User login
Outcomes of Arthroscopic Versus Open Rotator Cuff Repair: A Systematic Review of the Literature
UPDATE: URINARY INCONTINENCE
Pelvic organ prolapse (POP) is no small problem. With a prevalence thought to range as high as 30%, the condition challenges us to manage resources in a way that is mindful of cost—both financial expense and cost to the patient in terms of recovery and quality of life.
Although a large percentage of women who have POP also complain of symptomatic incontinence, a substantial number of continent women who have severe POP become incontinent after surgical repair. One reason may be that advanced POP sometimes causes urethral kinking and external urethral compression, fixing a hypermobile urethra in place. Once normal anatomy is restored and the urethra is no longer kinked, the urinary incontinence is “unmasked.”
Women who develop de novo incontinence after POP repair are thought to have “occult” urinary incontinence. Occult stress incontinence is urinary leakage that is prevented by POP and becomes symptomatic only after restoration of pelvic anatomy.1 It has been reported that 36% to 80% of continent women who have POP will develop stress urinary incontinence once the prolapse is reduced, either preoperatively with a pessary or vaginal pack, or after surgical correction.2
This information prompts important questions: If a woman who has POP is continent at the time of her surgical repair, should she undergo a concomitant incontinence procedure “just in case”? Or should she be reevaluated postoperatively for a possible continence procedure at a later time?
The colpopexy and urinary reduction efforts (CARE) trial concluded that postoperative stress incontinence in continent women is significantly reduced when sacrocolpopexy is combined with Burch urethropexy (FIGURE).3 When women who underwent a concomitant Burch procedure were compared with those who didn’t, de novo stress incontinence after prolapse repair occurred in 24% and 44% of women, respectively.3,4 This finding suggests that Burch urethropexy provides a protective benefit for continent women when it is performed at the time of abdominal sacrocolpopexy, eliminating the need for an additional procedure in the future.
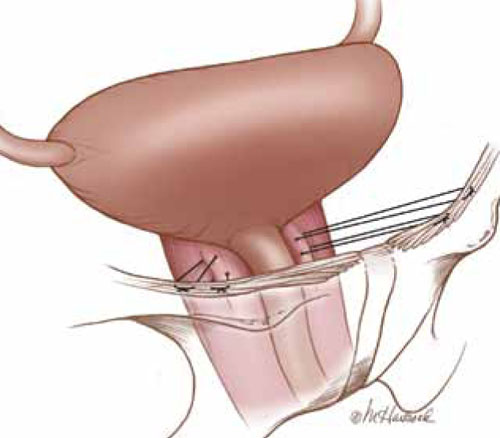
FIGURE: Burch urethropexy
Sutures are placed at the level of the bladder neck and passed through the Cooper’s ligaments to support the urethra and eliminate stress urinary incontinence.
Publication of the CARE findings sparked debate among pelvic surgeons. According to a recent survey of pelvic surgeons, only 50% changed their practice as a result of the CARE trial.5 Some argue that the addition of a continence procedure adds unnecessary surgical risk when the patient lacks subjective or objective evidence of stress incontinence. Besides the surgical risks—which, one might argue, are low—continence surgery may lead to new symptoms of urinary dysfunction, such as urinary obstruction or new-onset urge incontinence. The development of such symptoms can create significant dissatisfaction in a patient who was previously asymptomatic.
This article explores the issue in more depth, focusing on two recent studies:
- analysis of CARE trial data to determine the positive predictive value of preoperative prolapse reduction and urodynamic testing among continent women who have POP
- a retrospective comparison of women who had urodynamically confirmed occult incontinence with those who didn’t, along with their response to different interventions.
What’s the best way to assess women for occult stress incontinence?
Visco AG, Brubaker L, Nygaard I, et al; for Pelvic Floor Disorders Network. The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: the Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):607–614.
Elser DM, Moen MD, Stanford EJ, et al. Abdominal sacrocolpopexy and urinary incontinence: surgical planning based on urodynamics. Am J Obstet Gynecol. 2010;202(4):375.e1–5.
Preoperative urodynamic testing is often used to evaluate women undergoing pelvic and continence surgery. For adequate evaluation, the prolapse must be reduced sufficiently to simulate the support achieved with the planned surgery. The techniques used to reduce the prolapse during the testing are variable, as is the predictive value of the urodynamic evaluation.
Prolapse may be reduced using a large cotton swab, ring forceps, pessary, or split speculum. When these methods and the utility of urodynamics were evaluated as part of the CARE trial, Visco and colleagues demonstrated that reduction of the prolapse with a large swab yielded the highest positive predictive value. Women who had urodynamically confirmed stress incontinence after the prolapse was reduced with a swab were more likely to develop symptomatic stress incontinence after sacrocolpopexy.
In this study, 35% of women who did not demonstrate occult incontinence during preoperative testing with the swab also went on to develop postoperative incontinence. Overall, urodynamic testing was not helpful in the evaluation of women who had POP. However, asymptomatic women who leaked during preoperative evaluation were more likely to experience incontinence postoperatively, even if they underwent Burch urethropexy.
1. Long CY, Hsu SC, Wu TP, Sun DJ, Su JH, Tsai EM. Urodynamic comparison of continent and incontinent women with severe uterovaginal prolapse. J Reprod Med. 2004;49(1):33-37.
2. Roovers JP, Oelke M. Clinical relevance of urodynamic investigation tests prior to surgical correction of genital prolapse: a literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):455-460.
3. Brubaker L, Cundiff GW, Fine P, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354(15):1557-1566.
4. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112(1):49-55.
5. Aungst MJ, Mamienski TD, Albright TS, Zahn CM, Fischer JR. Prophylactic Burch colposuspension at the time of abdominal sacrocolpopexy: a survey of current practice patterns. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(8):897-904.
6. Ward KL, Hilton P. Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325(735):67-70.
7. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence. Obstet Gynecol. 2008;111(3):611-621.
8. Kennelly MJ, Moore R, Nguyen JN, Lukban JC, Siegel S. Prospective evaluation of a single incision sling for stress urinary incontinence. J Urol. 2010;184(2):604-609.
Pelvic organ prolapse (POP) is no small problem. With a prevalence thought to range as high as 30%, the condition challenges us to manage resources in a way that is mindful of cost—both financial expense and cost to the patient in terms of recovery and quality of life.
Although a large percentage of women who have POP also complain of symptomatic incontinence, a substantial number of continent women who have severe POP become incontinent after surgical repair. One reason may be that advanced POP sometimes causes urethral kinking and external urethral compression, fixing a hypermobile urethra in place. Once normal anatomy is restored and the urethra is no longer kinked, the urinary incontinence is “unmasked.”
Women who develop de novo incontinence after POP repair are thought to have “occult” urinary incontinence. Occult stress incontinence is urinary leakage that is prevented by POP and becomes symptomatic only after restoration of pelvic anatomy.1 It has been reported that 36% to 80% of continent women who have POP will develop stress urinary incontinence once the prolapse is reduced, either preoperatively with a pessary or vaginal pack, or after surgical correction.2
This information prompts important questions: If a woman who has POP is continent at the time of her surgical repair, should she undergo a concomitant incontinence procedure “just in case”? Or should she be reevaluated postoperatively for a possible continence procedure at a later time?
The colpopexy and urinary reduction efforts (CARE) trial concluded that postoperative stress incontinence in continent women is significantly reduced when sacrocolpopexy is combined with Burch urethropexy (FIGURE).3 When women who underwent a concomitant Burch procedure were compared with those who didn’t, de novo stress incontinence after prolapse repair occurred in 24% and 44% of women, respectively.3,4 This finding suggests that Burch urethropexy provides a protective benefit for continent women when it is performed at the time of abdominal sacrocolpopexy, eliminating the need for an additional procedure in the future.

FIGURE: Burch urethropexy
Sutures are placed at the level of the bladder neck and passed through the Cooper’s ligaments to support the urethra and eliminate stress urinary incontinence.
Publication of the CARE findings sparked debate among pelvic surgeons. According to a recent survey of pelvic surgeons, only 50% changed their practice as a result of the CARE trial.5 Some argue that the addition of a continence procedure adds unnecessary surgical risk when the patient lacks subjective or objective evidence of stress incontinence. Besides the surgical risks—which, one might argue, are low—continence surgery may lead to new symptoms of urinary dysfunction, such as urinary obstruction or new-onset urge incontinence. The development of such symptoms can create significant dissatisfaction in a patient who was previously asymptomatic.
This article explores the issue in more depth, focusing on two recent studies:
- analysis of CARE trial data to determine the positive predictive value of preoperative prolapse reduction and urodynamic testing among continent women who have POP
- a retrospective comparison of women who had urodynamically confirmed occult incontinence with those who didn’t, along with their response to different interventions.
What’s the best way to assess women for occult stress incontinence?
Visco AG, Brubaker L, Nygaard I, et al; for Pelvic Floor Disorders Network. The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: the Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):607–614.
Elser DM, Moen MD, Stanford EJ, et al. Abdominal sacrocolpopexy and urinary incontinence: surgical planning based on urodynamics. Am J Obstet Gynecol. 2010;202(4):375.e1–5.
Preoperative urodynamic testing is often used to evaluate women undergoing pelvic and continence surgery. For adequate evaluation, the prolapse must be reduced sufficiently to simulate the support achieved with the planned surgery. The techniques used to reduce the prolapse during the testing are variable, as is the predictive value of the urodynamic evaluation.
Prolapse may be reduced using a large cotton swab, ring forceps, pessary, or split speculum. When these methods and the utility of urodynamics were evaluated as part of the CARE trial, Visco and colleagues demonstrated that reduction of the prolapse with a large swab yielded the highest positive predictive value. Women who had urodynamically confirmed stress incontinence after the prolapse was reduced with a swab were more likely to develop symptomatic stress incontinence after sacrocolpopexy.
In this study, 35% of women who did not demonstrate occult incontinence during preoperative testing with the swab also went on to develop postoperative incontinence. Overall, urodynamic testing was not helpful in the evaluation of women who had POP. However, asymptomatic women who leaked during preoperative evaluation were more likely to experience incontinence postoperatively, even if they underwent Burch urethropexy.
Pelvic organ prolapse (POP) is no small problem. With a prevalence thought to range as high as 30%, the condition challenges us to manage resources in a way that is mindful of cost—both financial expense and cost to the patient in terms of recovery and quality of life.
Although a large percentage of women who have POP also complain of symptomatic incontinence, a substantial number of continent women who have severe POP become incontinent after surgical repair. One reason may be that advanced POP sometimes causes urethral kinking and external urethral compression, fixing a hypermobile urethra in place. Once normal anatomy is restored and the urethra is no longer kinked, the urinary incontinence is “unmasked.”
Women who develop de novo incontinence after POP repair are thought to have “occult” urinary incontinence. Occult stress incontinence is urinary leakage that is prevented by POP and becomes symptomatic only after restoration of pelvic anatomy.1 It has been reported that 36% to 80% of continent women who have POP will develop stress urinary incontinence once the prolapse is reduced, either preoperatively with a pessary or vaginal pack, or after surgical correction.2
This information prompts important questions: If a woman who has POP is continent at the time of her surgical repair, should she undergo a concomitant incontinence procedure “just in case”? Or should she be reevaluated postoperatively for a possible continence procedure at a later time?
The colpopexy and urinary reduction efforts (CARE) trial concluded that postoperative stress incontinence in continent women is significantly reduced when sacrocolpopexy is combined with Burch urethropexy (FIGURE).3 When women who underwent a concomitant Burch procedure were compared with those who didn’t, de novo stress incontinence after prolapse repair occurred in 24% and 44% of women, respectively.3,4 This finding suggests that Burch urethropexy provides a protective benefit for continent women when it is performed at the time of abdominal sacrocolpopexy, eliminating the need for an additional procedure in the future.

