood pressure was measured at 120/84 mm Hg, and her pulse, 96 beats/min. Findings in the skin/head/eyes/ears/nose/throat exam were within normal limits except for the presence of contact lenses and a subclavicular dialysis indwelling catheter. Neither thyroid enlargement nor supraclavicular adenopathy was noted. Her heart rate was regular without murmurs. The abdomen was soft and nontender without rebound. The extremities showed no edema. Neurologic and vascular findings were intact.
The most recent 24-hour urine study showed a urine creatinine clearance of 4 mL/min (normal, 85 to 125 mL/min), despite a very large urine volume. Renal ultrasonography revealed two small kidneys that were highly echogenic, with evidence of medullary nephrocalcinosis without obstruction bilaterally.
The presentation of a woman with a kidney stone load high enough to cause full kidney failure by age 26 led the nephrologist to suspect the presence of hyperoxaluria type 1 (primary) or type 2 (secondary). The patient’s urine oxalate level was 158 mcmol/L (normal, < 57 mcmol/L), and her plasma oxalate level was 73 mcmol/L (normal, < 10 mcmol/L).
In response to the patient’s high blood and urine oxalate levels and her interest in kidney transplantation, genetic testing was performed to determine whether she had type 1 or type 2 hyperoxaluria. If she was found to have type 1 hyperoxaluria, she would need a liver transplant before her body showered a newly transplanted kidney with stones, causing recurrent kidney failure.
Discussion
Primary hyperoxaluria (PHO) type 1 is a very rare recessive hereditary disease with a prevalence of one to three cases per one million persons.1 Patients typically present with kidney stones at an early age (as did the case patient) or in full kidney failure. It is calculated that PHO is responsible for 1% of all end-stage renal disease among pediatric patients.2,3
Stones are caused by a deficiency of the liver enzyme alanine-glyoxylate aminotransferase (AGXT), which ordinarily converts glyoxylate to glycine.2,4 When AGXT is absent, glyoxylate is converted instead to oxalate, which forms insoluble salts that accumulate in the kidney as oxalate kidney stones. Most patients (ie, 80% to 90%) present in late childhood or early adolescence with systems of recurrent stones and urinary tract infections resulting from blockage.5,6 The natural history of the disease is progression to kidney failure and death from end-stage renal disease unless dialysis is initiated.
While testing of oxalate-to-creatinine molar ratio in a random urine sample may be helpful, this measurement does not stabilize until age 14 to 18—often after kidney damage has already occurred.7 Liver biopsy can confirm whether the enzyme AGXT is absent. Differentiation between PHO and type 2 hyperoxaluria can only be confirmed by genetic testing in which the AGXT gene is identified.8
There is an increased incidence of PHO in Tunisia and Kuwait9-11 and in the Arab and Druze families of Israel12 as a result of intermarriages in this population. Since AGXT is a recessive gene, the child of parents who are both carriers has a 25% chance of having the disease. If either parent carries the genetic variant, there is a 50% chance that the recessive gene will be passed on.
Diagnosis
Early diagnosis of PHO is critical. However, because the disease is so rare, more than 40% of affected patients do not receive a diagnosis until three years after symptoms develop, and 30% are diagnosed only upon presentation with end-stage renal disease.2,13
If PHO is detected early, the key management goal is to minimize renal and skeletal oxalate deposition. Components of medical management are shown in the table.2,14-17 It is important to note that these strategies are effective only if initiated early, that is, before the patient’s glomerular filtration rate drops below 25 mL/min.18
Treatment
Organ transplantation remains the only definitive treatment for PHO14,19—to prevent severe systemic oxalosis or to manage the patient who has progressed to end-stage renal disease. Researchers from the Mayo Clinic in Rochester, Minnesota (where, it should be noted, a National Oxalosis and Hyperoxaluria Registry is maintained under the direction of Dawn S. Milliner, MD), recently published an observational study of outcomes in transplant graft survival among 203 PHO patients. Bergstralh et al20 reported high rates of recurrent oxalosis in patients undergoing kidney transplantation alone, and significantly improved outcomes in patients who underwent both liver and kidney transplantation.
Before 1990, according to a report by the Rare Kidney Stone Consortium,18 the prognosis for PHO transplant patients in the United States was so poor that a donor kidney was considered wasted on these patients. Since the year 2000, however, survival after transplantation has improved greatly, with rates similar to those of all kidney transplant patients nationwide. The explanation for increased survival rates among PHO patients undergoing transplantation was twofold:
• Increased preoperative stone control
• Use of combined liver-kidney transplants.21,22
Since the liver is responsible for the cascade of calcium oxalate stones, the native liver must be fully removed prior to transplantation of a new liver and kidney. Postoperatively, stones will also emerge from where they have lodged in the skeletal tissue to shower the new kidney. Thus, medical management of this cascade of new stones is vital if the transplanted grafts are to survive.23 Calcium oxalate blood levels can remain high for one to two years posttransplantation,2,24 so long-term medical management of oxalate is essential.
The Case Patient
Clinicians engaged in a discussion with the patient and her family regarding a possible diagnosis of PHO. Blood was drawn and sent to the Mayo Clinic for genetic analysis. It was found that the patient had an abnormality in the AGXT gene; with the diagnosis of type 1 hyperoxaluria confirmed, she was flown to Rochester for a full workup.
The patient was the only member of her family with the defective AGXT gene, and her genetic counselors considered this a single mutation. She was accepted for the liver/kidney transplantation list.
Due to the increase in reported survival among patients if they undergo transplantation early in the natural history of stone deposition, the average wait time for PHO patients is only three to four months. The case patient returned to the dialysis unit in Virginia, where she was placed on a dialysis regimen of five-hour treatments, five times per week (nighttime and day); this was determined to be the peak treatment duration for most efficient stone removal, as determined by calcium oxalate measurement during her workup at the Mayo Clinic.
This regimen was continued for three months, at which time the patient was nearing the top of the transplant waiting list. She returned to the Mayo Clinic in September 2010 and underwent transplantation in October; since then, she has regained excellent kidney function and experienced an immediate drop in her calcium oxalate levels. She remained in Rochester until late November, then returned to her home in South Carolina, where she continues to undergo follow-up at a local transplantation center.
The case patient was fortunate that an attending nephrologist at the nephrology office in Virginia developed a high clinical suspicion for her actual condition and started the workup that led to a diagnosis of PHO. She could well have been among the 19% of patients with PHO in whom the correct diagnosis is not reached until after a newly transplanted kidney has been showered with stones again,18,25 necessitating a second kidney transplant following the essential liver transplantation.
Before her current presentation, the patient had been under the care of another nephrologist and had spent six months on a transplant waiting list. If she had proceeded with her original plan, the scheduled kidney transplant (unaccompanied by the essential liver transplant) would have been ineffective, and her donor would have undergone major surgery to no good result.
Conclusion
Type 1 hyperoxaluria is a rare diagnosis that is frequently missed. According to data from the Rare Kidney Stone Consortium,18 nearly one-fifth of patients with PHO do not receive a correct diagnosis until after an unsuccessful kidney transplantation, as liver transplantation is initially required.
The author wishes to extend special thanks to Stephen G. Goldberger, MD, “for being such a good detective.”
References
1. Ajzensztejn MJ, Sebire NJ, Trompeter RS, Marks SD. Primary hyperoxaluria type 1. Arch Dis Child. 2007; 92(3):197.
2. Niaudet P. Primary hyperoxaluria (2010). www.uptodate.com/contents/primary-hyperoxaluria?source=search_result& selectedTitle=1%7E39. Accessed February 17, 2011.
3. Latta K, Brodehl J. Primary hyperoxaluria type I. Eur J Pediatr. 1990;149(8):518-522.
4. Danpure CJ. Advances in the enzymology and molecular genetics of primary hyperoxaluria type 1: prospects for gene therapy. Nephrol Dial Transplant. 1995;10 suppl 8:24-29.
5. Lieske JC, Monico CG, Holmes WS, et al. International registry for primary hyperoxaluria. Am J Nephrol. 2005;25(3):290-296.
6. Genetics Home Reference. Primary hyperoxaluria. www.ghr.nlm.nih.gov/condition/primary-hyperoxaluria. Accessed February 17, 2011.
7. Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002;75(3):561-569.
8. Danpure CJ. Molecular and clinical heterogeneity in primary hyperoxaluria type 1. Am J Kidney Dis. 1991;17(4):366-369.
9. Kamoun A, Lakhoua R. End-stage renal disease of the Tunisian child: epidemiology, etiologies, and outcome. Pediatr Nephrol. 1996;10(4):479-482.
10. Al-Eisa AA, Samhan M, Naseef M. End-stage renal disease in Kuwaiti children: an 8-year experience. Transplant Proc. 2004;36(6):1788-1791.
11. Cochat P, Liutkus A, Fargue S, et al. Primary hyperoxaluria type 1: still challenging! Pediatr Nephrol. 2006;21(8):1075-1081.
12. Rinat C, Wanders RJ, Drukker A, et al. Primary hyperoxaluria type I: a model for multiple mutations in a monogenic disease within a distinct ethnic group. J Am Soc Nephrol. 1999;10(11):2352-2358.
13. Hoppe B, Langman CB. A United States survey on diagnosis, treatment, and outcome of primary hyperoxaluria. Pediatr Nephrol. 2003;18(10):986-991.
14. Watts RW. Primary hyperoxaluria type I. QJM. 1994;87(10):593-600.
15. Hoppe B, Latta K, von Schnakenburg C, Kemper MJ. Primary hyperoxaluria: the German experience. Am J Nephrol. 2005;25(3):276-281.
16. Milliner DS, Eickholt JT, Bergstralh EJ, et al. Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N Engl J Med. 1994;331(23):1553-1558.
17. Danpure CJ. Primary hyperoxaluria: from gene defects to designer drugs? Nephrol Dial Transplant. 2005;20(8):1525-1529.
18. Rare Kidney Stone Consortium. Primary hyperoxaluria. www.rarekidneystones.org/hyperoxaluria. Accessed February 9, 2011.
19. Brinkert F, Ganschow R, Helmke, K, et al. Transplantation procedures in children with primary hyperoxaluria type 1: outcome and longitudinal growth. Transplantation. 2009;87(9):1415:1421.
20. Bergstralh EJ, Monico CG, Lieske JC, et al; IPHR Investigators. Transplantation outcomes in primary hyperoxaluria. Am J Transplant. 2010;10(11):2493-2501.
21. Millan MT, Berquist WE, So SK, et al. One hundred percent patient and kidney allograft survival with simultaneous liver and kidney transplantation in infants with primary hyperoxaluria: a single-center experience. Transplantation. 2003;76(10):1458-1463.
22. Watts RWE, Danpure CJ, De Pauw L, Toussaint C; European Study Group on Transplantation in Hyperoxaluria Type 1. Combined liver-kidney and isolated liver transplantations for primary hyperoxaluria type 1: the European experience. Nephrol Dial Transplant. 1991;6(7):502-511.
23. Broyer M, Jouvet P, Niaudet P, et al. Management of oxalosis. Kidney Int Suppl. 1996;53:S93-S98.
24. de Pauw L, Gelin M, Danpure CJ, et al. Combined liver-kidney transplantation in primary hyperoxaluria type 1. Transplantation. 1990;50(5):886-887.
25. Broyer M, Brunner FP, Brynger H, et al. Kidney transplantation in primary oxalosis: data from the EDTA Registry. Nephrol Dial Transplant. 1990;5(5):332-336.
Common Ocular Emergencies
UPDATE ON CERVICAL DISEASE
Over the past year, we have gained further insight into the efficacy and safety of the human papillomavirus (HPV) vaccines; received new, practical cervical screening guidance from the Centers for Disease Control and Prevention (CDC); and gathered further evidence that colposcopy is not as sensitive at detecting high-grade cervical disease as we once thought.
In this article, I describe each of these developments in depth.
Both HPV vaccines are safe and effective—
and both offer cross-protection
Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13.
HPV 16 accounts for about 55% of all cases of cervical cancer and HPV 18 for another 15%—and both HPV vaccines on the market provide coverage against these two types. Because vaccination stands to reduce the burden of cervical disease so dramatically, it behooves us to achieve the highest possible vaccination rate for girls and young women.
Regrettably, fewer than 40% of the eligible female population of the United States has received one or more injections of either the bivalent (HPV 16, 18) (Cervarix) or quadrivalent (HPV 6, 11, 16, 18) (Gardasil) vaccine—with the vaccination rate varying considerably by geographic location and socioeconomic status.1 Clearly, we have much work ahead of us to improve this rate.
What’s the big picture?
Each trial of the HPV vaccine to date has demonstrated high efficacy and safety. Drawing from the individual findings of these trials to develop a snapshot of overall efficacy
and safety has been difficult, however, owing to multiple clinical endpoints, differences in both the number of virus-like particle types and in the adjuvant used in each vaccine, variability of the populations, and different definitions of efficacy. These limitations have made it difficult for clinicians and patients to make an informed decision about which vaccine to choose.
To address these concerns, Lu and colleagues conducted a comprehensive systematic review and meta-analysis of seven unique randomized, controlled trials with a total enrollment of 44,141 females. Their goal: to assess the safety and efficacy of both vaccines against multiple virologic and clinical endpoints, including efficacy not only against the primary HPV vaccine types, but closely related types as well.
They focused on two groups of girls and women:
- The per protocol population (PPP) included females who were both DNA- and sero-negative to the HPV types contained in the vaccine at the start and end of the vaccination period. The PPP group received all three injections of the vaccine, with no protocol violations.
- The intention-to-treat cohort (ITT) included women and girls who had received one or more doses of the vaccine or placebo and who had follow-up data available, regardless of HPV status at enrollment.
The PPP more closely resembles the sexually naïve population that stands to benefit most from the full vaccination series, whereas the ITT is more similar to girls and women 18 to 26 years old who are seeking “catch-up” vaccination, most of them having initiated sexual activity or had less than perfect compliance with vaccination, or both.
In the ITT cohort, the pooled relative risk (RR) for HPV 16-related cervical intraepithelial neoplasia (CIN) grade 2 or worse was 0.47, corresponding to a pooled efficacy of 53%, a statistically significant benefit. In the PPP, the RR was 0.04, corresponding to a pooled efficacy of 96% for HPV 16-related CIN 2+. The RR was similar for HPV 18 (TABLE). The reduction in CIN 1 for women not previously infected with either of these high-risk HPV types was also high—95% for HPV 16 and 97% for HPV 18.
Effect of HPV vaccination on high-grade cervical disease
| Group | Relative risk of CIN 2+ | Reduction in CIN 2+ | ||
|---|---|---|---|---|
| HpV 16 | HpV 18 | HpV 16 | HpV 18 | |
| Intention to treat | 0.47 | 0.16 | 53% | 84% |
| Per protocol | 0.04 | 0.10 | 96% | 90% |
| CIN=cervical intraepithelial neoplasia SOURCE: Lu B, et al. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13. | ||||
Vaccines offer cross-protection against 3 additional HPV types
The possibility that the HPV vaccines provide cross-protection against closely related HPV types has generated considerable interest. Lu and colleagues assessed cross-protection against 6-month persistent infection related to five HPV types:
- HPV 31—relative risk (RR) of 0.47 and 0.30 in the ITT and PPP cohorts, respectively
- HPV 45—RR of 0.50 and 0.42 in the ITT and PPP cohorts, respectively. There was significant heterogeneity between the trials in efficacy against persistent HPV 45 infection.
- HPV 33—RR of 0.65 and 0.57 in the ITT and PPP cohorts, respectively
- HPV 52 and 58—no statistically significant cross-protection.
Adverse events are minimal
The most common systemic vaccine-related adverse events reported in all the trials were headache and fatigue, which were noted in 50% to 60% of participants. The most common serious adverse events were abnormal pregnancy outcomes, such as birth defects and spontaneous abortion, but the RR of 1.0 for all serious adverse events suggests a statistically insignificant difference in the risk of serious adverse events between vaccine and control groups. These findings are consistent with the most recent review by the CDC and FDA (October 2010), which concluded that Gardasil is safe and effective for the prevention of the four types covered in the vaccine.2 CDC updates on safety do not yet include the bivalent vaccine because of its more recent release to the US market.
At every opportunity, encourage HPV vaccination for girls and women who are 9 to 26 years old.
New STD guidelines from the CDC include tips
on cervical cancer screening
Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110.
The CDC’s most recent sexually transmitted disease (STD) guidelines, released at the end of 2010, cover all sexually transmitted infections, including genital HPV infection. In general, the recommendations on cervical cancer screening are consistent with ACOG’s 2009 guidelines, which I discussed in the March 2010 Update on Cervical Disease. The CDC also offers concrete, useful suggestions on how to counsel patients who have genital warts or who test positive for an oncogenic strain of HPV. Although the guidelines are aimed at STD and public health clinics, they include many recommendations useful to all health care providers. For that reason, discussion of the highlights seems appropriate.
Like ACOG, CDC says screening should start at 21 years
Screening should begin when the patient is 21 years old and continue at 2-year intervals until she is 30 years old, at which time it should switch to every 3 years—provided she has had three consecutive normal Pap tests or one normal cotest (Pap and HPV test combined).
Because a woman may sometimes assume that she has undergone a Pap test by virtue of having had a pelvic examination, inaccuracies in self-reported screening intervals may arise. Therefore, it is imperative to devise a protocol for cervical cancer screening among women who do not have documentation, in their medical record, of a normal Pap test within the preceding 12 months. Although some women will undoubtedly undergo screening sooner than necessary, this approach will protect women lacking adequate documentation from being underscreened.
When to use the HPV test (and when to avoid it)
The guidelines confirm that the HPV test is an appropriate tool in the management of atypical squamous cells of undetermined significance (ASC–US) among women 21 years and older and as a cotest with the Pap for women who are 30 years and older.
The CDC recommends against the HPV test in the following situations:
- when deciding whether to vaccinate against HPV
- as part of a screen for STD
- in the triage of low-grade squamous intraepithelial lesion (LSIL) Pap results, although 2006 guidelines from the American Society for Colposcopy and Cervical Pathology and 2007 guidelines from ACOG recommend, as an option, the use of the HPV test in the triage of postmenopausal women who have LSIL
- in women younger than 21 years
- as a stand-alone primary cervical cancer screen (without the Pap test).
These recommendations are consistent with earlier conclusions.3
Perhaps the most important insights offered in the CDC’s 2010 STD guidelines are the counseling messages for women who undergo cotesting with both the HPV and Pap tests. It often is a challenge to communicate the indications for and findings of this screening approach. Here is guidance offered by the CDC:
- HPV is very common. It can infect the genital areas of both men and women. It usually has no signs or symptoms.
- Most sexually active persons get HPV at some time in their life, although few will ever know it. Even a person who has had only one lifetime sex partner can get HPV if the partner was infected.
- Although the immune system clears HPV infection most of the time, the infection fails to resolve in some people
- No clinically validated test exists for men to determine whether they have HPV infection. The most common manifestation of HPV infection in men is genital warts. High-risk HPV types seldom cause genital warts.
- Partners who are in a long-term relationship tend to share HPV. Sexual partners of HPV-infected people also likely have HPV, even though they may have no signs or symptoms of infection.
- Detection of high-risk HPV infection in a woman does not mean that she or her partner is engaging in sexual activity outside of a relationship. HPV infection can be present for many years before it is detected, and no method can accurately confirm when HPV infection was acquired.
The pap test is not a screening test for Std
Other findings that may be useful for all clinicians, as well as for those who practice in an STD clinic:
- The Pap test is not a screening test for STD
- All eligible women should undergo cervical cancer screening, regardless of sexual orientation (i.e., heterosexual, lesbian, or bisexual)
- Conventional cytology should be delayed if the patient is menstruating, and she should be advised to undergo a Pap test at the earliest opportunity
- If specific infections other than HPV are identified, the patient may need to undergo a repeat Pap test after appropriate treatment for those infections. However, in most instances, the Pap test will be reported as satisfactory for evaluation, and a reliable final report can be produced without the need to repeat the Pap test after treatment.
- The presence of a mucopurulent discharge should not delay the Pap test. The test can be performed after careful removal of the discharge with a saline-soaked cotton swab.
- When the Pap test is repeated because the previous test was interpreted as unsatisfactory, the patient should not be returned to regular screening intervals until the Pap test is reported as satisfactory and negative
- Cervical screening should not be accelerated for women who have genital warts and no other indication.
CDC recommendations on cervical cancer prevention and screening are consistent with those of other organizations, including ACOG. the counseling messages should be adopted universally.
When colposcopic biopsy is indicated, take more than one sample
Stoler MH, Vichnin MD, Ferenczy A, et al; the FUTURE I, II and III Investigators. The accuracy of colposcopic biopsy: Analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer. 2011;128(6):1354–1362.
The progress we have made in cervical cancer prevention is largely due to our ability to detect and treat precancer, particularly CIN 3, before it gains the capacity to invade. Until recently, few experts would have questioned the value of the partnership between cervical cytology screening and treatment of lesions detected on colposcopically directed biopsy.4 However, over the past decade, the accuracy of colposcopy for detection of high-grade lesions has been widely questioned, first by studies assessing static digitized cervigrams or colposcopy photo images, and more recently by studies comparing “real-time” colposcopy to histology obtained during colposcopy or excisional biopsy, or both.
The largest of these studies was conducted by Stoler and colleagues to compare the results of colposcopically directed biopsy and subsequent cervical excision among 737 women (16 to 45 years old) in the placebo arm of the quadrivalent HPV vaccine (FUTURE) randomized, controlled trials. In these trials, all women were referred for colposcopy according to a Pap triage algorithm, and one or more biopsies was taken from the area with the greatest apparent abnormality, as viewed by colposcopy. When excisional treatment was indicated, a biopsy of the worst-appearing area was taken again just before the excision.
