User login
Hepatitis in pregnancy: Sorting through the alphabet
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
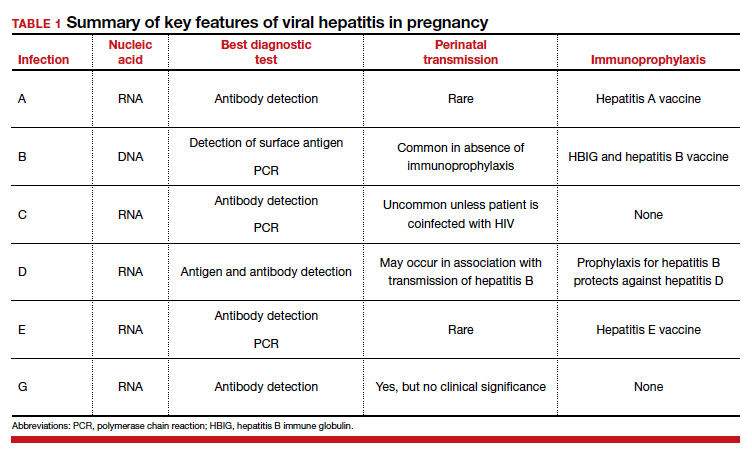
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).
Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
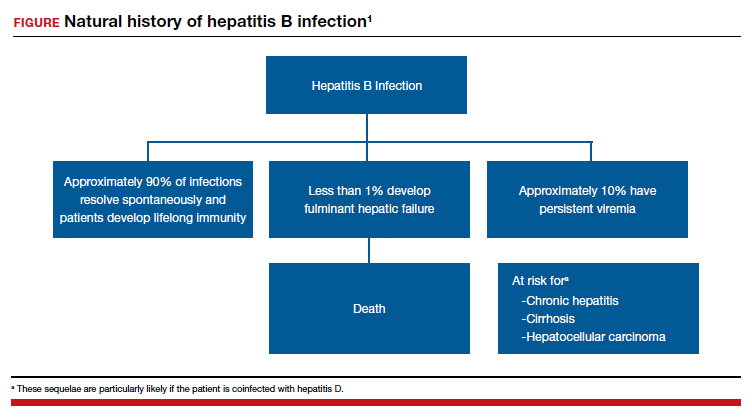
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).

All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).

Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).


All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).

Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).


All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
Dynamic ultrasonography: An idea whose time has come
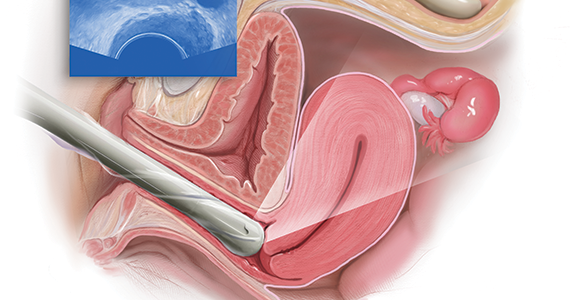
Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
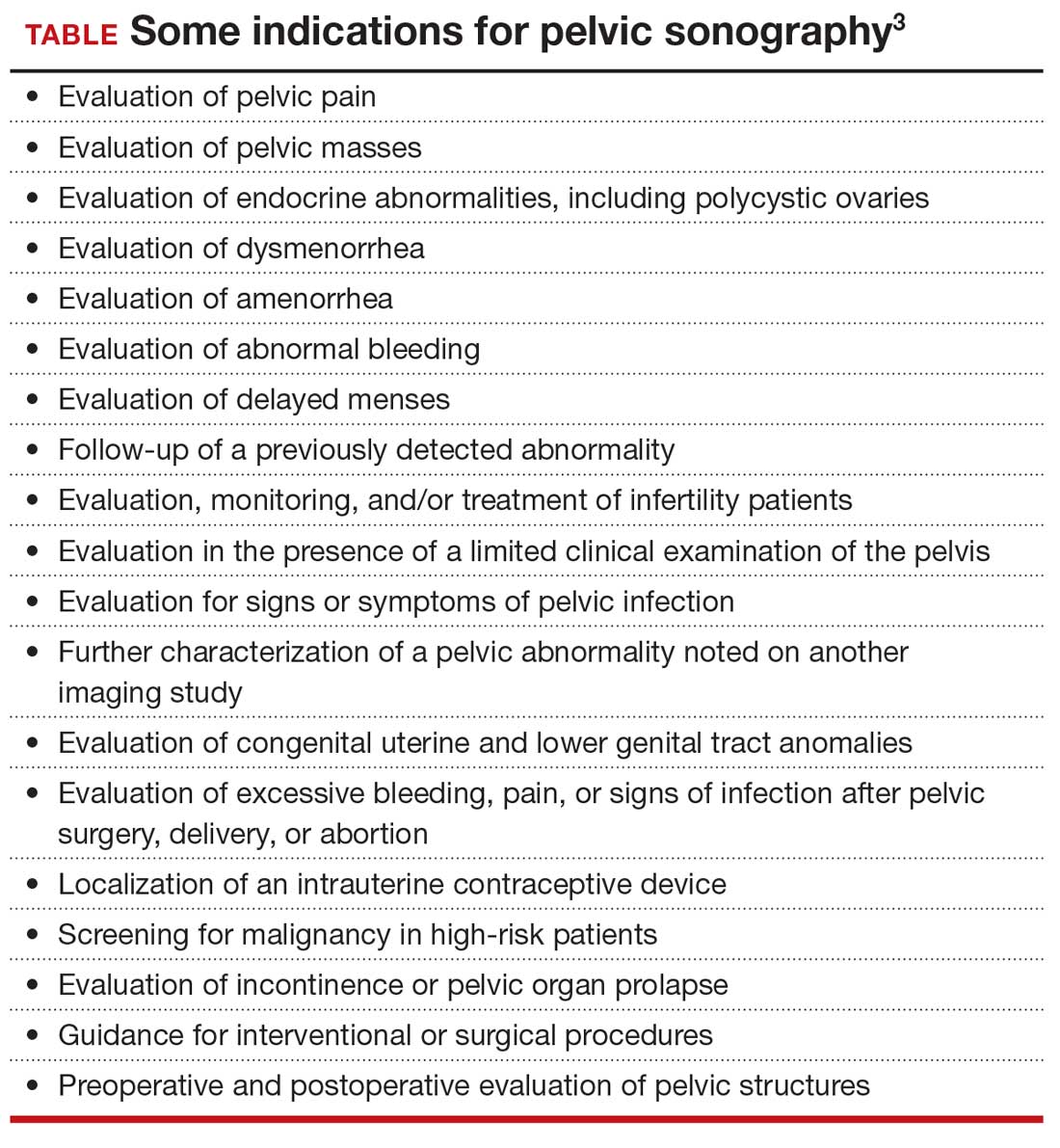
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3
Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
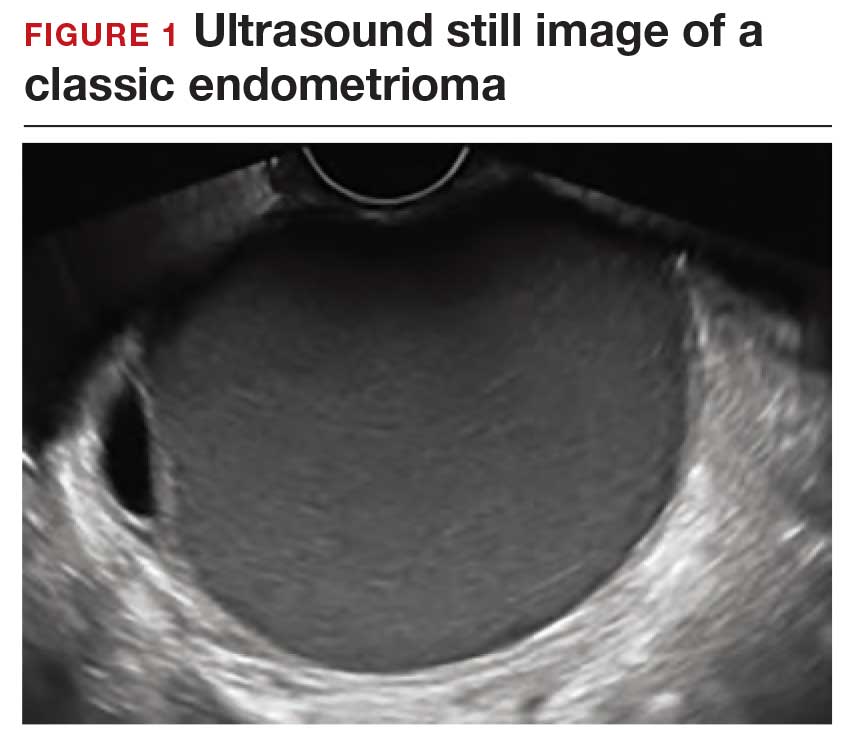
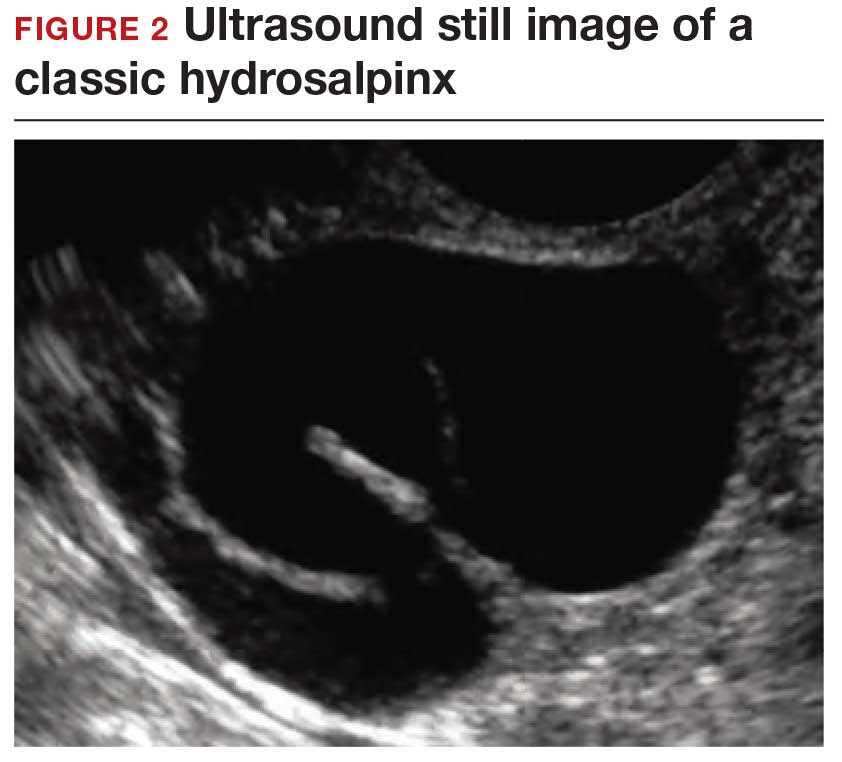
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.
Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.

Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3

Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.


Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation

Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3

Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.


Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.
Squamoid Eccrine Ductal Carcinoma
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).
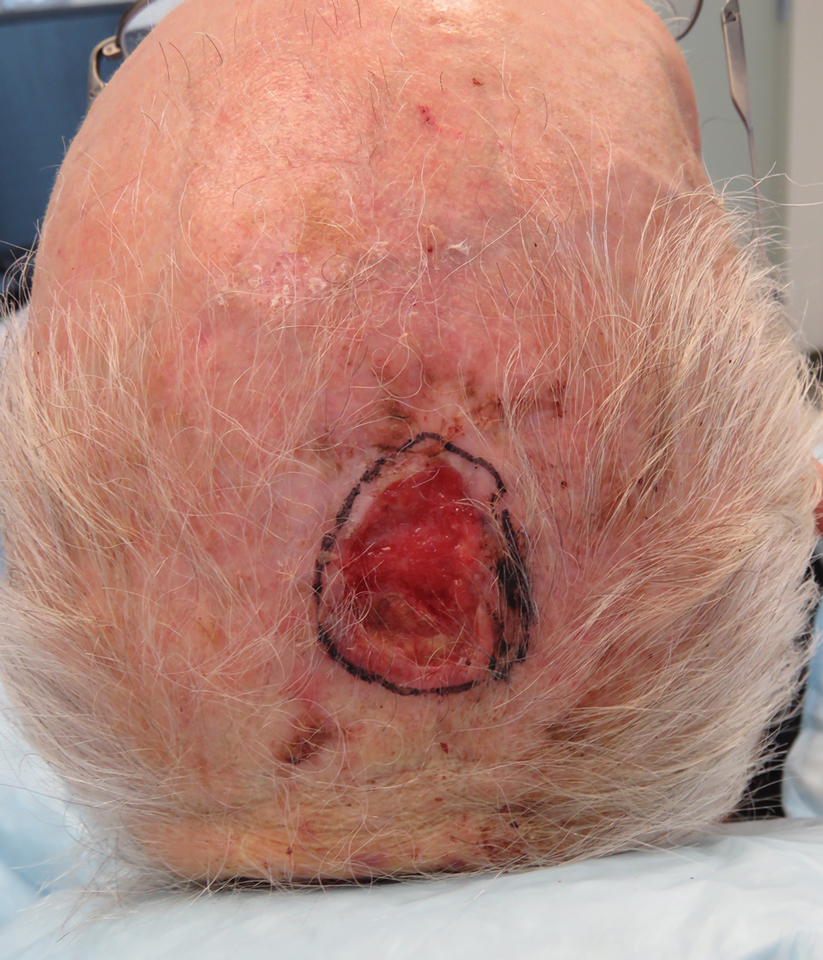
Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.
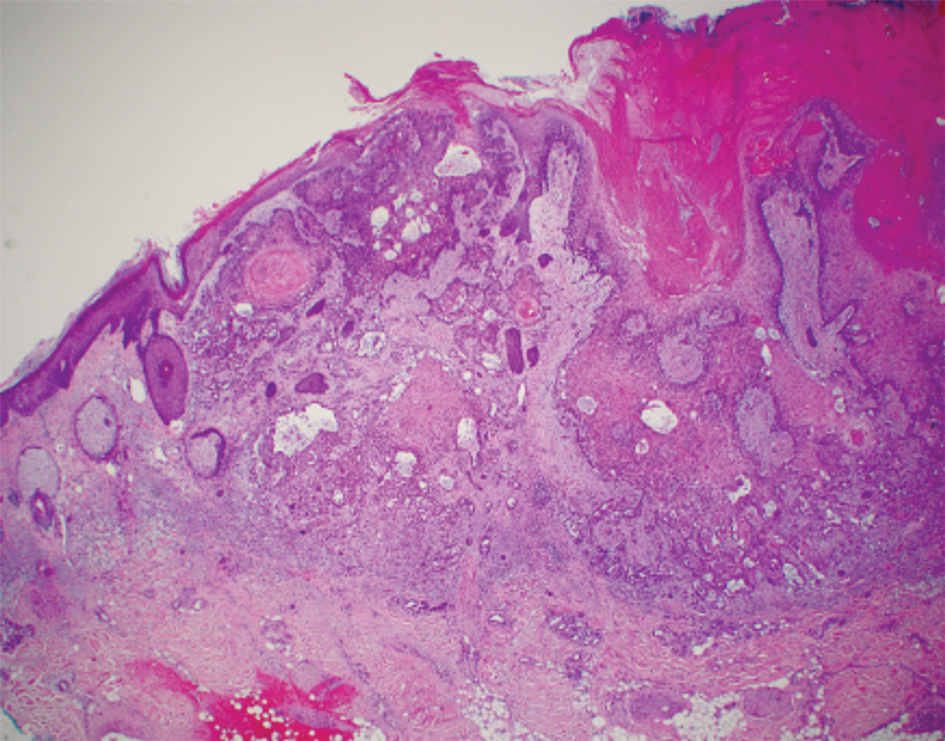


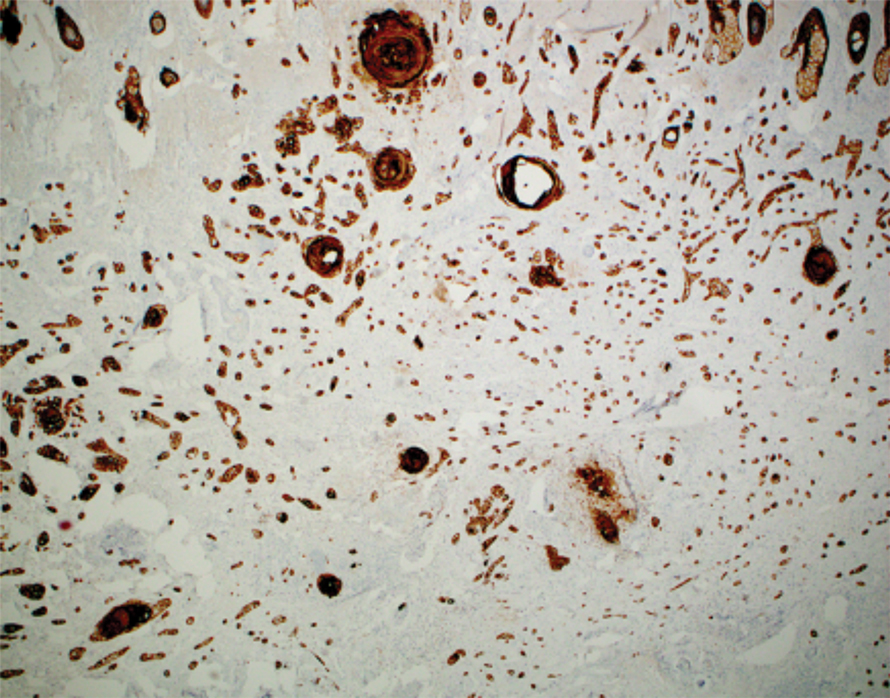
The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).

Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.




The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).

Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.




The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
PRACTICE POINTS
- Squamoid eccrine ductal carcinoma is an aggressive underrecognized cutaneous malignancy that often is misdiagnosed as squamous cell carcinoma (SCC) during initial biopsy.
- Squamoid eccrine ductal carcinoma has a biphasic histologic appearance with a superficial portion resembling well-differentiated SCC and a deeply invasive portion comprised of infiltrative irregular cords with ductal differentiation.
- Excision with complete circumferential peripheral and deep margin assessment with close follow-up is recommended for these patients because of the high risk for recurrence and metastasis.
2021 Update on menopause
Among the studies we review in this Update are a follow-up of the US Women’s Health Initiative clinical trials and a large observational study from the United Kingdom, which exlore the impact of different hormone therapies (HTs) on breast cancer risk. We look at the interesting patterns found by authors of a study in Canada that analyzed predictors of unnecessary bilateral salpingo-oophorectomy. In addition, we review a study that investigates whether hormone therapy can be effective, alone or adjunctively, in peri- and postmenopausal women with depression. Finally, Dr. Chrisandra Shufelt and Dr. JoAnn Manson summarize highlights from the recent American Heart Association’s scientific statement on the menopause transition and increasing risk factors for cardiovascular disease, and how this period can be viewed as an opportunity to encourage healthy, cardiovascular risk–reducing behaviors.
Studies clarify menopausal HT’s impact on breast cancer risk
Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
For many menopausal women, the most worrisome concern related to the use of HT is that it might increase breast cancer risk. In the summer and fall of 2020, 2 important articles were published that addressed how the use of menopausal HT impacts the risk of breast cancer.
The Women’s Health Initiative (WHI) represents the largest and longest-term randomized trial assessing the health impacts of systemic HT. A 2013 WHI report found that with a median of 13 years’ cumulative follow-up, estrogen-only HT (ET) reduced the risk for breast cancer while estrogen-progestin therapy (EPT) increased the risk.1 In a July 2020 issue of JAMA, WHI investigators analyzed longer-term data (cumulative median follow-up >20 years), which allowed assessment of whether these trends (breast cancer incidence) persisted and if they led to changes in mortality from breast cancer.2
WHI data on breast cancer risk trends in ET vs EPT users
In the ET trial, in which Chlebowski and colleagues studied 10,739 women with prior hysterectomy, 238 versus 296 new cases of breast cancer were diagnosed in women in the ET versus placebo groups, respectively (annualized incidence, 0.30% [ET] vs 0.37% [placebo]; hazard ratio [HR], 0.78; P = .005). ET also was associated with significantly lower mortality from breast cancer: 30 versus 46 deaths (annualized mortality, 0.031% [ET] vs 0.046% [placebo]; HR, 0.60; P = 0.04).
In the EPT trial, which included 16,608 participants with an intact uterus, EPT compared with placebo was associated with significantly elevated risk for incident breast cancer: 584 versus 447 new cases, respectively (annualized incidence, 0.45% [EPT] vs 0.36% [placebo]; HR, 1.28; P<.001). However, mortality from breast cancer was similar in the EPT and placebo groups: 71 and 53 deaths (annualized mortality, 0.045% [EPT] and 0.035% [placebo]; HR, 1.35; P = .11).2
For women with previous hysterectomy who are considering initiating or continuing ET for treatment of bothersome menopausal symptoms, the breast cancer mortality benefit documented in this long-term WHI analysis could, as editorialists point out, “tip the scales” in favor of ET.3 Furthermore, the mortality benefit raises the possibility that ET could be evaluated as a risk-reduction strategy for selected high-risk menopausal women who have undergone hysterectomy. Although tamoxifen and aromatase inhibitors are approved for breast cancer chemoprophylaxis in high-risk menopausal women, these agents have not been found to lower breast cancer mortality.2
UK data analysis and risk for breast cancer in HT users
In an October 2020 issue of BMJ, Vinogradova and colleagues described their analysis of 2 primary care databases in the United Kingdom that in aggregate included roughly 99,000 women with breast cancer diagnosed between 1998 and 2018 (age range, 50–79; mean age at diagnosis, 63; >95% White); these were matched with more than 450,000 women without breast cancer (controls).4 Analyses were adjusted for smoking, body mass index (BMI), ethnicity, and mammography.
In this study, ever-use of EPT was associated with an adjusted odds ratio (OR) for breast cancer of 1.26 (95% confidence interval [CI], 1.24–1.29), while ET had an OR of 1.06 (95% CI, 1.03–1.10). In women aged 50 to 59 who used EPT for 5 years or more, 15 additional breast cancers were diagnosed per 10,000 woman-years; for ET users, the attributable risk was 3. Although risk rose with longer HT duration, this trend was less evident with ET than EPT.
In addition, the increased risk associated with ET use was less pronounced in women with a BMI greater than 30 kg/m2. Among EPT users, risks were similar with the progestins medroxyprogesterone acetate (MPA), norethindrone (NET), and levonorgestrel (LNG). Likewise, risks were similar regardless of estrogen dose and route of administration (that is, oral vs transdermal). Vaginal estrogen was not associated with a higher or lower risk for breast cancer. Among past users of ET or EPT (with MPA), no increased risk was noted 5 years or more after stopping HT. For users of EPT (with NET or LNG), risks diminished 5 years or more after stopping HT but remained modestly elevated compared with risk in never-users.4
In this large observational UK study, ET was associated with minimally elevated risk for breast cancer, while in the WHI study, ET reduced the risk for breast cancer. For EPT, the excess risk in both studies was identical. As the authors note, mean BMI in the UK study participants was slightly lower than that in the WHI participants, a distinction that might explain the differing findings with ET use.
In our practice, for women with an intact uterus who are considering the use of EPT for treatment of bothersome menopausal symptoms, we counsel that long-term use of HT slightly elevates the risk for breast cancer. By contrast, we advise posthysterectomy women with bothersome menopausal symptoms that ET does not appear to increase the risk for breast cancer.
Continue to: Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women...
Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women
Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2020;28:8-11. doi: 10.1097/GME.0000000000001652.
While prevention of ovarian cancer is an important benefit of bilateral salpingo-oophorectomy (BSO), performing a BSO at the time of hysterectomy in pre- or perimenopausal patients not only will induce surgical menopause but also is associated with significantly increased overall mortality and an increased risk of mortality due to cardiovascular disease in patients younger than age 45.5,6 Earlier BSO also has been associated with diabetes, accelerated bone density loss, sexual dysfunction, mood disorders, and decreased cognitive function.7
BSO at hysterectomy: How many procedures are not indicated?
To evaluate the prevalence and predictors of unnecessary BSO at the time of hysterectomy, Wong and colleagues conducted a multicenter retrospective review of hysterectomy procedures completed at 6 Canadian hospitals.8 Criteria for unnecessary BSO included age younger than 51 years; benign preoperative diagnosis (other than endometriosis, premenstrual dysphoric disorder, and gender dysphoria); and absence of endometriosis and pelvic adhesions.
A total of 2,656 hysterectomies were performed by 75 surgeons (28 fellowship trained and 47 generalists) across 3 community and 3 tertiary care hospitals between 2016 and 2018. At the time of hysterectomy, 749 patients (28%) underwent BSO. Of these, 509 women (68%) had at least 1 indication for concurrent BSO based on preoperative diagnosis.
Key study findings. Concurrent BSO procedures performed at academic hospitals were more likely to have a preoperative indication compared with BSO performed at community sites (70% vs 63%; OR, 1.42; 95% CI, 1.02–1.97; P = .04). BSO was more likely to be indicated when performed by fellowship-trained surgeons compared with surgeries performed by generalist surgeons (75% vs 63%; OR, 1.76; 95% CI, 1.26–2.44, P = .001). BSO procedures performed with vaginal hysterectomy were less likely to be indicated (3 of 20, 15%) when compared with open hysterectomy (74 of 154, 48%) and laparoscopic hysterectomy (432 of 575, 75%).
Of the patients who lacked a preoperative indication for concomitant BSO, 105 of 239 (43.9%) were younger than age 51. Overall, 8% (59 of 749) of patients in the study cohort had an unnecessary BSO based on a combination of preoperative diagnosis, age younger than age 51, and intraoperative factors including absence of endometriosis and adhesions.
The retrospective study by Wong and colleagues provides the first assessment of Canadian practice patterns with respect to concurrent BSO at the time of hysterectomy. The authors found that, overall, more than two-thirds of BSO procedures were indicated. However, the proportion of BSO that was indicated was higher in teaching hospitals and in surgeries performed by fellowship-trained gynecologists. These important observations underscore the role of clinician education in reducing nonindicated BSO in pre- and perimenopausal women undergoing hysterectomy for benign disease.
Continue to: HT for menopausal depression: Which patients may benefit?
HT for menopausal depression: Which patients may benefit?
Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686- 705. doi:10.1176/appi.ajp.2020.19080848.
The cumulative lifetime prevalence of major depression in US women is 21%.9 An increased risk of mood symptoms and major depressive disorder occurs with the cessation of ovarian hormone production during menopause. In a review of both physiology and clinical studies, an American Psychiatric Association task force found support for several hormone-related strategies for treating depression and highlighted the rapidly advancing, but mixed, findings in this field.10
Clinical trials that examined mood in peri- and postmenopausal women treated with HT have produced mixed results for a variety of reasons, including differences in psychiatric symptomatology across studies and differences in treatment timing in relation to menopause onset.
HT effectiveness for depression depends on menopausal status
Five studies included in a meta-analysis by Rubinow and colleagues examined the use of ET and EPT as antidepressant monotherapy in peri- or postmenopausal women with major depression.11 Of the 3 higher-quality studies, 2 conducted in perimenopausal women demonstrated the antidepressant efficacy of transdermal estrogen patches compared with placebo. The third study included a mixed population of both peri- and postmenopausal women, and it found that increased estradiol levels (spontaneously occurring or due to ET) were associated with improvement in depression in perimenopausal women but not in postmenopausal women.11
ET also has been investigated as a potential adjunctive treatment to selective serotonin reuptake inhibitors (SSRIs). In a retrospective analysis of a multicenter randomized controlled trial of fluoxetine in patients with depression, women who received ET and fluoxetine demonstrated a greater improvement than those who received fluoxetine monotherapy.12 One small study that prospectively assessed ET in combination with an antidepressant in postmenopausal women demonstrated no benefit of ET in treating depression.13 Another small trial found that while combining transdermal ET with an SSRI accelerated symptom improvement, by the end of the 10-week study, treatment efficacy in the HT plus SSRI group was no greater than that observed in the SSRI-only group.14
Nineteen studies included in the metaanalysis by Rubinow and colleagues, which examined mood after ET or EPT treatment in nondepressed women, found little evidence of benefit, particularly in women without other physical symptoms of menopause.11
The Kronos Early Estrogen Prevention Study (KEEPS) followed 661 women who received either oral estrogen plus progesterone, transdermal estrogen plus progesterone, or placebo over 4 years.15 Women with clinical depression were excluded from the study; however, women with mild to moderate mood symptoms who were being treated with an antidepressant were included. Improvements in depressive symptoms and anxiety were observed only in the oral estrogen plus progesterone group compared with the placebo group.15
In a study of 172 euthymic peri- and postmenopausal women treated for 12 months with transdermal estrogen plus oral progesterone, investigators found that, unlike postmenopausal women and those in the late perimenopausal transition, only women in the early perimenopausal transition had a lower risk of developing depressive symptoms.16
Bottom line
This complex literature suggests that ET/HT interventions are most likely to be successful when implemented early in the menopausal transition. The clearest indication for the use of HT is for perimenopausal women experiencing depression who are also experiencing menopausal symptoms (for example, bothersome hot flashes). There is little evidence that the use of ET/HT in late perimenopausal or postmenopausal women effectively treats depression; accordingly, HT is not recommended for the treatment of mood disorders in this population. The more ambiguous cases are those of perimenopausal women who are depressed but do not have classic vasomotor symptoms; some evidence supports the antidepressant efficacy of HT in this setting.11 Although some studies suggest that HT can be effective in preventing depression in perimenopausal women, more evidence is needed.16
A trial of ET/EPT is reasonable in perimenopausal women with depression and classic menopausal symptoms. Use of HT also can be considered either alone or in combination with an SSRI in perimenopausal women with depression who do not have significant classic menopausal symptoms. However, HT is not recommended as prophylaxis against depression in euthymic perimenopausal women. Finally, keep in mind that the use of HT to address mood issues constitutes off-label use.
The menopause transition: A key period for strategizing CVD risk factor reduction

