User login
Vulvovaginal disorders: 4 challenging conditions
The symptoms are recited every day in gynecologists’ offices around the world: itching, irritation, burning, rawness, pain, dyspareunia. The challenge is tracing these general symptoms to a specific pathology, a task harder than one might expect, because vulvovaginal conditions often represent a complex mix of several problems. Candida and bacterial invasion frequently complicate genital dermatologic conditions. Atrophy and loss of the epithelial barrier worsen the problem. Over-the-counter (OTC) and prescription remedies can lead to contact dermatitis. Vulvodynia may be the ultimate outcome, possibly from central sensitization after chronic inflammation, which in turn can mislead the clinician into thinking appropriate therapy “doesn’t work.” And it is important to remember that any genital complaint has the potential to dampen a woman’s self-esteem and hamper sexual function.
This article covers the fine points of diagnosis and treatment of 4 common vulvovaginal problems:
- Candidiasis
- Contact dermatitis
- Lichen sclerosus
- Vestibulodynia
Could all 4 problems coexist in 1 patient? They frequently do. As always, a careful history and physical examination with appropriate use of yeast cultures make it possible to manage the complexity.
1. CandidiasisTelephone and self-diagnosis are a waste of time
In vulvovaginal candidiasis (VVC), symptoms can range from none to recurrent. VVC can complicate genital dermatologic conditions and interfere with the treatment of illnesses that call for steroids or antibiotics. Because the symptoms of VVC are nonspecific, diagnosis necessitates consideration of a long list of other potential causes, both infectious and noninfectious.
Candida albicans predominates in 85% to 90% of positive vaginal yeast cultures. Non-albicans species such as C glabrata, parapsilosis, krusei, lusitaniae, and tropicalis are more difficult to treat.
Not all episodes are the same
VVC is uncomplicated when it occurs sporadically or infrequently in a woman in good overall health and involves mild to moderate symptoms; albicans species are likely. VVC is complicated when it is severe or recurrent or occurs in a debilitated, unhealthy, or pregnant woman; non-albicans species often are involved. Proper classification is essential to successful treatment.1
Phone diagnosis is usually inaccurate
Although phone diagnosis is unreliable,2 it is still fairly common, and fewer offices use microscopy and vaginal pH to diagnose vaginal infections because of the tightened (though still simple) requirements of the Clinical Laboratory Improvement Amendment. (Clinicians who do wet mounts and KOH are required to pass a simple test each year to continue the practice.)
Women are poor self-diagnosticians when it comes to Candida infection; only one third of a group purchasing OTC antifungals had accurately identified their condition.3
Clinicians are not exempt from error, either. About 50% of the time, Candida is misdiagnosed,4 largely because of the assumption that the wet mount is more specific than it actually is (it is only 40% specific for Candida).
Ask about sex habits, douching, drugs, and diseases
Accurate diagnosis requires a careful history, focusing on risk factors for Candida: a new sexual partner; oral sex; douching; use of antibiotics, steroids, or exogenous estrogen; and uncontrolled diabetes.
Look for signs of vulvar and vaginal erythema, edema, and excoriation.
Classic “cottage cheese” discharge may not be present, and the amount has no correlation with symptom severity.
Vaginal pH of less than 4.5 excludes bacterial vaginosis, trichomoniasis, atrophic vaginitis, desquamative inflammatory vaginitis, and vaginal lichen planus.
Blastospores or pseudohyphae are diagnostic (on 10% KOH microscopy). If they are absent, a yeast culture is essential and will allow speciation. A vaginal culture is especially important in women with recurrent or refractory symptoms.
Always consider testing for sexually transmitted diseases.
Azole antifungals are usual treatment
For uncomplicated VVC, azole antifungals are best (TABLE 1). For complicated VVC, follow this therapy with maintenance fluconazole (150 mg weekly for 6 months), which clears Candida in 90.8% of cases.5
Non-albicans infection can be treated with boric acid capsules (inserted vaginally at bedtime for 14 days) or terconazole cream (7 days) or suppositories (3 days). A culture to confirm cure is essential, since non-albicans infection can be difficult to eradicate.
Note that boric acid is not approved for pregnancy.
TABLE 1
CDC guidelines for treatment of candidiasis
Any of these intravaginal or oral regimens may be used
| DOSE (NUMBER OF DAYS) | |
|---|---|
| INTRAVAGINAL AGENTS | |
| Butoconazole | |
| 2% cream* | 5 g (3) |
| Butoconazole-1 sustained-release cream | 5 g (1) |
| Clotrimazole | |
| 1% cream* | 5 g (7–14) |
| 100 mg | 1 tablet (7) |
| 2 tablets (3) | |
| 500 mg | 1 tablet (1) |
| Miconazole | |
| 2% cream* | 5 g (7) |
| 100 mg* | 1 suppository (7) |
| 200 mg* | 1 suppository (3) |
| Nystatin | |
| 100,000 U | 1 tablet (14) |
| Tioconazole | |
| 6.5% ointment* | 5 g (1) |
| Terconazole | |
| 0.4% cream | 5 g (7) |
| 0.8% cream | 5 g (3) |
| 80 mg | 1 suppository (3) |
| ORAL AGENT | |
| Fluconazole | |
| 150 mg | 1 tablet (1) |
| * Over-the-counter | |
2. Contact DermatitisNine essentials of treatment
Contact dermatitis, the most common form of vulvar dermatitis, is inflammation of the skin caused by an external agent that acts as an irritant or allergen. The skin reaction may escape notice because changes ranging from minor to extreme are often superimposed on complex preexisting conditions such as lichen simplex chronicus, lichen planus, and lichen sclerosus.6
Contact dermatitis occurs readily in the vulvar area because the skin of the vulva reacts more intensely to irritants than other skin, and its barrier function is easily weakened by moisture, friction, urine, and vaginal discharge. The 3 main types of irritant dermatitis are7:
- A potent irritant, which may produce the equivalent of a chemical burn.
- A weaker irritant, which may be applied repeatedly before inflammation manifests.
- Stinging and burning, which can occur without detectable skin change, due to chemical exposure.
Many products can cause dermatitis. Even typically harmless products can cause dermatitis if combined with lack of estrogen or use of pads, panty hose, or girdles.
No typical pattern
Patients complain of varying degrees of itching, burning, and irritation. Depending on the agent involved, onset may be sudden or gradual, and the woman may be aware or oblivious of the cause. New reactions to “old” practices or products are also possible.
Ask about personal hygiene, care during menses and after intercourse, and about soap, cleansers, and any product applied to the genital skin, as well as clothing types and exercise habits. Review prescription and OTC products, including topicals, and note which products or actions improve or aggravate symptoms. A history of allergy and atopy should heighten suspicion.
The physical exam may reveal erythema and edema; scaling is possible. Severe cases manifest as erosion, ulceration, or pigment changes. Secondary infection, if any, may involve pustules, crusting, and fissuring. The dermatitis may be localized, but often extends over the area of product spread to the mons, labiocrural folds, and anus. C albicans often complicates genital dermatologic conditions.
9-step treatment
- Stop the offending product and/or practices.
- Restore the skin barrier with sitz baths in plain lukewarm water for 5 to 10 minutes twice daily. Compresses or a handheld shower are alternatives.
- Provide moisture. After hydration, have the patient pat dry and apply a thin film of plain petrolatum.
- Replace local estrogen if necessary.
- Control any concomitant Candida with oral fluconazole 150 mg weekly, avoiding the potential irritation caused by topical antifungals.
- Treat itching and scratching with cool gel packs from the refrigerator, not the freezer (frozen packs can burn). Stop involuntary nighttime scratching with sedation: doxepin or hydroxyzine (10–75 mg at 6 PM).
- Use topical steroids for dermatitis:
- Moderate: Triamcinolone, 0.1% ointment twice daily.
- Severe: A super-potent steroid such as clobetasol, 0.05% ointment, twice daily for 1 to 3 weeks.
- Extreme: Burst and taper prednisone (0.5–1 mg/kg/day decreased over 14–21 days) or a single dose of intramuscular triamcinolone (1 mg/kg).
- Order patch testing to rule out or define allergens.
- Educate the patient about the many potential causes of dermatitis, to prevent recurrence.
CAUSTIC AGENTS
Bichloracetic acid
Trichloroacetic acid
5-Fluorouracil
Lye (in soap)
Phenol
Podophyllin
Sodium hypochlorite
Solvents
WEAK CUMULATIVE IRRITANTS
Alcohol
Deodorants
Diapers
Feces
Feminine spray
Pads
Perfume
Povidone iodine
Powders
Propylene glycol
Semen
Soap
Sweat
Urine
Vaginal secretions
Water
Wipes
PHYSICALLY ABRASIVE CONTACTANTS
Face cloths
Sponges
THERMAL IRRITANTS
Hot water bottles
Hair dryers
Source: Lynette Margesson,MD26
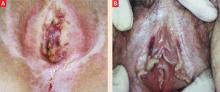
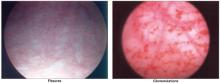
FIGURE 1 A mutilating disease of mysterious origin
Though lichen sclerosus is a disfiguring disease, the intensity of symptoms does not necessarily correlate with clinical appearance. Generally, the first change is (A) whitening of an irregular area on the labia, near the clitoris, on the perineum, and/or other vulvar areas. In some cases (A and B), inflammation can alter the anatomy of the vulva by flattening the labia minora, fusing the hood over the clitoris, effectively burying it beneath the skin, and shrinking the skin around the vaginal opening. Images courtesy Lynette Margesson, MD
3. Lichen SclerosusLifelong follow-up is a must
Although it has long been described in medical journals and textbooks, information on lichen sclerosus was often unreliable until recently, and adequate treatment guidelines were lacking. The cause still has not been fully elucidated, but a wealth of information now allows for considerable expertise in the management of this disease.
Lichen sclerosus is a chronic inflammatory and scarring disease that preferentially affects the anogenital area and is 6 to 10 times more prevalent in women than men.8 Any cutaneous site may also be affected, but the vagina is never involved.
Infection? Autoimmunity? An infectious cause has been proposed but never proven. In some women, an autoimmune component is recognized: Immunoglobulin G antibodies to extracellular matrix protein I have been found in 67% of patients with lichen sclerosus, but whether these antibodies are secondary or pathogenic is unclear.9 A genetic component is suggested by the association with autoimmunity and by the link with human leukocyte antigen DQ7 in women10 and girls.11
Affects 1.7%, or 1 in 60 women.12 In females, lichen sclerosus peaks in 2 populations: prepubertal girls and postmenopausal women.
No remission after age 70. Although remission of the disease has been reported, a recent study concluded that lichen sclerosus never remits after the age of 70; the average length of remission is 4.7 years, although this figure is still in question.13 Only close follow-up can determine if disease is in remission.
Main symptom is itching
Pruritus is the most common symptom, but dysuria and a sore or burning sensation have also been reported. Some women have no symptoms. When erosions, fissures, or introital narrowing are present, dyspareunia may also occur.
Typical lesions are porcelain-white papules and plaques, often with areas of fissuring or ecchymosis on the vulva or extending around the anus in a figure-of-8 pattern.
Both lichen sclerosus and lichen planus may be seen on the same vulva.
Squamous cell carcinoma can arise in anogenital lichen sclerosus; risk is thought to be 5%. Instruct women in regular self-examination because carcinoma can arise between annual or semiannual visits.
Ultrapotent steroids: Good control, but risk of malignancy persists
Renaud-Vilmer C, Cavalier-Balloy B, Porcher R, Dubertret L. Vulvar lichen sclerosus. Arch Dermatol. 2004;140:709-712.
If we gynecologists have been assuming that lichen sclerosus is one of those nebulous, little-explored diseases out there, we need to think again. Lichen sclerosus is a chronic and mutilating condition, an obstacle to quality of life, a threat to body image, a destroyer of sexual function, and a risk for malignancy.
Cancer developed only in untreated or irregularly treated lesions
In a key study, Renaud-Vilmer and colleagues13 explored remission and recurrence rates after treatment with 0.05% clobetasol propionate ointment, as well as whether the treatment reduces risk of malignant evolution. They determined that the rate of clinical and histologic remission is related to age. Although 72% of women under age 50 had complete remission, only 23% of women between 50 and 70 years of age had complete remission, and none of the women older than 70 did. Relapse was noted in most women over time (50% by 18 months), and 9.6% of women were later diagnosed with invasive squamous cell carcinoma.
Although we have known since 1988 that ultrapotent steroids offer outstanding relief of symptoms and some control over the disease, the optimal length of treatment has never been clear. The prospective study by Renaud-Vilmer et al has impressive power that derives from its 20-year duration. They demonstrated that ultrapotent steroids do not cure lichen sclerosus in women over 70. Complete remission in younger women is only temporary, and steroid therapy offers no significant reduction in the risk of vulvar cancer—although carcinoma developed only in untreated or irregularly treated lesions.
Histologic and clinical findings were used to judge efficacy. Because only 83 women were studied, the cohort is too small for the findings on carcinoma to be significant, but the authors emphasized that lifelong follow-up is necessary in all cases.
Lichen sclerosus never backs down after menopause
In more than 15 years of vulvovaginal specialization, I have found similar results. Older women detest the need to apply topicals to the genital area, but they are the ones who need ongoing use, because the disease never backs down after menopause.
I follow a cohort of young women in whom I detected early disease. Their clinical signs regressed and scarring was prevented by steroid treatment, but disease recurrence appears to be inevitable: The longest remission has been just over 4 years.
I have seen cancer arise quickly even in closely supervised patients, although many cases of squamous cell carcinoma have occurred in women with undetected or poorly treated disease.
Use tacrolimus with caution
Although small trials have produced some enthusiasm for therapeutic use of tacrolimus, treatment should proceed with extreme caution, as the drug inhibits an arm of the immune system and women with lichen sclerosus are at risk for malignancy. The agent now carries a warning based on the development of malignancy in animals.
Consider treatment even without biopsy proof
Although a biopsy generally makes the diagnosis, treatment should be considered even in the face of an inconclusive or negative finding if the clinician suspects that lichen sclerosus is present. The reason treatment should proceed in these cases: Loss of the labia or fusion over the clitoris can occur if the disease progresses, as shown in the photos on page.
Powerful corticosteroids are treatment of choice
Treatment can control lichen sclerosus, relieve symptoms, and prevent further anatomical changes. Potent or ultrapotent topical corticosteroids in an ointment base are preferred. These drugs are now widely recognized for their efficacy and minimal adverse effects, although no regimen is universally advocated.14 The patient applies ointment once daily for 1 to 3 months, depending on severity, and then once or twice a week.
Ointments are preferred over creams for vulvar treatment, because creams frequently contain allergens or irritants such as fragrance and propylene glycol preservative.
I continue once-weekly therapy indefinitely in postmenopausal women. If a premenopausal woman is not comfortable using the ointment indefinitely, I will allow her to discontinue treatment but follow her every 3 to 6 months.
Treatment also requires educating the patient about the disease, instructing her in gentle local care, and showing her exactly where to apply the ointment.
In all cases, lifelong follow-up is necessary. Hyperkeratosis, ecchymoses, fissuring, and erosions resolve, but atrophy and color change remain. Scarring usually remains unchanged, but may resolve if treated early in the course of the disease.15
Testosterone is not as effective as an ultrapotent steroid,16 and is no more effective than an emollient.17
Estrogen is valuable for skin integrity, but has no role in the treatment of lichen sclerosus.
Dilator work may be necessary for dyspareunia, once the disease is controlled.
Refer for help with depression and/or negative body image, if present.
4. VestibulodyniaEight treatment options to try
The prevalence of pain in an ethnically diverse population is 16%, and approximately half of this figure (8%) represents vulvodynia.18 The International Society for the Study of Vulvovaginal Disease now classifies vulvar pain in 3 categories:
- vulvar pain with a known cause, including infection, trauma, and systemic disease (TABLE 2),
- generalized vulvodynia, also known as dysesthetic vulvodynia, essential vulvodynia, or pudendal neuralgia, and
- localized vulvodynia or vestibulodynia, formerly called vestibulitis, vulvar vestibulitis syndrome, and vestibular adenitis.
Terminology is likely to evolve with further study.
Vestibulodynia, the leading cause of dyspareunia in women under age 50,19 refers to pain on touch within the vestibule. It is primary pain if it has been present since the first tampon use or sexual experience, and it is secondary if it arises after a period of comfortable sexual function.
TABLE 2
Rule out these known causes of vulvar pain
| INFECTIONS |
|
| TRAUMA |
|
| SYSTEMIC DISEASE |
|
| CANCER AND PRE-CANCER |
|
| IRRITANTS |
|
| SKIN CONDITIONS |
|
| Source: Haefner and Pearlman27 |
Inflammation starts the cascade
Current theory suggests that inflammatory events such as yeast infection, seminal plasma allergy, and local chemical application release a cascade of cytokines that sensitizes nociceptors in the vestibular epithelium. Prolonged neural firing in turn alters neurons in the dorsal horn, allowing sensitization of mechanoreceptive fibers in the vestibule with sensory allodynia (pain on touch). The proliferation of introital nociceptive fibers is well documented.20
Diagnosis: Report of pain and a positive Q-tip test
When a woman reports superficial dyspareunia with introital contact and clinical examination reveals pain on touch in the vestibule (using a cotton swab), vestibulodynia is diagnosed, provided no other known causes of the vulvar pain are detected during a careful history and examination or after pH measurement, a wet mount, and any indicated cultures.
A careful psychosexual history can help the clinician identify current sexual practices, prior sexual issues, and the impact of the current sexual dysfunction with an eye toward guiding support and counseling.
Multifactorial treatment: 8 options
Because the cause of vestibulodynia is unclear, a multifactorial approach generally is accepted and involves the following:
- Patient education about the problem and instruction in gentle local care and the elimination of contactants.
- Referral for support, counseling, and treatment of depression, as indicated.
- Elimination of any known trigger such as C albicans, although this generally does not lead to remission unless the pain is also treated.
- Suppression of nociceptor afferent input using topical lidocaine hydrochloride, for which good results have been reported.21
- Systemic oral analgesia with a tricyclic antidepressant modulates the serotonin and epinephrine imbalance associated with persistent pain (TABLE 3).22
- Use of anticonvulsants such as gabapentin to increase the amount of stimuli needed for nerves to fire and elevate the central pain threshold.23
- Reduction of muscle tension and spasm in the pelvic floor, using physical therapy and biofeedback.24
- When medical management fails, vulvar vestibulectomy with vaginal advancement yields excellent long-term results,25 but should be a last resort.
TABLE 3
Tricyclic antidepressants modify chemical imbalance associated with persistent pain of vestibulodynia
| Names of standard agents used | Amitriptyline (Elavil) |
| Nortriptyline (Aventyl, Pamelor) | |
| Desipramine (Norpramin) | |
| Imipramine (Tofranil) | |
| Standard dosing | Start with 10 mg; increase by 10 mg weekly to 100–150 mg |
| Side effects | Sedation, often transient |
| Constipation, must be actively managed | |
| Dry mouth | |
| Palpitations, tachycardia | |
| Sun sensitivity | |
| Cautions | Obtain EKG over 50 years or with cardiac history |
| Contraindicated in glaucoma | |
| Levels must be monitored if combined with SSRI | |
| Success | Slow change over 6–12 months in sensitivity to touch; ability to use tampon. Minimal pain with dilator use, penetration |
| Failure | Over 3 months at max dose without any improvement |
1. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Obstet Gynecol. 1998;178:203-211.
2. Spinollo A, Pizzoli G, Colonna L, Nicola S, DeSeta F, Guashino S. Epidemiologic characteristics of women with idiopathic recurrent vulvovaginal candidiasis. Obstet Gynecol. 1999;180:14-17.
3. Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol. 2002;99:419-425.
4. Ledger WJ, Polaneczky MM, Yih MC. Difficulties in the diagnosis of Candida vaginitis. Inf Dis Clin Pract. 2000;9:66-69.
5. Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;35:876-883.
6. Margesson LJ. Contact dermatitis of the vulva. Dermatol Ther. 2004;17:20-27.
7. Kamarashev JA, Vassileva SG. Dermatologic diseases of the vulva. Clin Dermatol. 1997;15:53-65.
8. Powell J, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
9. Oyama N, Chan I, Neill SM, et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet. 2003;362:118-213.
10. Marren P, Yell J, Charnock FM, Bunce M, Welsh K, Wojnarowska F. The association between lichen sclerosus and antigens of the HLA system. Br J Dermatol. 1995;132:197-203.
11. Powell J, Wojnarowska F, Winsey S, Marren P, Welsh K. Lichen sclerosus premenarche: autoimmunity and immunogenetics. Br J Dermatol. 2000;142:481-484.
12. Goldstein AT, Marinoff SC, Cristopher K, Srodon M. Prevalence of vulvar lichen sclerosus in a general gynecology practice. J Reprod Med. 2005;50:477-480.
13. Renaud-Vilmer C, Cavalier-Balloy B, Porcher R, Dubertret L. Vulvar lichen sclerosus. Arch Dermatol. 2004;140:709-712.
14. Dalziel KL, Millard PR, Wojnarowska F. The treatment of vulval lichen sclerosus with a very potent topical steroid (clobetasol propionate 0.05%) cream. Br J Dermatol. 1991;124:461-464.
15. Cooper SM, Gao XH, Powell JJ, Wojnarowska F. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004;140:702-706.
16. Bornstein J, Heifetz S, Kellner Y, et al. Clobetasol dipropionate 0.05% versus 2% testosterone application for severe vulvar lichen sclerosus. Am J Obstet Gynecol. 1998;178:80-84.
17. Sideri M, Origoni M, Spinaci L, Ferrari A. Topical testosterone in the treatment of vulvar lichen sclerosus. Int J Gynecol Obstet. 1994;46:53-56.
18. Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58:82-88.
19. Meana M, Binik YM, Khalife S, et al. Biopsychosocial profile of women with dyspareunia. Obstet Gynecol. 1997;90:583-589.
20. Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Neurochemical characterization of the vestibular nerves in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest. 1999;48:270-275.
21. Zolnoun DA, Hartmann KE, Steege JF. Overnight 5% lidocaine ointment for treatment of vulvar vestibulitis. Obstet Gynecol. 2003;102:84-87.
22. Mariani L. Vulvar vestibulitis syndrome: an overview of nonsurgical treatment. Eur J Obstet Gynecol. 2002;101:109-112.
23. Graziottin A, Vincenti E. Analgesic treatment of intractable pain due to vulvar vestibulitis syndrome: preliminary results with oral gabapentin and anesthetic block of ganglion impar. Poster presented at: International Meeting of the International Society for the Study of Women’s Sexual Health; 2002; Vancouver, Canada.
24. Glazer HI, Rodke G, Swencionis C, Hertz R, Young AW. Treatment of vulvar vestibulitis syndrome with electromyographic biofeedback of pelvic floor musculature. J Reprod Med. 1995;40:283-290.
25. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Gen Tract Dis. 2005;9:40-45.
26. Margesson LJ. Inflammatory disorders of the vulva. In: Fisher BK, Margesson LJ, eds. Genital Skin Disorders Diagnosis and Treatment. St Louis: Mosby; 1998:155-157.
27. Haefner HK, Pearlman MD. Diagnosing and managing vulvodynia. Contemp ObGyn. 1999;2:110.-
The author reports no financial relationships relevant to this article.
The symptoms are recited every day in gynecologists’ offices around the world: itching, irritation, burning, rawness, pain, dyspareunia. The challenge is tracing these general symptoms to a specific pathology, a task harder than one might expect, because vulvovaginal conditions often represent a complex mix of several problems. Candida and bacterial invasion frequently complicate genital dermatologic conditions. Atrophy and loss of the epithelial barrier worsen the problem. Over-the-counter (OTC) and prescription remedies can lead to contact dermatitis. Vulvodynia may be the ultimate outcome, possibly from central sensitization after chronic inflammation, which in turn can mislead the clinician into thinking appropriate therapy “doesn’t work.” And it is important to remember that any genital complaint has the potential to dampen a woman’s self-esteem and hamper sexual function.
This article covers the fine points of diagnosis and treatment of 4 common vulvovaginal problems:
- Candidiasis
- Contact dermatitis
- Lichen sclerosus
- Vestibulodynia
Could all 4 problems coexist in 1 patient? They frequently do. As always, a careful history and physical examination with appropriate use of yeast cultures make it possible to manage the complexity.
1. CandidiasisTelephone and self-diagnosis are a waste of time
In vulvovaginal candidiasis (VVC), symptoms can range from none to recurrent. VVC can complicate genital dermatologic conditions and interfere with the treatment of illnesses that call for steroids or antibiotics. Because the symptoms of VVC are nonspecific, diagnosis necessitates consideration of a long list of other potential causes, both infectious and noninfectious.
Candida albicans predominates in 85% to 90% of positive vaginal yeast cultures. Non-albicans species such as C glabrata, parapsilosis, krusei, lusitaniae, and tropicalis are more difficult to treat.
Not all episodes are the same
VVC is uncomplicated when it occurs sporadically or infrequently in a woman in good overall health and involves mild to moderate symptoms; albicans species are likely. VVC is complicated when it is severe or recurrent or occurs in a debilitated, unhealthy, or pregnant woman; non-albicans species often are involved. Proper classification is essential to successful treatment.1
Phone diagnosis is usually inaccurate
Although phone diagnosis is unreliable,2 it is still fairly common, and fewer offices use microscopy and vaginal pH to diagnose vaginal infections because of the tightened (though still simple) requirements of the Clinical Laboratory Improvement Amendment. (Clinicians who do wet mounts and KOH are required to pass a simple test each year to continue the practice.)
Women are poor self-diagnosticians when it comes to Candida infection; only one third of a group purchasing OTC antifungals had accurately identified their condition.3
Clinicians are not exempt from error, either. About 50% of the time, Candida is misdiagnosed,4 largely because of the assumption that the wet mount is more specific than it actually is (it is only 40% specific for Candida).
Ask about sex habits, douching, drugs, and diseases
Accurate diagnosis requires a careful history, focusing on risk factors for Candida: a new sexual partner; oral sex; douching; use of antibiotics, steroids, or exogenous estrogen; and uncontrolled diabetes.
Look for signs of vulvar and vaginal erythema, edema, and excoriation.
Classic “cottage cheese” discharge may not be present, and the amount has no correlation with symptom severity.
Vaginal pH of less than 4.5 excludes bacterial vaginosis, trichomoniasis, atrophic vaginitis, desquamative inflammatory vaginitis, and vaginal lichen planus.
Blastospores or pseudohyphae are diagnostic (on 10% KOH microscopy). If they are absent, a yeast culture is essential and will allow speciation. A vaginal culture is especially important in women with recurrent or refractory symptoms.
Always consider testing for sexually transmitted diseases.
Azole antifungals are usual treatment
For uncomplicated VVC, azole antifungals are best (TABLE 1). For complicated VVC, follow this therapy with maintenance fluconazole (150 mg weekly for 6 months), which clears Candida in 90.8% of cases.5
Non-albicans infection can be treated with boric acid capsules (inserted vaginally at bedtime for 14 days) or terconazole cream (7 days) or suppositories (3 days). A culture to confirm cure is essential, since non-albicans infection can be difficult to eradicate.
Note that boric acid is not approved for pregnancy.
TABLE 1
CDC guidelines for treatment of candidiasis
Any of these intravaginal or oral regimens may be used
| DOSE (NUMBER OF DAYS) | |
|---|---|
| INTRAVAGINAL AGENTS | |
| Butoconazole | |
| 2% cream* | 5 g (3) |
| Butoconazole-1 sustained-release cream | 5 g (1) |
| Clotrimazole | |
| 1% cream* | 5 g (7–14) |
| 100 mg | 1 tablet (7) |
| 2 tablets (3) | |
| 500 mg | 1 tablet (1) |
| Miconazole | |
| 2% cream* | 5 g (7) |
| 100 mg* | 1 suppository (7) |
| 200 mg* | 1 suppository (3) |
| Nystatin | |
| 100,000 U | 1 tablet (14) |
| Tioconazole | |
| 6.5% ointment* | 5 g (1) |
| Terconazole | |
| 0.4% cream | 5 g (7) |
| 0.8% cream | 5 g (3) |
| 80 mg | 1 suppository (3) |
| ORAL AGENT | |
| Fluconazole | |
| 150 mg | 1 tablet (1) |
| * Over-the-counter | |
2. Contact DermatitisNine essentials of treatment
Contact dermatitis, the most common form of vulvar dermatitis, is inflammation of the skin caused by an external agent that acts as an irritant or allergen. The skin reaction may escape notice because changes ranging from minor to extreme are often superimposed on complex preexisting conditions such as lichen simplex chronicus, lichen planus, and lichen sclerosus.6
Contact dermatitis occurs readily in the vulvar area because the skin of the vulva reacts more intensely to irritants than other skin, and its barrier function is easily weakened by moisture, friction, urine, and vaginal discharge. The 3 main types of irritant dermatitis are7:
- A potent irritant, which may produce the equivalent of a chemical burn.
- A weaker irritant, which may be applied repeatedly before inflammation manifests.
- Stinging and burning, which can occur without detectable skin change, due to chemical exposure.
Many products can cause dermatitis. Even typically harmless products can cause dermatitis if combined with lack of estrogen or use of pads, panty hose, or girdles.
No typical pattern
Patients complain of varying degrees of itching, burning, and irritation. Depending on the agent involved, onset may be sudden or gradual, and the woman may be aware or oblivious of the cause. New reactions to “old” practices or products are also possible.
Ask about personal hygiene, care during menses and after intercourse, and about soap, cleansers, and any product applied to the genital skin, as well as clothing types and exercise habits. Review prescription and OTC products, including topicals, and note which products or actions improve or aggravate symptoms. A history of allergy and atopy should heighten suspicion.
The physical exam may reveal erythema and edema; scaling is possible. Severe cases manifest as erosion, ulceration, or pigment changes. Secondary infection, if any, may involve pustules, crusting, and fissuring. The dermatitis may be localized, but often extends over the area of product spread to the mons, labiocrural folds, and anus. C albicans often complicates genital dermatologic conditions.
9-step treatment
- Stop the offending product and/or practices.
- Restore the skin barrier with sitz baths in plain lukewarm water for 5 to 10 minutes twice daily. Compresses or a handheld shower are alternatives.
- Provide moisture. After hydration, have the patient pat dry and apply a thin film of plain petrolatum.
- Replace local estrogen if necessary.
- Control any concomitant Candida with oral fluconazole 150 mg weekly, avoiding the potential irritation caused by topical antifungals.
- Treat itching and scratching with cool gel packs from the refrigerator, not the freezer (frozen packs can burn). Stop involuntary nighttime scratching with sedation: doxepin or hydroxyzine (10–75 mg at 6 PM).
- Use topical steroids for dermatitis:
- Moderate: Triamcinolone, 0.1% ointment twice daily.
- Severe: A super-potent steroid such as clobetasol, 0.05% ointment, twice daily for 1 to 3 weeks.
- Extreme: Burst and taper prednisone (0.5–1 mg/kg/day decreased over 14–21 days) or a single dose of intramuscular triamcinolone (1 mg/kg).
- Order patch testing to rule out or define allergens.
- Educate the patient about the many potential causes of dermatitis, to prevent recurrence.
CAUSTIC AGENTS
Bichloracetic acid
Trichloroacetic acid
5-Fluorouracil
Lye (in soap)
Phenol
Podophyllin
Sodium hypochlorite
Solvents
WEAK CUMULATIVE IRRITANTS
Alcohol
Deodorants
Diapers
Feces
Feminine spray
Pads
Perfume
Povidone iodine
Powders
Propylene glycol
Semen
Soap
Sweat
Urine
Vaginal secretions
Water
Wipes
PHYSICALLY ABRASIVE CONTACTANTS
Face cloths
Sponges
THERMAL IRRITANTS
Hot water bottles
Hair dryers
Source: Lynette Margesson,MD26
FIGURE 1 A mutilating disease of mysterious origin
Though lichen sclerosus is a disfiguring disease, the intensity of symptoms does not necessarily correlate with clinical appearance. Generally, the first change is (A) whitening of an irregular area on the labia, near the clitoris, on the perineum, and/or other vulvar areas. In some cases (A and B), inflammation can alter the anatomy of the vulva by flattening the labia minora, fusing the hood over the clitoris, effectively burying it beneath the skin, and shrinking the skin around the vaginal opening. Images courtesy Lynette Margesson, MD
3. Lichen SclerosusLifelong follow-up is a must
Although it has long been described in medical journals and textbooks, information on lichen sclerosus was often unreliable until recently, and adequate treatment guidelines were lacking. The cause still has not been fully elucidated, but a wealth of information now allows for considerable expertise in the management of this disease.
Lichen sclerosus is a chronic inflammatory and scarring disease that preferentially affects the anogenital area and is 6 to 10 times more prevalent in women than men.8 Any cutaneous site may also be affected, but the vagina is never involved.
Infection? Autoimmunity? An infectious cause has been proposed but never proven. In some women, an autoimmune component is recognized: Immunoglobulin G antibodies to extracellular matrix protein I have been found in 67% of patients with lichen sclerosus, but whether these antibodies are secondary or pathogenic is unclear.9 A genetic component is suggested by the association with autoimmunity and by the link with human leukocyte antigen DQ7 in women10 and girls.11
Affects 1.7%, or 1 in 60 women.12 In females, lichen sclerosus peaks in 2 populations: prepubertal girls and postmenopausal women.
No remission after age 70. Although remission of the disease has been reported, a recent study concluded that lichen sclerosus never remits after the age of 70; the average length of remission is 4.7 years, although this figure is still in question.13 Only close follow-up can determine if disease is in remission.
Main symptom is itching
Pruritus is the most common symptom, but dysuria and a sore or burning sensation have also been reported. Some women have no symptoms. When erosions, fissures, or introital narrowing are present, dyspareunia may also occur.
Typical lesions are porcelain-white papules and plaques, often with areas of fissuring or ecchymosis on the vulva or extending around the anus in a figure-of-8 pattern.
Both lichen sclerosus and lichen planus may be seen on the same vulva.
Squamous cell carcinoma can arise in anogenital lichen sclerosus; risk is thought to be 5%. Instruct women in regular self-examination because carcinoma can arise between annual or semiannual visits.
Ultrapotent steroids: Good control, but risk of malignancy persists
Renaud-Vilmer C, Cavalier-Balloy B, Porcher R, Dubertret L. Vulvar lichen sclerosus. Arch Dermatol. 2004;140:709-712.
If we gynecologists have been assuming that lichen sclerosus is one of those nebulous, little-explored diseases out there, we need to think again. Lichen sclerosus is a chronic and mutilating condition, an obstacle to quality of life, a threat to body image, a destroyer of sexual function, and a risk for malignancy.
Cancer developed only in untreated or irregularly treated lesions
In a key study, Renaud-Vilmer and colleagues13 explored remission and recurrence rates after treatment with 0.05% clobetasol propionate ointment, as well as whether the treatment reduces risk of malignant evolution. They determined that the rate of clinical and histologic remission is related to age. Although 72% of women under age 50 had complete remission, only 23% of women between 50 and 70 years of age had complete remission, and none of the women older than 70 did. Relapse was noted in most women over time (50% by 18 months), and 9.6% of women were later diagnosed with invasive squamous cell carcinoma.
Although we have known since 1988 that ultrapotent steroids offer outstanding relief of symptoms and some control over the disease, the optimal length of treatment has never been clear. The prospective study by Renaud-Vilmer et al has impressive power that derives from its 20-year duration. They demonstrated that ultrapotent steroids do not cure lichen sclerosus in women over 70. Complete remission in younger women is only temporary, and steroid therapy offers no significant reduction in the risk of vulvar cancer—although carcinoma developed only in untreated or irregularly treated lesions.
Histologic and clinical findings were used to judge efficacy. Because only 83 women were studied, the cohort is too small for the findings on carcinoma to be significant, but the authors emphasized that lifelong follow-up is necessary in all cases.
Lichen sclerosus never backs down after menopause
In more than 15 years of vulvovaginal specialization, I have found similar results. Older women detest the need to apply topicals to the genital area, but they are the ones who need ongoing use, because the disease never backs down after menopause.
I follow a cohort of young women in whom I detected early disease. Their clinical signs regressed and scarring was prevented by steroid treatment, but disease recurrence appears to be inevitable: The longest remission has been just over 4 years.
I have seen cancer arise quickly even in closely supervised patients, although many cases of squamous cell carcinoma have occurred in women with undetected or poorly treated disease.
Use tacrolimus with caution
Although small trials have produced some enthusiasm for therapeutic use of tacrolimus, treatment should proceed with extreme caution, as the drug inhibits an arm of the immune system and women with lichen sclerosus are at risk for malignancy. The agent now carries a warning based on the development of malignancy in animals.
Consider treatment even without biopsy proof
Although a biopsy generally makes the diagnosis, treatment should be considered even in the face of an inconclusive or negative finding if the clinician suspects that lichen sclerosus is present. The reason treatment should proceed in these cases: Loss of the labia or fusion over the clitoris can occur if the disease progresses, as shown in the photos on page.
Powerful corticosteroids are treatment of choice
Treatment can control lichen sclerosus, relieve symptoms, and prevent further anatomical changes. Potent or ultrapotent topical corticosteroids in an ointment base are preferred. These drugs are now widely recognized for their efficacy and minimal adverse effects, although no regimen is universally advocated.14 The patient applies ointment once daily for 1 to 3 months, depending on severity, and then once or twice a week.
Ointments are preferred over creams for vulvar treatment, because creams frequently contain allergens or irritants such as fragrance and propylene glycol preservative.
I continue once-weekly therapy indefinitely in postmenopausal women. If a premenopausal woman is not comfortable using the ointment indefinitely, I will allow her to discontinue treatment but follow her every 3 to 6 months.
Treatment also requires educating the patient about the disease, instructing her in gentle local care, and showing her exactly where to apply the ointment.
In all cases, lifelong follow-up is necessary. Hyperkeratosis, ecchymoses, fissuring, and erosions resolve, but atrophy and color change remain. Scarring usually remains unchanged, but may resolve if treated early in the course of the disease.15
Testosterone is not as effective as an ultrapotent steroid,16 and is no more effective than an emollient.17
Estrogen is valuable for skin integrity, but has no role in the treatment of lichen sclerosus.
Dilator work may be necessary for dyspareunia, once the disease is controlled.
Refer for help with depression and/or negative body image, if present.
4. VestibulodyniaEight treatment options to try
The prevalence of pain in an ethnically diverse population is 16%, and approximately half of this figure (8%) represents vulvodynia.18 The International Society for the Study of Vulvovaginal Disease now classifies vulvar pain in 3 categories:
- vulvar pain with a known cause, including infection, trauma, and systemic disease (TABLE 2),
- generalized vulvodynia, also known as dysesthetic vulvodynia, essential vulvodynia, or pudendal neuralgia, and
- localized vulvodynia or vestibulodynia, formerly called vestibulitis, vulvar vestibulitis syndrome, and vestibular adenitis.
Terminology is likely to evolve with further study.
Vestibulodynia, the leading cause of dyspareunia in women under age 50,19 refers to pain on touch within the vestibule. It is primary pain if it has been present since the first tampon use or sexual experience, and it is secondary if it arises after a period of comfortable sexual function.
TABLE 2
Rule out these known causes of vulvar pain
| INFECTIONS |
|
| TRAUMA |
|
| SYSTEMIC DISEASE |
|
| CANCER AND PRE-CANCER |
|
| IRRITANTS |
|
| SKIN CONDITIONS |
|
| Source: Haefner and Pearlman27 |
Inflammation starts the cascade
Current theory suggests that inflammatory events such as yeast infection, seminal plasma allergy, and local chemical application release a cascade of cytokines that sensitizes nociceptors in the vestibular epithelium. Prolonged neural firing in turn alters neurons in the dorsal horn, allowing sensitization of mechanoreceptive fibers in the vestibule with sensory allodynia (pain on touch). The proliferation of introital nociceptive fibers is well documented.20
Diagnosis: Report of pain and a positive Q-tip test
When a woman reports superficial dyspareunia with introital contact and clinical examination reveals pain on touch in the vestibule (using a cotton swab), vestibulodynia is diagnosed, provided no other known causes of the vulvar pain are detected during a careful history and examination or after pH measurement, a wet mount, and any indicated cultures.
A careful psychosexual history can help the clinician identify current sexual practices, prior sexual issues, and the impact of the current sexual dysfunction with an eye toward guiding support and counseling.
Multifactorial treatment: 8 options
Because the cause of vestibulodynia is unclear, a multifactorial approach generally is accepted and involves the following:
- Patient education about the problem and instruction in gentle local care and the elimination of contactants.
- Referral for support, counseling, and treatment of depression, as indicated.
- Elimination of any known trigger such as C albicans, although this generally does not lead to remission unless the pain is also treated.
- Suppression of nociceptor afferent input using topical lidocaine hydrochloride, for which good results have been reported.21
- Systemic oral analgesia with a tricyclic antidepressant modulates the serotonin and epinephrine imbalance associated with persistent pain (TABLE 3).22
- Use of anticonvulsants such as gabapentin to increase the amount of stimuli needed for nerves to fire and elevate the central pain threshold.23
- Reduction of muscle tension and spasm in the pelvic floor, using physical therapy and biofeedback.24
- When medical management fails, vulvar vestibulectomy with vaginal advancement yields excellent long-term results,25 but should be a last resort.
TABLE 3
Tricyclic antidepressants modify chemical imbalance associated with persistent pain of vestibulodynia
| Names of standard agents used | Amitriptyline (Elavil) |
| Nortriptyline (Aventyl, Pamelor) | |
| Desipramine (Norpramin) | |
| Imipramine (Tofranil) | |
| Standard dosing | Start with 10 mg; increase by 10 mg weekly to 100–150 mg |
| Side effects | Sedation, often transient |
| Constipation, must be actively managed | |
| Dry mouth | |
| Palpitations, tachycardia | |
| Sun sensitivity | |
| Cautions | Obtain EKG over 50 years or with cardiac history |
| Contraindicated in glaucoma | |
| Levels must be monitored if combined with SSRI | |
| Success | Slow change over 6–12 months in sensitivity to touch; ability to use tampon. Minimal pain with dilator use, penetration |
| Failure | Over 3 months at max dose without any improvement |
The symptoms are recited every day in gynecologists’ offices around the world: itching, irritation, burning, rawness, pain, dyspareunia. The challenge is tracing these general symptoms to a specific pathology, a task harder than one might expect, because vulvovaginal conditions often represent a complex mix of several problems. Candida and bacterial invasion frequently complicate genital dermatologic conditions. Atrophy and loss of the epithelial barrier worsen the problem. Over-the-counter (OTC) and prescription remedies can lead to contact dermatitis. Vulvodynia may be the ultimate outcome, possibly from central sensitization after chronic inflammation, which in turn can mislead the clinician into thinking appropriate therapy “doesn’t work.” And it is important to remember that any genital complaint has the potential to dampen a woman’s self-esteem and hamper sexual function.
This article covers the fine points of diagnosis and treatment of 4 common vulvovaginal problems:
- Candidiasis
- Contact dermatitis
- Lichen sclerosus
- Vestibulodynia
Could all 4 problems coexist in 1 patient? They frequently do. As always, a careful history and physical examination with appropriate use of yeast cultures make it possible to manage the complexity.
1. CandidiasisTelephone and self-diagnosis are a waste of time
In vulvovaginal candidiasis (VVC), symptoms can range from none to recurrent. VVC can complicate genital dermatologic conditions and interfere with the treatment of illnesses that call for steroids or antibiotics. Because the symptoms of VVC are nonspecific, diagnosis necessitates consideration of a long list of other potential causes, both infectious and noninfectious.
Candida albicans predominates in 85% to 90% of positive vaginal yeast cultures. Non-albicans species such as C glabrata, parapsilosis, krusei, lusitaniae, and tropicalis are more difficult to treat.
Not all episodes are the same
VVC is uncomplicated when it occurs sporadically or infrequently in a woman in good overall health and involves mild to moderate symptoms; albicans species are likely. VVC is complicated when it is severe or recurrent or occurs in a debilitated, unhealthy, or pregnant woman; non-albicans species often are involved. Proper classification is essential to successful treatment.1
Phone diagnosis is usually inaccurate
Although phone diagnosis is unreliable,2 it is still fairly common, and fewer offices use microscopy and vaginal pH to diagnose vaginal infections because of the tightened (though still simple) requirements of the Clinical Laboratory Improvement Amendment. (Clinicians who do wet mounts and KOH are required to pass a simple test each year to continue the practice.)
Women are poor self-diagnosticians when it comes to Candida infection; only one third of a group purchasing OTC antifungals had accurately identified their condition.3
Clinicians are not exempt from error, either. About 50% of the time, Candida is misdiagnosed,4 largely because of the assumption that the wet mount is more specific than it actually is (it is only 40% specific for Candida).
Ask about sex habits, douching, drugs, and diseases
Accurate diagnosis requires a careful history, focusing on risk factors for Candida: a new sexual partner; oral sex; douching; use of antibiotics, steroids, or exogenous estrogen; and uncontrolled diabetes.
Look for signs of vulvar and vaginal erythema, edema, and excoriation.
Classic “cottage cheese” discharge may not be present, and the amount has no correlation with symptom severity.
Vaginal pH of less than 4.5 excludes bacterial vaginosis, trichomoniasis, atrophic vaginitis, desquamative inflammatory vaginitis, and vaginal lichen planus.
Blastospores or pseudohyphae are diagnostic (on 10% KOH microscopy). If they are absent, a yeast culture is essential and will allow speciation. A vaginal culture is especially important in women with recurrent or refractory symptoms.
Always consider testing for sexually transmitted diseases.
Azole antifungals are usual treatment
For uncomplicated VVC, azole antifungals are best (TABLE 1). For complicated VVC, follow this therapy with maintenance fluconazole (150 mg weekly for 6 months), which clears Candida in 90.8% of cases.5
Non-albicans infection can be treated with boric acid capsules (inserted vaginally at bedtime for 14 days) or terconazole cream (7 days) or suppositories (3 days). A culture to confirm cure is essential, since non-albicans infection can be difficult to eradicate.
Note that boric acid is not approved for pregnancy.
TABLE 1
CDC guidelines for treatment of candidiasis
Any of these intravaginal or oral regimens may be used
| DOSE (NUMBER OF DAYS) | |
|---|---|
| INTRAVAGINAL AGENTS | |
| Butoconazole | |
| 2% cream* | 5 g (3) |
| Butoconazole-1 sustained-release cream | 5 g (1) |
| Clotrimazole | |
| 1% cream* | 5 g (7–14) |
| 100 mg | 1 tablet (7) |
| 2 tablets (3) | |
| 500 mg | 1 tablet (1) |
| Miconazole | |
| 2% cream* | 5 g (7) |
| 100 mg* | 1 suppository (7) |
| 200 mg* | 1 suppository (3) |
| Nystatin | |
| 100,000 U | 1 tablet (14) |
| Tioconazole | |
| 6.5% ointment* | 5 g (1) |
| Terconazole | |
| 0.4% cream | 5 g (7) |
| 0.8% cream | 5 g (3) |
| 80 mg | 1 suppository (3) |
| ORAL AGENT | |
| Fluconazole | |
| 150 mg | 1 tablet (1) |
| * Over-the-counter | |
2. Contact DermatitisNine essentials of treatment
Contact dermatitis, the most common form of vulvar dermatitis, is inflammation of the skin caused by an external agent that acts as an irritant or allergen. The skin reaction may escape notice because changes ranging from minor to extreme are often superimposed on complex preexisting conditions such as lichen simplex chronicus, lichen planus, and lichen sclerosus.6
Contact dermatitis occurs readily in the vulvar area because the skin of the vulva reacts more intensely to irritants than other skin, and its barrier function is easily weakened by moisture, friction, urine, and vaginal discharge. The 3 main types of irritant dermatitis are7:
- A potent irritant, which may produce the equivalent of a chemical burn.
- A weaker irritant, which may be applied repeatedly before inflammation manifests.
- Stinging and burning, which can occur without detectable skin change, due to chemical exposure.
Many products can cause dermatitis. Even typically harmless products can cause dermatitis if combined with lack of estrogen or use of pads, panty hose, or girdles.
No typical pattern
Patients complain of varying degrees of itching, burning, and irritation. Depending on the agent involved, onset may be sudden or gradual, and the woman may be aware or oblivious of the cause. New reactions to “old” practices or products are also possible.
Ask about personal hygiene, care during menses and after intercourse, and about soap, cleansers, and any product applied to the genital skin, as well as clothing types and exercise habits. Review prescription and OTC products, including topicals, and note which products or actions improve or aggravate symptoms. A history of allergy and atopy should heighten suspicion.
The physical exam may reveal erythema and edema; scaling is possible. Severe cases manifest as erosion, ulceration, or pigment changes. Secondary infection, if any, may involve pustules, crusting, and fissuring. The dermatitis may be localized, but often extends over the area of product spread to the mons, labiocrural folds, and anus. C albicans often complicates genital dermatologic conditions.
9-step treatment
- Stop the offending product and/or practices.
- Restore the skin barrier with sitz baths in plain lukewarm water for 5 to 10 minutes twice daily. Compresses or a handheld shower are alternatives.
- Provide moisture. After hydration, have the patient pat dry and apply a thin film of plain petrolatum.
- Replace local estrogen if necessary.
- Control any concomitant Candida with oral fluconazole 150 mg weekly, avoiding the potential irritation caused by topical antifungals.
- Treat itching and scratching with cool gel packs from the refrigerator, not the freezer (frozen packs can burn). Stop involuntary nighttime scratching with sedation: doxepin or hydroxyzine (10–75 mg at 6 PM).
- Use topical steroids for dermatitis:
- Moderate: Triamcinolone, 0.1% ointment twice daily.
- Severe: A super-potent steroid such as clobetasol, 0.05% ointment, twice daily for 1 to 3 weeks.
- Extreme: Burst and taper prednisone (0.5–1 mg/kg/day decreased over 14–21 days) or a single dose of intramuscular triamcinolone (1 mg/kg).
- Order patch testing to rule out or define allergens.
- Educate the patient about the many potential causes of dermatitis, to prevent recurrence.
