User login
Female genital cutting: Caring for patients through the lens of health care equity
Female genital cutting (FGC), also known as female circumcision or female genital mutilation, is defined by the World Health Organization (WHO) as “the partial or total removal of the external female genitalia, or other injury to the female genital organs for non-medical reasons.”1 It is a culturally determined practice that is mainly concentrated in certain parts of Africa, the Middle East, and Asia and now is observed worldwide among migrants from those areas.1 Approximately 200 million women and girls alive today have undergone FGC in 31 countries, although encouragingly the practice’s prevalence seems to be declining, especially among younger women.2
Too often, FGC goes unrecognized in women who present for medical care, even in cases where a genitourinary exam is performed and documented.3,4 As a result, patients face delays in diagnosis and management of associated complications and symptoms. Female genital cutting is usually excluded from medical school or residency training curricula,5 and physicians often lack familiarity with the necessary clinical or surgical management of patients who have had the procedure.6 It is crucial, however, that ObGyns feel comfortable recognizing FGC and clinically caring for pregnant and nonpregnant patients who have undergone the procedure. The obstetric-gynecologic setting should be the clinical space in which FGC is correctly diagnosed and from where patients with complications can be referred for appropriate care.
FGC: Through the lens of inequity
Providing culturally competent and sensitive care to women who have undergone FGC is paramount to reducing health care inequities for these patients. Beyond the medical recommendations we review below, we suggest the following considerations when approaching care for these patients.
Acknowledge our biases. It is paramount for us, as providers, to acknowledge our own biases and how these might affect our relationship with the patient and how our care is received. This starts with our language and terminology: The term female genital mutilation can be judgmental or offensive to our patients, many of whom do not consider themselves to have been mutilated. This is why we prefer to use the term female genital cutting, or whichever word the patient uses, so as not to alienate a patient who might already face many other barriers and microaggressions in seeking health care.
Control our responses. Another way we must check our bias is by controlling our reactions during history taking or examining patients who have undergone FGC. Understandably, providers might be shocked to hear patients recount their childhood experiences of FGC or by examining an infibulated scar, but patients report noticing and experiencing hurt, distress, and shame when providers display judgment, horror, or disgust.7 Patients have reported that they are acutely aware that they might be viewed as “backward” and “primitive” in US health care settings.8 These kinds of feelings and experiences can further exacerbate patients’ distrust and avoidance of the health care system altogether. Therefore, providers should acknowledge their own biases regarding the issue as well as those of their staff and work to mitigate them.
Avoid stigmatization. While FGC can have long-term effects (discussed below), it is important to remember that many women who have undergone FGC do not experience symptoms that are bothersome or feel that FGC is central to their lives or lived experiences. While we must be thorough in our history taking to explore possible urinary, gynecologic, and sexual symptoms of concern and bother to the patient, we must avoid stigmatizing our patients by assuming that all who have undergone FGC are “sexually disabled,” which may lead a provider to recommend medically unindicated intervention, such as clitoral reconstruction.9
Continue to: Classifying FGC types...
Classifying FGC types
The WHO has classified FGC into 4 different types1:
- type 1, partial or total removal of the clitoris or prepuce
- type 2, partial or total removal of part of the clitoris and labia minora
- type 3 (also known as infibulation), the narrowing of the vaginal orifice by cutting, removing, and/or repositioning the labia, and
- type 4, all other procedures to the female genitalia for nonmedical reasons.
Long-term complications
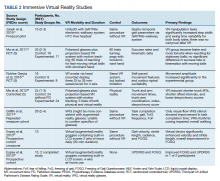
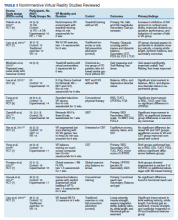
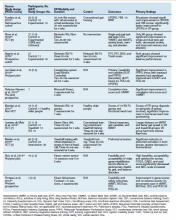
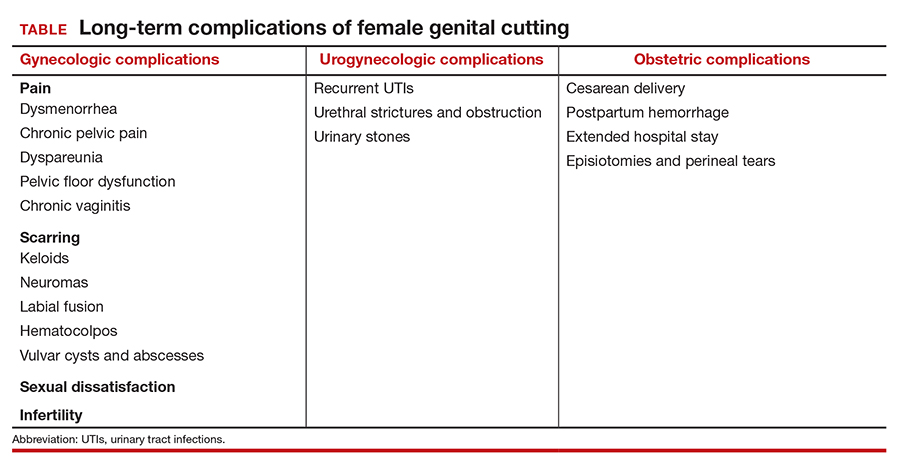
Female genital cutting, especially types 2 and 3, can lead to long-term obstetric and gynecologic complications that the ObGyn should be able to diagnose and manage (TABLE).
The most common long-term complications of FGC are dysmenorrhea, dyspareunia, recurrent vaginal and urinary tract infections, and sexual dysfunction/dissatisfaction.10 One recent cross-sectional study that used validated questionnaires on pelvic floor and psychosexual symptoms found that women with FGC had higher distress scores than women who had not undergone FGC, indicating various pelvic floor symptoms responsible for impact on their daily lives.11
Infertility can result from a combination of physical barriers (vaginal stenosis and an infibulated scar) and psychologic barriers secondary to dyspareunia, for example.12 Labor and delivery also presents a challenge to both patients and providers, especially in cases of infibulation. Studies show that patients who have undergone FGC are at increased risk of adverse obstetric outcomes, including postpartum hemorrhage, episiotomy, cesarean delivery, and extended hospital stay.13 Neonatal complications, including infant resuscitation and perinatal death, are more commonly reported in studies outside the United States.13
Clinical management recommendations
It is important to be aware of the WHO FCG classifications and be able to recognize evidence of the procedure on examination. The ObGyn should perform a detailed physical exam of the external genitalia as well as a pelvic floor exam of every patient. If the patient does not disclose a history of FGC but it is suspected based on the examination, the clinician should inquire sensitively if the patient is aware of having undergone any genital procedures.
Especially when a history of FGC has been confirmed, clinicians should ask patients sensitively about their urinary and sexual function and satisfaction. Validated tools, such as the Female Sexual Function Index, the Female Sexual Distress Scale, and the Pelvic Floor Disability Index, may be helpful in gathering an objective and detailed assessment of the patient’s symptoms and level of distress.14 Clinicians also should ask about the patient’s detailed obstetric history, particularly regarding the second stage, delivery, and postpartum complications. The clinician also should specifically inquire about a history of defibulation or additional genital procedures.
Patients with urethral strictures or stenosis may require an exam under anesthesia, cystoscopy, urethral dilation, or urethroplasty.12 Those with chronic urinary tract or vaginal infections may require chronic oral suppressive therapy or defibulation (described below). Defibulation also may be considered for relief of severe dysmenorrhea and menorrhagia that may be resulting from hematocolpos. The ObGyn also should make certain to evaluate for other common causes of these symptoms that may be unrelated to FGC, such as endometriosis.
Many women who have undergone FGC do not report dyspareunia or sexual dissatisfaction; however, infibulation especially has been associated with higher rates of these sequelae.12 In addition to defibulation, pelvic floor physical therapy with an experienced therapist may be helpful for patients with pelvic floor dysfunction, vaginismus, and/or dyspareunia.
The defibulation procedure
Defibulation (or deinfibulation) is a surgical reconstructive procedure that opens the infibulated scar of patients who have undergone type 3 FGC (infibulation), thus exposing the urethra and introitus, and in almost half of cases an intact clitoris.15 Defibulation may be specifically requested by a patient or it may be recommended by the ObGyn either for reducing complications of pregnancy or to address the patient’s gynecologic, sexual, or urogynecologic symptoms by allowing penetrative intercourse, urinary flow, physiologic delivery, and menstruation.16
Defibulation should be performed under regional or general anesthesia and can be performed during pregnancy (or even in labor). An anterior incision is made on the infibulated scar, creating a new labia major, and the edges are sutured separately. Postoperatively, patients should be instructed to perform sitz baths and to expect a change in their urinary voiding stream.12 The few studies that have evaluated defibulation have shown high rates of success in addressing preoperative symptoms; the complication rates of defibulation are low and the satisfaction rates are high.16
The ethical conundrum of reinfibulation
Reinfibulation is defined as the restitching or reapproximation of scar tissue or the labia after delivery or a gynecologic procedure, and it is often performed routinely after every delivery in patients’ countries of origin.17
Postpartum reinfibulation on patient request raises legal and ethical issues for the ObGyn. In the United Kingdom, reinfibulation is illegal, and some international organizations, including the International Federation of Gynecology and Obstetrics and the WHO, have recommended against the practice. In the United States, reinfibulation of an adult is legal, as it falls under the umbrella of elective female genital cosmetic surgery.18,19
The procedure could create or exacerbate long-term complications and should generally be discouraged. However, if despite extensive counseling (preferably in the prenatal period) a patient insists on having the procedure, the ObGyn may need to elevate the principle of patient autonomy and either comply or find a practitioner who is comfortable performing it. One retrospective review in Switzerland suggested that specific care and informative counseling prenatally with the inclusion of a patient’s partner in the discussion can improve the acceptability of defibulation without reinfibulation.20
Conclusion
It is important for ObGyns to be familiar with the practice of FGC and to be trained in its recognition on examination and care for the long-term complications that can result from the practice. At the same time, ObGyns should be especially conscious of their biases in order to provide culturally competent care and reduce health care stigmatization and inequities for these patients.
- World Health Organization. Female genital mutilation. February 3, 2020. https://www.who.int/news-room/fact-sheets/detail/female-genital-mutilation. Accessed February 22, 2021.
- UNICEF. Female genital mutilation (FGM). February 2020. https://data.unicef.org/topic/child-protection/female-genital-mutilation/. Accessed February 22, 2021.
- Stoklosa H, Nour NM. The eye cannot see what the mind does not know: female genital mutilation. Emerg Med J. 2018;35:585-586. doi: 10.1136/emermed-2018-207994.
- Abdulcadir J, Dugerdil A, Boulvain M, et al. Missed opportunities for diagnosis of female genital mutilation. Int J Gynaecol Obstet. 2014;125:256-260. doi: 10.1016/j.ijgo.2013.11.016.
- Jäger F, Schulze S, Hohlfeld P. Female genital mutilation in Switzerland: a survey among gynaecologists. Swiss Med Wkly. 2002;132:259-264.
- Zaidi N, Khalil A, Roberts C, et al. Knowledge of female genital mutilation among healthcare professionals. J Obstet Gynaecol. 2007;27:161-164. doi: 10.1080/01443610601124257.
- Chalmers B, Hashi KO. 432 Somali women’s birth experiences in Canada after earlier female genital mutilation. Birth. 2000;27:227-234. doi: 10.1046/j.1523-536x.2000.00227.x.
- Shahawy S, Amanuel H, Nour NM. Perspectives on female genital cutting among immigrant women and men in Boston. Soc Sci Med. 2019;220:331-339. doi: 10.1016/j.socscimed.2018.11.030.
- Sharif Mohamed F, Wild V, Earp BD, et al. Clitoral reconstruction after female genital mutilation/cutting: a review of surgical techniques and ethical debate. J Sex Med. 2020;17:531-542. doi: 10.1016/j.jsxm.2019.12.004.
- Nour NM. Female genital cutting: a persisting practice. Rev Obstet Gynecol. 2008 Summer;1(3):135-139.
- Binkova A, Uebelhart M, Dällenbach P, et al. A cross-sectional study on pelvic floor symptoms in women living with female genital mutilation/cutting. Reprod Health. 2021;18:39. doi: 10.1186/s12978-021-01097-9.
- Nour NM. Female genital cutting: clinical and cultural guidelines. Obstet Gynecol Surv. 2004;59:272-279. doi: 10.1097/01.ogx.0000118939.19371.af.
- WHO Study Group on Female Genital Mutilation and Obstetric Outcome; Banks E, Meirik O, Farley T, et al. Female genital mutilation and obstetric outcome: WHO collaborative prospective study in six African countries. Lancet. 2006;367:1835-1841. doi: 10.1016/S0140-6736(06)68805-3.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 119: female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007. doi: 10.1097/AOG.0b013e31821921ce.
- Nour NM, Michels KB, Bryant AE. Defibulation to treat female genital cutting: effect on symptoms and sexual function. Obstet Gynecol. 2006;108:55-60. doi: 10.1097/01.AOG.0000224613.72892.77.
- Johnson C, Nour NM. Surgical techniques: defibulation of type III female genital cutting. J Sex Med. 2007;4:1544-1547. doi: 10.1111/j.1743-6109.2007.00616.x.
- Serour GI. The issue of reinfibulation. Int J Gynaecol Obstet. 2010;109:93-96. doi: 10.1016/j.ijgo.2010.01.001.
- Shahawy S, Deshpande NA, Nour NM. Cross-cultural obstetric and gynecologic care of Muslim patients. Obstet Gynecol. 2015;126:969-973. doi: 10.1097/AOG.0000000000001112.
- American College of Obstetricians and Gynecologists. Elective female genital cosmetic surgery: ACOG committee opinion summary, number 795. Obstet Gynecol. 2020;135:249-250. doi: 10.1097/AOG.0000000000003617.
- Abdulcadir J, McLaren S, Boulvain M, et al. Health education and clinical care of immigrant women with female genital mutilation/cutting who request postpartum reinfibulation. Int J Gynaecol Obstet. 2016;135:69-72. doi: 10.1016/j.ijgo.2016.03.027.
Female genital cutting (FGC), also known as female circumcision or female genital mutilation, is defined by the World Health Organization (WHO) as “the partial or total removal of the external female genitalia, or other injury to the female genital organs for non-medical reasons.”1 It is a culturally determined practice that is mainly concentrated in certain parts of Africa, the Middle East, and Asia and now is observed worldwide among migrants from those areas.1 Approximately 200 million women and girls alive today have undergone FGC in 31 countries, although encouragingly the practice’s prevalence seems to be declining, especially among younger women.2
Too often, FGC goes unrecognized in women who present for medical care, even in cases where a genitourinary exam is performed and documented.3,4 As a result, patients face delays in diagnosis and management of associated complications and symptoms. Female genital cutting is usually excluded from medical school or residency training curricula,5 and physicians often lack familiarity with the necessary clinical or surgical management of patients who have had the procedure.6 It is crucial, however, that ObGyns feel comfortable recognizing FGC and clinically caring for pregnant and nonpregnant patients who have undergone the procedure. The obstetric-gynecologic setting should be the clinical space in which FGC is correctly diagnosed and from where patients with complications can be referred for appropriate care.
FGC: Through the lens of inequity
Providing culturally competent and sensitive care to women who have undergone FGC is paramount to reducing health care inequities for these patients. Beyond the medical recommendations we review below, we suggest the following considerations when approaching care for these patients.
Acknowledge our biases. It is paramount for us, as providers, to acknowledge our own biases and how these might affect our relationship with the patient and how our care is received. This starts with our language and terminology: The term female genital mutilation can be judgmental or offensive to our patients, many of whom do not consider themselves to have been mutilated. This is why we prefer to use the term female genital cutting, or whichever word the patient uses, so as not to alienate a patient who might already face many other barriers and microaggressions in seeking health care.
Control our responses. Another way we must check our bias is by controlling our reactions during history taking or examining patients who have undergone FGC. Understandably, providers might be shocked to hear patients recount their childhood experiences of FGC or by examining an infibulated scar, but patients report noticing and experiencing hurt, distress, and shame when providers display judgment, horror, or disgust.7 Patients have reported that they are acutely aware that they might be viewed as “backward” and “primitive” in US health care settings.8 These kinds of feelings and experiences can further exacerbate patients’ distrust and avoidance of the health care system altogether. Therefore, providers should acknowledge their own biases regarding the issue as well as those of their staff and work to mitigate them.
Avoid stigmatization. While FGC can have long-term effects (discussed below), it is important to remember that many women who have undergone FGC do not experience symptoms that are bothersome or feel that FGC is central to their lives or lived experiences. While we must be thorough in our history taking to explore possible urinary, gynecologic, and sexual symptoms of concern and bother to the patient, we must avoid stigmatizing our patients by assuming that all who have undergone FGC are “sexually disabled,” which may lead a provider to recommend medically unindicated intervention, such as clitoral reconstruction.9
Continue to: Classifying FGC types...
Classifying FGC types
The WHO has classified FGC into 4 different types1:
- type 1, partial or total removal of the clitoris or prepuce
- type 2, partial or total removal of part of the clitoris and labia minora
- type 3 (also known as infibulation), the narrowing of the vaginal orifice by cutting, removing, and/or repositioning the labia, and
- type 4, all other procedures to the female genitalia for nonmedical reasons.
Long-term complications
Female genital cutting, especially types 2 and 3, can lead to long-term obstetric and gynecologic complications that the ObGyn should be able to diagnose and manage (TABLE).
The most common long-term complications of FGC are dysmenorrhea, dyspareunia, recurrent vaginal and urinary tract infections, and sexual dysfunction/dissatisfaction.10 One recent cross-sectional study that used validated questionnaires on pelvic floor and psychosexual symptoms found that women with FGC had higher distress scores than women who had not undergone FGC, indicating various pelvic floor symptoms responsible for impact on their daily lives.11
Infertility can result from a combination of physical barriers (vaginal stenosis and an infibulated scar) and psychologic barriers secondary to dyspareunia, for example.12 Labor and delivery also presents a challenge to both patients and providers, especially in cases of infibulation. Studies show that patients who have undergone FGC are at increased risk of adverse obstetric outcomes, including postpartum hemorrhage, episiotomy, cesarean delivery, and extended hospital stay.13 Neonatal complications, including infant resuscitation and perinatal death, are more commonly reported in studies outside the United States.13

Clinical management recommendations
It is important to be aware of the WHO FCG classifications and be able to recognize evidence of the procedure on examination. The ObGyn should perform a detailed physical exam of the external genitalia as well as a pelvic floor exam of every patient. If the patient does not disclose a history of FGC but it is suspected based on the examination, the clinician should inquire sensitively if the patient is aware of having undergone any genital procedures.
Especially when a history of FGC has been confirmed, clinicians should ask patients sensitively about their urinary and sexual function and satisfaction. Validated tools, such as the Female Sexual Function Index, the Female Sexual Distress Scale, and the Pelvic Floor Disability Index, may be helpful in gathering an objective and detailed assessment of the patient’s symptoms and level of distress.14 Clinicians also should ask about the patient’s detailed obstetric history, particularly regarding the second stage, delivery, and postpartum complications. The clinician also should specifically inquire about a history of defibulation or additional genital procedures.
Patients with urethral strictures or stenosis may require an exam under anesthesia, cystoscopy, urethral dilation, or urethroplasty.12 Those with chronic urinary tract or vaginal infections may require chronic oral suppressive therapy or defibulation (described below). Defibulation also may be considered for relief of severe dysmenorrhea and menorrhagia that may be resulting from hematocolpos. The ObGyn also should make certain to evaluate for other common causes of these symptoms that may be unrelated to FGC, such as endometriosis.
Many women who have undergone FGC do not report dyspareunia or sexual dissatisfaction; however, infibulation especially has been associated with higher rates of these sequelae.12 In addition to defibulation, pelvic floor physical therapy with an experienced therapist may be helpful for patients with pelvic floor dysfunction, vaginismus, and/or dyspareunia.
The defibulation procedure
Defibulation (or deinfibulation) is a surgical reconstructive procedure that opens the infibulated scar of patients who have undergone type 3 FGC (infibulation), thus exposing the urethra and introitus, and in almost half of cases an intact clitoris.15 Defibulation may be specifically requested by a patient or it may be recommended by the ObGyn either for reducing complications of pregnancy or to address the patient’s gynecologic, sexual, or urogynecologic symptoms by allowing penetrative intercourse, urinary flow, physiologic delivery, and menstruation.16
Defibulation should be performed under regional or general anesthesia and can be performed during pregnancy (or even in labor). An anterior incision is made on the infibulated scar, creating a new labia major, and the edges are sutured separately. Postoperatively, patients should be instructed to perform sitz baths and to expect a change in their urinary voiding stream.12 The few studies that have evaluated defibulation have shown high rates of success in addressing preoperative symptoms; the complication rates of defibulation are low and the satisfaction rates are high.16
The ethical conundrum of reinfibulation
Reinfibulation is defined as the restitching or reapproximation of scar tissue or the labia after delivery or a gynecologic procedure, and it is often performed routinely after every delivery in patients’ countries of origin.17
Postpartum reinfibulation on patient request raises legal and ethical issues for the ObGyn. In the United Kingdom, reinfibulation is illegal, and some international organizations, including the International Federation of Gynecology and Obstetrics and the WHO, have recommended against the practice. In the United States, reinfibulation of an adult is legal, as it falls under the umbrella of elective female genital cosmetic surgery.18,19
The procedure could create or exacerbate long-term complications and should generally be discouraged. However, if despite extensive counseling (preferably in the prenatal period) a patient insists on having the procedure, the ObGyn may need to elevate the principle of patient autonomy and either comply or find a practitioner who is comfortable performing it. One retrospective review in Switzerland suggested that specific care and informative counseling prenatally with the inclusion of a patient’s partner in the discussion can improve the acceptability of defibulation without reinfibulation.20
Conclusion
It is important for ObGyns to be familiar with the practice of FGC and to be trained in its recognition on examination and care for the long-term complications that can result from the practice. At the same time, ObGyns should be especially conscious of their biases in order to provide culturally competent care and reduce health care stigmatization and inequities for these patients.
Female genital cutting (FGC), also known as female circumcision or female genital mutilation, is defined by the World Health Organization (WHO) as “the partial or total removal of the external female genitalia, or other injury to the female genital organs for non-medical reasons.”1 It is a culturally determined practice that is mainly concentrated in certain parts of Africa, the Middle East, and Asia and now is observed worldwide among migrants from those areas.1 Approximately 200 million women and girls alive today have undergone FGC in 31 countries, although encouragingly the practice’s prevalence seems to be declining, especially among younger women.2
Too often, FGC goes unrecognized in women who present for medical care, even in cases where a genitourinary exam is performed and documented.3,4 As a result, patients face delays in diagnosis and management of associated complications and symptoms. Female genital cutting is usually excluded from medical school or residency training curricula,5 and physicians often lack familiarity with the necessary clinical or surgical management of patients who have had the procedure.6 It is crucial, however, that ObGyns feel comfortable recognizing FGC and clinically caring for pregnant and nonpregnant patients who have undergone the procedure. The obstetric-gynecologic setting should be the clinical space in which FGC is correctly diagnosed and from where patients with complications can be referred for appropriate care.
FGC: Through the lens of inequity
Providing culturally competent and sensitive care to women who have undergone FGC is paramount to reducing health care inequities for these patients. Beyond the medical recommendations we review below, we suggest the following considerations when approaching care for these patients.
Acknowledge our biases. It is paramount for us, as providers, to acknowledge our own biases and how these might affect our relationship with the patient and how our care is received. This starts with our language and terminology: The term female genital mutilation can be judgmental or offensive to our patients, many of whom do not consider themselves to have been mutilated. This is why we prefer to use the term female genital cutting, or whichever word the patient uses, so as not to alienate a patient who might already face many other barriers and microaggressions in seeking health care.
Control our responses. Another way we must check our bias is by controlling our reactions during history taking or examining patients who have undergone FGC. Understandably, providers might be shocked to hear patients recount their childhood experiences of FGC or by examining an infibulated scar, but patients report noticing and experiencing hurt, distress, and shame when providers display judgment, horror, or disgust.7 Patients have reported that they are acutely aware that they might be viewed as “backward” and “primitive” in US health care settings.8 These kinds of feelings and experiences can further exacerbate patients’ distrust and avoidance of the health care system altogether. Therefore, providers should acknowledge their own biases regarding the issue as well as those of their staff and work to mitigate them.
Avoid stigmatization. While FGC can have long-term effects (discussed below), it is important to remember that many women who have undergone FGC do not experience symptoms that are bothersome or feel that FGC is central to their lives or lived experiences. While we must be thorough in our history taking to explore possible urinary, gynecologic, and sexual symptoms of concern and bother to the patient, we must avoid stigmatizing our patients by assuming that all who have undergone FGC are “sexually disabled,” which may lead a provider to recommend medically unindicated intervention, such as clitoral reconstruction.9
Continue to: Classifying FGC types...
Classifying FGC types
The WHO has classified FGC into 4 different types1:
- type 1, partial or total removal of the clitoris or prepuce
- type 2, partial or total removal of part of the clitoris and labia minora
- type 3 (also known as infibulation), the narrowing of the vaginal orifice by cutting, removing, and/or repositioning the labia, and
- type 4, all other procedures to the female genitalia for nonmedical reasons.
Long-term complications
Female genital cutting, especially types 2 and 3, can lead to long-term obstetric and gynecologic complications that the ObGyn should be able to diagnose and manage (TABLE).
The most common long-term complications of FGC are dysmenorrhea, dyspareunia, recurrent vaginal and urinary tract infections, and sexual dysfunction/dissatisfaction.10 One recent cross-sectional study that used validated questionnaires on pelvic floor and psychosexual symptoms found that women with FGC had higher distress scores than women who had not undergone FGC, indicating various pelvic floor symptoms responsible for impact on their daily lives.11
Infertility can result from a combination of physical barriers (vaginal stenosis and an infibulated scar) and psychologic barriers secondary to dyspareunia, for example.12 Labor and delivery also presents a challenge to both patients and providers, especially in cases of infibulation. Studies show that patients who have undergone FGC are at increased risk of adverse obstetric outcomes, including postpartum hemorrhage, episiotomy, cesarean delivery, and extended hospital stay.13 Neonatal complications, including infant resuscitation and perinatal death, are more commonly reported in studies outside the United States.13

Clinical management recommendations
It is important to be aware of the WHO FCG classifications and be able to recognize evidence of the procedure on examination. The ObGyn should perform a detailed physical exam of the external genitalia as well as a pelvic floor exam of every patient. If the patient does not disclose a history of FGC but it is suspected based on the examination, the clinician should inquire sensitively if the patient is aware of having undergone any genital procedures.
Especially when a history of FGC has been confirmed, clinicians should ask patients sensitively about their urinary and sexual function and satisfaction. Validated tools, such as the Female Sexual Function Index, the Female Sexual Distress Scale, and the Pelvic Floor Disability Index, may be helpful in gathering an objective and detailed assessment of the patient’s symptoms and level of distress.14 Clinicians also should ask about the patient’s detailed obstetric history, particularly regarding the second stage, delivery, and postpartum complications. The clinician also should specifically inquire about a history of defibulation or additional genital procedures.
Patients with urethral strictures or stenosis may require an exam under anesthesia, cystoscopy, urethral dilation, or urethroplasty.12 Those with chronic urinary tract or vaginal infections may require chronic oral suppressive therapy or defibulation (described below). Defibulation also may be considered for relief of severe dysmenorrhea and menorrhagia that may be resulting from hematocolpos. The ObGyn also should make certain to evaluate for other common causes of these symptoms that may be unrelated to FGC, such as endometriosis.
Many women who have undergone FGC do not report dyspareunia or sexual dissatisfaction; however, infibulation especially has been associated with higher rates of these sequelae.12 In addition to defibulation, pelvic floor physical therapy with an experienced therapist may be helpful for patients with pelvic floor dysfunction, vaginismus, and/or dyspareunia.
The defibulation procedure
Defibulation (or deinfibulation) is a surgical reconstructive procedure that opens the infibulated scar of patients who have undergone type 3 FGC (infibulation), thus exposing the urethra and introitus, and in almost half of cases an intact clitoris.15 Defibulation may be specifically requested by a patient or it may be recommended by the ObGyn either for reducing complications of pregnancy or to address the patient’s gynecologic, sexual, or urogynecologic symptoms by allowing penetrative intercourse, urinary flow, physiologic delivery, and menstruation.16
Defibulation should be performed under regional or general anesthesia and can be performed during pregnancy (or even in labor). An anterior incision is made on the infibulated scar, creating a new labia major, and the edges are sutured separately. Postoperatively, patients should be instructed to perform sitz baths and to expect a change in their urinary voiding stream.12 The few studies that have evaluated defibulation have shown high rates of success in addressing preoperative symptoms; the complication rates of defibulation are low and the satisfaction rates are high.16
The ethical conundrum of reinfibulation
Reinfibulation is defined as the restitching or reapproximation of scar tissue or the labia after delivery or a gynecologic procedure, and it is often performed routinely after every delivery in patients’ countries of origin.17
Postpartum reinfibulation on patient request raises legal and ethical issues for the ObGyn. In the United Kingdom, reinfibulation is illegal, and some international organizations, including the International Federation of Gynecology and Obstetrics and the WHO, have recommended against the practice. In the United States, reinfibulation of an adult is legal, as it falls under the umbrella of elective female genital cosmetic surgery.18,19
The procedure could create or exacerbate long-term complications and should generally be discouraged. However, if despite extensive counseling (preferably in the prenatal period) a patient insists on having the procedure, the ObGyn may need to elevate the principle of patient autonomy and either comply or find a practitioner who is comfortable performing it. One retrospective review in Switzerland suggested that specific care and informative counseling prenatally with the inclusion of a patient’s partner in the discussion can improve the acceptability of defibulation without reinfibulation.20
Conclusion
It is important for ObGyns to be familiar with the practice of FGC and to be trained in its recognition on examination and care for the long-term complications that can result from the practice. At the same time, ObGyns should be especially conscious of their biases in order to provide culturally competent care and reduce health care stigmatization and inequities for these patients.
- World Health Organization. Female genital mutilation. February 3, 2020. https://www.who.int/news-room/fact-sheets/detail/female-genital-mutilation. Accessed February 22, 2021.
- UNICEF. Female genital mutilation (FGM). February 2020. https://data.unicef.org/topic/child-protection/female-genital-mutilation/. Accessed February 22, 2021.
- Stoklosa H, Nour NM. The eye cannot see what the mind does not know: female genital mutilation. Emerg Med J. 2018;35:585-586. doi: 10.1136/emermed-2018-207994.
- Abdulcadir J, Dugerdil A, Boulvain M, et al. Missed opportunities for diagnosis of female genital mutilation. Int J Gynaecol Obstet. 2014;125:256-260. doi: 10.1016/j.ijgo.2013.11.016.
- Jäger F, Schulze S, Hohlfeld P. Female genital mutilation in Switzerland: a survey among gynaecologists. Swiss Med Wkly. 2002;132:259-264.
- Zaidi N, Khalil A, Roberts C, et al. Knowledge of female genital mutilation among healthcare professionals. J Obstet Gynaecol. 2007;27:161-164. doi: 10.1080/01443610601124257.
- Chalmers B, Hashi KO. 432 Somali women’s birth experiences in Canada after earlier female genital mutilation. Birth. 2000;27:227-234. doi: 10.1046/j.1523-536x.2000.00227.x.
- Shahawy S, Amanuel H, Nour NM. Perspectives on female genital cutting among immigrant women and men in Boston. Soc Sci Med. 2019;220:331-339. doi: 10.1016/j.socscimed.2018.11.030.
- Sharif Mohamed F, Wild V, Earp BD, et al. Clitoral reconstruction after female genital mutilation/cutting: a review of surgical techniques and ethical debate. J Sex Med. 2020;17:531-542. doi: 10.1016/j.jsxm.2019.12.004.
- Nour NM. Female genital cutting: a persisting practice. Rev Obstet Gynecol. 2008 Summer;1(3):135-139.
- Binkova A, Uebelhart M, Dällenbach P, et al. A cross-sectional study on pelvic floor symptoms in women living with female genital mutilation/cutting. Reprod Health. 2021;18:39. doi: 10.1186/s12978-021-01097-9.
- Nour NM. Female genital cutting: clinical and cultural guidelines. Obstet Gynecol Surv. 2004;59:272-279. doi: 10.1097/01.ogx.0000118939.19371.af.
- WHO Study Group on Female Genital Mutilation and Obstetric Outcome; Banks E, Meirik O, Farley T, et al. Female genital mutilation and obstetric outcome: WHO collaborative prospective study in six African countries. Lancet. 2006;367:1835-1841. doi: 10.1016/S0140-6736(06)68805-3.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 119: female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007. doi: 10.1097/AOG.0b013e31821921ce.
- Nour NM, Michels KB, Bryant AE. Defibulation to treat female genital cutting: effect on symptoms and sexual function. Obstet Gynecol. 2006;108:55-60. doi: 10.1097/01.AOG.0000224613.72892.77.
- Johnson C, Nour NM. Surgical techniques: defibulation of type III female genital cutting. J Sex Med. 2007;4:1544-1547. doi: 10.1111/j.1743-6109.2007.00616.x.
- Serour GI. The issue of reinfibulation. Int J Gynaecol Obstet. 2010;109:93-96. doi: 10.1016/j.ijgo.2010.01.001.
- Shahawy S, Deshpande NA, Nour NM. Cross-cultural obstetric and gynecologic care of Muslim patients. Obstet Gynecol. 2015;126:969-973. doi: 10.1097/AOG.0000000000001112.
- American College of Obstetricians and Gynecologists. Elective female genital cosmetic surgery: ACOG committee opinion summary, number 795. Obstet Gynecol. 2020;135:249-250. doi: 10.1097/AOG.0000000000003617.
- Abdulcadir J, McLaren S, Boulvain M, et al. Health education and clinical care of immigrant women with female genital mutilation/cutting who request postpartum reinfibulation. Int J Gynaecol Obstet. 2016;135:69-72. doi: 10.1016/j.ijgo.2016.03.027.
- World Health Organization. Female genital mutilation. February 3, 2020. https://www.who.int/news-room/fact-sheets/detail/female-genital-mutilation. Accessed February 22, 2021.
- UNICEF. Female genital mutilation (FGM). February 2020. https://data.unicef.org/topic/child-protection/female-genital-mutilation/. Accessed February 22, 2021.
- Stoklosa H, Nour NM. The eye cannot see what the mind does not know: female genital mutilation. Emerg Med J. 2018;35:585-586. doi: 10.1136/emermed-2018-207994.
- Abdulcadir J, Dugerdil A, Boulvain M, et al. Missed opportunities for diagnosis of female genital mutilation. Int J Gynaecol Obstet. 2014;125:256-260. doi: 10.1016/j.ijgo.2013.11.016.
- Jäger F, Schulze S, Hohlfeld P. Female genital mutilation in Switzerland: a survey among gynaecologists. Swiss Med Wkly. 2002;132:259-264.
- Zaidi N, Khalil A, Roberts C, et al. Knowledge of female genital mutilation among healthcare professionals. J Obstet Gynaecol. 2007;27:161-164. doi: 10.1080/01443610601124257.
- Chalmers B, Hashi KO. 432 Somali women’s birth experiences in Canada after earlier female genital mutilation. Birth. 2000;27:227-234. doi: 10.1046/j.1523-536x.2000.00227.x.
- Shahawy S, Amanuel H, Nour NM. Perspectives on female genital cutting among immigrant women and men in Boston. Soc Sci Med. 2019;220:331-339. doi: 10.1016/j.socscimed.2018.11.030.
- Sharif Mohamed F, Wild V, Earp BD, et al. Clitoral reconstruction after female genital mutilation/cutting: a review of surgical techniques and ethical debate. J Sex Med. 2020;17:531-542. doi: 10.1016/j.jsxm.2019.12.004.
- Nour NM. Female genital cutting: a persisting practice. Rev Obstet Gynecol. 2008 Summer;1(3):135-139.
- Binkova A, Uebelhart M, Dällenbach P, et al. A cross-sectional study on pelvic floor symptoms in women living with female genital mutilation/cutting. Reprod Health. 2021;18:39. doi: 10.1186/s12978-021-01097-9.
- Nour NM. Female genital cutting: clinical and cultural guidelines. Obstet Gynecol Surv. 2004;59:272-279. doi: 10.1097/01.ogx.0000118939.19371.af.
- WHO Study Group on Female Genital Mutilation and Obstetric Outcome; Banks E, Meirik O, Farley T, et al. Female genital mutilation and obstetric outcome: WHO collaborative prospective study in six African countries. Lancet. 2006;367:1835-1841. doi: 10.1016/S0140-6736(06)68805-3.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 119: female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007. doi: 10.1097/AOG.0b013e31821921ce.
- Nour NM, Michels KB, Bryant AE. Defibulation to treat female genital cutting: effect on symptoms and sexual function. Obstet Gynecol. 2006;108:55-60. doi: 10.1097/01.AOG.0000224613.72892.77.
- Johnson C, Nour NM. Surgical techniques: defibulation of type III female genital cutting. J Sex Med. 2007;4:1544-1547. doi: 10.1111/j.1743-6109.2007.00616.x.
- Serour GI. The issue of reinfibulation. Int J Gynaecol Obstet. 2010;109:93-96. doi: 10.1016/j.ijgo.2010.01.001.
- Shahawy S, Deshpande NA, Nour NM. Cross-cultural obstetric and gynecologic care of Muslim patients. Obstet Gynecol. 2015;126:969-973. doi: 10.1097/AOG.0000000000001112.
- American College of Obstetricians and Gynecologists. Elective female genital cosmetic surgery: ACOG committee opinion summary, number 795. Obstet Gynecol. 2020;135:249-250. doi: 10.1097/AOG.0000000000003617.
- Abdulcadir J, McLaren S, Boulvain M, et al. Health education and clinical care of immigrant women with female genital mutilation/cutting who request postpartum reinfibulation. Int J Gynaecol Obstet. 2016;135:69-72. doi: 10.1016/j.ijgo.2016.03.027.
2021 Update on sequencing in prenatal genetics
Prenatal diagnosis has expanded from identification of aneuploidy to include copy number variants detected on microarray (such as 22q11 deletion syndrome) and now single-gene disorders identified by targeted or exome and genome sequencing. How and when different sequencing tests should be used clinically are questions faced by every provider engaged in modern prenatal diagnosis.
In this Update, we highlight new clinical insights into prenatal sequencing and explore how information gained from sequencing may help us understand some of the unanswered questions in obstetrics.
What is the yield of a RASopathy gene panel with specific prenatal findings?
Scott A, Di Giosaffatte N, Pinna V, et al. When to test fetuses for RASopathies? Proposition from a systematic analysis of 352 multicenter cases and a postnatal cohort. Genet Med. Published online February 10, 2021. doi:10.1038/s41436-020-01093-7.
RASopathies, a group of genetic conditions caused by mutations in the RAS/mitogen-activated protein kinase (RAS-MAPK) pathway, are common, occurring in 1:1,000 to 1:2,500 live births. RASopathies are much more common than 22q11 deletion syndrome, or DiGeorge syndrome, which occurs in 1.4:10,000 live births.1
RASopathy disorders include Noonan syndrome, Noonan syndrome with multiple lentigines, Costello syndrome, cardiofaciocutaneous syndrome, and Noonan-like syndrome with loose anagen hair. These are autosomal dominant disorders caused by a pathogenic variant (or mutation) in 1 of more than 20 genes in the RAS-MAPK signaling pathway in the body. Clinical features include congenital anomalies of the kidney and urinary tract, lymphatic anomalies, congenital heart disease (CHD), hypertrophic cardiomyopathy (HCM), postnatal growth disorders, neurodevelopmental disorders, and more rarely hematologic malignancies. Prenatal clues include an increased nuchal translucency (NT), CHD, cystic hygroma, lymphatic anomalies, anomalies of the kidney and urinary tract, hydrops, and HCM.
Cohort of pregnancies that received a RASopathy panel
Scott and colleagues sought to clarify the utility of testing for RASopathies with a prenatal gene panel. They conducted a multicenter retrospective cohort study with cases from 2 hospitals in Italy and Canada; data were collected between 2012 and 2019.
Eligible fetuses were those referred to the prenatal genetics clinic because of an increased NT, increased nuchal fold (NF), hydrops, ascites, thoracic effusions, chylothorax, other lymphatic anomalies, CHD, or HCM with a nondiagnostic (negative) microarray or karyotype. All eligible cases had RASopathy molecular testing in the prenatal or neonatal period.
Among the 352 referrals to clinic, 50 cases of a RASopathy disorder were diagnosed. Additionally, to complement this cohort over the same time period, 25 postnatal diagnoses were made after retrospective review performed to ascertain additional prenatal findings. The size of the testing panel ranged from 9 to 20 genes, which were sent to clinical laboratories that performed sequencing based on standard protocols.
Study outcomes
Overall, 14% of fetuses with an indication for testing had a pathogenic or likely pathogenic variant (diagnostic) on panel testing among 11 genes (notably, all presented results are after excluding copy number variants and aneuploidy). Fetuses with only 1 ultrasonography finding were much less likely to have a positive result than those with more than 1 ultrasonography finding, 3% versus 18%. The highest diagnostic yields were for HCM at 69%; thoracic effusions and ascites, 41%; persistent hydrops, 39%; cystic hygroma combined with another suggestive ultrasonography finding, 28%; CHD, 23%; and persistent cystic hygroma, 21%. Five fetuses were affected with CHD and HCM, and 44% had an intrauterine fetal demise.
Importance of NT size. An isolated increased NT had a diagnostic yield of 1% overall (1/90); however, the size of the NT mattered. Seventeen fetuses had an NT between 3 and 3.5 mm and none of these had diagnostic sequencing, whereas 26% with an NT greater than 6 mm had a diagnostic result (11/43). An increased NF had a diagnostic yield of 25%.
Other findings. Of fetuses with a cystic hygroma, 16% had a pathogenic or likely pathogenic variant, and when these persisted into the second trimester or were associated with other anomalies, the percentages increased to 21% and 28%, respectively. Of prenatal patients, 20.6% had variants of uncertain significance, and 12% of the pathogenic and likely pathogenic variants were inherited, which is less than previously reported series. Additionally, 48% of the postnatal RASopathy diagnosis group did not have an ultrasonography finding on record review.
Continue to: Study strengths and limitations...
Study strengths and limitations
This study presents a large cohort of prenatal and neonatal patients tested for RASopathies at 2 international centers with very granular and clinically useful data about ultrasonography findings and yield of panel testing. Prenatal care providers, geneticists, and computational biologists may find this study of great interest and take away useful information and ideas due to the authors’ presentation and details.
The number of genes tested changed over the inclusion time period, but this is an inescapable reality of retrospective clinical research in an advancing field. The authors presented the prenatal and postnatal diagnoses ultrasonography findings separately and together. Given the different nature of cohort ascertainment, we prefer to consider these groups separately and have presented the data for the prenatal group.
Prenatal sequencing panels and exome sequencing are detecting disorders with important implications for prenatal care. If your practice is not testing for RASopathies in prenatal patients with concerning ultrasonography features, you are missing cases. In this study, the most concerning ultrasonography features (more than 20% diagnosis) were HCM, thoracic effusions and ascites, persistent hydrops, cystic hygroma combined with another suggestive ultrasonography finding, CHD, and persistent cystic hygroma. Isolated ultrasonography findings or findings that resolved had a lower diagnostic yield, and an isolated enlarged NT had a 1% diagnostic yield, with most cases having an NT larger than 6 mm.
For pretest counseling, in this study 20% of patients had a variant of uncertain significance, and preparing patients for this possibility is crucial. Most variants of uncertain significance are reclassified to benign when more information is available. Providers can consider sending parental samples concurrently with the fetal sample to help obtain useful information quickly, although the possibility of an inherited pathogenic variant still exists (12% in this study).
Prenatal diagnosis gives your patients the opportunity to learn about the disorder, plan for treatment and delivery location, and establish their care team before birth or consider pregnancy termination.
Sequencing provides insights into twin pregnancies
Jonsson H, Magnusdottir E, Eggertsson HP, et al. Differences between germline genomes of monozygotic twins. Nat Genet. 2021;53:27-34. doi:10.1038/s41588 -020-00755-1.
You have a monozygotic twin pair with an anomaly and intend to do diagnostic testing for prenatal diagnosis. The question always arises: Do you sample both twins or just one? Surely, they are genetically identical? A wise mentor once instilled a valuable lesson: Monozygotic twins are more likely to have an anomaly. Their existence is already out of the realm of normal. Finally, we now have an engaging and interesting answer to this and other fascinating embryology questions through the work of Jonsson and colleagues.
Study eligibility criteria and treatment protocol
The authors enrolled 381 twin pairs and 2 monozygotic triplets and compared genome sequencing of different tissues (cheek cells and blood). They went further to assess what other tissues might share the genetic change. To do this, they sequenced the children and the partners of 181 of the pairs. Presumably, if a twin and their offspring shared a genetic change that was not present in the spouse or twin, this genetic change must be present in the oocytes or sperm of the parent twin. The goal of sequencing multiple tissue sources in each twin was to help determine when the genetic change occurred in embryonic development.
Study outcomes
The authors found that 15% of twins had mutations that were absent in the other twin. Because of the extent of tissues that had the genetic change, the authors asserted that these changes must have occurred very early in embryonic development (even from one cell after twinning) for the changes to be near-constitutional (among sampled tissues).
An average of 14 genetic differences were found between twin pairs that developed after twinning. However, the number of differences varied. For example, 39 pairs of twins differed by more than 100 changes, and 38 did not differ at all. Differences between twins were more likely in blood samples than in cheek swabs, suggesting that some differences were due to acquired genetic changes in hematologic cell lines, or clonal hematopoiesis.
The authors also looked at what percentage of sequenced DNA contained the variants (or mutations) and found that many of these DNA differences were present at high amounts in sequencing reads. This suggests that the DNA changes happened very early after twinning in about one-third of pairs. Additionally, if one twin had a near-constitutional change, in 42% of pairs the other twin had a different near-constitutional change. Among the triplets, 2 of a triplet pair shared more genetic similarity and were likely descendent from a single split cell and the third likely was formed from a different set of cells.
By examining the offspring of twins, Jonsson and colleagues found that there were 2.6 early embryonic mutations, and this did not differ when blood or buccal DNA was compared. The rate of transmission of a variant to offspring was proportional to the variant allele frequency (proportion of alternate alleles) in the blood or buccal cells. This is an important counseling point when considering patients with mosaic genetic disorders and counseling about the likelihood of inheritance or transmission to future offspring. If the rate of mosaicism was higher in blood or buccal cells, the likelihood of transmission was higher. Additionally, the mutations did not differ by sex, and there was no relationship to whether the chromosome was maternally or paternally inherited.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors did not have access to information about chorionicity of the monozygotic twin pairs. Consequently, they were unable to correlate chorionicity with the degree of noted genetic difference between the monozygotic twin pairs. Additionally, although the authors were thoughtful in their utilization of offspring and spouses to infer germline genomic content, the study had a limited number of tissues sampled, which could reduce the applicability. However, the sample size, clinically accessible tissue sampling, and thoughtful analysis used in this study make it an interesting and relevant contribution to reproductive medicine and evolutionary biology.
We all accumulate changes to our DNA throughout life. The study by Jonsson and colleagues illustrates that for many, this accumulation of genetic changes starts very early in gestation. In the early zygote, the authors observed roughly 1 mutation per cell division prior to the point of twinning. In the realm of prenatal diagnosis, one should consider that monochorionic twins with different phenotypes (that is, an ultrasonography anomaly in 1 of the twin pair) could represent a genetic change rather than an environmental difference. This genetic change may not be shared by the other twin despite originating from the same primordial cell line. The genetic changes that the authors investigated were detected on genome sequencing, which is much more comprehensive than the exome sequencing that is increasingly utilized in rare disease diagnosis. The clinical utility of this observation in prenatal diagnosis has yet to be proven, but this study provides preliminary data that 15% of monozygotic twins have genetic differences and may warrant individualized testing.
The genetic landscape of the placenta
Coorens TH, Oliver TR, Sanghvi R, et al. Inherent mosaicism and extensive mutation of human placentas. Nature. Published online March 10, 2021. doi:10.1038/ s41586-021-03345-1.
Confined placental mosaicism (CPM) is a phenomenon in which the genetics of the placenta are different from those of the fetus. Historically, this phenomenon has been described in 1% to 2% of pregnancies based on karyotype data obtained from chorionic villus sampling. Some studies have demonstrated adverse pregnancy outcomes in the setting of CPM, thought to be secondary to aneuploid cells in the placenta leading to insufficiency or dysfunction.
Although our sophistication and level of detail in prenatal genetic testing has rapidly expanded to include information about copy number variants and singlenucleotide changes, their contribution to CPM has been understudied. Coorens and colleagues recently published a landmark study that describes a surprisingly high rate of mosaicism for these smaller genetic changes.
A cohort study of placentas
The authors performed whole genome sequencing on placental samples obtained from 37 term pregnancies. Umbilical cord tissue and maternal blood also were collected and served as controls for fetal and maternal genetic profiles, respectively.
In a subgroup of 5 placentas, lasercapture microscopy was used to separate placental cells of different origins, including trophoblastic cells, mesenchymal core cells, and cells originating from the inner cell mass. To investigate variation within different geographic regions of a single placenta, these cell lines were derived multiple times from each quadrant of the 5 placentas.
Placental biopsies revealed “bottlenecks” of genetic differentiation
Genome sequencing was used uniquely in this study to help delineate the phylogeny of placental cells by tracking somatic mutations both in different geographic locations of each placenta and between different cells of origin within 1 placenta.
The authors concluded that bottlenecks of differentiation in placental development led to unique genetic signatures in every bulk placental sample studied. Their findings led them to describe the placenta as a “patchwork” of independent genetic units resulting from clonal expansion at different stages of embryonic development.
Early insights into human placental cells
This study provides fascinating insight into the surprisingly high rates of copy number variants and single-gene changes that exist, in mosaic form, within human placentas. The authors distinguish the placenta from other human organs (such as the colon, endometrium, liver, and skin) in which many fewer genetic changes exist. In fact, they suggest parallels between the “mutational signature” of the placenta with rapidly dividing neoplastic cells.
As one of the first investigations into the variation and complexity of genetic changes within the placenta, this study was not designed to draw conclusions regarding the clinical impact of the numerous genetic changes described. Further studies will elucidate the potential contribution of genetically mosaic placentas to common adverse obstetric outcomes. ●
With a new appreciation for the smaller genetic alterations that exist within placental tissue, it appears that the rate of CPM has been vastly underestimated. We know that aneuploid placental cells increase the risk of adverse pregnancy outcomes and we may learn more about the contribution of copy number variants and single-nucleotide changes to preeclampsia, growth restriction, and pregnancy loss. Furthermore, as the applications of cell-free fetal DNA (cffDNA) in genetic screening continue to expand, we must exercise caution in assuming that copy number variants or single-nucleotide changes detected by cffDNA reflect those of the developing fetus.
- Roberts AE, Allanson JE, Tartaglia M, et al. Noonan syndrome. Lancet. 2013;381:333-342. doi:10.1016/S0140-6736(12)61023-X.
Prenatal diagnosis has expanded from identification of aneuploidy to include copy number variants detected on microarray (such as 22q11 deletion syndrome) and now single-gene disorders identified by targeted or exome and genome sequencing. How and when different sequencing tests should be used clinically are questions faced by every provider engaged in modern prenatal diagnosis.
In this Update, we highlight new clinical insights into prenatal sequencing and explore how information gained from sequencing may help us understand some of the unanswered questions in obstetrics.
What is the yield of a RASopathy gene panel with specific prenatal findings?
Scott A, Di Giosaffatte N, Pinna V, et al. When to test fetuses for RASopathies? Proposition from a systematic analysis of 352 multicenter cases and a postnatal cohort. Genet Med. Published online February 10, 2021. doi:10.1038/s41436-020-01093-7.
RASopathies, a group of genetic conditions caused by mutations in the RAS/mitogen-activated protein kinase (RAS-MAPK) pathway, are common, occurring in 1:1,000 to 1:2,500 live births. RASopathies are much more common than 22q11 deletion syndrome, or DiGeorge syndrome, which occurs in 1.4:10,000 live births.1
RASopathy disorders include Noonan syndrome, Noonan syndrome with multiple lentigines, Costello syndrome, cardiofaciocutaneous syndrome, and Noonan-like syndrome with loose anagen hair. These are autosomal dominant disorders caused by a pathogenic variant (or mutation) in 1 of more than 20 genes in the RAS-MAPK signaling pathway in the body. Clinical features include congenital anomalies of the kidney and urinary tract, lymphatic anomalies, congenital heart disease (CHD), hypertrophic cardiomyopathy (HCM), postnatal growth disorders, neurodevelopmental disorders, and more rarely hematologic malignancies. Prenatal clues include an increased nuchal translucency (NT), CHD, cystic hygroma, lymphatic anomalies, anomalies of the kidney and urinary tract, hydrops, and HCM.
Cohort of pregnancies that received a RASopathy panel
Scott and colleagues sought to clarify the utility of testing for RASopathies with a prenatal gene panel. They conducted a multicenter retrospective cohort study with cases from 2 hospitals in Italy and Canada; data were collected between 2012 and 2019.
Eligible fetuses were those referred to the prenatal genetics clinic because of an increased NT, increased nuchal fold (NF), hydrops, ascites, thoracic effusions, chylothorax, other lymphatic anomalies, CHD, or HCM with a nondiagnostic (negative) microarray or karyotype. All eligible cases had RASopathy molecular testing in the prenatal or neonatal period.
Among the 352 referrals to clinic, 50 cases of a RASopathy disorder were diagnosed. Additionally, to complement this cohort over the same time period, 25 postnatal diagnoses were made after retrospective review performed to ascertain additional prenatal findings. The size of the testing panel ranged from 9 to 20 genes, which were sent to clinical laboratories that performed sequencing based on standard protocols.
Study outcomes
Overall, 14% of fetuses with an indication for testing had a pathogenic or likely pathogenic variant (diagnostic) on panel testing among 11 genes (notably, all presented results are after excluding copy number variants and aneuploidy). Fetuses with only 1 ultrasonography finding were much less likely to have a positive result than those with more than 1 ultrasonography finding, 3% versus 18%. The highest diagnostic yields were for HCM at 69%; thoracic effusions and ascites, 41%; persistent hydrops, 39%; cystic hygroma combined with another suggestive ultrasonography finding, 28%; CHD, 23%; and persistent cystic hygroma, 21%. Five fetuses were affected with CHD and HCM, and 44% had an intrauterine fetal demise.
Importance of NT size. An isolated increased NT had a diagnostic yield of 1% overall (1/90); however, the size of the NT mattered. Seventeen fetuses had an NT between 3 and 3.5 mm and none of these had diagnostic sequencing, whereas 26% with an NT greater than 6 mm had a diagnostic result (11/43). An increased NF had a diagnostic yield of 25%.
Other findings. Of fetuses with a cystic hygroma, 16% had a pathogenic or likely pathogenic variant, and when these persisted into the second trimester or were associated with other anomalies, the percentages increased to 21% and 28%, respectively. Of prenatal patients, 20.6% had variants of uncertain significance, and 12% of the pathogenic and likely pathogenic variants were inherited, which is less than previously reported series. Additionally, 48% of the postnatal RASopathy diagnosis group did not have an ultrasonography finding on record review.
Continue to: Study strengths and limitations...
Study strengths and limitations
This study presents a large cohort of prenatal and neonatal patients tested for RASopathies at 2 international centers with very granular and clinically useful data about ultrasonography findings and yield of panel testing. Prenatal care providers, geneticists, and computational biologists may find this study of great interest and take away useful information and ideas due to the authors’ presentation and details.
The number of genes tested changed over the inclusion time period, but this is an inescapable reality of retrospective clinical research in an advancing field. The authors presented the prenatal and postnatal diagnoses ultrasonography findings separately and together. Given the different nature of cohort ascertainment, we prefer to consider these groups separately and have presented the data for the prenatal group.
Prenatal sequencing panels and exome sequencing are detecting disorders with important implications for prenatal care. If your practice is not testing for RASopathies in prenatal patients with concerning ultrasonography features, you are missing cases. In this study, the most concerning ultrasonography features (more than 20% diagnosis) were HCM, thoracic effusions and ascites, persistent hydrops, cystic hygroma combined with another suggestive ultrasonography finding, CHD, and persistent cystic hygroma. Isolated ultrasonography findings or findings that resolved had a lower diagnostic yield, and an isolated enlarged NT had a 1% diagnostic yield, with most cases having an NT larger than 6 mm.
For pretest counseling, in this study 20% of patients had a variant of uncertain significance, and preparing patients for this possibility is crucial. Most variants of uncertain significance are reclassified to benign when more information is available. Providers can consider sending parental samples concurrently with the fetal sample to help obtain useful information quickly, although the possibility of an inherited pathogenic variant still exists (12% in this study).
Prenatal diagnosis gives your patients the opportunity to learn about the disorder, plan for treatment and delivery location, and establish their care team before birth or consider pregnancy termination.
Sequencing provides insights into twin pregnancies
Jonsson H, Magnusdottir E, Eggertsson HP, et al. Differences between germline genomes of monozygotic twins. Nat Genet. 2021;53:27-34. doi:10.1038/s41588 -020-00755-1.
You have a monozygotic twin pair with an anomaly and intend to do diagnostic testing for prenatal diagnosis. The question always arises: Do you sample both twins or just one? Surely, they are genetically identical? A wise mentor once instilled a valuable lesson: Monozygotic twins are more likely to have an anomaly. Their existence is already out of the realm of normal. Finally, we now have an engaging and interesting answer to this and other fascinating embryology questions through the work of Jonsson and colleagues.
Study eligibility criteria and treatment protocol
The authors enrolled 381 twin pairs and 2 monozygotic triplets and compared genome sequencing of different tissues (cheek cells and blood). They went further to assess what other tissues might share the genetic change. To do this, they sequenced the children and the partners of 181 of the pairs. Presumably, if a twin and their offspring shared a genetic change that was not present in the spouse or twin, this genetic change must be present in the oocytes or sperm of the parent twin. The goal of sequencing multiple tissue sources in each twin was to help determine when the genetic change occurred in embryonic development.
Study outcomes
The authors found that 15% of twins had mutations that were absent in the other twin. Because of the extent of tissues that had the genetic change, the authors asserted that these changes must have occurred very early in embryonic development (even from one cell after twinning) for the changes to be near-constitutional (among sampled tissues).
An average of 14 genetic differences were found between twin pairs that developed after twinning. However, the number of differences varied. For example, 39 pairs of twins differed by more than 100 changes, and 38 did not differ at all. Differences between twins were more likely in blood samples than in cheek swabs, suggesting that some differences were due to acquired genetic changes in hematologic cell lines, or clonal hematopoiesis.
The authors also looked at what percentage of sequenced DNA contained the variants (or mutations) and found that many of these DNA differences were present at high amounts in sequencing reads. This suggests that the DNA changes happened very early after twinning in about one-third of pairs. Additionally, if one twin had a near-constitutional change, in 42% of pairs the other twin had a different near-constitutional change. Among the triplets, 2 of a triplet pair shared more genetic similarity and were likely descendent from a single split cell and the third likely was formed from a different set of cells.
By examining the offspring of twins, Jonsson and colleagues found that there were 2.6 early embryonic mutations, and this did not differ when blood or buccal DNA was compared. The rate of transmission of a variant to offspring was proportional to the variant allele frequency (proportion of alternate alleles) in the blood or buccal cells. This is an important counseling point when considering patients with mosaic genetic disorders and counseling about the likelihood of inheritance or transmission to future offspring. If the rate of mosaicism was higher in blood or buccal cells, the likelihood of transmission was higher. Additionally, the mutations did not differ by sex, and there was no relationship to whether the chromosome was maternally or paternally inherited.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors did not have access to information about chorionicity of the monozygotic twin pairs. Consequently, they were unable to correlate chorionicity with the degree of noted genetic difference between the monozygotic twin pairs. Additionally, although the authors were thoughtful in their utilization of offspring and spouses to infer germline genomic content, the study had a limited number of tissues sampled, which could reduce the applicability. However, the sample size, clinically accessible tissue sampling, and thoughtful analysis used in this study make it an interesting and relevant contribution to reproductive medicine and evolutionary biology.
We all accumulate changes to our DNA throughout life. The study by Jonsson and colleagues illustrates that for many, this accumulation of genetic changes starts very early in gestation. In the early zygote, the authors observed roughly 1 mutation per cell division prior to the point of twinning. In the realm of prenatal diagnosis, one should consider that monochorionic twins with different phenotypes (that is, an ultrasonography anomaly in 1 of the twin pair) could represent a genetic change rather than an environmental difference. This genetic change may not be shared by the other twin despite originating from the same primordial cell line. The genetic changes that the authors investigated were detected on genome sequencing, which is much more comprehensive than the exome sequencing that is increasingly utilized in rare disease diagnosis. The clinical utility of this observation in prenatal diagnosis has yet to be proven, but this study provides preliminary data that 15% of monozygotic twins have genetic differences and may warrant individualized testing.
The genetic landscape of the placenta
Coorens TH, Oliver TR, Sanghvi R, et al. Inherent mosaicism and extensive mutation of human placentas. Nature. Published online March 10, 2021. doi:10.1038/ s41586-021-03345-1.
Confined placental mosaicism (CPM) is a phenomenon in which the genetics of the placenta are different from those of the fetus. Historically, this phenomenon has been described in 1% to 2% of pregnancies based on karyotype data obtained from chorionic villus sampling. Some studies have demonstrated adverse pregnancy outcomes in the setting of CPM, thought to be secondary to aneuploid cells in the placenta leading to insufficiency or dysfunction.
Although our sophistication and level of detail in prenatal genetic testing has rapidly expanded to include information about copy number variants and singlenucleotide changes, their contribution to CPM has been understudied. Coorens and colleagues recently published a landmark study that describes a surprisingly high rate of mosaicism for these smaller genetic changes.
A cohort study of placentas
The authors performed whole genome sequencing on placental samples obtained from 37 term pregnancies. Umbilical cord tissue and maternal blood also were collected and served as controls for fetal and maternal genetic profiles, respectively.
In a subgroup of 5 placentas, lasercapture microscopy was used to separate placental cells of different origins, including trophoblastic cells, mesenchymal core cells, and cells originating from the inner cell mass. To investigate variation within different geographic regions of a single placenta, these cell lines were derived multiple times from each quadrant of the 5 placentas.
Placental biopsies revealed “bottlenecks” of genetic differentiation
Genome sequencing was used uniquely in this study to help delineate the phylogeny of placental cells by tracking somatic mutations both in different geographic locations of each placenta and between different cells of origin within 1 placenta.
The authors concluded that bottlenecks of differentiation in placental development led to unique genetic signatures in every bulk placental sample studied. Their findings led them to describe the placenta as a “patchwork” of independent genetic units resulting from clonal expansion at different stages of embryonic development.
Early insights into human placental cells
This study provides fascinating insight into the surprisingly high rates of copy number variants and single-gene changes that exist, in mosaic form, within human placentas. The authors distinguish the placenta from other human organs (such as the colon, endometrium, liver, and skin) in which many fewer genetic changes exist. In fact, they suggest parallels between the “mutational signature” of the placenta with rapidly dividing neoplastic cells.
As one of the first investigations into the variation and complexity of genetic changes within the placenta, this study was not designed to draw conclusions regarding the clinical impact of the numerous genetic changes described. Further studies will elucidate the potential contribution of genetically mosaic placentas to common adverse obstetric outcomes. ●
With a new appreciation for the smaller genetic alterations that exist within placental tissue, it appears that the rate of CPM has been vastly underestimated. We know that aneuploid placental cells increase the risk of adverse pregnancy outcomes and we may learn more about the contribution of copy number variants and single-nucleotide changes to preeclampsia, growth restriction, and pregnancy loss. Furthermore, as the applications of cell-free fetal DNA (cffDNA) in genetic screening continue to expand, we must exercise caution in assuming that copy number variants or single-nucleotide changes detected by cffDNA reflect those of the developing fetus.
Prenatal diagnosis has expanded from identification of aneuploidy to include copy number variants detected on microarray (such as 22q11 deletion syndrome) and now single-gene disorders identified by targeted or exome and genome sequencing. How and when different sequencing tests should be used clinically are questions faced by every provider engaged in modern prenatal diagnosis.
In this Update, we highlight new clinical insights into prenatal sequencing and explore how information gained from sequencing may help us understand some of the unanswered questions in obstetrics.
What is the yield of a RASopathy gene panel with specific prenatal findings?
Scott A, Di Giosaffatte N, Pinna V, et al. When to test fetuses for RASopathies? Proposition from a systematic analysis of 352 multicenter cases and a postnatal cohort. Genet Med. Published online February 10, 2021. doi:10.1038/s41436-020-01093-7.
RASopathies, a group of genetic conditions caused by mutations in the RAS/mitogen-activated protein kinase (RAS-MAPK) pathway, are common, occurring in 1:1,000 to 1:2,500 live births. RASopathies are much more common than 22q11 deletion syndrome, or DiGeorge syndrome, which occurs in 1.4:10,000 live births.1
RASopathy disorders include Noonan syndrome, Noonan syndrome with multiple lentigines, Costello syndrome, cardiofaciocutaneous syndrome, and Noonan-like syndrome with loose anagen hair. These are autosomal dominant disorders caused by a pathogenic variant (or mutation) in 1 of more than 20 genes in the RAS-MAPK signaling pathway in the body. Clinical features include congenital anomalies of the kidney and urinary tract, lymphatic anomalies, congenital heart disease (CHD), hypertrophic cardiomyopathy (HCM), postnatal growth disorders, neurodevelopmental disorders, and more rarely hematologic malignancies. Prenatal clues include an increased nuchal translucency (NT), CHD, cystic hygroma, lymphatic anomalies, anomalies of the kidney and urinary tract, hydrops, and HCM.
Cohort of pregnancies that received a RASopathy panel
Scott and colleagues sought to clarify the utility of testing for RASopathies with a prenatal gene panel. They conducted a multicenter retrospective cohort study with cases from 2 hospitals in Italy and Canada; data were collected between 2012 and 2019.
Eligible fetuses were those referred to the prenatal genetics clinic because of an increased NT, increased nuchal fold (NF), hydrops, ascites, thoracic effusions, chylothorax, other lymphatic anomalies, CHD, or HCM with a nondiagnostic (negative) microarray or karyotype. All eligible cases had RASopathy molecular testing in the prenatal or neonatal period.
Among the 352 referrals to clinic, 50 cases of a RASopathy disorder were diagnosed. Additionally, to complement this cohort over the same time period, 25 postnatal diagnoses were made after retrospective review performed to ascertain additional prenatal findings. The size of the testing panel ranged from 9 to 20 genes, which were sent to clinical laboratories that performed sequencing based on standard protocols.
Study outcomes
Overall, 14% of fetuses with an indication for testing had a pathogenic or likely pathogenic variant (diagnostic) on panel testing among 11 genes (notably, all presented results are after excluding copy number variants and aneuploidy). Fetuses with only 1 ultrasonography finding were much less likely to have a positive result than those with more than 1 ultrasonography finding, 3% versus 18%. The highest diagnostic yields were for HCM at 69%; thoracic effusions and ascites, 41%; persistent hydrops, 39%; cystic hygroma combined with another suggestive ultrasonography finding, 28%; CHD, 23%; and persistent cystic hygroma, 21%. Five fetuses were affected with CHD and HCM, and 44% had an intrauterine fetal demise.
Importance of NT size. An isolated increased NT had a diagnostic yield of 1% overall (1/90); however, the size of the NT mattered. Seventeen fetuses had an NT between 3 and 3.5 mm and none of these had diagnostic sequencing, whereas 26% with an NT greater than 6 mm had a diagnostic result (11/43). An increased NF had a diagnostic yield of 25%.
Other findings. Of fetuses with a cystic hygroma, 16% had a pathogenic or likely pathogenic variant, and when these persisted into the second trimester or were associated with other anomalies, the percentages increased to 21% and 28%, respectively. Of prenatal patients, 20.6% had variants of uncertain significance, and 12% of the pathogenic and likely pathogenic variants were inherited, which is less than previously reported series. Additionally, 48% of the postnatal RASopathy diagnosis group did not have an ultrasonography finding on record review.
Continue to: Study strengths and limitations...
Study strengths and limitations
This study presents a large cohort of prenatal and neonatal patients tested for RASopathies at 2 international centers with very granular and clinically useful data about ultrasonography findings and yield of panel testing. Prenatal care providers, geneticists, and computational biologists may find this study of great interest and take away useful information and ideas due to the authors’ presentation and details.
The number of genes tested changed over the inclusion time period, but this is an inescapable reality of retrospective clinical research in an advancing field. The authors presented the prenatal and postnatal diagnoses ultrasonography findings separately and together. Given the different nature of cohort ascertainment, we prefer to consider these groups separately and have presented the data for the prenatal group.
Prenatal sequencing panels and exome sequencing are detecting disorders with important implications for prenatal care. If your practice is not testing for RASopathies in prenatal patients with concerning ultrasonography features, you are missing cases. In this study, the most concerning ultrasonography features (more than 20% diagnosis) were HCM, thoracic effusions and ascites, persistent hydrops, cystic hygroma combined with another suggestive ultrasonography finding, CHD, and persistent cystic hygroma. Isolated ultrasonography findings or findings that resolved had a lower diagnostic yield, and an isolated enlarged NT had a 1% diagnostic yield, with most cases having an NT larger than 6 mm.
For pretest counseling, in this study 20% of patients had a variant of uncertain significance, and preparing patients for this possibility is crucial. Most variants of uncertain significance are reclassified to benign when more information is available. Providers can consider sending parental samples concurrently with the fetal sample to help obtain useful information quickly, although the possibility of an inherited pathogenic variant still exists (12% in this study).
Prenatal diagnosis gives your patients the opportunity to learn about the disorder, plan for treatment and delivery location, and establish their care team before birth or consider pregnancy termination.
Sequencing provides insights into twin pregnancies
Jonsson H, Magnusdottir E, Eggertsson HP, et al. Differences between germline genomes of monozygotic twins. Nat Genet. 2021;53:27-34. doi:10.1038/s41588 -020-00755-1.
You have a monozygotic twin pair with an anomaly and intend to do diagnostic testing for prenatal diagnosis. The question always arises: Do you sample both twins or just one? Surely, they are genetically identical? A wise mentor once instilled a valuable lesson: Monozygotic twins are more likely to have an anomaly. Their existence is already out of the realm of normal. Finally, we now have an engaging and interesting answer to this and other fascinating embryology questions through the work of Jonsson and colleagues.
Study eligibility criteria and treatment protocol
The authors enrolled 381 twin pairs and 2 monozygotic triplets and compared genome sequencing of different tissues (cheek cells and blood). They went further to assess what other tissues might share the genetic change. To do this, they sequenced the children and the partners of 181 of the pairs. Presumably, if a twin and their offspring shared a genetic change that was not present in the spouse or twin, this genetic change must be present in the oocytes or sperm of the parent twin. The goal of sequencing multiple tissue sources in each twin was to help determine when the genetic change occurred in embryonic development.
Study outcomes
The authors found that 15% of twins had mutations that were absent in the other twin. Because of the extent of tissues that had the genetic change, the authors asserted that these changes must have occurred very early in embryonic development (even from one cell after twinning) for the changes to be near-constitutional (among sampled tissues).
An average of 14 genetic differences were found between twin pairs that developed after twinning. However, the number of differences varied. For example, 39 pairs of twins differed by more than 100 changes, and 38 did not differ at all. Differences between twins were more likely in blood samples than in cheek swabs, suggesting that some differences were due to acquired genetic changes in hematologic cell lines, or clonal hematopoiesis.
The authors also looked at what percentage of sequenced DNA contained the variants (or mutations) and found that many of these DNA differences were present at high amounts in sequencing reads. This suggests that the DNA changes happened very early after twinning in about one-third of pairs. Additionally, if one twin had a near-constitutional change, in 42% of pairs the other twin had a different near-constitutional change. Among the triplets, 2 of a triplet pair shared more genetic similarity and were likely descendent from a single split cell and the third likely was formed from a different set of cells.
By examining the offspring of twins, Jonsson and colleagues found that there were 2.6 early embryonic mutations, and this did not differ when blood or buccal DNA was compared. The rate of transmission of a variant to offspring was proportional to the variant allele frequency (proportion of alternate alleles) in the blood or buccal cells. This is an important counseling point when considering patients with mosaic genetic disorders and counseling about the likelihood of inheritance or transmission to future offspring. If the rate of mosaicism was higher in blood or buccal cells, the likelihood of transmission was higher. Additionally, the mutations did not differ by sex, and there was no relationship to whether the chromosome was maternally or paternally inherited.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors did not have access to information about chorionicity of the monozygotic twin pairs. Consequently, they were unable to correlate chorionicity with the degree of noted genetic difference between the monozygotic twin pairs. Additionally, although the authors were thoughtful in their utilization of offspring and spouses to infer germline genomic content, the study had a limited number of tissues sampled, which could reduce the applicability. However, the sample size, clinically accessible tissue sampling, and thoughtful analysis used in this study make it an interesting and relevant contribution to reproductive medicine and evolutionary biology.
We all accumulate changes to our DNA throughout life. The study by Jonsson and colleagues illustrates that for many, this accumulation of genetic changes starts very early in gestation. In the early zygote, the authors observed roughly 1 mutation per cell division prior to the point of twinning. In the realm of prenatal diagnosis, one should consider that monochorionic twins with different phenotypes (that is, an ultrasonography anomaly in 1 of the twin pair) could represent a genetic change rather than an environmental difference. This genetic change may not be shared by the other twin despite originating from the same primordial cell line. The genetic changes that the authors investigated were detected on genome sequencing, which is much more comprehensive than the exome sequencing that is increasingly utilized in rare disease diagnosis. The clinical utility of this observation in prenatal diagnosis has yet to be proven, but this study provides preliminary data that 15% of monozygotic twins have genetic differences and may warrant individualized testing.
The genetic landscape of the placenta
Coorens TH, Oliver TR, Sanghvi R, et al. Inherent mosaicism and extensive mutation of human placentas. Nature. Published online March 10, 2021. doi:10.1038/ s41586-021-03345-1.
Confined placental mosaicism (CPM) is a phenomenon in which the genetics of the placenta are different from those of the fetus. Historically, this phenomenon has been described in 1% to 2% of pregnancies based on karyotype data obtained from chorionic villus sampling. Some studies have demonstrated adverse pregnancy outcomes in the setting of CPM, thought to be secondary to aneuploid cells in the placenta leading to insufficiency or dysfunction.
Although our sophistication and level of detail in prenatal genetic testing has rapidly expanded to include information about copy number variants and singlenucleotide changes, their contribution to CPM has been understudied. Coorens and colleagues recently published a landmark study that describes a surprisingly high rate of mosaicism for these smaller genetic changes.
A cohort study of placentas
The authors performed whole genome sequencing on placental samples obtained from 37 term pregnancies. Umbilical cord tissue and maternal blood also were collected and served as controls for fetal and maternal genetic profiles, respectively.
In a subgroup of 5 placentas, lasercapture microscopy was used to separate placental cells of different origins, including trophoblastic cells, mesenchymal core cells, and cells originating from the inner cell mass. To investigate variation within different geographic regions of a single placenta, these cell lines were derived multiple times from each quadrant of the 5 placentas.
Placental biopsies revealed “bottlenecks” of genetic differentiation
Genome sequencing was used uniquely in this study to help delineate the phylogeny of placental cells by tracking somatic mutations both in different geographic locations of each placenta and between different cells of origin within 1 placenta.
The authors concluded that bottlenecks of differentiation in placental development led to unique genetic signatures in every bulk placental sample studied. Their findings led them to describe the placenta as a “patchwork” of independent genetic units resulting from clonal expansion at different stages of embryonic development.
Early insights into human placental cells
This study provides fascinating insight into the surprisingly high rates of copy number variants and single-gene changes that exist, in mosaic form, within human placentas. The authors distinguish the placenta from other human organs (such as the colon, endometrium, liver, and skin) in which many fewer genetic changes exist. In fact, they suggest parallels between the “mutational signature” of the placenta with rapidly dividing neoplastic cells.
As one of the first investigations into the variation and complexity of genetic changes within the placenta, this study was not designed to draw conclusions regarding the clinical impact of the numerous genetic changes described. Further studies will elucidate the potential contribution of genetically mosaic placentas to common adverse obstetric outcomes. ●
With a new appreciation for the smaller genetic alterations that exist within placental tissue, it appears that the rate of CPM has been vastly underestimated. We know that aneuploid placental cells increase the risk of adverse pregnancy outcomes and we may learn more about the contribution of copy number variants and single-nucleotide changes to preeclampsia, growth restriction, and pregnancy loss. Furthermore, as the applications of cell-free fetal DNA (cffDNA) in genetic screening continue to expand, we must exercise caution in assuming that copy number variants or single-nucleotide changes detected by cffDNA reflect those of the developing fetus.
- Roberts AE, Allanson JE, Tartaglia M, et al. Noonan syndrome. Lancet. 2013;381:333-342. doi:10.1016/S0140-6736(12)61023-X.
- Roberts AE, Allanson JE, Tartaglia M, et al. Noonan syndrome. Lancet. 2013;381:333-342. doi:10.1016/S0140-6736(12)61023-X.
Patient-centered contraceptive care for medically complex patients
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).
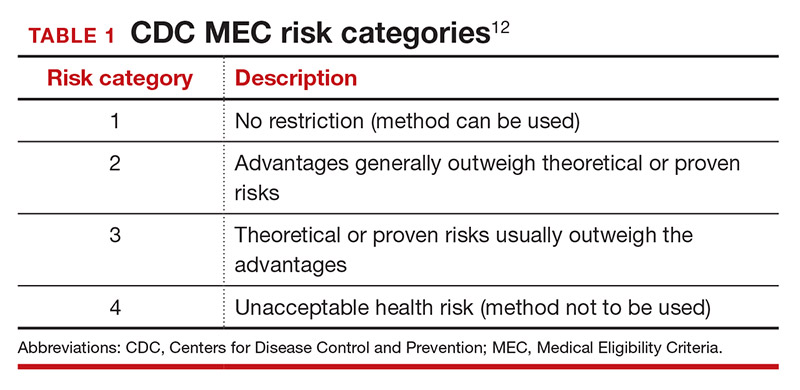
In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
How to craft your approach
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.
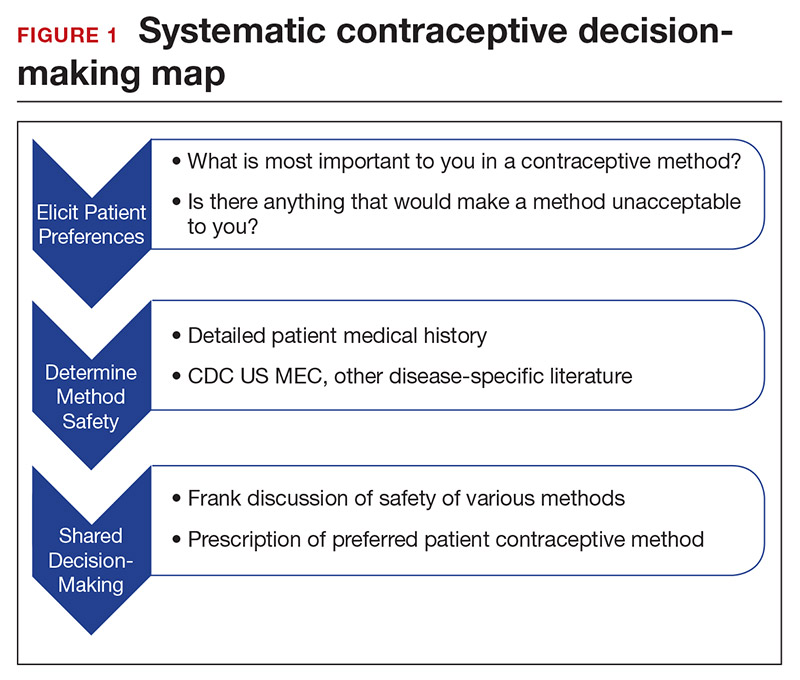
CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12
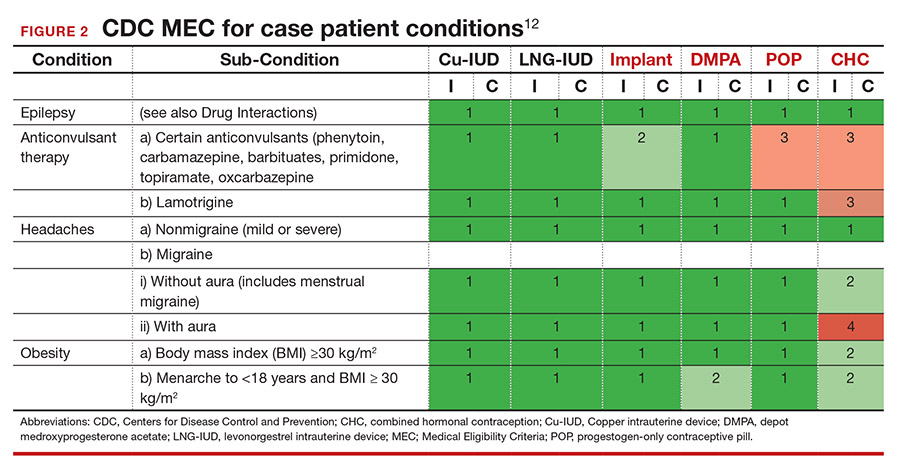
Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).


In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
How to craft your approach
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.

CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12

Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).


In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
How to craft your approach
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.

CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12

Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
Managing the second stage of labor: An evidence-based approach

CASE Woman in second stage with prolonged pushing
Ms. J. is an 18-year-old woman (G1P0) at 39 weeks’ gestation whose cervix is completely dilated; she has been actively pushing for 60 minutes. The estimated fetal weight is 8 lb, and electronic fetal monitoring shows a Category I fetal heart rate (FHR) tracing. The presenting part remains at 0 station and occiput transverse despite great pushing effort.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. During the third hour of the second stage, Ms. J. is diagnosed with chorioamnionitis and the fetus remains at 0 station. She undergoes a primary cesarean delivery (CD) complicated by bilateral lower uterine extensions and postpartum hemorrhage. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 4 and 8, respectively. The umbilical cord arterial pH was 7.03.
Ms. J. and her baby were discharged home on postoperative day 4.
In 2014, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine jointly released a document, “Safe prevention of the primary cesarean delivery,” in response to the sharp rise in cesarean births from 1996 to 2011.1 It described management strategies to safely reduce the most common indications for a primary CD in nulliparous women. Specifically, it recommended that the second stage of labor—defined as the interval from complete cervical dilation through delivery of the neonate—may be prolonged, as “longer durations may be appropriate on an individualized basis (eg, with the use of epidural analgesia or with fetal malposition) as long as progress is being documented.”1
A prolonged second stage was defined as 3 hours of pushing in nulliparous women and 2 hours in multiparous women, with 1 additional hour (or longer) in those receiving epidural analgesia. Indeed, the primary CD rate decreased slightly to 21.7% in 2018, down from 21.9% in 2017.2 More recent evidence, however, has shown an increase in maternal and neonatal morbidity with prolonged second stage.3-8
Efforts to manage the second stage from an evidence-based perspective are critical to balance the desired outcome of a safe vaginal delivery against the risks of prolonged second stage and operative vaginal delivery or CD.
Perspectives on the “ideal” labor duration
It is important to consider the historical context that led to the 2014 change in recommendations for duration of the second stage.9 In 1955, Dr. Emanuel Friedman published a prospective observational study of 622 consecutive primigravid parturients at term, of which 500 were included in the analysis that led to the graphicostatistical labor curve, or the well-known “Friedman’s curve.”10 The mean duration of the second stage was 0.95 hour. The statistical maximum for “ideal labor” for the second stage was set at 2 hours, with an additional hour allotted for patients receiving epidural analgesia.
In 2010, Zhang and colleagues published contemporary labor curves using data from the Consortium on Safe Labor, a multicenter retrospective observational study of 62,415 parturients.11 Among more than 25,000 nulliparous women, the median duration (95th percentile) of the second stage in hours was 1.1 (3.6), respectively. Notably, this analysis included only women with a spontaneous vaginal delivery and normal neonatal outcome.
Prior to the publication of the “Safe prevention of primary cesarean delivery,” multiple investigations examined the relationship between the duration of the second stage and adverse maternal and neonatal outcomes, and the findings have been inconsistent.12-15
For example, Cheng and colleagues noted increased maternal complications that included postpartum hemorrhage, third- and fourth-degree perineal lacerations, and chorioamnionitis, but not neonatal morbidity, with each increasing hour within the second stage.12 By contrast, a large, population-based cohort study among low-risk women showed an increase in low 5-minute Apgar scores, admission to the neonatal intensive care unit (NICU), and composite perinatal morbidity with prolonged second stage.15 Furthermore, a secondary analysis of the Pushing Early or Pushing Late with Epidural (PEOPLE) trial showed that the chances of a vaginal delivery with a newborn without signs of asphyxia decreased significantly every hour after the first hour, and the risk of postpartum hemorrhage and intrapartum fever increased significantly after 2 hours of pushing.14
While these findings may represent the risks inherent with the intervention of operative delivery and not the duration of second stage of labor per se, one could posit that if the intervention were initiated earlier, could it prevent or at least reduce maternal and neonatal morbidity?
Continue to: Factors to assess and monitor in the second stage...
Factors to assess and monitor in the second stage
When assessing progress in the second stage of labor, consider:
- maternal factors
- fetal/neonatal factors, and
- modifiable factors.
Maternal factors that influence the second stage of labor include parity, body mass index (BMI), age, and clinical pelvimetry.11,16-19 Fetal/neonatal factors that impact the second stage include the estimated fetal weight, fetal presentation (cephalic, face, and so on), position, and station, as well as the FHR Category.20, 21 Factors that can be modified in the second stage include the effect of epidural analgesia (turning it down to reduce motor blockade while maintaining sensory pain relief so that patients feel the “urge” to push), maternal pushing position and technique, the presence of maternal support person(s), manual rotation for a fetal position that is not optimal, immediate versus delayed pushing, and prevention of perineal tears.22-32 Interestingly, epidural analgesia, parity, birth weight, and station at complete dilation predicted second stage duration but accounted for only 25% of the variability in second stage length, leaving 75% of the variance unexplained.16
A specific absolute maximum length of time spent in the second stage of labor beyond which all women should undergo operative delivery has not been identified.1 Therefore, maternal, fetal/neonatal, and modifiable factors need to be critically assessed and continually monitored to determine whether a prolonged second stage or an operative delivery is warranted to prevent or minimize adverse maternal and neonatal outcomes.
Maternal factors
Maternal age correlates directly with the length of the second stage. That is, the length of the second stage increases with increasing age.17
Multiparous women have a shorter length of the second stage, regardless of epidural analgesia, compared with nulliparous women.11 In the Consortium for Safe Labor, multiparous women had a significantly shorter median second stage compared with nulliparous women.11
In adjusted analyses, maternal obesity was associated with an increased risk for CD, with the risk of CD more than 3 times greater in women with a BMI higher than 40 kg/m2 compared with those who had a BMI less than 25 kg/m2.18 There were no significant differences in the length of the second stage of labor by BMI catgeories.19
Fetal factors
Birth weight greater than 4,000 g was associated with an increased risk for arrest of descent during the second stage.33
Persistent fetal occiput posterior or transverse position may impact the duration of the second stage. A retrospective cohort study in women who underwent a trial of manual rotation compared with expectant management during the second stage of labor with the fetus in occiput posterior or occiput transverse position found that women with manual rotation were less likely to have a CD, severe perineal laceration, postpartum hemorrhage, and chorioamnionitis. However, an increased risk of cervical laceration was associated with manual rotation.20
Regarding FHR status, FHR abnormalities occurred in 91% of second stage labor patterns, with Category II being the most common.21 The fetal status should remain reassuring to allow for continuation of the second stage.
Continue to: Epidural analgesia...
Epidural analgesia
About 60% of women receive neuraxial analgesia in the United States,22 although rates vary widely across different populations. A Cochrane review showed no difference in the duration of the second stage among women who had early versus late initiation of epidural analgesia in labor.23 Epidural analgesia has no impact on the risk of CD; however, women with epidural analgesia experienced more hypotension, motor blockade, fever, and urinary retention.24
One management practice has been to discontinue epidural analgesia to allow resumption of sensory and motor nerve function. Another Cochrane systematic review found no difference in mode of delivery or neonatal outcomes.25 Rather than discontinuing epidural analgesia, which results in a profound increase in inadequate pain relief, one may consider titrating the dose with joint patient decision-making to allow for greater motor capability while maintaining adequate analgesia.34
Immediate vs delayed pushing
The 2 most common approaches to managing the second stage were either to initiate pushing with contractions once complete dilation occurred (immediate pushing) or to allow for a rest period in which the fetus passively rotated and descended while conserving a woman’s energy for pushing efforts (delayed pushing, laboring down, or passive descent). Since the publication of “Safe prevention of primary cesarean delivery,” however, studies have shown a concerning association between maternal and neonatal complications and prolonged second stage (which may occur with delayed pushing).3-8,35 An observational study of nearly 44,000 nulliparous women without epidural analgesia found that prolonged second stage was associated with increased chorioamnionitis, third- and fourth-degree lacerations, neonatal sepsis, neonatal asphyxia, and perinatal mortality.35
A pragmatic multicenter randomized clinical trial on the optimal management of second stage of labor across the United States recently was conducted.7 More than 2,000 nulliparous women at term in spontaneous or induced labor with epidural analgesia were randomly assigned at complete dilation to immediate pushing or delayed pushing (1 hour after complete dilation). There was no difference in the rate of vaginal delivery. The rate of postpartum hemorrhage was significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (2.3% vs 4.0%, respectively; relative risk [RR], 0.6; 95% confidence interval [CI], 0.3–0.9; P = .03). Furthermore, rates of chorioamnionitis were significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (6.7% vs 9.1%, respectively; RR, 0.70; 95% CI, 0.66–0.90; P = .005). No significant difference occurred in the composite outcome of neonatal morbidity between the groups. However neonatal acidemia (umbilical cord arterial pH <7.1) and confirmed or suspected sepsis were significantly increased in the delayed-pushing group.
The evidence supports active pushing at the start of the second stage. Women who consider delayed pushing should be informed that delayed pushing has not been shown to increase the likelihood of vaginal birth and that it is associated with increased risks of infection, hemorrhage, and neonatal acidemia.36
Maternal pushing position and technique
Spontaneous pushing (in which women are free to follow their instincts and generally push 3 to 5 times per contraction) versus directed pushing (women are encouraged to take a deep breath at the beginning of a contraction then hold it and bear down throughout the contraction) demonstrated no clear difference in duration of the second stage, perineal laceration, episiotomy, time spent pushing, or number of women with spontaneous vaginal birth. There was no difference in 5-minute Apgar score less than 7 or admission to the NICU.26
With regard to maternal positioning during the second stage, a Cochrane systematic review found benefits for upright posture, including a very small reduction in the duration of the second stage, reduction in episiotomy rates, and reduction in assisted deliveries.37 There was an increased risk of blood loss greater than 500 mL and possibly an increased risk in second-degree tears.37 Compared with women allocated to lying down, women in the upright position during the second stage with epidural analgesia had significantly fewer spontaneous vaginal births. There was no difference in operative vaginal delivery, obstetric anal sphincter injury (OASI), infant Apgar score of less than 4 at 5 minutes, and maternal fecal incontinence at 1 year.28
Continue to: Maternal support person...
Maternal support person
Continuous support during labor may improve outcomes for women and infants, including increased spontaneous vaginal birth, shorter duration of labor, and decreased cesarean birth.29 In a randomized trial of 412 healthy nulliparous women, women in labor were assigned to either a support group that received continuous support from a doula or an observed group that was monitored by an inconspicuous observer. Continuous labor support significantly reduced the rate of CDs and forceps deliveries.30,36 Indeed, during the COVID-19 pandemic, doulas have found innovative ways to continue to provide this essential support through virtual health.38
Prevention of perineal tears
Evidence suggests that warm compresses, and massage, may reduce third- and fourth-degree perineal tears.31 A meta-analysis of observational studies showed a significant reduction in the risk of OASI.32
Second stage steps: Recap
Throughout the second stage of labor, the decision to continue with expectant management or intervene with either an operative vaginal delivery or a CD is complex and requires consistent assessment and integration of multiple factors. An evidence-based approach to second stage labor management includes active pushing that is either Valsalva pushing or spontaneous, coached or uncoached, but most importantly, at the start of the second stage when a patient reaches complete dilation. Reassessment should occur at regular intervals to determine progress, after ensuring maternal and fetal well-being.
If there has been no advancement in station, an attempt at manual rotation or titration of epidural analgesia should be considered. Importantly, fetal descent with adequate pushing should be demonstrated throughout the second stage.
Additional considerations that improve outcomes include warm compresses or perineal massage to prevent third- and fourth-degree tears and the presence of a continuous support person to reduce the risk for an operative delivery.
Delivery should be expected within 2 hours for multiparous women and 3 hours for nulliparous women in the second stage. Prolonging the second stage beyond these thresholds should be individualized and occur only in the setting of assured maternal and fetal well-being.

CASE An alternative management strategy
Despite Ms. J.’s great active pushing effort for 60 minutes, the presenting part remains at 0 station and occiput transverse. Ms. J. is counseled regarding the risks and benefits of an attempt at manual rotation of the fetal head, and she wishes to proceed. The fetal position remains occiput transverse.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. At this time, Ms. J. is informed that there has been no descent, and she is counseled on the risks and benefits of continued pushing versus CD. Through shared decision-making, she consents to a CD. She undergoes a primary CD without complication. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 8 and 9, respectively. The umbilical cord arterial pH was 7.13.
Ms. J. and her baby were discharged home on postoperative day 4. ●
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine; Caughey AB, Cahill AG, Guise JM, et al. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210:179-193. doi:10.1016/j.ajog.2014.01.026.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2018. Natl Vital Stat Rep. 2019;68:1-47.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127:667-673. doi:10.1097/aog.0000000000001354.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-6. doi:10.1016/j.ajog.2015.12.042.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8. doi:10.1016/j.ajog.2017.10.007.
- Grantz KL, Sundaram R, Ma L, et al. Reassessing the duration of the second stage of labor in relation to maternal and neonatal morbidity. Obstet Gynecol. 2018;131:345-353. doi:10.1097/aog.0000000000002431.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454. doi:10.1001/jama.2018.13986.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019;220:191.e1-191.e7. doi:10.1016/j.ajog.2018.10.028.
- Leveno KJ, Nelson DB, McIntire DD. Second-stage labor: how long is too long? Am J Obstet Gynecol. 2016;214:484-489. doi:10.1016/j.ajog.2015.10.926.
- Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567-589. doi:10.1097/00006250-195512000-00001.
- Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281-1287. doi:10.1097/AOG.0b013e3181fdef6e.
- Cheng YW, Hopkins LM, Caughey AB. How long is too long: does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcomes? Am J Obstet Gynecol. 2004;191:933-938. doi:10.1016/j.ajog.2004.05.044.
- Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357.e1-7. doi:10.1016/j.ajog.2009.08.003.
- Le Ray C, Audibert F, Goffinet F, et al. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol. 2009;201:361.e1-7. doi:10.1016/j.ajog.2009.08.002.
- Allen VM, Baskett TF, O’Connell CM, et al. Maternal and perinatal outcomes with increasing duration of the second stage of labor. Obstet Gynecol. 2009;113:1248-1258. doi:10.1097/AOG.0b013e3181a722d6.
- Piper JM, Bolling DR, Newton ER. The second stage of labor: factors influencing duration. Am J Obstet Gynecol. 1991;165(4 pt 1):976-979. doi:10.1016/0002-9378(91)90452-w.
- Zaki MN, Hibbard JU, Kominiarek MA. Contemporary labor patterns and maternal age. Obstet Gynecol. 2013;122:1018-1024. doi:10.1097/AOG.0b013e3182a9c92c.
- Kominiarek MA, Vanveldhuisen P, Hibbard J, et al; Consortium on Safe Labor. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203:264.e1-7. doi:10.1016/j.ajog.2010.06.024.
- Kominiarek MA, Zhang J, Vanveldhuisen P, et al. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205:244.e1-8. doi:10.1016/j.ajog.2011.06.014.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72. doi:10.3109/14767051003710276.
- Krebs HB, Petres RE, Dunn LJ. Intrapartum fetal heart rate monitoring. V. Fetal heart rate patterns in the second stage of labor. Am J Obstet Gynecol. 1981;140:435-439. doi:10.1016/0002-9378(81)90041-7.
- Grant EN, Tao W, Craig M, et al. Neuraxial analgesia effects on labour progression: facts, fallacies, uncertainties and the future. BJOG. 2015;122:288-293. doi:10.1111/1471-0528.12966.
- Sng BL, Leong WL, Zeng Y, et al. Early versus late initiation of epidural analgesia for labour. Cochrane Database Syst Rev. 2014;(10):CD007238. doi:10.1002/14651858.CD007238.pub2.
- Anim-Somuah M, Smyth RM, Cyna AM, et al. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331. doi:10.1002/14651858.CD000331.pub4.
- Torvaldsen S, Roberts CL, Bell JC, et al. Discontinuation of epidural analgesia late in labour for reducing the adverse delivery outcomes associated with epidural analgesia. Cochrane Database Syst Rev. 2004;(4):CD004457. doi:10.1002/14651858.CD004457.pub2.
- Lemos A, Amorim MM, Dornelas de Andrade A, et al. Pushing/bearing down methods for the second stage of labour. Cochrane Database Syst Rev. 2017;3(3):CD009124. doi:10.1002/14651858.CD009124.pub3.
- Prins M, Boxem J, Lucas C, et al. Effect of spontaneous pushing versus Valsalva pushing in the second stage of labour on mother and fetus: a systematic review of randomised trials. BJOG. 2011;118:662-670. doi:10.1111/j.1471-0528.2011 .02910.x.
- Epidural and Position Trial Collaborative Group. Upright versus lying down position in second stage of labour in nulliparous women with low dose epidural: BUMPES randomised controlled trial. BMJ. 2017;359:j4471. doi:10.1136/bmj.j4471.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7(7):CD003766. doi:10.1002/14651858.CD003766.pub6.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital. A randomized controlled trial. JAMA. 1991;265:2197-2201.
- Aasheim V, Nilsen AB, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;6(6):CD006672. doi:10.1002/14651858.CD006672.pub3.
- Bulchandani S, Watts E, Sucharitha A, et al. Manual perineal support at the time of childbirth: a systematic review and meta-analysis. BJOG. 2015;122:1157-1165. doi:10.1111/1471-0528.13431.
- Feinstein U, Sheiner E, Levy A, et al. Risk factors for arrest of descent during the second stage of labor. Int J Gynaecol Obstet. 2002;77:7-14. doi:10.1016/s0020-7292(02)00007-3.
- Cheng YW, Caughey AB. Defining and managing normal and abnormal second stage of labor. Obstet Gynecol Clin North Am. 2017;44:547-566. doi:10.1016/j.ogc.2017.08.009.
- Laughon SK, Berghella V, Reddy UM, et al. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol. 2014;124:57-67. doi:10.1097/aog.0000000000000278.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 766: approaches to limit intervention during labor and birth. Obstet Gynecol. 2019;133:e164-e173. doi:10.1097/aog.0000000000003074.
- Gupta JK, Sood A, Hofmeyr GJ, et al. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev. 2017;5(5):CD002006. doi:10.1002/14651858.CD002006.pub4.
- Castaneda AN, Searcy JJ. Practising intimate labour: birth doulas respond during COVID-19. Anthropol Action. 2021;28:21-24. https://www.berghahnjournals.com/view/ journals/aia/28/1/aia280104.xml. Accessed February 8, 2021.

CASE Woman in second stage with prolonged pushing
Ms. J. is an 18-year-old woman (G1P0) at 39 weeks’ gestation whose cervix is completely dilated; she has been actively pushing for 60 minutes. The estimated fetal weight is 8 lb, and electronic fetal monitoring shows a Category I fetal heart rate (FHR) tracing. The presenting part remains at 0 station and occiput transverse despite great pushing effort.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. During the third hour of the second stage, Ms. J. is diagnosed with chorioamnionitis and the fetus remains at 0 station. She undergoes a primary cesarean delivery (CD) complicated by bilateral lower uterine extensions and postpartum hemorrhage. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 4 and 8, respectively. The umbilical cord arterial pH was 7.03.
Ms. J. and her baby were discharged home on postoperative day 4.
In 2014, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine jointly released a document, “Safe prevention of the primary cesarean delivery,” in response to the sharp rise in cesarean births from 1996 to 2011.1 It described management strategies to safely reduce the most common indications for a primary CD in nulliparous women. Specifically, it recommended that the second stage of labor—defined as the interval from complete cervical dilation through delivery of the neonate—may be prolonged, as “longer durations may be appropriate on an individualized basis (eg, with the use of epidural analgesia or with fetal malposition) as long as progress is being documented.”1
A prolonged second stage was defined as 3 hours of pushing in nulliparous women and 2 hours in multiparous women, with 1 additional hour (or longer) in those receiving epidural analgesia. Indeed, the primary CD rate decreased slightly to 21.7% in 2018, down from 21.9% in 2017.2 More recent evidence, however, has shown an increase in maternal and neonatal morbidity with prolonged second stage.3-8
Efforts to manage the second stage from an evidence-based perspective are critical to balance the desired outcome of a safe vaginal delivery against the risks of prolonged second stage and operative vaginal delivery or CD.
Perspectives on the “ideal” labor duration
It is important to consider the historical context that led to the 2014 change in recommendations for duration of the second stage.9 In 1955, Dr. Emanuel Friedman published a prospective observational study of 622 consecutive primigravid parturients at term, of which 500 were included in the analysis that led to the graphicostatistical labor curve, or the well-known “Friedman’s curve.”10 The mean duration of the second stage was 0.95 hour. The statistical maximum for “ideal labor” for the second stage was set at 2 hours, with an additional hour allotted for patients receiving epidural analgesia.
In 2010, Zhang and colleagues published contemporary labor curves using data from the Consortium on Safe Labor, a multicenter retrospective observational study of 62,415 parturients.11 Among more than 25,000 nulliparous women, the median duration (95th percentile) of the second stage in hours was 1.1 (3.6), respectively. Notably, this analysis included only women with a spontaneous vaginal delivery and normal neonatal outcome.
Prior to the publication of the “Safe prevention of primary cesarean delivery,” multiple investigations examined the relationship between the duration of the second stage and adverse maternal and neonatal outcomes, and the findings have been inconsistent.12-15
For example, Cheng and colleagues noted increased maternal complications that included postpartum hemorrhage, third- and fourth-degree perineal lacerations, and chorioamnionitis, but not neonatal morbidity, with each increasing hour within the second stage.12 By contrast, a large, population-based cohort study among low-risk women showed an increase in low 5-minute Apgar scores, admission to the neonatal intensive care unit (NICU), and composite perinatal morbidity with prolonged second stage.15 Furthermore, a secondary analysis of the Pushing Early or Pushing Late with Epidural (PEOPLE) trial showed that the chances of a vaginal delivery with a newborn without signs of asphyxia decreased significantly every hour after the first hour, and the risk of postpartum hemorrhage and intrapartum fever increased significantly after 2 hours of pushing.14
While these findings may represent the risks inherent with the intervention of operative delivery and not the duration of second stage of labor per se, one could posit that if the intervention were initiated earlier, could it prevent or at least reduce maternal and neonatal morbidity?
Continue to: Factors to assess and monitor in the second stage...
Factors to assess and monitor in the second stage
When assessing progress in the second stage of labor, consider:
- maternal factors
- fetal/neonatal factors, and
- modifiable factors.
Maternal factors that influence the second stage of labor include parity, body mass index (BMI), age, and clinical pelvimetry.11,16-19 Fetal/neonatal factors that impact the second stage include the estimated fetal weight, fetal presentation (cephalic, face, and so on), position, and station, as well as the FHR Category.20, 21 Factors that can be modified in the second stage include the effect of epidural analgesia (turning it down to reduce motor blockade while maintaining sensory pain relief so that patients feel the “urge” to push), maternal pushing position and technique, the presence of maternal support person(s), manual rotation for a fetal position that is not optimal, immediate versus delayed pushing, and prevention of perineal tears.22-32 Interestingly, epidural analgesia, parity, birth weight, and station at complete dilation predicted second stage duration but accounted for only 25% of the variability in second stage length, leaving 75% of the variance unexplained.16
A specific absolute maximum length of time spent in the second stage of labor beyond which all women should undergo operative delivery has not been identified.1 Therefore, maternal, fetal/neonatal, and modifiable factors need to be critically assessed and continually monitored to determine whether a prolonged second stage or an operative delivery is warranted to prevent or minimize adverse maternal and neonatal outcomes.
Maternal factors
Maternal age correlates directly with the length of the second stage. That is, the length of the second stage increases with increasing age.17
Multiparous women have a shorter length of the second stage, regardless of epidural analgesia, compared with nulliparous women.11 In the Consortium for Safe Labor, multiparous women had a significantly shorter median second stage compared with nulliparous women.11
In adjusted analyses, maternal obesity was associated with an increased risk for CD, with the risk of CD more than 3 times greater in women with a BMI higher than 40 kg/m2 compared with those who had a BMI less than 25 kg/m2.18 There were no significant differences in the length of the second stage of labor by BMI catgeories.19
Fetal factors
Birth weight greater than 4,000 g was associated with an increased risk for arrest of descent during the second stage.33
Persistent fetal occiput posterior or transverse position may impact the duration of the second stage. A retrospective cohort study in women who underwent a trial of manual rotation compared with expectant management during the second stage of labor with the fetus in occiput posterior or occiput transverse position found that women with manual rotation were less likely to have a CD, severe perineal laceration, postpartum hemorrhage, and chorioamnionitis. However, an increased risk of cervical laceration was associated with manual rotation.20
Regarding FHR status, FHR abnormalities occurred in 91% of second stage labor patterns, with Category II being the most common.21 The fetal status should remain reassuring to allow for continuation of the second stage.
Continue to: Epidural analgesia...
Epidural analgesia
About 60% of women receive neuraxial analgesia in the United States,22 although rates vary widely across different populations. A Cochrane review showed no difference in the duration of the second stage among women who had early versus late initiation of epidural analgesia in labor.23 Epidural analgesia has no impact on the risk of CD; however, women with epidural analgesia experienced more hypotension, motor blockade, fever, and urinary retention.24
One management practice has been to discontinue epidural analgesia to allow resumption of sensory and motor nerve function. Another Cochrane systematic review found no difference in mode of delivery or neonatal outcomes.25 Rather than discontinuing epidural analgesia, which results in a profound increase in inadequate pain relief, one may consider titrating the dose with joint patient decision-making to allow for greater motor capability while maintaining adequate analgesia.34
Immediate vs delayed pushing
The 2 most common approaches to managing the second stage were either to initiate pushing with contractions once complete dilation occurred (immediate pushing) or to allow for a rest period in which the fetus passively rotated and descended while conserving a woman’s energy for pushing efforts (delayed pushing, laboring down, or passive descent). Since the publication of “Safe prevention of primary cesarean delivery,” however, studies have shown a concerning association between maternal and neonatal complications and prolonged second stage (which may occur with delayed pushing).3-8,35 An observational study of nearly 44,000 nulliparous women without epidural analgesia found that prolonged second stage was associated with increased chorioamnionitis, third- and fourth-degree lacerations, neonatal sepsis, neonatal asphyxia, and perinatal mortality.35
A pragmatic multicenter randomized clinical trial on the optimal management of second stage of labor across the United States recently was conducted.7 More than 2,000 nulliparous women at term in spontaneous or induced labor with epidural analgesia were randomly assigned at complete dilation to immediate pushing or delayed pushing (1 hour after complete dilation). There was no difference in the rate of vaginal delivery. The rate of postpartum hemorrhage was significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (2.3% vs 4.0%, respectively; relative risk [RR], 0.6; 95% confidence interval [CI], 0.3–0.9; P = .03). Furthermore, rates of chorioamnionitis were significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (6.7% vs 9.1%, respectively; RR, 0.70; 95% CI, 0.66–0.90; P = .005). No significant difference occurred in the composite outcome of neonatal morbidity between the groups. However neonatal acidemia (umbilical cord arterial pH <7.1) and confirmed or suspected sepsis were significantly increased in the delayed-pushing group.
The evidence supports active pushing at the start of the second stage. Women who consider delayed pushing should be informed that delayed pushing has not been shown to increase the likelihood of vaginal birth and that it is associated with increased risks of infection, hemorrhage, and neonatal acidemia.36
Maternal pushing position and technique
Spontaneous pushing (in which women are free to follow their instincts and generally push 3 to 5 times per contraction) versus directed pushing (women are encouraged to take a deep breath at the beginning of a contraction then hold it and bear down throughout the contraction) demonstrated no clear difference in duration of the second stage, perineal laceration, episiotomy, time spent pushing, or number of women with spontaneous vaginal birth. There was no difference in 5-minute Apgar score less than 7 or admission to the NICU.26
With regard to maternal positioning during the second stage, a Cochrane systematic review found benefits for upright posture, including a very small reduction in the duration of the second stage, reduction in episiotomy rates, and reduction in assisted deliveries.37 There was an increased risk of blood loss greater than 500 mL and possibly an increased risk in second-degree tears.37 Compared with women allocated to lying down, women in the upright position during the second stage with epidural analgesia had significantly fewer spontaneous vaginal births. There was no difference in operative vaginal delivery, obstetric anal sphincter injury (OASI), infant Apgar score of less than 4 at 5 minutes, and maternal fecal incontinence at 1 year.28
Continue to: Maternal support person...
Maternal support person
Continuous support during labor may improve outcomes for women and infants, including increased spontaneous vaginal birth, shorter duration of labor, and decreased cesarean birth.29 In a randomized trial of 412 healthy nulliparous women, women in labor were assigned to either a support group that received continuous support from a doula or an observed group that was monitored by an inconspicuous observer. Continuous labor support significantly reduced the rate of CDs and forceps deliveries.30,36 Indeed, during the COVID-19 pandemic, doulas have found innovative ways to continue to provide this essential support through virtual health.38
Prevention of perineal tears
Evidence suggests that warm compresses, and massage, may reduce third- and fourth-degree perineal tears.31 A meta-analysis of observational studies showed a significant reduction in the risk of OASI.32
Second stage steps: Recap
Throughout the second stage of labor, the decision to continue with expectant management or intervene with either an operative vaginal delivery or a CD is complex and requires consistent assessment and integration of multiple factors. An evidence-based approach to second stage labor management includes active pushing that is either Valsalva pushing or spontaneous, coached or uncoached, but most importantly, at the start of the second stage when a patient reaches complete dilation. Reassessment should occur at regular intervals to determine progress, after ensuring maternal and fetal well-being.
If there has been no advancement in station, an attempt at manual rotation or titration of epidural analgesia should be considered. Importantly, fetal descent with adequate pushing should be demonstrated throughout the second stage.
Additional considerations that improve outcomes include warm compresses or perineal massage to prevent third- and fourth-degree tears and the presence of a continuous support person to reduce the risk for an operative delivery.
Delivery should be expected within 2 hours for multiparous women and 3 hours for nulliparous women in the second stage. Prolonging the second stage beyond these thresholds should be individualized and occur only in the setting of assured maternal and fetal well-being.

CASE An alternative management strategy
Despite Ms. J.’s great active pushing effort for 60 minutes, the presenting part remains at 0 station and occiput transverse. Ms. J. is counseled regarding the risks and benefits of an attempt at manual rotation of the fetal head, and she wishes to proceed. The fetal position remains occiput transverse.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. At this time, Ms. J. is informed that there has been no descent, and she is counseled on the risks and benefits of continued pushing versus CD. Through shared decision-making, she consents to a CD. She undergoes a primary CD without complication. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 8 and 9, respectively. The umbilical cord arterial pH was 7.13.
Ms. J. and her baby were discharged home on postoperative day 4. ●

CASE Woman in second stage with prolonged pushing
Ms. J. is an 18-year-old woman (G1P0) at 39 weeks’ gestation whose cervix is completely dilated; she has been actively pushing for 60 minutes. The estimated fetal weight is 8 lb, and electronic fetal monitoring shows a Category I fetal heart rate (FHR) tracing. The presenting part remains at 0 station and occiput transverse despite great pushing effort.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. During the third hour of the second stage, Ms. J. is diagnosed with chorioamnionitis and the fetus remains at 0 station. She undergoes a primary cesarean delivery (CD) complicated by bilateral lower uterine extensions and postpartum hemorrhage. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 4 and 8, respectively. The umbilical cord arterial pH was 7.03.
Ms. J. and her baby were discharged home on postoperative day 4.
In 2014, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine jointly released a document, “Safe prevention of the primary cesarean delivery,” in response to the sharp rise in cesarean births from 1996 to 2011.1 It described management strategies to safely reduce the most common indications for a primary CD in nulliparous women. Specifically, it recommended that the second stage of labor—defined as the interval from complete cervical dilation through delivery of the neonate—may be prolonged, as “longer durations may be appropriate on an individualized basis (eg, with the use of epidural analgesia or with fetal malposition) as long as progress is being documented.”1
A prolonged second stage was defined as 3 hours of pushing in nulliparous women and 2 hours in multiparous women, with 1 additional hour (or longer) in those receiving epidural analgesia. Indeed, the primary CD rate decreased slightly to 21.7% in 2018, down from 21.9% in 2017.2 More recent evidence, however, has shown an increase in maternal and neonatal morbidity with prolonged second stage.3-8
Efforts to manage the second stage from an evidence-based perspective are critical to balance the desired outcome of a safe vaginal delivery against the risks of prolonged second stage and operative vaginal delivery or CD.
Perspectives on the “ideal” labor duration
It is important to consider the historical context that led to the 2014 change in recommendations for duration of the second stage.9 In 1955, Dr. Emanuel Friedman published a prospective observational study of 622 consecutive primigravid parturients at term, of which 500 were included in the analysis that led to the graphicostatistical labor curve, or the well-known “Friedman’s curve.”10 The mean duration of the second stage was 0.95 hour. The statistical maximum for “ideal labor” for the second stage was set at 2 hours, with an additional hour allotted for patients receiving epidural analgesia.
In 2010, Zhang and colleagues published contemporary labor curves using data from the Consortium on Safe Labor, a multicenter retrospective observational study of 62,415 parturients.11 Among more than 25,000 nulliparous women, the median duration (95th percentile) of the second stage in hours was 1.1 (3.6), respectively. Notably, this analysis included only women with a spontaneous vaginal delivery and normal neonatal outcome.
Prior to the publication of the “Safe prevention of primary cesarean delivery,” multiple investigations examined the relationship between the duration of the second stage and adverse maternal and neonatal outcomes, and the findings have been inconsistent.12-15
For example, Cheng and colleagues noted increased maternal complications that included postpartum hemorrhage, third- and fourth-degree perineal lacerations, and chorioamnionitis, but not neonatal morbidity, with each increasing hour within the second stage.12 By contrast, a large, population-based cohort study among low-risk women showed an increase in low 5-minute Apgar scores, admission to the neonatal intensive care unit (NICU), and composite perinatal morbidity with prolonged second stage.15 Furthermore, a secondary analysis of the Pushing Early or Pushing Late with Epidural (PEOPLE) trial showed that the chances of a vaginal delivery with a newborn without signs of asphyxia decreased significantly every hour after the first hour, and the risk of postpartum hemorrhage and intrapartum fever increased significantly after 2 hours of pushing.14
While these findings may represent the risks inherent with the intervention of operative delivery and not the duration of second stage of labor per se, one could posit that if the intervention were initiated earlier, could it prevent or at least reduce maternal and neonatal morbidity?
Continue to: Factors to assess and monitor in the second stage...
Factors to assess and monitor in the second stage
When assessing progress in the second stage of labor, consider:
- maternal factors
- fetal/neonatal factors, and
- modifiable factors.
Maternal factors that influence the second stage of labor include parity, body mass index (BMI), age, and clinical pelvimetry.11,16-19 Fetal/neonatal factors that impact the second stage include the estimated fetal weight, fetal presentation (cephalic, face, and so on), position, and station, as well as the FHR Category.20, 21 Factors that can be modified in the second stage include the effect of epidural analgesia (turning it down to reduce motor blockade while maintaining sensory pain relief so that patients feel the “urge” to push), maternal pushing position and technique, the presence of maternal support person(s), manual rotation for a fetal position that is not optimal, immediate versus delayed pushing, and prevention of perineal tears.22-32 Interestingly, epidural analgesia, parity, birth weight, and station at complete dilation predicted second stage duration but accounted for only 25% of the variability in second stage length, leaving 75% of the variance unexplained.16
A specific absolute maximum length of time spent in the second stage of labor beyond which all women should undergo operative delivery has not been identified.1 Therefore, maternal, fetal/neonatal, and modifiable factors need to be critically assessed and continually monitored to determine whether a prolonged second stage or an operative delivery is warranted to prevent or minimize adverse maternal and neonatal outcomes.
Maternal factors
Maternal age correlates directly with the length of the second stage. That is, the length of the second stage increases with increasing age.17
Multiparous women have a shorter length of the second stage, regardless of epidural analgesia, compared with nulliparous women.11 In the Consortium for Safe Labor, multiparous women had a significantly shorter median second stage compared with nulliparous women.11
In adjusted analyses, maternal obesity was associated with an increased risk for CD, with the risk of CD more than 3 times greater in women with a BMI higher than 40 kg/m2 compared with those who had a BMI less than 25 kg/m2.18 There were no significant differences in the length of the second stage of labor by BMI catgeories.19
Fetal factors
Birth weight greater than 4,000 g was associated with an increased risk for arrest of descent during the second stage.33
Persistent fetal occiput posterior or transverse position may impact the duration of the second stage. A retrospective cohort study in women who underwent a trial of manual rotation compared with expectant management during the second stage of labor with the fetus in occiput posterior or occiput transverse position found that women with manual rotation were less likely to have a CD, severe perineal laceration, postpartum hemorrhage, and chorioamnionitis. However, an increased risk of cervical laceration was associated with manual rotation.20
Regarding FHR status, FHR abnormalities occurred in 91% of second stage labor patterns, with Category II being the most common.21 The fetal status should remain reassuring to allow for continuation of the second stage.
Continue to: Epidural analgesia...
Epidural analgesia
About 60% of women receive neuraxial analgesia in the United States,22 although rates vary widely across different populations. A Cochrane review showed no difference in the duration of the second stage among women who had early versus late initiation of epidural analgesia in labor.23 Epidural analgesia has no impact on the risk of CD; however, women with epidural analgesia experienced more hypotension, motor blockade, fever, and urinary retention.24
One management practice has been to discontinue epidural analgesia to allow resumption of sensory and motor nerve function. Another Cochrane systematic review found no difference in mode of delivery or neonatal outcomes.25 Rather than discontinuing epidural analgesia, which results in a profound increase in inadequate pain relief, one may consider titrating the dose with joint patient decision-making to allow for greater motor capability while maintaining adequate analgesia.34
Immediate vs delayed pushing
The 2 most common approaches to managing the second stage were either to initiate pushing with contractions once complete dilation occurred (immediate pushing) or to allow for a rest period in which the fetus passively rotated and descended while conserving a woman’s energy for pushing efforts (delayed pushing, laboring down, or passive descent). Since the publication of “Safe prevention of primary cesarean delivery,” however, studies have shown a concerning association between maternal and neonatal complications and prolonged second stage (which may occur with delayed pushing).3-8,35 An observational study of nearly 44,000 nulliparous women without epidural analgesia found that prolonged second stage was associated with increased chorioamnionitis, third- and fourth-degree lacerations, neonatal sepsis, neonatal asphyxia, and perinatal mortality.35
A pragmatic multicenter randomized clinical trial on the optimal management of second stage of labor across the United States recently was conducted.7 More than 2,000 nulliparous women at term in spontaneous or induced labor with epidural analgesia were randomly assigned at complete dilation to immediate pushing or delayed pushing (1 hour after complete dilation). There was no difference in the rate of vaginal delivery. The rate of postpartum hemorrhage was significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (2.3% vs 4.0%, respectively; relative risk [RR], 0.6; 95% confidence interval [CI], 0.3–0.9; P = .03). Furthermore, rates of chorioamnionitis were significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (6.7% vs 9.1%, respectively; RR, 0.70; 95% CI, 0.66–0.90; P = .005). No significant difference occurred in the composite outcome of neonatal morbidity between the groups. However neonatal acidemia (umbilical cord arterial pH <7.1) and confirmed or suspected sepsis were significantly increased in the delayed-pushing group.
The evidence supports active pushing at the start of the second stage. Women who consider delayed pushing should be informed that delayed pushing has not been shown to increase the likelihood of vaginal birth and that it is associated with increased risks of infection, hemorrhage, and neonatal acidemia.36
Maternal pushing position and technique
Spontaneous pushing (in which women are free to follow their instincts and generally push 3 to 5 times per contraction) versus directed pushing (women are encouraged to take a deep breath at the beginning of a contraction then hold it and bear down throughout the contraction) demonstrated no clear difference in duration of the second stage, perineal laceration, episiotomy, time spent pushing, or number of women with spontaneous vaginal birth. There was no difference in 5-minute Apgar score less than 7 or admission to the NICU.26
With regard to maternal positioning during the second stage, a Cochrane systematic review found benefits for upright posture, including a very small reduction in the duration of the second stage, reduction in episiotomy rates, and reduction in assisted deliveries.37 There was an increased risk of blood loss greater than 500 mL and possibly an increased risk in second-degree tears.37 Compared with women allocated to lying down, women in the upright position during the second stage with epidural analgesia had significantly fewer spontaneous vaginal births. There was no difference in operative vaginal delivery, obstetric anal sphincter injury (OASI), infant Apgar score of less than 4 at 5 minutes, and maternal fecal incontinence at 1 year.28
Continue to: Maternal support person...
Maternal support person
Continuous support during labor may improve outcomes for women and infants, including increased spontaneous vaginal birth, shorter duration of labor, and decreased cesarean birth.29 In a randomized trial of 412 healthy nulliparous women, women in labor were assigned to either a support group that received continuous support from a doula or an observed group that was monitored by an inconspicuous observer. Continuous labor support significantly reduced the rate of CDs and forceps deliveries.30,36 Indeed, during the COVID-19 pandemic, doulas have found innovative ways to continue to provide this essential support through virtual health.38
Prevention of perineal tears
Evidence suggests that warm compresses, and massage, may reduce third- and fourth-degree perineal tears.31 A meta-analysis of observational studies showed a significant reduction in the risk of OASI.32
Second stage steps: Recap
Throughout the second stage of labor, the decision to continue with expectant management or intervene with either an operative vaginal delivery or a CD is complex and requires consistent assessment and integration of multiple factors. An evidence-based approach to second stage labor management includes active pushing that is either Valsalva pushing or spontaneous, coached or uncoached, but most importantly, at the start of the second stage when a patient reaches complete dilation. Reassessment should occur at regular intervals to determine progress, after ensuring maternal and fetal well-being.
If there has been no advancement in station, an attempt at manual rotation or titration of epidural analgesia should be considered. Importantly, fetal descent with adequate pushing should be demonstrated throughout the second stage.
Additional considerations that improve outcomes include warm compresses or perineal massage to prevent third- and fourth-degree tears and the presence of a continuous support person to reduce the risk for an operative delivery.
Delivery should be expected within 2 hours for multiparous women and 3 hours for nulliparous women in the second stage. Prolonging the second stage beyond these thresholds should be individualized and occur only in the setting of assured maternal and fetal well-being.

CASE An alternative management strategy
Despite Ms. J.’s great active pushing effort for 60 minutes, the presenting part remains at 0 station and occiput transverse. Ms. J. is counseled regarding the risks and benefits of an attempt at manual rotation of the fetal head, and she wishes to proceed. The fetal position remains occiput transverse.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. At this time, Ms. J. is informed that there has been no descent, and she is counseled on the risks and benefits of continued pushing versus CD. Through shared decision-making, she consents to a CD. She undergoes a primary CD without complication. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 8 and 9, respectively. The umbilical cord arterial pH was 7.13.
Ms. J. and her baby were discharged home on postoperative day 4. ●
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine; Caughey AB, Cahill AG, Guise JM, et al. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210:179-193. doi:10.1016/j.ajog.2014.01.026.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2018. Natl Vital Stat Rep. 2019;68:1-47.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127:667-673. doi:10.1097/aog.0000000000001354.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-6. doi:10.1016/j.ajog.2015.12.042.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8. doi:10.1016/j.ajog.2017.10.007.
- Grantz KL, Sundaram R, Ma L, et al. Reassessing the duration of the second stage of labor in relation to maternal and neonatal morbidity. Obstet Gynecol. 2018;131:345-353. doi:10.1097/aog.0000000000002431.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454. doi:10.1001/jama.2018.13986.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019;220:191.e1-191.e7. doi:10.1016/j.ajog.2018.10.028.
- Leveno KJ, Nelson DB, McIntire DD. Second-stage labor: how long is too long? Am J Obstet Gynecol. 2016;214:484-489. doi:10.1016/j.ajog.2015.10.926.
- Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567-589. doi:10.1097/00006250-195512000-00001.
- Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281-1287. doi:10.1097/AOG.0b013e3181fdef6e.
- Cheng YW, Hopkins LM, Caughey AB. How long is too long: does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcomes? Am J Obstet Gynecol. 2004;191:933-938. doi:10.1016/j.ajog.2004.05.044.
- Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357.e1-7. doi:10.1016/j.ajog.2009.08.003.
- Le Ray C, Audibert F, Goffinet F, et al. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol. 2009;201:361.e1-7. doi:10.1016/j.ajog.2009.08.002.
- Allen VM, Baskett TF, O’Connell CM, et al. Maternal and perinatal outcomes with increasing duration of the second stage of labor. Obstet Gynecol. 2009;113:1248-1258. doi:10.1097/AOG.0b013e3181a722d6.
- Piper JM, Bolling DR, Newton ER. The second stage of labor: factors influencing duration. Am J Obstet Gynecol. 1991;165(4 pt 1):976-979. doi:10.1016/0002-9378(91)90452-w.
- Zaki MN, Hibbard JU, Kominiarek MA. Contemporary labor patterns and maternal age. Obstet Gynecol. 2013;122:1018-1024. doi:10.1097/AOG.0b013e3182a9c92c.
- Kominiarek MA, Vanveldhuisen P, Hibbard J, et al; Consortium on Safe Labor. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203:264.e1-7. doi:10.1016/j.ajog.2010.06.024.
- Kominiarek MA, Zhang J, Vanveldhuisen P, et al. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205:244.e1-8. doi:10.1016/j.ajog.2011.06.014.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72. doi:10.3109/14767051003710276.
- Krebs HB, Petres RE, Dunn LJ. Intrapartum fetal heart rate monitoring. V. Fetal heart rate patterns in the second stage of labor. Am J Obstet Gynecol. 1981;140:435-439. doi:10.1016/0002-9378(81)90041-7.
- Grant EN, Tao W, Craig M, et al. Neuraxial analgesia effects on labour progression: facts, fallacies, uncertainties and the future. BJOG. 2015;122:288-293. doi:10.1111/1471-0528.12966.
- Sng BL, Leong WL, Zeng Y, et al. Early versus late initiation of epidural analgesia for labour. Cochrane Database Syst Rev. 2014;(10):CD007238. doi:10.1002/14651858.CD007238.pub2.
- Anim-Somuah M, Smyth RM, Cyna AM, et al. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331. doi:10.1002/14651858.CD000331.pub4.
- Torvaldsen S, Roberts CL, Bell JC, et al. Discontinuation of epidural analgesia late in labour for reducing the adverse delivery outcomes associated with epidural analgesia. Cochrane Database Syst Rev. 2004;(4):CD004457. doi:10.1002/14651858.CD004457.pub2.
- Lemos A, Amorim MM, Dornelas de Andrade A, et al. Pushing/bearing down methods for the second stage of labour. Cochrane Database Syst Rev. 2017;3(3):CD009124. doi:10.1002/14651858.CD009124.pub3.
- Prins M, Boxem J, Lucas C, et al. Effect of spontaneous pushing versus Valsalva pushing in the second stage of labour on mother and fetus: a systematic review of randomised trials. BJOG. 2011;118:662-670. doi:10.1111/j.1471-0528.2011 .02910.x.
- Epidural and Position Trial Collaborative Group. Upright versus lying down position in second stage of labour in nulliparous women with low dose epidural: BUMPES randomised controlled trial. BMJ. 2017;359:j4471. doi:10.1136/bmj.j4471.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7(7):CD003766. doi:10.1002/14651858.CD003766.pub6.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital. A randomized controlled trial. JAMA. 1991;265:2197-2201.
- Aasheim V, Nilsen AB, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;6(6):CD006672. doi:10.1002/14651858.CD006672.pub3.
- Bulchandani S, Watts E, Sucharitha A, et al. Manual perineal support at the time of childbirth: a systematic review and meta-analysis. BJOG. 2015;122:1157-1165. doi:10.1111/1471-0528.13431.
- Feinstein U, Sheiner E, Levy A, et al. Risk factors for arrest of descent during the second stage of labor. Int J Gynaecol Obstet. 2002;77:7-14. doi:10.1016/s0020-7292(02)00007-3.
- Cheng YW, Caughey AB. Defining and managing normal and abnormal second stage of labor. Obstet Gynecol Clin North Am. 2017;44:547-566. doi:10.1016/j.ogc.2017.08.009.
- Laughon SK, Berghella V, Reddy UM, et al. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol. 2014;124:57-67. doi:10.1097/aog.0000000000000278.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 766: approaches to limit intervention during labor and birth. Obstet Gynecol. 2019;133:e164-e173. doi:10.1097/aog.0000000000003074.
- Gupta JK, Sood A, Hofmeyr GJ, et al. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev. 2017;5(5):CD002006. doi:10.1002/14651858.CD002006.pub4.
- Castaneda AN, Searcy JJ. Practising intimate labour: birth doulas respond during COVID-19. Anthropol Action. 2021;28:21-24. https://www.berghahnjournals.com/view/ journals/aia/28/1/aia280104.xml. Accessed February 8, 2021.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine; Caughey AB, Cahill AG, Guise JM, et al. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210:179-193. doi:10.1016/j.ajog.2014.01.026.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2018. Natl Vital Stat Rep. 2019;68:1-47.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127:667-673. doi:10.1097/aog.0000000000001354.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-6. doi:10.1016/j.ajog.2015.12.042.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8. doi:10.1016/j.ajog.2017.10.007.
- Grantz KL, Sundaram R, Ma L, et al. Reassessing the duration of the second stage of labor in relation to maternal and neonatal morbidity. Obstet Gynecol. 2018;131:345-353. doi:10.1097/aog.0000000000002431.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454. doi:10.1001/jama.2018.13986.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019;220:191.e1-191.e7. doi:10.1016/j.ajog.2018.10.028.
- Leveno KJ, Nelson DB, McIntire DD. Second-stage labor: how long is too long? Am J Obstet Gynecol. 2016;214:484-489. doi:10.1016/j.ajog.2015.10.926.
- Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567-589. doi:10.1097/00006250-195512000-00001.
- Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281-1287. doi:10.1097/AOG.0b013e3181fdef6e.
- Cheng YW, Hopkins LM, Caughey AB. How long is too long: does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcomes? Am J Obstet Gynecol. 2004;191:933-938. doi:10.1016/j.ajog.2004.05.044.
- Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357.e1-7. doi:10.1016/j.ajog.2009.08.003.
- Le Ray C, Audibert F, Goffinet F, et al. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol. 2009;201:361.e1-7. doi:10.1016/j.ajog.2009.08.002.
- Allen VM, Baskett TF, O’Connell CM, et al. Maternal and perinatal outcomes with increasing duration of the second stage of labor. Obstet Gynecol. 2009;113:1248-1258. doi:10.1097/AOG.0b013e3181a722d6.
- Piper JM, Bolling DR, Newton ER. The second stage of labor: factors influencing duration. Am J Obstet Gynecol. 1991;165(4 pt 1):976-979. doi:10.1016/0002-9378(91)90452-w.
- Zaki MN, Hibbard JU, Kominiarek MA. Contemporary labor patterns and maternal age. Obstet Gynecol. 2013;122:1018-1024. doi:10.1097/AOG.0b013e3182a9c92c.
- Kominiarek MA, Vanveldhuisen P, Hibbard J, et al; Consortium on Safe Labor. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203:264.e1-7. doi:10.1016/j.ajog.2010.06.024.
- Kominiarek MA, Zhang J, Vanveldhuisen P, et al. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205:244.e1-8. doi:10.1016/j.ajog.2011.06.014.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72. doi:10.3109/14767051003710276.
- Krebs HB, Petres RE, Dunn LJ. Intrapartum fetal heart rate monitoring. V. Fetal heart rate patterns in the second stage of labor. Am J Obstet Gynecol. 1981;140:435-439. doi:10.1016/0002-9378(81)90041-7.
- Grant EN, Tao W, Craig M, et al. Neuraxial analgesia effects on labour progression: facts, fallacies, uncertainties and the future. BJOG. 2015;122:288-293. doi:10.1111/1471-0528.12966.
- Sng BL, Leong WL, Zeng Y, et al. Early versus late initiation of epidural analgesia for labour. Cochrane Database Syst Rev. 2014;(10):CD007238. doi:10.1002/14651858.CD007238.pub2.
- Anim-Somuah M, Smyth RM, Cyna AM, et al. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331. doi:10.1002/14651858.CD000331.pub4.
- Torvaldsen S, Roberts CL, Bell JC, et al. Discontinuation of epidural analgesia late in labour for reducing the adverse delivery outcomes associated with epidural analgesia. Cochrane Database Syst Rev. 2004;(4):CD004457. doi:10.1002/14651858.CD004457.pub2.
- Lemos A, Amorim MM, Dornelas de Andrade A, et al. Pushing/bearing down methods for the second stage of labour. Cochrane Database Syst Rev. 2017;3(3):CD009124. doi:10.1002/14651858.CD009124.pub3.
- Prins M, Boxem J, Lucas C, et al. Effect of spontaneous pushing versus Valsalva pushing in the second stage of labour on mother and fetus: a systematic review of randomised trials. BJOG. 2011;118:662-670. doi:10.1111/j.1471-0528.2011 .02910.x.
- Epidural and Position Trial Collaborative Group. Upright versus lying down position in second stage of labour in nulliparous women with low dose epidural: BUMPES randomised controlled trial. BMJ. 2017;359:j4471. doi:10.1136/bmj.j4471.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7(7):CD003766. doi:10.1002/14651858.CD003766.pub6.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital. A randomized controlled trial. JAMA. 1991;265:2197-2201.
- Aasheim V, Nilsen AB, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;6(6):CD006672. doi:10.1002/14651858.CD006672.pub3.
- Bulchandani S, Watts E, Sucharitha A, et al. Manual perineal support at the time of childbirth: a systematic review and meta-analysis. BJOG. 2015;122:1157-1165. doi:10.1111/1471-0528.13431.
- Feinstein U, Sheiner E, Levy A, et al. Risk factors for arrest of descent during the second stage of labor. Int J Gynaecol Obstet. 2002;77:7-14. doi:10.1016/s0020-7292(02)00007-3.
- Cheng YW, Caughey AB. Defining and managing normal and abnormal second stage of labor. Obstet Gynecol Clin North Am. 2017;44:547-566. doi:10.1016/j.ogc.2017.08.009.
- Laughon SK, Berghella V, Reddy UM, et al. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol. 2014;124:57-67. doi:10.1097/aog.0000000000000278.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 766: approaches to limit intervention during labor and birth. Obstet Gynecol. 2019;133:e164-e173. doi:10.1097/aog.0000000000003074.
- Gupta JK, Sood A, Hofmeyr GJ, et al. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev. 2017;5(5):CD002006. doi:10.1002/14651858.CD002006.pub4.
- Castaneda AN, Searcy JJ. Practising intimate labour: birth doulas respond during COVID-19. Anthropol Action. 2021;28:21-24. https://www.berghahnjournals.com/view/ journals/aia/28/1/aia280104.xml. Accessed February 8, 2021.
Systemic Literature Review of the Use of Virtual Reality for Rehabilitation in Parkinson Disease
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease.1 Age-standardized incidence rates of PD in population-based studies in Europe and the United States range from 8.6 to 19.0 per 100,000 individuals, using a strict diagnostic criterion for PD.2 The negative impact of PD on health-related quality of life imposes a heavy burden on veterans. According to the US Department of Veterans Affairs (VA) National Parkinson’s Disease Consortium, the VA has as many as 50,000 patients with PD under its care. Because of this demand, the VA has strived to revolutionize available services for veterans with PD and related movement disorders.3
The classic motor symptoms of resting tremors, bradykinesia, postural instability, and rigidity of this progressive neurodegenerative disorder is a significant cause of functional limitations that lead to increased falls and inability to perform activities of daily living that challenges the individual and caregiver. 4 Rehabilitation has been considered as an adjuvant to surgical and medical treatments for PD to maximize function and minimize complications. High-intensity multimodal exercise boot camps and therapy that focuses on intensely exercising high-amplitude movements, have been shown to improve motor performance in PD.5,6 Available evidence has shown that exercise-dependent plasticity is the main mechanism underlying the effects of physiotherapy because it increases synaptic strength and affects neurotransmission.7 Although there is no consensus on the optimal approach for rehabilitation, innovative techniques have been proposed and studied. One such approach involves virtual reality (VR), which has begun to attract attention for its potential use during rehabilitation.8
VR is a simulated experience created by computer-based technology that grants users access to a virtual environment. There are 2 categories of VR: immersive and nonimmersive. Immersive VR is the most direct experience of virtual environments and usually is implemented through a head-mounted display. These displays have monitors in front of each eye, which can provide monocular or biocular imaging with the most common display being small liquid crystal display (LCD) panels.
Nonimmersive VR typically allows a participant to view a virtual environment by using standard high-resolution monitors rather than a headset or an immersive screen room. Many systems are readily available to the general public as electronic interactive entertainment (ie, video games). Interaction with the virtual world happens through interfaces such as keyboards and controllers while viewing a television or computer monitor. These systems often are more accessible and affordable when compared with immersive VR, although this is changing rapidly.
VR therapy is a noninvasive therapeutic alternative modality for PD. This review aims to study the use of VR to treat PD from a rehabilitative standpoint. Although not the only review on the topic, this systematic review is the first to examine the differences between immersive and nonimmersive VR rehabilitation for PD. VR technology is evolving rapidly and the research behind its clinical applications is steadily growing, especially as accessibility improves. This review also is an updated summary of the current literature on the effectiveness of VR therapy during PD rehabilitation.
Methods
Starting in July 2019, the authors searched several databases (PubMed, Google Scholar, Cochrane, and the Physiotherapy Evidence Database [PEDro]) for articles by using the keyword “Parkinson’s disease” combined with either “virtual reality” or “video games.” To find studies specific to rehabilitation, searches included the additional keyword: “rehabilitation.” After compiling an initial set of 89 articles, titles were reviewed to eliminate duplicates. The authors then read the abstracts to exclude study protocols, systematic reviews, and studies that used VR but did not focus on PD or any therapeutic outcome.
Articles were sorted into immersive or nonimmersive virtual reality categories. To be included as immersive VR, studies had to use any type of VR headset or full-scale VR room. Anything less immersive or similar to a traditional video game was included in the nonimmersive VR category. Articles that met inclusion criteria were selected for the systematic review. Criteria for inclusion in this review were: (1) English language; (2) included a study population focused on PD; (3) used some form of VR therapy; and (4) assessed potential rehabilitation by quantitative outcome measures. Only articles published in peer-reviewed journals were included.
Data were extracted into 2 tables specifically modified for this review: immersive and nonimmersive VR. Extracted data included study author name and publication date, study design, methodologic quality, sample size and group allocation, symptom progression via the Hoehn and Yahr Scale (1 to 5), VR modality, presence of control groups, primary outcomes, and primary findings.
Two of the authors (AS, BC) assessed the quality of each study by using the 11-point PEDro scale for randomized controlled trials (RCTs) (Table 1). Most criterion relate to the design and conduct of the study, but 3 focus on eligibility criteria (item 1), between-group statistical comparisons (item 10), and measures of variability (item 11). The total possible score was 10 because only 2 out of the 3 items on reporting quality contributed points to the total score (eligibility criteria specified did not).9
Results
This review is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA).10 After screening and assessment, 28 articles met inclusion criteria for this review: 7 using immersive VR and 21 using nonimmersive VR (Figure). The immersive studies included 2 RCTs (both with PEDro scores of 5), 1 controlled study with a PEDro score of 5, 1 pre-post pilot study, and 3 cohort studies (Table 2). The nonimmersive studies included 13 RCTs with an average PEDro score of 5.8; 2 pre-post pilot studies, 1 repeated measures study with a historic control, 1 non-RCT, 2 pre-post prospective studies, and 2 cohort studies (1 retrospective and 1 prospective) (Table 3).
Several outcome and assessment tools were used; the most common measures were related to gait, balance, kinematics, and VR feasibility. Studies varied in VR modalities and protocol, ranging from 21 sessions of Nintendo Wii Fit gaming for 7 weeks to 1 session of VR headset use.
Immersive VR
There were fewer immersive VR studies and these studies had lower mean PEDro scores when compared with nonimmersive VR studies. The VR modalities in the immersive studies used a VR headset or a multisensory immersive system that included polarized glasses. All the studies showed positive improvement in primary outcomes with the exception of Ma and colleagues,which showed no difference in success rates or kinematics with moving balls, and only showed improvement in reaching for stationary balls.11 The mean number of participants in the studies was 18.4.
All 7 studies had each participant complete tasks without VR then with the VR therapy. None of the studies compared immersive VR therapy with more conventional therapies. Robles-Garcia and colleagues compared 2 VR groups where the experimental group imitated an avatar’s finger tapping in the VR system while the control group lacked this imitation.12 The authors found that adding that imitation to the VR group lead to an increase in movement amplitude.
Among the immersive VR studies, only Janeh and colleagues commented on possible adverse effects (AEs) and found that VR was a safe method without AEs of discomfort or simulator sickness.13 The other 6 studies did not make any mention or discussion of AEs related to the training.
Nonimmersive VR
VR modalities used in nonimmersive studies included consumer video gaming systems. Nintendo Wii and Microsoft Xbox Kinect were most commonly used. Among the 21 studies, 14 compared VR therapy with a type of traditional exercise (eg, treadmill training, stretching exercises, balance training). The mean number of participants of the studies was 28.3.
Five studies showed a difference between the VR and traditional training groups.14-18 However, 9 studies showed positive improvement in both groups and found no between-group differences.19-25 Among the remaining 7 studies, all showed improvement in primary outcomes after adding VR interventional therapy. In 1 RCT, 3 groups were compared (no intervention, Nintendo Wii, and Xbox Kinect) for gait tests, anxiety levels, memory, and attention.26 The authors found that only the Nintendo Wii group showed improvement in outcomes. A prospective cohort study was the only one to compare different doses of VR therapy (10 sessions vs 15 sessions of Nintendo Wii Fit).27 The authors found that both groups demonstrated the same amount of improvement on balance performances with no group effect.
Ten studiesreported no AEs during the training, but also did not define what was considered an AE.15,16,19,22-25,27-29 Eight studies did not make any mention of AEs.14,17,21,26,27,30-32 Yen and colleagues reported no AEs during training except for the patients’ tendency to fall.20 However, therapists supervised the patients to avoid falls and no falls occurred. Nuic and colleaguesreported 3 serious AEs, unrelated to the training: severe pneumonia (n = 1) and deep-brain stimulation generator replacement (n = 2).33 During the video game training sessions no specific AEs occurred. Only Pompeu and colleagues defined an AE as any untoward medical occurrence such as convulsion, syncope, dizziness, vertigo, falls, or any medical condition that required hospitalization or disability.34 One researcher registered the occurrence of any AE; however, none occurred during the study period.
Discussion
This systematic review demonstrates that VR therapy is a promising addition to rehabilitation for PD. Evidence supporting VR therapy is limited, but is continually expanding, and current evidence has shown improvement in assessments and rehabilitative outcomes involving PD. Most nonimmersive studies have shown that VR therapy does not lead to better outcomes when compared with traditional therapy but also is not harmful and does provide similar improvement. Immersive VR studies, on the other hand, have not compared therapy with conventional training extensively, and tend to focus more on time for task completion or movement.
There were fewer immersive VR studies than nonimmersive VR studies. This could be because of the increased technological difficulty and demand to correctly execute immersive VR modalities, as well as the—until recently—substantial expense. This might be another reason why the mean PEDro scores for immersive VR RCTs were lower than the mean scores found in nonimmersive RCTs.
Limitations
This review was limited by several factors related to the included studies. A variety of rating scales were used in the immersive and nonimmersive VR studies. Although there was some general overlap with common measurements such as gait, balance, kinematics, and VR feasibility, no studies had the same primary and secondary outcomes. Such heterogeneity in protocols and outcomes limited our ability to draw conclusions from these differing studies. Additionally, the average number of participants of both immersive and nonimmersive studies were small and the statistical significance of findings should be interpreted with caution. Finally, VR devices and systems differed between studies, further limiting comparisons. Although these factors limit this systematic review, we can still identify treatment and research implications. Adequately powered future studies with standardized protocols would further improve the available evidence and support for VR as an intervention.
Conclusions
VR therapy is a promising rehabilitation modality for PD. Additional investigations of VR therapy and PD should include direct comparisons between immersive and nonimmersive VR therapies. It could be hypothesized that the greater immersion and engagement potential of immersive VR would demonstrate greater functional improvement compared with nonimmersive VR, but there is no data to support this for PD. VR therapy for PD appears to be a relatively safe alternative or adjunct to traditional therapy with a potentially positive impact on a variety of symptoms and is growing as an innovative therapeutic approach for PD patients.
1. de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525-535. doi:10.1016/S1474-4422(06)70471-9
2. Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP. Epidemiology of Parkinson’s disease. J Neurol. 2008;255 Suppl 5:18-32. doi:10.1007/s00415-008-5004-3
3. US Department of Veterans Affairs. Parkinson’s Disease Research, Education and Clinical Centers. Updated March 4, 2021. Accessed March 5, 2021. https://www.parkinsons.va.gov/index.asp.
4. Raza C, Anjum R, Shakeel NUA. Parkinson’s disease: mechanisms, translational models and management strategies. Life Sci. 2019;226:77-90. doi:10.1016/j.lfs.2019.03.057
5. Landers MR, Navalta JW, Murtishaw AS, Kinney JW, Pirio Richardson S. A high-intensity exercise boot camp for persons with Parkinson disease: a phase ii, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J Neurol Phys Ther. 2019;43(1):12-25. doi:10.1097/NPT.0000000000000249
6. Ebersbach G, Ebersbach A, Edler D, et al. Comparing exercise in Parkinson’s disease--the Berlin LSVT®BIG study [published correction appears in Mov Disord. 2010 Oct 30;25(14):2478]. Mov Disord. 2010;25(12):1902-1908. doi:10.1002/mds.23212
7. Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson’s disease: current outlook and future challenges. Parkinsonism Relat Disord. 2016;22(suppl 1):S60-S64. doi:10.1016/j.parkreldis.2015.09.005
8. Weiss PL, Katz N. The potential of virtual reality for rehabilitation. J Rehabil Res Dev. 2004;41(5):vii-x.
9. da Costa BR, Hilfiker R, Egger M. PEDro’s bias: summary quality scores should not be used in meta-analysis. J Clin Epidemiol. 2013;66(1):75-77.doi:10.1016/j.jclinepi.2012.08.003
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
11. Ma HI, Hwang WJ, Fang JJ, et al. Effects of virtual reality training on functional reaching movements in people with Parkinson’s disease: a randomized controlled pilot trial. Clin Rehabil. 2011;25(10):892-902. doi:10.1177/0269215511406757
12. Robles-García V, Corral-Bergantiños Y, Espinosa N, et al. Effects of movement imitation training in Parkinson’s disease: a virtual reality pilot study. Parkinsonism Relat Disord. 2016;26:17-23. doi:10.1016/j.parkreldis.2016.02.022
13. Janeh O, Fründt O, Schönwald B, et al. Gait Training in virtual reality: short-term effects of different virtual manipulation techniques in Parkinson’s Disease. Cells. 2019;8(5):419. Published 2019 May 6.doi:10.3390/cells8050419
14. Pelosin E, Cerulli C, Ogliastro C, et al. A multimodal training modulates short afferent inhibition and improves complex walking in a cohort of faller older adults with an increased prevalence of Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2020;75(4):722-728.doi:10.1093/gerona/glz072
15. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
16. Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease?. J Gerontol A Biol Sci Med Sci. 2011;66(2):234-240.doi:10.1093/gerona/glq201
17. Lee NY, Lee DK, Song HS. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J Phys Ther Sci. 2015;27(1):145-147. doi:10.1589/jpts.27.145
18. Feng H, Li C, Liu J, et al. Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson’s disease patients: a randomized controlled trial. Med Sci Monit. 2019;25:4186-4192. Published 2019 Jun 5. doi:10.12659/MSM.916455
19. Gandolfi M, Geroin C, Dimitrova E, et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: a multicenter, single-blind, randomized, controlled trial. Biomed Res Int. 2017;2017:7962826. doi:10.1155/2017/7962826
20. Yen CY, Lin KH, Hu MH, Wu RM, Lu TW, Lin CH. Effects of virtual reality-augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: a randomized controlled trial. Phys Ther. 2011;91(6):862-874. doi:10.2522/ptj.20100050
21. Yang WC, Wang HK, Wu RM, Lo CS, Lin KH. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: a randomized controlled trial. J Formos Med Assoc. 2016;115(9):734-743. doi:10.1016/j.jfma.2015.07.012
22. Pompeu JE, Mendes FA, Silva KG, et al. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy. 2012;98(3):196-204. doi:10.1016/j.physio.2012.06.004
23. van den Heuvel MR, Kwakkel G, Beek PJ, Berendse HW, Daffertshofer A, van Wegen EE. Effects of augmented visual feedback during balance training in Parkinson’s disease: a pilot randomized clinical trial. Parkinsonism Relat Disord. 2014;20(12):1352-1358. doi:10.1016/j.parkreldis.2014.09.022
24. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
25. Fundarò C, Maestri R, Ferriero G, Chimento P, Taveggia G, Casale R. Self-selected speed gait training in Parkinson’s disease: robot-assisted gait training with virtual reality versus gait training on the ground. Eur J Phys Rehabil Med. 2019;55(4):456-462. doi:10.23736/S1973-9087.18.05368-6
26. Alves MLM, Mesquita BS, Morais WS, Leal JC, Satler CE, Dos Santos Mendes FA. Nintendo Wii™ versus Xbox Kinect™ for assisting people with Parkinson’s disease. Percept Mot Skills. 2018;125(3):546-565. doi:10.1177/0031512518769204
27. Negrini S, Bissolotti L, Ferraris A, Noro F, Bishop MD, Villafañe JH. Nintendo Wii Fit for balance rehabilitation in patients with Parkinson’s disease: A comparative study. J Bodyw Mov Ther. 2017;21(1):117-123. doi:10.1016/j.jbmt.2016.06.001
28. van Beek JJW, van Wegen EEH, Bohlhalter S, Vanbellingen T. Exergaming-based dexterity training in persons with Parkinson disease: a pilot feasibility study. J Neurol Phys Ther. 2019;43(3):168-174. doi:10.1097/NPT.0000000000000278
29. Palacios-Navarro G, García-Magariño I, Ramos-Lorente P. A kinect-based system for lower limb rehabilitation in Parkinson’s disease patients: a pilot study. J Med Syst. 2015;39(9):103. doi:10.1007/s10916-015-0289-0
30. dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease--effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy. 2012;98(3):217-223. doi:10.1016/j.physio.2012.06.001
31. de Melo GEL, Kleiner AFR, Lopes JBP, et al. Effect of virtual reality training on walking distance and physical fitness in individuals with Parkinson’s disease. Neuro Rehabilitation. 2018;42(4):473-480. doi:10.3233/NRE-172355
32. Maidan I, Nieuwhof F, Bernad-Elazari H, et al. Evidence for differential effects of 2 forms of exercise on prefrontal plasticity during walking in Parkinson’s disease. Neurorehabil Neural Repair. 2018;32(3):200-208. doi:10.1177/1545968318763750
33. Nuic D, Vinti M, Karachi C, Foulon P, Van Hamme A, Welter ML. The feasibility and positive effects of a customised videogame rehabilitation programme for freezing of gait and falls in Parkinson’s disease patients: a pilot study. J Neuroeng Rehabil. 2018;15(1):31. Published 2018 Apr 10. doi:10.1186/s12984-018-0375-x
34. Pompeu JE, Arduini LA, Botelho AR, et al. Feasibility, safety and outcomes of playing Kinect Adventures!™ for people with Parkinson’s disease: a pilot study. Physiotherapy. 2014;100(2):162-168. doi:10.1016/j.physio.2013.10.003
35. Ma HI, Hwang WJ, Wang CY, Fang JJ, Leong IF, Wang TY. Trunk-arm coordination in reaching for moving targets in people with Parkinson’s disease: comparison between virtual and physical reality. Hum Mov Sci. 2012;31(5):1340-1352. doi:10.1016/j.humov.2011.11.004
36. Griffin HJ, Greenlaw R, Limousin P, Bhatia K, Quinn NP, Jahanshahi M. The effect of real and virtual visual cues on walking in Parkinson’s disease. J Neurol. 2011;258(6):991-1000. doi:10.1007/s00415-010-5866-z
37. Espay AJ, Baram Y, Dwivedi AK, et al. At-home training with closed-loop augmented-reality cueing device for improving gait in patients with Parkinson disease. J Rehabil Res Dev. 2010;47(6):573-581. doi:10.1682/jrrd.2009.10.0165
38. Espay AJ, Gaines L, Gupta R. Sensory feedback in Parkinson’s disease patients with “on”-predominant freezing of gait. Front Neurol. 2013;4:14. Published 2013 Feb 25. doi:10.3389/fneur.2013.00014
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease.1 Age-standardized incidence rates of PD in population-based studies in Europe and the United States range from 8.6 to 19.0 per 100,000 individuals, using a strict diagnostic criterion for PD.2 The negative impact of PD on health-related quality of life imposes a heavy burden on veterans. According to the US Department of Veterans Affairs (VA) National Parkinson’s Disease Consortium, the VA has as many as 50,000 patients with PD under its care. Because of this demand, the VA has strived to revolutionize available services for veterans with PD and related movement disorders.3
The classic motor symptoms of resting tremors, bradykinesia, postural instability, and rigidity of this progressive neurodegenerative disorder is a significant cause of functional limitations that lead to increased falls and inability to perform activities of daily living that challenges the individual and caregiver. 4 Rehabilitation has been considered as an adjuvant to surgical and medical treatments for PD to maximize function and minimize complications. High-intensity multimodal exercise boot camps and therapy that focuses on intensely exercising high-amplitude movements, have been shown to improve motor performance in PD.5,6 Available evidence has shown that exercise-dependent plasticity is the main mechanism underlying the effects of physiotherapy because it increases synaptic strength and affects neurotransmission.7 Although there is no consensus on the optimal approach for rehabilitation, innovative techniques have been proposed and studied. One such approach involves virtual reality (VR), which has begun to attract attention for its potential use during rehabilitation.8
VR is a simulated experience created by computer-based technology that grants users access to a virtual environment. There are 2 categories of VR: immersive and nonimmersive. Immersive VR is the most direct experience of virtual environments and usually is implemented through a head-mounted display. These displays have monitors in front of each eye, which can provide monocular or biocular imaging with the most common display being small liquid crystal display (LCD) panels.
Nonimmersive VR typically allows a participant to view a virtual environment by using standard high-resolution monitors rather than a headset or an immersive screen room. Many systems are readily available to the general public as electronic interactive entertainment (ie, video games). Interaction with the virtual world happens through interfaces such as keyboards and controllers while viewing a television or computer monitor. These systems often are more accessible and affordable when compared with immersive VR, although this is changing rapidly.
VR therapy is a noninvasive therapeutic alternative modality for PD. This review aims to study the use of VR to treat PD from a rehabilitative standpoint. Although not the only review on the topic, this systematic review is the first to examine the differences between immersive and nonimmersive VR rehabilitation for PD. VR technology is evolving rapidly and the research behind its clinical applications is steadily growing, especially as accessibility improves. This review also is an updated summary of the current literature on the effectiveness of VR therapy during PD rehabilitation.
Methods
Starting in July 2019, the authors searched several databases (PubMed, Google Scholar, Cochrane, and the Physiotherapy Evidence Database [PEDro]) for articles by using the keyword “Parkinson’s disease” combined with either “virtual reality” or “video games.” To find studies specific to rehabilitation, searches included the additional keyword: “rehabilitation.” After compiling an initial set of 89 articles, titles were reviewed to eliminate duplicates. The authors then read the abstracts to exclude study protocols, systematic reviews, and studies that used VR but did not focus on PD or any therapeutic outcome.
Articles were sorted into immersive or nonimmersive virtual reality categories. To be included as immersive VR, studies had to use any type of VR headset or full-scale VR room. Anything less immersive or similar to a traditional video game was included in the nonimmersive VR category. Articles that met inclusion criteria were selected for the systematic review. Criteria for inclusion in this review were: (1) English language; (2) included a study population focused on PD; (3) used some form of VR therapy; and (4) assessed potential rehabilitation by quantitative outcome measures. Only articles published in peer-reviewed journals were included.
Data were extracted into 2 tables specifically modified for this review: immersive and nonimmersive VR. Extracted data included study author name and publication date, study design, methodologic quality, sample size and group allocation, symptom progression via the Hoehn and Yahr Scale (1 to 5), VR modality, presence of control groups, primary outcomes, and primary findings.
Two of the authors (AS, BC) assessed the quality of each study by using the 11-point PEDro scale for randomized controlled trials (RCTs) (Table 1). Most criterion relate to the design and conduct of the study, but 3 focus on eligibility criteria (item 1), between-group statistical comparisons (item 10), and measures of variability (item 11). The total possible score was 10 because only 2 out of the 3 items on reporting quality contributed points to the total score (eligibility criteria specified did not).9
Results
This review is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA).10 After screening and assessment, 28 articles met inclusion criteria for this review: 7 using immersive VR and 21 using nonimmersive VR (Figure). The immersive studies included 2 RCTs (both with PEDro scores of 5), 1 controlled study with a PEDro score of 5, 1 pre-post pilot study, and 3 cohort studies (Table 2). The nonimmersive studies included 13 RCTs with an average PEDro score of 5.8; 2 pre-post pilot studies, 1 repeated measures study with a historic control, 1 non-RCT, 2 pre-post prospective studies, and 2 cohort studies (1 retrospective and 1 prospective) (Table 3).
Several outcome and assessment tools were used; the most common measures were related to gait, balance, kinematics, and VR feasibility. Studies varied in VR modalities and protocol, ranging from 21 sessions of Nintendo Wii Fit gaming for 7 weeks to 1 session of VR headset use.
Immersive VR
There were fewer immersive VR studies and these studies had lower mean PEDro scores when compared with nonimmersive VR studies. The VR modalities in the immersive studies used a VR headset or a multisensory immersive system that included polarized glasses. All the studies showed positive improvement in primary outcomes with the exception of Ma and colleagues,which showed no difference in success rates or kinematics with moving balls, and only showed improvement in reaching for stationary balls.11 The mean number of participants in the studies was 18.4.
All 7 studies had each participant complete tasks without VR then with the VR therapy. None of the studies compared immersive VR therapy with more conventional therapies. Robles-Garcia and colleagues compared 2 VR groups where the experimental group imitated an avatar’s finger tapping in the VR system while the control group lacked this imitation.12 The authors found that adding that imitation to the VR group lead to an increase in movement amplitude.
Among the immersive VR studies, only Janeh and colleagues commented on possible adverse effects (AEs) and found that VR was a safe method without AEs of discomfort or simulator sickness.13 The other 6 studies did not make any mention or discussion of AEs related to the training.
Nonimmersive VR
VR modalities used in nonimmersive studies included consumer video gaming systems. Nintendo Wii and Microsoft Xbox Kinect were most commonly used. Among the 21 studies, 14 compared VR therapy with a type of traditional exercise (eg, treadmill training, stretching exercises, balance training). The mean number of participants of the studies was 28.3.
Five studies showed a difference between the VR and traditional training groups.14-18 However, 9 studies showed positive improvement in both groups and found no between-group differences.19-25 Among the remaining 7 studies, all showed improvement in primary outcomes after adding VR interventional therapy. In 1 RCT, 3 groups were compared (no intervention, Nintendo Wii, and Xbox Kinect) for gait tests, anxiety levels, memory, and attention.26 The authors found that only the Nintendo Wii group showed improvement in outcomes. A prospective cohort study was the only one to compare different doses of VR therapy (10 sessions vs 15 sessions of Nintendo Wii Fit).27 The authors found that both groups demonstrated the same amount of improvement on balance performances with no group effect.
Ten studiesreported no AEs during the training, but also did not define what was considered an AE.15,16,19,22-25,27-29 Eight studies did not make any mention of AEs.14,17,21,26,27,30-32 Yen and colleagues reported no AEs during training except for the patients’ tendency to fall.20 However, therapists supervised the patients to avoid falls and no falls occurred. Nuic and colleaguesreported 3 serious AEs, unrelated to the training: severe pneumonia (n = 1) and deep-brain stimulation generator replacement (n = 2).33 During the video game training sessions no specific AEs occurred. Only Pompeu and colleagues defined an AE as any untoward medical occurrence such as convulsion, syncope, dizziness, vertigo, falls, or any medical condition that required hospitalization or disability.34 One researcher registered the occurrence of any AE; however, none occurred during the study period.
Discussion
This systematic review demonstrates that VR therapy is a promising addition to rehabilitation for PD. Evidence supporting VR therapy is limited, but is continually expanding, and current evidence has shown improvement in assessments and rehabilitative outcomes involving PD. Most nonimmersive studies have shown that VR therapy does not lead to better outcomes when compared with traditional therapy but also is not harmful and does provide similar improvement. Immersive VR studies, on the other hand, have not compared therapy with conventional training extensively, and tend to focus more on time for task completion or movement.
There were fewer immersive VR studies than nonimmersive VR studies. This could be because of the increased technological difficulty and demand to correctly execute immersive VR modalities, as well as the—until recently—substantial expense. This might be another reason why the mean PEDro scores for immersive VR RCTs were lower than the mean scores found in nonimmersive RCTs.
Limitations
This review was limited by several factors related to the included studies. A variety of rating scales were used in the immersive and nonimmersive VR studies. Although there was some general overlap with common measurements such as gait, balance, kinematics, and VR feasibility, no studies had the same primary and secondary outcomes. Such heterogeneity in protocols and outcomes limited our ability to draw conclusions from these differing studies. Additionally, the average number of participants of both immersive and nonimmersive studies were small and the statistical significance of findings should be interpreted with caution. Finally, VR devices and systems differed between studies, further limiting comparisons. Although these factors limit this systematic review, we can still identify treatment and research implications. Adequately powered future studies with standardized protocols would further improve the available evidence and support for VR as an intervention.
Conclusions
VR therapy is a promising rehabilitation modality for PD. Additional investigations of VR therapy and PD should include direct comparisons between immersive and nonimmersive VR therapies. It could be hypothesized that the greater immersion and engagement potential of immersive VR would demonstrate greater functional improvement compared with nonimmersive VR, but there is no data to support this for PD. VR therapy for PD appears to be a relatively safe alternative or adjunct to traditional therapy with a potentially positive impact on a variety of symptoms and is growing as an innovative therapeutic approach for PD patients.
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease.1 Age-standardized incidence rates of PD in population-based studies in Europe and the United States range from 8.6 to 19.0 per 100,000 individuals, using a strict diagnostic criterion for PD.2 The negative impact of PD on health-related quality of life imposes a heavy burden on veterans. According to the US Department of Veterans Affairs (VA) National Parkinson’s Disease Consortium, the VA has as many as 50,000 patients with PD under its care. Because of this demand, the VA has strived to revolutionize available services for veterans with PD and related movement disorders.3
The classic motor symptoms of resting tremors, bradykinesia, postural instability, and rigidity of this progressive neurodegenerative disorder is a significant cause of functional limitations that lead to increased falls and inability to perform activities of daily living that challenges the individual and caregiver. 4 Rehabilitation has been considered as an adjuvant to surgical and medical treatments for PD to maximize function and minimize complications. High-intensity multimodal exercise boot camps and therapy that focuses on intensely exercising high-amplitude movements, have been shown to improve motor performance in PD.5,6 Available evidence has shown that exercise-dependent plasticity is the main mechanism underlying the effects of physiotherapy because it increases synaptic strength and affects neurotransmission.7 Although there is no consensus on the optimal approach for rehabilitation, innovative techniques have been proposed and studied. One such approach involves virtual reality (VR), which has begun to attract attention for its potential use during rehabilitation.8
VR is a simulated experience created by computer-based technology that grants users access to a virtual environment. There are 2 categories of VR: immersive and nonimmersive. Immersive VR is the most direct experience of virtual environments and usually is implemented through a head-mounted display. These displays have monitors in front of each eye, which can provide monocular or biocular imaging with the most common display being small liquid crystal display (LCD) panels.
Nonimmersive VR typically allows a participant to view a virtual environment by using standard high-resolution monitors rather than a headset or an immersive screen room. Many systems are readily available to the general public as electronic interactive entertainment (ie, video games). Interaction with the virtual world happens through interfaces such as keyboards and controllers while viewing a television or computer monitor. These systems often are more accessible and affordable when compared with immersive VR, although this is changing rapidly.
VR therapy is a noninvasive therapeutic alternative modality for PD. This review aims to study the use of VR to treat PD from a rehabilitative standpoint. Although not the only review on the topic, this systematic review is the first to examine the differences between immersive and nonimmersive VR rehabilitation for PD. VR technology is evolving rapidly and the research behind its clinical applications is steadily growing, especially as accessibility improves. This review also is an updated summary of the current literature on the effectiveness of VR therapy during PD rehabilitation.
Methods
Starting in July 2019, the authors searched several databases (PubMed, Google Scholar, Cochrane, and the Physiotherapy Evidence Database [PEDro]) for articles by using the keyword “Parkinson’s disease” combined with either “virtual reality” or “video games.” To find studies specific to rehabilitation, searches included the additional keyword: “rehabilitation.” After compiling an initial set of 89 articles, titles were reviewed to eliminate duplicates. The authors then read the abstracts to exclude study protocols, systematic reviews, and studies that used VR but did not focus on PD or any therapeutic outcome.
Articles were sorted into immersive or nonimmersive virtual reality categories. To be included as immersive VR, studies had to use any type of VR headset or full-scale VR room. Anything less immersive or similar to a traditional video game was included in the nonimmersive VR category. Articles that met inclusion criteria were selected for the systematic review. Criteria for inclusion in this review were: (1) English language; (2) included a study population focused on PD; (3) used some form of VR therapy; and (4) assessed potential rehabilitation by quantitative outcome measures. Only articles published in peer-reviewed journals were included.
Data were extracted into 2 tables specifically modified for this review: immersive and nonimmersive VR. Extracted data included study author name and publication date, study design, methodologic quality, sample size and group allocation, symptom progression via the Hoehn and Yahr Scale (1 to 5), VR modality, presence of control groups, primary outcomes, and primary findings.
Two of the authors (AS, BC) assessed the quality of each study by using the 11-point PEDro scale for randomized controlled trials (RCTs) (Table 1). Most criterion relate to the design and conduct of the study, but 3 focus on eligibility criteria (item 1), between-group statistical comparisons (item 10), and measures of variability (item 11). The total possible score was 10 because only 2 out of the 3 items on reporting quality contributed points to the total score (eligibility criteria specified did not).9
Results
This review is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA).10 After screening and assessment, 28 articles met inclusion criteria for this review: 7 using immersive VR and 21 using nonimmersive VR (Figure). The immersive studies included 2 RCTs (both with PEDro scores of 5), 1 controlled study with a PEDro score of 5, 1 pre-post pilot study, and 3 cohort studies (Table 2). The nonimmersive studies included 13 RCTs with an average PEDro score of 5.8; 2 pre-post pilot studies, 1 repeated measures study with a historic control, 1 non-RCT, 2 pre-post prospective studies, and 2 cohort studies (1 retrospective and 1 prospective) (Table 3).
Several outcome and assessment tools were used; the most common measures were related to gait, balance, kinematics, and VR feasibility. Studies varied in VR modalities and protocol, ranging from 21 sessions of Nintendo Wii Fit gaming for 7 weeks to 1 session of VR headset use.
Immersive VR
There were fewer immersive VR studies and these studies had lower mean PEDro scores when compared with nonimmersive VR studies. The VR modalities in the immersive studies used a VR headset or a multisensory immersive system that included polarized glasses. All the studies showed positive improvement in primary outcomes with the exception of Ma and colleagues,which showed no difference in success rates or kinematics with moving balls, and only showed improvement in reaching for stationary balls.11 The mean number of participants in the studies was 18.4.
All 7 studies had each participant complete tasks without VR then with the VR therapy. None of the studies compared immersive VR therapy with more conventional therapies. Robles-Garcia and colleagues compared 2 VR groups where the experimental group imitated an avatar’s finger tapping in the VR system while the control group lacked this imitation.12 The authors found that adding that imitation to the VR group lead to an increase in movement amplitude.
Among the immersive VR studies, only Janeh and colleagues commented on possible adverse effects (AEs) and found that VR was a safe method without AEs of discomfort or simulator sickness.13 The other 6 studies did not make any mention or discussion of AEs related to the training.
Nonimmersive VR
VR modalities used in nonimmersive studies included consumer video gaming systems. Nintendo Wii and Microsoft Xbox Kinect were most commonly used. Among the 21 studies, 14 compared VR therapy with a type of traditional exercise (eg, treadmill training, stretching exercises, balance training). The mean number of participants of the studies was 28.3.
Five studies showed a difference between the VR and traditional training groups.14-18 However, 9 studies showed positive improvement in both groups and found no between-group differences.19-25 Among the remaining 7 studies, all showed improvement in primary outcomes after adding VR interventional therapy. In 1 RCT, 3 groups were compared (no intervention, Nintendo Wii, and Xbox Kinect) for gait tests, anxiety levels, memory, and attention.26 The authors found that only the Nintendo Wii group showed improvement in outcomes. A prospective cohort study was the only one to compare different doses of VR therapy (10 sessions vs 15 sessions of Nintendo Wii Fit).27 The authors found that both groups demonstrated the same amount of improvement on balance performances with no group effect.
Ten studiesreported no AEs during the training, but also did not define what was considered an AE.15,16,19,22-25,27-29 Eight studies did not make any mention of AEs.14,17,21,26,27,30-32 Yen and colleagues reported no AEs during training except for the patients’ tendency to fall.20 However, therapists supervised the patients to avoid falls and no falls occurred. Nuic and colleaguesreported 3 serious AEs, unrelated to the training: severe pneumonia (n = 1) and deep-brain stimulation generator replacement (n = 2).33 During the video game training sessions no specific AEs occurred. Only Pompeu and colleagues defined an AE as any untoward medical occurrence such as convulsion, syncope, dizziness, vertigo, falls, or any medical condition that required hospitalization or disability.34 One researcher registered the occurrence of any AE; however, none occurred during the study period.
Discussion
This systematic review demonstrates that VR therapy is a promising addition to rehabilitation for PD. Evidence supporting VR therapy is limited, but is continually expanding, and current evidence has shown improvement in assessments and rehabilitative outcomes involving PD. Most nonimmersive studies have shown that VR therapy does not lead to better outcomes when compared with traditional therapy but also is not harmful and does provide similar improvement. Immersive VR studies, on the other hand, have not compared therapy with conventional training extensively, and tend to focus more on time for task completion or movement.
There were fewer immersive VR studies than nonimmersive VR studies. This could be because of the increased technological difficulty and demand to correctly execute immersive VR modalities, as well as the—until recently—substantial expense. This might be another reason why the mean PEDro scores for immersive VR RCTs were lower than the mean scores found in nonimmersive RCTs.
Limitations
This review was limited by several factors related to the included studies. A variety of rating scales were used in the immersive and nonimmersive VR studies. Although there was some general overlap with common measurements such as gait, balance, kinematics, and VR feasibility, no studies had the same primary and secondary outcomes. Such heterogeneity in protocols and outcomes limited our ability to draw conclusions from these differing studies. Additionally, the average number of participants of both immersive and nonimmersive studies were small and the statistical significance of findings should be interpreted with caution. Finally, VR devices and systems differed between studies, further limiting comparisons. Although these factors limit this systematic review, we can still identify treatment and research implications. Adequately powered future studies with standardized protocols would further improve the available evidence and support for VR as an intervention.
Conclusions
VR therapy is a promising rehabilitation modality for PD. Additional investigations of VR therapy and PD should include direct comparisons between immersive and nonimmersive VR therapies. It could be hypothesized that the greater immersion and engagement potential of immersive VR would demonstrate greater functional improvement compared with nonimmersive VR, but there is no data to support this for PD. VR therapy for PD appears to be a relatively safe alternative or adjunct to traditional therapy with a potentially positive impact on a variety of symptoms and is growing as an innovative therapeutic approach for PD patients.
1. de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525-535. doi:10.1016/S1474-4422(06)70471-9
2. Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP. Epidemiology of Parkinson’s disease. J Neurol. 2008;255 Suppl 5:18-32. doi:10.1007/s00415-008-5004-3
3. US Department of Veterans Affairs. Parkinson’s Disease Research, Education and Clinical Centers. Updated March 4, 2021. Accessed March 5, 2021. https://www.parkinsons.va.gov/index.asp.
4. Raza C, Anjum R, Shakeel NUA. Parkinson’s disease: mechanisms, translational models and management strategies. Life Sci. 2019;226:77-90. doi:10.1016/j.lfs.2019.03.057
5. Landers MR, Navalta JW, Murtishaw AS, Kinney JW, Pirio Richardson S. A high-intensity exercise boot camp for persons with Parkinson disease: a phase ii, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J Neurol Phys Ther. 2019;43(1):12-25. doi:10.1097/NPT.0000000000000249
6. Ebersbach G, Ebersbach A, Edler D, et al. Comparing exercise in Parkinson’s disease--the Berlin LSVT®BIG study [published correction appears in Mov Disord. 2010 Oct 30;25(14):2478]. Mov Disord. 2010;25(12):1902-1908. doi:10.1002/mds.23212
7. Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson’s disease: current outlook and future challenges. Parkinsonism Relat Disord. 2016;22(suppl 1):S60-S64. doi:10.1016/j.parkreldis.2015.09.005
8. Weiss PL, Katz N. The potential of virtual reality for rehabilitation. J Rehabil Res Dev. 2004;41(5):vii-x.
9. da Costa BR, Hilfiker R, Egger M. PEDro’s bias: summary quality scores should not be used in meta-analysis. J Clin Epidemiol. 2013;66(1):75-77.doi:10.1016/j.jclinepi.2012.08.003
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
11. Ma HI, Hwang WJ, Fang JJ, et al. Effects of virtual reality training on functional reaching movements in people with Parkinson’s disease: a randomized controlled pilot trial. Clin Rehabil. 2011;25(10):892-902. doi:10.1177/0269215511406757
12. Robles-García V, Corral-Bergantiños Y, Espinosa N, et al. Effects of movement imitation training in Parkinson’s disease: a virtual reality pilot study. Parkinsonism Relat Disord. 2016;26:17-23. doi:10.1016/j.parkreldis.2016.02.022
13. Janeh O, Fründt O, Schönwald B, et al. Gait Training in virtual reality: short-term effects of different virtual manipulation techniques in Parkinson’s Disease. Cells. 2019;8(5):419. Published 2019 May 6.doi:10.3390/cells8050419
14. Pelosin E, Cerulli C, Ogliastro C, et al. A multimodal training modulates short afferent inhibition and improves complex walking in a cohort of faller older adults with an increased prevalence of Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2020;75(4):722-728.doi:10.1093/gerona/glz072
15. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
16. Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease?. J Gerontol A Biol Sci Med Sci. 2011;66(2):234-240.doi:10.1093/gerona/glq201
17. Lee NY, Lee DK, Song HS. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J Phys Ther Sci. 2015;27(1):145-147. doi:10.1589/jpts.27.145
18. Feng H, Li C, Liu J, et al. Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson’s disease patients: a randomized controlled trial. Med Sci Monit. 2019;25:4186-4192. Published 2019 Jun 5. doi:10.12659/MSM.916455
19. Gandolfi M, Geroin C, Dimitrova E, et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: a multicenter, single-blind, randomized, controlled trial. Biomed Res Int. 2017;2017:7962826. doi:10.1155/2017/7962826
20. Yen CY, Lin KH, Hu MH, Wu RM, Lu TW, Lin CH. Effects of virtual reality-augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: a randomized controlled trial. Phys Ther. 2011;91(6):862-874. doi:10.2522/ptj.20100050
21. Yang WC, Wang HK, Wu RM, Lo CS, Lin KH. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: a randomized controlled trial. J Formos Med Assoc. 2016;115(9):734-743. doi:10.1016/j.jfma.2015.07.012
22. Pompeu JE, Mendes FA, Silva KG, et al. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy. 2012;98(3):196-204. doi:10.1016/j.physio.2012.06.004
23. van den Heuvel MR, Kwakkel G, Beek PJ, Berendse HW, Daffertshofer A, van Wegen EE. Effects of augmented visual feedback during balance training in Parkinson’s disease: a pilot randomized clinical trial. Parkinsonism Relat Disord. 2014;20(12):1352-1358. doi:10.1016/j.parkreldis.2014.09.022
24. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
25. Fundarò C, Maestri R, Ferriero G, Chimento P, Taveggia G, Casale R. Self-selected speed gait training in Parkinson’s disease: robot-assisted gait training with virtual reality versus gait training on the ground. Eur J Phys Rehabil Med. 2019;55(4):456-462. doi:10.23736/S1973-9087.18.05368-6
26. Alves MLM, Mesquita BS, Morais WS, Leal JC, Satler CE, Dos Santos Mendes FA. Nintendo Wii™ versus Xbox Kinect™ for assisting people with Parkinson’s disease. Percept Mot Skills. 2018;125(3):546-565. doi:10.1177/0031512518769204
27. Negrini S, Bissolotti L, Ferraris A, Noro F, Bishop MD, Villafañe JH. Nintendo Wii Fit for balance rehabilitation in patients with Parkinson’s disease: A comparative study. J Bodyw Mov Ther. 2017;21(1):117-123. doi:10.1016/j.jbmt.2016.06.001
28. van Beek JJW, van Wegen EEH, Bohlhalter S, Vanbellingen T. Exergaming-based dexterity training in persons with Parkinson disease: a pilot feasibility study. J Neurol Phys Ther. 2019;43(3):168-174. doi:10.1097/NPT.0000000000000278
29. Palacios-Navarro G, García-Magariño I, Ramos-Lorente P. A kinect-based system for lower limb rehabilitation in Parkinson’s disease patients: a pilot study. J Med Syst. 2015;39(9):103. doi:10.1007/s10916-015-0289-0
30. dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease--effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy. 2012;98(3):217-223. doi:10.1016/j.physio.2012.06.001
31. de Melo GEL, Kleiner AFR, Lopes JBP, et al. Effect of virtual reality training on walking distance and physical fitness in individuals with Parkinson’s disease. Neuro Rehabilitation. 2018;42(4):473-480. doi:10.3233/NRE-172355
32. Maidan I, Nieuwhof F, Bernad-Elazari H, et al. Evidence for differential effects of 2 forms of exercise on prefrontal plasticity during walking in Parkinson’s disease. Neurorehabil Neural Repair. 2018;32(3):200-208. doi:10.1177/1545968318763750
33. Nuic D, Vinti M, Karachi C, Foulon P, Van Hamme A, Welter ML. The feasibility and positive effects of a customised videogame rehabilitation programme for freezing of gait and falls in Parkinson’s disease patients: a pilot study. J Neuroeng Rehabil. 2018;15(1):31. Published 2018 Apr 10. doi:10.1186/s12984-018-0375-x
34. Pompeu JE, Arduini LA, Botelho AR, et al. Feasibility, safety and outcomes of playing Kinect Adventures!™ for people with Parkinson’s disease: a pilot study. Physiotherapy. 2014;100(2):162-168. doi:10.1016/j.physio.2013.10.003
35. Ma HI, Hwang WJ, Wang CY, Fang JJ, Leong IF, Wang TY. Trunk-arm coordination in reaching for moving targets in people with Parkinson’s disease: comparison between virtual and physical reality. Hum Mov Sci. 2012;31(5):1340-1352. doi:10.1016/j.humov.2011.11.004
36. Griffin HJ, Greenlaw R, Limousin P, Bhatia K, Quinn NP, Jahanshahi M. The effect of real and virtual visual cues on walking in Parkinson’s disease. J Neurol. 2011;258(6):991-1000. doi:10.1007/s00415-010-5866-z
37. Espay AJ, Baram Y, Dwivedi AK, et al. At-home training with closed-loop augmented-reality cueing device for improving gait in patients with Parkinson disease. J Rehabil Res Dev. 2010;47(6):573-581. doi:10.1682/jrrd.2009.10.0165
38. Espay AJ, Gaines L, Gupta R. Sensory feedback in Parkinson’s disease patients with “on”-predominant freezing of gait. Front Neurol. 2013;4:14. Published 2013 Feb 25. doi:10.3389/fneur.2013.00014
1. de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525-535. doi:10.1016/S1474-4422(06)70471-9
2. Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP. Epidemiology of Parkinson’s disease. J Neurol. 2008;255 Suppl 5:18-32. doi:10.1007/s00415-008-5004-3
3. US Department of Veterans Affairs. Parkinson’s Disease Research, Education and Clinical Centers. Updated March 4, 2021. Accessed March 5, 2021. https://www.parkinsons.va.gov/index.asp.
4. Raza C, Anjum R, Shakeel NUA. Parkinson’s disease: mechanisms, translational models and management strategies. Life Sci. 2019;226:77-90. doi:10.1016/j.lfs.2019.03.057
5. Landers MR, Navalta JW, Murtishaw AS, Kinney JW, Pirio Richardson S. A high-intensity exercise boot camp for persons with Parkinson disease: a phase ii, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J Neurol Phys Ther. 2019;43(1):12-25. doi:10.1097/NPT.0000000000000249
6. Ebersbach G, Ebersbach A, Edler D, et al. Comparing exercise in Parkinson’s disease--the Berlin LSVT®BIG study [published correction appears in Mov Disord. 2010 Oct 30;25(14):2478]. Mov Disord. 2010;25(12):1902-1908. doi:10.1002/mds.23212
7. Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson’s disease: current outlook and future challenges. Parkinsonism Relat Disord. 2016;22(suppl 1):S60-S64. doi:10.1016/j.parkreldis.2015.09.005
8. Weiss PL, Katz N. The potential of virtual reality for rehabilitation. J Rehabil Res Dev. 2004;41(5):vii-x.
9. da Costa BR, Hilfiker R, Egger M. PEDro’s bias: summary quality scores should not be used in meta-analysis. J Clin Epidemiol. 2013;66(1):75-77.doi:10.1016/j.jclinepi.2012.08.003
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
11. Ma HI, Hwang WJ, Fang JJ, et al. Effects of virtual reality training on functional reaching movements in people with Parkinson’s disease: a randomized controlled pilot trial. Clin Rehabil. 2011;25(10):892-902. doi:10.1177/0269215511406757
12. Robles-García V, Corral-Bergantiños Y, Espinosa N, et al. Effects of movement imitation training in Parkinson’s disease: a virtual reality pilot study. Parkinsonism Relat Disord. 2016;26:17-23. doi:10.1016/j.parkreldis.2016.02.022
13. Janeh O, Fründt O, Schönwald B, et al. Gait Training in virtual reality: short-term effects of different virtual manipulation techniques in Parkinson’s Disease. Cells. 2019;8(5):419. Published 2019 May 6.doi:10.3390/cells8050419
14. Pelosin E, Cerulli C, Ogliastro C, et al. A multimodal training modulates short afferent inhibition and improves complex walking in a cohort of faller older adults with an increased prevalence of Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2020;75(4):722-728.doi:10.1093/gerona/glz072
15. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
16. Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease?. J Gerontol A Biol Sci Med Sci. 2011;66(2):234-240.doi:10.1093/gerona/glq201
17. Lee NY, Lee DK, Song HS. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J Phys Ther Sci. 2015;27(1):145-147. doi:10.1589/jpts.27.145
18. Feng H, Li C, Liu J, et al. Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson’s disease patients: a randomized controlled trial. Med Sci Monit. 2019;25:4186-4192. Published 2019 Jun 5. doi:10.12659/MSM.916455
19. Gandolfi M, Geroin C, Dimitrova E, et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: a multicenter, single-blind, randomized, controlled trial. Biomed Res Int. 2017;2017:7962826. doi:10.1155/2017/7962826
20. Yen CY, Lin KH, Hu MH, Wu RM, Lu TW, Lin CH. Effects of virtual reality-augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: a randomized controlled trial. Phys Ther. 2011;91(6):862-874. doi:10.2522/ptj.20100050
21. Yang WC, Wang HK, Wu RM, Lo CS, Lin KH. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: a randomized controlled trial. J Formos Med Assoc. 2016;115(9):734-743. doi:10.1016/j.jfma.2015.07.012
22. Pompeu JE, Mendes FA, Silva KG, et al. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy. 2012;98(3):196-204. doi:10.1016/j.physio.2012.06.004
23. van den Heuvel MR, Kwakkel G, Beek PJ, Berendse HW, Daffertshofer A, van Wegen EE. Effects of augmented visual feedback during balance training in Parkinson’s disease: a pilot randomized clinical trial. Parkinsonism Relat Disord. 2014;20(12):1352-1358. doi:10.1016/j.parkreldis.2014.09.022
24. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
25. Fundarò C, Maestri R, Ferriero G, Chimento P, Taveggia G, Casale R. Self-selected speed gait training in Parkinson’s disease: robot-assisted gait training with virtual reality versus gait training on the ground. Eur J Phys Rehabil Med. 2019;55(4):456-462. doi:10.23736/S1973-9087.18.05368-6
26. Alves MLM, Mesquita BS, Morais WS, Leal JC, Satler CE, Dos Santos Mendes FA. Nintendo Wii™ versus Xbox Kinect™ for assisting people with Parkinson’s disease. Percept Mot Skills. 2018;125(3):546-565. doi:10.1177/0031512518769204
27. Negrini S, Bissolotti L, Ferraris A, Noro F, Bishop MD, Villafañe JH. Nintendo Wii Fit for balance rehabilitation in patients with Parkinson’s disease: A comparative study. J Bodyw Mov Ther. 2017;21(1):117-123. doi:10.1016/j.jbmt.2016.06.001
28. van Beek JJW, van Wegen EEH, Bohlhalter S, Vanbellingen T. Exergaming-based dexterity training in persons with Parkinson disease: a pilot feasibility study. J Neurol Phys Ther. 2019;43(3):168-174. doi:10.1097/NPT.0000000000000278
29. Palacios-Navarro G, García-Magariño I, Ramos-Lorente P. A kinect-based system for lower limb rehabilitation in Parkinson’s disease patients: a pilot study. J Med Syst. 2015;39(9):103. doi:10.1007/s10916-015-0289-0
30. dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease--effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy. 2012;98(3):217-223. doi:10.1016/j.physio.2012.06.001
31. de Melo GEL, Kleiner AFR, Lopes JBP, et al. Effect of virtual reality training on walking distance and physical fitness in individuals with Parkinson’s disease. Neuro Rehabilitation. 2018;42(4):473-480. doi:10.3233/NRE-172355
32. Maidan I, Nieuwhof F, Bernad-Elazari H, et al. Evidence for differential effects of 2 forms of exercise on prefrontal plasticity during walking in Parkinson’s disease. Neurorehabil Neural Repair. 2018;32(3):200-208. doi:10.1177/1545968318763750
33. Nuic D, Vinti M, Karachi C, Foulon P, Van Hamme A, Welter ML. The feasibility and positive effects of a customised videogame rehabilitation programme for freezing of gait and falls in Parkinson’s disease patients: a pilot study. J Neuroeng Rehabil. 2018;15(1):31. Published 2018 Apr 10. doi:10.1186/s12984-018-0375-x
34. Pompeu JE, Arduini LA, Botelho AR, et al. Feasibility, safety and outcomes of playing Kinect Adventures!™ for people with Parkinson’s disease: a pilot study. Physiotherapy. 2014;100(2):162-168. doi:10.1016/j.physio.2013.10.003
35. Ma HI, Hwang WJ, Wang CY, Fang JJ, Leong IF, Wang TY. Trunk-arm coordination in reaching for moving targets in people with Parkinson’s disease: comparison between virtual and physical reality. Hum Mov Sci. 2012;31(5):1340-1352. doi:10.1016/j.humov.2011.11.004
36. Griffin HJ, Greenlaw R, Limousin P, Bhatia K, Quinn NP, Jahanshahi M. The effect of real and virtual visual cues on walking in Parkinson’s disease. J Neurol. 2011;258(6):991-1000. doi:10.1007/s00415-010-5866-z
37. Espay AJ, Baram Y, Dwivedi AK, et al. At-home training with closed-loop augmented-reality cueing device for improving gait in patients with Parkinson disease. J Rehabil Res Dev. 2010;47(6):573-581. doi:10.1682/jrrd.2009.10.0165
38. Espay AJ, Gaines L, Gupta R. Sensory feedback in Parkinson’s disease patients with “on”-predominant freezing of gait. Front Neurol. 2013;4:14. Published 2013 Feb 25. doi:10.3389/fneur.2013.00014
The Future of Progressive Multiple Sclerosis Therapies (FULL)
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system, with recent estimates of around 1 million people living with MS in the US.1 In many countries, MS is a leading cause of disability among young adults, second only to trauma.2 Clinically, neurologic worsening (ie, disability) in MS can occur in the relapsing-remitting (RRMS) phase of disease due to incomplete recovery from neuroinflammatory relapses. However, in the 15% of patients with a progressive course from onset (PPMS), and in those with RRMS who transition to a secondary progressive phenotype (SPMS), neurologic worsening follows a slowly progressive pattern.3 A progressive disease course—either PPMS at onset or transitioning to SPMS—is the dominant factor affecting MS-related neurologic disability accumulation. In particular, epidemiologic studies have shown that, in the absence of transitioning to a progressive disease course, < 5% of individuals with MS will accumulate sufficient disability to necessitate use of a cane for ambulation.4-6 Therefore, developing disease modifying therapies (DMTs) that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS represent a critical unmet need.
Research into the development of DMTs for progressive MS has been hindered by a number of factors. In particular, the clinical definition and diagnosis of progressive MS has been an evolving concept. Diagnostic criteria for MS, which help facilitate the enrollment of appropriate subjects into clinical trials, have only recently clarified the current consensus definition for progressive MS—steadily increasing neurologic disability independent of clinical relapses. Looking back to the Schumacher criteria in 1965 and Poser criteria in 1983, it was acknowledged that neurologic symptoms in MS may follow a relapsing-remitting or progressive pattern, but little attempt was made to define progressive MS.7,8 The original McDonald criteria in 2001 defined diagnostic criteria for progressive MS.9 These criteria continued to evolve through subsequent revisions (eg, cerebrospinal fluid [CSF] specific oligoclonal bands no longer are an absolute requirement), and only in the 2017 revision was it emphasized that disability progression must occur independent of clinical relapses, concordant with similar emphasis in the 2013 revision of MS clinical course definitions.3,10
The interpretation of prior clinical trials of DMT for progressive MS must consider this evolving clinical definition. The US Food and Drug Administration (FDA) approved mitoxantrone in 2000—making it the first DMT to carry an approved label for SPMS. While achieving significant clinical efficacy, it is clear from the details of the trial that the enrolled subjects had a high degree of inflammatory disease activity, which suggests that mitoxantrone treats neuroinflammation and not relapse-independent worsening. More recently, disparate results were seen in the anti-CD20 (rituximab, ocrelizumab) and S1P receptor modulator (fingolimod, siponimod) trials targeted at patients with primary and secondary progressive MS.11-14 Although there are differences between these therapies, they are more similar than not within the same therapeutic class. Taken together, these trials illustrate the critical impact the narrower inclusion/exclusion criteria (namely age and extent of inflammatory activity) had on attaining positive outcomes. Other considerations, such as confounding illness, also may impact trial recruitment and generalizability of findings.
The lack of known biological targets in progressive MS, which is a complex disease with an incompletely understood and heterogeneous pathology, also hinders DMT development. Decades of research has characterized multifocal central nervous system (CNS) lesions that exhibit features of demyelination, inflammation, reactive gliosis, axonal loss, and neuronal damage. Until recently, however, much of this research focused on the relapsing phase of disease, and so the understanding of the pathologic underpinnings of progressive disease has remained limited. Current areas of investigation encompass a broad range of pathological processes, such as microglial activation, meningeal lymphoid follicles, remyelination failure, vulnerability of chronically demyelinated axons, oxidative injury, iron accumulation, mitochondrial damage, and others. There is the added complication that the pathologic processes underlying progressive MS are superimposed on the CNS aging process. In particular, the transition to progressive MS and the rate of disability accumulation during progressive MS show strong correlation with age.6,15-17
Finally, DMT development for progressive MS also is hindered by the lack of specific surrogate and clinical outcome measures. Trials for relapsing MS have benefited greatly from the relatively straightforward assessment of clinical relapses and inflammatory disease activity on magnetic resonance imaging (MRI). With the goal of developing DMTs that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS, which by definition occurs independent of clinical relapses, these measures are not directly relevant. The longitudinal clinical disability outcome measures change at a slower rate than in early, relapsing disease. The use of standardized scales (eg, the Expanded Disability Status Scale [EDSS]), lower limb function, upper limb function, cognition, or a combination is a subject of ongoing debate. For example, the ASCEND and IMPACT trials (placebo-controlled trials for SPMS with natalizumab and interferon β-1a, respectively) showed no significant impact in EDSS progression—but in both of these trials, the 9-hole peg test (9-HPT), a performance measure for upper limb function, showed significant improvement.10,18 Particularly in those with an EDSS of > 6.5, who are unlikely to have measurable EDSS progression, functional tests such as the 9-HPT or timed 25-foot walk may be more sensitive as measures for disability progression.11 MRI measures of brain atrophy is the current gold standard surrogate outcome for clinical trials in progressive MS, but others that may warrant consideration include optical coherence tomography (OCT) or CSF markers of axonal degeneration.
DMT for Progressive MS
Current diagnostic nomenclature separates patients with active (superimposed clinical and/or radiographic relapses) from those with inactive disease.3,12 Relapsing forms of MS include all RRMS and those with SPMS with superimposed relapses (ie, active SPMS). Following this paradigm shift, the FDA changed the indication for already approved DMT from RRMS to relapsing forms of MS. Below is a discussion of DMT that specifically use the term SPMS and PPMS in the indication, where phase 3 trial data for progressive MS is available.
In 2019, the FDA approved the first oral medication (siponimod) for active SPMS. Subsequently, updates to the labels of the older DMT expanded to include active SPMS. Until 2019, the only FDA approved medication for SPMS was mitoxantrone, and use of this medication was limited due to unfavorable adverse effects (AEs). No medications had obtained FDA approval for inactive SPMS to this point, which represented an unmet need for a considerable number of patients.
Mitoxantrone became the first DMT approved for use in SPMS in 2000 following early trials that showed reductions in EDSS worsening, change in ambulation index, reduced number of treated relapses, and prolonged time to first treated relapse. However, as with some of the other positive trials in progressive MS, it is difficult to discern the impact of suppression of relapses as opposed to direct impact on progressive pathophysiology. Within the placebo arm, for example, there were 129 relapses among the 64 subjects, which suggests that these cases had particularly active disease or were in the early stages of SPMS.13 Furthermore, the use of this medication was limited due to concerns of cardiotoxicity and hematologic malignancy as serious AEs.
The trials of interferon β-1b illustrate the same difficulty of isolating possible benefits in disease progression from disability accumulated from relapses. The first interferon β-1b trial for SPMS, was conducted in Europe using fingolimod and showed a delay in confirmed disability progression compared to placebo as measured with the EDSS.14 However, a North American trial that followed in 2004 was unable to replicate this finding.15 The patients in the European trial appeared to be in an earlier phase of SPMS with more active disease, and a post-hoc pooled analysis suggested that patients with active disease and those with more pronounced disability progression were more likely to benefit from treatment.16 Overall, interferons do not appear to appreciably alter disability in the long-term for patients with SPMS, though they may modify short-term, relapse-related disability.
Perhaps the most encouraging data for SPMS was found in the EXPAND trial, which investigated siponimod, an S1P receptor modulator that is more selective than fingolimod. The trial identified a 21% reduction in 3-month confirmed disability progression for SPMS patients taking siponimod compared with those taking a placebo.17 Although the patients in EXPAND did seem to have relatively less disease activity at baseline than those who participated in other SPMS trials, those who benefitted from siponimod were primarily patients who had clinical and/or radiographic relapses over the prior 2 years. Based on this, the FDA approved siponimod for active SPMS. The extent to which siponimod exerts a true neuroprotective effect beyond reducing inflammation has not been clearly established.
B-cell depleting therapies rituximab and ocrelizumab have been evaluated in both primary and secondary progressive MS populations. Early investigations of the chimeric rituximab in PPMS did not show benefits on disability (EDSS) progression; however, benefits were seen in analysis of some subgroups.18 With this in mind, the ORATORIO trial for the humanized version, ocreluzimab, included PPMS patients that were younger (aged < 55 years) and had cutoffs for disease duration (< 15 years for those with EDSS more than 5 years, < 10 years for those with EDSS less than 5 years). The study showed statistically significant changes on disability progression, which led to ocrelizumab receiving the first FDA indication for PPMS.11 There are substantial pathophysiologic similarities between PPMS and SPMS in the progressive phase.19 While these medications may have similar effects across these disease processes, these benefits have not yet been demonstrated in a prospective trial for the SPMS population. Regardless, B-cell depleting therapy is a reasonable consideration for select patients with active SPMS, consistent with a relapsing form of MS.
Therapies in Development
DMT development for progressive MS is a high priority area. Current immunomodulatory therapies for RRMS have consistently been ineffective in the inactive forms of MS, with the possible exceptions of ocrelizumab and siponimod. Therefore, instead of immunosuppression, many agents currently in phase 2 and 3 clinical trials target alternative pathophysiological processes in order to provide neuroprotection, and/or promote remyelination and neurogenesis. These targets include oxidative stress (OS), non-T cell mediated inflammation, and mitochondrial/energy failure.20 Below we review a selection of clinical trials testing agents following these approaches. Many agents have more than one potential mechanism of action (MOA) that could benefit progressive MS.
Lipoic acid (LA), also known as α-lipoic acid and thiotic acid, is one such agent targeting alternative pathophysiology in SPMS. LA is an endogenous agent synthesized de novo from fatty acids and cysteine as well as obtained in small amounts from foods.21 It has antioxidant (AO) properties including direct radical scavenging, regeneration of glutathione, and upregulation of AO enzymes via the NrF2 pathway.22 It supports mitochondria as a key cofactor for pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, and it also aids mitochondrial DNA synthesis.21,22 Studies in experimental autoimmune encephalomyelitis, a widely used experimental mouse model of inflammatory demyelinating disease, also indicate a reduction in excessive microglial activation.23 A phase 2 pilot randomized controlled trial (RCT) of 1200 mg LA in SPMS (n = 51) resulted in significantly less whole brain atrophy by SIENA (Structural Image Evaluation, Using Normalization, of Atrophy) at 2 years.24 A follow-up multicenter trial is ongoing.
Simvastatin also targets alternative pathophysiology in SPMS. It has anti-inflammatory effects, improves vascular function, and promotes neuroprotection by reducing excitotoxicity. A phase 2 RCT demonstrated a reduction in whole brain atrophy in SPMS (n = 140), and a phase 3 trial is underway.25 Ibudilast is another repurposed drug that targets alternative inflammation by inhibiting several cyclic nucleotide phosphodiesterases, macrophage migration inhibitory factor and toll-like receptor 4. A phase 2 trial (n = 225) in both SPMS and PPMS also demonstrated a reduction in brain atrophy, but participants had high rates of AEs.26
Lithium and riluzole promote neuroprotection by reducing excitotoxicity. Lithium is a pharmacologic active cation used as a mood stabilizer and causes inhibition of glycogen synthase kinase-3β. Animal models also indicate that lithium may decrease inflammation and positively impact neurogenesis.27 A crossover pilot trial demonstrated tolerability with trends toward stabilization of EDSS and reductions in brain atrophy.28 Three neuroprotective agents, riluzole (reduces glutamate excitotoxicity), fluoxetine (stimulates glycogenolysis and improves mitochondrial energy production), and amiloride (an acid-sensing ion channel blocker that opens in response to inflammation) were tested in a phase 2b multi-arm, multi-site parallel group RCT in SPMS (n = 445). The study failed to yield differences from placebo for any agent in reduction of brain volume loss.29 A prior study of lamotrigine, a sodium channel blocker, also failed to find changes in brain volume loss.30 These studies highlight the large sample sizes and/or long study durations needed to test agents using brain atrophy as primary outcome. In the future, precise surrogate markers of neuroprotection will be a great need for earlier phase trials. These results also suggest that targeting > 1 MOA may be necessary to treat SPMS effectively.
Efforts to promote remyelination target one hallmark of MS damage. High dose biotin (about 10,000× usual dose) may promote myelin repair as a cofactor for fatty acid synthesis and support mitochondrial oxidative phosphorylation. While a RCT yielded a greater proportion of participants with either PPMS or SPMS with improvement in disability than placebo at 12 months, an open label trial suggested otherwise indicating a need for a more definitive trial.31,32
Anti-LINGO-1 (opicinumab) is a monoclonal antibody that targets LINGO, a potent negative regulator of oligodendrocyte differentiation and myelination.33 Although this agent failed in a phase 2 trial in relapsing MS, and is thus unlikely to be tested in progressive forms, the innovative approach to stimulating oligodendrocytes is ongoing. One such effort is to use thyroid hormone, crucial to myelin formation during development, as a repair agent in MS.34 A dose-finding study of thyroid hormone was completed and efforts to develop a thyromimetic agent are ongoing.
Finally, efforts to promote neurogenesis remain a goal for many neurodegenerative diseases. Exercise appears to prevent age-related atrophy of the hippocampus in animals and humans and help maintain neuronal health.35 In patients with RRMS, cortical thickness is impacted positively by resistance training, which suggests a neuroprotective effect.36 A multi-center trial of exercise in patients with progressive MS investigating cognitive outcomes is ongoing.
Discontinuing DMT
In the early 1990s, the successful development of immune modulating therapies that reliably reduced disease activity in RRMS led to widespread initiation in patients with relapsing disease. However, guidance on when or if to discontinue DMT, even in those who have transitioned to SPMS, remains largely absent at this time. Requests to discontinue DMT may come from patients weary of taking medication (especially injections), bothered by AEs, or those who no longer perceive efficacy from their treatments. Clinicians also may question the benefit of immune modulation in patients with longstanding freedom from relapses or changes in MRI lesion burden.
To inform discussion centered on treatment discontinuation, a clinical trial is currently underway to better answer the question of when and how to withdraw MS therapy. Discontinuation of Disease Modifying Therapies in Multiple Sclerosis (DISCO-MS) is a prospective, placebo-controlled RCT and its primary endpoint is recurrence of disease activity over a 2 year follow-up period.37 Eligibility requirements for the trial include age > 55 years, 5-year freedom from relapses, and 3-year freedom from new MRI lesions (criteria informed by progressive MS cohort studies).31 In addition to demonstrating the active disease recurrence rates in this patient population, the trial also aims to identify risk factors for recurrent disease activity among treated MS patients.37 DISCO-MS builds upon a series of retrospective and observational studies that partially answered these questions, albeit in the context of biases inherent in retrospective or observational studies.
A Minneapolis MS Treatment and Research Center single-center study identified 77 SPMS patients with no acute CNS inflammatory events over 2 to 20 years and advised these patients to stop taking DMT.32 In this group, 11.7% of subjects experienced recurrent active disease. Age was the primary discriminating factor. The mean age of those who experienced disease activity was 56 years vs 61 years those who did not. A second observational study from France found that among 100 SPMS patients treated either with interferon β or glatiramer acetate for at least 6 months, 35% experienced some form of inflammatory disease upon discontinuation.38 Sixteen patients relapsed and 19 developed gadolinium-enhancing MR lesions after DMT discontinuation. However, the age of the cohort was younger than the Minneapolis study (47.2 years vs 61 years), and reasons for discontinuation (eg, AEs or lack of disease activity) were not considered in the analysis.
Other studies examining the safety of DMT discontinuation have not considered MS subtype or excluded patients with progressive subtypes of MS. The largest studies to date on DMT discontinuation utilized the international MSBase global patient registry, which identified nearly 5,000 patients who discontinued interferons (73%), glatiramer acetate (18%), natalizumab (6%), or fingolimod (3%), without specifying the reasons for discontinuation.39 Despite these shortcomings, data reveal trends that are helpful in predicting how MS tends to behave in patients who have discontinued therapy. Not surprisingly, disability progression was most likely among patients already characterized as having a progressive phenotype, while relapses were less likely to occur among older, progressive patients.
Although clinicians may be increasingly willing to discuss DMT discontinuation with their patients, at least 1 study exploring patient perspectives on stopping treatment suggests widespread reluctance to stop treatment. A survey conducted with participants in the North American Research Committee on Multiple Sclerosis patient-report registry found that among survey respondents, only 11.9% would discontinue their MS medication if deemed stable, while 66.3% stated they were unlikely to stop treatment.40
These results suggest that before clinicians incorporate DMT discontinuation into the normal course of discussion with patients, they should be prepared to provide both education (on the wisdom of stopping under the right circumstances) and evidence to support when and how to make the recommendation. Based on existing evidence, criteria for recommending treatment discontinuation might include prolonged freedom from disease activity (≥ 5 years), age > 55 years or 60 years, and a progressive disease course. Thus far, no combination of factors has been shown to completely predict an event-free transition off of medicine. Since no fixed algorithm yet exists to guide DMT stoppage in MS, reasonable suggestions for monitoring patients might include surveillance MRIs, more frequent clinic visits, and possible transitional treatment for patients coming off of natalizumab or fingolimod, since these drugs have been associated with rebound disease activity when discontinued.41,42
Clinicians wishing to maximize function and quality of life for their patients at any age or stage of disease should look to nonpharmacologic interventions to lessen disability and maximize quality of life. While beyond the scope of this discussion, preliminary evidence suggests multimodal (aerobic, resistance, balance) exercise may enhance endurance and cognitive processing speed, and that treatment of comorbid diseases affecting vascular health benefits MS. 43
Conclusions
The development of numerous treatments for RRMS has established an entirely new landscape and disease course for those with MS. While this benefit has not entirely extended to those with progressive MS, those with active disease with superimposed relapses may receive limited benefit from these medications. New insights into the pathophysiology of progressive MS may lead us to new treatments through multiple alternative pathophysiologic pathways. Some early studies using this strategy show promise in slowing the progressive phase. Medication development for progressive MS faces multiple challenges due to lack of a single animal model demonstrating both pathology and clinical effects, absence of phase 1 surrogate biomarkers, and later phase trial endpoints that require large sample sizes and extended study durations. Nevertheless, the increase in number of trials and diversity of therapeutic approaches for progressive MS provides hope for effective therapy. Currently, the heterogeneity of the population with progressive MS requires an individualized treatment approach, and in some of these patients, stopping therapy may be a reasonable consideration. Symptomatic management remains critical for all patients with progressive MS as well as non-pharmacologic approaches that maximize quality of life.
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data [published correction appears in Neurology. 2019;93(15):688]. Neurology. 2019;92(10):e1029-e1040.
2. Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022-1024.
3. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
4. Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112(Pt 1):133-146. 5. Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129(Pt 3):595-605.
6. Tutuncu M, Tang J, Zeid NA, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler. 2013;19(2):188-198.
7. Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;122:552-568.
8. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
9. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
10. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
11. Montalban X, Hauser SL, Kappos L, et al; ORATORIO Clinical Investigators. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209-220.
12. Hawker K, O’Connor P, Freedman MS, et al; OLYMPUS trial group. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
13. Kappos L, Bar-Or A, Cree BAC, et al; EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study [published correction appears in Lancet. 2018;392(10160):2170]. Lancet. 2018;391(10127):1263-1273.
14. Lublin F, Miller DH, Freedman MS, et al; INFORMS study investigators. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial [published correction appears in Lancet. 2017;389(10066):254]. Lancet. 2016;387(10023):1075-1084.
15. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430-1438.
16. Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129(Pt 3):584-594.
17. Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain. 2010;133(Pt 7):1900–1913.
18. Kapoor R, Ho PR, Campbell N, et al; ASCEND investigators. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405-415.
19. Koch MW, Mostert J, Uitdehaag B, Cutter G. Clinical outcome measures in SPMS trials: an analysis of the IMPACT and ASCEND original trial data sets [published online ahead of print, 2019 Sep 13]. Mult Scler. 2019;1352458519876701.
20. Hartung HP, Gonsette R, König N, et al; Mitoxantrone in Multiple Sclerosis Study Group (MIMS). Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018-2025.
21. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet. 1998;352(9139):1491-1497.
22. Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid - biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849-858.
23. Chaudhary P, Marracci G, Pocius E, Galipeau D, Morris B, Bourdette D. Effects of lipoic acid on primary murine microglial cells. J Neuroimmunol. 2019;334:576972.
24. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4:e374.
25. Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383:2213-2221.
26. Fox RJ, Coffey CS, Conwit R, et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N Engl J Med. 2018;379:846-855.
27. Rinker JR, 2nd, Cossey TC, Cutter GR, Culpepper WJ. A retrospective review of lithium usage in veterans with multiple sclerosis. Mult Scler Relat Disord. 2013;2:327-333.
28. Rinker JR, W Meador, V Sung, A Nicholas, G Cutter. Results of a pilot trial of lithium in progressive multiple sclerosis. ECTRIMS Online Library. 09/16/16; 145965; P12822016.
29. Chataway J, De Angelis F, Connick P, et al; MS-SMART Investigators. Efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis (MS-SMART): a phase 2b, multiarm, double-blind, randomised placebo-controlled trial. Lancet Neurol. 2020;19(3):214-225.
30. Kapoor R, Furby J, Hayton T, et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:681-688.
31. Paz Soldan MM, Novotna M, Abou Zeid N, et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology. 2015;84:81-88.
32. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19:11-14.
33. Ruggieri S, Tortorella C, Gasperini C. Anti lingo 1 (opicinumab) a new monoclonal antibody tested in relapsing remitting multiple sclerosis. Expert Rev Neurother 2017;17:1081-1089.
34. Hartley MD, Banerji T, Tagge IJ, et al. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insight. 2019;4(8):e126329.
35. Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. 2018;166:230-238.
36. Kjolhede T, Siemonsen S, Wenzel D, et al. Can resistance training impact MRI outcomes in relapsing-remitting multiple sclerosis? Mult Scler. 2018;24:1356-1365.
37. US National Library of Medicine, Clinicaltrials.gov. Discontinuation of Disease Modifying Therapies (DMTs) in Multiple Sclerosis (MS) (DISCOMS). https://clinicaltrials.gov/ct2/show/NCT03073603. Updated February 10, 2020. Accessed March 26, 2020.
38. Bonenfant J, Bajeux E, Deburghgraeve V, Le Page E, Edan G, Kerbrat A. Can we stop immunomodulatory treatments in secondary progressive multiple sclerosis? Eur J Neurol. 2017;24:237-244.
39. Kister I, Spelman T, Patti F, et al. Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. J Neurol Sci. 2018;391:72-76.
40. McGinley MP, Cola PA, Fox RJ, Cohen JA, Corboy JJ, Miller D. Perspectives of individuals with multiple sclerosis on discontinuation of disease-modifying therapies. Mult Scler. 2019:1352458519867314.
41. Hatcher SE, Waubant E, Graves JS. Rebound Syndrome in Multiple Sclerosis After Fingolimod Cessation-Reply. JAMA Neurol. 2016;73:1376.
42. Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH. Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology. 2008;70:1150-1151.
43. Sandroff BM, Bollaert RE, Pilutti LA, et al. Multimodal exercise training in multiple sclerosis: A randomized controlled trial in persons with substantial mobility disability. Contemp Clin Trials 2017;61:39-47.
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system, with recent estimates of around 1 million people living with MS in the US.1 In many countries, MS is a leading cause of disability among young adults, second only to trauma.2 Clinically, neurologic worsening (ie, disability) in MS can occur in the relapsing-remitting (RRMS) phase of disease due to incomplete recovery from neuroinflammatory relapses. However, in the 15% of patients with a progressive course from onset (PPMS), and in those with RRMS who transition to a secondary progressive phenotype (SPMS), neurologic worsening follows a slowly progressive pattern.3 A progressive disease course—either PPMS at onset or transitioning to SPMS—is the dominant factor affecting MS-related neurologic disability accumulation. In particular, epidemiologic studies have shown that, in the absence of transitioning to a progressive disease course, < 5% of individuals with MS will accumulate sufficient disability to necessitate use of a cane for ambulation.4-6 Therefore, developing disease modifying therapies (DMTs) that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS represent a critical unmet need.
Research into the development of DMTs for progressive MS has been hindered by a number of factors. In particular, the clinical definition and diagnosis of progressive MS has been an evolving concept. Diagnostic criteria for MS, which help facilitate the enrollment of appropriate subjects into clinical trials, have only recently clarified the current consensus definition for progressive MS—steadily increasing neurologic disability independent of clinical relapses. Looking back to the Schumacher criteria in 1965 and Poser criteria in 1983, it was acknowledged that neurologic symptoms in MS may follow a relapsing-remitting or progressive pattern, but little attempt was made to define progressive MS.7,8 The original McDonald criteria in 2001 defined diagnostic criteria for progressive MS.9 These criteria continued to evolve through subsequent revisions (eg, cerebrospinal fluid [CSF] specific oligoclonal bands no longer are an absolute requirement), and only in the 2017 revision was it emphasized that disability progression must occur independent of clinical relapses, concordant with similar emphasis in the 2013 revision of MS clinical course definitions.3,10
The interpretation of prior clinical trials of DMT for progressive MS must consider this evolving clinical definition. The US Food and Drug Administration (FDA) approved mitoxantrone in 2000—making it the first DMT to carry an approved label for SPMS. While achieving significant clinical efficacy, it is clear from the details of the trial that the enrolled subjects had a high degree of inflammatory disease activity, which suggests that mitoxantrone treats neuroinflammation and not relapse-independent worsening. More recently, disparate results were seen in the anti-CD20 (rituximab, ocrelizumab) and S1P receptor modulator (fingolimod, siponimod) trials targeted at patients with primary and secondary progressive MS.11-14 Although there are differences between these therapies, they are more similar than not within the same therapeutic class. Taken together, these trials illustrate the critical impact the narrower inclusion/exclusion criteria (namely age and extent of inflammatory activity) had on attaining positive outcomes. Other considerations, such as confounding illness, also may impact trial recruitment and generalizability of findings.
The lack of known biological targets in progressive MS, which is a complex disease with an incompletely understood and heterogeneous pathology, also hinders DMT development. Decades of research has characterized multifocal central nervous system (CNS) lesions that exhibit features of demyelination, inflammation, reactive gliosis, axonal loss, and neuronal damage. Until recently, however, much of this research focused on the relapsing phase of disease, and so the understanding of the pathologic underpinnings of progressive disease has remained limited. Current areas of investigation encompass a broad range of pathological processes, such as microglial activation, meningeal lymphoid follicles, remyelination failure, vulnerability of chronically demyelinated axons, oxidative injury, iron accumulation, mitochondrial damage, and others. There is the added complication that the pathologic processes underlying progressive MS are superimposed on the CNS aging process. In particular, the transition to progressive MS and the rate of disability accumulation during progressive MS show strong correlation with age.6,15-17
Finally, DMT development for progressive MS also is hindered by the lack of specific surrogate and clinical outcome measures. Trials for relapsing MS have benefited greatly from the relatively straightforward assessment of clinical relapses and inflammatory disease activity on magnetic resonance imaging (MRI). With the goal of developing DMTs that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS, which by definition occurs independent of clinical relapses, these measures are not directly relevant. The longitudinal clinical disability outcome measures change at a slower rate than in early, relapsing disease. The use of standardized scales (eg, the Expanded Disability Status Scale [EDSS]), lower limb function, upper limb function, cognition, or a combination is a subject of ongoing debate. For example, the ASCEND and IMPACT trials (placebo-controlled trials for SPMS with natalizumab and interferon β-1a, respectively) showed no significant impact in EDSS progression—but in both of these trials, the 9-hole peg test (9-HPT), a performance measure for upper limb function, showed significant improvement.10,18 Particularly in those with an EDSS of > 6.5, who are unlikely to have measurable EDSS progression, functional tests such as the 9-HPT or timed 25-foot walk may be more sensitive as measures for disability progression.11 MRI measures of brain atrophy is the current gold standard surrogate outcome for clinical trials in progressive MS, but others that may warrant consideration include optical coherence tomography (OCT) or CSF markers of axonal degeneration.
DMT for Progressive MS
Current diagnostic nomenclature separates patients with active (superimposed clinical and/or radiographic relapses) from those with inactive disease.3,12 Relapsing forms of MS include all RRMS and those with SPMS with superimposed relapses (ie, active SPMS). Following this paradigm shift, the FDA changed the indication for already approved DMT from RRMS to relapsing forms of MS. Below is a discussion of DMT that specifically use the term SPMS and PPMS in the indication, where phase 3 trial data for progressive MS is available.
In 2019, the FDA approved the first oral medication (siponimod) for active SPMS. Subsequently, updates to the labels of the older DMT expanded to include active SPMS. Until 2019, the only FDA approved medication for SPMS was mitoxantrone, and use of this medication was limited due to unfavorable adverse effects (AEs). No medications had obtained FDA approval for inactive SPMS to this point, which represented an unmet need for a considerable number of patients.
Mitoxantrone became the first DMT approved for use in SPMS in 2000 following early trials that showed reductions in EDSS worsening, change in ambulation index, reduced number of treated relapses, and prolonged time to first treated relapse. However, as with some of the other positive trials in progressive MS, it is difficult to discern the impact of suppression of relapses as opposed to direct impact on progressive pathophysiology. Within the placebo arm, for example, there were 129 relapses among the 64 subjects, which suggests that these cases had particularly active disease or were in the early stages of SPMS.13 Furthermore, the use of this medication was limited due to concerns of cardiotoxicity and hematologic malignancy as serious AEs.
The trials of interferon β-1b illustrate the same difficulty of isolating possible benefits in disease progression from disability accumulated from relapses. The first interferon β-1b trial for SPMS, was conducted in Europe using fingolimod and showed a delay in confirmed disability progression compared to placebo as measured with the EDSS.14 However, a North American trial that followed in 2004 was unable to replicate this finding.15 The patients in the European trial appeared to be in an earlier phase of SPMS with more active disease, and a post-hoc pooled analysis suggested that patients with active disease and those with more pronounced disability progression were more likely to benefit from treatment.16 Overall, interferons do not appear to appreciably alter disability in the long-term for patients with SPMS, though they may modify short-term, relapse-related disability.
Perhaps the most encouraging data for SPMS was found in the EXPAND trial, which investigated siponimod, an S1P receptor modulator that is more selective than fingolimod. The trial identified a 21% reduction in 3-month confirmed disability progression for SPMS patients taking siponimod compared with those taking a placebo.17 Although the patients in EXPAND did seem to have relatively less disease activity at baseline than those who participated in other SPMS trials, those who benefitted from siponimod were primarily patients who had clinical and/or radiographic relapses over the prior 2 years. Based on this, the FDA approved siponimod for active SPMS. The extent to which siponimod exerts a true neuroprotective effect beyond reducing inflammation has not been clearly established.
B-cell depleting therapies rituximab and ocrelizumab have been evaluated in both primary and secondary progressive MS populations. Early investigations of the chimeric rituximab in PPMS did not show benefits on disability (EDSS) progression; however, benefits were seen in analysis of some subgroups.18 With this in mind, the ORATORIO trial for the humanized version, ocreluzimab, included PPMS patients that were younger (aged < 55 years) and had cutoffs for disease duration (< 15 years for those with EDSS more than 5 years, < 10 years for those with EDSS less than 5 years). The study showed statistically significant changes on disability progression, which led to ocrelizumab receiving the first FDA indication for PPMS.11 There are substantial pathophysiologic similarities between PPMS and SPMS in the progressive phase.19 While these medications may have similar effects across these disease processes, these benefits have not yet been demonstrated in a prospective trial for the SPMS population. Regardless, B-cell depleting therapy is a reasonable consideration for select patients with active SPMS, consistent with a relapsing form of MS.
Therapies in Development
DMT development for progressive MS is a high priority area. Current immunomodulatory therapies for RRMS have consistently been ineffective in the inactive forms of MS, with the possible exceptions of ocrelizumab and siponimod. Therefore, instead of immunosuppression, many agents currently in phase 2 and 3 clinical trials target alternative pathophysiological processes in order to provide neuroprotection, and/or promote remyelination and neurogenesis. These targets include oxidative stress (OS), non-T cell mediated inflammation, and mitochondrial/energy failure.20 Below we review a selection of clinical trials testing agents following these approaches. Many agents have more than one potential mechanism of action (MOA) that could benefit progressive MS.
Lipoic acid (LA), also known as α-lipoic acid and thiotic acid, is one such agent targeting alternative pathophysiology in SPMS. LA is an endogenous agent synthesized de novo from fatty acids and cysteine as well as obtained in small amounts from foods.21 It has antioxidant (AO) properties including direct radical scavenging, regeneration of glutathione, and upregulation of AO enzymes via the NrF2 pathway.22 It supports mitochondria as a key cofactor for pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, and it also aids mitochondrial DNA synthesis.21,22 Studies in experimental autoimmune encephalomyelitis, a widely used experimental mouse model of inflammatory demyelinating disease, also indicate a reduction in excessive microglial activation.23 A phase 2 pilot randomized controlled trial (RCT) of 1200 mg LA in SPMS (n = 51) resulted in significantly less whole brain atrophy by SIENA (Structural Image Evaluation, Using Normalization, of Atrophy) at 2 years.24 A follow-up multicenter trial is ongoing.
Simvastatin also targets alternative pathophysiology in SPMS. It has anti-inflammatory effects, improves vascular function, and promotes neuroprotection by reducing excitotoxicity. A phase 2 RCT demonstrated a reduction in whole brain atrophy in SPMS (n = 140), and a phase 3 trial is underway.25 Ibudilast is another repurposed drug that targets alternative inflammation by inhibiting several cyclic nucleotide phosphodiesterases, macrophage migration inhibitory factor and toll-like receptor 4. A phase 2 trial (n = 225) in both SPMS and PPMS also demonstrated a reduction in brain atrophy, but participants had high rates of AEs.26
Lithium and riluzole promote neuroprotection by reducing excitotoxicity. Lithium is a pharmacologic active cation used as a mood stabilizer and causes inhibition of glycogen synthase kinase-3β. Animal models also indicate that lithium may decrease inflammation and positively impact neurogenesis.27 A crossover pilot trial demonstrated tolerability with trends toward stabilization of EDSS and reductions in brain atrophy.28 Three neuroprotective agents, riluzole (reduces glutamate excitotoxicity), fluoxetine (stimulates glycogenolysis and improves mitochondrial energy production), and amiloride (an acid-sensing ion channel blocker that opens in response to inflammation) were tested in a phase 2b multi-arm, multi-site parallel group RCT in SPMS (n = 445). The study failed to yield differences from placebo for any agent in reduction of brain volume loss.29 A prior study of lamotrigine, a sodium channel blocker, also failed to find changes in brain volume loss.30 These studies highlight the large sample sizes and/or long study durations needed to test agents using brain atrophy as primary outcome. In the future, precise surrogate markers of neuroprotection will be a great need for earlier phase trials. These results also suggest that targeting > 1 MOA may be necessary to treat SPMS effectively.
Efforts to promote remyelination target one hallmark of MS damage. High dose biotin (about 10,000× usual dose) may promote myelin repair as a cofactor for fatty acid synthesis and support mitochondrial oxidative phosphorylation. While a RCT yielded a greater proportion of participants with either PPMS or SPMS with improvement in disability than placebo at 12 months, an open label trial suggested otherwise indicating a need for a more definitive trial.31,32
Anti-LINGO-1 (opicinumab) is a monoclonal antibody that targets LINGO, a potent negative regulator of oligodendrocyte differentiation and myelination.33 Although this agent failed in a phase 2 trial in relapsing MS, and is thus unlikely to be tested in progressive forms, the innovative approach to stimulating oligodendrocytes is ongoing. One such effort is to use thyroid hormone, crucial to myelin formation during development, as a repair agent in MS.34 A dose-finding study of thyroid hormone was completed and efforts to develop a thyromimetic agent are ongoing.
Finally, efforts to promote neurogenesis remain a goal for many neurodegenerative diseases. Exercise appears to prevent age-related atrophy of the hippocampus in animals and humans and help maintain neuronal health.35 In patients with RRMS, cortical thickness is impacted positively by resistance training, which suggests a neuroprotective effect.36 A multi-center trial of exercise in patients with progressive MS investigating cognitive outcomes is ongoing.
Discontinuing DMT
In the early 1990s, the successful development of immune modulating therapies that reliably reduced disease activity in RRMS led to widespread initiation in patients with relapsing disease. However, guidance on when or if to discontinue DMT, even in those who have transitioned to SPMS, remains largely absent at this time. Requests to discontinue DMT may come from patients weary of taking medication (especially injections), bothered by AEs, or those who no longer perceive efficacy from their treatments. Clinicians also may question the benefit of immune modulation in patients with longstanding freedom from relapses or changes in MRI lesion burden.
To inform discussion centered on treatment discontinuation, a clinical trial is currently underway to better answer the question of when and how to withdraw MS therapy. Discontinuation of Disease Modifying Therapies in Multiple Sclerosis (DISCO-MS) is a prospective, placebo-controlled RCT and its primary endpoint is recurrence of disease activity over a 2 year follow-up period.37 Eligibility requirements for the trial include age > 55 years, 5-year freedom from relapses, and 3-year freedom from new MRI lesions (criteria informed by progressive MS cohort studies).31 In addition to demonstrating the active disease recurrence rates in this patient population, the trial also aims to identify risk factors for recurrent disease activity among treated MS patients.37 DISCO-MS builds upon a series of retrospective and observational studies that partially answered these questions, albeit in the context of biases inherent in retrospective or observational studies.
A Minneapolis MS Treatment and Research Center single-center study identified 77 SPMS patients with no acute CNS inflammatory events over 2 to 20 years and advised these patients to stop taking DMT.32 In this group, 11.7% of subjects experienced recurrent active disease. Age was the primary discriminating factor. The mean age of those who experienced disease activity was 56 years vs 61 years those who did not. A second observational study from France found that among 100 SPMS patients treated either with interferon β or glatiramer acetate for at least 6 months, 35% experienced some form of inflammatory disease upon discontinuation.38 Sixteen patients relapsed and 19 developed gadolinium-enhancing MR lesions after DMT discontinuation. However, the age of the cohort was younger than the Minneapolis study (47.2 years vs 61 years), and reasons for discontinuation (eg, AEs or lack of disease activity) were not considered in the analysis.
Other studies examining the safety of DMT discontinuation have not considered MS subtype or excluded patients with progressive subtypes of MS. The largest studies to date on DMT discontinuation utilized the international MSBase global patient registry, which identified nearly 5,000 patients who discontinued interferons (73%), glatiramer acetate (18%), natalizumab (6%), or fingolimod (3%), without specifying the reasons for discontinuation.39 Despite these shortcomings, data reveal trends that are helpful in predicting how MS tends to behave in patients who have discontinued therapy. Not surprisingly, disability progression was most likely among patients already characterized as having a progressive phenotype, while relapses were less likely to occur among older, progressive patients.
Although clinicians may be increasingly willing to discuss DMT discontinuation with their patients, at least 1 study exploring patient perspectives on stopping treatment suggests widespread reluctance to stop treatment. A survey conducted with participants in the North American Research Committee on Multiple Sclerosis patient-report registry found that among survey respondents, only 11.9% would discontinue their MS medication if deemed stable, while 66.3% stated they were unlikely to stop treatment.40
These results suggest that before clinicians incorporate DMT discontinuation into the normal course of discussion with patients, they should be prepared to provide both education (on the wisdom of stopping under the right circumstances) and evidence to support when and how to make the recommendation. Based on existing evidence, criteria for recommending treatment discontinuation might include prolonged freedom from disease activity (≥ 5 years), age > 55 years or 60 years, and a progressive disease course. Thus far, no combination of factors has been shown to completely predict an event-free transition off of medicine. Since no fixed algorithm yet exists to guide DMT stoppage in MS, reasonable suggestions for monitoring patients might include surveillance MRIs, more frequent clinic visits, and possible transitional treatment for patients coming off of natalizumab or fingolimod, since these drugs have been associated with rebound disease activity when discontinued.41,42
Clinicians wishing to maximize function and quality of life for their patients at any age or stage of disease should look to nonpharmacologic interventions to lessen disability and maximize quality of life. While beyond the scope of this discussion, preliminary evidence suggests multimodal (aerobic, resistance, balance) exercise may enhance endurance and cognitive processing speed, and that treatment of comorbid diseases affecting vascular health benefits MS. 43
Conclusions
The development of numerous treatments for RRMS has established an entirely new landscape and disease course for those with MS. While this benefit has not entirely extended to those with progressive MS, those with active disease with superimposed relapses may receive limited benefit from these medications. New insights into the pathophysiology of progressive MS may lead us to new treatments through multiple alternative pathophysiologic pathways. Some early studies using this strategy show promise in slowing the progressive phase. Medication development for progressive MS faces multiple challenges due to lack of a single animal model demonstrating both pathology and clinical effects, absence of phase 1 surrogate biomarkers, and later phase trial endpoints that require large sample sizes and extended study durations. Nevertheless, the increase in number of trials and diversity of therapeutic approaches for progressive MS provides hope for effective therapy. Currently, the heterogeneity of the population with progressive MS requires an individualized treatment approach, and in some of these patients, stopping therapy may be a reasonable consideration. Symptomatic management remains critical for all patients with progressive MS as well as non-pharmacologic approaches that maximize quality of life.
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system, with recent estimates of around 1 million people living with MS in the US.1 In many countries, MS is a leading cause of disability among young adults, second only to trauma.2 Clinically, neurologic worsening (ie, disability) in MS can occur in the relapsing-remitting (RRMS) phase of disease due to incomplete recovery from neuroinflammatory relapses. However, in the 15% of patients with a progressive course from onset (PPMS), and in those with RRMS who transition to a secondary progressive phenotype (SPMS), neurologic worsening follows a slowly progressive pattern.3 A progressive disease course—either PPMS at onset or transitioning to SPMS—is the dominant factor affecting MS-related neurologic disability accumulation. In particular, epidemiologic studies have shown that, in the absence of transitioning to a progressive disease course, < 5% of individuals with MS will accumulate sufficient disability to necessitate use of a cane for ambulation.4-6 Therefore, developing disease modifying therapies (DMTs) that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS represent a critical unmet need.
Research into the development of DMTs for progressive MS has been hindered by a number of factors. In particular, the clinical definition and diagnosis of progressive MS has been an evolving concept. Diagnostic criteria for MS, which help facilitate the enrollment of appropriate subjects into clinical trials, have only recently clarified the current consensus definition for progressive MS—steadily increasing neurologic disability independent of clinical relapses. Looking back to the Schumacher criteria in 1965 and Poser criteria in 1983, it was acknowledged that neurologic symptoms in MS may follow a relapsing-remitting or progressive pattern, but little attempt was made to define progressive MS.7,8 The original McDonald criteria in 2001 defined diagnostic criteria for progressive MS.9 These criteria continued to evolve through subsequent revisions (eg, cerebrospinal fluid [CSF] specific oligoclonal bands no longer are an absolute requirement), and only in the 2017 revision was it emphasized that disability progression must occur independent of clinical relapses, concordant with similar emphasis in the 2013 revision of MS clinical course definitions.3,10
The interpretation of prior clinical trials of DMT for progressive MS must consider this evolving clinical definition. The US Food and Drug Administration (FDA) approved mitoxantrone in 2000—making it the first DMT to carry an approved label for SPMS. While achieving significant clinical efficacy, it is clear from the details of the trial that the enrolled subjects had a high degree of inflammatory disease activity, which suggests that mitoxantrone treats neuroinflammation and not relapse-independent worsening. More recently, disparate results were seen in the anti-CD20 (rituximab, ocrelizumab) and S1P receptor modulator (fingolimod, siponimod) trials targeted at patients with primary and secondary progressive MS.11-14 Although there are differences between these therapies, they are more similar than not within the same therapeutic class. Taken together, these trials illustrate the critical impact the narrower inclusion/exclusion criteria (namely age and extent of inflammatory activity) had on attaining positive outcomes. Other considerations, such as confounding illness, also may impact trial recruitment and generalizability of findings.
The lack of known biological targets in progressive MS, which is a complex disease with an incompletely understood and heterogeneous pathology, also hinders DMT development. Decades of research has characterized multifocal central nervous system (CNS) lesions that exhibit features of demyelination, inflammation, reactive gliosis, axonal loss, and neuronal damage. Until recently, however, much of this research focused on the relapsing phase of disease, and so the understanding of the pathologic underpinnings of progressive disease has remained limited. Current areas of investigation encompass a broad range of pathological processes, such as microglial activation, meningeal lymphoid follicles, remyelination failure, vulnerability of chronically demyelinated axons, oxidative injury, iron accumulation, mitochondrial damage, and others. There is the added complication that the pathologic processes underlying progressive MS are superimposed on the CNS aging process. In particular, the transition to progressive MS and the rate of disability accumulation during progressive MS show strong correlation with age.6,15-17
Finally, DMT development for progressive MS also is hindered by the lack of specific surrogate and clinical outcome measures. Trials for relapsing MS have benefited greatly from the relatively straightforward assessment of clinical relapses and inflammatory disease activity on magnetic resonance imaging (MRI). With the goal of developing DMTs that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS, which by definition occurs independent of clinical relapses, these measures are not directly relevant. The longitudinal clinical disability outcome measures change at a slower rate than in early, relapsing disease. The use of standardized scales (eg, the Expanded Disability Status Scale [EDSS]), lower limb function, upper limb function, cognition, or a combination is a subject of ongoing debate. For example, the ASCEND and IMPACT trials (placebo-controlled trials for SPMS with natalizumab and interferon β-1a, respectively) showed no significant impact in EDSS progression—but in both of these trials, the 9-hole peg test (9-HPT), a performance measure for upper limb function, showed significant improvement.10,18 Particularly in those with an EDSS of > 6.5, who are unlikely to have measurable EDSS progression, functional tests such as the 9-HPT or timed 25-foot walk may be more sensitive as measures for disability progression.11 MRI measures of brain atrophy is the current gold standard surrogate outcome for clinical trials in progressive MS, but others that may warrant consideration include optical coherence tomography (OCT) or CSF markers of axonal degeneration.
DMT for Progressive MS
Current diagnostic nomenclature separates patients with active (superimposed clinical and/or radiographic relapses) from those with inactive disease.3,12 Relapsing forms of MS include all RRMS and those with SPMS with superimposed relapses (ie, active SPMS). Following this paradigm shift, the FDA changed the indication for already approved DMT from RRMS to relapsing forms of MS. Below is a discussion of DMT that specifically use the term SPMS and PPMS in the indication, where phase 3 trial data for progressive MS is available.
In 2019, the FDA approved the first oral medication (siponimod) for active SPMS. Subsequently, updates to the labels of the older DMT expanded to include active SPMS. Until 2019, the only FDA approved medication for SPMS was mitoxantrone, and use of this medication was limited due to unfavorable adverse effects (AEs). No medications had obtained FDA approval for inactive SPMS to this point, which represented an unmet need for a considerable number of patients.
Mitoxantrone became the first DMT approved for use in SPMS in 2000 following early trials that showed reductions in EDSS worsening, change in ambulation index, reduced number of treated relapses, and prolonged time to first treated relapse. However, as with some of the other positive trials in progressive MS, it is difficult to discern the impact of suppression of relapses as opposed to direct impact on progressive pathophysiology. Within the placebo arm, for example, there were 129 relapses among the 64 subjects, which suggests that these cases had particularly active disease or were in the early stages of SPMS.13 Furthermore, the use of this medication was limited due to concerns of cardiotoxicity and hematologic malignancy as serious AEs.
The trials of interferon β-1b illustrate the same difficulty of isolating possible benefits in disease progression from disability accumulated from relapses. The first interferon β-1b trial for SPMS, was conducted in Europe using fingolimod and showed a delay in confirmed disability progression compared to placebo as measured with the EDSS.14 However, a North American trial that followed in 2004 was unable to replicate this finding.15 The patients in the European trial appeared to be in an earlier phase of SPMS with more active disease, and a post-hoc pooled analysis suggested that patients with active disease and those with more pronounced disability progression were more likely to benefit from treatment.16 Overall, interferons do not appear to appreciably alter disability in the long-term for patients with SPMS, though they may modify short-term, relapse-related disability.
Perhaps the most encouraging data for SPMS was found in the EXPAND trial, which investigated siponimod, an S1P receptor modulator that is more selective than fingolimod. The trial identified a 21% reduction in 3-month confirmed disability progression for SPMS patients taking siponimod compared with those taking a placebo.17 Although the patients in EXPAND did seem to have relatively less disease activity at baseline than those who participated in other SPMS trials, those who benefitted from siponimod were primarily patients who had clinical and/or radiographic relapses over the prior 2 years. Based on this, the FDA approved siponimod for active SPMS. The extent to which siponimod exerts a true neuroprotective effect beyond reducing inflammation has not been clearly established.
B-cell depleting therapies rituximab and ocrelizumab have been evaluated in both primary and secondary progressive MS populations. Early investigations of the chimeric rituximab in PPMS did not show benefits on disability (EDSS) progression; however, benefits were seen in analysis of some subgroups.18 With this in mind, the ORATORIO trial for the humanized version, ocreluzimab, included PPMS patients that were younger (aged < 55 years) and had cutoffs for disease duration (< 15 years for those with EDSS more than 5 years, < 10 years for those with EDSS less than 5 years). The study showed statistically significant changes on disability progression, which led to ocrelizumab receiving the first FDA indication for PPMS.11 There are substantial pathophysiologic similarities between PPMS and SPMS in the progressive phase.19 While these medications may have similar effects across these disease processes, these benefits have not yet been demonstrated in a prospective trial for the SPMS population. Regardless, B-cell depleting therapy is a reasonable consideration for select patients with active SPMS, consistent with a relapsing form of MS.
Therapies in Development
DMT development for progressive MS is a high priority area. Current immunomodulatory therapies for RRMS have consistently been ineffective in the inactive forms of MS, with the possible exceptions of ocrelizumab and siponimod. Therefore, instead of immunosuppression, many agents currently in phase 2 and 3 clinical trials target alternative pathophysiological processes in order to provide neuroprotection, and/or promote remyelination and neurogenesis. These targets include oxidative stress (OS), non-T cell mediated inflammation, and mitochondrial/energy failure.20 Below we review a selection of clinical trials testing agents following these approaches. Many agents have more than one potential mechanism of action (MOA) that could benefit progressive MS.
Lipoic acid (LA), also known as α-lipoic acid and thiotic acid, is one such agent targeting alternative pathophysiology in SPMS. LA is an endogenous agent synthesized de novo from fatty acids and cysteine as well as obtained in small amounts from foods.21 It has antioxidant (AO) properties including direct radical scavenging, regeneration of glutathione, and upregulation of AO enzymes via the NrF2 pathway.22 It supports mitochondria as a key cofactor for pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, and it also aids mitochondrial DNA synthesis.21,22 Studies in experimental autoimmune encephalomyelitis, a widely used experimental mouse model of inflammatory demyelinating disease, also indicate a reduction in excessive microglial activation.23 A phase 2 pilot randomized controlled trial (RCT) of 1200 mg LA in SPMS (n = 51) resulted in significantly less whole brain atrophy by SIENA (Structural Image Evaluation, Using Normalization, of Atrophy) at 2 years.24 A follow-up multicenter trial is ongoing.
Simvastatin also targets alternative pathophysiology in SPMS. It has anti-inflammatory effects, improves vascular function, and promotes neuroprotection by reducing excitotoxicity. A phase 2 RCT demonstrated a reduction in whole brain atrophy in SPMS (n = 140), and a phase 3 trial is underway.25 Ibudilast is another repurposed drug that targets alternative inflammation by inhibiting several cyclic nucleotide phosphodiesterases, macrophage migration inhibitory factor and toll-like receptor 4. A phase 2 trial (n = 225) in both SPMS and PPMS also demonstrated a reduction in brain atrophy, but participants had high rates of AEs.26
Lithium and riluzole promote neuroprotection by reducing excitotoxicity. Lithium is a pharmacologic active cation used as a mood stabilizer and causes inhibition of glycogen synthase kinase-3β. Animal models also indicate that lithium may decrease inflammation and positively impact neurogenesis.27 A crossover pilot trial demonstrated tolerability with trends toward stabilization of EDSS and reductions in brain atrophy.28 Three neuroprotective agents, riluzole (reduces glutamate excitotoxicity), fluoxetine (stimulates glycogenolysis and improves mitochondrial energy production), and amiloride (an acid-sensing ion channel blocker that opens in response to inflammation) were tested in a phase 2b multi-arm, multi-site parallel group RCT in SPMS (n = 445). The study failed to yield differences from placebo for any agent in reduction of brain volume loss.29 A prior study of lamotrigine, a sodium channel blocker, also failed to find changes in brain volume loss.30 These studies highlight the large sample sizes and/or long study durations needed to test agents using brain atrophy as primary outcome. In the future, precise surrogate markers of neuroprotection will be a great need for earlier phase trials. These results also suggest that targeting > 1 MOA may be necessary to treat SPMS effectively.
Efforts to promote remyelination target one hallmark of MS damage. High dose biotin (about 10,000× usual dose) may promote myelin repair as a cofactor for fatty acid synthesis and support mitochondrial oxidative phosphorylation. While a RCT yielded a greater proportion of participants with either PPMS or SPMS with improvement in disability than placebo at 12 months, an open label trial suggested otherwise indicating a need for a more definitive trial.31,32
Anti-LINGO-1 (opicinumab) is a monoclonal antibody that targets LINGO, a potent negative regulator of oligodendrocyte differentiation and myelination.33 Although this agent failed in a phase 2 trial in relapsing MS, and is thus unlikely to be tested in progressive forms, the innovative approach to stimulating oligodendrocytes is ongoing. One such effort is to use thyroid hormone, crucial to myelin formation during development, as a repair agent in MS.34 A dose-finding study of thyroid hormone was completed and efforts to develop a thyromimetic agent are ongoing.
Finally, efforts to promote neurogenesis remain a goal for many neurodegenerative diseases. Exercise appears to prevent age-related atrophy of the hippocampus in animals and humans and help maintain neuronal health.35 In patients with RRMS, cortical thickness is impacted positively by resistance training, which suggests a neuroprotective effect.36 A multi-center trial of exercise in patients with progressive MS investigating cognitive outcomes is ongoing.
Discontinuing DMT
In the early 1990s, the successful development of immune modulating therapies that reliably reduced disease activity in RRMS led to widespread initiation in patients with relapsing disease. However, guidance on when or if to discontinue DMT, even in those who have transitioned to SPMS, remains largely absent at this time. Requests to discontinue DMT may come from patients weary of taking medication (especially injections), bothered by AEs, or those who no longer perceive efficacy from their treatments. Clinicians also may question the benefit of immune modulation in patients with longstanding freedom from relapses or changes in MRI lesion burden.
To inform discussion centered on treatment discontinuation, a clinical trial is currently underway to better answer the question of when and how to withdraw MS therapy. Discontinuation of Disease Modifying Therapies in Multiple Sclerosis (DISCO-MS) is a prospective, placebo-controlled RCT and its primary endpoint is recurrence of disease activity over a 2 year follow-up period.37 Eligibility requirements for the trial include age > 55 years, 5-year freedom from relapses, and 3-year freedom from new MRI lesions (criteria informed by progressive MS cohort studies).31 In addition to demonstrating the active disease recurrence rates in this patient population, the trial also aims to identify risk factors for recurrent disease activity among treated MS patients.37 DISCO-MS builds upon a series of retrospective and observational studies that partially answered these questions, albeit in the context of biases inherent in retrospective or observational studies.
A Minneapolis MS Treatment and Research Center single-center study identified 77 SPMS patients with no acute CNS inflammatory events over 2 to 20 years and advised these patients to stop taking DMT.32 In this group, 11.7% of subjects experienced recurrent active disease. Age was the primary discriminating factor. The mean age of those who experienced disease activity was 56 years vs 61 years those who did not. A second observational study from France found that among 100 SPMS patients treated either with interferon β or glatiramer acetate for at least 6 months, 35% experienced some form of inflammatory disease upon discontinuation.38 Sixteen patients relapsed and 19 developed gadolinium-enhancing MR lesions after DMT discontinuation. However, the age of the cohort was younger than the Minneapolis study (47.2 years vs 61 years), and reasons for discontinuation (eg, AEs or lack of disease activity) were not considered in the analysis.
Other studies examining the safety of DMT discontinuation have not considered MS subtype or excluded patients with progressive subtypes of MS. The largest studies to date on DMT discontinuation utilized the international MSBase global patient registry, which identified nearly 5,000 patients who discontinued interferons (73%), glatiramer acetate (18%), natalizumab (6%), or fingolimod (3%), without specifying the reasons for discontinuation.39 Despite these shortcomings, data reveal trends that are helpful in predicting how MS tends to behave in patients who have discontinued therapy. Not surprisingly, disability progression was most likely among patients already characterized as having a progressive phenotype, while relapses were less likely to occur among older, progressive patients.
Although clinicians may be increasingly willing to discuss DMT discontinuation with their patients, at least 1 study exploring patient perspectives on stopping treatment suggests widespread reluctance to stop treatment. A survey conducted with participants in the North American Research Committee on Multiple Sclerosis patient-report registry found that among survey respondents, only 11.9% would discontinue their MS medication if deemed stable, while 66.3% stated they were unlikely to stop treatment.40
These results suggest that before clinicians incorporate DMT discontinuation into the normal course of discussion with patients, they should be prepared to provide both education (on the wisdom of stopping under the right circumstances) and evidence to support when and how to make the recommendation. Based on existing evidence, criteria for recommending treatment discontinuation might include prolonged freedom from disease activity (≥ 5 years), age > 55 years or 60 years, and a progressive disease course. Thus far, no combination of factors has been shown to completely predict an event-free transition off of medicine. Since no fixed algorithm yet exists to guide DMT stoppage in MS, reasonable suggestions for monitoring patients might include surveillance MRIs, more frequent clinic visits, and possible transitional treatment for patients coming off of natalizumab or fingolimod, since these drugs have been associated with rebound disease activity when discontinued.41,42
Clinicians wishing to maximize function and quality of life for their patients at any age or stage of disease should look to nonpharmacologic interventions to lessen disability and maximize quality of life. While beyond the scope of this discussion, preliminary evidence suggests multimodal (aerobic, resistance, balance) exercise may enhance endurance and cognitive processing speed, and that treatment of comorbid diseases affecting vascular health benefits MS. 43
Conclusions
The development of numerous treatments for RRMS has established an entirely new landscape and disease course for those with MS. While this benefit has not entirely extended to those with progressive MS, those with active disease with superimposed relapses may receive limited benefit from these medications. New insights into the pathophysiology of progressive MS may lead us to new treatments through multiple alternative pathophysiologic pathways. Some early studies using this strategy show promise in slowing the progressive phase. Medication development for progressive MS faces multiple challenges due to lack of a single animal model demonstrating both pathology and clinical effects, absence of phase 1 surrogate biomarkers, and later phase trial endpoints that require large sample sizes and extended study durations. Nevertheless, the increase in number of trials and diversity of therapeutic approaches for progressive MS provides hope for effective therapy. Currently, the heterogeneity of the population with progressive MS requires an individualized treatment approach, and in some of these patients, stopping therapy may be a reasonable consideration. Symptomatic management remains critical for all patients with progressive MS as well as non-pharmacologic approaches that maximize quality of life.
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data [published correction appears in Neurology. 2019;93(15):688]. Neurology. 2019;92(10):e1029-e1040.
2. Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022-1024.
3. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
4. Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112(Pt 1):133-146. 5. Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129(Pt 3):595-605.
6. Tutuncu M, Tang J, Zeid NA, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler. 2013;19(2):188-198.
7. Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;122:552-568.
8. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
9. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
10. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
11. Montalban X, Hauser SL, Kappos L, et al; ORATORIO Clinical Investigators. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209-220.
12. Hawker K, O’Connor P, Freedman MS, et al; OLYMPUS trial group. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
13. Kappos L, Bar-Or A, Cree BAC, et al; EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study [published correction appears in Lancet. 2018;392(10160):2170]. Lancet. 2018;391(10127):1263-1273.
14. Lublin F, Miller DH, Freedman MS, et al; INFORMS study investigators. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial [published correction appears in Lancet. 2017;389(10066):254]. Lancet. 2016;387(10023):1075-1084.
15. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430-1438.
16. Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129(Pt 3):584-594.
17. Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain. 2010;133(Pt 7):1900–1913.
18. Kapoor R, Ho PR, Campbell N, et al; ASCEND investigators. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405-415.
19. Koch MW, Mostert J, Uitdehaag B, Cutter G. Clinical outcome measures in SPMS trials: an analysis of the IMPACT and ASCEND original trial data sets [published online ahead of print, 2019 Sep 13]. Mult Scler. 2019;1352458519876701.
20. Hartung HP, Gonsette R, König N, et al; Mitoxantrone in Multiple Sclerosis Study Group (MIMS). Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018-2025.
21. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet. 1998;352(9139):1491-1497.
22. Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid - biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849-858.
23. Chaudhary P, Marracci G, Pocius E, Galipeau D, Morris B, Bourdette D. Effects of lipoic acid on primary murine microglial cells. J Neuroimmunol. 2019;334:576972.
24. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4:e374.
25. Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383:2213-2221.
26. Fox RJ, Coffey CS, Conwit R, et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N Engl J Med. 2018;379:846-855.
27. Rinker JR, 2nd, Cossey TC, Cutter GR, Culpepper WJ. A retrospective review of lithium usage in veterans with multiple sclerosis. Mult Scler Relat Disord. 2013;2:327-333.
28. Rinker JR, W Meador, V Sung, A Nicholas, G Cutter. Results of a pilot trial of lithium in progressive multiple sclerosis. ECTRIMS Online Library. 09/16/16; 145965; P12822016.
29. Chataway J, De Angelis F, Connick P, et al; MS-SMART Investigators. Efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis (MS-SMART): a phase 2b, multiarm, double-blind, randomised placebo-controlled trial. Lancet Neurol. 2020;19(3):214-225.
30. Kapoor R, Furby J, Hayton T, et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:681-688.
31. Paz Soldan MM, Novotna M, Abou Zeid N, et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology. 2015;84:81-88.
32. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19:11-14.
33. Ruggieri S, Tortorella C, Gasperini C. Anti lingo 1 (opicinumab) a new monoclonal antibody tested in relapsing remitting multiple sclerosis. Expert Rev Neurother 2017;17:1081-1089.
34. Hartley MD, Banerji T, Tagge IJ, et al. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insight. 2019;4(8):e126329.
35. Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. 2018;166:230-238.
36. Kjolhede T, Siemonsen S, Wenzel D, et al. Can resistance training impact MRI outcomes in relapsing-remitting multiple sclerosis? Mult Scler. 2018;24:1356-1365.
37. US National Library of Medicine, Clinicaltrials.gov. Discontinuation of Disease Modifying Therapies (DMTs) in Multiple Sclerosis (MS) (DISCOMS). https://clinicaltrials.gov/ct2/show/NCT03073603. Updated February 10, 2020. Accessed March 26, 2020.
38. Bonenfant J, Bajeux E, Deburghgraeve V, Le Page E, Edan G, Kerbrat A. Can we stop immunomodulatory treatments in secondary progressive multiple sclerosis? Eur J Neurol. 2017;24:237-244.
39. Kister I, Spelman T, Patti F, et al. Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. J Neurol Sci. 2018;391:72-76.
40. McGinley MP, Cola PA, Fox RJ, Cohen JA, Corboy JJ, Miller D. Perspectives of individuals with multiple sclerosis on discontinuation of disease-modifying therapies. Mult Scler. 2019:1352458519867314.
41. Hatcher SE, Waubant E, Graves JS. Rebound Syndrome in Multiple Sclerosis After Fingolimod Cessation-Reply. JAMA Neurol. 2016;73:1376.
42. Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH. Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology. 2008;70:1150-1151.
43. Sandroff BM, Bollaert RE, Pilutti LA, et al. Multimodal exercise training in multiple sclerosis: A randomized controlled trial in persons with substantial mobility disability. Contemp Clin Trials 2017;61:39-47.
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data [published correction appears in Neurology. 2019;93(15):688]. Neurology. 2019;92(10):e1029-e1040.
2. Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022-1024.
3. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
4. Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112(Pt 1):133-146. 5. Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129(Pt 3):595-605.
6. Tutuncu M, Tang J, Zeid NA, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler. 2013;19(2):188-198.
7. Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;122:552-568.
8. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
9. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
10. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
11. Montalban X, Hauser SL, Kappos L, et al; ORATORIO Clinical Investigators. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209-220.
12. Hawker K, O’Connor P, Freedman MS, et al; OLYMPUS trial group. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
13. Kappos L, Bar-Or A, Cree BAC, et al; EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study [published correction appears in Lancet. 2018;392(10160):2170]. Lancet. 2018;391(10127):1263-1273.
14. Lublin F, Miller DH, Freedman MS, et al; INFORMS study investigators. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial [published correction appears in Lancet. 2017;389(10066):254]. Lancet. 2016;387(10023):1075-1084.
15. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430-1438.
16. Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129(Pt 3):584-594.
17. Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain. 2010;133(Pt 7):1900–1913.
18. Kapoor R, Ho PR, Campbell N, et al; ASCEND investigators. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405-415.
19. Koch MW, Mostert J, Uitdehaag B, Cutter G. Clinical outcome measures in SPMS trials: an analysis of the IMPACT and ASCEND original trial data sets [published online ahead of print, 2019 Sep 13]. Mult Scler. 2019;1352458519876701.
20. Hartung HP, Gonsette R, König N, et al; Mitoxantrone in Multiple Sclerosis Study Group (MIMS). Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018-2025.
21. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet. 1998;352(9139):1491-1497.
22. Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid - biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849-858.
23. Chaudhary P, Marracci G, Pocius E, Galipeau D, Morris B, Bourdette D. Effects of lipoic acid on primary murine microglial cells. J Neuroimmunol. 2019;334:576972.
24. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4:e374.
25. Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383:2213-2221.
26. Fox RJ, Coffey CS, Conwit R, et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N Engl J Med. 2018;379:846-855.
27. Rinker JR, 2nd, Cossey TC, Cutter GR, Culpepper WJ. A retrospective review of lithium usage in veterans with multiple sclerosis. Mult Scler Relat Disord. 2013;2:327-333.
28. Rinker JR, W Meador, V Sung, A Nicholas, G Cutter. Results of a pilot trial of lithium in progressive multiple sclerosis. ECTRIMS Online Library. 09/16/16; 145965; P12822016.
29. Chataway J, De Angelis F, Connick P, et al; MS-SMART Investigators. Efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis (MS-SMART): a phase 2b, multiarm, double-blind, randomised placebo-controlled trial. Lancet Neurol. 2020;19(3):214-225.
30. Kapoor R, Furby J, Hayton T, et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:681-688.
31. Paz Soldan MM, Novotna M, Abou Zeid N, et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology. 2015;84:81-88.
32. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19:11-14.
33. Ruggieri S, Tortorella C, Gasperini C. Anti lingo 1 (opicinumab) a new monoclonal antibody tested in relapsing remitting multiple sclerosis. Expert Rev Neurother 2017;17:1081-1089.
34. Hartley MD, Banerji T, Tagge IJ, et al. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insight. 2019;4(8):e126329.
35. Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. 2018;166:230-238.
36. Kjolhede T, Siemonsen S, Wenzel D, et al. Can resistance training impact MRI outcomes in relapsing-remitting multiple sclerosis? Mult Scler. 2018;24:1356-1365.
37. US National Library of Medicine, Clinicaltrials.gov. Discontinuation of Disease Modifying Therapies (DMTs) in Multiple Sclerosis (MS) (DISCOMS). https://clinicaltrials.gov/ct2/show/NCT03073603. Updated February 10, 2020. Accessed March 26, 2020.
38. Bonenfant J, Bajeux E, Deburghgraeve V, Le Page E, Edan G, Kerbrat A. Can we stop immunomodulatory treatments in secondary progressive multiple sclerosis? Eur J Neurol. 2017;24:237-244.
39. Kister I, Spelman T, Patti F, et al. Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. J Neurol Sci. 2018;391:72-76.
40. McGinley MP, Cola PA, Fox RJ, Cohen JA, Corboy JJ, Miller D. Perspectives of individuals with multiple sclerosis on discontinuation of disease-modifying therapies. Mult Scler. 2019:1352458519867314.
41. Hatcher SE, Waubant E, Graves JS. Rebound Syndrome in Multiple Sclerosis After Fingolimod Cessation-Reply. JAMA Neurol. 2016;73:1376.
42. Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH. Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology. 2008;70:1150-1151.
43. Sandroff BM, Bollaert RE, Pilutti LA, et al. Multimodal exercise training in multiple sclerosis: A randomized controlled trial in persons with substantial mobility disability. Contemp Clin Trials 2017;61:39-47.
Multiple Sclerosis Medications in the VHA: Delivering Specialty, High-Cost, Pharmacy Care in a National System (FULL)
Prior to the first approved disease modifying therapy (DMT) in the 1990s, treatment approaches for multiple sclerosis (MS) were not well understood. The discovery that MS was an immune mediated inflammatory disease paved the way for the treatments we know today. In 1993, interferon β‐1b became the first DMT for MS approved by the US Food and Drug Administration (FDA). Approvals for interferon β‐1a as well as glatiramer acetate (GA) soon followed. Today, we consider these the mildest immunosuppressant DMTs; however, their success verified that suppressing the immune system had a positive effect on the MS disease process.
Following these approvals, the disease process in MS is now better understood. Recently approved therapies include monoclonal antibodies, which affect other immune pathways. Today, there are 14 approved DMTs (Table 1). Although the advent of these newer DMTs has revolutionized care for patients with MS, it has been accompanied by increasing costs for the agents. Direct medical costs associated with MS management, coupled with indirect costs from lost productivity, have been estimated to be $24.2 billion annually in the US.1 These increases have been seen across many levels of insurance coverage—private payer, Medicare, and the Veterans Health Administration (VHA).2,3
The Figure demonstrates the cost increase that have been seen across VHA between 2004 and 2019 for the DMTs identified in Table 1. Indeed, this compound annual growth rate may be an underestimate because infusion therapies (eg, natalizumab, ocrelizumab, and alemtuzumab) are difficult to track as they may be dispensed directly via a Risk Evaluation Medication Strategy (REMS) program. According to the VHA Pharmacy Benefit Management Service (PBM), in September 2019, dimethyl fumarate (DMF) had the 13th highest total outpatient drug cost for the US Department of Veterans Affairs (VA), interferon β‐1a ranked 62nd and 83rd (prefilled pen and syringe, respectively), and GA 40 mg ranked 89th.
The DMT landscape has demonstrated significant price fluctuations and given rise to a class of medications that requires extensive oversight in terms of efficacy, safety, and cost minimization. The purpose of this article is to show how delivery of this specialty group of medications can be optimized with safety, efficacy, and cost value within a large health care system.
Factors Impacting DMT Use
Recent changes to MS typing have impacted utilization of DMTs. Traditionally, there were 4 subtypes of MS: relapsing remitting (RRMS), secondary progressive (SPMS), progressive relapsing (PRMS), and primary progressive (PPMS). These subtypes are now viewed more broadly and grouped as either relapsing or progressive. The traditional subtypes fall under these broader definitions. Additionally, SPMS has been broken into active SPMS, characterized by continued worsening of disability unrelated to acute relapses, superimposed with activity that can be seen on magnetic resonance images (MRIs), and nonactive SPMS, which has the same disability progression as active SPMS but without MRI-visible activity.4-6 In 2019, these supplementary designations to SPMS made their first appearance in FDA-approved indications. All existing DMTs now include this terminology in their labelling and are indicated in active SPMS. There remain no DMTs that treat nonactive SPMS.
The current landscape of DMTs is highly varied in method of administration, risks, and benefits. As efficacy of these medications often is marked by how well they can prevent the immune system from attacking myelin, an inverse relationship between safety and efficacy results. The standard treatment outcomes in MS have evolved over time. The following are the commonly used primary outcomes in clinical trials: relapse reduction; increased time between relapses; decreased severity of relapses; prevention or extend time to disability milestones as measured by the Expanded Disability Status Scale (EDSS) and other disability measures; prevention or extension of time to onset of secondary progressive disease; prevention or reduction of the number and size of new and enhancing lesions on MRI; and limitation of overall MRI lesion burden in the central nervous system (CNS).
Newer treatment outcomes employed in more recent trials include: measures of axonal damage, CNS atrophy, evidence of microscopic disease via conventional MRI and advanced imaging modalities, biomarkers associated with inflammatory disease activity and neurodegeneration in MS, and the use of no evidence of disease activity (NEDA). These outcomes also must be evaluated by the safety concerns of each agent. Short- and long-term safety are critical factors in the selection of DMTs for MS. The injectable therapies for MS (interferon β‐1a, interferon β‐1b, and GA) have established long-term safety profiles from > 20 years of continuous use. The long-term safety profiles of oral immunomodulatory agents and monoclonal antibodies for these drugs in MS have yet to be determined. Safety concerns associated with some therapies and added requirements for safety monitoring may increase the complexity of a therapeutic selection.
Current cost minimization strategies for DMT include limiting DMT agents on formularies, tier systems that incentivize patients/prescribers to select the lowest priced agents on the formulary, negotiating arrangements with manufacturers to freeze prices or provide discounts in exchange for a priority position in the formulary, and requiring prior authorization to initiate or switch therapy. The use of generic medications and interchange to these agents from a brand name formulation can help reduce expense. Several of these strategies have been implemented in VHA.
Disease-Modifying Therapies
In 2019, 18,645 veterans with MS had either a MS-specific DMT or ≥ 1 annual encounters with a primary diagnosis of MS. Of this population, 4,720 were female and 13,357 were service connected according to VA data. About 50% of veterans with MS take a DMT. This percentage has remained stable over the past decade (Table 2). Although it appears the number of unique veterans prescribed an outpatient DMT is decreasing, this does not include the growing use of infused DMTs or DMTs obtained through the Veterans Choice Program (VCP)/Community Care (CC).
The overall outpatient pharmacy costs for veterans have remained constant despite the reduction in outpatient pharmacy prescription numbers. This may be due to increases in DMT cost to the VHA and the use of more expensive oral agents over the previously used platform injection DMTs.
Generic Conversion
GA is available in 20 mg daily and 40 mg3 times weekly subcutaneous injection dosing. The first evidence of clinical efficacy for a generic formulation for GA was evaluated by the GATE trial.7 This trial was a multicenter, randomized, double-blind, active- and placebo-controlled phase 3 trial. Eligible participants were randomized to receive daily SC injection for 9 months of 20 mg generic GA (n = 5,353), 20 mg brand GA (n = 5,357), or placebo (n = 584). The primary endpoint was the mean number of gadolinium (Gd1) lesions visible on MRIs during months 7, 8, and 9, which were significantly reduced in the combined GA-treated group and in each GA group individually when compared with the placebo group, confirming the study sensitivity (ie, GA was effective under the conditions of the study). Tolerability (including injection site reactions) and safety (incidence, spectrum, and severity of adverse events [AEs]) were similar in the generic and brand GA groups. These results demonstrated that generic and brand GA had equivalent efficacy, tolerability, and safety over a 9-month period.7
Results of a 15-month extension of the study were presented in 2015 and showed similar efficacy, safety, and tolerability in participants treated with generic GA for 2 years and patients switched from brand to generic GA.8 Multiple shifts for GA occurred, most notably the conversion from branded Copaxone (Teva Pharmaceutical Industries) to generic Glatopa (Sandoz). Subsequently, Sandoz released a generic 40 mg 3 times weekly formulation. Additionally, Mylan entered the generic GA market. With 3 competing manufacturers, internal data from the VHA indicated that it was able to negotiate a single source contract for this medication that provided a savings of $32,088,904.69 between September 2016 and May 2019.
The impact of generic conversions is just being realized. Soon, patents will begin to expire for oral DMTs, leading to an expected growth of generic alternatives. Already the FDA has approved 4 generic alternatives for teriflunomide, 3 for fingolimod (with 13 tentative approvals), and 15 generic alternatives for dimethyl fumarate (DMF). Implementation of therapeutic interchanges will be pursued by VHA as clinically supported by evidence.
Criteria for Use
PBM supports utilizing criteria to help guide providers on DMT options and promote safe, effective, and value-based selection of a DMT. The PBM creates monographs and criteria for use (CFU) for new medications. The monograph contains a literature evaluation of all studies available to date that concern both safety and efficacy of the new medication. Therapeutic alternatives also are presented and assessed for key elements that may determine the most safe and effective use. Additional safety areas for the new medications such as look-alike, sound-alike potential, special populations use (ie, those who are pregnant, the elderly, and those with liver or renal dysfunction), and drug-drug interactions are presented. Lastly, and possibly most importantly in an ever-growing growing world of DMTs, the monograph describes a reasonable place in therapy for the new DMT.
CFU are additional guidance for some DMTs. The development of CFU are based on several questions that arise during the monograph development for a DMT. These include, but are not limited to:
- Are there safety concerns that require the drug to receive a review to ensure safe prescribing (eg, agents with REMS programs, or safety concerns in specific populations)?
- Does the drug require a specialty provider type with knowledge and experience in those disease states to ensure appropriate and safe prescribing (eg restricted to infectious diseases)?
- Do VHA or non-VHA guidelines suggest alternative therapy be used prior to the agent?
- Is a review deemed necessary to ensure the preferred agent is used first (eg, second-line therapy)?
The CFU defines parameters of drug use consistent with high quality and evidence-based patient care. CFUs also serve as a basis for monitoring local, regional, and national patterns of pharmacologic care and help guide health care providers (HCPs) on appropriate use of medication.
CFUs are designed to ensure the HCP is safely starting a medication that has evidence for efficacy for their patient. For example, alemtuzumab is a high-risk, high-efficacy DMT. The alemtuzumab CFU acknowledges this by having exclusion criteria that prevent a veteran at high risk (ie, on another immunosuppressant) from being exposed to severe AEs (ie, severe leukopenia) that are associated with the medication. On the other hand, the inclusion criteria recognize the benefits of alemtuzumab and allows those with highly active MS who have failed other DMTs to receive the medication.
The drug monograph and CFU process is an important part of VHA efforts to optimize patient care. After a draft version is developed, HCPs can provide feedback on the exclusion/inclusion criteria and describe how they anticipate using the medication in their practice. This insight can be beneficial for MS treatment as diverse HCPs may have distinct viewpoints on how DMTs should be started. Pharmacists and physicians on a national level then discuss and decide together what to include in the final drafts of the drug monograph and CFU. Final documents are disseminated to all sites, which encourages consistent practices across the VHA.9 These documents are reviewed on a regular basis and updated as needed based on available literature evidence.
It is well accepted that early use of DMT correlates with lower accumulated long-term disability.10 However, discontinuation of DMT should be treated with equal importance. This benefits the patient by reducing their risk of AEs from DMTs and provides cost savings. Age and disease stability are factors to consider for DMT discontinuation. In a study with patients aged > 45 years and another with patients aged > 60 years, discontinuing DMT rarely had a negative impact and improved quality of life.11,12 A retrospective meta-analysis of age-dependent efficacy of current DMTs predicted that DMT loses efficacy at age 53 years. In addition, higher efficacy DMT only outperforms lower efficacy DMT in patients aged < 40.5 years.13 Stability of disease and lack of relapses for ≥ 2 years also may be a positive predictor to safely discontinue DMT.14,15 The growing literature to support safe discontinuation of DMT makes this a more convincing strategy to avoid unnecessary costs associated with current DMTs. With an average age of 59 years for veterans with MS, this may be one of the largest areas of cost avoidance to consider.
Off-Label Use
Other potential ways to reduce DMT costs is to consider off-label treatments. The OLYMPUS trial studied off-label use of rituximab, an anti-CD20 antibody like ocrelizumab. It did not meet statistical significance for its primary endpoint; however, in a subgroup analysis, off-label use was found to be more effective in a population aged < 51 years.16 Other case reports and smaller scale studies also describe rituximab’s efficacy in MS.17,18 In 2018, the FDA approved the first rituximab biosimilar.19 Further competition from biosimilars likely will make rituximab an even more cost-effective choice when compared with ocrelizumab.
Alternate Dosing Regimens
Extended interval dosing of natalizumab has been studied, extending the standard infusion interval from every 4 weeks to 5- to 8-week intervals. One recent article compared these interval extensions and found that all extended intervals of up to 56 days did not increase new or enhancing lesions on MRI when compared with standard interval dosing.20 Another larger randomized trial is underway to evaluate efficacy and safety of extended interval dosing of natalizumab (NCT03689972). Utilization of this dosing may reduce natalizumab annual costs by up to 50%.
Safety Monitoring
DMF is an oral DMT on the VHA formulary with CFU. Since leukopenia is a known AE, baseline and quarterly monitoring of the complete blood count (CBC) is recommended for patients taking DMF. Additionally, DMF should be held if white blood cell count (WBC) falls below 2,000/mm3.21 There have been recent reports of death secondary to progressive multifocal leukoencephalopathy (PML) among European patients taking DMF.22-24 This has raised concerns about adherence to recommended CBC monitoring in veterans taking DMF. The association of DMF and leukopenia has been evident since early clinical trials.25 Leukopenia in immunocompromised patients increases the risk of PML.
In the long-term extension study ENDORSE, 6% to 7% of patients continuing DMF had WBC counts of 3.0×109/L compared with 7% to 10% in the new to DMF group.26 In addition 6% to 8% of patients continuing DMF had lymphocyte counts of 0.5×109/L, compared with 5% to 9% in the new to DMF group. The cases of PML occurred in patients who had low lymphocyte counts over an extended period with no adjustment to DMF therapy, such as holding the drug until WBC counts returned to normal levels or stopping the drug. Discussion and review within VHA resulted in the recommendation for quarterly WBC monitoring criteria.
PBM and VA Center for Medication Safety (MedSafe) conducted a medication usage evaluation (MUE) on adherence to the WBC monitoring set forth in the CFU. Data collection began in fourth quarter of fiscal year (FY) 2015 with the most recent reporting period of fourth quarter of FY 2017. The Medication Utilization Evaluation Tool tracks patients with no reported WBC in 90 days and WBC < 2,000/mm3. Over the reporting period, 20% to 23% of patients have not received appropriate quarterly monitoring. Additionally, there have been 4 cases where the WBC decreased below the threshold limit. To ensure safe and effective use of DMF, it is important to adhere to the monitoring requirements set forth in the CFU.
Impact of REMS and Special Distribution
As DMTs increase in efficacy, there are often more risks associated with them. Some of these high-risk medications, including natalizumab and alemtuzumab, have REMS programs and/or have special distribution procedures. Although REMS are imperative for patient safety, the complexity of these programs can be difficult to navigate, which can create a barrier to access. The PBM helps to assist all sites with navigating and adhering to required actions to dispense and administer these medications through a national Special Handling Drugs Microsoft SharePoint site, which provides access to REMS forms and procurement information when drugs are dispensed from specialty pharmacies. Easing this process nationwide empowers more sites to be confident they can dispense specialty medications appropriately.
Clinical Pharmacists
The VHA is unique in its utilization of pharmacists in outpatient clinic settings. Utilization of an interdisciplinary team for medication management has been highly used in VHA for areas like primary care; however, pharmacist involvement in specialty areas is on the rise and MS is no exception. Pharmacists stationed in clinics, such as neurology or spinal cord injury, can impact care for veterans with MS. Interdisciplinary teams that include a pharmacist have been shown to increase patient adherence to DMTs.27 However, pharmacists often assist with medication education and monitoring, which adds an additional layer of safety to DMT treatment. At the VHA, pharmacists also can obtain a scope of practice that allows them to prescribe medications and increase access to care for veterans with MS.
Education
The VHA demonstrates how education on a disease state like MS can be distributed on a large, national scale through drug monographs, CFU, and Microsoft SharePoint sites. In addition, VHA has created the MS Centers of Excellence (MSCoE) that serve as a hub of specialized health care providers in all aspects of MS care.
A core function of the MSCoE is to provide education to both HCPs and patients. The MSCoE and its regional hubs support sites that may not have an HCP who specializes in MS by providing advice on DMT selection, how to obtain specialty medications, and monitoring that needs to be completed to ensure veterans’ safety. The MSCoE also has partnered with the National MS Society to hold a lecture series on topics in MS. This free series is available online to all HCPs who interact with patients who have MS and is a way that VA is extending its best practices and expertise beyond its own health care system. There also is a quarterly newsletter for veterans with MS that highlights new information on DMTs that can affect their care.
Conclusion
It is an exciting and challenging period in MS treatment. New DMTs are being approved and entering clinical trials at a rapid pace. These new DMT agents may offer increased efficacy, improvements in AE profiles, and the possibility of increased medication adherence—but often at a higher cost. The utilization of CFU and formulary management provides the ability to ensure the safe and appropriate use of medications by veterans, with a secondary outcome of controlling pharmacy expenditures.
The VHA had expenditures of $142,135,938 for DMT use in FY 2018. As the VHA sees the new contract prices for DMT in January 2020, we are reminded that costs will continue to rise with some pharmaceutical manufacturers implementing prices 8% to 11% higher than 2019 prices, when the consumer price index defines an increase of 1.0% for 2020 and 1.4% in 2021.28 It is imperative that the VHA formulary be managed judiciously and the necessary measures be in place for VHA practitioners to enable effective, safe and value-based care to the veteran population.
1. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81(4):479-484.
2. Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? [published correction appears in Neurology. 2015;85(19):1728]. Neurology. 2015;84(21):2185–2192.
3. San-Juan-Rodriguez A, Good CB, Heyman RA, Parekh N, Shrank WH, Hernandez I. Trends in prices, market share, and spending on self-administered disease-modifying therapies for multiple sclerosis in Medicare Part D. JAMA Neurol. 2019;76(11):1386-1390.
4. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
5. Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis [published correction appears in Mult Scler. 2003;9(6):641]. Mult Scler. 2003;9(3):260-274.
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
7. Cohen J, Belova A, Selmaj K, et al. Equivalence of generic glatiramer acetate in multiple sclerosis: a randomized clinical trial. JAMA Neurol. 2015;72(12):1433-1441.
8. Selmaj K, Barkhof F, Belova AN, et al; GATE study group. Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult Scler. 2017;23(14):1909-1917.
9. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063.
10. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175-187.
11. Hua LH, Harris H, Conway D, Thompson NR. Changes in patient-reported outcomes between continuers and discontinuers of disease modifying therapy in patients with multiple sclerosis over age 60 [published correction appears in Mult Scler Relat Disord. 2019;30:293]. Mult Scler Relat Disord. 2019;30:252-256.
12. Bsteh G, Feige J, Ehling R, et al. Discontinuation of disease-modifying therapies in multiple sclerosis - Clinical outcome and prognostic factors. Mult Scler. 2017;23(9):1241-1248.
13. Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
14. Kister I, Spelman T, Alroughani R, et al; MSBase Study Group. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study [published correction appears in J Neurol Neurosurg Psychiatry. 2019;90(4):e2]. J Neurol Neurosurg Psychiatry. 2016;87(10):1133-1137.
15. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19(1):11-14.
16. Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
17. Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688.
18. Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950–958.
19. Rituximab-abbs [package insert]. North Wales, PA: Teva Pharmaceuticals; 2018.
20. Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(8):885-889.
21. Dimethyl fumarate [package insert]. Cambridge, MA: Biogen Inc; 2015.
22. van Kester MS, Bouwes Bavinck JN, Quint KD. PML in Patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):583-584.
23. Nieuwkamp DJ, Murk JL, van Oosten BW. PML in patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):584.
24. Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372(15):1476-1478.
25. Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler. 2015;21(6):796-797.
26. Gold R, Arnold DL, Bar-Or A, et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: Interim analysis of ENDORSE, a randomized extension study. Mult Scler. 2017;23(2):253–265.
27. Hanson RL, Habibi M, Khamo N, Abdou S, Stubbings J. Integrated clinical and specialty pharmacy practice model for management of patients with multiple sclerosis. Am J Health Syst Pharm. 2014;71(6):463-469.
28. Federal Planning Bureau. Consumer Price Index - Inflation forecasts. https://www.plan.be/databases/17-en-consumer+price+index+inflation+forecasts. Updated March 3, 2020. Accessed March 9, 2020.
Prior to the first approved disease modifying therapy (DMT) in the 1990s, treatment approaches for multiple sclerosis (MS) were not well understood. The discovery that MS was an immune mediated inflammatory disease paved the way for the treatments we know today. In 1993, interferon β‐1b became the first DMT for MS approved by the US Food and Drug Administration (FDA). Approvals for interferon β‐1a as well as glatiramer acetate (GA) soon followed. Today, we consider these the mildest immunosuppressant DMTs; however, their success verified that suppressing the immune system had a positive effect on the MS disease process.
Following these approvals, the disease process in MS is now better understood. Recently approved therapies include monoclonal antibodies, which affect other immune pathways. Today, there are 14 approved DMTs (Table 1). Although the advent of these newer DMTs has revolutionized care for patients with MS, it has been accompanied by increasing costs for the agents. Direct medical costs associated with MS management, coupled with indirect costs from lost productivity, have been estimated to be $24.2 billion annually in the US.1 These increases have been seen across many levels of insurance coverage—private payer, Medicare, and the Veterans Health Administration (VHA).2,3
The Figure demonstrates the cost increase that have been seen across VHA between 2004 and 2019 for the DMTs identified in Table 1. Indeed, this compound annual growth rate may be an underestimate because infusion therapies (eg, natalizumab, ocrelizumab, and alemtuzumab) are difficult to track as they may be dispensed directly via a Risk Evaluation Medication Strategy (REMS) program. According to the VHA Pharmacy Benefit Management Service (PBM), in September 2019, dimethyl fumarate (DMF) had the 13th highest total outpatient drug cost for the US Department of Veterans Affairs (VA), interferon β‐1a ranked 62nd and 83rd (prefilled pen and syringe, respectively), and GA 40 mg ranked 89th.
The DMT landscape has demonstrated significant price fluctuations and given rise to a class of medications that requires extensive oversight in terms of efficacy, safety, and cost minimization. The purpose of this article is to show how delivery of this specialty group of medications can be optimized with safety, efficacy, and cost value within a large health care system.
Factors Impacting DMT Use
Recent changes to MS typing have impacted utilization of DMTs. Traditionally, there were 4 subtypes of MS: relapsing remitting (RRMS), secondary progressive (SPMS), progressive relapsing (PRMS), and primary progressive (PPMS). These subtypes are now viewed more broadly and grouped as either relapsing or progressive. The traditional subtypes fall under these broader definitions. Additionally, SPMS has been broken into active SPMS, characterized by continued worsening of disability unrelated to acute relapses, superimposed with activity that can be seen on magnetic resonance images (MRIs), and nonactive SPMS, which has the same disability progression as active SPMS but without MRI-visible activity.4-6 In 2019, these supplementary designations to SPMS made their first appearance in FDA-approved indications. All existing DMTs now include this terminology in their labelling and are indicated in active SPMS. There remain no DMTs that treat nonactive SPMS.
The current landscape of DMTs is highly varied in method of administration, risks, and benefits. As efficacy of these medications often is marked by how well they can prevent the immune system from attacking myelin, an inverse relationship between safety and efficacy results. The standard treatment outcomes in MS have evolved over time. The following are the commonly used primary outcomes in clinical trials: relapse reduction; increased time between relapses; decreased severity of relapses; prevention or extend time to disability milestones as measured by the Expanded Disability Status Scale (EDSS) and other disability measures; prevention or extension of time to onset of secondary progressive disease; prevention or reduction of the number and size of new and enhancing lesions on MRI; and limitation of overall MRI lesion burden in the central nervous system (CNS).
Newer treatment outcomes employed in more recent trials include: measures of axonal damage, CNS atrophy, evidence of microscopic disease via conventional MRI and advanced imaging modalities, biomarkers associated with inflammatory disease activity and neurodegeneration in MS, and the use of no evidence of disease activity (NEDA). These outcomes also must be evaluated by the safety concerns of each agent. Short- and long-term safety are critical factors in the selection of DMTs for MS. The injectable therapies for MS (interferon β‐1a, interferon β‐1b, and GA) have established long-term safety profiles from > 20 years of continuous use. The long-term safety profiles of oral immunomodulatory agents and monoclonal antibodies for these drugs in MS have yet to be determined. Safety concerns associated with some therapies and added requirements for safety monitoring may increase the complexity of a therapeutic selection.
Current cost minimization strategies for DMT include limiting DMT agents on formularies, tier systems that incentivize patients/prescribers to select the lowest priced agents on the formulary, negotiating arrangements with manufacturers to freeze prices or provide discounts in exchange for a priority position in the formulary, and requiring prior authorization to initiate or switch therapy. The use of generic medications and interchange to these agents from a brand name formulation can help reduce expense. Several of these strategies have been implemented in VHA.
Disease-Modifying Therapies
In 2019, 18,645 veterans with MS had either a MS-specific DMT or ≥ 1 annual encounters with a primary diagnosis of MS. Of this population, 4,720 were female and 13,357 were service connected according to VA data. About 50% of veterans with MS take a DMT. This percentage has remained stable over the past decade (Table 2). Although it appears the number of unique veterans prescribed an outpatient DMT is decreasing, this does not include the growing use of infused DMTs or DMTs obtained through the Veterans Choice Program (VCP)/Community Care (CC).
The overall outpatient pharmacy costs for veterans have remained constant despite the reduction in outpatient pharmacy prescription numbers. This may be due to increases in DMT cost to the VHA and the use of more expensive oral agents over the previously used platform injection DMTs.
Generic Conversion
GA is available in 20 mg daily and 40 mg3 times weekly subcutaneous injection dosing. The first evidence of clinical efficacy for a generic formulation for GA was evaluated by the GATE trial.7 This trial was a multicenter, randomized, double-blind, active- and placebo-controlled phase 3 trial. Eligible participants were randomized to receive daily SC injection for 9 months of 20 mg generic GA (n = 5,353), 20 mg brand GA (n = 5,357), or placebo (n = 584). The primary endpoint was the mean number of gadolinium (Gd1) lesions visible on MRIs during months 7, 8, and 9, which were significantly reduced in the combined GA-treated group and in each GA group individually when compared with the placebo group, confirming the study sensitivity (ie, GA was effective under the conditions of the study). Tolerability (including injection site reactions) and safety (incidence, spectrum, and severity of adverse events [AEs]) were similar in the generic and brand GA groups. These results demonstrated that generic and brand GA had equivalent efficacy, tolerability, and safety over a 9-month period.7
Results of a 15-month extension of the study were presented in 2015 and showed similar efficacy, safety, and tolerability in participants treated with generic GA for 2 years and patients switched from brand to generic GA.8 Multiple shifts for GA occurred, most notably the conversion from branded Copaxone (Teva Pharmaceutical Industries) to generic Glatopa (Sandoz). Subsequently, Sandoz released a generic 40 mg 3 times weekly formulation. Additionally, Mylan entered the generic GA market. With 3 competing manufacturers, internal data from the VHA indicated that it was able to negotiate a single source contract for this medication that provided a savings of $32,088,904.69 between September 2016 and May 2019.
The impact of generic conversions is just being realized. Soon, patents will begin to expire for oral DMTs, leading to an expected growth of generic alternatives. Already the FDA has approved 4 generic alternatives for teriflunomide, 3 for fingolimod (with 13 tentative approvals), and 15 generic alternatives for dimethyl fumarate (DMF). Implementation of therapeutic interchanges will be pursued by VHA as clinically supported by evidence.
Criteria for Use
PBM supports utilizing criteria to help guide providers on DMT options and promote safe, effective, and value-based selection of a DMT. The PBM creates monographs and criteria for use (CFU) for new medications. The monograph contains a literature evaluation of all studies available to date that concern both safety and efficacy of the new medication. Therapeutic alternatives also are presented and assessed for key elements that may determine the most safe and effective use. Additional safety areas for the new medications such as look-alike, sound-alike potential, special populations use (ie, those who are pregnant, the elderly, and those with liver or renal dysfunction), and drug-drug interactions are presented. Lastly, and possibly most importantly in an ever-growing growing world of DMTs, the monograph describes a reasonable place in therapy for the new DMT.
CFU are additional guidance for some DMTs. The development of CFU are based on several questions that arise during the monograph development for a DMT. These include, but are not limited to:
- Are there safety concerns that require the drug to receive a review to ensure safe prescribing (eg, agents with REMS programs, or safety concerns in specific populations)?
- Does the drug require a specialty provider type with knowledge and experience in those disease states to ensure appropriate and safe prescribing (eg restricted to infectious diseases)?
- Do VHA or non-VHA guidelines suggest alternative therapy be used prior to the agent?
- Is a review deemed necessary to ensure the preferred agent is used first (eg, second-line therapy)?
The CFU defines parameters of drug use consistent with high quality and evidence-based patient care. CFUs also serve as a basis for monitoring local, regional, and national patterns of pharmacologic care and help guide health care providers (HCPs) on appropriate use of medication.
CFUs are designed to ensure the HCP is safely starting a medication that has evidence for efficacy for their patient. For example, alemtuzumab is a high-risk, high-efficacy DMT. The alemtuzumab CFU acknowledges this by having exclusion criteria that prevent a veteran at high risk (ie, on another immunosuppressant) from being exposed to severe AEs (ie, severe leukopenia) that are associated with the medication. On the other hand, the inclusion criteria recognize the benefits of alemtuzumab and allows those with highly active MS who have failed other DMTs to receive the medication.
The drug monograph and CFU process is an important part of VHA efforts to optimize patient care. After a draft version is developed, HCPs can provide feedback on the exclusion/inclusion criteria and describe how they anticipate using the medication in their practice. This insight can be beneficial for MS treatment as diverse HCPs may have distinct viewpoints on how DMTs should be started. Pharmacists and physicians on a national level then discuss and decide together what to include in the final drafts of the drug monograph and CFU. Final documents are disseminated to all sites, which encourages consistent practices across the VHA.9 These documents are reviewed on a regular basis and updated as needed based on available literature evidence.
It is well accepted that early use of DMT correlates with lower accumulated long-term disability.10 However, discontinuation of DMT should be treated with equal importance. This benefits the patient by reducing their risk of AEs from DMTs and provides cost savings. Age and disease stability are factors to consider for DMT discontinuation. In a study with patients aged > 45 years and another with patients aged > 60 years, discontinuing DMT rarely had a negative impact and improved quality of life.11,12 A retrospective meta-analysis of age-dependent efficacy of current DMTs predicted that DMT loses efficacy at age 53 years. In addition, higher efficacy DMT only outperforms lower efficacy DMT in patients aged < 40.5 years.13 Stability of disease and lack of relapses for ≥ 2 years also may be a positive predictor to safely discontinue DMT.14,15 The growing literature to support safe discontinuation of DMT makes this a more convincing strategy to avoid unnecessary costs associated with current DMTs. With an average age of 59 years for veterans with MS, this may be one of the largest areas of cost avoidance to consider.
Off-Label Use
Other potential ways to reduce DMT costs is to consider off-label treatments. The OLYMPUS trial studied off-label use of rituximab, an anti-CD20 antibody like ocrelizumab. It did not meet statistical significance for its primary endpoint; however, in a subgroup analysis, off-label use was found to be more effective in a population aged < 51 years.16 Other case reports and smaller scale studies also describe rituximab’s efficacy in MS.17,18 In 2018, the FDA approved the first rituximab biosimilar.19 Further competition from biosimilars likely will make rituximab an even more cost-effective choice when compared with ocrelizumab.
Alternate Dosing Regimens
Extended interval dosing of natalizumab has been studied, extending the standard infusion interval from every 4 weeks to 5- to 8-week intervals. One recent article compared these interval extensions and found that all extended intervals of up to 56 days did not increase new or enhancing lesions on MRI when compared with standard interval dosing.20 Another larger randomized trial is underway to evaluate efficacy and safety of extended interval dosing of natalizumab (NCT03689972). Utilization of this dosing may reduce natalizumab annual costs by up to 50%.
Safety Monitoring
DMF is an oral DMT on the VHA formulary with CFU. Since leukopenia is a known AE, baseline and quarterly monitoring of the complete blood count (CBC) is recommended for patients taking DMF. Additionally, DMF should be held if white blood cell count (WBC) falls below 2,000/mm3.21 There have been recent reports of death secondary to progressive multifocal leukoencephalopathy (PML) among European patients taking DMF.22-24 This has raised concerns about adherence to recommended CBC monitoring in veterans taking DMF. The association of DMF and leukopenia has been evident since early clinical trials.25 Leukopenia in immunocompromised patients increases the risk of PML.
In the long-term extension study ENDORSE, 6% to 7% of patients continuing DMF had WBC counts of 3.0×109/L compared with 7% to 10% in the new to DMF group.26 In addition 6% to 8% of patients continuing DMF had lymphocyte counts of 0.5×109/L, compared with 5% to 9% in the new to DMF group. The cases of PML occurred in patients who had low lymphocyte counts over an extended period with no adjustment to DMF therapy, such as holding the drug until WBC counts returned to normal levels or stopping the drug. Discussion and review within VHA resulted in the recommendation for quarterly WBC monitoring criteria.
PBM and VA Center for Medication Safety (MedSafe) conducted a medication usage evaluation (MUE) on adherence to the WBC monitoring set forth in the CFU. Data collection began in fourth quarter of fiscal year (FY) 2015 with the most recent reporting period of fourth quarter of FY 2017. The Medication Utilization Evaluation Tool tracks patients with no reported WBC in 90 days and WBC < 2,000/mm3. Over the reporting period, 20% to 23% of patients have not received appropriate quarterly monitoring. Additionally, there have been 4 cases where the WBC decreased below the threshold limit. To ensure safe and effective use of DMF, it is important to adhere to the monitoring requirements set forth in the CFU.
Impact of REMS and Special Distribution
As DMTs increase in efficacy, there are often more risks associated with them. Some of these high-risk medications, including natalizumab and alemtuzumab, have REMS programs and/or have special distribution procedures. Although REMS are imperative for patient safety, the complexity of these programs can be difficult to navigate, which can create a barrier to access. The PBM helps to assist all sites with navigating and adhering to required actions to dispense and administer these medications through a national Special Handling Drugs Microsoft SharePoint site, which provides access to REMS forms and procurement information when drugs are dispensed from specialty pharmacies. Easing this process nationwide empowers more sites to be confident they can dispense specialty medications appropriately.
Clinical Pharmacists
The VHA is unique in its utilization of pharmacists in outpatient clinic settings. Utilization of an interdisciplinary team for medication management has been highly used in VHA for areas like primary care; however, pharmacist involvement in specialty areas is on the rise and MS is no exception. Pharmacists stationed in clinics, such as neurology or spinal cord injury, can impact care for veterans with MS. Interdisciplinary teams that include a pharmacist have been shown to increase patient adherence to DMTs.27 However, pharmacists often assist with medication education and monitoring, which adds an additional layer of safety to DMT treatment. At the VHA, pharmacists also can obtain a scope of practice that allows them to prescribe medications and increase access to care for veterans with MS.
Education
The VHA demonstrates how education on a disease state like MS can be distributed on a large, national scale through drug monographs, CFU, and Microsoft SharePoint sites. In addition, VHA has created the MS Centers of Excellence (MSCoE) that serve as a hub of specialized health care providers in all aspects of MS care.
A core function of the MSCoE is to provide education to both HCPs and patients. The MSCoE and its regional hubs support sites that may not have an HCP who specializes in MS by providing advice on DMT selection, how to obtain specialty medications, and monitoring that needs to be completed to ensure veterans’ safety. The MSCoE also has partnered with the National MS Society to hold a lecture series on topics in MS. This free series is available online to all HCPs who interact with patients who have MS and is a way that VA is extending its best practices and expertise beyond its own health care system. There also is a quarterly newsletter for veterans with MS that highlights new information on DMTs that can affect their care.
Conclusion
It is an exciting and challenging period in MS treatment. New DMTs are being approved and entering clinical trials at a rapid pace. These new DMT agents may offer increased efficacy, improvements in AE profiles, and the possibility of increased medication adherence—but often at a higher cost. The utilization of CFU and formulary management provides the ability to ensure the safe and appropriate use of medications by veterans, with a secondary outcome of controlling pharmacy expenditures.
The VHA had expenditures of $142,135,938 for DMT use in FY 2018. As the VHA sees the new contract prices for DMT in January 2020, we are reminded that costs will continue to rise with some pharmaceutical manufacturers implementing prices 8% to 11% higher than 2019 prices, when the consumer price index defines an increase of 1.0% for 2020 and 1.4% in 2021.28 It is imperative that the VHA formulary be managed judiciously and the necessary measures be in place for VHA practitioners to enable effective, safe and value-based care to the veteran population.
Prior to the first approved disease modifying therapy (DMT) in the 1990s, treatment approaches for multiple sclerosis (MS) were not well understood. The discovery that MS was an immune mediated inflammatory disease paved the way for the treatments we know today. In 1993, interferon β‐1b became the first DMT for MS approved by the US Food and Drug Administration (FDA). Approvals for interferon β‐1a as well as glatiramer acetate (GA) soon followed. Today, we consider these the mildest immunosuppressant DMTs; however, their success verified that suppressing the immune system had a positive effect on the MS disease process.
Following these approvals, the disease process in MS is now better understood. Recently approved therapies include monoclonal antibodies, which affect other immune pathways. Today, there are 14 approved DMTs (Table 1). Although the advent of these newer DMTs has revolutionized care for patients with MS, it has been accompanied by increasing costs for the agents. Direct medical costs associated with MS management, coupled with indirect costs from lost productivity, have been estimated to be $24.2 billion annually in the US.1 These increases have been seen across many levels of insurance coverage—private payer, Medicare, and the Veterans Health Administration (VHA).2,3
The Figure demonstrates the cost increase that have been seen across VHA between 2004 and 2019 for the DMTs identified in Table 1. Indeed, this compound annual growth rate may be an underestimate because infusion therapies (eg, natalizumab, ocrelizumab, and alemtuzumab) are difficult to track as they may be dispensed directly via a Risk Evaluation Medication Strategy (REMS) program. According to the VHA Pharmacy Benefit Management Service (PBM), in September 2019, dimethyl fumarate (DMF) had the 13th highest total outpatient drug cost for the US Department of Veterans Affairs (VA), interferon β‐1a ranked 62nd and 83rd (prefilled pen and syringe, respectively), and GA 40 mg ranked 89th.
The DMT landscape has demonstrated significant price fluctuations and given rise to a class of medications that requires extensive oversight in terms of efficacy, safety, and cost minimization. The purpose of this article is to show how delivery of this specialty group of medications can be optimized with safety, efficacy, and cost value within a large health care system.
Factors Impacting DMT Use
Recent changes to MS typing have impacted utilization of DMTs. Traditionally, there were 4 subtypes of MS: relapsing remitting (RRMS), secondary progressive (SPMS), progressive relapsing (PRMS), and primary progressive (PPMS). These subtypes are now viewed more broadly and grouped as either relapsing or progressive. The traditional subtypes fall under these broader definitions. Additionally, SPMS has been broken into active SPMS, characterized by continued worsening of disability unrelated to acute relapses, superimposed with activity that can be seen on magnetic resonance images (MRIs), and nonactive SPMS, which has the same disability progression as active SPMS but without MRI-visible activity.4-6 In 2019, these supplementary designations to SPMS made their first appearance in FDA-approved indications. All existing DMTs now include this terminology in their labelling and are indicated in active SPMS. There remain no DMTs that treat nonactive SPMS.
The current landscape of DMTs is highly varied in method of administration, risks, and benefits. As efficacy of these medications often is marked by how well they can prevent the immune system from attacking myelin, an inverse relationship between safety and efficacy results. The standard treatment outcomes in MS have evolved over time. The following are the commonly used primary outcomes in clinical trials: relapse reduction; increased time between relapses; decreased severity of relapses; prevention or extend time to disability milestones as measured by the Expanded Disability Status Scale (EDSS) and other disability measures; prevention or extension of time to onset of secondary progressive disease; prevention or reduction of the number and size of new and enhancing lesions on MRI; and limitation of overall MRI lesion burden in the central nervous system (CNS).
Newer treatment outcomes employed in more recent trials include: measures of axonal damage, CNS atrophy, evidence of microscopic disease via conventional MRI and advanced imaging modalities, biomarkers associated with inflammatory disease activity and neurodegeneration in MS, and the use of no evidence of disease activity (NEDA). These outcomes also must be evaluated by the safety concerns of each agent. Short- and long-term safety are critical factors in the selection of DMTs for MS. The injectable therapies for MS (interferon β‐1a, interferon β‐1b, and GA) have established long-term safety profiles from > 20 years of continuous use. The long-term safety profiles of oral immunomodulatory agents and monoclonal antibodies for these drugs in MS have yet to be determined. Safety concerns associated with some therapies and added requirements for safety monitoring may increase the complexity of a therapeutic selection.
Current cost minimization strategies for DMT include limiting DMT agents on formularies, tier systems that incentivize patients/prescribers to select the lowest priced agents on the formulary, negotiating arrangements with manufacturers to freeze prices or provide discounts in exchange for a priority position in the formulary, and requiring prior authorization to initiate or switch therapy. The use of generic medications and interchange to these agents from a brand name formulation can help reduce expense. Several of these strategies have been implemented in VHA.
Disease-Modifying Therapies
In 2019, 18,645 veterans with MS had either a MS-specific DMT or ≥ 1 annual encounters with a primary diagnosis of MS. Of this population, 4,720 were female and 13,357 were service connected according to VA data. About 50% of veterans with MS take a DMT. This percentage has remained stable over the past decade (Table 2). Although it appears the number of unique veterans prescribed an outpatient DMT is decreasing, this does not include the growing use of infused DMTs or DMTs obtained through the Veterans Choice Program (VCP)/Community Care (CC).
The overall outpatient pharmacy costs for veterans have remained constant despite the reduction in outpatient pharmacy prescription numbers. This may be due to increases in DMT cost to the VHA and the use of more expensive oral agents over the previously used platform injection DMTs.
Generic Conversion
GA is available in 20 mg daily and 40 mg3 times weekly subcutaneous injection dosing. The first evidence of clinical efficacy for a generic formulation for GA was evaluated by the GATE trial.7 This trial was a multicenter, randomized, double-blind, active- and placebo-controlled phase 3 trial. Eligible participants were randomized to receive daily SC injection for 9 months of 20 mg generic GA (n = 5,353), 20 mg brand GA (n = 5,357), or placebo (n = 584). The primary endpoint was the mean number of gadolinium (Gd1) lesions visible on MRIs during months 7, 8, and 9, which were significantly reduced in the combined GA-treated group and in each GA group individually when compared with the placebo group, confirming the study sensitivity (ie, GA was effective under the conditions of the study). Tolerability (including injection site reactions) and safety (incidence, spectrum, and severity of adverse events [AEs]) were similar in the generic and brand GA groups. These results demonstrated that generic and brand GA had equivalent efficacy, tolerability, and safety over a 9-month period.7
Results of a 15-month extension of the study were presented in 2015 and showed similar efficacy, safety, and tolerability in participants treated with generic GA for 2 years and patients switched from brand to generic GA.8 Multiple shifts for GA occurred, most notably the conversion from branded Copaxone (Teva Pharmaceutical Industries) to generic Glatopa (Sandoz). Subsequently, Sandoz released a generic 40 mg 3 times weekly formulation. Additionally, Mylan entered the generic GA market. With 3 competing manufacturers, internal data from the VHA indicated that it was able to negotiate a single source contract for this medication that provided a savings of $32,088,904.69 between September 2016 and May 2019.
The impact of generic conversions is just being realized. Soon, patents will begin to expire for oral DMTs, leading to an expected growth of generic alternatives. Already the FDA has approved 4 generic alternatives for teriflunomide, 3 for fingolimod (with 13 tentative approvals), and 15 generic alternatives for dimethyl fumarate (DMF). Implementation of therapeutic interchanges will be pursued by VHA as clinically supported by evidence.
Criteria for Use
PBM supports utilizing criteria to help guide providers on DMT options and promote safe, effective, and value-based selection of a DMT. The PBM creates monographs and criteria for use (CFU) for new medications. The monograph contains a literature evaluation of all studies available to date that concern both safety and efficacy of the new medication. Therapeutic alternatives also are presented and assessed for key elements that may determine the most safe and effective use. Additional safety areas for the new medications such as look-alike, sound-alike potential, special populations use (ie, those who are pregnant, the elderly, and those with liver or renal dysfunction), and drug-drug interactions are presented. Lastly, and possibly most importantly in an ever-growing growing world of DMTs, the monograph describes a reasonable place in therapy for the new DMT.
CFU are additional guidance for some DMTs. The development of CFU are based on several questions that arise during the monograph development for a DMT. These include, but are not limited to:
- Are there safety concerns that require the drug to receive a review to ensure safe prescribing (eg, agents with REMS programs, or safety concerns in specific populations)?
- Does the drug require a specialty provider type with knowledge and experience in those disease states to ensure appropriate and safe prescribing (eg restricted to infectious diseases)?
- Do VHA or non-VHA guidelines suggest alternative therapy be used prior to the agent?
- Is a review deemed necessary to ensure the preferred agent is used first (eg, second-line therapy)?
The CFU defines parameters of drug use consistent with high quality and evidence-based patient care. CFUs also serve as a basis for monitoring local, regional, and national patterns of pharmacologic care and help guide health care providers (HCPs) on appropriate use of medication.
CFUs are designed to ensure the HCP is safely starting a medication that has evidence for efficacy for their patient. For example, alemtuzumab is a high-risk, high-efficacy DMT. The alemtuzumab CFU acknowledges this by having exclusion criteria that prevent a veteran at high risk (ie, on another immunosuppressant) from being exposed to severe AEs (ie, severe leukopenia) that are associated with the medication. On the other hand, the inclusion criteria recognize the benefits of alemtuzumab and allows those with highly active MS who have failed other DMTs to receive the medication.
The drug monograph and CFU process is an important part of VHA efforts to optimize patient care. After a draft version is developed, HCPs can provide feedback on the exclusion/inclusion criteria and describe how they anticipate using the medication in their practice. This insight can be beneficial for MS treatment as diverse HCPs may have distinct viewpoints on how DMTs should be started. Pharmacists and physicians on a national level then discuss and decide together what to include in the final drafts of the drug monograph and CFU. Final documents are disseminated to all sites, which encourages consistent practices across the VHA.9 These documents are reviewed on a regular basis and updated as needed based on available literature evidence.
It is well accepted that early use of DMT correlates with lower accumulated long-term disability.10 However, discontinuation of DMT should be treated with equal importance. This benefits the patient by reducing their risk of AEs from DMTs and provides cost savings. Age and disease stability are factors to consider for DMT discontinuation. In a study with patients aged > 45 years and another with patients aged > 60 years, discontinuing DMT rarely had a negative impact and improved quality of life.11,12 A retrospective meta-analysis of age-dependent efficacy of current DMTs predicted that DMT loses efficacy at age 53 years. In addition, higher efficacy DMT only outperforms lower efficacy DMT in patients aged < 40.5 years.13 Stability of disease and lack of relapses for ≥ 2 years also may be a positive predictor to safely discontinue DMT.14,15 The growing literature to support safe discontinuation of DMT makes this a more convincing strategy to avoid unnecessary costs associated with current DMTs. With an average age of 59 years for veterans with MS, this may be one of the largest areas of cost avoidance to consider.
Off-Label Use
Other potential ways to reduce DMT costs is to consider off-label treatments. The OLYMPUS trial studied off-label use of rituximab, an anti-CD20 antibody like ocrelizumab. It did not meet statistical significance for its primary endpoint; however, in a subgroup analysis, off-label use was found to be more effective in a population aged < 51 years.16 Other case reports and smaller scale studies also describe rituximab’s efficacy in MS.17,18 In 2018, the FDA approved the first rituximab biosimilar.19 Further competition from biosimilars likely will make rituximab an even more cost-effective choice when compared with ocrelizumab.
Alternate Dosing Regimens
Extended interval dosing of natalizumab has been studied, extending the standard infusion interval from every 4 weeks to 5- to 8-week intervals. One recent article compared these interval extensions and found that all extended intervals of up to 56 days did not increase new or enhancing lesions on MRI when compared with standard interval dosing.20 Another larger randomized trial is underway to evaluate efficacy and safety of extended interval dosing of natalizumab (NCT03689972). Utilization of this dosing may reduce natalizumab annual costs by up to 50%.
Safety Monitoring
DMF is an oral DMT on the VHA formulary with CFU. Since leukopenia is a known AE, baseline and quarterly monitoring of the complete blood count (CBC) is recommended for patients taking DMF. Additionally, DMF should be held if white blood cell count (WBC) falls below 2,000/mm3.21 There have been recent reports of death secondary to progressive multifocal leukoencephalopathy (PML) among European patients taking DMF.22-24 This has raised concerns about adherence to recommended CBC monitoring in veterans taking DMF. The association of DMF and leukopenia has been evident since early clinical trials.25 Leukopenia in immunocompromised patients increases the risk of PML.
In the long-term extension study ENDORSE, 6% to 7% of patients continuing DMF had WBC counts of 3.0×109/L compared with 7% to 10% in the new to DMF group.26 In addition 6% to 8% of patients continuing DMF had lymphocyte counts of 0.5×109/L, compared with 5% to 9% in the new to DMF group. The cases of PML occurred in patients who had low lymphocyte counts over an extended period with no adjustment to DMF therapy, such as holding the drug until WBC counts returned to normal levels or stopping the drug. Discussion and review within VHA resulted in the recommendation for quarterly WBC monitoring criteria.
PBM and VA Center for Medication Safety (MedSafe) conducted a medication usage evaluation (MUE) on adherence to the WBC monitoring set forth in the CFU. Data collection began in fourth quarter of fiscal year (FY) 2015 with the most recent reporting period of fourth quarter of FY 2017. The Medication Utilization Evaluation Tool tracks patients with no reported WBC in 90 days and WBC < 2,000/mm3. Over the reporting period, 20% to 23% of patients have not received appropriate quarterly monitoring. Additionally, there have been 4 cases where the WBC decreased below the threshold limit. To ensure safe and effective use of DMF, it is important to adhere to the monitoring requirements set forth in the CFU.
Impact of REMS and Special Distribution
As DMTs increase in efficacy, there are often more risks associated with them. Some of these high-risk medications, including natalizumab and alemtuzumab, have REMS programs and/or have special distribution procedures. Although REMS are imperative for patient safety, the complexity of these programs can be difficult to navigate, which can create a barrier to access. The PBM helps to assist all sites with navigating and adhering to required actions to dispense and administer these medications through a national Special Handling Drugs Microsoft SharePoint site, which provides access to REMS forms and procurement information when drugs are dispensed from specialty pharmacies. Easing this process nationwide empowers more sites to be confident they can dispense specialty medications appropriately.
Clinical Pharmacists
The VHA is unique in its utilization of pharmacists in outpatient clinic settings. Utilization of an interdisciplinary team for medication management has been highly used in VHA for areas like primary care; however, pharmacist involvement in specialty areas is on the rise and MS is no exception. Pharmacists stationed in clinics, such as neurology or spinal cord injury, can impact care for veterans with MS. Interdisciplinary teams that include a pharmacist have been shown to increase patient adherence to DMTs.27 However, pharmacists often assist with medication education and monitoring, which adds an additional layer of safety to DMT treatment. At the VHA, pharmacists also can obtain a scope of practice that allows them to prescribe medications and increase access to care for veterans with MS.
Education
The VHA demonstrates how education on a disease state like MS can be distributed on a large, national scale through drug monographs, CFU, and Microsoft SharePoint sites. In addition, VHA has created the MS Centers of Excellence (MSCoE) that serve as a hub of specialized health care providers in all aspects of MS care.
A core function of the MSCoE is to provide education to both HCPs and patients. The MSCoE and its regional hubs support sites that may not have an HCP who specializes in MS by providing advice on DMT selection, how to obtain specialty medications, and monitoring that needs to be completed to ensure veterans’ safety. The MSCoE also has partnered with the National MS Society to hold a lecture series on topics in MS. This free series is available online to all HCPs who interact with patients who have MS and is a way that VA is extending its best practices and expertise beyond its own health care system. There also is a quarterly newsletter for veterans with MS that highlights new information on DMTs that can affect their care.
Conclusion
It is an exciting and challenging period in MS treatment. New DMTs are being approved and entering clinical trials at a rapid pace. These new DMT agents may offer increased efficacy, improvements in AE profiles, and the possibility of increased medication adherence—but often at a higher cost. The utilization of CFU and formulary management provides the ability to ensure the safe and appropriate use of medications by veterans, with a secondary outcome of controlling pharmacy expenditures.
The VHA had expenditures of $142,135,938 for DMT use in FY 2018. As the VHA sees the new contract prices for DMT in January 2020, we are reminded that costs will continue to rise with some pharmaceutical manufacturers implementing prices 8% to 11% higher than 2019 prices, when the consumer price index defines an increase of 1.0% for 2020 and 1.4% in 2021.28 It is imperative that the VHA formulary be managed judiciously and the necessary measures be in place for VHA practitioners to enable effective, safe and value-based care to the veteran population.
1. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81(4):479-484.
2. Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? [published correction appears in Neurology. 2015;85(19):1728]. Neurology. 2015;84(21):2185–2192.
3. San-Juan-Rodriguez A, Good CB, Heyman RA, Parekh N, Shrank WH, Hernandez I. Trends in prices, market share, and spending on self-administered disease-modifying therapies for multiple sclerosis in Medicare Part D. JAMA Neurol. 2019;76(11):1386-1390.
4. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
5. Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis [published correction appears in Mult Scler. 2003;9(6):641]. Mult Scler. 2003;9(3):260-274.
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
7. Cohen J, Belova A, Selmaj K, et al. Equivalence of generic glatiramer acetate in multiple sclerosis: a randomized clinical trial. JAMA Neurol. 2015;72(12):1433-1441.
8. Selmaj K, Barkhof F, Belova AN, et al; GATE study group. Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult Scler. 2017;23(14):1909-1917.
9. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063.
10. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175-187.
11. Hua LH, Harris H, Conway D, Thompson NR. Changes in patient-reported outcomes between continuers and discontinuers of disease modifying therapy in patients with multiple sclerosis over age 60 [published correction appears in Mult Scler Relat Disord. 2019;30:293]. Mult Scler Relat Disord. 2019;30:252-256.
12. Bsteh G, Feige J, Ehling R, et al. Discontinuation of disease-modifying therapies in multiple sclerosis - Clinical outcome and prognostic factors. Mult Scler. 2017;23(9):1241-1248.
13. Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
14. Kister I, Spelman T, Alroughani R, et al; MSBase Study Group. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study [published correction appears in J Neurol Neurosurg Psychiatry. 2019;90(4):e2]. J Neurol Neurosurg Psychiatry. 2016;87(10):1133-1137.
15. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19(1):11-14.
16. Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
17. Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688.
18. Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950–958.
19. Rituximab-abbs [package insert]. North Wales, PA: Teva Pharmaceuticals; 2018.
20. Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(8):885-889.
21. Dimethyl fumarate [package insert]. Cambridge, MA: Biogen Inc; 2015.
22. van Kester MS, Bouwes Bavinck JN, Quint KD. PML in Patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):583-584.
23. Nieuwkamp DJ, Murk JL, van Oosten BW. PML in patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):584.
24. Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372(15):1476-1478.
25. Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler. 2015;21(6):796-797.
26. Gold R, Arnold DL, Bar-Or A, et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: Interim analysis of ENDORSE, a randomized extension study. Mult Scler. 2017;23(2):253–265.
27. Hanson RL, Habibi M, Khamo N, Abdou S, Stubbings J. Integrated clinical and specialty pharmacy practice model for management of patients with multiple sclerosis. Am J Health Syst Pharm. 2014;71(6):463-469.
28. Federal Planning Bureau. Consumer Price Index - Inflation forecasts. https://www.plan.be/databases/17-en-consumer+price+index+inflation+forecasts. Updated March 3, 2020. Accessed March 9, 2020.
1. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81(4):479-484.
2. Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? [published correction appears in Neurology. 2015;85(19):1728]. Neurology. 2015;84(21):2185–2192.
3. San-Juan-Rodriguez A, Good CB, Heyman RA, Parekh N, Shrank WH, Hernandez I. Trends in prices, market share, and spending on self-administered disease-modifying therapies for multiple sclerosis in Medicare Part D. JAMA Neurol. 2019;76(11):1386-1390.
4. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
5. Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis [published correction appears in Mult Scler. 2003;9(6):641]. Mult Scler. 2003;9(3):260-274.
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
7. Cohen J, Belova A, Selmaj K, et al. Equivalence of generic glatiramer acetate in multiple sclerosis: a randomized clinical trial. JAMA Neurol. 2015;72(12):1433-1441.
8. Selmaj K, Barkhof F, Belova AN, et al; GATE study group. Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult Scler. 2017;23(14):1909-1917.
9. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063.
10. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175-187.
11. Hua LH, Harris H, Conway D, Thompson NR. Changes in patient-reported outcomes between continuers and discontinuers of disease modifying therapy in patients with multiple sclerosis over age 60 [published correction appears in Mult Scler Relat Disord. 2019;30:293]. Mult Scler Relat Disord. 2019;30:252-256.
12. Bsteh G, Feige J, Ehling R, et al. Discontinuation of disease-modifying therapies in multiple sclerosis - Clinical outcome and prognostic factors. Mult Scler. 2017;23(9):1241-1248.
13. Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
14. Kister I, Spelman T, Alroughani R, et al; MSBase Study Group. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study [published correction appears in J Neurol Neurosurg Psychiatry. 2019;90(4):e2]. J Neurol Neurosurg Psychiatry. 2016;87(10):1133-1137.
15. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19(1):11-14.
16. Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
17. Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688.
18. Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950–958.
19. Rituximab-abbs [package insert]. North Wales, PA: Teva Pharmaceuticals; 2018.
20. Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(8):885-889.
21. Dimethyl fumarate [package insert]. Cambridge, MA: Biogen Inc; 2015.
22. van Kester MS, Bouwes Bavinck JN, Quint KD. PML in Patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):583-584.
23. Nieuwkamp DJ, Murk JL, van Oosten BW. PML in patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):584.
24. Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372(15):1476-1478.
25. Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler. 2015;21(6):796-797.
26. Gold R, Arnold DL, Bar-Or A, et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: Interim analysis of ENDORSE, a randomized extension study. Mult Scler. 2017;23(2):253–265.
27. Hanson RL, Habibi M, Khamo N, Abdou S, Stubbings J. Integrated clinical and specialty pharmacy practice model for management of patients with multiple sclerosis. Am J Health Syst Pharm. 2014;71(6):463-469.
28. Federal Planning Bureau. Consumer Price Index - Inflation forecasts. https://www.plan.be/databases/17-en-consumer+price+index+inflation+forecasts. Updated March 3, 2020. Accessed March 9, 2020.
Behavioral Interventions in Multiple Sclerosis
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.