Chrisandra L. Shufelt, MD, MS, NCMP
Dr. Shufelt is Associate Director of the Barbra
Streisand Women’s Heart Center, Smidt
Heart Institute, Cedars-Sinai Medical Center,
Los Angeles, California.

JoAnn E. Manson, MD, DrPH, NCMP
Dr. Manson is Professor of Medicine and the
Michael and Lee Bell Professor of Women’s
Health at Harvard Medical School; Professor
in the Department of Epidemiology, Harvard
T.H. Chan School of Public Health; and Chief
of the Division of Preventive Medicine
at Brigham and Women’s Hospital, Boston,
Massachusetts.
The authors report no financial relationships relevant to this article. Dr. Manson is a coauthor of the AHA Scientific Statement discussed in this article.
In the United States, nearly one-half of a woman’s life, on average, will be lived after menopause. For women with natural menopause, the menopause transition (MT) can begin 2 to 7 years before and may extend 1 year past the final menstrual period, which occurs at an average age of 51 years. For women with surgical menopause, the MT occurs abruptly with the sudden loss of endogenous ovarian hormones. Both types of transitions mark a critical time period when reproduction and endogenous sex hormone levels diminish and when cardiovascular disease (CVD) risk factors begin to rise.
The 2020 American Heart Association (AHA) scientific statement, “Menopause transition and cardiovascular disease risk: Implications for timing of early prevention,” highlights the MT as a window of opportunity for CVD prevention.1
CVD risk factors associated with ovarian aging
In the AHA scientific statement, data from several longitudinal women’s health studies were used to identify which CVD risk factor changes during the MT are related to ovarian aging as opposed to chronologic aging. Independent of aging, those associated with reproductive or ovarian aging included an increase in serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B. Changes in high-density lipoprotein cholesterol (HDL-C) particles and function also occur during the MT, which may explain why higher HDL-C levels during the MT and the postmenopausal years are not as cardioprotective as during the premenopausal period.
Changes in body composition and adipose tissue distribution also are associated with ovarian aging, with reduction in muscle mass and lean body mass and an increase in abdominal/visceral fat and subcutaneous adipose tissue. Although these body composition changes reflect ovarian aging, midlife weight gain is more closely related to chronologic aging.
The risk of the metabolic syndrome constellation of risk factors was found to be more closely associated with ovarian aging, whereas changes in blood pressure, insulin, and glucose individually tracked more closely with chronologic aging. Additionally, the AHA statement notes the research that identified several symptoms during the MT—including vasomotor symptoms, sleep disturbance, and depression—as being associated with more adverse CVD risk factor status and with subclinical measures of atherosclerosis. Additional research on the mechanistic basis for these associations is needed.
Chronologic age and type of menopause
Notably, a woman’s age and type of menopause matter with respect to CVD risk. Higher CVD risk is seen in women with premature onset (age < 40 years) or early onset (age < 45 years) of menopause and in women undergoing surgical menopause (bilateral oophorectomy) before age 45. In general, menopausal hormone therapy (HT) is recommended for women with premature or early menopause, whether natural or surgical, with continuation through at least the average age of natural menopause. In other women, although not recommended for the express purpose of CVD prevention, menopausal HT is appropriate for the treatment of bothersome vasomotor or other menopausal symptoms, especially when therapy is started before age 60 or within 10 years of menopause among women who are not at elevated risk of CVD.
While the AHA statement suggests that some women who begin estrogen early in menopause may experience reduced coronary heart disease risk, major research gaps remain with regard to HT dose, formulation, route of delivery, and recommended duration of treatment.
An opportunity to promote healthy lifestyle behaviors
Translating the AHA’s first-of-its-kind scientific statement into clinical practice requires recognition and awareness of the MT as a unique phase in a woman’s life associated with myriad changes in CVD risk factors. The statement underscores that the MT is an important time to target behavioral changes to promote CVD risk reduction, including lifestyle modifications in the AHA’s Life’s Simple 7 components (increased physical activity, smoking cessation, healthy diet, avoidance of weight gain) as well as vigilant control of blood pressure, cholesterol, and glucose levels. The MT is truly a window of opportunity for reinvigorated efforts to lower women’s CVD risk. ●
Reference
1. El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532. doi: 10.1161/CIR.000000000000912.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353- 1368. doi: 10.1001/jama.2013.278040.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Minami CA, Freedman RA. Menopausal hormone therapy and long-term breast cancer risk: further data from the Women’s Health Initiative trials. JAMA. 2020;324:347-349. doi: 10.1001/jama.2020.9620.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
- Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279. doi: 10.1016/j.ajog.2017.07.037.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15-23. doi: 10.1097/gme.0b013e31818888f7.
- Karp NE, Fenner DE, Burgunder-Zdravkovski L, et al. Removal of normal ovaries in women under age 51 at the time of hysterectomy. Am J Obstetr Gynecol. 2015;213:716.e1-6. doi: 10.1016/j.ajog.2015.05.062.
- Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2021;28:8-11. doi: 10.1097/GME.0000000000001652.
- Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity, and recurrence. J Affect Disord. 1993;29:85- 96. doi: 10.1016/0165-0327(93)00026-g.
- Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686-705. doi:10.1176/appi. ajp.2020.19080848.
- Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32:539-549. doi: 10.1002/ da.22391.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Dias RS, Kerr-Corrêa F, Moreno RA, et al. Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause. 2006;13:202-211. doi:10.1097/01.gme.0000198491.34371.9c.
- Rasgon NL, Dunkin J, Fairbanks L, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41:338- 343. doi: 10.1016/j.jpsychires.2006.03.009.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS–cognitive and affective study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833.
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75:149–157. doi:10.1001/jamapsychiatry.2017.3998.
Among the studies we review in this Update are a follow-up of the US Women’s Health Initiative clinical trials and a large observational study from the United Kingdom, which exlore the impact of different hormone therapies (HTs) on breast cancer risk. We look at the interesting patterns found by authors of a study in Canada that analyzed predictors of unnecessary bilateral salpingo-oophorectomy. In addition, we review a study that investigates whether hormone therapy can be effective, alone or adjunctively, in peri- and postmenopausal women with depression. Finally, Dr. Chrisandra Shufelt and Dr. JoAnn Manson summarize highlights from the recent American Heart Association’s scientific statement on the menopause transition and increasing risk factors for cardiovascular disease, and how this period can be viewed as an opportunity to encourage healthy, cardiovascular risk–reducing behaviors.
Studies clarify menopausal HT’s impact on breast cancer risk
Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
For many menopausal women, the most worrisome concern related to the use of HT is that it might increase breast cancer risk. In the summer and fall of 2020, 2 important articles were published that addressed how the use of menopausal HT impacts the risk of breast cancer.
The Women’s Health Initiative (WHI) represents the largest and longest-term randomized trial assessing the health impacts of systemic HT. A 2013 WHI report found that with a median of 13 years’ cumulative follow-up, estrogen-only HT (ET) reduced the risk for breast cancer while estrogen-progestin therapy (EPT) increased the risk.1 In a July 2020 issue of JAMA, WHI investigators analyzed longer-term data (cumulative median follow-up >20 years), which allowed assessment of whether these trends (breast cancer incidence) persisted and if they led to changes in mortality from breast cancer.2
WHI data on breast cancer risk trends in ET vs EPT users
In the ET trial, in which Chlebowski and colleagues studied 10,739 women with prior hysterectomy, 238 versus 296 new cases of breast cancer were diagnosed in women in the ET versus placebo groups, respectively (annualized incidence, 0.30% [ET] vs 0.37% [placebo]; hazard ratio [HR], 0.78; P = .005). ET also was associated with significantly lower mortality from breast cancer: 30 versus 46 deaths (annualized mortality, 0.031% [ET] vs 0.046% [placebo]; HR, 0.60; P = 0.04).
In the EPT trial, which included 16,608 participants with an intact uterus, EPT compared with placebo was associated with significantly elevated risk for incident breast cancer: 584 versus 447 new cases, respectively (annualized incidence, 0.45% [EPT] vs 0.36% [placebo]; HR, 1.28; P<.001). However, mortality from breast cancer was similar in the EPT and placebo groups: 71 and 53 deaths (annualized mortality, 0.045% [EPT] and 0.035% [placebo]; HR, 1.35; P = .11).2
For women with previous hysterectomy who are considering initiating or continuing ET for treatment of bothersome menopausal symptoms, the breast cancer mortality benefit documented in this long-term WHI analysis could, as editorialists point out, “tip the scales” in favor of ET.3 Furthermore, the mortality benefit raises the possibility that ET could be evaluated as a risk-reduction strategy for selected high-risk menopausal women who have undergone hysterectomy. Although tamoxifen and aromatase inhibitors are approved for breast cancer chemoprophylaxis in high-risk menopausal women, these agents have not been found to lower breast cancer mortality.2
UK data analysis and risk for breast cancer in HT users
In an October 2020 issue of BMJ, Vinogradova and colleagues described their analysis of 2 primary care databases in the United Kingdom that in aggregate included roughly 99,000 women with breast cancer diagnosed between 1998 and 2018 (age range, 50–79; mean age at diagnosis, 63; >95% White); these were matched with more than 450,000 women without breast cancer (controls).4 Analyses were adjusted for smoking, body mass index (BMI), ethnicity, and mammography.
In this study, ever-use of EPT was associated with an adjusted odds ratio (OR) for breast cancer of 1.26 (95% confidence interval [CI], 1.24–1.29), while ET had an OR of 1.06 (95% CI, 1.03–1.10). In women aged 50 to 59 who used EPT for 5 years or more, 15 additional breast cancers were diagnosed per 10,000 woman-years; for ET users, the attributable risk was 3. Although risk rose with longer HT duration, this trend was less evident with ET than EPT.
In addition, the increased risk associated with ET use was less pronounced in women with a BMI greater than 30 kg/m2. Among EPT users, risks were similar with the progestins medroxyprogesterone acetate (MPA), norethindrone (NET), and levonorgestrel (LNG). Likewise, risks were similar regardless of estrogen dose and route of administration (that is, oral vs transdermal). Vaginal estrogen was not associated with a higher or lower risk for breast cancer. Among past users of ET or EPT (with MPA), no increased risk was noted 5 years or more after stopping HT. For users of EPT (with NET or LNG), risks diminished 5 years or more after stopping HT but remained modestly elevated compared with risk in never-users.4
In this large observational UK study, ET was associated with minimally elevated risk for breast cancer, while in the WHI study, ET reduced the risk for breast cancer. For EPT, the excess risk in both studies was identical. As the authors note, mean BMI in the UK study participants was slightly lower than that in the WHI participants, a distinction that might explain the differing findings with ET use.
In our practice, for women with an intact uterus who are considering the use of EPT for treatment of bothersome menopausal symptoms, we counsel that long-term use of HT slightly elevates the risk for breast cancer. By contrast, we advise posthysterectomy women with bothersome menopausal symptoms that ET does not appear to increase the risk for breast cancer.
Continue to: Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women...
Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women
Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2020;28:8-11. doi: 10.1097/GME.0000000000001652.
While prevention of ovarian cancer is an important benefit of bilateral salpingo-oophorectomy (BSO), performing a BSO at the time of hysterectomy in pre- or perimenopausal patients not only will induce surgical menopause but also is associated with significantly increased overall mortality and an increased risk of mortality due to cardiovascular disease in patients younger than age 45.5,6 Earlier BSO also has been associated with diabetes, accelerated bone density loss, sexual dysfunction, mood disorders, and decreased cognitive function.7
BSO at hysterectomy: How many procedures are not indicated?
To evaluate the prevalence and predictors of unnecessary BSO at the time of hysterectomy, Wong and colleagues conducted a multicenter retrospective review of hysterectomy procedures completed at 6 Canadian hospitals.8 Criteria for unnecessary BSO included age younger than 51 years; benign preoperative diagnosis (other than endometriosis, premenstrual dysphoric disorder, and gender dysphoria); and absence of endometriosis and pelvic adhesions.
A total of 2,656 hysterectomies were performed by 75 surgeons (28 fellowship trained and 47 generalists) across 3 community and 3 tertiary care hospitals between 2016 and 2018. At the time of hysterectomy, 749 patients (28%) underwent BSO. Of these, 509 women (68%) had at least 1 indication for concurrent BSO based on preoperative diagnosis.
Key study findings. Concurrent BSO procedures performed at academic hospitals were more likely to have a preoperative indication compared with BSO performed at community sites (70% vs 63%; OR, 1.42; 95% CI, 1.02–1.97; P = .04). BSO was more likely to be indicated when performed by fellowship-trained surgeons compared with surgeries performed by generalist surgeons (75% vs 63%; OR, 1.76; 95% CI, 1.26–2.44, P = .001). BSO procedures performed with vaginal hysterectomy were less likely to be indicated (3 of 20, 15%) when compared with open hysterectomy (74 of 154, 48%) and laparoscopic hysterectomy (432 of 575, 75%).
Of the patients who lacked a preoperative indication for concomitant BSO, 105 of 239 (43.9%) were younger than age 51. Overall, 8% (59 of 749) of patients in the study cohort had an unnecessary BSO based on a combination of preoperative diagnosis, age younger than age 51, and intraoperative factors including absence of endometriosis and adhesions.
The retrospective study by Wong and colleagues provides the first assessment of Canadian practice patterns with respect to concurrent BSO at the time of hysterectomy. The authors found that, overall, more than two-thirds of BSO procedures were indicated. However, the proportion of BSO that was indicated was higher in teaching hospitals and in surgeries performed by fellowship-trained gynecologists. These important observations underscore the role of clinician education in reducing nonindicated BSO in pre- and perimenopausal women undergoing hysterectomy for benign disease.
Continue to: HT for menopausal depression: Which patients may benefit?
HT for menopausal depression: Which patients may benefit?
Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686- 705. doi:10.1176/appi.ajp.2020.19080848.
The cumulative lifetime prevalence of major depression in US women is 21%.9 An increased risk of mood symptoms and major depressive disorder occurs with the cessation of ovarian hormone production during menopause. In a review of both physiology and clinical studies, an American Psychiatric Association task force found support for several hormone-related strategies for treating depression and highlighted the rapidly advancing, but mixed, findings in this field.10
Clinical trials that examined mood in peri- and postmenopausal women treated with HT have produced mixed results for a variety of reasons, including differences in psychiatric symptomatology across studies and differences in treatment timing in relation to menopause onset.
HT effectiveness for depression depends on menopausal status
Five studies included in a meta-analysis by Rubinow and colleagues examined the use of ET and EPT as antidepressant monotherapy in peri- or postmenopausal women with major depression.11 Of the 3 higher-quality studies, 2 conducted in perimenopausal women demonstrated the antidepressant efficacy of transdermal estrogen patches compared with placebo. The third study included a mixed population of both peri- and postmenopausal women, and it found that increased estradiol levels (spontaneously occurring or due to ET) were associated with improvement in depression in perimenopausal women but not in postmenopausal women.11
ET also has been investigated as a potential adjunctive treatment to selective serotonin reuptake inhibitors (SSRIs). In a retrospective analysis of a multicenter randomized controlled trial of fluoxetine in patients with depression, women who received ET and fluoxetine demonstrated a greater improvement than those who received fluoxetine monotherapy.12 One small study that prospectively assessed ET in combination with an antidepressant in postmenopausal women demonstrated no benefit of ET in treating depression.13 Another small trial found that while combining transdermal ET with an SSRI accelerated symptom improvement, by the end of the 10-week study, treatment efficacy in the HT plus SSRI group was no greater than that observed in the SSRI-only group.14
Nineteen studies included in the metaanalysis by Rubinow and colleagues, which examined mood after ET or EPT treatment in nondepressed women, found little evidence of benefit, particularly in women without other physical symptoms of menopause.11
The Kronos Early Estrogen Prevention Study (KEEPS) followed 661 women who received either oral estrogen plus progesterone, transdermal estrogen plus progesterone, or placebo over 4 years.15 Women with clinical depression were excluded from the study; however, women with mild to moderate mood symptoms who were being treated with an antidepressant were included. Improvements in depressive symptoms and anxiety were observed only in the oral estrogen plus progesterone group compared with the placebo group.15
In a study of 172 euthymic peri- and postmenopausal women treated for 12 months with transdermal estrogen plus oral progesterone, investigators found that, unlike postmenopausal women and those in the late perimenopausal transition, only women in the early perimenopausal transition had a lower risk of developing depressive symptoms.16
Bottom line
This complex literature suggests that ET/HT interventions are most likely to be successful when implemented early in the menopausal transition. The clearest indication for the use of HT is for perimenopausal women experiencing depression who are also experiencing menopausal symptoms (for example, bothersome hot flashes). There is little evidence that the use of ET/HT in late perimenopausal or postmenopausal women effectively treats depression; accordingly, HT is not recommended for the treatment of mood disorders in this population. The more ambiguous cases are those of perimenopausal women who are depressed but do not have classic vasomotor symptoms; some evidence supports the antidepressant efficacy of HT in this setting.11 Although some studies suggest that HT can be effective in preventing depression in perimenopausal women, more evidence is needed.16
A trial of ET/EPT is reasonable in perimenopausal women with depression and classic menopausal symptoms. Use of HT also can be considered either alone or in combination with an SSRI in perimenopausal women with depression who do not have significant classic menopausal symptoms. However, HT is not recommended as prophylaxis against depression in euthymic perimenopausal women. Finally, keep in mind that the use of HT to address mood issues constitutes off-label use.
The menopause transition: A key period for strategizing CVD risk factor reduction

Chrisandra L. Shufelt, MD, MS, NCMP
Dr. Shufelt is Associate Director of the Barbra
Streisand Women’s Heart Center, Smidt
Heart Institute, Cedars-Sinai Medical Center,
Los Angeles, California.

