User login
A guide to management: Adnexal masses in pregnancy
CASE 1 An enlarging cystic tumor
A 20-year-old gravida 3 para 1011 visits the emergency department with persistent right flank pain. Although ultrasonography (US) shows a 21-week gestation, the patient has had no prenatal care. Imaging also reveals a right-sided ovarian tumor, 14×11×8 cm, that is mainly cystic with some internal echogenicity.
At 30 weeks’ gestation, a gynecologic oncologist is consulted. Repeat US reveals the mass to be about 20 cm in diameter and cystic, without internal papillation. The patient’s CA-125 level is 12 U/mL. Based on this information, the physicians decide the likely finding is a benign ovarian cystadenoma.
How should they proceed?
The discovery of an adnexal mass during pregnancy isn’t as rare as you might think—depending on when and how closely you look, it occurs in about 1 in 100 gestations. In most cases, we have found, the mass is clearly benign (TABLE 1), warranting only observation.
TABLE 1
Adnexal masses removed during pregnancy: Histologic profile
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Cystadenoma | 549 (33) |
| Dermoid | 451 (27) |
| Paraovarian/paratubal | 204 (12) |
| Functional | 237 (14) |
| Endometrioma | 55 (3) |
| Benign stromal | 28 (2) |
| Leiomyoma | 23 (1.5) |
| Luteoma | 8 (0.5) |
| Miscellaneous | 55 (3) |
| Malignant | 68 (4) |
| Total | 1,678 |
| Data supplied by the authors from surgical experience | |
In the case described above, the physicians followed the patient and removed the mass at term because it was cystic with no other indications of malignancy. At 37 weeks’ gestation, a cesarean section was performed through a midline laparotomy incision, followed by removal of the ovarian tumor, which was benign. The pathologist measured the tumor at 16×12×4 cm and determined that it was a corpus luteum cyst.
Presence of mass raises questions
Despite the rarity of malignancy, the discovery of an ovarian mass during pregnancy prompts several important questions:
How should the mass be assessed? How can the likelihood of malignancy be determined as quickly and efficiently as possible, without jeopardy to the pregnancy?
When is surgical intervention warranted? And when can it be postponed? Specifically, is elective operative intervention for a tumor that is probably benign appropriate during pregnancy?
When is the best time to operate? And what is the optimal surgical route?
In this article, we address these questions with a focus on intervention. As we’ll explain, only a small percentage of gravidas who have an adnexal mass require surgery during pregnancy. When surgery is necessary, it is usually indicated for an emergent problem or suspicion of malignancy. Even when ovarian cancer is confirmed, we have found that it is usually in its early stages and therefore has a favorable prognosis (TABLE 2).
TABLE 2
Malignant adnexal masses removed during pregnancy
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Epithelial | 101 (28) |
| Borderline epithelial | 147 (40) |
| Germ-cell dysgerminoma | 47 (13) |
| Other | 34 (9) |
| Stromal | 24 (7) |
| Undifferentiated | 5 (1.4) |
| Sarcoma | 2 (0.5) |
| Metastatic | 4 (1.1) |
| Total | 364 |
| Data supplied by the authors from surgical experience | |
How should a mass be assessed?
Ultrasonography and other imaging often reveal the presence of a mass and help determine whether it is benign or malignant. In fact, most adnexal masses discovered during pregnancy are incidental findings at the time of routine prenatal US. (see the most commonly found tumors.) Operative intervention is required in 3 situations:
- malignancy is suspected
- an acute complication develops
- the sheer size of the tumor is likely to cause difficulty.
Corpus luteum
A persistent corpus luteum is a normal component of pregnancy. Although it usually appears as a small cystic structure on ultrasonographic imaging, the corpus luteum of pregnancy can reach 10 cm in size. Other types of “functional” ovarian cysts may also be found during pregnancy. Most functional cysts resolve by the early second trimester.4,6 In rare cases, a cyst may develop complications such as torsion or rupture, causing acute pain or hemorrhage. Otherwise, a cystic tumor identified in the first trimester should be characterized and followed using ultrasonography (US).
Benign neoplasm
An adnexal mass that persists beyond the first trimester is more likely to be a neoplasm.3-5,10,11,22 Such a mass is generally considered clinically significant if it exceeds 5 cm in diameter and has a complex sonographic appearance. Usually such a neoplasm will be a benign cystadenoma or cystic teratoma.5,10-13,19,23,24
Benign cystic teratoma
This tumor can be identified with a fairly high degree of specificity using a variety of imaging techniques, with management based on the presumptive diagnosis. This tumor is unlikely to grow substantially during pregnancy. When it is smaller than 6 cm, such a tumor can simply be observed.14 A larger tumor can occasionally rupture or lead to torsion or obstruction of labor, but such occurrences are rare.
Benign cystadenoma
In an asymptomatic patient with imaging that suggests a benign cystadenoma (see sonogram), benign cystic teratoma, or other benign tumor, observation is reasonable in most cases.4,6,7,9-11,14,19 Operative intervention is required when there is less certainty regarding the benign nature of the tumor, an acute complication develops, or the tumor is expected to pose problems because of its large size alone.
Uterine leiomyoma
It is rare for an ovarian tumor detected during pregnancy to have a solid appearance on US. When it does, it may be a uterine leiomyoma mimicking an adnexal tumor (see intraoperative photograph). It should be reevaluated with more detailed US or magnetic resonance imaging.25
Malignancy
About 10% of adnexal masses that persist during pregnancy are malignant, according to recent series.4,5,7-10,12,13,24,26
Most of the ovarian cancers diagnosed during pregnancy are epithelial, and a substantial portion of these are low-malignant-potential (LMP) tumors.5,10,11,13,19,23,24,26,27 This ratio is in keeping with the age of these women, which also explains the stage distribution (most are stage 1) and the large percentage of germ-cell tumors detected. The majority of ovarian cancers discovered in pregnant women have a favorable prognosis.
Benign-appearing cystadenoma
A morphologically benign-appearing, large, cystic adnexal mass can be seen in association with an 11-week gestation.
Leiomyoma mimics an ovarian tumor
This 17-week gestation was marked by a large pedunculated leiomyoma that at fist appeared to be a right adnexal tumor.
Appearance of adnexal masses on US
A functional cyst such as a follicular cyst, corpus luteum cyst, or theca lutein cyst usually has smooth borders and a fluid center. Other cysts may sometimes contain debris, such as clotted blood, that suggests endometriosis or a simple cyst with bleeding into it.
A benign cystic teratoma often has multiple tissue lines, evidence of calcification, and layering of fat and fluid contents.
A benign cystadenoma usually has the appearance of a simple cyst without large septates, whereas a cystadenocarcinoma often contains septates, abnormal blood flow, increased vascularity, or all of these. However, it is impossible to definitively distinguish a cystadenoma from a cystadenocarcinoma using US imaging alone.
Functional cysts usually resolve by the second trimester. A cyst warrants closer scrutiny when it persists, is larger than 5 cm in diameter, or has a complex appearance on US.
CA-125 may be useful after the first trimester
The serum CA-125 level is typically elevated during the first trimester, but may be useful during later assessment or for follow-up of a malignancy.1
A markedly elevated serum level of alpha-fetoprotein (fractionated in some cases) has been reported in some gravidas with an endodermal sinus or mixed germ-cell ovarian tumor.2 Alpha-fetoprotein should be measured when there is suspicion for a germ-cell tumor based on clinical or US findings.
When a mass is discovered during cesarean section
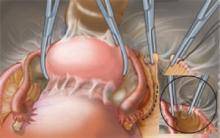
Occasionally, an adnexal mass is detected at the time of cesarean section (FIGURE 1).3 This phenomenon is increasingly common, given the large number of cesarean deliveries in the United States. To eliminate the need for future surgery and avoid a delay in the diagnosis of an ovarian malignancy, inspect the adnexa routinely after closing the uterine incision in all women who deliver by cesarean section.
FIGURE 1 Mass discovered at cesarean section
This cystic tumor was discovered at cesarean section that was undertaken for obstetric indications.
CASE 2 LMP tumor is suspected
A 36-year-old gravida 3 para 1011 makes a prenatal visit during the first trimester. Her previous delivery was a cesarean section through a Pfannenstiel incision for a breech presentation. US imaging reveals a 6-week, 5-day fetus and a complex left adnexal mass, 4.5×3.9×4.1 cm. Imaging is repeated 1 month later at a tertiary-care center and shows an 11-week viable fetus, a right ovary with a corpus luteum cyst, and a left ovary with a 6.6×4 cm cystic mass with extensive vascular surface papillations that is suspicious for a low-malignant-potential (LMP) tumor. In several sonograms prior to the pregnancy, this mass appeared to be solid and was 3 cm in size.
When is surgery warranted?
Surgery is indicated when physical examination or imaging of a pregnant woman reveals an adnexal mass that is suspicious for malignancy, but the physician must weigh the benefit of prompt surgery against the risk to the pregnancy. This equation can be complicated in several ways. For example, surgical staging of clinically early ovarian cancer is more difficult due to the pregnant uterus, which is more extensively manipulated during these procedures. In addition, an optimal operation sometimes necessitates removal of the uterus.
At 13 weeks’ gestation, the patient described in case 2 underwent laparoscopy with peritoneal washings and left salpingo-oophorectomy, but the tumor ruptured during removal. Final pathology showed it to be a serous LMP tumor involving the surface of the left ovary. Washings were in line with this diagnosis.
The pregnancy continued uneventfully, and a repeat cesarean section was performed at 37 weeks through the Pfannenstiel scar, followed by limited surgical staging. Exploration and all biopsies were negative, and the final diagnosis was a stage 1C serous LMP tumor of the ovary.
The patient articulated a desire to preserve her fertility and was monitored with US imaging of the remaining ovary every 6 months.
Does ‘indolent’ behavior of malignancy justify watchful waiting?
LMP tumors comprise a relatively large percentage of ovarian “cancers” encountered during pregnancy. Some authors report the accurate identification of these tumors prospectively, based on ultrasonographic characteristics.4,5 When an LMP tumor is the likely diagnosis, serial observation during pregnancy may be appropriate because of the indolent nature of the tumor. Further studies are needed to refine preoperative diagnosis and determine the overall safety of this approach.
When the problem is acute
In rare cases, a pregnant patient will have (or develop during observation) an acute problem due to torsion or rupture of an adnexal mass. Some ovarian cancers may present acutely, such as a rapidly growing malignant germ-cell tumor or a ruptured and hemorrhaging granulosecell tumor. Emergent surgery is necessary to manage the acute adnexal disease and reduce the likelihood of pregnancy loss. These events are infrequent, occurring in less than 10% of women with a known, persistent adnexal mass during pregnancy.4-14 Furthermore, recent studies have not found a substantial pregnancy complication rate associated with such emergency surgeries.
CASE 3 Suspicious mass, ascites signal need for surgery
A 19-year-old gravida 1 para 0 seeks prenatal care at 17 weeks’ gestation, complaining of rapidly enlarging abdominal girth. The physical examination estimates gestational size to be considerably greater than dates, but US is consistent with a 17-week intrauterine pregnancy. Imaging also reveals a 12-cm heterogenous left adnexal mass and a large amount of ascites.
Surgery is clearly warranted, but how extensive should it be?
When a malignancy is detected, a thorough staging procedure may be justified, depending on gestational age, exposure, desires of the patient, and operative findings. A midline incision is preferred.
Pregnant and nonpregnant women with stage 1A or 1C epithelial ovarian cancer who undergo fertility-preserving surgery (with chemotherapy in selected patients) have a good prognosis and a high likelihood of achieving a subsequent normal pregnancy.15 The same is true for women with a malignant germ-cell tumor of the ovary, even when disease is advanced.16 However, careful surgical staging is necessary.
The most important consideration when deciding whether to continue the pregnancy is the need for adjuvant chemotherapy. Depending on the gestational age and diagnosis, a short delay (4 to 6 weeks) may be appropriate to allow the pregnancy to progress beyond the first trimester or to maturity.
In case 3, a laparotomy was performed at 19 weeks’ gestation via a midline incision, and approximately 5.3 L of ascites was evacuated. A large, nonadherent left ovarian tumor was removed. The right ovary appeared to be normal, as did the gravid uterus, which was minimally manipulated. The rest of the surgical exploration was normal, and the distal portion of the omentum was excised. The frozen-section diagnosis was a malignant stromal tumor. Final pathology showed an 18×13.5×8.8 cm, poorly differentiated, SertoliLeydig-cell tumor with heterologous elements in the form of mucinous epithelium. The omentum was negative for tumor.
Chemotherapy was initiated in the third trimester, based on the limited data available, with intravenous etoposide and platinum administered every 21 days. The patient received 3 cycles of chemotherapy prior to delivery.
At 37 weeks’ gestation, labor was successfully induced. After delivery, bleomycin was added to the chemotherapy regimen, and 3 additional courses with all 3 agents were administered. The patient was lost to follow-up shortly after completing chemotherapy.
Clearly, an informed discussion of the options with the patient is imperative before any surgery, especially when chemotherapy may be delayed. Pregnancy does not appear to alter the prognosis for the patient with an ovarian malignancy, and ovarian cancer has not been reported to metastasize to the fetus.
Pregnant women have a very low rate of ovarian cancer
Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol. 2006;101:315–321.
Ovarian malignancies are rare during pregnancy. When they do occur, they are likely to be early stage and to have a favorable outcome, according to this recent population-based study.
Using 3 large databases containing records on 4,846,505 California obstetric patients between 1991 and 1999, Leiserowitz and colleagues identified 9,375 women who had an ovarian mass associated with pregnancy. Of these, 87 had ovarian cancer and 115 had a low-malignant-potential (LMP) tumor, for a cancer occurrence rate of 0.93%, or 0.0179 per 1,000 deliveries. Thirty-four of the 87 cancers were germ-cell tumors.
Of the 87 ovarian cancers, 65.5% were localized, 6.9% regional, 23% remote, and 4.6% of unknown stage. The respective rates for LMP tumors were 81.7%, 7.8%, 4.4%, and 6.1%.
Women with malignant tumors were more likely than pregnant controls without cancer to undergo cesarean delivery, hysterectomy, transfusion, and prolonged hospitalization. These women did not, however, have a higher rate of adverse neonatal outcomes.
When cancer is advanced
Few data shed light on whether a pregnancy should continue when ovarian cancer is advanced.17 The definitive surgical approach must be highly individualized.
It is not always possible to make an accurate diagnosis based on a frozen section. In such a case, the pregnancy should be preserved until the time of definitive diagnosis. As always, the patient’s wishes and gestational age must be considered.
How factors besides malignancy can influence care
Most persistent adnexal masses move well out of the pelvis as pregnancy progresses. Occasionally, however, an ovarian tumor may be located in the posterior cul-de-sac even at term, a fact easily confirmed by examination or US.4,7 A tumor in the posterior cul-de-sac can obstruct delivery or rupture. When it has a benign cystic appearance on US, it may be decompressed via transvaginal aspiration. Otherwise, the best approach is cesarean section and concomitant management of the mass.
When size alone is the problem
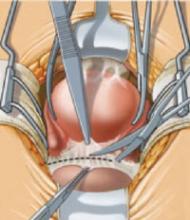
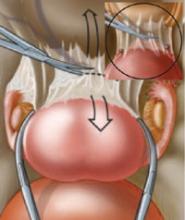
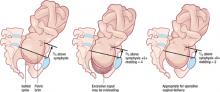
Some ovarian tumors are so large they seem incompatible with an advancing pregnancy. Tumors up to 20 cm in diameter have been removed intact at the time of cesarean section (FIGURE 2).18 The tumor may accommodate in shape and become less problematic as it is gradually pushed into the upper abdomen (FIGURE 3).
The ability of the peritoneal cavity to accommodate a tumor varies greatly among women. As pregnancy advances, the likelihood that a large cystic mass will rupture tends to increase. Depending on the circumstances, percutaneous aspiration7,18 or removal of a benign-appearing cystic tumor may be appropriate.
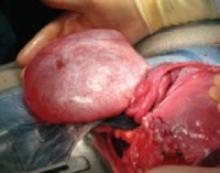
FIGURE 2 Even a very large tumor may coexist with advancing pregnancy
This benign serous cystadenoma was exteriorized at the time of cesarean section at term.
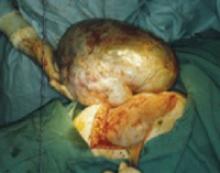
FIGURE 3 Large ovarian tumor has accommodated to the pregnancy
Laparotomy—performed at term for cesarean section and to manage this large tumor—revealed that the tumor had accommodated in shape between the enlarging pregnant uterus and the abdominal wall.
When is the best time to operate?
Surgery is generally not recommended during the first trimester.5-11 Among the reasons are the high likelihood of a corpus luteum cyst, the low likelihood of an invasive malignancy, the low risk of adnexal complications associated with observation, and the potential for pregnancy loss or teratogenicity. However, as pregnancy progresses beyond the first trimester, surgery poses other problems: Operative exposure diminishes and the need to manipulate the pregnant uterus increases.
Can surgery be delayed when a mass is detected?
Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098–1103.
Close observation is a reasonable alternative to operative intervention during pregnancy, unless a malignancy is suspected.
Schmeler and colleagues reviewed the cases of 59 women who had an adnexal mass larger than 5 cm in diameter detected during pregnancy, out of a total of 127,177 deliveries at a single institution between 1990 and 2003. Antepartum surgery was performed in 17 women (29%). Of these, 13 cases had ultrasonographic findings suggesting malignancy, and 4 had ovarian torsion. The remaining women were observed, with surgery delayed until the time of cesarean section or later.
Twenty-five of the 59 masses (42%) were dermoid cysts. Cancer was diagnosed in 4 patients (6.8%), and 1 patient (1.7%) had an LMP tumor. All 5 cases (100%) involving a malignancy had a suspicious US appearance and were identified during antepartum surgery, whereas only 12 patients with a benign tumor (22%) underwent surgery prior to delivery.
Surgery poses risks to the pregnancy
Elective surgery for an adnexal mass any time during pregnancy increases the risk of pregnancy loss and the likelihood of intrauterine growth restriction (IUGR) and preterm delivery.5,7,10,13,19 A 1989 study from Sweden20 defined a cohort of 5,405 women (from 720,000 births) who were known to have a nonobstetric operation while pregnant, with the following results:
- Congenital malformation and stillbirth were not increased in the women undergoing surgery
- The number of very-low- and low-birth-weight infants did rise, however—the result of both prematurity and IUGR
- Also elevated was the incidence of infants born alive but dying within 168 hours; these risks increased regardless of trimester
- No specific type of anesthesia or operation was associated with adverse reproductive outcomes, and the cause of those adverse outcomes was not determined.
Some recent data suggest that adnexal surgery during the late second or early third trimester poses the greatest risk of preterm delivery or IUGR, or both.13
Window of opportunity: early to mid- second trimester
During this time frame, elective surgery for an adnexal mass still affords some pelvic exposure without the need for significant uterine manipulation and has been associated with a lower risk of pregnancy complications.
The other window for operation is at the time of cesarean section. An elective cesarean section is sometimes performed specifically to manage a persistent adnexal mass. Among the factors that warrant consideration when contemplating this approach are the elective uterine incision (with its attendant implications for future pregnancies), the higher risks associated with cesarean delivery in general, the type of skin incision (a vertical incision is appropriate in the event of ovarian malignancy), the potential for better exposure or laparoscopy at a later date, the increased difficulty of ovarian cystectomy at the time of cesarean section, and the patient’s wishes.
The data on laparoscopy during the first and second trimesters of pregnancy indicate that it is as safe as laparotomy. A 1997 Swedish study21 identified cohorts of 2,181 women undergoing laparoscopy and 1,522 women undergoing laparotomy (from a total of 2,015,000 deliveries) between the fourth and 20th weeks of pregnancy. In both groups there was an increased risk for the infant to weigh less than 2,500 g, to be delivered before 37 weeks, and to have IUGR. There were no differences between the 2 groups for these and other adverse outcomes.
Small series of laparoscopic procedures to manage an adnexal mass during pregnancy suggest that this approach is most applicable during the first (for highly selected emergent cases) or early second trimester to manage masses less than 10 cm in diameter, particularly when adnexectomy is planned.
Laparoscopy may be considered “minimally invasive” because it reduces manipulation of the pregnant uterus during adnexal surgery. However, it is more difficult to assess and remove ovarian cysts laparoscopically, although an early ovarian malignancy could be staged via laparoscopy by an experienced surgeon.
Considerations during laparotomy
When performing a laparotomy or cesarean section for an adnexal mass, the surgeon must take into account a number of variables when selecting the type of incision (ie, vertical vs transverse). In general, if malignancy is suspected, or if uterine manipulation is to be minimized, a vertical incision is best. Other considerations include a prior scar, body habitus, obstetric issues, and the patient’s wishes.
The author reports no financial relationships relevant to this article.
1. Spitzer M, Kaushal N, Benjamin F. Maternal CA-125 levels in pregnancy and the puerperium. J Reprod Med. 1998;43:387-392.
2. Aoki Y, Higashino M, Ishii S, Tanaka K. Yolk sac tumor of the ovary during pregnancy: a case report. Gynecol Oncol. 2005;99:497-499.
3. Koonings PP, Platt LD, Wallace R. Incidental adnexal neoplasms at cesarean section. Obstet Gynecol. 1988;72:767-769.
4. Zanetta G, Mariani E, Lissoni A, et al. A prospective study of the role of ultrasound in the management of adnexal masses in pregnancy. BJOG. 2003;110:578-583.
5. Sherard GB, 3rd, Hodson CA, Williams HJ, et al. Adnexal masses and pregnancy: a 12 year experience. Am J Obstet Gynecol. 2003;189:358-363.
6. Bernhard LM, Klebba PK, Gray DL, Mutch DG. Predictors of persistence of adnexal masses in pregnancy. Obstet Gynecol. 1999;93:585-589.
7. Platek DN, Henderson CE, Goldberg GL. The management of a persistent adnexal mass in pregnancy. Am J Obstet Gynecol. 1995;173:1236-1240.
8. Bromley B, Benacerraf B. Adnexal masses during pregnancy: accuracy of sonographic diagnosis and outcome. J Ultrasound Med. 1997;16:447-452.
9. Hill LM, Connors-Beatty DJ, Nowak A, Tush B. The role of ultrasonography in the detection and management of adnexal mass during the second and third trimesters of pregnancy. Am J Obstet Gynecol. 1998;179:703-707.
10. Agarwal N, Parul, Kriplani A, Bhatla N, Gupta A. Management and outcome of pregnancies complicated with adnexal masses. Arch Gynecol Obstet. 2003;267:148-152.
11. Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098-1103.
12. Coenen VH, Dunton C, Cardonick E, Berghella V. Persistent adnexal masses during pregnancy. Obstet Gynecol. 1999;93:66S.-
13. Whitecar P, Turner S, Higby K. Adnexal masses in pregnancy: a review of 130 cases undergoing surgical management. Am J Obstet Gynecol. 1999;181:19-24.
14. Caspi B, Levi R, Appelman Z, Rabinerson D, Goldman G, Hagay Z. Conservative management of ovarian cystic teratoma during pregnancy and labor. Am J Obstet Gynecol. 2000;182:503-505.
15. Schilder JM, Thompson AM, DePriest PD, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87:1-7.
16. Tangir J, Zelterman D, Ma W, Schwartz PE. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101:251-257.
17. Ferrandina G, Distefano M, Testa A, De Vincenzo R, Scambia G. Management of an advanced ovarian cancer at 15 weeks of gestation: case report and literature review. Gynecol Oncol. 2005;97:693-696.
18. Caspi B, Ben-Arie A, Appelman Z, Or Y, Hagay Z. Aspiration of simple pelvic cysts during pregnancy. Gynecol Obstet Invest. 2000;49:102-105.
19. Usui R, Minakami H, Kosuge S, et al. A retrospective survey of clinical, pathologic, and prognostic features of adnexal masses operated on during pregnancy. J Obstet Gynaecol Res. 2000;26(2):89-93.
20. Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy; a registry study of 5,405 cases. Am J Obstet Gynecol. 1989;161:1178-1185.
21. Reedy MB, Källén B, Kuehl TJ. Laparoscopy during pregnancy: a study of five fetal outcome parameters with use of the Swedish health registry. Am J Obstet Gynecol. 1997;177:673-679.
22. Hermans RHM, Fischer D-C, van der Putten HWHM, et al. Adnexal masses in pregnancy. Onkologie. 2003;26:167-172.
23. Hoffman MS. Primary ovarian carcinoma during pregnancy. Clin Consul Obstet Gynecol. 1995;7:237-241.
24. Ueda M, Ueki M. Ovarian tumors associated with pregnancy. Int J Obstet Gynaecol. 1996;55:59-65.
25. Curtis M, Hopkins MP, Zarlingo T, et al. Magnetic resonance imaging to avoid laparotomy in pregnancy. Obstet Gynecol. 1993;82:833-836.
26. Leiserowitz GS, Xing G, Cress R, et al. Adnexal masses in pregnancy; how often are they malignant? Gynecol Oncol. 2006;101:315-321.
27. Rahman MS, Al-Sibai MH, Rahman J, et al. Ovarian carcinoma associated with pregnancy. A review of 9 cases. Acta Obstet Gynecol Scand. 2002;81:260-264.
CASE 1 An enlarging cystic tumor
A 20-year-old gravida 3 para 1011 visits the emergency department with persistent right flank pain. Although ultrasonography (US) shows a 21-week gestation, the patient has had no prenatal care. Imaging also reveals a right-sided ovarian tumor, 14×11×8 cm, that is mainly cystic with some internal echogenicity.
At 30 weeks’ gestation, a gynecologic oncologist is consulted. Repeat US reveals the mass to be about 20 cm in diameter and cystic, without internal papillation. The patient’s CA-125 level is 12 U/mL. Based on this information, the physicians decide the likely finding is a benign ovarian cystadenoma.
How should they proceed?
The discovery of an adnexal mass during pregnancy isn’t as rare as you might think—depending on when and how closely you look, it occurs in about 1 in 100 gestations. In most cases, we have found, the mass is clearly benign (TABLE 1), warranting only observation.
TABLE 1
Adnexal masses removed during pregnancy: Histologic profile
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Cystadenoma | 549 (33) |
| Dermoid | 451 (27) |
| Paraovarian/paratubal | 204 (12) |
| Functional | 237 (14) |
| Endometrioma | 55 (3) |
| Benign stromal | 28 (2) |
| Leiomyoma | 23 (1.5) |
| Luteoma | 8 (0.5) |
| Miscellaneous | 55 (3) |
| Malignant | 68 (4) |
| Total | 1,678 |
| Data supplied by the authors from surgical experience | |
In the case described above, the physicians followed the patient and removed the mass at term because it was cystic with no other indications of malignancy. At 37 weeks’ gestation, a cesarean section was performed through a midline laparotomy incision, followed by removal of the ovarian tumor, which was benign. The pathologist measured the tumor at 16×12×4 cm and determined that it was a corpus luteum cyst.
Presence of mass raises questions
Despite the rarity of malignancy, the discovery of an ovarian mass during pregnancy prompts several important questions:
How should the mass be assessed? How can the likelihood of malignancy be determined as quickly and efficiently as possible, without jeopardy to the pregnancy?
When is surgical intervention warranted? And when can it be postponed? Specifically, is elective operative intervention for a tumor that is probably benign appropriate during pregnancy?
When is the best time to operate? And what is the optimal surgical route?
In this article, we address these questions with a focus on intervention. As we’ll explain, only a small percentage of gravidas who have an adnexal mass require surgery during pregnancy. When surgery is necessary, it is usually indicated for an emergent problem or suspicion of malignancy. Even when ovarian cancer is confirmed, we have found that it is usually in its early stages and therefore has a favorable prognosis (TABLE 2).
TABLE 2
Malignant adnexal masses removed during pregnancy
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Epithelial | 101 (28) |
| Borderline epithelial | 147 (40) |
| Germ-cell dysgerminoma | 47 (13) |
| Other | 34 (9) |
| Stromal | 24 (7) |
| Undifferentiated | 5 (1.4) |
| Sarcoma | 2 (0.5) |
| Metastatic | 4 (1.1) |
| Total | 364 |
| Data supplied by the authors from surgical experience | |
How should a mass be assessed?
Ultrasonography and other imaging often reveal the presence of a mass and help determine whether it is benign or malignant. In fact, most adnexal masses discovered during pregnancy are incidental findings at the time of routine prenatal US. (see the most commonly found tumors.) Operative intervention is required in 3 situations:
- malignancy is suspected
- an acute complication develops
- the sheer size of the tumor is likely to cause difficulty.
Corpus luteum
A persistent corpus luteum is a normal component of pregnancy. Although it usually appears as a small cystic structure on ultrasonographic imaging, the corpus luteum of pregnancy can reach 10 cm in size. Other types of “functional” ovarian cysts may also be found during pregnancy. Most functional cysts resolve by the early second trimester.4,6 In rare cases, a cyst may develop complications such as torsion or rupture, causing acute pain or hemorrhage. Otherwise, a cystic tumor identified in the first trimester should be characterized and followed using ultrasonography (US).
Benign neoplasm
An adnexal mass that persists beyond the first trimester is more likely to be a neoplasm.3-5,10,11,22 Such a mass is generally considered clinically significant if it exceeds 5 cm in diameter and has a complex sonographic appearance. Usually such a neoplasm will be a benign cystadenoma or cystic teratoma.5,10-13,19,23,24
Benign cystic teratoma
This tumor can be identified with a fairly high degree of specificity using a variety of imaging techniques, with management based on the presumptive diagnosis. This tumor is unlikely to grow substantially during pregnancy. When it is smaller than 6 cm, such a tumor can simply be observed.14 A larger tumor can occasionally rupture or lead to torsion or obstruction of labor, but such occurrences are rare.
Benign cystadenoma
In an asymptomatic patient with imaging that suggests a benign cystadenoma (see sonogram), benign cystic teratoma, or other benign tumor, observation is reasonable in most cases.4,6,7,9-11,14,19 Operative intervention is required when there is less certainty regarding the benign nature of the tumor, an acute complication develops, or the tumor is expected to pose problems because of its large size alone.
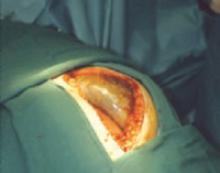
Uterine leiomyoma
It is rare for an ovarian tumor detected during pregnancy to have a solid appearance on US. When it does, it may be a uterine leiomyoma mimicking an adnexal tumor (see intraoperative photograph). It should be reevaluated with more detailed US or magnetic resonance imaging.25
Malignancy
About 10% of adnexal masses that persist during pregnancy are malignant, according to recent series.4,5,7-10,12,13,24,26
Most of the ovarian cancers diagnosed during pregnancy are epithelial, and a substantial portion of these are low-malignant-potential (LMP) tumors.5,10,11,13,19,23,24,26,27 This ratio is in keeping with the age of these women, which also explains the stage distribution (most are stage 1) and the large percentage of germ-cell tumors detected. The majority of ovarian cancers discovered in pregnant women have a favorable prognosis.
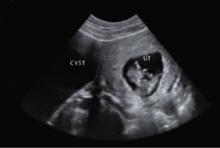
Benign-appearing cystadenoma
A morphologically benign-appearing, large, cystic adnexal mass can be seen in association with an 11-week gestation.
Leiomyoma mimics an ovarian tumor
This 17-week gestation was marked by a large pedunculated leiomyoma that at fist appeared to be a right adnexal tumor.
Appearance of adnexal masses on US
A functional cyst such as a follicular cyst, corpus luteum cyst, or theca lutein cyst usually has smooth borders and a fluid center. Other cysts may sometimes contain debris, such as clotted blood, that suggests endometriosis or a simple cyst with bleeding into it.
A benign cystic teratoma often has multiple tissue lines, evidence of calcification, and layering of fat and fluid contents.
A benign cystadenoma usually has the appearance of a simple cyst without large septates, whereas a cystadenocarcinoma often contains septates, abnormal blood flow, increased vascularity, or all of these. However, it is impossible to definitively distinguish a cystadenoma from a cystadenocarcinoma using US imaging alone.
Functional cysts usually resolve by the second trimester. A cyst warrants closer scrutiny when it persists, is larger than 5 cm in diameter, or has a complex appearance on US.
CA-125 may be useful after the first trimester
The serum CA-125 level is typically elevated during the first trimester, but may be useful during later assessment or for follow-up of a malignancy.1
A markedly elevated serum level of alpha-fetoprotein (fractionated in some cases) has been reported in some gravidas with an endodermal sinus or mixed germ-cell ovarian tumor.2 Alpha-fetoprotein should be measured when there is suspicion for a germ-cell tumor based on clinical or US findings.
When a mass is discovered during cesarean section
Occasionally, an adnexal mass is detected at the time of cesarean section (FIGURE 1).3 This phenomenon is increasingly common, given the large number of cesarean deliveries in the United States. To eliminate the need for future surgery and avoid a delay in the diagnosis of an ovarian malignancy, inspect the adnexa routinely after closing the uterine incision in all women who deliver by cesarean section.
FIGURE 1 Mass discovered at cesarean section
This cystic tumor was discovered at cesarean section that was undertaken for obstetric indications.
CASE 2 LMP tumor is suspected
A 36-year-old gravida 3 para 1011 makes a prenatal visit during the first trimester. Her previous delivery was a cesarean section through a Pfannenstiel incision for a breech presentation. US imaging reveals a 6-week, 5-day fetus and a complex left adnexal mass, 4.5×3.9×4.1 cm. Imaging is repeated 1 month later at a tertiary-care center and shows an 11-week viable fetus, a right ovary with a corpus luteum cyst, and a left ovary with a 6.6×4 cm cystic mass with extensive vascular surface papillations that is suspicious for a low-malignant-potential (LMP) tumor. In several sonograms prior to the pregnancy, this mass appeared to be solid and was 3 cm in size.
When is surgery warranted?
Surgery is indicated when physical examination or imaging of a pregnant woman reveals an adnexal mass that is suspicious for malignancy, but the physician must weigh the benefit of prompt surgery against the risk to the pregnancy. This equation can be complicated in several ways. For example, surgical staging of clinically early ovarian cancer is more difficult due to the pregnant uterus, which is more extensively manipulated during these procedures. In addition, an optimal operation sometimes necessitates removal of the uterus.
At 13 weeks’ gestation, the patient described in case 2 underwent laparoscopy with peritoneal washings and left salpingo-oophorectomy, but the tumor ruptured during removal. Final pathology showed it to be a serous LMP tumor involving the surface of the left ovary. Washings were in line with this diagnosis.
The pregnancy continued uneventfully, and a repeat cesarean section was performed at 37 weeks through the Pfannenstiel scar, followed by limited surgical staging. Exploration and all biopsies were negative, and the final diagnosis was a stage 1C serous LMP tumor of the ovary.
The patient articulated a desire to preserve her fertility and was monitored with US imaging of the remaining ovary every 6 months.
Does ‘indolent’ behavior of malignancy justify watchful waiting?
LMP tumors comprise a relatively large percentage of ovarian “cancers” encountered during pregnancy. Some authors report the accurate identification of these tumors prospectively, based on ultrasonographic characteristics.4,5 When an LMP tumor is the likely diagnosis, serial observation during pregnancy may be appropriate because of the indolent nature of the tumor. Further studies are needed to refine preoperative diagnosis and determine the overall safety of this approach.
