User login
The Multiple Sclerosis Surveillance Registry: A Novel Interactive Database Within the Veterans Health Administration (FULL)
The VA MS Surveillance Registry combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US.
A number of large registries exist for multiple sclerosis (MS) in North America and Europe. The Scandinavian countries have some of the longest running and integrated MS registries to date. The Danish MS Registry was initiated in 1948 and has been consistently maintained to track MS epidemiologic trends.2 Similar databases exist in Swedenand Norway that were created in the later 20th century.3,4 The Rochester Epidemiology Project, launched by Len Kurland at the Mayo Clinic, has tracked the morbidity of MS and many other conditions in Olmsted county Minnesota for > 60 years.5
The Canadian provinces of British Columbia, Ontario, and Manitoba also have long standing MS registries.6-8 Other North American MS registries have gathered state-wide cases, such as the New York State MS Consortium.9 Some registries have gathered a population-based sample throughout the US, such as the Sonya Slifka MS Study.10 The North American Research Consortium on MS (NARCOMS) registry is a patient-driven registry within the US that has enrolled > 30,000 cases.11 The MSBase is the largest online registry to date utilizing data from several countries.12 The MS Bioscreen, based at the University of California San Francisco, is a recent effort to create a longitudinal clinical dataset.13 This electronic registry integrates clinical disease morbidity scales, neuroimaging, genetics and laboratory data for individual patients with the goal of providing predictive tools.
The US military provides a unique population to study MS and has the oldest and largest nation-wide MS cohort in existence starting with World War I service members and continuing through the recent Gulf War Era.14 With the advent of EHRs in the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) in the mid-1990s and large clinical databases, the possibility of an integrated registry for chronic conditions was created. In this report, we describe the creation of the VA MS Surveillance Registry (MSSR) and the initial roll out to several VA medical centers within the MS Center of Excellence (MSCoE). The MSSR is a unique platform with potential for improving MS patient care and clinical research.
Methods
The MSSR was designed by MSCoE health care providers in conjunction with IT specialists from the VA Northwest Innovation Center. Between 2012 and 2013, the team developed and tested a core template for data entry and refined an efficient data dashboard display to optimize clinical decisions. IT programmers created data entry templates that were tested by 4 to 5 clinicians who provided feedback in biweekly meetings. Technical problems were addressed and enhancements added and the trial process was repeated.
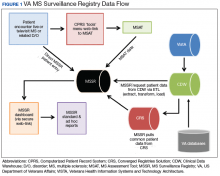
After creation of the prototype MS Assessment Tool (MSAT) data entry template that fed into the prototype MSSR, our team received a grant in 2013 for national development and sustainment. The MSSR was established on the VA Converged Registries Solution (CRS) platform, which is a hardware and software architecture designed to host individual clinical registries and eliminate duplicative development effort while maximizing the ability to create new patient registries. The common platform includes a relational database, Health Level 7 messaging, software classes, security modules, extraction services, and other components. The CR obtains data from the VA Corporate Data Warehouse (CDW), directly from the Veterans Health Information Systems and Technology Architecture (VISTA) and via direct user input using MSAT.
From 2016 to 2019, data from patients with MS followed in several VA MS regional programs were inputted into MSSR. A roll-out process to start patient data entry at VA medical centers began in 2017 that included an orientation, technical support, and quality assurance review. Twelve sites from Veteran Integrated Service Network (VISN) 5 (mid-Atlantic) and VISN 20 (Pacific Northwest) were included in the initial roll-out.
Results
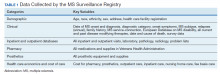
After a live or remote telehealth or telephone visit, a clinician can access MSAT from the Computerized Patient Record System (CPRS) or directly from the MSSR online portal (Figure 1). The tool uses radio buttons and pull-down menus and takes about 5 to 15 minutes to complete with a list of required variables. Data is auto-saved for efficiency, and the key variables that are collected in MSAT are noted in Table 1. The MSAT subsequently creates a text integration utility progress note with health factors that is processed through an integration engine and eventually transmitted to VISTA and becomes part of the EHR and available to all health care providers involved in that patient’s care. Additionally, data from VA outpatient and inpatient utilization files, pharmacy, prosthetics, laboratory, and radiology databases are included in the CDW and are included in MSSR. With data from 1998 to the present, the MSAT and CDW databases can provide longitudinal data analysis.
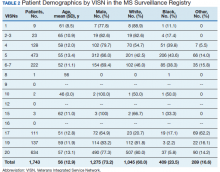
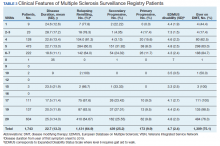
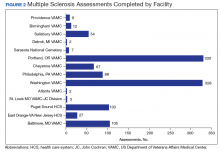
Between 18,000 and 20,000 patients with MS are evaluated in the VHA annually, and 56,000 unique patients have been assessed since 1998. From 2016 to 2019, 1,743 patients with MS or related disorders were enrolled in MSSR (Table 2 and Figure 2). The mean (SD) age of patients was 56.0 (12.9) years and the male:female ratio was 2.7. Racial minorities make up 40% of the cohort. Among those with definite and possible MS, the mean disease duration was 22.7 years and the mean (SD) European Database for MS disability score was 4.7 (2.4) (Table 3). Three-quarters of the MSSR cohort have used ≥ 1 MS disease modifying therapy and 65% were classified as relapsing-remitting MS. An electronic dashboard was developed for health care providers to easily access demographic and clinical data for individuals and groups of patients (Figure 3). Standard and ad hoc reports can be generated from the MSSR. Larger longitudinal analyses can be performed with MSAT and clinical data from CDW. Data on comorbid conditions, pharmacy, radiology and prosthetics utilization, outpatient clinic and inpatient admission can be accessed for each patient or a group of patients.
In 2015, MSCoE published a larger national survey of the VA MS population.15 This study revealed that the majority of clinical features and demographics of the MSSR were not significantly different from other major US MS registries including the North American Research Committee on MS, the New York State MS Consortium, and the Sonya Slifka Study.16-18
Discussion
The MSSR is novel in that it combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US. This new registry leverages the existing databases related to cost of care, utilization, and pharmacy services to provide surveillance tools for longitudinal follow-up of the MS population within the VHA. Because the structure of the MSAT and MSSR were developed in a partnership between IT developers and clinicians, there has been mutual buy-in for those who use it and maintain it. This registry can be a test bed for standardized patient outcomes including the recently released MS Quality measures from the American Academy of Neurology.19
To achieve greater numbers across populations, there has been efforts in Europe to combine registries into a common European Register for MS. A recent survey found that although many European registries were heterogeneous, it would be possible to have a minimum common data set for limited epidemiologic studies.20 Still many registries do not have environmental or genetic data to evaluate etiologic questions.21 Additionally, most registries are not set up to evaluate cost or quality of care within a health care system.
Recommendations for maximizing the impact of existing MS registries were recently released by a panel of MS clinicians and researchers.22 The first recommendation was to create a broad network of registries that would communicate and collaborate. This group of MS registries would have strategic oversight and direction that would greatly streamline and leverage existing and future efforts. Second, registries should standardize data collection and management thereby enhancing the ability to share data and perform meta-analyses with aggregated data. Third, the collection of physician- and patient-reported outcomes should be encouraged to provide a more complete picture of MS. Finally, registries should prioritize research questions and utilize new technologies for data collection. These recommendations would help to coordinate existing registries and accelerate knowledge discovery.
The MSSR will contribute to the growing registry network of data. The MSSR can address questions about clinical outcomes, cost, quality with a growing data repository and linked biobank. Based on the CR platform, the MSSR allows for integration with other VA clinical registries, including registries for traumatic brain injuries, oncology, HIV, hepatitis C virus, and eye injuries. Identifying case outcomes related to other registries is optimized with the CR common structure.
Conclusion
The MSSR has been a useful tool for clinicians managing individual patients and their regional referral populations with real-time access to clinical and utilization data. It will also be a useful research tool in tracking epidemiological trends for the military population. The MSSR has enhanced clinical management of MS and serves as a national source for clinical outcomes.
1. Flachenecker P. Multiple sclerosis databases: present and future. Eur Neurol. 2014;72(suppl 1):29-31.
2. Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand. 2015;132(199):4-10.
3. Alping P, Piehl F, Langer-Gould A, Frisell T; COMBAT-MS Study Group. Validation of the Swedish Multiple Sclerosis Register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology. 2019;30(2):230-233.
4. Benjaminsen E, Myhr KM, Grytten N, Alstadhaug KB. Validation of the multiple sclerosis diagnosis in the Norwegian Patient Registry. Brain Behav. 2019;9(11):e01422.
5. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213.
6. Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991-2010). J Neurol. 2015;262(10):2352-2363.
7. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914-1929.
8. Mahmud SM, Bozat-Emre S, Mostaço-Guidolin LC, Marrie RA. Registry cohort study to determine risk for multiple sclerosis after vaccination for pandemic influenza A(H1N1) with Arepanrix, Manitoba, Canada. Emerg Infect Dis. 2018;24(7):1267-1274.
9. Kister I, Chamot E, Bacon JH, Cutter G, Herbert J; New York State Multiple Sclerosis Consortium. Trend for decreasing Multiple Sclerosis Severity Scores (MSSS) with increasing calendar year of enrollment into the New York State Multiple Sclerosis Consortium. Mult Scler. 2011;17(6):725-733.
10. Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006;12(1):24-38.
11. Fox RJ, Salter A, Alster JM, et al. Risk tolerance to MS therapies: survey results from the NARCOMS registry. Mult Scler Relat Disord. 2015;4(3):241-249.
12. Kalincik T, Butzkueven H. The MSBase registry: Informing clinical practice. Mult Scler. 2019;25(14):1828-1834.
13. Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol. 2014;76(5):633-642.
14. Wallin MT, Culpepper WJ, Coffman P, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain. 2012;135(Pt 6):1778-1785.
15. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272.
16. Salter A, Stahmann A, Ellenberger D, et al. Data harmonization for collaborative research among MS registries: a case study in employment [published online ahead of print, 2020 Mar 12]. Mult Scler. 2020;1352458520910499.
17. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Mult Scler. 2020;26(1):99-108.
18. Minden SL, Kinkel RP, Machado HT, et al. Use and cost of disease-modifying therapies by Sonya Slifka Study participants: has anything really changed since 2000 and 2009? Mult Scler J Exp Transl Clin. 2019;5(1):2055217318820888.
19. Rae-Grant A, Bennett A, Sanders AE, Phipps M, Cheng E, Bever C. Quality improvement in neurology: multiple sclerosis quality measures: Executive summary [published correction appears in Neurology. 2016;86(15):1465]. Neurology. 2015;85(21):1904-1908.
20. Flachenecker P, Buckow K, Pugliatti M, et al; EUReMS Consortium. Multiple sclerosis registries in Europe - results of a systematic survey. Mult Scler. 2014;20(11):1523-1532.
21. Traboulsee A, McMullen K. How useful are MS registries?. Mult Scler. 2014;20(11):1423-1424.
22. Bebo BF Jr, Fox RJ, Lee K, Utz U, Thompson AJ. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Mult Scler. 2018;24(5):579-586.
The VA MS Surveillance Registry combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US.
The VA MS Surveillance Registry combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US.
A number of large registries exist for multiple sclerosis (MS) in North America and Europe. The Scandinavian countries have some of the longest running and integrated MS registries to date. The Danish MS Registry was initiated in 1948 and has been consistently maintained to track MS epidemiologic trends.2 Similar databases exist in Swedenand Norway that were created in the later 20th century.3,4 The Rochester Epidemiology Project, launched by Len Kurland at the Mayo Clinic, has tracked the morbidity of MS and many other conditions in Olmsted county Minnesota for > 60 years.5
The Canadian provinces of British Columbia, Ontario, and Manitoba also have long standing MS registries.6-8 Other North American MS registries have gathered state-wide cases, such as the New York State MS Consortium.9 Some registries have gathered a population-based sample throughout the US, such as the Sonya Slifka MS Study.10 The North American Research Consortium on MS (NARCOMS) registry is a patient-driven registry within the US that has enrolled > 30,000 cases.11 The MSBase is the largest online registry to date utilizing data from several countries.12 The MS Bioscreen, based at the University of California San Francisco, is a recent effort to create a longitudinal clinical dataset.13 This electronic registry integrates clinical disease morbidity scales, neuroimaging, genetics and laboratory data for individual patients with the goal of providing predictive tools.
The US military provides a unique population to study MS and has the oldest and largest nation-wide MS cohort in existence starting with World War I service members and continuing through the recent Gulf War Era.14 With the advent of EHRs in the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) in the mid-1990s and large clinical databases, the possibility of an integrated registry for chronic conditions was created. In this report, we describe the creation of the VA MS Surveillance Registry (MSSR) and the initial roll out to several VA medical centers within the MS Center of Excellence (MSCoE). The MSSR is a unique platform with potential for improving MS patient care and clinical research.
Methods
The MSSR was designed by MSCoE health care providers in conjunction with IT specialists from the VA Northwest Innovation Center. Between 2012 and 2013, the team developed and tested a core template for data entry and refined an efficient data dashboard display to optimize clinical decisions. IT programmers created data entry templates that were tested by 4 to 5 clinicians who provided feedback in biweekly meetings. Technical problems were addressed and enhancements added and the trial process was repeated.
After creation of the prototype MS Assessment Tool (MSAT) data entry template that fed into the prototype MSSR, our team received a grant in 2013 for national development and sustainment. The MSSR was established on the VA Converged Registries Solution (CRS) platform, which is a hardware and software architecture designed to host individual clinical registries and eliminate duplicative development effort while maximizing the ability to create new patient registries. The common platform includes a relational database, Health Level 7 messaging, software classes, security modules, extraction services, and other components. The CR obtains data from the VA Corporate Data Warehouse (CDW), directly from the Veterans Health Information Systems and Technology Architecture (VISTA) and via direct user input using MSAT.
From 2016 to 2019, data from patients with MS followed in several VA MS regional programs were inputted into MSSR. A roll-out process to start patient data entry at VA medical centers began in 2017 that included an orientation, technical support, and quality assurance review. Twelve sites from Veteran Integrated Service Network (VISN) 5 (mid-Atlantic) and VISN 20 (Pacific Northwest) were included in the initial roll-out.
Results
After a live or remote telehealth or telephone visit, a clinician can access MSAT from the Computerized Patient Record System (CPRS) or directly from the MSSR online portal (Figure 1). The tool uses radio buttons and pull-down menus and takes about 5 to 15 minutes to complete with a list of required variables. Data is auto-saved for efficiency, and the key variables that are collected in MSAT are noted in Table 1. The MSAT subsequently creates a text integration utility progress note with health factors that is processed through an integration engine and eventually transmitted to VISTA and becomes part of the EHR and available to all health care providers involved in that patient’s care. Additionally, data from VA outpatient and inpatient utilization files, pharmacy, prosthetics, laboratory, and radiology databases are included in the CDW and are included in MSSR. With data from 1998 to the present, the MSAT and CDW databases can provide longitudinal data analysis.
Between 18,000 and 20,000 patients with MS are evaluated in the VHA annually, and 56,000 unique patients have been assessed since 1998. From 2016 to 2019, 1,743 patients with MS or related disorders were enrolled in MSSR (Table 2 and Figure 2). The mean (SD) age of patients was 56.0 (12.9) years and the male:female ratio was 2.7. Racial minorities make up 40% of the cohort. Among those with definite and possible MS, the mean disease duration was 22.7 years and the mean (SD) European Database for MS disability score was 4.7 (2.4) (Table 3). Three-quarters of the MSSR cohort have used ≥ 1 MS disease modifying therapy and 65% were classified as relapsing-remitting MS. An electronic dashboard was developed for health care providers to easily access demographic and clinical data for individuals and groups of patients (Figure 3). Standard and ad hoc reports can be generated from the MSSR. Larger longitudinal analyses can be performed with MSAT and clinical data from CDW. Data on comorbid conditions, pharmacy, radiology and prosthetics utilization, outpatient clinic and inpatient admission can be accessed for each patient or a group of patients.
In 2015, MSCoE published a larger national survey of the VA MS population.15 This study revealed that the majority of clinical features and demographics of the MSSR were not significantly different from other major US MS registries including the North American Research Committee on MS, the New York State MS Consortium, and the Sonya Slifka Study.16-18
Discussion
The MSSR is novel in that it combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US. This new registry leverages the existing databases related to cost of care, utilization, and pharmacy services to provide surveillance tools for longitudinal follow-up of the MS population within the VHA. Because the structure of the MSAT and MSSR were developed in a partnership between IT developers and clinicians, there has been mutual buy-in for those who use it and maintain it. This registry can be a test bed for standardized patient outcomes including the recently released MS Quality measures from the American Academy of Neurology.19
To achieve greater numbers across populations, there has been efforts in Europe to combine registries into a common European Register for MS. A recent survey found that although many European registries were heterogeneous, it would be possible to have a minimum common data set for limited epidemiologic studies.20 Still many registries do not have environmental or genetic data to evaluate etiologic questions.21 Additionally, most registries are not set up to evaluate cost or quality of care within a health care system.
Recommendations for maximizing the impact of existing MS registries were recently released by a panel of MS clinicians and researchers.22 The first recommendation was to create a broad network of registries that would communicate and collaborate. This group of MS registries would have strategic oversight and direction that would greatly streamline and leverage existing and future efforts. Second, registries should standardize data collection and management thereby enhancing the ability to share data and perform meta-analyses with aggregated data. Third, the collection of physician- and patient-reported outcomes should be encouraged to provide a more complete picture of MS. Finally, registries should prioritize research questions and utilize new technologies for data collection. These recommendations would help to coordinate existing registries and accelerate knowledge discovery.
The MSSR will contribute to the growing registry network of data. The MSSR can address questions about clinical outcomes, cost, quality with a growing data repository and linked biobank. Based on the CR platform, the MSSR allows for integration with other VA clinical registries, including registries for traumatic brain injuries, oncology, HIV, hepatitis C virus, and eye injuries. Identifying case outcomes related to other registries is optimized with the CR common structure.
Conclusion
The MSSR has been a useful tool for clinicians managing individual patients and their regional referral populations with real-time access to clinical and utilization data. It will also be a useful research tool in tracking epidemiological trends for the military population. The MSSR has enhanced clinical management of MS and serves as a national source for clinical outcomes.
A number of large registries exist for multiple sclerosis (MS) in North America and Europe. The Scandinavian countries have some of the longest running and integrated MS registries to date. The Danish MS Registry was initiated in 1948 and has been consistently maintained to track MS epidemiologic trends.2 Similar databases exist in Swedenand Norway that were created in the later 20th century.3,4 The Rochester Epidemiology Project, launched by Len Kurland at the Mayo Clinic, has tracked the morbidity of MS and many other conditions in Olmsted county Minnesota for > 60 years.5
The Canadian provinces of British Columbia, Ontario, and Manitoba also have long standing MS registries.6-8 Other North American MS registries have gathered state-wide cases, such as the New York State MS Consortium.9 Some registries have gathered a population-based sample throughout the US, such as the Sonya Slifka MS Study.10 The North American Research Consortium on MS (NARCOMS) registry is a patient-driven registry within the US that has enrolled > 30,000 cases.11 The MSBase is the largest online registry to date utilizing data from several countries.12 The MS Bioscreen, based at the University of California San Francisco, is a recent effort to create a longitudinal clinical dataset.13 This electronic registry integrates clinical disease morbidity scales, neuroimaging, genetics and laboratory data for individual patients with the goal of providing predictive tools.
The US military provides a unique population to study MS and has the oldest and largest nation-wide MS cohort in existence starting with World War I service members and continuing through the recent Gulf War Era.14 With the advent of EHRs in the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) in the mid-1990s and large clinical databases, the possibility of an integrated registry for chronic conditions was created. In this report, we describe the creation of the VA MS Surveillance Registry (MSSR) and the initial roll out to several VA medical centers within the MS Center of Excellence (MSCoE). The MSSR is a unique platform with potential for improving MS patient care and clinical research.
Methods
The MSSR was designed by MSCoE health care providers in conjunction with IT specialists from the VA Northwest Innovation Center. Between 2012 and 2013, the team developed and tested a core template for data entry and refined an efficient data dashboard display to optimize clinical decisions. IT programmers created data entry templates that were tested by 4 to 5 clinicians who provided feedback in biweekly meetings. Technical problems were addressed and enhancements added and the trial process was repeated.
After creation of the prototype MS Assessment Tool (MSAT) data entry template that fed into the prototype MSSR, our team received a grant in 2013 for national development and sustainment. The MSSR was established on the VA Converged Registries Solution (CRS) platform, which is a hardware and software architecture designed to host individual clinical registries and eliminate duplicative development effort while maximizing the ability to create new patient registries. The common platform includes a relational database, Health Level 7 messaging, software classes, security modules, extraction services, and other components. The CR obtains data from the VA Corporate Data Warehouse (CDW), directly from the Veterans Health Information Systems and Technology Architecture (VISTA) and via direct user input using MSAT.
From 2016 to 2019, data from patients with MS followed in several VA MS regional programs were inputted into MSSR. A roll-out process to start patient data entry at VA medical centers began in 2017 that included an orientation, technical support, and quality assurance review. Twelve sites from Veteran Integrated Service Network (VISN) 5 (mid-Atlantic) and VISN 20 (Pacific Northwest) were included in the initial roll-out.
Results
After a live or remote telehealth or telephone visit, a clinician can access MSAT from the Computerized Patient Record System (CPRS) or directly from the MSSR online portal (Figure 1). The tool uses radio buttons and pull-down menus and takes about 5 to 15 minutes to complete with a list of required variables. Data is auto-saved for efficiency, and the key variables that are collected in MSAT are noted in Table 1. The MSAT subsequently creates a text integration utility progress note with health factors that is processed through an integration engine and eventually transmitted to VISTA and becomes part of the EHR and available to all health care providers involved in that patient’s care. Additionally, data from VA outpatient and inpatient utilization files, pharmacy, prosthetics, laboratory, and radiology databases are included in the CDW and are included in MSSR. With data from 1998 to the present, the MSAT and CDW databases can provide longitudinal data analysis.
Between 18,000 and 20,000 patients with MS are evaluated in the VHA annually, and 56,000 unique patients have been assessed since 1998. From 2016 to 2019, 1,743 patients with MS or related disorders were enrolled in MSSR (Table 2 and Figure 2). The mean (SD) age of patients was 56.0 (12.9) years and the male:female ratio was 2.7. Racial minorities make up 40% of the cohort. Among those with definite and possible MS, the mean disease duration was 22.7 years and the mean (SD) European Database for MS disability score was 4.7 (2.4) (Table 3). Three-quarters of the MSSR cohort have used ≥ 1 MS disease modifying therapy and 65% were classified as relapsing-remitting MS. An electronic dashboard was developed for health care providers to easily access demographic and clinical data for individuals and groups of patients (Figure 3). Standard and ad hoc reports can be generated from the MSSR. Larger longitudinal analyses can be performed with MSAT and clinical data from CDW. Data on comorbid conditions, pharmacy, radiology and prosthetics utilization, outpatient clinic and inpatient admission can be accessed for each patient or a group of patients.
In 2015, MSCoE published a larger national survey of the VA MS population.15 This study revealed that the majority of clinical features and demographics of the MSSR were not significantly different from other major US MS registries including the North American Research Committee on MS, the New York State MS Consortium, and the Sonya Slifka Study.16-18
Discussion
The MSSR is novel in that it combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US. This new registry leverages the existing databases related to cost of care, utilization, and pharmacy services to provide surveillance tools for longitudinal follow-up of the MS population within the VHA. Because the structure of the MSAT and MSSR were developed in a partnership between IT developers and clinicians, there has been mutual buy-in for those who use it and maintain it. This registry can be a test bed for standardized patient outcomes including the recently released MS Quality measures from the American Academy of Neurology.19
To achieve greater numbers across populations, there has been efforts in Europe to combine registries into a common European Register for MS. A recent survey found that although many European registries were heterogeneous, it would be possible to have a minimum common data set for limited epidemiologic studies.20 Still many registries do not have environmental or genetic data to evaluate etiologic questions.21 Additionally, most registries are not set up to evaluate cost or quality of care within a health care system.
Recommendations for maximizing the impact of existing MS registries were recently released by a panel of MS clinicians and researchers.22 The first recommendation was to create a broad network of registries that would communicate and collaborate. This group of MS registries would have strategic oversight and direction that would greatly streamline and leverage existing and future efforts. Second, registries should standardize data collection and management thereby enhancing the ability to share data and perform meta-analyses with aggregated data. Third, the collection of physician- and patient-reported outcomes should be encouraged to provide a more complete picture of MS. Finally, registries should prioritize research questions and utilize new technologies for data collection. These recommendations would help to coordinate existing registries and accelerate knowledge discovery.