JoAnn E. Manson, MD, DrPH, NCMP
Dr. Manson is Professor of Medicine and the
Michael and Lee Bell Professor of Women’s
Health at Harvard Medical School; Professor
in the Department of Epidemiology, Harvard
T.H. Chan School of Public Health; and Chief
of the Division of Preventive Medicine
at Brigham and Women’s Hospital, Boston,
Massachusetts.
The authors report no financial relationships relevant to this article. Dr. Manson is a coauthor of the AHA Scientific Statement discussed in this article.
In the United States, nearly one-half of a woman’s life, on average, will be lived after menopause. For women with natural menopause, the menopause transition (MT) can begin 2 to 7 years before and may extend 1 year past the final menstrual period, which occurs at an average age of 51 years. For women with surgical menopause, the MT occurs abruptly with the sudden loss of endogenous ovarian hormones. Both types of transitions mark a critical time period when reproduction and endogenous sex hormone levels diminish and when cardiovascular disease (CVD) risk factors begin to rise.
The 2020 American Heart Association (AHA) scientific statement, “Menopause transition and cardiovascular disease risk: Implications for timing of early prevention,” highlights the MT as a window of opportunity for CVD prevention.1
CVD risk factors associated with ovarian aging
In the AHA scientific statement, data from several longitudinal women’s health studies were used to identify which CVD risk factor changes during the MT are related to ovarian aging as opposed to chronologic aging. Independent of aging, those associated with reproductive or ovarian aging included an increase in serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B. Changes in high-density lipoprotein cholesterol (HDL-C) particles and function also occur during the MT, which may explain why higher HDL-C levels during the MT and the postmenopausal years are not as cardioprotective as during the premenopausal period.
Changes in body composition and adipose tissue distribution also are associated with ovarian aging, with reduction in muscle mass and lean body mass and an increase in abdominal/visceral fat and subcutaneous adipose tissue. Although these body composition changes reflect ovarian aging, midlife weight gain is more closely related to chronologic aging.
The risk of the metabolic syndrome constellation of risk factors was found to be more closely associated with ovarian aging, whereas changes in blood pressure, insulin, and glucose individually tracked more closely with chronologic aging. Additionally, the AHA statement notes the research that identified several symptoms during the MT—including vasomotor symptoms, sleep disturbance, and depression—as being associated with more adverse CVD risk factor status and with subclinical measures of atherosclerosis. Additional research on the mechanistic basis for these associations is needed.
Chronologic age and type of menopause
Notably, a woman’s age and type of menopause matter with respect to CVD risk. Higher CVD risk is seen in women with premature onset (age < 40 years) or early onset (age < 45 years) of menopause and in women undergoing surgical menopause (bilateral oophorectomy) before age 45. In general, menopausal hormone therapy (HT) is recommended for women with premature or early menopause, whether natural or surgical, with continuation through at least the average age of natural menopause. In other women, although not recommended for the express purpose of CVD prevention, menopausal HT is appropriate for the treatment of bothersome vasomotor or other menopausal symptoms, especially when therapy is started before age 60 or within 10 years of menopause among women who are not at elevated risk of CVD.
While the AHA statement suggests that some women who begin estrogen early in menopause may experience reduced coronary heart disease risk, major research gaps remain with regard to HT dose, formulation, route of delivery, and recommended duration of treatment.
An opportunity to promote healthy lifestyle behaviors
Translating the AHA’s first-of-its-kind scientific statement into clinical practice requires recognition and awareness of the MT as a unique phase in a woman’s life associated with myriad changes in CVD risk factors. The statement underscores that the MT is an important time to target behavioral changes to promote CVD risk reduction, including lifestyle modifications in the AHA’s Life’s Simple 7 components (increased physical activity, smoking cessation, healthy diet, avoidance of weight gain) as well as vigilant control of blood pressure, cholesterol, and glucose levels. The MT is truly a window of opportunity for reinvigorated efforts to lower women’s CVD risk. ●
Reference
1. El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532. doi: 10.1161/CIR.000000000000912.
Among the studies we review in this Update are a follow-up of the US Women’s Health Initiative clinical trials and a large observational study from the United Kingdom, which exlore the impact of different hormone therapies (HTs) on breast cancer risk. We look at the interesting patterns found by authors of a study in Canada that analyzed predictors of unnecessary bilateral salpingo-oophorectomy. In addition, we review a study that investigates whether hormone therapy can be effective, alone or adjunctively, in peri- and postmenopausal women with depression. Finally, Dr. Chrisandra Shufelt and Dr. JoAnn Manson summarize highlights from the recent American Heart Association’s scientific statement on the menopause transition and increasing risk factors for cardiovascular disease, and how this period can be viewed as an opportunity to encourage healthy, cardiovascular risk–reducing behaviors.
Studies clarify menopausal HT’s impact on breast cancer risk
Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
For many menopausal women, the most worrisome concern related to the use of HT is that it might increase breast cancer risk. In the summer and fall of 2020, 2 important articles were published that addressed how the use of menopausal HT impacts the risk of breast cancer.
The Women’s Health Initiative (WHI) represents the largest and longest-term randomized trial assessing the health impacts of systemic HT. A 2013 WHI report found that with a median of 13 years’ cumulative follow-up, estrogen-only HT (ET) reduced the risk for breast cancer while estrogen-progestin therapy (EPT) increased the risk.1 In a July 2020 issue of JAMA, WHI investigators analyzed longer-term data (cumulative median follow-up >20 years), which allowed assessment of whether these trends (breast cancer incidence) persisted and if they led to changes in mortality from breast cancer.2
WHI data on breast cancer risk trends in ET vs EPT users
In the ET trial, in which Chlebowski and colleagues studied 10,739 women with prior hysterectomy, 238 versus 296 new cases of breast cancer were diagnosed in women in the ET versus placebo groups, respectively (annualized incidence, 0.30% [ET] vs 0.37% [placebo]; hazard ratio [HR], 0.78; P = .005). ET also was associated with significantly lower mortality from breast cancer: 30 versus 46 deaths (annualized mortality, 0.031% [ET] vs 0.046% [placebo]; HR, 0.60; P = 0.04).
In the EPT trial, which included 16,608 participants with an intact uterus, EPT compared with placebo was associated with significantly elevated risk for incident breast cancer: 584 versus 447 new cases, respectively (annualized incidence, 0.45% [EPT] vs 0.36% [placebo]; HR, 1.28; P<.001). However, mortality from breast cancer was similar in the EPT and placebo groups: 71 and 53 deaths (annualized mortality, 0.045% [EPT] and 0.035% [placebo]; HR, 1.35; P = .11).2
For women with previous hysterectomy who are considering initiating or continuing ET for treatment of bothersome menopausal symptoms, the breast cancer mortality benefit documented in this long-term WHI analysis could, as editorialists point out, “tip the scales” in favor of ET.3 Furthermore, the mortality benefit raises the possibility that ET could be evaluated as a risk-reduction strategy for selected high-risk menopausal women who have undergone hysterectomy. Although tamoxifen and aromatase inhibitors are approved for breast cancer chemoprophylaxis in high-risk menopausal women, these agents have not been found to lower breast cancer mortality.2
UK data analysis and risk for breast cancer in HT users
In an October 2020 issue of BMJ, Vinogradova and colleagues described their analysis of 2 primary care databases in the United Kingdom that in aggregate included roughly 99,000 women with breast cancer diagnosed between 1998 and 2018 (age range, 50–79; mean age at diagnosis, 63; >95% White); these were matched with more than 450,000 women without breast cancer (controls).4 Analyses were adjusted for smoking, body mass index (BMI), ethnicity, and mammography.
In this study, ever-use of EPT was associated with an adjusted odds ratio (OR) for breast cancer of 1.26 (95% confidence interval [CI], 1.24–1.29), while ET had an OR of 1.06 (95% CI, 1.03–1.10). In women aged 50 to 59 who used EPT for 5 years or more, 15 additional breast cancers were diagnosed per 10,000 woman-years; for ET users, the attributable risk was 3. Although risk rose with longer HT duration, this trend was less evident with ET than EPT.
In addition, the increased risk associated with ET use was less pronounced in women with a BMI greater than 30 kg/m2. Among EPT users, risks were similar with the progestins medroxyprogesterone acetate (MPA), norethindrone (NET), and levonorgestrel (LNG). Likewise, risks were similar regardless of estrogen dose and route of administration (that is, oral vs transdermal). Vaginal estrogen was not associated with a higher or lower risk for breast cancer. Among past users of ET or EPT (with MPA), no increased risk was noted 5 years or more after stopping HT. For users of EPT (with NET or LNG), risks diminished 5 years or more after stopping HT but remained modestly elevated compared with risk in never-users.4
In this large observational UK study, ET was associated with minimally elevated risk for breast cancer, while in the WHI study, ET reduced the risk for breast cancer. For EPT, the excess risk in both studies was identical. As the authors note, mean BMI in the UK study participants was slightly lower than that in the WHI participants, a distinction that might explain the differing findings with ET use.
In our practice, for women with an intact uterus who are considering the use of EPT for treatment of bothersome menopausal symptoms, we counsel that long-term use of HT slightly elevates the risk for breast cancer. By contrast, we advise posthysterectomy women with bothersome menopausal symptoms that ET does not appear to increase the risk for breast cancer.
Continue to: Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women...
Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women
Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2020;28:8-11. doi: 10.1097/GME.0000000000001652.
While prevention of ovarian cancer is an important benefit of bilateral salpingo-oophorectomy (BSO), performing a BSO at the time of hysterectomy in pre- or perimenopausal patients not only will induce surgical menopause but also is associated with significantly increased overall mortality and an increased risk of mortality due to cardiovascular disease in patients younger than age 45.5,6 Earlier BSO also has been associated with diabetes, accelerated bone density loss, sexual dysfunction, mood disorders, and decreased cognitive function.7
BSO at hysterectomy: How many procedures are not indicated?
To evaluate the prevalence and predictors of unnecessary BSO at the time of hysterectomy, Wong and colleagues conducted a multicenter retrospective review of hysterectomy procedures completed at 6 Canadian hospitals.8 Criteria for unnecessary BSO included age younger than 51 years; benign preoperative diagnosis (other than endometriosis, premenstrual dysphoric disorder, and gender dysphoria); and absence of endometriosis and pelvic adhesions.
A total of 2,656 hysterectomies were performed by 75 surgeons (28 fellowship trained and 47 generalists) across 3 community and 3 tertiary care hospitals between 2016 and 2018. At the time of hysterectomy, 749 patients (28%) underwent BSO. Of these, 509 women (68%) had at least 1 indication for concurrent BSO based on preoperative diagnosis.
Key study findings. Concurrent BSO procedures performed at academic hospitals were more likely to have a preoperative indication compared with BSO performed at community sites (70% vs 63%; OR, 1.42; 95% CI, 1.02–1.97; P = .04). BSO was more likely to be indicated when performed by fellowship-trained surgeons compared with surgeries performed by generalist surgeons (75% vs 63%; OR, 1.76; 95% CI, 1.26–2.44, P = .001). BSO procedures performed with vaginal hysterectomy were less likely to be indicated (3 of 20, 15%) when compared with open hysterectomy (74 of 154, 48%) and laparoscopic hysterectomy (432 of 575, 75%).
Of the patients who lacked a preoperative indication for concomitant BSO, 105 of 239 (43.9%) were younger than age 51. Overall, 8% (59 of 749) of patients in the study cohort had an unnecessary BSO based on a combination of preoperative diagnosis, age younger than age 51, and intraoperative factors including absence of endometriosis and adhesions.
The retrospective study by Wong and colleagues provides the first assessment of Canadian practice patterns with respect to concurrent BSO at the time of hysterectomy. The authors found that, overall, more than two-thirds of BSO procedures were indicated. However, the proportion of BSO that was indicated was higher in teaching hospitals and in surgeries performed by fellowship-trained gynecologists. These important observations underscore the role of clinician education in reducing nonindicated BSO in pre- and perimenopausal women undergoing hysterectomy for benign disease.
Continue to: HT for menopausal depression: Which patients may benefit?
HT for menopausal depression: Which patients may benefit?
Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686- 705. doi:10.1176/appi.ajp.2020.19080848.
The cumulative lifetime prevalence of major depression in US women is 21%.9 An increased risk of mood symptoms and major depressive disorder occurs with the cessation of ovarian hormone production during menopause. In a review of both physiology and clinical studies, an American Psychiatric Association task force found support for several hormone-related strategies for treating depression and highlighted the rapidly advancing, but mixed, findings in this field.10
Clinical trials that examined mood in peri- and postmenopausal women treated with HT have produced mixed results for a variety of reasons, including differences in psychiatric symptomatology across studies and differences in treatment timing in relation to menopause onset.
HT effectiveness for depression depends on menopausal status
Five studies included in a meta-analysis by Rubinow and colleagues examined the use of ET and EPT as antidepressant monotherapy in peri- or postmenopausal women with major depression.11 Of the 3 higher-quality studies, 2 conducted in perimenopausal women demonstrated the antidepressant efficacy of transdermal estrogen patches compared with placebo. The third study included a mixed population of both peri- and postmenopausal women, and it found that increased estradiol levels (spontaneously occurring or due to ET) were associated with improvement in depression in perimenopausal women but not in postmenopausal women.11
ET also has been investigated as a potential adjunctive treatment to selective serotonin reuptake inhibitors (SSRIs). In a retrospective analysis of a multicenter randomized controlled trial of fluoxetine in patients with depression, women who received ET and fluoxetine demonstrated a greater improvement than those who received fluoxetine monotherapy.12 One small study that prospectively assessed ET in combination with an antidepressant in postmenopausal women demonstrated no benefit of ET in treating depression.13 Another small trial found that while combining transdermal ET with an SSRI accelerated symptom improvement, by the end of the 10-week study, treatment efficacy in the HT plus SSRI group was no greater than that observed in the SSRI-only group.14
Nineteen studies included in the metaanalysis by Rubinow and colleagues, which examined mood after ET or EPT treatment in nondepressed women, found little evidence of benefit, particularly in women without other physical symptoms of menopause.11
The Kronos Early Estrogen Prevention Study (KEEPS) followed 661 women who received either oral estrogen plus progesterone, transdermal estrogen plus progesterone, or placebo over 4 years.15 Women with clinical depression were excluded from the study; however, women with mild to moderate mood symptoms who were being treated with an antidepressant were included. Improvements in depressive symptoms and anxiety were observed only in the oral estrogen plus progesterone group compared with the placebo group.15
In a study of 172 euthymic peri- and postmenopausal women treated for 12 months with transdermal estrogen plus oral progesterone, investigators found that, unlike postmenopausal women and those in the late perimenopausal transition, only women in the early perimenopausal transition had a lower risk of developing depressive symptoms.16
Bottom line
This complex literature suggests that ET/HT interventions are most likely to be successful when implemented early in the menopausal transition. The clearest indication for the use of HT is for perimenopausal women experiencing depression who are also experiencing menopausal symptoms (for example, bothersome hot flashes). There is little evidence that the use of ET/HT in late perimenopausal or postmenopausal women effectively treats depression; accordingly, HT is not recommended for the treatment of mood disorders in this population. The more ambiguous cases are those of perimenopausal women who are depressed but do not have classic vasomotor symptoms; some evidence supports the antidepressant efficacy of HT in this setting.11 Although some studies suggest that HT can be effective in preventing depression in perimenopausal women, more evidence is needed.16
A trial of ET/EPT is reasonable in perimenopausal women with depression and classic menopausal symptoms. Use of HT also can be considered either alone or in combination with an SSRI in perimenopausal women with depression who do not have significant classic menopausal symptoms. However, HT is not recommended as prophylaxis against depression in euthymic perimenopausal women. Finally, keep in mind that the use of HT to address mood issues constitutes off-label use.
The menopause transition: A key period for strategizing CVD risk factor reduction

Chrisandra L. Shufelt, MD, MS, NCMP
Dr. Shufelt is Associate Director of the Barbra
Streisand Women’s Heart Center, Smidt
Heart Institute, Cedars-Sinai Medical Center,
Los Angeles, California.