FIGURE: Burch urethropexy
Sutures are placed at the level of the bladder neck and passed through the Cooper’s ligaments to support the urethra and eliminate stress urinary incontinence.
Publication of the CARE findings sparked debate among pelvic surgeons. According to a recent survey of pelvic surgeons, only 50% changed their practice as a result of the CARE trial.5 Some argue that the addition of a continence procedure adds unnecessary surgical risk when the patient lacks subjective or objective evidence of stress incontinence. Besides the surgical risks—which, one might argue, are low—continence surgery may lead to new symptoms of urinary dysfunction, such as urinary obstruction or new-onset urge incontinence. The development of such symptoms can create significant dissatisfaction in a patient who was previously asymptomatic.
This article explores the issue in more depth, focusing on two recent studies:
- analysis of CARE trial data to determine the positive predictive value of preoperative prolapse reduction and urodynamic testing among continent women who have POP
- a retrospective comparison of women who had urodynamically confirmed occult incontinence with those who didn’t, along with their response to different interventions.
What’s the best way to assess women for occult stress incontinence?
Visco AG, Brubaker L, Nygaard I, et al; for Pelvic Floor Disorders Network. The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: the Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):607–614.
Elser DM, Moen MD, Stanford EJ, et al. Abdominal sacrocolpopexy and urinary incontinence: surgical planning based on urodynamics. Am J Obstet Gynecol. 2010;202(4):375.e1–5.
Preoperative urodynamic testing is often used to evaluate women undergoing pelvic and continence surgery. For adequate evaluation, the prolapse must be reduced sufficiently to simulate the support achieved with the planned surgery. The techniques used to reduce the prolapse during the testing are variable, as is the predictive value of the urodynamic evaluation.
Prolapse may be reduced using a large cotton swab, ring forceps, pessary, or split speculum. When these methods and the utility of urodynamics were evaluated as part of the CARE trial, Visco and colleagues demonstrated that reduction of the prolapse with a large swab yielded the highest positive predictive value. Women who had urodynamically confirmed stress incontinence after the prolapse was reduced with a swab were more likely to develop symptomatic stress incontinence after sacrocolpopexy.
In this study, 35% of women who did not demonstrate occult incontinence during preoperative testing with the swab also went on to develop postoperative incontinence. Overall, urodynamic testing was not helpful in the evaluation of women who had POP. However, asymptomatic women who leaked during preoperative evaluation were more likely to experience incontinence postoperatively, even if they underwent Burch urethropexy.
1. Long CY, Hsu SC, Wu TP, Sun DJ, Su JH, Tsai EM. Urodynamic comparison of continent and incontinent women with severe uterovaginal prolapse. J Reprod Med. 2004;49(1):33-37.
2. Roovers JP, Oelke M. Clinical relevance of urodynamic investigation tests prior to surgical correction of genital prolapse: a literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):455-460.
3. Brubaker L, Cundiff GW, Fine P, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354(15):1557-1566.
4. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112(1):49-55.
5. Aungst MJ, Mamienski TD, Albright TS, Zahn CM, Fischer JR. Prophylactic Burch colposuspension at the time of abdominal sacrocolpopexy: a survey of current practice patterns. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(8):897-904.
6. Ward KL, Hilton P. Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325(735):67-70.
7. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence. Obstet Gynecol. 2008;111(3):611-621.
8. Kennelly MJ, Moore R, Nguyen JN, Lukban JC, Siegel S. Prospective evaluation of a single incision sling for stress urinary incontinence. J Urol. 2010;184(2):604-609.
1. Long CY, Hsu SC, Wu TP, Sun DJ, Su JH, Tsai EM. Urodynamic comparison of continent and incontinent women with severe uterovaginal prolapse. J Reprod Med. 2004;49(1):33-37.
2. Roovers JP, Oelke M. Clinical relevance of urodynamic investigation tests prior to surgical correction of genital prolapse: a literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):455-460.
3. Brubaker L, Cundiff GW, Fine P, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354(15):1557-1566.
4. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112(1):49-55.
5. Aungst MJ, Mamienski TD, Albright TS, Zahn CM, Fischer JR. Prophylactic Burch colposuspension at the time of abdominal sacrocolpopexy: a survey of current practice patterns. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(8):897-904.
6. Ward KL, Hilton P. Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325(735):67-70.
7. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence. Obstet Gynecol. 2008;111(3):611-621.
8. Kennelly MJ, Moore R, Nguyen JN, Lukban JC, Siegel S. Prospective evaluation of a single incision sling for stress urinary incontinence. J Urol. 2010;184(2):604-609.
Sound strategies to avoid malpractice hazards on labor and delivery
CASE: Is TOLAC feasible?
Your patient is a 33-year-old gravida 3, para 2002, with a previous cesarean delivery who was admitted to labor and delivery with premature ruptured membranes at term. She is not contracting. Fetal status is reassuring.
Her obstetric history is of one normal, spontaneous delivery followed by one cesarean delivery, both occurring at term.
She wants to know if she can safely undergo a trial of labor, or if she must have a repeat cesarean delivery. How should you counsel her?
At the start of any discussion about how to reduce your risk of being sued for malpractice because of your work as an obstetrician, in particular during labor and delivery, two distinct, underlying avenues of concern need to be addressed. Before moving on to discuss strategy, then, let’s consider what they are and how they arise: Allegation (perception). You are at risk of an allegation of malpractice (or of a perception of malpractice) because of an unexpected event or outcome for mother or baby. Allegation and perception can arise apart from any specific clinical action you undertook, or did not undertake. An example? Counseling about options for care that falls short of full understanding by the patient.
Allegation and perception are the subjects of this first installment of our two-part article on strategies for avoiding claims of malpractice in L & D that begin with the first prenatal visit.
Causation. Your actions—what you do in the course of providing prenatal care and delivering a baby—put you at risk of a charge of malpractice when you have provided medical care that 1) is inconsistent with current medical practice and thus 2) harmed the mother or newborn.
For a medical malpractice case to go forward, it must meet a well-defined paradigm that teases apart components of causation, beginning with your duty to the patient (TABLE 1).
TABLE 1 Signposts in the medical malpractice paradigm
| When the clinical issue at hand is … | … Then the legal term is … |
|---|---|
| A health-care professional’s obligation to provide care | “Duty” |
| A deviation in the care that was provided | “Standard of care” |
| An allegation that a breach in the standard of care resulted in injury | “Proximate cause” |
| An assertion or finding that an injury is “compensable” | “Damages” |
| Source: Yale New Haven Medical Center, 1997.5 | |
Allegation of malpractice arises from a range of sources, as we’ll discuss, but it is causation that reflects the actual, hands-on practice of medicine. We’ll examine strategies for avoiding charges of causation in the second part of this article.
(For now, we’ll just note that a recent excellent review of intrapartum interventions and their basis in evidence1 offers a model for evaluating a number of widely utilized practices in obstetrics. The goal, of course, is to minimize bad outcomes that follow from causation. Regrettably, that evidence-based approach is a limited one, because of a paucity of adequately controlled studies about OB practice.)
CASE: Continued
You consider your patient’s comment that she would like to avoid a repeat cesarean delivery, and advise her that she may safely attempt vaginal birth.
When spontaneous labor does not occur in 6 hours, oxytocin is administered. She dilates to 9 cm and begins to push spontaneously.
The fetal heart rate then drops to 70/min; fetal station, which had been +2, is now -1. A Stat cesarean delivery is performed. Uterine rupture with partial fetal expulsion is found. Apgar scores are 1, 3, and 5 at 1, 5, and 10 minutes.
Your patient requires a hysterectomy to control bleeding.
Some broad considerations for the physician arising from this CASE
- The counseling that you provide to a patient should be nondirective; it should include your opinion, however, about the best option available to her. Insert yourself into this hypothetical case, for discussion’s sake: Did you provide that important opinion to her?
- You must make certain that she clearly understands the risks and benefits of a procedure or other action, and the available alternatives. Did you undertake a check of her comprehension, given the anxiety and confusion of the moment?
- When an adverse outcome ensues—however unlikely it was to occur—it is necessary for you to review the circumstances with the patient as soon as clinically possible. Did you “debrief” and counsel her before and after the hysterectomy?
No more “perfect outcomes”: Our role changed, so did our risk
From the moment an OB patient enters triage, until her arrival home with her infant, this crucial period of her life is colored by concern, curiosity, myth, and fear.
Every woman anticipates the birth of a healthy infant. In an earlier era, the patient and her family relied on the sage advice of their physician to ensure this outcome. To an extent, physicians themselves reinforced this reliance, embracing the notion that they were, in fact, able to provide such a perfect outcome.
With advances that have been made in reproductive medicine, pregnancy has become more readily available to women with increasingly advanced disease; this has made labor and delivery more challenging to them and to their physicians. Realistically, our role as physicians is now better expressed as providing advice to help a woman achieve the best possible outcome, recognizing her individual clinical circumstances, instead of ensuring a perfect outcome.
Every woman anticipates the birth of a healthy baby. But the role of the OB is better expressed as helping her achieve the best possible outcome, not a perfect outcome. ABOVE: Shoulder dystocia is one of the most treacherous and frightening—and litigated—complications of childbirth, yet it is, for the most part, unpredictable and unpreventable in the course of even routine delivery.
Key concept #1
COMMUNICATION
Communication is central to patients’ comprehension about the care that you provide to them. But to enter a genuine dialogue with a patient under your care, and with her family, can challenge your communication skills.
First, you need written and verbal skills. Second, you need to know how to read visual cues.
Third, the messages that you deliver to the patient are influenced by:
- your style of communication
- your cultural background
- the setting in which you’re providing care (office, hospital).
Where are such skills developed? For one, biopsychosocial models that are employed in medical student education and resident training aid the physician in developing appropriate communication skills.
But training alone cannot overcome the fact that communication is a double-sided activity: Patients bring many of their own variables to a dialogue. How patients understand and interact with you—and with other providers and the health-care system—is not, therefore, directly or strictly within your sphere of influence.
Yet your sensitivity to a patient’s issues can go a long way toward ameliorating her misconceptions and prejudices. Here are several suggestions, developed by others, to optimize patients’ understanding of their care2,3:
- Apply what’s known as flip default. Assume the patient does not understand the information that you’re providing. Ask her to repeat your instructions back to you (as is done with a verbal order in the hospital).
- Manage face-to-face time effectively. Don’t attempt to teach a patient everything about her care at once. Focus on the critical aspects of her case and on providing understanding; use a strategy of sequential learning.
- Reduce the “overwhelm” factor. Periodically, stop and ask the patient if she has questions. Don’t wait until the end of the appointment to do this.
- Eliminate jargon. When you notify a patient about the results of testing, for example, clarify what the results say about her health and mean for her care. Do so in plain language.
- Recognize her preconceptions. Discuss any psychosocial issues head on with the patient. Use an interpreter or a social worker, or counselors from other fields, as appropriate.
Remember: All health-care personnel need to understand the importance of making the patient comfortable in the often foreign, and sometimes sterile, milieu of the medical office and hospital.
Key concept #2
TRUST
Trust between patient and clinician is, we believe, the most basic necessity for ameliorating allegations of malpractice—secondary only, perhaps, to your knowledge of medicine.
Trust can be enhanced by interactions that demonstrate to both parties the advisability of working together to resolve a problem. Any aspect of the physician-patient interaction that is potentially adversarial does not serve the interests of either.
We encourage you to construct a communication bridge, so to speak, with your patient. Begin by:
- introducing yourself to her and explaining your role in her care
- making appropriate eye contact with her
- maintaining a positive attitude
- dressing appropriately
- making her feel that she is your No. 1 priority.
There is more.
Recognize the duality of respect
- Ask the patient how she wishes to be addressed
- Ask about her belief system
- Explain the specifics of her care without arrogance.
Engender trust
- Be honest with her
- Be on her side
- Take time with her
- Allow her the right that she has to select from the options or to refuse treatment
- Disclose to the patient your status as a student or resident, if that is your rank.
Recognize the benefits of partnership
Forging a partnership with the patient:
- improves the accuracy of information
- eases ongoing communication
- facilitates informed consent
- provides an opportunity for you to educate her.
TABLE 2 When building trust, both patient and physician
are charged with responsibilities
| In regard to … | The patient’s responsibility is to … | The physician’s responsibility is to … |
|---|---|---|
| Gathering an honest and complete medical history | Know and report | Question completely |
| Being adherent to prescribed care | Follow through | Make reasonable demands |
| Making decisions about care | Ask questions and actively participate in choices Make realistic requests | Be knowledgeable about available alternatives Individualize options |
Key concept #3
SHARED RESPONSIBILITY
Patient and physician both have responsibilities that are important to achieving an optimal outcome; so does the hospital (TABLE 2 and TABLE 3). Both patient and physician should practice full disclosure throughout the course of care; this will benefit both of you.4 Here are a few select examples.
TABLE 3 Relative degrees of responsibility for a good outcome
vary across interested parties, but none are exempt
| Area of emphasis | Hospital’s responsibility | Physician’s responsibility | Patient’s responsibility |
|---|---|---|---|
| Creating a positive environment for care | 3+ | 2+ | 1+ |
| Providing clear communication | 3+ | 3+ | 3+ |
| Obtaining informed consent | 3+ | 3+ | 3+ |
| Making reasonable requests | 1+ | 1+ | 3+ |
| Compliance | 3+ | 3+ | 3+ |
| Key to the relative scale: 1+: at the least, minimally responsible; 2+: at the least, somewhat responsible; 3+, responsible to the greatest degree. | |||
The importance of the intake form
At the outset of OB care, in most practices, the patient provides the initial detailed medical history by completing a form in the waiting room. In reviewing and completing this survey with her during the appointment, pay particular attention to those questions for which the response has been left blank.
Patients need to understand that key recommendations about their care, and a proper analysis of their concerns, are based on the information that they provide on this survey. In our practices, we find that patients answer most of these early questions without difficulty—even inquiries of a personal nature, such as the number of prior pregnancies, or drug, alcohol, and smoking habits—as long as they understand why it’s in their best interests for you to have this information. If they leave a question blank and you do not follow up verbally, you may have lost invaluable information that can affect the outcome of her pregnancy.
What should you do when, occasionally, a patient refuses to answer one of your questions? We recommend that you record her refusal on the form itself, where the note remains part of the record.
Keep in mind that all necessary and useful information about a patient may not be available, or may not be appropriate to consider, at the initial prenatal visit. In that case, you have an ongoing opportunity—at subsequent visits during the pregnancy—to develop her full medical profile and algorithm.
The necessity of adherence
It almost goes without saying: To provide the care that our patients need, we sometimes require the unpleasant of them—to undergo evaluations, or testing, or to take medications that may be inconvenient or costly.
After you explain the specific course of care to a patient—whether you’re ordering a test or writing a prescription—your follow-up must include notation in the record of adherence. The fact is that both of you share responsibility for having her understand the importance of adherence to your instructions and the consequences of limited adherence or nonadherence.
Recall one of the lessons from the case that introduced this article: For the patient to make an informed decision about her care, the clinician must have thorough knowledge of 1) the risks and benefits of whatever intervention is being proposed in the particular clinical scenario and 2) the available alternatives. It is key that you communicate your risk-benefit assessment accurately to the patient.
Follow-up
Sometimes, new medical problems arise during subsequent prenatal visits. Follow-up appointments also provide an opportunity for you to expand your attention to problems identified earlier. Regardless of what the patient reported about her history and current health at the initial prenatal visit, listen for her to bring new issues to light for resolution later in the pregnancy that will have an impact on L & D. Again, it goes without saying but needs to be said: The OB clinician needs to have whatever skills are necessary to 1) fully evaluate the progress of a pregnancy and 2) make recommendations for care in light of changes in the status of mother and fetus along the way.
TABLE 4 Examples of the cardinal rule of “Be specific”
when you document care
| Instead of noting … | … Use alternative wording |
|---|---|
| “Mild vaginal bleeding” | “Vaginal bleeding requiring two pads an hour” |
| “Gentle traction” | “The shoulders were rotated before assisting the patient’s expulsive efforts” |
| “Patient refuses…” [or “declines…”] | “Patient voiced the nature of the problem and the alternatives that i have explained to her” |
| “Expedited cesarean section” | “The time from decision to incision was 35 minutes” |
Basic principles of documentation
The medical record is the best witness to interactions between a physician and a patient. In the record, we’re required to write a “5-C” description of events—namely, one that is:
- correct
- comprehensive
- conscientious
- clear
- contemporaneous.
Avoid medical jargon in the record. Be careful not to use vague terminology or descriptions, such as “mild vaginal bleeding,” “gentle traction,” or “patient refuses and accepts the consequences.” Specificity is the key to accuracy with respect to documentation (TABLE 4).
Editor’s note: Part 2 of this article will appear in the January 2011 issue of OBG Management. The authors’ analysis of L & D malpractice claims moves to a discussion of causation—by way of 4 troubling cases.
You’ll find a rich, useful archive of expert analysis of your professional liability and malpractice risk, at www.obgmanagement.com
• 10 keys to defending (or, better, keeping clear of) a shoulder dystocia suit
Andrew K. Worek, Esq (March 2008)
• After a patient’s unexpected death, First Aid for the emotionally wounded
Ronald A. Chez, MD, and Wayne Fortin, MS (April 2010)
• Afraid of getting sued? A plaintiff attorney offers counsel (but no sympathy)
Janelle Yates, Senior Editor, with Lewis Laska, JD, PhD (October 2009)
• Can a change in practice patterns reduce the number of OB malpractice claims?
Jason K. Baxter, MD, MSCP, and Louis Weinstein, MD (April 2009)
• Strategies for breaking bad news to patients
Barry Bub, MD (September 2008)
• Stuff of nightmares: Criminal prosecution for malpractice
Gary Steinman, MD, PhD (August 2008)
• Deposition Dos and Don’ts: How to answer 8 tricky questions
James L. Knoll, IV, MD, and Phillip J. Resnick, MD (May 2008)
• Playing high-stakes poker: Do you fight—or settle—that malpractice lawsuit?
Jeffrey Segal, MD (April 2008)
We want to hear from you! Tell us what you think.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based labor and delivery management. Am J Obstet Gynecol. 2008;199(5):445-454.
2. Paasche-Orlow MK, Riekert KA, Bilderback A, et al. Tailored education may reduce health literacy disparities in asthma self-management. Am J Respir Crit Care Med. 2005;172:980-986.
3. Huvane K. Health literacy: reading is just the beginning. Focus on multicultural healthcare. 2007;3(4):16-19.
4. Giordano K. Legal Principles. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello L, Giordano K, eds. Operative Obstetrics. 2nd ed. New York: Cambridge University Press; 2008.
5. The Four Elements of Medical Malpractice Yale New Haven Medical Center: Issues in Risk Management. 1997.
CASE: Is TOLAC feasible?
Your patient is a 33-year-old gravida 3, para 2002, with a previous cesarean delivery who was admitted to labor and delivery with premature ruptured membranes at term. She is not contracting. Fetal status is reassuring.
Her obstetric history is of one normal, spontaneous delivery followed by one cesarean delivery, both occurring at term.
She wants to know if she can safely undergo a trial of labor, or if she must have a repeat cesarean delivery. How should you counsel her?
At the start of any discussion about how to reduce your risk of being sued for malpractice because of your work as an obstetrician, in particular during labor and delivery, two distinct, underlying avenues of concern need to be addressed. Before moving on to discuss strategy, then, let’s consider what they are and how they arise: Allegation (perception). You are at risk of an allegation of malpractice (or of a perception of malpractice) because of an unexpected event or outcome for mother or baby. Allegation and perception can arise apart from any specific clinical action you undertook, or did not undertake. An example? Counseling about options for care that falls short of full understanding by the patient.
Allegation and perception are the subjects of this first installment of our two-part article on strategies for avoiding claims of malpractice in L & D that begin with the first prenatal visit.
Causation. Your actions—what you do in the course of providing prenatal care and delivering a baby—put you at risk of a charge of malpractice when you have provided medical care that 1) is inconsistent with current medical practice and thus 2) harmed the mother or newborn.
For a medical malpractice case to go forward, it must meet a well-defined paradigm that teases apart components of causation, beginning with your duty to the patient (TABLE 1).
TABLE 1 Signposts in the medical malpractice paradigm
| When the clinical issue at hand is … | … Then the legal term is … |
|---|---|
| A health-care professional’s obligation to provide care | “Duty” |
| A deviation in the care that was provided | “Standard of care” |
| An allegation that a breach in the standard of care resulted in injury | “Proximate cause” |
| An assertion or finding that an injury is “compensable” | “Damages” |
| Source: Yale New Haven Medical Center, 1997.5 | |
Allegation of malpractice arises from a range of sources, as we’ll discuss, but it is causation that reflects the actual, hands-on practice of medicine. We’ll examine strategies for avoiding charges of causation in the second part of this article.
(For now, we’ll just note that a recent excellent review of intrapartum interventions and their basis in evidence1 offers a model for evaluating a number of widely utilized practices in obstetrics. The goal, of course, is to minimize bad outcomes that follow from causation. Regrettably, that evidence-based approach is a limited one, because of a paucity of adequately controlled studies about OB practice.)
CASE: Continued
You consider your patient’s comment that she would like to avoid a repeat cesarean delivery, and advise her that she may safely attempt vaginal birth.
When spontaneous labor does not occur in 6 hours, oxytocin is administered. She dilates to 9 cm and begins to push spontaneously.
The fetal heart rate then drops to 70/min; fetal station, which had been +2, is now -1. A Stat cesarean delivery is performed. Uterine rupture with partial fetal expulsion is found. Apgar scores are 1, 3, and 5 at 1, 5, and 10 minutes.
Your patient requires a hysterectomy to control bleeding.
Some broad considerations for the physician arising from this CASE
- The counseling that you provide to a patient should be nondirective; it should include your opinion, however, about the best option available to her. Insert yourself into this hypothetical case, for discussion’s sake: Did you provide that important opinion to her?
- You must make certain that she clearly understands the risks and benefits of a procedure or other action, and the available alternatives. Did you undertake a check of her comprehension, given the anxiety and confusion of the moment?
- When an adverse outcome ensues—however unlikely it was to occur—it is necessary for you to review the circumstances with the patient as soon as clinically possible. Did you “debrief” and counsel her before and after the hysterectomy?
No more “perfect outcomes”: Our role changed, so did our risk
From the moment an OB patient enters triage, until her arrival home with her infant, this crucial period of her life is colored by concern, curiosity, myth, and fear.
Every woman anticipates the birth of a healthy infant. In an earlier era, the patient and her family relied on the sage advice of their physician to ensure this outcome. To an extent, physicians themselves reinforced this reliance, embracing the notion that they were, in fact, able to provide such a perfect outcome.
With advances that have been made in reproductive medicine, pregnancy has become more readily available to women with increasingly advanced disease; this has made labor and delivery more challenging to them and to their physicians. Realistically, our role as physicians is now better expressed as providing advice to help a woman achieve the best possible outcome, recognizing her individual clinical circumstances, instead of ensuring a perfect outcome.