The MSSR will contribute to the growing registry network of data. The MSSR can address questions about clinical outcomes, cost, quality with a growing data repository and linked biobank. Based on the CR platform, the MSSR allows for integration with other VA clinical registries, including registries for traumatic brain injuries, oncology, HIV, hepatitis C virus, and eye injuries. Identifying case outcomes related to other registries is optimized with the CR common structure.
Conclusion
The MSSR has been a useful tool for clinicians managing individual patients and their regional referral populations with real-time access to clinical and utilization data. It will also be a useful research tool in tracking epidemiological trends for the military population. The MSSR has enhanced clinical management of MS and serves as a national source for clinical outcomes.
1. Flachenecker P. Multiple sclerosis databases: present and future. Eur Neurol. 2014;72(suppl 1):29-31.
2. Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand. 2015;132(199):4-10.
3. Alping P, Piehl F, Langer-Gould A, Frisell T; COMBAT-MS Study Group. Validation of the Swedish Multiple Sclerosis Register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology. 2019;30(2):230-233.
4. Benjaminsen E, Myhr KM, Grytten N, Alstadhaug KB. Validation of the multiple sclerosis diagnosis in the Norwegian Patient Registry. Brain Behav. 2019;9(11):e01422.
5. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213.
6. Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991-2010). J Neurol. 2015;262(10):2352-2363.
7. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914-1929.
8. Mahmud SM, Bozat-Emre S, Mostaço-Guidolin LC, Marrie RA. Registry cohort study to determine risk for multiple sclerosis after vaccination for pandemic influenza A(H1N1) with Arepanrix, Manitoba, Canada. Emerg Infect Dis. 2018;24(7):1267-1274.
9. Kister I, Chamot E, Bacon JH, Cutter G, Herbert J; New York State Multiple Sclerosis Consortium. Trend for decreasing Multiple Sclerosis Severity Scores (MSSS) with increasing calendar year of enrollment into the New York State Multiple Sclerosis Consortium. Mult Scler. 2011;17(6):725-733.
10. Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006;12(1):24-38.
11. Fox RJ, Salter A, Alster JM, et al. Risk tolerance to MS therapies: survey results from the NARCOMS registry. Mult Scler Relat Disord. 2015;4(3):241-249.
12. Kalincik T, Butzkueven H. The MSBase registry: Informing clinical practice. Mult Scler. 2019;25(14):1828-1834.
13. Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol. 2014;76(5):633-642.
14. Wallin MT, Culpepper WJ, Coffman P, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain. 2012;135(Pt 6):1778-1785.
15. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272.
16. Salter A, Stahmann A, Ellenberger D, et al. Data harmonization for collaborative research among MS registries: a case study in employment [published online ahead of print, 2020 Mar 12]. Mult Scler. 2020;1352458520910499.
17. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Mult Scler. 2020;26(1):99-108.
18. Minden SL, Kinkel RP, Machado HT, et al. Use and cost of disease-modifying therapies by Sonya Slifka Study participants: has anything really changed since 2000 and 2009? Mult Scler J Exp Transl Clin. 2019;5(1):2055217318820888.
19. Rae-Grant A, Bennett A, Sanders AE, Phipps M, Cheng E, Bever C. Quality improvement in neurology: multiple sclerosis quality measures: Executive summary [published correction appears in Neurology. 2016;86(15):1465]. Neurology. 2015;85(21):1904-1908.
20. Flachenecker P, Buckow K, Pugliatti M, et al; EUReMS Consortium. Multiple sclerosis registries in Europe - results of a systematic survey. Mult Scler. 2014;20(11):1523-1532.
21. Traboulsee A, McMullen K. How useful are MS registries?. Mult Scler. 2014;20(11):1423-1424.
22. Bebo BF Jr, Fox RJ, Lee K, Utz U, Thompson AJ. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Mult Scler. 2018;24(5):579-586.
1. Flachenecker P. Multiple sclerosis databases: present and future. Eur Neurol. 2014;72(suppl 1):29-31.
2. Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand. 2015;132(199):4-10.
3. Alping P, Piehl F, Langer-Gould A, Frisell T; COMBAT-MS Study Group. Validation of the Swedish Multiple Sclerosis Register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology. 2019;30(2):230-233.
4. Benjaminsen E, Myhr KM, Grytten N, Alstadhaug KB. Validation of the multiple sclerosis diagnosis in the Norwegian Patient Registry. Brain Behav. 2019;9(11):e01422.
5. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213.
6. Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991-2010). J Neurol. 2015;262(10):2352-2363.
7. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914-1929.
8. Mahmud SM, Bozat-Emre S, Mostaço-Guidolin LC, Marrie RA. Registry cohort study to determine risk for multiple sclerosis after vaccination for pandemic influenza A(H1N1) with Arepanrix, Manitoba, Canada. Emerg Infect Dis. 2018;24(7):1267-1274.
9. Kister I, Chamot E, Bacon JH, Cutter G, Herbert J; New York State Multiple Sclerosis Consortium. Trend for decreasing Multiple Sclerosis Severity Scores (MSSS) with increasing calendar year of enrollment into the New York State Multiple Sclerosis Consortium. Mult Scler. 2011;17(6):725-733.
10. Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006;12(1):24-38.
11. Fox RJ, Salter A, Alster JM, et al. Risk tolerance to MS therapies: survey results from the NARCOMS registry. Mult Scler Relat Disord. 2015;4(3):241-249.
12. Kalincik T, Butzkueven H. The MSBase registry: Informing clinical practice. Mult Scler. 2019;25(14):1828-1834.
13. Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol. 2014;76(5):633-642.
14. Wallin MT, Culpepper WJ, Coffman P, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain. 2012;135(Pt 6):1778-1785.
15. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272.
16. Salter A, Stahmann A, Ellenberger D, et al. Data harmonization for collaborative research among MS registries: a case study in employment [published online ahead of print, 2020 Mar 12]. Mult Scler. 2020;1352458520910499.
17. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Mult Scler. 2020;26(1):99-108.
18. Minden SL, Kinkel RP, Machado HT, et al. Use and cost of disease-modifying therapies by Sonya Slifka Study participants: has anything really changed since 2000 and 2009? Mult Scler J Exp Transl Clin. 2019;5(1):2055217318820888.
19. Rae-Grant A, Bennett A, Sanders AE, Phipps M, Cheng E, Bever C. Quality improvement in neurology: multiple sclerosis quality measures: Executive summary [published correction appears in Neurology. 2016;86(15):1465]. Neurology. 2015;85(21):1904-1908.
20. Flachenecker P, Buckow K, Pugliatti M, et al; EUReMS Consortium. Multiple sclerosis registries in Europe - results of a systematic survey. Mult Scler. 2014;20(11):1523-1532.
21. Traboulsee A, McMullen K. How useful are MS registries?. Mult Scler. 2014;20(11):1423-1424.
22. Bebo BF Jr, Fox RJ, Lee K, Utz U, Thompson AJ. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Mult Scler. 2018;24(5):579-586.
Cutaneous Manifestation as Initial Presentation of Metastatic Breast Cancer: A Systematic Review
Breast cancer is the second most common malignancy in women (after primary skin cancer) and is the second leading cause of cancer-related death in this population. In 2020, the American Cancer Society reported an estimated 276,480 new breast cancer diagnoses and 42,170 breast cancer–related deaths.1 Despite the fact that routine screening with mammography and sonography is standard, the incidence of advanced breast cancer at the time of diagnosis has remained stable over time, suggesting that life-threatening breast cancers are not being caught at an earlier stage. The number of breast cancers with distant metastases at the time of diagnosis also has not decreased.2 Therefore, although screening tests are valuable, they are imperfect and not without limitations.
Cutaneous metastasis is defined as the spread of malignant cells from an internal neoplasm to the skin, which can occur either by contiguous invasion or by distant metastasis through hematogenous or lymphatic routes.3 The diagnosis of cutaneous metastasis requires a high index of suspicion on the part of the clinician.4 Of the various internal malignancies in women, breast cancer most frequently results in metastasis to the skin,5 with up to 24% of patients with metastatic breast cancer developing cutaneous lesions.6
In recent years, there have been multiple reports of skin lesions prompting the diagnosis of a previously unknown breast cancer. In a study by Lookingbill et al,6 6.3% of patients with breast cancer presented with cutaneous involvement at the time of diagnosis, with 3.5% having skin symptoms as the presenting sign. Although there have been studies analyzing cutaneous metastasis from various internal malignancies, none thus far have focused on cutaneous metastasis as a presenting sign of breast cancer. This systematic review aimed to highlight the diverse clinical presentations of cutaneous metastatic breast cancer and their clinical implications.
Methods
Study Selection
This study utilized the PRISMA guidelines for systematic reviews.7 A review of the literature was conducted using the following databases: MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO.
Search Strategy and Analysis
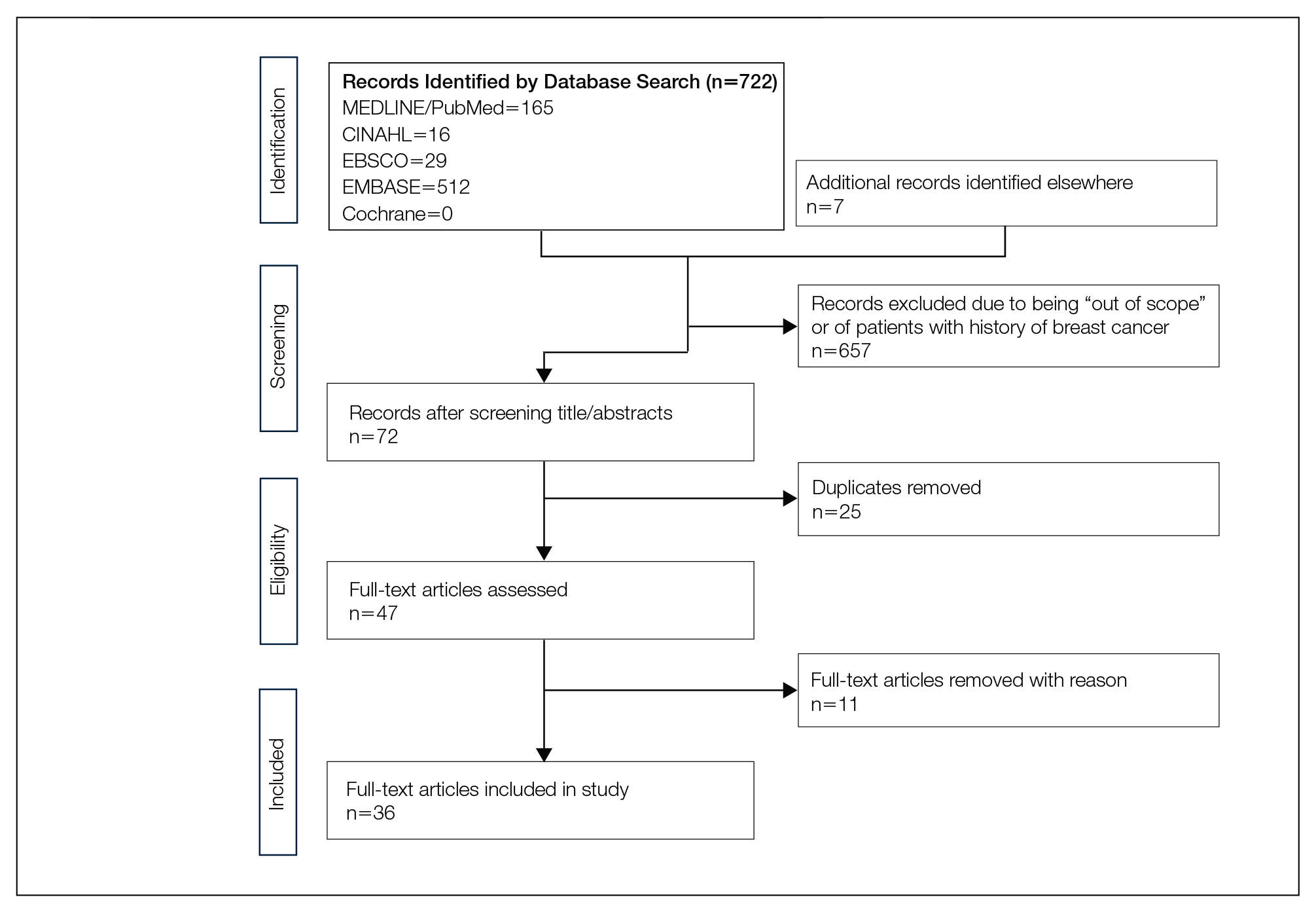
We completed our search of each of the databases on December 16, 2017, using the phrases cutaneous metastasis and breast cancer to find relevant case reports and retrospective studies. Three authors (C.J., S.R., and M.A.) manually reviewed the resulting abstracts. If an abstract did not include enough information to determine inclusion, the full-text version was reviewed by 2 of the authors (C.J. and S.R.). Two of the authors (C.J. and M.A.) also assessed each source for relevancy and included the articles deemed eligible (Figure 1).
Inclusion criteria were the following: case reports and retrospective studies published in the prior 10 years (January 1, 2007, to December 16, 2017) with human female patients who developed metastatic cutaneous lesions due to a previously unknown primary breast malignancy. Studies published in other languages were included; these articles were translated into English using a human translator or computer translation program (Google Translate). Exclusion criteria were the following: male patients, patients with a known diagnosis of primary breast malignancy prior to the appearance of a metastatic cutaneous lesion, articles focusing on the treatment of breast cancer, and articles without enough details to draw meaningful conclusions.
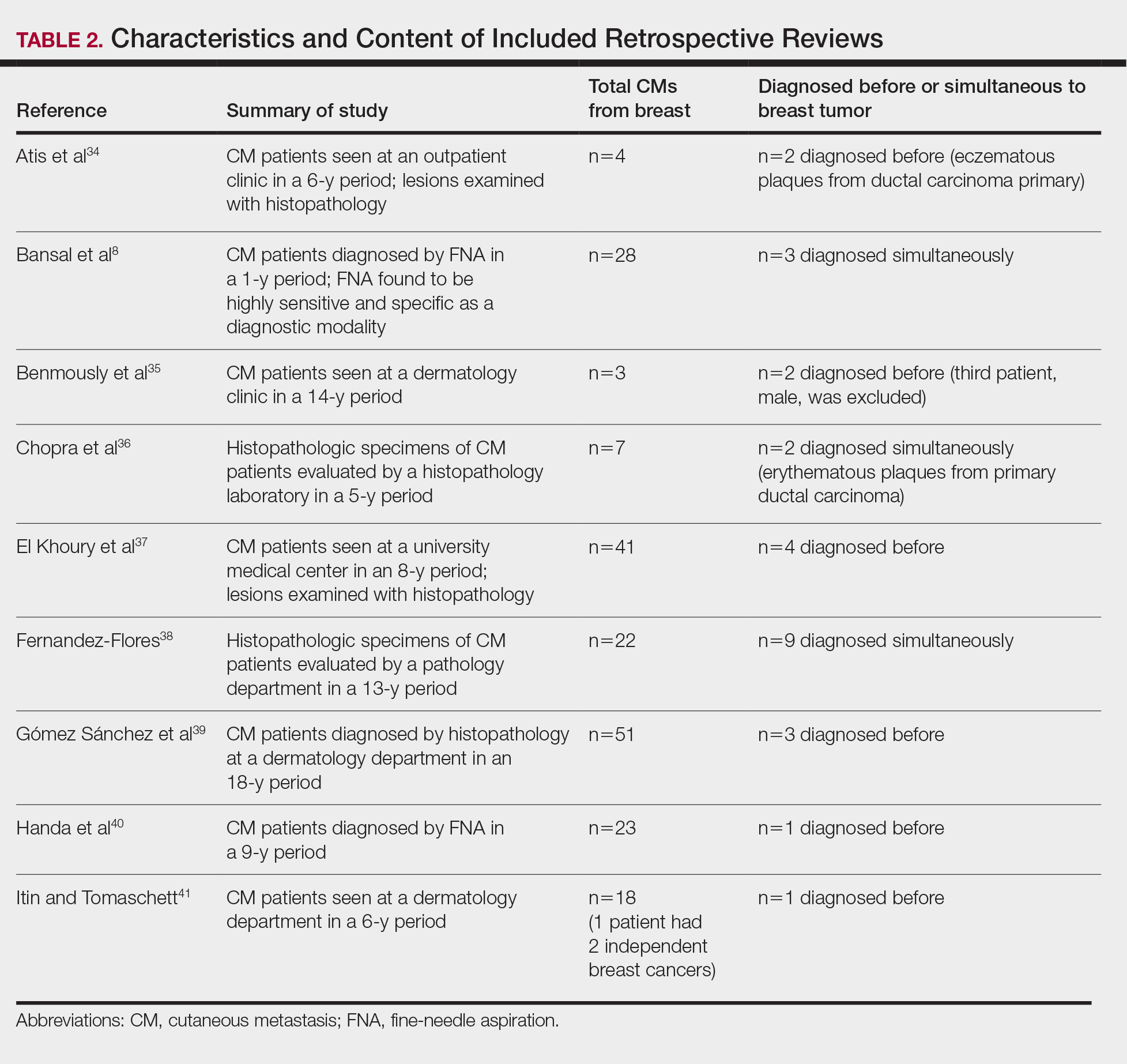
For a retrospective review to be included, it must have specified the number of breast cancer cases and the number of cutaneous metastases presenting initially or simultaneously to the breast cancer diagnosis. Bansal et al8 defined a simultaneous diagnosis as a skin lesion presenting with other concerns associated with the primary malignancy.
Results
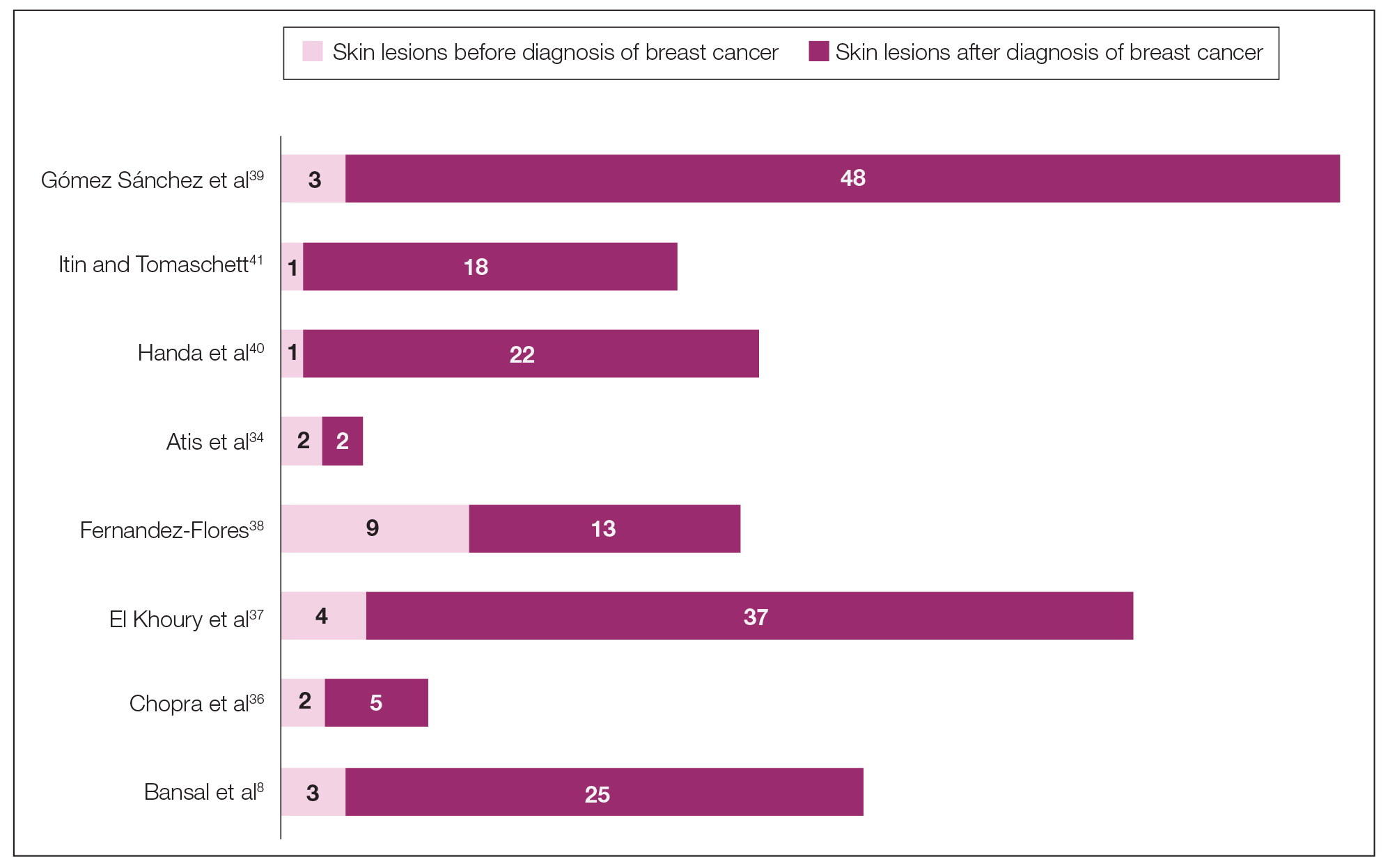
The initial search of MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO yielded a total of 722 articles. Seven other articles found separately while undergoing our initial research were added to this total. Abstracts were manually screened, with 657 articles discarded after failing to meet the predetermined inclusion criteria. After removal of 25 duplicate articles, the full text of the remaining 47 articles were reviewed, leading to the elimination of an additional 11 articles that did not meet the necessary criteria. This resulted in 36 articles (Figure 1), including 27 individual case reports (Table 1) and 9 retrospective reviews (Table 2). Approximately 13.7% of patients in the 9 retrospective reviews presented with a skin lesion before or simultaneous to the diagnosis of breast cancer (Figure 2).
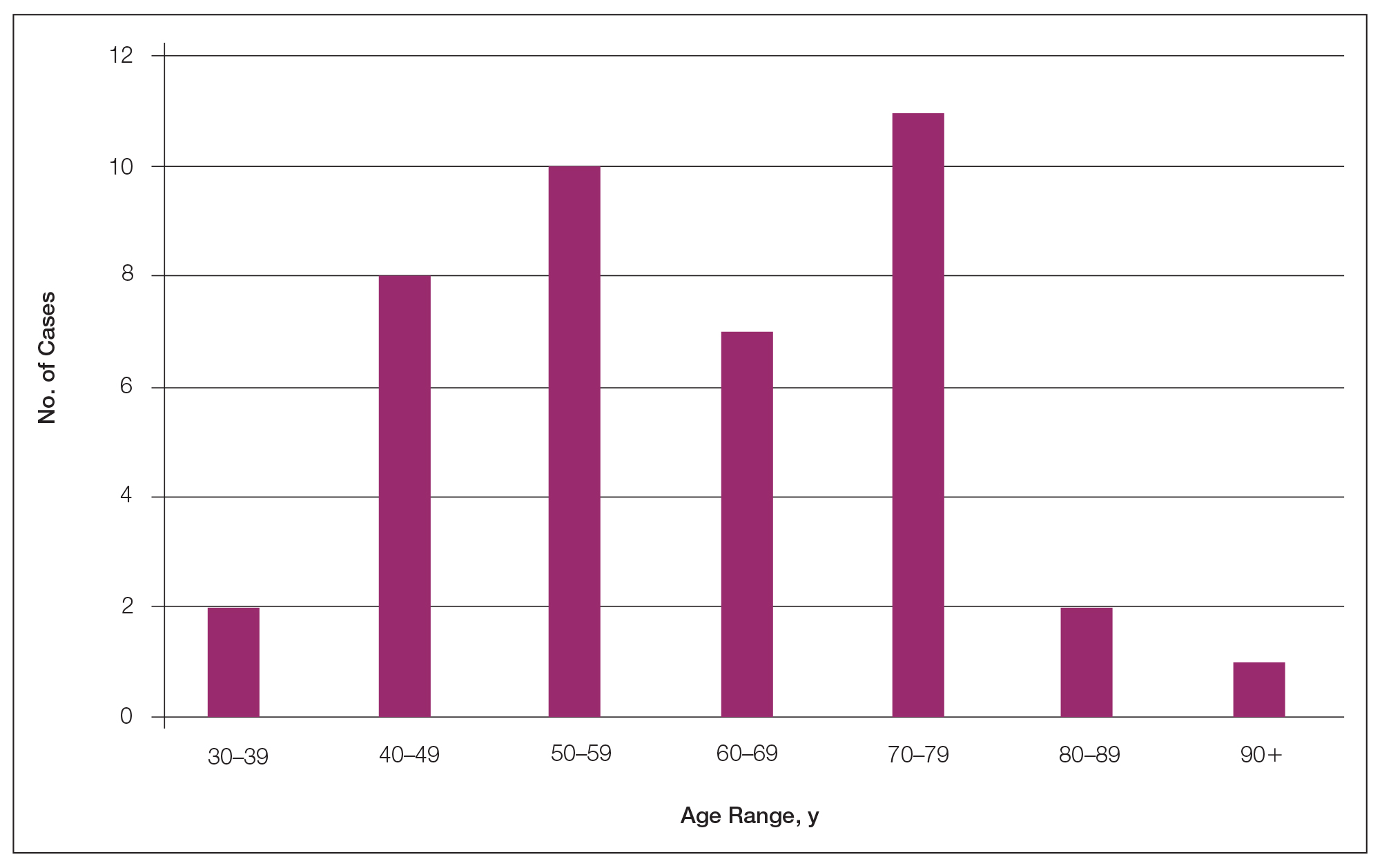
Forty-one percent (17/41) of the patients with cutaneous metastasis as a presenting feature of their breast cancer fell outside the age range for breast cancer screening recommended by the US Preventive Services Task Force,42 with 24% of the patients younger than 50 years and 17% older than 74 years (Figure 3).