When the problem is acute
In rare cases, a pregnant patient will have (or develop during observation) an acute problem due to torsion or rupture of an adnexal mass. Some ovarian cancers may present acutely, such as a rapidly growing malignant germ-cell tumor or a ruptured and hemorrhaging granulosecell tumor. Emergent surgery is necessary to manage the acute adnexal disease and reduce the likelihood of pregnancy loss. These events are infrequent, occurring in less than 10% of women with a known, persistent adnexal mass during pregnancy.4-14 Furthermore, recent studies have not found a substantial pregnancy complication rate associated with such emergency surgeries.
CASE 3 Suspicious mass, ascites signal need for surgery
A 19-year-old gravida 1 para 0 seeks prenatal care at 17 weeks’ gestation, complaining of rapidly enlarging abdominal girth. The physical examination estimates gestational size to be considerably greater than dates, but US is consistent with a 17-week intrauterine pregnancy. Imaging also reveals a 12-cm heterogenous left adnexal mass and a large amount of ascites.
Surgery is clearly warranted, but how extensive should it be?
When a malignancy is detected, a thorough staging procedure may be justified, depending on gestational age, exposure, desires of the patient, and operative findings. A midline incision is preferred.
Pregnant and nonpregnant women with stage 1A or 1C epithelial ovarian cancer who undergo fertility-preserving surgery (with chemotherapy in selected patients) have a good prognosis and a high likelihood of achieving a subsequent normal pregnancy.15 The same is true for women with a malignant germ-cell tumor of the ovary, even when disease is advanced.16 However, careful surgical staging is necessary.
The most important consideration when deciding whether to continue the pregnancy is the need for adjuvant chemotherapy. Depending on the gestational age and diagnosis, a short delay (4 to 6 weeks) may be appropriate to allow the pregnancy to progress beyond the first trimester or to maturity.
In case 3, a laparotomy was performed at 19 weeks’ gestation via a midline incision, and approximately 5.3 L of ascites was evacuated. A large, nonadherent left ovarian tumor was removed. The right ovary appeared to be normal, as did the gravid uterus, which was minimally manipulated. The rest of the surgical exploration was normal, and the distal portion of the omentum was excised. The frozen-section diagnosis was a malignant stromal tumor. Final pathology showed an 18×13.5×8.8 cm, poorly differentiated, SertoliLeydig-cell tumor with heterologous elements in the form of mucinous epithelium. The omentum was negative for tumor.
Chemotherapy was initiated in the third trimester, based on the limited data available, with intravenous etoposide and platinum administered every 21 days. The patient received 3 cycles of chemotherapy prior to delivery.
At 37 weeks’ gestation, labor was successfully induced. After delivery, bleomycin was added to the chemotherapy regimen, and 3 additional courses with all 3 agents were administered. The patient was lost to follow-up shortly after completing chemotherapy.
Clearly, an informed discussion of the options with the patient is imperative before any surgery, especially when chemotherapy may be delayed. Pregnancy does not appear to alter the prognosis for the patient with an ovarian malignancy, and ovarian cancer has not been reported to metastasize to the fetus.
Pregnant women have a very low rate of ovarian cancer
Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol. 2006;101:315–321.
Ovarian malignancies are rare during pregnancy. When they do occur, they are likely to be early stage and to have a favorable outcome, according to this recent population-based study.
Using 3 large databases containing records on 4,846,505 California obstetric patients between 1991 and 1999, Leiserowitz and colleagues identified 9,375 women who had an ovarian mass associated with pregnancy. Of these, 87 had ovarian cancer and 115 had a low-malignant-potential (LMP) tumor, for a cancer occurrence rate of 0.93%, or 0.0179 per 1,000 deliveries. Thirty-four of the 87 cancers were germ-cell tumors.
Of the 87 ovarian cancers, 65.5% were localized, 6.9% regional, 23% remote, and 4.6% of unknown stage. The respective rates for LMP tumors were 81.7%, 7.8%, 4.4%, and 6.1%.
Women with malignant tumors were more likely than pregnant controls without cancer to undergo cesarean delivery, hysterectomy, transfusion, and prolonged hospitalization. These women did not, however, have a higher rate of adverse neonatal outcomes.
When cancer is advanced
Few data shed light on whether a pregnancy should continue when ovarian cancer is advanced.17 The definitive surgical approach must be highly individualized.
It is not always possible to make an accurate diagnosis based on a frozen section. In such a case, the pregnancy should be preserved until the time of definitive diagnosis. As always, the patient’s wishes and gestational age must be considered.
How factors besides malignancy can influence care
Most persistent adnexal masses move well out of the pelvis as pregnancy progresses. Occasionally, however, an ovarian tumor may be located in the posterior cul-de-sac even at term, a fact easily confirmed by examination or US.4,7 A tumor in the posterior cul-de-sac can obstruct delivery or rupture. When it has a benign cystic appearance on US, it may be decompressed via transvaginal aspiration. Otherwise, the best approach is cesarean section and concomitant management of the mass.
When size alone is the problem
Some ovarian tumors are so large they seem incompatible with an advancing pregnancy. Tumors up to 20 cm in diameter have been removed intact at the time of cesarean section (FIGURE 2).18 The tumor may accommodate in shape and become less problematic as it is gradually pushed into the upper abdomen (FIGURE 3).
The ability of the peritoneal cavity to accommodate a tumor varies greatly among women. As pregnancy advances, the likelihood that a large cystic mass will rupture tends to increase. Depending on the circumstances, percutaneous aspiration7,18 or removal of a benign-appearing cystic tumor may be appropriate.
FIGURE 2 Even a very large tumor may coexist with advancing pregnancy
This benign serous cystadenoma was exteriorized at the time of cesarean section at term.
FIGURE 3 Large ovarian tumor has accommodated to the pregnancy
Laparotomy—performed at term for cesarean section and to manage this large tumor—revealed that the tumor had accommodated in shape between the enlarging pregnant uterus and the abdominal wall.
When is the best time to operate?
Surgery is generally not recommended during the first trimester.5-11 Among the reasons are the high likelihood of a corpus luteum cyst, the low likelihood of an invasive malignancy, the low risk of adnexal complications associated with observation, and the potential for pregnancy loss or teratogenicity. However, as pregnancy progresses beyond the first trimester, surgery poses other problems: Operative exposure diminishes and the need to manipulate the pregnant uterus increases.
Can surgery be delayed when a mass is detected?
Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098–1103.
Close observation is a reasonable alternative to operative intervention during pregnancy, unless a malignancy is suspected.
Schmeler and colleagues reviewed the cases of 59 women who had an adnexal mass larger than 5 cm in diameter detected during pregnancy, out of a total of 127,177 deliveries at a single institution between 1990 and 2003. Antepartum surgery was performed in 17 women (29%). Of these, 13 cases had ultrasonographic findings suggesting malignancy, and 4 had ovarian torsion. The remaining women were observed, with surgery delayed until the time of cesarean section or later.
Twenty-five of the 59 masses (42%) were dermoid cysts. Cancer was diagnosed in 4 patients (6.8%), and 1 patient (1.7%) had an LMP tumor. All 5 cases (100%) involving a malignancy had a suspicious US appearance and were identified during antepartum surgery, whereas only 12 patients with a benign tumor (22%) underwent surgery prior to delivery.
Surgery poses risks to the pregnancy
Elective surgery for an adnexal mass any time during pregnancy increases the risk of pregnancy loss and the likelihood of intrauterine growth restriction (IUGR) and preterm delivery.5,7,10,13,19 A 1989 study from Sweden20 defined a cohort of 5,405 women (from 720,000 births) who were known to have a nonobstetric operation while pregnant, with the following results:
- Congenital malformation and stillbirth were not increased in the women undergoing surgery
- The number of very-low- and low-birth-weight infants did rise, however—the result of both prematurity and IUGR
- Also elevated was the incidence of infants born alive but dying within 168 hours; these risks increased regardless of trimester
- No specific type of anesthesia or operation was associated with adverse reproductive outcomes, and the cause of those adverse outcomes was not determined.
Some recent data suggest that adnexal surgery during the late second or early third trimester poses the greatest risk of preterm delivery or IUGR, or both.13
Window of opportunity: early to mid- second trimester
During this time frame, elective surgery for an adnexal mass still affords some pelvic exposure without the need for significant uterine manipulation and has been associated with a lower risk of pregnancy complications.
The other window for operation is at the time of cesarean section. An elective cesarean section is sometimes performed specifically to manage a persistent adnexal mass. Among the factors that warrant consideration when contemplating this approach are the elective uterine incision (with its attendant implications for future pregnancies), the higher risks associated with cesarean delivery in general, the type of skin incision (a vertical incision is appropriate in the event of ovarian malignancy), the potential for better exposure or laparoscopy at a later date, the increased difficulty of ovarian cystectomy at the time of cesarean section, and the patient’s wishes.
The data on laparoscopy during the first and second trimesters of pregnancy indicate that it is as safe as laparotomy. A 1997 Swedish study21 identified cohorts of 2,181 women undergoing laparoscopy and 1,522 women undergoing laparotomy (from a total of 2,015,000 deliveries) between the fourth and 20th weeks of pregnancy. In both groups there was an increased risk for the infant to weigh less than 2,500 g, to be delivered before 37 weeks, and to have IUGR. There were no differences between the 2 groups for these and other adverse outcomes.
Small series of laparoscopic procedures to manage an adnexal mass during pregnancy suggest that this approach is most applicable during the first (for highly selected emergent cases) or early second trimester to manage masses less than 10 cm in diameter, particularly when adnexectomy is planned.
Laparoscopy may be considered “minimally invasive” because it reduces manipulation of the pregnant uterus during adnexal surgery. However, it is more difficult to assess and remove ovarian cysts laparoscopically, although an early ovarian malignancy could be staged via laparoscopy by an experienced surgeon.
Considerations during laparotomy
When performing a laparotomy or cesarean section for an adnexal mass, the surgeon must take into account a number of variables when selecting the type of incision (ie, vertical vs transverse). In general, if malignancy is suspected, or if uterine manipulation is to be minimized, a vertical incision is best. Other considerations include a prior scar, body habitus, obstetric issues, and the patient’s wishes.
The author reports no financial relationships relevant to this article.
CASE 1 An enlarging cystic tumor
A 20-year-old gravida 3 para 1011 visits the emergency department with persistent right flank pain. Although ultrasonography (US) shows a 21-week gestation, the patient has had no prenatal care. Imaging also reveals a right-sided ovarian tumor, 14×11×8 cm, that is mainly cystic with some internal echogenicity.
At 30 weeks’ gestation, a gynecologic oncologist is consulted. Repeat US reveals the mass to be about 20 cm in diameter and cystic, without internal papillation. The patient’s CA-125 level is 12 U/mL. Based on this information, the physicians decide the likely finding is a benign ovarian cystadenoma.
How should they proceed?
The discovery of an adnexal mass during pregnancy isn’t as rare as you might think—depending on when and how closely you look, it occurs in about 1 in 100 gestations. In most cases, we have found, the mass is clearly benign (TABLE 1), warranting only observation.
TABLE 1
Adnexal masses removed during pregnancy: Histologic profile
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Cystadenoma | 549 (33) |
| Dermoid | 451 (27) |
| Paraovarian/paratubal | 204 (12) |
| Functional | 237 (14) |
| Endometrioma | 55 (3) |
| Benign stromal | 28 (2) |
| Leiomyoma | 23 (1.5) |
| Luteoma | 8 (0.5) |
| Miscellaneous | 55 (3) |
| Malignant | 68 (4) |
| Total | 1,678 |
| Data supplied by the authors from surgical experience | |
In the case described above, the physicians followed the patient and removed the mass at term because it was cystic with no other indications of malignancy. At 37 weeks’ gestation, a cesarean section was performed through a midline laparotomy incision, followed by removal of the ovarian tumor, which was benign. The pathologist measured the tumor at 16×12×4 cm and determined that it was a corpus luteum cyst.
Presence of mass raises questions
Despite the rarity of malignancy, the discovery of an ovarian mass during pregnancy prompts several important questions:
How should the mass be assessed? How can the likelihood of malignancy be determined as quickly and efficiently as possible, without jeopardy to the pregnancy?
When is surgical intervention warranted? And when can it be postponed? Specifically, is elective operative intervention for a tumor that is probably benign appropriate during pregnancy?
When is the best time to operate? And what is the optimal surgical route?
In this article, we address these questions with a focus on intervention. As we’ll explain, only a small percentage of gravidas who have an adnexal mass require surgery during pregnancy. When surgery is necessary, it is usually indicated for an emergent problem or suspicion of malignancy. Even when ovarian cancer is confirmed, we have found that it is usually in its early stages and therefore has a favorable prognosis (TABLE 2).
TABLE 2
Malignant adnexal masses removed during pregnancy
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Epithelial | 101 (28) |
| Borderline epithelial | 147 (40) |
| Germ-cell dysgerminoma | 47 (13) |
| Other | 34 (9) |
| Stromal | 24 (7) |
| Undifferentiated | 5 (1.4) |
| Sarcoma | 2 (0.5) |
| Metastatic | 4 (1.1) |
| Total | 364 |
| Data supplied by the authors from surgical experience | |
How should a mass be assessed?
Ultrasonography and other imaging often reveal the presence of a mass and help determine whether it is benign or malignant. In fact, most adnexal masses discovered during pregnancy are incidental findings at the time of routine prenatal US. (see the most commonly found tumors.) Operative intervention is required in 3 situations:
- malignancy is suspected
- an acute complication develops
- the sheer size of the tumor is likely to cause difficulty.
Corpus luteum
A persistent corpus luteum is a normal component of pregnancy. Although it usually appears as a small cystic structure on ultrasonographic imaging, the corpus luteum of pregnancy can reach 10 cm in size. Other types of “functional” ovarian cysts may also be found during pregnancy. Most functional cysts resolve by the early second trimester.4,6 In rare cases, a cyst may develop complications such as torsion or rupture, causing acute pain or hemorrhage. Otherwise, a cystic tumor identified in the first trimester should be characterized and followed using ultrasonography (US).
Benign neoplasm
An adnexal mass that persists beyond the first trimester is more likely to be a neoplasm.3-5,10,11,22 Such a mass is generally considered clinically significant if it exceeds 5 cm in diameter and has a complex sonographic appearance. Usually such a neoplasm will be a benign cystadenoma or cystic teratoma.5,10-13,19,23,24
Benign cystic teratoma
This tumor can be identified with a fairly high degree of specificity using a variety of imaging techniques, with management based on the presumptive diagnosis. This tumor is unlikely to grow substantially during pregnancy. When it is smaller than 6 cm, such a tumor can simply be observed.14 A larger tumor can occasionally rupture or lead to torsion or obstruction of labor, but such occurrences are rare.
Benign cystadenoma
In an asymptomatic patient with imaging that suggests a benign cystadenoma (see sonogram), benign cystic teratoma, or other benign tumor, observation is reasonable in most cases.4,6,7,9-11,14,19 Operative intervention is required when there is less certainty regarding the benign nature of the tumor, an acute complication develops, or the tumor is expected to pose problems because of its large size alone.
Uterine leiomyoma
It is rare for an ovarian tumor detected during pregnancy to have a solid appearance on US. When it does, it may be a uterine leiomyoma mimicking an adnexal tumor (see intraoperative photograph). It should be reevaluated with more detailed US or magnetic resonance imaging.25
Malignancy
About 10% of adnexal masses that persist during pregnancy are malignant, according to recent series.4,5,7-10,12,13,24,26
Most of the ovarian cancers diagnosed during pregnancy are epithelial, and a substantial portion of these are low-malignant-potential (LMP) tumors.5,10,11,13,19,23,24,26,27 This ratio is in keeping with the age of these women, which also explains the stage distribution (most are stage 1) and the large percentage of germ-cell tumors detected. The majority of ovarian cancers discovered in pregnant women have a favorable prognosis.
Benign-appearing cystadenoma
A morphologically benign-appearing, large, cystic adnexal mass can be seen in association with an 11-week gestation.
Leiomyoma mimics an ovarian tumor
This 17-week gestation was marked by a large pedunculated leiomyoma that at fist appeared to be a right adnexal tumor.
Appearance of adnexal masses on US
A functional cyst such as a follicular cyst, corpus luteum cyst, or theca lutein cyst usually has smooth borders and a fluid center. Other cysts may sometimes contain debris, such as clotted blood, that suggests endometriosis or a simple cyst with bleeding into it.
A benign cystic teratoma often has multiple tissue lines, evidence of calcification, and layering of fat and fluid contents.
A benign cystadenoma usually has the appearance of a simple cyst without large septates, whereas a cystadenocarcinoma often contains septates, abnormal blood flow, increased vascularity, or all of these. However, it is impossible to definitively distinguish a cystadenoma from a cystadenocarcinoma using US imaging alone.
Functional cysts usually resolve by the second trimester. A cyst warrants closer scrutiny when it persists, is larger than 5 cm in diameter, or has a complex appearance on US.
CA-125 may be useful after the first trimester
The serum CA-125 level is typically elevated during the first trimester, but may be useful during later assessment or for follow-up of a malignancy.1
A markedly elevated serum level of alpha-fetoprotein (fractionated in some cases) has been reported in some gravidas with an endodermal sinus or mixed germ-cell ovarian tumor.2 Alpha-fetoprotein should be measured when there is suspicion for a germ-cell tumor based on clinical or US findings.
When a mass is discovered during cesarean section
Occasionally, an adnexal mass is detected at the time of cesarean section (FIGURE 1).3 This phenomenon is increasingly common, given the large number of cesarean deliveries in the United States. To eliminate the need for future surgery and avoid a delay in the diagnosis of an ovarian malignancy, inspect the adnexa routinely after closing the uterine incision in all women who deliver by cesarean section.
FIGURE 1 Mass discovered at cesarean section
This cystic tumor was discovered at cesarean section that was undertaken for obstetric indications.
CASE 2 LMP tumor is suspected
A 36-year-old gravida 3 para 1011 makes a prenatal visit during the first trimester. Her previous delivery was a cesarean section through a Pfannenstiel incision for a breech presentation. US imaging reveals a 6-week, 5-day fetus and a complex left adnexal mass, 4.5×3.9×4.1 cm. Imaging is repeated 1 month later at a tertiary-care center and shows an 11-week viable fetus, a right ovary with a corpus luteum cyst, and a left ovary with a 6.6×4 cm cystic mass with extensive vascular surface papillations that is suspicious for a low-malignant-potential (LMP) tumor. In several sonograms prior to the pregnancy, this mass appeared to be solid and was 3 cm in size.
When is surgery warranted?
Surgery is indicated when physical examination or imaging of a pregnant woman reveals an adnexal mass that is suspicious for malignancy, but the physician must weigh the benefit of prompt surgery against the risk to the pregnancy. This equation can be complicated in several ways. For example, surgical staging of clinically early ovarian cancer is more difficult due to the pregnant uterus, which is more extensively manipulated during these procedures. In addition, an optimal operation sometimes necessitates removal of the uterus.
At 13 weeks’ gestation, the patient described in case 2 underwent laparoscopy with peritoneal washings and left salpingo-oophorectomy, but the tumor ruptured during removal. Final pathology showed it to be a serous LMP tumor involving the surface of the left ovary. Washings were in line with this diagnosis.
The pregnancy continued uneventfully, and a repeat cesarean section was performed at 37 weeks through the Pfannenstiel scar, followed by limited surgical staging. Exploration and all biopsies were negative, and the final diagnosis was a stage 1C serous LMP tumor of the ovary.
The patient articulated a desire to preserve her fertility and was monitored with US imaging of the remaining ovary every 6 months.
Does ‘indolent’ behavior of malignancy justify watchful waiting?
LMP tumors comprise a relatively large percentage of ovarian “cancers” encountered during pregnancy. Some authors report the accurate identification of these tumors prospectively, based on ultrasonographic characteristics.4,5 When an LMP tumor is the likely diagnosis, serial observation during pregnancy may be appropriate because of the indolent nature of the tumor. Further studies are needed to refine preoperative diagnosis and determine the overall safety of this approach.
When the problem is acute
In rare cases, a pregnant patient will have (or develop during observation) an acute problem due to torsion or rupture of an adnexal mass. Some ovarian cancers may present acutely, such as a rapidly growing malignant germ-cell tumor or a ruptured and hemorrhaging granulosecell tumor. Emergent surgery is necessary to manage the acute adnexal disease and reduce the likelihood of pregnancy loss. These events are infrequent, occurring in less than 10% of women with a known, persistent adnexal mass during pregnancy.4-14 Furthermore, recent studies have not found a substantial pregnancy complication rate associated with such emergency surgeries.
CASE 3 Suspicious mass, ascites signal need for surgery
A 19-year-old gravida 1 para 0 seeks prenatal care at 17 weeks’ gestation, complaining of rapidly enlarging abdominal girth. The physical examination estimates gestational size to be considerably greater than dates, but US is consistent with a 17-week intrauterine pregnancy. Imaging also reveals a 12-cm heterogenous left adnexal mass and a large amount of ascites.
Surgery is clearly warranted, but how extensive should it be?
When a malignancy is detected, a thorough staging procedure may be justified, depending on gestational age, exposure, desires of the patient, and operative findings. A midline incision is preferred.
Pregnant and nonpregnant women with stage 1A or 1C epithelial ovarian cancer who undergo fertility-preserving surgery (with chemotherapy in selected patients) have a good prognosis and a high likelihood of achieving a subsequent normal pregnancy.15 The same is true for women with a malignant germ-cell tumor of the ovary, even when disease is advanced.16 However, careful surgical staging is necessary.
The most important consideration when deciding whether to continue the pregnancy is the need for adjuvant chemotherapy. Depending on the gestational age and diagnosis, a short delay (4 to 6 weeks) may be appropriate to allow the pregnancy to progress beyond the first trimester or to maturity.
In case 3, a laparotomy was performed at 19 weeks’ gestation via a midline incision, and approximately 5.3 L of ascites was evacuated. A large, nonadherent left ovarian tumor was removed. The right ovary appeared to be normal, as did the gravid uterus, which was minimally manipulated. The rest of the surgical exploration was normal, and the distal portion of the omentum was excised. The frozen-section diagnosis was a malignant stromal tumor. Final pathology showed an 18×13.5×8.8 cm, poorly differentiated, SertoliLeydig-cell tumor with heterologous elements in the form of mucinous epithelium. The omentum was negative for tumor.
Chemotherapy was initiated in the third trimester, based on the limited data available, with intravenous etoposide and platinum administered every 21 days. The patient received 3 cycles of chemotherapy prior to delivery.
At 37 weeks’ gestation, labor was successfully induced. After delivery, bleomycin was added to the chemotherapy regimen, and 3 additional courses with all 3 agents were administered. The patient was lost to follow-up shortly after completing chemotherapy.
Clearly, an informed discussion of the options with the patient is imperative before any surgery, especially when chemotherapy may be delayed. Pregnancy does not appear to alter the prognosis for the patient with an ovarian malignancy, and ovarian cancer has not been reported to metastasize to the fetus.
Pregnant women have a very low rate of ovarian cancer
Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol. 2006;101:315–321.
Ovarian malignancies are rare during pregnancy. When they do occur, they are likely to be early stage and to have a favorable outcome, according to this recent population-based study.
Using 3 large databases containing records on 4,846,505 California obstetric patients between 1991 and 1999, Leiserowitz and colleagues identified 9,375 women who had an ovarian mass associated with pregnancy. Of these, 87 had ovarian cancer and 115 had a low-malignant-potential (LMP) tumor, for a cancer occurrence rate of 0.93%, or 0.0179 per 1,000 deliveries. Thirty-four of the 87 cancers were germ-cell tumors.
Of the 87 ovarian cancers, 65.5% were localized, 6.9% regional, 23% remote, and 4.6% of unknown stage. The respective rates for LMP tumors were 81.7%, 7.8%, 4.4%, and 6.1%.
Women with malignant tumors were more likely than pregnant controls without cancer to undergo cesarean delivery, hysterectomy, transfusion, and prolonged hospitalization. These women did not, however, have a higher rate of adverse neonatal outcomes.
When cancer is advanced
Few data shed light on whether a pregnancy should continue when ovarian cancer is advanced.17 The definitive surgical approach must be highly individualized.
It is not always possible to make an accurate diagnosis based on a frozen section. In such a case, the pregnancy should be preserved until the time of definitive diagnosis. As always, the patient’s wishes and gestational age must be considered.
How factors besides malignancy can influence care
Most persistent adnexal masses move well out of the pelvis as pregnancy progresses. Occasionally, however, an ovarian tumor may be located in the posterior cul-de-sac even at term, a fact easily confirmed by examination or US.4,7 A tumor in the posterior cul-de-sac can obstruct delivery or rupture. When it has a benign cystic appearance on US, it may be decompressed via transvaginal aspiration. Otherwise, the best approach is cesarean section and concomitant management of the mass.
When size alone is the problem
Some ovarian tumors are so large they seem incompatible with an advancing pregnancy. Tumors up to 20 cm in diameter have been removed intact at the time of cesarean section (FIGURE 2).18 The tumor may accommodate in shape and become less problematic as it is gradually pushed into the upper abdomen (FIGURE 3).
The ability of the peritoneal cavity to accommodate a tumor varies greatly among women. As pregnancy advances, the likelihood that a large cystic mass will rupture tends to increase. Depending on the circumstances, percutaneous aspiration7,18 or removal of a benign-appearing cystic tumor may be appropriate.
FIGURE 2 Even a very large tumor may coexist with advancing pregnancy
This benign serous cystadenoma was exteriorized at the time of cesarean section at term.
FIGURE 3 Large ovarian tumor has accommodated to the pregnancy
Laparotomy—performed at term for cesarean section and to manage this large tumor—revealed that the tumor had accommodated in shape between the enlarging pregnant uterus and the abdominal wall.
When is the best time to operate?
Surgery is generally not recommended during the first trimester.5-11 Among the reasons are the high likelihood of a corpus luteum cyst, the low likelihood of an invasive malignancy, the low risk of adnexal complications associated with observation, and the potential for pregnancy loss or teratogenicity. However, as pregnancy progresses beyond the first trimester, surgery poses other problems: Operative exposure diminishes and the need to manipulate the pregnant uterus increases.
Can surgery be delayed when a mass is detected?
Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098–1103.
Close observation is a reasonable alternative to operative intervention during pregnancy, unless a malignancy is suspected.
Schmeler and colleagues reviewed the cases of 59 women who had an adnexal mass larger than 5 cm in diameter detected during pregnancy, out of a total of 127,177 deliveries at a single institution between 1990 and 2003. Antepartum surgery was performed in 17 women (29%). Of these, 13 cases had ultrasonographic findings suggesting malignancy, and 4 had ovarian torsion. The remaining women were observed, with surgery delayed until the time of cesarean section or later.
Twenty-five of the 59 masses (42%) were dermoid cysts. Cancer was diagnosed in 4 patients (6.8%), and 1 patient (1.7%) had an LMP tumor. All 5 cases (100%) involving a malignancy had a suspicious US appearance and were identified during antepartum surgery, whereas only 12 patients with a benign tumor (22%) underwent surgery prior to delivery.
Surgery poses risks to the pregnancy
Elective surgery for an adnexal mass any time during pregnancy increases the risk of pregnancy loss and the likelihood of intrauterine growth restriction (IUGR) and preterm delivery.5,7,10,13,19 A 1989 study from Sweden20 defined a cohort of 5,405 women (from 720,000 births) who were known to have a nonobstetric operation while pregnant, with the following results:
- Congenital malformation and stillbirth were not increased in the women undergoing surgery
- The number of very-low- and low-birth-weight infants did rise, however—the result of both prematurity and IUGR
- Also elevated was the incidence of infants born alive but dying within 168 hours; these risks increased regardless of trimester
- No specific type of anesthesia or operation was associated with adverse reproductive outcomes, and the cause of those adverse outcomes was not determined.
Some recent data suggest that adnexal surgery during the late second or early third trimester poses the greatest risk of preterm delivery or IUGR, or both.13
Window of opportunity: early to mid- second trimester
During this time frame, elective surgery for an adnexal mass still affords some pelvic exposure without the need for significant uterine manipulation and has been associated with a lower risk of pregnancy complications.
The other window for operation is at the time of cesarean section. An elective cesarean section is sometimes performed specifically to manage a persistent adnexal mass. Among the factors that warrant consideration when contemplating this approach are the elective uterine incision (with its attendant implications for future pregnancies), the higher risks associated with cesarean delivery in general, the type of skin incision (a vertical incision is appropriate in the event of ovarian malignancy), the potential for better exposure or laparoscopy at a later date, the increased difficulty of ovarian cystectomy at the time of cesarean section, and the patient’s wishes.
The data on laparoscopy during the first and second trimesters of pregnancy indicate that it is as safe as laparotomy. A 1997 Swedish study21 identified cohorts of 2,181 women undergoing laparoscopy and 1,522 women undergoing laparotomy (from a total of 2,015,000 deliveries) between the fourth and 20th weeks of pregnancy. In both groups there was an increased risk for the infant to weigh less than 2,500 g, to be delivered before 37 weeks, and to have IUGR. There were no differences between the 2 groups for these and other adverse outcomes.
Small series of laparoscopic procedures to manage an adnexal mass during pregnancy suggest that this approach is most applicable during the first (for highly selected emergent cases) or early second trimester to manage masses less than 10 cm in diameter, particularly when adnexectomy is planned.
Laparoscopy may be considered “minimally invasive” because it reduces manipulation of the pregnant uterus during adnexal surgery. However, it is more difficult to assess and remove ovarian cysts laparoscopically, although an early ovarian malignancy could be staged via laparoscopy by an experienced surgeon.
Considerations during laparotomy
When performing a laparotomy or cesarean section for an adnexal mass, the surgeon must take into account a number of variables when selecting the type of incision (ie, vertical vs transverse). In general, if malignancy is suspected, or if uterine manipulation is to be minimized, a vertical incision is best. Other considerations include a prior scar, body habitus, obstetric issues, and the patient’s wishes.
The author reports no financial relationships relevant to this article.
1. Spitzer M, Kaushal N, Benjamin F. Maternal CA-125 levels in pregnancy and the puerperium. J Reprod Med. 1998;43:387-392.
2. Aoki Y, Higashino M, Ishii S, Tanaka K. Yolk sac tumor of the ovary during pregnancy: a case report. Gynecol Oncol. 2005;99:497-499.
3. Koonings PP, Platt LD, Wallace R. Incidental adnexal neoplasms at cesarean section. Obstet Gynecol. 1988;72:767-769.
4. Zanetta G, Mariani E, Lissoni A, et al. A prospective study of the role of ultrasound in the management of adnexal masses in pregnancy. BJOG. 2003;110:578-583.
5. Sherard GB, 3rd, Hodson CA, Williams HJ, et al. Adnexal masses and pregnancy: a 12 year experience. Am J Obstet Gynecol. 2003;189:358-363.
6. Bernhard LM, Klebba PK, Gray DL, Mutch DG. Predictors of persistence of adnexal masses in pregnancy. Obstet Gynecol. 1999;93:585-589.
7. Platek DN, Henderson CE, Goldberg GL. The management of a persistent adnexal mass in pregnancy. Am J Obstet Gynecol. 1995;173:1236-1240.
8. Bromley B, Benacerraf B. Adnexal masses during pregnancy: accuracy of sonographic diagnosis and outcome. J Ultrasound Med. 1997;16:447-452.
9. Hill LM, Connors-Beatty DJ, Nowak A, Tush B. The role of ultrasonography in the detection and management of adnexal mass during the second and third trimesters of pregnancy. Am J Obstet Gynecol. 1998;179:703-707.
10. Agarwal N, Parul, Kriplani A, Bhatla N, Gupta A. Management and outcome of pregnancies complicated with adnexal masses. Arch Gynecol Obstet. 2003;267:148-152.
11. Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098-1103.
12. Coenen VH, Dunton C, Cardonick E, Berghella V. Persistent adnexal masses during pregnancy. Obstet Gynecol. 1999;93:66S.-
13. Whitecar P, Turner S, Higby K. Adnexal masses in pregnancy: a review of 130 cases undergoing surgical management. Am J Obstet Gynecol. 1999;181:19-24.
14. Caspi B, Levi R, Appelman Z, Rabinerson D, Goldman G, Hagay Z. Conservative management of ovarian cystic teratoma during pregnancy and labor. Am J Obstet Gynecol. 2000;182:503-505.
15. Schilder JM, Thompson AM, DePriest PD, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87:1-7.
16. Tangir J, Zelterman D, Ma W, Schwartz PE. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101:251-257.
17. Ferrandina G, Distefano M, Testa A, De Vincenzo R, Scambia G. Management of an advanced ovarian cancer at 15 weeks of gestation: case report and literature review. Gynecol Oncol. 2005;97:693-696.
18. Caspi B, Ben-Arie A, Appelman Z, Or Y, Hagay Z. Aspiration of simple pelvic cysts during pregnancy. Gynecol Obstet Invest. 2000;49:102-105.
19. Usui R, Minakami H, Kosuge S, et al. A retrospective survey of clinical, pathologic, and prognostic features of adnexal masses operated on during pregnancy. J Obstet Gynaecol Res. 2000;26(2):89-93.
20. Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy; a registry study of 5,405 cases. Am J Obstet Gynecol. 1989;161:1178-1185.
21. Reedy MB, Källén B, Kuehl TJ. Laparoscopy during pregnancy: a study of five fetal outcome parameters with use of the Swedish health registry. Am J Obstet Gynecol. 1997;177:673-679.
22. Hermans RHM, Fischer D-C, van der Putten HWHM, et al. Adnexal masses in pregnancy. Onkologie. 2003;26:167-172.
23. Hoffman MS. Primary ovarian carcinoma during pregnancy. Clin Consul Obstet Gynecol. 1995;7:237-241.
24. Ueda M, Ueki M. Ovarian tumors associated with pregnancy. Int J Obstet Gynaecol. 1996;55:59-65.
25. Curtis M, Hopkins MP, Zarlingo T, et al. Magnetic resonance imaging to avoid laparotomy in pregnancy. Obstet Gynecol. 1993;82:833-836.
26. Leiserowitz GS, Xing G, Cress R, et al. Adnexal masses in pregnancy; how often are they malignant? Gynecol Oncol. 2006;101:315-321.
27. Rahman MS, Al-Sibai MH, Rahman J, et al. Ovarian carcinoma associated with pregnancy. A review of 9 cases. Acta Obstet Gynecol Scand. 2002;81:260-264.
1. Spitzer M, Kaushal N, Benjamin F. Maternal CA-125 levels in pregnancy and the puerperium. J Reprod Med. 1998;43:387-392.
2. Aoki Y, Higashino M, Ishii S, Tanaka K. Yolk sac tumor of the ovary during pregnancy: a case report. Gynecol Oncol. 2005;99:497-499.
3. Koonings PP, Platt LD, Wallace R. Incidental adnexal neoplasms at cesarean section. Obstet Gynecol. 1988;72:767-769.
4. Zanetta G, Mariani E, Lissoni A, et al. A prospective study of the role of ultrasound in the management of adnexal masses in pregnancy. BJOG. 2003;110:578-583.
5. Sherard GB, 3rd, Hodson CA, Williams HJ, et al. Adnexal masses and pregnancy: a 12 year experience. Am J Obstet Gynecol. 2003;189:358-363.
6. Bernhard LM, Klebba PK, Gray DL, Mutch DG. Predictors of persistence of adnexal masses in pregnancy. Obstet Gynecol. 1999;93:585-589.
7. Platek DN, Henderson CE, Goldberg GL. The management of a persistent adnexal mass in pregnancy. Am J Obstet Gynecol. 1995;173:1236-1240.
8. Bromley B, Benacerraf B. Adnexal masses during pregnancy: accuracy of sonographic diagnosis and outcome. J Ultrasound Med. 1997;16:447-452.