Each patient’s most severe pathology-panel diagnosis for the excisional specimen was compared with:
- the most severe biopsy result from the preceding 6 months (excluding the biopsy taken on the same day as the excisional procedure) (Analysis 1)
- the biopsy taken on the same day as the definitive excisional procedure (Analysis 2).
When CIN 2 and CIN 3 are managed similarly, a discrepancy of one degree between colposcopically directed biopsy and the excisional specimen is considered sufficient agreement. Therefore, in this study, a difference of one degree in histologic diagnosis was considered agreement.
High-grade disease was more likely to be underestimated
on the same-day biopsy
Colposcopically directed biopsies obtained within 6 months before definitive treatment (Analysis 1) had lower overall agreement with the excisional specimen than biopsies collected on the same day as definitive treatment (Analysis 2). However, underestimation of high-grade disease was lower (26% overall underestimation of CIN 2 or 3 or adenocarcinoma in situ [AIS]) on earlier biopsy specimens than on those collected on the same day as definitive treatment (57% overall underestimation of CIN 2 or 3 or AIS).
Conversely, overestimation, or removal, of disease was higher (36%) in biopsies collected within 6 months before the excisional treatment, compared with biopsies collected on the same day as definitive treatment (5%).
The investigators suggested that any discrepancy in accuracy between the biopsy obtained at treatment and the biopsy obtained earlier might be the result of less diligent colposcopic evaluation and biopsy placement when the colposcopist knew that definitive therapy would immediately follow. Another possibility, they noted, is that lesions biopsied as early as 6 months before definitive treatment may have regressed in the process of tissue repair or were completely removed by the biopsy.
When all biopsies were compared with the final diagnosis of the excisional specimen, the colposcopically directed biopsy was less severe 42% to 66% of the time when the excisional histology was read as CIN 3 or AIS. However, when one degree of discrepancy was allowed, as it is in clinical practice, agreement was 92%. This suggests that women in the FUTURE trials, as well as those in real clinical practice, are typically managed appropriately under current protocols that combine cytology and colposcopy results to properly identify women who have cervical lesions that require surgical intervention.
Most CIN 3 lesions were small
Many of the CIN 3 lesions in this trial were small, as they were in the ASCUS LSIL Triage Study (ALTS), in which the median length of CIN 3 lesions was only 6.5 mm. Also in ALTS, lesions in one third of patients were so small that colposcopically directed biopsy did not leave any residual disease to be detected in the loop electrosurgical excision specimen.5 The size of a CIN 3 lesion that has associated invasion is, on average, seven times larger than without invasion.6 Although colposcopy is much less likely to miss large lesions, it is important to miss as little high-grade disease as possible because the risk of invasion is cumulative over time and unpredictable in a given patient.

Multiple biopsies boost detection
Sampling more than one area improves the accuracy of colposcopically directed biopsy, even when one area looks most abnormal. This colposcopy photo shows potential biopsy sites (within the ovals), although other choices may also be reasonable. Several studies have shown that colposcopically directed biopsy of even normal-appearing areas at the squamocolumnar junction or within large ectopies can improve detection of high-grade cervical intraepithelial neoplasia or adenocarcinoma in situ.Studies have shown that it is possible to increase the accuracy of detection of CIN 2+ by increasing the number of biopsies. In this study by Stoler and colleagues, the sensitivity of initial colposcopy improved from 47% (for one biopsy) to 65% (two biopsies) and 77% (three or more) (FIGURE). Overall agreement increased with increasing age, which is consistent with the likelihood that CIN 3 lesions expand with age and become increasingly detectable by colposcopy.
Colposcopy does work, but the era of biopsying only the most abnormal-appearing area is over. Take more biopsies.
We want to hear from you! Tell us what you think.
1. Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38(5):525-533.
2. Centers for Disease Control and Prevention. Frequently asked questions about HPV vaccine safety. http://www.cdc.gov/vaccinesafety/Vaccines/HPV/hpv_faqs.html. Accessed February 1, 2011.
3. Cox JT, Moriarty AT, Castle PE. Commentary on: Statement on HPV DNA test utilization. Diagn Cytopathol. 2009;37(7):471-474.
4. Cox JT. More questions about the accuracy of colposcopy: what does this mean for cervical cancer prevention? Obstet Gynecol. 2008;111(6):1266-1267.
5. Sherman ME, Wang SS, Tarone R, Rich L, Schiffman M. Histopathologic extent of CIN 3 lesions in ALTS: implications for subject safety and lead-time bias. Cancer Epidemiol Biomarkers Prev. 2003;12(4):372-379.
6. Tidbury P, Singer A, Jenkins D. CIN 3: the role of lesion size in invasion. Br J Obstet Gynaecol. 1992;99(7):583-586.
Over the past year, we have gained further insight into the efficacy and safety of the human papillomavirus (HPV) vaccines; received new, practical cervical screening guidance from the Centers for Disease Control and Prevention (CDC); and gathered further evidence that colposcopy is not as sensitive at detecting high-grade cervical disease as we once thought.
In this article, I describe each of these developments in depth.
Both HPV vaccines are safe and effective—
and both offer cross-protection
Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13.
HPV 16 accounts for about 55% of all cases of cervical cancer and HPV 18 for another 15%—and both HPV vaccines on the market provide coverage against these two types. Because vaccination stands to reduce the burden of cervical disease so dramatically, it behooves us to achieve the highest possible vaccination rate for girls and young women.
Regrettably, fewer than 40% of the eligible female population of the United States has received one or more injections of either the bivalent (HPV 16, 18) (Cervarix) or quadrivalent (HPV 6, 11, 16, 18) (Gardasil) vaccine—with the vaccination rate varying considerably by geographic location and socioeconomic status.1 Clearly, we have much work ahead of us to improve this rate.
What’s the big picture?
Each trial of the HPV vaccine to date has demonstrated high efficacy and safety. Drawing from the individual findings of these trials to develop a snapshot of overall efficacy
and safety has been difficult, however, owing to multiple clinical endpoints, differences in both the number of virus-like particle types and in the adjuvant used in each vaccine, variability of the populations, and different definitions of efficacy. These limitations have made it difficult for clinicians and patients to make an informed decision about which vaccine to choose.
To address these concerns, Lu and colleagues conducted a comprehensive systematic review and meta-analysis of seven unique randomized, controlled trials with a total enrollment of 44,141 females. Their goal: to assess the safety and efficacy of both vaccines against multiple virologic and clinical endpoints, including efficacy not only against the primary HPV vaccine types, but closely related types as well.
They focused on two groups of girls and women:
- The per protocol population (PPP) included females who were both DNA- and sero-negative to the HPV types contained in the vaccine at the start and end of the vaccination period. The PPP group received all three injections of the vaccine, with no protocol violations.
- The intention-to-treat cohort (ITT) included women and girls who had received one or more doses of the vaccine or placebo and who had follow-up data available, regardless of HPV status at enrollment.
The PPP more closely resembles the sexually naïve population that stands to benefit most from the full vaccination series, whereas the ITT is more similar to girls and women 18 to 26 years old who are seeking “catch-up” vaccination, most of them having initiated sexual activity or had less than perfect compliance with vaccination, or both.
In the ITT cohort, the pooled relative risk (RR) for HPV 16-related cervical intraepithelial neoplasia (CIN) grade 2 or worse was 0.47, corresponding to a pooled efficacy of 53%, a statistically significant benefit. In the PPP, the RR was 0.04, corresponding to a pooled efficacy of 96% for HPV 16-related CIN 2+. The RR was similar for HPV 18 (TABLE). The reduction in CIN 1 for women not previously infected with either of these high-risk HPV types was also high—95% for HPV 16 and 97% for HPV 18.
Effect of HPV vaccination on high-grade cervical disease
| Group | Relative risk of CIN 2+ | Reduction in CIN 2+ | ||
|---|---|---|---|---|
| HpV 16 | HpV 18 | HpV 16 | HpV 18 | |
| Intention to treat | 0.47 | 0.16 | 53% | 84% |
| Per protocol | 0.04 | 0.10 | 96% | 90% |
| CIN=cervical intraepithelial neoplasia SOURCE: Lu B, et al. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13. | ||||
Vaccines offer cross-protection against 3 additional HPV types
The possibility that the HPV vaccines provide cross-protection against closely related HPV types has generated considerable interest. Lu and colleagues assessed cross-protection against 6-month persistent infection related to five HPV types:
- HPV 31—relative risk (RR) of 0.47 and 0.30 in the ITT and PPP cohorts, respectively
- HPV 45—RR of 0.50 and 0.42 in the ITT and PPP cohorts, respectively. There was significant heterogeneity between the trials in efficacy against persistent HPV 45 infection.
- HPV 33—RR of 0.65 and 0.57 in the ITT and PPP cohorts, respectively
- HPV 52 and 58—no statistically significant cross-protection.
Adverse events are minimal
The most common systemic vaccine-related adverse events reported in all the trials were headache and fatigue, which were noted in 50% to 60% of participants. The most common serious adverse events were abnormal pregnancy outcomes, such as birth defects and spontaneous abortion, but the RR of 1.0 for all serious adverse events suggests a statistically insignificant difference in the risk of serious adverse events between vaccine and control groups. These findings are consistent with the most recent review by the CDC and FDA (October 2010), which concluded that Gardasil is safe and effective for the prevention of the four types covered in the vaccine.2 CDC updates on safety do not yet include the bivalent vaccine because of its more recent release to the US market.
At every opportunity, encourage HPV vaccination for girls and women who are 9 to 26 years old.
New STD guidelines from the CDC include tips
on cervical cancer screening
Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110.
The CDC’s most recent sexually transmitted disease (STD) guidelines, released at the end of 2010, cover all sexually transmitted infections, including genital HPV infection. In general, the recommendations on cervical cancer screening are consistent with ACOG’s 2009 guidelines, which I discussed in the March 2010 Update on Cervical Disease. The CDC also offers concrete, useful suggestions on how to counsel patients who have genital warts or who test positive for an oncogenic strain of HPV. Although the guidelines are aimed at STD and public health clinics, they include many recommendations useful to all health care providers. For that reason, discussion of the highlights seems appropriate.
Like ACOG, CDC says screening should start at 21 years
Screening should begin when the patient is 21 years old and continue at 2-year intervals until she is 30 years old, at which time it should switch to every 3 years—provided she has had three consecutive normal Pap tests or one normal cotest (Pap and HPV test combined).
Because a woman may sometimes assume that she has undergone a Pap test by virtue of having had a pelvic examination, inaccuracies in self-reported screening intervals may arise. Therefore, it is imperative to devise a protocol for cervical cancer screening among women who do not have documentation, in their medical record, of a normal Pap test within the preceding 12 months. Although some women will undoubtedly undergo screening sooner than necessary, this approach will protect women lacking adequate documentation from being underscreened.
When to use the HPV test (and when to avoid it)
The guidelines confirm that the HPV test is an appropriate tool in the management of atypical squamous cells of undetermined significance (ASC–US) among women 21 years and older and as a cotest with the Pap for women who are 30 years and older.
The CDC recommends against the HPV test in the following situations:
- when deciding whether to vaccinate against HPV
- as part of a screen for STD
- in the triage of low-grade squamous intraepithelial lesion (LSIL) Pap results, although 2006 guidelines from the American Society for Colposcopy and Cervical Pathology and 2007 guidelines from ACOG recommend, as an option, the use of the HPV test in the triage of postmenopausal women who have LSIL
- in women younger than 21 years
- as a stand-alone primary cervical cancer screen (without the Pap test).
These recommendations are consistent with earlier conclusions.3
Perhaps the most important insights offered in the CDC’s 2010 STD guidelines are the counseling messages for women who undergo cotesting with both the HPV and Pap tests. It often is a challenge to communicate the indications for and findings of this screening approach. Here is guidance offered by the CDC:
- HPV is very common. It can infect the genital areas of both men and women. It usually has no signs or symptoms.
- Most sexually active persons get HPV at some time in their life, although few will ever know it. Even a person who has had only one lifetime sex partner can get HPV if the partner was infected.
- Although the immune system clears HPV infection most of the time, the infection fails to resolve in some people
- No clinically validated test exists for men to determine whether they have HPV infection. The most common manifestation of HPV infection in men is genital warts. High-risk HPV types seldom cause genital warts.
- Partners who are in a long-term relationship tend to share HPV. Sexual partners of HPV-infected people also likely have HPV, even though they may have no signs or symptoms of infection.
- Detection of high-risk HPV infection in a woman does not mean that she or her partner is engaging in sexual activity outside of a relationship. HPV infection can be present for many years before it is detected, and no method can accurately confirm when HPV infection was acquired.
The pap test is not a screening test for Std
Other findings that may be useful for all clinicians, as well as for those who practice in an STD clinic:
- The Pap test is not a screening test for STD
- All eligible women should undergo cervical cancer screening, regardless of sexual orientation (i.e., heterosexual, lesbian, or bisexual)
- Conventional cytology should be delayed if the patient is menstruating, and she should be advised to undergo a Pap test at the earliest opportunity
- If specific infections other than HPV are identified, the patient may need to undergo a repeat Pap test after appropriate treatment for those infections. However, in most instances, the Pap test will be reported as satisfactory for evaluation, and a reliable final report can be produced without the need to repeat the Pap test after treatment.
- The presence of a mucopurulent discharge should not delay the Pap test. The test can be performed after careful removal of the discharge with a saline-soaked cotton swab.
- When the Pap test is repeated because the previous test was interpreted as unsatisfactory, the patient should not be returned to regular screening intervals until the Pap test is reported as satisfactory and negative
- Cervical screening should not be accelerated for women who have genital warts and no other indication.
CDC recommendations on cervical cancer prevention and screening are consistent with those of other organizations, including ACOG. the counseling messages should be adopted universally.
When colposcopic biopsy is indicated, take more than one sample
Stoler MH, Vichnin MD, Ferenczy A, et al; the FUTURE I, II and III Investigators. The accuracy of colposcopic biopsy: Analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer. 2011;128(6):1354–1362.
The progress we have made in cervical cancer prevention is largely due to our ability to detect and treat precancer, particularly CIN 3, before it gains the capacity to invade. Until recently, few experts would have questioned the value of the partnership between cervical cytology screening and treatment of lesions detected on colposcopically directed biopsy.4 However, over the past decade, the accuracy of colposcopy for detection of high-grade lesions has been widely questioned, first by studies assessing static digitized cervigrams or colposcopy photo images, and more recently by studies comparing “real-time” colposcopy to histology obtained during colposcopy or excisional biopsy, or both.
The largest of these studies was conducted by Stoler and colleagues to compare the results of colposcopically directed biopsy and subsequent cervical excision among 737 women (16 to 45 years old) in the placebo arm of the quadrivalent HPV vaccine (FUTURE) randomized, controlled trials. In these trials, all women were referred for colposcopy according to a Pap triage algorithm, and one or more biopsies was taken from the area with the greatest apparent abnormality, as viewed by colposcopy. When excisional treatment was indicated, a biopsy of the worst-appearing area was taken again just before the excision.
Each patient’s most severe pathology-panel diagnosis for the excisional specimen was compared with:
- the most severe biopsy result from the preceding 6 months (excluding the biopsy taken on the same day as the excisional procedure) (Analysis 1)
- the biopsy taken on the same day as the definitive excisional procedure (Analysis 2).
When CIN 2 and CIN 3 are managed similarly, a discrepancy of one degree between colposcopically directed biopsy and the excisional specimen is considered sufficient agreement. Therefore, in this study, a difference of one degree in histologic diagnosis was considered agreement.
High-grade disease was more likely to be underestimated
on the same-day biopsy
Colposcopically directed biopsies obtained within 6 months before definitive treatment (Analysis 1) had lower overall agreement with the excisional specimen than biopsies collected on the same day as definitive treatment (Analysis 2). However, underestimation of high-grade disease was lower (26% overall underestimation of CIN 2 or 3 or adenocarcinoma in situ [AIS]) on earlier biopsy specimens than on those collected on the same day as definitive treatment (57% overall underestimation of CIN 2 or 3 or AIS).
Conversely, overestimation, or removal, of disease was higher (36%) in biopsies collected within 6 months before the excisional treatment, compared with biopsies collected on the same day as definitive treatment (5%).
The investigators suggested that any discrepancy in accuracy between the biopsy obtained at treatment and the biopsy obtained earlier might be the result of less diligent colposcopic evaluation and biopsy placement when the colposcopist knew that definitive therapy would immediately follow. Another possibility, they noted, is that lesions biopsied as early as 6 months before definitive treatment may have regressed in the process of tissue repair or were completely removed by the biopsy.
When all biopsies were compared with the final diagnosis of the excisional specimen, the colposcopically directed biopsy was less severe 42% to 66% of the time when the excisional histology was read as CIN 3 or AIS. However, when one degree of discrepancy was allowed, as it is in clinical practice, agreement was 92%. This suggests that women in the FUTURE trials, as well as those in real clinical practice, are typically managed appropriately under current protocols that combine cytology and colposcopy results to properly identify women who have cervical lesions that require surgical intervention.
Most CIN 3 lesions were small
Many of the CIN 3 lesions in this trial were small, as they were in the ASCUS LSIL Triage Study (ALTS), in which the median length of CIN 3 lesions was only 6.5 mm. Also in ALTS, lesions in one third of patients were so small that colposcopically directed biopsy did not leave any residual disease to be detected in the loop electrosurgical excision specimen.5 The size of a CIN 3 lesion that has associated invasion is, on average, seven times larger than without invasion.6 Although colposcopy is much less likely to miss large lesions, it is important to miss as little high-grade disease as possible because the risk of invasion is cumulative over time and unpredictable in a given patient.

Multiple biopsies boost detection
Sampling more than one area improves the accuracy of colposcopically directed biopsy, even when one area looks most abnormal. This colposcopy photo shows potential biopsy sites (within the ovals), although other choices may also be reasonable. Several studies have shown that colposcopically directed biopsy of even normal-appearing areas at the squamocolumnar junction or within large ectopies can improve detection of high-grade cervical intraepithelial neoplasia or adenocarcinoma in situ.Studies have shown that it is possible to increase the accuracy of detection of CIN 2+ by increasing the number of biopsies. In this study by Stoler and colleagues, the sensitivity of initial colposcopy improved from 47% (for one biopsy) to 65% (two biopsies) and 77% (three or more) (FIGURE). Overall agreement increased with increasing age, which is consistent with the likelihood that CIN 3 lesions expand with age and become increasingly detectable by colposcopy.
Colposcopy does work, but the era of biopsying only the most abnormal-appearing area is over. Take more biopsies.
We want to hear from you! Tell us what you think.
Over the past year, we have gained further insight into the efficacy and safety of the human papillomavirus (HPV) vaccines; received new, practical cervical screening guidance from the Centers for Disease Control and Prevention (CDC); and gathered further evidence that colposcopy is not as sensitive at detecting high-grade cervical disease as we once thought.
In this article, I describe each of these developments in depth.
Both HPV vaccines are safe and effective—
and both offer cross-protection
Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13.
HPV 16 accounts for about 55% of all cases of cervical cancer and HPV 18 for another 15%—and both HPV vaccines on the market provide coverage against these two types. Because vaccination stands to reduce the burden of cervical disease so dramatically, it behooves us to achieve the highest possible vaccination rate for girls and young women.
Regrettably, fewer than 40% of the eligible female population of the United States has received one or more injections of either the bivalent (HPV 16, 18) (Cervarix) or quadrivalent (HPV 6, 11, 16, 18) (Gardasil) vaccine—with the vaccination rate varying considerably by geographic location and socioeconomic status.1 Clearly, we have much work ahead of us to improve this rate.
What’s the big picture?
Each trial of the HPV vaccine to date has demonstrated high efficacy and safety. Drawing from the individual findings of these trials to develop a snapshot of overall efficacy
and safety has been difficult, however, owing to multiple clinical endpoints, differences in both the number of virus-like particle types and in the adjuvant used in each vaccine, variability of the populations, and different definitions of efficacy. These limitations have made it difficult for clinicians and patients to make an informed decision about which vaccine to choose.
To address these concerns, Lu and colleagues conducted a comprehensive systematic review and meta-analysis of seven unique randomized, controlled trials with a total enrollment of 44,141 females. Their goal: to assess the safety and efficacy of both vaccines against multiple virologic and clinical endpoints, including efficacy not only against the primary HPV vaccine types, but closely related types as well.
They focused on two groups of girls and women:
- The per protocol population (PPP) included females who were both DNA- and sero-negative to the HPV types contained in the vaccine at the start and end of the vaccination period. The PPP group received all three injections of the vaccine, with no protocol violations.
- The intention-to-treat cohort (ITT) included women and girls who had received one or more doses of the vaccine or placebo and who had follow-up data available, regardless of HPV status at enrollment.
The PPP more closely resembles the sexually naïve population that stands to benefit most from the full vaccination series, whereas the ITT is more similar to girls and women 18 to 26 years old who are seeking “catch-up” vaccination, most of them having initiated sexual activity or had less than perfect compliance with vaccination, or both.
In the ITT cohort, the pooled relative risk (RR) for HPV 16-related cervical intraepithelial neoplasia (CIN) grade 2 or worse was 0.47, corresponding to a pooled efficacy of 53%, a statistically significant benefit. In the PPP, the RR was 0.04, corresponding to a pooled efficacy of 96% for HPV 16-related CIN 2+. The RR was similar for HPV 18 (TABLE). The reduction in CIN 1 for women not previously infected with either of these high-risk HPV types was also high—95% for HPV 16 and 97% for HPV 18.