Lesion Characteristics
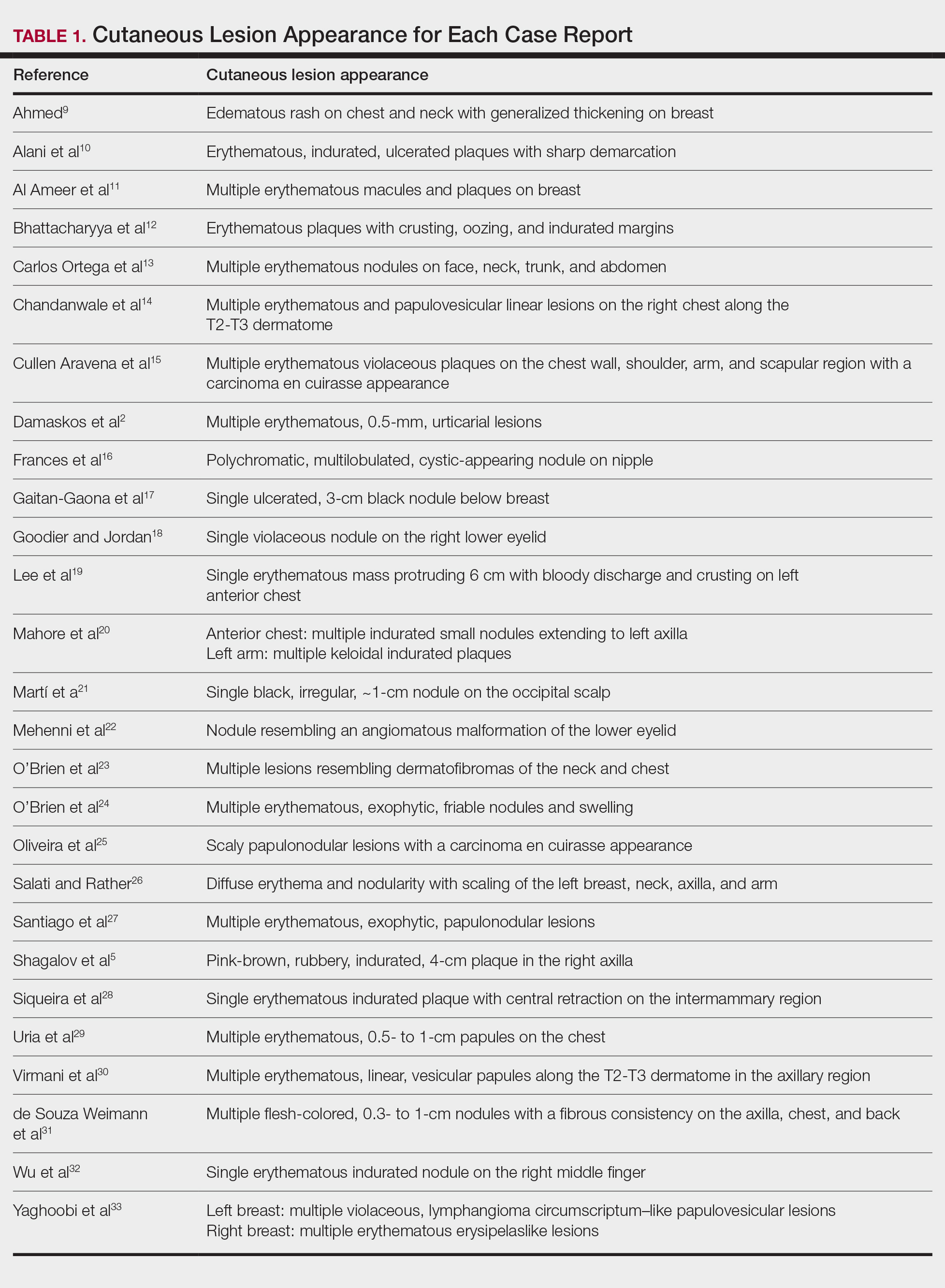
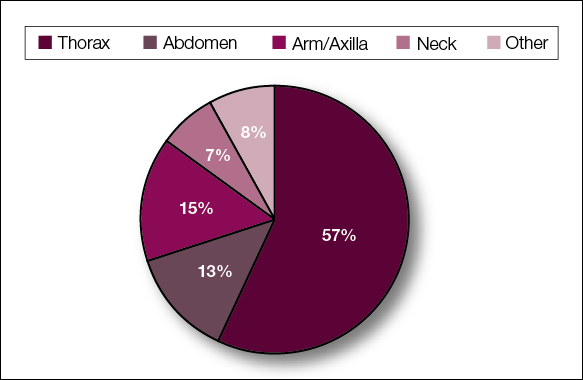
The most common cutaneous lesions were erythematous nodules and plaques, with a few reports of black17,21 or flesh-colored5,20,31 lesions, as well as ulceration.8,17,32 The most common location for skin lesions was on the thorax (chest or breast), accounting for 57% of the cutaneous metastases, with the arms and axillae being second most commonly involved (15%)(Figure 4). Some cases presented with skin lesions extending to multiple regions. In these cases, each location of the lesion was recorded separately when analyzing the data. An additional 5 cases, shown as “Other” in Figure 4, included the eyelids, occiput, and finger. Eight case reports described symptoms associated with the cutaneous lesions, with painful or tender lesions reported in 7 cases5,9,14,17,20,30,32 and pruritus in 2 cases.12,20 Moreover, 6 case reports presented patients denying any systemic or associated symptoms with their skin lesions.2,5,9,16,17,28 Multiple cases were initially treated as other conditions due to misdiagnosis, including herpes zoster14,30 and dermatitis.11,12
Diagnostic Data
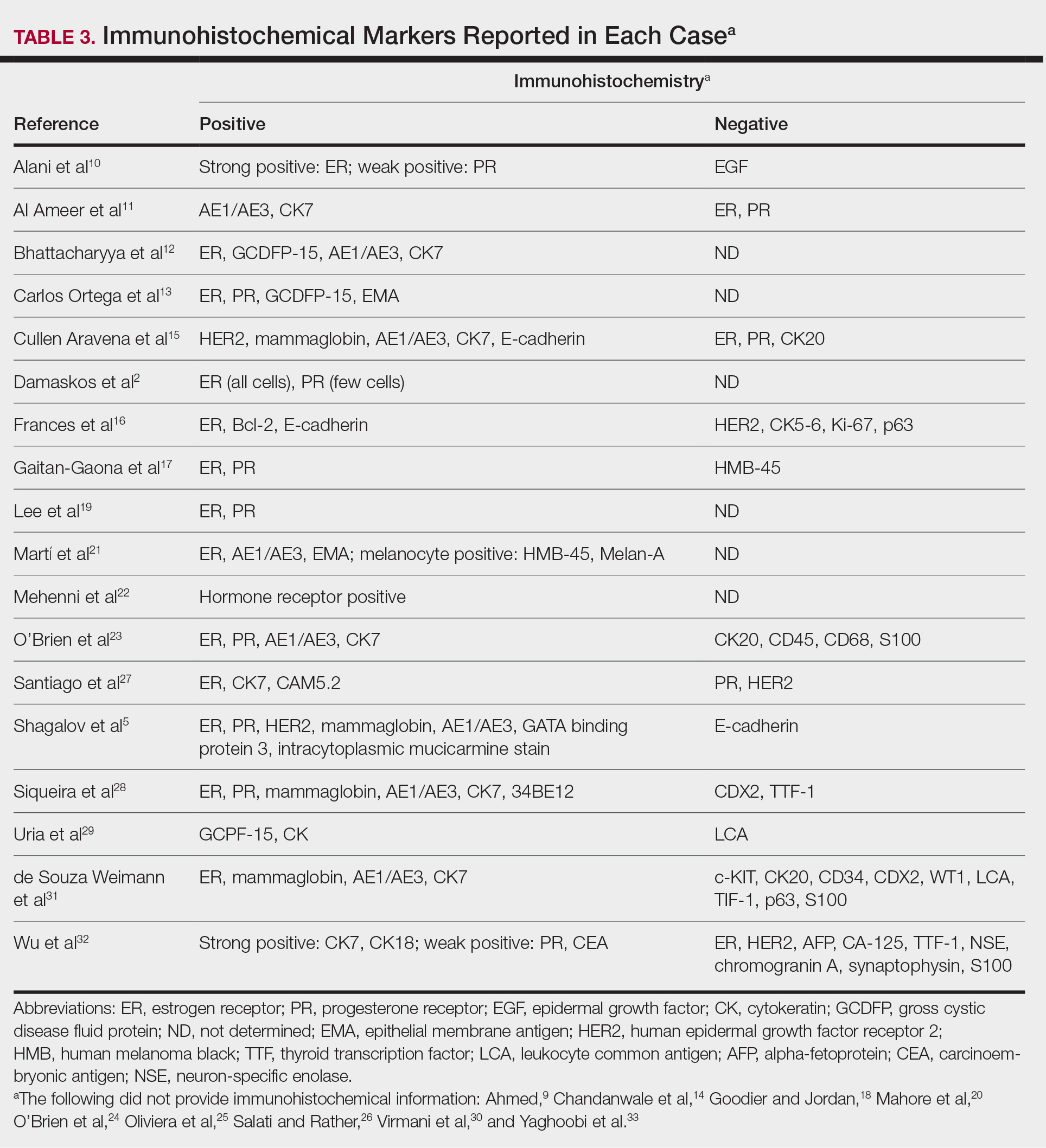
Eighteen cases reported positive immunohistochemistry from cutaneous biopsy (Table 3), given its high specificity in determining the origin of cutaneous metastases, while 8 case reports only performed hematoxylin and eosin staining. One case did not report hematoxylin and eosin or immunohistochemical staining. Table 4 lists the final breast cancer diagnosis for each case.
As per the standard of care, patients were evaluated with mammography or ultrasonography, combined with fine-needle aspiration of a suspected primary tumor, to give a definitive diagnosis of breast cancer. However, 4 cases reported negative mammography and ultrasonography.13,22,28,31 In 3 of these cases, no primary tumor was ever found.13,22,31
Comment
Our systematic review demonstrated that cutaneous lesions may be the first clinical manifestation of an undetected primary malignancy.40 These lesions often occur on the chest but may involve the face, abdomen, or extremities. Although asymptomatic erythematous nodules and plaques are the most common clinical presentations, lesions may be tender or pruritic or may even resemble benign skin conditions, including dermatitis, cellulitis, urticaria, and papulovesicular eruptions, causing them to go unrecognized.
Nevertheless, cutaneous metastasis of a visceral malignancy generally is observed late in the disease course, often following the diagnosis of a primary malignancy.14 Breast cancer is the most common internal malignancy to feature cutaneous spread, with the largest case series revealing a 23.9% rate of cutaneous metastases in females with breast carcinoma.6 Because of its proximity, the chest wall is the most common location for cutaneous lesions of metastatic breast cancer.
Malignant cells from a primary breast tumor may spread to the skin via lymphatic, hematogenous, or contiguous tissue dissemination, as well as iatrogenically through direct implantation during surgical procedures.3 The mechanism of neoplasm spread may likewise influence the clinical appearance of the resulting lesions. The localized lymphedema with a peau d’orange appearance of inflammatory metastatic breast carcinoma or the erythematous plaques of carcinoma erysipeloides are caused by embolized tumor cells obstructing dermal lymphatic vessels.3,11 On the other hand, the indurated erythematous plaques of carcinoma en cuirasse are caused by diffuse cutaneous and subcutaneous infiltration of tumor cells that also may be associated with marked reduction in breast volume.3
A primary breast cancer is classically diagnosed with a combination of clinical breast examination, radiologic imaging (ultrasound, mammogram, breast magnetic resonance imaging, or computed tomography), and fine-needle aspiration or lesional biopsy with histopathology.9 Given that in 20% of metastasized breast cancers the primary tumor may not be identified, a negative breast examination and imaging do not rule out breast cancer, especially if cutaneous biopsy reveals a primary malignancy.43 Histopathology and immunohistochemistry can thereby confirm the presence of metastatic cutaneous lesions and help characterize the breast cancer type involved, with adenocarcinomas being most commonly implicated.28 Although both ductal and lobular adenocarcinomas stain positive for cytokeratin 7, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15, carcinoembryonic antigen, and mammaglobin, only the former shows positivity for e-cadherin markers.3 Conversely, inflammatory carcinoma stains positive for CD31 and podoplanin, telangiectatic carcinoma stains positive for CD31, and mammary Paget disease stains positive for cytokeratin 7 and mucin 1, cell surface associated.3 Apart from cutaneous biopsy, fine-needle aspiration cytology can likewise provide a simple and rapid method of diagnosis with high sensitivity and specificity.14
Conclusion
Although cutaneous metastasis as the presenting sign of a breast malignancy is rare, a high index of suspicion should be exercised when encountering rapid-onset, out-of-place nodules or plaques in female patients, particularly nodules or plaques presenting on the chest.
- Siegel R, Miller K, Jemal A. Cancer statistics, 2020 [published online January 8, 2020]. CA Cancer J Clin. 2020;70:7-30.
- Damaskos C, Dimitroulis D, Pergialiotis V, et al. An unexpected metastasis of breast cancer mimicking wheal rush. G Chir. 2016;37:136-138.
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
- Shagalov D, Xu M, Liebman T, et al. Unilateral indurated plaque in the axilla: a case of metastatic breast carcinoma. Dermatol Online J. 2016;22:13030/qt8vw382nx.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-e34.
- Bansal R, Patel T, Sarin J, et al. Cutaneous and subcutaneous metastases from internal malignancies: an analysis of cases diagnosed by fine needle aspiration. Diagn Cytopathol. 2011;39:882-887.
- Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011:bcr0620114398.
- Alani A, Roberts G, Kerr O. Carcinoma en cuirasse. BMJ Case Rep. 2017;2017:bcr2017222121.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Bhattacharyya A, Gangopadhyay M, Ghosh K, et al. Wolf in sheep’s clothing: a case of carcinoma erysipeloides. Oxf Med Case Rep. 2016;2016:97-100.
- Carlos Ortega B, Alfaro Mejia A, Gómez-Campos G, et al. Metástasis de carcinoma de mama que simula prototecosis. Dermatol Rev Mex. 2012;56:55-61.
- Chandanwale SS, Gore CR, Buch AC, et al. Zosteriform cutaneous metastasis: a primary manifestation of carcinoma breast, rare case report. Indian J Pathol Microbiol. 2011;54:863-864.
- Cullen Aravena R, Cullen Aravena D, Velasco MJ, et al. Carcinoma hemorrhagiectoides: case report of an uncommon presentation of cutaneous metastatic breast carcinoma. Dermatol Online J. 2017;23:13030/qt3hm3z850.
- Frances L, Cuesta L, Leiva-Salinas M, et al. Secondary mucinous carcinoma of the skin. Dermatol Online J. 2014;20:22361.
- Gaitan-Gaona F, Said MC, Valdes-Rodriguez R. Cutaneous metastatic pigmented breast carcinoma. Dermatol Online J. 2016;22:13030/qt0sv018ck.
- Goodier MA, Jordan JR. Metastatic breast cancer to the lower eyelid. Laryngoscope. 2010;120(suppl 4):S129.
- Lee H-J, Kim J-M, Kim G-W, et al. A unique cutaneous presentation of breast cancer: a red apple stuck in the breast. Ann Dermatol. 2016;28:499-501.
- Mahore SD, Bothale KA, Patrikar AD, et al. Carcinoma en cuirasse : a rare presentation of breast cancer. Indian J Pathol Microbiol. 2010;53:351-358.
- Martí N, Molina I, Monteagudo C, et al. Cutaneous metastasis of breast carcinoma mimicking malignant melanoma in scalp. Dermatol Online J. 2008;14:12.
- Mehenni NN, Gamaz-Bensaou M, Bouzid K. Metastatic breast carcinoma to the gallbladder and the lower eyelid with no malignant lesion in the breast: an unusual case report with a short review of the literature [abstract]. Ann Oncol. 2013;24(suppl 3):iii49.
- O’Brien OA, AboGhaly E, Heffron C. An unusual presentation of a common malignancy [abstract]. J Pathol. 2013;231:S33.
- O’Brien R, Porto DA, Friedman BJ, et al. Elderly female with swelling of the right breast. Ann Emerg Med. 2016;67:e25-e26.
- Oliveira GM de, Zachetti DBC, Barros HR, et al. Breast carcinoma en Cuirasse—case report. An Bras Dermatol. 2013;88:608-610.
- Salati SA, Rather AA. Carcinoma en cuirasse. J Pak Assoc Derma. 2013;23:452-454.
- Santiago F, Saleiro S, Brites MM, et al. A remarkable case of cutaneous metastatic breast carcinoma. Dermatol Online J. 2009;15:10.
- Siqueira VR, Frota AS, Maia IL, et al. Cutaneous involvement as the initial presentation of metastatic breast adenocarcinoma - case report. An Bras Dermatol. 2014;89:960-963.
- Uria M, Chirino C, Rivas D. Inusual clinical presentation of cutaneous metastasis from breast carcinoma. A case report. Rev Argent Dermatol. 2009;90:230-236.
- Virmani NC, Sharma YK, Panicker NK, et al. Zosteriform skin metastases: clue to an undiagnosed breast cancer. Indian J Dermatol. 2011;56:726-727.
- de Souza Weimann ET, Botero EB, Mendes C, et al. Cutaneous metastasis as the first manifestation of occult malignant breast neoplasia. An Bras Dermatol. 2016;91(5 suppl 1):105-107.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:e409-e410.
- Yaghoobi R, Talaizade A, Lal K, et al. Inflammatory breast carcinoma presenting with two different patterns of cutaneous metastases: carcinoma telangiectaticum and carcinoma erysipeloides. J Clin Aesthet Dermatol. 2015;8:47-51.
- Atis G, Demirci GT, Atunay IK, et al. The clinical characteristics and the frequency of metastatic cutaneous tumors among primary skin tumors. Turkderm. 2013;47:166-169.
- Benmously R, Souissi A, Badri T, et al. Cutaneous metastases from internal cancers. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:167-170.
- Chopra R, Chhabra S, Samra SG, et al. Cutaneous metastases of internal malignancies: a clinicopathologic study. Indian J Dermatol Venereol Leprol. 2010;76:125-131.
- El Khoury J, Khalifeh I, Kibbi AG, et al. Cutaneous metastasis: clinicopathological study of 72 patients from a tertiary care center in Lebanon. Int J Dermatol. 2014;53:147-158.
- Fernandez-Flores A. Cutaneous metastases: a study of 78 biopsies from 69 patients. Am J Dermatopathol. 2010;32:222-239.
- Gómez Sánchez ME, Martinez Martinez ML, Martín De Hijas MC, et al. Metástasis cutáneas de tumores sólidos. Estudio descriptivo retrospectivo. Piel. 2014;29:207-212
- Handa U, Kundu R, Dimri K. Cutaneous metastasis: a study of 138 cases diagnosed by fine-needle aspiration cytology. Acta Cytol. 2017;61:47-54.
- Itin P, Tomaschett S. Cutaneous metastases from malignancies which do not originate from the skin. An epidemiological study. Article in German. Internist (Berl). 2009;50:179-186.
- Siu AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Torres HA, Bodey GP, Tarrand JJ, et al. Protothecosis in patients with cancer: case series and literature review. Clin Microbiol Infect. 2003;9:786-792.
Breast cancer is the second most common malignancy in women (after primary skin cancer) and is the second leading cause of cancer-related death in this population. In 2020, the American Cancer Society reported an estimated 276,480 new breast cancer diagnoses and 42,170 breast cancer–related deaths.1 Despite the fact that routine screening with mammography and sonography is standard, the incidence of advanced breast cancer at the time of diagnosis has remained stable over time, suggesting that life-threatening breast cancers are not being caught at an earlier stage. The number of breast cancers with distant metastases at the time of diagnosis also has not decreased.2 Therefore, although screening tests are valuable, they are imperfect and not without limitations.
Cutaneous metastasis is defined as the spread of malignant cells from an internal neoplasm to the skin, which can occur either by contiguous invasion or by distant metastasis through hematogenous or lymphatic routes.3 The diagnosis of cutaneous metastasis requires a high index of suspicion on the part of the clinician.4 Of the various internal malignancies in women, breast cancer most frequently results in metastasis to the skin,5 with up to 24% of patients with metastatic breast cancer developing cutaneous lesions.6
In recent years, there have been multiple reports of skin lesions prompting the diagnosis of a previously unknown breast cancer. In a study by Lookingbill et al,6 6.3% of patients with breast cancer presented with cutaneous involvement at the time of diagnosis, with 3.5% having skin symptoms as the presenting sign. Although there have been studies analyzing cutaneous metastasis from various internal malignancies, none thus far have focused on cutaneous metastasis as a presenting sign of breast cancer. This systematic review aimed to highlight the diverse clinical presentations of cutaneous metastatic breast cancer and their clinical implications.
Methods
Study Selection
This study utilized the PRISMA guidelines for systematic reviews.7 A review of the literature was conducted using the following databases: MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO.
Search Strategy and Analysis
We completed our search of each of the databases on December 16, 2017, using the phrases cutaneous metastasis and breast cancer to find relevant case reports and retrospective studies. Three authors (C.J., S.R., and M.A.) manually reviewed the resulting abstracts. If an abstract did not include enough information to determine inclusion, the full-text version was reviewed by 2 of the authors (C.J. and S.R.). Two of the authors (C.J. and M.A.) also assessed each source for relevancy and included the articles deemed eligible (Figure 1).
Inclusion criteria were the following: case reports and retrospective studies published in the prior 10 years (January 1, 2007, to December 16, 2017) with human female patients who developed metastatic cutaneous lesions due to a previously unknown primary breast malignancy. Studies published in other languages were included; these articles were translated into English using a human translator or computer translation program (Google Translate). Exclusion criteria were the following: male patients, patients with a known diagnosis of primary breast malignancy prior to the appearance of a metastatic cutaneous lesion, articles focusing on the treatment of breast cancer, and articles without enough details to draw meaningful conclusions.
For a retrospective review to be included, it must have specified the number of breast cancer cases and the number of cutaneous metastases presenting initially or simultaneously to the breast cancer diagnosis. Bansal et al8 defined a simultaneous diagnosis as a skin lesion presenting with other concerns associated with the primary malignancy.
Results
The initial search of MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO yielded a total of 722 articles. Seven other articles found separately while undergoing our initial research were added to this total. Abstracts were manually screened, with 657 articles discarded after failing to meet the predetermined inclusion criteria. After removal of 25 duplicate articles, the full text of the remaining 47 articles were reviewed, leading to the elimination of an additional 11 articles that did not meet the necessary criteria. This resulted in 36 articles (Figure 1), including 27 individual case reports (Table 1) and 9 retrospective reviews (Table 2). Approximately 13.7% of patients in the 9 retrospective reviews presented with a skin lesion before or simultaneous to the diagnosis of breast cancer (Figure 2).
Forty-one percent (17/41) of the patients with cutaneous metastasis as a presenting feature of their breast cancer fell outside the age range for breast cancer screening recommended by the US Preventive Services Task Force,42 with 24% of the patients younger than 50 years and 17% older than 74 years (Figure 3).