JoAnn E. Manson, MD, DrPH, NCMP
Dr. Manson is Professor of Medicine and the
Michael and Lee Bell Professor of Women’s
Health at Harvard Medical School; Professor
in the Department of Epidemiology, Harvard
T.H. Chan School of Public Health; and Chief
of the Division of Preventive Medicine
at Brigham and Women’s Hospital, Boston,
Massachusetts.
The authors report no financial relationships relevant to this article. Dr. Manson is a coauthor of the AHA Scientific Statement discussed in this article.
In the United States, nearly one-half of a woman’s life, on average, will be lived after menopause. For women with natural menopause, the menopause transition (MT) can begin 2 to 7 years before and may extend 1 year past the final menstrual period, which occurs at an average age of 51 years. For women with surgical menopause, the MT occurs abruptly with the sudden loss of endogenous ovarian hormones. Both types of transitions mark a critical time period when reproduction and endogenous sex hormone levels diminish and when cardiovascular disease (CVD) risk factors begin to rise.
The 2020 American Heart Association (AHA) scientific statement, “Menopause transition and cardiovascular disease risk: Implications for timing of early prevention,” highlights the MT as a window of opportunity for CVD prevention.1
CVD risk factors associated with ovarian aging
In the AHA scientific statement, data from several longitudinal women’s health studies were used to identify which CVD risk factor changes during the MT are related to ovarian aging as opposed to chronologic aging. Independent of aging, those associated with reproductive or ovarian aging included an increase in serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B. Changes in high-density lipoprotein cholesterol (HDL-C) particles and function also occur during the MT, which may explain why higher HDL-C levels during the MT and the postmenopausal years are not as cardioprotective as during the premenopausal period.
Changes in body composition and adipose tissue distribution also are associated with ovarian aging, with reduction in muscle mass and lean body mass and an increase in abdominal/visceral fat and subcutaneous adipose tissue. Although these body composition changes reflect ovarian aging, midlife weight gain is more closely related to chronologic aging.
The risk of the metabolic syndrome constellation of risk factors was found to be more closely associated with ovarian aging, whereas changes in blood pressure, insulin, and glucose individually tracked more closely with chronologic aging. Additionally, the AHA statement notes the research that identified several symptoms during the MT—including vasomotor symptoms, sleep disturbance, and depression—as being associated with more adverse CVD risk factor status and with subclinical measures of atherosclerosis. Additional research on the mechanistic basis for these associations is needed.
Chronologic age and type of menopause
Notably, a woman’s age and type of menopause matter with respect to CVD risk. Higher CVD risk is seen in women with premature onset (age < 40 years) or early onset (age < 45 years) of menopause and in women undergoing surgical menopause (bilateral oophorectomy) before age 45. In general, menopausal hormone therapy (HT) is recommended for women with premature or early menopause, whether natural or surgical, with continuation through at least the average age of natural menopause. In other women, although not recommended for the express purpose of CVD prevention, menopausal HT is appropriate for the treatment of bothersome vasomotor or other menopausal symptoms, especially when therapy is started before age 60 or within 10 years of menopause among women who are not at elevated risk of CVD.
While the AHA statement suggests that some women who begin estrogen early in menopause may experience reduced coronary heart disease risk, major research gaps remain with regard to HT dose, formulation, route of delivery, and recommended duration of treatment.
An opportunity to promote healthy lifestyle behaviors
Translating the AHA’s first-of-its-kind scientific statement into clinical practice requires recognition and awareness of the MT as a unique phase in a woman’s life associated with myriad changes in CVD risk factors. The statement underscores that the MT is an important time to target behavioral changes to promote CVD risk reduction, including lifestyle modifications in the AHA’s Life’s Simple 7 components (increased physical activity, smoking cessation, healthy diet, avoidance of weight gain) as well as vigilant control of blood pressure, cholesterol, and glucose levels. The MT is truly a window of opportunity for reinvigorated efforts to lower women’s CVD risk. ●
Reference
1. El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532. doi: 10.1161/CIR.000000000000912.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353- 1368. doi: 10.1001/jama.2013.278040.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Minami CA, Freedman RA. Menopausal hormone therapy and long-term breast cancer risk: further data from the Women’s Health Initiative trials. JAMA. 2020;324:347-349. doi: 10.1001/jama.2020.9620.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
- Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279. doi: 10.1016/j.ajog.2017.07.037.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15-23. doi: 10.1097/gme.0b013e31818888f7.
- Karp NE, Fenner DE, Burgunder-Zdravkovski L, et al. Removal of normal ovaries in women under age 51 at the time of hysterectomy. Am J Obstetr Gynecol. 2015;213:716.e1-6. doi: 10.1016/j.ajog.2015.05.062.
- Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2021;28:8-11. doi: 10.1097/GME.0000000000001652.
- Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity, and recurrence. J Affect Disord. 1993;29:85- 96. doi: 10.1016/0165-0327(93)00026-g.
- Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686-705. doi:10.1176/appi. ajp.2020.19080848.
- Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32:539-549. doi: 10.1002/ da.22391.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Dias RS, Kerr-Corrêa F, Moreno RA, et al. Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause. 2006;13:202-211. doi:10.1097/01.gme.0000198491.34371.9c.
- Rasgon NL, Dunkin J, Fairbanks L, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41:338- 343. doi: 10.1016/j.jpsychires.2006.03.009.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS–cognitive and affective study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833.
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75:149–157. doi:10.1001/jamapsychiatry.2017.3998.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353- 1368. doi: 10.1001/jama.2013.278040.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Minami CA, Freedman RA. Menopausal hormone therapy and long-term breast cancer risk: further data from the Women’s Health Initiative trials. JAMA. 2020;324:347-349. doi: 10.1001/jama.2020.9620.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
- Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279. doi: 10.1016/j.ajog.2017.07.037.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15-23. doi: 10.1097/gme.0b013e31818888f7.
- Karp NE, Fenner DE, Burgunder-Zdravkovski L, et al. Removal of normal ovaries in women under age 51 at the time of hysterectomy. Am J Obstetr Gynecol. 2015;213:716.e1-6. doi: 10.1016/j.ajog.2015.05.062.
- Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2021;28:8-11. doi: 10.1097/GME.0000000000001652.
- Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity, and recurrence. J Affect Disord. 1993;29:85- 96. doi: 10.1016/0165-0327(93)00026-g.
- Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686-705. doi:10.1176/appi. ajp.2020.19080848.
- Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32:539-549. doi: 10.1002/ da.22391.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Dias RS, Kerr-Corrêa F, Moreno RA, et al. Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause. 2006;13:202-211. doi:10.1097/01.gme.0000198491.34371.9c.
- Rasgon NL, Dunkin J, Fairbanks L, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41:338- 343. doi: 10.1016/j.jpsychires.2006.03.009.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS–cognitive and affective study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833.
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75:149–157. doi:10.1001/jamapsychiatry.2017.3998.
How to choose the right vaginal moisturizer or lubricant for your patient
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.
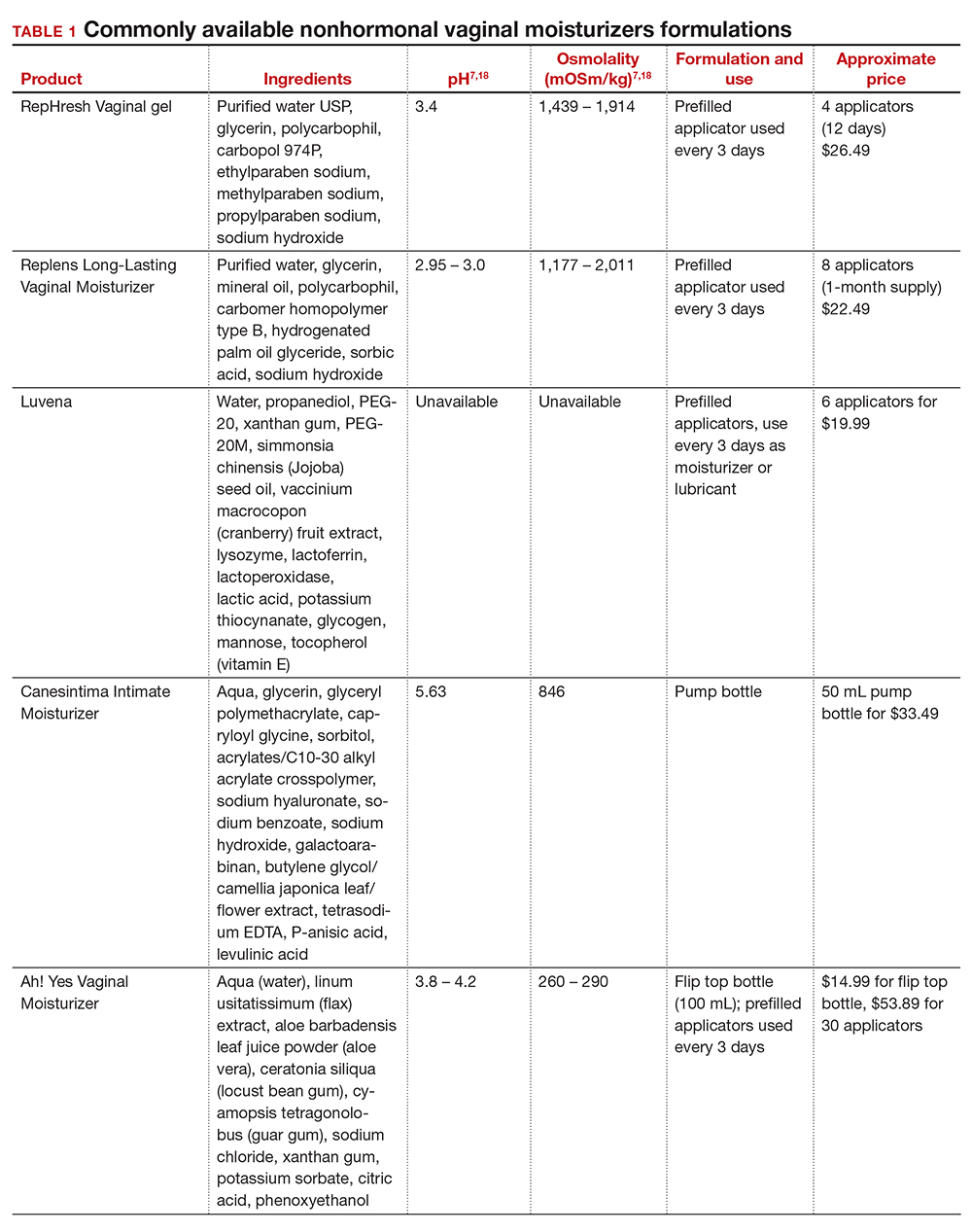
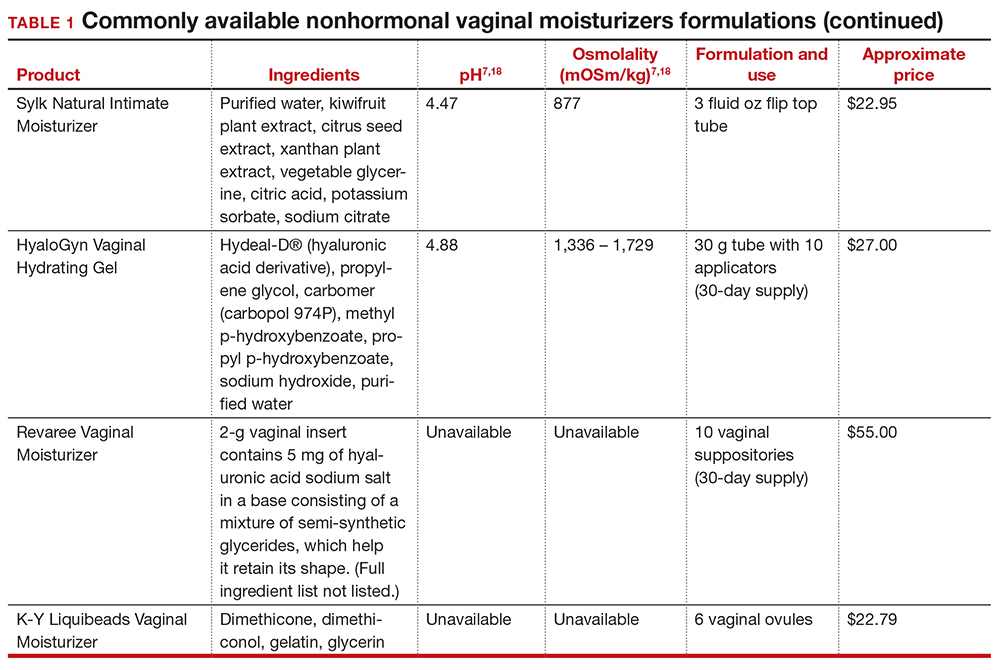
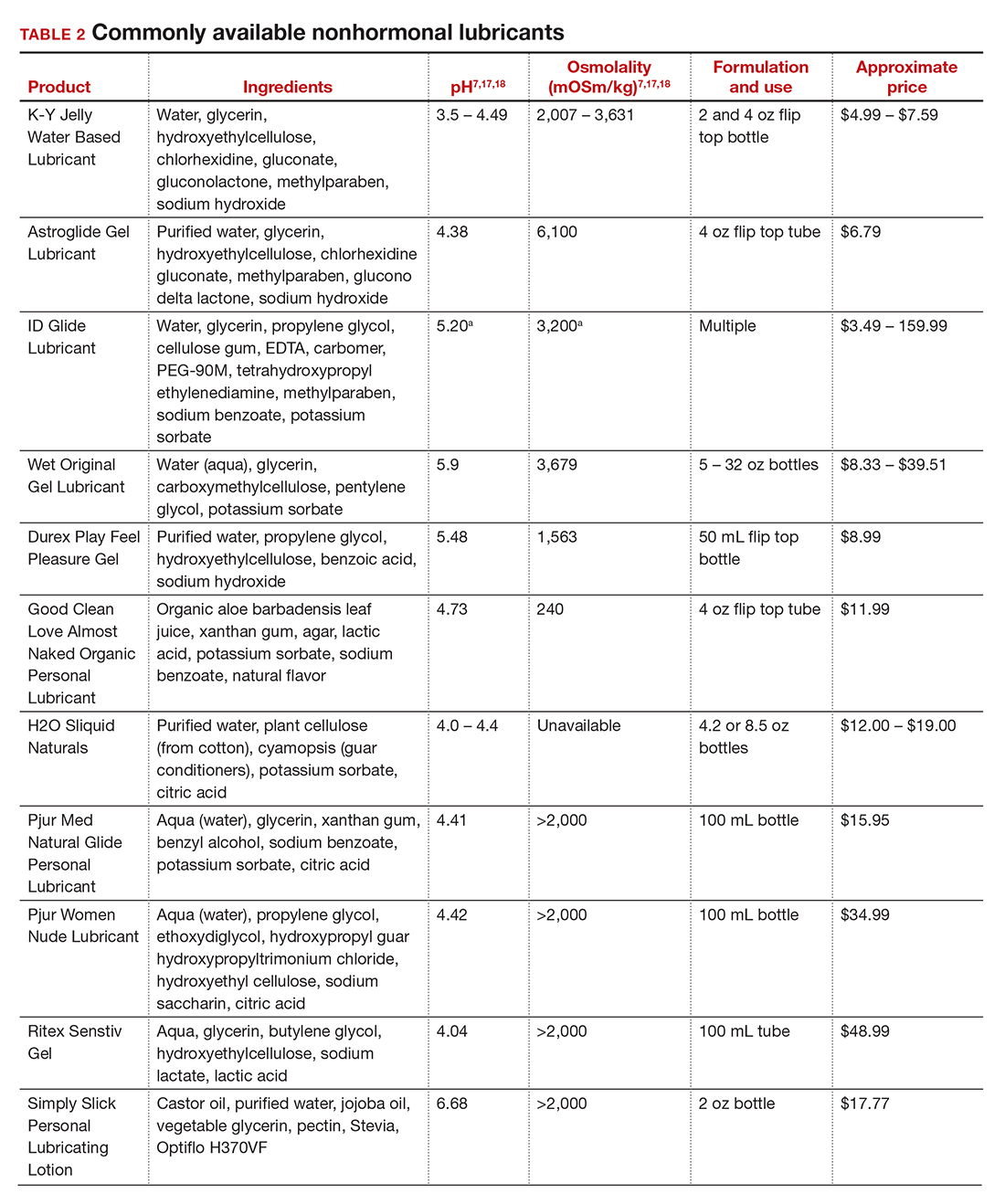
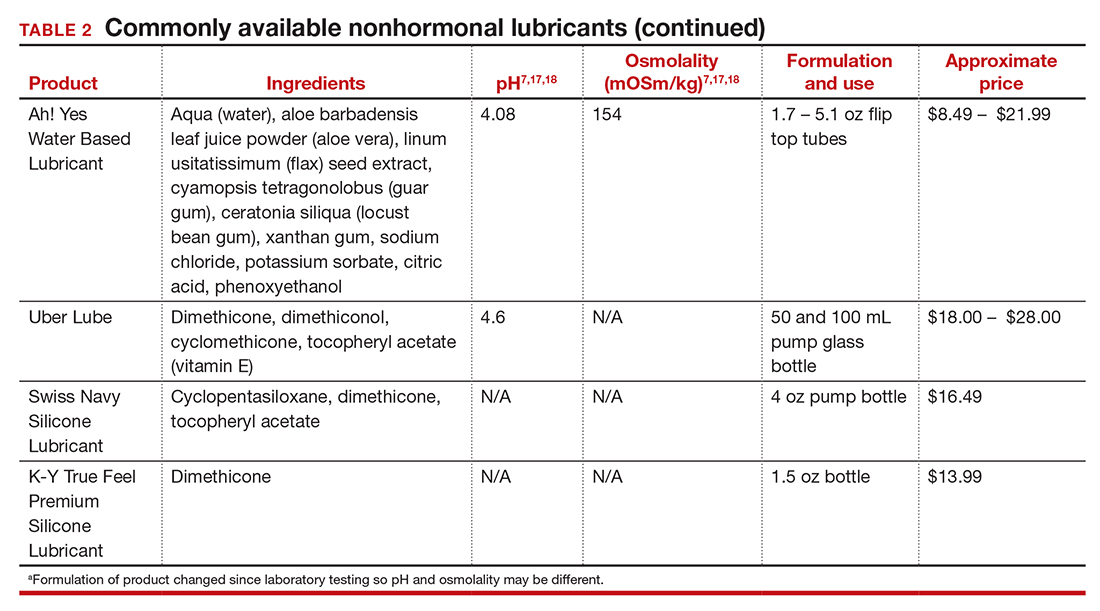
The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
COVID-19 vaccination during pregnancy: Expert guidance on counseling your patients
Each clinician has had to develop his or her individual approach and follow their institutional guidance regarding counseling and managing all of their patients’ expectations in this quickly changing climate of vaccination availability and recommendations. For pregnant women specifically, who are at increased risk for severe SARS-COV-2 disease,1 the availability of varied types of vaccines for COVID-19 is promising, but with limited data available to discuss safety for the mother and the baby, what is the best discussion to guide decision making? OBG Management reached out to several experts, who share here what they do in their practices.
Addressing an uncharted front in the war on COVID-19
Ashley S. Roman, MD, MPH
In December 2020, the US Food and Drug Administration (FDA)’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.2 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.3 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.4 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.5,6
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- the presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes
- potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- the ability to take precautions (social distancing, wearing a mask, etc).
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
Continue to: Benefits outweigh the risks, for now...
Benefits outweigh the risks, for now
Ashley S. Coggins, MD, and Jeanne S. Sheffield, MD
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.7 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers— and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.8
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the FDA are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention (CDC)’s V-safe Vaccine Pregnancy Registry.9 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.10 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices. These organizations advise that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered. As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
- Zambrano LD, Ellington S, Strid S, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. 2020;69:1641–1647.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. New Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
- Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-datatracker/#pregnant-population. Accessed April 19, 2021.
- Centers for Disease Control and Prevention. V-safe COVID19 Vaccine Pregnancy Registry. https://www.cdc.gov /coronavirus/2019-ncov/vaccines/safety/vsafepregnancy registry.html. Updated May 3, 2021. Accessed April 19, 2021.
- Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j. ajog.2021.03.023.
Each clinician has had to develop his or her individual approach and follow their institutional guidance regarding counseling and managing all of their patients’ expectations in this quickly changing climate of vaccination availability and recommendations. For pregnant women specifically, who are at increased risk for severe SARS-COV-2 disease,1 the availability of varied types of vaccines for COVID-19 is promising, but with limited data available to discuss safety for the mother and the baby, what is the best discussion to guide decision making? OBG Management reached out to several experts, who share here what they do in their practices.
Addressing an uncharted front in the war on COVID-19
Ashley S. Roman, MD, MPH
In December 2020, the US Food and Drug Administration (FDA)’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.2 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.3 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.4 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.5,6
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- the presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes
- potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- the ability to take precautions (social distancing, wearing a mask, etc).
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
Continue to: Benefits outweigh the risks, for now...
Benefits outweigh the risks, for now
Ashley S. Coggins, MD, and Jeanne S. Sheffield, MD
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.7 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers— and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.8
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the FDA are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention (CDC)’s V-safe Vaccine Pregnancy Registry.9 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.10 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices. These organizations advise that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered. As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
Each clinician has had to develop his or her individual approach and follow their institutional guidance regarding counseling and managing all of their patients’ expectations in this quickly changing climate of vaccination availability and recommendations. For pregnant women specifically, who are at increased risk for severe SARS-COV-2 disease,1 the availability of varied types of vaccines for COVID-19 is promising, but with limited data available to discuss safety for the mother and the baby, what is the best discussion to guide decision making? OBG Management reached out to several experts, who share here what they do in their practices.
Addressing an uncharted front in the war on COVID-19
Ashley S. Roman, MD, MPH
In December 2020, the US Food and Drug Administration (FDA)’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.2 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.3 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.4 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.5,6
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- the presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes
- potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- the ability to take precautions (social distancing, wearing a mask, etc).
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
Continue to: Benefits outweigh the risks, for now...
Benefits outweigh the risks, for now
Ashley S. Coggins, MD, and Jeanne S. Sheffield, MD
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.7 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers— and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.8
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the FDA are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention (CDC)’s V-safe Vaccine Pregnancy Registry.9 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.10 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices. These organizations advise that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered. As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
- Zambrano LD, Ellington S, Strid S, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. 2020;69:1641–1647.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. New Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
- Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-datatracker/#pregnant-population. Accessed April 19, 2021.
- Centers for Disease Control and Prevention. V-safe COVID19 Vaccine Pregnancy Registry. https://www.cdc.gov /coronavirus/2019-ncov/vaccines/safety/vsafepregnancy registry.html. Updated May 3, 2021. Accessed April 19, 2021.
- Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j. ajog.2021.03.023.
- Zambrano LD, Ellington S, Strid S, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. 2020;69:1641–1647.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. New Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
- Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-datatracker/#pregnant-population. Accessed April 19, 2021.
- Centers for Disease Control and Prevention. V-safe COVID19 Vaccine Pregnancy Registry. https://www.cdc.gov /coronavirus/2019-ncov/vaccines/safety/vsafepregnancy registry.html. Updated May 3, 2021. Accessed April 19, 2021.
- Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j. ajog.2021.03.023.
Relationship-Centered Care in the Physician-Patient Interaction: Improving Your Understanding of Metacognitive Interventions
Communication and relationships cannot be taken for granted, particularly in the physician-patient relationship, where life-altering diagnoses may be given. With one diagnosis, someone’s life may be changed, and both physicians and patients need to be cognizant of the importance of a strong relationship and clear communication.
In the current US health care system, both physicians and patients often are not getting their needs met, and studies that include factors of race, ethnicity, and socioeconomic status suggest that physician-patient relationship barriers contribute to racial disparities in health care.1,2 Although patient-centered care is a widely recognized and upheld model, relationship-centered care between physician and patient involves focusing on the patient and the physician-patient relationship through recognizing personhood, affect (being empathic), and reciprocal influence.3,4 Although it is not necessarily intuitive because it can appear to be yet another task for busy physicians, relationship-centered care improves health care delivery for both physicians and patients through decreased physician burnout, reduced medical errors, and better patient outcomes and satisfaction.5,6
Every physician, patient, and physician-patient relationship is different; unlike the standard questions directed at a routine patient history focused on gathering data, there is no one-size-fits-all relationship-centered conversation.7-10 As with any successful interaction between 2 people, there is a certain amount of necessary self-awareness (Table 1)11 that allows for improvisation and appropriate responsiveness to what is seen, heard, and felt. Rather than attending solely to disease states, the focus of relationship-centered care is on patients, interpersonal interaction, and promoting health and well-being.15
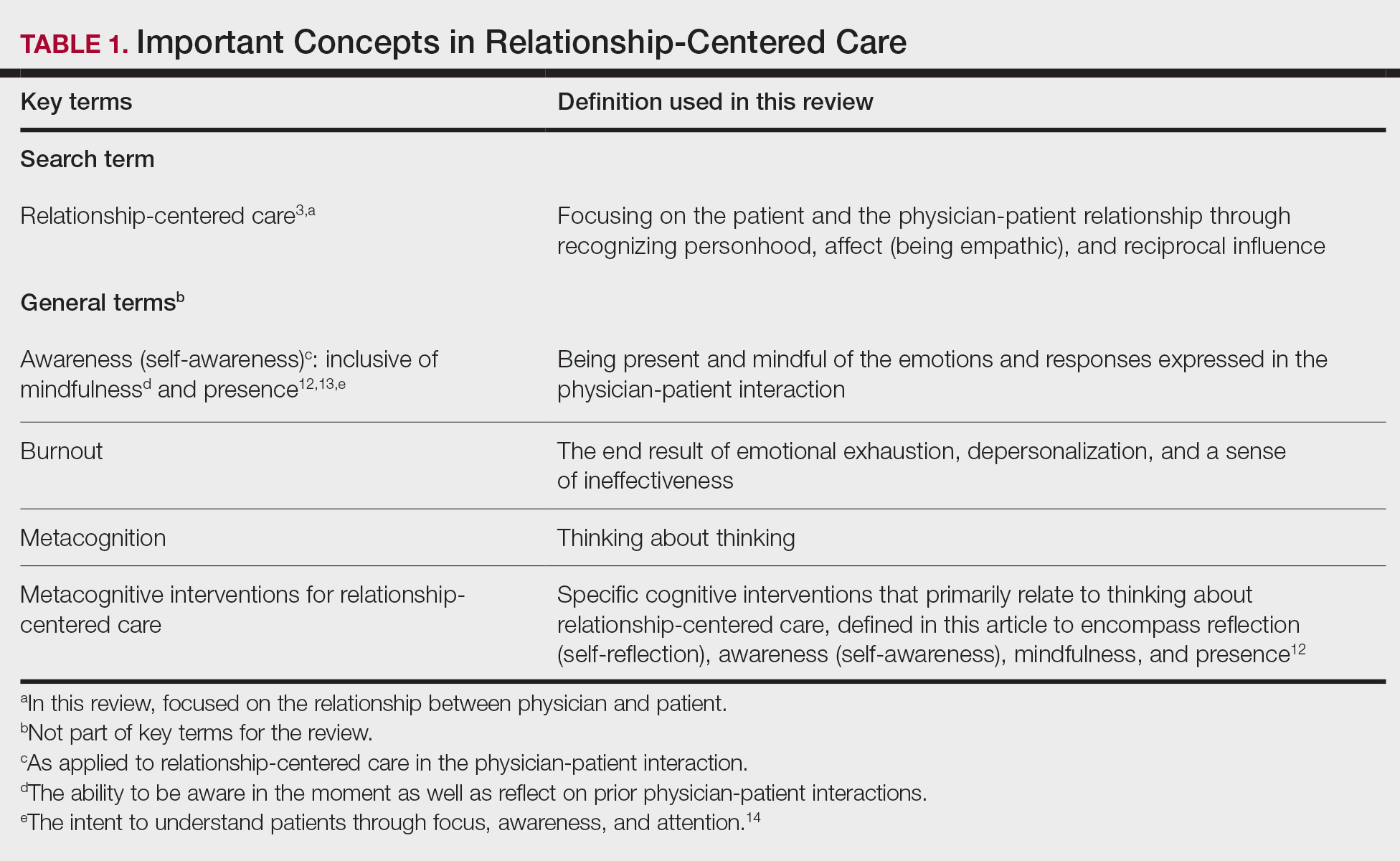
This review summarizes the existing literature on relationship-centered care, introduces the use of metacognition (Table 1), and suggests creating simple habits to promote such care. The following databases were searched from inception through November 23, 2020, using the term relationship-centered care: MEDLINE (Ovid), EMBASE (Ovid), APA PsycInfo (Ovid), Scopus, Web of Science Core Collection, CINAHL Complete (EBSCO), Academic Search Premier (EBSCOhost), and ERIC (ProQuest). A total of 1772 records were retrieved through searches, and after deduplication of 1116 studies, 350 records were screened through a 2-part process. Articles were first screened by title and abstract for relevance to the relationship between physician and patient, with 185 studies deemed irrelevant (eg, pertaining to the relationship of veterinarian to animal). The remaining 165 studies were assessed for eligibility, with 69 further studies excluded for various reasons. The screening process resulted in 96 articles considered in this review.
Definitions/key terms, as used in this article, are listed in Table 1.
Background of Relationship-Centered Care
Given time constraints, the diagnosis and treatment of medical problems often are the focus of physicians. Although proper medical diagnosis and treatment are important, and their delivery is made possible by the physician having the appropriate knowledge, a physician-patient relationship that focuses solely on disease without acknowledging the patient creates a system that ultimately neglects both patients and physicians.15 This prevailing physician-patient relationship paradigm is suboptimal, and a proposed remedy is relationship-centered care, which focuses on relationships among the human beings in health care interactions.3 Relationship-centered care has 4 principles: (1) the personhood of each party must be recognized, (2) emotion is part of relationships, (3) relationships are reciprocal and not just one way, and (4) creating these types of relationships is morally valuable3 and beneficial to patient care.16
Assessment of the Need for Relationship-Centered Care
Relationship-centered care has been studied in physician-patient interactions in various health care settings.17-23 For at least 2 decades, relationship-centered care has been set forth as a model,4,24,25 but there are challenges. Physicians tend to overrate or underrate their communication skills in patient interactions.26,27 A given physician’s preferences often still seem to supersede those of the patient.3,28,29 The impetus to develop relationship-centered care skills generally needs to be internally driven,4,30 as, ultimately, physicians and patients have varying needs.4,31 However, providing physicians with a potential structure is helpful.32
A Solution: Metacognition in the Physician-Patient Interaction
Metacognition is important to integrating basic science knowledge into medical learning and practice,33,34 and it is no less important in translating interpersonal knowledge to the physician-patient interaction. Decreased metacognitive effort35 may underpin the decline in empathy seen with increasing medical training.36,37 Understanding how metacognitive practices foster relationship-centered care is important for teaching, developing, and maintaining that care.
Metacognition is already embedded in the fabric of the physician-patient interaction.33,34 The complex interplay of the physician-patient interview, patient examination, and integration of physical as well as ancillary data requires higher-order thinking and the ability to parse out that thinking successfully. As a concrete example, coming to a diagnosis requires thinking about what has been presented during the physician-patient interaction and considering what supports and suggests the disease while a list of potential differential diagnosis alternatives is being generated. Physicians are trained to apply this clinical reasoning approach to their patient care.
Conversely, although communication skills are a key component of doctoring,38 both between physician and patient as well as among other colleagues and staff, many physicians have never received formal training in communication skills,26,32,39 though it is now an integral part of medical school curricula.40 When such training is mandatory, less than 1% of physicians continue to believe that there was no benefit, even from a single 8-hour communications skills training session.41 Communication cannot be taught comprehensively in 8 hours; thus, the benefit of such training may be the end result of metacognition and increased self-awareness (Table 1).42,43
Building Relationship-Centered Care Through Metacognitive Attention
Metacognition as manifested by such self-awareness can build relationship-centered care.4 Self-awareness can be taught through mentorship or role models.44 Journaling,40 meditation, and appreciation of beauty and the arts45 can contribute, as well as more formal training programs,32,38,42 as offered by the Academy of Communication in Healthcare. Creating opportunities for patient empowerment also supports relationship-centered care, as does applying knowledge of implicit bias.46
Even without formal training, relationship-centered care can be built through attention to cues9—visual (eg, sitting down, other body language),47,48 auditory (eg, knocking, language, tone, conversational flow),48,49 and emotional (eg, clinical empathy, emotional intelligence)(Table 2). Such attention is familiar to everyone, not just physicians or patients, through interactions outside of health care; inattention may be due to the hidden curriculum or culture of medicine40 as well as real-time changes, such as the introduction of the electronic health record.51 Inattention to these cues also may be a result of context-specific knowledge, in which a physician’s real-life communication skills are not applied to the unique context of patient care.
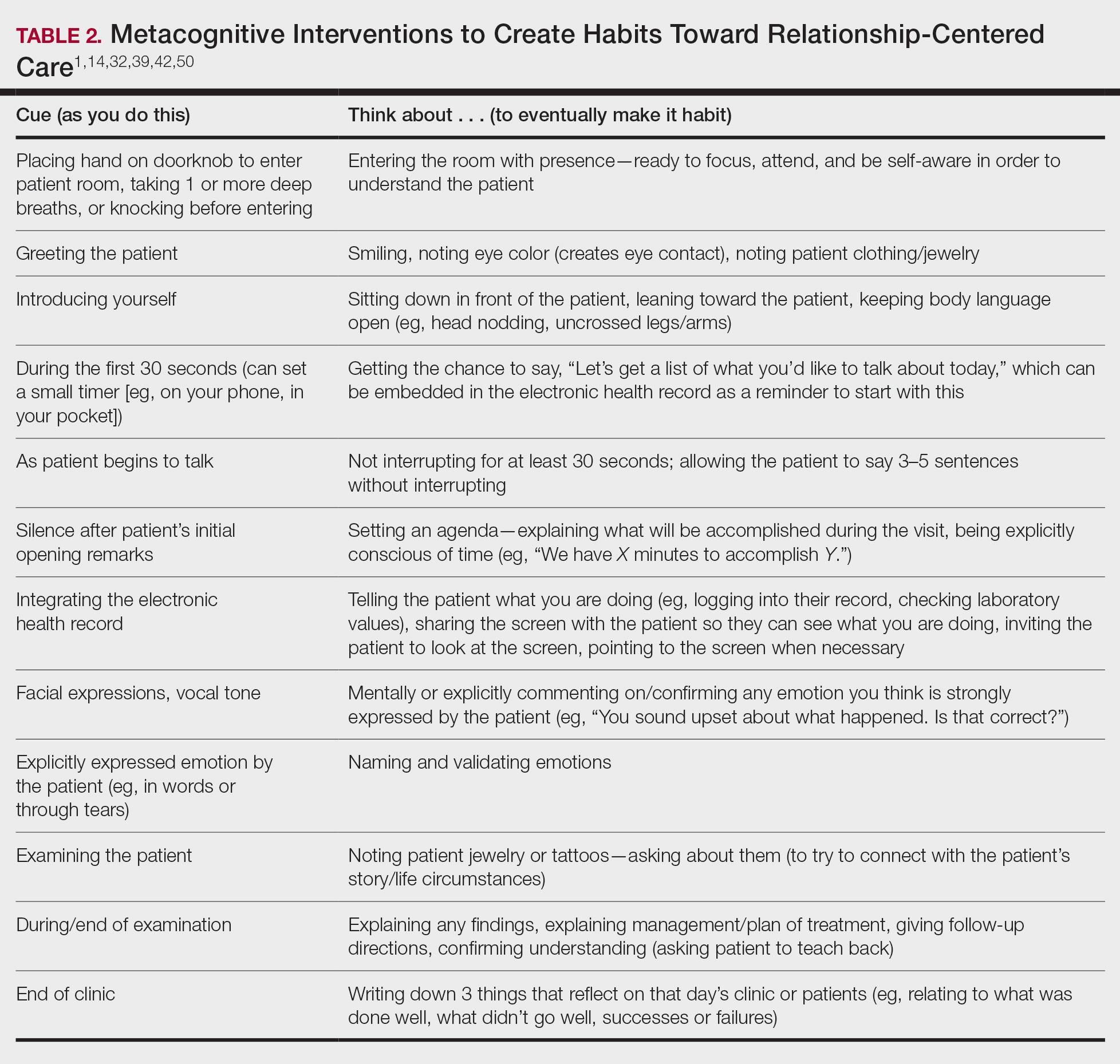
Although the theoretical foundation of relationship-centered care is relatively complex,9 a simple formula that has improved patient experience is “The Big 3,” which entails (1) simply knocking before entering the examination room, (2) sitting, and (3) asking, “What is your main concern?”30 Another relatively simple technique would be to involve the patient with the electronic health record by sharing the screen with them.52 Learning about narrative medicine and developing skills to appreciate each patient’s story is another method to increase relationship-centered care,40,53 as is emotional intelligence.54 These interventions are simple to implement, and good relationship-centered care will save time, help manage patient visits more effectively, and aid in avoiding the urgent new concern that the patient adds at the end of the visit.55 The positive effect of these different interventions highlights that small changes (Table 2) can shift the prevailing culture of medicine to become more relationship centered.56
Metacognitive Attention Can Generate Habit
Taking metacognition a step further, these small interventions can become habit11,14,39 through self-awareness, deliberate practice, and feedback.43 Habit is generated by linking a given intervention to another defined cue. For example, placing a hand on a doorknob to enter an examination room can be the cue to generate a habit of entering with presence.14 Alternatively, before entering an examination room, taking 3 deep breaths can be the cue to trigger presence.14 Habits can be created in just 3 weeks,57 and other proposed cues to generate habits toward relationship-centered care are listed in Table 2. By creating habit through metacognitive attention, relationship-centered care will become something that happens subconsciously without further burdening physicians with another task. Asking patients for permission to record video of an interaction also can create opportunities for self-awareness and self-evaluation through rewatching the video.58
Final Thoughts
Physicians already have the tools to create relationship-centered care in physician-patient interactions. A critical mental shift is to develop habits and apply thinking patterns toward understanding and responding appropriately to patients of all ethnicities and their emotions in the physician-patient interaction. This shift is aided by metacognitive awareness (Table 1) and the development of useful habits (Table 2).
- Sanders L, Fortin AH VI, Schiff GD. Connecting with patients—the missing links. JAMA. 2020;323:33-34.
- Peck BM, Denney M. Disparities in the conduct of the medical encounter: the effects of physician and patient race and gender. SAGE Open. 2012;2:1-14.
- Beach MC, Inui T. Relationship-centered care. a constructive reframing. J Gen Intern Med. 2006;21(suppl 1):S3-S8.
- Tresolini CP, Pew-Fetzer Task Force. Health Professions Education and Relationship-Centered Care. Pew Health Professions Commission; 1994.
- Hojat M. Empathy in Health Professions Education and Patient Care. Springer; 2016.
- Wilkinson H, Whittington R, Perry L, et al. Examining the relationship between burnout and empathy in healthcare professionals: a systematic review. Burn Res. 2017;6:18-29.
- Frankel RM, Quill T. Integrating biopsychosocial and relationship-centered care into mainstream medical practice: a challenge that continues to produce positive results. Fam Syst Health. 2005;23:413-421.
- Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19:1163-1165.
- Ventres WB, Frankel RM. Shared presence in physician-patient communication: a graphic representation. Fam Syst Health. 2015;33:270-279.
- Cooper LA, Beach MC, Johnson RL, et al. Delving below the surface: understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21(suppl 1):S21-S27.
- Epstein RM. Mindful practice. JAMA. 1999;282:833-839.
- Dobie S. Viewpoint: reflections on a well-traveled path: self-awareness, mindful practice, and relationship-centered care as foundations for medical education. Acad Med. 2007;82:422-427.
- Rabow MW. Meaning and relationship-centered care: recommendations for clinicians attending to the spiritual distress of patients at the end of life. Ethics Med Public Health. 2019;9:57-62.
- Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323:70-81.
- Rakel DP, Guerrera MP, Bayles BP, et al. CAM education: promoting a salutogenic focus in health care. J Altern Complement Med. 2008;14:87-93.
- Olaisen RH, Schluchter MD, Flocke SA, et al. Assessing the longitudinal impact of physician-patient relationship on functional health. Ann Fam Med. 2020;18:422-429.
- Berg GM, Ekengren F, Lee FA, et al. Patient satisfaction with surgeons in a trauma population: testing a structural equation model using perceptions of interpersonal and technical care. J Trauma Acute Care Surg. 2012;72:1316-1322.
- Nassar A, Weimer-Elder B, Kline M, et al. Developing an inpatient relationship-centered communication curriculum for surgical teams: pilot study. J Am Coll Surg. 2019;229(4 suppl 2):E48.
- Caldicott CV, Dunn KA, Frankel RM. Can patients tell when they are unwanted? “turfing” in residency training. Patient Educ Couns. 2005;56:104-111.
- Tucker Edmonds B, Mogul M, Shea JA. Understanding low-income African American women’s expectations, preferences, and priorities in prenatal care. Fam Community Health. 2015;38:149-157.
- Sundstrom B, Szabo C, Dempsey A. “My body. my choice:” a qualitative study of the influence of trust and locus of control on postpartum contraceptive choice. J Health Commun. 2018;23:162-169.
- Block S, Billings JA. Nurturing humanism through teaching palliative care. Acad Med. 1998;73:763-765.
- Hebert RS, Schulz R, Copeland VC, et al. Preparing family caregivers for death and bereavement. insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37:3-12.
- Nundy S, Oswald J. Relationship-centered care: a new paradigm for population health management. Healthc (Amst). 2014;2:216-219.
- Sprague S. Relationship centered care. J S C Med Assoc. 2009;105:135-136.
- Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. mechanisms and outcomes. J Gen Intern Med. 2006;21(suppl 1):S28-S34.
- Kenny DA, Veldhuijzen W, van der Weijden T, et al. Interpersonal perception in the context of doctor-patient relationships: a dyadic analysis of doctor-patient communication. Soc Sci Med. 2010;70:763-768.
- Tarzian AJ, Neal MT, O’Neil JA. Attitudes, experiences, and beliefs affecting end-of-life decision-making among homeless individuals. J Palliat Med. 2005;8:36-48.
- Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ Couns. 2000;39:5-15.
- Sharieff GQ. MD to MD coaching: improving physician-patient experience scores: what works, what doesn’t. J Patient Exp. 2017;4:210-212.
- Duggan AP, Bradshaw YS, Swergold N, et al. When rapport building extends beyond affiliation: communication overaccommodation toward patients with disabilities. Perm J. 2011;15:23-30.
- Hirschmann K, Rosler G, Fortin AH VI. “For me, this has been transforming”: a qualitative analysis of interprofessional relationship-centered communication skills training. J Patient Exp. 2020;7:1007-1014.
- Hennrikus EF, Skolka MP, Hennrikus N. Applying metacognition through patient encounters and illness scripts to create a conceptual framework for basic science integration, storage, and retrieval. J Med Educ Curric Dev. 2018;5:2382120518777770.
- Eichbaum QG. Thinking about thinking and emotion: the metacognitive approach to the medical humanities that integrates the humanities with the basic and clinical sciences. Perm J. 2014;18:64-75.
- Stansfield RB, Schwartz A, O’Brien CL, et al. Development of a metacognitive effort construct of empathy during clinical training: a longitudinal study of the factor structure of the Jefferson Scale of Empathy. Adv Health Sci Educ Theory Pract. 2016;21:5-17.
- Hojat M, Vergare MJ, Maxwell K, et al. The devil is in the third year: a longitudinal study of erosion of empathy in medical school. Acad Med. 2009;84:1182-1191.
- Neumann M, Edelhäuser F, Tauschel D, et al. Empathy decline and its reasons: a systematic review of studies with medical students and residents. Acad Med. 2011;86:996-1009.
- Chou CL, Hirschmann K, Fortin AHT, et al. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89:1051-1056.
- Rider EA. Advanced communication strategies for relationship-centered care. Pediatr Ann. 2011;40:447-453.
- Reichman JAH. Narrative competence, mindfulness,and relationship-centered care in medical education: an innovative approach to teaching medical interviewing. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2015;75(8-A(E)).
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31:755-761.
- Hatem DS, Barrett SV, Hewson M, et al. Teaching the medical interview: methods and key learning issues in a faculty development course. J Gen Intern Med. 2007;22:1718-1724.
- Gilligan TD, Baile WF. ASCO patient-clinician communication guideline: fostering relationship-centered care. ASCO Connection. November 20, 2017. Accessed March 5, 2021. https://connection.asco.org/blogs/asco-patient-clinician-communication-guideline-fostering-relationship-centered-care
- Haidet P, Stein HF. The role of the student-teacher relationship in the formation of physicians. The hidden curriculum as process. J Gen Intern Med. 2006;(suppl 1):S16-S20.
- Puchalski CM, Guenther M. Restoration and re-creation: spirituality in the lives of healthcare professionals. Curr Opin Support Palliat Care. 2012;6:254-258.
- Williams SW, Hanson LC, Boyd C, et al. Communication, decision making, and cancer: what African Americans want physicians to know. J Palliative Med. 2008;11:1221-1226.
- Lindsley I, Woodhead S, Micallef C, et al. The concept of body language in the medical consultation. Psychiatr Danub. 2015;27(suppl 1):S41-S47.
- Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
- Ness DE, Kiesling SF. Language and connectedness in the medical and psychiatric interview. Patient Educ Couns. 2007;68:139-144.
- Miller WL. The clinical hand: a curricular map for relationship-centered care. Fam Med. 2004;36:330-335.
- Wald HS, George P, Reis SP, et al. Electronic health record training in undergraduate medical education: bridging theory to practice with curricula for empowering patient- and relationship-centered care in the computerized setting. Acad Med. 2014;89:380-386.
- Silverman H, Ho YX, Kaib S, et al. A novel approach to supporting relationship-centered care through electronic health record ergonomic training in preclerkship medical education. Acad Med. 2014;89:1230-1234.
- Weiss T, Swede MJ. Transforming preprofessional health education through relationship-centered care and narrative medicine. Teach Learn Med. 2019;31:222-233.
- Blanch-Hartigan D. An effective training to increase accurate recognition of patient emotion cues. Patient Educ Couns. 2012;89:274-280.
- White J, Levinson W, Roter D. “Oh, by the way ...”: the closing moments of the medical visit. J Gen Intern Med. 1994;9:24-28.
- Suchman AL, Williamson PR, Litzelman DK, et al. Toward an informal curriculum that teaches professionalism. Transforming the social environment of a medical school. J Gen Intern Med. 2004;19:501-504.
- Lally P, van Jaarsveld CHM, Potts HWW, et al. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol. 2010;40:998-1009.
- Little P, White P, Kelly J, et al. Randomised controlled trial of a brief intervention targeting predominantly non-verbal communication in general practice consultations. Br J Gen Pract. 2015;65:E351-E356.
Communication and relationships cannot be taken for granted, particularly in the physician-patient relationship, where life-altering diagnoses may be given. With one diagnosis, someone’s life may be changed, and both physicians and patients need to be cognizant of the importance of a strong relationship and clear communication.
In the current US health care system, both physicians and patients often are not getting their needs met, and studies that include factors of race, ethnicity, and socioeconomic status suggest that physician-patient relationship barriers contribute to racial disparities in health care.1,2 Although patient-centered care is a widely recognized and upheld model, relationship-centered care between physician and patient involves focusing on the patient and the physician-patient relationship through recognizing personhood, affect (being empathic), and reciprocal influence.3,4 Although it is not necessarily intuitive because it can appear to be yet another task for busy physicians, relationship-centered care improves health care delivery for both physicians and patients through decreased physician burnout, reduced medical errors, and better patient outcomes and satisfaction.5,6
Every physician, patient, and physician-patient relationship is different; unlike the standard questions directed at a routine patient history focused on gathering data, there is no one-size-fits-all relationship-centered conversation.7-10 As with any successful interaction between 2 people, there is a certain amount of necessary self-awareness (Table 1)11 that allows for improvisation and appropriate responsiveness to what is seen, heard, and felt. Rather than attending solely to disease states, the focus of relationship-centered care is on patients, interpersonal interaction, and promoting health and well-being.15