9. Hill LM, Connors-Beatty DJ, Nowak A, Tush B. The role of ultrasonography in the detection and management of adnexal mass during the second and third trimesters of pregnancy. Am J Obstet Gynecol. 1998;179:703-707.
10. Agarwal N, Parul, Kriplani A, Bhatla N, Gupta A. Management and outcome of pregnancies complicated with adnexal masses. Arch Gynecol Obstet. 2003;267:148-152.
11. Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098-1103.
12. Coenen VH, Dunton C, Cardonick E, Berghella V. Persistent adnexal masses during pregnancy. Obstet Gynecol. 1999;93:66S.-
13. Whitecar P, Turner S, Higby K. Adnexal masses in pregnancy: a review of 130 cases undergoing surgical management. Am J Obstet Gynecol. 1999;181:19-24.
14. Caspi B, Levi R, Appelman Z, Rabinerson D, Goldman G, Hagay Z. Conservative management of ovarian cystic teratoma during pregnancy and labor. Am J Obstet Gynecol. 2000;182:503-505.
15. Schilder JM, Thompson AM, DePriest PD, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87:1-7.
16. Tangir J, Zelterman D, Ma W, Schwartz PE. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101:251-257.
17. Ferrandina G, Distefano M, Testa A, De Vincenzo R, Scambia G. Management of an advanced ovarian cancer at 15 weeks of gestation: case report and literature review. Gynecol Oncol. 2005;97:693-696.
18. Caspi B, Ben-Arie A, Appelman Z, Or Y, Hagay Z. Aspiration of simple pelvic cysts during pregnancy. Gynecol Obstet Invest. 2000;49:102-105.
19. Usui R, Minakami H, Kosuge S, et al. A retrospective survey of clinical, pathologic, and prognostic features of adnexal masses operated on during pregnancy. J Obstet Gynaecol Res. 2000;26(2):89-93.
20. Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy; a registry study of 5,405 cases. Am J Obstet Gynecol. 1989;161:1178-1185.
21. Reedy MB, Källén B, Kuehl TJ. Laparoscopy during pregnancy: a study of five fetal outcome parameters with use of the Swedish health registry. Am J Obstet Gynecol. 1997;177:673-679.
22. Hermans RHM, Fischer D-C, van der Putten HWHM, et al. Adnexal masses in pregnancy. Onkologie. 2003;26:167-172.
23. Hoffman MS. Primary ovarian carcinoma during pregnancy. Clin Consul Obstet Gynecol. 1995;7:237-241.
24. Ueda M, Ueki M. Ovarian tumors associated with pregnancy. Int J Obstet Gynaecol. 1996;55:59-65.
25. Curtis M, Hopkins MP, Zarlingo T, et al. Magnetic resonance imaging to avoid laparotomy in pregnancy. Obstet Gynecol. 1993;82:833-836.
26. Leiserowitz GS, Xing G, Cress R, et al. Adnexal masses in pregnancy; how often are they malignant? Gynecol Oncol. 2006;101:315-321.
27. Rahman MS, Al-Sibai MH, Rahman J, et al. Ovarian carcinoma associated with pregnancy. A review of 9 cases. Acta Obstet Gynecol Scand. 2002;81:260-264.
Surgical strategies to untangle a frozen pelvis
For a surgeon, the “frozen” pelvis can be as hazardous as the icy tundra that its name evokes: The reproductive organs and adjacent structures are distorted by extensive adhesive disease and fibrosis, which obscure the normal anatomic landmarks and surgical planes, making dissection extremely difficult and increasing the risk of damage to vital organs.
Despite these very real challenges, few training programs provide gynecologic residents with sufficient surgical experience to operate safely in this setting. The overall keys to success:
- Solid grounding in pelvic anatomy, with live experience involving varying degrees of pelvic distortion
- A realistic expectation that the operation will be difficult and fraught with hazards
- Flexibility to change course when a particular pathway proves too risky
- Patience to take things as slowly as necessary.
Most important is a retroperitoneal approach—not to mention complete knowledge of the retroperitoneal spaces, where the structures that nourish and support the uterus and lymphatic system lie, as well as the ureters and rectum (FIGURE 1).
It may not be sufficient to learn the anatomy of the pelvis and the steps of the operation from an atlas of surgical technique; “real-life” findings can vary greatly from those described in a textbook. The surgeon needs ample experience to recognize the appearance and tactile characteristics of disease processes that afflict the female pelvis—and to know how to manage them.
FIGURE 1 Areolar tissue fills the pelvic spaces
The pelvic spaces contain areolar tissue and can be exposed by applying traction and deeply placed retractors.
This article describes the challenges posed by the frozen pelvis, so the surgeon can confront the condition with greater confidence and understanding. There is no substitute for hands-on experience, however. Do not begin the operation if you do not think you can complete it. Seek help beforehand, not during the procedure.
Undertake a multipronged diagnostic evaluation
The potential for a frozen pelvis, as well as its causes, can usually be identified by taking a careful history and documenting previous surgeries or pelvic problems (see “Five culprits: Which one is to blame?”).
The physical examination also can be revealing. Be alert for any anatomic changes apparent at the pelvic examination, which should include a rectovaginal assessment. If a lesion is palpated, attempt to define its size and determine whether it is fixed or mobile. Also asccertain whether the cul-de-sac is free, the uterus can be lifted out of the pelvis, and the disease process is predominantly uterine, adnexal, or involves adjacent organs. Although imaging studies may be useful, a careful pelvic examination may yield more practical information about potential difficulties.
Five major causes of extensive pelvic disease lead to a frozen pelvis: infection, surgery, benign growths, malignant growths, and radiation therapy. When evaluating a patient, it is important to determine which of these conditions exist.
Infection. Adhesions and fibrosis secondary to infectious processes such as gonococcal salpingitis, tubo-ovarian abscess, a ruptured diverticulum, infected pelvic hematoma, and ruptured appendix can create anatomic abnormalities.
Surgery. The type of surgery a patient has undergone may provide important clues to potential problems. For example, pelvic distortion that arises from cesarean section and tubal reconstructive surgery differs considerably from that found in women who have undergone abdominal hysterectomy with preservation of one or both ovaries. Removal of a retained left ovary may require extensive dissection of the ureter and bowel.4
Benign and malignant growths. Uterine myomata, endometriosis, and adenomyosis are the most common benign growths that can lead to a frozen pelvis. Malignant growths of the adnexa, such as ovarian carcinoma, can necessitate en bloc resection of portions of the gastrointestinal tract along with the tumor. In contrast, carcinomas of the endometrium and cervix generally do not present with a frozen pelvis, although they occasionally require extensive or radical surgery.
Radiation therapy. When a woman has undergone radiation, pelvic structures are commonly adherent to the uterus and each other, making hysterectomy a challenge. The intestinal and urinary tracts also must be handled with great care. Even a small degree of intraoperative trauma to these structures can lead to postoperative complications including fistula formation.
Imaging studies—useful tool
Preoperative imaging can be of inestimable value. Pelvic ultrasonography,1 computed tomography, or magnetic resonance imaging may be worthwhile, as well as evaluation of the urinary and intestinal tracts. It is particularly important to learn preoperatively whether there is involvement of the ureters, bowel, and pelvic sidewalls.
Diagnostic laparoscopy may aid in planning the definitive surgery
When there is doubt about the extent of pelvic disease, diagnostic laparoscopy is a prudent way to assess the potential difficulties of surgery. The information it provides makes it possible to plan the definitive procedure and determine whether other specialists may be needed.
Other diagnostic steps, such as cystoscopy and sigmoidoscopy, can be performed at the time of diagnostic laparoscopy or postponed until the actual surgery.
Preparing for surgery
Level with the patient
Give the patient as much information as possible about potential problems with pelvic structures such as the ureters, bowel, and bladder. Also advise her that other surgeons may be called in to assist or to help repair damage to surrounding structures. In particular, counsel her about the very real possibility that a temporary diverting colostomy or ileostomy will be required. As usual, document details of these discussions in the record.
Bowel prep is imperative
In anticipation of possible enterolysis or intestinal tract surgery, all patients should undergo preoperative bowel preparation.
Consider ureteral catheterization
The possible need for preoperative ureteral catheterization should be discussed with a urologist, particularly if imaging reveals any significant ureteral deviation, fixation, constriction, or dilatation.
The use of catheters also helps the surgeon identify the ureters intraoperatively and may therefore prevent their injury.
Prepare for blood replacement
Advise the patient of the possible need for transfusion of red blood cells or other blood products during surgery. Whether it would be best to store her own blood (or that of a designated donor) or rely on the hospital blood bank depends on the circumstances of her case.
Insert a 3-way catheter
This precaution permits the instillation of retrograde dye intraoperatively to assess the integrity of the bladder.
Prophylactic anticoagulation and antibiotics? Absolutely
Postoperative wound infections and deep venous thrombosis, with the potential for life-threatening pulmonary embolization, are both significantly increased in patients who undergo pelvic surgery.2 The prophylactic use of antibiotics and blood thinners has been shown to reduce both complications and is strongly advised.
I prefer subcutaneous heparin because some newer agents, such as low-molecular-weight heparin, have been associated with significant postoperative bleeding.3
Choose an incision that guarantees broad exposure
The extreme care necessary during surgery in a frozen pelvis begins with the incision. If chosen wisely, it can help the surgeon avoid injury to the intestines upon abdominal entry.
In general, I prefer a vertical midline incision because it allows for maximum flexibility and exposure, particularly when used in conjunction with a Book-walter retractor. However, if the patient has had a prior paramedian or midline incision, extensive omental and intestinal adhesions are likely and can make entry difficult and increase the risk of intestinal injury. In such a case, an incision in a different location or direction may be wise.
For example, a transverse muscle-dividing incision may make it possible to find an area lateral to the original incision where the peritoneum, omentum, and intestinal tract are not adherent. Then, under direct vision, the incision can be extended and any adherent bowel near the midline incision can be safely dissected.
Once the fascia is incised, grasp it with a Kocher clamp. After entering the peritoneal cavity, include the peritoneum in the clamp. This allows for maximal traction during dissection of the bowel and omentum with scissors.
The most important action to take at the time of incision is to make it large enough to allow for excellent exposure.
An adequate incision and the appropriate retractor will minimize operative time and facilitate completion. The old adage that “wounds heal from side to side, not end to end” is particularly applicable.
First steps: Get oriented, assess adhesions
After entering the abdomen, identify pelvic structures and their location in relation to one another. In patients who have undergone previous surgery or had inflammatory disease, the omentum may be adherent to these structures. If the omental adhesions are filmy and easy to reduce, cut them free. However, if the omentum is densely adherent to the parietal peritoneum or other pelvic organs or bowel, it may be helpful to cut across the omentum, leaving a portion attached to the structures to be removed.
Identify landmarks
After omental or intestinal adhesions have been separated, move the small and large intestines up as far as possible from the pelvis and pack them away. Then identify the following pelvic structures: uterine fundus, round ligaments, infundibulopelvic (IP) ligaments, posterior cul-de-sac, anterior cul-de-sac, prevesical peritoneum, and pelvic brim. These structures may be difficult to recognize and to mobilize because of fibrosis and adhesions.
At one time or another, every surgeon finds it necessary to rethink a planned procedure after the operation begins—a not uncommon scenario during surgery in the frozen pelvis. It can occur at the beginning of a procedure, once the incision is made and the pathology is surveyed, or it can arise when the surgeon is well into an operation, when all the usual landmarks are indistinguishable.
When the problem is clear from the get-go
When confronted with an impossible situation upon opening the abdomen, the surgeon has 2 options:
- close the abdomen and refer the patient
- call for the aid of a surgical colleague who has the necessary experience and skill.
When the operation is under way
This situation may not lend itself to so easy a solution. When the surgeon becomes overwhelmed by an unfamiliar operative field, he or she should stop operating, take stock of what has been accomplished and what remains to be done, check the status of the patient, and reevaluate the case. Again, 2 options are available:
- change the original goal and terminate the procedure at that point, scheduling reoperation for a later date
- call for help, particularly if arrangements have been made beforehand.
Either way, a compromised patient is too high a price to pay for the sake of the surgeon’s vanity, and the dictum of “primum non nocere” should become the guiding principle.
Choose entry into the retroperitoneum with care
Once you have identified the structures, determine how you will be entering the retroperitoneum. This decision is important because the blood supply to the uterus and adnexa lies in the retroperitoneum, as do the ureters, which must be identified and kept under direct vision during ligation of the IP ligaments and dissection of the peritoneum around the uterus.
Retroperitoneal entry and elaboration of the retroperitoneal spaces are keys to the safe performance of a difficult hysterectomy or removal of retained adnexa in a patient with a frozen pelvis. The retroperitoneal approach makes it possible to reach around structures that are fixed in the pelvis, to identify the blood supply and other vital structures, and to proceed safely.
Several entry sites are possible. In the frozen pelvis, I believe the round ligament is the ideal location. Identify and divide this ligament as it enters the internal ring, and incise the peritoneum cephalad along the course of the IP ligaments (FIGURE 2).
FIGURE 2 Transect the round ligaments
To facilitate entry into the retroperitoneum, transect the round ligaments. Then develop the retroperitoneal spaces using blunt dissection.Next, open the pararectal space to visualize the iliac vessels and ureter, which can be identified on the medial leaf of the peritoneum. The IP ligaments can then be visualized and safely divided. Extending the dissection caudad allows entry into the retrovesical space and into areas of the broad ligament adjacent to the uterus. This approach also makes it possible to produce traction on the peritoneum and facilitate separation of the areolar tissue between the bladder and cervix to enter the vesicovaginal space. Continue dissection of the bladder from the cervix and vagina by operating from one side of the pelvis to the other.
The ureter, a surgeon’s nemesis
Never assume you know the position of the ureter without confirming it; a major deviation of its course can occur secondary to pathologic processes in the pelvis. The ureter can be identified by direct visualization, peristalsis, and palpation. If it is rolled between the thumb and forefinger, the ureter produces a snapping sensation.
After entering the retroperitoneum, identify the ureter between the pelvic brim and Wertheim’s tunnel. Although it may, at times, be seen through the thin peritoneum as it travels over the common iliac artery down into the pelvis, the ureter is generally not clearly visualized in the frozen pelvis and may become apparent only after the peritoneum has been opened and the retroperitoneal spaces elaborated.
Near the level of the pelvic brim on the left side of the body, the ureter will be closer to the IP ligament than it is on the right side, due to the location of the sigmoid colon and its mesentery on the left side, which elevate the ureter in the ventral direction.
Once the ureter has been identified, leave it attached to the peritoneum as much as possible. When the ureter is adherent to pelvic pathology, lateralize it from the medial leaf of the broad ligament as far down into the pelvis as necessary to allow complete removal of the peritoneum along with the pathology. The extent of dissection necessary will vary with the pathology.
Handle the ureter gently
Never handle the ureter excessively or unnecessarily. When it is dissected free, let it lie free rather than retracting it with umbilical tapes or Penrose drains, which can slide up and down, damaging the vasculature that lies in the adventitia of the ureteral wall.
Freeing the bladder can be difficult
A history of surgery in the area of the bladder, such as cesarean section or bladder advancement with uterine suspension, may leave the bladder adherent to or hard to separate from the cervix and vagina. Normally, the vesicouterine peritoneum is flexible, mobile, and easy to free from the cervix and vagina. A history of disease processes such as endometriosis, infection, or tumors makes this dissection difficult, with a real risk of inadvertent cystotomy.
One technique to make this dissection easier and safer is to enter the retroperitoneum laterally near the round ligament. In this location, the bladder may not have been involved in the prior dissection, and the tissue may be more areolar and less dense than it is in the midline. After entering the retroperitoneum, elevate the bladder by incising the vesicouterine peritoneum transversely. By rolling a finger or an instrument cephalad in the anterior leaf of the broad ligament, the bladder can be sharply separated from the cervix.
When the bladder is densely adherent, make an incision into its dome away from the cervix to visualize the interior of the bladder. Place the index finger into the bladder to identify its reflection, and cut through the dense adhesions between it and the cervix and vagina (FIGURE 3).
FIGURE 3 Incise the broad-ligament peritoneum
Incise the peritoneum of the avascular layer of the anterior broad ligament bilaterally between the divided round ligaments to develop the vesical space bilaterally. One way to do this is to gently tease away the areolar tissue using a finger, pushing the tissue medially. Then divide the peritoneum between the 2 spaces and sweep the bladder off the cervix using blunt or sharp dissection.
Cul-de-sac of Douglas may be hard to recognize
In a disease-free pelvis, the posterior cul-de-sac is bounded laterally by the uterosacral ligaments, posteriorly by the rectum and sacrum, and caudally by the vagina—but these relationships are usually lost in the frozen pelvis. Extensive inflammatory disease, tumors of the tubes and ovaries, extensive pelvic endometriosis, and prior infection due to a diverticular abscess or ruptured appendix can obscure the normal confines of the cul-de-sac. It can also be obliterated by large myomas, which can fill the pelvic cavity and extend upward beyond the sacral promontory into the abdominal cavity, thereby displacing the intestines and creating potential deviation and compression of the ureters.
Freeing the peritoneal attachments both anteriorly and posteriorly, as well as at the sides of the pelvis, may release the pelvic contents and allow elevation of the uterus into the abdominal cavity. Then the ureter, uterine vasculature, and supporting ligaments can be identified. Dissection becomes simpler after this point.
However, when the rectum and ureters are densely adherent, as they often are in the frozen pelvis, dissection can become difficult, with a real danger of rectal perforation. A basic principle in any hysterectomy is to hug the uterus as closely as possible, staying near the posterior surface of the uterus and cervix using both blunt and sharp dissection. This eventually makes it possible to find a reasonable plane to enter the rectovaginal space at the superior portion of the cul-de-sac between the uterosacral ligaments.
The tissue below this level is not usually involved in the frozen pelvis and will give way readily once the uterosacral ligaments are divided. It is unnecessary to operate beyond this level to any great extent because the surgery already extends distal to the cervicovaginal junction. In some circumstances, it may be necessary to enter the vagina anteriorly to define the relationship between the posterior cervix and adherent bowel (FIGURE 4).
FIGURE 4 Mobilize the uterus
To free the uterus from its posterior visceral attachments and render it mobile, develop the rectovaginal space.
Identifying and repairing bowel injury
If the bowel has been prepped, and rectal enterotomy occurs during dissection, closure and drainage are the only necessary steps, and a temporary colostomy can be avoided. Bowel resection may be necessary if the bowel has sustained damage.
If bowel injury is suspected, irrigate the bowel through the rectum with sterile milk or dye solution using a Foley catheter with a 30-cc bag. Another widely used option to assess rectosigmoid integrity is to insufflate the submersed rectosigmoid with air. Bubbles signal a breach in the integrity of the bowel wall.
Postoperative care
After surgery, the principles of early mobilization and ambulation are key. The single most important postoperative intervention is early ambulation, which can minimize pulmonary problems, ileus, bladder atony, and thromboembolic disease secondary to deep venous thrombosis.
To protect against venous thromboembolic disease, use pneumatic compression devices and early ambulation. In women with other risk factors for deep venous thrombosis, such as malignancy, diabetes, obesity, and smoking, consider subcutaneous heparin.
Unless the bowel was resected or extensive adhesiolysis was performed, I generally allow the patient to have clear liquids on the first postoperative day. I then advance oral intake after gastrointestinal motility returns or when the patient complains of hunger.
Remove the urethral catheter on the first postoperative day unless cystotomy or extensive dissection of the ureters or bladder was performed.
The author reports no financial relationships relevant to this article.
1. Willson JR. Ultrasonography in the diagnosis of gynecologic disorders. Am J Obstet Gynecol. 1991;160:1064-1071.
2. Polk HC, Jr. Continuing refinements in surgical antibiotic prophylaxis. Arch Surg. 2005;140:1066-1067.
3. Fejgin MD, Lourwood DL. Low-molecular-weight heparins and their use in obstetrics and gynecology. Obstet Gynecol Surv. 1994;49:424-426.
4. Magitbay PM, Nyholm JL, Hernandez JL, Podratz KC. Ovarian remnant syndrome. Am J Obstet Gynecol. 2005;193:2062-2066.
For a surgeon, the “frozen” pelvis can be as hazardous as the icy tundra that its name evokes: The reproductive organs and adjacent structures are distorted by extensive adhesive disease and fibrosis, which obscure the normal anatomic landmarks and surgical planes, making dissection extremely difficult and increasing the risk of damage to vital organs.
Despite these very real challenges, few training programs provide gynecologic residents with sufficient surgical experience to operate safely in this setting. The overall keys to success:
- Solid grounding in pelvic anatomy, with live experience involving varying degrees of pelvic distortion
- A realistic expectation that the operation will be difficult and fraught with hazards
- Flexibility to change course when a particular pathway proves too risky
- Patience to take things as slowly as necessary.
Most important is a retroperitoneal approach—not to mention complete knowledge of the retroperitoneal spaces, where the structures that nourish and support the uterus and lymphatic system lie, as well as the ureters and rectum (FIGURE 1).
It may not be sufficient to learn the anatomy of the pelvis and the steps of the operation from an atlas of surgical technique; “real-life” findings can vary greatly from those described in a textbook. The surgeon needs ample experience to recognize the appearance and tactile characteristics of disease processes that afflict the female pelvis—and to know how to manage them.
FIGURE 1 Areolar tissue fills the pelvic spaces
The pelvic spaces contain areolar tissue and can be exposed by applying traction and deeply placed retractors.
This article describes the challenges posed by the frozen pelvis, so the surgeon can confront the condition with greater confidence and understanding. There is no substitute for hands-on experience, however. Do not begin the operation if you do not think you can complete it. Seek help beforehand, not during the procedure.
Undertake a multipronged diagnostic evaluation
The potential for a frozen pelvis, as well as its causes, can usually be identified by taking a careful history and documenting previous surgeries or pelvic problems (see “Five culprits: Which one is to blame?”).
The physical examination also can be revealing. Be alert for any anatomic changes apparent at the pelvic examination, which should include a rectovaginal assessment. If a lesion is palpated, attempt to define its size and determine whether it is fixed or mobile. Also asccertain whether the cul-de-sac is free, the uterus can be lifted out of the pelvis, and the disease process is predominantly uterine, adnexal, or involves adjacent organs. Although imaging studies may be useful, a careful pelvic examination may yield more practical information about potential difficulties.
Five major causes of extensive pelvic disease lead to a frozen pelvis: infection, surgery, benign growths, malignant growths, and radiation therapy. When evaluating a patient, it is important to determine which of these conditions exist.
Infection. Adhesions and fibrosis secondary to infectious processes such as gonococcal salpingitis, tubo-ovarian abscess, a ruptured diverticulum, infected pelvic hematoma, and ruptured appendix can create anatomic abnormalities.
Surgery. The type of surgery a patient has undergone may provide important clues to potential problems. For example, pelvic distortion that arises from cesarean section and tubal reconstructive surgery differs considerably from that found in women who have undergone abdominal hysterectomy with preservation of one or both ovaries. Removal of a retained left ovary may require extensive dissection of the ureter and bowel.4
Benign and malignant growths. Uterine myomata, endometriosis, and adenomyosis are the most common benign growths that can lead to a frozen pelvis. Malignant growths of the adnexa, such as ovarian carcinoma, can necessitate en bloc resection of portions of the gastrointestinal tract along with the tumor. In contrast, carcinomas of the endometrium and cervix generally do not present with a frozen pelvis, although they occasionally require extensive or radical surgery.
Radiation therapy. When a woman has undergone radiation, pelvic structures are commonly adherent to the uterus and each other, making hysterectomy a challenge. The intestinal and urinary tracts also must be handled with great care. Even a small degree of intraoperative trauma to these structures can lead to postoperative complications including fistula formation.
Imaging studies—useful tool
Preoperative imaging can be of inestimable value. Pelvic ultrasonography,1 computed tomography, or magnetic resonance imaging may be worthwhile, as well as evaluation of the urinary and intestinal tracts. It is particularly important to learn preoperatively whether there is involvement of the ureters, bowel, and pelvic sidewalls.
Diagnostic laparoscopy may aid in planning the definitive surgery
When there is doubt about the extent of pelvic disease, diagnostic laparoscopy is a prudent way to assess the potential difficulties of surgery. The information it provides makes it possible to plan the definitive procedure and determine whether other specialists may be needed.
Other diagnostic steps, such as cystoscopy and sigmoidoscopy, can be performed at the time of diagnostic laparoscopy or postponed until the actual surgery.
Preparing for surgery
Level with the patient
Give the patient as much information as possible about potential problems with pelvic structures such as the ureters, bowel, and bladder. Also advise her that other surgeons may be called in to assist or to help repair damage to surrounding structures. In particular, counsel her about the very real possibility that a temporary diverting colostomy or ileostomy will be required. As usual, document details of these discussions in the record.
Bowel prep is imperative
In anticipation of possible enterolysis or intestinal tract surgery, all patients should undergo preoperative bowel preparation.
Consider ureteral catheterization
The possible need for preoperative ureteral catheterization should be discussed with a urologist, particularly if imaging reveals any significant ureteral deviation, fixation, constriction, or dilatation.
The use of catheters also helps the surgeon identify the ureters intraoperatively and may therefore prevent their injury.
Prepare for blood replacement
Advise the patient of the possible need for transfusion of red blood cells or other blood products during surgery. Whether it would be best to store her own blood (or that of a designated donor) or rely on the hospital blood bank depends on the circumstances of her case.
Insert a 3-way catheter
This precaution permits the instillation of retrograde dye intraoperatively to assess the integrity of the bladder.
Prophylactic anticoagulation and antibiotics? Absolutely
Postoperative wound infections and deep venous thrombosis, with the potential for life-threatening pulmonary embolization, are both significantly increased in patients who undergo pelvic surgery.2 The prophylactic use of antibiotics and blood thinners has been shown to reduce both complications and is strongly advised.
I prefer subcutaneous heparin because some newer agents, such as low-molecular-weight heparin, have been associated with significant postoperative bleeding.3
Choose an incision that guarantees broad exposure
The extreme care necessary during surgery in a frozen pelvis begins with the incision. If chosen wisely, it can help the surgeon avoid injury to the intestines upon abdominal entry.
In general, I prefer a vertical midline incision because it allows for maximum flexibility and exposure, particularly when used in conjunction with a Book-walter retractor. However, if the patient has had a prior paramedian or midline incision, extensive omental and intestinal adhesions are likely and can make entry difficult and increase the risk of intestinal injury. In such a case, an incision in a different location or direction may be wise.
For example, a transverse muscle-dividing incision may make it possible to find an area lateral to the original incision where the peritoneum, omentum, and intestinal tract are not adherent. Then, under direct vision, the incision can be extended and any adherent bowel near the midline incision can be safely dissected.
Once the fascia is incised, grasp it with a Kocher clamp. After entering the peritoneal cavity, include the peritoneum in the clamp. This allows for maximal traction during dissection of the bowel and omentum with scissors.
The most important action to take at the time of incision is to make it large enough to allow for excellent exposure.
An adequate incision and the appropriate retractor will minimize operative time and facilitate completion. The old adage that “wounds heal from side to side, not end to end” is particularly applicable.
First steps: Get oriented, assess adhesions
After entering the abdomen, identify pelvic structures and their location in relation to one another. In patients who have undergone previous surgery or had inflammatory disease, the omentum may be adherent to these structures. If the omental adhesions are filmy and easy to reduce, cut them free. However, if the omentum is densely adherent to the parietal peritoneum or other pelvic organs or bowel, it may be helpful to cut across the omentum, leaving a portion attached to the structures to be removed.
Identify landmarks
After omental or intestinal adhesions have been separated, move the small and large intestines up as far as possible from the pelvis and pack them away. Then identify the following pelvic structures: uterine fundus, round ligaments, infundibulopelvic (IP) ligaments, posterior cul-de-sac, anterior cul-de-sac, prevesical peritoneum, and pelvic brim. These structures may be difficult to recognize and to mobilize because of fibrosis and adhesions.
At one time or another, every surgeon finds it necessary to rethink a planned procedure after the operation begins—a not uncommon scenario during surgery in the frozen pelvis. It can occur at the beginning of a procedure, once the incision is made and the pathology is surveyed, or it can arise when the surgeon is well into an operation, when all the usual landmarks are indistinguishable.
When the problem is clear from the get-go
When confronted with an impossible situation upon opening the abdomen, the surgeon has 2 options:
- close the abdomen and refer the patient
- call for the aid of a surgical colleague who has the necessary experience and skill.
When the operation is under way
This situation may not lend itself to so easy a solution. When the surgeon becomes overwhelmed by an unfamiliar operative field, he or she should stop operating, take stock of what has been accomplished and what remains to be done, check the status of the patient, and reevaluate the case. Again, 2 options are available:
- change the original goal and terminate the procedure at that point, scheduling reoperation for a later date
- call for help, particularly if arrangements have been made beforehand.
Either way, a compromised patient is too high a price to pay for the sake of the surgeon’s vanity, and the dictum of “primum non nocere” should become the guiding principle.
Choose entry into the retroperitoneum with care
Once you have identified the structures, determine how you will be entering the retroperitoneum. This decision is important because the blood supply to the uterus and adnexa lies in the retroperitoneum, as do the ureters, which must be identified and kept under direct vision during ligation of the IP ligaments and dissection of the peritoneum around the uterus.
Retroperitoneal entry and elaboration of the retroperitoneal spaces are keys to the safe performance of a difficult hysterectomy or removal of retained adnexa in a patient with a frozen pelvis. The retroperitoneal approach makes it possible to reach around structures that are fixed in the pelvis, to identify the blood supply and other vital structures, and to proceed safely.
Several entry sites are possible. In the frozen pelvis, I believe the round ligament is the ideal location. Identify and divide this ligament as it enters the internal ring, and incise the peritoneum cephalad along the course of the IP ligaments (FIGURE 2).
FIGURE 2 Transect the round ligaments
To facilitate entry into the retroperitoneum, transect the round ligaments. Then develop the retroperitoneal spaces using blunt dissection.Next, open the pararectal space to visualize the iliac vessels and ureter, which can be identified on the medial leaf of the peritoneum. The IP ligaments can then be visualized and safely divided. Extending the dissection caudad allows entry into the retrovesical space and into areas of the broad ligament adjacent to the uterus. This approach also makes it possible to produce traction on the peritoneum and facilitate separation of the areolar tissue between the bladder and cervix to enter the vesicovaginal space. Continue dissection of the bladder from the cervix and vagina by operating from one side of the pelvis to the other.
The ureter, a surgeon’s nemesis
Never assume you know the position of the ureter without confirming it; a major deviation of its course can occur secondary to pathologic processes in the pelvis. The ureter can be identified by direct visualization, peristalsis, and palpation. If it is rolled between the thumb and forefinger, the ureter produces a snapping sensation.
After entering the retroperitoneum, identify the ureter between the pelvic brim and Wertheim’s tunnel. Although it may, at times, be seen through the thin peritoneum as it travels over the common iliac artery down into the pelvis, the ureter is generally not clearly visualized in the frozen pelvis and may become apparent only after the peritoneum has been opened and the retroperitoneal spaces elaborated.
Near the level of the pelvic brim on the left side of the body, the ureter will be closer to the IP ligament than it is on the right side, due to the location of the sigmoid colon and its mesentery on the left side, which elevate the ureter in the ventral direction.
Once the ureter has been identified, leave it attached to the peritoneum as much as possible. When the ureter is adherent to pelvic pathology, lateralize it from the medial leaf of the broad ligament as far down into the pelvis as necessary to allow complete removal of the peritoneum along with the pathology. The extent of dissection necessary will vary with the pathology.
Handle the ureter gently
Never handle the ureter excessively or unnecessarily. When it is dissected free, let it lie free rather than retracting it with umbilical tapes or Penrose drains, which can slide up and down, damaging the vasculature that lies in the adventitia of the ureteral wall.
Freeing the bladder can be difficult
A history of surgery in the area of the bladder, such as cesarean section or bladder advancement with uterine suspension, may leave the bladder adherent to or hard to separate from the cervix and vagina. Normally, the vesicouterine peritoneum is flexible, mobile, and easy to free from the cervix and vagina. A history of disease processes such as endometriosis, infection, or tumors makes this dissection difficult, with a real risk of inadvertent cystotomy.
One technique to make this dissection easier and safer is to enter the retroperitoneum laterally near the round ligament. In this location, the bladder may not have been involved in the prior dissection, and the tissue may be more areolar and less dense than it is in the midline. After entering the retroperitoneum, elevate the bladder by incising the vesicouterine peritoneum transversely. By rolling a finger or an instrument cephalad in the anterior leaf of the broad ligament, the bladder can be sharply separated from the cervix.
When the bladder is densely adherent, make an incision into its dome away from the cervix to visualize the interior of the bladder. Place the index finger into the bladder to identify its reflection, and cut through the dense adhesions between it and the cervix and vagina (FIGURE 3).
FIGURE 3 Incise the broad-ligament peritoneum
Incise the peritoneum of the avascular layer of the anterior broad ligament bilaterally between the divided round ligaments to develop the vesical space bilaterally. One way to do this is to gently tease away the areolar tissue using a finger, pushing the tissue medially. Then divide the peritoneum between the 2 spaces and sweep the bladder off the cervix using blunt or sharp dissection.
Cul-de-sac of Douglas may be hard to recognize
In a disease-free pelvis, the posterior cul-de-sac is bounded laterally by the uterosacral ligaments, posteriorly by the rectum and sacrum, and caudally by the vagina—but these relationships are usually lost in the frozen pelvis. Extensive inflammatory disease, tumors of the tubes and ovaries, extensive pelvic endometriosis, and prior infection due to a diverticular abscess or ruptured appendix can obscure the normal confines of the cul-de-sac. It can also be obliterated by large myomas, which can fill the pelvic cavity and extend upward beyond the sacral promontory into the abdominal cavity, thereby displacing the intestines and creating potential deviation and compression of the ureters.
Freeing the peritoneal attachments both anteriorly and posteriorly, as well as at the sides of the pelvis, may release the pelvic contents and allow elevation of the uterus into the abdominal cavity. Then the ureter, uterine vasculature, and supporting ligaments can be identified. Dissection becomes simpler after this point.
However, when the rectum and ureters are densely adherent, as they often are in the frozen pelvis, dissection can become difficult, with a real danger of rectal perforation. A basic principle in any hysterectomy is to hug the uterus as closely as possible, staying near the posterior surface of the uterus and cervix using both blunt and sharp dissection. This eventually makes it possible to find a reasonable plane to enter the rectovaginal space at the superior portion of the cul-de-sac between the uterosacral ligaments.
The tissue below this level is not usually involved in the frozen pelvis and will give way readily once the uterosacral ligaments are divided. It is unnecessary to operate beyond this level to any great extent because the surgery already extends distal to the cervicovaginal junction. In some circumstances, it may be necessary to enter the vagina anteriorly to define the relationship between the posterior cervix and adherent bowel (FIGURE 4).
FIGURE 4 Mobilize the uterus
To free the uterus from its posterior visceral attachments and render it mobile, develop the rectovaginal space.
Identifying and repairing bowel injury
If the bowel has been prepped, and rectal enterotomy occurs during dissection, closure and drainage are the only necessary steps, and a temporary colostomy can be avoided. Bowel resection may be necessary if the bowel has sustained damage.
If bowel injury is suspected, irrigate the bowel through the rectum with sterile milk or dye solution using a Foley catheter with a 30-cc bag. Another widely used option to assess rectosigmoid integrity is to insufflate the submersed rectosigmoid with air. Bubbles signal a breach in the integrity of the bowel wall.
Postoperative care
After surgery, the principles of early mobilization and ambulation are key. The single most important postoperative intervention is early ambulation, which can minimize pulmonary problems, ileus, bladder atony, and thromboembolic disease secondary to deep venous thrombosis.