Every woman anticipates the birth of a healthy baby. But the role of the OB is better expressed as helping her achieve the best possible outcome, not a perfect outcome. ABOVE: Shoulder dystocia is one of the most treacherous and frightening—and litigated—complications of childbirth, yet it is, for the most part, unpredictable and unpreventable in the course of even routine delivery.
Key concept #1
COMMUNICATION
Communication is central to patients’ comprehension about the care that you provide to them. But to enter a genuine dialogue with a patient under your care, and with her family, can challenge your communication skills.
First, you need written and verbal skills. Second, you need to know how to read visual cues.
Third, the messages that you deliver to the patient are influenced by:
- your style of communication
- your cultural background
- the setting in which you’re providing care (office, hospital).
Where are such skills developed? For one, biopsychosocial models that are employed in medical student education and resident training aid the physician in developing appropriate communication skills.
But training alone cannot overcome the fact that communication is a double-sided activity: Patients bring many of their own variables to a dialogue. How patients understand and interact with you—and with other providers and the health-care system—is not, therefore, directly or strictly within your sphere of influence.
Yet your sensitivity to a patient’s issues can go a long way toward ameliorating her misconceptions and prejudices. Here are several suggestions, developed by others, to optimize patients’ understanding of their care2,3:
- Apply what’s known as flip default. Assume the patient does not understand the information that you’re providing. Ask her to repeat your instructions back to you (as is done with a verbal order in the hospital).
- Manage face-to-face time effectively. Don’t attempt to teach a patient everything about her care at once. Focus on the critical aspects of her case and on providing understanding; use a strategy of sequential learning.
- Reduce the “overwhelm” factor. Periodically, stop and ask the patient if she has questions. Don’t wait until the end of the appointment to do this.
- Eliminate jargon. When you notify a patient about the results of testing, for example, clarify what the results say about her health and mean for her care. Do so in plain language.
- Recognize her preconceptions. Discuss any psychosocial issues head on with the patient. Use an interpreter or a social worker, or counselors from other fields, as appropriate.
Remember: All health-care personnel need to understand the importance of making the patient comfortable in the often foreign, and sometimes sterile, milieu of the medical office and hospital.
Key concept #2
TRUST
Trust between patient and clinician is, we believe, the most basic necessity for ameliorating allegations of malpractice—secondary only, perhaps, to your knowledge of medicine.
Trust can be enhanced by interactions that demonstrate to both parties the advisability of working together to resolve a problem. Any aspect of the physician-patient interaction that is potentially adversarial does not serve the interests of either.
We encourage you to construct a communication bridge, so to speak, with your patient. Begin by:
- introducing yourself to her and explaining your role in her care
- making appropriate eye contact with her
- maintaining a positive attitude
- dressing appropriately
- making her feel that she is your No. 1 priority.
There is more.
Recognize the duality of respect
- Ask the patient how she wishes to be addressed
- Ask about her belief system
- Explain the specifics of her care without arrogance.
Engender trust
- Be honest with her
- Be on her side
- Take time with her
- Allow her the right that she has to select from the options or to refuse treatment
- Disclose to the patient your status as a student or resident, if that is your rank.
Recognize the benefits of partnership
Forging a partnership with the patient:
- improves the accuracy of information
- eases ongoing communication
- facilitates informed consent
- provides an opportunity for you to educate her.
TABLE 2 When building trust, both patient and physician
are charged with responsibilities
| In regard to … | The patient’s responsibility is to … | The physician’s responsibility is to … |
|---|---|---|
| Gathering an honest and complete medical history | Know and report | Question completely |
| Being adherent to prescribed care | Follow through | Make reasonable demands |
| Making decisions about care | Ask questions and actively participate in choices Make realistic requests | Be knowledgeable about available alternatives Individualize options |
Key concept #3
SHARED RESPONSIBILITY
Patient and physician both have responsibilities that are important to achieving an optimal outcome; so does the hospital (TABLE 2 and TABLE 3). Both patient and physician should practice full disclosure throughout the course of care; this will benefit both of you.4 Here are a few select examples.
TABLE 3 Relative degrees of responsibility for a good outcome
vary across interested parties, but none are exempt
| Area of emphasis | Hospital’s responsibility | Physician’s responsibility | Patient’s responsibility |
|---|---|---|---|
| Creating a positive environment for care | 3+ | 2+ | 1+ |
| Providing clear communication | 3+ | 3+ | 3+ |
| Obtaining informed consent | 3+ | 3+ | 3+ |
| Making reasonable requests | 1+ | 1+ | 3+ |
| Compliance | 3+ | 3+ | 3+ |
| Key to the relative scale: 1+: at the least, minimally responsible; 2+: at the least, somewhat responsible; 3+, responsible to the greatest degree. | |||
The importance of the intake form
At the outset of OB care, in most practices, the patient provides the initial detailed medical history by completing a form in the waiting room. In reviewing and completing this survey with her during the appointment, pay particular attention to those questions for which the response has been left blank.
Patients need to understand that key recommendations about their care, and a proper analysis of their concerns, are based on the information that they provide on this survey. In our practices, we find that patients answer most of these early questions without difficulty—even inquiries of a personal nature, such as the number of prior pregnancies, or drug, alcohol, and smoking habits—as long as they understand why it’s in their best interests for you to have this information. If they leave a question blank and you do not follow up verbally, you may have lost invaluable information that can affect the outcome of her pregnancy.
What should you do when, occasionally, a patient refuses to answer one of your questions? We recommend that you record her refusal on the form itself, where the note remains part of the record.
Keep in mind that all necessary and useful information about a patient may not be available, or may not be appropriate to consider, at the initial prenatal visit. In that case, you have an ongoing opportunity—at subsequent visits during the pregnancy—to develop her full medical profile and algorithm.
The necessity of adherence
It almost goes without saying: To provide the care that our patients need, we sometimes require the unpleasant of them—to undergo evaluations, or testing, or to take medications that may be inconvenient or costly.
After you explain the specific course of care to a patient—whether you’re ordering a test or writing a prescription—your follow-up must include notation in the record of adherence. The fact is that both of you share responsibility for having her understand the importance of adherence to your instructions and the consequences of limited adherence or nonadherence.
Recall one of the lessons from the case that introduced this article: For the patient to make an informed decision about her care, the clinician must have thorough knowledge of 1) the risks and benefits of whatever intervention is being proposed in the particular clinical scenario and 2) the available alternatives. It is key that you communicate your risk-benefit assessment accurately to the patient.
Follow-up
Sometimes, new medical problems arise during subsequent prenatal visits. Follow-up appointments also provide an opportunity for you to expand your attention to problems identified earlier. Regardless of what the patient reported about her history and current health at the initial prenatal visit, listen for her to bring new issues to light for resolution later in the pregnancy that will have an impact on L & D. Again, it goes without saying but needs to be said: The OB clinician needs to have whatever skills are necessary to 1) fully evaluate the progress of a pregnancy and 2) make recommendations for care in light of changes in the status of mother and fetus along the way.
TABLE 4 Examples of the cardinal rule of “Be specific”
when you document care
| Instead of noting … | … Use alternative wording |
|---|---|
| “Mild vaginal bleeding” | “Vaginal bleeding requiring two pads an hour” |
| “Gentle traction” | “The shoulders were rotated before assisting the patient’s expulsive efforts” |
| “Patient refuses…” [or “declines…”] | “Patient voiced the nature of the problem and the alternatives that i have explained to her” |
| “Expedited cesarean section” | “The time from decision to incision was 35 minutes” |
Basic principles of documentation
The medical record is the best witness to interactions between a physician and a patient. In the record, we’re required to write a “5-C” description of events—namely, one that is:
- correct
- comprehensive
- conscientious
- clear
- contemporaneous.
Avoid medical jargon in the record. Be careful not to use vague terminology or descriptions, such as “mild vaginal bleeding,” “gentle traction,” or “patient refuses and accepts the consequences.” Specificity is the key to accuracy with respect to documentation (TABLE 4).
Editor’s note: Part 2 of this article will appear in the January 2011 issue of OBG Management. The authors’ analysis of L & D malpractice claims moves to a discussion of causation—by way of 4 troubling cases.
You’ll find a rich, useful archive of expert analysis of your professional liability and malpractice risk, at www.obgmanagement.com
• 10 keys to defending (or, better, keeping clear of) a shoulder dystocia suit
Andrew K. Worek, Esq (March 2008)
• After a patient’s unexpected death, First Aid for the emotionally wounded
Ronald A. Chez, MD, and Wayne Fortin, MS (April 2010)
• Afraid of getting sued? A plaintiff attorney offers counsel (but no sympathy)
Janelle Yates, Senior Editor, with Lewis Laska, JD, PhD (October 2009)
• Can a change in practice patterns reduce the number of OB malpractice claims?
Jason K. Baxter, MD, MSCP, and Louis Weinstein, MD (April 2009)
• Strategies for breaking bad news to patients
Barry Bub, MD (September 2008)
• Stuff of nightmares: Criminal prosecution for malpractice
Gary Steinman, MD, PhD (August 2008)
• Deposition Dos and Don’ts: How to answer 8 tricky questions
James L. Knoll, IV, MD, and Phillip J. Resnick, MD (May 2008)
• Playing high-stakes poker: Do you fight—or settle—that malpractice lawsuit?
Jeffrey Segal, MD (April 2008)
We want to hear from you! Tell us what you think.
CASE: Is TOLAC feasible?
Your patient is a 33-year-old gravida 3, para 2002, with a previous cesarean delivery who was admitted to labor and delivery with premature ruptured membranes at term. She is not contracting. Fetal status is reassuring.
Her obstetric history is of one normal, spontaneous delivery followed by one cesarean delivery, both occurring at term.
She wants to know if she can safely undergo a trial of labor, or if she must have a repeat cesarean delivery. How should you counsel her?
At the start of any discussion about how to reduce your risk of being sued for malpractice because of your work as an obstetrician, in particular during labor and delivery, two distinct, underlying avenues of concern need to be addressed. Before moving on to discuss strategy, then, let’s consider what they are and how they arise: Allegation (perception). You are at risk of an allegation of malpractice (or of a perception of malpractice) because of an unexpected event or outcome for mother or baby. Allegation and perception can arise apart from any specific clinical action you undertook, or did not undertake. An example? Counseling about options for care that falls short of full understanding by the patient.
Allegation and perception are the subjects of this first installment of our two-part article on strategies for avoiding claims of malpractice in L & D that begin with the first prenatal visit.
Causation. Your actions—what you do in the course of providing prenatal care and delivering a baby—put you at risk of a charge of malpractice when you have provided medical care that 1) is inconsistent with current medical practice and thus 2) harmed the mother or newborn.
For a medical malpractice case to go forward, it must meet a well-defined paradigm that teases apart components of causation, beginning with your duty to the patient (TABLE 1).
TABLE 1 Signposts in the medical malpractice paradigm
| When the clinical issue at hand is … | … Then the legal term is … |
|---|---|
| A health-care professional’s obligation to provide care | “Duty” |
| A deviation in the care that was provided | “Standard of care” |
| An allegation that a breach in the standard of care resulted in injury | “Proximate cause” |
| An assertion or finding that an injury is “compensable” | “Damages” |
| Source: Yale New Haven Medical Center, 1997.5 | |
Allegation of malpractice arises from a range of sources, as we’ll discuss, but it is causation that reflects the actual, hands-on practice of medicine. We’ll examine strategies for avoiding charges of causation in the second part of this article.
(For now, we’ll just note that a recent excellent review of intrapartum interventions and their basis in evidence1 offers a model for evaluating a number of widely utilized practices in obstetrics. The goal, of course, is to minimize bad outcomes that follow from causation. Regrettably, that evidence-based approach is a limited one, because of a paucity of adequately controlled studies about OB practice.)
CASE: Continued
You consider your patient’s comment that she would like to avoid a repeat cesarean delivery, and advise her that she may safely attempt vaginal birth.
When spontaneous labor does not occur in 6 hours, oxytocin is administered. She dilates to 9 cm and begins to push spontaneously.
The fetal heart rate then drops to 70/min; fetal station, which had been +2, is now -1. A Stat cesarean delivery is performed. Uterine rupture with partial fetal expulsion is found. Apgar scores are 1, 3, and 5 at 1, 5, and 10 minutes.
Your patient requires a hysterectomy to control bleeding.
Some broad considerations for the physician arising from this CASE
- The counseling that you provide to a patient should be nondirective; it should include your opinion, however, about the best option available to her. Insert yourself into this hypothetical case, for discussion’s sake: Did you provide that important opinion to her?
- You must make certain that she clearly understands the risks and benefits of a procedure or other action, and the available alternatives. Did you undertake a check of her comprehension, given the anxiety and confusion of the moment?
- When an adverse outcome ensues—however unlikely it was to occur—it is necessary for you to review the circumstances with the patient as soon as clinically possible. Did you “debrief” and counsel her before and after the hysterectomy?
No more “perfect outcomes”: Our role changed, so did our risk
From the moment an OB patient enters triage, until her arrival home with her infant, this crucial period of her life is colored by concern, curiosity, myth, and fear.
Every woman anticipates the birth of a healthy infant. In an earlier era, the patient and her family relied on the sage advice of their physician to ensure this outcome. To an extent, physicians themselves reinforced this reliance, embracing the notion that they were, in fact, able to provide such a perfect outcome.
With advances that have been made in reproductive medicine, pregnancy has become more readily available to women with increasingly advanced disease; this has made labor and delivery more challenging to them and to their physicians. Realistically, our role as physicians is now better expressed as providing advice to help a woman achieve the best possible outcome, recognizing her individual clinical circumstances, instead of ensuring a perfect outcome.

