User login
The case for chemoprevention as a tool to avert breast cancer
The author reports that he is a consultant to Eli Lilly, Pfizer, and Wyeth, and a speaker for Eli Lilly and Wyeth.
CASE 1: Premenopausal woman
at high risk of breast cancer
R. J. is a 43-year-old, nulliparous woman who reached menarche at age 11. She has undergone two breast biopsies, the most recent of which revealed ductal hyperplasia with marked atypia.
R. J.’s sister had breast cancer at 49 years of age; her mother had breast cancer at 66 years. Because of R. J.’s family history, she underwent testing for a BRCA mutation. The result was negative.
R. J. has come to your office today to find out if she can do anything to reduce her risk of breast cancer. What options can you offer?
The most common method of “prevention” of breast cancer involves early detection and assessment of abnormalities through frequent surveillance with mammography. Some women who have dense breasts, a history of breast biopsy, or other risk factors for breast cancer may benefit from intensive surveillance with both mammography and ultrasonography—and, in some cases, magnetic resonance imaging.
More aggressive options include:
- the use of a chemopreventive agent such as tamoxifen or raloxifene
- in rare cases—usually when a BRCA mutation is present—prophylactic mastectomy.
Before it is possible to determine the optimal approach for a particular woman, it is necessary to conduct an individualized assessment of her risk—that is, to estimate the probability that she will develop breast cancer over a defined period of time. Such an estimate is also useful for designing prevention trials in high-risk subsets of the population. (Prevention trials differ from therapeutic clinical trials in that asymptomatic healthy women are exposed to potentially toxic interventions for prolonged periods to reduce their risk of breast cancer.)
This article describes chemopreventive options for women at high risk, based on individualized risk assessment using the Gail model.
(Editor’s note: For additional discussion of the important role ObGyns play in the fight against breast cancer, see Editor in Chief Dr. Robert L. Barbieri’s Editorial.)
You can estimate the likelihood that a woman like your patient may develop breast cancer using various individual risk factors ( TABLE 1 ), but estimates for combinations of risk factors are preferable. The Gail model takes into account some nongenetic factors, such as parity and age at menarche, but also genetic factors, such as family history. The model calculates a woman’s individualized breast cancer probability and yields a numerical risk (a percentage) that she will develop invasive breast cancer over the next 5 years; it also yields an estimate of her risk of developing the malignancy over the remainder of her life.1,2
A Gail-model 5-year estimate of 1.66% or higher denotes a high risk of developing breast cancer. That benchmark was the one employed in the Breast Cancer Prevention Trial (BCPT), conducted as part of the National Surgical Adjuvant Breast and Bowel Project (NSABP).3
TABLE 1
What are the risk factors for breast cancer?
And what degree of relative risk do they confer?
| Relative risk | ||
|---|---|---|
| <2 | 2–4 | >4 |
| • Age 25–34 years at first live birth • Early menarche • Late menopause • Benign proliferative disease • Postmenopausal obesity • Alcohol use • Hormone replacement therapy | • Age >35 years at first live birth • First-degree relative with breast cancer • Nulliparity • Radiation exposure • Personal history of breast cancer | • Gene mutation (BRCA 1 or 2) • Lobular carcinoma in situ • Ductal carcinoma in situ • Atypical hyperplasia |
| Adapted from Bilimoria and Morrow23 | ||
Weaknesses of the Gail model
The Gail model’s approach to estimating risk has some limitations. The model uses the number of prior breast biopsies in its assessment—but the relative risk associated with prior biopsy is smaller for women older than 50 years than it is for younger women.
Furthermore, data on which Gail bases its estimates were collected in the late 1970s and early 1980s. Since then, the increasing ease of breast histopathologic assessment—through fine-needle aspiration and outpatient core-needle biopsy—has confused the issue of just what constitutes a breast “biopsy.” (Most patients surveyed consider it to be any histologic sampling of the breast.)
As a result, the 1.66% cutoff becomes somewhat difficult to interpret in light of current practice.
Consider the following example. A 50-year-old nulliparous Caucasian woman reached menarche when she was 11 years old, has never had a biopsy, and has no first-degree relatives with breast cancer. According to the Gail model, her risk of developing breast cancer is 1.2% over the next 5 years and 10.8% in her lifetime. Therefore, she is not considered at high risk. If she were to give a history of three previous breast biopsies, however, none of them showing hyperplasia, her 5-year risk would rise to 1.8% and push her over the line into the high-risk category.
Compare her situation to that of R. J., the nulliparous woman described in Case 1. R. J. also reached menarche at 11 years, but she has had two breast biopsies (one of which showed atypical hyperplasia) and has two first-degree relatives who have had breast cancer. Her Gail score shows a 5-year risk of breast cancer of 13.5% (the norm for a 43-year-old woman is 0.8%), and a lifetime risk of 69.2%. Clearly, she has a high risk of breast cancer.
How do we improve an imperfect science?
We need to identify objective findings that are patient-specific but highly correlative with the development of breast cancer. Patient-specific biomarkers have been proposed, such as ultrasensitive measurement of the serum estradiol level in postmenopausal women. In the Multiple Outcomes of Raloxifene Evaluation, also known as the MORE trial, women who experienced the greatest reduction in the rate of breast cancer during treatment with raloxifene were a subgroup who had the highest baseline level of serum estradiol—although, overall, all patients had an estradiol level well within the postmenopausal range (≤20 pmol/L).4,5
How tamoxifen became a chemopreventive agent
Tamoxifen inhibits mammary tumors in mice and rats and suppresses hormone-dependent breast cancer cell lines in vitro.6 Clinical data from the Early Breast Cancer Trialists’ Collaborative Group yielded additional motivation for prevention trials with tamoxifen: Besides reducing the rate of recurrent breast cancer, tamoxifen reduced the risk of contralateral new-onset breast cancer by 47% after 5 years of adjuvant treatment.7 Preclinical findings in vitro and in animal models, coupled with clinical data and evidence of tamoxifen’s favorable effects on skeleton remodeling and lipid levels, led to a series of chemoprevention trials in the United States and Europe using tamoxifen.
In the aforementioned BCPT, launched in 1992, 13,388 women 35 years and older who were deemed to be at high risk of developing breast cancer were enrolled at numerous sites throughout the United States and Canada.3 The Gail model was used to select women for the trial—only those who had a 5-year risk of 1.66% or higher were included. Participants were randomly assigned to receive tamoxifen 20 mg or placebo daily for 5 years. The trial was terminated early because of the dramatic reduction in new-onset breast cancer with tamoxifen, compared with placebo.
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases for every 1,000 women, compared with 6.8 cases for every 1,000 women receiving placebo.3 Overall, the reduction in invasive breast cancer with tamoxifen was 49% (P<.00001). When broken down by age group, the reduction was:
- 44% in women 35 to 49 years old
- 51% in women 50 to 59 years old
- 55% in women 60 years and older.
Even noninvasive breast cancer was reduced with tamoxifen
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ [DCIS]) by 50%. Expanded use of mammography has increased the detection of DCIS. Most DCIS lesions appear to be estrogen-receptor positive.8
In addition, tamoxifen reduced breast cancer risk in women who had a history of lobular carcinoma in situ (LCIS), a precancer, by 56%, and it reduced the risk of breast cancer in women who had a history of atypical hyperplasia by 86%. Overall, tamoxifen reduced the occurrence of estrogen-positive tumors by 69%, but had no impact on the incidence of estrogen-receptor–negative tumors.
The BCPT was stopped 14 months before planned because the Data and Safety Monitoring Board felt it was unethical to continue to allow one half of such high-risk participants to take placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer among women who took tamoxifen.
In postmenopausal women, tamoxifen increases some risks
Two secondary endpoints of the BCPT are worthy of consideration:
- The overall relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence interval [CI], 1.35, 4.97). However, further analysis by age yielded a RR of 4.01 in women who were older than 50 years (95% CI, 1.70, 10.90), compared with a RR of 1.21 in women 49 years and younger (95% CI, 0.41, 3.60).
- The same age distinction held true for deep venous thrombosis (DVT) and pulmonary embolus, with no statistically significant increases in either in women 49 years and younger, but a RR of 1.71 and 3.19, respectively, in women 50 years and older. It is unclear whether the trial was sufficiently powered for this particular secondary endpoint.
These findings suggest that serious adverse events do not occur at the same magnitude in women younger than 50 years that they do in women 50 and older. The difference in the risk–benefit profile between younger and older women has significant clinical implications for the care of perimenopausal patients.
Risk of other malignancies was not affected by tamoxifen
Overall, invasive cancers other than those of the breast and uterus occurred at the same rate in the tamoxifen and placebo groups of the BCPT. The RR of death from any cause was 0.81 (95% CI, 0.56–1.16). There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the risk of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these risks was statistically significant.
The overall RR of fracture of the hip, spine, or radius was 0.81 (95% CI, 0.63–1.05). There was a statistically significant increase in the number of women who had cataracts who then underwent cataract surgery in the tamoxifen group (RR, 1.57; 95% CI, 1.16–2.14).
Tamoxifen is approved as a preventive for high-risk women only
Based on the results of the BCPT, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for the primary prevention of breast cancer in women who are at high risk of the disease. The FDA recommends that use of tamoxifen be limited to women at high risk because of the potentially serious side effects seen in clinical trials, including the BCPT.
The FDA did not define “high risk,” but it did recommend that the decision to use tamoxifen as chemopreventive therapy be based on thorough evaluation of the patient’s personal, family, and medical histories; her age; and her understanding of the risks and benefits of treatment.
The FDA also required the following language in the package insert:
- You should not take tamoxifen to reduce the risk of breast cancer unless you are at high risk of breast cancer. Certain conditions put women at high risk, and it is possible to calculate this risk for any woman. Breast cancer risk-assessment tools to help calculate your risk of breast cancer have been developed and are available to your health-care professional. You should discuss your risk with your healthcare professional.
CASE 1 RESOLVED
You determine that R. J. is an excellent candidate for tamoxifen by virtue of her significant risk of breast cancer. You are able to reassure her that, as the BCPT demonstrated, tamoxifen should not increase the risk of uterine cancer, DVT, or pulmonary embolism in a woman her age.
Raloxifene
CASE 2: Patient worries about breasts and bones
S. T. is a 58-year-old Caucasian mother of two whose own mother had breast cancer when she was 74 years old, and whose older sister was given a diagnosis of the malignancy 4 years ago.
S. T. had her first period when she was 11 years old, delivered her first child when she was 31, and entered menopause when she was 52. She is 5 ft 5 in tall and weighs 144 lb.
Her main reason for visiting you today is a breast Mammotome biopsy that showed ductal hyperplasia with atypia. She has been tested for a BRCA mutation, but the result was negative. Her Gail-model score is a 9.7% risk of developing breast cancer over the next 5 years, and a lifetime risk of 44.2%.
She also asks about osteoporosis prevention, given that a dual-energy x-ray absorptiometry (DXA) scan 1 year ago yielded a T-score of –1.3 for her hip and –1.1 for her spine. Her World Health Organization FRAX 10-year risk of hip fracture is 0.7%, and her risk of major osteoporotic fracture is 8.6%.
How do you respond to her concerns?
This patient has a high risk of invasive breast cancer but does not meet criteria for pharmacotherapy for osteoporosis prevention. A good option for her would be raloxifene, a selective estrogen-receptor modulator (SERM) that has been shown to reduce the risk of breast cancer as well as osteoporosis. S. T. would benefit from it on the basis of its breast benefit alone.
The genesis of a drug with multiple benefits
Raloxifene is a benzothiophene derivative, unlike the triphenylethylene family from which tamoxifen is derived. Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer.
Preclinical studies indicated that raloxifene had an antiproliferative effect on both estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.9 In the 1980s, however, a small, phase-II trial revealed that raloxifene had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen had failed.10 After information surfaced about the neoplastic effect of tamoxifen on the uteri of postmenopausal women, interest in raloxifene revived.11
Raloxifene has estrogen-agonistic activity on bone remodeling and lipid metabolism and was approved by the FDA for prevention of osteoporosis in postmenopausal women in December 1997. Its indication was extended to treatment of osteoporosis 2 years later.
Raloxifene appears to have no effect on the endometrium of postmenopausal women, compared with placebo. In a 12-month comparative trial, there was no difference in endometrial thickness, endoluminal masses, proliferation, or hyperplasia between the raloxifene and placebo groups.12 This finding corroborates earlier evidence that raloxifene does not cause endometrial hyperplasia or cancer and is not associated with vaginal bleeding or increased endometrial thickness, as measured by transvaginal ultrasonography.
A big difference between raloxifene and tamoxifen, therefore, is their varying effect on the uterus of postmenopausal women.
Additional clinical trials confirm anticancer action of raloxifene
Preclinical data in animal models suggested that, like tamoxifen, raloxifene has potent antiestrogenic effects on breast tissue.9 The MORE trial involved 7,705 postmenopausal women up to 80 years old who had established osteoporosis.13 In that trial, participants were randomized to raloxifene or placebo. Bone mineral density (BMD) and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint.
Over the 4 years of the trial, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR, 0.28; 95% CI, 0.17–0.46). Raloxifene also significantly reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR, 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors. The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from that of the placebo group.
Like tamoxifen, raloxifene appeared to be associated with an increased risk of thromboembolic disease, including DVT and pulmonary embolism, which developed in 1.1% of women taking raloxifene, compared with 0.5% of women in the placebo group (P=.003).
In a 4-year continuation of the MORE trial, known as the Continuing Outcomes Relevant to Evista, or CORE, trial, 5,231 women were randomized to continue raloxifene or placebo.14 Over the 8 years of the combined trials, the incidence of invasive breast cancer was reduced by 66% in the raloxifene group (RR, 0.34; 95% CI, 0.22–0.50). The 8-year data are extremely clinically relevant, in that raloxifene has no time limit, whereas tamoxifen is usually prescribed for no longer than 5 years.
Raloxifene is not approved for use in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as evidenced by tamoxifen’s differing effects by age in the BCPT.
Other investigations of raloxifene confirm its value in high-risk women
To compare the clinical safety and efficacy of tamoxifen and raloxifene in reducing the risk of breast cancer among healthy women, the Study of Tamoxifen and Raloxifene (STAR) was initiated in 1999.15 In that trial, 19,747 postmenopausal women older than 35 years were blindly assigned to raloxifene 60 mg or tamoxifen 20 mg daily.
Baseline characteristics of subjects in STAR are summarized in TABLE 2 . Mean age was 58.5 years. All women had a 5-year risk of developing breast cancer that exceeded 1.66%, according to the Gail model. The average Gail score was 4.03% (standard deviation, ±2.17%). Because it would have been unethical to subject high-risk women to a placebo group in light of the findings of the BCPT, there was no placebo control.
TABLE 2
Baseline characteristics of women
in the Study of Tamoxifen and Raloxifene (STAR) trial
| Characteristic | Value |
|---|---|
| Age (mean) | 58.5 years |
| Caucasian | 93% |
| Hysterectomy | 51% |
| At least one first-degree relative with breast cancer | 71% |
| Lobular carcinoma in situ | 9% |
| Atypical hyperplasia | 23% |
| 5-year risk of invasive breast cancer (mean)* | 4.03% |
| *As estimated with the Gail model Risk Calculator. | |
Here are noteworthy findings of the STAR trial:
- 163 cases of invasive breast cancer occurred in the tamoxifen group, compared with 168 among women taking raloxifene (RR, 1.02; 95% CI, 0.82–1.28).
- 36 cases of uterine cancer occurred in the tamoxifen group, compared with 23 among women taking raloxifene (RR, 0.62; 95% CI, 0.35–1.08). Earlier studies had shown a marked difference in the rate of uterine cancer between these agents. Although the difference here is not statistically significant, uterine cancer was not an endpoint of the study; nor was the study powered to explore this difference.
- The number of hysterectomies among women who were diagnosed with endometrial hyperplasia with or without atypia was, proportionally, significantly higher among women taking tamoxifen ( TABLE 3 ).
- No difference between groups was found for other invasive cancers, ischemic heart events, or stroke.
- Thromboembolic events occurred less frequently in the raloxifene group (RR, 0.70; 95% CI, 0.54–0.91). However, both raloxifene and tamoxifen have consistently been associated with a twofold to threefold increase in the risk of thromboembolic events, compared with placebo.
- Vasomotor symptoms and leg cramps increased in frequency and severity among women in both groups of the trial. These symptoms appear to be less common and less severe among women who are older and more remote from the onset of menopause.
TABLE 3
Relative risk of hysterectomy and uterine hyperplasia during STAR
| Characteristic | Women who took tamoxifen | Women who took raloxifene | Relative risk (95% confidence interval) |
|---|---|---|---|
| Hysterectomy during study | 246 | 92 | 0.37 (0.28, 0.47) |
| Hyperplasia • with atypia • without atypia | 100 15 85 | 17 2 15 | 0.17 (0.09, 0.28) 0.13 (0.01, 0.56) 0.17 (0.09, 0.30) |
What is raloxifene’s effect on the heart?
The Raloxifene Use for The Heart (RUTH) trial explored the primary endpoints of coronary artery disease (CAD) and breast cancer in more than 10,000 women who had CAD or multiple risk factors for it.16 This study began prior to the Women’s Health Initiative, at a time when hormone replacement therapy was widely believed to reduce CAD.
In the double-blinded, randomized, placebo-controlled RUTH trial, raloxifene had no significant effect on primary coronary events (533 vs 553; hazard ratio [HR], 0.95; 95% CI, 0.84–1.07). Even in this population, however, there was a 44% reduction in invasive breast cancer (40 vs 70 events; HR, 0.56; 95% CI, 0.38–0.83).
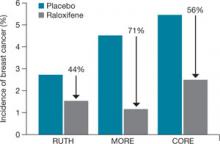
Based on these results, the FDA approved raloxifene for the “reduction in risk of invasive breast cancer in postmenopausal women at high risk for breast cancer,” as well as for the “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” ( FIGURE ).
FIGURE How raloxifene reduced invasive breast cancer in three trials
Raloxifene significantly reduced the risk of cancer, compared with placebo, in the Raloxifene Use for The Heart (RUTH), Multiple Outcomes of Raloxifene Evaluation (MORE), and Continuing Outcomes Relevant to Evista (CORE) trials.
CASE 2 RESOLVED
S. T. begins taking raloxifene 60 mg daily to lower her risk of invasive breast cancer. Although she temporarily experienced hot flashes after initiating the drug, they are only mildly bothersome, and she continues raloxifene therapy. She says she is grateful that there is an agent that can help her reduce the likelihood that she will develop breast cancer, and protection of her BMD is an added benefit.
CASE 3: At risk for both breast cancer and bone fracture
A. N., 63, is a nulliparous Caucasian woman who weighs 134 lb and stands 5 ft 4 in tall. She reached menarche when she was 12 years old and entered menopause at 49.
Although A. N. has never had a breast abnormality, her 59-year-old sister was just given a diagnosis of breast cancer. Her Gail score reveals that she has a 3.1% risk of developing breast cancer over the next 5 years.
In addition to her concerns about breast cancer, A. N. is worried about hip fracture—because her mother suffered one after menopause and because her T-score is –1.9 at the hip and –2.1 at the spine. A. N. has used steroids off and on for much of her life for asthma. Her FRAX score indicates that she has a 2.8% risk of hip fracture and a 25% risk of major osteoporotic fracture over the next 10 years.
What do you offer her?
Because of new FRAX criteria, this osteopenic woman is now a candidate for medication to reduce her risk of major osteoporotic fracture, and raloxifene is a good option. Her Gail score of 3.1% also makes her a good candidate for breast cancer risk reduction with raloxifene.
CASE 3 RESOLVED
Because A. N. needs an agent that benefits both breast and bone, you prescribe raloxifene. The drug should significantly reduce her risk of both invasive breast cancer and bone fracture, without increasing her risk of endometrial hyperplasia and cancer, both of which are associated with tamoxifen in her age group.
Aromatase inhibitors
A fairly new class of drugs being explored for their ability to reduce the risk of breast cancer is aromatase inhibitors. Substantial evidence suggests that estrogens facilitate the development of breast cancer in animals and in women, although the precise mechanism remains unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells and thereby increases the risk of genetic mutation that could lead to cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal women, the primary site of this action is in the ovary. In postmenopausal women, this conversion occurs primarily in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Aromatase inhibitors may be more effective than SERMs in preventing breast cancer because of their dual role: blocking both the initiation and promotion of breast cancer.18 These agents reduce levels of the genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. At the same time, aromatase inhibitors also block tumor promotion by lowering tissue levels of estrogen and preventing cell proliferation.
The main drawback of these agents—besides the fact that they are not FDA-approved for reducing risk—is their antiestrogenic effect on bone and lipid metabolism. They also induce vasomotor symptoms.
Studies of third-generation aromatase inhibitors in the prevention of breast cancer are under way in high-risk women. These agents include anastrozole, exemestane, and letrozole.
1. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
2. Breast Cancer Assessment Tool. Available at: www.cancer.gov/bcrisktool/Default.aspx. Accessed June 5, 2009.
3. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
4. Ruffin MT, 4th, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
5. Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. For the Multiple Outcomes of Raloxifene Evaluation (MORE) Trial. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
6. Jordan VC, Allen KE. Evaluation of the antitumor activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
7. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Womens Health. 1997;6:523-531.
10. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
11. Neven P, De Muylder X, Van Belle Y, Vanderick G, De Muylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
12. Goldstein SR, Scheele WH, Rajagopalan SK, Wilkie JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
13. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
14. Martino S, Cauley JA, Barrett-Connor E, et al. For the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751-1761.
15. Vogel VG, Costantino JP, Wickerham DL, et al. For the National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;21:2727-2741.
16. Barrett-Connor E, Mosca L, Collins P, et al. For the Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
17. Santen RJ, Yue W, Naftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocr Relat Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bryant HU, Dere WH. Selective estrogen receptor modulators: an alternative to hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:45-52.
20. Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304-313.
21. Miller BA, Feuer EJ, Hankey BF. The significance of the rising incidence of breast cancer in the United States. In: DeVita VT, Hellman S, Rosenberg SA, eds. Important Advances in Oncology. Philadelphia: Lippincott; 1994:193-207.
22. Spicer DV, Pike MC. Risk factors in breast cancer. In: Roses DF, ed. Breast Cancer. New York: Churchill Livingston; 1944.
23. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.
The author reports that he is a consultant to Eli Lilly, Pfizer, and Wyeth, and a speaker for Eli Lilly and Wyeth.
CASE 1: Premenopausal woman
at high risk of breast cancer
R. J. is a 43-year-old, nulliparous woman who reached menarche at age 11. She has undergone two breast biopsies, the most recent of which revealed ductal hyperplasia with marked atypia.