To protect against venous thromboembolic disease, use pneumatic compression devices and early ambulation. In women with other risk factors for deep venous thrombosis, such as malignancy, diabetes, obesity, and smoking, consider subcutaneous heparin.
Unless the bowel was resected or extensive adhesiolysis was performed, I generally allow the patient to have clear liquids on the first postoperative day. I then advance oral intake after gastrointestinal motility returns or when the patient complains of hunger.
Remove the urethral catheter on the first postoperative day unless cystotomy or extensive dissection of the ureters or bladder was performed.
The author reports no financial relationships relevant to this article.
For a surgeon, the “frozen” pelvis can be as hazardous as the icy tundra that its name evokes: The reproductive organs and adjacent structures are distorted by extensive adhesive disease and fibrosis, which obscure the normal anatomic landmarks and surgical planes, making dissection extremely difficult and increasing the risk of damage to vital organs.
Despite these very real challenges, few training programs provide gynecologic residents with sufficient surgical experience to operate safely in this setting. The overall keys to success:
- Solid grounding in pelvic anatomy, with live experience involving varying degrees of pelvic distortion
- A realistic expectation that the operation will be difficult and fraught with hazards
- Flexibility to change course when a particular pathway proves too risky
- Patience to take things as slowly as necessary.
Most important is a retroperitoneal approach—not to mention complete knowledge of the retroperitoneal spaces, where the structures that nourish and support the uterus and lymphatic system lie, as well as the ureters and rectum (FIGURE 1).
It may not be sufficient to learn the anatomy of the pelvis and the steps of the operation from an atlas of surgical technique; “real-life” findings can vary greatly from those described in a textbook. The surgeon needs ample experience to recognize the appearance and tactile characteristics of disease processes that afflict the female pelvis—and to know how to manage them.
FIGURE 1 Areolar tissue fills the pelvic spaces
The pelvic spaces contain areolar tissue and can be exposed by applying traction and deeply placed retractors.
This article describes the challenges posed by the frozen pelvis, so the surgeon can confront the condition with greater confidence and understanding. There is no substitute for hands-on experience, however. Do not begin the operation if you do not think you can complete it. Seek help beforehand, not during the procedure.
Undertake a multipronged diagnostic evaluation
The potential for a frozen pelvis, as well as its causes, can usually be identified by taking a careful history and documenting previous surgeries or pelvic problems (see “Five culprits: Which one is to blame?”).
The physical examination also can be revealing. Be alert for any anatomic changes apparent at the pelvic examination, which should include a rectovaginal assessment. If a lesion is palpated, attempt to define its size and determine whether it is fixed or mobile. Also asccertain whether the cul-de-sac is free, the uterus can be lifted out of the pelvis, and the disease process is predominantly uterine, adnexal, or involves adjacent organs. Although imaging studies may be useful, a careful pelvic examination may yield more practical information about potential difficulties.
Five major causes of extensive pelvic disease lead to a frozen pelvis: infection, surgery, benign growths, malignant growths, and radiation therapy. When evaluating a patient, it is important to determine which of these conditions exist.
Infection. Adhesions and fibrosis secondary to infectious processes such as gonococcal salpingitis, tubo-ovarian abscess, a ruptured diverticulum, infected pelvic hematoma, and ruptured appendix can create anatomic abnormalities.
Surgery. The type of surgery a patient has undergone may provide important clues to potential problems. For example, pelvic distortion that arises from cesarean section and tubal reconstructive surgery differs considerably from that found in women who have undergone abdominal hysterectomy with preservation of one or both ovaries. Removal of a retained left ovary may require extensive dissection of the ureter and bowel.4
Benign and malignant growths. Uterine myomata, endometriosis, and adenomyosis are the most common benign growths that can lead to a frozen pelvis. Malignant growths of the adnexa, such as ovarian carcinoma, can necessitate en bloc resection of portions of the gastrointestinal tract along with the tumor. In contrast, carcinomas of the endometrium and cervix generally do not present with a frozen pelvis, although they occasionally require extensive or radical surgery.
Radiation therapy. When a woman has undergone radiation, pelvic structures are commonly adherent to the uterus and each other, making hysterectomy a challenge. The intestinal and urinary tracts also must be handled with great care. Even a small degree of intraoperative trauma to these structures can lead to postoperative complications including fistula formation.
Imaging studies—useful tool
Preoperative imaging can be of inestimable value. Pelvic ultrasonography,1 computed tomography, or magnetic resonance imaging may be worthwhile, as well as evaluation of the urinary and intestinal tracts. It is particularly important to learn preoperatively whether there is involvement of the ureters, bowel, and pelvic sidewalls.
Diagnostic laparoscopy may aid in planning the definitive surgery
When there is doubt about the extent of pelvic disease, diagnostic laparoscopy is a prudent way to assess the potential difficulties of surgery. The information it provides makes it possible to plan the definitive procedure and determine whether other specialists may be needed.
Other diagnostic steps, such as cystoscopy and sigmoidoscopy, can be performed at the time of diagnostic laparoscopy or postponed until the actual surgery.
Preparing for surgery
Level with the patient
Give the patient as much information as possible about potential problems with pelvic structures such as the ureters, bowel, and bladder. Also advise her that other surgeons may be called in to assist or to help repair damage to surrounding structures. In particular, counsel her about the very real possibility that a temporary diverting colostomy or ileostomy will be required. As usual, document details of these discussions in the record.
Bowel prep is imperative
In anticipation of possible enterolysis or intestinal tract surgery, all patients should undergo preoperative bowel preparation.
Consider ureteral catheterization
The possible need for preoperative ureteral catheterization should be discussed with a urologist, particularly if imaging reveals any significant ureteral deviation, fixation, constriction, or dilatation.
The use of catheters also helps the surgeon identify the ureters intraoperatively and may therefore prevent their injury.
Prepare for blood replacement
Advise the patient of the possible need for transfusion of red blood cells or other blood products during surgery. Whether it would be best to store her own blood (or that of a designated donor) or rely on the hospital blood bank depends on the circumstances of her case.
Insert a 3-way catheter
This precaution permits the instillation of retrograde dye intraoperatively to assess the integrity of the bladder.
Prophylactic anticoagulation and antibiotics? Absolutely
Postoperative wound infections and deep venous thrombosis, with the potential for life-threatening pulmonary embolization, are both significantly increased in patients who undergo pelvic surgery.2 The prophylactic use of antibiotics and blood thinners has been shown to reduce both complications and is strongly advised.
I prefer subcutaneous heparin because some newer agents, such as low-molecular-weight heparin, have been associated with significant postoperative bleeding.3
Choose an incision that guarantees broad exposure
The extreme care necessary during surgery in a frozen pelvis begins with the incision. If chosen wisely, it can help the surgeon avoid injury to the intestines upon abdominal entry.
In general, I prefer a vertical midline incision because it allows for maximum flexibility and exposure, particularly when used in conjunction with a Book-walter retractor. However, if the patient has had a prior paramedian or midline incision, extensive omental and intestinal adhesions are likely and can make entry difficult and increase the risk of intestinal injury. In such a case, an incision in a different location or direction may be wise.
For example, a transverse muscle-dividing incision may make it possible to find an area lateral to the original incision where the peritoneum, omentum, and intestinal tract are not adherent. Then, under direct vision, the incision can be extended and any adherent bowel near the midline incision can be safely dissected.
Once the fascia is incised, grasp it with a Kocher clamp. After entering the peritoneal cavity, include the peritoneum in the clamp. This allows for maximal traction during dissection of the bowel and omentum with scissors.
The most important action to take at the time of incision is to make it large enough to allow for excellent exposure.
An adequate incision and the appropriate retractor will minimize operative time and facilitate completion. The old adage that “wounds heal from side to side, not end to end” is particularly applicable.
First steps: Get oriented, assess adhesions
After entering the abdomen, identify pelvic structures and their location in relation to one another. In patients who have undergone previous surgery or had inflammatory disease, the omentum may be adherent to these structures. If the omental adhesions are filmy and easy to reduce, cut them free. However, if the omentum is densely adherent to the parietal peritoneum or other pelvic organs or bowel, it may be helpful to cut across the omentum, leaving a portion attached to the structures to be removed.
Identify landmarks
After omental or intestinal adhesions have been separated, move the small and large intestines up as far as possible from the pelvis and pack them away. Then identify the following pelvic structures: uterine fundus, round ligaments, infundibulopelvic (IP) ligaments, posterior cul-de-sac, anterior cul-de-sac, prevesical peritoneum, and pelvic brim. These structures may be difficult to recognize and to mobilize because of fibrosis and adhesions.
At one time or another, every surgeon finds it necessary to rethink a planned procedure after the operation begins—a not uncommon scenario during surgery in the frozen pelvis. It can occur at the beginning of a procedure, once the incision is made and the pathology is surveyed, or it can arise when the surgeon is well into an operation, when all the usual landmarks are indistinguishable.
When the problem is clear from the get-go
When confronted with an impossible situation upon opening the abdomen, the surgeon has 2 options:
- close the abdomen and refer the patient
- call for the aid of a surgical colleague who has the necessary experience and skill.
When the operation is under way
This situation may not lend itself to so easy a solution. When the surgeon becomes overwhelmed by an unfamiliar operative field, he or she should stop operating, take stock of what has been accomplished and what remains to be done, check the status of the patient, and reevaluate the case. Again, 2 options are available:
- change the original goal and terminate the procedure at that point, scheduling reoperation for a later date
- call for help, particularly if arrangements have been made beforehand.
Either way, a compromised patient is too high a price to pay for the sake of the surgeon’s vanity, and the dictum of “primum non nocere” should become the guiding principle.
Choose entry into the retroperitoneum with care
Once you have identified the structures, determine how you will be entering the retroperitoneum. This decision is important because the blood supply to the uterus and adnexa lies in the retroperitoneum, as do the ureters, which must be identified and kept under direct vision during ligation of the IP ligaments and dissection of the peritoneum around the uterus.
Retroperitoneal entry and elaboration of the retroperitoneal spaces are keys to the safe performance of a difficult hysterectomy or removal of retained adnexa in a patient with a frozen pelvis. The retroperitoneal approach makes it possible to reach around structures that are fixed in the pelvis, to identify the blood supply and other vital structures, and to proceed safely.
Several entry sites are possible. In the frozen pelvis, I believe the round ligament is the ideal location. Identify and divide this ligament as it enters the internal ring, and incise the peritoneum cephalad along the course of the IP ligaments (FIGURE 2).
FIGURE 2 Transect the round ligaments
To facilitate entry into the retroperitoneum, transect the round ligaments. Then develop the retroperitoneal spaces using blunt dissection.Next, open the pararectal space to visualize the iliac vessels and ureter, which can be identified on the medial leaf of the peritoneum. The IP ligaments can then be visualized and safely divided. Extending the dissection caudad allows entry into the retrovesical space and into areas of the broad ligament adjacent to the uterus. This approach also makes it possible to produce traction on the peritoneum and facilitate separation of the areolar tissue between the bladder and cervix to enter the vesicovaginal space. Continue dissection of the bladder from the cervix and vagina by operating from one side of the pelvis to the other.
The ureter, a surgeon’s nemesis
Never assume you know the position of the ureter without confirming it; a major deviation of its course can occur secondary to pathologic processes in the pelvis. The ureter can be identified by direct visualization, peristalsis, and palpation. If it is rolled between the thumb and forefinger, the ureter produces a snapping sensation.
After entering the retroperitoneum, identify the ureter between the pelvic brim and Wertheim’s tunnel. Although it may, at times, be seen through the thin peritoneum as it travels over the common iliac artery down into the pelvis, the ureter is generally not clearly visualized in the frozen pelvis and may become apparent only after the peritoneum has been opened and the retroperitoneal spaces elaborated.
Near the level of the pelvic brim on the left side of the body, the ureter will be closer to the IP ligament than it is on the right side, due to the location of the sigmoid colon and its mesentery on the left side, which elevate the ureter in the ventral direction.
Once the ureter has been identified, leave it attached to the peritoneum as much as possible. When the ureter is adherent to pelvic pathology, lateralize it from the medial leaf of the broad ligament as far down into the pelvis as necessary to allow complete removal of the peritoneum along with the pathology. The extent of dissection necessary will vary with the pathology.
Handle the ureter gently
Never handle the ureter excessively or unnecessarily. When it is dissected free, let it lie free rather than retracting it with umbilical tapes or Penrose drains, which can slide up and down, damaging the vasculature that lies in the adventitia of the ureteral wall.
Freeing the bladder can be difficult
A history of surgery in the area of the bladder, such as cesarean section or bladder advancement with uterine suspension, may leave the bladder adherent to or hard to separate from the cervix and vagina. Normally, the vesicouterine peritoneum is flexible, mobile, and easy to free from the cervix and vagina. A history of disease processes such as endometriosis, infection, or tumors makes this dissection difficult, with a real risk of inadvertent cystotomy.
One technique to make this dissection easier and safer is to enter the retroperitoneum laterally near the round ligament. In this location, the bladder may not have been involved in the prior dissection, and the tissue may be more areolar and less dense than it is in the midline. After entering the retroperitoneum, elevate the bladder by incising the vesicouterine peritoneum transversely. By rolling a finger or an instrument cephalad in the anterior leaf of the broad ligament, the bladder can be sharply separated from the cervix.
When the bladder is densely adherent, make an incision into its dome away from the cervix to visualize the interior of the bladder. Place the index finger into the bladder to identify its reflection, and cut through the dense adhesions between it and the cervix and vagina (FIGURE 3).
FIGURE 3 Incise the broad-ligament peritoneum
Incise the peritoneum of the avascular layer of the anterior broad ligament bilaterally between the divided round ligaments to develop the vesical space bilaterally. One way to do this is to gently tease away the areolar tissue using a finger, pushing the tissue medially. Then divide the peritoneum between the 2 spaces and sweep the bladder off the cervix using blunt or sharp dissection.
Cul-de-sac of Douglas may be hard to recognize
In a disease-free pelvis, the posterior cul-de-sac is bounded laterally by the uterosacral ligaments, posteriorly by the rectum and sacrum, and caudally by the vagina—but these relationships are usually lost in the frozen pelvis. Extensive inflammatory disease, tumors of the tubes and ovaries, extensive pelvic endometriosis, and prior infection due to a diverticular abscess or ruptured appendix can obscure the normal confines of the cul-de-sac. It can also be obliterated by large myomas, which can fill the pelvic cavity and extend upward beyond the sacral promontory into the abdominal cavity, thereby displacing the intestines and creating potential deviation and compression of the ureters.
Freeing the peritoneal attachments both anteriorly and posteriorly, as well as at the sides of the pelvis, may release the pelvic contents and allow elevation of the uterus into the abdominal cavity. Then the ureter, uterine vasculature, and supporting ligaments can be identified. Dissection becomes simpler after this point.
However, when the rectum and ureters are densely adherent, as they often are in the frozen pelvis, dissection can become difficult, with a real danger of rectal perforation. A basic principle in any hysterectomy is to hug the uterus as closely as possible, staying near the posterior surface of the uterus and cervix using both blunt and sharp dissection. This eventually makes it possible to find a reasonable plane to enter the rectovaginal space at the superior portion of the cul-de-sac between the uterosacral ligaments.
The tissue below this level is not usually involved in the frozen pelvis and will give way readily once the uterosacral ligaments are divided. It is unnecessary to operate beyond this level to any great extent because the surgery already extends distal to the cervicovaginal junction. In some circumstances, it may be necessary to enter the vagina anteriorly to define the relationship between the posterior cervix and adherent bowel (FIGURE 4).
FIGURE 4 Mobilize the uterus
To free the uterus from its posterior visceral attachments and render it mobile, develop the rectovaginal space.
Identifying and repairing bowel injury
If the bowel has been prepped, and rectal enterotomy occurs during dissection, closure and drainage are the only necessary steps, and a temporary colostomy can be avoided. Bowel resection may be necessary if the bowel has sustained damage.
If bowel injury is suspected, irrigate the bowel through the rectum with sterile milk or dye solution using a Foley catheter with a 30-cc bag. Another widely used option to assess rectosigmoid integrity is to insufflate the submersed rectosigmoid with air. Bubbles signal a breach in the integrity of the bowel wall.
Postoperative care
After surgery, the principles of early mobilization and ambulation are key. The single most important postoperative intervention is early ambulation, which can minimize pulmonary problems, ileus, bladder atony, and thromboembolic disease secondary to deep venous thrombosis.
To protect against venous thromboembolic disease, use pneumatic compression devices and early ambulation. In women with other risk factors for deep venous thrombosis, such as malignancy, diabetes, obesity, and smoking, consider subcutaneous heparin.
Unless the bowel was resected or extensive adhesiolysis was performed, I generally allow the patient to have clear liquids on the first postoperative day. I then advance oral intake after gastrointestinal motility returns or when the patient complains of hunger.
Remove the urethral catheter on the first postoperative day unless cystotomy or extensive dissection of the ureters or bladder was performed.
The author reports no financial relationships relevant to this article.
1. Willson JR. Ultrasonography in the diagnosis of gynecologic disorders. Am J Obstet Gynecol. 1991;160:1064-1071.
2. Polk HC, Jr. Continuing refinements in surgical antibiotic prophylaxis. Arch Surg. 2005;140:1066-1067.
3. Fejgin MD, Lourwood DL. Low-molecular-weight heparins and their use in obstetrics and gynecology. Obstet Gynecol Surv. 1994;49:424-426.
4. Magitbay PM, Nyholm JL, Hernandez JL, Podratz KC. Ovarian remnant syndrome. Am J Obstet Gynecol. 2005;193:2062-2066.
1. Willson JR. Ultrasonography in the diagnosis of gynecologic disorders. Am J Obstet Gynecol. 1991;160:1064-1071.
2. Polk HC, Jr. Continuing refinements in surgical antibiotic prophylaxis. Arch Surg. 2005;140:1066-1067.
3. Fejgin MD, Lourwood DL. Low-molecular-weight heparins and their use in obstetrics and gynecology. Obstet Gynecol Surv. 1994;49:424-426.
4. Magitbay PM, Nyholm JL, Hernandez JL, Podratz KC. Ovarian remnant syndrome. Am J Obstet Gynecol. 2005;193:2062-2066.
Implementing the Eden Alternative on a VA Dementia Care Unit
Amniotic Fluid Embolism
Clinical Decision Making: Doctor, When Can I Drive?
Surgical Management of Radial Head Fractures
FERTILITY
Changes are occurring so quickly it is often difficult for the general ObGyn to know the most advanced and appropriate treatment for a given patient. The American Society for Reproductive Medicine (ASRM) Practice Committee establishes guidelines based upon well-designed studies to help physicians keep abreast of the best clinical practices. In this article, I focus on recent ASRM guidelines in 5 topical areas associated with substantial misinformation in both the professional and public sectors:
- when and how gynecologists should initiate infertility testing and treatment
- how to evaluate and manage recurrent pregnancy loss
- the need to reduce the rate of multiple gestation from IVF and ART
- the expanded applications for preimplantation genetic diagnosis
- the truth about fertility-sparing efforts in young women planning to undergo cancer therapy and other treatments.
When and how to evaluate patients complaining of infertility
Infertility is a disease, but there are different opinions about when a woman reporting this condition should be assessed (TABLE 1). According to the ASRM, a couple should not be considered infertile until they have tried to conceive spontaneously for at least 12 months, unless the medical history and physical findings dictate earlier evaluation and treatment.1
For example, approximately 25% of couples experience infertility when the woman is age 35, and about 50% experience it when the woman is age 40. Therefore, it is reasonable to investigate infertility after 6 months of attempted conception when the woman is over 35 and after 3 months if she is over age 40.2 The primary reason for this age-related reduction in fertility is the diminishing number and quality of oocytes over time.
Other risk factors for infertility include smoking, family history of premature ovarian failure, significant ovarian pathology, previous ovarian surgery, history of oligomenorrhea or amenorrhea, known or suspected disease of the uterus or fallopian tubes, endometriosis, or a partner known to be subfertile.3,4
TABLE 1
When to investigate infertility, treat, and refer
| INVESTIGATE |
| After 12 months of unprotected intercourse if age |
| After 6 months of unprotected intercourse if age 35–39 |
| After 3 months of unprotected intercourse if age ≥40 |
| After 0–6 months if patient has history of or risk factors for infertility |
| TREAT |
| Treat identifiable causes of infertility |
| Optimize factors influencing fertility: |
|
| Treat empirically (eg, clomiphene, insemination) for 3–6 months in patients |
| REFER |
| History of infertility or significant risk factors |
| Significant fertility problems identified during investigation |
| Age ≥40 |
| After 3–6 months of failed treatment for identifiable causes |
| After 3–6 months of failed empiric treatment |
How to evaluate ovarian function
A careful history and physical examination are key components of systematic, expeditious, and cost-effective identification of the cause of infertility (TABLE 2). A menstrual history and basal body temperature recordings are useful in the diagnosis of ovulatory dysfunction and are easy to obtain. Measurements of urinary luteinizing hormone (LH) using ovulation-prediction kits and mid-luteal-phase serum progesterone are also helpful.
Endometrial biopsy is rarely indicated because of its lack of clinical relevance.
Serial vaginal ultrasonography of the size and number of ovarian follicles may be indicated when simpler methods are inconclusive.
Other tests to evaluate ovarian function may include thyroid-stimulating hormone (TSH), serum prolactin, cycle day 3 follicle-stimulating hormone (FSH) and estradiol, and the clomiphene citrate challenge test in selected patients at higher risk of ovarian dysfunction.
TABLE 2
Current status of tests and treatments
| OLD, NOW RARELY INDICATED |
| Postcoital test |
| Endometrial biopsy |
| Antisperm antibodies testing |
| Intracervical insemination |
| Clomiphene for more than 3–6 cycles |
| Routine hCG injection to stimulate ovulation in clomiphene cycles |
| NEW AND HELPFUL |
| Clomiphene citrate challenge test in selected patients |
| Serial vaginal ultrasounds to evaluate response to ovarian stimulation |
| Saline sonohysterography |
| Preimplantation genetic diagnosis for single-gene defects |
| Embryo cryopreservation |
| Single-embryo transfer to reduce multiple pregnancy rates |
| NEW BUT STILL EXPERIMENTAL* |
| Preimplantation genetic screening for aneuploidy in older patients |
| Human lymphocyte antigen typing for recurrent pregnancy loss |
| Intravenous immunoglobulin for recurrent pregnancy loss |
| Ovarian tissue or oocyte cryopreservation for fertility preservation |
| * Should be performed only in clinical trials |
Clomiphene citrate is preferred
Ovarian dysfunction can be treated with clomiphene for 3 to 6 cycles5 starting at 50 mg per day from cycle day 5 to 9 and increasing to 100 and then 150 mg per day if ovulation does not occur. The drug may also be effective empiric treatment for unexplained infertility using 100 mg per day from cycle days 3 through 7 for a maximum of 3 to 4 cycles.
Only gynecologists experienced with ovarian stimulation drugs and with access to daily ultrasonographic monitoring and estradiol levels should use them, because of the risk of multiple pregnancy and ovarian hyperstimulation.
For women with polycystic ovary syndrome (PCOS), clomiphene alone is more effective than metformin alone. Ovarian drilling may be an effective surgical treatment for PCOS if clomiphene fails, but the cost and risk of adhesions must be considered.
Human chorionic gonadotropin (hCG) injections during clomiphene treatment to stimulate ovulation should be given only if the patient’s own urinary LH surge cannot be detected.
A single intrauterine insemination (IUI) improves the pregnancy rate slightly in conjunction with clomiphene, and by an odds ratio of approximately 2 in conjunction with gonadotropins. The gonadotropin dosage ranges from about 75 to 600 IU per day for 8 to 12 days, based on patient need and careful monitoring.
When to give up on ovarian stimulation. Failure to achieve pregnancy after 3 to 6 cycles signals the need to expand diagnostic evaluation or change treatment strategies.
Evaluate the uterus and tubes
Uterine factors rarely cause infertility but warrant thorough investigation all the same, including assessment of uterine cavity size and shape. A number of methods are available:
- hysterosalpingography (HSG)
- ultrasonography
- saline sonohysterography
Peritoneal factors such as endometriosis or pelvic or adnexal adhesions may occasionally be identified by ultrasonography if there is a mass, but are more likely to require laparoscopy.
When laparoscopy is indicated
If there is evidence or a strong suspicion of endometriosis, pelvic or adnexal adhesions, or significant tubal disease, laparoscopy is warranted. It also may be helpful in younger patients (eg,
Because they reduce pregnancy rates by 50%, hydrosalpinges should be removed or the fallopian tube should be ligated proximally before IVF. It also is important to consider the number of patients needed to treat by laparoscopy to obtain 1 additional pregnancy.
Only gynecologists with expertise should perform laparoscopy, because it is important to make the correct diagnosis and be capable of surgically treating conditions found during the surgery.
Skip the postcoital test, but keep the semen analysis
Abnormalities of the cervical mucus or sperm–mucus interaction rarely cause infertility. Therefore, the postcoital test has questionable predictive value and is probably only useful to confirm that the couple can have properly timed intercourse during the cycle.3
A male factor is solely responsible in about 20% of infertile couples and contributory in another 30% to 40%. For this reason, semen analysis is always warranted when the female is being evaluated for infertility.
Examination of the male partner should be performed by the gynecologist, or the male should be referred to a urologist interested in infertility.6
For recurrent pregnancy loss, best treatment is TLC
Recurrent pregnancy loss is challenging because it is so emotionally charged for the patient, the cause is often unclear, and we lack specific treatments. A methodical and empathetic approach is therefore recommended.
What the history can reveal
Many women with recurrent pregnancy loss will eventually have a live birth, but increasing numbers of miscarriages do predict a poorer overall chance of success, as does increasing age.
Lifestyle factors rarely, if ever, cause recurrent pregnancy loss, but the following factors may increase the risk of miscarriage: obesity, high daily caffeine intake, alcohol consumption, use of nonsteroidal anti-inflammatory drugs, and social class and occupation. A previous diagnosis of or treatment for infertility also increases the risk of recurrent loss.
Smoking should be discouraged and healthy lifestyles should be promoted.7
Causes of recurrent pregnancy loss
Definite causal factors include chromosomal abnormalities, such as translocations, in approximately 5% of couples with 2 or more losses.
Probable factors include uterine abnormalities (both congenital abnormalities such as septate, and acquired defects such as adhesions and intrauterine or submucous myomas), uncontrolled thyroid disease or diabetes, PCOS, and antiphospholipid antibody syndrome.
Other thrombophilias, such as those associated with factor V Leiden mutation, activated protein C resistance, and possibly prothrombin G20210A and protein S deficiency, have been found by some investigators to be associated with recurrent pregnancy loss. It is doubtful that antithyroid antibodies and sharing of parental human lymphocyte antigen (HLA) cause recurrent miscarriage.7
Genetic component likely. The risk of recurrent pregnancy loss in first-degree relatives of women with unexplained repeated pregnancy loss who have normal chromosomes is approximately 6 times higher than the risk in the background population, suggesting a polygenic mode of inheritance.7,8
Other possible causes include low plasma folate levels, which have been associated with an increased risk of first-trimester pregnancy loss. Environmental toxins such as ionizing radiation, organic solvents, alcohol, mercury, and lead are confirmed causes of recurrent pregnancy loss; hyperthermia is a suspected cause.8
Recommended evaluation
Investigations that have been proven in many studies include:
- HSG, hysteroscopy, and sonohysterography
- karyotyping of the couple
- measurement of thyroid hormone
- hemoglobin A1C and serum glucose assessment
- activated partial thromboplastin time, dilute Russell viper venom time, and lupus anticoagulant assessment
- measurement of immunoglobulin G and immunoglobulin M anticardiolipin antibodies
- test for factor V Leiden mutation
Examine products of conception?
Although it is routine practice to send products of conception for histologic examination, mainly to exclude a gestational trophoblastic disorder, the usefulness of this practice is unclear.8 In couples with recurrent pregnancy loss, chromosomal analysis of the products of conception indicates that a normal conceptus karyotype in a previous pregnancy is a predictor of a higher rate of miscarriage in a subsequent pregnancy.8 When stratified by maternal age, there is no difference in the distribution of cytogenetically abnormal miscarriages in couples with recurrent pregnancy loss, compared with controls.8 The cost-effectiveness of karyotyping is therefore unclear.
High levels of homocysteine (ie, hyperhomocysteinemia) can be associated with recurrent pregnancy loss. Among genetic causes is polymorphism at position 677 in the methylene tetrahydrofolate reductase (MTHFR) gene, which is often evaluated to rule out this condition.
Infections with bacteria, viruses, or parasites can all interfere with early pregnancy development, but none seem to be a significant cause of recurrent pregnancy loss.8 Testing is most useful in the context of an acute infectious episode.
Can recurrent loss be treated?
The hallmark of treatment is empathetic care, along with counseling emphasizing the complexity of this condition. Any endocrinologic, anatomic, or other abnormality that is identified during evaluation should be treated, if possible.
Progesterone supplementation is not proven treatment. This therapy is commonly prescribed but has not been proved to improve live birth rates.
Prednisone, aspirin, and NSAIDs have no benefits but potential risks and should not be used.
Current immunologic therapies for recurrent pregnancy loss have no sound scientific basis, except for the use of heparin and aspirin in patients with well-documented antiphospholipid antibodies.7 Specifically, intravenous immunoglobulin remains unproven, is experimental, and should be provided only in approved research settings.9 Paternal leukocyte immunization does not work, has been proscribed by the US Food and Drug Administration, and should be avoided.
Careful counseling and education of the patient about the history, pathophysiology, testing, test results, and treatment of recurrent pregnancy loss are necessary. Women with subfertility who have taken a long time to conceive should be treated empirically with ovarian stimulation in an attempt to shorten the time to conception.
Singleton births can be encouraged without jeopardizing IVF, ART
Multiple gestations have increased over the past 15 years, largely because of:
- ovulation induction for management of oligo-ovulation
- superovulation to produce more than 1 ovulated egg for fertility treatments
- assisted reproductive technologies (ART), in which more than 1 embryo is replaced to increase the pregnancy rate
Multiple gestations are a bad idea
Risks include a higher complication rate for gravidas and fetuses, as well as higher short- and long-term costs to patients and society. It is therefore important to reduce the incidence of multiple gestation associated with fertility treatments.10
How to reduce the likelihood of multiple fetuses
- Closely monitor cycles involving ovulation induction and superovulation for efficacy and safety, to avoid ovarian hyperstimulation and reduce the risk of multiple gestation. Although attempts to limit multiple gestation during ovulation induction or superovulation using ultrasonographic criteria and serum estradiol limits have been ineffective,10 it is my opinion that we should err on the side of conservatism, even though the optimal parameters for doing so have not been determined by high-quality trials. I recommend that hCG or IUI be avoided if more than 4 mature follicles (>15 mm) or 6 large follicles (>12 mm) are present on a sonogram, and the couple should be instructed to refrain from intercourse.
- Focus on the objective of a single healthy baby as the optimal outcome. Data published by the Society for Assisted Reproductive Technology (SART) clearly demonstrate the clinical impact of a reduction in the number of embryos transferred, which reduced triplet pregnancy rates in 2005 to less than half the rate in the late 1990s. Fewer embryos are transferred today than just a few years ago, and the trend is continuing. This will help reduce the triplet rate further and also reduce twin pregnancies. In the past 6 months, guidelines have recommended replacement of only 1 blastocyst at day 5 or 1 to 2 embryos at day 3 in women under age 35 with a favorable prognosis.
What the future holds
We can expect more elective single-embryo or single-blastocyst transfers as we gain further expertise in this area. However, this practice should be implemented carefully in selected patients to maintain adequate pregnancy rates while reducing multiple gestations.
The United States has the highest ART success rates in the world (approximately 40% higher than in Europe) despite a reduction in the number of triplet or higher-order pregnancies resulting in live births after ART—from 7.0% in 1996 to 2.4% in 2004. The twin rate has remained stable at approximately 30%, but should decrease as 1- and 2-embryo transfers become more common.11
Preimplantation genetic diagnosis now has multiple applications
Preimplantation genetic diagnosis (PGD) is over 15 years old, and at least 1,000 babies worldwide have been born after its use, with no reports of increased fetal malformation or other problems.12
Two basic techniques are employed to analyze the genomic status of the 1 or 2 blastomeres usually removed from the 8-cell embryo on day 3 after fertilization:
- Polymerase chain reaction (PCR) is used to amplify a specific DNA sequence harboring a mutation. A mismatch (eg, due to a genetic deletion) leads to differential migration on the gel, thus permitting diagnosis. The error rate, primarily due to allelic dropout, in which 1 of the 2 alleles selectively amplifies and thus contributes to diagnostic errors, is approximately 0.3% to 5.6%.
- Fluorescent in situ hybridization (FISH) allows determination of the ploidy of a blastomere. Labeled probes bind to chromosomes and are viewed under a fluorescent microscope. The error rate is 1% to 10% for a variety of technical reasons.12 Testing takes about 1 day while the embryos are developing to blastocysts, at which time those that are viable and tested to be normal are transferred back into the uterus.
Not just for gender determination
PGD initially was used to determine gender (by FISH) as an indirect method of avoiding X-linked genetic diseases such as hemophilia. The error rate for gender determination is less than 1%. Since then, single-gene-defect disorders have been diagnosed using PCR and heteroduplex formation or restriction endonuclease digestion, both of which distinguish normal from mutant alleles. PGD has been performed broadly to diagnose Tay-Sachs, Huntington’s disease, and hundreds of other diseases.
Testing for translocation by PGD has been especially useful and may reduce the risk of spontaneous abortion from as much as 95% to 13% if one of the parents is a known translocation carrier.
Still under investigation is the routine use of FISH to detect aneuploidy in cases of recurrent pregnancy loss. The use of FISH for gender selection for family balancing is not recommended by the ASRM.
More young women seek to preserve their fertility
Fertility preservation through ovarian tissue or oocyte cryopreservation or vitrification has recently been popularized by cancer survival consumer groups, the media, and other interests. In addition to cancer patients planning to undergo chemotherapy or radiotherapy, candidates for fertility preservation include women undergoing bone marrow or stem cell transplantation or oophorectomy (for a benign tumor, endometriosis, or prophylaxis) and patients with severe autoimmune disease needing chemotherapy.
In cancer patients, fertility is preserved using one of several methods:
- shielding or moving the ovaries to a different anatomic site during radiation
- use of gonadotropin-releasing hormone analogs or oral contraceptives during chemotherapy (unproven)
- changes to chemotherapy regimen
- IVF cycle followed by cryopreservation of embryos if the patient has a male partner or is prepared to use donor sperm (provided the oncologist confirms that ovarian stimulation and high estradiol levels are acceptable and there is time to undergo an IVF cycle before cancer treatment begins).
Unresolved issues
Concerns about cryopreservation of ovarian tissue in cancer patients13,14 include the possibility of reseeding tumor cells after ovarian transplantation, malignant transformation of transplanted ovarian tissue, and the possibility of congenital abnormalities as a result of cryopreservation—although no increase has been found in the patients studied so far.
The pregnancy rate is low
For cancer patients, the preservation of ovarian tissue or oocytes yields pregnancy rates significantly lower than those observed with standard IVF procedures. For cancer patients facing chemotherapy, however, oocyte cryopreservation may be one of the few options available and is acceptable in experimental protocols approved by the institutional review board.