CAUSTIC AGENTS
Bichloracetic acid
Trichloroacetic acid
5-Fluorouracil
Lye (in soap)
Phenol
Podophyllin
Sodium hypochlorite
Solvents
WEAK CUMULATIVE IRRITANTS
Alcohol
Deodorants
Diapers
Feces
Feminine spray
Pads
Perfume
Povidone iodine
Powders
Propylene glycol
Semen
Soap
Sweat
Urine
Vaginal secretions
Water
Wipes
PHYSICALLY ABRASIVE CONTACTANTS
Face cloths
Sponges
THERMAL IRRITANTS
Hot water bottles
Hair dryers
Source: Lynette Margesson,MD26
FIGURE 1 A mutilating disease of mysterious origin
Though lichen sclerosus is a disfiguring disease, the intensity of symptoms does not necessarily correlate with clinical appearance. Generally, the first change is (A) whitening of an irregular area on the labia, near the clitoris, on the perineum, and/or other vulvar areas. In some cases (A and B), inflammation can alter the anatomy of the vulva by flattening the labia minora, fusing the hood over the clitoris, effectively burying it beneath the skin, and shrinking the skin around the vaginal opening. Images courtesy Lynette Margesson, MD
3. Lichen SclerosusLifelong follow-up is a must
Although it has long been described in medical journals and textbooks, information on lichen sclerosus was often unreliable until recently, and adequate treatment guidelines were lacking. The cause still has not been fully elucidated, but a wealth of information now allows for considerable expertise in the management of this disease.
Lichen sclerosus is a chronic inflammatory and scarring disease that preferentially affects the anogenital area and is 6 to 10 times more prevalent in women than men.8 Any cutaneous site may also be affected, but the vagina is never involved.
Infection? Autoimmunity? An infectious cause has been proposed but never proven. In some women, an autoimmune component is recognized: Immunoglobulin G antibodies to extracellular matrix protein I have been found in 67% of patients with lichen sclerosus, but whether these antibodies are secondary or pathogenic is unclear.9 A genetic component is suggested by the association with autoimmunity and by the link with human leukocyte antigen DQ7 in women10 and girls.11
Affects 1.7%, or 1 in 60 women.12 In females, lichen sclerosus peaks in 2 populations: prepubertal girls and postmenopausal women.
No remission after age 70. Although remission of the disease has been reported, a recent study concluded that lichen sclerosus never remits after the age of 70; the average length of remission is 4.7 years, although this figure is still in question.13 Only close follow-up can determine if disease is in remission.
Main symptom is itching
Pruritus is the most common symptom, but dysuria and a sore or burning sensation have also been reported. Some women have no symptoms. When erosions, fissures, or introital narrowing are present, dyspareunia may also occur.
Typical lesions are porcelain-white papules and plaques, often with areas of fissuring or ecchymosis on the vulva or extending around the anus in a figure-of-8 pattern.
Both lichen sclerosus and lichen planus may be seen on the same vulva.
Squamous cell carcinoma can arise in anogenital lichen sclerosus; risk is thought to be 5%. Instruct women in regular self-examination because carcinoma can arise between annual or semiannual visits.
Ultrapotent steroids: Good control, but risk of malignancy persists
Renaud-Vilmer C, Cavalier-Balloy B, Porcher R, Dubertret L. Vulvar lichen sclerosus. Arch Dermatol. 2004;140:709-712.
If we gynecologists have been assuming that lichen sclerosus is one of those nebulous, little-explored diseases out there, we need to think again. Lichen sclerosus is a chronic and mutilating condition, an obstacle to quality of life, a threat to body image, a destroyer of sexual function, and a risk for malignancy.
Cancer developed only in untreated or irregularly treated lesions
In a key study, Renaud-Vilmer and colleagues13 explored remission and recurrence rates after treatment with 0.05% clobetasol propionate ointment, as well as whether the treatment reduces risk of malignant evolution. They determined that the rate of clinical and histologic remission is related to age. Although 72% of women under age 50 had complete remission, only 23% of women between 50 and 70 years of age had complete remission, and none of the women older than 70 did. Relapse was noted in most women over time (50% by 18 months), and 9.6% of women were later diagnosed with invasive squamous cell carcinoma.
Although we have known since 1988 that ultrapotent steroids offer outstanding relief of symptoms and some control over the disease, the optimal length of treatment has never been clear. The prospective study by Renaud-Vilmer et al has impressive power that derives from its 20-year duration. They demonstrated that ultrapotent steroids do not cure lichen sclerosus in women over 70. Complete remission in younger women is only temporary, and steroid therapy offers no significant reduction in the risk of vulvar cancer—although carcinoma developed only in untreated or irregularly treated lesions.
Histologic and clinical findings were used to judge efficacy. Because only 83 women were studied, the cohort is too small for the findings on carcinoma to be significant, but the authors emphasized that lifelong follow-up is necessary in all cases.
Lichen sclerosus never backs down after menopause
In more than 15 years of vulvovaginal specialization, I have found similar results. Older women detest the need to apply topicals to the genital area, but they are the ones who need ongoing use, because the disease never backs down after menopause.
I follow a cohort of young women in whom I detected early disease. Their clinical signs regressed and scarring was prevented by steroid treatment, but disease recurrence appears to be inevitable: The longest remission has been just over 4 years.
I have seen cancer arise quickly even in closely supervised patients, although many cases of squamous cell carcinoma have occurred in women with undetected or poorly treated disease.
Use tacrolimus with caution
Although small trials have produced some enthusiasm for therapeutic use of tacrolimus, treatment should proceed with extreme caution, as the drug inhibits an arm of the immune system and women with lichen sclerosus are at risk for malignancy. The agent now carries a warning based on the development of malignancy in animals.
Consider treatment even without biopsy proof
Although a biopsy generally makes the diagnosis, treatment should be considered even in the face of an inconclusive or negative finding if the clinician suspects that lichen sclerosus is present. The reason treatment should proceed in these cases: Loss of the labia or fusion over the clitoris can occur if the disease progresses, as shown in the photos on page.
Powerful corticosteroids are treatment of choice
Treatment can control lichen sclerosus, relieve symptoms, and prevent further anatomical changes. Potent or ultrapotent topical corticosteroids in an ointment base are preferred. These drugs are now widely recognized for their efficacy and minimal adverse effects, although no regimen is universally advocated.14 The patient applies ointment once daily for 1 to 3 months, depending on severity, and then once or twice a week.
Ointments are preferred over creams for vulvar treatment, because creams frequently contain allergens or irritants such as fragrance and propylene glycol preservative.
I continue once-weekly therapy indefinitely in postmenopausal women. If a premenopausal woman is not comfortable using the ointment indefinitely, I will allow her to discontinue treatment but follow her every 3 to 6 months.
Treatment also requires educating the patient about the disease, instructing her in gentle local care, and showing her exactly where to apply the ointment.
In all cases, lifelong follow-up is necessary. Hyperkeratosis, ecchymoses, fissuring, and erosions resolve, but atrophy and color change remain. Scarring usually remains unchanged, but may resolve if treated early in the course of the disease.15
Testosterone is not as effective as an ultrapotent steroid,16 and is no more effective than an emollient.17
Estrogen is valuable for skin integrity, but has no role in the treatment of lichen sclerosus.
Dilator work may be necessary for dyspareunia, once the disease is controlled.
Refer for help with depression and/or negative body image, if present.
4. VestibulodyniaEight treatment options to try
The prevalence of pain in an ethnically diverse population is 16%, and approximately half of this figure (8%) represents vulvodynia.18 The International Society for the Study of Vulvovaginal Disease now classifies vulvar pain in 3 categories:
- vulvar pain with a known cause, including infection, trauma, and systemic disease (TABLE 2),
- generalized vulvodynia, also known as dysesthetic vulvodynia, essential vulvodynia, or pudendal neuralgia, and
- localized vulvodynia or vestibulodynia, formerly called vestibulitis, vulvar vestibulitis syndrome, and vestibular adenitis.
Terminology is likely to evolve with further study.
Vestibulodynia, the leading cause of dyspareunia in women under age 50,19 refers to pain on touch within the vestibule. It is primary pain if it has been present since the first tampon use or sexual experience, and it is secondary if it arises after a period of comfortable sexual function.
TABLE 2
Rule out these known causes of vulvar pain
| INFECTIONS |
|
| TRAUMA |
|
| SYSTEMIC DISEASE |
|
| CANCER AND PRE-CANCER |
|
| IRRITANTS |
|
| SKIN CONDITIONS |
|
| Source: Haefner and Pearlman27 |
Inflammation starts the cascade
Current theory suggests that inflammatory events such as yeast infection, seminal plasma allergy, and local chemical application release a cascade of cytokines that sensitizes nociceptors in the vestibular epithelium. Prolonged neural firing in turn alters neurons in the dorsal horn, allowing sensitization of mechanoreceptive fibers in the vestibule with sensory allodynia (pain on touch). The proliferation of introital nociceptive fibers is well documented.20
Diagnosis: Report of pain and a positive Q-tip test
When a woman reports superficial dyspareunia with introital contact and clinical examination reveals pain on touch in the vestibule (using a cotton swab), vestibulodynia is diagnosed, provided no other known causes of the vulvar pain are detected during a careful history and examination or after pH measurement, a wet mount, and any indicated cultures.
A careful psychosexual history can help the clinician identify current sexual practices, prior sexual issues, and the impact of the current sexual dysfunction with an eye toward guiding support and counseling.
Multifactorial treatment: 8 options
Because the cause of vestibulodynia is unclear, a multifactorial approach generally is accepted and involves the following:
- Patient education about the problem and instruction in gentle local care and the elimination of contactants.
- Referral for support, counseling, and treatment of depression, as indicated.
- Elimination of any known trigger such as C albicans, although this generally does not lead to remission unless the pain is also treated.
- Suppression of nociceptor afferent input using topical lidocaine hydrochloride, for which good results have been reported.21
- Systemic oral analgesia with a tricyclic antidepressant modulates the serotonin and epinephrine imbalance associated with persistent pain (TABLE 3).22
- Use of anticonvulsants such as gabapentin to increase the amount of stimuli needed for nerves to fire and elevate the central pain threshold.23
- Reduction of muscle tension and spasm in the pelvic floor, using physical therapy and biofeedback.24
- When medical management fails, vulvar vestibulectomy with vaginal advancement yields excellent long-term results,25 but should be a last resort.
TABLE 3
Tricyclic antidepressants modify chemical imbalance associated with persistent pain of vestibulodynia
| Names of standard agents used | Amitriptyline (Elavil) |
| Nortriptyline (Aventyl, Pamelor) | |
| Desipramine (Norpramin) | |
| Imipramine (Tofranil) | |
| Standard dosing | Start with 10 mg; increase by 10 mg weekly to 100–150 mg |
| Side effects | Sedation, often transient |
| Constipation, must be actively managed | |
| Dry mouth | |
| Palpitations, tachycardia | |
| Sun sensitivity | |
| Cautions | Obtain EKG over 50 years or with cardiac history |
| Contraindicated in glaucoma | |
| Levels must be monitored if combined with SSRI | |
| Success | Slow change over 6–12 months in sensitivity to touch; ability to use tampon. Minimal pain with dilator use, penetration |
| Failure | Over 3 months at max dose without any improvement |
1. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Obstet Gynecol. 1998;178:203-211.
2. Spinollo A, Pizzoli G, Colonna L, Nicola S, DeSeta F, Guashino S. Epidemiologic characteristics of women with idiopathic recurrent vulvovaginal candidiasis. Obstet Gynecol. 1999;180:14-17.
3. Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol. 2002;99:419-425.
4. Ledger WJ, Polaneczky MM, Yih MC. Difficulties in the diagnosis of Candida vaginitis. Inf Dis Clin Pract. 2000;9:66-69.
5. Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;35:876-883.
6. Margesson LJ. Contact dermatitis of the vulva. Dermatol Ther. 2004;17:20-27.
7. Kamarashev JA, Vassileva SG. Dermatologic diseases of the vulva. Clin Dermatol. 1997;15:53-65.
8. Powell J, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
9. Oyama N, Chan I, Neill SM, et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet. 2003;362:118-213.
10. Marren P, Yell J, Charnock FM, Bunce M, Welsh K, Wojnarowska F. The association between lichen sclerosus and antigens of the HLA system. Br J Dermatol. 1995;132:197-203.
11. Powell J, Wojnarowska F, Winsey S, Marren P, Welsh K. Lichen sclerosus premenarche: autoimmunity and immunogenetics. Br J Dermatol. 2000;142:481-484.
12. Goldstein AT, Marinoff SC, Cristopher K, Srodon M. Prevalence of vulvar lichen sclerosus in a general gynecology practice. J Reprod Med. 2005;50:477-480.
13. Renaud-Vilmer C, Cavalier-Balloy B, Porcher R, Dubertret L. Vulvar lichen sclerosus. Arch Dermatol. 2004;140:709-712.
14. Dalziel KL, Millard PR, Wojnarowska F. The treatment of vulval lichen sclerosus with a very potent topical steroid (clobetasol propionate 0.05%) cream. Br J Dermatol. 1991;124:461-464.
15. Cooper SM, Gao XH, Powell JJ, Wojnarowska F. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004;140:702-706.
16. Bornstein J, Heifetz S, Kellner Y, et al. Clobetasol dipropionate 0.05% versus 2% testosterone application for severe vulvar lichen sclerosus. Am J Obstet Gynecol. 1998;178:80-84.
17. Sideri M, Origoni M, Spinaci L, Ferrari A. Topical testosterone in the treatment of vulvar lichen sclerosus. Int J Gynecol Obstet. 1994;46:53-56.
18. Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58:82-88.
19. Meana M, Binik YM, Khalife S, et al. Biopsychosocial profile of women with dyspareunia. Obstet Gynecol. 1997;90:583-589.
20. Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Neurochemical characterization of the vestibular nerves in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest. 1999;48:270-275.
21. Zolnoun DA, Hartmann KE, Steege JF. Overnight 5% lidocaine ointment for treatment of vulvar vestibulitis. Obstet Gynecol. 2003;102:84-87.
22. Mariani L. Vulvar vestibulitis syndrome: an overview of nonsurgical treatment. Eur J Obstet Gynecol. 2002;101:109-112.
23. Graziottin A, Vincenti E. Analgesic treatment of intractable pain due to vulvar vestibulitis syndrome: preliminary results with oral gabapentin and anesthetic block of ganglion impar. Poster presented at: International Meeting of the International Society for the Study of Women’s Sexual Health; 2002; Vancouver, Canada.
24. Glazer HI, Rodke G, Swencionis C, Hertz R, Young AW. Treatment of vulvar vestibulitis syndrome with electromyographic biofeedback of pelvic floor musculature. J Reprod Med. 1995;40:283-290.
25. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Gen Tract Dis. 2005;9:40-45.
26. Margesson LJ. Inflammatory disorders of the vulva. In: Fisher BK, Margesson LJ, eds. Genital Skin Disorders Diagnosis and Treatment. St Louis: Mosby; 1998:155-157.
27. Haefner HK, Pearlman MD. Diagnosing and managing vulvodynia. Contemp ObGyn. 1999;2:110.-
The author reports no financial relationships relevant to this article.
1. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Obstet Gynecol. 1998;178:203-211.
2. Spinollo A, Pizzoli G, Colonna L, Nicola S, DeSeta F, Guashino S. Epidemiologic characteristics of women with idiopathic recurrent vulvovaginal candidiasis. Obstet Gynecol. 1999;180:14-17.
3. Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol. 2002;99:419-425.
4. Ledger WJ, Polaneczky MM, Yih MC. Difficulties in the diagnosis of Candida vaginitis. Inf Dis Clin Pract. 2000;9:66-69.
5. Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;35:876-883.
6. Margesson LJ. Contact dermatitis of the vulva. Dermatol Ther. 2004;17:20-27.
7. Kamarashev JA, Vassileva SG. Dermatologic diseases of the vulva. Clin Dermatol. 1997;15:53-65.
8. Powell J, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
9. Oyama N, Chan I, Neill SM, et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet. 2003;362:118-213.
10. Marren P, Yell J, Charnock FM, Bunce M, Welsh K, Wojnarowska F. The association between lichen sclerosus and antigens of the HLA system. Br J Dermatol. 1995;132:197-203.
11. Powell J, Wojnarowska F, Winsey S, Marren P, Welsh K. Lichen sclerosus premenarche: autoimmunity and immunogenetics. Br J Dermatol. 2000;142:481-484.
12. Goldstein AT, Marinoff SC, Cristopher K, Srodon M. Prevalence of vulvar lichen sclerosus in a general gynecology practice. J Reprod Med. 2005;50:477-480.
13. Renaud-Vilmer C, Cavalier-Balloy B, Porcher R, Dubertret L. Vulvar lichen sclerosus. Arch Dermatol. 2004;140:709-712.
14. Dalziel KL, Millard PR, Wojnarowska F. The treatment of vulval lichen sclerosus with a very potent topical steroid (clobetasol propionate 0.05%) cream. Br J Dermatol. 1991;124:461-464.
15. Cooper SM, Gao XH, Powell JJ, Wojnarowska F. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004;140:702-706.
16. Bornstein J, Heifetz S, Kellner Y, et al. Clobetasol dipropionate 0.05% versus 2% testosterone application for severe vulvar lichen sclerosus. Am J Obstet Gynecol. 1998;178:80-84.
17. Sideri M, Origoni M, Spinaci L, Ferrari A. Topical testosterone in the treatment of vulvar lichen sclerosus. Int J Gynecol Obstet. 1994;46:53-56.
18. Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58:82-88.
19. Meana M, Binik YM, Khalife S, et al. Biopsychosocial profile of women with dyspareunia. Obstet Gynecol. 1997;90:583-589.
20. Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Neurochemical characterization of the vestibular nerves in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest. 1999;48:270-275.
21. Zolnoun DA, Hartmann KE, Steege JF. Overnight 5% lidocaine ointment for treatment of vulvar vestibulitis. Obstet Gynecol. 2003;102:84-87.
22. Mariani L. Vulvar vestibulitis syndrome: an overview of nonsurgical treatment. Eur J Obstet Gynecol. 2002;101:109-112.
23. Graziottin A, Vincenti E. Analgesic treatment of intractable pain due to vulvar vestibulitis syndrome: preliminary results with oral gabapentin and anesthetic block of ganglion impar. Poster presented at: International Meeting of the International Society for the Study of Women’s Sexual Health; 2002; Vancouver, Canada.
24. Glazer HI, Rodke G, Swencionis C, Hertz R, Young AW. Treatment of vulvar vestibulitis syndrome with electromyographic biofeedback of pelvic floor musculature. J Reprod Med. 1995;40:283-290.
25. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Gen Tract Dis. 2005;9:40-45.
26. Margesson LJ. Inflammatory disorders of the vulva. In: Fisher BK, Margesson LJ, eds. Genital Skin Disorders Diagnosis and Treatment. St Louis: Mosby; 1998:155-157.
27. Haefner HK, Pearlman MD. Diagnosing and managing vulvodynia. Contemp ObGyn. 1999;2:110.-
The author reports no financial relationships relevant to this article.
We’re on the way to ending cervical cancer
It could be the end of the affair with HPV!1 With this exclamation, Prof. Margaret Stanley, the noted human papillomavirus immunologist, expressed the optimism we all share, now that the possibility of conquering cervical cancer is within view. Not yet 25 years have passed since the first sequencing of a genital HPV type, and scarcely 10 years since the International Agency for Research on Cancer proclaimed that HPV causes cervical cancer. It has been 57 years since the discovery that launched an international quest to reduce the cervical cancer rate: George Papanicolaou’s test for early abnormal cell changes that, decades later, were found to be secondary to HPV. We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality.
But even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. And the Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab and country to country.
What is new in 2006 that we may soon be able to put into practice, bringing us closer to a new world—with respect to cervical cancer prevention—different from any we’ve known?
- 1 More sensitive and objective screening
- 2 Better management of screen positives
- 3 HPV vaccine, soon to be in our offices
1 More sensitive and more objective screening
A comforting combo: Negative Pap and HPV tests
ACOG Practice Bulletin, Number 61. Human papillomavirus. Washington, DC: American College of Obstetricians and Gynecologists; April 2005.
Because HPV testing is more sensitive than cervical cytology in detecting CIN 2 and CIN 3, women with concurrent negative Pap and HPV tests can be reassured that their risk of unidentified CIN 2, CIN 3, or cervical cancer is approximately 1 in 1,000. (Level A evidence)
The American Cancer Society and the American College of Obstetricians and Gynecologists have both provided as an option the screening of women age 30 and older with the combination of the Pap and a test for high-risk HPV types.2,3 These “sophisticated new tests for the detection of HPV…hold great promise for improved screening for cervical cancer precursors and invasive cancer, and for triage of cervical cytology,” the Bulletin states.
Not all women get annual screening, however, and even if they do, the IARC estimates, the lifetime risk for cervical cancer for women who have conventional Paps annually is approximately 216 per 100,000, if the Pap sensitivity is about 70%. The prospect of reducing the risk of missing significant cervical neoplasia at each screen to 1 per 1,000 should be of comfort to women and the clinicians who watch over their health.
Dilemma: Women over 30, with normal Pap and high-risk HPV
What about the approximately 4% of women aged 30 and older with normal cytology and high-risk HPV? How should these women be managed? A panel of experts on HPV and cervical screening published “interim guidance” in 2004, recommending that until further data are available, these women should be retested in 6 to 12 months for persistence of HPV or development of abnormal cytology, and referred to colposcopy if still HPV-positive or if Pap results show low-grade squamous intraepithelial lesion (LSIL) or worse, regardless of HPV result.4
Although the April 2005 ACOG Bulletin affirmed that guideline, concern persisted that, while some women so identified might be better evaluated immediately by colposcopy, the majority would not, and there was no good way to identify HPV-positive women most at risk. Several longitudinal studies (discussed in the following section) have now made the path clearer.
REFERENCES
1. Stanley M. The end for genital human papillomavirus infections? Lancet Oncol 2005;6:256-257.
2. Sasow D, Runowicz CD, Solomon D, et al, for the American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342-362.
3. ACOG Practice Bulletin, Number 45. Cervical cytology screening. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
4. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
Type-specific testing identifies highest risk
Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079.
HPV screening that distinguishes types 16 and 18 from other oncogenic (high-risk) HPV types identifies women at the greatest risk of CIN 2/3+ and may permit less aggressive management of women with other high-risk HPV infections.
The solution to the dilemma of having to wait 6 to 12 months to repeat Pap and HPV tests for women with a normal cytology but a positive HPV test before determining the need for colposcopy may be solved by type-specific HPV testing. The 10-year cumulative incidence of CIN 3 and cervical cancer (CIN 3+) in 20,810 women tested once for HPV at enrollment was only 0.8% in the women who tested negative for high-risk HPV by Hybrid Capture 2. In contrast, CIN 3+ developed in 17% of the HPV-16-positive women and 14% of the HPV-18-positive women within 10 years.
Women positive for other high-risk types of HPV, but negative for HPV 16 and 18 had far less risk: only 3% developed CIN 3+.
When stratified by age to limit the analysis to women aged 30 and older, the cumulative incidence of CIN 3+ was 20% in HPV-16-positive women and 15% in HPV-18-positive women (FIGURE 1). Contrast these results to the 10-year predictive value of 11% for an LSIL Pap for the same level of cervical neoplasia. In other words, a single positive HPV 16 or 18 test is almost twice as likely to identify women at high risk for CIN 3+ as an LSIL Pap result, over time.
FIGURE 1 Positive HPV 16 or 18 linked to 14% to 17% incidence of CIN3+
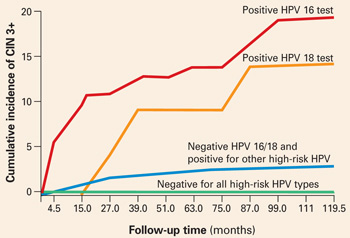
The cumulative incidence of CIN 3+ over a 10-year period, as a function of a single HPV test result at enrollment. Women positive for HPV 16 or 18 had a much greater incidence of CIN 3+, compared to women negative for HPV 16 and 18 but positive for other high-risk HPV types by Hybrid Capture 2, or negative for all high-risk HPV types. Adapted from Khan et al.
Follow-up according to risk
These findings support a follow-up strategy that would permit risk stratification of HPV-infected women for whom an optimal repeat screening interval has been unclear.
- Women positive for HPV 16 or 18 warrant referral to colposcopy, for they carry the majority of risk from a positive high-risk HPV test.
- Women positive only for other high-risk types could be reassured of the safety of a 12-month interval without colposcopy, and referred to colposcopy only if the repeat Pap shows worse than atypical squamous cells of undetermined significance (ASC-US) or the HPV test is again positive (FIGURE 2).
FIGURE 2 Type-specific testing in clinical practice
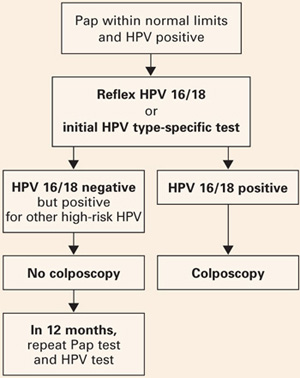
Proposed management of women aged 30 or older, who are screened concurrently with both a Pap test and an HPV test, with typing for HPV 16/18. Adapted from Khan et al.
2 type-specific tests in the pipeline
Currently, the only FDA-approved test for combined screening of women aged 30 and older is the Hybrid Capture 2 High-risk HPV test, which tests for a panel of the 13 most common HPV types known to cause cervical cancer, but does not report on individual types.
But 2 type-specific HPV tests may become available in 2006, which would enable clinicians to follow this strategy.
Digene is nearly ready to launch a 16, 18, 45 type-specific “reflex” test (to a positive Hybrid Capture 2 HPV panel), and Roche is preparing to get its type-specific Linear Array HPV test approved.
2 Better management of screen positives
New practice bulletin on managing abnormal tests
ACOG Practice Bulletin, Number 66. Management of abnormal cervical cytology and histology. Washington, DC: American College of Obstetricians and Gynecologists; September 2005.
The new Practice Bulletin published last September in most respects mirrors the most recent American Society for Colposcopy and Cervical Pathology (ASCCP) Consensus guidelines.1
Key points
- ASC-US may be managed by referral to immediate colposcopy, by repeat Pap, or by HPV testing. However, “reflex HPV testing” when ASC-US is derived from liquid-based cytology has advantages. (It is estimated that a large majority of ASC-US is now managed by HPV testing.)
- Initial management of all other Pap abnormalities is by immediate referral to colposcopy, ie, the finding of atypical squamous cells cannot rule out high-grade (ASC-H), atypical glandular cells (AGC), LSIL, and high-grade intraepithelial lesions (HSIL).
- Management of ASC-US and LSIL in adolescence and postmenopause: ACOG provides an alternative strategy for adolescents with either ASC-US or LSIL cytology, who may have either repeat cytology at 6 and 12 months or a single HPV test at 12 months. ACOG did not differentiate postmenopausal women with either ASC-US or LSIL as “special situations” with additional management strategies.
- CIN 2/3 should usually be treated, both guidelines say. The only exception is the adolescent with CIN 2, who may be followed with repeat cytology and colposcopy at 4 to 6 months if she is deemed reliable for follow-up, the colposcopy is adequate, and the endocervical sampling is negative.
- HPV-positive ASC-US, ASC-H, or LSIL and either CIN 1 or normal colposcopy findings should be followed by repeat Pap at 6 and 12 months, or a single HPV test at 12 months, with referral to colposcopy if either the Pap results show ASC-US or more advanced abnormality or the HPV test is positive.
- In contrast, an excisional procedure is required for normal findings, or an unsatisfactory colposcopy in nonpregnant women referred for atypical glandular cells “favor neoplasia” (AGC-H), or adenocarcinoma in situ (AIS), or repeat atypical glandular cells “not otherwise specified” (AGC-NOS), or HSIL. The only exception is an adolescent with HSIL cytology and a satisfactory and normal colposcopy and biopsy, who may be followed closely.
- Women treated for CIN 2/3 can be monitored after treatment by cytology screening at 6-month intervals 3 or 4 times or by a single HPV test at 6 months, before returning to annual screening. Any repeat abnormal Pap at the threshold of ASC-US or more advanced abnormality or a positive HPV test requires colposcopic evaluation.
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. American Society for Colposcopy and Cervical Pathology. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
3 HPV vaccine, soon to be in our offices
Vaccines will stop CIN 2/3 and cancer
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27.
The HPV 16 vaccine provided 100% protection against development of HPV-16-related CIN 2/3 during an average of 3.5 years of follow-up.
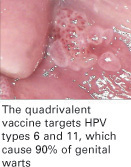
This preliminary study to the Quadrivalent HPV 6, 11, 16, 18 trial on an HPV 16 virus-like particle (VLP) vaccine reached an average of 3.5 years of follow-up. CIN 2/3 developed in 12 of the 750 women receiving placebo, in contrast to none of the 755 vaccine recipients. Persistent HPV 16 infections were defined as testing positive for type-specific HPV 16 on 2 or more visits, with the caveat that women testing positive on the last visit were considered persistent because they would have no further follow-up to determine that status. As a result, some women with a positive test only on the last visit were included as “persisters,” perhaps explaining why the efficacy in preventing persistent HPV 16 in the vaccine recipients was only 94%. Single-test positives can be transient infections, vaginal contamination with infected cells from a partner during recent intercourse, or early persistent infections. Although antibody titers to HPV 16 in vaccine recipients waned over time, they still exceeded titers in placebo recipients who already had natural immunity to HPV 16.
Benefit of the HPV 16 vaccine was also seen for women already HPV-16 positive at enrollment, but only if they were seronegative for HPV 16. It is possible that, if an immune response has not yet been mounted, the vaccine may still have a positive effect for women already HPV-16 infected.
Who will be vaccinated?
Although the primary target group for the HPV vaccine will be children before natural exposure can occur after the onset of sexual activity, many women already sexually active will likely want to be vaccinated.
It is this “catch-up” group that will challenge the OBGyn to become familiar with and to provide the HPV vaccine when it becomes available, likely later this year.
Quadrivalent vaccine 100% effective
Skjeldstad FE, Koutsky LA, for the Merck Phase 3 HPV Vaccine Steering Committee (Future II). Phase II trial of prophylactic quadrivalent HPV 6, 11, 16, 18 L1 virus-like particle (VLP) vaccine; prevention of cervical intraepithelial neoplasia (CIN) 2/3 including adeno- and squamous cell carcinoma in situ (CIS). Presented at: Infectious Diseases Society of America, Late Breaker Session 66, LB–8A; October 7, 2005; San Francisco, Calif.
The Quadrivalent HPV 6, 11, 16, 18 vaccine provided 100% protection against persistent HPV 16/18 and HPV 16/18-related CIN 2/3.
This trial became center stage in the world media in early October 2005, with headlines such as “First anti-cancer vaccine 100% effective.” The results are truly astounding, as there were no CIN 2/3 cases in the Per Protocol group, among the 5,301 women vaccinated, in contrast to 21 cases in the 5,258 women who received the placebo (TABLE 1).
In the group most likely to mirror a typical vaccinated population, only 1 case of HPV-16/18-positive CIN 2/3 occurred in the 5,736 vaccinated women, some of whom were already positive for 1 or more HPV types, or had serologic evidence of prior type-specific infection, or received fewer than the 3 recommended doses. In contrast, 36 HPV-16/18 CIN 2/3 occurred in the 5,766 women who received placebo.
TABLE 1
Efficacy of quadrivalent HPV 6, 11, 16, 18 vaccine in preventing CIN 2/3
| VACCINE GROUPS | PLACEBO GROUPS | EFFICACY | |||||
|---|---|---|---|---|---|---|---|
| NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | % | |
| Per protocol | 5,301 | 0 | 0.0 | 5,258 | 21 | 0.3 | 100 |
| Modified intention to treat | 5,301 | 1 | <0.1 | 5,766 | 36 | 0.3 | 97 |
| Source: Skjeldstad et al | |||||||
No warts, either
Subsequent analysis revealed similar protection from HPV 6 or 11 genital warts.
No serious adverse events were recorded in the entire trial.
Because HPV 16 and 18 together cause approximately 70% of all cervical cancers, and HPV 6 and 11 cause 90% of genital warts, these results are surely something about which to rejoice!
Gardasil and Cervarix vaccines
Now the challenge will be in getting the population vaccinated. Merck is expected to have its Gardasil Quadrivalent vaccine on the market mid- to late 2006. GlaxoSmithKline expects to put Cervarix Bivalent HPV 16, 18 vaccine on the market sometime in 2007.
How HPV vaccine will—and won’t—change practice
Franco EL, Harper DM. Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine. 2005;23:2388–2394.
Cervical screening will continue, but will be more accurate and more efficient.
Yes, we are on the verge of the possibility of reducing the risk of cervical cancer to close to zero, but it will take decades. Vaccinating young girls will not significantly reduce cervical cancer rates until these girls reach the median ages of microinvasive (early 40s) and invasive (late 40s) cervical cancer.
Even then, cervical cancer rates will depend on these factors:
- the extent of vaccination coverage
- the number of high-risk HPV types in the vaccine
- whether vaccination provides multidecade protection or falls off with time
- whether the medical community and the public continue to diligently follow recommended screening guidelines
If immune protection falls with time, booster HPV vaccine shots should provide ongoing protection, but population protection will depend on the percent of the population obtaining the booster. If the population becomes complacent about cervical screening as risk for cervical cancer decreases, then cancers will develop that would have otherwise been prevented.
Why screening will continue
Virus-like particle (VLP) vaccines for all of the important oncogenic HPV types could, theoretically, be produced. But until long after multivalent HPV vaccines that include all the important oncogenic types are available, women will require screening to prevent the 30% of cancers that occur from other high-risk HPV types not in the present vaccine. And, we will need screening to protect women who are not vaccinated, and those already infected.
As Franco and Harper stressed, “Although the future seems bright on the vaccine front, policy makers are strongly cautioned to avoid scaling back cervical cancer screening. Any premature relaxation of cervical cancer control measures already in place will bring a resurgence of the disease to the unacceptable levels of the not too distant past.”
In other words, cervical screening will continue for the foreseeable future.
A peek at a “new world”
Fewer abnormal Pap tests. The vaccine will likely steadily decrease the rate of abnormal Paps that are important, as an increasing proportion of women are vaccinated against the 2 most common types in high-grade CIN.
Colposcopies and cervical treatments will decline in number coincident with the proportion of the population vaccinated.
A training challenge? This change will decrease the number of significant lesions that a colposcopist may see, increasing the challenge of training and maintaining expertise in identification and treatment of these lesions. As significant Pap abnormalities decrease, maintaining expertise in cytologic interpretation, and even in maintaining attention to detail, may become more difficult.
Specific testing. Finding women with significant abnormalities may more and more be accomplished with the accuracy afforded by testing for specific HPV types known to be most at-risk for CIN 3+.
With respect to cervical cancer prevention, the years to come will surely be a new world, different from what we all have known.
Piyathilake CJ, Henao OL, Macaluso M, et al. Folate is associated with the natural history of high-risk human papillomaviruses. Cancer Res. 2004;64:8788–8793.
Improving folate status in women at risk of getting infected or already infected with high-risk HPV may help prevent cervical cancer. It is reasonable to advise women with HPV that folate supplements may be helpful.
Recommending oral folate supplements is one of the few things we can offer that can empower our patients with something positive that they can do for themselves.
A subset of women in the ASCUS LSIL Triage (ALT) study were evaluated prospectively to determine whether systemic levels of folic acid are associated with the occurrence and duration of HPV infections after controlling for other micronutrients (vitamins B12, A, E, C, and total carotene) and for known risk factors for high-risk HPV infections and cervical cancer. Hybrid Capture 2 and serum levels of these micronutrients were obtained at 6-month intervals throughout the trial’s 2-year follow-up.
Women with higher folate status were significantly less likely to be repeatedly HPV positive, more likely to become testnegative during the 2-year study, and 73% less likely to become newly HPV positive.
These associations held after controlling for other micronutrients and known risk factors for HPV. The authors reviewed a possible role of folate in preventing integration of HPV, thereby improving clearance of HPV infections, and documented that increased folate levels were also protective against the development of CIN 2/3.
Food fortification with folate became mandatory in the United States in 1998. The median folate level in women in this study mirrored the median post-fortification level for women in the United States—indicating that folate levels in food are not adequate to affect HPV status.
Therefore, it appears reasonable to advise women with HPV that taking folic acid supplementation in the levels usually advised for pregnant women may be helpful.
Dr. Cox served as a member of the American Cancer Society Cervical Guidelines Committee, the 2001 Bethesda Workshop, and was one of the primary authors of the 2001 ASCCP Consensus Guidelines for the management of women with abnormal cervical cytology and cervical cancer precursors. He is President-elect of the American Society for Colposcopy and Cervical Pathology and presently is on the ACS HPV Vaccine Advisory Committee, and the Data and Safety Monitoring Board of the HPV 6, 11, 16, 18 Quadrivalent Vaccine Trial. Dr. Cox is a consultant to Digene, GlaxoSmithKline, and the Merck Data and Safety Monitoring Board, and a speaker for Digene.
It could be the end of the affair with HPV!1 With this exclamation, Prof. Margaret Stanley, the noted human papillomavirus immunologist, expressed the optimism we all share, now that the possibility of conquering cervical cancer is within view. Not yet 25 years have passed since the first sequencing of a genital HPV type, and scarcely 10 years since the International Agency for Research on Cancer proclaimed that HPV causes cervical cancer. It has been 57 years since the discovery that launched an international quest to reduce the cervical cancer rate: George Papanicolaou’s test for early abnormal cell changes that, decades later, were found to be secondary to HPV. We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality.
But even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. And the Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab and country to country.
What is new in 2006 that we may soon be able to put into practice, bringing us closer to a new world—with respect to cervical cancer prevention—different from any we’ve known?
- 1 More sensitive and objective screening
- 2 Better management of screen positives
- 3 HPV vaccine, soon to be in our offices
1 More sensitive and more objective screening
A comforting combo: Negative Pap and HPV tests
ACOG Practice Bulletin, Number 61. Human papillomavirus. Washington, DC: American College of Obstetricians and Gynecologists; April 2005.
Because HPV testing is more sensitive than cervical cytology in detecting CIN 2 and CIN 3, women with concurrent negative Pap and HPV tests can be reassured that their risk of unidentified CIN 2, CIN 3, or cervical cancer is approximately 1 in 1,000. (Level A evidence)
The American Cancer Society and the American College of Obstetricians and Gynecologists have both provided as an option the screening of women age 30 and older with the combination of the Pap and a test for high-risk HPV types.2,3 These “sophisticated new tests for the detection of HPV…hold great promise for improved screening for cervical cancer precursors and invasive cancer, and for triage of cervical cytology,” the Bulletin states.
Not all women get annual screening, however, and even if they do, the IARC estimates, the lifetime risk for cervical cancer for women who have conventional Paps annually is approximately 216 per 100,000, if the Pap sensitivity is about 70%. The prospect of reducing the risk of missing significant cervical neoplasia at each screen to 1 per 1,000 should be of comfort to women and the clinicians who watch over their health.
Dilemma: Women over 30, with normal Pap and high-risk HPV
What about the approximately 4% of women aged 30 and older with normal cytology and high-risk HPV? How should these women be managed? A panel of experts on HPV and cervical screening published “interim guidance” in 2004, recommending that until further data are available, these women should be retested in 6 to 12 months for persistence of HPV or development of abnormal cytology, and referred to colposcopy if still HPV-positive or if Pap results show low-grade squamous intraepithelial lesion (LSIL) or worse, regardless of HPV result.4
Although the April 2005 ACOG Bulletin affirmed that guideline, concern persisted that, while some women so identified might be better evaluated immediately by colposcopy, the majority would not, and there was no good way to identify HPV-positive women most at risk. Several longitudinal studies (discussed in the following section) have now made the path clearer.
REFERENCES
1. Stanley M. The end for genital human papillomavirus infections? Lancet Oncol 2005;6:256-257.
2. Sasow D, Runowicz CD, Solomon D, et al, for the American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342-362.
3. ACOG Practice Bulletin, Number 45. Cervical cytology screening. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
4. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
Type-specific testing identifies highest risk
Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079.
HPV screening that distinguishes types 16 and 18 from other oncogenic (high-risk) HPV types identifies women at the greatest risk of CIN 2/3+ and may permit less aggressive management of women with other high-risk HPV infections.
The solution to the dilemma of having to wait 6 to 12 months to repeat Pap and HPV tests for women with a normal cytology but a positive HPV test before determining the need for colposcopy may be solved by type-specific HPV testing. The 10-year cumulative incidence of CIN 3 and cervical cancer (CIN 3+) in 20,810 women tested once for HPV at enrollment was only 0.8% in the women who tested negative for high-risk HPV by Hybrid Capture 2. In contrast, CIN 3+ developed in 17% of the HPV-16-positive women and 14% of the HPV-18-positive women within 10 years.
Women positive for other high-risk types of HPV, but negative for HPV 16 and 18 had far less risk: only 3% developed CIN 3+.
When stratified by age to limit the analysis to women aged 30 and older, the cumulative incidence of CIN 3+ was 20% in HPV-16-positive women and 15% in HPV-18-positive women (FIGURE 1). Contrast these results to the 10-year predictive value of 11% for an LSIL Pap for the same level of cervical neoplasia. In other words, a single positive HPV 16 or 18 test is almost twice as likely to identify women at high risk for CIN 3+ as an LSIL Pap result, over time.
FIGURE 1 Positive HPV 16 or 18 linked to 14% to 17% incidence of CIN3+

The cumulative incidence of CIN 3+ over a 10-year period, as a function of a single HPV test result at enrollment. Women positive for HPV 16 or 18 had a much greater incidence of CIN 3+, compared to women negative for HPV 16 and 18 but positive for other high-risk HPV types by Hybrid Capture 2, or negative for all high-risk HPV types. Adapted from Khan et al.
Follow-up according to risk
These findings support a follow-up strategy that would permit risk stratification of HPV-infected women for whom an optimal repeat screening interval has been unclear.
- Women positive for HPV 16 or 18 warrant referral to colposcopy, for they carry the majority of risk from a positive high-risk HPV test.
- Women positive only for other high-risk types could be reassured of the safety of a 12-month interval without colposcopy, and referred to colposcopy only if the repeat Pap shows worse than atypical squamous cells of undetermined significance (ASC-US) or the HPV test is again positive (FIGURE 2).
FIGURE 2 Type-specific testing in clinical practice

Proposed management of women aged 30 or older, who are screened concurrently with both a Pap test and an HPV test, with typing for HPV 16/18. Adapted from Khan et al.
2 type-specific tests in the pipeline
Currently, the only FDA-approved test for combined screening of women aged 30 and older is the Hybrid Capture 2 High-risk HPV test, which tests for a panel of the 13 most common HPV types known to cause cervical cancer, but does not report on individual types.
But 2 type-specific HPV tests may become available in 2006, which would enable clinicians to follow this strategy.
Digene is nearly ready to launch a 16, 18, 45 type-specific “reflex” test (to a positive Hybrid Capture 2 HPV panel), and Roche is preparing to get its type-specific Linear Array HPV test approved.
2 Better management of screen positives
New practice bulletin on managing abnormal tests
ACOG Practice Bulletin, Number 66. Management of abnormal cervical cytology and histology. Washington, DC: American College of Obstetricians and Gynecologists; September 2005.
The new Practice Bulletin published last September in most respects mirrors the most recent American Society for Colposcopy and Cervical Pathology (ASCCP) Consensus guidelines.1
Key points
- ASC-US may be managed by referral to immediate colposcopy, by repeat Pap, or by HPV testing. However, “reflex HPV testing” when ASC-US is derived from liquid-based cytology has advantages. (It is estimated that a large majority of ASC-US is now managed by HPV testing.)
- Initial management of all other Pap abnormalities is by immediate referral to colposcopy, ie, the finding of atypical squamous cells cannot rule out high-grade (ASC-H), atypical glandular cells (AGC), LSIL, and high-grade intraepithelial lesions (HSIL).
- Management of ASC-US and LSIL in adolescence and postmenopause: ACOG provides an alternative strategy for adolescents with either ASC-US or LSIL cytology, who may have either repeat cytology at 6 and 12 months or a single HPV test at 12 months. ACOG did not differentiate postmenopausal women with either ASC-US or LSIL as “special situations” with additional management strategies.
- CIN 2/3 should usually be treated, both guidelines say. The only exception is the adolescent with CIN 2, who may be followed with repeat cytology and colposcopy at 4 to 6 months if she is deemed reliable for follow-up, the colposcopy is adequate, and the endocervical sampling is negative.
- HPV-positive ASC-US, ASC-H, or LSIL and either CIN 1 or normal colposcopy findings should be followed by repeat Pap at 6 and 12 months, or a single HPV test at 12 months, with referral to colposcopy if either the Pap results show ASC-US or more advanced abnormality or the HPV test is positive.
- In contrast, an excisional procedure is required for normal findings, or an unsatisfactory colposcopy in nonpregnant women referred for atypical glandular cells “favor neoplasia” (AGC-H), or adenocarcinoma in situ (AIS), or repeat atypical glandular cells “not otherwise specified” (AGC-NOS), or HSIL. The only exception is an adolescent with HSIL cytology and a satisfactory and normal colposcopy and biopsy, who may be followed closely.
- Women treated for CIN 2/3 can be monitored after treatment by cytology screening at 6-month intervals 3 or 4 times or by a single HPV test at 6 months, before returning to annual screening. Any repeat abnormal Pap at the threshold of ASC-US or more advanced abnormality or a positive HPV test requires colposcopic evaluation.
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. American Society for Colposcopy and Cervical Pathology. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
3 HPV vaccine, soon to be in our offices
Vaccines will stop CIN 2/3 and cancer
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27.
The HPV 16 vaccine provided 100% protection against development of HPV-16-related CIN 2/3 during an average of 3.5 years of follow-up.

This preliminary study to the Quadrivalent HPV 6, 11, 16, 18 trial on an HPV 16 virus-like particle (VLP) vaccine reached an average of 3.5 years of follow-up. CIN 2/3 developed in 12 of the 750 women receiving placebo, in contrast to none of the 755 vaccine recipients. Persistent HPV 16 infections were defined as testing positive for type-specific HPV 16 on 2 or more visits, with the caveat that women testing positive on the last visit were considered persistent because they would have no further follow-up to determine that status. As a result, some women with a positive test only on the last visit were included as “persisters,” perhaps explaining why the efficacy in preventing persistent HPV 16 in the vaccine recipients was only 94%. Single-test positives can be transient infections, vaginal contamination with infected cells from a partner during recent intercourse, or early persistent infections. Although antibody titers to HPV 16 in vaccine recipients waned over time, they still exceeded titers in placebo recipients who already had natural immunity to HPV 16.

Benefit of the HPV 16 vaccine was also seen for women already HPV-16 positive at enrollment, but only if they were seronegative for HPV 16. It is possible that, if an immune response has not yet been mounted, the vaccine may still have a positive effect for women already HPV-16 infected.
Who will be vaccinated?
Although the primary target group for the HPV vaccine will be children before natural exposure can occur after the onset of sexual activity, many women already sexually active will likely want to be vaccinated.
It is this “catch-up” group that will challenge the OBGyn to become familiar with and to provide the HPV vaccine when it becomes available, likely later this year.
Quadrivalent vaccine 100% effective
Skjeldstad FE, Koutsky LA, for the Merck Phase 3 HPV Vaccine Steering Committee (Future II). Phase II trial of prophylactic quadrivalent HPV 6, 11, 16, 18 L1 virus-like particle (VLP) vaccine; prevention of cervical intraepithelial neoplasia (CIN) 2/3 including adeno- and squamous cell carcinoma in situ (CIS). Presented at: Infectious Diseases Society of America, Late Breaker Session 66, LB–8A; October 7, 2005; San Francisco, Calif.
The Quadrivalent HPV 6, 11, 16, 18 vaccine provided 100% protection against persistent HPV 16/18 and HPV 16/18-related CIN 2/3.
This trial became center stage in the world media in early October 2005, with headlines such as “First anti-cancer vaccine 100% effective.” The results are truly astounding, as there were no CIN 2/3 cases in the Per Protocol group, among the 5,301 women vaccinated, in contrast to 21 cases in the 5,258 women who received the placebo (TABLE 1).
In the group most likely to mirror a typical vaccinated population, only 1 case of HPV-16/18-positive CIN 2/3 occurred in the 5,736 vaccinated women, some of whom were already positive for 1 or more HPV types, or had serologic evidence of prior type-specific infection, or received fewer than the 3 recommended doses. In contrast, 36 HPV-16/18 CIN 2/3 occurred in the 5,766 women who received placebo.
TABLE 1
Efficacy of quadrivalent HPV 6, 11, 16, 18 vaccine in preventing CIN 2/3
| VACCINE GROUPS | PLACEBO GROUPS | EFFICACY | |||||
|---|---|---|---|---|---|---|---|
| NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | % | |
| Per protocol | 5,301 | 0 | 0.0 | 5,258 | 21 | 0.3 | 100 |
| Modified intention to treat | 5,301 | 1 | <0.1 | 5,766 | 36 | 0.3 | 97 |
| Source: Skjeldstad et al | |||||||
No warts, either
Subsequent analysis revealed similar protection from HPV 6 or 11 genital warts.
No serious adverse events were recorded in the entire trial.
Because HPV 16 and 18 together cause approximately 70% of all cervical cancers, and HPV 6 and 11 cause 90% of genital warts, these results are surely something about which to rejoice!
Gardasil and Cervarix vaccines
Now the challenge will be in getting the population vaccinated. Merck is expected to have its Gardasil Quadrivalent vaccine on the market mid- to late 2006. GlaxoSmithKline expects to put Cervarix Bivalent HPV 16, 18 vaccine on the market sometime in 2007.
How HPV vaccine will—and won’t—change practice
Franco EL, Harper DM. Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine. 2005;23:2388–2394.
Cervical screening will continue, but will be more accurate and more efficient.
Yes, we are on the verge of the possibility of reducing the risk of cervical cancer to close to zero, but it will take decades. Vaccinating young girls will not significantly reduce cervical cancer rates until these girls reach the median ages of microinvasive (early 40s) and invasive (late 40s) cervical cancer.
Even then, cervical cancer rates will depend on these factors:
- the extent of vaccination coverage
- the number of high-risk HPV types in the vaccine
- whether vaccination provides multidecade protection or falls off with time
- whether the medical community and the public continue to diligently follow recommended screening guidelines
If immune protection falls with time, booster HPV vaccine shots should provide ongoing protection, but population protection will depend on the percent of the population obtaining the booster. If the population becomes complacent about cervical screening as risk for cervical cancer decreases, then cancers will develop that would have otherwise been prevented.