Every woman anticipates the birth of a healthy baby. But the role of the OB is better expressed as helping her achieve the best possible outcome, not a perfect outcome. ABOVE: Shoulder dystocia is one of the most treacherous and frightening—and litigated—complications of childbirth, yet it is, for the most part, unpredictable and unpreventable in the course of even routine delivery.
Key concept #1
COMMUNICATION
Communication is central to patients’ comprehension about the care that you provide to them. But to enter a genuine dialogue with a patient under your care, and with her family, can challenge your communication skills.
First, you need written and verbal skills. Second, you need to know how to read visual cues.
Third, the messages that you deliver to the patient are influenced by:
- your style of communication
- your cultural background
- the setting in which you’re providing care (office, hospital).
Where are such skills developed? For one, biopsychosocial models that are employed in medical student education and resident training aid the physician in developing appropriate communication skills.
But training alone cannot overcome the fact that communication is a double-sided activity: Patients bring many of their own variables to a dialogue. How patients understand and interact with you—and with other providers and the health-care system—is not, therefore, directly or strictly within your sphere of influence.
Yet your sensitivity to a patient’s issues can go a long way toward ameliorating her misconceptions and prejudices. Here are several suggestions, developed by others, to optimize patients’ understanding of their care2,3:
- Apply what’s known as flip default. Assume the patient does not understand the information that you’re providing. Ask her to repeat your instructions back to you (as is done with a verbal order in the hospital).
- Manage face-to-face time effectively. Don’t attempt to teach a patient everything about her care at once. Focus on the critical aspects of her case and on providing understanding; use a strategy of sequential learning.
- Reduce the “overwhelm” factor. Periodically, stop and ask the patient if she has questions. Don’t wait until the end of the appointment to do this.
- Eliminate jargon. When you notify a patient about the results of testing, for example, clarify what the results say about her health and mean for her care. Do so in plain language.
- Recognize her preconceptions. Discuss any psychosocial issues head on with the patient. Use an interpreter or a social worker, or counselors from other fields, as appropriate.
Remember: All health-care personnel need to understand the importance of making the patient comfortable in the often foreign, and sometimes sterile, milieu of the medical office and hospital.
Key concept #2
TRUST
Trust between patient and clinician is, we believe, the most basic necessity for ameliorating allegations of malpractice—secondary only, perhaps, to your knowledge of medicine.
Trust can be enhanced by interactions that demonstrate to both parties the advisability of working together to resolve a problem. Any aspect of the physician-patient interaction that is potentially adversarial does not serve the interests of either.
We encourage you to construct a communication bridge, so to speak, with your patient. Begin by:
- introducing yourself to her and explaining your role in her care
- making appropriate eye contact with her
- maintaining a positive attitude
- dressing appropriately
- making her feel that she is your No. 1 priority.
There is more.
Recognize the duality of respect
- Ask the patient how she wishes to be addressed
- Ask about her belief system
- Explain the specifics of her care without arrogance.
Engender trust
- Be honest with her
- Be on her side
- Take time with her
- Allow her the right that she has to select from the options or to refuse treatment
- Disclose to the patient your status as a student or resident, if that is your rank.
Recognize the benefits of partnership
Forging a partnership with the patient:
- improves the accuracy of information
- eases ongoing communication
- facilitates informed consent
- provides an opportunity for you to educate her.
TABLE 2 When building trust, both patient and physician
are charged with responsibilities
| In regard to … | The patient’s responsibility is to … | The physician’s responsibility is to … |
|---|---|---|
| Gathering an honest and complete medical history | Know and report | Question completely |
| Being adherent to prescribed care | Follow through | Make reasonable demands |
| Making decisions about care | Ask questions and actively participate in choices Make realistic requests | Be knowledgeable about available alternatives Individualize options |
Key concept #3
SHARED RESPONSIBILITY
Patient and physician both have responsibilities that are important to achieving an optimal outcome; so does the hospital (TABLE 2 and TABLE 3). Both patient and physician should practice full disclosure throughout the course of care; this will benefit both of you.4 Here are a few select examples.
TABLE 3 Relative degrees of responsibility for a good outcome
vary across interested parties, but none are exempt
| Area of emphasis | Hospital’s responsibility | Physician’s responsibility | Patient’s responsibility |
|---|---|---|---|
| Creating a positive environment for care | 3+ | 2+ | 1+ |
| Providing clear communication | 3+ | 3+ | 3+ |
| Obtaining informed consent | 3+ | 3+ | 3+ |
| Making reasonable requests | 1+ | 1+ | 3+ |
| Compliance | 3+ | 3+ | 3+ |
| Key to the relative scale: 1+: at the least, minimally responsible; 2+: at the least, somewhat responsible; 3+, responsible to the greatest degree. | |||
The importance of the intake form
At the outset of OB care, in most practices, the patient provides the initial detailed medical history by completing a form in the waiting room. In reviewing and completing this survey with her during the appointment, pay particular attention to those questions for which the response has been left blank.
Patients need to understand that key recommendations about their care, and a proper analysis of their concerns, are based on the information that they provide on this survey. In our practices, we find that patients answer most of these early questions without difficulty—even inquiries of a personal nature, such as the number of prior pregnancies, or drug, alcohol, and smoking habits—as long as they understand why it’s in their best interests for you to have this information. If they leave a question blank and you do not follow up verbally, you may have lost invaluable information that can affect the outcome of her pregnancy.
What should you do when, occasionally, a patient refuses to answer one of your questions? We recommend that you record her refusal on the form itself, where the note remains part of the record.
Keep in mind that all necessary and useful information about a patient may not be available, or may not be appropriate to consider, at the initial prenatal visit. In that case, you have an ongoing opportunity—at subsequent visits during the pregnancy—to develop her full medical profile and algorithm.
The necessity of adherence
It almost goes without saying: To provide the care that our patients need, we sometimes require the unpleasant of them—to undergo evaluations, or testing, or to take medications that may be inconvenient or costly.
After you explain the specific course of care to a patient—whether you’re ordering a test or writing a prescription—your follow-up must include notation in the record of adherence. The fact is that both of you share responsibility for having her understand the importance of adherence to your instructions and the consequences of limited adherence or nonadherence.
Recall one of the lessons from the case that introduced this article: For the patient to make an informed decision about her care, the clinician must have thorough knowledge of 1) the risks and benefits of whatever intervention is being proposed in the particular clinical scenario and 2) the available alternatives. It is key that you communicate your risk-benefit assessment accurately to the patient.
Follow-up
Sometimes, new medical problems arise during subsequent prenatal visits. Follow-up appointments also provide an opportunity for you to expand your attention to problems identified earlier. Regardless of what the patient reported about her history and current health at the initial prenatal visit, listen for her to bring new issues to light for resolution later in the pregnancy that will have an impact on L & D. Again, it goes without saying but needs to be said: The OB clinician needs to have whatever skills are necessary to 1) fully evaluate the progress of a pregnancy and 2) make recommendations for care in light of changes in the status of mother and fetus along the way.
TABLE 4 Examples of the cardinal rule of “Be specific”
when you document care
| Instead of noting … | … Use alternative wording |
|---|---|
| “Mild vaginal bleeding” | “Vaginal bleeding requiring two pads an hour” |
| “Gentle traction” | “The shoulders were rotated before assisting the patient’s expulsive efforts” |
| “Patient refuses…” [or “declines…”] | “Patient voiced the nature of the problem and the alternatives that i have explained to her” |
| “Expedited cesarean section” | “The time from decision to incision was 35 minutes” |
Basic principles of documentation
The medical record is the best witness to interactions between a physician and a patient. In the record, we’re required to write a “5-C” description of events—namely, one that is:
- correct
- comprehensive
- conscientious
- clear
- contemporaneous.
Avoid medical jargon in the record. Be careful not to use vague terminology or descriptions, such as “mild vaginal bleeding,” “gentle traction,” or “patient refuses and accepts the consequences.” Specificity is the key to accuracy with respect to documentation (TABLE 4).
Editor’s note: Part 2 of this article will appear in the January 2011 issue of OBG Management. The authors’ analysis of L & D malpractice claims moves to a discussion of causation—by way of 4 troubling cases.
You’ll find a rich, useful archive of expert analysis of your professional liability and malpractice risk, at www.obgmanagement.com
• 10 keys to defending (or, better, keeping clear of) a shoulder dystocia suit
Andrew K. Worek, Esq (March 2008)
• After a patient’s unexpected death, First Aid for the emotionally wounded
Ronald A. Chez, MD, and Wayne Fortin, MS (April 2010)
• Afraid of getting sued? A plaintiff attorney offers counsel (but no sympathy)
Janelle Yates, Senior Editor, with Lewis Laska, JD, PhD (October 2009)
• Can a change in practice patterns reduce the number of OB malpractice claims?
Jason K. Baxter, MD, MSCP, and Louis Weinstein, MD (April 2009)
• Strategies for breaking bad news to patients
Barry Bub, MD (September 2008)
• Stuff of nightmares: Criminal prosecution for malpractice
Gary Steinman, MD, PhD (August 2008)
• Deposition Dos and Don’ts: How to answer 8 tricky questions
James L. Knoll, IV, MD, and Phillip J. Resnick, MD (May 2008)
• Playing high-stakes poker: Do you fight—or settle—that malpractice lawsuit?
Jeffrey Segal, MD (April 2008)
We want to hear from you! Tell us what you think.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based labor and delivery management. Am J Obstet Gynecol. 2008;199(5):445-454.
2. Paasche-Orlow MK, Riekert KA, Bilderback A, et al. Tailored education may reduce health literacy disparities in asthma self-management. Am J Respir Crit Care Med. 2005;172:980-986.
3. Huvane K. Health literacy: reading is just the beginning. Focus on multicultural healthcare. 2007;3(4):16-19.
4. Giordano K. Legal Principles. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello L, Giordano K, eds. Operative Obstetrics. 2nd ed. New York: Cambridge University Press; 2008.
5. The Four Elements of Medical Malpractice Yale New Haven Medical Center: Issues in Risk Management. 1997.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based labor and delivery management. Am J Obstet Gynecol. 2008;199(5):445-454.
2. Paasche-Orlow MK, Riekert KA, Bilderback A, et al. Tailored education may reduce health literacy disparities in asthma self-management. Am J Respir Crit Care Med. 2005;172:980-986.
3. Huvane K. Health literacy: reading is just the beginning. Focus on multicultural healthcare. 2007;3(4):16-19.
4. Giordano K. Legal Principles. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello L, Giordano K, eds. Operative Obstetrics. 2nd ed. New York: Cambridge University Press; 2008.
5. The Four Elements of Medical Malpractice Yale New Haven Medical Center: Issues in Risk Management. 1997.
Skilled US imaging of the adnexae: The fallopian tubes
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).
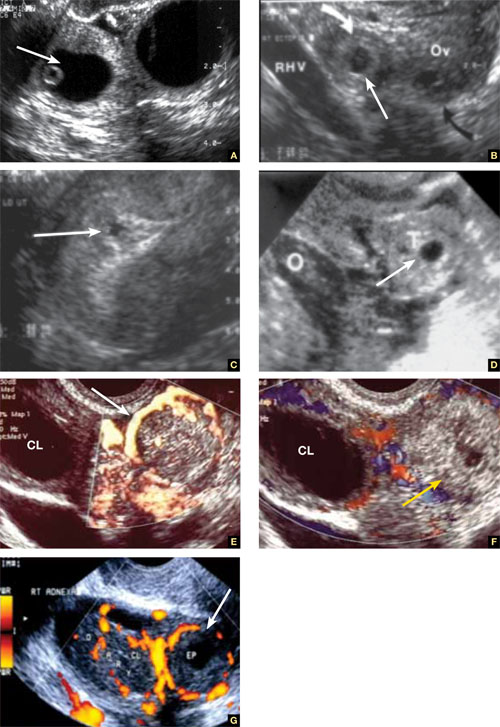
FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).
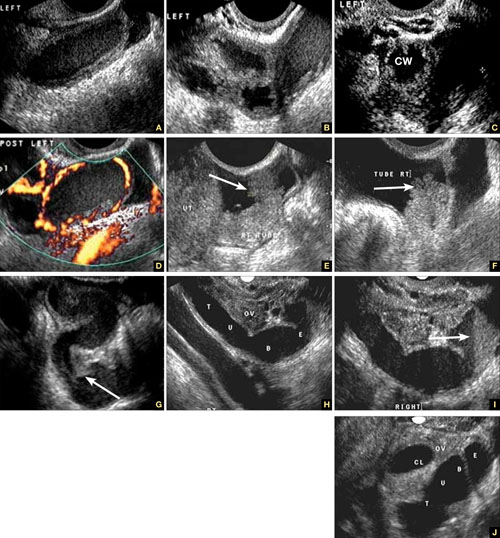
FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.
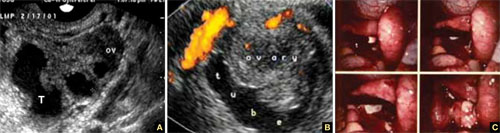
FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).
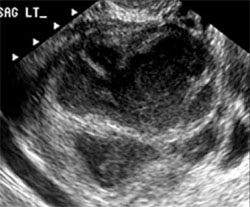
FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).
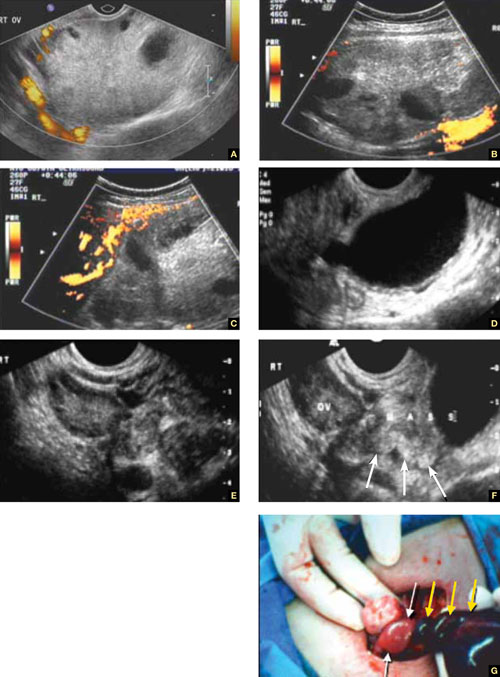
FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.
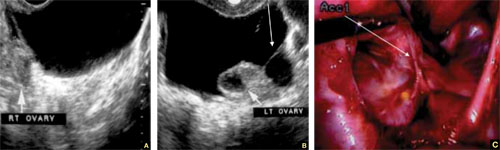
FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.
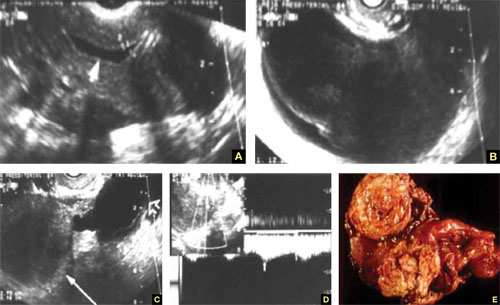
FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).

FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).

FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.

FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).

FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).

FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.

FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.

FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).

FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).

FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.

FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).

FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).

FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.

FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.

FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.
Quality-of-Life Consequences of Obesity
Monitoring Lipids in Patients Treated With Ziprasidone or Aripiprazole
Supraclavicular Lymphadenopathy Secondary to Smallpox Vaccination
Is Gulf War Illness "Real"? The Jury Is Still Out
Understanding Lactose Intolerance
Lactose intolerance (LI), a term closely associated with hypolactasia (lactase deficiency) and lactose malabsorption,1 is a common syndrome composed of diarrhea, abdominal pain, flatulence and/or bloating, and sometimes nausea and vomiting in severe cases, after ingestion of dairy products.2 This common disorder results from a deficiency in the enzyme lactase, which makes affected patients unable to digest lactose, a sugar found in milk and other dairy products.3 Malabsorption of lactose produces the symptoms associated with LI.
The level of LI varies among affected individuals, depending on many nutritional and genetic factors, the amount of lactose consumed, the patient’s degree of lactase deficiency, and the substance in which the lactose is ingested.4
Epidemiology
LI prevalence is difficult to ascertain because the symptoms are vague and can be attributed to a number of conditions; additionally, there is no gold standard for diagnosis of LI. It is estimated that about 70% of the world population is affected by LI—with great variation among ethnicities and races.5,6 Some degree of LI is reported in up to 80% of African-Americans and Latinos, and almost 100% of Native Americans and Asian Americans. LI is least common in people of northern European descent (and is unlikely to develop before adulthood7), although it has been suggested that 30 million American adults experienced lactose malabsorption to some degree by age 20.3,8 Heyman4 estimates that approximately 2% of people of northern European descent have LI.
Regardless of ethnicity or race, older patients are more susceptible to LI than are younger adults, largely as a result of the normal processes of aging.2
Pathophysiology and Patient Presentation
Cells of the inner lumen of the small intestines, enterocytes, are covered with a membrane that has a brush border composed of microvilli.9 The microvilli produce lactase, the enzyme necessary to split and hydrolyze dietary lactose into glucose and galactose for transport across the cell membrane.6 Unfortunately, lactase is produced in the upper, most shallow section of the villi, which is exceedingly prone to damage by secondary insult.
If the lactase enzymes are absent or deficient, unabsorbed sugars osmotically attract fluid into the bowel lumen. The amount of fluid influx into the bowel is approximately triple the normal amount, based on the osmolality of sugar alone. In addition, the unabsorbed lactose entering the colon is fermented by bacteria, producing gas and resulting in the cleavage of lactose into monosaccharides. Monosaccharides cannot be absorbed by the colonic mucosa; as a result, osmotic pressure increases, and fluid levels rise in the bowel. This process explains the most common symptoms of flatulence, diarrhea, abdominal pain, and bloating.6
Most mammalian babies, including human infants, produce enough lactase to digest milk, including breast milk. This ability persists until the child is weaned. In humans, lactase activity drops at age 2 to 3 years and may cease altogether by age 5 to 10.9 Worldwide, most humans lose 90% to 95% of birth lactase levels by early childhood, followed by a continuing decline during the course of a lifetime.6 This may help explain why many elderly people are affected by LI.
Typically, development of LI progresses subtly over many years, but onset can also be relatively acute.4
Lactose Malabsorption
The three main types of lactose malabsorption are primary, secondary, and congenital. The latter is a rare, genetic form of LI in which the lactase enzyme is entirely absent; for the purposes of this article, congenital lactose malabsorption will not be discussed.
Primary lactase deficiency is the most common form and the focus of this article. It is the normal, gradual reduction in lactase enzyme that a maturing individual experiences through adulthood, and the rate of reduction is genetically determined. Secondary lactose malabsorption occurs following an insult to the small bowel, as in severe diarrhea, infection (eg, rotavirus), chemotherapy, or acute gastroenteritis.4 In these situations, lactase is the first enzyme to be negatively affected and the last to return as the insult resolves.10 Secondary hypolactasia is transient and reversible.11,12
LI is not to be confused with cow’s milk allergy—an immune response to the protein in cow’s milk, which can be a life-threatening event. A true milk allergy most commonly appears within the first year of life, whereas LI occurs more often in adulthood.5,8
LI is not considered life threatening, but its symptoms can severely affect a person’s quality of life and productivity. In addition to ethnicity and age, the type and amount of lactose ingested and the amount that the patient is unable to digest all affect the severity of LI symptoms.13
Lactose makes up between 2% and 8% of the solids in milk; 1 mL of milk (0.03 fl oz) contains 47.2 mg of lactose. No amount of lactose has been specified to produce symptoms, but most adults can tolerate as much as 8 fl oz of milk without problems,1 and patients can tolerate more lactose if the food containing it is consumed with a meal.11 Some adults may be able to ingest only 2 to 4 fl oz before symptoms appear4; in highly sensitive adults, as little as 200 mg of lactose (0.13 fl oz of milk) can produce symptoms.5
Also playing a role are the patient’s gastric emptying time and intestinal transit time.12 Symptoms of LI can be produced between 30 minutes and two hours after ingestion of milk or a milk product.9
Diagnosis
Most patients do not require specialized, sophisticated testing for a diagnosis of LI. A thorough medical history and physical examination are needed to rule out other conditions in the differential diagnosis (see Table 16,14). For the primary care provider, a basic workup should include a complete blood count, a comprehensive metabolic panel, erythrocyte sedimentation rate, a thyroid-stimulating hormone level, a stool culture, and if symptoms are severe, abdominal/pelvic radiography and CT.
In the absence of accepted guidelines, a common therapeutic approach is to exclude milk and dairy products from the patient’s diet.11 Generally, a two-week trial of a strict lactose-free diet leading to resolution of symptoms, followed by reintroduction of dairy foods and recurrence of symptoms, can be considered diagnostic.4
It is important to instruct the patient that while he or she follows this diagnostic diet, all sources of lactose must be eliminated; food labels must be read carefully to identify “hidden” lactose sources (see Table 28). Additionally, many patients (and even clinicians) may not realize that many commonly used prescription and OTC medications contain lactose, including certain agents indicated for gastrointestinal problems5 (see Table 35).
During the diagnostic diet, patients may find it helpful to keep a diary of food choices and note any symptoms that may occur. This helps empower them to be an active participant in food choices, using self-experimentation to identify which foods they can and cannot tolerate.
Gastroenterology Consult
Referral to a gastroenterologist is needed if the diagnosis is unclear or if other illnesses are suspected. Tests the specialist may perform include the hydrogen breath test, a small-intestine biopsy, the lactose tolerance test, and/or the stool acidity test for infants and children,4 although these tests vary in sensitivity and specificity.13
The hydrogen breath test, by which enzymatic activity is confirmed after the patient consumes 25 to 50 g of lactose,6 is the most widely used formal test for confirming a diagnosis of LI because it is relatively inexpensive and is the most sensitive and the most specific for LI, according to Hovde and Farup13 and Eadala et al.5 The test has been shown to yield positive results in 90% of patients with lactose malabsorption.6,15 False-negative results may signify absence of bacterial flora, as in the case of recent antibiotic use or a recent high-colonic enema. Previous aspirin use, sleep, exercise, and smoking may increase breath hydrogen secretion unrelated to lactose consumption.6
Management of Lactose Intolerance
Although the body’s ability (or inability) to produce lactase cannot be changed, the symptoms of LI can be managed with dietary restrictions. The extent of change needed depends on how much lactose the patient is able to consume before experiencing symptoms.8
In patients with secondary LI, a complete lactose-free diet is recommended until the causative pathologic condition has resolved. Patients with primary LI can opt to exclude all milk and dairy products, at least initially, until symptoms have resolved; they can then reintroduce certain milk and dairy products gradually and in small amounts, according to their individual tolerance threshold. Certain lactose-containing foods may be easier to digest than others (see Table 42).
Ingesting lactose-containing foods with a meal helps decrease gastric transit time and can lessen the symptoms of LI.11 Additionally, people who cannot drink milk may find they can eat yogurt because it contains lactase-producing bacteria,9 although clinical trials examining consumption of yogurt or probiotics in patients with LI had inconclusive results.1
Lactose-free milk or soy milk is available at most major grocery stores. These products tend to be more expensive and taste somewhat sweeter than regular milk but can be used as a reasonable substitute.9
Some patients may benefit from taking lactase enzyme supplements,1,16 which are taken with any ingestion of lactose. The enzymes may not completely prevent symptoms because the lactose is not completely digested or because it is difficult to determine an effective dose of the enzyme. Therefore, enzyme supplementation should be an adjunct to, not a substitute for, dietary restrictions.6 This may help patients when they eat at restaurants, where they do not know how food is prepared and which are unlikely to offer lactose-free food selections.
Instead of taking lactase enzyme supplements in tablet form, patients may prefer to mix lactase liquid with regular milk, producing lactose-free milk. A waiting period of 24 hours is needed before the mixture can be considered lactose-free. A trial-and-error period should be expected when enzyme supplementation or any dietary approach is tried.11
The theory of adaptative phenomena suggests that most people with LI can teach themselves to ingest more lactose gradually, leading over time to beneficial changes in the microflora of the gut and in improved colonic function.11,17 The ultimate result, whether the explanation is reduced hydrogen excretion or increased gas absorption, is less severe gastrointestinal symptoms. This strategy is not a cure for LI, nor has it been found effective for all patients with LI,1 but it can help manage symptoms to some extent.
Information for the Patient
Patients often need instruction in reading food labels to identify foods that contain milk, milk products, lactose, whey, curds, milk byproducts, dry milk solids, or even nonfat dry milk powder.8 Follow-up with the primary care provider should be arranged on an as-needed basis.
Simply excluding all dairy products from the diet does raise some health concerns. Milk and other dairy products are important sources of calcium and vitamin D, which are needed for growth and bone health in patients of all ages. A decrease in calcium consumption is one of the primary risk factors for osteoporosis, although research examining a possible association between LI and osteoporosis has yielded conflicting results. According to Kudlacek et al,18 even individuals with severe LI do not appear to be at risk for accelerated bone loss. By contrast, other research groups7,19 studied patients with LI from various age-groups and concluded that low calcium intake and impaired vitamin D status could lead to increased bone turnover and decreased bone mass, especially in men and postmenopausal women. No guidelines have been published regarding screening for osteoporosis in patients with LI.
According to a consensus statement from the NIH,3 both men and women younger than 50 should consume 1,000 mg/d of calcium, and older persons, 1,500 mg/d. In addition to calcium supplements, patients can obtain the necessary calcium through certain foods, including leafy green vegetables (spinach, kale, broccoli), sardines, calcium-fortified cereal bars, calcium-enriched soy or lactose-free milk and other soy products, fruit juices, dried beans, and tuna.
Calcium is absorbed only when enough vitamin D is present; vitamin D intake should be 400 to 600 IU/d for both women and men.3 Foods that contain vitamin D include eggs, liver, vitamin D–enriched soy or lactose-free milk, and vitamin D–fortified cereals and other processed foods. Regular exposure to sunlight helps the body synthesize vitamin D naturally.3
For optimal bone health, the NIH3 continues to recommend a combination of cardiovascular exercise, weight-bearing exercise, smoking cessation, and a well-balanced diet (including foods that are rich in calcium and vitamin D).
In addition to its role in bone health, calcium has been suggested to improve cardiac and vascular smooth muscle contractility,20 and clinical research is under way to investigate the role of calcium in reducing the risk for adenomatous colon polyps.21
Conclusion
Primary LI is a common disorder resulting from a deficiency in the enzyme lactase, making affected patients unable to digest lactose. LI is widespread in varying degrees across all races and ethnicities, affecting people of all ages; however, it is more common among older adults due to natural pathophysiologic processes.
In LI-affected patients, consuming lactose leads to troublesome symptoms of diarrhea, abdominal pain, flatulence, and/or bloating, and sometimes nausea and vomiting.
No tool is considered a “gold standard” for making a diagnosis of LI, so it is important to rule out other gastrointestinal conditions first. Oftentimes a diagnosis of LI is confirmed by the effectiveness of a lactose-free trial diet. When diagnosis is uncertain, referral to a gastroenterologist is required.
Without formal treatment guidelines, the primary form of therapy for LI is to adjust the amount of ingested lactose, with careful attention to adequate calcium and vitamin D intake. Patient education is crucial for management of LI and improvement in the patient’s quality of life.
1. Shaukat A, Levitt MD, Taylor BC, et al. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med. 2010;152(12):797-803.
2. Lomer MC, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice—myths and realities. Aliment Pharmacol Ther. 2008;27(2):93-103.
3. NIH Consensus Development Conference. Lactose intolerance and health: final statement. February 22–24, 2010; Bethesda, MD.
4. Heyman MB; American Academy of Pediatrics Committee on Nutrition. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118(3):1279-1286.
5. Eadala P, Waud JP, Matthews SB, et al. Quantifying the ‘hidden’ lactose in drugs used for the treatment of gastrointestinal conditions. Aliment Pharmacol Ther. 2009;29(6):677-687.
6. Swagerty DL Jr, Walling AD, Klein RM. Lactose intolerance. Am Fam Physician. 2002;65(9): 1845-1850.
7. Wilt TJ, Shaukat A, Shamliyan T, et al. Lactose intolerance and health. Evid Rep Technol Assess (Full Rep). 2010 Feb(192):1-410.
8. National Digestive Diseases Information Clearinghouse, National Institute of Diabetes and Digestive and Kidney Diseases, NIH. Lactose intolerance (2009). http://digestive.niddk.nih.gov/ddiseases/pubs/lactoseintolerance. Accessed October 25, 2010.
9. Pray WS, Pray JJ. Lactose intolerance. US Pharmacist. 2004;29(6). www.medscape.com/viewarticle/482131. Accessed October 25, 2010.
10. Host A. Clinical course of cow’s milk protein allergy and intolerance. Pediatr Allergy Immunol. 1998;9(11 suppl):48-52.
11. Montalto M, Curigliano V, Santoro L, et al. Management and treatment of lactose malabsorption. World J Gastroenterol. 2006;12(2): 187-191.
12. Labayen I, Forga L, Gonzalez A, et al. Relationship between lactose digestion, gastrointestinal transit time and symptoms in lactose malabsorbers after dairy consumption. Aliment Pharmacol Ther. 2001;15(4):543-549.
13. Hovde O, Farup PG. A comparison of diagnostic tests for lactose malabsorption: which one is best? BMC Gastroenterol. 2009;9 82-88.
14. Srinivasan R, Minocha A. When to suspect lactose intolerance: symptomatic, ethnic, and laboratory clues. Postgrad Med. 1998;104(3): 109-111,115-116,122-123.
15. Arola H. Diagnosis of hypolactasia and lactose malabsorption. Scand J Gastroenterol Suppl. 1994;202:26-35.
16. Lin MY, Dipalma JA, Martini MC, et al. Comparative effects of exogenous lactase (beta-galactosidase) preparations on in vivo lactose digestion. Dig Dis Sci. 1993;38(11):2022-2027.
17. Hertzler SR, Savaiano DA. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am J Clin Nutr. 1996;64(2):232-236.