Physicians should inform cancer patients about the options for fertility preservation prior to treatment.14 We lack data to recommend ovarian tissue or oocyte cryopreservation for the sole purpose of circumventing reproductive aging in healthy women.13
1. Practice Committee. American Society for Reproductive Medicine. Definition of “infertility.” Fertil Steril. 2006;86(Suppl 4):S228.-
2. Practice Committee, American Society for Reproductive Medicine. Aging and infertility in women. Fertil Steril. 2006;86(Suppl 4):S248-252.
3. Practice Committee, American Society for Reproductive Medicine. Optimal evaluation of the infertile female. Fertil Steril. 2006;86(Suppl 4):S264-267.
4. Practice Committee, American Society for Reproductive Medicine. Smoking and infertility. Fertil Steril. 2006;86(Suppl 4):S172-177.
5. Practice committee, American Society for Reproductive Medicine. Use of clomiphene citrate in women. Fertil Steril. 2006;86(Suppl 4):S187-193.
6. Male Infertility Best Practice Committee, American Urological Association, and Practice Committee, American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86(Suppl 4):S202-209.
7. Christiansen OB, Andersen A-MN, Bosch E, et al. Evidence-based investigations and treatments of recurrent pregnancy loss. Fertil Steril. 2005;83:821-839.
8. Jauniauz E, Farquharson RG, Christiansen OB, et al. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod. 2006;21:2216-2222.
9. Practice Committee, American Society for Reproductive Medicine. Intravenous immunoglobulin (IVIG) and recurrent spontaneous pregnancy loss. Fertil Steril. 2006;86(Suppl 4):S226-227.
10. Practice committee, American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(Suppl 4):S106-110.
11. Practice Committee, Society for Assisted Reproductive Technology and the Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2006;86(Suppl 4):S51-52.
12. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Preimplantation genetic diagnosis. Fertil Steril. 2006;86(Suppl 4):S257-258.
13. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2006;86(Suppl 4):S142-147.
14. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622-1628.
Dr. Adamson reports no financial relationships with any company whose products are mentioned in this article. He receives grant/research support from IBSA, Serono, and ViaCell and is a consultant to ViaCell.
Changes are occurring so quickly it is often difficult for the general ObGyn to know the most advanced and appropriate treatment for a given patient. The American Society for Reproductive Medicine (ASRM) Practice Committee establishes guidelines based upon well-designed studies to help physicians keep abreast of the best clinical practices. In this article, I focus on recent ASRM guidelines in 5 topical areas associated with substantial misinformation in both the professional and public sectors:
- when and how gynecologists should initiate infertility testing and treatment
- how to evaluate and manage recurrent pregnancy loss
- the need to reduce the rate of multiple gestation from IVF and ART
- the expanded applications for preimplantation genetic diagnosis
- the truth about fertility-sparing efforts in young women planning to undergo cancer therapy and other treatments.
When and how to evaluate patients complaining of infertility
Infertility is a disease, but there are different opinions about when a woman reporting this condition should be assessed (TABLE 1). According to the ASRM, a couple should not be considered infertile until they have tried to conceive spontaneously for at least 12 months, unless the medical history and physical findings dictate earlier evaluation and treatment.1
For example, approximately 25% of couples experience infertility when the woman is age 35, and about 50% experience it when the woman is age 40. Therefore, it is reasonable to investigate infertility after 6 months of attempted conception when the woman is over 35 and after 3 months if she is over age 40.2 The primary reason for this age-related reduction in fertility is the diminishing number and quality of oocytes over time.
Other risk factors for infertility include smoking, family history of premature ovarian failure, significant ovarian pathology, previous ovarian surgery, history of oligomenorrhea or amenorrhea, known or suspected disease of the uterus or fallopian tubes, endometriosis, or a partner known to be subfertile.3,4
TABLE 1
When to investigate infertility, treat, and refer
| INVESTIGATE |
| After 12 months of unprotected intercourse if age |
| After 6 months of unprotected intercourse if age 35–39 |
| After 3 months of unprotected intercourse if age ≥40 |
| After 0–6 months if patient has history of or risk factors for infertility |
| TREAT |
| Treat identifiable causes of infertility |
| Optimize factors influencing fertility: |
|
| Treat empirically (eg, clomiphene, insemination) for 3–6 months in patients |
| REFER |
| History of infertility or significant risk factors |
| Significant fertility problems identified during investigation |
| Age ≥40 |
| After 3–6 months of failed treatment for identifiable causes |
| After 3–6 months of failed empiric treatment |
How to evaluate ovarian function
A careful history and physical examination are key components of systematic, expeditious, and cost-effective identification of the cause of infertility (TABLE 2). A menstrual history and basal body temperature recordings are useful in the diagnosis of ovulatory dysfunction and are easy to obtain. Measurements of urinary luteinizing hormone (LH) using ovulation-prediction kits and mid-luteal-phase serum progesterone are also helpful.
Endometrial biopsy is rarely indicated because of its lack of clinical relevance.
Serial vaginal ultrasonography of the size and number of ovarian follicles may be indicated when simpler methods are inconclusive.
Other tests to evaluate ovarian function may include thyroid-stimulating hormone (TSH), serum prolactin, cycle day 3 follicle-stimulating hormone (FSH) and estradiol, and the clomiphene citrate challenge test in selected patients at higher risk of ovarian dysfunction.
TABLE 2
Current status of tests and treatments
| OLD, NOW RARELY INDICATED |
| Postcoital test |
| Endometrial biopsy |
| Antisperm antibodies testing |
| Intracervical insemination |
| Clomiphene for more than 3–6 cycles |
| Routine hCG injection to stimulate ovulation in clomiphene cycles |
| NEW AND HELPFUL |
| Clomiphene citrate challenge test in selected patients |
| Serial vaginal ultrasounds to evaluate response to ovarian stimulation |
| Saline sonohysterography |
| Preimplantation genetic diagnosis for single-gene defects |
| Embryo cryopreservation |
| Single-embryo transfer to reduce multiple pregnancy rates |
| NEW BUT STILL EXPERIMENTAL* |
| Preimplantation genetic screening for aneuploidy in older patients |
| Human lymphocyte antigen typing for recurrent pregnancy loss |
| Intravenous immunoglobulin for recurrent pregnancy loss |
| Ovarian tissue or oocyte cryopreservation for fertility preservation |
| * Should be performed only in clinical trials |
Clomiphene citrate is preferred
Ovarian dysfunction can be treated with clomiphene for 3 to 6 cycles5 starting at 50 mg per day from cycle day 5 to 9 and increasing to 100 and then 150 mg per day if ovulation does not occur. The drug may also be effective empiric treatment for unexplained infertility using 100 mg per day from cycle days 3 through 7 for a maximum of 3 to 4 cycles.
Only gynecologists experienced with ovarian stimulation drugs and with access to daily ultrasonographic monitoring and estradiol levels should use them, because of the risk of multiple pregnancy and ovarian hyperstimulation.
For women with polycystic ovary syndrome (PCOS), clomiphene alone is more effective than metformin alone. Ovarian drilling may be an effective surgical treatment for PCOS if clomiphene fails, but the cost and risk of adhesions must be considered.
Human chorionic gonadotropin (hCG) injections during clomiphene treatment to stimulate ovulation should be given only if the patient’s own urinary LH surge cannot be detected.
A single intrauterine insemination (IUI) improves the pregnancy rate slightly in conjunction with clomiphene, and by an odds ratio of approximately 2 in conjunction with gonadotropins. The gonadotropin dosage ranges from about 75 to 600 IU per day for 8 to 12 days, based on patient need and careful monitoring.
When to give up on ovarian stimulation. Failure to achieve pregnancy after 3 to 6 cycles signals the need to expand diagnostic evaluation or change treatment strategies.
Evaluate the uterus and tubes
Uterine factors rarely cause infertility but warrant thorough investigation all the same, including assessment of uterine cavity size and shape. A number of methods are available:
- hysterosalpingography (HSG)
- ultrasonography
- saline sonohysterography
Peritoneal factors such as endometriosis or pelvic or adnexal adhesions may occasionally be identified by ultrasonography if there is a mass, but are more likely to require laparoscopy.
When laparoscopy is indicated
If there is evidence or a strong suspicion of endometriosis, pelvic or adnexal adhesions, or significant tubal disease, laparoscopy is warranted. It also may be helpful in younger patients (eg,
Because they reduce pregnancy rates by 50%, hydrosalpinges should be removed or the fallopian tube should be ligated proximally before IVF. It also is important to consider the number of patients needed to treat by laparoscopy to obtain 1 additional pregnancy.
Only gynecologists with expertise should perform laparoscopy, because it is important to make the correct diagnosis and be capable of surgically treating conditions found during the surgery.
Skip the postcoital test, but keep the semen analysis
Abnormalities of the cervical mucus or sperm–mucus interaction rarely cause infertility. Therefore, the postcoital test has questionable predictive value and is probably only useful to confirm that the couple can have properly timed intercourse during the cycle.3
A male factor is solely responsible in about 20% of infertile couples and contributory in another 30% to 40%. For this reason, semen analysis is always warranted when the female is being evaluated for infertility.
Examination of the male partner should be performed by the gynecologist, or the male should be referred to a urologist interested in infertility.6
For recurrent pregnancy loss, best treatment is TLC
Recurrent pregnancy loss is challenging because it is so emotionally charged for the patient, the cause is often unclear, and we lack specific treatments. A methodical and empathetic approach is therefore recommended.
What the history can reveal
Many women with recurrent pregnancy loss will eventually have a live birth, but increasing numbers of miscarriages do predict a poorer overall chance of success, as does increasing age.
Lifestyle factors rarely, if ever, cause recurrent pregnancy loss, but the following factors may increase the risk of miscarriage: obesity, high daily caffeine intake, alcohol consumption, use of nonsteroidal anti-inflammatory drugs, and social class and occupation. A previous diagnosis of or treatment for infertility also increases the risk of recurrent loss.
Smoking should be discouraged and healthy lifestyles should be promoted.7
Causes of recurrent pregnancy loss
Definite causal factors include chromosomal abnormalities, such as translocations, in approximately 5% of couples with 2 or more losses.
Probable factors include uterine abnormalities (both congenital abnormalities such as septate, and acquired defects such as adhesions and intrauterine or submucous myomas), uncontrolled thyroid disease or diabetes, PCOS, and antiphospholipid antibody syndrome.
Other thrombophilias, such as those associated with factor V Leiden mutation, activated protein C resistance, and possibly prothrombin G20210A and protein S deficiency, have been found by some investigators to be associated with recurrent pregnancy loss. It is doubtful that antithyroid antibodies and sharing of parental human lymphocyte antigen (HLA) cause recurrent miscarriage.7
Genetic component likely. The risk of recurrent pregnancy loss in first-degree relatives of women with unexplained repeated pregnancy loss who have normal chromosomes is approximately 6 times higher than the risk in the background population, suggesting a polygenic mode of inheritance.7,8
Other possible causes include low plasma folate levels, which have been associated with an increased risk of first-trimester pregnancy loss. Environmental toxins such as ionizing radiation, organic solvents, alcohol, mercury, and lead are confirmed causes of recurrent pregnancy loss; hyperthermia is a suspected cause.8
Recommended evaluation
Investigations that have been proven in many studies include:
- HSG, hysteroscopy, and sonohysterography
- karyotyping of the couple
- measurement of thyroid hormone
- hemoglobin A1C and serum glucose assessment
- activated partial thromboplastin time, dilute Russell viper venom time, and lupus anticoagulant assessment
- measurement of immunoglobulin G and immunoglobulin M anticardiolipin antibodies
- test for factor V Leiden mutation
Examine products of conception?
Although it is routine practice to send products of conception for histologic examination, mainly to exclude a gestational trophoblastic disorder, the usefulness of this practice is unclear.8 In couples with recurrent pregnancy loss, chromosomal analysis of the products of conception indicates that a normal conceptus karyotype in a previous pregnancy is a predictor of a higher rate of miscarriage in a subsequent pregnancy.8 When stratified by maternal age, there is no difference in the distribution of cytogenetically abnormal miscarriages in couples with recurrent pregnancy loss, compared with controls.8 The cost-effectiveness of karyotyping is therefore unclear.
High levels of homocysteine (ie, hyperhomocysteinemia) can be associated with recurrent pregnancy loss. Among genetic causes is polymorphism at position 677 in the methylene tetrahydrofolate reductase (MTHFR) gene, which is often evaluated to rule out this condition.
Infections with bacteria, viruses, or parasites can all interfere with early pregnancy development, but none seem to be a significant cause of recurrent pregnancy loss.8 Testing is most useful in the context of an acute infectious episode.
Can recurrent loss be treated?
The hallmark of treatment is empathetic care, along with counseling emphasizing the complexity of this condition. Any endocrinologic, anatomic, or other abnormality that is identified during evaluation should be treated, if possible.
Progesterone supplementation is not proven treatment. This therapy is commonly prescribed but has not been proved to improve live birth rates.
Prednisone, aspirin, and NSAIDs have no benefits but potential risks and should not be used.
Current immunologic therapies for recurrent pregnancy loss have no sound scientific basis, except for the use of heparin and aspirin in patients with well-documented antiphospholipid antibodies.7 Specifically, intravenous immunoglobulin remains unproven, is experimental, and should be provided only in approved research settings.9 Paternal leukocyte immunization does not work, has been proscribed by the US Food and Drug Administration, and should be avoided.
Careful counseling and education of the patient about the history, pathophysiology, testing, test results, and treatment of recurrent pregnancy loss are necessary. Women with subfertility who have taken a long time to conceive should be treated empirically with ovarian stimulation in an attempt to shorten the time to conception.
Singleton births can be encouraged without jeopardizing IVF, ART
Multiple gestations have increased over the past 15 years, largely because of:
- ovulation induction for management of oligo-ovulation
- superovulation to produce more than 1 ovulated egg for fertility treatments
- assisted reproductive technologies (ART), in which more than 1 embryo is replaced to increase the pregnancy rate
Multiple gestations are a bad idea
Risks include a higher complication rate for gravidas and fetuses, as well as higher short- and long-term costs to patients and society. It is therefore important to reduce the incidence of multiple gestation associated with fertility treatments.10
How to reduce the likelihood of multiple fetuses
- Closely monitor cycles involving ovulation induction and superovulation for efficacy and safety, to avoid ovarian hyperstimulation and reduce the risk of multiple gestation. Although attempts to limit multiple gestation during ovulation induction or superovulation using ultrasonographic criteria and serum estradiol limits have been ineffective,10 it is my opinion that we should err on the side of conservatism, even though the optimal parameters for doing so have not been determined by high-quality trials. I recommend that hCG or IUI be avoided if more than 4 mature follicles (>15 mm) or 6 large follicles (>12 mm) are present on a sonogram, and the couple should be instructed to refrain from intercourse.
- Focus on the objective of a single healthy baby as the optimal outcome. Data published by the Society for Assisted Reproductive Technology (SART) clearly demonstrate the clinical impact of a reduction in the number of embryos transferred, which reduced triplet pregnancy rates in 2005 to less than half the rate in the late 1990s. Fewer embryos are transferred today than just a few years ago, and the trend is continuing. This will help reduce the triplet rate further and also reduce twin pregnancies. In the past 6 months, guidelines have recommended replacement of only 1 blastocyst at day 5 or 1 to 2 embryos at day 3 in women under age 35 with a favorable prognosis.
What the future holds
We can expect more elective single-embryo or single-blastocyst transfers as we gain further expertise in this area. However, this practice should be implemented carefully in selected patients to maintain adequate pregnancy rates while reducing multiple gestations.
The United States has the highest ART success rates in the world (approximately 40% higher than in Europe) despite a reduction in the number of triplet or higher-order pregnancies resulting in live births after ART—from 7.0% in 1996 to 2.4% in 2004. The twin rate has remained stable at approximately 30%, but should decrease as 1- and 2-embryo transfers become more common.11
Preimplantation genetic diagnosis now has multiple applications
Preimplantation genetic diagnosis (PGD) is over 15 years old, and at least 1,000 babies worldwide have been born after its use, with no reports of increased fetal malformation or other problems.12
Two basic techniques are employed to analyze the genomic status of the 1 or 2 blastomeres usually removed from the 8-cell embryo on day 3 after fertilization:
- Polymerase chain reaction (PCR) is used to amplify a specific DNA sequence harboring a mutation. A mismatch (eg, due to a genetic deletion) leads to differential migration on the gel, thus permitting diagnosis. The error rate, primarily due to allelic dropout, in which 1 of the 2 alleles selectively amplifies and thus contributes to diagnostic errors, is approximately 0.3% to 5.6%.
- Fluorescent in situ hybridization (FISH) allows determination of the ploidy of a blastomere. Labeled probes bind to chromosomes and are viewed under a fluorescent microscope. The error rate is 1% to 10% for a variety of technical reasons.12 Testing takes about 1 day while the embryos are developing to blastocysts, at which time those that are viable and tested to be normal are transferred back into the uterus.
Not just for gender determination
PGD initially was used to determine gender (by FISH) as an indirect method of avoiding X-linked genetic diseases such as hemophilia. The error rate for gender determination is less than 1%. Since then, single-gene-defect disorders have been diagnosed using PCR and heteroduplex formation or restriction endonuclease digestion, both of which distinguish normal from mutant alleles. PGD has been performed broadly to diagnose Tay-Sachs, Huntington’s disease, and hundreds of other diseases.
Testing for translocation by PGD has been especially useful and may reduce the risk of spontaneous abortion from as much as 95% to 13% if one of the parents is a known translocation carrier.
Still under investigation is the routine use of FISH to detect aneuploidy in cases of recurrent pregnancy loss. The use of FISH for gender selection for family balancing is not recommended by the ASRM.
More young women seek to preserve their fertility
Fertility preservation through ovarian tissue or oocyte cryopreservation or vitrification has recently been popularized by cancer survival consumer groups, the media, and other interests. In addition to cancer patients planning to undergo chemotherapy or radiotherapy, candidates for fertility preservation include women undergoing bone marrow or stem cell transplantation or oophorectomy (for a benign tumor, endometriosis, or prophylaxis) and patients with severe autoimmune disease needing chemotherapy.
In cancer patients, fertility is preserved using one of several methods:
- shielding or moving the ovaries to a different anatomic site during radiation
- use of gonadotropin-releasing hormone analogs or oral contraceptives during chemotherapy (unproven)
- changes to chemotherapy regimen
- IVF cycle followed by cryopreservation of embryos if the patient has a male partner or is prepared to use donor sperm (provided the oncologist confirms that ovarian stimulation and high estradiol levels are acceptable and there is time to undergo an IVF cycle before cancer treatment begins).
Unresolved issues
Concerns about cryopreservation of ovarian tissue in cancer patients13,14 include the possibility of reseeding tumor cells after ovarian transplantation, malignant transformation of transplanted ovarian tissue, and the possibility of congenital abnormalities as a result of cryopreservation—although no increase has been found in the patients studied so far.
The pregnancy rate is low
For cancer patients, the preservation of ovarian tissue or oocytes yields pregnancy rates significantly lower than those observed with standard IVF procedures. For cancer patients facing chemotherapy, however, oocyte cryopreservation may be one of the few options available and is acceptable in experimental protocols approved by the institutional review board.
Physicians should inform cancer patients about the options for fertility preservation prior to treatment.14 We lack data to recommend ovarian tissue or oocyte cryopreservation for the sole purpose of circumventing reproductive aging in healthy women.13
Changes are occurring so quickly it is often difficult for the general ObGyn to know the most advanced and appropriate treatment for a given patient. The American Society for Reproductive Medicine (ASRM) Practice Committee establishes guidelines based upon well-designed studies to help physicians keep abreast of the best clinical practices. In this article, I focus on recent ASRM guidelines in 5 topical areas associated with substantial misinformation in both the professional and public sectors:
- when and how gynecologists should initiate infertility testing and treatment
- how to evaluate and manage recurrent pregnancy loss
- the need to reduce the rate of multiple gestation from IVF and ART
- the expanded applications for preimplantation genetic diagnosis
- the truth about fertility-sparing efforts in young women planning to undergo cancer therapy and other treatments.
When and how to evaluate patients complaining of infertility
Infertility is a disease, but there are different opinions about when a woman reporting this condition should be assessed (TABLE 1). According to the ASRM, a couple should not be considered infertile until they have tried to conceive spontaneously for at least 12 months, unless the medical history and physical findings dictate earlier evaluation and treatment.1
For example, approximately 25% of couples experience infertility when the woman is age 35, and about 50% experience it when the woman is age 40. Therefore, it is reasonable to investigate infertility after 6 months of attempted conception when the woman is over 35 and after 3 months if she is over age 40.2 The primary reason for this age-related reduction in fertility is the diminishing number and quality of oocytes over time.
Other risk factors for infertility include smoking, family history of premature ovarian failure, significant ovarian pathology, previous ovarian surgery, history of oligomenorrhea or amenorrhea, known or suspected disease of the uterus or fallopian tubes, endometriosis, or a partner known to be subfertile.3,4
TABLE 1
When to investigate infertility, treat, and refer
| INVESTIGATE |
| After 12 months of unprotected intercourse if age |
| After 6 months of unprotected intercourse if age 35–39 |
| After 3 months of unprotected intercourse if age ≥40 |
| After 0–6 months if patient has history of or risk factors for infertility |
| TREAT |
| Treat identifiable causes of infertility |
| Optimize factors influencing fertility: |
|
| Treat empirically (eg, clomiphene, insemination) for 3–6 months in patients |
| REFER |
| History of infertility or significant risk factors |
| Significant fertility problems identified during investigation |
| Age ≥40 |
| After 3–6 months of failed treatment for identifiable causes |
| After 3–6 months of failed empiric treatment |
How to evaluate ovarian function
A careful history and physical examination are key components of systematic, expeditious, and cost-effective identification of the cause of infertility (TABLE 2). A menstrual history and basal body temperature recordings are useful in the diagnosis of ovulatory dysfunction and are easy to obtain. Measurements of urinary luteinizing hormone (LH) using ovulation-prediction kits and mid-luteal-phase serum progesterone are also helpful.
Endometrial biopsy is rarely indicated because of its lack of clinical relevance.
Serial vaginal ultrasonography of the size and number of ovarian follicles may be indicated when simpler methods are inconclusive.
Other tests to evaluate ovarian function may include thyroid-stimulating hormone (TSH), serum prolactin, cycle day 3 follicle-stimulating hormone (FSH) and estradiol, and the clomiphene citrate challenge test in selected patients at higher risk of ovarian dysfunction.
TABLE 2
Current status of tests and treatments
| OLD, NOW RARELY INDICATED |
| Postcoital test |
| Endometrial biopsy |
| Antisperm antibodies testing |
| Intracervical insemination |
| Clomiphene for more than 3–6 cycles |
| Routine hCG injection to stimulate ovulation in clomiphene cycles |
| NEW AND HELPFUL |
| Clomiphene citrate challenge test in selected patients |
| Serial vaginal ultrasounds to evaluate response to ovarian stimulation |
| Saline sonohysterography |
| Preimplantation genetic diagnosis for single-gene defects |
| Embryo cryopreservation |
| Single-embryo transfer to reduce multiple pregnancy rates |
| NEW BUT STILL EXPERIMENTAL* |
| Preimplantation genetic screening for aneuploidy in older patients |
| Human lymphocyte antigen typing for recurrent pregnancy loss |
| Intravenous immunoglobulin for recurrent pregnancy loss |
| Ovarian tissue or oocyte cryopreservation for fertility preservation |
| * Should be performed only in clinical trials |
Clomiphene citrate is preferred
Ovarian dysfunction can be treated with clomiphene for 3 to 6 cycles5 starting at 50 mg per day from cycle day 5 to 9 and increasing to 100 and then 150 mg per day if ovulation does not occur. The drug may also be effective empiric treatment for unexplained infertility using 100 mg per day from cycle days 3 through 7 for a maximum of 3 to 4 cycles.
Only gynecologists experienced with ovarian stimulation drugs and with access to daily ultrasonographic monitoring and estradiol levels should use them, because of the risk of multiple pregnancy and ovarian hyperstimulation.
For women with polycystic ovary syndrome (PCOS), clomiphene alone is more effective than metformin alone. Ovarian drilling may be an effective surgical treatment for PCOS if clomiphene fails, but the cost and risk of adhesions must be considered.
Human chorionic gonadotropin (hCG) injections during clomiphene treatment to stimulate ovulation should be given only if the patient’s own urinary LH surge cannot be detected.
A single intrauterine insemination (IUI) improves the pregnancy rate slightly in conjunction with clomiphene, and by an odds ratio of approximately 2 in conjunction with gonadotropins. The gonadotropin dosage ranges from about 75 to 600 IU per day for 8 to 12 days, based on patient need and careful monitoring.
When to give up on ovarian stimulation. Failure to achieve pregnancy after 3 to 6 cycles signals the need to expand diagnostic evaluation or change treatment strategies.
Evaluate the uterus and tubes
Uterine factors rarely cause infertility but warrant thorough investigation all the same, including assessment of uterine cavity size and shape. A number of methods are available:
- hysterosalpingography (HSG)
- ultrasonography
- saline sonohysterography
Peritoneal factors such as endometriosis or pelvic or adnexal adhesions may occasionally be identified by ultrasonography if there is a mass, but are more likely to require laparoscopy.
When laparoscopy is indicated
If there is evidence or a strong suspicion of endometriosis, pelvic or adnexal adhesions, or significant tubal disease, laparoscopy is warranted. It also may be helpful in younger patients (eg,
Because they reduce pregnancy rates by 50%, hydrosalpinges should be removed or the fallopian tube should be ligated proximally before IVF. It also is important to consider the number of patients needed to treat by laparoscopy to obtain 1 additional pregnancy.
Only gynecologists with expertise should perform laparoscopy, because it is important to make the correct diagnosis and be capable of surgically treating conditions found during the surgery.
Skip the postcoital test, but keep the semen analysis
Abnormalities of the cervical mucus or sperm–mucus interaction rarely cause infertility. Therefore, the postcoital test has questionable predictive value and is probably only useful to confirm that the couple can have properly timed intercourse during the cycle.3
A male factor is solely responsible in about 20% of infertile couples and contributory in another 30% to 40%. For this reason, semen analysis is always warranted when the female is being evaluated for infertility.
Examination of the male partner should be performed by the gynecologist, or the male should be referred to a urologist interested in infertility.6
For recurrent pregnancy loss, best treatment is TLC
Recurrent pregnancy loss is challenging because it is so emotionally charged for the patient, the cause is often unclear, and we lack specific treatments. A methodical and empathetic approach is therefore recommended.
What the history can reveal
Many women with recurrent pregnancy loss will eventually have a live birth, but increasing numbers of miscarriages do predict a poorer overall chance of success, as does increasing age.
Lifestyle factors rarely, if ever, cause recurrent pregnancy loss, but the following factors may increase the risk of miscarriage: obesity, high daily caffeine intake, alcohol consumption, use of nonsteroidal anti-inflammatory drugs, and social class and occupation. A previous diagnosis of or treatment for infertility also increases the risk of recurrent loss.
Smoking should be discouraged and healthy lifestyles should be promoted.7
Causes of recurrent pregnancy loss
Definite causal factors include chromosomal abnormalities, such as translocations, in approximately 5% of couples with 2 or more losses.
Probable factors include uterine abnormalities (both congenital abnormalities such as septate, and acquired defects such as adhesions and intrauterine or submucous myomas), uncontrolled thyroid disease or diabetes, PCOS, and antiphospholipid antibody syndrome.
Other thrombophilias, such as those associated with factor V Leiden mutation, activated protein C resistance, and possibly prothrombin G20210A and protein S deficiency, have been found by some investigators to be associated with recurrent pregnancy loss. It is doubtful that antithyroid antibodies and sharing of parental human lymphocyte antigen (HLA) cause recurrent miscarriage.7
Genetic component likely. The risk of recurrent pregnancy loss in first-degree relatives of women with unexplained repeated pregnancy loss who have normal chromosomes is approximately 6 times higher than the risk in the background population, suggesting a polygenic mode of inheritance.7,8
Other possible causes include low plasma folate levels, which have been associated with an increased risk of first-trimester pregnancy loss. Environmental toxins such as ionizing radiation, organic solvents, alcohol, mercury, and lead are confirmed causes of recurrent pregnancy loss; hyperthermia is a suspected cause.8
Recommended evaluation
Investigations that have been proven in many studies include:
- HSG, hysteroscopy, and sonohysterography
- karyotyping of the couple
- measurement of thyroid hormone
- hemoglobin A1C and serum glucose assessment
- activated partial thromboplastin time, dilute Russell viper venom time, and lupus anticoagulant assessment
- measurement of immunoglobulin G and immunoglobulin M anticardiolipin antibodies
- test for factor V Leiden mutation
Examine products of conception?
Although it is routine practice to send products of conception for histologic examination, mainly to exclude a gestational trophoblastic disorder, the usefulness of this practice is unclear.8 In couples with recurrent pregnancy loss, chromosomal analysis of the products of conception indicates that a normal conceptus karyotype in a previous pregnancy is a predictor of a higher rate of miscarriage in a subsequent pregnancy.8 When stratified by maternal age, there is no difference in the distribution of cytogenetically abnormal miscarriages in couples with recurrent pregnancy loss, compared with controls.8 The cost-effectiveness of karyotyping is therefore unclear.
High levels of homocysteine (ie, hyperhomocysteinemia) can be associated with recurrent pregnancy loss. Among genetic causes is polymorphism at position 677 in the methylene tetrahydrofolate reductase (MTHFR) gene, which is often evaluated to rule out this condition.
Infections with bacteria, viruses, or parasites can all interfere with early pregnancy development, but none seem to be a significant cause of recurrent pregnancy loss.8 Testing is most useful in the context of an acute infectious episode.
Can recurrent loss be treated?
The hallmark of treatment is empathetic care, along with counseling emphasizing the complexity of this condition. Any endocrinologic, anatomic, or other abnormality that is identified during evaluation should be treated, if possible.
Progesterone supplementation is not proven treatment. This therapy is commonly prescribed but has not been proved to improve live birth rates.
Prednisone, aspirin, and NSAIDs have no benefits but potential risks and should not be used.
Current immunologic therapies for recurrent pregnancy loss have no sound scientific basis, except for the use of heparin and aspirin in patients with well-documented antiphospholipid antibodies.7 Specifically, intravenous immunoglobulin remains unproven, is experimental, and should be provided only in approved research settings.9 Paternal leukocyte immunization does not work, has been proscribed by the US Food and Drug Administration, and should be avoided.
Careful counseling and education of the patient about the history, pathophysiology, testing, test results, and treatment of recurrent pregnancy loss are necessary. Women with subfertility who have taken a long time to conceive should be treated empirically with ovarian stimulation in an attempt to shorten the time to conception.
Singleton births can be encouraged without jeopardizing IVF, ART
Multiple gestations have increased over the past 15 years, largely because of:
- ovulation induction for management of oligo-ovulation
- superovulation to produce more than 1 ovulated egg for fertility treatments
- assisted reproductive technologies (ART), in which more than 1 embryo is replaced to increase the pregnancy rate
Multiple gestations are a bad idea
Risks include a higher complication rate for gravidas and fetuses, as well as higher short- and long-term costs to patients and society. It is therefore important to reduce the incidence of multiple gestation associated with fertility treatments.10
How to reduce the likelihood of multiple fetuses
- Closely monitor cycles involving ovulation induction and superovulation for efficacy and safety, to avoid ovarian hyperstimulation and reduce the risk of multiple gestation. Although attempts to limit multiple gestation during ovulation induction or superovulation using ultrasonographic criteria and serum estradiol limits have been ineffective,10 it is my opinion that we should err on the side of conservatism, even though the optimal parameters for doing so have not been determined by high-quality trials. I recommend that hCG or IUI be avoided if more than 4 mature follicles (>15 mm) or 6 large follicles (>12 mm) are present on a sonogram, and the couple should be instructed to refrain from intercourse.
- Focus on the objective of a single healthy baby as the optimal outcome. Data published by the Society for Assisted Reproductive Technology (SART) clearly demonstrate the clinical impact of a reduction in the number of embryos transferred, which reduced triplet pregnancy rates in 2005 to less than half the rate in the late 1990s. Fewer embryos are transferred today than just a few years ago, and the trend is continuing. This will help reduce the triplet rate further and also reduce twin pregnancies. In the past 6 months, guidelines have recommended replacement of only 1 blastocyst at day 5 or 1 to 2 embryos at day 3 in women under age 35 with a favorable prognosis.
What the future holds
We can expect more elective single-embryo or single-blastocyst transfers as we gain further expertise in this area. However, this practice should be implemented carefully in selected patients to maintain adequate pregnancy rates while reducing multiple gestations.
The United States has the highest ART success rates in the world (approximately 40% higher than in Europe) despite a reduction in the number of triplet or higher-order pregnancies resulting in live births after ART—from 7.0% in 1996 to 2.4% in 2004. The twin rate has remained stable at approximately 30%, but should decrease as 1- and 2-embryo transfers become more common.11
Preimplantation genetic diagnosis now has multiple applications
Preimplantation genetic diagnosis (PGD) is over 15 years old, and at least 1,000 babies worldwide have been born after its use, with no reports of increased fetal malformation or other problems.12
Two basic techniques are employed to analyze the genomic status of the 1 or 2 blastomeres usually removed from the 8-cell embryo on day 3 after fertilization:
- Polymerase chain reaction (PCR) is used to amplify a specific DNA sequence harboring a mutation. A mismatch (eg, due to a genetic deletion) leads to differential migration on the gel, thus permitting diagnosis. The error rate, primarily due to allelic dropout, in which 1 of the 2 alleles selectively amplifies and thus contributes to diagnostic errors, is approximately 0.3% to 5.6%.
- Fluorescent in situ hybridization (FISH) allows determination of the ploidy of a blastomere. Labeled probes bind to chromosomes and are viewed under a fluorescent microscope. The error rate is 1% to 10% for a variety of technical reasons.12 Testing takes about 1 day while the embryos are developing to blastocysts, at which time those that are viable and tested to be normal are transferred back into the uterus.
Not just for gender determination
PGD initially was used to determine gender (by FISH) as an indirect method of avoiding X-linked genetic diseases such as hemophilia. The error rate for gender determination is less than 1%. Since then, single-gene-defect disorders have been diagnosed using PCR and heteroduplex formation or restriction endonuclease digestion, both of which distinguish normal from mutant alleles. PGD has been performed broadly to diagnose Tay-Sachs, Huntington’s disease, and hundreds of other diseases.
Testing for translocation by PGD has been especially useful and may reduce the risk of spontaneous abortion from as much as 95% to 13% if one of the parents is a known translocation carrier.
Still under investigation is the routine use of FISH to detect aneuploidy in cases of recurrent pregnancy loss. The use of FISH for gender selection for family balancing is not recommended by the ASRM.
More young women seek to preserve their fertility
Fertility preservation through ovarian tissue or oocyte cryopreservation or vitrification has recently been popularized by cancer survival consumer groups, the media, and other interests. In addition to cancer patients planning to undergo chemotherapy or radiotherapy, candidates for fertility preservation include women undergoing bone marrow or stem cell transplantation or oophorectomy (for a benign tumor, endometriosis, or prophylaxis) and patients with severe autoimmune disease needing chemotherapy.
In cancer patients, fertility is preserved using one of several methods:
- shielding or moving the ovaries to a different anatomic site during radiation
- use of gonadotropin-releasing hormone analogs or oral contraceptives during chemotherapy (unproven)
- changes to chemotherapy regimen
- IVF cycle followed by cryopreservation of embryos if the patient has a male partner or is prepared to use donor sperm (provided the oncologist confirms that ovarian stimulation and high estradiol levels are acceptable and there is time to undergo an IVF cycle before cancer treatment begins).