Effect of HPV vaccination on high-grade cervical disease
| Group | Relative risk of CIN 2+ | Reduction in CIN 2+ | ||
|---|---|---|---|---|
| HpV 16 | HpV 18 | HpV 16 | HpV 18 | |
| Intention to treat | 0.47 | 0.16 | 53% | 84% |
| Per protocol | 0.04 | 0.10 | 96% | 90% |
| CIN=cervical intraepithelial neoplasia SOURCE: Lu B, et al. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis. 2011;11(1):13. | ||||
Vaccines offer cross-protection against 3 additional HPV types
The possibility that the HPV vaccines provide cross-protection against closely related HPV types has generated considerable interest. Lu and colleagues assessed cross-protection against 6-month persistent infection related to five HPV types:
- HPV 31—relative risk (RR) of 0.47 and 0.30 in the ITT and PPP cohorts, respectively
- HPV 45—RR of 0.50 and 0.42 in the ITT and PPP cohorts, respectively. There was significant heterogeneity between the trials in efficacy against persistent HPV 45 infection.
- HPV 33—RR of 0.65 and 0.57 in the ITT and PPP cohorts, respectively
- HPV 52 and 58—no statistically significant cross-protection.
Adverse events are minimal
The most common systemic vaccine-related adverse events reported in all the trials were headache and fatigue, which were noted in 50% to 60% of participants. The most common serious adverse events were abnormal pregnancy outcomes, such as birth defects and spontaneous abortion, but the RR of 1.0 for all serious adverse events suggests a statistically insignificant difference in the risk of serious adverse events between vaccine and control groups. These findings are consistent with the most recent review by the CDC and FDA (October 2010), which concluded that Gardasil is safe and effective for the prevention of the four types covered in the vaccine.2 CDC updates on safety do not yet include the bivalent vaccine because of its more recent release to the US market.
At every opportunity, encourage HPV vaccination for girls and women who are 9 to 26 years old.
New STD guidelines from the CDC include tips
on cervical cancer screening
Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110.
The CDC’s most recent sexually transmitted disease (STD) guidelines, released at the end of 2010, cover all sexually transmitted infections, including genital HPV infection. In general, the recommendations on cervical cancer screening are consistent with ACOG’s 2009 guidelines, which I discussed in the March 2010 Update on Cervical Disease. The CDC also offers concrete, useful suggestions on how to counsel patients who have genital warts or who test positive for an oncogenic strain of HPV. Although the guidelines are aimed at STD and public health clinics, they include many recommendations useful to all health care providers. For that reason, discussion of the highlights seems appropriate.
Like ACOG, CDC says screening should start at 21 years
Screening should begin when the patient is 21 years old and continue at 2-year intervals until she is 30 years old, at which time it should switch to every 3 years—provided she has had three consecutive normal Pap tests or one normal cotest (Pap and HPV test combined).
Because a woman may sometimes assume that she has undergone a Pap test by virtue of having had a pelvic examination, inaccuracies in self-reported screening intervals may arise. Therefore, it is imperative to devise a protocol for cervical cancer screening among women who do not have documentation, in their medical record, of a normal Pap test within the preceding 12 months. Although some women will undoubtedly undergo screening sooner than necessary, this approach will protect women lacking adequate documentation from being underscreened.
When to use the HPV test (and when to avoid it)
The guidelines confirm that the HPV test is an appropriate tool in the management of atypical squamous cells of undetermined significance (ASC–US) among women 21 years and older and as a cotest with the Pap for women who are 30 years and older.
The CDC recommends against the HPV test in the following situations:
- when deciding whether to vaccinate against HPV
- as part of a screen for STD
- in the triage of low-grade squamous intraepithelial lesion (LSIL) Pap results, although 2006 guidelines from the American Society for Colposcopy and Cervical Pathology and 2007 guidelines from ACOG recommend, as an option, the use of the HPV test in the triage of postmenopausal women who have LSIL
- in women younger than 21 years
- as a stand-alone primary cervical cancer screen (without the Pap test).
These recommendations are consistent with earlier conclusions.3
Perhaps the most important insights offered in the CDC’s 2010 STD guidelines are the counseling messages for women who undergo cotesting with both the HPV and Pap tests. It often is a challenge to communicate the indications for and findings of this screening approach. Here is guidance offered by the CDC:
- HPV is very common. It can infect the genital areas of both men and women. It usually has no signs or symptoms.
- Most sexually active persons get HPV at some time in their life, although few will ever know it. Even a person who has had only one lifetime sex partner can get HPV if the partner was infected.
- Although the immune system clears HPV infection most of the time, the infection fails to resolve in some people
- No clinically validated test exists for men to determine whether they have HPV infection. The most common manifestation of HPV infection in men is genital warts. High-risk HPV types seldom cause genital warts.
- Partners who are in a long-term relationship tend to share HPV. Sexual partners of HPV-infected people also likely have HPV, even though they may have no signs or symptoms of infection.
- Detection of high-risk HPV infection in a woman does not mean that she or her partner is engaging in sexual activity outside of a relationship. HPV infection can be present for many years before it is detected, and no method can accurately confirm when HPV infection was acquired.
The pap test is not a screening test for Std
Other findings that may be useful for all clinicians, as well as for those who practice in an STD clinic:
- The Pap test is not a screening test for STD
- All eligible women should undergo cervical cancer screening, regardless of sexual orientation (i.e., heterosexual, lesbian, or bisexual)
- Conventional cytology should be delayed if the patient is menstruating, and she should be advised to undergo a Pap test at the earliest opportunity
- If specific infections other than HPV are identified, the patient may need to undergo a repeat Pap test after appropriate treatment for those infections. However, in most instances, the Pap test will be reported as satisfactory for evaluation, and a reliable final report can be produced without the need to repeat the Pap test after treatment.
- The presence of a mucopurulent discharge should not delay the Pap test. The test can be performed after careful removal of the discharge with a saline-soaked cotton swab.
- When the Pap test is repeated because the previous test was interpreted as unsatisfactory, the patient should not be returned to regular screening intervals until the Pap test is reported as satisfactory and negative
- Cervical screening should not be accelerated for women who have genital warts and no other indication.
CDC recommendations on cervical cancer prevention and screening are consistent with those of other organizations, including ACOG. the counseling messages should be adopted universally.
When colposcopic biopsy is indicated, take more than one sample
Stoler MH, Vichnin MD, Ferenczy A, et al; the FUTURE I, II and III Investigators. The accuracy of colposcopic biopsy: Analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer. 2011;128(6):1354–1362.
The progress we have made in cervical cancer prevention is largely due to our ability to detect and treat precancer, particularly CIN 3, before it gains the capacity to invade. Until recently, few experts would have questioned the value of the partnership between cervical cytology screening and treatment of lesions detected on colposcopically directed biopsy.4 However, over the past decade, the accuracy of colposcopy for detection of high-grade lesions has been widely questioned, first by studies assessing static digitized cervigrams or colposcopy photo images, and more recently by studies comparing “real-time” colposcopy to histology obtained during colposcopy or excisional biopsy, or both.
The largest of these studies was conducted by Stoler and colleagues to compare the results of colposcopically directed biopsy and subsequent cervical excision among 737 women (16 to 45 years old) in the placebo arm of the quadrivalent HPV vaccine (FUTURE) randomized, controlled trials. In these trials, all women were referred for colposcopy according to a Pap triage algorithm, and one or more biopsies was taken from the area with the greatest apparent abnormality, as viewed by colposcopy. When excisional treatment was indicated, a biopsy of the worst-appearing area was taken again just before the excision.
Each patient’s most severe pathology-panel diagnosis for the excisional specimen was compared with:
- the most severe biopsy result from the preceding 6 months (excluding the biopsy taken on the same day as the excisional procedure) (Analysis 1)
- the biopsy taken on the same day as the definitive excisional procedure (Analysis 2).
When CIN 2 and CIN 3 are managed similarly, a discrepancy of one degree between colposcopically directed biopsy and the excisional specimen is considered sufficient agreement. Therefore, in this study, a difference of one degree in histologic diagnosis was considered agreement.
High-grade disease was more likely to be underestimated
on the same-day biopsy
Colposcopically directed biopsies obtained within 6 months before definitive treatment (Analysis 1) had lower overall agreement with the excisional specimen than biopsies collected on the same day as definitive treatment (Analysis 2). However, underestimation of high-grade disease was lower (26% overall underestimation of CIN 2 or 3 or adenocarcinoma in situ [AIS]) on earlier biopsy specimens than on those collected on the same day as definitive treatment (57% overall underestimation of CIN 2 or 3 or AIS).
Conversely, overestimation, or removal, of disease was higher (36%) in biopsies collected within 6 months before the excisional treatment, compared with biopsies collected on the same day as definitive treatment (5%).
The investigators suggested that any discrepancy in accuracy between the biopsy obtained at treatment and the biopsy obtained earlier might be the result of less diligent colposcopic evaluation and biopsy placement when the colposcopist knew that definitive therapy would immediately follow. Another possibility, they noted, is that lesions biopsied as early as 6 months before definitive treatment may have regressed in the process of tissue repair or were completely removed by the biopsy.
When all biopsies were compared with the final diagnosis of the excisional specimen, the colposcopically directed biopsy was less severe 42% to 66% of the time when the excisional histology was read as CIN 3 or AIS. However, when one degree of discrepancy was allowed, as it is in clinical practice, agreement was 92%. This suggests that women in the FUTURE trials, as well as those in real clinical practice, are typically managed appropriately under current protocols that combine cytology and colposcopy results to properly identify women who have cervical lesions that require surgical intervention.
Most CIN 3 lesions were small
Many of the CIN 3 lesions in this trial were small, as they were in the ASCUS LSIL Triage Study (ALTS), in which the median length of CIN 3 lesions was only 6.5 mm. Also in ALTS, lesions in one third of patients were so small that colposcopically directed biopsy did not leave any residual disease to be detected in the loop electrosurgical excision specimen.5 The size of a CIN 3 lesion that has associated invasion is, on average, seven times larger than without invasion.6 Although colposcopy is much less likely to miss large lesions, it is important to miss as little high-grade disease as possible because the risk of invasion is cumulative over time and unpredictable in a given patient.

Multiple biopsies boost detection
Sampling more than one area improves the accuracy of colposcopically directed biopsy, even when one area looks most abnormal. This colposcopy photo shows potential biopsy sites (within the ovals), although other choices may also be reasonable. Several studies have shown that colposcopically directed biopsy of even normal-appearing areas at the squamocolumnar junction or within large ectopies can improve detection of high-grade cervical intraepithelial neoplasia or adenocarcinoma in situ.Studies have shown that it is possible to increase the accuracy of detection of CIN 2+ by increasing the number of biopsies. In this study by Stoler and colleagues, the sensitivity of initial colposcopy improved from 47% (for one biopsy) to 65% (two biopsies) and 77% (three or more) (FIGURE). Overall agreement increased with increasing age, which is consistent with the likelihood that CIN 3 lesions expand with age and become increasingly detectable by colposcopy.
Colposcopy does work, but the era of biopsying only the most abnormal-appearing area is over. Take more biopsies.
We want to hear from you! Tell us what you think.
1. Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38(5):525-533.
2. Centers for Disease Control and Prevention. Frequently asked questions about HPV vaccine safety. http://www.cdc.gov/vaccinesafety/Vaccines/HPV/hpv_faqs.html. Accessed February 1, 2011.
3. Cox JT, Moriarty AT, Castle PE. Commentary on: Statement on HPV DNA test utilization. Diagn Cytopathol. 2009;37(7):471-474.
4. Cox JT. More questions about the accuracy of colposcopy: what does this mean for cervical cancer prevention? Obstet Gynecol. 2008;111(6):1266-1267.
5. Sherman ME, Wang SS, Tarone R, Rich L, Schiffman M. Histopathologic extent of CIN 3 lesions in ALTS: implications for subject safety and lead-time bias. Cancer Epidemiol Biomarkers Prev. 2003;12(4):372-379.
6. Tidbury P, Singer A, Jenkins D. CIN 3: the role of lesion size in invasion. Br J Obstet Gynaecol. 1992;99(7):583-586.
1. Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38(5):525-533.
2. Centers for Disease Control and Prevention. Frequently asked questions about HPV vaccine safety. http://www.cdc.gov/vaccinesafety/Vaccines/HPV/hpv_faqs.html. Accessed February 1, 2011.
3. Cox JT, Moriarty AT, Castle PE. Commentary on: Statement on HPV DNA test utilization. Diagn Cytopathol. 2009;37(7):471-474.
4. Cox JT. More questions about the accuracy of colposcopy: what does this mean for cervical cancer prevention? Obstet Gynecol. 2008;111(6):1266-1267.
5. Sherman ME, Wang SS, Tarone R, Rich L, Schiffman M. Histopathologic extent of CIN 3 lesions in ALTS: implications for subject safety and lead-time bias. Cancer Epidemiol Biomarkers Prev. 2003;12(4):372-379.
6. Tidbury P, Singer A, Jenkins D. CIN 3: the role of lesion size in invasion. Br J Obstet Gynaecol. 1992;99(7):583-586.
How to improve outcomes in gestational diabetes— for mother and baby
- Are oral hypoglycemic agents equivalent to insulin in the treatment of gestational diabetes?
Aaron B. Caughey, MD, PhD; (Examining the Evidence, March 2011)
Gestational diabetes mellitus (GDM) was once thought to be a mild condition that had few lasting consequences. Now, we know that it carries significant short- and long-term implications for women and their offspring. A growing body of evidence suggests that early detection and aggressive monitoring and management of GDM can greatly improve outcomes for pregnant women and their babies. This article outlines the parameters of this approach.
GDM increases maternal risks even after pregnancy
Even mild degrees of hyperglycemia during pregnancy can harm mother and baby. Hyperglycemia is associated with an elevated risk of hypertensive disorders during pregnancy, as well as preterm labor, cesarean delivery, and later metabolic disorders—but there is no obvious threshold of hyperglycemia at which these risks increase.1
GDM is a strong predictor that a woman will later develop type 2 diabetes.2 One study found that GDM increases that risk as much as sevenfold over a woman’s lifetime.3 GDM is also associated with an elevated risk of cardiovascular disease, particularly if the woman has a family history of type 2 diabetes.4
Obesity appears to worsen the consequences of GDM for women.5 A recent literature review found that the risk of GDM is positively associated with the prepregnancy body mass index (BMI).6
One of the most common and serious types of morbidity affecting infants born to women who have GDM is large size for gestational age, which imparts a significantly elevated risk of injury at the time of vaginal birth and increases the risk of trauma to the mother during cesarean delivery.
GDM is not benign in the fetus, either
Evidence is increasing that GDM raises the risk of adverse clinical consequences in the fetus. The two most frequent and serious types of morbidity affecting infants born to mothers who have GDM are:
- large size for gestational age
- respiratory distress syndrome.7
Infants who are large for gestational age (LGA) face a significantly elevated risk of injury at the time of vaginal birth, such as shoulder dystocia and newborn asphyxia.8 Cesarean delivery is the preferred route for the LGA infant, but it often increases the risk of trauma to the mother, compared with the vaginal route.8
Respiratory distress syndrome, common among premature infants, also affects many infants born to women who have GDM— even near-term infants—because hyperglycemia appears to delay fetal lung maturity.9
Recent studies indicate that exposure to maternal hyperglycemia also increases a child’s risk of long-term complications. Children born to mothers who have GDM have nearly twice the risk of childhood obesity and metabolic syndrome, compared with children born to mothers who do not have GDM.10 In addition, several studies have found that children born to obese mothers who have GDM are more likely to develop type 2 diabetes than are children of non-obese mothers without GDM.3,11
Occasionally, infants of women who have GDM are born with hypoglycemia; this condition arises from an insulin surge in response to maternal hyperglycemia. In an infant, hypoglycemia can lead to seizures and death, and maternal hypoglycemia can cause neuro-psychological deficits in the infant.12
Other health problems related to GDM include jaundice and developmental delays in walking and other motor skills.13
The two-step, 100-g, 3-hour oral glucose tolerance test (OGTT) has been the gold standard for diagnosis of GDM in the United States for many years. However, this approach is expensive—rendering it impractical in some settings. Moreover, reproducibility is only approximately 78%.14
The World Health Organization recently reviewed evidence underlying various diagnostic techniques and recommended a one-step, 2-hour, 75-g OGTT for GDM.14 Another recent review of the literature on the various screening protocols underscores the validity of this approach.15
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of more than 23,000 nondiabetic women incorporated the 2-hour, 75-g OGTT.16 Investigators found that elevated glucose levels on this test are highly predictive of birth weight above the 90th percentile and a cord-blood serum C-peptide level above the 90th percentile. However, the test has weaker predictive value for primary cesarean delivery and clinical neonatal hypoglycemia.
Based on the work of HAPO, the American Diabetes Association (ADA) revised its guidelines for diabetes assessment and now recommends that physicians perform a 75-g OGTT at 24 to 28 weeks’ gestation, with plasma glucose measurement in the fasting state and at 1 and 2 hours. A single abnormal level merits a diagnosis of GDM in women not previously diagnosed with overt diabetes.17
Any diagnosis of GDM warrants aggressive treatment
Perhaps the single greatest controversy in the field of diabetes centers on the level of hyperglycemia at which aggressive treatment of GDM should begin. Traditionally, aggressive therapy (i.e., insulin) was not initiated until the fasting plasma glucose level reached 95 mg/dL or higher or the 1-hour glucose level reached 130 mg/dL or higher (ALGORITHM). However, recent studies suggest that aggressive treatment should be administered for any diagnosis of GDM.
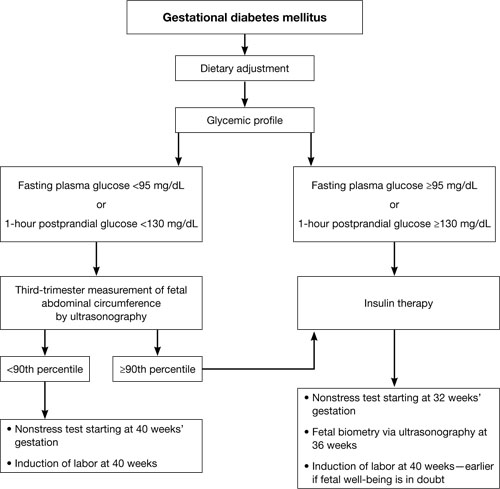
Typical management plan for gestational diabetesFor example, the HAPO study was designed to determine the level of glucose intolerance during pregnancy, short of diabetes, associated with adverse outcomes.16 It found that even mild hyperglycemia is associated with adverse fetal outcomes and that diagnostic criteria for GDM cannot easily be based on any particular level of hyperglycemia.
Several other studies have demonstrated that aggressive treatment of mild GDM can ameliorate many of its negative effects. In 2005, for instance, Bonomo and coworkers explored the effect on newborns of treating a very mild level of gestational glucose intolerance among 300 women.18 The randomized trial involved three groups:
- Group A – standard management, which entailed no special care, diet, or pharmacotherapy
- Group B – dietary treatment and regular monitoring
- Group C – randomly selected pregnant women who were matched by BMI and age and who had normal screening test results.
The women in Group B experienced significant improvements in fasting and 2-hour postprandial glucose levels. In addition, the fasting glucose level at delivery was significantly lower in Group B, compared with the other two groups. More important, fewer LGA infants were born to the women in Group B (6.0%) than in Group A (14.0%) and Group C (9.1%).
Landon and colleagues obtained similar findings when they randomized almost 1,000 pregnant women who had mild GDM to 1) usual prenatal care or 2) dietary intervention, self-monitoring of blood glucose, and, if necessary, insulin therapy.19
Insulin analogs have joined the treatment options
Standard treatment for GDM involves diet and nutritional therapy and, when needed, insulin. A diet that limits carbohydrate in-take can significantly reduce glycemia after meals in women who have GDM.20
For years, human insulin was the only option for treating diabetes that cannot be controlled by diet and lifestyle modifications alone. Recently, however, several insulin analogs have come on the market. Only two of them have been well studied in pregnancy:
- 28B-L-lysine-29B-L-proline insulin (lispro)
- 28B-aspartic acid insulin (aspart).
These two analogs have been tested primarily in the setting of type 1 diabetes, but both improve postprandial glucose excursions, compared with human regular insulin, and both may be associated with a lower risk of delayed postprandial hypoglycemia.21,22
Some oral agents appear to be safe
Several oral antihyperglycemic agents are available for the management of diabetes (TABLE). However, in the past, oral agents were not used in pregnant women out of concern over reports of fetal anomalies and other adverse outcomes in animal studies and some human cases. More recent evidence suggests that glyburide and metformin are safe and effective for use in GDM.23-25
Oral antihyperglycemic agents and their potential side effects
| Class | Agents | Effects |
|---|---|---|
| Insulin secretagogue | Sulfonylureas and meglitinides such as glyburide, glipizide, glimepiride, repaglinide, nateglinide | Hypoglycemia if caloric intake is reduced Some are long-acting (increasing risk of prolonged hypoglycemia) |
| Biguanide | Metformin | Risk of lactic acidosis when used in the setting of renal dysfunction, circulatory compromise, or hypoxemia Relatively slow onset of action GI complications: nausea, diarrhea |
| Thiazoladinedione | Rosiglitazone, pioglitazone | Long delay to onset of action (2–3 weeks) Associated with fluid retention (particularly when used with insulin) and increased risk of congestive heart failure Use contraindicated in presence of liver disease or elevated transaminases |
| Alpha-glucosidase inhibitor | Acarbose, miglitol | Prandial/meal agent (no effect in the fasting patient) Abdominal bloating and flatus Pure dextrose is required to treat hypoglycemia that occurs in the setting of these agents |
| Glucagon-like peptide–1 mimetic | Exenatide | Newer agents with limited inpatient experience Abdominal bloating and nausea secondary to delayed gastric emptying |
| Dipeptidyl peptidase IV inhibitor | Sitagliptin | Newer agent with limited inpatient experience |
Langer and coworkers compared glyburide with insulin in the management of GDM and found the agents to be equally effective, with comparable levels of risk of large size for gestational age, macrosomia, hypoglycemia (in infants), NICU admission, and fetal anomaly.23 Subsequent studies have confirmed these findings, although at least one suggests that women who have a high fasting plasma glucose level may not respond adequately to glyburide.26 None of these studies has been large enough or long enough to truly assess whether these oral medications are equivalent to insulin in the management of GDM without posing significant long-term complications for mothers or babies, or both.