This review summarizes the existing literature on relationship-centered care, introduces the use of metacognition (Table 1), and suggests creating simple habits to promote such care. The following databases were searched from inception through November 23, 2020, using the term relationship-centered care: MEDLINE (Ovid), EMBASE (Ovid), APA PsycInfo (Ovid), Scopus, Web of Science Core Collection, CINAHL Complete (EBSCO), Academic Search Premier (EBSCOhost), and ERIC (ProQuest). A total of 1772 records were retrieved through searches, and after deduplication of 1116 studies, 350 records were screened through a 2-part process. Articles were first screened by title and abstract for relevance to the relationship between physician and patient, with 185 studies deemed irrelevant (eg, pertaining to the relationship of veterinarian to animal). The remaining 165 studies were assessed for eligibility, with 69 further studies excluded for various reasons. The screening process resulted in 96 articles considered in this review.
Definitions/key terms, as used in this article, are listed in Table 1.
Background of Relationship-Centered Care
Given time constraints, the diagnosis and treatment of medical problems often are the focus of physicians. Although proper medical diagnosis and treatment are important, and their delivery is made possible by the physician having the appropriate knowledge, a physician-patient relationship that focuses solely on disease without acknowledging the patient creates a system that ultimately neglects both patients and physicians.15 This prevailing physician-patient relationship paradigm is suboptimal, and a proposed remedy is relationship-centered care, which focuses on relationships among the human beings in health care interactions.3 Relationship-centered care has 4 principles: (1) the personhood of each party must be recognized, (2) emotion is part of relationships, (3) relationships are reciprocal and not just one way, and (4) creating these types of relationships is morally valuable3 and beneficial to patient care.16
Assessment of the Need for Relationship-Centered Care
Relationship-centered care has been studied in physician-patient interactions in various health care settings.17-23 For at least 2 decades, relationship-centered care has been set forth as a model,4,24,25 but there are challenges. Physicians tend to overrate or underrate their communication skills in patient interactions.26,27 A given physician’s preferences often still seem to supersede those of the patient.3,28,29 The impetus to develop relationship-centered care skills generally needs to be internally driven,4,30 as, ultimately, physicians and patients have varying needs.4,31 However, providing physicians with a potential structure is helpful.32
A Solution: Metacognition in the Physician-Patient Interaction
Metacognition is important to integrating basic science knowledge into medical learning and practice,33,34 and it is no less important in translating interpersonal knowledge to the physician-patient interaction. Decreased metacognitive effort35 may underpin the decline in empathy seen with increasing medical training.36,37 Understanding how metacognitive practices foster relationship-centered care is important for teaching, developing, and maintaining that care.
Metacognition is already embedded in the fabric of the physician-patient interaction.33,34 The complex interplay of the physician-patient interview, patient examination, and integration of physical as well as ancillary data requires higher-order thinking and the ability to parse out that thinking successfully. As a concrete example, coming to a diagnosis requires thinking about what has been presented during the physician-patient interaction and considering what supports and suggests the disease while a list of potential differential diagnosis alternatives is being generated. Physicians are trained to apply this clinical reasoning approach to their patient care.
Conversely, although communication skills are a key component of doctoring,38 both between physician and patient as well as among other colleagues and staff, many physicians have never received formal training in communication skills,26,32,39 though it is now an integral part of medical school curricula.40 When such training is mandatory, less than 1% of physicians continue to believe that there was no benefit, even from a single 8-hour communications skills training session.41 Communication cannot be taught comprehensively in 8 hours; thus, the benefit of such training may be the end result of metacognition and increased self-awareness (Table 1).42,43
Building Relationship-Centered Care Through Metacognitive Attention
Metacognition as manifested by such self-awareness can build relationship-centered care.4 Self-awareness can be taught through mentorship or role models.44 Journaling,40 meditation, and appreciation of beauty and the arts45 can contribute, as well as more formal training programs,32,38,42 as offered by the Academy of Communication in Healthcare. Creating opportunities for patient empowerment also supports relationship-centered care, as does applying knowledge of implicit bias.46
Even without formal training, relationship-centered care can be built through attention to cues9—visual (eg, sitting down, other body language),47,48 auditory (eg, knocking, language, tone, conversational flow),48,49 and emotional (eg, clinical empathy, emotional intelligence)(Table 2). Such attention is familiar to everyone, not just physicians or patients, through interactions outside of health care; inattention may be due to the hidden curriculum or culture of medicine40 as well as real-time changes, such as the introduction of the electronic health record.51 Inattention to these cues also may be a result of context-specific knowledge, in which a physician’s real-life communication skills are not applied to the unique context of patient care.

Although the theoretical foundation of relationship-centered care is relatively complex,9 a simple formula that has improved patient experience is “The Big 3,” which entails (1) simply knocking before entering the examination room, (2) sitting, and (3) asking, “What is your main concern?”30 Another relatively simple technique would be to involve the patient with the electronic health record by sharing the screen with them.52 Learning about narrative medicine and developing skills to appreciate each patient’s story is another method to increase relationship-centered care,40,53 as is emotional intelligence.54 These interventions are simple to implement, and good relationship-centered care will save time, help manage patient visits more effectively, and aid in avoiding the urgent new concern that the patient adds at the end of the visit.55 The positive effect of these different interventions highlights that small changes (Table 2) can shift the prevailing culture of medicine to become more relationship centered.56
Metacognitive Attention Can Generate Habit
Taking metacognition a step further, these small interventions can become habit11,14,39 through self-awareness, deliberate practice, and feedback.43 Habit is generated by linking a given intervention to another defined cue. For example, placing a hand on a doorknob to enter an examination room can be the cue to generate a habit of entering with presence.14 Alternatively, before entering an examination room, taking 3 deep breaths can be the cue to trigger presence.14 Habits can be created in just 3 weeks,57 and other proposed cues to generate habits toward relationship-centered care are listed in Table 2. By creating habit through metacognitive attention, relationship-centered care will become something that happens subconsciously without further burdening physicians with another task. Asking patients for permission to record video of an interaction also can create opportunities for self-awareness and self-evaluation through rewatching the video.58
Final Thoughts
Physicians already have the tools to create relationship-centered care in physician-patient interactions. A critical mental shift is to develop habits and apply thinking patterns toward understanding and responding appropriately to patients of all ethnicities and their emotions in the physician-patient interaction. This shift is aided by metacognitive awareness (Table 1) and the development of useful habits (Table 2).
Communication and relationships cannot be taken for granted, particularly in the physician-patient relationship, where life-altering diagnoses may be given. With one diagnosis, someone’s life may be changed, and both physicians and patients need to be cognizant of the importance of a strong relationship and clear communication.
In the current US health care system, both physicians and patients often are not getting their needs met, and studies that include factors of race, ethnicity, and socioeconomic status suggest that physician-patient relationship barriers contribute to racial disparities in health care.1,2 Although patient-centered care is a widely recognized and upheld model, relationship-centered care between physician and patient involves focusing on the patient and the physician-patient relationship through recognizing personhood, affect (being empathic), and reciprocal influence.3,4 Although it is not necessarily intuitive because it can appear to be yet another task for busy physicians, relationship-centered care improves health care delivery for both physicians and patients through decreased physician burnout, reduced medical errors, and better patient outcomes and satisfaction.5,6
Every physician, patient, and physician-patient relationship is different; unlike the standard questions directed at a routine patient history focused on gathering data, there is no one-size-fits-all relationship-centered conversation.7-10 As with any successful interaction between 2 people, there is a certain amount of necessary self-awareness (Table 1)11 that allows for improvisation and appropriate responsiveness to what is seen, heard, and felt. Rather than attending solely to disease states, the focus of relationship-centered care is on patients, interpersonal interaction, and promoting health and well-being.15

This review summarizes the existing literature on relationship-centered care, introduces the use of metacognition (Table 1), and suggests creating simple habits to promote such care. The following databases were searched from inception through November 23, 2020, using the term relationship-centered care: MEDLINE (Ovid), EMBASE (Ovid), APA PsycInfo (Ovid), Scopus, Web of Science Core Collection, CINAHL Complete (EBSCO), Academic Search Premier (EBSCOhost), and ERIC (ProQuest). A total of 1772 records were retrieved through searches, and after deduplication of 1116 studies, 350 records were screened through a 2-part process. Articles were first screened by title and abstract for relevance to the relationship between physician and patient, with 185 studies deemed irrelevant (eg, pertaining to the relationship of veterinarian to animal). The remaining 165 studies were assessed for eligibility, with 69 further studies excluded for various reasons. The screening process resulted in 96 articles considered in this review.
Definitions/key terms, as used in this article, are listed in Table 1.
Background of Relationship-Centered Care
Given time constraints, the diagnosis and treatment of medical problems often are the focus of physicians. Although proper medical diagnosis and treatment are important, and their delivery is made possible by the physician having the appropriate knowledge, a physician-patient relationship that focuses solely on disease without acknowledging the patient creates a system that ultimately neglects both patients and physicians.15 This prevailing physician-patient relationship paradigm is suboptimal, and a proposed remedy is relationship-centered care, which focuses on relationships among the human beings in health care interactions.3 Relationship-centered care has 4 principles: (1) the personhood of each party must be recognized, (2) emotion is part of relationships, (3) relationships are reciprocal and not just one way, and (4) creating these types of relationships is morally valuable3 and beneficial to patient care.16
Assessment of the Need for Relationship-Centered Care
Relationship-centered care has been studied in physician-patient interactions in various health care settings.17-23 For at least 2 decades, relationship-centered care has been set forth as a model,4,24,25 but there are challenges. Physicians tend to overrate or underrate their communication skills in patient interactions.26,27 A given physician’s preferences often still seem to supersede those of the patient.3,28,29 The impetus to develop relationship-centered care skills generally needs to be internally driven,4,30 as, ultimately, physicians and patients have varying needs.4,31 However, providing physicians with a potential structure is helpful.32
A Solution: Metacognition in the Physician-Patient Interaction
Metacognition is important to integrating basic science knowledge into medical learning and practice,33,34 and it is no less important in translating interpersonal knowledge to the physician-patient interaction. Decreased metacognitive effort35 may underpin the decline in empathy seen with increasing medical training.36,37 Understanding how metacognitive practices foster relationship-centered care is important for teaching, developing, and maintaining that care.
Metacognition is already embedded in the fabric of the physician-patient interaction.33,34 The complex interplay of the physician-patient interview, patient examination, and integration of physical as well as ancillary data requires higher-order thinking and the ability to parse out that thinking successfully. As a concrete example, coming to a diagnosis requires thinking about what has been presented during the physician-patient interaction and considering what supports and suggests the disease while a list of potential differential diagnosis alternatives is being generated. Physicians are trained to apply this clinical reasoning approach to their patient care.
Conversely, although communication skills are a key component of doctoring,38 both between physician and patient as well as among other colleagues and staff, many physicians have never received formal training in communication skills,26,32,39 though it is now an integral part of medical school curricula.40 When such training is mandatory, less than 1% of physicians continue to believe that there was no benefit, even from a single 8-hour communications skills training session.41 Communication cannot be taught comprehensively in 8 hours; thus, the benefit of such training may be the end result of metacognition and increased self-awareness (Table 1).42,43
Building Relationship-Centered Care Through Metacognitive Attention
Metacognition as manifested by such self-awareness can build relationship-centered care.4 Self-awareness can be taught through mentorship or role models.44 Journaling,40 meditation, and appreciation of beauty and the arts45 can contribute, as well as more formal training programs,32,38,42 as offered by the Academy of Communication in Healthcare. Creating opportunities for patient empowerment also supports relationship-centered care, as does applying knowledge of implicit bias.46
Even without formal training, relationship-centered care can be built through attention to cues9—visual (eg, sitting down, other body language),47,48 auditory (eg, knocking, language, tone, conversational flow),48,49 and emotional (eg, clinical empathy, emotional intelligence)(Table 2). Such attention is familiar to everyone, not just physicians or patients, through interactions outside of health care; inattention may be due to the hidden curriculum or culture of medicine40 as well as real-time changes, such as the introduction of the electronic health record.51 Inattention to these cues also may be a result of context-specific knowledge, in which a physician’s real-life communication skills are not applied to the unique context of patient care.