Unresolved issues
Concerns about cryopreservation of ovarian tissue in cancer patients13,14 include the possibility of reseeding tumor cells after ovarian transplantation, malignant transformation of transplanted ovarian tissue, and the possibility of congenital abnormalities as a result of cryopreservation—although no increase has been found in the patients studied so far.
The pregnancy rate is low
For cancer patients, the preservation of ovarian tissue or oocytes yields pregnancy rates significantly lower than those observed with standard IVF procedures. For cancer patients facing chemotherapy, however, oocyte cryopreservation may be one of the few options available and is acceptable in experimental protocols approved by the institutional review board.
Physicians should inform cancer patients about the options for fertility preservation prior to treatment.14 We lack data to recommend ovarian tissue or oocyte cryopreservation for the sole purpose of circumventing reproductive aging in healthy women.13
1. Practice Committee. American Society for Reproductive Medicine. Definition of “infertility.” Fertil Steril. 2006;86(Suppl 4):S228.-
2. Practice Committee, American Society for Reproductive Medicine. Aging and infertility in women. Fertil Steril. 2006;86(Suppl 4):S248-252.
3. Practice Committee, American Society for Reproductive Medicine. Optimal evaluation of the infertile female. Fertil Steril. 2006;86(Suppl 4):S264-267.
4. Practice Committee, American Society for Reproductive Medicine. Smoking and infertility. Fertil Steril. 2006;86(Suppl 4):S172-177.
5. Practice committee, American Society for Reproductive Medicine. Use of clomiphene citrate in women. Fertil Steril. 2006;86(Suppl 4):S187-193.
6. Male Infertility Best Practice Committee, American Urological Association, and Practice Committee, American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86(Suppl 4):S202-209.
7. Christiansen OB, Andersen A-MN, Bosch E, et al. Evidence-based investigations and treatments of recurrent pregnancy loss. Fertil Steril. 2005;83:821-839.
8. Jauniauz E, Farquharson RG, Christiansen OB, et al. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod. 2006;21:2216-2222.
9. Practice Committee, American Society for Reproductive Medicine. Intravenous immunoglobulin (IVIG) and recurrent spontaneous pregnancy loss. Fertil Steril. 2006;86(Suppl 4):S226-227.
10. Practice committee, American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(Suppl 4):S106-110.
11. Practice Committee, Society for Assisted Reproductive Technology and the Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2006;86(Suppl 4):S51-52.
12. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Preimplantation genetic diagnosis. Fertil Steril. 2006;86(Suppl 4):S257-258.
13. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2006;86(Suppl 4):S142-147.
14. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622-1628.
Dr. Adamson reports no financial relationships with any company whose products are mentioned in this article. He receives grant/research support from IBSA, Serono, and ViaCell and is a consultant to ViaCell.
1. Practice Committee. American Society for Reproductive Medicine. Definition of “infertility.” Fertil Steril. 2006;86(Suppl 4):S228.-
2. Practice Committee, American Society for Reproductive Medicine. Aging and infertility in women. Fertil Steril. 2006;86(Suppl 4):S248-252.
3. Practice Committee, American Society for Reproductive Medicine. Optimal evaluation of the infertile female. Fertil Steril. 2006;86(Suppl 4):S264-267.
4. Practice Committee, American Society for Reproductive Medicine. Smoking and infertility. Fertil Steril. 2006;86(Suppl 4):S172-177.
5. Practice committee, American Society for Reproductive Medicine. Use of clomiphene citrate in women. Fertil Steril. 2006;86(Suppl 4):S187-193.
6. Male Infertility Best Practice Committee, American Urological Association, and Practice Committee, American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86(Suppl 4):S202-209.
7. Christiansen OB, Andersen A-MN, Bosch E, et al. Evidence-based investigations and treatments of recurrent pregnancy loss. Fertil Steril. 2005;83:821-839.
8. Jauniauz E, Farquharson RG, Christiansen OB, et al. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod. 2006;21:2216-2222.
9. Practice Committee, American Society for Reproductive Medicine. Intravenous immunoglobulin (IVIG) and recurrent spontaneous pregnancy loss. Fertil Steril. 2006;86(Suppl 4):S226-227.
10. Practice committee, American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(Suppl 4):S106-110.
11. Practice Committee, Society for Assisted Reproductive Technology and the Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2006;86(Suppl 4):S51-52.
12. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Preimplantation genetic diagnosis. Fertil Steril. 2006;86(Suppl 4):S257-258.
13. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2006;86(Suppl 4):S142-147.
14. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622-1628.
Dr. Adamson reports no financial relationships with any company whose products are mentioned in this article. He receives grant/research support from IBSA, Serono, and ViaCell and is a consultant to ViaCell.
Operative vaginal delivery: 10 components of success
Operative vaginal delivery is a dying art. National databases in the United States and elsewhere have shown this trend for decades.1 Women no longer can be reliably predicted to prefer operative vaginal delivery over cesarean section, and providers caring for delivering mothers (and their families) should not assume that they do. Nor does the 20th century paradigm of operative vaginal delivery as the accepted “next step” between spontaneous vaginal delivery and cesarean section hold up, given the decreased maternal and neonatal morbidity and mortality associated with modern techniques of cesarean section. Nevertheless, operative vaginal delivery remains a viable option in some cases.
This article—based on personal opinion and experience, as well as published data whenever possible—describes 10 selected aspects of operative vaginal delivery, offering recommendations for each.
1. Consider obstetric history
How a woman fared in previous deliveries has a bearing on the current delivery. For example, if she has a history of persistent occiput posterior position, as in the case described on page 56, she may have an anthropoid pelvis, placing her at increased risk for another malposition.1 In such cases, the patient should be counseled about the potential for operative vaginal delivery, and the risks and benefits should be discussed prenatally.
A history of obesity, excessive weight gain, and glucose intolerance should be considered warning signs of a large-for-gestational-age infant.
2. Ensure adequate informed consent
Patients should be informed of the risks of any procedure they are offered, and operative vaginal delivery—like any operative procedure—has definite risks.
It is unbalanced to mention only the perceived benefits of a procedure and to avoid the discomfort of discussing the potential significant fetal and maternal injury that may result from a procedure. It is far better for the patient and her family to learn—before an adverse outcome occurs—that forceps delivery sometimes leads to maternal and fetal lacerations, and that operative vaginal delivery can be associated with an increased risk for shoulder dystocia in some circumstances.
The best way to educate patients about operative vaginal delivery is during prenatal care. I recommend a written informed consent document similar to the one used for cesarean section. If such a form is not signed during the course of office prenatal care, it should be offered upon admission for delivery.
In some cases, operative vaginal delivery may be safer than cesarean
Operative vaginal delivery clearly increases the risk of neonatal intracranial bleeds when compared with normal spontaneous vaginal delivery or elective cesarean section.2 However, a patient should understand that cesarean section carries a risk of neonatal intracranial hemorrhage similar to that of operative vaginal delivery once a woman has labored to complete dilation and pushed for some time.2 In fact, a baby with a well-engaged head can experience significant increases in intracranial pressure during cesarean delivery when concerted efforts have to be used to deliver a deeply engaged fetal head out of a hysterotomy incision. Such maneuvering can also injure the fetal neck and brachial plexus.
3. The abdominal examination is critical
Examination of the maternal abdomen helps to confirm the fetal lie and presentation and may give an idea of the position of the fetal back in relation to the uterine midline. If the fetal back cannot be felt or is palpated far laterally, the fetus may be in an occiput posterior or transverse position. Often this knowledge helps the examiner make sense of an otherwise difficult vaginal examination.
Estimate fetal weight
Fetal weight estimations from a careful abdominal examination can be as accurate as ultrasonographic evaluation.3 It is strongly recommended that fetal weight be estimated and considered in context with maternal diabetes, obesity, excessive weight gain, and previous ultrasound examinations before operative vaginal delivery is undertaken.
Is the fetal head engaged?
The average term (3,200 g) fetus has a basovertical head diameter of approximately 9 to 10 cm,4,5 and the average adult finger has a diameter of 2 cm (one fifth of the head). Using this information, an estimate of how many “fifths” of the fetal head are above the pelvic brim can be made by evaluating how many fingerbreadths of fetal head can be palpated above the symphysis pubis on abdominal examination.
Crichton4 described this method in 1974, and it is an extremely useful and underutilized technique, in my opinion. He stated that no more than two fifths (2 fingerbreadths) of an unmolded fetal head should be palpated abdominally once the occiput is felt at the ischial spines. If three fifths or more of the fetal head is still palpable above the pubic symphysis, regardless of whether there is bone palpated at or below 0 station on vaginal examination, consider the head unengaged and avoid operative delivery.
It is quite possible to feel the fetal skull bone below the ischial spines and still have an unengaged head.5 This is due to molding of the head and elongation of the basovertical diameter. When this occurs, the widest diameter of the fetal skull remains above the plain of the pelvic brim (unengaged), even though the lowermost point is felt below the spines on vaginal examination. A graphic example of such an elongated basovertical diameter can be seen in the so-called cone-head baby.
At examination, fetal head should be in occiput anterior position
In order to best use the abdominal examination to assess the amount of fetal head above the pelvic brim, the fetal head must be in an occiput anterior position. This is because the occiput is sometimes difficult to palpate in a posterior or transverse position, and the obstetrician may incorrectly assume full engagement. This further underscores the importance of a careful maternal abdominal examination and the location of the fetal spine.
Abdominal examination is more informative than vaginal examination
Knight and colleagues6 studied the relative value of abdominal and vaginal examinations in the determination of fetal head engagement. They examined the records of 104 women who had been evaluated by both methods prior to attempted operative vaginal delivery. Successful vaginal delivery was correctly predicted using abdominal criteria (94%) more often than using vaginal criteria (80%) (P<.01).
E.D., a 32-year-old gravida 4 para 3, presents at 39 weeks’ gestation with spontaneous rupture of membranes in early labor. Her 3 deliveries thus far have all been vaginal, with the infants ranging in weight from 3,700 to 3,900 g. Two of these infants were delivered with vacuum extraction because of occiput posterior position and a prolonged second stage.
E.D.’s prenatal course has been relatively uncomplicated except for a 43-lb weight gain (she weighs 240 lb) and a borderline 1-hour glucose challenge test. She also had 1 abnormal value on a 3-hour glucose tolerance test. Her prenatal pelvic examination was documented as “adequate.”
In early stages, all appears normal
On admission, E.D. is dilated 4 cm with 70% effacement and a cephalic presentation at -2 station. Electronic fetal monitoring is reassuring, and she is contracting regularly every 6 minutes, with moderate pain. The physician on call instructs the nurse to start oxytocin if there is no progress in 2 hours, and to call anesthesia to give an epidural if the patient requests it. E.D. asks for, and is given, an epidural 2 hours later, when her cervix is dilated 5 cm.
The next morning, a different physician examines her and reports a rim of cervix remaining, with the fetal head at 0 to +1 station. He asks E.D. to push, and the rim is reduced over the infant’s head. The patient is instructed to continue pushing with contractions. The physician writes the admission (and only predelivery) note: “32 yr old G4P3, term, SROM, good FHTs, good progress, complete, 1+ station, clear fluid. Anticipate vaginal delivery.”
When progress stalls, mother tires
E.D. pushes well with adequate contractions for 2.5 hours, with minimal descent of the head and increasing caput and molding. The physician examines her again and reports that the baby is at +2 station. He also suggests the use of the vacuum extractor, because the patient is becoming exhausted and the baby is “quite big.” The obstetrician appears somewhat hesitant when applying the vacuum and remarks to the nurse that he “thinks the baby is in a left occiput anterior position” but is not “100% sure.”
When vacuum fails, a switch to forceps
After 2 attempts with the vacuum extractor, during which there are 2 “pop-offs,” the physician asks for Simpson forceps, adding that he thinks the baby is now in right occiput posterior position and he needs to “get a better grip on the baby’s head.” The forceps are applied with some difficulty, necessitating 2 reapplications.
After 5 contractions (and 6 pulling efforts), a baby boy is delivered. Because of a delay in delivery of the shoulders after delivery of the head, the physician places the patient in McRoberts position and has a nurse apply suprapubic pressure, and no further difficulties are encountered.
Large baby has brachial plexus injury
The infant weighs 4,200 g and has Apgar scores of 3 and 8, as well as a small laceration on his forehead, moderate flaccidity of the left arm, and an elongated head. The mother has a 4th-degree laceration that is repaired with some difficulty.
The delivery note reads: “Assisted vaginal delivery, 4,200 g male, 3 vessel cord, 600 cc estimated blood loss, 4th-degree laceration repaired in layers.” E.D. ultimately requires 2 U of blood on postpartum day 2 for symptomatic anemia.
Mother and baby are discharged on postpartum day 4 in stable condition. The infant has a brachial plexus injury that resolves within 6 weeks.
Lessons learned
Among the mistakes the obstetrician made in this case are a failure to take the obstetric history into account, omission of a comprehensive abdominal exam, ignoring signs of a large baby, and lack of a plan for emergent cesarean section.
4. Keep molding in mind
Some (up to +2) occipito-parietal molding may be normal in the late stages of delivery (ie, the occipital bone slips under the 2 parietal bones, but can be easily reduced), but severe parieto-parieto molding is never normal and should be interpreted as a sign of relative or absolute cephalopelvic disproportion. FIGURE 1 shows a classification system for molding.
FIGURE 1 How to characterize the degree of molding
Excessive molding may lead to tears in dura and underlying vessels.
Traction plus severe molding may increase the risk of intracranial injury
The most frequent causes of molding are asynclitism and deflexion of the head, commonly seen in occiput posterior and transverse positions. Correction of the asynclitism and malposition may correct the molding and allow safe vaginal delivery. Traction on a head with severe molding may increase the risk of intracranial injury.
Using maximum likelihood logistic regression analyses, Knight and colleagues6 demonstrated that the factor of greatest importance in determining whether a case would be allocated to engaged versus unengaged groups was molding (odds ratio 2.17; 95% confidence intervals 0.75–6.27). The authors concluded that when abdominal and vaginal assessments produce different findings, the major factor responsible is molding. They noted that data derived from vaginal examination alone may be misleading when molding is present.
The rule of 3’s
With the fetus in an occiput anterior position, determine the number of fifths of the fetal head that can be palpated above the pelvic brim abdominally, add it to the degree of molding palpated vaginally, and avoid operative vaginal delivery if the sum is 3 or higher (FIGURE 2).7
FIGURE 2 Abdominopelvic assessment using the rule of 3’s
If the sum of the number of fifths of the fetal head palpated above the pelvic brim abdominally and the degree of molding palpated vaginally equals or exceeds 3, operative vaginal delivery is unlikely to be successful.For example, if two fifths (~4 cm) of the fetal head is palpated above the maternal pubic symphysis, and there is already +1 of parieto-parieto molding, significant cephalopelvic disproportion is likely and operative vaginal delivery will probably fail, with an increased risk of fetal and maternal damage. Obviously, if three fifths or more of the fetal head is palpated abdominally, the head is not engaged and operative vaginal delivery is contraindicated, regardless of whether the scalp is felt at or below the ischial spines.
Knowledge of fetal head diameters is useful
Using measurements of fetal head diameters (FIGURE 3), it is easy to see why a vertex presentation in an occiput anterior position (presenting diameter=suboccipitobregmatic diameter=9.5 cm) will deliver more easily than a baby in a deflexed occiput posterior position (presenting diameter=occipitofrontal diameter ≥ 11.5 cm). The presenting diameter of a brow presentation will never negotiate a normally proportioned female pelvis, whereas that of a mentum anterior face presentation is clearly adequate for a vaginal delivery if all other factors are favorable.
FIGURE 3 Know the basic term fetal head diameters
Depending on the presentation, the fetal head will deliver easily, as in occiput anterior position, when the presenting diameter is 9.5 cm, or with difficulty, as when the presenting diameter is 11.5 cm or more.
5. Be aware of fetal head position throughout labor
Early documentation of fetal head position during labor may help tremendously when decisions regarding the mode of delivery have to be made in a hurry. If one is aware that the head has been persistently in a deflexed occiput posterior position (or a transverse position) throughout the labor, a prolongation or arrest of descent can be explained by progressive deflexion of the head and increasing presenting diameters (or deep transverse arrest, as the case may be).
In such a case, if sudden fetal decompensation necessitates emergent delivery, operative vaginal delivery is a much less viable option than it would be with a well-flexed occiput anterior position at the same station.
When there is knowledge of a fetal malposition, cesarean section may be the wisest choice in an emergency, even if the fetal head is at an appropriate station, unless the operator has the requisite skills at operative vaginal delivery and is certain of a high chance of a successful outcome.
6. Have a valid indication
Operative vaginal delivery should not proceed without a valid indication8 that conforms to accepted guidelines.
Consider the pathology underlying the indication
For example, although maternal exhaustion is clearly a valid indication for operative vaginal delivery, it is important to examine the underlying reason for the exhaustion. A diabetic mother who is known to have a large-for-gestational-age infant, who has a prolonged active phase (8 hours) and is exhausted after 3 hours of excellent pushing with adequate contractions, may appear to have, on the surface, a valid indication—but clearly this situation calls for extreme caution. The size of the infant and the lack of progress suggest at the least the potential for cephalopelvic disproportion.
In my opinion, operative vaginal delivery for maternal exhaustion should probably be reserved for someone who has progressed at a normal rate to crowning and who simply does not have the energy to push out a normal-sized baby.
Judicious use is key
Operative vaginal delivery can be used judiciously to remedy situations that have the potential to escalate. For example, a persistent transverse fetal head position in a primigravida with a platypelloid pelvis who has pushed for 1 hour with increasing caput and molding is highly unlikely to resolve. If the fetus is an appropriate candidate for rotational forceps, and the physician has the requisite training and experience, a rotational delivery after only 1 hour is entirely appropriate to avoid potential maternal and fetal injury that could follow 3 hours of pushing.
Persistent variable decelerations are another indication of potential fetal compromise and justify judicious use of operative vaginal delivery in appropriate candidates.
7. Do not use instruments sequentially
The use of sequential operative vaginal delivery methods to complete a vaginal delivery is no longer acceptable. Significantly increased neonatal and maternal risks have been demonstrated in at least 3 well-designed studies. Data indicate that a failed operative vaginal delivery attempt,2,9,10 more than 3 hours of maternal pushing,10 and more than 3 traction episodes (regardless of ultimate success with the instrument)10 are associated with an increased risk of neonatal intracranial hemorrhage.
Because we lack a standard of care for the optimal number of traction efforts or “pop-offs” for operative vaginal delivery, I suggest that any practitioners be familiar with, and adhere to, the manufacturer’s suggested guidelines. These guidelines will usually be designed to fall on the conservative side of safety issues.
I have heard of physicians who sometimes use the vacuum extractor to bring the head down to a place where they feel more comfortable applying forceps. This practice is unacceptable. By the same token, proceeding with vacuum extraction after concluding there is too much molding or caput for forceps is untenable.
8. Have a clear endpoint and exit strategy
Resist the temptation to persist with operative vaginal delivery in the face of inadequate descent or progress. It may sometimes seem as though “just one more pull” will effect delivery, but exceeding the recommended number of attempts can lead to excessive traction and maternal or fetal damage. It can be easy to become fixated on achieving vaginal delivery, and rational thought can become clouded.
I recommend that each department establish clear and agreed-upon limits for their practitioners. To this end, there should be an appropriately cooperative atmosphere in each delivery unit that encourages the provider team to work together to prevent adverse outcomes from operative vaginal delivery. Protocols or checklists that help the nursing staff keep the physician informed of the number of traction efforts and/or pop-offs that occur will help prevent inadvertent exceeding of the limits established for that unit.
Prior to attempting an operative vaginal delivery, the obstetrician should have a clear exit strategy, and this strategy should be outlined to the patient and the nursing/ancillary staff. When the predetermined criteria are met, operative vaginal delivery should be abandoned without delay, and cesarean section should be performed expeditiously. Obviously, this requires that preparations for emergent cesarean section be made prior to use of the forceps or vacuum extractor.
The necessary anesthesia and neonatal and operating room personnel should be ready and in position at the time the operative vaginal delivery is attempted.
Never resume maternal pushing after failed forceps or vacuum
There is no place for “rest and descend” protocols or further attempts at spontaneous vaginal delivery after a failed operative vaginal delivery. Once an easy operative vaginal delivery becomes impossible, immediate cesarean section is the best option.
- A valid indication documented preoperatively
- Unambiguous knowledge of the fetal head position
- Complete dilatation of the cervix
- Confirmed engagement of the fetal head
- Station at or below +2, unless the operator is experienced and there is a justifiable reason for a midpelvic delivery
- Rule of 3’s satisfied
- A documented estimate of appropriate fetal weight and adequate maternal pelvic anatomy
- Adequate anesthesia
- Preparations in place for immediate cesarean section and resuscitation of the neonate, if needed
- An informed, willing, and cooperative patient who understands that cesarean section may be an appropriate alternative mode of delivery (depending on the circumstances)
In addition, the person intending to perform the delivery should personally examine the patient before the attempt to confirm that the prerequisites have been met. I would go so far as to state that, unless there is a high expectation of an easy operative vaginal delivery, it should not be attempted.
9. Document, document
Under ideal circumstances, the obstetrician initiates a discussion with the patient during prenatal care and mentions the possibility of vacuum extraction or forceps delivery. This discussion is documented in the prenatal chart. The note includes a statement discussing the relative risks and benefits of the alternative delivery modes, the patient’s expressed desire for a vaginal delivery, including operative vaginal delivery, and why, in the physician’s best judgment, an operative vaginal delivery is a reasonable option.
Document the events of labor and delivery
Clear, concise progress notes from nursing and obstetric care providers are extremely important. All pertinent maternal and fetal information should be addressed at each examination of the patient, and some comment on the rate of progress, threshold limits, management plan, and preparations should be included.
In my opinion, each progress note should describe maternal vital signs, adequacy of contractions, use of labor augmentation and the dose, fetal tolerance of contractions, reassuring nature of the monitoring, cervical dilation, fetal head position (if discernible), station, and any molding and caput. If maternal or fetal monitoring is inadequate with external devices, the notes should include details of the plan to improve the situation.
Include a preoperative note
I strongly recommend a preoperative note if there is time. It should clearly document the pertinent obstetric and prenatal care the patient has received, the progress of labor, the indication for operative vaginal delivery, estimated fetal weight, adequacy of the maternal pelvis for an infant of the anticipated weight, fetal head position, degree of molding, complete dilation of the cervix, station of the fetal head, and some assessment of flexion of the neck, if possible.
Once the decision to proceed has been made, I would add a statement indicating that the chances of success are high and, in your considered opinion, operative vaginal delivery is a safe and indicated option.
Write a detailed postoperative note
I suggest a dictated postoperative note for every operative vaginal delivery, successful or not. The elements included in the preoperative note should be reiterated and details of the delivery explained. The position and station of the fetal head at the time the instrument was applied (especially if this contrasts with what was stated in the preoperative note), the degree of caput and molding, the number and duration of traction efforts, progress of the fetal head with each traction effort, duration of the procedure, personnel present, and the preparations made for the delivery should all be documented. Physicians and nurses should agree on what constitutes a traction effort, to avoid conflicts in the various sets of notes.
Document postdelivery vaginal and rectal examinations, which should alert you to the presence of any retained sponges, vaginal hematomas or sulcus tears, or a previously unidentified rectovaginal fistula.
10. Handle bad outcomes with compassion
Do not avoid contact with the family in the event of a bad outcome. Rather, confront the outcome as honestly and compassionately as possible. If you correctly assessed and informed the patient and proceeded to operative vaginal delivery with her full understanding of the indication, she will have accepted a small risk of an untoward outcome. In general, if she perceives your behavior to have been professional and caring, she is much less likely to seek retribution.
The author reports no financial relationships relevant to this article.
1. Gardberg M, Stenwall O, Laakkonen E. Recurrent persistent occipito-posterior position in subsequent deliveries. BJOG. 2004;111:170-171.
2. Towner D, Castro MA, Eby-Wilkens E, Golbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
3. Fetal macrosomia. American College of Obstetricians and Gynecologists Practice Bulletin #22. Washington, DC: ACOG; November 2000.
4. Crichton D. A reliable method of establishing the level of the fetal head in obstetrics. S Afr Med J. 1974;48:784-787.
5. Crichton D. The accuracy and value of cephalopelvimetry. J Obstet Gynaecol Br Emp. 1962;69:366-378.
6. Knight D, Newnham JP, McKenna M, Evans S. A comparison of abdominal and vaginal examinations for diagnosis of engagement of the fetal head. Aust N Z J Obstet Gynaecol. 1993;33:154-158.
7. Philpott RH. The recognition of cephalopelvic disproportion. Clin Obstet Gynecol. 1982;9:609-624.
8. Operative vaginal delivery. American College of Obstetricians and Gynecologists Practice Bulletin #17. Washington, DC: ACOG; June 2000.
9. Gardella C, Taylor M, Benedetti T, Hitti J, Critchlow C. The effect of sequential use of vacuum and forceps for assisted vaginal delivery on neonatal and maternal outcomes. Am J Obstet Gynecol. 2001;185:896-902.
10. Murphy DJ, Liebling RE, Patel R, Verity L, Swingler R. Cohort study of operative delivery in the second stage of labour and standard of obstetric care. BJOG. 2003;110:610-615.
Operative vaginal delivery is a dying art. National databases in the United States and elsewhere have shown this trend for decades.1 Women no longer can be reliably predicted to prefer operative vaginal delivery over cesarean section, and providers caring for delivering mothers (and their families) should not assume that they do. Nor does the 20th century paradigm of operative vaginal delivery as the accepted “next step” between spontaneous vaginal delivery and cesarean section hold up, given the decreased maternal and neonatal morbidity and mortality associated with modern techniques of cesarean section. Nevertheless, operative vaginal delivery remains a viable option in some cases.
This article—based on personal opinion and experience, as well as published data whenever possible—describes 10 selected aspects of operative vaginal delivery, offering recommendations for each.
1. Consider obstetric history
How a woman fared in previous deliveries has a bearing on the current delivery. For example, if she has a history of persistent occiput posterior position, as in the case described on page 56, she may have an anthropoid pelvis, placing her at increased risk for another malposition.1 In such cases, the patient should be counseled about the potential for operative vaginal delivery, and the risks and benefits should be discussed prenatally.
A history of obesity, excessive weight gain, and glucose intolerance should be considered warning signs of a large-for-gestational-age infant.
2. Ensure adequate informed consent
Patients should be informed of the risks of any procedure they are offered, and operative vaginal delivery—like any operative procedure—has definite risks.
It is unbalanced to mention only the perceived benefits of a procedure and to avoid the discomfort of discussing the potential significant fetal and maternal injury that may result from a procedure. It is far better for the patient and her family to learn—before an adverse outcome occurs—that forceps delivery sometimes leads to maternal and fetal lacerations, and that operative vaginal delivery can be associated with an increased risk for shoulder dystocia in some circumstances.
The best way to educate patients about operative vaginal delivery is during prenatal care. I recommend a written informed consent document similar to the one used for cesarean section. If such a form is not signed during the course of office prenatal care, it should be offered upon admission for delivery.
In some cases, operative vaginal delivery may be safer than cesarean
Operative vaginal delivery clearly increases the risk of neonatal intracranial bleeds when compared with normal spontaneous vaginal delivery or elective cesarean section.2 However, a patient should understand that cesarean section carries a risk of neonatal intracranial hemorrhage similar to that of operative vaginal delivery once a woman has labored to complete dilation and pushed for some time.2 In fact, a baby with a well-engaged head can experience significant increases in intracranial pressure during cesarean delivery when concerted efforts have to be used to deliver a deeply engaged fetal head out of a hysterotomy incision. Such maneuvering can also injure the fetal neck and brachial plexus.
3. The abdominal examination is critical
Examination of the maternal abdomen helps to confirm the fetal lie and presentation and may give an idea of the position of the fetal back in relation to the uterine midline. If the fetal back cannot be felt or is palpated far laterally, the fetus may be in an occiput posterior or transverse position. Often this knowledge helps the examiner make sense of an otherwise difficult vaginal examination.
Estimate fetal weight
Fetal weight estimations from a careful abdominal examination can be as accurate as ultrasonographic evaluation.3 It is strongly recommended that fetal weight be estimated and considered in context with maternal diabetes, obesity, excessive weight gain, and previous ultrasound examinations before operative vaginal delivery is undertaken.
Is the fetal head engaged?
The average term (3,200 g) fetus has a basovertical head diameter of approximately 9 to 10 cm,4,5 and the average adult finger has a diameter of 2 cm (one fifth of the head). Using this information, an estimate of how many “fifths” of the fetal head are above the pelvic brim can be made by evaluating how many fingerbreadths of fetal head can be palpated above the symphysis pubis on abdominal examination.
Crichton4 described this method in 1974, and it is an extremely useful and underutilized technique, in my opinion. He stated that no more than two fifths (2 fingerbreadths) of an unmolded fetal head should be palpated abdominally once the occiput is felt at the ischial spines. If three fifths or more of the fetal head is still palpable above the pubic symphysis, regardless of whether there is bone palpated at or below 0 station on vaginal examination, consider the head unengaged and avoid operative delivery.
It is quite possible to feel the fetal skull bone below the ischial spines and still have an unengaged head.5 This is due to molding of the head and elongation of the basovertical diameter. When this occurs, the widest diameter of the fetal skull remains above the plain of the pelvic brim (unengaged), even though the lowermost point is felt below the spines on vaginal examination. A graphic example of such an elongated basovertical diameter can be seen in the so-called cone-head baby.
At examination, fetal head should be in occiput anterior position
In order to best use the abdominal examination to assess the amount of fetal head above the pelvic brim, the fetal head must be in an occiput anterior position. This is because the occiput is sometimes difficult to palpate in a posterior or transverse position, and the obstetrician may incorrectly assume full engagement. This further underscores the importance of a careful maternal abdominal examination and the location of the fetal spine.
Abdominal examination is more informative than vaginal examination
Knight and colleagues6 studied the relative value of abdominal and vaginal examinations in the determination of fetal head engagement. They examined the records of 104 women who had been evaluated by both methods prior to attempted operative vaginal delivery. Successful vaginal delivery was correctly predicted using abdominal criteria (94%) more often than using vaginal criteria (80%) (P<.01).
E.D., a 32-year-old gravida 4 para 3, presents at 39 weeks’ gestation with spontaneous rupture of membranes in early labor. Her 3 deliveries thus far have all been vaginal, with the infants ranging in weight from 3,700 to 3,900 g. Two of these infants were delivered with vacuum extraction because of occiput posterior position and a prolonged second stage.
E.D.’s prenatal course has been relatively uncomplicated except for a 43-lb weight gain (she weighs 240 lb) and a borderline 1-hour glucose challenge test. She also had 1 abnormal value on a 3-hour glucose tolerance test. Her prenatal pelvic examination was documented as “adequate.”
In early stages, all appears normal
On admission, E.D. is dilated 4 cm with 70% effacement and a cephalic presentation at -2 station. Electronic fetal monitoring is reassuring, and she is contracting regularly every 6 minutes, with moderate pain. The physician on call instructs the nurse to start oxytocin if there is no progress in 2 hours, and to call anesthesia to give an epidural if the patient requests it. E.D. asks for, and is given, an epidural 2 hours later, when her cervix is dilated 5 cm.
The next morning, a different physician examines her and reports a rim of cervix remaining, with the fetal head at 0 to +1 station. He asks E.D. to push, and the rim is reduced over the infant’s head. The patient is instructed to continue pushing with contractions. The physician writes the admission (and only predelivery) note: “32 yr old G4P3, term, SROM, good FHTs, good progress, complete, 1+ station, clear fluid. Anticipate vaginal delivery.”
When progress stalls, mother tires
E.D. pushes well with adequate contractions for 2.5 hours, with minimal descent of the head and increasing caput and molding. The physician examines her again and reports that the baby is at +2 station. He also suggests the use of the vacuum extractor, because the patient is becoming exhausted and the baby is “quite big.” The obstetrician appears somewhat hesitant when applying the vacuum and remarks to the nurse that he “thinks the baby is in a left occiput anterior position” but is not “100% sure.”
When vacuum fails, a switch to forceps
After 2 attempts with the vacuum extractor, during which there are 2 “pop-offs,” the physician asks for Simpson forceps, adding that he thinks the baby is now in right occiput posterior position and he needs to “get a better grip on the baby’s head.” The forceps are applied with some difficulty, necessitating 2 reapplications.
After 5 contractions (and 6 pulling efforts), a baby boy is delivered. Because of a delay in delivery of the shoulders after delivery of the head, the physician places the patient in McRoberts position and has a nurse apply suprapubic pressure, and no further difficulties are encountered.
Large baby has brachial plexus injury
The infant weighs 4,200 g and has Apgar scores of 3 and 8, as well as a small laceration on his forehead, moderate flaccidity of the left arm, and an elongated head. The mother has a 4th-degree laceration that is repaired with some difficulty.
The delivery note reads: “Assisted vaginal delivery, 4,200 g male, 3 vessel cord, 600 cc estimated blood loss, 4th-degree laceration repaired in layers.” E.D. ultimately requires 2 U of blood on postpartum day 2 for symptomatic anemia.
Mother and baby are discharged on postpartum day 4 in stable condition. The infant has a brachial plexus injury that resolves within 6 weeks.
Lessons learned
Among the mistakes the obstetrician made in this case are a failure to take the obstetric history into account, omission of a comprehensive abdominal exam, ignoring signs of a large baby, and lack of a plan for emergent cesarean section.
4. Keep molding in mind
Some (up to +2) occipito-parietal molding may be normal in the late stages of delivery (ie, the occipital bone slips under the 2 parietal bones, but can be easily reduced), but severe parieto-parieto molding is never normal and should be interpreted as a sign of relative or absolute cephalopelvic disproportion. FIGURE 1 shows a classification system for molding.
FIGURE 1 How to characterize the degree of molding
Excessive molding may lead to tears in dura and underlying vessels.
Traction plus severe molding may increase the risk of intracranial injury
The most frequent causes of molding are asynclitism and deflexion of the head, commonly seen in occiput posterior and transverse positions. Correction of the asynclitism and malposition may correct the molding and allow safe vaginal delivery. Traction on a head with severe molding may increase the risk of intracranial injury.
Using maximum likelihood logistic regression analyses, Knight and colleagues6 demonstrated that the factor of greatest importance in determining whether a case would be allocated to engaged versus unengaged groups was molding (odds ratio 2.17; 95% confidence intervals 0.75–6.27). The authors concluded that when abdominal and vaginal assessments produce different findings, the major factor responsible is molding. They noted that data derived from vaginal examination alone may be misleading when molding is present.
The rule of 3’s
With the fetus in an occiput anterior position, determine the number of fifths of the fetal head that can be palpated above the pelvic brim abdominally, add it to the degree of molding palpated vaginally, and avoid operative vaginal delivery if the sum is 3 or higher (FIGURE 2).7
FIGURE 2 Abdominopelvic assessment using the rule of 3’s
If the sum of the number of fifths of the fetal head palpated above the pelvic brim abdominally and the degree of molding palpated vaginally equals or exceeds 3, operative vaginal delivery is unlikely to be successful.For example, if two fifths (~4 cm) of the fetal head is palpated above the maternal pubic symphysis, and there is already +1 of parieto-parieto molding, significant cephalopelvic disproportion is likely and operative vaginal delivery will probably fail, with an increased risk of fetal and maternal damage. Obviously, if three fifths or more of the fetal head is palpated abdominally, the head is not engaged and operative vaginal delivery is contraindicated, regardless of whether the scalp is felt at or below the ischial spines.