For more on the use of oral agents in GDM, see Dr. Aaron B. Caughey’s commentary on the subject of this issue.
Continuous monitoring may detect occult hyperglycemia and hypoglycemia
The traditional method of monitoring the blood glucose level is to stick a finger to obtain a blood sample and use a test strip and a meter to measure the concentration of glucose in the sample. Most meters on the market are reasonably accurate. However, research has demonstrated that they are least accurate during episodes of hypoglycemia.27
Automated continuous glucose-monitoring systems are less intrusive than the traditional method, but they are usually reserved for people who have type 1 diabetes requiring intensive insulin therapy. However, because data suggest that even short periods of hyperglycemia or hypoglycemia can be detrimental to a developing fetus, there is increasing interest in utilizing continuous glucose monitoring for GDM.
Several research groups have compared continuous glucose monitoring with finger-stick monitoring and found that women randomized to continuous monitoring have lower mean hemoglobin A1c levels from 32 to 36 weeks’ gestation.28,29 (See “Exploring the value of continuous glucose monitoring in gestational diabetes?”) Women undergoing continuous monitoring also have:
- lower mean birth-weight standard- deviation scores
- lower median customized birth-weight centiles
- a reduced risk of macrosomia.
One study found that information gleaned from continuous glucose monitoring provided additional information that altered clinical management in 42 of 68 (62%) cases. These additional data included evidence of undetected and potentially dangerous postprandial hyperglycemia and overnight hypoglycemia.29
Yogev and colleagues found that continuous glucose monitoring is significantly more sensitive than traditional methods in detecting periods of hypoglycemia in women who have GDM. They also found that asymptomatic hypoglycemic events are common during pharmacotherapy in gestations affected by GDM.30 The same group used continuous glucose monitoring at night in obese, nondiabetic women to identify previously undetected:
- high postprandial glucose peak values
- increased 1- and 2-hour postprandial glucose levels
- increased time to the glucose peak
- significantly lower mean blood glucose levels.31
Insurers were reluctant to cover continuous glucose monitoring devices when they first became available. Since then, however, much progress has been made. Nevertheless, inadequate reimbursement for the time it takes a clinician to change a patient’s treatment regimen and her subsequent management remains a significant barrier to adoption of these systems.32 The key to success with continuous glucose monitoring is to train the patient to use it properly.
Exploring the value of continuous glucose monitoring in gestational diabetes
Tanenberg R, Bode B, Lane W, et al. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526.
The American Diabetes association recommends that patients on insulin self-monitor blood glucose three or four times daily to guide adjustments in therapy and ensure a stable and optimal hemoglobin A1c level. “however, adherence to frequent blood-glucose monitoring is low, and less than 54% of patients with insulin-treated diabetes are reported to self-monitor their blood glucose at least three times each day,” say tanenberg and coworkers.
To determine whether use of a continuous glucose-monitoring system improves metabolic control, the investigators randomized 109 patients who had insulin-treated diabetes to continuous monitoring or frequent self-monitoring. at enrollment, all patients had insulin-treated diabetes and inadequate metabolic control. at the end of the study, both groups used continuous monitoring for 3 days; these values were used to calculate measures of hypoglycemia.
In the study, the women in the self-monitoring group were counseled to measure capillary blood glucose a minimum of four times daily, as well as when they experienced symptoms of hypoglycemia, which was defined as a blood glucose measurement of 60 mg/dL or lower. any hypoglycemic event was considered to be over when the measurement exceeded 60 mg/dL for at least 30 minutes.
Findings
Hemoglobin A1c levels were similar between groups at baseline, and both groups showed significant (P < .001) and similar (P=.95) improvement in these levels after 12 weeks of study. however, the continuous-monitoring group had a significantly shorter duration of hypoglycemic events than the self-monitoring group at week 12 (49.4±40.8 minutes vs 81.0±61.1 minutes per event, respectively; P=.009).
Tanenberg and coworkers hypothesize that the improvement in hemoglobin A1c in the self-monitoring group was a result of monitoring that was more frequent (7 times a day) than is typical. they concluded that use of continuous monitoring to guide therapy adjustments in patients who use insulin significantly reduces the duration of hypoglycemia, compared with adjustments guided by self-monitoring values alone.
We want to hear from you! Tell us what you think.
1. Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: determinants outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188(5):1372-1378.
2. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668-1669.
3. Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9(1):83-88.
4. Gunderson EP, Jacobs DR, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201(2):177.e1-9.
5. van Hoorn J, Dekker G, Jeffries B. Gestational diabetes versus obesity as risk factors for pregnancy-induced hypertensive disorders and fetal macrosomia. Aust N Z J Obstet Gynaecol. 2002;42(1):29-34.
6. Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203.
7. Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can. 2006;28(2):122-127.
8. Jones CW. Gestational diabetes and its impact on the neonate. Neonatal Netw. 2001;20(6):17-23
9. De Luca AK, Nakazawa CY, Azevedo BC, Rudge MV, De Araujo Costa RA, Calderon IM. Influence of glycemic control on fetal lung maturity in gestations affected by diabetes or mild hyperglycemia. Acta Obstet Gynecol Scand. 2009;88(9):1036-1040.
10. Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672.-e1–4.
11. Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21(3):149-157
12. ter Braak EW, Evers IM, Willem Erkelens D, Visser GH. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev. 2002;18(2):96-105.
13. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3):199-203.
14. Harlass FE, Brady K, Read JA. Reproducibility of the oral glucose tolerance test in pregnancy. Am J Obstet Gynecol. 1991;164(2):564-568.
15. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31(8):1650-1655.
16. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New Engl J Med. 2008;358(19):1991-2002.
17. American Diabetes Association. Executive summary: standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S4-10.
18. Bonomo M, Corica D, Mion E, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med. 2005;22(11):1536-1541.
19. Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
20. 20Cypryk K, Kaminska P, Kosinski M, Pertynska-Marczewska M, Lewinski A. A comparison of the effectiveness tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol. 2007;58(4):314-319.
21. Lapolla A, Dalfrà MG, Spezia R, et al. Outcome of pregnancy in type 1 diabetic patients treated with insulin lispro or regular insulin: an Italian experience. Acta Diabetol. 2008;45(1):61-66.
22. Hod M, Damm P, Kaaja R, et al. Insulin Aspart Pregnancy Study Group. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198(2):186-187.
23. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzles O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343(16):1134-1138.
24. Ho FL, Liew CF, Cunanan EC, Lee KO. Oral hypoglycaemic agents for diabetes in pregnancy—an appraisal of the current evidence for oral anti-diabetic drug use in pregnancy. Ann Acad Med Singapore. 2007;36(8):672-678.
25. Nicholson W, Bolen S, Witkop CT, et al. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):193-205.
26. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. Matern Fetal Neonatal Med. 2004;15(1):51-55.
27. Carr S, Coustan DR, Martelly P, et al. Precision of reflectance meters in screening for gestational diabetes. Obstet Gynecol. 1989;73(5 Pt 1):727-731.
28. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680.-doi: 10.1136/bmj.a1680.
29. McLachlan K, Jenkins A, O’Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol. 2007;47(3):186-190.
30. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Undiagnosed asymptomatic hypoglycemia: diet insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104(1):88-93.
31. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949-953.
32. Bartelme A, Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3(4):992-995.
- Are oral hypoglycemic agents equivalent to insulin in the treatment of gestational diabetes?
Aaron B. Caughey, MD, PhD; (Examining the Evidence, March 2011)
Gestational diabetes mellitus (GDM) was once thought to be a mild condition that had few lasting consequences. Now, we know that it carries significant short- and long-term implications for women and their offspring. A growing body of evidence suggests that early detection and aggressive monitoring and management of GDM can greatly improve outcomes for pregnant women and their babies. This article outlines the parameters of this approach.
GDM increases maternal risks even after pregnancy
Even mild degrees of hyperglycemia during pregnancy can harm mother and baby. Hyperglycemia is associated with an elevated risk of hypertensive disorders during pregnancy, as well as preterm labor, cesarean delivery, and later metabolic disorders—but there is no obvious threshold of hyperglycemia at which these risks increase.1
GDM is a strong predictor that a woman will later develop type 2 diabetes.2 One study found that GDM increases that risk as much as sevenfold over a woman’s lifetime.3 GDM is also associated with an elevated risk of cardiovascular disease, particularly if the woman has a family history of type 2 diabetes.4
Obesity appears to worsen the consequences of GDM for women.5 A recent literature review found that the risk of GDM is positively associated with the prepregnancy body mass index (BMI).6

One of the most common and serious types of morbidity affecting infants born to women who have GDM is large size for gestational age, which imparts a significantly elevated risk of injury at the time of vaginal birth and increases the risk of trauma to the mother during cesarean delivery.
GDM is not benign in the fetus, either
Evidence is increasing that GDM raises the risk of adverse clinical consequences in the fetus. The two most frequent and serious types of morbidity affecting infants born to mothers who have GDM are:
- large size for gestational age
- respiratory distress syndrome.7
Infants who are large for gestational age (LGA) face a significantly elevated risk of injury at the time of vaginal birth, such as shoulder dystocia and newborn asphyxia.8 Cesarean delivery is the preferred route for the LGA infant, but it often increases the risk of trauma to the mother, compared with the vaginal route.8
Respiratory distress syndrome, common among premature infants, also affects many infants born to women who have GDM— even near-term infants—because hyperglycemia appears to delay fetal lung maturity.9
Recent studies indicate that exposure to maternal hyperglycemia also increases a child’s risk of long-term complications. Children born to mothers who have GDM have nearly twice the risk of childhood obesity and metabolic syndrome, compared with children born to mothers who do not have GDM.10 In addition, several studies have found that children born to obese mothers who have GDM are more likely to develop type 2 diabetes than are children of non-obese mothers without GDM.3,11
Occasionally, infants of women who have GDM are born with hypoglycemia; this condition arises from an insulin surge in response to maternal hyperglycemia. In an infant, hypoglycemia can lead to seizures and death, and maternal hypoglycemia can cause neuro-psychological deficits in the infant.12
Other health problems related to GDM include jaundice and developmental delays in walking and other motor skills.13
The two-step, 100-g, 3-hour oral glucose tolerance test (OGTT) has been the gold standard for diagnosis of GDM in the United States for many years. However, this approach is expensive—rendering it impractical in some settings. Moreover, reproducibility is only approximately 78%.14
The World Health Organization recently reviewed evidence underlying various diagnostic techniques and recommended a one-step, 2-hour, 75-g OGTT for GDM.14 Another recent review of the literature on the various screening protocols underscores the validity of this approach.15
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of more than 23,000 nondiabetic women incorporated the 2-hour, 75-g OGTT.16 Investigators found that elevated glucose levels on this test are highly predictive of birth weight above the 90th percentile and a cord-blood serum C-peptide level above the 90th percentile. However, the test has weaker predictive value for primary cesarean delivery and clinical neonatal hypoglycemia.
Based on the work of HAPO, the American Diabetes Association (ADA) revised its guidelines for diabetes assessment and now recommends that physicians perform a 75-g OGTT at 24 to 28 weeks’ gestation, with plasma glucose measurement in the fasting state and at 1 and 2 hours. A single abnormal level merits a diagnosis of GDM in women not previously diagnosed with overt diabetes.17
Any diagnosis of GDM warrants aggressive treatment
Perhaps the single greatest controversy in the field of diabetes centers on the level of hyperglycemia at which aggressive treatment of GDM should begin. Traditionally, aggressive therapy (i.e., insulin) was not initiated until the fasting plasma glucose level reached 95 mg/dL or higher or the 1-hour glucose level reached 130 mg/dL or higher (ALGORITHM). However, recent studies suggest that aggressive treatment should be administered for any diagnosis of GDM.

Typical management plan for gestational diabetesFor example, the HAPO study was designed to determine the level of glucose intolerance during pregnancy, short of diabetes, associated with adverse outcomes.16 It found that even mild hyperglycemia is associated with adverse fetal outcomes and that diagnostic criteria for GDM cannot easily be based on any particular level of hyperglycemia.
Several other studies have demonstrated that aggressive treatment of mild GDM can ameliorate many of its negative effects. In 2005, for instance, Bonomo and coworkers explored the effect on newborns of treating a very mild level of gestational glucose intolerance among 300 women.18 The randomized trial involved three groups:
- Group A – standard management, which entailed no special care, diet, or pharmacotherapy
- Group B – dietary treatment and regular monitoring
- Group C – randomly selected pregnant women who were matched by BMI and age and who had normal screening test results.
The women in Group B experienced significant improvements in fasting and 2-hour postprandial glucose levels. In addition, the fasting glucose level at delivery was significantly lower in Group B, compared with the other two groups. More important, fewer LGA infants were born to the women in Group B (6.0%) than in Group A (14.0%) and Group C (9.1%).
Landon and colleagues obtained similar findings when they randomized almost 1,000 pregnant women who had mild GDM to 1) usual prenatal care or 2) dietary intervention, self-monitoring of blood glucose, and, if necessary, insulin therapy.19
Insulin analogs have joined the treatment options
Standard treatment for GDM involves diet and nutritional therapy and, when needed, insulin. A diet that limits carbohydrate in-take can significantly reduce glycemia after meals in women who have GDM.20
For years, human insulin was the only option for treating diabetes that cannot be controlled by diet and lifestyle modifications alone. Recently, however, several insulin analogs have come on the market. Only two of them have been well studied in pregnancy:
- 28B-L-lysine-29B-L-proline insulin (lispro)
- 28B-aspartic acid insulin (aspart).
These two analogs have been tested primarily in the setting of type 1 diabetes, but both improve postprandial glucose excursions, compared with human regular insulin, and both may be associated with a lower risk of delayed postprandial hypoglycemia.21,22
Some oral agents appear to be safe
Several oral antihyperglycemic agents are available for the management of diabetes (TABLE). However, in the past, oral agents were not used in pregnant women out of concern over reports of fetal anomalies and other adverse outcomes in animal studies and some human cases. More recent evidence suggests that glyburide and metformin are safe and effective for use in GDM.23-25
Oral antihyperglycemic agents and their potential side effects
| Class | Agents | Effects |
|---|---|---|
| Insulin secretagogue | Sulfonylureas and meglitinides such as glyburide, glipizide, glimepiride, repaglinide, nateglinide | Hypoglycemia if caloric intake is reduced Some are long-acting (increasing risk of prolonged hypoglycemia) |
| Biguanide | Metformin | Risk of lactic acidosis when used in the setting of renal dysfunction, circulatory compromise, or hypoxemia Relatively slow onset of action GI complications: nausea, diarrhea |
| Thiazoladinedione | Rosiglitazone, pioglitazone | Long delay to onset of action (2–3 weeks) Associated with fluid retention (particularly when used with insulin) and increased risk of congestive heart failure Use contraindicated in presence of liver disease or elevated transaminases |
| Alpha-glucosidase inhibitor | Acarbose, miglitol | Prandial/meal agent (no effect in the fasting patient) Abdominal bloating and flatus Pure dextrose is required to treat hypoglycemia that occurs in the setting of these agents |
| Glucagon-like peptide–1 mimetic | Exenatide | Newer agents with limited inpatient experience Abdominal bloating and nausea secondary to delayed gastric emptying |
| Dipeptidyl peptidase IV inhibitor | Sitagliptin | Newer agent with limited inpatient experience |
Langer and coworkers compared glyburide with insulin in the management of GDM and found the agents to be equally effective, with comparable levels of risk of large size for gestational age, macrosomia, hypoglycemia (in infants), NICU admission, and fetal anomaly.23 Subsequent studies have confirmed these findings, although at least one suggests that women who have a high fasting plasma glucose level may not respond adequately to glyburide.26 None of these studies has been large enough or long enough to truly assess whether these oral medications are equivalent to insulin in the management of GDM without posing significant long-term complications for mothers or babies, or both.
For more on the use of oral agents in GDM, see Dr. Aaron B. Caughey’s commentary on the subject of this issue.
Continuous monitoring may detect occult hyperglycemia and hypoglycemia
The traditional method of monitoring the blood glucose level is to stick a finger to obtain a blood sample and use a test strip and a meter to measure the concentration of glucose in the sample. Most meters on the market are reasonably accurate. However, research has demonstrated that they are least accurate during episodes of hypoglycemia.27
Automated continuous glucose-monitoring systems are less intrusive than the traditional method, but they are usually reserved for people who have type 1 diabetes requiring intensive insulin therapy. However, because data suggest that even short periods of hyperglycemia or hypoglycemia can be detrimental to a developing fetus, there is increasing interest in utilizing continuous glucose monitoring for GDM.
Several research groups have compared continuous glucose monitoring with finger-stick monitoring and found that women randomized to continuous monitoring have lower mean hemoglobin A1c levels from 32 to 36 weeks’ gestation.28,29 (See “Exploring the value of continuous glucose monitoring in gestational diabetes?”) Women undergoing continuous monitoring also have:
- lower mean birth-weight standard- deviation scores
- lower median customized birth-weight centiles
- a reduced risk of macrosomia.
One study found that information gleaned from continuous glucose monitoring provided additional information that altered clinical management in 42 of 68 (62%) cases. These additional data included evidence of undetected and potentially dangerous postprandial hyperglycemia and overnight hypoglycemia.29
Yogev and colleagues found that continuous glucose monitoring is significantly more sensitive than traditional methods in detecting periods of hypoglycemia in women who have GDM. They also found that asymptomatic hypoglycemic events are common during pharmacotherapy in gestations affected by GDM.30 The same group used continuous glucose monitoring at night in obese, nondiabetic women to identify previously undetected:
- high postprandial glucose peak values
- increased 1- and 2-hour postprandial glucose levels
- increased time to the glucose peak
- significantly lower mean blood glucose levels.31
Insurers were reluctant to cover continuous glucose monitoring devices when they first became available. Since then, however, much progress has been made. Nevertheless, inadequate reimbursement for the time it takes a clinician to change a patient’s treatment regimen and her subsequent management remains a significant barrier to adoption of these systems.32 The key to success with continuous glucose monitoring is to train the patient to use it properly.
Exploring the value of continuous glucose monitoring in gestational diabetes
Tanenberg R, Bode B, Lane W, et al. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526.
The American Diabetes association recommends that patients on insulin self-monitor blood glucose three or four times daily to guide adjustments in therapy and ensure a stable and optimal hemoglobin A1c level. “however, adherence to frequent blood-glucose monitoring is low, and less than 54% of patients with insulin-treated diabetes are reported to self-monitor their blood glucose at least three times each day,” say tanenberg and coworkers.
To determine whether use of a continuous glucose-monitoring system improves metabolic control, the investigators randomized 109 patients who had insulin-treated diabetes to continuous monitoring or frequent self-monitoring. at enrollment, all patients had insulin-treated diabetes and inadequate metabolic control. at the end of the study, both groups used continuous monitoring for 3 days; these values were used to calculate measures of hypoglycemia.
In the study, the women in the self-monitoring group were counseled to measure capillary blood glucose a minimum of four times daily, as well as when they experienced symptoms of hypoglycemia, which was defined as a blood glucose measurement of 60 mg/dL or lower. any hypoglycemic event was considered to be over when the measurement exceeded 60 mg/dL for at least 30 minutes.
Findings
Hemoglobin A1c levels were similar between groups at baseline, and both groups showed significant (P < .001) and similar (P=.95) improvement in these levels after 12 weeks of study. however, the continuous-monitoring group had a significantly shorter duration of hypoglycemic events than the self-monitoring group at week 12 (49.4±40.8 minutes vs 81.0±61.1 minutes per event, respectively; P=.009).
Tanenberg and coworkers hypothesize that the improvement in hemoglobin A1c in the self-monitoring group was a result of monitoring that was more frequent (7 times a day) than is typical. they concluded that use of continuous monitoring to guide therapy adjustments in patients who use insulin significantly reduces the duration of hypoglycemia, compared with adjustments guided by self-monitoring values alone.
We want to hear from you! Tell us what you think.
- Are oral hypoglycemic agents equivalent to insulin in the treatment of gestational diabetes?
Aaron B. Caughey, MD, PhD; (Examining the Evidence, March 2011)
Gestational diabetes mellitus (GDM) was once thought to be a mild condition that had few lasting consequences. Now, we know that it carries significant short- and long-term implications for women and their offspring. A growing body of evidence suggests that early detection and aggressive monitoring and management of GDM can greatly improve outcomes for pregnant women and their babies. This article outlines the parameters of this approach.
GDM increases maternal risks even after pregnancy
Even mild degrees of hyperglycemia during pregnancy can harm mother and baby. Hyperglycemia is associated with an elevated risk of hypertensive disorders during pregnancy, as well as preterm labor, cesarean delivery, and later metabolic disorders—but there is no obvious threshold of hyperglycemia at which these risks increase.1
GDM is a strong predictor that a woman will later develop type 2 diabetes.2 One study found that GDM increases that risk as much as sevenfold over a woman’s lifetime.3 GDM is also associated with an elevated risk of cardiovascular disease, particularly if the woman has a family history of type 2 diabetes.4
Obesity appears to worsen the consequences of GDM for women.5 A recent literature review found that the risk of GDM is positively associated with the prepregnancy body mass index (BMI).6

One of the most common and serious types of morbidity affecting infants born to women who have GDM is large size for gestational age, which imparts a significantly elevated risk of injury at the time of vaginal birth and increases the risk of trauma to the mother during cesarean delivery.