Lesion Characteristics
The most common cutaneous lesions were erythematous nodules and plaques, with a few reports of black17,21 or flesh-colored5,20,31 lesions, as well as ulceration.8,17,32 The most common location for skin lesions was on the thorax (chest or breast), accounting for 57% of the cutaneous metastases, with the arms and axillae being second most commonly involved (15%)(Figure 4). Some cases presented with skin lesions extending to multiple regions. In these cases, each location of the lesion was recorded separately when analyzing the data. An additional 5 cases, shown as “Other” in Figure 4, included the eyelids, occiput, and finger. Eight case reports described symptoms associated with the cutaneous lesions, with painful or tender lesions reported in 7 cases5,9,14,17,20,30,32 and pruritus in 2 cases.12,20 Moreover, 6 case reports presented patients denying any systemic or associated symptoms with their skin lesions.2,5,9,16,17,28 Multiple cases were initially treated as other conditions due to misdiagnosis, including herpes zoster14,30 and dermatitis.11,12
Diagnostic Data
Eighteen cases reported positive immunohistochemistry from cutaneous biopsy (Table 3), given its high specificity in determining the origin of cutaneous metastases, while 8 case reports only performed hematoxylin and eosin staining. One case did not report hematoxylin and eosin or immunohistochemical staining. Table 4 lists the final breast cancer diagnosis for each case.
As per the standard of care, patients were evaluated with mammography or ultrasonography, combined with fine-needle aspiration of a suspected primary tumor, to give a definitive diagnosis of breast cancer. However, 4 cases reported negative mammography and ultrasonography.13,22,28,31 In 3 of these cases, no primary tumor was ever found.13,22,31
Comment
Our systematic review demonstrated that cutaneous lesions may be the first clinical manifestation of an undetected primary malignancy.40 These lesions often occur on the chest but may involve the face, abdomen, or extremities. Although asymptomatic erythematous nodules and plaques are the most common clinical presentations, lesions may be tender or pruritic or may even resemble benign skin conditions, including dermatitis, cellulitis, urticaria, and papulovesicular eruptions, causing them to go unrecognized.
Nevertheless, cutaneous metastasis of a visceral malignancy generally is observed late in the disease course, often following the diagnosis of a primary malignancy.14 Breast cancer is the most common internal malignancy to feature cutaneous spread, with the largest case series revealing a 23.9% rate of cutaneous metastases in females with breast carcinoma.6 Because of its proximity, the chest wall is the most common location for cutaneous lesions of metastatic breast cancer.
Malignant cells from a primary breast tumor may spread to the skin via lymphatic, hematogenous, or contiguous tissue dissemination, as well as iatrogenically through direct implantation during surgical procedures.3 The mechanism of neoplasm spread may likewise influence the clinical appearance of the resulting lesions. The localized lymphedema with a peau d’orange appearance of inflammatory metastatic breast carcinoma or the erythematous plaques of carcinoma erysipeloides are caused by embolized tumor cells obstructing dermal lymphatic vessels.3,11 On the other hand, the indurated erythematous plaques of carcinoma en cuirasse are caused by diffuse cutaneous and subcutaneous infiltration of tumor cells that also may be associated with marked reduction in breast volume.3
A primary breast cancer is classically diagnosed with a combination of clinical breast examination, radiologic imaging (ultrasound, mammogram, breast magnetic resonance imaging, or computed tomography), and fine-needle aspiration or lesional biopsy with histopathology.9 Given that in 20% of metastasized breast cancers the primary tumor may not be identified, a negative breast examination and imaging do not rule out breast cancer, especially if cutaneous biopsy reveals a primary malignancy.43 Histopathology and immunohistochemistry can thereby confirm the presence of metastatic cutaneous lesions and help characterize the breast cancer type involved, with adenocarcinomas being most commonly implicated.28 Although both ductal and lobular adenocarcinomas stain positive for cytokeratin 7, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15, carcinoembryonic antigen, and mammaglobin, only the former shows positivity for e-cadherin markers.3 Conversely, inflammatory carcinoma stains positive for CD31 and podoplanin, telangiectatic carcinoma stains positive for CD31, and mammary Paget disease stains positive for cytokeratin 7 and mucin 1, cell surface associated.3 Apart from cutaneous biopsy, fine-needle aspiration cytology can likewise provide a simple and rapid method of diagnosis with high sensitivity and specificity.14
Conclusion
Although cutaneous metastasis as the presenting sign of a breast malignancy is rare, a high index of suspicion should be exercised when encountering rapid-onset, out-of-place nodules or plaques in female patients, particularly nodules or plaques presenting on the chest.
Breast cancer is the second most common malignancy in women (after primary skin cancer) and is the second leading cause of cancer-related death in this population. In 2020, the American Cancer Society reported an estimated 276,480 new breast cancer diagnoses and 42,170 breast cancer–related deaths.1 Despite the fact that routine screening with mammography and sonography is standard, the incidence of advanced breast cancer at the time of diagnosis has remained stable over time, suggesting that life-threatening breast cancers are not being caught at an earlier stage. The number of breast cancers with distant metastases at the time of diagnosis also has not decreased.2 Therefore, although screening tests are valuable, they are imperfect and not without limitations.
Cutaneous metastasis is defined as the spread of malignant cells from an internal neoplasm to the skin, which can occur either by contiguous invasion or by distant metastasis through hematogenous or lymphatic routes.3 The diagnosis of cutaneous metastasis requires a high index of suspicion on the part of the clinician.4 Of the various internal malignancies in women, breast cancer most frequently results in metastasis to the skin,5 with up to 24% of patients with metastatic breast cancer developing cutaneous lesions.6
In recent years, there have been multiple reports of skin lesions prompting the diagnosis of a previously unknown breast cancer. In a study by Lookingbill et al,6 6.3% of patients with breast cancer presented with cutaneous involvement at the time of diagnosis, with 3.5% having skin symptoms as the presenting sign. Although there have been studies analyzing cutaneous metastasis from various internal malignancies, none thus far have focused on cutaneous metastasis as a presenting sign of breast cancer. This systematic review aimed to highlight the diverse clinical presentations of cutaneous metastatic breast cancer and their clinical implications.
Methods
Study Selection
This study utilized the PRISMA guidelines for systematic reviews.7 A review of the literature was conducted using the following databases: MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO.
Search Strategy and Analysis
We completed our search of each of the databases on December 16, 2017, using the phrases cutaneous metastasis and breast cancer to find relevant case reports and retrospective studies. Three authors (C.J., S.R., and M.A.) manually reviewed the resulting abstracts. If an abstract did not include enough information to determine inclusion, the full-text version was reviewed by 2 of the authors (C.J. and S.R.). Two of the authors (C.J. and M.A.) also assessed each source for relevancy and included the articles deemed eligible (Figure 1).
Inclusion criteria were the following: case reports and retrospective studies published in the prior 10 years (January 1, 2007, to December 16, 2017) with human female patients who developed metastatic cutaneous lesions due to a previously unknown primary breast malignancy. Studies published in other languages were included; these articles were translated into English using a human translator or computer translation program (Google Translate). Exclusion criteria were the following: male patients, patients with a known diagnosis of primary breast malignancy prior to the appearance of a metastatic cutaneous lesion, articles focusing on the treatment of breast cancer, and articles without enough details to draw meaningful conclusions.
For a retrospective review to be included, it must have specified the number of breast cancer cases and the number of cutaneous metastases presenting initially or simultaneously to the breast cancer diagnosis. Bansal et al8 defined a simultaneous diagnosis as a skin lesion presenting with other concerns associated with the primary malignancy.
Results
The initial search of MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO yielded a total of 722 articles. Seven other articles found separately while undergoing our initial research were added to this total. Abstracts were manually screened, with 657 articles discarded after failing to meet the predetermined inclusion criteria. After removal of 25 duplicate articles, the full text of the remaining 47 articles were reviewed, leading to the elimination of an additional 11 articles that did not meet the necessary criteria. This resulted in 36 articles (Figure 1), including 27 individual case reports (Table 1) and 9 retrospective reviews (Table 2). Approximately 13.7% of patients in the 9 retrospective reviews presented with a skin lesion before or simultaneous to the diagnosis of breast cancer (Figure 2).
Forty-one percent (17/41) of the patients with cutaneous metastasis as a presenting feature of their breast cancer fell outside the age range for breast cancer screening recommended by the US Preventive Services Task Force,42 with 24% of the patients younger than 50 years and 17% older than 74 years (Figure 3).
Lesion Characteristics
The most common cutaneous lesions were erythematous nodules and plaques, with a few reports of black17,21 or flesh-colored5,20,31 lesions, as well as ulceration.8,17,32 The most common location for skin lesions was on the thorax (chest or breast), accounting for 57% of the cutaneous metastases, with the arms and axillae being second most commonly involved (15%)(Figure 4). Some cases presented with skin lesions extending to multiple regions. In these cases, each location of the lesion was recorded separately when analyzing the data. An additional 5 cases, shown as “Other” in Figure 4, included the eyelids, occiput, and finger. Eight case reports described symptoms associated with the cutaneous lesions, with painful or tender lesions reported in 7 cases5,9,14,17,20,30,32 and pruritus in 2 cases.12,20 Moreover, 6 case reports presented patients denying any systemic or associated symptoms with their skin lesions.2,5,9,16,17,28 Multiple cases were initially treated as other conditions due to misdiagnosis, including herpes zoster14,30 and dermatitis.11,12
Diagnostic Data
Eighteen cases reported positive immunohistochemistry from cutaneous biopsy (Table 3), given its high specificity in determining the origin of cutaneous metastases, while 8 case reports only performed hematoxylin and eosin staining. One case did not report hematoxylin and eosin or immunohistochemical staining. Table 4 lists the final breast cancer diagnosis for each case.
As per the standard of care, patients were evaluated with mammography or ultrasonography, combined with fine-needle aspiration of a suspected primary tumor, to give a definitive diagnosis of breast cancer. However, 4 cases reported negative mammography and ultrasonography.13,22,28,31 In 3 of these cases, no primary tumor was ever found.13,22,31
Comment
Our systematic review demonstrated that cutaneous lesions may be the first clinical manifestation of an undetected primary malignancy.40 These lesions often occur on the chest but may involve the face, abdomen, or extremities. Although asymptomatic erythematous nodules and plaques are the most common clinical presentations, lesions may be tender or pruritic or may even resemble benign skin conditions, including dermatitis, cellulitis, urticaria, and papulovesicular eruptions, causing them to go unrecognized.
Nevertheless, cutaneous metastasis of a visceral malignancy generally is observed late in the disease course, often following the diagnosis of a primary malignancy.14 Breast cancer is the most common internal malignancy to feature cutaneous spread, with the largest case series revealing a 23.9% rate of cutaneous metastases in females with breast carcinoma.6 Because of its proximity, the chest wall is the most common location for cutaneous lesions of metastatic breast cancer.
Malignant cells from a primary breast tumor may spread to the skin via lymphatic, hematogenous, or contiguous tissue dissemination, as well as iatrogenically through direct implantation during surgical procedures.3 The mechanism of neoplasm spread may likewise influence the clinical appearance of the resulting lesions. The localized lymphedema with a peau d’orange appearance of inflammatory metastatic breast carcinoma or the erythematous plaques of carcinoma erysipeloides are caused by embolized tumor cells obstructing dermal lymphatic vessels.3,11 On the other hand, the indurated erythematous plaques of carcinoma en cuirasse are caused by diffuse cutaneous and subcutaneous infiltration of tumor cells that also may be associated with marked reduction in breast volume.3
A primary breast cancer is classically diagnosed with a combination of clinical breast examination, radiologic imaging (ultrasound, mammogram, breast magnetic resonance imaging, or computed tomography), and fine-needle aspiration or lesional biopsy with histopathology.9 Given that in 20% of metastasized breast cancers the primary tumor may not be identified, a negative breast examination and imaging do not rule out breast cancer, especially if cutaneous biopsy reveals a primary malignancy.43 Histopathology and immunohistochemistry can thereby confirm the presence of metastatic cutaneous lesions and help characterize the breast cancer type involved, with adenocarcinomas being most commonly implicated.28 Although both ductal and lobular adenocarcinomas stain positive for cytokeratin 7, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15, carcinoembryonic antigen, and mammaglobin, only the former shows positivity for e-cadherin markers.3 Conversely, inflammatory carcinoma stains positive for CD31 and podoplanin, telangiectatic carcinoma stains positive for CD31, and mammary Paget disease stains positive for cytokeratin 7 and mucin 1, cell surface associated.3 Apart from cutaneous biopsy, fine-needle aspiration cytology can likewise provide a simple and rapid method of diagnosis with high sensitivity and specificity.14
Conclusion
Although cutaneous metastasis as the presenting sign of a breast malignancy is rare, a high index of suspicion should be exercised when encountering rapid-onset, out-of-place nodules or plaques in female patients, particularly nodules or plaques presenting on the chest.
- Siegel R, Miller K, Jemal A. Cancer statistics, 2020 [published online January 8, 2020]. CA Cancer J Clin. 2020;70:7-30.
- Damaskos C, Dimitroulis D, Pergialiotis V, et al. An unexpected metastasis of breast cancer mimicking wheal rush. G Chir. 2016;37:136-138.
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
- Shagalov D, Xu M, Liebman T, et al. Unilateral indurated plaque in the axilla: a case of metastatic breast carcinoma. Dermatol Online J. 2016;22:13030/qt8vw382nx.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-e34.
- Bansal R, Patel T, Sarin J, et al. Cutaneous and subcutaneous metastases from internal malignancies: an analysis of cases diagnosed by fine needle aspiration. Diagn Cytopathol. 2011;39:882-887.
- Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011:bcr0620114398.
- Alani A, Roberts G, Kerr O. Carcinoma en cuirasse. BMJ Case Rep. 2017;2017:bcr2017222121.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Bhattacharyya A, Gangopadhyay M, Ghosh K, et al. Wolf in sheep’s clothing: a case of carcinoma erysipeloides. Oxf Med Case Rep. 2016;2016:97-100.
- Carlos Ortega B, Alfaro Mejia A, Gómez-Campos G, et al. Metástasis de carcinoma de mama que simula prototecosis. Dermatol Rev Mex. 2012;56:55-61.
- Chandanwale SS, Gore CR, Buch AC, et al. Zosteriform cutaneous metastasis: a primary manifestation of carcinoma breast, rare case report. Indian J Pathol Microbiol. 2011;54:863-864.
- Cullen Aravena R, Cullen Aravena D, Velasco MJ, et al. Carcinoma hemorrhagiectoides: case report of an uncommon presentation of cutaneous metastatic breast carcinoma. Dermatol Online J. 2017;23:13030/qt3hm3z850.
- Frances L, Cuesta L, Leiva-Salinas M, et al. Secondary mucinous carcinoma of the skin. Dermatol Online J. 2014;20:22361.
- Gaitan-Gaona F, Said MC, Valdes-Rodriguez R. Cutaneous metastatic pigmented breast carcinoma. Dermatol Online J. 2016;22:13030/qt0sv018ck.
- Goodier MA, Jordan JR. Metastatic breast cancer to the lower eyelid. Laryngoscope. 2010;120(suppl 4):S129.
- Lee H-J, Kim J-M, Kim G-W, et al. A unique cutaneous presentation of breast cancer: a red apple stuck in the breast. Ann Dermatol. 2016;28:499-501.
- Mahore SD, Bothale KA, Patrikar AD, et al. Carcinoma en cuirasse : a rare presentation of breast cancer. Indian J Pathol Microbiol. 2010;53:351-358.
- Martí N, Molina I, Monteagudo C, et al. Cutaneous metastasis of breast carcinoma mimicking malignant melanoma in scalp. Dermatol Online J. 2008;14:12.
- Mehenni NN, Gamaz-Bensaou M, Bouzid K. Metastatic breast carcinoma to the gallbladder and the lower eyelid with no malignant lesion in the breast: an unusual case report with a short review of the literature [abstract]. Ann Oncol. 2013;24(suppl 3):iii49.
- O’Brien OA, AboGhaly E, Heffron C. An unusual presentation of a common malignancy [abstract]. J Pathol. 2013;231:S33.
- O’Brien R, Porto DA, Friedman BJ, et al. Elderly female with swelling of the right breast. Ann Emerg Med. 2016;67:e25-e26.
- Oliveira GM de, Zachetti DBC, Barros HR, et al. Breast carcinoma en Cuirasse—case report. An Bras Dermatol. 2013;88:608-610.
- Salati SA, Rather AA. Carcinoma en cuirasse. J Pak Assoc Derma. 2013;23:452-454.
- Santiago F, Saleiro S, Brites MM, et al. A remarkable case of cutaneous metastatic breast carcinoma. Dermatol Online J. 2009;15:10.
- Siqueira VR, Frota AS, Maia IL, et al. Cutaneous involvement as the initial presentation of metastatic breast adenocarcinoma - case report. An Bras Dermatol. 2014;89:960-963.
- Uria M, Chirino C, Rivas D. Inusual clinical presentation of cutaneous metastasis from breast carcinoma. A case report. Rev Argent Dermatol. 2009;90:230-236.
- Virmani NC, Sharma YK, Panicker NK, et al. Zosteriform skin metastases: clue to an undiagnosed breast cancer. Indian J Dermatol. 2011;56:726-727.
- de Souza Weimann ET, Botero EB, Mendes C, et al. Cutaneous metastasis as the first manifestation of occult malignant breast neoplasia. An Bras Dermatol. 2016;91(5 suppl 1):105-107.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:e409-e410.
- Yaghoobi R, Talaizade A, Lal K, et al. Inflammatory breast carcinoma presenting with two different patterns of cutaneous metastases: carcinoma telangiectaticum and carcinoma erysipeloides. J Clin Aesthet Dermatol. 2015;8:47-51.
- Atis G, Demirci GT, Atunay IK, et al. The clinical characteristics and the frequency of metastatic cutaneous tumors among primary skin tumors. Turkderm. 2013;47:166-169.
- Benmously R, Souissi A, Badri T, et al. Cutaneous metastases from internal cancers. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:167-170.
- Chopra R, Chhabra S, Samra SG, et al. Cutaneous metastases of internal malignancies: a clinicopathologic study. Indian J Dermatol Venereol Leprol. 2010;76:125-131.
- El Khoury J, Khalifeh I, Kibbi AG, et al. Cutaneous metastasis: clinicopathological study of 72 patients from a tertiary care center in Lebanon. Int J Dermatol. 2014;53:147-158.
- Fernandez-Flores A. Cutaneous metastases: a study of 78 biopsies from 69 patients. Am J Dermatopathol. 2010;32:222-239.
- Gómez Sánchez ME, Martinez Martinez ML, Martín De Hijas MC, et al. Metástasis cutáneas de tumores sólidos. Estudio descriptivo retrospectivo. Piel. 2014;29:207-212
- Handa U, Kundu R, Dimri K. Cutaneous metastasis: a study of 138 cases diagnosed by fine-needle aspiration cytology. Acta Cytol. 2017;61:47-54.
- Itin P, Tomaschett S. Cutaneous metastases from malignancies which do not originate from the skin. An epidemiological study. Article in German. Internist (Berl). 2009;50:179-186.
- Siu AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Torres HA, Bodey GP, Tarrand JJ, et al. Protothecosis in patients with cancer: case series and literature review. Clin Microbiol Infect. 2003;9:786-792.
- Siegel R, Miller K, Jemal A. Cancer statistics, 2020 [published online January 8, 2020]. CA Cancer J Clin. 2020;70:7-30.
- Damaskos C, Dimitroulis D, Pergialiotis V, et al. An unexpected metastasis of breast cancer mimicking wheal rush. G Chir. 2016;37:136-138.
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
- Shagalov D, Xu M, Liebman T, et al. Unilateral indurated plaque in the axilla: a case of metastatic breast carcinoma. Dermatol Online J. 2016;22:13030/qt8vw382nx.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-e34.
- Bansal R, Patel T, Sarin J, et al. Cutaneous and subcutaneous metastases from internal malignancies: an analysis of cases diagnosed by fine needle aspiration. Diagn Cytopathol. 2011;39:882-887.
- Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011:bcr0620114398.
- Alani A, Roberts G, Kerr O. Carcinoma en cuirasse. BMJ Case Rep. 2017;2017:bcr2017222121.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Bhattacharyya A, Gangopadhyay M, Ghosh K, et al. Wolf in sheep’s clothing: a case of carcinoma erysipeloides. Oxf Med Case Rep. 2016;2016:97-100.
- Carlos Ortega B, Alfaro Mejia A, Gómez-Campos G, et al. Metástasis de carcinoma de mama que simula prototecosis. Dermatol Rev Mex. 2012;56:55-61.
- Chandanwale SS, Gore CR, Buch AC, et al. Zosteriform cutaneous metastasis: a primary manifestation of carcinoma breast, rare case report. Indian J Pathol Microbiol. 2011;54:863-864.
- Cullen Aravena R, Cullen Aravena D, Velasco MJ, et al. Carcinoma hemorrhagiectoides: case report of an uncommon presentation of cutaneous metastatic breast carcinoma. Dermatol Online J. 2017;23:13030/qt3hm3z850.
- Frances L, Cuesta L, Leiva-Salinas M, et al. Secondary mucinous carcinoma of the skin. Dermatol Online J. 2014;20:22361.
- Gaitan-Gaona F, Said MC, Valdes-Rodriguez R. Cutaneous metastatic pigmented breast carcinoma. Dermatol Online J. 2016;22:13030/qt0sv018ck.
- Goodier MA, Jordan JR. Metastatic breast cancer to the lower eyelid. Laryngoscope. 2010;120(suppl 4):S129.
- Lee H-J, Kim J-M, Kim G-W, et al. A unique cutaneous presentation of breast cancer: a red apple stuck in the breast. Ann Dermatol. 2016;28:499-501.
- Mahore SD, Bothale KA, Patrikar AD, et al. Carcinoma en cuirasse : a rare presentation of breast cancer. Indian J Pathol Microbiol. 2010;53:351-358.
- Martí N, Molina I, Monteagudo C, et al. Cutaneous metastasis of breast carcinoma mimicking malignant melanoma in scalp. Dermatol Online J. 2008;14:12.
- Mehenni NN, Gamaz-Bensaou M, Bouzid K. Metastatic breast carcinoma to the gallbladder and the lower eyelid with no malignant lesion in the breast: an unusual case report with a short review of the literature [abstract]. Ann Oncol. 2013;24(suppl 3):iii49.
- O’Brien OA, AboGhaly E, Heffron C. An unusual presentation of a common malignancy [abstract]. J Pathol. 2013;231:S33.
- O’Brien R, Porto DA, Friedman BJ, et al. Elderly female with swelling of the right breast. Ann Emerg Med. 2016;67:e25-e26.
- Oliveira GM de, Zachetti DBC, Barros HR, et al. Breast carcinoma en Cuirasse—case report. An Bras Dermatol. 2013;88:608-610.
- Salati SA, Rather AA. Carcinoma en cuirasse. J Pak Assoc Derma. 2013;23:452-454.
- Santiago F, Saleiro S, Brites MM, et al. A remarkable case of cutaneous metastatic breast carcinoma. Dermatol Online J. 2009;15:10.
- Siqueira VR, Frota AS, Maia IL, et al. Cutaneous involvement as the initial presentation of metastatic breast adenocarcinoma - case report. An Bras Dermatol. 2014;89:960-963.
- Uria M, Chirino C, Rivas D. Inusual clinical presentation of cutaneous metastasis from breast carcinoma. A case report. Rev Argent Dermatol. 2009;90:230-236.
- Virmani NC, Sharma YK, Panicker NK, et al. Zosteriform skin metastases: clue to an undiagnosed breast cancer. Indian J Dermatol. 2011;56:726-727.
- de Souza Weimann ET, Botero EB, Mendes C, et al. Cutaneous metastasis as the first manifestation of occult malignant breast neoplasia. An Bras Dermatol. 2016;91(5 suppl 1):105-107.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:e409-e410.
- Yaghoobi R, Talaizade A, Lal K, et al. Inflammatory breast carcinoma presenting with two different patterns of cutaneous metastases: carcinoma telangiectaticum and carcinoma erysipeloides. J Clin Aesthet Dermatol. 2015;8:47-51.
- Atis G, Demirci GT, Atunay IK, et al. The clinical characteristics and the frequency of metastatic cutaneous tumors among primary skin tumors. Turkderm. 2013;47:166-169.
- Benmously R, Souissi A, Badri T, et al. Cutaneous metastases from internal cancers. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:167-170.
- Chopra R, Chhabra S, Samra SG, et al. Cutaneous metastases of internal malignancies: a clinicopathologic study. Indian J Dermatol Venereol Leprol. 2010;76:125-131.
- El Khoury J, Khalifeh I, Kibbi AG, et al. Cutaneous metastasis: clinicopathological study of 72 patients from a tertiary care center in Lebanon. Int J Dermatol. 2014;53:147-158.
- Fernandez-Flores A. Cutaneous metastases: a study of 78 biopsies from 69 patients. Am J Dermatopathol. 2010;32:222-239.
- Gómez Sánchez ME, Martinez Martinez ML, Martín De Hijas MC, et al. Metástasis cutáneas de tumores sólidos. Estudio descriptivo retrospectivo. Piel. 2014;29:207-212
- Handa U, Kundu R, Dimri K. Cutaneous metastasis: a study of 138 cases diagnosed by fine-needle aspiration cytology. Acta Cytol. 2017;61:47-54.
- Itin P, Tomaschett S. Cutaneous metastases from malignancies which do not originate from the skin. An epidemiological study. Article in German. Internist (Berl). 2009;50:179-186.
- Siu AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Torres HA, Bodey GP, Tarrand JJ, et al. Protothecosis in patients with cancer: case series and literature review. Clin Microbiol Infect. 2003;9:786-792.
PRACTICE POINTS
- Dermatologists may play a role in diagnosing breast cancer through cutaneous metastasis, even in patients without a history of breast cancer.
- Clinicians should consider breast cancer metastasis in the differential for any erythematous lesion on the trunk.
2021 Update on gynecologic cancer
Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).
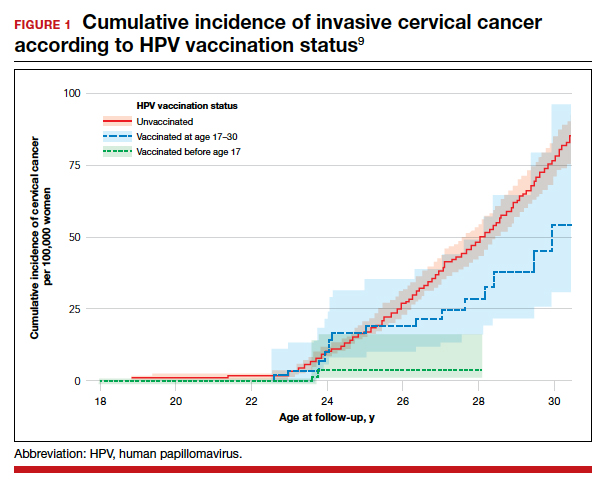
The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●
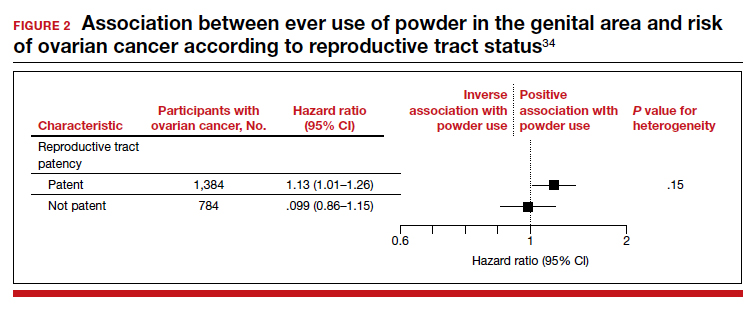
The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.

Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).

The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●

The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.

Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).

The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●

The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Caring for women with pelvic floor disorders during pregnancy and postpartum: Expert guidance
Pelvic floor disorders (PFDs) affect many pregnant and newly postpartum women. These conditions, including urinary incontinence, anal incontinence, and pelvic organ prolapse (POP), can be overshadowed by common pregnancy and postpartum concerns (TABLE 1).1 With the use of a few quick screening questions, however, PFDs easily can be identified in this at-risk population. Active management need not be delayed until after delivery for women experiencing bother, as options exist for women with PFDs during pregnancy as well as postpartum.
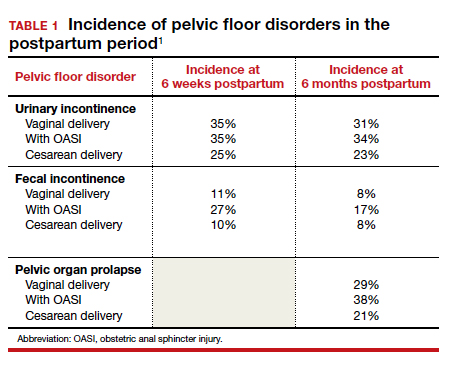
In this article, we discuss the common PFDs that obstetric clinicians face in the context of case scenarios and review how you can be better equipped to care for affected individuals.
CASE 1 Screening
A 30-year-old woman (G1P1) presents for her routine postpartum visit after an operative vaginal delivery with a second-degree laceration.
How would you screen this patient for PFDs?
Why screening for PFDs matters
While there are no validated PFD screening tools for this patient population, clinicians can ask a series of brief open-ended questions as part of the review of systems to efficiently evaluate for the common PFDs in peripartum patients (see “Screening questions to evaluate patients for peripartum pelvic floor disorders” below).
Pelvic floor disorders in the peripartum period can have a significant negative impact. In pregnancy, nearly half of women report psychological strain due to the presence of bowel, bladder, prolapse, or sexual dysfunction symptoms.2 Postpartum, PFDs have negative effects on overall health, well-being, and self-esteem, with significantly increased rates of postpartum depression in women who experience urinary incontinence.3,4 Proactively inquiring about PFD symptoms, providing anticipatory guidance, and recommending treatment options can positively impact a patient in multiple domains.
Sometimes during pregnancy or after having a baby, a woman experiences pelvic floor symptoms. Do you have any of the following?
- leakage with coughing, laughing, sneezing, or physical activity
- urgency to urinate or leakage due to urgency
- bulging or pressure within the vagina
- pain with intercourse
- accidental bowel leakage of stool or flatus
CASE 2 Stress urinary incontinence
A 27-year-old woman (G1P1) presents 2 months following spontaneous vaginal delivery with symptoms of urine leakage with laughing and running. Her urinary incontinence has been improving since delivery, but it continues to be bothersome.
What would you recommend for this patient?
Conservative SUI management strategies in pregnancy
Urinary tract symptoms are common in pregnancy, with up to 41.8% of women reporting urinary symptom distress in the third trimester.5 During pregnancy, estrogen and progesterone decrease urethral pressure that, together with increased intra-abdominal pressure from the gravid uterus, can cause or worsen stress urinary incontinence (SUI).6
During pregnancy, women should be offered conservative therapies for SUI. For women who can perform a pelvic floor contraction (a Kegel exercise), self-guided pelvic floor muscle exercises (PFMEs) may be helpful (see “Pelvic floor muscle exercises” below). We recommend that women start with 1 to 2 sets of 10 Kegel exercises per day and that they hold the squeeze for 2 to 3 seconds, working up to holding for 10 seconds. The goal is to strengthen and improve muscle control so that the Kegel squeeze can be paired with activities that cause SUI.
For women who are unable to perform a Kegel exercise or are not improving with a home PFME regimen, referral to pelvic floor physical therapy (PFPT) can be considered. While data support the efficacy of PFPT for SUI treatment in nonpregnant women,7 data are lacking on PFME in pregnancy.
In women without urinary incontinence, PFME in early pregnancy can prevent the onset of incontinence in late pregnancy and the postpartum period.8 By contrast, the same 2020 Cochrane Review found no evidence that antenatal pelvic floor muscle therapy in incontinent women decreases incontinence in mid- or late-pregnancy or in the postpartum period.8 As the quality of this evidence is very low and there is no evidence of harm with PFME, we continue to recommend it for women with bothersome SUI.
Incontinence pessaries or vaginal inserts (such as Poise Impressa bladder supports) can be helpful for SUI treatment. An incontinence pessary can be fitted in the office, and fitting kits are available for both. Pessaries can safely be used in pregnancy, but there are no data on the efficacy of pessaries for treating SUI in pregnancy. In nonpregnant women, evidence demonstrates 63% satisfaction 3 months post–pessary placement for SUI.7
We do not recommend invasive procedures for the treatment of SUI during pregnancy or in the first 6 months following delivery. There is no evidence that elective cesarean delivery prevents persistent SUI postpartum.9
To identify and engage the proper pelvic floor muscles:
- Insert a finger in the vagina and squeeze the vaginal muscles around your finger.
- Imagine you are sitting on a marble and have to pick it up with the vaginal muscles.
- Squeeze the muscles you would use to stop the flow of urine or hold back flatulence.
Perform sets of 10, 2 to 3 times per day as follows:
- Squeeze: Engage the pelvic floor muscles as described above; avoid performing Kegels while voiding.
- Hold: For 2 to 10 seconds; increase the duration to 10 seconds as able.
- Relax: Completely relax muscles before initating the next squeeze.
Reference
1. UpToDate. Patient education: pelvic muscle (Kegel) exercises (the basics). 2018. https://uptodatefree.ir/topic.htm?path=pelvic-muscle-kegel-exercises-the-basics. Accessed February 24, 2021.
Continue to: Managing SUI in the postpartum period...
Managing SUI in the postpartum period
After the first 6 months postpartum and exhaustion of conservative measures, we offer surgical interventions for women with persistent, bothersome incontinence. Surgery for SUI typically is not recommended until childbearing is complete, but it can be considered if the patient’s bother is significant.
For women with bothersome SUI who still desire future pregnancy, management options include periurethral bulking, a retropubic urethropexy (Burch procedure), or a midurethral sling procedure. Women who undergo an anti-incontinence procedure have an increased risk for urinary retention during a subsequent pregnancy.10 Most women with a midurethral sling will continue to be continent following an obstetric delivery.
Anticipatory guidance
At 3 months postpartum, the incidence of urinary incontinence is 6% to 40%, depending on parity and delivery type. Postpartum urinary incontinence is most common after instrumented vaginal delivery (32%) followed by spontaneous vaginal delivery (28%) and cesarean delivery (15%). The mean prevalence of any type of urinary incontinence is 33% at 3 months postpartum, and only small changes in the rate of urinary incontinence occur over the first postpartum year.11 While urinary incontinence is common postpartum, it should not be considered normal. We counsel that symptoms may improve spontaneously, but treatment can be initiated if the patient experiences significant bother.
A longitudinal cohort study that followed women from 3 months to 12 years postpartum found that, of women with urinary incontinence at 3 months postpartum, 76% continued to report incontinence at 12 years postpartum.12 We recommend that women be counseled that, even when symptoms resolve, they remain at increased risk for urinary incontinence in the future. Invasive therapies should be used to treat bothersome urinary incontinence, not to prevent future incontinence.
CASE 3 Fecal incontinence
A 24-year-old woman (G1P1) presents 3 weeks postpartum following a forceps-assisted vaginal delivery complicated by a 3c laceration. She reports fecal urgency, inability to control flatus, and once-daily fecal incontinence.
How would you evaluate these symptoms?
Steps in evaluation
The initial evaluation should include an inquiry regarding the patient’s stool consistency and bowel regimen. The Bristol stool form scale can be used to help patients describe their typical bowel movements (TABLE 2).13 During healing, the goal is to achieve a Bristol type 4 stool, both to avoid straining and to improve continence, as loose stool is the most difficult to control.
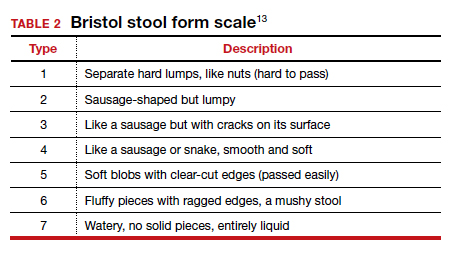
A physical examination can evaluate healing and sphincter integrity; it should include inspection of the distal vagina and perineal body and a digital rectal exam. Anal canal resting tone and squeeze strength should be evaluated, and the digital rectal examination scoring system (DRESS) can be useful for quantification (TABLE 3).14 Lack of tone at rest in the anterolateral portion of the sphincter complex can indicate an internal anal sphincter defect, as 80% of the resting tone comes from this muscle (FIGURE).15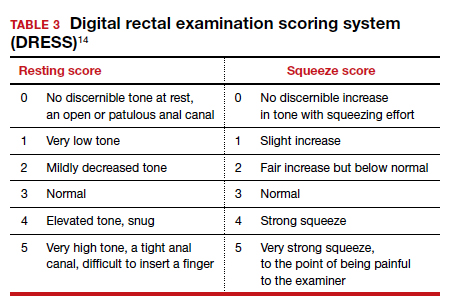
The rectovaginal septum should be assessed given the increased risk of rectovaginal fistula in women with obstetric anal sphincter injury (OASI). The patient should be instructed to contract the anal sphincter, allowing evaluation of muscular contraction. Lack of contraction anteriolaterally may indicate external anal sphincter separation.
Continue to: Conservative options for improving fecal incontinence symptoms...
Conservative options for improving fecal incontinence symptoms
The patient can be counseled regarding stool bulking, first with insoluble fiber supplementation and cessation of stool softeners if she is incontinent of liquid stool. If these measures are not effective, use of a constipating agent, such as loperamide, can improve stool consistency and thereby decrease incontinence episodes. PFPT with biofeedback can be offered as well. While typically we do not recommend initiating PFPT before 6 weeks postpartum, so the initial phases of healing can occur, early referral enables the patient to avoid a delay in access to care.
The patient also can be counseled about a referral to a pelvic floor specialist for further evaluation. A variety of peripartum pelvic floor disorder clinics are being established by Female Pelvic Medicine and Reconstructive Surgery (FPMRS) physicians. These clinics provide the benefit of comprehensive care for pelvic floor disorders in this unique population.
When conservative measures fail. If a patient has persistent bowel control issues despite conservative measures, a referral to an FPMRS physician should be initiated.
Delivery route in future pregnancies
The risk of a subsequent OASI is low. While this means that many women can safely pursue a future vaginal delivery, a scheduled cesarean delivery is indicated for women with persistent bowel control issues, wound healing complications, and those who experienced psychological trauma from their delivery.16 We recommend a shared-decision making approach, reviewing modifiable and nonmodifiable risk factors to help determine whether or not a future vaginal birth is appropriate. It is important to highlight that a cesarean delivery does not protect against fecal incontinence in women with a history of OASI; however, there is benefit in preventing worsening of anal incontinence, if present.17
CASE 4 Uterovaginal prolapse
A 36-year-old woman (G3P3) presents for her routine postpartum visit at 6 weeks after a spontaneous vaginal delivery without lacerations. She reports a persistent feeling of vaginal pressure and fullness. She thinks she felt a bulge with wiping after a bowel movement.
What options are available for this patient?
Prolapse in the peripartum population
Previous studies have revealed an increased prevalence of POP in pregnant women on examination compared with their nulligravid counterparts (47.6% vs 0%).18 With the changes in the hormonal milieu in pregnancy, as well as the weight of the gravid uterus on the pelvic floor, it is not surprising that pregnancy may be the inciting event to expose even transient defects in pelvic organ support.19
It is well established that increasing parity and, to a lesser extent, larger babies are associated with increased risk for future POP and surgery for prolapse. In the first year postpartum, nearly one-third of women have stage 2 or greater prolapse on exam, with studies demonstrating an increased prevalence of postpartum POP in women who delivered vaginally compared with those who delivered by cesarean.20,21
Initial evaluation
Diagnosis can be made during a routine pelvic exam by having the patient perform a Valsalva maneuver while in the lithotomy position. Using half of a speculum permits evaluation of the anterior and posterior vaginal walls separately, and Valsalva during a bimanual exam can aid in evaluating descensus of the uterus and cervix.
Excellent free patient education resources available online through the American Urogynecologic Society and the International Urogynecological Association can be used to direct counseling.
Continue to: Treatments you can offer for POP...
Treatments you can offer for POP
For pregnant or postpartum patients with bothersome prolapse, initial management options include pessary fitting and/or PFPT referral. In pregnancy, women often can be successfully fitted with a pessary for POP; however, as expulsion is a common issue, selection of a stiffer or space-occupying device may be more efficacious.
Often, early onset POP in pregnancy resolves as the gravid uterus lifts out of the pelvis in the second trimester, at which time the pessary can be discontinued. In the postpartum period, a pessary fitting can be undertaken similarly to that in nonpregnant patients. While data are lacking in the peripartum population, evidence supports the positive impact of PFPT on improving POP symptom bother.22 Additionally, for postpartum women who experience OASI, PFPT can produce significant improvement in subjective POP and associated bother.23
Impact of future childbearing wishes on treatment
The desire for future childbearing does not preclude treatment of patients experiencing bother from POP after conservative management options have failed. Both vaginal native tissue and mesh-augmented uterine-sparing repairs are performed by many FPMRS specialists and are associated with good outcomes. As with SUI, we do not recommend invasive treatment for POP during pregnancy or before 6 months postpartum.
In conclusion
Obstetric specialists play an essential role in caring for women with PFDs in the peripartum period. Basic evaluation, counseling, and management can be initiated using many of the resources already available in an obstetric ambulatory practice. Important adjunctive resources include those available for both providers and patients through the American Urogynecologic Society and the International Urogynecological Association. In addition, clinicians can partner with pelvic floor specialists through the growing number of FPMRS-run peripartum pelvic floor disorder clinics across the country and pelvic floor physical therapists.
If these specialty clinics and therapists are not available in your area, FPMRS specialists, urologists, gastroenterologists, and/or colorectal surgeons can aid in patient diagnosis and management to reach the ultimate goal of improving PFDs in this at-risk population. ●
- Madsen AM, Hickman LC, Propst K. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. Forthcoming 2021.
- Bodner-Adler B, Kimberger O, Laml T, et al. Prevalence and risk factors for pelvic floor disorders during early and late pregnancy in a cohort of Austrian women. Arch Gynecol Obstet. 2019;300:1325-1330.
- Swenson CW, DePorre JA, Haefner JK, et al. Postpartum depression screening and pelvic floor symptoms among women referred to a specialty postpartum perineal clinic. Am J Obstet Gynecol. 2018;218:335.e1-335.e6.
- Skinner EM, Dietz HP. Psychological and somatic sequelae of traumatic vaginal delivery: a literature review. Aust N Z J Obstet Gynaecol. 2015;55:309-314.
- Yohay D, Weintraub AY, Mauer-Perry N, et al. Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Eur J Obstet Gynecol Reprod Biol. 2016;200:35-39.
- Gregory WT, Sibai BM. Obstetrics and pelvic floor disorders. In: Walters M, Karram M, eds. Urogynecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Saunders; 2015:224-237.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Woodley SJ, Lawrenson P, Boyle R, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;6:CD007471.
- Foldspang A, Hvidman L, Mommsen S, et al. Risk of postpartum urinary incontinence associated with pregnancy and mode of delivery. Acta Obstet Gynecol Scand. 2004;83:923-927.
- Wieslander CK, Weinstein MM, Handa V, et al. Pregnancy in women with prior treatments for pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2020;26:299-305.
- Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89:1511-1522.
- MacArthur C, Wilson D, Herbison P, et al; Prolong Study Group. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG. 2016;123:1022-1029.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693-703
- Orkin BA, Sinykin SB, Lloyd PC. The digital rectal examination scoring system (DRESS). Dis Colon Rectum. 2010;53:1656-1660.
- UpToDate. Repair of episiotomy and perineal lacerations associated with childbirth. 2020. https://www-uptodate-com .ccmain.ohionet.org/contents/repair-of-perineal-and-other -lacerations-associated-with-childbirth?search=repair%20 episiotomy&source=search_result&selectedTitle=1~150&usa ge_type=default&display_rank=1. Accessed February 28, 2021.
- Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 198: prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2018;132:e87-e102.
- Jangö H, Langhoff-Roos J, Rosthøj S, et al. Long-term anal incontinence after obstetric anal sphincter injury—does grade of tear matter? Am J Obstet Gynecol. 2018;218:232.e1-232.e10.
- O’Boyle AL, Woodman PJ, O’Boyle JD, et al. Pelvic organ support in nulliparous pregnant and nonpregnant women: a case control study. Am J Obstet Gynecol. 2002;187:99-102.
- Handa VL, Blomquist JL, McDermott KC, et al. Pelvic floor disorders after vaginal birth. Obstet Gynecol. 2012;119 (2, pt 1):233-239.
- Handa VL, Nygaard I, Kenton K, et al; Pelvic Floor Disorders Network. Pelvic organ support among primiparous women in the first year after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1407-1411.
- O’Boyle AL, O’Boyle JD, Calhoun B, et al. Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:69-72.
- Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014;383:796-806.
- Von Bargen E, Haviland MJ, Chang OH, et al. Evaluation of postpartum pelvic floor physical therapy on obstetrical anal sphincter injury: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020. doi: 10.1097/SPV.0000000000000849.
Pelvic floor disorders (PFDs) affect many pregnant and newly postpartum women. These conditions, including urinary incontinence, anal incontinence, and pelvic organ prolapse (POP), can be overshadowed by common pregnancy and postpartum concerns (TABLE 1).1 With the use of a few quick screening questions, however, PFDs easily can be identified in this at-risk population. Active management need not be delayed until after delivery for women experiencing bother, as options exist for women with PFDs during pregnancy as well as postpartum.

In this article, we discuss the common PFDs that obstetric clinicians face in the context of case scenarios and review how you can be better equipped to care for affected individuals.
CASE 1 Screening
A 30-year-old woman (G1P1) presents for her routine postpartum visit after an operative vaginal delivery with a second-degree laceration.
How would you screen this patient for PFDs?
Why screening for PFDs matters
While there are no validated PFD screening tools for this patient population, clinicians can ask a series of brief open-ended questions as part of the review of systems to efficiently evaluate for the common PFDs in peripartum patients (see “Screening questions to evaluate patients for peripartum pelvic floor disorders” below).
Pelvic floor disorders in the peripartum period can have a significant negative impact. In pregnancy, nearly half of women report psychological strain due to the presence of bowel, bladder, prolapse, or sexual dysfunction symptoms.2 Postpartum, PFDs have negative effects on overall health, well-being, and self-esteem, with significantly increased rates of postpartum depression in women who experience urinary incontinence.3,4 Proactively inquiring about PFD symptoms, providing anticipatory guidance, and recommending treatment options can positively impact a patient in multiple domains.
Sometimes during pregnancy or after having a baby, a woman experiences pelvic floor symptoms. Do you have any of the following?
- leakage with coughing, laughing, sneezing, or physical activity
- urgency to urinate or leakage due to urgency
- bulging or pressure within the vagina
- pain with intercourse
- accidental bowel leakage of stool or flatus
CASE 2 Stress urinary incontinence
A 27-year-old woman (G1P1) presents 2 months following spontaneous vaginal delivery with symptoms of urine leakage with laughing and running. Her urinary incontinence has been improving since delivery, but it continues to be bothersome.
What would you recommend for this patient?
Conservative SUI management strategies in pregnancy
Urinary tract symptoms are common in pregnancy, with up to 41.8% of women reporting urinary symptom distress in the third trimester.5 During pregnancy, estrogen and progesterone decrease urethral pressure that, together with increased intra-abdominal pressure from the gravid uterus, can cause or worsen stress urinary incontinence (SUI).6
During pregnancy, women should be offered conservative therapies for SUI. For women who can perform a pelvic floor contraction (a Kegel exercise), self-guided pelvic floor muscle exercises (PFMEs) may be helpful (see “Pelvic floor muscle exercises” below). We recommend that women start with 1 to 2 sets of 10 Kegel exercises per day and that they hold the squeeze for 2 to 3 seconds, working up to holding for 10 seconds. The goal is to strengthen and improve muscle control so that the Kegel squeeze can be paired with activities that cause SUI.
For women who are unable to perform a Kegel exercise or are not improving with a home PFME regimen, referral to pelvic floor physical therapy (PFPT) can be considered. While data support the efficacy of PFPT for SUI treatment in nonpregnant women,7 data are lacking on PFME in pregnancy.
In women without urinary incontinence, PFME in early pregnancy can prevent the onset of incontinence in late pregnancy and the postpartum period.8 By contrast, the same 2020 Cochrane Review found no evidence that antenatal pelvic floor muscle therapy in incontinent women decreases incontinence in mid- or late-pregnancy or in the postpartum period.8 As the quality of this evidence is very low and there is no evidence of harm with PFME, we continue to recommend it for women with bothersome SUI.
Incontinence pessaries or vaginal inserts (such as Poise Impressa bladder supports) can be helpful for SUI treatment. An incontinence pessary can be fitted in the office, and fitting kits are available for both. Pessaries can safely be used in pregnancy, but there are no data on the efficacy of pessaries for treating SUI in pregnancy. In nonpregnant women, evidence demonstrates 63% satisfaction 3 months post–pessary placement for SUI.7
We do not recommend invasive procedures for the treatment of SUI during pregnancy or in the first 6 months following delivery. There is no evidence that elective cesarean delivery prevents persistent SUI postpartum.9
To identify and engage the proper pelvic floor muscles:
- Insert a finger in the vagina and squeeze the vaginal muscles around your finger.
- Imagine you are sitting on a marble and have to pick it up with the vaginal muscles.
- Squeeze the muscles you would use to stop the flow of urine or hold back flatulence.
Perform sets of 10, 2 to 3 times per day as follows:
- Squeeze: Engage the pelvic floor muscles as described above; avoid performing Kegels while voiding.
- Hold: For 2 to 10 seconds; increase the duration to 10 seconds as able.
- Relax: Completely relax muscles before initating the next squeeze.
Reference
1. UpToDate. Patient education: pelvic muscle (Kegel) exercises (the basics). 2018. https://uptodatefree.ir/topic.htm?path=pelvic-muscle-kegel-exercises-the-basics. Accessed February 24, 2021.
Continue to: Managing SUI in the postpartum period...
Managing SUI in the postpartum period
After the first 6 months postpartum and exhaustion of conservative measures, we offer surgical interventions for women with persistent, bothersome incontinence. Surgery for SUI typically is not recommended until childbearing is complete, but it can be considered if the patient’s bother is significant.
For women with bothersome SUI who still desire future pregnancy, management options include periurethral bulking, a retropubic urethropexy (Burch procedure), or a midurethral sling procedure. Women who undergo an anti-incontinence procedure have an increased risk for urinary retention during a subsequent pregnancy.10 Most women with a midurethral sling will continue to be continent following an obstetric delivery.
Anticipatory guidance
At 3 months postpartum, the incidence of urinary incontinence is 6% to 40%, depending on parity and delivery type. Postpartum urinary incontinence is most common after instrumented vaginal delivery (32%) followed by spontaneous vaginal delivery (28%) and cesarean delivery (15%). The mean prevalence of any type of urinary incontinence is 33% at 3 months postpartum, and only small changes in the rate of urinary incontinence occur over the first postpartum year.11 While urinary incontinence is common postpartum, it should not be considered normal. We counsel that symptoms may improve spontaneously, but treatment can be initiated if the patient experiences significant bother.
A longitudinal cohort study that followed women from 3 months to 12 years postpartum found that, of women with urinary incontinence at 3 months postpartum, 76% continued to report incontinence at 12 years postpartum.12 We recommend that women be counseled that, even when symptoms resolve, they remain at increased risk for urinary incontinence in the future. Invasive therapies should be used to treat bothersome urinary incontinence, not to prevent future incontinence.
CASE 3 Fecal incontinence
A 24-year-old woman (G1P1) presents 3 weeks postpartum following a forceps-assisted vaginal delivery complicated by a 3c laceration. She reports fecal urgency, inability to control flatus, and once-daily fecal incontinence.
How would you evaluate these symptoms?
Steps in evaluation
The initial evaluation should include an inquiry regarding the patient’s stool consistency and bowel regimen. The Bristol stool form scale can be used to help patients describe their typical bowel movements (TABLE 2).13 During healing, the goal is to achieve a Bristol type 4 stool, both to avoid straining and to improve continence, as loose stool is the most difficult to control.

A physical examination can evaluate healing and sphincter integrity; it should include inspection of the distal vagina and perineal body and a digital rectal exam. Anal canal resting tone and squeeze strength should be evaluated, and the digital rectal examination scoring system (DRESS) can be useful for quantification (TABLE 3).14 Lack of tone at rest in the anterolateral portion of the sphincter complex can indicate an internal anal sphincter defect, as 80% of the resting tone comes from this muscle (FIGURE).15

The rectovaginal septum should be assessed given the increased risk of rectovaginal fistula in women with obstetric anal sphincter injury (OASI). The patient should be instructed to contract the anal sphincter, allowing evaluation of muscular contraction. Lack of contraction anteriolaterally may indicate external anal sphincter separation.
Continue to: Conservative options for improving fecal incontinence symptoms...
Conservative options for improving fecal incontinence symptoms
The patient can be counseled regarding stool bulking, first with insoluble fiber supplementation and cessation of stool softeners if she is incontinent of liquid stool. If these measures are not effective, use of a constipating agent, such as loperamide, can improve stool consistency and thereby decrease incontinence episodes. PFPT with biofeedback can be offered as well. While typically we do not recommend initiating PFPT before 6 weeks postpartum, so the initial phases of healing can occur, early referral enables the patient to avoid a delay in access to care.
The patient also can be counseled about a referral to a pelvic floor specialist for further evaluation. A variety of peripartum pelvic floor disorder clinics are being established by Female Pelvic Medicine and Reconstructive Surgery (FPMRS) physicians. These clinics provide the benefit of comprehensive care for pelvic floor disorders in this unique population.
When conservative measures fail. If a patient has persistent bowel control issues despite conservative measures, a referral to an FPMRS physician should be initiated.
Delivery route in future pregnancies
The risk of a subsequent OASI is low. While this means that many women can safely pursue a future vaginal delivery, a scheduled cesarean delivery is indicated for women with persistent bowel control issues, wound healing complications, and those who experienced psychological trauma from their delivery.16 We recommend a shared-decision making approach, reviewing modifiable and nonmodifiable risk factors to help determine whether or not a future vaginal birth is appropriate. It is important to highlight that a cesarean delivery does not protect against fecal incontinence in women with a history of OASI; however, there is benefit in preventing worsening of anal incontinence, if present.17
CASE 4 Uterovaginal prolapse
A 36-year-old woman (G3P3) presents for her routine postpartum visit at 6 weeks after a spontaneous vaginal delivery without lacerations. She reports a persistent feeling of vaginal pressure and fullness. She thinks she felt a bulge with wiping after a bowel movement.
What options are available for this patient?
Prolapse in the peripartum population
Previous studies have revealed an increased prevalence of POP in pregnant women on examination compared with their nulligravid counterparts (47.6% vs 0%).18 With the changes in the hormonal milieu in pregnancy, as well as the weight of the gravid uterus on the pelvic floor, it is not surprising that pregnancy may be the inciting event to expose even transient defects in pelvic organ support.19
It is well established that increasing parity and, to a lesser extent, larger babies are associated with increased risk for future POP and surgery for prolapse. In the first year postpartum, nearly one-third of women have stage 2 or greater prolapse on exam, with studies demonstrating an increased prevalence of postpartum POP in women who delivered vaginally compared with those who delivered by cesarean.20,21
Initial evaluation
Diagnosis can be made during a routine pelvic exam by having the patient perform a Valsalva maneuver while in the lithotomy position. Using half of a speculum permits evaluation of the anterior and posterior vaginal walls separately, and Valsalva during a bimanual exam can aid in evaluating descensus of the uterus and cervix.
Excellent free patient education resources available online through the American Urogynecologic Society and the International Urogynecological Association can be used to direct counseling.
Continue to: Treatments you can offer for POP...
Treatments you can offer for POP
For pregnant or postpartum patients with bothersome prolapse, initial management options include pessary fitting and/or PFPT referral. In pregnancy, women often can be successfully fitted with a pessary for POP; however, as expulsion is a common issue, selection of a stiffer or space-occupying device may be more efficacious.
Often, early onset POP in pregnancy resolves as the gravid uterus lifts out of the pelvis in the second trimester, at which time the pessary can be discontinued. In the postpartum period, a pessary fitting can be undertaken similarly to that in nonpregnant patients. While data are lacking in the peripartum population, evidence supports the positive impact of PFPT on improving POP symptom bother.22 Additionally, for postpartum women who experience OASI, PFPT can produce significant improvement in subjective POP and associated bother.23
Impact of future childbearing wishes on treatment
The desire for future childbearing does not preclude treatment of patients experiencing bother from POP after conservative management options have failed. Both vaginal native tissue and mesh-augmented uterine-sparing repairs are performed by many FPMRS specialists and are associated with good outcomes. As with SUI, we do not recommend invasive treatment for POP during pregnancy or before 6 months postpartum.
In conclusion
Obstetric specialists play an essential role in caring for women with PFDs in the peripartum period. Basic evaluation, counseling, and management can be initiated using many of the resources already available in an obstetric ambulatory practice. Important adjunctive resources include those available for both providers and patients through the American Urogynecologic Society and the International Urogynecological Association. In addition, clinicians can partner with pelvic floor specialists through the growing number of FPMRS-run peripartum pelvic floor disorder clinics across the country and pelvic floor physical therapists.
If these specialty clinics and therapists are not available in your area, FPMRS specialists, urologists, gastroenterologists, and/or colorectal surgeons can aid in patient diagnosis and management to reach the ultimate goal of improving PFDs in this at-risk population. ●
Pelvic floor disorders (PFDs) affect many pregnant and newly postpartum women. These conditions, including urinary incontinence, anal incontinence, and pelvic organ prolapse (POP), can be overshadowed by common pregnancy and postpartum concerns (TABLE 1).1 With the use of a few quick screening questions, however, PFDs easily can be identified in this at-risk population. Active management need not be delayed until after delivery for women experiencing bother, as options exist for women with PFDs during pregnancy as well as postpartum.

In this article, we discuss the common PFDs that obstetric clinicians face in the context of case scenarios and review how you can be better equipped to care for affected individuals.
CASE 1 Screening
A 30-year-old woman (G1P1) presents for her routine postpartum visit after an operative vaginal delivery with a second-degree laceration.
How would you screen this patient for PFDs?
Why screening for PFDs matters
While there are no validated PFD screening tools for this patient population, clinicians can ask a series of brief open-ended questions as part of the review of systems to efficiently evaluate for the common PFDs in peripartum patients (see “Screening questions to evaluate patients for peripartum pelvic floor disorders” below).
Pelvic floor disorders in the peripartum period can have a significant negative impact. In pregnancy, nearly half of women report psychological strain due to the presence of bowel, bladder, prolapse, or sexual dysfunction symptoms.2 Postpartum, PFDs have negative effects on overall health, well-being, and self-esteem, with significantly increased rates of postpartum depression in women who experience urinary incontinence.3,4 Proactively inquiring about PFD symptoms, providing anticipatory guidance, and recommending treatment options can positively impact a patient in multiple domains.
Sometimes during pregnancy or after having a baby, a woman experiences pelvic floor symptoms. Do you have any of the following?
- leakage with coughing, laughing, sneezing, or physical activity
- urgency to urinate or leakage due to urgency
- bulging or pressure within the vagina
- pain with intercourse
- accidental bowel leakage of stool or flatus
CASE 2 Stress urinary incontinence
A 27-year-old woman (G1P1) presents 2 months following spontaneous vaginal delivery with symptoms of urine leakage with laughing and running. Her urinary incontinence has been improving since delivery, but it continues to be bothersome.
What would you recommend for this patient?
Conservative SUI management strategies in pregnancy
Urinary tract symptoms are common in pregnancy, with up to 41.8% of women reporting urinary symptom distress in the third trimester.5 During pregnancy, estrogen and progesterone decrease urethral pressure that, together with increased intra-abdominal pressure from the gravid uterus, can cause or worsen stress urinary incontinence (SUI).6
During pregnancy, women should be offered conservative therapies for SUI. For women who can perform a pelvic floor contraction (a Kegel exercise), self-guided pelvic floor muscle exercises (PFMEs) may be helpful (see “Pelvic floor muscle exercises” below). We recommend that women start with 1 to 2 sets of 10 Kegel exercises per day and that they hold the squeeze for 2 to 3 seconds, working up to holding for 10 seconds. The goal is to strengthen and improve muscle control so that the Kegel squeeze can be paired with activities that cause SUI.
For women who are unable to perform a Kegel exercise or are not improving with a home PFME regimen, referral to pelvic floor physical therapy (PFPT) can be considered. While data support the efficacy of PFPT for SUI treatment in nonpregnant women,7 data are lacking on PFME in pregnancy.
In women without urinary incontinence, PFME in early pregnancy can prevent the onset of incontinence in late pregnancy and the postpartum period.8 By contrast, the same 2020 Cochrane Review found no evidence that antenatal pelvic floor muscle therapy in incontinent women decreases incontinence in mid- or late-pregnancy or in the postpartum period.8 As the quality of this evidence is very low and there is no evidence of harm with PFME, we continue to recommend it for women with bothersome SUI.
Incontinence pessaries or vaginal inserts (such as Poise Impressa bladder supports) can be helpful for SUI treatment. An incontinence pessary can be fitted in the office, and fitting kits are available for both. Pessaries can safely be used in pregnancy, but there are no data on the efficacy of pessaries for treating SUI in pregnancy. In nonpregnant women, evidence demonstrates 63% satisfaction 3 months post–pessary placement for SUI.7
We do not recommend invasive procedures for the treatment of SUI during pregnancy or in the first 6 months following delivery. There is no evidence that elective cesarean delivery prevents persistent SUI postpartum.9
To identify and engage the proper pelvic floor muscles:
- Insert a finger in the vagina and squeeze the vaginal muscles around your finger.
- Imagine you are sitting on a marble and have to pick it up with the vaginal muscles.
- Squeeze the muscles you would use to stop the flow of urine or hold back flatulence.
Perform sets of 10, 2 to 3 times per day as follows:
- Squeeze: Engage the pelvic floor muscles as described above; avoid performing Kegels while voiding.
- Hold: For 2 to 10 seconds; increase the duration to 10 seconds as able.
- Relax: Completely relax muscles before initating the next squeeze.
Reference
1. UpToDate. Patient education: pelvic muscle (Kegel) exercises (the basics). 2018. https://uptodatefree.ir/topic.htm?path=pelvic-muscle-kegel-exercises-the-basics. Accessed February 24, 2021.
Continue to: Managing SUI in the postpartum period...
Managing SUI in the postpartum period
After the first 6 months postpartum and exhaustion of conservative measures, we offer surgical interventions for women with persistent, bothersome incontinence. Surgery for SUI typically is not recommended until childbearing is complete, but it can be considered if the patient’s bother is significant.
For women with bothersome SUI who still desire future pregnancy, management options include periurethral bulking, a retropubic urethropexy (Burch procedure), or a midurethral sling procedure. Women who undergo an anti-incontinence procedure have an increased risk for urinary retention during a subsequent pregnancy.10 Most women with a midurethral sling will continue to be continent following an obstetric delivery.
Anticipatory guidance
At 3 months postpartum, the incidence of urinary incontinence is 6% to 40%, depending on parity and delivery type. Postpartum urinary incontinence is most common after instrumented vaginal delivery (32%) followed by spontaneous vaginal delivery (28%) and cesarean delivery (15%). The mean prevalence of any type of urinary incontinence is 33% at 3 months postpartum, and only small changes in the rate of urinary incontinence occur over the first postpartum year.11 While urinary incontinence is common postpartum, it should not be considered normal. We counsel that symptoms may improve spontaneously, but treatment can be initiated if the patient experiences significant bother.
A longitudinal cohort study that followed women from 3 months to 12 years postpartum found that, of women with urinary incontinence at 3 months postpartum, 76% continued to report incontinence at 12 years postpartum.12 We recommend that women be counseled that, even when symptoms resolve, they remain at increased risk for urinary incontinence in the future. Invasive therapies should be used to treat bothersome urinary incontinence, not to prevent future incontinence.
CASE 3 Fecal incontinence
A 24-year-old woman (G1P1) presents 3 weeks postpartum following a forceps-assisted vaginal delivery complicated by a 3c laceration. She reports fecal urgency, inability to control flatus, and once-daily fecal incontinence.
How would you evaluate these symptoms?
Steps in evaluation
The initial evaluation should include an inquiry regarding the patient’s stool consistency and bowel regimen. The Bristol stool form scale can be used to help patients describe their typical bowel movements (TABLE 2).13 During healing, the goal is to achieve a Bristol type 4 stool, both to avoid straining and to improve continence, as loose stool is the most difficult to control.

A physical examination can evaluate healing and sphincter integrity; it should include inspection of the distal vagina and perineal body and a digital rectal exam. Anal canal resting tone and squeeze strength should be evaluated, and the digital rectal examination scoring system (DRESS) can be useful for quantification (TABLE 3).14 Lack of tone at rest in the anterolateral portion of the sphincter complex can indicate an internal anal sphincter defect, as 80% of the resting tone comes from this muscle (FIGURE).15

The rectovaginal septum should be assessed given the increased risk of rectovaginal fistula in women with obstetric anal sphincter injury (OASI). The patient should be instructed to contract the anal sphincter, allowing evaluation of muscular contraction. Lack of contraction anteriolaterally may indicate external anal sphincter separation.
Continue to: Conservative options for improving fecal incontinence symptoms...
Conservative options for improving fecal incontinence symptoms
The patient can be counseled regarding stool bulking, first with insoluble fiber supplementation and cessation of stool softeners if she is incontinent of liquid stool. If these measures are not effective, use of a constipating agent, such as loperamide, can improve stool consistency and thereby decrease incontinence episodes. PFPT with biofeedback can be offered as well. While typically we do not recommend initiating PFPT before 6 weeks postpartum, so the initial phases of healing can occur, early referral enables the patient to avoid a delay in access to care.
The patient also can be counseled about a referral to a pelvic floor specialist for further evaluation. A variety of peripartum pelvic floor disorder clinics are being established by Female Pelvic Medicine and Reconstructive Surgery (FPMRS) physicians. These clinics provide the benefit of comprehensive care for pelvic floor disorders in this unique population.
When conservative measures fail. If a patient has persistent bowel control issues despite conservative measures, a referral to an FPMRS physician should be initiated.
Delivery route in future pregnancies
The risk of a subsequent OASI is low. While this means that many women can safely pursue a future vaginal delivery, a scheduled cesarean delivery is indicated for women with persistent bowel control issues, wound healing complications, and those who experienced psychological trauma from their delivery.16 We recommend a shared-decision making approach, reviewing modifiable and nonmodifiable risk factors to help determine whether or not a future vaginal birth is appropriate. It is important to highlight that a cesarean delivery does not protect against fecal incontinence in women with a history of OASI; however, there is benefit in preventing worsening of anal incontinence, if present.17
CASE 4 Uterovaginal prolapse
A 36-year-old woman (G3P3) presents for her routine postpartum visit at 6 weeks after a spontaneous vaginal delivery without lacerations. She reports a persistent feeling of vaginal pressure and fullness. She thinks she felt a bulge with wiping after a bowel movement.
What options are available for this patient?
Prolapse in the peripartum population
Previous studies have revealed an increased prevalence of POP in pregnant women on examination compared with their nulligravid counterparts (47.6% vs 0%).18 With the changes in the hormonal milieu in pregnancy, as well as the weight of the gravid uterus on the pelvic floor, it is not surprising that pregnancy may be the inciting event to expose even transient defects in pelvic organ support.19
It is well established that increasing parity and, to a lesser extent, larger babies are associated with increased risk for future POP and surgery for prolapse. In the first year postpartum, nearly one-third of women have stage 2 or greater prolapse on exam, with studies demonstrating an increased prevalence of postpartum POP in women who delivered vaginally compared with those who delivered by cesarean.20,21
Initial evaluation
Diagnosis can be made during a routine pelvic exam by having the patient perform a Valsalva maneuver while in the lithotomy position. Using half of a speculum permits evaluation of the anterior and posterior vaginal walls separately, and Valsalva during a bimanual exam can aid in evaluating descensus of the uterus and cervix.
Excellent free patient education resources available online through the American Urogynecologic Society and the International Urogynecological Association can be used to direct counseling.
Continue to: Treatments you can offer for POP...
Treatments you can offer for POP
For pregnant or postpartum patients with bothersome prolapse, initial management options include pessary fitting and/or PFPT referral. In pregnancy, women often can be successfully fitted with a pessary for POP; however, as expulsion is a common issue, selection of a stiffer or space-occupying device may be more efficacious.
Often, early onset POP in pregnancy resolves as the gravid uterus lifts out of the pelvis in the second trimester, at which time the pessary can be discontinued. In the postpartum period, a pessary fitting can be undertaken similarly to that in nonpregnant patients. While data are lacking in the peripartum population, evidence supports the positive impact of PFPT on improving POP symptom bother.22 Additionally, for postpartum women who experience OASI, PFPT can produce significant improvement in subjective POP and associated bother.23
Impact of future childbearing wishes on treatment
The desire for future childbearing does not preclude treatment of patients experiencing bother from POP after conservative management options have failed. Both vaginal native tissue and mesh-augmented uterine-sparing repairs are performed by many FPMRS specialists and are associated with good outcomes. As with SUI, we do not recommend invasive treatment for POP during pregnancy or before 6 months postpartum.
In conclusion
Obstetric specialists play an essential role in caring for women with PFDs in the peripartum period. Basic evaluation, counseling, and management can be initiated using many of the resources already available in an obstetric ambulatory practice. Important adjunctive resources include those available for both providers and patients through the American Urogynecologic Society and the International Urogynecological Association. In addition, clinicians can partner with pelvic floor specialists through the growing number of FPMRS-run peripartum pelvic floor disorder clinics across the country and pelvic floor physical therapists.
If these specialty clinics and therapists are not available in your area, FPMRS specialists, urologists, gastroenterologists, and/or colorectal surgeons can aid in patient diagnosis and management to reach the ultimate goal of improving PFDs in this at-risk population. ●
- Madsen AM, Hickman LC, Propst K. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. Forthcoming 2021.
- Bodner-Adler B, Kimberger O, Laml T, et al. Prevalence and risk factors for pelvic floor disorders during early and late pregnancy in a cohort of Austrian women. Arch Gynecol Obstet. 2019;300:1325-1330.
- Swenson CW, DePorre JA, Haefner JK, et al. Postpartum depression screening and pelvic floor symptoms among women referred to a specialty postpartum perineal clinic. Am J Obstet Gynecol. 2018;218:335.e1-335.e6.
- Skinner EM, Dietz HP. Psychological and somatic sequelae of traumatic vaginal delivery: a literature review. Aust N Z J Obstet Gynaecol. 2015;55:309-314.
- Yohay D, Weintraub AY, Mauer-Perry N, et al. Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Eur J Obstet Gynecol Reprod Biol. 2016;200:35-39.
- Gregory WT, Sibai BM. Obstetrics and pelvic floor disorders. In: Walters M, Karram M, eds. Urogynecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Saunders; 2015:224-237.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Woodley SJ, Lawrenson P, Boyle R, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;6:CD007471.
- Foldspang A, Hvidman L, Mommsen S, et al. Risk of postpartum urinary incontinence associated with pregnancy and mode of delivery. Acta Obstet Gynecol Scand. 2004;83:923-927.
- Wieslander CK, Weinstein MM, Handa V, et al. Pregnancy in women with prior treatments for pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2020;26:299-305.
- Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89:1511-1522.
- MacArthur C, Wilson D, Herbison P, et al; Prolong Study Group. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG. 2016;123:1022-1029.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693-703
- Orkin BA, Sinykin SB, Lloyd PC. The digital rectal examination scoring system (DRESS). Dis Colon Rectum. 2010;53:1656-1660.
- UpToDate. Repair of episiotomy and perineal lacerations associated with childbirth. 2020. https://www-uptodate-com .ccmain.ohionet.org/contents/repair-of-perineal-and-other -lacerations-associated-with-childbirth?search=repair%20 episiotomy&source=search_result&selectedTitle=1~150&usa ge_type=default&display_rank=1. Accessed February 28, 2021.
- Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 198: prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2018;132:e87-e102.
- Jangö H, Langhoff-Roos J, Rosthøj S, et al. Long-term anal incontinence after obstetric anal sphincter injury—does grade of tear matter? Am J Obstet Gynecol. 2018;218:232.e1-232.e10.
- O’Boyle AL, Woodman PJ, O’Boyle JD, et al. Pelvic organ support in nulliparous pregnant and nonpregnant women: a case control study. Am J Obstet Gynecol. 2002;187:99-102.
- Handa VL, Blomquist JL, McDermott KC, et al. Pelvic floor disorders after vaginal birth. Obstet Gynecol. 2012;119 (2, pt 1):233-239.
- Handa VL, Nygaard I, Kenton K, et al; Pelvic Floor Disorders Network. Pelvic organ support among primiparous women in the first year after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1407-1411.
- O’Boyle AL, O’Boyle JD, Calhoun B, et al. Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:69-72.
- Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014;383:796-806.
- Von Bargen E, Haviland MJ, Chang OH, et al. Evaluation of postpartum pelvic floor physical therapy on obstetrical anal sphincter injury: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020. doi: 10.1097/SPV.0000000000000849.
- Madsen AM, Hickman LC, Propst K. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. Forthcoming 2021.
- Bodner-Adler B, Kimberger O, Laml T, et al. Prevalence and risk factors for pelvic floor disorders during early and late pregnancy in a cohort of Austrian women. Arch Gynecol Obstet. 2019;300:1325-1330.
- Swenson CW, DePorre JA, Haefner JK, et al. Postpartum depression screening and pelvic floor symptoms among women referred to a specialty postpartum perineal clinic. Am J Obstet Gynecol. 2018;218:335.e1-335.e6.
- Skinner EM, Dietz HP. Psychological and somatic sequelae of traumatic vaginal delivery: a literature review. Aust N Z J Obstet Gynaecol. 2015;55:309-314.
- Yohay D, Weintraub AY, Mauer-Perry N, et al. Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Eur J Obstet Gynecol Reprod Biol. 2016;200:35-39.
- Gregory WT, Sibai BM. Obstetrics and pelvic floor disorders. In: Walters M, Karram M, eds. Urogynecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Saunders; 2015:224-237.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Woodley SJ, Lawrenson P, Boyle R, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;6:CD007471.
- Foldspang A, Hvidman L, Mommsen S, et al. Risk of postpartum urinary incontinence associated with pregnancy and mode of delivery. Acta Obstet Gynecol Scand. 2004;83:923-927.
- Wieslander CK, Weinstein MM, Handa V, et al. Pregnancy in women with prior treatments for pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2020;26:299-305.
- Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89:1511-1522.
- MacArthur C, Wilson D, Herbison P, et al; Prolong Study Group. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG. 2016;123:1022-1029.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693-703
- Orkin BA, Sinykin SB, Lloyd PC. The digital rectal examination scoring system (DRESS). Dis Colon Rectum. 2010;53:1656-1660.
- UpToDate. Repair of episiotomy and perineal lacerations associated with childbirth. 2020. https://www-uptodate-com .ccmain.ohionet.org/contents/repair-of-perineal-and-other -lacerations-associated-with-childbirth?search=repair%20 episiotomy&source=search_result&selectedTitle=1~150&usa ge_type=default&display_rank=1. Accessed February 28, 2021.
- Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 198: prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2018;132:e87-e102.
- Jangö H, Langhoff-Roos J, Rosthøj S, et al. Long-term anal incontinence after obstetric anal sphincter injury—does grade of tear matter? Am J Obstet Gynecol. 2018;218:232.e1-232.e10.
- O’Boyle AL, Woodman PJ, O’Boyle JD, et al. Pelvic organ support in nulliparous pregnant and nonpregnant women: a case control study. Am J Obstet Gynecol. 2002;187:99-102.
- Handa VL, Blomquist JL, McDermott KC, et al. Pelvic floor disorders after vaginal birth. Obstet Gynecol. 2012;119 (2, pt 1):233-239.
- Handa VL, Nygaard I, Kenton K, et al; Pelvic Floor Disorders Network. Pelvic organ support among primiparous women in the first year after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1407-1411.
- O’Boyle AL, O’Boyle JD, Calhoun B, et al. Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:69-72.
- Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014;383:796-806.
- Von Bargen E, Haviland MJ, Chang OH, et al. Evaluation of postpartum pelvic floor physical therapy on obstetrical anal sphincter injury: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020. doi: 10.1097/SPV.0000000000000849.
Management of a Child vs an Adult Presenting With Acral Lesions During the COVID-19 Pandemic: A Practical Review
There has been a rise in the prevalence of perniolike lesions—erythematous to violaceous, edematous papules or nodules on the fingers or toes—during the coronavirus disease 2019 (COVID-19) pandemic. These lesions are referred to as “COVID toes.” Although several studies have suggested an association with these lesions and COVID-19, and coronavirus particles have been identified in endothelial cells of biopsies of pernio lesions, questions remain on the management, pathophysiology, and implications of these lesions.1 We provide a practical review for primary care clinicians and dermatologists on the current management, recommendations, and remaining questions, with particular attention to the distinctions for children vs adults presenting with pernio lesions.
Hypothetical Case of a Child Presenting With Acral Lesions
A 7-year-old boy presents with acute-onset, violaceous, mildly painful and pruritic macules on the distal toes that began 3 days earlier and have progressed to involve more toes and appear more purpuric. A review of symptoms reveals no fever, cough, fatigue, or viral symptoms. He has been staying at home for the last few weeks with his brother, mother, and father. His father is working in delivery services and is social distancing at work but not at home. His mother is concerned about the lesions, if they could be COVID toes, and if testing is needed for the patient or family. In your assessment and management of this patient, you consider the following questions.
What Is the Relationship Between These Clinical Findings and COVID-19?
Despite negative polymerase chain reaction (PCR) tests reported in cases of chilblains during the COVID-19 pandemic as well as the possibility that these lesions are an indirect result of environmental factors or behavioral changes during quarantine, the majority of studies favor an association between these chilblains lesions and COVID-19 infection.2,3 Most compellingly, COVID-19 viral particles have been identified by immunohistochemistry and electron microscopy in the endothelial cells of biopsies of these lesions.1 Additionally, there is evidence for possible associations of other viruses, including Epstein-Barr virus and parvovirus B19, with chilblains lesions.4,5 In sum, with the lack of any large prospective study, the weight of current evidence suggests that these perniolike skin lesions are not specific markers of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).6
Published studies differ in reporting the coincidence of perniolike lesions with typical COVID-19 symptoms, including fever, dyspnea, cough, fatigue, myalgia, headache, and anosmia, among others. Some studies have reported that up to 63% of patients with reported perniolike lesions developed typical COVID-19 symptoms, but other studies found that no patients with these lesions developed symptoms.6-11 Studies with younger cohorts tended to report lower prevalence of COVID-19 symptoms, and within cohorts, younger patients tended to have less severe symptoms. For example, 78.8% of patients in a cohort (n=58) with an average age of 14 years did not experience COVID-19–related symptoms.6 Based on these data, it has been hypothesized that patients with chilblainslike lesions may represent a subpopulation who will have a robust interferon response that is protective from more symptomatic and severe COVID-19.12-14
Current evidence suggests that these lesions are most likely to occur between 9 days and 2 months after the onset of COVID-19 symptoms.4,9,10 Most cases have been only mildly symptomatic, with an overall favorable prognosis of both lesions and any viral symptoms.8,10 The lesions typically resolve without treatment within a few days of initial onset.15,16
What Should Be the Workup and Management of These Lesions?
Given the currently available information and favorable prognosis, usually no further workup specific to the perniolike lesions is required in the case of an asymptomatic child presenting with acral lesions, and the majority of management will center around patient and parent/guardian education and reassurance. When asked by the patient’s parent, “What does it mean that my child has these lesions?”, clinicians can provide information on the possible association with COVID-19 and the excellent, self-resolving prognosis. An example of honest and reasonable phrasing with current understanding might be, “We are currently not certain if COVID-19 causes these lesions, although there are data to suggest that they are associated. There are a lot of data showing that children with these lesions either do not have any symptoms or have very mild symptoms that resolve without treatment.”
For management, important considerations include how painful the lesions are to the individual patient and how they affect quality of life. If less severe, clinicians can reassure patients and parents/guardians that the lesions will likely self-resolve without treatment. If worsening or symptomatic, clinicians can try typical treatments for chilblains, such as topical steroids, whole-body warming, and nifedipine.17-19 Obtaining a review of symptoms, including COVID-19 symptoms and general viral symptoms, is important given the rare cases of children with severe COVID-19.20,21
The question of COVID-19 testing as related to these lesions remains controversial, and currently there are still differing perspectives on the need for biopsy, PCR for COVID-19, or serologies for COVID-19 in patients presenting with these lesions. Some experts report that additional testing is not needed in the pediatric population because of the high frequency of negative testing reported to date.22,23 However, these children may be silent carriers, and until more is known about their potential to transmit the virus, testing may be considered if resources allow, particularly if the patient has a known exposure.10,12,16,24 The ultimate decision to pursue biopsy or serologic workup for COVID-19 remains up to clinical discretion with consideration of symptoms, severity, and immunocompromised household contacts. If lesions developed after infection, PCR likely will result negative, whereas serologic testing may reveal antibodies.
Hypothetical Case of an Adult Presenting With Acral Lesions and COVID-19 Symptoms
A 50-year-old man presents with acute-onset, violaceous, painful, edematous plaques on the distal toes that began 3 days earlier and have progressed to include the soles. A review of symptoms reveals fever (temperature, 38.4 °C [101 °F]), cough, dyspnea, diarrhea, and severe asthenia. He has had interactions with a coworker who recently tested positive for COVID-19.
How Should You Consider These Lesions in the Context of the Other Symptoms Concerning for COVID-19?
In contrast to the asymptomatic child above, this adult has chilblainslike lesions and viral symptoms. In adults, chilblainslike lesions have been associated with relatively mild COVID-19, and patients with these lesions who are otherwise asymptomatic have largely tested negative for COVID-19 by PCR and serologic antibody testing.11,25,26
True acral ischemia, which is more severe and should be differentiated from chilblains, has been reported in critically ill patients.9 Additionally, studies have found that retiform purpura is the most common cutaneous finding in patients with severe COVID-19.27 For this patient, who has an examination consistent with progressive and severe chilblainslike lesions and suspicion for COVID-19 infection, it is important to observe and monitor these lesions, as clinical progression suggestive of acral ischemia or retiform purpura should be taken seriously and may indicate worsening of the underlying disease. Early intervention with anticoagulation might be considered, though there currently is no evidence of successful treatment.28
What Causes These Lesions in a Patient With COVID-19?
The underlying pathophysiology has been proposed to be a monocytic-macrophage–induced hyperinflammatory systemic state that damages the lungs, as well as the gastrointestinal, renal, and endothelial systems. The activation of the innate immune system triggers a cytokine storm that creates a hypercoagulable state that ultimately can manifest as superficial thromboses, leading to gangrene of the extremities. Additionally, interferon response and resulting hypercytokinemia may cause direct cytopathic damage to the endothelium of arterioles and capillaries, causing the development of papulovesicular lesions that resemble the chilblainslike lesions observed in children.29 In contrast to children, who typically have no or mild COVID-19 symptoms, adults may have a delayed interferon response, which has been proposed to allow for more severe manifestations of infection.12,30
How Should an Adult With Perniolike Lesions Be Managed?
Adults with chilblainslike lesions and no other signs or symptoms of COVID-19 infection do not necessarily need be tested for COVID-19, given the reports demonstrating most patients in this clinical situation will have negative PCRs and serologies for antibodies. However, there have been several reports of adults with acro-ischemic skin findings who also had severe COVID-19, with an observed incidence of 23% in intensive care unit patients with COVID-19.27,28,31,32 If there is suspicion of infection with COVID-19, it is advisable to first obtain workup for COVID-19 and other viruses that can cause acral lesions, including Epstein-Barr virus and parvovirus. Other pertinent laboratory tests may include D-dimer, fibrinogen, prothrombin time, activated partial thromboplastin time, antithrombin activity, platelet count, neutrophil count, procalcitonin, triglycerides, ferritin, C-reactive protein, and hemoglobin. For patients with evidence of worsening acro-ischemia, regular monitoring of these values up to several times per week can allow for initiation of vascular intervention, including angiontensin-converting enzyme inhibitors, statins, or antiplatelet drugs.32 The presence of antiphospholipid antibodies also has been associated with critically ill patients who develop digit ischemia as part of the sequelae of COVID-19 infection and therefore may act as an important marker for the potential to develop disseminated intravascular coagulation in this patient.33 Even if COVID-19 infection is not suspected, a thorough review of systems is important to look for an underlying connective tissue disease, such as systemic lupus erythematosus, which is associated with pernio. Associated symptoms may warrant workup with antinuclear antibodies and other appropriate autoimmune serologies.
If there is any doubt of the diagnosis, the patient is experiencing symptoms from the lesion, or the patient is experiencing other viral symptoms, it is appropriate to biopsy immediately to confirm the diagnosis. Prior studies have identified fibrin clots, angiocentric and eccrinotropic lymphocytic infiltrates, lymphocytic vasculopathy, and papillary dermal edema as the most common features in chilblainslike lesions during the COVID-19 pandemic.9
For COVID-19 testing, many studies have revealed adult patients with an acute hypercoagulable state testing positive by SARS-CoV-2 PCR. These same patients also experienced thromboembolic events shortly after testing positive for COVID-19, which suggests that patients with elevated D-dimer and fibrinogen likely will have a viral load that is sufficient to test positive for COVID-19.32,34-36 It is appropriate to test all patients with suspected COVID-19, especially adults who are more likely to experience adverse complications secondary to infection.
This patient experiencing COVID-19 symptoms with signs of acral ischemia is likely to test positive by PCR, and additional testing for serologic antibodies is unlikely to be clinically meaningful in this patient’s state. Furthermore, there is little evidence that serology is reliable because of the markedly high levels of both false-negative and false-positive results when using the available antibody testing kits.37 The latter evidence makes serology testing of little value for the general population, but particularly for patients with acute COVID-19.
Conclusion and Outstanding Questions
There is evidence suggesting an association between chilblainslike lesions and COVID-19.11,22,38,39 Children presenting with these lesions have an excellent prognosis and only need a workup or treatment if there are other symptoms, as the lesions self-resolve in the majority of reported cases.7-9 Adults presenting with these lesions and without symptoms likewise are unlikely to test positive for COVID-19, and the lesions typically resolve spontaneously or with first-line treatment. However, adults presenting with these lesions and COVID-19 symptoms should raise clinical concern for evolving skin manifestations of acro-ischemia. If the diagnosis is uncertain or systemic symptoms are concerning, biopsy, COVID-19 PCR, and other appropriate laboratory workup should be obtained.
There remains controversy and uncertainty over the relationship between these skin findings and SARS-CoV-2 infection, with clinical evidence to support both a direct relationship representing convalescent-phase cutaneous reaction as well as an indirect epiphenomenon. If there was a direct relationship, we would have expected to see a rise in the incidence of acral lesions proportionate to the rising caseload of COVID-19 after the reopening of many states in the summer of 2020. Similarly, because young adults represent the largest demographic of increasing cases and as some schools have remained open for in-person instruction during the current academic year, we also would have expected the incidence of chilblains-like lesions presenting to dermatologists and pediatricians to increase alongside these cases. Continued evaluation of emerging literature and ongoing efforts to understand the cause of this observed phenomenon will hopefully help us arrive at a future understanding of the pathophysiology of this puzzling skin manifestation.40
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737. doi:10.1111/bjd.19327
- Neri I, Virdi A, Corsini I, et al. Major cluster of paediatric “true” primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635. doi:10.1111/jdv.16751
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions [letter]. Lancet Infect Dis. June 18, 2020. doi:10.1016/S1473-3099(20)30518-1
- Pistorius MA, Blaise S, Le Hello C, et al. Chilblains and COVID19 infection: causality or coincidence? How to proceed? J Med Vasc. 2020;45:221-223. doi:10.1016/j.jdmv.2020.05.002
- Massey PR, Jones KM. Going viral: a brief history of Chilblain-like skin lesions (“COVID toes”) amidst the COVID-19 pandemic. Semin Oncol. 2020;47:330-334. doi:10.1053/j.seminoncol.2020.05.012
- Docampo-Simón A, Sánchez-Pujol MJ, Juan-Carpena G, et al. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review [letter]. J Eur Acad Dermatol Venereol. 2020;34:e445-e446. doi:10.1111/jdv.16665
- El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:2620-2629. doi:10.1111/jdv.16682
- Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346-e347. doi:10.1111/jdv.16533
- Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406-411. doi:10.1111/pde.14215
- Casas CG, Català A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489-492. doi:10.1016/j.jdcr.2020.04.011
- Damsky W, Peterson D, King B. When interferon tiptoes through COVID-19: pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J Am Acad Dermatol. 2020;83:E269-E270. doi:10.1016/j.jaad.2020.06.052
- Lipsker D. A chilblain epidemic during the COVID-19 pandemic. A sign of natural resistance to SARS-CoV-2? Med Hypotheses. 2020;144:109959. doi:10.1016/j.mehy.2020.109959
- Kaya G, Kaya A, Saurat J-H. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology. 2020;7:3-16. doi:10.3390/dermatopathology7010002
- Pavone P, Marino S, Marino L, et al. Chilblains-like lesions and SARS-CoV-2 in children: An overview in therapeutic approach. Dermatol Ther. 2021;34:E14502. doi:https://doi.org/10.1111/dth.14502
- Dowd PM, Rustin MH, Lanigan S. Nifedipine in the treatment of chilblains. Br Med J (Clin Res Ed). 1986;293:923-924. doi:10.1136/bmj.293.6552.923-a
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol. 1989;120:267-275. doi:10.1111/j.1365-2133.1989.tb07792.x
- Almahameed A, Pinto DS. Pernio (chilblains). Curr Treat Options Cardiovasc Med. 2008;10:128-135. doi:10.1007/s11936-008-0014-0
- Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58:179-182. doi:10.3760/cma.j.issn.0578-1310.2020.03.003
- Choi S-H, Kim HW, Kang J-M, et al. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63:125-132. doi:10.3345/cep.2020.00535
- Piccolo V, Neri I, Manunza F, et al. Chilblain-like lesions during the COVID-19 pandemic: should we really worry? Int J Dermatol. 2020;59:1026-1027. doi:10.1111/ijd.1499
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. JAMA Dermatol. 2020;156:992-997. doi:10.1001/jamadermatol.2020.2340
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998-1003. doi:10.1001/jamadermatol.2020.2368
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897. doi:10.1016/j.jdcr.2020.07.009
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi:10.3760/cma.j.issn.0253-2727.2020.0006
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756. doi:10.1007/s00011-020-01370-w
- Park A, Iwasaki A. Type I and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870-878. doi:10.1016/j.chom.2020.05.008
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:E13549. doi:10.1111/dth.13549
- Alonso MN, Mata-Forte T, García-León N, et al. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag. 2020;16:467-478. doi:10.2147/VHRM.S276530
- Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324-1329. doi:10.1111/jth.14859
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. doi:10.1007/s00134-020-06062-x
- Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725-733. doi:10.1093/ajcp/aqaa062
- Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268-277. doi:10.7326/M20-2003
- Bastos ML, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi:10.1136/bmj.m2516
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of
COVID -19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163 - Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Deutsch A, Blasiak R, Keyes A, et al. COVID toes: phenomenon or epiphenomenon? J Am Acad Dermatol. 2020;83:E347-E348. doi:10.1016/j.jaad.2020.07.037
There has been a rise in the prevalence of perniolike lesions—erythematous to violaceous, edematous papules or nodules on the fingers or toes—during the coronavirus disease 2019 (COVID-19) pandemic. These lesions are referred to as “COVID toes.” Although several studies have suggested an association with these lesions and COVID-19, and coronavirus particles have been identified in endothelial cells of biopsies of pernio lesions, questions remain on the management, pathophysiology, and implications of these lesions.1 We provide a practical review for primary care clinicians and dermatologists on the current management, recommendations, and remaining questions, with particular attention to the distinctions for children vs adults presenting with pernio lesions.
Hypothetical Case of a Child Presenting With Acral Lesions
A 7-year-old boy presents with acute-onset, violaceous, mildly painful and pruritic macules on the distal toes that began 3 days earlier and have progressed to involve more toes and appear more purpuric. A review of symptoms reveals no fever, cough, fatigue, or viral symptoms. He has been staying at home for the last few weeks with his brother, mother, and father. His father is working in delivery services and is social distancing at work but not at home. His mother is concerned about the lesions, if they could be COVID toes, and if testing is needed for the patient or family. In your assessment and management of this patient, you consider the following questions.
What Is the Relationship Between These Clinical Findings and COVID-19?
Despite negative polymerase chain reaction (PCR) tests reported in cases of chilblains during the COVID-19 pandemic as well as the possibility that these lesions are an indirect result of environmental factors or behavioral changes during quarantine, the majority of studies favor an association between these chilblains lesions and COVID-19 infection.2,3 Most compellingly, COVID-19 viral particles have been identified by immunohistochemistry and electron microscopy in the endothelial cells of biopsies of these lesions.1 Additionally, there is evidence for possible associations of other viruses, including Epstein-Barr virus and parvovirus B19, with chilblains lesions.4,5 In sum, with the lack of any large prospective study, the weight of current evidence suggests that these perniolike skin lesions are not specific markers of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).6
Published studies differ in reporting the coincidence of perniolike lesions with typical COVID-19 symptoms, including fever, dyspnea, cough, fatigue, myalgia, headache, and anosmia, among others. Some studies have reported that up to 63% of patients with reported perniolike lesions developed typical COVID-19 symptoms, but other studies found that no patients with these lesions developed symptoms.6-11 Studies with younger cohorts tended to report lower prevalence of COVID-19 symptoms, and within cohorts, younger patients tended to have less severe symptoms. For example, 78.8% of patients in a cohort (n=58) with an average age of 14 years did not experience COVID-19–related symptoms.6 Based on these data, it has been hypothesized that patients with chilblainslike lesions may represent a subpopulation who will have a robust interferon response that is protective from more symptomatic and severe COVID-19.12-14
Current evidence suggests that these lesions are most likely to occur between 9 days and 2 months after the onset of COVID-19 symptoms.4,9,10 Most cases have been only mildly symptomatic, with an overall favorable prognosis of both lesions and any viral symptoms.8,10 The lesions typically resolve without treatment within a few days of initial onset.15,16
What Should Be the Workup and Management of These Lesions?
Given the currently available information and favorable prognosis, usually no further workup specific to the perniolike lesions is required in the case of an asymptomatic child presenting with acral lesions, and the majority of management will center around patient and parent/guardian education and reassurance. When asked by the patient’s parent, “What does it mean that my child has these lesions?”, clinicians can provide information on the possible association with COVID-19 and the excellent, self-resolving prognosis. An example of honest and reasonable phrasing with current understanding might be, “We are currently not certain if COVID-19 causes these lesions, although there are data to suggest that they are associated. There are a lot of data showing that children with these lesions either do not have any symptoms or have very mild symptoms that resolve without treatment.”
For management, important considerations include how painful the lesions are to the individual patient and how they affect quality of life. If less severe, clinicians can reassure patients and parents/guardians that the lesions will likely self-resolve without treatment. If worsening or symptomatic, clinicians can try typical treatments for chilblains, such as topical steroids, whole-body warming, and nifedipine.17-19 Obtaining a review of symptoms, including COVID-19 symptoms and general viral symptoms, is important given the rare cases of children with severe COVID-19.20,21
The question of COVID-19 testing as related to these lesions remains controversial, and currently there are still differing perspectives on the need for biopsy, PCR for COVID-19, or serologies for COVID-19 in patients presenting with these lesions. Some experts report that additional testing is not needed in the pediatric population because of the high frequency of negative testing reported to date.22,23 However, these children may be silent carriers, and until more is known about their potential to transmit the virus, testing may be considered if resources allow, particularly if the patient has a known exposure.10,12,16,24 The ultimate decision to pursue biopsy or serologic workup for COVID-19 remains up to clinical discretion with consideration of symptoms, severity, and immunocompromised household contacts. If lesions developed after infection, PCR likely will result negative, whereas serologic testing may reveal antibodies.
Hypothetical Case of an Adult Presenting With Acral Lesions and COVID-19 Symptoms
A 50-year-old man presents with acute-onset, violaceous, painful, edematous plaques on the distal toes that began 3 days earlier and have progressed to include the soles. A review of symptoms reveals fever (temperature, 38.4 °C [101 °F]), cough, dyspnea, diarrhea, and severe asthenia. He has had interactions with a coworker who recently tested positive for COVID-19.
How Should You Consider These Lesions in the Context of the Other Symptoms Concerning for COVID-19?
In contrast to the asymptomatic child above, this adult has chilblainslike lesions and viral symptoms. In adults, chilblainslike lesions have been associated with relatively mild COVID-19, and patients with these lesions who are otherwise asymptomatic have largely tested negative for COVID-19 by PCR and serologic antibody testing.11,25,26
True acral ischemia, which is more severe and should be differentiated from chilblains, has been reported in critically ill patients.9 Additionally, studies have found that retiform purpura is the most common cutaneous finding in patients with severe COVID-19.27 For this patient, who has an examination consistent with progressive and severe chilblainslike lesions and suspicion for COVID-19 infection, it is important to observe and monitor these lesions, as clinical progression suggestive of acral ischemia or retiform purpura should be taken seriously and may indicate worsening of the underlying disease. Early intervention with anticoagulation might be considered, though there currently is no evidence of successful treatment.28
What Causes These Lesions in a Patient With COVID-19?
The underlying pathophysiology has been proposed to be a monocytic-macrophage–induced hyperinflammatory systemic state that damages the lungs, as well as the gastrointestinal, renal, and endothelial systems. The activation of the innate immune system triggers a cytokine storm that creates a hypercoagulable state that ultimately can manifest as superficial thromboses, leading to gangrene of the extremities. Additionally, interferon response and resulting hypercytokinemia may cause direct cytopathic damage to the endothelium of arterioles and capillaries, causing the development of papulovesicular lesions that resemble the chilblainslike lesions observed in children.29 In contrast to children, who typically have no or mild COVID-19 symptoms, adults may have a delayed interferon response, which has been proposed to allow for more severe manifestations of infection.12,30
How Should an Adult With Perniolike Lesions Be Managed?
Adults with chilblainslike lesions and no other signs or symptoms of COVID-19 infection do not necessarily need be tested for COVID-19, given the reports demonstrating most patients in this clinical situation will have negative PCRs and serologies for antibodies. However, there have been several reports of adults with acro-ischemic skin findings who also had severe COVID-19, with an observed incidence of 23% in intensive care unit patients with COVID-19.27,28,31,32 If there is suspicion of infection with COVID-19, it is advisable to first obtain workup for COVID-19 and other viruses that can cause acral lesions, including Epstein-Barr virus and parvovirus. Other pertinent laboratory tests may include D-dimer, fibrinogen, prothrombin time, activated partial thromboplastin time, antithrombin activity, platelet count, neutrophil count, procalcitonin, triglycerides, ferritin, C-reactive protein, and hemoglobin. For patients with evidence of worsening acro-ischemia, regular monitoring of these values up to several times per week can allow for initiation of vascular intervention, including angiontensin-converting enzyme inhibitors, statins, or antiplatelet drugs.32 The presence of antiphospholipid antibodies also has been associated with critically ill patients who develop digit ischemia as part of the sequelae of COVID-19 infection and therefore may act as an important marker for the potential to develop disseminated intravascular coagulation in this patient.33 Even if COVID-19 infection is not suspected, a thorough review of systems is important to look for an underlying connective tissue disease, such as systemic lupus erythematosus, which is associated with pernio. Associated symptoms may warrant workup with antinuclear antibodies and other appropriate autoimmune serologies.
If there is any doubt of the diagnosis, the patient is experiencing symptoms from the lesion, or the patient is experiencing other viral symptoms, it is appropriate to biopsy immediately to confirm the diagnosis. Prior studies have identified fibrin clots, angiocentric and eccrinotropic lymphocytic infiltrates, lymphocytic vasculopathy, and papillary dermal edema as the most common features in chilblainslike lesions during the COVID-19 pandemic.9
For COVID-19 testing, many studies have revealed adult patients with an acute hypercoagulable state testing positive by SARS-CoV-2 PCR. These same patients also experienced thromboembolic events shortly after testing positive for COVID-19, which suggests that patients with elevated D-dimer and fibrinogen likely will have a viral load that is sufficient to test positive for COVID-19.32,34-36 It is appropriate to test all patients with suspected COVID-19, especially adults who are more likely to experience adverse complications secondary to infection.
This patient experiencing COVID-19 symptoms with signs of acral ischemia is likely to test positive by PCR, and additional testing for serologic antibodies is unlikely to be clinically meaningful in this patient’s state. Furthermore, there is little evidence that serology is reliable because of the markedly high levels of both false-negative and false-positive results when using the available antibody testing kits.37 The latter evidence makes serology testing of little value for the general population, but particularly for patients with acute COVID-19.
Conclusion and Outstanding Questions
There is evidence suggesting an association between chilblainslike lesions and COVID-19.11,22,38,39 Children presenting with these lesions have an excellent prognosis and only need a workup or treatment if there are other symptoms, as the lesions self-resolve in the majority of reported cases.7-9 Adults presenting with these lesions and without symptoms likewise are unlikely to test positive for COVID-19, and the lesions typically resolve spontaneously or with first-line treatment. However, adults presenting with these lesions and COVID-19 symptoms should raise clinical concern for evolving skin manifestations of acro-ischemia. If the diagnosis is uncertain or systemic symptoms are concerning, biopsy, COVID-19 PCR, and other appropriate laboratory workup should be obtained.
There remains controversy and uncertainty over the relationship between these skin findings and SARS-CoV-2 infection, with clinical evidence to support both a direct relationship representing convalescent-phase cutaneous reaction as well as an indirect epiphenomenon. If there was a direct relationship, we would have expected to see a rise in the incidence of acral lesions proportionate to the rising caseload of COVID-19 after the reopening of many states in the summer of 2020. Similarly, because young adults represent the largest demographic of increasing cases and as some schools have remained open for in-person instruction during the current academic year, we also would have expected the incidence of chilblains-like lesions presenting to dermatologists and pediatricians to increase alongside these cases. Continued evaluation of emerging literature and ongoing efforts to understand the cause of this observed phenomenon will hopefully help us arrive at a future understanding of the pathophysiology of this puzzling skin manifestation.40
There has been a rise in the prevalence of perniolike lesions—erythematous to violaceous, edematous papules or nodules on the fingers or toes—during the coronavirus disease 2019 (COVID-19) pandemic. These lesions are referred to as “COVID toes.” Although several studies have suggested an association with these lesions and COVID-19, and coronavirus particles have been identified in endothelial cells of biopsies of pernio lesions, questions remain on the management, pathophysiology, and implications of these lesions.1 We provide a practical review for primary care clinicians and dermatologists on the current management, recommendations, and remaining questions, with particular attention to the distinctions for children vs adults presenting with pernio lesions.
Hypothetical Case of a Child Presenting With Acral Lesions
A 7-year-old boy presents with acute-onset, violaceous, mildly painful and pruritic macules on the distal toes that began 3 days earlier and have progressed to involve more toes and appear more purpuric. A review of symptoms reveals no fever, cough, fatigue, or viral symptoms. He has been staying at home for the last few weeks with his brother, mother, and father. His father is working in delivery services and is social distancing at work but not at home. His mother is concerned about the lesions, if they could be COVID toes, and if testing is needed for the patient or family. In your assessment and management of this patient, you consider the following questions.
What Is the Relationship Between These Clinical Findings and COVID-19?
Despite negative polymerase chain reaction (PCR) tests reported in cases of chilblains during the COVID-19 pandemic as well as the possibility that these lesions are an indirect result of environmental factors or behavioral changes during quarantine, the majority of studies favor an association between these chilblains lesions and COVID-19 infection.2,3 Most compellingly, COVID-19 viral particles have been identified by immunohistochemistry and electron microscopy in the endothelial cells of biopsies of these lesions.1 Additionally, there is evidence for possible associations of other viruses, including Epstein-Barr virus and parvovirus B19, with chilblains lesions.4,5 In sum, with the lack of any large prospective study, the weight of current evidence suggests that these perniolike skin lesions are not specific markers of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).6
Published studies differ in reporting the coincidence of perniolike lesions with typical COVID-19 symptoms, including fever, dyspnea, cough, fatigue, myalgia, headache, and anosmia, among others. Some studies have reported that up to 63% of patients with reported perniolike lesions developed typical COVID-19 symptoms, but other studies found that no patients with these lesions developed symptoms.6-11 Studies with younger cohorts tended to report lower prevalence of COVID-19 symptoms, and within cohorts, younger patients tended to have less severe symptoms. For example, 78.8% of patients in a cohort (n=58) with an average age of 14 years did not experience COVID-19–related symptoms.6 Based on these data, it has been hypothesized that patients with chilblainslike lesions may represent a subpopulation who will have a robust interferon response that is protective from more symptomatic and severe COVID-19.12-14
Current evidence suggests that these lesions are most likely to occur between 9 days and 2 months after the onset of COVID-19 symptoms.4,9,10 Most cases have been only mildly symptomatic, with an overall favorable prognosis of both lesions and any viral symptoms.8,10 The lesions typically resolve without treatment within a few days of initial onset.15,16
What Should Be the Workup and Management of These Lesions?
Given the currently available information and favorable prognosis, usually no further workup specific to the perniolike lesions is required in the case of an asymptomatic child presenting with acral lesions, and the majority of management will center around patient and parent/guardian education and reassurance. When asked by the patient’s parent, “What does it mean that my child has these lesions?”, clinicians can provide information on the possible association with COVID-19 and the excellent, self-resolving prognosis. An example of honest and reasonable phrasing with current understanding might be, “We are currently not certain if COVID-19 causes these lesions, although there are data to suggest that they are associated. There are a lot of data showing that children with these lesions either do not have any symptoms or have very mild symptoms that resolve without treatment.”
For management, important considerations include how painful the lesions are to the individual patient and how they affect quality of life. If less severe, clinicians can reassure patients and parents/guardians that the lesions will likely self-resolve without treatment. If worsening or symptomatic, clinicians can try typical treatments for chilblains, such as topical steroids, whole-body warming, and nifedipine.17-19 Obtaining a review of symptoms, including COVID-19 symptoms and general viral symptoms, is important given the rare cases of children with severe COVID-19.20,21
The question of COVID-19 testing as related to these lesions remains controversial, and currently there are still differing perspectives on the need for biopsy, PCR for COVID-19, or serologies for COVID-19 in patients presenting with these lesions. Some experts report that additional testing is not needed in the pediatric population because of the high frequency of negative testing reported to date.22,23 However, these children may be silent carriers, and until more is known about their potential to transmit the virus, testing may be considered if resources allow, particularly if the patient has a known exposure.10,12,16,24 The ultimate decision to pursue biopsy or serologic workup for COVID-19 remains up to clinical discretion with consideration of symptoms, severity, and immunocompromised household contacts. If lesions developed after infection, PCR likely will result negative, whereas serologic testing may reveal antibodies.
Hypothetical Case of an Adult Presenting With Acral Lesions and COVID-19 Symptoms
A 50-year-old man presents with acute-onset, violaceous, painful, edematous plaques on the distal toes that began 3 days earlier and have progressed to include the soles. A review of symptoms reveals fever (temperature, 38.4 °C [101 °F]), cough, dyspnea, diarrhea, and severe asthenia. He has had interactions with a coworker who recently tested positive for COVID-19.
How Should You Consider These Lesions in the Context of the Other Symptoms Concerning for COVID-19?
In contrast to the asymptomatic child above, this adult has chilblainslike lesions and viral symptoms. In adults, chilblainslike lesions have been associated with relatively mild COVID-19, and patients with these lesions who are otherwise asymptomatic have largely tested negative for COVID-19 by PCR and serologic antibody testing.11,25,26
True acral ischemia, which is more severe and should be differentiated from chilblains, has been reported in critically ill patients.9 Additionally, studies have found that retiform purpura is the most common cutaneous finding in patients with severe COVID-19.27 For this patient, who has an examination consistent with progressive and severe chilblainslike lesions and suspicion for COVID-19 infection, it is important to observe and monitor these lesions, as clinical progression suggestive of acral ischemia or retiform purpura should be taken seriously and may indicate worsening of the underlying disease. Early intervention with anticoagulation might be considered, though there currently is no evidence of successful treatment.28
What Causes These Lesions in a Patient With COVID-19?
The underlying pathophysiology has been proposed to be a monocytic-macrophage–induced hyperinflammatory systemic state that damages the lungs, as well as the gastrointestinal, renal, and endothelial systems. The activation of the innate immune system triggers a cytokine storm that creates a hypercoagulable state that ultimately can manifest as superficial thromboses, leading to gangrene of the extremities. Additionally, interferon response and resulting hypercytokinemia may cause direct cytopathic damage to the endothelium of arterioles and capillaries, causing the development of papulovesicular lesions that resemble the chilblainslike lesions observed in children.29 In contrast to children, who typically have no or mild COVID-19 symptoms, adults may have a delayed interferon response, which has been proposed to allow for more severe manifestations of infection.12,30
How Should an Adult With Perniolike Lesions Be Managed?
Adults with chilblainslike lesions and no other signs or symptoms of COVID-19 infection do not necessarily need be tested for COVID-19, given the reports demonstrating most patients in this clinical situation will have negative PCRs and serologies for antibodies. However, there have been several reports of adults with acro-ischemic skin findings who also had severe COVID-19, with an observed incidence of 23% in intensive care unit patients with COVID-19.27,28,31,32 If there is suspicion of infection with COVID-19, it is advisable to first obtain workup for COVID-19 and other viruses that can cause acral lesions, including Epstein-Barr virus and parvovirus. Other pertinent laboratory tests may include D-dimer, fibrinogen, prothrombin time, activated partial thromboplastin time, antithrombin activity, platelet count, neutrophil count, procalcitonin, triglycerides, ferritin, C-reactive protein, and hemoglobin. For patients with evidence of worsening acro-ischemia, regular monitoring of these values up to several times per week can allow for initiation of vascular intervention, including angiontensin-converting enzyme inhibitors, statins, or antiplatelet drugs.32 The presence of antiphospholipid antibodies also has been associated with critically ill patients who develop digit ischemia as part of the sequelae of COVID-19 infection and therefore may act as an important marker for the potential to develop disseminated intravascular coagulation in this patient.33 Even if COVID-19 infection is not suspected, a thorough review of systems is important to look for an underlying connective tissue disease, such as systemic lupus erythematosus, which is associated with pernio. Associated symptoms may warrant workup with antinuclear antibodies and other appropriate autoimmune serologies.
If there is any doubt of the diagnosis, the patient is experiencing symptoms from the lesion, or the patient is experiencing other viral symptoms, it is appropriate to biopsy immediately to confirm the diagnosis. Prior studies have identified fibrin clots, angiocentric and eccrinotropic lymphocytic infiltrates, lymphocytic vasculopathy, and papillary dermal edema as the most common features in chilblainslike lesions during the COVID-19 pandemic.9
For COVID-19 testing, many studies have revealed adult patients with an acute hypercoagulable state testing positive by SARS-CoV-2 PCR. These same patients also experienced thromboembolic events shortly after testing positive for COVID-19, which suggests that patients with elevated D-dimer and fibrinogen likely will have a viral load that is sufficient to test positive for COVID-19.32,34-36 It is appropriate to test all patients with suspected COVID-19, especially adults who are more likely to experience adverse complications secondary to infection.
This patient experiencing COVID-19 symptoms with signs of acral ischemia is likely to test positive by PCR, and additional testing for serologic antibodies is unlikely to be clinically meaningful in this patient’s state. Furthermore, there is little evidence that serology is reliable because of the markedly high levels of both false-negative and false-positive results when using the available antibody testing kits.37 The latter evidence makes serology testing of little value for the general population, but particularly for patients with acute COVID-19.
Conclusion and Outstanding Questions
There is evidence suggesting an association between chilblainslike lesions and COVID-19.11,22,38,39 Children presenting with these lesions have an excellent prognosis and only need a workup or treatment if there are other symptoms, as the lesions self-resolve in the majority of reported cases.7-9 Adults presenting with these lesions and without symptoms likewise are unlikely to test positive for COVID-19, and the lesions typically resolve spontaneously or with first-line treatment. However, adults presenting with these lesions and COVID-19 symptoms should raise clinical concern for evolving skin manifestations of acro-ischemia. If the diagnosis is uncertain or systemic symptoms are concerning, biopsy, COVID-19 PCR, and other appropriate laboratory workup should be obtained.
There remains controversy and uncertainty over the relationship between these skin findings and SARS-CoV-2 infection, with clinical evidence to support both a direct relationship representing convalescent-phase cutaneous reaction as well as an indirect epiphenomenon. If there was a direct relationship, we would have expected to see a rise in the incidence of acral lesions proportionate to the rising caseload of COVID-19 after the reopening of many states in the summer of 2020. Similarly, because young adults represent the largest demographic of increasing cases and as some schools have remained open for in-person instruction during the current academic year, we also would have expected the incidence of chilblains-like lesions presenting to dermatologists and pediatricians to increase alongside these cases. Continued evaluation of emerging literature and ongoing efforts to understand the cause of this observed phenomenon will hopefully help us arrive at a future understanding of the pathophysiology of this puzzling skin manifestation.40
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737. doi:10.1111/bjd.19327
- Neri I, Virdi A, Corsini I, et al. Major cluster of paediatric “true” primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635. doi:10.1111/jdv.16751
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions [letter]. Lancet Infect Dis. June 18, 2020. doi:10.1016/S1473-3099(20)30518-1
- Pistorius MA, Blaise S, Le Hello C, et al. Chilblains and COVID19 infection: causality or coincidence? How to proceed? J Med Vasc. 2020;45:221-223. doi:10.1016/j.jdmv.2020.05.002
- Massey PR, Jones KM. Going viral: a brief history of Chilblain-like skin lesions (“COVID toes”) amidst the COVID-19 pandemic. Semin Oncol. 2020;47:330-334. doi:10.1053/j.seminoncol.2020.05.012
- Docampo-Simón A, Sánchez-Pujol MJ, Juan-Carpena G, et al. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review [letter]. J Eur Acad Dermatol Venereol. 2020;34:e445-e446. doi:10.1111/jdv.16665
- El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:2620-2629. doi:10.1111/jdv.16682
- Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346-e347. doi:10.1111/jdv.16533
- Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406-411. doi:10.1111/pde.14215
- Casas CG, Català A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489-492. doi:10.1016/j.jdcr.2020.04.011
- Damsky W, Peterson D, King B. When interferon tiptoes through COVID-19: pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J Am Acad Dermatol. 2020;83:E269-E270. doi:10.1016/j.jaad.2020.06.052
- Lipsker D. A chilblain epidemic during the COVID-19 pandemic. A sign of natural resistance to SARS-CoV-2? Med Hypotheses. 2020;144:109959. doi:10.1016/j.mehy.2020.109959
- Kaya G, Kaya A, Saurat J-H. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology. 2020;7:3-16. doi:10.3390/dermatopathology7010002
- Pavone P, Marino S, Marino L, et al. Chilblains-like lesions and SARS-CoV-2 in children: An overview in therapeutic approach. Dermatol Ther. 2021;34:E14502. doi:https://doi.org/10.1111/dth.14502
- Dowd PM, Rustin MH, Lanigan S. Nifedipine in the treatment of chilblains. Br Med J (Clin Res Ed). 1986;293:923-924. doi:10.1136/bmj.293.6552.923-a
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol. 1989;120:267-275. doi:10.1111/j.1365-2133.1989.tb07792.x
- Almahameed A, Pinto DS. Pernio (chilblains). Curr Treat Options Cardiovasc Med. 2008;10:128-135. doi:10.1007/s11936-008-0014-0
- Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58:179-182. doi:10.3760/cma.j.issn.0578-1310.2020.03.003
- Choi S-H, Kim HW, Kang J-M, et al. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63:125-132. doi:10.3345/cep.2020.00535
- Piccolo V, Neri I, Manunza F, et al. Chilblain-like lesions during the COVID-19 pandemic: should we really worry? Int J Dermatol. 2020;59:1026-1027. doi:10.1111/ijd.1499
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. JAMA Dermatol. 2020;156:992-997. doi:10.1001/jamadermatol.2020.2340
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998-1003. doi:10.1001/jamadermatol.2020.2368
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897. doi:10.1016/j.jdcr.2020.07.009
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi:10.3760/cma.j.issn.0253-2727.2020.0006
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756. doi:10.1007/s00011-020-01370-w
- Park A, Iwasaki A. Type I and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870-878. doi:10.1016/j.chom.2020.05.008
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:E13549. doi:10.1111/dth.13549
- Alonso MN, Mata-Forte T, García-León N, et al. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag. 2020;16:467-478. doi:10.2147/VHRM.S276530
- Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324-1329. doi:10.1111/jth.14859
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. doi:10.1007/s00134-020-06062-x
- Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725-733. doi:10.1093/ajcp/aqaa062
- Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268-277. doi:10.7326/M20-2003
- Bastos ML, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi:10.1136/bmj.m2516
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of
COVID -19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163 - Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Deutsch A, Blasiak R, Keyes A, et al. COVID toes: phenomenon or epiphenomenon? J Am Acad Dermatol. 2020;83:E347-E348. doi:10.1016/j.jaad.2020.07.037
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737. doi:10.1111/bjd.19327
- Neri I, Virdi A, Corsini I, et al. Major cluster of paediatric “true” primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635. doi:10.1111/jdv.16751
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions [letter]. Lancet Infect Dis. June 18, 2020. doi:10.1016/S1473-3099(20)30518-1
- Pistorius MA, Blaise S, Le Hello C, et al. Chilblains and COVID19 infection: causality or coincidence? How to proceed? J Med Vasc. 2020;45:221-223. doi:10.1016/j.jdmv.2020.05.002
- Massey PR, Jones KM. Going viral: a brief history of Chilblain-like skin lesions (“COVID toes”) amidst the COVID-19 pandemic. Semin Oncol. 2020;47:330-334. doi:10.1053/j.seminoncol.2020.05.012
- Docampo-Simón A, Sánchez-Pujol MJ, Juan-Carpena G, et al. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review [letter]. J Eur Acad Dermatol Venereol. 2020;34:e445-e446. doi:10.1111/jdv.16665
- El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:2620-2629. doi:10.1111/jdv.16682
- Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346-e347. doi:10.1111/jdv.16533
- Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406-411. doi:10.1111/pde.14215
- Casas CG, Català A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489-492. doi:10.1016/j.jdcr.2020.04.011
- Damsky W, Peterson D, King B. When interferon tiptoes through COVID-19: pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J Am Acad Dermatol. 2020;83:E269-E270. doi:10.1016/j.jaad.2020.06.052
- Lipsker D. A chilblain epidemic during the COVID-19 pandemic. A sign of natural resistance to SARS-CoV-2? Med Hypotheses. 2020;144:109959. doi:10.1016/j.mehy.2020.109959
- Kaya G, Kaya A, Saurat J-H. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology. 2020;7:3-16. doi:10.3390/dermatopathology7010002
- Pavone P, Marino S, Marino L, et al. Chilblains-like lesions and SARS-CoV-2 in children: An overview in therapeutic approach. Dermatol Ther. 2021;34:E14502. doi:https://doi.org/10.1111/dth.14502
- Dowd PM, Rustin MH, Lanigan S. Nifedipine in the treatment of chilblains. Br Med J (Clin Res Ed). 1986;293:923-924. doi:10.1136/bmj.293.6552.923-a
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol. 1989;120:267-275. doi:10.1111/j.1365-2133.1989.tb07792.x
- Almahameed A, Pinto DS. Pernio (chilblains). Curr Treat Options Cardiovasc Med. 2008;10:128-135. doi:10.1007/s11936-008-0014-0
- Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58:179-182. doi:10.3760/cma.j.issn.0578-1310.2020.03.003
- Choi S-H, Kim HW, Kang J-M, et al. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63:125-132. doi:10.3345/cep.2020.00535
- Piccolo V, Neri I, Manunza F, et al. Chilblain-like lesions during the COVID-19 pandemic: should we really worry? Int J Dermatol. 2020;59:1026-1027. doi:10.1111/ijd.1499
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. JAMA Dermatol. 2020;156:992-997. doi:10.1001/jamadermatol.2020.2340
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998-1003. doi:10.1001/jamadermatol.2020.2368
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897. doi:10.1016/j.jdcr.2020.07.009
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi:10.3760/cma.j.issn.0253-2727.2020.0006
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756. doi:10.1007/s00011-020-01370-w
- Park A, Iwasaki A. Type I and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870-878. doi:10.1016/j.chom.2020.05.008
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:E13549. doi:10.1111/dth.13549
- Alonso MN, Mata-Forte T, García-León N, et al. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag. 2020;16:467-478. doi:10.2147/VHRM.S276530
- Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324-1329. doi:10.1111/jth.14859
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. doi:10.1007/s00134-020-06062-x
- Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725-733. doi:10.1093/ajcp/aqaa062
- Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268-277. doi:10.7326/M20-2003
- Bastos ML, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi:10.1136/bmj.m2516
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of
COVID -19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163 - Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Deutsch A, Blasiak R, Keyes A, et al. COVID toes: phenomenon or epiphenomenon? J Am Acad Dermatol. 2020;83:E347-E348. doi:10.1016/j.jaad.2020.07.037
Practice Points
- Children with chilblainslike lesions generally have a favorable prognosis. As lesions self-resolve, treatment should focus on symptom management and education.
- In children with chilblainslike lesions and no systemic symptoms, further workup for coronavirus disease 2019 (COVID-19) is not necessary for the care of the individual patient.
- In adults with acral lesions, it is important to distinguish between chilblainslike lesions, true acral ischemia, and retiform purpura. Chilblainslike lesions have been associated with mild COVID-19 disease, whereas acral ischemia and retiform purpura have been associated with severe and fatal disease.
- Biopsy and COVID-19 testing should be obtained in adults if there is diagnostic uncertainty or if there are worsening symptoms.
Comparison of Shave and Punch Biopsy Utilization Among Dermatology Practices
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
Practice Points
- Dermatologists should be aware that skin biopsy billing codes and reimbursements were changed in 2019 to reflect their level of complexity, which may impact how often each type of biopsy is used.
- Even small shifts in biopsy utilization behavior among dermatologists in the context of reimbursement changes can have a large impact on net reimbursements.
Upper Lip Anatomy, Mechanics of Local Flaps, and Considerations for Reconstruction
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.
Vibrio vulnificus: Review of Mild to Life-threatening Skin Infections
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
Practice Points
- Vibrio vulnificus infection should be high on the differential for patients who present with chronic liver disease and immunosuppression; a history of raw seafood consumption or exposure to brackish water; and bullae, cellulitis, necrotic lesions, or sepsis.
- Time to treatment is directly proportional to mortality rates in V vulnificus infections, and prompt treatment with antibiotics, wound care, debridement, and supportive measures is necessary to decrease mortality rates.
- The incidence of V vulnificus infection is rising in the United States, likely due to a combination of factors, including an aging population with multiple comorbidities, improvements in diagnosis, and climate change.