Why screening will continue
Virus-like particle (VLP) vaccines for all of the important oncogenic HPV types could, theoretically, be produced. But until long after multivalent HPV vaccines that include all the important oncogenic types are available, women will require screening to prevent the 30% of cancers that occur from other high-risk HPV types not in the present vaccine. And, we will need screening to protect women who are not vaccinated, and those already infected.
As Franco and Harper stressed, “Although the future seems bright on the vaccine front, policy makers are strongly cautioned to avoid scaling back cervical cancer screening. Any premature relaxation of cervical cancer control measures already in place will bring a resurgence of the disease to the unacceptable levels of the not too distant past.”
In other words, cervical screening will continue for the foreseeable future.
A peek at a “new world”
Fewer abnormal Pap tests. The vaccine will likely steadily decrease the rate of abnormal Paps that are important, as an increasing proportion of women are vaccinated against the 2 most common types in high-grade CIN.
Colposcopies and cervical treatments will decline in number coincident with the proportion of the population vaccinated.
A training challenge? This change will decrease the number of significant lesions that a colposcopist may see, increasing the challenge of training and maintaining expertise in identification and treatment of these lesions. As significant Pap abnormalities decrease, maintaining expertise in cytologic interpretation, and even in maintaining attention to detail, may become more difficult.
Specific testing. Finding women with significant abnormalities may more and more be accomplished with the accuracy afforded by testing for specific HPV types known to be most at-risk for CIN 3+.
With respect to cervical cancer prevention, the years to come will surely be a new world, different from what we all have known.
Piyathilake CJ, Henao OL, Macaluso M, et al. Folate is associated with the natural history of high-risk human papillomaviruses. Cancer Res. 2004;64:8788–8793.
Improving folate status in women at risk of getting infected or already infected with high-risk HPV may help prevent cervical cancer. It is reasonable to advise women with HPV that folate supplements may be helpful.
Recommending oral folate supplements is one of the few things we can offer that can empower our patients with something positive that they can do for themselves.
A subset of women in the ASCUS LSIL Triage (ALT) study were evaluated prospectively to determine whether systemic levels of folic acid are associated with the occurrence and duration of HPV infections after controlling for other micronutrients (vitamins B12, A, E, C, and total carotene) and for known risk factors for high-risk HPV infections and cervical cancer. Hybrid Capture 2 and serum levels of these micronutrients were obtained at 6-month intervals throughout the trial’s 2-year follow-up.
Women with higher folate status were significantly less likely to be repeatedly HPV positive, more likely to become testnegative during the 2-year study, and 73% less likely to become newly HPV positive.
These associations held after controlling for other micronutrients and known risk factors for HPV. The authors reviewed a possible role of folate in preventing integration of HPV, thereby improving clearance of HPV infections, and documented that increased folate levels were also protective against the development of CIN 2/3.
Food fortification with folate became mandatory in the United States in 1998. The median folate level in women in this study mirrored the median post-fortification level for women in the United States—indicating that folate levels in food are not adequate to affect HPV status.
Therefore, it appears reasonable to advise women with HPV that taking folic acid supplementation in the levels usually advised for pregnant women may be helpful.
It could be the end of the affair with HPV!1 With this exclamation, Prof. Margaret Stanley, the noted human papillomavirus immunologist, expressed the optimism we all share, now that the possibility of conquering cervical cancer is within view. Not yet 25 years have passed since the first sequencing of a genital HPV type, and scarcely 10 years since the International Agency for Research on Cancer proclaimed that HPV causes cervical cancer. It has been 57 years since the discovery that launched an international quest to reduce the cervical cancer rate: George Papanicolaou’s test for early abnormal cell changes that, decades later, were found to be secondary to HPV. We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality.
But even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. And the Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab and country to country.
What is new in 2006 that we may soon be able to put into practice, bringing us closer to a new world—with respect to cervical cancer prevention—different from any we’ve known?
- 1 More sensitive and objective screening
- 2 Better management of screen positives
- 3 HPV vaccine, soon to be in our offices
1 More sensitive and more objective screening
A comforting combo: Negative Pap and HPV tests
ACOG Practice Bulletin, Number 61. Human papillomavirus. Washington, DC: American College of Obstetricians and Gynecologists; April 2005.
Because HPV testing is more sensitive than cervical cytology in detecting CIN 2 and CIN 3, women with concurrent negative Pap and HPV tests can be reassured that their risk of unidentified CIN 2, CIN 3, or cervical cancer is approximately 1 in 1,000. (Level A evidence)
The American Cancer Society and the American College of Obstetricians and Gynecologists have both provided as an option the screening of women age 30 and older with the combination of the Pap and a test for high-risk HPV types.2,3 These “sophisticated new tests for the detection of HPV…hold great promise for improved screening for cervical cancer precursors and invasive cancer, and for triage of cervical cytology,” the Bulletin states.
Not all women get annual screening, however, and even if they do, the IARC estimates, the lifetime risk for cervical cancer for women who have conventional Paps annually is approximately 216 per 100,000, if the Pap sensitivity is about 70%. The prospect of reducing the risk of missing significant cervical neoplasia at each screen to 1 per 1,000 should be of comfort to women and the clinicians who watch over their health.
Dilemma: Women over 30, with normal Pap and high-risk HPV
What about the approximately 4% of women aged 30 and older with normal cytology and high-risk HPV? How should these women be managed? A panel of experts on HPV and cervical screening published “interim guidance” in 2004, recommending that until further data are available, these women should be retested in 6 to 12 months for persistence of HPV or development of abnormal cytology, and referred to colposcopy if still HPV-positive or if Pap results show low-grade squamous intraepithelial lesion (LSIL) or worse, regardless of HPV result.4
Although the April 2005 ACOG Bulletin affirmed that guideline, concern persisted that, while some women so identified might be better evaluated immediately by colposcopy, the majority would not, and there was no good way to identify HPV-positive women most at risk. Several longitudinal studies (discussed in the following section) have now made the path clearer.
REFERENCES
1. Stanley M. The end for genital human papillomavirus infections? Lancet Oncol 2005;6:256-257.
2. Sasow D, Runowicz CD, Solomon D, et al, for the American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342-362.
3. ACOG Practice Bulletin, Number 45. Cervical cytology screening. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
4. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
Type-specific testing identifies highest risk
Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079.
HPV screening that distinguishes types 16 and 18 from other oncogenic (high-risk) HPV types identifies women at the greatest risk of CIN 2/3+ and may permit less aggressive management of women with other high-risk HPV infections.
The solution to the dilemma of having to wait 6 to 12 months to repeat Pap and HPV tests for women with a normal cytology but a positive HPV test before determining the need for colposcopy may be solved by type-specific HPV testing. The 10-year cumulative incidence of CIN 3 and cervical cancer (CIN 3+) in 20,810 women tested once for HPV at enrollment was only 0.8% in the women who tested negative for high-risk HPV by Hybrid Capture 2. In contrast, CIN 3+ developed in 17% of the HPV-16-positive women and 14% of the HPV-18-positive women within 10 years.
Women positive for other high-risk types of HPV, but negative for HPV 16 and 18 had far less risk: only 3% developed CIN 3+.
When stratified by age to limit the analysis to women aged 30 and older, the cumulative incidence of CIN 3+ was 20% in HPV-16-positive women and 15% in HPV-18-positive women (FIGURE 1). Contrast these results to the 10-year predictive value of 11% for an LSIL Pap for the same level of cervical neoplasia. In other words, a single positive HPV 16 or 18 test is almost twice as likely to identify women at high risk for CIN 3+ as an LSIL Pap result, over time.
FIGURE 1 Positive HPV 16 or 18 linked to 14% to 17% incidence of CIN3+

The cumulative incidence of CIN 3+ over a 10-year period, as a function of a single HPV test result at enrollment. Women positive for HPV 16 or 18 had a much greater incidence of CIN 3+, compared to women negative for HPV 16 and 18 but positive for other high-risk HPV types by Hybrid Capture 2, or negative for all high-risk HPV types. Adapted from Khan et al.
Follow-up according to risk
These findings support a follow-up strategy that would permit risk stratification of HPV-infected women for whom an optimal repeat screening interval has been unclear.
- Women positive for HPV 16 or 18 warrant referral to colposcopy, for they carry the majority of risk from a positive high-risk HPV test.
- Women positive only for other high-risk types could be reassured of the safety of a 12-month interval without colposcopy, and referred to colposcopy only if the repeat Pap shows worse than atypical squamous cells of undetermined significance (ASC-US) or the HPV test is again positive (FIGURE 2).
FIGURE 2 Type-specific testing in clinical practice

Proposed management of women aged 30 or older, who are screened concurrently with both a Pap test and an HPV test, with typing for HPV 16/18. Adapted from Khan et al.
2 type-specific tests in the pipeline
Currently, the only FDA-approved test for combined screening of women aged 30 and older is the Hybrid Capture 2 High-risk HPV test, which tests for a panel of the 13 most common HPV types known to cause cervical cancer, but does not report on individual types.
But 2 type-specific HPV tests may become available in 2006, which would enable clinicians to follow this strategy.
Digene is nearly ready to launch a 16, 18, 45 type-specific “reflex” test (to a positive Hybrid Capture 2 HPV panel), and Roche is preparing to get its type-specific Linear Array HPV test approved.
2 Better management of screen positives
New practice bulletin on managing abnormal tests
ACOG Practice Bulletin, Number 66. Management of abnormal cervical cytology and histology. Washington, DC: American College of Obstetricians and Gynecologists; September 2005.
The new Practice Bulletin published last September in most respects mirrors the most recent American Society for Colposcopy and Cervical Pathology (ASCCP) Consensus guidelines.1
Key points
- ASC-US may be managed by referral to immediate colposcopy, by repeat Pap, or by HPV testing. However, “reflex HPV testing” when ASC-US is derived from liquid-based cytology has advantages. (It is estimated that a large majority of ASC-US is now managed by HPV testing.)
- Initial management of all other Pap abnormalities is by immediate referral to colposcopy, ie, the finding of atypical squamous cells cannot rule out high-grade (ASC-H), atypical glandular cells (AGC), LSIL, and high-grade intraepithelial lesions (HSIL).
- Management of ASC-US and LSIL in adolescence and postmenopause: ACOG provides an alternative strategy for adolescents with either ASC-US or LSIL cytology, who may have either repeat cytology at 6 and 12 months or a single HPV test at 12 months. ACOG did not differentiate postmenopausal women with either ASC-US or LSIL as “special situations” with additional management strategies.
- CIN 2/3 should usually be treated, both guidelines say. The only exception is the adolescent with CIN 2, who may be followed with repeat cytology and colposcopy at 4 to 6 months if she is deemed reliable for follow-up, the colposcopy is adequate, and the endocervical sampling is negative.
- HPV-positive ASC-US, ASC-H, or LSIL and either CIN 1 or normal colposcopy findings should be followed by repeat Pap at 6 and 12 months, or a single HPV test at 12 months, with referral to colposcopy if either the Pap results show ASC-US or more advanced abnormality or the HPV test is positive.
- In contrast, an excisional procedure is required for normal findings, or an unsatisfactory colposcopy in nonpregnant women referred for atypical glandular cells “favor neoplasia” (AGC-H), or adenocarcinoma in situ (AIS), or repeat atypical glandular cells “not otherwise specified” (AGC-NOS), or HSIL. The only exception is an adolescent with HSIL cytology and a satisfactory and normal colposcopy and biopsy, who may be followed closely.
- Women treated for CIN 2/3 can be monitored after treatment by cytology screening at 6-month intervals 3 or 4 times or by a single HPV test at 6 months, before returning to annual screening. Any repeat abnormal Pap at the threshold of ASC-US or more advanced abnormality or a positive HPV test requires colposcopic evaluation.
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. American Society for Colposcopy and Cervical Pathology. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
3 HPV vaccine, soon to be in our offices
Vaccines will stop CIN 2/3 and cancer
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27.
The HPV 16 vaccine provided 100% protection against development of HPV-16-related CIN 2/3 during an average of 3.5 years of follow-up.

This preliminary study to the Quadrivalent HPV 6, 11, 16, 18 trial on an HPV 16 virus-like particle (VLP) vaccine reached an average of 3.5 years of follow-up. CIN 2/3 developed in 12 of the 750 women receiving placebo, in contrast to none of the 755 vaccine recipients. Persistent HPV 16 infections were defined as testing positive for type-specific HPV 16 on 2 or more visits, with the caveat that women testing positive on the last visit were considered persistent because they would have no further follow-up to determine that status. As a result, some women with a positive test only on the last visit were included as “persisters,” perhaps explaining why the efficacy in preventing persistent HPV 16 in the vaccine recipients was only 94%. Single-test positives can be transient infections, vaginal contamination with infected cells from a partner during recent intercourse, or early persistent infections. Although antibody titers to HPV 16 in vaccine recipients waned over time, they still exceeded titers in placebo recipients who already had natural immunity to HPV 16.

Benefit of the HPV 16 vaccine was also seen for women already HPV-16 positive at enrollment, but only if they were seronegative for HPV 16. It is possible that, if an immune response has not yet been mounted, the vaccine may still have a positive effect for women already HPV-16 infected.
Who will be vaccinated?
Although the primary target group for the HPV vaccine will be children before natural exposure can occur after the onset of sexual activity, many women already sexually active will likely want to be vaccinated.
It is this “catch-up” group that will challenge the OBGyn to become familiar with and to provide the HPV vaccine when it becomes available, likely later this year.
Quadrivalent vaccine 100% effective
Skjeldstad FE, Koutsky LA, for the Merck Phase 3 HPV Vaccine Steering Committee (Future II). Phase II trial of prophylactic quadrivalent HPV 6, 11, 16, 18 L1 virus-like particle (VLP) vaccine; prevention of cervical intraepithelial neoplasia (CIN) 2/3 including adeno- and squamous cell carcinoma in situ (CIS). Presented at: Infectious Diseases Society of America, Late Breaker Session 66, LB–8A; October 7, 2005; San Francisco, Calif.
The Quadrivalent HPV 6, 11, 16, 18 vaccine provided 100% protection against persistent HPV 16/18 and HPV 16/18-related CIN 2/3.
This trial became center stage in the world media in early October 2005, with headlines such as “First anti-cancer vaccine 100% effective.” The results are truly astounding, as there were no CIN 2/3 cases in the Per Protocol group, among the 5,301 women vaccinated, in contrast to 21 cases in the 5,258 women who received the placebo (TABLE 1).
In the group most likely to mirror a typical vaccinated population, only 1 case of HPV-16/18-positive CIN 2/3 occurred in the 5,736 vaccinated women, some of whom were already positive for 1 or more HPV types, or had serologic evidence of prior type-specific infection, or received fewer than the 3 recommended doses. In contrast, 36 HPV-16/18 CIN 2/3 occurred in the 5,766 women who received placebo.
TABLE 1
Efficacy of quadrivalent HPV 6, 11, 16, 18 vaccine in preventing CIN 2/3
| VACCINE GROUPS | PLACEBO GROUPS | EFFICACY | |||||
|---|---|---|---|---|---|---|---|
| NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | % | |
| Per protocol | 5,301 | 0 | 0.0 | 5,258 | 21 | 0.3 | 100 |
| Modified intention to treat | 5,301 | 1 | <0.1 | 5,766 | 36 | 0.3 | 97 |
| Source: Skjeldstad et al | |||||||
No warts, either
Subsequent analysis revealed similar protection from HPV 6 or 11 genital warts.
No serious adverse events were recorded in the entire trial.
Because HPV 16 and 18 together cause approximately 70% of all cervical cancers, and HPV 6 and 11 cause 90% of genital warts, these results are surely something about which to rejoice!
Gardasil and Cervarix vaccines
Now the challenge will be in getting the population vaccinated. Merck is expected to have its Gardasil Quadrivalent vaccine on the market mid- to late 2006. GlaxoSmithKline expects to put Cervarix Bivalent HPV 16, 18 vaccine on the market sometime in 2007.
How HPV vaccine will—and won’t—change practice
Franco EL, Harper DM. Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine. 2005;23:2388–2394.
Cervical screening will continue, but will be more accurate and more efficient.
Yes, we are on the verge of the possibility of reducing the risk of cervical cancer to close to zero, but it will take decades. Vaccinating young girls will not significantly reduce cervical cancer rates until these girls reach the median ages of microinvasive (early 40s) and invasive (late 40s) cervical cancer.
Even then, cervical cancer rates will depend on these factors:
- the extent of vaccination coverage
- the number of high-risk HPV types in the vaccine
- whether vaccination provides multidecade protection or falls off with time
- whether the medical community and the public continue to diligently follow recommended screening guidelines
If immune protection falls with time, booster HPV vaccine shots should provide ongoing protection, but population protection will depend on the percent of the population obtaining the booster. If the population becomes complacent about cervical screening as risk for cervical cancer decreases, then cancers will develop that would have otherwise been prevented.
Why screening will continue
Virus-like particle (VLP) vaccines for all of the important oncogenic HPV types could, theoretically, be produced. But until long after multivalent HPV vaccines that include all the important oncogenic types are available, women will require screening to prevent the 30% of cancers that occur from other high-risk HPV types not in the present vaccine. And, we will need screening to protect women who are not vaccinated, and those already infected.
As Franco and Harper stressed, “Although the future seems bright on the vaccine front, policy makers are strongly cautioned to avoid scaling back cervical cancer screening. Any premature relaxation of cervical cancer control measures already in place will bring a resurgence of the disease to the unacceptable levels of the not too distant past.”
In other words, cervical screening will continue for the foreseeable future.
A peek at a “new world”
Fewer abnormal Pap tests. The vaccine will likely steadily decrease the rate of abnormal Paps that are important, as an increasing proportion of women are vaccinated against the 2 most common types in high-grade CIN.
Colposcopies and cervical treatments will decline in number coincident with the proportion of the population vaccinated.
A training challenge? This change will decrease the number of significant lesions that a colposcopist may see, increasing the challenge of training and maintaining expertise in identification and treatment of these lesions. As significant Pap abnormalities decrease, maintaining expertise in cytologic interpretation, and even in maintaining attention to detail, may become more difficult.
Specific testing. Finding women with significant abnormalities may more and more be accomplished with the accuracy afforded by testing for specific HPV types known to be most at-risk for CIN 3+.
With respect to cervical cancer prevention, the years to come will surely be a new world, different from what we all have known.
Piyathilake CJ, Henao OL, Macaluso M, et al. Folate is associated with the natural history of high-risk human papillomaviruses. Cancer Res. 2004;64:8788–8793.
Improving folate status in women at risk of getting infected or already infected with high-risk HPV may help prevent cervical cancer. It is reasonable to advise women with HPV that folate supplements may be helpful.
Recommending oral folate supplements is one of the few things we can offer that can empower our patients with something positive that they can do for themselves.
A subset of women in the ASCUS LSIL Triage (ALT) study were evaluated prospectively to determine whether systemic levels of folic acid are associated with the occurrence and duration of HPV infections after controlling for other micronutrients (vitamins B12, A, E, C, and total carotene) and for known risk factors for high-risk HPV infections and cervical cancer. Hybrid Capture 2 and serum levels of these micronutrients were obtained at 6-month intervals throughout the trial’s 2-year follow-up.
Women with higher folate status were significantly less likely to be repeatedly HPV positive, more likely to become testnegative during the 2-year study, and 73% less likely to become newly HPV positive.
These associations held after controlling for other micronutrients and known risk factors for HPV. The authors reviewed a possible role of folate in preventing integration of HPV, thereby improving clearance of HPV infections, and documented that increased folate levels were also protective against the development of CIN 2/3.
Food fortification with folate became mandatory in the United States in 1998. The median folate level in women in this study mirrored the median post-fortification level for women in the United States—indicating that folate levels in food are not adequate to affect HPV status.
Therefore, it appears reasonable to advise women with HPV that taking folic acid supplementation in the levels usually advised for pregnant women may be helpful.
Dr. Cox served as a member of the American Cancer Society Cervical Guidelines Committee, the 2001 Bethesda Workshop, and was one of the primary authors of the 2001 ASCCP Consensus Guidelines for the management of women with abnormal cervical cytology and cervical cancer precursors. He is President-elect of the American Society for Colposcopy and Cervical Pathology and presently is on the ACS HPV Vaccine Advisory Committee, and the Data and Safety Monitoring Board of the HPV 6, 11, 16, 18 Quadrivalent Vaccine Trial. Dr. Cox is a consultant to Digene, GlaxoSmithKline, and the Merck Data and Safety Monitoring Board, and a speaker for Digene.
Dr. Cox served as a member of the American Cancer Society Cervical Guidelines Committee, the 2001 Bethesda Workshop, and was one of the primary authors of the 2001 ASCCP Consensus Guidelines for the management of women with abnormal cervical cytology and cervical cancer precursors. He is President-elect of the American Society for Colposcopy and Cervical Pathology and presently is on the ACS HPV Vaccine Advisory Committee, and the Data and Safety Monitoring Board of the HPV 6, 11, 16, 18 Quadrivalent Vaccine Trial. Dr. Cox is a consultant to Digene, GlaxoSmithKline, and the Merck Data and Safety Monitoring Board, and a speaker for Digene.
Bone loss in young women
- In young women, many cases of osteoporosis are caused by hypoestrogenism resulting from hormone treatment (eg, GnRH agonists, aromatase inhibitors) or lifestyle adaptations (elite athletics, eating disorders).
- Treatment of osteoporosis in young women can often be successful with the use of estrogen or “androgenic” progestins.
- Chronic glucocorticoid treatment is a common cause of clinically significant osteoporosis in young women. Glucocorticoid-induced osteoporosis is an important cause of premenopausal osteoporotic fractures.
- ObGyns play a key role in ensuring that women enter midlife with strong bones. A focus on young women at very high risk for osteoporosis will help to ensure that our patients build their future bone health on a strong foundation.
ObGyns are very well trained to diagnose and treat women with osteoporosis, most of whom are perimenopausal and menopausal. We are also treating a significant number of young women at risk for osteoporosis because of lifestyle choices or medical treatment of endometriosis or rheumatic diseases. Treatment of this population poses unique challenges and requires specialized approaches.
An important caveat in any discussion of bone loss in young women: Few randomized clinical trials have assessed the efficacy of the various treatments available. In most treatment studies of osteoporosis in young women, bone mineral density (BMD)—an intermediate biometric endpoint—is the primary treatment outcome. In contrast, in the best studies in the menopausal population, the primary treatment outcome is bone fracture—a clinically important endpoint.
In addition, fewer consensus recommendations are available on the management of osteoporosis in young women than for its treatment in menopausal women. In this article, 3 different kinds of cases of bone loss in young women are used to develop key clinical points.
CASE 1 HISTORY
A teen with pelvic pain, bone loss
Therapy eased pain but decreased BMD
A 19-year-old woman has a 3-year history of severe, disabling dysmenorrhea. For 2 years she was treated with nonsteroidal anti-inflammatory drugs and cyclic estrogen-progestin contraceptives before switching to continuous oral contraceptives. These interventions did not relieve her pain. When she was 18, laparoscopy revealed stage I endometriosis, which was resected, providing 6 months of relief. When her pain recurred, the patient was started on leuprolide acetate depot, which caused amenorrhea and provided excellent pain relief. The patient said the leuprolide had “given back her life”. After 6 months of leuprolide therapy, a DXA bone scan demonstrated osteoporosis with a lumbar spine Z score of–2.6.
What treatment do you recommend?
Under age 25, use Z score, not T score
The diagnosis of osteoporosis in very young women is complex and continues to evolve. Physicians have been educated to use the T score (comparison to the mean peak bone mass of young adults) to assess BMD in menopausal women, in whom a decrease in T score of 1 standard deviation is associated with a 2- to 4-fold increase in fracture risk. However, teenage women are often still gaining bone mineral mass as the skeleton develops, so their T scores are normally below that of the peak bone mass of an adult woman. The Z score gives a good comparison of their bone mass with that of teens of the same sex at a similar developmental stage. Most experts agree that, when using DXA test results to assess bone density in women younger than 25, the Z score should be used.1 In one recent study, peak bone mass in the spine was achieved at approximately 23 years of age.2 As women reach age 25, the T and Z scores converge (FIGURE 1).
FIGURE 1 The Z score is more informative when the patient is under 25
The Z score compares the patient with persons of the same age and sex. The T score compares the patient with young normal adults of the same sex. Since bone mass increases until approximately age 20 to 25 years, it is best to use the Z score to evaluate the bone mineral density of women younger than 25 years.1 Reprinted from Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253–257. ©2004 with permission from Elsevier.
Treatment
For this patient with osteoporosis, there are several approaches to treatment:
Ensure adequate vitamin D and calcium. Vitamin D should be prescribed in doses of 400 to 800 IU daily. A recent meta-analysis reported that 800 IU vitamin D daily appears to provide better protection against osteoporotic fractures than 400 IU in menopausal women.3 Consider measuring 25-hydroxy vitamin D levels to assess the patient’s stores of this important pre-vitamin. Many young women are vitamin D-deficient.
Calcium intake should be in the range of 1,500 mg daily. To achieve this level of calcium intake, a calcium supplement, 500 to 1,000 mg daily, will probably need to be prescribed.
Discontinue leuprolide treatment. Typically, menses and ovarian estrogen production resume once leuprolide is stopped, and bone mass begins to recover. If pain recurs, the patient could be treated with cyclic or continuous estrogen-progestin contraceptives, depot-subQ Provera, a progestinreleasing intrauterine device, or a second laparoscopy procedure.
Continue leuprolide therapy and initiate steroid add-back treatment. Options shown to be effective in preserving bone mass in young women taking a gonadotropin-releasing hormone (GnRH) analogue include:
- norethindrone acetate, 5 mg daily,
- conjugated equine estrogen (Premarin), 0.3 or 0.625 mg daily, plus a progestin, and
- low-dose transdermal estrogen, 25 μg daily, plus a progestin.
Key trials
Lupron Add-Back Study. Women with endometriosis and chronic pelvic pain were randomly assigned to 1 of 4 treatment groups:
- leuprolide alone,
- leuprolide plus an oral synthetic progestin, norethindrone acetate, 5 mg daily,
- leuprolide plus “low-dose” conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, or
- leuprolide plus “high-dose” conjugated equine estrogen, 1.25 mg, plus norethindrone acetate, 5 mg daily.4
Women in all 4 groups received depotleuprolide, 3.75 mg intramuscularly every 4 weeks for 1 year. Over 1 year of treatment, BMD decreased significantly in the women who received the GnRH agonist alone. Bone density was preserved in the 3 groups that received steroid add-back.
Vasomotor symptoms were significantly reduced in all 3 groups receiving steroid add-back therapy, compared with the placebo group. However, more women in the group that received the larger dose of estrogen dropped out of the study than from the other groups because of more significant pelvic pain.
In summary, norethindrone, 5 mg daily, or low-dose conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, were both effective steroid add-back regimens for prevention of bone loss in young women with endometriosis being treated with long-term GnRH analogues (TABLE 1).
GnRH analogue vs GnRH plus estradiolprogestin. In another trial, women who experienced endometriosis and pelvic pain were randomized to receive a GnRH agonist alone or a GnRH agonist plus low-dose transdermal estradiol, 25 μg daily, plus medroxyprogesterone acetate, 2.5 mg daily, for 6 months. Women in both groups had similar improvement in pelvic pain symptoms and a similar decrease in endometriosis surgical staging scores as determined by laparoscopy before and after treatment. However, the women who received the GnRH agonist alone, without add-back therapy, had more vasomotor symptoms and a greater decline in BMD than the women who received the GnRH agonist plus low-dose transdermal estradiol plus progestin.5
This study supports the estrogenthreshold hypothesis6 that there is a “sweet spot” in estradiol concentration where vasomotor symptoms and bone loss can be attenuated, but where endometriosis lesion activity is suppressed. This “sweet spot” appears to be at an estradiol concentration of about 30 pg/mL (FIGURE 2).
GnRH analogue vs GnRH plus teriparatide. A synthetic parathyroid analogue (PTH 1-34, teriparatide, Forteo) has been demonstrated to prevent bone loss in hypoestrogenic women. In this study, women with pelvic pain and endometriosis were randomized to treatment with a GnRH agonist alone or a GnRH agonist plus teriparatide for 6 months. Women receiving the GnRH agonist alone had a decrease in BMD at the spine of 3.5%, when measured in the lateral plane. Women receiving combined treatment had an increase in BMD at the spine of 3.4%.7
The Food and Drug Administration (FDA) has approved teriparatide at a dose of 20 μg daily by subcutaneous injection for the treatment of osteoporosis. A major advantage of PTH is that it appears to stimulate both osteoblasts and osteoclasts. In contrast, estrogen may preferentially block osteoclast activity without significantly stimulating osteoblasts. A major disadvantage of PTH: It is currently available only as a daily injection. It also is more expensive than estrogen or a synthetic progestin.
CASE 1 MANAGEMENT
A teen with pelvic pain, bone loss
Long-term combined therapy improved her BMD
This patient strongly preferred to continue the leuprolide treatment because it was so effective in treating her pelvic pain. She continued her leuprolide treatment and started norethindrone acetate 5 mg daily. She reported continued excellent control of her pelvic pain on the combined regimen of leuprolide plus norethindrone acetate. Follow-up bone density measurement demonstrated significant improvement. She continues on long-term combined therapy and is scheduled for annual BMD measurements.
CASE 2 HISTORY
The female athlete triad
A college-age dancer with an eating disorder
A 21-year-old elite ballet dancer, height 5’4” and weight 104 lb (BMI 17.8 kg/m2), presents with a history of a stress fracture of her foot. Besides being amenorrheic, she is on a high-fiber macrobiotic diet. DXA measurement demonstrates a Z score consistent with osteoporosis. She refuses to take estrogen-progestin contraceptives because she claims they impair her ability to train for her dance performances. She wonders if she should start alendronate therapy.
The female athlete triad is the combination of amenorrhea, disordered eating, and osteoporosis.8 In young female athletes, exercise and disordered eating may cause menstrual irregularity, and either disordered eating or menstrual irregularity, or both, may cause decreased BMD.9
Strenuous exercise has divergent effects on trabecular and cortical bone. By causing hypoestrogenism, strenuous exercise decreases bone density at cortical sites (vertebral spine). However, the weight-bearing exercise itself can lead to an increase in bone density at weight-bearing sites.10
Many elite athletes with the female athlete triad do not want to alter the intensity of their training or their diet, which might result in weight gain and resolution of the osteoporosis. Some elite athletes report that they do not want to take standard birth control pills because they believe the pill may impair their training and peak competitive performance. The clinician is often challenged by these strongly held beliefs to identify a treatment plan that will reverse the bone loss.
Is systemic disease involved? Occasionally, systemic disease causes or contributes to low bone mass in a young woman. Laboratory tests may help in screening some women for these diseases (TABLE 2).
FIGURE 2 Aim for the “sweet spot” in estradiol concentrations
According to the estrogen-threshold hypothesis, there is a range, or “sweet spot,” of estradiol concentrations where endometriosis lesion activity is suppressed and vasomotor symptoms and bone loss are not excessive. In humans, estrogen concentrations in the range of 30 pg/mL appear to be associated with decreased endometriosis pain and minimal degrees of bone loss. At estradiol levels of less than 10 pg/mL, bone loss is markedly accelerated.6 Reprinted from Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740–745. ©1992 with permission from Elsevier.TABLE 1
Effective steroid hormone add-back regimens
Regimens that suppressed vasomotor symptoms and bone loss associated with long-term GnRH analogue use
| INVESTIGATOR | RANDOMIZATION SCHEME | STEROID HORMONE ADD-BACK REGIMEN |
|---|---|---|
| Howell et al5 | 50 women with endometriosis and pelvic pain randomized to GnRH agonist alone or GnRH agonist plus add-back regimen | Transdermal estradiol, 25 μg/d, plus oral medroxyprogesterone acetate, 2.5 mg/d |
| Lupron Add-Back Study Group4 | 201 women with endometriosis and pelvic pain randomized to GnRH agonist plus placebo or GnRH agonist plus 1 of 3 add-back regimens | Oral norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 0.625 mg/d plus norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 1.25 mg/d plus norethindrone, 5 mg/d |
TABLE 2
Screening for secondary causes of bone loss
| The following serum tests may be useful in screening women with osteoporosis for secondary causes of disordered bone metabolism |
| Calcium and albumin |
| Phosphorus |
| 25-Hydroxy vitamin D |
| Thyroid-stimulating hormone |
| Alkaline phosphatase |
| Creatinine |
Treatment of elite athletes
Peak bone mass depends on many factors, including genetics11; ovarian estrogen production12; adequate intake of calories, protein, vitamin D, and calcium9; exercise13; and achievement of target height and weight.14 When combinations of risk factors for osteoporosis are present, such as an eating disorder plus amenorrhea, correction of only one factor may not result in improved BMD.15
There are few clinical trials of the treatment of osteoporosis in women with the female athlete triad or an eating disorder. Treatment recommendations are largely based on clinical experience. If the woman resists lifestyle and dietary changes that will result in weight gain, other treatment recommendations to pursue include:
- vitamin D and calcium supplements,
- increased dietary protein,
- weight training,16 and
- smoking cessation.
Psychological counseling may help many young women with disordered eating.
Hormonal therapy has not been documented to reliably increase bone density in young amenorrheic women with an eating disorder. In 3 clinical trials, the effect of estrogen replacement on bone density in such patients was negligible or minimal.17-19 Given our understanding of the positive effect of estrogen on spinal BMD, it is difficult to understand these findings. Two possibilities are that compliance with estrogen replacement was modest because the women did not want a return of menses, or an insufficient dose of estrogen was prescribed. Another is that being underweight blocks the positive effect of estrogen on bone density. One small clinical trial did report that treatment of hypoestrogenic women with an estrogen-progestin contraceptive containing 35 μg of ethinyl estradiol resulted in a significant increase in lumbar spine BMD (5.4%) and a nonsignificant increase in femur BMD (3.6%).20 A logical recommendation, based on clinical experience, would be to prescribe an estrogen-progestin contraceptive to this woman to help preserve or improve her bone density.
Bisphosphonate therapy has been studied in small groups of women with disordered eating and osteoporosis. In one small clinical trial, 32 women with anorexia nervosa were given 10 mg alendronate daily or placebo for 1 year. Alendronate treatment was associated with a nonsignificant increase in femoral neck and spine bone density of 4.4% and 3.5%, respectively. Weight gain was an important predictor of improved BMD.21
The young woman in this case probably should not be prescribed alendronate. The FDA drug information for alendronate warns: “Safety and efficacy have not been established in pregnant women. Animal studies have shown delays in delivery and fetal/neonatal death (secondary to hypocalcemia). Bisphosphonates are incorporated into the bone matrix and gradually released over time. Theoretically, there may be a risk of fetal harm when pregnancy follows the completion of therapy. Based on limited case reports with pamidronate, serum calcium levels in the newborn may be altered if administered during pregnancy.”
Although there are minimal data in humans that alendronate will have adverse effects on fetal bone development and function, findings in rats treated with very high doses of alendronate leave some concern about the potential risk for human fetuses.
The young woman in this case may want to have children in the near future. Bisphosphonates may be incorporated into the bone and have a long residual half-life, resulting in potential exposure of the fetus to low doses of the compound. Very few malformations in human pregnancy have been reported after treatment with bisphosphonates.22 Based on a cautious approach to this situation, however, I would recommend that this woman of reproductive age who plans future child-bearing probably should not be treated with alendronate.
CASE 2 MANAGEMENT
The female athlete triad
Estrogen-progestin ring and mental health evaluation
Many women with the female athlete triad do not meet formal criteria for anorexia nervosa, but have disordered eating patterns. Questions in the medical history such as “Do you think you should be dieting?”23 may help initiate a conversation about eating practices and attitudes. The woman in Case 2 was screened for clinical depression. She reported that she was not blue over the past 2 weeks, but noted that she worried that she measured too much of her self-worth by her body image—a diagnostic criterion for anorexia nervosa. After extensive counseling, she was willing to start an estrogen-progestin contraceptive ring. She was also referred to a mental health provider for further evaluation.
CASE 3 HISTORY
Glucocorticoid-related bone loss
A 35-year-old woman with rheumatoid arthritis
This woman (G1P1) has been treated with various doses of prednisone over the past 10 years. Currently she is taking prednisone, 20 mg daily. She is oligomenorrheic with menstrual cycles every 45 days. She has undergone both bilateral hip replacement and bilateral tubal ligation. Her BMI is 20.5 kg/m2. DXA shows osteoporosis at her lumbar spine.
Glucocorticoids are a common cause of osteoporosis in young women. These drugs inhibit osteoblast activity and increase osteoclast activity. They also increase renal calcium excretion and decrease intestinal calcium absorption and adrenal androgen production. A meta-analysis of 89 publications reported that chronic glucocorticoid use increased both the risk of osteoporosis and clinically significant fractures.24 Chronic glucocorticoid treatment was associated with an increased relative risk of vertebral and hip fracture of 2.60 (95% confidence interval [CI], 2.31–2.92) and 1.61 (95% CI, 1.47–1.76), respectively. Daily doses of prednisone 10 mg or greater were clearly associated with an increased risk of fracture. The onset of the increased fracture risk occurred within 3 months of initiating therapy.
Treatment
Treatment for this woman should include:
- vitamin D and calcium supplementation,25 and
- consideration of estrogen-progestin therapy26 and/or bisphosphonates.
PTH analogue treatment could also be utilized if neither estrogen replacement nor bisphosphonate treatment were possible.27 If the patient could discontinue the glucocorticoid therapy and begin another treatment for rheumatoid arthritis, such as etanercept (Enbrel), her BMD might improve.
Bisphosphonates are a first-line option, since she has had a bilateral tubal ligation and is at very low risk for a future pregnancy. Many clinical trials demonstrate the efficacy of bisphosphonates in this clinical situation. Bisphosphonates are approved by the FDA for the prevention and treatment of osteoporosis in women receiving glucocorticoids.
In a study of alendronate, the rate of new vertebral fractures was 0.7% in the alendronate group and 6.8% in the placebo group.28 Subjects who were taking at least 7.5 mg prednisone daily were randomized to receive alendronate, 5 or 10 mg daily, or a placebo. After 2 years of treatment, there was a 3.7% increase in lumbar spine BMD in the group treated with alendronate, 10 mg daily, and a 0.8% loss in bone denisty in the placebo group (FIGURE 3).
In a study of risedronate, 5 mg daily versus placebo, bisphosphonate treatment was associated with a 70% decrease in fracture risk.29
These clinical trials were completed before the development of once-weekly bisphosphonate treatment. Now that such treatment is available, it makes sense to prescribe either alendronate, 70 mg once weekly, or risedronate, 35 mg once weekly. Ibandronate, 150 mg once monthly, is also likely to be effective.
FIGURE 3 Alendronate improves BMD in women taking glucocorticoids
Effects of 2 years of alendronate treatment on bone mineral density of the lumbar spine in patients receiving average daily doses of prednisone of at least 7.5 mg. Alendronate treatment significantly improved bone mineral density (P<.0001). *Alendronate 2.5 mg was switched to 10 mg after it was determined that 2.5 mg was a suboptimal dose.
28 From Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, doubleblind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. Copyright ©2001. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Do US women get adequate treatment?
Surveys of community-based practices report that approximately 40% of women taking chronic glucocorticoid therapy are not receiving any intervention to prevent osteoporosis.30
Gynecologists are well positioned to ensure that these young women are fully apprised of their options and receive appropriate preventive intervention.
CASE 3 MANAGEMENT
Glucocorticoid-related bone loss
Estrogen-progestin patch, a bisphosphonate, and a rheumatology consult
This patient was started on an estrogen-progestin contraceptive patch and a bisphosphonate. An annual BMD measurement is planned.
In addition, a rheumatologist was consulted to determine if etanercept (Enbrel) could be initiated for the treatment of her rheumatoid arthritis, with a concomitant reduction in her prednisone dosage.
SUMMARYAim treatment toward future bone health
Many controversies remain unresolved concerning the best approach to bone loss in young women. On the one hand, most young women with osteoporosis are at low risk of vertebral and hip fracture, so a large number would need to be treated to prevent one fracture. On the other hand, most hope to become middle-aged; if young women begin adult life with osteoporosis, it is possible that they will have a poor foundation upon which to achieve optimal bone health. Until this key issue is resolved, it is probably best to try to help young women with osteoporosis achieve the best bone health possible. Bone health in young women is likely to be the foundation determining their future bone health and fracture risk.
1. Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253-257.
2. Lin YC, Lyle RM, Weaver CM, et al. Peak spine and femoral neck bone mass in young women. Bone. 2003;32:546-253.
3. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation. JAMA. 2005;293:2257-2264.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
5. Howell R, Edmonds D, Dowsett M. Gonadotropin releasing hormone analogue plus hormone replacement therapy for the treatment of endometriosis: a randomized controlled trial. Fertil Steril. 1996;66:666-668.
6. Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
7. Finkelstein JS, Klibanski A, Shaefer EH, Hornstein MD, Schiff I, Neer RM. Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med. 1994;331:1618-1623.
8. Warren MP, Brooks-Gunn J, Fox RP, Holderness C, Hyle EP, Hamilton WG. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab. 2002;87:3162-3168.
9. Cobb KL, Bachrach LK, Greendale G, et al. Disordered eating, menstrual irregularity and bone mineral density in female runners. Med Sci Sports Exerc. 2003;35:711-719.
10. Young N, Formica C, Szmukler, Seeman E. Bone density at weight-bearing and non weight-bearing sites in ballet dancers: the effects of exercise, hypogonadism and body weight. J Clin Endocrinol Metab. 1994;78:449-454.
11. Young D, Hopper JL, Nowson CA, et al. Determinants of bone mineral mass in 10- to 26- year old females: a twin study. J Bone Miner Res. 1995;10:558-567.
12. Drinkwater BL, Bruemner B, Chestnut CH. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263:545-548.
13. Fehily AM, Coles RJ, Evans WD, Elwood PC. Factors affecting bone density in young adults. Am J Clin Nutr. 1992;56:579-586.
14. Lloyd T, Rollings N, Andon MB, et al. Determinants of bone density in young women. J Clin Endocrinol Metab. 1992;75:383-387.
15. Jonnavithula S, Warren MP, Fox RP, Lazaro MI. Bone mineral density is compromised in amenorrheic women despite return of menses: a 2-year study. Obstet Gynecol. 1993;81:669-674.
16. Friedlander AL, Genant HK, Sadowsky S, Byl NN, Gluer CC. A two-year program of aerobics and weight training enhances bone mineral density of young women. J Bone Mineral Res. 1995;10:574-585.
17. Klibanski A, Biller BMK, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898-904.
18. Golden NH, Lanzkowsky L, Schebendach J, et al. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15:135-143.
19. Gordon CM, Grace E, Emans SJ, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935-4941.
20. Hergenroeder AC, Smith EO, Shypailo R, et al. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone acetate or placebo over 12 months. Am J Obstet Gynecol. 1997;176:1017-1025.
21. Golden NH, Iglesias EA, Jacobsen MS, et al. Alendronate for the treatment of osteopenia in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:3179-3185.
22. Ott SM. Letter re: alendronate in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:5508.-
23. Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Phys. 2003;67:297-304.
24. van Staa TP, Leufkens HGM, Cooper C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int. 2002;13:624-629.
25. Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med. 1998;339:292-299.
26. Hall GM, Daniels M, Doyle DV, Spector TD. Effect of hormone replacement therapy on bone mass in rheumatoid arthritis patients treated with and without steroids. Arthritis Rheum. 1994;37:1499-1505.
27. Lane NE, Roe B, Genant HK, Arnaud C. Parathyroid hormone treatment reverses glucocorticoid-induced osteoporosis: results of a randomized controlled trial. J Clin Invest. 1998;102:1627-1633.
28. Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202-211.
29. Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000;15:1006-1013.
30. Yood RA, Harrold L, Fish L, et al. Prevention of glucocorticoid-induced osteoporosis: experience in a managed care setting. Arch Intern Med. 2001;161:1322-1327.
The author reports no financial relationships relevant to this article.
- In young women, many cases of osteoporosis are caused by hypoestrogenism resulting from hormone treatment (eg, GnRH agonists, aromatase inhibitors) or lifestyle adaptations (elite athletics, eating disorders).
- Treatment of osteoporosis in young women can often be successful with the use of estrogen or “androgenic” progestins.
- Chronic glucocorticoid treatment is a common cause of clinically significant osteoporosis in young women. Glucocorticoid-induced osteoporosis is an important cause of premenopausal osteoporotic fractures.
- ObGyns play a key role in ensuring that women enter midlife with strong bones. A focus on young women at very high risk for osteoporosis will help to ensure that our patients build their future bone health on a strong foundation.
ObGyns are very well trained to diagnose and treat women with osteoporosis, most of whom are perimenopausal and menopausal. We are also treating a significant number of young women at risk for osteoporosis because of lifestyle choices or medical treatment of endometriosis or rheumatic diseases. Treatment of this population poses unique challenges and requires specialized approaches.
An important caveat in any discussion of bone loss in young women: Few randomized clinical trials have assessed the efficacy of the various treatments available. In most treatment studies of osteoporosis in young women, bone mineral density (BMD)—an intermediate biometric endpoint—is the primary treatment outcome. In contrast, in the best studies in the menopausal population, the primary treatment outcome is bone fracture—a clinically important endpoint.
In addition, fewer consensus recommendations are available on the management of osteoporosis in young women than for its treatment in menopausal women. In this article, 3 different kinds of cases of bone loss in young women are used to develop key clinical points.
CASE 1 HISTORY
A teen with pelvic pain, bone loss
Therapy eased pain but decreased BMD
A 19-year-old woman has a 3-year history of severe, disabling dysmenorrhea. For 2 years she was treated with nonsteroidal anti-inflammatory drugs and cyclic estrogen-progestin contraceptives before switching to continuous oral contraceptives. These interventions did not relieve her pain. When she was 18, laparoscopy revealed stage I endometriosis, which was resected, providing 6 months of relief. When her pain recurred, the patient was started on leuprolide acetate depot, which caused amenorrhea and provided excellent pain relief. The patient said the leuprolide had “given back her life”. After 6 months of leuprolide therapy, a DXA bone scan demonstrated osteoporosis with a lumbar spine Z score of–2.6.
What treatment do you recommend?
Under age 25, use Z score, not T score
The diagnosis of osteoporosis in very young women is complex and continues to evolve. Physicians have been educated to use the T score (comparison to the mean peak bone mass of young adults) to assess BMD in menopausal women, in whom a decrease in T score of 1 standard deviation is associated with a 2- to 4-fold increase in fracture risk. However, teenage women are often still gaining bone mineral mass as the skeleton develops, so their T scores are normally below that of the peak bone mass of an adult woman. The Z score gives a good comparison of their bone mass with that of teens of the same sex at a similar developmental stage. Most experts agree that, when using DXA test results to assess bone density in women younger than 25, the Z score should be used.1 In one recent study, peak bone mass in the spine was achieved at approximately 23 years of age.2 As women reach age 25, the T and Z scores converge (FIGURE 1).
FIGURE 1 The Z score is more informative when the patient is under 25
The Z score compares the patient with persons of the same age and sex. The T score compares the patient with young normal adults of the same sex. Since bone mass increases until approximately age 20 to 25 years, it is best to use the Z score to evaluate the bone mineral density of women younger than 25 years.1 Reprinted from Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253–257. ©2004 with permission from Elsevier.
Treatment
For this patient with osteoporosis, there are several approaches to treatment:
Ensure adequate vitamin D and calcium. Vitamin D should be prescribed in doses of 400 to 800 IU daily. A recent meta-analysis reported that 800 IU vitamin D daily appears to provide better protection against osteoporotic fractures than 400 IU in menopausal women.3 Consider measuring 25-hydroxy vitamin D levels to assess the patient’s stores of this important pre-vitamin. Many young women are vitamin D-deficient.
Calcium intake should be in the range of 1,500 mg daily. To achieve this level of calcium intake, a calcium supplement, 500 to 1,000 mg daily, will probably need to be prescribed.
Discontinue leuprolide treatment. Typically, menses and ovarian estrogen production resume once leuprolide is stopped, and bone mass begins to recover. If pain recurs, the patient could be treated with cyclic or continuous estrogen-progestin contraceptives, depot-subQ Provera, a progestinreleasing intrauterine device, or a second laparoscopy procedure.
Continue leuprolide therapy and initiate steroid add-back treatment. Options shown to be effective in preserving bone mass in young women taking a gonadotropin-releasing hormone (GnRH) analogue include:
- norethindrone acetate, 5 mg daily,
- conjugated equine estrogen (Premarin), 0.3 or 0.625 mg daily, plus a progestin, and
- low-dose transdermal estrogen, 25 μg daily, plus a progestin.
Key trials
Lupron Add-Back Study. Women with endometriosis and chronic pelvic pain were randomly assigned to 1 of 4 treatment groups:
- leuprolide alone,
- leuprolide plus an oral synthetic progestin, norethindrone acetate, 5 mg daily,
- leuprolide plus “low-dose” conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, or
- leuprolide plus “high-dose” conjugated equine estrogen, 1.25 mg, plus norethindrone acetate, 5 mg daily.4
Women in all 4 groups received depotleuprolide, 3.75 mg intramuscularly every 4 weeks for 1 year. Over 1 year of treatment, BMD decreased significantly in the women who received the GnRH agonist alone. Bone density was preserved in the 3 groups that received steroid add-back.
Vasomotor symptoms were significantly reduced in all 3 groups receiving steroid add-back therapy, compared with the placebo group. However, more women in the group that received the larger dose of estrogen dropped out of the study than from the other groups because of more significant pelvic pain.
In summary, norethindrone, 5 mg daily, or low-dose conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, were both effective steroid add-back regimens for prevention of bone loss in young women with endometriosis being treated with long-term GnRH analogues (TABLE 1).
GnRH analogue vs GnRH plus estradiolprogestin. In another trial, women who experienced endometriosis and pelvic pain were randomized to receive a GnRH agonist alone or a GnRH agonist plus low-dose transdermal estradiol, 25 μg daily, plus medroxyprogesterone acetate, 2.5 mg daily, for 6 months. Women in both groups had similar improvement in pelvic pain symptoms and a similar decrease in endometriosis surgical staging scores as determined by laparoscopy before and after treatment. However, the women who received the GnRH agonist alone, without add-back therapy, had more vasomotor symptoms and a greater decline in BMD than the women who received the GnRH agonist plus low-dose transdermal estradiol plus progestin.5
This study supports the estrogenthreshold hypothesis6 that there is a “sweet spot” in estradiol concentration where vasomotor symptoms and bone loss can be attenuated, but where endometriosis lesion activity is suppressed. This “sweet spot” appears to be at an estradiol concentration of about 30 pg/mL (FIGURE 2).