GDM is not benign in the fetus, either
Evidence is increasing that GDM raises the risk of adverse clinical consequences in the fetus. The two most frequent and serious types of morbidity affecting infants born to mothers who have GDM are:
- large size for gestational age
- respiratory distress syndrome.7
Infants who are large for gestational age (LGA) face a significantly elevated risk of injury at the time of vaginal birth, such as shoulder dystocia and newborn asphyxia.8 Cesarean delivery is the preferred route for the LGA infant, but it often increases the risk of trauma to the mother, compared with the vaginal route.8
Respiratory distress syndrome, common among premature infants, also affects many infants born to women who have GDM— even near-term infants—because hyperglycemia appears to delay fetal lung maturity.9
Recent studies indicate that exposure to maternal hyperglycemia also increases a child’s risk of long-term complications. Children born to mothers who have GDM have nearly twice the risk of childhood obesity and metabolic syndrome, compared with children born to mothers who do not have GDM.10 In addition, several studies have found that children born to obese mothers who have GDM are more likely to develop type 2 diabetes than are children of non-obese mothers without GDM.3,11
Occasionally, infants of women who have GDM are born with hypoglycemia; this condition arises from an insulin surge in response to maternal hyperglycemia. In an infant, hypoglycemia can lead to seizures and death, and maternal hypoglycemia can cause neuro-psychological deficits in the infant.12
Other health problems related to GDM include jaundice and developmental delays in walking and other motor skills.13
The two-step, 100-g, 3-hour oral glucose tolerance test (OGTT) has been the gold standard for diagnosis of GDM in the United States for many years. However, this approach is expensive—rendering it impractical in some settings. Moreover, reproducibility is only approximately 78%.14
The World Health Organization recently reviewed evidence underlying various diagnostic techniques and recommended a one-step, 2-hour, 75-g OGTT for GDM.14 Another recent review of the literature on the various screening protocols underscores the validity of this approach.15
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of more than 23,000 nondiabetic women incorporated the 2-hour, 75-g OGTT.16 Investigators found that elevated glucose levels on this test are highly predictive of birth weight above the 90th percentile and a cord-blood serum C-peptide level above the 90th percentile. However, the test has weaker predictive value for primary cesarean delivery and clinical neonatal hypoglycemia.
Based on the work of HAPO, the American Diabetes Association (ADA) revised its guidelines for diabetes assessment and now recommends that physicians perform a 75-g OGTT at 24 to 28 weeks’ gestation, with plasma glucose measurement in the fasting state and at 1 and 2 hours. A single abnormal level merits a diagnosis of GDM in women not previously diagnosed with overt diabetes.17
Any diagnosis of GDM warrants aggressive treatment
Perhaps the single greatest controversy in the field of diabetes centers on the level of hyperglycemia at which aggressive treatment of GDM should begin. Traditionally, aggressive therapy (i.e., insulin) was not initiated until the fasting plasma glucose level reached 95 mg/dL or higher or the 1-hour glucose level reached 130 mg/dL or higher (ALGORITHM). However, recent studies suggest that aggressive treatment should be administered for any diagnosis of GDM.

Typical management plan for gestational diabetesFor example, the HAPO study was designed to determine the level of glucose intolerance during pregnancy, short of diabetes, associated with adverse outcomes.16 It found that even mild hyperglycemia is associated with adverse fetal outcomes and that diagnostic criteria for GDM cannot easily be based on any particular level of hyperglycemia.
Several other studies have demonstrated that aggressive treatment of mild GDM can ameliorate many of its negative effects. In 2005, for instance, Bonomo and coworkers explored the effect on newborns of treating a very mild level of gestational glucose intolerance among 300 women.18 The randomized trial involved three groups:
- Group A – standard management, which entailed no special care, diet, or pharmacotherapy
- Group B – dietary treatment and regular monitoring
- Group C – randomly selected pregnant women who were matched by BMI and age and who had normal screening test results.
The women in Group B experienced significant improvements in fasting and 2-hour postprandial glucose levels. In addition, the fasting glucose level at delivery was significantly lower in Group B, compared with the other two groups. More important, fewer LGA infants were born to the women in Group B (6.0%) than in Group A (14.0%) and Group C (9.1%).
Landon and colleagues obtained similar findings when they randomized almost 1,000 pregnant women who had mild GDM to 1) usual prenatal care or 2) dietary intervention, self-monitoring of blood glucose, and, if necessary, insulin therapy.19
Insulin analogs have joined the treatment options
Standard treatment for GDM involves diet and nutritional therapy and, when needed, insulin. A diet that limits carbohydrate in-take can significantly reduce glycemia after meals in women who have GDM.20
For years, human insulin was the only option for treating diabetes that cannot be controlled by diet and lifestyle modifications alone. Recently, however, several insulin analogs have come on the market. Only two of them have been well studied in pregnancy:
- 28B-L-lysine-29B-L-proline insulin (lispro)
- 28B-aspartic acid insulin (aspart).
These two analogs have been tested primarily in the setting of type 1 diabetes, but both improve postprandial glucose excursions, compared with human regular insulin, and both may be associated with a lower risk of delayed postprandial hypoglycemia.21,22
Some oral agents appear to be safe
Several oral antihyperglycemic agents are available for the management of diabetes (TABLE). However, in the past, oral agents were not used in pregnant women out of concern over reports of fetal anomalies and other adverse outcomes in animal studies and some human cases. More recent evidence suggests that glyburide and metformin are safe and effective for use in GDM.23-25
Oral antihyperglycemic agents and their potential side effects
| Class | Agents | Effects |
|---|---|---|
| Insulin secretagogue | Sulfonylureas and meglitinides such as glyburide, glipizide, glimepiride, repaglinide, nateglinide | Hypoglycemia if caloric intake is reduced Some are long-acting (increasing risk of prolonged hypoglycemia) |
| Biguanide | Metformin | Risk of lactic acidosis when used in the setting of renal dysfunction, circulatory compromise, or hypoxemia Relatively slow onset of action GI complications: nausea, diarrhea |
| Thiazoladinedione | Rosiglitazone, pioglitazone | Long delay to onset of action (2–3 weeks) Associated with fluid retention (particularly when used with insulin) and increased risk of congestive heart failure Use contraindicated in presence of liver disease or elevated transaminases |
| Alpha-glucosidase inhibitor | Acarbose, miglitol | Prandial/meal agent (no effect in the fasting patient) Abdominal bloating and flatus Pure dextrose is required to treat hypoglycemia that occurs in the setting of these agents |
| Glucagon-like peptide–1 mimetic | Exenatide | Newer agents with limited inpatient experience Abdominal bloating and nausea secondary to delayed gastric emptying |
| Dipeptidyl peptidase IV inhibitor | Sitagliptin | Newer agent with limited inpatient experience |
Langer and coworkers compared glyburide with insulin in the management of GDM and found the agents to be equally effective, with comparable levels of risk of large size for gestational age, macrosomia, hypoglycemia (in infants), NICU admission, and fetal anomaly.23 Subsequent studies have confirmed these findings, although at least one suggests that women who have a high fasting plasma glucose level may not respond adequately to glyburide.26 None of these studies has been large enough or long enough to truly assess whether these oral medications are equivalent to insulin in the management of GDM without posing significant long-term complications for mothers or babies, or both.
For more on the use of oral agents in GDM, see Dr. Aaron B. Caughey’s commentary on the subject of this issue.
Continuous monitoring may detect occult hyperglycemia and hypoglycemia
The traditional method of monitoring the blood glucose level is to stick a finger to obtain a blood sample and use a test strip and a meter to measure the concentration of glucose in the sample. Most meters on the market are reasonably accurate. However, research has demonstrated that they are least accurate during episodes of hypoglycemia.27
Automated continuous glucose-monitoring systems are less intrusive than the traditional method, but they are usually reserved for people who have type 1 diabetes requiring intensive insulin therapy. However, because data suggest that even short periods of hyperglycemia or hypoglycemia can be detrimental to a developing fetus, there is increasing interest in utilizing continuous glucose monitoring for GDM.
Several research groups have compared continuous glucose monitoring with finger-stick monitoring and found that women randomized to continuous monitoring have lower mean hemoglobin A1c levels from 32 to 36 weeks’ gestation.28,29 (See “Exploring the value of continuous glucose monitoring in gestational diabetes?”) Women undergoing continuous monitoring also have:
- lower mean birth-weight standard- deviation scores
- lower median customized birth-weight centiles
- a reduced risk of macrosomia.
One study found that information gleaned from continuous glucose monitoring provided additional information that altered clinical management in 42 of 68 (62%) cases. These additional data included evidence of undetected and potentially dangerous postprandial hyperglycemia and overnight hypoglycemia.29
Yogev and colleagues found that continuous glucose monitoring is significantly more sensitive than traditional methods in detecting periods of hypoglycemia in women who have GDM. They also found that asymptomatic hypoglycemic events are common during pharmacotherapy in gestations affected by GDM.30 The same group used continuous glucose monitoring at night in obese, nondiabetic women to identify previously undetected:
- high postprandial glucose peak values
- increased 1- and 2-hour postprandial glucose levels
- increased time to the glucose peak
- significantly lower mean blood glucose levels.31
Insurers were reluctant to cover continuous glucose monitoring devices when they first became available. Since then, however, much progress has been made. Nevertheless, inadequate reimbursement for the time it takes a clinician to change a patient’s treatment regimen and her subsequent management remains a significant barrier to adoption of these systems.32 The key to success with continuous glucose monitoring is to train the patient to use it properly.
Exploring the value of continuous glucose monitoring in gestational diabetes
Tanenberg R, Bode B, Lane W, et al. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526.
The American Diabetes association recommends that patients on insulin self-monitor blood glucose three or four times daily to guide adjustments in therapy and ensure a stable and optimal hemoglobin A1c level. “however, adherence to frequent blood-glucose monitoring is low, and less than 54% of patients with insulin-treated diabetes are reported to self-monitor their blood glucose at least three times each day,” say tanenberg and coworkers.
To determine whether use of a continuous glucose-monitoring system improves metabolic control, the investigators randomized 109 patients who had insulin-treated diabetes to continuous monitoring or frequent self-monitoring. at enrollment, all patients had insulin-treated diabetes and inadequate metabolic control. at the end of the study, both groups used continuous monitoring for 3 days; these values were used to calculate measures of hypoglycemia.
In the study, the women in the self-monitoring group were counseled to measure capillary blood glucose a minimum of four times daily, as well as when they experienced symptoms of hypoglycemia, which was defined as a blood glucose measurement of 60 mg/dL or lower. any hypoglycemic event was considered to be over when the measurement exceeded 60 mg/dL for at least 30 minutes.
Findings
Hemoglobin A1c levels were similar between groups at baseline, and both groups showed significant (P < .001) and similar (P=.95) improvement in these levels after 12 weeks of study. however, the continuous-monitoring group had a significantly shorter duration of hypoglycemic events than the self-monitoring group at week 12 (49.4±40.8 minutes vs 81.0±61.1 minutes per event, respectively; P=.009).
Tanenberg and coworkers hypothesize that the improvement in hemoglobin A1c in the self-monitoring group was a result of monitoring that was more frequent (7 times a day) than is typical. they concluded that use of continuous monitoring to guide therapy adjustments in patients who use insulin significantly reduces the duration of hypoglycemia, compared with adjustments guided by self-monitoring values alone.
We want to hear from you! Tell us what you think.
1. Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: determinants outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188(5):1372-1378.
2. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668-1669.
3. Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9(1):83-88.
4. Gunderson EP, Jacobs DR, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201(2):177.e1-9.
5. van Hoorn J, Dekker G, Jeffries B. Gestational diabetes versus obesity as risk factors for pregnancy-induced hypertensive disorders and fetal macrosomia. Aust N Z J Obstet Gynaecol. 2002;42(1):29-34.
6. Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203.
7. Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can. 2006;28(2):122-127.
8. Jones CW. Gestational diabetes and its impact on the neonate. Neonatal Netw. 2001;20(6):17-23
9. De Luca AK, Nakazawa CY, Azevedo BC, Rudge MV, De Araujo Costa RA, Calderon IM. Influence of glycemic control on fetal lung maturity in gestations affected by diabetes or mild hyperglycemia. Acta Obstet Gynecol Scand. 2009;88(9):1036-1040.
10. Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672.-e1–4.
11. Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21(3):149-157
12. ter Braak EW, Evers IM, Willem Erkelens D, Visser GH. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev. 2002;18(2):96-105.
13. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3):199-203.
14. Harlass FE, Brady K, Read JA. Reproducibility of the oral glucose tolerance test in pregnancy. Am J Obstet Gynecol. 1991;164(2):564-568.
15. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31(8):1650-1655.
16. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New Engl J Med. 2008;358(19):1991-2002.
17. American Diabetes Association. Executive summary: standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S4-10.
18. Bonomo M, Corica D, Mion E, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med. 2005;22(11):1536-1541.
19. Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
20. 20Cypryk K, Kaminska P, Kosinski M, Pertynska-Marczewska M, Lewinski A. A comparison of the effectiveness tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol. 2007;58(4):314-319.
21. Lapolla A, Dalfrà MG, Spezia R, et al. Outcome of pregnancy in type 1 diabetic patients treated with insulin lispro or regular insulin: an Italian experience. Acta Diabetol. 2008;45(1):61-66.
22. Hod M, Damm P, Kaaja R, et al. Insulin Aspart Pregnancy Study Group. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198(2):186-187.
23. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzles O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343(16):1134-1138.
24. Ho FL, Liew CF, Cunanan EC, Lee KO. Oral hypoglycaemic agents for diabetes in pregnancy—an appraisal of the current evidence for oral anti-diabetic drug use in pregnancy. Ann Acad Med Singapore. 2007;36(8):672-678.
25. Nicholson W, Bolen S, Witkop CT, et al. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):193-205.
26. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. Matern Fetal Neonatal Med. 2004;15(1):51-55.
27. Carr S, Coustan DR, Martelly P, et al. Precision of reflectance meters in screening for gestational diabetes. Obstet Gynecol. 1989;73(5 Pt 1):727-731.
28. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680.-doi: 10.1136/bmj.a1680.
29. McLachlan K, Jenkins A, O’Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol. 2007;47(3):186-190.
30. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Undiagnosed asymptomatic hypoglycemia: diet insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104(1):88-93.
31. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949-953.
32. Bartelme A, Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3(4):992-995.
1. Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: determinants outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188(5):1372-1378.
2. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668-1669.
3. Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9(1):83-88.
4. Gunderson EP, Jacobs DR, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201(2):177.e1-9.
5. van Hoorn J, Dekker G, Jeffries B. Gestational diabetes versus obesity as risk factors for pregnancy-induced hypertensive disorders and fetal macrosomia. Aust N Z J Obstet Gynaecol. 2002;42(1):29-34.
6. Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203.
7. Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can. 2006;28(2):122-127.
8. Jones CW. Gestational diabetes and its impact on the neonate. Neonatal Netw. 2001;20(6):17-23
9. De Luca AK, Nakazawa CY, Azevedo BC, Rudge MV, De Araujo Costa RA, Calderon IM. Influence of glycemic control on fetal lung maturity in gestations affected by diabetes or mild hyperglycemia. Acta Obstet Gynecol Scand. 2009;88(9):1036-1040.
10. Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672.-e1–4.
11. Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21(3):149-157
12. ter Braak EW, Evers IM, Willem Erkelens D, Visser GH. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev. 2002;18(2):96-105.
13. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3):199-203.
14. Harlass FE, Brady K, Read JA. Reproducibility of the oral glucose tolerance test in pregnancy. Am J Obstet Gynecol. 1991;164(2):564-568.
15. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31(8):1650-1655.
16. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New Engl J Med. 2008;358(19):1991-2002.
17. American Diabetes Association. Executive summary: standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S4-10.
18. Bonomo M, Corica D, Mion E, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med. 2005;22(11):1536-1541.
19. Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
20. 20Cypryk K, Kaminska P, Kosinski M, Pertynska-Marczewska M, Lewinski A. A comparison of the effectiveness tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol. 2007;58(4):314-319.
21. Lapolla A, Dalfrà MG, Spezia R, et al. Outcome of pregnancy in type 1 diabetic patients treated with insulin lispro or regular insulin: an Italian experience. Acta Diabetol. 2008;45(1):61-66.
22. Hod M, Damm P, Kaaja R, et al. Insulin Aspart Pregnancy Study Group. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198(2):186-187.
23. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzles O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343(16):1134-1138.
24. Ho FL, Liew CF, Cunanan EC, Lee KO. Oral hypoglycaemic agents for diabetes in pregnancy—an appraisal of the current evidence for oral anti-diabetic drug use in pregnancy. Ann Acad Med Singapore. 2007;36(8):672-678.
25. Nicholson W, Bolen S, Witkop CT, et al. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):193-205.
26. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. Matern Fetal Neonatal Med. 2004;15(1):51-55.
27. Carr S, Coustan DR, Martelly P, et al. Precision of reflectance meters in screening for gestational diabetes. Obstet Gynecol. 1989;73(5 Pt 1):727-731.
28. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680.-doi: 10.1136/bmj.a1680.
29. McLachlan K, Jenkins A, O’Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol. 2007;47(3):186-190.
30. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Undiagnosed asymptomatic hypoglycemia: diet insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104(1):88-93.
31. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949-953.
32. Bartelme A, Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3(4):992-995.
Probing Postpartum Depression
Care of the postpartum patient is an important component of primary care practice—particularly for clinicians who provide women’s health care and are called upon to effectively screen, identify, and manage patients who may be at risk for postpartum depression (PPD). Effective management of the postpartum patient also extends to care of her infant(s) and ideally should involve a multidisciplinary team, including the primary care provider, the pediatrician, and a psychologist or a psychiatrist.1
PPD is commonly described as depression that begins within the first month after delivery.2 It is diagnosed using the same criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)3 as are used to identify major depressive disorder. According to the DSM-IV, PPD begins within the first four weeks after the infant’s birth.2,3 Although onset can occur at any time between 24 hours after a woman gives birth to several months later, PPD most commonly occurs within the first six weeks after delivery.1
The woman with PPD may be reluctant to report her symptoms for a number of reasons, including lack of motivation or energy, fatigue, embarrassment that she is experiencing such symptoms during a presumably happy time in her life—even fear that her child may be removed from her care.4 In addition to depressive symptoms (eg, sleeping difficulty, appetite changes, anhedonia, and guilt), women affected by PPD often experience anxiety and may become obsessed with the health, feeding, and sleeping behaviors of their infants.1,5
Postpartum mood disorders occur in a spectrum, ranging from postpartum baby blues to postpartum psychosis. By DSM-IV definition, a diagnosis of major or minor PPD requires that depressed mood, loss of interest or pleasure, or other characteristic symptoms be present most of the day, nearly every day, for at least two weeks.3,6 PPD must be differentiated from the more common and less severe baby blues, which usually start two to three days after delivery and last less than two weeks.2,7 Between 40% and 80% of women who have given birth experience baby blues, which are characterized by transient mood swings, irritability, crying spells, difficulty sleeping, and difficulty concentrating.8 Experiencing baby blues may place women at increased risk for PPD.9
Postpartum, or puerperal, psychosis occurs in approximately 0.2% of women,10 with an early and sudden onset—that is, within the first week to four weeks postpartum. This severe condition is characterized by hallucinations and delusions often centered on the infant, in addition to insomnia, agitation, and extreme behavioral changes. Postpartum psychosis, which can recur in subsequent pregnancies, may be a manifestation of a preexisting affective disorder, such as bipolar disorder.10 Postpartum psychosis is considered an emergent condition because the safety and well-being of both mother and infant may be at serious risk.2,10
EPIDEMIOLOGY AND RISK FACTORS
PPD is often cited as affecting 10% to 15% of women within the first year after childbirth.11,12 Currently, reported prevalence rates of PPD range from 5% to 20% of women who have recently given birth, depending on the source of information. Among 17 US states participating in the Pregnancy Risk Assessment Monitoring System, a CDC surveillance project, prevalence of self-reported PPD symptoms ranged from 11.7% in Maine to 20.4% in New Mexico.11,13 According to the Agency for Healthcare Research and Quality (AHRQ),14 the prevalence of major or minor depression ranges from 6.5% to 12.9% at different times during the year following delivery, although study design varied throughout the research used, and confidence intervals were deemed wide.14,15
Nevertheless, according to results from a systematic review of 28 studies, the prevalence of minor or major depression is estimated at up to 19.2% of women during the first three months postpartum, and major depression in up to 7% during that time.16 A 2007 chart review of 4,398 US women experiencing live births identified PPD in 10.4%.17
Incidence of PPD is “much higher than the quoted rate of 10% to 15%,” concludes Almond18 after a comprehensive literature review. The condition also affects women globally, the British researcher reports: Not only did she find numerous data on the incidence of PPD in high-income countries, including the US, the United Kingdom, and Australia, but she concluded that incidence rates of PPD in developing countries are grossly underestimated, according to epidemiologic studies in low- and middle-income countries (eg, Pakistan, Indonesia, Vietnam). Additionally, the risk factors for PPD are likely to be influenced by cultural differences, and attempts to identify PPD must be culturally sensitive.18
Several risk factors have been associated with PPD. Perhaps the most significant risk factor is a personal history of depression (prior to pregnancy or postpartum); at least one-half of women with PPD experience onset of depressive symptoms before or during their pregnancies,19,20 and one research group reported a relative risk (RR) of 1.87 for PPD in women with a history of depression, compared with those without such a history.21 Thus, women previously affected by depression should be carefully monitored in the immediate postpartum period for any signs of depressed mood, anxiety, sleep difficulties, loss of appetite or energy, and psychomotor changes.
According to McCoy et al,21 neither patient age nor marital status nor method of delivery appeared to be associated with PPD at four weeks postpartum. Women who were feeding by formula alone were more likely to experience PPD (RR, 2.04) than were those who breastfed their infants. Women who smoked were more likely to be affected by PPD (RR, 1.58) than were nonsmokers.
Additional risk factors for PPD identified by Dennis et al22 included pregnancy-induced hypertension and immigration within the previous five years. Various psychosocial stressors may also represent risk factors for PPD, including lack of social support, financial concerns, miscarriage or fetal demise, limited partner support, physical abuse before or during pregnancy, lack of readiness for hospital discharge, and complications during pregnancy and delivery (eg, low birth weight, premature birth, admission of the infant to the neonatal ICU).11,22,23
It is important to identify women who are at highest risk for PPD as soon as possible. High-risk patients should be screened upon discharge from the hospital and certainly on or before the first postpartum visit.