18. Kudlacek S, Freudenthaler O, Weissboeck H, et al. Lactose intolerance: a risk factor for reduced bone mineral density and vertebral fractures? J Gastroenterol. 2002;37(12):1014-1019.
19. Segal E, Dvorkin L, Lavy A, et al. Bone density in axial and appendicular skeleton in patients with lactose intolerance: influence of calcium intake and vitamin D status. J Am Coll Nutr. 2003;22(3):201-207.
20. Johns A, Leijten P, Yamamoto H, et al. Calcium regulation in vascular smooth muscle contractility. Am J Cardiol. 1987;59(2):A18-A23.
21. Emory University, National Cancer Institute. Calcium/vitamin D, biomarkers, and colon polyp prevention (PPS4B). clinicaltrials.gov/ct2/show/NCT00399607. Accessed October 25, 2010.
Lactose intolerance (LI), a term closely associated with hypolactasia (lactase deficiency) and lactose malabsorption,1 is a common syndrome composed of diarrhea, abdominal pain, flatulence and/or bloating, and sometimes nausea and vomiting in severe cases, after ingestion of dairy products.2 This common disorder results from a deficiency in the enzyme lactase, which makes affected patients unable to digest lactose, a sugar found in milk and other dairy products.3 Malabsorption of lactose produces the symptoms associated with LI.
The level of LI varies among affected individuals, depending on many nutritional and genetic factors, the amount of lactose consumed, the patient’s degree of lactase deficiency, and the substance in which the lactose is ingested.4
Epidemiology
LI prevalence is difficult to ascertain because the symptoms are vague and can be attributed to a number of conditions; additionally, there is no gold standard for diagnosis of LI. It is estimated that about 70% of the world population is affected by LI—with great variation among ethnicities and races.5,6 Some degree of LI is reported in up to 80% of African-Americans and Latinos, and almost 100% of Native Americans and Asian Americans. LI is least common in people of northern European descent (and is unlikely to develop before adulthood7), although it has been suggested that 30 million American adults experienced lactose malabsorption to some degree by age 20.3,8 Heyman4 estimates that approximately 2% of people of northern European descent have LI.
Regardless of ethnicity or race, older patients are more susceptible to LI than are younger adults, largely as a result of the normal processes of aging.2
Pathophysiology and Patient Presentation
Cells of the inner lumen of the small intestines, enterocytes, are covered with a membrane that has a brush border composed of microvilli.9 The microvilli produce lactase, the enzyme necessary to split and hydrolyze dietary lactose into glucose and galactose for transport across the cell membrane.6 Unfortunately, lactase is produced in the upper, most shallow section of the villi, which is exceedingly prone to damage by secondary insult.
If the lactase enzymes are absent or deficient, unabsorbed sugars osmotically attract fluid into the bowel lumen. The amount of fluid influx into the bowel is approximately triple the normal amount, based on the osmolality of sugar alone. In addition, the unabsorbed lactose entering the colon is fermented by bacteria, producing gas and resulting in the cleavage of lactose into monosaccharides. Monosaccharides cannot be absorbed by the colonic mucosa; as a result, osmotic pressure increases, and fluid levels rise in the bowel. This process explains the most common symptoms of flatulence, diarrhea, abdominal pain, and bloating.6
Most mammalian babies, including human infants, produce enough lactase to digest milk, including breast milk. This ability persists until the child is weaned. In humans, lactase activity drops at age 2 to 3 years and may cease altogether by age 5 to 10.9 Worldwide, most humans lose 90% to 95% of birth lactase levels by early childhood, followed by a continuing decline during the course of a lifetime.6 This may help explain why many elderly people are affected by LI.
Typically, development of LI progresses subtly over many years, but onset can also be relatively acute.4
Lactose Malabsorption
The three main types of lactose malabsorption are primary, secondary, and congenital. The latter is a rare, genetic form of LI in which the lactase enzyme is entirely absent; for the purposes of this article, congenital lactose malabsorption will not be discussed.
Primary lactase deficiency is the most common form and the focus of this article. It is the normal, gradual reduction in lactase enzyme that a maturing individual experiences through adulthood, and the rate of reduction is genetically determined. Secondary lactose malabsorption occurs following an insult to the small bowel, as in severe diarrhea, infection (eg, rotavirus), chemotherapy, or acute gastroenteritis.4 In these situations, lactase is the first enzyme to be negatively affected and the last to return as the insult resolves.10 Secondary hypolactasia is transient and reversible.11,12
LI is not to be confused with cow’s milk allergy—an immune response to the protein in cow’s milk, which can be a life-threatening event. A true milk allergy most commonly appears within the first year of life, whereas LI occurs more often in adulthood.5,8
LI is not considered life threatening, but its symptoms can severely affect a person’s quality of life and productivity. In addition to ethnicity and age, the type and amount of lactose ingested and the amount that the patient is unable to digest all affect the severity of LI symptoms.13
Lactose makes up between 2% and 8% of the solids in milk; 1 mL of milk (0.03 fl oz) contains 47.2 mg of lactose. No amount of lactose has been specified to produce symptoms, but most adults can tolerate as much as 8 fl oz of milk without problems,1 and patients can tolerate more lactose if the food containing it is consumed with a meal.11 Some adults may be able to ingest only 2 to 4 fl oz before symptoms appear4; in highly sensitive adults, as little as 200 mg of lactose (0.13 fl oz of milk) can produce symptoms.5
Also playing a role are the patient’s gastric emptying time and intestinal transit time.12 Symptoms of LI can be produced between 30 minutes and two hours after ingestion of milk or a milk product.9
Diagnosis
Most patients do not require specialized, sophisticated testing for a diagnosis of LI. A thorough medical history and physical examination are needed to rule out other conditions in the differential diagnosis (see Table 16,14). For the primary care provider, a basic workup should include a complete blood count, a comprehensive metabolic panel, erythrocyte sedimentation rate, a thyroid-stimulating hormone level, a stool culture, and if symptoms are severe, abdominal/pelvic radiography and CT.
In the absence of accepted guidelines, a common therapeutic approach is to exclude milk and dairy products from the patient’s diet.11 Generally, a two-week trial of a strict lactose-free diet leading to resolution of symptoms, followed by reintroduction of dairy foods and recurrence of symptoms, can be considered diagnostic.4
It is important to instruct the patient that while he or she follows this diagnostic diet, all sources of lactose must be eliminated; food labels must be read carefully to identify “hidden” lactose sources (see Table 28). Additionally, many patients (and even clinicians) may not realize that many commonly used prescription and OTC medications contain lactose, including certain agents indicated for gastrointestinal problems5 (see Table 35).
During the diagnostic diet, patients may find it helpful to keep a diary of food choices and note any symptoms that may occur. This helps empower them to be an active participant in food choices, using self-experimentation to identify which foods they can and cannot tolerate.
Gastroenterology Consult
Referral to a gastroenterologist is needed if the diagnosis is unclear or if other illnesses are suspected. Tests the specialist may perform include the hydrogen breath test, a small-intestine biopsy, the lactose tolerance test, and/or the stool acidity test for infants and children,4 although these tests vary in sensitivity and specificity.13
The hydrogen breath test, by which enzymatic activity is confirmed after the patient consumes 25 to 50 g of lactose,6 is the most widely used formal test for confirming a diagnosis of LI because it is relatively inexpensive and is the most sensitive and the most specific for LI, according to Hovde and Farup13 and Eadala et al.5 The test has been shown to yield positive results in 90% of patients with lactose malabsorption.6,15 False-negative results may signify absence of bacterial flora, as in the case of recent antibiotic use or a recent high-colonic enema. Previous aspirin use, sleep, exercise, and smoking may increase breath hydrogen secretion unrelated to lactose consumption.6
Management of Lactose Intolerance
Although the body’s ability (or inability) to produce lactase cannot be changed, the symptoms of LI can be managed with dietary restrictions. The extent of change needed depends on how much lactose the patient is able to consume before experiencing symptoms.8
In patients with secondary LI, a complete lactose-free diet is recommended until the causative pathologic condition has resolved. Patients with primary LI can opt to exclude all milk and dairy products, at least initially, until symptoms have resolved; they can then reintroduce certain milk and dairy products gradually and in small amounts, according to their individual tolerance threshold. Certain lactose-containing foods may be easier to digest than others (see Table 42).
Ingesting lactose-containing foods with a meal helps decrease gastric transit time and can lessen the symptoms of LI.11 Additionally, people who cannot drink milk may find they can eat yogurt because it contains lactase-producing bacteria,9 although clinical trials examining consumption of yogurt or probiotics in patients with LI had inconclusive results.1
Lactose-free milk or soy milk is available at most major grocery stores. These products tend to be more expensive and taste somewhat sweeter than regular milk but can be used as a reasonable substitute.9
Some patients may benefit from taking lactase enzyme supplements,1,16 which are taken with any ingestion of lactose. The enzymes may not completely prevent symptoms because the lactose is not completely digested or because it is difficult to determine an effective dose of the enzyme. Therefore, enzyme supplementation should be an adjunct to, not a substitute for, dietary restrictions.6 This may help patients when they eat at restaurants, where they do not know how food is prepared and which are unlikely to offer lactose-free food selections.
Instead of taking lactase enzyme supplements in tablet form, patients may prefer to mix lactase liquid with regular milk, producing lactose-free milk. A waiting period of 24 hours is needed before the mixture can be considered lactose-free. A trial-and-error period should be expected when enzyme supplementation or any dietary approach is tried.11
The theory of adaptative phenomena suggests that most people with LI can teach themselves to ingest more lactose gradually, leading over time to beneficial changes in the microflora of the gut and in improved colonic function.11,17 The ultimate result, whether the explanation is reduced hydrogen excretion or increased gas absorption, is less severe gastrointestinal symptoms. This strategy is not a cure for LI, nor has it been found effective for all patients with LI,1 but it can help manage symptoms to some extent.
Information for the Patient
Patients often need instruction in reading food labels to identify foods that contain milk, milk products, lactose, whey, curds, milk byproducts, dry milk solids, or even nonfat dry milk powder.8 Follow-up with the primary care provider should be arranged on an as-needed basis.
Simply excluding all dairy products from the diet does raise some health concerns. Milk and other dairy products are important sources of calcium and vitamin D, which are needed for growth and bone health in patients of all ages. A decrease in calcium consumption is one of the primary risk factors for osteoporosis, although research examining a possible association between LI and osteoporosis has yielded conflicting results. According to Kudlacek et al,18 even individuals with severe LI do not appear to be at risk for accelerated bone loss. By contrast, other research groups7,19 studied patients with LI from various age-groups and concluded that low calcium intake and impaired vitamin D status could lead to increased bone turnover and decreased bone mass, especially in men and postmenopausal women. No guidelines have been published regarding screening for osteoporosis in patients with LI.
According to a consensus statement from the NIH,3 both men and women younger than 50 should consume 1,000 mg/d of calcium, and older persons, 1,500 mg/d. In addition to calcium supplements, patients can obtain the necessary calcium through certain foods, including leafy green vegetables (spinach, kale, broccoli), sardines, calcium-fortified cereal bars, calcium-enriched soy or lactose-free milk and other soy products, fruit juices, dried beans, and tuna.
Calcium is absorbed only when enough vitamin D is present; vitamin D intake should be 400 to 600 IU/d for both women and men.3 Foods that contain vitamin D include eggs, liver, vitamin D–enriched soy or lactose-free milk, and vitamin D–fortified cereals and other processed foods. Regular exposure to sunlight helps the body synthesize vitamin D naturally.3
For optimal bone health, the NIH3 continues to recommend a combination of cardiovascular exercise, weight-bearing exercise, smoking cessation, and a well-balanced diet (including foods that are rich in calcium and vitamin D).
In addition to its role in bone health, calcium has been suggested to improve cardiac and vascular smooth muscle contractility,20 and clinical research is under way to investigate the role of calcium in reducing the risk for adenomatous colon polyps.21
Conclusion
Primary LI is a common disorder resulting from a deficiency in the enzyme lactase, making affected patients unable to digest lactose. LI is widespread in varying degrees across all races and ethnicities, affecting people of all ages; however, it is more common among older adults due to natural pathophysiologic processes.
In LI-affected patients, consuming lactose leads to troublesome symptoms of diarrhea, abdominal pain, flatulence, and/or bloating, and sometimes nausea and vomiting.
No tool is considered a “gold standard” for making a diagnosis of LI, so it is important to rule out other gastrointestinal conditions first. Oftentimes a diagnosis of LI is confirmed by the effectiveness of a lactose-free trial diet. When diagnosis is uncertain, referral to a gastroenterologist is required.
Without formal treatment guidelines, the primary form of therapy for LI is to adjust the amount of ingested lactose, with careful attention to adequate calcium and vitamin D intake. Patient education is crucial for management of LI and improvement in the patient’s quality of life.
Lactose intolerance (LI), a term closely associated with hypolactasia (lactase deficiency) and lactose malabsorption,1 is a common syndrome composed of diarrhea, abdominal pain, flatulence and/or bloating, and sometimes nausea and vomiting in severe cases, after ingestion of dairy products.2 This common disorder results from a deficiency in the enzyme lactase, which makes affected patients unable to digest lactose, a sugar found in milk and other dairy products.3 Malabsorption of lactose produces the symptoms associated with LI.
The level of LI varies among affected individuals, depending on many nutritional and genetic factors, the amount of lactose consumed, the patient’s degree of lactase deficiency, and the substance in which the lactose is ingested.4
Epidemiology
LI prevalence is difficult to ascertain because the symptoms are vague and can be attributed to a number of conditions; additionally, there is no gold standard for diagnosis of LI. It is estimated that about 70% of the world population is affected by LI—with great variation among ethnicities and races.5,6 Some degree of LI is reported in up to 80% of African-Americans and Latinos, and almost 100% of Native Americans and Asian Americans. LI is least common in people of northern European descent (and is unlikely to develop before adulthood7), although it has been suggested that 30 million American adults experienced lactose malabsorption to some degree by age 20.3,8 Heyman4 estimates that approximately 2% of people of northern European descent have LI.
Regardless of ethnicity or race, older patients are more susceptible to LI than are younger adults, largely as a result of the normal processes of aging.2
Pathophysiology and Patient Presentation
Cells of the inner lumen of the small intestines, enterocytes, are covered with a membrane that has a brush border composed of microvilli.9 The microvilli produce lactase, the enzyme necessary to split and hydrolyze dietary lactose into glucose and galactose for transport across the cell membrane.6 Unfortunately, lactase is produced in the upper, most shallow section of the villi, which is exceedingly prone to damage by secondary insult.
If the lactase enzymes are absent or deficient, unabsorbed sugars osmotically attract fluid into the bowel lumen. The amount of fluid influx into the bowel is approximately triple the normal amount, based on the osmolality of sugar alone. In addition, the unabsorbed lactose entering the colon is fermented by bacteria, producing gas and resulting in the cleavage of lactose into monosaccharides. Monosaccharides cannot be absorbed by the colonic mucosa; as a result, osmotic pressure increases, and fluid levels rise in the bowel. This process explains the most common symptoms of flatulence, diarrhea, abdominal pain, and bloating.6