Although the theoretical foundation of relationship-centered care is relatively complex,9 a simple formula that has improved patient experience is “The Big 3,” which entails (1) simply knocking before entering the examination room, (2) sitting, and (3) asking, “What is your main concern?”30 Another relatively simple technique would be to involve the patient with the electronic health record by sharing the screen with them.52 Learning about narrative medicine and developing skills to appreciate each patient’s story is another method to increase relationship-centered care,40,53 as is emotional intelligence.54 These interventions are simple to implement, and good relationship-centered care will save time, help manage patient visits more effectively, and aid in avoiding the urgent new concern that the patient adds at the end of the visit.55 The positive effect of these different interventions highlights that small changes (Table 2) can shift the prevailing culture of medicine to become more relationship centered.56
Metacognitive Attention Can Generate Habit
Taking metacognition a step further, these small interventions can become habit11,14,39 through self-awareness, deliberate practice, and feedback.43 Habit is generated by linking a given intervention to another defined cue. For example, placing a hand on a doorknob to enter an examination room can be the cue to generate a habit of entering with presence.14 Alternatively, before entering an examination room, taking 3 deep breaths can be the cue to trigger presence.14 Habits can be created in just 3 weeks,57 and other proposed cues to generate habits toward relationship-centered care are listed in Table 2. By creating habit through metacognitive attention, relationship-centered care will become something that happens subconsciously without further burdening physicians with another task. Asking patients for permission to record video of an interaction also can create opportunities for self-awareness and self-evaluation through rewatching the video.58
Final Thoughts
Physicians already have the tools to create relationship-centered care in physician-patient interactions. A critical mental shift is to develop habits and apply thinking patterns toward understanding and responding appropriately to patients of all ethnicities and their emotions in the physician-patient interaction. This shift is aided by metacognitive awareness (Table 1) and the development of useful habits (Table 2).
- Sanders L, Fortin AH VI, Schiff GD. Connecting with patients—the missing links. JAMA. 2020;323:33-34.
- Peck BM, Denney M. Disparities in the conduct of the medical encounter: the effects of physician and patient race and gender. SAGE Open. 2012;2:1-14.
- Beach MC, Inui T. Relationship-centered care. a constructive reframing. J Gen Intern Med. 2006;21(suppl 1):S3-S8.
- Tresolini CP, Pew-Fetzer Task Force. Health Professions Education and Relationship-Centered Care. Pew Health Professions Commission; 1994.
- Hojat M. Empathy in Health Professions Education and Patient Care. Springer; 2016.
- Wilkinson H, Whittington R, Perry L, et al. Examining the relationship between burnout and empathy in healthcare professionals: a systematic review. Burn Res. 2017;6:18-29.
- Frankel RM, Quill T. Integrating biopsychosocial and relationship-centered care into mainstream medical practice: a challenge that continues to produce positive results. Fam Syst Health. 2005;23:413-421.
- Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19:1163-1165.
- Ventres WB, Frankel RM. Shared presence in physician-patient communication: a graphic representation. Fam Syst Health. 2015;33:270-279.
- Cooper LA, Beach MC, Johnson RL, et al. Delving below the surface: understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21(suppl 1):S21-S27.
- Epstein RM. Mindful practice. JAMA. 1999;282:833-839.
- Dobie S. Viewpoint: reflections on a well-traveled path: self-awareness, mindful practice, and relationship-centered care as foundations for medical education. Acad Med. 2007;82:422-427.
- Rabow MW. Meaning and relationship-centered care: recommendations for clinicians attending to the spiritual distress of patients at the end of life. Ethics Med Public Health. 2019;9:57-62.
- Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323:70-81.
- Rakel DP, Guerrera MP, Bayles BP, et al. CAM education: promoting a salutogenic focus in health care. J Altern Complement Med. 2008;14:87-93.
- Olaisen RH, Schluchter MD, Flocke SA, et al. Assessing the longitudinal impact of physician-patient relationship on functional health. Ann Fam Med. 2020;18:422-429.
- Berg GM, Ekengren F, Lee FA, et al. Patient satisfaction with surgeons in a trauma population: testing a structural equation model using perceptions of interpersonal and technical care. J Trauma Acute Care Surg. 2012;72:1316-1322.
- Nassar A, Weimer-Elder B, Kline M, et al. Developing an inpatient relationship-centered communication curriculum for surgical teams: pilot study. J Am Coll Surg. 2019;229(4 suppl 2):E48.
- Caldicott CV, Dunn KA, Frankel RM. Can patients tell when they are unwanted? “turfing” in residency training. Patient Educ Couns. 2005;56:104-111.
- Tucker Edmonds B, Mogul M, Shea JA. Understanding low-income African American women’s expectations, preferences, and priorities in prenatal care. Fam Community Health. 2015;38:149-157.
- Sundstrom B, Szabo C, Dempsey A. “My body. my choice:” a qualitative study of the influence of trust and locus of control on postpartum contraceptive choice. J Health Commun. 2018;23:162-169.
- Block S, Billings JA. Nurturing humanism through teaching palliative care. Acad Med. 1998;73:763-765.
- Hebert RS, Schulz R, Copeland VC, et al. Preparing family caregivers for death and bereavement. insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37:3-12.
- Nundy S, Oswald J. Relationship-centered care: a new paradigm for population health management. Healthc (Amst). 2014;2:216-219.
- Sprague S. Relationship centered care. J S C Med Assoc. 2009;105:135-136.
- Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. mechanisms and outcomes. J Gen Intern Med. 2006;21(suppl 1):S28-S34.
- Kenny DA, Veldhuijzen W, van der Weijden T, et al. Interpersonal perception in the context of doctor-patient relationships: a dyadic analysis of doctor-patient communication. Soc Sci Med. 2010;70:763-768.
- Tarzian AJ, Neal MT, O’Neil JA. Attitudes, experiences, and beliefs affecting end-of-life decision-making among homeless individuals. J Palliat Med. 2005;8:36-48.
- Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ Couns. 2000;39:5-15.
- Sharieff GQ. MD to MD coaching: improving physician-patient experience scores: what works, what doesn’t. J Patient Exp. 2017;4:210-212.
- Duggan AP, Bradshaw YS, Swergold N, et al. When rapport building extends beyond affiliation: communication overaccommodation toward patients with disabilities. Perm J. 2011;15:23-30.
- Hirschmann K, Rosler G, Fortin AH VI. “For me, this has been transforming”: a qualitative analysis of interprofessional relationship-centered communication skills training. J Patient Exp. 2020;7:1007-1014.
- Hennrikus EF, Skolka MP, Hennrikus N. Applying metacognition through patient encounters and illness scripts to create a conceptual framework for basic science integration, storage, and retrieval. J Med Educ Curric Dev. 2018;5:2382120518777770.
- Eichbaum QG. Thinking about thinking and emotion: the metacognitive approach to the medical humanities that integrates the humanities with the basic and clinical sciences. Perm J. 2014;18:64-75.
- Stansfield RB, Schwartz A, O’Brien CL, et al. Development of a metacognitive effort construct of empathy during clinical training: a longitudinal study of the factor structure of the Jefferson Scale of Empathy. Adv Health Sci Educ Theory Pract. 2016;21:5-17.
- Hojat M, Vergare MJ, Maxwell K, et al. The devil is in the third year: a longitudinal study of erosion of empathy in medical school. Acad Med. 2009;84:1182-1191.
- Neumann M, Edelhäuser F, Tauschel D, et al. Empathy decline and its reasons: a systematic review of studies with medical students and residents. Acad Med. 2011;86:996-1009.
- Chou CL, Hirschmann K, Fortin AHT, et al. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89:1051-1056.