CLINICAL MANIFESTATIONS
Clinical manifestations of PPD include depressed mood for at least two weeks with changes in somatic functions, such as sleep, energy level, appetite, weight, gastrointestinal functioning, and decrease in libido.3,24 These manifestations are more severe and prolonged than those associated with baby blues (which almost always resolve within two weeks postpartum).2,7
On physical examination, the patient with PPD may appear tearful and disheveled, with psychomotor retardation. She may report that she is unable to sleep even when her infant is sleeping, or that she has a significant lack of energy despite sufficient sleep; she may admit being unable to get out of bed for hours.2 The patient may report a significant decrease in appetite and little enjoyment in eating, which may lead to rapid weight loss.
Other symptoms may include obsessive thoughts about the infant and his or her care, significant anxiety (possibly manifested in panic attacks), uncontrollable crying, guilt, feelings of being overwhelmed or unable to care for the infant, mood swings, and severe irritability or even anger.2
Severe fatigue may warrant hemoglobin/hematocrit evaluation and possibly measurement of serum thyroid-stimulating hormone (TSH).25,26
It is essential to rule out postpartum psychosis, which is associated with prolonged lack of sleep, confusion, lapsed insight, cognitive impairment, “grossly disorganized behavior,”10 and delusions or hallucinations.5,10 The patient should be asked specifically about unusual or bizarre thoughts or beliefs concerning the infant, in addition to thoughts of harming herself or others, particularly the infant.10
SCREENING
Numerous researchers have suggested that PPD is underrecognized and undertreated.5,6,18,27,28 Screening for PPD in the United States is not standardized and is highly variable.29 The American Academy of Family Physicians supports universal screening for PPD at the first postpartum visit, between two and six weeks.30,31 According to a 2010 Committee Opinion from the American College of Obstetricians and Gynecologists, “at this time, there is insufficient evidence to support a firm recommendation for universal antepartum or postpartum screening; however, screening for depression has the potential to benefit a woman and her family and should be strongly considered.”32
As the AHRQ14 notes, symptoms of PPD may not peak in some women until after their first postpartum visit, and providers of family medicine, internal medicine, and pediatric care may also be in a position to provide screening. The initial well-baby examination by the pediatric primary care provider, for example, presents an important opportunity to screen new mothers for PPD. According to Chaudron et al,33 the well-being of the infant should outweigh any scope-of-practice concerns, practitioner time limitations, or reimbursement issues; rather, screening efforts can be considered “tools to enhance [mothers’] ability to care for their children in a way that is supportive and not punitive.”33
In 2009, Sheeder and colleagues28 reported on a prospective study of 199 mothers in an adolescent maternity clinic who were screened using the Edinburgh Postnatal Depression Scale (EPDS)34 at each well-baby visit during the first six months postpartum. The authors concluded that the optimal time for screening for PPD in this setting is two months after delivery, although repeated screening may identify worsening of depressive symptoms.28
Several screening tools are available for the detection of PPD, particularly the EPDS,34 the Postpartum Depression Screening Scale,35 and the Patient Health Questionnaire–936-38 (see table,11,13,34-37 ). The EPDS, a widely used and well-validated formal screening tool,27,37 is a 10-item self-report questionnaire designed to detect depression in the postpartum period. Cox et al,34 who developed the scale, initially reported its sensitivity at 86% and specificity at 78%21; since then, the tool’s reported sensitivity for detecting major depression in the postpartum period has ranged from 60% to 96%, and specificity from 45% to 97%.6,39 The EPDS has been shown to result in a diagnosis of PPD in significantly more women than routine clinical evaluation (35.4% vs 6.3%, respectively).40 It is possible to administer the EPDS by telephone.41
The validity of the EPDS tool in detecting PPD was recently examined in a systematic review of 37 studies. Gibson et al42 concluded that the heterogeneity of these studies (ie, differences in study methodology, language used, and diagnostic criteria) precluded meta-analysis and did not provide clear support of EPDS as an accurate screening tool for PPD, especially across diverse cultures. In a similar review, Hewitt and colleagues6 sought to “provide an overview of all available methods to identify postnatal depression in primary care and to assess their validity.” They concluded that the EPDS is the most frequently reported screening tool and, with an overall sensitivity of 86% and overall specificity of 87%, its diagnostic performance seems “reasonably good.”6 Of note, fewer data have been collected to demonstrate the effectiveness of other screening tools.35,36,38,41
Cost-effectiveness is a consideration in the use of screening programs for PPD. According to a hypothetical cohort analysis conducted in the UK, the costs of treating women with false-positive screening results made implementation of a formal screening strategy for PPD not cost-effective, compared with usual care only, for use by the British National Health Service.43
MANAGEMENT
Once PPD is identified, treatment should be initiated as quickly as possible; referral for psychological counseling is an appropriate initial strategy for mild to moderate symptoms of PPD.5 A clinical care manager can be a valuable resource to provide education and coordination of care for women affected by PPD. NPs and PAs in primary care, obstetrics/gynecology, women’s health, and psychiatry or psychology can play an important role in the identification and management of PPD.
Treatment of PPD involves combination therapy—short-term psychological therapy combined with pharmacotherapy. According to investigators in a Cochrane Review of nine trials reporting short-term outcomes for 956 women with PPD, their findings suggest that psychosocial and psychological interventions are an effective option for reducing symptoms of PPD.44 Compared with usual care, the types of psychological therapy that were found most effective included cognitive behavioral therapy, interpersonal therapy, and psychodynamic therapy. As most trials’ follow-up periods were limited to six months, however, neither the long-term effects of psychological therapy nor the relative effectiveness of each type of therapy was made clear by these studies.44,45
Pharmacotherapy
Although antidepressant drugs are known to be effective for the treatment of major depressive disorder, well-designed clinical trials demonstrating the overall effectiveness of antidepressants in treatment of PPD have been limited.46 According to Ng et al,46 who in 2010 performed a systematic review of studies examining pharmacologic interventions for PPD, preliminary evidence showing the effectiveness of antidepressants and hormone therapy should prompt the initiation of larger, more rigorous randomized and controlled trials.
The choice of antidepressants will be influenced by the mother’s breastfeeding status and whether PPD represents her first episode of depression or a recurrence of previous major depression. If the patient is not breastfeeding, the choice among antidepressants is similar to those used for treatment of nonpuerperal major depression. If PPD is a relapse of a prior depression, the therapeutic agent that was most effective and best-tolerated for previous depression should be prescribed.47
Generally, the SSRIs are considered first-line agents because of their superior safety profile.47 Fluoxetine has been shown in a small randomized trial (n = 87) to be significantly more effective than placebo and as effective as a full course of cognitive-behavioral counseling.45 In non–placebo-controlled studies, sertraline, fluvoxamine, and venlafaxine all produced improvement in PPD symptoms.48,49
Whether the mother is breastfeeding her infant will influence the use and choice of antidepressants for PPD. Although barely detectable levels of certain antidepressant medications (including the SSRIs sertraline and paroxetine, and the tricyclic antidepressant nortriptyline) have been reported in breast milk or in infant serum,50,51 it is recommended that the lowest possible therapeutic dose be prescribed, and that infants be carefully monitored for adverse effects.51
Fluoxetine, it should be noted, has been found to be transmitted through breast milk and was associated with reduced infant weight gain (specifically, by 392 g over six months) in a comparison between 64 fluoxetine-treated mothers and 38 non-treated mother-infant pairs.52 Thus, fluoxetine use should be avoided in women who are breastfeeding.
In small studies, paroxetine and fluvoxamine were not detected in infant serum, and although low levels of sertraline were detected in one-fourth of infants whose mothers received doses exceeding 100 mg/d, no adverse infant outcomes were noted.52-54 Paroxetine, nortriptyline, and sertraline appear to be relatively safer antidepressant choices in breastfeeding women with PPD.50,52,53
Antidepressants should be continued for six months after full remission of depressive symptoms. Longer courses of therapy may be necessary in patients who experience recurrent major depressive episodes.55
Few researchers have reported on the use of hormonal therapy for PPD. Yet significant hormonal fluctuations,56,57 including “estrogen withdrawal at parturition,”56 are known to occur after childbirth; in their study of women with severe PPD, Ahokas et al57 found that two-thirds of participants had serum estradiol concentrations below the cutoff for gonadal failure. Such a deficiency is likely to contribute to mood disturbances.56,57
In a small, double-blind, placebo-controlled study that enrolled women with severe, persistent PPD (mean EPDS score, 21.8), six months’ treatment with transdermally administered estradiol was associated with significantly greater relief of depressive symptoms than was found in controls (mean EPDS scores at one month, 13.3 vs 16.5, respectively). Of note, more than half of the women studied were concurrently receiving antidepressants.58
Additional studies may elucidate the role of estrogen therapy in the treatment of PPD.
Nonpharmacologic Options
An effective nonpharmacologic option for rapid resolution of severe symptoms of PPD is electroconvulsive therapy (ECT).59-61 Its use is safe in nursing mothers because it does not affect breast milk. ECT is considered especially useful for women who have not responded to pharmacotherapy, those experiencing severe psychotic depression, and those who are considered at high risk for suicide or infanticide.
Patients typically receive three treatments per week; three to six treatments often produce an effective response.59 Anesthesia administered to women who undergo ECT has not been shown to have a negative effect on infants who are being breastfed.60,61
Interpersonal strategies to address PPD should not be overlooked. A pilot study conducted in Canada showed promising results when women who had previously experienced PPD were trained to provide peer support by telephone to mothers who were deemed at high risk for PPD (ie, those with EPDS scores > 12). At four weeks and eight weeks postpartum, follow-up EPDS scores exceeded 12 in 10% and 15%, respectively, of women receiving peer support, compared with 41% and 52%, respectively, of controls.62
Participation in one of numerous support groups that exist for women with PPD (see box, for online information) may reduce isolation in these women and possibly offer additional benefits.
COMPLICATIONS
PPD may be associated with significant complications, underscoring the importance of prompt identification and treatment.5 Maternal depressive symptoms in the critical postnatal period, for example, have been associated with long-term impairment of mother-child bonding. In one study of 101 women, lower-quality maternal bonding was found in women who had symptoms of depression at two weeks, six weeks, and four months postpartum—but not in those with depression at 14 months.63 Additionally, it was found in a systematic review of 49 studies that women with PPD were likely to discontinue breastfeeding earlier than women not affected.64
Delayed growth and development has been reported in infants of mothers with untreated or inadequately treated PPD.65 It has also been suggested that children of depressed mothers may have an increased risk for anxiety, depression, hyperactivity, and other behavioral disorders later in childhood.65-67
PROGNOSIS
Untreated PPD may resolve spontaneously within three to six months, but in about one-quarter of PPD patients, depressive symptoms persist one year after delivery.24,68,69 PPD increases a woman’s risk for future episodes of major depression.2,5
PREVENTION
As previously discussed, the risk for PPD is greatest in women with a history of mood disorders (25%) and PPD (50%).2 Although several approaches have been studied to prevent PPD, no clear optimal strategy has been revealed. In one Cochrane review, insufficient evidence was found to justify prophylactic use of antidepressants.70 Similarly, findings in a second review fell short of confirming the effectiveness of prenatal psychosocial or psychological interventions to prevent antenatal depression.71
Additional studies are needed to make recommendations on prevention of PPD in high-risk patients. Until such recommendations emerge, close monitoring, screening, and follow-up are essential for these women.
CONCLUSION
Postpartum depression is an important concern among childbearing women, as it is associated with adverse maternal and infant outcomes. A personal history of depression is a major risk factor for PPD. It is imperative to question women about signs and symptoms of depression during the immediate postpartum period; it is particularly important to inquire about thoughts of harm to self or to the infant.
Pharmacotherapy combined with adjunctive psychological therapy is indicated for new mothers with significant depressive symptoms. The choice of antidepressants is based on previous response to antidepressants and the woman’s breastfeeding status. Generally, SSRIs are effective and well tolerated for major depression; based on results from small studies, they appear to be safe for breastfeeding mothers.
Electroconvulsive therapy is considered a safe and effective option for women with severe symptoms of PPD.
REFERENCES
1. Muzik M, Marcus SM, Heringhausen JE, Flynn H. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788.
2. Wisner KL, Parry BL, Piontek CM. Clinical practice: postpartum depression. N Engl J Med. 2002;347(3):194-199.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
4. Whitton A, Warner R, Appleby L. The pathway to care in post-natal depression: women’s attitudes to post-natal depression and its treatment. Br J Gen Pract. 1996;46(408):427-428.
5. Sit DK, Wisner KL. Identification of postpartum depression. Clin Obstet Gynecol. 2009; 52(3):456-468.
6. Hewitt C, Gilbody S, Brealey S, et al. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess. 2009;13(36):1-145, 147-230.
7. Epperson CN. Postpartum major depression: detection and treatment. Am Fam Physician. 1999;59(8):2247-2254, 2259-2260.
8. O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
9. Hannah P, Adams D, Lee A, et al. Links between early post-partum mood and post-natal depression. Br J Psychiatry. 1992;160:777-780.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
11. CDC. Prevalence of self-reported postpartum depressive symptoms—17 states, 2004–2005. MMWR Weekly. 2008;57(14):361-366.
12. O’Hara MW, Swain AM. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8:37-54.
13. Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med. 1997;12(7):439-445.
14. Agency for Healthcare Research and Quality. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes: Summary. Evidence Report/Technology Assessment No. 119. www.ahrq.gov/clinic/epcsums/peridep sum.htm. Accessed January 24, 2011.
15. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005 Feb;(119):1-8.
16. Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005; 106(5 pt 1):1071-1083.
17. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10): 1515-1520.
18. Almond P. Postnatal depression a global public health perspective. Perspect Public Health. 2009;129(5):221-227.
19. Lee D, Yip A, Chiu H, et al. A psychiatric epidemiological study of postpartum Chinese women. Am J Psychiatry. 2001;158(2):220-226.
20. Cooper PJ, Tomlinson M, Swartz L, et al. Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry. 1999;175:554-558.
21. McCoy SJB, Beal JM, Miller SBM, et al. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. J Am Osteopath Assoc. 2006;106(4):193-198.
22. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
23. Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108(1-2):147-157.
24. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59 suppl 2:34-40.
25. Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133(12):4139-4142.
26. McCoy SJ, Beal JM, Payton ME, et al. Postpartum thyroid measures and depressive symptomology: a pilot study. J Am Osteopath Assoc. 2008;108(9):503-507.
27. Seehusen DA, Baldwin LM, Runkle GP, Clark G. Are family physicians appropriately screening for postpartum depression? J Am Board Fam Pract. 2005;18(2):104-112.
28. Sheeder J, Kabir K, Stafford B. Screening for postpartum depression at well-child visits: is once enough during the first 6 months of life? Pediatrics. 2009;123(6):e982-e988.
29. Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. Am J Obstet Gynecol. 2009;200(4):357-364.
30. Shaver K. Treating postpartum depression during lactation. Prescriber’s Letter. 2001 Aug;8(8):47.
31. Georgiopoulos AM, Bryan TL, Wollan P, Yawn BP. Routine screening for postpartum depression. J Fam Pract. 2001;50(20):117-122.
32. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 453: Screening for depression during and after pregnancy. Obstet Gynecol. 2010;115(2 pt 1):394-395.
33. Chaudron LH, Szilagyi PG, Campbell AT, et al. Legal and ethical considerations: risks and benefits of postpartum depression screening at well-child visits. Pediatrics. 2007;119(1):123-128.
34. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
35. Beck CT, Gable RK. Postpartum Depression Screening Scale: development and psychometric testing. Nurs Res. 2000;49(5):272-282.
36. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders, Patient Health Questionnaire. JAMA. 1999; 282(18):1737-44.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Yawn BP, Pace W, Wollan PC, et al. Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. J Am Board Fam Med. 2009;22(5):483-491.
39. Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157: 288-290.
40. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
41. Hanusa BH, Scholle SH, Haskett RF, et al. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585-596.
42. Gibson J, McKenzie-McHarg K, Shakespeare J, et al. A systematic review of studies validating the Edinburgh postnatal depression scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350-364.
43. Paulden M, Palmer S, Hewitt C, Gilbody S. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009; 339:b5203.
44. Dennis CL,Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev. 2007;(4):CD006116.
45. Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085): 932-936.
46. Ng RC, Hirata CK, Yeung W, et al. Pharmacologic treatment for postpartum depression: a systematic review. Pharmacotherapy. 2010; 30(9):928-941.
47. Whitby DH, Smith KM. The use of tricyclic antidepressants and selective serotonin reuptake inhibitors in women who are breastfeeding. Phamacotherapy. 2005;25(3):411-425.
48. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
49. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
50. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353-360.
51. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;79(9):e35.
52. Gjerdingen D. The effectiveness of various postpartum depression treatments and the impact of antidepressant drugs on nursing infants. J Am Board Fam Pract. 2003;16(5): 372-382.
53. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry. 1996;153(9):1132-1137.
54. Hendrick V, Fukuchi A, Altshuler L, et al. Use of sertraline, paroxetine and fluvoxamine by nursing women. Br J Psychiatry. 2001;179: 163-166.
55. Davidson JR. Major depressive disorder treatment guidelines in America and Europe.
J Clin Psychiatry. 2010;71 suppl E1:e04.
56. Moses-Kolko EL, Berga SL, Kalro B, et al. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52(3):516-529.
57. Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62(5):
332-336.
58. Gregoire AJ, Kumar R, Everitt B, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006): 930-933.
59. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
60. Altshuler LL, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592-606.
61. Rabheru K. The use of electroconvulsive therapy in special patient populations. Can J Psychiatry. 2001;46(8):710-719.
62. Dennis CL. The effect of peer support on postpartum depression: a pilot randomized controlled trial. Can J Psychiatry. 2003;48(2): 115-124.
63. Moehler E, Brunner R, Wiebel A, et al. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Arch Womens Ment Health. 2006;9(5):273-278.
64. Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123(4):e736-e751.
65. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82(8): 926-933.
66. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12(1):12-20.
67. Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295(12):1389-1398.
68. Cooper PJ, Murray L. Course and recurrence of postnatal depression: evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166(2):191-195.
69. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
70. Howard LM, Hoffbrand S, Henshaw C, et al. Antidepressant prevention of postnatal depression. Cochrane Database Syst Rev. 2005;(2):CD00436.
71. Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database Syst Rev. 2007;(3):CD006309.
Care of the postpartum patient is an important component of primary care practice—particularly for clinicians who provide women’s health care and are called upon to effectively screen, identify, and manage patients who may be at risk for postpartum depression (PPD). Effective management of the postpartum patient also extends to care of her infant(s) and ideally should involve a multidisciplinary team, including the primary care provider, the pediatrician, and a psychologist or a psychiatrist.1
PPD is commonly described as depression that begins within the first month after delivery.2 It is diagnosed using the same criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)3 as are used to identify major depressive disorder. According to the DSM-IV, PPD begins within the first four weeks after the infant’s birth.2,3 Although onset can occur at any time between 24 hours after a woman gives birth to several months later, PPD most commonly occurs within the first six weeks after delivery.1
The woman with PPD may be reluctant to report her symptoms for a number of reasons, including lack of motivation or energy, fatigue, embarrassment that she is experiencing such symptoms during a presumably happy time in her life—even fear that her child may be removed from her care.4 In addition to depressive symptoms (eg, sleeping difficulty, appetite changes, anhedonia, and guilt), women affected by PPD often experience anxiety and may become obsessed with the health, feeding, and sleeping behaviors of their infants.1,5
Postpartum mood disorders occur in a spectrum, ranging from postpartum baby blues to postpartum psychosis. By DSM-IV definition, a diagnosis of major or minor PPD requires that depressed mood, loss of interest or pleasure, or other characteristic symptoms be present most of the day, nearly every day, for at least two weeks.3,6 PPD must be differentiated from the more common and less severe baby blues, which usually start two to three days after delivery and last less than two weeks.2,7 Between 40% and 80% of women who have given birth experience baby blues, which are characterized by transient mood swings, irritability, crying spells, difficulty sleeping, and difficulty concentrating.8 Experiencing baby blues may place women at increased risk for PPD.9
Postpartum, or puerperal, psychosis occurs in approximately 0.2% of women,10 with an early and sudden onset—that is, within the first week to four weeks postpartum. This severe condition is characterized by hallucinations and delusions often centered on the infant, in addition to insomnia, agitation, and extreme behavioral changes. Postpartum psychosis, which can recur in subsequent pregnancies, may be a manifestation of a preexisting affective disorder, such as bipolar disorder.10 Postpartum psychosis is considered an emergent condition because the safety and well-being of both mother and infant may be at serious risk.2,10
EPIDEMIOLOGY AND RISK FACTORS
PPD is often cited as affecting 10% to 15% of women within the first year after childbirth.11,12 Currently, reported prevalence rates of PPD range from 5% to 20% of women who have recently given birth, depending on the source of information. Among 17 US states participating in the Pregnancy Risk Assessment Monitoring System, a CDC surveillance project, prevalence of self-reported PPD symptoms ranged from 11.7% in Maine to 20.4% in New Mexico.11,13 According to the Agency for Healthcare Research and Quality (AHRQ),14 the prevalence of major or minor depression ranges from 6.5% to 12.9% at different times during the year following delivery, although study design varied throughout the research used, and confidence intervals were deemed wide.14,15
Nevertheless, according to results from a systematic review of 28 studies, the prevalence of minor or major depression is estimated at up to 19.2% of women during the first three months postpartum, and major depression in up to 7% during that time.16 A 2007 chart review of 4,398 US women experiencing live births identified PPD in 10.4%.17
Incidence of PPD is “much higher than the quoted rate of 10% to 15%,” concludes Almond18 after a comprehensive literature review. The condition also affects women globally, the British researcher reports: Not only did she find numerous data on the incidence of PPD in high-income countries, including the US, the United Kingdom, and Australia, but she concluded that incidence rates of PPD in developing countries are grossly underestimated, according to epidemiologic studies in low- and middle-income countries (eg, Pakistan, Indonesia, Vietnam). Additionally, the risk factors for PPD are likely to be influenced by cultural differences, and attempts to identify PPD must be culturally sensitive.18
Several risk factors have been associated with PPD. Perhaps the most significant risk factor is a personal history of depression (prior to pregnancy or postpartum); at least one-half of women with PPD experience onset of depressive symptoms before or during their pregnancies,19,20 and one research group reported a relative risk (RR) of 1.87 for PPD in women with a history of depression, compared with those without such a history.21 Thus, women previously affected by depression should be carefully monitored in the immediate postpartum period for any signs of depressed mood, anxiety, sleep difficulties, loss of appetite or energy, and psychomotor changes.