GnRH analogue vs GnRH plus teriparatide. A synthetic parathyroid analogue (PTH 1-34, teriparatide, Forteo) has been demonstrated to prevent bone loss in hypoestrogenic women. In this study, women with pelvic pain and endometriosis were randomized to treatment with a GnRH agonist alone or a GnRH agonist plus teriparatide for 6 months. Women receiving the GnRH agonist alone had a decrease in BMD at the spine of 3.5%, when measured in the lateral plane. Women receiving combined treatment had an increase in BMD at the spine of 3.4%.7
The Food and Drug Administration (FDA) has approved teriparatide at a dose of 20 μg daily by subcutaneous injection for the treatment of osteoporosis. A major advantage of PTH is that it appears to stimulate both osteoblasts and osteoclasts. In contrast, estrogen may preferentially block osteoclast activity without significantly stimulating osteoblasts. A major disadvantage of PTH: It is currently available only as a daily injection. It also is more expensive than estrogen or a synthetic progestin.
CASE 1 MANAGEMENT
A teen with pelvic pain, bone loss
Long-term combined therapy improved her BMD
This patient strongly preferred to continue the leuprolide treatment because it was so effective in treating her pelvic pain. She continued her leuprolide treatment and started norethindrone acetate 5 mg daily. She reported continued excellent control of her pelvic pain on the combined regimen of leuprolide plus norethindrone acetate. Follow-up bone density measurement demonstrated significant improvement. She continues on long-term combined therapy and is scheduled for annual BMD measurements.
CASE 2 HISTORY
The female athlete triad
A college-age dancer with an eating disorder
A 21-year-old elite ballet dancer, height 5’4” and weight 104 lb (BMI 17.8 kg/m2), presents with a history of a stress fracture of her foot. Besides being amenorrheic, she is on a high-fiber macrobiotic diet. DXA measurement demonstrates a Z score consistent with osteoporosis. She refuses to take estrogen-progestin contraceptives because she claims they impair her ability to train for her dance performances. She wonders if she should start alendronate therapy.
The female athlete triad is the combination of amenorrhea, disordered eating, and osteoporosis.8 In young female athletes, exercise and disordered eating may cause menstrual irregularity, and either disordered eating or menstrual irregularity, or both, may cause decreased BMD.9
Strenuous exercise has divergent effects on trabecular and cortical bone. By causing hypoestrogenism, strenuous exercise decreases bone density at cortical sites (vertebral spine). However, the weight-bearing exercise itself can lead to an increase in bone density at weight-bearing sites.10
Many elite athletes with the female athlete triad do not want to alter the intensity of their training or their diet, which might result in weight gain and resolution of the osteoporosis. Some elite athletes report that they do not want to take standard birth control pills because they believe the pill may impair their training and peak competitive performance. The clinician is often challenged by these strongly held beliefs to identify a treatment plan that will reverse the bone loss.
Is systemic disease involved? Occasionally, systemic disease causes or contributes to low bone mass in a young woman. Laboratory tests may help in screening some women for these diseases (TABLE 2).
FIGURE 2 Aim for the “sweet spot” in estradiol concentrations
According to the estrogen-threshold hypothesis, there is a range, or “sweet spot,” of estradiol concentrations where endometriosis lesion activity is suppressed and vasomotor symptoms and bone loss are not excessive. In humans, estrogen concentrations in the range of 30 pg/mL appear to be associated with decreased endometriosis pain and minimal degrees of bone loss. At estradiol levels of less than 10 pg/mL, bone loss is markedly accelerated.6 Reprinted from Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740–745. ©1992 with permission from Elsevier.TABLE 1
Effective steroid hormone add-back regimens
Regimens that suppressed vasomotor symptoms and bone loss associated with long-term GnRH analogue use
| INVESTIGATOR | RANDOMIZATION SCHEME | STEROID HORMONE ADD-BACK REGIMEN |
|---|---|---|
| Howell et al5 | 50 women with endometriosis and pelvic pain randomized to GnRH agonist alone or GnRH agonist plus add-back regimen | Transdermal estradiol, 25 μg/d, plus oral medroxyprogesterone acetate, 2.5 mg/d |
| Lupron Add-Back Study Group4 | 201 women with endometriosis and pelvic pain randomized to GnRH agonist plus placebo or GnRH agonist plus 1 of 3 add-back regimens | Oral norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 0.625 mg/d plus norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 1.25 mg/d plus norethindrone, 5 mg/d |
TABLE 2
Screening for secondary causes of bone loss
| The following serum tests may be useful in screening women with osteoporosis for secondary causes of disordered bone metabolism |
| Calcium and albumin |
| Phosphorus |
| 25-Hydroxy vitamin D |
| Thyroid-stimulating hormone |
| Alkaline phosphatase |
| Creatinine |
Treatment of elite athletes
Peak bone mass depends on many factors, including genetics11; ovarian estrogen production12; adequate intake of calories, protein, vitamin D, and calcium9; exercise13; and achievement of target height and weight.14 When combinations of risk factors for osteoporosis are present, such as an eating disorder plus amenorrhea, correction of only one factor may not result in improved BMD.15
There are few clinical trials of the treatment of osteoporosis in women with the female athlete triad or an eating disorder. Treatment recommendations are largely based on clinical experience. If the woman resists lifestyle and dietary changes that will result in weight gain, other treatment recommendations to pursue include:
- vitamin D and calcium supplements,
- increased dietary protein,
- weight training,16 and
- smoking cessation.
Psychological counseling may help many young women with disordered eating.
Hormonal therapy has not been documented to reliably increase bone density in young amenorrheic women with an eating disorder. In 3 clinical trials, the effect of estrogen replacement on bone density in such patients was negligible or minimal.17-19 Given our understanding of the positive effect of estrogen on spinal BMD, it is difficult to understand these findings. Two possibilities are that compliance with estrogen replacement was modest because the women did not want a return of menses, or an insufficient dose of estrogen was prescribed. Another is that being underweight blocks the positive effect of estrogen on bone density. One small clinical trial did report that treatment of hypoestrogenic women with an estrogen-progestin contraceptive containing 35 μg of ethinyl estradiol resulted in a significant increase in lumbar spine BMD (5.4%) and a nonsignificant increase in femur BMD (3.6%).20 A logical recommendation, based on clinical experience, would be to prescribe an estrogen-progestin contraceptive to this woman to help preserve or improve her bone density.
Bisphosphonate therapy has been studied in small groups of women with disordered eating and osteoporosis. In one small clinical trial, 32 women with anorexia nervosa were given 10 mg alendronate daily or placebo for 1 year. Alendronate treatment was associated with a nonsignificant increase in femoral neck and spine bone density of 4.4% and 3.5%, respectively. Weight gain was an important predictor of improved BMD.21
The young woman in this case probably should not be prescribed alendronate. The FDA drug information for alendronate warns: “Safety and efficacy have not been established in pregnant women. Animal studies have shown delays in delivery and fetal/neonatal death (secondary to hypocalcemia). Bisphosphonates are incorporated into the bone matrix and gradually released over time. Theoretically, there may be a risk of fetal harm when pregnancy follows the completion of therapy. Based on limited case reports with pamidronate, serum calcium levels in the newborn may be altered if administered during pregnancy.”
Although there are minimal data in humans that alendronate will have adverse effects on fetal bone development and function, findings in rats treated with very high doses of alendronate leave some concern about the potential risk for human fetuses.
The young woman in this case may want to have children in the near future. Bisphosphonates may be incorporated into the bone and have a long residual half-life, resulting in potential exposure of the fetus to low doses of the compound. Very few malformations in human pregnancy have been reported after treatment with bisphosphonates.22 Based on a cautious approach to this situation, however, I would recommend that this woman of reproductive age who plans future child-bearing probably should not be treated with alendronate.
CASE 2 MANAGEMENT
The female athlete triad
Estrogen-progestin ring and mental health evaluation
Many women with the female athlete triad do not meet formal criteria for anorexia nervosa, but have disordered eating patterns. Questions in the medical history such as “Do you think you should be dieting?”23 may help initiate a conversation about eating practices and attitudes. The woman in Case 2 was screened for clinical depression. She reported that she was not blue over the past 2 weeks, but noted that she worried that she measured too much of her self-worth by her body image—a diagnostic criterion for anorexia nervosa. After extensive counseling, she was willing to start an estrogen-progestin contraceptive ring. She was also referred to a mental health provider for further evaluation.
CASE 3 HISTORY
Glucocorticoid-related bone loss
A 35-year-old woman with rheumatoid arthritis
This woman (G1P1) has been treated with various doses of prednisone over the past 10 years. Currently she is taking prednisone, 20 mg daily. She is oligomenorrheic with menstrual cycles every 45 days. She has undergone both bilateral hip replacement and bilateral tubal ligation. Her BMI is 20.5 kg/m2. DXA shows osteoporosis at her lumbar spine.
Glucocorticoids are a common cause of osteoporosis in young women. These drugs inhibit osteoblast activity and increase osteoclast activity. They also increase renal calcium excretion and decrease intestinal calcium absorption and adrenal androgen production. A meta-analysis of 89 publications reported that chronic glucocorticoid use increased both the risk of osteoporosis and clinically significant fractures.24 Chronic glucocorticoid treatment was associated with an increased relative risk of vertebral and hip fracture of 2.60 (95% confidence interval [CI], 2.31–2.92) and 1.61 (95% CI, 1.47–1.76), respectively. Daily doses of prednisone 10 mg or greater were clearly associated with an increased risk of fracture. The onset of the increased fracture risk occurred within 3 months of initiating therapy.
Treatment
Treatment for this woman should include:
- vitamin D and calcium supplementation,25 and
- consideration of estrogen-progestin therapy26 and/or bisphosphonates.
PTH analogue treatment could also be utilized if neither estrogen replacement nor bisphosphonate treatment were possible.27 If the patient could discontinue the glucocorticoid therapy and begin another treatment for rheumatoid arthritis, such as etanercept (Enbrel), her BMD might improve.
Bisphosphonates are a first-line option, since she has had a bilateral tubal ligation and is at very low risk for a future pregnancy. Many clinical trials demonstrate the efficacy of bisphosphonates in this clinical situation. Bisphosphonates are approved by the FDA for the prevention and treatment of osteoporosis in women receiving glucocorticoids.
In a study of alendronate, the rate of new vertebral fractures was 0.7% in the alendronate group and 6.8% in the placebo group.28 Subjects who were taking at least 7.5 mg prednisone daily were randomized to receive alendronate, 5 or 10 mg daily, or a placebo. After 2 years of treatment, there was a 3.7% increase in lumbar spine BMD in the group treated with alendronate, 10 mg daily, and a 0.8% loss in bone denisty in the placebo group (FIGURE 3).
In a study of risedronate, 5 mg daily versus placebo, bisphosphonate treatment was associated with a 70% decrease in fracture risk.29
These clinical trials were completed before the development of once-weekly bisphosphonate treatment. Now that such treatment is available, it makes sense to prescribe either alendronate, 70 mg once weekly, or risedronate, 35 mg once weekly. Ibandronate, 150 mg once monthly, is also likely to be effective.
FIGURE 3 Alendronate improves BMD in women taking glucocorticoids
Effects of 2 years of alendronate treatment on bone mineral density of the lumbar spine in patients receiving average daily doses of prednisone of at least 7.5 mg. Alendronate treatment significantly improved bone mineral density (P<.0001). *Alendronate 2.5 mg was switched to 10 mg after it was determined that 2.5 mg was a suboptimal dose.
28 From Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, doubleblind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. Copyright ©2001. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Do US women get adequate treatment?
Surveys of community-based practices report that approximately 40% of women taking chronic glucocorticoid therapy are not receiving any intervention to prevent osteoporosis.30
Gynecologists are well positioned to ensure that these young women are fully apprised of their options and receive appropriate preventive intervention.
CASE 3 MANAGEMENT
Glucocorticoid-related bone loss
Estrogen-progestin patch, a bisphosphonate, and a rheumatology consult
This patient was started on an estrogen-progestin contraceptive patch and a bisphosphonate. An annual BMD measurement is planned.
In addition, a rheumatologist was consulted to determine if etanercept (Enbrel) could be initiated for the treatment of her rheumatoid arthritis, with a concomitant reduction in her prednisone dosage.
SUMMARYAim treatment toward future bone health
Many controversies remain unresolved concerning the best approach to bone loss in young women. On the one hand, most young women with osteoporosis are at low risk of vertebral and hip fracture, so a large number would need to be treated to prevent one fracture. On the other hand, most hope to become middle-aged; if young women begin adult life with osteoporosis, it is possible that they will have a poor foundation upon which to achieve optimal bone health. Until this key issue is resolved, it is probably best to try to help young women with osteoporosis achieve the best bone health possible. Bone health in young women is likely to be the foundation determining their future bone health and fracture risk.
- In young women, many cases of osteoporosis are caused by hypoestrogenism resulting from hormone treatment (eg, GnRH agonists, aromatase inhibitors) or lifestyle adaptations (elite athletics, eating disorders).
- Treatment of osteoporosis in young women can often be successful with the use of estrogen or “androgenic” progestins.
- Chronic glucocorticoid treatment is a common cause of clinically significant osteoporosis in young women. Glucocorticoid-induced osteoporosis is an important cause of premenopausal osteoporotic fractures.
- ObGyns play a key role in ensuring that women enter midlife with strong bones. A focus on young women at very high risk for osteoporosis will help to ensure that our patients build their future bone health on a strong foundation.
ObGyns are very well trained to diagnose and treat women with osteoporosis, most of whom are perimenopausal and menopausal. We are also treating a significant number of young women at risk for osteoporosis because of lifestyle choices or medical treatment of endometriosis or rheumatic diseases. Treatment of this population poses unique challenges and requires specialized approaches.
An important caveat in any discussion of bone loss in young women: Few randomized clinical trials have assessed the efficacy of the various treatments available. In most treatment studies of osteoporosis in young women, bone mineral density (BMD)—an intermediate biometric endpoint—is the primary treatment outcome. In contrast, in the best studies in the menopausal population, the primary treatment outcome is bone fracture—a clinically important endpoint.
In addition, fewer consensus recommendations are available on the management of osteoporosis in young women than for its treatment in menopausal women. In this article, 3 different kinds of cases of bone loss in young women are used to develop key clinical points.
CASE 1 HISTORY
A teen with pelvic pain, bone loss
Therapy eased pain but decreased BMD
A 19-year-old woman has a 3-year history of severe, disabling dysmenorrhea. For 2 years she was treated with nonsteroidal anti-inflammatory drugs and cyclic estrogen-progestin contraceptives before switching to continuous oral contraceptives. These interventions did not relieve her pain. When she was 18, laparoscopy revealed stage I endometriosis, which was resected, providing 6 months of relief. When her pain recurred, the patient was started on leuprolide acetate depot, which caused amenorrhea and provided excellent pain relief. The patient said the leuprolide had “given back her life”. After 6 months of leuprolide therapy, a DXA bone scan demonstrated osteoporosis with a lumbar spine Z score of–2.6.
What treatment do you recommend?
Under age 25, use Z score, not T score
The diagnosis of osteoporosis in very young women is complex and continues to evolve. Physicians have been educated to use the T score (comparison to the mean peak bone mass of young adults) to assess BMD in menopausal women, in whom a decrease in T score of 1 standard deviation is associated with a 2- to 4-fold increase in fracture risk. However, teenage women are often still gaining bone mineral mass as the skeleton develops, so their T scores are normally below that of the peak bone mass of an adult woman. The Z score gives a good comparison of their bone mass with that of teens of the same sex at a similar developmental stage. Most experts agree that, when using DXA test results to assess bone density in women younger than 25, the Z score should be used.1 In one recent study, peak bone mass in the spine was achieved at approximately 23 years of age.2 As women reach age 25, the T and Z scores converge (FIGURE 1).
FIGURE 1 The Z score is more informative when the patient is under 25
The Z score compares the patient with persons of the same age and sex. The T score compares the patient with young normal adults of the same sex. Since bone mass increases until approximately age 20 to 25 years, it is best to use the Z score to evaluate the bone mineral density of women younger than 25 years.1 Reprinted from Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253–257. ©2004 with permission from Elsevier.
Treatment
For this patient with osteoporosis, there are several approaches to treatment:
Ensure adequate vitamin D and calcium. Vitamin D should be prescribed in doses of 400 to 800 IU daily. A recent meta-analysis reported that 800 IU vitamin D daily appears to provide better protection against osteoporotic fractures than 400 IU in menopausal women.3 Consider measuring 25-hydroxy vitamin D levels to assess the patient’s stores of this important pre-vitamin. Many young women are vitamin D-deficient.
Calcium intake should be in the range of 1,500 mg daily. To achieve this level of calcium intake, a calcium supplement, 500 to 1,000 mg daily, will probably need to be prescribed.
Discontinue leuprolide treatment. Typically, menses and ovarian estrogen production resume once leuprolide is stopped, and bone mass begins to recover. If pain recurs, the patient could be treated with cyclic or continuous estrogen-progestin contraceptives, depot-subQ Provera, a progestinreleasing intrauterine device, or a second laparoscopy procedure.
Continue leuprolide therapy and initiate steroid add-back treatment. Options shown to be effective in preserving bone mass in young women taking a gonadotropin-releasing hormone (GnRH) analogue include:
- norethindrone acetate, 5 mg daily,
- conjugated equine estrogen (Premarin), 0.3 or 0.625 mg daily, plus a progestin, and
- low-dose transdermal estrogen, 25 μg daily, plus a progestin.
Key trials
Lupron Add-Back Study. Women with endometriosis and chronic pelvic pain were randomly assigned to 1 of 4 treatment groups:
- leuprolide alone,
- leuprolide plus an oral synthetic progestin, norethindrone acetate, 5 mg daily,
- leuprolide plus “low-dose” conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, or
- leuprolide plus “high-dose” conjugated equine estrogen, 1.25 mg, plus norethindrone acetate, 5 mg daily.4
Women in all 4 groups received depotleuprolide, 3.75 mg intramuscularly every 4 weeks for 1 year. Over 1 year of treatment, BMD decreased significantly in the women who received the GnRH agonist alone. Bone density was preserved in the 3 groups that received steroid add-back.
Vasomotor symptoms were significantly reduced in all 3 groups receiving steroid add-back therapy, compared with the placebo group. However, more women in the group that received the larger dose of estrogen dropped out of the study than from the other groups because of more significant pelvic pain.
In summary, norethindrone, 5 mg daily, or low-dose conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, were both effective steroid add-back regimens for prevention of bone loss in young women with endometriosis being treated with long-term GnRH analogues (TABLE 1).
GnRH analogue vs GnRH plus estradiolprogestin. In another trial, women who experienced endometriosis and pelvic pain were randomized to receive a GnRH agonist alone or a GnRH agonist plus low-dose transdermal estradiol, 25 μg daily, plus medroxyprogesterone acetate, 2.5 mg daily, for 6 months. Women in both groups had similar improvement in pelvic pain symptoms and a similar decrease in endometriosis surgical staging scores as determined by laparoscopy before and after treatment. However, the women who received the GnRH agonist alone, without add-back therapy, had more vasomotor symptoms and a greater decline in BMD than the women who received the GnRH agonist plus low-dose transdermal estradiol plus progestin.5
This study supports the estrogenthreshold hypothesis6 that there is a “sweet spot” in estradiol concentration where vasomotor symptoms and bone loss can be attenuated, but where endometriosis lesion activity is suppressed. This “sweet spot” appears to be at an estradiol concentration of about 30 pg/mL (FIGURE 2).
GnRH analogue vs GnRH plus teriparatide. A synthetic parathyroid analogue (PTH 1-34, teriparatide, Forteo) has been demonstrated to prevent bone loss in hypoestrogenic women. In this study, women with pelvic pain and endometriosis were randomized to treatment with a GnRH agonist alone or a GnRH agonist plus teriparatide for 6 months. Women receiving the GnRH agonist alone had a decrease in BMD at the spine of 3.5%, when measured in the lateral plane. Women receiving combined treatment had an increase in BMD at the spine of 3.4%.7
The Food and Drug Administration (FDA) has approved teriparatide at a dose of 20 μg daily by subcutaneous injection for the treatment of osteoporosis. A major advantage of PTH is that it appears to stimulate both osteoblasts and osteoclasts. In contrast, estrogen may preferentially block osteoclast activity without significantly stimulating osteoblasts. A major disadvantage of PTH: It is currently available only as a daily injection. It also is more expensive than estrogen or a synthetic progestin.
CASE 1 MANAGEMENT
A teen with pelvic pain, bone loss
Long-term combined therapy improved her BMD
This patient strongly preferred to continue the leuprolide treatment because it was so effective in treating her pelvic pain. She continued her leuprolide treatment and started norethindrone acetate 5 mg daily. She reported continued excellent control of her pelvic pain on the combined regimen of leuprolide plus norethindrone acetate. Follow-up bone density measurement demonstrated significant improvement. She continues on long-term combined therapy and is scheduled for annual BMD measurements.
CASE 2 HISTORY
The female athlete triad
A college-age dancer with an eating disorder
A 21-year-old elite ballet dancer, height 5’4” and weight 104 lb (BMI 17.8 kg/m2), presents with a history of a stress fracture of her foot. Besides being amenorrheic, she is on a high-fiber macrobiotic diet. DXA measurement demonstrates a Z score consistent with osteoporosis. She refuses to take estrogen-progestin contraceptives because she claims they impair her ability to train for her dance performances. She wonders if she should start alendronate therapy.
The female athlete triad is the combination of amenorrhea, disordered eating, and osteoporosis.8 In young female athletes, exercise and disordered eating may cause menstrual irregularity, and either disordered eating or menstrual irregularity, or both, may cause decreased BMD.9
Strenuous exercise has divergent effects on trabecular and cortical bone. By causing hypoestrogenism, strenuous exercise decreases bone density at cortical sites (vertebral spine). However, the weight-bearing exercise itself can lead to an increase in bone density at weight-bearing sites.10
Many elite athletes with the female athlete triad do not want to alter the intensity of their training or their diet, which might result in weight gain and resolution of the osteoporosis. Some elite athletes report that they do not want to take standard birth control pills because they believe the pill may impair their training and peak competitive performance. The clinician is often challenged by these strongly held beliefs to identify a treatment plan that will reverse the bone loss.
Is systemic disease involved? Occasionally, systemic disease causes or contributes to low bone mass in a young woman. Laboratory tests may help in screening some women for these diseases (TABLE 2).
FIGURE 2 Aim for the “sweet spot” in estradiol concentrations
According to the estrogen-threshold hypothesis, there is a range, or “sweet spot,” of estradiol concentrations where endometriosis lesion activity is suppressed and vasomotor symptoms and bone loss are not excessive. In humans, estrogen concentrations in the range of 30 pg/mL appear to be associated with decreased endometriosis pain and minimal degrees of bone loss. At estradiol levels of less than 10 pg/mL, bone loss is markedly accelerated.6 Reprinted from Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740–745. ©1992 with permission from Elsevier.TABLE 1
Effective steroid hormone add-back regimens
Regimens that suppressed vasomotor symptoms and bone loss associated with long-term GnRH analogue use
| INVESTIGATOR | RANDOMIZATION SCHEME | STEROID HORMONE ADD-BACK REGIMEN |
|---|---|---|
| Howell et al5 | 50 women with endometriosis and pelvic pain randomized to GnRH agonist alone or GnRH agonist plus add-back regimen | Transdermal estradiol, 25 μg/d, plus oral medroxyprogesterone acetate, 2.5 mg/d |
| Lupron Add-Back Study Group4 | 201 women with endometriosis and pelvic pain randomized to GnRH agonist plus placebo or GnRH agonist plus 1 of 3 add-back regimens | Oral norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 0.625 mg/d plus norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 1.25 mg/d plus norethindrone, 5 mg/d |
TABLE 2
Screening for secondary causes of bone loss
| The following serum tests may be useful in screening women with osteoporosis for secondary causes of disordered bone metabolism |
| Calcium and albumin |
| Phosphorus |
| 25-Hydroxy vitamin D |
| Thyroid-stimulating hormone |
| Alkaline phosphatase |
| Creatinine |
Treatment of elite athletes
Peak bone mass depends on many factors, including genetics11; ovarian estrogen production12; adequate intake of calories, protein, vitamin D, and calcium9; exercise13; and achievement of target height and weight.14 When combinations of risk factors for osteoporosis are present, such as an eating disorder plus amenorrhea, correction of only one factor may not result in improved BMD.15
There are few clinical trials of the treatment of osteoporosis in women with the female athlete triad or an eating disorder. Treatment recommendations are largely based on clinical experience. If the woman resists lifestyle and dietary changes that will result in weight gain, other treatment recommendations to pursue include:
- vitamin D and calcium supplements,
- increased dietary protein,
- weight training,16 and
- smoking cessation.
Psychological counseling may help many young women with disordered eating.
Hormonal therapy has not been documented to reliably increase bone density in young amenorrheic women with an eating disorder. In 3 clinical trials, the effect of estrogen replacement on bone density in such patients was negligible or minimal.17-19 Given our understanding of the positive effect of estrogen on spinal BMD, it is difficult to understand these findings. Two possibilities are that compliance with estrogen replacement was modest because the women did not want a return of menses, or an insufficient dose of estrogen was prescribed. Another is that being underweight blocks the positive effect of estrogen on bone density. One small clinical trial did report that treatment of hypoestrogenic women with an estrogen-progestin contraceptive containing 35 μg of ethinyl estradiol resulted in a significant increase in lumbar spine BMD (5.4%) and a nonsignificant increase in femur BMD (3.6%).20 A logical recommendation, based on clinical experience, would be to prescribe an estrogen-progestin contraceptive to this woman to help preserve or improve her bone density.
Bisphosphonate therapy has been studied in small groups of women with disordered eating and osteoporosis. In one small clinical trial, 32 women with anorexia nervosa were given 10 mg alendronate daily or placebo for 1 year. Alendronate treatment was associated with a nonsignificant increase in femoral neck and spine bone density of 4.4% and 3.5%, respectively. Weight gain was an important predictor of improved BMD.21
The young woman in this case probably should not be prescribed alendronate. The FDA drug information for alendronate warns: “Safety and efficacy have not been established in pregnant women. Animal studies have shown delays in delivery and fetal/neonatal death (secondary to hypocalcemia). Bisphosphonates are incorporated into the bone matrix and gradually released over time. Theoretically, there may be a risk of fetal harm when pregnancy follows the completion of therapy. Based on limited case reports with pamidronate, serum calcium levels in the newborn may be altered if administered during pregnancy.”
Although there are minimal data in humans that alendronate will have adverse effects on fetal bone development and function, findings in rats treated with very high doses of alendronate leave some concern about the potential risk for human fetuses.
The young woman in this case may want to have children in the near future. Bisphosphonates may be incorporated into the bone and have a long residual half-life, resulting in potential exposure of the fetus to low doses of the compound. Very few malformations in human pregnancy have been reported after treatment with bisphosphonates.22 Based on a cautious approach to this situation, however, I would recommend that this woman of reproductive age who plans future child-bearing probably should not be treated with alendronate.
CASE 2 MANAGEMENT
The female athlete triad
Estrogen-progestin ring and mental health evaluation
Many women with the female athlete triad do not meet formal criteria for anorexia nervosa, but have disordered eating patterns. Questions in the medical history such as “Do you think you should be dieting?”23 may help initiate a conversation about eating practices and attitudes. The woman in Case 2 was screened for clinical depression. She reported that she was not blue over the past 2 weeks, but noted that she worried that she measured too much of her self-worth by her body image—a diagnostic criterion for anorexia nervosa. After extensive counseling, she was willing to start an estrogen-progestin contraceptive ring. She was also referred to a mental health provider for further evaluation.
CASE 3 HISTORY
Glucocorticoid-related bone loss
A 35-year-old woman with rheumatoid arthritis
This woman (G1P1) has been treated with various doses of prednisone over the past 10 years. Currently she is taking prednisone, 20 mg daily. She is oligomenorrheic with menstrual cycles every 45 days. She has undergone both bilateral hip replacement and bilateral tubal ligation. Her BMI is 20.5 kg/m2. DXA shows osteoporosis at her lumbar spine.
Glucocorticoids are a common cause of osteoporosis in young women. These drugs inhibit osteoblast activity and increase osteoclast activity. They also increase renal calcium excretion and decrease intestinal calcium absorption and adrenal androgen production. A meta-analysis of 89 publications reported that chronic glucocorticoid use increased both the risk of osteoporosis and clinically significant fractures.24 Chronic glucocorticoid treatment was associated with an increased relative risk of vertebral and hip fracture of 2.60 (95% confidence interval [CI], 2.31–2.92) and 1.61 (95% CI, 1.47–1.76), respectively. Daily doses of prednisone 10 mg or greater were clearly associated with an increased risk of fracture. The onset of the increased fracture risk occurred within 3 months of initiating therapy.
Treatment
Treatment for this woman should include:
- vitamin D and calcium supplementation,25 and
- consideration of estrogen-progestin therapy26 and/or bisphosphonates.
PTH analogue treatment could also be utilized if neither estrogen replacement nor bisphosphonate treatment were possible.27 If the patient could discontinue the glucocorticoid therapy and begin another treatment for rheumatoid arthritis, such as etanercept (Enbrel), her BMD might improve.
Bisphosphonates are a first-line option, since she has had a bilateral tubal ligation and is at very low risk for a future pregnancy. Many clinical trials demonstrate the efficacy of bisphosphonates in this clinical situation. Bisphosphonates are approved by the FDA for the prevention and treatment of osteoporosis in women receiving glucocorticoids.
In a study of alendronate, the rate of new vertebral fractures was 0.7% in the alendronate group and 6.8% in the placebo group.28 Subjects who were taking at least 7.5 mg prednisone daily were randomized to receive alendronate, 5 or 10 mg daily, or a placebo. After 2 years of treatment, there was a 3.7% increase in lumbar spine BMD in the group treated with alendronate, 10 mg daily, and a 0.8% loss in bone denisty in the placebo group (FIGURE 3).
In a study of risedronate, 5 mg daily versus placebo, bisphosphonate treatment was associated with a 70% decrease in fracture risk.29
These clinical trials were completed before the development of once-weekly bisphosphonate treatment. Now that such treatment is available, it makes sense to prescribe either alendronate, 70 mg once weekly, or risedronate, 35 mg once weekly. Ibandronate, 150 mg once monthly, is also likely to be effective.
FIGURE 3 Alendronate improves BMD in women taking glucocorticoids
Effects of 2 years of alendronate treatment on bone mineral density of the lumbar spine in patients receiving average daily doses of prednisone of at least 7.5 mg. Alendronate treatment significantly improved bone mineral density (P<.0001). *Alendronate 2.5 mg was switched to 10 mg after it was determined that 2.5 mg was a suboptimal dose.
28 From Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, doubleblind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. Copyright ©2001. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Do US women get adequate treatment?
Surveys of community-based practices report that approximately 40% of women taking chronic glucocorticoid therapy are not receiving any intervention to prevent osteoporosis.30
Gynecologists are well positioned to ensure that these young women are fully apprised of their options and receive appropriate preventive intervention.
CASE 3 MANAGEMENT
Glucocorticoid-related bone loss
Estrogen-progestin patch, a bisphosphonate, and a rheumatology consult
This patient was started on an estrogen-progestin contraceptive patch and a bisphosphonate. An annual BMD measurement is planned.
In addition, a rheumatologist was consulted to determine if etanercept (Enbrel) could be initiated for the treatment of her rheumatoid arthritis, with a concomitant reduction in her prednisone dosage.
SUMMARYAim treatment toward future bone health
Many controversies remain unresolved concerning the best approach to bone loss in young women. On the one hand, most young women with osteoporosis are at low risk of vertebral and hip fracture, so a large number would need to be treated to prevent one fracture. On the other hand, most hope to become middle-aged; if young women begin adult life with osteoporosis, it is possible that they will have a poor foundation upon which to achieve optimal bone health. Until this key issue is resolved, it is probably best to try to help young women with osteoporosis achieve the best bone health possible. Bone health in young women is likely to be the foundation determining their future bone health and fracture risk.
1. Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253-257.
2. Lin YC, Lyle RM, Weaver CM, et al. Peak spine and femoral neck bone mass in young women. Bone. 2003;32:546-253.
3. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation. JAMA. 2005;293:2257-2264.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
5. Howell R, Edmonds D, Dowsett M. Gonadotropin releasing hormone analogue plus hormone replacement therapy for the treatment of endometriosis: a randomized controlled trial. Fertil Steril. 1996;66:666-668.
6. Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
7. Finkelstein JS, Klibanski A, Shaefer EH, Hornstein MD, Schiff I, Neer RM. Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med. 1994;331:1618-1623.
8. Warren MP, Brooks-Gunn J, Fox RP, Holderness C, Hyle EP, Hamilton WG. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab. 2002;87:3162-3168.
9. Cobb KL, Bachrach LK, Greendale G, et al. Disordered eating, menstrual irregularity and bone mineral density in female runners. Med Sci Sports Exerc. 2003;35:711-719.
10. Young N, Formica C, Szmukler, Seeman E. Bone density at weight-bearing and non weight-bearing sites in ballet dancers: the effects of exercise, hypogonadism and body weight. J Clin Endocrinol Metab. 1994;78:449-454.
11. Young D, Hopper JL, Nowson CA, et al. Determinants of bone mineral mass in 10- to 26- year old females: a twin study. J Bone Miner Res. 1995;10:558-567.
12. Drinkwater BL, Bruemner B, Chestnut CH. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263:545-548.
13. Fehily AM, Coles RJ, Evans WD, Elwood PC. Factors affecting bone density in young adults. Am J Clin Nutr. 1992;56:579-586.
14. Lloyd T, Rollings N, Andon MB, et al. Determinants of bone density in young women. J Clin Endocrinol Metab. 1992;75:383-387.
15. Jonnavithula S, Warren MP, Fox RP, Lazaro MI. Bone mineral density is compromised in amenorrheic women despite return of menses: a 2-year study. Obstet Gynecol. 1993;81:669-674.
16. Friedlander AL, Genant HK, Sadowsky S, Byl NN, Gluer CC. A two-year program of aerobics and weight training enhances bone mineral density of young women. J Bone Mineral Res. 1995;10:574-585.
17. Klibanski A, Biller BMK, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898-904.
18. Golden NH, Lanzkowsky L, Schebendach J, et al. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15:135-143.
19. Gordon CM, Grace E, Emans SJ, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935-4941.
20. Hergenroeder AC, Smith EO, Shypailo R, et al. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone acetate or placebo over 12 months. Am J Obstet Gynecol. 1997;176:1017-1025.
21. Golden NH, Iglesias EA, Jacobsen MS, et al. Alendronate for the treatment of osteopenia in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:3179-3185.
22. Ott SM. Letter re: alendronate in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:5508.-
23. Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Phys. 2003;67:297-304.
24. van Staa TP, Leufkens HGM, Cooper C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int. 2002;13:624-629.
25. Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med. 1998;339:292-299.
26. Hall GM, Daniels M, Doyle DV, Spector TD. Effect of hormone replacement therapy on bone mass in rheumatoid arthritis patients treated with and without steroids. Arthritis Rheum. 1994;37:1499-1505.
27. Lane NE, Roe B, Genant HK, Arnaud C. Parathyroid hormone treatment reverses glucocorticoid-induced osteoporosis: results of a randomized controlled trial. J Clin Invest. 1998;102:1627-1633.
28. Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202-211.
29. Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000;15:1006-1013.
30. Yood RA, Harrold L, Fish L, et al. Prevention of glucocorticoid-induced osteoporosis: experience in a managed care setting. Arch Intern Med. 2001;161:1322-1327.
The author reports no financial relationships relevant to this article.
1. Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253-257.
2. Lin YC, Lyle RM, Weaver CM, et al. Peak spine and femoral neck bone mass in young women. Bone. 2003;32:546-253.
3. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation. JAMA. 2005;293:2257-2264.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
5. Howell R, Edmonds D, Dowsett M. Gonadotropin releasing hormone analogue plus hormone replacement therapy for the treatment of endometriosis: a randomized controlled trial. Fertil Steril. 1996;66:666-668.
6. Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
7. Finkelstein JS, Klibanski A, Shaefer EH, Hornstein MD, Schiff I, Neer RM. Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med. 1994;331:1618-1623.
8. Warren MP, Brooks-Gunn J, Fox RP, Holderness C, Hyle EP, Hamilton WG. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab. 2002;87:3162-3168.
9. Cobb KL, Bachrach LK, Greendale G, et al. Disordered eating, menstrual irregularity and bone mineral density in female runners. Med Sci Sports Exerc. 2003;35:711-719.
10. Young N, Formica C, Szmukler, Seeman E. Bone density at weight-bearing and non weight-bearing sites in ballet dancers: the effects of exercise, hypogonadism and body weight. J Clin Endocrinol Metab. 1994;78:449-454.
11. Young D, Hopper JL, Nowson CA, et al. Determinants of bone mineral mass in 10- to 26- year old females: a twin study. J Bone Miner Res. 1995;10:558-567.
12. Drinkwater BL, Bruemner B, Chestnut CH. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263:545-548.
13. Fehily AM, Coles RJ, Evans WD, Elwood PC. Factors affecting bone density in young adults. Am J Clin Nutr. 1992;56:579-586.
14. Lloyd T, Rollings N, Andon MB, et al. Determinants of bone density in young women. J Clin Endocrinol Metab. 1992;75:383-387.
15. Jonnavithula S, Warren MP, Fox RP, Lazaro MI. Bone mineral density is compromised in amenorrheic women despite return of menses: a 2-year study. Obstet Gynecol. 1993;81:669-674.
16. Friedlander AL, Genant HK, Sadowsky S, Byl NN, Gluer CC. A two-year program of aerobics and weight training enhances bone mineral density of young women. J Bone Mineral Res. 1995;10:574-585.
17. Klibanski A, Biller BMK, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898-904.
18. Golden NH, Lanzkowsky L, Schebendach J, et al. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15:135-143.
19. Gordon CM, Grace E, Emans SJ, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935-4941.
20. Hergenroeder AC, Smith EO, Shypailo R, et al. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone acetate or placebo over 12 months. Am J Obstet Gynecol. 1997;176:1017-1025.
21. Golden NH, Iglesias EA, Jacobsen MS, et al. Alendronate for the treatment of osteopenia in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:3179-3185.
22. Ott SM. Letter re: alendronate in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:5508.-
23. Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Phys. 2003;67:297-304.
24. van Staa TP, Leufkens HGM, Cooper C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int. 2002;13:624-629.
25. Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med. 1998;339:292-299.
26. Hall GM, Daniels M, Doyle DV, Spector TD. Effect of hormone replacement therapy on bone mass in rheumatoid arthritis patients treated with and without steroids. Arthritis Rheum. 1994;37:1499-1505.
27. Lane NE, Roe B, Genant HK, Arnaud C. Parathyroid hormone treatment reverses glucocorticoid-induced osteoporosis: results of a randomized controlled trial. J Clin Invest. 1998;102:1627-1633.
28. Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202-211.
29. Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000;15:1006-1013.
30. Yood RA, Harrold L, Fish L, et al. Prevention of glucocorticoid-induced osteoporosis: experience in a managed care setting. Arch Intern Med. 2001;161:1322-1327.
The author reports no financial relationships relevant to this article.
Chronic Invasive Sinus Aspergillosis in an Immunocompetent Patient
FERTILITY
- Metformin as frontline treatment for ovulation induction and other PCOS-related disorders
- New PCOS diagnostic criteria
It seems that most advances in the field of infertility entail high-tech, high-risk therapies and expensive diagnostics that are the domain of subspecialists. But this year brought compelling evidence of a better “low-tech” treatment for a common dilemma that has challenged generalists and subspecialists alike for decades: PCOS-related infertility.
A more effective primary-care based strategy is all the more welcome because the difficulties encountered in helping women with PCOS achieve pregnancy have prompted many generalists to routinely refer these patients to subspecialty care.
There’s more: The same new findings that generalists can apply to management of infertility also apply to other PCOS-caused problems: abnormal bleeding, obesity, and cosmetic concerns.
The new research on PCOS also points out our need to stay up-to-date on the current definition and diagnostic criteria for PCOS—both have changed within the past 2 years.
In addition, as a follow-up to my comments in this column a year ago: new and exciting information on oogonial stem cells, though not of immediate clinical utility, may nevertheless be of interest for patients who desire to preserve their fertility.
Try metformin first for PCOS-related infertility
Palomba S, Orio F Jr, Falbo A, et al. Prospective parallel randomized, double-blind, double-dummy controlled clinical trial comparing clomiphene citrate and metformin as the first-line treatment for ovulation induction in nonobese anovulatory women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4068–4074.
This study heralds a shift away from our typical approach. It argues that a trial of metformin for up to 6 months prior to an alternative strategy is very reasonable, and easily managed by any ObGyn.
Metformin has been utilized increasingly over the past decade to improve ovulation and conception rates in women with PCOS who wish to conceive. Traditionally, primary therapy has involved ovulation induction, initially with clomiphene citrate, frequently followed by gonadotropins due to failure of the initial therapy. Palomba and colleagues conducted the first well-designed, well-controlled head-to-head trial of metformin versus clomiphene citrate as frontline therapy to induce ovulation.
A total of 100 women with nonobese PCOS were randomly assigned to metformin (850 mg twice a day) or clomiphene citrate (150 mg on days 3–7 of each cycle) for 6 months. The main outcome measures were ovulation, pregnancy, abortion, and live-birth rates. More than 200 potential conception cycles were studied in each group.
Although metformin and clomiphene resulted in statistically similar rates of ovulation in the treatment groups (about 2 out of 3 cycles), there was a big difference in the pregnancy rates. The percycle pregnancy rate was twice as high in the metformin group (15.1% vs 7.2%, P=.009). The cumulative pregnancy rate was also far higher in the metformin group (68.9% vs 34.0%, P<.001), and the abortion rate was much lower (9.7% vs 37.5%).
Metformin’s benefits increase over time
Equally interesting was the progressive increase in both ovulation and conception rates in the metformin group during the course of the 6-month trial, compared with a progressive decrease in both the ovulation and conception rates in the clomiphene group—suggesting a cumulative benefit with ongoing metformin therapy. Although the trial was conducted in nonobese PCOS patients, it is reasonable to extrapolate the approach to the larger sub-group of women with PCOS who are obese.
Side effects. Metformin is relatively safe and well tolerated, except for a small percentage of women with intractable gastrointestinal (GI) side effects. The risk of multiple gestations does not increase.
Metformin for hirsutism and abnormal bleeding
While the Palomba study deals specifically with treatment of PCOS to induce pregnancy, the metabolic implications of inducing normal ovulation make this strategy applicable to the treatment of PCOS in women who are not attempting conception. It now appears reasonable to consider the use of metformin to help manage other issues such as hirsutism and abnormal bleeding, particularly when more conventional therapies have been insufficient.
Treatment tips
I now use metformin as first-line therapy in all patients with a confirmed diagnosis of PCOS regardless of the reason for therapy.
- Minimal pretreatment screening is appropriate to rule out thyroid or pituitary disorders, unsuspected renal disease, or actual diabetes mellitus.
- I titrate the dose over several weeks from an initial 500 mg daily with food to a target of 1,500 mg daily to help reduce GI symptoms.
- I prefer the extended-release preparation for its ease of use and (anecdotally) fewer side effects.
When to start additional therapy. If regular menses do not occur within 3 months on metformin alone, I add additional therapy as indicated.
A new way to define PCOS
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25.
The addition of ultrasonographic criteria for the diagnosis has effectively nearly doubled the prevalence of PCOS in the United States—and justifies the use of ultrasound to make the diagnosis.
In conjunction with the shift toward metformin as first-line therapy for ovulation induction in women with PCOS, it is important that ObGyns incorporate the latest clinical criteria for the diagnosis of PCOS.
3 clinical indicators
The “Rotterdam Criteria” from the 2003 Consensus Conference require 2 out of 3 clinical indicators to make the diagnosis:
- Oligo- or anovulation,
- Clinical and/or biochemical signs of hyperandrogenism, and
- Polycystic ovaries as evidenced on ultrasound or histology. Ultrasound criteria for polycystic ovaries are specific: increased stroma-to-follicle ratio with multiple subcapsular early antral follicles.
It is also important to note that the diagnosis is primarily clinical, not biochemical, thereby shifting the emphasis to history, physical examination and ultrasound. It also requires the exclusion of other endocrinologic diseases such as thyroid or prolactin disorders, Cushing’s syndrome, or adult onset congenital adrenal hyperplasia.
The addition of ultrasonographic criteria for the diagnosis has effectively nearly doubled the prevalence of PCOS in the United States—and justifies the use of ultrasound to make the diagnosis.
When to use ultrasound in the diagnosis of PCOS
If you suspect PCOS but the patient has only oligo/anovulation or evidence of hyperandrogenism, it is quite reasonable to use vaginal ultrasound to establish the diagnosis, if the strict sonographic criteria are met.
If she has both oligo/anovulation and evidence of hyperandrogenism, however, the diagnosis is established and ultrasound is not necessary.
More promise for fertility preservation
Johnson J, Bagley J, Skaznik-Wikiel M, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315.
For women interested in preserving their fertility, this finding suggests the possibility of harvesting and storing oogonial stem cells from a simple blood draw.
In a follow-up from Jonathan Tilly’s lab as reported in this column last year, this paper describes the latest advance in the oogonial stem cell story. The authors report that both bone marrow transplantation and peripheral blood transplantation restored oocytes in the ovaries of mice sterilized with chemotherapy. While the reproductive competence of the restored oocytes has not yet been determined, these findings suggest that germ line stem cells may reside in bone marrow and circulate in the blood stream.
Although a great deal of work is required to verify these findings and demonstrate them in humans, they highlight the astounding progress in the field of stem cell biology and emphasize the promise for fertility preservation in the future.
The author reports no financial relationships relevant to this article.
- Metformin as frontline treatment for ovulation induction and other PCOS-related disorders
- New PCOS diagnostic criteria
It seems that most advances in the field of infertility entail high-tech, high-risk therapies and expensive diagnostics that are the domain of subspecialists. But this year brought compelling evidence of a better “low-tech” treatment for a common dilemma that has challenged generalists and subspecialists alike for decades: PCOS-related infertility.
A more effective primary-care based strategy is all the more welcome because the difficulties encountered in helping women with PCOS achieve pregnancy have prompted many generalists to routinely refer these patients to subspecialty care.
There’s more: The same new findings that generalists can apply to management of infertility also apply to other PCOS-caused problems: abnormal bleeding, obesity, and cosmetic concerns.
The new research on PCOS also points out our need to stay up-to-date on the current definition and diagnostic criteria for PCOS—both have changed within the past 2 years.
In addition, as a follow-up to my comments in this column a year ago: new and exciting information on oogonial stem cells, though not of immediate clinical utility, may nevertheless be of interest for patients who desire to preserve their fertility.
Try metformin first for PCOS-related infertility
Palomba S, Orio F Jr, Falbo A, et al. Prospective parallel randomized, double-blind, double-dummy controlled clinical trial comparing clomiphene citrate and metformin as the first-line treatment for ovulation induction in nonobese anovulatory women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4068–4074.
This study heralds a shift away from our typical approach. It argues that a trial of metformin for up to 6 months prior to an alternative strategy is very reasonable, and easily managed by any ObGyn.
Metformin has been utilized increasingly over the past decade to improve ovulation and conception rates in women with PCOS who wish to conceive. Traditionally, primary therapy has involved ovulation induction, initially with clomiphene citrate, frequently followed by gonadotropins due to failure of the initial therapy. Palomba and colleagues conducted the first well-designed, well-controlled head-to-head trial of metformin versus clomiphene citrate as frontline therapy to induce ovulation.
A total of 100 women with nonobese PCOS were randomly assigned to metformin (850 mg twice a day) or clomiphene citrate (150 mg on days 3–7 of each cycle) for 6 months. The main outcome measures were ovulation, pregnancy, abortion, and live-birth rates. More than 200 potential conception cycles were studied in each group.
Although metformin and clomiphene resulted in statistically similar rates of ovulation in the treatment groups (about 2 out of 3 cycles), there was a big difference in the pregnancy rates. The percycle pregnancy rate was twice as high in the metformin group (15.1% vs 7.2%, P=.009). The cumulative pregnancy rate was also far higher in the metformin group (68.9% vs 34.0%, P<.001), and the abortion rate was much lower (9.7% vs 37.5%).
Metformin’s benefits increase over time
Equally interesting was the progressive increase in both ovulation and conception rates in the metformin group during the course of the 6-month trial, compared with a progressive decrease in both the ovulation and conception rates in the clomiphene group—suggesting a cumulative benefit with ongoing metformin therapy. Although the trial was conducted in nonobese PCOS patients, it is reasonable to extrapolate the approach to the larger sub-group of women with PCOS who are obese.
Side effects. Metformin is relatively safe and well tolerated, except for a small percentage of women with intractable gastrointestinal (GI) side effects. The risk of multiple gestations does not increase.
Metformin for hirsutism and abnormal bleeding
While the Palomba study deals specifically with treatment of PCOS to induce pregnancy, the metabolic implications of inducing normal ovulation make this strategy applicable to the treatment of PCOS in women who are not attempting conception. It now appears reasonable to consider the use of metformin to help manage other issues such as hirsutism and abnormal bleeding, particularly when more conventional therapies have been insufficient.
Treatment tips
I now use metformin as first-line therapy in all patients with a confirmed diagnosis of PCOS regardless of the reason for therapy.
- Minimal pretreatment screening is appropriate to rule out thyroid or pituitary disorders, unsuspected renal disease, or actual diabetes mellitus.
- I titrate the dose over several weeks from an initial 500 mg daily with food to a target of 1,500 mg daily to help reduce GI symptoms.
- I prefer the extended-release preparation for its ease of use and (anecdotally) fewer side effects.
When to start additional therapy. If regular menses do not occur within 3 months on metformin alone, I add additional therapy as indicated.
A new way to define PCOS
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25.
The addition of ultrasonographic criteria for the diagnosis has effectively nearly doubled the prevalence of PCOS in the United States—and justifies the use of ultrasound to make the diagnosis.
In conjunction with the shift toward metformin as first-line therapy for ovulation induction in women with PCOS, it is important that ObGyns incorporate the latest clinical criteria for the diagnosis of PCOS.
3 clinical indicators
The “Rotterdam Criteria” from the 2003 Consensus Conference require 2 out of 3 clinical indicators to make the diagnosis:
- Oligo- or anovulation,
- Clinical and/or biochemical signs of hyperandrogenism, and
- Polycystic ovaries as evidenced on ultrasound or histology. Ultrasound criteria for polycystic ovaries are specific: increased stroma-to-follicle ratio with multiple subcapsular early antral follicles.
It is also important to note that the diagnosis is primarily clinical, not biochemical, thereby shifting the emphasis to history, physical examination and ultrasound. It also requires the exclusion of other endocrinologic diseases such as thyroid or prolactin disorders, Cushing’s syndrome, or adult onset congenital adrenal hyperplasia.
The addition of ultrasonographic criteria for the diagnosis has effectively nearly doubled the prevalence of PCOS in the United States—and justifies the use of ultrasound to make the diagnosis.
When to use ultrasound in the diagnosis of PCOS
If you suspect PCOS but the patient has only oligo/anovulation or evidence of hyperandrogenism, it is quite reasonable to use vaginal ultrasound to establish the diagnosis, if the strict sonographic criteria are met.
If she has both oligo/anovulation and evidence of hyperandrogenism, however, the diagnosis is established and ultrasound is not necessary.
More promise for fertility preservation
Johnson J, Bagley J, Skaznik-Wikiel M, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315.
For women interested in preserving their fertility, this finding suggests the possibility of harvesting and storing oogonial stem cells from a simple blood draw.
In a follow-up from Jonathan Tilly’s lab as reported in this column last year, this paper describes the latest advance in the oogonial stem cell story. The authors report that both bone marrow transplantation and peripheral blood transplantation restored oocytes in the ovaries of mice sterilized with chemotherapy. While the reproductive competence of the restored oocytes has not yet been determined, these findings suggest that germ line stem cells may reside in bone marrow and circulate in the blood stream.
Although a great deal of work is required to verify these findings and demonstrate them in humans, they highlight the astounding progress in the field of stem cell biology and emphasize the promise for fertility preservation in the future.
The author reports no financial relationships relevant to this article.
- Metformin as frontline treatment for ovulation induction and other PCOS-related disorders
- New PCOS diagnostic criteria
It seems that most advances in the field of infertility entail high-tech, high-risk therapies and expensive diagnostics that are the domain of subspecialists. But this year brought compelling evidence of a better “low-tech” treatment for a common dilemma that has challenged generalists and subspecialists alike for decades: PCOS-related infertility.
A more effective primary-care based strategy is all the more welcome because the difficulties encountered in helping women with PCOS achieve pregnancy have prompted many generalists to routinely refer these patients to subspecialty care.
There’s more: The same new findings that generalists can apply to management of infertility also apply to other PCOS-caused problems: abnormal bleeding, obesity, and cosmetic concerns.
The new research on PCOS also points out our need to stay up-to-date on the current definition and diagnostic criteria for PCOS—both have changed within the past 2 years.
In addition, as a follow-up to my comments in this column a year ago: new and exciting information on oogonial stem cells, though not of immediate clinical utility, may nevertheless be of interest for patients who desire to preserve their fertility.
Try metformin first for PCOS-related infertility
Palomba S, Orio F Jr, Falbo A, et al. Prospective parallel randomized, double-blind, double-dummy controlled clinical trial comparing clomiphene citrate and metformin as the first-line treatment for ovulation induction in nonobese anovulatory women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4068–4074.
This study heralds a shift away from our typical approach. It argues that a trial of metformin for up to 6 months prior to an alternative strategy is very reasonable, and easily managed by any ObGyn.
Metformin has been utilized increasingly over the past decade to improve ovulation and conception rates in women with PCOS who wish to conceive. Traditionally, primary therapy has involved ovulation induction, initially with clomiphene citrate, frequently followed by gonadotropins due to failure of the initial therapy. Palomba and colleagues conducted the first well-designed, well-controlled head-to-head trial of metformin versus clomiphene citrate as frontline therapy to induce ovulation.
A total of 100 women with nonobese PCOS were randomly assigned to metformin (850 mg twice a day) or clomiphene citrate (150 mg on days 3–7 of each cycle) for 6 months. The main outcome measures were ovulation, pregnancy, abortion, and live-birth rates. More than 200 potential conception cycles were studied in each group.
Although metformin and clomiphene resulted in statistically similar rates of ovulation in the treatment groups (about 2 out of 3 cycles), there was a big difference in the pregnancy rates. The percycle pregnancy rate was twice as high in the metformin group (15.1% vs 7.2%, P=.009). The cumulative pregnancy rate was also far higher in the metformin group (68.9% vs 34.0%, P<.001), and the abortion rate was much lower (9.7% vs 37.5%).
Metformin’s benefits increase over time
Equally interesting was the progressive increase in both ovulation and conception rates in the metformin group during the course of the 6-month trial, compared with a progressive decrease in both the ovulation and conception rates in the clomiphene group—suggesting a cumulative benefit with ongoing metformin therapy. Although the trial was conducted in nonobese PCOS patients, it is reasonable to extrapolate the approach to the larger sub-group of women with PCOS who are obese.
Side effects. Metformin is relatively safe and well tolerated, except for a small percentage of women with intractable gastrointestinal (GI) side effects. The risk of multiple gestations does not increase.
Metformin for hirsutism and abnormal bleeding
While the Palomba study deals specifically with treatment of PCOS to induce pregnancy, the metabolic implications of inducing normal ovulation make this strategy applicable to the treatment of PCOS in women who are not attempting conception. It now appears reasonable to consider the use of metformin to help manage other issues such as hirsutism and abnormal bleeding, particularly when more conventional therapies have been insufficient.
Treatment tips
I now use metformin as first-line therapy in all patients with a confirmed diagnosis of PCOS regardless of the reason for therapy.
- Minimal pretreatment screening is appropriate to rule out thyroid or pituitary disorders, unsuspected renal disease, or actual diabetes mellitus.
- I titrate the dose over several weeks from an initial 500 mg daily with food to a target of 1,500 mg daily to help reduce GI symptoms.
- I prefer the extended-release preparation for its ease of use and (anecdotally) fewer side effects.
When to start additional therapy. If regular menses do not occur within 3 months on metformin alone, I add additional therapy as indicated.
A new way to define PCOS
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25.
The addition of ultrasonographic criteria for the diagnosis has effectively nearly doubled the prevalence of PCOS in the United States—and justifies the use of ultrasound to make the diagnosis.
In conjunction with the shift toward metformin as first-line therapy for ovulation induction in women with PCOS, it is important that ObGyns incorporate the latest clinical criteria for the diagnosis of PCOS.
3 clinical indicators
The “Rotterdam Criteria” from the 2003 Consensus Conference require 2 out of 3 clinical indicators to make the diagnosis:
- Oligo- or anovulation,
- Clinical and/or biochemical signs of hyperandrogenism, and
- Polycystic ovaries as evidenced on ultrasound or histology. Ultrasound criteria for polycystic ovaries are specific: increased stroma-to-follicle ratio with multiple subcapsular early antral follicles.
It is also important to note that the diagnosis is primarily clinical, not biochemical, thereby shifting the emphasis to history, physical examination and ultrasound. It also requires the exclusion of other endocrinologic diseases such as thyroid or prolactin disorders, Cushing’s syndrome, or adult onset congenital adrenal hyperplasia.
The addition of ultrasonographic criteria for the diagnosis has effectively nearly doubled the prevalence of PCOS in the United States—and justifies the use of ultrasound to make the diagnosis.
When to use ultrasound in the diagnosis of PCOS
If you suspect PCOS but the patient has only oligo/anovulation or evidence of hyperandrogenism, it is quite reasonable to use vaginal ultrasound to establish the diagnosis, if the strict sonographic criteria are met.
If she has both oligo/anovulation and evidence of hyperandrogenism, however, the diagnosis is established and ultrasound is not necessary.
More promise for fertility preservation
Johnson J, Bagley J, Skaznik-Wikiel M, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315.
For women interested in preserving their fertility, this finding suggests the possibility of harvesting and storing oogonial stem cells from a simple blood draw.
In a follow-up from Jonathan Tilly’s lab as reported in this column last year, this paper describes the latest advance in the oogonial stem cell story. The authors report that both bone marrow transplantation and peripheral blood transplantation restored oocytes in the ovaries of mice sterilized with chemotherapy. While the reproductive competence of the restored oocytes has not yet been determined, these findings suggest that germ line stem cells may reside in bone marrow and circulate in the blood stream.
Although a great deal of work is required to verify these findings and demonstrate them in humans, they highlight the astounding progress in the field of stem cell biology and emphasize the promise for fertility preservation in the future.
The author reports no financial relationships relevant to this article.
Controlling chronic hypertension in pregnancy
One unhappy effect of the obesity epidemic and the increasing age of women at childbirth is the rising prevalence of chronic hypertension, which climbed from 4.6% to 22.3% in women aged 30 to 39 years, and from 0.6% to 2.0% in women aged 18 to 29 years, according to the National Health and Nutrition Examination Survey for 1988–1991. These trends are expected to continue, and so are the rates of chronic hypertension in pregnancy, with its increased possibility of super-imposed preeclampsia.
This article outlines diagnosis and management, including:
- how to tell when drug therapy is needed
- how to detect superimposed preeclampsia
- when to discontinue drug regimens
- which drugs and doses should be used during pregnancy and after delivery.
When is hypertension “chronic”?
Hypertension is chronic if the elevated blood pressure was documented before pregnancy. If prepregnancy blood pressure is unknown, the patient is thought to have chronic hypertension if it is consistently elevated before 20 weeks of gestation.
Blood pressure is elevated if systolic pressure is at least 140 mm Hg or diastolic pressure is at least 90 mm Hg. These blood pressure ranges should be documented on at least 2 occasions at least 4 hours apart.1
Diagnosis may be difficult in women with previously undiagnosed chronic hypertension who begin prenatal care after 16 weeks’ gestation, because a physiologic decrease in blood pressure usually begins at that time. These women are more likely to be erroneously diagnosed as having gestational hypertension.2
Chronic hypertension is primary (essential) in approximately 80% to 90% of cases, and, in 10% to 20% of cases, secondary to collagen vascular disease, or renal, endocrine, or vascular disorders.
Outside of pregnancy, hypertension is categorized into 3 stages: prehypertension, stage 1 hypertension, and stage 2 hypertension.3
Mild vs severe, low-risk vs high-risk
During pregnancy, chronic hypertension is classified according to its severity, depending on the systolic and diastolic blood pressures. Systolic pressures of at least 160 mm Hg and/or diastolic pressures of at least 110 mm Hg constitute severe hypertension (Korotkoff phase V). The diagnosis requires documented evidence of hypertension before pregnancy and/or before 20 weeks’ gestation.
Korotkoff phase V readings are more precise. This phase occurs when the sound disappears, as opposed to phase IV, in which the sound is muffled. Phase V is more accurate because it correlates with actual intra-arterial pressure. Moreover, phase IV cannot be recorded in at least 10% of gravidas because of hemodynamic changes of pregnancy.
Low vs high risk. For management and counseling purposes, chronic hypertension in pregnancy also is classified as low- or high-risk (TABLE 1). A gravida has a low risk when she has mild essential hypertension without any organ involvement.
Blood pressure criteria are based on measurements at the initial visit regardless of whether the patient is taking antihypertensive drugs. For example, if the patient has blood pressure of 140/80 mm Hg and is taking antihypertensive agents, she is nevertheless classified as low-risk. Her medications are discontinued, and blood pressure is monitored very closely. If readings reach severe levels, she is then classified as high-risk and managed as such.
Risk classification may change. A woman initially classified as low-risk early in pregnancy may become high-risk if preeclampsia or severe hypertension develops.
TABLE 1
Low- and high-risk criteria
| LOW RISK | HIGH RISK |
|---|---|
| Uncomplicated essential hypertension | Secondary hypertension |
| Target organ damage* | |
| No previous perinatal loss | Previous perinatal loss |
| Systolic pressure <160 mm Hg and diastolic pressure <110 mm Hg | Maternal age >40 years |
| Systolic pressure ≥160 mm Hg or diastolic pressure ≥110 mm Hg | |
| *Left ventricular dysfunction, retinopathy, dyslipidemia, microvascular disease, or stroke. | |
Risk factors for preeclampsia
Pregnancies complicated by chronic hypertension carry a heightened risk of superimposed preeclampsia, which is associated with high rates of adverse maternal and perinatal outcomes.4 Sibai and colleagues4 documented the rate of superimposed preeclampsia among 763 women with chronic hypertension who were followed prospectively at several medical centers in the United States. The overall rate of superimposed preeclampsia was 25%.
Specific characteristics affected the risk of preeclampsia: age, previous preeclampsia, duration of hypertension, diastolic blood pressure, thrombophilia, diabetes, proteinuria, multifetal gestation, and use of assisted reproductive technology (TABLE 2).
Diagnostic criteria
In women with hypertension only, superimposed preeclampsia is diagnosed when there is proteinuria of at least 500 mg in 24 hours or thrombocytopenia or abnormal liver enzymes (TABLE 3).
In women with hypertension and proteinuria (renal disease or class F diabetes), new onset of persistent symptoms (severe headache, visual changes) and/or thrombocytopenia, and/or elevated liver enzymes makes the diagnosis of preeclampsia.
Risk of abruption and other complications
Gravidas with chronic hypertension also have an increased risk for abruptio placentae.
In addition, women with high-risk chronic hypertension are at increased risk for life-threatening maternal complications such as pulmonary edema, hypertensive encephalopathy, retinopathy, cerebral hemorrhage, and acute renal failure.5 These risks are particularly acute in women with uncontrolled severe hypertension, renal dysfunction early in pregnancy, or left ventricular dysfunction prior to conception. The risk of these and other complications increases further when superimposed preeclampsia develops (TABLE 4).
Fetal and neonatal complications in women with chronic hypertension are 3 to 4 times more likely than in the general obstetric population.1 These complications include premature delivery and small-for-gestational-age infants (TABLE 5).
Benefits vs risks of drug treatment
Although long-term blood pressure control greatly reduces stroke, cardiovascular morbidity, and mortality in nonpregnant persons,3 hypertension in pregnancy is different because the duration of therapy is shorter. In people with mild to moderate hypertension, the benefit is achieved after at least 5 years of treatment.2 In pregnancy, the benefits to the mother may not be obvious during the short time of treatment, and exposure to drugs includes both mother and fetus.6 Thus, in pregnancy, one must balance the potential short-term maternal benefits against possible short- and long-term benefits and risks to the fetus and infant.1,5,6
Low-risk: No benefit
We lack compelling evidence that shortterm antihypertensive therapy is beneficial in these women except for a reduction in the exacerbation of hypertension.5,7
High-risk: Drug therapy is needed
We lack placebo-controlled trials of antihypertensive therapy in gravidas with severe hypertension, and none are likely to be performed because of the potential risks of untreated severe hypertension.
In these women, drug therapy reduces the acute risk of stroke, congestive heart failure, and renal failure.2 Control of severe hypertension may also prolong the pregnancy and thereby improve perinatal outcome. However, there is no evidence that control of severe hypertension reduces the rates of superimposed preeclampsia or abruptio placentae.2,4,5
Adverse effects
The potential adverse effects of the most commonly prescribed antihypertensive agents are poorly established or unclearly quantified.1 In general, we have limited and selective information about teratogenicity except in laboratory animals, and minimal data on the benefits and risks of most antihypertensive drugs when used during pregnancy. Nevertheless, the limited data available suggest that some drugs carry the potential for adverse fetal effects and should be avoided (TABLE 6).
Chronic hypertension heightens risk of placental abruption
Gravidas with chronic hypertension are at increased risk for abruptio placentae, which ranges from 0.7% to 1.5% in women with mild chronic hypertension, and from 5% to 10% in women with severe or high-risk hypertension. The rate increases to 30% with superimposed preeclampsia.
Drug treatment of comorbidities
According to data from clinical trials in nonpregnant subjects, selected comorbidities can be treated as follows:
- Ischemic heart disease. Beta-blockers are the first line of treatment, particularly labetalol. Alternatively, calciumchannel blockers can be used.
- Heart failure. In asymptomatic gravidas, beta-blockers should be used. In symptomatic gravidas, both beta-blockers and diuretics are recommended.
- Diabetes. Two or more drugs are usually needed to control blood pressure in this population. Although angiotensin-converting enzyme (ACE) inhibitors have a beneficial effect outside of pregnancy, calcium-channel blockers and diuretics are safer for gravidas.
- Chronic kidney disease warrants aggressive management, typically with 3 or more medications. Again, while ACE inhibitors have a favorable effect outside of pregnancy, calcium-channel blockers, beta-blockers, and diuretics are better choices.
ACE inhibitors are contraindicated in pregnancy due to the risk of oligohydramnios, renal dysplasia, pulmonary hypoplasia, and intrauterine growth restriction.8
Management goals
The primary objectives in managing chronic hypertension in pregnancy are to reduce maternal risks and achieve optimal perinatal survival. These objectives call for a rational approach that includes:
- preconception education and counseling,
- early antenatal care,
- frequent antepartum visits to monitor both mother and fetus,
- timely delivery with intensive intrapartum monitoring, and
- proper postpartum care.
Ideally, management should begin prior to pregnancy, with extensive evaluation and a complete workup to assess the cause and severity of the hypertension, determine whether other medical illnesses are present, and rule out target organ damage associated with longstanding hypertension (TABLE 7).
TABLE 2
Characteristics that affect risk of preeclampsia
| CHARACTERISTIC | PREECLAMPSIA (%) |
|---|---|
| Age (yr) | |
| ≤35 | 26 |
| >35 | 25 |
| Previous preeclampsia | |
| Yes | 32 |
| No | 23 |
| Duration of hypertension | |
| <4 years | 23 |
| ≥4 years | 32 |
| Diastolic blood pressure (mm Hg) | |
| <100 | 24 |
| 100-109 | 25 |
| ≥110 | 40-50 |
| Thrombophilia | 40-50 |
| Diabetes mellitus | 30-40 |
| Proteinuria | |
| No | 25 |
| Yes | 27 |
| Note: Risk is also increased in women with multifetal gestation and in those who have conceived using assisted reproductive technology. | |
| Source: Sibai BM, et al4 | |
Low-risk hypertension
Stop drugs at first visit
Women with low-risk chronic hypertension without superimposed preeclampsia usually have pregnancy outcomes similar to those in the general obstetric population.2,5,9
Discontinuation of antihypertensive therapy early in pregnancy does not increase the rates of preeclampsia, abruptio placentae, and preterm delivery in these women.2,9
Our policy is to discontinue antihypertensive treatment in low-risk women at the first prenatal visit, because most of these women have good outcomes without such therapy.
Follow-up strategy
During subsequent visits, we educate the patient about nutritional requirements, weight gain, and sodium intake (maximum of 2.4 g sodium per day). We also remind them that alcohol use and smoking during pregnancy can aggravate maternal hypertension and cause adverse effects in the fetus such as fetal growth restriction and abruptio placentae.
During the remainder of the pregnancy, we observe the gravida very closely for appropriate fetal growth and early signs of preeclampsia.
Fetal evaluation should include an ultrasound examination at 16 to 20 weeks’ gestation, to be repeated at 32 to 34 weeks and monthly thereafter until term. In addition, all women with low-risk hypertension should undergo growth scans starting at 32 to 34 weeks, especially obese women in whom fundal height measurements are unreliable, because of the increased risk of intrauterine growth restriction.
If severe hypertension develops before term, start either nifedipine or labetalol (TABLE 6).
Immediate fetal testing with the nonstress test or biophysical profile is necessary if severe hypertension, preeclampsia, abnormal fetal growth, or evidence of oligohydramnios develops.
Hospitalization and delivery are necessary if severe hypertension, fetal growth restriction documented by ultrasound, or superimposed preeclampsia develops at or beyond 37 weeks.
If none of these complications is present, pregnancy can continue until 40 weeks’ gestation.5
TABLE 3
Diagnosis of preeclampsia in women with preexisting conditions
| PREEXISTING CONDITION | PREECLAMPSIA IS PRESENT IF SHE HAS… |
|---|---|
| Hypertension | Proteinuria ≥500 mg/24 hours or thrombocytopenia or abnormal liver enzymes |
| Proteinuria | New onset hypertension plus symptoms and/or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | New onset of persistent symptoms (severe headache, visual changes) or thrombocytopenia or elevated liver enzymes |
TABLE 4
Complication rates in women with superimposed preeclampsia vs women without hypertension*
| COMPLICATION | WITHOUT HYPERTENSION (PER 1,000 CASES) | PREECLAMPSIA SUPERIMPOSED ON CHRONIC HYPERTENSION (PER 1,000 CASES) |
|---|---|---|
| Abruptio placentae | 9.6 | 30.6 |
| Thrombocytopenia | 1.6 | 11.5 |
| Disseminated intravascular coagulation | 2.9 | 17.4 |
| Pulmonary edema | 0.2 | 6.4 |
| Blood transfusion | 1.5 | 16.3 |
| Mechanical ventilation | 0.2 | 17.0 |
| *US women, 1988–1997 | ||
| Source: Zhang J, et al15 | ||
High-risk hypertension
The frequency and nature of maternalfetal adverse effects depends on the cause of the hypertension and the extent of target organ damage.
Realistic preconception counseling
Women with substantial renal insufficiency (ie, serum creatinine >1.4 mg/dL), diabetes with vascular involvement (class R/F), severe collagen vascular disease, cardiomyopathy, or coarctation of the aorta should be advised that the pregnancy might exacerbate their condition. These patients should be made aware of the potential for congestive heart failure, acute renal failure requiring dialysis, and even death. In addition, perinatal loss and neonatal complications are markedly increased in these women.
Refer or consult a specialist
All women with severe hypertension should be managed in consultation with a subspecialist in maternal-fetal medicine, as well as any other specialists who may be indicated.
They also should be observed and delivered at a tertiary care center with adequate maternal-neonatal care facilities.5
TABLE 5
Adverse pregnancy outcomes in women with mild chronic hypertension
| OBSERVATIONAL STUDY | PREECLAMPSIA (%) | ABRUPTIO PLACENTAE (%) | DELIVERY AT <37 WEEKS (%) | SMALL FOR GESTATIONAL AGE (%) |
|---|---|---|---|---|
| Sibai et al2 (n=211) | 10.0 | 1.4 | 12.0 | 8.0 |
| Rey and Couturier16 (n=337) | 21.0 | 0.7 | 34.4 | 15.5 |
| McCowan et al17 (n=142) | 14.0 | Not reported | 16.0 | 11.0 |
| Sibai et al4 (n=763) | 25.0 | 1.5 | 33.3 | 11.1 |
| August et al18 (n=110) | 34.0 | Not reported | Not reported | 8.0 |
Management strategy
Our policy is to hospitalize women with high-risk hypertension at the time of the first prenatal visit to evaluate their cardiovascular and renal status and regulate antihypertensive medications, as well as other prescribed drugs (eg, insulin, cardiac drugs, thyroid drugs). Women receiving atenolol, ACE inhibitors, or angiotensin II receptor antagonists should have these medications discontinued under close observation.
In women without target organ damage, the aim of antihypertensive therapy is to keep systolic pressure between 140 and 150 mm Hg and diastolic pressure between 90 and 100 mm Hg.
In women with target organ damage and mild hypertension, antihypertensive therapy is also indicated, because there are short-term maternal benefits to lowering blood pressure. We recommend keeping systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg.
Early, frequent visits.Women with high-risk chronic hypertension need close observation throughout pregnancy and may require serial evaluation of 24-hour urine protein excretion and a complete blood count with a metabolic profile at least once every trimester. Further laboratory testing depends on the clinical progress of the pregnancy. At each visit, remind the woman about the adverse effects of smoking and alcohol use, and counsel her about the importance of diet and minimal salt intake.5
Fetal surveillance includes ultrasound, growth scans, and nonstress testing (TABLE 8).
Hospitalization is warranted if uncontrolled severe hypertension, preeclampsia, or evidence of fetal growth restriction develops, so that more frequent evaluation of maternal and fetal well-being can be performed.
Delivery is indicated if any of these complications develop at or beyond 34 weeks’ gestation. If there are none of these complications, consider delivery at 36 to 37 weeks after documenting fetal lung maturity.5
Postpartum care
Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.10,11 These risks are heightened in women with target organ involvement, superimposed preeclampsia, or abruptio placentae.10
Blood pressure must be closely controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be used as needed, and diuretics may be appropriate in women with circulatory congestion and pulmonary edema.12 Oral therapy may be needed to control blood pressure after delivery. In some women, it may be necessary to switch to a new agent such as an ACE inhibitor, particularly in women who had pregestational diabetes or cardiomyopathy.
All antihypertensive drugs are found in breast milk, although differences in the milk-to-plasma ratio do occur. The longterm effects of maternal antihypertensive drugs on breastfeeding infants has not been studied. However, methyldopa appears to be a reasonable first-line oral therapy (if it is contraindicated, use labetalol). Milk concentrations of methyldopa appear to be low and are considered safe. Beta-blockers (atenolol and metoprolol) are concentrated in breast milk, whereas labetalol or propanolol have low concentrations.13,14 Concentrations of diuretics in breast milk are low, but may diminish milk production.13 Little is known about the transfer of calcium-channel blockers to breast milk, but there are no apparent side effects. ACE inhibitors and angiotensin II receptor antagonists should be avoided because of their effects on neonatal renal function, even though their concentrations in breast milk appear to be low.
The authors report no financial relationships relevant to this article.
TABLE 6
Acute and long-term drug treatment
| DRUG | STARTING DOSE | MAXIMUM DOSE | COMMENTS |
|---|---|---|---|
| ACUTE TREATMENT OF SEVERE HYPERTENSION | |||
| Hydralazine | 5-10 mg IV every 20 min | 30 mg* | |
| Labetalol | 20-40 mg IV every 10-15 min | 220 mg* | Avoid in women with asthma or congestive heart failure |
| Nifedipine | 10-20 mg orally every 30 min | 50 mg* | |
| LONG-TERM TREATMENT OF HYPERTENSION | |||
| Methyldopa | 250 mg BID | 4 g/day | Rarely indicated |
| Labetalol | 100 mg BID | 2,400 mg/day | First choice |
| Atenolol | 50 mg/day | 100 mg/day | Associated with intrauterine growth restriction |
| Propanolol | 40 mg BID | 640 mg/day | Use with associated thyroid disease |
| Hydralazine | 10 mg TID | 100 mg/day | Use in cases of left ventricular hypertrophy |
| Nifedipine | 10 mg BID | 120 mg/day | Use in women with diabetes |
| Diltiazem | 120-180 mg/day | 540 mg/day | |
| Thiazide diuretic | 12.5 mg BID | 50 mg/day | Use in salt-sensitive hypertension and/or congestive heart failure |
| May be added as second agent | |||
| Avoid if preeclampsia develops or intrauterine growth restriction is present | |||
| Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers | — | — | Do not use after 16-18 weeks |
| *If desired blood pressure levels are not achieved, switch to another drug. | |||
TABLE 7
How to evaluate gravidas with chronic hypertension
| POPULATION | RECOMMENDED TESTS |
|---|---|
| All |
|
| Gravidas with longstanding hypertension, poor compliance, or poor control |
|
TABLE 8
Recommended antenatal testing
| LEVEL OF RISK | TEST |
|---|---|
| Low (uncomplicated) |
|
| High (complicated) |
|
1. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy:a review. Obstet Gynecol. 2000;96:849-860.
2. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
3. Chobanian AB, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289:2560-2572.
4. Sibai BM, Lindheimer M, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. N Engl J Med. 1998;339:667-671.
5. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
6. Umans JG, Lindheimer MD. Antihypertensive treatment. In:Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn:Appleton and Lange; 1998:581-604.
7. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. In: The Cochrane Library, Issue 12, 2003. Oxford:Update Software.
8. How HY, Sibai BM. Use of angiotensin-converting enzyme inhibitors in patients with diabetic nephropathy. J Matern Fetal Neonatal Med. 2002;12:402-407.
9. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
10. Sibai BM, Villar MA, Mabie BC. Acute renal failure in hypertensive disorders of pregnancy:pregnancy outcome and remote prognosis in thirty-one consecutive cases. Am J Obstet Gynecol. 1990;62:777.-
11. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women:a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
12. Lie RT, Rasmussen S, Brunborg H, et al. Fetal and maternal contributions to risk of pre-eclampsia:a population based study. Br Med J. 1998;316:1343-1347.
13. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation:A Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore:Williams & Wilkins;1998.
14. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
15. Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203-212.
16. Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol. 1994;171:410-416.
17. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
18. August P, Helseth G, Cook EF, Silson C. A prediction model for superimposed preeclampsia in women with chronic hypertension during pregnancy. Am J Obstet Gynecol. 2004;191:1666-1672.
One unhappy effect of the obesity epidemic and the increasing age of women at childbirth is the rising prevalence of chronic hypertension, which climbed from 4.6% to 22.3% in women aged 30 to 39 years, and from 0.6% to 2.0% in women aged 18 to 29 years, according to the National Health and Nutrition Examination Survey for 1988–1991. These trends are expected to continue, and so are the rates of chronic hypertension in pregnancy, with its increased possibility of super-imposed preeclampsia.
This article outlines diagnosis and management, including:
- how to tell when drug therapy is needed
- how to detect superimposed preeclampsia
- when to discontinue drug regimens
- which drugs and doses should be used during pregnancy and after delivery.
When is hypertension “chronic”?
Hypertension is chronic if the elevated blood pressure was documented before pregnancy. If prepregnancy blood pressure is unknown, the patient is thought to have chronic hypertension if it is consistently elevated before 20 weeks of gestation.
Blood pressure is elevated if systolic pressure is at least 140 mm Hg or diastolic pressure is at least 90 mm Hg. These blood pressure ranges should be documented on at least 2 occasions at least 4 hours apart.1
Diagnosis may be difficult in women with previously undiagnosed chronic hypertension who begin prenatal care after 16 weeks’ gestation, because a physiologic decrease in blood pressure usually begins at that time. These women are more likely to be erroneously diagnosed as having gestational hypertension.2
Chronic hypertension is primary (essential) in approximately 80% to 90% of cases, and, in 10% to 20% of cases, secondary to collagen vascular disease, or renal, endocrine, or vascular disorders.
Outside of pregnancy, hypertension is categorized into 3 stages: prehypertension, stage 1 hypertension, and stage 2 hypertension.3
Mild vs severe, low-risk vs high-risk
During pregnancy, chronic hypertension is classified according to its severity, depending on the systolic and diastolic blood pressures. Systolic pressures of at least 160 mm Hg and/or diastolic pressures of at least 110 mm Hg constitute severe hypertension (Korotkoff phase V). The diagnosis requires documented evidence of hypertension before pregnancy and/or before 20 weeks’ gestation.
Korotkoff phase V readings are more precise. This phase occurs when the sound disappears, as opposed to phase IV, in which the sound is muffled. Phase V is more accurate because it correlates with actual intra-arterial pressure. Moreover, phase IV cannot be recorded in at least 10% of gravidas because of hemodynamic changes of pregnancy.
Low vs high risk. For management and counseling purposes, chronic hypertension in pregnancy also is classified as low- or high-risk (TABLE 1). A gravida has a low risk when she has mild essential hypertension without any organ involvement.
Blood pressure criteria are based on measurements at the initial visit regardless of whether the patient is taking antihypertensive drugs. For example, if the patient has blood pressure of 140/80 mm Hg and is taking antihypertensive agents, she is nevertheless classified as low-risk. Her medications are discontinued, and blood pressure is monitored very closely. If readings reach severe levels, she is then classified as high-risk and managed as such.
Risk classification may change. A woman initially classified as low-risk early in pregnancy may become high-risk if preeclampsia or severe hypertension develops.
TABLE 1
Low- and high-risk criteria
| LOW RISK | HIGH RISK |
|---|---|
| Uncomplicated essential hypertension | Secondary hypertension |
| Target organ damage* | |
| No previous perinatal loss | Previous perinatal loss |
| Systolic pressure <160 mm Hg and diastolic pressure <110 mm Hg | Maternal age >40 years |
| Systolic pressure ≥160 mm Hg or diastolic pressure ≥110 mm Hg | |
| *Left ventricular dysfunction, retinopathy, dyslipidemia, microvascular disease, or stroke. | |
Risk factors for preeclampsia
Pregnancies complicated by chronic hypertension carry a heightened risk of superimposed preeclampsia, which is associated with high rates of adverse maternal and perinatal outcomes.4 Sibai and colleagues4 documented the rate of superimposed preeclampsia among 763 women with chronic hypertension who were followed prospectively at several medical centers in the United States. The overall rate of superimposed preeclampsia was 25%.
Specific characteristics affected the risk of preeclampsia: age, previous preeclampsia, duration of hypertension, diastolic blood pressure, thrombophilia, diabetes, proteinuria, multifetal gestation, and use of assisted reproductive technology (TABLE 2).
Diagnostic criteria
In women with hypertension only, superimposed preeclampsia is diagnosed when there is proteinuria of at least 500 mg in 24 hours or thrombocytopenia or abnormal liver enzymes (TABLE 3).
In women with hypertension and proteinuria (renal disease or class F diabetes), new onset of persistent symptoms (severe headache, visual changes) and/or thrombocytopenia, and/or elevated liver enzymes makes the diagnosis of preeclampsia.
Risk of abruption and other complications
Gravidas with chronic hypertension also have an increased risk for abruptio placentae.
In addition, women with high-risk chronic hypertension are at increased risk for life-threatening maternal complications such as pulmonary edema, hypertensive encephalopathy, retinopathy, cerebral hemorrhage, and acute renal failure.5 These risks are particularly acute in women with uncontrolled severe hypertension, renal dysfunction early in pregnancy, or left ventricular dysfunction prior to conception. The risk of these and other complications increases further when superimposed preeclampsia develops (TABLE 4).
Fetal and neonatal complications in women with chronic hypertension are 3 to 4 times more likely than in the general obstetric population.1 These complications include premature delivery and small-for-gestational-age infants (TABLE 5).
Benefits vs risks of drug treatment
Although long-term blood pressure control greatly reduces stroke, cardiovascular morbidity, and mortality in nonpregnant persons,3 hypertension in pregnancy is different because the duration of therapy is shorter. In people with mild to moderate hypertension, the benefit is achieved after at least 5 years of treatment.2 In pregnancy, the benefits to the mother may not be obvious during the short time of treatment, and exposure to drugs includes both mother and fetus.6 Thus, in pregnancy, one must balance the potential short-term maternal benefits against possible short- and long-term benefits and risks to the fetus and infant.1,5,6
Low-risk: No benefit
We lack compelling evidence that shortterm antihypertensive therapy is beneficial in these women except for a reduction in the exacerbation of hypertension.5,7
High-risk: Drug therapy is needed
We lack placebo-controlled trials of antihypertensive therapy in gravidas with severe hypertension, and none are likely to be performed because of the potential risks of untreated severe hypertension.
In these women, drug therapy reduces the acute risk of stroke, congestive heart failure, and renal failure.2 Control of severe hypertension may also prolong the pregnancy and thereby improve perinatal outcome. However, there is no evidence that control of severe hypertension reduces the rates of superimposed preeclampsia or abruptio placentae.2,4,5
Adverse effects
The potential adverse effects of the most commonly prescribed antihypertensive agents are poorly established or unclearly quantified.1 In general, we have limited and selective information about teratogenicity except in laboratory animals, and minimal data on the benefits and risks of most antihypertensive drugs when used during pregnancy. Nevertheless, the limited data available suggest that some drugs carry the potential for adverse fetal effects and should be avoided (TABLE 6).
Chronic hypertension heightens risk of placental abruption
Gravidas with chronic hypertension are at increased risk for abruptio placentae, which ranges from 0.7% to 1.5% in women with mild chronic hypertension, and from 5% to 10% in women with severe or high-risk hypertension. The rate increases to 30% with superimposed preeclampsia.
Drug treatment of comorbidities
According to data from clinical trials in nonpregnant subjects, selected comorbidities can be treated as follows:
- Ischemic heart disease. Beta-blockers are the first line of treatment, particularly labetalol. Alternatively, calciumchannel blockers can be used.
- Heart failure. In asymptomatic gravidas, beta-blockers should be used. In symptomatic gravidas, both beta-blockers and diuretics are recommended.
- Diabetes. Two or more drugs are usually needed to control blood pressure in this population. Although angiotensin-converting enzyme (ACE) inhibitors have a beneficial effect outside of pregnancy, calcium-channel blockers and diuretics are safer for gravidas.
- Chronic kidney disease warrants aggressive management, typically with 3 or more medications. Again, while ACE inhibitors have a favorable effect outside of pregnancy, calcium-channel blockers, beta-blockers, and diuretics are better choices.
ACE inhibitors are contraindicated in pregnancy due to the risk of oligohydramnios, renal dysplasia, pulmonary hypoplasia, and intrauterine growth restriction.8
Management goals
The primary objectives in managing chronic hypertension in pregnancy are to reduce maternal risks and achieve optimal perinatal survival. These objectives call for a rational approach that includes:
- preconception education and counseling,
- early antenatal care,
- frequent antepartum visits to monitor both mother and fetus,
- timely delivery with intensive intrapartum monitoring, and
- proper postpartum care.
Ideally, management should begin prior to pregnancy, with extensive evaluation and a complete workup to assess the cause and severity of the hypertension, determine whether other medical illnesses are present, and rule out target organ damage associated with longstanding hypertension (TABLE 7).
TABLE 2
Characteristics that affect risk of preeclampsia
| CHARACTERISTIC | PREECLAMPSIA (%) |
|---|---|
| Age (yr) | |
| ≤35 | 26 |
| >35 | 25 |
| Previous preeclampsia | |
| Yes | 32 |
| No | 23 |
| Duration of hypertension | |
| <4 years | 23 |
| ≥4 years | 32 |
| Diastolic blood pressure (mm Hg) | |
| <100 | 24 |
| 100-109 | 25 |
| ≥110 | 40-50 |
| Thrombophilia | 40-50 |
| Diabetes mellitus | 30-40 |
| Proteinuria | |
| No | 25 |
| Yes | 27 |
| Note: Risk is also increased in women with multifetal gestation and in those who have conceived using assisted reproductive technology. | |
| Source: Sibai BM, et al4 | |
Low-risk hypertension
Stop drugs at first visit
Women with low-risk chronic hypertension without superimposed preeclampsia usually have pregnancy outcomes similar to those in the general obstetric population.2,5,9
Discontinuation of antihypertensive therapy early in pregnancy does not increase the rates of preeclampsia, abruptio placentae, and preterm delivery in these women.2,9
Our policy is to discontinue antihypertensive treatment in low-risk women at the first prenatal visit, because most of these women have good outcomes without such therapy.
Follow-up strategy
During subsequent visits, we educate the patient about nutritional requirements, weight gain, and sodium intake (maximum of 2.4 g sodium per day). We also remind them that alcohol use and smoking during pregnancy can aggravate maternal hypertension and cause adverse effects in the fetus such as fetal growth restriction and abruptio placentae.
During the remainder of the pregnancy, we observe the gravida very closely for appropriate fetal growth and early signs of preeclampsia.
Fetal evaluation should include an ultrasound examination at 16 to 20 weeks’ gestation, to be repeated at 32 to 34 weeks and monthly thereafter until term. In addition, all women with low-risk hypertension should undergo growth scans starting at 32 to 34 weeks, especially obese women in whom fundal height measurements are unreliable, because of the increased risk of intrauterine growth restriction.
If severe hypertension develops before term, start either nifedipine or labetalol (TABLE 6).
Immediate fetal testing with the nonstress test or biophysical profile is necessary if severe hypertension, preeclampsia, abnormal fetal growth, or evidence of oligohydramnios develops.
Hospitalization and delivery are necessary if severe hypertension, fetal growth restriction documented by ultrasound, or superimposed preeclampsia develops at or beyond 37 weeks.
If none of these complications is present, pregnancy can continue until 40 weeks’ gestation.5
TABLE 3
Diagnosis of preeclampsia in women with preexisting conditions
| PREEXISTING CONDITION | PREECLAMPSIA IS PRESENT IF SHE HAS… |
|---|---|
| Hypertension | Proteinuria ≥500 mg/24 hours or thrombocytopenia or abnormal liver enzymes |
| Proteinuria | New onset hypertension plus symptoms and/or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | New onset of persistent symptoms (severe headache, visual changes) or thrombocytopenia or elevated liver enzymes |
TABLE 4
Complication rates in women with superimposed preeclampsia vs women without hypertension*
| COMPLICATION | WITHOUT HYPERTENSION (PER 1,000 CASES) | PREECLAMPSIA SUPERIMPOSED ON CHRONIC HYPERTENSION (PER 1,000 CASES) |
|---|---|---|
| Abruptio placentae | 9.6 | 30.6 |
| Thrombocytopenia | 1.6 | 11.5 |
| Disseminated intravascular coagulation | 2.9 | 17.4 |
| Pulmonary edema | 0.2 | 6.4 |
| Blood transfusion | 1.5 | 16.3 |
| Mechanical ventilation | 0.2 | 17.0 |
| *US women, 1988–1997 | ||
| Source: Zhang J, et al15 | ||
High-risk hypertension
The frequency and nature of maternalfetal adverse effects depends on the cause of the hypertension and the extent of target organ damage.
Realistic preconception counseling
Women with substantial renal insufficiency (ie, serum creatinine >1.4 mg/dL), diabetes with vascular involvement (class R/F), severe collagen vascular disease, cardiomyopathy, or coarctation of the aorta should be advised that the pregnancy might exacerbate their condition. These patients should be made aware of the potential for congestive heart failure, acute renal failure requiring dialysis, and even death. In addition, perinatal loss and neonatal complications are markedly increased in these women.
Refer or consult a specialist
All women with severe hypertension should be managed in consultation with a subspecialist in maternal-fetal medicine, as well as any other specialists who may be indicated.
They also should be observed and delivered at a tertiary care center with adequate maternal-neonatal care facilities.5
TABLE 5
Adverse pregnancy outcomes in women with mild chronic hypertension
| OBSERVATIONAL STUDY | PREECLAMPSIA (%) | ABRUPTIO PLACENTAE (%) | DELIVERY AT <37 WEEKS (%) | SMALL FOR GESTATIONAL AGE (%) |
|---|---|---|---|---|
| Sibai et al2 (n=211) | 10.0 | 1.4 | 12.0 | 8.0 |
| Rey and Couturier16 (n=337) | 21.0 | 0.7 | 34.4 | 15.5 |
| McCowan et al17 (n=142) | 14.0 | Not reported | 16.0 | 11.0 |
| Sibai et al4 (n=763) | 25.0 | 1.5 | 33.3 | 11.1 |
| August et al18 (n=110) | 34.0 | Not reported | Not reported | 8.0 |
Management strategy
Our policy is to hospitalize women with high-risk hypertension at the time of the first prenatal visit to evaluate their cardiovascular and renal status and regulate antihypertensive medications, as well as other prescribed drugs (eg, insulin, cardiac drugs, thyroid drugs). Women receiving atenolol, ACE inhibitors, or angiotensin II receptor antagonists should have these medications discontinued under close observation.
In women without target organ damage, the aim of antihypertensive therapy is to keep systolic pressure between 140 and 150 mm Hg and diastolic pressure between 90 and 100 mm Hg.
In women with target organ damage and mild hypertension, antihypertensive therapy is also indicated, because there are short-term maternal benefits to lowering blood pressure. We recommend keeping systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg.
Early, frequent visits.Women with high-risk chronic hypertension need close observation throughout pregnancy and may require serial evaluation of 24-hour urine protein excretion and a complete blood count with a metabolic profile at least once every trimester. Further laboratory testing depends on the clinical progress of the pregnancy. At each visit, remind the woman about the adverse effects of smoking and alcohol use, and counsel her about the importance of diet and minimal salt intake.5
Fetal surveillance includes ultrasound, growth scans, and nonstress testing (TABLE 8).
Hospitalization is warranted if uncontrolled severe hypertension, preeclampsia, or evidence of fetal growth restriction develops, so that more frequent evaluation of maternal and fetal well-being can be performed.
Delivery is indicated if any of these complications develop at or beyond 34 weeks’ gestation. If there are none of these complications, consider delivery at 36 to 37 weeks after documenting fetal lung maturity.5
Postpartum care
Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.10,11 These risks are heightened in women with target organ involvement, superimposed preeclampsia, or abruptio placentae.10
Blood pressure must be closely controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be used as needed, and diuretics may be appropriate in women with circulatory congestion and pulmonary edema.12 Oral therapy may be needed to control blood pressure after delivery. In some women, it may be necessary to switch to a new agent such as an ACE inhibitor, particularly in women who had pregestational diabetes or cardiomyopathy.
All antihypertensive drugs are found in breast milk, although differences in the milk-to-plasma ratio do occur. The longterm effects of maternal antihypertensive drugs on breastfeeding infants has not been studied. However, methyldopa appears to be a reasonable first-line oral therapy (if it is contraindicated, use labetalol). Milk concentrations of methyldopa appear to be low and are considered safe. Beta-blockers (atenolol and metoprolol) are concentrated in breast milk, whereas labetalol or propanolol have low concentrations.13,14 Concentrations of diuretics in breast milk are low, but may diminish milk production.13 Little is known about the transfer of calcium-channel blockers to breast milk, but there are no apparent side effects. ACE inhibitors and angiotensin II receptor antagonists should be avoided because of their effects on neonatal renal function, even though their concentrations in breast milk appear to be low.
The authors report no financial relationships relevant to this article.
TABLE 6
Acute and long-term drug treatment
| DRUG | STARTING DOSE | MAXIMUM DOSE | COMMENTS |
|---|---|---|---|
| ACUTE TREATMENT OF SEVERE HYPERTENSION | |||
| Hydralazine | 5-10 mg IV every 20 min | 30 mg* | |
| Labetalol | 20-40 mg IV every 10-15 min | 220 mg* | Avoid in women with asthma or congestive heart failure |
| Nifedipine | 10-20 mg orally every 30 min | 50 mg* | |
| LONG-TERM TREATMENT OF HYPERTENSION | |||
| Methyldopa | 250 mg BID | 4 g/day | Rarely indicated |
| Labetalol | 100 mg BID | 2,400 mg/day | First choice |
| Atenolol | 50 mg/day | 100 mg/day | Associated with intrauterine growth restriction |
| Propanolol | 40 mg BID | 640 mg/day | Use with associated thyroid disease |
| Hydralazine | 10 mg TID | 100 mg/day | Use in cases of left ventricular hypertrophy |
| Nifedipine | 10 mg BID | 120 mg/day | Use in women with diabetes |
| Diltiazem | 120-180 mg/day | 540 mg/day | |
| Thiazide diuretic | 12.5 mg BID | 50 mg/day | Use in salt-sensitive hypertension and/or congestive heart failure |
| May be added as second agent | |||
| Avoid if preeclampsia develops or intrauterine growth restriction is present | |||
| Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers | — | — | Do not use after 16-18 weeks |
| *If desired blood pressure levels are not achieved, switch to another drug. | |||
TABLE 7
How to evaluate gravidas with chronic hypertension
| POPULATION | RECOMMENDED TESTS |
|---|---|
| All |
|
| Gravidas with longstanding hypertension, poor compliance, or poor control |
|
TABLE 8
Recommended antenatal testing
| LEVEL OF RISK | TEST |
|---|---|
| Low (uncomplicated) |
|
| High (complicated) |
|
One unhappy effect of the obesity epidemic and the increasing age of women at childbirth is the rising prevalence of chronic hypertension, which climbed from 4.6% to 22.3% in women aged 30 to 39 years, and from 0.6% to 2.0% in women aged 18 to 29 years, according to the National Health and Nutrition Examination Survey for 1988–1991. These trends are expected to continue, and so are the rates of chronic hypertension in pregnancy, with its increased possibility of super-imposed preeclampsia.
This article outlines diagnosis and management, including:
- how to tell when drug therapy is needed
- how to detect superimposed preeclampsia
- when to discontinue drug regimens
- which drugs and doses should be used during pregnancy and after delivery.
When is hypertension “chronic”?
Hypertension is chronic if the elevated blood pressure was documented before pregnancy. If prepregnancy blood pressure is unknown, the patient is thought to have chronic hypertension if it is consistently elevated before 20 weeks of gestation.
Blood pressure is elevated if systolic pressure is at least 140 mm Hg or diastolic pressure is at least 90 mm Hg. These blood pressure ranges should be documented on at least 2 occasions at least 4 hours apart.1
Diagnosis may be difficult in women with previously undiagnosed chronic hypertension who begin prenatal care after 16 weeks’ gestation, because a physiologic decrease in blood pressure usually begins at that time. These women are more likely to be erroneously diagnosed as having gestational hypertension.2
Chronic hypertension is primary (essential) in approximately 80% to 90% of cases, and, in 10% to 20% of cases, secondary to collagen vascular disease, or renal, endocrine, or vascular disorders.
Outside of pregnancy, hypertension is categorized into 3 stages: prehypertension, stage 1 hypertension, and stage 2 hypertension.3
Mild vs severe, low-risk vs high-risk
During pregnancy, chronic hypertension is classified according to its severity, depending on the systolic and diastolic blood pressures. Systolic pressures of at least 160 mm Hg and/or diastolic pressures of at least 110 mm Hg constitute severe hypertension (Korotkoff phase V). The diagnosis requires documented evidence of hypertension before pregnancy and/or before 20 weeks’ gestation.
Korotkoff phase V readings are more precise. This phase occurs when the sound disappears, as opposed to phase IV, in which the sound is muffled. Phase V is more accurate because it correlates with actual intra-arterial pressure. Moreover, phase IV cannot be recorded in at least 10% of gravidas because of hemodynamic changes of pregnancy.
Low vs high risk. For management and counseling purposes, chronic hypertension in pregnancy also is classified as low- or high-risk (TABLE 1). A gravida has a low risk when she has mild essential hypertension without any organ involvement.
Blood pressure criteria are based on measurements at the initial visit regardless of whether the patient is taking antihypertensive drugs. For example, if the patient has blood pressure of 140/80 mm Hg and is taking antihypertensive agents, she is nevertheless classified as low-risk. Her medications are discontinued, and blood pressure is monitored very closely. If readings reach severe levels, she is then classified as high-risk and managed as such.
Risk classification may change. A woman initially classified as low-risk early in pregnancy may become high-risk if preeclampsia or severe hypertension develops.
TABLE 1
Low- and high-risk criteria
| LOW RISK | HIGH RISK |
|---|---|
| Uncomplicated essential hypertension | Secondary hypertension |
| Target organ damage* | |
| No previous perinatal loss | Previous perinatal loss |
| Systolic pressure <160 mm Hg and diastolic pressure <110 mm Hg | Maternal age >40 years |
| Systolic pressure ≥160 mm Hg or diastolic pressure ≥110 mm Hg | |
| *Left ventricular dysfunction, retinopathy, dyslipidemia, microvascular disease, or stroke. | |
Risk factors for preeclampsia
Pregnancies complicated by chronic hypertension carry a heightened risk of superimposed preeclampsia, which is associated with high rates of adverse maternal and perinatal outcomes.4 Sibai and colleagues4 documented the rate of superimposed preeclampsia among 763 women with chronic hypertension who were followed prospectively at several medical centers in the United States. The overall rate of superimposed preeclampsia was 25%.
Specific characteristics affected the risk of preeclampsia: age, previous preeclampsia, duration of hypertension, diastolic blood pressure, thrombophilia, diabetes, proteinuria, multifetal gestation, and use of assisted reproductive technology (TABLE 2).
Diagnostic criteria
In women with hypertension only, superimposed preeclampsia is diagnosed when there is proteinuria of at least 500 mg in 24 hours or thrombocytopenia or abnormal liver enzymes (TABLE 3).
In women with hypertension and proteinuria (renal disease or class F diabetes), new onset of persistent symptoms (severe headache, visual changes) and/or thrombocytopenia, and/or elevated liver enzymes makes the diagnosis of preeclampsia.
Risk of abruption and other complications
Gravidas with chronic hypertension also have an increased risk for abruptio placentae.
In addition, women with high-risk chronic hypertension are at increased risk for life-threatening maternal complications such as pulmonary edema, hypertensive encephalopathy, retinopathy, cerebral hemorrhage, and acute renal failure.5 These risks are particularly acute in women with uncontrolled severe hypertension, renal dysfunction early in pregnancy, or left ventricular dysfunction prior to conception. The risk of these and other complications increases further when superimposed preeclampsia develops (TABLE 4).
Fetal and neonatal complications in women with chronic hypertension are 3 to 4 times more likely than in the general obstetric population.1 These complications include premature delivery and small-for-gestational-age infants (TABLE 5).
Benefits vs risks of drug treatment
Although long-term blood pressure control greatly reduces stroke, cardiovascular morbidity, and mortality in nonpregnant persons,3 hypertension in pregnancy is different because the duration of therapy is shorter. In people with mild to moderate hypertension, the benefit is achieved after at least 5 years of treatment.2 In pregnancy, the benefits to the mother may not be obvious during the short time of treatment, and exposure to drugs includes both mother and fetus.6 Thus, in pregnancy, one must balance the potential short-term maternal benefits against possible short- and long-term benefits and risks to the fetus and infant.1,5,6
Low-risk: No benefit
We lack compelling evidence that shortterm antihypertensive therapy is beneficial in these women except for a reduction in the exacerbation of hypertension.5,7
High-risk: Drug therapy is needed
We lack placebo-controlled trials of antihypertensive therapy in gravidas with severe hypertension, and none are likely to be performed because of the potential risks of untreated severe hypertension.
In these women, drug therapy reduces the acute risk of stroke, congestive heart failure, and renal failure.2 Control of severe hypertension may also prolong the pregnancy and thereby improve perinatal outcome. However, there is no evidence that control of severe hypertension reduces the rates of superimposed preeclampsia or abruptio placentae.2,4,5
Adverse effects
The potential adverse effects of the most commonly prescribed antihypertensive agents are poorly established or unclearly quantified.1 In general, we have limited and selective information about teratogenicity except in laboratory animals, and minimal data on the benefits and risks of most antihypertensive drugs when used during pregnancy. Nevertheless, the limited data available suggest that some drugs carry the potential for adverse fetal effects and should be avoided (TABLE 6).
Chronic hypertension heightens risk of placental abruption
Gravidas with chronic hypertension are at increased risk for abruptio placentae, which ranges from 0.7% to 1.5% in women with mild chronic hypertension, and from 5% to 10% in women with severe or high-risk hypertension. The rate increases to 30% with superimposed preeclampsia.
Drug treatment of comorbidities
According to data from clinical trials in nonpregnant subjects, selected comorbidities can be treated as follows:
- Ischemic heart disease. Beta-blockers are the first line of treatment, particularly labetalol. Alternatively, calciumchannel blockers can be used.
- Heart failure. In asymptomatic gravidas, beta-blockers should be used. In symptomatic gravidas, both beta-blockers and diuretics are recommended.
- Diabetes. Two or more drugs are usually needed to control blood pressure in this population. Although angiotensin-converting enzyme (ACE) inhibitors have a beneficial effect outside of pregnancy, calcium-channel blockers and diuretics are safer for gravidas.
- Chronic kidney disease warrants aggressive management, typically with 3 or more medications. Again, while ACE inhibitors have a favorable effect outside of pregnancy, calcium-channel blockers, beta-blockers, and diuretics are better choices.
ACE inhibitors are contraindicated in pregnancy due to the risk of oligohydramnios, renal dysplasia, pulmonary hypoplasia, and intrauterine growth restriction.8
Management goals
The primary objectives in managing chronic hypertension in pregnancy are to reduce maternal risks and achieve optimal perinatal survival. These objectives call for a rational approach that includes:
- preconception education and counseling,
- early antenatal care,
- frequent antepartum visits to monitor both mother and fetus,
- timely delivery with intensive intrapartum monitoring, and
- proper postpartum care.
Ideally, management should begin prior to pregnancy, with extensive evaluation and a complete workup to assess the cause and severity of the hypertension, determine whether other medical illnesses are present, and rule out target organ damage associated with longstanding hypertension (TABLE 7).
TABLE 2
Characteristics that affect risk of preeclampsia
| CHARACTERISTIC | PREECLAMPSIA (%) |
|---|---|
| Age (yr) | |
| ≤35 | 26 |
| >35 | 25 |
| Previous preeclampsia | |
| Yes | 32 |
| No | 23 |
| Duration of hypertension | |
| <4 years | 23 |
| ≥4 years | 32 |
| Diastolic blood pressure (mm Hg) | |
| <100 | 24 |
| 100-109 | 25 |
| ≥110 | 40-50 |
| Thrombophilia | 40-50 |
| Diabetes mellitus | 30-40 |
| Proteinuria | |
| No | 25 |
| Yes | 27 |
| Note: Risk is also increased in women with multifetal gestation and in those who have conceived using assisted reproductive technology. | |
| Source: Sibai BM, et al4 | |
Low-risk hypertension
Stop drugs at first visit
Women with low-risk chronic hypertension without superimposed preeclampsia usually have pregnancy outcomes similar to those in the general obstetric population.2,5,9
Discontinuation of antihypertensive therapy early in pregnancy does not increase the rates of preeclampsia, abruptio placentae, and preterm delivery in these women.2,9
Our policy is to discontinue antihypertensive treatment in low-risk women at the first prenatal visit, because most of these women have good outcomes without such therapy.
Follow-up strategy
During subsequent visits, we educate the patient about nutritional requirements, weight gain, and sodium intake (maximum of 2.4 g sodium per day). We also remind them that alcohol use and smoking during pregnancy can aggravate maternal hypertension and cause adverse effects in the fetus such as fetal growth restriction and abruptio placentae.
During the remainder of the pregnancy, we observe the gravida very closely for appropriate fetal growth and early signs of preeclampsia.
Fetal evaluation should include an ultrasound examination at 16 to 20 weeks’ gestation, to be repeated at 32 to 34 weeks and monthly thereafter until term. In addition, all women with low-risk hypertension should undergo growth scans starting at 32 to 34 weeks, especially obese women in whom fundal height measurements are unreliable, because of the increased risk of intrauterine growth restriction.
If severe hypertension develops before term, start either nifedipine or labetalol (TABLE 6).
Immediate fetal testing with the nonstress test or biophysical profile is necessary if severe hypertension, preeclampsia, abnormal fetal growth, or evidence of oligohydramnios develops.
Hospitalization and delivery are necessary if severe hypertension, fetal growth restriction documented by ultrasound, or superimposed preeclampsia develops at or beyond 37 weeks.
If none of these complications is present, pregnancy can continue until 40 weeks’ gestation.5
TABLE 3
Diagnosis of preeclampsia in women with preexisting conditions
| PREEXISTING CONDITION | PREECLAMPSIA IS PRESENT IF SHE HAS… |
|---|---|
| Hypertension | Proteinuria ≥500 mg/24 hours or thrombocytopenia or abnormal liver enzymes |
| Proteinuria | New onset hypertension plus symptoms and/or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | New onset of persistent symptoms (severe headache, visual changes) or thrombocytopenia or elevated liver enzymes |
TABLE 4
Complication rates in women with superimposed preeclampsia vs women without hypertension*
| COMPLICATION | WITHOUT HYPERTENSION (PER 1,000 CASES) | PREECLAMPSIA SUPERIMPOSED ON CHRONIC HYPERTENSION (PER 1,000 CASES) |
|---|---|---|
| Abruptio placentae | 9.6 | 30.6 |
| Thrombocytopenia | 1.6 | 11.5 |
| Disseminated intravascular coagulation | 2.9 | 17.4 |
| Pulmonary edema | 0.2 | 6.4 |
| Blood transfusion | 1.5 | 16.3 |
| Mechanical ventilation | 0.2 | 17.0 |
| *US women, 1988–1997 | ||
| Source: Zhang J, et al15 | ||
High-risk hypertension
The frequency and nature of maternalfetal adverse effects depends on the cause of the hypertension and the extent of target organ damage.
Realistic preconception counseling
Women with substantial renal insufficiency (ie, serum creatinine >1.4 mg/dL), diabetes with vascular involvement (class R/F), severe collagen vascular disease, cardiomyopathy, or coarctation of the aorta should be advised that the pregnancy might exacerbate their condition. These patients should be made aware of the potential for congestive heart failure, acute renal failure requiring dialysis, and even death. In addition, perinatal loss and neonatal complications are markedly increased in these women.
Refer or consult a specialist
All women with severe hypertension should be managed in consultation with a subspecialist in maternal-fetal medicine, as well as any other specialists who may be indicated.
They also should be observed and delivered at a tertiary care center with adequate maternal-neonatal care facilities.5
TABLE 5
Adverse pregnancy outcomes in women with mild chronic hypertension
| OBSERVATIONAL STUDY | PREECLAMPSIA (%) | ABRUPTIO PLACENTAE (%) | DELIVERY AT <37 WEEKS (%) | SMALL FOR GESTATIONAL AGE (%) |
|---|---|---|---|---|
| Sibai et al2 (n=211) | 10.0 | 1.4 | 12.0 | 8.0 |
| Rey and Couturier16 (n=337) | 21.0 | 0.7 | 34.4 | 15.5 |
| McCowan et al17 (n=142) | 14.0 | Not reported | 16.0 | 11.0 |
| Sibai et al4 (n=763) | 25.0 | 1.5 | 33.3 | 11.1 |
| August et al18 (n=110) | 34.0 | Not reported | Not reported | 8.0 |
Management strategy
Our policy is to hospitalize women with high-risk hypertension at the time of the first prenatal visit to evaluate their cardiovascular and renal status and regulate antihypertensive medications, as well as other prescribed drugs (eg, insulin, cardiac drugs, thyroid drugs). Women receiving atenolol, ACE inhibitors, or angiotensin II receptor antagonists should have these medications discontinued under close observation.
In women without target organ damage, the aim of antihypertensive therapy is to keep systolic pressure between 140 and 150 mm Hg and diastolic pressure between 90 and 100 mm Hg.
In women with target organ damage and mild hypertension, antihypertensive therapy is also indicated, because there are short-term maternal benefits to lowering blood pressure. We recommend keeping systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg.
Early, frequent visits.Women with high-risk chronic hypertension need close observation throughout pregnancy and may require serial evaluation of 24-hour urine protein excretion and a complete blood count with a metabolic profile at least once every trimester. Further laboratory testing depends on the clinical progress of the pregnancy. At each visit, remind the woman about the adverse effects of smoking and alcohol use, and counsel her about the importance of diet and minimal salt intake.5
Fetal surveillance includes ultrasound, growth scans, and nonstress testing (TABLE 8).
Hospitalization is warranted if uncontrolled severe hypertension, preeclampsia, or evidence of fetal growth restriction develops, so that more frequent evaluation of maternal and fetal well-being can be performed.
Delivery is indicated if any of these complications develop at or beyond 34 weeks’ gestation. If there are none of these complications, consider delivery at 36 to 37 weeks after documenting fetal lung maturity.5
Postpartum care
Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.10,11 These risks are heightened in women with target organ involvement, superimposed preeclampsia, or abruptio placentae.10
Blood pressure must be closely controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be used as needed, and diuretics may be appropriate in women with circulatory congestion and pulmonary edema.12 Oral therapy may be needed to control blood pressure after delivery. In some women, it may be necessary to switch to a new agent such as an ACE inhibitor, particularly in women who had pregestational diabetes or cardiomyopathy.
All antihypertensive drugs are found in breast milk, although differences in the milk-to-plasma ratio do occur. The longterm effects of maternal antihypertensive drugs on breastfeeding infants has not been studied. However, methyldopa appears to be a reasonable first-line oral therapy (if it is contraindicated, use labetalol). Milk concentrations of methyldopa appear to be low and are considered safe. Beta-blockers (atenolol and metoprolol) are concentrated in breast milk, whereas labetalol or propanolol have low concentrations.13,14 Concentrations of diuretics in breast milk are low, but may diminish milk production.13 Little is known about the transfer of calcium-channel blockers to breast milk, but there are no apparent side effects. ACE inhibitors and angiotensin II receptor antagonists should be avoided because of their effects on neonatal renal function, even though their concentrations in breast milk appear to be low.
The authors report no financial relationships relevant to this article.
TABLE 6
Acute and long-term drug treatment
| DRUG | STARTING DOSE | MAXIMUM DOSE | COMMENTS |
|---|---|---|---|
| ACUTE TREATMENT OF SEVERE HYPERTENSION | |||
| Hydralazine | 5-10 mg IV every 20 min | 30 mg* | |
| Labetalol | 20-40 mg IV every 10-15 min | 220 mg* | Avoid in women with asthma or congestive heart failure |
| Nifedipine | 10-20 mg orally every 30 min | 50 mg* | |
| LONG-TERM TREATMENT OF HYPERTENSION | |||
| Methyldopa | 250 mg BID | 4 g/day | Rarely indicated |
| Labetalol | 100 mg BID | 2,400 mg/day | First choice |
| Atenolol | 50 mg/day | 100 mg/day | Associated with intrauterine growth restriction |
| Propanolol | 40 mg BID | 640 mg/day | Use with associated thyroid disease |
| Hydralazine | 10 mg TID | 100 mg/day | Use in cases of left ventricular hypertrophy |
| Nifedipine | 10 mg BID | 120 mg/day | Use in women with diabetes |
| Diltiazem | 120-180 mg/day | 540 mg/day | |
| Thiazide diuretic | 12.5 mg BID | 50 mg/day | Use in salt-sensitive hypertension and/or congestive heart failure |
| May be added as second agent | |||
| Avoid if preeclampsia develops or intrauterine growth restriction is present | |||
| Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers | — | — | Do not use after 16-18 weeks |
| *If desired blood pressure levels are not achieved, switch to another drug. | |||
TABLE 7
How to evaluate gravidas with chronic hypertension
| POPULATION | RECOMMENDED TESTS |
|---|---|
| All |
|
| Gravidas with longstanding hypertension, poor compliance, or poor control |
|
TABLE 8
Recommended antenatal testing
| LEVEL OF RISK | TEST |
|---|---|
| Low (uncomplicated) |
|
| High (complicated) |
|
1. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy:a review. Obstet Gynecol. 2000;96:849-860.
2. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
3. Chobanian AB, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289:2560-2572.
4. Sibai BM, Lindheimer M, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. N Engl J Med. 1998;339:667-671.
5. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
6. Umans JG, Lindheimer MD. Antihypertensive treatment. In:Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn:Appleton and Lange; 1998:581-604.
7. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. In: The Cochrane Library, Issue 12, 2003. Oxford:Update Software.
8. How HY, Sibai BM. Use of angiotensin-converting enzyme inhibitors in patients with diabetic nephropathy. J Matern Fetal Neonatal Med. 2002;12:402-407.
9. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
10. Sibai BM, Villar MA, Mabie BC. Acute renal failure in hypertensive disorders of pregnancy:pregnancy outcome and remote prognosis in thirty-one consecutive cases. Am J Obstet Gynecol. 1990;62:777.-
11. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women:a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
12. Lie RT, Rasmussen S, Brunborg H, et al. Fetal and maternal contributions to risk of pre-eclampsia:a population based study. Br Med J. 1998;316:1343-1347.
13. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation:A Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore:Williams & Wilkins;1998.
14. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
15. Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203-212.
16. Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol. 1994;171:410-416.
17. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
18. August P, Helseth G, Cook EF, Silson C. A prediction model for superimposed preeclampsia in women with chronic hypertension during pregnancy. Am J Obstet Gynecol. 2004;191:1666-1672.
1. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy:a review. Obstet Gynecol. 2000;96:849-860.
2. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
3. Chobanian AB, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289:2560-2572.
4. Sibai BM, Lindheimer M, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. N Engl J Med. 1998;339:667-671.
5. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
6. Umans JG, Lindheimer MD. Antihypertensive treatment. In:Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn:Appleton and Lange; 1998:581-604.
7. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. In: The Cochrane Library, Issue 12, 2003. Oxford:Update Software.
8. How HY, Sibai BM. Use of angiotensin-converting enzyme inhibitors in patients with diabetic nephropathy. J Matern Fetal Neonatal Med. 2002;12:402-407.
9. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
10. Sibai BM, Villar MA, Mabie BC. Acute renal failure in hypertensive disorders of pregnancy:pregnancy outcome and remote prognosis in thirty-one consecutive cases. Am J Obstet Gynecol. 1990;62:777.-
11. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women:a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
12. Lie RT, Rasmussen S, Brunborg H, et al. Fetal and maternal contributions to risk of pre-eclampsia:a population based study. Br Med J. 1998;316:1343-1347.
13. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation:A Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore:Williams & Wilkins;1998.
14. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
15. Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203-212.
16. Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol. 1994;171:410-416.
17. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
18. August P, Helseth G, Cook EF, Silson C. A prediction model for superimposed preeclampsia in women with chronic hypertension during pregnancy. Am J Obstet Gynecol. 2004;191:1666-1672.
The generalist’s guide to interstitial cystitis
The typical patient voids 16 times a day and 2 or more times at night. In later stages of the disease, she may urinate as often as 60 times a day and every half hour at night, severely eroding her ability to hold a job, travel, or lead a normal life. In fact, her quality of life may be impaired as much as that of a person with end-stage renal disease.1,2 She sees an average of 4 doctors and endures irritative voiding symptoms for 4 years before her disease is identified. The cause is unknown.
Interstitial cystitis produces a wide spectrum of symptom severity, occurring episodically with spontaneous flare-ups and remission, or with continuous, intractable urinary urgency and pain. Until recently, women presenting with urinary urgency, frequency, and pain were presumed to have a urinary tract infection (UTI) or overactive bladder, and were often treated—to no avail—with multiple courses of antibiotics or anticholinergics.
Fortunately, interstitial cystitis is gaining recognition, and effective treatments are emerging. Usually the ObGyn—often the first physician a woman consults—need refer only the refractory cases to a specialist. This article describes the components of diagnosis and the most effective treatments, including use of the first-line agents amitriptyline (Elavil) and pentosan polysulfate sodium (Elmiron).
CASE Is overactive bladder the cause of stubborn symptoms?
“R.H.,” a healthy 48-year-old G2P2 with a 5-year history of urinary urgency and frequency, reports that she voids “at least 15 times per day.” She denies any urge incontinence, but says she experiences occasional stress incontinence if she has a bad cold. Four years ago, she saw a urologist for these symptoms, after her husband said he was tired of having to stop the car so she could go to the bathroom. The urologist diagnosed a “small bladder,” performed urethral “stretching,” and prescribed oxybutynin.
Her symptoms improved for about 6 months, but then progressed and have now worsened. She began taking tolterodine, 4 mg daily, 2 months ago, as prescribed by her primary care physician. The sensation of painful urgency has eased, but there has been no change in frequency. R.H. used to wake as often as 4 times a night with the urge to urinate, but since she began taking zolpidem tartrate (Ambien) as a sleep aid, she now wakes only 2 times every night.
Why are her symptoms so persistent?
This woman’s case is a classic example of interstitial cystitis masquerading as overactive bladder. Treatment with anticholinergic drugs may ease urgency symptoms slightly, but has no real effect on frequency.
This case has 5 hallmarks of the syndrome of interstitial cystitis:
- Urgency
- Frequency (more than 8 voids/day, taking fluid intake into account)
- Bladder pain
- Nocturia (more than twice)
- Absence of a genitourinary tract infection
Patients show signs of “battle fatigue”
Women with interstitial cystitis may be anxious, depressed, angry, and sleep-deprived. In some women, stress exacerbates the urinary symptoms and pain (as do certain common foods and beverages, especially citrus, tomatoes, and caffeine).
Approximately 60% of patients report dyspareunia, and many report chronic pelvic pain. In fact, 75% of women who report chronic pelvic pain also have irritative voiding symptoms. Therefore, it is important to ask about lower urinary tract symptoms whenever a woman presents with pelvic pain.3,4
Pain may be suprapubic, vaginal, perineal, or originate in the groin or lower back. Although 16% of patients present solely with pain, and 30% have only urinary frequency, most patients suffer from both symptoms.
Approximately 40% report premenstrual or ovulatory exacerbation of symptoms, although symptoms may improve during pregnancy.5 Voided volumes are usually small, despite the strong urgency, which does not always resolve. Pelvic pain may ease after voiding but recurs shortly.
Insidious, worsening course
Symptoms appear insidiously and worsen to a “final” stage within 5 to 15 years, at which point a plateau is reached with little further progression.6 Some experts suggest that the disease be classified as “early non-ulcerous” or “classic ulcerous.”
- In early disease, bladder capacity exceeds 450 cc under anesthesia, with glomerulations and hemorrhage.
- In classic disease, bladder capacity is less than 450 cc under anesthesia, and Hunner’s ulcers and fissures are evident. Hunner’s ulcers are described as “a central scar with small fibrin deposits before distension, and post-distension edema.”7
For now, however, there are no agreed-upon markers to distinguish the 2 types of disease.
The female-to-male ratio is 9:1, and about 500,000 to more than 1 million adults in the United States are thought to have interstitial cystitis.8 Caucasian women constitute 95% of patients, and the average age at diagnosis is 45 years. Thirty percent of women with interstitial cystitis are 30 years old or younger. Significantly more women with interstitial cystitis have had a hysterectomy than controls.9
For a diagnosis, skip the NIH criteria
Symptoms
Although the National Institutes of Health (NIH) established diagnostic criteria for research subjects, the criteria are overly stringent—60% of women with symptoms typical of interstitial cystitis do not qualify, but should not necessarily be excluded from diagnosis and treatment.
When a woman has the hallmark symptoms listed on page 57, but also reports continuous pain or dysmenorrhea, other pelvic pathology such as endometriosis should be considered, although interstitial cystitis should be included in the differential diagnosis of any woman reporting pelvic pain.
Incontinence is atypical. If present, it merits an incontinence evaluation to detect detrusor hyperreflexia or detrusor-sphincter dyssynergia.
Dysuria suggests a UTI, urethral diverticulum, urogenital atrophy, or vaginitis. Many patients present with an erroneous diagnosis of “recurrent UTIs.”
Diagnostic tools
Voiding diaries are useful and can be revealing. The Pelvic Pain and Urinary Frequency (PUF) scale, developed by Parsons, is helpful in predicting interstitial cystitis (see the Clip-and-save chart). The higher the score, the greater the likelihood of interstitial cystitis, particularly with a score of more than 8.
Another tool is the O’Leary-Sant Index, which measures pain, voiding symptoms, and quality of life.
Physical examination and laboratory studies
Perform a pelvic exam to rule out other diseases and pelvic pathology, including sexually transmitted diseases, urethral diverticulum, and pelvic masses. Typically, the pelvic exam in women with interstitial cystitis is negative except for suprapubic and/or trigonal tenderness.
Urinalysis, culture, and sensitivity are warranted but are usually negative.
Cytology should be analyzed if microscopic hematuria is present, or with other risk factors such as a history of smoking or age over 40.
Obtain cultures for sexually transmitted diseases if clinically indicated.
Urodynamic studies are not necessary to diagnose interstitial cystitis. However, if incontinence is present, a cystometrogram can confirm detrusor hyperreflexia. Otherwise the cystometrogram is normal except for heightened sensation or pain with bladder filling, or a bladder capacity of less than 350 cc.
The potassium sensitivity test: Useful but painful
Women with interstitial cystitis are thought to have increased bladder permeability that allows potassium to pass through to the detrusor muscle. Thus, the potassium sensitivity test often is used to diagnose the condition. The test is an office procedure in which 2 separate solutions are instilled into the bladder: 40 cc sterile water followed by 40 cc of a solution of 400 mEq potassium per liter of water. After each solution is instilled, the patient is monitored for symptoms. The test is positive when the patient responds only to the potassium.
The response may be marked and painful, and the bladder should be emptied immediately. Subsequent irrigation with sterile water may be necessary to alleviate the discomfort caused by the potassium solution. Symptoms provoked by the test generally subside after bladder emptying, but can persist and cause moderate distress, which limits the utility of this office-based test.
Parsons et al10 demonstrated an 81% positive response (197 of 244 women) to the test among women with pelvic pain, compared with 0 of 47 patients with no pelvic pain. They also found that 70% of patients with interstitial cystitis and 4% of controls had a positive response.
If a woman is extremely volume-sensitive during the water phase, the potassium phase may not be accurate. A false-positive response can be caused by infection or prior exposure to radiation or chemotherapy. A thorough history is imperative.
The gold standard: Cystoscopy under anesthesia
Cystoscopy with hydrodistention under general anesthesia is the surest way to diagnose interstitial cystitis or rule it out. Sterile water or saline is infused until bladder capacity is reached. Bladder rupture occurs in up to 10% of patients, so careful inspection during filling is crucial. After 5 minutes of distension, bladder volume is measured into a calibrated beaker. Terminal hematuria (the last 50 cc of effluent) often is noted.
Normal bladder capacity under anesthesia is 1,000 cc, but it is reduced in women with interstitial cystitis. Bladder capacity of 450 cc or less under anesthesia indicates a more contracted bladder and a later-stage disease. Glomerulations, petechiae, fissures, or (rarely) Hunner’s ulcers typically are visible, regardless of bladder volume. However, the presence of glomerulations does not necessarily make the diagnosis, because they can be found in asymptomatic women. Further, cystoscopic observations do not always correlate with the severity of symptoms (nor does positive biopsy always reflect interstitial cystitis).
Hydrodistention is not only diagnostic, but also can be therapeutic, as sympathetic nerve fiber density decreases afterward.11 However, the need for this procedure is under debate, due to the limitations described above. A bladder capacity less than 1,000 cc with the presence of glomerulations or petechiae and fissures, with or without the Hunner’s ulcers, constitutes a definitive diagnosis.
Cystoscopy under anesthesia is recommended because medical treatment can be costly and cause significant side effects. An accurate diagnosis should precede therapy to avoid misdirected therapy in a patient who does not have interstitial cystitis. Moreover, cystoscopy can rule out bladder neoplasms or other diseases. Some bladder carcinomas have been missed in women treated empirically for interstitial cystitis.12
Cystoscopic images “paint a thousand words.” When a woman sees her cystoscopy images, the picture indeed “paints a thousand words.” For many women, the images “justify” their symptoms and confirm that the disease is real.
CASE Don’t treat a UTI without a positive culture
“M.P.” is a healthy 44-year-old G2P2 with a history of recurrent UTIs. Approximately 14 months ago, while on vacation, she began having symptoms of urinary frequency, urgency, and lower abdominal pain that were relieved with voiding. She called her primary care physician, who prescribed levofloxacin and phenazopyridine over the phone for a presumed UTI. Since the patient was out of town, a urine culture was not obtained.
When M.P. returned from vacation, her symptoms recurred, so she underwent urinalysis, including culture and sensitivity, and began a 7-day course of nitrofurantoin (100 mg twice daily). When her symptoms did not improve by day 4, a second course of levofloxacin was given. The urine culture was sterile. As her physician recommended, M.P. increased her fluid intake, including water and cranberry juice. She also avoided sexual relations, since they exacerbated her symptoms, which improved overall but did not clearly abate.
Three months later her symptoms returned in full force.
How would you treat this patient?
Interstitial cystitis can produce symptoms consistent with a lower UTI, but urine cultures will be negative and the response to antibiotics will be minimal. Many patients call their physicians and report “another UTI.” However, if the woman is healthy with no history of renal disease or diabetes, consider interstitial cystitis. Obtain urine culture results from other physicians, if possible, to determine whether bacterial infection was ever confirmed.
Cranberry juice is acidic and may exacerbate urgency and pain.
What to tell patients
The Interstitial Cystitis Association (ICA) encourages patients to become involved in their own care. The ICA was formed in 1984 by women with painful bladder symptoms who had been told by their physicians that nothing was wrong. The organization provides patients with clinical research updates, clinical trial opportunities, and literature and information.
Once the diagnosis is confirmed, patient education and counseling are imperative. Compliance is critical.
There is no cure for interstitial cystitis; the disease is chronic, with relapses and remissions. Although it does not progress once it develops fully, improvement is slow, usually occurring after 3 months or more of treatment. No single treatment works for all patients, so empiric trials with various agents may be needed. Treatment often is multimodal, and the rationale for each therapy should be explained.
Have the patient keep a voiding diary before and after treatment, as well as during any flare-up, to provide evidence of improvement and identify triggers. Also instruct her to pay attention to any foods or activities that exacerbate her symptoms (eg, caffeine, sexual activity).
Treatment
Does a change in diet help?
Some foods and beverages apparently exacerbate symptoms, although the link between foods and symptoms has not been fully investigated. About 53% of patients with interstitial cystitis associate symptom aggravation with dietary factors, especially acidic foods and beverages.5 Dietary restrictions should be attempted for 1 to 2 weeks to determine which foods to avoid.
Gillespie13 found elevated urine levels of tryptophan metabolites in women with hypersensitive bladders, compared with controls.
Tryptophan metabolites may disrupt the glycosaminoglycan layer of the bladder epithelium, as seen in a study involving rabbit bladders.14
Urge suppression
Another helpful strategy is having the patient increase the time between voids using distraction techniques and by contracting the pelvic floor muscles and overriding the first urge to void.
Oral medications
Pentosan polysulfate sodium is a glycosaminoglycan with an affinity for mucosal membranes. It is approved by the Food and Drug Administration (FDA) for the treatment of interstitial cystitis. The mechanism by which it reduces pain and urinary frequency is unclear, but it may replace the deficient glycosaminoglycan layer in the bladder epithelium.
Pain relief occurs in approximately 40% to 60% of patients after 3 months of therapy (100 mg orally 3 times daily).15
The patient should clearly understand that beneficial effects may not occur for 3 to 6 months, and that patience is necessary to give the drug an adequate trial. The response is maintained over the long term, and the drug should be used indefinitely. Pentosan polysulfate sodium is well tolerated, although gastrointestinal side effects and reversible alopecia occur in 4% of patients.
Performing a cystoscopy under anesthesia with hydrodistension is not always necessary prior to starting pentosan polysulfate, as long as the patient is not at risk for bladder neoplasms. However, prior to starting the drug the minimal evaluation should include a voiding diary and either the PUF questionnaire or the potassium sensitivity test.
Antihistamines. If the patient has a history of allergies, or mast cells were confirmed on bladder biopsy, an antihistamine such as hydroxyzine should be given along with pentosan polysulfate sodium. Hydroxyzine has an inhibitory effect on bladder mast cells, as well as anticholinergic and analgesic properties, which improve typical symptoms of interstitial cystitis.
Initiate hydroxyzine at a dose of 10 to 25 mg at bedtime for 1 week, gradually increasing to 50 to 75 mg. Side effects include drowsiness, which is beneficial for women who have nocturia. Other effects are dry mouth and a bitter taste.
Amitriptyline hydrochloride also has analgesic, antihistaminic, anticholinergic, and sedative effects. Amitriptyline is a noradrenaline and serotonin reuptake inhibitor that blocks nociception in the central nervous system.
Compared with placebo, amitriptyline significantly improved symptom scores, pain, and urgency intensity. In a study by van Ophoven and colleagues,16 50 patients (44 women, 6 men) were randomly assigned to amitriptyline at self-titrating doses or placebo. O’Leary-Sant symptom scores, pain, and urgency intensity improved significantly in the amitriptyline group, compared with placebo.
Anticholinergic side effects (eg, dry mouth, constipation), weight gain, and sedation occur in 20% to 80% of patients. In an open-label study of amitriptyline for interstitial cystitis,17 long-term efficacy (mean of 17 months) revealed a 64% response rate (60 of 94 patients) using the global response assessment questionnaire.
Start amitriptyline at a dose of 10 to 25 mg at bedtime, gradually increasing to 75 mg as tolerated. Sedation becomes a limiting factor in the higher doses. Other tricyclic antidepressants have not been studied to any significant extent in treating interstitial cystitis. When used as part of a multimodal treatment in addition to pentosan polysulfate sodium, amitriptyline may be tapered off once remission is attained. No studies have compared treatment response using pentosan polysulfate sodium with and without amitriptyline.
Calcium channel blockers (nifedipine) and drugs for neuropathic pain (gabapentin) are being investigated.
Anticholinergic and antispasmodic agents are typically ineffective in women with interstitial cystitis. In fact, if a patient has no improvement in her symptoms after these drugs are tried, interstitial cystitis should be strongly considered.
Oral L-arginine (1,500–3,000 mg per day, divided doses) improved symptoms in a small study by increasing nitric oxide synthase activity.18
NSAIDs are used adjunctively and may help reduce pain.
Intravesical therapy
Patients unable to tolerate oral medications may benefit from intravesical therapy. It can also be used as an adjunct to oral therapy. Intravesical therapy delivers drugs directly to the bladder wall with a low incidence of side effects. Risks include a potential for UTI via catheterization, as well as transient chemical cystitis, which exacerbates symptoms. A variety of therapeutic “cocktails” are used.
Dimethyl sulfoxide (DMSO) is the only other drug, besides pentosan polysulfate, approved by the FDA for treatment of interstitial cystitis. DMSO has antiinflammatory, analgesic, and muscle-relaxant effects, and inhibits mast-cell activity. DMSO induces remission in 50% to 70% of patients for up to 24 months.19
Lidocaine jelly is injected intraurethrally, followed by instillation of 50 cc of DMSO (alone or with heparin, sodium bicarbonate, and Solu-Cortef). This solution is held in the bladder for 20 to 30 minutes before voiding.
DMSO is secreted through the lungs and skin and has a garlic-like odor. Treatments are administered every 1 to 2 weeks for a total of 4 to 8 treatments. If the condition relapses, DMSO can be reinstituted on a long-term basis. Motivated patients can be taught to administer this treatment themselves.
Heparin is another option. It is administered at a dose of 10,000 U thrice weekly.
Hyaluronic acid. In a small study involving 20 patients, weekly intravesical hyaluronic acid improved symptoms in 65% of patients, with a 40% and 30% decrease in nocturia and pain, respectively.20
Intravesical pentosan polysulfate sodium is another option that improves symptoms and increases bladder capacity,21 although larger studies of its efficacy are lacking.
Bacillus Calmette-Guérin (BCG) solution intravesically had a 60% response rate in 1 study (versus 27% for placebo).22 The mechanism of action is unknown, but the solution may modulate the bladder immune response. Additional studies are pending.
Although their link to interstitial cystitis has not been proven, these foods are thought to be implicated and should be limited or avoided
ACIDIC FOODS
All alcoholic beverages
Apples
Apple juice
Cantaloupe
Carbonated drinks
Chilies/spicy foods
Citrus fruits (lemons, limes, oranges, etc)
Coffee
Cranberries
Grapes
Guava
Lemon juice
Peaches
Pineapples
Plums
Strawberries
Tea
Tomatoes
Vinegar
FOODS HIGH IN TYROSINE, TRYPTOPHAN, OR ASPARTATE
Aspartame
Avocado
Bananas
Beer
Brewer’s yeast
Canned figs
Champagne
Cheese
Chicken livers
Chocolate
Corned beef
Cranberries
Fava beans
Lima beans
Mayonnaise
Nuts
Onions
Pickled herring
Pineapple
Prunes
Raisins
Rye bread
Saccharine
Sour cream
Soy sauce
Wines
Yogurt
Vitamins buffered with aspartate
Adapted from You Don’t Have to Live with Cystitis! by Larrian Gillespie, MD (revised and updated 1996)
1. Altered bladder permeability
In interstitial cystitis, the protective glycosaminoglycan layer of the bladder epithelium may be deficient, increasing bladder permeability. Antiproliferative factor in the urine may impair the proliferation and repair of urothelium, increasing bladder permeability further.28 This breakdown allows potassium to penetrate the urothelium, stimulating pain receptors and causing an inflammatory response in the detrusor muscle.
Loss of the normal protective layer
In a normal bladder, the epithelium is protected by an ionic, hydrophilic, glycosaminoglycan (GAG) layer that serves as a barrier against urine, which is hyperosmolar and rich in acid and potassium. When the GAG layer is deficient, as it is thought to be in interstitial cystitis, bladder permeability increases, triggering an inflammatory response that inhibits repair of the GAG layer, creating a vicious cycle of exposure, inflammation, and pain.
2. Mast cell activation
Mast cell degranulation may cause or contribute to interstitial cystitis and produce its hallmark symptoms. Mast cells also may be activated in response to a noxious factor. These cells secrete histamine, prostaglandins, leukotrienes, cytokines, and chemotactic factors. Urine from women with interstitial cystitis contains histamine, histamine metabolites, and tryptase,29,30 and electron microscopy of bladder biopsies from affected women shows degranulating mast cells adjacent to sensory nerve fibers. When these fibers are stimulated, they release neuropeptides (such as substance P) and may promote inflammation by activating mast cells and nearby nerve terminals.31
3. Inflammation
Inflammation clearly plays a role in interstitial cystitis. Bladder biopsies reveal mild to severe inflammation, and the presence of T cells, B cells, plasma cells, neutrophils, eosinophils, and mast cells. Inflammatory mediators such as kallikrein, interleukin-6, interleukin-2 inhibitor, and neutrophil chemotactic factor are increased in the urine of individuals with the disease.
4. Autoimmunity
Clinical features of interstitial cystitis that mimic those of other autoimmune diseases include chronic symptoms that wax and wane; higher prevalence in women; immunological deposits in bladder biopsies with mononuclear cell infiltrates, which suggests the presence of bladder autoantigens; association with other autoimmune disorders such as Sjögren’s syndrome and lupus; and, in some cases, a positive response to steroids or other immunosuppressants.32
Glomerulations and fissures are telltale signs
When the bladder is distended under anesthesia, examination may reveal a pattern of fissures and glomerulations, which are hallmarks of interstitial cystitis. These findings are sometimes present in asymptomatic women as well, and do not always correlate with severity of symptoms.
5. Infection
Some experts have postulated that occult infection with fastidious organisms, fungi, or viruses plays a role in the development of interstitial cystitis. However, special cultures, serology, and electron microscopy have not shown any organisms consistently associated with the disease.33
The use of polymerase chain reaction techniques to test for bacterial DNA in bladder biopsies of patients with interstitial cystitis has yielded conflicting results.34,35 Infection may be the inciting event that injures the bladder epithelium, causing a cascade of inflammation.
6. Neurologic changes
Studies have revealed increased sympathetic nerve fiber density in the bladders of patients with interstitial cystitis.31,36 The disease may also be a type of reflex-sympathetic dystrophy with increased and abnormal spinal sympathetic activity. Butrick describes interstitial cystitis as a “visceral pain syndrome.”37 C-fibers (silent afferents) transmit pain when activated by a prolonged or noxious stimulus. This leads to neuroplastic changes that lower the threshold of nociceptive nerves, thus reducing the amount of stimulus needed to provoke pain (allodynia). Pelvic viscera share innervation, which may explain the association between
interstitial cystitis and irritable bowel syndrome and endometriosis. Interstitial cystitis is not limited to the bladder; it involves chronic neuropathic inflammation, afferent overactivity, and central sensitization. Increased pain perception may cause pelvic floor muscle instability, spasm, and hypertonic state.38
Alternative therapies
Electrical nerve stimulation
This transcutaneous modality improves symptoms in 25% to 50% of patients.23 It is thought to stimulate the afferent nerves, thereby activating the inhibitory circuits and decreasing the sensation of pain.
Sacral neuromodulation is another modality being studied for the treatment of interstitial cystitis. So far it has significantly reduced urinary urgency-frequency symptoms as well as pain.24
Other new therapies under investigation include intravesical injection of botulinum toxin, resiniferatoxin, gene therapy, and nerve growth-factor inhibitors.
Surgery: High relapse rates make it a last resort
The treatment of visible ulcers by resection or laser ablation improves symptoms but carries a relapse rate of more than 50%.25 More aggressive surgeries, with cure rates ranging from 50% to 80%, include denervation procedures, augmentation cystoplasty for severely contracted bladders (not necessarily due to interstitial cystitis), cecocystoplasty (where a segment of cecum is excised and reanastomosed with the bladder to increase bladder capacity), and total cystourethrectomy and urinary diversion. However, persistent pain has been reported after these invasive procedures, and permanent intermittent self-catheterization and/or reoperation is often required.26,27
Dr. LaSala is a speaker for Pfizer, Inc.
1. Held PJ, Janno PM, Wein AJ, et al. Epidemiology of IC. In: Hanno P, Staskin DR, Krane RJ, et al, eds. Interstitial Cystitis. New York: Springer-Verlag; 1990:29–48.
2. Ratner V, Slade D, Greene G. Interstitial cystitis: a patient’s perspective. Urol Clin North Am. 1994;21:1-5.
3. Parson CL, Bullen M, Kahn BS, et al. Gynecologic presentation of interstitial cystitis as detected by intravesical potassium sensitivity. Obstet Gynecol. 2001;98:127-132.
4. Clemons JL, Arya LA, Myers DL. Diagnosing interstitial cystitis in women with chronic pelvic pain. Obstet Gynecol. 2002;100:337-341.
5. Whitmore KE. Self-care regimens for patients with interstitial cystitis. Urol Clin North Am. 1994;21:121-130.
6. Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993;149:465-469.
7. Parsons CL. Interstitial cystitis: clinical manifestations and diagnostic criteria in over 200 cases. Neurourol Urodyn. 1990;9:241-250.
8. Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49:2-9.
9. Kozial JA. Epidemiology of interstitial cystitis. Urol Clin North Am. 1994;21:7-20.
10. Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Willems JJ. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187:1395-1400.
11. Ruggieri MR, Chelsky MJ, Rosen SI, Shickley TJ, Hanno PM. Current findings and future research avenues in the study of interstitial cystitis. Urol Clin North Am. 1994;21:163-176.
12. Tissot WD, Diokno AC, Peters KM. A referral center’s experience with transitional cell carcinoma misdiag-nosed as interstitial cystitis. J Urol. 2004;172:478-480.
13. Gillespie L. Metabolic appraisal of the effects of dietary modification on hypersensitive bladder symptoms. Br J Urol. 1993;72:293-297.
14. Kaufman JE, Anderson K, Parsons CL. Inactivation of antiadherence effect of bladder surface glycosaminoglycans as possible mechanism for carcinogenesis. Urology. 1987;30:255-258.
15. Hanno PM. Analysis of long-term Elmiron therapy for interstitial cystitis. Urology. 1997;49(suppl 5A):93-99.
16. Van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172:533-536.
17. van Ophoven, Hertle L. Long-term results of amitripty-line treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
18. Smity SD, Ehheler MA, Foster HE, Weiss RM. Improvement in interstitial cystitis symptom scores during treatment with L-arginine. J Urol. 1997;158:703-708.
19. Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol. 1988;140:36-39.
20. Kallestrup EB, Jorgensen SS, Nordling J, et al. Treatment of interstitial cystitis with Cystistat: a hyaluronic acid product. Scand J Urol Nephrol. 2005;39:143-147.
21. Bade JJ, Laseur M, Nieuwenburg A, van der Weele LT, Mensink HJ. A placebo-controlled study of intravesical pentosan polysulphate for the treatment of interstitial cystitis. Br J Urol. 1997;79:168-171.
22. Peters K, Diokno A, Steinart B, et al. The efficacy of intravesical Tice strain Bacillus Calmette-Guérin in the treatment of interstitial cystitis: a double-blind, prospective, placebo controlled trial. J Urol. 1997;157:2090-2094.
23. Fall M, Lindstrom S. Transcutaneous electrical nerve stimulation in classic and nonulcer interstitial cystitis. Urol Clin North Am. 1994;21:133-139.
24. Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: a prospective study. J Urol. 2003;169:1369-1373.
25. Malloy TR, Shanberg AM. Laser therapy for interstitial cystitis. Urol Clin North Am. 1994;21:141-144.
26. Smith RB, Van Caugh P, Skinner DG, et al. Augmentation enterocystoplasty: a critical review. J Urol. 1977;118:35-39.
27. Chakravarti A, Ganta S, Somani B, Jones MA. Caecocystoplasty for intractable interstitial cystitis: long-term results. Eur Urol. 2004;46:114-117.
28. Erickson DR, Zie SX, Bhavanandan VP, et al. A comparison of multiple urine markers for interstitial cystitis. J Urol. 2002;167:2461.-
29. El-Mansoury M, Boucher W, Sant GR, Theoharides TC. Increased urine histamine and methylhistamine in interstitial cystitis. J Urol. 1994;152:250-353.
30. Boucher W, El-Mansoury M, Pang X, Sant GR. Elevated mast cell tryptase in the urine of patients with interstitial cystitis. Br J Urol. 1995;76:94-100.
31. Pang X, Marchand J, Sant GR, et al. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. 1995;75:744-750.
32. Oravisto KJ. Interstitial cystitis as an autoimmune disease. Eur Urol. 1980;6:10-13.
33. Duncan JL, Schaeffer AJ. Do infectious agents cause interstitial cystitis? Urology. 1997;49:48-51.
34. Haarala M, Jalava J, Laato M, Kilholma P, Nurmi M, Alanen A. Absence of bacterial DNA in the bladder of patients with interstitial cystitis. J Urol. 1996;156:1843-1845.
35. Keay S, Zhang C-O, Bladwin BR, Jacobs SC, Warren JW. Polymerase chain reaction amplification of bacterial 16S rRNA genes in interstitial cystitis and control patient bladder biopsies. J Urol. 1998;159:280-283.
36. Hohenfeller M, Nunes L, Schmidt RA, Lampel A, Thuroff JW, Tanagho EA. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol. 1992;147:587-591.
37. Butrick CW. Interstitial cystitis and chronic pelvic pain: new insights in neuropathology, diagnosis and treatment. Clin Obstet Gynecol. 2003;46:812-814.
38. Moldwin RM, Fried-Siegel J, Mendelowitz F. Pelvic floor dysfunction and interstitial cystitis. J Urol. 1994;151:285A.-
The typical patient voids 16 times a day and 2 or more times at night. In later stages of the disease, she may urinate as often as 60 times a day and every half hour at night, severely eroding her ability to hold a job, travel, or lead a normal life. In fact, her quality of life may be impaired as much as that of a person with end-stage renal disease.1,2 She sees an average of 4 doctors and endures irritative voiding symptoms for 4 years before her disease is identified. The cause is unknown.
Interstitial cystitis produces a wide spectrum of symptom severity, occurring episodically with spontaneous flare-ups and remission, or with continuous, intractable urinary urgency and pain. Until recently, women presenting with urinary urgency, frequency, and pain were presumed to have a urinary tract infection (UTI) or overactive bladder, and were often treated—to no avail—with multiple courses of antibiotics or anticholinergics.
Fortunately, interstitial cystitis is gaining recognition, and effective treatments are emerging. Usually the ObGyn—often the first physician a woman consults—need refer only the refractory cases to a specialist. This article describes the components of diagnosis and the most effective treatments, including use of the first-line agents amitriptyline (Elavil) and pentosan polysulfate sodium (Elmiron).
CASE Is overactive bladder the cause of stubborn symptoms?
“R.H.,” a healthy 48-year-old G2P2 with a 5-year history of urinary urgency and frequency, reports that she voids “at least 15 times per day.” She denies any urge incontinence, but says she experiences occasional stress incontinence if she has a bad cold. Four years ago, she saw a urologist for these symptoms, after her husband said he was tired of having to stop the car so she could go to the bathroom. The urologist diagnosed a “small bladder,” performed urethral “stretching,” and prescribed oxybutynin.
Her symptoms improved for about 6 months, but then progressed and have now worsened. She began taking tolterodine, 4 mg daily, 2 months ago, as prescribed by her primary care physician. The sensation of painful urgency has eased, but there has been no change in frequency. R.H. used to wake as often as 4 times a night with the urge to urinate, but since she began taking zolpidem tartrate (Ambien) as a sleep aid, she now wakes only 2 times every night.
Why are her symptoms so persistent?
This woman’s case is a classic example of interstitial cystitis masquerading as overactive bladder. Treatment with anticholinergic drugs may ease urgency symptoms slightly, but has no real effect on frequency.
This case has 5 hallmarks of the syndrome of interstitial cystitis:
- Urgency
- Frequency (more than 8 voids/day, taking fluid intake into account)
- Bladder pain
- Nocturia (more than twice)
- Absence of a genitourinary tract infection
Patients show signs of “battle fatigue”
Women with interstitial cystitis may be anxious, depressed, angry, and sleep-deprived. In some women, stress exacerbates the urinary symptoms and pain (as do certain common foods and beverages, especially citrus, tomatoes, and caffeine).
Approximately 60% of patients report dyspareunia, and many report chronic pelvic pain. In fact, 75% of women who report chronic pelvic pain also have irritative voiding symptoms. Therefore, it is important to ask about lower urinary tract symptoms whenever a woman presents with pelvic pain.3,4
Pain may be suprapubic, vaginal, perineal, or originate in the groin or lower back. Although 16% of patients present solely with pain, and 30% have only urinary frequency, most patients suffer from both symptoms.
Approximately 40% report premenstrual or ovulatory exacerbation of symptoms, although symptoms may improve during pregnancy.5 Voided volumes are usually small, despite the strong urgency, which does not always resolve. Pelvic pain may ease after voiding but recurs shortly.
Insidious, worsening course
Symptoms appear insidiously and worsen to a “final” stage within 5 to 15 years, at which point a plateau is reached with little further progression.6 Some experts suggest that the disease be classified as “early non-ulcerous” or “classic ulcerous.”
- In early disease, bladder capacity exceeds 450 cc under anesthesia, with glomerulations and hemorrhage.
- In classic disease, bladder capacity is less than 450 cc under anesthesia, and Hunner’s ulcers and fissures are evident. Hunner’s ulcers are described as “a central scar with small fibrin deposits before distension, and post-distension edema.”7
For now, however, there are no agreed-upon markers to distinguish the 2 types of disease.
The female-to-male ratio is 9:1, and about 500,000 to more than 1 million adults in the United States are thought to have interstitial cystitis.8 Caucasian women constitute 95% of patients, and the average age at diagnosis is 45 years. Thirty percent of women with interstitial cystitis are 30 years old or younger. Significantly more women with interstitial cystitis have had a hysterectomy than controls.9
For a diagnosis, skip the NIH criteria
Symptoms
Although the National Institutes of Health (NIH) established diagnostic criteria for research subjects, the criteria are overly stringent—60% of women with symptoms typical of interstitial cystitis do not qualify, but should not necessarily be excluded from diagnosis and treatment.
When a woman has the hallmark symptoms listed on page 57, but also reports continuous pain or dysmenorrhea, other pelvic pathology such as endometriosis should be considered, although interstitial cystitis should be included in the differential diagnosis of any woman reporting pelvic pain.
Incontinence is atypical. If present, it merits an incontinence evaluation to detect detrusor hyperreflexia or detrusor-sphincter dyssynergia.
Dysuria suggests a UTI, urethral diverticulum, urogenital atrophy, or vaginitis. Many patients present with an erroneous diagnosis of “recurrent UTIs.”
Diagnostic tools
Voiding diaries are useful and can be revealing. The Pelvic Pain and Urinary Frequency (PUF) scale, developed by Parsons, is helpful in predicting interstitial cystitis (see the Clip-and-save chart). The higher the score, the greater the likelihood of interstitial cystitis, particularly with a score of more than 8.
Another tool is the O’Leary-Sant Index, which measures pain, voiding symptoms, and quality of life.
Physical examination and laboratory studies
Perform a pelvic exam to rule out other diseases and pelvic pathology, including sexually transmitted diseases, urethral diverticulum, and pelvic masses. Typically, the pelvic exam in women with interstitial cystitis is negative except for suprapubic and/or trigonal tenderness.
Urinalysis, culture, and sensitivity are warranted but are usually negative.
Cytology should be analyzed if microscopic hematuria is present, or with other risk factors such as a history of smoking or age over 40.
Obtain cultures for sexually transmitted diseases if clinically indicated.
Urodynamic studies are not necessary to diagnose interstitial cystitis. However, if incontinence is present, a cystometrogram can confirm detrusor hyperreflexia. Otherwise the cystometrogram is normal except for heightened sensation or pain with bladder filling, or a bladder capacity of less than 350 cc.
The potassium sensitivity test: Useful but painful
Women with interstitial cystitis are thought to have increased bladder permeability that allows potassium to pass through to the detrusor muscle. Thus, the potassium sensitivity test often is used to diagnose the condition. The test is an office procedure in which 2 separate solutions are instilled into the bladder: 40 cc sterile water followed by 40 cc of a solution of 400 mEq potassium per liter of water. After each solution is instilled, the patient is monitored for symptoms. The test is positive when the patient responds only to the potassium.
The response may be marked and painful, and the bladder should be emptied immediately. Subsequent irrigation with sterile water may be necessary to alleviate the discomfort caused by the potassium solution. Symptoms provoked by the test generally subside after bladder emptying, but can persist and cause moderate distress, which limits the utility of this office-based test.
Parsons et al10 demonstrated an 81% positive response (197 of 244 women) to the test among women with pelvic pain, compared with 0 of 47 patients with no pelvic pain. They also found that 70% of patients with interstitial cystitis and 4% of controls had a positive response.
If a woman is extremely volume-sensitive during the water phase, the potassium phase may not be accurate. A false-positive response can be caused by infection or prior exposure to radiation or chemotherapy. A thorough history is imperative.
The gold standard: Cystoscopy under anesthesia
Cystoscopy with hydrodistention under general anesthesia is the surest way to diagnose interstitial cystitis or rule it out. Sterile water or saline is infused until bladder capacity is reached. Bladder rupture occurs in up to 10% of patients, so careful inspection during filling is crucial. After 5 minutes of distension, bladder volume is measured into a calibrated beaker. Terminal hematuria (the last 50 cc of effluent) often is noted.
Normal bladder capacity under anesthesia is 1,000 cc, but it is reduced in women with interstitial cystitis. Bladder capacity of 450 cc or less under anesthesia indicates a more contracted bladder and a later-stage disease. Glomerulations, petechiae, fissures, or (rarely) Hunner’s ulcers typically are visible, regardless of bladder volume. However, the presence of glomerulations does not necessarily make the diagnosis, because they can be found in asymptomatic women. Further, cystoscopic observations do not always correlate with the severity of symptoms (nor does positive biopsy always reflect interstitial cystitis).
Hydrodistention is not only diagnostic, but also can be therapeutic, as sympathetic nerve fiber density decreases afterward.11 However, the need for this procedure is under debate, due to the limitations described above. A bladder capacity less than 1,000 cc with the presence of glomerulations or petechiae and fissures, with or without the Hunner’s ulcers, constitutes a definitive diagnosis.
Cystoscopy under anesthesia is recommended because medical treatment can be costly and cause significant side effects. An accurate diagnosis should precede therapy to avoid misdirected therapy in a patient who does not have interstitial cystitis. Moreover, cystoscopy can rule out bladder neoplasms or other diseases. Some bladder carcinomas have been missed in women treated empirically for interstitial cystitis.12
Cystoscopic images “paint a thousand words.” When a woman sees her cystoscopy images, the picture indeed “paints a thousand words.” For many women, the images “justify” their symptoms and confirm that the disease is real.
CASE Don’t treat a UTI without a positive culture
“M.P.” is a healthy 44-year-old G2P2 with a history of recurrent UTIs. Approximately 14 months ago, while on vacation, she began having symptoms of urinary frequency, urgency, and lower abdominal pain that were relieved with voiding. She called her primary care physician, who prescribed levofloxacin and phenazopyridine over the phone for a presumed UTI. Since the patient was out of town, a urine culture was not obtained.
When M.P. returned from vacation, her symptoms recurred, so she underwent urinalysis, including culture and sensitivity, and began a 7-day course of nitrofurantoin (100 mg twice daily). When her symptoms did not improve by day 4, a second course of levofloxacin was given. The urine culture was sterile. As her physician recommended, M.P. increased her fluid intake, including water and cranberry juice. She also avoided sexual relations, since they exacerbated her symptoms, which improved overall but did not clearly abate.
Three months later her symptoms returned in full force.
How would you treat this patient?
Interstitial cystitis can produce symptoms consistent with a lower UTI, but urine cultures will be negative and the response to antibiotics will be minimal. Many patients call their physicians and report “another UTI.” However, if the woman is healthy with no history of renal disease or diabetes, consider interstitial cystitis. Obtain urine culture results from other physicians, if possible, to determine whether bacterial infection was ever confirmed.
Cranberry juice is acidic and may exacerbate urgency and pain.
What to tell patients
The Interstitial Cystitis Association (ICA) encourages patients to become involved in their own care. The ICA was formed in 1984 by women with painful bladder symptoms who had been told by their physicians that nothing was wrong. The organization provides patients with clinical research updates, clinical trial opportunities, and literature and information.
Once the diagnosis is confirmed, patient education and counseling are imperative. Compliance is critical.
There is no cure for interstitial cystitis; the disease is chronic, with relapses and remissions. Although it does not progress once it develops fully, improvement is slow, usually occurring after 3 months or more of treatment. No single treatment works for all patients, so empiric trials with various agents may be needed. Treatment often is multimodal, and the rationale for each therapy should be explained.
Have the patient keep a voiding diary before and after treatment, as well as during any flare-up, to provide evidence of improvement and identify triggers. Also instruct her to pay attention to any foods or activities that exacerbate her symptoms (eg, caffeine, sexual activity).
Treatment
Does a change in diet help?
Some foods and beverages apparently exacerbate symptoms, although the link between foods and symptoms has not been fully investigated. About 53% of patients with interstitial cystitis associate symptom aggravation with dietary factors, especially acidic foods and beverages.5 Dietary restrictions should be attempted for 1 to 2 weeks to determine which foods to avoid.
Gillespie13 found elevated urine levels of tryptophan metabolites in women with hypersensitive bladders, compared with controls.
Tryptophan metabolites may disrupt the glycosaminoglycan layer of the bladder epithelium, as seen in a study involving rabbit bladders.14
Urge suppression
Another helpful strategy is having the patient increase the time between voids using distraction techniques and by contracting the pelvic floor muscles and overriding the first urge to void.
Oral medications
Pentosan polysulfate sodium is a glycosaminoglycan with an affinity for mucosal membranes. It is approved by the Food and Drug Administration (FDA) for the treatment of interstitial cystitis. The mechanism by which it reduces pain and urinary frequency is unclear, but it may replace the deficient glycosaminoglycan layer in the bladder epithelium.
Pain relief occurs in approximately 40% to 60% of patients after 3 months of therapy (100 mg orally 3 times daily).15
The patient should clearly understand that beneficial effects may not occur for 3 to 6 months, and that patience is necessary to give the drug an adequate trial. The response is maintained over the long term, and the drug should be used indefinitely. Pentosan polysulfate sodium is well tolerated, although gastrointestinal side effects and reversible alopecia occur in 4% of patients.
Performing a cystoscopy under anesthesia with hydrodistension is not always necessary prior to starting pentosan polysulfate, as long as the patient is not at risk for bladder neoplasms. However, prior to starting the drug the minimal evaluation should include a voiding diary and either the PUF questionnaire or the potassium sensitivity test.
Antihistamines. If the patient has a history of allergies, or mast cells were confirmed on bladder biopsy, an antihistamine such as hydroxyzine should be given along with pentosan polysulfate sodium. Hydroxyzine has an inhibitory effect on bladder mast cells, as well as anticholinergic and analgesic properties, which improve typical symptoms of interstitial cystitis.
Initiate hydroxyzine at a dose of 10 to 25 mg at bedtime for 1 week, gradually increasing to 50 to 75 mg. Side effects include drowsiness, which is beneficial for women who have nocturia. Other effects are dry mouth and a bitter taste.
Amitriptyline hydrochloride also has analgesic, antihistaminic, anticholinergic, and sedative effects. Amitriptyline is a noradrenaline and serotonin reuptake inhibitor that blocks nociception in the central nervous system.
Compared with placebo, amitriptyline significantly improved symptom scores, pain, and urgency intensity. In a study by van Ophoven and colleagues,16 50 patients (44 women, 6 men) were randomly assigned to amitriptyline at self-titrating doses or placebo. O’Leary-Sant symptom scores, pain, and urgency intensity improved significantly in the amitriptyline group, compared with placebo.
Anticholinergic side effects (eg, dry mouth, constipation), weight gain, and sedation occur in 20% to 80% of patients. In an open-label study of amitriptyline for interstitial cystitis,17 long-term efficacy (mean of 17 months) revealed a 64% response rate (60 of 94 patients) using the global response assessment questionnaire.
Start amitriptyline at a dose of 10 to 25 mg at bedtime, gradually increasing to 75 mg as tolerated. Sedation becomes a limiting factor in the higher doses. Other tricyclic antidepressants have not been studied to any significant extent in treating interstitial cystitis. When used as part of a multimodal treatment in addition to pentosan polysulfate sodium, amitriptyline may be tapered off once remission is attained. No studies have compared treatment response using pentosan polysulfate sodium with and without amitriptyline.
Calcium channel blockers (nifedipine) and drugs for neuropathic pain (gabapentin) are being investigated.
Anticholinergic and antispasmodic agents are typically ineffective in women with interstitial cystitis. In fact, if a patient has no improvement in her symptoms after these drugs are tried, interstitial cystitis should be strongly considered.
Oral L-arginine (1,500–3,000 mg per day, divided doses) improved symptoms in a small study by increasing nitric oxide synthase activity.18
NSAIDs are used adjunctively and may help reduce pain.
Intravesical therapy
Patients unable to tolerate oral medications may benefit from intravesical therapy. It can also be used as an adjunct to oral therapy. Intravesical therapy delivers drugs directly to the bladder wall with a low incidence of side effects. Risks include a potential for UTI via catheterization, as well as transient chemical cystitis, which exacerbates symptoms. A variety of therapeutic “cocktails” are used.
Dimethyl sulfoxide (DMSO) is the only other drug, besides pentosan polysulfate, approved by the FDA for treatment of interstitial cystitis. DMSO has antiinflammatory, analgesic, and muscle-relaxant effects, and inhibits mast-cell activity. DMSO induces remission in 50% to 70% of patients for up to 24 months.19
Lidocaine jelly is injected intraurethrally, followed by instillation of 50 cc of DMSO (alone or with heparin, sodium bicarbonate, and Solu-Cortef). This solution is held in the bladder for 20 to 30 minutes before voiding.
DMSO is secreted through the lungs and skin and has a garlic-like odor. Treatments are administered every 1 to 2 weeks for a total of 4 to 8 treatments. If the condition relapses, DMSO can be reinstituted on a long-term basis. Motivated patients can be taught to administer this treatment themselves.
Heparin is another option. It is administered at a dose of 10,000 U thrice weekly.
Hyaluronic acid. In a small study involving 20 patients, weekly intravesical hyaluronic acid improved symptoms in 65% of patients, with a 40% and 30% decrease in nocturia and pain, respectively.20
Intravesical pentosan polysulfate sodium is another option that improves symptoms and increases bladder capacity,21 although larger studies of its efficacy are lacking.
Bacillus Calmette-Guérin (BCG) solution intravesically had a 60% response rate in 1 study (versus 27% for placebo).22 The mechanism of action is unknown, but the solution may modulate the bladder immune response. Additional studies are pending.
Although their link to interstitial cystitis has not been proven, these foods are thought to be implicated and should be limited or avoided
ACIDIC FOODS
All alcoholic beverages
Apples
Apple juice
Cantaloupe
Carbonated drinks
Chilies/spicy foods
Citrus fruits (lemons, limes, oranges, etc)
Coffee
Cranberries
Grapes
Guava
Lemon juice
Peaches
Pineapples
Plums
Strawberries
Tea
Tomatoes
Vinegar
FOODS HIGH IN TYROSINE, TRYPTOPHAN, OR ASPARTATE
Aspartame
Avocado
Bananas
Beer
Brewer’s yeast
Canned figs
Champagne
Cheese
Chicken livers
Chocolate
Corned beef
Cranberries
Fava beans
Lima beans
Mayonnaise
Nuts
Onions
Pickled herring
Pineapple
Prunes
Raisins
Rye bread
Saccharine
Sour cream
Soy sauce
Wines
Yogurt
Vitamins buffered with aspartate
Adapted from You Don’t Have to Live with Cystitis! by Larrian Gillespie, MD (revised and updated 1996)
1. Altered bladder permeability
In interstitial cystitis, the protective glycosaminoglycan layer of the bladder epithelium may be deficient, increasing bladder permeability. Antiproliferative factor in the urine may impair the proliferation and repair of urothelium, increasing bladder permeability further.28 This breakdown allows potassium to penetrate the urothelium, stimulating pain receptors and causing an inflammatory response in the detrusor muscle.
Loss of the normal protective layer
In a normal bladder, the epithelium is protected by an ionic, hydrophilic, glycosaminoglycan (GAG) layer that serves as a barrier against urine, which is hyperosmolar and rich in acid and potassium. When the GAG layer is deficient, as it is thought to be in interstitial cystitis, bladder permeability increases, triggering an inflammatory response that inhibits repair of the GAG layer, creating a vicious cycle of exposure, inflammation, and pain.
2. Mast cell activation
Mast cell degranulation may cause or contribute to interstitial cystitis and produce its hallmark symptoms. Mast cells also may be activated in response to a noxious factor. These cells secrete histamine, prostaglandins, leukotrienes, cytokines, and chemotactic factors. Urine from women with interstitial cystitis contains histamine, histamine metabolites, and tryptase,29,30 and electron microscopy of bladder biopsies from affected women shows degranulating mast cells adjacent to sensory nerve fibers. When these fibers are stimulated, they release neuropeptides (such as substance P) and may promote inflammation by activating mast cells and nearby nerve terminals.31
3. Inflammation
Inflammation clearly plays a role in interstitial cystitis. Bladder biopsies reveal mild to severe inflammation, and the presence of T cells, B cells, plasma cells, neutrophils, eosinophils, and mast cells. Inflammatory mediators such as kallikrein, interleukin-6, interleukin-2 inhibitor, and neutrophil chemotactic factor are increased in the urine of individuals with the disease.
4. Autoimmunity
Clinical features of interstitial cystitis that mimic those of other autoimmune diseases include chronic symptoms that wax and wane; higher prevalence in women; immunological deposits in bladder biopsies with mononuclear cell infiltrates, which suggests the presence of bladder autoantigens; association with other autoimmune disorders such as Sjögren’s syndrome and lupus; and, in some cases, a positive response to steroids or other immunosuppressants.32
Glomerulations and fissures are telltale signs
When the bladder is distended under anesthesia, examination may reveal a pattern of fissures and glomerulations, which are hallmarks of interstitial cystitis. These findings are sometimes present in asymptomatic women as well, and do not always correlate with severity of symptoms.
5. Infection
Some experts have postulated that occult infection with fastidious organisms, fungi, or viruses plays a role in the development of interstitial cystitis. However, special cultures, serology, and electron microscopy have not shown any organisms consistently associated with the disease.33
The use of polymerase chain reaction techniques to test for bacterial DNA in bladder biopsies of patients with interstitial cystitis has yielded conflicting results.34,35 Infection may be the inciting event that injures the bladder epithelium, causing a cascade of inflammation.
6. Neurologic changes
Studies have revealed increased sympathetic nerve fiber density in the bladders of patients with interstitial cystitis.31,36 The disease may also be a type of reflex-sympathetic dystrophy with increased and abnormal spinal sympathetic activity. Butrick describes interstitial cystitis as a “visceral pain syndrome.”37 C-fibers (silent afferents) transmit pain when activated by a prolonged or noxious stimulus. This leads to neuroplastic changes that lower the threshold of nociceptive nerves, thus reducing the amount of stimulus needed to provoke pain (allodynia). Pelvic viscera share innervation, which may explain the association between
interstitial cystitis and irritable bowel syndrome and endometriosis. Interstitial cystitis is not limited to the bladder; it involves chronic neuropathic inflammation, afferent overactivity, and central sensitization. Increased pain perception may cause pelvic floor muscle instability, spasm, and hypertonic state.38
Alternative therapies
Electrical nerve stimulation
This transcutaneous modality improves symptoms in 25% to 50% of patients.23 It is thought to stimulate the afferent nerves, thereby activating the inhibitory circuits and decreasing the sensation of pain.
Sacral neuromodulation is another modality being studied for the treatment of interstitial cystitis. So far it has significantly reduced urinary urgency-frequency symptoms as well as pain.24
Other new therapies under investigation include intravesical injection of botulinum toxin, resiniferatoxin, gene therapy, and nerve growth-factor inhibitors.
Surgery: High relapse rates make it a last resort
The treatment of visible ulcers by resection or laser ablation improves symptoms but carries a relapse rate of more than 50%.25 More aggressive surgeries, with cure rates ranging from 50% to 80%, include denervation procedures, augmentation cystoplasty for severely contracted bladders (not necessarily due to interstitial cystitis), cecocystoplasty (where a segment of cecum is excised and reanastomosed with the bladder to increase bladder capacity), and total cystourethrectomy and urinary diversion. However, persistent pain has been reported after these invasive procedures, and permanent intermittent self-catheterization and/or reoperation is often required.26,27
Dr. LaSala is a speaker for Pfizer, Inc.
The typical patient voids 16 times a day and 2 or more times at night. In later stages of the disease, she may urinate as often as 60 times a day and every half hour at night, severely eroding her ability to hold a job, travel, or lead a normal life. In fact, her quality of life may be impaired as much as that of a person with end-stage renal disease.1,2 She sees an average of 4 doctors and endures irritative voiding symptoms for 4 years before her disease is identified. The cause is unknown.
Interstitial cystitis produces a wide spectrum of symptom severity, occurring episodically with spontaneous flare-ups and remission, or with continuous, intractable urinary urgency and pain. Until recently, women presenting with urinary urgency, frequency, and pain were presumed to have a urinary tract infection (UTI) or overactive bladder, and were often treated—to no avail—with multiple courses of antibiotics or anticholinergics.
Fortunately, interstitial cystitis is gaining recognition, and effective treatments are emerging. Usually the ObGyn—often the first physician a woman consults—need refer only the refractory cases to a specialist. This article describes the components of diagnosis and the most effective treatments, including use of the first-line agents amitriptyline (Elavil) and pentosan polysulfate sodium (Elmiron).
CASE Is overactive bladder the cause of stubborn symptoms?
“R.H.,” a healthy 48-year-old G2P2 with a 5-year history of urinary urgency and frequency, reports that she voids “at least 15 times per day.” She denies any urge incontinence, but says she experiences occasional stress incontinence if she has a bad cold. Four years ago, she saw a urologist for these symptoms, after her husband said he was tired of having to stop the car so she could go to the bathroom. The urologist diagnosed a “small bladder,” performed urethral “stretching,” and prescribed oxybutynin.
Her symptoms improved for about 6 months, but then progressed and have now worsened. She began taking tolterodine, 4 mg daily, 2 months ago, as prescribed by her primary care physician. The sensation of painful urgency has eased, but there has been no change in frequency. R.H. used to wake as often as 4 times a night with the urge to urinate, but since she began taking zolpidem tartrate (Ambien) as a sleep aid, she now wakes only 2 times every night.
Why are her symptoms so persistent?
This woman’s case is a classic example of interstitial cystitis masquerading as overactive bladder. Treatment with anticholinergic drugs may ease urgency symptoms slightly, but has no real effect on frequency.
This case has 5 hallmarks of the syndrome of interstitial cystitis:
- Urgency
- Frequency (more than 8 voids/day, taking fluid intake into account)
- Bladder pain
- Nocturia (more than twice)
- Absence of a genitourinary tract infection
Patients show signs of “battle fatigue”
Women with interstitial cystitis may be anxious, depressed, angry, and sleep-deprived. In some women, stress exacerbates the urinary symptoms and pain (as do certain common foods and beverages, especially citrus, tomatoes, and caffeine).
Approximately 60% of patients report dyspareunia, and many report chronic pelvic pain. In fact, 75% of women who report chronic pelvic pain also have irritative voiding symptoms. Therefore, it is important to ask about lower urinary tract symptoms whenever a woman presents with pelvic pain.3,4
Pain may be suprapubic, vaginal, perineal, or originate in the groin or lower back. Although 16% of patients present solely with pain, and 30% have only urinary frequency, most patients suffer from both symptoms.
Approximately 40% report premenstrual or ovulatory exacerbation of symptoms, although symptoms may improve during pregnancy.5 Voided volumes are usually small, despite the strong urgency, which does not always resolve. Pelvic pain may ease after voiding but recurs shortly.
Insidious, worsening course
Symptoms appear insidiously and worsen to a “final” stage within 5 to 15 years, at which point a plateau is reached with little further progression.6 Some experts suggest that the disease be classified as “early non-ulcerous” or “classic ulcerous.”
- In early disease, bladder capacity exceeds 450 cc under anesthesia, with glomerulations and hemorrhage.
- In classic disease, bladder capacity is less than 450 cc under anesthesia, and Hunner’s ulcers and fissures are evident. Hunner’s ulcers are described as “a central scar with small fibrin deposits before distension, and post-distension edema.”7
For now, however, there are no agreed-upon markers to distinguish the 2 types of disease.
The female-to-male ratio is 9:1, and about 500,000 to more than 1 million adults in the United States are thought to have interstitial cystitis.8 Caucasian women constitute 95% of patients, and the average age at diagnosis is 45 years. Thirty percent of women with interstitial cystitis are 30 years old or younger. Significantly more women with interstitial cystitis have had a hysterectomy than controls.9
For a diagnosis, skip the NIH criteria
Symptoms
Although the National Institutes of Health (NIH) established diagnostic criteria for research subjects, the criteria are overly stringent—60% of women with symptoms typical of interstitial cystitis do not qualify, but should not necessarily be excluded from diagnosis and treatment.
When a woman has the hallmark symptoms listed on page 57, but also reports continuous pain or dysmenorrhea, other pelvic pathology such as endometriosis should be considered, although interstitial cystitis should be included in the differential diagnosis of any woman reporting pelvic pain.
Incontinence is atypical. If present, it merits an incontinence evaluation to detect detrusor hyperreflexia or detrusor-sphincter dyssynergia.
Dysuria suggests a UTI, urethral diverticulum, urogenital atrophy, or vaginitis. Many patients present with an erroneous diagnosis of “recurrent UTIs.”
Diagnostic tools
Voiding diaries are useful and can be revealing. The Pelvic Pain and Urinary Frequency (PUF) scale, developed by Parsons, is helpful in predicting interstitial cystitis (see the Clip-and-save chart). The higher the score, the greater the likelihood of interstitial cystitis, particularly with a score of more than 8.
Another tool is the O’Leary-Sant Index, which measures pain, voiding symptoms, and quality of life.
Physical examination and laboratory studies
Perform a pelvic exam to rule out other diseases and pelvic pathology, including sexually transmitted diseases, urethral diverticulum, and pelvic masses. Typically, the pelvic exam in women with interstitial cystitis is negative except for suprapubic and/or trigonal tenderness.
Urinalysis, culture, and sensitivity are warranted but are usually negative.
Cytology should be analyzed if microscopic hematuria is present, or with other risk factors such as a history of smoking or age over 40.
Obtain cultures for sexually transmitted diseases if clinically indicated.
Urodynamic studies are not necessary to diagnose interstitial cystitis. However, if incontinence is present, a cystometrogram can confirm detrusor hyperreflexia. Otherwise the cystometrogram is normal except for heightened sensation or pain with bladder filling, or a bladder capacity of less than 350 cc.
The potassium sensitivity test: Useful but painful
Women with interstitial cystitis are thought to have increased bladder permeability that allows potassium to pass through to the detrusor muscle. Thus, the potassium sensitivity test often is used to diagnose the condition. The test is an office procedure in which 2 separate solutions are instilled into the bladder: 40 cc sterile water followed by 40 cc of a solution of 400 mEq potassium per liter of water. After each solution is instilled, the patient is monitored for symptoms. The test is positive when the patient responds only to the potassium.
The response may be marked and painful, and the bladder should be emptied immediately. Subsequent irrigation with sterile water may be necessary to alleviate the discomfort caused by the potassium solution. Symptoms provoked by the test generally subside after bladder emptying, but can persist and cause moderate distress, which limits the utility of this office-based test.
Parsons et al10 demonstrated an 81% positive response (197 of 244 women) to the test among women with pelvic pain, compared with 0 of 47 patients with no pelvic pain. They also found that 70% of patients with interstitial cystitis and 4% of controls had a positive response.
If a woman is extremely volume-sensitive during the water phase, the potassium phase may not be accurate. A false-positive response can be caused by infection or prior exposure to radiation or chemotherapy. A thorough history is imperative.
The gold standard: Cystoscopy under anesthesia
Cystoscopy with hydrodistention under general anesthesia is the surest way to diagnose interstitial cystitis or rule it out. Sterile water or saline is infused until bladder capacity is reached. Bladder rupture occurs in up to 10% of patients, so careful inspection during filling is crucial. After 5 minutes of distension, bladder volume is measured into a calibrated beaker. Terminal hematuria (the last 50 cc of effluent) often is noted.
Normal bladder capacity under anesthesia is 1,000 cc, but it is reduced in women with interstitial cystitis. Bladder capacity of 450 cc or less under anesthesia indicates a more contracted bladder and a later-stage disease. Glomerulations, petechiae, fissures, or (rarely) Hunner’s ulcers typically are visible, regardless of bladder volume. However, the presence of glomerulations does not necessarily make the diagnosis, because they can be found in asymptomatic women. Further, cystoscopic observations do not always correlate with the severity of symptoms (nor does positive biopsy always reflect interstitial cystitis).
Hydrodistention is not only diagnostic, but also can be therapeutic, as sympathetic nerve fiber density decreases afterward.11 However, the need for this procedure is under debate, due to the limitations described above. A bladder capacity less than 1,000 cc with the presence of glomerulations or petechiae and fissures, with or without the Hunner’s ulcers, constitutes a definitive diagnosis.
Cystoscopy under anesthesia is recommended because medical treatment can be costly and cause significant side effects. An accurate diagnosis should precede therapy to avoid misdirected therapy in a patient who does not have interstitial cystitis. Moreover, cystoscopy can rule out bladder neoplasms or other diseases. Some bladder carcinomas have been missed in women treated empirically for interstitial cystitis.12
Cystoscopic images “paint a thousand words.” When a woman sees her cystoscopy images, the picture indeed “paints a thousand words.” For many women, the images “justify” their symptoms and confirm that the disease is real.
CASE Don’t treat a UTI without a positive culture
“M.P.” is a healthy 44-year-old G2P2 with a history of recurrent UTIs. Approximately 14 months ago, while on vacation, she began having symptoms of urinary frequency, urgency, and lower abdominal pain that were relieved with voiding. She called her primary care physician, who prescribed levofloxacin and phenazopyridine over the phone for a presumed UTI. Since the patient was out of town, a urine culture was not obtained.
When M.P. returned from vacation, her symptoms recurred, so she underwent urinalysis, including culture and sensitivity, and began a 7-day course of nitrofurantoin (100 mg twice daily). When her symptoms did not improve by day 4, a second course of levofloxacin was given. The urine culture was sterile. As her physician recommended, M.P. increased her fluid intake, including water and cranberry juice. She also avoided sexual relations, since they exacerbated her symptoms, which improved overall but did not clearly abate.
Three months later her symptoms returned in full force.
How would you treat this patient?
Interstitial cystitis can produce symptoms consistent with a lower UTI, but urine cultures will be negative and the response to antibiotics will be minimal. Many patients call their physicians and report “another UTI.” However, if the woman is healthy with no history of renal disease or diabetes, consider interstitial cystitis. Obtain urine culture results from other physicians, if possible, to determine whether bacterial infection was ever confirmed.
Cranberry juice is acidic and may exacerbate urgency and pain.
What to tell patients
The Interstitial Cystitis Association (ICA) encourages patients to become involved in their own care. The ICA was formed in 1984 by women with painful bladder symptoms who had been told by their physicians that nothing was wrong. The organization provides patients with clinical research updates, clinical trial opportunities, and literature and information.
Once the diagnosis is confirmed, patient education and counseling are imperative. Compliance is critical.
There is no cure for interstitial cystitis; the disease is chronic, with relapses and remissions. Although it does not progress once it develops fully, improvement is slow, usually occurring after 3 months or more of treatment. No single treatment works for all patients, so empiric trials with various agents may be needed. Treatment often is multimodal, and the rationale for each therapy should be explained.
Have the patient keep a voiding diary before and after treatment, as well as during any flare-up, to provide evidence of improvement and identify triggers. Also instruct her to pay attention to any foods or activities that exacerbate her symptoms (eg, caffeine, sexual activity).
Treatment
Does a change in diet help?
Some foods and beverages apparently exacerbate symptoms, although the link between foods and symptoms has not been fully investigated. About 53% of patients with interstitial cystitis associate symptom aggravation with dietary factors, especially acidic foods and beverages.5 Dietary restrictions should be attempted for 1 to 2 weeks to determine which foods to avoid.
Gillespie13 found elevated urine levels of tryptophan metabolites in women with hypersensitive bladders, compared with controls.
Tryptophan metabolites may disrupt the glycosaminoglycan layer of the bladder epithelium, as seen in a study involving rabbit bladders.14
Urge suppression
Another helpful strategy is having the patient increase the time between voids using distraction techniques and by contracting the pelvic floor muscles and overriding the first urge to void.
Oral medications
Pentosan polysulfate sodium is a glycosaminoglycan with an affinity for mucosal membranes. It is approved by the Food and Drug Administration (FDA) for the treatment of interstitial cystitis. The mechanism by which it reduces pain and urinary frequency is unclear, but it may replace the deficient glycosaminoglycan layer in the bladder epithelium.
Pain relief occurs in approximately 40% to 60% of patients after 3 months of therapy (100 mg orally 3 times daily).15
The patient should clearly understand that beneficial effects may not occur for 3 to 6 months, and that patience is necessary to give the drug an adequate trial. The response is maintained over the long term, and the drug should be used indefinitely. Pentosan polysulfate sodium is well tolerated, although gastrointestinal side effects and reversible alopecia occur in 4% of patients.
Performing a cystoscopy under anesthesia with hydrodistension is not always necessary prior to starting pentosan polysulfate, as long as the patient is not at risk for bladder neoplasms. However, prior to starting the drug the minimal evaluation should include a voiding diary and either the PUF questionnaire or the potassium sensitivity test.
Antihistamines. If the patient has a history of allergies, or mast cells were confirmed on bladder biopsy, an antihistamine such as hydroxyzine should be given along with pentosan polysulfate sodium. Hydroxyzine has an inhibitory effect on bladder mast cells, as well as anticholinergic and analgesic properties, which improve typical symptoms of interstitial cystitis.
Initiate hydroxyzine at a dose of 10 to 25 mg at bedtime for 1 week, gradually increasing to 50 to 75 mg. Side effects include drowsiness, which is beneficial for women who have nocturia. Other effects are dry mouth and a bitter taste.
Amitriptyline hydrochloride also has analgesic, antihistaminic, anticholinergic, and sedative effects. Amitriptyline is a noradrenaline and serotonin reuptake inhibitor that blocks nociception in the central nervous system.
Compared with placebo, amitriptyline significantly improved symptom scores, pain, and urgency intensity. In a study by van Ophoven and colleagues,16 50 patients (44 women, 6 men) were randomly assigned to amitriptyline at self-titrating doses or placebo. O’Leary-Sant symptom scores, pain, and urgency intensity improved significantly in the amitriptyline group, compared with placebo.
Anticholinergic side effects (eg, dry mouth, constipation), weight gain, and sedation occur in 20% to 80% of patients. In an open-label study of amitriptyline for interstitial cystitis,17 long-term efficacy (mean of 17 months) revealed a 64% response rate (60 of 94 patients) using the global response assessment questionnaire.
Start amitriptyline at a dose of 10 to 25 mg at bedtime, gradually increasing to 75 mg as tolerated. Sedation becomes a limiting factor in the higher doses. Other tricyclic antidepressants have not been studied to any significant extent in treating interstitial cystitis. When used as part of a multimodal treatment in addition to pentosan polysulfate sodium, amitriptyline may be tapered off once remission is attained. No studies have compared treatment response using pentosan polysulfate sodium with and without amitriptyline.
Calcium channel blockers (nifedipine) and drugs for neuropathic pain (gabapentin) are being investigated.
Anticholinergic and antispasmodic agents are typically ineffective in women with interstitial cystitis. In fact, if a patient has no improvement in her symptoms after these drugs are tried, interstitial cystitis should be strongly considered.
Oral L-arginine (1,500–3,000 mg per day, divided doses) improved symptoms in a small study by increasing nitric oxide synthase activity.18
NSAIDs are used adjunctively and may help reduce pain.
Intravesical therapy
Patients unable to tolerate oral medications may benefit from intravesical therapy. It can also be used as an adjunct to oral therapy. Intravesical therapy delivers drugs directly to the bladder wall with a low incidence of side effects. Risks include a potential for UTI via catheterization, as well as transient chemical cystitis, which exacerbates symptoms. A variety of therapeutic “cocktails” are used.
Dimethyl sulfoxide (DMSO) is the only other drug, besides pentosan polysulfate, approved by the FDA for treatment of interstitial cystitis. DMSO has antiinflammatory, analgesic, and muscle-relaxant effects, and inhibits mast-cell activity. DMSO induces remission in 50% to 70% of patients for up to 24 months.19
Lidocaine jelly is injected intraurethrally, followed by instillation of 50 cc of DMSO (alone or with heparin, sodium bicarbonate, and Solu-Cortef). This solution is held in the bladder for 20 to 30 minutes before voiding.
DMSO is secreted through the lungs and skin and has a garlic-like odor. Treatments are administered every 1 to 2 weeks for a total of 4 to 8 treatments. If the condition relapses, DMSO can be reinstituted on a long-term basis. Motivated patients can be taught to administer this treatment themselves.
Heparin is another option. It is administered at a dose of 10,000 U thrice weekly.
Hyaluronic acid. In a small study involving 20 patients, weekly intravesical hyaluronic acid improved symptoms in 65% of patients, with a 40% and 30% decrease in nocturia and pain, respectively.20
Intravesical pentosan polysulfate sodium is another option that improves symptoms and increases bladder capacity,21 although larger studies of its efficacy are lacking.
Bacillus Calmette-Guérin (BCG) solution intravesically had a 60% response rate in 1 study (versus 27% for placebo).22 The mechanism of action is unknown, but the solution may modulate the bladder immune response. Additional studies are pending.
Although their link to interstitial cystitis has not been proven, these foods are thought to be implicated and should be limited or avoided
ACIDIC FOODS
All alcoholic beverages
Apples
Apple juice
Cantaloupe
Carbonated drinks
Chilies/spicy foods
Citrus fruits (lemons, limes, oranges, etc)
Coffee
Cranberries
Grapes
Guava
Lemon juice
Peaches
Pineapples
Plums
Strawberries
Tea
Tomatoes
Vinegar
FOODS HIGH IN TYROSINE, TRYPTOPHAN, OR ASPARTATE
Aspartame
Avocado
Bananas
Beer
Brewer’s yeast
Canned figs
Champagne
Cheese
Chicken livers
Chocolate
Corned beef
Cranberries
Fava beans
Lima beans
Mayonnaise
Nuts
Onions
Pickled herring
Pineapple
Prunes
Raisins
Rye bread
Saccharine
Sour cream
Soy sauce
Wines
Yogurt
Vitamins buffered with aspartate
Adapted from You Don’t Have to Live with Cystitis! by Larrian Gillespie, MD (revised and updated 1996)
1. Altered bladder permeability
In interstitial cystitis, the protective glycosaminoglycan layer of the bladder epithelium may be deficient, increasing bladder permeability. Antiproliferative factor in the urine may impair the proliferation and repair of urothelium, increasing bladder permeability further.28 This breakdown allows potassium to penetrate the urothelium, stimulating pain receptors and causing an inflammatory response in the detrusor muscle.
Loss of the normal protective layer
In a normal bladder, the epithelium is protected by an ionic, hydrophilic, glycosaminoglycan (GAG) layer that serves as a barrier against urine, which is hyperosmolar and rich in acid and potassium. When the GAG layer is deficient, as it is thought to be in interstitial cystitis, bladder permeability increases, triggering an inflammatory response that inhibits repair of the GAG layer, creating a vicious cycle of exposure, inflammation, and pain.
2. Mast cell activation
Mast cell degranulation may cause or contribute to interstitial cystitis and produce its hallmark symptoms. Mast cells also may be activated in response to a noxious factor. These cells secrete histamine, prostaglandins, leukotrienes, cytokines, and chemotactic factors. Urine from women with interstitial cystitis contains histamine, histamine metabolites, and tryptase,29,30 and electron microscopy of bladder biopsies from affected women shows degranulating mast cells adjacent to sensory nerve fibers. When these fibers are stimulated, they release neuropeptides (such as substance P) and may promote inflammation by activating mast cells and nearby nerve terminals.31
3. Inflammation
Inflammation clearly plays a role in interstitial cystitis. Bladder biopsies reveal mild to severe inflammation, and the presence of T cells, B cells, plasma cells, neutrophils, eosinophils, and mast cells. Inflammatory mediators such as kallikrein, interleukin-6, interleukin-2 inhibitor, and neutrophil chemotactic factor are increased in the urine of individuals with the disease.
4. Autoimmunity
Clinical features of interstitial cystitis that mimic those of other autoimmune diseases include chronic symptoms that wax and wane; higher prevalence in women; immunological deposits in bladder biopsies with mononuclear cell infiltrates, which suggests the presence of bladder autoantigens; association with other autoimmune disorders such as Sjögren’s syndrome and lupus; and, in some cases, a positive response to steroids or other immunosuppressants.32
Glomerulations and fissures are telltale signs
When the bladder is distended under anesthesia, examination may reveal a pattern of fissures and glomerulations, which are hallmarks of interstitial cystitis. These findings are sometimes present in asymptomatic women as well, and do not always correlate with severity of symptoms.
5. Infection
Some experts have postulated that occult infection with fastidious organisms, fungi, or viruses plays a role in the development of interstitial cystitis. However, special cultures, serology, and electron microscopy have not shown any organisms consistently associated with the disease.33
The use of polymerase chain reaction techniques to test for bacterial DNA in bladder biopsies of patients with interstitial cystitis has yielded conflicting results.34,35 Infection may be the inciting event that injures the bladder epithelium, causing a cascade of inflammation.
6. Neurologic changes
Studies have revealed increased sympathetic nerve fiber density in the bladders of patients with interstitial cystitis.31,36 The disease may also be a type of reflex-sympathetic dystrophy with increased and abnormal spinal sympathetic activity. Butrick describes interstitial cystitis as a “visceral pain syndrome.”37 C-fibers (silent afferents) transmit pain when activated by a prolonged or noxious stimulus. This leads to neuroplastic changes that lower the threshold of nociceptive nerves, thus reducing the amount of stimulus needed to provoke pain (allodynia). Pelvic viscera share innervation, which may explain the association between
interstitial cystitis and irritable bowel syndrome and endometriosis. Interstitial cystitis is not limited to the bladder; it involves chronic neuropathic inflammation, afferent overactivity, and central sensitization. Increased pain perception may cause pelvic floor muscle instability, spasm, and hypertonic state.38
Alternative therapies
Electrical nerve stimulation
This transcutaneous modality improves symptoms in 25% to 50% of patients.23 It is thought to stimulate the afferent nerves, thereby activating the inhibitory circuits and decreasing the sensation of pain.
Sacral neuromodulation is another modality being studied for the treatment of interstitial cystitis. So far it has significantly reduced urinary urgency-frequency symptoms as well as pain.24
Other new therapies under investigation include intravesical injection of botulinum toxin, resiniferatoxin, gene therapy, and nerve growth-factor inhibitors.
Surgery: High relapse rates make it a last resort
The treatment of visible ulcers by resection or laser ablation improves symptoms but carries a relapse rate of more than 50%.25 More aggressive surgeries, with cure rates ranging from 50% to 80%, include denervation procedures, augmentation cystoplasty for severely contracted bladders (not necessarily due to interstitial cystitis), cecocystoplasty (where a segment of cecum is excised and reanastomosed with the bladder to increase bladder capacity), and total cystourethrectomy and urinary diversion. However, persistent pain has been reported after these invasive procedures, and permanent intermittent self-catheterization and/or reoperation is often required.26,27
Dr. LaSala is a speaker for Pfizer, Inc.
1. Held PJ, Janno PM, Wein AJ, et al. Epidemiology of IC. In: Hanno P, Staskin DR, Krane RJ, et al, eds. Interstitial Cystitis. New York: Springer-Verlag; 1990:29–48.
2. Ratner V, Slade D, Greene G. Interstitial cystitis: a patient’s perspective. Urol Clin North Am. 1994;21:1-5.
3. Parson CL, Bullen M, Kahn BS, et al. Gynecologic presentation of interstitial cystitis as detected by intravesical potassium sensitivity. Obstet Gynecol. 2001;98:127-132.
4. Clemons JL, Arya LA, Myers DL. Diagnosing interstitial cystitis in women with chronic pelvic pain. Obstet Gynecol. 2002;100:337-341.
5. Whitmore KE. Self-care regimens for patients with interstitial cystitis. Urol Clin North Am. 1994;21:121-130.
6. Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993;149:465-469.
7. Parsons CL. Interstitial cystitis: clinical manifestations and diagnostic criteria in over 200 cases. Neurourol Urodyn. 1990;9:241-250.
8. Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49:2-9.
9. Kozial JA. Epidemiology of interstitial cystitis. Urol Clin North Am. 1994;21:7-20.
10. Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Willems JJ. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187:1395-1400.
11. Ruggieri MR, Chelsky MJ, Rosen SI, Shickley TJ, Hanno PM. Current findings and future research avenues in the study of interstitial cystitis. Urol Clin North Am. 1994;21:163-176.
12. Tissot WD, Diokno AC, Peters KM. A referral center’s experience with transitional cell carcinoma misdiag-nosed as interstitial cystitis. J Urol. 2004;172:478-480.
13. Gillespie L. Metabolic appraisal of the effects of dietary modification on hypersensitive bladder symptoms. Br J Urol. 1993;72:293-297.
14. Kaufman JE, Anderson K, Parsons CL. Inactivation of antiadherence effect of bladder surface glycosaminoglycans as possible mechanism for carcinogenesis. Urology. 1987;30:255-258.
15. Hanno PM. Analysis of long-term Elmiron therapy for interstitial cystitis. Urology. 1997;49(suppl 5A):93-99.
16. Van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172:533-536.
17. van Ophoven, Hertle L. Long-term results of amitripty-line treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
18. Smity SD, Ehheler MA, Foster HE, Weiss RM. Improvement in interstitial cystitis symptom scores during treatment with L-arginine. J Urol. 1997;158:703-708.
19. Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol. 1988;140:36-39.
20. Kallestrup EB, Jorgensen SS, Nordling J, et al. Treatment of interstitial cystitis with Cystistat: a hyaluronic acid product. Scand J Urol Nephrol. 2005;39:143-147.
21. Bade JJ, Laseur M, Nieuwenburg A, van der Weele LT, Mensink HJ. A placebo-controlled study of intravesical pentosan polysulphate for the treatment of interstitial cystitis. Br J Urol. 1997;79:168-171.
22. Peters K, Diokno A, Steinart B, et al. The efficacy of intravesical Tice strain Bacillus Calmette-Guérin in the treatment of interstitial cystitis: a double-blind, prospective, placebo controlled trial. J Urol. 1997;157:2090-2094.
23. Fall M, Lindstrom S. Transcutaneous electrical nerve stimulation in classic and nonulcer interstitial cystitis. Urol Clin North Am. 1994;21:133-139.
24. Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: a prospective study. J Urol. 2003;169:1369-1373.
25. Malloy TR, Shanberg AM. Laser therapy for interstitial cystitis. Urol Clin North Am. 1994;21:141-144.
26. Smith RB, Van Caugh P, Skinner DG, et al. Augmentation enterocystoplasty: a critical review. J Urol. 1977;118:35-39.
27. Chakravarti A, Ganta S, Somani B, Jones MA. Caecocystoplasty for intractable interstitial cystitis: long-term results. Eur Urol. 2004;46:114-117.
28. Erickson DR, Zie SX, Bhavanandan VP, et al. A comparison of multiple urine markers for interstitial cystitis. J Urol. 2002;167:2461.-
29. El-Mansoury M, Boucher W, Sant GR, Theoharides TC. Increased urine histamine and methylhistamine in interstitial cystitis. J Urol. 1994;152:250-353.
30. Boucher W, El-Mansoury M, Pang X, Sant GR. Elevated mast cell tryptase in the urine of patients with interstitial cystitis. Br J Urol. 1995;76:94-100.
31. Pang X, Marchand J, Sant GR, et al. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. 1995;75:744-750.
32. Oravisto KJ. Interstitial cystitis as an autoimmune disease. Eur Urol. 1980;6:10-13.
33. Duncan JL, Schaeffer AJ. Do infectious agents cause interstitial cystitis? Urology. 1997;49:48-51.
34. Haarala M, Jalava J, Laato M, Kilholma P, Nurmi M, Alanen A. Absence of bacterial DNA in the bladder of patients with interstitial cystitis. J Urol. 1996;156:1843-1845.
35. Keay S, Zhang C-O, Bladwin BR, Jacobs SC, Warren JW. Polymerase chain reaction amplification of bacterial 16S rRNA genes in interstitial cystitis and control patient bladder biopsies. J Urol. 1998;159:280-283.
36. Hohenfeller M, Nunes L, Schmidt RA, Lampel A, Thuroff JW, Tanagho EA. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol. 1992;147:587-591.
37. Butrick CW. Interstitial cystitis and chronic pelvic pain: new insights in neuropathology, diagnosis and treatment. Clin Obstet Gynecol. 2003;46:812-814.
38. Moldwin RM, Fried-Siegel J, Mendelowitz F. Pelvic floor dysfunction and interstitial cystitis. J Urol. 1994;151:285A.-
1. Held PJ, Janno PM, Wein AJ, et al. Epidemiology of IC. In: Hanno P, Staskin DR, Krane RJ, et al, eds. Interstitial Cystitis. New York: Springer-Verlag; 1990:29–48.
2. Ratner V, Slade D, Greene G. Interstitial cystitis: a patient’s perspective. Urol Clin North Am. 1994;21:1-5.
3. Parson CL, Bullen M, Kahn BS, et al. Gynecologic presentation of interstitial cystitis as detected by intravesical potassium sensitivity. Obstet Gynecol. 2001;98:127-132.
4. Clemons JL, Arya LA, Myers DL. Diagnosing interstitial cystitis in women with chronic pelvic pain. Obstet Gynecol. 2002;100:337-341.
5. Whitmore KE. Self-care regimens for patients with interstitial cystitis. Urol Clin North Am. 1994;21:121-130.
6. Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993;149:465-469.
7. Parsons CL. Interstitial cystitis: clinical manifestations and diagnostic criteria in over 200 cases. Neurourol Urodyn. 1990;9:241-250.
8. Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49:2-9.
9. Kozial JA. Epidemiology of interstitial cystitis. Urol Clin North Am. 1994;21:7-20.
10. Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Willems JJ. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187:1395-1400.
11. Ruggieri MR, Chelsky MJ, Rosen SI, Shickley TJ, Hanno PM. Current findings and future research avenues in the study of interstitial cystitis. Urol Clin North Am. 1994;21:163-176.
12. Tissot WD, Diokno AC, Peters KM. A referral center’s experience with transitional cell carcinoma misdiag-nosed as interstitial cystitis. J Urol. 2004;172:478-480.
13. Gillespie L. Metabolic appraisal of the effects of dietary modification on hypersensitive bladder symptoms. Br J Urol. 1993;72:293-297.
14. Kaufman JE, Anderson K, Parsons CL. Inactivation of antiadherence effect of bladder surface glycosaminoglycans as possible mechanism for carcinogenesis. Urology. 1987;30:255-258.
15. Hanno PM. Analysis of long-term Elmiron therapy for interstitial cystitis. Urology. 1997;49(suppl 5A):93-99.
16. Van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172:533-536.
17. van Ophoven, Hertle L. Long-term results of amitripty-line treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
18. Smity SD, Ehheler MA, Foster HE, Weiss RM. Improvement in interstitial cystitis symptom scores during treatment with L-arginine. J Urol. 1997;158:703-708.
19. Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol. 1988;140:36-39.
20. Kallestrup EB, Jorgensen SS, Nordling J, et al. Treatment of interstitial cystitis with Cystistat: a hyaluronic acid product. Scand J Urol Nephrol. 2005;39:143-147.
21. Bade JJ, Laseur M, Nieuwenburg A, van der Weele LT, Mensink HJ. A placebo-controlled study of intravesical pentosan polysulphate for the treatment of interstitial cystitis. Br J Urol. 1997;79:168-171.
22. Peters K, Diokno A, Steinart B, et al. The efficacy of intravesical Tice strain Bacillus Calmette-Guérin in the treatment of interstitial cystitis: a double-blind, prospective, placebo controlled trial. J Urol. 1997;157:2090-2094.
23. Fall M, Lindstrom S. Transcutaneous electrical nerve stimulation in classic and nonulcer interstitial cystitis. Urol Clin North Am. 1994;21:133-139.
24. Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: a prospective study. J Urol. 2003;169:1369-1373.
25. Malloy TR, Shanberg AM. Laser therapy for interstitial cystitis. Urol Clin North Am. 1994;21:141-144.
26. Smith RB, Van Caugh P, Skinner DG, et al. Augmentation enterocystoplasty: a critical review. J Urol. 1977;118:35-39.
27. Chakravarti A, Ganta S, Somani B, Jones MA. Caecocystoplasty for intractable interstitial cystitis: long-term results. Eur Urol. 2004;46:114-117.
28. Erickson DR, Zie SX, Bhavanandan VP, et al. A comparison of multiple urine markers for interstitial cystitis. J Urol. 2002;167:2461.-
29. El-Mansoury M, Boucher W, Sant GR, Theoharides TC. Increased urine histamine and methylhistamine in interstitial cystitis. J Urol. 1994;152:250-353.
30. Boucher W, El-Mansoury M, Pang X, Sant GR. Elevated mast cell tryptase in the urine of patients with interstitial cystitis. Br J Urol. 1995;76:94-100.
31. Pang X, Marchand J, Sant GR, et al. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. 1995;75:744-750.
32. Oravisto KJ. Interstitial cystitis as an autoimmune disease. Eur Urol. 1980;6:10-13.
33. Duncan JL, Schaeffer AJ. Do infectious agents cause interstitial cystitis? Urology. 1997;49:48-51.
34. Haarala M, Jalava J, Laato M, Kilholma P, Nurmi M, Alanen A. Absence of bacterial DNA in the bladder of patients with interstitial cystitis. J Urol. 1996;156:1843-1845.
35. Keay S, Zhang C-O, Bladwin BR, Jacobs SC, Warren JW. Polymerase chain reaction amplification of bacterial 16S rRNA genes in interstitial cystitis and control patient bladder biopsies. J Urol. 1998;159:280-283.
36. Hohenfeller M, Nunes L, Schmidt RA, Lampel A, Thuroff JW, Tanagho EA. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol. 1992;147:587-591.
37. Butrick CW. Interstitial cystitis and chronic pelvic pain: new insights in neuropathology, diagnosis and treatment. Clin Obstet Gynecol. 2003;46:812-814.
38. Moldwin RM, Fried-Siegel J, Mendelowitz F. Pelvic floor dysfunction and interstitial cystitis. J Urol. 1994;151:285A.-
Managing Endometriosis in the Primary Care Setting
Developing an Integrative Health Care Program
PRENATAL COUNSELING
- Screening for fragile X, the most common cause of mental retardation
- Fetal RhD genotyping using maternal plasma
Genetic medicine is fast gaining recognition as an essential component of clinical care. Since this Update last year, 2 areas have seen notable progress:
- New guidelines were issued for fragile X screening in women of reproductive age, and
- Research on free fetal DNA in the maternal circulation deepened our understanding of its diagnostic potential. Clinical applications are emerging in both avenues, and there will be further investigation and refinement. Be sure to check this column again next year!
New guidelines on who to test for mental retardation marker
Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7:584–587.
McConkie-Rosell A, Finucane B, Cronister A, Abrams L, Bennett RL, Petterson BJ. Genetic counseling for fragile X syndrome: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2005;14:249–270.
Fragile X syndrome is the most common inherited cause of mental retardation. The condition can occur in both males and females and is characterized by a range of behavioral changes consistent with autism spectrum, mental retardation, and developmental delay, as well as a facial phenotype that tends to become more recognizable as the individual ages.
Test is not for everybody
New guidelines issued by the American College of Medical Genetics recommended general population screening only within the constructs of research protocols. In selected populations, however, screening should be considered (TABLE 1). Among preconception and prenatal patients, directed interrogation of the family history for findings suggestive of fragile X syndrome can be guided by these recommendations.
Prevalence. ObGyns should be aware of the increasing spectrum of full and premutation fragile X phenotypes and the relatively high prevalence of premutations among women.
Anatomy of fragile X
Changes in a specific region of the X chromosome known as the fragile X mental retardation-1 (FMR-1) gene are responsible for the syndrome. Elongation of an unstable CGG repeat sequence at the 5′ end of FMR-1 leads to hypermethylation, impaired translation, and altered production of the fragile X mental retardation protein. Investigations of knock-out mice reveal that this protein plays an important role in prenatal and postnatal brain development, especially in the area of dendrite maturation.
Among Caucasians, the characteristic features of fragile X syndrome occur in approximately 1 in 4,000 males and 1 in 8,000 females and are associated with elongation of the FMR-1 gene to more than 200 CGG repeats (a full mutation). Initial studies of other races suggest a similar range of full mutations in males and females.
TABLE 1
Fragile X syndrome: Diagnostic and carrier testing guidelines
| Both women and men with |
|
| Physical or behavioral characteristics of fragile X |
| or family history of fragile X |
| or a relative with undiagnosed mental retardation |
| Persons seeking reproductive counseling who have |
|
| Fetuses of carrier mothers |
| Affected individuals or relatives in whom the diagnosis was made by cytogenetic studies |
| Women with elevated follicle-stimulating hormone, especially with family history of |
|
| Men or women with late-onset intention tremor or ataxia…especially with family history of |
|
| Source: Sherman et al. |
Which offspring will inherit the gene?
In the general population, the FMR-1 region has variable lengths.1 In most individuals, 40 or fewer CGG repeats are present and the region remains stable when passed from either parent to the child. Occasionally, however, individuals inherit expansions of this repeat region—either slight (41–60 repeats, intermediate range) or larger (61–200, premutation range). Repeats in the premutation range are carried by 1 in 700 to 1,000 males and 1 in 113 to 350 females.
Expansion of the premutation to a full FMR-1 mutation depends on the sex of the transmitting parent, the length of repeats, and the frequency of AGG interspersion. Only the first 2 criteria are available for clinical interpretation. FMR-1 expansion occurs only in the X originating from the maternal cell line. The larger the premutation, the more likely it will expand to a full mutation (TABLE 2).
Timing of the maternal FMR-1 expansion can vary, with meiotic, postzygotic, and mitotic instability of CGG length all reported.
- Typically, all sons who inherit an expanded, full mutation exhibit features of fragile X syndrome.
- In daughters, however, a full mutation causes a range of features. In daughters with a full mutation, prognostication is limited. Studies indicate that at least 50%, and in some series 75%, have IQs in the borderline or mentally retarded range.
Longitudinal studies of asymptomatic females with full mutations have not been reported. Fathers with premutations pass the FMR-1 gene in a stable fashion to all offspring, occasionally with contraction to a smaller repeat size.
Note that the complex inheritance pattern, with premutations transmitted through both sexes but expansion limited to the maternal X chromosome, can confound interpretation of family histories of mental retardation or developmental delay.
TABLE 2
Number of CGG repeats influences mutation status
| PERCENT RISK OF EXPANSION TO FULL MUTATION (>200 REPEATS) | ||||
|---|---|---|---|---|
| MATERNAL REPEATS | NOLIN, 19962 | PESSO, 20003 | TOLEDANO-ALHADEF, 20014 | NOLIN, 20035 |
| 55–59 | 13 (3/22) | 0 (0/11) | 0 (0/22) | 4 (1/27) |
| 60–69 | 21 (7/34) | 12 (1/8) | 10 (2/20) | 5 (6/113) |
| 70–79 | 58 (59/102) | 50 (1/2) | 17 (1/6) | 31 (28/90) |
| 80–89 | 73 (78/107) | 50 (1/2) | — | 58 (81/140) |
| 90–99 | 94 (83/88) | 100 (1/1) | — | 80 (89/111) |
| 100–200 | 99 (177/179) | 75 (3/4) | — | 98 (194/197) |
| Values presented as percent (n/N). | ||||
| Data modified from Nolin et al. | ||||
Screening populations
Testing for the FMR-1 gene by determining the expansion size is possible via DNA analysis. Most labs utilize both Southern analyses, to measure the degree of methylation, and polymerase chain reaction, to discriminate at a more refined level the subtle differences in repeat sizes that distinguish intermediate and premutation sizes.
Screening the general population for premutations of the FMR-1 gene is not yet the standard of care. However, several authorities advocate fragile X screening among prenatal and preconception populations, given the relatively high rate of the carrier state (1 in 113 to 350), the sensitivity of testing, and the implications for mental retardation and disability in offspring.
In the United States, even assuming a relatively conservative premutation rate of 1 in 300 and an expansion rate of only 11.3%, such testing would be cost-effective, ranging from $99 to $300 per test.6
What are the risks?
Concerns include the implications of intermediate expansions and the substantial patient education needed to convey the risk of expansion to premutation (but not full mutation). No child with a full mutation has been born to a mother with 59 or fewer repeats. Also needing study is the variability of fragile X syndrome in women with a full mutation. These women are at substantial risk for learning impairment, but the degree of disability varies unpredictably.
Prenatal diagnosis requires DNA from amniocytes or chorionic villus sampling. If the latter, follow-up amniocentesis may be needed because methylation begins at variable times during placental development.7
Preimplantation genetic assessment for fragile X premutation carriers has been reported using a system of closely linked markers, circumventing the need to assess onset of methylation abnormalities.
Spectrum of symptoms
Previously, individuals with premutations were considered clinically asymptomatic. However, we now know that phenotypic expression of expansion sizes occurs along a spectrum.
Recent data indicate 2 phenotypes associated with premutations:
- In women, premature ovarian failure, defined as menopause before the age of 40 years, occurs in 13% to 24% of those with premutations of the FMR-1 gene, among families with fragile X syndrome. Conversely, among women with premature ovarian failure, premutations are found in 2% and 14% of sporadic and familial cases, respectively. Further, the size of the premutation may be directly correlated to the risk of premature ovarian failure.
- In men with premutations, a neurologic syndrome of tremors and ataxia is a newly described phenomenon. The fragile X-associated tremor/ataxia syndrome (FXTAS) is a progressive, neurodegenerative process with Parkinsonism and peripheral neuropathy, and penetrance appears to increase with age.8 The frequency of this diagnosis among older men with premutations is under study.
Fetal RhD genotyping now possible using maternal plasma
Gautier E, Benachi A, Giovangrandi Y, et al. Fetal RhD genotyping by maternal serum analyses: a two-year experience. Am J Obstet Gynecol. 2005;192:666–669.
Moise K. Fetal RhD typing with free DNA in maternal plasma. Am J Obstet Gynecol. 2005;192:663–665.
Fetal RhD typing using free fetal DNA (ffDNA) is routine in the United Kingdom but not yet in this country. Since 1997, when Lo identified ffDNA in maternal plasma,9 numerous studies have focused on the physiology, timing, and clinical application of fetal RhD typing using ffDNA. Previously, the focus was detection of fetal cells in maternal circulation.
Unlike intact fetal cells, ffDNA fragments are present in the maternal plasma in sufficient quantities to allow extensive investigation. While most maternal free DNA is composed of longer DNA fragments, shorter DNA fragments of fetal origin appear as the pregnancy advances and in some studies are first detectable as early as 32 days after conception.
Free fetal DNA increases throughout gestation, representing 3% of total DNA in maternal plasma during the second trimester, and increasing to 6% in the third trimester. Free fetal DNA fragments are cleared rapidly by the renal system, with a half-life of 16 minutes and no discernable levels as soon as 2 hours after delivery.
We now understand that ffDNA fragments are continuously deposited in the maternal circulation from early in pregnancy, perhaps even before fetal circulation develops. We also know that maternal levels of ffDNA depend on 2 forces: rate of deposition and rate of removal.10
Trophoblastic origin?
A placental source is suggested by evidence that ffDNA can be retrieved from maternal plasma prior to the development of fetal circulation. A trophoblastic origin is supported by identification in maternal plasma of fetal mRNA with specificity for genes expressed by the placenta. Moreover, ffDNA has been detected in maternal circulation as early as 14 days after conception, corroborating a trophoblastic origin, with programmed apoptosis of placental cells a likely mechanism.
Further increases in ffDNA throughout gestation may reflect direct contributions from the fetal circulation that are transferred to maternal circulation via the placenta. In later gestation, destruction of fetal cells within the maternal circulation may contribute to the pool of ffDNA in maternal plasma. The exact proportions of each contribution are unknown.11
ffDNA may help diagnose these disorders
Placental abnormalities
Recent work suggests sufficient quantities of ffDNA can be obtained for both quantitative and qualitative assessments.12 Used quantitatively, ffDNA reflects placental integrity, an active area of investigation.
Autosomal trisomies, preeclampsia, and fetal growth restriction are conditions thought to involve abnormal placental function. Increased levels of ffDNA have been found in these entities. Increases have been documented even before onset of preeclampsia.10
Gene defects
Use of ffDNA to identify specific gene defects is also under study. Sensitive microarray technology will likely be needed to assess fetal chromosome aneuploidy from maternal plasma.
The detection of single gene defects from ffDNA has been reported for paternally inherited myotonic dystrophy, Huntington disease, and achondroplasia.
For autosomal recessive disorders, genetic testing of ffDNA may be a first step to exclude inheritance of a paternal allele. For this application, discordant parental alleles will be needed so that exclusion of the paternal mutation in the ffDNA signifies an unaffected fetus or a heterozygotic carrier of the maternal allele. If the paternal allele is detected by ffDNA, further genetic testing by chorionic villus sampling or amniocentesis would be needed to differentiate heterozygotic carriers of the paternal mutation from homozygotic, affected fetuses.
RhD genotyping
Since 2001, ffDNA has been used clinically in the United Kingdom for fetal blood group genotyping in isoimmunized gravidas with heterozygous partners, through the International Blood Group Reference Laboratory (part of the National Blood Service), which brings us to the highlighted study. Gautier and colleagues added data affirming that the RhD genotype can be detected through ffDNA with high sensitivity and specificity. Among 285 RhD-negative women, the fetal RhD genotype was determined in 283. In 2 cases, the maternal RhD-negative phenotype did not result from a complete gene deletion; thus, the genotypes of fetus and mother could not be differentiated. Among the women with RhD-negative genotypes, all fetuses were accurately genotyped through ffDNA.
This study differs from prior investigations in its use of RhD-negative women who were not already sensitized, and suggests that ffDNA genotyping in RhD-negative women is sensitive enough to be incorporated into the distribution of Rh immune globulin.
2 problems
As Moise points out in an editorial accompanying the study, a robust, automated system for ffDNA assessment prior to administration of Rh immune globulin likely would be cost-effective. The Moise editorial also points out these 2 concerns:
False positives are a real possibility, as the 2 cases in the Gautier study illustrate. Free fetal DNA analysis for RhD genotyping assumes that the serologic finding that indicates RhD-negative status (lack of RhD on the fetal red blood cells) is due to deletion of the RhD locus. Thus, when RhD DNA fragments are detected in maternal plasma, they are presumed to be fetal in origin. However, we now know that pseudogene regions of the RhD locus occur with relatively high frequency—in particular, in more than half of African Americans, who serologically type as RhD-negative. Such pseudogenes cause a stop codon that effectively diminishes production of RhD antigen. Serologic typing of such individuals indicates an RhD-negative phenotype. Because the most common pseudogenes are within exon 4, inclusion of primers that assess multiple exons can reduce these false positives.
False negatives have graver clinical implications. Misidentification of an RhD-positive fetus as RhD-negative could prevent that fetus from receiving appropriate surveillance and intervention. False-negative assessments from ffDNA are probably caused by poor amplification of the test sample.
Safeguards have been used in most protocols, including tracer mouse DNA as an internal control to assure amplification. Simultaneous SRY gene testing assures amplification of male fetal DNA. For females, incorporation in the amplification assessment of highly polymorphic markers different from those of the maternal sample may verify fetal DNA amplification.
What is ahead?
Protocols to refine use of ffDNA for RhD genotyping are likely. Meanwhile, techniques are being modified to assure extraction of sufficient quantities of fetal DNA.
Future research will focus on quantitative changes in ffDNA as a marker for pregnancy complications, and development of noninvasive prenatal assessment of specific genes. Successful development of a noninvasive ffDNA diagnostic test will enhance prenatal evaluations without the risk of pregnancy loss currently associated with amniocentesis and chorionic villus sampling.
1. Hagerman PJ, Hagerman RJ. The fragile-X premutation: a maturing perspective [published correction appears in: Am J Hum Genet. 2004;75:352]. Am J Hum Genet. 2004;74:805-816.
2. Nolin SL, Lewis FA, 3rd, Ye LL, et al. Familial transmission of the FMR1 CGG repeat. Am J Hum Genet. 1996;59:1252-12561.
3. Pesso R, Berkenstadt M, Cuckle H, et al. Screening for fragile X syndrome in women of reproductive age. Prenat Diagn. 2000;20:611-614.
4. Toledano-Alhadef H, Basel-Vanagaite L, Magal N, et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001;69:351-360.
5. Nolin SL, Brown WT, Glicksman A, et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am J Hum Genet. 2003;72:454-464.
6. Musci TJ, Caughey AB. Cost-effectiveness analysis of prenatal population-based fragile X carrier screening. Am J Obstet Gynecol. 2005;192:1905-1915.
7. Willemsen R, Bontekoe CJ, Severijnen LA, Oostra BA. Timing of the absence of FMR1 expression in full mutation chorionic villi. Hum Genet. 2002;110:601-605.
8. Willemsen R, Mientjes E, Oostra BA. FXTAS: a progressive neurologic syndrome associated with fragile X premutation. Curr Neurol Neurosci Rep. 2005;5:405-410.
9. Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485-487.
10. Lo YM. Recent advances in fetal nucleic acids in maternal plasma. J Histochem Cytochem. 2005;53:293-296.
11. Illanes S, Avent N, Soothill PW. Cell-free fetal DNA in maternal plasma: an important advance to link fetal genetics to obstetric ultrasound. Ultrasound Obstet Gynecol. 2005;25:317-322.
12. Galbiati S, Smid M, Gambini D, et al. Fetal DNA detection in maternal plasma throughout gestation. Hum Genet. 2005;117:243-248.
- Screening for fragile X, the most common cause of mental retardation
- Fetal RhD genotyping using maternal plasma
Genetic medicine is fast gaining recognition as an essential component of clinical care. Since this Update last year, 2 areas have seen notable progress:
- New guidelines were issued for fragile X screening in women of reproductive age, and
- Research on free fetal DNA in the maternal circulation deepened our understanding of its diagnostic potential. Clinical applications are emerging in both avenues, and there will be further investigation and refinement. Be sure to check this column again next year!
New guidelines on who to test for mental retardation marker
Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7:584–587.
McConkie-Rosell A, Finucane B, Cronister A, Abrams L, Bennett RL, Petterson BJ. Genetic counseling for fragile X syndrome: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2005;14:249–270.
Fragile X syndrome is the most common inherited cause of mental retardation. The condition can occur in both males and females and is characterized by a range of behavioral changes consistent with autism spectrum, mental retardation, and developmental delay, as well as a facial phenotype that tends to become more recognizable as the individual ages.
Test is not for everybody
New guidelines issued by the American College of Medical Genetics recommended general population screening only within the constructs of research protocols. In selected populations, however, screening should be considered (TABLE 1). Among preconception and prenatal patients, directed interrogation of the family history for findings suggestive of fragile X syndrome can be guided by these recommendations.
Prevalence. ObGyns should be aware of the increasing spectrum of full and premutation fragile X phenotypes and the relatively high prevalence of premutations among women.
Anatomy of fragile X
Changes in a specific region of the X chromosome known as the fragile X mental retardation-1 (FMR-1) gene are responsible for the syndrome. Elongation of an unstable CGG repeat sequence at the 5′ end of FMR-1 leads to hypermethylation, impaired translation, and altered production of the fragile X mental retardation protein. Investigations of knock-out mice reveal that this protein plays an important role in prenatal and postnatal brain development, especially in the area of dendrite maturation.
Among Caucasians, the characteristic features of fragile X syndrome occur in approximately 1 in 4,000 males and 1 in 8,000 females and are associated with elongation of the FMR-1 gene to more than 200 CGG repeats (a full mutation). Initial studies of other races suggest a similar range of full mutations in males and females.
TABLE 1
Fragile X syndrome: Diagnostic and carrier testing guidelines
| Both women and men with |
|
| Physical or behavioral characteristics of fragile X |
| or family history of fragile X |
| or a relative with undiagnosed mental retardation |
| Persons seeking reproductive counseling who have |
|
| Fetuses of carrier mothers |
| Affected individuals or relatives in whom the diagnosis was made by cytogenetic studies |
| Women with elevated follicle-stimulating hormone, especially with family history of |
|
| Men or women with late-onset intention tremor or ataxia…especially with family history of |
|
| Source: Sherman et al. |
Which offspring will inherit the gene?
In the general population, the FMR-1 region has variable lengths.1 In most individuals, 40 or fewer CGG repeats are present and the region remains stable when passed from either parent to the child. Occasionally, however, individuals inherit expansions of this repeat region—either slight (41–60 repeats, intermediate range) or larger (61–200, premutation range). Repeats in the premutation range are carried by 1 in 700 to 1,000 males and 1 in 113 to 350 females.
Expansion of the premutation to a full FMR-1 mutation depends on the sex of the transmitting parent, the length of repeats, and the frequency of AGG interspersion. Only the first 2 criteria are available for clinical interpretation. FMR-1 expansion occurs only in the X originating from the maternal cell line. The larger the premutation, the more likely it will expand to a full mutation (TABLE 2).
Timing of the maternal FMR-1 expansion can vary, with meiotic, postzygotic, and mitotic instability of CGG length all reported.
- Typically, all sons who inherit an expanded, full mutation exhibit features of fragile X syndrome.
- In daughters, however, a full mutation causes a range of features. In daughters with a full mutation, prognostication is limited. Studies indicate that at least 50%, and in some series 75%, have IQs in the borderline or mentally retarded range.
Longitudinal studies of asymptomatic females with full mutations have not been reported. Fathers with premutations pass the FMR-1 gene in a stable fashion to all offspring, occasionally with contraction to a smaller repeat size.
Note that the complex inheritance pattern, with premutations transmitted through both sexes but expansion limited to the maternal X chromosome, can confound interpretation of family histories of mental retardation or developmental delay.
TABLE 2
Number of CGG repeats influences mutation status
| PERCENT RISK OF EXPANSION TO FULL MUTATION (>200 REPEATS) | ||||
|---|---|---|---|---|
| MATERNAL REPEATS | NOLIN, 19962 | PESSO, 20003 | TOLEDANO-ALHADEF, 20014 | NOLIN, 20035 |
| 55–59 | 13 (3/22) | 0 (0/11) | 0 (0/22) | 4 (1/27) |
| 60–69 | 21 (7/34) | 12 (1/8) | 10 (2/20) | 5 (6/113) |
| 70–79 | 58 (59/102) | 50 (1/2) | 17 (1/6) | 31 (28/90) |
| 80–89 | 73 (78/107) | 50 (1/2) | — | 58 (81/140) |
| 90–99 | 94 (83/88) | 100 (1/1) | — | 80 (89/111) |
| 100–200 | 99 (177/179) | 75 (3/4) | — | 98 (194/197) |
| Values presented as percent (n/N). | ||||
| Data modified from Nolin et al. | ||||
Screening populations
Testing for the FMR-1 gene by determining the expansion size is possible via DNA analysis. Most labs utilize both Southern analyses, to measure the degree of methylation, and polymerase chain reaction, to discriminate at a more refined level the subtle differences in repeat sizes that distinguish intermediate and premutation sizes.
Screening the general population for premutations of the FMR-1 gene is not yet the standard of care. However, several authorities advocate fragile X screening among prenatal and preconception populations, given the relatively high rate of the carrier state (1 in 113 to 350), the sensitivity of testing, and the implications for mental retardation and disability in offspring.
In the United States, even assuming a relatively conservative premutation rate of 1 in 300 and an expansion rate of only 11.3%, such testing would be cost-effective, ranging from $99 to $300 per test.6
What are the risks?
Concerns include the implications of intermediate expansions and the substantial patient education needed to convey the risk of expansion to premutation (but not full mutation). No child with a full mutation has been born to a mother with 59 or fewer repeats. Also needing study is the variability of fragile X syndrome in women with a full mutation. These women are at substantial risk for learning impairment, but the degree of disability varies unpredictably.
Prenatal diagnosis requires DNA from amniocytes or chorionic villus sampling. If the latter, follow-up amniocentesis may be needed because methylation begins at variable times during placental development.7
Preimplantation genetic assessment for fragile X premutation carriers has been reported using a system of closely linked markers, circumventing the need to assess onset of methylation abnormalities.
Spectrum of symptoms
Previously, individuals with premutations were considered clinically asymptomatic. However, we now know that phenotypic expression of expansion sizes occurs along a spectrum.
Recent data indicate 2 phenotypes associated with premutations:
- In women, premature ovarian failure, defined as menopause before the age of 40 years, occurs in 13% to 24% of those with premutations of the FMR-1 gene, among families with fragile X syndrome. Conversely, among women with premature ovarian failure, premutations are found in 2% and 14% of sporadic and familial cases, respectively. Further, the size of the premutation may be directly correlated to the risk of premature ovarian failure.
- In men with premutations, a neurologic syndrome of tremors and ataxia is a newly described phenomenon. The fragile X-associated tremor/ataxia syndrome (FXTAS) is a progressive, neurodegenerative process with Parkinsonism and peripheral neuropathy, and penetrance appears to increase with age.8 The frequency of this diagnosis among older men with premutations is under study.
Fetal RhD genotyping now possible using maternal plasma
Gautier E, Benachi A, Giovangrandi Y, et al. Fetal RhD genotyping by maternal serum analyses: a two-year experience. Am J Obstet Gynecol. 2005;192:666–669.
Moise K. Fetal RhD typing with free DNA in maternal plasma. Am J Obstet Gynecol. 2005;192:663–665.
Fetal RhD typing using free fetal DNA (ffDNA) is routine in the United Kingdom but not yet in this country. Since 1997, when Lo identified ffDNA in maternal plasma,9 numerous studies have focused on the physiology, timing, and clinical application of fetal RhD typing using ffDNA. Previously, the focus was detection of fetal cells in maternal circulation.
Unlike intact fetal cells, ffDNA fragments are present in the maternal plasma in sufficient quantities to allow extensive investigation. While most maternal free DNA is composed of longer DNA fragments, shorter DNA fragments of fetal origin appear as the pregnancy advances and in some studies are first detectable as early as 32 days after conception.
Free fetal DNA increases throughout gestation, representing 3% of total DNA in maternal plasma during the second trimester, and increasing to 6% in the third trimester. Free fetal DNA fragments are cleared rapidly by the renal system, with a half-life of 16 minutes and no discernable levels as soon as 2 hours after delivery.
We now understand that ffDNA fragments are continuously deposited in the maternal circulation from early in pregnancy, perhaps even before fetal circulation develops. We also know that maternal levels of ffDNA depend on 2 forces: rate of deposition and rate of removal.10
Trophoblastic origin?
A placental source is suggested by evidence that ffDNA can be retrieved from maternal plasma prior to the development of fetal circulation. A trophoblastic origin is supported by identification in maternal plasma of fetal mRNA with specificity for genes expressed by the placenta. Moreover, ffDNA has been detected in maternal circulation as early as 14 days after conception, corroborating a trophoblastic origin, with programmed apoptosis of placental cells a likely mechanism.
Further increases in ffDNA throughout gestation may reflect direct contributions from the fetal circulation that are transferred to maternal circulation via the placenta. In later gestation, destruction of fetal cells within the maternal circulation may contribute to the pool of ffDNA in maternal plasma. The exact proportions of each contribution are unknown.11
ffDNA may help diagnose these disorders
Placental abnormalities
Recent work suggests sufficient quantities of ffDNA can be obtained for both quantitative and qualitative assessments.12 Used quantitatively, ffDNA reflects placental integrity, an active area of investigation.
Autosomal trisomies, preeclampsia, and fetal growth restriction are conditions thought to involve abnormal placental function. Increased levels of ffDNA have been found in these entities. Increases have been documented even before onset of preeclampsia.10
Gene defects
Use of ffDNA to identify specific gene defects is also under study. Sensitive microarray technology will likely be needed to assess fetal chromosome aneuploidy from maternal plasma.
The detection of single gene defects from ffDNA has been reported for paternally inherited myotonic dystrophy, Huntington disease, and achondroplasia.
For autosomal recessive disorders, genetic testing of ffDNA may be a first step to exclude inheritance of a paternal allele. For this application, discordant parental alleles will be needed so that exclusion of the paternal mutation in the ffDNA signifies an unaffected fetus or a heterozygotic carrier of the maternal allele. If the paternal allele is detected by ffDNA, further genetic testing by chorionic villus sampling or amniocentesis would be needed to differentiate heterozygotic carriers of the paternal mutation from homozygotic, affected fetuses.
RhD genotyping
Since 2001, ffDNA has been used clinically in the United Kingdom for fetal blood group genotyping in isoimmunized gravidas with heterozygous partners, through the International Blood Group Reference Laboratory (part of the National Blood Service), which brings us to the highlighted study. Gautier and colleagues added data affirming that the RhD genotype can be detected through ffDNA with high sensitivity and specificity. Among 285 RhD-negative women, the fetal RhD genotype was determined in 283. In 2 cases, the maternal RhD-negative phenotype did not result from a complete gene deletion; thus, the genotypes of fetus and mother could not be differentiated. Among the women with RhD-negative genotypes, all fetuses were accurately genotyped through ffDNA.
This study differs from prior investigations in its use of RhD-negative women who were not already sensitized, and suggests that ffDNA genotyping in RhD-negative women is sensitive enough to be incorporated into the distribution of Rh immune globulin.
2 problems
As Moise points out in an editorial accompanying the study, a robust, automated system for ffDNA assessment prior to administration of Rh immune globulin likely would be cost-effective. The Moise editorial also points out these 2 concerns:
False positives are a real possibility, as the 2 cases in the Gautier study illustrate. Free fetal DNA analysis for RhD genotyping assumes that the serologic finding that indicates RhD-negative status (lack of RhD on the fetal red blood cells) is due to deletion of the RhD locus. Thus, when RhD DNA fragments are detected in maternal plasma, they are presumed to be fetal in origin. However, we now know that pseudogene regions of the RhD locus occur with relatively high frequency—in particular, in more than half of African Americans, who serologically type as RhD-negative. Such pseudogenes cause a stop codon that effectively diminishes production of RhD antigen. Serologic typing of such individuals indicates an RhD-negative phenotype. Because the most common pseudogenes are within exon 4, inclusion of primers that assess multiple exons can reduce these false positives.
False negatives have graver clinical implications. Misidentification of an RhD-positive fetus as RhD-negative could prevent that fetus from receiving appropriate surveillance and intervention. False-negative assessments from ffDNA are probably caused by poor amplification of the test sample.
Safeguards have been used in most protocols, including tracer mouse DNA as an internal control to assure amplification. Simultaneous SRY gene testing assures amplification of male fetal DNA. For females, incorporation in the amplification assessment of highly polymorphic markers different from those of the maternal sample may verify fetal DNA amplification.
What is ahead?
Protocols to refine use of ffDNA for RhD genotyping are likely. Meanwhile, techniques are being modified to assure extraction of sufficient quantities of fetal DNA.
Future research will focus on quantitative changes in ffDNA as a marker for pregnancy complications, and development of noninvasive prenatal assessment of specific genes. Successful development of a noninvasive ffDNA diagnostic test will enhance prenatal evaluations without the risk of pregnancy loss currently associated with amniocentesis and chorionic villus sampling.
- Screening for fragile X, the most common cause of mental retardation
- Fetal RhD genotyping using maternal plasma
Genetic medicine is fast gaining recognition as an essential component of clinical care. Since this Update last year, 2 areas have seen notable progress:
- New guidelines were issued for fragile X screening in women of reproductive age, and
- Research on free fetal DNA in the maternal circulation deepened our understanding of its diagnostic potential. Clinical applications are emerging in both avenues, and there will be further investigation and refinement. Be sure to check this column again next year!
New guidelines on who to test for mental retardation marker
Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7:584–587.
McConkie-Rosell A, Finucane B, Cronister A, Abrams L, Bennett RL, Petterson BJ. Genetic counseling for fragile X syndrome: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2005;14:249–270.
Fragile X syndrome is the most common inherited cause of mental retardation. The condition can occur in both males and females and is characterized by a range of behavioral changes consistent with autism spectrum, mental retardation, and developmental delay, as well as a facial phenotype that tends to become more recognizable as the individual ages.
Test is not for everybody
New guidelines issued by the American College of Medical Genetics recommended general population screening only within the constructs of research protocols. In selected populations, however, screening should be considered (TABLE 1). Among preconception and prenatal patients, directed interrogation of the family history for findings suggestive of fragile X syndrome can be guided by these recommendations.
Prevalence. ObGyns should be aware of the increasing spectrum of full and premutation fragile X phenotypes and the relatively high prevalence of premutations among women.
Anatomy of fragile X
Changes in a specific region of the X chromosome known as the fragile X mental retardation-1 (FMR-1) gene are responsible for the syndrome. Elongation of an unstable CGG repeat sequence at the 5′ end of FMR-1 leads to hypermethylation, impaired translation, and altered production of the fragile X mental retardation protein. Investigations of knock-out mice reveal that this protein plays an important role in prenatal and postnatal brain development, especially in the area of dendrite maturation.
Among Caucasians, the characteristic features of fragile X syndrome occur in approximately 1 in 4,000 males and 1 in 8,000 females and are associated with elongation of the FMR-1 gene to more than 200 CGG repeats (a full mutation). Initial studies of other races suggest a similar range of full mutations in males and females.
TABLE 1
Fragile X syndrome: Diagnostic and carrier testing guidelines
| Both women and men with |
|
| Physical or behavioral characteristics of fragile X |
| or family history of fragile X |
| or a relative with undiagnosed mental retardation |
| Persons seeking reproductive counseling who have |
|
| Fetuses of carrier mothers |
| Affected individuals or relatives in whom the diagnosis was made by cytogenetic studies |
| Women with elevated follicle-stimulating hormone, especially with family history of |
|
| Men or women with late-onset intention tremor or ataxia…especially with family history of |
|
| Source: Sherman et al. |
Which offspring will inherit the gene?
In the general population, the FMR-1 region has variable lengths.1 In most individuals, 40 or fewer CGG repeats are present and the region remains stable when passed from either parent to the child. Occasionally, however, individuals inherit expansions of this repeat region—either slight (41–60 repeats, intermediate range) or larger (61–200, premutation range). Repeats in the premutation range are carried by 1 in 700 to 1,000 males and 1 in 113 to 350 females.
Expansion of the premutation to a full FMR-1 mutation depends on the sex of the transmitting parent, the length of repeats, and the frequency of AGG interspersion. Only the first 2 criteria are available for clinical interpretation. FMR-1 expansion occurs only in the X originating from the maternal cell line. The larger the premutation, the more likely it will expand to a full mutation (TABLE 2).
Timing of the maternal FMR-1 expansion can vary, with meiotic, postzygotic, and mitotic instability of CGG length all reported.
- Typically, all sons who inherit an expanded, full mutation exhibit features of fragile X syndrome.
- In daughters, however, a full mutation causes a range of features. In daughters with a full mutation, prognostication is limited. Studies indicate that at least 50%, and in some series 75%, have IQs in the borderline or mentally retarded range.
Longitudinal studies of asymptomatic females with full mutations have not been reported. Fathers with premutations pass the FMR-1 gene in a stable fashion to all offspring, occasionally with contraction to a smaller repeat size.
Note that the complex inheritance pattern, with premutations transmitted through both sexes but expansion limited to the maternal X chromosome, can confound interpretation of family histories of mental retardation or developmental delay.
TABLE 2
Number of CGG repeats influences mutation status
| PERCENT RISK OF EXPANSION TO FULL MUTATION (>200 REPEATS) | ||||
|---|---|---|---|---|
| MATERNAL REPEATS | NOLIN, 19962 | PESSO, 20003 | TOLEDANO-ALHADEF, 20014 | NOLIN, 20035 |
| 55–59 | 13 (3/22) | 0 (0/11) | 0 (0/22) | 4 (1/27) |
| 60–69 | 21 (7/34) | 12 (1/8) | 10 (2/20) | 5 (6/113) |
| 70–79 | 58 (59/102) | 50 (1/2) | 17 (1/6) | 31 (28/90) |
| 80–89 | 73 (78/107) | 50 (1/2) | — | 58 (81/140) |
| 90–99 | 94 (83/88) | 100 (1/1) | — | 80 (89/111) |
| 100–200 | 99 (177/179) | 75 (3/4) | — | 98 (194/197) |
| Values presented as percent (n/N). | ||||
| Data modified from Nolin et al. | ||||
Screening populations
Testing for the FMR-1 gene by determining the expansion size is possible via DNA analysis. Most labs utilize both Southern analyses, to measure the degree of methylation, and polymerase chain reaction, to discriminate at a more refined level the subtle differences in repeat sizes that distinguish intermediate and premutation sizes.
Screening the general population for premutations of the FMR-1 gene is not yet the standard of care. However, several authorities advocate fragile X screening among prenatal and preconception populations, given the relatively high rate of the carrier state (1 in 113 to 350), the sensitivity of testing, and the implications for mental retardation and disability in offspring.
In the United States, even assuming a relatively conservative premutation rate of 1 in 300 and an expansion rate of only 11.3%, such testing would be cost-effective, ranging from $99 to $300 per test.6
What are the risks?
Concerns include the implications of intermediate expansions and the substantial patient education needed to convey the risk of expansion to premutation (but not full mutation). No child with a full mutation has been born to a mother with 59 or fewer repeats. Also needing study is the variability of fragile X syndrome in women with a full mutation. These women are at substantial risk for learning impairment, but the degree of disability varies unpredictably.
Prenatal diagnosis requires DNA from amniocytes or chorionic villus sampling. If the latter, follow-up amniocentesis may be needed because methylation begins at variable times during placental development.7
Preimplantation genetic assessment for fragile X premutation carriers has been reported using a system of closely linked markers, circumventing the need to assess onset of methylation abnormalities.
Spectrum of symptoms
Previously, individuals with premutations were considered clinically asymptomatic. However, we now know that phenotypic expression of expansion sizes occurs along a spectrum.
Recent data indicate 2 phenotypes associated with premutations:
- In women, premature ovarian failure, defined as menopause before the age of 40 years, occurs in 13% to 24% of those with premutations of the FMR-1 gene, among families with fragile X syndrome. Conversely, among women with premature ovarian failure, premutations are found in 2% and 14% of sporadic and familial cases, respectively. Further, the size of the premutation may be directly correlated to the risk of premature ovarian failure.
- In men with premutations, a neurologic syndrome of tremors and ataxia is a newly described phenomenon. The fragile X-associated tremor/ataxia syndrome (FXTAS) is a progressive, neurodegenerative process with Parkinsonism and peripheral neuropathy, and penetrance appears to increase with age.8 The frequency of this diagnosis among older men with premutations is under study.
Fetal RhD genotyping now possible using maternal plasma
Gautier E, Benachi A, Giovangrandi Y, et al. Fetal RhD genotyping by maternal serum analyses: a two-year experience. Am J Obstet Gynecol. 2005;192:666–669.
Moise K. Fetal RhD typing with free DNA in maternal plasma. Am J Obstet Gynecol. 2005;192:663–665.
Fetal RhD typing using free fetal DNA (ffDNA) is routine in the United Kingdom but not yet in this country. Since 1997, when Lo identified ffDNA in maternal plasma,9 numerous studies have focused on the physiology, timing, and clinical application of fetal RhD typing using ffDNA. Previously, the focus was detection of fetal cells in maternal circulation.
Unlike intact fetal cells, ffDNA fragments are present in the maternal plasma in sufficient quantities to allow extensive investigation. While most maternal free DNA is composed of longer DNA fragments, shorter DNA fragments of fetal origin appear as the pregnancy advances and in some studies are first detectable as early as 32 days after conception.
Free fetal DNA increases throughout gestation, representing 3% of total DNA in maternal plasma during the second trimester, and increasing to 6% in the third trimester. Free fetal DNA fragments are cleared rapidly by the renal system, with a half-life of 16 minutes and no discernable levels as soon as 2 hours after delivery.
We now understand that ffDNA fragments are continuously deposited in the maternal circulation from early in pregnancy, perhaps even before fetal circulation develops. We also know that maternal levels of ffDNA depend on 2 forces: rate of deposition and rate of removal.10
Trophoblastic origin?
A placental source is suggested by evidence that ffDNA can be retrieved from maternal plasma prior to the development of fetal circulation. A trophoblastic origin is supported by identification in maternal plasma of fetal mRNA with specificity for genes expressed by the placenta. Moreover, ffDNA has been detected in maternal circulation as early as 14 days after conception, corroborating a trophoblastic origin, with programmed apoptosis of placental cells a likely mechanism.
Further increases in ffDNA throughout gestation may reflect direct contributions from the fetal circulation that are transferred to maternal circulation via the placenta. In later gestation, destruction of fetal cells within the maternal circulation may contribute to the pool of ffDNA in maternal plasma. The exact proportions of each contribution are unknown.11
ffDNA may help diagnose these disorders
Placental abnormalities
Recent work suggests sufficient quantities of ffDNA can be obtained for both quantitative and qualitative assessments.12 Used quantitatively, ffDNA reflects placental integrity, an active area of investigation.
Autosomal trisomies, preeclampsia, and fetal growth restriction are conditions thought to involve abnormal placental function. Increased levels of ffDNA have been found in these entities. Increases have been documented even before onset of preeclampsia.10
Gene defects
Use of ffDNA to identify specific gene defects is also under study. Sensitive microarray technology will likely be needed to assess fetal chromosome aneuploidy from maternal plasma.
The detection of single gene defects from ffDNA has been reported for paternally inherited myotonic dystrophy, Huntington disease, and achondroplasia.
For autosomal recessive disorders, genetic testing of ffDNA may be a first step to exclude inheritance of a paternal allele. For this application, discordant parental alleles will be needed so that exclusion of the paternal mutation in the ffDNA signifies an unaffected fetus or a heterozygotic carrier of the maternal allele. If the paternal allele is detected by ffDNA, further genetic testing by chorionic villus sampling or amniocentesis would be needed to differentiate heterozygotic carriers of the paternal mutation from homozygotic, affected fetuses.
RhD genotyping
Since 2001, ffDNA has been used clinically in the United Kingdom for fetal blood group genotyping in isoimmunized gravidas with heterozygous partners, through the International Blood Group Reference Laboratory (part of the National Blood Service), which brings us to the highlighted study. Gautier and colleagues added data affirming that the RhD genotype can be detected through ffDNA with high sensitivity and specificity. Among 285 RhD-negative women, the fetal RhD genotype was determined in 283. In 2 cases, the maternal RhD-negative phenotype did not result from a complete gene deletion; thus, the genotypes of fetus and mother could not be differentiated. Among the women with RhD-negative genotypes, all fetuses were accurately genotyped through ffDNA.
This study differs from prior investigations in its use of RhD-negative women who were not already sensitized, and suggests that ffDNA genotyping in RhD-negative women is sensitive enough to be incorporated into the distribution of Rh immune globulin.
2 problems
As Moise points out in an editorial accompanying the study, a robust, automated system for ffDNA assessment prior to administration of Rh immune globulin likely would be cost-effective. The Moise editorial also points out these 2 concerns:
False positives are a real possibility, as the 2 cases in the Gautier study illustrate. Free fetal DNA analysis for RhD genotyping assumes that the serologic finding that indicates RhD-negative status (lack of RhD on the fetal red blood cells) is due to deletion of the RhD locus. Thus, when RhD DNA fragments are detected in maternal plasma, they are presumed to be fetal in origin. However, we now know that pseudogene regions of the RhD locus occur with relatively high frequency—in particular, in more than half of African Americans, who serologically type as RhD-negative. Such pseudogenes cause a stop codon that effectively diminishes production of RhD antigen. Serologic typing of such individuals indicates an RhD-negative phenotype. Because the most common pseudogenes are within exon 4, inclusion of primers that assess multiple exons can reduce these false positives.
False negatives have graver clinical implications. Misidentification of an RhD-positive fetus as RhD-negative could prevent that fetus from receiving appropriate surveillance and intervention. False-negative assessments from ffDNA are probably caused by poor amplification of the test sample.
Safeguards have been used in most protocols, including tracer mouse DNA as an internal control to assure amplification. Simultaneous SRY gene testing assures amplification of male fetal DNA. For females, incorporation in the amplification assessment of highly polymorphic markers different from those of the maternal sample may verify fetal DNA amplification.
What is ahead?
Protocols to refine use of ffDNA for RhD genotyping are likely. Meanwhile, techniques are being modified to assure extraction of sufficient quantities of fetal DNA.
Future research will focus on quantitative changes in ffDNA as a marker for pregnancy complications, and development of noninvasive prenatal assessment of specific genes. Successful development of a noninvasive ffDNA diagnostic test will enhance prenatal evaluations without the risk of pregnancy loss currently associated with amniocentesis and chorionic villus sampling.
1. Hagerman PJ, Hagerman RJ. The fragile-X premutation: a maturing perspective [published correction appears in: Am J Hum Genet. 2004;75:352]. Am J Hum Genet. 2004;74:805-816.
2. Nolin SL, Lewis FA, 3rd, Ye LL, et al. Familial transmission of the FMR1 CGG repeat. Am J Hum Genet. 1996;59:1252-12561.
3. Pesso R, Berkenstadt M, Cuckle H, et al. Screening for fragile X syndrome in women of reproductive age. Prenat Diagn. 2000;20:611-614.
4. Toledano-Alhadef H, Basel-Vanagaite L, Magal N, et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001;69:351-360.
5. Nolin SL, Brown WT, Glicksman A, et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am J Hum Genet. 2003;72:454-464.
6. Musci TJ, Caughey AB. Cost-effectiveness analysis of prenatal population-based fragile X carrier screening. Am J Obstet Gynecol. 2005;192:1905-1915.
7. Willemsen R, Bontekoe CJ, Severijnen LA, Oostra BA. Timing of the absence of FMR1 expression in full mutation chorionic villi. Hum Genet. 2002;110:601-605.
8. Willemsen R, Mientjes E, Oostra BA. FXTAS: a progressive neurologic syndrome associated with fragile X premutation. Curr Neurol Neurosci Rep. 2005;5:405-410.
9. Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485-487.
10. Lo YM. Recent advances in fetal nucleic acids in maternal plasma. J Histochem Cytochem. 2005;53:293-296.
11. Illanes S, Avent N, Soothill PW. Cell-free fetal DNA in maternal plasma: an important advance to link fetal genetics to obstetric ultrasound. Ultrasound Obstet Gynecol. 2005;25:317-322.
12. Galbiati S, Smid M, Gambini D, et al. Fetal DNA detection in maternal plasma throughout gestation. Hum Genet. 2005;117:243-248.
1. Hagerman PJ, Hagerman RJ. The fragile-X premutation: a maturing perspective [published correction appears in: Am J Hum Genet. 2004;75:352]. Am J Hum Genet. 2004;74:805-816.
2. Nolin SL, Lewis FA, 3rd, Ye LL, et al. Familial transmission of the FMR1 CGG repeat. Am J Hum Genet. 1996;59:1252-12561.
3. Pesso R, Berkenstadt M, Cuckle H, et al. Screening for fragile X syndrome in women of reproductive age. Prenat Diagn. 2000;20:611-614.
4. Toledano-Alhadef H, Basel-Vanagaite L, Magal N, et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001;69:351-360.
5. Nolin SL, Brown WT, Glicksman A, et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am J Hum Genet. 2003;72:454-464.
6. Musci TJ, Caughey AB. Cost-effectiveness analysis of prenatal population-based fragile X carrier screening. Am J Obstet Gynecol. 2005;192:1905-1915.
7. Willemsen R, Bontekoe CJ, Severijnen LA, Oostra BA. Timing of the absence of FMR1 expression in full mutation chorionic villi. Hum Genet. 2002;110:601-605.
8. Willemsen R, Mientjes E, Oostra BA. FXTAS: a progressive neurologic syndrome associated with fragile X premutation. Curr Neurol Neurosci Rep. 2005;5:405-410.
9. Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485-487.
10. Lo YM. Recent advances in fetal nucleic acids in maternal plasma. J Histochem Cytochem. 2005;53:293-296.
11. Illanes S, Avent N, Soothill PW. Cell-free fetal DNA in maternal plasma: an important advance to link fetal genetics to obstetric ultrasound. Ultrasound Obstet Gynecol. 2005;25:317-322.
12. Galbiati S, Smid M, Gambini D, et al. Fetal DNA detection in maternal plasma throughout gestation. Hum Genet. 2005;117:243-248.