Knowledge of fetal head diameters is useful
Using measurements of fetal head diameters (FIGURE 3), it is easy to see why a vertex presentation in an occiput anterior position (presenting diameter=suboccipitobregmatic diameter=9.5 cm) will deliver more easily than a baby in a deflexed occiput posterior position (presenting diameter=occipitofrontal diameter ≥ 11.5 cm). The presenting diameter of a brow presentation will never negotiate a normally proportioned female pelvis, whereas that of a mentum anterior face presentation is clearly adequate for a vaginal delivery if all other factors are favorable.
FIGURE 3 Know the basic term fetal head diameters
Depending on the presentation, the fetal head will deliver easily, as in occiput anterior position, when the presenting diameter is 9.5 cm, or with difficulty, as when the presenting diameter is 11.5 cm or more.
5. Be aware of fetal head position throughout labor
Early documentation of fetal head position during labor may help tremendously when decisions regarding the mode of delivery have to be made in a hurry. If one is aware that the head has been persistently in a deflexed occiput posterior position (or a transverse position) throughout the labor, a prolongation or arrest of descent can be explained by progressive deflexion of the head and increasing presenting diameters (or deep transverse arrest, as the case may be).
In such a case, if sudden fetal decompensation necessitates emergent delivery, operative vaginal delivery is a much less viable option than it would be with a well-flexed occiput anterior position at the same station.
When there is knowledge of a fetal malposition, cesarean section may be the wisest choice in an emergency, even if the fetal head is at an appropriate station, unless the operator has the requisite skills at operative vaginal delivery and is certain of a high chance of a successful outcome.
6. Have a valid indication
Operative vaginal delivery should not proceed without a valid indication8 that conforms to accepted guidelines.
Consider the pathology underlying the indication
For example, although maternal exhaustion is clearly a valid indication for operative vaginal delivery, it is important to examine the underlying reason for the exhaustion. A diabetic mother who is known to have a large-for-gestational-age infant, who has a prolonged active phase (8 hours) and is exhausted after 3 hours of excellent pushing with adequate contractions, may appear to have, on the surface, a valid indication—but clearly this situation calls for extreme caution. The size of the infant and the lack of progress suggest at the least the potential for cephalopelvic disproportion.
In my opinion, operative vaginal delivery for maternal exhaustion should probably be reserved for someone who has progressed at a normal rate to crowning and who simply does not have the energy to push out a normal-sized baby.
Judicious use is key
Operative vaginal delivery can be used judiciously to remedy situations that have the potential to escalate. For example, a persistent transverse fetal head position in a primigravida with a platypelloid pelvis who has pushed for 1 hour with increasing caput and molding is highly unlikely to resolve. If the fetus is an appropriate candidate for rotational forceps, and the physician has the requisite training and experience, a rotational delivery after only 1 hour is entirely appropriate to avoid potential maternal and fetal injury that could follow 3 hours of pushing.
Persistent variable decelerations are another indication of potential fetal compromise and justify judicious use of operative vaginal delivery in appropriate candidates.
7. Do not use instruments sequentially
The use of sequential operative vaginal delivery methods to complete a vaginal delivery is no longer acceptable. Significantly increased neonatal and maternal risks have been demonstrated in at least 3 well-designed studies. Data indicate that a failed operative vaginal delivery attempt,2,9,10 more than 3 hours of maternal pushing,10 and more than 3 traction episodes (regardless of ultimate success with the instrument)10 are associated with an increased risk of neonatal intracranial hemorrhage.
Because we lack a standard of care for the optimal number of traction efforts or “pop-offs” for operative vaginal delivery, I suggest that any practitioners be familiar with, and adhere to, the manufacturer’s suggested guidelines. These guidelines will usually be designed to fall on the conservative side of safety issues.
I have heard of physicians who sometimes use the vacuum extractor to bring the head down to a place where they feel more comfortable applying forceps. This practice is unacceptable. By the same token, proceeding with vacuum extraction after concluding there is too much molding or caput for forceps is untenable.
8. Have a clear endpoint and exit strategy
Resist the temptation to persist with operative vaginal delivery in the face of inadequate descent or progress. It may sometimes seem as though “just one more pull” will effect delivery, but exceeding the recommended number of attempts can lead to excessive traction and maternal or fetal damage. It can be easy to become fixated on achieving vaginal delivery, and rational thought can become clouded.
I recommend that each department establish clear and agreed-upon limits for their practitioners. To this end, there should be an appropriately cooperative atmosphere in each delivery unit that encourages the provider team to work together to prevent adverse outcomes from operative vaginal delivery. Protocols or checklists that help the nursing staff keep the physician informed of the number of traction efforts and/or pop-offs that occur will help prevent inadvertent exceeding of the limits established for that unit.
Prior to attempting an operative vaginal delivery, the obstetrician should have a clear exit strategy, and this strategy should be outlined to the patient and the nursing/ancillary staff. When the predetermined criteria are met, operative vaginal delivery should be abandoned without delay, and cesarean section should be performed expeditiously. Obviously, this requires that preparations for emergent cesarean section be made prior to use of the forceps or vacuum extractor.
The necessary anesthesia and neonatal and operating room personnel should be ready and in position at the time the operative vaginal delivery is attempted.
Never resume maternal pushing after failed forceps or vacuum
There is no place for “rest and descend” protocols or further attempts at spontaneous vaginal delivery after a failed operative vaginal delivery. Once an easy operative vaginal delivery becomes impossible, immediate cesarean section is the best option.
- A valid indication documented preoperatively
- Unambiguous knowledge of the fetal head position
- Complete dilatation of the cervix
- Confirmed engagement of the fetal head
- Station at or below +2, unless the operator is experienced and there is a justifiable reason for a midpelvic delivery
- Rule of 3’s satisfied
- A documented estimate of appropriate fetal weight and adequate maternal pelvic anatomy
- Adequate anesthesia
- Preparations in place for immediate cesarean section and resuscitation of the neonate, if needed
- An informed, willing, and cooperative patient who understands that cesarean section may be an appropriate alternative mode of delivery (depending on the circumstances)
In addition, the person intending to perform the delivery should personally examine the patient before the attempt to confirm that the prerequisites have been met. I would go so far as to state that, unless there is a high expectation of an easy operative vaginal delivery, it should not be attempted.
9. Document, document
Under ideal circumstances, the obstetrician initiates a discussion with the patient during prenatal care and mentions the possibility of vacuum extraction or forceps delivery. This discussion is documented in the prenatal chart. The note includes a statement discussing the relative risks and benefits of the alternative delivery modes, the patient’s expressed desire for a vaginal delivery, including operative vaginal delivery, and why, in the physician’s best judgment, an operative vaginal delivery is a reasonable option.
Document the events of labor and delivery
Clear, concise progress notes from nursing and obstetric care providers are extremely important. All pertinent maternal and fetal information should be addressed at each examination of the patient, and some comment on the rate of progress, threshold limits, management plan, and preparations should be included.
In my opinion, each progress note should describe maternal vital signs, adequacy of contractions, use of labor augmentation and the dose, fetal tolerance of contractions, reassuring nature of the monitoring, cervical dilation, fetal head position (if discernible), station, and any molding and caput. If maternal or fetal monitoring is inadequate with external devices, the notes should include details of the plan to improve the situation.
Include a preoperative note
I strongly recommend a preoperative note if there is time. It should clearly document the pertinent obstetric and prenatal care the patient has received, the progress of labor, the indication for operative vaginal delivery, estimated fetal weight, adequacy of the maternal pelvis for an infant of the anticipated weight, fetal head position, degree of molding, complete dilation of the cervix, station of the fetal head, and some assessment of flexion of the neck, if possible.
Once the decision to proceed has been made, I would add a statement indicating that the chances of success are high and, in your considered opinion, operative vaginal delivery is a safe and indicated option.
Write a detailed postoperative note
I suggest a dictated postoperative note for every operative vaginal delivery, successful or not. The elements included in the preoperative note should be reiterated and details of the delivery explained. The position and station of the fetal head at the time the instrument was applied (especially if this contrasts with what was stated in the preoperative note), the degree of caput and molding, the number and duration of traction efforts, progress of the fetal head with each traction effort, duration of the procedure, personnel present, and the preparations made for the delivery should all be documented. Physicians and nurses should agree on what constitutes a traction effort, to avoid conflicts in the various sets of notes.
Document postdelivery vaginal and rectal examinations, which should alert you to the presence of any retained sponges, vaginal hematomas or sulcus tears, or a previously unidentified rectovaginal fistula.
10. Handle bad outcomes with compassion
Do not avoid contact with the family in the event of a bad outcome. Rather, confront the outcome as honestly and compassionately as possible. If you correctly assessed and informed the patient and proceeded to operative vaginal delivery with her full understanding of the indication, she will have accepted a small risk of an untoward outcome. In general, if she perceives your behavior to have been professional and caring, she is much less likely to seek retribution.
The author reports no financial relationships relevant to this article.
Operative vaginal delivery is a dying art. National databases in the United States and elsewhere have shown this trend for decades.1 Women no longer can be reliably predicted to prefer operative vaginal delivery over cesarean section, and providers caring for delivering mothers (and their families) should not assume that they do. Nor does the 20th century paradigm of operative vaginal delivery as the accepted “next step” between spontaneous vaginal delivery and cesarean section hold up, given the decreased maternal and neonatal morbidity and mortality associated with modern techniques of cesarean section. Nevertheless, operative vaginal delivery remains a viable option in some cases.
This article—based on personal opinion and experience, as well as published data whenever possible—describes 10 selected aspects of operative vaginal delivery, offering recommendations for each.
1. Consider obstetric history
How a woman fared in previous deliveries has a bearing on the current delivery. For example, if she has a history of persistent occiput posterior position, as in the case described on page 56, she may have an anthropoid pelvis, placing her at increased risk for another malposition.1 In such cases, the patient should be counseled about the potential for operative vaginal delivery, and the risks and benefits should be discussed prenatally.
A history of obesity, excessive weight gain, and glucose intolerance should be considered warning signs of a large-for-gestational-age infant.
2. Ensure adequate informed consent
Patients should be informed of the risks of any procedure they are offered, and operative vaginal delivery—like any operative procedure—has definite risks.
It is unbalanced to mention only the perceived benefits of a procedure and to avoid the discomfort of discussing the potential significant fetal and maternal injury that may result from a procedure. It is far better for the patient and her family to learn—before an adverse outcome occurs—that forceps delivery sometimes leads to maternal and fetal lacerations, and that operative vaginal delivery can be associated with an increased risk for shoulder dystocia in some circumstances.
The best way to educate patients about operative vaginal delivery is during prenatal care. I recommend a written informed consent document similar to the one used for cesarean section. If such a form is not signed during the course of office prenatal care, it should be offered upon admission for delivery.
In some cases, operative vaginal delivery may be safer than cesarean
Operative vaginal delivery clearly increases the risk of neonatal intracranial bleeds when compared with normal spontaneous vaginal delivery or elective cesarean section.2 However, a patient should understand that cesarean section carries a risk of neonatal intracranial hemorrhage similar to that of operative vaginal delivery once a woman has labored to complete dilation and pushed for some time.2 In fact, a baby with a well-engaged head can experience significant increases in intracranial pressure during cesarean delivery when concerted efforts have to be used to deliver a deeply engaged fetal head out of a hysterotomy incision. Such maneuvering can also injure the fetal neck and brachial plexus.
3. The abdominal examination is critical
Examination of the maternal abdomen helps to confirm the fetal lie and presentation and may give an idea of the position of the fetal back in relation to the uterine midline. If the fetal back cannot be felt or is palpated far laterally, the fetus may be in an occiput posterior or transverse position. Often this knowledge helps the examiner make sense of an otherwise difficult vaginal examination.
Estimate fetal weight
Fetal weight estimations from a careful abdominal examination can be as accurate as ultrasonographic evaluation.3 It is strongly recommended that fetal weight be estimated and considered in context with maternal diabetes, obesity, excessive weight gain, and previous ultrasound examinations before operative vaginal delivery is undertaken.
Is the fetal head engaged?
The average term (3,200 g) fetus has a basovertical head diameter of approximately 9 to 10 cm,4,5 and the average adult finger has a diameter of 2 cm (one fifth of the head). Using this information, an estimate of how many “fifths” of the fetal head are above the pelvic brim can be made by evaluating how many fingerbreadths of fetal head can be palpated above the symphysis pubis on abdominal examination.
Crichton4 described this method in 1974, and it is an extremely useful and underutilized technique, in my opinion. He stated that no more than two fifths (2 fingerbreadths) of an unmolded fetal head should be palpated abdominally once the occiput is felt at the ischial spines. If three fifths or more of the fetal head is still palpable above the pubic symphysis, regardless of whether there is bone palpated at or below 0 station on vaginal examination, consider the head unengaged and avoid operative delivery.
It is quite possible to feel the fetal skull bone below the ischial spines and still have an unengaged head.5 This is due to molding of the head and elongation of the basovertical diameter. When this occurs, the widest diameter of the fetal skull remains above the plain of the pelvic brim (unengaged), even though the lowermost point is felt below the spines on vaginal examination. A graphic example of such an elongated basovertical diameter can be seen in the so-called cone-head baby.
At examination, fetal head should be in occiput anterior position
In order to best use the abdominal examination to assess the amount of fetal head above the pelvic brim, the fetal head must be in an occiput anterior position. This is because the occiput is sometimes difficult to palpate in a posterior or transverse position, and the obstetrician may incorrectly assume full engagement. This further underscores the importance of a careful maternal abdominal examination and the location of the fetal spine.
Abdominal examination is more informative than vaginal examination
Knight and colleagues6 studied the relative value of abdominal and vaginal examinations in the determination of fetal head engagement. They examined the records of 104 women who had been evaluated by both methods prior to attempted operative vaginal delivery. Successful vaginal delivery was correctly predicted using abdominal criteria (94%) more often than using vaginal criteria (80%) (P<.01).
E.D., a 32-year-old gravida 4 para 3, presents at 39 weeks’ gestation with spontaneous rupture of membranes in early labor. Her 3 deliveries thus far have all been vaginal, with the infants ranging in weight from 3,700 to 3,900 g. Two of these infants were delivered with vacuum extraction because of occiput posterior position and a prolonged second stage.
E.D.’s prenatal course has been relatively uncomplicated except for a 43-lb weight gain (she weighs 240 lb) and a borderline 1-hour glucose challenge test. She also had 1 abnormal value on a 3-hour glucose tolerance test. Her prenatal pelvic examination was documented as “adequate.”
In early stages, all appears normal
On admission, E.D. is dilated 4 cm with 70% effacement and a cephalic presentation at -2 station. Electronic fetal monitoring is reassuring, and she is contracting regularly every 6 minutes, with moderate pain. The physician on call instructs the nurse to start oxytocin if there is no progress in 2 hours, and to call anesthesia to give an epidural if the patient requests it. E.D. asks for, and is given, an epidural 2 hours later, when her cervix is dilated 5 cm.
The next morning, a different physician examines her and reports a rim of cervix remaining, with the fetal head at 0 to +1 station. He asks E.D. to push, and the rim is reduced over the infant’s head. The patient is instructed to continue pushing with contractions. The physician writes the admission (and only predelivery) note: “32 yr old G4P3, term, SROM, good FHTs, good progress, complete, 1+ station, clear fluid. Anticipate vaginal delivery.”
When progress stalls, mother tires
E.D. pushes well with adequate contractions for 2.5 hours, with minimal descent of the head and increasing caput and molding. The physician examines her again and reports that the baby is at +2 station. He also suggests the use of the vacuum extractor, because the patient is becoming exhausted and the baby is “quite big.” The obstetrician appears somewhat hesitant when applying the vacuum and remarks to the nurse that he “thinks the baby is in a left occiput anterior position” but is not “100% sure.”
When vacuum fails, a switch to forceps
After 2 attempts with the vacuum extractor, during which there are 2 “pop-offs,” the physician asks for Simpson forceps, adding that he thinks the baby is now in right occiput posterior position and he needs to “get a better grip on the baby’s head.” The forceps are applied with some difficulty, necessitating 2 reapplications.
After 5 contractions (and 6 pulling efforts), a baby boy is delivered. Because of a delay in delivery of the shoulders after delivery of the head, the physician places the patient in McRoberts position and has a nurse apply suprapubic pressure, and no further difficulties are encountered.
Large baby has brachial plexus injury
The infant weighs 4,200 g and has Apgar scores of 3 and 8, as well as a small laceration on his forehead, moderate flaccidity of the left arm, and an elongated head. The mother has a 4th-degree laceration that is repaired with some difficulty.
The delivery note reads: “Assisted vaginal delivery, 4,200 g male, 3 vessel cord, 600 cc estimated blood loss, 4th-degree laceration repaired in layers.” E.D. ultimately requires 2 U of blood on postpartum day 2 for symptomatic anemia.
Mother and baby are discharged on postpartum day 4 in stable condition. The infant has a brachial plexus injury that resolves within 6 weeks.
Lessons learned
Among the mistakes the obstetrician made in this case are a failure to take the obstetric history into account, omission of a comprehensive abdominal exam, ignoring signs of a large baby, and lack of a plan for emergent cesarean section.
4. Keep molding in mind
Some (up to +2) occipito-parietal molding may be normal in the late stages of delivery (ie, the occipital bone slips under the 2 parietal bones, but can be easily reduced), but severe parieto-parieto molding is never normal and should be interpreted as a sign of relative or absolute cephalopelvic disproportion. FIGURE 1 shows a classification system for molding.
FIGURE 1 How to characterize the degree of molding
Excessive molding may lead to tears in dura and underlying vessels.
Traction plus severe molding may increase the risk of intracranial injury
The most frequent causes of molding are asynclitism and deflexion of the head, commonly seen in occiput posterior and transverse positions. Correction of the asynclitism and malposition may correct the molding and allow safe vaginal delivery. Traction on a head with severe molding may increase the risk of intracranial injury.
Using maximum likelihood logistic regression analyses, Knight and colleagues6 demonstrated that the factor of greatest importance in determining whether a case would be allocated to engaged versus unengaged groups was molding (odds ratio 2.17; 95% confidence intervals 0.75–6.27). The authors concluded that when abdominal and vaginal assessments produce different findings, the major factor responsible is molding. They noted that data derived from vaginal examination alone may be misleading when molding is present.
The rule of 3’s
With the fetus in an occiput anterior position, determine the number of fifths of the fetal head that can be palpated above the pelvic brim abdominally, add it to the degree of molding palpated vaginally, and avoid operative vaginal delivery if the sum is 3 or higher (FIGURE 2).7
FIGURE 2 Abdominopelvic assessment using the rule of 3’s
If the sum of the number of fifths of the fetal head palpated above the pelvic brim abdominally and the degree of molding palpated vaginally equals or exceeds 3, operative vaginal delivery is unlikely to be successful.For example, if two fifths (~4 cm) of the fetal head is palpated above the maternal pubic symphysis, and there is already +1 of parieto-parieto molding, significant cephalopelvic disproportion is likely and operative vaginal delivery will probably fail, with an increased risk of fetal and maternal damage. Obviously, if three fifths or more of the fetal head is palpated abdominally, the head is not engaged and operative vaginal delivery is contraindicated, regardless of whether the scalp is felt at or below the ischial spines.
Knowledge of fetal head diameters is useful
Using measurements of fetal head diameters (FIGURE 3), it is easy to see why a vertex presentation in an occiput anterior position (presenting diameter=suboccipitobregmatic diameter=9.5 cm) will deliver more easily than a baby in a deflexed occiput posterior position (presenting diameter=occipitofrontal diameter ≥ 11.5 cm). The presenting diameter of a brow presentation will never negotiate a normally proportioned female pelvis, whereas that of a mentum anterior face presentation is clearly adequate for a vaginal delivery if all other factors are favorable.
FIGURE 3 Know the basic term fetal head diameters
Depending on the presentation, the fetal head will deliver easily, as in occiput anterior position, when the presenting diameter is 9.5 cm, or with difficulty, as when the presenting diameter is 11.5 cm or more.
5. Be aware of fetal head position throughout labor
Early documentation of fetal head position during labor may help tremendously when decisions regarding the mode of delivery have to be made in a hurry. If one is aware that the head has been persistently in a deflexed occiput posterior position (or a transverse position) throughout the labor, a prolongation or arrest of descent can be explained by progressive deflexion of the head and increasing presenting diameters (or deep transverse arrest, as the case may be).
In such a case, if sudden fetal decompensation necessitates emergent delivery, operative vaginal delivery is a much less viable option than it would be with a well-flexed occiput anterior position at the same station.
When there is knowledge of a fetal malposition, cesarean section may be the wisest choice in an emergency, even if the fetal head is at an appropriate station, unless the operator has the requisite skills at operative vaginal delivery and is certain of a high chance of a successful outcome.
6. Have a valid indication
Operative vaginal delivery should not proceed without a valid indication8 that conforms to accepted guidelines.
Consider the pathology underlying the indication
For example, although maternal exhaustion is clearly a valid indication for operative vaginal delivery, it is important to examine the underlying reason for the exhaustion. A diabetic mother who is known to have a large-for-gestational-age infant, who has a prolonged active phase (8 hours) and is exhausted after 3 hours of excellent pushing with adequate contractions, may appear to have, on the surface, a valid indication—but clearly this situation calls for extreme caution. The size of the infant and the lack of progress suggest at the least the potential for cephalopelvic disproportion.
In my opinion, operative vaginal delivery for maternal exhaustion should probably be reserved for someone who has progressed at a normal rate to crowning and who simply does not have the energy to push out a normal-sized baby.
Judicious use is key
Operative vaginal delivery can be used judiciously to remedy situations that have the potential to escalate. For example, a persistent transverse fetal head position in a primigravida with a platypelloid pelvis who has pushed for 1 hour with increasing caput and molding is highly unlikely to resolve. If the fetus is an appropriate candidate for rotational forceps, and the physician has the requisite training and experience, a rotational delivery after only 1 hour is entirely appropriate to avoid potential maternal and fetal injury that could follow 3 hours of pushing.
Persistent variable decelerations are another indication of potential fetal compromise and justify judicious use of operative vaginal delivery in appropriate candidates.
7. Do not use instruments sequentially
The use of sequential operative vaginal delivery methods to complete a vaginal delivery is no longer acceptable. Significantly increased neonatal and maternal risks have been demonstrated in at least 3 well-designed studies. Data indicate that a failed operative vaginal delivery attempt,2,9,10 more than 3 hours of maternal pushing,10 and more than 3 traction episodes (regardless of ultimate success with the instrument)10 are associated with an increased risk of neonatal intracranial hemorrhage.
Because we lack a standard of care for the optimal number of traction efforts or “pop-offs” for operative vaginal delivery, I suggest that any practitioners be familiar with, and adhere to, the manufacturer’s suggested guidelines. These guidelines will usually be designed to fall on the conservative side of safety issues.
I have heard of physicians who sometimes use the vacuum extractor to bring the head down to a place where they feel more comfortable applying forceps. This practice is unacceptable. By the same token, proceeding with vacuum extraction after concluding there is too much molding or caput for forceps is untenable.
8. Have a clear endpoint and exit strategy
Resist the temptation to persist with operative vaginal delivery in the face of inadequate descent or progress. It may sometimes seem as though “just one more pull” will effect delivery, but exceeding the recommended number of attempts can lead to excessive traction and maternal or fetal damage. It can be easy to become fixated on achieving vaginal delivery, and rational thought can become clouded.
I recommend that each department establish clear and agreed-upon limits for their practitioners. To this end, there should be an appropriately cooperative atmosphere in each delivery unit that encourages the provider team to work together to prevent adverse outcomes from operative vaginal delivery. Protocols or checklists that help the nursing staff keep the physician informed of the number of traction efforts and/or pop-offs that occur will help prevent inadvertent exceeding of the limits established for that unit.
Prior to attempting an operative vaginal delivery, the obstetrician should have a clear exit strategy, and this strategy should be outlined to the patient and the nursing/ancillary staff. When the predetermined criteria are met, operative vaginal delivery should be abandoned without delay, and cesarean section should be performed expeditiously. Obviously, this requires that preparations for emergent cesarean section be made prior to use of the forceps or vacuum extractor.
The necessary anesthesia and neonatal and operating room personnel should be ready and in position at the time the operative vaginal delivery is attempted.
Never resume maternal pushing after failed forceps or vacuum
There is no place for “rest and descend” protocols or further attempts at spontaneous vaginal delivery after a failed operative vaginal delivery. Once an easy operative vaginal delivery becomes impossible, immediate cesarean section is the best option.
- A valid indication documented preoperatively
- Unambiguous knowledge of the fetal head position
- Complete dilatation of the cervix
- Confirmed engagement of the fetal head
- Station at or below +2, unless the operator is experienced and there is a justifiable reason for a midpelvic delivery
- Rule of 3’s satisfied
- A documented estimate of appropriate fetal weight and adequate maternal pelvic anatomy
- Adequate anesthesia
- Preparations in place for immediate cesarean section and resuscitation of the neonate, if needed
- An informed, willing, and cooperative patient who understands that cesarean section may be an appropriate alternative mode of delivery (depending on the circumstances)
In addition, the person intending to perform the delivery should personally examine the patient before the attempt to confirm that the prerequisites have been met. I would go so far as to state that, unless there is a high expectation of an easy operative vaginal delivery, it should not be attempted.
9. Document, document
Under ideal circumstances, the obstetrician initiates a discussion with the patient during prenatal care and mentions the possibility of vacuum extraction or forceps delivery. This discussion is documented in the prenatal chart. The note includes a statement discussing the relative risks and benefits of the alternative delivery modes, the patient’s expressed desire for a vaginal delivery, including operative vaginal delivery, and why, in the physician’s best judgment, an operative vaginal delivery is a reasonable option.
Document the events of labor and delivery
Clear, concise progress notes from nursing and obstetric care providers are extremely important. All pertinent maternal and fetal information should be addressed at each examination of the patient, and some comment on the rate of progress, threshold limits, management plan, and preparations should be included.
In my opinion, each progress note should describe maternal vital signs, adequacy of contractions, use of labor augmentation and the dose, fetal tolerance of contractions, reassuring nature of the monitoring, cervical dilation, fetal head position (if discernible), station, and any molding and caput. If maternal or fetal monitoring is inadequate with external devices, the notes should include details of the plan to improve the situation.
Include a preoperative note
I strongly recommend a preoperative note if there is time. It should clearly document the pertinent obstetric and prenatal care the patient has received, the progress of labor, the indication for operative vaginal delivery, estimated fetal weight, adequacy of the maternal pelvis for an infant of the anticipated weight, fetal head position, degree of molding, complete dilation of the cervix, station of the fetal head, and some assessment of flexion of the neck, if possible.
Once the decision to proceed has been made, I would add a statement indicating that the chances of success are high and, in your considered opinion, operative vaginal delivery is a safe and indicated option.
Write a detailed postoperative note
I suggest a dictated postoperative note for every operative vaginal delivery, successful or not. The elements included in the preoperative note should be reiterated and details of the delivery explained. The position and station of the fetal head at the time the instrument was applied (especially if this contrasts with what was stated in the preoperative note), the degree of caput and molding, the number and duration of traction efforts, progress of the fetal head with each traction effort, duration of the procedure, personnel present, and the preparations made for the delivery should all be documented. Physicians and nurses should agree on what constitutes a traction effort, to avoid conflicts in the various sets of notes.
Document postdelivery vaginal and rectal examinations, which should alert you to the presence of any retained sponges, vaginal hematomas or sulcus tears, or a previously unidentified rectovaginal fistula.
10. Handle bad outcomes with compassion
Do not avoid contact with the family in the event of a bad outcome. Rather, confront the outcome as honestly and compassionately as possible. If you correctly assessed and informed the patient and proceeded to operative vaginal delivery with her full understanding of the indication, she will have accepted a small risk of an untoward outcome. In general, if she perceives your behavior to have been professional and caring, she is much less likely to seek retribution.
The author reports no financial relationships relevant to this article.
1. Gardberg M, Stenwall O, Laakkonen E. Recurrent persistent occipito-posterior position in subsequent deliveries. BJOG. 2004;111:170-171.
2. Towner D, Castro MA, Eby-Wilkens E, Golbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
3. Fetal macrosomia. American College of Obstetricians and Gynecologists Practice Bulletin #22. Washington, DC: ACOG; November 2000.
4. Crichton D. A reliable method of establishing the level of the fetal head in obstetrics. S Afr Med J. 1974;48:784-787.
5. Crichton D. The accuracy and value of cephalopelvimetry. J Obstet Gynaecol Br Emp. 1962;69:366-378.
6. Knight D, Newnham JP, McKenna M, Evans S. A comparison of abdominal and vaginal examinations for diagnosis of engagement of the fetal head. Aust N Z J Obstet Gynaecol. 1993;33:154-158.
7. Philpott RH. The recognition of cephalopelvic disproportion. Clin Obstet Gynecol. 1982;9:609-624.
8. Operative vaginal delivery. American College of Obstetricians and Gynecologists Practice Bulletin #17. Washington, DC: ACOG; June 2000.
9. Gardella C, Taylor M, Benedetti T, Hitti J, Critchlow C. The effect of sequential use of vacuum and forceps for assisted vaginal delivery on neonatal and maternal outcomes. Am J Obstet Gynecol. 2001;185:896-902.
10. Murphy DJ, Liebling RE, Patel R, Verity L, Swingler R. Cohort study of operative delivery in the second stage of labour and standard of obstetric care. BJOG. 2003;110:610-615.
1. Gardberg M, Stenwall O, Laakkonen E. Recurrent persistent occipito-posterior position in subsequent deliveries. BJOG. 2004;111:170-171.
2. Towner D, Castro MA, Eby-Wilkens E, Golbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
3. Fetal macrosomia. American College of Obstetricians and Gynecologists Practice Bulletin #22. Washington, DC: ACOG; November 2000.
4. Crichton D. A reliable method of establishing the level of the fetal head in obstetrics. S Afr Med J. 1974;48:784-787.
5. Crichton D. The accuracy and value of cephalopelvimetry. J Obstet Gynaecol Br Emp. 1962;69:366-378.
6. Knight D, Newnham JP, McKenna M, Evans S. A comparison of abdominal and vaginal examinations for diagnosis of engagement of the fetal head. Aust N Z J Obstet Gynaecol. 1993;33:154-158.
7. Philpott RH. The recognition of cephalopelvic disproportion. Clin Obstet Gynecol. 1982;9:609-624.
8. Operative vaginal delivery. American College of Obstetricians and Gynecologists Practice Bulletin #17. Washington, DC: ACOG; June 2000.
9. Gardella C, Taylor M, Benedetti T, Hitti J, Critchlow C. The effect of sequential use of vacuum and forceps for assisted vaginal delivery on neonatal and maternal outcomes. Am J Obstet Gynecol. 2001;185:896-902.
10. Murphy DJ, Liebling RE, Patel R, Verity L, Swingler R. Cohort study of operative delivery in the second stage of labour and standard of obstetric care. BJOG. 2003;110:610-615.
Sialorrhea in an Adult Taking Aripiprazole
Prenatal Counseling
Prenatal diagnosis became a reality almost 40 years ago, when advances in microscopy and cell culture made it possible to examine chromosomes in fetal cells drawn from amniotic fluid—the familiar karyotype analysis. Technical advances continue to sharpen the resolution of routine karyotype analysis on amniotic fluid or a specimen of chorionic villus, and to raise the level of detail obtained from such a study.
Yet examining chromosomes by light microscopy remains time- and labor-intensive. A cell culture typically requires 2 weeks before growth of cells is sufficient to undertake a karyotype analysis—after which the microscopic evaluation requires further time and significant skill to perform.
Change is coming to practice
Over the past 10 years, however, the human genome project has produced technologies that allow us to examine DNA at a level of resolution unattainable when chromosomes are evaluated under a light microscope. The exciting news is that these research technologies are being transferred to the clinical arena, where they will transform prenatal diagnosis and counseling in your practice.
One such technology that will have such a far-reaching effect, and that I focus on in this “Update,” is known as molecular karyotyping.
What is “molecular karyotyping”? How is it performed?
Refinements to hybridization technology yield new tools; a new term enters the lexicon of prenatal diagnosis
Vermeesch JR, Melotte C, Froyen G, et al. Molecular karyotyping: array CGH quality criteria for constitutional genetic diagnosis, J Histochem Cytochem. 2005;53:413–422.
Van den Veyver I, Beaudet A. Comparative genomic hybridization and prenatal diagnosis. Curr Opin Obstet Gynecol. 2006;18:185–191.
So-called molecular karyotyping utilizes the evolving technology of comparative genomic hybridization by microarray (or, simply, array CGH), which is a refinement of older CGH technology. Initial work with whole-genome hybridization involved applying fragmented and fluorescently labeled subject DNA to a normal metaphase chromosome spread. Deletions or duplications within the subject DNA were then made evident by reduced, or increased, fluorescence at complementary sites along the metaphase chromosomes. The resolution afforded by this approach was comparable to that of light microscopy—namely, alterations of at least 5 to 10 megabases (Mb) could be detected.
Array technology emerged in the late 1990s and increased the resolution of genome hybridization by at least 10-fold. How does it work?
FIGURE 1
Sample display of array CGH and corresponding FISH analysis
At left: Hybridization ratios of normal sex-matched control DNA (Cy5) to sample DNA (Cy3) are plotted as a function of Cy5/Cy3 signal intensity. (Note that ratios of deleted clones are greater than +3SD.)
At right: Fluorescence in situ hybridization (FISH) analysis demonstrates intact (arrows) and deleted (arrowheads) signals.
Bottom: Clones are summarized schematically.
Modified from Yamagata et al. Am J Med Genet. 2006;140A:205–211.
FIGURE 2
Array CGH reveals a duplicated chromosome 15q
At left: Analysis by array CGH demonstrates trisomy 16 and duplication of the Prader-Willi/Angelman syndrome region on chromosome 15q in this patient. Each clone is spotted in triplicate on the array; clones with a gain in the specimen are represented in green; those with a loss, in red; and those with a normal copy number, in gray. Green boxes mark chromosome 16 clones that demonstrate trisomy. White boxes highlight clones from the Prader-Willi/Angelman syndrome region that are duplicated; corresponding ratios are shown next to each target. Other red and green signals correspond to clones from, respectively, the X and Y chromosomes.
At right: Interphase FISH analysis confirms the interstitial duplication of chromosome 15q that was identified by array CGH. The small arrow in each cell points to the normal signal for the SNRPN (Prader-Willi) gene; the large arrow indicates duplicated chromosome 15q, which shows two hybridization signals for SNRPN.
Modified from Schaffer. Am J Human Genet. 2004;74:1168.
Array CGH is still new but already being improved
The 1st generation of array CGH slides covered the entire human genome with DNA fragments spaced approximately 1 Mb apart. Refinements have produced arrays of more than 30,000 overlapping DNA fragments. Such resolution allows detection of a gain or loss of segments as small as 100 to 200 kilobases (Kb). Compare this resolution with the best resolution of traditional microscopic cytogenetic analysis: approximately 5 Mb.
Into the clinical realm
Specialized “targeted” arrays can be applied to clinical work in several ways, including:
And consider what is anticipated: highly dense arrays that are capable of assessing single nucleotide alterations, making it possible to detect single-gene mutations.
Because array-CGH technology utilizes DNA and does not require cell culture, the time to results is significantly shorter. Furthermore, many aspects of the assessment are automated, providing both high resolution and rapid processing and reporting.
Array CGH uncovers genomic problems in the young
Causes of mental retardation, developmental deficits, congenital anomalies, and more are localized
Miyake N, Shimaokawa O, Harada N, et al. BAC array CGH genomic aberrations in idiopathic mental retardation. Am J Med Genet. 2006;140A:205–211.