According to McCoy et al,21 neither patient age nor marital status nor method of delivery appeared to be associated with PPD at four weeks postpartum. Women who were feeding by formula alone were more likely to experience PPD (RR, 2.04) than were those who breastfed their infants. Women who smoked were more likely to be affected by PPD (RR, 1.58) than were nonsmokers.
Additional risk factors for PPD identified by Dennis et al22 included pregnancy-induced hypertension and immigration within the previous five years. Various psychosocial stressors may also represent risk factors for PPD, including lack of social support, financial concerns, miscarriage or fetal demise, limited partner support, physical abuse before or during pregnancy, lack of readiness for hospital discharge, and complications during pregnancy and delivery (eg, low birth weight, premature birth, admission of the infant to the neonatal ICU).11,22,23
It is important to identify women who are at highest risk for PPD as soon as possible. High-risk patients should be screened upon discharge from the hospital and certainly on or before the first postpartum visit.
CLINICAL MANIFESTATIONS
Clinical manifestations of PPD include depressed mood for at least two weeks with changes in somatic functions, such as sleep, energy level, appetite, weight, gastrointestinal functioning, and decrease in libido.3,24 These manifestations are more severe and prolonged than those associated with baby blues (which almost always resolve within two weeks postpartum).2,7
On physical examination, the patient with PPD may appear tearful and disheveled, with psychomotor retardation. She may report that she is unable to sleep even when her infant is sleeping, or that she has a significant lack of energy despite sufficient sleep; she may admit being unable to get out of bed for hours.2 The patient may report a significant decrease in appetite and little enjoyment in eating, which may lead to rapid weight loss.
Other symptoms may include obsessive thoughts about the infant and his or her care, significant anxiety (possibly manifested in panic attacks), uncontrollable crying, guilt, feelings of being overwhelmed or unable to care for the infant, mood swings, and severe irritability or even anger.2
Severe fatigue may warrant hemoglobin/hematocrit evaluation and possibly measurement of serum thyroid-stimulating hormone (TSH).25,26
It is essential to rule out postpartum psychosis, which is associated with prolonged lack of sleep, confusion, lapsed insight, cognitive impairment, “grossly disorganized behavior,”10 and delusions or hallucinations.5,10 The patient should be asked specifically about unusual or bizarre thoughts or beliefs concerning the infant, in addition to thoughts of harming herself or others, particularly the infant.10
SCREENING
Numerous researchers have suggested that PPD is underrecognized and undertreated.5,6,18,27,28 Screening for PPD in the United States is not standardized and is highly variable.29 The American Academy of Family Physicians supports universal screening for PPD at the first postpartum visit, between two and six weeks.30,31 According to a 2010 Committee Opinion from the American College of Obstetricians and Gynecologists, “at this time, there is insufficient evidence to support a firm recommendation for universal antepartum or postpartum screening; however, screening for depression has the potential to benefit a woman and her family and should be strongly considered.”32
As the AHRQ14 notes, symptoms of PPD may not peak in some women until after their first postpartum visit, and providers of family medicine, internal medicine, and pediatric care may also be in a position to provide screening. The initial well-baby examination by the pediatric primary care provider, for example, presents an important opportunity to screen new mothers for PPD. According to Chaudron et al,33 the well-being of the infant should outweigh any scope-of-practice concerns, practitioner time limitations, or reimbursement issues; rather, screening efforts can be considered “tools to enhance [mothers’] ability to care for their children in a way that is supportive and not punitive.”33
In 2009, Sheeder and colleagues28 reported on a prospective study of 199 mothers in an adolescent maternity clinic who were screened using the Edinburgh Postnatal Depression Scale (EPDS)34 at each well-baby visit during the first six months postpartum. The authors concluded that the optimal time for screening for PPD in this setting is two months after delivery, although repeated screening may identify worsening of depressive symptoms.28
Several screening tools are available for the detection of PPD, particularly the EPDS,34 the Postpartum Depression Screening Scale,35 and the Patient Health Questionnaire–936-38 (see table,11,13,34-37 ). The EPDS, a widely used and well-validated formal screening tool,27,37 is a 10-item self-report questionnaire designed to detect depression in the postpartum period. Cox et al,34 who developed the scale, initially reported its sensitivity at 86% and specificity at 78%21; since then, the tool’s reported sensitivity for detecting major depression in the postpartum period has ranged from 60% to 96%, and specificity from 45% to 97%.6,39 The EPDS has been shown to result in a diagnosis of PPD in significantly more women than routine clinical evaluation (35.4% vs 6.3%, respectively).40 It is possible to administer the EPDS by telephone.41
The validity of the EPDS tool in detecting PPD was recently examined in a systematic review of 37 studies. Gibson et al42 concluded that the heterogeneity of these studies (ie, differences in study methodology, language used, and diagnostic criteria) precluded meta-analysis and did not provide clear support of EPDS as an accurate screening tool for PPD, especially across diverse cultures. In a similar review, Hewitt and colleagues6 sought to “provide an overview of all available methods to identify postnatal depression in primary care and to assess their validity.” They concluded that the EPDS is the most frequently reported screening tool and, with an overall sensitivity of 86% and overall specificity of 87%, its diagnostic performance seems “reasonably good.”6 Of note, fewer data have been collected to demonstrate the effectiveness of other screening tools.35,36,38,41
Cost-effectiveness is a consideration in the use of screening programs for PPD. According to a hypothetical cohort analysis conducted in the UK, the costs of treating women with false-positive screening results made implementation of a formal screening strategy for PPD not cost-effective, compared with usual care only, for use by the British National Health Service.43
MANAGEMENT
Once PPD is identified, treatment should be initiated as quickly as possible; referral for psychological counseling is an appropriate initial strategy for mild to moderate symptoms of PPD.5 A clinical care manager can be a valuable resource to provide education and coordination of care for women affected by PPD. NPs and PAs in primary care, obstetrics/gynecology, women’s health, and psychiatry or psychology can play an important role in the identification and management of PPD.
Treatment of PPD involves combination therapy—short-term psychological therapy combined with pharmacotherapy. According to investigators in a Cochrane Review of nine trials reporting short-term outcomes for 956 women with PPD, their findings suggest that psychosocial and psychological interventions are an effective option for reducing symptoms of PPD.44 Compared with usual care, the types of psychological therapy that were found most effective included cognitive behavioral therapy, interpersonal therapy, and psychodynamic therapy. As most trials’ follow-up periods were limited to six months, however, neither the long-term effects of psychological therapy nor the relative effectiveness of each type of therapy was made clear by these studies.44,45
Pharmacotherapy
Although antidepressant drugs are known to be effective for the treatment of major depressive disorder, well-designed clinical trials demonstrating the overall effectiveness of antidepressants in treatment of PPD have been limited.46 According to Ng et al,46 who in 2010 performed a systematic review of studies examining pharmacologic interventions for PPD, preliminary evidence showing the effectiveness of antidepressants and hormone therapy should prompt the initiation of larger, more rigorous randomized and controlled trials.
The choice of antidepressants will be influenced by the mother’s breastfeeding status and whether PPD represents her first episode of depression or a recurrence of previous major depression. If the patient is not breastfeeding, the choice among antidepressants is similar to those used for treatment of nonpuerperal major depression. If PPD is a relapse of a prior depression, the therapeutic agent that was most effective and best-tolerated for previous depression should be prescribed.47
Generally, the SSRIs are considered first-line agents because of their superior safety profile.47 Fluoxetine has been shown in a small randomized trial (n = 87) to be significantly more effective than placebo and as effective as a full course of cognitive-behavioral counseling.45 In non–placebo-controlled studies, sertraline, fluvoxamine, and venlafaxine all produced improvement in PPD symptoms.48,49
Whether the mother is breastfeeding her infant will influence the use and choice of antidepressants for PPD. Although barely detectable levels of certain antidepressant medications (including the SSRIs sertraline and paroxetine, and the tricyclic antidepressant nortriptyline) have been reported in breast milk or in infant serum,50,51 it is recommended that the lowest possible therapeutic dose be prescribed, and that infants be carefully monitored for adverse effects.51
Fluoxetine, it should be noted, has been found to be transmitted through breast milk and was associated with reduced infant weight gain (specifically, by 392 g over six months) in a comparison between 64 fluoxetine-treated mothers and 38 non-treated mother-infant pairs.52 Thus, fluoxetine use should be avoided in women who are breastfeeding.
In small studies, paroxetine and fluvoxamine were not detected in infant serum, and although low levels of sertraline were detected in one-fourth of infants whose mothers received doses exceeding 100 mg/d, no adverse infant outcomes were noted.52-54 Paroxetine, nortriptyline, and sertraline appear to be relatively safer antidepressant choices in breastfeeding women with PPD.50,52,53
Antidepressants should be continued for six months after full remission of depressive symptoms. Longer courses of therapy may be necessary in patients who experience recurrent major depressive episodes.55
Few researchers have reported on the use of hormonal therapy for PPD. Yet significant hormonal fluctuations,56,57 including “estrogen withdrawal at parturition,”56 are known to occur after childbirth; in their study of women with severe PPD, Ahokas et al57 found that two-thirds of participants had serum estradiol concentrations below the cutoff for gonadal failure. Such a deficiency is likely to contribute to mood disturbances.56,57
In a small, double-blind, placebo-controlled study that enrolled women with severe, persistent PPD (mean EPDS score, 21.8), six months’ treatment with transdermally administered estradiol was associated with significantly greater relief of depressive symptoms than was found in controls (mean EPDS scores at one month, 13.3 vs 16.5, respectively). Of note, more than half of the women studied were concurrently receiving antidepressants.58
Additional studies may elucidate the role of estrogen therapy in the treatment of PPD.
Nonpharmacologic Options
An effective nonpharmacologic option for rapid resolution of severe symptoms of PPD is electroconvulsive therapy (ECT).59-61 Its use is safe in nursing mothers because it does not affect breast milk. ECT is considered especially useful for women who have not responded to pharmacotherapy, those experiencing severe psychotic depression, and those who are considered at high risk for suicide or infanticide.
Patients typically receive three treatments per week; three to six treatments often produce an effective response.59 Anesthesia administered to women who undergo ECT has not been shown to have a negative effect on infants who are being breastfed.60,61
Interpersonal strategies to address PPD should not be overlooked. A pilot study conducted in Canada showed promising results when women who had previously experienced PPD were trained to provide peer support by telephone to mothers who were deemed at high risk for PPD (ie, those with EPDS scores > 12). At four weeks and eight weeks postpartum, follow-up EPDS scores exceeded 12 in 10% and 15%, respectively, of women receiving peer support, compared with 41% and 52%, respectively, of controls.62
Participation in one of numerous support groups that exist for women with PPD (see box, for online information) may reduce isolation in these women and possibly offer additional benefits.
COMPLICATIONS
PPD may be associated with significant complications, underscoring the importance of prompt identification and treatment.5 Maternal depressive symptoms in the critical postnatal period, for example, have been associated with long-term impairment of mother-child bonding. In one study of 101 women, lower-quality maternal bonding was found in women who had symptoms of depression at two weeks, six weeks, and four months postpartum—but not in those with depression at 14 months.63 Additionally, it was found in a systematic review of 49 studies that women with PPD were likely to discontinue breastfeeding earlier than women not affected.64
Delayed growth and development has been reported in infants of mothers with untreated or inadequately treated PPD.65 It has also been suggested that children of depressed mothers may have an increased risk for anxiety, depression, hyperactivity, and other behavioral disorders later in childhood.65-67
PROGNOSIS
Untreated PPD may resolve spontaneously within three to six months, but in about one-quarter of PPD patients, depressive symptoms persist one year after delivery.24,68,69 PPD increases a woman’s risk for future episodes of major depression.2,5
PREVENTION
As previously discussed, the risk for PPD is greatest in women with a history of mood disorders (25%) and PPD (50%).2 Although several approaches have been studied to prevent PPD, no clear optimal strategy has been revealed. In one Cochrane review, insufficient evidence was found to justify prophylactic use of antidepressants.70 Similarly, findings in a second review fell short of confirming the effectiveness of prenatal psychosocial or psychological interventions to prevent antenatal depression.71
Additional studies are needed to make recommendations on prevention of PPD in high-risk patients. Until such recommendations emerge, close monitoring, screening, and follow-up are essential for these women.
CONCLUSION
Postpartum depression is an important concern among childbearing women, as it is associated with adverse maternal and infant outcomes. A personal history of depression is a major risk factor for PPD. It is imperative to question women about signs and symptoms of depression during the immediate postpartum period; it is particularly important to inquire about thoughts of harm to self or to the infant.
Pharmacotherapy combined with adjunctive psychological therapy is indicated for new mothers with significant depressive symptoms. The choice of antidepressants is based on previous response to antidepressants and the woman’s breastfeeding status. Generally, SSRIs are effective and well tolerated for major depression; based on results from small studies, they appear to be safe for breastfeeding mothers.
Electroconvulsive therapy is considered a safe and effective option for women with severe symptoms of PPD.
REFERENCES
1. Muzik M, Marcus SM, Heringhausen JE, Flynn H. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788.
2. Wisner KL, Parry BL, Piontek CM. Clinical practice: postpartum depression. N Engl J Med. 2002;347(3):194-199.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
4. Whitton A, Warner R, Appleby L. The pathway to care in post-natal depression: women’s attitudes to post-natal depression and its treatment. Br J Gen Pract. 1996;46(408):427-428.
5. Sit DK, Wisner KL. Identification of postpartum depression. Clin Obstet Gynecol. 2009; 52(3):456-468.
6. Hewitt C, Gilbody S, Brealey S, et al. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess. 2009;13(36):1-145, 147-230.
7. Epperson CN. Postpartum major depression: detection and treatment. Am Fam Physician. 1999;59(8):2247-2254, 2259-2260.
8. O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
9. Hannah P, Adams D, Lee A, et al. Links between early post-partum mood and post-natal depression. Br J Psychiatry. 1992;160:777-780.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
11. CDC. Prevalence of self-reported postpartum depressive symptoms—17 states, 2004–2005. MMWR Weekly. 2008;57(14):361-366.
12. O’Hara MW, Swain AM. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8:37-54.
13. Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med. 1997;12(7):439-445.
14. Agency for Healthcare Research and Quality. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes: Summary. Evidence Report/Technology Assessment No. 119. www.ahrq.gov/clinic/epcsums/peridep sum.htm. Accessed January 24, 2011.
15. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005 Feb;(119):1-8.
16. Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005; 106(5 pt 1):1071-1083.
17. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10): 1515-1520.
18. Almond P. Postnatal depression a global public health perspective. Perspect Public Health. 2009;129(5):221-227.
19. Lee D, Yip A, Chiu H, et al. A psychiatric epidemiological study of postpartum Chinese women. Am J Psychiatry. 2001;158(2):220-226.
20. Cooper PJ, Tomlinson M, Swartz L, et al. Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry. 1999;175:554-558.
21. McCoy SJB, Beal JM, Miller SBM, et al. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. J Am Osteopath Assoc. 2006;106(4):193-198.
22. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
23. Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108(1-2):147-157.
24. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59 suppl 2:34-40.
25. Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133(12):4139-4142.
26. McCoy SJ, Beal JM, Payton ME, et al. Postpartum thyroid measures and depressive symptomology: a pilot study. J Am Osteopath Assoc. 2008;108(9):503-507.
27. Seehusen DA, Baldwin LM, Runkle GP, Clark G. Are family physicians appropriately screening for postpartum depression? J Am Board Fam Pract. 2005;18(2):104-112.
28. Sheeder J, Kabir K, Stafford B. Screening for postpartum depression at well-child visits: is once enough during the first 6 months of life? Pediatrics. 2009;123(6):e982-e988.
29. Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. Am J Obstet Gynecol. 2009;200(4):357-364.
30. Shaver K. Treating postpartum depression during lactation. Prescriber’s Letter. 2001 Aug;8(8):47.
31. Georgiopoulos AM, Bryan TL, Wollan P, Yawn BP. Routine screening for postpartum depression. J Fam Pract. 2001;50(20):117-122.
32. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 453: Screening for depression during and after pregnancy. Obstet Gynecol. 2010;115(2 pt 1):394-395.
33. Chaudron LH, Szilagyi PG, Campbell AT, et al. Legal and ethical considerations: risks and benefits of postpartum depression screening at well-child visits. Pediatrics. 2007;119(1):123-128.
34. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
35. Beck CT, Gable RK. Postpartum Depression Screening Scale: development and psychometric testing. Nurs Res. 2000;49(5):272-282.
36. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders, Patient Health Questionnaire. JAMA. 1999; 282(18):1737-44.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Yawn BP, Pace W, Wollan PC, et al. Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. J Am Board Fam Med. 2009;22(5):483-491.
39. Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157: 288-290.
40. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
41. Hanusa BH, Scholle SH, Haskett RF, et al. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585-596.
42. Gibson J, McKenzie-McHarg K, Shakespeare J, et al. A systematic review of studies validating the Edinburgh postnatal depression scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350-364.
43. Paulden M, Palmer S, Hewitt C, Gilbody S. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009; 339:b5203.
44. Dennis CL,Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev. 2007;(4):CD006116.
45. Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085): 932-936.
46. Ng RC, Hirata CK, Yeung W, et al. Pharmacologic treatment for postpartum depression: a systematic review. Pharmacotherapy. 2010; 30(9):928-941.
47. Whitby DH, Smith KM. The use of tricyclic antidepressants and selective serotonin reuptake inhibitors in women who are breastfeeding. Phamacotherapy. 2005;25(3):411-425.
48. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
49. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
50. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353-360.
51. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;79(9):e35.
52. Gjerdingen D. The effectiveness of various postpartum depression treatments and the impact of antidepressant drugs on nursing infants. J Am Board Fam Pract. 2003;16(5): 372-382.
53. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry. 1996;153(9):1132-1137.
54. Hendrick V, Fukuchi A, Altshuler L, et al. Use of sertraline, paroxetine and fluvoxamine by nursing women. Br J Psychiatry. 2001;179: 163-166.
55. Davidson JR. Major depressive disorder treatment guidelines in America and Europe.
J Clin Psychiatry. 2010;71 suppl E1:e04.
56. Moses-Kolko EL, Berga SL, Kalro B, et al. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52(3):516-529.
57. Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62(5):
332-336.
58. Gregoire AJ, Kumar R, Everitt B, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006): 930-933.
59. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
60. Altshuler LL, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592-606.
61. Rabheru K. The use of electroconvulsive therapy in special patient populations. Can J Psychiatry. 2001;46(8):710-719.
62. Dennis CL. The effect of peer support on postpartum depression: a pilot randomized controlled trial. Can J Psychiatry. 2003;48(2): 115-124.
63. Moehler E, Brunner R, Wiebel A, et al. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Arch Womens Ment Health. 2006;9(5):273-278.
64. Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123(4):e736-e751.
65. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82(8): 926-933.
66. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12(1):12-20.
67. Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295(12):1389-1398.
68. Cooper PJ, Murray L. Course and recurrence of postnatal depression: evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166(2):191-195.
69. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
70. Howard LM, Hoffbrand S, Henshaw C, et al. Antidepressant prevention of postnatal depression. Cochrane Database Syst Rev. 2005;(2):CD00436.
71. Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database Syst Rev. 2007;(3):CD006309.