R. J.’s sister had breast cancer at 49 years of age; her mother had breast cancer at 66 years. Because of R. J.’s family history, she underwent testing for a BRCA mutation. The result was negative.
R. J. has come to your office today to find out if she can do anything to reduce her risk of breast cancer. What options can you offer?
The most common method of “prevention” of breast cancer involves early detection and assessment of abnormalities through frequent surveillance with mammography. Some women who have dense breasts, a history of breast biopsy, or other risk factors for breast cancer may benefit from intensive surveillance with both mammography and ultrasonography—and, in some cases, magnetic resonance imaging.
More aggressive options include:
- the use of a chemopreventive agent such as tamoxifen or raloxifene
- in rare cases—usually when a BRCA mutation is present—prophylactic mastectomy.
Before it is possible to determine the optimal approach for a particular woman, it is necessary to conduct an individualized assessment of her risk—that is, to estimate the probability that she will develop breast cancer over a defined period of time. Such an estimate is also useful for designing prevention trials in high-risk subsets of the population. (Prevention trials differ from therapeutic clinical trials in that asymptomatic healthy women are exposed to potentially toxic interventions for prolonged periods to reduce their risk of breast cancer.)
This article describes chemopreventive options for women at high risk, based on individualized risk assessment using the Gail model.
(Editor’s note: For additional discussion of the important role ObGyns play in the fight against breast cancer, see Editor in Chief Dr. Robert L. Barbieri’s Editorial.)
You can estimate the likelihood that a woman like your patient may develop breast cancer using various individual risk factors ( TABLE 1 ), but estimates for combinations of risk factors are preferable. The Gail model takes into account some nongenetic factors, such as parity and age at menarche, but also genetic factors, such as family history. The model calculates a woman’s individualized breast cancer probability and yields a numerical risk (a percentage) that she will develop invasive breast cancer over the next 5 years; it also yields an estimate of her risk of developing the malignancy over the remainder of her life.1,2
A Gail-model 5-year estimate of 1.66% or higher denotes a high risk of developing breast cancer. That benchmark was the one employed in the Breast Cancer Prevention Trial (BCPT), conducted as part of the National Surgical Adjuvant Breast and Bowel Project (NSABP).3
TABLE 1
What are the risk factors for breast cancer?
And what degree of relative risk do they confer?
| Relative risk | ||
|---|---|---|
| <2 | 2–4 | >4 |
| • Age 25–34 years at first live birth • Early menarche • Late menopause • Benign proliferative disease • Postmenopausal obesity • Alcohol use • Hormone replacement therapy | • Age >35 years at first live birth • First-degree relative with breast cancer • Nulliparity • Radiation exposure • Personal history of breast cancer | • Gene mutation (BRCA 1 or 2) • Lobular carcinoma in situ • Ductal carcinoma in situ • Atypical hyperplasia |
| Adapted from Bilimoria and Morrow23 | ||
Weaknesses of the Gail model
The Gail model’s approach to estimating risk has some limitations. The model uses the number of prior breast biopsies in its assessment—but the relative risk associated with prior biopsy is smaller for women older than 50 years than it is for younger women.
Furthermore, data on which Gail bases its estimates were collected in the late 1970s and early 1980s. Since then, the increasing ease of breast histopathologic assessment—through fine-needle aspiration and outpatient core-needle biopsy—has confused the issue of just what constitutes a breast “biopsy.” (Most patients surveyed consider it to be any histologic sampling of the breast.)
As a result, the 1.66% cutoff becomes somewhat difficult to interpret in light of current practice.
Consider the following example. A 50-year-old nulliparous Caucasian woman reached menarche when she was 11 years old, has never had a biopsy, and has no first-degree relatives with breast cancer. According to the Gail model, her risk of developing breast cancer is 1.2% over the next 5 years and 10.8% in her lifetime. Therefore, she is not considered at high risk. If she were to give a history of three previous breast biopsies, however, none of them showing hyperplasia, her 5-year risk would rise to 1.8% and push her over the line into the high-risk category.
Compare her situation to that of R. J., the nulliparous woman described in Case 1. R. J. also reached menarche at 11 years, but she has had two breast biopsies (one of which showed atypical hyperplasia) and has two first-degree relatives who have had breast cancer. Her Gail score shows a 5-year risk of breast cancer of 13.5% (the norm for a 43-year-old woman is 0.8%), and a lifetime risk of 69.2%. Clearly, she has a high risk of breast cancer.
How do we improve an imperfect science?
We need to identify objective findings that are patient-specific but highly correlative with the development of breast cancer. Patient-specific biomarkers have been proposed, such as ultrasensitive measurement of the serum estradiol level in postmenopausal women. In the Multiple Outcomes of Raloxifene Evaluation, also known as the MORE trial, women who experienced the greatest reduction in the rate of breast cancer during treatment with raloxifene were a subgroup who had the highest baseline level of serum estradiol—although, overall, all patients had an estradiol level well within the postmenopausal range (≤20 pmol/L).4,5
How tamoxifen became a chemopreventive agent
Tamoxifen inhibits mammary tumors in mice and rats and suppresses hormone-dependent breast cancer cell lines in vitro.6 Clinical data from the Early Breast Cancer Trialists’ Collaborative Group yielded additional motivation for prevention trials with tamoxifen: Besides reducing the rate of recurrent breast cancer, tamoxifen reduced the risk of contralateral new-onset breast cancer by 47% after 5 years of adjuvant treatment.7 Preclinical findings in vitro and in animal models, coupled with clinical data and evidence of tamoxifen’s favorable effects on skeleton remodeling and lipid levels, led to a series of chemoprevention trials in the United States and Europe using tamoxifen.
In the aforementioned BCPT, launched in 1992, 13,388 women 35 years and older who were deemed to be at high risk of developing breast cancer were enrolled at numerous sites throughout the United States and Canada.3 The Gail model was used to select women for the trial—only those who had a 5-year risk of 1.66% or higher were included. Participants were randomly assigned to receive tamoxifen 20 mg or placebo daily for 5 years. The trial was terminated early because of the dramatic reduction in new-onset breast cancer with tamoxifen, compared with placebo.
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases for every 1,000 women, compared with 6.8 cases for every 1,000 women receiving placebo.3 Overall, the reduction in invasive breast cancer with tamoxifen was 49% (P<.00001). When broken down by age group, the reduction was:
- 44% in women 35 to 49 years old
- 51% in women 50 to 59 years old
- 55% in women 60 years and older.
Even noninvasive breast cancer was reduced with tamoxifen
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ [DCIS]) by 50%. Expanded use of mammography has increased the detection of DCIS. Most DCIS lesions appear to be estrogen-receptor positive.8
In addition, tamoxifen reduced breast cancer risk in women who had a history of lobular carcinoma in situ (LCIS), a precancer, by 56%, and it reduced the risk of breast cancer in women who had a history of atypical hyperplasia by 86%. Overall, tamoxifen reduced the occurrence of estrogen-positive tumors by 69%, but had no impact on the incidence of estrogen-receptor–negative tumors.
The BCPT was stopped 14 months before planned because the Data and Safety Monitoring Board felt it was unethical to continue to allow one half of such high-risk participants to take placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer among women who took tamoxifen.
In postmenopausal women, tamoxifen increases some risks
Two secondary endpoints of the BCPT are worthy of consideration:
- The overall relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence interval [CI], 1.35, 4.97). However, further analysis by age yielded a RR of 4.01 in women who were older than 50 years (95% CI, 1.70, 10.90), compared with a RR of 1.21 in women 49 years and younger (95% CI, 0.41, 3.60).
- The same age distinction held true for deep venous thrombosis (DVT) and pulmonary embolus, with no statistically significant increases in either in women 49 years and younger, but a RR of 1.71 and 3.19, respectively, in women 50 years and older. It is unclear whether the trial was sufficiently powered for this particular secondary endpoint.
These findings suggest that serious adverse events do not occur at the same magnitude in women younger than 50 years that they do in women 50 and older. The difference in the risk–benefit profile between younger and older women has significant clinical implications for the care of perimenopausal patients.
Risk of other malignancies was not affected by tamoxifen
Overall, invasive cancers other than those of the breast and uterus occurred at the same rate in the tamoxifen and placebo groups of the BCPT. The RR of death from any cause was 0.81 (95% CI, 0.56–1.16). There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the risk of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these risks was statistically significant.
The overall RR of fracture of the hip, spine, or radius was 0.81 (95% CI, 0.63–1.05). There was a statistically significant increase in the number of women who had cataracts who then underwent cataract surgery in the tamoxifen group (RR, 1.57; 95% CI, 1.16–2.14).
Tamoxifen is approved as a preventive for high-risk women only
Based on the results of the BCPT, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for the primary prevention of breast cancer in women who are at high risk of the disease. The FDA recommends that use of tamoxifen be limited to women at high risk because of the potentially serious side effects seen in clinical trials, including the BCPT.
The FDA did not define “high risk,” but it did recommend that the decision to use tamoxifen as chemopreventive therapy be based on thorough evaluation of the patient’s personal, family, and medical histories; her age; and her understanding of the risks and benefits of treatment.
The FDA also required the following language in the package insert:
- You should not take tamoxifen to reduce the risk of breast cancer unless you are at high risk of breast cancer. Certain conditions put women at high risk, and it is possible to calculate this risk for any woman. Breast cancer risk-assessment tools to help calculate your risk of breast cancer have been developed and are available to your health-care professional. You should discuss your risk with your healthcare professional.
CASE 1 RESOLVED
You determine that R. J. is an excellent candidate for tamoxifen by virtue of her significant risk of breast cancer. You are able to reassure her that, as the BCPT demonstrated, tamoxifen should not increase the risk of uterine cancer, DVT, or pulmonary embolism in a woman her age.
Raloxifene
CASE 2: Patient worries about breasts and bones
S. T. is a 58-year-old Caucasian mother of two whose own mother had breast cancer when she was 74 years old, and whose older sister was given a diagnosis of the malignancy 4 years ago.
S. T. had her first period when she was 11 years old, delivered her first child when she was 31, and entered menopause when she was 52. She is 5 ft 5 in tall and weighs 144 lb.
Her main reason for visiting you today is a breast Mammotome biopsy that showed ductal hyperplasia with atypia. She has been tested for a BRCA mutation, but the result was negative. Her Gail-model score is a 9.7% risk of developing breast cancer over the next 5 years, and a lifetime risk of 44.2%.
She also asks about osteoporosis prevention, given that a dual-energy x-ray absorptiometry (DXA) scan 1 year ago yielded a T-score of –1.3 for her hip and –1.1 for her spine. Her World Health Organization FRAX 10-year risk of hip fracture is 0.7%, and her risk of major osteoporotic fracture is 8.6%.
How do you respond to her concerns?
This patient has a high risk of invasive breast cancer but does not meet criteria for pharmacotherapy for osteoporosis prevention. A good option for her would be raloxifene, a selective estrogen-receptor modulator (SERM) that has been shown to reduce the risk of breast cancer as well as osteoporosis. S. T. would benefit from it on the basis of its breast benefit alone.
The genesis of a drug with multiple benefits
Raloxifene is a benzothiophene derivative, unlike the triphenylethylene family from which tamoxifen is derived. Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer.
Preclinical studies indicated that raloxifene had an antiproliferative effect on both estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.9 In the 1980s, however, a small, phase-II trial revealed that raloxifene had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen had failed.10 After information surfaced about the neoplastic effect of tamoxifen on the uteri of postmenopausal women, interest in raloxifene revived.11
Raloxifene has estrogen-agonistic activity on bone remodeling and lipid metabolism and was approved by the FDA for prevention of osteoporosis in postmenopausal women in December 1997. Its indication was extended to treatment of osteoporosis 2 years later.
Raloxifene appears to have no effect on the endometrium of postmenopausal women, compared with placebo. In a 12-month comparative trial, there was no difference in endometrial thickness, endoluminal masses, proliferation, or hyperplasia between the raloxifene and placebo groups.12 This finding corroborates earlier evidence that raloxifene does not cause endometrial hyperplasia or cancer and is not associated with vaginal bleeding or increased endometrial thickness, as measured by transvaginal ultrasonography.
A big difference between raloxifene and tamoxifen, therefore, is their varying effect on the uterus of postmenopausal women.
Additional clinical trials confirm anticancer action of raloxifene
Preclinical data in animal models suggested that, like tamoxifen, raloxifene has potent antiestrogenic effects on breast tissue.9 The MORE trial involved 7,705 postmenopausal women up to 80 years old who had established osteoporosis.13 In that trial, participants were randomized to raloxifene or placebo. Bone mineral density (BMD) and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint.
Over the 4 years of the trial, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR, 0.28; 95% CI, 0.17–0.46). Raloxifene also significantly reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR, 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors. The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from that of the placebo group.
Like tamoxifen, raloxifene appeared to be associated with an increased risk of thromboembolic disease, including DVT and pulmonary embolism, which developed in 1.1% of women taking raloxifene, compared with 0.5% of women in the placebo group (P=.003).
In a 4-year continuation of the MORE trial, known as the Continuing Outcomes Relevant to Evista, or CORE, trial, 5,231 women were randomized to continue raloxifene or placebo.14 Over the 8 years of the combined trials, the incidence of invasive breast cancer was reduced by 66% in the raloxifene group (RR, 0.34; 95% CI, 0.22–0.50). The 8-year data are extremely clinically relevant, in that raloxifene has no time limit, whereas tamoxifen is usually prescribed for no longer than 5 years.
Raloxifene is not approved for use in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as evidenced by tamoxifen’s differing effects by age in the BCPT.
Other investigations of raloxifene confirm its value in high-risk women
To compare the clinical safety and efficacy of tamoxifen and raloxifene in reducing the risk of breast cancer among healthy women, the Study of Tamoxifen and Raloxifene (STAR) was initiated in 1999.15 In that trial, 19,747 postmenopausal women older than 35 years were blindly assigned to raloxifene 60 mg or tamoxifen 20 mg daily.
Baseline characteristics of subjects in STAR are summarized in TABLE 2 . Mean age was 58.5 years. All women had a 5-year risk of developing breast cancer that exceeded 1.66%, according to the Gail model. The average Gail score was 4.03% (standard deviation, ±2.17%). Because it would have been unethical to subject high-risk women to a placebo group in light of the findings of the BCPT, there was no placebo control.
TABLE 2
Baseline characteristics of women
in the Study of Tamoxifen and Raloxifene (STAR) trial
| Characteristic | Value |
|---|---|
| Age (mean) | 58.5 years |
| Caucasian | 93% |
| Hysterectomy | 51% |
| At least one first-degree relative with breast cancer | 71% |
| Lobular carcinoma in situ | 9% |
| Atypical hyperplasia | 23% |
| 5-year risk of invasive breast cancer (mean)* | 4.03% |
| *As estimated with the Gail model Risk Calculator. | |
Here are noteworthy findings of the STAR trial:
- 163 cases of invasive breast cancer occurred in the tamoxifen group, compared with 168 among women taking raloxifene (RR, 1.02; 95% CI, 0.82–1.28).
- 36 cases of uterine cancer occurred in the tamoxifen group, compared with 23 among women taking raloxifene (RR, 0.62; 95% CI, 0.35–1.08). Earlier studies had shown a marked difference in the rate of uterine cancer between these agents. Although the difference here is not statistically significant, uterine cancer was not an endpoint of the study; nor was the study powered to explore this difference.
- The number of hysterectomies among women who were diagnosed with endometrial hyperplasia with or without atypia was, proportionally, significantly higher among women taking tamoxifen ( TABLE 3 ).
- No difference between groups was found for other invasive cancers, ischemic heart events, or stroke.
- Thromboembolic events occurred less frequently in the raloxifene group (RR, 0.70; 95% CI, 0.54–0.91). However, both raloxifene and tamoxifen have consistently been associated with a twofold to threefold increase in the risk of thromboembolic events, compared with placebo.
- Vasomotor symptoms and leg cramps increased in frequency and severity among women in both groups of the trial. These symptoms appear to be less common and less severe among women who are older and more remote from the onset of menopause.
TABLE 3
Relative risk of hysterectomy and uterine hyperplasia during STAR
| Characteristic | Women who took tamoxifen | Women who took raloxifene | Relative risk (95% confidence interval) |
|---|---|---|---|
| Hysterectomy during study | 246 | 92 | 0.37 (0.28, 0.47) |
| Hyperplasia • with atypia • without atypia | 100 15 85 | 17 2 15 | 0.17 (0.09, 0.28) 0.13 (0.01, 0.56) 0.17 (0.09, 0.30) |
What is raloxifene’s effect on the heart?
The Raloxifene Use for The Heart (RUTH) trial explored the primary endpoints of coronary artery disease (CAD) and breast cancer in more than 10,000 women who had CAD or multiple risk factors for it.16 This study began prior to the Women’s Health Initiative, at a time when hormone replacement therapy was widely believed to reduce CAD.
In the double-blinded, randomized, placebo-controlled RUTH trial, raloxifene had no significant effect on primary coronary events (533 vs 553; hazard ratio [HR], 0.95; 95% CI, 0.84–1.07). Even in this population, however, there was a 44% reduction in invasive breast cancer (40 vs 70 events; HR, 0.56; 95% CI, 0.38–0.83).
Based on these results, the FDA approved raloxifene for the “reduction in risk of invasive breast cancer in postmenopausal women at high risk for breast cancer,” as well as for the “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” ( FIGURE ).
FIGURE How raloxifene reduced invasive breast cancer in three trials
Raloxifene significantly reduced the risk of cancer, compared with placebo, in the Raloxifene Use for The Heart (RUTH), Multiple Outcomes of Raloxifene Evaluation (MORE), and Continuing Outcomes Relevant to Evista (CORE) trials.
CASE 2 RESOLVED
S. T. begins taking raloxifene 60 mg daily to lower her risk of invasive breast cancer. Although she temporarily experienced hot flashes after initiating the drug, they are only mildly bothersome, and she continues raloxifene therapy. She says she is grateful that there is an agent that can help her reduce the likelihood that she will develop breast cancer, and protection of her BMD is an added benefit.
CASE 3: At risk for both breast cancer and bone fracture
A. N., 63, is a nulliparous Caucasian woman who weighs 134 lb and stands 5 ft 4 in tall. She reached menarche when she was 12 years old and entered menopause at 49.
Although A. N. has never had a breast abnormality, her 59-year-old sister was just given a diagnosis of breast cancer. Her Gail score reveals that she has a 3.1% risk of developing breast cancer over the next 5 years.
In addition to her concerns about breast cancer, A. N. is worried about hip fracture—because her mother suffered one after menopause and because her T-score is –1.9 at the hip and –2.1 at the spine. A. N. has used steroids off and on for much of her life for asthma. Her FRAX score indicates that she has a 2.8% risk of hip fracture and a 25% risk of major osteoporotic fracture over the next 10 years.
What do you offer her?
Because of new FRAX criteria, this osteopenic woman is now a candidate for medication to reduce her risk of major osteoporotic fracture, and raloxifene is a good option. Her Gail score of 3.1% also makes her a good candidate for breast cancer risk reduction with raloxifene.
CASE 3 RESOLVED
Because A. N. needs an agent that benefits both breast and bone, you prescribe raloxifene. The drug should significantly reduce her risk of both invasive breast cancer and bone fracture, without increasing her risk of endometrial hyperplasia and cancer, both of which are associated with tamoxifen in her age group.
Aromatase inhibitors
A fairly new class of drugs being explored for their ability to reduce the risk of breast cancer is aromatase inhibitors. Substantial evidence suggests that estrogens facilitate the development of breast cancer in animals and in women, although the precise mechanism remains unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells and thereby increases the risk of genetic mutation that could lead to cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal women, the primary site of this action is in the ovary. In postmenopausal women, this conversion occurs primarily in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Aromatase inhibitors may be more effective than SERMs in preventing breast cancer because of their dual role: blocking both the initiation and promotion of breast cancer.18 These agents reduce levels of the genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. At the same time, aromatase inhibitors also block tumor promotion by lowering tissue levels of estrogen and preventing cell proliferation.
The main drawback of these agents—besides the fact that they are not FDA-approved for reducing risk—is their antiestrogenic effect on bone and lipid metabolism. They also induce vasomotor symptoms.
Studies of third-generation aromatase inhibitors in the prevention of breast cancer are under way in high-risk women. These agents include anastrozole, exemestane, and letrozole.
The author reports that he is a consultant to Eli Lilly, Pfizer, and Wyeth, and a speaker for Eli Lilly and Wyeth.
CASE 1: Premenopausal woman
at high risk of breast cancer
R. J. is a 43-year-old, nulliparous woman who reached menarche at age 11. She has undergone two breast biopsies, the most recent of which revealed ductal hyperplasia with marked atypia.
R. J.’s sister had breast cancer at 49 years of age; her mother had breast cancer at 66 years. Because of R. J.’s family history, she underwent testing for a BRCA mutation. The result was negative.
R. J. has come to your office today to find out if she can do anything to reduce her risk of breast cancer. What options can you offer?
The most common method of “prevention” of breast cancer involves early detection and assessment of abnormalities through frequent surveillance with mammography. Some women who have dense breasts, a history of breast biopsy, or other risk factors for breast cancer may benefit from intensive surveillance with both mammography and ultrasonography—and, in some cases, magnetic resonance imaging.
More aggressive options include:
- the use of a chemopreventive agent such as tamoxifen or raloxifene
- in rare cases—usually when a BRCA mutation is present—prophylactic mastectomy.
Before it is possible to determine the optimal approach for a particular woman, it is necessary to conduct an individualized assessment of her risk—that is, to estimate the probability that she will develop breast cancer over a defined period of time. Such an estimate is also useful for designing prevention trials in high-risk subsets of the population. (Prevention trials differ from therapeutic clinical trials in that asymptomatic healthy women are exposed to potentially toxic interventions for prolonged periods to reduce their risk of breast cancer.)
This article describes chemopreventive options for women at high risk, based on individualized risk assessment using the Gail model.
(Editor’s note: For additional discussion of the important role ObGyns play in the fight against breast cancer, see Editor in Chief Dr. Robert L. Barbieri’s Editorial.)
You can estimate the likelihood that a woman like your patient may develop breast cancer using various individual risk factors ( TABLE 1 ), but estimates for combinations of risk factors are preferable. The Gail model takes into account some nongenetic factors, such as parity and age at menarche, but also genetic factors, such as family history. The model calculates a woman’s individualized breast cancer probability and yields a numerical risk (a percentage) that she will develop invasive breast cancer over the next 5 years; it also yields an estimate of her risk of developing the malignancy over the remainder of her life.1,2
A Gail-model 5-year estimate of 1.66% or higher denotes a high risk of developing breast cancer. That benchmark was the one employed in the Breast Cancer Prevention Trial (BCPT), conducted as part of the National Surgical Adjuvant Breast and Bowel Project (NSABP).3
TABLE 1
What are the risk factors for breast cancer?