Ming J, Geiger E, James A, et al. Rapid detection of submicroscopic chromosomal rearrangements in children with multiple congenital anomalies using high density oligonucleotide arrays. Hum Mutat. 2006;27:467–473.
Early use of array CGH in the study of solid tumors was followed closely by its clinical application to children with mental retardation or developmental deficits, with or without birth defects. Historically, suspicion of a duplication or deletion syndrome despite a normal chromosome analysis in these children could prompt specific testing for that disorder. More often, however, it was impossible to delineate a specific syndrome, and disorder-by-disorder testing was not feasible. Today, estimates are that submicroscopic duplications and deletions on chromosomes, detected primarily by array CGH, occur in 1 of every 1, 000 births.
Initial work in the pediatric population by Vissers, in 2003, and Shaw-Smith, in 2004, showed that, with array CGH at a resolution of 1 Mb, 14% to 20% of children who were mentally retarded had duplications or deletions that could not be detected by routine karyotype analysis. Further detail on this approach, using an array with 1.4-Mb coverage, appears in the article by Miyake and co-workers. Among 30 children with idiopathic mental retardation and dysmorphic features, 17% (5 of 30) had submicroscopic deletions or duplications by array CGH. The imbalances ranged from 0.7 Mb to 1.0 Mb and spanned numerous and various chromosomes. The investigators emphasized the need to:
Numerous “copy number polymorphisms” have been uncovered—do they always matter?
Work with array CGH among the pediatric population was expanded by Ming and colleagues, who obtained greater resolution and coverage of the genome by utilizing a 2nd-generation array of oligonucleotides with >100,000 single-nucleotide polymorphisms. With this array, intermarker distance is estimated at 25 Kb—a resolution at which very small genomic imbalances can be identified. Of 10 children evaluated using this greater-density array, 2 (20%) had a previously unidentified genomic imbalance—both deletions.
Ming also put forward concerns that more non–disease-causing “copy number polymorphisms” (CNPs) will be uncovered as higher-density arrays increase the resolution of array CGH. These polymorphisms are encountered in healthy persons and are considered clinically insignificant. Consequently, when a copy number imbalance is detected by array, several actions are warranted: comparison with normal controls, evaluation of published CNP databases, and—most important—array CGH analysis of both parents’ DNA.
Such an approach adds to the labor-intensity of array CGH, but is necessary to ensure that imbalances that are clinically relevant and causative are distinguished from normal variants. With more than 250 discrete CNPs reported in normal controls, the use of denser arrays will uncover more CNPs than arrays targeted to significant fetal and pediatric disorders. Applying array CGH to clinical practice will entail (1) ongoing assessment of the technology and the results it provides and (2) perhaps, targeting of arrays to particular populations—the goal being to balance the yield of useful information against the increase in reported CNPs.
Where is the potential of array CGH in prenatal diagnosis?
Le Caignec C, Boceno M, Saugier-Veber P, et al. Detection of genomic imbalances by array based comparative genomic hybridization in fetuses with multiple malformations. J Med Genet. 2005;42:121–128.
Rickman L, Fiegler H, Shaw-Smith C, et al. Prenatal detection of unbalanced chromosomal rearrangements by array CGH. J Med Genet. 2006;43:353–361.
Sahoo T, Cheung S, Ward P, et al. Prenatal diagnosis of chromosomal abnormalities using array-based comparative genomic hybridization. Genet Med. 2006;8:719–727.
Prenatal diagnosis can be enhanced by array CGH
If ongoing research on array CGH can accomplish any of the following goals, it is likely that the technology will be propelled into clinical use as part of prenatal counseling within the next 5 years:
Le Caignec and colleagues’ work on applying array CGH to DNA specimens from fetuses that had multiple malformations—but in whom cytogenetic study was normal—have provided a foundation for subsequent prenatal studies. Using an array that targeted subtelomeres and specific DNA loci that are important in cytogenetic deletion–duplication syndromes, Le Caignec found that 5 of 49 (10.2%) fetuses studied had clinically significant genomic imbalances. These included:
The fetuses studied by Le Caignec had at least three malformations—variously in the cardiovascular, urogenital, skeletal, digestive, and central nervous systems. But when the list of identified anomalies was assessed, most of those fetuses, if examined by high-resolution ultrasonography, would have had anomalies identified in only 2 systems; the 3rd involved system would have been detectable only on fetopsy.
Rickman and colleagues used a custom array that focused on prenatal and pediatric abnormalities to examine the sensitivity and specificity of array CGH for detecting common aneuploidies in amniotic fluid specimens. All but 1 of the 30 subjects’ unbalanced chromosome rearrangements could be detected by array CGH—in some cases, from a specimen of amniotic fluid as small as 1 cc.
In Rickman’s hands, as well as in the hands of others, triploidy could not be detected, however—a problem that has been addressed in newer array platforms. In an additional 30 cases, no false positives were noted.
Similar results were obtained by Sahoo and co-workers: In 98 prenatal specimens (obtained by CVS or amniocentesis), there was complete concordance between the results of karyotype analysis and array CGH studies. In most cases, specimens were obtained because of advanced maternal age; only 19% represented concern over a sonographic abnormality. This study population included 4 cases of trisomy 21 and 1 case of an unbalanced translocation.
Notably, among the 98 specimens, 30 were thought to be characterized by gain or loss of copy number of 1 or more clones. Because these copy number repeats are recognized as normal variants (based on analyses of normal populations), they were considered copy number polymorphisms (CNPs) and without clinical significance to the fetus.
In addition, 12 cases contained a copy number imbalance that had not been recognized among normal controls. In 9 of those cases, the same loss or gain was demonstrated in 1 parent. In 1 other case, the parents elected not to be studied and, in the 2 others, the array finding was not confirmed on further testing (although low-level mosaicism could not be excluded). Sahoo’s team emphasizes both the targeted specificity of their custom array for well-characterized disorders, the reference to normal population databases being constructed for CNPs, the use of at least 3 clones for each disease locus, and the necessity for parental specimens to appropriately counsel the family about the presence of CNPs.
The work of Rickman and Sahoo reveals the potential for applying array CGH to a small volume of amniotic fluid or a specimen from direct CVS—a process that begins with whole-genome amplification. As this approach is refined to decrease the sample size and shorten the time to results even more, we can expect to see array CGH applied to areas where analysis has been constrained by the fact of small specimen size—such as preimplantation genetic screening.
Analysis of fetal loss will mean better counseling about recurrence
Fritz B, Hallerman C, Olert J, et al. Cytogenetic analyses of culture failures by comparative genomic hybridization (CGH)—re-evaluation of chromosome aberration rates in early spontaneous abortions. Eur J Hum Genet. 2001;9:539–547.
Schaeffer A, Chung J, Heretis K, et al. Comparative genomic hybridization-array analysis enhances the detection of aneuploidy and submicroscopic imbalances in spontaneous miscarriages. Am J Hum Genet. 2004;74:1168–1174.
Approximately 50% of 1st-trimester pregnancy losses are considered to be the results of chromosomal abnormalities. Often, however, it isn’t productive to analyze the products of conception because fetal cells fail to grow in culture or are overgrown by maternal cells. And, although chromosomal abnormalities play less of a role in 2nd-trimester fetal loss or in stillbirth, the rate of nondiagnostic results from classical cytogenetic study in such cases is high.
Sampling of the placenta or amniocentesis at the time fetal loss/stillbirth is recognized can lower the no-growth rate, but these methods have not been incorporated into practice universally. With array CGH, however, results can be obtained from uncultured cells, and that capability offers the opportunity to assess a demised fetus for common aneuplodies.
Array CGH will also provide an assessment of genomic imbalances that aren’t otherwise detectable at the resolution of metaphase chromosomes. Identification of a genomic imbalance—during a 1st- or 2nd-trimester loss—would facilitate an appropriate workup and lead to more accurate counseling about the risk of recurrence.
Assessment of nondividing cells reveals an unexpectedly high rate of chromosomal abnormality
Fritz and co-workers used array CGH to assess 60 cases of 1st-trimester spontaneous loss in which culture did not yield a karyotype result. Utilizing the older methodology of genomic CGH (ie, resolution is comparable to that of karyotype analysis), 72% of fetuses were found to have an underlying chromosome abnormality.
The work of this team supports what is increasingly reported:
These data warrant expanding array CGH to the evaluation of loss in 2nd and 3rd trimesters.
Schaffer and colleagues assessed a population of 41 products of conception using conventional cytogenetic analysis and array CGH. The conventional karyotype study and the array CGH were concordant in 37 of 41 cases—with 100% concordance for normal karyotypes, 10 cases of trisomy, 2 cases of sex chromosome aneuploidy, and 2 cases of deletion. More important, 4 cases (9.8%) that had been interpreted as normal on a conventional karyotype study were found by array CGH to have submicroscopic genomic imbalances, including trisomic mosaicism, interstitial deletion, and subtelomeric deletion.
The author reports no financial relationships relevant to this article.
Prenatal diagnosis became a reality almost 40 years ago, when advances in microscopy and cell culture made it possible to examine chromosomes in fetal cells drawn from amniotic fluid—the familiar karyotype analysis. Technical advances continue to sharpen the resolution of routine karyotype analysis on amniotic fluid or a specimen of chorionic villus, and to raise the level of detail obtained from such a study.
Yet examining chromosomes by light microscopy remains time- and labor-intensive. A cell culture typically requires 2 weeks before growth of cells is sufficient to undertake a karyotype analysis—after which the microscopic evaluation requires further time and significant skill to perform.
Change is coming to practice
Over the past 10 years, however, the human genome project has produced technologies that allow us to examine DNA at a level of resolution unattainable when chromosomes are evaluated under a light microscope. The exciting news is that these research technologies are being transferred to the clinical arena, where they will transform prenatal diagnosis and counseling in your practice.
One such technology that will have such a far-reaching effect, and that I focus on in this “Update,” is known as molecular karyotyping.
What is “molecular karyotyping”? How is it performed?
Refinements to hybridization technology yield new tools; a new term enters the lexicon of prenatal diagnosis
Vermeesch JR, Melotte C, Froyen G, et al. Molecular karyotyping: array CGH quality criteria for constitutional genetic diagnosis, J Histochem Cytochem. 2005;53:413–422.
Van den Veyver I, Beaudet A. Comparative genomic hybridization and prenatal diagnosis. Curr Opin Obstet Gynecol. 2006;18:185–191.
So-called molecular karyotyping utilizes the evolving technology of comparative genomic hybridization by microarray (or, simply, array CGH), which is a refinement of older CGH technology. Initial work with whole-genome hybridization involved applying fragmented and fluorescently labeled subject DNA to a normal metaphase chromosome spread. Deletions or duplications within the subject DNA were then made evident by reduced, or increased, fluorescence at complementary sites along the metaphase chromosomes. The resolution afforded by this approach was comparable to that of light microscopy—namely, alterations of at least 5 to 10 megabases (Mb) could be detected.
Array technology emerged in the late 1990s and increased the resolution of genome hybridization by at least 10-fold. How does it work?
FIGURE 1
Sample display of array CGH and corresponding FISH analysis
At left: Hybridization ratios of normal sex-matched control DNA (Cy5) to sample DNA (Cy3) are plotted as a function of Cy5/Cy3 signal intensity. (Note that ratios of deleted clones are greater than +3SD.)
At right: Fluorescence in situ hybridization (FISH) analysis demonstrates intact (arrows) and deleted (arrowheads) signals.
Bottom: Clones are summarized schematically.
Modified from Yamagata et al. Am J Med Genet. 2006;140A:205–211.
FIGURE 2
Array CGH reveals a duplicated chromosome 15q
At left: Analysis by array CGH demonstrates trisomy 16 and duplication of the Prader-Willi/Angelman syndrome region on chromosome 15q in this patient. Each clone is spotted in triplicate on the array; clones with a gain in the specimen are represented in green; those with a loss, in red; and those with a normal copy number, in gray. Green boxes mark chromosome 16 clones that demonstrate trisomy. White boxes highlight clones from the Prader-Willi/Angelman syndrome region that are duplicated; corresponding ratios are shown next to each target. Other red and green signals correspond to clones from, respectively, the X and Y chromosomes.
At right: Interphase FISH analysis confirms the interstitial duplication of chromosome 15q that was identified by array CGH. The small arrow in each cell points to the normal signal for the SNRPN (Prader-Willi) gene; the large arrow indicates duplicated chromosome 15q, which shows two hybridization signals for SNRPN.
Modified from Schaffer. Am J Human Genet. 2004;74:1168.
Array CGH is still new but already being improved
The 1st generation of array CGH slides covered the entire human genome with DNA fragments spaced approximately 1 Mb apart. Refinements have produced arrays of more than 30,000 overlapping DNA fragments. Such resolution allows detection of a gain or loss of segments as small as 100 to 200 kilobases (Kb). Compare this resolution with the best resolution of traditional microscopic cytogenetic analysis: approximately 5 Mb.
Into the clinical realm
Specialized “targeted” arrays can be applied to clinical work in several ways, including:
And consider what is anticipated: highly dense arrays that are capable of assessing single nucleotide alterations, making it possible to detect single-gene mutations.
Because array-CGH technology utilizes DNA and does not require cell culture, the time to results is significantly shorter. Furthermore, many aspects of the assessment are automated, providing both high resolution and rapid processing and reporting.
Array CGH uncovers genomic problems in the young
Causes of mental retardation, developmental deficits, congenital anomalies, and more are localized
Miyake N, Shimaokawa O, Harada N, et al. BAC array CGH genomic aberrations in idiopathic mental retardation. Am J Med Genet. 2006;140A:205–211.
Ming J, Geiger E, James A, et al. Rapid detection of submicroscopic chromosomal rearrangements in children with multiple congenital anomalies using high density oligonucleotide arrays. Hum Mutat. 2006;27:467–473.
Early use of array CGH in the study of solid tumors was followed closely by its clinical application to children with mental retardation or developmental deficits, with or without birth defects. Historically, suspicion of a duplication or deletion syndrome despite a normal chromosome analysis in these children could prompt specific testing for that disorder. More often, however, it was impossible to delineate a specific syndrome, and disorder-by-disorder testing was not feasible. Today, estimates are that submicroscopic duplications and deletions on chromosomes, detected primarily by array CGH, occur in 1 of every 1, 000 births.
Initial work in the pediatric population by Vissers, in 2003, and Shaw-Smith, in 2004, showed that, with array CGH at a resolution of 1 Mb, 14% to 20% of children who were mentally retarded had duplications or deletions that could not be detected by routine karyotype analysis. Further detail on this approach, using an array with 1.4-Mb coverage, appears in the article by Miyake and co-workers. Among 30 children with idiopathic mental retardation and dysmorphic features, 17% (5 of 30) had submicroscopic deletions or duplications by array CGH. The imbalances ranged from 0.7 Mb to 1.0 Mb and spanned numerous and various chromosomes. The investigators emphasized the need to:
Numerous “copy number polymorphisms” have been uncovered—do they always matter?
Work with array CGH among the pediatric population was expanded by Ming and colleagues, who obtained greater resolution and coverage of the genome by utilizing a 2nd-generation array of oligonucleotides with >100,000 single-nucleotide polymorphisms. With this array, intermarker distance is estimated at 25 Kb—a resolution at which very small genomic imbalances can be identified. Of 10 children evaluated using this greater-density array, 2 (20%) had a previously unidentified genomic imbalance—both deletions.
Ming also put forward concerns that more non–disease-causing “copy number polymorphisms” (CNPs) will be uncovered as higher-density arrays increase the resolution of array CGH. These polymorphisms are encountered in healthy persons and are considered clinically insignificant. Consequently, when a copy number imbalance is detected by array, several actions are warranted: comparison with normal controls, evaluation of published CNP databases, and—most important—array CGH analysis of both parents’ DNA.
Such an approach adds to the labor-intensity of array CGH, but is necessary to ensure that imbalances that are clinically relevant and causative are distinguished from normal variants. With more than 250 discrete CNPs reported in normal controls, the use of denser arrays will uncover more CNPs than arrays targeted to significant fetal and pediatric disorders. Applying array CGH to clinical practice will entail (1) ongoing assessment of the technology and the results it provides and (2) perhaps, targeting of arrays to particular populations—the goal being to balance the yield of useful information against the increase in reported CNPs.
Where is the potential of array CGH in prenatal diagnosis?
Le Caignec C, Boceno M, Saugier-Veber P, et al. Detection of genomic imbalances by array based comparative genomic hybridization in fetuses with multiple malformations. J Med Genet. 2005;42:121–128.
Rickman L, Fiegler H, Shaw-Smith C, et al. Prenatal detection of unbalanced chromosomal rearrangements by array CGH. J Med Genet. 2006;43:353–361.
Sahoo T, Cheung S, Ward P, et al. Prenatal diagnosis of chromosomal abnormalities using array-based comparative genomic hybridization. Genet Med. 2006;8:719–727.
Prenatal diagnosis can be enhanced by array CGH
If ongoing research on array CGH can accomplish any of the following goals, it is likely that the technology will be propelled into clinical use as part of prenatal counseling within the next 5 years:
Le Caignec and colleagues’ work on applying array CGH to DNA specimens from fetuses that had multiple malformations—but in whom cytogenetic study was normal—have provided a foundation for subsequent prenatal studies. Using an array that targeted subtelomeres and specific DNA loci that are important in cytogenetic deletion–duplication syndromes, Le Caignec found that 5 of 49 (10.2%) fetuses studied had clinically significant genomic imbalances. These included:
The fetuses studied by Le Caignec had at least three malformations—variously in the cardiovascular, urogenital, skeletal, digestive, and central nervous systems. But when the list of identified anomalies was assessed, most of those fetuses, if examined by high-resolution ultrasonography, would have had anomalies identified in only 2 systems; the 3rd involved system would have been detectable only on fetopsy.
Rickman and colleagues used a custom array that focused on prenatal and pediatric abnormalities to examine the sensitivity and specificity of array CGH for detecting common aneuploidies in amniotic fluid specimens. All but 1 of the 30 subjects’ unbalanced chromosome rearrangements could be detected by array CGH—in some cases, from a specimen of amniotic fluid as small as 1 cc.
In Rickman’s hands, as well as in the hands of others, triploidy could not be detected, however—a problem that has been addressed in newer array platforms. In an additional 30 cases, no false positives were noted.
Similar results were obtained by Sahoo and co-workers: In 98 prenatal specimens (obtained by CVS or amniocentesis), there was complete concordance between the results of karyotype analysis and array CGH studies. In most cases, specimens were obtained because of advanced maternal age; only 19% represented concern over a sonographic abnormality. This study population included 4 cases of trisomy 21 and 1 case of an unbalanced translocation.
Notably, among the 98 specimens, 30 were thought to be characterized by gain or loss of copy number of 1 or more clones. Because these copy number repeats are recognized as normal variants (based on analyses of normal populations), they were considered copy number polymorphisms (CNPs) and without clinical significance to the fetus.
In addition, 12 cases contained a copy number imbalance that had not been recognized among normal controls. In 9 of those cases, the same loss or gain was demonstrated in 1 parent. In 1 other case, the parents elected not to be studied and, in the 2 others, the array finding was not confirmed on further testing (although low-level mosaicism could not be excluded). Sahoo’s team emphasizes both the targeted specificity of their custom array for well-characterized disorders, the reference to normal population databases being constructed for CNPs, the use of at least 3 clones for each disease locus, and the necessity for parental specimens to appropriately counsel the family about the presence of CNPs.
The work of Rickman and Sahoo reveals the potential for applying array CGH to a small volume of amniotic fluid or a specimen from direct CVS—a process that begins with whole-genome amplification. As this approach is refined to decrease the sample size and shorten the time to results even more, we can expect to see array CGH applied to areas where analysis has been constrained by the fact of small specimen size—such as preimplantation genetic screening.
Analysis of fetal loss will mean better counseling about recurrence
Fritz B, Hallerman C, Olert J, et al. Cytogenetic analyses of culture failures by comparative genomic hybridization (CGH)—re-evaluation of chromosome aberration rates in early spontaneous abortions. Eur J Hum Genet. 2001;9:539–547.
Schaeffer A, Chung J, Heretis K, et al. Comparative genomic hybridization-array analysis enhances the detection of aneuploidy and submicroscopic imbalances in spontaneous miscarriages. Am J Hum Genet. 2004;74:1168–1174.
Approximately 50% of 1st-trimester pregnancy losses are considered to be the results of chromosomal abnormalities. Often, however, it isn’t productive to analyze the products of conception because fetal cells fail to grow in culture or are overgrown by maternal cells. And, although chromosomal abnormalities play less of a role in 2nd-trimester fetal loss or in stillbirth, the rate of nondiagnostic results from classical cytogenetic study in such cases is high.
Sampling of the placenta or amniocentesis at the time fetal loss/stillbirth is recognized can lower the no-growth rate, but these methods have not been incorporated into practice universally. With array CGH, however, results can be obtained from uncultured cells, and that capability offers the opportunity to assess a demised fetus for common aneuplodies.
Array CGH will also provide an assessment of genomic imbalances that aren’t otherwise detectable at the resolution of metaphase chromosomes. Identification of a genomic imbalance—during a 1st- or 2nd-trimester loss—would facilitate an appropriate workup and lead to more accurate counseling about the risk of recurrence.
Assessment of nondividing cells reveals an unexpectedly high rate of chromosomal abnormality
Fritz and co-workers used array CGH to assess 60 cases of 1st-trimester spontaneous loss in which culture did not yield a karyotype result. Utilizing the older methodology of genomic CGH (ie, resolution is comparable to that of karyotype analysis), 72% of fetuses were found to have an underlying chromosome abnormality.
The work of this team supports what is increasingly reported:
These data warrant expanding array CGH to the evaluation of loss in 2nd and 3rd trimesters.
Schaffer and colleagues assessed a population of 41 products of conception using conventional cytogenetic analysis and array CGH. The conventional karyotype study and the array CGH were concordant in 37 of 41 cases—with 100% concordance for normal karyotypes, 10 cases of trisomy, 2 cases of sex chromosome aneuploidy, and 2 cases of deletion. More important, 4 cases (9.8%) that had been interpreted as normal on a conventional karyotype study were found by array CGH to have submicroscopic genomic imbalances, including trisomic mosaicism, interstitial deletion, and subtelomeric deletion.
The author reports no financial relationships relevant to this article.
Prenatal diagnosis became a reality almost 40 years ago, when advances in microscopy and cell culture made it possible to examine chromosomes in fetal cells drawn from amniotic fluid—the familiar karyotype analysis. Technical advances continue to sharpen the resolution of routine karyotype analysis on amniotic fluid or a specimen of chorionic villus, and to raise the level of detail obtained from such a study.
Yet examining chromosomes by light microscopy remains time- and labor-intensive. A cell culture typically requires 2 weeks before growth of cells is sufficient to undertake a karyotype analysis—after which the microscopic evaluation requires further time and significant skill to perform.
Change is coming to practice
Over the past 10 years, however, the human genome project has produced technologies that allow us to examine DNA at a level of resolution unattainable when chromosomes are evaluated under a light microscope. The exciting news is that these research technologies are being transferred to the clinical arena, where they will transform prenatal diagnosis and counseling in your practice.
One such technology that will have such a far-reaching effect, and that I focus on in this “Update,” is known as molecular karyotyping.
What is “molecular karyotyping”? How is it performed?
Refinements to hybridization technology yield new tools; a new term enters the lexicon of prenatal diagnosis
Vermeesch JR, Melotte C, Froyen G, et al. Molecular karyotyping: array CGH quality criteria for constitutional genetic diagnosis, J Histochem Cytochem. 2005;53:413–422.
Van den Veyver I, Beaudet A. Comparative genomic hybridization and prenatal diagnosis. Curr Opin Obstet Gynecol. 2006;18:185–191.
So-called molecular karyotyping utilizes the evolving technology of comparative genomic hybridization by microarray (or, simply, array CGH), which is a refinement of older CGH technology. Initial work with whole-genome hybridization involved applying fragmented and fluorescently labeled subject DNA to a normal metaphase chromosome spread. Deletions or duplications within the subject DNA were then made evident by reduced, or increased, fluorescence at complementary sites along the metaphase chromosomes. The resolution afforded by this approach was comparable to that of light microscopy—namely, alterations of at least 5 to 10 megabases (Mb) could be detected.
Array technology emerged in the late 1990s and increased the resolution of genome hybridization by at least 10-fold. How does it work?
FIGURE 1
Sample display of array CGH and corresponding FISH analysis
At left: Hybridization ratios of normal sex-matched control DNA (Cy5) to sample DNA (Cy3) are plotted as a function of Cy5/Cy3 signal intensity. (Note that ratios of deleted clones are greater than +3SD.)
At right: Fluorescence in situ hybridization (FISH) analysis demonstrates intact (arrows) and deleted (arrowheads) signals.
Bottom: Clones are summarized schematically.
Modified from Yamagata et al. Am J Med Genet. 2006;140A:205–211.
FIGURE 2
Array CGH reveals a duplicated chromosome 15q
At left: Analysis by array CGH demonstrates trisomy 16 and duplication of the Prader-Willi/Angelman syndrome region on chromosome 15q in this patient. Each clone is spotted in triplicate on the array; clones with a gain in the specimen are represented in green; those with a loss, in red; and those with a normal copy number, in gray. Green boxes mark chromosome 16 clones that demonstrate trisomy. White boxes highlight clones from the Prader-Willi/Angelman syndrome region that are duplicated; corresponding ratios are shown next to each target. Other red and green signals correspond to clones from, respectively, the X and Y chromosomes.
At right: Interphase FISH analysis confirms the interstitial duplication of chromosome 15q that was identified by array CGH. The small arrow in each cell points to the normal signal for the SNRPN (Prader-Willi) gene; the large arrow indicates duplicated chromosome 15q, which shows two hybridization signals for SNRPN.
Modified from Schaffer. Am J Human Genet. 2004;74:1168.
Array CGH is still new but already being improved
The 1st generation of array CGH slides covered the entire human genome with DNA fragments spaced approximately 1 Mb apart. Refinements have produced arrays of more than 30,000 overlapping DNA fragments. Such resolution allows detection of a gain or loss of segments as small as 100 to 200 kilobases (Kb). Compare this resolution with the best resolution of traditional microscopic cytogenetic analysis: approximately 5 Mb.
Into the clinical realm
Specialized “targeted” arrays can be applied to clinical work in several ways, including:
And consider what is anticipated: highly dense arrays that are capable of assessing single nucleotide alterations, making it possible to detect single-gene mutations.
Because array-CGH technology utilizes DNA and does not require cell culture, the time to results is significantly shorter. Furthermore, many aspects of the assessment are automated, providing both high resolution and rapid processing and reporting.
Array CGH uncovers genomic problems in the young
Causes of mental retardation, developmental deficits, congenital anomalies, and more are localized
Miyake N, Shimaokawa O, Harada N, et al. BAC array CGH genomic aberrations in idiopathic mental retardation. Am J Med Genet. 2006;140A:205–211.
Ming J, Geiger E, James A, et al. Rapid detection of submicroscopic chromosomal rearrangements in children with multiple congenital anomalies using high density oligonucleotide arrays. Hum Mutat. 2006;27:467–473.
Early use of array CGH in the study of solid tumors was followed closely by its clinical application to children with mental retardation or developmental deficits, with or without birth defects. Historically, suspicion of a duplication or deletion syndrome despite a normal chromosome analysis in these children could prompt specific testing for that disorder. More often, however, it was impossible to delineate a specific syndrome, and disorder-by-disorder testing was not feasible. Today, estimates are that submicroscopic duplications and deletions on chromosomes, detected primarily by array CGH, occur in 1 of every 1, 000 births.
Initial work in the pediatric population by Vissers, in 2003, and Shaw-Smith, in 2004, showed that, with array CGH at a resolution of 1 Mb, 14% to 20% of children who were mentally retarded had duplications or deletions that could not be detected by routine karyotype analysis. Further detail on this approach, using an array with 1.4-Mb coverage, appears in the article by Miyake and co-workers. Among 30 children with idiopathic mental retardation and dysmorphic features, 17% (5 of 30) had submicroscopic deletions or duplications by array CGH. The imbalances ranged from 0.7 Mb to 1.0 Mb and spanned numerous and various chromosomes. The investigators emphasized the need to:
Numerous “copy number polymorphisms” have been uncovered—do they always matter?
Work with array CGH among the pediatric population was expanded by Ming and colleagues, who obtained greater resolution and coverage of the genome by utilizing a 2nd-generation array of oligonucleotides with >100,000 single-nucleotide polymorphisms. With this array, intermarker distance is estimated at 25 Kb—a resolution at which very small genomic imbalances can be identified. Of 10 children evaluated using this greater-density array, 2 (20%) had a previously unidentified genomic imbalance—both deletions.
Ming also put forward concerns that more non–disease-causing “copy number polymorphisms” (CNPs) will be uncovered as higher-density arrays increase the resolution of array CGH. These polymorphisms are encountered in healthy persons and are considered clinically insignificant. Consequently, when a copy number imbalance is detected by array, several actions are warranted: comparison with normal controls, evaluation of published CNP databases, and—most important—array CGH analysis of both parents’ DNA.
Such an approach adds to the labor-intensity of array CGH, but is necessary to ensure that imbalances that are clinically relevant and causative are distinguished from normal variants. With more than 250 discrete CNPs reported in normal controls, the use of denser arrays will uncover more CNPs than arrays targeted to significant fetal and pediatric disorders. Applying array CGH to clinical practice will entail (1) ongoing assessment of the technology and the results it provides and (2) perhaps, targeting of arrays to particular populations—the goal being to balance the yield of useful information against the increase in reported CNPs.
Where is the potential of array CGH in prenatal diagnosis?
Le Caignec C, Boceno M, Saugier-Veber P, et al. Detection of genomic imbalances by array based comparative genomic hybridization in fetuses with multiple malformations. J Med Genet. 2005;42:121–128.
Rickman L, Fiegler H, Shaw-Smith C, et al. Prenatal detection of unbalanced chromosomal rearrangements by array CGH. J Med Genet. 2006;43:353–361.
Sahoo T, Cheung S, Ward P, et al. Prenatal diagnosis of chromosomal abnormalities using array-based comparative genomic hybridization. Genet Med. 2006;8:719–727.
Prenatal diagnosis can be enhanced by array CGH
If ongoing research on array CGH can accomplish any of the following goals, it is likely that the technology will be propelled into clinical use as part of prenatal counseling within the next 5 years:
Le Caignec and colleagues’ work on applying array CGH to DNA specimens from fetuses that had multiple malformations—but in whom cytogenetic study was normal—have provided a foundation for subsequent prenatal studies. Using an array that targeted subtelomeres and specific DNA loci that are important in cytogenetic deletion–duplication syndromes, Le Caignec found that 5 of 49 (10.2%) fetuses studied had clinically significant genomic imbalances. These included:
The fetuses studied by Le Caignec had at least three malformations—variously in the cardiovascular, urogenital, skeletal, digestive, and central nervous systems. But when the list of identified anomalies was assessed, most of those fetuses, if examined by high-resolution ultrasonography, would have had anomalies identified in only 2 systems; the 3rd involved system would have been detectable only on fetopsy.
Rickman and colleagues used a custom array that focused on prenatal and pediatric abnormalities to examine the sensitivity and specificity of array CGH for detecting common aneuploidies in amniotic fluid specimens. All but 1 of the 30 subjects’ unbalanced chromosome rearrangements could be detected by array CGH—in some cases, from a specimen of amniotic fluid as small as 1 cc.
In Rickman’s hands, as well as in the hands of others, triploidy could not be detected, however—a problem that has been addressed in newer array platforms. In an additional 30 cases, no false positives were noted.
Similar results were obtained by Sahoo and co-workers: In 98 prenatal specimens (obtained by CVS or amniocentesis), there was complete concordance between the results of karyotype analysis and array CGH studies. In most cases, specimens were obtained because of advanced maternal age; only 19% represented concern over a sonographic abnormality. This study population included 4 cases of trisomy 21 and 1 case of an unbalanced translocation.
Notably, among the 98 specimens, 30 were thought to be characterized by gain or loss of copy number of 1 or more clones. Because these copy number repeats are recognized as normal variants (based on analyses of normal populations), they were considered copy number polymorphisms (CNPs) and without clinical significance to the fetus.
In addition, 12 cases contained a copy number imbalance that had not been recognized among normal controls. In 9 of those cases, the same loss or gain was demonstrated in 1 parent. In 1 other case, the parents elected not to be studied and, in the 2 others, the array finding was not confirmed on further testing (although low-level mosaicism could not be excluded). Sahoo’s team emphasizes both the targeted specificity of their custom array for well-characterized disorders, the reference to normal population databases being constructed for CNPs, the use of at least 3 clones for each disease locus, and the necessity for parental specimens to appropriately counsel the family about the presence of CNPs.
The work of Rickman and Sahoo reveals the potential for applying array CGH to a small volume of amniotic fluid or a specimen from direct CVS—a process that begins with whole-genome amplification. As this approach is refined to decrease the sample size and shorten the time to results even more, we can expect to see array CGH applied to areas where analysis has been constrained by the fact of small specimen size—such as preimplantation genetic screening.
Analysis of fetal loss will mean better counseling about recurrence
Fritz B, Hallerman C, Olert J, et al. Cytogenetic analyses of culture failures by comparative genomic hybridization (CGH)—re-evaluation of chromosome aberration rates in early spontaneous abortions. Eur J Hum Genet. 2001;9:539–547.
Schaeffer A, Chung J, Heretis K, et al. Comparative genomic hybridization-array analysis enhances the detection of aneuploidy and submicroscopic imbalances in spontaneous miscarriages. Am J Hum Genet. 2004;74:1168–1174.
Approximately 50% of 1st-trimester pregnancy losses are considered to be the results of chromosomal abnormalities. Often, however, it isn’t productive to analyze the products of conception because fetal cells fail to grow in culture or are overgrown by maternal cells. And, although chromosomal abnormalities play less of a role in 2nd-trimester fetal loss or in stillbirth, the rate of nondiagnostic results from classical cytogenetic study in such cases is high.
Sampling of the placenta or amniocentesis at the time fetal loss/stillbirth is recognized can lower the no-growth rate, but these methods have not been incorporated into practice universally. With array CGH, however, results can be obtained from uncultured cells, and that capability offers the opportunity to assess a demised fetus for common aneuplodies.
Array CGH will also provide an assessment of genomic imbalances that aren’t otherwise detectable at the resolution of metaphase chromosomes. Identification of a genomic imbalance—during a 1st- or 2nd-trimester loss—would facilitate an appropriate workup and lead to more accurate counseling about the risk of recurrence.
Assessment of nondividing cells reveals an unexpectedly high rate of chromosomal abnormality
Fritz and co-workers used array CGH to assess 60 cases of 1st-trimester spontaneous loss in which culture did not yield a karyotype result. Utilizing the older methodology of genomic CGH (ie, resolution is comparable to that of karyotype analysis), 72% of fetuses were found to have an underlying chromosome abnormality.
The work of this team supports what is increasingly reported:
These data warrant expanding array CGH to the evaluation of loss in 2nd and 3rd trimesters.
Schaffer and colleagues assessed a population of 41 products of conception using conventional cytogenetic analysis and array CGH. The conventional karyotype study and the array CGH were concordant in 37 of 41 cases—with 100% concordance for normal karyotypes, 10 cases of trisomy, 2 cases of sex chromosome aneuploidy, and 2 cases of deletion. More important, 4 cases (9.8%) that had been interpreted as normal on a conventional karyotype study were found by array CGH to have submicroscopic genomic imbalances, including trisomic mosaicism, interstitial deletion, and subtelomeric deletion.
The author reports no financial relationships relevant to this article.