Care of the postpartum patient is an important component of primary care practice—particularly for clinicians who provide women’s health care and are called upon to effectively screen, identify, and manage patients who may be at risk for postpartum depression (PPD). Effective management of the postpartum patient also extends to care of her infant(s) and ideally should involve a multidisciplinary team, including the primary care provider, the pediatrician, and a psychologist or a psychiatrist.1
PPD is commonly described as depression that begins within the first month after delivery.2 It is diagnosed using the same criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)3 as are used to identify major depressive disorder. According to the DSM-IV, PPD begins within the first four weeks after the infant’s birth.2,3 Although onset can occur at any time between 24 hours after a woman gives birth to several months later, PPD most commonly occurs within the first six weeks after delivery.1
The woman with PPD may be reluctant to report her symptoms for a number of reasons, including lack of motivation or energy, fatigue, embarrassment that she is experiencing such symptoms during a presumably happy time in her life—even fear that her child may be removed from her care.4 In addition to depressive symptoms (eg, sleeping difficulty, appetite changes, anhedonia, and guilt), women affected by PPD often experience anxiety and may become obsessed with the health, feeding, and sleeping behaviors of their infants.1,5
Postpartum mood disorders occur in a spectrum, ranging from postpartum baby blues to postpartum psychosis. By DSM-IV definition, a diagnosis of major or minor PPD requires that depressed mood, loss of interest or pleasure, or other characteristic symptoms be present most of the day, nearly every day, for at least two weeks.3,6 PPD must be differentiated from the more common and less severe baby blues, which usually start two to three days after delivery and last less than two weeks.2,7 Between 40% and 80% of women who have given birth experience baby blues, which are characterized by transient mood swings, irritability, crying spells, difficulty sleeping, and difficulty concentrating.8 Experiencing baby blues may place women at increased risk for PPD.9
Postpartum, or puerperal, psychosis occurs in approximately 0.2% of women,10 with an early and sudden onset—that is, within the first week to four weeks postpartum. This severe condition is characterized by hallucinations and delusions often centered on the infant, in addition to insomnia, agitation, and extreme behavioral changes. Postpartum psychosis, which can recur in subsequent pregnancies, may be a manifestation of a preexisting affective disorder, such as bipolar disorder.10 Postpartum psychosis is considered an emergent condition because the safety and well-being of both mother and infant may be at serious risk.2,10
EPIDEMIOLOGY AND RISK FACTORS
PPD is often cited as affecting 10% to 15% of women within the first year after childbirth.11,12 Currently, reported prevalence rates of PPD range from 5% to 20% of women who have recently given birth, depending on the source of information. Among 17 US states participating in the Pregnancy Risk Assessment Monitoring System, a CDC surveillance project, prevalence of self-reported PPD symptoms ranged from 11.7% in Maine to 20.4% in New Mexico.11,13 According to the Agency for Healthcare Research and Quality (AHRQ),14 the prevalence of major or minor depression ranges from 6.5% to 12.9% at different times during the year following delivery, although study design varied throughout the research used, and confidence intervals were deemed wide.14,15
Nevertheless, according to results from a systematic review of 28 studies, the prevalence of minor or major depression is estimated at up to 19.2% of women during the first three months postpartum, and major depression in up to 7% during that time.16 A 2007 chart review of 4,398 US women experiencing live births identified PPD in 10.4%.17
Incidence of PPD is “much higher than the quoted rate of 10% to 15%,” concludes Almond18 after a comprehensive literature review. The condition also affects women globally, the British researcher reports: Not only did she find numerous data on the incidence of PPD in high-income countries, including the US, the United Kingdom, and Australia, but she concluded that incidence rates of PPD in developing countries are grossly underestimated, according to epidemiologic studies in low- and middle-income countries (eg, Pakistan, Indonesia, Vietnam). Additionally, the risk factors for PPD are likely to be influenced by cultural differences, and attempts to identify PPD must be culturally sensitive.18
Several risk factors have been associated with PPD. Perhaps the most significant risk factor is a personal history of depression (prior to pregnancy or postpartum); at least one-half of women with PPD experience onset of depressive symptoms before or during their pregnancies,19,20 and one research group reported a relative risk (RR) of 1.87 for PPD in women with a history of depression, compared with those without such a history.21 Thus, women previously affected by depression should be carefully monitored in the immediate postpartum period for any signs of depressed mood, anxiety, sleep difficulties, loss of appetite or energy, and psychomotor changes.
According to McCoy et al,21 neither patient age nor marital status nor method of delivery appeared to be associated with PPD at four weeks postpartum. Women who were feeding by formula alone were more likely to experience PPD (RR, 2.04) than were those who breastfed their infants. Women who smoked were more likely to be affected by PPD (RR, 1.58) than were nonsmokers.
Additional risk factors for PPD identified by Dennis et al22 included pregnancy-induced hypertension and immigration within the previous five years. Various psychosocial stressors may also represent risk factors for PPD, including lack of social support, financial concerns, miscarriage or fetal demise, limited partner support, physical abuse before or during pregnancy, lack of readiness for hospital discharge, and complications during pregnancy and delivery (eg, low birth weight, premature birth, admission of the infant to the neonatal ICU).11,22,23
It is important to identify women who are at highest risk for PPD as soon as possible. High-risk patients should be screened upon discharge from the hospital and certainly on or before the first postpartum visit.
CLINICAL MANIFESTATIONS
Clinical manifestations of PPD include depressed mood for at least two weeks with changes in somatic functions, such as sleep, energy level, appetite, weight, gastrointestinal functioning, and decrease in libido.3,24 These manifestations are more severe and prolonged than those associated with baby blues (which almost always resolve within two weeks postpartum).2,7
On physical examination, the patient with PPD may appear tearful and disheveled, with psychomotor retardation. She may report that she is unable to sleep even when her infant is sleeping, or that she has a significant lack of energy despite sufficient sleep; she may admit being unable to get out of bed for hours.2 The patient may report a significant decrease in appetite and little enjoyment in eating, which may lead to rapid weight loss.
Other symptoms may include obsessive thoughts about the infant and his or her care, significant anxiety (possibly manifested in panic attacks), uncontrollable crying, guilt, feelings of being overwhelmed or unable to care for the infant, mood swings, and severe irritability or even anger.2
Severe fatigue may warrant hemoglobin/hematocrit evaluation and possibly measurement of serum thyroid-stimulating hormone (TSH).25,26
It is essential to rule out postpartum psychosis, which is associated with prolonged lack of sleep, confusion, lapsed insight, cognitive impairment, “grossly disorganized behavior,”10 and delusions or hallucinations.5,10 The patient should be asked specifically about unusual or bizarre thoughts or beliefs concerning the infant, in addition to thoughts of harming herself or others, particularly the infant.10
SCREENING
Numerous researchers have suggested that PPD is underrecognized and undertreated.5,6,18,27,28 Screening for PPD in the United States is not standardized and is highly variable.29 The American Academy of Family Physicians supports universal screening for PPD at the first postpartum visit, between two and six weeks.30,31 According to a 2010 Committee Opinion from the American College of Obstetricians and Gynecologists, “at this time, there is insufficient evidence to support a firm recommendation for universal antepartum or postpartum screening; however, screening for depression has the potential to benefit a woman and her family and should be strongly considered.”32
As the AHRQ14 notes, symptoms of PPD may not peak in some women until after their first postpartum visit, and providers of family medicine, internal medicine, and pediatric care may also be in a position to provide screening. The initial well-baby examination by the pediatric primary care provider, for example, presents an important opportunity to screen new mothers for PPD. According to Chaudron et al,33 the well-being of the infant should outweigh any scope-of-practice concerns, practitioner time limitations, or reimbursement issues; rather, screening efforts can be considered “tools to enhance [mothers’] ability to care for their children in a way that is supportive and not punitive.”33
In 2009, Sheeder and colleagues28 reported on a prospective study of 199 mothers in an adolescent maternity clinic who were screened using the Edinburgh Postnatal Depression Scale (EPDS)34 at each well-baby visit during the first six months postpartum. The authors concluded that the optimal time for screening for PPD in this setting is two months after delivery, although repeated screening may identify worsening of depressive symptoms.28
Several screening tools are available for the detection of PPD, particularly the EPDS,34 the Postpartum Depression Screening Scale,35 and the Patient Health Questionnaire–936-38 (see table,11,13,34-37 ). The EPDS, a widely used and well-validated formal screening tool,27,37 is a 10-item self-report questionnaire designed to detect depression in the postpartum period. Cox et al,34 who developed the scale, initially reported its sensitivity at 86% and specificity at 78%21; since then, the tool’s reported sensitivity for detecting major depression in the postpartum period has ranged from 60% to 96%, and specificity from 45% to 97%.6,39 The EPDS has been shown to result in a diagnosis of PPD in significantly more women than routine clinical evaluation (35.4% vs 6.3%, respectively).40 It is possible to administer the EPDS by telephone.41
The validity of the EPDS tool in detecting PPD was recently examined in a systematic review of 37 studies. Gibson et al42 concluded that the heterogeneity of these studies (ie, differences in study methodology, language used, and diagnostic criteria) precluded meta-analysis and did not provide clear support of EPDS as an accurate screening tool for PPD, especially across diverse cultures. In a similar review, Hewitt and colleagues6 sought to “provide an overview of all available methods to identify postnatal depression in primary care and to assess their validity.” They concluded that the EPDS is the most frequently reported screening tool and, with an overall sensitivity of 86% and overall specificity of 87%, its diagnostic performance seems “reasonably good.”6 Of note, fewer data have been collected to demonstrate the effectiveness of other screening tools.35,36,38,41
Cost-effectiveness is a consideration in the use of screening programs for PPD. According to a hypothetical cohort analysis conducted in the UK, the costs of treating women with false-positive screening results made implementation of a formal screening strategy for PPD not cost-effective, compared with usual care only, for use by the British National Health Service.43
MANAGEMENT
Once PPD is identified, treatment should be initiated as quickly as possible; referral for psychological counseling is an appropriate initial strategy for mild to moderate symptoms of PPD.5 A clinical care manager can be a valuable resource to provide education and coordination of care for women affected by PPD. NPs and PAs in primary care, obstetrics/gynecology, women’s health, and psychiatry or psychology can play an important role in the identification and management of PPD.
Treatment of PPD involves combination therapy—short-term psychological therapy combined with pharmacotherapy. According to investigators in a Cochrane Review of nine trials reporting short-term outcomes for 956 women with PPD, their findings suggest that psychosocial and psychological interventions are an effective option for reducing symptoms of PPD.44 Compared with usual care, the types of psychological therapy that were found most effective included cognitive behavioral therapy, interpersonal therapy, and psychodynamic therapy. As most trials’ follow-up periods were limited to six months, however, neither the long-term effects of psychological therapy nor the relative effectiveness of each type of therapy was made clear by these studies.44,45
Pharmacotherapy
Although antidepressant drugs are known to be effective for the treatment of major depressive disorder, well-designed clinical trials demonstrating the overall effectiveness of antidepressants in treatment of PPD have been limited.46 According to Ng et al,46 who in 2010 performed a systematic review of studies examining pharmacologic interventions for PPD, preliminary evidence showing the effectiveness of antidepressants and hormone therapy should prompt the initiation of larger, more rigorous randomized and controlled trials.
The choice of antidepressants will be influenced by the mother’s breastfeeding status and whether PPD represents her first episode of depression or a recurrence of previous major depression. If the patient is not breastfeeding, the choice among antidepressants is similar to those used for treatment of nonpuerperal major depression. If PPD is a relapse of a prior depression, the therapeutic agent that was most effective and best-tolerated for previous depression should be prescribed.47
Generally, the SSRIs are considered first-line agents because of their superior safety profile.47 Fluoxetine has been shown in a small randomized trial (n = 87) to be significantly more effective than placebo and as effective as a full course of cognitive-behavioral counseling.45 In non–placebo-controlled studies, sertraline, fluvoxamine, and venlafaxine all produced improvement in PPD symptoms.48,49
Whether the mother is breastfeeding her infant will influence the use and choice of antidepressants for PPD. Although barely detectable levels of certain antidepressant medications (including the SSRIs sertraline and paroxetine, and the tricyclic antidepressant nortriptyline) have been reported in breast milk or in infant serum,50,51 it is recommended that the lowest possible therapeutic dose be prescribed, and that infants be carefully monitored for adverse effects.51
Fluoxetine, it should be noted, has been found to be transmitted through breast milk and was associated with reduced infant weight gain (specifically, by 392 g over six months) in a comparison between 64 fluoxetine-treated mothers and 38 non-treated mother-infant pairs.52 Thus, fluoxetine use should be avoided in women who are breastfeeding.
In small studies, paroxetine and fluvoxamine were not detected in infant serum, and although low levels of sertraline were detected in one-fourth of infants whose mothers received doses exceeding 100 mg/d, no adverse infant outcomes were noted.52-54 Paroxetine, nortriptyline, and sertraline appear to be relatively safer antidepressant choices in breastfeeding women with PPD.50,52,53
Antidepressants should be continued for six months after full remission of depressive symptoms. Longer courses of therapy may be necessary in patients who experience recurrent major depressive episodes.55
Few researchers have reported on the use of hormonal therapy for PPD. Yet significant hormonal fluctuations,56,57 including “estrogen withdrawal at parturition,”56 are known to occur after childbirth; in their study of women with severe PPD, Ahokas et al57 found that two-thirds of participants had serum estradiol concentrations below the cutoff for gonadal failure. Such a deficiency is likely to contribute to mood disturbances.56,57
In a small, double-blind, placebo-controlled study that enrolled women with severe, persistent PPD (mean EPDS score, 21.8), six months’ treatment with transdermally administered estradiol was associated with significantly greater relief of depressive symptoms than was found in controls (mean EPDS scores at one month, 13.3 vs 16.5, respectively). Of note, more than half of the women studied were concurrently receiving antidepressants.58
Additional studies may elucidate the role of estrogen therapy in the treatment of PPD.
Nonpharmacologic Options
An effective nonpharmacologic option for rapid resolution of severe symptoms of PPD is electroconvulsive therapy (ECT).59-61 Its use is safe in nursing mothers because it does not affect breast milk. ECT is considered especially useful for women who have not responded to pharmacotherapy, those experiencing severe psychotic depression, and those who are considered at high risk for suicide or infanticide.
Patients typically receive three treatments per week; three to six treatments often produce an effective response.59 Anesthesia administered to women who undergo ECT has not been shown to have a negative effect on infants who are being breastfed.60,61
Interpersonal strategies to address PPD should not be overlooked. A pilot study conducted in Canada showed promising results when women who had previously experienced PPD were trained to provide peer support by telephone to mothers who were deemed at high risk for PPD (ie, those with EPDS scores > 12). At four weeks and eight weeks postpartum, follow-up EPDS scores exceeded 12 in 10% and 15%, respectively, of women receiving peer support, compared with 41% and 52%, respectively, of controls.62
Participation in one of numerous support groups that exist for women with PPD (see box, for online information) may reduce isolation in these women and possibly offer additional benefits.
COMPLICATIONS
PPD may be associated with significant complications, underscoring the importance of prompt identification and treatment.5 Maternal depressive symptoms in the critical postnatal period, for example, have been associated with long-term impairment of mother-child bonding. In one study of 101 women, lower-quality maternal bonding was found in women who had symptoms of depression at two weeks, six weeks, and four months postpartum—but not in those with depression at 14 months.63 Additionally, it was found in a systematic review of 49 studies that women with PPD were likely to discontinue breastfeeding earlier than women not affected.64
Delayed growth and development has been reported in infants of mothers with untreated or inadequately treated PPD.65 It has also been suggested that children of depressed mothers may have an increased risk for anxiety, depression, hyperactivity, and other behavioral disorders later in childhood.65-67
PROGNOSIS
Untreated PPD may resolve spontaneously within three to six months, but in about one-quarter of PPD patients, depressive symptoms persist one year after delivery.24,68,69 PPD increases a woman’s risk for future episodes of major depression.2,5
PREVENTION
As previously discussed, the risk for PPD is greatest in women with a history of mood disorders (25%) and PPD (50%).2 Although several approaches have been studied to prevent PPD, no clear optimal strategy has been revealed. In one Cochrane review, insufficient evidence was found to justify prophylactic use of antidepressants.70 Similarly, findings in a second review fell short of confirming the effectiveness of prenatal psychosocial or psychological interventions to prevent antenatal depression.71
Additional studies are needed to make recommendations on prevention of PPD in high-risk patients. Until such recommendations emerge, close monitoring, screening, and follow-up are essential for these women.
CONCLUSION
Postpartum depression is an important concern among childbearing women, as it is associated with adverse maternal and infant outcomes. A personal history of depression is a major risk factor for PPD. It is imperative to question women about signs and symptoms of depression during the immediate postpartum period; it is particularly important to inquire about thoughts of harm to self or to the infant.
Pharmacotherapy combined with adjunctive psychological therapy is indicated for new mothers with significant depressive symptoms. The choice of antidepressants is based on previous response to antidepressants and the woman’s breastfeeding status. Generally, SSRIs are effective and well tolerated for major depression; based on results from small studies, they appear to be safe for breastfeeding mothers.
Electroconvulsive therapy is considered a safe and effective option for women with severe symptoms of PPD.
REFERENCES
1. Muzik M, Marcus SM, Heringhausen JE, Flynn H. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788.
2. Wisner KL, Parry BL, Piontek CM. Clinical practice: postpartum depression. N Engl J Med. 2002;347(3):194-199.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
4. Whitton A, Warner R, Appleby L. The pathway to care in post-natal depression: women’s attitudes to post-natal depression and its treatment. Br J Gen Pract. 1996;46(408):427-428.
5. Sit DK, Wisner KL. Identification of postpartum depression. Clin Obstet Gynecol. 2009; 52(3):456-468.
6. Hewitt C, Gilbody S, Brealey S, et al. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess. 2009;13(36):1-145, 147-230.
7. Epperson CN. Postpartum major depression: detection and treatment. Am Fam Physician. 1999;59(8):2247-2254, 2259-2260.
8. O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
9. Hannah P, Adams D, Lee A, et al. Links between early post-partum mood and post-natal depression. Br J Psychiatry. 1992;160:777-780.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
11. CDC. Prevalence of self-reported postpartum depressive symptoms—17 states, 2004–2005. MMWR Weekly. 2008;57(14):361-366.
12. O’Hara MW, Swain AM. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8:37-54.
13. Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med. 1997;12(7):439-445.
14. Agency for Healthcare Research and Quality. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes: Summary. Evidence Report/Technology Assessment No. 119. www.ahrq.gov/clinic/epcsums/peridep sum.htm. Accessed January 24, 2011.
15. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005 Feb;(119):1-8.
16. Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005; 106(5 pt 1):1071-1083.
17. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10): 1515-1520.
18. Almond P. Postnatal depression a global public health perspective. Perspect Public Health. 2009;129(5):221-227.
19. Lee D, Yip A, Chiu H, et al. A psychiatric epidemiological study of postpartum Chinese women. Am J Psychiatry. 2001;158(2):220-226.
20. Cooper PJ, Tomlinson M, Swartz L, et al. Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry. 1999;175:554-558.
21. McCoy SJB, Beal JM, Miller SBM, et al. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. J Am Osteopath Assoc. 2006;106(4):193-198.
22. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
23. Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108(1-2):147-157.
24. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59 suppl 2:34-40.
25. Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133(12):4139-4142.
26. McCoy SJ, Beal JM, Payton ME, et al. Postpartum thyroid measures and depressive symptomology: a pilot study. J Am Osteopath Assoc. 2008;108(9):503-507.
27. Seehusen DA, Baldwin LM, Runkle GP, Clark G. Are family physicians appropriately screening for postpartum depression? J Am Board Fam Pract. 2005;18(2):104-112.
28. Sheeder J, Kabir K, Stafford B. Screening for postpartum depression at well-child visits: is once enough during the first 6 months of life? Pediatrics. 2009;123(6):e982-e988.
29. Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. Am J Obstet Gynecol. 2009;200(4):357-364.
30. Shaver K. Treating postpartum depression during lactation. Prescriber’s Letter. 2001 Aug;8(8):47.
31. Georgiopoulos AM, Bryan TL, Wollan P, Yawn BP. Routine screening for postpartum depression. J Fam Pract. 2001;50(20):117-122.
32. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 453: Screening for depression during and after pregnancy. Obstet Gynecol. 2010;115(2 pt 1):394-395.
33. Chaudron LH, Szilagyi PG, Campbell AT, et al. Legal and ethical considerations: risks and benefits of postpartum depression screening at well-child visits. Pediatrics. 2007;119(1):123-128.
34. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
35. Beck CT, Gable RK. Postpartum Depression Screening Scale: development and psychometric testing. Nurs Res. 2000;49(5):272-282.
36. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders, Patient Health Questionnaire. JAMA. 1999; 282(18):1737-44.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Yawn BP, Pace W, Wollan PC, et al. Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. J Am Board Fam Med. 2009;22(5):483-491.
39. Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157: 288-290.
40. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
41. Hanusa BH, Scholle SH, Haskett RF, et al. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585-596.
42. Gibson J, McKenzie-McHarg K, Shakespeare J, et al. A systematic review of studies validating the Edinburgh postnatal depression scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350-364.
43. Paulden M, Palmer S, Hewitt C, Gilbody S. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009; 339:b5203.
44. Dennis CL,Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev. 2007;(4):CD006116.
45. Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085): 932-936.
46. Ng RC, Hirata CK, Yeung W, et al. Pharmacologic treatment for postpartum depression: a systematic review. Pharmacotherapy. 2010; 30(9):928-941.
47. Whitby DH, Smith KM. The use of tricyclic antidepressants and selective serotonin reuptake inhibitors in women who are breastfeeding. Phamacotherapy. 2005;25(3):411-425.
48. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
49. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
50. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353-360.
51. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;79(9):e35.
52. Gjerdingen D. The effectiveness of various postpartum depression treatments and the impact of antidepressant drugs on nursing infants. J Am Board Fam Pract. 2003;16(5): 372-382.
53. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry. 1996;153(9):1132-1137.
54. Hendrick V, Fukuchi A, Altshuler L, et al. Use of sertraline, paroxetine and fluvoxamine by nursing women. Br J Psychiatry. 2001;179: 163-166.
55. Davidson JR. Major depressive disorder treatment guidelines in America and Europe.
J Clin Psychiatry. 2010;71 suppl E1:e04.
56. Moses-Kolko EL, Berga SL, Kalro B, et al. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52(3):516-529.
57. Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62(5):
332-336.
58. Gregoire AJ, Kumar R, Everitt B, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006): 930-933.
59. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
60. Altshuler LL, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592-606.
61. Rabheru K. The use of electroconvulsive therapy in special patient populations. Can J Psychiatry. 2001;46(8):710-719.
62. Dennis CL. The effect of peer support on postpartum depression: a pilot randomized controlled trial. Can J Psychiatry. 2003;48(2): 115-124.
63. Moehler E, Brunner R, Wiebel A, et al. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Arch Womens Ment Health. 2006;9(5):273-278.
64. Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123(4):e736-e751.
65. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82(8): 926-933.
66. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12(1):12-20.
67. Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295(12):1389-1398.
68. Cooper PJ, Murray L. Course and recurrence of postnatal depression: evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166(2):191-195.
69. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
70. Howard LM, Hoffbrand S, Henshaw C, et al. Antidepressant prevention of postnatal depression. Cochrane Database Syst Rev. 2005;(2):CD00436.
71. Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database Syst Rev. 2007;(3):CD006309.