And what degree of relative risk do they confer?
| Relative risk | ||
|---|---|---|
| <2 | 2–4 | >4 |
| • Age 25–34 years at first live birth • Early menarche • Late menopause • Benign proliferative disease • Postmenopausal obesity • Alcohol use • Hormone replacement therapy | • Age >35 years at first live birth • First-degree relative with breast cancer • Nulliparity • Radiation exposure • Personal history of breast cancer | • Gene mutation (BRCA 1 or 2) • Lobular carcinoma in situ • Ductal carcinoma in situ • Atypical hyperplasia |
| Adapted from Bilimoria and Morrow23 | ||
Weaknesses of the Gail model
The Gail model’s approach to estimating risk has some limitations. The model uses the number of prior breast biopsies in its assessment—but the relative risk associated with prior biopsy is smaller for women older than 50 years than it is for younger women.
Furthermore, data on which Gail bases its estimates were collected in the late 1970s and early 1980s. Since then, the increasing ease of breast histopathologic assessment—through fine-needle aspiration and outpatient core-needle biopsy—has confused the issue of just what constitutes a breast “biopsy.” (Most patients surveyed consider it to be any histologic sampling of the breast.)
As a result, the 1.66% cutoff becomes somewhat difficult to interpret in light of current practice.
Consider the following example. A 50-year-old nulliparous Caucasian woman reached menarche when she was 11 years old, has never had a biopsy, and has no first-degree relatives with breast cancer. According to the Gail model, her risk of developing breast cancer is 1.2% over the next 5 years and 10.8% in her lifetime. Therefore, she is not considered at high risk. If she were to give a history of three previous breast biopsies, however, none of them showing hyperplasia, her 5-year risk would rise to 1.8% and push her over the line into the high-risk category.
Compare her situation to that of R. J., the nulliparous woman described in Case 1. R. J. also reached menarche at 11 years, but she has had two breast biopsies (one of which showed atypical hyperplasia) and has two first-degree relatives who have had breast cancer. Her Gail score shows a 5-year risk of breast cancer of 13.5% (the norm for a 43-year-old woman is 0.8%), and a lifetime risk of 69.2%. Clearly, she has a high risk of breast cancer.
How do we improve an imperfect science?
We need to identify objective findings that are patient-specific but highly correlative with the development of breast cancer. Patient-specific biomarkers have been proposed, such as ultrasensitive measurement of the serum estradiol level in postmenopausal women. In the Multiple Outcomes of Raloxifene Evaluation, also known as the MORE trial, women who experienced the greatest reduction in the rate of breast cancer during treatment with raloxifene were a subgroup who had the highest baseline level of serum estradiol—although, overall, all patients had an estradiol level well within the postmenopausal range (≤20 pmol/L).4,5
How tamoxifen became a chemopreventive agent
Tamoxifen inhibits mammary tumors in mice and rats and suppresses hormone-dependent breast cancer cell lines in vitro.6 Clinical data from the Early Breast Cancer Trialists’ Collaborative Group yielded additional motivation for prevention trials with tamoxifen: Besides reducing the rate of recurrent breast cancer, tamoxifen reduced the risk of contralateral new-onset breast cancer by 47% after 5 years of adjuvant treatment.7 Preclinical findings in vitro and in animal models, coupled with clinical data and evidence of tamoxifen’s favorable effects on skeleton remodeling and lipid levels, led to a series of chemoprevention trials in the United States and Europe using tamoxifen.
In the aforementioned BCPT, launched in 1992, 13,388 women 35 years and older who were deemed to be at high risk of developing breast cancer were enrolled at numerous sites throughout the United States and Canada.3 The Gail model was used to select women for the trial—only those who had a 5-year risk of 1.66% or higher were included. Participants were randomly assigned to receive tamoxifen 20 mg or placebo daily for 5 years. The trial was terminated early because of the dramatic reduction in new-onset breast cancer with tamoxifen, compared with placebo.
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases for every 1,000 women, compared with 6.8 cases for every 1,000 women receiving placebo.3 Overall, the reduction in invasive breast cancer with tamoxifen was 49% (P<.00001). When broken down by age group, the reduction was:
- 44% in women 35 to 49 years old
- 51% in women 50 to 59 years old
- 55% in women 60 years and older.
Even noninvasive breast cancer was reduced with tamoxifen
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ [DCIS]) by 50%. Expanded use of mammography has increased the detection of DCIS. Most DCIS lesions appear to be estrogen-receptor positive.8
In addition, tamoxifen reduced breast cancer risk in women who had a history of lobular carcinoma in situ (LCIS), a precancer, by 56%, and it reduced the risk of breast cancer in women who had a history of atypical hyperplasia by 86%. Overall, tamoxifen reduced the occurrence of estrogen-positive tumors by 69%, but had no impact on the incidence of estrogen-receptor–negative tumors.
The BCPT was stopped 14 months before planned because the Data and Safety Monitoring Board felt it was unethical to continue to allow one half of such high-risk participants to take placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer among women who took tamoxifen.
In postmenopausal women, tamoxifen increases some risks
Two secondary endpoints of the BCPT are worthy of consideration:
- The overall relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence interval [CI], 1.35, 4.97). However, further analysis by age yielded a RR of 4.01 in women who were older than 50 years (95% CI, 1.70, 10.90), compared with a RR of 1.21 in women 49 years and younger (95% CI, 0.41, 3.60).
- The same age distinction held true for deep venous thrombosis (DVT) and pulmonary embolus, with no statistically significant increases in either in women 49 years and younger, but a RR of 1.71 and 3.19, respectively, in women 50 years and older. It is unclear whether the trial was sufficiently powered for this particular secondary endpoint.
These findings suggest that serious adverse events do not occur at the same magnitude in women younger than 50 years that they do in women 50 and older. The difference in the risk–benefit profile between younger and older women has significant clinical implications for the care of perimenopausal patients.
Risk of other malignancies was not affected by tamoxifen
Overall, invasive cancers other than those of the breast and uterus occurred at the same rate in the tamoxifen and placebo groups of the BCPT. The RR of death from any cause was 0.81 (95% CI, 0.56–1.16). There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the risk of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these risks was statistically significant.
The overall RR of fracture of the hip, spine, or radius was 0.81 (95% CI, 0.63–1.05). There was a statistically significant increase in the number of women who had cataracts who then underwent cataract surgery in the tamoxifen group (RR, 1.57; 95% CI, 1.16–2.14).
Tamoxifen is approved as a preventive for high-risk women only
Based on the results of the BCPT, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for the primary prevention of breast cancer in women who are at high risk of the disease. The FDA recommends that use of tamoxifen be limited to women at high risk because of the potentially serious side effects seen in clinical trials, including the BCPT.
The FDA did not define “high risk,” but it did recommend that the decision to use tamoxifen as chemopreventive therapy be based on thorough evaluation of the patient’s personal, family, and medical histories; her age; and her understanding of the risks and benefits of treatment.
The FDA also required the following language in the package insert:
- You should not take tamoxifen to reduce the risk of breast cancer unless you are at high risk of breast cancer. Certain conditions put women at high risk, and it is possible to calculate this risk for any woman. Breast cancer risk-assessment tools to help calculate your risk of breast cancer have been developed and are available to your health-care professional. You should discuss your risk with your healthcare professional.
CASE 1 RESOLVED
You determine that R. J. is an excellent candidate for tamoxifen by virtue of her significant risk of breast cancer. You are able to reassure her that, as the BCPT demonstrated, tamoxifen should not increase the risk of uterine cancer, DVT, or pulmonary embolism in a woman her age.
Raloxifene
CASE 2: Patient worries about breasts and bones
S. T. is a 58-year-old Caucasian mother of two whose own mother had breast cancer when she was 74 years old, and whose older sister was given a diagnosis of the malignancy 4 years ago.
S. T. had her first period when she was 11 years old, delivered her first child when she was 31, and entered menopause when she was 52. She is 5 ft 5 in tall and weighs 144 lb.
Her main reason for visiting you today is a breast Mammotome biopsy that showed ductal hyperplasia with atypia. She has been tested for a BRCA mutation, but the result was negative. Her Gail-model score is a 9.7% risk of developing breast cancer over the next 5 years, and a lifetime risk of 44.2%.
She also asks about osteoporosis prevention, given that a dual-energy x-ray absorptiometry (DXA) scan 1 year ago yielded a T-score of –1.3 for her hip and –1.1 for her spine. Her World Health Organization FRAX 10-year risk of hip fracture is 0.7%, and her risk of major osteoporotic fracture is 8.6%.
How do you respond to her concerns?
This patient has a high risk of invasive breast cancer but does not meet criteria for pharmacotherapy for osteoporosis prevention. A good option for her would be raloxifene, a selective estrogen-receptor modulator (SERM) that has been shown to reduce the risk of breast cancer as well as osteoporosis. S. T. would benefit from it on the basis of its breast benefit alone.
The genesis of a drug with multiple benefits
Raloxifene is a benzothiophene derivative, unlike the triphenylethylene family from which tamoxifen is derived. Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer.
Preclinical studies indicated that raloxifene had an antiproliferative effect on both estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.9 In the 1980s, however, a small, phase-II trial revealed that raloxifene had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen had failed.10 After information surfaced about the neoplastic effect of tamoxifen on the uteri of postmenopausal women, interest in raloxifene revived.11
Raloxifene has estrogen-agonistic activity on bone remodeling and lipid metabolism and was approved by the FDA for prevention of osteoporosis in postmenopausal women in December 1997. Its indication was extended to treatment of osteoporosis 2 years later.
Raloxifene appears to have no effect on the endometrium of postmenopausal women, compared with placebo. In a 12-month comparative trial, there was no difference in endometrial thickness, endoluminal masses, proliferation, or hyperplasia between the raloxifene and placebo groups.12 This finding corroborates earlier evidence that raloxifene does not cause endometrial hyperplasia or cancer and is not associated with vaginal bleeding or increased endometrial thickness, as measured by transvaginal ultrasonography.
A big difference between raloxifene and tamoxifen, therefore, is their varying effect on the uterus of postmenopausal women.
Additional clinical trials confirm anticancer action of raloxifene
Preclinical data in animal models suggested that, like tamoxifen, raloxifene has potent antiestrogenic effects on breast tissue.9 The MORE trial involved 7,705 postmenopausal women up to 80 years old who had established osteoporosis.13 In that trial, participants were randomized to raloxifene or placebo. Bone mineral density (BMD) and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint.
Over the 4 years of the trial, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR, 0.28; 95% CI, 0.17–0.46). Raloxifene also significantly reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR, 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors. The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from that of the placebo group.
Like tamoxifen, raloxifene appeared to be associated with an increased risk of thromboembolic disease, including DVT and pulmonary embolism, which developed in 1.1% of women taking raloxifene, compared with 0.5% of women in the placebo group (P=.003).
In a 4-year continuation of the MORE trial, known as the Continuing Outcomes Relevant to Evista, or CORE, trial, 5,231 women were randomized to continue raloxifene or placebo.14 Over the 8 years of the combined trials, the incidence of invasive breast cancer was reduced by 66% in the raloxifene group (RR, 0.34; 95% CI, 0.22–0.50). The 8-year data are extremely clinically relevant, in that raloxifene has no time limit, whereas tamoxifen is usually prescribed for no longer than 5 years.
Raloxifene is not approved for use in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as evidenced by tamoxifen’s differing effects by age in the BCPT.
Other investigations of raloxifene confirm its value in high-risk women
To compare the clinical safety and efficacy of tamoxifen and raloxifene in reducing the risk of breast cancer among healthy women, the Study of Tamoxifen and Raloxifene (STAR) was initiated in 1999.15 In that trial, 19,747 postmenopausal women older than 35 years were blindly assigned to raloxifene 60 mg or tamoxifen 20 mg daily.
Baseline characteristics of subjects in STAR are summarized in TABLE 2 . Mean age was 58.5 years. All women had a 5-year risk of developing breast cancer that exceeded 1.66%, according to the Gail model. The average Gail score was 4.03% (standard deviation, ±2.17%). Because it would have been unethical to subject high-risk women to a placebo group in light of the findings of the BCPT, there was no placebo control.
TABLE 2
Baseline characteristics of women
in the Study of Tamoxifen and Raloxifene (STAR) trial
| Characteristic | Value |
|---|---|
| Age (mean) | 58.5 years |
| Caucasian | 93% |
| Hysterectomy | 51% |
| At least one first-degree relative with breast cancer | 71% |
| Lobular carcinoma in situ | 9% |
| Atypical hyperplasia | 23% |
| 5-year risk of invasive breast cancer (mean)* | 4.03% |
| *As estimated with the Gail model Risk Calculator. | |
Here are noteworthy findings of the STAR trial:
- 163 cases of invasive breast cancer occurred in the tamoxifen group, compared with 168 among women taking raloxifene (RR, 1.02; 95% CI, 0.82–1.28).
- 36 cases of uterine cancer occurred in the tamoxifen group, compared with 23 among women taking raloxifene (RR, 0.62; 95% CI, 0.35–1.08). Earlier studies had shown a marked difference in the rate of uterine cancer between these agents. Although the difference here is not statistically significant, uterine cancer was not an endpoint of the study; nor was the study powered to explore this difference.
- The number of hysterectomies among women who were diagnosed with endometrial hyperplasia with or without atypia was, proportionally, significantly higher among women taking tamoxifen ( TABLE 3 ).
- No difference between groups was found for other invasive cancers, ischemic heart events, or stroke.
- Thromboembolic events occurred less frequently in the raloxifene group (RR, 0.70; 95% CI, 0.54–0.91). However, both raloxifene and tamoxifen have consistently been associated with a twofold to threefold increase in the risk of thromboembolic events, compared with placebo.
- Vasomotor symptoms and leg cramps increased in frequency and severity among women in both groups of the trial. These symptoms appear to be less common and less severe among women who are older and more remote from the onset of menopause.
TABLE 3
Relative risk of hysterectomy and uterine hyperplasia during STAR
| Characteristic | Women who took tamoxifen | Women who took raloxifene | Relative risk (95% confidence interval) |
|---|---|---|---|
| Hysterectomy during study | 246 | 92 | 0.37 (0.28, 0.47) |
| Hyperplasia • with atypia • without atypia | 100 15 85 | 17 2 15 | 0.17 (0.09, 0.28) 0.13 (0.01, 0.56) 0.17 (0.09, 0.30) |
What is raloxifene’s effect on the heart?
The Raloxifene Use for The Heart (RUTH) trial explored the primary endpoints of coronary artery disease (CAD) and breast cancer in more than 10,000 women who had CAD or multiple risk factors for it.16 This study began prior to the Women’s Health Initiative, at a time when hormone replacement therapy was widely believed to reduce CAD.
In the double-blinded, randomized, placebo-controlled RUTH trial, raloxifene had no significant effect on primary coronary events (533 vs 553; hazard ratio [HR], 0.95; 95% CI, 0.84–1.07). Even in this population, however, there was a 44% reduction in invasive breast cancer (40 vs 70 events; HR, 0.56; 95% CI, 0.38–0.83).
Based on these results, the FDA approved raloxifene for the “reduction in risk of invasive breast cancer in postmenopausal women at high risk for breast cancer,” as well as for the “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” ( FIGURE ).
FIGURE How raloxifene reduced invasive breast cancer in three trials
Raloxifene significantly reduced the risk of cancer, compared with placebo, in the Raloxifene Use for The Heart (RUTH), Multiple Outcomes of Raloxifene Evaluation (MORE), and Continuing Outcomes Relevant to Evista (CORE) trials.
CASE 2 RESOLVED
S. T. begins taking raloxifene 60 mg daily to lower her risk of invasive breast cancer. Although she temporarily experienced hot flashes after initiating the drug, they are only mildly bothersome, and she continues raloxifene therapy. She says she is grateful that there is an agent that can help her reduce the likelihood that she will develop breast cancer, and protection of her BMD is an added benefit.
CASE 3: At risk for both breast cancer and bone fracture
A. N., 63, is a nulliparous Caucasian woman who weighs 134 lb and stands 5 ft 4 in tall. She reached menarche when she was 12 years old and entered menopause at 49.
Although A. N. has never had a breast abnormality, her 59-year-old sister was just given a diagnosis of breast cancer. Her Gail score reveals that she has a 3.1% risk of developing breast cancer over the next 5 years.
In addition to her concerns about breast cancer, A. N. is worried about hip fracture—because her mother suffered one after menopause and because her T-score is –1.9 at the hip and –2.1 at the spine. A. N. has used steroids off and on for much of her life for asthma. Her FRAX score indicates that she has a 2.8% risk of hip fracture and a 25% risk of major osteoporotic fracture over the next 10 years.
What do you offer her?
Because of new FRAX criteria, this osteopenic woman is now a candidate for medication to reduce her risk of major osteoporotic fracture, and raloxifene is a good option. Her Gail score of 3.1% also makes her a good candidate for breast cancer risk reduction with raloxifene.
CASE 3 RESOLVED
Because A. N. needs an agent that benefits both breast and bone, you prescribe raloxifene. The drug should significantly reduce her risk of both invasive breast cancer and bone fracture, without increasing her risk of endometrial hyperplasia and cancer, both of which are associated with tamoxifen in her age group.
Aromatase inhibitors
A fairly new class of drugs being explored for their ability to reduce the risk of breast cancer is aromatase inhibitors. Substantial evidence suggests that estrogens facilitate the development of breast cancer in animals and in women, although the precise mechanism remains unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells and thereby increases the risk of genetic mutation that could lead to cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal women, the primary site of this action is in the ovary. In postmenopausal women, this conversion occurs primarily in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Aromatase inhibitors may be more effective than SERMs in preventing breast cancer because of their dual role: blocking both the initiation and promotion of breast cancer.18 These agents reduce levels of the genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. At the same time, aromatase inhibitors also block tumor promotion by lowering tissue levels of estrogen and preventing cell proliferation.
The main drawback of these agents—besides the fact that they are not FDA-approved for reducing risk—is their antiestrogenic effect on bone and lipid metabolism. They also induce vasomotor symptoms.
Studies of third-generation aromatase inhibitors in the prevention of breast cancer are under way in high-risk women. These agents include anastrozole, exemestane, and letrozole.
1. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
2. Breast Cancer Assessment Tool. Available at: www.cancer.gov/bcrisktool/Default.aspx. Accessed June 5, 2009.
3. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
4. Ruffin MT, 4th, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
5. Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. For the Multiple Outcomes of Raloxifene Evaluation (MORE) Trial. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
6. Jordan VC, Allen KE. Evaluation of the antitumor activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
7. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Womens Health. 1997;6:523-531.
10. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
11. Neven P, De Muylder X, Van Belle Y, Vanderick G, De Muylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
12. Goldstein SR, Scheele WH, Rajagopalan SK, Wilkie JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
13. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
14. Martino S, Cauley JA, Barrett-Connor E, et al. For the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751-1761.
15. Vogel VG, Costantino JP, Wickerham DL, et al. For the National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;21:2727-2741.
16. Barrett-Connor E, Mosca L, Collins P, et al. For the Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
17. Santen RJ, Yue W, Naftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocr Relat Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bryant HU, Dere WH. Selective estrogen receptor modulators: an alternative to hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:45-52.
20. Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304-313.
21. Miller BA, Feuer EJ, Hankey BF. The significance of the rising incidence of breast cancer in the United States. In: DeVita VT, Hellman S, Rosenberg SA, eds. Important Advances in Oncology. Philadelphia: Lippincott; 1994:193-207.
22. Spicer DV, Pike MC. Risk factors in breast cancer. In: Roses DF, ed. Breast Cancer. New York: Churchill Livingston; 1944.
23. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.
1. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
2. Breast Cancer Assessment Tool. Available at: www.cancer.gov/bcrisktool/Default.aspx. Accessed June 5, 2009.
3. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
4. Ruffin MT, 4th, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
5. Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. For the Multiple Outcomes of Raloxifene Evaluation (MORE) Trial. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
6. Jordan VC, Allen KE. Evaluation of the antitumor activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
7. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Womens Health. 1997;6:523-531.
10. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
11. Neven P, De Muylder X, Van Belle Y, Vanderick G, De Muylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
12. Goldstein SR, Scheele WH, Rajagopalan SK, Wilkie JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
13. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
14. Martino S, Cauley JA, Barrett-Connor E, et al. For the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751-1761.
15. Vogel VG, Costantino JP, Wickerham DL, et al. For the National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;21:2727-2741.
16. Barrett-Connor E, Mosca L, Collins P, et al. For the Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
17. Santen RJ, Yue W, Naftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocr Relat Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bryant HU, Dere WH. Selective estrogen receptor modulators: an alternative to hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:45-52.
20. Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304-313.
21. Miller BA, Feuer EJ, Hankey BF. The significance of the rising incidence of breast cancer in the United States. In: DeVita VT, Hellman S, Rosenberg SA, eds. Important Advances in Oncology. Philadelphia: Lippincott; 1994:193-207.
22. Spicer DV, Pike MC. Risk factors in breast cancer. In: Roses DF, ed. Breast Cancer. New York: Churchill Livingston; 1944.
23. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.
A VA-Based, Multidisciplinary Weight Management Program
Grand Rounds: Boy, 10, With Knee Pain
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.
His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).
The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13
The Case Patient
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.
At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.

His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).

The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13

The Case Patient
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.

At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.

His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).

The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13

The Case Patient
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.

At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.
Capturing Elusive Foreign Bodies With Ultrasound
Significant Causes of Pediatric Abdominal Pain
Decompression Illness in Scuba Divers: What You Need to Know
How to Reverse an Antithrombotic Agent
Management of Acute Glenohumeral Dislocations
INFECTIOUS DISEASE
Dr. Duff reports no financial relationships relevant to this article.
Four important developments have marked the past year in infectious disease:
- A promising vaccine against cytomegalovirus (CMV) was tested in women of reproductive age
- Extended-spectrum antibiotic prophylaxis proved to be effective in reducing the incidence of wound infection following cesarean delivery
- Investigators developed a simple but effective method to prevent wound complications following repair of a third- or fourth-degree perineal laceration
- The incidence of severe Clostridium difficile-associated diarrhea crept upward, emerging as a threat to pregnant women.
CMV vaccine makes an auspicious debut—but isn’t ready for practice
Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009; 360:1191–1199 [Classification of evidence – Level I].
This Phase-2, randomized, double-blind, placebo-controlled trial of a new cytomegalovirus (CMV) vaccine in women found an overall efficacy rate of 50% (95% confidence interval, 7% to 73%), with no unusually serious reactions among women who were vaccinated. This efficacy rate is disappointing, but it isn’t entirely surprising; even the immune response resulting from natural infection is not fully protective against reactivated infection or recurrent infection with a different strain of virus. Nor is natural immunity completely effective in preventing severe fetal injury in recurrent infection.1
Virus poses greatest risk to pregnant women and their fetuses
CMV is the most common perinatally transmitted infection, affecting 0.6% to 0.7% of infants ( FIGURE 1 ). The greatest risk of fetal injury occurs when the mother develops primary infection during pregnancy, which raises her infant’s risk of infection to 40% to 50%. Of infants delivered to mothers with primary infection, approximately 10% to 15% will be acutely symptomatic at birth.
Clinical manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, intracranial calcification, chorioretinitis, hearing impairment, hepatitis, and thrombocytopenia.
Because the morbidity and mortality associated with these conditions are alarmingly high, development of a safe, effective vaccine against CMV would be most welcome.2,3
Recurrent or reactivated maternal CMV infection poses a much lower risk to the fetus. Infected infants are rarely symptomatic at birth. Clinical manifestations of infection typically occur later in childhood and include hearing and visual deficits, dental anomalies, and learning or behavioral disorders.2,3
FIGURE 1 Cytomegalovirus
Cytomegalovirus is a member of the herpesvirus family. It is shed intermittently in bodily fluids, without detectable signs and symptoms.
Details of the trial
Women were eligible for the study if they were seronegative for CMV antibody, in good health, 14 to 40 years old, and not pregnant or lactating. Participants received three doses of vaccine or placebo at 0, 1, and 6 months. (The vaccine was composed of CMV envelope glycoprotein B with MF59 adjuvant.) The women were then tested for CMV infection every 3 months for as long as 42 months, using an assay for IgG antibodies directed against viral proteins other than glycoprotein B. Infection was confirmed by viral culture or immuno blotting. The primary endpoint was time until detection of CMV infection.
The vaccine was given to 234 patients, and 230 received placebo. Eighteen infections occurred in the vaccine group, compared with 31 in the placebo group. Vaccinated patients were more likely to remain uninfected during follow-up (p=.02).
One of 81 infants (1%) born to mothers in the vaccinated group had congenital CMV infection, compared with three of 97 (3%) infants born to mothers in the placebo group (p=.41). One infant in the placebo group had severe infection that was evident at birth. The other three infants were asymptomatic at birth and free of sequelae 3 to 5 years later.
The most promising preventive remains experimental
No drug is uniformly effective in treating maternal CMV infection and preventing congenital infection. The most promising intervention for prevention of congenital CMV infection is administration of hyperimmune anti-CMV antibody to the mother. A recent report by Nigro and colleagues4 found this agent to be of great value for both treating and preventing congenital CMV. However, because of limitations in the design of this study, administration of hyperimmune globulin still must be regarded as experimental.3
Key questions remain unanswered
The goal of a large-scale vaccination program is to ensure that women enter reproductive age with preexisting immunity to infection. In that light, the study by Pass and colleagues is only partially encouraging. Despite vaccination, 18 infections occurred, and the follow-up period was relatively short. We do not yet know whether the protective effect of the vaccine will be of extended duration. Moreover, one vaccinated mother delivered an infant who had congenital CMV infection.
Until additional trials of the CMV vaccine are reported, we must focus on helping patients prevent acquisition of infection during pregnancy. Preventive measures include:
- safe sex practices
- use of CMV-negative blood for transfusion to pregnant women and their fetuses
- strict hand-washing procedures for mothers when changing diapers and caring for young children.
Extended-spectrum antibiotics reduce the rate of postcesarean wound infection
Tita ATN, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199:303e.1–303e.3 [Classification of evidence – Level II].
This prospective study describes surveillance for postcesarean wound infection during three different periods at the University of Alabama:
- 1992–1996, during which patients undergoing cesarean delivery routinely received prophylaxis with a first- or second-generation cephalosporin. Overall incidence of wound infection: 3.1%
- 1997–1999, during which patients were randomized to standard prophylaxis with cefazolin or to cefazolin plus either intravenous (IV) doxycycline or oral azithromycin. Overall incidence of wound infection: 2.4%
- 2001–2006, during which patients routinely received IV cefazolin plus IV azithromycin. Overall incidence of wound infection: 1.3%.
In each time period, the prophylactic antibiotics were administered after the infant’s umbilical cord was clamped. The p value for test of trend was highly significant (p<.002). The same significant trend was noted when superficial and deep wound infections were examined separately.
This evidence is a “practice changer”
For almost 20 years, the standard of practice has been to routinely administer prophylactic antibiotics to all women having cesarean delivery. Essentially, every published study has demonstrated a highly significant reduction in the frequency of postcesarean endometritis when patients received prophylaxis. Multiple studies also confirmed that a more limited-spectrum cephalosporin was as effective as an extended-spectrum agent in reducing the frequency of endometritis.5
Many of these earlier reports were unable to demonstrate a consistently beneficial effect of prophylaxis on the incidence of postoperative wound infection. That is why the present study is of such interest and importance. Tita and colleagues previously demonstrated an improved effect of extended-spectrum prophylaxis on the incidence of postcesarean endometritis.6 Now they have confirmed that this method of prophylaxis is also effective in lowering the rate of surgical wound infection.
Wound infections are more troublesome than endometritis
Wound infections—either incisional abscess or cellulitis—are even more likely than post-cesarean endometritis to prolong a patient’s postoperative stay and create the potential for severe morbidity, such as fascial dehiscence and necrotizing fasciitis. With the increasing prevalence of obesity in the US population, wound infections are likely to become even more frequent.
These infections typically are caused by aerobic streptococci and staphylococci from the skin, combined with coliform organisms and anaerobes from the pelvic flora. Incisional abscesses require surgical drainage; cellulitis usually will respond to a change in antibiotic therapy that specifically targets streptococci and staphylococci, along with the coliforms and anaerobes.
I strongly recommend routine prophylaxis with IV cefazolin (1 g) plus azithromycin (500 mg) in all women having cesarean delivery. Moreover, in view of several recent investigations that evaluated the timing of antibiotic administration (immediately preoperative versus after the umbilical cord is clamped), I recommend that extended-spectrum prophylaxis be given before the start of surgery.7
Duggal N, Mercado C, Daniels K, Bujor A, Caughey AB, El-Sayed YY. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized control trial. Obstet Gynecol. 2008;111:1268–1273 [Classification of evidence – Level I].
Take note of this prospective, randomized, placebo-controlled trial of prophylactic antibiotics in women who sustained a third-or fourth-degree perineal laceration during vaginal delivery: It is the first, and only, well-designed trial of antibiotic prophylaxis for prevention of complications after repair of a major perineal laceration. Among patients in the study, 8% who received antibiotics developed a wound complication, compared with 24% of patients who received placebo, a statistically and clinically significant difference.
Details of the study
Eighty-three women received placebo, and 64 received a single IV dose of either cefotetan (1 g) or cefoxitin (1 g) before their perineal laceration was repaired. Patients who were allergic to penicillin received clindamycin (900 mg). The primary endpoints of the study were gross disruption of the wound or purulent drainage from the wound site 2 weeks after delivery.
Forty patients (27%) did not return for their post-partum appointment. Of the remaining patients, four of 49 (8%) who received antibiotics developed a wound complication, compared with 14 of 58 (24%) of those who received placebo (p=.037).
Sequelae of major perineal laceration can be severe
Major perineal laceration occurs in approximately 2% to 20% of vaginal deliveries in the United States. The principal risk factors for third- and fourth-degree lacerations are nulliparity, midline episiotomy, and operative vaginal delivery, especially forceps extraction. Both types of laceration may lead to serious morbidity, such as prolonged pain, fecal incontinence, and perineal infection, including necrotizing fasciitis. These infections typically are polymicrobial, mixed aerobic–anaerobic. Moreover, fourth-degree lacerations may result in rectovaginal fistula if they are not repaired properly. This complication causes considerable debility and major social inconvenience for the patient.
Although the sample size was relatively small, this study clearly demonstrated that a single dose of extended-spectrum cephalosporin (cefotetan or cefoxitin) was highly effective in reducing the rate of perineal infection and perineal wound disruption. Whether a more limited-spectrum agent such as cefazolin would be as effective is not certain.
I strongly recommend routine antibiotic prophylaxis for any patient who sustains a third- or fourth-degree perineal laceration.
If the patient has a mild allergy to penicillin (morbilliform rash), I would administer cefotetan because it is less expensive than cefoxitin. If the patient has a severe reaction to penicillin (urticaria, anaphylaxis), I would administer both clindamycin and gentamicin in order to ensure adequate coverage of the multiple organisms likely to cause soft-tissue infection of the genital tract.
Clindamycin alone covers only aerobic gram-positive cocci and offers no protection against the coliform organisms that are so prevalent in perineal wound infection.8
Diarrhea linked to Clostridium difficile emerges as a potent threat to pregnant women
Rouphael NG, O’Donnell JA, Bhatnagar J, et al. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198:635.e1–635.e6 [Classification of evidence – Level III].
This report describes 10 cases of severe Clostridium difficile-associated diarrhea (CDAD) in pregnant women during 2005 and 2006. CDAD usually affects elderly debilitated patients in hospitals and nursing homes. This report is of great concern because the affected patients were otherwise healthy, young, pregnant women. The observations are even more alarming because the mortality rate in this small series was 30% for both mothers and babies.
Details of the series
The women developed signs of severe CDAD 3 to 60 days after receiving antibiotics; the median was 5 days. CDAD was considered severe if the patient required hospitalization, ICU admission, or colectomy, or if she died. These cases occurred in California, Georgia, Oklahoma, and Pennsylvania and were reported to the Centers for Disease Control and Prevention (CDC).
Six women became ill before delivery, and four developed symptoms postpartum. The most common manifestations of infection were diarrhea, abdominal pain and distention, and fever. The peripheral white blood cell count was in the range of 11–72 × 103/μL. In nine patients, the diagnosis was confirmed by a positive test for toxin A/B; seven of these patients also had visible pseudomembranes in the colon. One patient had a positive stool sample for C difficile ( FIGURE 2 ).
Six patients required admission to the ICU. Six developed toxic megacolon, and five required subtotal colectomy. Three had sepsis; three had acute renal failure; two had disseminated intravascular coagulation. Three patients died, and three had stillbirths. Two patients relapsed following treatment.
One patient had no treatment and died. The others received either metronidazole or vancomycin or a combination of the two. One of the patients who died received metronidazole, vancomycin, and cholestyramine.
FIGURE 2 Clostridium difficile
C difficile is a spore-forming, gram-positive anaerobic bacillus that is a common cause of antibiotic-associated diarrhea.
An epidemic strain appears
The incidence of C difficile infection in acute care US hospitals has increased to 84 for every 100,000 patients in recent years, about three times the rate of 31 for every 100,000 that was reported in 1996, as the authors note. Many of the most severe cases of CDAD are caused by a new epidemic strain of bacteria, termed North American Pulsed Field type 1 (NAP1) and PCR ribotype 027.
This new strain is characterized by three key virulence factors:
- increased production of toxins A and B
- resistance to fluoroquinolones
- production of binary toxin.
Toxins A and B bind to the surface of intestinal epithelial cells, stimulate tissue injury and inflammation, and, ultimately, lead to cell death. Binary toxin appears to act synergistically with toxins A and B to cause severe colitis.9
Metronidazole is no longer the treatment of choice for severe CDAD
Before 2000, treatment of CDAD with vancomycin or metronidazole was 97% to 98% effective.9 In recent years, however, a failure rate as high as 26% has been reported among patients who are treated with metronidazole.10 One prospective, randomized clinical trial demonstrated that, in patients who had severe CDAD, vancomycin, 125 mg four times daily, was superior to metronidazole, 250 mg four times daily (97% success rate vs 76%; p=.02).11 The efficacy of the two drugs was comparable in treating milder cases of CDAD (98% for vancomycin, 90% for metronidazole; p=.36).
The clinical implications of this case series and the reports cited above are clear: When we administer broad-spectrum antibiotics to our patients, we must be ever watchful for signs of toxicity. If the patient develops diarrhea, the offending drug should be discontinued. If the diarrhea does not promptly resolve, tests to isolate C difficile in the stool and identify toxins unique to this organism are advised.
In addition, anoscopy or sigmoidoscopy should be performed to assess the patient for pseudomembranes.
If mild CDAD is confirmed, the patient may be treated with vancomycin or metronidazole. If severe CDAD is identified, vancomycin should be administered, and the patient should be transferred to the ICU for close monitoring and supportive care.
1. Dekker CL, Arvin AM. One step closer to a CMV vaccine. N Engl J Med. 2009;360:1250-1252.
2. Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1402-1404.
3. Nigro G, Adler SP, LaTorre RL, Best AM. Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362.
4. Duff P, Barth WH, Jr, Post MD. Case records of the Massachusetts General Hospital. Case 4-2009: A 39-year-old pregnant woman with fever after a trip to Africa. N Engl J Med. 2009;360:508-516.
5. Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost-effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157(4 Pt 1):794-798.
6. Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
7. Tita ATN, Rouse DJ, Blackwell S, Saade GR, Spong CY, Andrews WW. Emerging concepts in antibiotic prophylaxis for cesarean delivery: a systematic review. Obstet Gynecol. 2009;113:675-682.
8. Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
9. Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932-1940.
10. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
11. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
Dr. Duff reports no financial relationships relevant to this article.
Four important developments have marked the past year in infectious disease:
- A promising vaccine against cytomegalovirus (CMV) was tested in women of reproductive age
- Extended-spectrum antibiotic prophylaxis proved to be effective in reducing the incidence of wound infection following cesarean delivery
- Investigators developed a simple but effective method to prevent wound complications following repair of a third- or fourth-degree perineal laceration
- The incidence of severe Clostridium difficile-associated diarrhea crept upward, emerging as a threat to pregnant women.
CMV vaccine makes an auspicious debut—but isn’t ready for practice
Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009; 360:1191–1199 [Classification of evidence – Level I].
This Phase-2, randomized, double-blind, placebo-controlled trial of a new cytomegalovirus (CMV) vaccine in women found an overall efficacy rate of 50% (95% confidence interval, 7% to 73%), with no unusually serious reactions among women who were vaccinated. This efficacy rate is disappointing, but it isn’t entirely surprising; even the immune response resulting from natural infection is not fully protective against reactivated infection or recurrent infection with a different strain of virus. Nor is natural immunity completely effective in preventing severe fetal injury in recurrent infection.1
Virus poses greatest risk to pregnant women and their fetuses
CMV is the most common perinatally transmitted infection, affecting 0.6% to 0.7% of infants ( FIGURE 1 ). The greatest risk of fetal injury occurs when the mother develops primary infection during pregnancy, which raises her infant’s risk of infection to 40% to 50%. Of infants delivered to mothers with primary infection, approximately 10% to 15% will be acutely symptomatic at birth.
Clinical manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, intracranial calcification, chorioretinitis, hearing impairment, hepatitis, and thrombocytopenia.
Because the morbidity and mortality associated with these conditions are alarmingly high, development of a safe, effective vaccine against CMV would be most welcome.2,3
Recurrent or reactivated maternal CMV infection poses a much lower risk to the fetus. Infected infants are rarely symptomatic at birth. Clinical manifestations of infection typically occur later in childhood and include hearing and visual deficits, dental anomalies, and learning or behavioral disorders.2,3
FIGURE 1 Cytomegalovirus
Cytomegalovirus is a member of the herpesvirus family. It is shed intermittently in bodily fluids, without detectable signs and symptoms.
Details of the trial
Women were eligible for the study if they were seronegative for CMV antibody, in good health, 14 to 40 years old, and not pregnant or lactating. Participants received three doses of vaccine or placebo at 0, 1, and 6 months. (The vaccine was composed of CMV envelope glycoprotein B with MF59 adjuvant.) The women were then tested for CMV infection every 3 months for as long as 42 months, using an assay for IgG antibodies directed against viral proteins other than glycoprotein B. Infection was confirmed by viral culture or immuno blotting. The primary endpoint was time until detection of CMV infection.
The vaccine was given to 234 patients, and 230 received placebo. Eighteen infections occurred in the vaccine group, compared with 31 in the placebo group. Vaccinated patients were more likely to remain uninfected during follow-up (p=.02).
One of 81 infants (1%) born to mothers in the vaccinated group had congenital CMV infection, compared with three of 97 (3%) infants born to mothers in the placebo group (p=.41). One infant in the placebo group had severe infection that was evident at birth. The other three infants were asymptomatic at birth and free of sequelae 3 to 5 years later.
The most promising preventive remains experimental
No drug is uniformly effective in treating maternal CMV infection and preventing congenital infection. The most promising intervention for prevention of congenital CMV infection is administration of hyperimmune anti-CMV antibody to the mother. A recent report by Nigro and colleagues4 found this agent to be of great value for both treating and preventing congenital CMV. However, because of limitations in the design of this study, administration of hyperimmune globulin still must be regarded as experimental.3
Key questions remain unanswered
The goal of a large-scale vaccination program is to ensure that women enter reproductive age with preexisting immunity to infection. In that light, the study by Pass and colleagues is only partially encouraging. Despite vaccination, 18 infections occurred, and the follow-up period was relatively short. We do not yet know whether the protective effect of the vaccine will be of extended duration. Moreover, one vaccinated mother delivered an infant who had congenital CMV infection.
Until additional trials of the CMV vaccine are reported, we must focus on helping patients prevent acquisition of infection during pregnancy. Preventive measures include:
- safe sex practices
- use of CMV-negative blood for transfusion to pregnant women and their fetuses
- strict hand-washing procedures for mothers when changing diapers and caring for young children.
Extended-spectrum antibiotics reduce the rate of postcesarean wound infection
Tita ATN, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199:303e.1–303e.3 [Classification of evidence – Level II].
This prospective study describes surveillance for postcesarean wound infection during three different periods at the University of Alabama:
- 1992–1996, during which patients undergoing cesarean delivery routinely received prophylaxis with a first- or second-generation cephalosporin. Overall incidence of wound infection: 3.1%
- 1997–1999, during which patients were randomized to standard prophylaxis with cefazolin or to cefazolin plus either intravenous (IV) doxycycline or oral azithromycin. Overall incidence of wound infection: 2.4%
- 2001–2006, during which patients routinely received IV cefazolin plus IV azithromycin. Overall incidence of wound infection: 1.3%.
In each time period, the prophylactic antibiotics were administered after the infant’s umbilical cord was clamped. The p value for test of trend was highly significant (p<.002). The same significant trend was noted when superficial and deep wound infections were examined separately.
This evidence is a “practice changer”
For almost 20 years, the standard of practice has been to routinely administer prophylactic antibiotics to all women having cesarean delivery. Essentially, every published study has demonstrated a highly significant reduction in the frequency of postcesarean endometritis when patients received prophylaxis. Multiple studies also confirmed that a more limited-spectrum cephalosporin was as effective as an extended-spectrum agent in reducing the frequency of endometritis.5
Many of these earlier reports were unable to demonstrate a consistently beneficial effect of prophylaxis on the incidence of postoperative wound infection. That is why the present study is of such interest and importance. Tita and colleagues previously demonstrated an improved effect of extended-spectrum prophylaxis on the incidence of postcesarean endometritis.6 Now they have confirmed that this method of prophylaxis is also effective in lowering the rate of surgical wound infection.
Wound infections are more troublesome than endometritis
Wound infections—either incisional abscess or cellulitis—are even more likely than post-cesarean endometritis to prolong a patient’s postoperative stay and create the potential for severe morbidity, such as fascial dehiscence and necrotizing fasciitis. With the increasing prevalence of obesity in the US population, wound infections are likely to become even more frequent.
These infections typically are caused by aerobic streptococci and staphylococci from the skin, combined with coliform organisms and anaerobes from the pelvic flora. Incisional abscesses require surgical drainage; cellulitis usually will respond to a change in antibiotic therapy that specifically targets streptococci and staphylococci, along with the coliforms and anaerobes.
I strongly recommend routine prophylaxis with IV cefazolin (1 g) plus azithromycin (500 mg) in all women having cesarean delivery. Moreover, in view of several recent investigations that evaluated the timing of antibiotic administration (immediately preoperative versus after the umbilical cord is clamped), I recommend that extended-spectrum prophylaxis be given before the start of surgery.7
Duggal N, Mercado C, Daniels K, Bujor A, Caughey AB, El-Sayed YY. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized control trial. Obstet Gynecol. 2008;111:1268–1273 [Classification of evidence – Level I].
Take note of this prospective, randomized, placebo-controlled trial of prophylactic antibiotics in women who sustained a third-or fourth-degree perineal laceration during vaginal delivery: It is the first, and only, well-designed trial of antibiotic prophylaxis for prevention of complications after repair of a major perineal laceration. Among patients in the study, 8% who received antibiotics developed a wound complication, compared with 24% of patients who received placebo, a statistically and clinically significant difference.
Details of the study
Eighty-three women received placebo, and 64 received a single IV dose of either cefotetan (1 g) or cefoxitin (1 g) before their perineal laceration was repaired. Patients who were allergic to penicillin received clindamycin (900 mg). The primary endpoints of the study were gross disruption of the wound or purulent drainage from the wound site 2 weeks after delivery.
Forty patients (27%) did not return for their post-partum appointment. Of the remaining patients, four of 49 (8%) who received antibiotics developed a wound complication, compared with 14 of 58 (24%) of those who received placebo (p=.037).
Sequelae of major perineal laceration can be severe
Major perineal laceration occurs in approximately 2% to 20% of vaginal deliveries in the United States. The principal risk factors for third- and fourth-degree lacerations are nulliparity, midline episiotomy, and operative vaginal delivery, especially forceps extraction. Both types of laceration may lead to serious morbidity, such as prolonged pain, fecal incontinence, and perineal infection, including necrotizing fasciitis. These infections typically are polymicrobial, mixed aerobic–anaerobic. Moreover, fourth-degree lacerations may result in rectovaginal fistula if they are not repaired properly. This complication causes considerable debility and major social inconvenience for the patient.
Although the sample size was relatively small, this study clearly demonstrated that a single dose of extended-spectrum cephalosporin (cefotetan or cefoxitin) was highly effective in reducing the rate of perineal infection and perineal wound disruption. Whether a more limited-spectrum agent such as cefazolin would be as effective is not certain.
I strongly recommend routine antibiotic prophylaxis for any patient who sustains a third- or fourth-degree perineal laceration.
If the patient has a mild allergy to penicillin (morbilliform rash), I would administer cefotetan because it is less expensive than cefoxitin. If the patient has a severe reaction to penicillin (urticaria, anaphylaxis), I would administer both clindamycin and gentamicin in order to ensure adequate coverage of the multiple organisms likely to cause soft-tissue infection of the genital tract.
Clindamycin alone covers only aerobic gram-positive cocci and offers no protection against the coliform organisms that are so prevalent in perineal wound infection.8
Diarrhea linked to Clostridium difficile emerges as a potent threat to pregnant women
Rouphael NG, O’Donnell JA, Bhatnagar J, et al. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198:635.e1–635.e6 [Classification of evidence – Level III].
This report describes 10 cases of severe Clostridium difficile-associated diarrhea (CDAD) in pregnant women during 2005 and 2006. CDAD usually affects elderly debilitated patients in hospitals and nursing homes. This report is of great concern because the affected patients were otherwise healthy, young, pregnant women. The observations are even more alarming because the mortality rate in this small series was 30% for both mothers and babies.
Details of the series
The women developed signs of severe CDAD 3 to 60 days after receiving antibiotics; the median was 5 days. CDAD was considered severe if the patient required hospitalization, ICU admission, or colectomy, or if she died. These cases occurred in California, Georgia, Oklahoma, and Pennsylvania and were reported to the Centers for Disease Control and Prevention (CDC).
Six women became ill before delivery, and four developed symptoms postpartum. The most common manifestations of infection were diarrhea, abdominal pain and distention, and fever. The peripheral white blood cell count was in the range of 11–72 × 103/μL. In nine patients, the diagnosis was confirmed by a positive test for toxin A/B; seven of these patients also had visible pseudomembranes in the colon. One patient had a positive stool sample for C difficile ( FIGURE 2 ).
Six patients required admission to the ICU. Six developed toxic megacolon, and five required subtotal colectomy. Three had sepsis; three had acute renal failure; two had disseminated intravascular coagulation. Three patients died, and three had stillbirths. Two patients relapsed following treatment.
One patient had no treatment and died. The others received either metronidazole or vancomycin or a combination of the two. One of the patients who died received metronidazole, vancomycin, and cholestyramine.
FIGURE 2 Clostridium difficile
C difficile is a spore-forming, gram-positive anaerobic bacillus that is a common cause of antibiotic-associated diarrhea.
An epidemic strain appears
The incidence of C difficile infection in acute care US hospitals has increased to 84 for every 100,000 patients in recent years, about three times the rate of 31 for every 100,000 that was reported in 1996, as the authors note. Many of the most severe cases of CDAD are caused by a new epidemic strain of bacteria, termed North American Pulsed Field type 1 (NAP1) and PCR ribotype 027.
This new strain is characterized by three key virulence factors:
- increased production of toxins A and B
- resistance to fluoroquinolones
- production of binary toxin.
Toxins A and B bind to the surface of intestinal epithelial cells, stimulate tissue injury and inflammation, and, ultimately, lead to cell death. Binary toxin appears to act synergistically with toxins A and B to cause severe colitis.9
Metronidazole is no longer the treatment of choice for severe CDAD
Before 2000, treatment of CDAD with vancomycin or metronidazole was 97% to 98% effective.9 In recent years, however, a failure rate as high as 26% has been reported among patients who are treated with metronidazole.10 One prospective, randomized clinical trial demonstrated that, in patients who had severe CDAD, vancomycin, 125 mg four times daily, was superior to metronidazole, 250 mg four times daily (97% success rate vs 76%; p=.02).11 The efficacy of the two drugs was comparable in treating milder cases of CDAD (98% for vancomycin, 90% for metronidazole; p=.36).
The clinical implications of this case series and the reports cited above are clear: When we administer broad-spectrum antibiotics to our patients, we must be ever watchful for signs of toxicity. If the patient develops diarrhea, the offending drug should be discontinued. If the diarrhea does not promptly resolve, tests to isolate C difficile in the stool and identify toxins unique to this organism are advised.
In addition, anoscopy or sigmoidoscopy should be performed to assess the patient for pseudomembranes.
If mild CDAD is confirmed, the patient may be treated with vancomycin or metronidazole. If severe CDAD is identified, vancomycin should be administered, and the patient should be transferred to the ICU for close monitoring and supportive care.
Dr. Duff reports no financial relationships relevant to this article.
Four important developments have marked the past year in infectious disease:
- A promising vaccine against cytomegalovirus (CMV) was tested in women of reproductive age
- Extended-spectrum antibiotic prophylaxis proved to be effective in reducing the incidence of wound infection following cesarean delivery
- Investigators developed a simple but effective method to prevent wound complications following repair of a third- or fourth-degree perineal laceration
- The incidence of severe Clostridium difficile-associated diarrhea crept upward, emerging as a threat to pregnant women.
CMV vaccine makes an auspicious debut—but isn’t ready for practice
Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009; 360:1191–1199 [Classification of evidence – Level I].
This Phase-2, randomized, double-blind, placebo-controlled trial of a new cytomegalovirus (CMV) vaccine in women found an overall efficacy rate of 50% (95% confidence interval, 7% to 73%), with no unusually serious reactions among women who were vaccinated. This efficacy rate is disappointing, but it isn’t entirely surprising; even the immune response resulting from natural infection is not fully protective against reactivated infection or recurrent infection with a different strain of virus. Nor is natural immunity completely effective in preventing severe fetal injury in recurrent infection.1
Virus poses greatest risk to pregnant women and their fetuses
CMV is the most common perinatally transmitted infection, affecting 0.6% to 0.7% of infants ( FIGURE 1 ). The greatest risk of fetal injury occurs when the mother develops primary infection during pregnancy, which raises her infant’s risk of infection to 40% to 50%. Of infants delivered to mothers with primary infection, approximately 10% to 15% will be acutely symptomatic at birth.
Clinical manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, intracranial calcification, chorioretinitis, hearing impairment, hepatitis, and thrombocytopenia.
Because the morbidity and mortality associated with these conditions are alarmingly high, development of a safe, effective vaccine against CMV would be most welcome.2,3
Recurrent or reactivated maternal CMV infection poses a much lower risk to the fetus. Infected infants are rarely symptomatic at birth. Clinical manifestations of infection typically occur later in childhood and include hearing and visual deficits, dental anomalies, and learning or behavioral disorders.2,3
FIGURE 1 Cytomegalovirus
Cytomegalovirus is a member of the herpesvirus family. It is shed intermittently in bodily fluids, without detectable signs and symptoms.
Details of the trial
Women were eligible for the study if they were seronegative for CMV antibody, in good health, 14 to 40 years old, and not pregnant or lactating. Participants received three doses of vaccine or placebo at 0, 1, and 6 months. (The vaccine was composed of CMV envelope glycoprotein B with MF59 adjuvant.) The women were then tested for CMV infection every 3 months for as long as 42 months, using an assay for IgG antibodies directed against viral proteins other than glycoprotein B. Infection was confirmed by viral culture or immuno blotting. The primary endpoint was time until detection of CMV infection.
The vaccine was given to 234 patients, and 230 received placebo. Eighteen infections occurred in the vaccine group, compared with 31 in the placebo group. Vaccinated patients were more likely to remain uninfected during follow-up (p=.02).
One of 81 infants (1%) born to mothers in the vaccinated group had congenital CMV infection, compared with three of 97 (3%) infants born to mothers in the placebo group (p=.41). One infant in the placebo group had severe infection that was evident at birth. The other three infants were asymptomatic at birth and free of sequelae 3 to 5 years later.
The most promising preventive remains experimental
No drug is uniformly effective in treating maternal CMV infection and preventing congenital infection. The most promising intervention for prevention of congenital CMV infection is administration of hyperimmune anti-CMV antibody to the mother. A recent report by Nigro and colleagues4 found this agent to be of great value for both treating and preventing congenital CMV. However, because of limitations in the design of this study, administration of hyperimmune globulin still must be regarded as experimental.3
Key questions remain unanswered
The goal of a large-scale vaccination program is to ensure that women enter reproductive age with preexisting immunity to infection. In that light, the study by Pass and colleagues is only partially encouraging. Despite vaccination, 18 infections occurred, and the follow-up period was relatively short. We do not yet know whether the protective effect of the vaccine will be of extended duration. Moreover, one vaccinated mother delivered an infant who had congenital CMV infection.
Until additional trials of the CMV vaccine are reported, we must focus on helping patients prevent acquisition of infection during pregnancy. Preventive measures include:
- safe sex practices
- use of CMV-negative blood for transfusion to pregnant women and their fetuses
- strict hand-washing procedures for mothers when changing diapers and caring for young children.
Extended-spectrum antibiotics reduce the rate of postcesarean wound infection
Tita ATN, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199:303e.1–303e.3 [Classification of evidence – Level II].
This prospective study describes surveillance for postcesarean wound infection during three different periods at the University of Alabama:
- 1992–1996, during which patients undergoing cesarean delivery routinely received prophylaxis with a first- or second-generation cephalosporin. Overall incidence of wound infection: 3.1%
- 1997–1999, during which patients were randomized to standard prophylaxis with cefazolin or to cefazolin plus either intravenous (IV) doxycycline or oral azithromycin. Overall incidence of wound infection: 2.4%
- 2001–2006, during which patients routinely received IV cefazolin plus IV azithromycin. Overall incidence of wound infection: 1.3%.
In each time period, the prophylactic antibiotics were administered after the infant’s umbilical cord was clamped. The p value for test of trend was highly significant (p<.002). The same significant trend was noted when superficial and deep wound infections were examined separately.
This evidence is a “practice changer”
For almost 20 years, the standard of practice has been to routinely administer prophylactic antibiotics to all women having cesarean delivery. Essentially, every published study has demonstrated a highly significant reduction in the frequency of postcesarean endometritis when patients received prophylaxis. Multiple studies also confirmed that a more limited-spectrum cephalosporin was as effective as an extended-spectrum agent in reducing the frequency of endometritis.5
Many of these earlier reports were unable to demonstrate a consistently beneficial effect of prophylaxis on the incidence of postoperative wound infection. That is why the present study is of such interest and importance. Tita and colleagues previously demonstrated an improved effect of extended-spectrum prophylaxis on the incidence of postcesarean endometritis.6 Now they have confirmed that this method of prophylaxis is also effective in lowering the rate of surgical wound infection.
Wound infections are more troublesome than endometritis
Wound infections—either incisional abscess or cellulitis—are even more likely than post-cesarean endometritis to prolong a patient’s postoperative stay and create the potential for severe morbidity, such as fascial dehiscence and necrotizing fasciitis. With the increasing prevalence of obesity in the US population, wound infections are likely to become even more frequent.
These infections typically are caused by aerobic streptococci and staphylococci from the skin, combined with coliform organisms and anaerobes from the pelvic flora. Incisional abscesses require surgical drainage; cellulitis usually will respond to a change in antibiotic therapy that specifically targets streptococci and staphylococci, along with the coliforms and anaerobes.
I strongly recommend routine prophylaxis with IV cefazolin (1 g) plus azithromycin (500 mg) in all women having cesarean delivery. Moreover, in view of several recent investigations that evaluated the timing of antibiotic administration (immediately preoperative versus after the umbilical cord is clamped), I recommend that extended-spectrum prophylaxis be given before the start of surgery.7
Duggal N, Mercado C, Daniels K, Bujor A, Caughey AB, El-Sayed YY. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized control trial. Obstet Gynecol. 2008;111:1268–1273 [Classification of evidence – Level I].
Take note of this prospective, randomized, placebo-controlled trial of prophylactic antibiotics in women who sustained a third-or fourth-degree perineal laceration during vaginal delivery: It is the first, and only, well-designed trial of antibiotic prophylaxis for prevention of complications after repair of a major perineal laceration. Among patients in the study, 8% who received antibiotics developed a wound complication, compared with 24% of patients who received placebo, a statistically and clinically significant difference.
Details of the study
Eighty-three women received placebo, and 64 received a single IV dose of either cefotetan (1 g) or cefoxitin (1 g) before their perineal laceration was repaired. Patients who were allergic to penicillin received clindamycin (900 mg). The primary endpoints of the study were gross disruption of the wound or purulent drainage from the wound site 2 weeks after delivery.
Forty patients (27%) did not return for their post-partum appointment. Of the remaining patients, four of 49 (8%) who received antibiotics developed a wound complication, compared with 14 of 58 (24%) of those who received placebo (p=.037).
Sequelae of major perineal laceration can be severe
Major perineal laceration occurs in approximately 2% to 20% of vaginal deliveries in the United States. The principal risk factors for third- and fourth-degree lacerations are nulliparity, midline episiotomy, and operative vaginal delivery, especially forceps extraction. Both types of laceration may lead to serious morbidity, such as prolonged pain, fecal incontinence, and perineal infection, including necrotizing fasciitis. These infections typically are polymicrobial, mixed aerobic–anaerobic. Moreover, fourth-degree lacerations may result in rectovaginal fistula if they are not repaired properly. This complication causes considerable debility and major social inconvenience for the patient.
Although the sample size was relatively small, this study clearly demonstrated that a single dose of extended-spectrum cephalosporin (cefotetan or cefoxitin) was highly effective in reducing the rate of perineal infection and perineal wound disruption. Whether a more limited-spectrum agent such as cefazolin would be as effective is not certain.
I strongly recommend routine antibiotic prophylaxis for any patient who sustains a third- or fourth-degree perineal laceration.
If the patient has a mild allergy to penicillin (morbilliform rash), I would administer cefotetan because it is less expensive than cefoxitin. If the patient has a severe reaction to penicillin (urticaria, anaphylaxis), I would administer both clindamycin and gentamicin in order to ensure adequate coverage of the multiple organisms likely to cause soft-tissue infection of the genital tract.
Clindamycin alone covers only aerobic gram-positive cocci and offers no protection against the coliform organisms that are so prevalent in perineal wound infection.8
Diarrhea linked to Clostridium difficile emerges as a potent threat to pregnant women
Rouphael NG, O’Donnell JA, Bhatnagar J, et al. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198:635.e1–635.e6 [Classification of evidence – Level III].
This report describes 10 cases of severe Clostridium difficile-associated diarrhea (CDAD) in pregnant women during 2005 and 2006. CDAD usually affects elderly debilitated patients in hospitals and nursing homes. This report is of great concern because the affected patients were otherwise healthy, young, pregnant women. The observations are even more alarming because the mortality rate in this small series was 30% for both mothers and babies.
Details of the series
The women developed signs of severe CDAD 3 to 60 days after receiving antibiotics; the median was 5 days. CDAD was considered severe if the patient required hospitalization, ICU admission, or colectomy, or if she died. These cases occurred in California, Georgia, Oklahoma, and Pennsylvania and were reported to the Centers for Disease Control and Prevention (CDC).
Six women became ill before delivery, and four developed symptoms postpartum. The most common manifestations of infection were diarrhea, abdominal pain and distention, and fever. The peripheral white blood cell count was in the range of 11–72 × 103/μL. In nine patients, the diagnosis was confirmed by a positive test for toxin A/B; seven of these patients also had visible pseudomembranes in the colon. One patient had a positive stool sample for C difficile ( FIGURE 2 ).
Six patients required admission to the ICU. Six developed toxic megacolon, and five required subtotal colectomy. Three had sepsis; three had acute renal failure; two had disseminated intravascular coagulation. Three patients died, and three had stillbirths. Two patients relapsed following treatment.
One patient had no treatment and died. The others received either metronidazole or vancomycin or a combination of the two. One of the patients who died received metronidazole, vancomycin, and cholestyramine.
FIGURE 2 Clostridium difficile
C difficile is a spore-forming, gram-positive anaerobic bacillus that is a common cause of antibiotic-associated diarrhea.
An epidemic strain appears
The incidence of C difficile infection in acute care US hospitals has increased to 84 for every 100,000 patients in recent years, about three times the rate of 31 for every 100,000 that was reported in 1996, as the authors note. Many of the most severe cases of CDAD are caused by a new epidemic strain of bacteria, termed North American Pulsed Field type 1 (NAP1) and PCR ribotype 027.
This new strain is characterized by three key virulence factors:
- increased production of toxins A and B
- resistance to fluoroquinolones
- production of binary toxin.
Toxins A and B bind to the surface of intestinal epithelial cells, stimulate tissue injury and inflammation, and, ultimately, lead to cell death. Binary toxin appears to act synergistically with toxins A and B to cause severe colitis.9
Metronidazole is no longer the treatment of choice for severe CDAD
Before 2000, treatment of CDAD with vancomycin or metronidazole was 97% to 98% effective.9 In recent years, however, a failure rate as high as 26% has been reported among patients who are treated with metronidazole.10 One prospective, randomized clinical trial demonstrated that, in patients who had severe CDAD, vancomycin, 125 mg four times daily, was superior to metronidazole, 250 mg four times daily (97% success rate vs 76%; p=.02).11 The efficacy of the two drugs was comparable in treating milder cases of CDAD (98% for vancomycin, 90% for metronidazole; p=.36).
The clinical implications of this case series and the reports cited above are clear: When we administer broad-spectrum antibiotics to our patients, we must be ever watchful for signs of toxicity. If the patient develops diarrhea, the offending drug should be discontinued. If the diarrhea does not promptly resolve, tests to isolate C difficile in the stool and identify toxins unique to this organism are advised.
In addition, anoscopy or sigmoidoscopy should be performed to assess the patient for pseudomembranes.
If mild CDAD is confirmed, the patient may be treated with vancomycin or metronidazole. If severe CDAD is identified, vancomycin should be administered, and the patient should be transferred to the ICU for close monitoring and supportive care.
1. Dekker CL, Arvin AM. One step closer to a CMV vaccine. N Engl J Med. 2009;360:1250-1252.
2. Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1402-1404.
3. Nigro G, Adler SP, LaTorre RL, Best AM. Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362.
4. Duff P, Barth WH, Jr, Post MD. Case records of the Massachusetts General Hospital. Case 4-2009: A 39-year-old pregnant woman with fever after a trip to Africa. N Engl J Med. 2009;360:508-516.
5. Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost-effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157(4 Pt 1):794-798.
6. Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
7. Tita ATN, Rouse DJ, Blackwell S, Saade GR, Spong CY, Andrews WW. Emerging concepts in antibiotic prophylaxis for cesarean delivery: a systematic review. Obstet Gynecol. 2009;113:675-682.
8. Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
9. Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932-1940.
10. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
11. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
1. Dekker CL, Arvin AM. One step closer to a CMV vaccine. N Engl J Med. 2009;360:1250-1252.
2. Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1402-1404.
3. Nigro G, Adler SP, LaTorre RL, Best AM. Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362.
4. Duff P, Barth WH, Jr, Post MD. Case records of the Massachusetts General Hospital. Case 4-2009: A 39-year-old pregnant woman with fever after a trip to Africa. N Engl J Med. 2009;360:508-516.
5. Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost-effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157(4 Pt 1):794-798.
6. Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
7. Tita ATN, Rouse DJ, Blackwell S, Saade GR, Spong CY, Andrews WW. Emerging concepts in antibiotic prophylaxis for cesarean delivery: a systematic review. Obstet Gynecol. 2009;113:675-682.
8. Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
9. Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932-1940.
10. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
11. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
Does your OB patient have a psychiatric complaint? And can you manage it?
There’s a full moon tonight—and you’re the obstetrician on call. Not that you should expect any more funny business than usual. Despite stories of werewolves and other deviants coming out of the woodwork, there is no “full moon effect”—at least not one that can be documented. Nevertheless, chances are good that you will encounter at least one of the following psychiatric challenges as you end your day in the clinic and move on to an extended vigil:
- postpartum depression
- leaving against medical advice
- agitation
- antenatal illicit drug use
- denial or concealment of pregnancy.
In this article, we describe the management of these challenges and make recommendations to help increase your comfort level with patients who exhibit psychiatric problems. In some situations, our suggestions may help you manage the problem without a psychiatric consult.
Postpartum depression
CASE 1: Is it just the blues?
It is the end of your day in the clinic, and your last patient is a 30-year-old G3P3 who is 6 weeks postpartum. She describes repeated tearful episodes over the course of several weeks, decreased concentration, and poor appetite. She feels guilty because she is tired all the time and not bonding with her baby. She denies having suicidal or homicidal thoughts, or any hallucinations. She had expected her energy to return to normal over the first few postpartum weeks, but it has not. She is worried because she will soon be returning to work as a medical resident.
Does this patient have postpartum depression? Or is it another condition with overlapping symptoms?
If a mother tells you that she is suicidal or having thoughts of harming her child or others, she should be sent immediately to the nearest emergency department for psychiatric evaluation. Short of such a dramatic situation, how do you know when you should manage a patient’s depression on your own and when she should see a psychiatrist? Thorough assessment is the key.
Don’t mistake transient feelings for depression
Transient feelings of sadness, bereavement, and grief are not the same as depression, which must last 2 weeks or longer to confirm the diagnosis.
A quick mnemonic for symptoms of depression is SIG: E CAPS (as if writing a prescription for energy capsules) (TABLE 1).1 This mnemonic helps remind you to assess the patient’s sleep, interest, guilt, energy, concentration, appetite, and psychomotor function, as well as identify any suicidal ideation.
It is important to assess a woman’s sleep and appetite in addition to mood. However, differences may be difficult to ascertain due to normal changes in the postpartum period. One useful question is whether the mother is able to sleep when the baby sleeps. If she isn’t, this wakefulness may be a symptom of depression.
The Edinburgh Postnatal Depression Scale is an easy, 10-question screening tool that is completed by the patient; it can be used both during pregnancy and postpartum. It is available on the Web at a number of sites, including www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf.
TABLE 1
SIG: E CAPS—a mnemonic to assess for depression1
| Decreased (sometimes increased) Sleep |
| Decreased Interests |
| Feelings of Guilt |
| Decreased Energy |
| Decreased Concentration |
| Decreased (sometimes increased) Appetite |
| Psychomotor retardation, slowness |
| Suicidal thoughts, plans, or intent |
Differential diagnosis
Besides postpartum depression, the differential diagnosis for altered mood in the postpartum period includes several entities.
Baby blues generally occurs quite soon after birth and resolves within 2 weeks. It involves crying, emotional lability, and irritability.2 It occurs in around 50% to 75% of new mothers (compared with postpartum depression, which affects 10% to 20%).3-5
Postpartum psychosis often involves the onset of psychotic symptoms within 1 week after delivery. The patient may exhibit both mood symptoms and psychosis. For example, she may believe that the baby is not hers or hear voices commanding her to kill the baby or warning her not to trust her healthcare providers.6 Postpartum psychosis has a prevalence of about 0.2%.3-6
This psychosis can be organic in nature or can arise from a preexisting mood disorder or schizophrenia. Because treatment varies, depending on the cause, a thorough medical workup is needed.
Bipolar disorder may present as depression, but it also consists of manic periods of elevated, expansive, or irritable mood that last several days to weeks. Many symptoms appear to be the opposite of depression, such as increased energy and elevated self-esteem.7,8
It is important to consider bipolar disorder in the differential diagnosis. If a woman who has unrecognized bipolar disorder is given an antidepressant, a manic state could be precipitated. Women who have bipolar disorder require different drugs than women who have depression only, and they should be evaluated by a psychiatrist, at least initially.
Start treatment as soon as possible
Once you confirm that the patient has postpartum depression—and not another psychiatric disorder—prescribing an antidepressant may be the next step. Keep in mind that these drugs take several weeks before their benefits are felt. Therefore, it is best to start an antidepressant before depression becomes severe. The mother may also benefit from psychotherapy.
The selective serotonin reuptake inhibitor sertraline (Zoloft) is a reasonable first choice in pregnancy and lactation when the depression is of new onset.9,10 Start it gradually (e.g., 25 mg for sertraline, which can cause nausea if it is initiated too rapidly) and titrate it over time, if necessary. When there is comorbid anxiety, it sometimes is helpful to prescribe low dosages of lorazepam (Ativan, Temesta) on an as-needed basis, while the patient is waiting for the antidepressant to “kick in.” Also consider follow-up—do you plan to follow her frequently or refer her to psychiatry?
Adjunctive or alternative options include psychotherapy, group therapy, and music therapy. Referral to a psychiatrist is warranted if the patient does not respond to the initial antidepressant agent.
Also be aware that untreated depression can become so severe that a woman can begin to experience psychosis, warranting rapid referral. Also refer any woman who reports a complex history of previous depression—unless the previous episode was easily controlled with a medicine safe for use during pregnancy and lactation.
If the patient is not lactating, a greater range of agents may be considered. (A full discussion of the risks and benefits of antidepressant use in pregnancy is outside the scope of this article. The interested reader is referred to an article on the subject by Wisner and colleagues.11)
CASE 1 RESOLVED
A comprehensive discussion with the mother reveals that she is suffering from postpartum depression. No history of bipolar or psychotic symptoms is discovered. After discussing treatment options, you prescribe sertraline. Over the next 2 months, the patient’s symptoms improve, and she bonds with her infant and successfully returns to work. She is also referred to a psychologist to work through some underlying issues.
Leaving against medical advice
CASE 2: Patient threatens to leave the hospital
At midnight, you are paged to attend to a 32-year-old G1P0 at 27 weeks’ gestation who is threatening to leave against medical advice. She was admitted earlier in the day with uncontrolled gestational diabetes and is refusing her insulin.
How do you respond?
Use the relationship that you have established with this patient to the best of your ability. Make sure that you have explained fully, and in language she can easily comprehend, the reasons she needs to stay for treatment.
Don’t overlook the obvious, either: Why does she want to leave? Sometimes the reason makes sense (e.g., one mother wanted to leave to protect her daughter from an abusive husband). Other reasons may be related to psychosis, addiction, lack of sleep in the hospital, or a desire to smoke, drink, or use drugs. Can you convince her to postpone her decision until morning, when her physician will be available?
It is important to document in the medical record your explanations and her reasoning. Can she coherently verbalize an understanding of the consequences of her decision to leave, including the risks and implications to herself and the fetus?12 Can she describe alternatives and the reasoning against them?
If she is able to do these things, and you find her thought processing and reasoning to be lucid, then she may have the capacity to leave against medical advice. Keep in mind that rational persons do have the right, constitutionally, to refuse treatment, even if doing so will lead to morbidity. (A Jehovah’s Witness who refuses treatment is the typical example.12) Contact the hospital’s attorney—tonight—and document that you did so. The attorney may recommend that the patient sign a letter stating that she recognizes the maternal and fetal risks of leaving.
Sometimes a patient must be held against her will
Some mothers lack the capacity to refuse treatment. They may be unable to verbalize an understanding of the situation and its risks. Their reasoning may be abnormal, with disorganized or delusional thinking, or both. The patient may be tangential or talk “in circles” rather than answer your questions.
Try to ascertain whether mood symptoms are contributing to her irrational thinking. For example, is her rationale for going home—“just to be with my husband because I don’t want to be alone”—due to her depression, despite the risk to herself and the fetus? Try to be flexible and creative. For example, you could call the husband and ask him to come to the hospital to sit with the patient.
Is the patient psychotic? For example, does she believe she has to leave now because the staff has been replaced by aliens who plan to kill her and her fetus? If so, you have the authority to continue her hospitalization—but contact the psychiatry department for medication recommendations. A urine toxicology screen would also be prudent.
If the patient is irrational and lacks the capacity to decide whether to stay or leave, document your conversation with her, as well as the reasoning behind your decision to intervene further. Other steps include:
- contacting the hospital’s attorney
- completing an emergency detention form
- calling security
- ensuring that the patient’s environment is safe for her and others (TABLE 2).13
TABLE 2
5 steps to sound management of a patient who wants to leave
against medical advice
| 1. Ask the patient why she wants to leave now |
| 2. Inform her of the risks to herself and to her fetus |
| 3. Ask her to verbalize the risks to herself and to her fetus |
4. Determine whether the patient’s request is rational
|
| 5. Document the medical explanation and reasoning in the chart |
CASE 2 RESOLVED
After building some rapport with the patient, you ask why she wants to leave right now. During this conversation, the patient reveals that she has not slept in three nights, and says she believes that the insulin is keeping her up. You are able to assure her that this is not the case and offer her something to help her sleep. She decides not to leave against medical advice.
Unexplained agitation
CASE 3: Patient becomes abusive
At 1 AM, you are called to the seventh floor, where a 20-year-old G2P1 at 26 weeks’ gestation is yelling at staff and hitting anyone who comes near. She was admitted earlier in the day for management of threatened abortion and a dilated cervix. She has no documented psychiatric history, but is flushed, disheveled, and hostile, accusing the staff of sabotaging her life, and is seen picking at imaginary things. You notify psychiatry, but no one is available.
What do you do?
Determining the origin of these symptoms will help determine the appropriate course of action. Among the possibilities are:
- drug intoxication or withdrawal
- delirium
- psychosis
- a chronic problem such as a personality disorder (TABLE 3).
Psychosis means that a patient is out of touch with reality. A psychotic patient may experience delusions, auditory and visual hallucinations, and gross disorganization. Brief psychotic episodes usually last for 1 day to 1 month, with eventual recovery to premorbid functioning.7
Substances such as medications or illicit drugs also can induce psychosis. Major offenders include steroids and narcotic agents. Alternatively, sudden withdrawal of illicit substances (due to hospitalization) could manifest as delirium or psychosis.
Personality disorder. If the patient’s behavior is not new but a long-term problem, she may have a chronic personality disorder rather than acute illness. Personality problems involve pervasive response patterns and dysfunctional coping patterns that affect daily life. For example, a patient who has borderline personality disorder may have emotional instability presenting as intense episodic dysphoria or irritability. Such patients have a hard time empathizing with others, poor impulse control, and a desire for instant gratification. They may also misinterpret the behavior of other people and take offense easily as a result. Lacking stress-management skills, they regress to unhealthy defense mechanisms such as acting out, complaining, passive aggressiveness, and splitting of the staff (thinking that people are all good or all bad).
Dementia may also be on the differential diagnosis, but this chronic condition is unlikely in such a young patient (unless she were in the late stages of HIV/AIDS, for example). Dementia has a gradual onset and is irreversible.
TABLE 3
Some causes of agitation
Delirium
|
Psychosis
|
Dementia
|
Personality dysfunction
|
Workup for the agitated patient
Assess vital signs and basic laboratory studies, particularly a complete blood count, thyroid testing, metabolic screens, glucose, serum chemistry panel, and urine toxicology, to rule out causes of delirium and detect any substances the patient may have used. Also consider that the patient may have initiated a new medication recently.
Imaging of the brain or chest or electroencephalography may be necessary, such as in the setting of infection or concerns regarding seizure activity.
Gather collateral information about the patient from her relatives, friends, and the support staff. Search her chart for recent contacts. Did she have a visitor or phone call that may have upset her? Explore whether she is having problems with her partner or family and friends. Also confirm that her agitated behavior is an acute change.
Investigate the patient’s paranoia. Why does she believe that the staff is against her? Does she believe they are trying to harm, kill, or poison her? Assess her reasoning to determine whether her behavior is psychotic or a personality problem.
Ask her about hallucinations, keeping in mind that hallucinations are different from illusions, in which a patient misinterprets what she sees. What are the voices saying to her?
Also ask about any suicidal or homicidal commands. If she acknowledges that she is hearing them, get a sitter for her immediately and make her environment safe so that she is unable to harm herself. Then contact the psychiatry department again.
How to intervene
Talk gently and quietly in an attempt to calm the situation. Try to make yourself “small”: Stand back and stay at the patient’s eye level, not in her personal space or towering over her.
Also, protect yourself. Don’t challenge her complaints immediately or you will alienate her. Medically evaluate and treat the cause of her agitation, and, if medications are necessary for psychosis or sedation, contact psychiatry for assistance.
CASE 3 RESOLVED
The patient does not respond to your attempts to reason and refuses to allow the nurses to check her vital signs. Security is called to stand by while her vital signs are reassessed. The nurses inform you that the patient’s family is in the waiting room. Though you find no documented history of substance use or abnormal labs, the family reports that the patient had a history of alcohol abuse but quit drinking about 3 days earlier. They also report that, before she quit, she drank approximately 25 oz of vodka and a sixpack of beer nightly. They deny knowledge of any other illicit drug use. Because her vital signs suggest alcohol withdrawal, you offer oral lorazepam and treat her according to the hospital’s alcohol withdrawal protocol. She recovers without any further complications and is referred to the chemical dependency service for evaluation.
Drug abuse during pregnancy
CASE 4: Patient skips prenatal care
It is 2 AM, and you are about to get some rest when you are paged by the emergency room about a 26-year-old G5P4, who is in active labor with no dates. You check the database and discover that her previous pregnancies were complicated by chronic substance abuse.
How do you respond?
You might feel frustrated and angry with this patient. Considering that you have not met her, these feelings would be based on previous contacts with patients who had a similar history. This is countertransference. We all experience it. It can be helpful or harmful, but you cannot control it unless you are aware of it. Pay attention to the anger, happiness, or pride that a patient triggers in you, and acknowledge that it is your issue.
Your frustration may also stem from personal feelings about mothers who repeatedly expose their fetuses to drugs and neglect prenatal care, as well as anxiety about what you are legally and ethically bound to do.
In Ferguson v City of Charleston, the Supreme Court found that drug testing of a pregnant woman for the purpose of criminal prosecution is a violation of Fourth Amendment rights.14 However, there have been cases in which a state prosecuted a woman for using an illegal substance during pregnancy.
These cases involved:
- child neglect15
- delivery of a stillborn fetus whose autopsy revealed traces of cocaine by-products16
- reckless endangerment after a newborn tested positive for cocaine.17
What is drug abuse?
According to the 4th edition of the Diagnostic and Statistical Manual of Mental Health Disorders, it is a “maladaptive pattern of substance use manifested by recurrent and significant adverse consequences related to the repeated use of substances” within a 12-month period, or persistently.7 To meet criteria for substance dependence, in addition to the criteria just mentioned, the individual must be tolerant to the drug, experience withdrawal when the drug is cut back or stopped, continue to use the drug despite knowledge of its dangers, or all of the above. Mothers who match this description often lose custody of their child—sometimes to foster care, sometimes to other family members.20,21
Some states use positive serum and urine toxicology as evidence to remove a child from the mother’s custody.19
It is important to attempt to build a doctor–patient relationship. You cannot solve the patient’s substance dependence problem in one night, but you can refer her to drug treatment. A urine toxicology screen can help you and the pediatricians know what the patient has been exposed to (TABLE 4).
TABLE 4
Commonly abused drugs and their potential effects
| Drug | Withdrawal effects on mother | Drug effects on fetus or infant |
|---|---|---|
| Alcohol | Sweating, increased heart rate, hand tremor, nausea/vomiting, physical agitation, hallucination (tactile, visual, auditory), illusions, grand mal seizures25 | Fetal alcohol syndrome. Withdrawal symptoms similar to those of the mother |
| Cocaine | Agitation, anxiety, anger, nausea/vomiting, muscle pain, disturbed sleep, depression, intense cravings for the drug, irritability25 | Risk of abruptio placenta, small-for-gestational-age infant, microcephaly, congenital anomaly (cardiac and genitourinary abnormality, necrotizing enterocolitis), central nervous system stroke or hemorrhage. Withdrawal effects include hypertonia, jitteriness, and seizures.26 |
| Crystal methamphetamine | Anxiety, psychotic reaction, intense hunger, irritability, restlessness, fatigue, depression, sleep disturbance, cravings25 | Premature birth, abruptio placenta, small-for-gestational age, hypertonia, tremors, poor feeding, abnormal sleep patterns26 |
| Marijuana | Irritability, anxiety, physical tension, decreased appetite and mood25 | Irritability, increase in bodily motility, tremors, startles, poor habituation to visual stimuli, abnormal reflexes, symptoms similar to mild withdrawal27 |
| Opioids (Heroin, methadone) | Dilated pupils, watery eyes, runny nose, diarrhea, nausea/vomiting, muscle cramps, piloerection, chills or profuse sweating, yawning, loss of appetite, tremor, jitteriness, panic, insomnia, stomach ache, irritability26 | Risk of prematurity, small-for-gestational age, adult withdrawal symptoms, irritability, hypertonia, wakefulness, jitteriness, diarrhea, increased hiccups, yawning and sneezing, excessive sucking and seizures. Withdrawal effects occur earlier in heroin-exposed babies than in methadone-exposed infants.26 |
CASE 4 RESOLVED
After delivery, a test indicates that the newborn has been exposed to cocaine. The mother admits to cocaine use during pregnancy. She says she did not seek prenatal care because she was afraid of being prosecuted and sent to jail. A social work consult is requested, and the mother is referred to a substance abuse treatment program. State law requires the case to be reported to child protective services. Upon hospital discharge, the newborn is initially placed with the paternal grandmother.
Denial of pregnancy
CASE 5: Patient’s labor takes her by surprise
The night is nearing its end, but it isn’t over yet. At 5:30 AM, you are called to a precipitous delivery involving a 17-year-old who has had no prenatal care. She denies knowing that she was pregnant, and says she thought her labor pains were a bowel movement. Her parents were similarly unaware that their daughter was pregnant, and are threatening to disown her.
How do you defuse the situation?
In a study of women who denied or concealed pregnancy, patients presented to the hospital for various reasons.22 For example, one woman went to the ER because she was seizing and her workup revealed that she had eclampsia. A number of women did not even recognize when they were in labor. The infants born to these women are at risk for a poor neonatal outcome.21,22
How can psychiatry help in such a case? By determining whether the patient denied her pregnancy—even to herself—or actively concealed it from others. Obviously, these circumstances have differing implications.
Denial is not a simple entity. It may involve a psychotic schizophrenic woman who is out of touch with the reality of her pregnancy; a woman who “affectively” denies her pregnancy, keeping the significance of her condition from herself and behaving as though she is not gravid (perhaps because she plans to give the baby up for adoption); or a woman who has pervasive denial and does not know that she is pregnant.22,23 In contrast, a woman who conceals her pregnancy is quite aware that she is gravid but consciously hides the gestation from others, begging the question of what she had planned for the future.22
Psychological issues abound, and may include a history of sexual and psychological trauma, an attempt to avoid religious prohibitions against unwed intercourse, anger at the father of the infant, and even homicidal urges toward the baby.24 There may be more going on under the surface than “only” a failure to recognize the pregnancy, and the patient may need further mental health treatment.
Consider how well this young woman can be a mother. When she did not even recognize that she was pregnant for 9 months, how well will she be able to attend to her baby’s needs? Psychiatry can evaluate the patient to help determine her capacity for parenting and whether child protective services should be alerted. Of additional concern is the distress of the patient’s parents. Family support will be extremely important.
Be sure to conduct thorough contraceptive education and planning at the time of discharge because this patient is at risk for future denied or concealed pregnancies.22
CASE 5 RESOLVED
The patient is seen by psychiatry. She has no major mental illness, but her denial appears to be related to problems with her boyfriend, her attempts to be the perfect daughter, and fear of being disowned. After the initial shock, the patient’s parents become more supportive and begin to bond with their new grandchild. The new mom is educated about birth control and agrees to follow up with a counselor and take parenting classes. The baby is discharged to his mother and grandparents.
1. What does SIGECAPS stand for? Available at: www.acronymfinder.com/Sleep,-Interest,-Guilt,-Energy,-Concentration,-Appetite,-Psychomotor,-Suicidal-(mnemonic-for-characteristics-of-major-depression)-(SIGECAPS).html. Accessed May 18, 2009.
2. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry. 2003;64:1284-1292.
3. Grigoriadis S, Romans S. Postpartum psychiatric disorders: what do we know and where do we go? Curr Psychiatry Rev. 2006;2:151-158.
4. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23:188-193.
5. Sadock BJ, Sadock VA. Psychiatry and reproductive medicine. In: Kaplan HI, Sadock BJ, eds. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. New York: Lippincott Williams & Wilkins; 2003:868-878.
6. Friedman SH, Resnick PJ, Rosenthal M. Postpartum psychosis: strategies to protect infant and mother from harm. Curr Psychiatry. 2009;8(2):40-46.
7. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Text revision. Washington, DC: American Psychiatric; 2000.
8. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
9. Gentile S. Use of contemporary antidepressants during breastfeeding: a proposal for a specific safety index. Drug Saf. 2007;30:107-121.
10. Rahimi R, Nikfar S, Abdollahi M. Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials. Reprod Toxicol. 2006;22:571-575.
11. Wisner KL, Zarin DA, Holmboe ES, et al. Riskbenefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157:1933.-
12. Roberts LW, Hoop JG. Professionalism and ethics. Washington, DC: American Psychiatric Publishing; 2008.
13. Ohio Criteria for Commitment, Section 5122.01.
14. Harris LH, Paltrow L. The status of pregnant women and fetuses in US criminal law. JAMA. 2003;289:1697-1699.
15. Drugs, Police & The Law: Whitner vs. the State of South Carolina. June 23, 2004. Available at: www.drugpolicy.org/law/womenpregnan/whitnervsth_/. Accessed April 30, 2009.
16. Gray P. Prosecution of prenatal substance abuse allowed to stand in McKnight case. Available at: www.law.uh.edu/healthlaw/perspectives/reproductive/040202prosecution.pdf. Accessed April 30, 2009.
17. Parks AW. New mothers fight endangerment convictions. Public Justice Center. April 10, 2006. Available at: www.publicjustice.org/news/index.cfm?newsid=106. Accessed April 30, 2009.
18. Parks AW. Using cocaine while pregnant is not reckless endangerment. The Daily Record. August 4, 2006. Available at: www.publicjustice.org/pdf/Cruzdailyrecord.pdf. Accessed April 30, 2009.
19. American Pregnancy Association. Using illegal street drugs during pregnancy. May 2007. Available at: www.americanpregnancy.org/pregnancyhealth/illegaldrugs.html. Accessed April 30, 2009.
20. Neuspiel DR, Zingman TM, Templeton VH, et al. Custody of cocaine-exposed newborns: determinants of discharge decisions. Am J Public Health. 1993;83:1726-1729.
21. Friedman SH, Heneghan A, Rosenthal M. Disposition and health outcomes among infants born to mothers with no prenatal care. Child Abuse Negl. 2009;33:116-122.
22. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48:117-122.
23. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. Washington, DC: American Psychiatric Publishing; 2003.
24. Bonnet C. Adoption at birth: prevention against abandonment or neonaticide. Child Abuse Negl. 1993;17:501-513.
25. Heroin withdrawal. Available at: www.addictionwithdrawal.com/heroin.htm. Accessed April 29, 2009.
26. Kwong TC, Ryan RM. Detection of intrauterine illicit drug exposure by newborn drug testing. Clin Chem. 1997;43:235-242.
27. Bada HS, Reynolds EW, Hansen WF. Marijuana use, adolescent pregnancy, and alteration in new born behavior: how complex can it get? J Pediatr. 2006;149:742.-
There’s a full moon tonight—and you’re the obstetrician on call. Not that you should expect any more funny business than usual. Despite stories of werewolves and other deviants coming out of the woodwork, there is no “full moon effect”—at least not one that can be documented. Nevertheless, chances are good that you will encounter at least one of the following psychiatric challenges as you end your day in the clinic and move on to an extended vigil:
- postpartum depression
- leaving against medical advice
- agitation
- antenatal illicit drug use
- denial or concealment of pregnancy.
In this article, we describe the management of these challenges and make recommendations to help increase your comfort level with patients who exhibit psychiatric problems. In some situations, our suggestions may help you manage the problem without a psychiatric consult.
Postpartum depression
CASE 1: Is it just the blues?
It is the end of your day in the clinic, and your last patient is a 30-year-old G3P3 who is 6 weeks postpartum. She describes repeated tearful episodes over the course of several weeks, decreased concentration, and poor appetite. She feels guilty because she is tired all the time and not bonding with her baby. She denies having suicidal or homicidal thoughts, or any hallucinations. She had expected her energy to return to normal over the first few postpartum weeks, but it has not. She is worried because she will soon be returning to work as a medical resident.
Does this patient have postpartum depression? Or is it another condition with overlapping symptoms?
If a mother tells you that she is suicidal or having thoughts of harming her child or others, she should be sent immediately to the nearest emergency department for psychiatric evaluation. Short of such a dramatic situation, how do you know when you should manage a patient’s depression on your own and when she should see a psychiatrist? Thorough assessment is the key.
Don’t mistake transient feelings for depression
Transient feelings of sadness, bereavement, and grief are not the same as depression, which must last 2 weeks or longer to confirm the diagnosis.
A quick mnemonic for symptoms of depression is SIG: E CAPS (as if writing a prescription for energy capsules) (TABLE 1).1 This mnemonic helps remind you to assess the patient’s sleep, interest, guilt, energy, concentration, appetite, and psychomotor function, as well as identify any suicidal ideation.
It is important to assess a woman’s sleep and appetite in addition to mood. However, differences may be difficult to ascertain due to normal changes in the postpartum period. One useful question is whether the mother is able to sleep when the baby sleeps. If she isn’t, this wakefulness may be a symptom of depression.