Most mammalian babies, including human infants, produce enough lactase to digest milk, including breast milk. This ability persists until the child is weaned. In humans, lactase activity drops at age 2 to 3 years and may cease altogether by age 5 to 10.9 Worldwide, most humans lose 90% to 95% of birth lactase levels by early childhood, followed by a continuing decline during the course of a lifetime.6 This may help explain why many elderly people are affected by LI.
Typically, development of LI progresses subtly over many years, but onset can also be relatively acute.4
Lactose Malabsorption
The three main types of lactose malabsorption are primary, secondary, and congenital. The latter is a rare, genetic form of LI in which the lactase enzyme is entirely absent; for the purposes of this article, congenital lactose malabsorption will not be discussed.
Primary lactase deficiency is the most common form and the focus of this article. It is the normal, gradual reduction in lactase enzyme that a maturing individual experiences through adulthood, and the rate of reduction is genetically determined. Secondary lactose malabsorption occurs following an insult to the small bowel, as in severe diarrhea, infection (eg, rotavirus), chemotherapy, or acute gastroenteritis.4 In these situations, lactase is the first enzyme to be negatively affected and the last to return as the insult resolves.10 Secondary hypolactasia is transient and reversible.11,12
LI is not to be confused with cow’s milk allergy—an immune response to the protein in cow’s milk, which can be a life-threatening event. A true milk allergy most commonly appears within the first year of life, whereas LI occurs more often in adulthood.5,8
LI is not considered life threatening, but its symptoms can severely affect a person’s quality of life and productivity. In addition to ethnicity and age, the type and amount of lactose ingested and the amount that the patient is unable to digest all affect the severity of LI symptoms.13
Lactose makes up between 2% and 8% of the solids in milk; 1 mL of milk (0.03 fl oz) contains 47.2 mg of lactose. No amount of lactose has been specified to produce symptoms, but most adults can tolerate as much as 8 fl oz of milk without problems,1 and patients can tolerate more lactose if the food containing it is consumed with a meal.11 Some adults may be able to ingest only 2 to 4 fl oz before symptoms appear4; in highly sensitive adults, as little as 200 mg of lactose (0.13 fl oz of milk) can produce symptoms.5
Also playing a role are the patient’s gastric emptying time and intestinal transit time.12 Symptoms of LI can be produced between 30 minutes and two hours after ingestion of milk or a milk product.9
Diagnosis
Most patients do not require specialized, sophisticated testing for a diagnosis of LI. A thorough medical history and physical examination are needed to rule out other conditions in the differential diagnosis (see Table 16,14). For the primary care provider, a basic workup should include a complete blood count, a comprehensive metabolic panel, erythrocyte sedimentation rate, a thyroid-stimulating hormone level, a stool culture, and if symptoms are severe, abdominal/pelvic radiography and CT.
In the absence of accepted guidelines, a common therapeutic approach is to exclude milk and dairy products from the patient’s diet.11 Generally, a two-week trial of a strict lactose-free diet leading to resolution of symptoms, followed by reintroduction of dairy foods and recurrence of symptoms, can be considered diagnostic.4
It is important to instruct the patient that while he or she follows this diagnostic diet, all sources of lactose must be eliminated; food labels must be read carefully to identify “hidden” lactose sources (see Table 28). Additionally, many patients (and even clinicians) may not realize that many commonly used prescription and OTC medications contain lactose, including certain agents indicated for gastrointestinal problems5 (see Table 35).
During the diagnostic diet, patients may find it helpful to keep a diary of food choices and note any symptoms that may occur. This helps empower them to be an active participant in food choices, using self-experimentation to identify which foods they can and cannot tolerate.
Gastroenterology Consult
Referral to a gastroenterologist is needed if the diagnosis is unclear or if other illnesses are suspected. Tests the specialist may perform include the hydrogen breath test, a small-intestine biopsy, the lactose tolerance test, and/or the stool acidity test for infants and children,4 although these tests vary in sensitivity and specificity.13
The hydrogen breath test, by which enzymatic activity is confirmed after the patient consumes 25 to 50 g of lactose,6 is the most widely used formal test for confirming a diagnosis of LI because it is relatively inexpensive and is the most sensitive and the most specific for LI, according to Hovde and Farup13 and Eadala et al.5 The test has been shown to yield positive results in 90% of patients with lactose malabsorption.6,15 False-negative results may signify absence of bacterial flora, as in the case of recent antibiotic use or a recent high-colonic enema. Previous aspirin use, sleep, exercise, and smoking may increase breath hydrogen secretion unrelated to lactose consumption.6
Management of Lactose Intolerance
Although the body’s ability (or inability) to produce lactase cannot be changed, the symptoms of LI can be managed with dietary restrictions. The extent of change needed depends on how much lactose the patient is able to consume before experiencing symptoms.8
In patients with secondary LI, a complete lactose-free diet is recommended until the causative pathologic condition has resolved. Patients with primary LI can opt to exclude all milk and dairy products, at least initially, until symptoms have resolved; they can then reintroduce certain milk and dairy products gradually and in small amounts, according to their individual tolerance threshold. Certain lactose-containing foods may be easier to digest than others (see Table 42).
Ingesting lactose-containing foods with a meal helps decrease gastric transit time and can lessen the symptoms of LI.11 Additionally, people who cannot drink milk may find they can eat yogurt because it contains lactase-producing bacteria,9 although clinical trials examining consumption of yogurt or probiotics in patients with LI had inconclusive results.1
Lactose-free milk or soy milk is available at most major grocery stores. These products tend to be more expensive and taste somewhat sweeter than regular milk but can be used as a reasonable substitute.9
Some patients may benefit from taking lactase enzyme supplements,1,16 which are taken with any ingestion of lactose. The enzymes may not completely prevent symptoms because the lactose is not completely digested or because it is difficult to determine an effective dose of the enzyme. Therefore, enzyme supplementation should be an adjunct to, not a substitute for, dietary restrictions.6 This may help patients when they eat at restaurants, where they do not know how food is prepared and which are unlikely to offer lactose-free food selections.
Instead of taking lactase enzyme supplements in tablet form, patients may prefer to mix lactase liquid with regular milk, producing lactose-free milk. A waiting period of 24 hours is needed before the mixture can be considered lactose-free. A trial-and-error period should be expected when enzyme supplementation or any dietary approach is tried.11
The theory of adaptative phenomena suggests that most people with LI can teach themselves to ingest more lactose gradually, leading over time to beneficial changes in the microflora of the gut and in improved colonic function.11,17 The ultimate result, whether the explanation is reduced hydrogen excretion or increased gas absorption, is less severe gastrointestinal symptoms. This strategy is not a cure for LI, nor has it been found effective for all patients with LI,1 but it can help manage symptoms to some extent.
Information for the Patient
Patients often need instruction in reading food labels to identify foods that contain milk, milk products, lactose, whey, curds, milk byproducts, dry milk solids, or even nonfat dry milk powder.8 Follow-up with the primary care provider should be arranged on an as-needed basis.
Simply excluding all dairy products from the diet does raise some health concerns. Milk and other dairy products are important sources of calcium and vitamin D, which are needed for growth and bone health in patients of all ages. A decrease in calcium consumption is one of the primary risk factors for osteoporosis, although research examining a possible association between LI and osteoporosis has yielded conflicting results. According to Kudlacek et al,18 even individuals with severe LI do not appear to be at risk for accelerated bone loss. By contrast, other research groups7,19 studied patients with LI from various age-groups and concluded that low calcium intake and impaired vitamin D status could lead to increased bone turnover and decreased bone mass, especially in men and postmenopausal women. No guidelines have been published regarding screening for osteoporosis in patients with LI.
According to a consensus statement from the NIH,3 both men and women younger than 50 should consume 1,000 mg/d of calcium, and older persons, 1,500 mg/d. In addition to calcium supplements, patients can obtain the necessary calcium through certain foods, including leafy green vegetables (spinach, kale, broccoli), sardines, calcium-fortified cereal bars, calcium-enriched soy or lactose-free milk and other soy products, fruit juices, dried beans, and tuna.
Calcium is absorbed only when enough vitamin D is present; vitamin D intake should be 400 to 600 IU/d for both women and men.3 Foods that contain vitamin D include eggs, liver, vitamin D–enriched soy or lactose-free milk, and vitamin D–fortified cereals and other processed foods. Regular exposure to sunlight helps the body synthesize vitamin D naturally.3
For optimal bone health, the NIH3 continues to recommend a combination of cardiovascular exercise, weight-bearing exercise, smoking cessation, and a well-balanced diet (including foods that are rich in calcium and vitamin D).
In addition to its role in bone health, calcium has been suggested to improve cardiac and vascular smooth muscle contractility,20 and clinical research is under way to investigate the role of calcium in reducing the risk for adenomatous colon polyps.21
Conclusion
Primary LI is a common disorder resulting from a deficiency in the enzyme lactase, making affected patients unable to digest lactose. LI is widespread in varying degrees across all races and ethnicities, affecting people of all ages; however, it is more common among older adults due to natural pathophysiologic processes.
In LI-affected patients, consuming lactose leads to troublesome symptoms of diarrhea, abdominal pain, flatulence, and/or bloating, and sometimes nausea and vomiting.
No tool is considered a “gold standard” for making a diagnosis of LI, so it is important to rule out other gastrointestinal conditions first. Oftentimes a diagnosis of LI is confirmed by the effectiveness of a lactose-free trial diet. When diagnosis is uncertain, referral to a gastroenterologist is required.
Without formal treatment guidelines, the primary form of therapy for LI is to adjust the amount of ingested lactose, with careful attention to adequate calcium and vitamin D intake. Patient education is crucial for management of LI and improvement in the patient’s quality of life.
1. Shaukat A, Levitt MD, Taylor BC, et al. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med. 2010;152(12):797-803.
2. Lomer MC, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice—myths and realities. Aliment Pharmacol Ther. 2008;27(2):93-103.
3. NIH Consensus Development Conference. Lactose intolerance and health: final statement. February 22–24, 2010; Bethesda, MD.
4. Heyman MB; American Academy of Pediatrics Committee on Nutrition. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118(3):1279-1286.
5. Eadala P, Waud JP, Matthews SB, et al. Quantifying the ‘hidden’ lactose in drugs used for the treatment of gastrointestinal conditions. Aliment Pharmacol Ther. 2009;29(6):677-687.
6. Swagerty DL Jr, Walling AD, Klein RM. Lactose intolerance. Am Fam Physician. 2002;65(9): 1845-1850.
7. Wilt TJ, Shaukat A, Shamliyan T, et al. Lactose intolerance and health. Evid Rep Technol Assess (Full Rep). 2010 Feb(192):1-410.
8. National Digestive Diseases Information Clearinghouse, National Institute of Diabetes and Digestive and Kidney Diseases, NIH. Lactose intolerance (2009). http://digestive.niddk.nih.gov/ddiseases/pubs/lactoseintolerance. Accessed October 25, 2010.
9. Pray WS, Pray JJ. Lactose intolerance. US Pharmacist. 2004;29(6). www.medscape.com/viewarticle/482131. Accessed October 25, 2010.
10. Host A. Clinical course of cow’s milk protein allergy and intolerance. Pediatr Allergy Immunol. 1998;9(11 suppl):48-52.
11. Montalto M, Curigliano V, Santoro L, et al. Management and treatment of lactose malabsorption. World J Gastroenterol. 2006;12(2): 187-191.
12. Labayen I, Forga L, Gonzalez A, et al. Relationship between lactose digestion, gastrointestinal transit time and symptoms in lactose malabsorbers after dairy consumption. Aliment Pharmacol Ther. 2001;15(4):543-549.
13. Hovde O, Farup PG. A comparison of diagnostic tests for lactose malabsorption: which one is best? BMC Gastroenterol. 2009;9 82-88.
14. Srinivasan R, Minocha A. When to suspect lactose intolerance: symptomatic, ethnic, and laboratory clues. Postgrad Med. 1998;104(3): 109-111,115-116,122-123.
15. Arola H. Diagnosis of hypolactasia and lactose malabsorption. Scand J Gastroenterol Suppl. 1994;202:26-35.
16. Lin MY, Dipalma JA, Martini MC, et al. Comparative effects of exogenous lactase (beta-galactosidase) preparations on in vivo lactose digestion. Dig Dis Sci. 1993;38(11):2022-2027.
17. Hertzler SR, Savaiano DA. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am J Clin Nutr. 1996;64(2):232-236.
18. Kudlacek S, Freudenthaler O, Weissboeck H, et al. Lactose intolerance: a risk factor for reduced bone mineral density and vertebral fractures? J Gastroenterol. 2002;37(12):1014-1019.
19. Segal E, Dvorkin L, Lavy A, et al. Bone density in axial and appendicular skeleton in patients with lactose intolerance: influence of calcium intake and vitamin D status. J Am Coll Nutr. 2003;22(3):201-207.
20. Johns A, Leijten P, Yamamoto H, et al. Calcium regulation in vascular smooth muscle contractility. Am J Cardiol. 1987;59(2):A18-A23.
21. Emory University, National Cancer Institute. Calcium/vitamin D, biomarkers, and colon polyp prevention (PPS4B). clinicaltrials.gov/ct2/show/NCT00399607. Accessed October 25, 2010.
1. Shaukat A, Levitt MD, Taylor BC, et al. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med. 2010;152(12):797-803.
2. Lomer MC, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice—myths and realities. Aliment Pharmacol Ther. 2008;27(2):93-103.
3. NIH Consensus Development Conference. Lactose intolerance and health: final statement. February 22–24, 2010; Bethesda, MD.
4. Heyman MB; American Academy of Pediatrics Committee on Nutrition. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118(3):1279-1286.
5. Eadala P, Waud JP, Matthews SB, et al. Quantifying the ‘hidden’ lactose in drugs used for the treatment of gastrointestinal conditions. Aliment Pharmacol Ther. 2009;29(6):677-687.
6. Swagerty DL Jr, Walling AD, Klein RM. Lactose intolerance. Am Fam Physician. 2002;65(9): 1845-1850.
7. Wilt TJ, Shaukat A, Shamliyan T, et al. Lactose intolerance and health. Evid Rep Technol Assess (Full Rep). 2010 Feb(192):1-410.
8. National Digestive Diseases Information Clearinghouse, National Institute of Diabetes and Digestive and Kidney Diseases, NIH. Lactose intolerance (2009). http://digestive.niddk.nih.gov/ddiseases/pubs/lactoseintolerance. Accessed October 25, 2010.
9. Pray WS, Pray JJ. Lactose intolerance. US Pharmacist. 2004;29(6). www.medscape.com/viewarticle/482131. Accessed October 25, 2010.
10. Host A. Clinical course of cow’s milk protein allergy and intolerance. Pediatr Allergy Immunol. 1998;9(11 suppl):48-52.
11. Montalto M, Curigliano V, Santoro L, et al. Management and treatment of lactose malabsorption. World J Gastroenterol. 2006;12(2): 187-191.
12. Labayen I, Forga L, Gonzalez A, et al. Relationship between lactose digestion, gastrointestinal transit time and symptoms in lactose malabsorbers after dairy consumption. Aliment Pharmacol Ther. 2001;15(4):543-549.
13. Hovde O, Farup PG. A comparison of diagnostic tests for lactose malabsorption: which one is best? BMC Gastroenterol. 2009;9 82-88.
14. Srinivasan R, Minocha A. When to suspect lactose intolerance: symptomatic, ethnic, and laboratory clues. Postgrad Med. 1998;104(3): 109-111,115-116,122-123.
15. Arola H. Diagnosis of hypolactasia and lactose malabsorption. Scand J Gastroenterol Suppl. 1994;202:26-35.
16. Lin MY, Dipalma JA, Martini MC, et al. Comparative effects of exogenous lactase (beta-galactosidase) preparations on in vivo lactose digestion. Dig Dis Sci. 1993;38(11):2022-2027.
17. Hertzler SR, Savaiano DA. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am J Clin Nutr. 1996;64(2):232-236.
18. Kudlacek S, Freudenthaler O, Weissboeck H, et al. Lactose intolerance: a risk factor for reduced bone mineral density and vertebral fractures? J Gastroenterol. 2002;37(12):1014-1019.
19. Segal E, Dvorkin L, Lavy A, et al. Bone density in axial and appendicular skeleton in patients with lactose intolerance: influence of calcium intake and vitamin D status. J Am Coll Nutr. 2003;22(3):201-207.
20. Johns A, Leijten P, Yamamoto H, et al. Calcium regulation in vascular smooth muscle contractility. Am J Cardiol. 1987;59(2):A18-A23.
21. Emory University, National Cancer Institute. Calcium/vitamin D, biomarkers, and colon polyp prevention (PPS4B). clinicaltrials.gov/ct2/show/NCT00399607. Accessed October 25, 2010.