- Rider EA. Advanced communication strategies for relationship-centered care. Pediatr Ann. 2011;40:447-453.
- Reichman JAH. Narrative competence, mindfulness,and relationship-centered care in medical education: an innovative approach to teaching medical interviewing. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2015;75(8-A(E)).
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31:755-761.
- Hatem DS, Barrett SV, Hewson M, et al. Teaching the medical interview: methods and key learning issues in a faculty development course. J Gen Intern Med. 2007;22:1718-1724.
- Gilligan TD, Baile WF. ASCO patient-clinician communication guideline: fostering relationship-centered care. ASCO Connection. November 20, 2017. Accessed March 5, 2021. https://connection.asco.org/blogs/asco-patient-clinician-communication-guideline-fostering-relationship-centered-care
- Haidet P, Stein HF. The role of the student-teacher relationship in the formation of physicians. The hidden curriculum as process. J Gen Intern Med. 2006;(suppl 1):S16-S20.
- Puchalski CM, Guenther M. Restoration and re-creation: spirituality in the lives of healthcare professionals. Curr Opin Support Palliat Care. 2012;6:254-258.
- Williams SW, Hanson LC, Boyd C, et al. Communication, decision making, and cancer: what African Americans want physicians to know. J Palliative Med. 2008;11:1221-1226.
- Lindsley I, Woodhead S, Micallef C, et al. The concept of body language in the medical consultation. Psychiatr Danub. 2015;27(suppl 1):S41-S47.
- Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
- Ness DE, Kiesling SF. Language and connectedness in the medical and psychiatric interview. Patient Educ Couns. 2007;68:139-144.
- Miller WL. The clinical hand: a curricular map for relationship-centered care. Fam Med. 2004;36:330-335.
- Wald HS, George P, Reis SP, et al. Electronic health record training in undergraduate medical education: bridging theory to practice with curricula for empowering patient- and relationship-centered care in the computerized setting. Acad Med. 2014;89:380-386.
- Silverman H, Ho YX, Kaib S, et al. A novel approach to supporting relationship-centered care through electronic health record ergonomic training in preclerkship medical education. Acad Med. 2014;89:1230-1234.
- Weiss T, Swede MJ. Transforming preprofessional health education through relationship-centered care and narrative medicine. Teach Learn Med. 2019;31:222-233.
- Blanch-Hartigan D. An effective training to increase accurate recognition of patient emotion cues. Patient Educ Couns. 2012;89:274-280.
- White J, Levinson W, Roter D. “Oh, by the way ...”: the closing moments of the medical visit. J Gen Intern Med. 1994;9:24-28.
- Suchman AL, Williamson PR, Litzelman DK, et al. Toward an informal curriculum that teaches professionalism. Transforming the social environment of a medical school. J Gen Intern Med. 2004;19:501-504.
- Lally P, van Jaarsveld CHM, Potts HWW, et al. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol. 2010;40:998-1009.
- Little P, White P, Kelly J, et al. Randomised controlled trial of a brief intervention targeting predominantly non-verbal communication in general practice consultations. Br J Gen Pract. 2015;65:E351-E356.
- Sanders L, Fortin AH VI, Schiff GD. Connecting with patients—the missing links. JAMA. 2020;323:33-34.
- Peck BM, Denney M. Disparities in the conduct of the medical encounter: the effects of physician and patient race and gender. SAGE Open. 2012;2:1-14.
- Beach MC, Inui T. Relationship-centered care. a constructive reframing. J Gen Intern Med. 2006;21(suppl 1):S3-S8.
- Tresolini CP, Pew-Fetzer Task Force. Health Professions Education and Relationship-Centered Care. Pew Health Professions Commission; 1994.
- Hojat M. Empathy in Health Professions Education and Patient Care. Springer; 2016.
- Wilkinson H, Whittington R, Perry L, et al. Examining the relationship between burnout and empathy in healthcare professionals: a systematic review. Burn Res. 2017;6:18-29.
- Frankel RM, Quill T. Integrating biopsychosocial and relationship-centered care into mainstream medical practice: a challenge that continues to produce positive results. Fam Syst Health. 2005;23:413-421.
- Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19:1163-1165.
- Ventres WB, Frankel RM. Shared presence in physician-patient communication: a graphic representation. Fam Syst Health. 2015;33:270-279.
- Cooper LA, Beach MC, Johnson RL, et al. Delving below the surface: understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21(suppl 1):S21-S27.
- Epstein RM. Mindful practice. JAMA. 1999;282:833-839.
- Dobie S. Viewpoint: reflections on a well-traveled path: self-awareness, mindful practice, and relationship-centered care as foundations for medical education. Acad Med. 2007;82:422-427.
- Rabow MW. Meaning and relationship-centered care: recommendations for clinicians attending to the spiritual distress of patients at the end of life. Ethics Med Public Health. 2019;9:57-62.
- Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323:70-81.
- Rakel DP, Guerrera MP, Bayles BP, et al. CAM education: promoting a salutogenic focus in health care. J Altern Complement Med. 2008;14:87-93.
- Olaisen RH, Schluchter MD, Flocke SA, et al. Assessing the longitudinal impact of physician-patient relationship on functional health. Ann Fam Med. 2020;18:422-429.
- Berg GM, Ekengren F, Lee FA, et al. Patient satisfaction with surgeons in a trauma population: testing a structural equation model using perceptions of interpersonal and technical care. J Trauma Acute Care Surg. 2012;72:1316-1322.
- Nassar A, Weimer-Elder B, Kline M, et al. Developing an inpatient relationship-centered communication curriculum for surgical teams: pilot study. J Am Coll Surg. 2019;229(4 suppl 2):E48.
- Caldicott CV, Dunn KA, Frankel RM. Can patients tell when they are unwanted? “turfing” in residency training. Patient Educ Couns. 2005;56:104-111.
- Tucker Edmonds B, Mogul M, Shea JA. Understanding low-income African American women’s expectations, preferences, and priorities in prenatal care. Fam Community Health. 2015;38:149-157.
- Sundstrom B, Szabo C, Dempsey A. “My body. my choice:” a qualitative study of the influence of trust and locus of control on postpartum contraceptive choice. J Health Commun. 2018;23:162-169.
- Block S, Billings JA. Nurturing humanism through teaching palliative care. Acad Med. 1998;73:763-765.
- Hebert RS, Schulz R, Copeland VC, et al. Preparing family caregivers for death and bereavement. insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37:3-12.
- Nundy S, Oswald J. Relationship-centered care: a new paradigm for population health management. Healthc (Amst). 2014;2:216-219.
- Sprague S. Relationship centered care. J S C Med Assoc. 2009;105:135-136.
- Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. mechanisms and outcomes. J Gen Intern Med. 2006;21(suppl 1):S28-S34.
- Kenny DA, Veldhuijzen W, van der Weijden T, et al. Interpersonal perception in the context of doctor-patient relationships: a dyadic analysis of doctor-patient communication. Soc Sci Med. 2010;70:763-768.
- Tarzian AJ, Neal MT, O’Neil JA. Attitudes, experiences, and beliefs affecting end-of-life decision-making among homeless individuals. J Palliat Med. 2005;8:36-48.
- Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ Couns. 2000;39:5-15.
- Sharieff GQ. MD to MD coaching: improving physician-patient experience scores: what works, what doesn’t. J Patient Exp. 2017;4:210-212.
- Duggan AP, Bradshaw YS, Swergold N, et al. When rapport building extends beyond affiliation: communication overaccommodation toward patients with disabilities. Perm J. 2011;15:23-30.
- Hirschmann K, Rosler G, Fortin AH VI. “For me, this has been transforming”: a qualitative analysis of interprofessional relationship-centered communication skills training. J Patient Exp. 2020;7:1007-1014.
- Hennrikus EF, Skolka MP, Hennrikus N. Applying metacognition through patient encounters and illness scripts to create a conceptual framework for basic science integration, storage, and retrieval. J Med Educ Curric Dev. 2018;5:2382120518777770.