The Edinburgh Postnatal Depression Scale is an easy, 10-question screening tool that is completed by the patient; it can be used both during pregnancy and postpartum. It is available on the Web at a number of sites, including www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf.
TABLE 1
SIG: E CAPS—a mnemonic to assess for depression1
| Decreased (sometimes increased) Sleep |
| Decreased Interests |
| Feelings of Guilt |
| Decreased Energy |
| Decreased Concentration |
| Decreased (sometimes increased) Appetite |
| Psychomotor retardation, slowness |
| Suicidal thoughts, plans, or intent |
Differential diagnosis
Besides postpartum depression, the differential diagnosis for altered mood in the postpartum period includes several entities.
Baby blues generally occurs quite soon after birth and resolves within 2 weeks. It involves crying, emotional lability, and irritability.2 It occurs in around 50% to 75% of new mothers (compared with postpartum depression, which affects 10% to 20%).3-5
Postpartum psychosis often involves the onset of psychotic symptoms within 1 week after delivery. The patient may exhibit both mood symptoms and psychosis. For example, she may believe that the baby is not hers or hear voices commanding her to kill the baby or warning her not to trust her healthcare providers.6 Postpartum psychosis has a prevalence of about 0.2%.3-6
This psychosis can be organic in nature or can arise from a preexisting mood disorder or schizophrenia. Because treatment varies, depending on the cause, a thorough medical workup is needed.
Bipolar disorder may present as depression, but it also consists of manic periods of elevated, expansive, or irritable mood that last several days to weeks. Many symptoms appear to be the opposite of depression, such as increased energy and elevated self-esteem.7,8
It is important to consider bipolar disorder in the differential diagnosis. If a woman who has unrecognized bipolar disorder is given an antidepressant, a manic state could be precipitated. Women who have bipolar disorder require different drugs than women who have depression only, and they should be evaluated by a psychiatrist, at least initially.
Start treatment as soon as possible
Once you confirm that the patient has postpartum depression—and not another psychiatric disorder—prescribing an antidepressant may be the next step. Keep in mind that these drugs take several weeks before their benefits are felt. Therefore, it is best to start an antidepressant before depression becomes severe. The mother may also benefit from psychotherapy.
The selective serotonin reuptake inhibitor sertraline (Zoloft) is a reasonable first choice in pregnancy and lactation when the depression is of new onset.9,10 Start it gradually (e.g., 25 mg for sertraline, which can cause nausea if it is initiated too rapidly) and titrate it over time, if necessary. When there is comorbid anxiety, it sometimes is helpful to prescribe low dosages of lorazepam (Ativan, Temesta) on an as-needed basis, while the patient is waiting for the antidepressant to “kick in.” Also consider follow-up—do you plan to follow her frequently or refer her to psychiatry?
Adjunctive or alternative options include psychotherapy, group therapy, and music therapy. Referral to a psychiatrist is warranted if the patient does not respond to the initial antidepressant agent.
Also be aware that untreated depression can become so severe that a woman can begin to experience psychosis, warranting rapid referral. Also refer any woman who reports a complex history of previous depression—unless the previous episode was easily controlled with a medicine safe for use during pregnancy and lactation.
If the patient is not lactating, a greater range of agents may be considered. (A full discussion of the risks and benefits of antidepressant use in pregnancy is outside the scope of this article. The interested reader is referred to an article on the subject by Wisner and colleagues.11)
CASE 1 RESOLVED
A comprehensive discussion with the mother reveals that she is suffering from postpartum depression. No history of bipolar or psychotic symptoms is discovered. After discussing treatment options, you prescribe sertraline. Over the next 2 months, the patient’s symptoms improve, and she bonds with her infant and successfully returns to work. She is also referred to a psychologist to work through some underlying issues.
Leaving against medical advice
CASE 2: Patient threatens to leave the hospital
At midnight, you are paged to attend to a 32-year-old G1P0 at 27 weeks’ gestation who is threatening to leave against medical advice. She was admitted earlier in the day with uncontrolled gestational diabetes and is refusing her insulin.
How do you respond?
Use the relationship that you have established with this patient to the best of your ability. Make sure that you have explained fully, and in language she can easily comprehend, the reasons she needs to stay for treatment.
Don’t overlook the obvious, either: Why does she want to leave? Sometimes the reason makes sense (e.g., one mother wanted to leave to protect her daughter from an abusive husband). Other reasons may be related to psychosis, addiction, lack of sleep in the hospital, or a desire to smoke, drink, or use drugs. Can you convince her to postpone her decision until morning, when her physician will be available?
It is important to document in the medical record your explanations and her reasoning. Can she coherently verbalize an understanding of the consequences of her decision to leave, including the risks and implications to herself and the fetus?12 Can she describe alternatives and the reasoning against them?
If she is able to do these things, and you find her thought processing and reasoning to be lucid, then she may have the capacity to leave against medical advice. Keep in mind that rational persons do have the right, constitutionally, to refuse treatment, even if doing so will lead to morbidity. (A Jehovah’s Witness who refuses treatment is the typical example.12) Contact the hospital’s attorney—tonight—and document that you did so. The attorney may recommend that the patient sign a letter stating that she recognizes the maternal and fetal risks of leaving.
Sometimes a patient must be held against her will
Some mothers lack the capacity to refuse treatment. They may be unable to verbalize an understanding of the situation and its risks. Their reasoning may be abnormal, with disorganized or delusional thinking, or both. The patient may be tangential or talk “in circles” rather than answer your questions.
Try to ascertain whether mood symptoms are contributing to her irrational thinking. For example, is her rationale for going home—“just to be with my husband because I don’t want to be alone”—due to her depression, despite the risk to herself and the fetus? Try to be flexible and creative. For example, you could call the husband and ask him to come to the hospital to sit with the patient.
Is the patient psychotic? For example, does she believe she has to leave now because the staff has been replaced by aliens who plan to kill her and her fetus? If so, you have the authority to continue her hospitalization—but contact the psychiatry department for medication recommendations. A urine toxicology screen would also be prudent.
If the patient is irrational and lacks the capacity to decide whether to stay or leave, document your conversation with her, as well as the reasoning behind your decision to intervene further. Other steps include:
- contacting the hospital’s attorney
- completing an emergency detention form
- calling security
- ensuring that the patient’s environment is safe for her and others (TABLE 2).13
TABLE 2
5 steps to sound management of a patient who wants to leave
against medical advice
| 1. Ask the patient why she wants to leave now |
| 2. Inform her of the risks to herself and to her fetus |
| 3. Ask her to verbalize the risks to herself and to her fetus |
4. Determine whether the patient’s request is rational
|
| 5. Document the medical explanation and reasoning in the chart |
CASE 2 RESOLVED
After building some rapport with the patient, you ask why she wants to leave right now. During this conversation, the patient reveals that she has not slept in three nights, and says she believes that the insulin is keeping her up. You are able to assure her that this is not the case and offer her something to help her sleep. She decides not to leave against medical advice.
Unexplained agitation
CASE 3: Patient becomes abusive
At 1 AM, you are called to the seventh floor, where a 20-year-old G2P1 at 26 weeks’ gestation is yelling at staff and hitting anyone who comes near. She was admitted earlier in the day for management of threatened abortion and a dilated cervix. She has no documented psychiatric history, but is flushed, disheveled, and hostile, accusing the staff of sabotaging her life, and is seen picking at imaginary things. You notify psychiatry, but no one is available.
What do you do?
Determining the origin of these symptoms will help determine the appropriate course of action. Among the possibilities are:
- drug intoxication or withdrawal
- delirium
- psychosis
- a chronic problem such as a personality disorder (TABLE 3).
Psychosis means that a patient is out of touch with reality. A psychotic patient may experience delusions, auditory and visual hallucinations, and gross disorganization. Brief psychotic episodes usually last for 1 day to 1 month, with eventual recovery to premorbid functioning.7
Substances such as medications or illicit drugs also can induce psychosis. Major offenders include steroids and narcotic agents. Alternatively, sudden withdrawal of illicit substances (due to hospitalization) could manifest as delirium or psychosis.
Personality disorder. If the patient’s behavior is not new but a long-term problem, she may have a chronic personality disorder rather than acute illness. Personality problems involve pervasive response patterns and dysfunctional coping patterns that affect daily life. For example, a patient who has borderline personality disorder may have emotional instability presenting as intense episodic dysphoria or irritability. Such patients have a hard time empathizing with others, poor impulse control, and a desire for instant gratification. They may also misinterpret the behavior of other people and take offense easily as a result. Lacking stress-management skills, they regress to unhealthy defense mechanisms such as acting out, complaining, passive aggressiveness, and splitting of the staff (thinking that people are all good or all bad).
Dementia may also be on the differential diagnosis, but this chronic condition is unlikely in such a young patient (unless she were in the late stages of HIV/AIDS, for example). Dementia has a gradual onset and is irreversible.
TABLE 3
Some causes of agitation
Delirium
|
Psychosis
|
Dementia
|
Personality dysfunction
|
Workup for the agitated patient
Assess vital signs and basic laboratory studies, particularly a complete blood count, thyroid testing, metabolic screens, glucose, serum chemistry panel, and urine toxicology, to rule out causes of delirium and detect any substances the patient may have used. Also consider that the patient may have initiated a new medication recently.
Imaging of the brain or chest or electroencephalography may be necessary, such as in the setting of infection or concerns regarding seizure activity.
Gather collateral information about the patient from her relatives, friends, and the support staff. Search her chart for recent contacts. Did she have a visitor or phone call that may have upset her? Explore whether she is having problems with her partner or family and friends. Also confirm that her agitated behavior is an acute change.
Investigate the patient’s paranoia. Why does she believe that the staff is against her? Does she believe they are trying to harm, kill, or poison her? Assess her reasoning to determine whether her behavior is psychotic or a personality problem.
Ask her about hallucinations, keeping in mind that hallucinations are different from illusions, in which a patient misinterprets what she sees. What are the voices saying to her?
Also ask about any suicidal or homicidal commands. If she acknowledges that she is hearing them, get a sitter for her immediately and make her environment safe so that she is unable to harm herself. Then contact the psychiatry department again.
How to intervene
Talk gently and quietly in an attempt to calm the situation. Try to make yourself “small”: Stand back and stay at the patient’s eye level, not in her personal space or towering over her.
Also, protect yourself. Don’t challenge her complaints immediately or you will alienate her. Medically evaluate and treat the cause of her agitation, and, if medications are necessary for psychosis or sedation, contact psychiatry for assistance.
CASE 3 RESOLVED
The patient does not respond to your attempts to reason and refuses to allow the nurses to check her vital signs. Security is called to stand by while her vital signs are reassessed. The nurses inform you that the patient’s family is in the waiting room. Though you find no documented history of substance use or abnormal labs, the family reports that the patient had a history of alcohol abuse but quit drinking about 3 days earlier. They also report that, before she quit, she drank approximately 25 oz of vodka and a sixpack of beer nightly. They deny knowledge of any other illicit drug use. Because her vital signs suggest alcohol withdrawal, you offer oral lorazepam and treat her according to the hospital’s alcohol withdrawal protocol. She recovers without any further complications and is referred to the chemical dependency service for evaluation.
Drug abuse during pregnancy
CASE 4: Patient skips prenatal care
It is 2 AM, and you are about to get some rest when you are paged by the emergency room about a 26-year-old G5P4, who is in active labor with no dates. You check the database and discover that her previous pregnancies were complicated by chronic substance abuse.
How do you respond?
You might feel frustrated and angry with this patient. Considering that you have not met her, these feelings would be based on previous contacts with patients who had a similar history. This is countertransference. We all experience it. It can be helpful or harmful, but you cannot control it unless you are aware of it. Pay attention to the anger, happiness, or pride that a patient triggers in you, and acknowledge that it is your issue.
Your frustration may also stem from personal feelings about mothers who repeatedly expose their fetuses to drugs and neglect prenatal care, as well as anxiety about what you are legally and ethically bound to do.
In Ferguson v City of Charleston, the Supreme Court found that drug testing of a pregnant woman for the purpose of criminal prosecution is a violation of Fourth Amendment rights.14 However, there have been cases in which a state prosecuted a woman for using an illegal substance during pregnancy.
These cases involved:
- child neglect15
- delivery of a stillborn fetus whose autopsy revealed traces of cocaine by-products16
- reckless endangerment after a newborn tested positive for cocaine.17
What is drug abuse?
According to the 4th edition of the Diagnostic and Statistical Manual of Mental Health Disorders, it is a “maladaptive pattern of substance use manifested by recurrent and significant adverse consequences related to the repeated use of substances” within a 12-month period, or persistently.7 To meet criteria for substance dependence, in addition to the criteria just mentioned, the individual must be tolerant to the drug, experience withdrawal when the drug is cut back or stopped, continue to use the drug despite knowledge of its dangers, or all of the above. Mothers who match this description often lose custody of their child—sometimes to foster care, sometimes to other family members.20,21
Some states use positive serum and urine toxicology as evidence to remove a child from the mother’s custody.19
It is important to attempt to build a doctor–patient relationship. You cannot solve the patient’s substance dependence problem in one night, but you can refer her to drug treatment. A urine toxicology screen can help you and the pediatricians know what the patient has been exposed to (TABLE 4).
TABLE 4
Commonly abused drugs and their potential effects
| Drug | Withdrawal effects on mother | Drug effects on fetus or infant |
|---|---|---|
| Alcohol | Sweating, increased heart rate, hand tremor, nausea/vomiting, physical agitation, hallucination (tactile, visual, auditory), illusions, grand mal seizures25 | Fetal alcohol syndrome. Withdrawal symptoms similar to those of the mother |
| Cocaine | Agitation, anxiety, anger, nausea/vomiting, muscle pain, disturbed sleep, depression, intense cravings for the drug, irritability25 | Risk of abruptio placenta, small-for-gestational-age infant, microcephaly, congenital anomaly (cardiac and genitourinary abnormality, necrotizing enterocolitis), central nervous system stroke or hemorrhage. Withdrawal effects include hypertonia, jitteriness, and seizures.26 |
| Crystal methamphetamine | Anxiety, psychotic reaction, intense hunger, irritability, restlessness, fatigue, depression, sleep disturbance, cravings25 | Premature birth, abruptio placenta, small-for-gestational age, hypertonia, tremors, poor feeding, abnormal sleep patterns26 |
| Marijuana | Irritability, anxiety, physical tension, decreased appetite and mood25 | Irritability, increase in bodily motility, tremors, startles, poor habituation to visual stimuli, abnormal reflexes, symptoms similar to mild withdrawal27 |
| Opioids (Heroin, methadone) | Dilated pupils, watery eyes, runny nose, diarrhea, nausea/vomiting, muscle cramps, piloerection, chills or profuse sweating, yawning, loss of appetite, tremor, jitteriness, panic, insomnia, stomach ache, irritability26 | Risk of prematurity, small-for-gestational age, adult withdrawal symptoms, irritability, hypertonia, wakefulness, jitteriness, diarrhea, increased hiccups, yawning and sneezing, excessive sucking and seizures. Withdrawal effects occur earlier in heroin-exposed babies than in methadone-exposed infants.26 |
CASE 4 RESOLVED
After delivery, a test indicates that the newborn has been exposed to cocaine. The mother admits to cocaine use during pregnancy. She says she did not seek prenatal care because she was afraid of being prosecuted and sent to jail. A social work consult is requested, and the mother is referred to a substance abuse treatment program. State law requires the case to be reported to child protective services. Upon hospital discharge, the newborn is initially placed with the paternal grandmother.
Denial of pregnancy
CASE 5: Patient’s labor takes her by surprise
The night is nearing its end, but it isn’t over yet. At 5:30 AM, you are called to a precipitous delivery involving a 17-year-old who has had no prenatal care. She denies knowing that she was pregnant, and says she thought her labor pains were a bowel movement. Her parents were similarly unaware that their daughter was pregnant, and are threatening to disown her.
How do you defuse the situation?
In a study of women who denied or concealed pregnancy, patients presented to the hospital for various reasons.22 For example, one woman went to the ER because she was seizing and her workup revealed that she had eclampsia. A number of women did not even recognize when they were in labor. The infants born to these women are at risk for a poor neonatal outcome.21,22
How can psychiatry help in such a case? By determining whether the patient denied her pregnancy—even to herself—or actively concealed it from others. Obviously, these circumstances have differing implications.
Denial is not a simple entity. It may involve a psychotic schizophrenic woman who is out of touch with the reality of her pregnancy; a woman who “affectively” denies her pregnancy, keeping the significance of her condition from herself and behaving as though she is not gravid (perhaps because she plans to give the baby up for adoption); or a woman who has pervasive denial and does not know that she is pregnant.22,23 In contrast, a woman who conceals her pregnancy is quite aware that she is gravid but consciously hides the gestation from others, begging the question of what she had planned for the future.22
Psychological issues abound, and may include a history of sexual and psychological trauma, an attempt to avoid religious prohibitions against unwed intercourse, anger at the father of the infant, and even homicidal urges toward the baby.24 There may be more going on under the surface than “only” a failure to recognize the pregnancy, and the patient may need further mental health treatment.
Consider how well this young woman can be a mother. When she did not even recognize that she was pregnant for 9 months, how well will she be able to attend to her baby’s needs? Psychiatry can evaluate the patient to help determine her capacity for parenting and whether child protective services should be alerted. Of additional concern is the distress of the patient’s parents. Family support will be extremely important.
Be sure to conduct thorough contraceptive education and planning at the time of discharge because this patient is at risk for future denied or concealed pregnancies.22
CASE 5 RESOLVED
The patient is seen by psychiatry. She has no major mental illness, but her denial appears to be related to problems with her boyfriend, her attempts to be the perfect daughter, and fear of being disowned. After the initial shock, the patient’s parents become more supportive and begin to bond with their new grandchild. The new mom is educated about birth control and agrees to follow up with a counselor and take parenting classes. The baby is discharged to his mother and grandparents.
There’s a full moon tonight—and you’re the obstetrician on call. Not that you should expect any more funny business than usual. Despite stories of werewolves and other deviants coming out of the woodwork, there is no “full moon effect”—at least not one that can be documented. Nevertheless, chances are good that you will encounter at least one of the following psychiatric challenges as you end your day in the clinic and move on to an extended vigil:
- postpartum depression
- leaving against medical advice
- agitation
- antenatal illicit drug use
- denial or concealment of pregnancy.
In this article, we describe the management of these challenges and make recommendations to help increase your comfort level with patients who exhibit psychiatric problems. In some situations, our suggestions may help you manage the problem without a psychiatric consult.
Postpartum depression
CASE 1: Is it just the blues?
It is the end of your day in the clinic, and your last patient is a 30-year-old G3P3 who is 6 weeks postpartum. She describes repeated tearful episodes over the course of several weeks, decreased concentration, and poor appetite. She feels guilty because she is tired all the time and not bonding with her baby. She denies having suicidal or homicidal thoughts, or any hallucinations. She had expected her energy to return to normal over the first few postpartum weeks, but it has not. She is worried because she will soon be returning to work as a medical resident.
Does this patient have postpartum depression? Or is it another condition with overlapping symptoms?
If a mother tells you that she is suicidal or having thoughts of harming her child or others, she should be sent immediately to the nearest emergency department for psychiatric evaluation. Short of such a dramatic situation, how do you know when you should manage a patient’s depression on your own and when she should see a psychiatrist? Thorough assessment is the key.
Don’t mistake transient feelings for depression
Transient feelings of sadness, bereavement, and grief are not the same as depression, which must last 2 weeks or longer to confirm the diagnosis.
A quick mnemonic for symptoms of depression is SIG: E CAPS (as if writing a prescription for energy capsules) (TABLE 1).1 This mnemonic helps remind you to assess the patient’s sleep, interest, guilt, energy, concentration, appetite, and psychomotor function, as well as identify any suicidal ideation.
It is important to assess a woman’s sleep and appetite in addition to mood. However, differences may be difficult to ascertain due to normal changes in the postpartum period. One useful question is whether the mother is able to sleep when the baby sleeps. If she isn’t, this wakefulness may be a symptom of depression.
The Edinburgh Postnatal Depression Scale is an easy, 10-question screening tool that is completed by the patient; it can be used both during pregnancy and postpartum. It is available on the Web at a number of sites, including www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf.
TABLE 1
SIG: E CAPS—a mnemonic to assess for depression1
| Decreased (sometimes increased) Sleep |
| Decreased Interests |
| Feelings of Guilt |
| Decreased Energy |
| Decreased Concentration |
| Decreased (sometimes increased) Appetite |
| Psychomotor retardation, slowness |
| Suicidal thoughts, plans, or intent |
Differential diagnosis
Besides postpartum depression, the differential diagnosis for altered mood in the postpartum period includes several entities.
Baby blues generally occurs quite soon after birth and resolves within 2 weeks. It involves crying, emotional lability, and irritability.2 It occurs in around 50% to 75% of new mothers (compared with postpartum depression, which affects 10% to 20%).3-5
Postpartum psychosis often involves the onset of psychotic symptoms within 1 week after delivery. The patient may exhibit both mood symptoms and psychosis. For example, she may believe that the baby is not hers or hear voices commanding her to kill the baby or warning her not to trust her healthcare providers.6 Postpartum psychosis has a prevalence of about 0.2%.3-6
This psychosis can be organic in nature or can arise from a preexisting mood disorder or schizophrenia. Because treatment varies, depending on the cause, a thorough medical workup is needed.
Bipolar disorder may present as depression, but it also consists of manic periods of elevated, expansive, or irritable mood that last several days to weeks. Many symptoms appear to be the opposite of depression, such as increased energy and elevated self-esteem.7,8
It is important to consider bipolar disorder in the differential diagnosis. If a woman who has unrecognized bipolar disorder is given an antidepressant, a manic state could be precipitated. Women who have bipolar disorder require different drugs than women who have depression only, and they should be evaluated by a psychiatrist, at least initially.
Start treatment as soon as possible
Once you confirm that the patient has postpartum depression—and not another psychiatric disorder—prescribing an antidepressant may be the next step. Keep in mind that these drugs take several weeks before their benefits are felt. Therefore, it is best to start an antidepressant before depression becomes severe. The mother may also benefit from psychotherapy.
The selective serotonin reuptake inhibitor sertraline (Zoloft) is a reasonable first choice in pregnancy and lactation when the depression is of new onset.9,10 Start it gradually (e.g., 25 mg for sertraline, which can cause nausea if it is initiated too rapidly) and titrate it over time, if necessary. When there is comorbid anxiety, it sometimes is helpful to prescribe low dosages of lorazepam (Ativan, Temesta) on an as-needed basis, while the patient is waiting for the antidepressant to “kick in.” Also consider follow-up—do you plan to follow her frequently or refer her to psychiatry?
Adjunctive or alternative options include psychotherapy, group therapy, and music therapy. Referral to a psychiatrist is warranted if the patient does not respond to the initial antidepressant agent.
Also be aware that untreated depression can become so severe that a woman can begin to experience psychosis, warranting rapid referral. Also refer any woman who reports a complex history of previous depression—unless the previous episode was easily controlled with a medicine safe for use during pregnancy and lactation.
If the patient is not lactating, a greater range of agents may be considered. (A full discussion of the risks and benefits of antidepressant use in pregnancy is outside the scope of this article. The interested reader is referred to an article on the subject by Wisner and colleagues.11)
CASE 1 RESOLVED
A comprehensive discussion with the mother reveals that she is suffering from postpartum depression. No history of bipolar or psychotic symptoms is discovered. After discussing treatment options, you prescribe sertraline. Over the next 2 months, the patient’s symptoms improve, and she bonds with her infant and successfully returns to work. She is also referred to a psychologist to work through some underlying issues.
Leaving against medical advice
CASE 2: Patient threatens to leave the hospital
At midnight, you are paged to attend to a 32-year-old G1P0 at 27 weeks’ gestation who is threatening to leave against medical advice. She was admitted earlier in the day with uncontrolled gestational diabetes and is refusing her insulin.
How do you respond?
Use the relationship that you have established with this patient to the best of your ability. Make sure that you have explained fully, and in language she can easily comprehend, the reasons she needs to stay for treatment.
Don’t overlook the obvious, either: Why does she want to leave? Sometimes the reason makes sense (e.g., one mother wanted to leave to protect her daughter from an abusive husband). Other reasons may be related to psychosis, addiction, lack of sleep in the hospital, or a desire to smoke, drink, or use drugs. Can you convince her to postpone her decision until morning, when her physician will be available?
It is important to document in the medical record your explanations and her reasoning. Can she coherently verbalize an understanding of the consequences of her decision to leave, including the risks and implications to herself and the fetus?12 Can she describe alternatives and the reasoning against them?
If she is able to do these things, and you find her thought processing and reasoning to be lucid, then she may have the capacity to leave against medical advice. Keep in mind that rational persons do have the right, constitutionally, to refuse treatment, even if doing so will lead to morbidity. (A Jehovah’s Witness who refuses treatment is the typical example.12) Contact the hospital’s attorney—tonight—and document that you did so. The attorney may recommend that the patient sign a letter stating that she recognizes the maternal and fetal risks of leaving.
Sometimes a patient must be held against her will
Some mothers lack the capacity to refuse treatment. They may be unable to verbalize an understanding of the situation and its risks. Their reasoning may be abnormal, with disorganized or delusional thinking, or both. The patient may be tangential or talk “in circles” rather than answer your questions.
Try to ascertain whether mood symptoms are contributing to her irrational thinking. For example, is her rationale for going home—“just to be with my husband because I don’t want to be alone”—due to her depression, despite the risk to herself and the fetus? Try to be flexible and creative. For example, you could call the husband and ask him to come to the hospital to sit with the patient.
Is the patient psychotic? For example, does she believe she has to leave now because the staff has been replaced by aliens who plan to kill her and her fetus? If so, you have the authority to continue her hospitalization—but contact the psychiatry department for medication recommendations. A urine toxicology screen would also be prudent.
If the patient is irrational and lacks the capacity to decide whether to stay or leave, document your conversation with her, as well as the reasoning behind your decision to intervene further. Other steps include:
- contacting the hospital’s attorney
- completing an emergency detention form
- calling security
- ensuring that the patient’s environment is safe for her and others (TABLE 2).13
TABLE 2
5 steps to sound management of a patient who wants to leave
against medical advice
| 1. Ask the patient why she wants to leave now |
| 2. Inform her of the risks to herself and to her fetus |
| 3. Ask her to verbalize the risks to herself and to her fetus |
4. Determine whether the patient’s request is rational
|
| 5. Document the medical explanation and reasoning in the chart |
CASE 2 RESOLVED
After building some rapport with the patient, you ask why she wants to leave right now. During this conversation, the patient reveals that she has not slept in three nights, and says she believes that the insulin is keeping her up. You are able to assure her that this is not the case and offer her something to help her sleep. She decides not to leave against medical advice.
Unexplained agitation
CASE 3: Patient becomes abusive
At 1 AM, you are called to the seventh floor, where a 20-year-old G2P1 at 26 weeks’ gestation is yelling at staff and hitting anyone who comes near. She was admitted earlier in the day for management of threatened abortion and a dilated cervix. She has no documented psychiatric history, but is flushed, disheveled, and hostile, accusing the staff of sabotaging her life, and is seen picking at imaginary things. You notify psychiatry, but no one is available.
What do you do?
Determining the origin of these symptoms will help determine the appropriate course of action. Among the possibilities are:
- drug intoxication or withdrawal
- delirium
- psychosis
- a chronic problem such as a personality disorder (TABLE 3).
Psychosis means that a patient is out of touch with reality. A psychotic patient may experience delusions, auditory and visual hallucinations, and gross disorganization. Brief psychotic episodes usually last for 1 day to 1 month, with eventual recovery to premorbid functioning.7
Substances such as medications or illicit drugs also can induce psychosis. Major offenders include steroids and narcotic agents. Alternatively, sudden withdrawal of illicit substances (due to hospitalization) could manifest as delirium or psychosis.
Personality disorder. If the patient’s behavior is not new but a long-term problem, she may have a chronic personality disorder rather than acute illness. Personality problems involve pervasive response patterns and dysfunctional coping patterns that affect daily life. For example, a patient who has borderline personality disorder may have emotional instability presenting as intense episodic dysphoria or irritability. Such patients have a hard time empathizing with others, poor impulse control, and a desire for instant gratification. They may also misinterpret the behavior of other people and take offense easily as a result. Lacking stress-management skills, they regress to unhealthy defense mechanisms such as acting out, complaining, passive aggressiveness, and splitting of the staff (thinking that people are all good or all bad).
Dementia may also be on the differential diagnosis, but this chronic condition is unlikely in such a young patient (unless she were in the late stages of HIV/AIDS, for example). Dementia has a gradual onset and is irreversible.
TABLE 3
Some causes of agitation
Delirium
|
Psychosis
|
Dementia
|
Personality dysfunction
|
Workup for the agitated patient
Assess vital signs and basic laboratory studies, particularly a complete blood count, thyroid testing, metabolic screens, glucose, serum chemistry panel, and urine toxicology, to rule out causes of delirium and detect any substances the patient may have used. Also consider that the patient may have initiated a new medication recently.
Imaging of the brain or chest or electroencephalography may be necessary, such as in the setting of infection or concerns regarding seizure activity.
Gather collateral information about the patient from her relatives, friends, and the support staff. Search her chart for recent contacts. Did she have a visitor or phone call that may have upset her? Explore whether she is having problems with her partner or family and friends. Also confirm that her agitated behavior is an acute change.
Investigate the patient’s paranoia. Why does she believe that the staff is against her? Does she believe they are trying to harm, kill, or poison her? Assess her reasoning to determine whether her behavior is psychotic or a personality problem.
Ask her about hallucinations, keeping in mind that hallucinations are different from illusions, in which a patient misinterprets what she sees. What are the voices saying to her?
Also ask about any suicidal or homicidal commands. If she acknowledges that she is hearing them, get a sitter for her immediately and make her environment safe so that she is unable to harm herself. Then contact the psychiatry department again.
How to intervene
Talk gently and quietly in an attempt to calm the situation. Try to make yourself “small”: Stand back and stay at the patient’s eye level, not in her personal space or towering over her.
Also, protect yourself. Don’t challenge her complaints immediately or you will alienate her. Medically evaluate and treat the cause of her agitation, and, if medications are necessary for psychosis or sedation, contact psychiatry for assistance.
CASE 3 RESOLVED
The patient does not respond to your attempts to reason and refuses to allow the nurses to check her vital signs. Security is called to stand by while her vital signs are reassessed. The nurses inform you that the patient’s family is in the waiting room. Though you find no documented history of substance use or abnormal labs, the family reports that the patient had a history of alcohol abuse but quit drinking about 3 days earlier. They also report that, before she quit, she drank approximately 25 oz of vodka and a sixpack of beer nightly. They deny knowledge of any other illicit drug use. Because her vital signs suggest alcohol withdrawal, you offer oral lorazepam and treat her according to the hospital’s alcohol withdrawal protocol. She recovers without any further complications and is referred to the chemical dependency service for evaluation.
Drug abuse during pregnancy
CASE 4: Patient skips prenatal care
It is 2 AM, and you are about to get some rest when you are paged by the emergency room about a 26-year-old G5P4, who is in active labor with no dates. You check the database and discover that her previous pregnancies were complicated by chronic substance abuse.
How do you respond?
You might feel frustrated and angry with this patient. Considering that you have not met her, these feelings would be based on previous contacts with patients who had a similar history. This is countertransference. We all experience it. It can be helpful or harmful, but you cannot control it unless you are aware of it. Pay attention to the anger, happiness, or pride that a patient triggers in you, and acknowledge that it is your issue.
Your frustration may also stem from personal feelings about mothers who repeatedly expose their fetuses to drugs and neglect prenatal care, as well as anxiety about what you are legally and ethically bound to do.
In Ferguson v City of Charleston, the Supreme Court found that drug testing of a pregnant woman for the purpose of criminal prosecution is a violation of Fourth Amendment rights.14 However, there have been cases in which a state prosecuted a woman for using an illegal substance during pregnancy.
These cases involved:
- child neglect15
- delivery of a stillborn fetus whose autopsy revealed traces of cocaine by-products16
- reckless endangerment after a newborn tested positive for cocaine.17
What is drug abuse?
According to the 4th edition of the Diagnostic and Statistical Manual of Mental Health Disorders, it is a “maladaptive pattern of substance use manifested by recurrent and significant adverse consequences related to the repeated use of substances” within a 12-month period, or persistently.7 To meet criteria for substance dependence, in addition to the criteria just mentioned, the individual must be tolerant to the drug, experience withdrawal when the drug is cut back or stopped, continue to use the drug despite knowledge of its dangers, or all of the above. Mothers who match this description often lose custody of their child—sometimes to foster care, sometimes to other family members.20,21
Some states use positive serum and urine toxicology as evidence to remove a child from the mother’s custody.19
It is important to attempt to build a doctor–patient relationship. You cannot solve the patient’s substance dependence problem in one night, but you can refer her to drug treatment. A urine toxicology screen can help you and the pediatricians know what the patient has been exposed to (TABLE 4).
TABLE 4
Commonly abused drugs and their potential effects
| Drug | Withdrawal effects on mother | Drug effects on fetus or infant |
|---|---|---|
| Alcohol | Sweating, increased heart rate, hand tremor, nausea/vomiting, physical agitation, hallucination (tactile, visual, auditory), illusions, grand mal seizures25 | Fetal alcohol syndrome. Withdrawal symptoms similar to those of the mother |
| Cocaine | Agitation, anxiety, anger, nausea/vomiting, muscle pain, disturbed sleep, depression, intense cravings for the drug, irritability25 | Risk of abruptio placenta, small-for-gestational-age infant, microcephaly, congenital anomaly (cardiac and genitourinary abnormality, necrotizing enterocolitis), central nervous system stroke or hemorrhage. Withdrawal effects include hypertonia, jitteriness, and seizures.26 |
| Crystal methamphetamine | Anxiety, psychotic reaction, intense hunger, irritability, restlessness, fatigue, depression, sleep disturbance, cravings25 | Premature birth, abruptio placenta, small-for-gestational age, hypertonia, tremors, poor feeding, abnormal sleep patterns26 |
| Marijuana | Irritability, anxiety, physical tension, decreased appetite and mood25 | Irritability, increase in bodily motility, tremors, startles, poor habituation to visual stimuli, abnormal reflexes, symptoms similar to mild withdrawal27 |
| Opioids (Heroin, methadone) | Dilated pupils, watery eyes, runny nose, diarrhea, nausea/vomiting, muscle cramps, piloerection, chills or profuse sweating, yawning, loss of appetite, tremor, jitteriness, panic, insomnia, stomach ache, irritability26 | Risk of prematurity, small-for-gestational age, adult withdrawal symptoms, irritability, hypertonia, wakefulness, jitteriness, diarrhea, increased hiccups, yawning and sneezing, excessive sucking and seizures. Withdrawal effects occur earlier in heroin-exposed babies than in methadone-exposed infants.26 |
CASE 4 RESOLVED
After delivery, a test indicates that the newborn has been exposed to cocaine. The mother admits to cocaine use during pregnancy. She says she did not seek prenatal care because she was afraid of being prosecuted and sent to jail. A social work consult is requested, and the mother is referred to a substance abuse treatment program. State law requires the case to be reported to child protective services. Upon hospital discharge, the newborn is initially placed with the paternal grandmother.
Denial of pregnancy
CASE 5: Patient’s labor takes her by surprise
The night is nearing its end, but it isn’t over yet. At 5:30 AM, you are called to a precipitous delivery involving a 17-year-old who has had no prenatal care. She denies knowing that she was pregnant, and says she thought her labor pains were a bowel movement. Her parents were similarly unaware that their daughter was pregnant, and are threatening to disown her.
How do you defuse the situation?
In a study of women who denied or concealed pregnancy, patients presented to the hospital for various reasons.22 For example, one woman went to the ER because she was seizing and her workup revealed that she had eclampsia. A number of women did not even recognize when they were in labor. The infants born to these women are at risk for a poor neonatal outcome.21,22
How can psychiatry help in such a case? By determining whether the patient denied her pregnancy—even to herself—or actively concealed it from others. Obviously, these circumstances have differing implications.
Denial is not a simple entity. It may involve a psychotic schizophrenic woman who is out of touch with the reality of her pregnancy; a woman who “affectively” denies her pregnancy, keeping the significance of her condition from herself and behaving as though she is not gravid (perhaps because she plans to give the baby up for adoption); or a woman who has pervasive denial and does not know that she is pregnant.22,23 In contrast, a woman who conceals her pregnancy is quite aware that she is gravid but consciously hides the gestation from others, begging the question of what she had planned for the future.22
Psychological issues abound, and may include a history of sexual and psychological trauma, an attempt to avoid religious prohibitions against unwed intercourse, anger at the father of the infant, and even homicidal urges toward the baby.24 There may be more going on under the surface than “only” a failure to recognize the pregnancy, and the patient may need further mental health treatment.
Consider how well this young woman can be a mother. When she did not even recognize that she was pregnant for 9 months, how well will she be able to attend to her baby’s needs? Psychiatry can evaluate the patient to help determine her capacity for parenting and whether child protective services should be alerted. Of additional concern is the distress of the patient’s parents. Family support will be extremely important.
Be sure to conduct thorough contraceptive education and planning at the time of discharge because this patient is at risk for future denied or concealed pregnancies.22
CASE 5 RESOLVED
The patient is seen by psychiatry. She has no major mental illness, but her denial appears to be related to problems with her boyfriend, her attempts to be the perfect daughter, and fear of being disowned. After the initial shock, the patient’s parents become more supportive and begin to bond with their new grandchild. The new mom is educated about birth control and agrees to follow up with a counselor and take parenting classes. The baby is discharged to his mother and grandparents.
1. What does SIGECAPS stand for? Available at: www.acronymfinder.com/Sleep,-Interest,-Guilt,-Energy,-Concentration,-Appetite,-Psychomotor,-Suicidal-(mnemonic-for-characteristics-of-major-depression)-(SIGECAPS).html. Accessed May 18, 2009.
2. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry. 2003;64:1284-1292.
3. Grigoriadis S, Romans S. Postpartum psychiatric disorders: what do we know and where do we go? Curr Psychiatry Rev. 2006;2:151-158.
4. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23:188-193.
5. Sadock BJ, Sadock VA. Psychiatry and reproductive medicine. In: Kaplan HI, Sadock BJ, eds. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. New York: Lippincott Williams & Wilkins; 2003:868-878.
6. Friedman SH, Resnick PJ, Rosenthal M. Postpartum psychosis: strategies to protect infant and mother from harm. Curr Psychiatry. 2009;8(2):40-46.
7. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Text revision. Washington, DC: American Psychiatric; 2000.
8. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
9. Gentile S. Use of contemporary antidepressants during breastfeeding: a proposal for a specific safety index. Drug Saf. 2007;30:107-121.
10. Rahimi R, Nikfar S, Abdollahi M. Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials. Reprod Toxicol. 2006;22:571-575.
11. Wisner KL, Zarin DA, Holmboe ES, et al. Riskbenefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157:1933.-
12. Roberts LW, Hoop JG. Professionalism and ethics. Washington, DC: American Psychiatric Publishing; 2008.
13. Ohio Criteria for Commitment, Section 5122.01.
14. Harris LH, Paltrow L. The status of pregnant women and fetuses in US criminal law. JAMA. 2003;289:1697-1699.
15. Drugs, Police & The Law: Whitner vs. the State of South Carolina. June 23, 2004. Available at: www.drugpolicy.org/law/womenpregnan/whitnervsth_/. Accessed April 30, 2009.
16. Gray P. Prosecution of prenatal substance abuse allowed to stand in McKnight case. Available at: www.law.uh.edu/healthlaw/perspectives/reproductive/040202prosecution.pdf. Accessed April 30, 2009.
17. Parks AW. New mothers fight endangerment convictions. Public Justice Center. April 10, 2006. Available at: www.publicjustice.org/news/index.cfm?newsid=106. Accessed April 30, 2009.
18. Parks AW. Using cocaine while pregnant is not reckless endangerment. The Daily Record. August 4, 2006. Available at: www.publicjustice.org/pdf/Cruzdailyrecord.pdf. Accessed April 30, 2009.
19. American Pregnancy Association. Using illegal street drugs during pregnancy. May 2007. Available at: www.americanpregnancy.org/pregnancyhealth/illegaldrugs.html. Accessed April 30, 2009.
20. Neuspiel DR, Zingman TM, Templeton VH, et al. Custody of cocaine-exposed newborns: determinants of discharge decisions. Am J Public Health. 1993;83:1726-1729.
21. Friedman SH, Heneghan A, Rosenthal M. Disposition and health outcomes among infants born to mothers with no prenatal care. Child Abuse Negl. 2009;33:116-122.
22. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48:117-122.
23. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. Washington, DC: American Psychiatric Publishing; 2003.
24. Bonnet C. Adoption at birth: prevention against abandonment or neonaticide. Child Abuse Negl. 1993;17:501-513.
25. Heroin withdrawal. Available at: www.addictionwithdrawal.com/heroin.htm. Accessed April 29, 2009.
26. Kwong TC, Ryan RM. Detection of intrauterine illicit drug exposure by newborn drug testing. Clin Chem. 1997;43:235-242.
27. Bada HS, Reynolds EW, Hansen WF. Marijuana use, adolescent pregnancy, and alteration in new born behavior: how complex can it get? J Pediatr. 2006;149:742.-
1. What does SIGECAPS stand for? Available at: www.acronymfinder.com/Sleep,-Interest,-Guilt,-Energy,-Concentration,-Appetite,-Psychomotor,-Suicidal-(mnemonic-for-characteristics-of-major-depression)-(SIGECAPS).html. Accessed May 18, 2009.
2. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry. 2003;64:1284-1292.
3. Grigoriadis S, Romans S. Postpartum psychiatric disorders: what do we know and where do we go? Curr Psychiatry Rev. 2006;2:151-158.
4. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23:188-193.
5. Sadock BJ, Sadock VA. Psychiatry and reproductive medicine. In: Kaplan HI, Sadock BJ, eds. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. New York: Lippincott Williams & Wilkins; 2003:868-878.
6. Friedman SH, Resnick PJ, Rosenthal M. Postpartum psychosis: strategies to protect infant and mother from harm. Curr Psychiatry. 2009;8(2):40-46.
7. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Text revision. Washington, DC: American Psychiatric; 2000.
8. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
9. Gentile S. Use of contemporary antidepressants during breastfeeding: a proposal for a specific safety index. Drug Saf. 2007;30:107-121.
10. Rahimi R, Nikfar S, Abdollahi M. Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials. Reprod Toxicol. 2006;22:571-575.
11. Wisner KL, Zarin DA, Holmboe ES, et al. Riskbenefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157:1933.-
12. Roberts LW, Hoop JG. Professionalism and ethics. Washington, DC: American Psychiatric Publishing; 2008.
13. Ohio Criteria for Commitment, Section 5122.01.
14. Harris LH, Paltrow L. The status of pregnant women and fetuses in US criminal law. JAMA. 2003;289:1697-1699.
15. Drugs, Police & The Law: Whitner vs. the State of South Carolina. June 23, 2004. Available at: www.drugpolicy.org/law/womenpregnan/whitnervsth_/. Accessed April 30, 2009.
16. Gray P. Prosecution of prenatal substance abuse allowed to stand in McKnight case. Available at: www.law.uh.edu/healthlaw/perspectives/reproductive/040202prosecution.pdf. Accessed April 30, 2009.
17. Parks AW. New mothers fight endangerment convictions. Public Justice Center. April 10, 2006. Available at: www.publicjustice.org/news/index.cfm?newsid=106. Accessed April 30, 2009.
18. Parks AW. Using cocaine while pregnant is not reckless endangerment. The Daily Record. August 4, 2006. Available at: www.publicjustice.org/pdf/Cruzdailyrecord.pdf. Accessed April 30, 2009.
19. American Pregnancy Association. Using illegal street drugs during pregnancy. May 2007. Available at: www.americanpregnancy.org/pregnancyhealth/illegaldrugs.html. Accessed April 30, 2009.
20. Neuspiel DR, Zingman TM, Templeton VH, et al. Custody of cocaine-exposed newborns: determinants of discharge decisions. Am J Public Health. 1993;83:1726-1729.
21. Friedman SH, Heneghan A, Rosenthal M. Disposition and health outcomes among infants born to mothers with no prenatal care. Child Abuse Negl. 2009;33:116-122.
22. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48:117-122.
23. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. Washington, DC: American Psychiatric Publishing; 2003.
24. Bonnet C. Adoption at birth: prevention against abandonment or neonaticide. Child Abuse Negl. 1993;17:501-513.
25. Heroin withdrawal. Available at: www.addictionwithdrawal.com/heroin.htm. Accessed April 29, 2009.
26. Kwong TC, Ryan RM. Detection of intrauterine illicit drug exposure by newborn drug testing. Clin Chem. 1997;43:235-242.
27. Bada HS, Reynolds EW, Hansen WF. Marijuana use, adolescent pregnancy, and alteration in new born behavior: how complex can it get? J Pediatr. 2006;149:742.-