- Eichbaum QG. Thinking about thinking and emotion: the metacognitive approach to the medical humanities that integrates the humanities with the basic and clinical sciences. Perm J. 2014;18:64-75.
- Stansfield RB, Schwartz A, O’Brien CL, et al. Development of a metacognitive effort construct of empathy during clinical training: a longitudinal study of the factor structure of the Jefferson Scale of Empathy. Adv Health Sci Educ Theory Pract. 2016;21:5-17.
- Hojat M, Vergare MJ, Maxwell K, et al. The devil is in the third year: a longitudinal study of erosion of empathy in medical school. Acad Med. 2009;84:1182-1191.
- Neumann M, Edelhäuser F, Tauschel D, et al. Empathy decline and its reasons: a systematic review of studies with medical students and residents. Acad Med. 2011;86:996-1009.
- Chou CL, Hirschmann K, Fortin AHT, et al. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89:1051-1056.
- Rider EA. Advanced communication strategies for relationship-centered care. Pediatr Ann. 2011;40:447-453.
- Reichman JAH. Narrative competence, mindfulness,and relationship-centered care in medical education: an innovative approach to teaching medical interviewing. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2015;75(8-A(E)).
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31:755-761.
- Hatem DS, Barrett SV, Hewson M, et al. Teaching the medical interview: methods and key learning issues in a faculty development course. J Gen Intern Med. 2007;22:1718-1724.
- Gilligan TD, Baile WF. ASCO patient-clinician communication guideline: fostering relationship-centered care. ASCO Connection. November 20, 2017. Accessed March 5, 2021. https://connection.asco.org/blogs/asco-patient-clinician-communication-guideline-fostering-relationship-centered-care
- Haidet P, Stein HF. The role of the student-teacher relationship in the formation of physicians. The hidden curriculum as process. J Gen Intern Med. 2006;(suppl 1):S16-S20.
- Puchalski CM, Guenther M. Restoration and re-creation: spirituality in the lives of healthcare professionals. Curr Opin Support Palliat Care. 2012;6:254-258.
- Williams SW, Hanson LC, Boyd C, et al. Communication, decision making, and cancer: what African Americans want physicians to know. J Palliative Med. 2008;11:1221-1226.
- Lindsley I, Woodhead S, Micallef C, et al. The concept of body language in the medical consultation. Psychiatr Danub. 2015;27(suppl 1):S41-S47.
- Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
- Ness DE, Kiesling SF. Language and connectedness in the medical and psychiatric interview. Patient Educ Couns. 2007;68:139-144.
- Miller WL. The clinical hand: a curricular map for relationship-centered care. Fam Med. 2004;36:330-335.
- Wald HS, George P, Reis SP, et al. Electronic health record training in undergraduate medical education: bridging theory to practice with curricula for empowering patient- and relationship-centered care in the computerized setting. Acad Med. 2014;89:380-386.
- Silverman H, Ho YX, Kaib S, et al. A novel approach to supporting relationship-centered care through electronic health record ergonomic training in preclerkship medical education. Acad Med. 2014;89:1230-1234.
- Weiss T, Swede MJ. Transforming preprofessional health education through relationship-centered care and narrative medicine. Teach Learn Med. 2019;31:222-233.
- Blanch-Hartigan D. An effective training to increase accurate recognition of patient emotion cues. Patient Educ Couns. 2012;89:274-280.
- White J, Levinson W, Roter D. “Oh, by the way ...”: the closing moments of the medical visit. J Gen Intern Med. 1994;9:24-28.
- Suchman AL, Williamson PR, Litzelman DK, et al. Toward an informal curriculum that teaches professionalism. Transforming the social environment of a medical school. J Gen Intern Med. 2004;19:501-504.
- Lally P, van Jaarsveld CHM, Potts HWW, et al. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol. 2010;40:998-1009.
- Little P, White P, Kelly J, et al. Randomised controlled trial of a brief intervention targeting predominantly non-verbal communication in general practice consultations. Br J Gen Pract. 2015;65:E351-E356.
Practice Points
- Relationship-centered care emphasizes that all relationships in health care are important, including not only relationships between physicians and patients but also among physicians and colleagues, staff, students, community, and self.
- The physician-patient relationship can be complex, and metacognition can lead to habitual practice of simple techniques to optimize the interaction
Dynamic ultrasonography: An idea whose time has come (videos)
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
--
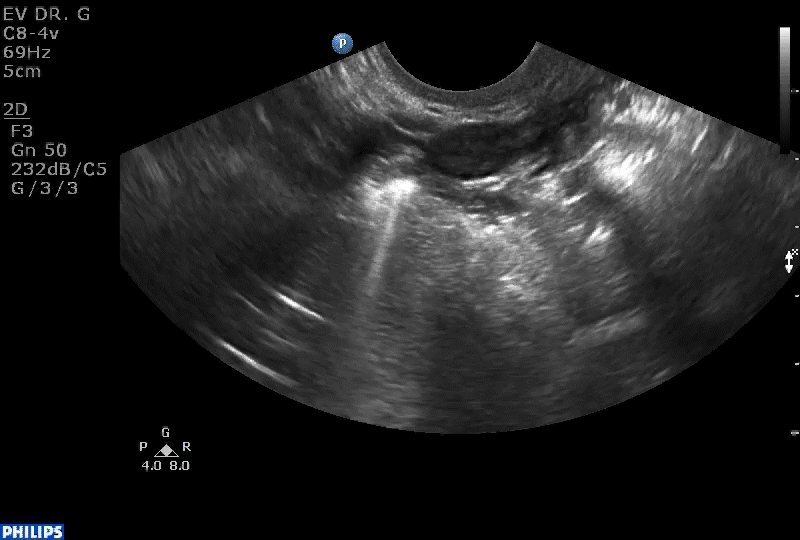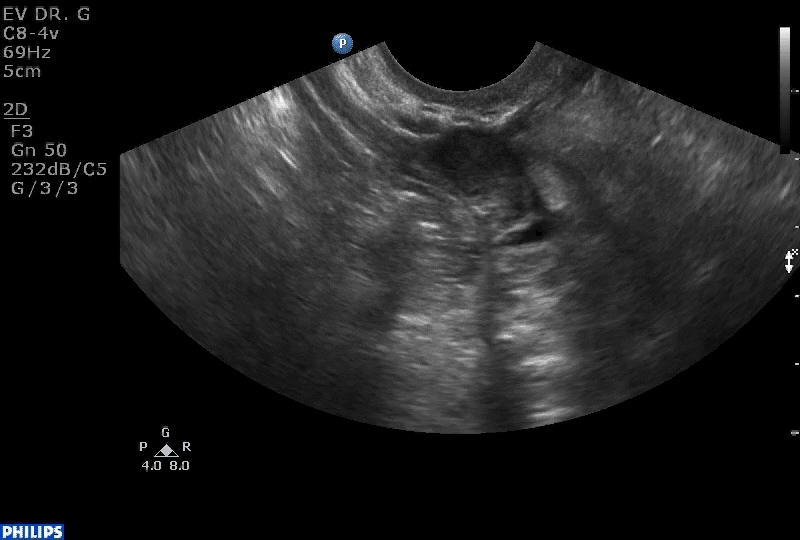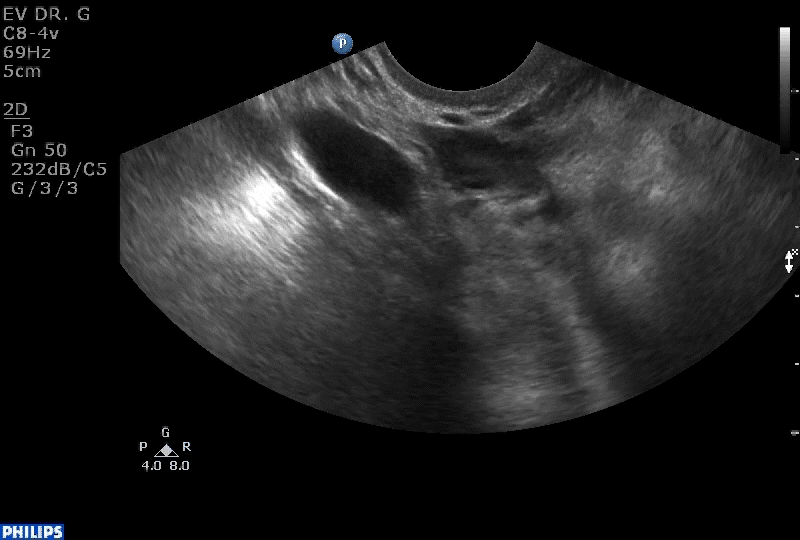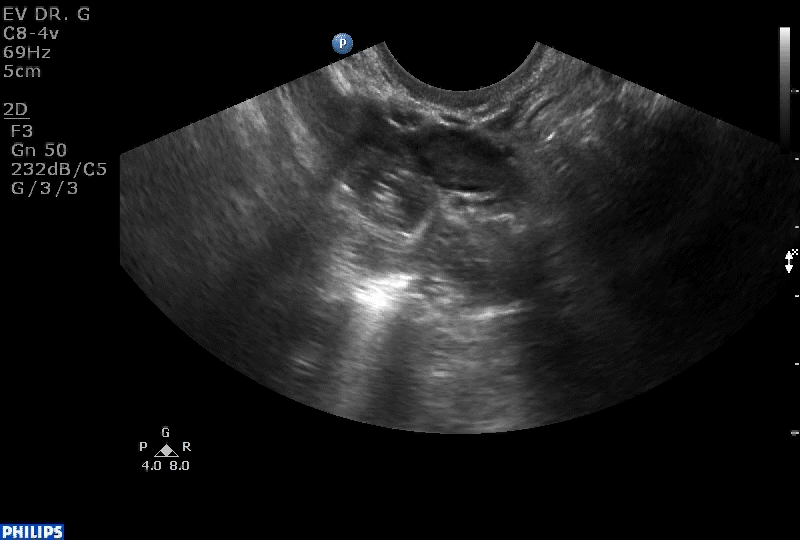
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
--
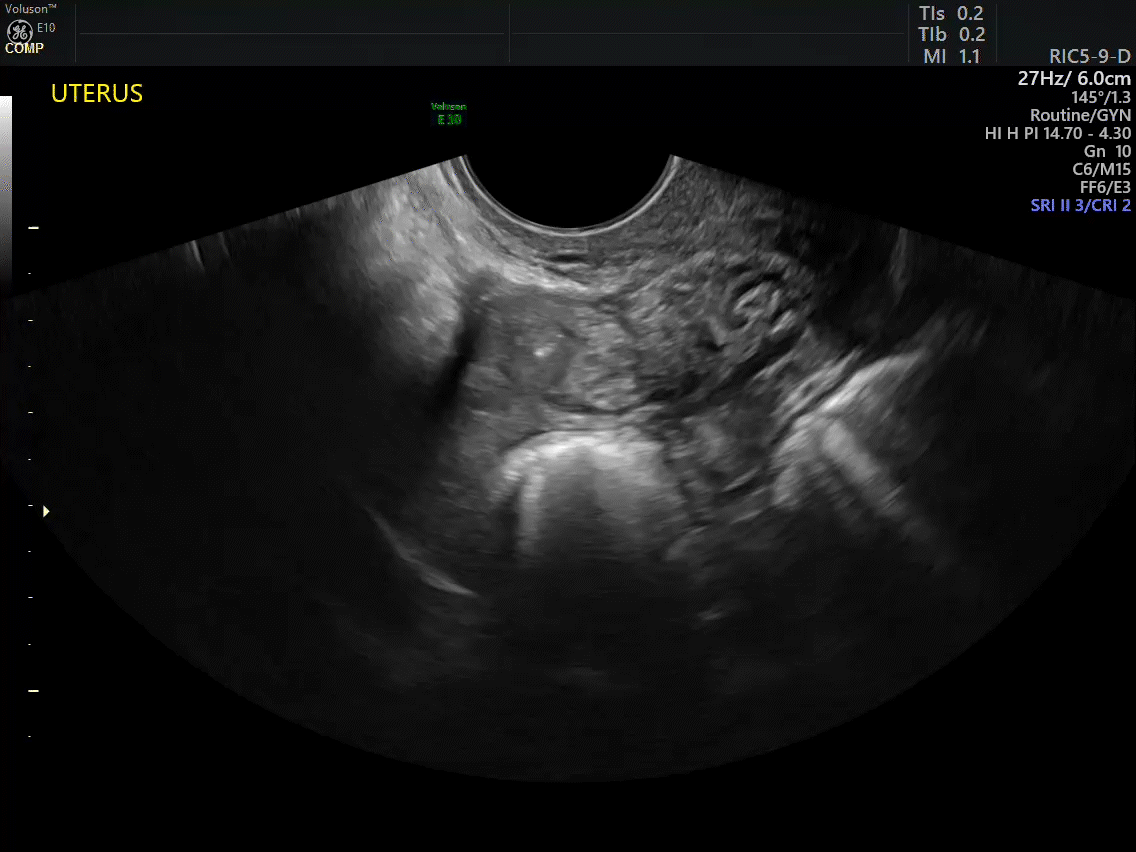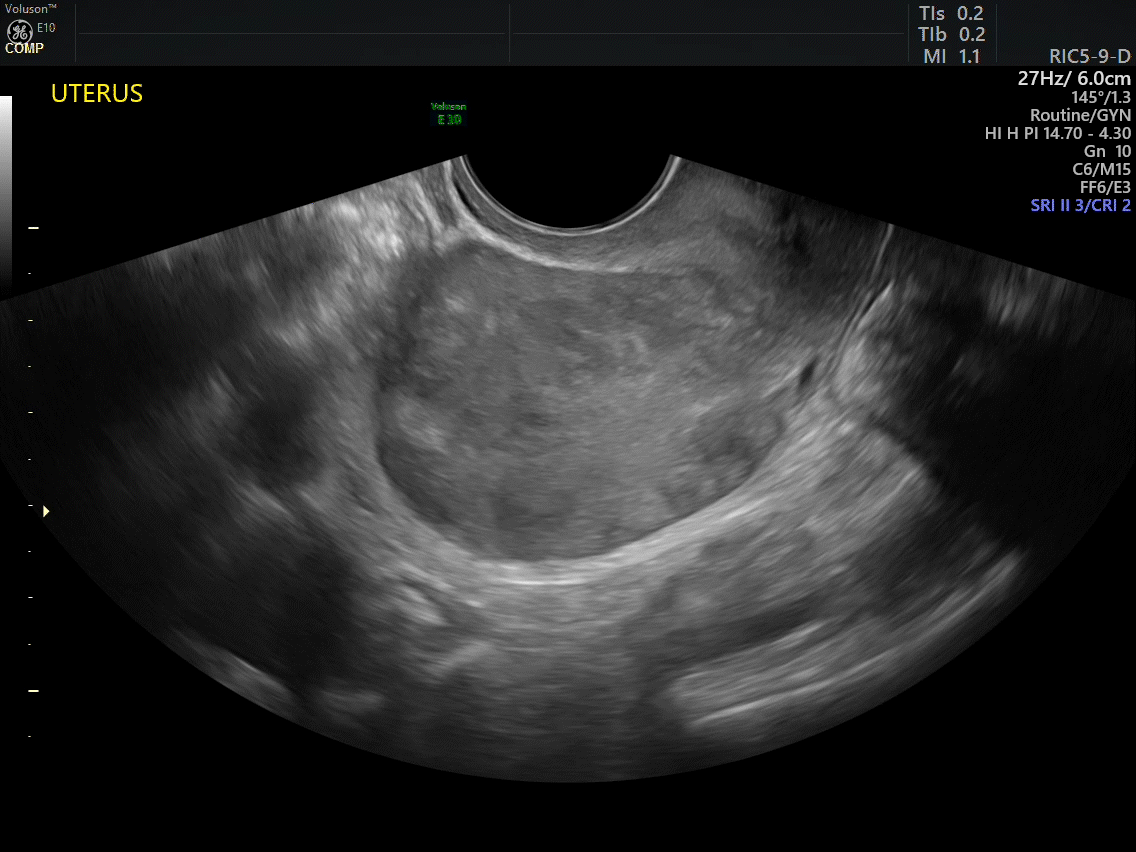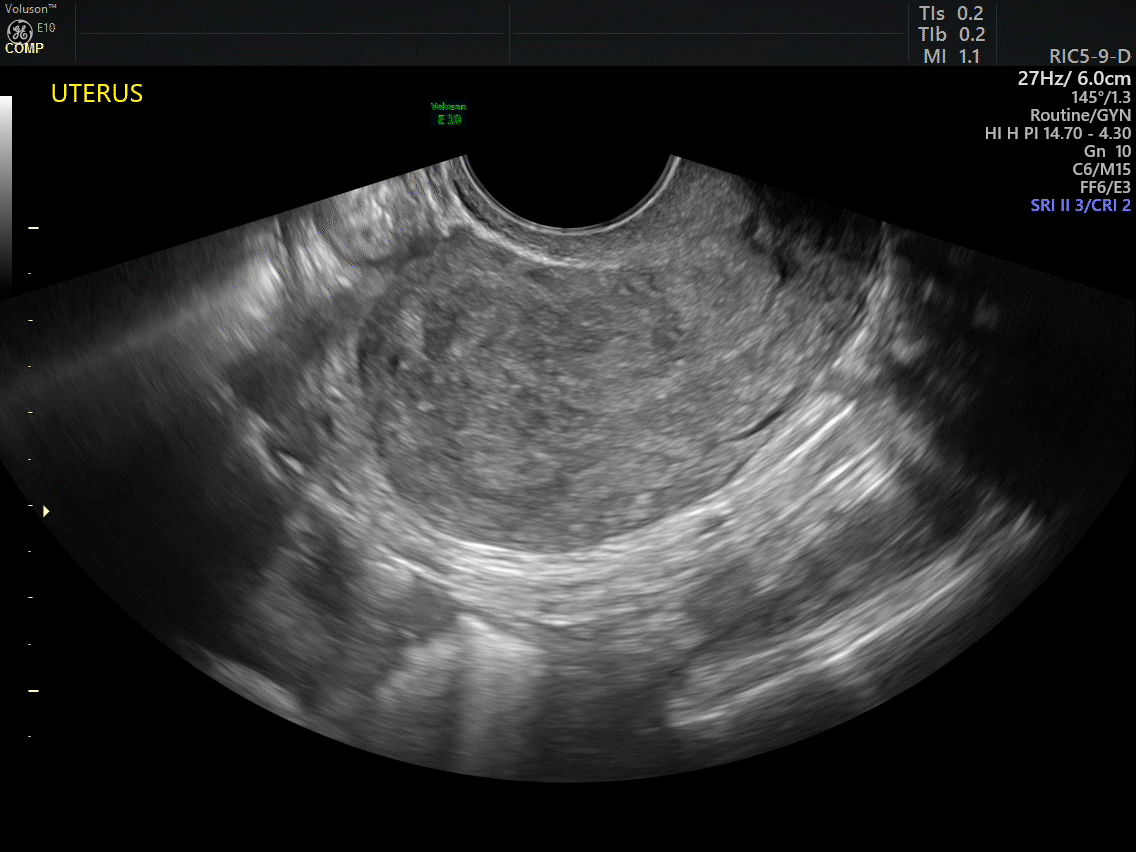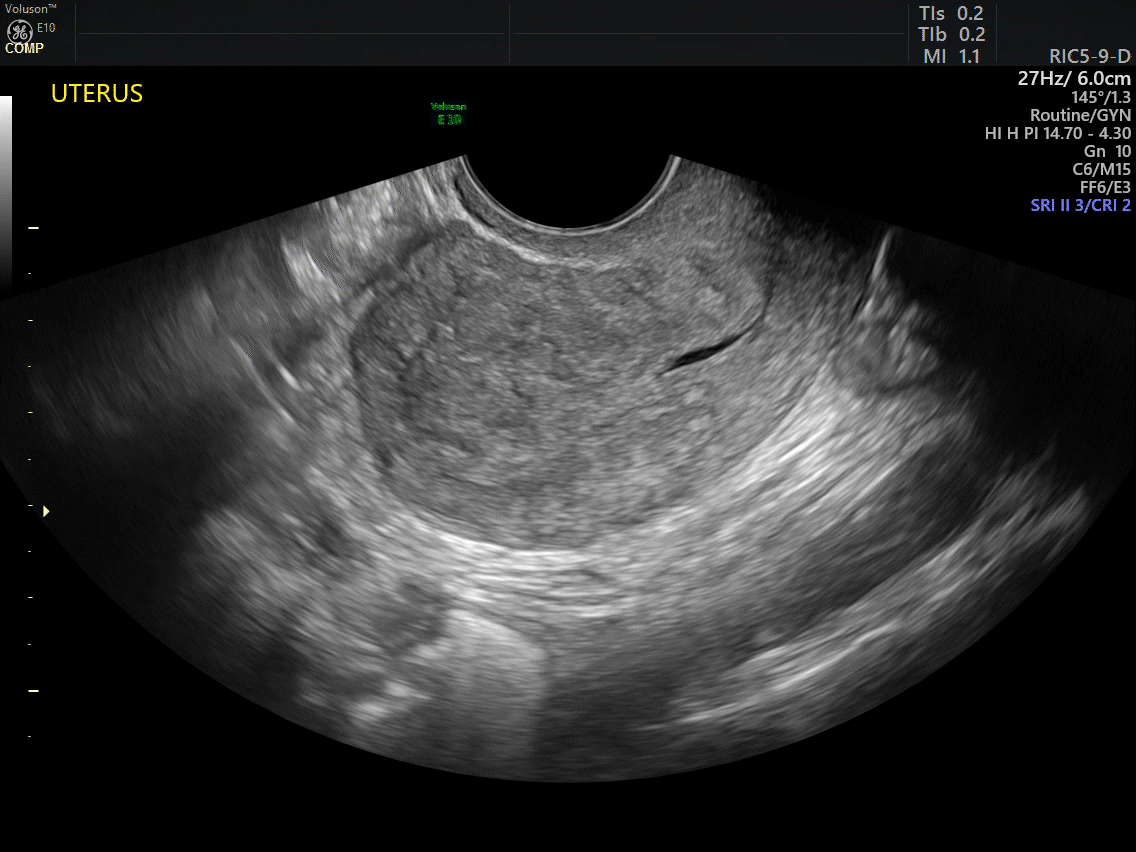
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
--
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in Video 2A showing what appears to be a solid adnexal mass
--
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
--
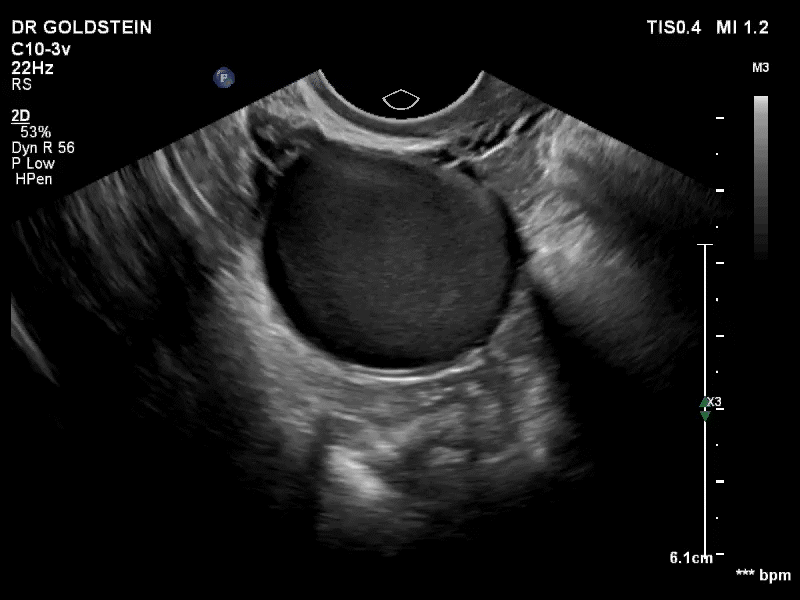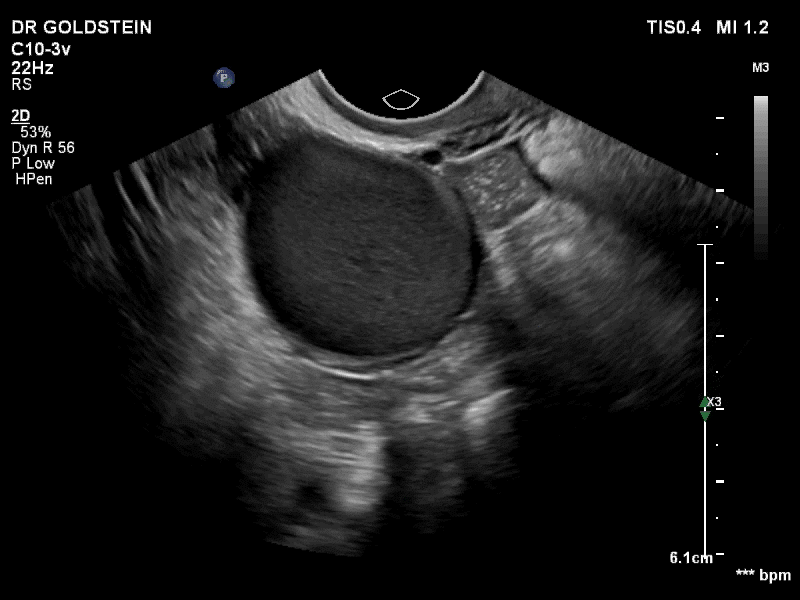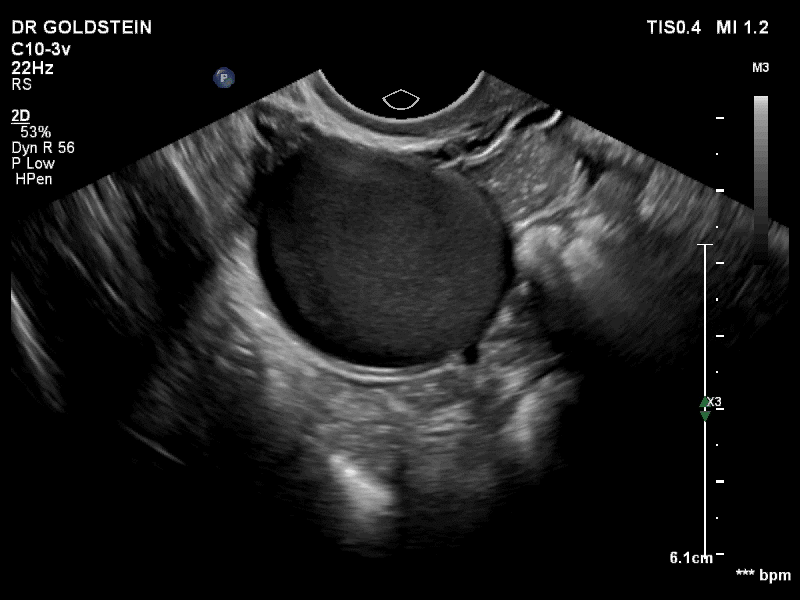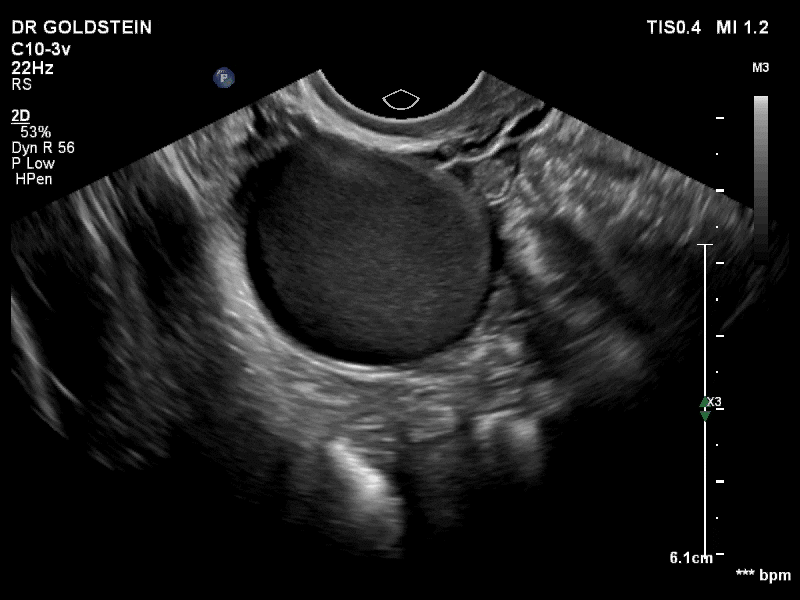
VIDEO 3A Video clip of a classic endometrioma
--
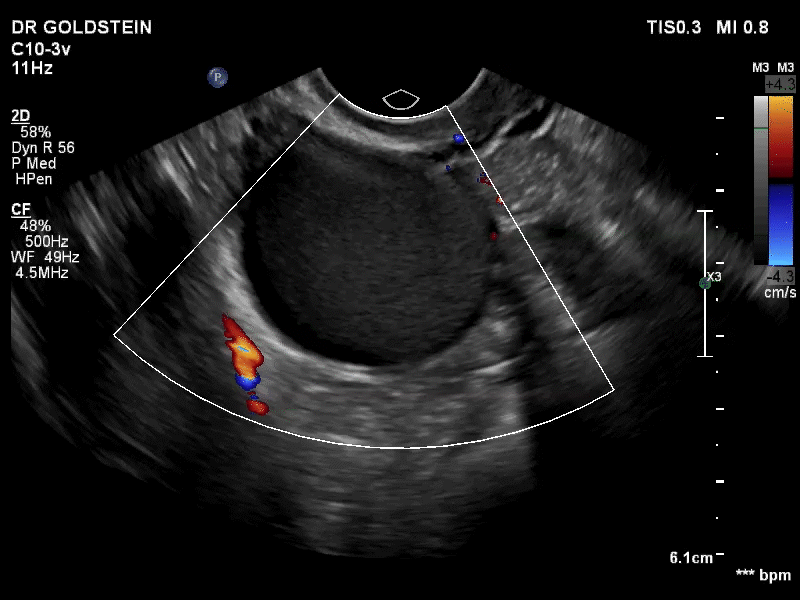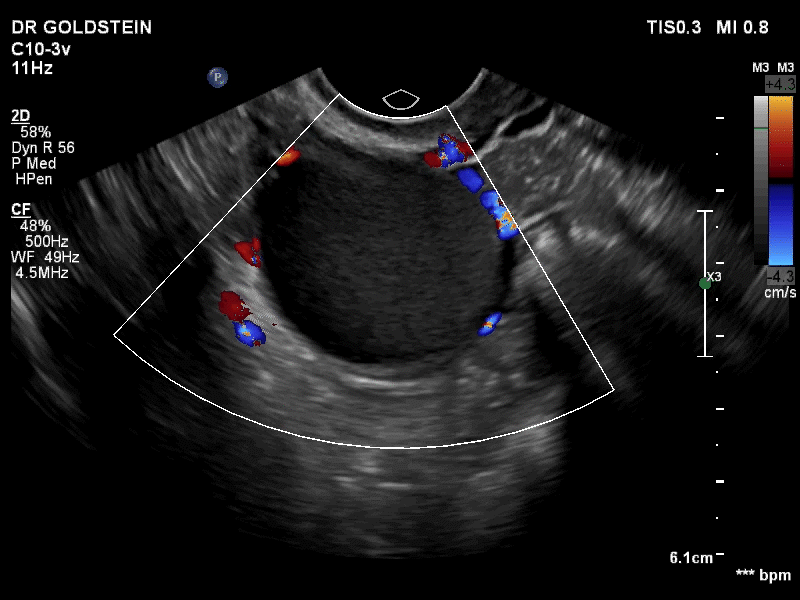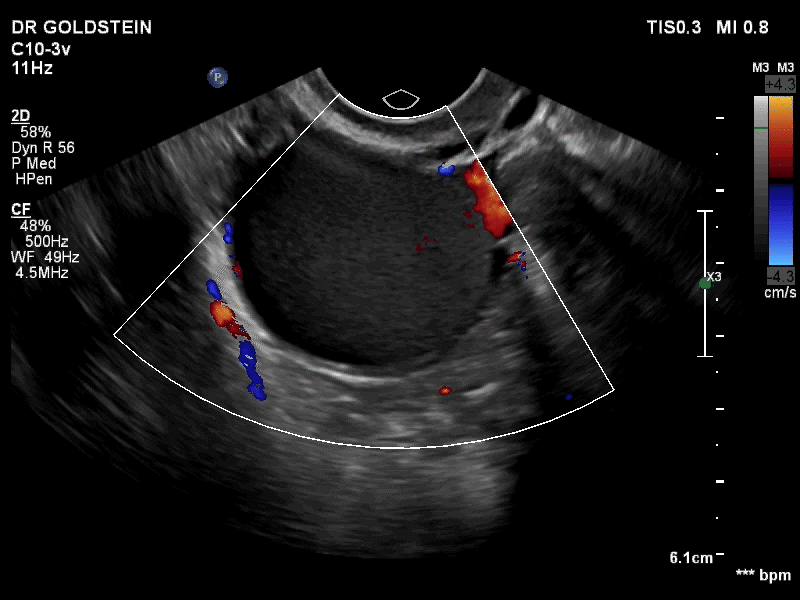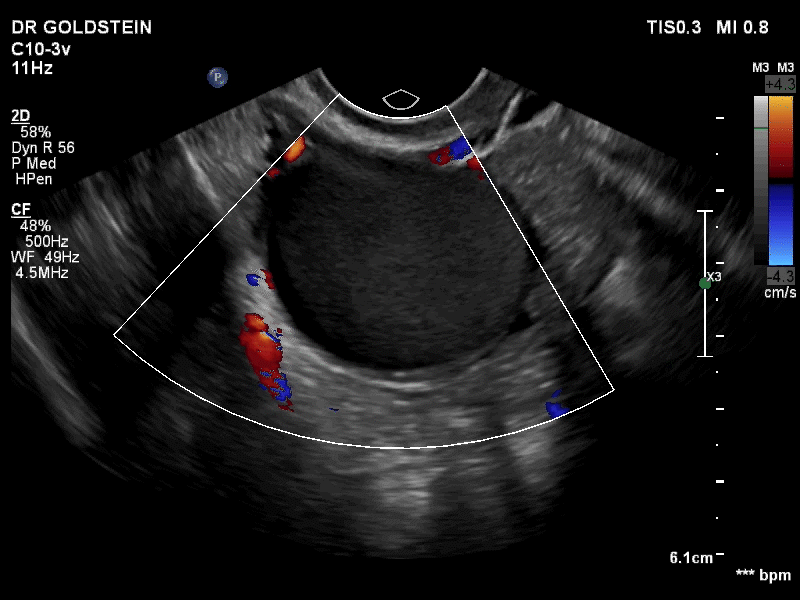
VIDEO 3B Classic endometrioma showing no Doppler flow internally
--
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--
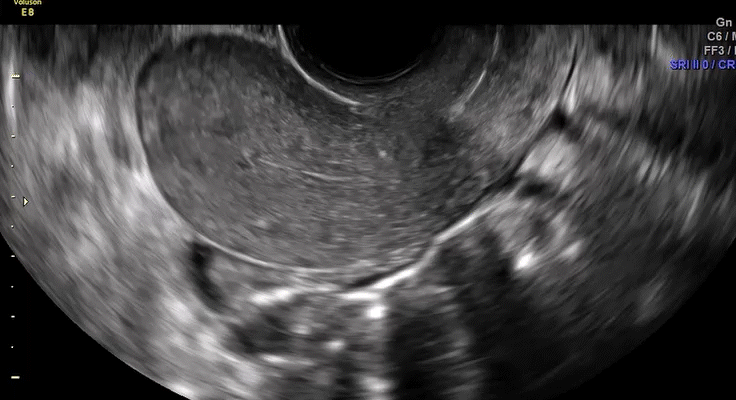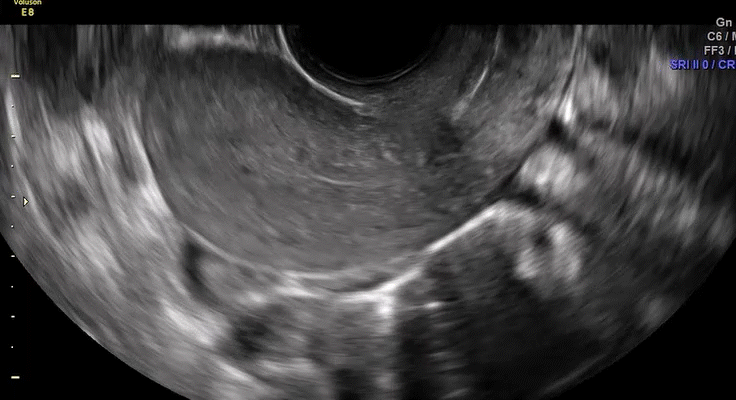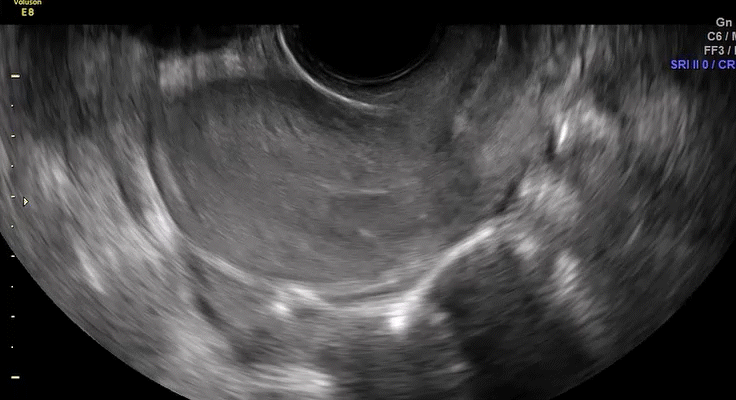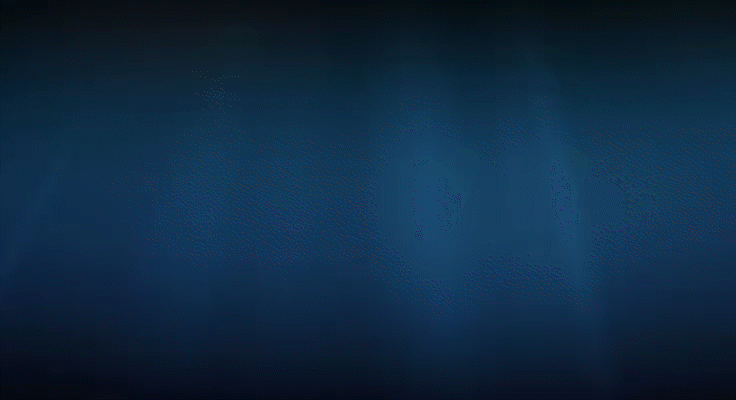
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
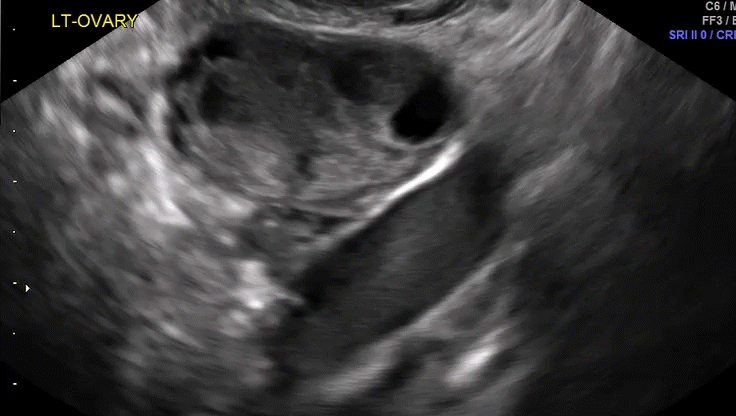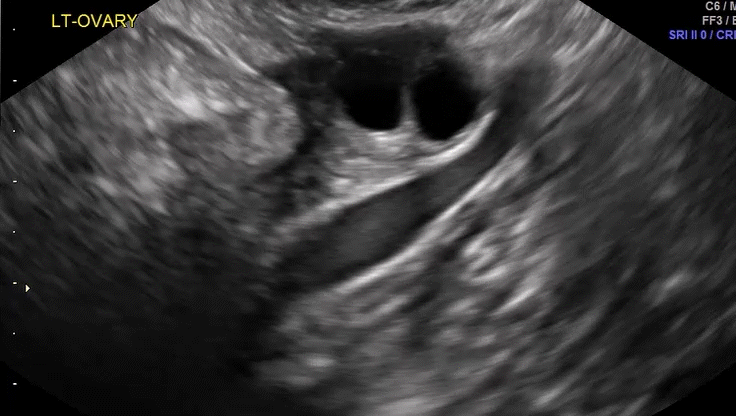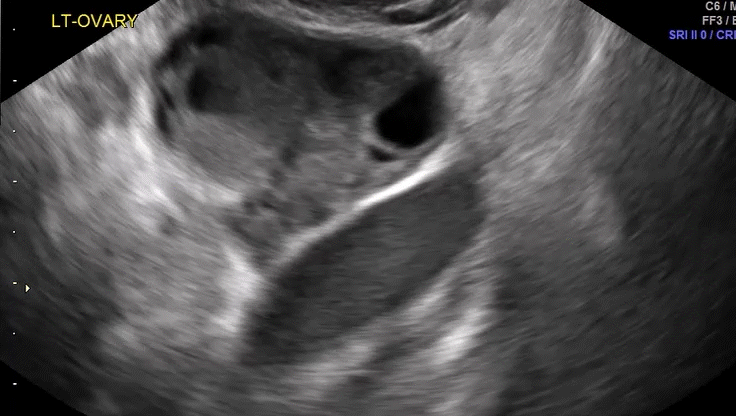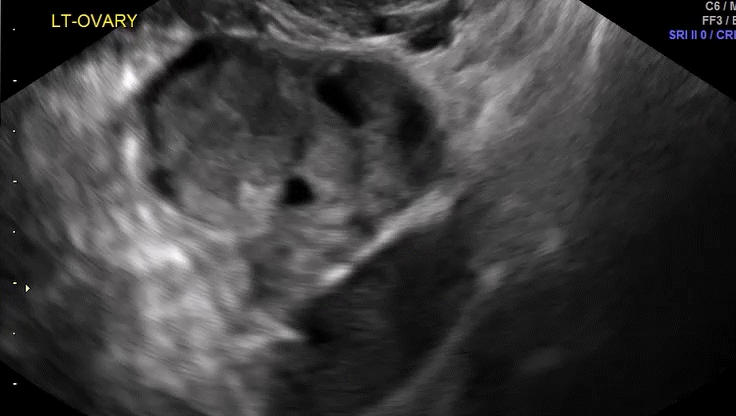
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
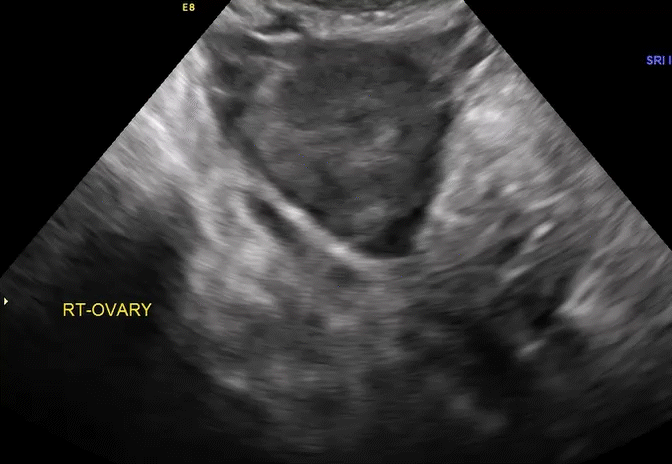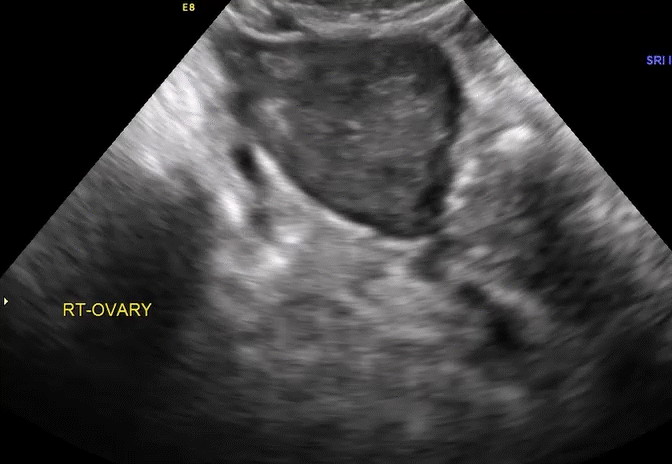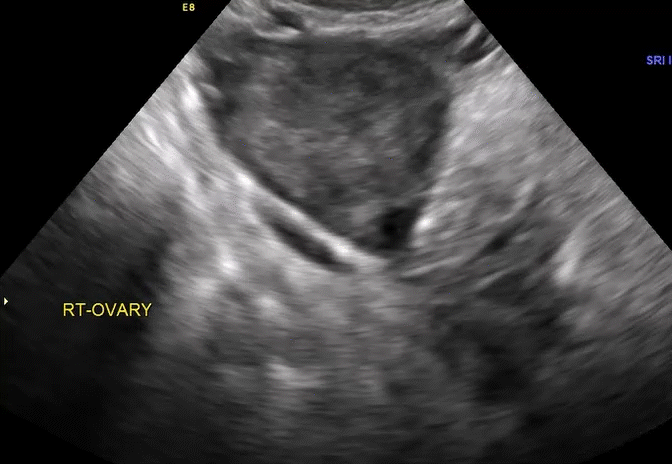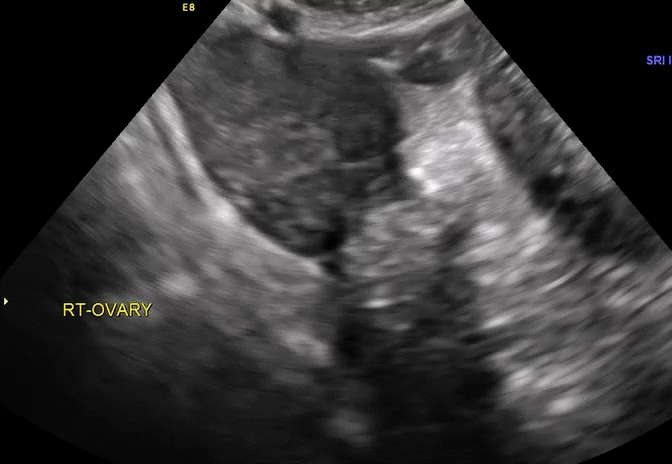
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
--
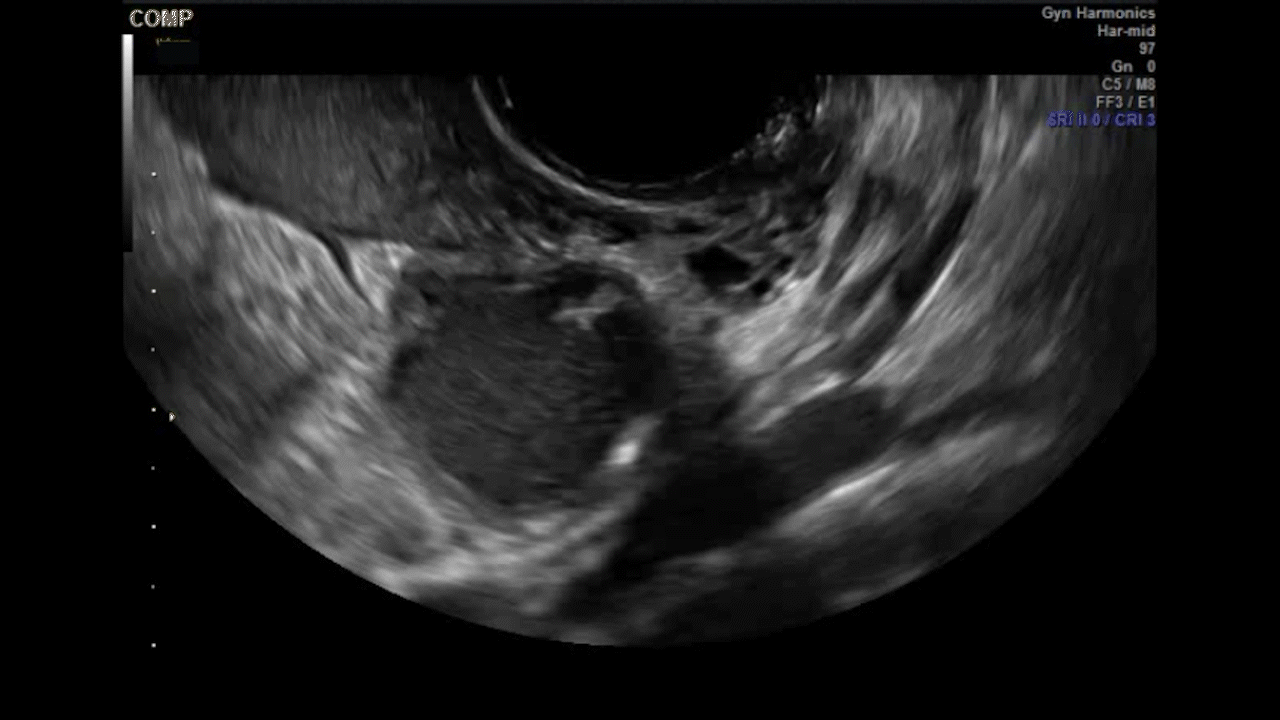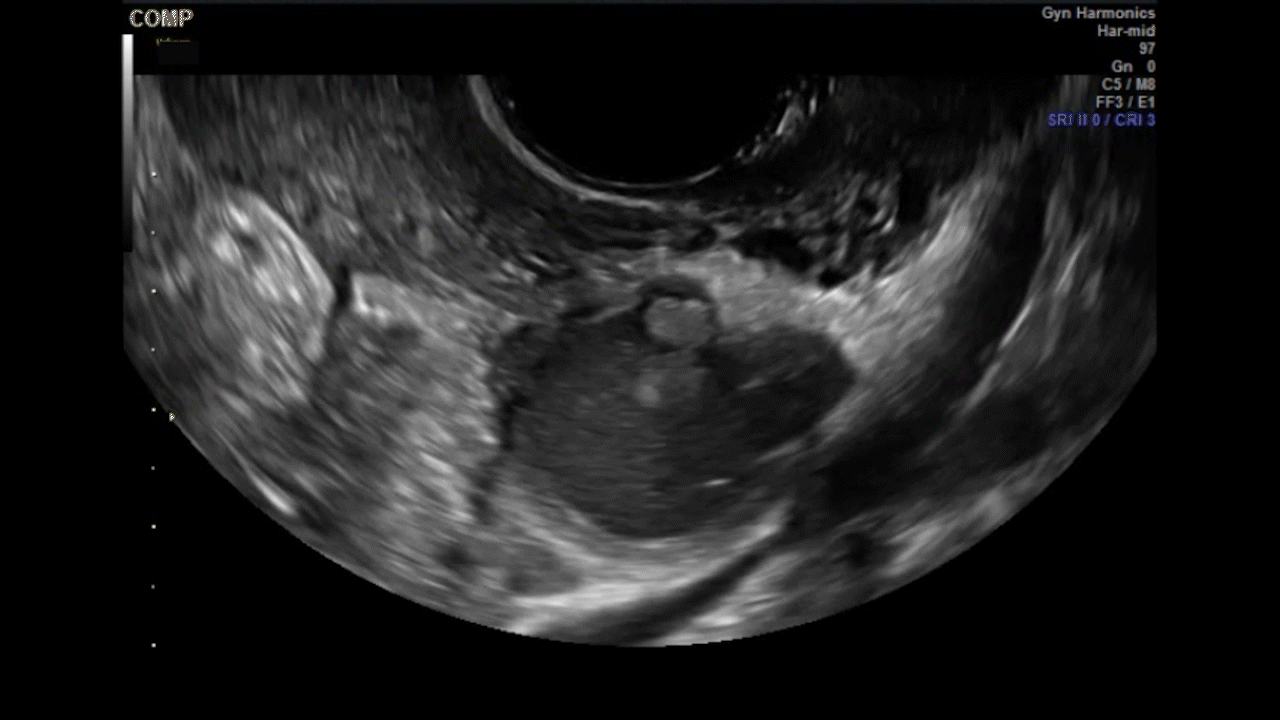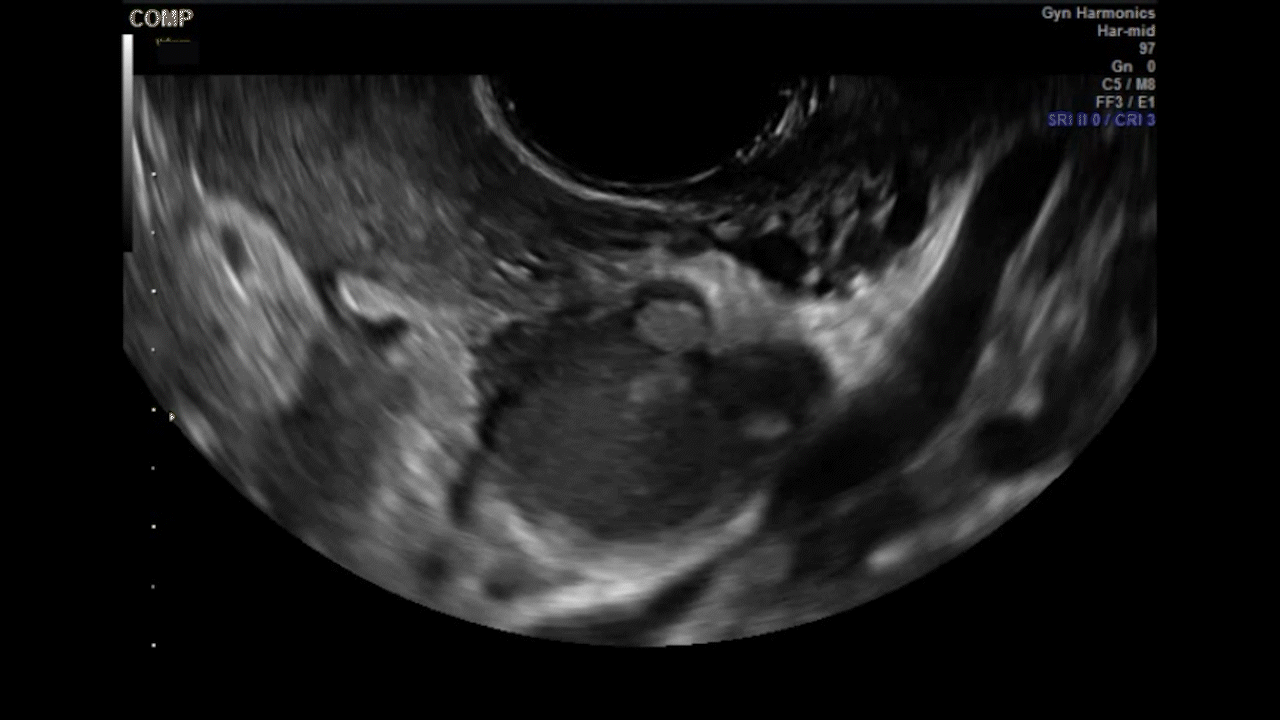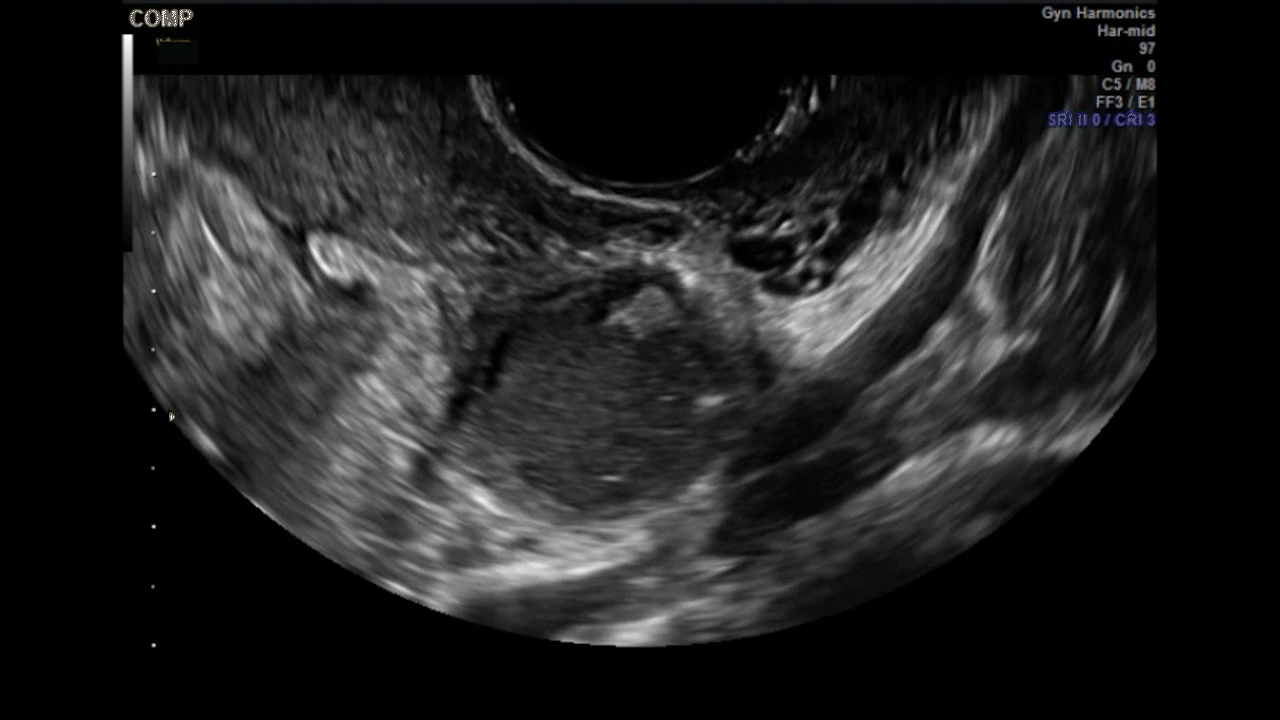
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
--
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--
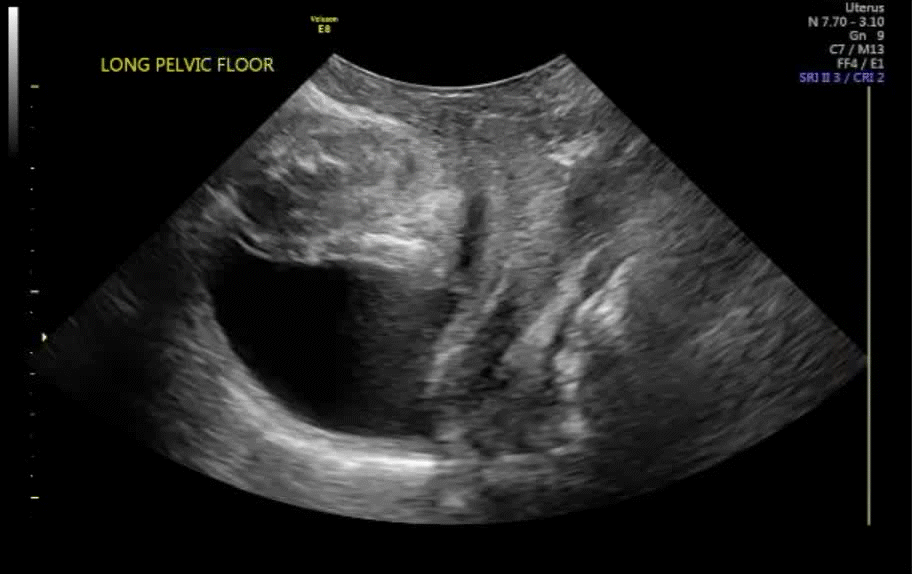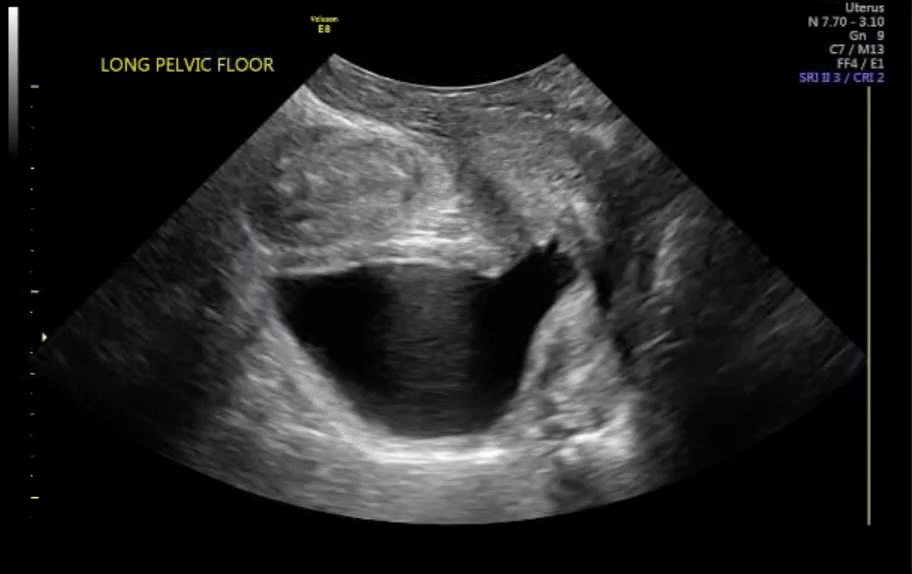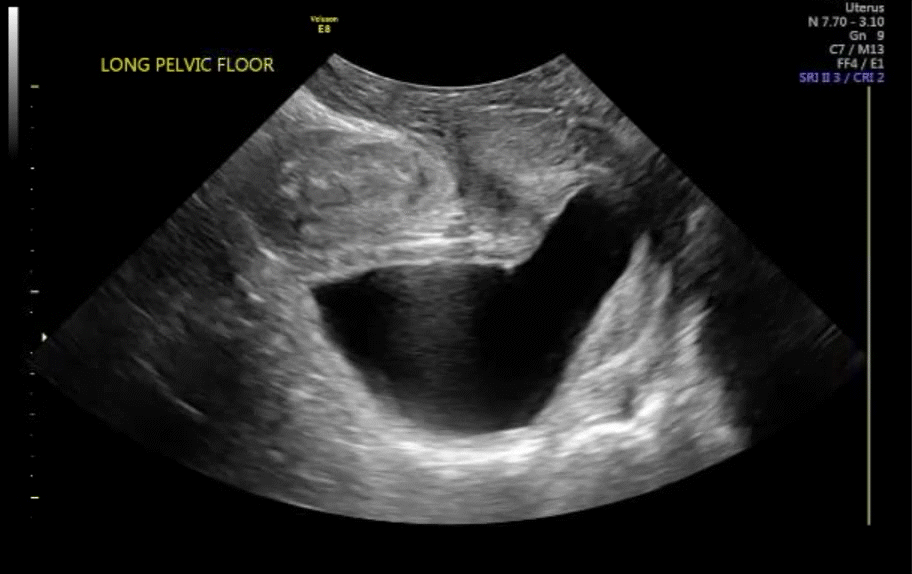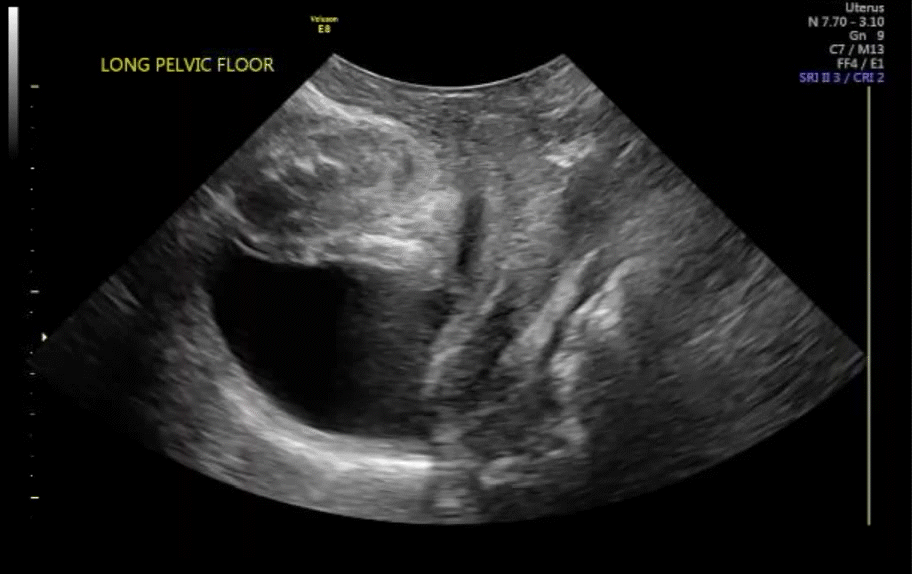
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
--
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation

VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
--

VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
--

VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
--

VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in Video 2A showing what appears to be a solid adnexal mass
--

VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
--

VIDEO 3A Video clip of a classic endometrioma
--

VIDEO 3B Classic endometrioma showing no Doppler flow internally
--

VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
--

VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
--

VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation

VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
--

VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
--

VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
--

VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in Video 2A showing what appears to be a solid adnexal mass
--

VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
--

VIDEO 3A Video clip of a classic endometrioma
--

VIDEO 3B Classic endometrioma showing no Doppler flow internally
--

VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
--

VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
--

VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation